REVIEW article
Checking agriculture’s pulse: field pea ( pisum sativum l.), sustainability, and phosphorus use efficiency.

- Plant and Environmental Sciences, 270 Poole Agricultural Center, Clemson University, Clemson, SC, United States
Investigations regarding the incorporation of better sustainable production strategies into current agricultural-food systems are necessary to grow crops that reduce negative impacts on the environment yet will meet the production and nutritional demand of 10 billion people by 2050. The introduction of organic, alternative staple food crops, such as nutrient-dense field pea ( Pisum sativum L.), to the everyday diet, may alleviate micronutrient malnutrition and incorporate more sustainable agriculture practices globally. Varieties are grown in organic systems currently yield less than conventionally produced foods, with less bioavailable nutrients, due to poor soil nutrient content. One of the most limiting nutrients for field pea is phosphorus (P) because this legume crop requires significant inputs for nodule formation. Therefore, P use efficiency (PUE) should be a breeding target for sustainable agriculture and biofortification efforts; the important role of the soil microbiome in nutrient acquisition should also be examined. The objectives of this review are to highlight the benefits of field pea for organic agriculture and human health, and discuss nutritional breeding strategies to increase field pea production in organic systems. Field pea and other pulse crops are underrepresented in agricultural research, yet are important crops for a sustainable future and better food systems. Furthermore, because field pea is consumed globally by both developed and at-risk populations, research efforts could help increase global health overall and combat micronutrient malnutrition.

Introduction
The Green Revolution is indisputably one of the most critical feats in recent agricultural history, but what has it cost our soils, crops, and environment as a whole? One result of the focus on mass production in monocultural systems for prolonged periods is the over-application of fertilizers and pesticides, which is a prevalent issue associated with conventional farming methods ( Ponisio et al., 2015 ). As a result, soil fertility and microbial biodiversity have decreased while rates of environmental pollution and greenhouse gas emissions continue to increase ( Reganold et al., 1987 ; Amundson et al., 2015 ; Reganold and Wachter, 2016 ; Peoples et al., 2019 ). Organic agriculture offers a potential solution to these problems, as organic production relies on environmentally friendly practices to increase soil fertility. However, current varieties bred for conventional systems do not perform as well in organic soils, resulting in reduced yield and nutritional quality. The agriculture industry as a whole has also begun to deplete the natural mineral deposits on which crops depend, such as phosphate rock, which is a nonrenewable resource ( van de Wiel et al., 2016 ). Organic and conventional agriculture both use phosphorus rock for around 90% of the phosphorus (P) found in fertilizers, feed, and other food additives; however, most P is subsequently lost from the food system due to mining and field practices ( Cordell and White, 2014 ; Amundson et al., 2015 ). The P that is applied as a fertilizer is also often mismanaged; specifically, it is over-applied to fields, leading to a build-up of the element in the soil where it is inaccessible to plants due to its immobile nature and affinity to form insoluble complexes with other minerals ( Vance et al., 2003 ; MacDonald et al., 2011 ). Experts cannot seem to agree on when phosphorus reserves will run out, with estimates between the years 2030 and 2100 ( van de Wiel et al., 2016 ); regardless, agriculture must still address its current P problem for future crop production.
Phosphorus is vital to agriculture because it is required by all plants, being involved in seed germination, root growth, structure development, and numerous metabolic processes such as photosynthesis and nutrient formation ( van de Wiel et al., 2016 ). Therefore, when P is limited in soils it negatively affects not only plant growth and yield but also the nutrient concentration and bioavailability in food crops, leading to micronutrient deficiencies or “hidden hunger” ( Assuero et al., 2004 ; Welch and Graham, 2004 ; Rehman et al., 2018 ). Potential solutions to hidden hunger include: 1) biofortification to increase bioavailable micronutrients in staple crops through agronomic, plant breeding, and biotechnology efforts ( Welch and Graham, 2004 ) and 2) diversifying staple crops to include cheaper, environmentally sustainable, and more nutrient-dense foods, such as field pea ( Pisum sativum L .) and other pulse crops ( Foyer et al., 2016 ).
Field pea is a member of the Leguminosae family, along with faba bean ( Vicia faba ), grass pea ( Lathyrus sativus ), white lupin ( Lupinus albus ), lentils ( Lenis culinaris ), mung bean ( Vigna radiata ), soybean ( Glycine max ), cow pea ( Vigna ungulicata ), and common bean ( Phaseolus vulgaris ) among others ( Foyer et al., 2016 ). Additionally, Leguminosae consists of the subfamily Papilionideae which splits into two distinct clades of cultivated legumes: 1) Hologalegina, evolving 50 million years ago and 2) Phaseoloid, evolving 45 million years ago ( Foyer et al., 2016 ). These clades evolved separately, as Hologalegina is comprised of all cool season legumes, such as field pea, lentil, faba bean, and grass pea, while Phaseoloids consists of warm-season legumes (pigeon pea, soybean, common bean, mung bean, and cowpea) ( Foyer et al., 2016 ). Cool season legumes are critical to sustainable agriculture, as they are planted during winter, complementing the growing season of cereals, and providing essential nitrogen and other nutrients back to the soil.
Field pea is a critical economic and nutritive crop and is often called “poor man’s meat” due to its high protein, vitamin and mineral, and prebiotic carbohydrate content yet affordability for poorer consumers ( Amarakoon et al., 2012 ). More specifically, field pea is naturally rich in iron and zinc and thus, could address two of the most common micronutrient deficiencies in the world ( Amarakoon et al., 2012 ). Despite the potential for the higher consumption of field pea to help alleviate hidden hunger, little advancement has been made to increase production and yields have lagged behind those of cereals ( Amarakoon et al., 2012 ). One of the main issues with field pea, and legumes, in general, is that they require much more P input than other crops due to their nodules, which require P for energy transformation ( Vance et al., 2003 ); this presents an issue for sustainable agriculture.
Field Pea Benefits Agriculture
Field pea is one of the oldest domesticated pulse crops, appearing in the Mediterranean between 7000 and 6000 BC and persisting in current agriculture ( Helback and Hopf, 1959 ). Pulse crops are a category of legumes, with seeds specifically harvested at full maturity ( FAO, 1994 ). Pulses are very beneficial to agriculture systems, achieving large success in sustainable agriculture systems through intercropping and crop rotations with cereals. Pulses are able to break disease and weed cycles associated with cereals, while replenishing nitrogen (N) in the soil through their ability to fix N from the atmosphere through their nodules and symbioses with rhizobia. Globally, 21 Mt of nitrogen is fixed by legumes, with 5–7 Mt returned to the soil by pulses, specifically, which saves U.S. farmers $8-12 billion in total ( Foyer et al., 2016 ). In Australia, farmers reported a 30% increase in wheat after adding a legume rotation compared to monocropped wheat ( Stagnari et al., 2017 ). Studies from Denmark also report nitrogen uptake of various crops increases between 23–59% after rotations with field pea and lupin ( Stagnari et al., 2017 ). As N is another of the most limiting nutrients for cereal and crop production, this legume-mediated increase in nitrogen use efficiency offers a sustainable and cost-effective alternative to high input fertilizer regiments. Pulses also foster other beneficial properties for soil health, such as increased biodiversity, soil organic carbon (SOC) levels, and soil water retention, while decreasing greenhouse gas emissions (GHG) ( Foyer et al., 2016 ; Stagnari et al., 2017 ; Peoples et al., 2019 ). Field pea has the most positive effect on SOC by improving humus levels and supplying organic C as a result of bacterial nitrogen fixation ( Stagnari et al., 2017 ).
In 2017, a total of 8,141,031 hectares of field pea were harvested globally ( Figure 1 ), with the top producers consisting of Canada, Russia, China, India, and the United States ( FAOSTAT, 2019 ); however, this is only a minimal fraction compared to cereal production. Cultivated land acreage for field pea and other pulses has been in steady decline over the past 30 years ( Stagnari et al., 2017 ). Average yields have increased about 70–84% since 1974 for staple legumes, such as soybean, lentil, chickpea, and groundnut; in contrast, yields for field pea have increased but resulted in no net production gains due to decreasing land acreage ( Foyer et al., 2016 ). The minimal expansion of pulses in agriculture is due to smaller and unpredictable yields, caused by susceptibility to environmental factors, and has resulted in a less-developed global market with decreased profits, disincentivizing farmers from using pulses for income while policymakers focus more attention and resources on cereals in developing countries ( Foyer et al., 2016 ; Stagnari et al., 2017 ). These practices have compromised human nutrition, as cereals have less protein than field pea and pulse crops as well as inadequate levels of micronutrients, contributing to hidden hunger ( Pingali, 2012 ). Pulses are also good sources of prebiotic carbohydrates (essential for gut health), fiber, minerals, vitamins, carotenoids, and polyphenols, allowing them to address health problems such as malnutrition, prenatal care, cardiovascular disease, diabetes, cancer, obesity, and gastrointestinal (GI)-related issues that plague both developing and developed nations ( Welch, 2002 ; Foyer et al., 2016 ).
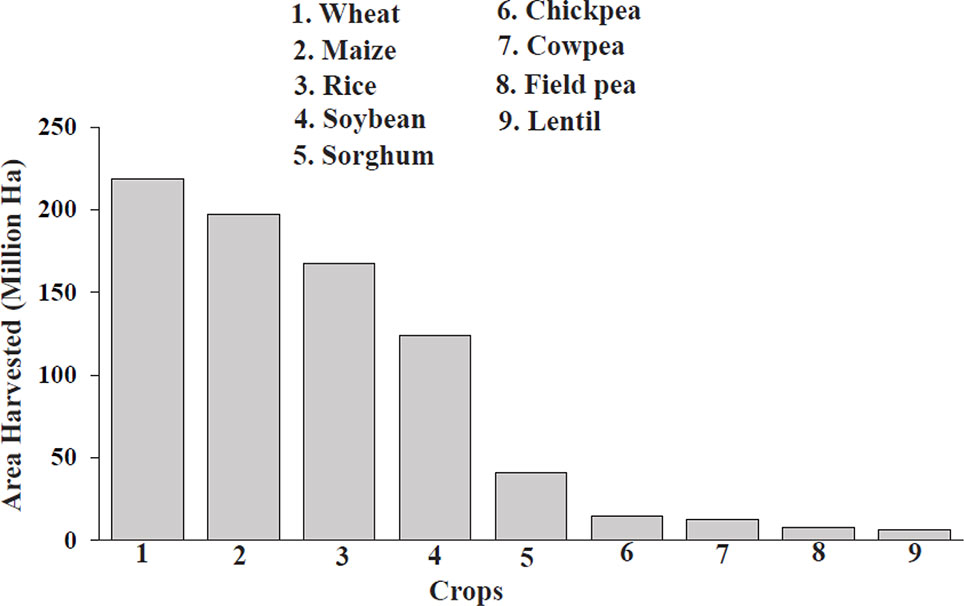
Figure 1 Comparison of area harvested for cereals and field pea in 2017 ( FAOSTAT, 2019 ). The numbers above each bar represent the individual harvest totals of each crop.
An additional hindrance to legume production is the high phosphorus requirement for nodule formation and function. Intensive mineral P fertilization has caused P surpluses in the soil of many countries, but deficits still exist in parts of Africa, the Northern U.S., South America, Eastern Europe, and Asia, likely due to multiple cycles of mono-crop farming or limited access to mineral fertilizers ( MacDonald et al., 2011 ). Many resource-poor farmers practice subsistence agriculture, which utilizes organic principles such as no pesticides, chemical fertilizers, or industrial equipment. The soils they farm are generally poorer in nutrients, resulting in poorer yields and possibly poorer nutritional quality of the crop. For pulses such as field pea to be effective in combating hidden hunger, breeding efforts should be conducted to prepare varieties for these limiting environments. In addition, more specific breeding efforts should also focus on breeding field pea varieties solely for the organic environment to resolve the yield and nutritional discrepancies between conventional and organic agriculture.
Organic Soil Vs. Conventional Soil
Organic agriculture is regarded as having healthier soils than conventional systems. Indeed, organic soil has higher soil organic matter, soil organic carbon, soil aggregate stability, and soil moisture content than conventional soils—all values that increase soil health and fertility ( Schrama et al., 2018 ). Organic soils also have an increased level of biodiversity, as in a wider range of pollinators, insects, and earthworms, along with high microbial biomass and enzymatic activity ( Hole et al., 2005 ). Despite healthier soils, limited herbicide and pesticide use along with additional weed pressure are limiting factors to productivity in organic agriculture. Additionally, N is a limiting nutrient in both conventional and organic production, but especially in organic systems that do not allow synthetic fertilizers as a source of N. Organic crops actually require twice as much N as conventional systems to achieve comparable yields ( Seufert et al., 2012 ). Therefore, legumes are critical in organic systems, as they fix and efficiently use their own N, and supply it back to the soil from biomass after harvest at a rate of 40 million tons per year ( Seufert et al., 2012 ; Udvardi and Poole, 2013 ).
Pulses face other nutrient constraints in organic agriculture due to their high P demand. Organic systems do not adequately replenish P supplies after harvest, leading to a deficit for the incoming crop ( Oehl et al., 2002 ; Seufert et al., 2012 ). Additionally, sources of P for organic farming in the U.S. are restricted to FDA-approved manures and bone meal, as well as phosphate rock ( Möller et al., 2018 ). For farmers that convert from conventional to organic management, decreases in available P in soils have been reported, meaning that the fertilizer is not adequate as a single source, and plants utilize P built up in the soil from previous fertilizer applications ( Oehl et al., 2002 ). Overall, this means that organic agriculture is still dependent on nonrenewable sources of phosphorus, which decreases the sustainability of organic production.
One strategy to combat the negative impact of increased weed and disease pressure and nutrient limitations in organic environments is to identify breeding targets that fortify varieties to cope with these stressors. There are cereal organic breeding programs already underway ( Wolfe et al., 2008 ; Jones et al., 2011 ). In field pea, genetic variation may also exist for phosphorus use efficiency (PUE), which would allow for the development of cultivars that are less dependent on P fertilizer input; this would benefit both conventional and organic growing systems. Additionally, PUE should be the main consideration for organic legumes, so that they can maintain nitrogen-fixing activity, yield stability, and adequate biomass under phosphorus-deficient conditions ( van de Wiel et al., 2016 ). This will also prolong the period residual P can contribute to production, allowing it to be used more efficiently ( van de Wiel et al., 2016 ). PUE is a complex trait, involving multiple pathways and gene networks, but can be broken into the ability of the plant roots to acquire P from the soil and the plant’s ability to remobilize and allocate P to sustain productivity ( van de Wiel et al., 2016 ). Unfortunately for field pea, there is a dearth of genomic information and resources regarding these processes, so more research should be conducted to identify these genomic regions as field pea becomes more popular in the health food market.
Phosphorus Physiology of Legumes
Phosphorus is only available to plants in its inorganic forms (Pi) as H 2 PO 4 − and HPO 4 2– which exist in very small concentrations in the soil (< 10 µm) ( Figure 2 ) ( Schachtman et al., 1998 ). Availability is also highly dependent on soil pH, as P forms insoluble complexes with Al and Fe under acidic conditions and Ca under alkaline conditions ( Seufert et al., 2012 ). This presents an issue for all forms of agriculture worldwide, as most soils are acidic ( Reganold and Wachter, 2016 ; Slessarev et al., 2016 ). All plants have adopted mechanisms to combat the unavailable nature of P, such as altered root architecture, organic acid exudation, specialized transport systems, lipid remodelling, and symbiosis with arbuscular mycorrhizal fungi (AMF) ( Figure 2 ) ( Vance et al., 2003 ; Oehl et al., 2004 ). AMF are especially important in organic systems, where less is P available, and plants rely more heavily on these fungi to gather and supply P and other nutrients ( Oehl et al., 2004 ). P is applied to soils from phosphate rock sources, and becomes immobile in the soil, with small concentrations accessible to the roots. Plants will form symbiotic relationships with Mycorrhizal fungi for greater P acquisition. For legumes specifically, high concentrations of P exist in the nodules to maintain nitrogen-fixing function. Due to stress or senescence, P is remobilized from younger tissues and moves into upper leaves and seeds for storage as phytic acid.
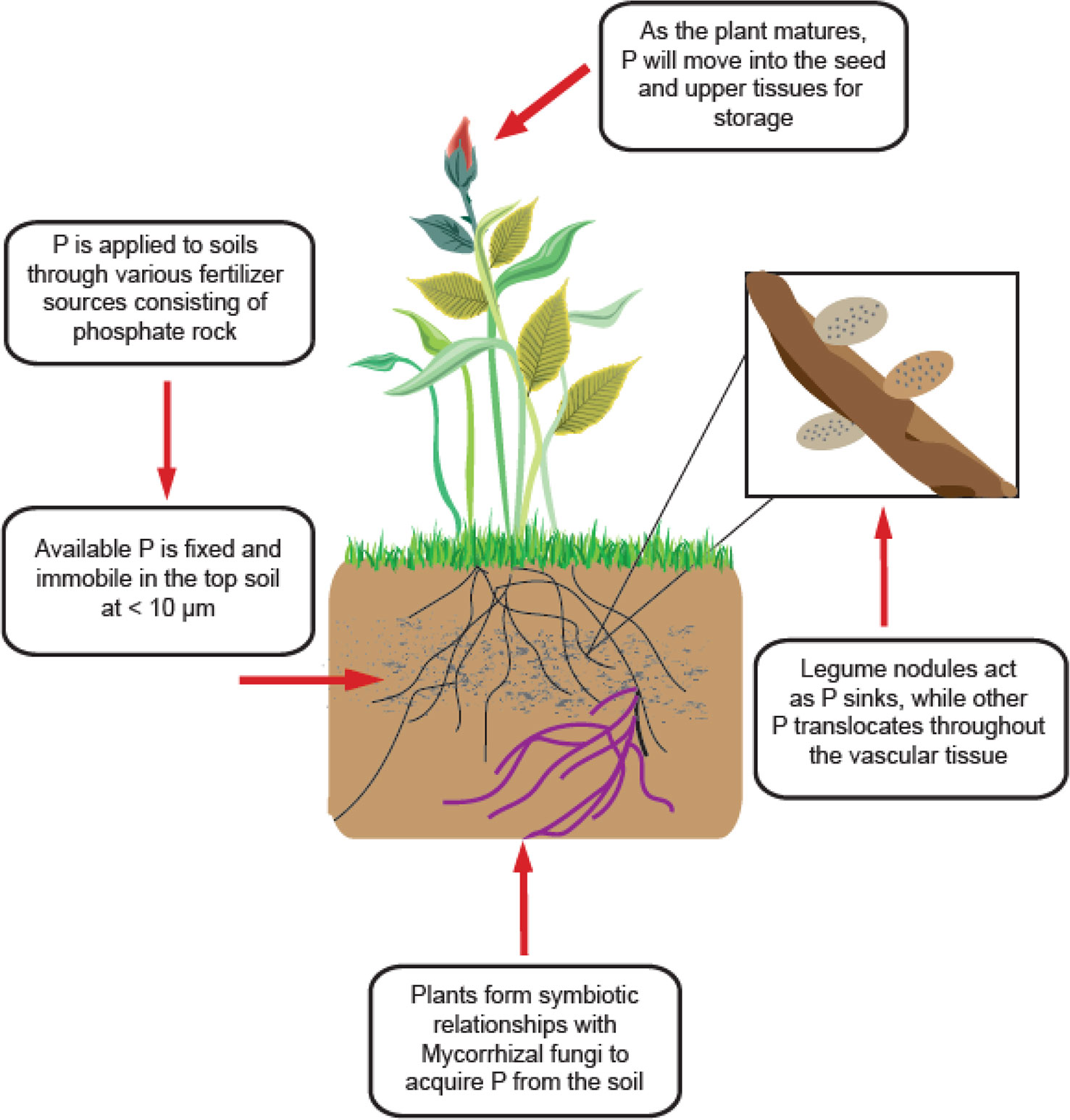
Figure 2 General scheme of P acquisition and utilization of legume plants.
P uptake is regulated by high and low affinity transporters located throughout the vascular system of the plant. The root hair mediates the uptake of Pi from the soil, where it is transported across the root plasma membrane by a P-type H+-ATPase pump ( Vance et al., 2003 ). From there, the Pi is transported into the nodules or upward into the shoot by the xylem where it goes to individual cells ( Vance et al., 2003 ). The cytoplasm maintains a strict Pi concentration of around 5–10 mM ( Schachtman et al., 1998 ); if no deficiency is detected, the Pi will be stored in the vacuole until P stress signals are detected or senescence begins. If Pi becomes limited throughout the plant, vacuolar Pi will efflux into the cell cytoplasm and be allocated to other vital tissues, such as legume nodules. Additionally, during senescence Pi is again effluxed from the vacuole, where it is transported from older leaves to younger leaves and seeds by the xylem and various transporters ( Robinson et al., 2012 ; Yang et al., 2017 ; Xu et al., 2019 ). Once Pi reaches the seed, it is stored as phytic acid or phytate and utilized during seed germination to establish enough growth until the seedling can take up nutrients on its own ( Robinson et al., 2012 ; Yang et al., 2017 ; Xu et al., 2019 ). Some crops have more phytic acid than others, with field pea containing a high amount. Phytic acid is an antinutrient, meaning it binds to other minerals and decreases bioavailability, making crops high in phytic acid undesirable for animal and human consumption.
Adaptations for P limitation in legumes specifically involve preferential allocation of P to nodules to maintain N fixation, rhizosphere acidification through root exudation, formation of proteoid roots, and modified carbon metabolism and mycorrhizae formation to control for competition with nodule development ( Vance et al., 2003 ). Therefore, legumes will suffer greatly if P is limited, as they will be unable to maintain nodule function and overall productivity due to decreased photosynthetic ability, tissue expansion, and flower formation ( Sa and Israel, 1991 ; Vance et al., 2003 ; Sulieman and Tran, 2015 ). Most research on these processes has been conducted in soybean ( Glycine max ), so there is a need to investigate responses in field pea specifically. There is also a gap in the literature with respect to specific links between phosphorus deficiency and nutrient bioavailability. The nutritional value of organic crops compared to conventional crops is an additional gray area; however, it can be inferred that limited P not only affects human health through reduced yield, but could also decrease protein, carbohydrate, and lipid content due to P’s involvement in plant metabolic activities.
P Efficiency and Plant-Soil-Microbe Interactions
As previously discussed, most vascular land plants have formed evolutionary beneficial relationships with AMF, but increasing evidence indicates the entire soil microbiome is a mediator for plant health. This relationship is caused by the secretion of photosynthates and carbon sources into the rhizosphere, acting as a tradeoff for various microbes ( Bakker et al., 2018 ), which then provide the plant with various health benefits such as nutrient availability. The composition of soil microbiomes is largely dependent on the soil type and the environment, but plant genotype can also influence microbial populations depending on the type of root exudate and hormones it produces. For example, Arabidopsis accessions differ in their ability to colonize Pseudomonas bacteria, leading to some accessions being more disease resistant than others ( Bakker et al., 2018 ). Additionally, salicylic acid exudation by Arabidopsis influences the composition of root microbiomes, again demonstrating the plant has some influence over the rhizosphere ( Bakker et al., 2018 ).
The soil microbiome is implicated in P acquisition, and AMF and rhizobia interactions with legumes are well characterized. Legumes exude flavonoids into the rhizosphere that attract rhizobia to stimulate nodule formation as well as allow for AMF interaction; both lead to enhanced P availability for the legume ( Jacoby et al., 2017 ). Another mechanism is the modification of root exudates under P limitation. Maize and rice alter their exudates to contain more carbohydrates and sugars to provide an energy source for AMF formation ( Carvalhais et al., 2011 ). Increased sugar exudation has also been identified in Pisum sativum , which then increased the mineralization of insoluble P by microbial activity ( Schilling et al., 1998 ).
A result of conventional farming is the notion that breeders have inadvertently selected for traits that weaken plant-microbe interactions due to intensive fertilizer and pesticide use ( Bakker et al., 2018 ). Studies in barley, maize, and Arabidopsis indicate differences in rhizospheres between wild and domesticated material as well as natural variation among accessions ( Bakker et al., 2018 ). More studies must be done to dissect the contribution of genetic variability to microbial communities, as these could be targets for organic plant breeding initiatives. The lack of efficient plant-soil-microbe interactions in conventionally bred crops could help explain reduced yields when these varieties are introduced to organic environments, where a stronger soil microbiome relationship would be beneficial due to the lack of fertilizers and pesticides ( Jones et al., 2011 ; Bakker et al., 2018 ). Organic pulse breeding should focus on microbial interactions to improve P acquisition, and genomic studies should be performed in the diverse germplasm to discover any beneficial traits that may have been lost from modern day varieties over time.
Field Pea and Phosphorus Use Efficiency
PUE is defined as the total biomass per unit of P taken up and encompasses the plant’s ability to acquire P from the soil then translocate, remobilize, and efficiently utilize it for various physiological processes ( Shenoy and Kalagudi, 2005 ; Veneklaas et al., 2012 ). The overall goal of PUE breeding is to determine genomic regions that contribute to these processes and allow crops to grow and yield at optimal levels under low P conditions. Generally, a greater focus has been placed on improving P acquisition from the soil by identifying genes and processes associated with root systems architecture and rhizosphere modifications under P deficiency through quantitative trait loci (QTL), genome-wide association study (GWAS), and biotechnological methods ( Rose and Wissuwa, 2012 ; Veneklaas et al., 2012 ; van de Wiel et al., 2016 ). While these aims would allow crops to scavenge residual P built up in the soil from the over-application of fertilizer, this strategy may deplete P from nutrient-poor soils and further upset the balance of fertilizer input to uptake, again leading to P depletion ( van de Wiel et al., 2016 ). Remobilization of P from vegetative tissues is the main source of P for reproductive tissues, impacting yield and seed quality, so understanding and improving allocation efficiency is a necessary goal for crops with better PUE. Additionally, increased P acquisition for field pea may lead to a greater accumulation of phytate in seeds, thus decreasing the bioavailability of nutrients upon consumption. Therefore, in terms of field pea, a balance must be achieved between P acquisition and internal P utilization to avoid excess accumulation of phytate.
Several studies in grain crops have concerned PUE ( Rose and Wissuwa, 2012 ), but none, as far as we are aware, have been conducted in field pea. Genetic variation is visible among pulse crop varieties, so field pea studies should not be ignored. A recent study in chickpea ( Cicer arietinum L .) showed great variation among diverse germplasm and commercial varieties for various aspects of PUE, such as total biomass, photosynthetic rate, root structure, and root acquisition under limited P conditions ( Pang et al., 2018 ). Several accessions from the diverse germplasm were shown to outperform commercial chickpea varieties in terms of these criteria, indicating genetic variation that may be exploited for PUE breeding purposes ( Pang et al., 2018 ).
Another challenge for PUE in organic agriculture is that most studies are conducted in greenhouses or under conventional management, which differs significantly from organic practices ( Rose and Wissuwa, 2012 ). Therefore, more PUE studies should be conducted with the field and growing environment in mind to generate more realistic results. Studies for PUE in field pea and other pulses should be increased in general and can be aided by recent genotypic data for the diverse field pea germplasm ( Holdsworth et al., 2017 ). Breeding for PUE will significantly benefit organic agriculture as it is a P-limited environment, where the ratio of P input to P uptake is already off-balance and inadequate. Research concerning field pea PUE should be prioritized in biofortification programs, as adequate phosphorus utilization will aid in increasing the amount and bioavailability of nutrients.
Biofortification Potential of Legumes
As previously stated, agriculture not only faces the issue of yield deficits for a growing population but also increased incidences of hidden hunger as more people develop micronutrient deficiencies. A potential solution to overcome micronutrient deficiencies is to increase consumption of pulses, which contain superior protein, carbohydrate, fiber, and micronutrient content compared to cereals, as well as complementary amino acid profiles to those found in cereals ( Rehman et al., 2018 ). Field pea is also attracting positive attention in health food markets, as they are rich in protein (23.5 g protein per 100 g) and a viable substitution to wheat and egg-based products. Protein extraction is reported to be most successful from field pea, and the protein structure of peas is the most similar to egg and stabilizes snacks and cereals most similarly to gluten when compared to other alternative protein sources. By increasing protein content of field pea, a more significant profit and expansion of the field pea market may take place, paving the way for more initiatives to support growers of field pea and other pulse crops. Another solution is to boost biofortification breeding efforts to increase nutritional value where legumes lack to supplement a low diversity diet due to climate change and crop availability.
However, an issue relating both biofortification and phosphorus use efficiency is the conversion of P to myo -inositol-1,2,3,4,5,6-hexa kis phosphate (InsP6), also known as phytic acid, which acts as an antinutritional factor by decreasing the bioavailability of nutrients in pulses when consumed ( Rehman et al., 2018 ) ( Raboy, 2003 ). As P is taken up by the roots from the soil, it is converted to glucose 6-phosphate (G6P) before entering the inositol phosphate pathway through the conversion of G6P to inositol 3-phosphate (Ins3P) by myo-inositol(3)P1 synthase (MIPS) ( Raboy, 2003 ). From there, every carbon of the 6-carbon ring is phosphorylated until it becomes InsP6 or phytic acid ( Raboy, 2003 ). Phytic acid is the primary storage form of P in seed and is often bound in phytate salts to Ca or Fe ( Raboy, 2003 ). These structures cannot be broken down by humans as they lack the necessary enzymes ( Raboy, 2003 ). As P is found throughout the plant and stored in various tissues during vegetative and reproductive growth, biofortification efforts should aim to understand the mobilization of P throughout the growing cycle. Additionally, more research should be dedicated to the speciation of P within the plant to identify genetic variation for P conversion and phytic acid content. These are concerns of both PUE and biofortification research as plants must efficiently take up P for growth, as well as convert P selectively for nutrient availability.
Several low phytic acid varieties have been developed in wheat, maize, barley, rice, and soybean through transgenic and biotechnological methods ( Wilcox et al., 2000 ; Larson et al., 2000 ; Raboy et al., 2000 ; Guttieri et al., 2004 ; Rasmussen and Hatzack, 2004 ). Warkentin et al. developed low phytate field pea variety CDC Bronco via EMS to produce the desired mutation in MIPS to halt conversion to higher inositol phosphate molecules. Overall grain phytic acid is reduced, but there are reports of several agronomic issues, such as decreased stress tolerance, germination, growth, and seed weight, as the plant cannot store enough P to use during vegetative processes, in addition to reports of reduced protein content in winter wheat ( Raboy et al., 1991 ; Oltmans et al., 2005 ; Bregitzer and Raboy, 2006 ; Warkentin et al., 2012 ; Rehman et al., 2018 ). While requiring less P overall, these lines are often stunted, with lower biomass and yield compared to commercial varieties, further illustrating the problem of less P uptake vs. high productivity ( Raboy, 2009 ; Warkentin et al., 2012 ; Sparvoli and Cominelli, 2015 ). Additionally, for organic systems, transgenic and chemical mutants are not currently allowed, so they have no use in sustainable agriculture. Furthermore, transgenics and chemically modified seeds are not allowed in the food market, may be banned as in the EU, and consumer approval is generally considered negative or unclear ( Lucht, 2015 ). A more conventional plant breeding approach could be more successful in terms of developing varieties with reduced phytic acid accumulation and positively impact biofortification.
Future Directions
Field pea is highly nutritious and beneficial to agriculture systems, along with other pulses. However field pea is especially advantageous in terms of protein content and extractability. This is the powerful avenue to expand field pea production as consumer interest in health foods, and meatless alternatives grow. Field pea could be biofortified for protein as well as micronutrient content, to increase marketability, as well as ability to fight hidden hunger. The adoption of organic principles is necessary, and organic agriculture should expand, as nonrenewable resources like P begin to deplete. Field pea and other pulses are critical to sustainable agriculture but will suffer from soil P deficiencies, affecting their beneficial status in organic systems and negatively affecting crops that depend on their nitrogen-fixing capabilities.
To adequately prepare and avoid the negative impacts of phosphorus deficiency, a thorough investigation of the genetic diversity of field pea in terms of phosphorus use efficiency is necessary. We hypothesize that there will be variation in the ability of different field pea accessions to acquire, mobilize, and store P under P deficient conditions. Phenotyping could reveal superior yield, nutritional value, and other important agronomic characteristics of some accessions and physiological and genetic analyses would aid in elucidating the biological mechanism. It is possible that there are accessions containing genes that can be incorporated into elite breeding lines to confer benefit in P deficient environments. The investigation regarding natural genetic variation within the germplasm for differing rates of P speciation should also be considered. For example, one genotype may preferentially convert to Ins3P or other lower inositol phosphates over phytic acid, leading to increased Pi and nutrient bioavailability in the seed, and allowing for low phytic acid lines to be conventionally bred rather than mutagenized. This would increase field pea production sustainability and allow new varieties to be developed for consumer use. In terms of biofortification, identifying field pea genotypes with higher potential for micronutrient accumulation, especially under P and nutrient-deficient environments, will be critical, through the understanding of variation in acquisition and translocation of nutrients to the seed ( Welch and Graham, 2004 ). The common bean core collection has demonstrated variation in Fe and Zn uptake, as well as elucidated a negative correlation between the two during breeding efforts, so these antagonistic relationships must also be discovered and considered ( Welch and Graham, 2004 ).
Despite growing interest in field pea, at the time of this review, there is still no reference genome published, and when one is released, it will always be the first assembly, meaning it will likely need to undergo revisions as technology and genomic understanding of field pea and improve. A single core collection consisting of 431 pea accessions does exist and shows ample genetic variation between accessions, allowing for more genetic studies (( Holdsworth et al., 2017 ). By using GWAS and other omics methods, questions concerning organic and nutritional breeding may be answered. Additionally, more funding for field pea and pulse research is required, as it has been limited by unstable yields and forgotten by institutions, leading to little germplasm improvement. Government agencies must get involved to promote awareness and create funding opportunities to improve field pea and pulse germplasm, so that legume profitability may increase to better compete with cereals. This is a key measure to ensure people have access to a diverse nutritional diet to combat hidden hunger.
A primary focus of agriculture should be to increase sustainability and nutritional value to the human diet through the adoption of more organic practices; this includes diversification of staple crops to include more pulses such as field pea and decreased dependence on nonrenewable resources such as phosphorus. For these goals to be met, more organic-specific breeding initiatives should be undertaken, and more research should be conducted on field pea. The dearth of knowledge on pulses compared to cereals is detrimental to agricultural research and the human diet, so more genomic studies should be conducted to increase productivity and adoption of pulses. Research concerning PUE will benefit farmers and consumers of all types by decreasing reliance on fertilizers and maximizing productivity for already P-deficient soils. Because field pea is consumed globally by both developed and at-risk populations, these efforts could help increase global health overall and combat hidden hunger.
Author Contributions
SP: drafted the manuscript and the doctoral student working on the project. DT: edited the draft and the project PI.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding support for this project was provided by the Organic Agriculture Research and Extension Initiative (OREI) (award no. 2018-51300-28431/proposal no. 2018-02799) of the United States Department of Agriculture, National Institute of Food and Agriculture, and the USDA-NIFA National Needs Doctoral Fellowship.
Amarakoon, D., Thavarajah, D., McPhee, K., Thavarajah, P. (2012). Iron-, zinc-, and magnesium-rich field peas (Pisum sativum L.) with naturally low phytic acid: a potential food-based solution to global micronutrient malnutrition. J. Food Compost. Anal. 27 (1), 8–13. doi: 10.1016/J.JFCA.2012.05.007
CrossRef Full Text | Google Scholar
Amundson, R., Berhe, A. A., Hopmans, J. W., Olson, C., Sztein, A. E., Sparks, D. L. (2015). Soil and human security in the 21st century. Science 348 (6235), 1261071. doi: 10.1126/science.1261071
PubMed Abstract | CrossRef Full Text | Google Scholar
Assuero, S. G., Mollier, A., Pellerin, S. (2004). The decrease in growth of phosphorus-deficient maize leaves is related to a lower cell production. Plant Cell Environ. 27 (7), 887–895. doi: 10.1111/j.1365-3040.2004.01194.x
Bakker, P. A. H. M., Pieterse, C. M. J., de Jonge, R., Berendsen, R. L. (2018). The soil-borne legacy. Cell 172 (6), 1178–1180. doi: 10.1016/J.CELL.2018.02.024
Bregitzer, P., Raboy, V. (2006). Effects of four independent low-phytate mutations on barley agronomic performance. Crop Sci. 46 (3), 1318. doi: 10.2135/cropsci2005.09-0301
Carvalhais, L. C., Dennis, P. G., Fedoseyenko, D., Hajirezaei, M.-R., Borriss, R., von Wirén, N. (2011). Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 174 (1), 3–11. doi: 10.1002/jpln.201000085
Cordell, D., White, S. (2014). Life’s bottleneck: sustaining the world’s phosphorus for a food secure future. Annu. Rev. Environ. Resour. 39 (1), 161–188. doi: 10.1146/annurev-environ-010213-113300
FAO. (1994). Definition and Classification of Commodities. 4. Pulses and Derived Products. Rome, Italy: Food and Agriculture Organization of the United Nations. http://www.fao.org/es/faodef/fdef04e.htm#4.02 .
Google Scholar
FAOSTAT. (2019) http://www.fao.org/faostat/en/#data/QC .
Foyer, C. H., Lam, H.-M., Nguyen, H. T., Siddique, K. H. M., Varshney, R. K., Colmer, T. D., et al. (2016). Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2 (8), 16112. doi: 10.1038/nplants.2016.112
Guttieri, M., Bowen, D., Dorsch, J. A., Raboy, V., Souza, E. (2004). Identification and characterization of a low phytic acid wheat. Crop Sci. 44 (2), 418. doi: 10.2135/cropsci2004.4180
Helback, H., Hopf, M. (1959). Domestication of Food Plants in the Old World: Joint efforts by botanists and archeologists illuminate the obscure history of plant domestication. Science 130 (3372), 365–372. doi: 10.1126/science.130.3372.365
Holdsworth, W. L., Gazave, E., Cheng, P., Myers, J. R., Gore, M. A., Coyne, C. J., et al. (2017). A community resource for exploring and utilizing genetic diversity in the USDA pea single plant plus collection. Hortic. Res. 4, 17017. doi: 10.1038/hortres.2017.17
Hole, D. G., Perkins, A. J., Wilson, J. D., Alexander, I. H., Grice, P. V., Evans, A. D. (2005). Does organic farming benefit biodiversity?. Biol. Conserv. 122 (1), 113–130. doi: 10.1016/J.BIOCON.2004.07.018
Jacoby, R., Peukert, M., Succurro, A., Koprivova, A., Kopriva, S. (2017). The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Front. In Plant Sci. 8, 1617. doi: 10.3389/fpls.2017.01617
Jones, S. S., Murphy, K. M., Myers, J. R., Messmer, M. M. (2011). The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: A review. NJAS - Wageningen J. Life Sci. 58 (3–4), 193–205. doi: 10.1016/J.NJAS.2010.04.001
Larson, S. R., Rutger, J.N., Young, K.A., Raboy, V., (2000). Isolation and genetic mapping of a non-lethal rice (Oryza sativa L.) low phytic acid 1 mutation. Crop Science 40 (5), 1397–1405.
Lucht, J. M. (2015). Public Acceptance of Plant Biotechnology and GM Crops. Viruses 7 (8), 4254–4281. doi: 10.3390/v7082819
Möller, K., Oberson, A., Bünemann, E. K., Cooper, J., Friedel, J. K., Glæsner, N., et al. (2018). "Improved phosphorus recycling in organic farming: Navigating between constraints" in Advances in Agronomy , vol. 147. (Elsevier: Amsterdam), 159–237. doi: 10.1016/bs.agron.2017.10.004
MacDonald, G. K., Bennett, E. M., Potter, P. A., Ramankutty, N. (2011). Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. U.S.A. 108 (7), 3086–3091. doi: 10.1073/pnas.1010808108
Oehl, F., Oberson, A., Tagmann, H. U., Besson, J. M., Dubois, D., Mäder, P., et al. (2002). Phosphorus budget and phosphorus availability in soils under organic and conventional farming. Nutr. Cycl. In Agroecosys 62 (1), 25–35. doi: 10.1023/A:1015195023724
Oehl, F., Sieverding, E., Mäder, P., Dubois, D., Ineichen, K., Boller, T., et al. (2004). Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 138 (4), 574–583. doi: 10.1007/s00442-003-1458-2
Oltmans, S. E., Fehr, W. R., Welke, G. A., Raboy, V., Peterson, K. L. (2005). Agronomic and seed traits of soybean lines with low–phytate phosphorus. Crop Sci. 45 (2), 593. doi: 10.2135/cropsci2005.0593
Pang, J., Zhao, H., Bansal, R., Bohuon, E., Lambers, H., Ryan, M. H., et al. (2018). Leaf transpiration plays a role in phosphorus acquisition among a large set of chickpea genotypes. Plant Cell Environ. 41 (9), 2069–2079. doi: 10.1111/pce.13139
Peoples, M. B., Hauggaard-Nielsen, H., Huguenin-Elie, O., Jensen, E. S., Justes, E., Williams, M. (2019). "The contributions of legumes to reducing the environmental risk of agricultural production" in Agroecosystem Diversity: Reconciling Contemporary Agriculture and Environmental Quality. Eds. Lemaire, P. C., De Faccio Carvalho, S., Kronberg, S., Recous, G. (Amsterdam: Elsevier). doi: 10.1016/B978-0-12-811050-8.00008-X
Pingali, P. L. (2012). Green revolution: impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. U.S.A. 109 (31), 12302–12308. doi: 10.1073/pnas.0912953109
Ponisio, L. C., M’gonigle, L. K., Mace, K. C., Palomino, J., de Valpine, P., Kremen, C. (2015). Diversification practices reduce organic to conventional yield gap. Proc. R. Soc. B: Biol. Sci. 282 (1799), 20141396.
Raboy, V. (2009). Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci. 177 (4), 281–296. doi: 10.1016/j.plantsci.2009.06.012
Raboy, Victor, Noaman, M. M., Taylor, G. A., Pickett, S. G. (1991). Grain phytic acid and protein are highly correlated in winter wheat. Crop Sci. 31 (3), 631. doi: 10.2135/cropsci1991.0011183X003100030017x
Raboy, V., Gerbasi, P. F., Young, K. A., Stoneberg, S. D., Pickett, S. G., Bauman, A. T., et al. (2000). Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiol. 124 (1), 355–368. doi: 10.1104/pp.124.1.355
Raboy, V. (2003). myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry 64 (6), 1033–1043. doi: 10.1016/S0031-9422(03)00446-1
Rasmussen, S. K., Hatzack, F. (2004). Identification of two low-phytate barley (Hordeum Vulgare l.) grain mutants by TLC and genetic analysis. Hereditas 129 (2), 107–112. doi: 10.1111/j.1601-5223.1998.00107.x
Reganold, J. P., Wachter, J. M. (2016). Organic agriculture in the twenty-first century. Nat. Plants 2 (2), 15221. doi: 10.1038/nplants.2015.221
Reganold, J. P., Elliott, L. F., Unger, Y. L. (1987). Long-term effects of organic and conventional farming on soil erosion. Nature 330 (6146), 370–372. doi: 10.1038/330370a0
Rehman, H. M., Cooper, J. W., Lam, H.-M., Yang, S. H. (2018). Legume biofortification is an underexploited strategy for combatting hidden hunger. Plant Cell Environ. 42 (1), 52–70. doi: 10.1111/pce.13368
Robinson, W. D., Carson, I., Ying, S., Ellis, K., Plaxton, W. C. (2012). Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization. New Phytol. 196 (4), 1024–1029. doi: 10.1111/nph.12006
Rose, T. J., Wissuwa, M. (2012). Rethinking internal phosphorus utilization efficiency: A new approach is needed to improve PUE in grain crops. Adv. In Agron. 116, 185–217. doi: 10.1016/B978-0-12-394277-7.00005-1
Sa, T.-M., Israel, D. W. (1991). Energy status and functioning of phosphorus-deficient soybean nodules. Plant Physiol. 97, 928–935. doi: 10.1104/pp.97.3.928
Schachtman, D.P., Reid, R.J., Ayling, S.M. (1998). Phosphorus uptake by plants: from soil to cell. Plant Physiol. 116 (2), 447–453. doi: 10.1104/pp.116.2.447
Schilling, G., Gransee, A., Deuhel, A., Ležoviž, G., Ruppel, S. (1998). Phosphorus availability, root exudates, and microbial activity in the rhizosphere. Z. Für Pflanzenernährung Und Bodenkunde 161 (4), 465–478. doi: 10.1002/jpln.1998.3581610413
Schrama, M., de Haan, J. J., Kroonen, M., Verstegen, H., van der Putten, W. H. (2018). Crop yield gap and stability in organic and conventional farming systems. Agric. Ecosyst. Environ. 256, 123–130. doi: 10.1016/J.AGEE.2017.12.023
Seufert, V., Ramankutty, N., Foley, J. A. (2012). Comparing the yields of organic and conventional agriculture. Nature 485, 229–232. doi: 10.1038/nature11069
Shenoy, V. V., Kalagudi, G. M. (2005). Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 23 (7–8), 501–513. doi: 10.1016/j.biotechadv.2005.01.004
Slessarev, E. W., Lin, Y., Bingham, N. L., Johnson, J. E., Dai, Y., Schimel, J. P., et al. (2016). Water balance creates a threshold in soil pH at the global scale. Nature 540, 567–569. doi: 10.1038/nature20139
Sparvoli, F., Cominelli, E. (2015). Seed biofortification and phytic acid reduction: a conflict of interest for the plant?. Plants (Basel Switzerland) 4 (4), 728–755. doi: 10.3390/plants4040728
Stagnari, F., Maggio, A., Galieni, A., Pisante, M. (2017). Multiple benefits of legumes for agriculture sustainability: an overview. Chem. Biol. Technol. In Agric. 4 (2), 2. doi: 10.1186/s40538-016-0085-1
Sulieman, S., Tran, L.-S. P. (2015). Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 239, 36–43. doi: 10.1016/j.plantsci.2015.06.018
Udvardi, M., Poole, P.S. (2013). Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 64, 781–805. doi: 10.1146/annurev-arplant-050312-120235
van de Wiel, C. C. M., van der Linden, C. G., Scholten, O. E. (2016). Improving phosphorus use efficiency in agriculture: opportunities for breeding. Euphytica 207 (1), 1–22. doi: 10.1007/s10681-015-1572-3
Vance, C. P., Uhde-Stone, C., Allan, D. L. (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157 (3), 423–447. doi: 10.1046/j.1469-8137.2003.00695.x
Veneklaas, E. J., Lambers, H., Bragg, J., Finnegan, P. M., Lovelock, C. E., Plaxton, W. C., et al. (2012). Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 195 (2), 306–320. doi: 10.1111/j.1469-8137.2012.04190.x
Warkentin, T. D., Delgerjav, O., Arganosa, G., Rehman, A. U., Bett, K. E., Anbessa, Y., et al. (2012). Development and characterization of low-phytate pea. Crop Sci. 52 (1), 74–78. doi: 10.2135/cropsci2011.05.0285
Welch, R. M., Graham, R. D. (2004). Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 55 (396), 353–364. doi: 10.1093/jxb/erh064
Welch, R. M. (2002). The impact of mineral nutrients in food crops on global human health. Plant Soil 247 (1), 83–90. doi: 10.1023/A:1021140122921
Wilcox, J.R., Premachandra, G.S., Young, K.A., Raboy, V. (2000). Isolation of high seed inorganic P, low-phytate soybean mutants. Crop Sci. 40 (6), 1601–1605. doi:10.2135/cropsci2000.4061601x
Wolfe, M. S., Baresel, J. P., Desclaux, D., Goldringer, I., Hoad, S., Kovacs, G., et al. (2008). Developments in breeding cereals for organic agriculture. Euphytica 163 (3), 323. doi: 10.1007/s10681-008-9690-9
Xu, L., Zhao, H., Wan, R., Liu, Y., Xu, Z., Tian, W., et al. (2019). Identification of vacuolar phosphate efflux transporters in land plants. Nat. Plants 5 (1), 84–94. doi: 10.1038/s41477-018-0334-3
Yang, S. Y., Huang, T. K., Kuo, H. F., Chiou, T. J. (2017). Role of vacuoles in phosphorus storage and remobilization. J. Exp. Bot. 68 (12), 3045–3055. doi: 10.1093/jxb/erw481
Keywords: field pea, organic farming, phosphorus, pulse crop physiology, biofortification
Citation: Powers SE and Thavarajah D (2019) Checking Agriculture’s Pulse: Field Pea ( Pisum Sativum L.), Sustainability, and Phosphorus Use Efficiency. Front. Plant Sci. 10:1489. doi: 10.3389/fpls.2019.01489
Received: 02 July 2019; Accepted: 28 October 2019; Published: 15 November 2019.
Reviewed by:
Copyright © 2019 Powers and Thavarajah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dil Thavarajah, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A Comprehensive Review of Pea ( Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications
Affiliations.
- 1 Key Laboratory of Coarse Cereal Processing, Ministry of Agriculture and Rural Affairs, Sichuan Engineering & Technology Research Center of Coarse Cereal Industralization, School of Food and Biological Engineering, Chengdu University, Chengdu 610106, China.
- 2 Institute for Advanced Study, Chengdu University, Chengdu 610106, China.
- 3 Singapore Institute of Food and Biotechnology Innovation (SIFBI), Agency for Science, Technology and Research (A*STAR), Singapore 138669, Singapore.
- PMID: 37444265
- PMCID: PMC10341148
- DOI: 10.3390/foods12132527
Pisum sativum L., commonly referred to as dry, green, or field pea, is one of the most common legumes that is popular and economically important. Due to its richness in a variety of nutritional and bioactive ingredients, the consumption of pea has been suggested to be associated with a wide range of health benefits, and there has been increasing focus on its potential as a functional food. However, there have been limited literature reviews concerning the bioactive compounds, health-promoting effects, and potential applications of pea up to now. This review, therefore, summarizes the literature from the last ten years regarding the chemical composition, physicochemical properties, processing, health benefits, and potential applications of pea. Whole peas are rich in macronutrients, including proteins, starches, dietary fiber, and non-starch polysaccharides. In addition, polyphenols, especially flavonoids and phenolic acids, are important bioactive ingredients that are mainly distributed in the pea coats. Anti-nutritional factors, such as phytic acid, lectin, and trypsin inhibitors, may hinder nutrient absorption. Whole pea seeds can be processed by different techniques such as drying, milling, soaking, and cooking to improve their functional properties. In addition, physicochemical and functional properties of pea starches and pea proteins can be improved by chemical, physical, enzymatic, and combined modification methods. Owing to the multiple bioactive ingredients in peas, the pea and its products exhibit various health benefits, such as antioxidant, anti-inflammatory, antimicrobial, anti-renal fibrosis, and regulation of metabolic syndrome effects. Peas have been processed into various products such as pea beverages, germinated pea products, pea flour-incorporated products, pea-based meat alternatives, and encapsulation and packing materials. Furthermore, recommendations are also provided on how to better utilize peas to promote their development as a sustainable and functional grain. Pea and its components can be further developed into more valuable and nutritious products.
Keywords: bioactive compounds; dietary fiber; functional grain; functional properties; modifications; polyphenol.
PubMed Disclaimer
Conflict of interest statement
The authors declare no conflict of interest.
Pea seeds of different surfaces,…
Pea seeds of different surfaces, colors, and shapes [7] (reprinted with permission from…
Biological activities of pea and…
Biological activities of pea and its bioactive components. Pea and its bioactive components…
The potential mechanisms of pea…
The potential mechanisms of pea and its bioactive components on the regulation of…
Food-related applications of pea and…
Food-related applications of pea and its components. Pea and its main components are…
Similar articles
- Pea protein composition, functionality, modification, and food applications: A review. Shen Y, Hong S, Li Y. Shen Y, et al. Adv Food Nutr Res. 2022;101:71-127. doi: 10.1016/bs.afnr.2022.02.002. Epub 2022 Mar 17. Adv Food Nutr Res. 2022. PMID: 35940709 Review.
- The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Ge J, Sun CX, Corke H, Gul K, Gan RY, Fang Y. Ge J, et al. Compr Rev Food Sci Food Saf. 2020 Jul;19(4):1835-1876. doi: 10.1111/1541-4337.12573. Epub 2020 Jun 22. Compr Rev Food Sci Food Saf. 2020. PMID: 33337084 Review.
- Characterization of in vitro antioxidant activity, bioactive components, and nutrient digestibility in pigeon pea (Cajanus cajan) as influenced by germination time and temperature. Sharma S, Singh A, Singh B. Sharma S, et al. J Food Biochem. 2019 Feb;43(2):e12706. doi: 10.1111/jfbc.12706. Epub 2018 Nov 8. J Food Biochem. 2019. PMID: 31353645
- Genotype and environment effects on the chemical composition and rheological properties of field peas. Maharjan P, Penny J, Partington DL, Panozzo JF. Maharjan P, et al. J Sci Food Agric. 2019 Sep;99(12):5409-5416. doi: 10.1002/jsfa.9801. Epub 2019 Jun 14. J Sci Food Agric. 2019. PMID: 31077380
- In vitro digestibility, protein composition and techno-functional properties of Saskatchewan grown yellow field peas (Pisum sativum L.) as affected by processing. Ma Z, Boye JI, Hu X. Ma Z, et al. Food Res Int. 2017 Feb;92:64-78. doi: 10.1016/j.foodres.2016.12.012. Epub 2016 Dec 28. Food Res Int. 2017. PMID: 28290299
- Εleven Greek Legume Beans: Assessment of Genotypic Effect on Their Phytochemical Content and Antioxidant Properties. Myrtsi ED, Vlachostergios DN, Petsoulas C, Koulocheri SD, Evergetis E, Haroutounian SA. Myrtsi ED, et al. Antioxidants (Basel). 2024 Apr 13;13(4):459. doi: 10.3390/antiox13040459. Antioxidants (Basel). 2024. PMID: 38671907 Free PMC article.
- Identification of QTLs associated with seed protein concentration in two diverse recombinant inbred line populations of pea. Gali KK, Jha A, Tar'an B, Burstin J, Aubert G, Bing D, Arganosa G, Warkentin TD. Gali KK, et al. Front Plant Sci. 2024 Mar 11;15:1359117. doi: 10.3389/fpls.2024.1359117. eCollection 2024. Front Plant Sci. 2024. PMID: 38533398 Free PMC article.
- Fahmi R., Ryland D., Sopiwnyk E., Aliani M. Sensory and Physical Characteristics of Pan Bread Fortified with Thermally Treated Split Yellow Pea (Pisum sativum L.) Flour. J. Food Sci. 2019;84:3735–3745. doi: 10.1111/1750-3841.14908. - DOI - PubMed
- Han X., Akhov L., Ashe P., Lewis C., Deibert L., Irina Zaharia L., Forseille L., Xiang D., Datla R., Nosworthy M. Comprehensive Compositional Assessment of Bioactive Compounds in Diverse Pea Accessions. Food Res. Int. 2023;165:112455. doi: 10.1016/j.foodres.2022.112455. - DOI - PubMed
- Kumari T., Deka S.C. Potential Health Benefits of Garden Pea Seeds and Pods: A Review. Legume Sci. 2021;3:e82. doi: 10.1002/leg3.82. - DOI
- Liu M., Wu N.-N., Yu G.-P., Zhai X.-T., Chen X., Zhang M., Tian X.-H., Liu Y.-X., Wang L.-P., Tan B. Physicochemical Properties, Structural Properties, and in vitro Digestibility of Pea Starch Treated with High Hydrostatic Pressure. Starch-Starke. 2018;70:9. doi: 10.1002/star.201700082. - DOI
- Raghunathan R., Hoover R., Waduge R., Liu Q., Warkentin T.D. Impact of Molecular Structure on the Physicochemical Properties of Starches Isolated from Different Field Pea (Pisum sativum L.) Cultivars Grown in Saskatchewan, Canada. Food Chem. 2017;221:1514–1521. doi: 10.1016/j.foodchem.2016.10.142. - DOI - PubMed
Publication types
- Search in MeSH
Grants and funding
- 2022-YF05-00629-SN/Chengdu Science and Technology Bureau
- 2023YFN0011/Science and Technology Department of Sichuan Province
- 2021CC002/Chengdu University
- 2081921047/Chengdu University
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Advertisement
Effect of field pea ( Pisum sativum subsp. arvense (L.) Asch.) and pea-oat ( Avena sativa L.) biculture cover crops on high tunnel vegetable under organic production system
- Original research paper
- Open access
- Published: 01 February 2022
- Volume 12 , pages 91–106, ( 2022 )
Cite this article
You have full access to this open access article

- I. Domagała-Świątkiewicz 1 &
- P. Siwek 2
2429 Accesses
4 Citations
Explore all metrics
In general, adaptation of cover cropping in crop rotation practices to organic tunnels by methods supporting soil health and quality has not yet been fully optimized. Effect of field pea and pea-oat cover crops to soil physicochemical properties and cash crop quality was assessed in an organic high tunnel in southern Poland in 2016–2017, with the following planting sequence: spring cover crops/tomato/romaine lettuce/green bean/iceberg lettuce. The sole pea produced a lower aboveground biomass (3.06 t ha −1 ) than the pea-oat mixture (4.17 t ha −1 ), and the N content in their biomass was 155 kg N ha −1 and 136 kg N ha −1 , respectively. The results indicated that a high residue input from cover crops was important for soil organic carbon stock, for retaining plant-available N in organic matter, and for improving soil physical properties, especially wet aggregate stability. We observed an increase in soil pH and the availability of some mineral nutrients in the soil under cover crop treatments, especially Ca, Mg, K, and P. N uptake by the subsequent cash crop significantly ( p ≤ 0.05) increased with pea than with pea-oat biculture, and in the green manure formula than with the mulch treatment. Early spring cover cropping depressed the subsequent tomato yield, but enhanced green bean yield in the second year of cropping.
Similar content being viewed by others
Soil profile carbon, nitrogen, and crop yields affected by cover crops in semiarid regions

Diversified crop rotation improves continuous monocropping eggplant production by altering the soil microbial community and biochemical properties

Use of Organic and Biological Fertilizers as Strategies to Improve Crop Biomass, Yields and Physicochemical Parameters of Soil
Avoid common mistakes on your manuscript.
Introduction
Poland has a temperate climate and relatively short cropping seasons, so unheated high tunnels are regarded as season extension structures that allow growers to meet consumer demand for fresh, locally sourced vegetables at times which are usually off-season. Tunnel production is well suited to growing high-value crops, such as organic fruits or vegetables (O’Connell et al. 2012 ; Siwek and Libik 2012 ; Hecher et al. 2014 ; Janke et al. 2017 ). However, because of the specificity of the environment in a high plastic-covered tunnel, with no natural rainfall, limited space, and controlled growth conditions, a special set of crop management skills is required. In vegetable-based cropping systems, a lack of crop rotation within these structures leads to serious problems such as soil-borne diseases, high soil salinity, poor soil structure, decreasing microbial diversity, and consequently reduced crop yields (Nair et al. 2014 ; Rudisill et al. 2015 ). Among the cropping practices used for ecological intensification of production, incorporating cover crop residues and mulching the soil are common management actions in sustainable agriculture worldwide. In field experiment, the effects of rotation and cover crop (CC) residue amendment practices on vegetables have been demonstrated under conventional production system (Kołota and Adamczewska-Sowińska 2013 ; Kaye and Quemada 2017 ) as well as under organic system (Campigila et al. 2012 ; Lawson et al. 2015 ), but few studies have been conducted on how these soil conservation practices influence vegetable production systems in high tunnels (Araki 2009 ; O’Connell et al. 2012 ; Rudisill et al. 2015 ).
One of the important determinants of vegetable productivity in high tunnels is the soil (Montri and Biernbaum 2009 ). A stable soil structure and high soil organic matter content encourage nutrient cycling and improve soil fertility, which finally promotes vegetable growth and increases crop quality and value (Bronic and Lal 2005 ; Spaccini and Piccolo 2013 ; Rudisill et al. 2015 ). Favorable effects of cover crop residue retention on the soil, especially under conservation tillage, include, among other benefits, increased soil organic matter and soil aggregation (Hartwig and Ammon 2002 ; McDaniel et al. 2014 ; Poeplau and Don 2015 ; Haruna and Nkongolo 2015 ; Kaye and Quemada 2017 ). Soil physical properties, such as the dynamics of water-stable macro- and microaggregates, are vital factors for maintaining the overall soil quality. The aggregation and disaggregation processes play a key role in the porosity of soil systems, which determines air, water, and nutrient availability to plants (Spaccini and Piccolo 2013 ). While cover crops provide many benefits, such as to build up soil organic matter, improve soil physical properties, protect from soil erosion, and suppress weed growth, their effects on the yield of the subsequent crop vary (Kumar et al 2005 ; Zaniewicz-Bajkowska et al. 2012 ; Kołota and Adamczewska-Sowińska 2013 ; Rudisill et al. 2015 ). In some cases, CCs increased the biomass production or yield of the subsequent crop. However, in other cases, the cover crop delivered no benefit to the subsequent crop. Cover crops can affect subsequent crop yield mainly through their influence on the use of water stored in the soil, by immobilizing N, or, when producing excessive residues, by inhibiting crop stand establishment or harvest (Weil and Kremen 2007 ; Lawson et al. 2015 , Zaniewicz-Bajkowska et al. 2012 ; Gieske et al. 2016 ). Cover crops can be used to manage nitrogen in agricultural soils by changing N cycling and availability. Initially, a CC reduces N availability by removing nitrate and ammonium ions from the soil. After the CC is terminated, this N becomes increasingly available as residues decompose. If the mineralization of N from the residue occurs too slowly, the N which has first been taken from the soil by the cover crop may not be available when the subsequent crop needs it. Generally, the C/N ratio of CC residues influences the dynamics of organic matter mineralization (Jani et al. 2016 ). However, the rate of decomposition and N release are affected by environmental and soil characteristics (Ruffo and Bollero 2003 ). They reported that the decomposition of leguminous plant residue indicated that it was a potential source of available N, while the decomposition dynamics of cereal plants showed that they were more useful in soil organic matter conservation. Establishing grass and legume CC species in bicultures or mixtures reduces the C to N ratio of plant residues and increases the rate of residue decomposition and N release (Ranells and Wagger 1997 ).
Diversifying crop rotations has a great potential to enhance soil ecosystem functions and is a key to maintaining soil services in agricultural systems generally. A study by McDaniel et al. ( 2014 ) showed that, over time, crop diversity influenced the processing of newly added residues, microbial dynamics, and nutrient cycling. There is a great need to examine such systems under a range of locations and growing conditions.
Therefore, the objectives of this study were (a) to demonstrate the use of cover crops in an organically managed high tunnel with the sequence of plants (2016–2017) spring cover crops/tomato/romaine lettuce/green bean/iceberg lettuce; (b) to study the effects cover crops on the physicochemical properties of the soil; and (c) to quantify their effects on the composition of the component crops and their yield under the growing conditions of southern Poland. The sequence effect of using spring cover crops (field pea monoculture or pea-oat mixture) as well as two different methods of managing the cover plant biomass (left on the soil surface as a mulch or incorporated into the soil) on soil and plant quality parameters was examined. The comprehensive research was related to the effects of CCs on the physicochemical properties of soil and the mineral status of cash crop plants, as well as yields and their quality.
Material and methods
Experimental site.
The experiment was established in 2016 at the experimental station of the University of Agriculture in Kraków, situated in Mydlniki (N 51° 13′, E 22° 38′) near Kraków, Poland. According to the Köppen climate classification, the area in which the station is positioned (southern Poland) belongs to the Dfb climatic region, i.e., the humid continental climate (Peel et al. 2007 ).
A high tunnel (STN 070, 30 × 7.0 × 3.2 m) covered with low-density polyethylene (ld PE) film 0.165 mm thick was constructed in an east-to-west orientation at this site in spring 2014. Previous management history at this tunnel before certification consisted of a 2-year rotation with organic cucumbers ( Cucumis sativus L.). By the use of particle size analysis (PN-R- 04032 1998 ), the soil from the location was categorized as fine-grained; its soil group was silty clay, while clay particles amounted to 40%.
The microclimatic conditions in the high tunnel had been described in detail for the seasons 2014–2015 in a separate paper (Świątkiewicz et al. 2020 ). The microclimatic conditions (photosynthetic active radiation (PAR), air temperature, relative air humidity, air CO 2 content) in the STN 070 plastic tunnel depended significantly on the weather. In the hours before and after midday, the differences were greater on a cloudy day. No significant differences were observed between the zones of the tunnel for individual parameters, especially on a sunny day.
Experiment design
In the spring season of 2016, four replications of cultivation plots were created inside of the high tunnel. All of the replicate plots had 15 m 2 in size. Field pea ( Pisum sativum L.) and a mixture of pea and oat ( Avena sativa L.) were used as cover crops in organic high tunnel vegetable production. They were manually planted at a 10–15 cm distance in pea monoculture and pea-oat biculture on February 10, 2016.The early spring cover crops (CCs) were not harvested but were used as green manure (GM) incorporated into the soil, or as organic mulch (M) with the whole plants remaining on the ground as an additional organic matter input. The control plot in this experiment was bare soil covered with black plastic mulch until the start of a tomato plantation.
Crop husbandry
Tunnel operations in the vegetable production with cover crops in 2016–2017 are presented in Table 1 . Prior to the start of the experiment, the soil was cultivated using a mechanical rotary cultivator. From February (18.02.2016) to the following May (6.05.2016), the pea ( Pisum sativum L.) cultivar “Milwa” and a mixture of pea and cereal oat ( Avena sativa L.) were planted in two cover crop treatments without fertilization. The seeding rate in the monoculture of field pea was 250 kg ha −1 , and for the pea and oat biculture, the seeding density was 120 kg and 90 kg ha −1 , respectively. After cover crop termination, the plant biomass on each plot designated for CC was left on the soil surface of one half of the plot, and incorporated into the soil on the other half. The following substances were used during the soil fertility management: in 2014, 20 t of cow manure was applied to the area of 1 ha; in 2017, potash sulfate (500 kg ha −1 ) was added to the cow manure prior to the sowing of green beans in order to fulfill the recommended crop nutrient requirements. All of the fertilizers were applied during the autumn season 15 cm deep into the soil.
Seeds of “Country Taste” F1 (HILD) tomato were sown on March 16 in greenhouse conditions. The seedlings were transplanted into the high tunnel on April 29 directly into the organic mulch of pea or pea-oat, with row and plant spacing of 90 × 80 cm. Daily mean temperature outside the tunnel in April was 8.9°C and minimum 3.7°C. The low soil temperature delayed acclimatization of the seedlings in the tunnel environment and early yielding of tomato, especially in the plots without black plastic mulch.
Yielding started on July 9 and ended on September 12 in 2016. The plots were irrigated using dripper lines. In the early period of tomato growth, in addition to standard treatments (binding and cutting), there was a need to remove weeds and oat sprouts growing out after mowing. After the tomato harvest, a vertical rotary tiller was used for soil cultivation and elimination of weedy vegetation. The romaine lettuce ( Lactuca sativa var. Romana, var. Tantan) (HILD) was planted on September 16, 2016, at a spacing of 30 × 20 cm. Harvesting of the plants was conducted on November 28. In 2017, after mechanical elimination of weedy vegetation, seeds of the green bean ( Phaseolus vulgaris L.) cultivar “Speedy” (HILD) were sown on April 6, with row and plant spacing of 50 × 20 cm. The plants were harvested repeatedly, on June 14 and 26, and July 3, 2017.
The last plant species in the 2-year cycle of crop rotation with cover crops in the high tunnel was the iceberg lettuce ( Lactuca sativa var. capitata L.) cultivar “Vytalist” (Vitalis BZ). Seeds of the lettuce were sown on July 4, 2017, in greenhouse conditions and transplanted to the tunnel on July 26 at a spacing of 40 × 30 cm. The lettuce was harvested on September 27.
Cultivation of all the species was conducted according to the principles of organic farming (certificates Pl-03-02786-16; PL-03-002786-17).
Data collection
After both the cover crop termination (which occurred on June 8, 2016) and each cash crop harvest, soil core sampler was used in order to collect soil samples from soil (0–20 cm deep). By the usage of a Kopecký’s 250 cm 3 cylinder, undistributed samples were gathered in four replicates from soil (0–10 cm deep) for the purpose of measuring bulk density. Subsequently, the process of weighing, wetting (for capillary action), and drying of soil cores at the temperature of 105°C occurred. From all of the plots, aggregates from undisturbed soil (0–20 cm deep) were gathered in six replicates. Through the process of sieving the bulk soil, air-dried aggregates (<5 mm) were produced. On the top of a stacked construction of five sieves of 0.25-, 0.5-, 1.0-, 1.5-, and 2.5-mm mesh size, 40 g of each soil sample was placed and for 5 min soaked in distilled water. Afterwards, by the process of raising and lowering the sieves with a motor-driven holder, the soil samples were wet-sieved in a water container. The soil samples underwent the process of sieving for 20 min; the frequency of sieving totaled at five 1 cm cycles, while 5 cm was the stroke length. When the wet-sieving process was completed, the soil materials from every sieve that were water-stable were oven-dried at the temperature of 105°C and weighed. The aggregate percentage ratio in every sieve indicates water-stable aggregates for different size classes: 5.0–2.5, 2.5–1.5, 1.5–1.0, 1.0–0.50, and 0.50–0.25 mm.
At a soil to water ratio of 1:2, the pH of soil was determined. By the use of the dichromate oxidation method, the content of soil organic carbon (SOC) was measured (Ostrowska et al. 1991 ). Moreover, by using the universal method, the available form of macronutrients and sodium was analyzed in 0.03 mol dm −3 CH 3 COOH. Boron (the extractable form) was determined in 1 mol dm −3 HCl (Ostrowska et al. 1991 ). The inductively coupled argon plasma atomic emission spectroscopy (ICP-OES) technique was used in order to determine the available forms of nutrients.
In every combination, from an area of 4 × 1 m 2 , samples of the aboveground biomass were gathered before the termination of the spring cover crops. In order to estimate the total aboveground fresh biomass, plants were weighed. Green bean pods, tomato fruits, and, most importantly, mature leaves with petioles of tomato and green bean (in the half-grown vegetable stage of cash crop) were selected at random. The following procedure was applied to the gathered leaf tissue samples (cover crop and main cash crop): washing with distilled water, drying at the temperature of 65°C for 48 h, and grinding for the purpose of elemental analysis. By the use of the ICP-OES technique, macro- and micronutrients were determined after the process of microwave digestion in HNO 3 . A Prodigy High Dispersion ICP-OES Spectrometer (Teledyne Leeman Labs, Hudson NH, USA) was employed with the aim of conducting elemental analyses. The Kjeldahl method was carried out in order to determine the total nitrogen of plant material (Ostrowska et al. 1991 ).
The gravimetric method (PN-A-75101-03 1990 ) was used to measure the dry matter content of the cover crop biomass, tomato and green bean leaves and fruits, and lettuce heads. The total soluble sugars and the L-ascorbic acid content in fresh cash crop tissues were determined according to the anthrone method (Yemm and Willis 1954) and PN-A-04019 1998 , respectively.
Statistical analyses
In order to compare the main effects of soil treatments (type of cover crops followed by different cover crop biomass management), statistical analysis was used. Statistica 13.1 software (Statsoft Inc.) was employed for the purpose of analyzing data. A one-way ANOVA system was used in the process of analyzing all data. Considering the combinations marked with the same letters, there is no significant difference in the mean values at the significance level of p ≤ 0.05.
Cover crop biomass and chemical composition
The spring cover crop biomass was relatively greater for the field pea-oat mixture than the field pea monoculture, but not statistically significant (Table 2 ). The 2016 growing season of the cover crops (February 10 to April 26) lasted for only 75 days in the high tunnel conditions. The nitrogen content in the cover crop aboveground biomass was affected by the cover crop species, and it was higher in the field pea monoculture (155 kg N ha −1 ) than in the mixture with oat (136 kg N ha −1 ) (Table 2 ). In the presented study, the percentage nitrogen content in the cover crop biomass was 5.06% and 3.27%, respectively, for pea and pea+oat mix (Table 3 ). With the exception of K, Na, Fe, and Mn, higher concentrations of nutrient elements were found in the aboveground biomass of field pea than the pea-oat mixture, especially for boron, copper, and molybdenum.
Soil analysis
In 2016, the soil bulk density (BD) found after the cover crop had been terminated was lower in the soil under the pea-oat mixture than in the control and field pea monoculture soil treatment (Table 4 ). After the tomato harvest in 2016, the plots mulched with cover crop residues had lower BD values than the plots where CC residues were incorporated into the soil, and also lower than the control plots. In the 2-year rotation, we did not observe positive effects of CC treatments on soil water capacity in the high tunnel conditions.
After the cover crop termination in 2016, the highest soil organic carbon (SOC) content was determined in the soils under the cover crop treatment (Table 4 ). This trend was also found in the soils after the tomato harvest in 2016, especially for the plots where CC residues were incorporated into the soil. The highest SOC values were recorded for the soils treated with the pea-oat mixture. This significant effect was also evident after the romaine lettuce harvest in 2016 and in the second year of the experiment after green bean and iceberg lettuce cultivation in 2017. In autumn 2016, the soil under the monoculture pea treatment already contained an SOC amount comparable with that of the control soil.
In both years of the high tunnel experiment, the highest water-stable aggregate index (WSI), expressed as the sum of the 0.25–5.0 mm water-stable aggregate fractions, was found in the soils under the field pea-oat mixture treatment (Table 5 ). The distribution of water-stable aggregates characterizing the initial soil samples, before the start of the high tunnel experiment (autumn 2015), showed a significant effect of textural composition on soil structural properties. An increase in the stability of macroaggregates and a decrease in the amount of microaggregates were found after the cover crops had been terminated in 2016, in comparison with the initial soil and the control soil (Table 5 ). Macroaggregates (2.5–5.0 mm) constituted 18.5–28.3% of water-stable aggregates and varied significantly among the treatments. After the tomato harvest in 2016, the trend to increase the macroaggregate content in the soil under the field pea treatment was observed irrespective of plant residue management. This effect was also observed after the green bean harvest in 2017. The water-resistant soil structure under the pea-oat treatment was characterized by a higher number of aggregates 1.0–0.50 mm in diameter, especially in the first year of our experiment (Table 5 ).
The nutrient element content in the control soils (bare soil under black polypropylene mulch during CC plantation) or soils under CC treatments was determined after the cover crops had been terminated (Table 6 ). The concentrations of potassium, magnesium, and phosphorus (only in pea-oat plots) were higher in the CC-treated soil than in the plastic-mulched soil (control).
Soil analysis data obtained after the tomato harvest in September 2016 indicated that the plots under CC treatments had significantly higher pH values (pH 7.17–7.43) compared to the control soil (pH 6.94) (Table 6 ). The tendency to decrease the concentration of soluble salts in the soil solution was also evident. The concentrations of NH 4 + were very low and those of NO 3 − showed no statistically significant differences in the soils treated with CC residues. However, a tendency towards slightly elevated NO 3 − concentrations was detected in the soil under treatment with CCs as green manure formula (GM). The concentration of Ca and Mg in the soils was from low (the control and pea M) to high according to the guide values estimated using the universal method (Sady 2000 ). A high concentration of these nutrients was found in the soil under CCs incorporated into the soil, irrespective of the cover crop species. The extractable K content in the high tunnel soils ranged from very low (40 and 42 mg K dm −3 , respectively, in the control soil and pea-oat GM treatment) to low (88 mg K dm −3 of the pea GM soil). In general, the sufficient level of K, determined by the universal soil test with acetate extraction, for most vegetables ranges from 150 to 250 mg K dm −3 for high textured soil (Sady 2000 ). Soil phosphorus concentration was within the low range for vegetables (<30 mg P dm −3 of soil) for the control and pea-treated soils, and within the optimal range for the pea-oat treatments.
In autumn 2016, after the romaine lettuce harvest, the concentrations of nutrient elements in the soils under the pea CC treatment were elevated, particularly for nitrogen (43.18 mg NH 4 -N and NO 3 -N dm −3 of soil), calcium, magnesium, and potassium (Table 6 ). In the second year after the CC treatments, the concentrations of mineral nitrogen determined in the soils after the green bean harvest were high and ranged from 45.57 mg NH 4 -N and NO 3 -N (field pea) to 88.7 mg NH 4 -N and NO 3 -N (pea-oat). A higher concentration of P in the soil after the green bean harvest was found in the pea-oat CC treatment. The iceberg lettuce production in the high tunnel in autumn 2017 reduced the concentrations of soluble forms of macro-elements in the soil, especially those of N and K (Table 6 ).
After the tomato and romaine lettuce harvests in 2016, with the exception of B (low content), micronutrient concentrations were optimal for plant nutrition (Table 7 ). The highest B amount was found in the soil under the pea-oat treatments, irrespective of the methods of using CC residues (incorporating versus mulching). During the second cropping period (2017), the CC treatments slightly increased Cu and Mn concentrations in the high tunnel soil.
Cash crop yielding
Already before the first harvest of tomatoes, more leaves and female flowers were observed to appear on the control plants, which indicated an inhibitory effect of cover plants, especially where oat was involved. Harvesting of tomato fruit began on July 9 (control), July 13 (field pea), and July 18 (pea-oat). The yields of fruit > 4 cm in diameter were much higher where no green manure was used. As a result of the occurrence of viral diseases, the yields in the whole tunnel were not high and amounted to 4.60 kg m −2 , control; 2.46 kg m −2 , field pea; and 2.15 kg m −2 , pea-oat (Table 8 ). In the second growing season, the yield of green bean was significantly higher in the plot after field pea, whereas the yield of iceberg lettuce was higher in the control plot and after the pea-oat mixture. The quality of crops expressed by the amounts of dry matter, vitamin C, and sugars in tomatoes and romaine lettuce grown in 2016 was at a similar level (Table 8 ). Despite the lack of statistically significant differences, one can notice a tendency for those contents to decrease in the edible parts obtained after the pea-oat mixture. In the second year of the study, when the biomass of the ground cover plants had been largely mineralized, a significantly lower dry matter content was found in the green bean plants grown after the pea-oat mixture. The mineralized plant residues of field pea caused an increase in the dry matter content in iceberg lettuce. The level of vitamin C in iceberg lettuce was significantly higher in the case of cultivation in the plot after the pea-oat mixture. The sugar content in the vegetables grown in the second year after the cover plants showed a slight positive effect of these treatments.
Foliar analysis showed that all leaf macronutrient concentrations except K in pea GM and pea-oat GM treatments, Mg in the control, and sulfur were in the range considered below optimal for commercially grown tomato (Sainju et al. 2003 ). Generally, cover cropping lowered Ca, Mg, and S content in tomato leaves, compared with the control plants. With the exception of plants grown in the plots where residues of CCs were used as organic mulch, the concentrations of potassium and phosphorus in tomato leaves were higher than in the control ones. This can indicate that organic CC residue incorporated into the soil decomposed easily, becoming a good source of available K and P for tomato plants. The concentration of these elements in plant tissues is also linked with the higher available soil K and P (only for pea-oat GM treatment) in the treatments with CCs as green manure (GM).
The highest N concentration in tomato leaves in August was determined in the pea GM treatment with residues incorporated into the soil (Table 9 ). The soil mulched with pea-oat residues reduced tomato leaf N accumulation and tomato fruit yield, relative to the control and the other treatments. Generally, cover cropping lowered the concentrations of micronutrients in tomato leaves in relation to the control soil. The exception was the high B and Mo contents in the tomato leaves growing in the plots where the mulch of field pea was used (Table 9 ). A significantly higher concentration of K was found in tomato fruits harvested from the plots with CC treatments, in comparison with the control (Table 10 ). Potassium and phosphorus concentrations in fruits were higher in the mulched plots, irrespective of the CC species. A similar tendency was observed for boron and manganese.
Leaf analyses of green bean plants planted in the high tunnel in the second year of the study showed that the effects of field pea and pea-oat cover crops in rotation on subsequent nutrient dynamics were mostly short-lived. For all the nutrients, with the exception of N and B, the concentrations in legume plant tissues were similar regardless of the prior CC treatment. In the second year, the field pea treatment slightly decreased N and B concentrations in green bean leaves (Table 11 ).
In contrast to the leaves, green bean pods harvested from the plots under prior CC treatments had significantly higher K and P concentrations in relation to the control plants (Table 12 ). Also, the highest Mn content was found in pods following the field pea treatment, in comparison with the control and pea-oat treatment. The reverse was true for the molybdenum concentration in green been pods.
In this trial, we surveyed management strategies that included a cover crop system and vegetable crop rotation in a high tunnel during cropping periods 2016–2017 with a sequence of plants: spring field pea (pea-oat mixture/tomato/romaine lettuce/green bean/iceberg lettuce. Some species grow poorly in monocultures, but cooperate positively with other species in mixtures, for example, grass-legume interactions (Sainju et al. 2005 ; Forest et al. 2011 ). This regularity confirms our results with pea-oat bicultural as well as the research by Campigila et al. ( 2012 ) for vetch-oat mixture. Conversely, during a short growing season of a hairy vetch and wild oat mixture cover crop, Araki ( 2009 ) did not observe biomass to be greater than in the monoculture of wild oat. Neugschwandtner and Kaul ( 2014 ) demonstrated that oat-pea intercrops could not achieve higher yields than the corresponding pure stands on a fertile soil.
In this experiment, the field pea and field pea-oat mixtures grown in early spring in unheated high tunnel had aboveground biomass comparable to or higher than that in other regions of Poland in autumn open field conditions (Jabłońska-Ceglarek and Rosa 2002 ; Zaniewicz-Bajkowska et al. 2012 ). Moreover, relatively low temperatures during the times of CC establishment in the spring and biomass production in February to April likely favored the growing of oat over the field pea. In the study by Neugschwandtner and Kaul ( 2014 ), oat was the dominant partner in the mixtures, strongly outcompeting pea. The authors concluded that the sowing ratio and fertilization affected the yield component parameters of oat and pea compared to the corresponding pure stands. In the presented study, the sowing ratio for pea in the mixture with oat was 120:90 in kilogram of seed per hectare.
The pea monoculture had lower fresh biomass than the pea-oat mixture, and the N content in their biomass was 155 kg N ha −1 and 136 kg N ha −1 , respectively. In the study by Ranells and Wagger ( 1997 ), N accumulation values for the legume component followed the ranking winter pea > hairy vetch > common vetch > crimson clover, and ranged from 24 to 93 kg N ha −1 . Grass factor N content ranged from 18 to 39 kg N ha −1 in the order rye > oat > wheat. In the study by Jabłońska-Ceglarek and Rosa ( 2002 ), N accumulation in the biomass (20 t f.m. ha −1 ) of field pea+oat in mixed cultivation amounted to 72 kg N ha −1 .
In our study, we examined the effect/sequence effect of spring cover crops and two different methods of managing the cover plant biomass (left on the soil surface as a mulch or incorporated into the soil) on soil and plant quality parameters in organic high tunnel production. This is important to recognize the benefits of this system beyond yield which justify the adoption of CCs by farmers. In this study, cover crop improved some of important soil properties examined, including bulk density, organic matter content, and water-stable aggregate index compared to the control soil. The fertility of soil significantly depends on its compactness, which impacts the agronomic yield. The tendency for a lower bulk density attribute in the soils under a cover crop treatment persisted after the romaine lettuce harvest in 2016 and at the end of 2017, particularly for the pea-oat mix. This confirms the results of the study by Haruna and Nkongolo ( 2015 ) which indicated a 3.5% decrease in soil bulk density in cover crop plots as compared with no-cover crop soils. Similarly, Gabriel et al. ( 2017 ) found a positive cover crop effect consisting in an increase in soil micro- and macro-porosity. That study also showed an improvement in water retention in 20 intermediate layers of the soil profile. Nascente and Stone ( 2018 ) after 2 years (two cycles of cover crops) also promoted improvement in soil physical properties in the two soil layers 0.06–0.10 m and 0.11–0.20 m.
Schipanski et al. ( 2014 ) and McDaniel et al. ( 2014 ) showed that cover cropping increased soil C sequestration. Poeplau and Don ( 2015 ), during an observation period of up to 54 years, indicated that the cover crop was correlated with SOC content change, with an annual variation rate of 0.32±0.08 Mg ha −1 yr −1 in 22 cm of soil depth. In the presented study, after the cover crop termination (2016), the highest soil organic carbon (SOC) content was determined in the soils under the cover crop treatment. In the subsequent cropping season (2017), the trend of an increasing SOC content in the soils under cover crop treatment was still clearly evident. The highest SOC values were noted for the soils treated with the pea-oat mixture. Our results confirmed that the quantity and quality (C/N ratio) of crop residues returned to the soil regulate SOC sequestration. Soil amendment with bio-labile organic inputs has short-term effects on soil chemical and physical attributes (Spaccini and Piccolo 2013 ). Generally, mineral nitrogen availability for organic matter decomposition is responsible for a faster and more advanced decomposition (Coppens et al. 2007 ).
Soil aggregation, as well as soil structure, is essential for soil functioning and productivity. In both years of our experiment, the highest water-stable aggregate index was found in the soils under the field pea-oat mixture treatment. Spaccini and Piccolo ( 2013 ) had indicated that enriching the soil with bio-labile organic matter had short-term effects on soil aggregation processes, and that soil treatments with humified organic materials might considerably improve aggregate stability. This may explain the relatively poorest results obtained for the field pea monoculture treatment. However, soil structure analysis conducted in 2016 showed an increase in the water stability index for both cover crop treatments on the tomato plots in comparison with the untreated soil (Table 4 ). By conducting the 2-year experiment, we demonstrated that rotation and/or cover crop treatment can maintain suitable physical and chemical soil properties through macroaggregate water stabilization, which contributes to protecting SOC stocks and a stable soil structure. Particle size analysis (PN-R-04032 1998 ) classified the soil at this site as fine-grained, belonging to the silty clay group with clay particles constituting about 40%. A high amount of clay particles as well as high level of bivalent cations (>1000 mg Ca and >100 mg Mg dm −3 of soil) in the analyzed soils determined a high percentage of water-stable soil aggregates. Bivalent cations improve soil structure by binding clay particles and soil organic matter (Bronic and Lal 2005 ). The growth in macroaggregate formation can be explained with the root and microbial biomass production and the presence of root debris and microbial bio-products, which promote the association of microaggregates into meso- and macroaggregates (Wohlenberg et al. 2004 ; Spaccini and Piccolo 2013 ; Ontl et al. 2015 ). Ontl et al. ( 2015 ) suggest that the formation of macroaggregates can be a good indicator of potential future C stabilization due to their significance for protecting freshly deposited SOM and helping the formation of stable organo-mineral complexes. Macroaggregates are formed around fresh organic residues, which are subsequently integrated and become coarse intra-aggregate particulate organic matter (Bronic and Lal 2005 ).
For nutrient management in organic high tunnel systems, the effects of organic matter and soil nutrient cycling are crucial points (Rudisill et al. 2015 ). Moreti et al. ( 2007 ) reported that cover crops could significantly affect the soil chemical attributes. Our results confirm the effect found by Fatima et al. ( 2012 ), who determined a 28% higher potassium content in vetch-treated soil than in bare soil. Plants can modify nutrient availability by secreting numerous chemicals from their roots (root exudates). These exudates alter nutrient mobility through various mechanisms such as ion exchange, chemical desorption, or complexation (Hinsinger et al. 2005 ). N 2 -fixing legumes, which can grow without nitrate uptake from the soil solution, but via the profits of N 2 reduction in the root nodules, must secrete an excess of protons. These protons can markedly lower rhizosphere pH and can increase the availability of some mineral nutrients in the soil (Dakora and Phillips 2002 ; Sugiyama and Yazaki 2012 ). In our study, this phenomenon was observed for Ca, Mg, K, and P in the CC plots (only under the pea-oat treatment). In the study of Nascente and Stone ( 2018 ) in open field conditions, cover crops alone provided no changes in soil chemical properties. However, the rotation cover crops/cash crops/cover crops/cash crops decreased pH values, and increased Ca, Mg, K, and Fe contents in the soil.
Cover cropping did not modify the availability of micronutrients in the high tunnel soils analyzed after CC plant termination (Table 7 ), which had been expected. Variation in the concentration of trace elements in plant leaves during cultivation can indicate their different availability to plants from the experimental plots. A probable explanation for this could be the method of soil extraction. The extractable forms of trace metals, including micronutrients, were measured in 1 mol dm −3 HCl soil extract (Ostrowska et al. 1991 ). This technique with a relatively “aggressive extractant” removes more than the soluble, exchangeable, and weakly adsorbed fractions of trace elements. This soil extractant and procedure is currently used to estimate the availability and critical levels for micronutrient cations in Poland.
Cover crops affect subsequent crop yield mainly through their influence on nitrogen availability. In our study, the nitrate content in the soil had been depleted of the available N reserve by the cover crops. The inhibitory effect of cover plants, especially for pea-oat mixture, was observed for tomato plants which were transplanted into the high tunnel directly after pea and pea-oat termination. However, in the second growing season, the yield of green bean was significantly higher in the plot after field pea. Cover crops can improve nutrient use efficiency when the elements in the cover crops are cycled back into the soil as green manure and are taken from the soil by subsequent cash crops (Ruffo and Bollero 2003 ; Araki 2009 ; Jabłońska-Ceglarek and Rosa 2002 ). The chemical composition of the residues has an important impact on the decomposition processes. Araki ( 2009 ) had shown higher marketable yields of high tunnel tomato in hairy vetch plots than in those with mineral fertilization and control plots. Similar results had been obtained by Sainju et al. ( 2003 ) and Sugihara et al. ( 2013 ). The C/N ratio is often used as an indicator of either net N mineralization or immobilization during residue decomposition. In the Araki ( 2009 ) study with the 15N-labelling method, hairy vetch residues incorporated into the soil in greenhouse conditions decomposed rapidly for about 1 month and the N released from green manure was absorbed by tomato plants. This demonstrated that pea residues in the presented study can provide a substantial level of N to the subsequent tomato crop. Decomposition of cereal residues immobilizes soil N near the soil surface, which can suppress tomato growth. Araki ( 2009 ) and Fatima et al. ( 2012 ) found the highest N content in the tomato leaves produced with a hairy vetch monoculture cover crop. As mentioned earlier, the nitrogen concentration in plant residues is the main factor determining residue quality due to the influence of N availability on microbial metabolism (Coppens et al. 2007 ; Jani et al. 2016 ). A lower initial C/N ratio of a legume cover crop than that of non-legume or mixed crop residues favors the mineralization of nutrients for their uptake by plants (Rosecrance et al. 2000 ; Lawson et al. 2015 ).
In the presented study, in the soil covered with mown CC plants, the tomato plants developed and grew more slowly in relation to the control plants. Gent (1992) had demonstrated that earlier tomato plantings suffered from nutrient deficiencies, presumably as a result of soil low-temperature effects on nutrient uptake. The reason for the large differences in tomato yielding between CCs plots and the control may have been the positive influence of black film on soil moisture and temperature, and the increased availability of nutrients in the plots not sown with the cover plants. Knowledge of these relationships could permit development of more precise cover crop practices in organic high tunnel production, where the success of this solution largely depends on the proper establishment and management of CC biomass.
In 2017, after green bean harvest, we found the significantly higher soil Ca, Mg, and P concentration in pea-oat field compared to the control soil. Additionally, higher concentrations of N-NO 3 in cover pea-oat cropping soils were observed in relation to pea treatment. Green bean pods collected from the plots under prior pea-oat treatments had significantly higher K and P concentrations in relation to the control and pea treatment plants. The green bean legume plant represents a tripartite symbiotic relationship where diazotrophic bacteria ( Rhizobium ) fix N not only for the host plant but also for mycorrhizal fungi. This is particularly important in the uptake of nutrients required by plants living in a soil environment that is low in available nutrients. Legume plants establish symbiotic interactions with rhizobia and arbuscular mycorrhizal fungi to obtain several nutrients such as nitrogen and phosphates (Sugiyama and Yazaki 2012 ). Organic acids from legume root exudates can also solubilize unavailable soil Ca, Fe, and Al phosphates (Dakora and Phillips 2002 ). This may explain the increase in some available macronutrients in soil after growing pea-oat plants in our experiment (Table 6 ).
Interesting interactions between the cover crops and the tomato plant in the high tunnel organic production resulting in delayed leaf senescence and increased fungal disease tolerance were observed. This is in agreement with the results of studies by Kumar et al. ( 2005 ) and Fatima et al. ( 2012 ). Thus, a greater K concentration in CC-amended soils, and in the tomato leaves and fruit harvested from those plots, could be responsible for delayed leaf senescence. Potassium is essential for most of the biochemical and physiological plant processes and influences plant growth and metabolism. Deficiency of potassium may intensify early leaf senescence, probably via stimulation of ethylene biosynthesis (Wang et al. 2013 ).
Conclusions
There are many good reasons to use cover crops in sustainable horticulture. To understand how cover cropping management affects vegetable production in an unheated organic high tunnel, this experiment investigated the effect of rotation and spring cover crops (pea monoculture and pea-oat biculture) on physicochemical soil properties and on yield and its quality. Our results revealed an increase in soil pH and in the availability of some mineral nutrients in the soil under CC treatments, especially Ca, Mg, K, and P. Obtained results suggest that cover crops should be included in the agricultural practices in organic high tunnels so that they can be used to protect soil quality. However, there may be instances when water and nutrient use by cover crops or field operations associated with CC management could decrease cash crop yield in high tunnels, and local knowledge of these risks will need to be taken into account. In the presented study, early spring cover crops delivered no benefit to the subsequent summer crop. Significant differences between CC species and the method of using the biomass of green manure were observed. N uptake by the subsequent cash crop was greater with pea monoculture than with pea-oat-biculture and in the green manure formula with residues incorporated into the soil than being used as mulch. Because of the higher N content in the biomass, field pea cover crops may increase N uptake by the summer crop, compared to pea-oat CC. However, the potential of field pea CC to improve yield in the high tunnel was observed by us only in the second-year cash crop yield (green bean). Further research and extension efforts are required for adaptation of cover cropping in crop rotation practices to organic tunnels, to facilitate greater application at the farm level.
Araki H (2009) Tomatoes production with cover crops in greenhouse. Hortic Environ Biote 50:324–328
Google Scholar
Bronic CJ, Lal R (2005) Soil structure and management: a review. Geoderma 124:3–22
Article Google Scholar
Campigila E, Radicetti E, Mancinelli R (2012) Weed control strategies and yield response in a pepper crop ( Capsicum annuum L.) mulched with hairy vetch ( Vicia villosa Roth.) and oat ( Avena sativa L.) residues. Crop Prot 33:65–73
Coppens F, Garnier P, Findeling A, Merckx R, Recous S (2007) Decomposition of mulched versus incorporated crop residues: modelling with PASTIS clarifies interactions between residue quality and location. Soil Biol Biochem 39:2339–2350
Article CAS Google Scholar
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47
Gabriel JL, Quemada M, Martín-Lammerding D, Vanclooster M (2017) Assessing the cover crop effect on soil hydraulic properties by inverse modelling in a 10-year field trial. Hydrol Earth Sys Sc. https://doi.org/10.5194/hess-2017-643
Gieske MF, Ackroyd VJ, Baas DG, Mutch DR, Wyse DL, Durgan BR (2016) Brassica cover crop effects on nitrogen availability and oat and corn yield. Agron J 108:151–161
Fatima T, Teasdale JR, Bunce J, Mattoo AK (2012) Tomato response to legume cover crop and nitrogen: differing enhancement patterns of fruit yield, photosynthesis and gene expression. Funct Plant Biol 39(3):246–254
Forest I, Calcagno V, Hector A, Connolly J, Harpole WS, Reich PB, Scherer-Lorenzen M et al (2011) High plant diversity is needed to maintain ecosystem services. Nature 477:199–202
Hartwig NL, Ammon HU (2002) Cover crops and living mulches. Weed Sci. 50:688–699
Haruna SI, Nkongolo NV (2015) Cover crop management effects on soil physical and biological properties. Procedia Environ Sci 29:13–14
Hecher EAD, Falk CL, Steven JE, Guldan SJ, Uchanski ME (2014) The economics of low-cost high tunnels for winter vegetable production in the southwestern United States. HortTechnol 24:7–14
Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW (2005) Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol 168:293–303
Jabłońska-Ceglarek R. Rosa R (2002) Influence of different forms of organic fertilization on cropping and chemical composition of white cabbage. Acta Sci Pol-Hortu 1(2) 2:25–32
Jani A, Grossman J, Smyth T, Hu S (2016) Winter legume cover-crop root decomposition and N release dynamics under disking and roller-crimping termination approaches. Renew Agric Food Sys 31:214–229
Janke RR, Altamimi ME, Khan M (2017) The use of high tunnels to produce fruit and vegetable crops in North America. Agri Sci 8:692–715
Kaye JP, Quemada M (2017) Using cover crops to mitigate and adapt to climate change: a review. Agron Sustain Dev 37. https://doi.org/10.1007/s13593-016-0410-x
Kołota E, Adamczewska-Sowińska K (2013) Living mulches in vegetables crops production: perspectives and limitations. A review. Acta Sci Pol-Hortu 12:127–142
Kumar V, Abdul-Baki A, Anderson JD, Mattoo AK (2005) Cover crop residues enhance growth, improve yield, and delay leaf senescence in greenhouse-grown tomatoes. Hort Sci 40(5):1307–1311
Lawson A, Cogger C, Bary A, Fortuna AM (2015) Influence of seeding ratio, planting date, and termination date on rye-hairy vetch cover crop mixture performance under organic management. PLoS ONE 10. https://doi.org/10.1371/journal.pone.0129597
McDaniel MD, Grandy AS, Tiemann LK, Weintraub MN (2014) Crop rotation complexity regulates the decomposition of high and low-quality residues. Soil Biol Biochem 78:243–254
Montri A, Biernbaum JA (2009) Management of the soil environment in high tunnels. HortTech 19:34–36
Moreti D, Alves MC, Valério Filho WV, Carvalho MP (2007) Soil chemical attributes of a Red Latosol under different systems of preparation, management, and covering plants. Rev Bras Ci Solo 31(1):167–175
Nair A, Carpenter BH, Tillman JL, Jokela DL (2014) Integrating cover crops in high tunnel crop production. Iowa State Research Farm Progress Reports. 2009
Nascente AS, Stone LF (2018) Cover crops as affecting soil chemical and physical properties and development of upland rice and soybean cultivated in rotation. Rice Sci 25(6):340–349
Neugschwandtner RW, Kaul HP (2014) Sowing ratio and N fertilization affect yield and yield components of oat and pea in intercrops. Field Crops Res 155:159–163
O’Connell S, Rivard C, Peet MM, Harlow C, Louws F (2012) High tunnel and field production of organic heirloom tomatoes: yield, fruit quality, disease and microclimate. Hort Sci 47:1283–1290
Ontl TA, Cambardella CA, Schulte LA, Kolka RK (2015) Factors influencing soil aggregation and particulate organic matter responses to bioenergy crop across a topographic gradient. Geoderma 255–256:1–11
Ostrowska A, Gawliński S, Szczubiałka Z (1991) Methods of analysis and evaluation of soil properties and plant – catalogue. Warsaw: Institute of Environmental Protection (In: Polish).
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11:1633–1644
PN-A-75101-03 (1990) Fruit and vegetables. Sample preparation and methods of physicochemical tests - determination of dry matter content by gravimetric method. Polish Committee for Standardization.
PN-R-04032 (1998) Soil and mineral materials. Sampling and determination of particle size distribution. Polish Committee for Standardization.
PN-A-04019 (1998) Food products - determination of vitamin C content. Polish Committee for Standardization.
Poeplau C, Don A (2015) Carbon sequestration in agricultural soils via cultivation of cover crops – a meta-analysis. Agric Ecosyst Environ 200:33–41
Ranells NN, Wagger MG (1997) Grass-legume bicultures as winter annual cover crops. Agron J 89:659–665
Rosecrance RC, McCarty GW, Shelton DR, Teasdale JR (2000) Denitrification and N mineralization from hairy vetch ( Vicia villosa Roth) and rye ( Secale cereale L.) cover crop monocultures and bicultures. Plant Soil 227:283–290
Rudisill MA, Bordelon BP, Turco RF, Hoagland LA (2015) Sustaining soil quality in intensively managed high tunnel vegetable production systems: a role for green manures and chicken litter. Hort Sci 50:461–468
Ruffo ML, Bollero GA (2003) Modeling rye and hairy vetch residues decomposition as a function of degree-days and decomposition-days. Agron J 95:900–907
Sady W (2000) Field vegetable fertilization. Plantpress, Kraków [In Polish]
Sainju UM, Dris R, Singh B (2003) Mineral nutrition of tomato. J Food Agric Environ 1(2):176–183
CAS Google Scholar
Sainju UM, Whitehead WF, Singh BP (2005) Biculture legume-cereal cover crops for enhanced biomass yield and carbon and nitrogen. Agron J 97:1403–1412
Schipanski M, Barbercheck M, Douglas MR, Finney DM, Haider K, Kaye JP et al (2014) A conceptual framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agric Syst 125:12–22
Siwek P, Libik A (2012) Plastic covers in Polish horticulture. Plasticulture 131:65–73
Spaccini R, Piccolo A (2013) Effects of field managements for soil organic matter stabilization on water-stable aggregate distribution and aggregate stability in three agricultural soils. J Geochem Explor 129:45–51
Sugihara Y, Ueno H, Hirata T, Araki H (2013) Uptake and distribution of nitrogen derived from hairy vetch used as cover crop by tomato plant. J Jpn Soc Horti Sci 82:30–38
Sugiyama A, Yazaki K (2012) Root exudates of legume plants and their involvement in interactions with soil microbes. In: Vivanco JM, Baluška F (ed) Secretions and exudates in biological systems, Signaling and communication in plants 12:27-48.
Świątkiewicz I, Kalisz A, Bucki P (2020) Microclimatic conditions and physico-chemical properties of soil in intensive ecological vegetable crops rotation in high tunnel Acta Scien Pol Hort Cult 19(3):73–87
Thorup-Kristensen K, Magid J, Jensen LS (2003) Catch crops and green manures as biological tools in nitrogen management in temperate zones. Adv Agron 79:227–302
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14(4):7370–7390
Weil R, Kremen A (2007) Thinking across and beyond disciplines to make cover crops pay. J Sci Food Agric 87:551–557
Wohlenberg EV, Reichert JM, Reinert DJ, Blume E (2004) Aggregation dynamics of a sandy soil under five cropping systems in rotation and in succession. Rev Bras Ci Solo 28(5):891–900
Zaniewicz-Bajkowska A, Franczuk J, Rosa R, Kosterna E (2012) Green manure in Mazowsze. Formator, Toruń, Poland. [In Polish].
Download references
Acknowledgements
The research at the University of Agriculture in Krakow was subvented by the Polish Ministry of Science and Higher Education in 2020.
Author information
Authors and affiliations.
Institute of Plant Biology and Biotechnology, Unit of Plant Nutrition, University of Agriculture in Krakow, Al. 29 Listopada 54, 31-425, Krakow, Poland
I. Domagała-Świątkiewicz
Department of Vegetable and Herb Plants, University of Agriculture in Krakow, Krakow, Poland
You can also search for this author in PubMed Google Scholar
Contributions
Iwona Domagała-Świątkiewicz: conceptualization, formal analysis, investigation, writing (original draft), visualization, project administration. Piotr Siwek: conceptualization, methodology, resources, writing (review and editing).
Corresponding authors
Correspondence to I. Domagała-Świątkiewicz or P. Siwek .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Domagała-Świątkiewicz, I., Siwek, P. Effect of field pea ( Pisum sativum subsp. arvense (L.) Asch.) and pea-oat ( Avena sativa L.) biculture cover crops on high tunnel vegetable under organic production system. Org. Agr. 12 , 91–106 (2022). https://doi.org/10.1007/s13165-021-00383-x
Download citation
Received : 16 May 2019
Accepted : 28 December 2021
Published : 01 February 2022
Issue Date : March 2022
DOI : https://doi.org/10.1007/s13165-021-00383-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Soil organic carbon
- Aggregate stability
- Nutrient elements
- Find a journal
- Publish with us
- Track your research

Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
A comprehensive review of pea ( pisum sativum l.): chemical composition, processing, health benefits, and food applications.

1. Introduction
2. chemical composition of pea, 2.1. proximate composition, 2.2. starch, 2.3. dietary fiber, 2.4. protein, 2.5. lipids, 2.6. minerals and vitamins, 2.7. polyphenols, 2.7.1. total phenolic content, 2.7.2. flavonoids.
| Family | Compounds | Plant Part | Methods | References |
|---|---|---|---|---|
| Flavonols | Isorhamnetin 3-rutinoside, isorhamnetin glycoside, quercetin, quercetin 3-galattoside, rutin, quercetin triglucoside, quercetin diglucoside, kaempferol triglucoside, quercetin caffeoyl triglucoside, quercetin coumaroyl triglucoside, quercetin sinapoyl triglucoside, quercetin feruloyl triglucoside, isorhamnetin glycoside, kaempferol glucoside, kaempferol coumaroyl, kaempferol, dihydromyricetin, kaempferol 3-O-rutinoside-4′-glucoside, dihydroquercetin, myricetin 3-O-rhamnoside, kaempferol 3-O-glucoside, kaempferol hexoside, kaempferol-7-O-glucoside, kaempferol-7-O-rutinoside, kaempferol-3-O-rhamnoside, kaempferol dihexoside, isorhamnetin, dihydrokaempferol, kaempferol 3-O-glucopyranoside, fisetin, kaempferol 3-O-neohesperidoside, kaempferol 3-O-sophorotrioside, kaempferol 3-O-(6″″-O-trans-p-coumaroyl)-sophorotrioside, galangin, morin, quercetin 3-O-β-D-glucopyranoside, quercetin 3-O-sophorotrioside, quercetin 3-O-(6″″-O-trans-p-coumaroyl)-sophorotrioside, quercetin 3-O-(6″″-O-trans-caffeoyl)-sophorotrioside, quercetin 3-O-(6″″-O-trans-feruloyl)-sophorotrioside, quercetin 3-O-(6″″-O-trans-sinapoyl)-sophorotrioside, quercetin 3-O-(6″″-O-(4-hydroxy)-trans-cinnamoyl)-sophorotrioside, Pisumflflavonoside II [quercetin 3-O-(6″″-O-trans-p-coumaroyl)-sophorotrio-side 7-O-β-D-glucopyranoside], Pisumflflavonoside II [quercetin 3-O-(6″″-O-trans-p-coumaroyl)-sophorotrio- side 7-O-β-D-glucopyranoside] | Seed, seed coat, pod, sprout, leaf | LC-MS, LC-ESI-MS, LC-ESI-MS/MS, UHPLC-MS, UHPLC-LTQ-MS, UHPLC-Q-HRMS | [ , , , , , , , , , ] |
| Flavones | Phloretin, apigenin, luteolin-7-O-glucoside, eriodictyol glycoside, apigenin-7-O-glucoside, luteolin, luteolin 8′-O-glucoside, vitexin, luteolin 3′,7-di-O-glucoside, apigenin-6.8-di-C-glucoside, luteolin-8′-C-glucoside, tricin | Seed, seed coat, pod | LC-MS, LC-ESI-MS, LC-ESI-MS/MS, UHPLC-MS | [ , , , , , , ] |
| Flavanols | Catechin, (epi) catechin, gallocatechin, (epi) gallocatechin, fisetin, catechin gallate | Seed, seed coat, pod, sprout | LC-MS, LC-ESI-MS/MS, UHPLC-MS, UHPLC-LTQ-MS, UHPLC-Q-HRMS | [ , , , , , , , ] |
| Flavanones | Eriodictyol, naringenin, naringin, hesperidin, melitidin, pinocembrin, liquiritigenin, hesperetin | Seed, seed coat, pod, sprout | LC-MS, LC-ESI-MS, UHPLC-MS, UHPLC-Q-HRMS, UHPLC-LTQ-MS | [ , , , , , , ] |
| Isoflavones | Genistein, daidzein, cirsiliol, prunetin, afrormosina, formononetin, isoformononetin, pseudobaptigenina, sayanedin, | Seed, seed coat, pod, sprout | LC-ESI-MS, UHPLC-MS, UHPLC-LTQ-MS | [ , , , , ] |
| Anthocyanins | Cyanidin 3-sambubioside-5-glucoside, cyanidin 3-sophoroside-5-glucoside, delphinidin 3-sambubioside-5-glucoside, delphinidin 3-sophoroside-5-glucoside, delphinidin 3-O-(2-O-β-D-xylopyranosyl-β-D-galactopyranoside)-5-O-β-D-glucopyranoside, delphinidin 3-O-(2-O-β-D-xylopyranosyl-β-D-galactopyranoside)-5-O-(6-O-acetyl)-β-D-glucopyranoside, pelargonadin 3-glucoside, cyanidin 3,5-di-O-glucoside, malvidine-3-O-glucoside, | Seed, seed coat, pod | LC-MS, UHPLC-MS | [ , , ] |
| Phenolic acids | gallic acid, vanillin, syringic acid, quinic acid, protocatechuic acid, chlorogenic acid, 4-o-caffeoylquinic acid, p-coumaric acid, trans-ferulic acid, trans-cinnamic acid, p-hydroxybenzoic acid, dicaffeoyl quinic acid, caffeic acid, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, vanillin acid, ferulic acid, coumaroyl quinic acid, 5-feruloylquinic acid, vanillic acid-4-β-D-glucoside, cinnamic acid, o-coumaric acid, 2,3-dihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, ferulic acid, gentisic acid, m-hydroxybenzoic acid, p-hydroxybenzoic acid, 4-hydroxy-3-methoxybenzoic acid, p-hydroxyphenylacetic acid, rosmarinic acid, salicylic acid, sinapic acid, tannic acid, veratric acid | Seed, seed coat, pod, sprout | LC-MS, LC-ESI-MS/MS, LC-ESI-MS, UHPLC-MS, UHPLC-LTQ-MS | [ , , , , , , , ] |
2.7.3. Phenolic Acids
2.8. other beneficial components, 2.9. anti-nutritional factors, 3. processing of pea and its components, 3.1. processing of the whole pea seeds, 3.1.1. drying, 3.1.2. milling, 3.1.3. soaking, 3.1.4. cooking, 3.2. modification of pea starches, 3.3. modification of pea proteins, 4. health benefits of pea and its components, 4.1. antioxidant activity, 4.2. anti-inflammatory effect, 4.3. regulation of metabolic syndrome, 4.4. antimicrobial effect, 4.5. anti-renal fibrosis effect, 4.6. other beneficial effects, 5. applications of pea and its components, 5.1. pea beverages and yoghurts, 5.2. germinated pea products, 5.3. pea flour-incorporated products, 5.4. meat alternatives, 5.5. encapsulation and packing materials, 6. conclusions and perspectives, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest, abbreviations.
- Fahmi, R.; Ryland, D.; Sopiwnyk, E.; Aliani, M. Sensory and Physical Characteristics of Pan Bread Fortified with Thermally Treated Split Yellow Pea ( Pisum sativum L.) Flour. J. Food Sci. 2019 , 84 , 3735–3745. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Han, X.; Akhov, L.; Ashe, P.; Lewis, C.; Deibert, L.; Irina Zaharia, L.; Forseille, L.; Xiang, D.; Datla, R.; Nosworthy, M. Comprehensive Compositional Assessment of Bioactive Compounds in Diverse Pea Accessions. Food Res. Int. 2023 , 165 , 112455. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kumari, T.; Deka, S.C. Potential Health Benefits of Garden Pea Seeds and Pods: A Review. Legume Sci. 2021 , 3 , e82. [ Google Scholar ] [ CrossRef ]
- Liu, M.; Wu, N.-N.; Yu, G.-P.; Zhai, X.-T.; Chen, X.; Zhang, M.; Tian, X.-H.; Liu, Y.-X.; Wang, L.-P.; Tan, B. Physicochemical Properties, Structural Properties, and in vitro Digestibility of Pea Starch Treated with High Hydrostatic Pressure. Starch-Starke 2018 , 70 , 9. [ Google Scholar ] [ CrossRef ]
- Raghunathan, R.; Hoover, R.; Waduge, R.; Liu, Q.; Warkentin, T.D. Impact of Molecular Structure on the Physicochemical Properties of Starches Isolated from Different Field Pea ( Pisum sativum L.) Cultivars Grown in Saskatchewan, Canada. Food Chem. 2017 , 221 , 1514–1521. [ Google Scholar ] [ CrossRef ]
- Gao, L.C.; Wu, Y.X.; Wan, C.X.; Wang, P.K.; Yang, P.; Gao, X.L.; Eeckhout, M.; Gao, J.F. Structural and Physicochemical Properties of Pea Starch Affected by Germination Treatment. Food Hydrocolloid 2022 , 124 , 107303. [ Google Scholar ] [ CrossRef ]
- Santos, C.S.; Carbas, B.; Castanho, A.; Vasconcelos, M.W.; Patto MC, V.; Domoney, C.; Brites, C. Variation in Pea ( Pisum sativum L.) Seed Quality Traits Defined by Physicochemical Functional Properties. Foods 2019 , 8 , 570. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Devi, J.; Sanwal, S.K.; Koley, T.K.; Mishra, G.P.; Karmakar, P.; Singha, P.M.; Singh, B. Variations in the Total Phenolics and Antioxidant Activities among Garden Pea ( Pisum sativum L.) Genotypes Differing for Maturity Duration, Seed and Flower Traits and their Association with the Yield. Sci. Hortic. 2019 , 244 , 141–150. [ Google Scholar ] [ CrossRef ]
- Shi, L.; Arntfield, S.D.; Nickerson, M. Changes in Levels of Phytic Acid, Lectins and Oxalates during Soaking and Cooking of Canadian Pulses. Food Res. Int. 2018 , 107 , 660–668. [ Google Scholar ] [ CrossRef ]
- Wojdylo, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and their in vitro Bioactive Properties. Molecules 2020 , 25 , 4648. [ Google Scholar ] [ CrossRef ]
- Avezum, L.; Rondet, E.; Mestres, C.; Achir, N.; Madode, Y.; Gibert, O.; Lefevre, C.; Hemery, Y.; Verdeil, J.-L.; Rajjou, L. Improving the Nutritional Quality of Pulses via Germination. Food Rev. Int. 2022 , 1–34. [ Google Scholar ] [ CrossRef ]
- Babbitt, C.W.; Babbitt, G.A.; Oehman, J.M. Behavioral Impacts on Residential Food Provisioning, Use, and Waste during the COVID-19 Pandemic. Sustain. Prod. Consum. 2021 , 28 , 315–325. [ Google Scholar ] [ CrossRef ]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012 , 108 , S3–S10. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Yu, Z.; Fan, Y.S.; Wang, X.W.; Xia, M.; Cai, Y. In Vitro and In Vivo Digestibility of Pea and Chickpea Powder Prepared by Cooking and Drying Treatment. Int. J. Food Prop. 2020 , 23 , 1187–1199. [ Google Scholar ] [ CrossRef ]
- Jenkins, D.J.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Rangarajan, S.; Srichaikul, K.; Mohan, V.; Avezum, A.; Díaz, R.; Rosengren, A.; et al. Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N. Engl. J. Med. 2021 , 384 , 1312–1322. [ Google Scholar ] [ CrossRef ]
- Thakur, S.; Scanlon, M.G.; Tyler, R.T.; Milani, A.; Paliwal, J. Pulse Flour Characteristics from a Wheat Flour Miller’s Perspective: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019 , 18 , 775–797. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y.P. The Health Benefits, Functional Properties, Modifications, and Applications of Pea ( Pisum sativum L.) Protein: Current Status, Challenges, and Perspectives. Compr. Rev. Food Sci. Food Saf. 2020 , 19 , 1835–1876. [ Google Scholar ] [ CrossRef ]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K.; et al. Comparative Study on the Chemical Composition, Anthocyanins, Tocopherols and Carotenoids of Selected Legumes. Food Chem. 2018 , 260 , 317–326. [ Google Scholar ] [ CrossRef ]
- Ye, S.X.; Shah, B.R.; Li, J.; Liang, H.S.; Zhan, F.C.; Geng, F.; Li, B. A Critical Review on Interplay Between Dietary Fibers and Gut Microbiota. Trends Food Sci. Technol. 2022 , 124 , 237–249. [ Google Scholar ] [ CrossRef ]
- Ashokkumar, K.; Diapari, M.; Jha, A.B.; Tar’an, B.; Arganosa, G.; Warkentin, T.D. Genetic Diversity of Nutritionally Important Carotenoids in 94 Pea and 121 Chickpea Accessions. J. Food Compos. Anal. 2015 , 43 , 49–60. [ Google Scholar ] [ CrossRef ]
- Fahim, J.R.; Attia, E.Z.; Kamel, M.S. The Phenolic Profile of Pea ( Pisum sativum ): A Phytochemical and Pharmacological Overview. Phytochem. Rev. 2019 , 18 , 173–198. [ Google Scholar ] [ CrossRef ]
- Chen, S.-K.; Lin, H.-F.; Wang, X.; Yuan, Y.; Yin, J.-Y.; Song, X.-X. Comprehensive Analysis in the Nutritional Composition, Phenolic Species and in vitro Antioxidant Activities of Different Pea Cultivars. Food Chem. X 2023 , 17 , 100599. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining Protein Nutrition through Plant-Based Foods. Front. Nutr. 2022 , 8 , 772573. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary Intake of Total, Animal, and Plant Proteins and Risk of All Cause, Cardiovascular, and Cancer Mortality: Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. BMJ 2020 , 370 , m2412. [ Google Scholar ] [ CrossRef ]
- Ewy, M.W.; Patel, A.; Abdelmagid, M.G.; Elfadil, O.M.; Bonnes, S.L.; Salonen, B.R.; Hurt, R.T.; Mundi, M.S. Plant-Based Diet: Is it as Good as an Animal-Based Diet When it Comes to Protein? Curr. Nutr. Rep. 2022 , 11 , 337–346. [ Google Scholar ] [ CrossRef ]
- Arif, U.; Ahmed, M.J.; Rabbani, M.A.; Arif, A.A. Assessment of Genetic Diversity in Pea ( Pisum sativum L.) Landraces Based on Physico-Chemical and Nutritive Quality Using Cluster and Principal Component Analysis. Pak. J. Bot. 2020 , 52 , 575–580. [ Google Scholar ] [ CrossRef ]
- Abdel-Aal, E.-S.M.; Ragaee, S.; Rabalski, I.; Warkentin, T.; Vandenberg, A. Nutrient Content and Viscosity of Saskatchewan-Grown Pulses in Relation to their Cooking Quality. Can. J. Plant. Sci. 2019 , 99 , 67–77. [ Google Scholar ] [ CrossRef ]
- Aluko, R.E.; Girgih, A.T.; He, R.; Malomo, S.; Li, H.; Offengenden, M.; Wu, J.P. Structural and Functional Characterization of Yellow Field Pea Seed ( Pisum sativum L.) Protein-Derived Antihypertensive Peptides. Food Res. Int. 2015 , 77 , 10–16. [ Google Scholar ] [ CrossRef ]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and its Application in the Food Industry. Molecules 2022 , 27 , 5354. [ Google Scholar ] [ CrossRef ]
- Ge, J.; Sun, C.X.; Mata, A.; Corke, H.; Gan, R.Y.; Fang, Y.P. Physicochemical and pH-Dependent Functional Properties of Proteins Isolated from Eight Traditional Chinese Beans. Food Hydrocolloid 2021 , 112 , 106288. [ Google Scholar ] [ CrossRef ]
- Wang, N.; Hatcher, D.W.; Warkentin, T.D.; Toews, R. Effect of Cultivar and Environment on Physicochemical and Cooking Characteristics of Field Pea ( Pisum sativum ). Food Chem. 2010 , 118 , 109–115. [ Google Scholar ] [ CrossRef ]
- Chen, X.Y.; Ma, X.W.; Wen, J.Y.; Liu, X.C.; Yu, X.R.; Xiong, F. Morphological, Structural and Functional Properties of Starches from Different Legume Resources. Legume Res. 2021 , 44 , 818–823. [ Google Scholar ] [ CrossRef ]
- Ren, Y.K.; Setia, R.; Warkentin, T.D.; Ai, Y.F. Functionality and Starch Digestibility of Wrinkled and Round Pea Flours of Two Different Particle Sizes. Food Chem. 2021 , 336 , 127711. [ Google Scholar ] [ CrossRef ]
- Ren, Y.; Yuan, T.Z.; Chigwedere, C.M.; Ai, Y. A Current Review of Structure, Functional Properties, and Industrial Applications of Pulse Starches for Value-Added Utilization. Compr. Rev. Food Sci. Food Saf. 2021 , 20 , 3061–3092. [ Google Scholar ] [ CrossRef ]
- Petropoulou, K.; Salt, L.; Warren, F. A Seed Trait Studied by Gregor Mendel in Pisum sativum L. (Pea): Potential Prevention of Type 2 Diabetes. In Legumes for Global Food Security ; Nova Science Publishers: New York, NY, USA, 2017; Chapter 6; pp. 129–155. [ Google Scholar ]
- Chung, H.J.; Liu, Q. Physicochemical Properties and In Vitro Digestibility of Flour and Starch from Pea ( Pisum sativum L.) Cultivars. Int. J. Biol. Macromol. 2012 , 50 , 131–137. [ Google Scholar ] [ CrossRef ]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In Vitro Colonic Fermentation of Dietary Fibers: Fermentation Rate, Short-Chain Fatty Acid Production and Changes in Microbiota. Trends Food Sci. Technol. 2019 , 88 , 1–9. [ Google Scholar ] [ CrossRef ]
- Wang, S.J.; Sharp, P.; Copeland, L. Structural and Functional Properties of Starches from Field Peas. Food Chem. 2011 , 126 , 1546–1552. [ Google Scholar ] [ CrossRef ]
- Liu, C.; Wang, S.J.; Copeland, L.; Wang, S. Physicochemical Properties and in vitro Digestibility of Starches from Field Peas Grown in China. LWT-Food Sci. Technol. 2015 , 64 , 829–836. [ Google Scholar ] [ CrossRef ]
- Guo, K.; Lin, L.S.; Fan, X.X.; Zhang, L.; Wei, C.X. Comparison of Structural and Functional Properties of Starches from Five Fruit Kernels. Food Chem. 2018 , 257 , 75–82. [ Google Scholar ] [ CrossRef ]
- Ashogbon, A.O.; Akintayo, E.T.; Oladebeye, A.O.; Oluwafemi, A.D.; Akinsola, A.F.; Imanah, O.E. Developments in the Isolation, Composition, and Physicochemical Properties of Legume Starches. Crit. Rev. Food Sci. Nutr. 2021 , 61 , 2938–2959. [ Google Scholar ] [ CrossRef ]
- Wojeicchowski, J.P.; De Siqueira, G.L.D.A.; Lacerda, L.G.; Schnitzler, E.; Demiate, I.M. Physicochemical, Structural and Thermal Properties of Oxidized, Acetylated and Dual-Modified Common Bean ( Phaseolus vulgaris L.) Starch. Food Sci. Technol. 2018 , 38 , 318–327. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physico-Chemical Properties of Acetylated Starches from Indian Black Gram ( Phaseolus mungo L.) Cultivars. J. Food Sci. Technol. 2015 , 52 , 4078–4089. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Du, S.K.; Jiang, H.X.; Ai, Y.F.; Jane, J.L. Physicochemical Properties and Digestibility of Common Bean ( Phaseolus vulgaris L.) Starches. Carbohydr. Polym. 2014 , 108 , 200–205. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ma, M.T.; Wang, Y.J.; Wang, M.X.; Jane, J.L.; Du, S.K. Physicochemical Properties and In Vitro Digestibility of Legume Starches. Food Hydrocolloid 2017 , 63 , 249–255. [ Google Scholar ] [ CrossRef ]
- Andrabi, S.N.; Wani, I.A.; Gani, A.; Hamdani, A.M.; Masoodi, F.A. Comparative Study of Physico-Chemical and Functional Properties of Starch Extracted from Two Kidney Bean ( Phaseolus vulgaris L.) and Green Gram Cultivars ( Vigna radiata L.) Grown in India. Starch-Starke 2016 , 68 , 416–426. [ Google Scholar ] [ CrossRef ]
- Li, L.Y.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y.F. Characteristics of Pea, Lentil and Faba Bean Starches Isolated from Air-Classified Flours in Comparison with Commercial Starches. Food Chem. 2019 , 276 , 599–607. [ Google Scholar ] [ CrossRef ]
- Xu, M.J.; Saleh AS, M.; Liu, Y.; Jing, L.Z.; Zhao, K.; Wu, H.; Zhang, G.Q.; Yang, S.O.; Li, W.H. The Changes in Structural, Physicochemical, and Digestive Properties of Red Adzuki Bean Starch after Repeated and Continuous Annealing Treatments. Starch-Starke 2018 , 70 , 1700322. [ Google Scholar ] [ CrossRef ]
- Zhang, Z.S.; Tian, X.L.; Wang, P.; Jiang, H.; Li, W.H. Compositional, Morphological, and Physicochemical Properties of Starches from Red Adzuki Bean, Chickpea, Faba Bean, and Baiyue Bean Grown in China. Food Sci. Nutr. 2019 , 7 , 2485–2494. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Wang, M.; Chen, X.; Dong, L.; Nan, X.; Ji, W.; Wang, S.; Sun, W.; Zhou, Q. Modification of Pea Dietary Fiber by Ultrafine Grinding and Hypoglycemic Effect in Diabetes Mellitus Mice. J. Food Sci. 2021 , 86 , 1273–1282. [ Google Scholar ] [ CrossRef ]
- Mayengbam, S.; Lambert, J.E.; Parnell, J.A.; Tunnicliffe, J.M.; Nicolucci, A.C.; Han, J.; Sturzenegger, T.; Shearer, J.; Mickiewicz, B.; Vogel, H.J.; et al. Impact of Dietary Fiber Supplementation on Modulating Microbiota-Host-Metabolic Axes in Obesity. J. Nutr. Biochem. 2019 , 64 , 228–236. [ Google Scholar ] [ CrossRef ]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and Functional Characteristics of Dietary Fibre in Beans, Lentils, Peas and Chickpeas. Food Res. Int. 2015 , 67 , 117–125. [ Google Scholar ] [ CrossRef ]
- Wu, G.J.; Liu, D.; Wan, Y.J.; Huang, X.J.; Nie, S.P. Comparison of Hypoglycemic Effects of Polysaccharides from Four Legume Species. Food Hydrocolloid 2019 , 90 , 299–304. [ Google Scholar ] [ CrossRef ]
- Zhang, S.J.; Hu, T.T.; Chen, Y.Y.; Wang, S.Y.; Kang, Y.F. Analysis of the Polysaccharide Fractions Isolated from Pea ( Pisum sativum L.) at Different Levels of Purification. J. Food Biochem. 2020 , 44 , e13248. [ Google Scholar ] [ CrossRef ]
- Eslinger, A.J.; Eller, L.K.; Reimer, R.A. Yellow Pea Fiber Improves Glycemia and Reduces Clostridium Leptum in Diet-Induced Obese Rats. Nutr. Res. 2014 , 34 , 714–722. [ Google Scholar ] [ CrossRef ]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, Physicochemical Properties of Pea Protein and its Application in Functional Foods. Crit. Rev. Food Sci. Nutr. 2020 , 60 , 2593–2605. [ Google Scholar ] [ CrossRef ]
- Luo, X.; Fei, Y.; Xu, Q.; Lei, T.; Mo, X.; Wang, Z.; Zhang, L.; Mou, X.; Li, H. Isolation and Identification of Antioxidant Peptides from Tartary Buckwheat Albumin ( Fagopyrum tataricum Gaertn.) and their Antioxidant Activities. J. Food Sci. 2020 , 85 , 611–617. [ Google Scholar ] [ CrossRef ]
- Popp, J.; Trendelenburg, V.; Niggemann, B.; Randow, S.; Völker, E.; Vogel, L.; Reuter, A.; Spiric, J.; Schiller, D.; Beyer, K.; et al. Pea ( Pisum sativum ) Allergy in Children: Pis S 1 is an Immunodominant Major Pea Allergen and Presents Ige Binding Sites with Potential Diagnostic Value. Clin. Exp. Allergy 2020 , 50 , 625–635. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Taylor, S.L.; Marsh, J.T.; Koppelman, S.J.; Kabourek, J.L.; Johnson, P.E.; Baumert, J.L. A Perspective on Pea Allergy and Pea Allergens. Trends Food Sci. Technol. 2021 , 116 , 186–198. [ Google Scholar ] [ CrossRef ]
- Han, F.; Moughan, P.J.; Li, J.T.; Pang, S.J. Digestible Indispensable Amino Acid Scores (Diaas) of Six Cooked Chinese Pulses. Nutrients 2020 , 12 , 3831. [ Google Scholar ] [ CrossRef ]
- Zhao, H.F.; Shen, C.; Wu, Z.J.; Zhang, Z.; Xu, C.M. Comparison of Wheat, Soybean, Rice, and Pea Protein Properties for Effective Applications in Food Products. J. Food Biochem. 2020 , 44 , e13157. [ Google Scholar ] [ CrossRef ]
- Hall, A.E.; Moraru, C.I. Structure and Function of Pea, Lentil and Faba Bean Proteins Treated by High Pressure Processing and Heat Treatment. LWT-Food Sci. Technol. 2021 , 152 , 112349. [ Google Scholar ] [ CrossRef ]
- Ciurescu, G.; Toncea, I.; Ropota, M.; Habeanu, M. Seeds Composition and Their Nutrients Quality of Some Pea ( Pisum sativum L.) and Lentil ( Lens culinaris Medik.) Cultivars. Rom. Agric. Res. 2018 , 35 , 101–108. [ Google Scholar ] [ CrossRef ]
- Nadeem, M.A.; Cilesiz, Y.; Yüce, İ.; Baloch, F.S.; Karaköy, T. Macro and Micronutrients Diversity in the Seeds of Field Pea Germplasm. Pak. J. Bot. 2021 , 53 , 1655–1664. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, D.D.; Guan, X.; Huang, K.; Li, S.; Liu, J.; Yu, W.W.; Duan, R.Q. Protective Effects of Mung Bean ( Vigna radiata L. ) and Pea ( Pisum sativum L.) against High-Fat-Induced Oxidative Stress. Food Sci. Nutr. 2019 , 7 , 4063–4075. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Padhi, E.M.T.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total Polyphenol Content, Carotenoid, Tocopherol and Fatty Acid Composition of Commonly Consumed Canadian Pulses and their Contribution to Antioxidant Activity. J. Funct. Food 2017 , 38 , 602–611. [ Google Scholar ] [ CrossRef ]
- Gan, R.-Y.; Wang, M.-F.; Lui, W.-Y.; Wu, K.; Dai, S.-H.; Sui, Z.-Q.; Corke, H. Diversity in Antioxidant Capacity, Phenolic Contents, and Flavonoid Contents of 42 Edible Beans from China. Cereal Chem. 2017 , 94 , 291–297. [ Google Scholar ] [ CrossRef ]
- Zhao, T.Y.; Su, W.J.; Qin, Y.; Wang, L.Y.; Kang, Y.F. Phenotypic Diversity of Pea ( Pisum sativum L.) Varieties and the Polyphenols, Flavonoids, and Antioxidant Activity of their Seeds. Cienc. Rural. 2020 , 50 , e20190196. [ Google Scholar ] [ CrossRef ]
- Ma, Y.; Gao, J.; Wei, Z.; Shahidi, F. Effect of in vitro Digestion on Phenolics and Antioxidant Activity of Red and Yellow Colored Pea Hulls. Food Chem. 2021 , 337 , 127606. [ Google Scholar ] [ CrossRef ]
- Borges-Martinez, E.; Gallardo-Velazquez, T.; Cardador-Martinez, A.; Moguel-Concha, D.; Osorio-Revilla, G.; Ruiz-Ruiz, J.C.; Martinez, C.J. Phenolic Compounds Profile and Antioxidant Activity of Pea ( Pisum sativum L.) and Black Bean ( Phaseolus L.) sprouts. Food Sci. Technol. 2022 , 42 , e45920. [ Google Scholar ] [ CrossRef ]
- Castaldo, L.; Izzo, L.; Gaspari, A.; Lombardi, S.; Rodriguez-Carrasco, Y.; Narvaez, A.; Grosso, M.; Ritieni, A. Chemical Composition of Green Pea ( Pisum sativum L.) Pods Extracts and their Potential Exploitation as Ingredients in Nutraceutical Formulations. Antioxidants 2022 , 11 , 105. [ Google Scholar ] [ CrossRef ]
- Abdelghffar, E.A.; Obaid, W.A.; Elgamal, A.M.; Daoud, R.; Sobeh, M.; El Raey, M.A. Pea ( Pisum sativum ) Peel Extract Attenuates Dox-Induced Oxidative Myocardial Injury. Biomed. Pharm. 2021 , 143 , 112120. [ Google Scholar ] [ CrossRef ]
- Guo, F.H.; Tsao, R.; Wang, X.Y.; Jiang, L.; Sun, Y.; Xiong, H. Phenolics of Yellow Pea ( Pisum sativum L.) Hulls, their Plasma and Urinary Metabolites, Organ Distribution, and In Vivo Antioxidant Activities. J. Agric. Food Chem. 2021 , 69 , 5013–5025. [ Google Scholar ] [ CrossRef ]
- Chahbani, A.; Fakhfakh, N.; Balti, M.A.; Mabrouk, M.; El-Hatmi, H.; Zouari, N.; Kechaou, N. Microwave Drying Effects on Drying Kinetics, Bioactive Compounds and Antioxidant Activity of Green Peas ( Pisum sativum L.). Food Biosci. 2018 , 25 , 32–38. [ Google Scholar ] [ CrossRef ]
- Elessawy, F.M.; Bazghaleh, N.; Vandenberg, A.; Purves, R.W. Polyphenol Profile Comparisons of Seed Coats of Five Pulse Crops Using a Semi-Quantitative Liquid Chromatography-Mass Spectrometric Method. Phytochem. Anal. 2020 , 31 , 458–471. [ Google Scholar ] [ CrossRef ]
- Jha, A.B.; Purves, R.W.; Elessawy, F.M.; Zhang, H.X.; Vandenberg, A.; Warkentin, T.D. Polyphenolic Profile of Seed Components of White and Purple Flower Pea Lines. Crop. Sci. 2019 , 59 , 2711–2719. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Guo, F.H.; Tsao, R.; Li, C.Y.; Wang, X.Y.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Green Pea ( Pisum sativum L.) Hull Polyphenol Extracts Ameliorate Dss-Induced Colitis through Keap1/Nrf2 Pathway and Gut Microbiota Modulation. Foods 2021 , 10 , 2765. [ Google Scholar ] [ CrossRef ]
- Mejri, F.; Ben Khoud, H.; Njim, L.; Baati, T.; Selmi, S.; Martins, A.; Serralheiro, M.L.M.; Rauter, A.P.; Hosni, K. In Vitro and In Vivo Biological Properties of Pea Pods ( Pisum sativum L.). Food Biosci. 2019 , 32 , 100482. [ Google Scholar ] [ CrossRef ]
- Carpentier, J.; Conforto, E.; Chaigneau, C.; Vendeville, J.E.; Maugard, T. Microencapsulation and Controlled Release of Alpha-Tocopherol by Complex Coacervation between Pea Protein and Tragacanth Gum: A Comparative Study with Arabic and Tara Gums. Innov. Food Sci. Emerg. Technol. 2022 , 77 , 102951. [ Google Scholar ] [ CrossRef ]
- Nazir, N.; Nisar, M.; Ahmad, S.; Wadood, S.F.; Jan, T.; Zahoor, M.; Ahmad, M.; Ullah, A. Characterization of Phenolic Compounds in Two Novel Lines of Pisum sativum L. Along with their in vitro Antioxidant Potential. Environ. Sci. Pollut. Res. 2020 , 27 , 7639–7646. [ Google Scholar ] [ CrossRef ]
- Troszynska, A.; Ciska, E. Phenolic Compounds of Seed Coats of White and Coloured Varieties of Pea ( Pisum sativum L.) and their Total Antioxidant Activity. Czech J. Food Sci. 2002 , 20 , 15–22. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Costantini, M.; Summo, C.; Centrone, M.; Rybicka, I.; D’agostino, M.; Annicchiarico, P.; Caponio, F.; Pavan, S.; Tamma, G.; Pasqualone, A. Macro- and Micro-Nutrient Composition and Antioxidant Activity of Chickpea and Pea Accessions. Pol. J. Food Nutr. Sci. 2021 , 71 , 177–185. [ Google Scholar ] [ CrossRef ]
- Sharma, A. A Review on Traditional Technology and Safety Challenges with Regard to Antinutrients in Legume Foods. J. Food Sci. Technol. 2021 , 58 , 2863–2883. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sinkovic, L.; Pipan, B.; Sibul, F.; Nemes, I.; Horecki, A.T.; Meglic, V. Nutrients, Phytic Acid and Bioactive Compounds in Marketable Pulses. Plants 2023 , 12 , 170. [ Google Scholar ] [ CrossRef ]
- Hugman, J.; Wang, L.F.; Beltranena, E.; Htoo, J.K.; Zijlstra, R.T. Nutrient Digestibilityof Heat-Processed Field Pea in Weaned Pigs. Anim. Feed. Sci. Technol. 2021 , 274 , 114891. [ Google Scholar ] [ CrossRef ]
- Moore, K.L.; Rodríguez-Ramiro, I.; Jones, E.R.; Jones, E.J.; Rodríguez-Celma, J.; Halsey, K.; Domoney, C.; Shewry, P.R.; Fairweather-Tait, S.; Balk, J. The Stage of Seed Development Influences Iron Bioavailability in Pea ( Pisum sativum L.). Sci. Rep. 2018 , 8 , 6865. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Xu, B.; Chang, S.K.C. Comparative Study on Antiproliferation Properties and Cellular Antioxidant Activities of Commonly Consumed Food Legumes against Nine Human Cancer Cell Lines. Food Chem. 2012 , 134 , 1287–1296. [ Google Scholar ] [ CrossRef ]
- Kamalasundari, S.; Babu, R.; Umamaheswari, T. Effect of Domestic Processing Methods on Anti-Nutritional Factors and its Impact on the Bio-Availability Proteins and Starch in Commonly Consumed Whole Legumes. Asian J. Dairy. Food Res. 2019 , 38 , 67–72. [ Google Scholar ] [ CrossRef ]
- Moussou, N.; Ouazib, M.; Wanasundara, J.; Zaidi, F.; Rubio, L.A. Nutrients and Non-Nutrients Composition and in vitro Starch Digestibility of Five Algerian Legume Seed Flours. Int. Food Res. J. 2019 , 26 , 1339–1349. [ Google Scholar ]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health Effects, Sources, Utilization and Safety of Tannins: A Critical Review. Toxin Rev. 2021 , 40 , 432–444. [ Google Scholar ] [ CrossRef ]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review Of Tannins’ Biological Activities and their Potential for Valorization. Foods 2021 , 10 , 137. [ Google Scholar ] [ CrossRef ]
- Ge, G.; Guo, W.X.; Zheng, J.B.; Zhao, M.M.; Sun, W.Z. Effect of Interaction between Tea Polyphenols with Soymilk Protein on Inactivation of Soybean Trypsin Inhibitor. Food Hydrocolloid 2021 , 111 , 106177. [ Google Scholar ] [ CrossRef ]
- Zhou, J.J.; Li, M.H.; Bai, Q.; de Souza, T.S.P.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Effects of Different Processing Methods on Pulses Phytochemicals: An Overview. Food Rev. Int. 2023 . [ Google Scholar ] [ CrossRef ]
- Espinosa, D.C.P.; Cortina, J.R.; Carrión, M.H.; Mora, O.O. Drying and Cooking Effects on the Final Quality of Pea Grains ( Pisum sativum L.) Varieties. Food Sci. Technol. 2021 , 42 . [ Google Scholar ] [ CrossRef ]
- Yu, F.; Yang, Z.; Tao, Z.C.; Yang, Z.Y. Optimization of Pea Seed Intermittent Drying Assisted with Ultrasound Technology. Int. J. Food Eng. 2020 , 16 , 10. [ Google Scholar ] [ CrossRef ]
- Yang, Z.; Li, X.; Tao, Z.C.; Luo, N.; Yu, F. Ultrasound-Assisted Heat Pump Drying of Pea Seed. Dry. Technol. 2018 , 36 , 1958–1969. [ Google Scholar ] [ CrossRef ]
- Cappelli, A.; Oliva, N.; Cini, E. Stone Milling Versus Roller Milling: A Systematic Review of the Effects on Wheat Flour Quality, Dough Rheology, and Bread Characteristics. Trends Food Sci. Technol. 2020 , 97 , 147–155. [ Google Scholar ] [ CrossRef ]
- Gu, Z.X.; Jiang, H.Y.; Zha, F.C.; Manthey, F.; Rao, J.J.; Chen, B.C. Toward a Comprehensive Understanding of Ultracentrifugal Milling on the Physicochemical Properties and Aromatic Profile of Yellow Pea Flour. Food Chem. 2021 , 345 , 128760. [ Google Scholar ] [ CrossRef ]
- Schmidt, F.; Blankart, M.; Wanger, J.; Scharfe, M.; Scheuerer, T.; Hinrichs, J. Upscaling of Alkaline Pea Protein Extraction from Dry Milled and Pre-Treated Peas from Laboratory to Pilot Scale: Optimization of Process Parameters for Higher Protein Yields. J. Food Meas. Charact. 2022 , 16 , 4904–4913. [ Google Scholar ] [ CrossRef ]
- Kaiser, A.C.; Barber, N.; Manthey, F.; Hall, C. Physicochemical Properties of Hammer-Milled Yellow Split Pea ( Pisum sativum L.). Cereal Chem. 2019 , 96 , 313–323. [ Google Scholar ] [ CrossRef ]
- Nguyen, G.T.; Gidley, M.J.; Sopade, P.A. Dependence of In-Vitro Starch and Protein Digestions on Particle Size of Field Peas ( Pisum sativum L.). LWT-Food Sci. Technol. 2015 , 63 , 541–549. [ Google Scholar ] [ CrossRef ]
- Li, H.; Zou, L.; Li, X.Y.; Wu, D.T.; Liu, H.Y.; Li, H.B.; Gan, R.Y. Adzuki Bean ( Vigna angularis ): Chemical Compositions, Physicochemical Properties, Health Benefits, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2022 , 21 , 2335–2362. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Setia, R.; Dai, Z.X.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y.F. Impacts of Short-Term Germination on the Chemical Compositions, Technological Characteristics and Nutritional Quality of Yellow Pea and Faba Bean Flours. Food Res. Int. 2019 , 122 , 263–272. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shi, L.; Mu, K.W.; Arntfield, S.D.; Nickerson, M.T. Changes in Levels of Enzyme Inhibitors during Soaking and Cooking for Pulses Available in Canada. J. Food Sci. Technol. 2017 , 54 , 1014–1022. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Wang, N.; Hatcher, D.W.; Gawalko, E.J. Effect of Variety and Processing on Nutrients and Certain Anti-Nutrients in Field Peas ( Pisum sativum ). Food Chem. 2008 , 111 , 132–138. [ Google Scholar ] [ CrossRef ]
- Skalickova, S.; Ridoskova, A.; Slama, P.; Skladanka, J.; Skarpa, P.; Smykalova, I.; Horacek, J.; Dostalova, R.; Horky, P. Effect of Lactic Fermentation and Cooking on Nutrient and Mineral Digestibility of Peas. Front. Nutr. 2022 , 9 , 838963. [ Google Scholar ] [ CrossRef ]
- Stone, A.K.; Waelchli, K.N.; Cabuk, B.; Mcintosh, T.C.; Wanasundara, J.; Arntfield, S.D.; Nickerson, M.T. The Levels of Bioactive Compounds Found in Raw and Cooked Canadian Pulses. Food Sci. Technol. Int. 2021 , 27 , 528–538. [ Google Scholar ] [ CrossRef ]
- Liu, Y.H.; Ragaee, S.; Marcone, M.F.; Abdel-Aal, E.M. Composition of Phenolic Acids and Antioxidant Properties of Selected Pulses Cooked with Different Heating Conditions. Foods 2020 , 9 , 908. [ Google Scholar ] [ CrossRef ]
- Shin, J.A.; Heo, Y.; Seo, M.; Choi, Y.; Lee, K.T. Effects of Cooking Methods on the Beta-Carotene Levels of Selected Plant Food Materials. Food Sci. Biotechnol. 2016 , 25 , 955–963. [ Google Scholar ] [ CrossRef ]
- Liu, Y.H.; Ragaee, S.; Marcone, M.F.; Abdel-Aal, E.M. Effect of Different Cooking Methods and Heating Solutions on Nutritionally-Important Starch Fractions and Flatus Oligosaccharides in Selected Pulses. Cereal Chem. 2020 , 97 , 1216–1226. [ Google Scholar ] [ CrossRef ]
- Obadi, M.; Xu, B. Review on the Physicochemical Properties, Modifications, and Applications of Starches and its Common Modified Forms Used in Noodle Products. Food Hydrocolloid 2021 , 112 , 106286. [ Google Scholar ] [ CrossRef ]
- He, X.; Dai, T.; Liang, R.; Liu, W.; Cheng, Y.; Liu, C.; Chen, J. A New Partially-Gelatinized Granular Starch Prepared by Industry-Scale Microfluidization Treatment of Pea Starch. Innov. Food Sci. Emerg. Technol. 2023 , 86 , 103351. [ Google Scholar ] [ CrossRef ]
- Shi, M.M.; Gao, Q.Y.; Liu, Y.Q. Changes in the Structure and Digestibility of Wrinkled Pea Starch with Malic Acid Treatment. Polymers 2018 , 10 , 1359. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Han, L.H.; Cao, S.P.; Yu, Y.T.; Xu, X.C.; Cao, X.H.; Chen, W.J. Modification in Physicochemical, Structural and Digestive Properties of Pea Starch during Heat-Moisture Process Assisted by Pre- and Post-Treatment of Ultrasound. Food Chem. 2021 , 360 , 129929. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Majeed, T.; Majeed, T.; Wani, I.A.; Wani, I.A.; Hamdani, A.M.; Hamdani, A.M.; Bhat, N.A.; Bhat, N.A.; Majeed, T.; Majeed, T.; et al. Effect of Sonication and Gamma-Irradiation on the Properties of Pea ( Pisum sativum ) and Vetch ( Vida villosa ) Starches: A Comparative Study. Int. J. Biol. Macromol. 2018 , 114 , 1144–1150. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhou, D.T.; Ma, Z.; Yin, X.X.; Hu, X.Z.; Boye, J.I. Structural Characteristics and Physicochemical Properties of Field Pea Starch Modified by Physical, Enzymatic, and Acid Treatments. Food Hydrocolloid 2019 , 93 , 386–394. [ Google Scholar ] [ CrossRef ]
- Chen, X.Y.; Zhang, Z.W.; Ji, N.; Li, M.; Wang, Y.F.; Xiong, L.; Sun, Q.J. The Effect of Ethanol Solution Annealing on the Physicochemical Properties of Pea and Potato Starches. Food Hydrocolloid 2022 , 125 , 107428. [ Google Scholar ] [ CrossRef ]
- Yan, Y.Z.; Peng, B.X.; Niu, B.; Ji, X.L.; He, Y.; Shi, M.M. Understanding the Structure, Thermal, Pasting, and Rheological Properties of Potato and Pea Starches Affected by Annealing Using Plasma-Activated Water. Front. Nutr. 2022 , 9 , 842662. [ Google Scholar ] [ CrossRef ]
- Arteaga, V.G.; Guardia, M.A.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of Enzymatic Hydrolysis on Molecular Weight Distribution, Techno- Functional Properties and Sensory Perception of Pea Protein Isolates. Innov. Food Sci. Emerg. Technol. 2020 , 65 , 102449. [ Google Scholar ] [ CrossRef ]
- Vatansever, S.; Ohm, J.B.; Simsek, S.; Hall, C. A Novel Approach: Supercritical Carbon Dioxide Plus Ethanol Extraction to Improve Techno-Functionalities of Pea Protein Isolate. Cereal Chem. 2022 , 99 , 130–143. [ Google Scholar ] [ CrossRef ]
- Wang, F.; Zhang, Y.Z.; Xu, L.; Ma, H. An Efficient Ultrasound-Assisted Extraction Method of Pea Protein and its Effect on Protein Functional Properties and Biological Activities. LWT-Food Sci. Technol. 2020 , 127 , 109348. [ Google Scholar ] [ CrossRef ]
- Ozturk, O.K.; Turasan, H. Latest Developments in the Applications of Microfluidization to Modify the Structure of Macromolecules Leading to Improved Physicochemical and Functional Properties. Crit. Rev. Food Sci. Nutr. 2022 , 62 , 4481–4503. [ Google Scholar ] [ CrossRef ]
- He, X.H.; Chen, J.; He, X.M.; Feng, Z.; Li, C.H.; Liu, W.; Dai, T.T.; Liu, C.M. Industry-Scale Microfluidization as a Potential Technique to Improve Solubility and Modify Structure of Pea Protein. Innov. Food Sci. Emerg. Technol. 2021 , 67 , 102582. [ Google Scholar ] [ CrossRef ]
- Oliete, B.; Potin, F.; Cases, E.; Saurel, R. Microfluidization as Homogenization Technique in Pea Globulin-Based Emulsions. Food Bioprocess. Technol. 2019 , 12 , 877–882. [ Google Scholar ] [ CrossRef ]
- Stanisavljevic, N.S.; Ilic, M.; Jovanovic, Z.S.; Cupic, T.; Dabic, D.C.; Natic, M.M.; Tesic, Z.L.; Radovic, S.S. Identification of Seed Coat Phenolic Compounds from Differently Colored Pea Varieties and Characterization of their Antioxidant Activity. Arch. Biol. Sci. 2015 , 67 , 829–840. [ Google Scholar ] [ CrossRef ]
- Stanisavljevic, N.S.; Ilic, M.D.; Matic, I.Z.; Jovanovic, Z.S.; Cupic, T.; Dabic, D.C.; Natic, M.M.; Tesic, Z.L. Identification of Phenolic Compounds from Seed Coats of Differently Colored European Varieties of Pea ( Pisum sativum L.) and Characterization of their Antioxidant and In Vitro Anticancer Activities. Nutr. Cancer 2016 , 68 , 988–1000. [ Google Scholar ] [ CrossRef ]
- Hadrich, F.; El Arbi, M.; Boukhris, M.; Sayadi, S.; Cherif, S. Valorization of the Peel of Pea: Pisum sativum by Evaluation of its Antioxidant and Antimicrobial Activities. J. Oleo Sci. 2014 , 63 , 1177–1183. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Jalili Safaryan, M.; Ganjloo, A.; Bimakr, M.; Zarringhalami, S. Optimization of Ultrasound-Assisted Extraction, Preliminary Characterization and In Vitro Antioxidant Activity of Polysaccharides from Green Pea Pods. Foods 2016 , 5 , 78. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ding, J.; Liang, R.; Yang, Y.Y.; Sun, N.; Lin, S.Y. Optimization of Pea Protein Hydrolysate Preparation and Purification of Antioxidant Peptides Based on an In Silico Analytical Approach. LWT-Food Sci. Technol. 2020 , 123 , 109126. [ Google Scholar ] [ CrossRef ]
- Guo, F.H.; Xiong, H.; Wang, X.Y.; Jiang, L.; Yu, N.X.; Hu, Z.Y.; Sun, Y.; Tsao, R. Phenolics of Green Pea ( Pisum sativum L.) Hulls, their Plasma and Urinary Metabolites, Bioavailability, and In Vivo Antioxidant Activities in a Rat Model. J. Agric. Food Chem. 2019 , 67 , 11955–11968. [ Google Scholar ] [ CrossRef ]
- Guo, F.H.; Tsao, R.; Li, C.Y.; Wang, X.Y.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Polyphenol Content of Green Pea ( Pisum sativum L.) Hull Under In Vitro Digestion and Effects of Digestive Products on Anti-Inflammatory Activity and Intestinal Barrier in the Caco-2/Raw264.7 Coculture Model. J. Agric. Food Chem. 2022 , 70 , 3477–3488. [ Google Scholar ] [ CrossRef ]
- Ndiaye, F.; Vuong, T.; Duarte, J.; Aluko, R.E.; Matar, C. Anti-Oxidant, Anti-Inflammatory and Immunomodulating Properties of an Enzymatic Protein Hydrolysate from Yellow Field Pea Seeds. Eur. J. Nutr. 2012 , 51 , 29–37. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bibi, S.; Moraes LF, D.; Lebow, N.; Zhu, M.J. Dietary Green Pea Protects against Dss-Induced Colitis in Mice Challenged with High-Fat Diet. Nutrients 2017 , 9 , 509. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Utrilla, M.P.; Peinado, M.J.; Ruiz, R.; Rodriguez-Nogales, A.; Algieri, F.; Rodriguez-Cabezas, M.E.; Clemente, A.; Galvez, J.; Rubio, L.A. Pea ( Pisum sativum L.) Seed Albumin Extracts Show Anti-Inflammatory Effect in the Dss Model of Mouse Colitis. Mol. Nutr. Food Res. 2015 , 59 , 807–819. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jakubczyk, A.; Baraniak, B. Angiotensin I Converting Enzyme Inhibitory Peptides Obtained after in vitro Hydrolysis of Pea ( Pisum sativum Var. Bajka) Globulins. Biomed. Res. Int. 2014 , 2014 , 438459. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Liao, W.; Fan, H.B.; Liu, P.; Wu, J.P. Identification of Angiotensin Converting Enzyme 2 (Ace2) Up-Regulating Peptides from Pea Protein Hydrolysate. J. Funct. Food 2019 , 60 , 103395. [ Google Scholar ] [ CrossRef ]
- Wang, X.; Bhullar, K.S.; Fan, H.B.; Liao, W.; Qiao, Y.J.; Su, D.; Wu, J.P. Regulatory Effects of a Pea-Derived Peptide Leu-Arg-Trp (Lrw) on Dysfunction of Rat Aortic Vascular Smooth Muscle Cells Against Angiotensin Ii Stimulation. J. Agric. Food Chem. 2020 , 68 , 3947–3953. [ Google Scholar ] [ CrossRef ]
- Girgih, A.T.; Nwachukwu, I.D.; Onuh, J.O.; Malomo, S.A.; Aluko, R.E. Antihypertensive Properties of a Pea Protein Hydrolysate during Short-and Long-Term Oral Administration to Spontaneously Hypertensive Rats. J. Food Sci. 2016 , 81 , H1281–H1287. [ Google Scholar ] [ CrossRef ]
- Inagaki, K.; Nishimura, Y.; Iwata, E.; Manabe, S.; Goto, M.; Ogura, Y.; Hotta, H. Hypolipidemic Effect of the Autoclaved Extract Prepared from Pea ( Pisum sativum L.) Pods In Vivo and In Vitro. J. Nutr. Sci. Vitam. 2016 , 62 , 322–329. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Rigamonti, E.; Parolini, C.; Marchesi, M.; Diani, E.; Brambilla, S.; Sirtori, C.R.; Chiesa, G. Hypolipidemic Effect of Dietary Pea Proteins: Impact on Genes Regulating Hepatic Lipid Metabolism. Mol. Nutr. Food Res. 2010 , 54 , S24–S30. [ Google Scholar ] [ CrossRef ]
- Ruiz, R.; Olias, R.; Clemente, A.; Rubio, L.A. A Pea ( Pisum sativum L.) Seed Vicilins Hydrolysate Exhibits Ppar Gamma Ligand Activity and Modulates Adipocyte Differentiation in a 3t3-L1 Cell Culture Model. Foods 2020 , 9 , 793. [ Google Scholar ] [ CrossRef ]
- Awosika, T.O.; Aluko, R.E. Inhibition of the in vitro Activities of Alpha-Amylase, Alpha-Glucosidase and Pancreatic Lipase by Yellow Field Pea ( Pisum sativum L.) Protein Hydrolysates. Int. J. Food Sci. Technol. 2019 , 54 , 2021–2034. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Qin, G.; Xu, W.; Liu, J.; Zhao, L.; Chen, G. Purification, Characterization and Hypoglycemic Activity of Glycoproteins Obtained from Pea ( Pisum sativum L.). Food Sci. Hum. Wellness 2021 , 10 , 297–307. [ Google Scholar ] [ CrossRef ]
- Liu, J.P.; Qian, Y.F.; Qin GY, X.; Zhao, L.Y.; Chen, G.T. Antidiabetic Activities of Glycoprotein from Pea ( Pisum sativum L.) in Stz-Induced Diabetic Mice. Food Funct. 2021 , 12 , 5087–5095. [ Google Scholar ] [ CrossRef ]
- Wei, Y.; Zhang, R.X.; Fang, L.; Qin, X.Y.; Cai, M.Y.; Gu, R.Z.; Lu, J.; Wang, Y.Q. Hypoglycemic Effects and Biochemical Mechanisms of Pea Oligopeptide on High-Fat Diet and Streptozotocin-Induced Diabetic Mice. J. Food Biochem. 2019 , 43 , e13055. [ Google Scholar ] [ CrossRef ]
- Thondre, P.S.; Achebe, I.; Sampson, A.; Maher, T.; Guerin-Deremaux, L.; Lefranc-Millot, C.; Ahlstrom, E.; Lightowler, H. Co-Ingestion of Nutralys ® Pea Protein and a High-Carbohydrate Beverage Influences the Glycaemic, Insulinaemic, Glucose-Dependent Insulinotropic Polypeptide (Gip) and Glucagon-Like Peptide-1 (Glp-1) Responses: Preliminary Results of a Randomised Controlled Trial. Eur. J. Nutr. 2021 , 60 , 3085–3093. [ Google Scholar ] [ CrossRef ]
- El-Saadony, M.T.; El-Hack, M.E.A.; Swelum, A.A.; Al-Sultan, S.I.; El-Ghareeb, W.R.; Hussein, E.O.S.; Ba-Awadh, H.A.; Akl, B.A.; Nader, M.M. Enhancing Quality and Safety of Raw Buffalo Meat Using the Bioactive Peptides of Pea and Red Kidney Bean under Refrigeration Conditions. Ital. J. Anim. Sci. 2021 , 20 , 762–776. [ Google Scholar ] [ CrossRef ]
- El-Araby, M.M.; El-Shatoury, E.H.; Soliman, M.M.; Shaaban, H.F. Characterization and Antimicrobial Activity of Lectins Purified from Three Egyptian Leguminous Seeds. Amb. Express 2020 , 10 , 90. [ Google Scholar ] [ CrossRef ]
- Belghith-Fendri, L.; Chaari, F.; Ben Jeddou, K.; Kallel, F.; Bouaziz, F.; Helbert, C.B.; Abdelkefi-Mesrati, L.; Ellouz-Chaabouni, S.; Ghribi-Aydi, D. Identification of Polysaccharides Extracted from Pea Pod By-Products and Evaluation of their Biological and Functional Properties. Int. J. Biol. Macromol. 2018 , 116 , 947–954. [ Google Scholar ] [ CrossRef ]
- Hidayat, M.; Prahastuti, S.; Afifah, E.; Widowati, W.; Yusuf, M.; Hasan, K. The Role of Green Peas Protein Hydrolysate in Tgf/Smad Signaling to Prevent Renal Fibrosis. J. King Saud. Univ. Sci. 2022 , 34 , 101920. [ Google Scholar ] [ CrossRef ]
- Hidayat, M.; Prahastuti, S.; Yusuf, M.; Hasan, K. Nutrition Profile and Potency of Rgd Motif in Protein Hydrolysate of Green Peas as an Antifibrosis in Chronic Kidney Disease. Iran. J. Basic. Med. Sci. 2021 , 24 , 734–743. [ Google Scholar ] [ CrossRef ]
- Kabir, S.R.; Nabi, M.M.; Haque, A.; Zaman, R.U.; Mahmud, Z.H.; Abu Reza, M. Pea Lectin Inhibits Growth of Ehrlich Ascites Carcinoma Cells by Inducing Apoptosis and G 2 /M Cell Cycle Arrest In Vivo in Mice. Phytomedicine 2013 , 20 , 1288–1296. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Arora, H.; Shang, N.; Bhullar, K.S.; Wu, J.P. Pea Protein-Derived Tripeptide Lrw Shows Osteoblastic Activity on Mc3t3-E1 Cells via the Activation of the Akt/Runx2 Pathway. Food Funct. 2020 , 11 , 7197–7207. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Feng, T.; Huang, Y.Y.; Tang, Z.H.; Wei, D.D.; Mo, J.M. Anti-Fatigue Effects of Pea ( Pisum sativum L.) Peptides Prepared by Compound Protease. J. Food Sci. Technol. 2021 , 58 , 2265–2272. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, Y.P.; Su, Y.Y.; Yang, Q.; Zhou, T.B. Stem Cells in the Treatment Of Renal Fibrosis: A Review of Preclinical and Clinical Studies of Renal Fibrosis Pathogenesis. Stem Cell Res. Ther. 2021 , 12 , 333. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bi, S.; Xu, X.; Luo, D.; Lao, F.; Pang, X.; Shen, Q.; Hu, X.; Wu, J. Characterization of Key Aroma Compounds in Raw and Roasted Peas ( Pisum sativum L.) by Application of Instrumental and Sensory Techniques. J. Agric. Food Chem. 2020 , 68 , 2718–2727. [ Google Scholar ] [ CrossRef ]
- Trikusuma, M.; Paravisini, L.; Peterson, D.G. Identification of Aroma Compounds in Pea Protein Uht Beverages. Food Chem. 2020 , 312 , 126082. [ Google Scholar ] [ CrossRef ]
- Bi, S.; Lao, F.; Pan, X.; Shen, Q.; Liu, Y.; Wu, J.H. Flavor Formation and Regulation of Peas ( Pisum sativum L.) Seed Milk via Enzyme Activity Inhibition and Off-Flavor Compounds Control Release. Food Chem. 2022 , 380 , 132203. [ Google Scholar ] [ CrossRef ]
- Ma, W.Y.; Zhang, C.M.; Kong, X.Z.; Li, X.F.; Chen, Y.M.; Hua, Y.F. Effect of Pea Milk Preparation on the Quality of Non-Dairy Yoghurts. Food Biosci. 2021 , 44 , 101416. [ Google Scholar ] [ CrossRef ]
- Manus, J.; Millette, M.; Dridi, C.; Salmieri, S.; Uscanga BR, A.; Lacroix, M. Protein Quality of a Probiotic Beverage Enriched with Pea and Rice Protein. J. Food Sci. 2021 , 86 , 3698–3706. [ Google Scholar ] [ CrossRef ]
- Yousseef, M.; Lafarge, C.; Valentin, D.; Lubbers, S.; Husson, F. Fermentation of Cow Milk and/or Pea Milk Mixtures by Different Starter Cultures: Physico-Chemical and Sensorial Properties. LWT-Food Sci. Technol. 2016 , 69 , 430–437. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Sim, S.Y.J.; Hua, X.Y.; Henry, C.J. A Novel Approach to Structure Plant-Based Yogurts Using High Pressure Processing. Foods 2020 , 9 , 10. [ Google Scholar ] [ CrossRef ]
- Nawaz, M.A.; Tan, M.V.; Oiseth, S.; Buckow, R. An Emerging Segment of Functional Legume-Based Beverages: A Review. Food Rev. Int. 2022 , 38 , 1064–1102. [ Google Scholar ] [ CrossRef ]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive Compounds and Bioactivities of Germinated Edible Seeds and Sprouts: An Updated Review. Trends Food Sci. Technol. 2017 , 59 , 1–14. [ Google Scholar ] [ CrossRef ]
- Erba, D.; Angelino, D.; Marti, A.; Manini, F.; Faoro, F.; Morreale, F.; Pellegrini, N.; Casiraghi, M.C. Effect of Sprouting on Nutritional Quality of Pulses. Int. J. Food Sci. Nutr. 2019 , 70 , 30–40. [ Google Scholar ] [ CrossRef ]
- Elliott, H.; Woods, P.; Green, B.D.; Nugent, A.P. Can Sprouting Reduce Phytate and Improve the Nutritional Composition and Nutrient Bioaccessibility in Cereals and Legumes? Nutr. Bull. 2022 , 47 , 138–156. [ Google Scholar ] [ CrossRef ]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. A Review on Recent Advances in Cold Plasma Technology for the Food Industry: Current Applications and Future Trends. Trends Food Sci. Technol. 2017 , 69 , 46–58. [ Google Scholar ] [ CrossRef ]
- Svubova, R.; Kyzek, S.; Medvecka, V.; Slovakova, L.; Galova, E.; Zahoranova, A. Novel Insight at the Effect of Cold Atmospheric Pressure Plasma on the Activity of Enzymes Essential for the Germination of Pea ( Pisum sativum L. Cv. Prophet) Seeds. Plasma Chem. Plasma Process. 2020 , 40 , 1221–1240. [ Google Scholar ] [ CrossRef ]
- Zhang, C.L.; Zhang, Y.Y.; Zhao, Z.Y.; Liu, W.F.; Chen, Y.Q.; Yang, G.J.; Xia, X.D.; Cao, Y.F. The Application of Slightly Acidic Electrolyzed Water in Pea Sprout Production to Ensure Food Safety, Biological and Nutritional Quality of the Sprout. Food Control. 2019 , 104 , 83–90. [ Google Scholar ] [ CrossRef ]
- Jerse, A.; Kacjan-Marsic, N.; Sircelj, H.; Germ, M.; Kroflic, A.; Stibilj, V. Seed Soaking in I and Se Solutions Increases Concentrations of Both Elements and Changes Morphological and Some Physiological Parameters of Pea Sprouts. Plant. Physiol. Biochem. 2017 , 118 , 285–294. [ Google Scholar ] [ CrossRef ]
- Baczek-Kwinta, R.; Baran, A.; Simlat, M.; Lang, J.; Bieniek, M.; Florek, B. Enrichment of Different Plant Seeds with Zinc and Assessment of Health Risk of Zn-Fortified Sprouts Consumption. Agronomy 2020 , 10 , 937. [ Google Scholar ] [ CrossRef ]
- Tan, C.; Zhang, L.; Duan, X.; Chai, X.; Huang, R.; Kang, Y.; Yang, X. Effects of Exogenous Sucrose and Selenium on Plant Growth, Quality, and Sugar Metabolism of Pea Sprouts. J. Sci. Food Agric. 2022 , 102 , 2855–2863. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, Y.Q.; Hu, R.J.; Tilley, M.; Siliveru, K.; Li, Y.H. Effect of Pulse Type and Substitution Level on Dough Rheology and Bread Quality of Whole Wheat-Based Composite Flours. Processes 2021 , 9 , 1687. [ Google Scholar ] [ CrossRef ]
- Kotsiou, K.; Sacharidis, D.D.; Matsakidou, A.; Biliaderis, C.G.; Lazaridou, A. Impact of Roasted Yellow Split Pea Flour on Dough Rheology and Quality of Fortified Wheat Breads. Foods 2021 , 10 , 1832. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Millar, K.A.; Barry-Ryan, C.; Burke, R.; Mccarthy, S.; Gallagher, E. Dough Properties and Baking Characteristics of White Bread, as Affected by Addition of Raw, Germinated and Toasted Pea Flour. Innov. Food Sci. Emerg. Technol. 2019 , 56 , 102189. [ Google Scholar ] [ CrossRef ]
- Millar, K.A.; Barry-Ryan, C.; Burke, R.; Hussey, K.; Mccarthy, S.; Gallagher, E. Effect of Pulse Flours on the Physiochemical Characteristics and Sensory Acceptance of Baked Crackers. Int. J. Food Sci. Technol. 2017 , 52 , 1155–1163. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Krause, S.; Debon, S.; Palchen, K.; Jakobi, R.; Rega, B.; Bonazzi, C.; Grauwet, T. In Vitro Digestion of Protein and Starch in Sponge Cakes Formulated with Pea ( Pisum sativum L.) Ingredients. Food Funct. 2022 , 13 , 3206–3219. [ Google Scholar ] [ CrossRef ]
- Hanan, E.; Rudra, S.G.; Sagar, V.R.; Sharma, V. Utilizationof Pea Pod Powder for Formulation of Instant Pea Soup Powder. J. Food Process. Preserv. 2020 , 44 , e14888. [ Google Scholar ] [ CrossRef ]
- Polizer-Rocha, Y.J.; Lorenzo, J.M.; Pompeu, D.; Rodrigues, I.; Baldin, J.C.; Pires, M.A.; Freire MT, A.; Barba, F.J.; Trindade, M.A. Physicochemical and Technological Properties of Beef Burger as Influenced by the Addition of Pea Fibre. Int. J. Food Sci. Technol. 2020 , 55 , 1018–1024. [ Google Scholar ] [ CrossRef ]
- Pietrasik, Z.; Sigvaldson, M.; Soladoye, O.P.; Gaudette, N.J. Utilization of Pea Starch and Fibre Fractions for Replacement of Wheat Crumb in Beef Burgers. Meat Sci. 2020 , 161 , 107974. [ Google Scholar ] [ CrossRef ]
- Baugreet, S.; Kerry, J.P.; Botinestean, C.; Allen, P.; Hamill, R.M. Development of Novel Fortified Beef Patties with Added Functional Protein Ingredients for the Elderly. Meat Sci. 2016 , 122 , 40–47. [ Google Scholar ] [ CrossRef ]
- Shoaib, A.; Sahar, A.; Sameen, A.; Saleem, A.; Tahir, A.T. Use of Pea and Rice Protein Isolates as Source of Meat Extenders in the Development of Chicken Nuggets. J. Food Process. Preserv. 2018 , 42 , e13763. [ Google Scholar ] [ CrossRef ]
- Osen, R.; Toelstede, S.; Eisner, P.; Schweiggert-Weisz, U. Effect of High Moisture Extrusion Cooking on Protein-Protein Interactions of Pea ( Pisum sativum L.) Protein Isolates. Int. J. Food Sci. Technol. 2015 , 50 , 1390–1396. [ Google Scholar ] [ CrossRef ]
- Golge, O.; Kilincceker, O.; Koluman, A. Effects of Different Fibers on The Quality of Chicken Meatballs. J. Food Saf. Food Qual. 2018 , 69 , 177–183. [ Google Scholar ] [ CrossRef ]
- Kehlet, U.; Pagter, M.; Aaslyng, M.D.; Raben, A. Meatballs with 3% and 6% Dietary Fibre from Rye Bran or Pea Fibre—Effects on Sensory Quality and Subjective Appetite Sensations. Meat Sci. 2017 , 125 , 66–75. [ Google Scholar ] [ CrossRef ]
- Hadidi, M.; Boostani, S.; Jafari, S.M. Pea Proteins as Emerging Biopolymers for the Emulsification and Encapsulation of Food Bioactives. Food Hydrocolloid 2022 , 126 , 107474. [ Google Scholar ] [ CrossRef ]
- Caballero, S.; Li, Y.O.; Mcclements, D.J.; Davidov-Pardo, G. Hesperetin (Citrus Peel Flavonoid Aglycone) Encapsulation Using Pea Protein-High Methoxyl Pectin Electrostatic Complexes: Complex Optimization and Biological Activity. J. Sci. Food Agric. 2022 , 102 , 5554–5560. [ Google Scholar ] [ CrossRef ]
- Li, J.T.; Zhang, X.G.; Zhao, R.; Lu, Y.C.; Wang, C.; Wang, C.N. Encapsulation of Quercetin in Pea Protein-High Methoxyl Pectin Nanocomplexes: Formation, Stability, Antioxidant Capacity and In Vitro Release Profile. Food Biosci. 2022 , 48 , 101811. [ Google Scholar ] [ CrossRef ]
- Okagu, O.D.; Udenigwe, C.C. Molecular Interactions of Pea Globulin, Albumin and Glutelin with Curcumin: Formation and Gastric Release Mechanisms of Curcumin-Loaded Bio-Nanocomplexes. Food Biophys. 2022 , 17 , 10–25. [ Google Scholar ] [ CrossRef ]
- Zhang, X.G.; Wang, C.; Qi, Z.T.; Zhao, R.; Wang, C.N.; Zhang, T.H. Pea Protein Based Nanocarriers for Lipophilic Polyphenols: Spectroscopic Analysis, Characterization, Chemical Stability, Antioxidant and Molecular Docking. Food Res. Int. 2022 , 160 , 111713. [ Google Scholar ] [ CrossRef ]
- Mihalca, V.; Kerezsi, A.D.; Weber, A.; Gruber-Traub, C.; Schmucker, J.; Vodnar, D.C.; Dulf, F.V.; Socaci, S.A.; Fărcaș, A.; Mureșan, C.I.; et al. Protein-Based Films and Coatings for Food Industry Applications. Polymers 2021 , 13 , 769. [ Google Scholar ] [ CrossRef ]
- Cheng, J.J.; Li, Z.Z.; Wang, J.; Zhu, Z.B.; Yi, J.H.; Chen, B.C.; Cui, L.Q. Structural Characteristics of Pea Protein Isolate (PPI) Modified by High-Pressure Homogenization and its Relation to the Packaging Properties of Ppi Edible Film. Food Chem. 2022 , 388 , 132974. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Azevedo, V.M.; De Oliveira, A.C.S.; Borges, S.V.; Raguzzoni, J.C.; Dias, M.V.; Costa, A.L.R. Pea Protein Isolate Nanocomposite Films for Packaging Applications: Effect of Starch Nanocrystals on the Structural, Morphological, Thermal, Mechanical and Barrier Properties. Emir. J. Food Agric. 2020 , 32 , 495–504. [ Google Scholar ] [ CrossRef ]
- Li, H.; Shi, H.B.; He, Y.Q.; Fei, X.; Peng, L.C. Preparation and Characterization of Carboxymethyl Cellulose-Based Composite Films Reinforced by Cellulose Nanocrystals Derived from Pea Hull Waste for Food Packaging Applications. Int. J. Biol. Macromol. 2020 , 164 , 4104–4112. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Salim, M.H.; Abdellaoui, Y.; Benhamou, A.A.; Ablouh, E.H.; El Achaby, M.; Kassab, Z. Influence of Cellulose Nanocrystals from Pea Pod Waste on Mechanical, Thermal, Biodegradability, and Barrier Properties of Chitosan-Based Films. Cellulose 2022 , 29 , 5117–5135. [ Google Scholar ] [ CrossRef ]
- Zhou, X.; Cheng, R.; Wang, B.; Zeng, J.; Xu, J.; Li, J.; Kang, L.; Cheng, Z.; Gao, W.; Chen, K. Biodegradable Sandwich-Architectured Films Derived from Pea Starch and Polylactic Acid with Enhanced Shelf-Life for Fruit Preservation. Carbohydr. Polym. 2021 , 251 , 117117. [ Google Scholar ] [ CrossRef ]
- Zhou, X.M.; Yang, R.D.; Wang, B.; Chen, K.F. Development and Characterization of Bilayer Films Based on Pea Starch/Polylactic Acid and Use in the Cherry Tomatoes Packaging. Carbohydr. Polym. 2019 , 222 , 114912. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Sample Types | Experimental Models | Major Results | References |
|---|---|---|---|
| Seed flour extracted with 95% ethanol | In vitro (DPPH) | values for DPPH free radical scavenging activity were varied in different microwave drying, ranging from 0.1 to 0.9 mg/mL | [ ] |
| Seed flour extracted with 80% ethanol | In vitro (ABTS; DPPH; reducing power) In vitro (cell model, OA-induced HepG2 cells) | [ ] | |
| Seed flour extracted with 80% methanol | In vitro (ABTS; DPPH) | [ ] | |
| Seed flour extracted with mixed solution (acetone/water/acetic acid, 70:29.5:0.5, v/v/v) | In vitro (ABTS; FRAP) | /g DW | [ ] |
| Seed coat extracted with mixed solution (methanol/water/acetic acid mixture, 80:19:1, v/v/v) | In vitro (DPPH; FRC; FCC) | [ ] | |
| Seed coat extracted with mixed solution (acetone/water/acetic acid mixture, 80:19:1, v/v/v) | In vitro (DPPH; FRC; FCC) | [ ] | |
| Seed coat extracted with water, methanol, and ethyl acetate | In vitro (ABTS; DPPH; FRAP) | values of ABTS free radical scavenging activity of ethyl acetate extract (9.61 μM TEAC/g) was higher than that of the methanol extract (1.9 μM TEAC/g) values of DPPH free radical scavenging activity were 350 μg/mL and 650 μg/mL for ethyl acetate and methanol extract, respectively | [ ] |
| Red and yellow pea hull in vitro digestion products | In vitro (DPPH; ABTS; H O ; FRAP) | O ; FRAP) and TPC/TFC (r > 0.92) | [ ] |
| Pea sprout extracted with 80% methanol | In vitro (DPPH; ORAC; CUPRAC) | [ ] | |
| Pea hull extracted with 95% ethanol | In vitro (DPPH; reducing power; FRAP) | [ ] | |
| Peptides derived from pea protein hydrolysate | In vitro (DPPH; OH) | [ ] | |
| Whole seed flour | In vivo (HFD-induced Sprague–Dawley (SD) male rats) | [ ] | |
| Seed coat extracted with water | In vivo (DOX-induced albino male rats) | [ ] | |
| Green pea hull extracted with 80% methanol | In vivo (D-galactose-induced SD female rats) | [ ] | |
| Yellow pea hull extracted with 80% methanol | In vivo (D-galactose-induced SD female rats) | [ ] | |
| Green pea hull in vitro digestion products | In vitro (LPS-induced Caco-2/Raw264.7 cells coculture) | [ ] | |
| Peptides derived from pea protein hydrolysate | In vitro (LPS/IFN-γ-induced RAW 264.7 cells) | [ ] | |
| Whole seed flour | In vivo (DSS-induced colitis in HFD-fed C57BL/6J female mice) | [ ] | |
| Green pea hull extracted with 80% ethanol | In vivo (DSS-induced colitis in C57BL/6 male mice) | -butyric acid) , Lachnospiraceae, Firmicutes; ↓ Bacteroidetes | [ ] |
| Two pea seed albumin extracts (PSE/AF-PSE) | In vivo (DSS-induced colitis in C57BL/6J male mice) | [ ] | |
| Peptides derived from pea protein hydrolysate | In vitro (ACE inhibition assay) | = 0.073 mg/mL) among all tested samples | [ ] |
| Peptides derived from pea protein hydrolysate | In vitro (A7r5 cells) | [ ] | |
| Tripeptide (Leu-Arg-Trp) | In vitro (A7r5 cells) | [ ] | |
| Peptides derived from pea protein hydrolysate | In vitro (ACE and renin inhibition assays) In vivo (male SHRs) | [ ] | |
| Peptides derived from pea protein hydrolysate | In vitro (ACE and renin inhibition assays) In vivo (male SHRs) | values of inhibition on renin and ACE were 0.57 and 0.10 mg/mL, respectively /w) in the SHR diet, respectively | [ ] |
| Pea pod autoclaved extract (AE) | In vitro (pancreatic lipase inhibition and cholesterol adsorption capacity assay) In vivo (high-sucrose-induced SD male rats) | [ ] | |
| Pea seed flour | In vivo (HFD-induced male SD rats) | [ ] | |
| Pea protein isolate | In vivo (HFD-induced male SD rats) | [ ] | |
| Pea protein hydrolysate | In vitro (3T3-L1 preadipocytes subline) | [ ] | |
| Pea flour and dietary fiber | In vivo (HFHSD-induced obese SD male rats) | /Bacteroidetes ratio | [ ] |
| Pea fiber | Clinical trial (12-week single center, double-blind placebo-controlled trial with 53 adults with overweight or obesity) | ; ↓ Actinomyces, Holdermania, Oscillospira (r = −0.463) abundance | [ ] |
| Pea protein hydrolysate | In vitro (α-amylase and α-glucosidase inhibition assays) | [ ] | |
| Purified pea glycoproteins (PGP1, PGP2, and PGP3) | In vitro (α-amylase and α-glucosidase inhibition assays) | [ ] | |
| Purified pea glycoprotein (PGP2) | In vivo (STZ-induced diabetic ICR male mice) | [ ] | |
| Pea oligopeptide | In vivo (HFD and STZ-induced diabetic Kunming male mice) | [ ] | |
| Pea dietary fiber | In vivo (STZ-induced diabetic Balb/c male mice) | [ ] | |
| Pea protein | Clinical trial (a randomised controlled trial with a high-carbohydrate beverage intake in healthy individuals) | [ ] | |
| 11S pea globulin (11SGP) | In vitro Bacteria: Bacillus cereus, Listeria monocytogenes, Streptococcus pyogenes, Escherichia coli, Acinetobacter baumannii, and Pseudomonas aeruginosa; Fungi: Alternaria alternate, Aspergillus flavus, Fusarium oxysporum, and Monascus purpureus | [ ] | |
| Pea lectin | In vitro Bacteria: Staphylococcus aureus, Streptococcus mutants, Pseudomonas aeruginosa, and Klebsiella pneumonia Fungi: Candida albicans | was 250 μg/mL | [ ] |
| Pea peel extracted with water, methanol, and ethyl acetate | In vitro Bacteria: Staphylococcus aureus, Salmonella enterica, Escherichia coli, and Pseudomonas aeruginosa Fungi: Aspergillus niger and Candida albicans | [ ] | |
| Pea pod polysaccharide | In vitro Bacteria: Bacillus thuringiensis, B. subtilis, Actinomycete sp., Enterococcus faecalis, Listeria monocytogenes, Micrococcus luteus, Klebsiella pneumonia, Pseudomonas aeruginosa, and Salmonella Typhimirium | and M. luteus with inhibition zones of 16, 15, and 15 mm at the concentration of 50 mg/mL, respectively with an inhibition zone of 15 mm at the concentration of 50 mg/mL | [ ] |
| Peptides derived from pea protein hydrolysate | In vitro (glucose-induced MES13 SV40 cells) | [ ] | |
| Peptides derived from pea protein hydrolysate | In vitro (glucose-induced MES13 SV40 cells) | [ ] | |
| Pea seed coat extracted with water | In vitro (cell lines, human colon denocarcinoma LS174, breast carcinoma MDA-MB-453, lung carcinoma A594, and myelogenous leukemia K562) | values ranged from 0.89% up to above 10.0% values ranged from 1.84% up to above 10.0% values ranged from 1.17% up to above 10.0% values ranged from 0.41% up to above 10.0% | [ ] |
| Pea lectin | In vitro (cell line, Ehrlich ascites carcinoma (EAC) cells) In vivo (Ehrlich ascites carcinoma cells in adult Swiss albino mice) | gene expression; ↓ Bcl-2 and Bcl-X gene expression | [ ] |
| Peptides derived from pea protein hydrolysate | In vivo (BALB/c female mice) | [ ] | |
| Pea tripeptide (Leu-Arg-Trp) | In vitro (MC3T3-E1 cell) | [ ] | |
| Peptides derived from pea protein hydrolysate | In vivo (Kunming mice) | [ ] | |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Wu, D.-T.; Li, W.-X.; Wan, J.-J.; Hu, Y.-C.; Gan, R.-Y.; Zou, L. A Comprehensive Review of Pea ( Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications. Foods 2023 , 12 , 2527. https://doi.org/10.3390/foods12132527
Wu D-T, Li W-X, Wan J-J, Hu Y-C, Gan R-Y, Zou L. A Comprehensive Review of Pea ( Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications. Foods . 2023; 12(13):2527. https://doi.org/10.3390/foods12132527
Wu, Ding-Tao, Wen-Xing Li, Jia-Jia Wan, Yi-Chen Hu, Ren-You Gan, and Liang Zou. 2023. "A Comprehensive Review of Pea ( Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications" Foods 12, no. 13: 2527. https://doi.org/10.3390/foods12132527
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC10648759

Yellow Field Pea Protein ( Pisum sativum L.): Extraction Technologies, Functionalities, and Applications
Nancy d. asen.
1 Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, MB R3T 2N2, Canada; ac.abotinamuym@adnnesa (N.D.A.); [email protected] (R.E.A.)
Rotimi E. Aluko
2 Richardson Centre for Food Technology and Research, University of Manitoba, Winnipeg, MB R3T 2N2, Canada
Alex Martynenko
3 Department of Engineering, Dalhousie University, Agricultural Campus, P.O. Box 550, Truro, NS B2N 5E3, Canada; [email protected]
Alphonsus Utioh
4 ACU Food Technology Services Inc., 64 Laverendrye Crescent, Portage la Prairie, MB R1N 1B2, Canada; ac.hcetdoofuca@hoitua
Pankaj Bhowmik
5 Aquatic and Crop Resource Development, National Research Council Canada, 110 Gymnasium Place, Saskatoon, SK S7N 0W9, Canada
Associated Data
No new data was created for this work. Review was majorly based on peer reviewed articles from scientists.
Yellow field peas ( Pisum sativum L.) hold significant value for producers, researchers, and ingredient manufacturers due to their wealthy composition of protein, starch, and micronutrients. The protein quality in peas is influenced by both intrinsic factors like amino acid composition and spatial conformations and extrinsic factors including growth and processing conditions. The existing literature substantiates that the structural modulation and optimization of functional, organoleptic, and nutritional attributes of pea proteins can be obtained through a combination of chemical, physical, and enzymatic approaches, resulting in superior protein ingredients. This review underscores recent methodologies in pea protein extraction aimed at enhancing yield and functionality for diverse food systems and also delineates existing research gaps related to mitigating off-flavor issues in pea proteins. A comprehensive examination of conventional dry and wet methods is provided, in conjunction with environmentally friendly approaches like ultrafiltration and enzyme-assisted techniques. Additionally, the innovative application of hydrodynamic cavitation technology in protein extraction is explored, focusing on its prospective role in flavor amelioration. This overview offers a nuanced understanding of the advancements in pea protein extraction methods, catering to the interests of varied stakeholders in the field.
1. Introduction
Field peas are cool-season legume pulse crops globally grown for use in human and animal nutrition. In Canada and other countries, field peas are crops of economic interest and are used as export commodities. The most common field pea is the yellow cotyledon species, which is followed by the green cotyledon species and a few other species [ 1 ]. Pea seeds are high in starch, protein, and micronutrients such as vitamins and minerals but low in fats with varying proximate composition across different cultivars.
While pulse crops are the most popular protein sources for human consumption in tropical and subtropical countries, their utilization is still in its infancy in Western nations [ 2 , 3 ]. Until more recently, soybean had been the most consumed plant protein, but there is a concern about allergenicity and genetic modification of the crop. Field peas have gained popularity due to their beneficial health effects, low production cost, and environmental sustainability [ 4 , 5 ]. The value of raw field peas is improved by processing into starch, protein, and fiber-rich fractions, and these ingredients are used in food formulations for nutritional enrichment or enhancement of techno-functional properties. Dry and wet fractionation or hybrid methods isolate pea protein constituents at different purity and functionality levels [ 6 , 7 , 8 , 9 ]. The outcoming products are subsequently used as ingredients in food formulations (i.e., stabilizers, emulsifiers, film-forming agents, and meat replacers).
The nutritional value and functional properties of proteins are dependent on the quantity and quality of the protein. Pea proteins have a high potential to be utilized as ingredients in the food industry because of their relatively balanced amino acid profile when compared with other plant proteins like soybeans [ 10 ]. However, the use of pea protein as a food ingredient is impaired by limitations including the presence of antinutritional factors (impairs digestibility), objectionable flavor components, low net surface charge density, and a complex globular structure. Furthermore, extraction methods at high pH and temperature used in the preparation of commercial pea protein harm functionality and consequently lower performance in food applications when compared with the laboratory-prepared protein. High protein solubility is achieved under alkaline conditions; however, the probability of aggregation is also high, which leads to reduced protein solubility, especially at acidic pH conditions. A study by Malafronte et al. [ 11 ] showed that different protein morphologies are produced by varying the drying conditions. Shell formations of the protein could occur during spray drying, resulting in conformational buckling and rheological changes [ 11 ]. Several works have sought to valorize pea protein ingredients by the use of simple to cutting-edge technologies to improve functionalities and minimize adverse effects on the native protein conformation. This review will discuss the effect of different extraction and processing technologies on the functionalities of pea protein as well as its application in food formulations. We will review recent approaches in extraction methods to produce pea protein ingredients with higher yield, functionality, and applicability in food systems and identify technology gaps where information is needed to improve the flavor component. Furthermore, the functionality of pea protein will be compared with a standard plant protein ingredient like soybean. Statistics show five field pea market classifications in Canada; however, yellow field pea varieties account for >75% of the 3.8 million pea acreage [ 12 , 13 ]. Hence, a lot of our discussion will be around processing technologies for yellow field peas and some aspects of other varieties of field peas.
2. A Comparison of the Chemical Characteristics of Pea Protein and Soybean Protein
Yellow field peas, like other pulses, are a leguminous crop grown mainly for the consumption of dry seeds which are rich in macromolecules (protein, carbohydrates, dietary fiber, and resistant starch) and micromolecules (minerals, vitamins, and phytochemicals) but low in fats. The chemical composition of pea protein is affected by the cultivar (i.e., genotype), growth conditions, and the protein extraction methods used to produce concentrates and isolates. In addition, pea protein contains residual antinutritional substances (i.e., protease inhibitors) and natural pigments (i.e., tannins and anthocyanins). Maharjan et al. [ 14 ] analyzed the effect and interaction between genotype, rainfall, and temperature in different field pea genotypes grown in two different locations. The results showed variation in protein content across the different genotypes, and the effect on protein content came from the interaction between genotype and environment, but a significant difference was observed with the different growing environments. Phytic acid content was influenced by the growth environment as seeds grown in one location had 6.3–8.1 g/kg and those in another location had 4.8–7.5 g/kg. Phytic acid in the field of pea seeds affects the nutritional value by binding to essential minerals such as calcium, iron, magnesium, and zinc, causing reduced bioavailability of nutrients [ 14 ]. In this section, a detailed review of the chemical composition of pea protein will be carried out and compared with soybean, another major source of plant protein for the food industry. The effect of some processing methods on the chemical composition will be discussed.
2.1. Proximate Analysis
The chemical composition of yellow field pea flours as determined by Millar et al. [ 15 ] showed the following: protein content (21.0–22.0%), ash (2.76–3.5%), lipid (1.28–1.4%), moisture (10.6–13.35%), and total dietary fiber (14.0–15.0%). As a result of the low lipid content, the pea protein extraction process is relatively easy, faster, and cost-effective as the defatting process is not required [ 16 ]. However, Garcia Arteaga et al. [ 17 ] reported much higher values in the proximate composition of various pea protein species, as shown in Table 1 . Dehulled field seed flours from 12 cultivars grown in different countries and harvest years showed variations in protein content (21.3–27.2%), ash content (2.5–3.6%), fat (1.9–2.5%), and starch (32.5–56.2%), and protein isolates from the same cultivars showed variations in protein (83.5–90.3%), ash (5.3–8.5%), and fat (4.7–9.0%) [ 17 ]. Lam et al. [ 18 ] investigated the physicochemical and functional properties of protein isolates derived from six pea cultivars grown in two different locations. The authors spotted differences in protein content (89.7–92.5%), ash (6.2–7.3%), and total lipids (2.3–3.5%) but stated that the differences had no practical significance. The total energy contributed by proteins for yellow field pea seeds is reported to be 24.4 and 26.3% [ 18 ], which satisfies the definition of high-protein foods [ 19 ]. Yellow field pea flour was also reported to provide at least 30% of the recommended dietary intake for zinc (3.78 mg/100 g), magnesium (114.2 mg/100 g), and potassium (1099.0 mg/100 g) [ 15 ]. Nikolopoulou et al. [ 20 ] showed that there was a significant impact on the proximate composition of field peas grown in three different locations for two years and established that high phytic acid content in pea seeds had a relationship with growing soils rich in phosphorus and low rainfall.
Proximate composition of isolated pea proteins from different cultivars.
| Cultivar | Dry Matter (%) | Protein (%) | Ash (%) | Fat (%) | Protein Yield (g/kg) |
|---|---|---|---|---|---|
| Navarro | 93.0 ± 0.0 | 83.5 ± 0.4 | 5.3 ± 0.3 | 5.9 ± 0.0 | 33.8 |
| Dolores | 93.5 ± 0.1 | 89.5 ± 0.2 | 5.4 ± 0.1 | 4.7 ± 0.1 | 54.4 |
| Greenwich | 93.8 ± 0.0 | 83.6 ± 0.4 | 6.0 ± 0.6 | 9.0 ± 0.2 | 34.8 |
| Bluetime | 94.4 ± 0.0 | 84.1 ± 0.0 | 6.4 ± 0.4 | 8.4 ± 0.3 | 42.2 |
| Ostinato | 94.1 ± 0.0 | 86.0 ± 0.5 | 7.6 ± 0.4 | 7.1 ± 0.4 | 38.6 |
| Kalifa | 93.0 ± 0.0 | 86.9 ± 0.9 | 5.9 ± 0.1 | 7.0 ± 0.5 | 46.2 |
| Salamanca | 93.7 ± 0.6 | 85.0 ± 0.3 | 6.1 ± 1.0 | 8.7 ± 0.6 | 42.2 |
| Florida | 92.5 ± 0.0 | 87.4 ± 1.1 | 5.6 ± 0.1 | 7.4 ± 0.7 | 59.2 |
| RLPY 141091 | 93.4 ± 0.0 | 90.3 ± 0.0 | 8.5 ± 0.7 | 7.3 ± 0.8 | 53.6 |
| Orchestra | 92.8 ± 0.3 | 87.1 ± 0.1 | 6.7 ± 1.1 | 6.2 ± 0.9 | 62.2 |
| Astronaute | 96.0 ± 0.2 | 86.4 ± 0.1 | 5.4 ± 0.1 | 7.8 ± 0.1 | 42.1 |
| Croft | 92.5 ± 0.1 | 86.7 ± 0.6 | 6.2 ± 0.1 | 7.8 ± 0.1 | 47.3 |
a–d Different letters indicate significant differences at the p ≤ 0.05 level for each column. Result expressed as mean ± standard deviation ( n = 2) adapted from Garcia-Arteaga [ 17 ].
The crude protein content in a pea protein isolate obtained by alkaline extraction coupled with isoelectric pH precipitation (AE-IEP) was reported to be 83.33–84.67% (dry weight basis, dwb), but there were no significant differences between the protein content of isolates extracted at pH 8.5, 9.0, and 9.5 [ 21 ]. However, extraction at pH 9.0 produced isolates with low contents of lipoxygenase (beany flavor factor). Lipid content in this study was reported to be identical for all isolates (~1.47%), and the extraction method did not influence the ash and moisture contents. The composition of a commercial pea protein isolate prepared by AE-IEP and drum drying was reported to be as follows: total protein (68.85%), lipid (0.5%), total carbohydrate (26.6%), starch (0.31%), ash (3.53%), moisture (7.12), and legumin/vicilin (L/V) ratio (8.34) [ 22 ]. The protein content from the commercial isolate was low when compared to literature records of other wet-fractionated pea ingredients. Similarly, protein isolate compositions (dry weight basis, dwb) from different cultivars produced by isoelectric pH precipitation (IEP) after initial saline solubilization had protein content (81–89%), moisture (7.6–8.8%), ash (1.33–2.55%), lipids (0.5–5.5%), and carbohydrates (0.37–3.9%) [ 23 ].
As shown in Table 2 , the major protein fractions in peas are the storage proteins, which are globulins (11S and 7S) and albumins (2S), with globulins making up the largest group of proteins (~65–85%). The electrophoretic profile of pea protein isolates under non-reducing conditions shows bands of approx. 10–105 kDa, which are assigned to dissociated hexameric legumin ~60 kDa subunits (α + β), intact vicilin fractions (α + β + γ) with bands of approx. 50 kDa, dissociated vicilin subunits (α + β) of approx. 30–37 kDa, and α, β, and γ subunits (approx. 14–20 kDa) [ 18 ]. The convicilin fraction is assigned the 70 kDa band, while lipoxygenase is assigned approx. 94–100 kDa. The same study reported 0.36–0.79 legumin/vicilin (L/V) ratios for the isolates and observed that the interaction between the cultivar and environment had no impact on these values. The polypeptide and allergen composition of pea protein could vary within the same cultivar grown under similar environment, harvest, and storage conditions. One reason is that the L/V ratio could change during the growth and maturity of the yellow field pea seeds. Dziuba et al. [ 24 ] carried out a proteomic analysis of a pea protein isolate using 2D electrophoresis and classified pea albumins as a heterogeneous group with 73 proteome accumulated spots in three molecular weight ranges of 50–110, 20–35, and 13–17 kDa over a broad isoelectric point range (pH 4.2–8.1). The pea albumin group comprises albumins (PA1), lectins, proteases, and protease inhibitors [ 24 , 25 ].
Polypeptide composition of yellow field pea protein.
| Classification | Content | Protein Fraction | Polypeptide | Svedberg Unit | Features | Author |
|---|---|---|---|---|---|---|
| Globulins | 55–65% | Hexameric/quarternary legumin (300–600 kDa) | Six paired α and β (60–80 kDa) | 11S | α and β subunits linked by disulfide linkage | Gueguen and Cerletti [ ]; Lam et al. [ ]; Tzitzikas et al. [ ] |
| Trimeric vicilin (175–180 kDa) | α, β, and γ (14–20 kDa) | 7S | Non-covalent bonds between subunits and glycosylation | Chang et al. [ ]; Kaur Dhaliwal et al. [ ] | ||
| Trimeric convicilin (210 kDa) | ~70 kDa | 8S | 80% amino acid homology with 7S | Kaur Dhaliwal et al. [ ]; Mertens et al. [ ] | ||
| Albumins | 18–25% | Pea albumins | PA1a (5.8 kDa) | 2S | 53 amino acids and high Cys | Barbana and Boye [ ]; De Santis et al. [ ]; Kornet et al. [ ]; Park et al. [ ] |
| PA1b (4.0 kDa) | 2S | 37 amino acids and high Cys | ||||
| Lectins | n/a | n/a | n/a | |||
| Lipoxygenase | 90–100 kDa | n/a | n/a | |||
| Protease inhibitors | n/a | n/a | ||||
| Natural pigments (anthocyanins and tannins) | n/a | n/a | ||||
| Prolamin | 4–5% | n/a | n/a | n/a | High Glu and Pro | Adebiyi and Aluko [ ] |
| Glutelin | 3–4% | n/a | n/a | n/a | n/a | |
The colors of pea protein isolates differ and are dependent on the cultivar, as determined using the International Commission on Illumination (CIE) L*a*b* method. An analysis of 12 of the 2S proteins showed a range for L* (87–91), which signifies lightness; a* (−0.5–3.5) for the green cotyledon color; and b* (19–24), which is the yellow color. [ 17 ] In another study, pea protein isolate (PPI) was shown to be darker with L* (69.8), a* (2.21), and b* (19.25), when compared to the lighter soybean protein isolate (SPI) with L* (94.18), a* (0.09), and b* (−0.92); the color variations may occur due to presence of pigments in the seed flour or the protein drying method [ 35 , 36 ] The protein yield varies widely with cultivar and ranges from 34 to 62 g/kg, which is not dependent on the protein content of the isolate [ 17 ].
Soybean is an oil seed with approx. 20–30% lipid content depending on the cultivar [ 22 ]. The major proteins in soybean are glycinin (10.1S–14S) and β-conglycinin (7.1S–8.7S), making up >70% of the total protein [ 37 ]. A meta-analytical approach of data collected for 1944 samples of soybean meal obtained from 18 published papers (2002–2018) was used to quantify the chemical composition based on country of origin [ 38 ]. Similar to variations observed with peas, the results of the study showed that the country of origin affected the chemical composition and amino acid profile of the soybean products by great margins. The inconsistencies across the different locations arose from seed genotype, planting location, environmental growth and harvesting conditions, and storage and processing conditions. Analysis of the polypeptides showed that at ambient temperature and around neutral pH, the legumin-like protein is a hexamer with a molecular weight of 300–380 kDa and each subunit consists of a pair with an α or acidic unit (MW of ~35 kDa) and a β or basic unit (MW of ~20 kDa) linked together by a disulfide bond [ 39 ]. The glycinin hexamers are dissociable species that could fragment into smaller polypeptides and constituent molecules of trimers (3S–8S) under processing conditions like low ionic strength, heating, and pH. Glycinin can form a 7S trimer of ~180 kDa at pH 3.8 or low ionic strength (i.e., 0.1 M) and neutral pH [ 39 ]. Soybean has five classifications based on polymorphism of the glycinin fractions, but some compositional parameters differ within these groups, and since the proteins exhibit molecular heterogeneity, the groups differ in functional properties. The vicilin-like 7S soy protein consists of subunits α’ (57–72 kDa), α (57–68 kDa), and β (42–52 kDa); it exists as hexamers and trimers at low (<0.1M) and high (>0.5 M) concentrations, respectively, and is held together by non-covalent bonds [ 39 ]. The protein reversibly dissociates into 2S–6S subunits at low pH (<5) and ionic strength (<0.1 M). The content of 2S proteins in soybeans are very low and consist of protease inhibitors, cytochrome c, and α-conglycinin. All three subunits of β-conglycinin and α-conglycinin are recognized as potential food allergens in humans and different animal species [ 36 , 39 ].
2.2. Amino Acid Profile
As shown in Table 3 , pea protein is limited in leucine as well as sulfur-containing amino acids (SCAAs) such as methionine and cysteine [ 17 ]. However, the leucine content of pea protein (5.7%) is slightly higher than that of soybean (5.0%), oat (3.8%), and hemp (2.6%) proteins [ 10 ]. Similarly, other studies reported higher lysine (4.7%) and phenylalanine (3.7%) for pea protein compared to soybean protein with values of 3.4% and 3.2%, respectively [ 10 ]. The essential amino acid composition of pea protein (23.6%) is slightly higher than that of wheat (18.9%) and soybean (19.9%) proteins and meets the WHO/FAO/UNU daily intake recommendation for adults [ 10 ]. Variations in amino acid composition are commonplace in pea protein, and responsible factors could be growth environment, germination, cultivar, storage, extraction methods, and processing conditions. Wet-fractionated pea protein isolate is reported to contain fewer SCAAs than dry-fractionated pea protein isolate because water-soluble albumins might be lost during acid precipitation and cysteine and serine residues are converted to dehydroalanine which is subsequently converted to lysine [ 40 ]. However, the amino acid and chemical score of wet-fractionated pea protein is superior to that of dry-fractionated pea protein with essential amino acids (EAAs) exceeding the FAO/WHO daily recommended level of 277 mg/g protein [ 40 ]. Furthermore, optimal unfolding and disruption of protein aggregates during wet fractionation facilitates increased protein solubilization and the higher presence of hydrophobic amino acids [ 40 ].
The different protein fractions (globulin, albumin, prolamin, and glutelin) vary in amino acid composition, and the major non-essential amino acids in globulins are asparagine, glutamine, glycine, arginine, isoleucine, leucine, phenylalanine, lysine, and threonine, while albumins are rich in tryptophan, lysine, and threonine [ 23 , 41 ]. Protein isolates obtained by lactic acid-assisted extraction exhibited improved amino acid composition because of the proteolytic activity of bacteria on the globulin and albumin fractions, which led to increased solubilization through the production of smaller polypeptides, peptides, and free amino acids [ 42 ]. Slight differences (~5%) were reported in the amino acid composition of pea protein isolates arising from different cultivars and extraction methods by Stone et al. [ 23 ], while Kaur Dhawali et al. [ 29 ] gave an update of up to 40% variations observed with some amino acids. Osen et al. [ 43 ] reported that the amino acid composition of pea protein isolates was not affected by the thermal and mechanical energy of 40–140 °C and 150 rpm, respectively, during extrusion, which suggests that there was no degradation of amino acids. The protein quality of soybeans is comparable to the quality of animal proteins because of the essential amino acid content. However, Gorissen et al. [ 10 ] showed that both pea and soybean proteins meet the WHO/FAO/UNU requirement at approx. 30 and 27%, respectively, and like most plant proteins, soybean protein is also limiting in SCAAs.
2.3. Comparative Nutritional Aspects
The quality of any food protein is assessed by the amino acid composition and protein digestibility. The protein-digestibility-corrected amino acid score (PDCAAS) is an assessment model that has been in use for over 20 years, and it is based on the assumption that all amino acids have the same digestibility as crude protein and calculated using fecal digestibility. However, proteins are mostly digested in the small intestine, and an accurate way to determine amino acid release and availability is using ileal digestibility in a procedure called digestible indispensable amino acid score (DIAAS) [ 44 ]. The PDCAAS and DIAAS of soybean and pea proteins were determined in the ileum of growing rats, and the results showed no significant difference in both proteins with values of 98–99% and 94–97%, respectively [ 45 ]. Although SCAAs are limiting in both plant proteins, the authors showed relatively good availability and quality of methionine and cysteine in pea protein (92 and 98%, respectively) and soybean protein (89–91% and 94–97%, respectively) [ 45 ]. Another study determined the combined mean DIAAS and PDCAAS of soy products as 84.5 ± 11.4 and 85.6 ± 18.2%, respectively, using in vitro and in vivo assays [ 46 ]. Understandably, pea flour has lower protein quality than the extracted protein, and a study reported a low protein quality of pea flour (67.8%) when compared to cooked flour (69.19%) [ 47 ]. Commercial pea and soybean protein brands showed protein contents in the ranges of 77–81% and 61–91%, respectively [ 10 ].
2.4. Flavor Components
Volatile (e.g., aldehydes, ketones, acids, pyrazines, and sulfur compounds) and non-volatile compounds (e.g., saponins, phenolic and alkaloid compounds) make up the flavor components of pulses [ 48 , 49 ]. Flavor is a combination of taste (i.e., non-volatiles perceived on the tongue), aroma (i.e., volatiles perceived nasally), texture (i.e., smoothness, viscosity, and sliminess), and trigeminal responses (i.e., brain in response to tactile or temperature stimuli). The off-flavor is a perception of an unpleasant taste or aroma and could be inherent in pea protein or develop during harvesting, processing, and storage due to lipoxygenase (LOX) activity, cultivar, harvest conditions, germination, and extraction methods [ 36 , 50 , 51 ]. An important inherent off-flavor in peas is the beany flavor which could be described as bitter, mouthcoating, rusty, nutty, metallic, or pea-like [ 52 ]. Although research shows it is difficult to attribute off-flavor to a single molecule, the presence of substances such as 3-methyl-1-butanol, 1-pentanol, 1-octen-3-ol, ( E,E )-2,4-heptadienal, acetophenone, 1-octen-3-one, and 3-isopropyl-2-methoxypyrazine are reported to be responsible for off-flavor in peas [ 48 ]. The concentration of the substances in the pea ingredient contributes to the intensity or absence of the off-flavor, and only a few differences in flavor attributes were found among different cultivars [ 17 , 53 ]. Other compounds like hexanal can contribute to off-flavors but have no beany flavor in themselves [ 48 ]. The presence of beany flavor in pea ingredients is a challenge and limits their utilization in food applications. Similarly, the utilization of soybean products in the developed world has been limited by the presence of flavor compounds (i.e., ketones, aldehydes, furans, alcohols), and these compounds could interact with protein and turn on other flavor compounds [ 54 , 55 ]. Beany flavor in soybean is a result of the enzymatic oxidation of linoleic and linolenic acids catalyzed by lipoxygenase. Aromatic compounds linked with beany flavor are hexanal, hexanol, and trans , trans -2,4-nonadienal [ 54 , 55 ].
Protein quality and digestibility of emulsion stabilized with pea and soy protein (g/100 g protein) and products (g/100 amino acids).
| Essential Amino Acids | Pea Protein [ ] | Soybean Protein [ ] | DIAAS (Peas) | DIAAS (Soybeans) | FAO/WHO/UNU [ ] |
|---|---|---|---|---|---|
| Emulsions [ ] | Milk [ ] | ||||
| Threonine | 3.80 | 3.90 | 3.86 | 3.73 | 2.30 |
| Methionine | 0.90 | 1.40 | 0.42 | 1.42 | 1.60 |
| Phenylalanine | 5.70 | 5.50 | 5.95 | 5.30 | 1.36 |
| Histidine | 2.40 | 2.50 | 5.60 | 7.10 | 1.50 |
| Lysine | 6.70 | 5.60 | 7.10 | 5.65 | 4.50 |
| Valine | 4.90 | 5.10 | 4.95 | 4.70 | 3.90 |
| Isoleucine | 4.40 | 4.90 | 4.85 | 4.74 | 3.00 |
| Leucine | 7.60 | 5.60 | 8.74 | 7.46 | 5.90 |
| Tryptophan | 0.90 | 1.30 | 3.23 | 2.82 | 0.60 |
| Non-essential amino acids | |||||
| Serine | 5.40 | 5.20 | |||
| Glycine | 4.00 | 4.40 | |||
| Glutamic acid | 16.40 | 20.50 | |||
| Aspartic acid | 11.80 | 11.90 | |||
| Proline | 4.40 | 4.90 | |||
| Cysteine | 1.20 | 1.00 | 0.6 | ||
| Alanine | 0.71 | 4.20 | |||
| Tyrosine | 4.00 | 3.90 | |||
| Arginine | 7.80 | 8.40 | |||
Several studies have been carried out to identify and reduce the odor-active volatile agents responsible for beany flavor in pulses. An article by Trindler et al. [ 48 ] gave a comprehensive review of the current state of knowledge on aromas and flavors associated with field pea protein. Off-flavors caused by inherent factors can not only be removed, masked, or modified but can also be prevented by breeding new cultivars. Off-flavors that develop because of external factors (i.e., storage temperature and moisture) can be controlled by careful handling of the peas and tuning of extraction methods. Physical, chemical, and enzymatic methods have been engaged in dealing with off-flavors in pulses. Thermal processing such as blanching at 60–100 °C can deactivate peroxidases and lipoxygenases [ 59 , 60 ]. Lactic acid fermentation of pea protein led to reduced or masked off-flavors by decreasing the content of n-hexanal (a lipoxygenase-derived molecule) and other contributors to the beany flavors [ 59 , 61 , 62 , 63 ]. Hexanal is a product of the degradation of unsaturated fatty acids and is the most abundant aldehyde detected in peas [ 64 ]. The content of aromatic compounds increased in fermented yellow pea flour, and the quantity of aromatic compounds produced was dependent on the bacteria strain and fermentation time [ 65 ]. Sensory analysis to evaluate flavor in pita bread and tortillas made from oven-roasted and micronized flour showed pitas from treated flour had higher aroma acceptability scores than those from untreated flours [ 66 ]. This result means that thermal treatment improves the flavor profile of the pea ingredient. Glycation of pea protein isolates with gum Arabic by incubation for 24 h improved the flavor profile remarkably (<1 ppm) through reductions in beany flavor markers [ 67 ]. Electronic tongue and sensory evaluation showed that enzymatic hydrolysis and the low-temperature Maillard reaction of pea protein reduced the bitter taste and increased the umami and salty tastes [ 68 ].
Recent advances have been made in monitoring flavor development, profiling, and reduction. Benavides-Paz et al. [ 69 ] monitored the development of volatile compounds during pH-optimized extraction of PPI using solvent-assistant flavor evaporation (SAFE), gas chromatography–mass spectrometry olfactometry (GC-MS-O), and gas chromatography–time-of-flight mass spectrometry (GC-TOF-MS). Wang et al. [ 49 ] performed aqueous solvent washing of air-classified pea-protein-enriched flour using different concentrations of ethanol and isopropanol to eliminate off-flavors. The effect of alcohol washing on volatiles, non-volatiles, proximate composition, and functionalities was compared between the untreated and treated samples. The volatile compounds were analyzed using headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography/mass spectrometry (GC-MS). The results showed reductions in volatile compounds by 50 and 80% for ethanol and isopropanol washes, respectively. Some functionalities (i.e., protein content and in vitro protein digestibility) were enhanced by the alcohol washing while others (i.e., solubility and amino acid scores) were reduced after the washes. Another potential method for the elimination of flavors in plant protein sources was reported by Guldiken et al. [ 64 ], where different adsorbent resins, namely Amberlite-XAD16N, Amberlite-XAD7HP, Amberlite-XAD4, Sepabeads-SP207, and Diaion-HP20, were used to wash volatile and non-volatile compounds in lentil protein isolate. The results showed that treatments reduced the amounts of aldehydes, ketones, nitrogen compounds, alcohols, furans, terpenes, and enone in the protein, but total acids, aromatic compounds, and esters increased. However, the study concluded that the technique is a potential tool to be employed in the production of bland plant protein ingredients.
3. Technologies for Pea Seed Isolation
Field pea seeds contain 20–40% protein; 60% starch and dietary fibers; and other constituents, namely lipids (1.5–2%) minerals, vitamins, polyphenols, oxalates, saponins, and phytic acid [ 29 ]. Therefore, the separation of the protein fraction from starch and non-starch materials is necessary. Pea protein ingredients (flours, fiber, concentrates, and isolates) can be produced through dry, wet, or mild fractionation, and the different ingredients have varying protein contents ( Table 4 , Figure 1 and Figure 2 ). To maximize yield and enhance the nutritional, structural, and functional properties of the protein ingredient, it is essential to choose the appropriate methods matching the intent of the end-user. Before fractionation, the seeds are cleaned, dried, sorted, and then dehulled/split to preserve the functional properties of the derived protein ingredient [ 8 ]. Different fractionation techniques affect the protein ingredient differently; i.e., wet fractionation produces pulse protein with an essential amino acid content within the range of recommended daily consumption and enhanced emulsification and foaming properties, while dry fractionation preserves the native state of the protein and enhances hydration properties [ 70 , 71 ]. Higher protein purities are attained with wet fractionation, but the native structure and functional properties are altered to some degree. In this section, a few techniques in dry and wet fractionation will be discussed.
Summary of processing and fractionation techniques.
| Method | Plant Source | Objective | Summary of Finding | Author |
|---|---|---|---|---|
| Dry fractionation | Pea | Using dry milling in combination with air classification to improve protein enrichment | Approx. 50% purity and 77% protein yield were obtained using the method. The native functionality of the protein was preserved. | Pelgrom et al. [ ] |
| Peas, beans, chickpeas and lentils | Optimize milling using different settings to achieve maximum detachment of starch granules | Optimal detachment was achieved, but protein content was influenced by the intrinsic properties of the pulse. | Pelgrom et al. [ ] | |
| Pea, lentils, and chickpeas | Air classification and electrostatic separation for protein enrichment | Higher protein purity (>60%), improved yield, less energy consumption, and preserved native protein functionality. | Xing et al. [ ] | |
| Pea and faba beans | Effect of dehulling on physical, chemical, and technological properties of the fractions | Dehulling slightly increased the protein content of the fine fractions and improved starch enrichment of the coarse fractions. The techno-functional properties were not enhanced with dehulling. | Saldanha do Carmo et al. [ ] | |
| Pea | Enhanced pea protein separation using Lorentz force-assisted charge carrier and triboelectric separation. | Protein content was increased by >100%. | Zhu et al. [ ] | |
| Pea | Effect of the protein content of pea flour on physicochemical, antinutritional, and functional properties of air-classified protein fractions | Variations in protein content influenced the properties of air-classified pea flour. | Fenn et al. [ ] | |
| Pea and chickpea | Determine the effect of relative humidity on particle dispersibility and flowability | Relative humidity above 70% affected the milling and air classification due to reduced particle dispersibility and flowability. | Politiek et al. [ ] | |
| Mung bean, field pea, and cowpea | Compare the functional and rheological properties of dry-fractionated ingredients from mung bean, yellow pea, and cowpea | Protein content of the protein-rich fractions was dependent on the air classifier speed. | Schlangen et al. [ ] | |
| Wet and aqueous fractionation | ||||
| Aqueous/ultrafiltration | Pea | Mild wet fractionation using water only and continuous ultrafiltration | Method produced high-purity (75%) protein concentrates with improved solubility. | Möller et al. [ ] |
| Alkaline extraction and isoelectric point precipitation | Pea | Compare protein functionality of isolates obtained from dry and wet (IP) fractionation | Wet fractionation produced isolates with high protein content, the presence of essential amino acids, and improved emulsification and foaming properties. | Zhu et al. [ ] |
| Chickpeas and green peas | Functional properties of protein isolates obtained by AE-IP method combined with modified salt dissolution precipitation | The purity of the globulin fractions was improved to >90%, and the protein composition played a major role in the functional properties. | Chang et al. [ ] | |
| Pea | AE-IP extraction in conjunction with lactic acid fermentation | Protein content and yield were improved by 20–30%. | Emkani et al. [ ] | |
| Pea | Compare the gelling properties of isolates obtained from different fractionation techniques | Gels from AE-IP in conjunction with ultrafiltration had good gel strength, but weak gels formed with AI alone. | Yang et al. [ ] | |
| Pea | Mild wet fractionation coupled with isoelectric precipitation | Method produced both globulins and albumins; functionality was dependent on the dominant protein fraction in a sample. | Möller et al. [ ] | |
| Enzyme-assisted extraction method | Pea and flaxseed | Comparison of the properties of protein obtained from different extraction methods | Enzymatic solvent extraction produced high protein quality, and enzymatic extraction produced protein with good emulsifying properties. | Tirgar et al. [ ] |
| Pea | Investigate the effect of enzymatic hydrolysis on the techno-functional and sensory properties of pea protein isolates | The different proteases enhanced the properties of the protein and lowered bitterness. | Garcia-arteaga et al. [ ] | |
| Osborne fractionation | Commercial pea protein | Fractionation based on solubility in weak salt, water, alcohol, and weak acid or alkaline solution using Osborne fractionation with dialysis | Alkaline-soluble fractions (glutelins) were the most abundant (87.0%) while alcohol-soluble fraction (prolamins) was the lowest in both yield (1.52%) and protein content (57.7%). The other fractions had protein content >79.0%. | Adebiyi and Aluko [ ] |
| Pea flour | Fractionation of globulins and albumins using isoelectric point isolation | Albumins and globulins were isolated and showed good foam and emulsification properties, respectively. | Kornet et al. [ ] | |

Flowchart showing a mild fractionation method adapted from Kornet et al [ 33 ]. ALB RF: albumin-rich fraction; GLB RF: globulin-rich fractions.

Wet and dry fractionation of pea protein. Adapted from Pulse Canada [ 80 ].
3.1. Dry Fractionation
The two major steps involved in dry fractionation are milling (pin, roller, hammer, or stone) and air classification ( Table 4 ). Dehulling and dry milling are pre-processing techniques that optimize protein enrichment during air classification [ 8 ]. Dehulling of the seeds is carried out to remove the seed coat before milling into flour [ 81 ]. Dehulling could be dry or wet; dry dehulling involves pitting the seed surface by abrasion [ 81 ], microwave technology [ 82 ], and ultrasound treatment [ 83 ], while wet dehulling involves soaking or tempering seeds in water as well as chemical or enzymatic treatment [ 81 ]. Subsequently, the pea seeds are milled to particles with a diameter < 40 μm to detach the protein from other seed materials [ 8 , 9 ]. The particle size of the flour is important because too coarse or too fine milling could hinder the proper separation of proteins from the starch granules and other cellular materials. The particle size is dependent on the speed of the classifier wheel and retention times; for example, in the case of the milling of lupine seed flour, increased classifier speed reduced the particle size from 280 μm to 10–14 μm [ 8 ]. During milling, starch granules and cellular matrix rich in protein and fibers are released by grinding the cotyledon into powder [ 9 ]. This step is performed carefully to minimize the production of damaged starch. After milling, air classification is applied to separate the small protein bodies from the larger starch granules for protein enrichment, and the separation is based on the size, shape, and density of the particles [ 7 , 8 ]. Very fine milling impairs the efficiency of air classification because separation is easier when non-protein materials like starch granules and fibers have larger particle sizes than protein. Increased air classifier speed could produce protein-enriched fractions with higher protein content and enhanced gelation properties [ 75 ]. Combining air classification and electrostatic separation produced a pea protein product with higher purity (63–68%) than the 57% obtainable by air classification only [ 9 ]. An alternative method to air classification is the use of sieves, which is based on variations in particle size [ 81 ].
Several factors have been mentioned as affecting protein separation efficiency, namely seed hardness or softness and fiber, ash, and oil contents. High fat content in chickpeas (6%) was shown to increase the chances for flour particle agglomeration, which impaired separation, whereas low fat (1%) in lentils and peas promoted sufficient separation [ 9 ], suggesting that defatting of the flour before dry fractionation is one way to improve the protein content as the adhesive forces that impair flowability would be reduced [ 8 ]. De-agglomeration (DA) is a parameter that measures the flowability or dispersibility of the flours in the air during classification. The dispersibility of flours is influenced by particle–particle adhesion, high humidity, and size; i.e., finer flour particle sizes would disperse better under low pressure [ 8 , 74 ]. Dispersibility and air classification of oil-rich flours can be enhanced using food-grade flowability aids. Aerosil (12 nm) and potato starch (44 μm) were added to lupine flour (high oil content); the air classification was improved at low pressure, and increased protein content was observed [ 8 ].
Electrostatic separation has been used to improve the quality of protein ingredients produced by dry fractionation [ 8 , 9 , 73 ]. Different electrostatic methods have been adapted to combine with or replace air classification, and these include triboelectrification or tribo-charging, Lorentz force-assisted, electric and magnetic field separation [ 71 ]. The principle is the use of the triboelectric charging properties of the material to obtain protein concentrates. For example, proteins will carry higher charges on the ionizable R groups and the amino and carboxyl termini than carbohydrates. making separation easy. Combined electrostatic treatment and milling of lupine seeds produced a protein material with a purity 15% higher than that using air classification [ 84 ], and higher protein recovery was recorded in navy bean protein after two-stage triboelectric treatment [ 85 ]. Modest protein enrichment of fine-milled peas and lentils could also be achieved using electrostatic separation [ 9 ]. Dry pea protein with 72% purity was obtained by a hybrid method between air classification and electromagnetic processes [ 9 ]. Furthermore, the best selection of the charging wall tube material (aluminum, steel, nylon, and PTFE) and its effect on electrostatic selection in the protein enrichment of lupine were evaluated [ 86 ]. The finding was that the tube material did not affect the separation, but the hydrodynamic conditions of the process were important.
The advantage of dry fractionation over wet fractionation is that the native functionality of the protein is retained, and dry fractionation is a more sustainable technique in terms of water and energy uses ( Figure 3 ) [ 7 , 73 ]. For example, wet fractionation consumes >50 kg and 5.4 × 10 −5 kJ/kg of water and energy for spray drying, respectively, per kg of recovered protein while dry fractionation has negligible water and energy (3.6 × 10 −7 kJ/kg) usage [ 28 ]. During dry fractionation, more valuable components will be maintained in the protein matrix; however, antinutritional compounds such as protease inhibitors and lipoxygenases, which impair digestibility and contribute to the development of beany flavor, respectively, are also retained [ 87 ]. Overall, during dry fractionation, milder processing conditions (i.e., pH, temperature, and ionic strength) are required for fractionation [ 87 ].

Energy consumption of dry and wet fractionation is shown as Sankey’s model. Adapted from Schutyser et al. [ 87 ].
3.2. Wet Fractionation
Wet fractionation is a conventional protein extraction method with the potential of producing high-purity (up to 95%) and high-yield (~60–90%) protein ingredients depending on the source [ 88 ] ( Table 4 ). During dry milling, the small particles adhere to the larger ones after the structural break-up, which impairs the optimal separation into pure components [ 89 ]. However, the addition of water disentangles the particles to produce better separation [ 89 ]. However, this method has drawbacks such as the loss of the protein’s native state and functionality (e.g., solubility) resulting from the use of harsh processing conditions (salt, ionic strength, pH, and temperature) and the high cost of energy and water leading to an overall high production cost [ 90 , 91 ]. The final ingredient is dried into a fine powder (referred to as concentrate or isolate) by freeze-drying or spray-drying techniques for ease of storage and transportation. It is possible to strategically target steps in wet fractionation to optimize the production of protein ingredients with enhanced and varying functionalities [ 33 ].
3.2.1. Alkaline Solubilization Coupled with Isoelectric pH Precipitation (AE-IP)
Alkaline solubilization coupled with isoelectric pH precipitation is a popular wet fractionation technique mainly because of the high-purity protein ingredients obtained ( Table 4 ). This method is based on the solubilization of plant proteins, usually at pH 8–11, resulting from the increased electronegative charge on the protein surface; the solubilized proteins are then recovered by acid-induced precipitation at the isoelectric point, which is usually pH 4–6 for most pulses. At acidic pH values, the amide group of the protein gains an extra proton, which results in an electropositive charge, while the carboxyl group loses a proton at alkaline pH, producing an electronegative charge. To maximize protein yield and purity, processing conditions such as extraction pH, temperature, and flour–solvent ratio could be optimized [ 73 , 92 , 93 ]. Defatting is a pre-step in which the lipid content of the flour is reduced to improve hydrophilicity. Without defatting, protein–lipid interactions minimize solubility and impair protein yield during extraction [ 29 ]. During extraction, a mixture is prepared from pea flour by mixing with water and adjusted to alkaline pH; the mixture is then subjected to continuous stirring to dissolve the protein and other cellular constituents. The protein is then separated from the starch by passing the mixture through a centrifuge to obtain a protein-enriched supernatant. The supernatant is adjusted to the protein’s isoelectric point using hydrochloric acid (HCl), which causes protein precipitation that can be recovered as the solid portion after centrifugation. The precipitate is resuspended in water, neutralized with sodium hydroxide (NaOH), and frozen or spray-dried to obtain protein concentrates or isolates with purities of <90% or >90%, respectively, on a dry weight basis [ 94 , 95 ]. An alkaline pH environment during the extraction is achieved using the addition of KOH or NaOH, and because maximal solubilization is obtained, and a high protein yield is achieved [ 29 ]. Alkaline solubilization was shown to also cleave disulfide bonds, hence improving protein recovery and yield [ 96 ].
Conditions reported to affect AE-IEP protein extraction are the temperature, flour-to-solvent ratio, alkaline solution concentration, and processing time, and these conditions can be optimized to maximize protein yield and recovery [ 33 , 97 ]. The presence of compounds like phenolics, organic acids, lipids, and nucleic acids could cause protein degradation, which results in low protein yield and functionality [ 87 ]. The AE-IEP method reportedly depletes sulfur-containing amino acids (SCAAs) in the protein through the loss of albumins during solubilization at the isoelectric point, impairs the bioavailability of SCAAs (71%) and His (80%), and converts cysteine and serine residues in the protein to dehydroalanine, which could be transformed to lysine [ 98 ]. The electrophoretic profile of pea protein fractionated by wet methods showed weakly stained polypeptide bands around 11–30 kDa, which may be due to depleted albumins, and intense bands around 48–63 kDa indicating aggregate formation by the denatured proteins [ 98 ]. The secondary structure of wet-fractionated pea protein is also changed because hydrogen bonds are broken and electrostatic repulsion is induced during alkali treatment, which leads to the rearrangement of polypeptide chains to produce high contents of secondary structures like β-sheets and β-turns [ 98 , 99 ]. Similarly, the tertiary structure of pea protein is also altered by the action of the organic acids or alkali on the disulfide bonds that stabilize the internal structure leaving a loose spatial structure of the protein, which is reflected as exposed internal chromophores, i.e., Try, Tyr, and Phe [ 98 ]. The nitrogen solubility of dry-fractionated protein (i.e., 76 and 89% at acidic and alkaline pH, respectively) was found to be higher than that of wet-fractioned protein (i.e., 57 and 76% at acidic and alkaline pH, respectively), and this is directly linked to increased content of hydrophobic amino acids, increased surface hydrophobicity, and depleted content of water-soluble albumins [ 8 , 85 , 98 ]. Functional properties of different cultivars of spray-dried pea protein isolates extracted by AE-IP were determined by Cui et al. [ 51 ], and the result revealed that most of the functional properties were dependent on the cultivar. However, emulsion stability and foam properties (capacity and stability) were directly affected by the extraction method. In spite of the pitfalls linked with wet fractionation, modifications to this technique have been reported to produce protein isolates with preserved native structures, thereby maintaining the quality of functional properties. Chang et al. [ 28 ] used AE-IP extraction coupled with a modified salt dissolution precipitation method to extract legumin and vicilin fractions at a large scale from defatted green peas and chickpeas. The result showed that a high purity of the fractions was achieved (80 and 90% for legumin and vicilin, respectively). The result showed improved protein content of the pea globulin and fractions (~80–96%) and improvement in other functional properties (solubility, emulsion, and foam properties) when compared with the conventional AE-IP method [ 100 ].
Also, amino acid and chemical scores in wet-fractionated protein were reported to be higher than those in dry-fractionated pea protein [ 98 ]. This was evaluated by total amino acid content and total essential amino acid (EAA) content, which exceeded the FAO/WHO recommended level (277 mg/g), and the abundance of hydrophobic amino acids resulting from maximal structural deformation of the protein [ 98 ]. Lactic acid bacteria (LAB) were used to lower the pH during the AE/IEP extraction of pea protein [ 42 ]. The method resulted in a ~20–30% increase in the protein content and yield due to the increased solubility of the protein through the proteolytic activity of LAB.
3.2.2. Ultrafiltration Processing (UF)
Ultrafiltration processing is a non-thermal, pressure-driven, and membrane-based separation technique with applications in protein fractionation, concentration, desalting, and clarification [ 96 , 101 , 102 , 103 , 104 ]. Ultrafiltration is a mild method because the native structure and functionalities of the protein are preserved, and it could be termed a green technique due to the absence of harmful chemicals and effluents [ 90 ]. Membrane UF technology is commonly characterized by a molecular weight cut-off (MWCO) and utilizes membranes with pore sizes of 0.001–0.1 µm, which act as physical sieves capable of retaining molecules with a molecular weight of ~30,000 kDa [ 90 ]. The MWCO is defined as the molecular weight above which ~90% of molecules are rejected by the membrane [ 91 ]. To obtain fractions with distinct sizes, the solubilized protein is sequentially passed through a smaller-sized membrane (e.g., 10 kDa), and the permeate is collected as the <10 kDa fraction. The retained solution is further passed through a bigger membrane size (e.g., 30 kDa), and the permeate is collected as the 10–30 kDa fraction while the retentate is the >30 kDa fraction. A reversed technique could start with a larger molecular membrane and the retentate collected from one size to the other. An addition to membrane UF technology is diafiltration, which involves the periodic addition of distilled water to the retentate during the process to reduce solution viscosity and increase the permeation rate through the membrane. UF technology has been widely used in dairy processing to improve the concentration of milk proteins or reduce lactose content in milk [ 92 , 93 ].
Membrane UF is used in combination with other techniques during protein extraction to produce ingredients with high native content and functionality. A comprehensive review of the application of the ultrafiltration technique in food applications was published by Ratnaningsih et al. [ 94 ]. An earlier study by Boye et al. [ 95 ] showed that the protein content of pea protein concentrates extracted by membrane UF and diafiltration increased by 4-fold compared to the flour content and was slightly higher than the protein content of the protein isolates obtained by AE-IP. More recently, Yang et al. [ 70 ] reported higher albumin content in pea protein fractions obtained through membrane UF and diafiltration than that obtained by AE-IP and the micellar precipitation technique. Also, the gel properties (capacity, morphology, and strength) and solubility of pea protein obtained from membrane UF of alkaline or salt extracts were superior to those of gels obtained from soybean protein [ 70 ]. Other studies reported the use of membrane UF and diafiltration in size-based separation and purification of pea protein and peptides from enzymatic digest. [ 96 , 102 ]. Hansen et al. [ 105 ] prepared salt-extracted PPI by coupling UF and diafiltration with mild solubilization of the protein at pH 7.5. The results revealed that protein content, yield, and functional properties of the laboratory-prepared PPI were enhanced when compared with a commercial brand and the method had the potential to be scaled up [ 105 ]. Additionally, Amat et al. [ 101 ] used UF technology to investigate interactions and complex formations between phytic acid, calcium, and pea protein fractions which could impair digestibility and bioavailability.
Limitations to UF technology are membrane fouling and concentration polarization, which decrease permeate flux [ 91 ]. Fouling reduces the efficiency of the process and reduces protein yield, and the remedy is the selection of appropriate membranes for protein separation [ 106 ]. Although the review shows that not much has been done with the UF technology in the processing of pea protein, it is clear from the few studies reported in the literature that UF is a non-invasive, easy-to-use, and green technology with proven results in protein processing.
3.2.3. Micellar Precipitation
Micellar precipitation (MP) is a mild extraction method that produces proteins with a high native structure content. In this method, proteins are extracted in a salt solution at a neutral pH, and the insoluble materials are separated using centrifugation. Subsequently, the proteins are recovered through precipitation and the formation of micelles by the addition of cold water to the high-salt protein extract at different ratios. Micelles form in water as nanosized aggregates where the polar heads orient with the outer environment and the hydrophobic moieties are within the core. Another variation of this method is the reverse micellar precipitation which forms nanostructured aggregates of surfactant molecules in a non-polar environment containing water at the core of the structure. A thorough review of the process and applications of this method was carried out by Sánchez-Velázquez et al. [ 107 ] and Mondor and Hernandez-alvarez [ 108 ].
Although the MP technique has been reported as an efficient extraction method for proteins, the literature has scanty information about its use for pea protein extraction. A study by Yang et al. [ 70 ] showed that MP extraction favored the extraction of pea globulins as the albumins were lost in the supernatant. The same authors showed that MP-extracted PPI had high fluorescence intensity (unfolded), high protein content, and high surface charge when compared with PPI produced using other extraction methods. The high surface charge was attributed to the low albumin content of the MP isolates as albumins have higher isoelectric points. Also, the MP isolates formed gels with high mechanical strength (compressive stress = 80 kPa) comparable to that of gels from soybean protein isolate. Although the surface charge of the MP isolates was high, these isolates exhibited relatively low solubility (67%), which resulted from partial precipitation of proteins during centrifugation. However, an earlier study by Stone et al. [ 23 ] compared the functionalities of pea proteins from three cultivars extracted using AE-IP, MP, and salt extraction (SE). The result showed that the MP isolates for the three cultivars were low in solubility (43–49%), protein yield (31%), and surface hydrophobicity (14–16 arbitrary units). However, there was no statistically significant difference between the surface charge (−21 mV at pH 7) for all cultivars and extraction methods. Similarly, Tanger et al. [ 109 ] reported low protein yield with PPI extracted using the MP technique, which is due to high losses (28–40%) at the initial solubilization stage and others (23–36%) during the precipitation step when compared with AE-IP and SE. The authors further suggested that MP protein extracts had a high legumin/vicilin ratio and high native structure content compared to SE and AE-IP, which was attributed to the observed high denaturation temperature and enthalpy changes, respectively.
3.2.4. Salt Extraction—Dialysis
The salt extraction method employs the basic salt-in (solubilization) and salt-out (concentration) principles of proteins [ 110 ]. The concentration step of salting out could be replaced with dialysis or membrane ultrafiltration. Yang et al. [ 70 ] compared the gelling properties of pea protein extracted using AE-IP with or without membrane UF, SE coupled with dialysis (SD) or membrane UF (SU), and MP. The results showed that protein contents of the SD and SU were not significantly different (~86%), but at pH 7, SD had higher surface hydrophobicity (732.19) than SU (594.91), which in turn had a higher surface charge (−23.47 mV). Consequently, SU had higher solubility (87%) at pH 7 than SD (63%) and the other extraction methods. Understandably, the isolates from SD and SU emitted higher fluorescence, which signifies a more compact conformation than the isolates prepared by AE-IP. SD produced particulate and weaker gels from fewer junction zones due to low legumin content while SU formed highly interconnected polymer-like gels facilitated by the presence of disulfide linkages. Another study reported that SE is the only method when compared with AE-IP and MP that retains both globulins and albumins after extraction as this method has no precipitation step [ 109 ]. The authors reported a relatively high yield (40%) but very low enthalpy change at pH 7 with (10%) or without salt, which signifies an unordered structure resulting from solubilization at pH 11.6. The optimal solubilization conditions suggested by these authors to maintain high native structure content with SE were pH 8 as reported by Stone et al. [ 23 ] or the use of a more neutral salt as reported by Sun and Arntfield [ 111 ].
3.2.5. Water Extraction
Water extraction is a mild fractionation method that uses water with or without the adjustment of pH. This technology has the potential to produce proteins with high native structure content, but like all the methods discussed so far, there are limitations, which include low protein yield and recovery, low purity, and high water consumption. Geert et al. [ 112 ] showed that the use of less refined pea protein fractions may be an advantage because the presence of other compounds could enhance some functionalities like emulsion formation and stability, in addition to reduced consumption of water and energy as observed with other wet fractionation methods. Similarly, another study by Moller et al. [ 76 ] showed that multiple washing steps with water alone efficiently separate the proteins from the starch. Furthermore, the authors reported a higher protein purity of 75% after the ultrafiltration of the soluble proteins, and the recovered water was recirculated. This technique is not very popular but is worth studying because of the potential of sustainably producing protein ingredients with high functionality.
3.2.6. Enzyme-Assisted Extraction (EAE) Method
Enzyme-assisted methods are green and environmentally friendly techniques [ 99 , 113 ]. The degrading enzymes act on major components such as cell walls (cellulose, pectin, and hemicellulose) to release protein bodies and break down proteins into smaller molecular sizes for improved solubility and ease of fractionation [ 114 , 115 ]. Protease activities will reduce the chances of protein denaturation and prevent the formation of complexes between the released proteins and other cellular components [ 115 ]. For example, proteases working under alkaline conditions have an optimum pH of 8–10 and a temperature of 45–60 °C [ 116 ]. EAE is shown to be a beneficial recovery technique for plant proteins and has more advantages than the conventional methods because the products formed have high purity and low production of toxic residues [ 117 ]. This method has been widely used in protein fractionation from different plant sources using either single or multiple enzymes. The drawbacks of this technology are that it is time-consuming and difficult to scale up and has high operational costs, high energy consumption, irreversible matrix alteration, and stringent protocols to maintain optimum conditions for the enzymes. However, scaling up enzymatic methods is very promising for the industry, and although it is difficult, it is also possible [ 118 ].
3.3. Scaling Up of Laboratory Extraction of Pea Protein Isolates to Industrial Scale
Different extraction and processing techniques have been used at the laboratory scale where process controls are relatively easy to manipulate. Scaling up these techniques requires high optimization of the processes at larger scales to maintain proper exposure to processing conditions and the purity, yield, and quality of the end product. Although scale-up is essential for industrial production, the information in the literature about pilot plants and industrial-scale pea protein extraction methods is very scanty. However, a few recent studies have reported some progress in the scaling up of pea protein extraction methods. Hansen et al. [ 105 ] determined the scalability of mild AE-IP or salt extraction coupled with ultrafiltration from the bench scale to the pilot plant scale and evaluated the effect of scaling on the functional properties of PPI. The authors reported some unavoidable differences in the extraction processes such as varying separation powers of the centrifuges and varying parameters of the ultrafiltration process. Furthermore, the process spanned two days, and precautions had to be taken to prevent microbial growth. Schmidt et al. [ 119 ] showed that upscaling of protein extraction from a laboratory centrifuge to a pilot plant decanter centrifuge was feasible, and the protein yield increased. Overall, the future of the innovations and methods employed in the extraction of plant protein depends on the ability of the researchers to scale up to optimized industrial-scale production levels.
4. Functional Properties of Pea Proteins
The functional properties of pea proteins are the qualities exploited for food formulation and processing, and these properties are closely related to the physicochemical properties of the protein. To effectively use pea protein in food applications, some form of pretreatment is required to improve flexibility, surface properties, digestibility, and flavor attributes. These methods can be classified as chemical (e.g., glycation, conjugation, phosphorylation), physical (e.g., thermal, micro-fluidization, sonication, high-pressure homogenization, atmospheric cold plasma, hydrodynamic cavitation), and biological (e.g., enzymatic hydrolysis, fermentation, germination). In this section, the common functional properties of pea protein will be discussed along with conventional or cutting-edge technologies that have been employed in the modulation of the physicochemical properties and, consequentially, the functionalities. Also, we will discuss some novel processing methods already in use in soybean protein processing as potential techniques in pea protein processing.
4.1. Solubility
Solubility is a measure of protein–solvent and protein–protein interactions and is largely dependent on a combination of factors such as the surface properties (charge and hydrophobicity) and non-covalent interactions of the protein. Karaca et al. [ 120 ] reported a correlation between the surface charge and solubility of native pea protein isolates; however, no relationship was established between the solubility and surface charge of commercial pea protein, probably due to the presence of aggregates [ 121 ]. Pea protein exhibits a pH-dependent solubility pattern, which is based on its amphiphilicity whereby solubility is higher below and above the isoelectric point [ 122 ]. This is because carboxylic groups of the protein are protonated at acidic pH and deprotonated at alkaline pH. At high pH, a negative surface charge on the protein facilitates the presence of electrostatic repulsive forces, and optimal unfolding of the structure is obtained [ 121 ]. Other functional properties of protein hinge on solubility for ease of homogeneity, flexibility, and mobility. Macej et al. [ 4 ] reported a direct relationship between the solubility of six pea genotypes and the emulsification activity index and indicated that the solubility of laboratory-prepared pea protein was better than that of commercial brands. This variance comes from the denaturation and formation of protein aggregates through some extraction processes and/or during heat-dependent spray drying [ 121 ]. The native structure of pea protein is globular and compact with less flexibility, and the net surface charge density is low as some ionizable (charged) groups are hidden in the core of the structure. High contents of the α-helical structure in proteins improve flexibility and solubility, as seen in animal proteins [ 123 ]. The secondary structure of pulse proteins consists largely of β-sheets, β-strands, and β-turns and has only a relatively small proportion of α-helical structures [ 124 ]. To improve solubility, the flexibility of the structure must be enhanced to expose the hidden groups [ 122 ]. Like most plant proteins, the solubility of native pea proteins at neutral pH is low (~20%) when compared with animal proteins, e.g., native β-lactoglobulin with >80% solubility at pH 7.0 [ 125 ].
Another factor that affects the solubility of pea protein is variations in the ratio of the different subunits. Going by Osborne’s classification, globulins are weak-salt-soluble, albumins are water-soluble, prolamins are alcohol-soluble, and glutelins are acid-soluble. Vicilin proteins are more soluble than legumins because they have a low molecular weight (LMW), are glycosylated, and contain no disulfide linkages (increased flexibility), while the α and β subunits of the legumin proteins are linked together by disulfide linkage, which contributes to structural rigidity [ 28 , 126 ]. Conversely, Liang and Tang [ 127 ] reported that legumin exhibits better solubility at pH 5 than vicilin. Laboratory-produced native and modified pea proteins have been shown to possess improved solubility of up to >80% (>pH 7.0). Another study showed that the L/V ratio in pea protein could affect solubility because vicilin is glycosylated, more hydrophilic, and contains higher amounts of charged amino acids like aspartic and glutamic acids [ 18 ].
A study was carried out to bridge the functionality gap between commercial and laboratory-prepared pea protein isolates by the treatment of the commercial protein with high-pressure homogenization (HPH) at 205 and 500 psi [ 121 ]. The results showed a significant increase in the solubility of the commercial pea protein powders after HPH treatment but impaired solubility for the laboratory equivalent, which had relatively high solubility before the treatment [ 121 ]. The solubility loss could be because of the exposure of hydrophobic groups, which may have induced protein–protein interactions. On the contrary, treatment of a commercial pea protein isolate (5%, w / v ) with high pressure at 600 MPa for 5 min and heat treatment at 95 °C for 15 min was reported to impair its solubility [ 128 ]. Phosphorylation modification of PPI improved solubility by 171.21% as the hydration properties improved and the hydration layer was enlarged by the addition of polar phosphate groups. A combination of HPH and ultrasound-assisted Maillard reaction improved the solubility of pea protein by 80–98% at pH below and above the isoelectric point due to increased steric repulsion between protein molecules with attached carbohydrates on the surface [ 129 ]. A >50 kDa pea protein aggregate fraction obtained by heat treatment at pH 3 coupled with membrane ultrafiltration had better solubility at pH 3–9 than the native proteins [ 96 ]. This was because of enhanced protein and water interaction after the treatment when compared with the native protein. Another hybrid technique, which combined pH shifting (pH 7.0–12.0) with ultrasound and heating to modulate the structure of pea protein, led to an increase in solubility from 30% to 90% [ 130 ].
4.2. Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
The WHC and OHC represent the total amounts of water and oil, respectively, that 1 g of a protein powder can absorb without expulsion. These properties are related to the texture, mouthfeel, and flavor retention of products and are based on the interactions of the protein with water or oil and other solutes [ 124 , 131 ]. WHC is a vital prerequisite functionality for the use of proteins in food applications such as meats and bread and may not have a direct relationship with solubility but with gelation [ 7 , 132 , 133 ]. This relationship was seen with an increase in WHC and gel strength when the oil weight fractions of the PPI-stabilized emulsion gels prepared at 37 °C for 6 h increased [ 134 ]. The WHC and OHC of pea proteins are reported to be comparable with those of soybean protein but superior to those of kidney bean protein, which makes PPI a suitable ingredient in the processing of products that require hydration and shortening [ 134 ].
Treatments to improve WHC and OHC of pea protein aim at structural modulation of the protein to expose the hydrophilic and hydrophobic groups, respectively. High energy media mill (HEMM)-treated pea dietary fiber (PDF) improved the WHC (37%) and OHC (123%) of a pea protein beverage because the treatment increased viscosity and steric properties [ 135 ]. High-intensity ultrasound treatment of pea protein powder (amplitude 0–100%) improved OHC (approx. 56%) as the intensity increased with a concomitant decrease in WHC (approx. 38%) [ 136 ]. Infrared heating (120 or 140 °C) of pea seeds and tempering to 20 or 30% moisture content before milling improved the WHC and OHC. Another study reported that ethanol washing of PPC improved the WHC by approx. 54% while the OHC decreased by approx. 34% as a result of reduced non-polar group content [ 137 ]. Furthermore, solid-phase and submerged fermentation of pea-protein-enriched flour using Aspergillus oryzae, Rhizopus oryzae, Rhizopus oligosporus, Lactobacillus plantarum, and Bacillus subtilis strains significantly improved both WHC and OHC due to the optimal exposure of polar and non-polar groups [ 138 ]. Additionally, pea protein blended with other flours was shown to improve WHC or OHC. Another blend between PPI and brown rice protein isolate (ratio 4:6) crosslinked with microbial glutaminase (1 U/g) improved the WHC by 91.6% while the OHC was reduced by approx. 39.3% [ 139 ]. This is because crosslinking at optimal substrate concentration produced a continuous network structure that could trap water [ 139 ]. However, enzymatic treatment and crosslinking of blended pea protein (with hemp 1:1, rice 3:2, and oat protein 1:1) reduced WHC, and increased OHC was observed in a few combinations [ 140 ]. Another study incorporated oat β-glucan as a fat substitute in a 1% pea protein yogurt and improved the WHC by 6% as a result of the dense network structures formed by the polysaccharide [ 141 ].
4.3. Foaming Capacity and Stability
Foams are two-phase dispersion systems of air cells separated by a thin continuous liquid layer, the lamella [ 142 ]. Foaming capacity is the ability of a substance under certain conditions (pH, ionic strength, and temperature) to quickly form a film around air bubbles in a food system (e.g., whipped cream, ice cream, and meringue), and foam stability could be evaluated as the volume of foam and liquid drainage that occurs over a fixed period [ 23 , 143 ]. The interest in foam properties is driven by the sensory pleasure derived from foamed products (e.g., the feel of ice cream or meringue kisses), and the sensory property of the product is dependent on the size distribution of the air bubbles within the food system. Foam systems with smaller and evenly distributed air bubbles produce food products with more appealing sensory properties. The capability of a protein to facilitate foaming is directly dependent on performance at the water/air interface [ 144 ]. Factors that influence foam properties include flexibility, film formation, dispersibility, and solubility [ 145 , 146 , 147 ]. Pea albumin 1 (PA1) has a foaming ability superior to that of pea protein concentrate (PPC) and globulins because of the ability to form air bubbles at least four times smaller than globulins, which was observed as high foam overrun (258%) and stability after 272 min [ 33 ]. Lower foam overruns (<81%) and stability (<70 min) were produced by the PPC and the globulin fractions due to larger size and higher surface charge when compared to the PA1 [ 33 ]. The results may be attributed to the higher surface activity of PA1 in the first 10 s, which facilitates the formation of a stiffer interfacial layer, in addition to the formation and retention/stability of higher foam levels than the PPC and globulin fraction [ 33 ].
The processing environment and pretreatment techniques influence the foam properties of proteins. For example, treatment of PPI at 90–100 °C and pH 5.0 (isoelectric point) significantly reduced the foaming ability as both conditions reduced surface properties and increased electrostatic attractive forces [ 148 ]. The unfolded structure of proteins could optimize foam properties because of the exposure of hydrophobic groups to the surface. The use of high-pressure supercritical CO2 treatment improved foam stability by unfolding the structure and creating affinity between CO2 and the hydrophobic moieties to improve the surface properties [ 149 ]. However, Lam et al. [ 18 ] showed that foaming capacity is not dependent on the intrinsic properties of the protein (e.g., surface properties and L/V ratio) but on structure and conformation (e.g., flexibility), which allow for quick adsorption at the lamella. Variations in foam properties were observed for protein isolates derived from different cultivars and extraction methods by Stone et al. [ 23 ] with results that showed better foaming capacity for salt extraction (SE) isolates and better foam stability for AE-IEP isolates. This observation may be because SE isolates were able to unfold, quickly adsorb to the air/water interface, and reduce the surface tension while AE-IEP proteins produced stable foams indicating strong interfacial films were formed by the adsorbed proteins. Similarly, PPC from different cultivars obtained by air classification had a wide range of foaming capacity (208–455%), which was positively correlated with the protein content [ 73 ].
Chang et al. [ 150 ] treated pea vicilin by pH shifting, controlled heating, and high-intensity ultrasound or a combination of methods to determine their effects on functional properties. The results showed that pH shifting and controlled heating at 80 ° C for 30 min improved the foam capacity by approx. 105% when compared to the untreated pea vicilin (73.53%) while foam stability was not different from the control. During controlled heating, soluble aggregates could be formed, β-sheets were converted to α-helices, and the surface properties (i.e., surface hydrophobicity) were enhanced [ 150 ]. Similarly, Asen and Aluko [ 96 ] reported improved foam capacity and stability (>10 and >7%, respectively) for soluble pea protein aggregates prepared at a controlled temperature (100 ° C for 30 min) and at different pH values coupled with membrane UF (>50 kDa MWCO), especially at pH 3.0, 7.0, and 9.0. Another study showed that the addition of tea saponin to PPI (50 mg/mL) improved foam capacity (210%) when compared with PPI alone (113%), while stability was improved at a lower protein concentration (10 mg/mL) [ 151 ]. A high protein concentration would facilitate optimal adsorption at the air–water interface to form a thicker and larger surface area, but foam stability was favored by a low protein concentration due to cell coarsening and coalescence [ 151 ]. The combined surface activity of 0.4% saponin–PPI complexes at the air–water interface enhanced foam capacity (263.33%) [ 151 ]. Also, Shen et al. [ 152 ] showed that the addition of TWEEN 20 to PPI improved foam capacity via protein displacement by the more effective nonionic surfactant at the interface, and foam stability was achieved by network formation on the protein film.
4.4. Emulsification Properties
Emulsification properties are the most widely studied functional properties of pea proteins, and the reason is that oil-in-water (O/W) emulsions are common in several food applications (e.g., milk, yogurt, soups). Emulsifiers are required to reduce the surface tension at the oil–water interface to stabilize the emulsions, and pea proteins have been identified as potential natural emulsifiers because of their physicochemical properties. Research has shown that commercial pea proteins have limited use in emulsification as functionality is reduced due to greater denaturation and protein aggregation resulting from harsh processing conditions (e.g., AE-IEP, hot air oven, and spray drying); hence, pretreatment is required [ 121 , 153 ]. Pretreatment of native proteins is required because of the compact and globular conformation that prevents the optimal encapsulation of oil droplets. In previous reports, cultivar and extraction methods have been reported to play a role in determining the emulsification functions of proteins [ 154 ]. Intrinsic (molar mass, hydrophobicity/hydrophilicity ratio, charge, and conformational stability) and extrinsic factors (pH, ionic strength, and temperature) play an influential role in the determination of the emulsifying properties of proteins [ 155 ]. Unlike foam properties, the emulsification properties of proteins have been shown to be favored by the surface charge because flocculation can occur when the net charge around the oil droplets and the electrostatic repulsive force are reduced [ 16 ]. Monomodal distribution and small oil droplet sizes are some indices of a good emulsifier, and the stability of an emulsion is achieved by the formation of a thick viscoelastic film at the interface and the presence of steric hindrance and electrostatic interactions [ 156 ]. The larger size and higher net charge of PPC and the globulin fraction facilitated the formation of stable emulsion droplets, while albumin-stabilized emulsions were only stable at higher protein concentrations [ 33 ].
The literature is replete with studies on different pretreatment methods used to modulate the emulsification properties of pea protein. Just to mention a few, a study showed that grinding PPI to powder for 10–20 min significantly reduced the particle size of the droplets, increased the surface charge, and improved emulsion stability [ 157 ]. Combining pH shifting with ultrasound and heating led to improvements in the solubility and surface hydrophobicity of PPI by 33 and 30%, respectively, and a subsequent improvement in emulsion stability [ 130 ]. Heat treatment of PPI at 95 ° C for 30 min also improved emulsification properties compared to those of the unheated pea protein, and higher proportions of vicilin and the basic subunit of legumin became adsorbed to the oil–water interfacial layer of the emulsions [ 158 ]. In addition, ultrasonic drying at 30 ° C produced PPI with smaller protein aggregates and enhanced solubility and emulsification properties compared to those of the continuous sheet-like morphology formed by conventional hot air drying at 60 ° C [ 153 ]. High-intensity ultrasound treatment of water-soluble pea protein fractions at 200, 300, and 500 W for 5, 10, and 20 min changed the secondary and tertiary structures and improved solubility and foam stability but impaired the emulsification properties [ 159 ]. The interfacial and emulsification properties of pea proteins were enhanced by high-intensity ultrasound treatment (57–60 W.cm−2 for 5 min) at 50% amplitude [ 160 ]. PPI–κ-carrageenan-complex-stabilized emulsions exhibited enhanced emulsion activity and stability, which was influenced by the hydrophilic groups from the κ-carrageenan [ 161 ]. Likewise, emulsion activity indices of PPC and PPI extracted from roasted pea seed (150 ° C for 10–20 min) were enhanced at pH 7.0 due to improved solubility [ 162 ].
4.5. Gelation Properties
Gels are formed when large molecules crosslink to form a 3D structure that is an intermediate between a solid and a liquid; these structural changes can be induced by heat, chemical, and enzymatic treatments. The ability of proteins to form gels is evaluated as the least gelation concentration (LGC), which is the lowest protein concentration required to form a self-supporting gel; the lower the LGC, the better the gelation ability of the protein. During gelation, various steps occur, including denaturation, aggregation, and formation of a protein network. Functional groups such as the sulfhydryl are exposed when the structure is unfolded, and irreversible aggregates are formed through disulfide bridge, hydrogen bond, hydrophobic, and/or van der Waals interactions [ 132 ]. The formed gels become self-supporting at a sufficient protein concentration, which is dependent on the protein source. Protein gels modify the texture of foods (e.g., seafood and meat replacements), and pea protein gels have attracted attention as an alternative to soybean protein, but the weaker gelling properties of soybean proteins have limited applications in food formulations [ 163 , 164 ].
Gelation is influenced by factors that include the extraction method; relative ratio of protein subunits; solubility; protein content; and other cellular materials like carbohydrates, lipids, and fiber [ 34 , 70 , 165 ]. The vicilin fractions of pea protein have been shown to possess better gelation ability than the legumin fractions, and PPI with a higher vicilin proportion formed better gels [ 164 , 166 ]. Legumin subunits under heat treatment (90 ° C) will denature and form large insoluble aggregates through disulfide bonds, while vicilin forms smaller aggregates stabilized by non-covalent interactions [ 70 ]. Pea protein gels could be described as firm and flexible for meat analogs or weak gels for semi-solid foods like tofu and yogurt [ 70 , 164 ], and the optimal pH for gelation of pea protein has been reported to be around neutral [ 34 , 120 , 166 ]. Commercial PPI has been reported to produce weak gels and higher (20–23%) LGC [ 34 , 164 ]. However, various types of processing could improve the quality of gels prepared from pea proteins. Transparent and thermo-reversible pea protein gels like gelatin were prepared by the ammonium sulfate precipitation method at pH 2.4–4.2, 10–15% protein concentration, and a compressive stress of ~6.32 kPa, which formed gels dominated by hydrogen bonds but no disulfide bonds and hydrophobic interactions [ 98 ]. The rheological and structural properties of heat-induced pea protein gels were enhanced by pH-shifting treatment where the protein solution was prepared in a buffer at pH 7.4, adjusted to pH 12, and then reverted to pH 7 followed by heating at 92 ° C for 1–2 h to form gels [ 98 ]. A uniform polymer-like gel network microstructure was obtained by this method because the treatment enabled the optimal unfolding of the protein structure and exposure of reactive groups to facilitate intermolecular interactions [ 98 ].
An earlier work by Sun and Arntfield [ 111 ] reported better gel quality and lower LGC of native PPI (14.5%) after the addition of 0.3 M NaCl, which reduced electrostatic repulsive forces and increased intermolecular interactions between the protein molecules with subsequent formation of a network. Enzymatic treatment (microbial transglutaminase) of gels containing 20–23% pea protein improved the structural quality, producing firmer and more flexible gels suitable for formulating meats and seafood [ 164 ]. A study of the effects of different extraction methods on pea protein gel properties showed that PPI extracted by micellar precipitation or UF of alkaline- or salt-extracted isolates formed gels with good compressive strength (60–80 kPa) because of optimal unfolding and the formation of strong protein–protein interactions [ 70 ]. The physical properties of meat analogs prepared by high-moisture extrusion (50 g/100g) of pea protein were enhanced by the addition of Haematococcus pluvialis residue (HPR) at 10–40 g/100 g; HPR gave the extrudate a reddish meat color and a loosened layered fibrous structure [ 167 ]. Another study improved the properties of pea protein aggregates prepared at ≥90 ° C by the addition of κ-carrageenan (0.5%) and a low protein concentration (7.5%) [ 163 ]. The surface hydrophobicity of the aggregates was modulated, which increased the capacity of the proteins to act as building blocks to form a three-dimensional network and subsequently produce gels with superior mechanical strength, while the untreated protein could not form gels [ 163 ]. Additionally, treatment of PPI with novel cold atmospheric plasma sources produced soluble protein aggregates through disulfide linkages and increased the surface hydrophobicity and β-sheets, which resulted in the formation of a strong 3D gel network [ 168 ].
4.6. Digestibility of Pea Protein
The nutritional quality of a protein is defined by the FAO/WHO based on the amino acid content and in vivo bioassay digestibility (FAO/WHO/UNU 2007). The FAO/WHO proposed the description of the nutritional quality of protein using digestibility based on individual amino acids (digestible indispensable amino acid score, DIAAS) rather than digestion based on the whole amino acid composition (protein-digestibility-corrected amino acid score, PDCAAS) [ 169 ]. A factor responsible for the underutilization of pea protein is its low digestibility when compared to animal protein. A study compared the DIAAS of four dairy proteins and four plant proteins (pea protein concentrate, soybean protein isolate, soya flour, and whole grain wheat) in pigs [ 44 ]. The DIAAS values were calculated as recommended for PDCAAS, and the results showed greater PDCAAS-like values for the dairy proteins than for the plant proteins. Another study compared the nutritional quality of milk casein and pea protein isolate in 15 healthy humans, and the results showed significantly lower digestibility of pea leucine, valine, lysine, and phenylalanine [ 170 ]. However, the results showed that although pea protein had less DIAAS than milk casein (1.0 and 1.45, respectively), pea protein demonstrated the ability to meet all amino acid requirements. Also, the real ileal digestibility and net postprandial protein utilization (NPPU) of pea protein were not different from those of milk casein [ 170 ].
The presence of naturally occurring antinutritional materials such as antigenic proteins, protease inhibitors, α-amylase inhibitors, lectins, alkaloids, saponins, and tannins would reduce the digestibility of pea proteins and nutrient availability in the gut [ 171 ]. Other contributing factors could occur during heat or alkaline processing. Dehulling, soaking, germination, conventional/microwave cooking (e.g., boiling, roasting, or frying), and fermentation are methods commonly used to improve the palatability, digestibility, and bioavailability of pea protein by inactivation of the antinutritional materials. A comprehensive review of the effect of processing conditions and fractionation on pea protein and other pulses was conducted by Rivera del Rio et al. [ 172 ].
Another study evaluated the effect of different processing treatments on the antinutritional materials and digestibility of the same cultivar of yellow field peas grown around four different locations in Saskatchewan [ 173 ]. The result showed a significant reduction in the activity of trypsin inhibitors and tannin and increased digestibility in peas that were processed by conventional and microwave cooking and in germinated and roasted pea seeds. This is because tannins are highly labile and soluble and will leach out or degrade easily during processing. Also, completely dehulled seeds had lower tannin content (1.56 mg Ecat/g) than the native pea seeds (2.37 mg Ecat/g) because tannin content is predominant in the seed coat. However, dehulled seeds had a lower reduction in the activity of trypsin inhibitors compared with seeds processed by heat treatment and germination. Furthermore, fermentation has been used to reduce the presence of antinutritional materials in isolated pea protein. A study showed that 11 h fermentation of pea protein concentrate using Lactobacillus plantarum reduced the activity of protease inhibitors, but digestibility was reduced at the 11th hour from 67.0% to 54.6% due to a reduced score of sulfur amino acids [ 174 ]. On the contrary, Skalickova et al. [ 175 ] fermented pea flour for 72 h at 37 ° C, and the result showed that digestibility was enhanced and there were improved levels of glutamine, cysteine, and methionine. Limiting the SCAA level in pea protein will influence digestibility, which is another reason for the lower digestibility in plant proteins than in animal proteins [ 10 ], and digestibility could further be reduced during fermentation [ 174 ]. The solution to maintaining the content of SCAAs in pea protein during fermentation will be the use of bacteria that have less impact on SCAAs. Improved digestibility was also shown to increase the bioaccessibility of manganese and iron [ 175 ].
4.7. Functional Gap between Laboratory-Prepared Pea Protein Isolates and Commercial Brands
The food industry is one of the highest consumers of water and energy, and for this sector to remain in business and maintain sustainable growth, the use of processing techniques that offer increased efficiency and reduced water and energy consumption must be employed [ 176 ]. However, most of the processing methods that fit this description are carried out at the bench scale, as discussed in the previous sections. For example, the most common protein extraction method and drying technique at the industrial scale are AE-IP and spray drying, respectively. However, studies have shown a functional divide between laboratory-prepared pea protein isolates and commercial brands. Burger et al. [ 121 ] compared the physicochemical properties of spray-dried pea protein isolates, and the result showed low solubility, high surface hydrophobicity, and high aggregate formation in four out of the five protein samples. AE-IP is a relatively cheap and easy method of extraction when compared with other methods because of the favorable outcome of high protein purity and yield. However, the limitations of this method include the loss of some functionalities due to denaturation during the solubilization step at pH 8–11 [ 177 ]. The authors employed high-pressure homogenization to break up the aggregates which reduced surface hydrophobicity and solubility. Other authors have reported the use of heat pretreatment coupled with membrane ultrafiltration to improve the physicochemical and functional properties of commercial pea protein isolates [ 96 ]. High temperatures used in spray drying have been shown to alter the spatial conformations of the protein and impair functionalities; these effects are not seen in proteins prepared by freeze drying [ 178 ]. Bridging the divide between commercial and laboratory-prepared pea protein isolates or concentrates would require scaling up the lab protocols/methods and/or carrying out pretreatment steps to improve the spatial conformations of the protein.
4.8. Potential Processing Technologies Not Yet Applied to Pea Protein
Our discourse so far has been based on processing technologies that have been applied in the extraction and structural modulation of pea protein. Under chemical treatments, techniques like glycation, acylation, phosphorylation, and pH shifting have been widely used in pea protein processing. Other common methods are the biological class (i.e., fermentation, enzymatic hydrolysis, and germination) and physical class (i.e., thermal, ultrasound, extrusion, ultrafiltration, cold plasma, high-pressure treatment, and irradiation). However, our search reveals some physical methods (i.e., hydrodynamic cavitation and cavitation jet technology) that have been used in the processing of soybean protein but have not been applied to pea protein processing. A study showed that the application of hydrodynamic cavitation (HC at 550 W for 0, 15, 30 min) to soybean glycinin aggregates dissociated the aggregates and enhanced the physicochemical and functional properties of the protein [ 179 ]. Another study showed that cavitation jet technology improved the solubility of protein in soymilk flour [ 180 ].
This literature review indicates a notable gap in research related to the utilization of pressure-assisted protein processing technologies to augment yield and functionality. A promising avenue for future investigation is the exploration of the impacts of high pressure (HP) and abrupt pressure changes in hydrodynamic cavitation (HC) on the yield and quality of pea protein, as both methodologies are harmonious with the wet fractionation technique. Additionally, HC technology can offer controlled heating, which is crucial for the inactivation of the lipoxygenase enzyme responsible for the oxidation of polyunsaturated fatty acids and resultant off-flavors in pea proteins. Initial studies have demonstrated the positive influence of HP treatment, within 50–125 MPa, on soybean protein extraction yield, with conditions for HP attainable through high-pressure homogenizers (HPH) at pressures exceeding 35 MPa [ 181 ]. However, the limited throughput capacity of HP techniques restricts their commercial viability. Conversely, HC does not require pervasive high pressure and possesses superior throughput capacity. The localized pressure drop in the cavitation zone generates intense turbulence, shear stress, shock waves, and cell disruption, facilitating an increased yield of bioactive compounds, including proteins, when small particle raw material is dispersed in the liquid flow. Martynenko et al. [ 182 ] provided an extensive description of the principles and apparatuses related to hydrodynamic cavitation. This environmentally green approach offers several benefits such as diminished energy, extraction time, and solvent usage and yields higher-quality protein ingredients [ 183 ]. Regrettably, the capabilities of HC in pea protein extraction remain largely untapped and warrant further exploration.
5. Food Applications
Plant-based proteins like PPI are taking center stage in research and food formulations for several reasons (i.e., religious inclinations, cost, health, availability, nutritional, environmental sustainability, and functional properties). Using pea proteins as ingredients in food and beverage formulation was well captured in a review by Boukid et al. [ 184 ]. The functional properties of proteins such as solubility; water- and oil-holding capacity; and gelation, foaming, and emulsification properties are exploited for the formulation of various food products. Pea protein is reportedly the best-suited and most popular plant-based protein to replace animal proteins because of its cost efficiency and reduced adverse effects on health and the environment. One of the most utilized processing techniques in pea protein utilization is the extrusion cooking method using high moisture (HME; >40%) or low moisture (LME; <35%) to produce meat and seafood analogs or extruded puff snacks, respectively [ 185 ]. Pea protein flours, concentrates, and isolates have been widely used for the formulation of composite flours together with ingredients from cereals and other legumes or pulses to produce extruded food snacks [ 186 , 187 , 188 , 189 ]. These fortified extruded snacks exhibit superior nutritional quality (high protein and balanced amino acid content) compared to snacks extruded from cereals only [ 185 ]. Extruded snack balls made from pea flour (60–90%) and cereal flour (rice or wheat) were less appreciated for taste (based on the beany flavor imparted by pea) by consumers, especially at high pea concentration, but increased crispiness and puffiness were positive perceptions reported by consumers [ 190 ]. Crackers produced from dehulled oats and PPI (COP) showed superior textural and nutritional quality when compared with two commercial crackers [ 191 ]. For example, the protein contents of the COP and commercial crackers were 25 and 10%, respectively, and the COP crackers were chewier than the commercial brand. However, sensory analysis was not carried out to determine consumer acceptance.
Nowadays, there is a surge in the consumption of plant-protein-based meat or seafood analog products. Pea proteins can create HME fibrous-textured extrudates to mimic meat products [ 43 , 167 , 192 ]. Wheat protein has been the popular option for producing meat replacements due to the viscoelastic and rheological properties of gluten which acts as a binder and is used in composites with soybean protein isolates. Currently, gluten-free and hypoallergenic ingredients like peas, rice, oats, and other pulses are replacing the use of wheat and soybeans [ 192 , 193 ]. The incorporation of oat protein (30%) with pea protein improved the sensory characteristics of meat analogs [ 193 ], and trained panelists’ evaluation of vegetable patties showed that products from soybean protein had more favorable scores (taste and texture) than different pulse proteins, including pea protein [ 192 ]. However, no significant differences were observed in the taste and texture of veggie patties produced from pea, lentil, and fava bean proteins [ 192 ].
Research in the replacement of other protein sources with pea protein in the production of beverages is progressing and yielding positive outcomes. The application of different plant proteins (pea, soybean, rice, and almond) in fermented beverages showed that the product from pea protein exhibited the highest viscosity and coagulum strength with no syneresis [ 194 ]. Klost and Drusch [ 195 ] prepared a base formulation for plant-protein-based yogurt alternatives using 10% pea protein isolate with or without oil and fiber supplementation. However, sensory evaluation of the product was not reported. Partial replacement of milk protein by pea protein (0–40 g/100 g protein) and fermentation had no favorable outcome on sensory attributes of yogurt; an increase in the concentration of pea protein led to higher acidity, higher syneresis, and weak gels [ 196 ].
PPI has been widely used as a carrier in nanoencapsulation technology to preserve and transport labile nutrients and nutraceuticals. Akkam et al. [ 197 ] prepared pea protein nanoemulsions (10 mg/mL) with 1.0% ( w / w ) cholecalciferol/canola oil to improve the stability of vitamin D when fortified in food formulations such as milk and juices. Nanoemulsions that carried 20 μg vitamin D/mL exhibited superior water holding, foam, emulsification, and antioxidant properties when compared to the PPI prepared by ultrasound treatment [ 197 ]. The addition of the nanoemulsions and vitamin D did not alter the sensory properties of the juices and milk but enhanced the stability of vitamin D and nutritional value of the formulations, and consumer evaluation based on overall impression, aroma, consistency, and color was positive. Nanoparticles formed from a chitosan–PPI complex for the encapsulation of hyssop essential oil exhibited higher antioxidant activity than the free hyssop essential oil [ 198 ].
6. Concluding Remarks and Future Research Directions
Yellow field pea is a high-protein crop with variations in chemical composition from different cultivars, and even when the same cultivar is grown in the same environment, the harvest and storage conditions are important factors. Extensive work has been carried out on optimizing the functional properties of pea proteins for applications in the food industry. The major challenges in using pea protein as an ingredient are the intrinsic and extrinsic factors (i.e., native form, processing, and storage). These lead to discrepancies and gaps in the functional properties and flavor output of the ingredient. Notably, laboratory-prepared field pea proteins maintain most of their native functionality, while commercial pea ingredients mainly have high degrees of denaturation because of conventional extraction methods like AE-IEP and spray drying conditions. Research has shown that the existing conventional extraction methods (dry and wet fractionation) do not provide optimal results (yield, purity, and functionality) alone and may be more effective when used as a hybrid model. Dry fractionation could produce protein concentrates with preserved native properties, but their purity is low (40–50% protein content), while protein isolates obtained from wet fractionation have high purity but are denatured with impaired functionalities. The purity of dry-fractionated proteins could be improved by ~15% using electrostatic methods in combination with or as an alternative to air classification. Milder wet or aqueous fractionation methods (e.g., enzyme-assisted, ultrafiltration) have been used either as a standalone technology or in combination with the AE-IEP method to produce proteins with superior yield, purity, and functionality when compared to conventional methods. A divide exists between the functional properties of laboratory-prepared and commercially prepared pea protein products because of the harsh extraction methods used for the latter. This gap can be effectively bridged using milder technologies (hybrid or standalone) to maximize yield, purity, and protein functionalities. In addition to the choice of the appropriate extraction method, research has shown that the use of chemical, physical, and enzymatic methods to modulate the complex conformation and improve the organoleptic and nutritional properties of pea protein have great potential to produce proteins with a high quality that is suitable for industrial use and consumer acceptability. This literature review indicates a notable gap in research related to the utilization of feedstock pretreatment technologies to enhance pea protein extraction in terms of yields and functionalities. For example, cavitation technology, based on ultrasonication or hydrodynamic pressure changes in the flow stream, is widely accepted for cell disruption. It has been applied in the extraction of soybean protein and should be explored for pea protein extraction. The authors are currently working on a project to utilize hydrodynamic cavitation for the pretreatment of yellow peas for protein extraction. The impact of abrupt pressure changes produced by hydrodynamic cavitation on the yield and quality of pea protein will be explored. The economic advantages of the pretreatment technologies and hybrid methods inspire industry interest in commercial implementation.
Funding Statement
This work was supported by the Sustainable Protein Production program of the National Research Council Canada under grant funding in support of a Collaborative R&D Investment Collaborative Science, Technology, and Innovation Program (CSTIP).
Author Contributions
N.D.A.—writing, review, and editing; R.E.A.—review and editing; A.U.—project administration and conceptualization; A.M. and P.B.—project administration, funding acquisition, review, and editing. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Conflicts of interest.
Alphonsus Utioh is employed by the ACU Food Technology Services Inc. The authors certify that they have no conflict of interest to declare.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Publishing
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

IMAGES
VIDEO
COMMENTS
1. Introduction. Pisum sativum L., known as green pea, dry pea, or field pea, is an important legume crop that provides a good source of protein, vitamins, minerals, and bioactive compounds that are beneficial to human health [1,2,3].Peas are cultivated in almost all countries around the world and regarded as an essential part of the human diet []. ...
Field pea and other pulse crops are underrepresented in agricultural research, yet are important crops for a sustainable future and better food systems. Furthermore, because field pea is consumed globally by both developed and at-risk populations, research efforts could help increase global health overall and combat micronutrient malnutrition.
Field pea is a cool-season grain legume from the diverse Pisum genus, grown in over 100 countries for dry or fresh seed and fodder. The growing need for affordable, high-quality protein and the role of field pea in rotations for sustainable agriculture is driving renewed interest in this crop. ... However, field pea research in southern ...
Didymella pinodes and its management in field pea: Challenges and opportunities. T.N. Khan, ... M.J. Barbetti, in Field Crops Research, 2013 1 Introduction. Field pea (Pisum sativum L.) is a cool-season crop widely grown across cooler temperate zones of the world on about 6.2 m ha annually with total production generally ranging between 10 and 11 m tonnes (FAOSTAT, 2012) The leading pea ...
Pisum sativum L., commonly referred to as dry, green, or field pea, is one of the most common legumes that is popular and economically important. Due to its richness in a variety of nutritional and bioactive ingredients, the consumption of pea has been suggested to be associated with a wide range of health benefits, and there has been increasing focus on its potential as a functional food.
Findings: Pea protein content varies from 13% to 38% and is influenced by envi-. ronmental and genetic factors. Protein- regulating genes per se directly and genes. involved in starch biosynthesis ...
1. Introduction. Field pea (Pisum sativum L.) is one self-pollinated diploid (2n=14) annual of the most important annual cool season pulse crop and is valued as high protein food [].It is widely grown in the cooler temperate zones and in the highlands of tropical regions of the world. The crop is cultivated in a wide range of soil types from light sandy loams to heavy clays but it does not ...
Original research paper; Open access; Published: 01 February 2022; Volume 12, pages 91-106, (2022) Cite this article; Download PDF. You ... Field pea (Pisum sativum L.) and a mixture of pea and oat (Avena sativa L.) were used as cover crops in organic high tunnel vegetable production. They were manually planted at a 10-15 cm distance in pea ...
Yellow field peas (Pisum sativum L.) hold significant value for producers, researchers, and ingredient manufacturers due to their wealthy composition of protein, starch, and micronutrients. ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial ...
Six varieties of field pea ( Pisum sativum L.) were sown at two sowing rates over two years to assess the impact on disease occurrence in a Baltic climate. The varieties used were Bruno (Latvia), Capella (Sweden), Clara (Sweden), Kirke (Estonia), Onward (Greece), and Vitra (Latvia). The two sowing rates were 120 seeds per m 2 and 144 seeds per m 2.
It is generally believed that the Ethiopian field pea landraces include valuable genetic diversity based on farmers' selection for advantageous traits across highly heterogeneous landscapes (Keneni et al., 2007; Singh & Singh, 2015).Although field pea was most likely domesticated in West Asia, the diversity of field pea varieties found by Vavilov and others led them to identify Ethiopia as a ...
on growt h, yield and econo mics of field pea ( Pisum sativum. L.) and found that number of po ds/ plant, number of seeds/. pod, pod length and1000 grain weight (g) of pea increased. by co ...
Experimental design. In two-year field experiments peas and oats were sown in pure stands and in mixtures. The three mixtures were 25/75, 50/50 and 75/25% of normal seed rate (100 and 450 emerged plants per m 2 for peas and oats, respectively). The crops were grown in three replicates in randomized blocks with plot sizes of ca 20 m 2.The following year, oats in pure stands were grown on all plots.
Field pea, which is also known as common pea, dry than pea (Carranca et al., 1999; Bilalis et al., 2012). Field peas pea, yellow pea and garden pea, is a cool-season legume have the capacity to increase the availability of phosphorus for cultivated worldwide.
Dryland pea (Pisum sativum L.) is an important pulse crop that can replace fallow or be added to existing crop rotations to sustain crop yields in arid and semiarid regions.Yet, we lack management practices to enhance yield and quality of dryland pea. This study evaluated the effect of crop rotation and cultural practices on dryland pea growth, yield, and quality from 2006 to 2011 in the ...
Thirty field pea (Pisum sativum) genotypes were evaluated during rabi of 2009-10 for 12 quantitative traits to examine the nature and magnitude of variability, correlation and path coefficient ...
Kohajdova et al. (2013) [28] reported 7.92% moisture in pea flour. Total ash: The total ash content of the five improved varieties of field pea seed flour ranged from 2.86 to 3.22 per cent (mean 3.07 per cent). The total ash content was found to be maximum in Pant Pea-42 whereas minimum was found in Pant Pea-25.
Pisum sativum L., known as green pea, dry pea, or field pea, is an important legume crop that provides a good source of protein, vitamins, minerals, and bioactive compounds that are beneficial to human health [1,2,3].Peas are cultivated in almost all countries around the world and regarded as an essential part of the human diet [].Canada is the biggest producer of peas around the world ...
A meta-analytical approach of data collected for 1944 samples of soybean meal obtained from 18 published papers (2002-2018) ... Concluding Remarks and Future Research Directions . Yellow field pea is a high-protein crop with variations in chemical composition from different cultivars, and even when the same cultivar is grown in the same ...
In Ethiopia field pea is cultivated since ancient time Dawit et al., (1994) and its wild and primitive forms of the species were concealed in the highlands of Ethiopia. Due to this fact Ethiopia considered as one of the centers of diversity for field pea. Field pea grow around the world for its fresh green seeds, tender green
Abstract and Figures. Pea occupies 459 thousand hectare area in India and shares 21 percent production of the world. In Punjab, it ranks second in area among vegetable crops after potato and ...
Two experiments evaluated digestive and performance effects of field pea-based creep feed in nursing calf diets. In Exp.1, eight nursing steer calves (145 +/- 27 kg initial BW) with ruminal cannulas were used to evaluate effects of supplementation and advancing season on dietary composition, intake, digestion, and ruminal fermentation characteristics.
A field experiment was conducted Effect of phosphorus and zinc on growth and yield of field pea (Pisum sativum) during the rabi season of 2021-22 with 9 treatments (viz., P at 55, 60, 65kg/ha ...