The Nature of Light
Introduction.
Light is a transverse, electromagnetic wave that can be seen by the typical human. The wave nature of light was first illustrated through experiments on diffraction and interference . Like all electromagnetic waves, light can travel through a vacuum. The transverse nature of light can be demonstrated through polarization .
- In 1678, Christiaan Huygens (1629–1695) published Traité de la Lumiere , where he argued in favor of the wave nature of light. Huygens stated that an expanding sphere of light behaves as if each point on the wave front were a new source of radiation of the same frequency and phase.
- Thomas Young (1773–1829) and Augustin-Jean Fresnel (1788–1827) disproved Newton's corpuscular theory.
Light is produced by one of two methods…
- Incandescence is the emission of light from "hot" matter (T ≳ 800 K).
- Luminescence is the emission of light when excited electrons fall to lower energy levels (in matter that may or may not be "hot").
Just notes so far. The speed of light in a vacuum is represented by the letter c from the Latin celeritas — swiftness. Measurements of the speed of light.
Veramente non l'ho sperimentata, salvo che in lontananza piccola, cioè manco d'un miglio, dal che non ho potuto assicurarmi se veramente la comparsa del lume opposto sia instantanea; ma ben, se non instantanea, velocissima…. In fact I have tried the experiment only at a short distance, less than a mile, from which I have not been able to ascertain with certainty whether the appearance of the opposite light was instantaneous or not; but if not instantaneous it is extraordinarily rapid …. Galileo Galilei, 1638 Galileo Galilei, 1638
Ole Rømer (1644–1710) Denmark. "Démonstration touchant le mouvement de la lumière trouvé par M. Roemer de l'Académie des Sciences." Journal des Scavans . 7 December 1676. Rømer's idea was to use the transits of Jupiter's moon Io to determine the time. Not local time, which was already possible, but a "universal" time that would be the same for all observers on the Earth, Knowing the standard time would allow one to determine one's longitude on the Earth — a handy thing to know when navigating the featureless oceans.
Unfortunately, Io did not turn out to be a good clock. Rømer observed that times between eclipses got shorter as Earth approached Jupiter, and longer as Earth moved farther away. He hypothesized that this variation was due to the time it took for light to travel the lesser or greater distance, and estimated that the time for light to travel the diameter of the Earth's orbit, a distance of two astronomical units, was 22 minutes.
- The speed of light in a vacuum is a universal constant in all reference frames.
- The speed of light in a vacuum is fixed at 299,792,458 m/s by the current definition of the meter.
- The speed of light in a medium is always slower the speed of light in a vacuum.
- The speed of light depends upon the medium through which it travels.The speed of anything with mass is always less than the speed of light in a vacuum.

other characteristics
The amplitude of a light wave is related to its intensity.
- Intensity is the absolute measure of a light wave's power density.
- Brightness is the relative intensity as perceived by the average human eye.
The frequency of a light wave is related to its color.
- Color is such a complex topic that it has its own section in this book.
- Laser light is effectively monochromatic.
- There are six simple, named colors in English (and many other languages) each associated with a band of monochromatic light. In order of increasing frequency they are red, orange, yellow, green, blue, and violet .
- Light is sometimes also known as visible light to contrast it from "ultraviolet light" and "infrared light"
- Other forms of electromagnetic radiation that are not visible to humans are sometimes also known informally as "light"
- Nearly every light source is polychromatic.
- White light is polychromatic.
A graph of relative intensity vs. frequency is called a spectrum (plural: spectra ). Although frequently associated with light, the term can be applied to any wave phenomena.
- Blackbody radiators emit a continuous spectrum.
- The excited electrons in a gas emit a discrete spectrum.
The wavelength of a light wave is inversely proportional to its frequency.
- Light is often described by it's wavelength in a vacuum .
- Light ranges in wavelength from 400 nm on the violet end to 700 nm on the red end of the visible spectrum.
Phase differences between light waves can produce visible interference effects. (There are several sections in this book on interference phenomena and light.)
Leftovers about animals.
- Falcon can see a 10 cm. object from a distance of 1.5 km.
- Fly's Eye has a flicker fusion rate of 300/s. Humans have a flicker fusion rate of only 60/s in bright light and 24/s in dim light. The flicker fusion rate is the frequency with which the "flicker" of an image cannot be distinguished as an individual event. Like the frame of a movie… if you slowed it down, you would see individual frames. Speed it up and you see a constantly moving image. Octopus' eye has a flicker fusion frequency of 70/s in bright light.
- Penguin has a flat cornea that allows for clear vision underwater. Penguins can also see into the ultraviolet range of the electromagnetic spectrum.
- Sparrow Retina has 400,000 photoreceptors per square. mm.
- Reindeer can see ultraviolet wavelengths, which may help them view contrasts in their mostly white environment.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.1: The Nature of Light
- Last updated
- Save as PDF
- Page ID 111596
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Skills to Develop
- Explain the basic behavior of waves, including traveling waves and standing waves
- Describe the wave nature of light
- Use appropriate equations to calculate related light-wave properties such as period, frequency, wavelength, and energy
Video \(\PageIndex{1}\): Is light a particle or a wave? This section will explore the answers to this question.
The nature of light has been a subject of inquiry since antiquity. In the seventeenth century, Isaac Newton performed experiments with lenses and prisms and was able to demonstrate that white light consists of the individual colors of the rainbow combined together. Newton explained his optics findings in terms of a "corpuscular" view of light, in which light was composed of streams of extremely tiny particles travelling at high speeds according to Newton's laws of motion. Others in the seventeenth century, such as Christiaan Huygens , had shown that optical phenomena such as reflection and refraction could be equally well explained in terms of light as waves travelling at high speed through a medium called "luminiferous aether" that was thought to permeate all space. Early in the nineteenth century, Thomas Young demonstrated that light passing through narrow, closely spaced slits produced interference patterns that could not be explained in terms of Newtonian particles but could be easily explained in terms of waves. Later in the nineteenth century, after James Clerk Maxwell developed his theory of electromagnetic radiation and showed that light was the visible part of a vast spectrum of electromagnetic waves, the particle view of light became thoroughly discredited. By the end of the nineteenth century, scientists viewed the physical universe as roughly comprising two separate domains: matter composed of particles moving according to Newton's laws of motion, and electromagnetic radiation consisting of waves governed by Maxwell's equations. Today, these domains are referred to as classical mechanics and classical electrodynamics (or classical electromagnetism). Although there were a few physical phenomena that could not be explained within this framework, scientists at that time were so confident of the overall soundness of this framework that they viewed these aberrations as puzzling paradoxes that would ultimately be resolved somehow within this framework. As we shall see, these paradoxes led to a contemporary framework that intimately connects particles and waves at a fundamental level called wave-particle duality, which has superseded the classical view.
Visible light and other forms of electromagnetic radiation play important roles in chemistry, since they can be used to infer the energies of electrons within atoms and molecules. Much of modern technology is based on electromagnetic radiation. For example, radio waves from a mobile phone, X-rays used by dentists, the energy used to cook food in your microwave, the radiant heat from red-hot objects, and the light from your television screen are forms of electromagnetic radiation that all exhibit wavelike behavior.
Video \(\PageIndex{2}\): An exploration of light as a wave.
A wave is an oscillation or periodic movement that can transport energy from one point in space to another. Common examples of waves are all around us. Shaking the end of a rope transfers energy from your hand to the other end of the rope, dropping a pebble into a pond causes waves to ripple outward along the water's surface, and the expansion of air that accompanies a lightning strike generates sound waves (thunder) that can travel outward for several miles. In each of these cases, kinetic energy is transferred through matter (the rope, water, or air) while the matter remains essentially in place. An insightful example of a wave occurs in sports stadiums when fans in a narrow region of seats rise simultaneously and stand with their arms raised up for a few seconds before sitting down again while the fans in neighboring sections likewise stand up and sit down in sequence. While this wave can quickly encircle a large stadium in a few seconds, none of the fans actually travel with the wave-they all stay in or above their seats.
Waves need not be restricted to travel through matter. As Maxwell showed, electromagnetic waves consist of an electric field oscillating in step with a perpendicular magnetic field, both of which are perpendicular to the direction of travel. These waves can travel through a vacuum at a constant speed of 2.998 × 10 8 m/s, the speed of light (denoted by c ).
All waves, including forms of electromagnetic radiation, are characterized by, a wavelength (denoted by λ , the lowercase Greek letter lambda), a frequency (denoted by ν , the lowercase Greek letter nu), and an amplitude . As can be seen in Figure \(\PageIndex{1}\), the wavelength is the distance between two consecutive peaks or troughs in a wave (measured in meters in the SI system). Electromagnetic waves have wavelengths that fall within an enormous range-wavelengths of kilometers (10 3 m) to picometers (10 −12 m) have been observed. The frequency is the number of wave cycles that pass a specified point in space in a specified amount of time (in the SI system, this is measured in seconds). A cycle corresponds to one complete wavelength. The unit for frequency, expressed as cycles per second [s −1 ], is the hertz (Hz) . Common multiples of this unit are megahertz, (1 MHz = 1 × 10 6 Hz) and gigahertz (1 GHz = 1 × 10 9 Hz). The amplitude corresponds to the magnitude of the wave's displacement and so, in Figure, this corresponds to one-half the height between the peaks and troughs. The amplitude is related to the intensity of the wave, which for light is the brightness, and for sound is the loudness.
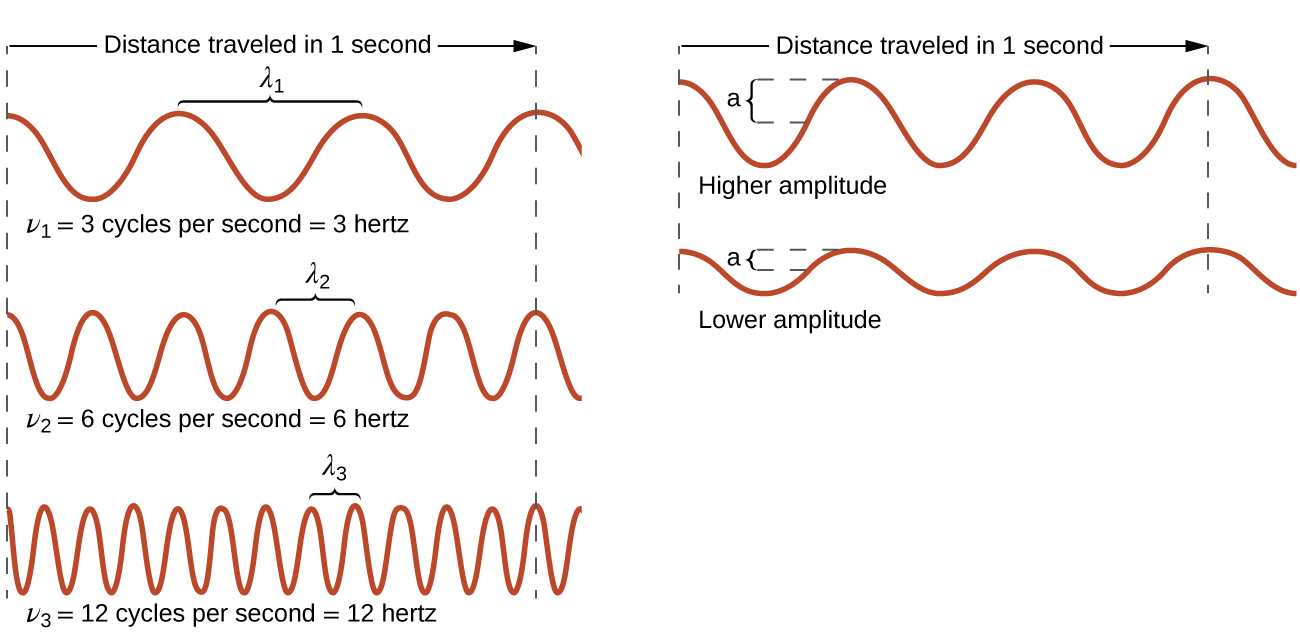
Figure \(\PageIndex{1}\): One-dimensional sinusoidal waves show the relationship among wavelength, frequency, and speed. The wave with the shortest wavelength has the highest frequency. Amplitude is one-half the height of the wave from peak to trough.
The product of a wave's wavelength ( λ ) and its frequency ( ν ), λν , is the speed of the wave. Thus, for electromagnetic radiation in a vacuum:
Wavelength and frequency are inversely proportional: As the wavelength increases, the frequency decreases. The inverse proportionality is illustrated in Figure \(\PageIndex{2}\). This figure also shows the electromagnetic spectrum , the range of all types of electromagnetic radiation. Each of the various colors of visible light has specific frequencies and wavelengths associated with them, and you can see that visible light makes up only a small portion of the electromagnetic spectrum. Because the technologies developed to work in various parts of the electromagnetic spectrum are different, for reasons of convenience and historical legacies, different units are typically used for different parts of the spectrum. For example, radio waves are usually specified as frequencies (typically in units of MHz), while the visible region is usually specified in wavelengths (typically in units of nm or angstroms).
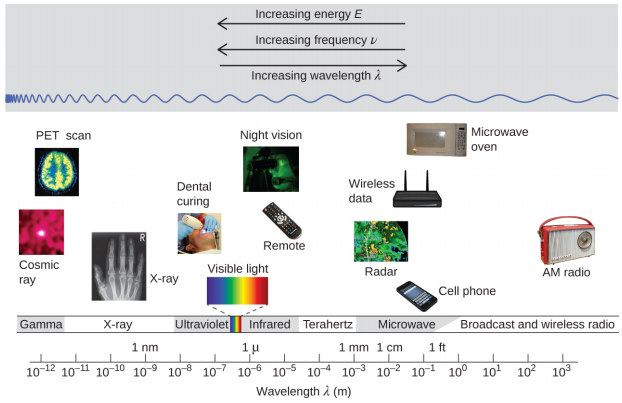
Figure \(\PageIndex{2}\): Portions of the electromagnetic spectrum are shown in order of decreasing frequency and increasing wavelength. Examples of some applications for various wavelengths include positron emission tomography (PET) scans, X-ray imaging, remote controls, wireless Internet, cellular telephones, and radios. (credit “Cosmic ray": modification of work by NASA; credit “PET scan": modification of work by the National Institute of Health; credit “X-ray": modification of work by Dr. Jochen Lengerke; credit “Dental curing": modification of work by the Department of the Navy; credit “Night vision": modification of work by the Department of the Army; credit “Remote": modification of work by Emilian Robert Vicol; credit “Cell phone": modification of work by Brett Jordan; credit “Microwave oven": modification of work by Billy Mabray; credit “Ultrasound": modification of work by Jane Whitney; credit “AM radio": modification of work by Dave Clausen)
The TeraHertz Region
Terahertz radiation is a region of the electromagnetic spectrum with frequencies of 0.3 to 3 THz (or 1 mm to 0.1 mm), and was previously defined as microwave radio waves or the far IR. Because of its seat into beteen microwaves and IR, scientists have begin refering to the region as the "TeraHertz gap" Due to the ability of several atmospheric gases to absorb energy in this region of the spectrum, it is unsuitable for radio communications, but a lot of research into uses of technology in this area of the spectrum have emerged over the last decade. To read more about it visit here or here .
Example \(\PageIndex{1}\): Determining the Frequency and Wavelength of Radiation
A sodium streetlight gives off yellow light that has a wavelength of 589 nm (1 nm = 1 × 10 −9 m). What is the frequency of this light?
We can rearrange the Equation \ref{6.2.1} to solve for the frequency:
\[\nu=\dfrac{c}{λ}\]
Since c is expressed in meters per second, we must also convert 589 nm to meters.
\[\nu=\mathrm{\left(\dfrac{2.998×10^8\:\cancel{m}s^{−1}}{589\cancel{nm}}\right)\left(\dfrac{1×10^9\cancel{nm}}{1\cancel{m}}\right)=5.09×10^{14}\,s^{−1}}\]
Exercise \(\PageIndex{1}\)
One of the frequencies used to transmit and receive cellular telephone signals in the United States is 850 MHz. What is the wavelength in meters of these radio waves?
0.353 m = 35.3 cm
Video \(\PageIndex{3}\): A summary of light's behavior as a wave.
Wireless Communication
Many valuable technologies operate in the radio (3 kHz-300 GHz) frequency region of the electromagnetic spectrum. At the low frequency (low energy, long wavelength) end of this region are AM (amplitude modulation) radio signals (540-2830 kHz) that can travel long distances. FM (frequency modulation) radio signals are used at higher frequencies (87.5-108.0 MHz). In AM radio, the information is transmitted by varying the amplitude of the wave (Figure \(\PageIndex{5}\)). In FM radio, by contrast, the amplitude is constant and the instantaneous frequency varies.

Figure \(\PageIndex{3}\): Radio and cell towers are typically used to transmit long-wavelength electromagnetic radiation. Increasingly, cell towers are designed to blend in with the landscape, as with the Tucson, Arizona, cell tower (right) disguised as a palm tree. (credit left: modification of work by Sir Mildred Pierce; credit middle: modification of work by M.O. Stevens)
Other technologies also operate in the radio-wave portion of the electromagnetic spectrum. For example, 4G cellular telephone signals are approximately 880 MHz, while Global Positioning System (GPS) signals operate at 1.228 and 1.575 GHz, local area wireless technology (Wi-Fi) networks operate at 2.4 to 5 GHz, and highway toll sensors operate at 5.8 GHz. The frequencies associated with these applications are convenient because such waves tend not to be absorbed much by common building materials.
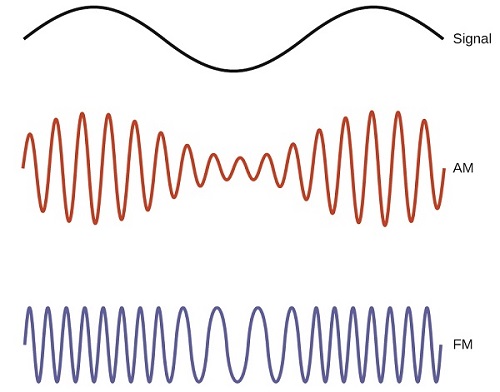
Figure \(\PageIndex{4}\): This schematic depicts how amplitude modulation (AM) and frequency modulation (FM) can be used to transmit a radio wave.
One particularly characteristic phenomenon of waves results when two or more waves come into contact: They interfere with each other. Figure \(\PageIndex{5}\) shows the interference patterns that arise when light passes through narrow slits closely spaced about a wavelength apart. The fringe patterns produced depend on the wavelength, with the fringes being more closely spaced for shorter wavelength light passing through a given set of slits. When the light passes through the two slits, each slit effectively acts as a new source, resulting in two closely spaced waves coming into contact at the detector (the camera in this case). The dark regions in Figure \(\PageIndex{5}\) correspond to regions where the peaks for the wave from one slit happen to coincide with the troughs for the wave from the other slit (destructive interference), while the brightest regions correspond to the regions where the peaks for the two waves (or their two troughs) happen to coincide (constructive interference). Likewise, when two stones are tossed close together into a pond, interference patterns are visible in the interactions between the waves produced by the stones. Such interference patterns cannot be explained by particles moving according to the laws of classical mechanics.

Figure \(\PageIndex{5}\): Interference fringe patterns are shown for light passing through two closely spaced, narrow slits. The spacing of the fringes depends on the wavelength, with the fringes being more closely spaced for the shorter-wavelength blue light. (credit: PASCO)
Dorothy Hodgkin
Because the wavelengths of X-rays (10-10,000 picometers [pm]) are comparable to the size of atoms, X-rays can be used to determine the structure of molecules. When a beam of X-rays is passed through molecules packed together in a crystal, the X-rays collide with the electrons and scatter. Constructive and destructive interference of these scattered X-rays creates a specific diffraction pattern. Calculating backward from this pattern, the positions of each of the atoms in the molecule can be determined very precisely. One of the pioneers who helped create this technology was Dorothy Crowfoot Hodgkin.
She was born in Cairo, Egypt, in 1910, where her British parents were studying archeology. Even as a young girl, she was fascinated with minerals and crystals. When she was a student at Oxford University, she began researching how X-ray crystallography could be used to determine the structure of biomolecules. She invented new techniques that allowed her and her students to determine the structures of vitamin B 12 , penicillin, and many other important molecules. Diabetes, a disease that affects 382 million people worldwide, involves the hormone insulin. Hodgkin began studying the structure of insulin in 1934, but it required several decades of advances in the field before she finally reported the structure in 1969. Understanding the structure has led to better understanding of the disease and treatment options.
Not all waves are travelling waves. Standing waves (also known as stationary waves ) remain constrained within some region of space. As we shall see, standing waves play an important role in our understanding of the electronic structure of atoms and molecules. The simplest example of a standing wave is a one-dimensional wave associated with a vibrating string that is held fixed at its two end points. Figure \(\PageIndex{6}\) shows the four lowest-energy standing waves (the fundamental wave and the lowest three harmonics) for a vibrating string at a particular amplitude. Although the string's motion lies mostly within a plane, the wave itself is considered to be one dimensional, since it lies along the length of the string. The motion of string segments in a direction perpendicular to the string length generates the waves and so the amplitude of the waves is visible as the maximum displacement of the curves seen in Figure \(\PageIndex{6}\). The key observation from the figure is that only those waves having an integer number, n, of half-wavelengths between the end points can form. A system with fixed end points such as this restricts the number and type of the possible waveforms. This is an example of quantization , in which only discrete values from a more general set of continuous values of some property are observed. Another important observation is that the harmonic waves (those waves displaying more than one-half wavelength) all have one or more points between the two end points that are not in motion. These special points are nodes . The energies of the standing waves with a given amplitude in a vibrating string increase with the number of half-wavelengths n . Since the number of nodes is n – 1, the energy can also be said to depend on the number of nodes, generally increasing as the number of nodes increases.
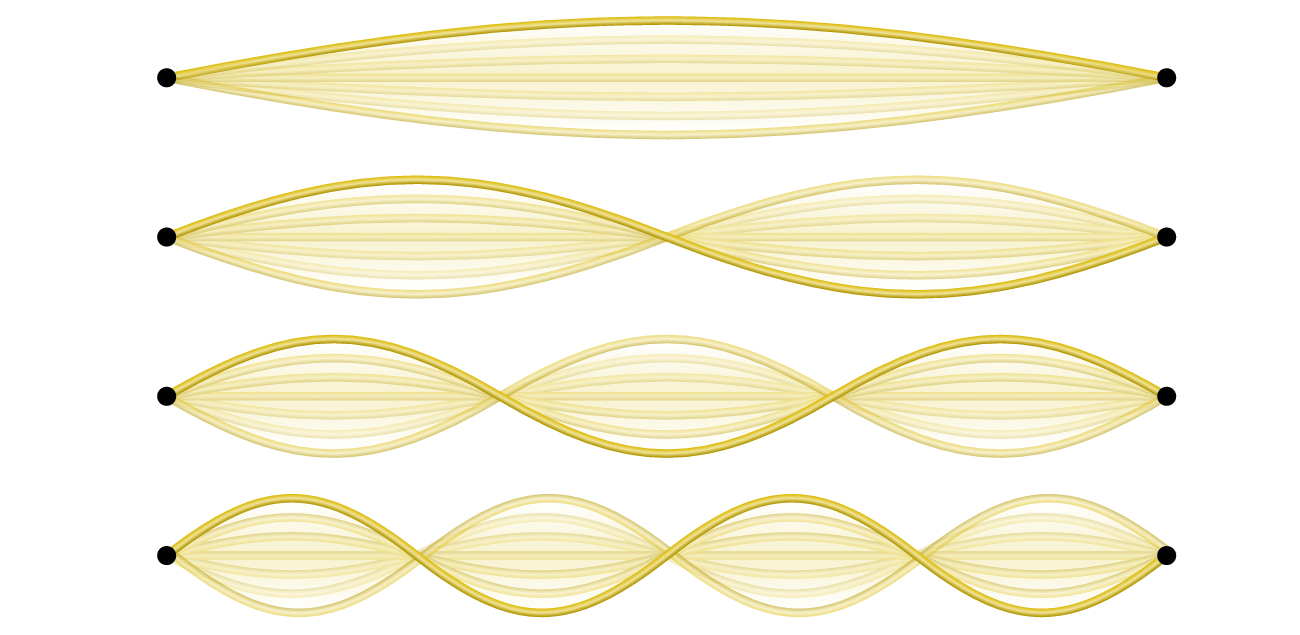
Figure \(\PageIndex{6}\): A vibrating string shows some one-dimensional standing waves. Since the two end points of the string are held fixed, only waves having an integer number of half-wavelengths can form. The points on the string between the end points that are not moving are called the nodes.
An example of two-dimensional standing waves is shown in Figure \(\PageIndex{7}\) which shows the vibrational patterns on a flat surface. Although the vibrational amplitudes cannot be seen like they could in the vibrating string, the nodes have been made visible by sprinkling the drum surface with a powder that collects on the areas of the surface that have minimal displacement. For one-dimensional standing waves, the nodes were points on the line, but for two-dimensional standing waves, the nodes are lines on the surface (for three-dimensional standing waves, the nodes are two-dimensional surfaces within the three-dimensional volume). Because of the circular symmetry of the drum surface, its boundary conditions (the drum surface being tightly constrained to the circumference of the drum) result in two types of nodes: radial nodes that sweep out all angles at constant radii and, thus, are seen as circles about the center, and angular nodes that sweep out all radii at constant angles and, thus, are seen as lines passing through the center. The upper left image in Figure \(\PageIndex{7}\) shows two radial nodes, while the image in the lower right shows the vibrational pattern associated with three radial nodes and two angular nodes.
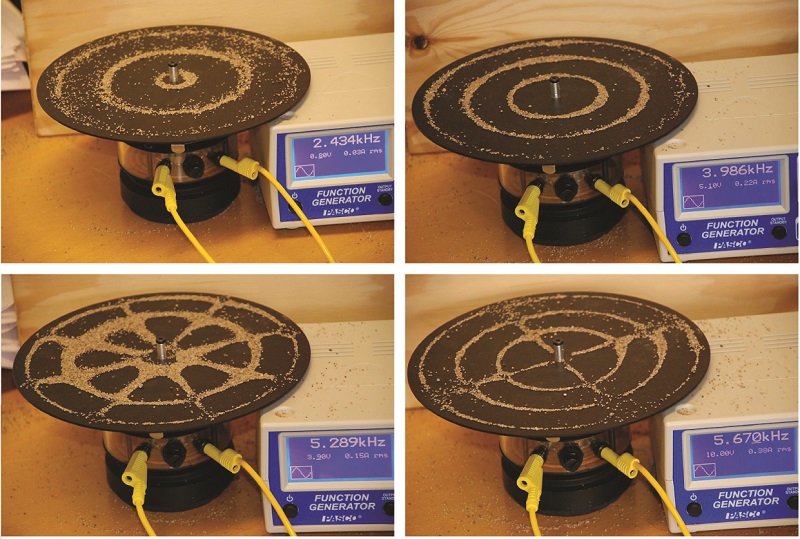
Figure \(\PageIndex{7}\) : Two-dimensional standing waves can be visualized on a vibrating surface. The surface has been sprinkled with a powder that collects near the nodal lines. There are two types of nodes visible: radial nodes (circles) and angular nodes (radii). For a more animated video, check this link out.
Radial Nodes & Imogen Heap
You can watch the formation of various radial nodes below as singer Imogen Heap projects her voice across a kettle drum.
Video \(\PageIndex{4}\): Singer Imogen Heap projects her voice across a kettle drum.
Blackbody Radiation and the Ultraviolet Catastrophe
The last few decades of the nineteenth century witnessed intense research activity in commercializing newly discovered electric lighting. This required obtaining a better understanding of the distributions of light emitted from various sources being considered. Artificial lighting is usually designed to mimic natural sunlight within the limitations of the underlying technology. Such lighting consists of a range of broadly distributed frequencies that form a continuous spe ctrum. Figure \(\PageIndex{8}\) shows the wavelength distribution for sunlight. The most intense radiation is in the visible region, with the intensity dropping off rapidly for shorter wavelength ultraviolet (UV) light, and more slowly for longer wavelength infrared (IR) light.
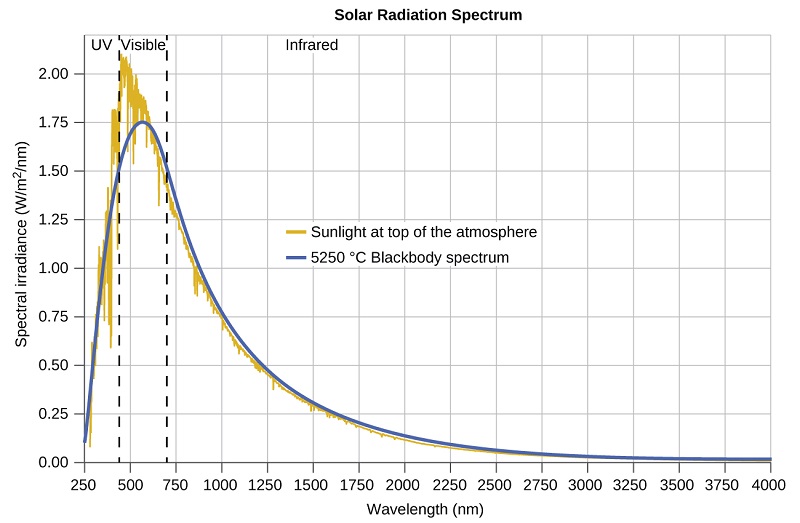
Figure \(\PageIndex{8}\): The spectral distribution (light intensity vs. wavelength) of sunlight reaches the Earth's atmosphere as UV light, visible light, and IR light. The unabsorbed sunlight at the top of the atmosphere has a distribution that approximately matches the theoretical distribution of a blackbody at 5250 °C, represented by the blue curve. (credit: modification of work by American Society for Testing and Materials (ASTM) Terrestrial Reference Spectra for Photovoltaic Performance Evaluation)
In Figure \(\PageIndex{8}\), the solar distribution is compared to a representative distribution, called a blackbody spectrum, that corresponds to a temperature of 5250 °C. The blackbody spectrum matches the solar spectrum quite well. A blackbody is a convenient, ideal emitter that approximates the behavior of many materials when heated. It is “ideal” in the same sense that an ideal gas is a convenient, simple representation of real gases that works well, provided that the pressure is not too high nor the temperature too low. A good approximation of a blackbody that can be used to observe blackbody radiation is a metal oven that can be heated to very high temperatures. The oven has a small hole allowing for the light being emitted within the oven to be observed with a spectrometer so that the wavelengths and their intensities can be measured. Figure \(\PageIndex{8}\) shows the resulting curves for some representative temperatures. Each distribution depends only on a single parameter: the temperature. The maxima in the blackbody curves, λ max , shift to shorter wavelengths as the temperature increases, reflecting the observation that metals being heated to high temperatures begin to glow a darker red that becomes brighter as the temperature increases, eventually becoming white hot at very high temperatures as the intensities of all of the visible wavelengths become appreciable. This common observation was at the heart of the first paradox that showed the fundamental limitations of classical physics that we will examine.
Physicists derived mathematical expressions for the blackbody curves using well-accepted concepts from the theories of classical mechanics and classical electromagnetism. The theoretical expressions as functions of temperature fit the observed experimental blackbody curves well at longer wavelengths, but showed significant discrepancies at shorter wavelengths. Not only did the theoretical curves not show a peak, they absurdly showed the intensity becoming infinitely large as the wavelength became smaller, which would imply that everyday objects at room temperature should be emitting large amounts of UV light. This became known as the “ultraviolet catastrophe” because no one could find any problems with the theoretical treatment that could lead to such unrealistic short-wavelength behavior. Finally, around 1900, Max Planck derived a theoretical expression for blackbody radiation that fit the experimental observations exactly (within experimental error). Planck developed his theoretical treatment by extending the earlier work that had been based on the premise that the atoms composing the oven vibrated at increasing frequencies (or decreasing wavelengths) as the temperature increased, with these vibrations being the source of the emitted electromagnetic radiation. But where the earlier treatments had allowed the vibrating atoms to have any energy values obtained from a continuous set of energies (perfectly reasonable, according to classical physics), Planck found that by restricting the vibrational energies to discrete values for each frequency, he could derive an expression for blackbody radiation that correctly had the intensity dropping rapidly for the short wavelengths in the UV region.
The quantity h is a constant now known as Planck's constant, in his honor. Although Planck was pleased he had resolved the blackbody radiation paradox, he was disturbed that to do so, he needed to assume the vibrating atoms required quantized energies, which he was unable to explain. The value of Planck's constant is very small, 6.626 × 10 −34 joule seconds (J s), which helps explain why energy quantization had not been observed previously in macroscopic phenomena.
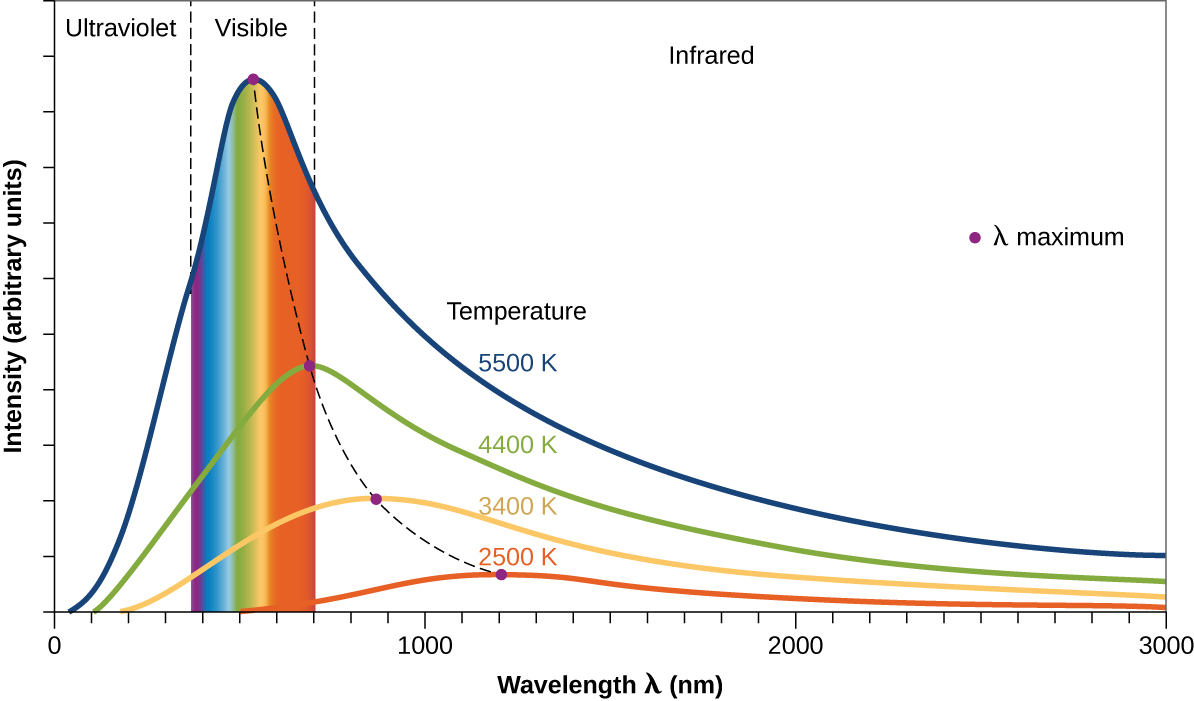
Figure \(\PageIndex{9}\): Blackbody spectral distribution curves are shown for some representative temperatures.
Video \(\PageIndex{5}\): An overview of the Ultraviolet Catastrophe.
The Photoelectric Effect
The next paradox in the classical theory to be resolved concerned the photoelectric effect (Figure \(\PageIndex{10}\)). It had been observed that electrons could be ejected from the clean surface of a metal when light having a frequency greater than some threshold frequency was shone on it. Surprisingly, the kinetic energy of the ejected electrons did not depend on the brightness of the light, but increased with increasing frequency of the light. Since the electrons in the metal had a certain amount of binding energy keeping them there, the incident light needed to have more energy to free the electrons. According to classical wave theory, a wave's energy depends on its intensity (which depends on its amplitude), not its frequency. One part of these observations was that the number of electrons ejected within in a given time period was seen to increase as the brightness increased. In 1905, Albert Einstein was able to resolve the paradox by incorporating Planck's quantization findings into the discredited particle view of light (Einstein actually won his Nobel prize for this work, and not for his theories of relativity for which he is most famous).
Einstein argued that the quantized energies that Planck had postulated in his treatment of blackbody radiation could be applied to the light in the photoelectric effect so that the light striking the metal surface should not be viewed as a wave, but instead as a stream of particles (later called photons ) whose energy depended on their frequency, according to Planck's formula, E = hν (or, in terms of wavelength using c = νλ , \(E=\dfrac{hc}{λ}\)). Electrons were ejected when hit by photons having sufficient energy (a frequency greater than the threshold). The greater the frequency, the greater the kinetic energy imparted to the escaping electrons by the collisions. Einstein also argued that the light intensity did not depend on the amplitude of the incoming wave, but instead corresponded to the number of photons striking the surface within a given time period. This explains why the number of ejected electrons increased with increasing brightness, since the greater the number of incoming photons, the greater the likelihood that they would collide with some of the electrons.
With Einstein's findings, the nature of light took on a new air of mystery. Although many light phenomena could be explained either in terms of waves or particles, certain phenomena, such as the interference patterns obtained when light passed through a double slit, were completely contrary to a particle view of light, while other phenomena, such as the photoelectric effect, were completely contrary to a wave view of light. Somehow, at a deep fundamental level still not fully understood, light is both wavelike and particle-like. This is known as wave-particle duality .
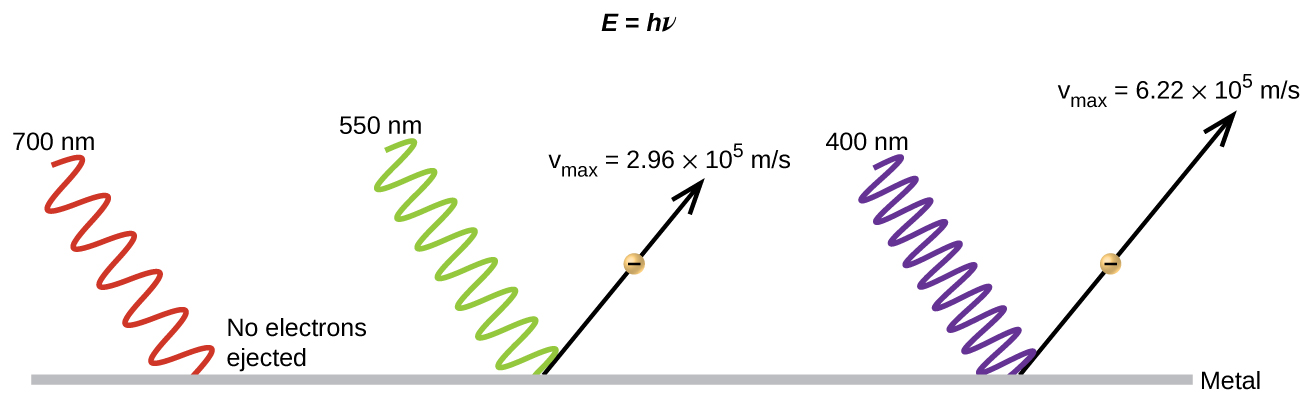
Figure \(\PageIndex{10}\): Photons with low frequencies do not have enough energy to cause electrons to be ejected via the photoelectric effect. For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon.
Example \(\PageIndex{2}\): Calculating the Energy of Radiation
When we see light from a neon sign, we are observing radiation from excited neon atoms. If this radiation has a wavelength of 640 nm, what is the energy of the photon being emitted?
We use the part of Planck's equation that includes the wavelength, λ , and convert units of nanometers to meters so that the units of λ and c are the same.
\[E=\dfrac{hc}{λ} \nonumber\]
\[\begin{align*} E&=\mathrm{\dfrac{(6.626×10^{−34}\:J\cancel{s})(2.998×10^{8}\:m\cancel{s}^{−1})}{(640\cancel{nm})\left(\dfrac{1\:m}{10^9\cancel{nm}}\right)}}\\ E&=\mathrm{3.10×10^{−19}\:J} \end{align*}\]
Exercise \(\PageIndex{2}\)
The microwaves in an oven are of a specific frequency that will heat the water molecules contained in food. (This is why most plastics and glass do not become hot in a microwave oven-they do not contain water molecules.) This frequency is about 3 × 10 9 Hz. What is the energy of one photon in these microwaves?
2 × 10 −24 J
Example \(\PageIndex{3}\): Photoelectric Effect
Identify which of the following statements are false and, where necessary, change the italicized word or phrase to make them true, consistent with Einstein's explanation of the photoelectric effect.
- Increasing the brightness of incoming light increases the kinetic energy of the ejected electrons.
- Increasing the wavelength of incoming light increases the kinetic energy of the ejected electrons.
- Increasing the brightness of incoming light increases the number of ejected electrons.
- Increasing the frequency of incoming light can increase the number of ejected electrons.
- False. Increasing the brightness of incoming light has no effect on the kinetic energy of the ejected electrons. Only energy, not the number or amplitude, of the photons influences the kinetic energy of the electrons.
- False. Increasing the frequency of incoming light increases the kinetic energy of the ejected electrons. Frequency is proportional to energy and inversely proportional to wavelength. Frequencies above the threshold value transfer the excess energy into the kinetic energy of the electrons.
- True. Because the number of collisions with photons increases with brighter light, the number of ejected electrons increases.
- True with regard to the threshold energy binding the electrons to the metal. Below this threshold, electrons are not emitted and above it they are. Once over the threshold value, further increasing the frequency does not increase the number of ejected electrons
Exercise \(\PageIndex{3}\)
Calculate the threshold energy in kJ/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Hz\).
\(3.94 \: kJ/mol\)
Video \(\PageIndex{6}\): An overview of the photoelectric effect.
Video \(\PageIndex{7}\): An overview of the wave nature of light.
Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c , of 2.998 × 10 8 m s −1 . This radiation shows wavelike behavior, which can be characterized by a frequency, ν , and a wavelength, λ , such that c = λν . Light is an example of a travelling wave. Other important wave phenomena include standing waves, periodic oscillations, and vibrations. Standing waves exhibit quantization, since their wavelengths are limited to discrete integer multiples of some characteristic lengths. Electromagnetic radiation that passes through two closely spaced narrow slits having dimensions roughly similar to the wavelength will show an interference pattern that is a result of constructive and destructive interference of the waves. Electromagnetic radiation also demonstrates properties of particles called photons. The energy of a photon is related to the frequency (or alternatively, the wavelength) of the radiation as E = hν (or \(E=\dfrac{hc}{λ}\) ), where h is Planck's constant. That light demonstrates both wavelike and particle-like behavior is known as wave-particle duality. All forms of electromagnetic radiation share these properties, although various forms including X-rays, visible light, microwaves, and radio waves interact differently with matter and have very different practical applications. Electromagnetic radiation can be generated by exciting matter to higher energies, such as by heating it. The emitted light can be either continuous (incandescent sources like the sun) or discrete (from specific types of excited atoms). Continuous spectra often have distributions that can be approximated as blackbody radiation at some appropriate temperature. The line spectrum of hydrogen can be obtained by passing the light from an electrified tube of hydrogen gas through a prism. This line spectrum was simple enough that an empirical formula called the Rydberg formula could be derived from the spectrum. Three historically important paradoxes from the late 19th and early 20th centuries that could not be explained within the existing framework of classical mechanics and classical electromagnetism were the blackbody problem, the photoelectric effect, and the discrete spectra of atoms. The resolution of these paradoxes ultimately led to quantum theories that superseded the classical theories.
Preview of Section 1.2
Video \(\PageIndex{8}\): Looking toward the next section...
Key Equations
- c = λν
- \(E=hν=\dfrac{hc}{λ}\), where h = 6.626 × 10 −34 J s
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/[email protected] ).
- Adelaide Clark, Oregon Institute of Technology
- Crash Course Physics: Crash Course is a division of Complexly and videos are free to stream for educational purposes.
- Crash Course Astronomy: Crash Course is a division of Complexly and videos are free to stream for educational purposes.
- TED-Ed’s commitment to creating lessons worth sharing is an extension of TED’s mission of spreading great ideas. Within TED-Ed’s growing library of TED-Ed animations, you will find carefully curated educational videos, many of which represent collaborations between talented educators and animators nominated through the TED-Ed website .
Have feedback to give about this text? Click here .
Found a typo and want extra credit? Click here .
- Search Menu
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Literature
- Classical Reception
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Papyrology
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Archaeology
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Emotions
- History of Agriculture
- History of Education
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Evolution
- Language Reference
- Language Acquisition
- Language Variation
- Language Families
- Lexicography
- Linguistic Anthropology
- Linguistic Theories
- Linguistic Typology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Modernism)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Media
- Music and Religion
- Music and Culture
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Science
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Politics
- Law and Society
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Clinical Neuroscience
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Toxicology
- Medical Oncology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Ethics
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Security
- Computer Games
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Psychology
- Cognitive Neuroscience
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Ethics
- Business Strategy
- Business History
- Business and Technology
- Business and Government
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic History
- Economic Systems
- Economic Methodology
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Behaviour
- Political Economy
- Political Institutions
- Political Sociology
- Political Methodology
- Political Communication
- Political Philosophy
- Political Theory
- Politics and Law
- Public Policy
- Public Administration
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Developmental and Physical Disabilities Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous
- Next chapter >
1 (page 1) p. 1 What is light?
- Published: September 2015
- Cite Icon Cite
- Permissions Icon Permissions
Light plays a fundamental role in life. ‘What is light?’ considers light’s physical properties—brightness, intensity, colour, polarization, and warmth—and how their application enables light to be used to discern, to measure, and to control properties of material substances. The origins of optics lie in the work of 4th-century bce Greek philosophers, but it was the fundamental work of several scientists in the 19th century—Michael Faraday, Hans Christian Oersted, André-Marie Ampère, Charles Augustin de Coulomb, Alessandro Volta, Georg Ohm, James Clerk Maxwell, and Heinrich Hertz—that made the connection between visible light and other parts of the light spectrum, including ultraviolet light, microwaves, and X-rays, enabling new technologies and further discoveries.
Signed in as
Institutional accounts.
- Google Scholar Indexing
- GoogleCrawler [DO NOT DELETE]
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Sign in through your institution
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

The Nature of Light
Properties of waves and light.
In many cases, the properties of light can be explained as a wave, as was shown in Young’s double-slit experiment.
Learning Objectives
Discuss how wave motion arises and its measurable properties, noting the conclusions of Young’s double slit experiment
Key Takeaways
- Wave motion arises when a periodic disturbance of some kind is propagated through an elastic medium. Pressure variations through air, transverse motions along a guitar string, or variations in the intensities of the local electric and magnetic fields in space, known as electromagnetic radiation, are all examples of waves.
- There are three measurable properties of wave motion: amplitude, wavelength, and frequency.
- A definitive experiment was Young’s double slit experiment, which demonstrated that light shined at two slits in a screen show an interference pattern characteristic of waves of light, rather than particles.
- The phase associated with a wave is also important in describing certain phenomena.
- The velocity of a wave is the product of the wavelength and the frequency.
- amplitude : The maximum value of the variable reached in either direction.
- wave : A shape that alternatively varies between a maximum in two opposite directions.
- frequency : The number of vibrations per second.
- wavelength : The distance traveled by the wave in a full period (1/frequency).
In this section, we will focus on the wave-like properties of light. While you will later learn about wave/particle duality (how light behaves as both a wave and a particle at the same time), here we shall discuss the wave nature of light and the experimental effects of this behavior.
Introduction to Wave Motion
Wave motion arises when a periodic disturbance of some kind is propagated through a medium. Pressure variations through air, transverse motions along a guitar string, or variations in the intensities of the local electric and magnetic fields in space, which constitute electromagnetic radiation, are all typical examples of wave motion. For each medium, there is a characteristic velocity at which the disturbance travels.

Sinusoidal wave : This image shows the anatomy of a sine curve: the crest is the peak of each wave, and the trough is the valley; the amplitude is the distance between the crest and the x-axis; and the wavelength is the distance between two crests (or two troughs).
There are three measurable properties of wave motion: amplitude, wavelength, and frequency (the number of vibrations per second). The relation between the wavelength λ (Greek lambda ) and frequency of a wave ν (Greek nu ) is determined by the propagation velocity v , such that
[latex]v=\nu \lambda[/latex]
For light, this equation becomes
[latex]\nu = \frac{c}{\lambda}[/latex]
where c is the speed of light, 2.998 x 10 8 m/s.
When utilizing these equations to determine wavelength, frequency, or velocity by manipulation of the equation, it is important to note that wavelengths are expressed in units of length, such as meters, centimeters, nanometers, etc; and frequency is typically expressed as megahertz or hertz (s –1 ).
What is the wavelength of the musical note A = 440 hz when it is propagated through air in which the velocity of sound is 343 m s–1?
λ = v (343 m s-1)/ v(440 s–1) = 0.780 m
Young’s Double-Slit Experiment

In the early 19th century, English scientist Thomas Young carried out the famous double-slit experiment (also known as Young’s experiment), which demonstrated that a beam of light, when split into two beams and then recombined, will show interference effects that can only be explained by assuming that light is a wavelike disturbance. If light consisted strictly of ordinary or classical particles, and these particles were fired in a straight line through a slit and allowed to strike a screen on the other side, we would expect to see a pattern corresponding to the size and shape of the slit. However, when this single-slit experiment is actually performed, the pattern on the screen is a diffraction pattern in which the light is spread out. The smaller the slit, the greater the angle of spread.
Similarly, if light consisted strictly of classical particles and we illuminated two parallel slits, the expected pattern on the screen would simply be the sum of the two single-slit patterns. In actuality, however, the pattern changes to one with a series of alternating light and dark bands. When Thomas Young first demonstrated this phenomenon, it indicated that light consists of waves, as the distribution of brightness can be explained by the alternately additive and subtractive interference of wavefronts. Young’s experiment, performed in the early 1800’s, played a vital part in the acceptance of the wave theory of light, superseding the corpuscular theory of light proposed by Isaac Newton, which had been the accepted model of light propagation in the 17th and 18th centuries. Almost a century later, in 1905, Albert Einstein’s Nobel-Prize winning research into the photoelectric effect demonstrated that light can behave as if it is composed of discrete particles under certain conditions. These seemingly contradictory discoveries made it necessary to go beyond classical physics and take the quantum nature of light into account.
Electromagnetic Spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation.
Calculate frequency or photon energy, identify the three physical properties of electromagnetic waves
- The electromagnetic spectrum includes common regimes such as ultraviolet, visible, microwave, and radio waves.
- Electromagnetic waves are typically described by any of the following three physical properties: frequency (f), wavelength (λ), or intensity (I). Light quanta are typically described by frequency (f), wavelength (λ), or photon energy (E). The spectrum can be ordered according to frequency or wavelength.
- Electromagnetic radiation interacts with matter in different ways in different parts of the spectrum. The types of interaction can range from electronic excitation to molecular vibration depending on the different types of radiation, such as ultraviolet, X-rays, microwaves, and infrared radiation.
- gamma ray : Electromagnetic radiation of high frequency and therefore high energy per photon.
- spectrum : A range of colors representing light (electromagnetic radiation) of contiguous frequencies; hence electromagnetic spectrum, visible spectrum, ultraviolet spectrum, etc.
- photon : The quantum of light and other electromagnetic energy, regarded as a discrete particle having zero rest mass, no electric charge, and an indefinitely long lifetime.
Range of the Electromagnetic Spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The electromagnetic spectrum of an object has a different meaning: it is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object.
Properties of the electromagnetic spectrum : The wavelengths of various regions of the electromagnetic spectrum are shown alongside an approximate proxy for size of the wavelength.
The electromagnetic spectrum extends from below the low frequencies used for modern radio communication to gamma radiation at the short-wavelength (high-frequency) end, covering wavelengths from thousands of kilometers down to a fraction of the size of an atom. The limit for long wavelengths is the size of the universe itself, while it is thought that the short wavelength limit is in the vicinity of the Planck length (1.616 x 10 -35 m), although in principle the spectrum is infinite and continuous.
Most parts of the electromagnetic spectrum are used in science for spectroscopic and other probing interactions, as ways to study and characterize matter. In general, if the wavelength of electromagnetic radiation is of a similar size to that of a particular object (atom, electron, etc.), then it is possible to probe that object with that frequency of light. In addition, radiation from various parts of the spectrum has been found to have many other uses in communications and manufacturing.
Energy of Photon
Electromagnetic waves are typically described by any of the following three physical properties: the frequency (f) (also sometimes represented by the Greek letter nu, ν), wavelength (λ), or photon energy (E). Frequencies observed in astronomy range from 2.4×10 23 Hz (1 GeV gamma rays ) down to the local plasma frequency of the ionized interstellar medium (~1 kHz). Wavelength is inversely proportional to wave frequency; hence, gamma rays have very short wavelengths that are a fraction of the size of atoms, whereas other wavelengths can be as long as the universe. Photon energy is directly proportional to the wave frequency, so gamma ray photons have the highest energy (around a billion electron volts), while radio wave photons have very low energy (around a femto-electron volt). These relations are illustrated by the following equations:
[latex]f = \frac{c}{\lambda} \,\,\,\text{ or }\,\,\, f= \frac{E}{h} \,\,\,\text{ or } \,\,\,E= \frac{hc}{\lambda}[/latex]
c = 299,792,458 m/s is the speed of light in vacuum
h = 6.62606896(33)×10 −34 J s = 4.13566733(10)×10 −15 eV s = Planck’s constant.
Whenever electromagnetic waves exist in a medium with matter, their wavelength is decreased. Wavelengths of electromagnetic radiation, no matter what medium they are traveling through, are usually quoted in terms of the vacuum wavelength, although this is not always explicitly stated. Generally, electromagnetic radiation is classified by wavelength into radio wave, microwave, terahertz (or sub-millimeter) radiation, infrared, the visible region we perceive as light, ultraviolet, X-rays, and gamma rays. The behavior of electromagnetic radiation depends on its wavelength. When electromagnetic radiation interacts with single atoms and molecules, its behavior also depends on the amount of energy per quantum (photon) it carries.
A.2.1 Describe the electromagnetic spectrum IB Chemistry SL – YouTube : This time with equations! Wave number = 1/wavelength in cm Speed of light = wavelength x frequency Energy = Planck’s constant x frequency. Dr Atkinson soon moved on to the un-needed gamma rays and improved them to delta rays!
Interaction of Elecromagnetic Radiation with Matter
Electromagnetic radiation interacts with matter in different ways in different parts of the spectrum. The types of interaction can be so different that it seems justified to refer to different types of radiation. At the same time, there is a continuum containing all these different kinds of electromagnetic radiation. Thus, we refer to a spectrum, but divide it up based on the different interactions with matter. Below are the regions of the spectrum and their main interactions with matter:
- Radio: Collective oscillation of charge carriers in bulk material (plasma oscillation). An example would be the oscillation of the electrons in an antenna.
- Microwave through far infrared: Plasma oscillation, molecular rotation.
- Near infrared: Molecular vibration, plasma oscillation (in metals only).
- Visible: Molecular electron excitation (including pigment molecules found in the human retina), plasma oscillations (in metals only).
- Ultraviolet: Excitation of molecular and atomic valence electrons, including ejection of the electrons (photoelectric effect).
- X-rays: Excitation and ejection of core atomic electrons, Compton scattering (for low atomic numbers).
- Gamma rays: Energetic ejection of core electrons in heavy elements, Compton scattering (for all atomic numbers), excitation of atomic nuclei, including dissociation of nuclei.
- High-energy gamma rays: Creation of particle-antiparticle pairs. At very high energies, a single photon can create a shower of high-energy particles and antiparticles upon interaction with matter.
This classification goes in the increasing order of frequency and decreasing order of wavelength, which is characteristic of the type of radiation. While, in general, the classification scheme is accurate, in reality there is often some overlap between neighboring types of electromagnetic energy. For example, SLF radio waves at 60 Hz may be received and studied by astronomers, or may be ducted along wires as electric power, although the latter is, in the strict sense, not electromagnetic radiation at all.
Interference and Diffraction
Interference and diffraction are terms that describe a wave interacting with something that changes its amplitude, such as another wave.
Recognize the difference between constructive and destructive interference, and between interference and diffraction
- In physics, interference is a phenomenon in which two waves superimpose to form a resultant wave of greater or lower amplitude.
- Constructive interference occurs when the phase difference between the waves is a multiple of 2π, whereas destructive interference occurs when the difference is π, 3π, 5π, etc.
- Diffraction refers to various phenomena that occur when a wave encounters an obstacle. In classical physics, the diffraction phenomenon is described as the apparent bending of waves around small obstacles and the spreading out of waves past small openings.
- interference : An effect caused by the superposition of two systems of waves, such as a distortion on a broadcast signal due to atmospheric or other effects. In physics, interference is a phenomenon in which two waves superimpose to form a resultant wave of greater or lower amplitude.
- diffraction : The breaking up of an electromagnetic wave as it passes a geometric structure (e.g., a slit), followed by reconstruction of the wave by interference.
- amplitude : The maximum absolute value of some quantity that varies, especially a wave.
In physics, interference is a phenomenon in which two waves superimpose to form a resultant wave of greater or lower amplitude. Interference usually refers to the interaction of waves that are correlated or coherent with each other, either because they come from the same source or because they have the same (or nearly the same) frequency. Interference effects can be observed with all types of waves, including light, radio, acoustic, and surface water waves. In chemistry, the applications of interference to light are the most relevant to the study of matter.
Mechanism of Interference
The principle of superposition of waves states that when two or more waves are incident on the same point, the total displacement at that point is equal to the vector sum of the displacements of the individual waves. If a crest of a wave meets a crest of another wave of the same frequency at the same point, then the magnitude of the displacement is the sum of the individual magnitudes; this is known as constructive interference. If a crest of one wave meets a trough of another wave, then the magnitude of the displacements is equal to the difference in the individual magnitudes; this is known as destructive interference.

Interference of two waves : These two examples represent constructive (left) and destructive interference (right) in wave phenomena. When the two waves are “in phase,” their periods are offset by 2nπ*period. However, when they are precisely out of phase, destructive interference results if the phase difference is nπ*period.
Constructive interference occurs when the phase difference between the waves is a multiple of 2π, whereas destructive interference occurs when the difference is π, 3π, 5π, etc. If the difference between the phases is intermediate between these two extremes, then the magnitude of the displacement of the summed waves lies between the minimum and maximum values.
Consider, for example, what happens when two identical stones are dropped into a still pool of water at different locations. Each stone generates a circular wave propagating outwards from the point where the stone was dropped. When the two waves overlap, the net displacement at a particular point is the sum of the displacements of the individual waves. At some points, these will be in phase and will produce a maximum displacement. In other places, the waves will be in anti-phase and there will be no net displacement at these points. Thus, parts of the surface will be stationary.
Diffraction
Diffraction refers to various phenomena that occur when a wave encounters an obstacle. In classical physics, the diffraction phenomenon is described as the apparent bending of waves around small obstacles and the spreading out of waves past small openings. Similar effects occur when light waves travel through a medium with a varying refractive index or a sound wave through one with varying acoustic impedance. Diffraction occurs with all waves, including sound waves, water waves, and electromagnetic waves such as visible light, X-rays, and radio waves. As physical objects have wave-like properties (at the atomic level), diffraction also occurs with matter and can be studied according to the principles of quantum mechanics. Italian scientist Francesco Maria Grimaldi coined the word diffraction and was the first to record accurate observations of the phenomenon in 1665.
The effects of diffraction are often seen in everyday life. The most striking examples of diffraction are those involving light; for example, the closely spaced tracks on a CD or DVD act as a diffraction grating to form the familiar rainbow pattern seen when looking at a disk. This principle can be extended to engineer a grating with a structure such that it will produce any diffraction pattern desired; the hologram on a credit card is an example. Diffraction in the atmosphere by small particles can cause a bright ring to be visible around a bright light source like the sun or the moon. A shadow of a solid object, using light from a compact source, shows small fringes near its edges. All these effects occur because light propagates as a wave.
Richard Feynman said, “No one has ever been able to define the difference between interference and diffraction satisfactorily. It is just a question of usage, and there is no specific, important physical difference between them.”
He suggested that when there are only a few sources, say two, we call it interference (as in Young’s slits), but with a large number of sources, the process can be labelled diffraction.
While diffraction occurs whenever propagating waves encounter such changes, its effects are generally most pronounced for waves where the wavelength is roughly similar to the dimensions of the diffracting objects. If the obstructing object provides multiple, closely spaced openings, a complex pattern of varying intensity can result. This is due to the superposition, or interference, of different parts of a wave that traveled to the observer by different paths (see diffraction grating).
Planck’s Quantum Theory
Max Planck suggested that the energy of light is proportional to its frequency, also showing that light exists in discrete quanta of energy.
Calculate the energy element E=hv, using Planck’s Quantum Theory
- Until the late 19th century, Newtonian physics dominated the scientific worldview. However, by the early 20th century, physicists discovered that the laws of classical mechanics do not apply at the atomic scale.
- The photoelectric effect could not be rationalized based on existing theories of light, as an increase in the intensity of light did not lead to the same outcome as an increase in the energy of the light.
- Planck postulated that the energy of light is proportional to the frequency, and the constant that relates them is known as Planck’s constant (h). His work led to Albert Einstein determining that light exists in discrete quanta of energy, or photons.
- photoelectric effect : The emission of electrons from the surface of a material following the absorption of electromagnetic radiation.
- electromagnetic radiation : Radiation (quantized as photons) consisting of oscillating electric and magnetic fields oriented perpendicularly to each other, moving through space.
In the late 18th century, great progress in physics had been made. Classical Newtonian physics at the time was widely accepted in the scientific community for its ability to accurately explain and predict many phenomena. However, by the early 20th century, physicists discovered that the laws of classical mechanics are not applicable at the atomic scale, and experiments such as the photoelectric effect completely contradicted the laws of classical physics. As a result of these observations, physicists articulated a set of theories now known as quantum mechanics. In some ways, quantum mechanics completely changed the way physicists viewed the universe, and it also marked the end of the idea of a clockwork universe (the idea that universe was predictable).
Electromagnetic radiation
Electromagnetic (EM) radiation is a form of energy with both wave -like and particle-like properties; visible light being a well-known example. From the wave perspective, all forms of EM radiation may be described in terms of their wavelength and frequency. Wavelength is the distance from one wave peak to the next, which can be measured in meters. Frequency is the number of waves that pass by a given point each second. While the wavelength and frequency of EM radiation may vary, its speed in a vacuum remains constant at 3.0 x 10 8 m/sec, the speed of light. The wavelength or frequency of any specific occurrence of EM radiation determine its position on the electromagnetic spectrum and can be calculated from the following equation:
[latex]c=\lambda\nu[/latex]
where c is the constant 3.0 x 10 8 m/sec (the speed of light in a vacuum), [latex]\lambda[/latex] = wavelength in meters, and [latex]\nu[/latex]=frequency in hertz (1/s). It is important to note that by using this equation, one can determine the wavelength of light from a given frequency and vice versa.

Wavelength of EM radiation : The distance used to determine the wavelength is shown. Light has many properties associated with its wave nature, and the wavelength in part determines these properties.
The Discovery of the Quantum
The wave model cannot account for something known as the photoelectric effect. This effect is observed when light focused on certain metals emits electrons. For each metal, there is a minimum threshold frequency of EM radiation at which the effect will occur. Replacement of light with twice the intensity and half the frequency will not produce the same outcome, contrary to what would be expected if light acted strictly as a wave. In that case, the effect of light would be cumulative—the light should add up, little by little, until it caused electrons to be emitted. Instead, there is a clear-cut minimum frequency of light that triggers electron ejection. The implication was that frequency is directly proportional to energy, with the higher light frequencies having more energy. This observation led to the discovery of the minimum amount of energy that could be gained or lost by an atom. Max Planck named this minimum amount the “quantum,” plural “quanta,” meaning “how much.” One photon of light carries exactly one quantum of energy.
Planck is considered the father of the Quantum Theory. According to Planck: E=h [latex]\nu[/latex] , where h is Planck’s constant (6.62606957(29) x 10 -34 J s), ν is the frequency, and E is energy of an electromagnetic wave. Planck (cautiously) insisted that this was simply an aspect of the processes of absorption and emission of radiation and had nothing to do with the physical reality of the radiation itself. However, in 1905, Albert Einstein reinterpreted Planck’s quantum hypothesis and used it to explain the photoelectric effect, in which shining light on certain materials can eject electrons from the material.
More Evidence for a Particle Theory of Energy
When an electric current is passed through a gas, some of the electrons in the gas molecules move from their ground energy state to an excited state that is further away from their nuclei. When the electrons return to the ground state, they emit energy of various wavelengths. A prism can be used to separate the wavelengths, making them easy to identify. If light acted only as a wave, then there should be a continuous rainbow created by the prism. Instead, there are discrete lines created by different wavelengths. This is because electrons release specific wavelengths of light when moving from an excited state to the ground state.

Emission spectrum of nitrogen gas : Each wavelength of light emitted (each colored line) corresponds to a transition of an electron from one energy level to another, releasing a quantum of light with defined energy (color).
The Photoelectric Effect
The photoelectric effect is the propensity of high-energy electromagnetic radiation to eject electrons from a given material.
Explain the the photoelectric effect and understand its mathematical description
- In the photoelectric effect, electrons are emitted from matter (typically metals and non-metallic solids ) as a consequence of their absorption of energy from electromagnetic radiation of high frequency (short wavelength), such as ultraviolet light.
- When electromagnetic radiation interacts with an atom, it either excites electrons to a higher energy level known as an excited state , or, if the energy of the light is sufficiently high, it can ionize the atom by removing the electron.
- For a given metal, there exists a certain minimum frequency of incident radiation below which no photoelectrons are emitted. This frequency is called the threshold frequency.
- work function : The minimum energy needed to remove an electron from the surface of a material.
- stopping voltage : The voltage required to completely balance the kinetic energy of electrons ejected from a material’s surface.
In the photoelectric effect, electrons are emitted from matter (metals and non-metallic solids, liquids, or gases) as a consequence of their absorption of energy from electromagnetic radiation of high frequency (short wavelength), such as ultraviolet radiation. Electrons emitted in this manner may be referred to as photoelectrons. This phenomenon was first observed by Heinrich Hertz in 1887.
The Photoelectric Effect : Electrons are emitted from matter by absorbed light.
The photoelectric effect has been demonstrated using light with energies from a few electronvolts (eV) to over 1 MeV in high atomic number elements. Study of the photoelectric effect led to an improved understanding of quantum mechanics as well as an appreciation of the wave-particle duality of light. It also led to Max Planck’s discovery of quanta (E=h[latex]\nu[/latex]), which links frequency ([latex]\nu[/latex]) with photon energy (E).
Planck’s constant, h, is also known as “the quantum of action.” It is a subatomic-scale constant and is one of the smallest constants used in physics. Other phenomena where light affects the movement of electric charges include the photoconductive effect (also known as photoconductivity or photoresistivity), the photovoltaic effect, and the photoelectrochemical effect.
Emission Mechanism
All atoms have their electrons in orbitals with well-defined energy levels. When electromagnetic radiation interacts with an atom, it can excite the electron to a higher energy level, which can then fall back down, returning to the ground state. However, if the energy of the light is such that the electron is excited above energy levels associated with the atom, the electron can actually break free from the atom leading to ionization of the atom. This, in essence, is the photoelectric effect.
The photons of a beam of light have a characteristic energy proportional to the frequency of the light. In the photoemission process, if an electron within some material absorbs the energy of one photon and acquires more energy than the work function of the material (the electron binding energy), it is ejected. If the photon energy is too low, the electron is unable to escape the material. Increasing the intensity of the light increases the number of photons in the beam of light and thus increases the number of electrons excited but does not increase the energy that each electron possesses. The energy of the emitted electrons does not depend on the intensity of the incoming light (the number of photons), only on the energy or frequency of the individual photons. It is strictly an interaction between the incident photon and the outermost electron.
Electrons can absorb energy from photons when irradiated, but they usually follow an all-or-nothing principle. Typically, one photon is either energetic enough to cause emission of an electron or the energy is lost as the atom returns back to the ground state. If excess photon energy is absorbed, some of the energy liberates the electron from the atom and the rest contributes to the electron’s kinetic energy as a free particle.
Experimental Observations of Photoelectric Emission
For a given metal, there exists a certain minimum frequency of incident radiation below which no photoelectrons are emitted. This frequency is called the threshold frequency. Increasing the frequency of the incident beam and keeping the number of incident photons fixed (resulting in a proportionate increase in energy) increases the maximum kinetic energy of the photoelectrons emitted. The number of electrons emitted also changes because the probability that each impacting photon results in an emitted electron is a function of the photon energy. However, if just the intensity of the incident radiation is increased, there is no effect on the kinetic energies of the photoelectrons.
For a given metal and frequency of incident radiation, the rate at which photoelectrons are ejected is directly proportional to the intensity of the incident light. An increase in the intensity of the incident beam (keeping the frequency fixed) increases the magnitude of the photoelectric current, though the stopping voltage remains the same. The time lag between the incidence of radiation and the emission of a photoelectron is very small, less than 10 −9 second, and is unaffected by intensity changes.
Mathematical Description
The maximum kinetic energy of an ejected electron is given by
[latex]K.E._{max}=hf-\varphi[/latex]
where h is the Planck constant (6.626 x 10 -34 m 2 kg/s) and f is the frequency of the incident photon. The term [latex]\varphi[/latex] is the work function (sometimes denoted W or ϕ), which gives the minimum energy required to remove a delocalized electron from the surface of the metal.
The work function satisfies [latex]\varphi = hf_{0}[/latex]
where f 0 is the threshold frequency for the metal. The maximum kinetic energy of an ejected electron is then
[latex]K.E._{max} = h(f-f_0)[/latex]
Kinetic energy must be positive for ejection to take place, so we must have f > f 0 for the photoelectric effect to occur.
Photomultipliers
Photomultipliers are extremely light-sensitive vacuum tubes with a photocathode coated onto part (an end or side) of the inside of the envelope. The photocathode contains combinations of materials, such as caesium, rubidium, and antimony, specially selected to provide a low work function, so when illuminated by even very low levels of light, the photocathode readily releases electrons. By means of a series of electrodes (dynodes) at ever-higher potentials, these electrons are accelerated and substantially increased in number through secondary emission to provide a readily detectable output current. Photomultipliers are still commonly used wherever low levels of light must be detected.
The Nature of Light Copyright © by Tulsa Community College is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

- Last updated
- Save as PDF
- Page ID 30460

- Kim Coble, Kevin McLin, & Lynn Cominsky
- San Francisco State University, Chico State University, & Sonoma State University
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Chapter 2 centers on the nature of light, which is humanity’s primary source of information about the Universe. It discusses the dual nature of light as both a particle and a wave; the relationship between wavelength, frequency, and speed; the wavebands of the electromagnetic spectrum; and continuum and line spectra.
- 2.0: Light Introduction Almost everything that we know about the Universe ultimately comes from the light we observe. Looking at the sky on a dark night is a breathtaking experience, and the Universe contains much more than is visible to the naked eye!
- 2.1: The Wave Nature of Light You will know that light can act like either a wave or a particle. You will know that all types of light travel at the same speed. You will be able to distinguish between wavelength, frequency, and speed You will be able to perform calculations and understand conceptually the relationship between wavelength, frequency, and speed.
- 2.2: The Particle Nature of Light ou will be able to perform calculations and understand conceptually the relationship between energy and frequency.
- 2.3: The Electromagnetic Spectrum You will be able to rank the different bandpasses in the EM Spectrum by energy, frequency, and wavelength.
- 2.4: What a Spectrum of Light Can Tell Us About Matter You will be able to describe how light can be measured as a function of energy (spectroscopy).
- 2.5: Continuous Spectra - a Planck Spectrum Tells us the Temperature of Objects You will know that most light seen in the universe is thermal and can be represented by a Planck spectrum. You will be able to perform calculations and understand conceptually the relationship between temperature and peak wavelength. You will be able to perform calculations and understand conceptually the relationship between flux and temperature.
- 2.6: Lines Spectra- Emission and Absorption Lines You will be able to distinguish between emission and absorption lines in a spectrum. You will know how spectral lines are produced. You will be able to calculate the energy/frequency/wavelength of a photon absorbed or emitted by a hydrogen atom.
- 2.7: Determining the Composition of an Unknown Gas You will know that chemical elements leave distinct “fingerprints” on the light from astronomical sources.
- 2.8: Wrapping It Up 2 - The Properties of Light
- 2.9: Mission Report 2 - The Properties of Light
Guide to Identifying Fake News
How to become an astronaut, openmind books, scientific anniversaries, animals on the verge of de-extinction, featured author, latest book, thomas young and the wave nature of light.
One of the milestones of the science of light commemorated during this International Year of Light and Light-based Technologies is «the notion of light as a wave proposed by Fresnel in 1815» that is, the celebration of the second centenary of the presentation of Augustin Fresnel’s paper titled Premier Mémoire sur la Diffraction de la Lumière before the Academy of Sciences in Paris on October 15, 1815 and which was published the following year. Fresnel contributed significantly to the establishment of the wave theory of light and thereafter this theory was very successful and resulted in a flood of new discoveries.
However, it was a British erudite and physician called Thomas Young who convincingly demonstrated the wave nature of light –contrary to the ideas of Newton who believed light was composed of a stream of particles– through the double-slit experiment , known today as Young’s light-interference experiment.
Thomas Young was born on June 13 th , 1773 in Milverton, in southwest England, into a Quaker family. He was the eldest of ten children and received a strict upbringing. He was a child prodigy. When he was two years old he was able to read and at six he had read the Bible twice from beginning to end. He knew a dozen languages including Latin and ancient Greek. Young studied medicine, but ultimately he did not succeed as a doctor, partly because of his inability to comfort patients . When he was 28, Young abandoned the medical practice to join the Royal Institution of London. In two years he delivered 91 lectures. He was one of the first people to decipher Egyptian hieroglyphics and played a key role in decoding the Rosetta stone . He was also a great linguist, the first in identifying numerous similarities between languages that he himself called Indo-European languages .

Young conducted studies on vision and the human eye and he proposed the three-colour theory of vision, only confirmed one hundred and fifty years later. He also conducted research on sound, on hearing and on the human voice and that was when he wondered if the sound and light would have the same wave nature. The Encyclopœdia Britannica summarizes Young as «English physician and physicist who established the principle of interference of light and thus resurrected the century-old wave theory of light. He was also an Egyptologist who helped decipher the Rosetta Stone.» In fact, Young held discoveries in virtually every field that he studied, including physics (the wave theory of light), engineering (the modulus of elasticity), physiology (the mechanism of vision), Egyptology, Linguistics and so on. For many people, Young is «the last man who knew everything.»
His major contribution to the field of light is the double-slit experiment, which has been considered not only «one of the most beautiful experiments in physics», but also «the favourite experiment with light.» With this experiment Young challenged the theories of Isaac Newton and proved that light is a wave, because light suffers the phenomenon of interference that is typical of the waves. Between 1801 and 1803, Young delivered a series of lectures to the Royal Society underlining the wave theory of light and adding to it a new fundamental concept, the so-called principle of interference. The double-slit experiment is a wonderfully simple experiment, which allowed Thomas Young to demonstrate convincingly the wave nature of light for the first time. When the waves emerging from two narrow slits are superimposed on a screen placed at some distance parallel to the line connecting these slits, a pattern of bright and dark fringes regularly spaced appears on the screen ( interference pattern ). This is the first clear proof that light added to light can produce darkness. Interference is accompanied by a spatial redistribution of the optical intensity without violation of power conservation. This phenomenon is known as interference and thanks to this experiment the intuitive ideas of Huygens regarding the wave nature of light were confirmed. Thomas Young had expected it because he believed in the wave theory of light and in his own judgment this was the most important of his many achievements.
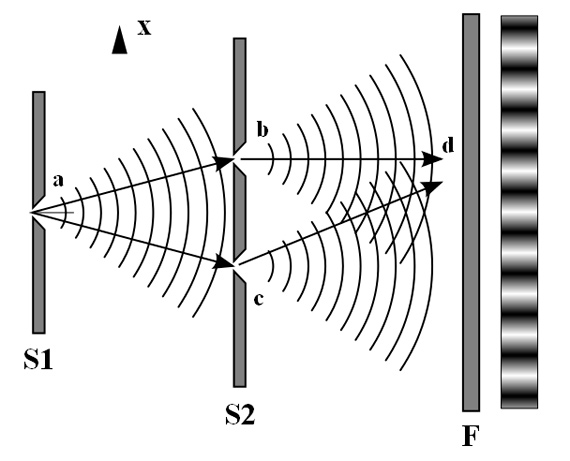
On November 12, 1801, Young gave the Bakerian Lecture titled On the Theory of Light and Colours to the Royal Society, and on November 24, 1803, also the Bakerian Lecture titled Experiments and Calculations relative to Physical Optics . In the latter he was able to proffer more than speculations presenting the «experimental demonstration of the general law of light interference» and an «argumentative inference respecting the nature of light», concluding that light is a wave. As all waves known at that period needed a material medium for their propagation, as happens with sound waves and water waves. Due to this, in his Lecture of 1801, Young pointed out that light propagated in a medium, the luminiferous ether, concluding that «A luminiferous Ether pervades Universe, rare and elastic in high degree» and he didn’t have a doubt that «Radiant light Consist in Undulations of the luminiferous Ether.» He also stated that «The Sensation of different Colours depends on the different frequency of Vibrations, excited by light in the Retina.»

In 1803, however, almost nobody accepted immediately Young’s radical ideas about the nature of light. Young published in 1807 his magnus opus , A Course of Lectures on Natural Philosophy and the Mechanical Arts , consisting of two quarto volumes running to more than fifteen hundred pages, which was described by the physicist Joseph Larmor (1857-1942) as «the greatest and most original of all general lecture courses.» The double-slit experiment is described in Lecture 39 on this book including a series of diagrams about the experiment in Plate XXX .
Thanks to the contributions made by Augustin Fresnel, the wave theory of light –experimentally demonstrated by Young in his famous experiment– was finally accepted.
Bibliography
Robert P. Crease, The prism and the pendulum: The ten most beautiful experiments in science (Random House Trade. New York, 2003).
Liesbeth Venema, Light, enchanted (of schemmes and memes, a community blog from nature.com). May 1, 2015.
Andrew Robinson, “Thomas Young: The Man Who Knew Everything”, History Today, vol. 56, pp. 53–57 (2006).
Andrew Robinson, The Last Man Who Knew Everything (Pi Press. New York, 2006).
Augusto Beléndez
Related publications.
- 2015, the Year of Light
- Dennis Gabor, “Father of Holography”
- Globalization and Science: One Physicist’s View
More about Science
Environment, leading figures, mathematics, scientific insights, more publications about augusto beléndez, comments on this publication.
Morbi facilisis elit non mi lacinia lacinia. Nunc eleifend aliquet ipsum, nec blandit augue tincidunt nec. Donec scelerisque feugiat lectus nec congue. Quisque tristique tortor vitae turpis euismod, vitae aliquam dolor pretium. Donec luctus posuere ex sit amet scelerisque. Etiam sed neque magna. Mauris non scelerisque lectus. Ut rutrum ex porta, tristique mi vitae, volutpat urna.
Sed in semper tellus, eu efficitur ante. Quisque felis orci, fermentum quis arcu nec, elementum malesuada magna. Nulla vitae finibus ipsum. Aenean vel sapien a magna faucibus tristique ac et ligula. Sed auctor orci metus, vitae egestas libero lacinia quis. Nulla lacus sapien, efficitur mollis nisi tempor, gravida tincidunt sapien. In massa dui, varius vitae iaculis a, dignissim non felis. Ut sagittis pulvinar nisi, at tincidunt metus venenatis a. Ut aliquam scelerisque interdum. Mauris iaculis purus in nulla consequat, sed fermentum sapien condimentum. Aliquam rutrum erat lectus, nec placerat nisl mollis id. Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Nam nisl nisi, efficitur et sem in, molestie vulputate libero. Quisque quis mattis lorem. Nunc quis convallis diam, id tincidunt risus. Donec nisl odio, convallis vel porttitor sit amet, lobortis a ante. Cras dapibus porta nulla, at laoreet quam euismod vitae. Fusce sollicitudin massa magna, eu dignissim magna cursus id. Quisque vel nisl tempus, lobortis nisl a, ornare lacus. Donec ac interdum massa. Curabitur id diam luctus, mollis augue vel, interdum risus. Nam vitae tortor erat. Proin quis tincidunt lorem.
The Second Curve
Do you want to stay up to date with our new publications.
Receive the OpenMind newsletter with all the latest contents published on our website
OpenMind Books
- The Search for Alternatives to Fossil Fuels
- View all books
About OpenMind
Connect with us.
- Keep up to date with our newsletter

Theories of Light
- First Online: 14 May 2023
Cite this chapter

- Steven S. Andrews 2
807 Accesses
Scientists and philosophers have studied light for thousands of years. The ancient Greeks thought that light came from people’s eyes, which is incorrect, but they nevertheless correctly described light rays, reflection, and refraction. Medieval Muslims corrected the direction of light travel and largely explained how human vision works. A persistent question regarded whether light is composed of particles or waves. Particle theories dominated up to the 16th century, based on essentially no evidence. Scientific results then favored waves, then Isaac Newton pushed consensus back to particles, and finally waves were proven at the end of the 19th century. Very shortly afterward, early quantum mechanics results promoted a particle view, which led to the current understanding of light as both particles and waves simultaneously.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Lindberg, David C. (1976) Theories of Vision from Al-Kindi to Keplar , University of Chicago Press: Chicago.
Winer, G.A., J.E. Cottrell, V. Gregg, J.S. Fournier, and L.A. Bica (2002) American Psychologist 67:417.
Descartes was correct that refraction arises from different wave speeds, but incorrectly believed that light travels faster in water than in air.
The letter reads, in part, “What Des-Cartes did was a good step. You have added much several ways, and especially in taking the colours of thin plates into philosophical consideration. If I have seen further it is by standing on the shoulders of Giants. But I make no question but you have divers very considerable experiments besides those you have published, ...” (Letter from Isaac Newton to Robert Hooke, 1675, available at https://digitallibrary.hsp.org/index.php/Detail/objects/9792 )
Quoted from F. Todd Baker, “Atoms and Photons and Quanta, Oh My!: Ask the Physicist about Atomic Nuclear, and Quantum Physics”, Morgan & Claypool Publishers, 2015, p. 2-4.
Author information
Authors and affiliations.
Department of Bioengineering, University of Washington, Seattle, WA, USA
Steven S. Andrews
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Steven S. Andrews .
Rights and permissions
Reprints and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Andrews, S.S. (2023). Theories of Light. In: Light and Waves. Springer, Cham. https://doi.org/10.1007/978-3-031-24097-3_1
Download citation
DOI : https://doi.org/10.1007/978-3-031-24097-3_1
Published : 14 May 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-24096-6
Online ISBN : 978-3-031-24097-3
eBook Packages : Physics and Astronomy Physics and Astronomy (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
The Enduring Mystery of Light

It goes through walls, but slows to a standstill in ultra-cold gases. It carries electronic information for radios and TVs, but destroys genetic information in cells. It bends around buildings and squeezes through pinholes, but ricochets off tiny electrons.
It's light. And although we know it primarily as the opposite of darkness, most of light is not visible to our eyes. From low energy radio waves to high energy gamma rays, light zips around us, bounces off us, and sometimes goes through us.
Because it is so many things, defining light is a bit of a philosophical quandary. It doesn't help that light continue to surprise us, with novel materials that alter light's speed and trajectory in unexpected ways.
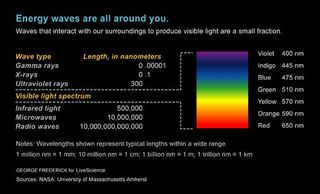
Is it a wave?
What ties together microwaves, X-rays and the colors of the rainbow is that they are all waves — electromagnetic waves to be exact. The substance that sloshes back and forth isn't water or air, but a combination of electric and magnetic fields.
These fluctuating fields exert forces on charged particles — sometimes causing them to bob up and down like buoys in the ocean.
What separates all the various forms of light is wavelength. Our eyes are sensitive to light with wavelengths between 750 nanometers (red) and 380 nanometers (violet), where a nanometer is one billionth of a meter, or about the size of a single molecule .
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
But the visible spectrum — seen through a prism — is only a small chunk of the entire electromagnetic spectrum. The wavelength of light ranges from hundreds of miles for long radio waves to one millionth of a nanometer for gamma rays.
The energy of light is inversely proportional to the wavelength, such that gamma rays are a billion billion times more energetic than radio waves.
Or is it a particle?
But waves are not the whole story. Light is composed of particles called photons. This is most obvious with higher energy light, like X-rays and gamma rays, but it is true all the way down to radio waves.
The classic example of particleness is the photoelectric effect, in which light hitting a metal sheet causes electrons to fly out of the surface. Surprisingly, light longer than a certain wavelength cannot liberate electrons, no matter how bright the source is.
A strict wave theory of light cannot explain this wavelength threshold, since many long waves should pack the same total energy as a few short waves.
Albert Einstein deciphered the mystery in 1905 by assuming that particles of light smacked into the electrons, like colliding billiard balls. Only particles from short wavelength light can give a hard enough kick.
Despite this success, the particle theory never replaced the wave theory, since only waves can describe how light interferes with itself when it passes through two slits. We therefore have to live with light being both a particle and a wave — sometimes acting as hard as a rock, sometimes as soft as a ripple.
Physicists rectify light's split personality by thinking in terms of wave packets, which one can imagine as a group of light waves traveling together in a tight, particle-like bundle.
Making a spectacle
Instead of worrying about what light is , it might be better to concentrate on what light does . Light shakes, twists and shoves the charged particles (like electrons) that reside in all materials.
These light actions are wavelength-specific. Or to say it another way, each material responds only to a particular set of wavelengths.
Take an apple, for instance. Radio waves and X-rays go essentially straight through it, whereas visible light is stopped by various apple molecules that either absorb the light as heat or reflect it back out.
If the reflected light enters our eyes, it will stimulate color receptors (cones) that are specifically "tuned" to either long, medium or short wavelengths. The brain compares the different cone responses to determine that the apple reflects "red" light.
Here are some other examples of light's specific activities.
- Radio waves from a local station cause the free electrons in a radio's antenna to oscillate. Electronics tuned to the station's frequency (or wavelength) can decode the oscillating signal into music or words.
- A microwave oven heats food from the inside out because microwaves penetrate the surface to rotate water molecules contained in the food. This molecular shuffling generates heat.
- Standing next to a camp fire, infrared light vibrates molecules in our skin to make us warm. Conversely, we constantly lose heat when these same molecules emit infrared light.
- In sunlight, several visible and ultraviolet wavelengths are missing, or dark. These "shadows" are due to the capture of photons by atoms, like hydrogen and helium, that make up the sun. The captured photon energy is used to boost the atoms' electrons from one energy level to another.
- An X-ray image of a skeleton is due to the fact that X-rays pass through soft tissue but are blocked by dense bone. However, even when just passing through, X-rays and gamma-rays ionize molecules along their path, meaning they strip electrons from the molecules. The ionized molecules can directly or indirectly damage DNA in a cell. Some of these genetic alterations may lead to cancer.
All this shows that light wears many different hats in its manipulation of matter. It is perhaps fitting then that light's true identity — wave or particle — is unanswerable.

Newfound 'glitch' in Einstein's relativity could rewrite the rules of the universe, study suggests
Atoms squished closer together than ever before, revealing seemingly impossible quantum effects
Long-lost branch of the Nile was 'indispensable for building the pyramids,' research shows
Most Popular
- 2 James Webb telescope measures the starlight around the universe's biggest, oldest black holes for 1st time ever
- 3 See stunning reconstruction of ancient Egyptian mummy that languished at an Australian high school for a century
- 4 China creates its largest ever quantum computing chip — and it could be key to building the nation's own 'quantum cloud'
- 5 James Webb telescope detects 1-of-a-kind atmosphere around 'Hell Planet' in distant star system
- 2 Newfound 'glitch' in Einstein's relativity could rewrite the rules of the universe, study suggests
- 3 Sun launches strongest solar flare of current cycle in monster X8.7-class eruption

The basic nature of light

Light is intimately involved with our daily lives. Many unique properties of light are extremely fascinating. Here, we will take one step closer to the wonders of light through its well-known basic properties.
Is Light a wave or a particle?
Light travels at a speed of 300,000 kilometers per second, interaction of light with matter, light “reflects”.
Light “scatters”
Light “refracts”
Light “interferes”
Light “disperses”
Light has the properties of a wave and a particle. The word “wavelength” is used to express the wave or undulating property of light. It is the distance that light travels in one oscillation, and is often expressed using a unit called "nanometer". One nanometer is equal to one billionth of a meter. Our eyes can only see light that is of a wavelength between approximately 400 to 700 nanometers. This range is called the visible light. The light of other wavelengths includes X-rays, ultraviolet rays, and infrared rays. Though we cannot see them directly, these are also members of the light family.
On the other hand, light also has the property of a particle. The intensity of the light varies depending on the number of particles. Bright light has many particles while dark light has fewer particles. These particles of light are called “photons”.
We can check out the particle property of light by comparing light with sound using a device called oscilloscope. Sound is known to have the characteristics of a wave. When the intensity or magnitude of sound gradually weakens, the signal of sound becomes smaller and eventually disappears. However, when light gradually weakens, the overall quantity of its signal becomes less yet the few remaining pulses (extremely short signals) can be detected and the size of these individual signals does not decrease. This tells us that light cannot become any smaller, and that light has a property of a “particle.”

Light travels at a speed of about 300,000 kilometers per second. Surprisingly, light can travel around the earth 7.5 times in a mere one second. This property of light is utilized in many technical applications such as optical communications which transfer huge data in a very short time. However, even light, which is faster than anything known to man, can move only 0.3 millimeters in a trillionth of a second (a picosecond – see note) in a vacuum. In recent years, research of such optical phenomenon that occur in these unbelievably short period of time, is becoming essential in new research fields of physics, chemistry, biology, and others.
Note: 1 millisecond = 1 thousandth of a second, 1 microsecond = 1 millionth of a second, 1 nanosecond = 1 billionth of a second, 1 picosecond = 1 trillionth of a second.

When in a vacuum such as outer space where no matter is present, light travels straightforward. However, light behaves in a variety of ways when it comes in contact with water, air, and other matters – it is "absorbed", "transmitted through", "reflected", and "scattered". When light strikes matter, a part of that light is absorbed into the matter (a) and is transformed into heat energy. If the matter that the light strikes is a transparent material, the light component that was not absorbed within the material is “transmitted” through (b) and exits to the outer side of the material. If the surface of the material is smooth (a mirror for example), “reflection” occurs (b), but if the surface is irregular having pits and protrusions, the light “scatters” (c).
The “transmitted,” “reflected,” or “scattered” light allows our eyes to see the colors and shapes of objects.

Why is a distant mountain sometimes seen clearly reflected on the surface of a lake or pond?
The sunlight striking a mountain bounces back in many directions. This is called reflected light. Our eyes see the mountain by capturing some of the light reflected from the mountain which directly reaches our eyes and then by forming an image of the reflected light on the retina through the lens of the eye. (Pink lines in the figure below represent the reflected light. To make it easier to describe, this figure shows a boy looking at a distant tree instead of a mountain.)
When there is a lake or pond between our eyes and a mountain, the light arriving there from the mountain reflects off the surface of the lake or pond (blue dotted lines in the figure). If the surface is calm with no wind and also flat and smooth such as on level surfaces with no irregularities like mirrors and glass, then the angle of the incident light (angle of incidence) and the angle of the light bouncing off the surface (angle of reflection) are equal to each other. This is referred to as specular reflection or mirror reflection. When the surface is located in an ideal location where the light bouncing off the surface by means of specular reflection directly reaches our eyes, then we can see a sharp, clear image of the mountain reflected on the surface.
On the other hand, if the surface is rough or irregular, then the direction of the reflected light varies depending on the position on the surface, resulting in a distorted image of the mountain reflecting on the water surface.

Light “scatters”
Why is it that on a clear day, the color of sky is blue but appears red in the evening?
Light from the sun reaches the earth after traveling through space, it “scatters” when striking the various particles and molecules in the atmosphere. A part of this light returns to the outer space and the remainder of the light reaches the surface of the earth after traveling through the atmosphere. The level of scattering of light depends on its wavelength, and of the lights that our eyes can see, blue light is more intensely dispersed or scattered. This is why the sky appears blue to our eyes during the day.
On the other hand, during sunrise and sunset, the sky can appear orange, pink, or red to our eyes. This is because when the position of the sun is lower, the distance that the light travels through the atmosphere becomes longer, and the blue light that is gradually scattered and weakens. Therefore, the remaining red or orange light reaches our eyes.

Light “refracts”
When you look into a straw placed in a glass, the portion of the straw inside the water appears bent. Why is that so?
Light “refracts” at the boundary between air and water in the glass. Refraction occurs because light travels at different speed in air and water. Our eyes catch the scattered light from the straw in the water, but refraction occurs when the light in the water enters the air. However, the light coming out from water appears to be moving straightforward to our eyes, and our eyes form a “virtual image” on the line extending from the refracted light. Thus the tip of the straw in the water appears to have deviated from its actual position.

Light “interferes”
How are those intriguing colors of blowing soap bubbles made?
Light moves in various directions so the light waves are constantly striking against each another. The phenomenon that occurs when the light waves collide with each other is called “interference.”
When the peaks of these waves overlap, the peaks become even larger. When the peaks and valleys of the waves collide, the waves cancel each other out. This interference is what causes us to see the various colors in soap bubbles.
A soap bubble is made of an extremely thin film. Light reflecting from the outer and inner sides of this film interferes with each other to cause the colors that we see. Moreover, the viewing angle of the light interference occurring at the soap bubble film changes due to the ceaseless movement of the soup bubble.
Due to the waves of light repeatedly intensifying and canceling each other out, our eyes see mysterious and constantly changing colors.

Light “disperses”
Why does rainbow appear in the sky after it rains?
The light from the sun is called white light beam, but it actually is a mixture of different colored lights which appear white to our eyes. Using a prism to separate the white light beam allows us to see the various colors of light.
This phenomenon is called “dispersion” of light. In the natural world, water droplets act like a prism then they remain in the air after the rain.
Light that strikes water droplets refracts and moves to the interior of the droplet, reflects within the droplet, and refracts when exiting the droplet. The water droplets in the air act just like a prism causing dispersion and the light reaching our eyes appears as continuous bands of different colors. That is what makes a rainbow.
If we look closely around the rainbow, we may sometimes see another rainbow (a secondary rainbow) whose color sequence is reversed, on the outer side of the first rainbow. This secondary rainbow appears due to light that reaches our eyes reflecting twice in the water droplet.

Related Links

The browser you use is not corresponded to javascript or is invalid. Turn on the javascript of browser before use.
The Beauty About The Nature
To go into solitude, a man needs to retire as much from his chamber as from society. I am not solitary whilst I read and write, though nobody is with me. But if a man would be alone, let him look at the stars. The rays that come from those heavenly worlds, will separate between him and what he touches. One might think the atmosphere was made transparent with this design, to give man, in the heavenly bodies, the perpetual presence of the sublime. Seen in the streets of cities, how great they are! If the stars should appear one night in a thousand years, how would men believe and adore; and preserve for many generations the remembrance of the city of God which had been shown! But every night come out these envoys of beauty and light the universe with their admonishing smile.
The Stars Awaken a Certain Reverence, Because Though Always Present, They Are Inaccessible;
but all natural objects make a kindred impression when the mind is open to their influence. Nature never wears a mean appearance. Neither does the wisest man extort her secret, and lose his curiosity by finding out all her perfection. Nature never became a toy to a wise spirit. The flowers, the animals, the mountains, reflected the wisdom of his best hour, as much as they had delighted the simplicity of his childhood. When we speak of nature in this manner, we have a distinct but most poetical sense in the mind. We mean the integrity of impression made by manifold natural objects. It is this which distinguishes the stick of timber of the wood-cutter, from the tree of the poet . The charming landscape which I saw this morning, is indubitably made up of some twenty or thirty farms. Miller owns this field, Locke that, and Manning the woodland beyond. But none of them owns the landscape. There is a property in the horizon which no man has but he whose eye can integrate all the parts, that is, the poet . This is the best part of these men's farms, yet to this, their warranty deeds give no title. To speak truly, few adult persons can see nature. Most persons do not see the sun. At least they have a very superficial seeing. The sun illuminates only the eye of the man but shines into the eye and the heart of the child.
The lover of nature is he whose inward and outward senses are still truly adjusted to each other;
who has retained the spirit of infancy even into the era of manhood. His intercourse with heaven and earth becomes part of his daily food. In the presence of nature, a wild delight runs through the man, in spite of real sorrows. Nature says, — he is my creature, and maugre all his impertinent griefs, he shall be glad with me. Not the sun or the summer alone, but every hour and season yields its tribute of delight; for every hour and change corresponds to and authorizes a different state of the mind, from breathless noon to grimmest midnight.
Nature is a setting that fits equally well a comic or a mourning piece. In good health, the air is a cordial of incredible virtue. Crossing a bare common, in snow puddles, at twilight, under a clouded sky, without having in my thoughts any occurrence of special good fortune, I have enjoyed a perfect exhilaration. I am glad to the brink of fear. In the woods too, a man casts off his years, as the snake his slough, and at what period soever of life, is always a child. In the woods, is perpetual youth. Within these plantations of God, a decorum and sanctity reign, a perennial festival is dressed, and the guest sees not how he should tire of them in a thousand years. In the woods, we return to reason and faith.
There I feel that nothing can befall me in life,
— no disgrace, no calamity, (leaving me my eyes,) which nature cannot repair. Standing on the bare ground, — my head bathed by the blithe air, and uplifted into infinite space, — all mean egotism vanishes. I become a transparent eye-ball; I am nothing; I see all; the currents of the Universal Being circulate through me; I am part or particle of God. The name of the nearest friend sounds then foreign and accidental: to be brothers, to be acquaintances, — master or servant, is then a trifle and a disturbance. I am the lover of uncontained and immortal beauty. In the wilderness, I find something more dear and connate than in streets or villages. In the tranquil landscape, and especially in the distant line of the horizon, man beholds somewhat as beautiful as his own nature.
The greatest delight which the fields and woods minister, is the suggestion of an occult relation between man and the vegetable.
I am not alone and unacknowledged. They nod to me, and I to them. The waving of the boughs in the storm is new to me and old. It takes me by surprise, and yet is not unknown. Its effect is like that of a higher thought or a better emotion coming over me, when I deemed I was thinking justly or doing right.
Yet it is certain that the power to produce this delight, does not reside in nature, but in man, or in a harmony of both. It is necessary to use these pleasures with great temperance. For, nature is not always tricked in holiday attire, but the same scene which yesterday breathed perfume and glittered as for the frolic of the nymphs, is overspread with melancholy today. Nature always wears the colors of the spirit. To a man laboring under calamity, the heat of his own fire hath sadness in it. Then, there is a kind of contempt of the landscape felt by him who has just lost by death a dear friend. The sky is less grand as it shuts down over less worth in the population.

Chapter I from Nature , published as part of Nature; Addresses and Lectures
What Is The Meaning Behind Nature, The Poem?
Emerson often referred to nature as the "Universal Being" in his many lectures. It was Emerson who deeply believed there was a spiritual sense of the natural world which felt was all around him.
Going deeper still in this discussion of the "Universal Being", Emerson writes, "The aspect of nature is devout. Like the figure of Jesus, she stands with bended head, and hands folded upon the breast. The happiest man is he who learns from nature the lesson of worship."
It's common sense that "nature" is everything you see that is NOT man-made, or changed by man (trees, foliage, mountains, etc.), but Emerson reminds us that nature was set forth to serve man. This is the essence of human will, for man to harness nature. Every object in nature has its own beauty. Therefore, Emerson advocates to view nature as a reality by building your own world and surrounding yourself with natural beauty.
- The purpose of science is to find the theory of nature.
- Nature wears the colors of the Spirit.
- A man is fed, not to fill his belly, but so he may work.
- Each natural action is graceful.
"Material objects are necessarily kinds of scoriae of the substantial thoughts of the Creator, which must always preserve an exact relation to their first origin; in other words, visible nature must have a spiritual and moral side."
This quote is cited in numerous works and it is attributed to a "French philosopher." However, no name can be found in association with this quote.
What is the main point of Nature, by Emerson?
The central theme of Emerson's famous essay "Nature" is the harmony that exists between the natural world and human beings. In "Nature," Ralph Waldo Emerson contends that man should rid himself of material cares and instead of being burdened by unneeded stress, he can enjoy an original relation with the universe and experience what Emerson calls "the sublime."
What is the central idea of the essay Nature, by Emerson?
For Emerson, nature is not literally God but the body of God’s soul. ”Nature,” he writes, is “mind precipitated.” Emerson feels that to realize one’s role in this respect fully is to be in paradise (similar to heaven itself).
What is Emerson's view of the Nature of humans?
Content is coming very soon

Ralph Waldo Emerson left the ministry to pursue a career in writing and public speaking. Emerson became one of America's best known and best-loved 19th-century figures. More About Emerson
Quick Links
Self-reliance.
- Address at Divinity College
- English Traits
- Representative Men
- The American Scholar
- The Conduct of Life
- Essays: First Series
- Essays: Second Series
- Nature: Addresses/Lectures
- Lectures / Biographies
- Letters and Social Aims
Early Emerson Poems
- Uncollected Prose
- Government of Children
Emerson Quotes
"Every man has his own courage, and is betrayed because he seeks in himself the courage of other persons." – Ralph Waldo Emerson
“Do not go where the path may lead, go instead where there is no path and leave a trail.” – Ralph Waldo Emerson
“The purpose of life is not to be happy. It is to be useful, to be honorable, to be compassionate, to have it make some difference that you have lived and lived well.” – Ralph Waldo Emerson
Emerson's Essays
Research the collective works of Ralph Waldo Emerson. Read More Essay
Emerson's most famous work that can truly change your life. Check it out
America's best known and best-loved poems. More Poems
Visiting Sleeping Beauties: Reawakening Fashion? You must join the virtual exhibition queue when you arrive. If capacity has been reached for the day, the queue will close early.

The Poetry of Nature: Edo Paintings from the Fishbein-Bender Collection
With a shared reverence for the arts of Japan, T. Richard Fishbein and his wife, Estelle P. Bender assembled an outstanding and diverse collection of paintings of the Edo period (1615–1868). The Poetry of Nature offers an in-depth look at more than forty works from their collection that together trace the development of the major schools and movements of the era—Rinpa, Nanga, Zen, Maruyama-Shijō, and Ukiyo-e—from their roots in Heian court culture and the Kano and Tosa artistic lineages that preceded them.
Insightful essays by John T. Carpenter and Midori Oka reveal a unifying theme—the celebration of the natural world—expressed in varied forms, from the bold, graphic manner of Rinpa to the muted sensitivity of Nanga. Lavishly illustrated, these works draw particular focus to the unique intertwinement of poetry and the pictorial arts that is fundamental to the Japanese tradition. In addition to providing new readings and translations of Japanese and Chinese poems, The Poetry of Nature sheds new light on the ways in which Edo artists used verse to transform their paintings into a hybrid literary and visual art.
Met Art in Publication
You May Also Like

Designing Nature: The Rinpa Aesthetic in Japanese Art

Japanese Lacquer, 1600–1900: Selections from the Charles A. Greeneld Collection

Kimono Style: Edo Traditions to Modern Design, The John C. Weber Collection

The Written Image: Japanese Calligraphy and Painting from the Sylvan Barnet and William Burto Collection

Bridge of Dreams: The Mary Griggs Burke Collection of Japanese Art
Related content.
- Essay Seasonal Imagery in Japanese Art
- Essay Art of the Edo Period (1615–1868)
- Essay Woodblock Prints in the _Ukiyo-e_ Style
- Essay Art of the Pleasure Quarters and the Ukiyo-e Style
- Essay Japanese Illustrated Handscrolls
- Essay Rinpa Painting Style
- Essay The Kano School of Painting
- Essay Momoyama Period (1573–1615)
- Essay Shōguns and Art
- Essay _Yamato-e_ Painting
Carpenter, John T., Midori Oka, and Metropolitan Museum of Art (New York, N.Y.), eds. 2018. The Poetry of Nature: Edo Paintings from the Fishbein-Bender Collection . New York: The Metropolitan Museum of Art.
To read this content please select one of the options below:
Please note you do not have access to teaching notes, regulatory framework on governing equity crowdfunding: a systematic literature review and future directions.
Journal of Financial Regulation and Compliance
ISSN : 1358-1988
Article publication date: 16 May 2024
The purpose of this study is to comprehensively analyse and compare equity crowdfunding (ECF) regulations across 26 countries, shedding light on the diverse regulatory frameworks, investor and issuer limits and the evolution of ECF globally. By addressing this research gap and providing consolidated insights, the study aims to inform policymakers, researchers and entrepreneurs about the regulatory landscape of ECF, fostering a deeper understanding of its potential and challenges in various economies. Ultimately, the study contributes to the advancement of ECF as an alternative financing method for small and medium enterprises (SMEs) and startups, empowering them to access much-needed capital for growth.
Design/methodology/approach
The study used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) model for a systematic literature review on global ECF regulations. Starting with 74 initial articles from Web of Sciences and Scopus databases, duplicates were removed and language criteria applied, leaving 42 articles. After a thorough full-text screening, 20 articles were excluded, resulting in the review of 22 papers from 2016 to 2022. PRISMA’s structured framework enhances the quality of systematic reviews, ensuring transparency and accessibility of findings for various stakeholders, including researchers, practitioners and policymakers, in the field of ECF regulations.
This study examines ECF regulations across various countries. Notably, the UK has advanced regulations, while the USA adopted them later through the Jumpstart Our Business Startups Act. Canada regulates at the provincial level. Malaysia and China were early adopters in Asia, but Hong Kong, Japan, Israel and India have bans. Turkey introduced regulations in 2019. New Zealand and Australia enacted laws, with Australia referring to it as “crowd-sourced equity funding”. Italy, Austria, France, Germany and Belgium have established regulations in Europe. These regulations vary in investor and issuer limits, disclosure requirements and anti-corruption measures, impacting the growth of ECF markets.
Research limitations/implications
This study’s findings underscore the diverse regulatory landscape governing ECF worldwide. It reveals that regulatory approaches vary from liberal to protectionist, reflecting each country’s unique economic and political context. The implications of this research highlight the need for cross-country analysis to inform practical implementation and the effectiveness of emerging ECF ecosystems. This knowledge can inspire regulatory adjustments, support startups and foster entrepreneurial growth in emerging economies, ultimately reshaping early-stage funding for new-age startups and SMEs on a global scale.
Originality/value
This study’s originality lies in its comprehensive analysis of ECF regulations across 26 diverse countries, shedding light on the intricate interplay between regulatory frameworks and a nation’s political-economic landscape. By delving into the nuanced variations in investor limits, investment types and regulatory strategies, it unveils the multifaceted nature of ECF regulation globally. Furthermore, this research adds value by comparing divergent perspectives on investment constraints and offering an understanding of their impact on ECF efficacy. Ultimately, the study’s unique contribution lies in its potential to inform practical implementation, shape legislative frameworks and catalyse entrepreneurial ecosystems in emerging economies, propelling the evolution of early-stage funding practices.
- Systematic literature review
- Crowdfunding
- Equity crowdfunding
- Regulations
- Entrepreneurial finance
Gupta, P. , Singh, S. , Ghosh, R. , Kumar, S. and Jain, C. (2024), "Regulatory framework on governing equity crowdfunding: a systematic literature review and future directions", Journal of Financial Regulation and Compliance , Vol. ahead-of-print No. ahead-of-print. https://doi.org/10.1108/JFRC-10-2023-0160
Emerald Publishing Limited
Copyright © 2024, Emerald Publishing Limited
Related articles
We’re listening — tell us what you think, something didn’t work….
Report bugs here
All feedback is valuable
Please share your general feedback
Join us on our journey
Platform update page.
Visit emeraldpublishing.com/platformupdate to discover the latest news and updates
Questions & More Information
Answers to the most commonly asked questions here

COMMENTS
introduction. Light is a transverse, electromagnetic wave that can be seen by the typical human. The wave nature of light was first illustrated through experiments on diffraction and interference. Like all electromagnetic waves, light can travel through a vacuum. The transverse nature of light can be demonstrated through polarization.
Light is a primary tool for perceiving the world and interacting with it for many organisms. Light from the Sun warms the Earth, drives global weather patterns, and initiates the life-sustaining process of photosynthesis; about 10 22 joules of solar radiant energy reach Earth each day. Light's interactions with matter have also helped shape the structure of the universe.
1.S: The Nature of Light (Summary) Thumbnail: An EM wave, such as light, is a transverse wave. The electric E→ E → and magnetic B→ B → fields are perpendicular to the direction of propagation. The direction of polarization of the wave is the direction of the electric field.
Summary. Video 1.1.7 1.1. 7: An overview of the wave nature of light. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. This radiation shows wavelike behavior, which can be characterized by a frequency, ν, and a wavelength, λ, such that c = λν.
Light - Electromagnetic, Wavelength, Spectrum: In spite of theoretical and experimental advances in the first half of the 19th century that established the wave properties of light, the nature of light was not yet revealed—the identity of the wave oscillations remained a mystery. This situation dramatically changed in the 1860s when the Scottish physicist James Clerk Maxwell, in a watershed ...
to the topic The Nature of Light: What is a Photon? In this issue, 1 a number of renowned scientists, through five pen-etrating essays, ac cepted the challenge of describing the photon.Said Arthur Zajonc, in his lead article titled Light Reconsidered : Light is an obvious feature of everyday life, and yet light s true nature has eluded us
An illustration of this is found in the experiments on the nature of colours undertaken by Sir Isaac Newton (Figure 3), a dominant figure of early 18th-century science, and whose book Opticks defined the view of light for two centuries, and by Johann Wolfgang von Goethe (Figure 3), a dominant figure of late 18th-century literature, who ...
1.4: Total Internal Reflection. The incident angle that produces an angle of refraction of 90° is called the critical angle.; Total internal reflection is a phenomenon that occurs at the boundary between two media, such that if the incident angle in the first medium is greater than the critical angle, then all the light is reflected back into that medium.
The relation between the wavelength λ (Greek lambda) and frequency of a wave ν (Greek nu) is determined by the propagation velocity v, such that. v = νλ v = ν λ. For light, this equation becomes. ν = c λ ν = c λ. where c is the speed of light, 2.998 x 10 8 m/s.
2.0: Light Introduction Almost everything that we know about the Universe ultimately comes from the light we observe. Looking at the sky on a dark night is a breathtaking experience, and the Universe contains much more than is visible to the naked eye! 2.1: The Wave Nature of Light You will know that light can act like either a wave or a particle.
The items that appeared in the same issue as Newton's included a traveller's description of Eastern India and an essay on music. At first glance, Newton's letter shares few obvious characteristics with modern scientific papers. ... Newton switches to a different and little-studied instrument—the prism—for exploring the nature of light ...
Its speed in vacuum, 299 792 458 m/s, is one of the fundamental constants of nature. ... A translation of Newton's essay on light appears in The large scale structure of space-time, by Stephen Hawking and George F. R. Ellis. The fact that light could be polarized was for the first time qualitatively explained by Newton using the particle theory.
1.1 The Nature of Light: a Brief Historical Overview. Until around 1800, most scientists thought that light consisted of streams of particles, in line with the view expressed by Newton in "Opticks" (Newton 1952 ). However, contemporaries of Newton, like Robert Hooke and Christian Huygens, conceived of light as waves.
Between 1801 and 1803, Young delivered a series of lectures to the Royal Society underlining the wave theory of light and adding to it a new fundamental concept, the so-called principle of interference. The double-slit experiment is a wonderfully simple experiment, which allowed Thomas Young to demonstrate convincingly the wave nature of light ...
Huygens, Wave Nature of Light. When Newton was expounding a corpuscular nature of light, his contemporary, Christian Huygens (1629-1695) suggested a wave picture of light. Huygens published his results in his Traite de la lumiere (Treatise on light) in 1690. Crucial to his wave theory was the result recently obtained by Olaus Romer (1679 ...
The first important discoveries about the nature of light that were based on careful analysis were made by Muslim scientists about a thousand years later. They were made during the Islamic Golden Age, which was an especially prolific period for Islamic culture, science, and mathematics and extended from about the 8th to 14th centuries. ...
published 26 February 2007. The Enduring Mystery of Light. It goes through walls, but slows to a standstill in ultra-cold gases. It carries electronic information for radios and TVs, but destroys ...
Abstract and Figures. Focusing on the unresolved debate between Newton and Huygens from 300 years ago, The Nature of Light: What is a Photon? discusses the reality behind enigmatic photons. It ...
Abstract: Light is sensed by rational beings. Only real entities can produce sensory perception. Matter alone provides substance needed for objective reality in space. Hence, light or its ...
Light that strikes water droplets refracts and moves to the interior of the droplet, reflects within the droplet, and refracts when exiting the droplet. The water droplets in the air act just like a prism causing dispersion and the light reaching our eyes appears as continuous bands of different colors. That is what makes a rainbow.
Nature Summary: "Nature" is an essay by Ralph Waldo Emerson that was first published in 1836. In this work, Emerson reflects on the beauty and power of nature and argues that it can serve as a source of inspiration and enlightenment for individuals. He encourages readers to look beyond the surface of nature and appreciate its underlying ...
Insightful essays by John T. Carpenter and Midori Oka reveal a unifying theme—the celebration of the natural world—expressed in varied forms, from the bold, graphic manner of Rinpa to the muted sensitivity of Nanga. ... The Poetry of Nature sheds new light on the ways in which Edo artists used verse to transform their paintings into a ...
By delving into the nuanced variations in investor limits, investment types and regulatory strategies, it unveils the multifaceted nature of ECF regulation globally. Furthermore, this research adds value by comparing divergent perspectives on investment constraints and offering an understanding of their impact on ECF efficacy.