Essay on Acid Rain for Students and Children
Essay on acid rain.
Acid Rain includes rain, snow, hail, fog, or dew that is high in acid pollutants, especially sulphuric and nitric acid. Acid Rain is mainly caused by emissions of sulphur dioxide and nitrogen oxide from various sources. They react with the water molecules in the atmosphere to produce acids. The problem of Acid Rain has not only increased with rapid growth in population and industrialization , but it has also become more harmful. In fact, the use of the tall chimneys on a factory, ship, has contributed to the spread of Acid Rain by releasing gases into the atmosphere. A large number of acid deposits are witnessed in Canada, the United States, Europe, portions of Sweden, Norway, and Germany. Some amount of acid deposits are found in parts of South Asia, South Africa, Sri Lanka and Southern parts of India like Bangalore, New Delhi, Mumbai.


Types of Acid Rain
There are two types of depositions in which acid rain occurs. They can be discussed as follows:
- Wet deposition: When the acid falls on the ground in the form of rain, snow, fog or mist, it removes acid from the atmosphere and settles them on the Earth’s surface. When this acid flows through the ground, it affects a large number of plants, animals and aquatic life. The water from drain flows into the water sources like rivers and canals which is then mixed up into seawater; thereby affecting the aquatic habitats.
- Dry deposition: When the acidic pollutants merge into dust or smoke and fall to the ground as dry particles, these stick to the ground and other surfaces such as buildings, cars, houses, trees, and monuments. Majority of the acidic pollutants in the atmosphere spread through dry deposition.
Causes of Acid Rain
The major causes of acid rain are Natural and Human-Instigated causes. However, Acid Rain is basically caused due to the combustion of fossil fuels which results in emissions of sulphur dioxide (SO 2 ) and nitrogen oxide (NO 2 ) in the atmosphere.
Natural Sources: The main nature causing agents for acid rain are volcanic eruptions. Volcanoes emit a large amount of lava, producing harmful gases which create a higher than normal amount of Acid Rain. Decaying vegetation, wildfires and other biological processes within the environment also generate the Acid Rain forming gases. Lighting strikes also produce nitric oxides that react with water molecules via electrical activity to produce nitric acid, thereby forming acid rain.
Get the huge list of more than 500 Essay Topics and Ideas
Human-Instigated Sources: Human activities leading to the emissions of chemical gas include sulphur and nitrogen gases from the factories, power generating premises and automobiles. Mainly, the use of coal for electrical power generation is the biggest contributor to gaseous emissions. These also lead to acid rain. These gases react with water, oxygen, and other chemicals to form various acidic compounds such as sulphuric acid, nitric acid, etc. As a result, those areas experience exceedingly high amounts of acid rain.
Harmful effects of acid rain
Acid Rain adversely affects the environment includes marine biodiversity, soil, architecture & infrastructure, forests, and forest wildlife, public health. For example, Taj Mahal is turning yellow mainly due to air pollution, discoloration of marble due to oxidation of its constituents is one of the harmful effects of acid rain.
Methods to Avoid Acid Rain
Acid Rain caused due to the natural reasons cannot be stopped. But there are ways following which we can avoid the same, caused due to man-made reasons. The ways by which acid rain can be avoided are by the use of limestone by which people can repair the damages caused by acid rain to lakes, rivers , brooks, and other water sources. By adding lime into acidic surface also we can avoid acid rain as water balances the acidity.
Use of hybrid vehicles with negligible NO 2 emissions is also a way out. Besides fossil fuels, there is a wide range of substitutable energy sources that can generate electrical power these include wind energy, solar energy, geothermal energy, nuclear power, and hydro energy. Using these energy sources can offer effective electrical power alternatives. Instead of using fossil fuels, use of natural gas, fuel cells and batteries can also substitute use of fossil fuels.
As you can see, there are many attempts to clean our air. Due to increase in population and rapid industrialization, we need to make efforts on a war footing to reduce the phenomenon of Acid Rain. The whole world needs to take a call to avoid inflicting colossal damage to the environment.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App


An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
JavaScript appears to be disabled on this computer. Please click here to see any active alerts .
- What is Acid Rain?
Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic.
What Causes Acid Rain?
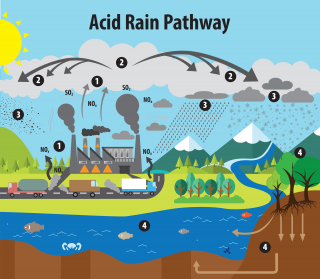
Acid rain results when sulfur dioxide (SO 2 ) and nitrogen oxides (NO X ) are emitted into the atmosphere and transported by wind and air currents. The SO 2 and NO X react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
While a small portion of the SO 2 and NO X that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. The major sources of SO 2 and NO X in the atmosphere are:
- Burning of fossil fuels to generate electricity. Two thirds of SO 2 and one fourth of NO X in the atmosphere come from electric power generators.
- Vehicles and heavy equipment.
- Manufacturing, oil refineries and other industries.
Winds can blow SO 2 and NO X over long distances and across borders making acid rain a problem for everyone and not just those who live close to these sources.
Forms of Acid Deposition
Wet deposition.
Wet deposition is what we most commonly think of as acid rain . The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with rain, snow, fog, or hail.
Dry Deposition
Acidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition . The acidic particles and gases may deposit to surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can be harmful to human health. When the accumulated acids are washed off a surface by the next rain, this acidic water flows over and through the ground, and can harm plants and wildlife, such as insects and fish.
The amount of acidity in the atmosphere that deposits to earth through dry deposition depends on the amount of rainfall an area receives. For example, in desert areas the ratio of dry to wet deposition is higher than an area that receives several inches of rain each year.
Measuring Acid Rain
Policymakers, research scientists, ecologists, and modelers rely on the National Atmospheric Deposition Program’s (NADP) National Trends Network (NTN) for measurements of wet deposition. The NADP/NTN collects acid rain at more than 250 monitoring sites throughout the US, Canada, Alaska, Hawaii and the US Virgin Islands. Unlike wet deposition, dry deposition is difficult and expensive to measure. Dry deposition estimates for nitrogen and sulfur pollutants are provided by the Clean Air Status and Trends Network (CASTNET). Air concentrations are measured by CASTNET at more than 90 locations.
When acid deposition is washed into lakes and streams, it can cause some to turn acidic. The Long-Term Monitoring (LTM) Network measures and monitors surface water chemistry at over 280 sites to provide valuable information on aquatic ecosystem health and how water bodies respond to changes in acid-causing emissions and acid deposition.
Next, learn about the Effects of Acid Rain .
Or, learn more about:
- Clean Air Markets
- Clean Air Status and Trends Network (CASTNET)
- National Atmospheric Deposition Program (NADP)
- Long-Term Monitoring (LTM) Network
- Acid Rain Home
- Effects of Acid Rain
- Acid Rain Program
- Acid Rain Program Results
Acid rain, explained
The fossil fuels that humans burn for energy can come back to haunt us as acid rain.
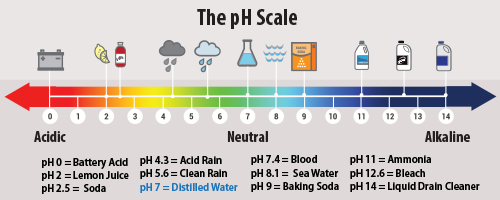
Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. It can also occur in the form of snow, fog, and tiny bits of dry material that settle to Earth. Normal rain is slightly acidic, with a pH of 5.6, while acid rain generally has a pH between 4.2 and 4.4 .
Causes of acid rain
Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a product of human activities. The biggest sources are coal-burning power plants , factories, and automobiles.
When humans burn fossil fuels , sulfur dioxide (SO 2 ) and nitrogen oxides (NO x ) are released into the atmosphere. Those air pollutants react with water, oxygen, and other substances to form airborne sulfuric and nitric acid. Winds may spread these acidic compounds through the atmosphere and over hundreds of miles . When acid rain reaches Earth, it flows across the surface in runoff water, enters water systems, and sinks into the soil.

A virtual tree graveyard of Norway spruce in Poland bears the scars of acid rain. Caused when rain droplets absorb air pollution like sulfur and nitrogen oxides, acid rain weakens trees by dissolving nutrients in the soil before plants can use them.
Effects of acid rain
Sulfur dioxide and nitrogen oxides are not primary greenhouse gases that contribute to global warming , one of the main effects of climate change ; in fact, sulfur dioxide has a cooling effect on the atmosphere. But nitrogen oxides contribute to the formation of ground-level ozone , a major pollutant that can be harmful to people. Both gases cause environmental and health concerns because they can spread easily via air pollution and acid rain.
Acid rain has many ecological effects, especially on lakes, streams, wetlands, and other aquatic environments. Acid rain makes such waters more acidic, which results in more aluminum absorption from soil, which is carried into lakes and streams. That combination makes waters toxic to aquatic animals. ( Learn more about the effects of water pollution .)
Some species can tolerate acidic waters better than others. However, in an interconnected ecosystem, what affects some species eventually affects many more throughout the food chain, including non-aquatic species such as birds .
Acid rain and fog also damage forests, especially those at higher elevations. The acid deposits rob the soil of essential nutrients such as calcium and cause aluminum to be released in the soil, which makes it hard for trees to take up water . Acids also harm tree leaves and needles.
The effects of acid rain, combined with other environmental stressors, leave trees and plants less healthy and more vulnerable to cold temperatures, insects, and disease. The pollutants may also inhibit trees' ability to reproduce. Some soils are better able to neutralize acids than others. But in areas where the soil's "buffering capacity" is low, such as parts of the U.S. Northeast, the harmful effects of acid rain are much greater.
Acid deposits damage physical structures such as limestone buildings and cars. And when it takes the form of inhalable fog, acid precipitation can cause health problems in people, including eye irritation and asthma.
What can be done?
The only way to fight acid rain is by curbing the release of the pollutants that cause it. This means burning fewer fossil fuels and setting air-quality standards.
In the U.S., the Clean Air Act of 1990 targeted acid rain, putting in place pollution limits that helped cut sulfur dioxide emissions 88 percent between 1990 and 2017. Air-quality standards have also driven U.S. emissions of nitrogen dioxide down 50 percent in the same time period. These trends have helped red spruce forests in New England and some fish populations , for example, recover from acid rain damage. But recovery takes time, and soils in the northeastern U.S. and eastern Canada have only recently shown signs of stabilizing nutrients .
Acid rain problems will persist as long as fossil fuel use does, and countries such as China that have relied heavily on coal for electricity and steel production are grappling with those effects. One study found that acid rain in China may have even contributed to a deadly 2009 landslide . China is implementing controls for sulfur dioxide emissions, which have fallen 75 percent since 2007 —but India's have increased by half.
For Hungry Minds
Related topics, you may also like.

Inside the Hidden World of Jaguars

This deadly fungus is hitchhiking its way across the world

Is a shoe-free home really better? Scientists may have an answer.

Are dust storms getting worse? Here’s why they’re so destructive.

Meet the marvelous creatures that bring soil to life
- Environment
- Perpetual Planet
History & Culture
- History & Culture
- History Magazine
- Mind, Body, Wonder
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved

Essay on Acid Rain
Students are often asked to write an essay on Acid Rain in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Acid Rain
Introduction.
Acid rain is a serious environmental issue. It’s rain containing harmful acids, created when pollutants like sulfur dioxide and nitrogen oxides mix with water in the atmosphere.
The main cause is the burning of fossil fuels like coal and oil, which release these pollutants. They rise into the atmosphere, combine with water, and fall as acid rain.
Acid rain damages ecosystems, including forests and lakes. It harms animals and plants, and can also damage buildings and monuments.
To combat acid rain, we must reduce our use of fossil fuels and promote cleaner energy sources like wind and solar power.
Also check:
- 10 Lines on Acid Rain
- Paragraph on Acid Rain
250 Words Essay on Acid Rain
Introduction to acid rain.
Acid rain is a significant environmental problem, characterized by the precipitation of harmful acidic substances from the atmosphere. It’s a consequence of industrialization, where the burning of fossil fuels releases sulfur dioxide (SO2) and nitrogen oxides (NOx) into the atmosphere. These gases react with water, oxygen, and other substances to form sulfuric and nitric acids.
Impact on the Environment
Acid rain has a profound impact on the environment. It can acidify soil and water bodies, leading to a loss of biodiversity. Acidic water can leach aluminum from soil clay particles, which can be toxic for many forms of aquatic life. Forests are also affected, as acid rain can strip essential nutrients from the soil, hindering tree growth.
Effects on Human Health
Acid rain also poses a risk to human health. It contributes to the formation of fine particulate matter, which can penetrate deep into the lungs, causing respiratory illnesses. Furthermore, acidified waters can lead to an increase in toxic metals in drinking water, posing additional health risks.
Addressing the Issue
Efforts to mitigate acid rain have included regulation of emissions, promotion of clean energy, and the use of limestone to neutralize acidity in lakes and rivers. However, the problem persists, calling for more robust, international cooperation to reduce emissions and promote sustainable practices.
In conclusion, acid rain is a complex issue with far-reaching implications for the environment and human health. Addressing it requires a comprehensive, multi-faceted approach that goes beyond national boundaries and involves global cooperation.
500 Words Essay on Acid Rain
Acid rain, a significant environmental issue, is a form of precipitation with elevated levels of hydrogen ions, thus having a low pH. It is primarily a result of human activities, particularly the burning of fossil fuels that release sulphur dioxide (SO2) and nitrogen oxides (NOx) into the atmosphere. These gases react with water molecules in the atmosphere to form sulphuric and nitric acids, which then fall to the ground as acid rain.
Causes of Acid Rain
The primary causes of acid rain are anthropogenic, although natural sources also contribute. The burning of coal and oil in power stations and residential homes releases large quantities of SO2 into the atmosphere. Similarly, NOx emissions mainly originate from vehicle exhausts and industrial processes. Volcanic eruptions and rotting vegetation also release these gases but to a lesser extent.
Effects of Acid Rain
Acid rain has numerous detrimental effects on the environment and human health. It can damage forests by acidifying the soil and inhibiting the growth of trees and other vegetation. Acid rain also acidifies bodies of water, harming aquatic life by disrupting their reproductive cycles and causing significant biodiversity loss.
From a human perspective, acid rain can corrode buildings, monuments, and statues, especially those made of limestone and marble, which contain large amounts of calcium carbonate. Human health is also affected as SO2 and NOx can cause respiratory problems and other health issues when inhaled.
Acid Rain and Climate Change
The relationship between acid rain and climate change is complex. While acid rain itself does not directly contribute to global warming, the same activities that produce the pollutants causing acid rain also emit greenhouse gases. Therefore, efforts to mitigate acid rain could also have a positive impact on reducing the effects of climate change.
Management and Mitigation
Several strategies can mitigate the effects of acid rain. These include reducing SO2 and NOx emissions by transitioning to cleaner energy sources, improving energy efficiency, and implementing emission control technologies. Legislative measures, such as the Clean Air Act in the U.S., have also been effective in reducing emissions.
In conclusion, acid rain is a serious environmental issue caused primarily by human activities, with far-reaching effects on ecosystems and human health. While it presents significant challenges, through concerted efforts in emission reduction and legislative control, it is a problem that can be effectively managed. Understanding and addressing the issue of acid rain is not just about preserving the environment; it is also about ensuring the health and well-being of future generations.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on My Favorite Season Monsoon
- Essay on Rainy Day
- Essay on Effects of Heavy Rainfall
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Environmental Chemistry
Acid Rain - Causes of Acid Rain
What is acid rain.
Acid Rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When atmospheric pollutants like oxides of nitrogen and sulphur react with rainwater and come down with the rain, then this results in Acid Rain.
Table of contents
- Definition Of Acid Rain
Recommended Videos Of Acid Rain
- Causes Of Acid Rain
- Effect Of Acid Rain
- Real-Life Examples Acid Rain
- Prevention Of Acid Rain
Acid Rain Definition
Acid rain is made up of highly acidic water droplets due to air emissions, most specifically the disproportionate levels of sulphur and nitrogen emitted by vehicles and manufacturing processes. It is often called acid rain as this concept contains many types of acidic precipitation.
The acidic deposition takes place in two ways: wet and dry. Wet deposition is any form of precipitation which removes acids from the atmosphere and places them on the surface of the earth. In the absence of precipitation, dry deposition of polluting particles and gases sticks to the ground through dust and smoke.

Causes of Acid Rain
The causes of acid rain are Sulphur and Nitrogen particles which get mixed with the wet components of rain . Sulphur and Nitrogen particles which get mixed with water are found in two ways either man-made i.e as the emissions that are given out from industries or by natural causes like lightning strike in the atmosphere releasing nitrogen oxides and volcanic eruptions releasing sulphur oxide.
According to the Royal Society of Chemistry, which considers him the “father of acid rain,” the word acid rain was invented in 1852 by Scottish chemist Robert Angus Smith. Smith decided on the word while studying rainwater chemistry near industrial towns in England and Scotland.
The regular clean rain we experience, even though it is not clean i.e water and carbon dioxide react together to form weak carbonic acid which essentially by itself is not extremely harmful. The reaction occurring is :
H 2 O (l) + CO 2 (g) ⇌ H 2 CO 3 (aq)
The pH value of regular rainwater is around 5.7, giving it an acidic nature. The oxides of nitrogen and sulphur are blown away by the wind along with the dust particles. They settle on the earth’s surface after coming down in the form of precipitation. Acid rain is essentially a by-product of human activities which emit oxides of nitrogen and sulphur in the atmosphere. Example – the burning of fossil fuels, unethical waste emission disposal techniques.
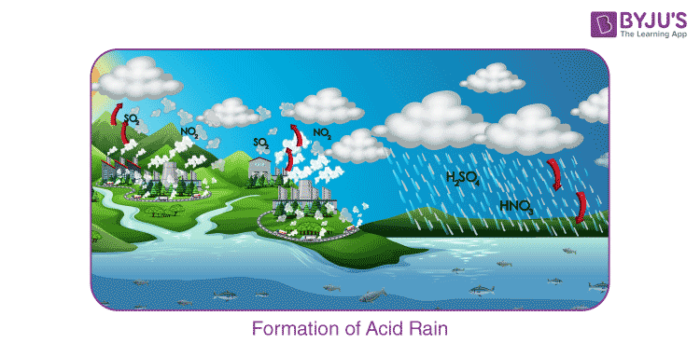
Sulphur dioxide and nitrogen dioxide undergo oxidation, and then they react with water resulting in the formation of sulphuric acid and nitric acid, respectively. The following reaction will clarify the acid formation reaction:
2SO 2 (g) + O 2 (g) + 2H 2 O (l) → 2H 2 SO 4 (aq) 4NO 2 (g) + O 2 (g) + 2H 2 O (l) → 4HNO 3 (aq)
Effects of Acid Rain
- Acid rain is very harmful to agriculture, plants, and animals. It washes away all nutrients which are required for the growth and survival of plants. Acid rain affects agriculture by the way it alters the composition of the soil.

- It causes respiratory issues in animals and humans.
- When acid rain falls down and flows into the rivers and ponds it affects the aquatic ecosystem. It alters the chemical composition of the water, to a form which is actually harmful to the aquatic ecosystem to survive and causes water pollution.
- Acid rain also causes the corrosion of water pipes, which further results in leaching of heavy metals such as iron, lead and copper into drinking water.
- It damages the buildings and monuments made up of stones and metals.

Real-Life Examples
- Taj Mahal , one of the 7 wonders of the world, is largely affected by acid rain. The city of Agra has many industries which emit the oxides of sulphur and nitrogen in the atmosphere. People continue to use low-quality coal and firewood as a domestic fuel, adding to this problem. Acid rain has the following reaction with the marble ( calcium carbonate ):
CaCO 3 (s) + H 2 SO 4 (l) → CaSO 4 (s) + H 2 O(l) + CO 2 (g)

The formation of calcium sulphate results in the corrosion of this beautiful monument.
- Statue of Liberty which is made of copper has also been damaged by the cumulative action of acid rain and oxidation for over 30 years and is, therefore, becoming green.

Prevention of Acid Rain
- The only precaution that we can take against acid rain is having a check at the emission of oxides of nitrogen and sulphur.
- Acid rain is harmful to animals, plants and the monuments.
- Being responsible citizens, one should be aware of the harmful effects they cause and of the industries which give out nitrogen and sulphur compound wastes unethically.
Frequently Asked Questions – FAQs
What is acid rain and how is it caused.
Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air. These substances can rise very high up into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants called acid rain.
What are the effects of acid rain?
The ecological consequences of acid rain are seen most strongly in marine habitats, such as streams, lakes and marshes where fish and other wildlife can be toxic. Acidic rainwater can leach aluminium from soil clay particles as it flows through the soil and then floods into streams and lakes.
What will happen if we don’t stop acid rain?
Sulphur dioxide and nitrogen oxide are the principal chemicals for acid rain. It can also influence humans since the acid goes into fruits, vegetables and animals. In other words, we can get really sick if acid rain doesn’t stop, and we eat those things. In general, acid rain affects men, but not directly.
What is acid rain? What are its harmful effects?
It has been shown that acid rain has detrimental effects on trees, freshwaters and soils, destroys insects and aquatic life-forms, causes paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and sculptures, as well as impacts on human health.
What are three ways to reduce acid rain?
Alternative energy sources should be used, such as solar and wind power. Renewable sources of energy are helping to reduce acid rain, as they produce much fewer emissions. There are other electricity sources as well, such as nuclear power, hydropower, and geothermal energy. Among these, the most extensive use is among nuclear and hydropower.
How does acid rain affect plants?
What is acid rain made of, what is the primary source of acid rain, can acid rain damage buildings, can acid rain burn your skin.
- Structure & Properties of Ozone
- Acids and Bases
- Global warming due to the greenhouse effect

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Nice informations
Really like the information tq
These notes are very helpful in my exams i scored 79/80 in science
these are very useful
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Talk to our experts
1800-120-456-456
- Essay on Acid Rain

Acid rain, or acid deposition, is a broad term that includes any form of acid component precipitation, with a pH of 5.2 or below, such as sulfuric or nitric acid, which, in wet or dry forms, falls from the atmosphere to the ground. This includes acidic rain, snow, fog, hail etc. These components are mainly produced due to human activities resulting in the emission of sulphur dioxide and Nitrogen oxides. In this essay, we shall discuss the causes and consequences of Acid rain.
Long and Short Acid Rain Essays in English for Students and Children
Acid rain is composed of extremely acidic water droplets that form as a result of air pollutants, notably the excessive quantities of sulphur and nitrogen produced by cars and manufacturing operations. Because this idea encompasses a wide range of acidic precipitation, it is commonly referred to as acid rain.
There are two primary types of acidic deposition- wet and dry. Wet deposition basically means the precipitation that occurs due to acids from the atmosphere and their deposits on the earth's surface. Dry deposition of harmful particles and gases refers to the deposition on the earth in the absence of precipitation via dust and smoke.
Acid rain is caused by Sulphur and Nitrogen particles that interact with moist components of rain. Sulphur and nitrogen particles undergo combination with the water primarily in two ways: man-made (emissions from industries) or natural (e.g., a lightning strike in the sky releases nitrogen ions and volcanic eruptions release sulphur).
Real-Life Examples
The Taj Mahal, one of the world's seven wonders, is severely damaged by acid rain. Agra has various factories that generate sulphur and nitrogen oxides into the environment. The deterioration of this wonderful monument is caused by the production of calcium sulphate.
The copper Statue of Liberty has also shown damages due to the continuous impact of acid rain and oxidation for over 30 years and is thus becoming green.
Acid rain causes severe damage to crops, vegetation, and animals. It wipes out the nutrients that plants require for growth and life. Acid precipitation has an impact on agriculture since it changes the soil's makeup. Because it changes the chemical makeup of the water, it is hazardous to the survival of the marine ecology and produces pollution. Acid precipitation also causes corrosion of water pipelines, which leads to the leaching of heavy metals such as iron, lead, and copper into the beverage. It causes harm to structures and monuments constructed of stone and metal.
The only preventative measure that can be taken up is the reduction of nitrogen and sulphur oxide emissions.
Short Essay on Acid Rain
Acid rain is damaging to animals, vegetation, and historic structures.
As responsible citizens, we take measures to spread awareness and counter the adverse impacts they produce, as well as the companies that are responsible for the unethical disposal of nitrogen and sulphur compound pollutants.
Acid rain has the greatest biological impact on coastal ecosystems, such as streams, lakes, and marshes, where fish and other species can be hazardous. As acidic rainfall runs through the soil and spills into streams and lakes, it can drain aluminium from soil clay particles.
Acid Rain is made up of extremely acidic water droplets that form as a result of air pollution, notably the excessive quantities of sulphur and nitrogen produced by cars and manufacturing operations. This notion is sometimes referred to as acid rain since it encompasses a wide range of acidic precipitation. Normal rainfall has a pH of roughly 5.7 indicating that it is acidic. Acid rain is a natural result of human activity.

FAQs on Essay on Acid Rain
1. Can acid rain directly affect people?
Humans are harmed when they breathe in polluted air, which may lead to lung difficulties and even cancer. Drinking water tainted by acid rain can cause brain damage over time. Apart from these, acid rain can have adverse effects on crop production and this, in turn, will have detrimental effects on the human body in the long run. Sulphur dioxide and oxide are the primary compounds involved in acid precipitation. In other words, if acid precipitation does not cease and we eat certain products, we will become unwell. In general, acid precipitation has an indirect impact on humans. Hence it is of utmost concern that the issue of acid rain is dealt with as soon as possible and with stringent countermeasures.
2. What are the disadvantages of acid rain?
The downsides of acid: Acid may cause skin irritation and serious burns.
The acid can cause eye discomfort and even blindness.
Acidity may be caused by an increase in hydrochloric acid output in the stomach.
The acid may cause clothing and materials to burn.
Apart from these, there are many other significant adverse effects of acid rain and hence stringent measures are the need of this hour to counter this problem of acid rain.
3. What is currently being done to counter acid rain?
The steps that are being taken to combat acid rain-
Alternative energy sources Stopping the use of nonrenewable fuels and switching to renewable energy sources such as solar, wind, and water energy is a good strategy to reduce acid rain. This alternative energy will become more available to the general people as technology advances.
The two methods undertook by the government at attempting to control acid rain.
Allowances and the selling of allowances Emissions monitoring and Continuous Emissions Monitoring systems Data on emissions and allowances were gathered.
4. Can acid rain damage buildings?
Acid rain can damage buildings, monuments, and statues with large amounts of carbonate, particularly those made from rocks, like limestone and marble. Acids react with the calcium compounds in the stones in the rain to form gypsum, which then flakes off. On old gravestones, acid rain can cause the inscriptions to become completely illegible. The corrosion rate of metals, especially iron, steel, copper, and bronze, is also increased by acid rain.
5. Is acid rain still an issue?
The phenomenon called acid rain was a well-known environmental issue in Europe and North America during the 1970s and '80s, appearing frequently in news features. Since that time, stories about climate change, global warming, biodiversity issues, and other environmental concerns have supplanted the visibility of acid rain in the media. Acid rain still occurs, but because of strong air pollution regulations in those regions, its impact on Europe and North America is far less than it was in the 1970s and '80s.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.49(4); 2020 Apr

Acid rain and air pollution: 50 years of progress in environmental science and policy
Peringe grennfelt.
1 IVL Swedish Environmental Research Institute, PO Box 53021, 40014 Gothenburg, Sweden

Anna Engleryd
2 Swedish Environmental Protection Agency, Virkesvägen 2F, 10648 Stockholm, Sweden
Martin Forsius
3 Finnish Environment Institute, Latokartanonkaari 11, 00790 Helsinki, Finland
Øystein Hov
4 The Norwegian Meteorological Institute, P.O. Box 43, Blindern, 0313 Oslo, Norway
Henning Rodhe
5 Department of Meteorology, Stockholm University, 10691 Stockholm, Sweden
Ellis Cowling
6 Department of Forestry and Environmental Resources, NC State University, 5211 Glenhope Court, Cary, NC 27511 USA
Because of its serious large-scale effects on ecosystems and its transboundary nature, acid rain received for a few decades at the end of the last century wide scientific and public interest, leading to coordinated policy actions in Europe and North America. Through these actions, in particular those under the UNECE Convention on Long-range Transboundary Air Pollution, air emissions were substantially reduced, and ecosystem impacts decreased. Widespread scientific research, long-term monitoring, and integrated assessment modelling formed the basis for the policy agreements. In this paper, which is based on an international symposium organised to commemorate 50 years of successful integration of air pollution research and policy, we briefly describe the scientific findings that provided the foundation for the policy development. We also discuss important characteristics of the science–policy interactions, such as the critical loads concept and the large-scale ecosystem field studies. Finally, acid rain and air pollution are set in the context of future societal developments and needs, e.g. the UN’s Sustainable Development Goals. We also highlight the need to maintain and develop supporting scientific infrastructures.
Introduction
Acid rain was one of the most important environmental issues during the last decades of the twentieth century. It became a game changer both scientifically and policy-wise. For some time, particularly during the 1980s, acid rain was by many considered to be one of the largest environmental threats of the time. Observations of fish extinction in Scandinavian surface waters and forest dieback on the European Continent were top stories in the news media. Even in North America acid rain received large public and policy attention.
During the cold war, with almost no contacts between East and West, acid rain broke the ice and formed an opening for scientific and political collaboration, resulting in a treaty under the United Nations’ Economic Commission for Europe (UNECE), the Convention on Long-range Transboundary Air Pollution (often mentioned as CLRTAP but in this paper we call it the Air Convention) signed in 1979. Eight protocols have been signed under the Air Convention committing parties to take far-reaching actions, not only with respect to acid rain but also with respect to several other air pollution problems (Table 1 ). Emissions of all key air pollutants have been reduced significantly and for the most important acidifying compound, sulphur dioxide, emissions in Europe have decreased by 80% or more since the peaks around 1980–1990 (Fig. 1 ).
Table 1
The Convention on Long-Range Transboundary Air Pollution and Its Protocols

European emissions of sulphur dioxide (SO 2 —black), nitrogen oxides (NO x , calculated as NO 2 —green) and ammonia (NH 3 —blue) 1880–2020 (updated from Fig. 2 in Schöpp et al. 2003 )
In this paper, we present and discuss how the acid rain problem became a key environmental issue among industrial countries from the late 1960s and the following decades (Fig. 2 ). We view the problem from a science-to-policy interaction perspective, based on a Symposium in Stockholm in the autumn 2017 organised to manifest 50 years of international air pollution science and policy development. The Symposium involved both a testimony from a number of those involved in science and policy during the first decades of the history but also a discussion of what we have learned and how the experience can be used in the future. Further information about the symposium and its outcome can be found at http://acidrain50years.ivl.se .

The timeline of science and policy interactions in Europe and North America 1967–2018. (updated from Driscoll et al. 2012). Abbreviations not occurring in text. NAAQS: National Ambient Air Quality Standards under the US Clean Air Act; CCAA: Canadian Clean Air Act; RADM: Regional Atmospheric Deposition Model; MAGIC Model of Acidification of Groundwater in Catchments. It should be mentioned that Canada and US are both parties to the Air Convention and they have also signed and ratified most of its protocols
Our historical review will be limited to some of the issues brought up at the Symposium. For more information on the early history see Cowling ( 1982 ). A comprehensive description of the acid rain history has recently been published by Rothschild ( 2018 ). The history of the first 30 years of the science–policy interactions under the Air Convention is also described in Sliggers and Kakebeeke ( 2004 ).
Short historical review
The discovery and the early acid rain history.
In a deliberatively provocative article in the Swedish newspaper Dagens Nyheter in October 1967, entitled “An Insidious Chemical Warfare Among the Nations of Europe”, the Swedish scientist Svante Odén (Fig. 3 ) described a new and threatening environmental problem—Acid Rain. He pointed to the significant decrease in pH of rainwater and surface waters that had occurred over the previous decade and linked it to the large and increasing emissions of sulphur dioxide in Europe.

Svante Odén around 1970 (photo Ellis B. Cowling)
The discovery received immediate attention by the Swedish government and, a few weeks after Odén’s article, the minister of industry presented the issue at the Organisation for Economic Cooperation and Development (OECD), but it did not receive any political attention at that time. The issue was also brought up in OECD’s Air Pollution Management Committee by the Swedish delegate Göran Persson. Also, here the message was met by scepticism and the common opinion among the members in the committee was that sulphur dioxide was a local problem, which easily could be solved by tall stacks. It was not until Persson felt he was going to “loose the case” he “played his last card” and pointed to the observations of intercontinental transport of radioactivity from the Chinese nuclear bomb experiments. The opinion then changed and the meeting agreed that acid rain might be an issue to look into. From now on, OECD and the western world realised that air pollution might be a problem of international political dimensions.
Odén’s discoveries were to a large extent based on the regional precipitation networks that were running in Sweden and Europe. In 1947, the Swedish scientist Hans Egnér set up a Swedish network to investigate the importance of atmospheric deposition for the fertilisation of crops. In 1954, the network was expanded forming the European Air Chemistry Network (EACN) through initiatives by Egnér, Carl Gustav Rossby, and Erik Eriksson (Egnér and Eriksson 1955 ; see also Engardt et al. 2017 ). Data from these networks together with a Scandinavian surface water network set up by Odén in 1961 formed the basis for Odén’s observations on the ongoing acidification (Odén 1968 ).
Acid rain and many of its ecological effects were, however, recognised long before 1967–1968. In fact, many features of the acid rain phenomenon were first discovered by an English chemist, Robert Angus Smith, in the middle of the nineteenth century! In 1852, Smith published a detailed report on the chemistry of rain in and around the city of Manchester, England. Twenty years later, in a very detailed book titled “Air and Rain: The Beginnings of a Chemical Climatology”, Smith first used the term “acid rain” and enunciated many of the principal ideas that are part of our present understanding of this phenomenon (Smith 1872 ). Unfortunately, however, Smith’s pioneering book was substantially ignored by nearly every subsequent investigator.
In Norway salmon catches decreased substantially in the early 1900s and in 1927, Professor Knut Dahl hypothesised that acidification of surface waters could be a factor of importance for the extinction of fish. Later Alf Dannevig assumed that “The acidity of a lake is dependent on the acidity of the rainwater and the contributions from the soil” (Dannevig 1959 ).
Based on detailed field observations and experimental studies both in England and in Canada, beginning in 1955 and continuing through 1963, Eville Gorham and his colleagues built a significant foundation for contemporary understanding of the causes of acid precipitation and its impacts on aquatic ecosystems, agricultural crops, soils, and even human health (Gorham 1981 ; Cowling 1982 ). Thus, Gorham and his colleagues as well as Dahl and Dannevig had discovered major aspects of the causes of contemporary changes in the chemistry of atmospheric emissions and deposition and their effects on aquatic ecosystems.
But these pioneering contributions, like those of Smith a century earlier, were not generally recognised—neither by scientists nor by society in general. Gorham’s researches, like those of Smith a century before, were met by what Gorham himself acknowledged as a “thundering silence”, not only by the scientific community, but also by the public at large.
It was not until 1967 and 1968 when Svante Oden published both his deliberatively provocative article in Dagens Nyheter and his carefully documented Ecological Committee Report (Odén 1968 ) that the acid rain problem was brought to both public and scientific considerations. The report included a huge body of scientific and policy-relevant evidence that long-distance transport and deposition of acidifying pollutants were causing significant environmental and ecological impacts, even in countries far away from pollutant-emitting source areas in other countries.
The Swedish case study and the OECD project
Two years after Odén’s article, the Swedish government decided to prepare a “case study” as a contribution to the UN Conference on the Human–Environment in Stockholm 1972 (Royal Ministry of Foreign Affairs and Royal Ministry of Agriculture 1972 ). Bert Bolin at the Stockholm University was appointed chair of the study, which included Svante Odén, Henning Rodhe, and Lennart Granat as authors. The report included a broad environmental assessment of the sulphur emission problem including sources, atmospheric and surface water chemistry, and effects on ecosystems and materials. Finally, it also included scenarios and estimated costs for environmental damage and control; in fact it was probably the first full systems analysis of an environmental problem.
In the report, a first estimate was made of the relative contributions of domestic and foreign emissions to the sulphur deposition in Sweden (Rodhe 1972 ). Estimates were also made of the effects of sulphur emissions on excess mortality and showed that 50% of the Swedish lakes and rivers would reach a critical pH level within 50 years (assuming continuation of present emission trends). Even if some aspects of the report received criticism, the overall case study was well received by the UN conference and in its final report (see http://www.un-documents.net/aconf48-14r1.pdf ) regional air pollution was explicitly mentioned (§85) with a citation of the Swedish study.
The Swedish initiative in the OECD resulted in a collaborative project to investigate the nature and magnitude of the transboundary transport of emitted sulphur dioxide over Western Europe, in which 11 countries participated. To initiate the project, a Nordic organisation on scientific research, Nordforsk, was asked to plan and develop methodologies for the investigation. Scientists and institutions from Norway, Sweden, Denmark, and Finland established an expert group in April 1970, which became central for the development and implementation of the OECD project. The Norwegian Institute for Air Research (NILU) offered through its director Brynulf Ottar to coordinate the project. The project included emission inventories, measurements of atmospheric concentrations, and deposition, together with model development and application for the assessment of the transport. A key part of the model calculations was to prepare the so-called “blame matrices”, through which the transport of pollutants between countries could be quantified.
The main conclusion from the OECD project, published in 1977, was that “Sulphur compounds do travel long distances in the atmosphere and the air quality in any European country is measurably affected by emissions from other European countries” (OECD 1977 ). Even if there still were hesitations about the magnitude of the transport, the common opinion was that transboundary transport of air pollution is an issue that needs collaboration across national borders. These conclusions paved the road for a pan-European scientific collaboration on air pollution, the European Monitoring and Evaluation Programme (EMEP) starting in 1977. The findings from the project also formed the basis for the Air Convention (Table 1 ). EMEP was already from the beginning included in the Convention as a key element, strongly contributing to the scientific credibility of the policy work.
Threats to forests boosted the interest
In 1980, the German scientist Bernhard Ulrich warned that European forests were seriously threatened from atmospheric deposition of sulphur. From his long-term experiments in the Solling area, he concluded that the high deposition of atmospheric pollutants had seriously changed the soil chemistry (Ulrich et al. 1980 ). Ulrich pointed to the links between sulphur deposition and the release of inorganic aluminium. His findings became a policy issue not only in Germany but in Europe as a whole, and even in North America. The alarms—often exaggerated—went like a wildfire through media and changed many attitudes throughout Europe. Newspapers were filled with photos of dying forests, in particular from “The Black Triangle”, the border areas between Poland, East Germany, and Czechoslovakia, characterised by large combustion of brown coal with high sulphur content. Forest inventories showed crown thinning and other effects on forests, but it became difficult to finally determine that acid deposition was the (only) cause for the observed effects.
The increasing interest in regional air pollution also paved the way for the first international agreement on emission control under the Air Convention. As a start, countries with a large interest in taking actions formed a “club” under the Convention, aiming for a 30% reduction in emissions. This ambition then became the basis for the first emission reduction protocol, the Sulphur Protocol signed in 1985. While Germany and some other West European countries acted almost immediately on the alarms, the progress in emission control in Eastern Europe was very slow during the 1980s, even though several of these countries signed the protocol. In fact, substantial decrease in emissions did not take place in the East until after the break-down of the communist regimes and the industrial collapse around 1990.
Critical loads and advanced policies
One of the most well-known characteristics for the control of the acid rain problem is the concept of Critical Loads (Nilsson 1986 ; Nilsson and Grennfelt 1988 ). The Executive Body, the highest decision-making body of the Air Convention, decided in 1988 that new negotiations on the control of sulphur and nitrogen emissions should be based on critical loads, and all parties to the Convention were requested to prepare their own critical load maps. The Netherlands offered to take a lead and prepared mapping manuals and initiated an international network, which became crucial for the scientific and policy acceptance of the concept (Hettelingh et al. 1991 ; De Vries et al. 2015 ; Fig. 4 ). (The critical loads concept is further discussed later in the paper)

The outcome of emission control of SO2, NOx, and NH3 between 1990 and 2010 presented as maps on exceedance of critical loads of acidity. Such maps have played an important role for illustrating outcomes of future policies as well as of actions taken (from Maas and Grennfelt 2016 )
When critical loads became a basis for further protocols, Integrated Assessment Models (IAMs) offered a method to calculate how to achieve a prescribed ecosystem effect reduction in the most cost-effective way. A couple of different approaches were developed, but the model at the International Institute for Applied Systems Analysis (IIASA) became the official model on which the Second Sulphur Protocol signed in 1994 was agreed (Hordijk 1995 ).
When revising or developing a new protocol for nitrogen oxides the concept could, however, not be used in the same way as for sulphur and acid deposition, since the NO x emissions contributed to several effects and, in addition, a strategy would need to take additional compounds into account. Instead, a more advanced approach was suggested by which both several effects and several compounds could be considered simultaneously (Grennfelt et al. 1994 , Fig. 5 ). IIASA and other bodies under the Air Convention were asked to develop an integrated assessment model that fitted into a broader approach and a more comprehensive model was developed, which made it possible to simultaneously take into account the effects of acidic deposition, nitrogen deposition, and ozone—the so-called multi-pollutant, multi-effect approach. The calculations became the basis for the Gothenburg Protocol (GP) that was signed in 1999 (Amann et al. 1999 ). The GP and the parallel EU National Emissions Ceilings (NEC) Directive from 2001 outlined control measures for 2010 and beyond.

Links between sources and effects used as an illustration in the preparation of the Gothenburg Protocol. From Grennfelt et al. 1994
After 2000—Health effects and integration with other policies became main drivers
The basis for the GP was almost entirely ecosystem effects. Around 2000, however, public health effects from air pollution became increasingly important. Large epidemiological studies indicated that air pollution was a significant source of premature deaths and that particles were a main cause of the health effects (WHO 2018 ). When the European Commission started its work to revise the NEC directive, health effects became central and the Air Convention followed. Further studies have supported the role of air pollution for health effects and when the GP was finally revised in 2012, health effects dominated as a policy driver for the establishment of national emission ceilings, and for the first time particulate matter was included in an international protocol (Reis et al. 2012 ).
When considering further actions after signing the GP in 1999, it was realised that for some pollutants under the Air Convention, emission control needed to be considered over larger geographic scales than Europe and North America alone. Ozone was of particular importance, since long-term objectives in the form of critical levels and public health standards could not be reached without taking into account sources outside the areas considered so far. Future policies therefore needed to include the ozone precursors methane and to some extent carbon monoxide. A task force on Hemispheric Transport of Air Pollution (HTAP) was set up under the Convention in 2004, with a primary objective to quantify the intercontinental transport of pollutants. The outcome of its work clearly showed the importance of considering air pollution in a wider geographic perspective than had been done so far (Dentener et al. 2010 ).
Climate change has for more than a decade become an issue of increasing interest for air pollution science and policy. In many cases, the emission sources are the same and there are obvious co-benefits (and some trade-offs) in handling them together. One aspect that has received large interest is the option to decrease short-term temperature increase through control measures directed towards atmospheric pollutants that also contribute to the warming of the atmosphere, in particular black carbon and methane (for methane both by itself but also as a tropospheric ozone precursor) (Ramanathan et al. 2001 ). Compounds contributing to both air pollution effects and to the radiation balance in the atmosphere have been named Short Lived Climate Pollutants (SLCPs). SLCPs thus also include compounds that are cooling the atmosphere, i.e. small secondary aerosols, e.g. sulphate particles. Recent research has focused on a better understanding of these compounds’ contribution to both air pollution and climate as well as on opportunities for selective control of these compounds (e.g. Sand et al. 2016 ).
Reactive nitrogen species are another group of compounds that has received increased attention after the turn of the century. Around 2006 several initiatives were taken in Europe, including a special task force on Reactive Nitrogen under the Air Convention, a large-scale EU project on nitrogen, and the preparation of a European Nitrogen Assessment (Sutton et al. 2011 ). Here nitrogen was considered both as a traditional atmospheric pollutant and within a societal and industrial context. A cascade perspective, where one fixed nitrogen molecule could contribute to a series of effects before it returns to molecular nitrogen again, was introduced (Galloway et al. 2003 ). The studies have pointed to the importance of the agricultural sector for the intensification of reactive nitrogen cycling, determined by food production mechanisms and dietary choices.
North America
In North America, the acid rain problem developed to a large extent in parallel with the situation in Europe. Lake acidification became already from the beginning a main driver, and monitoring programmes were set up both in the United States and Canada (Driscoll et al. 2010 ). The US National Atmospheric Deposition programme (NADP) started in 1976 and is still running. Both countries have taken part in the Air Convention activities and have signed most of the protocols and achieved decreases in SO 2 emissions of the order of 80% between 1980 and 2015. The US has however taken a different approach with respect to policy in comparison to Europe. Instead of developing a strategy based on integrated assessment modelling, it was decided to establish an emissions trading programme for the large electric generation sources under the Clean Air Act (See also UNECE 2016 ).
Characteristics of the science–policy interactions
In this section we will, from a science–policy perspective, briefly discuss some characteristics of the history of acid rain and transboundary air pollution that have become central for the international collaboration, not only on air pollution but also for international environmental collaboration in general. We will bring up monitoring, modelling, and data collection (including field experiments and long-term studies carried out in order to understand and quantify effects to ecosystems), development of bridging concepts that have served the implementation of strategies, and finally the dynamics in the science–policy interactions.
Monitoring, modelling, and data collection
Monitoring of atmospheric concentrations, deposition, and ecosystem effects has been a key for understanding the causes, impact, and trends in acid rain, both in Europe and North America and later in other geographic areas (Table 2 ). The original EMEP network has since the start over 40 years ago formed a broad atmospheric monitoring system. The originally established simple monitoring stations have over time been complemented with more advanced monitoring, and some stations are today advanced atmospheric chemistry platforms with continuous collection of a multitude of atmospheric parameters (Fig. 6 ). The EMEP database is nowadays widely used for a variety of scientific purposes including computation of long-term trends, exposure estimates, and as a basis for modelling. EMEP has also become a model for monitoring networks related to other geographical regions, conventions, and purposes. One example is the acid deposition monitoring network in East Asia (EANET). It is obvious that having a qualified centre for data collection and storage, standardisation, and intercalibration of methods has served the international policy system extremely well. Its open nature is part of the success. The financial support to EMEP, regulated through a separate protocol, has been fundamental for the development and progress of the monitoring activities.
Table 2
Long-term monitoring activities in relation to acid rain and other pollutants

Atmospheric monitoring stations have been of importance for understanding the long-range transport and chemical conversions of atmospheric pollutants. Pallas air pollution background station in Northern Finland (Photo Martin Forsius)
Monitoring of air pollution effects in a systematic way under the Air Convention started a few years later than EMEP and was organised through so-called International Cooperative Programmes (ICPs). Separate programmes were set up for forests, waters, vegetation (primarily ozone), materials, and integrated monitoring. A separate ICP was set up for developing critical load methodologies and coordinating European-scale mapping activities (ICP Modelling and Mapping). The ICPs are of great importance for general understanding of the magnitude and geographical distribution of the effects and for showing how decreases in emissions have led to beneficial conditions in ecosystems and decreased material corrosion (Maas and Grennfelt 2016 ). Ecosystem monitoring is also important for the development and verification of ecosystem models. Since their start, the responsibility for the ICPs has been taken by different parties of the Air Convention (Table 2 ). The distributed responsibility has been of large importance for the establishment of networks of monitoring sites among the Convention parties, but the system has not had a stable financial support in the same way as for EMEP. This has resulted in the lack of a common source for easily accessible data or adequate resources for standardisation and intercalibration.
Monitoring and other data collection (i.e. emissions and critical loads) under the Air Convention are responsibilities of every country, and data are then used for the assessments on the Convention level as well as for the development of EU air pollution policies. The bottom-up process in data collection is important for the development of national expertise and, not the least, for the establishment of national policies. In this way, direct communication links between the science and the policy levels within countries have evolved.
Numerical modelling of atmospheric pollution is also a long-term commitment under EMEP. The atmospheric chemistry models are necessary for the understanding of the nature of transboundary transport but also to make budget estimates of the exchange of pollutants over Europe and North America, and later on a hemispheric scale. The Meteorological Synthesizing Centre West at the Norwegian Meteorological Institute together with the Eastern Centre in Moscow took the lead in this work. In addition to calculating transboundary fluxes, the centres are important for coordinating modelling efforts done by other groups, forming a basis for scrutinising models and support further modelling.
Field experiments and long-term studies—a way to understand processes and trends, and to visualise the problems
Some of the most important and reliable findings regarding acid rain and its effects on ecosystems emanate from long-term field experiments. These experiments, which are known from the sites where they are run, include Hubbard Brook (US), Solling (Germany), Risdalsheia (Norway) and Lake Gårdsjön (Sweden) (Fig. 7 ). The studies there have shown how acid deposition and the impact of other air pollutants have changed the ecosystems, but also how ecosystems respond to decreased emissions (e.g. Wright et al. 1988 ; Likens et al. 1996 ). A central feature in all these field experiments was the establishment of ion budgets, from which the chemical effects on acid deposition can be analysed and understood (Reuss et al. 1987 ).

Field experiments have played an important role for the overall understanding of the interactions between atmospheric deposition and ecosystem effects. The photo illustrates the covered catchment experiment to study the recovery of ecosystems at reduced emissions in Risdalsheia Norway (Photo NIVA)
In the intense research period during the 1970s and 1980s, a number of large-scale research programmes and experiments of temporary nature were set up, some of them in connection with the above-mentioned sites. The first research programme of some magnitude was the Norwegian programme “Acid precipitation—effects on forest and fish” (SNSF), which run between 1972 and 1980 (Overrein et al. 1981 ). At that time the scientific understanding was limited, and the programme received a lot of attention. The results were important for the general acceptance that long-distance transport of sulphur caused acidification of surface waters, with a serious die-off of fresh water fish populations (salmon and trout) as a main consequence. On the other hand, the studies on Norwegian forests did not give any significant evidence for acid rain effects. The SNSF project was a joint effort across disciplinary and organisational boundaries, with scientists mainly from the research institute sectors outside of traditional academia. This project served as a model for later research programmes and provided educational opportunities for a new generation of scientists working together on all aspects of the acid rain issue—emissions and their control, atmospheric transport and deposition, impact on ecosystems, health and materials, and finally development of pollutant-control policies.
The long-term field experiments served another important task. The sites became exhibition platforms, at which policymakers, experts, scientific journalists, and leaders of non-governmental organisations (NGOs) and others can be informed about the problem directly on site. During the most intense period in the 1980s and early 1990s, politicians and industry leaders, often directly involved in decisions on the highest levels, visited many of these experimental sites. For example, US congress members travelled across Europe to see and understand the issue in preparation for the 1990 amendment of the Clean Air Act.
Bridging concepts and approaches
Concepts developed, such as critical loads and similar approaches, formed links between science and policy, and were essential for the understanding and scientific legitimacy of the policy measures. These concepts also formed a basis for priority setting in agreements under the Convention and the EU, but also to some extent for national policies. Even “acid rain” can be considered as a bridging concept. While the acidity from sulphur and nitrogen compounds is threatening ecosystems through a chemical change, the expression also gives the impression of a threat to the life-giving rain, a fundamental necessity for life on Earth.
The quantification of transboundary fluxes was very important politically. The establishment of national budgets and so-called blame matrices formed the first bridging concept. The development of mathematical models to calculate source–receptor relations was a scientific challenge but when the annual tables were prepared showing the interdependence between countries with respect to atmospheric emissions and deposition, they served as an important basis for the need for common action. Anton Eliassen, the leader of the modelling centre at the EMEP Meteorological Synthesising Centre West (MSC-W) during many years (the Eastern center is in Moscow—MSC-E), was key to this development as well as for the communication of the results to policymakers.
As earlier mentioned, critical loads played an outstanding role for the development of the more advanced strategies leading to the Second Sulphur Protocol and the GP. Critical loads formed a successful link between science and policy that became crucial for the negotiations and agreements. The concept, first discussed in 1982, was taken from the original idea to application quite quickly during the 1980s. The Swedish expert Jan Nilsson was a key leader for the success of the concept, and the Nordic Council of Ministers played a unique role for forming the links between science and policy. Through a series of workshops involving both key scientists and key policymakers, the concept gained the legitimacy on which policies were developed. According to Jan Nilsson, it all started with requests from both industry and negotiators to have a sounder base for emission control, something that could express the long-term objectives for emission control policies. The concept was first met by scepticism, not least from scientists, but after a couple of workshops, the interest turned around and the concept became widely accepted (Nilsson 1986 ; Nilsson and Grennfelt 1988 ). When critical loads were included in the plans for the next rounds of the sulphur and nitrogen protocols in 1988, it changed the way the Air Convention operated.
The application of the critical loads concept has encouraged intense research over several decades where the main objective has been to find simple chemical parameters that can mimic the (often biological) real effects or effect risks. For lake acidification, where the effects of dissolved aluminium on fish often were chosen as the main biological effect, the acidity of the water, mostly expressed as acid neutralising capacity (ANC), is used (e.g. Henriksen et al. 1989 ; Forsius et al. 2003 ; Posch et al. 2012 ). For forests, where the toxicity of aluminium to tree roots is considered as critical, the Al 3+ to Ca 2+ ratio in soil water has become the main effect parameter (Sverdrup et al. 1990 ; de Vries et al. 1994 ).
Integrated assessment modelling (IAM) also has been a bridging concept. The idea of applying systems analysis goes back to the work at IIASA in the beginning of 1980s. A conceptual model was formulated by Joseph Alcamo, Pekka Kauppi, and Maximilian Posch for the interactions between emissions, their control (including costs), and the effects on ecosystems (Alcamo et al. 1984 ). Their work of bringing together the scientific knowledge to a comprehensive systems analysis tool formed a new way of framing environmental policies. Under the leadership of Leen Hordijk, the new idea was introduced to and accepted by the policy side, which had asked for more targeted methods for policies than simple percentage decreases in amounts of emissions. IAMs as a policy-supporting concept was then taken further by Markus Amann, who led the development of the more advanced RAINS (later GAINS) models that were used as a basis for the GP and later agreements (Amann et al. 2011 ). From the strategies strictly directed at ecosystem effects, the approach is now widened to include health effects, local air pollution impact, climate policies, and reactive nitrogen.
All the bridging concepts are to varying degrees dependent on underlying models, assumptions, and simplifications. For these to be accepted among policymakers, it is important to keep transparency and confidence in the underlying data and to scientifically evaluate and scrutinise them. This is particularly important for the IAMs, which are the final step in a chain of inputs (Fig. 8 ). The models have often been criticised, not least from industry and other stakeholders that are questioning the priorities that result from the IAM calculations. IIASA, as a provider of the model calculations, has, however, been transparent, and countries and stakeholders have always had the option to re-check data and take this into account when developing their own negotiation positions.

The scientific support to regional air pollution policies consists today of a series of steps. The policy side may often only see the integrated assessment step and not realise that the legitimacy of the use of scientific support builds on an advanced system of underlying research and development
Forming science–policy credibility
In all interactions between science and policy, it becomes crucially important to maintain scientific credibility. The close involvement of scientists has been a signature of the Air Convention. Scientists have always had a role at the policy meetings, communicating results from basic scientific research over outcomes of monitoring and inventories to presenting options for control strategies. Scientists have in this way taken the responsibility to move scientific knowledge into the policy system and presenting results in a way that has been understandable and useful for the policy work. The role of the scientists has been as honest brokers , not that of issue advocates to follow the terminology of Pielke ( 2007 ). The leadership from the policy side and its sensitivity to changes in the underlying science and observations of new problems have also been important, and have resulted in repeated changes in the framings of the Air Convention to adapt to new situations: going from an initial framing around sulphur and acidification, through extension to eutrophication, human health, materials, crops, biological diversity, and finally to links to climate, urban air quality, and societal changes. A balanced interplay between the two communities has in this way been developed and maintained over time.
Another factor is the building of networks. The strong networks of scientists and policymakers pushed the politicians. The whole field of international diplomacy during these four decades of the Convention is built on incremental developments forming protocols of increasing capability to solve specific environmental issues by cutting emissions in a cost-effective way.
Future challenges
New approaches necessary.
International air pollution control is by many considered as a success story. However, the success is in many ways limited to Europe and North America and a few additional industrialised countries (including Japan and Australia), where emissions of sulphur dioxide, nitrogen oxides, VOCs, and some other compounds have been decreased significantly (Maas and Grennfelt 2016 ). But even in the areas, where air pollution has been a top priority for several decades, air pollution remains a problem. Ecosystem effects, which were the main reason for the establishment of the Convention, are to some extent reduced, but the acidification effects of historical emissions will remain for decades (Wright et al. 2005 ; Johnson et al. 2018 ) and the emissions of ammonia have so far only been reduced by 20–30% in Europe and even less in North America. Looking at health effects, it is difficult to talk about success, when hundreds of thousands of inhabitants on both continents are predicted to meet an earlier death due to air pollution.
But the problem is even larger and more urgent when looking outside the traditional industrialised world. The focus is today on the large urban regions in the countries that are facing rapid population growth and industrialisation. Although large efforts now are being made to decrease sulphur emissions in China—the world’s leading sulphur emitter—major challenges remain. In India and several other countries, sulphur emissions are still increasing. Estimates indicate that more than four million people die prematurely due to outdoor air pollution globally ( https://www.who.int/airpollution/ambient/health-impacts/en/ ). It is assumed that fine particles (PM2.5) are a main cause for the health effects. The new and great challenge is therefore to control air pollution in relation to health risks, in particular by decreasing exposure to the small particles.
There is, however, a risk that control measures will only to a limited extent focus on the right sources and the right measures. In Paris, several air pollution episodes with high concentrations of particles have occurred during recent years. At first, these episodes were considered to be caused essentially by local emissions. More thorough analysis has, however, shown that they were to a large extent caused by regional emissions and buildup of high concentrations over several days when urban emissions of oxides of nitrogen from traffic mix with ammonium emissions from surrounding agricultural areas to form particulate nitrate. Similar situations are also often encountered in urban regions in developing countries, e.g. by agricultural waste burning, and need to be considered. Air pollution problems are, as previously mentioned, also linked to intercontinental and hemispheric scales.
It is also obvious that the research communities within air pollution and climate change need to work more closely together. Health aspects are of importance both from air pollution and climate change perspectives, and heat waves carry poor air quality as winds are often very low and the atmospheric boundary layer stagnant. During heat waves, the soil and vegetation dry up and increase the likelihood of fires, which also can cause severe air pollution, as seen in wildfires around the world (e.g. California in 2018).
Despite the large progress in atmospheric and air pollution science, basic questions still need further investigations to develop the best policies. Such areas include a better understanding of health effects from air pollution, nitrogen effects to ecosystems, and air pollution interactions with climate through carbon storage in ecosystems and impacts on radiation balances. Modelling is a scientific area where much progress has been made and where increased computer power, as in climate change research, has allowed integration of atmospheric chemistry into the climate models formulated as Earth system models, coupling the atmosphere, ocean, the land surface, cryosphere, biogeochemical cycles, and human activities together. This has allowed studying air pollution and climate change simultaneously. The modelling approach can be further developed when observations are designed to map Earth system component boundaries to understand and quantify the flows and interactions between different compartments, including terrestrial and aquatic ecosystems. Air pollution should be an integrated part of such models. In this context, global-scale concepts such as “planetary boundaries” and “trajectories of the Earth system vs. planetary thresholds” have been developed (Rockström et al. 2009 ; Steffen et al. 2018 ).
Solutions are available; driving forces and investments are lacking
In 2016, the Air Convention launched a scientific report “Towards Cleaner Air”, in which the actual air pollution situation within the UNECE region was updated (Maas and Grennfelt 2016 ). The report also presented future challenges and ways forward to solve the air pollution problems. It also showed that solutions are available for most of the identified problems at affordable costs below the health and ecosystem benefits of the control actions.
Even if solutions are available, many parts of the world are facing large problems in implementing them. There are several reasons, but often there is a lack of knowledge and resources. This is particularly true in many developing countries. Another reason is the lack of political interest. Air pollution is still not of top priority among politicians, even if there is overwhelming evidence that air pollution is one of the most common causes of shortened life expectancies. Another reason may be that other interests (e.g., industry and agriculture) are forming strong lobbying forces delaying actions.
Air pollution is a problem that cannot be seen in isolation. Future policies need to take into account climate change and climate change policies. Whereas some air pollutants—in particular black carbon particles—contribute to warming, others, including sulphate particles, tend to cool the climate. A reduction in sulphur dioxide emissions, although highly desirable from health and ecosystems perspectives, will therefore contribute to warming. On the other hand, a reduction of black carbon will be a win–win solution. It is also important to see air pollution control in the perspective of sector policies, such as energy, agriculture, transportation, and urban planning in order to meet the challenges to decrease air pollution problems.
Internationally coordinated actions and infrastructures are keys for success
The perspective of international cooperation on air pollution is changing. Policy development is no longer limited to long-range transport in line with that developed under the Air Convention. The ranking of air pollution as a top ten cause of premature deaths in the world has given high priority to the issue within fora such as the WHO and UN Environment. Both organisations have adopted resolutions calling for actions (WHO 2015 ; UN Environment 2017 ). Additional initiatives are taken by other organisations, such as the World Meteorological Organisation (WMO), the Climate and Clean Air Coalition (CCAC), and the Arctic Monitoring and Assessment Programme (AMAP). WMO is particularly important as a global technical agency for weather and climate observations, research and services, and it is rapidly developing its regional and global capacities in Earth system observations, modelling, and predictions to the benefit of mitigating a range of environmental threats and for global use. The research is done in large programmes like Global Atmosphere Watch (GAW) and the World Weather Research Programme (WWRP). Even if the starting point and modes of action can be different, all initiatives are aiming for the same goal, cleaner air. It is also worth mentioning the initiative taken by the International Law Commission, under which a proposal for a Law for the Protection of the Atmosphere has been prepared ( http://legal.un.org/ilc/summaries/8_8.shtml ) but in the current international atmosphere there is a lack of political support to implement it. Our hope is that the situation will change soon—the initiative is too important to fail.
The UN has put forward a very strong agenda in order to reach the Sustainable Development Goals (SDGs), and air pollution is an integral part of several of the SGDs, like goal No 2: No Hunger, No 3: Good health and well-being, No 6: Clean Water, No 7: Affordable and clean energy, No 9: Industry, innovation and infrastructure, No 11: Sustainable cities and communities, No 13: Climate action, No 14: Life below water, No 15: Life on Land, No 16: Peace and Justice, and No 17: Partnerships for the Goals. The approach taken to develop multiple pollutant—multiple impacts protocols under the Air Convention can serve as important learning ground to meet the ambitions of many of the SDGs. Air pollution plays an integral role in the evolution of the food production and ecosystem services, the health of the population, the shape of the energy and transportation systems, and the availability of clean water. Climate change is a very significant common and cross-cutting factor.
The Air Convention has taken some steps in promoting air pollution on a wider scale. Due to its long history and well-developed structure, it has taken a role of making sure that international organisations having air pollution on its agenda are aware of each other and to invite to further collaboration and development. Initiatives are taken both within the formal Convention structure and through dedicated workshops (UNECE 2018 ; Engleryd and Grennfelt 2018 ). The approach developed under the Air Convention, which has proven successful in linking scientific evidence, monitoring, and integrated assessment modelling directed towards cost-effective solutions, may also serve as a working model for environmental problems in other fields.
These new international initiatives have a strong emphasis on policy development. The experience from the 50 years of international air pollution development is the value of well-defined scientific objectives and activities supporting policy. The increased interest from WHO and UN Environment is welcome and there are expectations of an active role from these organisations in combatting the situation in many parts of the world. However, for these organisations, air pollution is just one of several priority areas, and priorities may change. Further, none of these organisations are likely able to set up advanced infrastructures with respect to emission inventories, monitoring, and research. Here WMO needs to live up to its mission and capitalise on global research and development efforts and improve the global operational capability to observe, analyse, and forecast the development of the Earth system and its components, air pollution being an important part. This is in line with the WMO strategic plan and with fast growing capabilities in some countries and in global centres like The European Centre for Medium Range Weather Forecast (ECMWF). WMO, through GAW, is also developing a research-driven operational system (IG3IS) for top-down determination of greenhouse gas emissions, to complement the usual bottom-up-based inventories where emission factors and fuel consumption or production statistics form the basis for the emission estimates ( https://library.wmo.int/doc_num.php?explnum_id=4981 ). The Air Convention and the science support for the policy work there has been a model for the WMO ambitions on a global basis. However, current investments in these new capabilities are not enough to get the societal return they would offer.
Therefore, we see a need for developing long-lasting infrastructures that can continuously develop science-based control policy options, potentially as part of a wider network of global observatories for comprehensive monitoring of interactions between the planet’s surface and atmosphere (Kulmala 2018 ). Such a network should be able to support policies from local to the global levels. The challenge is how to organise and raise resources for scientific support on a wider scale. Financial institutions such as the World Bank and/or regional banks may step in and make sure that control measures and investments are made on a sound basis with respect to global air pollution.
There is also a need to mobilise new generations of scientists, scientists that are willing to cross boundaries and focus on thematic problems and to build legitimacy among policymakers (e.g. Bouma 2016 ). Today we have more developed and stronger political institutions to handle environmental problems, which may make it harder for scientists and individuals to influence and make a difference. It is also important to mobilise new generations of dedicated policymakers. Unfortunately, we also see that politicians often are questioning science and seeing science as just a special interest. Public awareness may be a key for forming stronger interests and put pressure on decision-makers. During the acid rain history, NGOs played an important role in driving the awareness at a wider scale than local or national actions and could be important for a more global movement towards cleaner air. We also see the need for a deeper responsibility not only from politicians but also from industry. The so-called “diesel gate” exposed the cynic view from parts of the industry to peoples’ health, which hopefully will not occur in the future. Instead we hope that it was an eye-opener and that industry instead can play a role as a forerunner and a positive power for a cleaner atmosphere.
Final remarks
The Acid Rain history taught us that when science, policy, industry, and the public worked together, the basis was formed for the successful control of, what was considered, one of the largest environmental problems towards the end of the last century. We learnt from experience that science-based policy advice worked well when the best available knowledge was provided, and used to understand the specific problems, generate, and evaluate the policy options and monitor the outcomes of policy implementation.
However, the world does not look the same today, and we cannot just apply the ways the international science community worked together then on today’s problems. But there are lessons to be learnt. Most important is the building of mutual trust between science advisers and policymakers, and that both communities are honest about their values and goals. In this way, a fruitful discussion around critical topics within society can be formed. The advice works best when it is guided by the ideal of co - creation of knowledge and policy options between scientists and policymakers (SAPEA 2019 ).
Acknowledgement
Open access funding provided by The Swedish Environmental Protection Agency. The Symposium could not have been arranged and this paper written without financial support from the Nordic Council of Ministers, the Swedish Environmental Protection Agency, the Mistra Foundation, and other organisations and institutes in the Nordic countries. We are also grateful to all participants of the Symposium and their contributions with background material for this paper. We also thank the two anonymous reviewers of the manuscript. Their comments greatly improved the quality of this paper.
Biographies
is a former Scientific Director at the Swedish Environmental Research Institute IVL. His main scientific activities include transboundary air pollution and environmental science–policy interactions.
is a Senior Policy Advisor at the Swedish Environmental Protection Agency. For the last 15 years, she has been a lead negotiator on air pollution for the Swedish Government in several international fora. Since 2014, she is the chair of the Executive Body to the UNECE convention on long-range transboundary air pollution. She has a background in energy efficiency and agronomy.
is a Research Professor at the Finnish Environment Institute SYKE. His research interests include impacts of air pollutants and climate change on biogeochemical processes.
is the Secretary General of The Norwegian Academy of Science and Letters and adviser to the Director General of the Norwegian Meteorological Institute. His research interests include atmospheric chemistry and earth system modelling.
is a Professor Emeritus at the Department of Meteorology and Bolin Centre for Climate Research at Stockholm University. His research interest includes atmospheric transport processes and climate impact of aerosol particles.
is University Distinguished Professor At-Large Emeritus at North Carolina State University in Raleigh, North Carolina. He was founding leader for the US National Atmospheric Deposition Program (NADP), which played a crucial role for the development of acid rain research and policy in North America from the 1970s and onwards.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peringe Grennfelt, Email: [email protected] .
Anna Engleryd, Email: [email protected] .
Martin Forsius, Email: [email protected] .
Øystein Hov, Email: on.tem@hnietsyo .
Henning Rodhe, Email: es.us.usim@ehdor .
Ellis Cowling, Email: ude.uscn@gnilwoc_sille .
- Amann M., I. Bertok, J. Cofala, F. Gyarfas, C. Heyes, Z. Klimont, and W. Schöpp. 1999. Integrated assessment modelling for the protocol to abate acidification, eutrophication and ground-level ozone in Europe, Lucht & Energie 132, November 1999.
- Amann M, Bertok I, Borken-Kleefeld J, Cofala J, Heyes C, Höglund-Isaksson L, Klimont Z, Nguyen B, et al. Cost-effective control of air quality and greenhouse gases in Europe: Modeling and policy applications. Environmental Modelling & Software. 2011; 26 (12):1489–1501. doi: 10.1016/j.envsoft.2011.07.012. [ CrossRef ] [ Google Scholar ]
- Alcamo, J., P. Kauppi, M. Posch and E. Runca. 1984. Acid rain in Europe: A framework to assist decision making. Working paper WP-84-32, International Institute for Applied Systems Analysis, Laxenburg, Austria.
- Bouma J. Implications of the nexus approach when assessing water and soil quality as a function of solid and liquid waste management. In: Hettiarachchi H, Ardakanian R, editors. Environmental resource management and the nexus approach. New York: Springer; 2016. pp. 179–209. [ Google Scholar ]
- Cowling EB. Acid precipitation in a historical perspective. Environmental Science & Technology. 1982; 16 :110A–123A. doi: 10.1021/es00096a725. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dannevig A. Influence of precipitation on river acidity and fish populations. Jeger og Fisker. 1959; 3 :116–118. [ Google Scholar ]
- Dentener, F., T. Keating, and H. Akimoto. 2010. Hemispheric transport of air pollution, part A. air pollution studies no. 17, Convention on long-range transboundary air pollution, UNECE.
- De Vries W, Reinds GJ, Posch M. Assessment of critical loads and their exceedance on European forests using a one-layer steady-state model. Water, Air, and Soil Pollution. 1994; 72 :357–394. doi: 10.1007/BF01257134. [ CrossRef ] [ Google Scholar ]
- De Vries, W., J.-P. Hettelingh, and M. Posch, eds. 2015. Critical loads and dynamic risk assessments: Nitrogen, acidity and metals in terrestrial and aquatic ecosystems. Environmental pollution series, Vol. 25. Dordrecht: Springer. ISBN 978-94-017-9507-4; 10.1007/978-94-017-9508-1.
- Driscoll, C.T., E.B. Cowling, P. Grennfelt, J.M. Galloway, and R.L. Dennis. 2010. Integrated assessment of ecosystem effects of atmospheric deposition: Lessons available to be learned. EM Magazine , November 2010: 6–13.
- Engleryd, A. and P. Grennfelt. 2018. Saltsjöbaden VI workshop. Clean air for a sustainable future—goals and challenges. Nordic Council of Ministers, TemaNord 2018:540, Copenhagen, ISSN 0908-6692. http://saltsjobaden6.ivl.se/finalreport.4.14bae12b164a305ba11c38f.html .
- Egnér H, Eriksson E. Current data on the chemical composition of air and precipitation. Tellus. 1955; 7 :134–139. doi: 10.3402/tellusa.v7i1.8763. [ CrossRef ] [ Google Scholar ]
- Engardt M, Simpson D, Schwikowski M, Granat L. Deposition of sulphur and nitrogen in Europe 1900-2050. Model calculations and comparison to historical observations. Tellus B. 2017; 69 :1328945. doi: 10.1080/16000889.2017.132. [ CrossRef ] [ Google Scholar ]
- Forsius M, Vuorenmaa J, Mannio J, Syri S. Recovery from acidification of Finnish lakes: Regional patterns and relations to emission reduction policy. Science of the Total Environment. 2003; 310 :121–132. doi: 10.1016/S0048-9697(02)00628-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ. The nitrogen cascade. BioScience. 2003; 53 (4):341–356. doi: 10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2. [ CrossRef ] [ Google Scholar ]
- Grennfelt P, Hov Ø, Derwent RG. Second generation abatement strategies for NO X , NH 3 , SO 2 and VOC. Ambio. 1994; 23 :425–433. [ Google Scholar ]
- Gorham, E. 1981. Scientific understanding of atmosphere-biosphere Interactions: A historical review. Chapter 2. In Toward a better understanding of the ecological consequences of fossil fuel combustion . National Research Council, 9–21. 1981. Washington, DC: The National Academies Press. 10.17226/135.
- Henriksen A, Lien L, Rosseland BO, Traaen TS, Sevaldrud IS. Lake acidification in Norway—present and predicted fish status. Ambio. 1989; 18 :314–321. [ Google Scholar ]
- Hettelingh, J.-P., R.J. Downing, and P.A.M. de Smet. 1991. Mapping critical loads for Europe, CCE Status Report 1 1991, Coordination Center for Effects, National Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
- Hordijk L. Integrated assessment models as a basis for air pollution negotiations. Water, Air, and Soil pollution. 1995; 85 :249–260. doi: 10.1007/BF00483705. [ CrossRef ] [ Google Scholar ]
- Johnson J, Graf Pannatier E, Carnicelli S, Cecchini C, Clarke N, Cools N, Hansen K, Meesenburg H, et al. The response of soil solution chemistry in European forests to decreasing acid deposition. Global Change Biology. 2018; 24 :3603–3619. doi: 10.1111/gcb.14156. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kulmala M. Build a global Earth observatory. Nature. 2018; 553 :21–23. doi: 10.1038/d41586-017-08967-y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Likens GE, Driscoll CT, Buso DC. Long-term effects of acid rain: Response and recovery of a forested ecosystem. Science. 1996; 272 :244–246. doi: 10.1126/science.272.5259.244. [ CrossRef ] [ Google Scholar ]
- Maas R. and P. Grennfelt, eds. 2016. Towards cleaner air. Scientific assessment report 2016. EMEP steering body and Working Group on effects of the convention on long-range transboundary air pollution, Oslo.
- Nilsson J., ed. 1986. Critical loads for nitrogen and sulphur. Report from a Nordic working group, Nordic Council of Ministers, Miljø rapport 1986:11, Stockholm.
- Nilsson, J. and P. Grennfelt, eds. 1988. Critical loads for sulphur and nitrogen. Report of a workshop held in Skokloster, Nordic Council of Ministers, Miljø rapport 1988:15, Stockholm.
- Odén, S. 1968. The acidification of air precipitation and its consequences in the natural environment. Bulletin Ecological Research Commission. NFR. Translation Consultants Ltd., Arlington VA.
- OECD . The OECD programme on long range transport of air pollutants. Paris: Measurements and Findings; 1977. [ Google Scholar ]
- Overrein, L.N., H.M. Seip, and A. Tollan, eds. 1981. Acid precipitation–effects on forest and fish: Final report of the SNSF-project 1972-1980. Oslo Norway.
- Pielke, R.A. Jr. 2007. The honest broker. Making sense of science in policy and politics. Cambridge: Cambridge University Press.
- Posch M, Aherne J, Forsius M, Rask M. Past, present and future exceedance of critical loads of acidity for surface waters in Finland. Environmental Science and Technology. 2012; 46 :4507–4514. doi: 10.1021/es300332r. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ramanathan V, Crutzen PJ, Kiehl JT, Rosenfeld D. Review paper: Aerosols, climate, and the hydrological cycle. Science. 2001; 294 :2119–2124. doi: 10.1126/science.1064034. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reis S, Grennfelt P, Klimont Z, Amann M, Apsimon H, Hettelingh J-P, Holland M, LeGall A-C, et al. From acid rain to climate change. Science. 2012; 338 (6111):1153–1154. doi: 10.1126/science.1226514. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reuss JO, Cosby BJ, Wright RF. Chemical processes governing soil and water acidification. Nature. 1987; 329 :27–32. doi: 10.1038/329027a0. [ CrossRef ] [ Google Scholar ]
- Rockström J, Steffen W, Noone K, et al. A safe operating space for humanity. Nature. 2009; 461 :472–475. doi: 10.1038/461472a. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rodhe H. A study of the sulfur budget for the atmosphere over Northern Europe. Tellus. 1972; 24 :128–138. [ Google Scholar ]
- Rothschild, R.E. 2018. Poisonous skies. Acid rain and the globalization of pollution . Chicago: University of Chicago Press. ISBN 9780226634852.
- Royal Ministry of Foreign Affairs and Royal Ministry of Agriculture. 1972. Air pollution across national boundaries. The impact on the environment of sulfur in air and precipitation. In Sweden’s case study for the United Nations’ Conference on the Human Environment, Stockholm.
- Sand M, Berntsen TK, von Salzen K, Flanner MG, Langner J, Victor DG. Response of Arctic temperature to changes in emissions of short-lived climate forcers. Nature Climate Change. 2016; 6 :286–289. doi: 10.1038/nclimate2880. [ CrossRef ] [ Google Scholar ]
- SAPEA. 2019. Making sense of science for policy under conditions of complexity and uncertainty. Science Advice for Policy by European Academies, 1–186, Berlin. 10.26356/MASOS.
- Schöpp W, Posch M, Mylona S, Johansson M. Long-term development of acid deposition (1880–2030) in sensitive freshwater regions in Europe. Hydrology and Earth System Sciences. 2003; 7 :436–446. doi: 10.5194/hess-7-436-2003. [ CrossRef ] [ Google Scholar ]
- Sliggers, J. and W. Kakebeeke, eds. 2004. Clearing the air. 25 years of the convention on long-range transboundary air pollution. United Nations Economic Commission for Europe, Geneva 1–167.
- Smith RA. Air and Rain, the Beginnings of a Chemical Climatology. London: Longmans Green; 1872. [ Google Scholar ]
- Steffen W, Rockström J, Richardson K, Lenton TM, Folke C, Liverman D, Summerhayes CP, Barnosky AD, et al. Trajectories of the earth system in the anthropocene. PNAS. 2018; 115 (33):8252–8259. doi: 10.1073/pnas.1810141115. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sverdrup H, de Vries W, Henriksen A. Mapping critical loads, Nord 1990:98. Copenhagen: Nordic Council of Ministers; 1990. [ Google Scholar ]
- Sutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt P, van Grinsven H, Grizzetti B, editors. The European nitrogen assessment. Cambridge: Cambridge University Press; 2011. [ Google Scholar ]
- Ulrich B, Mayer R, Khanna PK. Chemical changes due to acid precipitation in a loess‐derived soil in Central Europe. Soil Science. 1980; 130 :193–199. doi: 10.1097/00010694-198010000-00005. [ CrossRef ] [ Google Scholar ]
- UNECE. 2016. Towards cleaner air. Scientific assessment report 2016: North America. United Nations, Geneva. http://www.unece.org/index.php?id=42947&L=0 .
- UNECE. 2018. Report from 38th session of the executive body for the convention on long-range transboundary air pollution Geneva 10–14 December 2018, Annex, 18–21, http://www.unece.org/fileadmin/DAM/env/documents/2018/Air/EB/ECE_EB.AIR_142-1902903E.pdf .
- UN Environment. 2017. Preventing and reducing air pollution to improve air quality globally on air quality. Third UN Environment Assembly, 4–6 December 2017, Resolution 8. https://papersmart.unon.org/resolution/uploads/k1800222.english.pdf .
- WHO. 2015. Resolution WHA68.8 health and the environment: addressing the health impact of air pollution. 68th World Health Assembly World Health Organisation, http://apps.who.int/gb/ebwha/pdf_files/WHA68/A68_R8-en.pdf .
- WHO. 2018. https://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health .
- Wright RF, Lotse E, Semb A. Reversibility of acidification shown by whole-catchment experiments. Nature. 1988; 334 :670–675. doi: 10.1038/334670a0. [ CrossRef ] [ Google Scholar ]
- Wright RF, Camarero L, Cosby BJ, Ferrier RC, Forsius M, Helliwell R, Jenkins A, Kopáček J, et al. Recovery of acidified European surface waters. Environmental Science and Technology. 2005; 39 :64A–72A. doi: 10.1021/es0531778. [ PubMed ] [ CrossRef ] [ Google Scholar ]
Home — Essay Samples — Environment — Environmental Issues — Environmental Disasters: Causes and Effect of Acid Rain
Environmental Disasters: Causes and Effect of Acid Rain
- Categories: Environmental Issues Pollution Water Pollution
About this sample

Words: 1810 |
10 min read
Published: Aug 30, 2022
Words: 1810 | Pages: 4 | 10 min read
Table of contents
Introduction, what is acid rain, effect of acid rain, what is being done, ponds, lakes, and streams, effects of acid rain-causing pollutants on humans, effects of acid rain on man-made materials.

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Verified writer
- Expert in: Environment

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
3 pages / 1454 words
3 pages / 1362 words
3 pages / 1312 words
3 pages / 1381 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Environmental Issues
Deforestation is a complex and multifaceted issue that has far-reaching consequences for our planet. While it is commonly associated with negative impacts on the environment, such as the loss of biodiversity and increased [...]
The environment is the foundation of life on earth and ensures that humans and other living organisms can thrive. However, human activities have created significant environmental challenges in the modern world. This essay will [...]
There has been significant development in the car industry, particularly when comparing the current advancements to the first car models in the 19th century. Many individuals believe that electric cars are the future due to [...]
The natural environment plays a vital role in sustaining life on Earth, providing oxygen and water that are essential for all living beings.However, the movie The Lorax depicts a walled city called Thneedville where destructive [...]
Pakistan, a nation characterized by its rich cultural heritage and diverse landscapes, is grappling with a growing concern – the environmental challenges plaguing its big cities. As these urban centers continue to swell with [...]
The issue being discussed is desertification. This is a very big problems in some parts of the world, for example Africa, the Middle East, South America and more. This is an issue that mainly comes from climate change, [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
- About Project
- Testimonials
Business Management Ideas

Essay on Acid Rain
List of essays on acid rain in english, essay on acid rain – essay 1 (150 words), essay on acid rain: introduction, after-effects and conclusion – essay 2 (250 words), essay on acid rain: causes and effects – essay 3 (300 words), essay on acid rain – essay 4 (400 words), essay on acid rain: causes, effects and prevention – essay 5 (500 words), essay on acid rain: with conclusion – essay 6 (600 words), essay on acid rain: types, sources and harmful impacts – essay 7 (750 words), essay on acid rain: with solution – essay 8 (1000 words).
Introduction:
Acid rain simply means rain high in acidic contents such as a high rate of hydrogen ions. Acid rain is a cause of concern because of its effect on forest life and lakes. To a larger extent, acid rain is a big threat to our natural environment.
Causes of Acid Rain:
When we burn fossil fuel, we release a lot of sulfur dioxide and nitrogen oxide into the atmosphere. These compounds when mixed with air go straight into the atmosphere. At this level, they mix with water, oxygen, and other components to become acid rain.
Effect of Acid Rain:
For the aquatic animal, acid rain becomes deadly since it reduces the PH level in the water. Many animals that live in water need a particular PH level to survive. When that PH level is reduced, it becomes difficult to survive. For trees, acid rain leaves them exposed to diseases.
Conclusion:
Our animal and plant life should be of paramount importance to us. To end this, we must reduce our release of toxic gas into the atmosphere.
Advancement of science and mass industrialization has led to many man-induced phenomenon and acid rain is one of them. It is nothing but rain that is highly acidic in content. Poisonous gases emitted by chemical industries pollute the atmosphere with oxides of sulphur, nitrogen and hydrogen. When these oxides raise high into the clouds, they react with water molecules there and turn them into sulphuric acid, nitric acid and hydrochloric acid respectively. These acids are released into the atmosphere as rain, snow or precipitation. Natural phenomenon like volcanoes and fossil fuel combustion also produce harmful gases which can cause acid rain.
After-Effects of Acid Rain:
Acid rains have a multitude of effects on humans, animals and the environment. Breathing problems like asthma is caused in children and adults alike. Skin peeling is possible when there is direct contact with acid rain. Corrosion of metals, peeling of paints on buildings, erosion of natural stones like marbles, granites and limestones are all inclusive. In addition, rivers and oceans get polluted with high contents of acid resulting in death of plants, fishes and other sea creatures. Fresh water reserves turn toxic and harmful to consume. Trees die due to direct and continuous exposure. Many insects are killed instantaneously. And exposed agricultural lands will no longer be fit for cultivation.
Measures should be taken to avoid air pollution by industries. Environmentally friendly methods need to be adopted by everyone. Both the public and government need to realize the seriousness of the issue and work together to solve the problem of acid rain.
“Acid Rain”, according to the Royal Society of Chemistry this term was coined by Scottish chemist Robert Angus Smith in 1852. “Acid Rain” is a result of acidification of rainwater due to mixing relatively large quantities of oxides of sulphur and nitrogen emissions in the atmosphere. Interaction of this acidic water with other constituents of the atmosphere increases the soil acidity. It leaches away nutrients in the soil and heavily degrades quality of air. “Acid Rain” is posing a prospective threat to our ecosystem and environment.
Rain water is increasingly acidified by pollutants released from homes, factories, power stations and cars.
i. Man-made Causes:
Heavy industrialization and urbanization is the major cause of acid rain. The exhausts of industries and factories emit harmful gases like Sulphur Dioxide (SiO 2 ) and Nitrogen Dioxide (NO 2 ) in the atmosphere. These gases interact with the water precipitation in the atmosphere and the chemical reaction between these gases and water molecules form Sulphuric Acid and Nitric acid.
A Large number of industries are set up nearby the majestic Taj Mahal, which emit harmful pollutants in the air and cause acid rain. The “Acid rain” reacts with the marble of Taj Mahal, causing damage to this heritage structure. To protect Taj Mahal over 2000 industries situated near Taj Mahal had transferred. The situation still remains critical because of the nearby leather industries.
ii. Natural Causes:
Volcano eruption releases various harmful gases in the environment which results in “acid rain” in the nearby areas.
“Acid rain” causes heavy damage to the ecosystem in the affected areas. It has the largest impact on lakes, streams, wetlands, and other aquatic environments. Acidic water is absorbed into the soil and water bodies which turns them into toxic compounds. The toxic pollutants pose a huge risk to the survival of aquatic creatures like crayfish, clams, fish, and other aquatic animals. Forests are also destroyed by “acid rain” and the ecological balance of the affected area is hampered to a huge level.
The only way to mitigate “acid rain” is to impose censorship on heavy industries which cause damage to our ecosystem. All the citizens must adopt and practise eco-friendly lifestyle. We must encourage use of alternative energy sources which will prevent burning of fossil fuels which releases harmful gases.
Acid rain is any form of precipitation which has acidic components like sulfuric acid or nitric acid. It falls to ground from the atmosphere in either wet or dry form. The precipitation can be in many different forms – rain, snow, fog, hail or even dust. It has a pH less than 5.6. Normally, the clean rain water has a pH of 5.6 as there is carbonic acid present in the rain water. This is because of carbon dioxide dissolved in water. The term acid rain was coined by Robert Angus Smith in 1872. There are two types of acid rain depositions – wet deposition and dry deposition.
It is a form of air pollution that is very damaging to the environment. The reason behind acid rain is particles like sulphur dioxide and nitrogen oxides which are emitted from fuel combustion. These molecules move up the atmosphere with the help of air currents and then they react with water and oxygen to form sulphuric acids and nitric acids. These acids combine with water before falling to ground in any form of precipitation. There are other sources of sulphur oxides and nitric oxides like volcanoes, manufacturing industries and vehicles. Even the decaying vegetation and wild forest fires generate the gases that form acid rain.
There are many negative effects of acid rain. Acid rain can make water bodies like lakes and ponds poisonous. This makes these water bodies inhabitable for marine life and makes the drinking sources unfit for human consumption. The number of aquatic animals and plants reduces because water becomes more acidic. It also degrades the quality of soil because of the acidic pH when it is absorbed into soil. As the soil quality is affected, it leads to reduction in the crop yield. There is a reduced growth of plants and trees in areas that are affected by acid rain. It impacts human health by causing skin problems, heart and lung issues. Other than biotic components, it affects and damages buildings and property. It is corrosive in nature and this is reflected in the corrosion of Taj Mahal by acid rain.
The problem is increasing and becoming worse because of the rapid population growth and industrialization. Although natural sources cannot be contained or regulated, we can at least work on controlling the manmade causes. Acid rain can be reduced by choosing cleaner forms of energy like solar power. Afforestation is another thing that can help in reducing air pollution.
Acid rain is basically wet deposition of acids in rain or snow that is of low pH on to the surface of the earth. However, rain water is naturally acidic due to the carbonic acid rain is an environmental hazard brought about by pollution. Pollution of the environment especially air and water pollution result in formation of acid rain.
Formation and Causes of Acid Rain:
Acid rain occurs in two ways. Wet deposition occurs when there is a precipitation and raindrops deliver the acidic components to the earth’s surface whereas dry deposition occurs when no precipitation occurs and the pollutants stick to plants and the earth surface. Air pollution causes the accumulation of chemicals in the atmosphere. These chemicals form chemical reactions that react with water and oxygen in the atmosphere and form acid rain. Gases that are oxides like Sulphur dioxide and nitrogen oxide form reactions with oxygen and water and form acids. These chemical reactions increase with the increase in air pollution by human or natural activities. Natural activities like the eruption of volcanoes or hot springs in geothermal result in the release of Sulphur dioxide to the atmosphere while lightning strikes cause a release of nitrogen oxide to the atmosphere. Human activities especially industrialization, combustion of fuels and emission of greenhouse gases to the atmosphere also contribute to the accumulation of oxides in the atmosphere which result in acid rain formation. Air pollutants have greatly increased the formation of acid rain and the effects have affected the health of living things on the surface of the earth.
Effects of Acid Rain:
Acid rain affects both animate and inanimate components of the environment. Plants and animals are mostly affected health wise because of the direct interaction. Plants get scorched and growth is impaired because acid rain also affects the soil pH by acidifying it. Animals, including humans consume acid rain when harvested which affects their health negatively especially through heart and lung problems. Acid rain is deleterious to aquatic animals like fish because acidic water flows to water bodies. Inanimate objects like buildings are also negatively affected. Buildings and bridges made of stone, steel or sand are corroded by the acids.
Prevention of Acid Rain:
Reduction of pollutants released to the atmosphere will prevent acid rain formation. The industries will have to ensure that filtration of gases before release to the atmosphere is done. There is a technical method of flue-gas desulphurization that helps in preventing emissions of Sulphur dioxide and compounds to the atmosphere. The adherence to air quality agreements that have been put in place is important. Government effort in gaseous emissions control programs will enable the regulation of pollutants released to the atmosphere therefore acid rain formation will be reduced.
In conclusion, acid rain is an environmental hazard that is caused by acidic components that accumulate in the atmosphere. Acid rain has both deleterious and adverse effects to the environment. The control of air pollution will result in reduction of acid rain formation.
‘Acid Rain’ is not a term that we hear very commonly. Most of us would not even know that such a type of rain exists. Before we move any further, let’s first understand what acid rain means.
The literal term ‘Acid Rain’ will make us think that acid would pour from the sky as rain but it is not so. Acid rain is formed when certain gases mix up with the atmospheric moisture and create precipitation which is more acidic than the normal one. In chemical terms, when the pH level of rain water falls below 5.6, it turns acidic and changes as acid rain. Robert A. Smith was the first to use the term ‘Acid Rain’ in 1872 in his studies of air in Manchester, England. But the term was most widely recognized only in 1980.
On a general note, rain is welcomed by all since it refreshes the atmosphere and benefits the environment. But not all rains do this and acid rain is a best example to show the devastating effects that a rain can cause. Since most of us are unaware of the subject acid rain, we have only little knowledge about it. Acid rain can destroy anything over a short period of time. Acid rain is very dangerous to the environment.
Acid rain falling on rivers, lakes and other water bodies makes it poisonous and destroys marine life.
It damages the forests and kills the insects and other living creatures. Black Forest in Germany received its name due to the acid rain which turned the trunks and branches of all its trees into black.
Acid rain causes severe damage to buildings and monuments because these are made of Calcium Carbonate stone; acid rain reacts with Calcium Carbonate and destroys it. The paint peels off quickly and the statues lose their good appeal. Taj Mahal, one of the wonders of the world has faced the ill-effects of acid rain.
When the acid rain falls on a place, it changes the acidity level of that place causing great harm to both the living and non-living things existing there.
Acid rain is hazardous to humans also as it causes respiratory problems and various other health issues.
Both nature and humans are to be blamed for the occurrence of acid rain.
Normal rain occurs when Carbon dioxide and water present in the air react together and forms Carbonic acid which is a weak acid. Acid rain is caused when Sulphur dioxide and Nitrogen Oxide reacts with water molecules in the atmosphere and produces acid which is harmful in nature. So, the main cause of acid rain is the emission of Sulphur dioxide and Nitrogen oxides in the atmosphere. This emission occurs in two ways – emission through volcanic eruption and decaying vegetation, combustion of fossil fuels from transport, burning chimneys and industrialization.
Ways to Prevent Acid Rain:
Acid rain is very dangerous and it should be stopped from occurring. We cannot stop acid rain that occurs due to natural reasons but we can surely do our best to reduce the use of fossil fuels which leads to the emission of gases like Sulphur dioxide and Nitrogen Oxide. Air pollution is the main reason for almost all the environmental problems and humans should make all attempts to reduce pollution. Using solar power instead of electricity, recycling natural resources and planting more trees can help in purifying the air and can also help in preventing man-made disasters like acid rain.
Each of us must take vow to preserve the natural resources and maintain the environment as clean and healthy as possible. Causing damage to the environment will only lead to the extinction of this Planet and no technology can help us buy a beautiful home similar to Earth.
Acid Rain simply means a rainfall that is highly acidic and such rain causes environmental, atmospheric and material depletion. Acid rain affects infrastructure, aquatic creature, plants and so many more. When something is acidic, it means it has a high level of ions of hydrogen which also means that the pH of the object or substance is low. Rain water that is normal is already a little bit acidic and has pH between 5.3 and 6.0. This is so because the water and carbon dioxide that are together in the atmosphere react together and form an acid known as carbonic acid; carbonic acid is an acid that is weak. Whenever the pH of water drops below the above stipulated range, the rain water is said to be acid rain.
Acid rain formation isn’t due to just one reason; both man-made and natural sources are believed to influence acid rain. That said, the emissions and release of nitrogen oxides and sulphur dioxide as a result of fossil fuels being combusted is the major cause of acid rain. Acid rain can be in form of snow, rain, fog, hail or even dew that contains high level of acid pollutants, most especially nitric acid and sulphuric acid. When emitted nitrogen oxide and sulphur dioxide reacts and mix with water molecules that are present in the air and atmosphere, acid rains are produced.
Evolution of Acid Rain:
The name “acid rain” was given by a man called Robert Smith in 1872. In recent years, industrialisation and population has led to a sharp and exponential increase in acid rains with it becoming a lot more alarming. Acid rains are also being further influenced by the use of chimneys that are very tall also known as smokestacks on ships, factories, etc. Smokestacks are used to lower the rate of air pollution but they end up spreading and causing acid rains through the release of gases right into the atmospheric circulation of a region. There is a very large quantity of deposits of acid in the United States, Canada and almost all of Europe including parts of Germany, Norway and Sweden. Of recent, some parts of the southern part of Asia, Sri Lanka and South Africa have also witnessed deposits of acid.
Types of Acid Rain:
Acid rains are divided based on the type of deposition by which the occurrence of the acid rain takes place.
Basically, we have two depositions types and they are discussed below:
Wet Deposition:
When acid rain is in form of snow, rain, mist or fog when it falls to the surface of the earth, acid is removed from the air or atmosphere by these liquids and is dropped on the surface of the earth. The flow of this acid rain through the earth affects a great quantity of aquatic life, animals and plants. Water moves from the drains into small water bodies like canals and rivers which in turn flow into seas and ocean causing damage to marine life.
Dry Deposition:
When there is a merging together of various acidic pollutants and they form smoke and dust that comes to the surface of the earth in the form of dry particles that stick on the surface of the earth and many other surfaces like monuments, trees, houses, cars and buildings. It is important to note that almost all of pollutants that are in the air or atmosphere are spread through the method of dry deposition.
Sources of Acid Rain:
Acid rains are majorly caused by two sources and the sources are discussed below:
1. Natural Sources:
Volcanic eruptions are the major agents that cause acid rain. Huge quantities of lava that produces gases that are harmful are emitted by volcanoes and this causes acid rain that are quite more severe than usual. The gases that form the acid rain are also generated by biological processes including wildfires and decaying vegetation that occur within the environment.
2. Human Sources:
Activities of humans that lead to the release of gases like nitrogen and sulphur from automobile plants, power generating setups and factories are the causes of acid rain here. Coal when it is used to generate electric power is probably the greatest contribution to the emission of gases that can cause acid rain
The harmful impacts of Acid Rain:
Acid rain can bring serious harm to the environment and a few of the various wide segments of the environment where it can wreak havoc are listed below:
1. Public health
2. Wildlife and forests
3. Infrastructure and architecture
5. Marine life.
The presence of harmful hydrogen ions in the raindrops which fall on us is called acid rain. Acid rain is a prominent term alluding to the testimony of a mixture of wet (rain, snow, slush, mist, cloud water, and dew) and dry (acidifying particles and gases) acidic segments. Fluids with a pH under 7 are termed acidic, and those with a pH more prominent than 7 are alkaline.
History of Acid Rain:
The destructive effect of polluted, acidic nature of the air of a region on limestone and marble was noted in the seventeenth century by John Evelyn, who commented upon the poor state of the Arundel marbles. Since the Industrial Revolution, outflows of sulphur dioxide and nitrogen oxides into the environment have increased. In 1852, Robert Angus Smith was the first to demonstrate the connection between acid rain and climatic contamination in Manchester, England. He is also called as the Father of Acid rain due to his immense contribution towards the study of acid rain. Despite the fact that acid rain was found in 1853, it was not until the late 1960s that researchers started generally watching and observing the phenomenon. The expression acid rain was coined in 1872 by Robert Angus Smith.
The cause of acid rain is attributed to both man-made as well as natural causes.
Natural Causes – The major natural cause leading to acid rain is the volcanic eruptions. Volcanoes emanate acid releasing gases to make higher than ordinary measures of acid rain or some other type of precipitation, for example, mist and snow to a degree of influencing vegetation cover and wellbeing of inhabitants in an area. Decaying vegetation, out of control fires and natural procedures inside the earth likewise produce the acid rain-causing gases. Lightning strikes in addition normally create nitric oxides that respond with water particles by means of electrical action to deliver nitric acid and thus causing acid rain.
Man-Made Causes – Human activities prompting substance gas outflows, for example, sulphur and nitrogen are the essential causes of acid rain. The activities incorporate air contamination sources transmitting sulphur and nitrogen gases like power generation facilities and vehicular emissions. Specifically, utilization of coal for electrical power is the greatest cause of vaporous emanations prompting acid rain. Vehicles and manufacturing plants likewise discharge high scores of vaporous emanations on consistent schedule into the air, particularly in exceedingly industrialized zones and urban areas with huge quantities of vehicle movement. These gases respond in the air with water, oxygen, and different synthetic concoctions to shape different acidic mixes, for example, sulphuric acid, ammonium nitrate, and nitric corrosive. Accordingly, these areas encounter exceedingly high measures of acid rain.
Acid rain affects almost everything. Plants, soil, trees, structures and even statues can be changed by the precipitation. Acid rain has been observed to be very harmful to the trees. It debilitates them by washing ceaselessly the defensive film on leaves, and it stunts development. Acid rain can likewise change the creation of soil and waterways, making them appalling for nearby creatures and plants. For instance, normal lakes have a pH of 6.5 or higher. As acid rain raises the dimension of corrosiveness, the waters turn acidic. Most fish species can’t endure a water pH of underneath 5. At the point when the pH turns into a 4, the lake is viewed as dead. Such is the effect an acid rain can have on life. It can furthermore disintegrate and corrode limestone and marble structures and landmarks, similar to tombstones.
Solutions Ahead:
There are a few answers for ceasing artificial acid rain. Controlling the emanations originating from vehicles and structures is an essential step. This should be possible by confining the utilization of petroleum derivatives and concentrating on progressively maintainable vitality sources, for example, solar energy and wind control.
Additionally, every individual can do their part by decreasing their vehicle use. Utilizing open transportation, strolling, riding a bicycle or carpooling is a small yet important step. Individuals can likewise decrease their utilization of power, which is broadly made with petroleum by-products, or change to a sunlight based arrangement. Numerous power organizations offer sun oriented packs to their clients that require no establishment and low expenses.
Acid Rain Instances in India:
It has been discovered that potential neutralizer of the acidic parts of rainwater in the Indian subcontinent is Calcium which is predominantly available from the soil. As the soil of the most part of Indian land is Calcareous, it contains a bounty of calcium. Taj Mahal, one of the most beautiful historical monuments has been affected by acid rain. The city of Agra has numerous enterprises which transmit the oxides of sulphur and nitrogen in the environment. Individuals keep on utilizing low-quality coal and kindling as household fuel, adding to this issue. There have hardly been any measures taken in order to have a check on this increasing pollution and industrialization in Agra. Acid rain caused in this region is due to the interaction of sulphuric acid with calcium carbonate resulting in the formation of calcium sulphate. This has led to the damage of the surface of Taj Mahal and it has lost its shine owing to this acid rain.
There are natural causes of acid rain which are beyond our control. However, there are enough causes which have been instigated by us and we have all the means in this world to control them. What is, however, required is our willingness to do so. Only if we decide today that we are going to stop or at least minimise the use of petroleum by-products and instead switch to cleaner fuels, we can prevent a lot of current environmental issues such as air pollution, water pollution and acid rain. It is for all of us to understand that we need to this not for plants and animals but for our own good and for the betterment of our future generations. Some small measures by all individuals along with some measures in place by the government can do wonders for our country. At least we can make it a better place to live for all.
Acid Rain , Air Pollution , Environment , Pollution
Get FREE Work-at-Home Job Leads Delivered Weekly!

Join more than 50,000 subscribers receiving regular updates! Plus, get a FREE copy of How to Make Money Blogging!
Message from Sophia!
Like this post? Don’t forget to share it!
Here are a few recommended articles for you to read next:
- Essay on Noise Pollution
- Essay on Environmental Pollution
- Essay on Water Pollution
- Essay on Deforestation
No comments yet.
Leave a reply click here to cancel reply..
You must be logged in to post a comment.
Billionaires
- Donald Trump
- Warren Buffett
- Email Address
- Free Stock Photos
- Keyword Research Tools
- URL Shortener Tools
- WordPress Theme
Book Summaries
- How To Win Friends
- Rich Dad Poor Dad
- The Code of the Extraordinary Mind
- The Luck Factor
- The Millionaire Fastlane
- The ONE Thing
- Think and Grow Rich
- 100 Million Dollar Business
- Business Ideas
Digital Marketing
- Mobile Addiction
- Social Media Addiction
- Computer Addiction
- Drug Addiction
- Internet Addiction
- TV Addiction
- Healthy Habits
- Morning Rituals
- Wake up Early
- Cholesterol
- Reducing Cholesterol
- Fat Loss Diet Plan
- Reducing Hair Fall
- Sleep Apnea
- Weight Loss
Internet Marketing
- Email Marketing
Law of Attraction
- Subconscious Mind
- Vision Board
- Visualization
Law of Vibration
- Professional Life
Motivational Speakers
- Bob Proctor
- Robert Kiyosaki
- Vivek Bindra
- Inner Peace
Productivity
- Not To-do List
- Project Management Software
- Negative Energies
Relationship
- Getting Back Your Ex
Self-help 21 and 14 Days Course
Self-improvement.
- Body Language
- Complainers
- Emotional Intelligence
- Personality
Social Media
- Project Management
- Anik Singal
- Baba Ramdev
- Dwayne Johnson
- Jackie Chan
- Leonardo DiCaprio
- Narendra Modi
- Nikola Tesla
- Sachin Tendulkar
- Sandeep Maheshwari
- Shaqir Hussyin
Website Development
Wisdom post, worlds most.
- Expensive Cars
Our Portals: Gulf Canada USA Italy Gulf UK
Privacy Overview
- Acid Rain Essay Introduction
Featured Example Essay
This featured Acid Rain Essay Introduction is one of many example essays available on this topic.
Sample Essay Examples

Related Essay Topics
- Essay On Acid Rain And Its Effects
- Acid Rain Essay
- Essay On Acid Rain
Acid Rain and Ozone Pollution Research Paper
Introduction, the nature of acid rain and ozone pollution, causes of acid rain and ozone pollution, effects of acid rain and ozone pollution, works cited.
Pollution entails the introduction of substances into the environment in quantities that can change environmental conditions and in turn, harm organisms. Acid rain and ozone pollution are a form of pollution, which entails the release of gaseous and dust particles in quantities that destroy the integrity of the atmosphere and affect organisms in their respective habitats and ecosystems.
Essentially, the atmosphere is an integral natural resource of the earth because it contains and maintains gases in appropriate proportions, which are essential for the survival of organisms in nature. In this case, the occurrence of acid rain and ozone pollution is due to the emission of gases in huge quantities, which have the capacity to pollute the air. Singh and Agrawal state that human activities such as the burning of fossil fuels and natural causes such as volcanic eruptions release nitrogen oxides, sulfur dioxides, and ozone, which are precursors of acid rain (15).
These oxides combine with atmospheric water and form acid rain. Aggarwal et al. state that the interaction of nitrogen oxides and volatile organic compounds contributes to the formation of terrestrial ozone, which is a pollutant responsible for global warming (1991). In this view, to enhance understanding of air pollution, the research paper examines the nature of acid rain and ozone pollution and subsequently discusses its causes and effects.
Acid rain is a form of pollution characterized by the presence of nitric acid and sulfuric acid in the rain, snow, hailstones, dew, and fog. The presence of nitrogen oxides (NOx) and sulfur dioxide (SO2) in the atmosphere leads to the formation of acid rain. According to National Atmospheric Deposition Program, carbon dioxide, oxygen, sunlight, ozone, and water catalyze the conversion of nitrogen oxides and sulfur dioxide into nitric acid and sulfuric acid, respectively (par. 2).
These acids then accumulate in the atmosphere and fall to the earth’s surface as rain, snow, dew, fog, and hailstones. The amount of nitric acid and sulfuric acid is proportional to the number of nitrogen oxides and sulfur dioxide that are present in the atmosphere (Singh and Agrawal 15). Hence, acid rain occurs when there are high proportions of nitrogen oxides and sulfur dioxide in the atmosphere.
Ozone pollution is a form of air pollution, which occurs when the amount of ozone (O3) increases in the atmosphere. Although ozone that is present in the stratospheric layer is important because it protects humans and organisms against harmful ultra-violet radiation, its presence in the tropospheric layer is harmful. Aggarwal et al. argue that the presence of ozone in the tropospheric layer constitutes pollution because it acts as particulate matter that scatters sunlight, promotes absorption of ultra-violet radiation, and causes global warming (1990). Hence, terrestrial ozone is a very harmful pollutant to humanity and organisms.
Human activities and natural processes are the cause of acid rain. The human activities that emit nitrogen oxides and sulfur dioxide are exhaust fumes from motor vehicles, industrial emissions from smelters and fossil fuels, and power stations that use fossil fuels (Singh and Agrawal 15). Given that exhaust fumes from motor vehicles and industrial emissions are common in urban centers, the emissions of nitrogen oxides and sulfur dioxides are very high.
National Atmospheric Deposition Program states that urban centers with high population density, automobile traffic, and industrial activities experience high levels of nitrogen oxides and sulfur dioxide emissions (par. 9). Natural sources of nitrogen oxides and sulfur dioxide are lightenings, oceans, and volcanic eruptions (Singh and Agrawal 15). However, these natural sources do not contribute significantly to acid rain.
An increased amount of terrestrial ozone occurs due to human activities, which release nitrogen oxides and hydrocarbons into the atmosphere. According to Aggarwal et al., motor vehicles, industries, and power plants burn fossil fuels and emit nitrogen oxides and hydrocarbons, which interact in the presence of ultra-violet radiation and lead to the formation of ozone (1990). The number of ozone peaks late in the afternoon after the emitted gases have absorbed enough heat to catalyze the formation of ozone.
Acid rain and ozone pollution have harmful effects on organisms because they have scorching effects on the leaves of plants. Given that acids have scorching effects, they destroy the integrity of the leaves and interfere with their functions. National Atmospheric Deposition Program states that acid damages leave and make them susceptible to environmental stresses and diseases (p. 12).
Singh and Agrawal also indicate that ozone damages leave by causing desiccation and changing coloration (1992). The damaged leaves lose their physiological functions of photosynthesis and cause plants to experience retardation in their growth and development. The ability to regulate the loss of water is lost; hence, predisposing plants to physiological drought. Moreover, the scorching effects of acids destroy the protective membranes of plants and make them susceptible to diseases.
Since organisms in the environment live within a narrow range of pH, acid rain causes a significant drop in the normal pH. Singh and Agrawal explain that acid rain causes acidification of water bodies and results in massive deaths of aquatic organisms such as fishes, amphibians, planktons, and microorganisms (18).
A slight change in aquatic pH has deleterious effects on organisms because it affects their biochemical and physiological processes. A normal aquatic environment has a pH of 6.5 or more, but a few organisms can survive at a pH of 5; however, none can survive on a pH of less than 5 (National Atmospheric Deposition Program par. 15). Therefore, acid rain has the potential to kill all aquatic organisms if it occurs on a large scale.
Acid rain also has a considerable impact on agriculture because it affects the availability of nutrients in the soil. National Atmospheric Deposition Program reports that acid rain lowers agricultural production by reducing soil nutrients, changing the proportion of chemicals in the soil, and killing important microbes in the soil (par. 16). Acid rain reduces soil nutrients because it dissolves and leaches them away. Singh and Agrawal’s state explain that acid rain reduces the pH of the soil and causes the liberation of cations such as potassium, magnesium, and calcium, which are important in the growth and development of plants (18).
When leaching occurs, the proportion of nutrients in the soil reduces, while the proportion of toxic heavy metal increases. A decrease in pH harms microbes in the soil, hence, reducing the rate at which important microbial processes occur in the soil. Ozone is a greenhouse house gas, which has the capacity to cause global warming and affect the distribution of rainfall patterns in various places globally. Aggarwal et al. assert that the increased concentration of terrestrial ozone contributes to global warming because it has a greenhouse effect.
Acid rain has harmful effects on humanity because it dissolves heavy metals and causes respiratory diseases. National Atmospheric Deposition Program states that acid rain pollutes water by dissolving lead and copper, which are harmful metals, and inhalation of acidic fog causes respiratory illnesses such as asthma (par. 19). Exposure to lead and copper causes mental and systemic illnesses. The elderly are prone to respiratory diseases owing to their aging respiratory system.
Despite the fact that ozone in the stratosphere is protective against ultra-violet radiation from the sun, its presence in the troposphere is harmful to humanity and organisms. Ozone pollution has harmful effects on humanity because long-term exposure increases the occurrence of asthma, skin diseases, and lung cancer among individuals (“Green Facts: Air Pollution” par. 3). Aggarwal et al. argue that the combined effect of ozone and carbon monoxide causes acid rain and subsequently contributes to the damage of lung tissue (1990). In this view, acid rain and ozone pollution are responsible for the increasing cases of lung cancer and asthma.
Given that acid has corroding effects, acid rain corrodes human structures. National Atmospheric Deposition Program reports that buildings, statues, monuments, vehicles, metallic structures, and tombstones corrode faster in acid rain than in normal rain (par. 21). In this case, acid rain hastens deterioration of human structures, and thus, reduces their longevity.
Acid rain and ozone pollution are the dominant forms of air pollution because they emanate from human activities. Emissions of nitrogen oxides and sulfur dioxide do not only lead to the formation of acid rain but also act as catalysts in the formation of ozone.
The combined effect of acid rain and ozone leads to the destruction of terrestrial plants, death of aquatic organisms, reduced agricultural production, the emergence of human diseases such as lung cancer, asthma, and skin diseases, and deterioration of human structures. Therefore, acid rain and ozone pollution are public and environmental health issues that require effective mitigation measures.
Aggarwal, Anjali, Reeta Kumari, Neeti Mehla, Rishi Singh, Sonal Bhatnagar, Kameshwar Sharma, Kuldeep Sharma, Amit Vashishtha, and Brijesh Rathi. “Depletion of the ozone layer and its consequences: A review.” American Journal of Plant Sciences 4.10 (2013): 1990-1997. Print.
Singh, Anita, and Madhoolika Agrawal. “Acid rain and its ecological consequences.” Journal of Environmental Biology 29.1(2008): 15-24. Print.
National Atmospheric Deposition Program. Acid Rain. 2014. Web.
Green Facts: Air Pollution Ozone 2015. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2020, May 4). Acid Rain and Ozone Pollution. https://ivypanda.com/essays/acid-rain-and-ozone-pollution/
"Acid Rain and Ozone Pollution." IvyPanda , 4 May 2020, ivypanda.com/essays/acid-rain-and-ozone-pollution/.
IvyPanda . (2020) 'Acid Rain and Ozone Pollution'. 4 May.
IvyPanda . 2020. "Acid Rain and Ozone Pollution." May 4, 2020. https://ivypanda.com/essays/acid-rain-and-ozone-pollution/.
1. IvyPanda . "Acid Rain and Ozone Pollution." May 4, 2020. https://ivypanda.com/essays/acid-rain-and-ozone-pollution/.
Bibliography
IvyPanda . "Acid Rain and Ozone Pollution." May 4, 2020. https://ivypanda.com/essays/acid-rain-and-ozone-pollution/.
- Factory Emissions Leading to Acid Rain
- Automobile Pollution in the US
- The effects of acid rain on soil PH
- Reducing Carbon Emissions in USA
- Economic Impact of Industrial Pollution in China
- Expanding the Bayway Refinery
- Sustainability Policies in Slums
- Environmental Impact Statement
An anionic metal-organic framework decorated with tartaric acid for enhanced adsorption of indium ions
- Liu, Shengquan
- Zhao, Xudong
Adsorption and recovery of indium (In) ions is highly important for the sustainable use of this valuable metal resource. This work reports the synthesis of a novel metal-organic framework (MOF) adsorbent, MOF-808-TA (TA, tartaric acid). The introduction of TA molecules was achieved through a post-synthesis exchange of TA and acetic acid that is initially in the clusters of MOF-808. MOF-808-TA exhibits a larger adsorption amount for In(III) ions (173.3 mg g ‑1 ) than the unmodified MOF-808 (57.5 mg g ‑1 ). Meanwhile, adsorption equilibrium time is 1–120 min at the concentration range of 0.2–40 mg L ‑1 . Further experiments show that MOF-808-TA owns still a high removal ratio for In(III) ions upon the presence of some metal ions. Moreover, MOF-808-TA can be well regenerated with a slight loss of adsorption capacity after five adsorption-desorption cycles. X-ray photoelectron spectrometry (XPS), Fourier transform infrared (FTIR) spectroscopy, and density functional theory (DFT) calculation unveil the synergic chelation contribution of the aliphatic hydroxyl and carboxyl groups from TA molecules. Also, the use of TA leads to the formation of the negative charge property of MOF-808-TA, which induces an electrostatic interaction towards cationic In(III) ions. This work indicates the high potential of MOF-808-TA for the removal of In(III) ions from aqueous solutions.
- Metal-organic framework;
- Indium ion;
- Adsorption;
- Tartaric acid

IMAGES
VIDEO
COMMENTS
Essay on Acid Rain. Acid Rain includes rain, snow, hail, fog, or dew that is high in acid pollutants, especially sulphuric and nitric acid. Acid Rain is mainly caused by emissions of sulphur dioxide and nitrogen oxide from various sources. They react with the water molecules in the atmosphere to produce acids.
The phrase acid rain was first used in 1852 by Scottish chemist Robert Angus Smith during his investigation of rainwater chemistry near industrial cities in England and Scotland.The phenomenon became an important part of his book Air and Rain: The Beginnings of a Chemical Climatology (1872). It was not until the late 1960s and early 1970s, however, that acid rain was recognized as a regional ...
Essay on Acid Rain. ... Introduction to Acid Rain. Acid rain, a term first coined in 1852 by Scottish chemist Robert Angus Smith, refers to precipitation with acidic components, such as sulfuric or nitric acid. This phenomenon results primarily from human activities, notably burning fossil fuels and industrial processes, releasing sulfur ...
The effects of acid rain on man are serious and mainly impact negatively the air we breathe, the soil and the water. Sulfur dioxide and Nitrogen oxide emissions are major causes of respiratory complications that include asthma, lung damage, dry coughs, headaches, and eye, nose and throat irritations.
Acid rain is any precipitation with a very low pH, whereas normal rain water is slightly acidic with a pH range of 5-6, but the pH level of rain water falls below this range, becoming acid rain.
Acid rain results when sulfur dioxide (SO 2) and nitrogen oxides (NO X) are emitted into the atmosphere and transported by wind and air currents.The SO 2 and NO X react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground. While a small portion of the SO 2 and NO X that cause acid rain is from ...
February 28, 2019. • 5 min read. Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. It can also occur in the form of snow, fog, and tiny bits ...
500 Words Essay on Acid Rain Introduction. Acid rain, a significant environmental issue, is a form of precipitation with elevated levels of hydrogen ions, thus having a low pH. It is primarily a result of human activities, particularly the burning of fossil fuels that release sulphur dioxide (SO2) and nitrogen oxides (NOx) into the atmosphere.
Acid rain is also known as acidic atmospheric deposition. Acid rain is formed when acid forming substances interact with ozone and water forming strong acids which are then transferred to the earth's surface. Sulfuric acid is a major component of acid rain. Sulfuric acid is formed following emission of sulfur from combustion.
Introduction Acid rain, a term denoting precipitation with acidic properties, primarily characterized by the presence of nitric and sulfuric acids, is a pervasive environmental concern that demands comprehensive analysis. This essay seeks to delve deeply into the various dimensions of acid rain, encompassing its formation,...
Figure 13-2 Regions of North America with low soil alkalinity to neutralize acid rain. In areas where the biosphere is sensitive to acid rain, there has been ample evidence of the negative effects of acid rain on freshwater ecosystems. Elevated acidity in a lake or river is directly harmful to fish because it corrodes the organic gill material ...
article describes how acid rain is formed, what the difference is between acid rain and acid deposition, and the effects of acid rain on nature and humans. The article also provides access to a nationwide network of acid rain monitoring stations which are updated weekly. The article gives a good overview of the basics of acid deposition.
When atmospheric pollutants like oxides of nitrogen and sulphur react with rainwater and come down with the rain, then this results in Acid Rain. Acid rain is made up of highly acidic water droplets due to air emissions.To study the causes, effects of Acid rain, along with a few examples and understand the prevention measures, FAQs. Visit BYJU'S to learn more about it
Acid rain, or acid deposition, is a broad term that includes any form of acid component precipitation, with a pH of 5.2 or below, such as sulfuric or nitric acid, which, in wet or dry forms, falls from the atmosphere to the ground. This includes acidic rain, snow, fog, hail etc. These components are mainly produced due to human activities ...
Introduction. Acid rain was one of the most important environmental issues during the last decades of the twentieth century. It became a game changer both scientifically and policy-wise. For some time, particularly during the 1980s, acid rain was by many considered to be one of the largest environmental threats of the time.
Essay # Introduction to Acid Rain: Acid rain is the term used to describe the deposition of acidic air pollution. Although some air pollutants fall directly back to Earth, a lot of it returns in rain, snow, sleet, hail, mist or fog, hence the term "acid rain". When power stations, factories, houses and cars emit pollution into the air, it ...
Acid rain damages trees by dissolving the calcium in the soil and in the leaves of trees. This hurts the tree because calcium is a mineral that trees need to grow. Once the calcium is dissolved, the rain washes it away so the trees and other plants cannot use it to grow. Acid rain washes other minerals and nutrients from the soil in a similar ...
Essay on Acid Rain - Essay 1 (150 Words) Introduction: Acid rain simply means rain high in acidic contents such as a high rate of hydrogen ions. Acid rain is a cause of concern because of its effect on forest life and lakes. To a larger extent, acid rain is a big threat to our natural environment.
Acid Rain; Acid Rain Essay Introduction; Featured Example Essay Three Georges Dam The United States, China and the Three Gorges Dam: Toward A Sounder Foreign Environmental Policy Yumiko Kojima, Kyoko Murai, Howard Pang, and Elena Vitale
Acid rain is a form of pollution characterized by the presence of nitric acid and sulfuric acid in the rain, snow, hailstones, dew, and fog. The presence of nitrogen oxides (NOx) and sulfur dioxide (SO2) in the atmosphere leads to the formation of acid rain. According to National Atmospheric Deposition Program, carbon dioxide, oxygen, sunlight ...
Acid rain is one of the big poblems that could affect negatveily on the human, animals, environment and even nonliving things like buildings. In general it is rain that Formed when gases, such as CO2 and SO2 react with the water in the atmosphere. It can kill micro-organisms, poisons plants, damages metals and limestone and kills fish.
Acid rain looks, feels, and tastes just like clean rain. The harm to people from acid . rain is not direct. Walking in acid rain, or even swimming in an acid lake, is no more . dangerous than walking or swimming in clean water. However, the pollutants that cause acid rain sulfur dioxide (SO2) and nitrogen oxides (NOx) do damage human health.
Effects of acid rain. Acid rain causes a variety of changes in the ecosystems and man-made structures. It alters the chemical composition of soil, causes erratic growth of plants, and acidifies aquatic ecosystems. The effects are either direct or indirect. Direct effects include those that cause changes by themselves like alteration of soil ...
Adsorption and recovery of indium (In) ions is highly important for the sustainable use of this valuable metal resource. This work reports the synthesis of a novel metal-organic framework (MOF) adsorbent, MOF-808-TA (TA, tartaric acid). The introduction of TA molecules was achieved through a post-synthesis exchange of TA and acetic acid that is initially in the clusters of MOF-808. MOF-808-TA ...