

Journal of Cancer Research and Clinical Oncology Impact Factor & Key Scientometrics
Journal of cancer research and clinical oncology overview, impact factor.

I. Basic Journal Info

Journal ISSN: 01715216, 14321335
Publisher: springer verlag, history: 1979-ongoing, journal hompage: link, how to get published:, research categories, scope/description:.
--------------------------------
Best Academic Tools
- Academic Writing Tools
- Proofreading Tools
- Academic Search Engines
- Project Management Tools
- Survey Tools for Research
- Transcription Tools
- Reference Management Software
- AI-Based Summary Generators
- Academic Social Network Sites
- Plagiarism Checkers
- Science Communication Tools
- Jasper AI Review
II. Science Citation Report (SCR)
Journal of cancer research and clinical oncology scr impact factor, journal of cancer research and clinical oncology scr journal ranking, journal of cancer research and clinical oncology scimago sjr rank.
SCImago Journal Rank (SJR indicator) is a measure of scientific influence of scholarly journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from.
Journal of Cancer Research and Clinical Oncology Scopus 2-Year Impact Factor Trend
Journal of cancer research and clinical oncology scopus 3-year impact factor trend, journal of cancer research and clinical oncology scopus 4-year impact factor trend, journal of cancer research and clinical oncology impact factor history.
- 2022 Impact Factor 3.877 4.188 4.15
- 2021 Impact Factor 4.565 4.566 4.453
- 2020 Impact Factor 4.064 4.021 3.841
- 2019 Impact Factor 3.523 3.428 3.383
- 2018 Impact Factor 3.355 3.338 3.197
- 2017 Impact Factor 3.38 3.381 3.296
- 2016 Impact Factor 3.6 3.544 3.426
- 2015 Impact Factor 3.381 3.403 3.489
- 2014 Impact Factor 3.471 NA NA
- 2013 Impact Factor 3.485 NA NA
- 2012 Impact Factor 3.495 NA NA
- 2011 Impact Factor 2.923 NA NA
- 2010 Impact Factor 2.807 NA NA
- 2009 Impact Factor 2.529 NA NA
- 2008 Impact Factor 2.366 NA NA
- 2007 Impact Factor 2.697 NA NA
- 2006 Impact Factor 2.897 NA NA
- 2005 Impact Factor 2.879 NA NA
- 2004 Impact Factor 2.681 NA NA
- 2003 Impact Factor 2.284 NA NA
- 2002 Impact Factor 2.271 NA NA
- 2001 Impact Factor 2.164 NA NA
- 2000 Impact Factor 1.679 NA NA
See what other people are reading
HIGHEST PAID JOBS
- Highest Paying Nursing Jobs
- Highest Paying Non-Physician Jobs
- Highest Paying Immunology Jobs
- Highest Paying Microbiology Jobs
LATEX TUTORIALS
- LaTeX Installation Guide – Easy to Follow Steps to Install LaTeX
- 6 Easy Steps to Create Your First LaTeX Document
- How to Use LaTeX Paragraphs and Sections
- How to Use LaTeX Packages with Examples
MUST-READ BOOKS
- Multidisciplinary
- Health Science
Impact factor (IF) is a scientometric factor based on the yearly average number of citations on articles published by a particular journal in the last two years. A journal impact factor is frequently used as a proxy for the relative importance of a journal within its field. Find out more: What is a good impact factor?
III. Other Science Influence Indicators
Any impact factor or scientometric indicator alone will not give you the full picture of a science journal. There are also other factors such as H-Index, Self-Citation Ratio, SJR, SNIP, etc. Researchers may also consider the practical aspect of a journal such as publication fees, acceptance rate, review speed. ( Learn More )
Journal of Cancer Research and Clinical Oncology H-Index
The h-index is an author-level metric that attempts to measure both the productivity and citation impact of the publications of a scientist or scholar. The index is based on the set of the scientist's most cited papers and the number of citations that they have received in other publications
Journal of Cancer Research and Clinical Oncology H-Index History

scijournal.org is a platform dedicated to making the search and use of impact factors of science journals easier.
Advertisement
Impact Factor:
According to the Journal Citation Reports TM (JCR) from Clarivate TM , 2023, Impact Factors for the AACR Journals:
Five-Year Impact Factor:
While the standard Impact Factor measures citations to journal articles published within a 2-year period, the 5-year Impact Factor evaluates citations to journal articles published within a 5-year period to provide a measure of a journal's longer-term influence.
Eigenfactor:
The Eigenfactor uses methods from network theory to rank the influence of journals, much as Google's PageRank algorithm ranks the influence of web pages, by identifying journals as influential if they are cited often by other influential journals.
Immediacy Index:
An Immediacy Index is a measure of how topical and urgent work published in a scientific journal is. The Immediacy Index is calculated by dividing the number of citations to articles published in a given year by the number of articles published in that year.
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Journal of Experimental & Clinical Cancer Research
Featured article : regulatory mechanisms of immune checkpoints pd-l1 and ctla-4 in cancer.
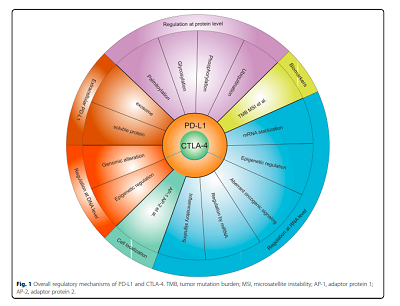
In this review , regulation of PD-L1 and CTLA-4 is discussed at the levels of DNA, RNA, and proteins, as well as indirect regulation of biomarkers, localization within the cell, and drugs. Specifically, some potential drugs have been developed to regulate PD-L1 and CTLA-4 expressions with high efficiency.
Aims and scope
Journal of Experimental & Clinical Cancer Research is an online peer-reviewed journal that provides a high-quality forum for all aspects of basic, clinical and translational work in oncology.
Please click here to read more.
Editor-in-Chief
Mauro Castelli, Regina Elena National Cancer Institute, Italy
If you would like to contact Journal of Experimental & Clinical Cancer Research, please send an email to:
- Most accessed
Analysis of the effect of CCR7 on the microenvironment of mouse oral squamous cell carcinoma by single-cell RNA sequencing technology
Authors: Zengxu Wang, Keith L. Kirkwood, Yao Wang, Weidong Du, Shanfeng Lin, Wanhang Zhou, Cong Yan, Jiaxing Gao, Zhenning Li, Changfu Sun and Fayu Liu
Bispecific aptamer-decorated and light-triggered nanoparticles targeting tumor and stromal cells in breast cancer derived organoids: implications for precision phototherapies
Authors: Simona Camorani, Alessandra Caliendo, Elena Morrone, Lisa Agnello, Matteo Martini, Monica Cantile, Margherita Cerrone, Antonella Zannetti, Massimo La Deda, Monica Fedele, Loredana Ricciardi and Laura Cerchia
PELI1: key players in the oncogenic characteristics of pancreatic Cancer
Authors: Xiaobin Fei, Changhao Zhu, Peng Liu, Songbai Liu, Likun Ren, Rishang Lu, Junyi Hou, Yongjia Gao, Xing Wang and Yaozhen Pan
Ropivacaine as a novel AKT1 specific inhibitor regulates the stemness of breast cancer
Authors: Lin Ding, Hui Jiang, Qiangwei Li, Qiushuang Li, Tian-Tian Zhang, Limeng Shang, Bin Xie, Yaling Zhu, Keshuo Ding, Xuanming Shi, Tao Zhu and Yong Zhu
Auranofin repurposing for lung and pancreatic cancer: low CA12 expression as a marker of sensitivity in patient-derived organoids, with potentiated efficacy by AKT inhibition
Authors: Christophe Deben, Laurie Freire Boullosa, Felicia Rodrigues Fortes, Edgar Cardenas De La Hoz, Maxim Le Compte, Sofie Seghers, Marc Peeters, Steve Vanlanduit, Abraham Lin, Krijn K. Dijkstra, Paul Van Schil, Jeroen M. H. Hendriks, Hans Prenen, Geert Roeyen, Filip Lardon and Evelien Smits
Most recent articles RSS
View all articles
Apoptosis in cancer: from pathogenesis to treatment
Authors: Rebecca SY Wong
High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer
Authors: Franziska Böttger, Andrea Vallés-Martí, Loraine Cahn and Connie R. Jimenez
Ivermectin reverses the drug resistance in cancer cells through EGFR/ERK/Akt/NF-κB pathway
Authors: Lu Jiang, Pan Wang, Ying-Jian Sun and Yi-Jun Wu
Effects of short-term fasting on cancer treatment
Authors: Stefanie de Groot, Hanno Pijl, Jacobus J. M. van der Hoeven and Judith R. Kroep
Iron and leukemia: new insights for future treatments
Authors: Fang Wang, Huanhuan Lv, Bin Zhao, Liangfu Zhou, Shenghang Wang, Jie Luo, Junyu Liu and Peng Shang
Most accessed articles RSS
Springer Nature Oncology Portfolio

Colorectal Cancer Awareness Month
Call for papers: Liquid Biopsy in Precision Oncology
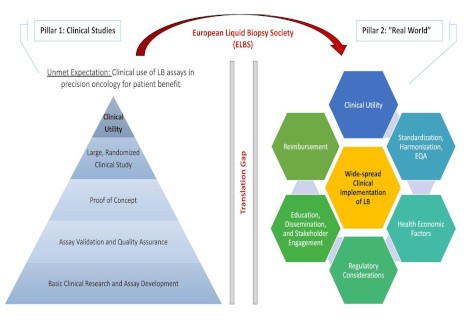
Journal of Experimental & Clinical Cancer Research is delighted to announce a new thematic series focused on:
Liquid Biopsy in Precision Oncology
The Special Issue will accept Research Articles and Reviews in this topic. Submit here .
- Submission opens: 1 st February 2024
- Submission deadline: 31 st January 2025
Click here to view this collection. Click here to access all thematic series published to date in Journal of Experimental & Clinical Cancer Research .
Call for papers: CRISPR-Cas9 system, the next generation in cancer therapy and target discovery

Journal of Experimental & Clinical Cancer Research is delighted to announce a new thematic series focused on:
CRISPR-Cas9 system, the next generation in cancer therapy and target discovery
The Special Issue will accept Research Articles and Review in this topic. Submit here .
- Submission opens: 1 st April 2023
- Submission deadline: 30 th June 2024
Click here to view this collection. Click here to access all thematic series published to date in Journal of Experimental & Clinical Cancer Research.
Follow JECCR on Social Media

Follow JECCR's social media accounts to be kept up-to-date with the latest articles, collections and journal news!
Recognising Editorial Excellence

Reviewer Acknowledgement and New Recruitment
The Editor-in-Chief of Journal of Experimental & Clinical Cancer Research would like to thank all of our reviewers who have contributed to the journal and is looking for new reviewers to assess manuscripts. For consideration, please send your CV with keywords and expertise to [email protected]

Archival content
Journal of Experimental & Clinical Cancer Research has been publishing since 1982. Prior to publishing with BioMed Central from 2008, Journal of Experimental & Clinical Cancer Research was published in print. For enquiries about previous content, please contact us on:

Owned by the Association for International Promotion & Study in Tumors (APSIT)

Official journal of the Regina Elena National Cancer Institute , Scientific Director Gennaro Ciliberto, Rome, Italy
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
- Submit manuscript
Annual Journal Metrics
2022 Citation Impact 11.3 - 2-year Impact Factor 11.5 - 5-year Impact Factor 1.870 - SNIP (Source Normalized Impact per Paper) 2.413 - SJR (SCImago Journal Rank)
2023 Speed 4 days submission to first editorial decision for all manuscripts (Median) 100 days submission to accept (Median)
2023 Usage 3,003,080 downloads 3,022 Altmetric mentions
- More about our metrics
ISSN: 1756-9966
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Journal of Clinical Oncology

Subject Area and Category
- Cancer Research
- Medicine (miscellaneous)
Lippincott Williams and Wilkins Ltd.
Publication type
15277755, 0732183X
Information
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2022. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy

- Source: Kantar Health (Cerner Enviza) estimate.
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666-75.
- Tian J, Sun J, Fu G, et al. Population-based outcome of muscle-invasive bladder cancer following radical cystectomy: who can benefit from adjuvant chemotherapy? Transl Androl Urol. 2021;10(1):356-373.
- Bajorin D, Witjes JA, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. N Engl J Med. 2021;384(22):2102-2114.
- Nivolumab Package Insert. https://packageinserts.bms.com/pi/pi_opdivo.pdf
- Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature . 2021;595(7867):432-437.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240401420903/en/
Related News
@ the bell: stocks level-out ahead of easter long weekend, recent u.s. press releases, schwab announces its spring business update, blackrock to report first quarter 2024 earnings on april 12th, a story of defiance… comcast's black experience on xfinity announces premiere of powerful..., featured news links, a green steel project with after-tax irr of 25% and the ability to generate us$173m of annual ebitda, this gambling tech stock is future-proofing the world’s casinos.
Get the latest news and updates from Stockhouse on social media
Follow STOCKHOUSE Today
Does the time interval from neoadjuvant camrelizumab combined with chemotherapy to surgery affect outcomes for locally advanced esophageal squamous cell carcinoma?
- Open access
- Published: 27 March 2024
- Volume 150 , article number 161 , ( 2024 )
Cite this article
You have full access to this open access article
- Jiacong Liu 1 na1 ,
- Linhai Zhu 1 na1 ,
- Xuhua Huang 1 na1 ,
- Zhongjie Lu 1 ,
- Yanye Wang 1 ,
- Yuhong Yang 1 ,
- Jiayue Ye 1 ,
- Chen Gu 1 ,
- Wang Lv 1 ,
- Chong Zhang 1 &
- Jian Hu 1 , 2
95 Accesses
Explore all metrics
There is currently no consensus on the optimal interval time between neoadjuvant therapy and surgery, and whether prolonged time interval from neoadjuvant therapy to surgery results in bad outcomes for locally advanced esophageal squamous cell carcinoma (ESCC). In this study, we aim to evaluate outcomes of time intervals ≤ 8 weeks and > 8 weeks in locally advanced ESCC.
This retrospective study consecutively included ESCC patients who received esophagectomy after neoadjuvant camrelizumab combined with chemotherapy at the Department of Thoracic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine. The primary endpoints were disease-free survival (DFS) and overall survival (OS), while the secondary endpoints were pathological response, surgical outcomes, and postoperative complications.
From 2019 to 2021, a total of 80 patients were included in our study and were divided into two groups according to the time interval from neoadjuvant immunochemotherapy to surgery: ≤ 8 weeks group ( n = 44) and > 8 weeks group ( n = 36). The rate of MPR in the ≤ 8 weeks group was 25.0% and 27.8% in the > 8 weeks group ( P = 0.779). The rate of pCR in the ≤ 8 weeks group was 11.4%, with 16.7% in the > 8 weeks group ( P = 0.493). The incidence of postoperative complications in the ≤ 8 weeks group was 27.3% and 19.4% in the > 8 weeks group ( P = 0.413). The median DFS in the two groups had not yet reached (hazard ratio [HR], 3.153; 95% confidence interval [CI] 1.383 to 6.851; P = 0.004). The median OS of ≤ 8 weeks group was not achieved (HR, 3.703; 95% CI 1.584 to 8.657; P = 0.0012), with the > 8 weeks group 31.6 months (95% CI 21.1 to 42.1). In multivariable analysis, inferior DFS and OS were observed in patients with interval time > 8 weeks (HR, 2.992; 95% CI 1.306 to 6.851; and HR, 3.478; 95% CI 1.481 to 8.170, respectively).
Conclusions
Locally advanced ESCC patients with time interval from neoadjuvant camrelizumab combined with chemotherapy to surgery > 8 weeks were associated with worse long-term survival.
Similar content being viewed by others
Effect of Time to Minimally Invasive Esophagectomy After Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma
Nguyen Vo Vinh Loc, Nguyen Lam Vuong, … Tran Thien Trung
Three-arm phase II trial comparing camrelizumab plus chemotherapy versus camrelizumab plus chemoradiation versus chemoradiation as preoperative treatment for locally advanced esophageal squamous cell carcinoma (NICE-2 Study)
Yang Yang, Li Zhu, … Zhigang Li

Safety and efficacy of camrelizumab combined with radiotherapy as neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma: a prospective single-arm phase II clinical trial protocol
Maohui Chen, Yizhou Huang, … Bin Zheng
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer (EC) is the seventh most prevalent tumor and the sixth most common cause of cancer-related death worldwide (Siegel et al. 2022 ). Among its two histological subtypes, esophageal squamous cell carcinoma (ESCC) is more common in Asia, accounting for approximately 90% of EC cases in China. (Arnold et al. 2015 ; Zhang 2013 ). And most patients are diagnosed with locally advanced EC (Yang et al. 2023 ). Nowadays, the standard treatment for patients with locally advanced EC is neoadjuvant therapy [chemotherapy (Ando et al. 2012 ), chemoradiotherapy (Hagen et al. 2012 ; Yang et al. 2018 ), immunochemotherapy (Yan et al. 2022 ; Liu et al. 2022 ), chemoradiotherapy plus immunotherapy (Li et al. 2021 )] followed by surgical resection. Neoadjuvant treatment can reduce the tumor size, lower the tumor stage, and subsequent surgical resection can remove the tumor more thoroughly and result in better outcomes.
Although neoadjuvant therapy followed by surgery has been recommended as the standard treatment for locally advanced EC, there still has been no consensus on the optimal interval time between neoadjuvant therapy and surgery. Surgical procedure was usually suggested after an interval of 4 to 8 weeks after completion of neoadjuvant treatment in current clinical studies (Hagen et al. 2012 ; Yang et al. 2018 ; Mukherjee et al. 2017 ; Haisley et al. 2016 ). However, surgical resection may sometimes be performed beyond this time frame owing to adverse events of neoadjuvant therapy, personal or logistic reasons. Some studies showed that a prolonged interval between neoadjuvant therapy and esophagectomy resulted in similar outcomes (Kim et al. 2012 ; Kathiravetpillai et al. 2016 ; Nilsson et al. 2020 ). Several studies reported that a prolonged interval between neoadjuvant therapy and esophagectomy was associated with a higher pathological response rate and similar long-term survival (Shapiro et al. 2014 ; Lee et al. 2016 ; Klevebro et al. 2020 ). Several studies revealed increased pathological response with prolonged interval, but worse long-term survival (Levinsky et al. 2020 ; Franko et al. 2016 ; Ranney et al. 2017 ). Additionally, some studies found that a prolonged interval following neoadjuvant therapy before esophagectomy was associated with increased incidence of postoperative complications (Teman et al. 2013 ), increased mortality (Wang et al. 2015 ), and poorer long-term survival (Chidambaram et al. 2023 ). Therefore, we launched this retrospective study to explore whether time interval from neoadjuvant therapy to surgery affect outcomes for locally advanced ESCC. And the cut off of 8 weeks were usually reserved to distinguish between early surgery group and delayed surgery group (Tie et al. 2018 ; Qin et al. 2018 ; Shang et al. 2020 ; Karthyarth et al. 2023 ). Therefore, we set the interval time at 8 weeks in the current study.
Study design and patients
Our study was a retrospective study, which consecutively enrolled ESCC patients who received esophagectomy after neoadjuvant camrelizumab combined with chemotherapy at the Department of Thoracic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine. It had been permitted by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (2021 IIT No. 742) and was in line with the Helsinki Declaration (revised in 2013) and Good Clinical Practice Guidelines.
Inclusion criteria included histopathologically diagnosed ESCC by gastroscopy, pre-treatment clinical stage II-IVA (according to the eighth edition of the AJCC TNM staging (Rice et al. 2017 )), receipt of 2–4 cycles (3 weeks per cycle) of neoadjuvant camrelizumab (200 mg) combined with platinum-containing dualdrug chemotherapy (platinum + paclitaxel), age over 18 and under 80 years and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Exclusion criteria were incomplete information at our hospital, previous anticancer treatment (such as radiotherapy, interventional therapy or drug treatment), autoimmune disease or infectious disease, ongoing systemic immunosuppressive treatment, other malignant tumors and distant metastases. These patients were split up into two groups according to time interval from neoadjuvant immunochemotherapy to surgery: ≤ 8 weeks group ( n = 44) and > 8 weeks group ( n = 36).
Treatment procedures and data collection
Immunotherapy regimen was camrelizumab 200 mg. Chemotherapy regimen included platinum (75 mg/m 2 of cisplatin, or area under the curve (AUC) of the plasma concentration–time curve after a single dose = 5 of carboplatin, or 80 mg/m 2 of nedaplatin) and paclitaxel (260 mg/m 2 of albumin-bound paclitaxel). Before neoadjuvant treatment, systematic imaging evaluations were performed for all patients, including computed tomography (CT) of the esophagus, endoscopic ultrasound, positron emission tomography (PET)–CT, brain magnetic resonance imaging and abdominal ultrasound. During neoadjuvant therapy, CT of the esophagus was performed every 2 cycles until the patient underwent surgery or withdrew from treatment. Moreover, routine blood and biochemical blood examinations were conducted every week. And myocardial enzyme spectrum, thyroid function, and coagulation function examinations were done every 3 weeks. We evaluated patients’ gastrointestinal reactions and skin reactions by their complaints. The response evaluation criteria in solid tumor version 1.1 (RECIST 1.1) (Eisenhauer et al. 2009 ) was used to evaluate the tumor treatment response–complete response (CR): disappearance of all target lesions, partial remission (PR): ≥ 30% decline in the total diameter of target lesions, progressive disease (PD): ≥ 20% enlargement in the total diameter of target lesions or the appearance of new lesions, stable disease (SD): neither CR, PR nor PD. Objective response rate (ORR) included CR and PR. Adverse events (AEs) were graded on the basis of Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (Common Terminology Criteria for Adverse Events (CTCAE), 2017 ).
Surgical approaches included open radical surgery, video-assisted thoracoscopic surgery (VATS), and robot-assisted thoracoscopic surgery (RATS). Surgical methods were comprised of Mc-Kewon and Ivor-Lewis. We considered Ivor-Lewis esophagectomy with at least a two-field lymph node dissection for inferior and medial ESCC, and McKeown esophagectomy with three-field lymph node dissection (neck, thoracic and abdominal lymph nodes) for superior ESCC. We adopted tumor regression grade (TRG) to express pathological response. TRG was divided into four categories according to the College of American Pathologists (CAP)/The National Comprehensive Cancer Network (NCCN) guidelines: TRG 0 (no remaining active tumor cells), TRG 1 (residual viable tumor cells ≤ 10%), TRG 2 (10% < residual viable tumor cells ≤ 50%) and TRG 3 (remaining active tumor cells > 50%). The pathological complete remission (pCR) rate and major pathological response (MPR) rate were considered as equal to TRG 0 and TRG 0–1 respectively. Postoperative complications were evaluated based on definitions proposed by the Esophagectomy Complications Consensus Group (ECCG) (Low et al. 2015 ).
After surgery, imaging assessments were conducted every 1–3 months. Patients continued to receive chemotherapy plus camrelizumab after surgery until the full 6 cycles, and then continued to receive camrelizumab alone for 1–2 years or until disease progression. And the follow-up date would not end until at least 1 year after surgery. The primary endpoints of this study were DFS and OS. DFS was defined as the time from surgery to disease progression according to the RECIST 1.1 or death, whichever occurred first. OS was defined as the time from surgery until death from any cause. Secondary endpoints of this study were pathological response (MPR and pCR), surgical outcomes and postoperative complications.
Statistical analysis
Categorical variables were expressed as frequencies (percentages), and continuous variables were shown as the median and interquartile range (IQR). Categorical variables were analyzed using the Chi-square test or Fisher’s exact test and continuous variables were compared with the t-test or Wilcoxon test. DFS and OS were estimated using the Kaplan–Meier method and compared with the stratified log-rank test. Median follow-up time was evaluated with the reverse Kaplan–Meier method. Stratified Cox proportional-hazards models were used to assess the correlation between each study variable and survival outcomes. Statistical analyses were performed using R software (version 4.1.2) and plotting was performed using GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA). A two-sided P value < 0.05 was considered to be statistically significant.
Baseline characteristics
From 2019 to 2021, a total of 80 patients were included in our study and were divided into two groups according to the time interval from neoadjuvant immunochemotherapy to surgery: ≤ 8 weeks group ( n = 44) and > 8 weeks group ( n = 36). The median time to surgery was 51.0 days (IQR, 49.0–54.0 days) in the ≤ 8 weeks group and 96.0 days (IQR, 81.3–101.8 days) in the > 8 weeks group. Characteristics of these patients at baseline are shown in Table 1 . There were no significant differences between the two groups in age, gender, ECOG performance status, smoking status, drinking status, comorbidities, pathological grade, tumor location, clinical stage, and treatment cycle. The ORR in the ≤ 8 weeks group was 77.3% and 86.1% in the > 8 weeks group ( P = 0.314, Fig. 1 A). The rate of T downstaging (assessed by CT before and after neoadjuvant immunochemotherapy) was 72.7% and 83.3% in the ≤ 8 weeks group and the > 8 weeks group, respectively ( P = 0.258, Fig. 1 B). The rate of N downstaging (assessed by CT before and after neoadjuvant immunochemotherapy) in the ≤ 8 weeks group was 18.2% and 27.8% in the > 8 weeks group ( P = 0.307, Fig. 1 C).
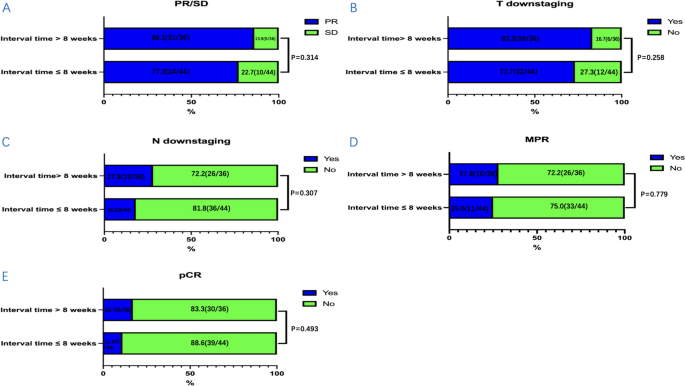
The distribution condition of clinical response, T downstaging, N downstaging and pathological response between the two groups: A PR/SD/PD, B T downstaging, C N downstaging, D MPR, and E pCR. Clinical response included partial remission (PR) and stable disease (SD). Pathological response included major pathological response (MPR) and pathological complete remission (pCR). T downstaging and N downstaging were assessed by CT before and after neoadjuvant immunochemotherapy. CT computed tomography
Adverse events
There were no previously unrecorded AEs in our study. Grade 3–4 AEs of neoadjuvant therapy were summarized in Table 2 . The incidence of grade 3–4 AEs in the ≤ 8 weeks group was 27.3% and 36.1% in the > 8 weeks group. Grade 3–4 AEs were mainly distributed in hematological abnormalities (anemia). There were no significant differences in the occurrence of grade 3–4 AEs between the two groups. These AEs were quickly resolved after symptomatic treatment.
Surgical outcomes and pathological response
The outcomes of surgery and the pathological response were summarized in Table 3 . There were no significant differences in the surgical approach, operation time, blood loss, length of hospital stays, TRG, and ypTNM stage between the two groups. The rate of R0 resection was 100.0% in the ≤ 8 weeks group and 97.2% in the > 8 weeks group. And more lymph nodes were removed during surgery in the ≤ 8 weeks group compared with the > 8 weeks group ( P = 0.034). The rate of MPR in the ≤ 8 weeks group was 25.0% and 27.8% in the > 8 weeks group ( P = 0.779, Fig. 1 D). The rate of pCR in the ≤ 8 weeks group was 11.4%, with 16.7% in the > 8 weeks group ( P = 0.493, Fig. 1 E). Overall, the incidence of postoperative complications in the ≤ 8 weeks group was 27.3% and 19.4% in the > 8 weeks group ( P = 0.413). There were no significant differences in the postoperative complications and no perioperative deaths occurred.
At the time of data cutoff (December 2023), the median follow-up time for the ≤ 8 weeks group was 35.7 months (95% confidence interval [CI] 32.5–39.0), while the median follow-up time for the > 8 weeks group was 31.0 months (95% CI 24.8–37.3). Among the ≤ 8 weeks group, 18.2% (8/44) patients experienced recurrence and metastasis, and 7 patients died due to recurrence and metastasis. Among the > 8 weeks group, 38.9% (14/36) patients experienced recurrence and metastasis, 1 patient died from COVID-19, and 14 patients died due to cancer recurrence and metastasis. The summary of recurrence and metastasis in the two groups is shown in Table 4 .
The median DFS in the two groups had not yet reached (hazard ratio [HR], 3.153; 95% CI 1.383 to 6.851; P = 0.004) (Fig. 2 A). The 1-year DFS rate, 2-year DFS rate, and 3-year DFS rate in the ≤ 8 weeks group were 97.7%, 84.1%, and 79.5%, with that in the > 8 weeks group 72.2%, 61.1% and 55.6%. The median OS of ≤ 8 weeks group was not achieved (HR, 3.703; 95% CI 1.584 to 8.657; P = 0.0012), with the > 8 weeks group 31.6 months (95% CI 21.1 to 42.1) (Fig. 2 B). The 1-year OS rate, 2-year OS rate and 3-year OS rate in the ≤ 8 weeks group were 95.5%, 88.6% and 81.8%, with that in the > 8 weeks group 80.6%, 63.9% and 52.8%.
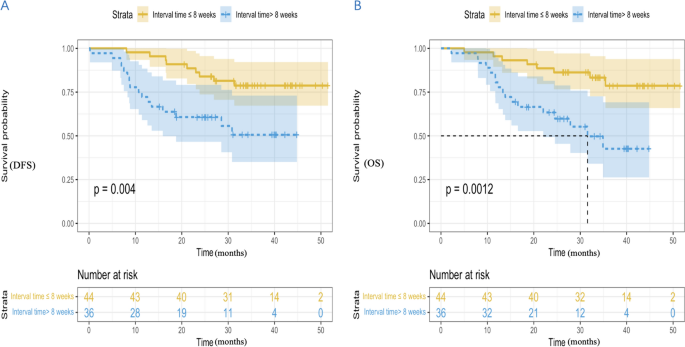
Kaplan Meier curves of DFS ( A ) and OS ( B ) between the two groups. DFS, disease-free survival; OS, overall survival
In univariable Cox regression analyses, there were no statistically significant correlations between these included factors and DFS (Fig. 3 A) or OS (Fig. 3 B), except for pathological grade and interval time. Patients with G3 had inferior DFS and OS (HR, 2.516; 95% CI 1.141 to 5.548; and HR, 2.292; 95% CI 1.040 to 5.051, respectively). Interval time > 8 weeks was associated with inferior DFS and OS (HR, 3.153; 95% CI 1.383 to 6.851; and HR, 3.703; 95% CI 1.584 to 8.657, respectively). Moreover, we performed multivariable Cox regression analyses on statistically significant factors identified through univariable analyses (Fig. 3 C and D). Inferior DFS and OS were observed in patients with interval time > 8 weeks (HR, 2.992; 95% CI 1.306 to 6.851; and HR, 3.478; 95% CI 1.481 to 8.170, respectively). Patients with G3 were associated with inferior DFS (HR, 2.327; 95% CI 1.051 to 5.152), but not inferior OS (HR, 2.032; 95% CI 0.919 to 4.496). It can be seen that interval time ≤ 8 weeks independently predicted better survival.
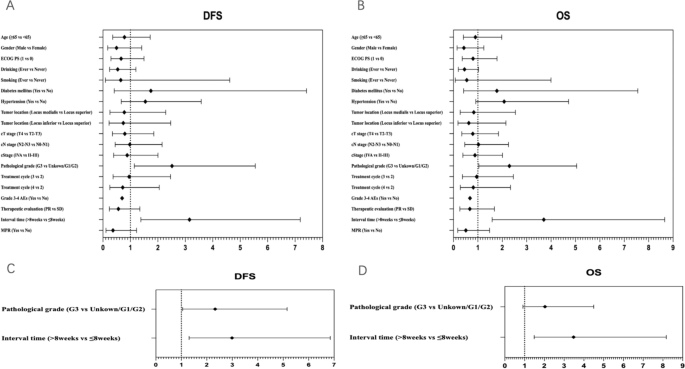
Forest plot of hazard ratio of univariable and multivariable Cox regression analyses for DFS ( A , C ) and OS ( B , D ). DFS , disease-free survival, OS overall survival, ECOG PS eastern cooperative oncology group performance status, PR partial remission, SD stable disease, AEs adverse events, MPR major pathologic response
Nowadays, there is still controversy over the outcomes of prolonged time intervals from neoadjuvant therapy to surgery for locally advanced esophageal squamous cell carcinoma (ESCC) (Kathiravetpillai et al. 2016 ; Shapiro et al. 2014 ; Franko et al. 2016 ; Teman et al. 2013 ). Therefore, we launched this retrospective study to explore whether the time interval from neoadjuvant therapy to surgery affects outcomes for locally advanced ESCC. Moreover, there is currently no consensus on the optimal interval time between neoadjuvant therapy and surgery. In clinical practice, the interval time has usually been set at 4 to 8 weeks (Yang et al. 2018 ; Haisley et al. 2016 ). In the current study, we set the interval time at 8 weeks. We found that the time interval from neoadjuvant camrelizumab combined with chemotherapy to surgery > 8 weeks was not associated with a difference in postoperative complications, postoperative morbidity, and pathological response. However, delaying surgery increases the risk of recurrence and metastasis for locally advanced ESCC patients. A longer interval between neoadjuvant therapy and surgery (> 8 weeks) was associated with worse long-term survival. Despite no significant differences in clinical oncologic factors (cStage) or surgical outcomes (R0 rate, complication) and tumor evaluation variables (pCR, TRG) between the two groups, the prognosis was poor in the surgery group after 8 weeks. In my opinion, the reasons for this result are as listed. Firstly, a longer waiting period may increase the risk of tumor repopulation, recurrence, and metastasis (Tessier et al. 2014 ; Chiu et al. 2013 ). Secondly, a longer waiting period was not a result of the patient’s preferences or opportunities, but rather because of their poor physical condition after neoadjuvant therapy, which may result in an inherent disadvantage in terms of survival. Finally, apart from clinical oncologic factors (cStage) or surgical outcomes (R0 rate, complication) and tumor evaluation variables (pCR, TRG), different factors have a significant impact on OS. Due to the various confounding factors of this issue, it may be necessary to conduct prospective randomized studies.
The findings of our study were different from the findings of other studies. Two studies and a meta-analysis showed there was no significant difference in the pathologic response and overall survival between timely esophagectomy and delayed esophagectomy (Kim et al. 2012 ; Tie et al. 2018 ; Tessier et al. 2014 ). A meta-analysis revealed a longer interval associated with unchanged pathological response and reduced overall survival (Lin et al. 2016 ). Three studies found a prolonged interval was associated with higher pathological response, without affecting survival (Haisley et al. 2016 ; Shapiro et al. 2014 ; Lee et al. 2016 ). Levinsky et al. and a meta-analysis showed that the delayed esophagectomy group (interval ≥ 90 days) had higher rates of pathological complete response and poorer overall survival (Levinsky et al. 2020 ; Qin et al. 2018 ). In our study, we found that there was no significant difference in the pathological response. The rates of MPR and pCR in the ≤ 8 weeks group and > 8 weeks group were similar (25.0% vs 27.8%, 11.4% vs 16.7%, P > 0.05). A longer interval (> 8 weeks) was associated with worse long-term survival. The median DFS in the two groups had not yet reached ( P = 0.004). The median OS of the ≤ 8 weeks group was not achieved ( P = 0.0012), with the > 8 weeks group at 31.6 months. The reasons for these differences may be different interval time, different neoadjuvant therapy regimens, and different treatment cycles.
In the study, we found that pathological grade (G3) and interval time > 8 weeks were associated with inferior DFS and OS in univariable Cox regression analyses. And after multivariable Cox regression analyses, inferior DFS and OS were observed in patients with interval time > 8 weeks. It can be seen that interval time ≤ 8 weeks independently predicted better survival. Therefore, it is not reasonable to delay esophagectomy beyond 8 weeks for patients who can tolerate surgery. However, patients with G3 were associated with inferior DFS (HR, 2.327; 95% CI 1.051 to 5.152), but not inferior OS (HR, 2.032; 95% CI 0.919 to 4.496). The reason for this may be the small sample size. Larger samples and randomized controlled trials are needed to confirm. Additionally, we found more lymph nodes were removed during surgery in the ≤ 8 weeks group compared with the > 8 weeks group ( P = 0.034). The reason we speculated was that delaying surgery made surgical dissection more difficult. A longer waiting period may lead to tumor repopulation or increase fibrosis and adhesion. In our study, there were no significant differences in the postoperative complications and no perioperative deaths occurred. The incidence of postoperative complications varied in different clinical researches. Nilsson et al. and Tie et al. found there were no significant differences in postoperative complications and 90-day mortality (Nilsson et al. 2020 ; Tie et al. 2018 ). Chidambaram et al. and Karthyarth et al. revealed that delay in surgery was associated with higher mortality and complications rates (Chidambaram et al. 2023 ; Karthyarth et al. 2023 ).
There are some limitations in this study
Firstly, our study is a retrospective study. The patients may be allocated to the two groups in a non-randomized manner. This may result in potential bias. And our sample size was small. This may limit our statistical ability for research. Therefore, our findings require larger scale randomized controlled trials to validate. Secondly, there was heterogeneity in patients in our study and our findings were based on a post-hoc analysis, which may cause some impacts on the results. Moreover, delaying surgery after neoadjuvant therapy is inevitable owing to adverse events of neoadjuvant therapy, poor physical condition, personal or logistic reasons. This may result in impacts in terms of survival. And the cutoff point of the interval was different in different studies. In the current study, we set the interval time at 8 weeks. This may result in potential bias. Finally, the postoperative follow-up time of this study was relatively short. Therefore, further follow-up actions are needed to evaluate long-term outcomes.
In conclusion, prolonged time interval from neoadjuvant camrelizumab combined with chemotherapy to surgery may increase the risk of recurrence and metastasis for locally advanced ESCC patients. And a longer interval time (> 8 weeks) was associated with worse long-term survival, but similar pathological response rate. It is not reasonable to delay esophagectomy beyond 8 weeks for patients who can tolerate surgery.
Data availability
The data of the current study are available from the corresponding author on reasonable request.
Ando N, Kato H, Igaki H et al (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19(1):68–74
Article PubMed Google Scholar
Arnold M, Soerjomataram I, Ferlay J, Forman D (2015) Global incidence of oesophageal cancer by histological subtype in 2012. Gut 64(3):381–387
Chidambaram S, Owen R, Sgromo B et al (2023) Delayed surgical intervention after chemoradiotherapy in esophageal cancer: (DICE) study. Ann Surg 278(5):701–708
Chiu CH, Chao YK, Chang HK et al (2013) Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: does delayed surgery impact outcome? Ann Surg Oncol 20:4245–4251
Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: November 27. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Article CAS PubMed Google Scholar
Franko J, Voynov G, Goldman CD (2016) Esophagectomy timing after neoadjuvant therapy for distal esophageal adenocarcinoma. Ann Thorac Surg 101(3):1123–1130
Haisley KR, Laird AE, Nabavizadeh N et al (2016) Association of intervals between neoadjuvant chemoradiation and surgical resection with pathologic complete response and survival in patients with esophageal cancer. JAMA Surg 151(11):e162743
Karthyarth MN, Mathew A, Ramachandra D et al (2023) Early versus delayed surgery following neoadjuvant chemoradiation for esophageal cancer: a systematic review and meta-analysis. Esophagus 20(3):390–401
Kathiravetpillai N, Koëter M, van der Sangen MJ et al (2016) Delaying surgery after neoadjuvant chemoradiotherapy does not significantly influence postoperative morbidity or oncological outcome in patients with oesophageal adenocarcinoma. Eur J Surg Oncol 42(8):1183–1190
Kim JY, Correa AM, Vaporciyan AA et al (2012) Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg 93(1):207–213
Klevebro F, Nilsson K, Lindblad M et al (2020) Association between time interval from neoadjuvant chemoradiotherapy to surgery and complete histological tumor response in esophageal and gastroesophageal junction cancer: a national cohort study. Dis Esophagus 33(5):1–8
Article Google Scholar
Lee A, Wong AT, Schwartz D, Weiner JP, Osborn VW, Schreiber D (2016) Is there a benefit to prolonging the interval between neoadjuvant chemoradiation and esophagectomy in esophageal cancer? Ann Thorac Surg 102(2):433–438
Levinsky NC, Wima K, Morris MC et al (2020) Outcome of delayed versus timely esophagectomy after chemoradiation for esophageal adenocarcinoma. J Thorac Cardiovasc Surg 159(6):2555–2566
Li C, Zhao S, Zheng Y et al (2021) Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer 144:232–241
Lin G, Han SY, Xu YP, Mao WM (2016) Increasing the interval between neoadjuvant chemoradiotherapy and surgery in esophageal cancer: a meta-analysis of published studies. Dis Esophagus 29(8):1107–1114
Liu J, Yang Y, Liu Z et al (2022) Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 10(3):e004291
Article PubMed PubMed Central Google Scholar
Low DE, Alderson D, Cecconello I et al (2015) International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg 262(2):286–294
Mukherjee S, Hurt CN, Gwynne S et al (2017) NEOSCOPE: A randomised phase II study of induction chemotherapy followed by oxaliplatin/capecitabine or carboplatin/paclitaxel based pre-operative chemoradiation for resectable oesophageal adenocarcinoma. Eur J Cancer 74:38–46
Article CAS PubMed PubMed Central Google Scholar
Nilsson K, Klevebro F, Rouvelas I et al (2020) Surgical morbidity and mortality from the multicenter randomized controlled neores II trial: standard versus prolonged time to surgery after neoadjuvant chemoradiotherapy for esophageal cancer. Ann Surg 272(5):684–689
Qin Q, Xu H, Liu J et al (2018) Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A Meta Anal Int J Surg 59:11–18
Ranney DN, Mulvihill MS, Yerokun BA et al (2017) Surgical resection after neoadjuvant chemoradiation for oesophageal adenocarcinoma: what is the optimal timing? Eur J Cardiothorac Surg 52(3):543–551
Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P (2017) Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol 12(1):36–42
Shang QX, Yang YS, Gu YM et al (2020) Timing of surgery after neoadjuvant chemoradiotherapy affects oncologic outcomes in patients with esophageal cancer. World J Gastrointest Oncol 12(6):687–698
Shapiro J, van Hagen P, Lingsma HF et al (2014) Prolonged time to surgery after neoadjuvant chemoradiotherapy increases histopathological response without affecting survival in patients with esophageal or junctional cancer. Ann Surg 260(5):807–814
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33
Teman NR, Silski L, Zhao L et al (2013) Delaying surgery for esophageal cancer increases postoperative complications. J Am Coll Surg 217(3):S35–S36
Tessier W, Gronnier C, Messager M et al (2014) Does timing of surgical procedure after neoadjuvant chemoradiation affect outcomes in esophageal cancer? Ann Thorac Surg 97(4):1181–1189
Tie H, He F, Shen J et al (2018) Prolonged interval between neoadjuvant chemoradiotherapy and esophagectomy does not benefit the outcome in esophageal cancer: a systematic review and meta-analysis. Dis Esophagus 31(1):1–9
van Hagen P, Hulshof MC, van Lanschot JJ et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366(22):2074–2084
Wang BY, Chen HS, Hsu PK et al (2015) Clinical impact of the interval between chemoradiotherapy and esophagectomy in esophageal squamous cell carcinoma patients. Ann Thorac Surg 99(3):947–955
Yan X, Duan H, Ni Y et al (2022) Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD–NICE). Int J Surg 103:106680
Yang H, Liu H, Chen Y et al (2018) Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized Open-Label Clinical Trial. J Clin Oncol 36(27):2796–2803
Yang W, Niu Y, Sun Y (2023) Current neoadjuvant therapy for operable locally advanced esophageal cancer. Med Oncol 40(9):252
Zhang Y (2013) Epidemiology of esophageal cancer. World J Gastroenterol 19(34):5598–5606
Download references
This research was supported by the the National Key Research and Development Program of China (2022YFC2407303); Major Science and Technology Projects of Zhejiang Province (2020C03058); Research Center for Lung Tumor Diagnosis and Treatment of Zhejiang Province (JBZX-202007).
Author information
Jiacong Liu, Linhai Zhu and Xuhua Huang have contributed equally to this work.
Authors and Affiliations
Department of Thoracic Surgery, The First Affiliated Hospital, School of Medicine, Zhejiang University, No. 79 Qingchun Road, Hangzhou, 310003, China
Jiacong Liu, Linhai Zhu, Xuhua Huang, Zhongjie Lu, Yanye Wang, Yuhong Yang, Jiayue Ye, Chen Gu, Wang Lv, Chong Zhang & Jian Hu
Key Laboratory of Clinical Evaluation Technology for Medical Device of Zhejiang Province, Hangzhou, 310003, China
You can also search for this author in PubMed Google Scholar
Contributions
Jiacong Liu (Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Methodology, Writing—original draft). Linhai Zhu (Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Methodology, Writing—review & editing). Xuhua Huang (Conceptualization, Data curation, Investigation, Visualization, Methodology, Writing—review & editing). Zhongjie Lu (Conceptualization, Investigation, Visualization, Methodology). Yanye Wang (Conceptualization, Investigation, Visualization, Methodology). Yuhong Yang (Conceptualization, Investigation, Visualization, Methodology). Jiayue Ye (Conceptualization, Investigation, Visualization, Methodology). Chen Gu (Conceptualization, Investigation, Visualization, Methodology). Wang Lv (Conceptualization, Investigation, Visualization, Methodology). Chong Zhang (Conceptualization, Investigation, Resources, Supervision, Validation). Jian Hu (Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Validation). All authors contributed to the article and approved the submitted version.
Corresponding authors
Correspondence to Linhai Zhu , Chong Zhang or Jian Hu .
Ethics declarations
Conflicts of interest.
The authors have no conflicts of interest to declare.
Ethical statement
This trial was permitted by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (2021 IIT No. 742), and was in line with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice Guidelines. What’s more, we gained written informed consent from enrolled patients so that we could get access to their electronic medical record information.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Liu, J., Zhu, L., Huang, X. et al. Does the time interval from neoadjuvant camrelizumab combined with chemotherapy to surgery affect outcomes for locally advanced esophageal squamous cell carcinoma?. J Cancer Res Clin Oncol 150 , 161 (2024). https://doi.org/10.1007/s00432-024-05696-4
Download citation
Received : 27 January 2024
Accepted : 11 March 2024
Published : 27 March 2024
DOI : https://doi.org/10.1007/s00432-024-05696-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Esophageal squamous cell carcinoma (ESCC)
- Locally advanced
- Neoadjuvant immunochemotherapy
- Interval time to surgery
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
The Journal of Cancer Research and Clinical Oncology publishes content within the fields of experimental and clinical oncology. Topics covered include, but are not limited to: carcinogenesis, molecular biology, developments in tumor therapy, general and laboratory diagnoses, diagnostic and experimental pathology, oncologic surgery, and epidemiology.
The Journal of Cancer Research and Clinical Oncology contains significant and up-to-date articles within the fields of experimental and clinical oncology. ... 2021: Q2: Oncology: 2022: Q2: ... three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric. Cites ...
The Impact IF 2022 of Journal of Cancer Research and Clinical Oncology is 3.88, which is computed in 2023 as per its definition. Journal of Cancer Research and Clinical Oncology IF is decreased by a factor of 0.68 and approximate percentage change is -14.91% when compared to preceding year 2021, which shows a falling trend. The impact IF, also denoted as Journal impact score (JIS), of an ...
The Journal of Cancer Research and Clinical Oncology is a monthly peer-reviewed medical journal covering oncology. ... According to the Journal Citation Reports, the journal has a 2021 impact factor of 4.322. The journal is abstracted and indexed in PubMed, MEDLINE, Scopus, and others.
Scope/Description: The "Journal of Cancer Research and Clinical Oncology" publishes significant and up-to-date articles within the fields of experimental and clinical oncology. The journal, which is chiefly devoted to Original papers and Rapid communications (given priority treatment by the Editors), also includes Reviews as well as Editorials ...
Journal. Five-Year Impact Factor Blood Cancer Discovery: 11.2 Cancer Discovery: 34.7 Cancer Epidemiology, Biomarkers & Prevention: 4.8 Cancer Immunology Research: 12.1 Cancer Prevention Research: 3.6 Cancer Research: 13.0 Clinical Cancer Research: 12.5 Molecular Cancer Research: 5.7 Molecular Cancer Therapeutics: 6.3
International Scientific Journal & Country Ranking. SCImago Institutions Rankings SCImago Media Rankings SCImago Iber SCImago Research Centers Ranking SCImago Graphica Ediciones Profesionales de la Información
Clinical Cancer Advances 2021: ASCO's Report on Progress Against Cancer highlights the most important clinical research advances of the past year and identifies priority areas where ASCO believes research efforts should be focused moving forward. This year's report also discusses the critical issue of health equity in cancer research and solutions to ensure that every patient with cancer ...
The ISSN (Online) of Journal of Cancer Research and Clinical Oncology is 1432-1335 . An ISSN is an 8-digit code used to identify newspapers, journals, magazines and periodicals of all kinds and on all media-print and electronic. Journal of Cancer Research and Clinical Oncology Key Factor Analysis
A world where cancer is prevented or cured. Founded in 1964, the American Society of Clinical Oncology, Inc. (ASCO®) is committed to making a world of difference in cancer care. Through research, education, and promotion of the highest-quality, equitable patient care, ASCO works to conquer cancer and create a world where cancer is prevented or ...
The article by Smith et al entitled "Clinical Cancer Advances 2021: ASCO's Report on Progress Against Cancer" (J Clin Oncol 10.1200/JCO.20.03420) was published online February 2, 2021, with errors. In the author and affiliation lists, Dr. Merry Jennifer Markham was omitted, and Dr. Muhammad S. Beg's middle initial was omitted.
International Scientific Journal & Country Ranking. SCImago Institutions Rankings SCImago Media Rankings SCImago Iber SCImago Research Centers Ranking SCImago Graphica Ediciones Profesionales de la Información
Journal of Experimental & Clinical Cancer Research is a top rated Springer Nature journal. Prof. Mauro Castelli and the editorial team performed in the top percentile of journals based on data collected from the Journal Author Satisfaction Survey. We are recognising extraordinary editors for their commitment and passion to their journals.
Yansu Chen. Yefei Huang. Jianwei Zhou. Correction 22 December 2021 Pages: 1007 - 1009. Volume 148, issue 4 articles listing for Journal of Cancer Research and Clinical Oncology.
During the most recent 2021 edition, 9.40% of publications had an unrecognized affiliation. Out of the publications with recognized affiliations, 14.43% were posted by at least one author from the top 10 institutions publishing in the journal. Another 7.85% included authors affiliated with research institutions from the top 11-20 affiliations. . Institutions from the 21-50 range included 19.75 ...
Clinical Oncology is essential reading for all those with an active interest in the treatment of cancer.Its multidisciplinary approach allows readers to keep up-to-date with developments in their own as well as related fields. Each issue is carefully selected to provide a combination of high quality original research, informative editorials and state-of-the-art reviews. The Journal covers all ...
The clinical validation cohort included 10,258 persons, 7861 of whom met eligibility criteria and were evaluable. A total of 83.1% of the participants with colorectal cancer detected by ...
Combination of treosulfan, fludarabine and cytarabine as conditioning in patients with acute myeloid leukemia, myelodysplastic syndrome and myeloproliferative neoplasms. Samantha O'Hagan Henderson. Jochen J. Frietsch. Jochen Casper. Original Article - Clinical Oncology Open access 21 October 2021 Pages: 2599 - 2609.
The Journal of Clinical Oncology serves its readers as the single most credible, authoritative resource for disseminating significant clinical oncology research. In print and in electronic format, JCO strives to publish the highest quality articles dedicated to clinical research. Original Reports remain the focus of JCO, but this scientific ...
AUSTIN, Texas, April 01, 2024--Natera, Inc. (NASDAQ: NTRA), a global leader in cell-free DNA testing, and the Alliance for Clinical Trials in Oncology, which is part of the National Clinical ...
The ISSN of Journal of Cancer Research and Clinical Oncology is 0171-5216 . An ISSN is an 8-digit code used to identify newspapers, journals, magazines and periodicals of all kinds and on all media-print and electronic. Journal of Cancer Research and Clinical Oncology Key Factor Analysis
Epithelial ovarian cancer (EOC) is the deadliest gynecological malignancy worldwide. Despite the latest advances, a major clinical issue in EOC is the disappointing prognosis related to chemoresistance in almost one-third of cases. Drug resistance relies on heterogeneous cancer stem cells (CSCs), endowed with tumor-initiating potential, leading to relapse. No biomarkers of chemoresistance have ...
During the most recent 2021 edition, 9.20% of publications had an unrecognized affiliation. Out of the publications with recognized affiliations, 32.91% were posted by at least one author from the top 10 institutions publishing in the journal. Another 8.86% included authors affiliated with research institutions from the top 11-20 affiliations. . Institutions from the 21-50 range included 15.50 ...
The Alliance is part of the National Clinical Trials Network (NCTN) funded by the National Cancer Institute (NCI) and serves as a research base for the NCI Community Research Oncology Program (NCORP). The Alliance comprises nearly 10,000 cancer specialists at hospitals, medical centers, and community clinics across the United States and Canada.
The striking variation in CRC globally reflects the large impact of lifestyle factors on cancer occurrence. 125 Similarly, wide differences within the United States in the prevalence of CRC risk factors, such as smoking and excess body weight, and access to high-quality health care, including screening, results in large geographic disparities ...
Baseline characteristics. From 2019 to 2021, a total of 80 patients were included in our study and were divided into two groups according to the time interval from neoadjuvant immunochemotherapy to surgery: ≤ 8 weeks group (n = 44) and > 8 weeks group (n = 36).The median time to surgery was 51.0 days (IQR, 49.0-54.0 days) in the ≤ 8 weeks group and 96.0 days (IQR, 81.3-101.8 days) in ...