Access provided by

Login to your account
If you don't remember your password, you can reset it by entering your email address and clicking the Reset Password button. You will then receive an email that contains a secure link for resetting your password
If the address matches a valid account an email will be sent to __email__ with instructions for resetting your password
Download started.
- PDF [334 KB] PDF [334 KB]
- Add To Online Library Powered By Mendeley
- Add To My Reading List
- Export Citation
- Create Citation Alert
A 100-Year Review: Progress on the chemistry of milk and its components
- John A. Lucey John A. Lucey Correspondence Corresponding author Contact Affiliations Center for Dairy Research, University of Wisconsin–Madison, Madison 53706 Search for articles by this author
- Don Otter Don Otter Affiliations Center for Dairy Research, University of Wisconsin–Madison, Madison 53706 Search for articles by this author
- David S. Horne David S. Horne Affiliations Center for Dairy Research, University of Wisconsin–Madison, Madison 53706 Search for articles by this author
- milk protein
- functionality
- dairy chemistry
INTRODUCTION
- Full Text PDF
- Google Scholar
- Harper W.J.
- Brunner R.J.
- Scopus (49)
- Larson B.L.
- McMeekin T.L.
- Swanson A.M.
- Whitnah C.H.
- Whitney R. McL.
- Scopus (25)
- Farrell Jr., H.M.
- Jimenez-Flores R.
- Butler J.E.
- Creamer L.K.
- Hollar C.M.
- Ng-Kwai-Hang K.F.
- Swaisgood H.E.
- Scopus (1009)
- Richmond H.D.
- Bauman D.E.
- Mather I.H.
- Scopus (294)
A CENTURY OF PROGRESS IN DAIRY CHEMISTRY
Advances in analytical techniques.
- Nitschmann H.
- Scopus (30)
- Bloomfield V.A.
- Scopus (98)
- Scopus (484)
- Kumosinski T.F.
- Scopus (168)
- Haugaard G.
- Pettinati J.D.
- Scopus (13)
Milk Proteins
- Gordon W.G.
- Scopus (65)
- Scopus (468)
- Scopus (157)
- Scopus (331)
- Brodkorb A.
- Scopus (300)
Fractionation and Identification of Individual Milk Proteins
- Linderstrøm-Lang K.
- Mellander O.
- von Hippel P.H.
- Scopus (171)
- Scopus (78)
- Aschaffenburg R.
- Scopus (153)
- Groves M.L.
- Scopus (28)
- Kaminogawa S.
- Mizobuchi H.
- Yamauchi K.
- Hofmann C.J.
- Chibber B.A.K.
- Tomich J.M.
- Keenan T.W.
- Scopus (112)
- Silanikove N.
- Rowland S.J.
- Scopus (369)
Physical and Chemical Properties of Proteins
- Hutton J.T.
- Scopus (57)
- Scopus (158)
- Scopus (62)
- Schauperl M.
- Podewitz M.
- Waldner B.J.
- Scopus (64)
- Scopus (22)
- Srinivasan M.
- Scopus (144)
Calcium Binding
- Scopus (91)
- Scopus (18)
- Scopus (87)
Micelle Models
- Shimmin P.D.
- Scopus (56)
- Scopus (100)
- Stothart P.H.
- Cebula D.J.
- McGann T.C.A.
- Buchheim W.
- Kearney R.D.
- Richardson T.
- Scopus (58)
- Schmidt D.G.
- De Kruif C.G.
- Scopus (432)
- Scopus (584)
Nutritional Aspects of Milk Proteins
- Pellegrino L.
- Cattaneo S.
- Hogenboom J.A.
- Rutherfurd S.M.
- Fanning A.C.
- Miller B.J.
- Moughan P.J.
- Scopus (270)
- Scopus (660)
- Beltrán-Barrientos L.M.
- Hernández-Mendoza A.
- Torres-Llanez M.J.
- González-Córdova A.F.
- Vallejo-Córdoba B.
- Scopus (123)
Functionality of Milk Proteins and Development of New Ingredients
- Mangino M.E.
- Scopus (59)
- Schmidt R.H.
- Packard V.S.
- Morris H.A.
- Scopus (127)
- de Wit J.N.
- Scopus (436)
- Melachouris N.
- Huffman L.M.
- Scopus (96)
- Scopus (29)
- Scopus (368)
- Jensen R.G.
- Sampugna J.
- Scopus (11)
- Scopus (200)
- Parodi P.W.
- Scopus (172)
- Lovegrove J.A.
- Gijsbers L.
- Givens D.I.
- Soedamah-Muthu A.S.
- Scopus (273)
- Palmquist D.L.
- Beaulieu A.D.
- Barbano D.M.
- Scopus (711)
- Whittier E.O.
- Scopus (47)
- Haworth W.N.
- Denton W.L.
- Brodbeck U.
- Whiteman M.
- Yarwood R.J.
- Scopus (37)
Other Milk Components
- McCollum E.V.
- Steenbock H.
- Scopus (90)
- Backstrand J.R.
- Pasteurized Milk Ordinance
- Shahani K.M.
- Parry Jr., R.M.
- Zittle C.A.
- Babcock S.M.
- Russell H.L.
- Jouan P.-N.
- Gauthier S.F.
- Laforest J.-P.
- Brossmer R.
- Scopus (61)
- Scopus (38)
Physical Equilibria and Chemistry of Milk
- Scopus (386)
- Sommer H.H.
- Scopus (17)
- Scopus (72)
- White J.C.D.
- Davies D.T.
- Scopus (43)
- Scopus (67)
- Scopus (51)
- Augustin M.A.
- Scopus (52)
SUMMARY AND FUTURE DIRECTIONS
Acknowledgments.
- Open table in a new tab
- Clarenburg R.
- Chaikoff I.L.
- Reinhardt T.A.
- Lippolis J.D.
- Scopus (222)
- Sorensen M.
- Sorensen S.P.L.
- DePeters E.J.
- German J.B.
- Lebrilla C.B.
- Scopus (207)
Article info
Publication history.
This review is part of a special issue of the Journal of Dairy Science commissioned to celebrate 100 years of publishing (1917–2017).
Identification
DOI: https://doi.org/10.3168/jds.2017-13250
User license

For non-commercial purposes:
- Read, print & download
- Text & data mine
- Translate the article
Not Permitted
- Reuse portions or extracts from the article in other works
- Redistribute or republish the final article
- Sell or re-use for commercial purposes
ScienceDirect
- View Large Image
- Download Hi-res image
- Download .PPT
Related Articles
- Access for Developing Countries
- Articles and Issues
- Articles In Press
- Current Issue
- List of Issues
- Supplements
- For Authors
- Instructions to Authors
- Permissions
- Researcher Academy
- Submit Manuscript
- For Reviewers
- Review a Manuscript
- Scientific Sections in the Journal
- Journal Info
- About the Journal
- About Open Access
- Abstracting/Indexing
- Contact Information
- Content Alerts
- Editorial Board
- Journal Editors
- Display Advertisers
- Recruitment Advertising
- Membership Benefits
- ADSA Meetings
- ADSA Member Access
- Collections
- Editor's Choice
- Meeting Abstracts
- JDS Club 100
- Twitter / X
The content on this site is intended for healthcare professionals and researchers across all fields of science.
- Privacy Policy
- Terms and Conditions
- Accessibility
- Help & Contact

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Data Descriptor
- Open access
- Published: 09 September 2022
MilkyBase, a database of human milk composition as a function of maternal-, infant- and measurement conditions
- Tünde Pacza ORCID: orcid.org/0000-0002-7743-5112 1 ,
- Mayara L. Martins ORCID: orcid.org/0000-0002-9207-1863 1 ,
- Maha Rockaya ORCID: orcid.org/0000-0001-6166-2997 1 ,
- Katalin Müller ORCID: orcid.org/0000-0001-5355-4224 2 , 3 ,
- Ayan Chatterjee 4 , 5 ,
- Albert-László Barabási ORCID: orcid.org/0000-0002-4028-3522 4 , 6 , 7 &
- József Baranyi ORCID: orcid.org/0000-0001-8360-6021 1
Scientific Data volume 9 , Article number: 557 ( 2022 ) Cite this article
2726 Accesses
2 Citations
10 Altmetric
Metrics details
- Data integration
This study describes the development of a database, called MilkyBase, of the biochemical composition of human milk. The data were selected, digitized and curated partly by machine-learning, partly manually from publications. The database can be used to find patterns in the milk composition as a function of maternal-, infant- and measurement conditions and as a platform for users to put their own data in the format shown here. The database is an Excel workbook of linked sheets, making it easy to input data by non-computationally minded nutritionists. The hierarchical organisation of the fields makes sure that statistical inference methods can be programmed to analyse the data. Uncertainty quantification and recording dynamic (time-dependent) compositions offer predictive potentials.
Similar content being viewed by others
Milk proteome from in silico data aggregation allows the identification of putative biomarkers of negative energy balance in dairy cows
Creation of a milk oligosaccharide database, MilkOligoDB, reveals common structural motifs and extensive diversity across mammals
The human milk microbiome aligns with lactation stage and not birth mode
Background & summary.
The effect of diet on health has primarily been analysed in a descriptive way. Widely acknowledged claims, such as garlic helps preventing cardiovascular diseases, are lacking mechanistic, biochemical explanations 1 . The main sources of such uncertainties are: (i) the complexity caused by thousands of chemical interactions; (ii) the inherent errors in the measurements and observations; (iii) many hitherto unknown other details 1 .
Human milk (HM) is the first nutrition an infant comes across and one of our most complex foods. Ideally, mothers should breastfeed their infants, but we need to acknowledge that in many cases this is not possible, and even when mothers try their best, breastfeeding is challenging and requires a strong supportive environment.
HM has been studied extensively, still its biochemical complexity is insufficiently explored 2 , 3 . It is the only food that meets all the nutritional requirements of infants and provides optimal adaptation, somatic growth, maturation, and development 4 . Beside the nutrients (carbohydrates, lipids, proteins, vitamins, and minerals), it provides bioactive components (hormones, cytokines, growth factors, antimicrobial substances, cells, etc.), which play important roles in the development of the central nervous system, metabolism, immune system, and microbiome 5 , 6 , 7 , 8 , 9 . Breastfeeding has been associated with improved health outcomes, including increased intelligence, reduced risks of infections and non-communicable diseases (obesity, atopic diseases, diabetes, inflammatory bowel diseases) 6 , 7 . This crucial role of HM in early life nutrition gains great clinical 5 , 6 , 7 , 10 , social and economic interest due to its impact on long-term health 10 , 11 .
HM is a biological system, where both nutritional and bioactive components are in constant interactions with one another 2 . The exact dynamics depend on characteristics related to the mother, the infant, and various environmental factors (such as the mother’s diet, the gestational age, the geographic location etc.), which are also responsible for the variability of the HM composition 3 . Our current knowledge is largely based on studies evaluating these components, typically analysing their variability and dynamics separately 2 , 3 , 7 . Therefore, explaining health outcomes directly by specific components is rarely satisfactory, due to the modifying effects of the interactions between the factors in question 2 , 3 , 7 .
As in any complex systems, the dynamics of HM cannot be predicted from the kinetics of its individual components 2 , 3 . A big-data platform is needed to help. More accurately than ever, an appropriately built database could provide objective, data- and science-based guidance on the diet and lifestyle of lactating women to optimize their children’s health. Besides, the development of HM substitutes could benefit enormously from the collective knowledge the database can store.
In this paper, we demonstrate that an adequately built database, combined with numerical/statistical tools, has huge potentials to unveil food complexity 1 , 12 and to benefit from the stored knowledge. A key to this is the basis of our database-building principle: it considers a record as a mapping from various, possibly dynamic explanatory conditions, under which observations have been made, to the composition of HM, a truly dynamic response variable. A vital means to realize this ontology-principle is that the temporal variation of the variables is represented by tables, and pointers to these tables make sure that time-dependence is a natural attribute of the respective fields.
Food composition data have already been collected in databases, following various ontology depending on the purpose and the wanted resolution of the database. Our MilkyBase is intended to be used by academia as well as industry and regulation, therefore many compromises had to be made to find a balance between the four-V-principle of Big Data: volume, velocity, veracity, and variety.
We have set up a database that hosts published measurements of molecular components of breast milk. With its ca 10,000 datapoints, MilkyBase is far from the volume that is expectable from a Big Data project. However, we hope to initiate an ontology that would be used by researchers as well as clinicians to input their own data, so to create a “periodic table” of other important food-types, as a pool for collective knowledge 13 . Therefore, the template for inputting the data must be user-friendly enough, on commonly used platform, easily handled by the data donors. This is the reason why Microsoft Excel was chosen, as the most ubiquitous package that can link tables and be programmed via the Visual Basic for Applications (VBA) language. The VBA programs will aid both input check and data analysis (such as comparing own and others’ similar observations) and serve as incentives to authors to submit relevant data. This is a kind of wiki-philosophy, which should result in a much bigger data volume than its current size.
With its current size, the navigation and data processing are running at an acceptable speed, but the Excel platform will not be practical as the volume of the data increases, therefore, with time, it will be imported into an SQL server and the Excel sheets will serve a transit area for data donors, for initial curation.
Variety and veracity
As these are closely related, we discuss them together. Our goal was to digitize published data in a rigorously organized database, ready to be analysed by considering the milk composition as a function of various conditions. Therefore we tried to avoid changing published data, except in trivial cases, such as conversion of units for the sake of uniformity. Many times we found ambiguity or controversy in the terminology used by authors. An example for this is the concentration of a particular fatty acid molecule, which was mostly reported as a proportion relative to the total fatty acid, but sometimes proportion in the total milk mass, and sometimes even just the proportion of the total measured fatty acids. In such cases, we used our best knowledge and expert help to make these concepts well-defined and quantified. Such efforts admittedly bear the footprint of the database developer’s judgement.
If there are trivial mistakes in the publication (such as conversion error from one unit to another one) that were easily correctable then we did so; otherwise, either we left the record out, or marked it as “suspicious”. Even so, the resultant database is inevitably imperfect. However, the discrepancies should get detected as the database is being used.
Note that the variety - veracity issue is closely related to the syntax and semantics of the fields of the database. While its syntax can be checked in an automated way, its semantics frequently reveals anomalies, affecting what data can be inputted (variety) and how can those be validated (veracity).
For compatibility, we fixed the “mass/volume milk” concentration of each biochemical component as the target response value. By “ Component” we mean either a molecule or a group of molecules, such as say “linoleic acid”; or “fatty acid”. Both are “ Components ”, while the first is a special case of the second. Grouping like this follows a hierarchical tree structure as published data suggest (see Fig. 1 ). This way, not only the density of a particular molecule, but any components from the level next to the HM root, can be inputted.

Tree-structure of components.
Many authors only publish rescaled or derived values as components. Examples for this are the 2FL and 2FL/OS components (concentration of 2-fucosylated lactose and its proportion to that of the total oligosaccharides). To deal with such scenarios, we call a numerical value for 2FL as direct , while that for the 2FL/OS ratio as indirect response . We considered the explanatory and response variables as vectors, where each entry in the first one is a (mostly quantified) value on a specific condition that resulted in the response variables, in either direct or indirect form. Then a measurement for an indirect variable, such as 2FL/OS, is analogous to an implicit relationship between two mathematical variables. Similarly, a variable with the name “C18:1n-9 + C18:3n-3” indicates that the two fatty acids were measured together. So, the name of a variable may contain the “:” character to make it close to their biochemical notations as much as possible, as well as the “/” and “+” special characters, as mnemonic codes for derived variables.
The recorded values for these response variables are given in a so-called “extended numerical” format. By this, we mean that the inputted number can be supplied with its ± standard deviation or with an interval around it (like minimum-maximum, or quantile), both characterizing the uncertainty of the data. What is more, we differentiate between raw observations and estimations. Both can be inputted as response values, in the latter case with standard errors or confidence intervals. Finally, the response can be also dynamic, i.e. its temporal variation is stored in a table, and a pointer to the table is the inputted entry for the variable.
The condition fields do not necessarily hold only (extended) numerical values as above. They can be Boolean values or (a list of) categories, too. In the same way how a number belongs to an interval, a category value can belong to a group or to several groups. An example for this is the geographical region, indicating where an observation was made: the category group for China , for example can be either “ Asia ” or “ FarEast ”. Similar ambiguous definitions can occur say with Vitamin-D , by which typically we mean Vitamin-D3 , but this is not necessarily stated in the publications explicitly. Therefore, an accurate analysis of the data may introduce a probabilistic weight when characterizing the HM components at molecular level.
The variety of the data is restricted by the significance of the conditions on which the publications report. For example, the HM composition is rarely studied as a function of the sex of the new-born, so there is no separate field for that explanatory variable in the database, but the sex is included in the cond_c variable that contains relevant infant characteristics.
The veracity is also affected by confusions on statistical/numerical concepts. For example, sometimes the standard deviation of the measured values is mistaken with the standard error of their mean. Several publications have drawn the attention on this 14 , 15 , but the mistake is still frequent. Similarly, either the publication or the person inputting the data may confuse quantiles (which is about the spread of the raw data), with confidence intervals (which is about the precision of the estimation). Whenever such errors are detected, we either correct them (if it is obvious) or mark them in the database (in less obvious situations).
The workflow can be overviewed as shown in Fig. 2 .
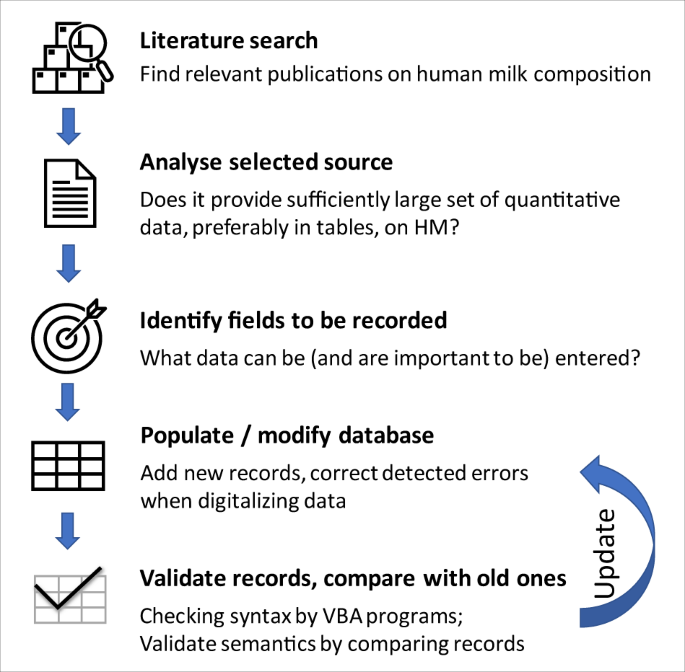
Workflow of building MilkyBase.
Literature search
The publication search was partly manual, partly performed by FoodMine, a natural-language processing algorithm that finds papers on the chemical composition of a target food from PubMed 16 . The manual search used MeSH terms and Boolean operators in PubMed, with the following searching descriptor: (“human milk” OR “breast milk” OR “mothers’ milk”) AND (“nutrients” OR “components” OR “composition” OR “biochemical” OR “quantification” OR “bioactive”). The search was focused on, but not limited to, English language.
Analyse source
The main selection criterium was quantitative data on the nutritional and/or non-nutritional components of HM. Priority was given to data (i) organized in a table format, in a systematic way; (ii) showing temporal variation (i.e., dynamic data); (iii) supplied with uncertainty quantification.
350 papers were selected by FoodMine and 201 were added from manual search. After elimination of irrelevant studies, a total of 365 potential papers were identified as suitable to enter the database. As of 1 st July 2022, MilkyBase contains data from 140 papers.
Identify components
More than 600 (possibly derived) components have been identified so far, which can be either nodes or leafs of the tree-structured value set, or relationships between them. In this set, some individual molecules are represented both explicitly and implicitly (such as a specific fatty acid with unit g/litre of milk, also with a ratio to the total fatty acids, which is measured in gram. Taking out such duplicates, explicit measurements exist on ca 400 “genuine” components. Out of these, ca 50 are groups, i.e. they can be divided into either further groups or into molecules as the final leaves of the tree.
Data Records
The MilkyBase database is a system of connected tables represented by sheets in a single Microsoft Excel workbook (Fig. 3 ). Each record of its core ( Master ) sheet is identified by a unique key. Filling the source of the information, the geographic region of the measurement, the size of the cohort, the analytical method(s) measuring the component of interest in HM, as well as at least one condition and at least one response value are compulsory. The values in the Component and Condition fields can be “extended numerical” (e.g., numbers supplied with uncertainty quantification) as well as time-dependent series of numerical values, i.e., dynamic values. The syntax and the descriptions of the fields can be followed in sheets called “definition sheets”. These are also used by the “Syntax check” macro, which is part of the MBmacros.xlsm macro-enabled Excel workbook 17 , a collection of useful macros assigned to the database.
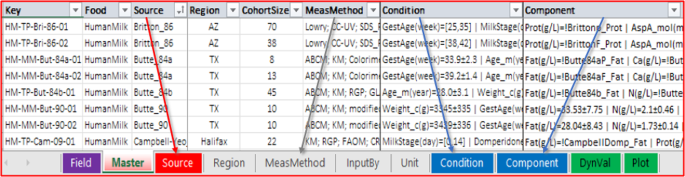
The MilkyBase database is a system of 10 linked tables. It The records of the core sheet are identified by a unique key and the possible values of a field are stored in the respective definition sheets with the same name.
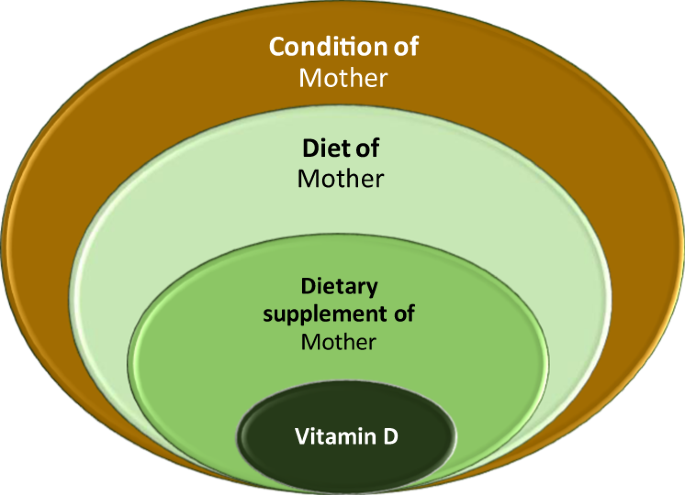
The relationships between the entries follow a tree-structure as before (Fig. 4 ). For example, the entries in the Conditions field can be numerical, just as the Component field, but also categories, which are defined in a nested way. An example for this is “ Vitamin D in the diet ”, which belongs to the Diet group, which in turn belongs to the mother-related “ condition_m” group.
Example for the nested grouping of condition values.
A big part of the implicit responses are proportions, mostly the concentration of a specific fatty acid molecule compared to the total fatty acid. From these, the concentration of the fatty acid molecule in question can be estimated only if the total fatty acid is known. The same holds for the situation when a molecule is measured in molecular weight; this can be converted to concentration only if the mol-weight is known; these are given in a separate field of the Master sheet. Therefore, it is possible that a certain molecule is measured in 2-3 ways. Deducing all these duplicates, the final number of explicitly recorded concentrations of molecules is currently 326. The list is expected to constantly expand as new data are coming in.
The information belonging to the CONDITION field have been organised in a similar way. 60 variables are identified and put in 6 main groups. The details are provided in the description file MBdescription.pdf 17 .
The MilkyBase.xlsx and its technical description MBdescription.pdf as well as the mentioned macros provided in a file called MBmacros.xlsm, were deposited in Figshare 17 .
Technical Validation
The database validation was helped by MS Excel VBA macros. The MBmacros.xlsm file containing them is available at the Figshare repository 17 .
It was straightforward to develop a “ Syntax check ” code but semantic check would require biochemical understanding. Various comparative plots were used to identify anomalies in the publications, such as wrong units, contradictions between figures and tables or misinterpreted data-scatter and uncertainty quantifications.
Usage Notes
The presented MilkyBase database hosts records on milk composition in linked Excel tables. Its main novelty is the ontology that focusses on the effect of conditions under which the milk composition was measured, and the dynamics and uncertainity characteristics of these data, which will be entered in the explanatory and response fields. Its purpose is to provide a resource for researchers and a template for laboratories to put their own data into this format, thus initiating a knowledge-share following a kind of Wiki-philosophy.
Though the job of digitizing published data is rather laborious, as not everything can be automated, the main challenge in the development is its variety and veracity. “What to record” is a major decision and can be even biased.
It is impossible to totally automate the task of verification, either. Despite all the programming efforts, the task and responsibility must remain in the hands of the inputter and will remain dependent on human skill and expertise.
An example for the multivariate dynamic response inputted in a record is shown by Fig. 5 . Such visualization is an aid to (i) recognize patterns and outliers in the data; (ii) identify data gaps; (iii) possibly identifying errors. For example this figure gives the idea, that the end of colostrum period can be defined as the time when the linear increase of fatty acid concentration is over.
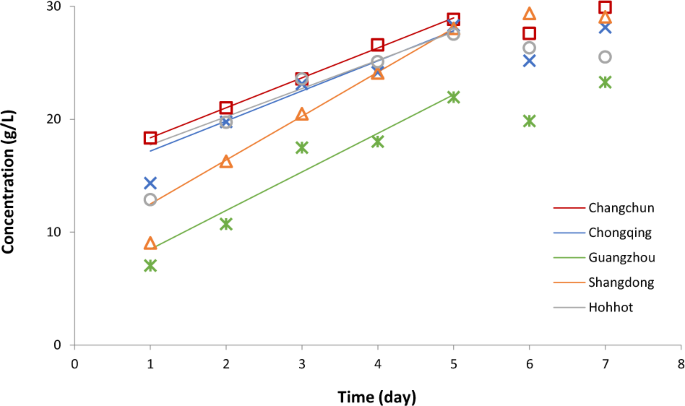
Temporal variation of fatty acid concentration in colostrum breast milk in five cities of China 19 . After the first day, the increase of the fatty acid is remarkably linear until day 5, with similar slopes, except in Shangdong (see the continuous lines, fitted to the data shown in respective colours). The fatty acid levels of breast milk (but not the rate of its increase) in Guangzhou are significantly different from those in the other four cities.
Figure 6 compares the temporal variations of the concentration of Lacto-N-tetraose (LNT) in human milk as found by different authors. Here the observations of Kunz et al . 18 show significant difference from other data, lending itself to an investigation what caused these differences.
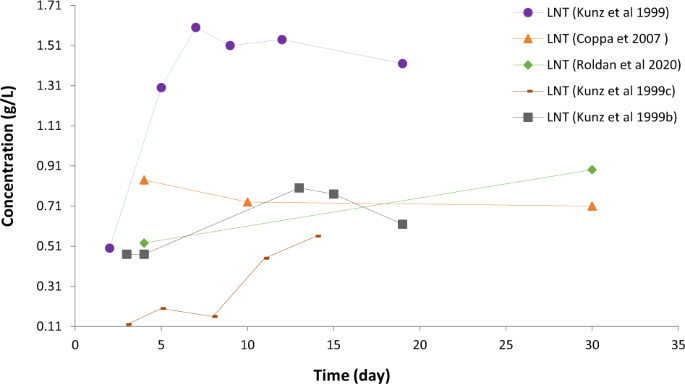
Visualisation gives ideas what relationships and patterns should be checked regarding the temporal variation of components.
MilkyBase demonstrates what benefits big data methods can bring for nutrition sciences. On a systematically organised database, users can run automated search and statistics that can help identifying data gaps (i.e., ideas for new research); finding mistakes in publications; and recognizing patterns, or possibly even model and optimize them for healthy infant and mother. A database like this needs to be of a relatively big volume (considering the complexity of the biochemical composition of milk), to get over a critical mass, from which we can consider the results as significant. Therefore, especially at the beginning of such database development, the amount of data that the authors make available in tables, plays a big role in the choice what papers should be digitized and recorded. Initially, the findings based on such database is inevitably more affected by what is derivable from the database, rather than what question is desirable to be solved by means of the database.
Code availability
MilkyBase.xlsx and its technical description MBdescription.pdf as well as the mentioned macros in an MBmacros.xlsm file, are available from the Figshare repository 17 .
Barabási, A.-L., Menichetti, G. & Loscalzo, J. The unmapped chemical complexity of our diet. Nature Food 1 , 33–37, https://doi.org/10.1038/s43016-019-0005-1 (2020).
Article CAS Google Scholar
Christian, P. et al . The need to study human milk as a biological system. The American Journal of Clinical Nutrition 113 , 1063–1072, https://doi.org/10.1093/ajcn/nqab075 (2021).
Article PubMed PubMed Central Google Scholar
Samuel, T. M. et al . Nutritional and Non-nutritional Composition of Human Milk Is Modulated by Maternal, Infant, and Methodological Factors. Frontiers in Nutrition 7 , https://doi.org/10.3389/fnut.2020.576133 (2020).
Eidelman, A. I. et al . Breastfeeding and the Use of Human Milk. Pediatrics 129 , e827–e841, https://doi.org/10.1542/peds.2011-3552 (2012).
Article Google Scholar
Gertosio, C., Meazza, C., Pagani, S. & Bozzola, M. Breastfeeding and its gamut of benefits. Minerva Pediatr 68 , 201–212 (2016).
PubMed Google Scholar
Carr, L. E. et al . Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front Immunol 12 , 604080, https://doi.org/10.3389/fimmu.2021.604080 (2021).
Article CAS PubMed PubMed Central Google Scholar
Boix-Amorós, A. et al . Reviewing the evidence on breast milk composition and immunological outcomes. Nutrition Reviews 77 , 541–556, https://doi.org/10.1093/nutrit/nuz019 (2019).
Victora, C. G. et al . Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387 , 475–490, https://doi.org/10.1016/s0140-6736(15)01024-7 (2016).
Article PubMed Google Scholar
Patro-Gołąb, B. et al . Nutritional interventions or exposures in infants and children aged up to 3 years and their effects on subsequent risk of overweight, obesity and body fat: a systematic review of systematic reviews. Obes Rev 17 , 1245–1257, https://doi.org/10.1111/obr.12476 (2016).
Who. Global Strategy for Infant and Young Child Feeding. Fifthy-fourth world health assembly , 8–8 (2003).
Rollins, N. C. et al . Why invest, and what it will take to improve breastfeeding practices? Lancet 387 , 491–504, https://doi.org/10.1016/s0140-6736(15)01044-2 (2016).
Morgenstern, J. D., Rosella, L. C., Costa, A. P., de Souza, R. J. & Anderson, L. N. Perspective: Big Data and Machine Learning Could Help Advance Nutritional Epidemiology. Advances in Nutrition 12 , 621–631, https://doi.org/10.1093/advances/nmaa183 (2021)
PTFI. Periodic Table of Food Initiative https://foodperiodictable.org/ (2021).
Vaux, D. L. Know when your numbers are significant. Nature 492 , 180–181, https://doi.org/10.1038/492180a (2012).
Article ADS CAS PubMed Google Scholar
Chavalarias, D., Wallach, J. D., Li, A. H. T. & Ioannidis, J. P. A. Evolution of ReportingPValues in the Biomedical Literature, 1990–2015. JAMA 315 , 1141, https://doi.org/10.1001/jama.2016.1952 (2016).
Article CAS PubMed Google Scholar
Hooton, F., Menichetti, G. & Barabási, A.-L. Exploring food contents in scientific literature with FoodMine. Scientific Reports 10 , https://doi.org/10.1038/s41598-020-73105-0 (2020).
Pacza, T. MilkyBase, a database of human milk composition as a function of maternal-, infant- and measurement conditions, figshare , https://doi.org/10.6084/m9.figshare.c.6160191.v1 (2022).
Kunz, C., Rudloff, S., Schad, W. & Braun, D. Lactose-derived oligosaccharides in the milk of elephants: comparison with human milk. British Journal of Nutrition 82 , 391–399, https://doi.org/10.1017/s0007114599001798 (1999).
Liu, Y., Liu, X. & Wang, L. The investigation of fatty acid composition of breast milk and its relationship with dietary fatty acid intake in 5 regions of China. Medicine 98, https://doi.org/10.1097/md.0000000000015855 (2019).
Download references
Acknowledgements
The authors would like to thank Anna Jánosity, Gyöngyi Kirschner, Bence Pecsenye, Luis Quevedo and Chyanne Rosenbaum for their technical help. A-L B work was partially supported by American Heart Association grant no. 151708, ERC grant no. 810115-DYNASET and Rockefeller Foundation grant no. 2019 FOD 026.
Open access funding provided by University of Debrecen.
Author information
Authors and affiliations.
Doctoral School of Food and Nutrition Science, Institute of Nutrition, University of Debrecen, Debrecen, Hungary
Tünde Pacza, Mayara L. Martins, Maha Rockaya & József Baranyi
Heim Pál National Paediatric Institute, Budapest, Hungary
Katalin Müller
Doctoral School of Clinical Medicine, University of Debrecen, Debrecen, Hungary
Center for Complex Network Research, Northeastern University, Boston, USA
Ayan Chatterjee & Albert-László Barabási
Network Science Institute, Northeastern University, Boston, USA
Ayan Chatterjee
Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA
Albert-László Barabási
Center for Network Science, Central European University, Budapest, Hungary
You can also search for this author in PubMed Google Scholar
Contributions
Tünde Pacza contributed to the design of the database structure, participated in the data acquisition and validation, and contributed to the writing up of the manuscript. Mayara L. Martins contributed to the design of the database, participated in the data acquisition, worked on the validation of the database, and contributed to the writing up of the manuscript. Maha Rockaya contributed to the data acquisition and participated in its validation. Katalin Müller contributed to the writing up of the paper and to the conceptualization and design of the database. Ayan Chatterjee was responsible for the automated literature search. Albert-László Barabási contributed to the conceptualization of the study. József Baranyi contributed to the conceptualization and design of the database, coordinated the validation efforts, largely wrote and edited the manuscript. All authors reviewed and commented on the manuscript and approved the final draft.
Corresponding author
Correspondence to József Baranyi .
Ethics declarations
Competing interests.
A.-L.B. is the founder of Scipher Medicine and Naring Health, companies that explore the use of network-based tools in health, and Datapolis, which focuses on urban data. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Pacza, T., Martins, M.L., Rockaya, M. et al. MilkyBase, a database of human milk composition as a function of maternal-, infant- and measurement conditions. Sci Data 9 , 557 (2022). https://doi.org/10.1038/s41597-022-01663-1
Download citation
Received : 06 May 2022
Accepted : 24 August 2022
Published : 09 September 2022
DOI : https://doi.org/10.1038/s41597-022-01663-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Reference Manager
- Simple TEXT file
People also looked at
Review article, composition, structure, and digestive dynamics of milk from different species—a review.

- Riddet Institute, Massey University, Palmerston North, New Zealand
Background: The traditional dairy-cattle-based industry is becoming increasingly diversified with milk and milk products from non-cattle dairy species. The interest in non-cattle milks has increased because there have been several anecdotal reports about the nutritional benefits of these milks and reports both of individuals tolerating and digesting some non-cattle milks better than cattle milk and of certain characteristics that non-cattle milks are thought to share in common with human milk. Thus, non-cattle milks are considered to have potential applications in infant, children, and elderly nutrition for the development of specialized products with better nutritional profiles. However, there is very little scientific information and understanding about the digestion behavior of non-cattle milks.
Scope and Approach: The general properties of some non-cattle milks, in comparison with human and cattle milks, particularly focusing on their protein profile, fat composition, hypoallergenic potential, and digestibility, are reviewed. The coagulation behaviors of different milks in the stomach and their impact on the rates of protein and fat digestion are reviewed in detail.
Key findings and Conclusions: Milk from different species vary in composition, structure, and physicochemical properties. This may be a key factor in their different digestion behaviors. The curds formed in the stomach during the gastric digestion of some non-cattle milks are considered to be relatively softer than those formed from cattle milk, which is thought to contribute to the degree to which non-cattle milks can be easily digested or tolerated. The rates of protein and fat delivery to the small intestine are likely to be a function of the macro- and micro-structure of the curd formed in the stomach, which in turn is affected by factors such as casein composition, fat globule and casein micelle size distribution, and protein-to-fat ratio. However, as no information on the coagulation behavior of non-cattle milks in the human stomach is available, in-depth scientific studies are needed in order to understand the impact of compositional and structural differences on the digestive dynamics of milk from different species.
Introduction
Milk has evolved to meet the nutritional and physiological requirements of the neonate. Milk is thus regarded as a high-quality food, nutritionally. Humans are known to have consumed cattle ( Bos taurus , cow) and non-cattle (such as goat and sheep) milks as part of their diet since prehistoric times ( 1 , 2 ). As a convenient source of nutrition, cattle milk is the most-consumed milk worldwide because of its widespread availability and large production volumes. Non-cattle milks are of nutritional importance to people in developing countries as well as in geographical areas in which the natural climate is unsuitable for the survival of dairy cattle ( 3 , 4 ). For example, buffalo milk in Asia, sheep milk in Europe and the Mediterranean basin (including the Middle East), camel milk (“the white gold of the desert”) in Africa, goat milk (“the cattle of the poor”) in Africa and southern Asia, horse milk in the steppe areas of central Asia, yak milk on the Tibetan plateau, reindeer milk in northern Scandinavia, musk ox milk in the Arctic, and mithun milk in the hilly regions of the Indian subcontinent ( 3 , 5 ).
Of the total world milk production, the proportion of total non-cattle milk production has increased from ~9% in 1961 to 19% in 2018 ( Figure 1 ). Of the total global non-cattle milk production, buffalo milk has nearly tripled, camel milk has nearly doubled, and goat milk has slightly increased during this period. No world statistics on the amounts of milk produced from other dairy species, such as yak, horse, donkey, deer, musk ox, and llama, are available. Much of the non-cattle milk production remains officially unreported because of the unknown amounts that are consumed locally at a farmer's home or are sold directly by farmers to local people, especially in developing countries ( 6 , 7 ).
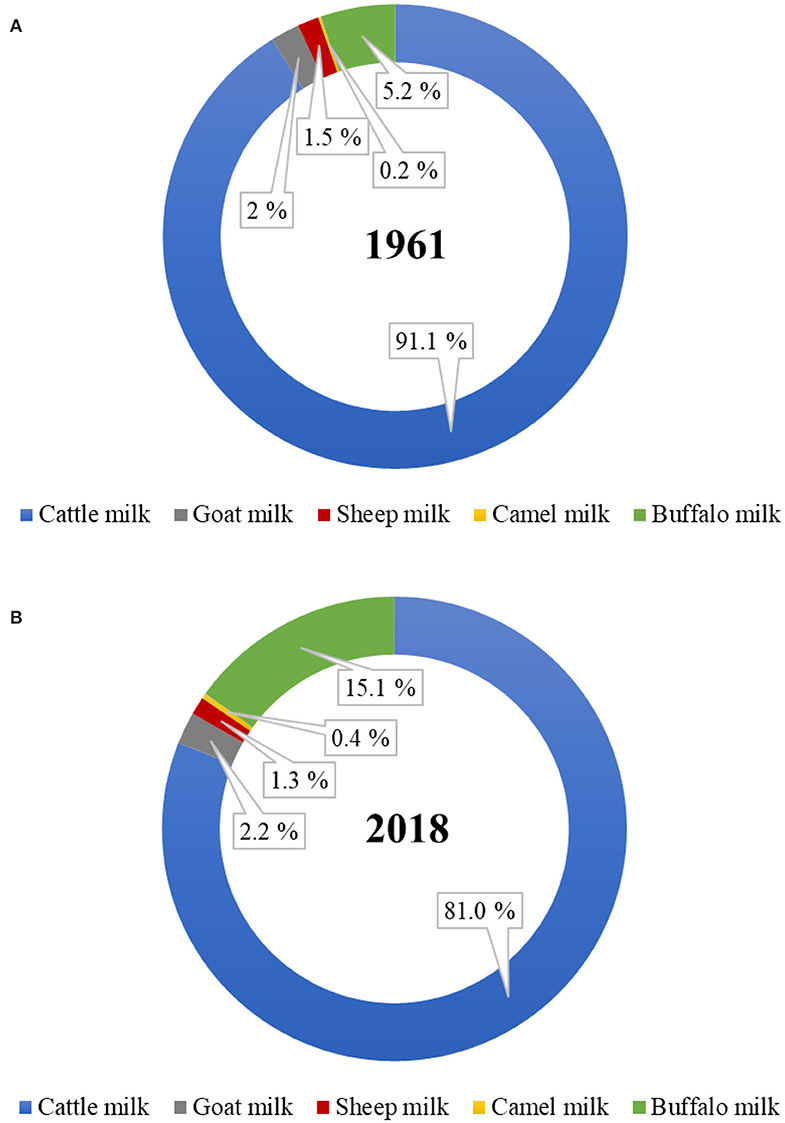
Figure 1 . Proportion of dairy cattle and non-cattle milks produced globally in the year (A) 1961 and (B) 2018. ( Source: FAOstat, March 2020).
The addition of milk as a product to non-cattle farm systems adds value and helps farmers in dealing with the fluctuating prices of meat, hair, and wool. The buffalo, goat, sheep, and camel milking industry is well set-up in many parts of the world, is gaining popularity, and is proving to be a profitable business for those who have already implemented it. Recently, New Zealand has introduced the development of a red deer dairy farming system. Large dairy companies as well as specialized small and medium enterprises (SMEs) are also interested in using non-cattle milks as a diversification strategy for their product portfolios. The regulatory requirements to ensure the safe production of cattle milk (and milk products) are well-defined in most of the world. However, the same regulatory limits may not be true to non-cattle milk and milk products. Thus, the emphasis on species-specific regulatory standards to guarantee the safety and quality of different milk for human consumption is needed ( 8 – 11 ). Also, understanding the significance of compliance to religious dietary laws (such as Kosher or Halal) will be of importance to the non-cattle milk-based dairy companies for gaining acceptance of their products from the various consumer groups ( 12 ).
In recent years, the opportunities for non-cattle milk production and the manufacture of products have expanded because the numbers of dairy cattle are perceived to be reaching their limit from environmental perspectives. Non-cattle milks are also believed to have certain nutritional benefits compared with cattle milks. For example, goat, sheep, camel, horse, and donkey milk are considered to be relatively more easily digestible, less allergenic, and more similar to human milk than cattle milk ( 4 , 13 , 14 ). In addition, non-cattle milks can be utilized for developing high value specialized dairy products of international as well as regional (local cultural) importance, such as cheese, yogurt, butter, ghee, ice-cream, fermented milk, probiotic dairy drinks, milk tablets, infant formulas ( 3 , 15 , 16 ). However, relatively little scientific information on the nutritional benefits of non-cattle milks is available. In addition, there is a significant gap in scientific knowledge on the detailed compositions, especially the minor components, and the protein and lipid structures in these milks.

Comparative Compositions of Cattle and Non-cattle Milks
The comparative compositions of milk from different species have been extensively reviewed in previous studies ( 5 , 17 – 19 ). The milk from different species vary in composition ( Table 1 ). Protein, fat, lactose, and minerals are the four major components in all milks, irrespective of the species ( 18 ); the composition of milk within the same species varies considerably because of various factors, such as breed, stage of lactation, milking interval, type of feed, and climate ( 7 , 19 ). For example, Li et al. ( 26 ) reported recently that the stage of lactation is a key factor responsible for differences in the compositional and physicochemical properties of dairy cattle milk in a seasonal calving system in New Zealand.
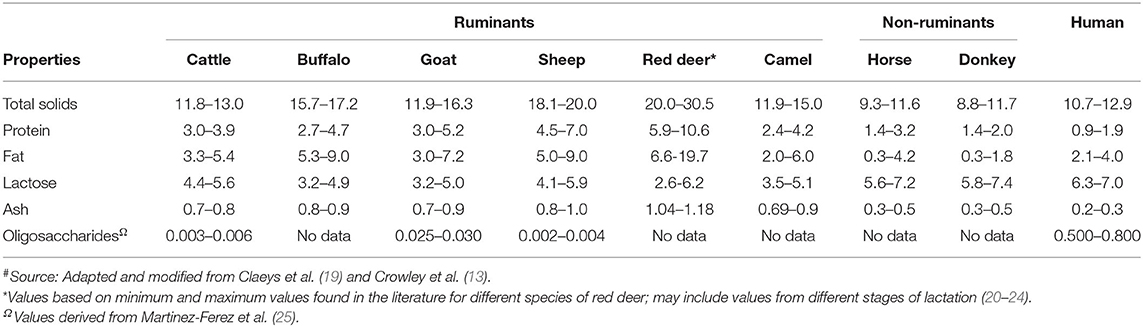
Table 1 . General composition (g 100 mL −1 ) of milk from different mammalian species # .
Non-ruminant milks (such as those from horse and donkey) are somewhat similar to human milk (in terms of protein, lactose, and ash contents), compared with dairy cattle milk and other ruminant milks ( Table 1 ). Ruminant milks have higher protein and fat contents, compared with human milk and other non-ruminant milks. Human milk contains much higher amounts of total lactose-derived oligosaccharides than milk from other species ( Table 1 ). Goat milk is also known to have a relatively higher oligosaccharide content, the composition of which is considered to be similar to that of human milk ( 27 , 28 ).
Proportions of Major Proteins
Compared with cattle milk and other ruminant milks, horse and donkey milk have a low casein-to-whey-protein ratio, similar to that in human milk. Among the ruminant milks, goat, sheep, and camel milk have a lower casein-to-whey-protein ratio as well as a relatively higher β-casein-to-α s -casein ratio compared with cattle milk ( Table 2 ). Thus, these non-cattle milks are an attractive alternative as a potential natural ingredient for infant formula ( 13 ); a lower casein-to-whey-protein ratio (i.e., a higher proportion of whey proteins) has been shown to be more desirable for faster digestion of the milk proteins in infant formula than a casein-dominant protein composition ( 31 , 32 ). As human milk has the lowest casein-to-whey-protein ratio, has a high β-casein-to-α s -casein ratio, and contains no β-lactoglobulin ( Table 2 ), milk from other species with similar properties are of great interest to the consumer as well as to the dairy industry for the development of specialized dairy products, not only for infants but also for people in other age groups.
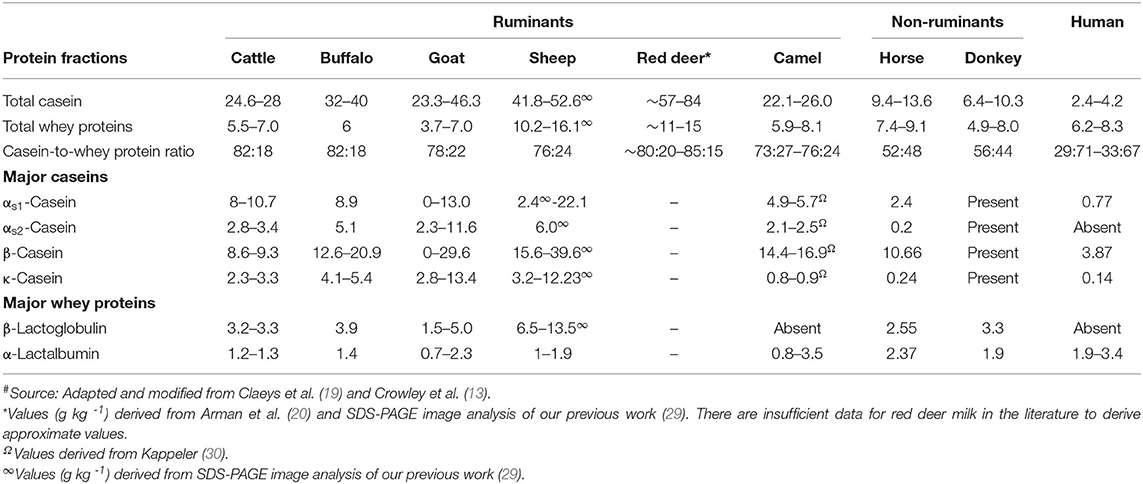
Table 2 . Protein profile (g L −1 ) of milk from different mammalian species # .
β-Lactoglobulin is considered to be one of the major allergens that is responsible for cattle milk allergy in children ( 33 ). Thus, milk from species that lack β-lactoglobulin or have lower β-lactoglobulin-to-α-lactalbumin ratios are of interest for human consumption. Camel milk, like human milk, does not contain β-lactoglobulin ( 34 , 35 ) or it may be present in trace amounts in different forms ( 36 – 38 ). Llama milk is also known to contain no β-lactoglobulin ( 5 , 39 ), but little detailed information on its protein composition is available.
Casein Micelle Characteristics
Individual caseins (α s1 -, α s2 -, β-, and κ-casein) are present in all milks as self-assembled particles known as “casein micelles” ( 40 ). The fundamental structure of the casein micelles in the milk from many species has not been studied in great detail, except in dairy cattle milk. Recently, Ingham et al. ( 41 ) used small-angle X-ray scattering and reported that the internal structures of the casein micelles of cattle, goat, and sheep milk had strong similarities with only slight differences, which may be due to the differences in casein composition, hydration, and physicochemical properties.
Apart from the differences in the proportions of different caseins ( Table 2 ), the casein micelles in the milk from different species differ in size, hydration, and mineralization ( Table 3 ). Among all mammalian milks, the casein micelles in human milk have the smallest diameter. The casein micelle sizes of goat, sheep, deer, camel, and horse milk are larger than that of human milk as well as cattle milk ( Table 3 ). Sood et al. ( 53 ) reported that the loss of micellar calcium from the skim milk casein micelles (when dialyzed against same skim milk sample containing ethylenediaminetetraacetic acid, EDTA) resulted in increased hydration (or swelling) of casein micelles. Based on this, it was considered that the hydration level of the casein micelles was negatively correlated with mineralization of micelles ( 54 ) i.e., when the mineralization of the casein micelle increases, the degree of hydration of casein micelle decreases. Thus, the lower hydration of goat and sheep milk casein micelles had been related to its higher mineralization than those of cattle milk casein micelles ( 55 , 56 ). Similarly, the casein micelles in buffalo milk ( 50 ) and donkey milk ( 51 ) are considered to be less hydrated and more mineralized than those in cattle milk.
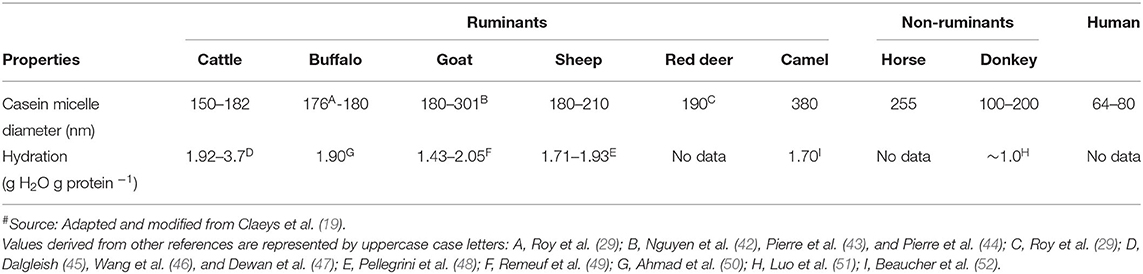
Table 3 . Casein characteristics of milk from different mammalian species # .
It should be highlighted that there is a high degree of variation in the results that have been reported for the casein micelle characteristics within the same species, which may be due to differences in the analytical methods used. In addition, differences in breeds, genetic variants, and phosphorylation sites of the caseins may also add to the variation in the characteristics of the casein micelles within and across species ( 13 ).
Milk Fat Composition
Compared with milk fat from other species (especially ruminants), human milk fat contains lower proportions of saturated fatty acids, higher proportions of monounsaturated fatty acids and polyunsaturated fatty acids, a higher ratio of ω-6 to ω-3 fatty acids, and higher levels of cholesterol ( Table 4 ). In general, horse and donkey milk contain lower proportions of saturated fatty acids and higher proportions of polyunsaturated fatty acids than ruminant milks. In contrast, ruminant milks contain higher proportions of monounsaturated fatty acids, a higher ratio of ω-6 to ω-3 fatty acids, and a higher cholesterol content than horse and donkey milk ( Table 4 ). The conjugated linoleic acid content is similar in human and ruminant milks but is lower in non-ruminant milks ( Table 4 ).
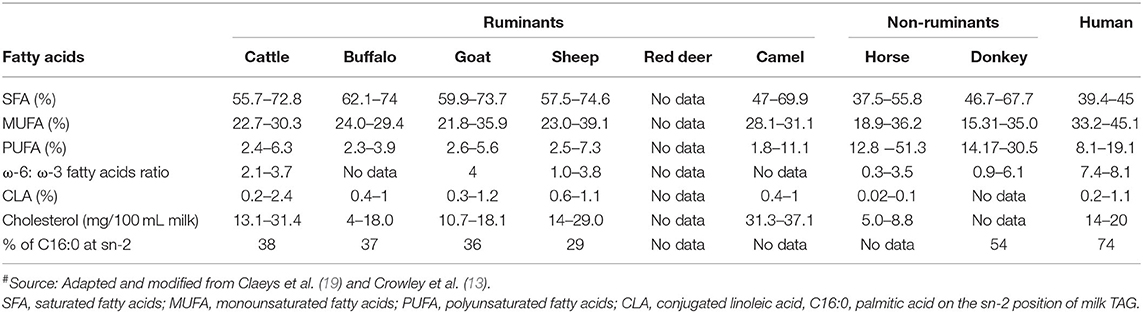
Table 4 . Fatty acid profile (% of total fatty acids) and cholesterol content of milk from different mammalian species # .
Sheep and goat milk fats are known to be rich in short chain (responsible for the distinct flavor of these milks) and medium chain triacylglycerols (TAGs); similarly, buffalo milk fat contains higher proportions of medium chain TAGs than cattle milk, which has high proportions of long chain TAGs ( 57 – 60 ). In contrast, camel milk contains a higher proportion of long chain fatty acids and a lower proportion of short chain fatty acids than cattle milk ( 61 ). Data for the fat composition of red deer milk are scarce, but this milk is considered to contain 5–10% fewer unsaturated fatty acids and higher proportions of shorter chain and saturated fatty acids than cattle milk ( 21 ). These differences may contribute to the different digestion behaviors of the milk fat from different species, as short or medium chain TAGs are considered to be more efficiently hydrolyzed by lipases ( 62 , 63 ).
Free long chain saturated fatty acids, such as palmitic acid (C16:0), are not considered to be efficiently absorbed in the body as they form insoluble fatty soaps with calcium in the small intestine ( 64 , 65 ). In this context, the TAG structure is considered to play a key role. Most of the long chain palmitic acid (C16:0) present in human milk (>70%) is located in the sn-2 position of the TAG structure; this position is considered to be suitable for the digestion and absorption of this fatty acid as well as other nutrients ( 18 , 62 , 66 ). German and Dillard ( 64 ) stated that the location of saturated fatty acids, such as long chain palmitic acid on the sn-2 position of TAGs, makes both the sn-1 and the sn-3 position fatty acids easily hydrolyzable by pancreatic lipases into free fatty acids, and produces sn-2 monoacylglycerols, which are easily absorbed in the small intestine; this also makes the milk calcium completely available and absorbable. Donkey milk has the closest proportion of palmitic acid located at the sn-2 position (i.e., 54%) to that of human milk (74%) ( Table 4 ). Thus, the modification of the TAG structure in milk from other species may help to deliver better milk fat digestion profiles; this could be an area of future interest.
Milk Fat Globule Size
The fat in the milk of all species is present as small spherical droplets, called globules, the diameter of which ranges from 0.2 to 15 μm ( 67 ). The size of these fat globules varies among milk from different species; goat, sheep, camel, and equine (horse and donkey) milk have higher proportions of smaller size fat globules compared to cattle milk ( Table 5 ). The differences in the sizes of the fat globules of milk from different species may influence the digestion of their fat differently ( 18 , 19 ). The TAG core of the fat globules from all species is surrounded, protected, and stabilized by a phospholipid trilayer (along with specific membrane proteins) called the milk fat globule membrane (MFGM) ( 68 , 69 ). The MFGM is unique to milk and its structure is considered to be similar in all milks, although the proportions of different proteins in the MFGM may differ among different species ( 70 ).
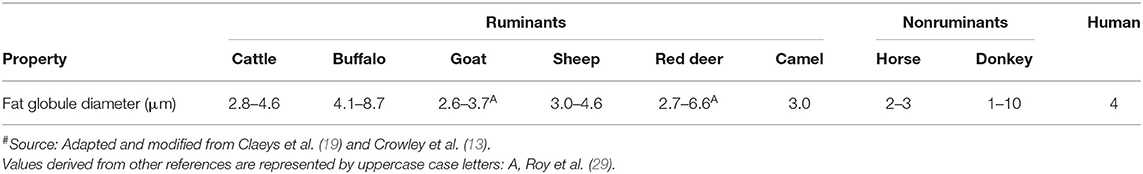
Table 5 . Fat globule size of milk from different mammalian species # .
In general, the differences in the characteristics of the casein micelles and the fat globules among different milks are considered to play important roles in influencing their coagulation behavior and nutrient delivery during digestion, which is discussed in the section on milk digestion.
Hypoallergenic Potential of Non-cattle Milks
More than 20 proteins in cattle milk are known to cause allergic reactions; of these, the casein fractions (especially α s2 -, α s1 -, and κ-caseins as well as, to some extent, β-casein), lactoferrin, serum albumin, and β-lactoglobulin are considered to be the most common cattle milk allergens ( 71 – 73 ).
There is increasing interest with respect to the suitability of non-cattle milks as a hypoallergenic option to cattle milk ( 74 ). A few studies have reported that horse milk ( 75 ), donkey milk ( 76 , 77 ), camel milk ( 78 , 79 ), and water buffalo milk ( 80 ) may be potential alternatives in cases of moderate allergenicity to cattle milk in children; however, this needs to be further investigated because weak cross-reactivity of non-cattle milk proteins with cattle milk proteins has been reported ( 81 – 83 ). Jenkins et al. ( 71 ) conducted a comprehensive study on the cross-reactivity of human and non-human milk proteins and found that the degree of allergenicity of a non-human milk protein is related to its extent of similarity with its human homologs. They found that, compared with cattle, goat, and sheep milk proteins, camel and horse milk proteins (i.e., α s1 - and β-caseins) are more homologous to their human milk counterparts, which may be a reason for their weak cross-reactivity or less allergenic nature compared with other non-cattle milks.
Infante et al. ( 84 ) reported that 25% of patients had a negative immunological test for adverse reactions to goat milk proteins; thus, goat milk cannot be considered to be a suitable alternative in cases of cattle milk allergy. Similarly, there is also strong evidence of allergenicity or positive cross-reactivities of goat, sheep, deer, and buffalo milk with cattle milk ( 83 , 85 – 87 ). In addition, reports concerning selective allergy to goat and sheep milk proteins, but not to cattle milk proteins, are also available ( 88 , 89 ). Bevilacqua et al. ( 90 ) found that goat milk with lower proportions of αs 1 -casein (and higher amounts of αs 2 -casein) was significantly less allergenic in guinea pigs than goat milk with high α s1 -casein content (and low α s2 -casein content); thus, different proportions of milk proteins may also play a key role in controlling milk protein allergy.
Overall, the scientific evidence indicates that there is little basis for promoting non-cattle milk or milk proteins as an alternative to cattle milk for people suffering from cattle (or cow) milk allergy.
Milk Digestion
Indispensable role of the gastric phase in milk digestion.
It is well-accepted that milk is a source of nutritionally balanced and highly digestible proteins ( 91 , 92 ). Previous studies have reported that the gastric emptying rates of two major fractions of milk protein (i.e., casein and whey protein) differ markedly; this has led to the concept of “slow” digested caseins and “fast” digested whey proteins ( 93 – 98 ).
The digestion of milk by the stomach enzymes (mainly pepsin and, to some extent, gastric lipases) in the presence of hydrochloric acid is considered to be the first key step, which is followed by further digestion in the small intestine by intestinal proteases and lipases ( 99 , 100 ). Some human infants may have chymosin like enzyme along with pepsin, which disappears from the gastric fluid by day 11 after birth ( 101 ). Chymosin and pepsin belong to the same group of aspartic proteinases that uses aspartic acid residues in their active center ( 102 ). Both the enzymes can preferentially hydrolyze the Phe105–Met106 bond of κ-casein, except that pepsin also exhibits unspecific proteolytic activity toward bonds with Trp, Tyr, Leu or Val residues, and thus have higher proteolytic activity relative to its milk clotting activity than chymosin ( 102 – 104 ). As the site of action of both chymosin and pepsin is the same, the mechanism of action of chymosin and pepsin is expected to be similar in relation to milk clotting. Chymosin is most stable in the pH range 5.3–6.3, but loses its activity rapidly under acidic conditions, i.e., below pH 3–4, as well as at high alkaline pH values, i.e., above pH 9.8 ( 105 ). Pepsin has maximum proteolytic activity at pH 2, with an optimum pH range of 2–5, and has activity in the pH range pH 5.5–7.5. Pepsin is irreversibly inactivated at pHs above 7.5 ( 106 ).
The protein hydrolysis sites of pepsin are different from those of the intestinal proteases (mainly trypsin and chymotrypsin). Pepsin acts preferentially on κ-casein on the casein micelles, leading to the coagulation of the casein fraction of milk proteins under acidic conditions, whereas the whey protein fraction remains soluble ( 107 ). Thus, the early role played by the stomach in milk digestion is an essential step in regulating the rate of digestion of the milk proteins in the gastrointestinal tract ( 108 ). In this respect, it is of great importance to understand the digestive dynamics and coagulation behavior of milk during gastric digestion, as milk coagulation can influence the delivery rates of proteins, fats, and associated milk constituents.
Evidence of Milk Coagulation
Human milk is known to form very soft and fragile curds in the infant stomach. Mason ( 109 ) investigated the changes in pH and the extent of protein hydrolysis in the stomach contents collected using a gastric tube at different time intervals from 25 healthy newborn infants (full-term, aged between 5 and 13 days). He reported the presence of casein curds in the stomach contents collected after 30 min of breastfeeding. He also reported that there was negligible protein hydrolysis in these samples. Similarly, recently, de Oliveira et al. ( 110 ) studied the gastric digestion of raw and pasteurized human milk in tube-fed preterm infants. The microstructural analysis in their study showed that human milk formed very soft and fragile protein aggregates in the infant's stomach.
Piglets and growing pigs have been regarded as a suitable animal model for human digestion research ( 111 – 113 ). Bottle-fed piglets have been used to study the digestion of human milk and infant formulas ( 114 – 116 ). Some evidence of clot (or curd) formation by cattle milk in pigs or piglets has been reported in the literature. Washburn and Jones ( 117 ) reported that cattle skim milk formed a tough or hard clot, whereas cattle whole milk formed a more friable and mellow curd in the stomach of baby pigs (28–35 days old), and that, the higher the fat content, the softer was the curd that formed. Braude et al. ( 118 ) found that the caseins from homogenized cattle milk clotted in the stomach of the 28-day-old pig after 15–30 min of feeding, whereas the “whey” fraction of the milk remained soluble and passed rapidly into the small intestine. Similarly, Decuypere et al. ( 119 ) reported the formation of firm casein clots in the stomachs of early weaned pigs (10–29 days of age) fed dry cattle-milk-based food; their gastric chyme had a long retention time and a low buffering capacity and stimulated more gastrin release, compared with the gastric contents of suckling piglets fed pig milk. They believed that these differences were due to the firm casein clot formed by a dry cattle-milk-based food in early weaned pigs in comparison with the soft casein aggregate formed from pig milk in suckling piglets.
Clotting Characteristics of Human Milk and Cattle Milk and Its Implications
Cattle milk is known to form firm curds (or clots) in the stomach, in comparison with human milk.
Nakai and Li-Chan ( 108 ) studied the coagulation characteristics of human and cattle milk using an in vitro acid precipitation test at 37°C, in which they added 0.2% acidic pepsin solution to 100 mL each of cattle milk and human milk at a flow rate of 15 mL/h. They found that human milk formed much finer protein aggregates (or clots) than cattle milk and reported that this could be the possible reason for the shorter gastric emptying time for human milk.
The differences in the structures of human and cattle milk curds could be related to the differences in their fat and protein compositions. The protein (casein)-to-fat ratio of human milk is very low ( Tables 1 , 2 ) compared with that of cattle milk (as well as of other non-cattle milks), which is likely to be a factor that is responsible for its soft (or fragile) curd formation. In addition, the higher β-casein-to-α s -casein ratio of human milk has been associated with the fine and loose curd formed by human milk in an infant stomach. Lichan and Nakai ( 120 ) performed an in vitro coagulation study with untreated cattle milk casein, rennin-modified cattle milk casein, and human milk casein. The rennin-modified cattle milk casein was a β-casein-rich cattle milk (similar to β-casein-rich human milk) that was produced by selectively eliminating the α s1 -casein fraction from cattle milk by a process involving rennet action. Upon acidification of the different casein solutions to pH 2 or pH 4, Lichan and Nakai ( 120 ) observed that the hardness of the clot formed from these different casein solutions decreased in the order: cattle milk casein > rennin-modified cattle milk casein (rich in β-casein) > human casein. In another study, Lichan and Nakai ( 121 ) also reported that moderate or partial dephosphorylation of cattle milk casein using different phosphatases (calf intestinal alkaline phosphatase and potato acid phosphatase) at pH 4 resulted in the acid-coagulating properties of these modified cattle milk casein solutions being similar to those of human milk as well as in a greater rate of proteolysis compared with the firm clots of untreated cattle milk casein. However, all these studies were in vitro physicochemical studies, and further studies in in vitro or in vivo digestion models need to be conducted to validate such findings.
Blakeborough et al. ( 122 ) studied the digestion of human milk, cattle milk, and reconstituted baby formula (based on full cream dry cattle milk powder) using 14-day-old piglets; cattle milk or baby formula formed firm solid curds, whereas human milk formed a very liquid-like coagulum (little solid material) in the piglet's upper gastrointestinal tract. They also determined the bioavailability of zinc (Zn) from these milk systems; they found that, for cattle milk (as well as baby formula), ~55–72 and ~60–66% of the Zn was retained in the curds present in the gastric chyme and the intestinal digesta, respectively, whereas, for human milk, ~43 and 7% of the Zn was retained in the curds present in the gastric chyme and the intestinal digesta, respectively. They suggested that these differences in the distribution and bioaccessibility of Zn in the gastrointestinal tract of piglets fed human milk or cattle milk may have been due to the differences found in the consistency of the casein curds formed by the different milks.
Digestion of Milk From Different Species
Protein digestion.
The lower protein content, lower casein-to-whey-protein ratio, and higher β-casein-to-α s -casein ratio of human milk compared with milk from other species have been related to its soft curdling properties in vitro as well as in vivo , as described earlier. Although none of the non-human milks match the composition of human milk, horse, and donkey milk are known to form very weak or fragile gels (or curds or flocs) when acidified or treated with rennet ( 123 – 125 ) and thus are expected to form soft or fragile curds in the stomach, in comparison with cattle milk, because of their lower casein content. Similarly, some of the ruminant milks, such as goat and camel milk ( 126 – 130 ), are also considered to form soft curds in the stomach when acidified or treated with rennet (or pepsin), because of their lower casein content or larger casein micelle size compared with cattle milk, even though they contain comparatively higher proportions of caseins than equine and human milk. However, no direct comparative in vitro or in vivo digestion studies between cattle and non-cattle milks, focusing on their curd formation characteristics in the stomach, have been reported to date. There are only a few comparative in vitro digestion studies on cattle and non-cattle milks, focusing on their protein or fat digestion.
Jasińska ( 131 ) compared the degrees of hydrolysis by pepsin and trypsin of micellar caseins obtained from cattle, human, goat, and horse skim milk; the peptic hydrolysis rates of the micellar caseins from cattle, human, goat, and horse milks were 23–42 (differed for different breeds of cattle), 80, 65, and 43%, respectively. The tryptic hydrolysis rates of the micellar caseins from cattle, human, goat, and horse milk were 76–90, 100, 96, and 92%, respectively. The higher susceptibility of human and goat milk was believed to be due to the smaller micellar aggregates and the presence of higher proportions of β-casein in their micellar structures, when compared with cattle milk (which had higher proportions of α s1 -casein).
Recently, Hodgkinson et al. ( 132 ) studied the in vitro static gastric digestion of cattle and goat whole milk (at pH 3.0) and reported that, after both 20 and 60 min of digestion, goat milk caseins were digested faster than cattle milk caseins (based on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) image analysis), possibly because of the relatively soft or fragile coagulum formed by goat milk. Tagliazucchi et al. ( 133 ) also studied the in vitro static gastrointestinal digestion of cattle, goat, sheep, and camel skim milk (as per the INFOGEST protocol) and reported that the extent of free amino groups generated during the gastric digestion was higher for goat, sheep, and camel milk proteins, indicating that the proteins in these non-cattle milks were hydrolyzed faster than cattle milk proteins during the gastric step. However, after the intestinal step, they reported that only the goat milk proteins were hydrolyzed faster than the milk proteins from the other species, all of which had similar hydrolysis rates. Tagliazucchi et al. ( 134 ) and Rutella et al. ( 135 ) reported similar findings in their previous studies, i.e., that the degree of hydrolysis of goat skim milk proteins during the gastric and intestinal steps was much higher than that of cattle skim milk proteins. The authors stated that the higher degree of hydrolysis of goat milk proteins observed in all studies was probably due to the higher susceptibility of goat milk proteins to pepsin.
Maathuis et al. ( 136 ) investigated the comparative protein digestibilities and qualities (based on bioaccessible nitrogen and amino acids) of human milk, cattle-milk-based infant formula, and goat-milk-based infant formula using the tiny-TIM model (a dynamic in vitro infant gastrointestinal model). They found that the protein digestibilities and qualities of all diets were similar; however, the rates of protein digestion were slower during the first 60 min of digestion for the cattle-milk-based formula than for the human milk and the goat-milk-based formula. They hypothesized that the differences in the clotting characteristics of different milks would have led to differences in their gastric emptying, as they found that the curds formed from the cattle-milk-based formula were retained for a longer duration in the gastric compartment of tiny-TIM compared with those from the human milk and the goat-milk-based infant formula. Similarly, Ye et al. ( 32 ) investigated the in vitro dynamic gastric digestion of goat- and cattle-milk-based formulas in a mini version of the human gastric simulator (HGS), simulating infant gastric digestion. The authors found that the goat-milk-based infant formula formed smaller protein aggregates in the mini-HGS, leading to faster hydrolysis of its proteins compared with those from the cattle milk formula. Based on the above-mentioned studies it appears that the differences in the structures of the curds formed from milk of different species during gastric digestion may be a key factor that is responsible for their different digestion behaviors.
In contrast, Almaas et al. ( 137 ) did not find any differences in the digestion of caseins and α-lactalbumin from cattle and goat skim milk (with high and low αs 1 -casein content) after static gastrointestinal digestion using human gastric juice (HGJ) and human duodenal juice (HDJ). They also did not find any differences between goat milk with high and low αs 1 -casein content after digestion with HGJ and HDJ. However, they observed (using SDS-PAGE image analysis) that goat milk β-lactoglobulin was rapidly digested during both gastric digestion and intestinal digestion, compared with cattle milk β-lactoglobulin. El-Zahar et al. ( 138 ) studied the hydrolysis of isolated β-lactoglobulin from sheep and cattle milk by porcine pepsin and found that β-lactoglobulin from sheep milk was hydrolyzed faster because of its slightly different tertiary structure and higher surface hydrophobicity. As β-lactoglobulin is considered to be one of the major allergens (as it is absent in human milk), the higher degree of hydrolysis by pepsin of the β-lactoglobulin in goat and sheep milk may be a possible reason that these non-cattle milks are better tolerated by some people than cattle milk.
Vithana et al. ( 23 ) studied the comparative in vitro gastrointestinal digestion of raw cattle and deer skim milk. They found that, after gastric digestion, nearly 49 and 27% of the deer and cattle milk caseins remained undigested (SDS-PAGE image analysis), respectively, whereas, after intestinal digestion, the caseins from both species were completely digested. This, indicated that, during the gastrointestinal digestion, deer milk caseins were digested at a faster rate than cattle milk caseins. We hypothesize that the higher amounts of caseins retained in the gastric phase for deer skim milk may have been due to the higher protein content (as well as casein content) of the deer milk used in their study, indicating that the inherent composition of milk also has a key role to play during gastric digestion. Vithana et al. ( 23 ) also found that α-lactalbumin was hydrolyzed faster in deer milk than in cattle milk. However, β-lactoglobulin from both species was found to be resistant to both gastric and duodenal digestion.
In contrast to the above studies, some studies have reported no differences or faster hydrolysis of cattle milk proteins than of goat milk proteins. For instance, Inglingstad et al. ( 139 ) reported (based on SDS-PAGE image analysis) that 69 and 82% of the caseins remained undigested after hydrolysis by HGJ of cattle and goat skim milks respectively; however, after further treatment with HDJ, almost all of the caseins from the milk of both species were digested. They found that the β-lactoglobulin and α-lactalbumin from both species were highly resistant to HGJ and that, after hydrolysis with HDJ, ~64% of the β-lactoglobulin from both species remained undigested and 91 and 65% of the α-lactalbumin from the cattle and goat skim milk respectively, remained undigested. Mros et al. ( 140 ) reported no differences in the protein digestion of cattle, goat, and sheep skim milk following hydrolysis by pepsin and pancreatin.
Similarly, Milan et al. ( 141 ) reported that whole goat-protein fortified milk, compared to whole cow-protein fortified milk, was digested and metabolized similarly (despite the differences in their inherent nutrient composition) in young adults (aged 18–28 years). However, they dissolved paracetamol in fortified milk drinks before giving it to the participants for consumption (plasma paracetamol levels were used as a marker for gastric emptying). It has to be noted that depending on the type of paracetamol used, it may have a buffering action during the gastric digestion in the stomach ( 142 ) and thus, careful consideration needs to be made while conducting human digestion studies to draw any firm conclusions.
Vaisman et al. ( 143 ) investigated the gastric emptying times in humans of camel and cattle milk using a scintigraphic technique and reported that the poor coagulation properties of camel milk (as observed during acid or rennet coagulation) did not provide any comparative advantage over cattle milk in terms of gastric emptying. It should be noted that the soft or fragile curd formed from non-cattle milks (such as camel, goat, horse, and donkey milk) during acid or rennet coagulation provides only an indication of how these non-cattle milks may behave in the human stomach during gastric digestion. The gastric digestion process is a complex and dynamic phenomenon, and in-depth comparative in vitro and in vivo studies on cattle and non-cattle milks that simulate the gastric digestion in humans need to be undertaken, to draw any definite conclusions.
Not only protein composition and (or) casein micelle structure, but also different processing temperature and time combinations may induce differences in the curd structure in the stomach, which may influence the rate of delivery of proteins to the small intestine and their subsequent absorption. For instance, Ye et al. ( 107 ) studied the dynamic gastric digestion behavior of raw and heated (90°C for 20 min) cattle skim milk using an HGS. The HGS is a dynamic stomach model that is capable of simulating the stomach contraction forces and the flow of gastric fluids that occur in vivo ( 144 ). Ye et al. ( 107 ) found that raw milk formed a “closely knitted” tight clot, whereas heated milk formed fine and loose protein aggregates ( Figure 2 ), leading to slow hydrolysis of caseins from raw milk, compared with heated milk. This was because, in raw milk, only the caseins were involved in clot formation, whereas, in heated milk, both the caseins and denatured whey proteins were involved in clot formation ( 145 ). Heating at 90°C for 20 min would have led to complex formation between fully denatured whey proteins and caseins via sulfhydryl groups and disulfide linkages ( Figure 3 ), hindering the formation of a firm clot ( 146 , 147 ). Kaufmann ( 148 ) reported that ultrahigh-temperature-treated (UHT) milk led to the formation of soft coagulates in the mini-pigs stomach, leading to higher levels of amino acids and urea in their blood serum compared to that of pasteurized and raw milk, which formed stronger coagulum. Thus, these differences in gastric restructuring induced by heating are expected to be a key possible reason for higher postprandial utilization of dietary nitrogen from defatted UHT milk (140°C for 5 s) compared to defatted pasteurized milk (72°C for 20 s) as well as defatted microfiltered milk in humans ( 149 ).
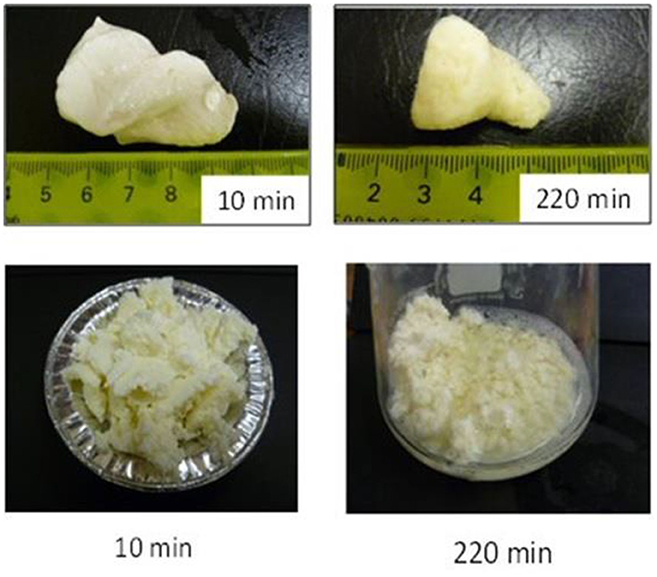
Figure 2 . Images of clots formed during the gastric digestion of 200 g of unheated (top row) and heated (bottom row) cattle skim milk at different digestion times. Source: Adapted from Ye et al. ( 107 ).
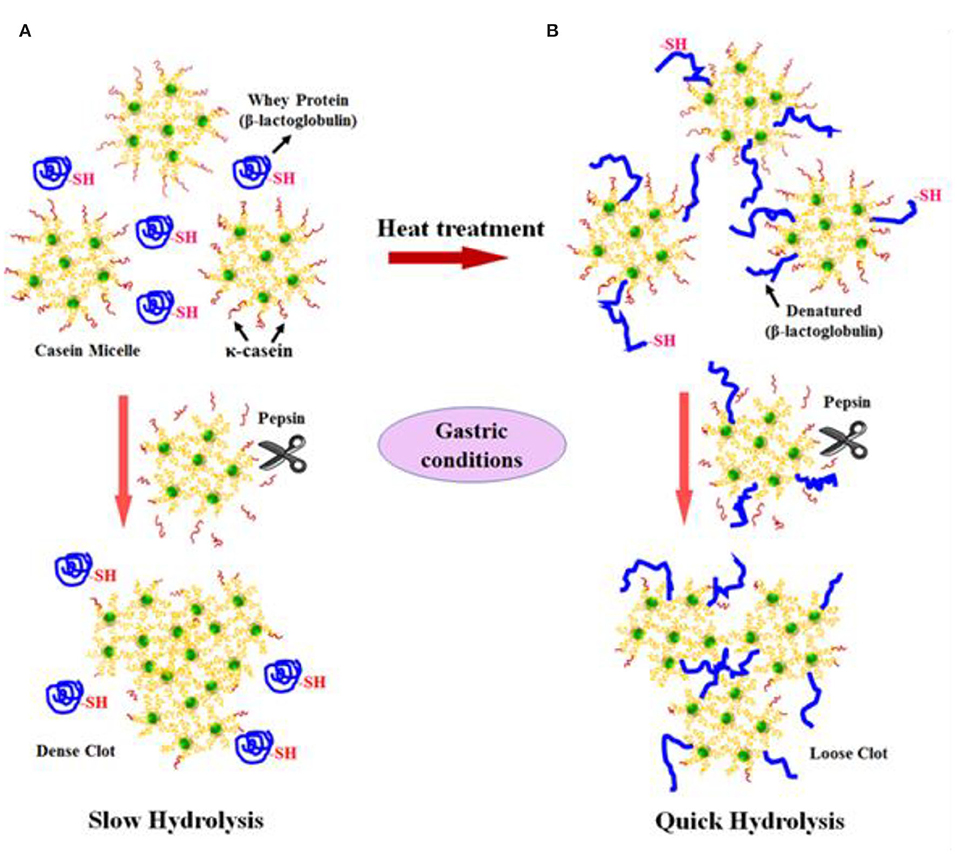
Figure 3 . Schematic diagram of the possible mechanism of events during the formation of protein curds from (A) raw milk (unheated) and (B) heated milk during gastric digestion. Source: Adapted from Ye et al. ( 145 ).
Doan ( 150 ) published a comprehensive review based on studies on the gastric digestion of processed (boiled, evaporated, or acidified) and raw cattle milk in the early 1900s, and reported that boiled, evaporated, or acidified milk were emptied rapidly from the human stomach because of the finer or softer curd that formed. It was suggested that the modification of raw cattle milk using different processing conditions may be a potential option in the development of dairy-based baby foods or beverages with properties similar to those of human milk.
To date, no studies on the impact of different heating or processing conditions on the digestion behaviors of non-cattle milks have been reported in the literature. It should be noted that the commercial processing or technological conditions needed for non-cattle milks may be different from those needed for cattle milk. In addition, the impact of different processing conditions on the digestion behaviors of non-cattle milks may be different from that on cattle milk because of the differences in their composition and structures.
The Influence of the Protein Network on Fat Digestion—The Whole Milk Matrix
During the gastric digestion of whole milk, the fat globules are known to be physically entrapped within the protein clot that is formed. Thus, the nature or structure of the protein network formed will influence the rate of release and the digestion of fat by gastrointestinal lipases ( 145 , 151 – 153 ). Previous studies have shown that the nature or structure of the protein network formed is, in turn, dependent on the protein composition (casein-to-whey-protein ratio), the protein-to-fat ratio, and the impact of different processing conditions ( 99 , 154 ). For instance, Mulet-Cabero et al. ( 154 ) studied the in vitro gastrointestinal digestion of model systems based on different casein-to-whey-protein ratios using a semidynamic gastric model, and reported that the viscosity or firmness of the coagulum formed increased as the casein-to-whey-protein ratio increased in the model protein systems, leading to slower gastric emptying, and slower digestion and absorption of nutrients. They also found that the addition of increasing amounts of fat to the casein-rich protein models produced more fragmented clots with a significant decrease in their firmness. This, indicates that the presence of fat hindered the aggregation of proteins, which may, in turn, influence the digestion rates of nutrients.
Ye et al. ( 151 ) studied the gastric digestion of raw (unheated) and heated (90°C for 20 min) cattle whole milk and reported that the release of fat globules was dependent on the disintegration characteristics of the protein clot and that the release of fat globules was higher from the finer aggregates of protein clots formed from the heated whole milk than from the firm clots formed from the raw whole milk ( Figure 4 ). Similarly, Ye et al. ( 145 ) studied the comparative in vitro and in vivo (in rats) gastric digestions of raw (nonhomogenized), pasteurized (homogenized), and UHT (homogenized) cattle whole milk, and reported that the UHT milk had faster rates of protein hydrolysis as well as release of fat globules during gastric digestion, compared with the raw and pasteurized milk; the differences were attributed to the smaller or fragmented protein aggregates formed from the UHT milk proteins in comparison with the aggregates from the other milks.
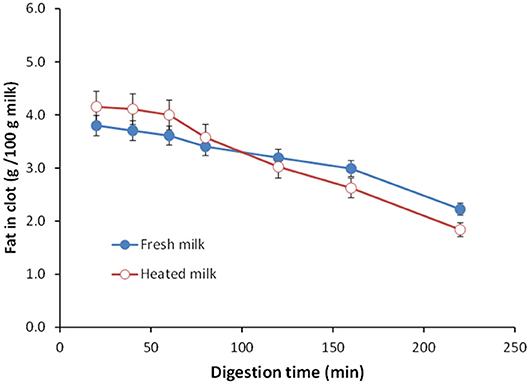
Figure 4 . Changes in the fat content (g/100 g milk) in clots obtained from (•) unheated (raw) and (°) heated cattle whole milk during gastric digestion. Source: Adapted from Ye et al. ( 151 ).
In another gastric digestion study, Ye et al. ( 152 ) reported that the release of fat globules was relatively higher in homogenized milk (20/5 MPa (primary/secondary pressure), 20°C) as well as heated, homogenized milk (20/5 MPa, 20°C + 90°C for 20 min) because of the fine and crumbled structure of the coagulum formed in these milks compared with the firm coagulum formed from raw cattle whole milk ( Figure 5 ). Similar results have been reported by Mulet-Cabero et al. ( 153 ) for processed cattle whole milks.

Figure 5 . Images of clots formed during the gastric digestion of raw (unheated), homogenized, and heated homogenized cattle whole milk during 20 and 160 min of gastric digestion. Source: Adapted from Ye et al. ( 152 ).
The coalescence of fat globules entrapped within the protein network as well as those present in the liquid phase of the gastric chyme has also been reported in the above-mentioned studies ( 145 , 152 , 153 ), which is expected to be due to the hydrolysis of the proteins present at the surface of the milk fat globule (present naturally in the MFGM or adsorbed proteins because of processing treatments), leading to destabilization of the fat globules and coalescence.
As the milk from different species are known to vary in fat content, protein-to-fat ratio, fat globule size, and structure, there may be differences in the consistency of the coagulum formed from milk of different species during gastric digestion, which may impact their overall digestion behavior differently.
Gastrointestinal Digestion of Fat
Little information is available on the gastric digestion of milk fat, irrespective of species. Lipolysis during the gastric phase was previously considered to be of less relevance during the overall digestion process as gastric lipolysis accounts for only 10–25% of the overall lipid digestion in adults ( 155 ). Therefore, most of the studies reported in the literature on fat digestion have focused mainly on intestinal digestion. However, it is now widely suggested that gastric lipases should be incorporated in in vitro digestion studies as their preliminary role may facilitate further breakdown of lipids by intestinal lipases ( 155 ). Also, in contrast to adults, gastric lipases play a significant role in infants because of their high postprandial gastric pH ( 156 ).
It is hypothesized that, the smaller the fat globule size, the higher will be the fat digestibility, because the higher surface area of smaller fat globules will help in rapid digestion via gastrointestinal lipases ( 13 , 18 , 19 , 157 ). Meena et al. ( 158 ) investigated the digestion of milk fat by pancreatic lipase in standardized raw cattle, buffalo, camel, and goat whole milk. The authors found that the amount of free fatty acids released followed the order: goat ~ camel > cattle > buffalo. The higher digestibility of goat and camel whole milk was believed to be due to the small size of their fat globules, as the fat globule sizes of the different milks were in the order: buffalo (3.9–7.7 μm) > cattle (1.6–4.9 μm) > goat (1.1–3.9 μm) ~ camel (1.1–2.1 μm). In addition to the fat globule size, the outer surface of the fat globule and its structure (i.e., the fat globule interface) have a crucial role to play in the digestion of fats. For example, the presence of adsorbed proteins (caused by processing such as heating and homogenization) at the interface of fat globules may result in providing easy access of lipases to the TAG core of the fat globules and thus in influencing the digestion of milk fat ( 157 ).
Some studies have also shown the influence of differences in the milk fat composition among different milks on their digestibility. For instance, Alférez et al. ( 159 ) studied the fat digestibility and metabolism in feces samples of male albino rats that were fed diets containing lyophilized goat and cattle whole milk. They found that, compared with the rats on the cattle-milk-based diet, the digestive utilization of fat was higher, and the levels of cholesterol were lower, in the rats on the goat-milk-based diet. The authors believed that the differences may have been due to the greater amounts of medium chain TAGs and the smaller fat globule sizes of the goat milk fat compared with the cattle milk fat used in their study. Similarly, Teng et al. ( 160 ) studied the in vitro gastric digestion of raw (non-homogenized) and homogenized cattle and sheep milk, and reported that the TAGs from both raw and homogenized sheep milk were digested by rabbit gastric lipases more rapidly than those from cattle milk; this was due to the presence of higher levels of medium chain fatty acids at the sn-1 or sn-3 position of the TAG structure in sheep milk compared with cattle milk, emphasizing that the structural characteristics of TAGs have an important role to play in their gastric digestion.
Overall, the digestibilities of the protein and fat in milk are likely to be functions of the unique compositions, protein profiles, fat compositions, casein micelle and fat globule structures, interfacial properties, mineral distributions, and physicochemical properties, all of which are likely to be affected to different degrees by the processing conditions, depending on the animal species. Although there are very few studies on the impact of the processing conditions and the milk composition of non-cattle milks in the literature, the principles of cattle milk protein coagulation and its impact on fat digestion are expected to also be applicable to non-cattle milks. However, as cattle and non-cattle milks vary in protein composition (proportion of different proteins) as well as protein-to-fat ratio, it is likely that there will be differences in the structure and consistency of the protein curd (or clot) formed from different milks, which may lead to further differences in the release of fat globules from the clot matrix of different milks. It should also be noted that the gastric and intestinal digestion conditions of infants (as well as the elderly) are different from those of adults in terms of acid secretions and enzyme (proteases and lipases) activities ( 155 , 156 , 161 ). Thus, relevant dynamic in vitro models need to be used to study the digestion of milks in different age groups, and in vitro results need to be ultimately corroborated based on in vivo observations.
Conclusions and Recommendations for Future Research
As non-cattle milk and milk products are highly regarded as a potential source of human nutrition, they can be utilized to develop specialized dairy products for people in all age groups. Non-cattle milks are of great interest to people as well as industries, because of their perceived better nutritional properties compared with cattle milk. However, most of these presumptions are based on anecdotal reports and only little scientific research has been conducted to understand the nutritional and physicochemical properties of non-cattle milks. One widely perceived notion is the formation of soft curds in the human stomach for some non-cattle milks (such as goat, camel, horse, and donkey milk). Because of this, these milks are considered to be better digested and tolerated by people of different age groups. However, to date, no direct scientific studies have been reported and there is a knowledge gap. As cattle and non-cattle milks vary in composition and structure of the casein micelles and fat globules, they are likely to behave differently in the gastrointestinal tract, possibly affecting the kinetics of digestion and the bioavailability of nutrients. Because of differences in milk composition and the structure of the casein micelles (or fat globules), there may be differences in the curds formed by the milk of each species in the stomach, which may further affect the delivery rates of macronutrients further down the gastrointestinal tract. Furthermore, different commercial processing conditions such as pasteurization or UHT (or other heat treatments) may influence the digestion behaviors of non-cattle milks differently. Thus, in-depth scientific studies need to be conducted to understand the impact of compositional as well as structural differences in milk from different species (in their natural form as well as processed forms) on their dynamic digestion behaviors, especially focusing on their differences in curd formation as well as their disintegration properties in the stomach. Such studies will often involve in vitro digestion models, which where possible should be dynamic and sophisticated enough to at least include the effects of key variables known to influence food digestion. Further, the physiological relevance of such phenomena needs to be investigated in animal and human studies focusing on different age groups or people in need of targeted personalized nutrition (such as infants, the elderly, athletes or malnourished people).
Author Contributions
DR prepared the original draft and edited the manuscript. HS critiqued and edited the original draft of the manuscript. AY and PM critically reviewed the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the conception and design of the manuscript and read and approved the final manuscript for publication.
This study was supported by Tertiary Education Commission–Center of Research Excellence (CoRE) funding, New Zealand.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the Riddet Institute (Massey University) for a PhD scholarship for DR.
1. Dunne J, Evershed RP, Salque M, Cramp L, Bruni S, Ryan K, et al. First dairying in green Saharan Africa in the fifth millennium BC. Nature. (2012) 486:390–94. doi: 10.1038/nature11186
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Evershed RP, Payne S, Sherratt AG, Copley MS, Coolidge J, Urem-Kotsu D, et al. Earliest date for milk use in the near East and southeastern Europe linked to cattle herding. Nature. (2008) 455:528–31. doi: 10.1038/nature07180
3. Faye B, Konuspayeva G. The sustainability challenge to the dairy sector – the growing importance of non-cattle milk production worldwide. Int Dairy J. (2012) 24:50–56. doi: 10.1016/j.idairyj.2011.12.011
CrossRef Full Text | Google Scholar
4. Park YW, Haenlein GFW. Overview of milk of non-bovine mammals. In: Park YW, Haenlein GFW, editors. Handbook of Milk of Non-Bovine Mammals. Oxford: Blackwell Publishing Professional (2006). p. 3–9. doi: 10.1002/9780470999738.ch1
5. Verduci E, D'Elios S, Cerrato L, Comberiati P, Calvani M, Palazzo S, et al. Cow's milk substitutes for children: nutritional aspects of milk from different mammalian species, special formula and plant-based beverages. Nutrients. (2019) 11:1739. doi: 10.3390/nu11081739
6. Ribeiro AC, Ribeiro SDA. Specialty products made from goat milk. Small Rumin. Res. (2010) 89:225–33. doi: 10.1016/j.smallrumres.2009.12.048
7. Haenlein GFW. Goat milk in human nutrition. Small Rumin Res. (2004) 51:155–63. doi: 10.1016/j.smallrumres.2003.08.010
8. Alichanidis E, Moatsou G, Polychroniadou A. Composition properties of non-cow milk products. In: Tsakalidou E, Papadimitriou K, editors. Non-Bovine Milk Milk Products. New York, NY: Academic Press (2016). p. 81–116. doi: 10.1016/B978-0-12-803361-6.00005-3
9. Park YW, Guo M. Goat milk products: types of products, manufacturing technology, chemical composition, and marketing. In: Park YW, Haenlein GFW, editors. Handbook of Milk of Non-Bovine Mammals . Oxford: Blackwell Publishing, Ltd (2006). p. 59–106. doi: 10.1002/9780470999738.ch4
10. Burgess K. Key requirements for milk quality and safety: a processor's perspective. In: Griffiths MW, editor. Improving the Safety and Quality of Milk–Volume 1 . Cambridge, UK: Woodhead Publishing Ltd (2010). p. 64–84. doi: 10.1533/9781845699420.1.64
11. Bencini R, Atzori AS, Nudda A, Battacone G, Pulina G. Improving the quality and safety of sheep milk. In: Griffiths MW, editor. Improving the Safety and Quality of Milk–Volume 2 . Cambridge, UK: Woodhead Publishing Ltd (2010). p. 347–401. doi: 10.1533/9781845699437.3.347
12. Regenstein JM, Chaudry MM, Regenstein CE. The kosher and halal food laws. Compr Rev Food Sci Food Saf. (2003) 2:111–27. doi: 10.1111/j.1541-4337.2003.tb00018.x
13. Crowley SV, Kelly AL, Lucey JA, O'Mahony JA. Potential applications of non-bovine mammalian milk in infant nutrition. In: Park YW, Haenlein GFW, Wendorff WL, editors. Handbook of Milk of Non-Bovine Mammals . Oxford: John Wiley & Sons, Ltd (2017). p. 625–54. doi: 10.1002/9781119110316.ch13
14. Potočnik K, Gantner V, Kuterovac K, Cividini A. Mare's milk: composition and protein fraction in comparison with different milk species. Mljekarstvo. (2011) 61:107–13. Available online at: https://hrcak.srce.hr/69078
Google Scholar
15. Balthazar CF, Pimentel TC, Ferrão LL, Almada CN, Santillo A, Albenzio M, et al. Sheep milk: physicochemical characteristics and relevance for functional food development. Compr Rev Food Sci Food Saf. (2017) 16:247–62. doi: 10.1111/1541-4337.12250
16. Ranadheera CS, Evans CA, Baines SK, Balthazar CF, Cruz AG, Esmerino EA, et al. Probiotics in goat milk products: delivery capacity and ability to improve sensory attributes. Compr Rev Food Sci Food Saf. (2019) 18:867–82. doi: 10.1111/1541-4337.12447
17. Barlowska J, Szwajkowska M, Litwinczuk Z, Krol J. Nutritional value and technological suitability of milk from various animal species used for dairy production. Compr Rev Food Sci Food Saf. (2011) 10:291–302. doi: 10.1111/j.1541-4337.2011.00163.x
18. Gantner V, Mijic P, Baban M, Skrtic Z, Turalija A. The overall and fat composition of milk of various species. Mljekarstvo. (2015) 65:223–31. doi: 10.15567/mljekarstvo.2015.0401
19. Claeys WL, Verraes C, Cardoen S, De Block J, Huyghebaert A, Raes K, et al. Consumption of raw or heated milk from different species: an evaluation of the nutritional and potential health benefits. Food Control. (2014) 42:188–201. doi: 10.1016/j.foodcont.2014.01.045
20. Arman P, Kay R, Goodall E, Sharman G. The composition and yield of milk from captive red deer (Cervus elaphus L.). J Reprod Fertil. (1974) 37:67–84. doi: 10.1530/jrf.0.0370067
21. Krzywiński A, Krzywińska K, Kisza J, Roskosz A, Kruk A. Milk composition, lactation and the artificial rearing of red deer. Acta Theriol. (1980) 25:341–7. doi: 10.4098/AT.arch.80-31
22. Landete-Castillejos T, Garcia A, Molina P, Vergara H, Garde J, Gallego L. Milk production and composition in captive Iberian red deer (Cervus elaphus hispanicus): effect of birth date. J Anim Sci. (2000) 78:2771–7. doi: 10.2527/2000.78112771x
23. Vithana NLO, Mason S, Bekhit A, Morton J. In vitro digestion of red deer (Cervus elaphus) and cow (Bos taurus) milk. Int Food Res J. (2012) 19:1367–74. Available online at: http://www.ifrj.upm.edu.my/19%20(04)%202012/10%20IFRJ%2019%20(04)%202012%20Nelum%20(074).pdf
24. Wang Y, Bekhit AEDA, Morton JD, Mason S. Nutritional value of deer milk. In: Watson RR, Collier RJ, Preedy V, editors. Nutrients in Dairy and their Implications for Health and Disease. 1st Edn. New York, NY: Academic Press (2017). p. 363–75. doi: 10.1016/B978-0-12-809762-5.00028-0
25. Martinez-Ferez A, Rudloff S, Guadix A, Henkel CA, Pohlentz G, Boza JJ, et al. Goats' milk as a natural source of lactose-derived oligosaccharides: isolation by membrane technology. Int Dairy J. (2006) 16:173–81. doi: 10.1016/j.idairyj.2005.02.003
26. Li S, Ye A, Singh H. Seasonal variations in composition, properties, and heat-induced changes in bovine milk in a seasonal calving system. J Dairy Sci. (2019) 102:7747–59. doi: 10.3168/jds.2019-16685
27. Oliveira DL, Wilbey RA, Grandison AS, Duarte LC, Roseiro LB. Separation of oligosaccharides from caprine milk whey, prior to prebiotic evaluation. Int Dairy J. (2012) 24:102–6. doi: 10.1016/j.idairyj.2011.12.012
28. Oliveira DL, Wilbey RA, Grandison AS, Roseiro LB. Milk oligosaccharides: a review. Int J Dairy Technol . (2015) 68:305–21. doi: 10.1111/1471-0307.12209
29. Roy D, Ye A, Moughan PJ, Singh H. Gelation of milks of different species (dairy cattle, goat, sheep, red deer, and water buffalo) using glucono-δ-lactone and pepsin. J Dairy Sci . (2020) 103:5844–62. doi: 10.3168/jds.2019-17571
30. Kappeler S. Compositional and structural analysis of camel milk proteins with emphasis on protective proteins . (thesis). Zurich, ETH Zurich (1998)
31. Tari NR, Fan MZ, Archbold T, Kristo E, Guri A, Arranz E, et al. Effect of milk protein composition of a model infant formula on the physicochemical properties of in vivo gastric digestates. J Dairy Sci. (2018) 101:2851–61. doi: 10.3168/jds.2017-13245
32. Ye A, Cui J, Carpenter E, Prosser C, Singh H. Dynamic in vitro gastric digestion of infant formulae made with goat milk and cow milk: influence of protein composition. Int Dairy J . (2019) 97:76–85. doi: 10.1016/j.idairyj.2019.06.002
33. Selo I, Clement G, Bernard H, Chatel J-M, Creminon C, Peltre G, et al. Allergy to bovine β-lactoglobulin: specificity of human IgE to tryptic peptides. Clin Exp Allergy. (1999) 29:1055–63. doi: 10.1046/j.1365-2222.1999.00612.x
34. Ereifej KI, Alu'datt MH, AlKhalidy HA, Alli I, Rababah T. Comparison and characterisation of fat and protein composition for camel milk from eight jordanian locations. Food Chem. (2011) 127:282–9. doi: 10.1016/j.foodchem.2010.12.112
35. El-Hatmi H, Jrad Z, Salhi I, Aguibi A, Nadri A, Khorchani T. Comparison of composition and whey protein fractions of human, camel, donkey, goat and cow milk. Mljekarstvo. (2015) 65:159–67. doi: 10.15567/mljekarstvo.2015.0302
36. Farah Z. Effect of heat treatment on whey proteins of camel milk. Milchwissenschaft. (1986) 41:763–65.
37. Beg OU, von Bahr-Lindström H, Zaidi ZH, Jörnvall H. Characterization of a heterogeneous camel milk whey non-casein protein. FEBS Lett. (1987) 216:270–74. doi: 10.1016/0014-5793(87)80704-4
38. Beg OU, von Bahr-Lindström H, Zaidi ZH, Jörnvall H. A small camel-milk protein rich in cysteine/half-cystine. Biosci Rep. (1984) 4:1065–70. doi: 10.1007/BF01116700
39. Park YW, Haenlein GFW. Other minor species milk (Reindeer, Caribou, Musk Ox, Llama, Alpaca, Moose, Elk, and Others). In: Park YW, Haenlein GFW, editors. Milk and Dairy Products in Human Nutrition . Oxford: Wiley-Blackwell Publishers (2013). p. 644–58. doi: 10.1002/9781118534168.ch30
40. De Kruif CG, Huppertz T, Urban VS, Petukhov AV. Casein micelles and their internal structure. Adv Colloid Interface Sci. (2012) 171:36–52. doi: 10.1016/j.cis.2012.01.002
41. Ingham B, Smialowska A, Kirby N, Wang C, Carr A. A structural comparison of casein micelles in cow, goat and sheep milk using X-ray scattering. Soft Matter. (2018) 14:3336–43. doi: 10.1039/C8SM00458G
42. Nguyen HTH, Afsar S, Day L. Differences in the microstructure and rheological properties of low-fat yoghurts from goat, sheep and cow milk. Food Res Int. (2018) 108:423–9. doi: 10.1016/j.foodres.2018.03.040
43. Pierre A, Michel F, Le Graët Y, Zahoute L. Casein micelle size in relation with casein composition and αs1, αs2, β and κ casein contents in goat milk. Lait. (1998) 78:591–605. doi: 10.1051/lait:1998653
44. Pierre A, Michel F, Le Graet Y. Variation in size of goat milk casein micelles related to casein genotype. Lait. (1995) 75:489–502. doi: 10.1051/lait:1995638
45. Dalgleish DG. The basis of structure in dairy-based foods: casein micelles and their properties. In: Boland M, Golding M, Singh H, editors. Food Structures, Digestion and Health. Amsterdam: Elsevier (2014). p. 83–105. doi: 10.1016/B978-0-12-404610-8.00003-7
46. Wang P, Liu H, Wen P, Zhang H, Guo H, Ren F. The composition, size and hydration of yak casein micelles. Int Dairy J. (2013) 31:107–10. doi: 10.1016/j.idairyj.2013.02.007
47. Dewan RK, Bloomfield VA, Chudgar A, Morr CV. Viscosity and voluminosity of bovine milk casein micelles. J Dairy Sci. (1973) 56:699–705. doi: 10.3168/jds.S0022-0302(73)85236-1
48. Pellegrini O, Remeuf F, Rivemale M. Évolution des caractéristiques physico-chimiques et des paramètres de coagulation du lait de brebis collecté dans la région de Roquefort. Lait. (1994) 74:425–42. doi: 10.1051/lait:1994635
49. Remeuf F, Lenoir J, Duby C, Letilly M-T, Normand A. Etude des relations entre les caractéristiques physico-chimiques des laits de chèvre et leur aptitude à la coagulation par la présure. Lait. (1989) 69:499–518. doi: 10.1051/lait:1989634
50. Ahmad S, Gaucher I, Rousseau F, Beaucher E, Piot M, Grongnet JF, et al. Effects of acidification on physico-chemical characteristics of buffalo milk: a comparison with cow's milk. Food Chem. (2008) 106:11–17. doi: 10.1016/j.foodchem.2007.04.021
51. Luo J, Jian S, Wang P, Ren F, Wang F, Chen S, et al. Thermal instability and characteristics of donkey casein micelles. Food Res Int. (2019) 119:436–43. doi: 10.1016/j.foodres.2019.02.023
52. Beaucher E, Nogueira N, Camier B, Jardin J, Briard-Bion V, Musaad A, et al. Physico-chemical characteristics of fresh and corresponding pasteurized camel milks from intensive dairy farm in Saudi Arabia: 479. In: JAM 2013, ADSA–ASAS . Indianapolis, IN (2013).
53. Sood SM, Gaind DK, Dewan R.K. Correlation between micelle salvation and calcium content. N Zeal J Dairy Sci. Technol. (1979) 14:32–44.
54. Remeuf F, Lenoir J. Relationship between the physico-chemical characteristics of goat's milk and its rennetability. Bull Fédér Int Lait. (1986) 202:68–72.
55. Park YW. Rheological characteristics of goat and sheep milk. Small Rumin Res. (2007) 68:73–87. doi: 10.1016/j.smallrumres.2006.09.015
56. Park YW, Juarez M, Ramos M, Haenlein GFW. Physico-chemical characteristics of goat and sheep milk. Small Rumin Res. (2007) 68:88–113. doi: 10.1016/j.smallrumres.2006.09.013
57. Ceballos LS, Morales ER, de la Torre Adarve G, Castro JD, Martínez LP, Sampelayo MRS. Composition of goat and cow milk produced under similar conditions and analyzed by identical methodology. J Food Compost Anal. (2009) 22:322–9. doi: 10.1016/j.jfca.2008.10.020
58. Ruiz-Sala P, Hierro M, Martinez-Castro I, Santa-Maria G. Triglyceride composition of ewe, cow, and goat milk fat. J Am Oil Chem Soc. (1996) 73:283–93. doi: 10.1007/BF02523421
59. Jenness R. Composition and characteristics of goat milk: review 1968–1979. J Dairy Sci. (1980) 63:1605–30. doi: 10.3168/jds.S0022-0302(80)83125-0
60. Abd El-Salam MH, El-Shibiny S. A comprehensive review on the composition and properties of buffalo milk. Dairy Sci Technol. (2011) 91:663. doi: 10.1007/s13594-011-0029-2
61. Kula JT, Tegegne D. Chemical composition and medicinal values of camel milk. Int J Res Stud Biosci. (2016) 4:13–25.
62. Park YW. Goat milk – chemistry and nutrition. In: Park YW, Haenlein GFW, editors. Handbook of Milk of Non-Bovine Mammals. Oxford: Blackwell Publishing Professional (2008). p. 34–58. doi: 10.1002/9780470999738.ch3
63. Jandal J. Comparative aspects of goat and sheep milk. Small Rumin Res. (1996) 22:177–85. doi: 10.1016/S0921-4488(96)00880-2
64. German JB, Dillard CJ. Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Crit Rev Food Sci Nutr. (2006) 46:57–92. doi: 10.1080/10408690590957098
65. Stroebinger N. The effect of dietary calcium and other nutritionally relevant divalent cations on fatty acid-soap formation . (thesis). Manawatu: Massey University, Manawatu, New Zealand (2016).
66. Innis SM. Dietary triacylglycerol structure and its role in infant nutrition. Adv Nutr. (2011) 2:275–83. doi: 10.3945/an.111.000448
67. Singh H. The milk fat globule membrane—a biophysical system for food applications. Curr Opin Colloid Interface Sci. (2006) 11:154–63. doi: 10.1016/j.cocis.2005.11.002
68. Lopez C, Cauty C, Guyomarc'h F. Unraveling the complexity of milk fat globules to tailor bioinspired emulsions providing health benefits: the key role played by the biological membrane. Eur J Lipid Sci Technol. (2019) 121:1800201. doi: 10.1002/ejlt.201800201
69. Lopez C, Madec M-N, Jimenez-Flores R. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chem. (2010) 120:22–33. doi: 10.1016/j.foodchem.2009.09.065
70. Nguyen HTH, Ong L, Hoque A, Kentish S, Williamson N, Ang C-S, et al. A proteomic characterization shows differences in the milk fat globule membrane of buffalo and bovine milk. Food Biosci. (2017) 19:7–16. doi: 10.1016/j.fbio.2017.05.004
71. Jenkins JA, Breiteneder H, Mills EN. Evolutionary distance from human homologs reflects allergenicity of animal food proteins. J Allergy Clin Immunol. (2007) 120:1399–405. doi: 10.1016/j.jaci.2007.08.019
72. El-Agamy EI. The challenge of cow milk protein allergy. Small Rumin Res. (2007) 68:64–72. doi: 10.1016/j.smallrumres.2006.09.016
73. Natale M, Bisson C, Monti G, Peltran A, Perono Garoffo L, Valentini S, et al. Cow's milk allergens identification by two-dimensional immunoblotting and mass spectrometry. Mol Nutr Food Res. (2004) 48:363–9. doi: 10.1002/mnfr.200400011
74. Hinz K, O'Connor PM, Huppertz T, Ross RP, Kelly AL. Comparison of the principal proteins in bovine, caprine, buffalo, equine and camel milk. J Dairy Res. (2012) 79:185–91. doi: 10.1017/S0022029912000015
75. Businco L, Giampietro PG, Lucenti P, Lucaroni F, Pini C, Di Felice G, et al. Allergenicity of mare's milk in children with cow's milk allergy. J Allergy Clin. Immunol. (2000) 105:1031–4. doi: 10.1067/mai.2000.106377
76. Tesse R, Paglialunga C, Braccio S, Armenio L. Adequacy and tolerance to ass's milk in an Italian cohort of children with cow's milk allergy. Ital J Pediatr. (2009) 35:19. doi: 10.1186/1824-7288-35-19
77. Monti G, Bertino E, Muratore MC, Coscia A, Cresi F, Silvestro L, et al. Efficacy of donkey's milk in treating highly problematic cow's milk allergic children: an in vivo and in vitro study. Pediatr Allergy Immunol. (2007) 18:258–64. doi: 10.1111/j.1399-3038.2007.00521.x
78. Ehlayel MS, Hazeima KA, Al-Mesaifri F, Bener A. Camel milk: an alternative for cow's milk allergy in children. Allergy Asthma Proc. (2011) 32:255–8. doi: 10.2500/aap.2011.32.3429
79. El-Agamy EI, Nawar M, Shamsia SM, Awad S, Haenlein GF. Are camel milk proteins convenient to the nutrition of cow milk allergic children? Small Rumin Res. (2009) 82:1–6. doi: 10.1016/j.smallrumres.2008.12.016
80. Sheehan WJ, Phipatanakul W. Tolerance to water buffalo milk in a child with cow milk allergy. Ann Allergy Asthma Immunol. (2009) 102:349. doi: 10.1016/S1081-1206(10)60342-0
81. Restani P, Beretta B, Fiocchi A, Ballabio C, Galli CL. Cross-reactivity between mammalian proteins. Ann Allergy Asthma Immunol. (2002) 89:11–15. doi: 10.1016/S1081-1206(10)62116-3
82. Katz Y, Goldberg MR, Zadik-Mnuhin G, Leshno M, Heyman E. Cross-sensitization between milk proteins: reactivity to a “kosher” epitope? Israel Med Assoc J. (2008) 10:85–8. Available online at: https://www.ima.org.il/MedicineIMAJ/viewarticle.aspx?year=2008&month=01&page=85
PubMed Abstract | Google Scholar
83. Restani P, Gaiaschi A, Plebani A, Beretta B, Cavagni G, Fiocchi A, et al. Cross-reactivity between milk proteins from different animal species. Clin Exp Allergy. (1999) 29:997–1004. doi: 10.1046/j.1365-2222.1999.00563.x
84. Infante PD, Tormo CR, Conde ZM. Use of goat's milk in patients with cow's milk allergy. An Pediatr. (2003) 9:138–42. doi: 10.1016/s1695-4033(03)78737-2
85. Bellioni-Businco B, Paganelli R, Lucenti P, Giampietro PG, Perbornc H, Businco L. Allergenicity of goat's milk in children with cow's milk allergy. J Allergy Clin Immunol. (1999) 103:1191–4. doi: 10.1016/S0091-6749(99)70198-3
86. Robinson F. Goats milk – a suitable hypoallergenic alternative? Br Food J. (2001) 103:198–208. doi: 10.1108/00070700110386746
87. Spuergin P, Walter M, Schiltz E, Deichmann K, Forster J, Mueller H. Allergenicity of α-caseins from cow, sheep, and goat. Allergy. (1997) 52:293–8. doi: 10.1111/j.1398-9995.1997.tb00993.x
88. Martín TM, De La Hoz Caballer B, Lizana FM, Mendiola RG, Montano PP, Cano MS. Selective allergy to sheep's and goat's milk proteins. Allergol Immunopathol. (2004) 32:39–42. doi: 10.1157/13057769
89. Ah-Leung S, Bernard H, Bidat E, Paty E, Rance F, Scheinmann P, et al. Allergy to goat and sheep milk without allergy to cow's milk. Allergy. (2006) 61:1358–65. doi: 10.1111/j.1398-9995.2006.01193.x
90. Bevilacqua C, Martin P, Candalh C, Fauquant J, Piot M, Roucayrol A-M, et al. Goats' milk of defective αs1-casein genotype decreases intestinal and systemic sensitization to β-lactoglobulin in guinea pigs. J Dairy Res. (2001) 68:217–27. doi: 10.1017/S0022029901004861
91. Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. (2015) 145:372–9. doi: 10.3945/jn.114.195438
92. Bos C, Mahé S, Gaudichon C, Benamouzig R, Gausserès N, Luengo C, et al. Assessment of net postprandial protein utilization of 15 N-labelled milk nitrogen in human subjects. Br J Nutr . (1999) 81:221–6. doi: 10.1017/S0007114599000410
93. Mahe S, Roos N, Benamouzig R, Davin L, Luengo C, Gagnon L, et al. Gastrojejunal kinetics and the digestion of [15N] beta-lactoglobulin and casein in humans: the influence of the nature and quantity of the protein. Am J Clin Nutr. (1996) 63:546–52. doi: 10.1093/ajcn/63.4.546
94. Boirie Y, Dangin M, Gachon P, Vasson M-P, Maubois J-L, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. (1997) 94:14930–5. doi: 10.1073/pnas.94.26.14930
95. Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. (2001) 280:E340–8. doi: 10.1152/ajpendo.2001.280.2.E340
96. Dangin M, Boirie Y, Guillet C, Beaufrère B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr. (2002) 132:3228S−33S. doi: 10.1093/jn/131.10.3228S
97. Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon, et al. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. (2011) 93:997–1005. doi: 10.3945/ajcn.110.008102
98. Fruhbeck G. Slow and fast dietary proteins. Nature. (1998) 391:843–5. doi: 10.1038/35993
99. Ye A, Roy D, Singh H. Structural changes to milk protein products during gastrointestinal digestion. In: Boland M, Singh H, editors. Milk Proteins. 3rd Edn. New York, NY: Academic Press (2020). p. 671–700. doi: 10.1016/B978-0-12-815251-5.00019-0
100. Mulet-Cabero A-I, Mackie AR, Brodkorb A, Wilde PJ. Dairy structures and physiological responses: a matter of gastric digestion. Crit Rev Food Sci Nutr . (2020). doi: 10.1080/10408398.2019.1707159. [Epub ahead of print].
101. Henschel MJ, Newport MJ, Parmar V. Gastric proteases in the human infant. Neonatology. (1987) 52:268–72. doi: 10.1159/000242719
102. Moschopoulou E. Characteristics of rennet and other enzymes from small ruminants used in cheese production. Small Rumin Res. (2011) 101:188–95. doi: 10.1016/j.smallrumres.2011.09.039
103. Guinee TP, Wilkinson MG. Rennet coagulation and coagulants in cheese manufacture. Int J Dairy Technol. (1992) 45:94–104. doi: 10.1111/j.1471-0307.1992.tb01791.x
104. Júnior BRCL, Tribst AAL, Cristianini M. High pressure homogenization of porcine pepsin protease: effects on enzyme activity, stability, milk coagulation profile and gel development. PLoS ONE. (2015) 10:e0125061. doi: 10.1371/journal.pone.0125061
105. Crabbe MJC. Rennets: general and molecular aspects. In: Fox PF, McSweeney PLH, Cogan TM, Guinee TP, editors. Cheese: Chemistry, Physics and Microbiology-Volume 1: General Aspects . 3rd Edn. Oxford: Academic Press (2004). p. 19–45. doi: 10.1016/S1874-558X(04)80061-7
106. Piper D, Fenton BH. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut. (1965) 6:506–8. doi: 10.1136/gut.6.5.506
107. Ye A, Cui J, Dalgleish D, Singh H. Formation of a structured clot during the gastric digestion of milk: impact on the rate of protein hydrolysis. Food Hydrocolloids . (2016) 52:478–86. doi: 10.1016/j.foodhyd.2015.07.023
108. Nakai S, Li-Chan E. Effect of clotting in stomachs of infants on protein digestibility of milk. Food Microstruct. (1987) 6:161–70.
109. Mason S. Some aspects of gastric function in the newborn. Arch Dis Child. (1962) 37:387–91. doi: 10.1136/adc.37.194.387
110. de Oliveira SC, Bellanger A, Ménard O, Pladys P, Le Gouar Y, Dirson E, et al. Impact of human milk pasteurization on gastric digestion in preterm infants: a randomized controlled trial. Am J Clin Nutr. (2017) 105:379–90. doi: 10.3945/ajcn.116.142539
111. Moughan PJ, Rowan A. The pig as a model animal for human nutrition research. Proc Nutr Soc NZ. (1989) 14:116–23. doi: 10.1146/annurev.nu.07.070187.002045
112. Moughan PJ, Cranwell P, Darragh A, Rowan A. The domestic pig as a model animal for studying digestion in humans. Eur Assoc Anim Prod. (1994) 80:389.
113. Moughan PJ, Birtles M, Cranwell P, Smith W, Pedraza M. The piglet as a model animal for studying aspects of digestion and absorption in milk-fed human infants. World Rev Nutr Diet. (1992) 67:40–113. doi: 10.1159/000419461
114. Moughan PJ, Pedraza M, Smith WC, Williams M, Wilson MN. An evaluation with piglets of bovine milk, hydrolyzed bovine milk, and isolated soybean proteins included in infant milk formulas. I. effect on organ development, digestive enzyme activities, and amino acid digestibility. J Pediatr Gastroenterol Nutr. (1990) 10:385–94. doi: 10.1097/00005176-199004000-00020
115. Moughan PJ, Cranwell PD, Smith WC. An evaluation with piglets of bovine milk, hydrolyzed bovine milk, and isolated soybean proteins included in infant milk forumlas. II. stomach-emptying rate and the postprandial change in gastric pH and milk-clotting enzyme activity. J Pediatr Gastroenterol Nutr. (1991) 12:253–9. doi: 10.1097/00005176-199102000-00019
116. Darragh AJ, Moughan PJ. The amino acid composition of human milk corrected for amino acid digestibility. Br J Nutr. (1998) 80:25–34. doi: 10.1017/S0007114598001731
117. Washburn RM, Jones CH. Studies of the values of different grades of milk in infant feeding. Vermont Agric Exp Station Bull. (1916) 195:94–101. Available online at: https://babel.hathitrust.org/cgi/pt?id=uiug.30112019892352&view=1up&seq=11
118. Braude R, Newport MJ, Porter JW. Artificial rearing of pigs: 2. The time course of milk protein digestion proteolytic enzyme secretion in the 28-day-old pig. Br J Nutr. (1970) 24:827–42. doi: 10.1079/BJN19700086
119. Decuypere JA, Bossuyt R, Henderickx HK. Gastric secretion in suckling pigs and early-weaned pigs given a dry cow's-milk formula ad lib. Br J Nutr . (1978) 40:91–102. doi: 10.1079/BJN19780099
120. Lichan E, Nakai S. Rennin modification of bovine casein to simulate human casein composition – effect on acid clotting and hydrolysis by pepsin. Can Inst Food Sci Technol J. (1988) 21:200–8. doi: 10.1016/S0315-5463(88)70777-4
121. Lichan E, Nakai S. Enzymic dephosphorylation of bovine casein to improve acid clotting properties and digestibility for infant formula. J Dairy Res. (1989) 56:381–90. doi: 10.1017/S0022029900028843
122. Blakeborough P, Gurr MI, Salter DN. Digestion of the zinc in human milk, cow's milk and a commercial babyfood: some implications for human infant nutrition. Br J Nutr . (1986) 55:209–17. doi: 10.1079/BJN19860027
123. Uniacke-Lowe T, Fox PF. Equid milk. In: Fuquay JW, Fox PF, McSweeney PLH, editors. Encyclopedia of Dairy Sciences . Vol. 3, 2nd Edn. San Diego, CA: Academic Press (2011). p. 518–29. doi: 10.1016/B978-0-12-374407-4.00318-6
124. Iannella G. Donkey cheese made through pure camel chymosin. Afr J Food Sci . (2015) 9:421–5. doi: 10.5897/AJFS2015.1322
125. Charfi I, Rezouga F, Makhlouf A, Bornaz S. The behaviour of Arabian donkey milk during acidification compared to bovine milk. Int J Dairy Technol. (2018) 71:439–45. doi: 10.1111/1471-0307.12447
126. Kamal M, Foukani M, Karoui R. Rheological and physical properties of camel and cow milk gels enriched with phosphate and calcium during acid-induced gelation. J Food Sci Technol. (2017) 54:439–46. doi: 10.1007/s13197-016-2480-9
127. Wang Y, Eastwood B, Yang Z, de Campo L, Knott R, Prosser C, et al. Rheological and structural characterization of acidified skim milks and infant formulae made from cow and goat milk. Food Hydrocolloids. (2019) 96:161–70. doi: 10.1016/j.foodhyd.2019.05.020
128. Ould Eleya MM, Desobry Banon S, Vetier N, Hardy J. Rheological study of acid gels from cow, goat and sheep milks. Lait. (1998) 78:453–9. doi: 10.1051/lait:1998443
129. Gamble JA, Besley AK, Ellis NR. Composition and properties of goat's milk as compared with cow's milk. US Dept Agric Tech Bull. (1939) 671:1–72.
130. Genene A, Hansen EB, Eshetu M, Hailu Y, Ipsen R. Effect of heat treatment on denaturation of whey protein and resultant rennetability of camel milk. LWT. (2019) 101:404–9. doi: 10.1016/j.lwt.2018.11.047
131. Jasińska B. The comparison of pepsin and trypsin action on goat, cow, mare and human caseins. Rocz Akad Med Bialymst. (1995) 40:486–93.
132. Hodgkinson AJ, Wallace OAM, Boggs I, Broadhurst M, Prosser CG. Gastric digestion of cow and goat milk: impact of infant and young child in vitro digestion conditions. Food Chem. (2018) 245:275–81. doi: 10.1016/j.foodchem.2017.10.028
133. Tagliazucchi D, Martini S, Shamsia S, Helal A, Conte A. Biological activities and peptidomic profile of in vitro -digested cow, camel, goat and sheep milk. Int Dairy J. (2018) 81:19–27. doi: 10.1016/j.idairyj.2018.01.014
134. Tagliazucchi D, Shamsia S, Helal A, Conte A. Angiotensin-converting enzyme inhibitory peptides from goats' milk released by in vitro gastro-intestinal digestion. Int Dairy J. (2017) 71:6–16. doi: 10.1016/j.idairyj.2017.03.001
135. Rutella GS, Solieri L, Martini S, Tagliazucchi D. Release of the antihypertensive tripeptides valine-proline-proline and isoleucine-proline-proline from bovine milk caseins during in vitro gastrointestinal digestion. J Agric Food Chem. (2016) 64:8509–15. doi: 10.1021/acs.jafc.6b03271
136. Maathuis A, Havenaar R, He T, Bellmann S. Protein digestion and quality of goat and cow milk infant formula and human milk under simulated infant conditions. J Pediatr Gastroenterol Nutr. (2017) 65:661–6. doi: 10.1097/MPG.0000000000001740
137. Almaas H, Cases A-L, Devold TG, Holm H, Langsrud T, Aabakken L, et al. In vitro digestion of bovine and caprine milk by human gastric and duodenal enzymes. Int Dairy J. (2006) 16:961–8. doi: 10.1016/j.idairyj.2005.10.029
138. El-Zahar K, Sitohy M, Choiset Y, Métro F, Haertlé T, Chobert J-M. Peptic hydrolysis of ovine β-lactoglobulin and α-lactalbumin. exceptional susceptibility of native ovine β-lactoglobulin to pepsinolysis. Int Dairy J. (2005) 15:17–27. doi: 10.1016/j.idairyj.2004.06.002
139. Inglingstad RA, Devold TG, Eriksen EK, Holm H, Jacobsen M, Liland KH, et al. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci Technol. (2010) 90:549–63. doi: 10.1051/dst/2010018
140. Mros S, Carne A, Ha M, Bekhit AE-D, Young W, McConnell, et al. Comparison of the bioactivity of whole and skimmed digested sheep milk with that of digested goat and cow milk in functional cell culture assays. Small Rumin Res. (2017) 149:202–8. doi: 10.1016/j.smallrumres.2017.02.018
141. Milan AM, Hodgkinson AJ, Mitchell SM, Prodhan UK, Prosser CG, Carpenter EA, et al. Digestive responses to fortified cow or goat dairy drinks: a randomised controlled trial. Nutrients. (2018) 10:1492. doi: 10.3390/nu10101492
142. Mills D. The in vitro buffering capacity of soluble paracetamol. Anaesthesia. (1989) 44:967–9. doi: 10.1111/j.1365-2044.1989.tb09197.x
143. Vaisman N, Reuven Y, Uzi M, Georgi G, Boehm G. Camel's milk and gastric emptying. Clin Nutr. (2006) 25:622–5. doi: 10.1016/j.clnu.2006.02.011
144. Kong F, Singh RP. A human gastric simulator (HGS) to study food digestion in human stomach. J Food Sci. (2010) 75:E627–35. doi: 10.1111/j.1750-3841.2010.01856.x
145. Ye A, Liu W, Cui J, Kong X, Roy D, Kong Y, et al. Coagulation behaviour of milk under gastric digestion: effect of pasteurization and ultra-high temperature treatment. Food Chem . (2019) 286:216–25. doi: 10.1016/j.foodchem.2019.02.010
146. Schorsch C, Wilkins DK, Jones MG, Norton IT. Gelation of casein-whey mixtures: effects of heating whey proteins alone or in the presence of casein micelles. J Dairy Res. (2001) 68:471–81. doi: 10.1017/S0022029901004915
147. Dannenberg F, Kessler HG. Reaction kinetics of the denaturation of whey proteins in milk. J Food Sci. (1988) 53:258–63. doi: 10.1111/j.1365-2621.1988.tb10223.x
148. Kaufmann W. Influences of different technological treatments of milk on the digestion in the stomach. VI. estimation of amino acid and urea concentrations in the blood: conclusions regarding the nutritional evaluation. Milchwissenschaft. (1984) 39:281–4.
149. Lacroix M, Bon C, Bos C, Léonil J, Benamouzig R, Luengo C, et al. Ultra high temperature treatment, but not pasteurization, affects the postprandial kinetics of milk proteins in humans. J Nutr. (2008) 138:2342–7. doi: 10.3945/jn.108.096990
150. Doan FJ. Soft curd milk: a critical review of the literature. J Dairy Sci. (1938) 21:739–56. doi: 10.3168/jds.S0022-0302(38)93028-0
151. Ye A, Cui J, Dalgleish D, Singh H. The formation and breakdown of structured clots from whole milk during gastric digestion. Food Funct . (2016) 7:4259–66. doi: 10.1039/C6FO00228E
152. Ye A, Cui J, Dalgleish D, Singh H. Effect of homogenization and heat treatment on the behavior of protein and fat globules during gastric digestion of milk. J Dairy Sci. (2017) 100:36–47. doi: 10.3168/jds.2016-11764
153. Mulet-Cabero A-I, Mackie AR, Wilde PJ, Fenelon MA, Brodkorb A. Structural mechanism and kinetics of in vitro gastric digestion are affected by process-induced changes in bovine milk. Food Hydrocolloids. (2019) 86:172–83. doi: 10.1016/j.foodhyd.2018.03.035
154. Mulet-Cabero A-I, Torcello-Gómez A, Saha S, Mackie AR, Wilde PJ, Brodkorb A. Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption. Food Chem . (2020) 319:126514. doi: 10.1016/j.foodchem.2020.126514
155. Mulet-Cabero A-I, Egger L, Portmann R, Ménard O, Marze S, Minekus M, et al. A standardised semi-dynamic in vitro digestion method suitable for food – an international consensus. Food Funct . (2020) 11:1702–20. doi: 10.1039/C9FO01293A
156. Ménard O, Bourlieu C, De Oliveira SCS, Dellarosa N, Laghi L, Carrière F, et al. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. (2018) 240:338–45. doi: 10.1016/j.foodchem.2017.07.145
157. Bourlieu C, Menard O, De La Chevasnerie A, Sams L, Rousseau F, Madec MN, et al. The structure of infant formulas impacts their lipolysis, proteolysis and disintegration during in vitro gastric digestion. Food Chem. (2015) 182:224–35. doi: 10.1016/j.foodchem.2015.03.001
158. Meena S, Rajput Y, Sharma R. Comparative fat digestibility of goat, camel, cow and buffalo milk. Int Dairy J. (2014) 35:153–6. doi: 10.1016/j.idairyj.2013.11.009
159. Alférez MJM, Barrionuevo M, López Aliaga I, Lisbona F, Robles JC, Campos MS, et al. Digestive utilization of goat and cow milk fat in malabsorption syndrome. J Dairy Res. (2001) 68:451–61. doi: 10.1017/S0022029901004903
160. Teng F, Reis MG, Yang L, Ma Y, Day L. Structural characteristics of triacylglycerols contribute to the distinct in vitro gastric digestibility of sheep and cow milk fat prior to and after homogenisation. Food Res Int. (2020) 130:108911. doi: 10.1016/j.foodres.2019.108911
161. Shani-Levi C, Alvito P, Andrés A, Assunção R, Barberá R, Blanquet-Diot S, et al. Extending in vitro digestion models to specific human populations: perspectives, practical tools and bio-relevant information. Trends Food Sci Technol. (2017) 60:52–63. doi: 10.1016/j.tifs.2016.10.017
Keywords: milk, composition, digestion, curd, protein, fat, structure, stomach
Citation: Roy D, Ye A, Moughan PJ and Singh H (2020) Composition, Structure, and Digestive Dynamics of Milk From Different Species—A Review. Front. Nutr. 7:577759. doi: 10.3389/fnut.2020.577759
Received: 29 June 2020; Accepted: 02 September 2020; Published: 06 October 2020.
Reviewed by:
Copyright © 2020 Roy, Ye, Moughan and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harjinder Singh, h.singh@massey.ac.nz
This article is part of the Research Topic
Milks Mean More: The Role of Milk in Nutrition, Digestion and Metabolism Across the Lifespan

Milk is the liquid produced by the mammary glands of mammals, including humans. Breast milk is the preferred food for infants, as it is well-tolerated while their digestive tracts develop and mature. Dairy milk may be introduced at later ages if tolerated well. Although dairy milk may come from any mammal, cows, goats, buffalo, and sheep are common producers. This section will focus on dairy milk from cows, and briefly discuss non-dairy plant milk alternatives.
Whole cow’s milk contains about 87% water. The remaining 13% contains protein, fat, carbohydrates, vitamins, and minerals. Processing techniques remove fat to produce lower fat varieties: “reduced fat” contains 2% milkfat, “lowfat” contains 1% milkfat, and “nonfat” or “skim” has virtually no milkfat. Cows are often pregnant while they are milked, so dairy milk contains hormones like insulin-like growth factor-1 (IGF-1), estrogens, and progestins. Some cows are given additional hormones to increase milk production.
- Vitamin B2 (riboflavin)
- Vitamin B12
- Vitamin A and Vitamin D (added during processing)
Milk and Health
The Dietary Guidelines for Americans recommends including three 8-ounce servings of milk daily (or equal portions of other dairy foods like cheese or yogurt), which is justified to increase calcium intake and reduce the risk of osteoporosis and fractures. Marketing efforts such as the iconic “Got Milk?” campaign with celebrities donning milk mustaches spread this message as well. However, research has not shown a consistent benefit on bone health with high intakes of milk, and furthermore has suggested potential harm with certain conditions like prostate cancer. [1]
Research on milk often produces contrary findings. Some reasons may be the wide range of different nutritional qualities in milk and how milk intake is measured, as seen in the following factors:
- The amount of milk that is considered a “high” or “low” intake can vary among populations studied. For example, people from Japan tend to drink about less than half of the milk consumed in Western countries [2]
- Are different classifications of milk included, or just one type? Whole, reduced-fat, fat-free, or organic?
- The composition of milk (fat, protein from varying amino acids) may differ depending on the breed and feed of the cows.
- Are other factors in the diet considered, such as if the participants are eating plenty of fruits and vegetables, or large amounts of processed meat or refined carbohydrates, which can confound the true health effects of milk?
- Different forms of dairy foods , such as cheese , or yogurt , may have health effects different than milk.
The connection of milk and cardiovascular disease (CVD) is unclear. Whole milk contains saturated fat, which is known to increase total cholesterol, raising both LDL “bad” and HDL “good” cholesterol levels. However, the minerals in milk, specifically calcium and potassium may help to control blood pressure.
Comparison of milk with other foods in the diet can affect study results. For example, if comparing milk with high saturated fat meats, milk may show no difference in heart health. However, if dairy fat in milk is compared with unsaturated plant oils, nuts, or seeds, milk may appear to increase CVD risk.
- A study following three large cohorts of men and women found that dairy fat including milk was not associated with overall CVD risk. However, when dairy fat was swapped with an equal amount of calories from polyunsaturated or vegetables oils, there was a 24% and 10% lower risk of CVD, respectively. There was a 6% increase in CVD risk when dairy fat was replaced with other animal fats (e.g., from red meat). [3]
- Whole milk was associated with 1.5 times the risk of heart disease in women drinking 1-2 glasses or more a day. Drinking skim milk was associated with a lower risk. [4]
- A meta-analysis of 15 prospective cohort studies found that low-fat and fermented milk were moderately protective of stroke, possibly due to a reduction in blood pressure. [5] Milk contains potassium, magnesium, and calcium, which helps to regulate blood pressure. However, randomized trials of low-fat milk have not consistently found milk to lower blood pressure.
- A review of 17 prospective studies found that milk intake was not associated with increased risk of mortality (early death) from cardiovascular diseases. There was no association when looking specifically at high-fat versus low-fat dairy products. [6]
- However, another study following three large cohorts of men and women found that whole milk was associated with higher risks of total mortality including mortality from cardiovascular disease. [7]
Milk contains nutrients important for bone health: calcium, phosphorus, vitamin D, and protein. However, an association with milk intake and decreased hip fractures has not been made.
Interestingly, the countries with the highest intakes of milk and calcium have the highest hip fracture rates. [8] Yet it’s unclear that milk alone is responsible because these studies are epidemiological and find associations rather than direct causes. It is known that higher milk intake tends to increase height, and a taller height is strongly related to fractures of the hip and other bones, particularly in men. [9]
Two meta-analyses of prospective cohort studies did not find an association between milk intake and risk of hip fractures. [10, 11] This result was found even with high milk intakes of 3-4 glasses a day.
Although it is believed that high calcium intakes at preadolescent and adolescent years may protect against bone loss later in life, studies have not supported this. The Recommended Daily Allowance for calcium in the U.S. for preadolescents ages 9-13 years is 1300 mg daily, but the calcium recommendations in other countries such as Japan and the United Kingdom are only on average 750 mg daily. Drinking three servings of milk daily for 18 months in adolescent boy and girls with a low calcium intake did not effect bone mass. [12] For further nutritional guidance for children and adolescents, visit Harvard’s Kid’s Healthy Eating Plate .
Contrary to widespread belief, research does not support that milk helps with weight control. Although a meta-analysis of 29 randomized controlled trials found that milk and other dairy foods were beneficial for body fat reduction in the short-term and if calories were restricted, no benefits on body weight were seen in the long-term and when calories were not restricted. [13] A later meta-analysis of 37 randomized controlled trials found similar beneficial effects of dairy intake on body weight and body fat when calories were limited, but without a calorie restriction, weight gain was likely. [14]
A large study of more than 12,000 adolescents looked to see if total milk helped to prevent weight gain. It found that dairy fat was not associated with weight gain, but a high intake of low-fat milk (more than 3 servings daily) was associated with weight gain and higher body mass index, mainly from the extra calories obtained. [15]
The association of milk and diabetes is unclear. Dairy foods are associated with a moderately lower risk of type 2 diabetes in cohort studies, but the benefit mainly appears with eating fermented milk products like yogurt rather than from milk. [16, 17] Although the action of yogurt in benefiting diabetes is unclear, it contains helpful bacteria that may reduce inflammation or improve the action of the body’s natural insulin.
Higher milk intake is associated with increased incidence of prostate cancer. The Physicians’ Health Study of 21,660 men found that an intake of more than 2.5 servings of dairy foods daily (compared with a half or less serving daily) was associated with a 12% increased risk prostate cancer. In men who consumed 1 or more servings of milk daily (compared with rarely consumed), skim milk was associated with an increased risk of early stage prostate cancer, and whole milk was associated with fatal advanced prostate cancer. [18]
A meta-analysis of 111 cohort studies by the World Cancer Research Fund found a decreased risk of colorectal cancer with higher milk intake but mostly in men. This is possibly due to the high calcium content in milk, a mineral found to be protective of colorectal cancer. [19]
Bottom line: The health benefits of dairy foods appear to be stronger for fermented types like yogurt , which play a role in the gut microbiome . Milk possesses several individual nutrients that can affect blood pressure and bone health, but some of their health-promoting effects may be weakened by whole milk’s high saturated fat content. Although popular media articles have speculated that whole milk is not less healthful than skim milk, research has not supported this statement in regards to diabetes and heart disease, and a high intake of any type of milk can lead to weight gain due to the extra calories.
The name A2 milk may sound like a futuristic space food, but it is a type of milk now sold in some stores. A1 and A2 are two types of gene variants of casein, a protein in cow’s milk. The digestion and breakdown of casein produces a peptide (BCM-7) that has been associated with inflammation, digestive discomfort, and even some chronic diseases. [20] Some animal studies have shown that A1 milk has 4 times the amount of BCM-7 than A2 milk. Certain cattle breeds possess more of the A1 gene variant than A2, and visa versa. [20]
Through epidemiological studies and animal studies, a high intake of A1 casein has been associated with increased risk of ischemic heart disease, increased incidence of type 1 diabetes, and some neurological disorders such as autism. [21]
However, a European Food Safety Authority (EFSA) published a summary report after conducting a review of the literature. Some of their findings:
- The effects of A1 and A2 genes on cardiovascular and neurological diseases and type 1 diabetes were inadequate to make health recommendations. [21] They noted inconsistent findings and weaknesses in study design that often did not adjust for confounders, included a small number of participants, or had a short intervention period.
- Fresh raw (unprocessed) milk from healthy cows does not contain BCM-7, and that in theory the fermentation process involved when producing fermented milks and cheeses may break down BCM-7. However little to no data are available on the actual BCM-7 content of fermented milk products.
A2 milk distributers market their product as beneficial for adults, children, and infants who experience milk intolerances, whether from milk allergy or lactose intolerance. Symptoms of these conditions include gas, bloating, constipation, or diarrhea. However, it is noted that the majority of clinical trials comparing A1 with A2 milk, with findings showing improved tolerance and less gastrointestinal side effects with A2 milk, are funded by the A2 Corporation, producer of the brand “a2 Milk” that is sold worldwide.
Bottom line: although milk with the A2 gene variant may be metabolized differently in the body than A1 milk, there is not enough evidence from research to show that these differences significantly affect health. If one has lactose intolerance or a milk allergy causing unpleasant symptoms, A2 milk may be tried. Plant-based milks are another option as they do not contain lactose or the milk protein casein.
Although fans of raw milk believe it tastes better and offers more nutrients than processed milk, raw milk is not pasteurized and may harbor harmful bacteria including Salmonella, Listeria and E. coli . Currently, twenty states prohibit the sale of raw milk. According to the Centers for Disease Control and Prevention, unpasteurized milk is 150 times more likely to cause foodborne illness than illnesses related to pasteurized dairy products. Between 1993 and 2006 more than 1,500 people in the U.S. became ill from drinking raw milk or eating cheese made from raw milk. [22] Raw milk and soft cheeses made from raw milk like Brie, Camembert, Queso Blanco, and Queso Fresco are especially dangerous for older adults, those with weakened immune systems, pregnant women, and children. In pregnant women, Listeria can cause miscarriage, or illness or death of a fetus or newborn.
For Your Health and the Planet’s Health

Milk is often sold in cartons or opaque containers because too much exposure to light can cause a loss of vitamin A and B2. Choose a carton with the latest sell-by or use-by date (indicating it is the freshest). Most milk sold in supermarkets is pasteurized and homogenized, processing techniques that use heat to kill most of the bacteria present and break down fat molecules so that texture of milk remains smooth and creamy.
What about plant-based milk?
*Some sugars are created in the processing of oats to make oat milk, which are listed as “added sugars” even if no other sweeteners are added.
Milk requires refrigeration at a temperature below 40 F. If it has been stored at room temperature for two hours or longer, it is recommended to discard it. Although pasteurization kills much of the bacteria in milk, any remaining bacteria can grow quickly in milk at room temperature or warmer. Once milk is opened, it will last about 3-5 days after the sell-by date on the label. Spoiled milk has a strong, sour odor and lumpy texture caused by excess bacteria producing lactic acid, which curdles the protein in milk and produces off odors.
Store milk towards the rear of the refrigerator rather than the front or side shelf door, where the temperature varies the most. Don’t forget to close the carton or recap the bottle to prevent the milk from absorbing the odors and flavors of other foods in the refrigerator.
Lactose-free milk undergoes pasteurization and the addition of an enzyme lactase, which breaks down the milk sugar lactose, so it generally lasts longer than regular milk. If refrigerated properly, lactose-free milk can last about 7 days after the sell-by date once it is opened.
Milk is not just for drinking by the glass or splashing onto cold cereal. Adding milk to foods can boost one’s intake of calcium, vitamin D, protein, and other nutrients.
- Blend 1 cup of milk, 1/2 cup of fresh or frozen berries, and 1 small banana for an easy breakfast drink or snack.
- Overnight oats. In a mason jar (you can also reuse a clean jelly or salsa jar), add 1 cup of milk, 1/2 cup of rolled oats, 1 tablespoon chia seeds, 3 tablespoons of chopped nuts, ½ sliced banana, and ¼ cup fresh or frozen berries. Secure the lid and shake the jar well until all the ingredients are mixed. Refrigerate overnight.
- Hot oatmeal. Cook old-fashioned rolled oats in milk (the ratio is generally ½ cup oats to 1 cup milk).
Did You Know?
Several other animals produce milk including sheep, goat, and yaks. These types of milk are more popular in European, Middle Eastern, and Asian countries than in the U.S. Sheep’s milk can be made into various cheeses like feta and ricotta, and goat’s milk produces a popular cheese called goat cheese or chevre.
How do they compare nutritionally? Sheep, goat, and yak milks contain about the same if not more calcium than cow’s milk. The amount of protein and carbohydrate are about the same. They all contain some lactose but less than found in cow’s milk, so they may be easier to digest for people with lactose sensitivity.
- Willett WC, Ludwig DS. Milk and Health. New England Journal of Medicine . 2020 Feb 13;382(7):644-54.
- Kondo I, Ojima T, Nakamura M, Hayasaka S, Hozawa A, Saitoh S, Ohnishi H, Akasaka H, Hayakawa T, Murakami Y, Okuda N. Consumption of dairy products and death from cardiovascular disease in the Japanese general population: the NIPPON DATA80. Journal of epidemiology . 2013 Jan 5;23(1):47-54.
- Chen M, Li Y, Sun Q, Pan A, Manson JE, Rexrode KM, Willett WC, Rimm EB, Hu FB. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. The American journal of clinical nutrition . 2016 Nov 1;104(5):1209-17.
- Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. The American journal of clinical nutrition . 1999 Dec 1;70(6):1001-8.
- Hu D, Huang J, Wang Y, Zhang D, Qu Y. Dairy foods and risk of stroke: a meta-analysis of prospective cohort studies. Nutrition, Metabolism and Cardiovascular Diseases . 2014 May 1;24(5):460-9.
- Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. The American journal of clinical nutrition . 2011 Jan 1;93(1):158-71.
- Ding M, Li J, Qi L, Ellervik C, Zhang X, Manson JE, Stampfer M, Chavarro JE, Rexrode KM, Kraft P, Chasman D. Associations of dairy intake with risk of mortality in women and men: three prospective cohort studies. BMJ . 2019 Nov 27;367.
- Hegsted DM. Fractures, calcium, and the modern diet. The American journal of clinical nutrition . 2001 Nov 1;74(5):571-3.
- Feskanich D, Bischoff-Ferrari HA, Frazier AL, Willett WC. Milk consumption during teenage years and risk of hip fractures in older adults. JAMA pediatrics . 2014 Jan 1;168(1):54-60.
- Bischoff‐Ferrari HA, Dawson‐Hughes B, Baron JA, Kanis JA, Orav EJ, Staehelin HB, Kiel DP, Burckhardt P, Henschkowski J, Spiegelman D, Li R. Milk intake and risk of hip fracture in men and women: a meta‐analysis of prospective cohort studies. Journal of Bone and Mineral Research . 2011 Apr;26(4):833-9.
- Matía-Martín P, Torrego-Ellacuría M, Larrad-Sainz A, Fernández-Pérez C, Cuesta-Triana F, Rubio-Herrera MÁ. Effects of milk and dairy products on the prevention of osteoporosis and osteoporotic fractures in Europeans and non-Hispanic Whites from North America: a systematic review and updated meta-analysis. Advances in Nutrition . 2019 May 1;10(suppl_2):S120-43.
- Vogel KA, Martin BR, McCabe LD, Peacock M, Warden SJ, McCabe GP, Weaver CM. The effect of dairy intake on bone mass and body composition in early pubertal girls and boys: a randomized controlled trial. The American journal of clinical nutrition . 2017 May 1;105(5):1214-29.
- Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. The American journal of clinical nutrition . 2012 Oct 1;96(4):735-47.
- Geng T, Qi L, Huang T. Effects of dairy products consumption on body weight and body composition among adults: an updated meta‐analysis of 37 randomized control trials. Molecular nutrition & food research . 2018 Jan;62(1):1700410.
- Berkey CS, Rockett HR, Willett WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain: a longitudinal study of adolescents. Archives of pediatrics & adolescent medicine . 2005 Jun 1;159(6):543-50.
- Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC medicine . 2014 Dec 1;12(1):215.
- Gijsbers L, Ding EL, Malik VS, De Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. The American journal of clinical nutrition . 2016 Apr 1;103(4):1111-24.
- Song Y, Chavarro JE, Cao Y, Qiu W, Mucci L, Sesso HD, Stampfer MJ, Giovannucci E, Pollak M, Liu S, Ma J. Whole milk intake is associated with prostate cancer-specific mortality among US male physicians. The Journal of nutrition . 2013 Feb 1;143(2):189-96.
- Vieira AR, Abar L, Chan DS, Vingeliene S, Polemiti E, Stevens C, Greenwood D, Norat T. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Annals of Oncology . 2017 Aug 1;28(8):1788-802.
- Kamiński S, Cieślińska A, Kostyra E. Polymorphism of bovine beta-casein and its potential effect on human health. Journal of applied genetics . 2007 Sep 1;48(3):189-98.
- European Food Safety Authority. Scientific Report of EFSA: Review of the potential health impact of beta-casomorphins and related peptides. EFSA Scientific Report 2009; 231: 1-107. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.231r Accessed 12/9/2019.
- U.S. Food and and Drug Administration. Food Facts: The Dangers of Raw Milk. August 2012. https://www.fda.gov/media/119383/download Accessed 11/25/2019 .
Last Reviewed July 2021
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
Infant formulae - Key components, nutritional value, and new perspectives
Affiliations.
- 1 State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu 214122, China.
- 2 State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu 214122, China; International Joint Laboratory on Food Safety, Jiangnan University, Wuxi 214122, China. Electronic address: [email protected].
- PMID: 37210844
- DOI: 10.1016/j.foodchem.2023.136393
Breastfeeding is the most effective strategy for meeting the nutritional demands of infants, whilst infant formulae are manufactured foods that mimic human milk and can be safely used to replace breastfeeding. In this paper, the compositional differences between human milk and other mammalian milk are reviewed, and thus nutritional profiles and compositions of standard bovine milk-based formulae as well as special formulae are discussed. Differences between breast milk and other mammalian milk in composition and content affect their digestion and absorption in infants. Characteristics and mimicking of breast milk have been intensively studied with the objective of narrowing the gap between human milk and infant formulae. The functions of the key nutritional components in infant formulae are examined. This review detailed recent developments in the formulation of different types of special infant formulae and efforts for their humanization, and summarized safety and quality control of infant formulae.
Keywords: Bioactive compounds; Composition; Humanization; Infant formulae.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Publication types
- Infant Food / analysis
- Infant Formula*
- Infant Nutritional Physiological Phenomena*
- Milk, Human
- Nutritive Value
- Open access
- Published: 11 May 2024
Molecular and phenotypic identification of bacterial species isolated from cows with mastitis from three regions of Poland
- Anna Dobrut 1 ,
- Izabela Siemińska 2 ,
- Agnieszka Sroka-Oleksiak 1 ,
- Kamil Drożdż 1 ,
- Joanna Sobońska 1 ,
- Urszula Mroczkowska 3 &
- Monika Brzychczy-Włoch 1
BMC Veterinary Research volume 20 , Article number: 193 ( 2024 ) Cite this article
Metrics details
Bovine mastitis is a widespread disease affecting dairy cattle worldwide and it generates substantial losses for dairy farmers. Mastitis may be caused by bacteria, fungi or algae. The most common species isolated from infected milk are, among others, Streptococcus spp. , Escherichia coli , Staphylococcus aureus and non-aureus staphylococci and mammaliicocci. The aim of this paper is to determine the frequency of occurrence of bacterial species in milk samples from cows with mastitis from three regions of Poland: the north-east, the south-west and the south. To this end 203 milk samples taken from cows with a clinical form (CM) of mastitis (n = 100) and healthy animals (n = 103) were examined, which included culture on an appropriate medium followed by molecular detection of E. coli , S. aureus , Streptococcus agalactiae and Streptococcus uberis , as one of the most common species isolated from mastitis milk.
The results obtained indicated that S. uberis was the most commonly cultivated CM species (38%, n = 38), followed by S. aureus (22%, n = 22), E. coli (21%, n = 21) and S. agalactiae (18%, n = 18). Similar frequencies in molecular methods were obtained for S. uberis (35.1%) and S. aureus (28.0%). The variation of sensitivity of both methods may be responsible for the differences in the E. coli (41.0%, p = 0.002) and S. agalactiae (5.0%, p = 0.004) detection rates. Significant differences in composition of species between three regions of Poland were noted for E. coli incidence (p < 0.001), in both the culture and molecular methods, but data obtained by the PCR method indicated that this species was the least common in north-eastern Poland, while the culture method showed that in north-eastern Poland E. coli was the most common species. Significant differences for the molecular method were also observed for S. uberis (p < 0.001) and S. aureus (p < 0.001). Both species were most common in southern and south-western Poland.
Conclusions
The results obtained confirm the need to introduce rapid molecular tests for veterinary diagnostics, as well as providing important epidemiological data, to the best of our knowledge data on Polish cows in selected areas of Poland is lacking.
Peer Review reports
Bovine mastitis is a major disease that affects dairy cattle worldwide and generates great losses for the dairy industry [ 1 ]. It is estimated that the economic losses generated by clinical forms of mastitis may reach as much as 240 euros per cow per year [ 2 , 3 ]. These financial losses tend to result from reduced volumes of milk, lower milk quality and the need to isolate sick animals, during which time they produce no milk and require treatment [ 1 ]. Mastitis may take the form of sub-clinical, clinical or chronic infections and this status may depend on the nature of the pathogen responsible and on the age, breed, immunological health and state of lactation of the animal [ 4 , 5 ]. The clinical form of mastitis demonstrates visible and palpable symptoms such as redness of the udder, pain, swelling, fever or elevated temperature, a change in the appearance of milk and a reduction in the production of milk [ 4 , 5 ]. The frequency of the clinical form of mastitis varies among regions and ranges from 12 to 30% [ 3 ]. The sub-clinical form generates the highest costs associated with difficulties in its detection due to the lack of visible symptoms. In turn, the chronic form is the least common but may lead to chronic mastitis [ 6 ].
Mastitis is generally caused by bacteria but also fungi and algae. The most common bacterial species responsible for mastitis are Streptococcus uberis , Staphylococcus aureus , Streptococcus dysgalactiae, Streptococcus agalactiae (group B streptococcus, GBS), Non-aureus staphylococci and mammaliicocci (NASM), Corynebacterium bovis , Mycoplasma bovis , Escherichia coli and Klebsiella pneumoniae [ 7 , 8 ]. The frequency of mastitis in countries and regions varies depending on latitude, management conditions and diagnostic methodology [ 9 ].
The current gold standard in the diagnosis of bovine mastitis is a method based on the determining the somatic cell count (SCC) in quarter milk, 200.000 cells/ml being considered a critical value [ 10 ]. This method allows the rapid detection of mastitis in animals examined directly in the field, undoubtedly its most important advantage. California Mastitis Test (CMT) [ 11 ] is one of the methods for the rapid detection of high SCC in milk on field, however other on-farm culture methods exist and can be used on field [ 12 , 13 ]. As a result, the infected animal may immediately be isolated from the healthy herd, thus thwarting the spread of the infection among the uninfected animals and helping avoid financial losses. This method, however, demonstrates a severe flaw: the inability of etiological factor identification. This lack of knowledge prevents veterinarians from introducing correct therapy which in turn leads to the increase of incorrect and unnecessary use of antibiotics [ 14 ], hence increase the adverse phenomenon leads to the increase of multidrug resistant bacteria. In response to this, scientists, supported by the biotechnology market, are therefore attempting to develop an alternative method, one that precisely, rapidly, and at limited costs allows the identification of the bacterial genus and species, and on the basis of which the administrated antibiotic would be selected precisely. Culture methods on an appropriated medium allows the identification of bacterial species, but waiting time for the results needs at least 24–72 h [ 15 ], and interpretation of the results may be ambiguous. Nucleic acid amplification techniques (NAATs) and serological methods are a promising alternative. As common advantages they share high selectivity and specificity, as the waiting time for the results is shorter than traditional culture. Another reliable and rapid technique to identify the most common microorganisms, which constantly develops is MALDI-TOF MS (Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry). This method allows obtaining results in 24 h and the database are continuously expanded and improved with additional species [ 16 ]. A crucial limitation, however, is the inability to perform the tests outside a laboratory equipped with specialist equipment [ 17 ]. Immunochromatographic assay (lateral flow assay, LFA), which does not demonstrate the above limitation mentioned above is a promising alternative. This rapid test allows the detection of pathogens in biological samples within a few minutes. It also requires no specialized equipment; it is easy to perform and simple to interpret. Crucially, however, the lateral flow assay may be performed directly in the field. The low costs, in comparison with other molecular and serological methods, also make this technique more accessible, not only to veterinary surgeons but also to dairy farmers and cattle breeders [ 18 ].
The aim of this research was to characterize the most common species of bacteria that cause mastitis among cows in Poland. The results obtained for three regions in Poland ( voivodeship s) may update the knowledge in the field of mastitis epidemiology among cows in these three regions Poland because, to the best of our knowledge, there is a lack of up-to-date data.
Phenotypic and molecular identification
In the course of identifying the most common species of bacteria in clinical samples from cows from three Polish voivodships, phenotypical and molecular identification were performed. Culture methods followed by phenotypic analyses indicated that the most common species in the studied CM samples was S. uberis (38.0%, n = 38), followed by S. aureus (22%, n = 22), E. coli (21%, n = 21), S. agalactiae (18%, n = 18), and K. pneumoniae (3%, n = 3) (Fig. 1 A). A greater diversity in the composition of bacterial species was observed in the pool of samples from animals without clinical signs of mastitis. Bacterial composition identified in the pool of non-clinical samples included the following species: Staphylococcus epidermidis (34.0%, n = 35), Viridans Group Streptococci (SV) (28.2%, n = 29), S. uberis (28.2%, n = 29), Corynebacterium spp. (19.4%, n = 20), other NASM (excluding S. epidermidis ) (9.7%, n = 10), E. coli (5.8%, n = 6), S. aureus (4.9%, n = 5), and K. pneumoniae (3.9%, n = 4) (Fig. 1 B). According to National Mastitis Council (NMC) in the control group, 13 samples were classified as sub-clinical and 11 were classified as a contamination.
The percentage of bacterial species identified by using culture methods in bovine milk from cows with clinical mastitis ( A ) and from cows from the control group ( B ). Legend: SA – Staphylococcus aureus , EC – Escherichia coli , GBS – Streptococcus agalactiae , SU – Streptococcus uberis , KP – Klebsiella pneumoniae , SE – Staphylococcus epidermidis , CS – Corynebacterium spp., SV – Viridians Group Streptococcus , NASM – Non-aureus staphylococci and mammaliicocci (exc. Staphylococcus epidermidis )
We also attempted to compare the accuracy between Columbia Blood Agar and Chromagar mastitis in detecting bacterial species that cause mastitis and we showed that Chromagar mastitis significantly increased the detectability of S. agalactiae (p < 0.001) more than Columbia Blood Agar. Detection levels for other species was comparable and statistically insignificant.
In the molecular analysis we began by comparing two methods of DNA isolation. In the first approach bacterial DNA was directly isolated and in the second samples were pre-incubated in TSB. The aim of this modification was to improve the amount of DNA obtained for further research. It was shown that pre-incubation in TSB improved sensitivity of the method for all species examined. Among the directly isolated CM pool of samples S. aureus was detected in 17% (n = 17) of the milk examined, while pre-incubation increased sensitivity by 11%. The introduction of the pre-incubation step for E. coli led to 9% growth in detection of the pathogen and the percentage of positive results increased from 41 to 50%. For S. agalactiae pre-incubation produced the detection of one more S. agalactiae -positive sample (to 5%). Most notable was the double growth observed for S. uberis , which allowed the detection of 35.1% of clinical milk samples and the differences were statistically significant (p = 0.005) (Fig. 2 ).
The results of a comparison between the two approaches of isolating the bacterial DNA from CM milk samples. Legend: without TSB – milk samples subjected to direct DNA isolation; with TSB – milk samples subjected to pre-incubation in TSB before further DNA isolation procedures; SA – Staphylococcus aureus , EC – Escherichia coli , GBS – Streptococcus agalactiae , SU – Streptococcus uberis
Combined results obtained by compilation of two methods of DNA isolation (direct and pre-incubation with TSB) followed by the PCR method indicated that E. coli was the most frequently identified species in the pool of the CM samples examined (41.0%, n = 41). The frequency of occurrence of S. uberis and S. aureus determined by molecular methods coincided with the culture methods and the percentage was as follow: 35.1% (n = 34) for S. uberis and 28.0% (n = 28) for S. aureus . S. agalactiae was detected in 5 samples (5.0%) (Fig. 3 A). In the control group the most common species was E. coli (53.4%, n = 55), followed by S. aureus (26.2%, n = 27) and S. uberis (17.5%, n = 18). S. agalactiae did not occur in the control group (Fig. 3 B).
The percentage of bacterial species identified by molecular methods in bovine milk from 100 cows with clinical mastitis ( A ) and from 103 cows from the control group ( B ). Legend: SA – Staphylococcus aureus , EC – Escherichia coli , GBS – Streptococcus agalactiae , SU – Streptococcus uberis
Results obtained by the culture methods and the molecular method demonstrated the variability in frequency of occurrence of individual species, which may result from differences in the sensitivity of the methods used, but overall the coverage (for CM and control groups) was 93.6% for S. agalactiae , 82.3% for S. aureus , 79.0% for S. uberis and 61.1% for E. coli . In-depth analysis into clinical and non-clinical samples showed that the highest coverage in CM was obtained for S. aureus (92.1%), followed by S. uberis (88.8%), S. agalactiae (87.1%) and E. coli (74.3%). In the control group the results were more divergent than for the clinical samples and the percentages obtained were as follows: the highest coverage was obtained for S. aureus (72.5%), for S. uberis 69.6%%, and for E. coli 48.0%. S. agalactiae was not found in any milk sample, so coverage reached 100.0%.
Species co-occurrence
The next step saw us compare the simultaneous co-occurrence of two, three and four species in the samples, both in clinical mastitis and in the control group. The most common combination found by the culture method was one species (33.5%). Two species were detected in 13.1% of samples and three species in 2.5%. Four species occurred in 0.5% of cases. In 50.7% no species were detected. In the PCR method more samples were positive, as only in 30.0% no species were detected. In 41.4% of milk samples only one species was detected. In 24.6% two species were detected and three species were detected in 3.9%. In no case were four samples detected simultaneously (Fig. 4 ). The differences in the combinations described were significant (p < 0.001).
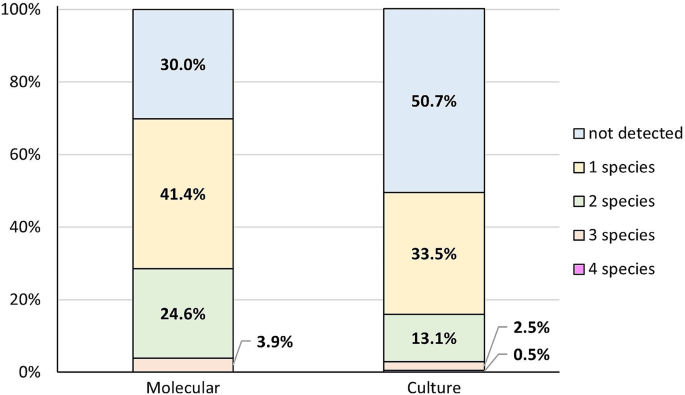
The percentage of simultaneous co-occurrence of species studied determined by the PCR method and in culture methods determined for all (N = 203) milk
Further in-depth analysis also demonstrated that the most frequent co-occurring species variant was S. uberis that simultaneously appeared with E. coli both according to the culture (12/203; 5.9%) and molecular (21/203; 10.3%) methods. The culture method also detected the common co-occurrence of S. uberis and S. aureus (8/203; 3.9%). The remaining combination of two, three or four co-occurred species were observed for 1 or 2 samples in the pool studied. Greater variability was noted for samples analysed by PCR in which 18/203 (8.8%) included both S. aureus and E. coli , 9/203 (4.4%) S. aureus and S. uberis , 7/203 (3.4%) were positive for the occurrence of three species S. aureus , S. uberis and E. coli. The simultaneous occurrence of S. aureus and S. agalactiae was shown in two samples and a variant consisting of S. aureus , S. uberis , and S. agalactiae was observed in one sample (Fig. 5 ).
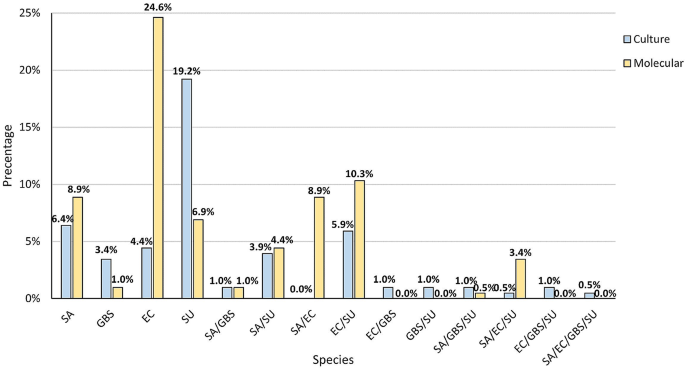
The combination of co-occurrence of the particular bacterial species of mastitis determined in the PCR method and in the culture method. Legend: SA – S. aureus , EC – E. coli , GBS – S. agalactiae , SU – S. uberis
Region-dependent distribution of species
This study also aimed to compare the composition of species between three regions of Poland: Region 1, north-eastern Poland (Podlasie); Region 2, south-western Poland (Upper Silesia); and Region 3, southern Poland (Małopolska) ( Fig. 6 ) . Significant differences were noted for E. coli incidence (p < 0.001), in both the culture and molecular methods, but data obtained by the PCR method indicated that this species was the least common in north-eastern Poland, while the culture method showed that in north-eastern Poland E. coli was the most common species. Significant differences for the molecular method were also observed for S. uberis (p < 0.001) and S. aureus (p < 0.001). Both species were most common in southern and south-western Poland (Fig. 7 ).
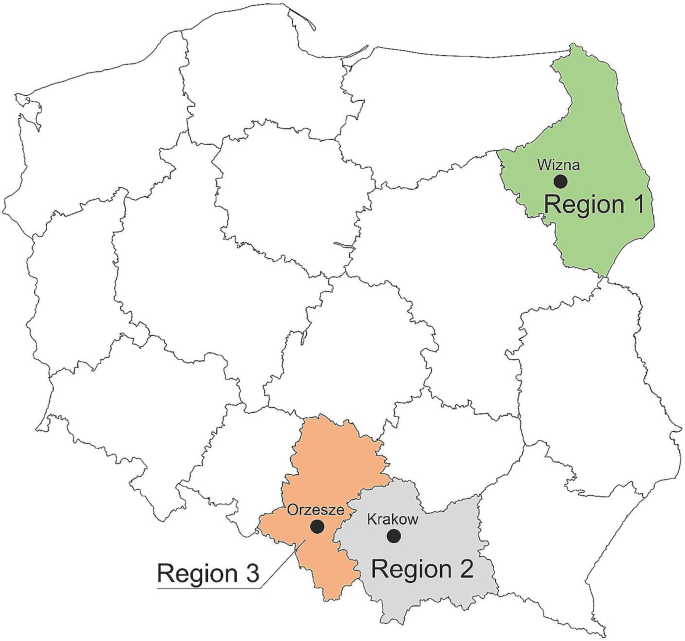
Map of Poland with marked regions and cities from which the tested milk samples came. Legend: Region 1 – the north-east, Region 2 – the south, Region 3 – the south-west
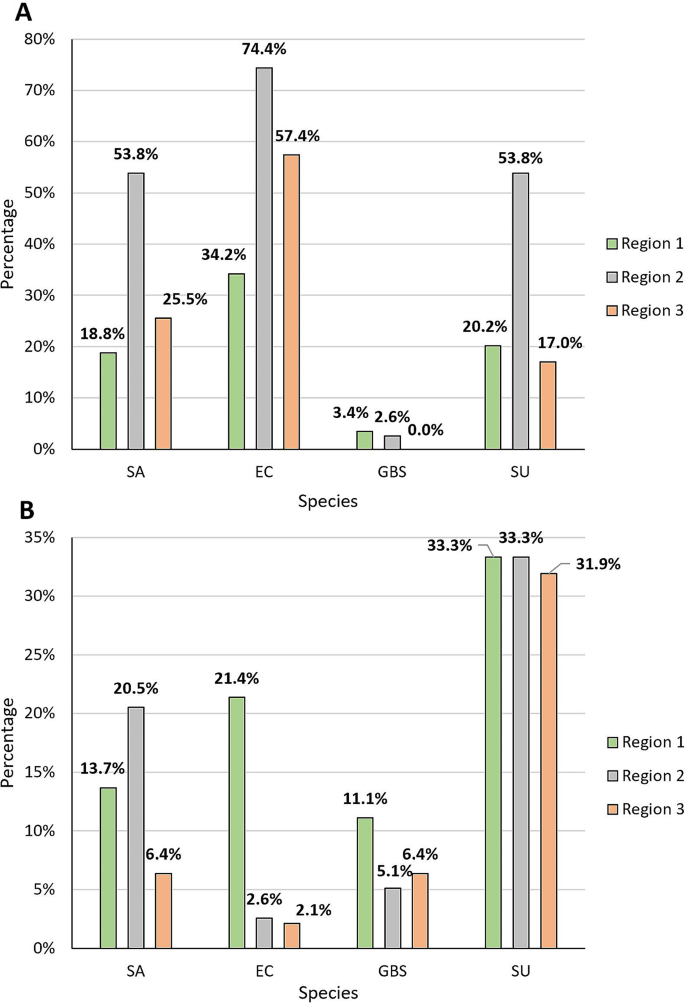
The distribution of S. aureus (SA), E. coli (EC), S. agalactiae (GBS), and S. uberis (SU) in the three regions of Poland: the north-east (Region 1), the south (Region 2) and the south-east (Region 3) determined by molecular ( A ) and culture ( B ) method
Clinical mastitis (CM) is one of the most widespread diseases affecting dairy cattle and more worryingly is tends to recur, which has a significant impact on the cost of the disease in dairy cows [ 19 , 20 , 21 ]. The issue of mastitis is not limited to bacterial or even microbiological causes, but indubitably bacteria dominate as a an etiological factor of this disease [ 22 ]. More than half the health asymptomatic cows demonstrated mastitis streptococci ( S. uberis , S. dysgalactiae , and S. agalactiae ) [ 23 ]. It is therefore unsurprising that even 70% of the anti-microbials used in dairy farms are used to prevent and treat mastitis [ 24 ]. This problem is not only associated with financial loss for farmers and harmful effects on animals, but also pose a potential risk to public health by dint of the transmission of zoonoses and increasing multidrug bacterial resistance [ 25 , 26 , 27 ]. The epidemiology of bovine mastitis varies from region to region, not only due to the prevalence of different bacteria but also different sensitivity of kept breeds of cows in particular regions [ 28 , 29 , 30 , 31 ]. In Poland previous studies have shown that streptococcus infections are more common than staphylococcus (38.5% vs. 17.9%) and the third bacterial cause is Gram-negative bacteria such as E. coli (16.4%) [ 8 ], but this has also changed over the years [ 32 ].
In the culture method the most common species detected in the pool of milk samples from the clinical cases of mastitis was S. uberis (38.0%), which is a significant problem both in Poland and elsewhere [ 33 ]. In our previous study, the percentage of S. uberis in the pool of clinical samples examined was higher, reaching 44% [ 34 ]. In turn the results obtained by Dyson et al., who carried out their investigation on Australian cattle and demonstrated that S. uberis was present in 39.2% of the population studied [ 35 ]. Al-Harbi et al. also showed that S. uberis was the most common species in their study, but the frequency of occurrence was almost fourfold lower than our results [ 36 ]. This knowledge provides us with the opportunity to react to and possible prevent cases. Our results showed that the most common cause of mastitis was S. uberis , which knowledge is even more important because of the environmental nature of this pathogen, however some S. uberis strains could show a contagious nature [ 37 ]. Improving the conditions in which cows are kept, including changing the flooring, the substrate, could play a significant role [ 38 ]. The prevalence of S. uberis mastitis shows that the dairy industry continues to face the challenge of combatting this pathogen despite the adoption of measures to control environmental pathogens [ 39 ]. To sum up, we should still put more emphasis on management practices and improve pre- and post-milking hygiene protocols to minimise S. uberis mastitis.
The most frequently detected species in the pool of samples examined was S. aureus , which generally causes sub-clinical mastitis with a high seeding rate of infected animals and chronic recurrent infections. The virulence of S. aureus results from its ability to produce biofilm, toxins and various enzymes that lead to cell damage in the host and allows the bacteria to initiate the host invasion [ 40 , 41 ]. Our studies confirmed the presence of S. aureus in 22% (culture method) and in 28% (PCR method) of CM samples. Polish studies carried out by Lassa et al. supported our results in which S. aureus accounted for 22.9% [ 8 ]. The percentages yielded in this study also corresponded with the results in a large-scale investigation carried out by Liu et al., who demonstrated that S. aureus , depending on the farm examined, reached up to 24.2% [ 42 ]. The prevalence of S. aureus is influenced by many factors, starting from sanitary and hygienic conditions, local strains/genotypes, cattle breeds, and bedding, which differs significantly between countries [ 43 , 44 ]. American studies showed that S. aureus was infrequent (2.8%) [ 45 ]. Unfortunately, in Poland, despite the constant improvement of sanitary and hygienic conditions, breeders’ awareness of mastitis prevention is insufficient. Constant screening and monitoring for S. aureus are vital because, even after many years of studying S. aureus , no effective therapy has been developed due to its rapid genetic variability [ 43 , 46 ]. It is therefore unsurprising that the drug-resistance of S. aureus prevails [ 47 ].
E. coli was third the most common CM species and is classified as an environmental pathogen. The frequency of E. coli infections increase during summer. This is explained by the seasonal rise in temperature and humidity. As a result of heat stress, the cows’ immunity also diminished, which may lead to an increase in the risk of infection [ 48 ]. In our study, the results of the culture method indicated that E. coli was present in 21% of pool of samples examined. In a previous study, on a smaller number of samples, E. coli was present in 18.2% of the milk examined [ 34 ]. Studies on Nepalese cattle showed the incidence of mastitis caused by E. coli was over 16% of samples examined [ 49 ]. Comparable results were obtained in France [ 50 ]. In Poland the incidence of mastitis caused by E. coli in 2020 was shown at only 2.7% [ 51 ]. The differences may result from the number of samples included in Krukowski et al.’s study, as they included over 38.000 milk samples, obtained from clinical and subclinical cases of mastitis. In our study percentage of E. coli was determined only for clinical cases. Infection caused by E. coli may be the result of a number of factors. Some strains of E. coli acquire specific virulence factors (VFs), which may enable them to infect the mammary glands and multiply in milk [ 52 , 53 ]. The host’s immunological system, however, demonstrates an ability to protect itself from E. coli infection thanks to components of innate immunity components, such as anti-microbial peptides, lysozyme, lactoferrin and other complements [ 54 , 55 ]. Some strains of E. coli associated with mastitis also evolved mechanisms that allow them to compete with other bacteria co-infecting the gland [ 48 , 56 ], which may explain that coinfection with E. coli is the most common in our study.
S. agalactiae , which is responsible for cases of contagious mastitis, was the fourth most common species identified in the CM milk sample studied. It was recognised as a highly contagious obligate bacterium of the bovine mammary gland, which tends to be incapable of surviving for long periods outside the mammary gland [ 57 ]. The latest research, however, confirms its ability to survive in extra-mammary sources [ 58 ]. In mammary glands S. agalactiae also has the ability to survive by dint of forming biofilms [ 59 ] and as a variety of other bacteria becomes increasingly resistant to appropriate treatment [ 60 ]. The prevalence of S. agalactiae in bovine mastitis due to the introduction of the mastitis control programme [ 61 , 62 ] has dwindled in recent decades. In the 1980s, however, it was the main cause of mastitis, being responsible for almost 50% of cases [ 36 , 57 , 63 ]. The decrease in the incidence of S. agalactiae detected in mastitis milk samples was also noted in Poland [ 64 ]. In our study S. agalactiae was present in 19% of CM milk and the results corresponded with other Polish studies carried out by Sztachańska at al. , who detected S. agalactiae in 15% of samples examined [ 65 ]. Malinowski et al. demonstrated that the presence of this bacterium varied between 2% and 25% on Polish farms [ 32 ].
In the control group the diversity in the combination bacterial species was greater than in the CM group. Whereas in the CM group 5 species were detected, in the milk from cows with no clinical signs of mastitis 8 species were detected. The species identified, especially these classified as environmental, e.g. Viridians group Streptococcus , may indicate that contamination of the samples with the skin the microbiota and may result from failure to follow the rules of sterile milk sampling. The high frequency of the occurrence of these species, as high as 1/3 of pool studied, in spite of our observing aseptic rules during the collection of biological material, indicates the need for even greater care when collecting material for examination. We are also aware that study material delivery on dry ice under deep-freeze conditions and storage at -80˚C could have an impact on the bacterial composition and the bacterial number.
The choice of suitable diagnostic methods significantly influences the reliability of the results and, consequently, epidemiological data. Many studies rely solely on culture techniques and biochemical tests [ 8 , 32 , 36 , 65 ]. Our research and other publications [ 66 , 67 , 68 , 69 ] confirm the necessity of complementing these methods with molecular techniques such as PCR or real-time PCR reactions. However, it should be considered that the higher sensibility of PCR can be related to the DNA amplification of a not significant number of bacterial colonies in milk (that cannot be considered as responsible for mastitis), or dead bacterial cells. However, in our study, for some milk samples, we observed that while positive results were obtained using culture methods, the result was negative with use of PCR amplification. Nevertheless, this observation shows that there are exceptions to the above assumption, for example due to the presence of PCR inhibitors. In this study, the use of molecular methods was intended to confirm the results obtained using culture methods and to additionally complement them. However, in order to achieve this goal, it was necessary to use 18 h of preincubation. In turn, the limitations of culture methods in relation to molecular methods are a topic discussed in many publications which emphasize, for example, the influence of inhibitory products of bacterial metabolism, the presence of dominant species, which influences the inhibition of the growth of subordinate species, unfavorable conditions during sample transport as factors that may reduce the viability of bacteria, which in turn may contribute to obtaining a false negative result in culture despite the occurrence of mastitis symptoms. In such cases, the use of molecular methods allows obtaining a positive result and complements culture methods. The crucial preliminary step before amplification is the extraction of bacterial DNA. In certain publications the standard isolation procedures [ 66 , 67 , 69 , 70 , 71 ] are described as involving direct extraction from milk samples by use of commercial kits. In our research, as well as employing the standard approach, we also evaluated the effect of an additional 18-hour pre-incubation of milk samples in TSB on the sensitivity of the detection of micro-organisms in the PCR reaction. The obtained results suggest the necessity of this step, as it led to an increased detection rate of Streptococcus uberis, Staphylococcus aureus, Escherichia coli and Streptococcus agalactiae by 18.1%, 11%, 9% and 1%, respectively in comparison to direct isolation. For S. aureus and S. uberis the results obtained after pre-incubation also align with those achieved through culture methods. A similar approach was described by Riffon et al. [ 72 ], who aimed of the research was to develop a sensitive and cheap PCR reaction enabling the detection of six main pathogens that cause mastitis ( Escherichia coli, Staphylococcus aureus, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus parauberis and Streptococcus uberis ), however the study was conducted, in opposition to ours, only on reference well characterized strains, with which sterile milk was infected, The developed PCR method, however, was not used to identify pathogens from milk samples collected from cows (with or without mastitis) or to compare the results with culture methods. Ding et al’s [ 73 ] also focused on the need to pre-incubate milk samples before bacterial DNA isolation and for this purpose five different liquid media were tested (alkaline peptone water (APW), Brain Heart Infusion broth (BHI), Luria-Bertani broth (LB), TSB and peptone water (PW)). The highest maximum population density of mastitis pathogens was achieved in LB, BHI and TSB, but these tests were conducted only for S. aureus , L. monocytogenes and Salmonella spp. In our research the use of enrichment TSB medium not only increased the concentration of micro-organisms but probably also enabled the dilution of PCR inhibitors contained in milk [ 74 ], e.g. the concentration of calcium or plasmin [ 75 ]. All of this may explain the observed quantitative differences in bacterial detection between enrichment and non-enrichment isolation. Pre-incubation in TSB, however, may introduce certain limitations, such as the potential for undesirable bacteria to grow and compete for nutrients with the targeted bacteria, or domination by the culture of fast-growing bacteria, which may mask the presence of slower-growing or more delicate target bacteria [ 73 ]. The identification of micro-organisms by PCR is difficult and has a direct impact on obtaining false negative results.
The above limitations of pre-incubation in TSB may explain our study’s lower percentage of positive results for S. agalactiae and S. uberis species after PCR than culture methods by 13% and 20.5% respectively (4% in the clinical group and 16.5% in the non-clinical group). The false-negative results concerned only streptococci, highlighting the necessity in future studies of employing a different pre-incubation medium that will be more selective towards streptococci. With regard to the remaining species, S. aureus and E. coli , molecular methods yielded a significantly higher number of positive results than culture methods, by 27.3% and 67.6% respectively (total values for the clinical and non-clinical groups). Higher PCR sensitivity in relation to culture for S. aureus or E. coli was also obtained in Nyman et al.’s [ 76 ], Graber et al.’s [ 77 ]and Koskinen et al.’s [ 78 ] research. In Koskinen’s work especially large discrepancies between these methods were observed. The use of PCR yielded an additional 53 and 68 positive samples for S. aureus and E. coli respectively, which were negative in culture. A significantly (p = 0.002) higher prevalence of this species in comparison with culture method may, however, be the result of sensitivity of the primers used and the PCR conditions that detect the remaining DNA in the sample rather than the DNA from the etiological factor that caused mastitis in individual cases. We hypothesise that the differences in results between methods may be caused by autolysis of E. coli , the presence of dead or damage bacterial cells in milk samples, which are unable to grow on the solid agar plate, but they still have bacterial DNA, detectable by molecular methods. These methods may also detect remaining DNA in samples, for example, after previous infections or as a contamination, hence not necessarily DNA from etiological factors causing mastitis in individual case.
As bovine mastitis constitutes to cause severe worldwide economic and epidemiologic issues, constant monitoring and management are crucial in order to curb this threat to the health and life of cattle. We are aware that a survey of more herds from more voivodeships would deliver even more informative results, but the percentage values obtained in our study correspond with the prevalence noted by other researchers. We therefore believe that further investigation would only confirm our observation. We believe that our results would increase and update epidemiological knowledge about mastitis among cows in three regions of Poland, including the Region 3 with the biggest concentration of cows in the country, and may encourage for further investigation including remaining Voivodeship as a relevant data is missing.
The investigation included 203 milk samples, obtained from 2 to 6 years old Holstein Friesian cattle, divided into two groups: (I) the study group, which included 100 milk samples from cattle with clinical signs of mastitis (CM) displaying the following symptoms: swelling, heat, hardness, redness or pain of the udder; watery appearance, flakes, clots or pus in milk; increased body temperature or lack of appetite, and (II) the control group, which included 103 milk samples from cattle with no clinical symptoms of mastitis. The number of samples was equal to the number of cows tested. From cows with active mastitis, a milk sample was collected only from the inflamed quarter, for healthy cows, milk from four quarters was pooled to one sample. According to the guidelines of National Mastitis Council (NMC) five or more colonies of an environmental species were considered as an infection while three or more species in one sample was treated as a contamination [ 79 ].
Milk samples were collected from udder quarters in the routine course of the submission of milk from different regions in Poland: Omniwet Veterinary Clinic, Orzesze (south-western Poland) (n = 47), Egida Veterinary Clinic, Wizna (north-eastern Poland) (n = 117), and University Centre for Veterinary Medicine Institute of Veterinary Sciences, Krakow (southern Poland) (n = 39). Cows included in the investigation came from different cowsheds, both stationary and free-standing cowsheds. The health status of the animals had been determined on the basis of physical parameters, such as the absence of edema or redness of the udder, as well as biochemical parameters by determining the number of somatic cells by California Mastitis Test (CMT) (animals with somatic cells > 100.000/ml and with clinical signs were classified as sick, whereas the lack of symptoms was a basis for classifying the animals as healthy). The herds studied were assessed by the Polish Federation of Cattle Breeders and Milk Producers, which requires monthly reports of milk parameters, such as the number of somatic cells per cow milked. According to the 1st local Ethical Committee at the Jagiellonian University Medical College in Cracow, Poland consent for the research project was not required, as the study has not direct involvement of animals. Milk samples that were delivered to the Department of Microbiology on dry ice under deep-freeze conditions were stored at -80˚C for further analyses.
Bacterial culture and phenotypic identification
100 µl of each milk sample was inoculated separately on each type of medium: on Columbia sheep blood agar (BioMaxima) [ 80 ] and CHROMagar™ Mastitis (BioMaxima) [ 81 ], and then cultured at 37˚C under aerobic conditions for 18 h. Individual bacterial colonies that grew on Columbia Agar and CHROMagar™ Mastitis and macroscopically corresponded to the pathogenic bacteria that constitute the most common etiological agent of mastitis were then inoculated onto selective media such as MacConkey agar (Graso) selective and differential medium for the identification of Gram-negative rods, Chapman’s (Graso) selective and differential medium for the identification of S. aureus [ 82 ], Granada chromogenic medium (Beckton Dickinson) [ 83 ] for the identification of S. agalactiae and esculin medium (Bio Maxima S. A.) [ 78 ] for the culture and differentiation of S. uberis from S. agalactiae . The culture was carried out at 37˚C under aerobic or microaerophilic conditions for 18 h. Isolated bacterial colonies were also suspended in 0.9% NaCl and spotted on glass slides until dry. Microscopic smears were then stained with the Gram method using standard dyes (STAMAR) and observed under a light microscope (OLYMPUS CX21) at 100x magnification using oil immersion (MERCK). In order to confirm cultivated bacterial species, some biochemical tests, such as the catalase test using 9% hydrogen peroxide (ALCHEM), the coagulase test using rabbit plasma (BIOMED) and the latex agglutination test using Streptococcal Grouping Kit (OXOID) [ 84 ] to confirm the species of the tested microorganisms were performed.
On the basis of the results obtained we also attempted to compare Columbia blood agar and Chromagar mastitis in precise identification of the bacterial species most common in mastitis.
Molecular identification
Species were identified by use of polymerase chain reaction (PCR) for four bacterial species: S. uberis , S. agalactiae , S. aureus , and E. coli , since they are the most common etiological factors in bovine mastitis.
The first step involved the original milk samples being thawed, intensively and pulse vortexed, before being transferred in volumes of 500 µl to sterile, screw-cap tubes with glass beads (Sigma-Aldrich). 2 isolation approaches were then performed, the first involving the isolation of bacterial DNA directly from the milk sample, and the second, involving the pre-incubation of the milk samples with tryptone-soy broth (TSB, Becton Dickinson) in 1:1 proportion for 18 h at 37˚C in aerobic conditions and before being subjected to bacterial isolation. As well as subjecting each sample to the first or second approach, 20 µl of lysozyme (50 mg/µl, Sigma-Aldrich), 15 µl of lysostaphin (1.0 mg/ml, A&A Biotechnology), and 5 µl of mutanolysin (10U/µl, Sigma-Aldrich) was added and the tubes were homogenised in a FastPrep device (Thermo Savant) for 1 min. After incubation for 30 min at 37˚C and vortexing, the samples were transferred to new tubes included in the GeneProof automated isolation kit (Imogena), 20 µl of proteinase was added and they were subjected to automatic isolation on CroBEE apparatus. The DNA isolates obtained were stored at -20 °C for further analysis.
The next step saw the amplification of isolated DNA by the use of four pairs of primers [ 58 , 71 , 72 ] (Table 1 ) dedicated to the detection of S. uberis , S. agalactiae , S. aureus , and E. coli in the material studied.
For each bacterial species, the PCR reaction conditions were optimized including the temperature range, oligonucleotide concentrations, DNA template volume [µl] and the number of amplification cycles. As a positive control, both in standardisation and amplification the DNA of reference strains (ATCC 25,923 for S. aureus , ATCC 25,922 for E. coli , ATCC 21,403 for S. agalactiae ) was used. Each time a reaction mixture consisting of sterile water free of Dnase and Rnase as a negative control was included. On the basis of the experiments conducted, the composition of the reaction mixtures for the following pairs of species S. agalactiae and S. aureus (pair 1), and E. coli and S. uberis (pair 2) were determined separately.
For S. agalactiae and S. aureus the following reagents were used: 5.0 µl of PCR Master Mix (A&A Biotechnology), 0.3 µl of GBS_F and GBS_R primers (10 µM, Genomed S. A.), 0.4 µl of SA_F and SA_R primers (10 µM, Genomed S. A.), 1.5 µl of studied DNA and 2.1 µl of DNAase free water (A&A Biotechnology). For E. coli and S. uberis reagents included: 5.0 µl of PCR Master Mix (A&A Biotechnology), 0.3 µl of EC_F and EC_R primers (10 µM, Genomed S. A.), 0.3 µl of SU_F and SU_R primers (10 µM, Genomed S. A.), 1.5 µl of the DNA studied and 2.3 µl of DNAase free water (A&A Biotechnology). The amplification procedure was carried out using the T100 Thermal Cycler (BioRad) according to the programme: preliminary denaturation at 92° C for 3 min prior to 40 repeated cycles: 1 min denaturation at 92° C, 1 min introduction of primers at 56° C and 1 min elongation at 72° C finished with 3-min final extension in 72° C. The products of amplifications were then separated on 2% agarose gel (Prona ABO) in 1× TBE buffer (Sigma-Aldrich) with the addition of ethidium bromide (Sigma-Aldrich) for 60 min at voltage 100 V. The results obtained from electrophoretic separation were analyzed in Gel Doc System FastGene (FAS-DIGI PRO).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 29. The relationships between categorical variables were assessed using the Pearson χ2 test and Fisher’s exact test. The results were presented as numbers and percentages. Statistical significance was defined as p < 0.05 for all tests.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Abbreviations
Clinical Mastitis
Group B Streptococcus
Non–aureus staphylococci and mammaliicocci
Somatic Cell Count
Nucleic Acid Amplification Techniques
Lateral Flow Assay
Viridans Group Streptococci
Polymerase Chain Reaction
Tryptone–Soy Broth
Tris–Borate–EDTA buffer
Hogeveen H, Huijps K, Lam TJ. G.M. Economic aspects of Mastitis: New Developments. N Z Vet J 2011, 59 .
van Soest FJS, Santman-Berends IMGA, Lam TJGM, Hogeveen H. Failure and preventive costs of Mastitis on Dutch dairy farms. J Dairy Sci. 2016;99. https://doi.org/10.3168/jds.2015-10561 .
Kalińska A, Wójcik A, Slósarz J, Kruzińska B, Michalczuk M, Jaworski S, Wierzbicki M, Gołębiewski M. Occurrence and Aetiology of Staphylococcal Mastitis – A Review. Anim Sci Pap Rep 2018, 36 .
Wilson DJ, Gonzalez RN, Das HH. Bovine Mastitis Pathogens in New York and Pennsylvania: Prevalence and effects on somatic cell count and milk production. J Dairy Sci. 1997;80:2592–8. https://doi.org/10.3168/JDS.S0022-0302(97)76215-5 .
Article CAS PubMed Google Scholar
Kulkarni AG, Kaliwal BB. Bovine mastitis: a review. Int J Recent Sci Res. 2013;4:543–8.
Google Scholar
Yalçin C. Cost of Mastitis in Scottish dairy herds with low and high subclinical mastitis problems. Turk J Vet Anim Sci. 2000;24:465–72.
Dufour S, Labrie J, Jacques M, Rasko D, Roy J-P. The Mastitis Pathogens Culture Collection CULTURE COLLECTIONS/ MUTANT LIBRARIES Crossm. 2019, 8 , 133–152, https://doi.org/10.1128/MRA.00133-19 .
Lassa H, Kubiak J, Małkińska-Horodyska M. Antibiotic susceptibility of the Bacteria most often isolated from clinical mastitis in Cows. Życie Weterynaryjne. 2013;88:651.
Ali T, Kamran; Raziq A, Wazir I, Ullah R, Shah P, Ali MI, Han B, Liu G. Prevalence of Mastitis pathogens and Antimicrobial susceptibility of isolates from cattle and buffaloes in Northwest of Pakistan. Front Vet Sci. 2021;8. https://doi.org/10.3389/fvets.2021.746755 .
Bach KD, Sipka A, McArt JAA, Case Study. Evaluating quarter and Composite Milk Sampling for Detection of Subclinical Intramammary Infections in dairy cattle. Prev Vet Med. 2019;163. https://doi.org/10.1016/j.prevetmed.2018.12.013 .
Huang C-H, Kusaba N. Association between Differential somatic cell count and California mastitis Test results in holstein cattle. JDS Commun. 2022;3. https://doi.org/10.3168/jdsc.2022-0249 .
Saila S, Bork O, Tucker IG, Cranefield S, Bryan MA. Evaluation of an On-Farm Culture System for the Detection of Subclinical Mastitis Pathogens in dairy cattle. JDS Commun. 2023;4. https://doi.org/10.3168/jdsc.2022-0312 .
Martins SAM, Martins VC, Cardoso FA, Germano J, Rodrigues M, Duarte C, Bexiga R, Cardoso S, Freitas PP. Biosensors for On-Farm Diagnosis of Mastitis. Front Bioeng Biotechnol 2019, 7 .
Ruegg PL. Making Antibiotic Treatment decisions for clinical Mastitis. Veterinary Clin North Am - Food Anim Pract 2018, 34 .
Wang Y, Jin Y, Bai Y, Song Z, Chu W, Zhao M, Hao Y, Lu Z. Rapid Method for Direct Identification of positive blood cultures by MALDITOF MS. Exp Ther Med. 2020;20. https://doi.org/10.3892/etm.2020.9365 .
Nonnemann B, Lyhs U, Svennesen L, Kristensen KA, Klaas IC, Pedersen K. Bovine mastitis Bacteria resolved by MALDI-TOF Mass Spectrometry. J Dairy Sci. 2019;102. https://doi.org/10.3168/jds.2018-15424 .
Kano R, Sato A, Sobukawa H, Sato Y, Ito T, Suzuki K, Hasegawa A, Kamata H. Short communication: ELISA system for screening of bovine mastitis caused by Prototheca Zopfii. J Dairy Sci. 2016;99:6590–3. https://doi.org/10.3168/jds.2016-11168 .
Koczula KM, Gallotta A. Lateral Flow assays. Essays Biochem. 2016;60:111–20. https://doi.org/10.1042/EBC20150012 .
Article PubMed PubMed Central Google Scholar
Ruegg PL. Investigation of mastitis problems on farms. Veterinary Clin North Am - Food Anim Pract 2003, 19 .
Halasa T, Huijps K, Østerås O, Hogeveen H. Economic effects of bovine mastitis and Mastitis Management: a review. Veterinary Q. 2007;29. https://doi.org/10.1080/01652176.2007.9695224 .
Jamali H, Barkema HW, Jacques M, Lavallée-Bourget EM, Malouin F, Saini V, Stryhn H, Dufour S. Invited review: incidence, risk factors, and effects of Clinical Mastitis recurrence in dairy cows. J Dairy Sci. 2018;101. https://doi.org/10.3168/jds.2017-13730 .
Contreras GA, Rodríguez JM, Mastitis. Comparative etiology and epidemiology. J Mammary Gland Biol Neoplasia. 2011;16. https://doi.org/10.1007/s10911-011-9234-0 .
Cervinkova D, Vlkova H, Borodacova I, Makovcova J, Babak V, Lorencova A, Vrtkova I, Marosevic D, Jaglic Z. Prevalence of Mastitis Pathogens in milk from clinically healthy cows. Vet Med (Praha). 2013;58. https://doi.org/10.17221/7138-VETMED .
Stevens M, Piepers S, De Vliegher S. Mastitis Prevention and Control Practices and Mastitis Treatment Strategies Associated with the consumption of (critically important) antimicrobials on dairy herds in Flanders, Belgium. J Dairy Sci. 2016;99. https://doi.org/10.3168/jds.2015-10496 .
Barlow J. Mastitis Therapy and Antimicrobial susceptibility: a multispecies review with a Focus on Antibiotic Treatment of Mastitis in dairy cattle. J Mammary Gland Biol Neoplasia. 2011;16. https://doi.org/10.1007/s10911-011-9235-z .
Zouharova M, Rysanek D, Multiplex PCR. Identification of Staphylococcus Aureus Enterotoxigenic strains from Bulk Tank Milk. Zoonoses Public Health. 2008;55. https://doi.org/10.1111/j.1863-2378.2008.01134.x .
Blum S, Heller ED, Krifucks O, Sela S, Hammer-Muntz O, Leitner G. Identification of a bovine Mastitis Escherichia Coli Subset. Vet Microbiol. 2008;132. https://doi.org/10.1016/j.vetmic.2008.05.012 .
Chen S, Zhang H, Zhai J, Wang H, Chen X, Qi Y. Prevalence of clinical mastitis and its Associated Risk factors among dairy cattle in Mainland China during 1982–2022: a systematic review and Meta-analysis. Front Vet Sci. 2023;10. https://doi.org/10.3389/fvets.2023.1185995 .
Fukushima Y, Kino E, Furutani A, Minamino T, Mikurino Y, Horii Y, Honkawa K, Sasaki Y. Epidemiological study to investigate the incidence and prevalence of clinical mastitis, Peracute Mastitis, metabolic disorders and Peripartum Disorders, on a dairy farm in a Temperate Zone in Japan. BMC Vet Res. 2020;16. https://doi.org/10.1186/s12917-020-02613-y .
Kurjogi MM, Kaliwal BB. Epidemiology of bovine mastitis in cows of Dharwad District. Int Sch Res Notices. 2014;2014. https://doi.org/10.1155/2014/968076 .
Audarya SD, Chhabra D, Sharda R, Gangil R, Sikrodia R, Jogi J, Shrivastava N. Epidemiology of Bovine Mastitis and Its Diagnosis, Prevention, and Control. In Mastitis in Dairy Cattle, Sheep and Goats ; 2022.
Malinowski E, Gajewski Z. Characteristics of Cows Mastitis Caused by Human Foodborne Pathogens. 2009, 84 .
Davies PL, Leigh JA, Bradley AJ, Archer SC, Emes RD, Green MJ. Molecular Epidemiology of Streptococcus Uberis clinical mastitis in dairy herds: strain heterogeneity and transmission. J Clin Microbiol. 2016;54. https://doi.org/10.1128/JCM.01583-15 .
Dobrut A, Wójcik-Grzybek D, Młodzińska A, Pietras-Ożga D, Michalak K, Tabacki A, Mroczkowska U, Brzychczy-Włoch M. Detection of Immunoreactive Proteins of Escherichia Coli, Streptococcus Uberis, and Streptococcus Agalactiae isolated from cows with diagnosed Mastitis. Front Cell Infect Microbiol. 2023;13. https://doi.org/10.3389/fcimb.2023.987842 .
Dyson R, Charman N, Hodge A, Rowe SM, Taylor LF. A survey of Mastitis pathogens Including Antimicrobial susceptibility in Southeastern Australian dairy herds. J Dairy Sci. 2022;105. https://doi.org/10.3168/jds.2021-20955 .
Al-harbi H, Ranjbar S, Moore RJ, Alawneh JI. Bacteria isolated from milk of dairy cows with and without clinical mastitis in different regions of Australia and their AMR profiles. Front Vet Sci. 2021;8. https://doi.org/10.3389/fvets.2021.743725 .
Monistero V, Barberio A, Cremonesi P, Castiglioni B, Morandi S, Lassen DCK, Astrup LB, Locatelli C, Piccinini R, Filippa Addis M et al. Genotyping and Antimicrobial Susceptibility Profiling of Streptococcus Uberis Isolated from a Clinical Bovine Mastitis Outbreak in a Dairy Farm. Antibiotics 2021, 10 , https://doi.org/10.3390/antibiotics10060644 .
Sherwin VE, Egan SA, Green MJ, Leigh JA. Survival of Streptococcus Uberis on Bedding substrates. Vet J. 2021;276. https://doi.org/10.1016/j.tvjl.2021.105731 .
Booth JM, Shearn MFH, Teverson RM, Langridge S, Booth JM. Effect of Pre-milking Teat dipping on clinical mastitis on dairy farms in England. J Dairy Res. 1993;60. https://doi.org/10.1017/S0022029900027321 .
Artursson K, Söderlund R, Liu L, Monecke S, Schelin J. Genotyping of Staphylococcus Aureus in bovine mastitis and correlation to phenotypic characteristics. Vet Microbiol. 2016;193. https://doi.org/10.1016/j.vetmic.2016.08.012 .
Brahma U, Suresh A, Murthy S, Bhandari V, Sharma P. Antibiotic Resistance and Molecular Profiling of the Clinical Isolates of Staphylococcus Aureus Causing Bovine Mastitis from India. Microorganisms 2022, 10 , https://doi.org/10.3390/microorganisms10040833 .
Liu K, Tao L, Li J, Fang L, Cui L, Li J, Meng X, Zhu G, Bi C, Wang H. Characterization of Staphylococcus Aureus isolates from cases of clinical bovine mastitis on large-scale Chinese dairy farms. Front Vet Sci. 2020;7. https://doi.org/10.3389/fvets.2020.580129 .
Campos B, Pickering AC, Rocha LS, Aguilar AP, Fabres-Klein MH, de Oliveira Mendes TA, de Fitzgerald JR. Oliveira Barros Ribon, A. Diversity and Pathogenesis of Staphylococcus Aureus from Bovine Mastitis: Current Understanding and Future Perspectives. BMC Vet Res 2022, 18 .
Patel K, Godden SM, Royster E, Crooker BA, Timmerman J, Fox L. Relationships among Bedding materials, bedding Bacteria counts, Udder Hygiene, milk quality, and Udder Health in US dairy herds. J Dairy Sci. 2019;102. https://doi.org/10.3168/jds.2019-16692 .
Oliveira L, Hulland C, Ruegg PL. Characterization of clinical mastitis Occurring in cows on 50 large dairy herds in Wisconsin. J Dairy Sci. 2013;96. https://doi.org/10.3168/jds.2012-6078 .
Messina JA, Thaden JT, Sharma-Kuinkel BK, Fowler VG. Impact of Bacterial and Human Genetic Variation on Staphylococcus Aureus Infections. PLoS Pathog 2016, 12 .
Wang D, Wang Z, Yan Z, Wu J, Ali T, Li J, Lv Y, Han B. Bovine Mastitis Staphylococcus Aureus: Antibiotic Susceptibility Profile, Resistance genes and molecular typing of Methicillin-Resistant and Methicillin-sensitive strains in China. Infect Genet Evol. 2015;31. https://doi.org/10.1016/j.meegid.2014.12.039 .
Rakib MRH, Zhou M, Xu S, Liu Y, Asfandyar Khan M, Han B, Gao J. Effect of heat stress on Udder Health of dairy cows. J Dairy Res. 2020;87. https://doi.org/10.1017/S0022029920000886 .
Bhandari S, Subedi D, Tiwari BB, Shrestha P, Shah S, Al-Mustapha AI. Prevalence and risk factors for Multidrug-Resistant Escherichia Coli isolated from subclinical mastitis in the Western Chitwan Region of Nepal. J Dairy Sci. 2021;104. https://doi.org/10.3168/jds.2020-19480 .
Botrel MA, Haenni M, Morignat E, Sulpice P, Madec JY, Calavas D. Distribution and Antimicrobial Resistance of Clinical and subclinical mastitis pathogens in dairy cows in Rhône-Alpes, France. Foodborne Pathog Dis. 2010;7. https://doi.org/10.1089/fpd.2009.0425 .
Krukowski H, Lassa H, Zastempowska E, Smulski S, Bis-Wencel H. Etiological agents of bovine mastitis in Poland. Med Weter. 2020;76. https://doi.org/10.21521/mw.6339 .
Zhou M, Yang Y, Wu M, Ma F, Xu Y, Deng B, Zhang J, Zhu G, Lu Y. Role of Long Polar Fimbriae Type 1 and 2 in Pathogenesis of mammary pathogenic Escherichia Coli. J Dairy Sci. 2021;104. https://doi.org/10.3168/jds.2021-20122 .
Salamon H, Nissim-Eliraz E, Ardronai O, Nissan I, Shpigel NY. The role of O-Polysaccharide chain and complement resistance of Escherichia Coli in Mammary Virulence. Vet Res. 2020;51. https://doi.org/10.1186/s13567-020-00804-x .
Alamdari EK, Ehsani MR. Antimicrobial peptides derived from milk: a review. J Food Biosci Technol 2017, 7 .
Kuhi Morteza AMTM. Antibacterial Action 0f Dextran conjugated lysozyme against Bacteria involved in bovine mastitis. Adv Dairy Res. 2021;9:1–6.
Goulart DB, Mellata M. Escherichia Coli Mastitis in dairy cattle: etiology, diagnosis, and Treatment challenges. Front Microbiol. 2022;13. https://doi.org/10.3389/fmicb.2022.928346 .
Keefe GP. Streptococcus Agalactiae mastitis: a review. Can Vet J 1997, 38 .
Cobo-Ángel C, Jaramillo-Jaramillo AS, Lasso-Rojas LM, Aguilar-Marin SB, Sanchez J, Rodriguez-Lecompte JC, Ceballos-Márquez A, Zadoks RN. Streptococcus Agalactiae is not always an Obligate Intramammary Pathogen: molecular epidemiology of GBS from milk, feces and environment in Colombian dairy herds. PLoS ONE. 2018;13. https://doi.org/10.1371/journal.pone.0208990 .
Cheng WN, Han SG, Bovine Mastitis. Risk factors, therapeutic strategies, and alternative treatments — a review. Asian-Australas J Anim Sci 2020, 33 .
Torres G, Macias D, Reyes-Vélez J, Rios-Agudelo P, Caraballo-Guzmán A. Streptococcus Agalactiae virulence factors isolated from bovine mastitis and Antibiotic Treatment Response. J Appl Microbiol. 2023;134. https://doi.org/10.1093/jambio/lxad116 .
Myllys V, Asplund K, Brofeldt E, Hirvelä-Koski V, Honkanen-Buzalski T, Junttila J, Kulkas L, Myllykangas O, Niskanen M, Saloniemi H, et al. Bovine mastitis in Finland in 1988 and 1995 - changes in prevalence and Antimicrobial Resistance. Acta Vet Scand. 1998;39. https://doi.org/10.1186/BF03547813 .
Pitkälä A, Haveri M, Pyörälä S, Myllys V, Honkanen-Buzalski T. Bovine mastitis in Finland 2001 - prevalence, distribution of Bacteria, and Antimicrobial Resistance. J Dairy Sci. 2004;87. https://doi.org/10.3168/jds.S0022-0302(04)73366-4 .
Keefe G. Update on control of Staphylococcus Aureus and Streptococcus Agalactiae for Management of Mastitis. Veterinary Clin North Am - Food Anim Pract 2012, 28 .
Malinowski E, Lassa H, Kłossowska A, Smulski S, Markiewicz H, Kaczmarowski M. Etiological Agents of Dairy Cows’ Mastitis in Western Part of Poland. Pol J Vet Sci 2006, 9 .
Sztachańska M, Barański W, Janowski T, Pogorzelska J, Zduńczyk S. Prevalence and Etiological Agents of Subclinical Mastitis at the end of Lactation in nine dairy herds in North-East Poland., https://doi.org/10.1515/pjvs-2016-0015 .
Shome BR, Das Mitra S, Bhuvana M, Krithiga N, Velu D, Shome R, Isloor S, Barbuddhe SB, Rahman H. Multiplex PCR assay for species identification of bovine mastitis pathogens. J Appl Microbiol. 2011;111. https://doi.org/10.1111/j.1365-2672.2011.05169.x .
SK K. Identification of bovine Mastitis Associated pathogens by Multiplex PCR. J Dairy Veterinary Sci. 2017;3. https://doi.org/10.19080/jdvs.2017.03.555622 .
Cressier B, Bissonnette N. Assessment of an extraction protocol to detect the Major Mastitis-Causing Pathogens in Bovine Milk. J Dairy Sci. 2011;94. https://doi.org/10.3168/jds.2010-3669 .
Keane OM, Budd KE, Flynn J, McCoy F. Increased detection of Mastitis pathogens by Real-Time PCR compared to Bacterial Culture. Vet Rec. 2013;173. https://doi.org/10.1136/vr.101598 .
Taponen S, Salmikivi L, Simojoki H, Koskinen MT, Pyörälä S. Real-time polymerase chain reaction-based identification of Bacteria in milk samples from bovine clinical mastitis with no growth in conventional culturing. J Dairy Sci. 2009;92. https://doi.org/10.3168/jds.2008-1729 .
Hiitiö H, Riva R, Autio T, Pohjanvirta T, Holopainen J, Pyörälä S, Pelkonen S. Performance of a real-time PCR assay in routine bovine Mastitis Diagnostics compared with in-depth Conventional Culture. J Dairy Res. 2015;82. https://doi.org/10.1017/S0022029915000084 .
Riffon R, Sayasith K, Khalil H, Dubreuil P, Drolet M, Lagacé J. Development of a Rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. J Clin Microbiol. 2001;39. https://doi.org/10.1128/JCM.39.7.2584-2589.2001 .
Ding T, Suo Y, Zhang Z, Liu D, Ye X, Chen S, Zhao YA, Multiplex RT-PCR, Assay S, Aureus L. Monocytogenes, and Salmonella Spp. Detection in Raw Milk with Pre-Enrichment. Front Microbiol 2017, 8 , https://doi.org/10.3389/fmicb.2017.00989 .
Gillespie BE, Oliver SP. Simultaneous Detection of Mastitis Pathogens, Staphylococcus Aureus, Streptococcus Uberis, and Streptococcus Agalactiae by Multiplex Real-Time polymerase chain reaction. J Dairy Sci. 2005;88. https://doi.org/10.3168/jds.S0022-0302(05)73036-8 .
Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors - occurrence, properties and removal. J Appl Microbiol 2012, 113 .
Nyman AK, Persson Waller K, Emanuelson U, Frössling J. Sensitivity and specificity of PCR analysis and Bacteriological Culture of Milk Samples for Identification of Intramammary Infections in dairy cows using latent class analysis. Prev Vet Med. 2016;135. https://doi.org/10.1016/j.prevetmed.2016.11.009 .
Graber HU, Casey MG, Naskova J, Stelner A, Schaeren W. Development of a highly sensitive and specific assay to detect Staphylococcus Aureus in bovine mastitic milk. J Dairy Sci. 2007;90. https://doi.org/10.3168/jds.2006-902 .
Koskinen MT, Wellenberg GJ, Sampimon OC, Holopainen J, Rothkamp A, Salmikivi L, van Haeringen WA, Lam TJGM, Pyörälä S. Field comparison of real-time polymerase chain reaction and bacterial culture for identification of bovine mastitis Bacteria. J Dairy Sci. 2010;93:5707–15. https://doi.org/10.3168/JDS.2010-3167 .
Dohoo IR, Smith J, Andersen S, Kelton DF, Godden S. Diagnosing Intramammary Infections: evaluation of definitions based on a single milk sample. J Dairy Sci. 2011;94. https://doi.org/10.3168/jds.2010-3559 .
Langhorne C, Gupta S, Das; Horsman S, Wood C, Wood BJ, Barker L, Deutscher A, Price R, McGowan MR, Humphris M, et al. Bacterial culture and Antimicrobial susceptibility results from bovine milk samples submitted to four Veterinary Diagnostic laboratories in Australia from 2015 to 2019. Front Vet Sci. 2023;10. https://doi.org/10.3389/fvets.2023.1232048 .
Griffioen K, Velthuis AGJ, Koop G, Lam TJGM. Effects of a Mastitis Treatment Strategy with or without On-Farm testing. J Dairy Sci. 2021;104. https://doi.org/10.3168/jds.2019-17871 .
Pascu C, Herman V, Iancu I, Costinar L. Etiology of Mastitis and Antimicrobial Resistance in dairy cattle farms in the western part of Romania. Antibiotics. 2022;11. https://doi.org/10.3390/antibiotics11010057 .
Rosa-Fraile M, Rodriguez-Granger J, Cueto-Lopez M, Sampedro A, Gaye EB, Haro JM, Andreu A. Use of Granada Medium to Detect Group B Streptococcal Colonization in pregnant women. J Clin Microbiol. 1999;37. https://doi.org/10.1128/jcm.37.8.2674-2677.1999 .
Pumipuntu N, Kulpeanprasit S, Santajit S, Tunyong W, Kong-ngoen T, Hinthong W, Indrawattana N. Screening method for Staphylococcus Aureus Identification in subclinical bovine mastitis from dairy farms. Vet World. 2017;10. https://doi.org/10.14202/vetworld.2017.721-726 .
Pereira EM, Schuenck RP, Malvar KL, Iorio NLP, Matos PDM, Olendzki AN, Oelemann WMR, dos Santos KRN. Staphylococcus Aureus, Staphylococcus Epidermidis and Staphylococcus Haemolyticus: Methicillin-Resistant isolates are detected directly in blood cultures by Multiplex PCR. Microbiol Res. 2010;165. https://doi.org/10.1016/j.micres.2009.03.003 .
Munari FM, De-Paris F, Salton GD, Lora PS, Giovanella P, Machado ABMP, Laybauer LS, Oliveira KRP, Ferri C, Silveira JLS, et al. A Combined Enrichment/Polymerase chain reaction based Method for the routine screening of Streptococcus Agalactiae in pregnant women. Brazilian J Microbiol. 2012;43. https://doi.org/10.1590/S1517-83822012000100029 .
Download references
Acknowledgements
The Authors would like to thank DVM Aleksander Tabacki from Omniwet Veterinary Clinic, Orzesze, Poland for the involvement in collecting milk samples and expert advice in the field of veterinary medicine. The results described in this paper were partially presented during the 16th MEEGID Molecular Epidemiology and Evolutionary Genetics of Infectious Diseases Conference in 2023 in Dresden, Germany under the title: “Molecular identification of bacterial species present in milk samples isolated from Polish cows with diagnosed mastitis” and during the Epidemics9 9th International Conference on Infectious Disease Dynamics 2023 in Bologna, Italy under the title: “Detection of the most common bacterial species present in milk samples isolated from Polish cows with diagnosed mastitis”.
This work was supported by the National Center for Research and Development [grant no: LIDER/9/0029/L-10/18/NCBR/2019].
Author information
Authors and affiliations.
Department of Molecular Medical Microbiology, Chair of Microbiology, Jagiellonian University Medical College, Krakow, Poland
Anna Dobrut, Agnieszka Sroka-Oleksiak, Kamil Drożdż, Joanna Sobońska & Monika Brzychczy-Włoch
Institute of Veterinary Sciences, University Center of Veterinary Medicine JU-AU, University of Agriculture in Krakow, Krakow, Poland
Izabela Siemińska
PetVet, Jedwabne, Poland
Urszula Mroczkowska
You can also search for this author in PubMed Google Scholar
Contributions
A.D. designed the concept of the study, obtained funds for research, coordinated the project, analysed results, drafted the manuscript; I.S. collected milk samples, drafted the manuscript, A.S-O carried out molecular analyses, drafted the manuscript; K.D. – carried out statistical analyses, prepared charts and graphics; J.S. – carried out phenotypic identifications, analysed results, U.M. – collected milk samples, analysed results; M.B-W – was a supervisor and helped to draft the manuscript. All authors reviewed the manuscript.
Corresponding author
Correspondence to Anna Dobrut .
Ethics declarations
Ethics approval and consent to participate.
According to the opinion of the 1st local Ethical Committee at the Jagiellonian University Medical College in Cracow, Poland the consent for the research obtained for the project was not required. All experiments were approved by the 1st local Ethical Committee at the Jagiellonian University Medical College in Cracow, Poland. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dobrut, A., Siemińska, I., Sroka-Oleksiak, A. et al. Molecular and phenotypic identification of bacterial species isolated from cows with mastitis from three regions of Poland. BMC Vet Res 20 , 193 (2024). https://doi.org/10.1186/s12917-023-03869-w
Download citation
Received : 28 September 2023
Accepted : 24 December 2023
Published : 11 May 2024
DOI : https://doi.org/10.1186/s12917-023-03869-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Streptococcus uberis
- Streptococcus agalactiae
- Staphylococcus aureus
- Escherichia coli
- Bacterial identification
- Epidemiological data
- Polish cattle
BMC Veterinary Research
ISSN: 1746-6148
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
1 INTRODUCTION. Milk provides a comprehensive source of nutrition for mammalian neonates. Although milk composition varies substantially between species, the milk of other species can be consumed by mature humans and children of sufficient renal development (Ziegler, 2007), providing a greater distribution of essential macro- and micro-nutrients than most other food sources (Drewnowski, 2005).
Comparative Compositions of Cattle and Non-cattle Milks. The comparative compositions of milk from different species have been extensively reviewed in previous studies (5, 17-19).The milk from different species vary in composition (Table 1).Protein, fat, lactose, and minerals are the four major components in all milks, irrespective of the species (); the composition of milk within the same ...
Milk is a considerable resource of products whose composition varies. Four components are dominant in quantitative terms: water, fat, protein and lactose; while the minor components are minerals ...
We identified 32 studies on the composition of human milk in the United States and Canada conducted in the 5-y period from 2017 to 2022, which suggests a rapid increase in human milk research compared to the 28 studies identified by Wu et al. [8] in the 36-y period from 1980 to 2016.
Understanding the chemistry of milk and its components is critical to the production of consistent, high-quality dairy products as well as the development of new dairy ingredients. Over the past 100 yr we have gone from believing that milk has only 3 protein fractions to identifying all the major and minor types of milk proteins as well as discovering that they have genetic variants.
Cow's milk: Composition, nutritional, biological an d cardioprotective benefits. Aicha BENYAHIA-MOSTEFAOUI, Myriem LAMRI-SENH ADJI. Laboratoire de Nutrition Clinique et Métabolique (LNCM ...
This paper will review the major scientific advances in manipulation of milk composition over the last 25 yr. A multitude of factors influence the final composition of milk including genetics and breed of animal, environment, stage of lactation, parity, and nutrition of the cow. Although all of these factors work in combination to determine the ...
Milk is one of the most valuable products in the food industry with most milk production throughout the world being carried out using conventional management, which includes intensive and traditional systems. The intensive use of fertilizers, antibiotics, pesticides and concerns regarding animal health and the environment have given increasing importance to organic dairy and dairy products in ...
This study describes the development of a database, called MilkyBase, of the biochemical composition of human milk. The data were selected, digitized and curated partly by machine-learning, partly ...
Milk composition determines the economic feasibility of processing (i.e., the yield of butter, or cheese obtained per kg of milk) and affects the quality of dairy products (Chen et al., 2014). Low protein percentage has been reported in a handful of studies investigating milk composition in Kenya (Kabui et al., 2015; Ondieki et al., 2017).
Milk is considered as a nearly complete food since it is a good source of protein and major research is to evaluate the nutrients composition, minerals, vitamins and bio-active components of camel ...
J. G. LINN. Milk composition is economically important to milk producers and processors and nutritionally important to consumers. It has been known for years that variations in milk composition occur; however, the composition of milk marketed nationally has been rather constant over the last 15 years, averaging 3.6 percent fat, 3.2 percent protein, and 4.7 percent lactose (Young et al., 1986).
1. Introduction. Human milk from the infant's own mother is the optimal source of nutrition for infants because: (1) it provides core required nutrients, including proteins, lipids, carbohydrates and minerals; (2) it provides bioactive factors, especially immunological factors, to protect infants from invading microorganisms (Ballard & Morrow, 2013; Lee et al., 2018); (3) it helps the ...
Research over the past 2-3 decades focused on increasing the understanding about the composition of human milk and different factors that influence the composition such as stage of lactation, impact of maternal diet, geographical location, gestational age at infant birth, and circadian rhythm. Presently, collaborative efforts are ongoing in ...
Background: The traditional dairy-cattle-based industry is becoming increasingly diversified with milk and milk products from non-cattle dairy species. The interest in non-cattle milks has increased because there have been several anecdotal reports about the nutritional benefits of these milks and reports both of individuals tolerating and digesting some non-cattle milks better than cattle ...
Weight. Contrary to widespread belief, research does not support that milk helps with weight control. Although a meta-analysis of 29 randomized controlled trials found that milk and other dairy foods were beneficial for body fat reduction in the short-term and if calories were restricted, no benefits on body weight were seen in the long-term and when calories were not restricted. [13]
For some people with digestive difficulties, goat's milk can be easily digested. Camel milk is an important source of proteins for the people living in the arid lands of the world camel milk is considered to have anti-cancer, hypo-allergenic and anti- diabetic properties. Buffalo milk is a natural product that can be consumed like any other milk.
1.1. The Level of Fat in Milk and its Significance. Fat is the most variable component in milk. It varies during lactation, during the day and depends on the individual characteristics of breastfeeding women or feeding animals [6,7,8].The mean fat content reported in our previous study in mature human milk was 3.8% (ranging from 1.63% to 6.40%) []. ...
Breastfeeding is the most effective strategy for meeting the nutritional demands of infants, whilst infant formulae are manufactured foods that mimic human milk and can be safely used to replace breastfeeding. In this paper, the compositional differences between human milk and other mammalian milk a …
Plant-based milk, the. aqueous liquid obtained from different plant materials such as cereals, legumes, nuts, seeds, and. pseudocereals, has been developed to replace animal milk. Therefore, the ...
Background Bovine mastitis is a widespread disease affecting dairy cattle worldwide and it generates substantial losses for dairy farmers. Mastitis may be caused by bacteria, fungi or algae. The most common species isolated from infected milk are, among others, Streptococcus spp., Escherichia coli, Staphylococcus aureus and non-aureus staphylococci and mammaliicocci. The aim of this paper is ...
This is the reason for including zinc in the composition of indicators assessing the nutritional quality of the diet i.e., calories-for-nutrient (CFN), and naturally nutrient rich (NNR) score [76,77,78,90]. According to our research, the share of milk and dairy products in the supply of potassium is almost 12%, with a significant share of milk.
The worker reported no contact with sick or dead wild birds, poultry, or other animals but reported direct and close exposure to dairy cows that appeared to be well and with sick cows that showed ...
The fatty acid composition of cow's milk depends on (1) synthesis de novo in the mammary gland; (2) gastrointestinal absorption from digestion; and (3) mobilization from body fat reserves [59,61], and can be influenced by the animals' feed, stage of lactation, breed, and season [59,60]. Saturated fatty acids (SFA) are the main group of ...
This research paper presents a comparative analysis of the physio-chemical properties of milk from three different buffalo breeds in Haryana, namely Murrah, Nilli-Ravi, and Surti.
English Literature and Composition. Comparative Government and Politics. Computer Science A. Thursday, May 9, 2024. Chinese Language and Culture. Environmental Science. Psychology. Friday, ... AP Seminar and AP Research students to submit performance tasks as final and their presentations to be scored by their AP Seminar or AP Research teachers.
Bactrian camel milk is a source of vitamin. A (approximat ely twic e that in cow milk) and is hig h in vitamin D and ribofla vin. Two cups of camel milk supp ly 16 0% of the recomme nded nutrient ...