COVID-19 and TDR
- Standing Committee
- Joint Coordinating Board
- Scientific and Technical Advisory Committee
- Scientific Working Groups

- Malaria research

Tuberculosis research
- Vector-borne diseases
- Neglected tropical diseases research
Implementation research training materials
- Massive open online course (MOOC) on implementation research
- Postgraduate training scheme
- Regional training centres
- Clinical Research Leadership fellowship programme
TDR Global profiles
- Women in science
- TDR impact research grants scheme
- Gender and infectious disease research
- Knowledge management
- SORT IT operational research and training
All feature stories
All Publications
TDR Strategy 2024-29
Global Health Matters podcast »
Grants and other funding opportunities
eTDR portal
Our partnerships
ESSENCE on Health Research
Social Innovation in Health Initiative
The Access and Delivery Partnership
- Research for implementation /
Tuberculosis is the leading cause of death from a single infectious agent and remains a global health emergency. In 2018 alone, there were 1.5 million deaths and 10 million new cases globally, among whom half a million had rifampicin resistant TB. The annual rate of decline in TB incidence is still much lower than what would be needed to end the TB epidemic by 2030.
It is critical to identify and overcome barriers to effective implementation of existing strategies and tools, which, if adequately employed, would drastically reduce the TB burden. Implementation research on best strategies for early diagnosis, treatment and prevention of TB, optimized and tailored to various socioeconomic contexts and responsive to local conditions, would help accelerate the decline in TB incidence rates globally. This is particularly essential in resource-limited settings where much remains to be done to achieve universal coverage for TB care. Implementation research on effective approaches that can mobilize sustained community engagement, address social determinants of TB and strengthen political commitment remains an important area to End TB.
WHO’s End TB Strategy, endorsed by the World Health Assembly in May 2014, distinctly recognized TB research as one of its three pillars. Locally owned and conducted implementation TB research within national TB programmes are needed in countries to identify more efficient ways of using existing tools and expeditiously scaling up new tools to End TB.
TDR aims to contribute to the End TB effort by conducting and enhancing capacity for implementation research at national, regional and global levels.
Active TB drug safety monitoring and management (aDSM)
TDA4Child initiative
African regional research networks for TB control
Calibrating computer-aided detection (CAD) for TB
ShORRT initiative
Impact Grants awarded for implementation research in South-East Asia Region
Even after full TB treatment, many people suffer disability, other illnesses
Improving childhood TB diagnosis in Nigeria and the Democratic Republic of the Congo: the TDA4Child initiative
Strengthening capacities for improving TB drug safety monitoring
Is tuberculosis treatment truly free? Insights from Pakistan
Exploring catastrophic household costs for tuberculosis treatment
Supporting a new regional network in Southern and East Africa for TB control
- 1 (current)
Featured publications

TDR annual report 2022
This report highlights the impact of research supported by the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases...

Good practices guidance handbook for national TB surveys
The purpose of this document is to describe and explain how to apply the principles of good clinical practice (GCP) and good data management practice (GDMP)...

Implementation research for digital technologies and tuberculosis
The Implementation research for digital technologies and tuberculosis (IR4DTB) toolkit has been designed for TB programme implementers (middle and senior-level...

Dialogues: a conversation with Vidya Krishnan

Research in times of COVID-19: the Cambodia experience

WARN/CARN-TB

Improving detection of TB in children: a smarter solution

A la recherche des « cas manquants » de tuberculose chez les enfants

The ADP Delivering New Health Technologies for TB, Malaria and NTDs
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Tuberculosis articles from across Nature Portfolio
Tuberculosis (TB) is an infectious disease caused by strains of bacteria known as mycobacteria. The disease most commonly affects the lungs and can be fatal if not treated. However, most infected individuals show no disease symptoms. One third of the worlds population is thought to have been infected with TB.
Latest Research and Reviews
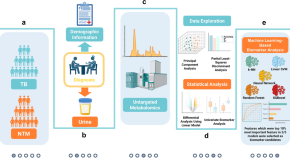
Discovery of urinary biosignatures for tuberculosis and nontuberculous mycobacteria classification using metabolomics and machine learning
- Nguyen Ky Anh
- Nguyen Ky Phat
- Jee Youn Oh

A new nomogram based on ultrasound and clinical features for distinguishing epididymal tuberculosis and nontuberculous epididymitis
A deep learning-based algorithm for pulmonary tuberculosis detection in chest radiography
- Chiu-Fan Chen
- Chun-Hsiang Hsu
- Po-Fan Chen
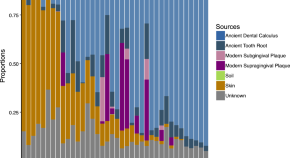
Metagenomic and paleopathological analyses of a historic documented collection explore ancient dental calculus as a diagnostic tool
- Rita M. Austin
- Tanvi P. Honap
- Courtney A. Hofman

Ocular tuberculosis with Mycobacterium tuberculosis DNA presence in ocular fluid: will post-COVID era bring a difference?
- Ikhwanuliman Putera
- Erica Widodo
- Rina La Distia Nora
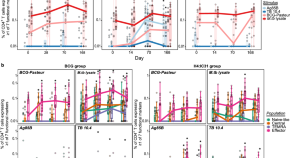
Adolescent BCG revaccination induces a phenotypic shift in CD4 + T cell responses to Mycobacterium tuberculosis
BCG revaccination of adolescents has been shown to provide some protection against sustained infection with Mycobacterium tuberculosis . Here, using intracellular cytokine staining and CITE-Seq, the authors identify several effector memory CD4 + T cell populations in adolescents revaccinated with BCG that can aid the search for correlates of protection.
- One B. Dintwe
- Lamar Ballweber Fleming
- M. Juliana McElrath
News and Comment
Restocking the tuberculosis drug arsenal
After many lean years, important progress has been made in updating the anti-tuberculosis drug armamentarium; a new drug that targets bacterial protein synthesis is one of several that could help transform the treatment of this neglected and deadly disease.
- Eric L. Nuermberger
- Richard E. Chaisson
Digital intervention improves tuberculosis treatment outcomes
An intervention that incorporates electronic pill boxes and remote adherence monitoring improved treatment success in patients with tuberculosis in Tibet — making this a promising strategy for low-resource settings.
- Karen O’Leary

A spotlight on the tuberculosis epidemic in South Africa
Tuberculosis is the leading cause of death from a single infectious agent, with over 25% of these occurring in the African region. Multi-drug resistant strains which do not respond to first-line antibiotics continue to emerge, putting at risk numerous public health strategies which aim to reduce incidence and mortality. Here, we speak with Professor Valerie Mizrahi, world-leading researcher and former director of the Institute of Infectious Disease and Molecular Medicine at the University of Cape Town, regarding the tuberculosis burden in South Africa. We discuss the challenges faced by researchers, the lessons that need to be learnt and current innovations to better understand the overall response required to accelerate progress.
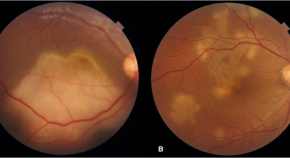
Presumed ocular tuberculosis – need for caution before considering anti-tubercular therapy
- Rohan Chawla
- Urvashi B. Singh
- Pradeep Venkatesh
Transforming tuberculosis diagnosis
Diagnosis is the weakest aspect of tuberculosis (TB) care and control. We describe seven critical transitions that can close the massive TB diagnostic gap and enable TB programmes worldwide to recover from the pandemic setbacks.
- Madhukar Pai
- Puneet K. Dewan
- Soumya Swaminathan
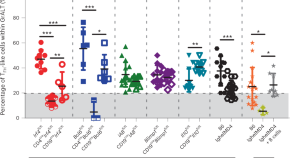
B cells and T follicular helper-like cells within lung granulomas are required for TB control
We show a crucial protective function for T follicular helper (T FH )-like cells localized within granuloma-associated lymphoid tissue for Mycobacterium tuberculosis control in mouse models of tuberculosis. Antigen-specific B cells contribute to this strategic localization and the maturation of cytokine-producing T FH -like cells.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
Media Advisory
Wednesday, September 26, 2018
NIH releases strategic plan to address tuberculosis research

Tuberculosis (TB) is the leading infectious cause of death worldwide, killing roughly 1.6 million people in 2017. In the past 200 years, TB claimed the lives of more than 1 billion people — more deaths than from malaria, influenza, smallpox, HIV/AIDS, cholera and plague combined.
Recently, the global health community has strengthened its efforts and resolve to tackle this ancient disease. Writing in the Journal of the American Medical Association , Anthony S. Fauci, M.D., director of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, details the institute’s new strategic plan for building on these current efforts by furthering the understanding of TB and developing and applying cutting-edge tools to fight the disease.
The NIAID Strategic Plan for Tuberculosis Research prioritizes expanding fundamental knowledge of TB by using modern tools, such as state-of-the-art imaging and systems biology methods, to better understand how TB infection remains latent in some individuals and then progresses to active disease, as well as the host and microbial factors that affect TB disease, transmission, and epidemiology. The plan is being released to coincide with the United Nations General Assembly High-Level Meeting on Ending TB on September 26.
In 2017, an estimated 3.6 million of 10 million new TB cases went undiagnosed or unreported, according to the World Health Organization. To address this gap, NIAID is calling for improved TB diagnostics, specifically, the development of tests that can be performed rapidly, accurately, inexpensively, and at the point-of-care for different forms of TB as well as for all populations, including children and people living with HIV. NIAID will support research on emerging technologies, such as nanotechnology, and identify host and microbial biomarkers that can be integrated into platforms that diagnose infection, indicate the risk of disease progression, or predict disease recurrence, Fauci writes.
Safe, broadly effective vaccines and short-course drug regimens to prevent TB infection, latent disease or the progression to active TB disease are also badly needed, according to Fauci. To that end, NIAID will support advanced technologies, assays, and animal models to facilitate the design of novel TB vaccines and to identify markers of protective immunity. Additionally, the institute will assess preventive drug regimens that interrupt transmission.
People living with TB currently must take lengthy and sometimes toxic treatment regimens for multidrug resistant and extensively drug-resistant TB. For people co-infected with TB and HIV, drug-drug interactions are particularly problematic. To address these challenges, NIAID will focus efforts on developing effective, shorter-duration therapeutics for latent and active drug-sensitive and drug-resistant TB, including host-directed therapies. Existing drugs approved for other indications will be assessed for repurposing to identify the most effective treatments with the shortest duration, according to Fauci. Further, research to understand the mechanisms of action and adverse effects of therapeutics will be conducted to prevent or reduce drug toxicity.
NIAID also will expand research resources and infrastructure, so that scientists can gain access to appropriate facilities and databases for sharing large, diverse data sets. Additionally, the institute will foster opportunities for early-stage investigators, support improved animal models, and facilitate development of assays, reagents and other tools.
Ending the TB epidemic will require an accelerated, collaborative global effort from diverse scientific disciplines, Fauci writes. NIAID will implement and lead a reinvigorated research program to build the tools for transformative improvements in TB diagnosis, prevention and treatment that are needed to end TB.
AS Fauci. Addressing the Tuberculosis Epidemic: 21 st Century Research for an Ancient Disease Journal of the American Medical Association DOI: 10.1001/jama.2018.12852.
The NIAID Strategic Plan for Tuberculosis Research is available online at: https://www.niaid.nih.gov/sites/default/files/TBStrategicPlan2018.pdf .
NIAID Director Anthony S. Fauci, M.D., is available to discuss the article and the NIAID Strategic Plan for Tuberculosis Research.
To schedule interviews, please contact the NIAID News Office at (301) 402-1663 or via e-mail at [email protected] .
NIAID conducts and supports research — at NIH, throughout the United States, and worldwide — to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
An Overview of Research Priorities in Tuberculosis
- First Online: 27 July 2021
Cite this chapter

- Christian Lienhardt 3 , 4 ,
- Dennis Falzon 5 ,
- Matteo Zignol 5 &
- Afranio Kritski 6
1241 Accesses
Intensified research and innovation is one of the three pillars of the End-TB Strategy launched by the World Health Organization in 2015. This underscores the essentiality of research and development to reach the ambitious targets of reducing tuberculosis (TB) incidence and deaths by 80% and 90%, respectively, by the year 2030. The development of new technologies to diagnose, treat and prevent TB requires intensification of research along the full spectrum, from fundamental research for better understanding of human TB and discovery of novel diagnostics, drugs and vaccines, to implementation/operational research, for their introduction in clinical and programmatic practices, ensuring access to those in highest need. At time of increasing antibiotic resistance and emergence of new infectious diseases, investments in TB research will enhance the feasibility and accessibility of innovations to the populations most vulnerable to this disease, and continue to produce broad benefits to health that extend well beyond the fight against TB.
The authors (D.F. and M.Z.) are a staff member of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the views, decisions or policies of the World Health Organization.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others
Tuberculosis: a persistent health challenge for india.
Global trends and gaps in research related to latent tuberculosis infection

Global Tuberculosis Epidemiology
Pfuetze KH, Pyle MM, Hinshaw HC, Feldman WH. The first clinical trial of streptomycin in human tuberculosis. Am Rev Tuberc. 1955;71(5):752–4.
CAS PubMed Google Scholar
Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3(10 Suppl 2):S231–79.
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–44.
Article CAS Google Scholar
Barry CE, Boshoff H, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–55.
Lienhardt C, Espinal M, Pai M, Maher D, Raviglione MC. What research is needed to stop TB? Introducing the TB research movement. PLoS Med. 2011 Nov;8(11):e1001135.
Article Google Scholar
World Health Organization and Stop TB Partnership. An international roadmap for tuberculosis research. Geneva: World Health Organization; 2011. http://www.stoptb.org/assets/documents/resources/publications/technical/tbresearchroadmap.pdf
Google Scholar
Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. WHO’s new end TB strategy. Lancet. 2015;385(9979):1799–801.
Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E, et al. Phase 2b controlled trial of M72/AS01 E vaccine to prevent tuberculosis. N Engl J Med. 2018;379(17):1621–34.
Treatment Action Group. Tuberculosis research funding trends 2005–2018 [Internet]. 2019. Available from: https://www.treatmentactiongroup.org/wp-content/uploads/2019/12/tbrd_2019_web.pdf .
Moscow Declaration to End TB. First WHO global ministerial conference - ending TB in the sustainable development era: a multisectoral response (WHO/HTM/TB/2017.11) [Internet]. Moscow: Russian Federation; 2017. Available from: https://www.who.int/tb/features_archive/Moscow_Declaration_to_End_TB_final_ENGLISH.pdf .
A draft global strategy for TB research and innovation. WHO/GTB/version_30 September 2019. Geneva: World Health Organization; 2019.
Resolution WHA71.3. Preparation for a high-level meeting of the General Assembly on ending tuberculosis. In: Seventy-first World Health Assembly, 26 May 2018. Geneva: World Health Organization; 2018. Available from: https://apps.who.int/gb/ebwha/pdf_files/WHA71/A71_R3-en.pdf .
Ending tuberculosis. Draft global strategy for tuberculosis research and innovation. Report by the Director-General. EB146/11. [Internet]. Geneva: World Health Organization; 2019. Available from: https://apps.who.int/gb/ebwha/pdf_files/EB146/B146_11-en.pdf .
World Health Organization. A global action framework for TB research in support of the third pillar of WHO’s end TB strategy. Geneva: World Health Organization; 2015 [cited 2017 Nov 24]. Available from: http://www.who.int/tb/publications/global-framework-research/en/ .
Kritski A, Ruffino-Netto A, Trajman A, Villa TCS, Lapa e Silva JR, Haddad DJ, et al. Brazilian tuberculosis research network - REDE- TB. An Inst Hig Med Trop. 2016;15(Suppl 1):S35–44.
Kritski A, Barreira D, Junqueira-Kipnis AP, Moraes MO, Campos MM, Degrave WM, et al. Brazilian response to “global end tb strategy”: national tuberculosis research. Rev Brasil Med Trop. 2016;49(1):135–45. https://doi.org/10.1590/0037-8682-0330-2015 .
World Health Organization. Global tuberculosis report 2016. Geneva: WHO; 2016 [cited 2017 Nov 24]. Available in http://www.who.int/tb/publications/global_report/en/ .
Download references
Author information
Authors and affiliations.
Institut de Recherche pour le Développement, Montpellier, France
Christian Lienhardt
London School of Hygiene and Tropical Medicine, London, UK
Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland
Dennis Falzon & Matteo Zignol
School of Medicine - Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
Afranio Kritski
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christian Lienhardt .
Editor information
Editors and affiliations.
Servizio di Epidemiologia Clinica delle Malattie Respiratorie, Istituti Clinici Scientifici Maugeri IRCCS, Tradate, Italy
Giovanni Battista Migliori
Centre for Multidisciplinary Research in Health Science (MACH), University of Milan, Milan, Italy
Mario C. Raviglione
Rights and permissions
Reprints and permissions
Copyright information
© 2021 World Health Organization
About this chapter
Lienhardt, C., Falzon, D., Zignol, M., Kritski, A. (2021). An Overview of Research Priorities in Tuberculosis. In: Migliori, G.B., Raviglione, M.C. (eds) Essential Tuberculosis. Springer, Cham. https://doi.org/10.1007/978-3-030-66703-0_42
Download citation
DOI : https://doi.org/10.1007/978-3-030-66703-0_42
Published : 27 July 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-66705-4
Online ISBN : 978-3-030-66703-0
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Fact sheets
- Facts in pictures
Publications
- Questions and answers
- Tools and toolkits
- HIV and AIDS
- Hypertension
- Mental disorders
- Top 10 causes of death
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Activities /
Intensifying TB research and innovation
Tuberculosis (TB) is a major public health threat worldwide and remains the world’s top infectious killer. This situation owes much to the neglect of TB research over several decades. Motivating progress in research and innovation to end the TB epidemic by 2030 – as called for by the United Nations Sustainable Development Goals (SDGs), WHO End TB Strategy and the UN High-Level Meeting on TB – requires decisive action by all countries and stakeholders.
WHO is driving advocacy for increased TB research and innovation, joining forces with Member States, civil society, and technical and funding partners. To enable scientific collaboration, WHO convenes international expert consultations and national workshops on topics of critical importance.
WHO is supporting the implementation of the global strategy for TB research and innovation to foster collaboration, improve efficiency and increase financing for research and innovation. The strategy will provide coherence and direction in reaching the targets of the WHO End TB Strategy and the UN High-Level Meeting on TB, ensuring that political commitments and initiatives are exploited to the full.
WHO is also supporting the BRICS TB research network and promoting the use of efficient, life-saving digital health innovations in TB care delivery in countries.
candidate vaccines
were in clinical trials as of August 2023
funding shortfall
of the US$ 2 billion needed for research in 2022, only 1 billion was available
Related technical units
- Global TB Programme
Compendium of research projects at the interface of tuberculosis and COVID-19
A meeting on digital innovations, TB and implementation research organized by WHO and the European Respiratory Society
Development of a Global Strategy for TB Research and Innovation
To end TB, we must invest in research and innovation

Evidence and research gaps identified during development of policy guidelines for tuberculosis, 2nd ed
Achieving the goals and targets of the WHO End TB Strategy requires innovative tools and strategies as well as rapid progress towards universal access....

Consolidated report of country success stories in mitigating the impact of the COVID-19 pandemic on TB...
The response to the COVID-19 pandemic continues to adversely affect essential TB services in many countries. A first report of case studies was published...

Evidence and research gaps identified during development of policy guidelines for tuberculosis

A Global Strategy for tuberculosis research and innovation
The Global Strategy for Tuberculosis Research and Innovation will support the efforts of governments and other stakeholders to accelerate TB research...

Global investments in tuberculosis research and development: past, present and future. A policy paper...
Tuberculosis is the leading cause of death from a single infectious agent globally. The present and future threat that TB poses to human health is mainly...
Meeting reports

Report of the technical consultation on innovative clinical trial designs for evaluating new TB preventive...
The World Health Organization (WHO) Global Tuberculosis Programme, in collaboration with University College London and other partners, convened a virtual...

WHO consultation on the translation of tuberculosis research into global policy guidelines
The Global Tuberculosis Programme of the World Health Organization (WHO/GTB) has the mandate to develop and disseminate evidence-based policy for tuberculosis...

Report of the technical consultation on Innovative Clinical Trial Designs for Development of New TB Treatments
The Global Tuberculosis Programme of the World Health Organization (WHO) convened a virtual technical consultation on “Innovative Clinical Trial...


Digital innovations, TB and implementation research
WHO's Global Tuberculosis Programme (WHO/GTB) and the European Respiratory Society convened a technical meeting on digital innovations, TB and implementation...

Technical Consultation on latent TB infection management: research in support of scale-up
The Global TB Programme of the World Health Organization (WHO/GTB), in partnership with the WHO Collaborating Centre in TB Research at McGill University...

M72/AS01E Tuberculosis Vaccine Candidate - consensus-generating consultation on the development pathway
On 30-31 July 2019, the WHO convened in Geneva a meeting to generate consensus on the clinical development pathway for the M72/AS01E tuberculosis (TB)...

Report of the high-level consultation on accelerating the development of the M72/AS01E tuberculosis vaccine...
Tuberculosis (TB) is the deadliest infectious disease in human history and remains the leading cause of death from a single infectious agent globally....

Report of the Technical Consultation on Advances in Clinical Trial Design for Development of New TB Treatments
The Global TB Programme of the World Health Organization (WHO) convened a technical consultation on “Advances in Clinical Trial Design for Development...

Digital health for the End TB strategy: progress since 2015 and future perspectives

First TB Research Funders’ Forum
Although there are a number of challenges to establishing a successful research programs in low/middle income countries, three universal concerns include...

The role of e/mHealth in tuberculosis and tobacco control
Tuberculosis (TB) and tobacco smoking represent two major, global public health concerns. About 9 million new cases of TB emerge each year and 1.5 million...
Harnessing digital technologies for the TB response
Preventing TB
Scaling up diagnosis of TB and drug-resistant TB

Global strategy

Health topics
Digital health
Tuberculosis
Vaccines and immunization
- Subscriptions
- Advanced search

Advanced Search
Tuberculosis research questions identified through the WHO policy guideline development process
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Figures & Data
- Info & Metrics
WHO guideline development groups identify research questions using systematic reviews, economic analyses and stakeholder consultations during policy guidance development to identify urgent research gaps in the policy/implementation interface http://ow.ly/lUUw30nQZRO
High-quality research evidence is critical for improving global health and health equity, and for achieving the World Health Organization (WHO)'s objective of the attainment of the highest possible level of health by all peoples [ 1 ]. This need is most apparent when responding to complex epidemics such as tuberculosis (TB). TB is the leading killer among diseases caused by an infectious agent worldwide, the leading killer of people with HIV infection and a leading cause of death from airborne anti-microbial resistant infections, taking heavy tolls on human lives, communities and health systems at large [ 2 , 3 ]. WHO estimates that TB caused illness in 10 million people and claimed an estimated 1.6 million lives in 2017 alone [ 2 ]. The WHO End TB Strategy, in the context of the Sustainable Development Goals (SDGs), lays ambitious goals and milestones to end the epidemic by reducing incidence and mortality by 80% and 90% in 2030 compared to 2015: such reductions can only be achieved if there are major technological breakthroughs by 2025 [ 4 ].
Critical research is needed to acquire rapid point-of-care TB diagnostics, including for drug resistance; shorter, safer and simpler regimens effective against drug-susceptible and drug-resistant TB, as well as latent TB infection (LTBI) that are appropriate for treatment of TB/HIV co-infection; and a new TB vaccine that is effective both before and after exposure. These require scientific advances in the discovery and development of new biomedical tools, together with innovative delivery mechanisms to effectively adapt and adopt new technologies and optimise the necessary linkages and integrations with other health services and sectors. For this reason, “Intensified research and innovation” has been identified as one of the three essential pillars of the End-TB Strategy. This editorial summarises the research questions identified through recent WHO TB policy guidance to increase the quality of evidence for policy-making. Based on evidence arising from research, WHO is mandated to produce recommendations to guide clinical practice and public health policy for TB prevention and care in response to demand from public health decision makers. WHO guideline development groups (GDGs), which include researchers, the health workforce, civil society, as well as end-users of the guidelines, such as policymakers from government, professional associations and other constituencies, are appointed by WHO to develop policy guidelines [ 5 ]. A GDG meets with the primary objective of agreeing on the scope of recommendations by reviewing evidence, structured according to the standard framework of population, intervention, control, outcomes (PICO). This permits a systematic study of relevant evidence, the formulation of recommendations and the identification of knowledge gaps that need to be addressed through high quality research conducted in various epidemiological, demographic and geographic settings. The research questions highlighted in this document arose because the respective GDGs agreed they were critical for increasing the certainty/strength of existing recommendation, and/or for stimulating the development or optimisation of new recommendations that can lead to improvement in patient health and welfare. This step is an integral part of the WHO guideline development process (see, for example, the discussion section of F alzon et al . [ 6 ]).
Among the major challenges facing global policy guidance development in TB are the shortage of good quality evidence exacerbated, for example, by lack of sufficient clinical trials with direct evidence of clinical benefit or improvement in an established surrogate for clinical benefit; data inaccessibility including for programmatic experiences of benefits and safety of interventions in real world setting; or when the evidence being presented does not address broader questions of values and priorities that go beyond medical interventions ( e.g. acceptability, feasibility, resource distribution and health equity). Evidence obtained from well-designed, large scale multidisciplinary studies with robust testing of interventions are therefore needed to improve the strength of future guidance.
The most up-to-date WHO policy guidance documents for TB prevention and care are summarised in a Compendium of TB Guidelines and Associated Standards [ 7 , 8 ]. Using this compendium as a reference, we compiled a list of 155 research questions across the continuum of TB prevention, diagnosis, treatment and care (also summarised in table 1 ): three related to early detection; 35 related to diagnosis of TB disease, 10 related to the diagnosis and management of latent TB infection, 38 related to treatment of TB disease, including drug-resistant TB; 38 related to the management of TB/HIV and malnutrition; and 31 related to childhood TB management [ 10 ]. Because these research questions are limited in scope to needs identified during guideline development processes, the majority of the questions highlight gaps at the policy/implementation interface ( figure 1 ). Systematically linking such research questions to public health goals requires collaboration among funders, researchers and end users to ensure that funded research represents value for money, not only through the generation of new knowledge but also by contributing to health and economic outcomes. There are several ways of accomplishing that. The National Institute for Health Research Public Health Research Programme (NIHR PHR Programme) in the UK, for example, includes public health decision makers in its decision-making committee, and subsequently, the research it funds has been shown to align with priorities highlighted in national guidelines [ 11 ]. However, this is not the practice across all research funders. An exploratory qualitative study of funding strategy among five high-profile public health research funding organisations showed limited involvement from end users/policymakers in the prioritisation of research questions for funding [ 12 ]. Considering the need for well-funded, timely and high quality research for policy, funders should capitalise on opportunities to strengthen participation of policymakers and other end users in generating priority-driven research funding streams.
- Download figure
- Open in new tab
- Download powerpoint
Representation of the research questions documented in World Health Organization tuberculosis (TB) policy guidance documents. BCG: bacille Calmette–Guerin; LTBI: latent TB infection; MDR-TB: multidrug-resistant TB; Hr-TB: isoniazid-resistant TB.
- View inline
Research questions from the World Health Organization (WHO) tuberculosis (TB) policy guidance documents
At a time when there are many competing demands on limited resources, the WHO and its partners, countries, civil society and affected communities have a joint responsibility to ensure that TB research investments help achieve the goals and targets of the End TB Strategy and the SDGs. In recognition of this need, a TB resolution adopted at the World Health Assembly in May 2018 requested WHO to develop a global strategy for TB research and innovation, “to make further progress in enhancing cooperation and coordination in respect of tuberculosis research and development” [ 13 ]. Considering the significant funding gap for TB research (USD 1.3 billion gap in 2017 when benchmarked against the targets outlined in the Global Plan to End TB 2016–2020: the Paradigm Shift ), such coordination and collaboration is envisioned to help direct time and resources to the most urgent evidence needs faced by TB policymakers [ 14 – 16 ].
Conflict of interest: N. Gebreselassie has nothing to disclose.
Conflict of interest: D. Falzon has nothing to disclose.
Conflict of interest: M. Zignol has nothing to disclose.
Conflict of interest: T. Kasaeva has nothing to disclose.
- Received November 6, 2018.
- Accepted February 8, 2019.
- The content of this work is copyright of the authors or their employers. Design and branding are copyright ©ERS 2019.
- ↵ Constitution of the World-Health-Organization . Public Health Rep 1946 ; 61 : 1268 – 1277 . OpenUrl
- World Health Organization
- The Review on Antimicrobial Resistance
- Uplekar M ,
- Lonnroth K , et al.
- Schunemann HJ ,
- Harausz E , et al.
- Korobitsyn A ,
- Migliori GB , et al.
- World Health Organization, TB/HIV Working Group/Stop TB Partnership
- Cartier Y ,
- Creatore MI ,
- Hoffman SJ , et al.
- ↵ Stop TB Partnership. The Global Plan to End TB, 2016–2020: the Paradigm Shift. Geneva, United Nations Office for Project Services/Stop TB Partnership, 2015 .

- Table of Contents
- Index by author
Thank you for your interest in spreading the word on European Respiratory Society .
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
More in this TOC Section
- Moving the needle on proteasome inhibitor-induced PAH
- Pleural mesothelioma: surgery questioned again?
- Advances in pleural diseases
Related Articles
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PLoS Pathog
- v.5(10); 2009 Oct

The Past and Future of Tuberculosis Research
Iñaki comas.
Division of Mycobacterial Research, MRC National Institute for Medical Research, London, United Kingdom
Sebastien Gagneux
Renewed efforts in tuberculosis (TB) research have led to important new insights into the biology and epidemiology of this devastating disease. Yet, in the face of the modern epidemics of HIV/AIDS, diabetes, and multidrug resistance—all of which contribute to susceptibility to TB—global control of the disease will remain a formidable challenge for years to come. New high-throughput genomics technologies are already contributing to studies of TB's epidemiology, comparative genomics, evolution, and host–pathogen interaction. We argue here, however, that new multidisciplinary approaches—especially the integration of epidemiology with systems biology in what we call “systems epidemiology”—will be required to eliminate TB.
Introduction
Tuberculosis (TB) remains an important public health problem [1] . With close to 10 million new cases per year, and a pool of two billion latently infected individuals, control efforts are struggling in many parts of the world ( Figure 1 ). Nevertheless, the renewed interest in research and improved funding for TB give reasons for optimism. Recently, the Stop TB Partnership, a network of concerned governments, organizations, and donors lead by the WHO ( http://www.stoptb.org/stop_tb_initiative/ ), outlined a global plan to halve TB prevalence and mortality by 2015 and eliminate the disease as a public health problem by 2050 [2] .Attaining these goals will depend on both strong government commitment and increased interdisciplinary research and development. As existing diagnostics, drugs, and vaccines will be insufficient to achieve these objectives, a substantial effort in both basic science and epidemiology will be necessary to develop better tools and strategies to control TB [3] . Here we review the recent history of TB research and some of the latest insights into the evolutionary history of the disease. We then discuss ways in which we could benefit from a more comprehensive systems approach to control TB in the future.

The number of new TB cases per 100,000 population for the year 2007 according to WHO estimates (adapted from [1] ).
Recent History of the Field
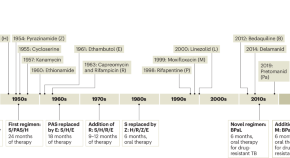
TB is caused by several species of gram-positive bacteria known as tubercle bacilli or Mycobacterium tuberculosis complex (MTBC). MTBC includes obligate human pathogens such as Mycobacterium tuberculosis and Mycobacterium africanum , as well as organisms adapted to various other species of mammal. In the developed world, TB incidence declined steadily during the second half of the 20th century and so funds available for research and control of TB decreased substantially during that time [4] . When TB started to reemerge in the early 1990s, fuelled by the growing pandemic of HIV/AIDS (Box 1), scientists and public health officials were caught off-guard; billions of dollars of emergency funds were necessary to control TB outbreaks [5] . Moreover, long-term neglect of basic TB research and product development meant that global TB control relied on a 100-year-old diagnostic method (i.e. sputum smear microscopy) of poor sensitivity, an 80-year-old and largely ineffective vaccine (Bacille Calmette-Guérin [BCG]), and just a few drugs that were decades old (streptomycin, rifampicin, isoniazid, ethambutol, pyrozinamide) [3] . Tragically, these are the tools still in use today in most parts of the world where TB remains one of the most important public health problems ( Figure 1 ).
Box 1. The Influence of Modern Epidemics on TB Incidence
HIV/AIDS and diabetes are important comorbidities that dramatically increase the susceptibility to TB. The synergy between TB and HIV/AIDS is a particular problem in sub-Saharan Africa, while the impact of diabetes on TB is increasing in many rapidly growing world economies; it may already be a more important risk factor for TB than HIV/AIDS in places like India and Mexico. The emergence of multidrug-resistant strains represents an additional threat to global TB control. The strong association between HIV/AIDS and drug-resistant TB has been well established, but whether similar interactions exist between drug-resistant TB and diabetes needs to be explored further.
In addition to the lack of appropriate tools to control TB globally, much about the disease was unknown in the early 1990s and many dogmas were guiding the field at the time. These included the view that differences in the clinical manifestation of TB were primarily driven by host variables and the environment as opposed to bacterial factors, a notion reinforced by early DNA sequencing studies that reported very limited genetic diversity in MTBC compared with other bacterial pathogens [6] . According to other dogmas, TB was mainly a consequence of reactivation of latent infections rather than ongoing disease transmission, and that mixed infections and exogenous reinfections with different strains were very unlikely.
The development of molecular techniques to differentiate between strains of MTBC made it possible to readdress some of these points. One of these methods, a DNA fingerprinting protocol based on the Mycobacterium insertion sequence IS6110, quickly evolved into the first international gold standard for genotyping of MTBC [7] . It also became a key component of pragmatic public health efforts, such as detecting disease outbreaks and ongoing TB transmission [8] , and allowed differentiation between patients who relapsed due to treatment failure and those reinfected with a different strain [9] . This latter finding demonstrated for the first time that previous exposure to MTBC does not protect against subsequent exogenous reinfection and TB disease, which is a phenomenon with implications for vaccine design. Many other new insights were gained through these molecular epidemiological studies [10] , which, for the most part, were performed in wealthy countries; corresponding data from most high-burden areas remained limited because of poor infrastructure and lack of funding.
Routine genotyping of MTBC for public health purposes also revived discussions about the role of pathogen variation in outcome of infection and disease. Some strains of MTBC appeared over-represented in particular patient populations, which suggested that strain diversity may have epidemiological implications. The completion of the first whole genome sequence of M. tuberculosis in 1998 [11] and the development of DNA microarrays offered a new opportunity to address this question by interrogating the entire genome of multiple clinical strains of MTBC. These comparative genomics studies revealed that genomic deletions, also known as large sequence polymorphisms (LSPs), are an important source of genome plasticity in MTBC [12] . Furthermore, statistical analyses of patient data suggested possible associations between strain genomic content and disease severity in humans [13] . Clinical phenotypes in TB are difficult to standardize, however, and whether MTBC genotype plays a meaningful role in TB severity remains controversial [14] .
Comparative genomics of MTBC also yielded interesting insights into the evolution and geographic distribution of the organism. Because MTBC has essentially no detectible horizontal gene transfer [15] , [16] , LSPs can be used as phylogenetic markers to trace the evolutionary relationships of different strain families. Following such an approach, studies have shown that humans did not, as previously believed, acquire MTBC from animals during the initiation of animal domestication, rather the human- and animal-adapted members of MTBC share a common ancestor, which might have infected humans even before the Neolithic transition [17] , [18] . LSPs also allowed researchers to define several discrete strain lineages within the human-adapted members of MTBC, which are associated with different human populations and geographical regions ( Figures 2 and and3) 3 ) [15] , [19] , [20] . Because of the lack of horizontal gene exchange in MTBC, phylogenetic trees derived using various molecular markers define the same phylogenetic groupings [21] , and several studies based on single nucleotide polymorphisms (SNPs) and other molecular makers have gathered additional support for the highly phylogeographical population structure of MTBC [22] – [25] .

Each dot represents the most frequent lineage(s) circulating in a country. Colours correspond to the lineages defined in Figure 3 (adapted from [20] ).

The phylogenic relationships between various human- and animal-adapted strains and species are largely consistent when defined by using either (A) large sequence polymorphisms (LSPs) or (B) single nucleotide polymorphisms (SNPs) identified by sequencing 89 genes in 108 MTBC strains. Numbers inside the squares in (A) refer to specific lineage-defining LSPs. Colors indicate congruent lineages (adapted from [20] and [29] ).
Ancient History of the Pathogen
Although LSPs have proven very useful for defining different lineages within MTBC, these markers do not reflect actual genetic distances, and the mode of molecular evolution in MTBC cannot be easily inferred from them [21] . By contrast, DNA sequence-based methods can provide important clues about the evolutionary forces shaping bacterial populations. Multilocus sequence typing (MLST), in which fragments of seven structural genes are sequenced for each strain [26] , has been used very successfully to define the genetic population structure of many bacterial species [27] . Because of the low degree of sequence polymorphisms in MTBC, however, standard MLST is uninformative [28] . A recent study of MTBC extended the traditional MLST scheme by sequencing 89 complete genes in 108 strains, covering 1.5% of the genome of each strain [29] . Phylogenetic analysis of this extended multilocus sequence dataset resulted in a tree that was highly congruent with that generated previously using LSPs ( Figure 3 ). The new sequence-based data also revealed that the MTBC strains that are adapted to various animal species represent just a subset of the global genetic diversity of MTBC that affects different human populations [29] . Furthermore, by comparing the geographical distribution of various human MTBC strains with their position on the phylogenetic tree, it became evident that MTBC most likely originated in Africa and that human MTBC originally spread out of Africa together with ancient human migrations along land routes. This view is further supported by the fact that the so-called “smooth tubercle bacilli,” which are the closest relatives of the human MTBC, are highly restricted to East Africa [30] . The multilocus sequence data reported by Hershberg et al. [29] further suggested a scenario in which the three “modern” lineages of MTBC (purple, blue, and red in Figure 3 ) seeded Eurasia, which experienced dramatic human population expansion in more recent times. These three lineages then spread globally out of Europe, India, and China, respectively, accompanying waves of colonization, trade and conquest. In contrast to the ancient human migrations, however, this more recent dispersal of human MTBC occurred primarily along water routes [29] .
The availability of comprehensive DNA sequence data has also allowed researchers to address questions about the molecular evolution of MTBC. In-depth population genetic analyses by Hershberg et al. highlight the fact that purifying selection against slightly deleterious mutations in this organism is strongly reduced compared to other bacteria [29] . As a consequence, nonsynonymous SNPs tend to accumulate in MTBC, leading to a high ratio of nonsynonymous to synonymous mutations (also known as dN/dS). The authors hypothesized that the high dN/dS in MTBC compared to most other bacteria might indicate increased random genetic drift associated with serial population bottlenecks during past human migrations and patient-to-patient transmission. If confirmed, this would indicate that “chance,” not just natural selection, has been driving the evolution of MTBC. Although these kinds of fundamental evolutionary questions are often underappreciated by clinicians and biomedical researchers, studying the evolution of a pathogen ultimately allows for better epidemiological predictions by contributing to our understanding of basic biology, particularly with respect to antibiotic resistance.
A Vision for the Future
Thanks to recent increases in research funding for TB [4] , substantial progress has been made in our understanding of the basic biology and epidemiology of the disease. Unfortunately, this increased knowledge has not yet had any noticeable impact on the current global trends of TB ( Figure 1 ). While TB incidence appears to have stabilized in many countries, the total number of cases is still increasing as a function of global human population growth [1] . Of particular concern are the ongoing epidemics of multidrug-resistant TB [31] , as well as the synergies between TB and the ongoing epidemics of HIV/AIDS and other comorbidities such as diabetes (Box 1).
As our understanding of TB improves, we would like to be able to make better predictions about the future trajectory of the disease and to develop new tools to control the disease better and ultimately reverse global trends. For this to be feasible, TB epidemiology needs to evolve into a more predictive, interdisciplinary endeavour; a discipline we might refer to as “systems epidemiology” ( Figure 4 ). Systems biology is already a rapidly emerging field, in which cycles of mathematical modelling and experiments using various large-scale “-omics” datasets are integrated in an iterative manner [32] . Novel biological processes are being discovered through these systems approaches, which might not have been possible using more traditional methods [33] – [35] .

The spread of TB is influenced by social and biological factors. On the one hand, the new discipline of systems biology integrates approaches that address the host, the pathogen, and interactions between the two. On the other hand, epidemiology addresses the burden of the disease and the social, economic, and ecological causes of its frequency and distribution. There is little crosstalk between these two disciplines at the moment. “Systems epidemiology” is an attempt to take into account the interactions between these various fields of research.
Last year, Young et al. argued that systems biology approaches will be necessary to elucidate some of the key aspects of host–pathogen interactions in TB [36] and to develop new drugs, vaccines, and biomarkers to evaluate new interventions [3] . For example, according to another dogma in the TB field, latent TB infections are caused by physiologically dormant bacilli and can thus be differentiated from active disease where MTBC is actively growing and dividing [37] . In reality, however, the phenomenon of TB latency most likely reflects a whole spectrum of responses to TB infection, involving phenotypically distinct bacterial subpopulations and spanning various degrees of bacterial burden and associated host immune responses [38] . We agree with Young et al. [36] that TB latency and similar biological complexities will only be adequately addressed using systems approaches, and we argue further that to comprehend the current TB epidemic as a whole, and to better predict its future trajectory, a complementary systems epidemiology approach will be necessary ( Figure 4 ).
Mathematical models are already being used extensively to study the epidemiology of TB and to guide control policies [39] . Recent applications have shown that socioeconomic factors are key drivers of today's TB epidemic [40] . In addition, much theoretical emphasis has been placed on trying to define the impact that drug resistance will have on the global TB epidemic [41] . Some of this theoretical work has become more complex by incorporating new biological insights obtained empirically and through targeted experimental studies. Early theoretical studies on the spread of drug-resistant MTBC were based on the assumption that all drug-resistant bacteria had an inherent fitness disadvantage compared to drug-susceptible strains [42] ; however, as is becoming clear from experimental and molecular epidemiological investigation, substantial heterogeneity exists with respect to the reproductive success of drug-resistant strains [43] – [46] . Newer mathematical models account for some of this heterogeneity [47] – [49] .
One could imagine an expansion of such mathematical approaches—much as systems biology operates—in which epidemiological modelling is combined with more comprehensive biological data related to the host, the pathogen, and their interactions ( Figure 4 ). Of course, environmental and sociological data would also need to be considered [40] . As mathematical models become more finely tuned, they could in turn inform future experimental work to test some of the specific predictions. The genomics revolution now offers the opportunity to study host–pathogen interactions at an unprecedented depth. To be able to make sense out of the current and upcoming deluge of -omics data, however, scientists will have to rely on a mathematically and statistically robust analytical framework. Ideally, some of these theoretical approaches will be able to accommodate increasingly diverse sets of data in order to capture the various biological, environmental, and social aspects of TB.
Among the newly emerging technologies, we believe that next-generation DNA sequencing will play an important role in improving our understanding of TB [50] . Whole-genome sequencing could potentially become the new gold standard for strain typing in routine molecular epidemiology [51] . For host genetics and TB susceptibility, too, de novo DNA sequencing based approaches could have advantages over traditional SNP typing [52] . For example, many of the human populations carrying the largest proportion of the global TB burden have not been sufficiently characterised genetically ( Figure 1 ) [53] , [54] , and screening for currently limited human SNP collections might have little relevance for these populations [55] . Furthermore, comprehensive DNA sequencing of TB patients and controls in various human populations could help unveil rare but biologically relevant mutations [56] . Another approach increasingly being used to study both the host and the pathogen is sequence-based transcriptomics, in which gene expression is measured by whole genome sequencing of RNA transcripts; a method referred to as RNA-seq [57] . One of the advantages of this approach over existing microarray-based methods is that changes in the expression of noncoding RNAs and other novel transcripts can be easily detected. RNA-seq is particularly useful for genome-wide studies of small regulatory RNAs, as such studies are more difficult to perform using standard DNA microarrays. Recent studies, for example, have reported a role for small regulatory RNAs in M. tuberculosis [58] , and there is little doubt more regulatory RNAs will soon be identified by RNA-seq [57] .
Challenges for the Future
Advances in TB research are hampered by the fact that MTBC is a Biosafety Level 3 pathogen with a long generation time, making it slow and complex to culture. Moreover, TB is a chronic disease that can develop over many years, and is characterised by extended periods of latency during which MTBC cannot be isolated from infected individuals. All of these factors complicate and prolong the development of new interventions and their assessment in clinical trials. As we have already mentioned, the field has been marked by a number of dogmas that, in some cases, might have contributed to the slow progress in TB research. New insights are now questioning some of these views, but at the same time, new opinions could well evolve into new dogmas. For example, we and others have spent much of our scientific careers seeking convincing evidence for the role of MTBC strain diversity in human disease. Although some pieces of evidence have recently started to emerge [59] – [61] , the subject needs more work. One of the problems has been that the macrophage and mouse infection models used in these studies relied on poorly characterised strains, and finding relevant links to human disease has been all but impossible [14] , [21] .
In TB control, too, potential new dogmas might emerge to limit future progress. A strong T cell–derived interferon gamma (INFγ) response appears to be crucial for the immunological control of TB, and many MTBC antigens have been identified based on their capacity to elicit INFγ responses in TB patients or their infected contacts [62] . Some of these antigens are being developed into new TB diagnostics and vaccines, but the potential impact of MTBC diversity on immune responses is not generally being considered [21] . A recent study in The Gambia showed that INFγ responses to one of the key MTBC antigens differed in an MTBC lineage–specific manner [63] . Developing a universally effective vaccine might be the only way to eliminate TB in the future [3] . This is particularly true given the large reservoir of latently infected individuals in the world, which would be impossible to eliminate through prophylactic drug treatment. Considering that natural TB infection does not protect against exogenous reinfection and disease, however, mimicking natural infection using attenuated strains or a cocktail of traditional INFγ-inducing antigens might not necessarily be the most promising vaccine strategy. Indeed, the largely unsuccessful implementation of BCG vaccination might serve as a warning [64] .
Acknowledgments
We thank Peter Small and Douglas Young for comments on the manuscript.
The authors have declared that no competing interests exist.
Work in our laboratory is supported by the Medical Research Council, UK, and the US National Institutes of Health grants HHSN266200700022C and AI034238. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Implications of subclinical tuberculosis for vaccine trial design and global effect
Affiliations.
- 1 Aurum Institute NPC, Houghton, Parktown, South Africa; Department of Medicine, Vanderbilt University, Nashville, TN, USA; School of Public Health, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa. Electronic address: [email protected].
- 2 Department of Infectious Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK; TB Modelling Group, TB Centre, London School of Hygiene & Tropical Medicine, London, UK.
- 3 Department of Infectious Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK.
- 4 University of Washington, Seattle, WA, USA.
- 5 MRC Clinical Trials Unit, University College London, London, United Kingdon; WHO Collaborating Centre for TB Research and Innovation, Institute for Global Health, University College London, London, UK.
- 6 TB Centre, London School of Hygiene & Tropical Medicine, London, UK; Department of Clinical Research, London School of Hygiene & Tropical Medicine, London, UK; Africa Health Research Institute, KwaZulu-Natal, Durban, South Africa.
- 7 MRC Clinical Trials Unit, University College London, London, United Kingdon; CIDRI-AFRICA, School of Public Health, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa.
- 8 McGill International TB Centre, McGill University, Montreal, QC, Canada.
- 9 ISGlobal, Hospital Clínic - Universitat de Barcelona, Barcelona, Spain; Centro de Investigação em Saúde de Manhiça (CISM), Maputo, Mozambique; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFECT), Barcelona, Spain.
- 10 Africa Health Research Institute, KwaZulu-Natal, Durban, South Africa; Division of Infectious Diseases, Heersink School of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA.
- 11 South African Tuberculosis Vaccine Initiative, Department of Pathology and Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa.
- 12 Byramjee-Jeejeebhoy Government Medical College, Johns Hopkins University Clinical Research Site, Pune, India.
- 13 Gates Medical Research Institute, Cambridge, MA, USA.
- 14 Division of Infection and Immunity, University College London, London, United Kingdon; Africa Health Research Institute, KwaZulu-Natal, Durban, South Africa.
- 15 Department of Global Health and Amsterdam Institute for Global Health and Development, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands.
- PMID: 38964359
- DOI: 10.1016/S2666-5247(24)00127-7
Tuberculosis is a leading cause of death from an infectious agent globally. Infectious subclinical tuberculosis accounts for almost half of all tuberculosis cases in national tuberculosis prevalence surveys, and possibly contributes to transmission and might be associated with morbidity. Modelling studies suggest that new tuberculosis vaccines could have substantial health and economic effects, partly based on the assumptions made regarding subclinical tuberculosis. Evaluating the efficacy of prevention of disease tuberculosis vaccines intended for preventing both clinical and subclinical tuberculosis is a priority. Incorporation of subclinical tuberculosis as a composite endpoint in tuberculosis vaccine trials can help to reduce the sample size and duration of follow-up and to evaluate the efficacy of tuberculosis vaccines in preventing clinical and subclinical tuberculosis. Several design options with various benefits, limitations, and ethical considerations are possible in this regard, which would allow for the generation of the evidence needed to estimate the positive global effects of tuberculosis vaccine trials, in addition to informing policy and vaccination strategies.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Declaration of interests The following authors declare grants to their respective institutions. GJC: Division of AIDS/NIH. ALF-G: Bill & Melinda Gates Foundation, US NIH for HIV Vaccine Trials Network Statistical Center. ALG-B: Barcelona Institute for Global Health tenure track associate research professorship. EBW: Burroughs Wellcome Fund, 1022002; Bill & Melinda Gates Foundation, INV-046520; NIH/National Institute of Allergy and Infectious Diseases, AI201700104 and AI147321. FC: Bill & Melinda Gates Foundation, EDCTP. HE: Canadian Institutes for Health Research, Tier 1 Canada Research Chair. VM: NIH, Centers for Disease Control and Prevention. MH: NIH, EDCTP, Gates Medical Research Institute. RMGJH: European Research Council. RGW: Wellcome Trust (218261/Z/19/Z), NIH (1R01AI147321-01, G-202303-69963), EDCTP (RIA208D-2505B), UK Medical Research Council (CCF17-7779 Bloomsbury), Economic and Social Research Council (ES/P008011/1), Bill & Melinda Gates Foundation (INV-004737, INV-035506), WHO (2020/985800-0). ADG, WAH, MB, ACS, KF, AFD, and MXR declare no competing interests. The following authors declare royalties. ALG-B: Royalties for the patent Molecular differences between species of the Mycobacterium tuberculosis complex in November, 2020 and November, 2021. HE: Royalties to Stanford University for the patent Molecular differences between species of the Mycobacterium tuberculosis complex in November, 2020 and November, 2021.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Elsevier Science

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
July 2, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
New biomarker identified for predicting adverse events of tuberculosis therapy
by Leibniz Lungenzentrum
Borstel researchers are the first to develop a biomarker to predict the occurrence of neuropathic adverse events during therapy for multidrug-resistant tuberculosis. The results have now been published in the journal Pathogens & Immunity .
Every year, an estimated 410,000 people worldwide contract a multidrug-resistant form of tuberculosis. During treatment, approximately a quarter of patients experience linezolid-associated adverse drug reactions (ADRs) associated with one of the medicines, linezolid. These include nerve disorders such as peripheral neuropathies and optic neuropathies. They cause significant impairment of the quality of life of those affected, weaken adherence to therapy and thus jeopardize the success of treatment.
To date, the underlying mechanisms of linezolid-associated ADRs are not fully understood. A team of researchers from Borstel investigated whether the risk of linezolid-associated neuropathies can be identified and predicted before the start of treatment using transcriptome analyses. The term transcriptome refers to the sum of ribonucleic acids (RNAs).
These are quasi photocopies from genes, which are located in the cell nucleus on chromosomes. As mobile units, the RNAs pass on the information of the genes to other parts of the cells. Their information is transcribed into so-called amino acids within the cells. Proteins are formed from amino acids and these in turn form structures and control elements in the metabolism.
When genes are switched on or off, more or fewer RNA copies are produced. Molecular methods can be used to simultaneously measure the copies of around 50,000 genes in a person's blood and thus determine whether metabolic pathways are switched on or off.
Dr. Maja Reimann from the Research Center Borstel, Leibniz Lung Center (FZB) is an expert in the field of reading this information from cells, processing it and reducing it to a few units of information using mathematical methods and models. This is how she develops biomarkers to predict clinical events.
As part of a team of researchers and doctors, she and Nika Zielinski, a doctoral student in the Clinical Infectiology Group at the Research Center Borstel, have discovered a biomarker that can predict the future occurrence of a serious side effect of tuberculosis therapy.
To this end, data from tuberculosis patients collected by the Borstel team since 2015 as part of studies by the German Center for Infection Research (DZIF) was analyzed. In addition to the systems biology determination of genes and metabolic pathways that were already significantly up- or downregulated in patients with and without Linezolid-associated neuropathies before the start of therapy, a machine learning algorithm was developed.
This identified the molecule Suprabasin (SBSN) as a biomarker for the prediction of neuropathies. The team then investigated the biomarker in an independent group of TB patients. Suprabasin was also able to predict the occurrence of side effects in this cohort.
"The accuracy for predicting neuropathies under tuberculosis therapy with linezolid using the Suprabasin biomarker is not outstanding, but it is the first biomarker ever that can be used to estimate the risk of developing these side effects under therapy," says Nika Zielinski, the first author of the study.
Explore further
Feedback to editors

Study links social and non-social synchrony to romantic attractiveness
17 hours ago

Diabetes drugs like Ozempic lower cancer risks: Study
21 hours ago

WHO agency says talc is 'probably' cancer-causing
Jul 5, 2024

New discovery reveals TRP14 is a crucial enzyme for cysteine metabolism, disease resistance

Researchers find biological clues to mental health impacts of prenatal cannabis exposure

Nanoscopic motor proteins in the brain build the physical structures of memory, study finds

Smoking is a key lifestyle factor linked to cognitive decline among older adults

Researchers aim to change contraceptive technology with new iron IUDs

Military service's hidden health toll: Servicewomen and their families endure increased chronic pain, finds study

Genomic variants study points to improved detection of thyroid cancer
Related stories.
Tuberculosis: New biomarker indicates individual treatment duration
Feb 19, 2021

Tailor-made therapy of multi-resistant tuberculosis
May 3, 2021

Eight-week bedaquiline-linezolid noninferior for TB
Feb 28, 2023

Study finds tuberculosis treatment during pregnancy is safe for mom and baby
Jun 13, 2022

Mozambique faces alarming multidrug-resistant tuberculosis epidemic
Nov 13, 2023

Biomarker could improve prediction of response to immunotherapy in melanoma
May 16, 2024
Recommended for you

Scientists identify thousands of high-risk cancer gene variants

Researchers map the effects of all potential changes in key cancer gene

Researchers identify unknown signaling pathway in the brain responsible for migraine with aura
Jul 4, 2024

Scientists discover new T cells and genes related to immune disorders

Target discovered for the treatment of pancreatic cancer
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Stanford Cancer Institute
Search stanford cancer institute, novartis call for pre-proposals: research project program.
Deadline: August 1, 2024
Novartis's objective is to work together with academic institutions to de-risk innovation and bridge the translational gap of early-stage research. We would like to collaborate and form long-term relationships with academic scholars who are deeply committed to translating their scientific discoveries into patients. We offer academic institutions new opportunities for scientific engagement with Novartis through in-licensing of academic technologies, research collaborations and opportunities for co-creation with our scientists, and long-term strategic partnerships. We are committed to working together with academic scholars to translate the next great innovative ideas into powerful medicines to change the future of medicine.
Please find detailed information and the Pre-Proposal Submission Template here: https://seedfunding.stanford.edu/opportunities/novartis-call-pre-proposals . There should be NO confidential information disclosed in the pre-proposals.
Amount of Funding: The budget amount is expected to be $100,000 to $300,000 for one year (including indirect costs). Novartis will rely on what the faculty, in collaboration with RMG, estimate as appropriate; however, details on budget would not be explored unless a pre-proposal is selected for a full proposal submission.
Eligibility: Stanford faculty with PI eligibility and CE faculty (with an approved CE faculty PI waiver). CE applicants must obtain an approved CE faculty PI waiver BEFORE the pre-proposal deadline. Instructions for CE faculty PI waiver requests: Submit the required, completed CE faculty PI waiver template and attachments directly to Kathleen Thompson at [email protected] by end of day on July 29, 2024. Please do not contact your RPM/RMG or CGO/OSR regarding waivers for this program.
Areas of Interest: Cardiovascular & metabolic Renal diseases Oncology Immunology Neuroscience Diseases of aging and regenerative medicines Gene therapies xRNA Please see the PDF attachments for specific thematic Novartis priorities under each area.
Timeline: July 29, 2024 – CE faculty PI waiver requests due (if applicable) August 1, 2024 – Pre-proposal submission deadline to Stanford Medicine - Industry Relations (SM-IR) team August 12, 2024 – Submission of completed pre-proposals to Novartis by SM-IR Deadlines for full proposal submission will be announced at a later time to selected faculty. This timeline will include the institutional representative (RPM/RMG or CGO/OSR) review deadline and Proposal Intake Form (PIF) requirement.
Submission Instructions for Pre-proposals: Fill out the attached pre-proposal template ( https://seedfunding.stanford.edu/opportunities/novartis-call-pre-proposals ).
Email the complete pre-proposal to Nour Malek at [email protected] and Nicole Kay at [email protected] before August 1, 2024. The Industry Relations team will review the submissions with the Office Technological Licensing (OTL) and Industry Contracting Office (ICO) and then formally submit all pre-proposals to Novartis on behalf of Stanford. There should be NO confidential information disclosed in the pre-proposals.
Institutional representative: you do not need to submit your pre-proposals through RMG or OSR for institutional approval; you may submit your pre-proposal directly to Nour Malek and Nicole Kay per the instructions above. If invited to submit a full proposal, please initiate your Proposal Intake Form (PIF) at your earliest convenience. Final proposal submission will require institutional approval prior to submission.
Any CE faculty PI waiver requests should be submitted directly to Kathleen Thompson per the instructions above.
Questions? Please contact Nour Malek at [email protected] and Nicole Kay at [email protected].
Stanford Resources Funding Information for the Stanford community Limited submission programs, NIH resources, DoD CDMRP, internal funding opportunities, searchable funding databases. Institutional representatives and
Internal Proposal Deadline Policy School of Medicine PIs: RPM Department Assignments and SoM internal proposal deadline policy
A Proposal Intake Form (PIF) in SeRA is required for all sponsored project proposal submissions in the School of Medicine (except for fellowships, clinical trials, and Internal Seed Funding NOT from sponsored projects).
PIs in all Other Schools: Office of Sponsored Research (OSR) Contract and Grant Officer Pre-Award & Post-award department assignments
- Patient Care
- Clinical Trials
- Health Equity
- Shared Resources
Stanford Medicine
Health care.

©2024 Stanford Medicine

IMAGES
VIDEO
COMMENTS
Tuberculosis (TB) research and innovation is essential to achieve the global TB targets of the United Nations (UN) Sustainable Development Goals (SDGs) and the World Health Organization (WHO) End TB Strategy. The SDG target is to "end the epidemic" by 2030; more specific targets for 2030 set in the End TB Strategy are a 90% reduction in TB ...
Tuberculosis research. Tuberculosis is the leading cause of death from a single infectious agent and remains a global health emergency. In 2018 alone, there were 1.5 million deaths and 10 million new cases globally, among whom half a million had rifampicin resistant TB.
Introduction. Tuberculosis (TB) continues to pose a major threat to global health , and research is a key component of the Global Plan to Stop TB2011-2015 .Research is particularly critical for developing new tools and approaches needed for eliminating TB by 2050 .Recognizing this, the Stop TB Partnership and the World Health Organization's (WHO) Stop TB Department have launched the TB ...
This NIAID Strategic Plan for Tuberculosis Research proposes building on current trans-NIAID efforts to understand better the immunology and pathogenesis of TB and expanding resources to quickly develop new tools to more effectively combat this disease. These tools include preventive vaccines and therapies, less-toxic treatment regimens of ...
Tuberculosis is the leading cause of death from a single infectious agent, with over 25% of these occurring in the African region. Multi-drug resistant strains which do not respond to first-line ...
The plan is being released to coincide with the United Nations General Assembly High-Level Meeting on Ending TB on September 26. In 2017, an estimated 3.6 million of 10 million new TB cases went undiagnosed or unreported, according to the World Health Organization. To address this gap, NIAID is calling for improved TB diagnostics, specifically ...
TB research and innovation. 7. TB research and innovation. Tuberculosis (TB) research and innovation is essential to achieve global TB targets for reductions in TB incidence and TB deaths. The targets of the WHO End TB Strategy (1), adopted in 2014, required a global rate of decline in TB incidence of 17% per year between 2025 and 2035 ...
Robert Koch isolated the pathogen Mycobacterium tuberculosis ( Mtb) in 1882 and formulated Koch's postulates, criteria for establishing any infectious agent as causative of disease ( Koch, 1982 ). In 2021, Mtb was estimated to be responsible for 10.6 million new cases of TB and 1.6 million deaths ( WHO, 2022a ).
Abstract. Intensified research and innovation is one of the three pillars of the End-TB Strategy launched by the World Health Organization in 2015. This underscores the essentiality of research and development to reach the ambitious targets of reducing tuberculosis (TB) incidence and deaths by 80% and 90%, respectively, by the year 2030.
Global mobilization of sufficient and sustainable financing for universal access to quality prevention, diagnosis, treatment and care of tuberculosis. At least US$ 13 billion annually by 2022. Mobilization globally of sufficient and sustainable financing for tuberculosis research. US$ 2 billion annually, over the period 2018-2022.
PRIORITIES FOR TUBERCULOSIS RESEARCH 7 Professor B. Xu Deputy Chair, Department of Epidemiology and Director, Tuberculosis Research Centre, School of Public Health, Fudan University, Shanghai, China. Professor C. Y. Yu Co-Chair, WHO Tropical Disease Reference Group (TB, Leprosy, Buruli Ulcer);
the commitments on research and innovation articulated in those declarations. 4. The Global Strategy for Tuberculosis Research and Innovation will support the efforts of governments and other stakeholders to accelerate TB research and innovation, and improve equitable accessto the benefits of research. It will do so through clear objectives and
To eliminate tuberculosis globally, a new, effective, and affordable vaccine is urgently needed, particularly for use in adults and adolescents in low-income and middle-income countries. We have created a roadmap that lists the actions needed to accelerate tuberculosis vaccine research and development using a participatory process. The vaccine pipeline needs more diverse immunological ...
We found 33 documents that specifically outline priorities in tuberculosis research. The top priority areas were drug development (28 articles), diagnosis and diagnostic tests (27), epidemiology (20), health services research (16), basic research (13), and vaccine development and use (13). The most focused questions were on the treatment and ...
Tuberculosis (TB) is a major public health threat worldwide and remains the world's top infectious killer. This situation owes much to the neglect of TB research over several decades. Motivating progress in research and innovation to end the TB epidemic by 2030 - as called for by the United Nations Sustainable Development Goals (SDGs), WHO End TB Strategy and the UN High-Level Meeting on ...
High-quality research evidence is critical for improving global health and health equity, and for achieving the World Health Organization (WHO)'s objective of the attainment of the highest possible level of health by all peoples [1]. This need is most apparent when responding to complex epidemics such as tuberculosis (TB). TB is the leading killer among diseases caused by an infectious agent ...
research methods such as focus groups, in-depth interviews and observation. Use of these methods is discussed in the section entitled Supplementary research activities. To guide survey design and data collection, TB programme managers need practical tools and guidelines. This guide presents practical guid-ance for conducting a KAP survey for TB ...
Mycobacterium tuberculosis, the bacterium that causes tuberculosis (TB), infects approximately a third of the global population [1,2]. M. tuberculosis is transmitted through the air via droplets when an infectious individual talks, sings [3], or coughs [4,5]. Tuberculosis infections can be classified as either "latent" (non-transmissible TB
Approximately 10·6 million people worldwide develop tuberculosis each year, representing a failure in epidemic control that is accentuated by the absence of effective vaccines to prevent infection or disease in adolescents and adults. Without effective vaccines, tuberculosis prevention has relied on testing for Mycobacterium tuberculosis infection and treating with antibiotics to prevent ...
To eliminate tuberculosis globally, a new, efective, and afordable vaccine is urgently needed, particularly for use in adults and adolescents in low-income and middle-income countries. We have created a roadmap that lists the actions needed to accelerate tuberculosis vaccine research and development using a participatory process.
ascertain the major funders of tuberculosis (TB) research and development (R&D) in 2005, what kinds of research activity they funded, and how much research activity is already taking place. This assessment will help policymakers, funders, researchers, and advocates understand the current state of research on TB, and it provides a
Introduction. Tuberculosis (TB) remains an important public health problem .With close to 10 million new cases per year, and a pool of two billion latently infected individuals, control efforts are struggling in many parts of the world (Figure 1).Nevertheless, the renewed interest in research and improved funding for TB give reasons for optimism.
Tuberculosis (TB) remains a significant global health challenge, with millions of new cases reported each year. Early detection and timely intervention are crucial in preventing the spread of the disease and reducing its impact on affected individuals and communities. This project proposal aims to implement a comprehensive strategy for the early detection and control of
Tuberculosis is a leading cause of death from an infectious agent globally. Infectious subclinical tuberculosis accounts for almost half of all tuberculosis cases in national tuberculosis prevalence surveys, and possibly contributes to transmission and might be associated with morbidity. ... 10 Africa Health Research Institute, KwaZulu-Natal ...
To this end, data from tuberculosis patients collected by the Borstel team since 2015 as part of studies by the German Center for Infection Research (DZIF) was analyzed.
Novartis Call for Pre-Proposals: Research Project Program. Deadline: August 1, 2024. Novartis's objective is to work together with academic institutions to de-risk innovation and bridge the translational gap of early-stage research. We would like to collaborate and form long-term relationships with academic scholars who are deeply committed to ...