Organizing Your Social Sciences Research Assignments
- Annotated Bibliography
- Analyzing a Scholarly Journal Article
- Group Presentations
- Dealing with Nervousness
- Using Visual Aids
- Grading Someone Else's Paper
- Types of Structured Group Activities
- Group Project Survival Skills
- Leading a Class Discussion
- Multiple Book Review Essay
- Reviewing Collected Works
- Writing a Case Analysis Paper
- Writing a Case Study
- About Informed Consent
- Writing Field Notes
- Writing a Policy Memo
- Writing a Reflective Paper
- Writing a Research Proposal
- Generative AI and Writing
- Acknowledgments
The goal of a research proposal is twofold: to present and justify the need to study a research problem and to present the practical ways in which the proposed study should be conducted. The design elements and procedures for conducting research are governed by standards of the predominant discipline in which the problem resides, therefore, the guidelines for research proposals are more exacting and less formal than a general project proposal. Research proposals contain extensive literature reviews. They must provide persuasive evidence that a need exists for the proposed study. In addition to providing a rationale, a proposal describes detailed methodology for conducting the research consistent with requirements of the professional or academic field and a statement on anticipated outcomes and benefits derived from the study's completion.
Krathwohl, David R. How to Prepare a Dissertation Proposal: Suggestions for Students in Education and the Social and Behavioral Sciences . Syracuse, NY: Syracuse University Press, 2005.

How to Approach Writing a Research Proposal
Your professor may assign the task of writing a research proposal for the following reasons:
- Develop your skills in thinking about and designing a comprehensive research study;
- Learn how to conduct a comprehensive review of the literature to determine that the research problem has not been adequately addressed or has been answered ineffectively and, in so doing, become better at locating pertinent scholarship related to your topic;
- Improve your general research and writing skills;
- Practice identifying the logical steps that must be taken to accomplish one's research goals;
- Critically review, examine, and consider the use of different methods for gathering and analyzing data related to the research problem; and,
- Nurture a sense of inquisitiveness within yourself and to help see yourself as an active participant in the process of conducting scholarly research.
A proposal should contain all the key elements involved in designing a completed research study, with sufficient information that allows readers to assess the validity and usefulness of your proposed study. The only elements missing from a research proposal are the findings of the study and your analysis of those findings. Finally, an effective proposal is judged on the quality of your writing and, therefore, it is important that your proposal is coherent, clear, and compelling.
Regardless of the research problem you are investigating and the methodology you choose, all research proposals must address the following questions:
- What do you plan to accomplish? Be clear and succinct in defining the research problem and what it is you are proposing to investigate.
- Why do you want to do the research? In addition to detailing your research design, you also must conduct a thorough review of the literature and provide convincing evidence that it is a topic worthy of in-depth study. A successful research proposal must answer the "So What?" question.
- How are you going to conduct the research? Be sure that what you propose is doable. If you're having difficulty formulating a research problem to propose investigating, go here for strategies in developing a problem to study.
Common Mistakes to Avoid
- Failure to be concise . A research proposal must be focused and not be "all over the map" or diverge into unrelated tangents without a clear sense of purpose.
- Failure to cite landmark works in your literature review . Proposals should be grounded in foundational research that lays a foundation for understanding the development and scope of the the topic and its relevance.
- Failure to delimit the contextual scope of your research [e.g., time, place, people, etc.]. As with any research paper, your proposed study must inform the reader how and in what ways the study will frame the problem.
- Failure to develop a coherent and persuasive argument for the proposed research . This is critical. In many workplace settings, the research proposal is a formal document intended to argue for why a study should be funded.
- Sloppy or imprecise writing, or poor grammar . Although a research proposal does not represent a completed research study, there is still an expectation that it is well-written and follows the style and rules of good academic writing.
- Too much detail on minor issues, but not enough detail on major issues . Your proposal should focus on only a few key research questions in order to support the argument that the research needs to be conducted. Minor issues, even if valid, can be mentioned but they should not dominate the overall narrative.
Procter, Margaret. The Academic Proposal. The Lab Report. University College Writing Centre. University of Toronto; Sanford, Keith. Information for Students: Writing a Research Proposal. Baylor University; Wong, Paul T. P. How to Write a Research Proposal. International Network on Personal Meaning. Trinity Western University; Writing Academic Proposals: Conferences, Articles, and Books. The Writing Lab and The OWL. Purdue University; Writing a Research Proposal. University Library. University of Illinois at Urbana-Champaign.
Structure and Writing Style
Beginning the Proposal Process
As with writing most college-level academic papers, research proposals are generally organized the same way throughout most social science disciplines. The text of proposals generally vary in length between ten and thirty-five pages, followed by the list of references. However, before you begin, read the assignment carefully and, if anything seems unclear, ask your professor whether there are any specific requirements for organizing and writing the proposal.
A good place to begin is to ask yourself a series of questions:
- What do I want to study?
- Why is the topic important?
- How is it significant within the subject areas covered in my class?
- What problems will it help solve?
- How does it build upon [and hopefully go beyond] research already conducted on the topic?
- What exactly should I plan to do, and can I get it done in the time available?
In general, a compelling research proposal should document your knowledge of the topic and demonstrate your enthusiasm for conducting the study. Approach it with the intention of leaving your readers feeling like, "Wow, that's an exciting idea and I can’t wait to see how it turns out!"
Most proposals should include the following sections:
I. Introduction
In the real world of higher education, a research proposal is most often written by scholars seeking grant funding for a research project or it's the first step in getting approval to write a doctoral dissertation. Even if this is just a course assignment, treat your introduction as the initial pitch of an idea based on a thorough examination of the significance of a research problem. After reading the introduction, your readers should not only have an understanding of what you want to do, but they should also be able to gain a sense of your passion for the topic and to be excited about the study's possible outcomes. Note that most proposals do not include an abstract [summary] before the introduction.
Think about your introduction as a narrative written in two to four paragraphs that succinctly answers the following four questions :
- What is the central research problem?
- What is the topic of study related to that research problem?
- What methods should be used to analyze the research problem?
- Answer the "So What?" question by explaining why this is important research, what is its significance, and why should someone reading the proposal care about the outcomes of the proposed study?
II. Background and Significance
This is where you explain the scope and context of your proposal and describe in detail why it's important. It can be melded into your introduction or you can create a separate section to help with the organization and narrative flow of your proposal. Approach writing this section with the thought that you can’t assume your readers will know as much about the research problem as you do. Note that this section is not an essay going over everything you have learned about the topic; instead, you must choose what is most relevant in explaining the aims of your research.
To that end, while there are no prescribed rules for establishing the significance of your proposed study, you should attempt to address some or all of the following:
- State the research problem and give a more detailed explanation about the purpose of the study than what you stated in the introduction. This is particularly important if the problem is complex or multifaceted .
- Present the rationale of your proposed study and clearly indicate why it is worth doing; be sure to answer the "So What? question [i.e., why should anyone care?].
- Describe the major issues or problems examined by your research. This can be in the form of questions to be addressed. Be sure to note how your proposed study builds on previous assumptions about the research problem.
- Explain the methods you plan to use for conducting your research. Clearly identify the key sources you intend to use and explain how they will contribute to your analysis of the topic.
- Describe the boundaries of your proposed research in order to provide a clear focus. Where appropriate, state not only what you plan to study, but what aspects of the research problem will be excluded from the study.
- If necessary, provide definitions of key concepts, theories, or terms.
III. Literature Review
Connected to the background and significance of your study is a section of your proposal devoted to a more deliberate review and synthesis of prior studies related to the research problem under investigation . The purpose here is to place your project within the larger whole of what is currently being explored, while at the same time, demonstrating to your readers that your work is original and innovative. Think about what questions other researchers have asked, what methodological approaches they have used, and what is your understanding of their findings and, when stated, their recommendations. Also pay attention to any suggestions for further research.
Since a literature review is information dense, it is crucial that this section is intelligently structured to enable a reader to grasp the key arguments underpinning your proposed study in relation to the arguments put forth by other researchers. A good strategy is to break the literature into "conceptual categories" [themes] rather than systematically or chronologically describing groups of materials one at a time. Note that conceptual categories generally reveal themselves after you have read most of the pertinent literature on your topic so adding new categories is an on-going process of discovery as you review more studies. How do you know you've covered the key conceptual categories underlying the research literature? Generally, you can have confidence that all of the significant conceptual categories have been identified if you start to see repetition in the conclusions or recommendations that are being made.
NOTE: Do not shy away from challenging the conclusions made in prior research as a basis for supporting the need for your proposal. Assess what you believe is missing and state how previous research has failed to adequately examine the issue that your study addresses. Highlighting the problematic conclusions strengthens your proposal. For more information on writing literature reviews, GO HERE .
To help frame your proposal's review of prior research, consider the "five C’s" of writing a literature review:
- Cite , so as to keep the primary focus on the literature pertinent to your research problem.
- Compare the various arguments, theories, methodologies, and findings expressed in the literature: what do the authors agree on? Who applies similar approaches to analyzing the research problem?
- Contrast the various arguments, themes, methodologies, approaches, and controversies expressed in the literature: describe what are the major areas of disagreement, controversy, or debate among scholars?
- Critique the literature: Which arguments are more persuasive, and why? Which approaches, findings, and methodologies seem most reliable, valid, or appropriate, and why? Pay attention to the verbs you use to describe what an author says/does [e.g., asserts, demonstrates, argues, etc.].
- Connect the literature to your own area of research and investigation: how does your own work draw upon, depart from, synthesize, or add a new perspective to what has been said in the literature?
IV. Research Design and Methods
This section must be well-written and logically organized because you are not actually doing the research, yet, your reader must have confidence that you have a plan worth pursuing . The reader will never have a study outcome from which to evaluate whether your methodological choices were the correct ones. Thus, the objective here is to convince the reader that your overall research design and proposed methods of analysis will correctly address the problem and that the methods will provide the means to effectively interpret the potential results. Your design and methods should be unmistakably tied to the specific aims of your study.
Describe the overall research design by building upon and drawing examples from your review of the literature. Consider not only methods that other researchers have used, but methods of data gathering that have not been used but perhaps could be. Be specific about the methodological approaches you plan to undertake to obtain information, the techniques you would use to analyze the data, and the tests of external validity to which you commit yourself [i.e., the trustworthiness by which you can generalize from your study to other people, places, events, and/or periods of time].
When describing the methods you will use, be sure to cover the following:
- Specify the research process you will undertake and the way you will interpret the results obtained in relation to the research problem. Don't just describe what you intend to achieve from applying the methods you choose, but state how you will spend your time while applying these methods [e.g., coding text from interviews to find statements about the need to change school curriculum; running a regression to determine if there is a relationship between campaign advertising on social media sites and election outcomes in Europe ].
- Keep in mind that the methodology is not just a list of tasks; it is a deliberate argument as to why techniques for gathering information add up to the best way to investigate the research problem. This is an important point because the mere listing of tasks to be performed does not demonstrate that, collectively, they effectively address the research problem. Be sure you clearly explain this.
- Anticipate and acknowledge any potential barriers and pitfalls in carrying out your research design and explain how you plan to address them. No method applied to research in the social and behavioral sciences is perfect, so you need to describe where you believe challenges may exist in obtaining data or accessing information. It's always better to acknowledge this than to have it brought up by your professor!
V. Preliminary Suppositions and Implications
Just because you don't have to actually conduct the study and analyze the results, doesn't mean you can skip talking about the analytical process and potential implications . The purpose of this section is to argue how and in what ways you believe your research will refine, revise, or extend existing knowledge in the subject area under investigation. Depending on the aims and objectives of your study, describe how the anticipated results will impact future scholarly research, theory, practice, forms of interventions, or policy making. Note that such discussions may have either substantive [a potential new policy], theoretical [a potential new understanding], or methodological [a potential new way of analyzing] significance. When thinking about the potential implications of your study, ask the following questions:
- What might the results mean in regards to challenging the theoretical framework and underlying assumptions that support the study?
- What suggestions for subsequent research could arise from the potential outcomes of the study?
- What will the results mean to practitioners in the natural settings of their workplace, organization, or community?
- Will the results influence programs, methods, and/or forms of intervention?
- How might the results contribute to the solution of social, economic, or other types of problems?
- Will the results influence policy decisions?
- In what way do individuals or groups benefit should your study be pursued?
- What will be improved or changed as a result of the proposed research?
- How will the results of the study be implemented and what innovations or transformative insights could emerge from the process of implementation?
NOTE: This section should not delve into idle speculation, opinion, or be formulated on the basis of unclear evidence . The purpose is to reflect upon gaps or understudied areas of the current literature and describe how your proposed research contributes to a new understanding of the research problem should the study be implemented as designed.
ANOTHER NOTE : This section is also where you describe any potential limitations to your proposed study. While it is impossible to highlight all potential limitations because the study has yet to be conducted, you still must tell the reader where and in what form impediments may arise and how you plan to address them.
VI. Conclusion
The conclusion reiterates the importance or significance of your proposal and provides a brief summary of the entire study . This section should be only one or two paragraphs long, emphasizing why the research problem is worth investigating, why your research study is unique, and how it should advance existing knowledge.
Someone reading this section should come away with an understanding of:
- Why the study should be done;
- The specific purpose of the study and the research questions it attempts to answer;
- The decision for why the research design and methods used where chosen over other options;
- The potential implications emerging from your proposed study of the research problem; and
- A sense of how your study fits within the broader scholarship about the research problem.
VII. Citations
As with any scholarly research paper, you must cite the sources you used . In a standard research proposal, this section can take two forms, so consult with your professor about which one is preferred.
- References -- a list of only the sources you actually used in creating your proposal.
- Bibliography -- a list of everything you used in creating your proposal, along with additional citations to any key sources relevant to understanding the research problem.
In either case, this section should testify to the fact that you did enough preparatory work to ensure the project will complement and not just duplicate the efforts of other researchers. It demonstrates to the reader that you have a thorough understanding of prior research on the topic.
Most proposal formats have you start a new page and use the heading "References" or "Bibliography" centered at the top of the page. Cited works should always use a standard format that follows the writing style advised by the discipline of your course [e.g., education=APA; history=Chicago] or that is preferred by your professor. This section normally does not count towards the total page length of your research proposal.
Develop a Research Proposal: Writing the Proposal. Office of Library Information Services. Baltimore County Public Schools; Heath, M. Teresa Pereira and Caroline Tynan. “Crafting a Research Proposal.” The Marketing Review 10 (Summer 2010): 147-168; Jones, Mark. “Writing a Research Proposal.” In MasterClass in Geography Education: Transforming Teaching and Learning . Graham Butt, editor. (New York: Bloomsbury Academic, 2015), pp. 113-127; Juni, Muhamad Hanafiah. “Writing a Research Proposal.” International Journal of Public Health and Clinical Sciences 1 (September/October 2014): 229-240; Krathwohl, David R. How to Prepare a Dissertation Proposal: Suggestions for Students in Education and the Social and Behavioral Sciences . Syracuse, NY: Syracuse University Press, 2005; Procter, Margaret. The Academic Proposal. The Lab Report. University College Writing Centre. University of Toronto; Punch, Keith and Wayne McGowan. "Developing and Writing a Research Proposal." In From Postgraduate to Social Scientist: A Guide to Key Skills . Nigel Gilbert, ed. (Thousand Oaks, CA: Sage, 2006), 59-81; Wong, Paul T. P. How to Write a Research Proposal. International Network on Personal Meaning. Trinity Western University; Writing Academic Proposals: Conferences , Articles, and Books. The Writing Lab and The OWL. Purdue University; Writing a Research Proposal. University Library. University of Illinois at Urbana-Champaign.
- << Previous: Writing a Reflective Paper
- Next: Generative AI and Writing >>
- Last Updated: Mar 6, 2024 1:00 PM
- URL: https://libguides.usc.edu/writingguide/assignments

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
6 Preparing Your Research Proposal
More often than not, there will be a few steps that you’ll have to take before you can start gathering and analyzing data in pursuit of an answer to your research question. Preparing a research proposal is a milestone in any research project and is often required by sponsoring institutions in order to transition from ‘the ‘planning’ phase to the ‘doing’ phase. So why, you might ask, are we talking about this step in phase III, ‘writing’? That’s a great question and it has to do, primarily, with the order of thought and the information that must be included in a research proposal. In this chapter, we’ll cover the basic requirements of most research proposals and address the requirements and responsibilities of a researcher.
Chapter 6: Learning Objectives
Before you prepare to implement your research methodology, it is likely that you’ll need to gain approval to continue. As we explore the development of the research proposal, you’ll be able to:
- Describe the individual elements of a research proposal
- Delineate between the rationale and implementation portions of a research proposal
- Discuss the ethical tenets which govern researchers
- Define the purpose of an institutional review board
- Compare categories of institutional review board applications
What is a research proposal?
A research proposal can be thought of as the general blueprint for a proposed research project. There are very few instances wherein research projects can be pursued without support of a sponsoring institution. That is, a healthcare system, hospital, or academic institution. To receive support from a sponsoring institution, a researcher must articulate a clear plan for their research process to include:
- An overview of the literature which supports the investigation
- A statement of the problem
- A statement of purpose
- A hypothesis or central question
- An overview of how participants will be identified, selected, contacted or data will be identified, analyzed, and protected
- An overview of the proposed methodology (i.e. approach to the study)
- An acknowledgment that participants, data, and results will be treated ethically throughout the study
- A timeline for the project
As Crawford, Burkholder, and Cox (2020) describe, these items can be split into separate portions of a research proposal, the rationale (i.e. Whye) and implementation (i.e. How).
As we discussed in previous chapters, developing a robust rationale for your research will help guide the entire research process. The introduction to your research proposal should include a general description of why the research should be conducted. Aside from your general interest, the introduction to the research should be firmly rooted in the available evidence which, first identifies a problem; second, identifies a purpose for the pursuit of inquiry into the problem; and finally, articulates a clear and focused research question which addresses the gap in current knowledge on the topic.
Implementation
Outlining your plan for implementation is essential to gain approval to conduct your research. Equally important to developing a well-articulated rationale, the identification of a clear methodology for how you will implement your approach is an important component of a research proposal.
A plan of implementation can be presented in several ways. However, an inclusive plan should include the following elements (Crawford, Burkholder, & Cox, 2020):
- How you will select participants or identify ‘what’ is included in your investigation
- How you will measure what you’re investigating
- What type of data you will collect and how
- How you will analyze the data
- Frame the terms that specify your investigation
- Qualities of the study that are inherent to the study, but may be overlooked as obvious unless addressed
- Delimitations narrow the scope of the study regarding what it does not include. Limitations are an acknowledgement of the weaknesses of the study design or methodology (Spoiler: there are limitations to EVERY study).
- Influence practice?
- Impact policy?
- Provide a foundation for future research?
We’ve spent a lot of time discussing how to identify a problem, a purpose, articulate a question, and identify a sample and the selection and implementation of an appropriate approach. Ethical considerations of the researcher is another essential topic for any researcher to cover. Here, we’ll provide a general overview of ethical considerations that are required of sponsoring institutions to ensure the ethical treatment of study participants and related data.
As a clinician, you’re likely familiar with the tenets of bedside bioethics that guide clinical practice:
- Autonomy : The right to self-direction and control
- Beneficence : The intention to do ‘good’, or what is in the best interest of the patient
- Non-Maleficence : The goal to ‘do no harm’ in practicing
- Justice : The pursuit of fairness and equity
These basic tenets of care do not change much when viewed through the lens of a researcher. However, it is important to note the foundation upon which research ethics were built. In 1974, the National Research Act was drafted in response to blatant abuse of research methods such as the Tuskegee study and resulted in the establishment of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research.The ethical principles which guide researchers are derived outlined by the Belmont Report (HHS.gov) and include:
- Autonomy : Respect for a person to make personal choices and provisions and protections to be provided for participants belonging to vulnerable populations
- Beneficence : The intention to do what is morally right; to minimize risk and maximize benefits
- Justice : To promote equity among the treatment of individuals and groups
Researchers must address the ways in which they intent to uphold these principles in their proposed research project. Methods by which they do this include:
- Voluntary Informed Consent : Informed consent is a process which ensures that a participant is educated in terms that they can understand about the risks inherent to their participation. This process underscores respect through the provision of consent for a voluntary act (HHS.gov, n.d.)
- Avoidance of Harm : Avoidance of harm is related to the ethical tenet of beneficence and is the primary responsibility of the researcher
- Assessment of Risk: The common rule mandates that researchers ensure that the risk to potential participants in a research study are minimized and that the research cannot impose risk that outweighs the potential benefit of the outcomes.
- Right to Withdrawal: Participants must be made aware of their rights to withdraw from the study at any time, for any reason, without consequences.
- Responsibility to Terminate: The principle investigator has the responsibility to terminate the research intervention should it be made clear that the intervention has either a detrimental effect on participants or an overwhelmingly positive effect such that it would be unethical to continue the study.
Universal research practices which promote these principles must be included in a research proposal in order to conduct research at most institutions and are outlined in the Common Rule which regulates the functions of institutional review boards (IRBs).
Institutional Review Board
An IRB is a formally designated group which has been established to protect the rights and welfare of human subjects recruited to participate in research; specifically research conducted at, or supported by, a specific institution. Here it is important to understand what is meant by the terms ‘research’ and ‘human subjects’. In regards to the requirement of IRB review, the term research means a systematic investigation, development, testing and evaluation designed to develop or contribute to generalizable knowledge (University of Southern California, n.d.). Human subjects in relation to research refers to a living individual who’s information or biospecimens are used or analyzed to generate either identifiable private information or biospecimens for the purpose of generalizable information (University of Southern California, n.d.).
Although there are some details which will differ between organizations, there are general categories of human subject research which must be reviewed by an IRB. These classifications are designated by the degree of risk assumed by the participants and the ability of the researcher to mitigate those risks. Minimal risk is described by the federal regulations as the probability and magnitude of physical or psychological harm that is normally encountered in the daily lives, or in the routine medical, dental, or psychological examination of healthy persons (Electronic Code of Federal Regulations, n.d). Generally, research proposals will fall into one of the following categories:
- Exempt : Exempt research poses no more than minimal risk to adult, non-vulnerable populations.
- Expedited : Research that poses no more than minimal risk to participants and fits into one of the expedited categories described in federal regulations 45 CFR 46.110 (HHS.gov)
- Full Board : Research that does not qualify for either exempt or expedited review and poses more than minimal risk to participants. This type of review requires the approval from a full membership of an IRB.
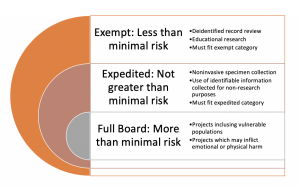
Projects that don’t need IRB approval
Projects which are not considered human subjects research are not required to be reviewed by an IRB. Quality improvement projects do not typically require formal IRB review. However, individual institutional requirements should be reviewed and followed; preferably, in the planning phase of your research project to ensure that the requirements of your specific review align with both your approach and your timeline.
Key Takeaways
- Research proposals can be split into two primary components: The rational and the plan of implementation
- The introduction of your research proposal should encompass a description of your problem, purpose, and research question
- The identification of your research approach should be firmly guided by the ethical tenets of autonomy, beneficence, and justice
- The researcher has an ethical responsibility to protect participants from risk
- An institutional review board is a formal board charged with reviewing risks associated with research projects
- There are differing levels of institutional review; assumption of risk is the primary factor in classifying level of IRB review
Crawford, L.M., Burkholder, G.J., Cox, K.A. (2020). Writing the Research Proposal. In G.J. Burkholder, K.A Cox, L.M. Crawford, and J.H. Hitchcock (Eds.), Research design and methods: An applied guide for the scholar-practitioner (pp. 309-334). Sage Publications
Electronic Code of Federal Regulations. (2020, August, 17). Protection of human subjects . Electronic Code of Federal Regulations. https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=83cd09e1c0f5c6937cd9d7513160fc3f&pitd=20180719&n=pt45.1.46&r=PART&ty=HTML#se45.1.46_1104
Health and Human Services. (2020, August, 14). The Belmont report . Health and Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/index.html
University of Southern California. (2020, August, 17). Office for the protection of research subjects . University of Southern California. https://oprs.usc.edu/irb-review/types-of-irb-review/
The right to self direction and control
The intention to do 'good'
The intention to do no harm
Pursuit of fairness and equity
A systematic investigation
Living persons participating in research
Probability of harm that does not exceed that encountered in every day life
IRB classification for research projects that do not pose more than minimal risk to adult, non-vulnerable populations
Classification of IRB approval for research that does not pose more than minimal risk, but fits into federally regulated categories.
IRB Classification for research that does pose more than minimal risk for participants
Practical Research: A Basic Guide to Planning, Doing, and Writing Copyright © by megankoster. All Rights Reserved.
Share This Book

Ch. 7 - Proposal Review, Approval, and Submission

7.1 Required Institutional Signature 7.2 Internal Review of Research Proposals 7.3 Internal Review of Proposals for Projects other than Research 7.4 Sponsor Limitation of Proposals from Institution 7.5 Procedures for the Submission of Proposals 7.6 Pre-Award Audit/Request for Additional Information
return to Table of Contents
7.1 Required Institutional Signature
Because Caltech certifies compliance with sponsor requirements and accepts certain responsibilities at the time a proposal is submitted, sponsors typically require a real or electronic signature from the applicant institution in addition to one from the PI. The signature of an institutional or authorized official indicates the organization complies with sponsor and governmental regulations, has adequate facilities and infrastructure to complete the project, and offers to perform the described work within the requested budget and any listed cost sharing commitment.
The Caltech Way
All proposals to sponsors for grants, contracts and cooperative agreements must be reviewed and approved by the Division Chair and submitted by the Office of Sponsored Research.
The Office of Sponsored Research is the Institute contact point for sponsored activities when a formal agreement (e.g., a grant, contract, or cooperative agreement) with the Institute is contemplated that includes terms and conditions, i.e., the funds will not be classified as a gift.
Caltech Faculty Handbook Chapter 7
7.2 Internal Review of Research Proposals
A formal proposal review process ensures key issues are reviewed by all stakeholders within an institution. Areas of review include: facilities and space commitments, conflict of interest, involvement of human subjects and/or vertebrate animals, and cost sharing commitments.
All institutional reviews must be completed prior to the submission of a proposal to a sponsor. The Division Approval Form for Sponsored Projects must be completed to document internal reviews and approvals of proposals prior to submission.
All applications for new, noncompeting continuation, renewal or supplemental awards or revisions to proposals or program budgets requested by the sponsor must be accompanied by a completed and signed Division Approval Form (DAF) .
The Administrative Committee on Sponsored Research is appointed by the President and reports to the Vice Provost for Research. The Committee is responsible for reviewing certain types of research proposals before an award can be accepted by the Institute. These include:
- Proposals submitted to for-profit sponsors (excluding federal flow-through funds)
- Proposals submitted to foreign entities
- Proposals requesting annual funding in excess of $5 million or total funding that exceeds $10 million
- Proposals referred to the Committee by the Vice Provost for Research or the Office of Sponsored Research
Caltech Faculty Handbook Chapter 7 (pg. 7/28)
Caltech Institute Research & Compliance Committees website
7.3 Internal Review of Proposals for Projects other than Research
The Institute seeks funding from government and private agencies for activities that are research-related, but not in themselves research. Examples include: instrumentation programs, training programs, student support, building renovations.
Research-related proposals follow the same procedures as research proposals, i.e., completion of a Divisional Approval Form, submission to the Office of Sponsored Research through the Division Chair. (see Section 7.2)
Proposals for testing programs require DAF approval from the Division Chair and/or other official responsible for the involved laboratory or facility, and the Office of Sponsored Research.
Proposals for graduate student fellowships, individual graduate student financial support and institutional training grants require approval of the Dean of Graduate Studies on the DAF.
Proposals for educational programs, summer institutes, equipment and renovation of facilities require a completed DAF.
7.4 Sponsor Limitation of Proposals from Institution
Public and private sponsors may limit the number of proposals that can be submitted by each institution for certain funding programs. These programs are referred to as "limited submissions." Information regarding such a restriction is usually found in the sponsor's program guidelines or announcement of availability of funding. Attention is required by the institution and PI in order to avoid disqualification or delays that could result from submissions exceeding the limitation.
The Provost's Office coordinates the selection of proposals in "limited submission" cases. When a limited submission announcement is received, the Provost's Office forwards the announcement to the Division Chairs and outlines the internal review process.
The Provost, in consultation with the Division Chairs and/or the Vice Provosts, determines which of the interested PIs may apply. The list of approved PIs is forwarded to the Office of Sponsored Research (OSR) with instructions to submit only these proposals for the limited submission deadline.
Proposals received by OSR without having been subject to the internal review process will be forwarded to the Provost's office for guidance. Investigators who were unaware of the Provost's review requirement will also be referred to the Provost's office.
7.5 Procedures for the Submission of Proposals
Once a proposal has been reviewed internally by various stakeholders, it is submitted to the Office of Sponsored Research (OSR), the Caltech office responsible for submitting it to the sponsor. This office is also the "office of record," the office assigned responsibility for retaining copies of proposals (and any resulting awards) as official records of the institution.
All proposals to sponsors for grants, contracts and cooperative agreements must be submitted along with the signed Division Approval Form (DAF) to the Office of Sponsored Research at least three (3) business days prior to the agency due date.
If the proposal is being submitted to a foreign entity, or to a commercial sponsor (other than for Federal flow through funds), OSR requires an electronic copy of the full proposal for review by the Administrative Committee on Sponsored Research. This review generally takes place following the submission of the proposal.
Sponsored Research, Frequently Asked Questions
The Office of Sponsored Research is often reviewing many proposals simultaneously for the same deadline, in addition to processing awards and miscellaneous award transactions, and assisting divisional staff. In order to provide a clearer idea to faculty and divisional staff regarding what OSR can do, we have prepared the following guideline:
With at least 3 days' lead time prior to submission, OSR will perform a complete review, including:
- Comparison of proposal to sponsors' guidelines (e.g. forms and formatting)
- Review of budget (calculation, rates, relevance to project description, cost-sharing)
- Division Approval Form
- Compliance issues (e.g., human/animal subjects, health and safety, conflict of interest)
- PI Eligibility to Submit
With1 to 2days' lead time prior to submission, OSR will review at least the following:
- Review of budget for correct rates and cost sharing
- Divisional Approval Form
With less than 1 day's time prior to submission,OSR may only be able to verify that the PI and Division Chair have signed the Divisional Approval Form (the minimum requirement for any proposal to be submitted).
This is not intended to represent the most OSR can do, but the minimum OSR will do. If possible, OSR will provide a more thorough review than these minima, time permitting. The OSR team will let PIs know when a proposal will receive less than a complete review due to time constraints.
The following are some tips to facilitate the proposal approval process:
- E-mail or fax a copy of the Division Approval Form, cover page, budget, and budget explanation to OSR for pre-approval. The budget is usually the most time consuming portion of a proposal to check, and advance copies allow OSR to help correct possible errors while the text is still in its draft stage.
- E-mail or call the Office of Sponsored Research if the proposal is being submitted in response to a specific Broad Agency Announcement, Request for Proposals, Research Announcement, etc. so that OSR can review the proposal along with the solicitation guidelines.
- E-mail or fax information regarding the submission of the proposal. This enables OSR to begin preparing the cover letter for the proposal.
- The end of the month is usually the busiest time for proposal submission.
- Allow time for corrections and make sure a contact is available. In many cases, proposals sent to OSR are ready to be signed and submitted without further correction or revision. However, there are times when corrections need to be made, particularly if a budget has not been sent in advance or if a PI is applying to an agency with which he/she is unfamiliar. Allowing time for corrections helps to ensure the proposal is at its best when it is sent out. The PI and or a divisional contact should be available to make corrections until the proposal is ready to submit to the agency.
Deadlines vary widely among sponsors and sponsor programs. Proposal deadlines can be based upon a postmark by the U.S. Postal Service or receipt in the sponsor's mail room. With electronic systems, an accepted proposal may mean the electronic proposal file has been transferred and processed by one or two systems. In most cases, sponsors will not consider proposals that miss a deadline.
7.6 Pre-Award Audit/Request for Additional Information
Some sponsors perform pre-award audits or request additional information concerning the project as part of the proposal review process. For this reason, it is advisable for PI's to maintain a complete copy of each proposal, along with all supporting documents used in the development of the proposal budget.
During a proposal review, a sponsor may indicate a willingness to support a project at a reduced level of funding. If requested by the sponsor, a revised budget should be submitted through the Office of Sponsored Research. It is important for investigators to be aware that a discussion should occur related to how the scope of work will be reduced to accommodate the revised budget.
Documents related to unfunded proposals should be retained while pending a funding decision and for 18 months after a proposal has been denied.
Once funded, the proposal record becomes part of the award file and falls under the disposition schedule related to the award. Documents related to funded proposals should be retained for one year after the expiration or termination of the award.
Record Retention and Disposition Policy (8/2008)
Records Retention Schedule (9/2011)
Click to go to Chapter 8
Writing Your Research Proposal
5 Essentials You Need To Keep In Mind
By: Derek Jansen (MBA) | Reviewer: Eunice Rautenbach (DTech) | June 2023
Writing a high-quality research proposal that “sells” your study and wins the favour (and approval) of your university is no small task. In this post, we’ll share five critical dos and don’ts to help you navigate the proposal writing process.
This post is based on an extract from our online course , Research Proposal Bootcamp . In the course, we walk you through the process of developing an A-grade proposal, step by step, with plain-language explanations and loads of examples. If it’s your first time writing a research proposal, you definitely want to check that out.
Overview: 5 Proposal Writing Essentials
- Understand your university’s requirements and restrictions
- Have a clearly articulated research problem
- Clearly communicate the feasibility of your research
- Pay very close attention to ethics policies
- Focus on writing critically and concisely
1. Understand the rules of the game
All too often, we see students going through all the effort of finding a unique and valuable topic and drafting a meaty proposal, only to realise that they’ve missed some critical information regarding their university’s requirements.
Every university is different, but they all have some sort of requirements or expectations regarding what students can and can’t research. For example:
- Restrictions regarding the topic area that can be research
- Restrictions regarding data sources – for example, primary or secondary
- Requirements regarding methodology – for example, qualitative, quantitative, or mixed methods-based research
- And most notably, there can be varying expectations regarding topic originality – does your topic need to be super original or not?
The key takeaway here is that you need to thoroughly read through any briefing documents provided by your university. Also, take a look at past dissertations or theses from your program to get a feel for what the norms are . Long story short, make sure you understand the rules of the game before you start playing.

2. Have a clearly articulated research problem
As we’ve explained many times on this blog, all good research starts with a strong research problem – without a problem, you don’t have a clear justification for your research. Therefore, it’s essential that you have clarity regarding the research problem you’re going to address before you start drafting your proposal. From the research problem , the research gap emerges and from the research gap, your research aims , objectives and research questions emerge. These then guide your entire dissertation from start to end.
Needless to say, all of this starts with the literature – in other words, you have to spend time reading the existing literature to understand the current state of knowledge. You can’t skip this all-important step. All too often, we see students make the mistake of trying to write up a proposal without having a clear understanding of the current state of the literature, which is just a recipe for disaster. You’ve got to take the time to understand what’s already been done before you can propose doing something new.

3. Demonstrate the feasibility of your research
One of the key concerns that reviewers or assessors have when deciding to approve or reject a research proposal is the practicality/feasibility of the proposed research , given the student’s resources (which are usually pretty limited). You can have a brilliant research topic that’s super original and valuable, but if there is any question about whether the project is something that you can realistically pull off, you’re going to run into issues when it comes to getting your proposal accepted.
So, what does this mean for you?
First, you need to make sure that the research topic you’ve chosen and the methodology you’re planning to use is 100% safe in terms of feasibility . In other words, you need to be super certain that you can actually pull off this study. Of greatest importance here is the data collection and analysis aspect – in other words, will you be able to get access to the data you need, and will you be able to analyse it?
Second, assuming you’re 100% confident that you can pull the research off, you need to clearly communicate that in your research proposal. To do this, you need to proactively think about all the concerns the reviewer or supervisor might have and ensure that you clearly address these in your proposal. Remember, the proposal is a one-way communication – you get one shot (per submission) to make your case, and there’s generally no Q&A opportunity . So, make it clear what you’ll be doing, what the potential risks are and how you’ll manage those risks to ensure that your study goes according to plan.
If you have the word count available, it’s a good idea to present a project plan , ideally using something like a Gantt chart. You can also consider presenting a risk register , where you detail the potential risks, their likelihood and impact, and your mitigation and response actions – this will show the assessor that you’ve really thought through the practicalities of your proposed project. If you want to learn more about project plans and risk registers, we cover these in detail in our proposal writing course, Research Proposal Bootcamp , and we also provide templates that you can use.
Need a helping hand?
4. Pay close attention to ethics policies
This one’s a biggy – and it can often be a dream crusher for students with lofty research ideas. If there’s one thing that will sink your research proposal faster than anything else, it’s non-compliance with your university’s research ethics policy . This is simply a non-negotiable, so don’t waste your time thinking you can convince your institution otherwise. If your proposed research runs against any aspect of your institution’s ethics policies, it’s a no-go.
The ethics requirements for dissertations can vary depending on the field of study, institution, and country, so we can’t give you a list of things you need to do, but some common requirements that you should be aware of include things like:
- Informed consent – in other words, getting permission/consent from your study’s participants and allowing them to opt out at any point
- Privacy and confidentiality – in other words, ensuring that you manage the data securely and respect people’s privacy
- If your research involves animals (as opposed to people), you’ll need to explain how you’ll ensure ethical treatment, how you’ll reduce harm or distress, etc.
One more thing to keep in mind is that certain types of research may be acceptable from an ethics perspective, but will require additional levels of approval . For example, if you’re planning to study any sort of vulnerable population (e.g., children, the elderly, people with mental health conditions, etc.), this may be allowed in principle but requires additional ethical scrutiny. This often involves some sort of review board or committee, which slows things down quite a bit. Situations like this aren’t proposal killers, but they can create a much more rigid environment , so you need to consider whether that works for you, given your timeline.

5. Write critically and concisely
The final item on the list is more generic but just as important to the success of your research proposal – that is, writing critically and concisely .
All too often, students fall short in terms of critical writing and end up writing in a very descriptive manner instead. We’ve got a detailed blog post and video explaining the difference between these two types of writing, so we won’t go into detail here. However, the simplest way to distinguish between the two types of writing is that descriptive writing focuses on the what , while analytical writing draws out the “so what” – in other words, what’s the impact and relevance of each point that you’re making to the bigger issue at hand.
In the case of a research proposal, the core task at hand is to convince the reader that your planned research deserves a chance . To do this, you need to show the reviewer that your research will (amongst other things) be original , valuable and practical . So, when you’re writing, you need to keep this core objective front of mind and write with purpose, taking every opportunity to link what you’re writing about to that core purpose of the proposal.
The second aspect in relation to writing is to write concisely . All too often, students ramble on and use far more word count than is necessary. Part of the problem here is that their writing is just too descriptive (the previous point) and part of the issue is just a lack of editing .
The keyword here is editing – in other words, you don’t need to write the most concise version possible on your first try – if anything, we encourage you to just thought vomit as much as you can in the initial stages of writing. Once you’ve got everything down on paper, then you can get down to editing and trimming down your writing . You need to get comfortable with this process of iteration and revision with everything you write – don’t try to write the perfect first draft. First, get the thoughts out of your head and onto the paper , then edit. This is a habit that will serve you well beyond your proposal, into your actual dissertation or thesis.

Wrapping Up
To recap, the five essentials to keep in mind when writing up your research proposal include:
If you want to learn more about how to craft a top-notch research proposal, be sure to check out our online course for a comprehensive, step-by-step guide. Alternatively, if you’d like to get hands-on help developing your proposal, be sure to check out our private coaching service , where we hold your hand through the research journey, step by step.

Psst… there’s more!
This post is an extract from our bestselling short course, Research Proposal Bootcamp . If you want to work smart, you don't want to miss this .
You Might Also Like:

Thanks alot, I am really getting you right with focus on how to approach my research work soon, Insha Allah, God blessed you.
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
SOC W 505/506 Foundations of Social Welfare Research
- What is a Research Proposal?
- Qualitative Research
- Quantitative Research
- General Research Methods
- IRB's and Research Ethics
- Data Management and Analysis
Information on Writing a Research Proposal
From the Sage Encyclopedia of Educational Research, Measurement and Evaluation:
Research proposals are written to propose a research project and oftentimes request funding, or sponsorship, for that research. The research proposal is used to assess the originality and quality of ideas and the feasibility of a proposed project. The goal of the research proposal is to convince others that the investigator has (a) an important idea; (b) the skills, knowledge, and resources to carry out the project; and (c) a plan to implement the project on time and within budget. This entry discusses the process of developing a research proposal and the elements of an effective proposal.
For a graduate student, a research proposal may be required to begin the dissertation process. This serves to communicate the research focus to others, such as members of the student’s dissertation committee. It also indicates the investigator’s plan of action, including a level of thoroughness and sufficient detail to replicate the study. The research proposal could also be considered as a contract, once members of the committee agree to the execution of the project.
Requirements may include: an abstract, introduction, literature review, method section, and conclusion. A research proposal has to clearly and concisely identify the proposed research and its importance. The background literature should support the need for the research and the potential impact of the findings.
The method section proposes a comprehensive explanation of the research design, including subjects, timeline, and data analysis. Research questions should be identified as well as measurement instruments and methods to answer the research questions. Proposals for research involving human subjects identify how the investigators will protect participants throughout their research project.
Proposals often require engaging in an external review either by an external evaluator or advisory board consisting of expert consultants in the field. References are included to provide documentation about the supporting literature identified in the proposal. Appendixes and supplemental materials may also be included, following the sponsoring organization’s guidelines. As a general rule, educational research proposals follow the American Psychological Association formatting guidelines and publishing standards. If funding is being requested, it is important for the proposal to identify how the research will benefit the sponsoring organization and its constituents.
The success of a research proposal depends on both the quality of the project and its presentation. A proposal may have specific goals, but if they are neither realistic nor desirable, the probability of obtaining funding is reduced. Similar to manuscripts being considered for journal articles, reviewers evaluate each research proposal to identify strengths and criticisms based on a general framework and scoring rubric determined by the sponsoring organization. Research proposals that meet the scoring criteria are considered for funding opportunities. If a proposal does not meet the scoring criteria, revisions may be necessary before resubmitting the proposal to the same or a different sponsoring organization.
Common mistakes and pitfalls can often be avoided in research proposal writing through awareness and careful planning. In an effective research proposal, the research idea is clearly stated as a problem and there is an explanation of how the proposed research addresses a demonstrable gap in the current literature. In addition, an effective proposal is well structured, frames the research question(s) within sufficient context supported by the literature, and has a timeline that is appropriate to address the focus and scope of the research project. All requirements of the sponsoring organization, including required project elements and document formatting, need to be met within the research proposal. Finally, an effective proposal is engaging and demonstrates the researcher’s passion and commitment to the research addressed.
- << Previous: Databases
- Next: Qualitative Research >>
- Last Updated: Aug 11, 2023 2:12 PM
- URL: https://guides.lib.uw.edu/hsl/sw505
Be boundless
1959 NE Pacific Street | T334 Health Sciences Building | Box 357155 | Seattle, WA 98195-7155 | 206-543-3390
© 2024 University of Washington | Seattle, WA

Writing a Research Proposal
- First Online: 10 April 2022
Cite this chapter

- Fahimeh Tabatabaei 3 &
- Lobat Tayebi 3
811 Accesses
A research proposal is a roadmap that brings the researcher closer to the objectives, takes the research topic from a purely subjective mind, and manifests an objective plan. It shows us what steps we need to take to reach the objective, what questions we should answer, and how much time we need. It is a framework based on which you can perform your research in a well-organized and timely manner. In other words, by writing a research proposal, you get a map that shows the direction to the destination (answering the research question). If the proposal is poorly prepared, after spending a lot of energy and money, you may realize that the result of the research has nothing to do with the initial objective, and the study may end up nowhere. Therefore, writing the proposal shows that the researcher is aware of the proper research and can justify the significance of his/her idea.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
A. Gholipour, E.Y. Lee, S.K. Warfield, The anatomy and art of writing a successful grant application: A practical step-by-step approach. Pediatr. Radiol. 44 (12), 1512–1517 (2014)
Article Google Scholar
L.S. Marshall, Research commentary: Grant writing: Part I first things first …. J. Radiol. Nurs. 31 (4), 154–155 (2012)
E.K. Proctor, B.J. Powell, A.A. Baumann, A.M. Hamilton, R.L. Santens, Writing implementation research grant proposals: Ten key ingredients. Implement. Sci. 7 (1), 96 (2012)
K.C. Chung, M.J. Shauver, Fundamental principles of writing a successful grant proposal. J. Hand Surg. Am. 33 (4), 566–572 (2008)
A.A. Monte, A.M. Libby, Introduction to the specific aims page of a grant proposal. Kline JA, editor. Acad. Emerg. Med. 25 (9), 1042–1047 (2018)
P. Kan, M.R. Levitt, W.J. Mack, R.M. Starke, K.N. Sheth, F.C. Albuquerque, et al., National Institutes of Health grant opportunities for the neurointerventionalist: Preparation and choosing the right mechanism. J. Neurointerv. Surg. 13 (3), 287–289 (2021)
A.M. Goldstein, S. Balaji, A.A. Ghaferi, A. Gosain, M. Maggard-Gibbons, B. Zuckerbraun, et al., An algorithmic approach to an impactful specific aims page. Surgery 169 (4), 816–820 (2021)
S. Engberg, D.Z. Bliss, Writing a grant proposal—Part 1. J. Wound Ostomy Cont. Nurs. 32 (3), 157–162 (2005)
D.Z. Bliss, K. Savik, Writing a grant proposal—Part 2. J. Wound Ostomy Cont. Nurs. 32 (4), 226–229 (2005)
D.Z. Bliss, Writing a grant proposal—Part 6. J. Wound Ostomy Cont. Nurs. 32 (6), 365–367 (2005)
J.C. Liu, M.A. Pynnonen, M. St John, E.L. Rosenthal, M.E. Couch, C.E. Schmalbach, Grant-writing pearls and pitfalls. Otolaryngol. Neck. Surg. 154 (2), 226–232 (2016)
R.J. Santen, E.J. Barrett, H.M. Siragy, L.S. Farhi, L. Fishbein, R.M. Carey, The jewel in the crown: Specific aims section of investigator-initiated grant proposals. J. Endocr. Soc. 1 (9), 1194–1202 (2017)
O.J. Arthurs, Think it through first: Questions to consider in writing a successful grant application. Pediatr. Radiol. 44 (12), 1507–1511 (2014)
M. Monavarian, Basics of scientific and technical writing. MRS Bull. 46 (3), 284–286 (2021)
Additional Resources
https://grants.nih.gov
https://grants.nih.gov/grants/oer.htm
https://www.ninr.nih.gov
https://www.niaid.nih.gov
http://www.grantcentral.com
http://www.saem.org/research
http://www.cfda.gov
http://www.ahrq.gov
http://www.nsf/gov
Download references
Author information
Authors and affiliations.
School of Dentistry, Marquette University, Milwaukee, WI, USA
Fahimeh Tabatabaei & Lobat Tayebi
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Tabatabaei, F., Tayebi, L. (2022). Writing a Research Proposal. In: Research Methods in Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-030-98028-3_4
Download citation
DOI : https://doi.org/10.1007/978-3-030-98028-3_4
Published : 10 April 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-98027-6
Online ISBN : 978-3-030-98028-3
eBook Packages : Engineering Engineering (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- louisville.edu
- PeopleSoft HR
- PeopleSoft Campus Solutions
- PeopleSoft Financials
- Business Ops
- Cardinal Careers

- Undergraduate
- International
- Online Learning
Office of Research and Innovation
- Events and Trainings
- News Stories
- Grand Challenges
- Research Challenge Trust Fund
- Publications and Reports
- University of Louisville Scholar and Distinguished Scholar Program
- Monthly Town Hall Recordings
- Find Funding
- Proposal Development and Submission
- Compliance and Regulatory
- IRB Submissions
- Non-Funded Agreements
- Grants Management
- Hiring and Purchasing
- Innovation and Technology Transfer
- Industry Partnerships
- Clinical Trials
- Research Centers and Institutes
- Core Facilities
- Publishing and Presenting
- Training and Workshops
- Research Handbook
- Grand Challenges / Research Priorities
- Research Systems and Tools
- Sponsoring Research
- Licensing Technologies
- Launching a Startup
- Co-location and Land
- Working with our Students
- Research Capabilities
- Get started
- Pay and Benefits
- Post-Doc Association - UofL Chapter
- Post-Doc Appointing Approval Form
- Trainings and Workshops
- Children in Research
- Open Trials
- Offices and Staff
- Unit Research Offices
- Newsletter Signup
- CHAPTER FOUR: Proposal Review, Approval, and Submission
- / Researchers
- / Research Handbook
- / CHAPTER FOUR: Proposal Review, Approval, and Submission
4.1 Review and Approval Responsibilities
4.2 Institutional Cover Sheet iRIS eProposal / Proposal Clearance Form (PCF) / Transmit for Review and Initial Assessment (TRIA)-Multi-Institutional Research Application (MIRA)
4.3 Other Pre-Submission Approvals and Requirements
4.4 Proposal Review
4.5 Signature Authority
4.6 Unfunded Proposals
Proposals must be reviewed and have the appropriate approvals prior to submission to an external funding agency. The review process includes review by the department chair/unit head, the respective dean or designee and potential review and approval by University compliance offices/committees such as the Human Subjects Protection Program/Institutional Review Board (IRB) and Animal Use/Institutional Animal Care and Use Committee (IACUC). Review and approval by the Office of Sponsored Programs Administration is the final step in the process prior to proposal submission.
All proposals submitted to external funding agencies must list the “University of Louisville Research Foundation, Inc.” as the award recipient unless the submission is required to be from the University. At least five (5) business days prior to submission to the Sponsor, the proposal must be submitted to OSPA for final review and approval.
Submission to OSPA is via the Integrated Research Information System (iRIS), by either the eProposal or the Proposal Short Form. The system’s eProposal is a comprehensive electronic packet which incorporates information required on the institutional cover sheet, proposal documents and sponsor forms. Alternately, the iRIS system offers a Proposal Short Form to which a conventional cover sheet and proposal documents can be uploaded. In this case, a completed and signed Proposal Clearance Form (PCF) or Transmit for Review and Initial Assessment (TRIA) – Multi-Institutional Research Application (MIRA) must accompany proposal submission to OSPA.
The institutional cover sheet - whether in the form of eProposal, Proposal Clearance Form (PCF) or Multi-Institutional Research Application TRIA-MIRA enables review of administrative, policy, and fiscal issues related to the proposal. The eProposal/PCF/TRIA-MIRA consists of a series of informational items and questions to assist the Principal Investigator/Project Director (PI/PD) and University reviewers in assessing potential risks and obligations should the proposal be funded. In addition to the signatures of the PI/PD and co-Investigators, signatures of the department chair/unit head and respective dean or designee are typically required.
The TRIA-MIRA or PCF Clinical Attachment is utilized for all clinical trials and sponsored research requiring approval of the Biomedical IRB and any other project/study that uses hospital facilities (for example, Jewish Hospital & St. Mary’s Healthcare Services, Norton Healthcare, or University of Louisville Hospital) or resources to conduct the research. The information on the PCF/TRIA-MIRA is shared with the respective hospital/study site and is used by the respective hospital/study site to grant approval for the research study to be conducted at their facility.
When University personnel from more than one academic department are participating in a proposed project, all appropriate department chairs/unit heads and deans must provide approval (by signing the PCF/MIRA) prior to submission of the proposal to OSPA for institutional approval.
If an award is received for which no proposal was submitted, an eProposal/PCF/TRIA-MIRA must be completed, signed, and submitted to OSPA prior to award establishment in PeopleSoft.
PIs/PDs are required to conduct research and manage the financial and regulatory aspects of sponsored projects in compliance with University policy, Federal and state law and Sponsor requirements. PIs/PDs must ensure that they and members of their research team(s) meet all compliance requirements, including any necessary disclosure(s) and training requirements.
Several areas of regulatory compliance may need to be considered when submitting proposals to an external funding agency. Examples include:
1) Conflict of Interest (COI);
2) Human Subjects Protection;
3) Animal Subjects Protection;
4) Biosafety and Radiation Safety;
5) Export and Secure Research Control.
See Chapter Nine of the Research Handbook for additional information on these and other research regulations.
If a proposed project requires UofL participants to interact with or handle human subjects, animals, or agents impacting environmental health and safety (e.g., recombinant DNA; pathogenic organisms; human blood, tissues, cell lines, or other potentially infectious materials [OPIM]), a proposal must be submitted to the appropriate committee(s) for internal review and approval prior to activation of an award. External Sponsors have different policies regarding the status of regulatory approvals at the time of proposal submission; while most will accept “pending review” or “pending approval,” some require full regulatory approval prior to submission. PIs/PDs should review the regulatory requirements of Sponsors when developing proposals for external funding.
Documentation of institutional approval (e.g., an approval letter) for actions “pending” at the time of proposal must be provided to OSPA prior to activation of an award (chartfield establishment). In limited situations OSPA may establish a chartfield prior to regulatory approval. As an example, for clinical trials, a chartfield may be established for site initiation visits and other limited startup activities prior to receiving final Institutional Review Board (IRB) approval [NOTE: no human subjects may be consented/enrolled into the trial until the IRB has granted formal approval].
All applications and proposals for external funding must be reviewed and approved by OSPA for consistency with Sponsor and Federal guidelines and University policies prior to submission. OSPA also reviews the budget for accuracy and proper format, ensure the correct application of fringe benefit and Facilities and Administrative Cost (aka F&A or indirect cost) rates, and verify University cost-sharing commitments.
Draft copies of all documents, including the draft budget may be submitted to OSPA for preliminary review and comment. This is particularly helpful for complex proposals, such as those with multi-year budgets, subagreements, and/or cost sharing commitments. It should be noted that preliminary review and comment does not constitute official approval for submission.
OSPA must receive the following items for review prior to granting approval for proposal submission:
1) eProposal, or completed and signed PCF and PCF Clinical Attachment (if applicable). Completed and signed TRIA-MIRA may be used for non-federal clinical proposals;
2) Proposal, including abstract, budget, budget justification, and Sponsor forms. A final copy of the proposal for OSPA files should be submitted at the time of proposal review (if electronic) or within two weeks following submission;
3) For proposals involving subrecipients, completed Subrecipient Commitment Form , budget, budget justification, and scope of work;
4) Indication of regulatory approval status (e.g., IRB, IACUC, DEHS);
5) Confirmation that all participants on the project, regardless of role, have completed the Attestation and Disclosure Form ; and
6) Approval in writing of all committed University cost sharing or matching obligations.
Only specific designees within OSPA have been granted signature authority by the President of the University. Under no circumstance is a PI/PD to sign a proposal to an external funding agency on behalf of the University and/or University of Louisville Research Foundation, Inc. without the prior approval of an Authorized Institutional Official.
Upon receipt of notification that a proposal will not be funded, the PI/PD should inform OSPA. OSPA retains copies of unfunded proposals for one year following notification that the proposal will not be funded.
1. General Information
2. Pre-Proposal Activities
3. Development, Budgeting
4. Proposal Review, Approval & Submission
5. Award Acceptance & Account Establishment
6. Financial Management of Awards
7. Admin/Non-Financial Management of Awards
8. Industry Awards & Agreements
9. Research Regulations
10. Service Centers

University of Louisville
Louisville, Ky. 40202
502.852.6512, 502.852.8361 (Fax)
Looking for a unit within the Office of Research and Innovation? Contact information here .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

How Do I Review Thee? Let Me Count the Ways: A Comparison of Research Grant Proposal Review Criteria Across US Federal Funding Agencies
While Elizabeth Barrett Browning counted 25 ways in which she loves her husband in her poem, “How Do I Love Thee? Let me Count the Ways,” we identified only eight ways to evaluate the potential for success of a federal research grant proposal. This may be surprising, as it seems upon initial glance of the review criteria used by various federal funding agencies that each has its own distinct set of “rules” regarding the review of grant proposals for research and scholarship. Much of the grantsmanship process is dependent upon the review criteria, which represent the funders’ desired impact of the research. But since most funders that offer research grants share the overarching goals of supporting research that (1) fits within its mission and (2) will bring a strong return on its financial investment, the review criteria used to evaluate research grant proposals are based on a similar set of fundamental questions. In this article, we compare the review criteria of 10 US federal agencies that support research through grant programs, and demonstrate that there are actually only a small and finite number of ways that a grant proposal can be evaluated. Though each funding agency may use slightly different wording, we found that the majority of the agencies’ criteria address eight key questions. Within the highly competitive landscape of research grant funding, new researchers must find support for their research agendas and established investigators and research development offices must consider ways to diversify their funding portfolios, yet all may be discouraged by the apparent myriad of differences in review criteria used by various funding agencies. Guided by research administrators and research development professionals, recognizing that grant proposal review criteria are similar across funding agencies may help lower the barrier to applying for federal funding for new and early career researchers, or facilitate funding portfolio diversification for experienced researchers. Grantmakers are furthermore provided valuable guidance to develop and refine their own proposal review criteria.

Introduction
The research funding landscape in the United States is highly competitive, with flat or shrinking budgets for investigator-initiated research programs at most federal agencies ( American Association for the Advancement of Science (AAAS), 2014) . Taking biomedical research as an example, in 2014, the National Institutes of Health (NIH) budgeted $15 billion to fund research project grants, an amount that has essentially remained the same since 2003 ( AAAS, 2014 ; Federation of American Societies for Experimental Biology, 2014 ). At the same time, the number of research grant applications has steadily increased, from close to 35,000 in 2003 to 51,000 in 2014. The result has been a stunning 30% drop in funding success rates, from 30.2% in 2003 to 18.8% in 2014. Other federal agencies that fund research, including the National Science Foundation (NSF), Office of Veterans Affairs (VA), and Department of Defense (DoD), are feeling the similar sting of budget restrictions.
Within this tenuous funding environment, it has become essential that investigators and research development offices sustain their research programs by continuing to encourage new researchers to apply for grant support and encouraging established researchers to diversify their funding portfolios. New researchers benefit from clear information about the federal grant process, and experienced researchers benefit from considering funding opportunities from federal funding agencies, national organizations and advocacy groups, state agencies, private philanthropic organizations, regional or local special interest groups, corporations, and internal institutional grant competitions that may not be their typical targets for support. With increasing competition for grant funding, investigators who might be accustomed to one set of rules for preparing grant proposals may become quickly overwhelmed by the prospect of learning entirely new sets of rules for different funding agencies.
Yet this process is not as daunting if we start from the perspective that any funder that offers research grants has essentially the same goal: to support research that fits within its mission and will bring a strong return on its financial investment ( Russell & Morrison, 2015 ). The review criteria used to evaluate research grant proposals reflect the funder’s approach to identifying the most relevant and impactful research to support ( Geever, 2012 ; Gerin & Kapelewski, 2010 ; Kiritz, 2007 ). Thus, planning and preparing a successful grant proposal depends on a clear understanding of the review criteria that will be used. These criteria directly inform how the proposal content should be presented and how much space should be afforded to each section of the proposal, as well as which keywords should be highlighted. It may seem that each funder—federal, state, local, private—has its own distinct set of rules regarding the preparation and review of grant proposals, and that each funder uses specific jargon in its review process. However, because all funders aim to support research that is relevant and impactful, we suggest that the mandatory review criteria used to evaluate research grant proposals are based on a set of fundamental questions, such as: Does this research fit within the funder’s mission? Will the results of this research fill a gap in knowledge or meet an unmet need? Do the investigators have the skills and resources necessary to carry out the research?
In this article, we examine the research grant proposal review criteria used by 10 US federal agencies to demonstrate that there exist only a small and finite number of ways that federal research grant proposals are actually evaluated. Our goal is to help research administrators and research development professionals empower investigators to more confidently navigate funder review criteria, thereby lowering the barrier to first-time applicants or to grant portfolio diversification for more established researchers. Recognizing that research proposal review criteria are aligned across federal funding agencies can also help proposal writers who might be faced with other funding opportunities in which the review criteria are not clearly defined. On the flip side of that equation, understanding that review criteria are based on the same core goals can help grantmakers as they develop and refine review criteria for their funding opportunities.
Observations
We performed an online search of 10 US federal agencies’ (NIH, NSF, VA, Department of Education [ED], DoD, National Aeronautics and Space Administration [NASA], Department of Energy [DOE], United States Department of Agriculture [USDA], National Endowment for the Humanities [NEH], and National Endowment for the Arts [NEA]) websites to identify policies and procedures related to their research grant proposal review process. The NIH Office of Extramural research (OER) website provided the greatest detail and transparency with regard to the review criteria and review process used for evaluating research grant proposals ( National Institutes of Health, 2008a ; 2008b ; 2015a ), and served as a starting point for our analysis of the review criteria for the other nine agencies. We developed key questions corresponding to each of the NIH review criteria, and then aligned the review criteria of the remaining nine agencies with these key questions.
Federal grant program guidance and policy changes occur frequently; the links to online resources for research grant proposal policies for each of the various funding agencies included in our analysis were current as of August 10, 2015. Note that our analysis includes information from the National Institute on Disability and Rehabilitation Research (NIDRR) program as administered by ED. On June 1, 2015, the NIDRR was transferred from ED to the Administration for Community Living (ACL) in the US Department of Health and Human Services (DHHS), and is now called the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) Field-Initiated Program. Our analysis of NIDRR was current as of May 4, 2015.
Also note that there is variability between different research grant programs within each federal agency. We included in our analysis review criteria from the DoD Congressionally Directed Medical Research Programs (CDMRP), the USDA National Institute of Food and Agriculture, the NEH Digital Humanities Start-up program, and the NEA ART WORKS program. Criteria for NASA research programs were compiled from numerous NASA Research Announcements.
The NIH review criteria
The NIH criteria emphasize clinical, interdisciplinary, and translational biomedical research ( National Institutes of Health, 2008a ). Reviewers are instructed to evaluate research grant proposals based on how well five core review criteria are met: Significance, Innovation, Approach, Investigator(s), and Environment ( Table 1 ) ( National Institutes of Health, 2015a ; 2015b ). Assigned reviewers consider each of the five core review criteria and assign a separate score for each using a 9-point scale. These ratings are included in a summary statement that is provided to the researcher, whether or not the entire study section ultimately discusses the proposal.
The NIH core review criteria for research project grant proposals a
NIH, National Institutes of Health.
Each of the five core review criteria can be simplified into a general question. The Significance criterion asks reviewers to consider “Why does the research matter?” Reviewers look for whether the proposed project will address an important problem or critical barrier to progress in the field, and whether the knowledge gained from the proposed research will advance scientific knowledge, technical capacity, or clinical practice to drive the field forward. Innovation translates into “How is the research new?” Reviewers consider how the proposed research challenges current thinking with novel concepts, approaches, tools, or treatments. Approach asks, “How will the research be done?” Reviewers assess the proposed research strategy, methodology, and analyses and determine whether they are appropriate to achieve the aims of the project, and how riskier aspects of the proposal might be handled with alternative approaches. The remaining two core criteria evaluate the context in which the research will be done—defined as the collective set of resources, equipment, institutional support, and facilities available (Environment)—and what is special about the people doing the research (Investigator). For the Environment criterion, reviewers evaluate whether the resources and institutional support available to the investigators are sufficient to ensure successful completion of the research aims, including any unique features such as access to specific subject populations or collaborative arrangements. For the Investigator criterion, reviewers determine whether the primary investigator (PI), other researchers, and any collaborators have the experience and training needed to complete the proposed research, as well as how collaborators will combine their skills and work together.
The five core review criteria ratings, in addition to other proposal-specific criteria, are then used to determine an Overall Impact/Priority Score ( National Institutes of Health, 2015a ; 2015b ). This score reflects the reviewers’ assessment of the “likelihood for the project to exert a sustained, powerful influence on the research field(s) involved.” An application does not need to have exemplary scores in all criteria in order to be judged as likely to have a high overall impact. For example, a project that by its nature is not highly innovative may nevertheless be deemed essential to advance knowledge within a field. A 2011 study by the National Institutes of General Medicine Science (NIGMS) examined the correlation between the core review criteria scores and the Overall Impact score and found that reviewers weighted certain criteria more heavily than others, in the following order: Approach > Significance > Innovation > Investigator > Environment ( Rockey, 2011 ). Thus, the quality of ideas appeared to matter more than investigator reputation, a particularly good finding for new investigators ( Berg, 2010a ; 2010b ; 2010c ). These findings of relative importance of the core review criteria by reviewers also suggest that, in terms of space, it makes sense for proposers to utilize more pages of the proposal narrative to address aspects of their approach and the research project’s significance than on the environment supporting the project.
Other agencies have formalized systems for weighting grant proposal review criteria. For example, the ED NIDRR standard selection criteria are weighted using a points designation ( US Department of Education, 2014 ): Design of Research Activities (50 pts); Importance of the Problem (15 pts); Project Staff (15 pts); Plan of Evaluation (10 pts); and Adequacy and Accessibility of Resources (10 pts). Similar to NIH reviewers, ED weights research design and the importance of the problem more heavily than staff or resources when evaluating grant proposals ( Committee on the External Evaluation of NIDRR and Its Grantees, National Research Council, Rivard, O’Connell, & Wegman, 2011 ).
How do the NIH review criteria compare to those of other federal agencies?
The most straightforward comparison of research grant review criteria is between the NIH and NSF, which together make up 25% of the research and development budget in the US ( AAAS, 2014 ). The NSF criteria emphasize transformative and interdisciplinary research ( National Science Foundation, 2007 ), and involve three (3) guiding principles , two (2) review criteria , and five (5) review elements ( National Science Foundation, 2014 ). The two review criteria used by the NSF are Intellectual Merit, which encompasses the potential to advance the field, and Broader Impacts, which encompasses the potential to benefit society and contribute to the achievement of specific, desired societal outcomes. Within each of these two review criteria are five review elements ( Figure 1 ). These five review elements line up remarkably well with the NIH core review criteria ( Table 2 ), with both agencies’ criteria addressing a similar set of concepts but using distinct language to describe each criterion.

NSF Merit Review Criteria ( National Science Foundation, 2014 )
Comparison of the NIH and NSF research grant proposal review criteria
NIH, National Institutes of Health; NSF, National Science Foundation; PD, program director; PI, principal investigator.
What about a non-science funding agency like the NEH? While there is some variability between individual NEH grant programs, the NEH application review criteria are: Humanities Significance, Project Feasibility and Work Plan, Quality of Innovation, Project Staff Qualifications, and Overall Value to Humanities Scholarship ( National Endowment for the Humanities, 2015a ; 2015b ). The significance of the project includes its potential to enhance research, teaching, and learning in the humanities. The quality of innovation is evaluated in terms of the idea, approach, method, or digital technology (and the appropriateness of the technology) that will be used in the project. Reviewers also examine the qualifications, expertise, and levels of commitment of the project director and key project staff or contributors. The quality of the conception, definition, organization, and description of the project and the applicant’s clarity of expression, as well as the feasibility of the plan of work are also assessed. Finally, reviewers consider the likelihood that the project will stimulate or facilitate new research of value to scholars and general audiences in the humanities. Table 3 shows the NEH review criteria compared with those used by the NIH and NSF. Though there is not an exact match for the key question “In what context will the research be done?” (i.e., the research environment and available resources), this is evaluated in NEH proposals as part of the Project Feasibility and Work Plan.
Comparison of research grant proposal review criteria used by the NIH, NSF, and NEH
NIH, National Institutes of Health; NSF, National Science Foundation; NEH, National Endowment for the Humanities.
Comparing review criteria across federal agencies: Eight key questions
In addition to the core review criteria mentioned above, funding agencies also typically ask reviewers to consider the project budget and the approach that will be used to evaluate project success. When we expanded the comparison of research grant proposal review criteria across 10 US federal agencies, and included the budget and evaluation criteria, we revealed that all of the agencies’ review criteria aligned with a consistent set of eight key questions that reviewers consider when evaluating any type of research proposal ( Table 4 ).
Eight key questions considered by reviewers of research grant proposals and the associated review criteria terms used by 10 US federal funding agencies
The research grant proposal review criteria used by the 10 federal funding agencies are associated with these eight key questions ( Table 5 ). We have already demonstrated that the question, “Why does it matter?”—which addresses the importance or significance of the proposed project— applies to similar review criteria from the NIH (Significance), NSF (Intellectual Merit), and the NEH (Humanities Significance) ( National Endowment for the Humanities, 2015a ; 2015b ; National Institutes of Health, 2015a , 2015b ; National Science Foundation, 2014 ). Likewise, ED evaluates the “Importance of the Problem” ( US Department of Education, 2014 ); the DoD application review criteria includes “Importance” ( Department of Defense, 2015 ); the VA and NASA each evaluate “Significance” ( National Aeronautics and Space Administration, 2015 ; US Department of Veterans Affairs, 2015 ); the DOE looks at “Scientific and Technological Merit” ( US Department of Energy, 2015 ); the USDA evaluates “Project Relevance” ( United States Department of Agriculture, 2015 ); and the NEA assesses “Artistic Excellence” ( National Endowment for the Arts, 2015 ). There are also parallels in the language used by each of the funders as they ask reviewers to assess proposed research project innovation or novelty, the approach or methodology to be used, the investigators or personnel involved, the environment and resources available, and the overall impact or value of the project ( Table 5 ).
Comparison of research grant proposal review criteria across 10 US federal funding agencies
NIH, National Institutes of Health; NSF, National Science Foundation; VA, Department of Veterans Affairs; ED, Department of Education; DoD, Department of Defense; NASA, National Aeronautics and Space Administration; DOE, Department of Education; USDA, US Department of Agriculture; NEH, National Endowment for the Humanities; NEA, National Endowment for the Arts; N/A, not applicable.
While all the agencies’ collective review criteria fall within the eight key questions, there is some variability across agencies. For example, the DOE does not have a clear review criterion for evaluating the overall impact or value of a project, equivalent to the key question “What is the return on investment?” Some agencies to do not explicitly include the budget as part of their review criteria, such as the NSF, VA, and USDA, while other agencies do not specifically ask for a plan to evaluate success of the project, including the NIH, VA, DoD, DOE, USDA, or NEH. Funders may also have unique review criteria. Unlike the other nine agencies evaluated, the DoD uses the review criterion “Application Presentation,” which assesses the writing, clarity, and presentation of the application components. Agencies may also have mission- or program-specific review criteria; for example, for certain applications, the NEA may evaluate the potential to reach underserved populations as part of “Artistic Merit.” Despite these differences, it is clear that for the 10 federal funding agencies examined, the review criteria used to evaluate research grant proposals are extraordinarily aligned.
If we remember that all funding agencies are trying to evaluate research grant proposals to reach the same goals—to determine which projects fit within their mission and will provide a return on their financial investment—it is perhaps not all that surprising that the review criteria that federal funding agencies use are aligned. We further propose that funding announcements from any funder, including state agencies, local groups, and private philanthropic organizations, similarly ask for research grant proposals to answer some, if not all, of the eight key questions that emerged from our analysis of US federal funding agencies. Keeping these key questions in mind can help research administrators and research development offices, as well as proposal writers, decipher research grant proposal review criteria from almost any funding agency, thereby facilitating proposal development.
For this article, we limited our analysis to the review criteria used across different US federal funders to evaluate research grant proposals, and did not include criteria used for other federal funding mechanisms, such as training grants or contract proposals. NIH has compared the review criteria used across their various funding mechanisms, including research grants, grants for conferences and scientific meetings, small business innovation or technology transfer grants, fellowship and career development grants, and training grants, among others ( National Institutes of Health, 2014 ). Again, while there are differences in the language used to describe each core review criterion across the various grant mechanisms, the concepts being reviewed—what is being done, why it is being done, how it is new, who is doing the work, and where it will be done—are essentially the same across each mechanism.
We have demonstrated that research grant proposal review criteria are remarkably aligned across 10 US federal funding agencies, despite the differences in their missions and the terminology each uses for its own review process ( Table 5 ). Moreover, a set of only eight key questions summarizes the collective research grant proposal review criteria across all these federal agencies. While the sheer number of non-federal funding opportunities makes a similar comparative analysis of their review criteria impractical, we suggest that the eight key questions emerging from our analysis provide a starting point for researchers, research administrators, and funders to assess the review criteria used by most, if not all, other research funding opportunities. This is reasonable given that each funder is trying to achieve the same goal during the grant review process: find those research projects that fit the funder’s mission and are worth its investment. Through this lens, the review criteria used for research proposals across agencies are easier to understand and address, which may encourage new investigators to apply for funding, and seasoned investigators and research development offices to consider a diversified set of funding sources for their research portfolios. We also hope that this analysis provides guidance to other grantmakers as they develop review criteria for their own funding opportunities. For the 10 US federal agencies included here, we hope that the analysis serves as a starting point to develop even greater consistency across the review criteria—perhaps even a single canonic, cross-agency set of review criteria—used to evaluate federal research grant proposals.
Acknowledgments
Author’s Note
The authors would like to thank Amy Lamborg, MS, MTSC, for providing invaluable insights and for reviewing the manuscript.
The work is based on material developed by HJF-K for the Grantsmanship for the Research Professionals course at Northwestern University School of Professional Studies (SCS PHIL_ NP 380-0), and was presented in part at the National Organization of Research Development Professionals 7th Annual Research Development Conference in Bethesda, MD, April 29- May 1, 2015.
- American Association for the Advancement of Science Intersociety Working Group. AAAS report XXXIX: Research and development FY 2015. 2014 Retrieved from http://www.aaas.org/page/aaas-report-xxxix-research-and-development-fy-2015 .
- Berg J. Even more on criterion scores: Full regression and principal component analysis. NIGMS Feedback Loop Blog. 2010a Retrieved June 17, 2015, from https://loop.nigms.nih.gov/2010/07/even-more-on-criterion-scores-full-regression-and-principal-component-analyses/
- Berg J. Model organisms and the significance of significance. NIGMS Feedback Loop Blog. 2010b Retrieved June 17, 2015, from https://loop.nigms.nih.gov/2010/07/model-organisms-and-the-significance-of-significance/
- Berg J. More on criterion scores. NIGMS Feedback Loop Blog. 2010c Retrieved June 17, 2015, from https://loop.nigms.nih.gov/2010/07/more-on-criterion-scores/
- Committee on the External Evaluation of NIDRR and Its Grantees, National Research Council . In: Review of disability and rehabilitation research: NIDRR grantmaking processes and products. Rivard JC, O’Connell ME, Wegman DH, editors. National Academies Press; Washington, DC: 2011. [ PubMed ] [ Google Scholar ]
- Department of Defense Congressionally directed medical research programs: Funding opportunities. 2015 Retrieved June 17, 2015, from http://cdmrp.army.mil/funding/ prgdefault.shtml .
- Federation of American Societies for Experimental Biology (FASEB) NIH research funding trends: FY1995-2014. 2014 Retrieved June 17, 2015, from http://www.faseb.org/Policy- and-Government-Affairs/Data-Compilations/NIH-Research-Funding-Trends.aspx .
- Geever JC. Guide to proposal writing. 6th The Foundation Center; New York, NY: 2012. [ Google Scholar ]
- Gerin W, Kapelewski CH. Writing the NIH grant proposal: A step-by-step guide. 2nd SAGE Publications, Inc.; Thousand Oaks, CA: 2010. [ Google Scholar ]
- Kiritz NJ. Program planning and proposal writing. Grantsmanship Center; Los Angeles, CA: 2007. [ Google Scholar ]
- National Aeronautics and Space Administration Guidebook for proposers responding to a NASA Research Announcement (NRA) or Cooperative Agreement Notice (CAN) 2015 Retrieved June 17, 2015, from http://www.hq.nasa.gov/office/procurement/nraguidebook/ proposer2015.pdf .
- National Endowment for the Arts ART WORKS guidelines: Application review. 2015 Retrieved June 17, 2015, from http://arts.gov/grants-organizations/art-works/ application-review .
- National Endowment for the Humanities NEH’s application review process. 2015a Retrieved June 17, 2015, from http://www.neh.gov/grants/application-process#panel .
- National Endowment for the Humanities Office of Digital Humanities: Digital humanities start-up grants. 2015b Retrieved August 10, 2015, from http://www.neh.gov/files/ grants/digital-humanities-start-sept-16-2015.pdf .
- National Institutes of Health Enhancing peer review: The NIH announces enhanced review criteria for evaluation of research applications received for potential FY2010 funding. 2008a Retrieved June 17, 2015, from https://grants.nih.gov/grants/guide/notice-files/NOT-OD-09-025.html .
- National Institutes of Health Enhancing peer review: The NIH announces new scoring procedures for evaluation of research applications received for potential FY2010 funding. 2008b Retrieved June 17, 2015, from http://grants.nih.gov/grants/guide/notice-files/NOT-OD-09-024.html .
- National Institutes of Health Review criteria at a glance. 2014 Retrieved June 17, 2015, from https://grants.nih.gov/grants/peer/Review_Criteria_at_a_Glance_MasterOA.pdf .
- National Institutes of Health Office of Extramural Research Support: Peer review process. 2015a Retrieved September 10, 2015, from http://grants.nih.gov/grants/peer_review_process.htm .
- National Institutes of Health Scoring system and procedure. 2015b Retrieved June 17, 2015, from https://grants.nih.gov/grants/peer/guidelines_general/scoring_system_and_procedure.pdf .
- National Science Foundation Important notice no. 130: Transformative research. 2007 Retrieved June 17, 2015, from http://www.nsf.gov/pubs/2007/in130/in130.jsp .
- National Science Foundation Chapter III - NSF proposal processing and review. Grant proposal guide. 2014 Retrieved September 10, 2015, from http://www.nsf.gov/pubs/policydocs/ pappguide/nsf15001/gpg_3.jsp#IIIA .
- Rockey S. Correlation between overall impact scores and criterion scores. Rock Talk. 2011 Retrieved June 17, 2015, from http://nexus.od.nih.gov/all/2011/03/08/overall-impact-and-criterion-scores/
- Russell SW, Morrison DC. The grant application writer’s workbook: Successful proposals to any agency. Grant Writers’ Seminars and Workshops, LLC; Buellton, CA: 2015. [ Google Scholar ]
- U.S. Department of Agriculture National Institute of Food and Agriculture (NIFA) peer review process for competitive grant applications. 2015 Retrieved June 17, 2015, from http://nifa.usda.gov/resource/nifa-peer-review-process-competitive-grant-applications .
- U.S. Department of Education. Office of Special Education and Rehabilitative Services FY 2014 application kit for new grants under the National Instutite on Disability and Rehabilitation: Field initiated program (research or development) 2014 Retrieved July 8, 2015, from https://www2.ed.gov/programs/fip/2014-133g1-2.doc .
- U.S. Department of Energy Merit review guide. 2015 Retrieved June 17, 2015, from http://energy.gov/management/downloads/merit-review-guide .
- U.S. Department of Veterans Affairs Office of Research & Development BLR&D/ CSR&D merit review program. 2015 Retrieved June 17, 2015, from http://www.research.va.gov/ services/shared_docs/merit_review.cfm .

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
Proposal Review Guidelines
The proposal review guidelines define the level of review performed by OSP based on the date the proposal was submitted to OSP vs. when the proposal is due to be submitted to the sponsor.
Your science/technical content does not have to be complete when you submit to OSP. You can keep working while OSP reviews the rest of the proposal. * A full business day is an official Cornell workday between 8:30 a.m. and 5:00 p.m.
**Adherence to Sponsor Guidelines includes; among other things, length, margins, line spacing, font size, file name and type, required information provided (e.g., Broader Impacts Statement); eligibility criteria; etc.
Risk Assessment
Pre-award research operations (pro), additional things to consider when preparing a proposal, find my gco (grant & contract officer), sample proposal library, limited submissions, pi eligibility, institutional profile, duns, and uei numbers, rass (form 10 is phased out), nfa (non-financial agreements).
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Regulatory Information
- Search for FDA Guidance Documents
- Institutional Review Boards Frequently Asked Questions
INFORMATION SHEET
Institutional Review Boards Frequently Asked Questions Guidance for Institutional Review Boards and Clinical Investigators January 1998
The following is a compilation of answers to questions asked of FDA regarding the protection of human subjects of research. For ease of reference, the numbers assigned to the questions are consecutive throughout this section. These questions and answers are organized as follows.
- IRB Organization
- IRB Membership
- IRB Procedures
- IRB Records
- Informed Consent Process
- Informed Consent Document Content
- Clinical Investigations
- General Questions
I. IRB Organization
1. What is an Institutional Review Board (IRB)?
Under FDA regulations, an IRB is an appropriately constituted group that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications in (to secure approval), or disapprove research. This group review serves an important role in the protection of the rights and welfare of human research subjects.
The purpose of IRB review is to assure, both in advance and by periodic review, that appropriate steps are taken to protect the rights and welfare of humans participating as subjects in the research. To accomplish this purpose, IRBs use a group process to review research protocols and related materials (e.g., informed consent documents and investigator brochures) to ensure protection of the rights and welfare of human subjects of research.
2. Do IRBs have to be formally called by that name?
No, "IRB" is a generic term used by FDA (and HHS) to refer to a group whose function is to review research to assure the protection of the rights and welfare of the human subjects. Each institution may use whatever name it chooses. Regardless of the name chosen, the IRB is subject to the Agency's IRB regulations when studies of FDA regulated products are reviewed and approved.
3. Does an IRB need to register with FDA before approving studies?
As published in the Federal Register on January 15, 2009, (74 FR 2358), 21 CFR Part 56, Institutional Review Boards, was amended with regard to IRB registration (21 CFR 56.106). This amendment requires each IRB in the United States (U.S.) that reviews FDA-regulated studies to register. IRB registration information is entered into an Internet-based registration system maintained by the Department of Health and Human Services (HHS). (See Guidance for Institutional Review Boards (IRBs) Frequently Asked Questions – IRB Registration ).
4. What is an "assurance" or a "multiple project assurance?"
An "assurance," is a document negotiated between an institution and the Department of Health and Human Services (HHS) in accordance with HHS regulations. For research involving human subjects conducted by HHS or supported in whole or in part by HHS, the HHS regulations require a written assurance from the performance-site institution that the institution will comply with the HHS protection of human subjects regulations [45 CFR part 46]. The assurance mechanism is described in 45 CFR 46.103. Once an institution's assurance has been approved by HHS, a number is assigned to the assurance. The assurance may be for a single grant or contract (a "single project assurance"); for multiple grants ("multiple project assurances" - formerly called "general assurances"); or for certain types of studies such as oncology group studies and AIDS research group studies ("cooperative project assurances"). The Office for Human Research Protection (OHRP) is responsible for implementing the HHS regulations. The address and telephone number for OHRP are: 1101 Wootton Parkway, The Tower Building, Suite 200, Rockville, MD 20852; Toll-Free Telephone within the U.S.: (866) 447-4777, Telephone: (240) 453-6900, FAX: (240) 453-6909.
5. Is an "assurance" required by FDA?
Currently, FDA regulations do not require an assurance. FDA regulations [21 CFR parts 50 and 56] apply to research involving products regulated by FDA - federal funds and/or support do not need to be involved for the FDA regulations to apply. When research studies involving products regulated by FDA are funded/supported by HHS, the research institution must comply with both the HHS and FDA regulations. [A table of significant differences between 45 CFR Part 46, Subpart A and 21 CFR Parts 50 and 56 is available on the FDA website.]
6. Must an institution establish its own IRB?
No. Although institutions engaged in research involving human subjects will usually have their own IRBs to oversee research conducted within the institution or by the staff of the institution, FDA regulations permit an institution without an IRB to arrange for an "outside" IRB to be responsible for initial and continuing review of studies conducted at the non-IRB institution. Such arrangements should be documented in writing. Individuals conducting research in a non-institutional setting often use established IRBs (independent or institutional) rather than form their own IRBs. Also see the information sheets entitled "Non-local IRB Review" and "Cooperative Research."
7. May a hospital IRB review a study that will be conducted outside of the hospital?
Yes. IRBs may agree to review research from affiliated or unaffiliated investigators, however, FDA does not require IRBs to assume this responsibility. If the IRB routinely conducts these reviews, the IRB policies should authorize such reviews and the process should be described in the IRB's written procedures. A hospital IRB may review outside studies on an individual basis when the minutes clearly show the members are aware of where the study is to be conducted and when the IRB possesses appropriate knowledge about the study site(s).
8. May IRB members be paid for their services?
The FDA regulations do not preclude a member from being compensated for services rendered. Payment to IRB members should not be related to or dependent upon a favorable decision. Expenses, such as travel costs, may also be reimbursed.
9. What is the FDA role in IRB liability in malpractice suits?
FDA regulations do not address the question of IRB or institutional liability in the case of malpractice suits. FDA does not have authority to limit liability of IRBs or their members. Compliance with FDA regulations may help minimize an IRB's exposure to liability.
10. Is the purpose of the IRB review of informed consent to protect the institution or the subject?
The fundamental purpose of IRB review of informed consent is to assure that the rights and welfare of subjects are protected. A signed informed consent document is evidence that the document has been provided to a prospective subject (and presumably, explained) and that the subject has agreed to participate in the research. IRB review of informed consent documents also ensures that the institution has complied with applicable regulations.
11. Does an IRB or institution have to compensate subjects if injury occurs as a result of participation in a research study?
Institutional policy, not FDA regulation, determines whether compensation and medical treatment(s) will be offered and the conditions that might be placed on subject eligibility for compensation or treatment(s). The FDA informed consent regulation on compensation [21 CFR 50.25(a)(6)] requires that, for research involving more than minimal risk, the subject must be told whether any compensation and any medical treatment(s) are available if injury occurs and, if so, what they are, or where further information may be obtained. Any statement that compensation is not offered must avoid waiving or appearing to waive any of the subject's rights or releasing or appearing to release the investigator, sponsor, or institution from liability for negligence [21 CFR 50.20].
II. IRB Membership
12. May a clinical investigator be an IRB member?
Yes, however, the IRB regulations [21 CFR 56.107(e)] prohibit any member from participating in the IRB's initial or continuing review of any study in which the member has a conflicting interest, except to provide information requested by the IRB. When selecting IRB members, the potential for conflicts of interest should be considered. When members frequently have conflicts and must absent themselves from deliberation and abstain from voting, their contributions to the group review process may be diminished and could hinder the review procedure. Even greater disruptions may result if this person is chairperson of the IRB.
13. The IRB regulations require an IRB to have a diverse membership. May one member satisfy more than one membership category?
Yes. For example, one member could be otherwise unaffiliated with the institution and have a primary concern in a non-scientific area. This individual would satisfy two of the membership requirements of the regulations. IRBs should strive, however, for a membership that has a diversity of representative capacities and disciplines. In fact, the FDA regulations [21 CFR 56.107(a)] require that, as part of being qualified as an IRB, the IRB must have "... diversity of members, including consideration of race, gender, cultural backgrounds and sensitivity to such issues as community attitudes ...."
14. When IRB members cannot attend a convened meeting, may they send someone from their department to vote for them?
No. Alternates who are formally appointed and listed in the membership roster may substitute, but ad hoc substitutes are not permissible as members of an IRB. However, a member who is unable to be present at the convened meeting may participate by video-conference or conference telephone call, when the member has received a copy of the documents that are to be reviewed at the meeting. Such members may vote and be counted as part of the quorum. If allowed by IRB procedures, ad hoc substitutes may attend as consultants and gather information for the absent member, but they may not be counted toward the quorum or participate in either deliberation or voting with the board. The IRB may, of course, ask questions of this representative just as they could of any non-member consultant. Opinions of the absent members that are transmitted by mail, telephone, telefax or e-mail may be considered by the attending IRB members but may not be counted as votes or the quorum for convened meetings.
15. May the IRB use alternate members?
The use of formally appointed alternate IRB members is acceptable to the FDA, provided that the IRB's written procedures describe the appointment and function of alternate members. The IRB roster should identify the primary member(s) for whom each alternate member may substitute. To ensure maintaining an appropriate quorum, the alternate's qualifications should be comparable to the primary member to be replaced. The IRB minutes should document when an alternate member replaces a primary member. When alternates substitute for a primary member, the alternate member should have received and reviewed the same material that the primary member received or would have received.
16. Does a non-affiliated member need to attend every IRB meeting?
No. Although 21 CFR 56.108(c) does not specifically require the presence of a member not otherwise affiliated with the institution to constitute a quorum, FDA considers the presence of such members an important element of the IRB's diversity. Therefore, frequent absence of all non-affiliated members is not acceptable to FDA. Acknowledging their important role, many IRBs have appointed more than one member who is not otherwise affiliated with the institution. FDA encourages IRBs to appoint members in accordance with 21 CFR 56.107(a) who will be able to participate fully in the IRB process.
17. Which IRB members should be considered to be scientists and non-scientists?
21 CFR 56.107(c) requires at least one member of the IRB to have primary concerns in the scientific area and at least one to have primary concerns in the non-scientific area. Most IRBs include physicians and Ph.D. level physical or biological scientists. Such members satisfy the requirement for at least one scientist. When an IRB encounters studies involving science beyond the expertise of the members, the IRB may use a consultant to assist in the review, as provided by 21 CFR 56.107(f).
FDA believes the intent of the requirement for diversity of disciplines was to include members who had little or no scientific or medical training or experience. Therefore, nurses, pharmacists and other biomedical health professionals should not be regarded to have "primary concerns in the non-scientific area." In the past, lawyers, clergy and ethicists have been cited as examples of persons whose primary concerns would be in non-scientific areas.
Some members have training in both scientific and non-scientific disciplines, such as a J.D., R.N. While such members are of great value to an IRB, other members who are unambiguously non-scientific should be appointed to satisfy the non-scientist requirement.
III. IRB Procedures
18. The FDA regulations [21 CFR 56.104(c)] exempt an emergency use of a test article from prospective IRB review, however, "... any subsequent use of the test article at the institution is subject to IRB review." What does the phrase "subsequent use" mean?
FDA regulations allow for one emergency use of a test article in an institution without prospective IRB review, provided that such emergency use is reported to the IRB within five working days after such use. An emergency use is defined as a single use (or single course of treatment, e.g., multiple doses of antibiotic) with one subject. "Subsequent use" would be a second use with that subject or the use with another subject.
In its review of the emergency use, if it is anticipated that the test article may be used again, the IRB should request a protocol and consent document(s) be developed so that an approved protocol would be in place when the next need arises. In spite of the best efforts of the clinical investigator and the IRB, a situation may occur where a second emergency use needs to be considered. FDA believes it is inappropriate to deny emergency treatment to an individual when the only obstacle is lack of time for the IRB to convene, review the use and give approval.
19. Are there any regulations that require clinical investigators to report to the IRB when a study has been completed?
IRBs are required to function under written procedures. One of these procedural requirements [21 CFR 56.108(a)(3)] requires ensuring "prompt reporting to the IRB of changes in a research activity." The completion of the study is a change in activity and should be reported to the IRB. Although subjects will no longer be "at risk" under the study, a final report/notice to the IRB allows it to close its files as well as providing information that may be used by the IRB in the evaluation and approval of related studies.
20. What is expedited review?
Expedited review is a procedure through which certain kinds of research may be reviewed and approved without convening a meeting of the IRB. The Agency's IRB regulations [21 CFR 56.110] permit, but do not require, an IRB to review certain categories of research through an expedited procedure if the research involves no more than minimal risk. A list of categories was last published in the Federal Register on January 27, 1981 [46 FR 8980].
The IRB may also use the expedited review procedure to review minor changes in previously approved research during the period covered by the original approval. Under an expedited review procedure, review of research may be carried out by the IRB chairperson or by one or more experienced members of the IRB designated by the chairperson. The reviewer(s) may exercise all the authorities of the IRB, except disapproval. Research may only be disapproved following review by the full committee. The IRB is required to adopt a method of keeping all members advised of research studies that have been approved by expedited review.
[See Conditions for IRB Use of Expedited Review - Federal Register: November 9, 1998 (Volume 63, Number 216), Notices ]
21. The number of studies we review has increased, and the size of the package of review materials we send to IRB members is becoming formidable. Must we send the full package to all IRB members?
The IRB system was designed to foster open discussion and debate at convened meetings of the full IRB membership. While it is preferable for every IRB member to have personal copies of all study materials, each member must be provided with sufficient information to be able to actively and constructively participate. Some institutions have developed a "primary reviewer" system to promote a thorough review. Under this system, studies are assigned to one or more IRB members for a full review of all materials. Then, at the convened IRB meeting the study is presented by the primary reviewer(s) and, after discussion by IRB members, a vote for an action is taken.
The "primary reviewer" procedure is acceptable to the FDA if each member receives, at a minimum; a copy of consent documents and a summary of the protocol in sufficient detail to determine the appropriateness of the study-specific statements in the consent documents. In addition, the complete documentation should be available to all members for their review, both before and at the meeting. The materials for review should be received by the membership sufficiently in advance of the meeting to allow for adequate review of the materials.
Some IRBs are also exploring the use of electronic submissions and computer access for IRB members. Whatever system the IRB develops and uses, it must ensure that each study receives an adequate review and that the rights and welfare of the subjects are protected.
22. Are sponsors allowed access to IRB written procedures, minutes and membership rosters?
The FDA regulations do not require public or sponsor access to IRB records. However, FDA does not prohibit the sponsor from requesting IRB records. The IRB and the institution may establish a policy on whether minutes or a pertinent portion of the minutes are provided to sponsors.
Because of variability, each IRB also needs to be aware of State and local laws regarding access to IRB records.
23. Must an investigator's brochure be included in the documentation when an IRB reviews an investigational drug study?
For studies conducted under an investigational new drug application, an investigator's brochure is usually required by FDA [21 CFR 312.23(a)(5) and 312.55]. Even though 21 CFR part 56 does not mention the investigator's brochure by name, much of the information contained in such brochures is clearly required to be reviewed by the IRB. The regulations do outline the criteria for IRB approval of research. 21 CFR 56.111(a)(1) requires the IRB to assure that risks to the subjects are minimized. 21 CFR 56.111(a)(2) requires the IRB to assure that the risks to subjects are reasonable in relation to the anticipated benefits. The risks cannot be adequately evaluated without review of the results of previous animal and human studies, which are summarized in the investigator's brochure.
There is no specific regulatory requirement that the Investigator's Brochure be submitted to the IRB. There are regulatory requirements for submission of information which normally is included in the Investigator's Brochure. It is common that the Investigator's Brochure is submitted to the IRB, and the IRB may establish written procedures which require its submission. Investigator's Brochures may be part of the investigational plan that the IRB reviews when reviewing medical device studies.
24. To what extent is the IRB expected to actively audit and monitor the performance of the investigator with respect to human subject protection issues?
FDA does not expect IRBs to routinely observe consent interviews, observe the conduct of the study or review study records. However, 21 CFR 56.109(f) gives the IRB the authority to observe, or have a third party observe, the consent process and the research. When and if the IRB is concerned about the conduct of the study or the process for obtaining consent, the IRB may consider whether, as part of providing adequate oversight of the study, an active audit is warranted.
25. How can a sponsor know whether an IRB has been inspected by FDA, and the results of the inspection?
The Division of Scientific Investigations, Center for Drug Evaluation and Research, maintains an inventory of the IRBs that have been inspected, including dates of inspection and classification. The Division recently began including the results of inspections assigned by the Center for Biologics Evaluation and Research and the Center for Devices and Radiological Health. This information is available through Freedom of Information Act (FOIA) procedures. Once an investigational file has been closed, the correspondence between FDA and the IRB and the narrative inspectional report are also available under FOI.
26. If an IRB disapproves a study submitted to it, and it is subsequently sent to another IRB for review, should the second IRB be told of the disapproval?
Yes. When an IRB disapproves a study, it must provide a written statement of the reasons for its decision to the investigator and the institution [21 CFR 56.109(e)]. If the study is submitted to a second IRB, a copy of this written statement should be included with the study documentation so that it can make an informed decision about the study. 21 CFR 56.109(a) requires an IRB to "... review ... all research activities [emphasis added] ...." The FDA regulations do not prohibit submission of a study to another IRB following disapproval. However, all pertinent information about the study should be provided to the second IRB.
27. May an independent IRB review a study to be conducted in an institution with an IRB?
Generally, no. Most institutional IRB have jurisdiction over all studies conducted within that institution. An independent IRB may become the IRB of record for such studies only upon written agreement with the administration of the institution or the in-house IRB.
28. Could an IRB lose its quorum when members with a conflict of interest leave the room for deliberation and voting on a study?
Yes. "The quorum is the count of the number of members present. If the number present falls below a majority, the quorum fails. The regulations only require that a member who is conflicted not participate in the deliberations and voting on a study on which he or she is conflicted. The IRB may decide whether an individual should remain in the room."
29. Does FDA expect the IRB chair to sign the approval letters?
FDA does not specify the procedure that IRBs must use regarding signature of the IRB approval letter. The written operating procedures for the IRB should outline the procedure that is followed.
30. Does FDA prohibit direct communication between sponsors and IRBs?
It is important that a formal line of communication be established between the clinical investigator and the IRB. Clinical investigators should report adverse events directly to the responsible IRB, and should send progress reports directly to that IRB. However, FDA does not prohibit direct communication between the sponsor and the IRB, and recognizes that doing so could result in more efficient resolution of some problems.
FDA does require direct communication between the sponsors and the IRBs for certain studies of medical devices and when the 21 CFR 50.24 informed consent waiver has been invoked. Sponsors and IRBs are required to communicate directly for medical device studies under 21 CFR 812.2, 812.66 and 812.150(b). For informed consent waiver studies, direct communication between sponsors and IRBs is required under 21 CFR 50.24(e), 56.109(e), 56.109(g), 312.54(b), 312.130(d), 812.38(b)(4) and 812.47(b).
IV. IRB Records
31. Are annual IRB reviews required when all studies are reviewed by the IRB each quarter?
The IRB records for each study's initial and continuing review should note the frequency (not to exceed one year) for the next continuing review in either months or other conditions, such as after a particular number of subjects are enrolled.
An IRB may decide, to review all studies on a quarterly basis. If every quarterly report contains sufficient information for an adequate continuing review and is reviewed by the IRB under procedures that meet FDA requirements for continuing review, FDA would not require an additional "annual" review.
32. 21 CFR 56.115(a)(1) requires that the IRB maintain copies of "research proposals reviewed." Is the "research proposal" the same as the formal study protocol that the investigator receives from the sponsor of the research?
Yes. The IRB should receive and review all research activities [21 CFR 56.109(a)]. The documents reviewed should include the complete documents received from the clinical investigator, such as the protocol, the investigator's brochure, a sample consent document and any advertising intended to be seen or heard by prospective study subjects. Some IRBs also require the investigator to submit an institutionally-developed protocol summary form. A copy of all documentation reviewed is to be maintained for at least three years after completion of the research at that institution [21 CFR 56.115(b)]. However, when the IRB makes changes, such as in the wording of the informed consent document, only the finally approved copy needs to be retained in the IRB records.
33. What IRB records are required for studies that are approved but never started?
When an IRB approves a study, continuing review should be performed at least annually. All of the records listed in 21 CFR 56.115(a)(1) - (4) are required to be maintained. The clock starts on the date of approval, whether or not subjects have been enrolled. Written progress reports should be received from the clinical investigator for all studies that are in approved status prior to the date of expiration of IRB approval. If subjects were never enrolled, the clinical investigator's progress report would be brief. Such studies may receive continuing IRB review using expedited procedures. If the study is finally canceled without subject enrollment, records should be maintained for at least three years after cancellation [21 CFR 56.115(b)].
V. Informed Consent Process
34. Is getting the subject to sign a consent document all that is required by the regulations?
No. The consent document is a written summary of the information that should be provided to the subject. Many clinical investigators use the consent document as a guide for the verbal explanation of the study. The subject's signature provides documentation of agreement to participate in a study, but is only one part of the consent process. The entire informed consent process involves giving a subject adequate information concerning the study, providing adequate opportunity for the subject to consider all options, responding to the subject's questions, ensuring that the subject has comprehended this information, obtaining the subject's voluntary agreement to participate and, continuing to provide information as the subject or situation requires. To be effective, the process should provide ample opportunity for the investigator and the subject to exchange information and ask questions.
35. May informed consent be obtained by telephone from a legally authorized representative?
A verbal approval does not satisfy the 21 CFR 56.109(c) requirement for a signed consent document, as outlined in 21 CFR 50.27(a). However, it is acceptable to send the informed consent document to the legally authorized representative (LAR) by facsimile and conduct the consent interview by telephone when the LAR can read the consent as it is discussed. If the LAR agrees, he/she can sign the consent and return the signed document to the clinical investigator by facsimile.
36. 21 CFR 50.27(a) requires that a copy of the consent document be given to the person signing the form. Does this copy have to be a photocopy of the form with the subject's signature affixed?
No. The regulation does not require the copy of the form given to the subject to be a copy of the document with the subject's signature, although this is encouraged. It must, however, be a copy of the IRB approved document that was given to the subject to obtain consent [21 CFR 50.27(a) or 21 CFR 50.27(b)(2)]. One purpose of providing the person signing the form with a copy of the consent document is to allow the subject to review the information with others, both before and after making a decision to participate in the study, as well as providing a continuing reference for items such as scheduling of procedures and emergency contacts.
37. If an IRB uses a standard "fill-in-the-blank" consent format, does the IRB need to review the filled out form for each study?
Yes. A fill-in-the-blank format provides only some standard wording and a framework for organizing the relevant study information. The IRB should review a completed sample form, individualized for each study, to ensure that the consent document, in its entirety, contains all the information required by 21 CFR 50.25 in language the subject can understand. The completed sample form should be typed to enhance its readability by the subjects. The form finally approved by the IRB should be an exact copy of the form that will be presented to the research subjects. The IRB should also review the "process" for conducting the consent interviews, i.e., the circumstances under which consent will be obtained, who will obtain consent, and so forth.
38. The informed consent regulations [21 CFR 50.25 (a)(5)] require the consent document to include a statement that notes the possibility that FDA may inspect the records. Is this statement a waiver of the subject's legal right to privacy?
No. FDA does not require any subject to "waive" a legal right. Rather, FDA requires that subjects be informed that complete privacy does not apply in the context of research involving FDA regulated products. Under the authority of the Federal Food, Drug, and Cosmetic Act, FDA may inspect and copy clinical records to verify information submitted by a sponsor. FDA generally will not copy a subject's name during the inspection unless a more detailed study of the case is required or there is reason to believe that the records do not represent the actual cases studied or results obtained.
The consent document should not state or imply that FDA needs clearance or permission from the clinical investigator, the subject or the IRB for such access. When clinical investigators conduct studies for submission to FDA, they agree to allow FDA access to the study records, as outlined in 21 CFR 312.68 and 812.145. Informed consent documents should make it clear that, by participating in research, the subject's records automatically become part of the research database. Subjects do not have the option to keep their records from being audited/reviewed by FDA.
When an individually identifiable medical record (usually kept by the clinical investigator, not by the IRB) is copied and reviewed by the Agency, proper confidentiality procedures are followed within FDA. Consistent with laws relating to public disclosure of information and the law enforcement responsibilities of the Agency, however, absolute confidentiality cannot be guaranteed.
39. Who should be present when the informed consent interview is conducted?
FDA does not require a third person to witness the consent interview unless the subject or representative is not given the opportunity to read the consent document before it is signed, see 21 CFR 50.27(b). The person who conducts the consent interview should be knowledgeable about the study and able to answer questions. FDA does not specify who this individual should be. Some sponsors and some IRBs require the clinical investigator to personally conduct the consent interview. However, if someone other than the clinical investigator conducts the interview and obtains consent, this responsibility should be formally delegated by the clinical investigator and the person so delegated should have received appropriate training to perform this activity.
40. How do you obtain informed consent from someone who speaks and understands English but cannot read?
Illiterate persons who understand English may have the consent read to them and "make their mark," if appropriate under applicable state law. The 21 CFR 50.27(b)(2) requirements for signature of a witness to the consent process and signature of the person conducting consent interview must be followed, if a "short form" is used. Clinical investigators should be cautious when enrolling subjects who may not truly understand what they have agreed to do. The IRB should consider illiterate persons as likely to be vulnerable to coercion and undue influence and should determine that appropriate additional safeguards are in place when enrollment of such persons is anticipated, see 21 CFR 56.111(b).
41. Must a witness observe the entire consent interview or only the signature of the subject?
FDA does not require the signature of a witness when the subject reads and is capable of understanding the consent document, as outlined in 21 CFR 50.27(b)(1). The intended purpose is to have the witness present during the entire consent interview and to attest to the accuracy of the presentation and the apparent understanding of the subject. If the intent of the regulation were only to attest to the validity of the subject's signature, witnessing would also be required when the subject reads the consent.
42. Should the sponsor prepare a model informed consent document?
Although not required by the IND regulations, the sponsor provides a service to the clinical investigator and the IRB when it prepares suggested study-specific wording for the scientific and technical content of the consent document. However, the IRB has the responsibility and authority to determine the adequacy and appropriateness of all of the wording in the consent, see 21 CFR 56.109(a), 111(a)(4) and 111(a)(5). If an IRB insists on wording the sponsor cannot accept, the sponsor may decide not to conduct the study at that site. For medical device studies that are conducted under an IDE, copies of all forms and informational materials to be provided to subjects to obtain informed consent must be submitted to FDA as part of the IDE, see 21 CFR 812.25(g).
43 . Is the sponsor required to review the consent form approved by the IRB to make sure all FDA requirements are met?
For investigational devices, the informed consent is a required part of the IDE submission. It is, therefore, approved by FDA as part of the IDE application. When an IRB makes substantive changes in the document, FDA reapproval is required and the sponsor is necessarily involved in this process.
FDA regulations for other products do not specifically require the sponsor to review IRB approved consent documents. However, most sponsors do conduct such reviews to assure the wording is acceptable to the sponsor.
44. Are there alternatives to obtaining informed consent from a subject?
The regulations generally require that the investigator obtain informed consent from subjects. Investigators also may obtain informed consent from a legally authorized representative of the subject. FDA recognizes that a durable power of attorney might suffice as identifying a legally authorized representative under some state and local laws. For example, a subject might have designated an individual to provide consent with regard to health care decisions through a durable power of attorney and have specified that the individual also has the power to make decisions on entry into research. FDA defers to state and local laws regarding who is a legally authorized representative. Therefore, the IRB should assure that the consent procedures comply with state and local laws, including assurance that the law applies to obtaining informed consent for subjects participating in research as well as for patients who require health care decisions."
Alternatives 1 and 2 are provided for in the regulations and are appropriate. Alternative 3 allows a designated individual to provide consent for a patient with regard to health care decisions and is appropriate when it specifically includes entry into research. FDA defers to state and local laws regarding substituted consent. Therefore, the IRB must assure itself that the substituted consent procedures comply with state and local law, including assurance the law applies to obtaining informed consent for subjects participating in research as well as for patients who require health care decisions.
45. When should study subjects be informed of changes in the study?
Protocol amendments must receive IRB review and approval before they are implemented, unless an immediate change is necessary to eliminate an apparent hazard to the subjects (21 CFR 56.108(a)(4)). Those subjects who are presently enrolled and actively participating in the study should be informed of the change if it might relate to the subjects' willingness to continue their participation in the study (21 CFR 50.25(b)(5)). FDA does not require reconsenting of subjects that have completed their active participation in the study, or of subjects who are still actively participating when the change will not affect their participation, for example when the change will be implemented only for subsequently enrolled subjects.
VI. Informed Consent Document Content
46. May an IRB require that the sponsor of the study and/or the clinical investigator be identified on the study's consent document?
Yes. The FDA requirements for informed consent are the minimum basic elements of informed consent that must be presented to a research subject [21 CFR 50.25]. An IRB may require inclusion of any additional information which it considers important to a subject's decision to participate in a research study [21 CFR 56.109(b)].
47. Does FDA require the informed consent document to contain a space for assent by children?
No, however, many investigators and IRBs consider it standard practice to obtain the agreement of older children who can understand the circumstances before enrolling them in research. While the FDA regulations do not specifically address enrollment of children (other than to include them as a class of vulnerable subjects), the basic requirement of 21 CFR 50.20 applies, i.e., the legally effective informed consent of the subject or the subject's legally authorized representative must be obtained before enrollment. Parents, legal guardians and/or others may have the ability to give permission to enroll children in research, depending on applicable state and local law of the jurisdiction in which the research is conducted. (Note: permission to enroll in research is not the same as permission to provide medical treatment.) IRBs generally require investigators to obtain the permission of one or both of the parents or guardian (as appropriate) and the assent of children who possess the intellectual and emotional ability to comprehend the concepts involved. Some IRBs require two documents, a fully detailed explanation for parents and older children to read and sign, and a shorter, simpler one for younger children. [For research supported by DHHS, the additional protections at 45 CFR 46 Subpart D are also required. The Subpart D regulations provide appropriate guidance for all other pediatric studies.]
On April 24, 2001, FDA issued an interim final rule, Additional Protections for Children, as subpart D to 21 CFR Part 50. Assent by children is addressed in subpart D. This interim final rule and its preamble are available at Additional Protections for Children .
48. Does FDA require the signature of children on informed consent documents?
As indicated above, researchers may seek assent of children of various ages. Older children may be well acquainted with signing documents through prior experience with testing, licensing and/or other procedures normally encountered in their lives. Signing a form to give their assent for research would not be perceived as unusual and would be reasonable. Younger children, however, may never have had the experience of signing a document. For these children requiring a signature may not be appropriate, and some other technique to verify assent could be used. For example, a third party may verify, by signature, that the assent of the child was obtained.
As noted in the previous answer, on April 24, 2001, FDA issued an interim final rule, Additional Protections for Children, as subpart D to 21 CFR Part 50. Informed consent of children who participate in clinical trials is addressed in subpart D. This interim final rule and its preamble are available at Additional Protections for Children .
49. Who should be listed on the consent as the contact to answer questions?
21 CFR 50.25(a)(7) requires contacts for questions about the research, the research subject's rights and in case of a research-related injury. It does not specify whom to contact. The same person may be listed for all three. However, FDA and most IRBs believe it is better to name a knowledgeable person other than the clinical investigator as the contact for study subject rights. Having the clinical investigator as the only contact may inhibit subjects from reporting concerns and/or possible abuses.
50. May the "compensation" for participation in a trial offered by a sponsor include a coupon good for a discount on the purchase price of the product once it has been approved for marketing?
No. This presumes, and inappropriately conveys to the subjects, a certainty of favorable outcome of the study and prompt approval for marketing. Also, if the product is approved, the coupon may financially coerce the subject to insist on that product, even though it may not be the most appropriate medically.
51. Must informed consent documents be translated into the written language native to study subjects who do not understand English?
The signed informed consent document is the written record of the consent interview. Study subjects are given a copy of the consent to be used as a reference document to reinforce their understanding of the study and, if desired, to consult with their physician or family members about the study.
In order to meet the requirements of 21 CFR 50.20, the consent document must be in language understandable to the subject. When the prospective subject is fluent in English, and the consent interview is conducted in English, the consent document should be in English. However, when the study subject population includes non-English speaking people so that the clinical investigator or the IRB anticipates that the consent interviews are likely to be conducted in a language other than English, the IRB should assure that a translated consent form is prepared and that the translation is accurate.
A consultant may be utilized to assure that the translation is correct. A copy of the translated consent document must be given to each appropriate subject. While a translator may be used to facilitate conversation with the subject, routine ad hoc translation of the consent document may not be substituted for a written translation.
Also see FDA Information Sheets: "A Guide to Informed Consent Documents" and "Informed Consent and the Clinical Investigator"
52. Is it acceptable for the consent document to say specimens are "donated"?
What about a separate donation statement? It would be acceptable for the consent to say that specimens are to be used for research purposes. However, the word "donation" implies abandonment of rights to the "property". 21 CFR 50.20 prohibits requiring subjects to waive or appear to waive any rights as a condition for participation in the study. Whether or not the wording is contained in "the actual consent form" is immaterial. All study-related documents must be submitted to the IRB for review. Any separate "donation" agreement is regarded to be part of the informed consent documentation, and must be in compliance with 21 CFR 50.
53. Do informed consent forms have to justify fees charged to study subjects?
FDA does not require the consent to contain justification of charges.
VII. Clinical Investigations
54. Does a physician, in private practice, conducting research with an FDA regulated product, need to obtain IRB approval?
Yes. The FDA regulations require IRB review and approval of regulated clinical investigations, whether or not the study involves institutionalized subjects. FDA has included non-institutionalized subjects because it is inappropriate to apply a double standard for the protection of research subjects based on whether or not they are institutionalized.
An investigator should be able to obtain IRB review by submitting the research proposal to a community hospital, a university/medical school, an independent IRB, a local or state government health agency or other organizations.
55. Does a clinical investigation involving a marketed product require IRB review and approval?
Yes, if the investigation is governed by FDA regulations [see 21 CFR 56.101, 56.102(c), 312.2(b)(1), 361.1, 601.2, and 812.2]. Also, see the information sheet entitled " 'Off-label' and Investigational Use of Marketed Drugs and Biologics" for more information.
VIII. General Questions
56. Which FDA office may an IRB contact to determine whether an investigational new drug application (IND) or investigational device exemption (IDE) is required for a study of a test article?
For drugs, the IRB may contact the Center for Drug Evaluation and Research (CDER), Office of Communications, Division of Drug Information at (301) 796-3400.
For biological products, contact the Center for Biologics Evaluation and Research (CBER), Office of Communication, Outreach and Development, at (800)-835-4709 or (301) 827-1800.
For medical devices, contact the Investigational Device Exemption (IDE) Staff, Office of Device Evaluation, Center for Devices and Radiological Health (CDRH), at (301) 796-5640.
57. What happens during an FDA inspection of an IRB?
FDA field investigators interview institutional officials and examine the IRB records to determine compliance with FDA regulations. Also, see the information sheet entitled "FDA Institutional Review Board Inspections" for a complete description of the inspection process.
58. Does a treatment IND/IDE [21 CFR 312.34/812.36 ] require prior IRB approval?
Test articles given to human subjects under a treatment IND/IDE require prior IRB approval, with two exceptions. If a life-threatening emergency exists, as defined by 21 CFR 56.102(d), the procedures described in 56.104(c) ("Exemptions from IRB Requirement") may be followed. In addition, FDA may grant the sponsor or sponsor/investigator a waiver of the IRB requirement in accord with 21 CFR 56.105. An IRB may still choose to review a study even if FDA has granted a waiver. For further information see the information sheets entitled "Emergency Use of an Investigational Drug or Biologic," "Emergency Use of Unapproved Medical Devices," "Waiver of IRB Requirements" and "Treatment use of Investigational Drugs and Biologics."
59. How have the FDA policies on enrollment of special populations changed?
On July 22, 1993, the FDA published the Guideline for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs, in the Federal Register [58 FR 39406]. The guideline was developed to ensure that the drug development process provides adequate information about the effects of drugs and biological products in women. For further information, see the information sheet entitled "Evaluation of Gender Differences in Clinical Investigations."
On December 13, 1994, FDA published a final rule on the labeling of prescription drugs for pediatric populations [59 FR 64240]. The rule [21 CFR 201.57] encourages sponsors to include pediatric subjects in clinical trials so that more complete information about the use of drugs and biological products in the pediatric population can be developed.
60. What is a medical device?
A medical device is any instrument, apparatus, or other similar or related article, including component, part, or accessory, which is: (a) recognized in the official National Formulary, or the United States Pharmacopeia, or any supplement to them; (b) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in humans or other animals; or (c) intended to affect the structure or any function of the human body or in animals; and does not achieve any of its principal intended purposes through chemical action within or on the human body or in animals and is not dependent upon being metabolized for the achievement of its principal intended purposes.
Approximately 1,700 types of medical devices are regulated by FDA. The range of devices is broad and diverse, including bandages, thermometers, ECG electrodes, IUDs, cardiac pacemakers, and hemodialysis machines. For further information, see the information sheets entitled "Medical Devices," "Frequently Asked Questions about IRB Review of Medical Devices" and "Significant Risk and Nonsignificant Risk Medical Device Studies."
61. Are in vitro diagnostic products medical devices?
Yes. The definition of a "device" includes in vitro diagnostic products - devices that aid in the diagnosis of disease or medical/physiological conditions (e.g., pregnancy) by using human or animal components to cause chemical reactions, fermentation, and the like. A few diagnostic products are intended for use in controlling other regulated products (such as those used to screen the blood supply for transfusion-transmitted diseases) and are regulated as biological products.
62. What are the IRB's general obligations towards intraocular lens (IOL) clinical investigations?
An IRB is responsible for the initial and continuing review of all IOL clinical investigations. Each individual IOL style is subject to a separate review by the IRB. This does not, however, preclude the IRB from using prior experience with other IOL investigations in considering the comparative merits of a new lens style. All IOL studies are also subject to FDA approval.
63. Considering the large number of IOL studies, how does an IRB approach the review of a new IOL style?
Full IRB review is required for all new IOLs that exhibit major departures from available lenses. Minor changes to existing lenses may be approved through expedited review. FDA designates new IOL styles as either major or minor changes based upon a predetermined classification scheme and advises the sponsor of its determination. The sponsor, through the investigator, should provide the IRB with the investigational plan which indicates the FDA study requirements, as well as the informed consent document and other comparative information on the proposed lens that describes its characteristics. It is the IRB's prerogative to request any relevant information on a new IOL to arrive at a decision or to be more rigorous in its evaluation than FDA considers minimally required.
64. Must a manufacturer comply with 21 CFR 50 and 56 when conducting trials within its own facility using employees as subjects?
Yes. This situation represents a prime example of a vulnerable subject population.
65. Do Radioactive Drug Research Committees (RDRCs) have authority to approve initial clinical studies in lieu of an IND?
No. An IND is required when the purpose of the study is to determine safety and efficacy of the drug or for immediate therapeutic, diagnostic or similar purposes. RDRCs are provided for in 21 CFR 361.1 Radioactive Drugs for Certain Research Uses . Radioactive drugs (as defined in 21 CFR 310.3(n)) may be administered to human research subjects without obtaining an IND when the purpose of the research project is to obtain basic information regarding the metabolism (including kinetics, distribution, and localization) of a radioactively labelled drug or regarding human physiology, pathophysiology, or biochemistry. Certain basic research studies, e.g., studies to determine whether a drug localizes in a particular organ or fluid space and to describe the kinetics of that localization, may have eventual therapeutic or diagnostic implications, but the initial studies are considered to be basic research within the meaning of 21 CFR 361.1. Such basic research studies must be conducted under the conditions set forth in 21 CFR 361.1(b).
All RDRC approved studies must also be approved by an IRB prior to initiation of the studies.
66. Does FDA approve RDRCs?
Yes. An RDRC must obtain and maintain approval by the Food and Drug Administration, as outlined in 21 CFR 361.1(c). RDRCs must register with the Division of Medical Imaging Products, Center for Drug Evaluation and Research (CDER), FDA, 5901-B Ammendale Road, Beltsville, MD 20105-1266, Attn: RDRC. The FDA contact for compliance issues is the Human Subject Protection Team, Division of Scientific Investigations (DSI),CDER, FDA,10903 New Hampshire Avenue, WO51, Room 5342, Silver Spring, MD 20993.
Submit Comments
Submit comments on this guidance document electronically via docket ID: FDA-2013-S-0610 - Specific Electronic Submissions Intended For FDA's Dockets Management Staff (i.e., Citizen Petitions, Draft Proposed Guidance Documents, Variances, and other administrative record submissions)
If unable to submit comments online, please mail written comments to:
Dockets Management Food and Drug Administration 5630 Fishers Lane, Rm 1061 Rockville, MD 20852
All comments should be identified with the title of the guidance.

Search This Site
- Budget & Planning
- Common Data Set
- Substantive change process
- Brown bags and presentations
- University Factbook
- Diversity dashboards
- Undergraduate
- Graduate School
- Professional Schools
- Medical Division
- Total Enrollment
- Undergraduate Enrollment
- Graduate School Enrollment
- Professional Schools Enrollment
- Medical Division Enrollment
- Tuition and Self-Help
- Degrees Conferred
- Academic Staff
- Non-academic Staff
- External Environment
- All undergraduate students
- Incoming undergraduates
- Graduating seniors
- Faculty and academics
- Submit survey proposals
- Survey calendar
- So you want to survey Cornell students…
- Academic program changes
- Academic program review
Formal Review of Research Proposals
When is Formal Review Required?
Student & Campus Life research projects that will use substantial resources of the Cornell community must be formally reviewed by the committee before they can be initiated. At a minimum, this includes research that draws participants from a major institutional data base, for example, those maintained by the University Registrar; Office of the Dean of Students; Fraternity, Sorority and Independent Living; and Class Councils. Regardless of how potential participants are to be identified, research that meets the following criteria will also require formal review by the committee:
- Involves more that 100 participants for a quantitative data collection method (e.g., survey research) or 25 participants for a qualitative data collection method (e.g., focus groups or interviews);
- Is broader in scope than program evaluation (e.g., asks about more than just program-based experiences or includes individuals who did not participate in the target program or event); and
- Will require a substantial amount of participants’ time (e.g., protocols that will take more than 10 or 15 minutes to complete, or longitudinal research designs).
Conversely, research projects that are very limited in scope, and research that is conducted exclusively for program evaluation purposes (i.e., research that examines the program-related experiences of students who participate in a specific program or event) will generally be exempt from formal review by the committee.
Submitting a Proposal for Formal Review
The committee meets monthly during the fall, winter and spring semesters to formally review research proposals and conduct related business. At least eight weeks before the anticipated launch date of the project, researchers should submit a SCLRG research proposal form to Leslie Meyerhoff or Marne Einarson . The proposal form asks for information about the purpose and proposed design of the study, as well as draft versions of data collection instruments. Samples of completed research proposals are available here and here .
The following criteria will be used by the committee to evaluate research proposals:
- Importance: Does the research address an important issue at Cornell? Will it provide useful information for academic planning or providing services to Cornell students?
- Content and Design : Does the proposed methodology fit the research question(s)? Are the questions well-constructed and easily understood? Is the instrument of reasonable length? Have the questions been pretested?
- Population and Sampling Methodology: Who is the target population? Is the sampling methodology appropriate to the research question(s)? Has the same student cohort and/or sample been used in other recent research? Could a smaller sample be drawn to achieve the same objective? How will the researcher(s) gain access to the proposed participants?
- Timing: Does the proposed timing of the research overlap with or follow closely upon other research directed toward the same population? When were data on this issue last collected at Cornell? Is the data collection period scheduled at a time when students are likely to respond?
- Data Management and Dissemination: Who will have access to the data? What are the provisions for secure storage of the data? Can data from this research be linked to other data sets? What is the plan for analyzing the data and disseminating the results? How will research results contribute to better decision making? How will research results be shared more broadly?
- Resources : What resources will be required to conduct this research (e.g., instrument design, Web application development, mail and/or e-mail services, data entry and analysis)? From where will these resources be obtained?
- Overall Impact: What will be the impact of the study? Are there any conceivable negative impacts on the University? Will the study overburden respondents? Overall, do the expected benefits of the study appear to outweigh the costs?
Based on their evaluation of the research proposal, the committee may decide to:
- Approve the project as submitted
- Approve the project with recommendations for changes that must be adopted before the project can be initiated
- Require revisions and re-submission of the project before approval is granted
- Reject the project (e.g., the potential benefits of the data do not justify the costs of collection; the research design has weaknesses that cannot be rectified)
IRB Approval
If research results will not be used exclusively for internal purposes (e.g., they will be presented or published beyond Cornell; or used for an undergraduate honors thesis, master’s thesis or doctoral dissertation), researchers may also be required to obtain approval from Cornell’s Institutional Review Board for Human Participants (IRB). IRB approval should be sought after the proposal has been reviewed by the SAS Research Group. The committee should subsequently be informed of the decision of the IRB.
© 2024 Cornell University
If you have a disability and are having trouble accessing information on this website or need materials in an alternate format, contact [email protected] for assistance.
We use essential cookies to make Venngage work. By clicking “Accept All Cookies”, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Manage Cookies
Cookies and similar technologies collect certain information about how you’re using our website. Some of them are essential, and without them you wouldn’t be able to use Venngage. But others are optional, and you get to choose whether we use them or not.
Strictly Necessary Cookies
These cookies are always on, as they’re essential for making Venngage work, and making it safe. Without these cookies, services you’ve asked for can’t be provided.
Show cookie providers
- Google Login
Functionality Cookies
These cookies help us provide enhanced functionality and personalisation, and remember your settings. They may be set by us or by third party providers.
Performance Cookies
These cookies help us analyze how many people are using Venngage, where they come from and how they're using it. If you opt out of these cookies, we can’t get feedback to make Venngage better for you and all our users.
- Google Analytics
Targeting Cookies
These cookies are set by our advertising partners to track your activity and show you relevant Venngage ads on other sites as you browse the internet.
- Google Tag Manager
- Infographics
- Daily Infographics
- Template Lists
- Graphic Design
- Graphs and Charts
- Data Visualization
- Human Resources
- Beginner Guides
Blog Business
How to Write a Research Proposal: A Step-by-Step
By Danesh Ramuthi , Nov 29, 2023

A research proposal is a structured outline for a planned study on a specific topic. It serves as a roadmap, guiding researchers through the process of converting their research idea into a feasible project.
The aim of a research proposal is multifold: it articulates the research problem, establishes a theoretical framework, outlines the research methodology and highlights the potential significance of the study. Importantly, it’s a critical tool for scholars seeking grant funding or approval for their research projects.
Crafting a good research proposal requires not only understanding your research topic and methodological approaches but also the ability to present your ideas clearly and persuasively. Explore Venngage’s Proposal Maker and Research Proposals Templates to begin your journey in writing a compelling research proposal.
What to include in a research proposal?
In a research proposal, include a clear statement of your research question or problem, along with an explanation of its significance. This should be followed by a literature review that situates your proposed study within the context of existing research.
Your proposal should also outline the research methodology, detailing how you plan to conduct your study, including data collection and analysis methods.
Additionally, include a theoretical framework that guides your research approach, a timeline or research schedule, and a budget if applicable. It’s important to also address the anticipated outcomes and potential implications of your study. A well-structured research proposal will clearly communicate your research objectives, methods and significance to the readers.

How to format a research proposal?
Formatting a research proposal involves adhering to a structured outline to ensure clarity and coherence. While specific requirements may vary, a standard research proposal typically includes the following elements:
- Title Page: Must include the title of your research proposal, your name and affiliations. The title should be concise and descriptive of your proposed research.
- Abstract: A brief summary of your proposal, usually not exceeding 250 words. It should highlight the research question, methodology and the potential impact of the study.
- Introduction: Introduces your research question or problem, explains its significance, and states the objectives of your study.
- Literature review: Here, you contextualize your research within existing scholarship, demonstrating your knowledge of the field and how your research will contribute to it.
- Methodology: Outline your research methods, including how you will collect and analyze data. This section should be detailed enough to show the feasibility and thoughtfulness of your approach.
- Timeline: Provide an estimated schedule for your research, breaking down the process into stages with a realistic timeline for each.
- Budget (if applicable): If your research requires funding, include a detailed budget outlining expected cost.
- References/Bibliography: List all sources referenced in your proposal in a consistent citation style.

How to write a research proposal in 11 steps?
Writing a research proposal in structured steps ensures a comprehensive and coherent presentation of your research project. Let’s look at the explanation for each of the steps here:
Step 1: Title and Abstract Step 2: Introduction Step 3: Research objectives Step 4: Literature review Step 5: Methodology Step 6: Timeline Step 7: Resources Step 8: Ethical considerations Step 9: Expected outcomes and significance Step 10: References Step 11: Appendices
Step 1: title and abstract.
Select a concise, descriptive title and write an abstract summarizing your research question, objectives, methodology and expected outcomes. The abstract should include your research question, the objectives you aim to achieve, the methodology you plan to employ and the anticipated outcomes.
Step 2: Introduction
In this section, introduce the topic of your research, emphasizing its significance and relevance to the field. Articulate the research problem or question in clear terms and provide background context, which should include an overview of previous research in the field.
Step 3: Research objectives
Here, you’ll need to outline specific, clear and achievable objectives that align with your research problem. These objectives should be well-defined, focused and measurable, serving as the guiding pillars for your study. They help in establishing what you intend to accomplish through your research and provide a clear direction for your investigation.
Step 4: Literature review
In this part, conduct a thorough review of existing literature related to your research topic. This involves a detailed summary of key findings and major contributions from previous research. Identify existing gaps in the literature and articulate how your research aims to fill these gaps. The literature review not only shows your grasp of the subject matter but also how your research will contribute new insights or perspectives to the field.
Step 5: Methodology
Describe the design of your research and the methodologies you will employ. This should include detailed information on data collection methods, instruments to be used and analysis techniques. Justify the appropriateness of these methods for your research.
Step 6: Timeline
Construct a detailed timeline that maps out the major milestones and activities of your research project. Break the entire research process into smaller, manageable tasks and assign realistic time frames to each. This timeline should cover everything from the initial research phase to the final submission, including periods for data collection, analysis and report writing.
It helps in ensuring your project stays on track and demonstrates to reviewers that you have a well-thought-out plan for completing your research efficiently.
Step 7: Resources
Identify all the resources that will be required for your research, such as specific databases, laboratory equipment, software or funding. Provide details on how these resources will be accessed or acquired.
If your research requires funding, explain how it will be utilized effectively to support various aspects of the project.
Step 8: Ethical considerations
Address any ethical issues that may arise during your research. This is particularly important for research involving human subjects. Describe the measures you will take to ensure ethical standards are maintained, such as obtaining informed consent, ensuring participant privacy, and adhering to data protection regulations.
Here, in this section you should reassure reviewers that you are committed to conducting your research responsibly and ethically.
Step 9: Expected outcomes and significance
Articulate the expected outcomes or results of your research. Explain the potential impact and significance of these outcomes, whether in advancing academic knowledge, influencing policy or addressing specific societal or practical issues.
Step 10: References
Compile a comprehensive list of all the references cited in your proposal. Adhere to a consistent citation style (like APA or MLA) throughout your document. The reference section not only gives credit to the original authors of your sourced information but also strengthens the credibility of your proposal.
Step 11: Appendices
Include additional supporting materials that are pertinent to your research proposal. This can be survey questionnaires, interview guides, detailed data analysis plans or any supplementary information that supports the main text.
Appendices provide further depth to your proposal, showcasing the thoroughness of your preparation.

Research proposal FAQs
1. how long should a research proposal be.
The length of a research proposal can vary depending on the requirements of the academic institution, funding body or specific guidelines provided. Generally, research proposals range from 500 to 1500 words or about one to a few pages long. It’s important to provide enough detail to clearly convey your research idea, objectives and methodology, while being concise. Always check
2. Why is the research plan pivotal to a research project?
The research plan is pivotal to a research project because it acts as a blueprint, guiding every phase of the study. It outlines the objectives, methodology, timeline and expected outcomes, providing a structured approach and ensuring that the research is systematically conducted.
A well-crafted plan helps in identifying potential challenges, allocating resources efficiently and maintaining focus on the research goals. It is also essential for communicating the project’s feasibility and importance to stakeholders, such as funding bodies or academic supervisors.

Mastering how to write a research proposal is an essential skill for any scholar, whether in social and behavioral sciences, academic writing or any field requiring scholarly research. From this article, you have learned key components, from the literature review to the research design, helping you develop a persuasive and well-structured proposal.
Remember, a good research proposal not only highlights your proposed research and methodology but also demonstrates its relevance and potential impact.
For additional support, consider utilizing Venngage’s Proposal Maker and Research Proposals Templates , valuable tools in crafting a compelling proposal that stands out.
Whether it’s for grant funding, a research paper or a dissertation proposal, these resources can assist in transforming your research idea into a successful submission.
Preparing a New Study: The Review Process
Types of irb review: how do i know which application to complete link.
There are three categories for IRB review as defined in the federal regulations. They are Exempt, Expedited and Full Board. The category of research is determined by the level of risk to the participant.
Types of Review/Review Categories
Exempt research studies present risks so benign, that the federal regulations determine these types of study to be exempt from review. This determination must be made by the SU IRB.
Research that qualifies for exemption involves no more than minimal risk and meets criteria outlined in one of the six categories specified by federal regulations.
Categories for Exemption
Examples of exempt research might include anonymous surveys, complete data sets already in existence and publicly available, research conducted in commonly accepted educational settings involving normal educational practices.
Exempt applications are reviewed by the ORIP Director, Tracy Cromp.
There are no deadline dates for the submission of exempt applications. They can be submitted electronically as an attachment to an email ( [email protected] ), via campus mail (IRB-214 Lyman Hall), via U.S. mail (IRB-Syracuse University, 214 Lyman Hall, Syracuse, NY 13244) or by hand delivery to our office located in Room 214 of Lyman Hall during regular business hours (Academic year hours are 8:30 am-5pm; summer hours are 8am-4:30 pm).
Turn-around time following initial review is generally 5-7 business days. Should modifications and/or clarifications be requested, additional review time will be required. On average the IRB advises you allow a minimum of 4 weeks for the IRB exempt review process. (This includes the investigators response time.)
All correspondence will flow through the Principal Investigator listed in the study protocol. Students/Research staff will be copied on all communications.
Exempt studies are authorized for a period of five years. They are not renewable. Two months before your research protocol expires you will be notified by the IRB. If you are still conducting the research, you will need to submit a new Exempt application for review and authorization.
If your research is complete prior to the expiration date, send an email to the IRB and request your study be closed. No further action is required.
Expedited research activities present no more than minimal risks to participants– no greater than those encountered in daily life
Research that qualifies for expedited review must be listed in one or more the 9 categories defined by the federal regulations. Categories for Expedited Review:
Examples-research on individual or group characteristics or behaviors; research employing the use of surveys, interviews, focus groups, program evaluation and/or the collection of data through non-evasive procedures (moderate exercise, BP screening, muscular strength, flexibility testing, body composition assessment).
Expedited applications are reviewed by the IRB Chair or one of the two IRB Co-Chairs and, when deemed necessary, an expert consultant (e.g. disabilities expert).
There are no deadlines for the submission of expedited applications. They can be submitted electronically as an attachment to an email ( [email protected] ), via campus mail (IRB-214 Lyman Hall), via U.S. mail (IRB-Syracuse University, 214 Lyman Hall, Syracuse, NY 13244) or by hand delivery to our office located in Room 214 of Lyman Hall during regular business hours (Academic year hours are 8:30 am-5pm; summer hours are 8am-4:30 pm).
Turn-around time for review is approximately 7-10 business days. Should modifications and/or clarifications be requested, additional review time will be required. On average the IRB advises you allow a minimum of 4-6 weeks for the IRB expedited review process. (This includes the investigators response time.)
Expedited studies are approved for a period of one year (unless the IRB determines more frequent review is necessary) and are renewable for up to 7 years.
Full Board Review
Your research requires full board review when:
- Involvement in the research exposes Human Subjects to discomfort or harassment beyond levels encountered in daily life.
- Disclosure of the subject’s responses outside of the research could place them at risk of criminal/civil liability, be damaging to their social and/or financial standing, employability or reputation.
- The research involves prisoners/legally restricted persons as participants.
Full board applications must be reviewed at a convened meeting of the IRB with a majority of IRB members in attendance.
There is a hard deadline for full board applications. Full board applications must be received two weeks prior to the scheduled IRB meeting.
The IRB meets monthly except during the month of July. The meeting schedule is posted on our website. Link to submission deadline schedule:
Full board applications cannot be submitted electronically. The application packet must include one original plus 12 copies of the signed application, including all appendices. The original copy should be single sided. The 12 copies can be double sided. The application packet can be submitted via campus mail (IRB-214 Lyman Hall), via U.S. mail (IRB-Syracuse University, 214 Lyman Hall, Syracuse, NY 13244) or by hand delivery to our office located in Room 214 of Lyman Hall during regular business hours (Academic year hours are 8:30 am-5pm; summer hours are 8am-4:30 pm).
The IRB advises you allow a minimum of eight weeks for the full board review approval process.
Full board applications can be approved for up to 365 days from the date of review (unless the IRB determines more frequent review is necessary) and are renewable for up to 7 years.
Recruitment Link
The Institutional Review Board is responsible for reviewing study recruitment procedures and materials to ensure protection of the rights and welfare of human subjects and equitable subject selection. All methods of recruitment must be approved by the IRB prior to implementation. Recruitment materials might include but are not limited to: oral scripts-for direct/in-person/telephone recruitment; posters/flyers/brochures; advertisements-newspaper/radio/television/SU Today/departmental research boards; postings-Internet websites/social media/twitter/Facebook, etc.
All recruitment materials should:
- Clearly state the purpose for the research
- Indicate that solicitation is for research purposes
- List the eligibility requirements for participation
- Specify the time commitment for participation
- Specify the location of the research
- Only when appropriate-include incentive/compensation information that is not emphasized in any manner such as larger or bolder type
If an agency/organization/institution/school/college will aid with recruitment by providing private contact information- listserv, phone numbers, email/home addresses, names, etc.-a letter of cooperation on official letterhead signed by the person who has the authority to share that information must be provided. In such instances, investigators are permitted to contact these sources in order to obtain the required permission.
If you ask the agency/organization/institution/school/college to simply share information regarding participation in your research by forwarding recruitment information, letters are not required.
No recruitment of participants may be initiated prior to formal notification from the IRB of Approval or Exemption of the research.
Informed Consent-Required Elements and Templates Link
Click here for complete list.
Privacy and Confidentiality Link
What is the difference between Privacy and Confidentiality?
The IRB is responsible for systematically evaluating proposed research for adequate provisions which protect the privacy interests of participants and to maintain the confidentiality of identifiable data.
The federal regulations differentiate between privacy and confidentiality, and it is important to understand the difference in order to determine whether these regulatory criteria for approval of human subject research are appropriately met.
Privacy refers to a person’s desire to control the access of others to themselves. For example, persons may not want to be seen entering a place that might stigmatize them, such as a pregnancy counseling center that is clearly identified as such by signs on the front of the building. Privacy concerns people, whereas confidentiality concerns data. The research proposal should outline strategies to protect privacy including how the investigator will access information from or about participants.
In developing strategies for the protection of subjects’ privacy, consideration should be given to:
- The methods used to identify and contact potential
- The settings in which an individual will be interacting with an
- The appropriateness of all personnel present for research
- The methods used to obtain information about
- The nature of the requested
- Information that is obtained about individuals other than the “target participants,” and whether such individuals meet the regulatory definition of “human participant” (e.g., a subject provides information about a family member for a survey)
- Privacy guidelines developed by relevant professional associations and scholarly disciplines (e.g., oral history, anthropology, psychology)
- How to access the minimum amount of information necessary to complete the study
Confidentiality refers to the researcher’s agreement with the participant about how the participant’s identifiable private information will be handled, managed, and disseminated. The research proposal should outline strategies to maintain confidentiality of identifiable data, including controls on storage, handling, and sharing of data. When appropriate, certificates of confidentiality can be used to maintain the confidentiality of identifiable data.
When the IRB evaluates research proposals for strategies for maintaining confidentiality, where appropriate, consideration will be given as to whether:
- Methods to shield participants’ identity adequately protect participant
- There is a long-range plan for protecting the confidentiality of research data, including a schedule for destruction of identifiers associated with the
- The consent form and other information presented to potential research participants adequately and clearly describe confidentiality
- The informed consent process and the informed consent document, and if applicable the Authorization Form, clearly delineates who will have access to the subject’s information and under what circumstances data may be shared (i.e., government agencies, sponsors).
- Answer each question in the application completely. If a questions is not applicable, it is better to state this than to leave sections of the application blank.
- Ensure all applicable attachments are included with the application including:
- All research instruments such as surveys/questionnaires/interview guide questions/focus group guide questions, etc. (The level of risk to the participant cannot be determined by the IRB without this information.)
- Copies of all consent and/or assent forms as applicable.
- Copies of all recruitment tools you plan to use to inform potential participants about your research such as oral recruitment scripts for direct (in-person) and/or telephone recruitment, letters, emails, newspaper/radio/television advertisements, flyers, brochures, posters, and information posted on departmental research boards, the Internet, Social Media, etc.
- When applicable:
- Letters of cooperation
- IRB approval from other institutions
- A list of references citing relevant background information (required for Expedited and Full Board only)
- CITI training certificates if training has been completed through an affiliation other than Syracuse University (required for Expedited and Full Board only).
- Vulnerable Populations Forms if the research involves Children/Minors, Cognitively Impaired Individuals, Prisoners, and/or Pregnant Women.
- A copy of the International Research Form if the research will be physically conducted internationally. Research conducted by a researcher in the U.S. via Skype with persons internationally, is not considered international research and does not require the submission of the International Form.
- The application must be signed by the Principal Investigator and when appropriate, the student/research staff listed on the first page of the Exempt application and in Section 1 of the Expedited/Full Board application.
Full Committee Review Dates and Deadlines Link
Learn more here.

IMAGES
VIDEO
COMMENTS
Research proposal examples. Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We've included a few for you below. Example research proposal #1: "A Conceptual Framework for Scheduling Constraint Management".
Your research advisor will help you determine which IRB should review your research protocol. JAASEP FALL, 2011 85. Information specific to your research institution may also be available via the Internet. Additionally, you may find that your advisor has received prior IRB approval via a grant-writing process.
The design elements and procedures for conducting research are governed by standards of the predominant discipline in which the problem resides, therefore, the guidelines for research proposals are more exacting and less formal than a general project proposal. Research proposals contain extensive literature reviews.
A proposal needs to show how your work fits into what is already known about the topic and what new paradigm will it add to the literature, while specifying the question that the research will answer, establishing its significance, and the implications of the answer. [ 2] The proposal must be capable of convincing the evaluation committee about ...
As we explore the development of the research proposal, you'll be able to: Describe the individual elements of a research proposal. Delineate between the rationale and implementation portions of a research proposal. Discuss the ethical tenets which govern researchers. Define the purpose of an institutional review board.
Ch. 7 - Proposal Review, Approval, and Submission. 7.1 Required Institutional Signature. 7.2 Internal Review of Research Proposals. 7.3 Internal Review of Proposals for Projects other than Research. 7.4 Sponsor Limitation of Proposals from Institution. 7.5 Procedures for the Submission of Proposals. 7.6 Pre-Award Audit/Request for Additional ...
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
Overview: 5 Proposal Writing Essentials. Understand your university's requirements and restrictions. Have a clearly articulated research problem. Clearly communicate the feasibility of your research. Pay very close attention to ethics policies. Focus on writing critically and concisely. 1. Understand the rules of the game.
It puts the proposal in context. 3. The introduction typically begins with a statement of the research problem in precise and clear terms. 1. The importance of the statement of the research problem 5: The statement of the problem is the essential basis for the construction of a research proposal (research objectives, hypotheses, methodology ...
The research proposal serves a triple goal, namely explaining why there is a for your need research, detailing why your research is feasible and perspectives your research. As such writing a research proposal is a valuable exercise even if you do not pursue a scientific career. You will have to
The research proposal could also be considered as a contract, once members of the committee agree to the execution of the project. Requirements may include: an abstract, introduction, literature review, method section, and conclusion. A research proposal has to clearly and concisely identify the proposed research and its importance.
As we explained in Chap. 2, the second step of the design cycle is designing a plan or the research map by writing the research proposal.The requirements for organizing different proposal sections depend on funding agencies and academic institutions [].For example, in National Institutes of Health (NIH) grants, research strategy which consists of significance, innovation, and approach ...
OSPA must receive the following items for review prior to granting approval for proposal submission: 1) eProposal, or completed and signed PCF and PCF Clinical Attachment (if applicable). Completed and signed TRIA-MIRA may be used for non-federal clinical proposals; 2) Proposal, including abstract, budget, budget justification, and Sponsor ...
We have demonstrated that research grant proposal review criteria are remarkably aligned across 10 US federal funding agencies, despite the differences in their missions and the terminology each uses for its own review process (Table 5). Moreover, a set of only eight key questions summarizes the collective research grant proposal review ...
Proposal Review Guidelines. Your science/technical content does not have to be complete when you submit to OSP. You can keep working while OSP reviews the rest of the proposal. * A full business day is an official Cornell workday between 8:30 a.m. and 5:00 p.m. MATTERS ENCOMPASSED IN THE REVIEW.
significant research projects and the research proposal is an essential part of this process. As a student, you may also be given an assignment task that requires you to write a proposal for a research project that may never take place, to familiarise you with the research proposal writing process. A research proposal should address the ...
Research may only be disapproved following review by the full committee. ... an investigator's brochure is usually required by FDA [21 CFR 312.23(a)(5) and 312.55]. ... be able to obtain IRB ...
Submitting a Proposal for Formal Review. The committee meets monthly during the fall, winter and spring semesters to formally review research proposals and conduct related business. At least eight weeks before the anticipated launch date of the project, researchers should submit a SCLRG research proposal form to Leslie Meyerhoff or Marne Einarson.
Writing a research proposal in structured steps ensures a comprehensive and coherent presentation of your research project. Let's look at the explanation for each of the steps here: Step 1: Title and Abstract. Step 2: Introduction. Step 3: Research objectives. Step 4: Literature review.
Figure 1 - Essential Steps in the Peer Review process Research Proposal/Report -This is usually a research funding proposal or a report intended for publication. Peer Review - The research proposal/report is sent out to two or more independent experts for review. Most journals/funding organisations have an assessment system in place, be it an
There are three categories for IRB review as defined in the federal regulations. They are Exempt, Expedited and Full Board. The category of research is determined by the level of risk to the participant. Types of Review/Review Categories. Exemption. Exempt research studies present risks so benign, that the federal regulations
Which of the following is usually required when an intervention will be tested in individuals or a community, data will be collected through interaction with individuals, or identifiable private information will be collected? ... Full review of the research proposal.
Study with Quizlet and memorize flashcards containing terms like A needs assessment answers which of the following questions?, Which of the following is usually required when an intervention will be tested in individuals or a community, data will be collected through interaction with individuals, or identifiable private information will be collected?, Which of the following, related to the ...