- Research article
- Open access
- Published: 21 July 2017

Treatment outcomes in schizophrenia: qualitative study of the views of family carers
- Joanne Lloyd 1 ,
- Helen Lloyd 2 ,
- Ray Fitzpatrick 3 &
- Michele Peters 3
BMC Psychiatry volume 17 , Article number: 266 ( 2017 ) Cite this article
28k Accesses
13 Citations
12 Altmetric
Metrics details
Schizophrenia is a complex, heterogeneous disorder, with highly variable treatment outcomes, and relatively little is known about what is important to patients. The aim of the study was to understand treatment outcomes informal carers perceive to be important to people with schizophrenia.
Qualitative interview study with 34 individuals and 8 couples who care for a person with schizophrenia/schizoaffective disorder. Interviews were transcribed verbatim and analysed by a thematic framework based approach.
Carers described well-recognised outcomes of importance, alongside more novel outcomes relating to: Safety (of the patient/others); insight (e.g. into non-reality of psychotic phenomena); respite from fear, distress or pain; socially acceptable behaviour; getting out of the house; attainment of life milestones; changes in personality and/or temperament; reduction of vulnerability to stress; and several aspects of physical health.
Conclusions
These findings have the potential to inform the development of patient- or carer- focused outcome measures that take into account the full range of domains that carers feel are important for patients.
Peer Review reports
Improving treatment outcomes and quality of life for people with long-term mental health conditions are key aims of health care policy [ 1 , 2 ]. Schizophrenia is a particularly important target, being associated with poor quality of life [ 3 ] and individual and societal impacts [ 4 , 5 , 6 ], and requiring long-term treatment [ 7 ]. Antipsychotic medications can ameliorate some symptoms and improve quality of life [ 3 , 8 , 9 ], but individual responses vary [ 10 , 11 ], and many discontinue medication due to poor efficacy or debilitating side effects [ 12 , 13 ]. Treatment outcomes are often assessed by clinician ratings, and/or symptom scales [ 14 ], but patients and carers may prioritise different outcomes to clinicians [ 15 , 16 , 17 ], and controlling symptoms is not the only outcome of importance [ 14 ]. The recovery literature draws attention to the importance of recognising a broad array of outcome domains in schizophrenia treatment, highlighting the relevance of improved social and domestic functioning, alongside subjective wellbeing, optimism and empowerment (e.g. [ 18 , 19 ]). Patients and relatives, in particular, refer to subjective wellbeing when defining ‘remission’, in contrast to traditional clinical definitions focused around reduced symptom scores [ 17 ]. People with schizophrenia value outcomes such as achieving life milestones, feeling safe, improved physical activity, employment, a positive sense of self and psychosocial outcomes [ 20 ]. Understanding the full range of treatment outcomes important to people with schizophrenia and their carers is key for ensuring that clinical practice, research and assessment are aligned with patient and carer priorities [ 4 , 21 ].
While people with schizophrenia can give valid and reliable accounts of outcomes [ 22 , 23 , 24 ], symptoms can make it difficult to participate in research [ 25 ], and carers represent a valuable additional resource [ 15 , 21 , 26 ]. Furthermore, carers have the potential to influence treatment decisions [ 26 ], and experience, indirectly, the impact of outcomes. This study sought to explore the treatment outcomes that carers feel are important for people with schizophrenia. It used a framework informed by a thematic review of the existing literature on treatment outcomes of importance to patients and carers, and a consensus conference with professionals, carers and patients, and aimed to identify whether carers report any outcome domains that have not been emphasised in the current literature.
Design of the study
A qualitative study using in-depth semi-structured interviews was conducted with self-identified ‘carers’ of a family member with a diagnosis of schizophrenia made at least 2 years previously. Ethical approval was obtained from NHS East of Scotland Research Ethics Service (EoSRES) REC 1 by proportionate review (Application Number 13/ES/0143). All participants gave written informed consent.
Participants and recruitment
A total of 34 individuals and 8 couples were interviewed (i.e. 50 people in 42 interviews). While qualitative methodology papers tend to avoid prescribing hard guidelines for sample sizes for qualitative studies, 25–30 participants have been deemed an acceptable minimum by Dworkin [ 27 ] and this number is usually sufficient for reaching data saturation. An email circulated by charity ‘Rethink Mental Illness’ was responded to by 102 people who were screened via telephone to confirm that they were the carer of someone with a ≥ 2-year diagnosis of schizophrenia or schizoaffective disorder. Within this self-selecting convenience sample, participants were then recruited purposively to generate a relatively heterogeneous final sample, consisting of 38 females and 12 males, aged from 20s–80s (48% in their 60s, 26% in their 50s, and the remainder in their 20s, 40s, 70s or 80s), and coming from urban (e.g. Greater London) and rural (e.g. Wiltshire) locations. Thirty-seven were the mother of a person with schizophrenia, 10 were the father or stepfather, one the husband, one the wife, and one the sibling. Duration of illness of the patients discussed ranged from 2 to 20+ years, with a modal duration of 11–15 years (42%). The majority ( n = 44) cared for someone with schizophrenia, and six cared for someone with schizoaffective disorder.
Most participants chose to be interviewed at home, but approximately 20% chose to come to the University. At the beginning of the interviews, carers re-confirmed that the patient had received a formal diagnosis of schizophrenia or schizoaffective disorder from a GP or psychiatrist, at least two years prior to the interview. Carers were then asked what they felt were important outcomes of treatment for the patient who they cared for: at present; at a time when the patient was particularly ill or unwell; at a time when they were more stable; and at a time when they were doing particularly well. Prompts were designed to encourage participants to discuss both directly-experienced outcomes, and important/desired but unattained outcomes. In addition, a series of prompts relating to key outcomes were compiled based on the conceptual review of the literature and feedback from a consensus conference, but were not in fact utilised in any of the interviews, as participants spontaneously discussed a broad array of outcomes of importance in response to the preliminary, general questions. After the initial 6 interviews, when it became apparent that participants identified multiple outcomes in response to the primary questions, without need for prompts, the researchers agreed that all future interviews in the study would proceed without prompts. Carers were encouraged to expand upon ideas that they themselves raised in relation to outcomes, rather than directed towards any specific topic. It was felt that this strengthened the data, as it reduced the potential for investigator bias. The topic guide, which was reviewed for tone and content prior to use by two carers and one person with schizophrenia, can be found in online Additional file 1 . Interview duration ranged from 40 to 125 min (average, approx. 60 min).
Interviews were transcribed verbatim by a professional transcriber, and anonymised. Transcripts were analysed in NVivo 8 by JL, using a thematic, framework based approach [ 28 ]. This involved the creation of a preliminary framework based on a literature review and consensus conference. Transcripts were then analysed, with themes being coded into appropriate categories within that framework, wherever appropriate categories existed. Where themes did not fit well into an existing category, novel categories were created. Interviews were continued until no further novel categories emerged, by which point all categories had been spontaneously mentioned by several participants, and saturation was deemed to have been reached. Once all interviews had been coded, the categories were reviewed by the research team, to ensure that they were representative of all the statements coded within them. Where categories were ambiguous, e.g. contained material that could potentially be better conceptualised within different domains, or could be better represented by different titles, they were revised, and the material coded within them was re-coded in order to ensure that it was coded within the most appropriate category. A final framework that encompassed the original and the novel categories was then agreed amongst the researchers. All of the interviews were then re-coded, using the final framework. In this second iteration, the majority of the material was coded into the same categories as during the initial coding. However, this process was important to ensure that any statements that had originally been coded into categories within the preliminary framework, but in retrospect better-reflected a novel category that had been added to the final framework, were coded appropriately. RF and MP independently cross-checked the final categorisation by coding a random selection of 6 transcripts, and no disagreements emerged. Categorized data were summarized and synthesized, and the resultant categories (and associations between them) were interpreted in relation to the categories already identified within the literature and consensus conference. After the final coding, the number of interviews in which each category occurred was calculated.
Outcomes of importance in schizophrenia reported by the carers included symptom related outcomes, quality of life, functional outcomes, personal recovery, physical health and lifestyle, and satisfaction with treatment. Table 1 lists these outcomes, and their sub-categories, and the proportion of interviews in which they occurred (using the conventions: ‘few’ for 2–10% ( n = 1–4), ‘some’ for 12–24% ( n = 5–10), ‘many’ for 25–50% ( n = 11–21), and ‘most’ for >50% ( n = 22–42)). It was not necessary for a participant to overtly state that an outcome had been experienced by the person they care for, in order to code their statement as an endorsement of that domain. While ‘endorsement’ of an outcome domain did, in some cases, take this form, any statement that either explicitly or implicitly indicated that a domain was relevant or important to that carer, was also coded within that domain. For example, where a carer identified that the person they cared for experienced ongoing difficulties with engaging in physical activity, or that they wished the person they cared for could have the energy to engage in physical activity, this was interpreted as the carer indicating that being able to engage in physical activity was an important outcome, and hence it was coded within the ‘physical activity’ category.
The categories in Table 1 were first identified through a literature review and consensus conference and subsequently adapted to include the newly identified and/or expanded categories from the interview data reported here. Standard font indicates categories which were pre-identified from the literature review (and replicated in the current study), and italic font indicates novel/ modified categories which emerged from the current study (which are illustrated by quotations in Tables 3 and 4 , and discussed below). All categories in Table 1 were identified as relevant by at least some of the carers interviewed, and the majority were mentioned in >50% of the interviews.
Symptom-related outcomes (Table 2 )
Safety was mentioned in most interviews, and encompassed safety from dangerous behaviours prompted by psychosis (such as absconding/ putting oneself or others into risky situations); from health risks linked to negative symptoms (e.g. not eating, living in squalor); and from potential for deliberate self-harm related to affective symptoms.
‘It's great for it to be diagnosed, to be put on your medication and you're safe’ [C41]
The importance of reduction of, or relief from fear, distress and emotional (or even physical) pain was raised in most interviews, often closely related to positive symptoms, but at the level of their physical and emotional consequences.
‘He was absolutely intimidated by his environment… he felt frightened and threatened’ [C25]
Insight was also mentioned in most interviews, encompassing both recognition that current/prior psychotic phenomena are not real, and understanding that one has a long-term illness. It was described as a gatekeeper to many other treatment benefits, partly through its impact upon treatment adherence, and was important in helping people deal with residual psychotic phenomena.
‘He can rationalise…although he hears the voices he has a sense of reality.’ [C40]
Side-effects are not described in detail here as they are well reported within existing literature (e.g. [ 29 ]), but they were identified as important in the majority of interviews, and in addition to commonly-reported side effects (e.g. weight gain and fatigue), a few participants mentioned negative impact of medication on imagination and/or creativity, and concerns over toxicity of medication during pregnancy and breastfeeding.
Quality of life (Table 2 )
The concept of ‘social acceptability’ was raised in most interviews, i.e. behaving in a socially appropriate way and avoiding bizarre/unconventional behaviour. Many discussed the importance of treatment in helping patients avoid illegal behaviour (sometimes precipitated by symptoms).
‘[When] he's not taking his medication, he occasionally offends people in the street’ [C25]
Functional outcomes (Table 3 )
The domain of ‘life milestones’ was added to encompass many carers’ reports of the importance of reaching key life/developmental milestones, such as attaining qualifications, learning to drive, moving out of the caregiver’s home, or having a family.
‘I think he missed out all his twenties and thirties so maybe catching up in some ways.’ [C03]
Simply ‘getting out’ of the house was mentioned in most interviews, and was consequently added as a sub-category of ‘leisure pursuits’. This encompassed the importance of being well enough to leave the house, which was something many patients needed to achieve before the more ambitious step of engaging in structured leisure activities or even activities of daily living.
‘The worst time that we've had was… when he was so unwell he didn’t go out the house for a year’ [C24]
A novel sub-category of ‘pets’ was added within the ‘role functioning and productivity’ category, because the importance of being able to care for a pet was raised in some interviews.
Personal recovery (Table 3 )
The importance of ‘personality/temperament’ was raised in most interviews, and was often particularly valued by carers themselves. This encompassed emergence of aspects of the patient’s character, such as sense of humour, consideration, and thoughtfulness, and of a generally calmer temperament, more ‘like oneself’.
‘He reverted to his old self. You could reason with him, you could have a laugh with him’ [C46]
The vast majority of carers also mentioned ‘vulnerability/sensitivity’ to all kinds of stress, in most cases as a residual difficulty that treatment failed to resolve, rather than a positive, attained outcome.
‘Although he seems fairly even I don’t think it would take a huge amount to kick him over the edge.’ [C06]
Physical health and lifestyle (Table 4 )
Exercise/physical activity and diet/weight were raised by the majority of carers, who sometimes described how treatment facilitated physical activity and healthy diet (by improving symptoms that create barriers), but also described how side-effects (such as alteration in appetite/metabolism, and fatigue) could act as barriers.
‘On such a high dose… of a sedating medication. Motivation is just not there’. [C46]
Many described the importance of outcomes related to drugs/alcohol/smoking, such as decreased reliance upon substances previously used to self-medicate positive or affective symptoms, or compensate for lack of social/functional activities.
‘She was drinking herself to sleep, I think, mostly because she had recurrent nightmares, and day time nightmares’ [C50]
Daily routine was mentioned in many interviews, in relation to sleep and waking, eating and self-care, and was described both as a factor that contributed to improving other outcomes, and as an outcome in itself.
Principle findings
All the schizophrenia treatment outcomes identified in the literature review and consensus conference preceding the study (i.e. symptom-related outcomes; functional outcomes; personal recovery; quality of life; and satisfaction with treatment) were confirmed in these qualitative interviews, along with several novel sub-categories within existing domains and a novel category of physical health and lifestyle, thus giving a deeper understanding of outcomes in this condition. While a large proportion of the sample endorsed most of the themes, it should be noted that frequency information are indicative of the frequency of these domains within our sample, and cannot be extrapolated from to estimate the prevalence of these concerns in carers of persons with schizophrenia.
While the importance of physical activity for persons with schizophrenia is recognised within the literature [ 30 ], and low levels of physical activity have been demonstrated empirically to be associated with poorer outcomes in schizophrenia [ 31 ], its importance as a treatment outcome is not expressed in existing outcome measures. This highlights the need to consider physical activity as a potentially relevant outcome domain in its own right. Designing interventions for schizophrenia that include attention to physical health and lifestyle, could help improve outcomes for many patients.
Safety of the patient (and those around them), and reduction of their fear, distress or pain, were considered important by most carers, and it is easy to see why they would value these outcomes, relating to resolution of negative practical and emotional consequences of symptoms. While the importance of these outcomes may be intuitive, they are not explicitly represented in current outcome measures, and this study is novel in highlighting their particular salience. These outcomes could be described as ‘secondary’, in the sense that they could be logically expected to follow on from the more ‘primary’ outcome of amelioration of (particularly, positive) symptoms. However, it could also be argued that there are other means of reducing patients’ fear, distress, or pain, aside from by symptom resolution, and thus outcome measures could benefit from assessing the extent to which treatments help to reduce a patient’s experience of these negative states. This could help professionals to gain a fuller understanding of how a given treatment programme is impacting on the individual’s level of fear and distress.
Most carers also valued insight which they often reported to be associated with improved communication with the person with schizophrenia, and a return of their personality and/or of a more favourable, ‘normal’ temperament. This is consistent with findings that insight in schizophrenia is associated with social cognition [ 32 ], and lower scores on an aggression scale [ 33 ]. Carers also described insight’s importance for enabling patients to apply cognitive strategies to counter paranoid thoughts, delusions or hallucinations, consistent with the finding that insight can be predictive of prognosis [ 34 ]. Monitoring level of insight may be beneficial in order to inform decisions about when cognitive interventions may be more effective. Exploring the value of educating carers in ways to cope with poor insight in the person for whom they care, could be another important target for future work.
Within functional outcomes, many carers talked of ‘getting out’ (i.e. leaving the house), similar to the existing domain of engaging in leisure pursuits, but at a more preliminary level. Caring for pets, similarly, could be conceptualised as a specific form of role functioning/productivity. Where residual difficulties are considerable and/or recovery is particularly limited, less ‘ambitious’ functional outcomes such as these may be particularly relevant. This is consistent with the observation that traditional social functioning measures may not be relevant to people with severe disabilities related to schizophrenia [ 35 ], and with carers’ comments about reduced potential and lowering of expectations. From carers’ references to a range of key developmental/life events such as moving out of the family home, getting a job, learning to drive, and having a romantic relationship, we identified ‘reaching life milestones’ as an important and novel outcome. Because schizophrenia onset is typically during adolescence or early adulthood [ 36 ], before traditional milestones have been reached, it is logical that the reaching of milestones would for many be the goal, rather than the resumption of familial, domestic, occupational or educational roles and responsibilities. This highlights the fact that functional outcome measures in schizophrenia may need to take subtle levels of attainment into account, in order to accurately capture small gains.
Within the realm of ‘personal recovery’ many carers highlighted the importance of changes in personality and temperament, and several described the return of the person they used to know as the most important outcome; understandably so, considering that these are good outward indicators of wellness and ‘personal recovery’ and directly impact upon the patient-carer relationship. Indeed, temperament has been linked with functional outcomes and psychological health [ 37 ]. Also relating to personal recovery, many carers discussed patients’ vulnerability (to stress, and in general) and sensitivity, consistent with empirical findings of increased biological reactivity to stress in schizophrenia [ 38 ]. These were typically described as residual unresolved difficulties, and several carers reported that they limited patients’ attainment of functional outcomes and acted as precipitants of relapse, requiring careful monitoring. This could indicate a potential benefit to be found in involving carers, where appropriate, in helping patients to monitor level of stress, and react quickly to try and reduce its impact.
In the sub-category of ‘leading a normal life’, a number of carers spoke of the importance of treatment for helping patients to avoid socially unacceptable/antisocial/illegal behaviours, (often precipitated by positive symptoms), in order to reduce risk of arrest or sectioning, facilitate social interactions and minimise stigma – consistent with findings that socially unacceptable behaviour is strongly associated with stigma in schizophrenia [ 39 ].
Consistent with other studies, many carers expressed desire for greater monitoring of physical health [ 40 ]. Exercise/physical activity, diet, and weight were all salient concerns; again consistent with findings of elevated obesity [ 41 ] and low activity [ 42 ] in schizophrenia/severe mental illness. A wide range of contributing factors were cited by the carers, including medication side effects, positive, negative and affective symptoms, and eating replacing less attainable leisure pursuits. Several also described patients who used alcohol or drugs to self-medicate and/or compensate for a lack of alternative leisure outlets; consistent with reported motivations for substance use in schizophrenia [ 43 ]. Some carers did describe physical health benefits of treatment, e.g. where it reduced use of drugs or alcohol for self-medication, or reduced symptoms enough to allow patients to exercise or shop for healthy food. In relation to lifestyle more generally, several carers emphasised the importance of routine, as a desirable outcome and useful intervention for facilitating the attainment of other outcomes (consistent with a study where people with schizophrenia rated organization of time as a useful coping strategy [ 44 ]). The discovery that physical health is an important concern in schizophrenia is not novel, but this study does support the growing body of work emphasising the importance of incorporating physical health interventions into schizophrenia treatment programmes (e.g. [ 45 ]).
Strengths and limitations
This study confirms the key treatment outcome categories found in the current literature, and contributes evidence of additional outcomes that carers feel are important for patients but are not apparently captured in current thinking about, and measurement of, schizophrenia outcomes. However, there are some possible biases in the sample. The majority of carers interviewed were parents of a person with schizophrenia, with a gender bias in the sample, such that around three quarters of participants were female. However, this is in line with the gender balance found in other convenience samples of carers of persons with schizophrenia [ 46 ], and reflects the fact that mothers are most frequently the primary carer in schizophrenia [ 47 ]. It is possible that spouses, siblings, or children (or those of a younger age in general) may have different perceptions of what the important outcomes are. Most participants were recruited via Rethink Mental Illness, which may have meant they were particularly well-informed about features of schizophrenia and issues around treatment. Finally, the patients discussed were typically quite advanced in chronicity (in most cases >10 years post-diagnosis). While carers were asked to discuss outcomes that they felt were important at different phases of illness, it is nevertheless possible that carers of patients more immediately post-diagnosis would report different outcomes. Future studies could benefit from exploring outcomes with younger carers with different relationships to the patient, from a range of backgrounds, and those caring for people more early post-diagnosis.
The outcomes carers identified as being important for patients may not be identical to the outcomes that patients themselves would identify. However, there is generally good agreement between the two [ 21 ], and as agents who potentially influence patients’ treatment decisions [ 16 ], and experience the consequences of the illness [ 48 ], carers’ views are important in their own right. Furthermore, we were able to gain insight into outcomes that might not otherwise have been represented, as most of the carers interviewed reported that the patients they were speaking about would have been unwilling/unable to participate (e.g. ‘he hates talking about it when he was really ill… he said, “It makes me feel so ill again” [C41]).
The findings from this study contribute to our understanding of the full range of treatment outcomes that carers feel are important to people with schizophrenia, and could contribute to ensuring research, treatment planning and assessment are aligned with the needs and priorities of patients [ 4 ]. The breadth of information gleaned from these interviews with family carers indicates what an important resource this population represents. Furthermore, it is clear that informal carers typically bear a high burden of care in schizophrenia [ 49 ]. Working with carers to gain insights and coordinate interventions, where appropriate, could be a valuable way for professionals to develop person-centred approaches in schizophrenia. Outcomes of treatment should ideally be assessed with measures that both complement existing clinical scales and incorporate patient and carer priorities. The domains and more specific experience emphasised here should inform the further development of such patient- or carer- focused outcome measures in order to ensure more appropriate and complete evaluation of interventions.
Centre for Mental Health, Department of Health, Mind, NHS Confederation Mental Health Network, R.M. Illness. Turning point: No health without mental health. Implementation framework 2012. London: Department of Health; 2012.
Department of Health. NHS Outcomes Framework 2012/13. London: Department of Health; 2012.
Google Scholar
Bobes J, Garcia-Portilla MP, Bascaran MT, Saiz PA, Bousono M. Quality of life in schizophrenic patients. Dialogues Clin Neurosci. 2007;9(2):215–26.
PubMed PubMed Central Google Scholar
Schizophrenia Commission. The abandoned illness: a report from the Schizophrenia Commission. London: Rethink Mental Illness; 2012.
Eack SM, Newhill CE. Psychiatric symptoms and quality of life in schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(5):1225–37.
Article PubMed PubMed Central Google Scholar
Jin H, Mosweu I. The Societal Cost of Schizophrenia: A Systematic Review. Pharmacoeconomics. 2016.
Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453–60.
Article PubMed Google Scholar
Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, Davis JM. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–71.
Article CAS PubMed Google Scholar
Wehmeier PM, Kluge M, Schneider E, Schacht A, Wagner T, Schreiber W. Quality of life and subjective well-being during treatment with antipsychotics in out-patients with schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2007;31(3):703–12.
Article CAS Google Scholar
Wehmeier PM, Kluge M, Schacht A, Helsberg K, Schreiber WG, Schimmelmann BG, Lambert M. Patterns of physician and patient rated quality of life during antipsychotic treatment in outpatients with schizophrenia. J Psychiatr Res. 2008;42(8):676–83.
Levine SZ, Rabinowitz J, Faries D, Lawson AH, Ascher-Svanum H. Treatment response trajectories and antipsychotic medications: examination of up to 18 smonths of treatment in the CATIE chronic schizophrenia trial. Schizophr Res. 2012;137(1–3):141–6.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz B, Hsiao J, Severe J. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia: primary efficacy and safety outcomes of the clinical antipsychotic trials of intervention effectiveness (CATIE) schizophrenia trial. Neuropsychopharmacology. 2005;30:S32.
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lassig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;392:951–62.
Article Google Scholar
Mortimer AM. Symptom rating scales and outcome in schizophrenia. Br J Psychiatry. 2007;191(50):s7–s14.
Shepherd G, Murray A, Muijen M. Perspectives on schizophrenia: a survey of user, family carer and professional views regarding effective care. J Ment Health. 1995;4(4):403–22.
Bridges JFP, Slawik L, Schmeding A, Reimer J, Naber D, Kuhnigk O. A test of concordance between patient and psychiatrist valuations of multiple treatment goals for schizophrenia. Health Expect. 2013;16(2):164–76.
Karow A, Naber D, Lambert M, Moritz S, Initiative E. Remission as perceived by people with schizophrenia, family members and psychiatrists. Eur Psychiatry. 2012;27(6):426–31.
Warner R. Recovery from schizophrenia and the recovery model. Curr Opin Psychiatry. 2009;22(4):374–80.
Karow A, Moritz S, Lambert M, Schottle D, Naber D, Initiative E. Remitted but still impaired? Symptomatic versus functional remission in patients with schizophrenia. Eur Psychiatry. 2012;27(6):401–5.
Lloyd H, Lloyd J, Fitzpatrick R, Peters M. The role of life context and self-defined well-being in the outcomes that matter to people with a diagnosis of schizophrenia. Health Expect. 2017; 1–12. doi: 10.1111/hex.12548 .
Balaji M, Chatterjee S, Brennan B, Rangaswamy T, Thornicroft G, Patel V. Outcomes that matter: a qualitative study with persons with schizophrenia and their primary caregivers in India. Asian J Psychiatr. 2012;5(3):258–65.
Voruganti L, Heslegrave R, Awad AG, Seeman MV. Quality of life measurement in schizophrenia: reconciling the quest for subjectivity with the question of reliability. Psychol Med. 1998;28(1):165–72.
Reininghaus U, Priebe S. Measuring patient-reported outcomes in psychosis: conceptual and methodological review. Br J Psychiatry. 2012;201(4):262–7.
Baumstarck K, Boyer L, Boucekine M, Aghababian V, Parola N, Lancon C, Auquier P. Self-reported quality of life measure is reliable and valid in adult patients suffering from schizophrenia with executive impairment. Schizophr Res. 2013;147:58–67.
Kaminsky A, Roberts LW, Brody JL. Influences upon willingness to participate in schizophrenia research: an analysis of narrative data from 63 people with schizophrenia. Ethics Behav. 2003;13(3):279–302.
Rettenbacher MA, Burns T, Kemmler G, Fleischhacker WW. Schizophrenia: attitudes of patients and professional Carers towards the illness and antipsychotic medication. Pharmacopsychiatry. 2004;37(03):103–9.
Dworkin SL. Sample size policy for qualitative studies using in-depth interviews. Arch Sex Behav. 2012;41(6):1319–20.
Ritchie J, Spencer L. In: Bryman A, Burgess B, editors. Qualitative data analysis for applied policy research, in Analyzing qualitative data. London: Routledge; 1993. p. 173–94.
Fischer EP, Shumway M, Owen RR. Priorities of consumers, providers, and family members in the treatment of schizophrenia. Psychiatr Serv. 2002;53(6):724–9.
Soundy A, Freeman P, Stubbs B, Probst M, Coffee P, Vancampfort D. The transcending benefits of physical activity for individuals with schizophrenia: a systematic review and meta-ethnography. Psychiatry Res. 2014;220(1–2):11–9.
Vancampfort D, Knapen J, Probst M, Scheewe T, Remans S, De Hert M. A systematic review of correlates of physical activity in patients with schizophrenia. Acta Psychiatr Scand. 2012;125(5):352–62.
Quee PJ, van der Meer L, Bruggeman R, de Haan L, Krabbendam L, Cahn W, Mulder NC, Wiersma D, Aleman A. Insight in psychosis: relationship with neurocognition, social cognition and clinical symptoms depends on phase of illness. Schizophr Bull. 2011;37(1):29–37.
Ekinci O, Ekinci A. Association between insight, cognitive insight, positive symptoms and violence in patients with schizophrenia. Nord J Psychiatry. 2013;67(2):116–23.
Saravanan B, Jacob KS, Johnson S, Prince M, Bhugra D, David AS. Outcome of first-episode schizophrenia in India: longitudinal study of effect of insight and psychopathology. Br J Psychiatry. 2010;196(6):454–9.
Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr Scand. 2007;116(6):403–18.
de Girolamo G, Dagani J, Purcell R, Cocchi A, McGorry PD. Age of onset of mental disorders and use of mental health services: needs, opportunities and obstacles. Epidemiol Psychiatr Sci. 2012;21(1):47–57.
Eklund M, Hansson L, Bengtsson-Tops A. The influence of temperament and character on functioning and aspects of psychological health among people with schizophrenia. Eur Psychiatry. 2004;19(1):34–41.
Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, Pruessner JC, Remington G, Houle S, Wilson AA. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71(6):561–7.
Loganathan S, Murthy SR. Experiences of stigma and discrimination endured by people suffering from schizophrenia. Indian J Psychiatry. 2008;50(1):39–46.
Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, Kane JM, Lieberman JA, Schooler NR, Covell N, Stroup S, Weissman EM, Wirshing DA, Hall CS, Pogach L, Pi-Sunyer X, Bigger JT Jr, Friedman A, Kleinberg D, Yevich SJ, Davis B, Shon S. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334–49.
Scott D, Happell B. The high prevalence of poor physical health and unhealthy lifestyle behaviours in individuals with severe mental illness. Issues Ment Health Nurs. 2011;32(9):589–97.
McNamee L, Mead G, MacGillivray S, Lawrie SM. Schizophrenia, poor physical health and physical activity: evidence-based interventions are required to reduce major health inequalities. Br J Psychiatry. 2013;203(3):239–41.
Gregg L, Barrowclough C, Haddock G. Development and validation of a scale for assessing reasons for substance use in schizophrenia: the ReSUS scale. Addict Behav. 2009;34(10):830–7.
Lee PW, Lieh-Mak F, Yu KK, Spinks JA. Coping strategies of schizophrenic patients and their relationship to outcome. Br J Psychiatry. 1993;163:177–82.
Stubbs B, Firth J, Berry A, Schuch FB, Rosenbaum S, Gaughran F, Veronesse N, Williams J, Craig T, Yung AR, Vancampfort D. How much physical activity do people with schizophrenia engage in? A systematic review, comparative meta-analysis and meta-regression. Schizophr Res. 2016;176(2–3):431–40.
Svettini A, Johnson B, Magro C, Saunders J, Jones K, Silk S, Hargarter L, Schreiner A. Schizophrenia through the carers’ eyes: results of a European cross-sectional survey. J Psychiatr Ment Health Nurs. 2015;22(7):472–83.
Wancata J, Freidl M, Krautgartner M, Friedrich F, Matschnig T, Unger A, Fruhwald S, Gossler R. Gender aspects of parents’ needs of schizophrenia patients. Soc Psychiatry Psychiatr Epidemiol. 2008;43(12):968–74.
Gutierrez-Maldonado J, Caqueo-Urizar A, Kavanagh DJ. Burden of care and general health in families of patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2005;40(11):899–904.
Nordstroem AL, Talbot D, Bernasconi C, Berardo CG, Lalonde J. Burden of illness of people with persistent symptoms of schizophrenia: a multinational cross-sectional study. Int J Soc Psychiatry. 2017;63(2):139–50.
Mojtabai R, Corey-Lisle PK, Ip EH, Kopeykina I, Haeri S, Cohen LJ, Shumaker S. The patient assessment questionnaire: initial validation of a measure of treatment effectiveness for patients with schizophrenia and schizoaffective disorder. Psychiatry Res. 2012;200(2–3):857–66.
Matza LS, Phillips GA, Revicki DA, Ascher-Svanum H, Malley KG, Palsgrove AC, Faries DE, Stauffer V, Kinon BJ, George Awad A, Keefe RSE, Naber D. Validation of a clinician questionnaire to assess reasons for antipsychotic discontinuation and continuation among patients with schizophrenia. Psychiatry Res. 2012;200(2–3):835–42.
Kitchen H, Rofail D, Heron L, Sacco P. Cognitive impairment associated with schizophrenia: a review of the humanistic burden. Adv Ther. 2012;29(2):148–62.
Mueser KT. Should psychosocial treatment for schizophrenia focus on the proximal or distal consequences of the disorder? J Ment Health. 2012;21(6):525–30.
Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–9.
Kikkert MJ, Schene AH, Koeter MW, Robson D, Born A, Helm H, Nose M, Goss C, Thornicroft G, Gray RJ. Medication adherence in schizophrenia: exploring patients’, carers’ and professionals’ views. Schizophr Bull. 2006;32(4):786–94.
Rogers A, Day JC, Williams B, Randall F, Wood P, Healy D, Bentall RP. The meaning and management of neuroleptic medication: a study of patients with a diagnosis of schizophrenia. Soc Sci Med. 1998;47(9):1313–23.
Weiden P, Rapkin B, Mott T, Zygmunt A, Goldman D, Horvitz-Lennon M, Frances A. Rating of medication influences (ROMI) scale in schizophrenia. Schizophr Bull. 1994;20(2):297–310.
Rosenheck R, Stroup S, Keefe RS, McEvoy J, Swartz M, Perkins D, Hsiao J, Shumway M, Lieberman J. Measuring outcome priorities and preferences in people with schizophrenia. Br J Psychiatry. 2005;187:529–36.
McCabe R, Saidi M, Priebe S. Patient-reported outcomes in schizophrenia. Br J Psychiatry. 2007;191(50):s21–8.
DiBonaventura M, Gabriel S, Dupclay L, Gupta S, Kim E. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12(1):20.
Naber D. A self-rating to measure subjective effects of neuroleptic drugs, relationships to objective psychopathology, quality of life, compliance and other clinical variables. Int Clin Psychopharmacol. 1995;10(Suppl 3):133–8.
PubMed Google Scholar
Wilkinson G, Hesdon B, Wild D, Cookson R, Farina C, Sharma V, Fitzpatrick R, Jenkinson C. Self-report quality of life measure for people with schizophrenia: The SQLS. British J Psychiatry. 2000;177:42–6.
Gerlinger G, Hauser M, De Hert M, Lacluyse K, Wampers M, Correll CU. Personal stigma in schizophrenia spectrum disorders: a systematic review of prevalence rates, correlates, impact and interventions. World Psychiatry. 2013;12(2):155–64.
Cuffel BJ, Fischer EP, Owen RR, Smith GR. An instrument for measurement of outcomes of Care for Schizophrenia: issues in development and implementation. Eval Health Prof. 1997;20(1):96–108.
Andresen R, Caputi P, Oades L. Stages of recovery instrument: development of a measure of recovery from serious mental illness. Aust N Z J Psychiatry. 2006;40(11–12):972–80.
Bullock WA, Young SL. The mental health recovery measure (MHRM). In: Bullock, et al., editors. Measuring the promise of recovery: a compendium of recovery and recovery-related instruments, Part II W.A. Cambridge: Evaluation Center@HSRI; 2005.
Giffort D, Schmook A, Woody C, Vollendorf C, Gervain M. The recovery assessment scale, in can we measure recovery? A compendium of recovery and recovery-related instruments, R.O. Ralph, K. Kidder, and D. Phillips, Editors. Cambridge: Human Services Research Institute; 2000. p. 7–8 52–55.
Resnick SG, Fontana A, Lehman AF, Rosenheck RA. An empirical conceptualization of the recovery orientation. Schizophr Res. 2005;75(1):119–28.
Bloom BL, Miller A. The consumer recovery outcomes system (CROS 3.0): assessing clinical status and progress in persons with severe and persistent mental illness. Colorado Springs: CROS, LLC/Colorado Health Networks; 2004.
Gibson S, Brand S, Burt S, Boden Z, Benson O. Understanding treatment non-adherence in schizophrenia and bipolar disorder: a survey of what service users do and why. BMC Psychiatry. 2013;13(1):153.
McCabe R, Bullenkamp J, Hansson L, Lauber C, Martinez-Leal R, Rossler W, Salize HJ, Svensson B, Torres-Gonzalez F, van den Brink R, Wiersma D, Priebe S, The Therapeutic Relationship and Adherence to Antipsychotic Medication in Schizophrenia. PLoS One. 2012;7(4).
Download references
Acknowledgments
The authors would like to acknowledge the support of Rethink Mental Illness in advertising the study, and the input of all the carers who took part.
This work was supported by EUFAMI, the European Federation of Associations of Families with Mental Illness.
Dr. Joanne Lloyd was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care Oxford whilst working on drafts of this article. Dr. Helen Lloyd was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula whilst commenting on drafts of this paper. Throughout this project, Prof Ray Fitzpatrick and Dr. Michele Peters were supported by the Department of Health funded Policy Research Unit on Quality and Outcomes of Person Centred Care (QORU), a collaboration between the London School of Economics and Political Science (LSE) and the Universities of Kent and Oxford. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Availability of data and materials
Anonymised transcripts are available from the corresponding author.
Author information
Authors and affiliations.
School of Psychology, Sport and Exercise, Staffordshire University, Stoke on Trent, UK
Joanne Lloyd
Peninsula Medical School, Plymouth University, Plymouth, Devon, UK
Helen Lloyd
Nuffield Department of Population Health, University of Oxford, Old Road Campus, Oxford, OX3 7LF, UK
Ray Fitzpatrick & Michele Peters
You can also search for this author in PubMed Google Scholar
Contributions
MP and RF conceived the study and raised the funding. JL and HL conducted the interviews and led the data analysis. MP and RF contributed to the analysis. All authors were involved in writing the publication. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Michele Peters .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained from NHS East of Scotland Research Ethics Service (EoSRES) REC 1 by proportionate review (Application Number 13/ES/0143). All participants gave written informed consent.
Consent for publication
Participants have given consent that anonymised quotes can be used in publications.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:.
Lloyd et al., Treatment outcomes in schizophrenia: qualitative study of the views of family carers. Interview schedule. (DOCX 12 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Lloyd, J., Lloyd, H., Fitzpatrick, R. et al. Treatment outcomes in schizophrenia: qualitative study of the views of family carers. BMC Psychiatry 17 , 266 (2017). https://doi.org/10.1186/s12888-017-1418-8
Download citation
Received : 16 December 2016
Accepted : 04 July 2017
Published : 21 July 2017
DOI : https://doi.org/10.1186/s12888-017-1418-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Schizophrenia
BMC Psychiatry
ISSN: 1471-244X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Original article
- Open access
- Published: 12 August 2022
Positive symptoms of schizophrenia and their relationship with cognitive and emotional executive functions
- Pamela Ruiz-Castañeda 1 , 2 ,
- Encarnación Santiago Molina 3 ,
- Haney Aguirre Loaiza 4 &
- María Teresa Daza González ORCID: orcid.org/0000-0002-6561-8982 1 , 2
Cognitive Research: Principles and Implications volume 7 , Article number: 78 ( 2022 ) Cite this article
10k Accesses
8 Citations
Metrics details
Positive symptoms of schizophrenia are associated with significant difficulties in daily functioning, and these difficulties have been associated with impaired executive functions (EEFF). However, specific cognitive and socio-emotional executive deficits have not been fully established.
The present study has several objectives. First, we aimed to examine the specific deficits in cognitive and socio-emotional EEFF in a group of patients with schizophrenia with a predominance of positive symptoms, as well as to determine if these patients present clinically significant scores in any of the three fronto-subcortical behavioral syndromes: Dorsolateral, Orbitofrontal, or Anterior Cingulate.
The sample consisted of 54 patients, 27 with a predominance of positive symptoms, and 27 healthy controls matched for gender, age, and education. The two groups completed four cognitive and three socio-emotional EEFF tasks. In the group of patients, positive symptoms were evaluated using the scale for the Evaluation of Positive Symptoms (SANS), while the behavioral alterations associated with the three fronto-subcortical syndromes were evaluated using the Frontal System Behavior Scale (FrSBe).
The patients, in comparison with a control group, presented specific deficits in cognitive and socio-emotional EEFF. In addition, a high percentage of patients presented clinically significant scores on the three fronto-subcortical syndromes.
The affectation that these patients present, in terms of both cognitive and emotional components, highlights the importance of developing a neuropsychological EEFF intervention that promotes the recovery of the affected cognitive capacities and improves the social and emotional functioning of the affected patients.
Introduction
The study of the positive symptoms (PS) of schizophrenia (such as prominent delusions, hallucinations, formal thought disorder, and bizarre behavior) is of particular interest both because of the severity of these symptoms and their consequences for the daily functioning of the patient and their impact on their caregivers. This psychotic clinic is usually associated with more significant social stigma and a higher rate of relapses and hospitalizations (Green, 1996 ; Holmén et al., 2012 ).
From a neuropsychological point of view, current research has realized that the study of neurocognition has important implications for understanding the prognosis, treatment, and neural systems of schizophrenia (Green et al., 2019 ; Molina & Tsuang, 2020 ; Seidman & Mirsky, 2017 ). Various investigations have suggested that the most pronounced neurocognitive deficits in these patients could occur in executive functions (EEFF) (Addington & Addington, 2000 ; Díaz-Caneja et al., 2019 ; Fonseca-Pedrero et al., 2013 ; Mingrone et al., 2013 ; Nieuwenstein et al., 2001 ). These functions are directly related to the quality of life and are considered significant predictors of the patient's prognosis (Bobes García & Saiz Ruiz, 2013 ). Several studies have highlighted these deficits as a strong predictor for the development of psychiatric disorders (Ancín et al., 2013 ; Sawada et al., 2017 ). Thus, the study carried out by Bolt et al. ( 2019 ) in patients with “ultra-high risk” of suffering from psychosis found that the EEFF were the only neurocognitive domain that emerged as a significant predictor of the transition to threshold psychosis full. The patients who had more pronounced deficits in this domain were those who developed psychosis in a mean period of 3.4 years. Similarly, Eslami et al. ( 2011 ) found that EEFF deficits at baseline were significant predictors of social functioning and occupational decline within one year. Therefore, these types of results could indicate that FFEE deficits may be a highly sensitive indicator of disease transition risk and poor functional outcomes.
Furthermore, in the scientific literature, a distinction has been established between the more cognitive aspects of EEFF, also called “ cool ” components, and the more socio-emotional, or “ hot ” components (Peterson & Welsh, 2014 ; Prencipe et al., 2011 ; Welsh & Peterson, 2014 ).
Cool EEFF include those cognitive processes manifested in analytical and non-emotional situations, primarily associated with the dorsolateral regions of the prefrontal cortex (Henri-Bhargava et al., 2018; Kamigaki, 2019). Within these EEFF, we would find at least three central components: (1) the processes of coding/maintenance and updating of information in working memory (WM); (2) inhibitory control; and (3) cognitive flexibility (Miyake & Friedman, 2013 ; Miyake et al., 2000 ). In addition, other more complex functions such as planning, abstract reasoning, or problem-solving are developed from these central components. In contrast, hot EEFF include those processes involved in contexts that require emotion, motivation, and tension between immediate gratification and long-term rewards (Zelazo & Carlson, 2012 ; Zelazo & Mller, 2007 ). Are mediated by the ventromedial and orbitofrontal cortices that support behaviors that require emotional regulation, decision-making in situations of uncertainty, recognition of facial expressions and their emotional content, as well as in the ability to infer the perspective of others, also known as mentalization or theory of mind (ToM) (Welsh & Peterson, 2014 ; Zimmerman et al., 2016).
Regarding decision-making in situations of uncertainty, it is a complex process that could be defined as the choice of an option among a set of alternatives, considering the possible results of the choices and their consequences on behavior (Kim & Lee, 2012 ; Xiao et al., 2012). Within this framework, Damasio ( 1994 ) postulates his “Somatic Marker” hypothesis to explain the role of emotions in reasoning and decision-making. In this sense, a Somatic Marker is an automatic emotional response that it is produced by the perception of a certain situation, and which in turn evokes past experiences. Specifically, the neural system for the acquisition of Somatic Marker signals is found in the orbitofrontal and ventromedial portion of the prefrontal cortex. Regarding the theory of the mind, authors such as Zimmerman et al. (2016) describe it as an emotional function that refers to the processes responsible for the perception and identification of emotions, such as empathizing with the affective state of another person. Specifically, the neuroanatomical network associated with ToM includes the medial prefrontal region of the prefrontal cortex, the posterior cingulate cortex, the amygdala, the temporoparietal junction, and the temporal sulcus, bilateral superior–posterior (Amodio & Frith, 2006 ; Ilzarbe et al., 2021 ; Zemánková et al., 2018 ).
Regarding the alterations in cool EEFF presented by patients with a predominance of PS, the results reported to date are inconclusive. On the one hand, studies that have analyzed EEFF through classical paper-and-pencil neuropsychological tests (e.g., Wisconsin Card Sorting Test; Trail Making Test A and B) have reported poor performance in these patients, suggesting general executive impairment (Addington et al., 1991 ; Zakzanis, 1998 ). Moreover, correlations have been reported between PS such as formal thought disorders and persistently bizarre behavior with cool executive components, such as inhibition and cognitive flexibility, pointing to a marked deficit in inhibitory control (Brazo et al., 2002 ; Laplante et al., 1992 ; Li et al., 2017 ; Subramaniam et al., 2008 ). On the other hand, other symptoms such as delusions and hallucinations have been moderately related to difficulties in processing speed, cognitive flexibility, and information updating processes in WM (Ibanez-Casas et al., 2013 ; Laloyaux et al., 2018 ). It has even been proposed that the PS are possible consequences of the deficits in self-monitoring capacity that are shown by these patients (Spironelli & Angrilli, 2015 ).
However, and in contrast to these investigations, other studies suggest conservation of EEFF in these patients (Berenbaum et al., 2008 ; Clark et al., 2010 ) or at least a minimal relationship with PS. Thus, some studies report low or null correlations between symptoms such as delusions or hallucinations and performance on verbal fluency, WM, and attention tasks (Berenbaum et al., 2008 ). Similarly, null correlations have been observed between delusions and hallucinations and performance on tasks that assess resolution problems, working memory, verbal and visual memory, and processing speed, and, using these same tasks, low or moderate correlations with symptoms such as formal thought disorders or bizarre behavior (Ventura et al., 2010 ).
An important question is whether these results could be influenced by the clinical or socio-demographic variables of the sample. In this regard, some studies (Addington et al., 1991 ; Zakzanis, 1998 ) have concluded that performance on EEFF tests is not related to the age of the participants, the number of admissions, the age of disease onset, or type of medication (chlorpromazine equivalents).
The literature on socio-emotional or hot EEFF has also yielded mixed results. Regarding decision-making in situations of uncertainty (participants do not have direct information about the disadvantages of their choices and do not have the opportunity to establish a reasonable strategy at the beginning of the task (Pedersen et al., 2017 )), the studies that have examined the performance of patients with a predominance of PS in the Iowa Gambling Task (IGT) show inconsistent results. Some studies have found negative correlations between symptoms such as hallucinations and prominent delusions and performance on this task compared to controls. In particular, a higher PS score was correlated with a lower Net Score (number of disadvantageous options minus the number of advantageous options), fewer advantageous choices (Struglia et al., 2011 ), and a greater number of disadvantageous choices (Pedersen et al., 2017 ). Other studies, however, using the same paradigm (IGT), did not find differences in performance compared to controls or correlations between IGT performance and symptomatology (Evans et al., 2005 ; Ritter et al., 2004 ; Wilder et al., 1998 ).
Regarding the ability to infer mental states or theory of mind, a generalized deterioration has been reported in these patients, particularly in those with marked PS such as delusions and hallucinations (Corcoran et al., 1995 ). However, in contrast, it has been hypothesized that for the development of certain PS such as persecutory delusions, an intact theory of mind is required, since this is necessary for inferring the intentions of others, even though these inferences are not correct (Peyroux et al., 2019 ; Walston et al., 2000 ).
When analyzing the possible influence of clinical and demographic variables on the results of these studies, although the studies have not considered this as a primary objective, the patients were matched with the control group in terms of age, gender, or education, which has led the authors to suggest that these variables are not the cause of the results and that patients perform the task in a different way to controls (Corcoran et al., 1995 ; Peyroux et al., 2019 ).
On the other hand, from a neuropsychological point of view, it has been suggested that the heterogeneity and diversity of symptoms shown by patients with schizophrenia could be a consequence of a malfunction of brain circuits of fronto-subcortical origin (Fornito et al., 2012 ; Penadés & Gastó, 2010 ). According to this approach, schizophrenia tends to be considered as a neuronal connectivity disorder and its different symptomatology could be explained by using the distributed neural network model (Goldman-Rakic, 1994 ; Pantelis & Brewer, 1995 ; Wang et al., 2014 ). This model posits that control of any cognitive function is distributed across several interconnected nuclei throughout the brain. The interruption of any of these nuclei or their interconnections would produce changes in cognitive function (Baars & Cage, 2010 ). In this sense, the involvement of these prefrontal areas and/or their connections with other subcortical regions (e.g., the fronto-subcortical circuits of prefrontal origin: Dorsolateral syndrome, related to executive deficits; Orbitofrontal syndrome, related to disinhibition; and syndrome Anterior Cingulate, related to apathetic behaviors (Bonelli & Cummings, 2007 ; Tekin & Cummings, 2002 )), could result in specific deficits in the different cool and hot components of the EEFF (Slachevsky Ch. et al., 2005 ).
In this sense, and regarding the brain areas involved in the PS of schizophrenia, these are not yet fully established. Some inferences in this regard have been obtained from patients with traumatic brain injury (TBI) who have developed clinical symptoms and behaviors like those presented in patients with PS in schizophrenia after the injury. Psychotic symptoms such as hallucinations, persecutory delusions, and thought disorders (loosening of associations, tangentiality, or thought blockage) occur more frequently in patients with TBI than in the general population (Fujii & Ahmed, 2002 ; Sachdev et al., 2001 ).
Similarly, a high percentage of patients with TBI also show significant alterations upon neuropsychological examination, similar to those presented by patients with psychotic symptoms, particularly in executive functions and memory (Berrios, 2013 ). These alterations have been associated with post-traumatic structural lesions located in different brain regions, such as the frontal cortex (dorsolateral and orbitofrontal), and, in those structures that form the so-called fronto-subcortical circuits (Alexander et al., 1986 ; Pettersson-Yeo et al., 2011 ).
Therefore, and in summary of the above, two main conclusions can be drawn. First, a review of the current literature has revealed inconclusive results regarding the level of alteration in cool and hot EEFF presented by schizophrenic patients with a predominance of PS. Moreover, there is no conclusive relationship between specific executive components and PS.
Second, the findings of neuroanatomical studies on the affectation of the fronto-subcortical circuits in TBI patients who develop behaviors and PS similar to those presented by patients with schizophrenia could suggest possible alterations of these circuits in schizophrenic patients. Therefore, it is possible that patients with schizophrenia with a predominance of PS present behaviors associated with the so-called fronto-subcortical syndromes (Dorsolateral Prefrontal Syndrome, related to executive deficits; Orbitofrontal syndrome, related to disinhibition; and Anterior or Mesial Cingulate Syndrome, related to apathic behaviors). However, to our knowledge, there is no previous study that has explored the possible involvement of the fronto-subcortical circuits in patients with positive symptoms from the presence of behaviors associated with fronto-subcortical syndromes.
Thus, the present study had several objectives. First, we aimed to study the specific deficits in cool and hot EEFFEF in a group of patients with schizophrenia with a predominance of PS, in comparison with a control group of healthy participants matched for age, gender, and educational level. Second, we set out to study the influence of the main clinical variables (years of evolution of the disease, clinical treatment device, and pharmacological treatment) on executive task performance shown by these patients. Third, we aimed to explore the possible relationship between the severity of PS (hallucinations, delusions, bizarre behavior, and formal thought disorders) with performance on both cool and hot EEFF tasks. And, finally, we wanted to confirm if these patients present clinically significant scores on any of the three fronto-subcortical behavioral syndromes: Dorsolateral, Orbitofrontal, or Anterior Cingulate. (These were measured through the self-reported version of the Frontal System Behavior Scale—FrSBe.)
Considering the previous literature concerning our first objective, we expect psychotic patients with a predominance of PS to show significantly poorer performance on the EEFFEF tasks in comparison with healthy controls. Moreover, in terms of clinical variables, we expect that the years of disease duration, the clinical treatment device, and the type of pharmacological treatment could affect the performance of patients on EEFF tasks.
Regarding the third objective, we expect that the patients with the highest scores on the scale for the Evaluation of Positive Symptoms (SAPS) also show poorer performance on the EEFFEF tasks. Regarding the fourth objective, we anticipate that these patients with a predominance of PS will present some of the frontal behavioral syndromes.
Materials and methods
Participants.
The initial sample consisted of 128 participants (age range: min = 20, max = 61, M age = 37.4, SD = 10.7). The selection process is shown in Fig. 1 . The final sample consisted of n = 54 participants (age range: min = 20, max = 60), of both genders: men ( n = 49, 74.2%, M age = 43.6, SD = 11.0), women ( n = 17, 25.8%, M age = 44.2, SD = 11.0); 27 patients with schizophrenia, and 27 participants assigned to the control group.
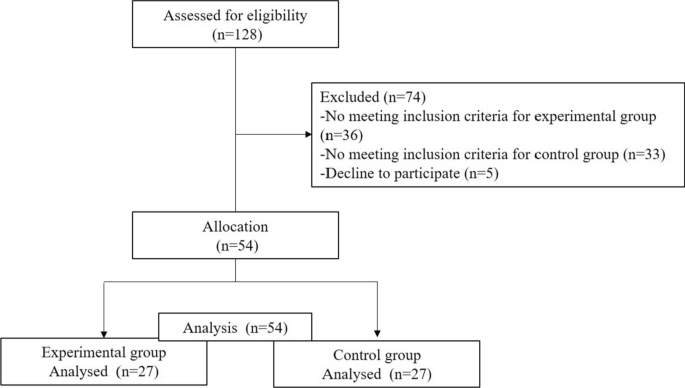
Flow of participants throughout the study
Criteria for inclusion and exclusion of the experimental group
Inclusion criteria.
Patients between 18 and 57 years.
Defined diagnosis of schizophrenia
Minimum of two years of evolution of the disease
PS predominance. For this, those patients who showed a higher percentage score in the Evaluation of Positive Symptoms (SAPS) than in the Scale for the Evaluation of Negative Symptoms (SANS) were selected.
Likewise, the psychopathological stability and motivation of the patient were considered, selecting psychopathologically stable patients to carry out the evaluation. The referral psychiatrist established this criterion based on prior knowledge of the patient's clinical status, ensuring sufficient compensation and motivation for participation in the study.
Exclusion criteria
Participants whose main diagnosis is an organic mental disorder, a different medical or psychological illness.
Electroconvulsive treatment in the last 2 years,
Patients with very low motivation for active participation in the study.
Criteria for inclusion and exclusion of the control group
Subjects between 18 and 57 years
Subjects who could be matched with the patients in age, gender, and educational level.
Have no history of mental, neurological, or substance abuse illness,
Not be medicated with any psychotropic medication.
Those participants who did not meet the inclusion criteria were excluded.
The patients were selected from the various medical facilities of the Mental Health unit of the reference Hospital Complex of the city. Regarding the socio-demographic variables, three levels were established according to the years of schooling: basic (6 years), medium (between 7 and 12 years), and high (more than 12 years). Regarding the clinical variables, for the duration of the illness, two levels were established according to the sample mean: a group with a shorter duration off illness (less than 11 years) and another group with a longer duration of illness (more than 11 years). Regarding clinical treatment service, two levels were established according to whether they received treatment in an inpatient or outpatient setting. For pharmacological treatment, two levels were established according to whether they took typical or/and atypical medications, and other medications unrelated to mental illness. The control group was matched with the patients in terms of age, gender, and years of schooling. The selected participants had no history of mental, neurological, or substance abuse illness and were not taking any psychotropic medications. The study was carried out in accordance with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of Centro-Almería belonging to the Torrecárdenas Hospital Complex in the city of Almería (protocol code 52,780. approval date: 26 / 10/2014). The patients/participants provided their written informed consent to participate in this study.
Execution tasks
For the study of cool EEFF, four different neuropsychological tasks were used: 1) the Sternberg-type task, which assesses the processes of encoding/maintaining information in working memory (WM); 2) the 2-back task, which evaluates the monitoring and updating processes of information in WM; 3) the Number–Letter task, which assesses cognitive flexibility or ability to change or alternate the mental set; and 4) a computerized version of the Tower of Hanoi (THO), which evaluates the planning processes involved in the preparation of ordered sequences of actions to achieve specific objectives.
Regarding the hot EEFF, the following three tasks were used: 1) a computerized version of the Iowa Gambling Task (IGT) which assesses decision-making processes in situations of uncertainty; 2) a computerized task for the recognition of facial emotional expressions, and 3) a pencil and paper version of the Hinting task that evaluates the theory of mind (ToM) (See Table 1 ). For a more detailed description of the cool and hot EEFF tasks used in the present study, see Ruiz-Castañeda et al. (Ruiz-Castañeda et al., 2020 ).
Scales for the evaluation of psychotic symptoms and frontal behavioral syndromes
To evaluate positive and negative symptoms, the Scale for the Evaluation of Positive Symptoms (SAPS) (Andreasen, 1984 ) and the Scale for the Evaluation of Negative Symptoms (SANS) (Beck & Chaudhari, 1976 ) were used. The behavioral alterations associated with the three frontal syndromes: Dorsolateral Syndrome (executive dysfunction); Orbitofrontal Syndrome (disinhibition); and Anterior or Mesial Cingulate Syndrome (apathy), were evaluated using the Spanish version of the Frontal System Behavior Scale (FrSBe) (Grace & Malloy, 2001 ; Pedrero-Pérez et al., 2009 ) .
For all participants (experimental and controls), the EEFF tasks were administered by two researchers so that one of them always carried out the evaluation, while the second investigator supervised these evaluations. For the patients, the evaluation took place across two individual sessions of approximately 50 min, each with the necessary breaks required by the participant. In the case of the control group, most of them required a single session of approximately 60 min, with the necessary breaks. The evaluation sessions were carried out individually in a quiet room using a laptop.
In the case of patients, the SANS and SAPS scales were administered by the referral physicians (psychiatrists or clinical psychologists). The self-reported version of the FrSBe Scale could be completed by the patient independently (in the researcher's presence) or by the researcher, always trying to ensure the maximum understanding of the questions.
To select psychotic patients with a predominance of PS, the following procedure was applied. Once the patients' referral psychiatrists or clinical psychologists completed the SAPS and SANS scales for each patient, the total scores for each scale were calculated. Each score was then transformed into a percentage. For the SAPS scale, the percentage is calculated based on the maximum score obtained on this scale (170), following the same procedure for the SANS scale (maximum score = 150). Finally, those patients who had a higher percentage on the SAPS scale ( M = 24.0, DT = 16,3) than on the SANS ( M = 15.1, SD = 14.4) were selected.
Statistical analysis
The data were processed through a descriptive and frequency analysis to characterize the socio-demographic and clinical variables. In the exploratory analysis of the data of the response variables, missing data were found, which were imputed to the median value of each group. Outliers were maintained to ensure consistency with the performance of the evaluated. Gender was matched in each group (n = 17 male, n = 10 female). Age was compared with the Mann–Whitney U test, and education level was assessed with X . 2
The direct scores of the neuropsychological tasks were transformed into Z scores. Two multivariate analysis models (MANOVA) were carried out, one with all the measures of the cool EEFF tasks and the other with the measures of the hot EEFF tasks. The first model was EEFF- cool * groups (9 × 2), and the second model was EEFF- hot * groups (6 × 2). Assumptions of normality for hypothesis testing were checked through standardized residuals in both groups. The assumption of equality of covariances was estimated with Box's test, and the multivariate Lambda test of Wilks (Λ) was used. The analysis of multiple comparisons between patients and controls was corrected with Sidak’s procedure. For the comparisons that showed significant differences, the confidence interval (95% CI) of the differences was reported. The effect size was estimated with eta squared ( η p 2 ), using the following values: < 0.01 small, 0.06 moderate, and > 0.14 strong (Cohen, 1988 ).
Pearson's r correlation analyses were conducted between PS and EEFF tasks. To check whether the patients with PS had clinically significant scores in any of the three frontal behavioral syndromes, the direct scores obtained on the FrSBe scale were converted into standardized scores ( T ) according to the age, education, and gender of the participant. With these T scores, three ranges of affectation can be obtained according to their cutoff point: no risk (< 59 points); high risk or borderline (60 to 64); and clinically significant (> 65). The data analyses were conducted using SPSS v.23.0. Post hoc statistical power ( 1-β ) was calculated with G * Power software (Faul et al., 2009 ).
No significant differences were found between patients and controls in age [U( N patients = 33, N controls ) = 542.0, z = − 0.03, p = 0.974], gender [ X 2 (1) = 0.79 , p = 0.778], or years of education [ X 2 (2) = 0.83 , p = 0.959]. The socio-demographic and clinical characteristics are shown in Table 2 .
Cool EEFF tasks
The descriptive data of the cool EEFF comparing patients with controls are shown in Table 3 . The MANOVA analysis revealed a significant interaction between the cool EEFF and the groups [ Wilks’ Λ = 0.498, F (9, 44) = 4.93, p < 0.001, ηp 2 = 0.50, 1-β = 0.99 ]. Better performance on the cool EEFF tasks was observed in the control group.
A main effect was found in the two conditions of the information coding/maintenance task in WM (Sternberg-type task) [low load: F (1, 52) = 4.86, p = 0.032, ηp 2 = 0.08, 1-β = 0.58 ; and high load: F (1, 52) = 8.19, p = 0.006, ηp 2 = 0.136, 1-β = 0.8 0]. Likewise, a main effect was found for the task of updating the information in WM (2-Back task) [ F (1, 52) = 16.69, p < 0.001, ηp 2 = 0.243, 1-β = 0.9 8].
Regarding performance on the task that assesses cognitive flexibility (Number–Letter task), only significant “task-switching costs” (TSC) were observed with reaction time (TSC TR ) [ F (1, 52) = 5.38, p = 0.024, ηp 2 = 0.094, 1-β = 0.6 24]. Regarding the planning task (Tower of Hanoi), only one main effect was observed with the latency measure in the short planning condition [ F (1, 52) = 5.27, p = 0.026, ηp 2 = 0.092, 1-β = 0.6 15] (See Fig. 2 ).

Cool EEFF compared between patients and controls. Note : TSC = Task-switching costs. RT = Response time. * p < 0.05. ** p < 0.01. *** p < 0.001
Hot EEFF tasks
The descriptive data of the hot EEFF comparing patients and controls are shown in Table 4 . The MANOVA analysis revealed a significant interaction between the hot EEFF tasks and the groups [ Wilks’ Λ = 0.475, F (6, 47) = 8.642, p < 0.001, ηp 2 = 0.52, 1-β = 1.0 ]. Better performance on the hot EF tasks was observed in the control group.
Regarding the task that assesses decision-making under conditions of uncertainty (Iowa Gambling Task), the analysis of the Net Score measure (Nº of Advantageous choices—Total Nº of disadvantageous choices) did not show a significant effect [ F (1, 52) = 0.19, p = 0.657, ηp 2 = 0.004, 1-β = 0.07 ].
In contrast, the task that measures the recognition of facial emotional expressions showed significant effects on errors, both in basic facial expressions [ F (1, 52) = 5.993, p = 0.018, ηp 2 = 0.10, 1-β = 0.67 ], as in complex facial expressions [ F (1, 52) = 9.34, p = 0.004, ηp 2 = 0.15, 1-β = 0.85 ]. Similarly, significant effects were also observed in reaction times, both for the condition of basic facial expressions [ F (1, 52) = 21.20, p < 0.001, ηp 2 = 0.29, 1-β = 0.99 ], as complex [ F (1, 52) = 16.34, p < 0.001, ηp 2 = 0.23, 1-β = 0.98 ]. Finally, the performance of the task that assesses the theory of mind (Hinting Task) was significant [ F (1, 52) = 29.06, p < 0.001, ηp 2 = 0.35, 1-β = 1.0 ] (See Fig. 3 ).
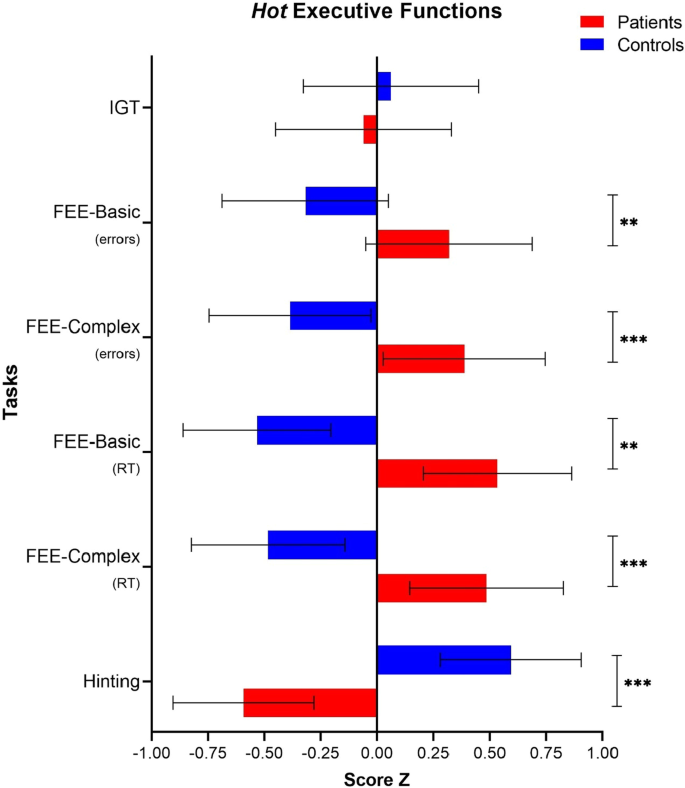
Hot EEFF compared between patients and controls. Note : IGT = Iowa Gambling Task. FEE = Facial emotional expressions. RT = Response time. * p < 0.05. ** p < 0.01. *** p < 0.001
Clinical variables and patient performance in hot and cool EEFF tasks
Regarding the variable years of disease evolution , differences were only observed in the errors of the planning task (Tower of Hanoi) in the condition of precision in short planning [ t (31) = − 2.51, p = 0.034, d = 0.71 95%CI (− 1.86, − 0.08)]; that is, patients with a short disease evolution (less than 11 years) showed better performance [ n = 15; M = − 0.25, SD = 0.62], than the patients with long disease evolution (more than 11 years) [ n = 12; M = 0.71, SD = 1.3]. Based on these results, we wanted to analyze whether the short evolution group showed similar performance to the control group [ n = 27; M = − 0.17, SD = 0.88] and found that these two groups did not differ.
Regarding the clinical device in which the patients received the intervention, no significant differences were found in performance between patients with an outpatient intervention (n = 17) and patients with in-hospital intervention (n = 10).
Regarding pharmacological treatment , no significant differences were found between the group of patients taking typical and/or atypical antipsychotics (n = 23) and those receiving other medication unrelated to mental illness (n = 4). Given these results, we wanted to check whether there were significant differences between those patients who were taking typical medications or a combination of typical and atypical ( n = 4), and those who were only taking atypical medications or other non-psychotropic medications ( n = 23), finding no significant differences between these two groups.
Correlations between positive symptoms and performance on the cool and hot EEFF tasks
The results of the correlation analysis between the severity of the PS and performance on the cool and hot EEFF tasks are shown in Table 5 . Regarding the cool EEFF tasks, both the hallucination symptoms ( r = − 0.47, p = 0.012) and delusions ( r = − 0.39, p = 0.044) were related to the planning task (the Tower of Hanoi), in the latency condition in short planning.
Regarding the symptoms of bizarre behavior , these correlated with the task of coding/maintaining the information in WM (Sternberg-type task) in the low load condition ( r = 0.42, p = 0.027). Formal thought disorder symptoms correlated with the cognitive flexibility task (Number–Letter task) in the TSC TR condition ( r = 0.44, p = 0.022), as well as the reaction times in the long planning condition ( r = 0.38, p = 0.047).
Regarding the hot EEFF, the symptoms of formal thought disorder correlated with performance on the theory of mind task (Hinting Task) ( r = − 0.46, p = 0.016).
Frontal Behavioral syndromes in patients with positive symptoms
Regarding the presence of the three frontal behavioral syndromes in patients with PS, we found that for Dorsolateral syndrome (executive dysfunction subscale), 81.5% presented a clinically significant score. For Orbitofrontal syndrome (Disinhibition subscale), 59.3% had a clinically significant score, while 77.8% had a clinically significant score for the anterior cingulate syndrome (Apathy subscale).
The objectives of this work were to (1) study the specific deficits in the cool and hot EEFF in a group of patients with schizophrenia with a predominance of PS, compared to a control group of healthy subjects matched for age, gender, and educational level; (2) study the influence of the main clinical variables (years of evolution of the disease, pharmacological treatment, and clinical service through which treatment is received) on the performance of patients on EEFF tasks; (3) explore the possible relationship between the severity of PS and the performance of patients on EEFF tasks; and finally (4) verify if the patients present clinically significant scores for any of the three frontal behavioral syndromes (Dorsolateral, Orbitofrontal, and Anterior Cingulate).
Alterations in cool EEFF
As we expected, the patient group showed significantly poorer performance than the control group on the cool EEFF tasks.
Regarding working memory , our data agree with findings in the previous literature (Forbes et al., 2009 ; Menon et al., 2001 ). In our study, patients showed poor performance on the two components of WM that we evaluated: coding/maintenance of information (Sternberg-type task) and updating of information in WM (2-Back task). Accordingly, various studies have highlighted the importance of WM in PS, such as hallucinations, formal thought disorders, or delusions (Díaz-Caneja et al., 2019 ).
Regarding hallucinations, a relationship has been observed between auditory hallucinations and deficits in verbal WM tasks (Bruder et al., 2011 ). Given these findings, it has been argued that WM deficits could predict the presence of auditory verbal hallucinations(Jenkins et al., 2018 ); even from a first psychotic episode (Gisselgård et al., 2014 ), or in the general population who have more frequently experienced psychotic experiences (hallucinations and delusions) but who have not been diagnosed with mental illness (Rossi et al., 2016 ). In this sense, it has been observed (in a group of adolescents with reports of psychotic experiences in the absence of clinical disorder) that increasing the WM load when moving from a 2-back task to an overload in the 3-back task was associated more strongly with a higher level of psychotic experiences. Similarly, and through signal detection theory (SDT), an increase in false alarms was found to be associated with stronger psychotic experiences, as well as greater false recognition of auditory signals and words (Rankin & O’Carroll, 1995 ), suggesting that decreased discrimination is a characteristic of positive psychotic phenomena (Bentall & Slade, 1985 ; Rossi et al., 2016 ).
Deficits in WM have also been implicated in formal positive thinking disorders. According to authors such as Goldman-Rakic (Goldman-Rakic, 1994 ), the derailment, the loss of logical associations in thought, the inability to perceive causal relationships, or typical behavior through internal mental representations are the product of weaknesses in WM. Similarly, symptoms such as tangentiality, poor planning, cohesion of discourse, and deficiencies in information processing have specifically been linked to a dysfunction in updating and retrieving information from verbal WM (McGrath et al., 1997 ).
Regarding the performance on the task that assesses the capacity for cognitive flexibility (Number–Letter task), our patients only showed higher task-switching costs in reaction times (TSC TR ) compared to controls, but not a higher cost of switching in terms of errors committed (TSC Error ) (categorizing a stimulus as consonant or vowel, according to the position of the squares in which it appears, compared to the performance when they do not have to make such a change).
In patients with PS, although some studies have found that a poorer ability to change the mental set allowed for distinguishing patients who presented auditory verbal hallucinations from those who did not (Siddi et al., 2017 ), other studies have found no evidence of this relationship (Berman et al., 1997 ) reporting a preserved capacity for cognitive flexibility in schizophrenia (Greenzang et al., 2007 ; Hilti et al., 2010 ). In this sense, Meiran et al. (Meiran et al., 2000 ) have proposed that the deficits in cognitive flexibility found in patients with schizophrenia (evaluated using task-switching paradigms (Allport et al., 1994 ) could reflect a poorer memory for remembering information from the context of the task rather than a deficit in cognitive flexibility. In their study, although the patients had a higher TSC TR , they were as efficient as controls when executing the task. To test this hypothesis regarding the difficulty to remember the keys that indicate change and their corresponding response, the authors evaluated healthy participants in conditions in which the information about the meaning of the response had to be acquired again on each trial. It was found that these participants showed a task-switching cost pattern similar to that of patients, suggesting that in patients with schizophrenia there could be a difficulty in remembering the instruction that signals the change in task, rather than dysfunction in the TSC.
Regarding the planning task (Tower of Hanoi), our patients only differed from the control group in terms of latency in the short planning trials. Still, they did not make more errors than the controls, suggesting a preserved ability, albeit with slower processing speed. A possible explanation for these results could be found in studies suggesting that cognitive deficits in schizophrenia may be mediated in part by a reduced processing speed that interferes with cognitive performance rather than by cognitive failure itself (Mathias et al., 2017 ; Rodríguez-Sánchez et al., 2007 ).
Alterations in hot EEFF
Regarding the most socio-emotional or hot EEFF, compared with the control group, the patients showed significantly poorer performance on two of the tasks studied: the recognition of facial emotional expressions and the task that evaluates the theory of mind (Hinting Task). These two processes—both the recognition of facial emotions and the recognition of intentions, emotions, and thoughts—are complementary processes that are necessary for adequate social functioning (Jáni & Kašpárek, 2018 ).
In our study, patients demonstrated a poor ability to identify and label facial emotions compared to controls; this was observed both for basic or innate facial expressions and those that are more complex. Therefore, our data suggest that patients with PS may present a marked deficit in identifying and categorizing emotions on the face. Although some studies have related these deficits more to negative symptoms than positive symptoms (Andrzejewska et al., 2017 ; Kohler et al., 2000 ), other studies have reported similar results. The latter found that in patients with PS, there was a generalized deficit in the perception of facial emotions, both in the earliest stages of the disease and in the more chronic stages, highlighting the possibility that this deterioration in the identification of emotions could represent a marker of trait susceptibility, rather than being a sequela of the disease (Barkl et al., 2014 ; Chan et al., 2010 ).
Mixed results can be found in the current scientific literature regarding the deficits that patients present in theory of mind (ToM). Some meta-analyses have found no clear affectation of ToM in patients with PS (Chan & Chen, 2011 ; Ventura et al., 2010 ), while other studies have found that patients show over mentalization in which an excessive and inaccurate attribution of mental state goes beyond the social cues provided (Abu-Akel, 1999 ; Fretland et al., 2015 ; Wastler & Lenzenweger, 2020 ). In a similar vein, the neurocognitive model developed by Frith (Frith, 2004 ) suggests that although patients with marked PS have an intact ToM in the sense of understanding that other people have mental states, they show poor performance due to difficulties in accurately monitoring and using contextual information, leading them to make incorrect inferences about the mental states of others. According to the model, these difficulties would lead to a breakdown in communication and eventually to a formal thought disorder and difficulties in distinguishing between subjectivity and objectivity, in addition to holding false beliefs in the form of delusional convictions. Our results, therefore, are in line with those studies that highlight ToM involvement in patients with a predominance of PS since, compared to the control group, our patients showed a significantly poorer ability to infer the true intention of indirect speech.
Regarding the clinical variables analyzed (years of evolution of the disease, clinical treatment device, and type of pharmacological treatment), we only found differences concerning the variable years of disease evolution . These differences were observed only in the planning task (Tower of Hanoi) of the cool , where patients with a short disease evolution (less than 11 years) made fewer errors in the short planning condition (less than five movements are required to complete the model) compared to the group with long disease evolution (more than 11 years). Subsequent analyzes with the control group revealed that patients with a short disease evolution showed similar performance to controls. This finding could suggest that, in patients with shorter disease evolution, the deficits in planning could be less severe or are more preserved in the earlier stages but deteriorates as the disease progresses, showing greater involvement.
Positive symptoms and hot and cool EEFF
Regarding the relationship between PS and performance on EEFF tasks, the Formal Thought Disorder symptom showed a significant correlation with performance on both cool and hot executive functions tasks. Specifically, this symptom was positively correlated with cognitive flexibility and planning and negatively correlated with ToM. The bizarre behavior symptom was only positively correlated with working memory, and the delusional symptom was negatively correlated with planning.
These results highlight the importance of EEFF of a more cognitive or cool type in PS, particularly in WM. Although we also found correlations with cognitive flexibility, and with planning, in this sense, it is also interesting to note that the correlation with planning was observed in the reaction time condition, which could suggest that in these patients, there is a marked decrease in the processing speed that could interfere with performance on the task (Mathias et al., 2017 ).
Regarding the correlation found between formal thought disorders and ToM, our results are in line with the suggestions of authors such as Frith (Frith, 2004 ) and Corcoran (Corcoran, 2004 ), where formal thought disorders, such as the use of neologisms, excessive use of pronominal referents, rigid thinking, and idiosyncratic speech, arise from not considering the state of knowledge of other people. These patients, therefore, do not recognize the difference between their state of knowledge about a subject and the state of knowledge of the other person. This difficulty in separating the two states of knowledge would thus be manifest in a significant failure of ToM.
Finally, it is worth highlighting our findings from the perspective of the three-dimensional model described by Liddle et al. (Liddle & Morris, 1991 ) In this model, the PS of schizophrenia include two different factors, one related to the distortion of reality ( hallucination symptoms and delusions ), and a disorganizing factor (e.g., formal thought disorder and bizarre behavior ). The disorganization symptoms are those that would present a stronger relationship with the neurocognitive deficits in comparison with distortion of reality symptoms (Cuesta & Peralta, 1995 ; Ventura et al., 2010 ). Similarly, in our study, disorganization symptoms were most strongly correlated with performance on both cool and hot executive EEFF tasks compared with distortion of reality symptoms. Therefore, these results could suggest that within the dimension of PS, there are two types of symptoms that differ in terms of cognitive functioning.
Frontal behavioral syndromes and positive symptoms
Concerning the issue of whether the patients with PS present any of the three frontal behavioral syndromes, we found that a large percentage of our patients presented a clinically significant score on the three syndromes. A high score (> 65) in the subscales that make up the FrSBe test is a robust indicator of behavioral abnormalities related to the frontal system (Grace & Malloy, 2001 ). Therefore, as we expected, our results point to a possible affectation of the three fronto-subcortical circuits in this population. A higher percentage of the patient group appeared to suffer from Dorsolateral syndrome (81.5%) and Anterior Cingulate syndrome (77.8%), while 59.3% also presented high scores for Orbitofrontal syndrome . Similar results were reported by Ruiz-Castañeda et al. (Ruiz-Castañeda et al., 2020 ) in patients with schizophrenia with a predominance of negative symptoms (see Appendix 1). In this study, a high percentage of patients with a predominance of negative symptoms also presented clinically significant scores for the three syndromes, particularly Dorsolateral syndrome (72.20%) and Anterior Cingulate syndrome (69.70%), while a lower percentage indicated the presence of Orbitofrontal syndrome (33.30%). This could suggest that in schizophrenia, patients also have a wide variety of behavioral abnormalities related to the involvement of the fronto-subcortical circuits.
Dorsolateral syndrome is mainly characterized by the presentation of problems in EEFF. Our patients, therefore, showed a wide variety of behaviors resulting from this syndrome, such as the difficulty to anticipate future events; the inability to use strategies to retain information and put it to proper use; in addition to difficulties when performing more than one task at the same time. Our patients also showed difficulty in self-reflection and monitoring of their behavior along with an inability to adjust their behavior according to the feedback provided by other people.
Regarding Anterior Cingulate syndrome, our patients presented behaviors related to poor initiation, psychomotor retardation, persistence, loss of energy and interest, personal hygiene problems, and apathetic behaviors. Regarding the Orbitofrontal syndrome, a part of our sample reported an inability to inhibit actions or behaviors appropriately; these patients reported impulsive, hyperactive, and socially inappropriate behaviors, as well as a difficulty to modulate their emotional states, presenting poor emotional control including emotional lability or irritability.
Implications and conclusions
The main findings of our study, following our proposed objectives, are described below. First, patients with a predominance of PS in schizophrenia presented specific deficits in cool and hot EEFF in comparison with healthy controls. The patients showed poorer performance on all the cool EEFF explored (WM, cognitive flexibility, and planning), with a larger effect size observed in WM. Regarding the hot EEFF, they showed worse performance in recognition of emotions and ToM. However, our patients did not show differences in the Iowa Gambling Task that assesses decision-making under conditions of uncertainty. Performance on this task has been consistently implicated in adequate functioning of the orbitofrontal area of the brain. In this sense, it is interesting to note that compared to the Dorsolateral and Anterior Cingulate syndrome, a lower percentage of our patients showed clinically significant behaviors associated with Orbitofrontal syndrome; therefore, a possible explanation for our results could be the conservation of this brain area in our sample of patients.
Regarding the influence of clinical variables , patients with a short disease evolution showed better execution of planning than patients with a long evolution. No difference was observed in the execution of the tasks depending on the type of clinical device to which the patients belonged or the psychopharmacological treatment.
Regarding the relationships between PS and poor performance in executive functioning, it was the formal thought disorder symptom that showed a significant correlation with performance on both cool and hot EEFF tasks. Specifically, this symptom correlated with cognitive flexibility, planning, and ToM. The bizarre behavior symptom only correlated with working memory, while both hallucinations and delusions were related to planning.
Concerning the three frontal behavioral syndromes (Dorsolateral, Orbitofrontal, and Anterior Cingulate), we found that a high percentage of our patients presented all three syndromes, the most prevalent being Dorsolateral syndrome (81.5%), followed by Anterior Cingulate (77.8%), and Orbitofrontal syndrome (59.3%).
Finally, we consider that our findings make a significant contribution to the literature in several ways:
There is a scarcity of studies in the literature that explore EEFF in patients with schizophrenia distinguished according to the predominance of positive versus negative symptoms. This approach offers the advantage of analyzing more precisely the relationship between clinical symptoms and EEFF, avoiding the rigidity implied by a nosological classification of schizophrenic disorder.
A further contribution of this work comes from our attempt to explore in more depth the EEFF in patients with schizophrenia by analyzing both the cool and hot components. The advantage of adopting this perspective is that it allows us to take a finer approach to determining the neuropsychological involvement in the functions studied, which will inform the development of appropriate neuropsychological and psychotherapeutic interventions for this patient population.
Another noteworthy aspect of this study is the measurement instruments used. We have employed a battery of computerized neuropsychological tasks based on experimental paradigms developed within cognitive neuroscience. These evaluative instruments allow us to obtain valid and precise measurements of the patient's performance under study. They also allow the study to be replicated with other populations for comparison of results.
Finally, another important aspect to emphasize is the involvement of fronto-subcortical circuits in patients with PS. Studies of other populations have reported that these circuits are altered in, for example, patients with brain damage. However, to our knowledge, this is the first study to explore the links between behavioral abnormalities related to the frontal system and the PS of schizophrenia.
Limitations
This study must be viewed in light of several limitations. First, a small sample was used, which could reduce the statistical power of our study. Second, the study was not carried out using the blind method because the recruitment and subsequent evaluation of the patients were carried out in the hospital context, so the evaluator knew the clinical characteristics of the participant. However, and to have greater control over the presentation of stimuli and the collection of responses and thus minimize the influence of evaluator biases, the study used an extensive battery of computerized neuropsychological tests to evaluate both hot and cool executive functions.
Finally, regarding the clinical variable of pharmacological treatment, the sample was not divided according to an estimate based on chlorpromazine equivalents. And although we found no differences in performance on the EEFF tasks according to the medication they were taking at the time of the evaluation ((1) medicated patients vs. patients without medication; (2) typical and atypical vs atypical/without medication), our results should be interpreted with caution, since some studies have highlighted the possible beneficial effects of atypical medications on general cognitive functioning (Buchanan et al., 1994 ; Meltzer & McGurk, 1999 ; Purdon et al., 2000 ). However, according to Harvey et al. (Harvey & Keefe, 2001 ), some of these studies used poor methodologies, and their results should be regarded as preliminary, requiring replication in further studies conducted with higher methodological standards.
Availability of data materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abu-Akel, A. (1999). Impaired theory of mind in schizophrenia. Pragmatics & CognitionPragmatics and Cognition, 7 (2), 247–282. https://doi.org/10.1075/pc.7.2.02abu
Article Google Scholar
Addington, J., & Addington, D. (2000). Neurocognitive and social functioning in schizophrenia: A 2.5 year follow-up study. Schizophrenia Research, 44 (1), 47–56. https://doi.org/10.1016/S0920-9964(99)00160-7
Article PubMed Google Scholar
Addington, J., Addington, D., & Maticka-Tyndale, E. (1991). Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophrenia Research, 5 (2), 123–134. https://doi.org/10.1016/0920-9964(91)90039-T
Alexander, G. E., Delong, M. R., & Strick, P. L. (1986). Parallel organisation of functionally separate circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9 (1), 357–381. https://doi.org/10.1146/annurev.ne.09.030186.002041
Allport, D. A., Styles, E. A., & Hsieh, S. (1994). Shifting intentional set: Exploring the dynamic control of tasks. In C. Umilta & M. Moscovitch (Eds.), Attention and Performance IV (pp. 421–452). MIT Press.
Google Scholar
Amodio, D. M., & Frith, C. D. (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7 (4), 268–277. https://doi.org/10.1038/nrn1884
Ancín, I., Cabranes, J. A., Santos, J. L., Sánchez-Morla, E., & Barabash, A. (2013). Executive deficits: A continuum schizophrenia-bipolar disorder or specific to schizophrenia? Journal of Psychiatric Research, 47 (11), 1564–1571. https://doi.org/10.1016/j.jpsychires.2013.07.008
Andreasen, N. C. (1984). Scale for the Assessment of Positive Symptoms (SAPS). British Journal of Psychiatry, 4 , 49–58. https://doi.org/10.1093/clinchem/22.4.528
Andrzejewska, M., Wójciak, P., Domowicz, K., & Rybakowski, J. (2017). Emotion recognition and theory of mind in chronicschizophrenia: Association with negative symptoms. Archives of Psychiatry and Psychotherapy, 19 (4), 7–12.
Baars, B. J., & Gage, N. M. (2010). Cognition, brain, and consciousness: Introduction to cognitive neuroscience . Elsevier/Academic Press. https://doi.org/10.1016/C2009-0-01556-6
Barkl, S. J., Lah, S., Harris, A. W. F., & Williams, L. M. (2014). Facial emotion identification in early-onset and first-episode psychosis: A systematic review with meta-analysis. Schizophrenia Research, 159 (1), 62–69. https://doi.org/10.1016/j.schres.2014.07.049
Baron-Cohen, S., Wheelwright, S., and Jolliffe, T. (1997). Is there a “language of the eyes”? Evidence from normal adults, and adults with autism or Asperger Syndrome. Visual Cognition , 4 , 311–331. https://doi.org/10.1080/713756761
Bechara, A., Damasio, A., Damasio, H., & Anderson, S. (1994). Insensitivity to furtur consequences following damage to human prefrontal cortex. Cognition, 50 (1–3), 7–15. https://doi.org/10.1016/0010-0277(94)90018-3
Beck, P. R., & Chaudhari, A. K. R. (1976). Effect of tobramycin on urinary γ-glutamyltransferase activity: Studies in a case of renal carcinoma. Clinical Chemistry, 22 (4), 528–531. https://doi.org/10.1093/clinchem/22.4.528
Bentall, R. P., & Slade, P. D. (1985). Reality testing and auditory hallucinations: A signal detection analysis. British Journal of Clinical Psychology, 24 (3), 159–169. https://doi.org/10.1111/j.2044-8260.1985.tb01331.x
Berenbaum, H., Kerns, J. G., Vernon, L. L., & Gomez, J. J. (2008). Cognitive correlates of schizophrenia signs and symptoms: III Hallucinations and Delusions. Psychiatry Research, 159 (1–2), 163–166. https://doi.org/10.1016/j.psychres.2007.08.017
Article PubMed PubMed Central Google Scholar
Berman, I., Viegner, B., Merson, A., Allan, E., Pappas, D., & Green, A. I. (1997). Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophrenia Research, 25 (1), 1–10. https://doi.org/10.1016/S0920-9964(96)00098-9
Berrios, G. E. (2013). Neuropsiquiatría del daño cerebral. Revista de Neuro-Psiquiatria, 59 (1–2), 41–56.
Bobes García, J., & Saiz Ruiz, J. (2013). El estigma social. In Impacto social de la esquizofrenia (Vol. 1, Issue 1). Editorial Glosa, S.L.
Bolt, L. K., Amminger, G. P., Farhall, J., McGorry, P. D., Nelson, B., Markulev, C., Yuen, H. P., Schäfer, M. R., Mossaheb, N., Schlögelhofer, M., Smesny, S., Hickie, I. B., Berger, G. E., Chen, E. Y. H., de Haan, L., Nieman, D. H., Nordentoft, M., Riecher-Rössler, A., Verma, S., & Allott, K. A. (2019). Neurocognition as a predictor of transition to psychotic disorder and functional outcomes in ultra-high risk participants: Findings from the NEURAPRO randomized clinical trial. Schizophrenia Research, 206 , 67–74. https://doi.org/10.1016/j.schres.2018.12.013
Bonelli, R. M., & Cummings, J. L. (2007). Frontal-subcortical circuitry and behavior. Dialogues in Clinical Neuroscience, 9 (2), 141–151. https://doi.org/10.1001/archneur.1993.00540080076020
Borys, S. V., Spitz, H. H., & Dorans, B. A. (1982). Tower of Hanoi performance of retarded young adults and nonretarded children as a function of solution length and goal state. Journal of Experimental Child Psychology, 33 (1), 87–110. https://doi.org/10.1016/0022-0965(82)90008-X
Brazo, P., Marié, R., Halbecq, I., Benali, K., Segard, L., Delamillieure, P., Langlois-Théry, S., Van Der Elst, A., Thibaut, F., Petit, M., & Dollfus, S. (2002). Cognitive patterns in subtypes of schizophrenia. European Psychiatry, 17 (3), 155–162. https://doi.org/10.1016/S0924-9338(02)00648-X
Bruder, G. E., Alschuler, D. M., Kroppmann, C. J., Fekri, S., Gil, R. B., Jarskog, L. F., Harkavy-Friedman, J. M., Goetz, R., Kayser, J., & Wexler, B. E. (2011). Heterogeneity of auditory verbal working memory in schizophrenia. Journal of Abnormal Psychology, 120 (1), 88–97. https://doi.org/10.1037/a0021661
Buchanan, R. W., Holstein, C., & Breier, A. (1994). The comparative efficacy and long-term effect of clozapine treatment on neuropsychological test performance. Biological Psychiatry, 36 (11), 717–725. https://doi.org/10.1016/0006-3223(94)90082-5
Chan, K. K. S., & Chen, E. Y. H. (2011). Theory of mind and paranoia in schizophrenia: A game theoretical investigation framework. Cognitive Neuropsychiatry, 16 (6), 505–529. https://doi.org/10.1080/13546805.2011.561576
Chan, R. C. K., Li, H., Cheung, E. F. C., Gong, Q., & yong. (2010). Impaired facial emotion perception in schizophrenia: A meta-analysis. Psychiatry Research, 178 (2), 381–390. https://doi.org/10.1016/j.psychres.2009.03.035
Clark, L. K., Warman, D., & Lysaker, P. H. (2010). The relationships between schizophrenia symptom dimensions and executive functioning components. Schizophrenia Research, 124 (1–3), 169–175. https://doi.org/10.1016/j.schres.2010.08.004
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Lawrence Earlbaum Associates. In Lawrence Earlbaum Associates.
Corcoran, R. (2004). Theory of mind and schizophrenia. In Social cognition and schizophrenia. (pp. 149–174). American Psychological Association. https://doi.org/10.1037/10407-005
Corcoran, R., Mercer, G., & Frith, C. D. (1995). Schizophrenia, symptomatology and social inference: Investigating “theory of mind” in people with schizophrenia. Schizophrenia Research, 17 (1), 5–13. https://doi.org/10.1016/0920-9964(95)00024-G
Cuesta, M. J., & Peralta, V. (1995). Cognitive disorders in the positive, negative, and disorganization syndromes of schizophrenia. Psychiatry Research, 58 (3), 227–235. https://doi.org/10.1016/0165-1781(95)02712-6
Damasio, A. R. (1994). Descartes’ error and the future of human life. Scientific American, 271 (4), 144.
Díaz-Caneja, C. M., Cervilla, J. A., Haro, J. M., Arango, C., & de Portugal, E. (2019). Cognition and functionality in delusional disorder. European Psychiatry, 55 , 52–60. https://doi.org/10.1016/j.eurpsy.2018.09.010
Eslami, A., Jahshan, C., & Cadenhead, K. S. (2011). Disorganized Symptoms and Executive Functioning Predict Impaired Social Functioning in Subjects at Risk for Psychosis. In The Journal of Neuropsychiatry and Clinical Neurosciences (Vol. 23).
Evans, C. E. Y., Bowman, C. H., & Turnbull, O. H. (2005). Subjective awareness on the Iowa Gambling Task: The key role of emotional experience in schizophrenia. Journal of Clinical and Experimental Neuropsychology, 27 (6), 656–664. https://doi.org/10.1081/13803390490918354
Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods . https://doi.org/10.3758/BRM.41.4.1149
Fletcher, P. C. (2001). Frontal lobes and human memory: Insights from functional neuroimaging. Brain, 124 (5), 849–881. https://doi.org/10.1093/brain/124.5.849
Fonseca-Pedrero, E., Paino, M., & Fraguas, D. (2013). DSM-5: ¿Síndrome de psicosis atenuada? Papeles Del Psicologo, 34 (3), 190–207. https://doi.org/10.1016/S0920-9964(01)00238-9
Forbes, N. F., Carrick, L. A., McIntosh, A. M., & Lawrie, S. M. (2009). Working memory in schizophrenia: A meta-analysis. Psychological Medicine, 39 (6), 889–905. https://doi.org/10.1017/S0033291708004558
Fornito, A., Zalesky, A., Pantelis, C., & Bullmore, E. T. (2012). Schizophrenia, neuroimaging and connectomics. In NeuroImage (Vol. 62, Issue 4, pp. 2296–2314). Academic Press. https://doi.org/10.1016/j.neuroimage.2011.12.090
Fretland, R. A., Andersson, S., Sundet, K., Andreassen, O. A., Melle, I., & Vaskinn, A. (2015). Theory of mind in schizophrenia: Error types and associations with symptoms. Schizophrenia Research, 162 (1–3), 42–46. https://doi.org/10.1016/j.schres.2015.01.024
Frith, C. D. (2004). Schizophrenia and theory of mind. In Psychological medicine (Vol. 34, Issue 3, pp. 385–389). https://doi.org/10.1017/S0033291703001326
Fujii, D., & Ahmed, I. (2002). Psychotic disorder following traumatic brain injury: A conceptual framework. Cognitive Neuropsychiatry, 7 (1), 41–62. https://doi.org/10.1080/135468000143000131
Gil, D., Fernández-Modamio, M., Bengochea, R., & Arrieta, M. (2012). Adaptación al español de la prueba de teoría de la mente Hinting Task. Revista De Psiquiatria y Salud Mental, 5 (2), 79–88. https://doi.org/10.1016/j.rpsm.2011.11.004
Gisselgård, J., Anda, L. G., Brønnick, K., Langeveld, J., Ten Velden Hegelstad, W., Joa, I., Johannessen, J. O., & Larsen, T. K. (2014). Verbal working memory deficits predict levels of auditory hallucination in first-episode psychosis. Schizophrenia Research, 153 (1–3), 38–41. https://doi.org/10.1016/j.schres.2013.12.018
Goldman-Rakic, P. S. (1994). Dysfunction Schizophrenia. American Psychiatric Press, 6 (4), 348–357.
Grace, J., & Malloy, P. (2001). Systems Behavior Scale (FrSBe): professional manual (Lutz, FL:).
Green, M. F. (1996). What are the functional consequences of neurocognitive deficits in schizophrenia? In American journal of psychiatry (Vol. 153, Issue 3, pp. 321–330). https://doi.org/10.1176/ajp.153.3.321
Greenzang, C., Manoach, D. S., Goff, D. C., & Barton, J. J. S. (2007). Task-switching in schizophrenia: Active switching costs and passive carry-over effects in an antisaccade paradigm. Experimental Brain Research, 181 (3), 493–502. https://doi.org/10.1007/s00221-007-0946-8
Green, M., Horan, W., & Lee, J. (2019). Nonsocial and social cognition in schizophrenia: current evidence and future directions. In World psychiatry (Vol. 18, Issue 2, pp. 146–161). John Wiley & Sons, Ltd. https://doi.org/10.1002/wps.20624
Harvey, P. D., & Keefe, R. S. E. (2001). Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. In American journal of psychiatry (Vol. 158, Issue 2, pp. 176–184). American Psychiatric Publishing. https://doi.org/10.1176/appi.ajp.158.2.176
Hilti, C. C., Delko, T., Orosz, A. T., Thomann, K., Ludewig, S., Geyer, M. A., Vollenweider, F. X., Feldon, J., & Cattapan-Ludewig, K. (2010). Sustained attention and planning deficits but intact attentional set-shifting in neuroleptic-naïve first-episode schizophrenia patients. Neuropsychobiology, 61 (2), 79–86. https://doi.org/10.1159/000265133
Holmén, A., Juuhl-Langseth, M., Thormodsen, R., Ueland, T., Agartz, I., Sundet, K., Andreassen, O. A., Rund, B. R., & Melle, I. (2012). Executive function in early- and adult onset schizophrenia. Schizophrenia Research, 142 (1–3), 177–182. https://doi.org/10.1016/j.schres.2012.10.006
Ibanez-Casas, I., De Portugal, E., Gonzalez, N., McKenney, K. A., Haro, J. M., Usall, J., Perez-Garcia, M., & Cervilla, J. A. (2013). Deficits in executive and memory processes in delusional disorder: A case-control study. PLoS ONE, 8 (7), 67341. https://doi.org/10.1371/journal.pone.0067341
Ilzarbe, D., Baeza, I., de la Serna, E., Fortea, A., Valli, I., Puig, O., Masias, M., Borras, R., Pariente, J. C., Dolz, M., Castro-Fornieles, J., & Sugranyes, G. (2021). Theory of mind performance and prefrontal connectivity in adolescents at clinical high risk for psychosis. Developmental Cognitive Neuroscience, 48 , 100940. https://doi.org/10.1016/j.dcn.2021.100940
Jáni, M., & Kašpárek, T. (2018). Emotion recognition and theory of mind in schizophrenia: A meta-analysis of neuroimaging studies. World Journal of Biological Psychiatry, 19 (sup3), S86–S96. https://doi.org/10.1080/15622975.2017.1324176
Jenkins, L. M., Bodapati, A. S., Sharma, R. P., & Rosen, C. (2018). Working memory predicts presence of auditory verbal hallucinations in schizophrenia and bipolar disorder with psychosis. Journal of Clinical and Experimental Neuropsychology, 40 (1), 84–94. https://doi.org/10.1080/13803395.2017.1321106
Kim, S., & Lee, D. (2012). Corteza prefrontal y toma de decisiones impulsiva. Psiquiatría Biológica, 19 (2), 54–61. https://doi.org/10.1016/J.PSIQ.2012.05.001
Kohler, C. G., Bilker, W., Hagendoorn, M., Gur, R. E., & Gur, R. C. (2000). Emotion recognition deficit in schizophrenia: Association with symptomatology and cognition. Biological Psychiatry, 48 (2), 127–136. https://doi.org/10.1016/S0006-3223(00)00847-7
Laloyaux, J., Della Libera, C., & Larøi, F. (2018). Source flexibility in schizophrenia: Specificity and role in auditory hallucinations. Cognitive Neuropsychiatry, 23 (6), 393–407. https://doi.org/10.1080/13546805.2018.1530648
Laplante, L., Everett, J., & Thomas, J. (1992). Inhibition through negative priming with Stroop stimuli in schizophrenia. British Journal of Clinical Psychology, 31 (3), 307–326. https://doi.org/10.1111/j.2044-8260.1992.tb00998.x
Liddle, P. F., & Morris, D. L. (1991). Schizophrenic syndromes and frontal lobe performance. British Journal of Psychiatry, 158 (Mar), 340–345. https://doi.org/10.1192/bjp.158.3.340
Li, X., Hu, D., Deng, W., Tao, Q., Hu, Y., Yang, X., Wang, Z., Tao, R., Yang, L., & Zhang, X. (2017). Pragmatic ability deficit in schizophrenia and associated theory of mind and executive function. Frontiers in Psychology . https://doi.org/10.3389/fpsyg.2017.02164
Mathias, S. R., Knowles, E. E. M., Barrett, J., Leach, O., Buccheri, S., Beetham, T., Blangero, J., Poldrack, R. A., & Glahn, D. C. (2017). The processing-speed impairment in psychosis is more than just accelerated aging. Schizophrenia Bulletin, 43 (4), 814–823. https://doi.org/10.1093/schbul/sbw168
McGrath, J., Scheldt, S., Hengstberger, P., & Dark, F. (1997). Thought disorder and executive ability. Cognitive Neuropsychiatry, 2 (4), 303–314. https://doi.org/10.1080/135468097396306
Meiran, N., Levine, J., Meiran, N., & Henik, A. (2000). Task set switching in schizophrenia. Neuropsychology, 14 (3), 471–482. https://doi.org/10.1037/0894-4105.14.3.471
Meltzer, H. Y., & McGurk, S. R. (1999). The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. In Schizophrenia bulletin (Vol. 25, Issue 2, pp. 233–255). DHHS Public Health Service. https://doi.org/10.1093/oxfordjournals.schbul.a033376
Menon, V., Anagnoson, R. T., Mathalon, D. H., Glover, G. H., & Pfefferbaum, A. (2001). Functional neuroanatomy of auditory working memory in schizophrenia: Relation to positive and negative symptoms. NeuroImage, 13 (3), 433–446. https://doi.org/10.1006/nimg.2000.0699
Mingrone, C., Rocca, P., Castagna, F., Montemagni, C., Sigaudo, M., Scalese, M., Rocca, G., & Bogetto, F. (2013). Insight in stable schizophrenia: Relations with psychopathology and cognition. Comprehensive Psychiatry, 54 (5), 484–492. https://doi.org/10.1016/j.comppsych.2012.12.014
Miyake, A., & Friedman, N. (2013). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21 (1), 8–14. https://doi.org/10.1177/0963721411429458.The
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology, 41 (1), 49–100. https://doi.org/10.1006/cogp.1999.0734
Molina, J., & Tsuang, M. T. (2020). Neurocognition and treatment outcomes in schizophrenia. Schizophrenia Treatment Outcomes . https://doi.org/10.1007/978-3-030-19847-3_5
Nieuwenstein, M. R., Aleman, A., & De Haan, E. H. F. (2001). Relationship between symptom dimensions and neurocognitive functioning in schizophrenia: A meta-analysis of WCST and CPT studies. Journal of Psychiatric Research, 35 (2), 119–125. https://doi.org/10.1016/S0022-3956(01)00014-0
Pantelis, C., & Brewer, W. (1995). Neuropsychological and olfactory dysfunction in schizophrenia: Relationship of frontal syndromes to syndromes of schizophrenia. Schizophrenia Research, 17 (1), 35–45.
Pedersen, A., Göder, R., Tomczyk, S., & Ohrmann, P. (2017). Risky decision-making under risk in schizophrenia: A deliberate choice? Journal of Behavior Therapy and Experimental Psychiatry, 56 , 57–64. https://doi.org/10.1016/j.jbtep.2016.08.004
Pedrero-Pérez, E. J., Ruiz-Sánchez De León, J. M., Llanero-Luque, M., Rojo-Mota, G., Olivar-Arroyo, A., & Puerta-García, C. (2009). Sintomatología frontal en adictos a sustancias en tratamiento mediante la versión española de la escala de comportamiento frontal. Revista De Neurologia, 48 (12), 624–631.
Pedrero, E., Ruíz, J., Rojo, G., Llanero, M., Olivar, A., Bouso, J., & Puerta, C. (2009). Versión española del Cuestionario Disejecutivo (DEX-Sp): Propiedades psicométricas en adictos y población no clínica. Adicciones , 21 (2), 155–166. https://doi.org/10.20882/adicciones.243
Penadés, R., & Gastó, C. (2010). El tratamiento de rehabilitación neurocognitiva en la esquizofrenia. In El tratamiento de rehabilitación neurocognitiva en la esquizofrenia . Herder Editorial. https://doi.org/10.2307/j.ctvt9k0h6
Peterson, E., & Welsh, M. C. (2014). The development of hot and cool executive functions in childhood and adolescence: Are we getting warmer? In Handbook of executive functioning (pp. 45–65). Springer New York. https://doi.org/10.1007/978-1-4614-8106-5_4
Pettersson-Yeo, W., Allen, P., Benetti, S., McGuire, P., & Mechelli, A. (2011). Dysconnectivity in schizophrenia: Where are we now? Neuroscience and Biobehavioral Reviews, 35 (5), 1110–1124. https://doi.org/10.1016/j.neubiorev.2010.11.004
Peyroux, E., Prost, Z., Danset-Alexandre, C., Brenugat-Herne, L., Carteau-Martin, I., Gaudelus, B., Jantac, C., Attali, D., Amado, I., Graux, J., Houy-Durand, E., Plasse, J., & Franck, N. (2019). From “under” to “over” social cognition in schizophrenia: Is there distinct profiles of impairments according to negative and positive symptoms? Schizophrenia Research: Cognition , 15 (October 2018), 21–29. https://doi.org/10.1016/j.scog.2018.10.001
Prencipe, A., Kesek, A., Cohen, J., Lamm, C., Lewis, M. D., & Zelazo, P. D. (2011). Development of hot and cool executive function during the transition to adolescence. Journal of Experimental Child Psychology, 108 (3), 621–637. https://doi.org/10.1016/j.jecp.2010.09.008
Purdon, S. E., Jones, B. D. W., Stip, E., Labelle, A., Addington, D., David, S. R., Breier, A., & Tollefson, G. D. (2000). Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. Archives of General Psychiatry, 57 (3), 249–258. https://doi.org/10.1001/archpsyc.57.3.249
Rankin, P. M., & O’Carroll, P. J. (1995). Reality discrimination, reality monitoring and disposition towards hallucination. British Journal of Clinical Psychology, 34 (4), 517–528. https://doi.org/10.1111/j.2044-8260.1995.tb01486.x
Ritter, L. M., Meador-Woodruff, J. H., & Dalack, G. W. (2004). Neurocognitive measures of prefrontal cortical dysfunction in schizophrenia. Schizophrenia Research, 68 (1), 65–73. https://doi.org/10.1016/S0920-9964(03)00086-0
Rodríguez-Sánchez, J. M., Crespo-Facorro, B., González-Blanch, C., Pérez-Iglesias, R., & Vázquez-Barquero, J. L. (2007). Cognitive dysfunction in first-episode psychosis: The processing speed hypothesis. British Journal of Psychiatry, 191 (SUPPL. 51), 7–10. https://doi.org/10.1192/bjp.191.51.s107
Rogers, R. D., & Monsell, S. (1995). Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General, 124 (2), 207–231. https://doi.org/10.1037/0096-3445.124.2.207
Rossi, R., Zammit, S., Button, K. S., Munafò, M. R., Lewis, G., & David, A. S. (2016). Psychotic experiences and working memory: A population-based study using signal-detection analysis. PLoS ONE, 11 (4), 1–16. https://doi.org/10.1371/journal.pone.0153148
Ruiz-Castañeda, P., Santiago-Molina, E., Aguirre-Loaiza, H., & Daza González, M. T. (2020). “Cool” and “Hot” executive functions in patients with a predominance of negative schizophrenic symptoms. Frontiers in Psychology, 11 , 2942. https://doi.org/10.3389/fpsyg.2020.571271
Sachdev, P., Smith, J. S., & Cathcart, S. (2001). Schizophrenia-like psychosis following traumatic brain injury: A chart-based descriptive and case-control study. Psychological Medicine, 31 (2), 231–239. https://doi.org/10.1017/S0033291701003336
Sawada, K., Kanehara, A., Sakakibara, E., Eguchi, S., Tada, M., Satomura, Y., Suga, M., Koike, S., & Kasai, K. (2017). Identifying neurocognitive markers for outcome prediction of global functioning in individuals with first-episode and ultra-high-risk for psychosis. Psychiatry and Clinical Neurosciences, 71 (5), 318–327. https://doi.org/10.1111/pcn.12522
Seidman, L. J., & Mirsky, A. F. (2017). Evolving notions of schizophrenia as a developmental neurocognitive disorder. Journal of the International Neuropsychological Society, 23 , 881–892. https://doi.org/10.1017/S1355617717001114
Siddi, S., Petretto, D. R., Burrai, C., Scanu, R., Baita, A., Trincas, P., Trogu, E., Campus, L., Contu, A., & Preti, A. (2017). The role of set-shifting in auditory verbal hallucinations. Comprehensive Psychiatry, 74 , 162–172. https://doi.org/10.1016/j.comppsych.2017.01.011
Slachevsky Ch., A., Pérez J., C., Silva C., J., Orellana, G., Prenafeta, M. L., Alegria, P., & Peña G., M. (2005). Córtex prefrontal y trastornos del comportamiento: Modelos explicativos y métodos de evaluación. In Revista Chilena de Neuro-Psiquiatria (Vol. 43, Issue 2, pp. 109–121). https://doi.org/10.4067/s0717-92272005000200004
Spironelli, C., & Angrilli, A. (2015). Language-related gamma EEG frontal reduction is associated with positive symptoms in schizophrenia patients. Schizophrenia Research, 165 (1), 22–29. https://doi.org/10.1016/j.schres.2015.04.003
Sternberg, S. (1966). High-speed scanning in human memory. Science, 153 (3736), 652–654. https://doi.org/10.1126/science.153.3736.652
Struglia, F., Stratta, P., Gianfelice, D., Pacifico, R., Riccardi, I., & Rossi, A. (2011). Decision-making impairment in schizophrenia: Relationships with positive symptomatology. Neuroscience Letters, 502 (2), 80–83. https://doi.org/10.1016/j.neulet.2011.07.017
Subramaniam, V., Poongodi, G. R., & Veena Sindhuja, V. (2008). Textile scaffolds for tissue engineering. Journal of the Textile Association, 69 (4), 180–183. https://doi.org/10.1016/j.biopsych.2004.06.023
Tekin, S., & Cummings, J. (2002). Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. In Journal of psychosomatic research (Vol. 53, Issue 2, pp. 647–654). https://doi.org/10.1016/S0022-3999(02)00428-2
Ventura, J., Thames, A. D., Wood, R. C., Guzik, L. H., & Hellemann, G. S. (2010). Disorganization and reality distortion in Schizophrenia: A meta-analysis of the relationship between positive symptoms and neurocognitive deficits. Schizophrenia research . https://doi.org/10.1016/j.schres.2010.05.033
Walston, F., Blennerhassett, R. C., & Charlton, B. G. (2000). “Theory of mind”, persecutory delusions and the somatic marker mechanism. Cognitive Neuropsychiatry, 5 (3), 161–174. https://doi.org/10.1080/13546800050083511
Wang, L., Zou, F., Shao, Y., Ye, E., Jin, X., Tan, S., Hu, D., & Yang, Z. (2014). Disruptive changes of cerebellar functional connectivity with the default mode network in schizophrenia. Schizophrenia Research, 160 (1–3), 67–72. https://doi.org/10.1016/j.schres.2014.09.034
Wastler, H. M., & Lenzenweger, M. F. (2020). Cognitive and affective theory of mind in positive Schizotypy: Relationship to schizotypal traits and psychosocial functioning. Journal of Personality Disorders . https://doi.org/10.1521/pedi_2020_34_473
Welsh, M., & Peterson, E. (2014). Issues in the conceptualization and assessment of hot executive functions in childhood. Journal of the International Neuropsychological Society, 20 (2), 152–156. https://doi.org/10.1017/S1355617713001379
Wilder, K. E., Weinberger, D. R., & Goldberg, T. E. (1998). Operant conditioning and the orbitofrontal cortex in schizophrenic patients: Unexpected evidence for intact functioning. Schizophrenia Research, 30 (2), 169–174. https://doi.org/10.1016/S0920-9964(97)00135-7
Zakzanis, K. K. (1998). Neuropsychological correlates of positive vs. negative schizophrenic symptomatology. Schizophrenia Research, 29 (3), 227–233. https://doi.org/10.1016/S0920-9964(97)00102-3
Zelazo, P. D., & Carlson, S. M. (2012). Hot and cool executive function in childhood and adolescence: Development and plasticity. Child Development Perspectives, 6 (4), 354–360. https://doi.org/10.1111/j.1750-8606.2012.00246.x
Zelazo, P. D., & Mller, U. (2007). Executive function in typical and atypical development. In Blackwell handbook of childhood cognitive development (pp. 445–469). Blackwell Publishers Ltd. https://doi.org/10.1002/9780470996652.ch20
Zemánková, P., Lošák, J., Czekóová, K., Lungu, O., Jáni, M., Kašpárek, T., & Bareš, M. (2018). Theory of mind skills are related to resting-state frontolimbic connectivity in Schizophrenia. Brain Connectivity, 8 (6), 350–361. https://doi.org/10.1089/brain.2017.0563
Download references
Acknowledgements
We thank the patients belonging to the mental health facility of the Torrecárdenas Hospital, who kindly decided to participate in this investigation. We also thank the mental health professionals for their cooperation in the referral of patients for this study.
Significance statement
Executive function (EEFF) deficits in schizophrenia have been associated with a deterioration in the quality of life of patients that affects their ability to lead an independent and socially productive life. However, to date, there is no clarity on the specific deficits that patients with positive symptoms (PS) present in FFEE. One way to deepen their study is to analyze the cognitive and socio-emotional components of these functions through experimental paradigms of cognitive neuroscience. On the other hand, EEFF have been associated with the functioning of the prefrontal cortex, so it would be expected that these patients would present clinically significant scores in any of the three fronto-subcortical behavioral syndromes: Dorsolateral, Orbitofrontal, or Anterior Cingulate. We present the first study that addresses the specific deficits of cognitive and socio-emotional EEFF and the presence of fronto-subcortical behavioral syndromes in patients with schizophrenia with a predominance of PS. Our results suggest the presence of specific executive deficits, presenting a greater deterioration of the cognitive component of working memory, and of the socio-emotional components of facial expression recognition and theory of mind. Symptoms of “disorganization” are those that are more closely related to FFEE than symptoms of “distortion of reality.” Finally, we report the presence of the three fronto-subcortical behavioral syndromes in this population. It shows the importance of implementing neuropsychological treatments that consider specific aspects of the FFEE that affect the adaptation of the patient to their environment and help to improve her quality of life.
This research was supported by the program for publication in open access journals from the Research and Transfer Plan 2021 of the University of Almería.
Author information
Authors and affiliations.
Neuropsychological Evaluation and Rehabilitation Center (CERNEP), University of Almeria, Carretera de Sacramento, s / n. La Cañada de San Urbano. 04120, Almeria, Spain
Pamela Ruiz-Castañeda & María Teresa Daza González
Department of Psychology, University of Almeria Spain, Carretera de Sacramento, s /n. La Cañada de San Urbano. 04120, Almeria, Spain
Mental Health Hospitalization Unit of Torrecárdenas University Hospital, Calle Hermandad de Donantes de Sangre, s/n, 04009, Almería, Spain
Encarnación Santiago Molina
Department of Psychology, Catholic University of Pereira, Avenida Sur/Las Americas Cra 21 # 49-95, Pereira, Colombia
Haney Aguirre Loaiza
You can also search for this author in PubMed Google Scholar
Contributions
PR-C, MD, and ES-MS formulated the original idea and designed the experiments, interpreted the data, and wrote the first draft of the manuscript. HA-L and PR-C conducted the statistical analysis. PR-C, MD, ES-M, and HA-L approved the final manuscript. All the authors contributed to the article and approved the submitted version. All authors read and approved the final manuscript.
Corresponding author
Correspondence to María Teresa Daza González .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
See Fig. 4 .
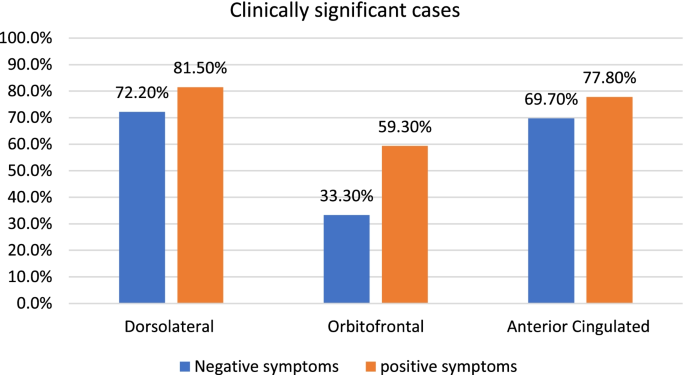
Percentage of clinically significant cases in fronto-subcortical syndromes in patients with positive and negative symptoms. The negative symptom scores have been adapted from the study of Ruiz-Castañeda et al. ( 2020 )
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ruiz-Castañeda, P., Santiago Molina, E., Aguirre Loaiza, H. et al. Positive symptoms of schizophrenia and their relationship with cognitive and emotional executive functions. Cogn. Research 7 , 78 (2022). https://doi.org/10.1186/s41235-022-00428-z
Download citation
Received : 02 August 2021
Accepted : 27 July 2022
Published : 12 August 2022
DOI : https://doi.org/10.1186/s41235-022-00428-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cognitive executive functions; Socio-emotional executive functions; Schizophrenia
- Positive symptoms
- Fronto-subcortical syndromes
Loading metrics
Open Access
Peer-reviewed
Research Article
A Systematic Review of the Prevalence of Schizophrenia
Affiliation Queensland Centre for Mental Health Research, The Park Centre for Mental Health, Wacol, Australia
Affiliations Queensland Centre for Mental Health Research, The Park Centre for Mental Health, Wacol, Australia, Department of Psychiatry, University of Queensland, St. Lucia, Australia
* To whom correspondence should be addressed. E-mail: [email protected]
- Sukanta Saha,
- David Chant,
- Joy Welham,
- John McGrath

- Published: May 31, 2005
- https://doi.org/10.1371/journal.pmed.0020141
- Reader Comments
Understanding the prevalence of schizophrenia has important implications for both health service planning and risk factor epidemiology. The aims of this review are to systematically identify and collate studies describing the prevalence of schizophrenia, to summarize the findings of these studies, and to explore selected factors that may influence prevalence estimates.
Methods and Findings
Studies with original data related to the prevalence of schizophrenia (published 1965–2002) were identified via searching electronic databases, reviewing citations, and writing to authors. These studies were divided into “core” studies, “migrant” studies, and studies based on “other special groups.” Between- and within-study filters were applied in order to identify discrete prevalence estimates. Cumulative plots of prevalence estimates were made and the distributions described when the underlying estimates were sorted according to prevalence type (point, period, lifetime, and lifetime morbid risk). Based on combined prevalence estimates, the influence of selected key variables was examined (sex, urbanicity, migrant status, country economic index, and study quality).
A total of 1,721 prevalence estimates from 188 studies were identified. These estimates were drawn from 46 countries, and were based on an estimated 154,140 potentially overlapping prevalent cases. We identified 132 core studies, 15 migrant studies, and 41 studies based on other special groups. The median values per 1,000 persons (10%–90% quantiles) for the distributions for point, period, lifetime, and lifetime morbid risk were 4.6 (1.9–10.0), 3.3 (1.3–8.2), 4.0 (1.6–12.1), and 7.2 (3.1–27.1), respectively. Based on combined prevalence estimates, we found no significant difference (a) between males and females, or (b) between urban, rural, and mixed sites. The prevalence of schizophrenia in migrants was higher compared to native-born individuals: the migrant-to-native-born ratio median (10%–90% quantile) was 1.8 (0.9–6.4). When sites were grouped by economic status, prevalence estimates from “least developed” countries were significantly lower than those from both “emerging” and “developed” sites ( p = 0.04). Studies that scored higher on a quality score had significantly higher prevalence estimates ( p = 0.02).
Conclusions
There is a wealth of data about the prevalence of schizophrenia. These gradients, and the variability found in prevalence estimate distributions, can provide direction for future hypothesis-driven research.
Citation: Saha S, Chant D, Welham J, McGrath J (2005) A Systematic Review of the Prevalence of Schizophrenia. PLoS Med 2(5): e141. https://doi.org/10.1371/journal.pmed.0020141
Academic Editor: Steven E. Hyman, Harvard University, United States of America
Received: February 15, 2005; Accepted: March 29, 2005; Published: May 31, 2005
Copyright: © 2005 Saha et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: LMR, lifetime morbid risk; NOS, not otherwise specified
Introduction
Schizophrenia is a disabling group of brain disorders characterized by symptoms such as hallucinations, delusions, disorganized communication, poor planning, reduced motivation, and blunted affect. While the incidence of the disorder is relatively low (median value 15.2 per 100,000 persons per year) [ 1 ], the condition is one of the major contributors to the global burden of disease [ 2 ]. The substantial burden of disease is a reflection of two features of schizophrenia: (a) the disorder usually has its onset in early adulthood, and (b) despite optimal treatment, approximately two-thirds of affected individuals have persisting or fluctuating symptoms [ 3 ].
Understanding the “epidemiological landscape” of schizophrenia requires many different types of descriptive studies [ 4 ]. Studies that estimate the incidence of schizophrenia are required in order to identify gradients across time and/or place. These gradients allow us to generate candidate risk factors that may underlie variations in the disorder. However, studies that report the prevalence of a disorder are also important. Estimating the proportion of a population affected with schizophrenia is central to health service planning. With respect to estimating the burden of disorder, prevalence proportions can provide insights into how incidence rates are refracted via different trajectories (e.g., recovery, chronicity, or early death). The statement “prevalence = incidence + course of illness” oversimplifies the dynamic matrix of factors influencing each component of the equation. Nevertheless, prevalence proportions can help us chart contours on the still-incomplete epidemiological map of schizophrenia.
Several scholarly narrative reviews of the prevalence of schizophrenia have been published in recent decades [ 4 – 8 ]. The sheer volume of data available on the prevalence of schizophrenia now requires a more systematic and orderly approach. As with many fields of medical knowledge, there is a growing appreciation that reviews should be based on data that are as complete and as free of bias as possible [ 9 ]. Systematic reviews have prespecified methods for locating studies and for extracting and synthesizing the data. Not all systematic reviews are accompanied by meta-analysis (i.e., pooling the data to provide one summary value) [ 10 ]. Even without pooling of data, the orderly sorting of data using meta-analytic techniques can provide useful insights into the structure of the relevant literature [ 11 ].
One systematic review of the prevalence of schizophrenia has been published to date [ 12 ]. This review (based solely on census and/or community survey data) identified 18 studies that provided estimates of either period and/or lifetime prevalence of schizophrenia. Goldner and colleagues reported pooled estimates for 1-y and lifetime prevalence of 3.4 and 5.5 per 1,000 persons, respectively. The authors commented on the heterogeneity of the data and suggested that this reflected “real variation” in the distribution of schizophrenia around the world.
Recently, we published a systematic review of the incidence of schizophrenia [ 1 ]. In brief, we found that the incidence of schizophrenia varied widely between sites (persons, media n = 15.2 per 100,000; 10%–90% quantiles = 7.7–43.0). In addition, the study identified that (a) males were more likely to develop schizophrenia than females (median male:female risk ratio = 1.4); (b) migrants were more likely to develop schizophrenia than native-born individuals (median risk ratio = 4.6); and (c) individuals in urban sites had a higher risk of developing schizophrenia than those in mixed urban/rural sites. Regardless of the factors that underpin these incidence gradients, would these same gradients also be found in the prevalence of schizophrenia? If so, then it might suggest, for example, that factors influencing the course of the illness were more evenly distributed across these groups than factors influencing the incidence of the disorder. If the prevalence gradients are not congruent with the incidence gradients, then we are faced with the challenging task of unraveling the factors that could influence the differential course of schizophrenia between risk groups.
In this paper we continue our cartography of the epidemiological landscape of schizophrenia by presenting a systematic review of the prevalence of this disorder.
Ways to Measure the Prevalence of Schizophrenia
Prevalence measures the proportion of individuals who manifest a disorder at a specified time, or during a specified period. Generally prevalence estimates are calculated as a proportion, by dividing the total number of individuals who manifest a disorder (the numerator) by the total population at risk, including those with the disorder (the denominator). Prevalence proportions vary according to temporal criteria (e.g., point, period, or lifetime), but are not reported as an index of events over time (i.e., they are not like incidence rates that report the number of new cases per background population per year). Prevalence proportions are often loosely referred to as “rates”; however, in this review we will refer to them as “prevalence estimates” or “estimates.” Tables S1 and S2 define the types of prevalence estimates used in this study, and provide descriptions of the variables that we have used to describe the studies.
Point prevalence is the proportion of individuals who manifest a disorder at a given point in time (e.g., 1 d or 1 wk), while period prevalence measures the proportion of individuals who manifest a disorder during a specified period of time (e.g., 1 y). Given that the course of schizophrenia extends over months to decades, estimates of point prevalence based on 1 d are comparable to those based on 1 mo [ 5 ]. Thus, in this review we have combined all estimates based on temporal criteria of 1 mo or less in “point prevalence,” while studies that reported prevalence estimates between 1 mo and 12 mo are included under the heading “period prevalence.”
“Lifetime prevalence” is the proportion of individuals in the population who have ever manifested a disorder, who are alive on a given day. It is important to emphasize that lifetime prevalence needs to be clearly distinguished from “lifetime morbid risk” (LMR; also described elsewhere as morbid risk or expectancy). LMR differs from lifetime prevalence in that it attempts to include the entire lifetime of a birth cohort both past and future, and includes those deceased at the time of the survey [ 13 ]. LMR is the probability of a person developing the disorder during a specified period of their life or up to a specified age. There are various ways to calculate LMR [ 14 , 15 ]. The reviews of Odegaard [ 15 ], and Larsson and Sjogren [ 16 ] noted that, for low-incidence disorders such as schizophrenia, summation of age-specific incidence rates gives almost the same result as other more complicated methods of calculation [ 17 ]. The World Health Organization ten-country study [ 18 ] used this so-called “summation method” for the approximation of LMR. If one were to apply Linnean principles in order to design a taxonomy of frequency measures of disease, prevalence measures such as point, period, and lifetime would be closely related species within the same genus. However, there is a case to allocate LMR to the Genus “Incidence” rather than the Genus “Prevalence.” Conceptually (but not mathematically), LMR is closely related to cumulative incidence proportions derived from birth cohort studies [ 19 ].
Traditional prevalence studies (henceforth referred to as “core” studies) generate an estimate based on the population residing within a defined catchment area. However, it should be noted that the boundaries chosen for epidemiological studies (e.g., health districts, cities, states, or nations) may not be optimal for the detection of variations of the disorder within or between various populations. Lumping populations into large but convenient administrative areas can obscure informative, fine-grained gradients. With respect to prevalence estimates, factors such as the age structure of the population, mortality rates, and migration patterns can influence the estimates, and these may vary within and between sites.
Apart from catchment-area-based studies of the general population, there are many studies that report prevalence estimates for subgroups of the population. These may include groups defined by narrow age strata (e.g., the elderly or children), migrant status, ethnic or religious status, or twin status, to name but a few. A recent paper has systematically reviewed the prevalence of schizophrenia in prison settings [ 20 ]; however, this will not be included in this review. Migrant studies will be collated separately for analysis , while the remaining subgroup prevalence estimates will be included in “other special groups.”
Some studies report inpatient census data over a period of time (e.g., 1 y) and use the count of unique individuals with schizophrenia to generate a proportion based on general population figures. While these studies may be useful for administrative purposes, it is important not to mistake these estimates as “true” prevalence proportions. Very few patients require prolonged and continuous inpatient care; therefore, prevalence proportions based on inpatient data alone grossly underestimate true prevalence proportions. This review will collate these studies separately (henceforth referred to as “inpatient-census-derived” data); however, they will not be included in any of the main analyses.
Key Research Questions about the Prevalence of Schizophrenia
First there is a need to examine the degree of variation in the prevalence estimates of schizophrenia between sites. The companion review on the incidence of schizophrenia [ 1 ] found that within the central 80% of incidence rates, the difference ranged from 7.7 to 43 per 100,000 (over a 5-fold difference). While there has been debate within the schizophrenia research community about whether this range of rates is “narrow” or “prominent” (see review [ 21 ]), variations in prevalence estimates have not been a focus of controversy. The World Health Organization ten-country study commented that the prognosis of schizophrenia [ 18 ] was better in developing than in developed nations, a finding that has been “clear and consistent” in general [ 22 ]. The present review will describe the distribution of the different types of prevalence rates, and specifically examine whether the “developed versus developing” status of the sites influences the distribution of estimates.
Are the gradients that were identified in the incidence of schizophrenia also reflected in the prevalence of the disorder? For example, based on the previous finding that males have a significantly higher incidence of schizophrenia [ 1 , 23 ], it would be predicted that this sex difference might also be reflected in prevalence estimates. In addition, a recent study from China [ 24 , 25 ] highlighted an apparently unusual higher prevalence of schizophrenia in females in this country. In light of this issue, the male:female prevalence ratio will also be compared when the sites are sorted by a measure of “developed versus developing” status. Similarly, the incidence review identified significantly higher rates for (a) urban place of residence when compared to mixed urban/rural sites, and (b) migrant groups when compared to native-born individuals. These gradients will also be explored regarding the prevalence of schizophrenia.
Finally, systematic reviews can explore possible sources of heterogeneity in data by sorting the data according to methodological features. We will compare the distributions of estimates based on the quality of the study (as assessed by design features and thoroughness of reporting).
Identification of Studies
This systematic review conforms to the guidelines outlined by the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) recommendations [ 26 ]. The search methodology for this review was identical to that of our previous review paper on incidence of schizophrenia [ 1 ]. As a first step, a broad (free text) search string ([schizo* OR psych*] AND [incidence OR prevalence]) was used in MEDLINE, PsychINFO, EMBASE, and LILACS. Potentially relevant papers (in all languages) were accessed in order to review the full text. The references cited by each potentially relevant paper, review, and book chapter were scrutinized in order to locate additional potential papers. Posters were presented at two international schizophrenia conferences [ 27 , 28 ] in order to encourage researchers to contribute studies, especially studies from the “grey literature” (e.g., conference reports, theses, government reports, and unpublished studies). Subsequently, letters or E-mails were sent to the senior authors of papers that met the inclusion criteria. These authors were provided with an interim list of included papers and asked to nominate missing studies.
Included Studies
We included studies that reported primary data on the prevalence of schizophrenia first published between January 1965 and December 2002. Where multiple publications presented identical data, the most “informative version” of the study was included. Studies published in a language other than English were translated, and relevant papers were included.
Excluded Studies
Studies that reported prevalence data on prison or forensic populations were excluded (see recent systematic review of these studies [ 20 ]). We did not include genetic epidemiological studies that reported prevalence estimates in family members of affected (index) probands. Some studies report the LMR within large, multiplex families. These were not included; however, if the prevalence estimates were based on the entire population within a catchment area (e.g., an isolated population living in a village), then they were included.
Potential studies that had not been located at the time of submission were allocated to the “awaiting assessment” category. Studies based on inpatient-census-derived proportions are presented in the tables and summarized for comparative purposes, but were excluded from the main analyses.
Data Extraction
Once a study was included, data were extracted and entered into a three-level normalized database (i.e., only the unique prevalence estimate identifier was allowed to occur in more than one level) that included study-level variables (e.g., authors, year of publication, and site), middle-level variables (e.g., urban/rural status, age group, recruitment duration, case finding method, and diagnostic criteria), and rate-level variables (e.g., sex-specific rates for persons, males, and females). Two or more of the authors checked all data used in the analysis. When disagreements arose, these were resolved by consensus. If required, we contacted the original authors for clarification of issues. The full electronic dataset is available as Dataset 1 .
Consistent with our previous systematic review of the incidence of schizophrenia [ 1 ], studies were given “quality points” based on operationalized features related to (a) optimal research design (e.g., higher scores for greater coverage, face-to-face interview versus chart diagnosis, and reliability of instruments), and (b) quality of reporting (e.g., provision of numerator and denominator, and description of diagnostic criteria). Details of the quality scores used in this review are provided in Table S3 .
Sorting Prevalence Estimates by the Application of Sequential Filters
In systematic reviews, it is important that individuals are not “double counted” by the same or different studies. Thus, a key feature of this study is the application of sequential filters in order to identify discrete prevalence estimates. We applied a similar sorting algorithm as in our previous review of incidence of schizophrenia [ 1 ]. Briefly, the first filter parsed prevalence estimates from the included studies into three groups: core, migrant, and other special groups. Next, as the second filter, the estimates were sorted into six main types: point (1 mo or less), period (between 1 and 12 mo), lifetime, LMR, not otherwise specified (NOS), and inpatient-census-derived data.
A third, study-level filter was applied in order to isolate discrete data from multiple studies that overlapped in both time and place. This third filter was used to select one representative prevalence estimate for inclusion in the cumulative distribution using the “most informative” rule. For example, if one study presented multiple overlapping estimates, the estimate based on the largest sample was preferred (e.g., the widest age range was preferred over narrower age strata). Furthermore, filter rules were defined in order to select discrete estimates such that they allowed the greatest number of estimates to be included.
Presentation and Analyses of the Data
Key details of the included studies are presented in tables sorted by country, year of publication and first author (Tables S4 , S5 , and S6 ). The distributions of prevalence estimates are presented in cumulative plots, with every estimate contributing to the distribution. The distribution of the data is shown in rank order for prevalence estimate (lowest to highest ranks) with the cumulative percent of estimates shown on the vertical axis. The plots show horizontal reference lines indicating the 50% (median), and 25% and 75% quantiles (between which lies the interquartile range). In order to aid visual interpretation, some plots have been truncated, excluding very high estimates. Key features of these distributions are presented in tables (e.g., median, mean, harmonic mean, standard deviation, and quantiles at 10%, 25%, 50%, 75%, and 90%). These summary characteristics are based on the entire distributions. Results are presented as prevalence estimates per 1,000. In plots of prevalence ratios (e.g., male:female ratio), a vertical reference at the line of unity is shown.
We wish to draw attention to several features of the graphs used in this review. First, the central, near-linear segment of the cumulative distributions may extend beyond the interquartile range (e.g., from the 10%–90% quantiles), thus shape features (where the tails start or the range of the linear central segment) can be more informative than traditional interquartile ranges. Second, steeper segments of the cumulative plots are underpinned by estimates that have a narrow distribution, while flatter (i.e., more horizontal) segments of the distribution are underpinned by data that are relatively more dispersed. Finally, some distributions are derived from more data than others. Regardless of slope (i.e., steep or flat), if many estimates underpin segments of the distributions, then inferences based on these segments are probably more reliable than those based on segments underpinned by less data.
Meta-analyses often display data points with confidence intervals, and formal tests of heterogeneity are usually applied before combining data. For several reasons, the data in this review do not lend themselves to this type of analysis. Among the discrete core studies (see below), no study provided confidence limits to accompany the prevalence estimate. One study, which was allocated to “other special groups,” did provide confidence limits [ 29 ]. Where studies provided the corresponding numerator and denominator for a prevalence estimate, we were able to derive standard errors. However, we were able to impute standard errors for only 26% of the prevalence estimates, which were drawn from less than half (45%) of the discrete core studies. Faced with such a restricted pool of standard errors, the ability to assess the heterogeneity of the estimates in a manner generalizable across all core studies is compromised. In addition, the issues that underlie the decision to combine data from randomized controlled trials or risk factor epidemiological studies are of less relevance to prevalence estimates, where estimates based on very large populations should not necessarily carry more weight than estimates based on small populations. Based on first principles, there is no reason to assume that prevalence estimates for a disease remain static across time or place. Thus, forcing individual prevalence estimates into one pooled estimate loses important information. In this review we wish to draw attention to several characteristics of the distribution of estimates (e.g., central tendency, shape and width of the distribution, and density of data), rather than provide one pooled estimate.
In keeping with our systematic review of the incidence of schizophrenia [ 1 ], we supplement the graphical presentation of the prevalence estimates with statistical analyses. These analyses take into account (a) the need to control for within-study variation (estimates drawn from the same study tend to be more alike than estimates drawn from different studies), and (b) the use of a log transformation of the data in order to analyze distributions that are often positively skewed. Note that the median value is more informative than the arithmetic mean to assess central tendency in a skewed distribution, as is the harmonic mean (which is calculated as the exponential of the arithmetic mean of the log-transformed data, also known as the geometric mean). The analyses were carried out in SAS 9.1 using proc univariate (for medians and other quantiles of the raw data) and proc mixed for comparisons of harmonic means (because one study may provide more than one estimate, it is important to control for within-study variation).
Faced with a large quantity of data, systematic reviewers need to keep a tight rein on the number of comparisons undertaken on the data [ 30 ]. While it is tempting to reanalyze data in the light of findings that emerge from the data, such reanalyses should be kept to a minimum. The analysis of prevalence estimates is particularly challenging because of the many different prevalence types (e.g., point, period, lifetime, and LMR). Thus, in order to minimize the number of statistical comparisons in the current review, we restricted the analyses to a limited set of planned sensitivity analyses, each with a priori directional hypotheses, and, for post hoc analyses, applied multiple comparison corrections to the nominal significance levels by a Bonferroni correction. Furthermore, these analyses were based on hybrid distributions, which merged four different prevalence estimate types (point, period, lifetime, and NOS; henceforth referred to as “combined prevalence estimates”). Apart from the specific analyses related to sex differences, we undertook these analyses on distributions for persons only (i.e., males and females combined).
Based on first principles, we predicted that the estimates for known prevalence types that include different temporal criteria would be significantly different. More specifically, we predicted the following: (a) prevalence estimates for persons would differ between lifetime, period and point (point being the lowest), and (b) LMR estimates would be higher than lifetime estimates.
There is now strong evidence that males have an increased risk of developing schizophrenia [ 1 , 23 ]. We compared the distribution for males versus females on the combined prevalence estimates, predicting that males would have distributions derived from higher estimates (i.e., distributions for males would be right-shifted compared to distributions for females).
In order to explore the influence of urbanicity of site on the prevalence of schizophrenia, we divided the combined prevalence estimates for persons into three categories (urban, rural, and mixed urban/rural). Allocation was based on the study descriptions of the area or, in the absence of these descriptors, the review authors' best estimate of this variable. There are several reasons to predict that the prevalence of schizophrenia would be higher in urban regions than in rural regions. First, the incidence of schizophrenia is higher in urban sites than mixed urban/rural sites [ 1 ]. Second, the “social drift” hypothesis suggests that the individuals with schizophrenia are more likely to move into urban regions in response to various factors related to poverty, the availability of services, and easier access to cheap accommodation [ 31 ]. Finally, some commentators suggest that less industrialized settings (e.g., rural regions and/or developing countries) may facilitate recovery via social connectedness and easier access to work [ 32 ]. Thus, we predicted that the prevalence of schizophrenia would be higher in urban sites than in rural or mixed urban/rural.
Migrants have a significantly increased risk of developing schizophrenia [ 1 , 33 ]. Assuming that the course of the illness does not vary according to migrant status, based on combined prevalence estimates for persons, we predicted that the prevalence of schizophrenia would be higher in migrants than in native-born individuals.
While there is a lack of evidence addressing whether the incidence of schizophrenia varies with the economic status of nations, there is solid evidence showing that people with schizophrenia from developing countries tend to have better outcomes than individuals in developed nations [ 18 , 22 ]. Mindful that there is a lack of consensus on how best to define the multidimensional concept of economic development, we have sorted prevalence estimates according to the per capita gross national product of the study site (2004 data) [ 34 ], and used standard World Bank definitions [ 35 ]: (a) least developed countries, = mean income of less than US$2,995; (b) emerging economy countries, = mean income between US$2,995 and $9,266; and (c) developed countries, = mean income of greater than US$9,266. Thus, based on combined prevalence estimates for persons, we predicted that the prevalence of schizophrenia would be significantly different across the three economic categories, and that the prevalence of schizophrenia would be significantly lower in least developed countries than in developed countries. Furthermore, a recent commentary drew attention to the apparent female excess in the prevalence of schizophrenia in developing nations, in contrast to the male excess thought to characterize the developed world [ 25 ]. Thus, based on combined prevalence estimates, we compared the male:female ratio when the prevalence estimates were classified by the three economic categories. We predicted that the ratio would be significantly different between the three economic levels, and specifically, that the male:female ratio in developed nations would be significantly higher than that of least developed countries.
Finally, methodological features can influence prevalence estimates. For example, studies that use comprehensive case ascertainment methods (e.g., “door-knock” surveys, inpatient and outpatient records, general practitioner surveys, and/or surveys based on other community sources), should identify more cases than those that rely on fewer recruitment sources. Based on the combined prevalence estimates for persons, we divided the estimates into quality score terciles. We predicted that the prevalence estimates would be significantly different when assessed by quality score. More specifically, we predicted that prevalence estimates from studies with the highest quality score tercile would be higher than those from the lowest tercile.
The “Epidemiology” of Prevalence Estimates
The results of the search strategy, including source of the studies, subsequent culling, and final distribution of the papers, are shown in Figure 1 . The electronic search identified 1,112 papers (85% of the total papers included in the study), while manual reference checking identified an additional 142 references (11%). We received responses from 31 authors (see Acknowledgments for full list), who provided an additional 53 references (4%). We identified 98 studies that were published in languages other than English. After translation 17 of these studies were included in this review.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Double asterisk indicates exclusion categories (number studies excluded in parentheses). Double asterisk indicates numbers that are not mutually exclusive. A few studies provided rates for more than one group (11 studies provided data for both core and migrant [ n = 3] or both core and other special groups [ n = 8]; details in Results). LOTE, language other than English.
https://doi.org/10.1371/journal.pmed.0020141.g001
The list of references arranged by various criteria can be found in Tables 1 – 4 . The systematic review identified 188 studies that provided prevalence estimates [ 18 , 29 , 36 – 223 ]. These studies provided 1,721 estimates and were drawn from 46 countries. There were 132 core studies, 15 migrant studies (of which three overlap with discrete core), and 41 studies that reported the prevalence of schizophrenia in other special groups.
https://doi.org/10.1371/journal.pmed.0020141.t001
https://doi.org/10.1371/journal.pmed.0020141.t004
https://doi.org/10.1371/journal.pmed.0020141.t002
https://doi.org/10.1371/journal.pmed.0020141.t003
Key features of these core, migrant, and special groups are provided in Tables S4 – S6 . We excluded 26 studies that were completely overlapping by time and place, and 19 studies that reported prevalence data on prison populations (see Figure 1 ). However, ten partially overlapping studies were included that provided at least one discrete rate for this review [ 37 , 40 , 45 , 60 , 64 , 100 , 148 , 153 , 187 , 220 ].
The prevalence estimates were based on an estimated total of 154,140 potentially overlapping cases. The 132 core studies provided from one to 13 prevalence estimates per study. Four studies [ 59 , 120 , 169 , 224 ] reported prevalence only within narrow age strata without providing an overall rate. These studies were not included in the discrete core analyses.
Of the 132 core studies, we identified 21 studies for point prevalence, 34 studies for period prevalence, and 24 studies for lifetime prevalence. Thirty-two studies provided no information on the type of prevalence they reported—these were allocated to NOS prevalence. There were nine studies that reported LMR. Finally there were 44 studies that reported inpatient-census-derived data.
The Distribution of Prevalence Estimates
Figures 2 – 7 and Tables 5 – 7 show the distribution of the different types of prevalence estimates, and quantiles and moments for persons, males, and females.
https://doi.org/10.1371/journal.pmed.0020141.g002
https://doi.org/10.1371/journal.pmed.0020141.g007
https://doi.org/10.1371/journal.pmed.0020141.t005
https://doi.org/10.1371/journal.pmed.0020141.t007
https://doi.org/10.1371/journal.pmed.0020141.g003
https://doi.org/10.1371/journal.pmed.0020141.g004
https://doi.org/10.1371/journal.pmed.0020141.g005
https://doi.org/10.1371/journal.pmed.0020141.g006
The median point prevalence for persons (based on 23 estimates) was 4.6 per 1,000, and the 10% and 90% quantiles ranged from 1.9 to 10.0 per 1,000 (a 5-fold difference). The median period prevalence for persons (based on 42 estimates) was 3.3 per 1,000, and the 10% and 90% quantiles ranged from 1.3 to 8.2 per 1,000 (a 6.5-fold difference). The median lifetime prevalence for persons (based on 29 estimates) was 4.0 per 1,000, and the 10% and 90% quantiles ranged from 1.8 to 11.6 per 1,000 (a 6.4-fold difference).
There were 32 prevalence estimates that could not be classified to the above criteria (NOS). Based on the distribution of these prevalence estimates, the median prevalence was 2.7 per 1,000 for persons, and the 10% and 90% quantiles ranged from 1.4 to 4.8 per 1,000 (a 3.4-fold difference).
The median LMR for persons (based on 27 estimates) was 7.2 per 1,000, and the 10% and 90% quantiles ranged from 3.1 to 27.1 per 1,000 (a 8.7-fold difference) (see Table 6 ).
https://doi.org/10.1371/journal.pmed.0020141.t006
The review identified 108 estimates based on inpatient-census-derived data. Based on the distribution of these estimates for persons, the median value was 2.4 per 1,000, and the 10% and 90% quantiles ranged from 0.07 to 10.0 per 1,000 (a 154-fold difference) (see Table 7 ). Inpatient-census-derived prevalence is not included for any subsequent analyses.
When point, period, and lifetime estimates were compared, the distributions were not significantly different ( F 2,75 = 2.48, p = 0.09) . Estimates based on LMR were significantly higher than estimates based on lifetime estimates ( F 1,25 = 4.53, p = 0.04).
Male Versus Female Prevalence
Table 8 shows the moments and quantiles for the combined prevalence estimates for persons, males, and females, and for a ratio derived from male:female estimates. Figure 8 shows the distribution of these data for males and females—these distributions were not significantly different ( F 1,72 = 0.68, p = 0.41). For the male:female estimate ratio (based on 57 ratios), the median value was 1.11, and the 10% and 90% quantiles were 0.50 to 1.70 (approximately a 3.4-fold difference) (see Figure 9 ).
https://doi.org/10.1371/journal.pmed.0020141.g008
https://doi.org/10.1371/journal.pmed.0020141.g009
https://doi.org/10.1371/journal.pmed.0020141.t008
Urbanicity of Sites
We identified 31 discrete-core studies with 73 rates from urban sites (see Table 4 ), 24 studies with 48 rates from rural sites, and 45 studies with 137 mixed urban/rural rates. There were four discrete-core studies providing rates for both urban ( n = 12) and rural ( n = 10) categories. Figure 10 and Table 9 show the distribution of overall prevalence based on rural, urban, and mixed urbanicity status for persons. While the mixed urban/rural estimates were higher than urban and rural rates, this difference was not statistically significant ( F 2,235 = 1.63, p = 0.20), nor were urban estimates significantly different from rural estimates ( F 1,120 = 0.95, p = 0.33).
https://doi.org/10.1371/journal.pmed.0020141.g010
https://doi.org/10.1371/journal.pmed.0020141.t009
https://doi.org/10.1371/journal.pmed.0020141.t010
https://doi.org/10.1371/journal.pmed.0020141.t011
Migrant Status
We identified 15 migrant studies from eight countries: Australia ( n = 2; [ 55 , 207 ]), Germany ( n = 1; [ 92 ]), India ( n = 1; [ 175 ]), Israel ( n = 1; [ 200 ]), Taiwan ( n = 1; [ 129 ]), the Netherlands ( n = 1; [ 173 ]); United Kingdom ( n = 7; [ 39 , 43 , 65 – 68 , 133 ]); and United States ( n = 1; [ 180 ]).
Table S5 presents a detailed list of migrant studies with key descriptive variables, prevalence rates, and within-study migrant:native-born estimate ratios.
The number of different migrant groups in one study ranged between one and 38. There were six studies that derived data from inpatient-census-derived prevalence [ 43 , 55 , 65 – 67 , 92 ] and thus could not used in this analysis. In addition, four migrant studies did not present data for native-born populations [ 92 , 133 , 173 , 200 ]. Therefore, our analysis was limited to five papers only [ 39 , 129 , 175 , 180 , 207 ]. Based on 22 prevalence ratios, the median migrant:native-born prevalence ratio was 1.84 and the 10% and 90% quantiles were 0.86 to 6.41 (approximately a 7.5-fold difference) (see Table 1 0; Figure 11 ). When the migrant versus the native-born prevalence estimates were compared, there was a significant difference ( F 1,2 = 5.57, p = 0.04).
https://doi.org/10.1371/journal.pmed.0020141.g011
Economic Status of Sites
Based on the three economic categories, we identified 19 estimates from least developed countries, 22 estimates from emerging economy countries, and 96 estimates from developed countries (see Table 1 1; Figure 12 ). When divided by this criterion, the prevalence estimate distributions were significantly different ( F 2,85 = 3.57, p = 0.03), with the difference attributed to the lower prevalence estimate distribution for the less developed economies (developed versus least developed, F 1,74 = 6.55, p = 0.04). Table 12 also shows the male:female prevalence estimate ratio when subdivided by economic status. The distributions of these ratios (see Figure 13 ) , were not significantly different ( F 2,42 = 0.44, p = 0.44).
https://doi.org/10.1371/journal.pmed.0020141.g012
https://doi.org/10.1371/journal.pmed.0020141.g013
https://doi.org/10.1371/journal.pmed.0020141.t012
Quality Score
When the combined prevalence estimates for persons were divided into quality score terciles, the prevalence estimate distributions were significantly different ( F 2,105 = 4.79 , p = 0.01), with the highest quality studies reporting significantly higher prevalence estimates than the other two terciles (highest versus lowest quality scores, p = 0.02) ( Table 13 ; Figure 14 ).
https://doi.org/10.1371/journal.pmed.0020141.g014
https://doi.org/10.1371/journal.pmed.0020141.t013
Other Special Group Studies
Details of these studies can be found in Table S6 . We identified 41 studies that reported the prevalence of schizophrenia in other special groups. These studies came from 14 countries: Australia ( n = 4), Canada ( n = 4), Denmark ( n = 3), Finland ( n = 1), Germany ( n = 3), India ( n = 2), Israel ( n = 1), Japan ( n = 3), Romania ( n = 1), Spain ( n = 1), Sweden ( n = 2), Taiwan ( n = 1), United Kingdom ( n = 2), and United States ( n = 5).
Prevalence estimates were obtained from a range of population subgroups including elderly individuals ( n = 10; [ 52 , 70 , 71 , 101 , 108 , 121 , 149 – 151 , 159 ]), ethnic groups ( n = 8; [ 58 , 134 , 139 , 140 , 166 , 199 , 213 , 218 ]), Aborigines ( n = 4; [ 105 , 106 , 115 , 164 ]), religious groups ( n = 5; [ 29 , 80 , 128 , 182 , 191 ]), homeless individuals ( n = 4; [ 118 , 161 , 192 , 194 ]), children and adolescents ( n = 3; [ 57 , 185 , 189 ]), students ( n = 2; [ 147 , 178 ]), twins ( n = 1; [ 61 ]), industrial workers ( n = 1; [ 172 ]), different castes ( n = 1; [ 145 ]), and an isolate pedigree ( n = 1; [ 99 ]).
The marked heterogeneity of these data does not make them suitable for combining. However, we note that prevalence estimates in some homeless populations were very high—300 per 1,000 persons for Sydney homeless individuals [ 194 ] and 131 per 1,000 persons for Los Angeles homeless individuals [ 118 ]. Conversely, some religious groups had very low prevalence estimates—0.36 per 1,000 persons for Amish individuals [ 80 ] and 1.29 per 1,000 persons for Hutterite individuals [ 29 ].
There is a wealth of data available on the prevalence of schizophrenia—a total of 1,721 estimates from 188 studies were identified in this systematic review. These estimates were drawn from 46 countries, and were based on an estimated 154,140 potentially overlapping prevalent cases.
The median prevalence estimates for persons were 4.6 per 1,000 for point prevalence, 3.3 for period prevalence, 4.0 for lifetime prevalence, and 7.2 for LMR. These estimates are congruent with an earlier narrative review of 70 studies by Torrey [ 8 ], who reported an overall prevalence estimate of 4.6 per 1,000. Key policy documents have correctly estimated the point prevalence of schizophrenia at about four per 1,000 [ 2 , 225 ]; however, the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) [ 3 ], reported that the lifetime prevalence of schizophrenia is “usually estimated to be between 0.5% and 1%.” This overestimate is often repeated in textbooks [ 226 ]. As with the misunderstandings about the incidence of schizophrenia [ 21 ], this is another example where the research community needs to review their belief systems in the face of data. It is reasonable to assume that lifetime prevalence estimates for schizophrenia would be higher than point estimates. Surprisingly, the data in this review do not support this assumption. While outside the scope of the current review, the findings raise interesting research questions about factors that may influence prevalence (e.g., recovery, suicide, or other forms of early mortality). Indeed, it is curious that the identification of the onset of psychotic disorders has received so much recent attention [ 227 , 228 ], while we still struggle to understand the offset of schizophrenia. Point and period prevalence estimates assume that we can identify when someone has recovered from an illness. Recovery from schizophrenia clearly occurs [ 229 – 231 ], but it is unclear whether those who are free of positive symptoms but who have mild residual disability should be counted as “active” cases or not. The definitions of recovery versus persistence are multidimensional, and future prevalence studies will benefit if these definitions can be operationalized.
The median LMR estimate was 7.2 per 1,000, which is consistent with two other narrative reviews. Fremming [ 232 ], who reviewed 18 studies conducted in central Europe between 1926 and 1938, reported a mean LMR of 7.4 per 1,000, while Gottesman and Shields [ 233 ] reported a mean LMR of 8.0 per 1,000 in their classic review. As predicted, LMR estimates were significantly higher than lifetime estimates, which reflects the different heritage of these two indices. It is reasonable to assume that the oft-quoted statistic that “schizophrenia affects about one in a hundred” derives from LMR data (see [ 234 ]). However, one in a hundred is an overestimate—our systematic review agrees with two previous reviews showing that the LMR for schizophrenia is between seven and eight per 1,000. While the arithmetic mean value of 11.9 per 1,000 is more consistent with the “one in a hundred” dogma, the median is a more appropriate measure of central tendency for this skewed distribution. If we wish to provide the general public with a measure of the likelihood that individuals will develop schizophrenia during their lifetime, then a more accurate statement would be that “about seven to eight individuals per 1,000 will be affected.”
While there has been considerable debate about whether or not the incidence of schizophrenia varies between sites [ 21 ], there is a tacit understanding that the prevalence of schizophrenia is variable. For example, in an earlier review by Eaton [ 5 ], a 12-fold variation in point and a 10-fold variation in lifetime prevalence were noted. A recent systematic review by Goldner et al. [ 12 ] also observed a 13-fold variation in lifetime prevalence of schizophrenia. Based on the central 80% of the estimates (10% to 90% quantiles), the present review found that the different types of prevalence estimates had from 3.4-fold (point) to 4.6-fold (period) variation. The use of the 10% and 90% quantiles to define the central segment of the distribution means that our reporting of the variability of estimates is more conservative than other commentators (i.e., we have ignored 20% of the distribution in the tails). If we had included all data points, the range of prevalence estimates would have been much higher. Regardless of whether this variability is labeled “narrow” or “prominent,” the task for the researchers is to determine how much of this variation is a function of measurement error versus “true” underlying variation. With respect to measurement error, it should be noted that this study found that quality of the study does significantly influence prevalence estimates. Future studies could explore the impact of quality on the variation in prevalence estimates.
Sex and Schizophrenia
One of the unexpected findings of this review was that there was no statistically significant difference in prevalence estimates between males and females. In our previous study of incidence of schizophrenia we found a male:female risk ratio of 1.40 [ 1 ]. Because narrative reviews conclude that the course of the illness tends to be more severe in men than in women [ 235 ], we assumed that this would be reflected in a higher prevalence in males than females. The lack of coherence between (a) the sex differences found in the incidence of schizophrenia, (b) the presumed difference in course of illness, and (c) the identified lack of difference in prevalence warrants closer scrutiny.
Economic Status and Schizophrenia
In keeping with our hypothesis, the prevalence of schizophrenia is lower in developing nations than in developed nations. However, we urge caution in the interpretation of these data. The use of a single economic variable is a crude way to assess a complex and multidimensional concept. Furthermore, the median prevalence estimates for emerging economies are numerically higher than those for the richest countries. While not statistically significant, the results did identify many prevalence studies from the developing world where females outnumbered males. Recently, a study from China examined whether this unexpected sex ratio was due to differential suicide rates in males with schizophrenia [ 24 ]; however, this did not seem to explain the female excess. Our findings lend weight to the commentary by Ran and Yu-Hai Chen [ 25 ], drawing attention to the different features of schizophrenia in the developing world. Overall, the findings suggest that factors that influence the course of illness of schizophrenia in men and women differ around the world. Regardless of the mechanisms underlying this possibility, the findings highlight the importance of using systematic techniques to identify data; 17 studies included in this review were only available in languages other than English. We speculate that the results of past narrative reviews may have been biased towards data from developed nations. From a wider perspective, the findings reinforce the importance of encouraging more research from poorer countries [ 236 ].
Urbanicity and the Prevalence of Schizophrenia
In the previous systematic review of the incidence of schizophrenia, we found that urban sites had significantly higher incidence rates of schizophrenia than mixed urban/rural sites (there were too few pure rural sites to make the direct urban versus rural comparison) [ 1 ]. Contrary to our expectations, the prevalence of schizophrenia did not differ according to urbanicity. While Figure 10 suggests that mixed urban/rural sites have higher prevalence estimates than pure urban and rural sites, this study found, in fact, that there was no significant difference between urban, rural, and mixed sites. Perhaps the inclusion of many sites from the developing world in this review has confounded the expected urban/rural gradient. This will be examined in more detail in future analyses.
Migrant Status and the Prevalence of Schizophrenia
As predicted, prevalence estimates for migrant groups tend to be higher than estimates for native-born populations. This finding is consistent with past systematic reviews of the incidence of schizophrenia [ 1 , 33 ]. Migrant studies are prone to a range of methodological issues (e.g., differential pathways to care, diagnostic inaccuracies due to language and cultural practices, and uncertainty about the denominator required for the calculation of proportions). While the prevalence estimates included in this systematic review may share common biases, the increased prevalence of schizophrenia in migrant groups found in this study adds weight to the argument that migrant status is an important risk factor for schizophrenia.
Quality Scores and Other Special Groups
Reassuringly, studies that had higher overall quality scores tended to identify more cases, and thus generate higher prevalence estimates than lower quality studies. Future studies will explore whether the findings based on the overall studies persist in the subgroup of studies in the highest quality tercile.
With respect to the studies included in the category “other special groups,” the estimates are not readily comparable, but it is interesting to note that these studies reported a wide range of prevalence estimates (e.g., high in homeless populations and low in certain religious groups). Future publications will examine these groups in more detail.
Based on our experience with previous systematic reviews, we acknowledge that we may have missed studies and/or made data entry errors. We encourage readers to inform us of missing studies or errors in the data. Updated lists of relevant studies and raw data will be available from the authors. Furthermore, in the absence of clear guidelines on how to synthesize descriptive studies [ 26 , 237 ], many of the rules we used to filter studies and extract data were necessarily ad hoc. In the future, researchers may wish to reanalyze the dataset using different criteria, and perform sensitivity analyses related to these choices.
Two of the prevalence types (LMR and inpatient-census-derived data) had distributions for persons that were higher than distributions for both males and females separately. This pattern, which is difficult to explain, was also noted in some of the previously published incidence distributions [ 1 ]. The impact of quality scores on this pattern will be assessed in future studies.
The planned sensitivity analyses were conducted on combined data, a strategy that reduced the number of comparisons substantially (one combined analysis versus five analyses on each of point, period, lifetime, LMR, and NOS data). However, the combined prevalence estimate included studies that contributed more than one prevalence type (e.g., one study could contribute both point and period prevalence estimates). Of the 94 studies, eight contributed more than one prevalence type to the combined prevalence estimates. While the analytic technique controlled for within-study variance, the combined dataset is not based on discrete data (in contrast to the prevalence-type-specific analyses).
It was disappointing that standard errors could be allocated to so few prevalence estimates (26%). Despite this, in the future we plan to undertake a traditional meta-analysis based on this subset of estimates in order to compare the pooled estimate values with those presented in the current study.
Concerning the analyses for urbanicity, the estimates from mixed urban/rural studies are likely to be very heterogeneous. Indeed, we allocated studies to the mixed category if there was any possibility that rural sectors were included. This bias would have made any true difference between urban versus mixed urban/rural more difficult to detect. There are good reasons to review the findings for both urbanicity and sex ratio more closely when categorized by economic status. Such analyses may help generate hypotheses for future analyses, but researchers need to be extremely cautious when systematic reviews are subjected to excessive data analyses (i.e., “data torturing” [ 238 ]). The contributing studies were not designed to test many of the hypotheses examined in this review, therefore researchers must be frugal in the use of planned sensitivity analyses, and cautious in the interpretation of the results. However, researchers are encouraged to freely explore the full data to examine additional research questions.
While there is substantial variation between sites, generally the prevalence of schizophrenia ranges from four to seven per 1,000 persons, depending on the type of prevalence estimate used. Countries from the developing world have a lower prevalence of schizophrenia. Overall, the prevalence of schizophrenia does not vary between the sexes; however, the data suggest that sex ratio of prevalence estimates may vary between sites more than previously believed. While the incidence of schizophrenia is higher in urban than rural settings, this is not reflected in the overall prevalence data. The prevalence of schizophrenia is higher in migrants than native-born individuals.
Regardless of the exact magnitude and precision of prevalence estimates, the numbers speak to a deeper, human dimension. Many people with schizophrenia have persisting symptoms, despite the best mix of interventions we can offer. This sobering reality has also emerged from research about “best buys” with respect to the cost of averting disability [ 239 ]. For schizophrenia, with the current mix of interventions we can only reduce 13% of the burden. If we improve efficiencies within the current services, we can do somewhat better (22%). In a utopian world, even if unlimited funding were available, three-quarters of the burden of schizophrenia would remain unavoidable [ 240 ]. This is a powerful argument for investing in applied and basic research.
As with its companion study on the incidence of schizophrenia [ 1 ], we hope that the current review will populate the “epidemiological landscape” with data, and that this enriched environment will select the fittest (most heuristic) hypotheses [ 21 ]. The epidemiological landscape of schizophrenia is no longer terra incognita—many of its contours have been mapped out. We can gain traction on this landscape and use the identified gradients to generate candidate risk factors for future research [ 241 ]. Equally, these systematic reviews have brought into focus the gaps in our knowledge—parts of the map “do not fit.” Paradoxes such as these can be powerful catalysts for advancing knowledge.
Supporting Information
Dataset s1. access dataset of prevalence studies.
https://doi.org/10.1371/journal.pmed.0020141.sd001
(272 KB ZIP).
Table S1. Definitions of Prevalence Estimate Types
https://doi.org/10.1371/journal.pmed.0020141.st001
(32 KB DOC).
Table S2. Definitions for the Variables Used to Characterize the Prevalence Studies
https://doi.org/10.1371/journal.pmed.0020141.st002
(43 KB DOC).
Table S3. Quality Score Criteria
https://doi.org/10.1371/journal.pmed.0020141.st003
(38 KB DOC).
Table S4. Characteristics of Core Prevalence Studies
https://doi.org/10.1371/journal.pmed.0020141.st004
(644 KB DOC).
Table S5. Characteristics of Migrant Prevalence Studies
https://doi.org/10.1371/journal.pmed.0020141.st005
(136 KB DOC).
Tables S6. Characteristics of Other Special Groups Prevalence Studies
https://doi.org/10.1371/journal.pmed.0020141.st006
(243 KB DOC).
Acknowledgments
The authors wish to express their gratitude to the following colleagues who assisted in the search for data and translation of the studies: R. C. Bland, D. Blazer, S. Caleo, G. Canino, B. Cooper, J. Copeland, J. Cullberg, H. Dominique, O. El Saadi, M. Fichter, R. Grawe, S. C. Gupta, H. Herrman, A. Isailovic, E. Jacko, F. Jacobi, H. Katchadourian, K. Kendler, B. Moreno-Kustner, I. Levav, C. MacCauley, D. McLean, R. McCreadie, P. Munk-Jorgensen, A. Preti, P. Rabins, J. Robertson, A. Robinson, S. Scheurer, P. Shrout, L. Teplin, P. Thomsen, E. F. Torrey, M. Von Korff, J. Waddington, A. Weeke, M. Weingarten, M. Weissman, Z. Welham, E. Wells, and H. Wittchen. The Stanley Medical Research Institute supported this project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
JM designed the study. SS, DC, JW, and JM analyzed the data and contributed to writing the paper.
- View Article
- Google Scholar
- 2. Murray CJ, Lopez AD, editors. (1996) The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Boston: Harvard School of Public Health. 990 p.
- 3. American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders: DSM-IV, 4th ed. Washington (DC): American Psychiatric Association. 886 p.
- 4. Jablensky A (2003) Schizophrenia: The epidemiological horizon. Hirsch SR, Weinberger DR, editors. Schizophrenia Oxford: Blackwell Science. pp. 203–231.
- 6. Eaton WW, Tien AY, Poeschla BDDen Boer JA, Westenberg HGM, van Praag HM (1995) Epidemiology of schizophrenia. Advances in the neurobiology of schizophrenia. Chichester (New York): John Wiley and Sons. 469 p.
- 13. Kleinbaum DG, Kupper LL, Morgenstern H (1982) Epidemiologic research: Principles and quantitative methods. Belmont (California): Lifetime Learning Publications. 560 p.
- 19. Cox DR, Oakes D (1984) Analysis of survival data. London: Chapman and Hall. 201 p.
- 22. Bresnahan M, Menezes P, Varma V, Susser EMurray RM>, Jones PB, Susser E, van Os J, Cannon M (2003) Geographical variation in incidence, course and outcome of schiozphrenia: A comparison of developing and developed countries. The epidemiology of schizophrenia. Cambridge: Cambridge University Press. pp. 18–33.
- 32. Warner R (1985) Recovery from schizophrenia: Psychiatry and political economy. London: Routledge and Kegan Paul. 380 p.
- 34. Central Intelligence Agency (2004) CIA World Factbook, 2004. Washington (DC): Central Intelligence Agency, Office of Public Affairs. 730 p.
- 35. Soubbotina TP (2004) Beyond economic growth: An introduction to sustainable development, 2nd ed. Washington (DC): World Bank. 205 p.
- 38. Babigian HKaplan H, Freedman A, Sadock B (1980) Schizophrenia: Epidemiology. Comprehensive textbook of psychiatry III. Baltimore: Williams and Wilkins. pp. 1113–1121.
- 59. Statistics Canada (1979) Mental Health Statistics, Volume II. Ottawa: Ministry of Industry, Trade and Commerce. 123 p.
- 103. Jayasundera MGLin WCT (1969) Mental health surveys in Ceylon. Mental health research in Asia and the Pacific. Honolulu: East-West Center Press. pp. 54–64.
- 123. Leaf PJ, Myers JK, McEvoy LTRobins LN, Regier DA (1991) Procedures used in the epidemiologic catchment area study. Psychiatric disorders in America. New York: The Free Press. pp. 11–32.
- 129. Lin TY, Rin H, Yeh EK, Hsu CC, Chu HM (1969) Mental disorders in Taiwan, fifteen years later. In: Caudill W, Lin T, editors. Mental health research in Asia and the Pacific. Honolulu: East-West Center Press. pp. 66–91.
- 184. Sundaram D, Sathyavathi K, Murthy HN (1967) Ecological aspects in schizophrenia. Transactions of All-India Institute of Mental Health. Bangladore: India Institute of Mental Health. pp. 43–53.
- 224. O'Hare A, Walsh D (1972) Activities of Irish Psychiatric Hospitals and Units 1965–1969, and 1970. Dublin: The Medico-Social Research Board. 33 p.
- 226. Murray RMMurray R, Hill P, McGuffin P (1997) Schizophrenia. The essentials of postgraduate psychiatry, 3rd ed. Cambridge: Cambridge University Press. pp. 281–309.
- 232. Fremming KH (1947) Morbidity risk of mental disease and other mental abnormalities in an average Danish population. Copenhagen: Munksgaard. 278 p.
- 233. Gottesman II, Shields J (1982) Schizophrenia: The epi-genetic puzzle. Cambridge: Cambridge University Press. 258 p.
Patient Summary
Background..
Schizophrenia is a very serious mental illness and a major contributor to the global burden of disease. The topic of this study is the question of how common schizophrenia is among different groups and in different countries around the world. “Prevalence” means the number of people who have the disease at a particular time. The study itself is a so-called systematic review, which means the researchers used prespecified methods for finding individual studies and for extracting and summarizing the data from these individual studies in as objective a way as possible.
Why Was This Study Done?
Health care planning is based on prevalence estimates, and as a result, many studies on schizophrenia prevalence have been done by researchers around the world. The authors decided to do a systematic review of these studies to come up with a scientifically sound view of the big picture.
What Did the Researchers Do?
They looked at a total of 1,721 estimates of the prevalence of schizophrenia from 188 studies and covering 46 countries. They then calculated median prevalence estimates (that is, the middle value of all estimates) over a variety of time periods (see below).
What Did They Find?
The take-home message from their study is that about seven to eight individuals out of 1,000 will be affected by schizophrenia. To be more precise, the researchers found the following median estimates for the prevalence of schizophrenia: 4.6 out of 1,000 people have the disease at a specific time point; 3.3 per 1,000 have the disease within a surveillance period one to 12 months long; the lifetime prevalence (the number of people in the population who have ever manifested the disease) is 4.0 per 1,000; and the lifetime morbid risk (the likelihood that a particular individual will develop schizophrenia in their lifetime) is 7.2 per 1,000. While previous research has shown that men have a higher risk of developing schizophrenia, the researchers found that the prevalence of schizophrenia was the same in men and women (suggesting that the course of the illness differs between the sexes). The prevalence of schizophrenia was lower in poorer countries than in richer countries.

What Does This Mean?
Based on these estimates, our textbook numbers on lifetime prevalence and overall risk for an individual to develop schizophrenia are probably too high. Taken together with estimates on the incidence of schizophrenia (that is, the annual number of new cases), it is also clear that current treatments fail to cure most patients with schizophrenia.
More Information Online.
Additional information on schizophrenia can be found at the following sources.
United States National Institutes of Mental Health (search for “schizophrenia”): http://www.nimh.nih.gov/
Schizophrenia.com, a not-for-profit Web site providing information and education on schizophrenia: http://www.schizophrenia.com
For an explanation of systematic reviews: http://www.shef.ac.uk/scharr/ir/units/systrev/definitions.htm ; http://www.cochrane.org/index0.htm
For definitions of incidence and prevalence: http://www.wrongdiagnosis.com/admin/preval.htm
For more information about the systematic reviews of the incidence and prevalence of schizophrenia: http://www.qcmhr.uq.edu.au/epi/
Cognitive behavioral therapy for schizophrenia: an empirical review
Affiliation.
- 1 Centre for Addiction and Mental Health, Clarke Institute of Psychiatry and Department of Psychiatry, University of Toronto, Ontario, Canada.
- PMID: 11379970
- DOI: 10.1097/00005053-200105000-00002
Early case studies and noncontrolled trial studies focusing on the treatment of delusions and hallucinations have laid the foundation for more recent developments in comprehensive cognitive behavioral therapy (CBT) interventions for schizophrenia. Seven randomized, controlled trial studies testing the efficacy of CBT for schizophrenia were identified by electronic search (MEDLINE and PsychInfo) and by personal correspondence. After a review of these studies, effect size (ES) estimates were computed to determine the statistical magnitude of clinical change in CBT and control treatment conditions. CBT has been shown to produce large clinical effects on measures of positive and negative symptoms of schizophrenia. Patients receiving routine care and adjunctive CBT have experienced additional benefits above and beyond the gains achieved with routine care and adjunctive supportive therapy. These results reveal promise for the role of CBT in the treatment of schizophrenia although additional research is required to test its efficacy, long-term durability, and impact on relapse rates and quality of life. Clinical refinements are needed also to help those who show only minimal benefit with the intervention.
Publication types
- Research Support, Non-U.S. Gov't
- Antipsychotic Agents / therapeutic use
- Cognitive Behavioral Therapy / methods*
- Combined Modality Therapy
- Delusions / drug therapy
- Delusions / psychology
- Delusions / therapy
- Hallucinations / drug therapy
- Hallucinations / psychology
- Hallucinations / therapy
- Psychotherapy / methods
- Randomized Controlled Trials as Topic
- Schizophrenia / drug therapy
- Schizophrenia / therapy*
- Schizophrenic Psychology
- Treatment Outcome
- Antipsychotic Agents
Multiresolution feature fusion for smart diagnosis of schizophrenia in adolescents using EEG signals
- Research Article
- Published: 11 May 2024
Cite this article

- Rakesh Ranjan ORCID: orcid.org/0000-0002-0089-1734 1 &
- Bikash Chandra Sahana 1
75 Accesses
Explore all metrics
Numerous studies on early detection of schizophrenia (SZ) have utilized all available channels or employed set of a few time domain or frequency domain features, while a limited number of features may not be sufficient enough to perform diagnosis efficiently. To encounter these problems, an automated diagnosis model is proposed for the efficient diagnosis of schizophrenia symptomatic adolescent subjects from electroencephalogram (EEG) signals via machine intelligence. A publicly accessible EEG dataset featuring 16-channels EEG obtained from 84 adolescents (45 SZ symptomatic and 39 healthy control) is used to demonstrate the work. Initially, the signals are decomposed into sub-bands using two multi-resolution signal analysis methods: Empirical Wavelet Transform and Empirical mode decomposition. 75 unique features from each sub-bands are extracted and the few selective prominent features are applied to machine learning classifiers for optimal sub-band selection. Subsequently, a hybrid model is proposed, combining convolutional neural network (CNN) and ensemble bagged tree, incorporating both deep learning and handcrafted features to perform SZ diagnosis. This innovative model achieved superior classification performance compared to existing methods, offering a promising approach for SZ diagnosis. Furthermore, the study explores the impact of different brain regions and combined regional data in SZ diagnosis comprehensively. Hence, this computer-assisted decision-making model minimizes the limitations of prior studies by providing a more robust and efficient diagnostic system for schizophrenia.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
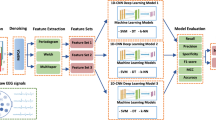
1D-convolutional neural network approach and feature extraction methods for automatic detection of schizophrenia
Diagnosis of schizophrenia based on transformation from EEG sub-bands to the image with deep learning architecture
Empirical mode decomposition and convolutional neural network-based approach for diagnosing psychotic disorders from eeg signals
Data availability.
The research is based solely on the analysis of publicly available data which is accessible at http://brain.bio.msu.ru/eeg_schizophrenia.htm .
Akar SA, Kara S, Latifoğlu F, Bilgiç V (2016) Analysis of the complexity measures in the EEG of schizophrenia patients. Int J Neural Syst 26:1–13. https://doi.org/10.1142/S0129065716500088
Article Google Scholar
Akbari H, Sadiq MT (2021) Detection of focal and non-focal EEG signals using non-linear features derived from empirical wavelet transform rhythms. Phys Eng Sci Med 44:157–171. https://doi.org/10.1007/S13246-020-00963-3/FIGURES/12
Article PubMed Google Scholar
Amezquita-Sanchez JP, Mammone N, Morabito FC, Adeli H (2021) A New dispersion entropy and fuzzy logic system methodology for automated classification of dementia stages using electroencephalograms. Clin Neurol Neurosurg 201:106446. https://doi.org/10.1016/j.clineuro.2020.106446
Amin HU, Mumtaz W, Subhani AR et al (2017) Classification of EEG signals based on pattern recognition approach. Front Comput Neurosci 11:1–12. https://doi.org/10.3389/fncom.2017.00103
Aslan Z, Akin M (2022) A deep learning approach in automated detection of schizophrenia using scalogram images of EEG signals. Phys Eng Sci Med 45:83–96. https://doi.org/10.1007/s13246-021-01083-2
Aslan Z, Akin M (2020) Automatic detection of schizophrenia by applying deep learning over spectrogram images of EEG signals. Traitement du Signal 37:235–244. https://doi.org/10.18280/ts.370209
Balasubramanian K, Ramya K, Gayathri Devi K (2022) Optimized adaptive neuro-fuzzy inference system based on hybrid grey wolf-bat algorithm for schizophrenia recognition from EEG signals. Cognit Neurodyn. https://doi.org/10.1007/s11571-022-09817-y
Borisov SV, Kaplan A. Ya., Gorbachevskaya NL, Kozlova IA (2005) Analysis of EEG structural synchrony in adolescents with schizophrenic disorders. Human Physiol 31(3):255–261. https://doi.org/10.1007/s10747-005-0042-z
Buckley PF, Miller BJ (2015) Schizophrenia research: a progress report. Psychiatr Clin North Am 38:373–377. https://doi.org/10.1016/J.PSC.2015.05.001
Budak U, Bajaj V, Akbulut Y et al (2019) An effective hybrid model for EEG-based drowsiness detection. IEEE Sens J 19:7624–7631. https://doi.org/10.1109/JSEN.2019.2917850
Bühlmann Peter (2012) Bagging, boosting and ensemble methods. In: Gentle James E, Härdle Wolfgang Karl, Mori Yuichi (eds) Handbook of computational statistics. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 985–1022. https://doi.org/10.1007/978-3-642-21551-3_33
Chapter Google Scholar
Calhas D, Romero E, Henriques R (2020) On the use of pairwise distance learning for brain signal classification with limited observations. Artif Intell Med 105:101852. https://doi.org/10.1016/J.ARTMED.2020.101852
Chen VCH, Chen CH, Chiu YH et al (2018) Leptin/Adiponectin ratio as a potential biomarker for metabolic syndrome in patients with schizophrenia. Psychoneuroendocrinology 92:34–40. https://doi.org/10.1016/J.PSYNEUEN.2018.03.021
Article CAS PubMed Google Scholar
Chernew M, Mintz H (2021) Administrative expenses in the US health care system: why so high? JAMA 326:1679–1680. https://doi.org/10.1001/JAMA.2021.17318
Cicone A, Pellegrino E (2022) Multivariate fast iterative filtering for the decomposition of nonstationary signals. IEEE Trans Signal Process 70:1521–1531. https://doi.org/10.1109/TSP.2022.3157482
Cortes-Briones JA, Tapia-Rivas NI, D’Souza DC, Estevez PA (2022) Going deep into schizophrenia with artificial intelligence. Schizophr Res 245:122–140. https://doi.org/10.1016/j.schres.2021.05.018
Das K, Pachori RB (2021) Schizophrenia detection technique using multivariate iterative filtering and multichannel EEG signals. Biomed Signal Process Control 67:102525. https://doi.org/10.1016/j.bspc.2021.102525
de Miras JR, Ibáñez-Molina AJ, Soriano MF, Iglesias-Parro S (2023) Schizophrenia classification using machine learning on resting state EEG signal. Biomed Signal Process Control 79:104233
Dogan S, Baygin M, Tasci B et al (2022) Primate brain pattern-based automated Alzheimer’s disease detection model using EEG signals. Cognitive Neurodyn. https://doi.org/10.1007/s11571-022-09859-2
Dvey-Aharon Z, Fogelson N, Peled A, Intrator N (2015) Schizophrenia detection and classification by advanced analysis of EEG recordings using a single electrode approach. PLoS ONE 10:e0123033. https://doi.org/10.1371/JOURNAL.PONE.0123033
Article PubMed PubMed Central Google Scholar
Dvorak D, Shang A, Abdel-Baki S et al (2018) Cognitive behavior classification from scalp EEG signals. IEEE Trans Neural Syst Rehabil Eng 26:729–739. https://doi.org/10.1109/TNSRE.2018.2797547
García-Gutiérrez MS, Navarrete F, Sala F et al (2020) Biomarkers in psychiatry: concept, definition, types and relevance to the clinical reality. Front Psych 11:432. https://doi.org/10.3389/FPSYT.2020.00432/BIBTEX
Gatouillat A, Badr Y, Massot B, Sejdic E (2018) Internet of medical things: a review of recent contributions dealing with cyber-physical systems in medicine. IEEE Internet Things J 5:3810–3822. https://doi.org/10.1109/JIOT.2018.2849014
Gilles J (2013) Empirical wavelet transform. IEEE Trans Signal Process 61:3999–4010. https://doi.org/10.1109/TSP.2013.2265222
Gogtay N, Vyas NS, Testa R et al (2011) Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull 37:504–513. https://doi.org/10.1093/SCHBUL/SBR030
Göker H (2023) Automatic detection of Parkinson’s disease from power spectral density of electroencephalography (EEG) signals using deep learning model. Phys Eng Sci Med 46:1163–1174. https://doi.org/10.1007/S13246-023-01284-X/TABLES/3
Goldblum M, Finzi M, Rowan K, Wilson AG The No Free Lunch Theorem, Kolmogorov Complexity, and the Role of Inductive Biases in Machine Learning
Goodfellow I, Bengio Y, Courville A (2016) Deep learning. MIT press
Google Scholar
Gosala B, Kapgate PD, Jain P et al (2023) Wavelet transforms for feature engineering in EEG data processing: An application on Schizophrenia. Biomed Signal Process Control 85:104811
Goshvarpour A, Goshvarpour A (2020) Schizophrenia diagnosis using innovative EEG feature-level fusion schemes. Phys Eng Sci Med 43:227–238
Hamaneh MB, Chitravas N, Kaiboriboon K et al (2014) Automated removal of EKG artifact from EEG data using independent component analysis and continuous wavelet transformation. IEEE Trans Biomed Eng 61:1634–1641. https://doi.org/10.1109/TBME.2013.2295173
Hassan F, Hussain SF, Qaisar SM (2023) Fusion of multivariate EEG signals for schizophrenia detection using CNN and machine learning techniques. Inf Fusion 92:466–478. https://doi.org/10.1016/j.inffus.2022.12.019
Hu B, Peng H, Zhao Q et al (2015) Signal quality assessment model for wearable EEG sensor on prediction of mental stress. IEEE Trans Nanobiosci 14:553–561. https://doi.org/10.1109/TNB.2015.2420576
Isham MF, Leong MS, Lim MH, Ahmad ZAB (2019) Optimized ELM based on whale optimization algorithm for gearbox diagnosis. MATEC Web Conf 255:02003. https://doi.org/10.1051/MATECCONF/201925502003
Jahmunah V, Lih OhS, Rajinikanth V et al (2019) Automated detection of schizophrenia using nonlinear signal processing methods. Artif Intell Med 100:101698. https://doi.org/10.1016/j.artmed.2019.07.006
Jana GC, Agrawal A, Pattnaik PK, Sain M (2022) DWT-EMD feature level fusion based approach over multi and single channel EEG signals for seizure detection. Diagnostics 12:324. https://doi.org/10.3390/DIAGNOSTICS12020324
Kasim Ö (2023) Identification of attention deficit hyperactivity disorder with deep learning model. Phys Eng Sci Med 46:1081–1090. https://doi.org/10.1007/S13246-023-01275-Y/TABLES/4
Khan SI, Pachori RB (2021) Automated classification of lung sound signals based on empirical mode decomposition. Expert Syst Appl 184:115456. https://doi.org/10.1016/J.ESWA.2021.115456
Khare SK, Bajaj V (2021) A self-learned decomposition and classification model for schizophrenia diagnosis. Comput Methods Programs Biomed 211:106450. https://doi.org/10.1016/j.cmpb.2021.106450
Khare SK, Bajaj V (2022) A hybrid decision support system for automatic detection of Schizophrenia using EEG signals. Comput Biol Med 141:105028. https://doi.org/10.1016/j.compbiomed.2021.105028
Khare SK, Bajaj V, Acharya UR (2021) Detection of Parkinson’s disease using automated tunable Q wavelet transform technique with EEG signals. Biocybern Biomed Eng 41:679–689. https://doi.org/10.1016/j.bbe.2021.04.008
Khodabakhsh A, Arabi H, Zaidi H (2021) U-Net Based Estimation of Functional Connectivity from Time Series Multi-Channel EEG from Schizophrenia Patients. In 2021 IEEE Nuclear Science Symposium and Medical Imaging Conference Record, NSS/MIC 2021 and 28th International Symposium on Room-Temperature Semiconductor Detectors, RTSD 2022.doi: https://doi.org/10.1109/NSS/MIC44867.2021.9875427
Krishnan PT, Joseph Raj AN, Balasubramanian P, Chen Y (2020) Schizophrenia detection using multivariateempirical mode decomposition and entropy measures from multichannel EEG signal. Biocybern Biomed Eng 40:1124–1139. https://doi.org/10.1016/j.bbe.2020.05.008
Kulkarni V, Joshi Y, Manthalkar R, Elamvazuthi I (2022) Band decomposition of asynchronous electroencephalogram signal for upper limb movement classification. Phys Eng Sci Med 45:643–656. https://doi.org/10.1007/S13246-022-01132-4/TABLES/8
Kumar G, Chander S, Almadhor A (2022) An intelligent epilepsy seizure detection system using adaptive mode decomposition of EEG signals. Phys Eng Sci Med 45:261–272. https://doi.org/10.1007/S13246-022-01111-9/TABLES/6
Kumar TS, Rajesh KN, Maheswari S et al (2023) Automated Schizophrenia detection using local descriptors with EEG signals. Eng Appl Artif Intell 117:105602
Kutepov IE, Dobriyan VV, Zhigalov MV et al (2020) EEG analysis in patients with schizophrenia based on Lyapunov exponents. Inf Med Unlocked 18:100289. https://doi.org/10.1016/j.imu.2020.100289
Lanillos P, Oliva D, Philippsen A et al (2020) A review on neural network models of schizophrenia and autism spectrum disorder. Neural Netw 122:338–363. https://doi.org/10.1016/j.neunet.2019.10.014
Laursen TM (2011) Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res 131:101–104. https://doi.org/10.1016/J.SCHRES.2011.06.008
Li P, Li C, Bore JC et al (2022) L1-norm based time-varying brain neural network and its application to dynamic analysis for motor imagery. J Neural Eng 19:026019. https://doi.org/10.1088/1741-2552/AC59A4
Lillo E, Mora M, Lucero B (2022) Automated diagnosis of schizophrenia using EEG microstates and Deep Convolutional Neural Network. Expert Syst Appl 209:118236. https://doi.org/10.1016/j.eswa.2022.118236
Messias Erick, Garcia-Rill Edgar (2019) Schizophrenia and arousal. Arousal in neurological and psychiatric diseases. Elsevier, pp 43–54. https://doi.org/10.1016/B978-0-12-817992-5.00003-9
Naira CAT, Del Alamo CJL (2019) Classification of people who suffer schizophrenia and healthy people by EEG signals using deep learning. Int J Adv Comput Sci Appl. https://doi.org/10.14569/IJACSA.2019.0101067
Najafzadeh H, Esmaeili M, Farhang S et al (2021) Automatic classification of schizophrenia patients using resting-state EEG signals. Phys Eng Sci Med 44:855–870
Nsugbe E, Samuel OW, Asogbon MG, Li G (2022) Intelligence combiner: a combination of deep learning and handcrafted features for an adolescent psychosis prediction using EEG signals. In 2022 IEEE International Workshop on Metrology for Industry 40 and IoT, MetroInd 40 and IoT 2022-Proceedings 92–97. doi: https://doi.org/10.1109/MetroInd4.0IoT54413.2022.9831741
Pan C, Shi C, Mu H et al (2020) EEG-based emotion recognition using logistic regression with gaussian kernel and laplacian prior and investigation of critical frequency bands. Appl Sci (Switz) 10:1619. https://doi.org/10.3390/app10051619
Article CAS Google Scholar
Phang CR, Noman F, Hussain H et al (2020) A multi-domain connectome convolutional neural network for identifying schizophrenia from EEG connectivity patterns. IEEE J Biomed Health Inform 24:1333–1343. https://doi.org/10.1109/JBHI.2019.2941222
Phang CR, Ting CM, Samdin SB, Ombao H (2019) Classification of EEG-based effective brain connectivity in Schizophrenia using deep neural networks. International IEEE/EMBS conference on neural engineering, NER 2019-March:401–406. doi: https://doi.org/10.1109/NER.2019.8717087
Preity, Ranjan R, Verma K, Sahana BC (2023) A Computer-aided prediagnosis system for health prediction based on personal health data. In 2023 IEEE 12th international conference on communication systems and network technologies (CSNT). pp 271–276
Raghavendra U, Acharya UR, Adeli H (2020) Artificial intelligence techniques for automated diagnosis of neurological disorders. Eur Neurol 82:41–64. https://doi.org/10.1159/000504292
Ranjan R, Arya R, Kshirsagar P et al (2018) Real time eye blink extraction circuit design from EEG signal for ALS patients. J Med Bio Eng 38:933–942. https://doi.org/10.1007/s40846-017-0357-7
Ranjan R, Chandra Sahana B, Kumar Bhandari A (2021) Ocular artifact elimination from electroencephalography signals: a systematic review. Biocyber Biomed Eng 41:960–996. https://doi.org/10.1016/j.bbe.2021.06.007
Ranjan R, Sahana BC, Bhandari AK (2022a) Cardiac artifact noise removal from sleep EEG signals using hybrid denoising model. IEEE Trans Instrum Meas 71:1–10. https://doi.org/10.1109/TIM.2022.3198441
Ranjan R, Sahana BC, Bhandari AK (2024) Deep learning models for diagnosis of schizophrenia using EEG signals: emerging trends, challenges, and prospects. Springer, Netherlands
Ranjan R, Sahana BC (2022) A machine learning framework for automatic diagnosis of schizophrenia using EEG signals. In INDICON 2022 - 2022 IEEE 19th India council international conference. IEEE, pp 1–6
Ranjan R, Sahana BC (2023) Automated alzheimer’s disease diagnosis using norm features extracted from EEG signals. In 2023 14th international conference on computing communication and networking technologies (ICCCNT). pp 1–6
Ranjan R, Sahana BC (2019) An efficient facial feature extraction method based supervised classification model for human facial emotion identification. In 2019 IEEE 19th international symposium on signal processing and information technology, ISSPIT 2019. https://doi.org/10.1109/ISSPIT47144.2019.9001839
Ranjan R, Sahana BC, Bhandari AK (2022) Motion artifacts suppression from EEG signals using an adaptive signal denoising method. IEEE trans instrument meas. https://doi.org/10.1109/TIM.2022.3142037
Reinertsen E, Clifford GD (2023) SchizoNET: a robust and accurate Margenau-Hill time-frequency distribution based deep neural network model for schizophrenia detection using EEG signals You may also like A review of physiological and behavioral monitoring with digital sensors for neurops. Physiol Meas 44:35005. https://doi.org/10.1088/1361-6579/acbc06
Riaz F, Hassan A, Rehman S et al (2016) EMD-based temporal and spectral features for the classification of EEG signals using supervised learning. IEEE Trans Neural Syst Rehabil Eng 24:28–35. https://doi.org/10.1109/TNSRE.2015.2441835
Richens JG, Lee CM, Johri S (2020) Improving the accuracy of medical diagnosis with causal machine learning. Nature Commun 11:1–9. https://doi.org/10.1038/s41467-020-17419-7
Roy Y, Banville H, Albuquerque I et al (2019) Deep learning-based electroencephalography analysis: a systematic review. J Neural Eng 16:051001. https://doi.org/10.1088/1741-2552/AB260C
Sadiq MT, Yu X, Yuan Z (2021) Exploiting dimensionality reduction and neural network techniques for the development of expert brain–computer interfaces. Expert Syst Appl 164:114031. https://doi.org/10.1016/J.ESWA.2020.114031
Saini M, Satija U, Upadhayay MD (2020) Wavelet based waveform distortion measures for assessment of denoised EEG quality with reference to noise-free EEG signal. IEEE Signal Process Lett 27:1260–1264. https://doi.org/10.1109/LSP.2020.3006417
Sairamya NJ, Subathra MSP, Thomas George S (2022) Automatic identification of schizophrenia using EEG signals based on discrete wavelet transform and RLNDiP technique with ANN. Expert Syst Appl 192:116230. https://doi.org/10.1016/j.eswa.2021.116230
Savas C, Dovis F (2019) The impact of different kernel functions on the performance of scintillation detection based on support vector machines. Sensors 19:5219. https://doi.org/10.3390/S19235219
Şen B, Peker M, Çavuşoğlu A, Çelebi FV (2014) A comparative study on classification of sleep stage based on EEG signals using feature selection and classification algorithms. J Med Syst. https://doi.org/10.1007/s10916-014-0018-0
Shalbaf A, Bagherzadeh S, Maghsoudi A (2020) Transfer learning with deep convolutional neural network for automated detection of schizophrenia from EEG signals. Phys Eng Sci Med 43:1229–1239. https://doi.org/10.1007/s13246-020-00925-9
Sharma M, Acharya UR (2021) Automated detection of schizophrenia using optimal wavelet-based l1 norm features extracted from single-channel EEG. Cogn Neurodyn 15:661–674. https://doi.org/10.1007/s11571-020-09655-w
Sharma G, Joshi AM (2022) SzHNN: a novel and scalable deep convolution hybrid neural network framework for schizophrenia detection using multichannel EEG. IEEE Trans Instrument Meas. https://doi.org/10.1109/TIM.2022.3212040
Shrestha A, Mahmood A (2019) Review of deep learning algorithms and architectures. IEEE Access 7:53040–53065
Singh K, Malhotra J (2021) Deep learning based smart health monitoring for automated prediction of epileptic seizures using spectral analysis of scalp EEG. Phys Eng Sci Med 44:1161–1173. https://doi.org/10.1007/S13246-021-01052-9
Singh K, Singh S, Malhotra J (2021) Spectral features based convolutional neural network for accurate and prompt identification of schizophrenic patients. Proc Inst Mech Eng [h] 235:167–184. https://doi.org/10.1177/0954411920966937
Siuly S, Li Y, Zhang Y (2016) EEG signal analysis and classification techniques and applications. Springer International Publishing, Cham
Book Google Scholar
Siuly S, Khare SK, Bajaj V et al (2020a) A computerized method for automatic detection of schizophrenia using EEG signals. IEEE Trans Neural Syst Rehabil Eng 28:2390–2400. https://doi.org/10.1109/TNSRE.2020.3022715
Siuly S, Khare SK, Bajaj V et al (2020b) A computerized method for automatic detection of schizophrenia using EEG signals. IEEE Trans Neural Syst Rehabil Eng 28:2390–2400
Sobahi N, Ari B, Cakar H et al (2022) A new signal to image mapping procedure and convolutional neural networks for efficient Schizophrenia detection in EEG recordings. IEEE Sens J 22:7913–7919. https://doi.org/10.1109/JSEN.2022.3151465
Sofri T, Rahim HA, Andrew AM et al (2023) Data normalization methods of hybridized multi-stage feature selection classification for 5G base station antenna health effect detection. J Adv Res Appl Sci Eng Technol 30:133–140
Song YY, Lu Y (2015) Decision tree methods: applications for classification and prediction. Shanghai Arch Psychiatry 27:130. https://doi.org/10.11919/J.ISSN.1002-0829.215044
Supakar R, Satvaya P, Chakrabarti P (2022) A deep learning based model using RNN-LSTM for the Detection of Schizophrenia from EEG data. Comput Biol Med 151:106225. https://doi.org/10.1016/j.compbiomed.2022.106225
Thilakavathi B, Shenbaga Devi S, Malaiappan M, Bhanu K (2019) EEG power spectrum analysis for schizophrenia during mental activity. Australas Phys Eng Sci Med 42:887–897. https://doi.org/10.1007/S13246-019-00779-W/TABLES/6
Thirumalaisamy MR, Ansell PJ (2018) Fast and adaptive empirical mode decomposition for multidimensional, multivariate signals. IEEE Signal Process Lett 25:1550–1554. https://doi.org/10.1109/LSP.2018.2867335
Wilches-Bernal F, Jiménez-Aparicio M, Reno MJ (2022) A machine learning-based method using the dynamic mode decomposition for fault location and classification. In 2022 IEEE Power & Energy Society Innovative Smart Grid Technologies Conference (ISGT). pp 1–5
Wu Y, Xia M, Wang X, Zhang Y (2023) Schizophrenia detection based on EEG using recurrent auto-encoder framework. In Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). pp 62–73
Yakoubi M, Hamdi R, Salah MB (2019) Abnormal brain detection and analysis of EEG signals.In 2018 International Conference on Signal, Image, Vision and their Applications, SIVA 2018. https://doi.org/10.1109/SIVA.2018.8661078
Yang J, Gao S, Shen T (2022) A two-branch CNN fusing temporal and frequency features for motor imagery EEG decoding. Entropy 24:376. https://doi.org/10.3390/E24030376
Zheng S, Tan J, Jiang C, et al (2022) L2-norm scaled transformer for 3D head and neck primary tumors segmentation in PET-CT. In 2022 IEEE International Conference on Systems, Man, and Cybernetics (SMC). pp 1186–1191
Zülfikar A, Mehmet A (2022) Empirical mode decomposition and convolutional neural network-based approach for diagnosing psychotic disorders from EEG signals. Appl Intell. https://doi.org/10.1007/s10489-022-03252-6
Download references
Author information
Authors and affiliations.
Department of Electronics and Communication Engineering, National Institute of Technology Patna, Patna-, 800005, India
Rakesh Ranjan & Bikash Chandra Sahana
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Rakesh Ranjan .
Ethics declarations
Conflict of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Ethical approval
This study did not involve the use of human participants or animals. The research is based solely on the analysis of publicly available data, and no new data were collected from humans or animals for the purposes of this study.
Informed consent
Not applicable.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Ranjan, R., Sahana, B.C. Multiresolution feature fusion for smart diagnosis of schizophrenia in adolescents using EEG signals. Cogn Neurodyn (2024). https://doi.org/10.1007/s11571-024-10120-1
Download citation
Received : 21 October 2023
Revised : 07 April 2024
Accepted : 22 April 2024
Published : 11 May 2024
DOI : https://doi.org/10.1007/s11571-024-10120-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Electroencephalogram
- Empirical mode decomposition
- Empirical wavelet transform
- Convolutional neural network
- Feature fusion
- Classification
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 16 May 2024
Effectiveness of a needs-tailored nurse-led recovery program for community-dwelling people with schizophrenia: a cluster-randomized controlled trial
- Wen-I Liu 1 ,
- Wen-Ling Hsieh 1 ,
- Ching-Ting Lai 1 ,
- Chia-Chen Liu 2 ,
- Yueh-Ming Tai 2 &
- Chieh-Yu Liu 3
BMC Nursing volume 23 , Article number: 329 ( 2024 ) Cite this article
60 Accesses
Metrics details
Meeting people’s needs is positively correlated with their recovery. However, recovery services rarely include nurse-led programs tailored to the needs of these people. This study aimed to evaluate the effectiveness of a new needs-tailored recovery program by using a cluster-randomized controlled trial design.
We conducted a parallel randomized controlled trial in two community psychiatric departments, employing nurse-level clustering for intervention delivery and selecting participants through convenience sampling. The participants were people diagnosed with schizophrenia that were receiving homecare services. The experimental group ( n = 82) received needs-tailored recovery program for six months. The control group ( n = 82) received traditional homecare. Data were collected at baseline, post-intervention, and the three-month follow-up (the study ran from February to December 2021). The outcomes were recovery, needs, hope, empowerment, psychotic symptoms, and medication adherence. We used repeated measures ANOVA tests to examine the effect of the group × time interaction.
The participants in the experimental group demonstrated statistically significant improvements in recovery, hope, and medication adherence compared to the control group, both immediately post-intervention and at the three-month follow-up. Moreover, they exhibited statistically significant reductions in needs compared to the control group at the three-month follow-up ( p < .05). While the interaction effect for psychotic symptoms was not significant, the time effect was significant ( p < .05). No significant interaction or time effect was observed for empowerment.
The findings increase our understanding of recovery-oriented care that prioritizes therapeutic alliance, integrated needs assessment, individual goals, hope, and empowerment.
Trial registration
The Clinicaltrials.gov identifier NCT05304780 retrospectively registered on 03/31/2022.
Peer Review reports
Recovery-oriented care has attracted considerable attention globally and is considered the goal of mental health care [ 1 ]. Personal recovery is a multifaceted concept and can be defined as both the personal process of living with serious mental illness and the results of mental health care [ 2 , 3 ]. Some developed countries have established related programs and evaluated their effectiveness in improving recovery rates [ 4 ]. Meeting the needs of community-dwelling people with schizophrenia is positively correlated with their recovery [ 5 , 6 ]. In a longitudinal study, it was demonstrated that systematically monitoring patients’ needs can improve their psychotic symptoms, thereby promoting their recovery. The degree to which patients’ needs are met is significantly and positively correlated with the extent of their recovery [ 6 ]. However, 25–50% of people with serious mental illness have unmet needs [ 7 ], which may exacerbate their psychotic symptoms and hinder their recovery [ 8 ].
In Taiwan, community psychiatric nurses play a vital role in delivering mental health care services to people with psychiatric disorders who reside in the community and experience residual symptoms, often without access to continuous treatment in mental health institutions. These services provided by community psychiatric nurses typically include medication treatment, health education, and family consultations, aiming to address the disease-oriented needs of people with psychiatric disorders. Despite the increasing demand for support in this population, existing care services have not adequately expanded to meet these needs. While temporary care gap solutions, such as community care quality promotion programs, have been implemented, formal, needs-tailored, and recovery-oriented home care services are notably absent. Moreover, the current community mental health care system primarily focuses on medical treatment and lacks an integrated care model that caters to individual needs. Consequently, there is an urgent imperative to develop integrated and needs-tailored recovery care services to bridge this gap and better support people with psychiatric disorders [ 9 ].
Based on a systematic review, the complex and multifaceted needs of community-dwelling people with schizophrenia are as follows: mental recovery, disease management, life management, crisis management, family support, social participation, and resource connection [ 10 ]. These services should be provided by multidisciplinary teams. Case management processes should provide integrated services that can effectively reduce the number of hospitalizations for community-dwelling patients, connect them with professional services, enhance the quality of care, and improve psychosocial outcomes [ 10 , 11 , 12 ]. Current recovery program providers are mostly psychologists, occupational therapists, and rehabilitation therapists, and nurse-led, recovery-oriented, and individualized care services are rare [ 2 , 4 ]. Psychiatric homecare nurses, who make up the largest group of community-based care professionals, have the most direct contact with this population. Their unique role in providing integrated care for community-based personal recovery should be expanded and the effectiveness of person-centered, individualized recovery programs must be developed and assessed [ 13 ].
In many countries, there is currently a trend of mental health services shifting from the hospital setting to community-based care. Emphasizing person-centered and recovery-oriented values is increasingly becoming a core concept in psychiatric care [ 14 , 15 ]. Psychiatric mental health nurses can work together with service users to support recovery processes [ 16 ]. However, the recovery services offered globally seldom comprise nurse-led, person-centered programs that are tailored to the needs of such people. Most Asian people with schizophrenia live at home in the community rather than in institutions [ 17 ]. As highlighted by the National Health Research Institute, the needs of people with schizophrenia in Taiwan have not been fully evaluated and community care services have been unable to respond to their needs for individualized care [ 13 ]. Consequently, the primary aim of this cluster-randomized controlled trial was to assess the effectiveness of a novel needs-tailored recovery program on various outcomes, including recovery, needs, hope, empowerment, medication adherence, and psychotic symptoms among community-dwelling people with schizophrenia. We hypothesized that participants receiving the needs-tailored recovery program, compared to those in the usual care control group, would demonstrate significantly enhanced levels of recovery, hope, empowerment, and medication adherence, alongside reduced levels of needs and psychotic symptoms. If successful, such a program would move community homecare services toward a forward-looking, evidence-based, and innovative model of care for community-dwelling people with schizophrenia.
As highlighted by the National Health Research Institute, the assessment of needs among people with schizophrenia in Taiwan remains incomplete, and existing community care services have struggled to meet their requirements for personalized care [ 13 ]. Consequently, the primary objective of this cluster-randomized controlled trial was to assess the impact of a novel needs-tailored recovery program on various outcomes, including recovery, needs, hope, empowerment, medication adherence, and psychotic symptoms, among people with schizophrenia residing in the community. Our hypothesis posited that participants receiving the needs-tailored recovery program, in contrast to those in the usual care control group, would exhibit significantly enhanced levels of recovery, hope, empowerment, and medication adherence, alongside reduced levels of needs and psychotic symptoms.
This was a cluster-randomized controlled trial with convenience sampling. The study was conducted over two years in two phases—development and evaluation. In the first phase, from August 2019 to June 2020, we developed the program and conducted expert content validity tests based on a systematic review and the Delphi method. The current manuscript describes the second phase, which was conducted between February and December 2021 in Taiwan and includes an effectiveness evaluation. We first conducted a pilot study to refine the program, followed by a parallel cluster-randomized controlled trial with a repeated measures design to evaluate the effectiveness of the intervention. The trial was registered at NCT05304780. This study was grounded in the CONSORT checklist for reports of randomized trials.
Participants
The participants were recruited from the community psychiatric departments of two research institutes in northern Taiwan. These institutes provide homecare for the largest number of people diagnosed with schizophrenia in Taipei City and New Taipei City. All research institutions are psychiatric teaching hospitals that share uniformity in terms of size, certification grade, competencies of community psychiatric nurses, and availability of psychiatric home care equipment. Each research institute typically employed six community psychiatric nurses. In total, approximately 1000 community-dwelling clients received care from the two research institutions, including around 600 diagnosed with schizophrenia, some of whom exhibit residual symptoms of psychosis.
People who met the following criteria were enrolled as study participants: (1) were living in the community and had a diagnosis of schizophrenia based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); (2) were aged 20–64 years; and (3) were able to communicate in Mandarin or Taiwanese. We applied these criteria to reduce participant heterogeneity and any potential communication difficulties.
Potential participants were excluded if they (1) were living in a community institution such as a recovery home, community rehabilitation center, nursing home, or day hospital; or (2) had a neurocognitive disorder, substance abuse disorder, or comorbidity.
Randomization
Given the primary nursing care model adopted in psychiatric home care, each community psychiatric nurse is responsible for providing home care within their assigned area. As a result, random assignment of patients was not feasible. Hence, this study employed nurse-level clustering for intervention delivery.
To ensure homogeneity in the education level and proficiency of the interventionists, as well as consistency in intervention delivery, selection criteria included holding a bachelor’s degree, possessing over five years of experience, and passing the community psychiatric mental health competency assessment.
To balance group sizes and ensure an adequate sample size across groups, a total of eight nurses, with four in each institution meeting the above criteria, were selected for this study. In each institution, two nurses were randomly selected as interventionists in the experimental group, while the other two were assigned to the control group. Random selection was performed using the RANDBETWEEN function in Microsoft Excel, and this process was conducted by a blinded allocator.
Each nurse screened and selected patients meeting the inclusion criteria for participation. Convenience sampling, based on patient availability or accessibility, streamlined data collection, saving time and costs. Nurses in the experimental group provided needs-tailored recovery interventions, while those in the control group delivered traditional home care.
The interventionists were aware of group allocation because they had to be given specific training and offer the intervention, but the participants were blinded to their group allocation. Both groups received home visits by the psychiatric homecare nurses, who were asked not to reveal their group assignment to the participants. The data were collected by two trained research assistants who were blinded to intervention to reduce potential bias in the data collection.
Sample size
As a post hoc validation procedure, we used G*Power version 3.10 to estimate the power of the study given our sample size and analysis methods. We employed repeated-measures ANOVA to compare changes between the two study groups at three time points. The statistical test utilized was the within-between interaction, assuming an effect size of 0.11 [ 18 ], with 80% power and a significance level of 5%. Prior to the trial, the estimated total sample size was 136, accounting for an anticipated 20% loss rate, resulting in an expected total of 164 participants.
Intervention: the needs-tailored recovery program
We conducted a systematic review, extracted the essential components for the recovery program, and developed an intervention manual with standardized workflows and service content. The content validity index of the program was assessed by eight clinical experts and scholars in community psychiatric mental health. The feasibility, efficacy, and cost-effectiveness of the intervention were considered adequate, based on its content validity index of 0.97. The recovery program integrated the following evidence-based essential components that we had identified as important for community-dwelling people with schizophrenia: care needs, empowerment, and medication adherence, as well as hope [ 3 , 19 ]. The intervention frequency comprised a minimum of one home visit every two weeks, spanning a duration of six months. The intervention process consisted of five steps (refer to Fig. 1 ), encompassing a total of 12 home visits, each lasting approximately 50 min. Initially, emphasis was placed on building relationships, followed by two visits dedicated to integrated needs assessment. Subsequently, eight visits focused on providing empowerment-oriented interventions and monitoring goals, culminating in a final visit for evaluations.
This intervention process adhered to a standard manual, with each step recorded using a checklist to ensure consistency. Interventionists conducted discussion and training meetings based on the standard manual and underwent consistency training before commencing the intervention. Further details are provided below:
Step 1: Build partnerships : create a partnering environment, develop self-awareness, show empathy and sincerity, and provide support.
Step 2: Conduct integrated needs assessment : use the needs assessment scale for community-dwelling people diagnosed with schizophrenia to identify their needs and priorities. The development of this scale was primarily informed by a systematic literature review and referenced the Camberwell Assessment of Need (CAN), a comprehensive evaluation of patients’ needs [ 20 ]. This tool comprises two parts: the first part is a score sheet ranging from 0 to 2 points, while the second part involves qualitative records obtained through visit interviews and interactions.
Step 3: Set needs-based recovery goals and provide empowerment-oriented interventions as follows :
Disease management: improve disease-response capacity, strengthen medication motivation, promote self-management of medications, and encourage alertness for and prevention of recurrence.
Crisis management: respond to stress, know how to deal with an immediate crisis affecting the client or their family, and activate crisis management through healthcare professionals.
Personal recovery: recognize the aspects of personal recovery, use self-empowerment, and overcome self-stigma.
Life management: promote motivation, planning, and implementation of life management and strengthen support.
Family support: promote consistent communication within the family, meeting family responsibilities, and family-based problem solving.
Social participation: improve social skills, provide peer support, and maintain appropriate social activities.
Resource connection: provide resource information, enhance motivation to use resources, and encourage the development of knowledge of and the ability to use resources.
Step 4: Conduct continuous monitoring of goals.
Step 5: Conduct effectiveness evaluations : treat recovery as the primary outcome and the other elements (needs, hope, empowerment, medication adherence, and psychotic symptoms) as the secondary outcome measures.
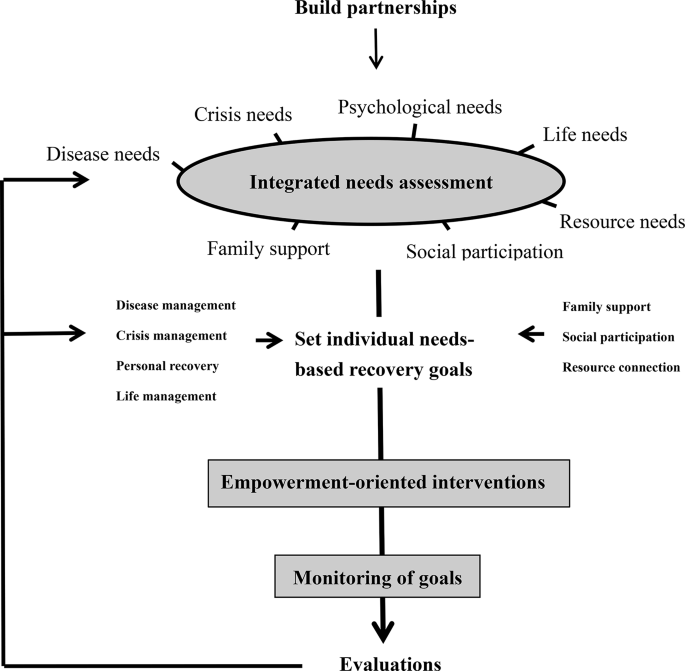
The needs-tailored nurse-led recovery program
Traditional homecare as usual care
Traditional homecare, serving as the usual care in this study, involved community psychiatric nurses and doctors conducting home visits for psychiatric patients. These visits encompassed the provision of medication treatment, health education, and family support to assist patients with living in the community. The frequency and duration of the visits were consistent with those of the experimental group, occurring every two weeks and lasting 50 min each, over a period of six months.
Data collection
Two nursing research assistants who were experienced in psychiatric care collected the data. They measured the effectiveness of the program by using reliable, validated questionnaires at the beginning and end of the intervention period and at a follow-up session three months later. The following basic demographic data were collected: gender, age, education level, marital status, living status, employment status, religion, economic status, duration of receiving homecare, total number of psychiatric hospitalizations, and number of such hospitalizations during the past year. Guided by the questionnaire, assessments of the participant’s personal recovery, empowerment, needs, hope, psychotic symptoms, and medication adherence were collected. The psychotic symptoms were assessed by the interviewers and the participants answered the remaining questions themselves. If the participants did not understand any of the questions, the interviewer provided assistance and instructions.
Outcome measurements
This study included seven outcome measures. The primary outcome was recovery, while the secondary outcomes consisted of needs, hope, empowerment, medication adherence, and psychotic symptoms. Assessments were conducted at baseline, post-intervention, and three-month follow-up. Nurses administered assessments for needs and psychotic symptoms, while participants self-assessed the other outcomes. Further details are provided below:
We used the Questionnaire about the Process of Recovery, developed and validated by Neil et al. (2009) [ 21 ], to evaluate the participants’ recovery. This instrument has been widely used internationally and was translated by Chien and Chan into Mandarin in 2013. The higher the score, the greater the recovery of the respondent. The total variance explained was > 48%, Cronbach’s α was 0.77–0.94, and the two-week test–retest reliability was 0.77–0.87 [ 22 ].
The development of this needs scale was based mainly on a systematic literature review of studies [ 10 ] and referencing the Camberwell Assessment of Need [ 23 , 24 , 25 ]. The associated questionnaire has a total of 22 questions, with each question scored from 0 to 2 points. The higher the score, the higher the level of needs. Cronbach’s α for this questionnaire was 0.81, and the total variance explained in the factor analysis was 61.23% [ 20 ].
We used the Herth Hope Index, which comprises 12 questions, to assess hope. These questions are answered using a four-point Likert scale, and the higher the score, the higher the level of hope [ 26 ]. The index is the most widely translated and thoroughly psychometrically tested tool in languages other than English [ 27 ]. Chan et al. (2011) translated this scale into Chinese and found that the reliability and validity of this version were good [ 28 ].
Empowerment
We used the Empowerment Scale developed by Rogers et al. (1997) [ 29 ]. We used the Mandarin version, which was translated by our research team and verified using people diagnosed as having schizophrenia. The content validity index was 0.88. The total variance explained was 59%. Cronbach’s α was 0.87 [ 30 ].
Medication adherence
We used the Medication Adherence Rating Scale developed by Thompson et al. (2000) [ 31 ]. The associated questionnaire consists of 10 questions and has a total score of 0–10 points. The higher the score, the better the adherence to medication. The questionnaire was translated into Mandarin by Kao and Liu (2010) [ 32 ]. Cronbach’s α for this questionnaire was 0.72, with a two-week test–retest reliability of 0.80 and 49.7% of the total variance explained [ 32 ].
Psychotic symptoms
We used the Brief Psychiatric Rating Scale developed by Overall and Gorham (1962) to evaluate the participants’ psychotic symptoms [ 33 ]. The score for each of the 16 questions is 0–7 points, and the higher the score, the more obvious the psychotic symptoms. Chang et al. (1986) translated the scale into Mandarin and found that the reliability and validity of the scale were good [ 34 ].
Data analysis
Loss of participants occurred primarily due to reasons such as rehospitalization and declining to respond. Despite the occurrence of some loss, it remained within an acceptable range and did not fall below the estimated total sample size of 136, ensuring a statistical power of over 0.8. Therefore, we chose not to utilize imputation methods to address missing data. Instead, we conducted a per-protocol (PP) analysis. We used IBM SPSS 25.0 (IBM, Armonk, NY, US) for data entry and analysis. We first conducted a descriptive analysis of the data using averages, standard deviations, numbers, and percentages. We then compared the homogeneity of the demographics and the pretest outcome measures between the groups using t -tests and chi-squared tests. We employed repeated-measures ANOVAs to examine differences between the two groups, focusing on group-by-time interactions for each outcome variable. We first tested the assumptions for the ANOVA (independence, normality, and sphericity), and where the data did not satisfy the requirement of sphericity, we applied the Huynh–Feldt correction.
Demographics, mental health status, and homogeneity tests of the two groups
Each research institution had four community psychiatric nurses, who were randomly selected as either experimental group interventionists or control group providers, with each nurse responsible for approximately 80 cases. Under the eight community psychiatric nurses, a total of 642 patients who received psychiatric home care were initially included to assess for eligibility. However, 478 patients either did not meet the inclusion criteria or did not provide the research consent form, as the required number of participants had already been reached. Consequently, a total of 164 participants were included in this study (Fig. 2 ). Each group contained 82 participants. During the study, 13 participants withdrew due to hospitalization or quarantine related to COVID-19, resulting in a subject loss rate of 7.9%. No statistically significant differences were observed between participants who completed the study and dropouts regarding demographics, clinical variables, and outcome variables ( p > .05). The analysis comprised 151 participants, with post-hoc analysis revealing a statistical power of 0.85.
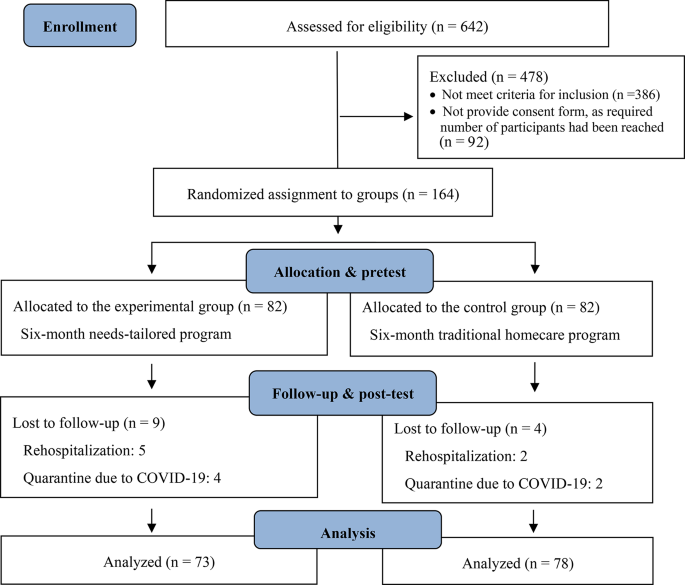
Flow diagram for participant recruitment and retention
The study had more male participants (58.9%) than female. The mean age of these participants was 49.53 ± 9.27 years. The majority of the participants were high school or vocational training graduates (43.0%) who were living with family members (90.7%), unemployed (76.8%), Buddhist (42.4%), and belonged to a low-income household (52.3%). Most participants (66.9%) had received homecare for more than two years. The average total number of psychiatric hospitalizations per participant was 3.59 ± 3.95. The average number of psychiatric hospitalizations per participant in the previous year was 0.15 ± 0.44 (Table 1 ).
At baseline, there were no statistically significant differences in demographics, clinical variables, and outcome variables between the experimental and control groups (Tables 1 and 2 ).
Effect on recovery, needs, hope, empowerment, medication adherence, and psychotic symptoms
Participants in the experimental group demonstrated statistically significant improvements in recovery compared to the control group, both immediately post-intervention ( F = 7.04, p = .009) and at the 3-month follow-up ( F = 8.34, p = .004). Furthermore, at the 3-month follow-up, the experimental group exhibited statistically significant decreases in needs compared to the control group (F = 22.56, p < .001).
Additionally, participants in the experimental group showed statistically significant improvements in hope compared to the control group, both immediately post-intervention ( F = 4.22, p = .037) and at the 3-month follow-up (F = 5.89, p = .016). Finally, participants in the experimental group displayed statistically significant improvements in medication adherence compared to the control group, both immediately post-intervention ( F = 7.20, p = .008) and at the 3-month follow-up ( F = 4.14, p = .044), as evidenced by repeated-measures ANOVA.
Although there were no statistically significant group differences in psychotic symptoms, there was a statistically significant time effect ( F = 4.10, p = .035). There were no statistically significant group or time effects on empowerment (Table 2 ; Fig. 3 ).
Group differences in outcomes over time. E = experimental group; C = control group; * = p -values < 0.05 of interaction effects; ** = p -values < 0.01 of interaction effects; *** = p -values < 0.001 of interaction effects
We found that a needs-tailored recovery program was more effective than traditional homecare for improving recovery, needs, hope, and medication adherence in people with schizophrenia, and that its effects were sustained. This finding aligns with systematic literature, indicating that adequate hope, family, and social support are key facilitators of recovery. This finding aligns with previous literature, which suggests that factors such as adequate hope, family support, social support, and reduced needs can contribute to facilitating recovery [ 9 , 35 ]. In a recovery program, the goal of care must align with and be tailored to the individual patients’ needs [ 8 , 36 ]. Our results support previous research that shows that people with schizophrenia have complex and multifaceted needs, which are influenced by a range of clinical, psychological, social, economic, and occupational factors [ 37 ]. Our needs-tailored, person-centered recovery program takes into account the aforementioned aspects and has proven to be effective in supporting patient recovery. Additionally, existing literature suggests that recovery is not only an outcome but also a continuous process [ 38 ]. Notably, our program demonstrated significant post-intervention effects, outperforming traditional homecare at the three-month follow-up. This underscores the program’s ability to support patients throughout their ongoing recovery journey and highlights its value as an intervention. Our needs-tailored, person-centered recovery program considers the abovementioned aspects and is effective in supporting patient recovery.
This confirms that this evidence-based program, which was designed based on quantitative literature [ 3 , 19 , 39 , 40 ] and whose effectiveness was evaluated using a randomized control trial design, is beneficial both in terms of its theoretical applications and for practical clinical work. It would be able to meet the disease-related, psychological, and social participation of more than 50% of existing people diagnosed as having schizophrenia and provide service strategies that respond to the needs assessed in this study. Schizophrenia is a substantial burden on the global community, and as an economic burden accounts for 0.02–1.65% of total production value. Given the limited available medical resources and other competing and urgent demands, effectively promoting patient recovery could reduce the burden placed on limited healthcare workforces [ 41 ]. This innovative recovery program is person-centered and individualized. It has good expert validity and empirical evidence supports its effectiveness. It could be used to guide future mental health policies and practices and serve as a leading nurse-led model for care practices.
One possible explanation for the program’s lack of significant impact on empowerment could be the cultural mismatch between the empowerment concepts utilized. The Chinese version of the empowerment scale, translated from Roger’s Empowerment Scale, may be more aligned with Western culture. However, literature suggests that in some Asian populations, empowerment may be perceived differently, with a focus on maintaining a satisfactory quality of life by remaining passive and minimizing stressors [ 35 ]. Additionally, the participants used to validate Roger’s Empowerment Scale or the Chinese version of the Empowerment Scale may have been individuals with stable chronic psychotic disorders in a community institution setting or chronic ward, whose characteristics may not fully align with those of the participants in our study [ 29 , 30 ]. Therefore, it is recommended to utilize measurement tools that are more aligned with Eastern culture when assessing empowerment among homecare psychiatric patients.
We also did not observe any statistically significant differences in psychotic symptoms between the two groups, although these symptoms were reduced over time in both. A possible reason for this is that since the participants were community-dwelling people with stable psychotic symptoms not requiring hospitalization [ 42 ], they were in the nonacute phase of the disease and had fewer symptoms anyway, although they did still require continuous care. The other possibility is that the usual homecare is already sufficient to provide statistically significant improvements in psychiatric symptoms [ 43 ]. Further, the nurses who provided the services to both groups had passed the competence assessment and were qualified to provide community mental healthcare. Consequently, both groups received high-quality care and achieved positive results [ 44 ]. The fact that the study used homecare provided by nurses also revealed the unique role of nurses in promoting clients’ medication adherence, thereby reducing disease recurrence and rehospitalization and enabling them to live a stable life in the community [ 45 , 46 ].
Adherence to medication involves multiple complex behaviors. In the future, continuous development and verification are required to design and apply empowerment-oriented, theory-based programs that combine diversified and empirical strategies to strengthen the motivation and attitudes toward medication adherence of people with schizophrenia [ 39 ]. In addition, ongoing evaluations may be necessary to track how effective these programs are in the long term.
Limitations
Although we used random assignment and research instruments with good reliability and validity, the study nevertheless had several limitations. First, we used convenience sampling, voluntary participation, and conducted the study in northern Taiwan. These aspects may be associated with a risk of sampling bias and the samples may not be representative of the wider population. However, we did recruit participants from two different institutes to reduce the threat to external validity. Further, the participants were all home-based clients, and the service providers were asked not to exchange information during the intervention training, so the risk of cross-contamination between clients and service providers was low.
Finally, we respected the participants’ right to withdraw from the study and were therefore unable to collect post-test data from several of them. We did not conduct an intention-to-treat analysis. However, both the participants and the data collectors were blinded to group assignment to maintain construct validity.
Conclusions
We developed an individualized recovery program in the form of a nurse-led homecare intervention that was tailored to the needs of community-dwelling people with schizophrenia. Compared with those who received the traditional homecare services, the recovery, hope, and medication adherence scores of the participants who received the experimental intervention were significantly improved. Additionally, they showed statistically significant decreases in needs compared to the control group.
This intervention is centered on addressing the diverse needs of people diagnosed with schizophrenia to promote recovery. It deviates from past disease-oriented care approaches by emphasizing the necessity of transitioning towards a recovery-oriented care model in the future. By focusing on holistic and individualized support, the intervention aims to empower patients and enhance their overall well-being, thereby facilitating long-term recovery outcomes.
Our findings contribute to the understanding of effective recovery-oriented care practices, which prioritize elements such as therapeutic alliance, integrated needs assessment, individual goal-setting, hope, and empowerment. This paradigm-shifting nurse-led program should be embraced as an innovative approach to community mental health care, serving as a practical guide for the care of people with schizophrenia residing in the community.
Data availability
The research data cannot be shared openly due to the necessity of safeguarding the privacy of individuals with schizophrenia and adhering to the regulations outlined in our Institutional Review Board’s agreement.
World Health Organization. Recovery practices for mental health and well-being. https://apps.who.int/iris/bitstream/handle/10665/329602/9789241516747-eng.pdf (2019). Accessed 21 Oct 2023.
Leendertse JCP, Wierdsma AI, van den Berg D, Ruissen AM, Slade M, Castelein S, Mulder CL. Personal recovery in people with a psychotic disorder: a systematic review and meta-analysis of associated factors. Front Psychiatry. 2021;12:622628. https://doi.org/10.3389/fpsyt.2021.622628 .
Article CAS PubMed PubMed Central Google Scholar
Van Weeghel J, van Zelst C, Boertien D, Hasson-Ohayon I. Conceptualizations, assessments, and implications of personal recovery in mental illness: a scoping review of systematic reviews and meta-analyses. Psychiatr Rehabil J. 2019;42(2):169–81. https://doi.org/10.1037/prj0000356 .
Article PubMed Google Scholar
Winsper C, Crawford-Docherty A, Weich S, Fenton SJ, Singh SP. How do recovery-oriented interventions contribute to personal mental health recovery? A systematic review and logic model. Clin Psychol Rev. 2020;76:101815. https://doi.org/10.1016/j.cpr.2020.101815 .
Isaacs A, Beauchamp A, Sutton K, Kocaali N. Care coordination can reduce unmet needs of persons with severe and persistent mental illness. Front Psychiatry. 2019;10:563. https://doi.org/10.3389/fpsyt.2019.00563 .
Article PubMed PubMed Central Google Scholar
Jorquera N, Alvarado R, Libuy N, de Angel V. Association between unmet needs and clinical status in patients with first episode of schizophrenia in Chile. Front Psychiatry. 2015;6:57. https://doi.org/10.3389/fpsyt.2015.00057 .
Wiersma D, van den Brink R, Wolters K, McCabe R, Bullenkamp J, Hansson L, Lauber C, Martinez-Leal R, Rössler W, Salize H, Björkman T, Torres-Gonzales F, Wright DJ, Priebe S. Individual unmet needs for care: are they sensitive as outcome criterion for the effectiveness of mental health services interventions? Soc Psychiatry Psychiatr Epidemiol. 2009;44(4):317–24. https://doi.org/10.1007/s00127-008-0432-z .
Chan KK, Mak WW. The mediating role of self-stigma and unmet needs on the recovery of people with schizophrenia living in the community. Qual Life Res. 2014;23(9):2559–68. https://doi.org/10.1007/s11136-014-0695-7 .
Liu WI, Yeh ST. Current status and prospects of community psychiatric home care. J Nurs. 2021;68(1):24–9. https://doi.org/10.6224/JN.202102_68(1).05 .
Article Google Scholar
Liu WI, Rong JR, Lee KT. A systematic review on care needs of mentally-ill patients in the community. J Psychiatric Mental Health Nurs. 2012;7(2):1–13. https://doi.org/10.6847/TJPMHN.201212_7(2).0002 .
Liu WI. Recovery-oriented integrated care-case management. National Health Research Institutes; 2020. pp. 256–63.
O’Donnell R, Savaglio M, Vicary D, Skouteris H. Effect of community mental health care programs in Australia: a systematic review. Aust J Prim Health. 2021;26(6):443–51. https://doi.org/10.1071/PY20147 .
Liu WI, Tsai SL. Difficulties in the development of community mental health care in Taiwan. National Health Research Institutes; 2020. pp. 40–8.
Gabrielsson S, Sävenstedt S, Olsson M. Taking personal responsibility: nurses’ and assistant nurses’ experiences of good nursing practice in psychiatric inpatient care. Int J Ment Health Nurs. 2016;25(5):434–43. https://doi.org/10.1111/inm.12230 .
Salberg J, Ekselius L, Hursti T, Öster C. Staff experiences related to implementation of a recovery-oriented nursing programme in psychiatric inpatient care. Int J Ment Health Nurs. 2022;31:731–42. https://doi.org/10.1111/inm.12995 .
Gabrielsson S, Tuvesson H, Wiklund Gustin L, Jormfeldt H. Positioning psychiatric and mental health nursing as a transformative force in health care. Issues Ment Health Nurs. 2020;41(11):976–84. https://doi.org/10.1080/01612840.2020.1756009 .
Article CAS PubMed Google Scholar
Yang CI, Hsieh MY, Lee LH, Chen SL. Experiences of caring for a sibling with schizophrenia in a Chinese context: a neglected issue. Int J Mental Health Nurs. 2017;26(4):409–17. https://doi.org/10.1111/inm.12269 .
Bitter N, Roeg D, van Assen M, van Nieuwenhuizen C, van Weeghel J. How effective is the comprehensive approach to rehabilitation (CARe) methodology? A cluster randomized controlled trial. BMC Psychiatry. 2017;17(1):396. https://doi.org/10.1186/s12888-017-1565-y .
Wood L, Alsawy S. Recovery in psychosis from a service user perspective: a systematic review and thematic synthesis of current qualitative evidence. Community Ment Health J. 2018;54(6):793–804. https://doi.org/10.1007/s10597-017-0185-9 .
Lin CC. Reliability and validity verification of the developed needs assessment instrument for the community-dwelling patients with schizophrenia in Taiwan [thesis]. Taiwan:National Taipei University of Nursing and Health Sciences; 2020.
Neil ST, Kilbride M, Pitt L, Nothard S, Welford M, Sellwood W, Morrison AP. The questionnaire about the process of recovery (QPR): a measurement tool developed in collaboration with service users. Psychosis. 2009;1(2):145–55. https://doi.org/10.1080/17522430902913450 .
Chien WT, Chan ZC. Chinese translation and validation of the questionnaire on the process of recovery in schizophrenia and other psychotic disorders. Res Nurs Health. 2013;36(4):400–11. https://doi.org/10.1002/nur.21549 .
Ke SM, Hsieh CJ, Liu CY, Liu WI. Reliability and validity of the camberwell assessment of needs for the community–dwelling patients with schizophrenia in Taiwan. J Psychiatric Mental Health Nurs. 2015;10(1):9–17. https://doi.org/10.6847/TJPMHN.201511_10(1).0002 .
Phelan M, Slade M, Thornicroft G, Dunn G, Holloway F, Wykes T, Strathdee G, Loftus L, McCrone P, Hayward P. The Camberwell Assessment of need: the validity and reliability of an instrument to assess the needs of people with severe mental illness. Br J Psychiatry. 1995;167(5):589–95. https://doi.org/10.1192/bjp.167.5.589 .
Yeh HS, Luh RL, Liu HJ, Lee YC, Slade M. Reliability of the camberwell assessment of need (Chinese version) for patients with schizophrenia at a daycare center of Taiwan. Soc Psychiatry Psychiatr Epidemiol. 2006;41(1):75–80.
Herth K. Development and refinement of an instrument to measure hope. Sch Inq Nurs Pract. 1991;5(1):39–51.
CAS PubMed Google Scholar
Nayeri ND, Goudarzian AH, Herth K, Naghavi N, Nia HS, Yaghoobzadeh A, Sharif SP, Allen KA. Construct validity of the Herth Hope Index: a systematic review. Int J Health Sci. 2020;14(5):50–7.
Google Scholar
Chan KS, Li HCW, Chan SWC, Lopez V. Herth Hope Index: psychometric testing of the Chinese version. J Adv Nurs. 2011;68(9):2079–85. https://doi.org/10.1111/j.1365-2648.2011.05887.x .
Rogers ES, Chamberlin J, Ellison ML. A consumer-constructed scale to measure empowerment among users of mental health services. Psychiatr Serv. 1997;48(8):1042–7. https://doi.org/10.1176/ps.48.8.1042 .
Chen SC, Liu CY, Hsieh CJ, Liu WI. Psychometric testing of a Chinese empowerment scale for the patients with chronic mental illness. J Psychiatric Mental Health Nurs. 2017;12(1):5–13. https://doi.org/10.6847/TJPMHN.201709_12(1).0001 .
Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale for the psychoses. Schizophr Res. 2000;42(3):241–7. https://doi.org/10.1016/s0920-9964(99)00130-9 .
Kao YC, Liu YP. Compliance and schizophrenia: the predictive potential of insight into illness, symptoms, and side effects. Compr Psychiatry. 2010;51(6):557–65. https://doi.org/10.1016/j.comppsych.2010.03.007 .
Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10(3):799–812.
Chang TJ, Hwu HG, Wei FW. Interrater reliability of brief Psychiatric Rating Scale (BPRS). Bull Chin Soc Neurol Psychiatry. 1986;12(1):2936. http://ntur.lib.ntu.edu.tw//handle/246246/130652 .
Murwasuminar B, Munro I, Recoche K. Mental health recovery for people with schizophrenia in Southeast Asia: a systematic review. J Psychiatr Ment Health Nurs. 2023;30(4):620–36. https://doi.org/10.1111/jpm.12902 .
Hancock N, Scanlan JN, Gillespie JA, Smith-Merry J, Yen I. Partners in recovery program evaluation: changes in unmet needs and recovery. Aust Health Rev. 2018;42(4):445–52. https://doi.org/10.1071/ah17004 .
Pompili M, Giordano G, Luciano M, et al. Unmet needs in schizophrenia. CNS Neurol Disord Drug Targets. 2017;16(8):870–84. https://doi.org/10.2174/1871527316666170803143927 .
Vita A, Barlati S. Recovery from schizophrenia: is it possible? Curr Opin Psychiatry. 2018;31(3):246–55. https://doi.org/10.1097/YCO.0000000000000407 .
Hsieh WL, Lee SK, Chien WT, Liu WI, Lai CY, Liu CY. Mediating effect of the motivation for medication use on disease management and medication adherence among community-dwelling patients with schizophrenia. Patient Prefer Adherence. 2019;13:1877–87. https://doi.org/10.2147/ppa.S218553 .
Li JB, Liu WI, Huang MW. Integrating evidence-based community-care services to improve schizophrenia outcomes: a preliminary trial. Arch Psychiatr Nurs. 2016;30(1):102–8. https://doi.org/10.1016/j.apnu.2015.08.018 .
Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357–73. https://doi.org/10.2147/ndt.S96649 .
National Health Research Institutes. Investigation and assessment on care needs of patients with psychotic disorders in community. https://reurl.cc/My24k4 (2020). Accessed 21 Oct 2023.
Klug G, Gallunder M, Hermann G, Singer M, Schulter G. Effectiveness of multidisciplinary psychiatric home treatment for elderly patients with mental illness: a systematic review of empirical studies. BMC Psychiatry. 2019;19:382. https://doi.org/10.1186/s12888-019-2369-z .
Chen HL, Li IL, Shiau SJ, Huang YH, Lee SK. An interpretation for the psychiatric mental health nursing competence in caring patients and their families. J Psychiatric Mental Health Nurs. 2017;12(1):47–58. https://doi.org/10.6847/TJPMHN.201709_12(1).0005 .
Chang YC, Chou FH. Effects of home visit intervention on re-hospitalization rates in psychiatric patients. Community Ment Health J. 2015;51(5):598–605. https://doi.org/10.1007/s10597-014-9807-7 .
Cheng J, Liu H, Geng T, Wang Y, Yu L, Li J, Li W, Chen C, Yao G. The effect of case management for schizophrenia clients in China. J Behav Brain Sci. 2020;10(8):297–310. https://www.scirp.org/journal/jbbs .
Download references
Acknowledgements
We would like to thank the the National Science and Technology Council for providing funding for the project [grant numbers MOST 108-2314-B-227-008-MY3], as well as all participants for their contributions.
This study has been funded by the National Science and Technology Council. Grant numbers MOST 108-2314-B-227-008-MY3.
Author information
Authors and affiliations.
School of Nursing, National Taipei University of Nursing and Health Sciences, Taipei City, Taiwan
Wen-I Liu, Wen-Ling Hsieh & Ching-Ting Lai
Tri-Service General Hospital Beitou Branch, Taipei City, Taiwan
Chia-Chen Liu & Yueh-Ming Tai
Department of Health Care Management, National Taipei University of Nursing and Health Sciences, Taipei City, Taiwan
Chieh-Yu Liu
You can also search for this author in PubMed Google Scholar
Contributions
Each author made substantial contributions to this study. WI designed the study framework, applied for the grant, prepared the writing draft, edited, and supervised. WL engaged in analyzing data and editing. CC conducted the investigation. CT, YM, and CY were responsible for data interpretation. All authors revised and approved the final manuscript.
Corresponding author
Correspondence to Wen-I Liu .
Ethics declarations
Ethics approval and consent to participate.
We obtained approval from the ethics committee of the institutional review board (IRB) of the relevant research institutes (TSGHIRB No. 1090324 and IRB1091209-01). The study strictly adhered to the principles outlined in the IRB agreement, ensuring confidentiality, non-maleficence, and justice throughout the research process. Prior to commencing the study, all participants provided written informed consent. Additionally, participants were informed that the intervention is non-invasive and carries minimal risk of harm.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, WI., Hsieh, WL., Lai, CT. et al. Effectiveness of a needs-tailored nurse-led recovery program for community-dwelling people with schizophrenia: a cluster-randomized controlled trial. BMC Nurs 23 , 329 (2024). https://doi.org/10.1186/s12912-024-01986-x
Download citation
Received : 13 December 2023
Accepted : 30 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1186/s12912-024-01986-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Community psychiatric nurses
- Needs-tailored
- Schizophrenia
- Cluster-randomized controlled trial
BMC Nursing
ISSN: 1472-6955
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Review Articles in 2023
Pharmaco-EEG of antipsychotic treatment response: a systematic review
- Marco De Pieri
- Vincent Rochas
- Stefan Kaiser
Satisfaction with cognitive remediation therapy: its effects on implementation and outcomes using the cognitive remediation satisfaction scale
- Joanne Evans
- Rose Tinch-Taylor
A systematic review of neuroimaging studies of clozapine-resistant schizophrenia
- Tiffanie Sze Wing Pang
- Johnny Siu Wah Chun
- Sherry Kit Wa Chan
The Brief negative Symptom Scale (BNSS): a systematic review of measurement properties
- Lucia Weigel
- Sophia Wehr
- Stefan Leucht
Microglia and cognitive impairment in schizophrenia: translating scientific progress into novel therapeutic interventions
- Chuanjun Zhuo
- Hongjun Tian
- Chunmian Chen
Psychosocial stress, interpersonal sensitivity, and social withdrawal in clinical high risk for psychosis: a systematic review
- A. Georgiades
- A. Almuqrin
- A. Mechelli
The association between psychosocial stress, interpersonal sensitivity, social withdrawal and psychosis relapse: a systematic review
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.335(7610); 2007 Jul 14
Schizophrenia
Marco m picchioni.
King's College London, Institute of Psychiatry, Division of Psychological Medicine, London SE5 8AF
Robin M Murray
Schizophrenia is one of the most serious and frightening of all mental illnesses. No other disorder arouses as much anxiety in the general public, the media, and doctors. Effective treatments are available, yet patients and their families often find it hard to access good care. In the United Kingdom, as in many parts of the world, this is often due to poor service provision, but sometimes it is simply down to misinformation. In this review, we clarify the causes and presentation of schizophrenia, summarise the treatments that are available, and try to clear up a few myths.
We searched the online electronic databases Web of Knowledge, the Cochrane Library, and the current National Institute for Health and Clinical Excellence (NICE) guidelines for suitable evidence based material.
What is schizophrenia?
The name schizophrenia derives from the early observation that the illness is typified by “the disconnection or splitting of the psychic functions.” w1 Unfortunately, this has led to the misconception that the illness is characterised by a “split personality,” which it is not. Box 1 lists the common symptoms of schizophrenia.
Box 1 Definitions of symptoms of schizophrenia
Positive symptoms, lack of insight.
- Failure to appreciate that symptoms are not real or caused by illness
Hallucination
- A perception without a stimulus
- Hallucinations can occur in any sense—touch, smell, taste, or vision—but auditory hallucinations are the most common (usually “hearing voices”)
- A fixedly held false belief that is not shared by others from the patient's community
- Delusions often develop along personal themes; for example:
- Persecution—patients think they are victims of some form of threat or are central to a conspiracy
- Passivity—patients think that their thoughts or actions are being controlled by an external force or person
- Other—delusions can develop along any theme; for instance grandiose, sexual, or religious
Thought disorder
- Manifests as distorted or illogical speech—a failure to use language in a logical and coherent way
- Typified by “knight's move” thinking—thoughts proceed in one direction but suddenly go off at right angles, like the knight in chess, with no logical chain of thought
Negative symptoms
- These include social withdrawal, self neglect, loss of motivation and initiative, emotional blunting, and paucity of speech
People with schizophrenia typically hear voices (auditory hallucinations), which often criticise or abuse them. The voices may speak directly to the patient, comment on the patient's actions, or discuss the patient among themselves. Not surprisingly, people who hear voices often try to make some sense of these hallucinations, and this can lead to the development of strange beliefs or delusions.
Many patients also have thought disorder and negative symptoms. While negative symptoms may be less troubling to the patient, they can be very distressing to relatives. Psychiatrists often classify schizophrenia into subtypes according to the balance of symptoms that a patient manifests (box 2).
Box 2 Common subtypes of schizophrenia
- Delusions or hallucinations are prominent
Hebephrenic
- Sustained flattened or incongruous affect
- Lack of goal directed behaviour
- Prominent thought disorder
- Sustained evidence over at least two weeks of catatonic behaviour including stupor, excitement, posturing, and rigidity
- Considerable loss of personal drive
- Progressive deepening of negative symptoms
- Pronounced decline in social, academic, or employment performance
While we often think of schizophrenia as a major departure from normal health, mild symptoms can occur in healthy people and are not associated with illness. 1 This has led to the conclusion that schizophrenia reflects a quantitative rather than qualitative deviation from normality, rather like hypertension or diabetes.
Dopamine: linking biology to symptoms
We have known for more than 40 years that excessive dopamine transmission in the brain's mesolimbic system plays a key role in schizophrenia. Support for this comes from several strands of evidence (box 3). How does dopamine excess lead patients to believe that their neighbours are talking about them, or that the Central Intelligence Agency (CIA) is after them? Shijit Kapur, a Canadian researcher, has proposed an attractive theory linking dopamine dysregulation to symptom formation. w2 He pointed out that one of dopamine's roles is to provide “salience” by converting a neutral mental representation into one with personal importance that grabs our attention. The theory proposes that, in psychosis, excessive dopamine adds salience to mundane and insignificant thoughts or perceptions. In this way, everyday events—eye contact with a stranger, a trivial sound, or the comments of a newsreader—are given personal importance. Delusions may then develop in an attempt to make sense of a world in which personally important events are going on around the patient all the time.
Box 3 Evidence linking excessive dopamine transmission to schizophrenia
- Amphetamine misuse, which increases synaptic dopamine release, can produce ideas of reference, delusions, and auditory hallucinations in healthy people
- Small doses of amphetamines make it harder to control symptoms in patients with schizophrenia
- Typical antipsychotic drugs cause extrapyramidal side effects by blocking dopamine in the substantia nigra
- The clinical efficacy of typical antipsychotics is closely correlated to their ability to block dopamine
- Patients with psychosis release excessive dopamine in response to an amphetamine challenge, and the degree of dopamine release correlates with the severity of their psychotic symptoms
- Dopamine receptor binding is increased in patients with psychosis
- COMT (which encodes catechol- O -methyltransferase, a dopamine metabolising enzyme) genotype moderates the future risk of developing psychosis in adolescent users of cannabis
How common is schizophrenia?
Systematic reviews show that despite its relatively low incidence (15.2/100 000), 2 the prevalence of schizophrenia (7.2/1000) 2 is relatively high, because it often starts in early adult life and becomes chronic. The incidence of schizophrenia varies; at present it is rising in some populations (such as South London w3 ) but falling in others. 3 A comprehensive global survey concluded that schizophrenia accounts for 1.1% of the total disability adjusted life years worldwide and 2.8% of the years lived with disability worldwide. w4
Who gets schizophrenia?
Schizophrenia typically presents in early adulthood or late adolescence. Men have an earlier age of onset than women, and also tend to experience a more serious form of the illness with more negative symptoms, less chance of a full recovery, and a generally worse outcome. 4 Systematic reviews show that it is more common in men than women (risk ratio 1.4:1 2 ) and is more frequent in people born in cities—the larger the city and the longer the person has lived there the greater the risk. 5 It is more common in migrants. 6 A large and comprehensive study showed that rates of schizophrenia in African-Caribbean people living in the UK are six to eight times higher than those of the native white population. w5 Rates remain high in the children of migrants, but this is not reflected in increased rates in their home country. w6 Environmental and social factors have been implicated in this increased risk, and intriguingly the risk of schizophrenia in migrants is greatest when they form a small proportion of their local community. 7
What causes schizophrenia?
Are genes important.
Schizophrenia is a multifactorial disorder, and the greatest risk factor is a positive family history (fig 1 1). ). While the lifetime risk in the general population in just below 1%, it is 6.5% in first degree relatives of patients, 8 and it rises to more than 40% in monozygotic twins of affected people. 9 Extended family, adoption, and twin studies show that this risk reflects the genetic proximity between relative and proband.

Fig 1 Aetiological model of schizophrenia
Recently, some progress has been made in identifying the genes that increase the risk for schizophrenia. In 2002, the deCODE genetics group in Iceland identified a haplotype in the neuregulin 1 (NRG1) gene w7 which seemed to double the risk of illness, a result later replicated in Scotland and Wales, South Africa, and China. Other susceptibility genes that have recently emerged are dysbindin (DTNBP1) and DISC1.
It seems likely that many risk genes exist—each of small effect and each relatively common in the general population. Patients probably inherit several risk genes, which interact with each other and the environment to cause schizophrenia once a critical threshold is crossed.
What environmental factors are important?
A meta-analysis has shown that patients with schizophrenia are more likely to have experienced obstetric complications, in particular premature birth, low birth weight, and perinatal hypoxia. w8 They are also slightly more likely to have been born in late winter and early spring, possibly reflecting intrauterine viral exposure. These early environmental hazards appear to have a subtle effect on brain development. In adulthood different environmental stressors act—including social isolation, migrant status, and urban life 10 —and this remains the case even when life events attributable to the incipient psychosis itself are excluded. The way parents raise their children does not seem to have a major impact on future vulnerability, but families do have an important part to play in the course of the illness; patients with supportive parents do much better than those with critical or hostile ones. Collectively, these risk factors point to an interaction between biological, psychological, and social risk factors that drive increasingly deviant development and finally frank psychosis. 11 w9
Can drug abuse cause schizophrenia?
We know that stimulants like cocaine and amphetamines can induce a picture clinically identical to paranoid schizophrenia, and recent reports have also implicated cannabis. The evidence that patients with established schizophrenia smoke more cannabis than the general population is overwhelming. Well conducted and comprehensive cohort studies, like that from Dunedin in New Zealand, 12 show that early cannabis use—long before psychotic symptoms appear—increases the future risk of schizophrenia fourfold, while a meta-analysis of prospective studies reported a doubling of the risk. 13 This effect is robust, even after controlling for any effect of self medication, 13 undermining the suggestion that early cannabis use is an attempt to alleviate distress caused by the developing illness. Only a small proportion of people who use cannabis develop schizophrenia, just as only a few of those who misuse alcohol develop cirrhosis. This probably reflects a genetically determined vulnerability to the environmental stressor, a gene-environment interaction. Indeed, variations in the dopamine metabolising COMT (catechol- O -methyltransferase) gene affect the propensity to develop psychosis in people who use cannabis. 14
Do recognisable changes occur in the brain?
A recent meta-analysis and systematic review has confirmed that patients with schizophrenia have smaller whole brain volumes and larger lateral ventricles. 15 Furthermore, these volume changes have greatest impact on grey matter in the frontal and temporal lobes. These deficits appear to be present even at the earliest stages of the illness, though whether they progressively worsen over the course of the illness remains contentious.
A patient's story
Henry grew up in Uganda and came to the UK about three years before he started to become unwell at the age of 25. He was living with his partner and had a young son. He initially became more irritable, argumentative, and more unpredictable; eventually the relationship ended and he moved out. He began to drift socially and at work.
Henry became increasingly preoccupied that he was the victim of a conspiracy that seemed to involve one of his neighbours. Over a period of months Henry became increasingly concerned that the neighbour was playing tricks on him—sending him messages and talking to him through the walls that separated their homes. Henry did not know who to turn to, and eventually he bought a knife for protection. One night Henry decided to confront the neighbour, and he was arrested shortly afterwards. Henry saw a prison psychiatrist who diagnosed paranoid schizophrenia, and he was transferred to hospital. Henry was convicted of threatening behaviour but received a hospital order under the Mental Health Act 1983. He was treated as an inpatient for the next two years with antipsychotic medication and insight oriented cognitive therapy. He is now treated and supervised by his local community forensic psychiatry team. He lives in his own housing association flat and works five days a week in a local supermarket; he was recently offered a promotion and sees his son every week.
Early diagnosis and management in primary care
Box 4 lists the most common positive symptoms of schizophrenia, and box 5 shows the ICD-10 (international classification of diseases, 10th revision) diagnostic criteria. However, few patients initially present with such florid symptoms. Patients are more likely to have more nebulous symptoms such as anxiety and depression, social problems, or changes in behaviour, particularly difficulties in concentrating or becoming withdrawn from their normal social life. Box 6 outlines useful screening questions for patients presenting in this manner.
Box 4 Most common positive symptoms of schizophrenia w17
- Lack of insight (97%)
- Auditory hallucinations (74%)
- Ideas of reference (70%)
- Delusions of reference (67%)
- Suspiciousness (66%)
- Flatness of affect (66%)
- Delusional mood (64%)
- Delusions of persecution (64%)
- Thought alienation (52%)
- Thoughts spoken aloud (50%)
Box 5 ICD-10 diagnostic criteria for schizophrenia
At least one present most of the time for a month.
- Thought echo, insertion or withdrawal, or thought broadcast
- Delusions of control referred to body parts, actions, or sensations
- Delusional perception
- Hallucinatory voices giving a running commentary, discussing the patient, or coming from some part of the patient's body
- Persistent bizarre or culturally inappropriate delusions
Or at least two present most of the time for a month
- Persistent daily hallucinations accompanied by delusions
- Incoherent or irrelevant speech
- Catatonic behaviour such as stupor or posturing
- Negative symptoms such as marked apathy, blunted or incongruous mood
Box 6 Suggested screening questions for patient presenting with possible psychosis
- Do you hear voices when no one is around? What do they say?
- Do you ever think that people are talking or gossiping about you, maybe even thinking about trying to get you?
- Do you ever think that somehow people can pick up on what you are thinking or can manipulate what you are thinking?
If the onset of psychosis is suspected, the patient should be rapidly referred to secondary care (box 7). This will be the local early intervention or home treatment team in many parts of the UK, or the generic catchment area community mental health team. The risk that patients pose to themselves and others must be assessed (table (table) ) at this first assessment and this information included in the referral. If the presence of psychotic symptoms is confirmed by a psychiatrist, then after discussion it may be appropriate for the general practitioner to prescribe an antipsychotic. Current NICE guidelines 16 recommend considering and offering an oral atypical antipsychotic such as amisulpiride, risperidone, quetiapine, or olanzapine in low doses. The need for hospital admission and even the use of the Mental Health Act will depend mainly on the patient's presentation, the risk assessment, and the availability of good community support. General practitioners can contribute greatly to this decision because of their long term relationship with the patient and family.
Brief risk assessment screen
Box 7 Early presentation of psychosis
John was in his mid-twenties when he was referred to the local early intervention in psychosis service on the advice of his counsellor. He lived with his partner and worked in a local shop at the time. For many years he had misused various illegal drugs including cannabis, amphetamine, LSD, and cocaine.
John's problems began a year or two earlier when he had a panic attack climbing a flight of stairs. He was treated with a β blocker and when this was unsuccessful he was given counselling. In the course of John's counselling sessions he revealed that he had experienced other unusual phenomena, particularly vivid dreams. He felt that he had some degree of control over these dreams though they were accompanied by a sense of not knowing whether he was really asleep.
When John was assessed by the early intervention service he reported that he sometimes thought that he could smell petrol and butane when others could not and that he could hear his phone ring when no one had called. He said he felt that people were murmuring about him, though he could not be sure, and if he checked he found nothing. He was also very worried about his physical health. Finally, he admitted that he had begun to notice unusual coincidences and links between events and people.
Paul met criteria for an “at risk mental state” for psychosis and was offered cognitive behaviour therapy with a clinical psychologist to deal with these symptoms. He declined treatment with an antipsychotic drug.
Nine months later John had made an excellent recovery—most of his symptoms had improved, he was not taking any illicit drugs, and he was still at work and with his girlfriend.
Is early recognition important?
Most general practitioners with a couple of thousand patients on their list will see one or two new cases of psychosis each year. The mean duration of untreated psychosis—the time between full symptoms emerging and starting continuous antipsychotic treatment—is currently around one to two years in the UK. w10 A systematic review and meta-analysis have shown that the longer this period, the worse the outcome. 17 w11 The idea that reducing the duration of untreated psychosis will be reflected in improved outcome has led to a recent expansion in first episode services in the UK and other countries. Whether or not this proves to be the case, 18 patients with psychotic symptoms should be identified and treated as quickly as possible.
Summary points
- Schizophrenia usually starts in late adolescence or early adulthood
- Genetic risk and environmental factors interact to cause the disorder
- The most common symptoms are lack of insight, auditory hallucinations, and delusions
- Clinicians should suspect the disorder in a young adult presenting with unusual symptoms and altered behaviour
- Treatments can alleviate symptoms, reduce distress, and improve functioning
- Delayed treatment worsens the prognosis
Long term management in primary care
An average general practitioner in the UK will look after about 12 patients with schizophrenia w12 and exclusively manage the care of about six. Once a patient has recovered from an acute episode of schizophrenia, current NICE guidelines recommend that they remain on prophylactic doses of antipsychotic for one to two years and continue to be supervised by specialist services. After that time, if they are well and symptom free, the drug dose can gradually be reduced and the patient carefully monitored to detect any signs of relapse; if such signs occur, then the dose must be increased until they disappear. Such a programme of careful monitoring may best be achieved by collaboration between primary and secondary care.
General practitioners are central to ensuring that patients with schizophrenia receive good quality physical health care (fig 2 2 ). 19 Current NICE guidelines encourage all practices to establish a mental health register and offer regular physical health checks tailored to the needs of the patient. Special attention should be paid to screening for endocrine disorders; hyperglycaemia and hyperprolactinaemia; cardiovascular risk factors such as smoking, hypertension, and hyperlipidaemia; and side effects of medication, particularly neurological, cardiovascular, and sexual ones (box 8).

Fig 2 Physical care algorithm: adapted from NICE guidelines 19
Box 8 Common side effects of antipsychotic drugs 20
First generation antipsychotics.
- Extrapyramidal effects:
- Pseudoparkinsonism
- Tardive dyskinesia
- Hyperprolactinaemia
- Reduced seizure threshold
- Postural hypotension
- Anticholinergic effects:
- Blurred vision
- Urinary retention
- Neuroleptic malignant syndrome
- Weight gain
- Sexual dysfunction
- Cardiotoxicity (including prolonged QTc)
Second generation antipsychotics
- Olanzapine:
- Glucose intolerance and frank diabetes mellitus
- Hypotension
- Risperidone:
- Extrapyramidal side effects at higher doses
- Amisulpiride:
- Extrapyramidal effects
- Quetiapine:
- Hypersalivation
- Constipation
- Hypotension and hypertension
- Tachycardia
- Glucose intolerance and diabetes mellitus
- Nocturnal enuresis
- Rare serious side effects:
- Neutropenia (93%)
- Agranulocytosis (0.8%)
- Thromboembolism
- Cardiomyopathy
- Myocarditis
- Aspiration pneumonia
Some patients will inevitably need to be referred back to secondary care. Guideline criteria for this decision include:
- Poor treatment compliance
- Poor treatment response
- Ongoing substance misuse
- Increase in risk profile.
How do primary and secondary care interface?
Specialist mental health services in the UK are obliged to structure patient care using the care programme approach. w13 w14 This approach provides a statutory framework that aims to ensure that clinicians comprehensively consider the patient's main areas of need. It demands regular assessment of various aspects of the patient's life including mental and physical health, relationships, accommodation, occupation, finances, etc. Although the framework is cumbersome and proscriptive, its strength lies in this rigid approach to structuring care and reviewing risk. Whether such a framework can be successful given the current attempts of government and primary care trusts to reduce the costs of mental health care remains to be seen.
What treatment can a patient expect in secondary care?
Pharmacological.
The first line drug for a patient with a first episode of psychosis is an oral atypical antipsychotic, such as risperidone or olanzapine (fig 3 3). ). Drug companies have emphasised the superior side effect profile of these drugs, but in reality the atypicals have different side effects from typical antipsychotics, and they can be just as debilitating. Well conducted randomised controlled trials have shown that, except for clozapine, they are no more effective than the older typical drugs. 21 22 Thus, patients with established illness who already take a typical antipsychotic, who are clinically well, and who have no troublesome side effects should not change to an atypical. 16 Clinicians should consider changing patients who take typical antipsychotics and have extrapyramidal side effects to an atypical drug. Intermittent dosing regimens and drug holidays to reduce side effects are not recommended because of the increased risk of relapse. Depot preparations are usually offered to prevent covert non-concordance with treatment and to facilitate dosing regimens. The lowest effective dose of antipsychotic should be used, and the concurrent use of two or more antipsychotics should be limited to specialist services. Anticholinergic drugs should not be routinely prescribed to prevent side effects because of their adverse effects on cognition and memory.

Fig 3 Pharmacological treatment algorithm. Adapted from the Maudsley prescribing guidelines 20
Meta-analysis has shown that clozapine is the best drug for 20-30% of patients who are resistant to treatment. 23 Treatment resistance is defined as failure to respond to two or more antipsychotics (one of which should be an atypical) when given at an adequate dose for at least six to eight weeks, and once confounding factors such as concordance failure or substance misuse have been excluded. To prevent agranulocytosis, which occurs in less than 1% of patients taking clozapine, a full blood count must be done regularly. Clozapine is the only antipsychotic that can reduce positive and negative symptoms in patients with treatment resistance, and it should be prescribed as soon as treatment resistance is confirmed.
Ongoing research questions
- Might there be better ways to define schizophrenia than by the presence of hallucinations and delusions? Are there other markers that are more closely related to the pathophysiological process than clinical symptoms?
- What are the biological underpinnings of schizophrenia? Can we gain a better understanding of the site of any pathophysiological lesions and their impact on cerebral function?
- Can we link these lesions to understand how symptoms develop?
- Can we identify the genes that increase vulnerability to schizophrenia? What neurotransmitter or developmental systems do these genes affect?
- What other factors in the environment increase vulnerability to schizophrenia?
- How does early substance misuse increase vulnerability to schizophrenia?
- What is the best model of care for schizophrenia?
- Can early intervention—particularly in people at high risk of developing the disorder—really prevent schizophrenia developing or improve the prognosis?
- Can we develop predictors of clinical response to treatment?
- Can we tailor treatment—especially drug treatment—to individual patients, to improve outcome and reduce the risk of side effects?
Psychological
Several psychological treatments can help ameliorate symptoms, improve functioning, and prevent relapse, although their availability is often limited by a lack of trained therapists. Systematic reviews show that cognitive behaviour therapy can reduce persistent symptoms and improve insight 19 24 ; NICE guidelines recommend that it should be provided for at least 10 sessions over three months. Family therapy provides support and education for families. It aims to improve communication between family members, raise awareness in all people involved, and reduce distress. It can help reduce relapse rates, admission rates, symptoms, and the burden on carers, as well as improve compliance with treatment. Systematic reviews have shown that psychoeducation can reduce relapse and readmission rates and is potentially cost efficient. Other treatments with less robustly established evidence include cognitive remediation therapy and social skills training. Psychodynamic psychotherapy may increase the risk of relapse. w15
What is the prognosis?
The common perception that schizophrenia has a poor prognosis is not true. More than 80% of patients with their first episode of psychosis will recover, although less than 20% will never have another episode. 25 While many patients with schizophrenia have a lifelong vulnerability to recurrent episodes of illness, a large proportion will have few relapses and make a good functional recovery. Poor premorbid adjustment, a slow insidious onset, and a long duration of untreated psychosis—together with prominent negative symptoms—tend to be associated with a worse prognosis. 17 w16 An acute onset, an obvious psychosocial precipitant, and good premorbid adjustment all improve the prognosis.
Additional educational resources
Information for healthcare professionals.
- Mental Health Care (www.mentalhealthcare.org.uk/)—A collaboration between the Institute of Psychiatry and Rethink providing clinical and up to the minute research evidence on a wide range of mental health matters
- EPPIC (www.eppic.org.au/index.asp)—Website produced by the EPPIC service in Melbourne with helpful advice and information sheets about the nature of first episode psychosis
- Early Intervention in Psychosis (www.iris-initiative.org.uk/index.shtml)—Website of IRIS, the Birmingham based partnership between NHS and non-statutory services, including pages of guidance for general practitioners
- Taylor D, Paton C, Kerwin R, eds. The 2005 South London and Maudsley Hospital prescribing guidelines. London: Martin Dunitz, 2005. This covers all aspects of psychiatric prescribing
- Frith C, Johnson EC. Schizophrenia: a very short introduction . Oxford: Oxford University Press, 2003
- Stein G, Wilkinson G, eds. Seminars in general adult psychiatry . London: Gaskell, 1998. A comprehensive textbook from the Royal College of Psychiatrists covering the major psychiatric diagnoses
Information for patients and carers
- Rethink (www.rethink.org/)—One of the major UK mental health charities that focus on psychotic illnesses
- Mind (www.mind.org.uk/)—Another UK mental health charity that focuses on a wide range of psychiatric illnesses
- Royal College of Psychiatrists (www.rcpsych.ac.uk/mentalhealthinformation.aspx)—A series of articles aimed at professionals, carers, and patients that provides comprehensive information on a variety of mental health problems
Thanks to Paul Tabraham and Penny Collins for help preparing this manuscript.
Contributors: Both authors contributed to the conception, planning, drafting and critical revision of the article and approved the final version. MMP is guarantor.
Competing interests: MMP has received travel awards from Pfizer, Janssen-Cilag, and Eli Lily. RMM has received honorariums for speaking at meetings organised by most major producers of antipsychotic drugs, and his research group has received funding from Eli Lilly and Astra Zeneca.
Provenance and peer review: Commissioned; externally peer reviewed.

IMAGES
VIDEO
COMMENTS
2. BACKGROUND. In 1985, Warner used empirical evidence to strongly challenge the prevailing view of schizophrenia, which largely arose through the influence of Kraepelin (Kendler, 2020), who suggested that psychosis was strongly characterized by poor clinical and social outcomes.Since then, evidence from epidemiological, sociological, psychological, and biological studies has made many aspects ...
Abstract. Schizophrenia is a highly disabling disorder whose causes remain to be better understood, and treatments have to be improved. However, several recent advances have been made in diagnosis, etiopathology, and treatment. Whereas reliability of diagnosis has improved with operational criteria, including Diagnostic and Statistical Manual ...
Schizophrenia has been conceptualized as a disorder of brain connectivity since the illness was first defined (9, 10, 44), ... Thus, research efforts that target the second decade of life are essential if we are to find mechanisms for intervention and cure. These studies are costly and time-consuming, but there is an example that is rapidly ...
Background Schizophrenia is a complex, heterogeneous disorder, with highly variable treatment outcomes, and relatively little is known about what is important to patients. The aim of the study was to understand treatment outcomes informal carers perceive to be important to people with schizophrenia. Method Qualitative interview study with 34 individuals and 8 couples who care for a person with ...
The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr. Bull. 36, 94-103 (2010). Article PubMed Google Scholar ...
Schizophrenia is a severe mental disorder associated with impairment in functioning. A multidisciplinary approach is essential to help individuals with this health condition, and psychological interventions are considered a priority. The International Classification of Functioning, Disability and Health (ICF) offers a theoretical framework for assessing functioning and disability. The ICF Core ...
The Choice of Drugs for Schizophrenia. J.M. DavisN Engl J Med 2006;354:518-520. Schizophrenia is a serious chronic illness that requires lifelong medication. In some patients, the illness is ...
Introduction. The goal of this guideline is to improve the quality of care and treatment outcomes for patients with schizophrenia, as defined by the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (American Psychiatric Association 2013).Since publication of the last full practice guideline (American Psychiatric Association 2004) and guideline watch (American Psychiatric ...
Discussion. In conclusion, it is possible to stage schizophrenia, but the models developed have several limitations. Empirical validation and inclusion of more specific biomarkers and measures of ...
Molecular Psychiatry - Epigenetic mechanisms in schizophrenia and other psychotic disorders: a systematic review of empirical human findings. ... Schizophrenia research. 2019;205:63-75.
Schizophrenia Research. Volume 228, February 2021, Pages 43-50. Social exclusion and rejection across the psychosis spectrum: A systematic review of empirical research ... and 3) was an empirical study. We did not include articles that studied related constructs such as discrimination, social alienation, and stigma. Additionally, articles were ...
The empirical study of ethical aspects of schizophrenia research has sought to clarify and resolve many of these questions. In this article we provide an overview of the existing data-based literature on schizophrenia research ethics and outline directions for future inquiry. We examine 5 broad categories of inquiry into the ethics of ...
Positive symptoms of schizophrenia are associated with significant difficulties in daily functioning, and these difficulties have been associated with impaired executive functions (EEFF). However, specific cognitive and socio-emotional executive deficits have not been fully established. The present study has several objectives. First, we aimed to examine the specific deficits in cognitive and ...
Schizophrenia is a debilitating disease that presents with both positive and negative symptoms affecting cognition and emotions. Extensive studies have analyzed the different factors that contribute to the disorder. There is evidence of significant genetic etiology involving multiple genes such as dystrobrevin binding protein 1 (DTNBP1) and ...
Abstract. Schizophrenia is a neurodevelopmental disorder that starts very early. In this review we describe the empirical evidence for the neurodevelopmental model. First, by outlining the roots of psychological research that laid the foundation of the model. Thereafter, describing cognitive dysfunction observed in schizophrenia, and the course ...
As official journal of the Schizophrenia International Research Society (SIRS) Schizophrenia Research is THE journal of choice for international researchers and clinicians to share their work with the …. View full aims & scope. $3800. Article publishing charge.
Key Research Questions about the Prevalence of Schizophrenia First there is a need to examine the degree of variation in the prevalence estimates of schizophrenia between sites. The companion review on the incidence of schizophrenia [ 1 ] found that within the central 80% of incidence rates, the difference ranged from 7.7 to 43 per 100,000 ...
Cognitive behavioral therapy for schizophrenia: an empirical review J Nerv Ment Dis. 2001 May;189(5):278-87. doi: 10.1097/00005053-200105000-00002. ... These results reveal promise for the role of CBT in the treatment of schizophrenia although additional research is required to test its efficacy, long-term durability, and impact on relapse ...
Practicing psychodynamically oriented clinicians need empirical evidence to support the use of individual psychodynamic psychotherapy for the treatment of individuals with schizophrenia. The purpose of this article is to provide psychodynamically oriented clinicians with that needed empirical evidence. A review of the meta-analytic research on the use of individual psychodynamic psychotherapy ...
The empirical study of ethical aspects of schizophrenia research has therefore sought to clarify and resolve many of these disagreements. 32,34,41,42. Consequently, schizophrenia research ethics has grown dramatically over the past decade, fueled by the collaborative efforts of multidisciplinary investigators, the receptiveness of editors to ...
Peripheral inflammation is implicated in the pathophysiology of schizophrenia, and provides a therapeutic target, but its intricate relationship with brain alterations were uncertain 3,4,5,6.A ...
Numerous studies on early detection of schizophrenia (SZ) have utilized all available channels or employed set of a few time domain or frequency domain features, while a limited number of features may not be sufficient enough to perform diagnosis efficiently. To encounter these problems, an automated diagnosis model is proposed for the efficient diagnosis of schizophrenia symptomatic ...
Most Asian people with schizophrenia live at home in the community rather than in institutions . As highlighted by the National Health Research Institute, the needs of people with schizophrenia in Taiwan have not been fully evaluated and community care services have been unable to respond to their needs for individualized care . Consequently ...
Schizophrenia Research. Supports open access. ... This journal offers authors two options (open access or subscription) to publish their research and is actively committed to transitioning to a fully open access journal. Year Total published Subscription Open access; 2018: 530: 499: 31: 2019: 356: 330: 26: 2020: 412: 377: 35:
Microglia and cognitive impairment in schizophrenia: translating scientific progress into novel therapeutic interventions. Chuanjun Zhuo. Hongjun Tian. Chunmian Chen. Review Article Open Access 10 ...
Thought disorder. Manifests as distorted or illogical speech—a failure to use language in a logical and coherent way. Typified by "knight's move" thinking—thoughts proceed in one direction but suddenly go off at right angles, like the knight in chess, with no logical chain of thought. Negative symptoms.