

Research Types Explained: Basic, Clinical, Translational
“Research” is a broad stroke of a word, the verbal equivalent of painting a wall instead of a masterpiece. There are important distinctions among the three principal types of medical research — basic, clinical and translational.
Whereas basic research is looking at questions related to how nature works, translational research aims to take what’s learned in basic research and apply that in the development of solutions to medical problems. Clinical research, then, takes those solutions and studies them in clinical trials. Together, they form a continuous research loop that transforms ideas into action in the form of new treatments and tests, and advances cutting-edge developments from the lab bench to the patient’s bedside and back again.
Basic Research
When it comes to science, the “basic” in basic research describes something that’s an essential starting point. “If you think of it in terms of construction, you can’t put up a beautiful, elegant house without first putting in a foundation,” says David Frank, MD , Associate Professor of Medicine, Medical Oncology, at Dana-Farber Cancer Institute. “In science, if you don’t first understand the basic research, then you can’t move on to advanced applications.”
Basic medical research is usually conducted by scientists with a PhD in such fields as biology and chemistry, among many others. They study the core building blocks of life — DNA, cells, proteins, molecules, etc. — to answer fundamental questions about their structures and how they work.
For example, oncologists now know that mutations in DNA enable the unchecked growth of cells in cancer. A scientist conducting basic research might ask: How does DNA work in a healthy cell? How do mutations occur? Where along the DNA sequence do mutations happen? And why?
“Basic research is fundamentally curiosity-driven research,” says Milka Kostic, Program Director, Chemical Biology at Dana-Farber Cancer Institute. “Think of that moment when an apple fell on Isaac Newton’s head. He thought to himself, ‘Why did that happen?’ and then went on to try to find the answer. That’s basic research.”

Clinical Research
Clinical research explores whether new treatments, medications and diagnostic techniques are safe and effective in patients. Physicians administer these to patients in rigorously controlled clinical trials, so that they can accurately and precisely monitor patients’ progress and evaluate the treatment’s efficacy, or measurable benefit.
“In clinical research, we’re trying to define the best treatment for a patient with a given condition,” Frank says. “We’re asking such questions as: Will this new treatment extend the life of a patient with a given type of cancer? Could this supportive medication diminish nausea, diarrhea or other side effects? Could this diagnostic test help physicians detect cancer earlier or distinguish between fast- and slow-growing cancers?”
Successful clinical researchers must draw on not only their medical training but also their knowledge of such areas as statistics, controls and regulatory compliance.
Translational Research
It’s neither practical nor safe to transition directly from studying individual cells to testing on patients. Translational research provides that crucial pivot point. It bridges the gap between basic and clinical research by bringing together a number of specialists to refine and advance the application of a discovery. “Biomedical science is so complex, and there’s so much knowledge available.” Frank says. “It’s through collaboration that advances are made.”
For example, let’s say a basic researcher has identified a gene that looks like a promising candidate for targeted therapy. Translational researchers would then evaluate thousands, if not millions, of potential compounds for the ideal combination that could be developed into a medicine to achieve the desired effect. They’d refine and test the compound on intermediate models, in laboratory and animal models. Then they would analyze those test results to determine proper dosage, side effects and other safety considerations before moving to first-in-human clinical trials. It’s the complex interplay of chemistry, biology, oncology, biostatistics, genomics, pharmacology and other specialties that makes such a translational study a success.
Collaboration and technology have been the twin drivers of recent quantum leaps in the quality and quantity of translational research. “Now, using modern molecular techniques,” Frank says, “we can learn so much from a tissue sample from a patient that we couldn’t before.”
Translational research provides a crucial pivot point after clinical trials as well. Investigators explore how the trial’s resulting treatment or guidelines can be implemented by physicians in their practice. And the clinical outcomes might also motivate basic researchers to reevaluate their original assumptions.
“Translational research is a two-way street,” Kostic says. “There is always conversation flowing in both directions. It’s a loop, a continuous cycle, with one research result inspiring another.”
Learn more about research at Dana-Farber .
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Clinical Trials: What Patients Need to Know
What Are the Different Types of Clinical Research?
Different types of clinical research are used depending on what the researchers are studying. Below are descriptions of some different kinds of clinical research.
Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to surgery or radiation therapy.
Prevention Research looks for better ways to prevent disorders from developing or returning. Different kinds of prevention research may study medicines, vitamins, vaccines, minerals, or lifestyle changes.
Diagnostic Research refers to the practice of looking for better ways to identify a particular disorder or condition.
Screening Research aims to find the best ways to detect certain disorders or health conditions.
Quality of Life Research explores ways to improve comfort and the quality of life for individuals with a chronic illness.
Genetic studies aim to improve the prediction of disorders by identifying and understanding how genes and illnesses may be related. Research in this area may explore ways in which a person’s genes make him or her more or less likely to develop a disorder. This may lead to development of tailor-made treatments based on a patient’s genetic make-up.
Epidemiological studies seek to identify the patterns, causes, and control of disorders in groups of people.
An important note: some clinical research is “outpatient,” meaning that participants do not stay overnight at the hospital. Some is “inpatient,” meaning that participants will need to stay for at least one night in the hospital or research center. Be sure to ask the researchers what their study requires.
Phases of clinical trials: when clinical research is used to evaluate medications and devices Clinical trials are a kind of clinical research designed to evaluate and test new interventions such as psychotherapy or medications. Clinical trials are often conducted in four phases. The trials at each phase have a different purpose and help scientists answer different questions.
Phase I trials Researchers test an experimental drug or treatment in a small group of people for the first time. The researchers evaluate the treatment’s safety, determine a safe dosage range, and identify side effects.
Phase II trials The experimental drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety.
Phase III trials The experimental study drug or treatment is given to large groups of people. Researchers confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug or treatment to be used safely.
Phase IV trials Post-marketing studies, which are conducted after a treatment is approved for use by the FDA, provide additional information including the treatment or drug’s risks, benefits, and best use.
Examples of other kinds of clinical research Many people believe that all clinical research involves testing of new medications or devices. This is not true, however. Some studies do not involve testing medications and a person’s regular medications may not need to be changed. Healthy volunteers are also needed so that researchers can compare their results to results of people with the illness being studied. Some examples of other kinds of research include the following:
A long-term study that involves psychological tests or brain scans
A genetic study that involves blood tests but no changes in medication
A study of family history that involves talking to family members to learn about people’s medical needs and history.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you.
The NIH Clinical Trials and You website is a resource for people who want to learn more about clinical trials. By expanding the below questions, you can read answers to common questions about taking part in a clinical trial.
What are clinical trials and why do people participate?
Clinical research is medical research that involves people like you. When you volunteer to take part in clinical research, you help doctors and researchers learn more about disease and improve health care for people in the future. Clinical research includes all research that involves people. Types of clinical research include:

- Epidemiology, which improves the understanding of a disease by studying patterns, causes, and effects of health and disease in specific groups.
- Behavioral, which improves the understanding of human behavior and how it relates to health and disease.
- Health services, which looks at how people access health care providers and health care services, how much care costs, and what happens to patients as a result of this care.
- Clinical trials, which evaluate the effects of an intervention on health outcomes.
What are clinical trials and why would I want to take part?
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Clinical trials can study:
- New drugs or new combinations of drugs
- New ways of doing surgery
- New medical devices
- New ways to use existing treatments
- New ways to change behaviors to improve health
- New ways to improve the quality of life for people with acute or chronic illnesses.
The goal of clinical trials is to determine if these treatment, prevention, and behavior approaches are safe and effective. People take part in clinical trials for many reasons. Healthy volunteers say they take part to help others and to contribute to moving science forward. People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff. Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future
Why is diversity and inclusion important in clinical trials?
People may experience the same disease differently. It’s essential that clinical trials include people with a variety of lived experiences and living conditions, as well as characteristics like race and ethnicity, age, sex, and sexual orientation, so that all communities benefit from scientific advances.
See Diversity & Inclusion in Clinical Trials for more information.
How does the research process work?
The idea for a clinical trial often starts in the lab. After researchers test new treatments or procedures in the lab and in animals, the most promising treatments are moved into clinical trials. As new treatments move through a series of steps called phases, more information is gained about the treatment, its risks, and its effectiveness.
What are clinical trial protocols?
Clinical trials follow a plan known as a protocol. The protocol is carefully designed to balance the potential benefits and risks to participants, and answer specific research questions. A protocol describes the following:
- The goal of the study
- Who is eligible to take part in the trial
- Protections against risks to participants
- Details about tests, procedures, and treatments
- How long the trial is expected to last
- What information will be gathered
A clinical trial is led by a principal investigator (PI). Members of the research team regularly monitor the participants’ health to determine the study’s safety and effectiveness.
What is an Institutional Review Board?
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are reduced and are outweighed by potential benefits. IRBs are committees that are responsible for reviewing research in order to protect the rights and safety of people who take part in research, both before the research starts and as it proceeds. You should ask the sponsor or research coordinator whether the research you are thinking about joining was reviewed by an IRB.
What is a clinical trial sponsor?
Clinical trial sponsors may be people, institutions, companies, government agencies, or other organizations that are responsible for initiating, managing or financing the clinical trial, but do not conduct the research.
What is informed consent?
Informed consent is the process of providing you with key information about a research study before you decide whether to accept the offer to take part. The process of informed consent continues throughout the study. To help you decide whether to take part, members of the research team explain the details of the study. If you do not understand English, a translator or interpreter may be provided. The research team provides an informed consent document that includes details about the study, such as its purpose, how long it’s expected to last, tests or procedures that will be done as part of the research, and who to contact for further information. The informed consent document also explains risks and potential benefits. You can then decide whether to sign the document. Taking part in a clinical trial is voluntary and you can leave the study at any time.
What are the types of clinical trials?
There are different types of clinical trials.
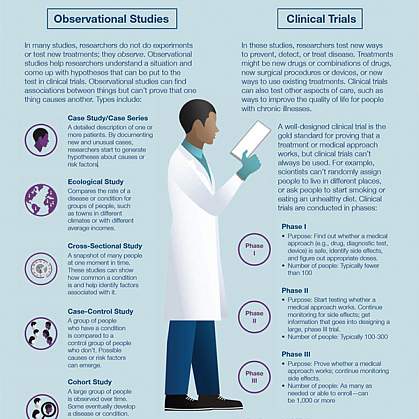
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning. Approaches may include medicines, vaccines, or lifestyle changes.
- Screening trials test new ways for detecting diseases or health conditions.
- Diagnostic trials study or compare tests or procedures for diagnosing a particular disease or condition.
- Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery or radiation therapy.
- Behavioral trials evaluate or compare ways to promote behavioral changes designed to improve health.
- Quality of life trials (or supportive care trials) explore and measure ways to improve the comfort and quality of life of people with conditions or illnesses.
What are the phases of clinical trials?
Clinical trials are conducted in a series of steps called “phases.” Each phase has a different purpose and helps researchers answer different questions.
- Phase I trials : Researchers test a drug or treatment in a small group of people (20–80) for the first time. The purpose is to study the drug or treatment to learn about safety and identify side effects.
- Phase II trials : The new drug or treatment is given to a larger group of people (100–300) to determine its effectiveness and to further study its safety.
- Phase III trials : The new drug or treatment is given to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or similar treatments, and collect information that will allow the new drug or treatment to be used safely.
- Phase IV trials : After a drug is approved by the FDA and made available to the public, researchers track its safety in the general population, seeking more information about a drug or treatment’s benefits, and optimal use.
What do the terms placebo, randomization, and blinded mean in clinical trials?
In clinical trials that compare a new product or therapy with another that already exists, researchers try to determine if the new one is as good, or better than, the existing one. In some studies, you may be assigned to receive a placebo (an inactive product that resembles the test product, but without its treatment value).
Comparing a new product with a placebo can be the fastest and most reliable way to show the new product’s effectiveness. However, placebos are not used if you would be put at risk — particularly in the study of treatments for serious illnesses — by not having effective therapy. You will be told if placebos are used in the study before entering a trial.
Randomization is the process by which treatments are assigned to participants by chance rather than by choice. This is done to avoid any bias in assigning volunteers to get one treatment or another. The effects of each treatment are compared at specific points during a trial. If one treatment is found superior, the trial is stopped so that the most volunteers receive the more beneficial treatment. This video helps explain randomization for all clinical trials .
" Blinded " (or " masked ") studies are designed to prevent members of the research team and study participants from influencing the results. Blinding allows the collection of scientifically accurate data. In single-blind (" single-masked ") studies, you are not told what is being given, but the research team knows. In a double-blind study, neither you nor the research team are told what you are given; only the pharmacist knows. Members of the research team are not told which participants are receiving which treatment, in order to reduce bias. If medically necessary, however, it is always possible to find out which treatment you are receiving.
Who takes part in clinical trials?
Many different types of people take part in clinical trials. Some are healthy, while others may have illnesses. Research procedures with healthy volunteers are designed to develop new knowledge, not to provide direct benefit to those taking part. Healthy volunteers have always played an important role in research.
Healthy volunteers are needed for several reasons. When developing a new technique, such as a blood test or imaging device, healthy volunteers help define the limits of "normal." These volunteers are the baseline against which patient groups are compared and are often matched to patients on factors such as age, gender, or family relationship. They receive the same tests, procedures, or drugs the patient group receives. Researchers learn about the disease process by comparing the patient group to the healthy volunteers.
Factors like how much of your time is needed, discomfort you may feel, or risk involved depends on the trial. While some require minimal amounts of time and effort, other studies may require a major commitment of your time and effort, and may involve some discomfort. The research procedure(s) may also carry some risk. The informed consent process for healthy volunteers includes a detailed discussion of the study's procedures and tests and their risks.
A patient volunteer has a known health problem and takes part in research to better understand, diagnose, or treat that disease or condition. Research with a patient volunteer helps develop new knowledge. Depending on the stage of knowledge about the disease or condition, these procedures may or may not benefit the study participants.
Patients may volunteer for studies similar to those in which healthy volunteers take part. These studies involve drugs, devices, or treatments designed to prevent,or treat disease. Although these studies may provide direct benefit to patient volunteers, the main aim is to prove, by scientific means, the effects and limitations of the experimental treatment. Therefore, some patient groups may serve as a baseline for comparison by not taking the test drug, or by receiving test doses of the drug large enough only to show that it is present, but not at a level that can treat the condition.
Researchers follow clinical trials guidelines when deciding who can participate, in a study. These guidelines are called Inclusion/Exclusion Criteria . Factors that allow you to take part in a clinical trial are called "inclusion criteria." Those that exclude or prevent participation are "exclusion criteria." These criteria are based on factors such as age, gender, the type and stage of a disease, treatment history, and other medical conditions. Before joining a clinical trial, you must provide information that allows the research team to determine whether or not you can take part in the study safely. Some research studies seek participants with illnesses or conditions to be studied in the clinical trial, while others need healthy volunteers. Inclusion and exclusion criteria are not used to reject people personally. Instead, the criteria are used to identify appropriate participants and keep them safe, and to help ensure that researchers can find new information they need.
What do I need to know if I am thinking about taking part in a clinical trial?

Risks and potential benefits
Clinical trials may involve risk, as can routine medical care and the activities of daily living. When weighing the risks of research, you can think about these important factors:
- The possible harms that could result from taking part in the study
- The level of harm
- The chance of any harm occurring
Most clinical trials pose the risk of minor discomfort, which lasts only a short time. However, some study participants experience complications that require medical attention. In rare cases, participants have been seriously injured or have died of complications resulting from their participation in trials of experimental treatments. The specific risks associated with a research protocol are described in detail in the informed consent document, which participants are asked to consider and sign before participating in research. Also, a member of the research team will explain the study and answer any questions about the study. Before deciding to participate, carefully consider risks and possible benefits.
Potential benefits
Well-designed and well-executed clinical trials provide the best approach for you to:
- Help others by contributing to knowledge about new treatments or procedures.
- Gain access to new research treatments before they are widely available.
- Receive regular and careful medical attention from a research team that includes doctors and other health professionals.
Risks to taking part in clinical trials include the following:
- There may be unpleasant, serious, or even life-threatening effects of experimental treatment.
- The study may require more time and attention than standard treatment would, including visits to the study site, more blood tests, more procedures, hospital stays, or complex dosage schedules.
What questions should I ask if offered a clinical trial?
If you are thinking about taking part in a clinical trial, you should feel free to ask any questions or bring up any issues concerning the trial at any time. The following suggestions may give you some ideas as you think about your own questions.
- What is the purpose of the study?
- Why do researchers think the approach may be effective?
- Who will fund the study?
- Who has reviewed and approved the study?
- How are study results and safety of participants being monitored?
- How long will the study last?
- What will my responsibilities be if I take part?
- Who will tell me about the results of the study and how will I be informed?
Risks and possible benefits
- What are my possible short-term benefits?
- What are my possible long-term benefits?
- What are my short-term risks, and side effects?
- What are my long-term risks?
- What other options are available?
- How do the risks and possible benefits of this trial compare with those options?
Participation and care
- What kinds of therapies, procedures and/or tests will I have during the trial?
- Will they hurt, and if so, for how long?
- How do the tests in the study compare with those I would have outside of the trial?
- Will I be able to take my regular medications while taking part in the clinical trial?
- Where will I have my medical care?
- Who will be in charge of my care?
Personal issues
- How could being in this study affect my daily life?
- Can I talk to other people in the study?
Cost issues
- Will I have to pay for any part of the trial such as tests or the study drug?
- If so, what will the charges likely be?
- What is my health insurance likely to cover?
- Who can help answer any questions from my insurance company or health plan?
- Will there be any travel or child care costs that I need to consider while I am in the trial?
Tips for asking your doctor about trials
- Consider taking a family member or friend along for support and for help in asking questions or recording answers.
- Plan what to ask — but don't hesitate to ask any new questions.
- Write down questions in advance to remember them all.
- Write down the answers so that they’re available when needed.
- Ask about bringing a tape recorder to make a taped record of what's said (even if you write down answers).
This information courtesy of Cancer.gov.
How is my safety protected?

Ethical guidelines
The goal of clinical research is to develop knowledge that improves human health or increases understanding of human biology. People who take part in clinical research make it possible for this to occur. The path to finding out if a new drug is safe or effective is to test it on patients in clinical trials. The purpose of ethical guidelines is both to protect patients and healthy volunteers, and to preserve the integrity of the science.
Informed consent
Informed consent is the process of learning the key facts about a clinical trial before deciding whether to participate. The process of providing information to participants continues throughout the study. To help you decide whether to take part, members of the research team explain the study. The research team provides an informed consent document, which includes such details about the study as its purpose, duration, required procedures, and who to contact for various purposes. The informed consent document also explains risks and potential benefits.
If you decide to enroll in the trial, you will need to sign the informed consent document. You are free to withdraw from the study at any time.
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are minimal when compared with potential benefits. An IRB is an independent committee that consists of physicians, statisticians, and members of the community who ensure that clinical trials are ethical and that the rights of participants are protected. You should ask the sponsor or research coordinator whether the research you are considering participating in was reviewed by an IRB.
Further reading
For more information about research protections, see:
- Office of Human Research Protection
- Children's Assent to Clinical Trial Participation
For more information on participants’ privacy and confidentiality, see:
- HIPAA Privacy Rule
- The Food and Drug Administration, FDA’s Drug Review Process: Ensuring Drugs Are Safe and Effective
For more information about research protections, see: About Research Participation
What happens after a clinical trial is completed?
After a clinical trial is completed, the researchers carefully examine information collected during the study before making decisions about the meaning of the findings and about the need for further testing. After a phase I or II trial, the researchers decide whether to move on to the next phase or to stop testing the treatment or procedure because it was unsafe or not effective. When a phase III trial is completed, the researchers examine the information and decide whether the results have medical importance.
Results from clinical trials are often published in peer-reviewed scientific journals. Peer review is a process by which experts review the report before it is published to ensure that the analysis and conclusions are sound. If the results are particularly important, they may be featured in the news, and discussed at scientific meetings and by patient advocacy groups before or after they are published in a scientific journal. Once a new approach has been proven safe and effective in a clinical trial, it may become a new standard of medical practice.
Ask the research team members if the study results have been or will be published. Published study results are also available by searching for the study's official name or Protocol ID number in the National Library of Medicine's PubMed® database .
How does clinical research make a difference to me and my family?

Only through clinical research can we gain insights and answers about the safety and effectiveness of treatments and procedures. Groundbreaking scientific advances in the present and the past were possible only because of participation of volunteers, both healthy and those with an illness, in clinical research. Clinical research requires complex and rigorous testing in collaboration with communities that are affected by the disease. As research opens new doors to finding ways to diagnose, prevent, treat, or cure disease and disability, clinical trial participation is essential to help us find the answers.
This page last reviewed on October 3, 2022
Connect with Us
- More Social Media from NIH
Evidence-Based Medicine: Types of Studies
- What is Evidence-Based Practice?
- Question Types and Corresponding Resources
- Types of Studies
- Practice Guidelines
- Step 3: Appraise This link opens in a new window
- Steps 4-5: Apply & Assess
Experimental vs. Observational Studies
An observational study is a study in which the investigator cannot control the assignment of treatment to subjects because the participants or conditions are not directly assigned by the researcher.
- Examines predetermined treatments, interventions, policies, and their effects
- Four main types: case series , case-control studies , cross-sectional studies , and cohort studies
In an experimental study , the investigators directly manipulate or assign participants to different interventions or environments
Experimental studies that involve humans are called clinical trials . They fall into two categories: those with controls, and those without controls.
- Controlled trials - studies in which the experimental drug or procedure is compared with another drug or procedure
- Uncontrolled trials - studies in which the investigators' experience with the experimental drug or procedure is described, but the treatment is not compared with another treatment
Definitions taken from: Dawson B, Trapp R.G. (2004). Chapter 2. Study Designs in Medical Research. In Dawson B, Trapp R.G. (Eds), Basic & Clinical Biostatistics, 4e . Retrieved September 15, 2014 from https://accessmedicine.mhmedical.com/book.aspx?bookid=2724
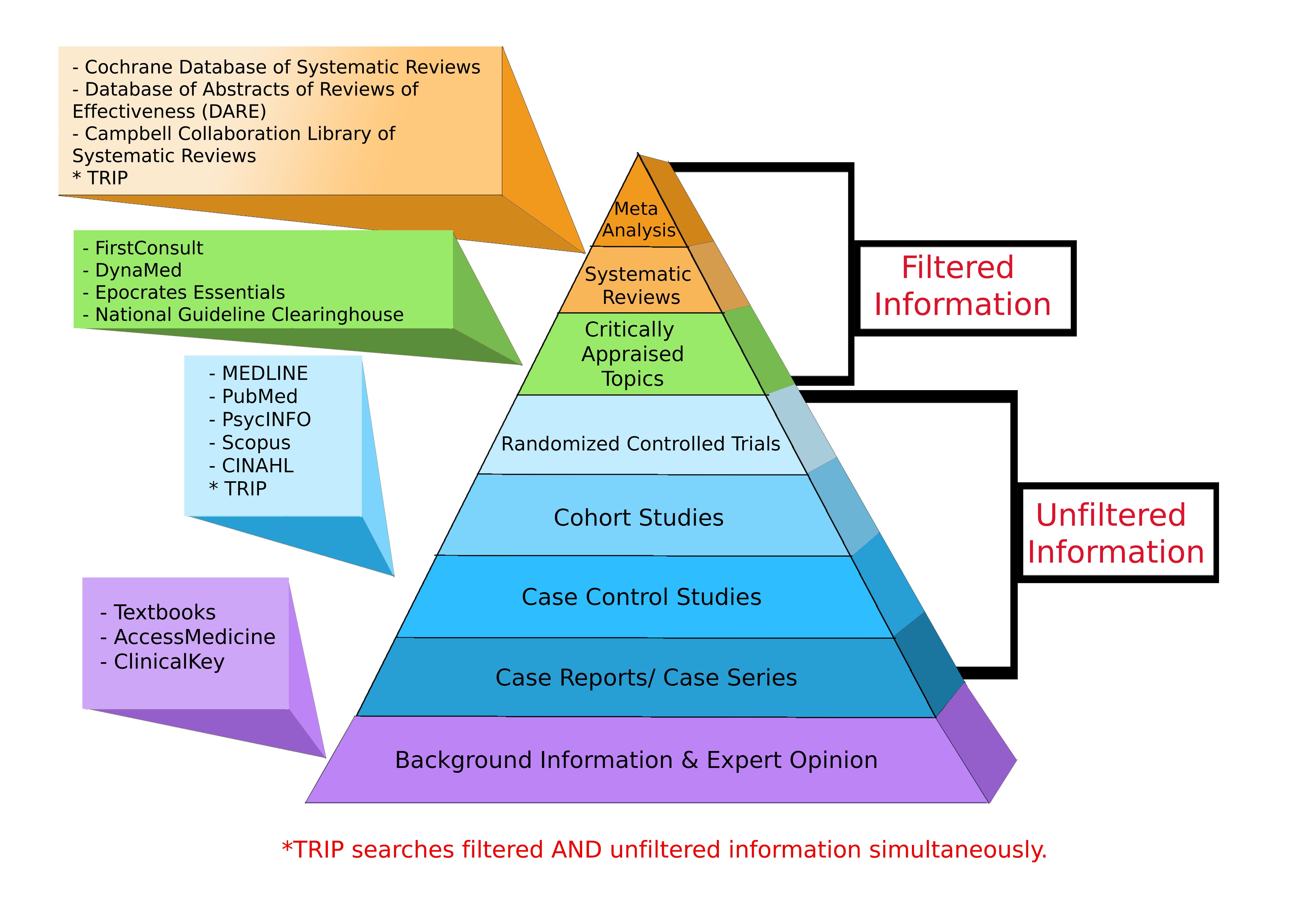
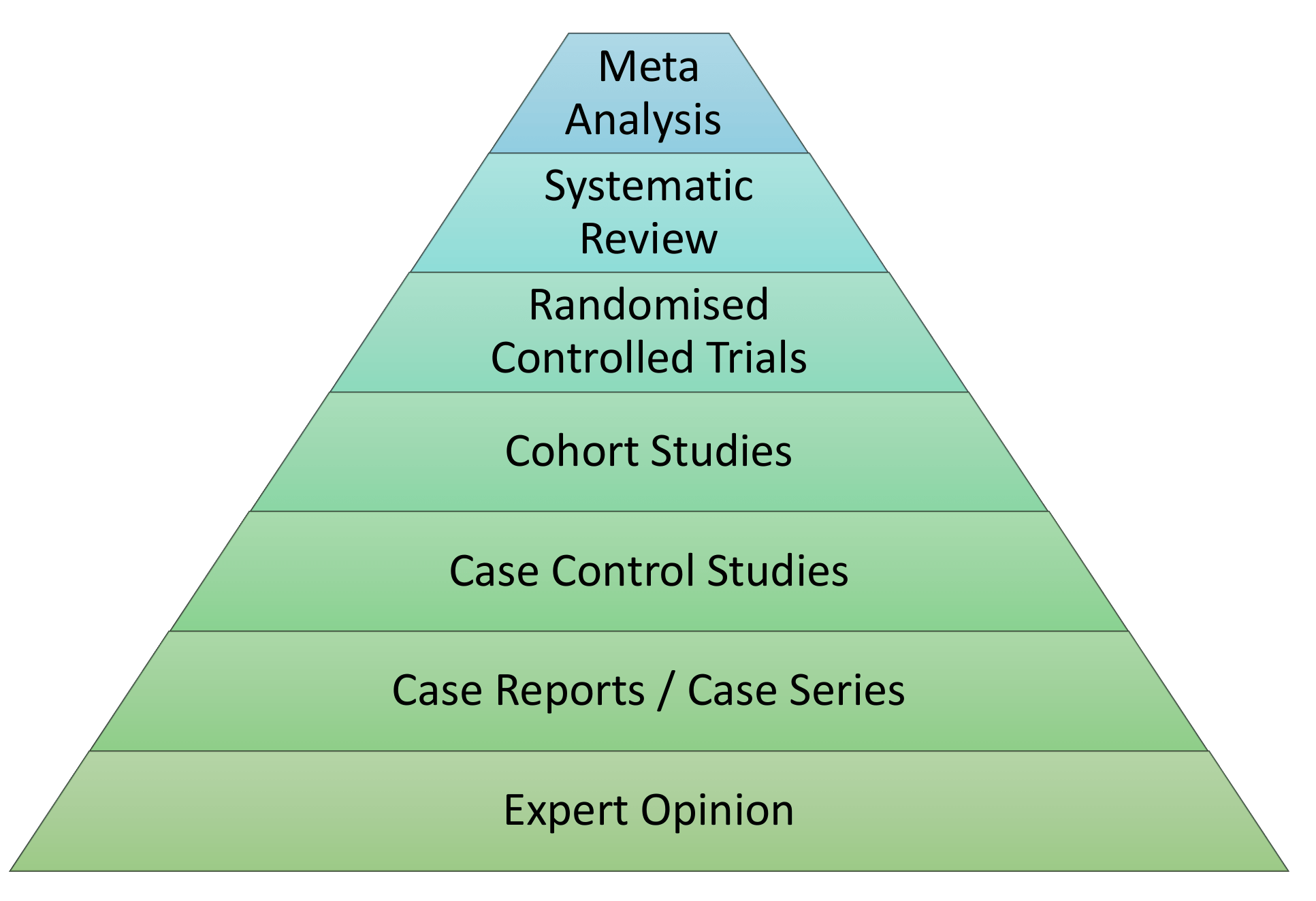
Levels of Evidence Pyramid
Levels of Evidence Pyramid created by Andy Puro, September 2014
Additional Study Design Resources
Study Design 101 : Himmelfarb's tutorial on study types and how to find them
Study Designs (Centre for Evidence Based Medicine, University of Oxford)
Learn about Clinical Studies (ClinicalTrials.gov, National Institutes of Health)
Study Designs Guide (Deakin University)
- << Previous: Step 2: Acquire
- Next: Practice Guidelines >>

- Last Updated: Dec 6, 2023 7:59 AM
- URL: https://guides.himmelfarb.gwu.edu/ebm

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2850
- [email protected]
- https://himmelfarb.gwu.edu

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Science News
- Meetings and Events
- Social Media
- Press Resources
- Email Updates
- Innovation Speaker Series
What are the different types of clinical research?
February 18, 2021
There are many different types of clinical research because researchers study many different things.
Treatment research usually tests an intervention such as medication, psychotherapy, new devices, or new approaches.
Prevention research looks for better ways to prevent disorders from developing or returning. Different kinds of prevention research may study medicines, vitamins, or lifestyle changes.
Diagnostic research refers to the practice of looking for better ways to identify a particular disorder or condition.
Screening research aims to find the best ways to detect certain disorders or health conditions.
Genetic studies aim to improve our ability to predict disorders by identifying and understanding how genes and illnesses may be related. Research in this area may explore ways in which a person’s genes make him or her more or less likely to develop a disorder. This may lead to development of tailor-made treatments based on a patient’s genetic make-up.
Epidemiological studies look at how often and why disorders happen in certain groups of people.
Research studies can be outpatient or inpatient. Outpatient means that participants do not stay overnight at the hospital or research center. Inpatient means that participants will need to stay at least one night in the hospital or research center.
Thank you for your interest in learning more about clinical research!
University Library, University of Illinois at Urbana-Champaign

Research & Publication in Medicine & Health
- Library Help & Services
- Literature Reviews This link opens in a new window
Clinical Research
- MedEd Research This link opens in a new window
- RESEARCH METHODS
- RESEARCH QUESTIONS
- Citation Management
- DATA ANALYSIS
- Data Sharing
- Where to Publish This link opens in a new window
- DOIs and PIDs
- GRANTS & FUNDING
- Informed Consent
- Reproducibility
- Staying Current in Major Databases
Related Guides
- Guide to Using Multimedia in Research This guide will introduce a couple of media platforms that can be used for research. We will go over images, videos and podcasts, and how you can use and cite them for research.
- Sociology Research Tips for Doing Research in Sociology
- Text Mining Tools and Methods This guide contains resources for researching with text mining
- Research in Global Studies - Global Health This guide is intended to assist Global Studies students in developing their research. Resources are organized by geographic area as well as by thematic areas that align with the Global Studies degree program's concentrations.
- Qualitative Data Analysis Resources on conducting qualitative data analysis
Major Types of Research for Medicine & Health
Basic sciences research.
Basic research asks fundamental questions about how life works. Scientists study cells, genes, proteins, and other building blocks of life. What they find can lead to better ways to predict, prevent, diagnose, and treat disease. more about Basic Science Research
Clinical research is research conducted with human subjects, or material of human origin, in which the researcher directly interacts with human subjects. Clinical researchers at the National Human Genome Research Institute (NHGRI) are developing advanced methods for studying the fundamental mechanisms of inherited and acquired genetic disorders. FAQs about Clinical Research
Epidemiological Research
The basic epidemiological study designs are cross-sectional, case-control, and cohort studies. Cross-sectional studies provide a snapshot of a population by determining both exposures and outcomes at one time point. Cohort studies identify the study groups based on the exposure and, then, the researchers follow up study participants to measure outcomes. Case-control studies identify the study groups based on the outcome, and the researchers retrospectively collect the exposure of interest. F rom: Introduction to Epidemiological Studies
Mixed Methods Research
- Glossary of Key Terms From Colorado State University: Members of the Research Methods Seminar (E600) taught by Mike Palmquist in the 1990s and 2000s. (1994-2022). Glossary of Key Terms. Writing@CSU. Colorado State University. https://writing.colostate.edu/guides/guide.cfm?guideid=90
- Mixed Methods in Health Sciences Research Sage Research Methods
- Mixed Methods Research : A guide to the Field From Sate Research Methods Conceptual Framework for the Field of Mixed Methods Research Welcome to the field of mixed methods research! We begin this book by considering the
- NIH Mixed Methods Research Pprovides guidance to NIH investigators on how to rigorously develop and evaluate mixed methods research applications. Pursuant to this, the team developed a report of “best practices” following three major objectives.
- SAGE Mixed Methods Research An Overview of Mixed Methods Research
Hypothesis Testing
- Hypothesis Testing Penn State University tutorial
- Hypothesis Testing Wolfram MathWorld overview
- GOHy Worksheet - Minimal experiemental design Worksheet for experimental design development
Historical Research
- History of Medicine (NLM) From National Library of Medicine - Resources and Services for history of medicine research
- Center for the History of Medicine (Harvard) Enabling the history of medicine and public health to inform healthcare, the health sciences, and the societies in which they are embedded
- << Previous: Library Help & Services
- Next: Literature Reviews >>
- Last Updated: Nov 9, 2023 11:46 AM
- URL: https://guides.library.illinois.edu/biomedresearch
- Fundamental Skills Education Improvement Leadership Research
- Additional Skills Communication Entrepreneur Finance Self Development
- Careers Training Pathways Out of Training Alternative Careers
Scroll to the bottom of the webpage to ensure images load completely before printing.
Types of Study
Introduction to Types of Medical Research
Evidence-based medicine may be defined as the systematic, quantitative and preferentially experimental approach to obtaining medical information. This information is obtained through medical research. Medical research encompasses a wide range of study techniques that can be used to understand diseases, uncover their causative factors and validate the treatments we have available for them. Each type of study technique comes with advantages as well as their own particular disadvantages. This article will introduce the different types of study commonly used within medical research and discuss their particular traits. The diagram below provides an overview of how the different types of study methodology relate to one another.
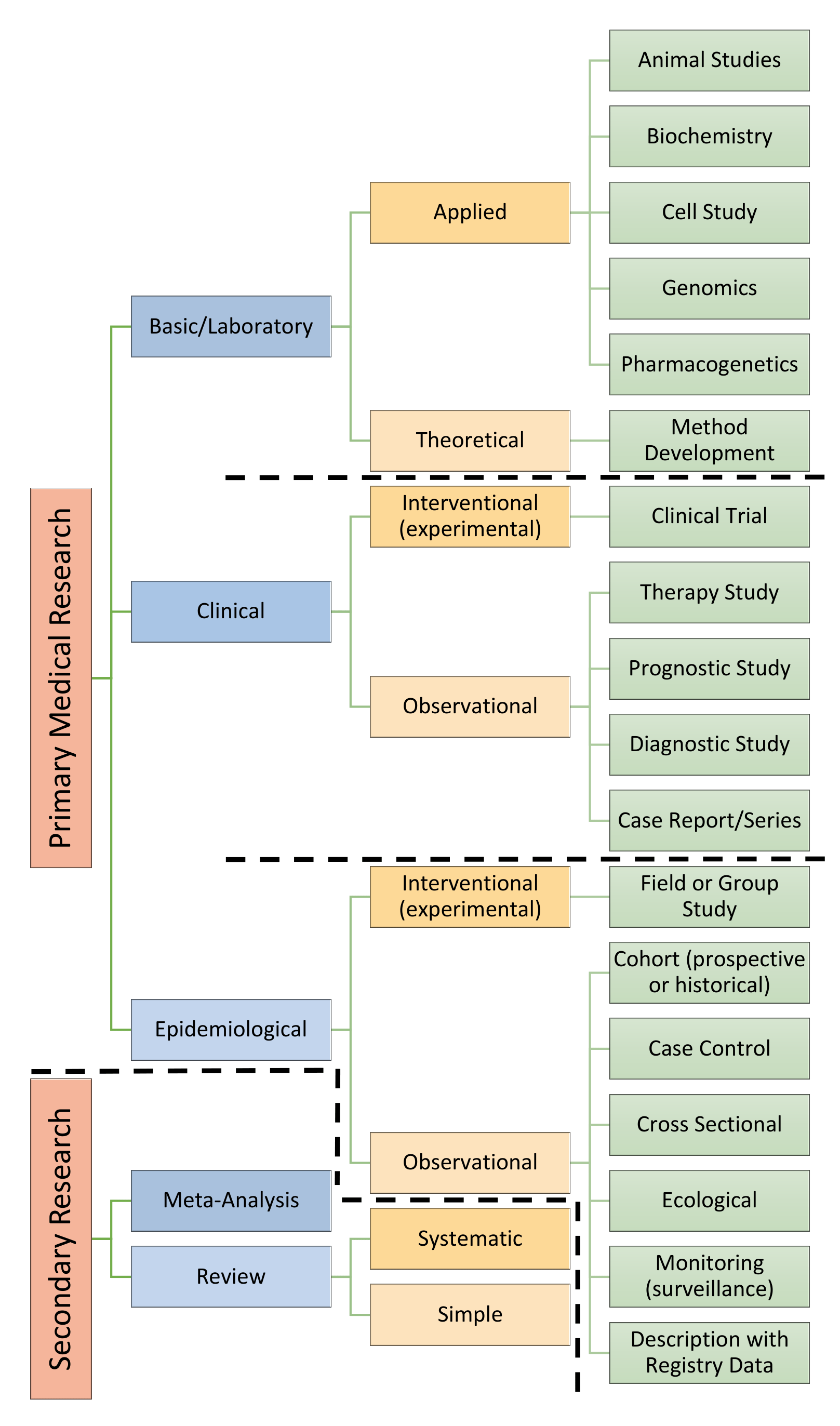
Primary vs. Secondary Research
Medical research can be classified as either primary or secondary research. Primary research involves performing studies and collecting raw data. Secondary research involves evaluating or synthesising data collected during primary research.
Observational vs. Experimental Research
An observational study is a study in which the investigator does not seek to control any of the variables nor the assignment of intervention to subjects. These decisions are usually made by the patient and their doctor. Examples include cohort, case-control, case-series and cross-sectional studies.
An experimental study involves direct manipulation or assignment of participants to different interventions or environments. Clinical experimental studies are known as clinical trials.
Prospective vs. Retrospective
In prospective studies , individuals are followed over a period of time and data is collected when their characteristics or circumstances change. Studies usually relate the outcome of interest to suspected risk factors. For these prospective studies, the outcome of interest should commonly occur to ensure statistical significance. Prospective studies allow precise estimation of the relative risk of an outcome based upon exposure.
In retrospective studies , individuals are sampled and information is collected about their past. These studies usually establish an outcome of interest and examine exposures to suspected risk or protective factors. Data is typically gathered from interviews or medical notes. The nature of retrospective studies makes them more susceptible to bias. Retrospective studies allow calculation of the odds ratio (this is an estimate of the relative risk) for uncommon outcomes. Retrospective studies are advantageous for studying rare diseases since prospective studies are unfeasible due to the large study sizes needed to reach statistical significance.
Randomised vs. Non-Randomised
Randomised studies involve the random allocation of individuals to intervention groups in order to minimise confounding variables. Allocation does not take into account any similarities or differences in the individuals. It usually involves use of a random number generator.
Non-randomised studies involve allocation of people to different interventions using methods which are not random.
Single-Blinded vs. Double Blinded vs. Triple-Blinded
Blinding is important to reduce bias and ensure a study’s internal validity. It prevents participants and researchers from affecting the outcomes of a study in a conscious or subconscious manner.
- Single-blind study – only the participants are blinded.
- Double-blind study – both participants and experimenters are blinded.
- Triple-blind study – participants, experimenters and researchers analysing the data are blinded.
The Levels of Evidence
Not all evidence is created equal with some forms of study technique thought to be superior in design. Studies which employ superior designs are felt to carry more weight when interpreting their conclusions. The result is the creation of a hierarchy based upon study technique. This has been outlined in the diagram below.
The ordering of evidence in this manner may be seen as simplistic because it does not take into account the methodological merit of individual study designs. Furthermore, the quality of systematic review evidence will depend largely upon the type of study included within the analysis and meta-analysis results can vary wildly depending upon the statistical methods employed. In the final instance, systematic reviews should be considered a lens through which evidence can be viewed.
Brief Description of Study Types
In this section we will cover the basics of the following study designs.
- Meta-Analysis
- Systematic Review
- Randomised Control Trial
- Cohort Study
- Case Control Series
- Case Report/Series
For further information on each of these study designs and how to perform them, have a look at the Equator Network .
Meta-Analysis - Secondary Research
Definition: A meta-analysis is a statistical procedure for systematically combining numerical (quantitative) data from multiple independent studies in the published literature. These data are assessed and used to derive conclusions about that body of research. It is a subset of systematic reviews (see below).
Uses: Meta-analyses can be used to provide more precise estimates than those given by any individual study included within the analysis. They may also answer questions not posed by individual studies or identify and examine the heterogeneity between the individual studies (including statistical significance where conflicting results are reported). Examples of alternative questions include providing a more complex analysis of harms/benefits or the examination of subgroups where individual study numbers were not large enough.
Brief Methodology: The Cochrane collaboration has developed a protocol which provides structure for literature search, analytic and diagnostic methods for evaluating the output of meta-analyses. These can be viewed within their handbook . Additional guidance can be found by using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). This is an evidence-based minimum set of items (checklist) for reporting in systematic reviews and meta-analyses.
- Provides greater statistical power and increased volume of data for more precise estimates.
- Hypothesis testing and biases within publications can be examined.
- Inconsistencies within research can be resolved.
- Provides better estimate of relationships.
Disadvantages
- It is difficult and time consuming to identify the correct studies.
- Not all studies may be appropriate for inclusion.
- An incomplete set of studies may have been analysed.
- Requires advanced statistical capabilities.
- Heterogeneity of methods used in studies may lead to erroneous inferences.
Systematic Review - Secondary Research
Definition: A systematic review is a detailed, systematic and transparent means of considering all published and unpublished material which fits within a prespecified eligibility criteria. The included material can be of varying study designs. Those materials which are judged to be methodologically sound are combined in either a quantitative or qualitative manner to answer a pre-defined research question. Meta-analyses are not required but many systematic reviews will include a meta-analysis.
Uses: Systematic reviews are used to deliver a meticulous summary of the available primary research in response to a research question.
Brief Methodology: Systematic reviews should have a clear set of objectives, predefined eligibility criteria, a reproducible methodology, a systemic search method, an assessment of the validity of the findings of included studies and a systematic presentation and synthesis of the attributes and findings from the studies used.
- Addresses a specific question.
- Explicit and bias limiting methods.
- More reliable and accurate than individual studies.
- Less costly than organising a new study.
- Requires less time than a new study.
- Results can be generalised and extrapolated into the general population.
- Time consuming.
- There may be difficulties combining different studies.
- May be composed of inadequate primary studies.
- May be poorly designed and executed.
- May mis-interpret results.
Randomised Controlled Trial - Primary Research, Experimental, Prospective
Definition: A randomised control trial involves one or more new treatments where participants are randomly assigned into an experimental or control group. The various groups are then followed up to see if there is any difference in the specified outcome. The results and subsequent analysis are used to evaluating the effectiveness of the intervention.
Uses: Randomised controlled trials are used to establish the effectiveness of a new intervention or treatment.
Brief Methodology: Interventions might include a medication or procedure. Control groups will either get a placebo treatment or receive the current ‘gold standard’ treatment. Randomisation seeks to evenly distribute baseline characteristics in order to reduce the effect of confounding variables. This process is usually performed using mathematical techniques.
The CONSORT (Consolidated Standards of Reporting Trials) Statement can be used as an evidence-based minimum set of recommendations (checklist) for reporting randomised trials. The Cochrane Library has formed a highly concentrated source of reports of randomised controlled trials which can be found within their CENTRAL (Cochrane Central Register of Controlled Trials) database.
- You can make direct comparisons between treatments.
- Effective randomisation removes selection bias.
- Randomisation reduces the impact of confounding factors and makes groups comparable with both known and unknown factors.
- Results can be reliably analysed with statistical tools.
- Blinding can be applied to reduce performance bias.
- Prospective design minimises recall error and selection bias.
- It is expensive and takes time.
- Participants must volunteer and so may not be representative of the whole population.
- Studies will have to be powered sufficiently to make significant outcomes.
- There is the risk of participants being lost to follow up.
- Ethical limitations. For example, informed consent is impossible to obtain, or some intervention arms would be ethically impossible.
- Results may not mimic realise and generalisability to the real world may be difficult.
Cohort Studies - Primary Research, Observational, Predominantly Prospective
Definition: Groups of disease-free individuals are identified, and baseline measurements are taken for a variety of variables (risk factors) that might be relevant to the development of the outcome of interest. These individuals are then followed over time to determine whether they develop the outcome of interest. Cohort studies are usually prospective but can be performed retrospectively with data collected for other purposes.
Uses: Cohort studies measure incidence rates and the relative risk for developing the outcome of interest for each measured variable. They are able to distinguish between cause and effect due to the temporal relationship between risk factor exposure and outcome occurrence.
Brief Methodology: In prospective cohort studies the risk exposure information is collected at the start of the study and new cases of disease identified from that point onwards. In retrospective cohort studies the exposure status was measured in the past and disease identification has already begun. Both methods enable calculation of the relative risk.
The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement (checklist) can be used to ensure observational studies are adequately described in research publications. This checklist has been designed for cohort studies, case-control studies and cross-sectional studies.
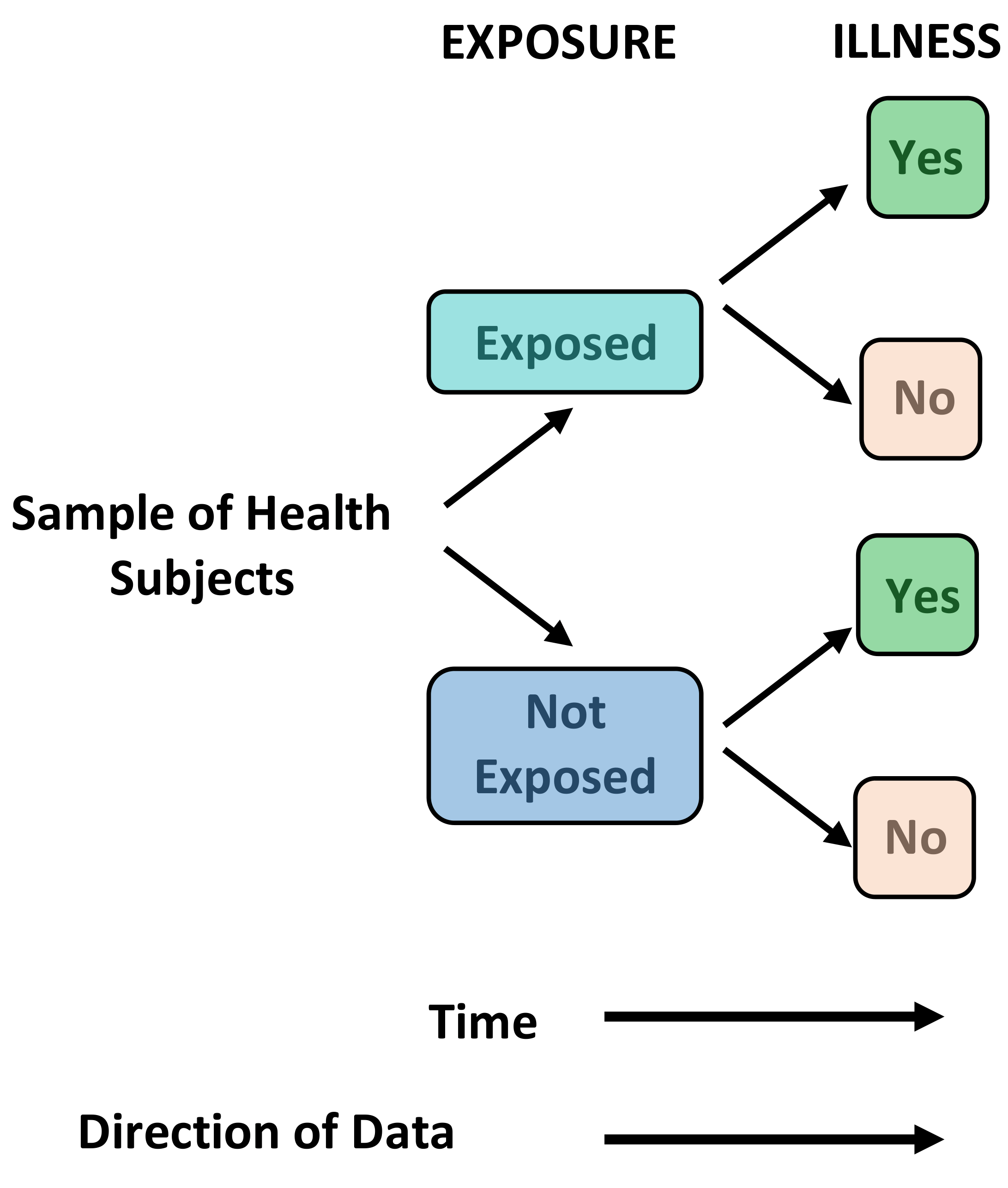
- It is cheaper and easier to implement than a randomised controlled trial.
- It is able to distinguish between cause and effect.
- Multiple outcomes can be studied.
- It may uncover unanticipated associations with the outcome.
- The efficiency of prospective cohort studies increases as the incidence of any particular outcome increases.
- Patients can be lost to follow up thereby introducing attrition bias.
- Subject selection can introduce bias due to an imbalance of patient characteristics.
- It is prone to change of methods over time.
- Confounding variables can be difficult to remove.
- It is difficult to blind researchers.
- Requires large numbers of patients.
- The outcome of interest can take a long time to occur.
Case Control Studies - Primary Research, Observational, Retrospective
Definition: A study that compares patients who have an outcome of interest (the disease in question) with those who do not. Case control studies are almost always retrospective. The researcher looks back in time to identify which individuals were exposed to a risk factor or treatment and thus the relation it has with the presence or absence of disease.
Uses: Good for studying rare diseases and outcomes. They can also be used where there is a long latent period between an exposure and disease occurrence. They are often used to generate hypotheses that can then be studied using other means.
Brief Methodology: Individuals with the outcome of interest (the disease in question) are selected (cases). A second group of similar individuals without the outcome of interest is constructed (controls). The researcher then looks at historical factors to identify if some exposures are found more commonly in the cases than the controls. If this is the case, a link can be established between the exposure and the outcome of interest. This produces an odds ratio that can be used to approximate the relative risk for each variable studied.
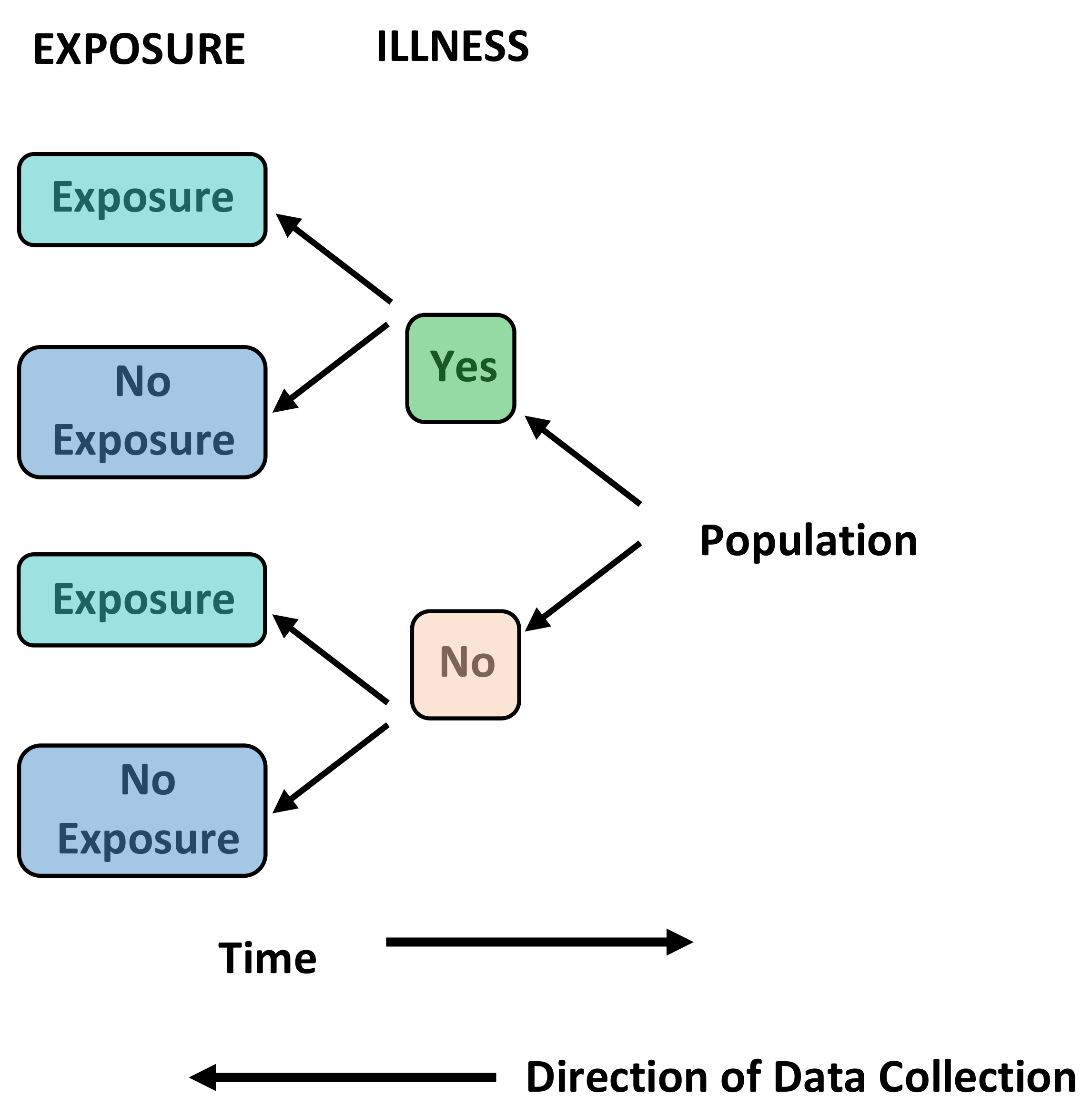
Case Report/Series - Primary Research, Observational, Retrospective
Definition: An article that describes and interprets an individual case or cases. It is often written as a detailed story.
Brief Methodology: An interesting case is identified, and the patient should be described in detail. Include the following: their age, sex, ethnicity, race, employment status, social situation, medical history, diagnosis, prognosis, previous treatments, diagnostic tests, medications, current intervention and the clinical and functional assessment.
Uses: Describe unique cases that cannot be explained by known diseases or syndromes. They may show an important variation from a known disease. They may show unexpected events that yield new information. They may include patients with two or more unexpected diseases or disorders.
- Stats Direct
- Cochrane Handbook
- CONSORT Statement: Cosolidated Standards of Reporting Trials
- PRISMA Statement: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- STROBE Statement: Strengthening the Reporting of Observational Studies in Epidemiology
- Equator Network: Enhancing the Quality and Transparency of Health Research
- Open MD: Medical Research
- Deutsches Ärzteblatt International: Types of Study in Medical Research
- Himmelfarb: Types of Studies
- Georgia State University: Literature Reviews: Types of Clinical Study Designs
- Deutsches Ärzteblatt International: Systematic Literature Reviews and Meta-Analyses
- Emergency Medicine Journal: Randomised Controlled Trials and Their Principles
Also in Research
Writing your First Research Article
The Anatomy of a Research Article
P-Values and Confidence Intervals
Introduction to Descriptive Statistics
Introduction to Inferential Statistics
Diagnostic Tables
Relative Risk and Odds Ratio
Data Visualisation

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.9: Types of Research Studies and How To Interpret Them
- Last updated
- Save as PDF
- Page ID 49296

- Alice Callahan, Heather Leonard, & Tamberly Powell
- Lane Community College via OpenOregon
The field of nutrition is dynamic, and our understanding and practices are always evolving. Nutrition scientists are continuously conducting new research and publishing their findings in peer-reviewed journals. This adds to scientific knowledge, but it’s also of great interest to the public, so nutrition research often shows up in the news and other media sources. You might be interested in nutrition research to inform your own eating habits, or if you work in a health profession, so that you can give evidence-based advice to others. Making sense of science requires that you understand the types of research studies used and their limitations.
The Hierarchy of Nutrition Evidence
Researchers use many different types of study designs depending on the question they are trying to answer, as well as factors such as time, funding, and ethical considerations. The study design affects how we interpret the results and the strength of the evidence as it relates to real-life nutrition decisions. It can be helpful to think about the types of studies within a pyramid representing a hierarchy of evidence, where the studies at the bottom of the pyramid usually give us the weakest evidence with the least relevance to real-life nutrition decisions, and the studies at the top offer the strongest evidence, with the most relevance to real-life nutrition decisions .
The pyramid also represents a few other general ideas. There tend to be more studies published using the methods at the bottom of the pyramid, because they require less time, money, and other resources. When researchers want to test a new hypothesis , they often start with the study designs at the bottom of the pyramid , such as in vitro, animal, or observational studies. Intervention studies are more expensive and resource-intensive, so there are fewer of these types of studies conducted. But they also give us higher quality evidence, so they’re an important next step if observational and non-human studies have shown promising results. Meta-analyses and systematic reviews combine the results of many studies already conducted, so they help researchers summarize scientific knowledge on a topic.

Non-Human Studies: In Vitro & Animal Studies
The simplest form of nutrition research is an in vitro study . In vitro means “within glass,” (although plastic is used more commonly today) and these experiments are conducted within flasks, dishes, plates, and test tubes. These studies are performed on isolated cells or tissue samples, so they’re less expensive and time-intensive than animal or human studies. In vitro studies are vital for zooming in on biological mechanisms, to see how things work at the cellular or molecular level. However, these studies shouldn’t be used to draw conclusions about how things work in humans (or even animals), because we can’t assume that the results will apply to a whole, living organism.

Animal studies are one form of in vivo research, which translates to “within the living.” Rats and mice are the most common animals used in nutrition research. Animals are often used in research that would be unethical to conduct in humans. Another advantage of animal dietary studies is that researchers can control exactly what the animals eat. In human studies, researchers can tell subjects what to eat and even provide them with the food, but they may not stick to the planned diet. People are also not very good at estimating, recording, or reporting what they eat and in what quantities. In addition, animal studies typically do not cost as much as human studies.
There are some important limitations of animal research. First, an animal’s metabolism and physiology are different from humans. Plus, animal models of disease (cancer, cardiovascular disease, etc.), although similar, are different from human diseases. Animal research is considered preliminary, and while it can be very important to the process of building scientific understanding and informing the types of studies that should be conducted in humans, animal studies shouldn’t be considered relevant to real-life decisions about how people eat.
Observational Studies
Observational studies in human nutrition collect information on people’s dietary patterns or nutrient intake and look for associations with health outcomes. Observational studies do not give participants a treatment or intervention; instead, they look at what they’re already doing and see how it relates to their health. These types of study designs can only identify correlations (relationships) between nutrition and health; they can’t show that one factor causes another. (For that, we need intervention studies, which we’ll discuss in a moment.) Observational studies that describe factors correlated with human health are also called epidemiological studies . 1
One example of a nutrition hypothesis that has been investigated using observational studies is that eating a Mediterranean diet reduces the risk of developing cardiovascular disease. (A Mediterranean diet focuses on whole grains, fruits and vegetables, beans and other legumes, nuts, olive oil, herbs, and spices. It includes small amounts of animal protein (mostly fish), dairy, and red wine. 2 ) There are three main types of observational studies, all of which could be used to test hypotheses about the Mediterranean diet:
- Cohort studies follow a group of people (a cohort) over time, measuring factors such as diet and health outcomes. A cohort study of the Mediterranean diet would ask a group of people to describe their diet, and then researchers would track them over time to see if those eating a Mediterranean diet had a lower incidence of cardiovascular disease.
- Case-control studies compare a group of cases and controls, looking for differences between the two groups that might explain their different health outcomes. For example, researchers might compare a group of people with cardiovascular disease with a group of healthy controls to see whether there were more controls or cases that followed a Mediterranean diet.
- Cross-sectional studies collect information about a population of people at one point in time. For example, a cross-sectional study might compare the dietary patterns of people from different countries to see if diet correlates with the prevalence of cardiovascular disease in the different countries.
Prospective cohort studies, which enroll a cohort and follow them into the future, are usually considered the strongest type of observational study design. Retrospective studies look at what happened in the past, and they’re considered weaker because they rely on people’s memory of what they ate or how they felt in the past. There are several well-known examples of prospective cohort studies that have described important correlations between diet and disease:
- Framingham Heart Study : Beginning in 1948, this study has followed the residents of Framingham, Massachusetts to identify risk factors for heart disease.
- Health Professionals Follow-Up Study : This study started in 1986 and enrolled 51,529 male health professionals (dentists, pharmacists, optometrists, osteopathic physicians, podiatrists, and veterinarians), who complete diet questionnaires every 2 years.
- Nurses Health Studies : Beginning in 1976, these studies have enrolled three large cohorts of nurses with a total of 280,000 participants. Participants have completed detailed questionnaires about diet, other lifestyle factors (smoking and exercise, for example), and health outcomes.
Observational studies have the advantage of allowing researchers to study large groups of people in the real world, looking at the frequency and pattern of health outcomes and identifying factors that correlate with them. But even very large observational studies may not apply to the population as a whole. For example, the Health Professionals Follow-Up Study and the Nurses Health Studies include people with above-average knowledge of health. In many ways, this makes them ideal study subjects, because they may be more motivated to be part of the study and to fill out detailed questionnaires for years. However, the findings of these studies may not apply to people with less baseline knowledge of health.
We’ve already mentioned another important limitation of observational studies—that they can only determine correlation, not causation. A prospective cohort study that finds that people eating a Mediterranean diet have a lower incidence of heart disease can only show that the Mediterranean diet is correlated with lowered risk of heart disease. It can’t show that the Mediterranean diet directly prevents heart disease. Why? There are a huge number of factors that determine health outcomes such as heart disease, and other factors might explain a correlation found in an observational study. For example, people who eat a Mediterranean diet might also be the same kind of people who exercise more, sleep more, have higher income (fish and nuts can be expensive!), or be less stressed. These are called confounding factors ; they’re factors that can affect the outcome in question (i.e., heart disease) and also vary with the factor being studied (i.e., Mediterranean diet).
Intervention Studies
Intervention studies , also sometimes called experimental studies or clinical trials, include some type of treatment or change imposed by the researcher. Examples of interventions in nutrition research include asking participants to change their diet, take a supplement, or change the time of day that they eat. Unlike observational studies, intervention studies can provide evidence of cause and effect , so they are higher in the hierarchy of evidence pyramid.
The gold standard for intervention studies is the randomized controlled trial (RCT) . In an RCT, study subjects are recruited to participate in the study. They are then randomly assigned into one of at least two groups, one of which is a control group (this is what makes the study controlled ). In an RCT to study the effects of the Mediterranean diet on cardiovascular disease development, researchers might ask the control group to follow a low-fat diet (typically recommended for heart disease prevention) and the intervention group to eat a Mediterrean diet. The study would continue for a defined period of time (usually years to study an outcome like heart disease), at which point the researchers would analyze their data to see if more people in the control or Mediterranean diet had heart attacks or strokes. Because the treatment and control groups were randomly assigned, they should be alike in every other way except for diet, so differences in heart disease could be attributed to the diet. This eliminates the problem of confounding factors found in observational research, and it’s why RCTs can provide evidence of causation, not just correlation.
Imagine for a moment what would happen if the two groups weren’t randomly assigned. What if the researchers let study participants choose which diet they’d like to adopt for the study? They might, for whatever reason, end up with more overweight people who smoke and have high blood pressure in the low-fat diet group, and more people who exercised regularly and had already been eating lots of olive oil and nuts for years in the Mediterranean diet group. If they found that the Mediterranean diet group had fewer heart attacks by the end of the study, they would have no way of knowing if this was because of the diet or because of the underlying differences in the groups. In other words, without randomization, their results would be compromised by confounding factors, with many of the same limitations as observational studies.
In an RCT of a supplement, the control group would receive a placebo—a “fake” treatment that contains no active ingredients, such as a sugar pill. The use of a placebo is necessary in medical research because of a phenomenon known as the placebo effect. The placebo effect results in a beneficial effect because of a subject’s belief in the treatment, even though there is no treatment actually being administered.

Blinding is a technique to prevent bias in intervention studies. In a study without blinding, the subject and the researchers both know what treatment the subject is receiving. This can lead to bias if the subject or researcher have expectations about the treatment working, so these types of trials are used less frequently. It’s best if a study is double-blind , meaning that neither the researcher nor the subject know what treatment the subject is receiving. It’s relatively simple to double-blind a study where subjects are receiving a placebo or treatment pill, because they could be formulated to look and taste the same. In a single-blind study , either the researcher or the subject knows what treatment they’re receiving, but not both. Studies of diets—such as the Mediterranean diet example—often can’t be double-blinded because the study subjects know whether or not they’re eating a lot of olive oil and nuts. However, the researchers who are checking participants’ blood pressure or evaluating their medical records could be blinded to their treatment group, reducing the chance of bias.
Like all studies, RCTs and other intervention studies do have some limitations. They can be difficult to carry on for long periods of time and require that participants remain compliant with the intervention. They’re also costly and often have smaller sample sizes. Furthermore, it is unethical to study certain interventions. (An example of an unethical intervention would be to advise one group of pregnant mothers to drink alcohol to determine its effects on pregnancy outcomes, because we know that alcohol consumption during pregnancy damages the developing fetus.)
VIDEO: “ Not all scientific studies are created equal ” by David H. Schwartz, YouTube (April 28, 2014), 4:26.
Meta-Analyses and Systematic Reviews
At the top of the hierarchy of evidence pyramid are systematic reviews and meta-analyses . You can think of these as “studies of studies.” They attempt to combine all of the relevant studies that have been conducted on a research question and summarize their overall conclusions. Researchers conducting a systematic review formulate a research question and then systematically and independently identify, select, evaluate, and synthesize all high-quality evidence that relates to the research question. Since systematic reviews combine the results of many studies, they help researchers produce more reliable findings. A meta-analysis is a type of systematic review that goes one step further, combining the data from multiple studies and using statistics to summarize it, as if creating a mega-study from many smaller studies . 4
However, even systematic reviews and meta-analyses aren’t the final word on scientific questions. For one thing, they’re only as good as the studies that they include. The Cochrane Collaboration is an international consortium of researchers who conduct systematic reviews in order to inform evidence-based healthcare, including nutrition, and their reviews are among the most well-regarded and rigorous in science. For the most recent Cochrane review of the Mediterranean diet and cardiovascular disease, two authors independently reviewed studies published on this question. Based on their inclusion criteria, 30 RCTs with a total of 12,461 participants were included in the final analysis. However, after evaluating and combining the data, the authors concluded that “despite the large number of included trials, there is still uncertainty regarding the effects of a Mediterranean‐style diet on cardiovascular disease occurrence and risk factors in people both with and without cardiovascular disease already.” Part of the reason for this uncertainty is that different trials found different results, and the quality of the studies was low to moderate. Some had problems with their randomization procedures, for example, and others were judged to have unreliable data. That doesn’t make them useless, but it adds to the uncertainty about this question, and uncertainty pushes the field forward towards more and better studies. The Cochrane review authors noted that they found seven ongoing trials of the Mediterranean diet, so we can hope that they’ll add more clarity to this question in the future. 5
Science is an ongoing process. It’s often a slow process, and it contains a lot of uncertainty, but it’s our best method of building knowledge of how the world and human life works. Many different types of studies can contribute to scientific knowledge. None are perfect—all have limitations—and a single study is never the final word on a scientific question. Part of what advances science is that researchers are constantly checking each other’s work, asking how it can be improved and what new questions it raises.
Attributions:
- “Chapter 1: The Basics” from Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR , CC BY-NC-SA 4.0
- “ The Broad Role of Nutritional Science ,” section 1.3 from the book An Introduction to Nutrition (v. 1.0), CC BY-NC-SA 3.0
References:
- 1 Thiese, M. S. (2014). Observational and interventional study design types; an overview. Biochemia Medica , 24 (2), 199–210. https://doi.org/10.11613/BM.2014.022
- 2 Harvard T.H. Chan School of Public Health. (2018, January 16). Diet Review: Mediterranean Diet . The Nutrition Source. https://www.hsph.harvard.edu/nutritionsource/healthy-weight/diet-reviews/mediterranean-diet/
- 3 Ross, R., Gray, C. M., & Gill, J. M. R. (2015). Effects of an Injected Placebo on Endurance Running Performance. Medicine and Science in Sports and Exercise , 47 (8), 1672–1681. https://doi.org/10.1249/MSS.0000000000000584
- 4 Hooper, A. (n.d.). LibGuides: Systematic Review Resources: Systematic Reviews vs Other Types of Reviews . Retrieved February 7, 2020, from //libguides.sph.uth.tmc.edu/c.php?g=543382&p=5370369
- 5 Rees, K., Takeda, A., Martin, N., Ellis, L., Wijesekara, D., Vepa, A., Das, A., Hartley, L., & Stranges, S. (2019). Mediterranean‐style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews , 3 . doi.org/10.1002/14651858.CD009825.pub3
- Figure 2.3. The hierarchy of evidence by Alice Callahan, is licensed under CC BY 4.0
- Research lab photo by National Cancer Institute on Unsplas h ; mouse photo by vaun0815 on Unsplash
- Figure 2.4. “Placebo effect example” by Lindshield, B. L. Kansas State University Human Nutrition (FNDH 400) Flexbook. goo.gl/vOAnR
Types of study in medical research: part 3 of a series on evaluation of scientific publications
Affiliation.
- 1 MDK Rheinland-Pfalz Referat Rehabilitation/Biometrie Albiger Str. 19 d 55232 Alzey, Germany. [email protected]
- PMID: 19547627
- PMCID: PMC2689572
- DOI: 10.3238/arztebl.2009.0262
Background: The choice of study type is an important aspect of the design of medical studies. The study design and consequent study type are major determinants of a study's scientific quality and clinical value.
Methods: This article describes the structured classification of studies into two types, primary and secondary, as well as a further subclassification of studies of primary type. This is done on the basis of a selective literature search concerning study types in medical research, in addition to the authors' own experience.
Results: Three main areas of medical research can be distinguished by study type: basic (experimental), clinical, and epidemiological research. Furthermore, clinical and epidemiological studies can be further subclassified as either interventional or noninterventional.
Conclusions: The study type that can best answer the particular research question at hand must be determined not only on a purely scientific basis, but also in view of the available financial resources, staffing, and practical feasibility (organization, medical prerequisites, number of patients, etc.).
Keywords: basic research; clinical research; epidemiology; literature search; study type.
- Biomedical Research*
- Clinical Trials as Topic*
- Periodicals as Topic*
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Why all doctors should...
Why all doctors should be involved in research
- Related content
- Peer review
- Hannah Jacob , academic clinical fellow
- 1 UCL Institute of Child Health, London WC1N 1EH
- hcjacob{at}gmail.com
Neena Modi tells Hannah Jacob about her career in research and why this is a fundamental part of every doctor’s job
Neena Modi is president of the Royal College of Paediatrics and Child Health and professor of neonatal medicine at Imperial College, London. She is a practising clinician and academic lead of a neonatal research programme focusing on nutritional and other perinatal determinants of lifelong metabolic health. After a period as vice president for science and research at the college, she was elected president in April 2015.
How did you become interested in research?
I realised that what I was being taught during my training was wrong, and my very enlightened consultant challenged me to design a trial to back my contention. There were no training posts in neonatal medicine when I started my paediatric training, but there were lots of opportunities to learn and undertake research because the rate of change was so great. That was really exciting.
Which research projects are you most proud of? Which do you think has had the biggest impact?
We did a series of studies to develop methods for measuring body water compartments in extremely preterm babies and to describe the postnatal alterations in fluid balance. We also tested the hypothesis that immediate sodium supplementation in babies with respiratory distress syndrome was harmful. That was a big achievement.
Most recently we have identified possible biological mechanisms that underpin the epidemiological associations between early onset of features of the metabolic syndrome and being born extremely preterm. That is of real interest as we learn more about the long term effects of extremely preterm birth.
How have you coped with the inevitable setbacks of a career in clinical research?
Real life is about being refused things and carrying on anyway, so I have developed resilience. There was no academic training route when I started out, so I have had to forge my own way. People will always tell you that it cannot be done. You have to pursue the things you are passionate about.
Do you have any advice for junior doctors interested in doing research?
Work out what interests you, and then find the person who is going to help you do it. Being approached by an enthusiastic junior doctor is always well received, and once you have found the right senior person they can support you in achieving your goals. Do not lose heart if you don’t get an academic training post as they are not the only way into research. Some of the best research students I have worked with have not come through the standard path.
What would you say to doctors who have no interest in doing research?
I would argue that they may not be thinking broadly enough about what research actually is. Every clinician is responsible for evaluating their own practice, and to do that in a robust and meaningful way you need to use the tools of research. We all need to be able to critically review research done by others. For example, the guidelines used in everyday clinical practice are based on meta-analyses and systematic reviews. So I think all doctors need to be involved in research in some way, and that may be different for different people.
How can undertaking research help doctors in their careers?
It’s not just a help, it’s essential. There are few absolutes in science, and without inquiring minds medicine will stand still. Participation in research enables doctors to evaluate their practice objectively and to be involved in advancing their discipline. You can learn so many skills that make you a better clinician around appraising the evidence and thinking critically about a situation.
What are the benefits and downsides of doing research—both on a personal and professional level?
The benefits come from knowing you are contributing to the science of medicine as well as the art, and are able to question, evaluate, and test different approaches objectively. Everyone has a role in supporting research—many will contribute, and some will be research leaders.
As for downsides, life has ups and downs, and research is no different. You have to not be too disheartened when a grant application gets rejected. When you want to achieve something, you have to keep speaking to the powers that be until you find someone who can be an advocate.
How do you juggle the research, clinical, and leadership aspects of your working life?
It is a balance that is evolving all the time and that provides me with a huge stimulus. Every time I have been presented with an opportunity I have had to evaluate its potential effect on the other components of my work. I always say yes to the things that interest me and follow my muse. We are very privileged as doctors to have such a range of tremendous opportunities available to us.
Do you have a particular philosophy that has guided you in your career?
When life offers you an opportunity, do not turn it down. I believe you must do what grabs your interest, and if you are still doing it years later you know you made the right decision. When you lose the excitement, it is time for a change. The future lies with junior doctors, and you can be a part of shaping it in the way you think is right.
Is there anything you would do differently if you had your career again?
I would have much greater confidence to fight for something I believed in.
Competing interests: I have read and understood BMJ policy on declaration of interests and declare that I am the academic officer for the Paediatric Educators Special Interest Group of the Royal College of Paediatrics and Child Health.
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Acute Cardiac Events in Hospitalized Older Adults With Respiratory Syncytial Virus Infection
- 1 Division for Heart Disease and Stroke Prevention, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia
- 2 Coronavirus and Other Respiratory Viruses Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia
- 3 US Public Health Service Commissioned Corps, Rockville, Maryland
- 4 California Emerging Infections Program, Oakland
- 5 Colorado Department of Public Health and Environment, Denver
- 6 Connecticut Emerging Infections Program, Yale School of Public Health, New Haven
- 7 Division of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia
- 8 Georgia Emerging Infections Program, Georgia Department of Public Health, Atlanta
- 9 Research, Atlanta Veterans Affairs Medical Center, Decatur, Georgia
- 10 Emerging Infections Program, Maryland Department of Health, Baltimore
- 11 Michigan Department of Health and Human Services, Lansing
- 12 Health Protection Bureau, Minnesota Department of Health, St. Paul
- 13 New Mexico Emerging Infections Program, University of New Mexico, Albuquerque
- 14 Division of Epidemiology, New York State Department of Health, Albany
- 15 School of Medicine and Dentistry, University of Rochester, Rochester, New York
- 16 Public Health Division, Oregon Health Authority, Portland
- 17 Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee
- 18 Epidemiology Bureau, Salt Lake County Health Department, Salt Lake City, Utah
- 19 Division of Global Health Protection, Global Health Center, Centers for Disease Control and Prevention, Atlanta, Georgia
- Editor's Note RSV Vaccination—The Juice Is Worth the Squeeze Tracy Y. Wang, MD, MHS, MSc JAMA Internal Medicine
Question What are the frequency and severity of acute cardiac events among hospitalized adults aged 50 years or older with laboratory-confirmed respiratory syncytial virus (RSV) infection?
Findings In this cross-sectional study of 6248 hospitalized adults with RSV infection, 22% of patients experienced an acute cardiac event, most often acute heart failure (16%). Acute cardiac events occurred more often among those with (33%) vs without (9%) underlying cardiovascular disease and were associated with nearly twice the risk of severe outcomes.
Meaning Findings of this study suggest acute cardiac events are common among hospitalized older adults with RSV infection and are associated with severe clinical outcomes.
Importance Respiratory syncytial virus (RSV) infection can cause severe respiratory illness in older adults. Less is known about the cardiac complications of RSV disease compared with those of influenza and SARS-CoV-2 infection.
Objective To describe the prevalence and severity of acute cardiac events during hospitalizations among adults aged 50 years or older with RSV infection.
Design, Setting, and Participants This cross-sectional study analyzed surveillance data from the RSV Hospitalization Surveillance Network, which conducts detailed medical record abstraction among hospitalized patients with RSV infection detected through clinician-directed laboratory testing. Cases of RSV infection in adults aged 50 years or older within 12 states over 5 RSV seasons (annually from 2014-2015 through 2017-2018 and 2022-2023) were examined to estimate the weighted period prevalence and 95% CIs of acute cardiac events.
Exposures Acute cardiac events, identified by International Classification of Diseases, 9th Revision, Clinical Modification or International Statistical Classification of Diseases, Tenth Revision, Clinical Modification discharge codes, and discharge summary review.
Main Outcomes and Measures Severe disease outcomes, including intensive care unit (ICU) admission, receipt of invasive mechanical ventilation, or in-hospital death. Adjusted risk ratios (ARR) were calculated to compare severe outcomes among patients with and without acute cardiac events.
Results The study included 6248 hospitalized adults (median [IQR] age, 72.7 [63.0-82.3] years; 59.6% female; 56.4% with underlying cardiovascular disease) with laboratory-confirmed RSV infection. The weighted estimated prevalence of experiencing a cardiac event was 22.4% (95% CI, 21.0%-23.7%). The weighted estimated prevalence was 15.8% (95% CI, 14.6%-17.0%) for acute heart failure, 7.5% (95% CI, 6.8%-8.3%) for acute ischemic heart disease, 1.3% (95% CI, 1.0%-1.7%) for hypertensive crisis, 1.1% (95% CI, 0.8%-1.4%) for ventricular tachycardia, and 0.6% (95% CI, 0.4%-0.8%) for cardiogenic shock. Adults with underlying cardiovascular disease had a greater risk of experiencing an acute cardiac event relative to those who did not (33.0% vs 8.5%; ARR, 3.51; 95% CI, 2.85-4.32). Among all hospitalized adults with RSV infection, 18.6% required ICU admission and 4.9% died during hospitalization. Compared with patients without an acute cardiac event, those who experienced an acute cardiac event had a greater risk of ICU admission (25.8% vs 16.5%; ARR, 1.54; 95% CI, 1.23-1.93) and in-hospital death (8.1% vs 4.0%; ARR, 1.77; 95% CI, 1.36-2.31).
Conclusions and Relevance In this cross-sectional study over 5 RSV seasons, nearly one-quarter of hospitalized adults aged 50 years or older with RSV infection experienced an acute cardiac event (most frequently acute heart failure), including 1 in 12 adults (8.5%) with no documented underlying cardiovascular disease. The risk of severe outcomes was nearly twice as high in patients with acute cardiac events compared with patients who did not experience an acute cardiac event. These findings clarify the baseline epidemiology of potential cardiac complications of RSV infection prior to RSV vaccine availability.
- Editor's Note RSV Vaccination—The Juice Is Worth the Squeeze JAMA Internal Medicine
Read More About
Woodruff RC , Melgar M , Pham H, et al. Acute Cardiac Events in Hospitalized Older Adults With Respiratory Syncytial Virus Infection. JAMA Intern Med. Published online April 15, 2024. doi:10.1001/jamainternmed.2024.0212
Manage citations:
© 2024
Artificial Intelligence Resource Center
Best of JAMA Network 2022
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Utility Menu
- Request an Appointment
Popular Searches
- Adult Primary Care
- Orthopedic Surgery
Boston Medical Center Study Furthers Understanding of Lung Regeneration
BOSTON - Researchers at Boston Medical Center (BMC) and Boston University (BU) today announced findings from a new research study, published in Cell Stem Cell, detailing the development of a method for generating human alveolar epithelial type I cells (AT1s) from pluripotent stem cells (iPSCs). The ability to recreate these cells in an iPSC-based model will allow researchers to analyze the historically difficult to isolate cells in greater detail, helping to further the understanding of human lung regeneration and may ultimately expedite progress in treatment and therapeutic options for people living with pulmonary diseases.
Pulmonary diseases, including pulmonary fibrosis and chronic obstructive pulmonary disease (COPD), cause significant mortality and morbidity worldwide, and many pulmonary diseases lack sufficient treatment options. As science and medicine have progressed, researchers have identified a clear need for additional knowledge about lung cells to help improve patient health.
The results of this study provide an in vitro model of human AT1 cells, which line the vast majority of the gas exchange barrier of the distal lung, and are a potential source of human AT1s to develop regenerative therapies. The new model will help researchers of pulmonary diseases deepen their understanding of lung regeneration, specifically after an infection or exposure to toxins, as well as diseases of the alveolar epithelium such as acute respiratory distress syndrome (ARDS) and pulmonary fibrosis.
“Uncovering the ability to generate human alveolar epithelial type I cells (AT1s), and similar cell types, from pluripotent stem cells (iPSCs), has expanded our knowledge of biological processes and can significantly improve disease understanding and management,” said Darrell Kotton, MD, Director, Center for Regenerative Medicine (CReM) of Boston University and Boston Medical Center.
This new study also furthers the CReM’s goal of generating every human lung cell type from iPSCs as a pathway to improving disease management and provides a source of cells for future transplantation to regenerate damaged lung tissues in vivo.
“We know that the respiratory system can respond to injury and regenerate lost or damaged cells, but the depth of that knowledge is currently limited,” said Claire Burgess, PhD, Boston University Chobanian and Avedisian School of Medicine, who is the study’s first author. “We anticipate this protocol will be used to further understand how AT1 cells react to toxins, bacteria, and viral exposures, and will be used in basic developmental studies, disease modeling, and potential engineering of future regenerative therapies."
The full study is published in Cell Stem Cell and can be located here .
About Boston Medical Center
Boston Medical Center models a new kind of excellence in healthcare, where innovative and equitable care empowers all patients to thrive. We combine world-class clinicians and cutting-edge treatments with compassionate, quality care that extends beyond our walls. As an award-winning health equity leader, our diverse clinicians and staff interrogate racial disparities in care and partner with our community to dismantle systemic inequities. And as a national leader in research and the teaching affiliate for Boston University Chobanian & Avedisian School of Medicine, we’re driving the future of care.
Media Contact:
Please reach out to the Boston Medical Center Media Relations team with any questions.
School of Medicine M.D./Ph.D. Training Program
- Enter keyword Search
M.D./Ph.D. Training Program
Elias (eli) wisdom.
Current Program Year: Grad 1 Current Student, Neuroscience Graduate Program, School of Medicine M.D./Ph.D. Program Students, School of Medicine
Research interests: neuroscience (neurobiological diseases, synapse biology, plasticity, engineered synapses, behavior).
Clinical interests: neurology and psychiatry
Eli is an MD-Ph.D. student studying the molecular nature of Alzheimer’s Disease, Parkinson’s Disease, and Dementia with Lewy bodies under mentorship from Dr. Vivek Unni, MD, Ph.D., and Andrew Emili, Ph.D. He grew up in La Grande, OR, where he developed a strong appreciation for the natural world and a deep passion for baseball. Following these, he attended Pacific University, in Forest Grove, OR, where he studied Biology while competing on the varsity baseball team all four years. During the summer before his senior year, he completed an internship at Fred Hutchinson Cancer Research Center in Seattle, WA, under mentorship from Harmit Malik, Ph.D., where he first learned that MD-PhD programs existed. Knowing that he could combine careers in medicine and scientific research with this path, he sought out further research and clinical training and moved to New Haven, CT, where he joined the lab of Daniel Colón-Ramos at Yale School of Medicine to study the cell biology of the synapse. His time in the Colón-Ramos Lab and volunteering at Yale New Haven Hospital affirmed his desire to have a career at the intersection of medicine and research. He was fortunate to matriculate into the MSTP program at Oregon Health & Science University in 2021, where he has been able to have outstanding training in his research and clinical interests while living close to family.
Parkinson’s Disease and Alzheimer’s Disease are the two most prevalent neurodegenerative diseases, with little to no treatments available to halt their progression. Having family members and friends afflicted with these diseases, and from his countless clinic visits with patients both before and during medical school, he is motivated to understand the basic biology that drives these conditions, and how to translate this into curative and preventative treatments.
Education and training
B.S., 2019, Pacific University
Fellowship : Postbaccalaureate Research Fellowship, Yale University, 2019-2021
Memberships and associations:
- American Physician Scientist Association
- American College of Physicians
- Beta-Beta-Beta Biological Honor Society
Honors and awards
Fred Hutchinson Intern Alumni Scholarship, 2019 $25,000 Inspired Ideas Competition Winner, Entrepreneur Award, 2018
Publications
Selected publications.
Hawk, J.D., Wisdom, E.M. , Sengupta, T. et al. "A genetically encoded tool for reconstituting synthetic modulatory neurotransmission and reconnect neural circuits in vivo". Nature Communications 12, 4795 (2021). https://doi.org/10.1038/s41467-021-24690-9
Ransey, E., Chesnov, K., Thomas, G., Wisdom, E. , Bowman, R., Rodriguez, T., Adamson, E., Almoril-Porras, A., Walder-Christensen, K., Hughes, D., Schwennesen, H., Mague, S., Colón-Ramos, D., Hultman, R., Bursac, N, Dzirasa, K., "Long-term precision editing of neural circuits in mammals using engineered gap junction hemichannels". bioRxiv, 2021, 08.24.457429; doi: https://doi.org/10.1101/2021.08.24.457429
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Balkan Med J
- v.31(4); 2014 Dec

Study Designs in Medicine
Scientific studies can be described as “ a planned and systematic effort based on evidence for the solution of any health problems using data with high degree of accuracy ” ( 1 ). The main aims are to quantify disease prevalence, and compare interventions, predictions, association assessments or etiology assessments ( 2 ). A scientific study requires good planning including research protocol, ethical approval, data collection, data analysis, interpretation of data analysis results and publication. This study can help authors understand study designs in medicine.
Scientific studies can be classified as “Basic Studies”, “Observational Studies”, “Experimental (Interventional) Studies”, “Economic Evaluations” and “Meta-Analysis – Systematic Review”, as shown in Figure 1 .

Study designs
BASIC STUDIES
Basic studies investigate the cause-outcome relationships between a dependent variable and independent variables, such as animal experiment, genetic and cell studies. Also, method development studies investigate the development and improvement of biochemical (e.g., enzymes, markers or genes), imaging (e.g., magnetic resonance) and biometric methods (e.g., statistical methods) ( 3 ). Several checklists have been developed to guide authors in the preparing, conducting and reporting stages of their studies. The ARRIVE checklist supplies transparency and accuracy in the animal experiments ( 1 ).
OBSERVATIONAL STUDIES
Observational studies can be defined as non-interventional and non-experimental ( 3 ). They do not contain any experiment or intervention methods. Investigated factors aren’t controlled, repetition of events aren’t generally possible and randomisation facilities are limited in these studies. However, their results are largely consistent with real life ( 4 ). They can be classified as descriptive or analytical, as shown in the Figure 1 .
Descriptive studies
Health problems or events as regards a particular disease or condition are detected and identified in these studies. They seek answers to the following questions about health problems or events: “What is it?”, “Where is it seen?”, “When is it seen?” and “Who are observed?” Descriptive statistics (mean, rate, etc.), frequency distributions and population parameters are determined by this kind of research.
Descriptive observational studies include case-report , case series and cross-sectional studies (descriptive or prevalence) . Patient and disease characteristics related to some interesting and remarkable type defined in a patient are called a “ case report ”. When the number of patients is more than one, this is called a “ case series ”. These are the most simple research types and do not contain a control group. Case series are usually starting points of the examined hypothesis in the case-control, cross-sectional or cohort studies ( 5 ). The use of CARE statement in the publication of a case report supplies transparency and accuracy ( 6 ).
Cross-sectional studies (descriptive or prevalence) can be described as prevalence studies and generally examine the prevalence, epidemiology or survey of a disease or clinical outcome. They reflect the situation of a disease or clinical outcome at a particular moment in a particular population ( 5 ).
Analytical (inferential) studies
Cross-sectional study.
Analytical cross-sectional studies are conducted in a specific time period which does not contain follow-up and enquires: “What is happening in a specific time period?” ( Figure 2 ) They try to explain potential causal associations between causes (exposures) and outcome (disease or clinical outcome). As a cohort study, they compare disease prevalence between exposure groups, and as a case-control study, they compare exposure between disease and healthy groups ( 2 ). Generally, they do not have a follow-up period.( 5 ) Checklists guide the authors in preparing, conducting and reporting stages of research. The STROBE statement for cross-sectional studies is a useful guideline for this design ( 1 ).

Cross-sectional study design
Case-control study
Case-Control Studies are conducted retrospectively and enquire: “What happened in the past?” ( Figure 3 ). The cases are subjects selected according to presence of disease or clinical outcome. However, the control subjects are selected without disease or clinical outcome. The case and control groups are compared in terms of the presence of certain factors. Case group should be matched to the control group except for investigated factors. These are matched case-control study ( 5 ). The STROBE statement for case-control studies guides authors ( 1 ).

Case-control study design
Diagnostic Accuracy Studies investigate the effect of a diagnostic method (such as imaging, pathological) compared with a gold standard method ( 3 ). They are similar to case-control studies. The STARD statement helps authors in designing, conducting and reporting diagnostic accuracy studies ( 1 ).
Cohort study
Cohort is a special group of people who have been selected according to some defining characteristics and they have certain disease risk factors or health outcome. Cohort Studies , also called follow-up studies, are generally prospective and enquire: “What will happen in the future?” ( Figure 4 ) Individuals are followed over time in cohort studies, and researchers assess exposure and outcome during follow-up ( 2 ). Cohort studies investigate the effect of prognostic factors (such as age, presence of hypertension and cholesterol level) on a clinical outcome (such as disease) ( 3 ).

Cohort study design
Moreover, cohort studies can be conducted retrospectively; these are called “Historical Cohort Study”. Cohort Studies produce the most reliable clinical evidence among the observational studies due to the fact that they identify clinical or health outcomes based on exposure ( 5 ). The STROBE statement for cohort studies helps authors ( 1 ).
EXPERIMENTAL (INTERVENTIONAL) STUDIES
Experimental or interventional studies compare the effect of treatments or interventions with control in humans. Placebo or different treatment(s) or intervention(s) may be used as control. Experimental studies have to be transparent and evidence-based. In these studies, randomisation methods can be used, investigated factors are controlled, cause-effect relationships are evidenced and an experiment can be repeated as much as desired. However, their results are always not appropriate for real life ( 4 ). They can be conducted in four phases ( 7 ).
Phase I study is conducted in a small number of healthy volunteers (e.g. 20–80) to determine whether a drug or treatment method is safe . Pharmacokinetic and pharmacodynamic measurements are done in these studies. Maximum safe dose, movement of the drug in the body and dose-response relationship are examined. Phase II study is conducted in a target population (75–300) to determine the treatment effect of a drug or treatment method. Standard treatment method has to be compared with placebo in Phase II clinical trials. Phase III study is conducted on many patients (e.g., 1000–2000) to determine whether the new drug is better than the standard drug. It is done in order to reveal that a drug is not only safe and effective, but also has better and less adverse effects than standard treatment . Usually, at least two RCTs are required in this phase.
Clinical trials (Phase IV) are called post-marketing product surveillance studies, which are conducted on patients in daily life; the new drug had been approved by the Ministry in this phase. They evaluate the adverse effect and various additional indications of a new drug ( 7 , 8 ).
Observational Drug Studies are other forms of Phase IV clinical trials. They collect the data about a spontaneously prescribed drug from the patients with diagnosed and ongoing treatment. In these studies, additional information from a larger population may be obtained in order to compare the results of experimental clinical drug trials ( 4 ).
Randomised controlled trial (RCT)
Randomised controlled trials produce the strongest evidence among clinical trials due to the fact that patients are allocated to treatments or interventions randomly (equal chance). In these studies, two or more clinical treatments or intervention are compared. RCTs are expensive and slow, however, their level of evidence is higher due to the fact that randomisation removes the allocation bias ( 2 ). Many respected journals endorse the CONSORT statement in order to improve the scientific quality and transparency of RCTs. Authors should be used to the CONSORT statement as a guideline in RCTs ( 1 ).
When the preference of participants is not to receive a placebo or control, randomisation procedure is not applied. These studies are called Non-Randomised Controlled Studies . They are inexpensive especially if they are conducted as retrospective and representative sample of patients in clinical practice. However, they are open to bias ( 2 ).
Self-controlled study
Self-Controlled Studies do not include an independent control group; they use the patients as their own controls. At least two measurements are obtained at different times from the same patients (e.g., preop, postop 1. month, and 6. month measurements) and the effect of treatment or intervention is determined ( 5 ).
Crossover study
Crossover Studies include both of self-control and independent groups. They are powerful, but not always possible to apply. In crossover studies, patients are assigned two groups (placebo or experimental treatment). After a time, the research is interrupted for a washout period (at least two weeks), and patients receive no treatments during this period. At the end of the washout period, the experimental treatment group receives the placebo and the placebo group receives to the experimental treatment ( 5 ). The effect of treatment or intervention is determined by comparisons of both self-control and independent groups in crossover design ( Figure 5 ).

Crossover study design
Properties of experimental studies
Direction of studies.
Studies can be classified as prospective or retrospective according to direction. In prospective studies, a specific sample is followed over a certain period in order to determine outcome from the reasons. The research question is: “What will happen in the future?” Retrospective studies generally compare the outcome of diagnostic and treatment methods. Data are obtained from patient records. The research question is: “What happened in the past?” ( 5 ).
Randomisation
In randomisation method the subjects or patients who will be included in the study are assigned to treatment groups with equal probability (chance) in the beginning of study. A computerized software is widely used for allocating the subjects/patients to the groups in the randomisation processes. Studies can be classified as i) randomised or ii) non-randomised. Randomised Controlled Studies (RCTs) produce the most reliable results among all research types.
Blinding describes that one or more of the physicians, researchers, patients and data analysts do not know which treatment subjects have received. It ensures reliable and objective results preventing bias. Blinding can be defined as three different types (single, double and triple). Single-blind: either subjects or researchers know which treatment subjects have received. Double-blind: both subjects and researchers do not know which treatment subjects have received. Triple-blind: in addition to the subjects and researcher(s), statisticians/monitors do not know which treatment subjects have received ( 5 ).
Confounding and interaction
Confounding can be defined as disruption of the relationships between two variables due to the effect of third variable. A confounder variable is associated both with causal and outcome variables ( 9 ). Two or higher independent variables have different effect on outcome variable to independent effect of each. This situation can be defined as interaction .
ECONOMIC EVALUATIONS
Cost Analysis is an economic analysis method that estimates total cost of a particular disease or health condition on society. Direct and indirect costs attributed to a specific disease are included in this method. It is also called “cost of illness”. Cost-Minimisation Analysis compares two alternative drugs’ (or interventions) costs and outcomes in order to determine the least costly drug (or intervention). However, it is quite difficult to find two alternative drugs which are equally effective and safe. Thus, it is rarely used in economic evaluations. Cost-Utility Analysis is an economic evaluation method comparing two alternative drugs (or interventions) costs and outcomes in order to determine the most useful drug (or intervention). Outcomes in these studies are measured in preference or utility of patients, and, generally, quality-adjusted life year (QALY) or disability-adjusted life year (DALY) are used as an outcome. Cost-Effectiveness Analysis compares two alternative drugs (or interventions) costs and clinical outcomes in order to determine the most effective drug (or intervention). Outcomes are measured by clinical parameters. It is the most widely used economic evaluation method. Cost-Benefit Analysis is an economic evaluation method, in which cost and benefit of alternative interventions are expressed in monetary units. Thus, it is rarely used in economic evaluations ( 8 ).
META-ANALYSIS AND SYSTEMATIC REVIEW
Several clinical studies (RCTs or Cohort) may be conducted in a clinical area over a period of years in different parts of the world. The results may be different and there may be different properties such as sample size and multicentre. Meta-Analysis combines the statistical results of different studies in a particular clinical area ( 7 , 9 ). The PRISMA statement guides the authors in the preparation of Meta-Analysis ( 1 ).
Systematic Review evaluates and interprets the evidence of all studies conducted in a clinical area ( 9 ). The main difference from Meta-Analysis is that it combines the evidence of different studies based on interpretation instead of combining statistical results.
Evidence level of the medical studies
The evidence pyramid shows the evidence level of a scientific study in clinical practices. The evidence pyramid of scientific medical studies is shown in Figure 6 . According to the evidence pyramid, the “Meta-Analysis/Systematic Review” produces the most reliable evidence, while “ in vitro study” produces the lowest reliable evidence ( 10 ).

Evidence pyramid for medical studies
In conclusion, authors should correctly report the study design in the method section of their studies. Also, if randomisation, stratification or blinding methods are used, they should be reported in this section. Generally, studies are conducted on a sample, so sample size should be a sufficient number and representative of population in structural terms. Thus, determination of sample size, selection method of sample, inclusion and exclusion criteria should be explained in detail in the method section. Use of the checklists ( CONSORT statement for RCTs, ARRIVE for animal experiments and STROBE statement for cross-sectional, case-control and cohort studies, CARE statement for case report and PRISMA statement for meta-analysis) may prevent bias and guide authors in the preparation of their studies.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Stanford Medicine-led study identifies novel target for epilepsy treatment
Researchers find that a little-understood part of the brain appears to be involved in starting seizures and keeping them going.
April 17, 2024 - By Kimberlee D'Ardenne
Stanford Medicine researchers and their colleagues found that removing or inhibiting the fasciola cinereum may help epilepsy patients who aren't helped by surgery. Tom - stock.adobe.com
Removing part of the brain’s temporal lobe is the only treatment available to the millions of people with a form of epilepsy that medications often don’t alleviate. But even that approach fails a third of the time.
A new study from Stanford Medicine researchers and their colleagues offers an explanation and suggests a more effective approach to treatment. They found that a previously overlooked region of the hippocampus, the fasciola cinereum, appears to be involved in instigating and propagating seizures. Removing or inhibiting the fasciola cinereum may help those patients who don’t find relief after surgery.
“The hippocampus is the best studied part of the brain by far, but there is shockingly little known about the fasciola cinereum,” said Ivan Soltesz , PhD, the James R. Doty Professor in Neurosurgery and Neurosciences and a senior author on the study. “This relatively small region was consistently involved in seizure activity in mice and in people undergoing pre-surgical electrical recordings. Our findings suggest that all patients with drug-resistant temporal lobe epilepsy should have depth electrodes placed in the fasciola cinereum as part of the surgery planning process.”
The work was published April 17 in Nature Medicine . Soltesz and Vivek Buch , MD, the Christina and Hamid Moghadam Faculty Scholar as well as the surgical director of the Stanford Comprehensive Epilepsy Center , are co-senior authors.
A tale of a tail
Worldwide, 65 million people live with epilepsy. Tens of millions have mesial temporal lobe epilepsy, with seizures originating, in part, from the amygdala, an almond-shaped structure involved in processing emotions, and the hippocampus, a region necessary for forming memories. When people with mesial temporal lobe epilepsy of just one hemisphere do not respond to anti-seizure drug therapies, the standard of care is surgery. In these procedures, the amygdala and most of the hippocampus in one hemisphere are either surgically removed or ablated, a technique that involves using a laser to heat up and destroy tissue. Because of the symmetry of the temporal lobe — both hemispheres of the brain have an amygdala and hippocampus — people who have these surgeries usually have minimal side effects, according to the researchers.

Ivan Soltesz
Before performing the surgery, physicians need to identify the brain tissue responsible for seizure activity. They do this by placing electrodes in areas of the brain suspected of starting or propagating seizures and taking recordings from the electrodes. This process, called stereoelectroencephalography, or sEEG, lets them map where in the brain seizure activity happens.
Though the amygdala and its next-door neighbor the hippocampus are common locations for sEEG recordings, the electrodes are typically placed in only the anterior and middle regions of the hippocampus. The human hippocampus, located deep in each hemisphere of the brain near the level of the ear, looks like a sea horse lying on its side, with its head pointing toward the front of the brain. sEEG electrodes are commonly placed in the anterior and medial regions, corresponding to the head, body and the beginnings of the tail of the sea horse.
The idea to record from the fasciola cinereum — the far tip of the sea horse’s tail — in patients with epilepsy undergoing sEEG for surgical planning first formed about three years ago, when Ryan Jamiolkowski , MD, PhD, co-lead author of the study and a resident in neurosurgery, joined the Soltesz lab.
At the time, Quynh-Anh Nguyen, PhD, co-lead author on the study and former postdoctoral scholar in the Soltesz lab who is now at Vanderbilt University, was screening for the hippocampal neurons that were active during seizures in mice. Unexpectedly, Nguyen discovered that neurons in a posterior region of the hippocampus, the fasciola cinereum, were involved in seizures.
Jamiolkowski and the research team used optogenetic techniques to test whether the fasciola cinereum could be a target for epilepsy interventions. The neurons in the fasciola cinereum were made to contain special proteins capable of shutting down neuronal activity when exposed to blue light. When electrical recordings from the hippocampus showed seizure activity, the researchers shined blue light onto the fasciola cinereum, shortening the duration of seizures in mice.
Recording from the human hippocampus tail
To understand the fasciola cinereum’s role in seizure activity in humans, Jamiolkowski and Buch recorded from the small region in six patients. All were undergoing sEEG to identify the source of their seizures in preparation for future surgeries to cure their epilepsy. The fasciola cinereum contributed recorded seizures in all six patients, including some episodes in which the head and body regions of the hippocampus were quiet.

Ryan Jamiolkowski
One of the patients with mesial temporal lobe epilepsy of the left hemisphere had already undergone laser ablation of the amygdala and anterior and middle regions of the hippocampus. The patient continued having seizures, and follow-up sEEG showed that the only part of the hippocampus that remained, the fasciola cinereum, was involved in all recorded seizures. The patient underwent a second surgical ablation that removed almost all of the fasciola cinereum, and the frequency of the seizures decreased by 83%, from one to two each month to once every three months.
The researchers said that patients whose seizures involve the fasciola cinereum may need to undergo two surgeries because of the shape of the hippocampus.
“The hippocampus curves like a banana, and the optical fiber used for laser ablation is a straight line. Reaching anterior and posterior regions requires different trajectories that are not currently feasible to combine into one procedure. The results of our study do not challenge the importance of ablating the amygdala and anterior hippocampus but suggest considering a second ablation targeting the posterior hippocampal tail for the patients whose seizures recur,” Jamiolkowski said.
Three of the patients had bilateral involvement of the mesial temporal lobe, which means the amygdala and hippocampus in both the right and left hemisphere showed seizure activity. Because new memories cannot be formed without at least one intact hippocampus, these patients instead received responsive neurostimulation from a device that detects and interrupts seizure activity. However, most responsive neurostimulation units can be configured to target only the anterior regions of the hippocampus on both sides of the brain. The findings from this study suggest that a more personalized approach that also allows the device to monitor and interrupt seizure activity in the posterior hippocampal tail region might be more beneficial to patients.
“Because one-third of patients — a high percentage — do not get seizure freedom from surgery, we should be putting sEEG electrodes in the fasciola cinereum in all temporal lobe epilepsy patients; seizure activity in this region could be a reason why these surgeries sometimes fail,” Jamiolkowski added. “Knowing which patients have seizures involving the fasciola cinereum would let us target it with either ablation or neurostimulation and help us treat patients better than a one-size-fits all approach.”
A researcher from Cambridge University contributed to the study.
Funding for this study was provided by the Stanford Maternal and Child Health Research Institute, the Tashia and John Morgridge Endowed Fellowship, the Lennox-Gastaut Syndrome Foundation Cure 365, the Stanford Neuroscience Scholars Program, and the National Institutes of Health (grants R25NS065741, K99NS121399, K99NS126725, NS121106 and P30AG066515).
- Kimberlee D'Ardenne Kimberlee D'Ardenne is a freelance writer.
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Artificial intelligence
Exploring ways AI is applied to health care
- Warning Signs and Symptoms
- Mental Health Conditions
- Common with Mental Illness
- Mental Health By the Numbers
- Individuals with Mental Illness
- Family Members and Caregivers
- Kids, Teens and Young Adults
- Veterans & Active Duty
- Identity and Cultural Dimensions
- Frontline Professionals
- Mental Health Education
- Support Groups
- NAMI HelpLine
- Publications & Reports
- Podcasts and Webinars
- Video Resource Library
- Justice Library
- Find Your Local NAMI
- Find a NAMIWalks
- Attend the NAMI National Convention
- Fundraise Your Way
- Create a Memorial Fundraiser
- Pledge to Be StigmaFree
- Awareness Events
- Share Your Story
- Partner with Us
- Advocate for Change
- Policy Priorities
- NAMI Advocacy Actions
- Policy Platform
- Crisis Intervention
- State Fact Sheets
- Public Policy Reports
- About Mental Illness
Types of Mental Health Professionals
Many types of mental health care professionals can help you achieve your recovery goals. These professionals work in inpatient facilities, such as general hospitals and psychiatric facilities, and outpatient facilities, such as community mental health clinics, schools and private practices.
Health care professional job titles and specialties can vary by state. The descriptions below give an overview of what to look for and what credentials to expect from a mental health professional. Finding the right professional is easier when you understand the different areas of expertise and training.
The NAMI HelpLine can provide information on how to find various mental health professionals and resources in your area. Please note that we are unable to provide specific recommendations to individual providers as we are unable to speak to the quality of their care.
Assessment And Therapy
Therapists can help someone better understand and cope with thoughts, feelings and behaviors. They can also offer guidance and help improve a person’s ability to achieve life goals. These mental health professionals may also help assess and diagnosis mental health conditions.
Psychologists
Psychologists hold a doctoral degree in clinical psychology or another specialty such as counseling or education. They are trained to evaluate a person’s mental health using clinical interviews, psychological evaluations and testing. They can make diagnoses and provide individual and group therapy. Some may have training in specific forms of therapy like cognitive behavioral therapy (CBT), dialectical behavior therapy (DBT) and other behavioral therapy interventions.
Degree requirements: Doctor of Philosophy (Ph.D.) in a field of psychology or Doctor of Psychology (Psy.D.). Licensure & credentials: Psychologists are licensed by licensure boards in each state.
Counselors, Clinicians, Therapists
These masters-level health care professionals are trained to evaluate a person’s mental health and use therapeutic techniques based on specific training programs. They operate under a variety of job titles—including counselor, clinician, therapist or something else—based on the treatment setting. Working with one of these mental health professionals can lead not only to symptom reduction but to better ways of thinking, feeling and living.
Degree requirements: master’s degree (M.S. or M.A.) in a mental health-related field such as psychology, counseling psychology, marriage or family therapy, among others. Licensure & Certification: Varies by specialty and state. Examples of licensure include:
- LPC, Licensed Professional Counselor
- LMFT, Licensed Marriage and Family Therapist
- LCADAC, Licensed Clinical Alcohol & Drug Abuse Counselor
Clinical Social Workers
Clinical social workers are trained to evaluate a person’s mental health and use therapeutic techniques based on specific training programs. They are also trained in case management and advocacy services.
Degree requirements: master’s degree in social work (MSW). Licensure & credentials: Examples of licensure include:
- LICSW, Licensed Independent Social Workers
- LCSW, Licensed Clinical Social Workers
- ACSW, Academy of Certified Social Workers
Prescribe And Monitor Medication
The following health care professionals can prescribe medication . They may also offer assessments, diagnoses and therapy.
Psychiatrists
Psychiatrists are licensed medical doctors who have completed psychiatric training. They can diagnose mental health conditions, prescribe and monitor medications and provide therapy. Some have completed additional training in child and adolescent mental health, substance use disorders or geriatric psychiatry.
Degree requirements: Doctor of Medicine (MD) or Doctor of Osteopathic Medicine (DO), plus completion of residency training in psychiatry. Licensure & credentials: Licensed physician in the state where they are practicing; may also be designated as a Board Certified Psychiatrist by the Board of Neurology and Psychiatry.
Psychiatric Or Mental Health Nurse Practitioners
Psychiatric or mental health nurse practitioners can provide assessment, diagnosis and therapy for mental health conditions or substance use disorders. In some states, they are also qualified to prescribe and monitor medications. Requirements also vary by state as to the degree of supervision necessary by a licensed psychiatrist.
Degree requirements: Master of Science (MS) or Doctor of Philosophy (Ph.D.) in nursing with specialized focus on psychiatry. Licensure & credentials: Licensed nurse in the state where they are practicing. Examples of credentials include, but are not limited to:
- NCLEX, National Council Licensure Examination
- PMHNP-BC, Board Certification in psychiatric nursing through the American Academy of Nurses Credentialing Center
Primary Care Physicians
Primary care physicians and pediatricians can prescribe medication, but you might consider visiting someone who specializes in mental health care. Primary care and mental health professionals should work together to determine an individual’s best treatment plan.
Degree requirements: Doctor of Medicine (M.D.) or Doctor of Osteopathic Medicine (DO). Licensure & credentials: Licensed physician in the state where they are practicing.
Family Nurse Practitioners
Family nurse practitioners (FNP) can provide general medical services like those of a primary care physician, based on each state’s laws. Like primary care physicians, they can prescribe medication, but you might consider visiting someone who specializes in mental health care. Family nurse practitioners and mental health professionals should work together to determine an individual’s best treatment plan.
Degree requirements: Master of Science (M.S.) or Doctor of Philosophy (Ph.D.) in nursing. Licensure & credentials: Licensed nurse in the state where they are practicing. Examples of credentials include:
- FNP-BC, Family Nurse Practitioner Board Certified
Psychiatric Pharmacists
Psychiatrist pharmacists are advanced-practice pharmacists who specialize in mental health care. They can prescribe or recommend appropriate medications if allowed in their state and practice setting. They are skilled at medication management—meaning they evaluate responses and modify treatment, manage medication reactions and drug interactions, and provide education about medications. Many have completed additional training in child/adolescent psychiatry, substance use disorders or geriatric psychiatry.
Degree requirements: Doctor of Pharmacy (PharmD). Completion of residency training in psychiatric pharmacy is not required, but is common. Licensure & credentials: Licensed pharmacist in the state where they practice; may also be designated a Board Certified Psychiatric Pharmacist by the Board of Pharmacy Specialties.
Other Professionals You May Encounter
Certified peer specialists.
These specialists have lived experience with a mental health condition or substance use disorder. They are often trained, certified and prepared to assist with recovery by helping a person set goals and develop strengths. They provide support, mentoring and guidance.
Social Workers
Social workers (B.A. or B.S.) provide case management, inpatient discharge planning services, placement services and other services to support healthy living.
Pastoral Counselors
Pastoral counselors are clergy members with training in clinical pastoral education. They are trained to diagnose and provide counseling. Pastoral counselors can have equivalents to a doctorate in counseling.
Updated April 2020

Know the warning signs of mental illness

Learn more about common mental health conditions
NAMI HelpLine is available M-F, 10 a.m. – 10 p.m. ET. Call 800-950-6264 , text “helpline” to 62640 , or chat online. In a crisis, call or text 988 (24/7).

COMMENTS
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. In almost all experiments, at least one independent variable is varied and the effects on the dependent variable are investigated.
Types of study design. Medical research is classified into primary and secondary research. Clinical/experimental studies are performed in primary research, whereas secondary research consolidates available studies as reviews, systematic reviews and meta-analyses. Three main areas in primary research are basic medical research, clinical research ...
There are various types of scientific studies such as experiments and comparative analyses, observational studies, surveys, or interviews. The choice of study type will mainly depend on the research question being asked. When making decisions, patients and doctors need reliable answers to a number of questions.
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
Basic medical research is usually conducted by scientists with a PhD in such fields as biology and chemistry, among many others. They study the core building blocks of life — DNA, cells, proteins, molecules, etc. — to answer fundamental questions about their structures and how they work. For example, oncologists now know that mutations in ...
Below are descriptions of some different kinds of clinical research. Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to ...
Clinical research is medical research that involves people like you. When you volunteer to take part in clinical research, you help doctors and researchers learn more about disease and improve health care for people in the future. Clinical research includes all research that involves people. Types of clinical research include:
Study Design 101: Himmelfarb's tutorial on study types and how to find them. Study Designs (Centre for Evidence Based Medicine, University of Oxford) Learn about Clinical Studies (ClinicalTrials.gov, National Institutes of Health) Study Designs Guide (Deakin University) Last Updated: Dec 6, 2023 7:59 AM. URL: https://guides.himmelfarb.gwu.edu/ebm.
Transcript. ANNOUNCER: There are many different types of clinical research because researchers study many different things. Treatment research usually tests an intervention such as medication, psychotherapy, new devices, or new approaches. Prevention research looks for better ways to prevent disorders from developing or returning.
Clinical research is research conducted with human subjects, or material of human origin, in which the researcher directly interacts with human subjects. Clinical researchers at the National Human Genome Research Institute (NHGRI) are developing advanced methods for studying the fundamental mechanisms of inherited and acquired genetic disorders.
Introduction to Types of Medical Research. Evidence-based medicine may be defined as the systematic, quantitative and preferentially experimental approach to obtaining medical information. This information is obtained through medical research. Medical research encompasses a wide range of study techniques that can be used to understand diseases ...
Primary medical research is categorized into three main fields: laboratorial, clinical, and epidemiological. Laboratory scientists analyze the fundamentals of diseases and treatments. Clinical researchers collaborate with participants to test new and established forms of treatment. Epidemiologists focus on populations to identify the cause and ...
A meta-analysis is a type of systematic review that goes one step further, combining the data from multiple studies and using statistics to summarize it, as if creating a mega-study from many smaller studies.4. However, even systematic reviews and meta-analyses aren't the final word on scientific questions.
Results: Three main areas of medical research can be distinguished by study type: basic (experimental), clinical, and epidemiological research. Furthermore, clinical and epidemiological studies can be further subclassified as either interventional or noninterventional. Conclusions: The study type that can best answer the particular research ...
In order to judge and evaluate medical research, people need, first, to have access to the biomedical literature and, second, the skill to search and find relevant articles. ... When using electronic entry, it is useful to define precisely the type of entered data (Table 15.3), e.g. enter data in the right format and group them according to the ...
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug ...
Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population.
Every clinician is responsible for evaluating their own practice, and to do that in a robust and meaningful way you need to use the tools of research. We all need to be able to critically review research done by others. For example, the guidelines used in everyday clinical practice are based on meta-analyses and systematic reviews.
The All of Us Research Program is a historic effort to gather data from one million or more people living in the United States to accelerate research and improve health. By taking into account individual differences in genes, environment, and lifestyle, researchers will uncover paths toward delivering precision medicine.
Cell lines, dissociated tumor cells, and primary cells all have their place in research. While there may be some overlap, each occupies a unique niche. Immortalized cell lines have been a ...
The study was funded by the National Institutes of Health (grants K24AR075060 and R01AR082109), Radiumhemmet Research, the Swedish Cancer Society and the Swedish Research Council. For more news about responsible AI in health and medicine, sign up for the RAISE Health newsletter. Register for the RAISE Health Symposium on May 14.
Despite having an overall survival rate of 94%, B-cell acute lymphoblastic leukemia (B-ALL), the most common childhood cancer, can prove challenging to treat, with survival among relapsed or resistant cases falling between 30-50%. Recent work by St. Jude Children's Research Hospital scientists discovered which tumor cells resist treatment and ...
Importance Respiratory syncytial virus (RSV) infection can cause severe respiratory illness in older adults. Less is known about the cardiac complications of RSV disease compared with those of influenza and SARS-CoV-2 infection. Objective To describe the prevalence and severity of acute cardiac events during hospitalizations among adults aged 50 years or older with RSV infection.
Boston Medical Center (BMC) is a 514-bed academic medical center located in Boston's historic South End, providing medical care for infants, children, teens and adults. One Boston Medical Center Place Boston, MA 02118 617.638.8000. 24 Hour Emergency Department 725 Albany Street Boston, MA 02118 617.414.4075. Social Links. Boston Medical Center ...
The study was funded by the Weston Family Foundation, the David Braley Centre for Antibiotic Discovery, the Canadian Institutes of Health Research, M. and M. Heersink, the Chan-Zuckerberg Biohub, and the Knight-Hennessy scholarship. For more news about responsible AI in health and medicine, sign up for the RAISE Health newsletter.
Clinical interests: neurology and psychiatry. Eli is an MD-Ph.D. student studying the molecular nature of Alzheimer's Disease, Parkinson's Disease, and Dementia with Lewy bodies under mentorship from Dr. Vivek Unni, MD, Ph.D., and Andrew Emili, Ph.D. He grew up in La Grande, OR, where he developed a strong appreciation for the natural world ...
Penn State researchers developed an robotic simulation training program to provide trainee physicians with more practice on the procedure. A year after deploying the program at the Penn State College of Medicine, the team found that all complication types — mechanical issues, infections and blood clots — were significantly lower.
Study Designs in Medicine. Scientific studies can be described as " a planned and systematic effort based on evidence for the solution of any health problems using data with high degree of accuracy " ( 1 ). The main aims are to quantify disease prevalence, and compare interventions, predictions, association assessments or etiology ...
The work was published April 17 in Nature Medicine. Soltesz and Vivek Buch, MD, ... Jamiolkowski and the research team used optogenetic techniques to test whether the fasciola cinereum could be a target for epilepsy interventions. The neurons in the fasciola cinereum were made to contain special proteins capable of shutting down neuronal ...
Many types of mental health care professionals can help you achieve your recovery goals. These professionals work in inpatient facilities, such as general hospitals and psychiatric facilities, and outpatient facilities, such as community mental health clinics, schools and private practices. Health care professional job titles and specialties can vary by state. The descriptions below give […]