An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.9(6); 2023 Jun
- PMC10320272


Advances in drug delivery systems, challenges and future directions
Tobechukwu christian ezike.
a Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria, Nsukka, 410001, Enugu State, Nigeria
b Department of Genetics and Biotechnology, Faculty of Biological Sciences, University of Nigeria, Nsukka, 410001, Enugu State, Nigeria
Ugochukwu Solomon Okpala
Ufedo lovet onoja, chinenye princess nwike, emmanuel chimeh ezeako, osinachi juliet okpara, charles chinkwere okoroafor, shadrach chinecherem eze.
c Department of Clinical Pharmacy and Pharmacy Management, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, 410001, Enugu State, Nigeria
Onyinyechi Loveth Kalu
Evaristus chinonso odoh.
d Department of Pharmacy, Federal Medical Center Bida, Niger State, Nigeria
Ugochukwu Gideon Nwadike
John onyebuchi ogbodo.
e Department of Science Laboratory Technology, Faculty of Physical Sciences, University of Nigeria, Nsukka, 410001, Enugu State, Nigeria
Bravo Udochukwu Umeh
Emmanuel chekwube ossai, bennett chima nwanguma, associated data.
No data was used for the research described in the article.
Advances in molecular pharmacology and an improved understanding of the mechanism of most diseases have created the need to specifically target the cells involved in the initiation and progression of diseases. This is especially true for most life-threatening diseases requiring therapeutic agents which have numerous side effects, thus requiring accurate tissue targeting to minimize systemic exposure. Recent drug delivery systems (DDS) are formulated using advanced technology to accelerate systemic drug delivery to the specific target site, maximizing therapeutic efficacy and minimizing off-target accumulation in the body. As a result, they play an important role in disease management and treatment. Recent DDS offer greater advantages when compared to conventional drug delivery systems due to their enhanced performance, automation, precision, and efficacy. They are made of nanomaterials or miniaturized devices with multifunctional components that are biocompatible, biodegradable, and have high viscoelasticity with an extended circulating half-life. This review, therefore, provides a comprehensive insight into the history and technological advancement of drug delivery systems. It updates the most recent drug delivery systems, their therapeutic applications, challenges associated with their use, and future directions for improved performance and use.
1. Introduction
Drug delivery systems are technological systems that formulate and store drug molecules into suitable forms like tablets or solutions for administration. They hasten the reach of drugs to the specific targeted site in the body, thereby maximizing therapeutic efficacy and minimizing off-target accumulation in the body [ 1 , 2 ]. Drugs have various routes through which they can be introduced into the body, they include but are not limited to the oral route of administration [ 3 , 4 ], buccal and sublingual routes of administration [ 5 ], nasal and ophthalmic [ 6 , 7 ], transdermal and subcutaneous [ 8 , 9 ], anal and transvaginal [ 10 , 11 ] and intravesical [ 12 , 13 ]. The components of the drug account for its physiochemical properties and are responsible for the changes it influences in the body system when taken.
Over the past few decades, DDS have been applied effectively in the treatment of diseases and improvement of health due to increased systemic circulation and control of the pharmacological effect of the drug. The advancement of pharmacology and pharmacokinetics showed the importance of drug release in determining therapeutic effectiveness, giving rise to the concept of controlled release [ 14 ]. The controlled-release formulation of a drug was first approved in the 1950s, and it has since attracted considerable attention due to its significant advantages over conventional drugs. It releases drugs at a predetermined rate and for a specific period of time. In addition, controlled drug delivery systems are not affected by physiological conditions and can thus last for days to years. It also provides spatial control over drug release, with constant or variable release rates [ 15 ]. Furthermore, it improves drug solubility, target site accumulation, efficacy, pharmacological activity, pharmacokinetic properties, patient acceptance, and compliance, and reduces drug toxicity [ 2 ].
Recently, several drug delivery systems (NDDS) have been developed using advanced systems for more convenient, controlled, and targeted delivery. Each drug delivery system has its own peculiarities that determine its release rate and mechanism. This is largely due to the differences in the physical, chemical, and morphological characteristics which will ultimately affect their affinities for various drug substances [ 16 ]. Studies on these have identified diffusion, chemical reaction, solvent reaction, and stimuli control as major release mechanisms [ 17 , 18 ]. For instance, since most cancer cells can proliferate the porous blood vessels and lymphatic system, the drug can easily permeate through this opening to reach the target tissues. This is referred to as Enhanced Permeability and Retention (EPR) [ 19 ]. EPR is a passive diffusion mechanism well researched and applied in the delivery of many chemotherapeutic agents. Although EPR is a controversial concept, this effect has been observed by many researchers in various types of human tumors and is the basis for the use of nanomedicine in cancer treatment. Though it has a drawback of lack of selectivity and increased toxicity [ 20 ]. Active targeting overcomes the lack of specificity and selectivity found in passive targeting. It involves attaching to the carriers, certain ligands, and molecules that can actively bind to the surface of target tissues. This prevents uptake by non-target cells thereby reducing side effects and toxicity [ 21 , 22 ]. Selectivity of ligands to target cells, immunogenicity, and chances of lysosomal degradation after macrophage endocytosis still pose solid challenges to the full development of actively targeting Drugs [ 23 ]. These delivery systems can also reach the target cells through the control of one or more physical or chemical properties in the process of responsive stimuli targeting [ 24 , 25 ]. These physical properties include pH, temperature, ultrasound, magnetic and electric field.
2. The early period of drug delivery systems
In the ancient period, people depended on medicinal plants. Although they were beneficial, they lacked consistency, homogeneity, and specificity in drug delivery [ 26 ]. Before the use of controlled drug delivery, all pharmaceuticals were produced and stored in pill or capsule formulations. It is dissolved when it comes in contact with gastrointestinal fluids, permeates the gut wall, and is then absorbed into the bloodstream through blood capillaries. There was no capacity to control the drug release kinetics. With the aim to hide the bitter taste of drugs, Rhazes and Avicenna, introduced coated technology. This coating method altered the rate of release of the drug itself. It was adopted in the 10th century however in the form of gold, silver, and pearl-coated tablets.
In the 20th century, advanced coating technology with keratin, shellac, sugar, enteric coating, and pearl coating, was also introduced, but keratin and shellac were ineffective due to storage instability and high pH for adequate dissolution in the small intestine. Malm et al. [ 27 ] introduced an enteric-coating material with polymeric cellulose acetate phthalate that is dissolved at a very weak alkaline pH, like that of the small intestine, which made it highly suitable to be applied for enteric controlled release.
The first generation was extremely productive, it focused on the development of numerous oral and transdermal controlled-release formulations for clinical use and the establishment of controlled drug-release mechanisms. In 1951, Lipowski first introduced a patent oral sustained-release formulation, when he coated pills with enteric polymers (like beads) such that the drug and coat were layered alternatively, resulting in slow release of the drug, regularly, and periodically [ 28 ]. This was further developed by Smith, Klein Beecham, and French (SKF) in 1952, they developed Spansule technology, an oral predetermined-release formulation that sustains and controls the kinetic release of a drug gradually [ 29 ]. This formula is composed of hundreds of micro pellets drug loaded beads with variable layers of natural water-soluble wax with dissimilar thicknesses on individual pellets. On ingestion, the outer capsule rapidly disintegrates, the waxy coating around the beads gradually dissolves as they transit down the GI tract, and liberates the drug-loaded beads. This improved patient compliance and convenience by reducing the dosing schedule, resulting in great popularity [ 30 ]. This technology was further developed by replacing the wax with more reproducible synthetic polymers [ 31 ].
In 1955, the first nanoparticle therapeutic was reported by Jatzkewitz when he prepared the first polymer-drug conjugate. In the 1960s, the first nanotechnology known as liposome (lipid vesicles) was discovered [ 32 , 33 ]. Polymer-drug conjugates and liposomes mark the birth of nanocarriers. In this decade, the ALZA Corporation did not create drugs they specialized in targeting and controlling the release of drugs at the right place and time [ 34 ]. In 1972, Scheffel and his colleague prepared the first protein-based microspheres. In 1976, “micelle” and “emulsion” polymerization techniques were used to prepare drug-loaded nanoparticles and microcapsules by Peter Paul Speiser's research group [ 35 ]. In 1977, Couvreur et al. [ 36 ] reported the lysosomotropic effects of the nanoparticles, and they produced the first rapidly biodegradable acrylic nanoparticles.
The drug delivery formulations developed during the second generation (2G) were impressive, but they did not produce the expected clinical results [ 37 ]. The researchers were interested in developing drug delivery systems with constant drug release rates, self-regulating, long-term depot formulations, and nanotechnology-based formulations, particularly nanoparticle formulations. In this era, long-term depot-sustained drug-release formulations of peptide/protein drugs were developed [ 38 ]. In addition, smart polymers and hydrogels were developed to stabilise drug delivery systems that are affected by physiological changes such as pH, temperature, electric field, and glucose. Furthermore, efforts were made to develop targeted nanotechnology DDS for tumors and gene delivery using biodegradable polymers in nanoparticle structures such as polymeric micelles, chitosan, lipids, and dendrimers. The idea was to modify the nanoparticles so that they could be administered directly into the body for increased drug accumulation at the site of action. Although this nanotechnology-based DDS demonstrated high efficacy in controlling tumor growth in animal models, the FDA only approved a few drugs [ 31 , 39 ].
The third generation of drug delivery systems is the modern era of controlled release technology. For it to be beneficial and successful, it has to overcome the hurdles of both physicochemical and biological barriers, associated with the earlier drug delivery systems. The physiochemical challenges are caused by poor water solubility, the high molecular weight of therapeutic proteins and peptides, and the difficulty in achieving targeted and controlled drug release, whereas the biological barrier challenges are associated with systemic drug distribution issues [ 31 , 40 ]. Many new drug delivery systems must be developed during this time period to meet the challenges associated with earlier forms of drug delivery in order to improve performance and sustainability. However, designing a suitable carrier system is often very difficult due to the challenges associated with targeting a drug to a specific site and continuous release over a specified period of time.
3. Recent drug delivery systems and applications
Significant progress has been made in recent years toward the successful development of drug delivery systems based on organic, inorganic, and hybrid nanoparticles as drug carriers for active targeting, particularly in chemotherapy. Recent drug delivery systems (DDS) are formulated with improved properties such as smaller particle size, increased permeability, increased solubility, efficacy, specific site targeting, stability, toxicity, and sustained delivery. They can significantly improve therapeutic agent performance over conventional dosage forms [ 19 , 41 ].
In the development of an optimal drug delivery system, recent drug delivery systems are recognized to be the newest developments and innovative understanding of the pharmacokinetic and pharmacodynamic behavior of pharmaceuticals. Because these DDS are transporters, they can keep medication concentrations in the therapeutic range for a long time while also delivering material to the site of action. The adoption of the delivery mechanism is directly tied to the commercial and therapeutic success of the innovation. This would entail involving patients early in the development process, recognizing any problems, and ensuring they receive the most out of the device. Improving delivery systems that reduce toxicity while increasing efficacy. The different types of drug delivery systems are depicted in Fig. 1 .

Several types of recent drug delivery systems for different therapeutic purposes.
3.1. Red blood cell membrane-camouflaged nanoparticles drug delivery system
Researchers have recognized the potential benefits of nanotechnology in vastly improving medicine delivery methods throughout time. Red blood cell membrane-camouflaged nanoparticles are a new class of drug delivery systems. The nature and biological significance of red blood cells (RBCs) allow for their use as an efficient system as a nanoparticle camouflaging material [ 42 ]. Because red blood cells (RBCs) are the most abundant circulating cells in the body, their biocompatibility (non-immunogenic), biodegradability, and extended circulating half-life, making them an ideal vehicle for drug delivery [ 43 ]. Engineered RBCs have been investigated and found to be an excellent carriers for a variety of bioactive chemicals, including enzymes, medications, proteins, and macromolecules [ 44 ]. Because of their abundance, red blood cell membranes serve as a “camouflage,” allowing nanoparticles to combine the benefits of native red blood cell membranes with those of the nanomaterial. Several strategies have been developed to load therapeutic agents onto RBCs without comprising the structure and the physiological function of RBCs. The coated nanoparticles will mimic RBCs and interact with the environment to establish long systemic circulation when injected. Sonication is the most common method for creating RBC camouflaged nanoparticles. Other methods of RBC fusion with nanoparticles include in-situ polymerization, microfluidic electroporation, and extrusion. However, each has advantages and disadvantages in terms of synthesis, scale-up challenges, reproducibility, and the nature of the final product [ 42 ]. Prior to the fusion, the RBC membrane-derived vesicle is obtained through hypotonic treatment (dialysis, hemolysis or dilutions) of fresh whole blood from an organism. The hypotonic treatment will help to remove unwanted cells and plasma ( Fig. 2 ).

Synthetic strategies for RBCM-NPs preparation for antitumor activity. Whole fresh blood is centrifuged and washed multiple times to remove the plasma and other unwanted cells. The resulting pure red blood cells are subjected to hypotonic hemolysis and are used to coat selected nanoparticles which are intravenously injected into the blood to maintain long systemic circulation. The RBCM-NPs permeate the tumor tissue via the EPR effect and finally enter into the tumor cells by endocytosis for therapeutic effect. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The use of RBCM-NPs drug delivery systems is extremely promising and offers numerous benefits due to their low immunogenicity and ability to maintain long systemic circulation (a lifespan of 120 days). Furthermore, because of the large number of cell membranes, RBC vesicles are inherently biocompatible and biodegradable, and can easily achieve high load capacity, resulting in higher accumulation at the target site. Remarkably, erythrocyte membrane-coated nano-formulations have been extensively applied in anticancer research to substantial accomplishment [ 45 , 46 ], cardiovascular diseases [ 47 ], and encephalopathy [ 48 ].
3.2. Hyaluronic acid-based drug nanocarriers drug delivery systems
The usage of hyaluronic acid is one of the drug delivery techniques. Hyaluronic acid is a novel polymer that can be used to make medication delivery systems [ 49 ]. It has a linear macromolecular mucopolysaccharide made up of proportionately connected glucuronic acid and N -acetylglucosamine saccharide units [ 50 ]. It exhibits biocompatibility, biodegradability, and high viscoelasticity, and it can be coupled with a specific cell surface receptor [ 51 ]. Because Hyaluronic acid is a natural component of eye tissue and plays an important function in wound healing, it makes sense to use it as a carrier for ocular drug delivery as long as the integrated pharmaceuticals are released consistently. They aid in the thickening, sustained release, and transdermal absorption of drugs, as well as improving drug targeting. Drug distribution to cancer cells was significantly improved using active targeted HA-based drug nanocarriers. In addition, lipid nanoparticles with an appropriate HA coating have been developed as biocompatible drug carriers with a great potential for targeted drug delivery to the target tissue while minimizing side effects and harming other tissues. Benefits of utilizing HA-based nanocarriers for cancers with elevated expression of the CD44 receptor include improved drug delivery, increased therapeutic efficacy, higher cytotoxicity, and considerable reduction of tumor development, as well as a high potential for targeted chemotherapy [ 52 ].
Another application is when an HA-based nanocarrier is combined with doxorubicin (DOX) and cisplatin (CDDP) and manufactured as a CD44-targeting anti-cancer drug delivery system, as well as its tumor inhibition activities in vitro and in vivo against CD44 + breast cancer cells. In 4T1 (CD44 + ) breast cancer cells, these dual drug-loaded HA micelles (HA-DOX-CDDP) showed significantly improved drug release under acidic circumstances, as well as higher cellular uptake and stronger cellular growth suppression than free medicines. HA-DOX-CDDP micelles are a potential drug delivery system with acid-sensitive drug release, CD44-targeted delivery, and high biocompatibility and biodegradability. These characteristics resulted in excellent tumor accumulation and less side effects, indicating that HA-DOX-CDDP micelles could be useful in breast cancer chemotherapy [ 53 , 54 ].
Hyaluronic acid and its derivatives are incorporated into various drug delivery systems (DDS) such as nanoparticle DDS, cationic polymer DDS, and gel DDS for active targeting of cancer cell CD44 receptors ( Fig. 3 ). According to studies, the use of HA and drug conjugates after administration aggregates at the tumor site, where it maintains sustained drug release. The surface of HA-based nanocarriers is generally negative, which helps in blocking the systemic clearance of nanocarriers by the reticuloendothelial system (RES). The Hyaluronic acid-based drug nanocarriers NDDs selectively enter into the cancer cells through the EPR effect and active targeting of CD44 receptors [ 55 , 56 ].

The application of hyaluronic acid-based nanocarriers in cancer treatment. (a) and (b) Direct conjugation of cytotoxic drug with HA or hydrophobic moiety results in self-assembly of nanoparticles (NPs) that can be administered intravenously for cancer cell targeting; (b) HA hydrogel formation using a cationic polymer; (c) Surface coating of NP with HA; (e) Hyaluronic acid-based drug nanocarriers permeate cancerous tissues via EPR effect and binds to the CD44 receptor site to elicit anticancer activity.
3.3. Hexagonal Boron Nitride nanosheet drug delivery system
As technology continues to advance and science evolves, more and more materials are being researched and studied to help improve drug delivery. Among these materials is Boron nitride (BN) which is a crystalline material with a balanced stoichiometry of nitrogen (N) and boron (B) atoms. This material occurs in various forms such as cubic BN (c-BN), hexagonal BN (h-BN), wurtzite BN (w-BN), and rhombohedral BN (r-BN). Hexagonal Boron Nitride is a two-dimensional (2D) layered-dense structure with sp2 hybridized B–N bonds. It can also be called white graphene sometimes regarded as an analogue of graphite [ 57 ]. The B–N atoms substitute the carbon atoms and are held together by a strong covalent bond forming interlocking rings. The layers of the compound are held together by van der Waals forces with a bond length of 1.466 Å and an interlayer space of 3.331 Å. This compound is partially ionic and as a result of this unique characteristic has its B–N bonds to be polar. H-BN is an insulator that has gained wide applications in various fields of life such as cosmetics, dental, cement, ceramics, and most especially in medicine as a drug carrier similar to graphene or graphene oxide [ 58 ].
Hexagonal Boron Nitride has been proven to be useful in drug research and delivery systems ( Fig. 4 ). The research by Jedrzejczak-Silicka and her colleagues reported a reduction in the proliferation of MCF-7 cell line cultures when compared with the normal L929 cell lines upon exposure to H-BN loaded with gold particles. H-BN was exfoliated via chemical treatment using modified Hummers' method, sonication treatment, and was finally functionalized with gold particles for the studies and analyzed using Neutral Red (NR) uptake assay [ 59 ]. In another study, H-BN nanosheets were conferred photothermal properties as a result of the in-situ deposition of Pd on its surface. This made the compound have a high loading capacity for doxorubicin which is an anticancer drug as well as functions effectively as a drug delivery carrier. The study reported a remarkable inhibition of tumor growth as the drug was administered in mice for two weeks. This was possible as a result of the decrease in pH which led to the release of doxorubicin from the nanohybrids and a concomitant increase in glutathione concentration as well as near infrared radiation (NIR) [ 60 ]. Another successful study showed that H-BN conjugated with DNA oligonucleotide and copper (II) phthalocyanine (CuPc) was effective as a therapeutic agent in photodynamic therapy (PDT) and in situ monitoring and miR-21 imaging [ 57 ]. Boron compound is being recognized today as an effective chemotherapeutic agent.

Schematic explanation of H-BN nanosheet drug delivery system. H-BN was exfoliated via chemical treatment and functionalized with Au particles resulting in high loading capacity of DOX, a chemotherapy drug which is released in tumor cells by decrease in pH.
3.4. Polymer-lipid hybrid nanoparticles drug delivery system
Nanocarriers are gaining wide usage as drug delivery systems because of their increased stability in storage, improved targeting ability on disease cells, sustained drug release, and higher encapsulation ability [ 61 ]. Amongst the widely accepted nanoparticles being used today for drug delivery, liposomes and polymeric nanoparticles are the most widely accepted. Liposomes which is a lipid-based nanoparticles though showed excellent biocompatibility still suffered drug leakage and instability upon storage while the polymeric nanoparticle which is a polymer-based nanoparticle was able to curb this limitation by exhibiting high encapsulation/drug loading ability as well as stability. However, it had its own shortfalls in that it showed lower biocompatibility [ 62 , 63 ]. In order to overcome these shortcomings and obtain an effective nanomaterial, researchers sought and developed a hybrid system that will combine the unique properties of the two classes of nanoparticles, which is known as Polymer-lipid hybrid nanoparticles (plhnps). This hybrid system was able to satisfy the requirements of biocompatibility, high storage stability, sustained drug release, minimal drug leakage, small particle size, and high encapsulation [ 64 ]. As a result of its efficacy, this system is being used today for different therapeutic purposes as well as diagnostic applications. Plhnps is made up of three distinct components which include: A polymeric core that encapsulates both hydrophilic and hydrophobic drugs effectively. This is possible as a result of the hydrophilic and hydrophobic nature of the core and results in a high sustained release, a lipid shell that provides biocompatibility and high stability and a lipid-polyethylene glycol (PEG) that is found in the outer part and covered by a lipid layer to provide increased steric stability, prevent immune recognition and increase time for circulation ( Fig. 5 ). Plhnps has wide applications such as in the delivery of various chemotherapeutic agents, in gene transfer (sirna, DNA) and in photothermal, photodynamic therapy and ultrasound. Studies have shown that they can be used in the delivery of vaccines and immune activation as well as in imaging and alternative magnetic field (AMF). Hence its wide application in the fast-growing medical environment [ 65 ].

The formation of a Polymeric-Lipid Hybrid Nanoparticle. The Hybrid contains three distinct components which include: A polymeric core that encapsulates both hydrophilic and hydrophobic drugs effectively. A lipid shell that provides biocompatibility and high stability and a lipid-polyethylene glycol (PEG) that is found in the outer part and covered by a lipid layer to provide increased steric stability, prevent immune recognition and increase time for circulation.
3.5. Self-microemulsifying drug-delivery system
Recently, lipid-based drug preparations have received a lot of interest, with a specific focus on self-microemulsifying drug-delivery systems (SMEDDS) [ 66 ]. Inadequate bioavailability is one of the most difficult aspects of developing oral dosage forms of drugs [ 67 ]. Accordingly, minimal hydrophilicity is an essential factor for bioavailability in this context, because drugs cannot be absorbed via the gastrointestinal tract (GIT) except it exists in solution forms [ 68 ]. Aqueous solubility is a problem for many chemical compounds having notable and favorable pharmacological effects [ 69 ]. Furthermore, almost 30% of widely marketed medicinal entities and nearly 50% of innovative drug compounds accessible for product manufacture are hydrophobic in nature, meaning they have low water solubility [ 70 ]. The utilization of a lipid-based carrier system to boost the bioavailability of less water-soluble medications has grown in popularity in recent years [ 71 ]. The main rationale behind this formulation is to sustain the hydrophobic components in solution all through the digestive system [ 70 ].
Lipid-based carriers come in a variety of forms, including suspensions, dry emulsions, microemulsions, and self-emulsifying drug-delivery systems (SEDDS) [ 72 ]. SEDDS' ability to incorporate hydrophobic drugs was previously reported. SEDDS has also been revised as self-microemulsifying drug-delivery systems (SMEDDS) and self-nanoemulsifying drug-delivery systems (SNEDDS). Emulsions, on the other hand, are created by dispersing a liquid phase containing macroscopic particles in a different liquid phase composed of surfactant [ 73 ]. They are also thermodynamically unstable solution that is semitransparent (occasionally hazy) and has properties that resemble viscous liquids [ 74 ]. Emulsions are of three types, viz; water-in-oil, oil-in-water, and multiple emulsions. Additionally, conventional micro- or nanoemulsions differ from SMEDDS, in that following oral ingestion, they self-emulsify [ 75 ].
Surfactants (S) and Co-surfactants (CoSs) are the two types of emulsifying agents used in microemulsions ( Fig. 6 ). However, surfactant is predominantly soluble in water, while CoS is mostly soluble in the oil phase. CoSs are essential for lowering the tension that exists between the two liquid phases to the optimal level required for the formation of a microemulsion [ 76 , 77 ]. The production of nanoemulsions with droplet sizes smaller than 100 nm, on the other hand, requires either mechanical or chemical energy [ 78 ]. Although nanoemulsions are classified as kinetically stable due to their extremely low destabilization rate, their long-duration stability (in months) is notable [ 79 ]. Consequently, nanoemulsion globules are shown to be stable across a variety of situations, including varied dilutions and temperatures, while microemulsions are mostly impacted by factors including dilutions and temperature [ 74 , 80 ]. The key distinctions between SMEDDS, SNEDDS, and SEDDS are shown in Table 1 .

Mechanism of Self-emulsification in aqueous environment. SEDDS comprise a mixture drugs, surfactants, oil, stabilizers, and cosolvents. Like conventional emulsification, SEDDS (ionotropic mixture) form (o/w) nano or microemulsion within the gastrointestinal tract with very small energy input.
Four basic types of lipid-based drug-delivery systems (LBDDS) with their merits and demerits.
HLB=Hydrophilic/lipophilic balance; LFCS = lipid formulation classification system; SEDDS = self-emulsifying drug-delivery systems; SMEDDS = self-microemulsifying drug-delivery systems; SNEDDS = self-nanoemulsifying drug-delivery system [ 70 ] (Rajpoot, Kuldeep 2020).
3.6. In-situ gel drug delivery system
The foremost goal of any drug delivery system is to change the drug's pharmacokinetic characteristics and tissue distribution in a meaningful way [ 81 ]. Over the last 60 years, there has been a lot of focus on developing controlled and well reliable drug delivery systems [ 82 ]. In-situ gel medication administration has emerged as one of the most innovative drug delivery systems. By virtue of its unique property of transitioning from Sol to Gel, the in-situ gel drug delivery system aids in the prolonged and regulated release of medications, as well as increased patient compliance and comfort [ 83 ]. In most case, formulations in solution form become transformed into gel form under certain physiological conditions before it enters the body [ 84 ]. A variety of stimuli, such as pH change, temperature modulation, and solvent exchange, combine to transform a solution into a gel form [ 85 ]. Oral, nasal, injectable, vaginal, rectal ocular, intraperitoneal, and parenteral routes have all been used in various research. Many polymeric methods capable of delivering pharmaceuticals have been created [ 86 ]. When these polymers come into touch with physiological stimuli, they go through a sol-gel transition. In situ gel drug delivery systems are made from a variety of natural and synthetic polymers [ 83 ]. Four processes are known to produce the formation of in-situ gel biomaterials, viz; (1) temperature and pH variations, (2) variations of the physical properties of the biomaterials such as solvent exchange and swelling, (3) Biochemical modification such as enzymatic and chemical reactions, and (4) photo-polymerization [ 87 ].
3.6.1. Applications of in-situ gel delivery system
3.6.1.1. oral in-situ gel delivery systems.
The application of pH-sensitive hydrogels to particular parts of the gastrointestinal tract for site-specific medicine delivery is the focus of this approach. With different amounts of Polyacrylic acid (PAA) derivatives and cross-linked Polyethylene glycol (PEG), silicone microsphere hydrogels that liberate prednisolone into the stomach media or exhibit gastroprotective properties have been produced. A possible colon-specific medicine delivery approach for decreasing edema at high pH has been developed using dextran hydrogels cross-linked with polysaccharides such as guar gum, inulin, and amidade pectin. Researchers produced gellan gum and sodium alginate formulations that utilized calcium ions as complexing agents and were gelatinized by releasing these ions into the stomach's acidic medium. The oral in situ gel distribution procedures employ natural polymers such as xyloglucan, pectin, and gellan gum. The pectin-based formulation was created to ensure that Metformin loaded pectin (PCM) was distributed continuously. Because pectin is water soluble, an organic solvent is not required [ 88 , 89 ].
3.6.1.2. Opthalmic in-situ gelling systems
Gellanic gum, alginic acid, and xyloglucan are examples of natural polymers employed in the ocular delivery system. Several combinations of anti-inflammatory, antibacterial, and autonomic medicines are utilized to reduce intraocular glaucoma stress in the local ophthalmic administration approach. Tear fluid has been devised to overcome the ocular in-situ gel bioavailability problem due to its rapid turnover and dynamics. Traditional delivery methods also result in limited availability and therapeutic response, making it easier to remove the medication from the eye. In ocular preparations, viscosity enhancers such as Carboxymethyl Cellulose, Polyvinyl alcohol, Carbomers, and Hydroxypropylmethyl cellulose are deployed to improve the viscosity of the drug formulations, resulting in enhanced bioavailability and precorneal residence duration. Chelating agents' penetration enhancers are utilized to promote the infiltration of corneal substances such as surfactants and preservers [ 90 , 91 ].
3.6.1.3. Nasal in-situ gelling systems
In nasal in-situ production, polymers such as gum gelan and gum xanthan are employed. The efficacy of Momethasone furoate as an in situ gel in the management of allergic rhinitis has been studied. The impact of in-situ gel on antigen-induced nasal symptoms was shown in vivo using sensitized rats as a model of allergic rhinitis. In situ gel was proven to resist an increase in nasal complications when compared to the commercialized nosonexex preparation [ 92 , 93 ].
3.6.1.4. Rectal in-situ gelling systems
This method can be used to prescribe a variety of pharmaceuticals that are packed as liquid, semi-solid (liniments, emulsions, and froths), or suppositories in solid administration formulations. During penetration, traditional suppositories can cause discomfort. Furthermore, because suppositories cannot be effectively kept at a single rectum site, they can migrate upward into the gut, allowing the drug's first-pass impact to be experienced. Indomethacin-loaded xyloglucan-based device administration to rabbits revealed a substantial medication immersion and a prolonged drug residence period when compared to commercial suppository administration [ 94 ].
3.6.1.5. Vaginal in-situ gelling systems
The vaginal canal is a possible route for medication delivery. A delivery system based on a thermoplastic graft copolymer undergoing in situ gelation has been formulated for the continuous liberation of active substances such as estrogens, peptides, progestins, and proteins. In a recent study, clotrimazole medication antifungal efficiency was boosted and sustained using a mix of poloxamers and polycarbophils mucoadhesive thermosensitive gels in contrast to conformist polyethylene glycol-based formulations [ 95 ].
3.6.1.6. Injectable in-situ gelling system
Formulating dose forms like injectable or implanted delivery systems is one of the only obvious ways to give drugs for prolonged release. Thermo-reversible gels, typically made of poloxamers, are the most commonly utilized. These dosage forms might be useful in the manufacture of controlled drug delivery for systemic absorption. Pluronic F127 gels with insulin or insulin-PLGA nanoparticles have been tested. Poloxamer gels have also been used to subcutaneously and intramuscularly deliver human growth hormone as well as to generate a single-dose lidocaine injectable with a longer duration of action. Pluronics recently developed a new type of depot protein injectables controlled release formulation made up of blends of poly (D, l -lactide)/1-methyl-2-pyrrolidone solutions. At a temperature of roughly 45 °C, the hydrogel develops into a gel after being injected subcutaneously, and fast body temperature cooling was achieved by employing biodegradable polymers of poly (ethylene oxide) and poly (propylene oxide) ( l -lactic acid). The injectable drug delivery method is utilized to cross-link pluronic acid-modified hydrazide with aldehyde-modified cellulose derivatives such as hydroxypropylmethyl cellulose, carboxymethyl cellulose, and methylcellulose, among others. In order to avoid postoperative peritoneal adhesion, this in-situ forming gel has been used to reduce pelvic discomfort, bowel blockage, and infertility [ 96 , 97 ].
3.7. Micro electro mechanical systems (MEMS) for drug delivery
MEMS technology has vast applications in fields such as actuators, drug delivery, motion sensing, accelerometers, and inkjet printing [ 98 ]. The devices produced through this technology incorporate microfabrication techniques to produce micro/nano-sized electromechanical and mechanical devices or implants [ 99 ]. Interestingly, the use of these techniques enhances the efficacy of these devices by allowing considerable control over their topography, microarchitecture, and size of the resulting devices [ 100 ].
Among the numerous materials and processes available for designing these MEMS-based devices, the most commonly used, incorporate creative blends of varying micromachining techniques such as; deposition (an addictive process), etching (a subtractive process), lithography (a patterning process), ink jetting, ion implantation, oxidation, and micromolding [ [101] , [102] , [103] , [104] ]. As drug delivery systems, MEMS technology fabricates miniaturized systems comprising of various materials such as silicon, glass, metals, and nitrides, as well as polymers, micropumps, sensors, microvalves, reservoirs, actuators, and high-performance processors [ 100 , 103 , 105 , 106 ]. These distinct components function synergistically, to provide the broadly reported multi-functionality and precision of MEMS devices, relative to other conventional drug delivery systems. Each of these features works strategically, for instance; actuators mostly play a vital role in the drug release process, as it pressurizes the drug reservoir to facilitate drug release [ 103 ]. Reservoirs provide port(s) to house the drug(s) and can be singular or multiple [ 107 ]. Single reservoir architecture comprises a relatively large port that can contain a single drug. It is thus able to contain a relatively larger amount of drug and is also suited for long-term usage as it can be refilled. On the other hand, multi-reservoirs comprise different ports (within the same substrate) that separately store drugs, and as such different drugs can be incorporated into these. However, they are less suitable for long-term usage as they would require repetitive replacement surgeries, due to a lack of refilling methods. Moreover, microvalves are employed to control fluid flow rate, sealing, as well as switching on/off of the delivery device [ 108 ]. Silicon is popularly used as a substrate or structural material during fabrication, due to its favorable mechanical and electrical properties [ 109 ]. Sensors, on their part, utilize electrical radiation, mechanical, thermal, magnetic, or biochemical mechanisms to monitor the flow measurements of fluid or gas being delivered [ [110] , [111] , [112] ]. As such, the choice of each feature during the design process is crucial to the overall functionality of the MEMS-based delivery device.
MEMS-based devices play essential roles in targeted and precise drug delivery by facilitating controlled and pulsatile release of enclosed pharmaceuticals [ 113 ]. For this purpose, these devices could be designed either as electric-powered or non-electric powered. The electric-powered devices allow selective drug release from the reservoir(s) by electric potential, while the non-powered devices utilize diffusion, and osmotic environmental stimulus mechanisms to enable drug release [ 105 ]. The most popular type of MEMS technology applicable in drug delivery is microchips, followed by microfluidic devices, majorly micropumps. These microchips are reservoir-based implantable devices, which are capable of delivering pharmaceutics in solid, gel, or liquid forms through trans/intradermal delivery [ 109 ]. On the other hand, micropumps which are either classified as mechanical or non-mechanical, depending on the presence of moving parts, are distinctly limited to the delivery of drug suspensions or solutions [ 106 ]. The major components of MEMs are shown in Fig. 7 .

Schematic representation of MEMS components. Generally, MEMS is made up of mechanical microstructures, microactuators, microsensors, and microelectronics all integrated onto a single silicon chip. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Drug delivery devices fabricated by MEMS technologies offer several advantages over conventional delivery methods, such as; enhanced performance, automation, precision, and efficacy due to the integration of their miniaturized sizes with multi-functional components. It also contributes to less painful and invasive attributes of the devices [ 105 ]. In addition, MEMS-based devices have the ability to maintain drug stability during encapsulation, adjustable and continuous delivery, and also facilitate the automated release of multiple drugs from reservoirs [ 114 ]. Furthermore, it enhances bioavailability and localized release of medication [ 115 ], sustainability over a long period of time for medications requiring complex dosing, as well as personalized dosing profile [ 109 ], and has the ability to function following sustained zero-order kinetics. However, MEMS-drug delivery devices may pose technical challenges; because the incorporation of wireless electronics to remotely control and track the device's operation, as well as patient's response, has also been projected to be capable of increasing device security risks, medical packaging, and regulatory complexities [ 105 ]. Moreover, surgeries would be required for the implantation and removal of these devices; highly stable products would be needed for long-term uses; and the technologies required for their fabrication are relatively expensive [ 114 ].
3.8. Combined drug delivery approach
Resistance to drugs has been a recurrent issue in medical therapy. Today, combination therapy proves more viable as a result of wider target specificity and complementarity in enhancing treatment efficiency and increasing clinical results. Combined drug delivery approach has been widely adopted in cancer research and therapy as a means to overcome multidrug resistance. It has been reported that combination drug delivery approach reduces therapeutic dosage as well as adverse reactions while efficiency and decrease in drug resistance are maintained [ 116 ].
A study conducted by Zamora-Mera et al. reported positive results in the use of combination therapy for magnetic hyperthermia therapy. They crosslinked chitosan nanoparticles (CSNPs) ionically with tripolyphosphate salts (TPP). The magnetic CSNPs were obtained by encapsulation with three different ferrofluid concentrations and a constant 5-Fluorouracil (5-FU) concentration. They used normal cells, fibroblasts (FHB) and cancer cells, human glioblastoma A-172 cells [ 117 ]. The CSNPs showed dose-dependent cytotoxicity and were successfully up-taken in both cell lines. The study reported that the MH-treatment in the A-172 cells produced a 67–75% cell viability whereas no cell viability was noticed in FHB. The study equally reported a 4-h regrowth of the population upon MH treatment with CSNPs loaded only with ferrofluid but a decreased amount of released 5-FU upon combination with the MH treatment and 5-FU demonstrating a positive result using a combination approach [ 117 ].
In a study conducted by Silva et al., they reported an increase in the application of combination approach in drug research and in therapeutic studies. They investigated the use of a combined method to remove endotoxins from protein nanocages for drug delivery approaches. They combined an affinity purification with Endotrap-HD resin and treatment with Triton X-144. The study yielded good results that showed combination treatment as a good potential in chemotherapy [ 118 ].
A review article conducted by Pang et al. on a particular combination drug delivery approach that focused on exploiting cells in combination with nanoparticles reported that nanoparticles loaded in cells were more effective than the nanoparticle drug delivery system. The cell-based therapy showed improved drug efficacy, extended half-lives, sustained drug release, and limited immunogenicity and cytotoxicity [ 119 ]. Combining nanoparticles with exploit cells did not affect its migration or chemotaxic ability. As a result, combination drug delivery approach is viewed as a promising approach in drug research and medical therapy [ 119 ].
3.9. Targeted drug delivery system
This approach is an advanced technique employed recently due to its efficiency and reduced side effects. It is a system that delivers drugs in a targeted sequence which in turn leads to an increase in the drug concentration as it is being delivered to its target site [ 120 ]. The dosage of the drug is reduced to minimize side effects but its efficacy and strength remain untouched. This approach employs other drug carriers such as soluble polymers, biodegradable microsphere polymers, neutrophils, liposomes, micelles, and artificial cells amongst others [ 120 ]. This technique is gaining wide acceptance as it proves useful, especially in the fight against cancer.
A study conducted by Murugun showed effectiveness in the use of this drug delivery system. Topotecan (TPT) and quercetin (QT) were delivered using polyacrylic acid chitosan surface-modified mesoporous silica nanoparticle (MSN) to target negative breast cancer cells (TNBC) (MDA-MB-231) and multidrug-resistant breast cancer cells (MCF-7) [ 121 ]. The surface of the nanoparticles was grafted with RGD-peptides which is an amino acid made up of Arg-Gly-Asp sequences. This was done to effectively target αvβ3 integrin. The RGD peptide led to an effective release of encapsulated drugs as well as cellular uptakes by the cancer cells. Both cell lines showed cell death, molecular and structural changes of cellular nucleus, endoplasmic reticulum, and mitochondria. A synergistic antiproliferative effect was also observed [ 121 ].
Another study conducted by Wu et al. showed an enhanced release of methrotrexate (MTX) from Fe 3 O 4 MgAl-LDH (layered double hydroxide) nanoparticles of ∼230 nm [ 122 ]. They reported 84.94% release in the tumor with a pH of 3.5 within 48 h. Their study showed higher antitumor activities across the cell lines that were investigated. Lin et al. used this approach to target HeLa cells. Mitomycin C (MMC) and 10-hydroxycamptothecin (HCPT) were co-delivered using a folate-functionalized soybean phosphatidylcholine micellar Nano formulation to determine their therapeutic effect on the HeLa Cells [ 123 ]. The study reported enhanced cellular uptake both in vitro and in vivo and an enhanced decrease in tumor burden compared to free drugs. These findings and much more suggest that a targeted drug delivery system is an area researchers’ ought to also pay more attention to. A comprehensive summary of recent drug delivery systems including their therapeutic uses, advantages, and disadvantages were presented in Table 2 .
The summary of recent drug delivery systems, their uses and advantages, and disadvantages.
4. Challenges associated with current drug delivery systems
Many delivery systems have been used successfully recently, there has been a great development in the quest to deliver drugs from various plant sources to their target sites for treatment in the body, however there are numerous limitations and challenges to what these systems can achieve in treatment, some of which are discussed below.
A major challenge facing the advancement of drug delivery systems is the limited amount of literature and variation in the available literature. Literature provides important information for the advancement of any research and in this case, nanomedicine approaches to treatment. The variation in published studies in relation to recorded characterization of reported experimental details is seen to also be a major difficulty in the progression of nanotechnology application in medicine [ 134 ]. The limited and varied information that should be a guide for industries could impede future breakthroughs of nanomedicines and delay the transformation from research and experimentation to clinical application [ 135 ]. Many researchers agree that nanoparticles can be either good or bad, the benefits of nanoparticles are more widely known and recognized but there is a scarcity of information when it comes to how safe these particles are, their level of interactions with proteins that are not specific to them and their movement and interaction with other organs that are not their target organs [ 136 ].
Some of these delivery systems make use of large particles as carriers which are not particularly favorable for treatment because they can constitute challenges such as poor absorption and solubility, in vivo instability , poor bioavailability, target-specific delivery complications, and several adverse side effects upon administration [ 23 ], the use of much smaller particles for delivery to the human biological system is a way out that solves the issues that come with using much larger particles.
Target-specific delivery complication is a challenge that faces all delivery systems. Even though target-specific delivery has been found to reduce toxicity and shows more effective treatment, its efficacy cannot be assured until it is able to reach the targeted site in sufficient amount, this is seen when siRNA is given systemically, rarely do they get to their target cell/organ because they are easily degraded by body enzymes, and when administered in large quantity their negative charge becomes an obstacle to absorption by the cells [ 137 ] resulting in little or no absorption by the body. Micelles and liposomes which are lipid nanoparticles are being studied for target drug delivery, but the downside to this is the lowering of their efficiency by their reaction with the body, these reactions include phagocytic absorption and hepatic filtration, which could lead to failure in target delivery, also the nanoparticles could show signs of toxicity [ 138 ]. The challenge to targeted delivery is that a patient that is unconscious cannot take a dose, there is low solubility and permeability at the target site, they could interact with food and may be degraded by gastro intestinal flora [ 139 ].
The toxicity of particles used in delivery is another great challenge that faces drug delivery systems in general, some of the nanomaterials used can be harmful to human health and also the environment [ 140 ]. In vivo and in vitro experimentation has shown the harmful effect of silver, gold, silica, and titanium used as nanoparticles for coupling and delivering drugs [ 136 ]. Carbon nanotubes (CNTs) have become widely used in gene therapy, bio-imaging, and drug delivery [ 141 ]. because they have been found to have the ability to cross cell membranes even when they are used as carriers for biomolecules [ 142 ] but the properties of carbon nanotubes have raised concerns among researchers especially in its use in drug delivery because experiments show that they can cause harm to embryos, genes, liver, heart, neurons and immune system [ 136 ]. Although carbon nanotubes have shown favorable results in their use, it is important to carry out crucial toxicity tests to ensure their safety before a widespread application in treatment [ 141 ]. In application, their effects have become an obstacle in their use for cancer treatment [ 143 ].
Scientists have successfully produced drugs that can serve as carriers and drugs as well, one of the major challenges facing drug delivery systems is biocompatibility (their ability to function with the body in specific situations) and acceptability (being received by the body without triggering the immune system), this is a problem because the way the body reacts to biological materials are very different from the way they react to synthetic materials [ 140 ]. In addition, because of the complex structure of the human system, there could be natural barriers to the functions of these delivery systems for example the blood brain-barrier (BBB) has a selectively permeable feature that makes it difficult in achieving therapeutic drug concentration in the brain tissues, the BBB prevents the entry of carrier particles into the brain and entire central nervous system causing ineffectiveness of therapeutic agents in the treatment of cerebral diseases due to inability to deliver and sustain intended drugs within the brain efficiently [ 144 ]. Also, one of the most abundant carriers in the body are the monoclonal antibodies (mAb), because they form immunoliposomes by binding to liposome surfaces however the functions of these immunoliposomes are limited because they can trigger an immune response and low levels of absorption, distribution, metabolism, and elimination by the body, this presents a challenge in the use of liposomes to achieve efficacy as a site-specific drug carrier [ 145 ].
The kidney and liver also have a natural ability to detox the body, these organs can treat the nanoparticles as potential waste products. Their function can constitute obstruction in drug delivery and lead to the accumulation of nanoparticles in these organs. In the liver, nanomaterials accumulate primarily in the Kupffer cells, macrophages in the liver, sinusoidal endothelial cells, hepatic stellate cells, and a little in the hepatocytes. Meanwhile the size, charge and shape of the kidney decide the fate of the nanomaterials once they get into the renal system [ 146 ].
5. Future directions and conclusion
Drug delivery and nanomedicine have become a very fascinating area of research in modern science, in the past years, it has gotten a lot of attention in both research, experimentation and in a number of clinical trials [ 147 ]. Despite the setback that have limited the clinical application of these delivery systems, the recent drug delivery system holds great potential, it would require a collaboration across academic theory, laboratory experimentation, the knowledge of medicine, pharmaceuticals, and great research to help achieve the efficiency we need to take, findings from bench to bedside [ 148 ]. Vargason et al. [ 2 ] believe that the use of cell therapies can go a long way to solve the bio-acceptability issues that drug delivery systems face, they also think it will create a single dose that is effective that avoids high accumulation of drugs in the system. As a matter of fact, cell therapies promise a seeming sustained source of complex biologics, break down innate biological barriers and create responses that appear natural within the system. Adepu [ 149 ] have suggested the use of inorganic mesoporous nanoparticles, micro fluids, and molecular imprinting polymers as some of the ways to combat some of the challenges that face drug delivery. According to Khalid et al. [ 137 ] a way to improve drug delivery efficacy is by the use of priming agents that can influence the biological environment where they are administered, especially those that can change the form and function of tissues in a way that makes the administered drug favorable without harming the patient. Also, cell-based drug systems should be considered in the field of biomaterials, this means the use of cells coupled with nano biomaterials since cells are indigenous to the human system, this is a novel method and still theoretical but appears to be most creative, encourages drug delivery method with hopes to achieve maximum drug delivery pattern. A lot of research and clinical trials are still needed to foster the efficiency of these modern drug delivery systems and the challenges that face their usage.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Declaration of competing interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- U.S. Department of Health & Human Services
- National Institutes of Health

En Español | Site Map | Staff Directory | Contact Us
Science Education
- Science Topics
- Drug Delivery Systems
What are drug delivery systems?
How are drug delivery systems used in current medical practice, what technologies are nibib-funded researchers developing for drug delivery.
Drug delivery systems describe technologies that carry drugs into or throughout the body. These technologies include the method of delivery, such as a pill that you swallow or a vaccine that is injected. Drug delivery systems can also describe the way that drugs are ‘packaged’—like a micelle or a nanoparticle —that protects the drug from degradation and allows it to travel wherever it needs to go in the body. The field of drug delivery has advanced dramatically in the past few decades, and even greater innovations are anticipated in the coming years. Biomedical engineers have contributed substantially to our understanding of the physiological barriers to efficient drug delivery and have also contributed to the development of several new modes of drug delivery that have entered clinical practice.
Yet, with all of this progress, many disease treatments still have unacceptable side effects. Side effects occur because drugs interact with healthy organs or tissues, and this can limit our ability to treat many diseases such as cancer, neurodegenerative diseases, and infectious diseases. Continuing advances in this space will help to facilitate the targeted delivery of drugs while also mitigating their side effects.
Clinicians historically have attempted to administer interventions to areas of the body directly affected by disease. Instead of delivering drugs systemically, which affects the whole body, some drugs can be administered locally, which can decrease side effects and drug toxicity while maximizing a treatment’s impact. A topical (used on the skin) antibacterial ointment for the treatment of a localized infection or a cortisone injection to relieve pain in a joint can avoid some of the systemic side effects of these medications. There are other ways to achieve targeted drug delivery, but some medications can only be given systemically.
Another example of a drug delivery system includes the components of a vaccine that helps it to travel inside the body. Vaccines work by providing our immune system with instructions to recognize and attack a pathogen. These ‘instructions’—such as mRNA , in the case of some COVID-19 vaccines—must be packaged so that it is not degraded by the body and can reach its target. The packaging used for COVID-19 mRNA vaccines are lipid nanoparticles, which protects the fragile mRNA cargo and facilitates its delivery into cells.
Current research on drug delivery systems can be described in two broad categories: routes of delivery and delivery vehicles.
Routes of Delivery
Medications can be taken in a variety of ways—by swallowing, by inhalation, by absorption through the skin, or by injection. Each method has advantages and disadvantages, and not all methods can be used for every medication. Improving current delivery methods or designing new ones can enhance the use of existing medications.
Microneedle patch for painless vaccinations: Microneedle arrays are one example of a new method to deliver medications through the skin. In these arrays, dozens of microscopic needles, each far thinner than a strand of human hair, can be fabricated to contain a medicine. The needles are so small that, although they penetrate the skin, they don’t reach the nerves and can deliver medications painlessly.
NIBIB-funded scientists are developing such a patch with an array of dissolvable microneedles for vaccine delivery. These patches are easy to use and do not require refrigeration or special disposal methods, so they could be used by patients at home. This technology could be especially helpful in low-resource communities that may not have many health care providers or adequate storage facilities for traditional, refrigerated medicines.

Robotic pill for oral drug delivery of complex drugs: Self-injections are used to manage some diseases, such as diabetes and Crohn’s disease. Medications for these conditions may require an injection because the drugs used are often complex and easily degradable, and therefore can’t be taken orally. However, self-injection can represent burdens for patients, including the frequency of the injections and the potential for needle stick injuries.
NIBIB-funded scientists are developing an alternative method for self-injection: a robotic pill that can be loaded with complex, liquid drugs. Once swallowed, this pill makes its way to the stomach, where the drug is injected into the stomach tissue. The pill is then excreted through the gastrointestinal tract. While these robotic pills have only been evaluated in animal models so far, they could potentially offer an alternative to self-injection across a range of conditions.
Delivery Vehicles
Drug delivery vehicles represent different ways that medications can be packaged so that the drug can safely travel within the body. Some common examples of drug delivery vehicles include micelles, liposomes , or nanoparticles. Different drug delivery vehicles can improve the targeting of the drug by helping the medication travel exactly where it needs to go. Additionally, research in this space allows for the development of new ways to package hard-to-use drugs, for reasons like size or fragility. These improvements in biotechnology are leading to improved medications that can target diseases more effectively and precisely.
Nanoparticle carriers for the treatment of eye disorders: Gene transfer therapies —where genetic material that codes for therapeutic proteins is introduced into cells—represent a promising way to treat a variety of eye disorders, including macular degeneration. Current delivery methods have limitations, such as the size of the genetic material that they can hold, and their tendency to initiate an immune response.
NIBIB-funded scientists are developing a nanoparticle to carry genetic material that overcomes these limitations. These nanoparticles are not readily detected by the immune system and can hold larger genes than current methods. In a mouse model of macular degeneration, the researchers found that injection with their gene-loaded nanoparticles resulted in a 60% reduction in abnormal blood vessels (a characteristic of the disease that causes vision impairments) compared with controls. While still in the early stages, this research could lead to better treatments for eye disorders.
Mimicking immune cells to combat inflammation: While inflammation is an essential part of our immune response to harmful substances, excessive inflammation in the vascular system can ultimately cause tissue injury, notably in the lungs. Acute respiratory distress syndrome (ARDS) is a life-threatening condition characterized by sudden and severe damage in the lungs which can cause low blood oxygen levels. Treatments typically include mechanical ventilation, but there are no recommended pharmacological interventions. Mortality rates for ARDS are high, ranging from 35-46%.
Part of the vascular inflammation process includes the migration and adhesion of neutrophils , a type of immune cell, into the lungs, where they bind to endothelial cells. Taking advantage of this behavior, NIBIB-funded researchers are designing nanovesicles that mimic neutrophils, which could be loaded with anti-inflammatory drugs that then deposit their therapeutic cargo in the lungs. This new drug delivery system, inspired by biology, is still early in development.
Updated July 2022
Explore More
- 60 Seconds of Science
- Understanding Medical Imaging Mobile App
- Science Fact Sheets
- Video Gallery
- Audio Library
Research Topics
Scientific Program Areas
- Division of Applied Science & Technology (Bioimaging)
- Division of Discovery Science & Technology (Bioengineering)
- Division of Health Informatics Technologies (Informatics)
- Division of Interdisciplinary Training (DIDT)
Inside NIBIB
- Director's Corner
- Funding Policies
- NIBIB Fact Sheets
- Press Releases
- Open access
- Published: 19 September 2018
Nano based drug delivery systems: recent developments and future prospects
- Jayanta Kumar Patra ORCID: orcid.org/0000-0003-4118-4355 1 ,
- Gitishree Das 1 ,
- Leonardo Fernandes Fraceto 2 , 3 ,
- Estefania Vangelie Ramos Campos 2 , 3 ,
- Maria del Pilar Rodriguez-Torres ORCID: orcid.org/0000-0001-9107-247X 4 ,
- Laura Susana Acosta-Torres ORCID: orcid.org/0000-0002-5959-9113 4 ,
- Luis Armando Diaz-Torres ORCID: orcid.org/0000-0002-1281-9916 5 ,
- Renato Grillo 6 ,
- Mallappa Kumara Swamy 7 ,
- Shivesh Sharma 8 ,
- Solomon Habtemariam 9 &
- Han-Seung Shin 10
Journal of Nanobiotechnology volume 16 , Article number: 71 ( 2018 ) Cite this article
445k Accesses
3595 Citations
60 Altmetric
Metrics details
Nanomedicine and nano delivery systems are a relatively new but rapidly developing science where materials in the nanoscale range are employed to serve as means of diagnostic tools or to deliver therapeutic agents to specific targeted sites in a controlled manner. Nanotechnology offers multiple benefits in treating chronic human diseases by site-specific, and target-oriented delivery of precise medicines. Recently, there are a number of outstanding applications of the nanomedicine (chemotherapeutic agents, biological agents, immunotherapeutic agents etc.) in the treatment of various diseases. The current review, presents an updated summary of recent advances in the field of nanomedicines and nano based drug delivery systems through comprehensive scrutiny of the discovery and application of nanomaterials in improving both the efficacy of novel and old drugs (e.g., natural products) and selective diagnosis through disease marker molecules. The opportunities and challenges of nanomedicines in drug delivery from synthetic/natural sources to their clinical applications are also discussed. In addition, we have included information regarding the trends and perspectives in nanomedicine area.
Since ancient times, humans have widely used plant-based natural products as medicines against various diseases. Modern medicines are mainly derived from herbs on the basis of traditional knowledge and practices. Nearly, 25% of the major pharmaceutical compounds and their derivatives available today are obtained from natural resources [ 1 , 2 ]. Natural compounds with different molecular backgrounds present a basis for the discovery of novel drugs. A recent trend in the natural product-based drug discovery has been the interest in designing synthetically amenable lead molecules, which mimic their counterpart’s chemistry [ 3 ]. Natural products exhibit remarkable characteristics such as extraordinary chemical diversity, chemical and biological properties with macromolecular specificity and less toxicity. These make them favorable leads in the discovery of novel drugs [ 4 ]. Further, computational studies have helped envisage molecular interactions of drugs and develop next-generation drug inventions such as target-based drug discovery and drug delivery.
Despite several advantages, pharmaceutical companies are hesitant to invest more in natural product-based drug discovery and drug delivery systems [ 5 ] and instead explore the available chemical compounds libraries to discover novel drugs. However, natural compounds are now being screened for treating several major diseases, including cancer, diabetes, cardiovascular, inflammatory, and microbial diseases. This is mainly because natural drugs possess unique advantages, such as lower toxicity and side effects, low-price, and good therapeutic potential. However, concerns associated with the biocompatibility, and toxicity of natural compounds presents a greater challenge of using them as medicine. Consequently, many natural compounds are not clearing the clinical trial phases because of these problems [ 6 , 7 , 8 ]. The use of large sized materials in drug delivery poses major challenges, including in vivo instability, poor bioavailability, and poor solubility, poor absorption in the body, issues with target-specific delivery, and tonic effectiveness, and probable adverse effects of drugs. Therefore, using new drug delivery systems for targeting drugs to specific body parts could be an option that might solve these critical issues [ 9 , 10 ]. Hence, nanotechnology plays a significant role in advanced medicine/drug formulations, targeting arena and their controlled drug release and delivery with immense success.
Nanotechnology is shown to bridge the barrier of biological and physical sciences by applying nanostructures and nanophases at various fields of science [ 11 ]; specially in nanomedicine and nano based drug delivery systems, where such particles are of major interest [ 12 , 13 ]. Nanomaterials can be well-defined as a material with sizes ranged between 1 and 100 nm, which influences the frontiers of nanomedicine starting from biosensors, microfluidics, drug delivery, and microarray tests to tissue engineering [ 14 , 15 , 16 ]. Nanotechnology employs curative agents at the nanoscale level to develop nanomedicines. The field of biomedicine comprising nanobiotechnology, drug delivery, biosensors, and tissue engineering has been powered by nanoparticles [ 17 ]. As nanoparticles comprise materials designed at the atomic or molecular level, they are usually small sized nanospheres [ 18 ]. Hence, they can move more freely in the human body as compared to bigger materials. Nanoscale sized particles exhibit unique structural, chemical, mechanical, magnetic, electrical, and biological properties. Nanomedicines have become well appreciated in recent times due to the fact that nanostructures could be utilized as delivery agents by encapsulating drugs or attaching therapeutic drugs and deliver them to target tissues more precisely with a controlled release [ 10 , 19 ]. Nanomedicine, is an emerging field implementing the use of knowledge and techniques of nanoscience in medical biology and disease prevention and remediation. It implicates the utilization of nanodimensional materials including nanorobots, nanosensors for diagnosis, delivery, and sensory purposes, and actuate materials in live cells (Fig. 1 ). For example, a nanoparticle-based method has been developed which combined both the treatment and imaging modalities of cancer diagnosis [ 20 ]. The very first generation of nanoparticle-based therapy included lipid systems like liposomes and micelles, which are now FDA-approved [ 21 ]. These liposomes and micelles can contain inorganic nanoparticles like gold or magnetic nanoparticles [ 22 ]. These properties let to an increase in the use of inorganic nanoparticles with an emphasis on drug delivery, imaging and therapeutics functions. In addition, nanostructures reportedly aid in preventing drugs from being tarnished in the gastrointestinal region and help the delivery of sparingly water-soluble drugs to their target location. Nanodrugs show higher oral bioavailability because they exhibit typical uptake mechanisms of absorptive endocytosis.
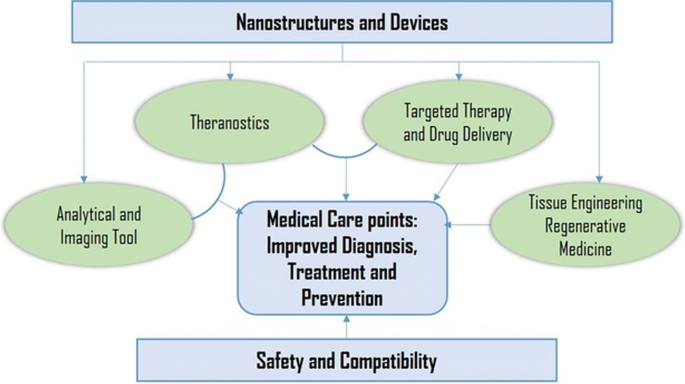
Application and goals of nanomedicine in different sphere of biomedical research
Nanostructures stay in the blood circulatory system for a prolonged period and enable the release of amalgamated drugs as per the specified dose. Thus, they cause fewer plasma fluctuations with reduced adverse effects [ 23 ]. Being nanosized, these structures penetrate in the tissue system, facilitate easy uptake of the drug by cells, permit an efficient drug delivery, and ensure action at the targeted location. The uptake of nanostructures by cells is much higher than that of large particles with size ranging between 1 and 10 µm [ 17 , 24 ]. Hence, they directly interact to treat the diseased cells with improved efficiency and reduced or negligible side effects.
At all stages of clinical practices, nanoparticles have been found to be useful in acquiring information owing to their use in numerous novel assays to treat and diagnose diseases. The main benefits of these nanoparticles are associated with their surface properties; as various proteins can be affixed to the surface. For instance, gold nanoparticles are used as biomarkers and tumor labels for various biomolecule detection procedural assays.
Regarding the use of nanomaterials in drug delivery, the selection of the nanoparticle is based on the physicochemical features of drugs. The combined use of nanoscience along with bioactive natural compounds is very attractive, and growing very rapidly in recent times. It presents several advantages when it comes to the delivery of natural products for treating cancer and many other diseases. Natural compounds have been comprehensively studied in curing diseases owing to their various characteristic activities, such as inducing tumor-suppressing autophagy and acting as antimicrobial agents. Autophagy has been observed in curcumin and caffeine [ 25 ], whereas antimicrobial effects have been shown by cinnamaldehyde, carvacrol, curcumin and eugenol [ 26 , 27 ]. The enrichment of their properties, such as bioavailability, targeting and controlled release were made by incorporating nanoparticles. For instance, thymoquinone, a bioactive compound in Nigella sativa , is studied after its encapsulation in lipid nanocarrier. After encapsulation, it showed sixfold increase in bioavailability in comparison to free thymoquinone and thus protects the gastrointestinal stuffs [ 28 ]. It also increased the pharmacokinetic characteristics of the natural product resulting in better therapeutic effects.
Metallic, organic, inorganic and polymeric nanostructures, including dendrimers, micelles, and liposomes are frequently considered in designing the target-specific drug delivery systems. In particular, those drugs having poor solubility with less absorption ability are tagged with these nanoparticles [ 17 , 29 ]. However, the efficacy of these nanostructures as drug delivery vehicles varies depending on the size, shape, and other inherent biophysical/chemical characteristics. For instance, polymeric nanomaterials with diameters ranging from 10 to 1000 nm, exhibit characteristics ideal for an efficient delivery vehicle [ 7 ]. Because of their high biocompatibility and biodegradability properties, various synthetic polymers such as polyvinyl alcohol, poly- l -lactic acid, polyethylene glycol, and poly(lactic- co -glycolic acid), and natural polymers, such as alginate and chitosan, are extensively used in the nanofabrication of nanoparticles [ 8 , 30 , 31 , 32 ]. Polymeric nanoparticles can be categorized into nanospheres and nanocapsules both of which are excellent drug delivery systems. Likewise, compact lipid nanostructures and phospholipids including liposomes and micelles are very useful in targeted drug delivery.
The use of ideal nano-drug delivery system is decided primarily based on the biophysical and biochemical properties of the targeted drugs being selected for the treatment [ 8 ]. However, problems such as toxicity exhibited by nanoparticles cannot be ignored when considering the use of nanomedicine. More recently, nanoparticles have mostly been used in combination with natural products to lower the toxicity issues. The green chemistry route of designing nanoparticles loaded with drugs is widely encouraged as it minimises the hazardous constituents in the biosynthetic process. Thus, using green nanoparticles for drug delivery can lessen the side-effects of the medications [ 19 ]. Moreover, adjustments in nanostructures size, shape, hydrophobicity, and surface changes can further enhance the bioactivity of these nanomaterials.
Thus, nanotechnology offers multiple benefits in treating chronic human diseases by site-specific, and target-oriented delivery of medicines. However, inadequate knowledge about nanostructures toxicity is a major worry and undoubtedly warrants further research to improve the efficacy with higher safety to enable safer practical implementation of these medicines. Therefore, cautiously designing these nanoparticles could be helpful in tackling the problems associated with their use. Considering the above facts, this review aims to report different nano based drug delivery systems, significant applications of natural compound-based nanomedicines, and bioavailability, targeting sites, and controlled release of nano-drugs, as well as other challenges associated with nanomaterials in medicines.
Nano based drug delivery systems
Recently, there has been enormous developments in the field of delivery systems to provide therapeutic agents or natural based active compounds to its target location for treatment of various aliments [ 33 , 34 ]. There are a number of drug delivery systems successfully employed in the recent times, however there are still certain challenges that need to be addresses and an advanced technology need to be developed for successful delivery of drugs to its target sites. Hence the nano based drug delivery systems are currently been studied that will facilitate the advanced system of drug delivery.
Fundamentals of nanotechnology based techniques in designing of drug
Nanomedicine is the branch of medicine that utilizes the science of nanotechnology in the preclusion and cure of various diseases using the nanoscale materials, such as biocompatible nanoparticles [ 35 ] and nanorobots [ 36 ], for various applications including, diagnosis [ 37 ], delivery [ 38 ], sensory [ 39 ], or actuation purposes in a living organism [ 40 ]. Drugs with very low solubility possess various biopharmaceutical delivery issues including limited bio accessibility after intake through mouth, less diffusion capacity into the outer membrane, require more quantity for intravenous intake and unwanted after-effects preceding traditional formulated vaccination process. However all these limitations could be overcome by the application of nanotechnology approaches in the drug delivery mechanism.
Drug designing at the nanoscale has been studied extensively and is by far, the most advanced technology in the area of nanoparticle applications because of its potential advantages such as the possibility to modify properties like solubility, drug release profiles, diffusivity, bioavailability and immunogenicity. This, can consequently lead to the improvement and development of convenient administration routes, lower toxicity, fewer side effects, improved biodistribution and extended drug life cycle [ 17 ]. The engineered drug delivery systems are either targeted to a particular location or are intended for the controlled release of therapeutic agents at a particular site. Their formation involves self-assembly where in well-defined structures or patterns spontaneously are formed from building blocks [ 41 ]. Additionally they need to overcome barriers like opsonization/sequestration by the mononuclear phagocyte system [ 42 ].
There are two ways through which nanostructures deliver drugs: passive and self-delivery. In the former, drugs are incorporated in the inner cavity of the structure mainly via the hydrophobic effect. When the nanostructure materials are targeted to a particular sites, the intended amount of the drug is released because of the low content of the drugs which is encapsulated in a hydrophobic environment [ 41 ]. Conversely, in the latter, the drugs intended for release are directly conjugated to the carrier nanostructure material for easy delivery. In this approach, the timing of release is crucial as the drug will not reach the target site and it dissociates from the carrier very quickly, and conversely, its bioactivity and efficacy will be decreased if it is released from its nanocarrier system at the right time [ 41 ]. Targeting of drugs is another significant aspect that uses nanomaterials or nanoformulations as the drug delivery systems and, is classified into active and passive. In active targeting, moieties, such as antibodies and peptides are coupled with drug delivery system to anchor them to the receptor structures expressed at the target site. In passive targeting, the prepared drug carrier complex circulates through the bloodstream and is driven to the target site by affinity or binding influenced by properties like pH, temperature, molecular site and shape. The main targets in the body are the receptors on cell membranes, lipid components of the cell membrane and antigens or proteins on the cell surfaces [ 43 ]. Currently, most nanotechnology-mediated drug delivery system are targeted towards the cancer disease and its cure.
Biopolymeric nanoparticles in diagnosis, detection and imaging
The integration of therapy and diagnosis is defined as theranostic and is being extensively utilized for cancer treatment [ 44 , 45 ]. Theranostic nanoparticles can help diagnose the disease, report the location, identify the stage of the disease, and provide information about the treatment response. In addition, such nanoparticles can carry a therapeutic agent for the tumor, which can provide the necessary concentrations of the therapeutic agent via molecular and/or external stimuli [ 44 , 45 ]. Chitosan is a biopolymer which possesses distinctive properties with biocompatibility and presence of functional groups [ 45 , 46 , 47 ]. It is used in the encapsulation or coating of various types of nanoparticles, thus producing different particles with multiple functions for their potential uses in the detection and diagnosis of different types of diseases [ 45 , 47 ].
Lee et al. [ 48 ] encapsulated oleic acid-coated FeO nanoparticles in oleic acid-conjugated chitosan (oleyl-chitosan) to examine the accretion of these nanoparticles in tumor cells through the penetrability and holding (EPR) consequence under the in vivo state for analytical uses by the near-infrared and magnetic resonance imaging (MRI) mechanisms. By the in vivo evaluations, both techniques showed noticeable signal strength and improvement in the tumor tissues through a higher EPR consequence after the injection of cyanine-5-attached oleyl-chitosan nanoparticles intravenously (Cyanine 5).
Yang et al. [ 49 ] prepared highly effective nanoparticles for revealing colorectal cancer (CC) cells via a light-mediated mechanism; these cells are visible owing to the physical conjugation of alginate with folic acid-modified chitosan leading to the formation of nanoparticles with enhanced 5-aminolevulinic (5-ALA) release in the cell lysosome. The results displayed that the engineered nanoparticles were voluntarily endocytosed by the CC cells by the folate receptor-based endocytosis process. Subsequently, the charged 5-ALA was dispersed into the lysosome which was triggered by less desirability strength between the 5-ALA and chitosan through deprotonated alginate that gave rise to the gathering of protoporphyrin IX (PpIX) for photodynamic detection within the cells. As per this research, chitosan-based nanoparticles in combination with alginate and folic acid are tremendous vectors for the definite delivery of 5-ALA to the CC cells to enable endoscopic fluorescent detection. Cathepsin B (CB) is strongly associated with the metastatic process and is available in surplus in the pericellular areas where this process occurs; thus, CB is important for the detection of metastasis. Ryu et al. [ 50 ] designed a CB-sensitive nanoprobe (CB-CNP) comprising a self-satisfied CB-CNP with a fluorogenic peptide attached to the tumor-targeting glycol chitosan nanoparticles (CNPs) on its surface. The designed nanoprobe is a sphere with a diameter of 280 nm, with spherical structure and its fluorescence capacity was completely extinguished under the biological condition. The evaluation of the usability of CB-sensitive nanoprobe in three rat metastatic models demonstrated the potential of these nonoprobes in discriminating metastatic cells from healthy ones through non-invasive imaging. Hyaluronic acid (HA) is another biopolymeric material. This is a biocompatible, negatively charged glycosaminoglycan, and is one of the main constituents of the extracellular matrix [ 51 , 52 ]. HA can bind to the CD44 receptor, which is mostly over articulated in various cancerous cells, through the receptor-linker interaction. Thus, HA-modified nanoparticles are intriguing for their use in the detection and cure of cancer [ 53 , 54 , 55 ]. Wang et al. [ 56 ], coated the surface of iron oxide nanoparticles (IONP) with dopamine-modified HA. These nanoparticles have a hydrophilic exterior and a hydrophobic interior where the chemotherapeutic homocamptothecin is encapsulated [ 56 ]. The biopotential of this process was investigated in both laboratory and in the live cells. Increased uptake of nanoparticles by tumor cells was observed by MRI when an external magnetic field was employed [ 56 ]. After the intravenous administration of the nano-vehicle in 3 mg/kg (relative to the free drug) rats, a large tumor ablation was observed and after treatment, the tumors almost disappeared [ 56 ].
Choi et al. [ 53 ] also synthesized nanoparticles of hyaluronic acid with different diameters by changing the degree of hydrophobic replacement of HA. The nanoparticles were systemically administered in the mice with tumor, and then, its effect was studied. This same research group developed a versatile thermostatic system using poly (ethylene glycol) conjugated hyaluronic acid (P-HA-NPs) nanoparticles for the early detection of colon cancer and targeted therapy. To assess the effectiveness of the nanoparticles, they were first attached to the near-infrared fluorescent dye (Cy 5.5) by chemical conjugation, and then, the irinotecan anticancer drug (IRT) was encapsulated within these systems. The therapeutic potential of P-HA-NP was then investigated in different systems of the mice colon cancer. Through the intravenous injection of the fluorescent dye attached nanoparticles (Cy 5.5-P-HA-NPs), minute and initial-stage tumors as well as liver-embedded colon tumors were efficiently pictured using an NIRF imaging method. Due to their extraordinary capability to target tumors, drug-containing nanoparticles (IRT-P-HA-NP) showed markedly decreased tumor development with decreased systemic harmfulness. In addition, healing effects could be examined concurrently with Cy 5.5-P-HA-NPs [ 57 ].
Another option that can be used is alginate, which is a natural polymer derived from the brown seaweed and has been expansively scrutinized for its potential uses in the biomedical field because of its several favorable characteristics, such as low cost of manufacture, harmonious nature, less harmfulness, and easy gelling in response to the addition of divalent cations [ 58 , 59 ]. Baghbani et al. [ 60 ] prepared perfluorohexane (PFH) nanodroplets stabilized with alginate to drive doxorubicin and then evaluated their sensitivity to ultrasound and imaging as well as their therapeutic properties. Further found that the ultrasound-facilitated treatment with PFH nanodroplets loaded with doxorubicin exhibited promising positive responses in the breast cancer rat models. The efficacy was characterized by the deterioration of the tumor [ 60 ]. In another study, Podgorna et al. [ 61 ] prepared gadolinium (GdNG) containing nanogels for hydrophilic drug loading and to enable screening by MRI. The gadolinium alginate nanogels had an average diameter of 110 nm with stability duration of 60 days. Because of their paramagnetic behavior, the gadolinium mixtures are normally used as positive contrast agents (T1) in the MRI images. Gadolinium nanogels significantly reduce the relaxation time (T1) compared to controls. Therefore, alginate nanogels act as contrast-enhancing agents and can be assumed as an appropriate material for pharmacological application.
Also, the polymeric material dextran is a neutral polymer and is assumed as the first notable example of microbial exopolysaccharides used in medical applications. A remarkable advantage of using dextran is that it is well-tolerated, non-toxic, and biodegradable in humans, with no reactions in the body [ 62 ]. Photodynamic therapy is a site-specific cancer cure with less damage to non-cancerous cells. Ding et al. [ 63 ] prepared a nanoparticulate multifunctional composite system by encapsulating Fe 3 O 4 nanoparticles in dextran nanoparticles conjugated to redox-responsive chlorine 6 (C6) for near infrared (NIR) and magnetic resonance (MR) imaging. The nanoparticles exhibited an “off/on” behavior of the redox cellular response of the fluorescence signal, thus resulting in accurate imaging of the tumor. In addition, excellent in vitro and in vivo magnetic targeting ability was observed, contributing to the efficacy of enhanced photodynamic therapy. Hong et al. [ 64 ] prepared theranostic nanoparticles or glioma cells of C6 mice. These particles comprised of gadolinium oxide nanoparticles coated with folic acid-conjugated dextran (FA) or paclitaxel (PTX). The bioprotective effects of dextran coating and the chemotherapeutic effect of PTX on the C6 glioma cells were evaluated by the MTT assay. The synthesized nanoparticles have been shown to enter C6 tumor cells by receptor-mediated endocytosis and provide enhanced contrast (MR) concentration-dependent activity due to the paramagnetic property of the gadolinium nanoparticle. Multifunctional nanoparticles were more effective in reducing cell viability than uncoated gadolinium nanoparticles. Therefore, FA and PTX conjugated nanoparticles can be used as theranostic agents with paramagnetic and chemotherapeutic properties.
Drug designing and drug delivery process and mechanism
With the progression of nanomedicine and, due to the advancement of drug discovery/design and drug delivery systems, numerous therapeutic procedures have been proposed and traditional clinical diagnostic methods have been studied, to increase the drug specificity and diagnostic accuracy. For instance, new routes of drug administration are being explored, and there is focus on ensuring their targeted action in specific regions, thus reducing their toxicity and increasing their bioavailability in the organism [ 65 ].
In this context, drug designing has been a promising feature that characterizes the discovery of novel lead drugs based on the knowledge of a biological target. The advancements in computer sciences, and the progression of experimental procedures for the categorization and purification of proteins, peptides, and biological targets are essential for the growth and development of this sector [ 66 , 67 ]. In addition, several studies and reviews have been found in this area; they focus on the rational design of different molecules and show the importance of studying different mechanisms of drug release [ 68 ]. Moreover, natural products can provide feasible and interesting solutions to address the drug design challenges, and can serve as an inspiration for drug discovery with desired physicochemical properties [ 3 , 69 , 70 ].
Also, the drug delivery systems have been gaining importance in the last few years. Such systems can be easily developed and are capable of promoting the modified release of the active ingredients in the body. For example, Chen et al. [ 70 ] described an interesting review using nanocarriers for imaging and sensory applications and discussed the, therapy effect of these systems. In addition, Pelaz et al. [ 71 ] provided an up-to-date overview of several applications of nanocarriers to nanomedicine and discussed new opportunities and challenges for this sector.
Interestingly, each of these drug delivery systems has its own chemical, physical and morphological characteristics, and may have affinity for different drugs polarities through chemical interactions (e.g., covalent bonds and hydrogen bonds) or physical interactions (e.g., electrostatic and van der Waals interactions). As an example, Mattos et al. [ 72 ] demonstrated that, the release profile of neem bark extract-grafted biogenic silica nanoparticles (chemical interactions) was lower than neem bark extract-loaded biogenic silica nanoparticles. Hence, all these factors influence the interaction of nanocarriers with biological systems [ 73 ], as well as the release kinetics of the active ingredient in the organism [ 68 ]. In addition, Sethi et al. [ 74 ] designed a crosslinkable lipid shell (CLS) containing docetaxel and wortmannin as the prototypical drugs used for controlling the drug discharge kinetics; then, they studied, its discharge profile, which was found to be affected in both in vivo and in vitro conditions. Apart from this, other parameters, such as the composition of the nanocarriers (e.g., organic, inorganic, and hybrid materials) and the form in which drugs are associated with them (such as core–shell system or matrix system) are also fundamental for understanding their drug delivery profile [ 75 , 76 ]. Taken together, several studies regarding release mechanisms of drugs in nanocarriers have been conducted. Diffusion, solvent, chemical reaction, and stimuli-controlled release are a few mechanisms that can represent the release of drugs in nanocarriers as shown in Fig. 2 [ 77 , 78 ]. Kamaly et al. [ 79 ] provided a widespread review of controlled-release systems with a focus on studies related to controlling drug release from polymeric nanocarriers.
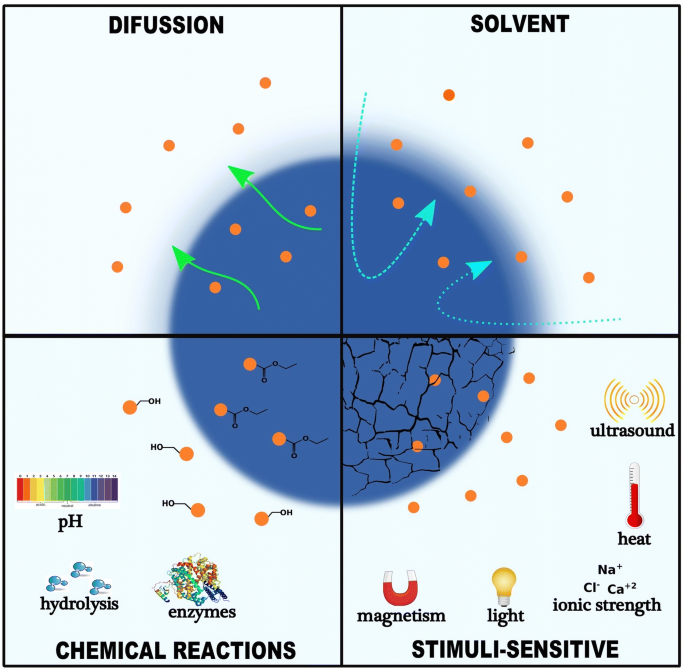
Mechanisms for controlled release of drugs using different types of nanocarriers
Although there are several nanocarriers with different drug release profiles, strategies are currently being formulated to improve the specificity of the nanostructures to target regions of the organism [ 80 ], and to reduce the immunogenicity through their coating or chemical functionalization with several substances, such as polymers [ 81 ], natural polysaccharides [ 82 , 83 ], antibodies [ 84 ], cell-membrane [ 85 ], and tunable surfactants [ 86 ], peptides [ 87 ], etc. In some cases where drugs do not display binding and affinity with a specific target or do not cross certain barriers (e.g. blood–brain barrier or the blood–cerebrospinal fluid barrier) [ 88 ], these ligand-modified nanocarriers have been used to pass through the cell membrane and allow a programmed drug delivery in a particular environment. For example, hyaluronic acid (a polysaccharide found in the extracellular matrix) has been used as a ligand-appended in several nanocarriers, showing promising results to boost antitumor action against the melanoma stem-like cells [ 89 ], breast cancer cells [ 90 ], pulmonary adenocarcinoma cells [ 91 ], as well as to facilitate intravitreal drug delivery for retinal gene therapy [ 83 ] and to reduce the immunogenicity of the formed protein corona [ 82 ]. However, the construction of the ligand-appended drug delivery systems is labor-intensive, and several targeting designs must be performed previously, taking into account the physiological variables of blood flow, disease status, and tissue architecture [ 92 ]. Moreover, few studies have been performed to evaluate the interaction of the ligand-appended in nanocarriers with cell membranes, and also their uptake mechanism is still unclear. Furthermore, has been known that the uptake of the nanoparticles by the cells occurs via phagocytic or non-phagocytic pathways (e.x. clathrin-mediated endocytosis, caveolae-mediated endocytosis, and others) [ 93 , 94 ], meanwhile due some particular physicochemical characteristics of each delivery systems have been difficult to standardize the mechanism of action/interaction of these systems in the cells. For example, Salatin and Khosroushahi [ 95 ], in a review highlighted the main endocytosis mechanisms responsible for the cellular uptake of polysaccharide nanoparticles containing active compounds.
On the other hand, stimuli-responsive nanocarriers have shown the ability to control the release profile of drugs (as a triggered release) using external factors such as ultrasound [ 96 ], heat [ 97 , 98 , 99 ], magnetism [ 100 , 101 ], light [ 102 ], pH [ 103 ], and ionic strength [ 104 ], which can improve the targeting and allow greater dosage control (Fig. 2 ). For example, superparamagnetic iron oxide nanoparticles are associated with polymeric nanocarriers [ 105 ] or lipids [ 106 ] to initially stimulate a controlled release system by the application of external magnetic field. In addition, Ulbrich et al. [ 107 ] revised recent achievements of drug delivery systems, in particular, on the basis of polymeric and magnetic nanoparticles, and also addressed the effect of covalently or noncovalently attached drugs for cancer cure [ 107 ]. Moreover, Au/Fe 3 O 4 @polymer nanoparticles have also been synthesized for the use in NIR-triggered chemo-photothermal therapy [ 108 ]. Therefore, hybrid nanocarriers are currently among the most promising tools for nanomedicine as they present a mixture of properties of different systems in a single system, thus ensuring materials with enhanced performance for both therapeutic and diagnostic applications (i.e., theranostic systems). Despite this, little is known about the real mechanisms of action and toxicity of drug delivery systems, which open opportunity for new studies. In addition, studies focusing on the synthesis of nanocarriers based on environmentally safe chemical reactions by implementing plant extracts and microorganisms have increased [ 10 ].
Nanoparticles used in drug delivery system
Biopolymeric nanoparticles.
There are numerous biopolymeric materials that are utilized in the drug delivery systems. These materials and their properties are discussed below.
Chitosan exhibits muco-adhesive properties and can be used to act in the tight epithelial junctions. Thus, chitosan-based nanomaterials are widely used for continued drug release systems for various types of epithelia, including buccal [ 109 ], intestinal [ 110 ], nasal [ 111 ], eye [ 112 ] and pulmonary [ 113 ]. Silva et al. [ 114 ] prepared and evaluated the efficacy of a 0.75% w/w isotonic solution of hydroxypropyl methylcellulose (HPMC) containing chitosan/sodium tripolyphosphate/hyaluronic acid nanoparticles to deliver the antibiotic ceftazidime to the eye. The rheological synergism parameter was calculated by calculating the viscosity of the nanoparticles in contact with mucin in different mass proportions. A minimum viscosity was observed when chitosan nanoparticles were placed in contact with mucin. However, the nanoparticles presented mucoadhesion which resulted in good interaction with the ocular mucosa and prolonged release of the antibiotic, and therefore, the nanoparticles can enhance the life span of the drug in the eyes. The nanoparticles did not show cytotoxicity for two cell lines tested (ARPE-19 and HEK 239T). The nanoparticles were also able to preserve the antibacterial activity, thus making them a promising formulations for the administration of ocular drugs with improved mucoadhesive properties.
Pistone et al. [ 115 ] prepared nanoparticles of chitosan, alginate and pectin as potential candidates for the administration of drugs into the oral cavity. The biocompatibility of the formulations was estimated based on the solubility of the nanoparticles in a salivary environment and its cytotoxicity potential was estimated in an oral cell line. Alginate nanoparticles were the most unwavering in the artificial saliva for at least 2 h, whereas pectin and especially chitosan nanoparticles were unstable. However, the chitosan nanoparticles were the most cyto-competitive, whereas alginate and pectin nanoparticles showed cytotoxicity under all tested conditions (concentration and time). The presence of Zn 2+ (cross-linking agent) may be the cause of the observed cytotoxicity. Each formulation presented advantage and limitations for release into the oral cavity, thus necessitating their further refinement.
In addition, Liu et al. [ 116 ] prepared nanoparticles of carboxymethyl chitosan for the release of intra-nasal carbamazepine (CBZ) to bypass the blood–brain barrier membrane, thus increasing the amount of the medication in the brain and refining the treatment efficacy, thereby reducing the systemic drug exposure. The nanoparticles had a mean diameter of 218.76 ± 2.41 nm, encapsulation efficiency of 80% and drug loading of 35%. Concentrations of CBZ remained higher (P < 0.05) in the brain than the plasma over 240 min.
In another example, Jain and Jain [ 117 ] investigated the discharge profile of 5-fluorouracil (5-FU) from hyaluronic acid-coated chitosan nanoparticles into the gut, via oral administration. Release assays in conditions mimicking the transit from the stomach to the colon indicated the release profile of 5-FU which was protected against discharge in the stomach and small intestine. Also, the high local concentration of drugs would be able to increase the exposure time and thus, enhance the capacity for antitumor efficacy and decrease the systemic toxicity in the treatment of colon cancer.
Another biopolymeric material that has been used as a drug delivery is alginate. This biopolymer presents final carboxyl groups, being classified as anionic mucoadhesive polymer and presents greater mucoadhesive strength when compared with cationic and neutral polymers [ 59 , 118 ]. Patil and Devarajan [ 119 ] developed insulin-containing alginate nanoparticles with nicotinamide as a permeation agent in order to lower the serum glucose levels and raise serum insulin levels in diabetic rats. Nanoparticles administered sublingually (5 IU/kg) in the presence of nicotinamide showed high availability pharmacology (> 100%) and bioavailability (> 80%). The fact that NPs are promising carriers of insulin via the sublingual route have been proved in case of the streptozotocin-induced diabetic mouse model by achieving a pharmacological high potential of 20.2% and bio-availability of 24.1% compared to the subcutaneous injection at 1 IU/kg [ 119 ].
Also, Haque et al. [ 120 ] prepared alginate nanoparticles to release venlafaxine (VLF) via intranasal for treatment of depression. The higher blood/brain ratios of the VLF concentration to the alginate nanoparticles administered intra-nasally when compared to the intranasal VLF and VLF solution intravenously indicated the superiority of the nano-formulation in directly transporting the VLF to the brain. In this way, these nanoparticles are promising for the treatment of depression. In another example, Román et al. [ 121 ] prepared alginate microcapsules containing epidermal growth factor bound on its exterior part to target the non-small cell lung cancer cells. Cisplatin (carcinogen drug) was also loaded in the nanoparticles. The addition of EGF significantly increased specificity of carrier systems and presented kinetics of cell death (H460-lung cancer strain) faster than the free drug.
In addition, Garrait et al. [ 122 ] prepared nanoparticles of chitosan containing Amaranth red (AR) and subsequently microencapsulated these nanoparticles in alginate microparticles and studied the release kinetics of this new system in simulated gastric and intestinal fluids. The microparticles had a mean diameter of 285 μm with a homogeneous distribution; it was observed that there was a release of less than 5% of the AR contained in the systems in the gastric pH conditions, whereas the discharge was fast and comprehensive in the intestinal pH conditions. Thus, the carrier showed promise to protect molecules for intestinal release after oral administration.
Costa et al. [ 123 ] prepared chitosan-coated alginate nanoparticles to enhance the permeation of daptomycin into the ocular epithelium aiming for an antibacterial effect. In vitro permeability was assessed using ocular epithelial cell culture models. The antimicrobial activity of nanoencapsulated daptomycin showed potential over the pathogens engaged in bacterial endophthalmitis. Also, the ocular permeability studies demonstrated that with 4 h of treatment from 9 to 12% in total of daptomycin encapsulated in chitosan/alginate nanoparticles, these were able to cross the HCE and ARPE-19 cells. These results indicated that with this system an increasing in the drug retention in the ocular epithelium has occurred.
Xanthan gum
Xanthan gum (XG) is a high molecular weight heteropolysaccharide produced by Xanthomonas campestris . It is a polyanionic polysaccharide and has good bioadhesive properties. Because it is considered non-toxic and non-irritating, xanthan gum is widely used as a pharmaceutical excipient [ 124 ].
Laffleur and Michalek [ 125 ] have prepared a carrier composed of xanthan gum thiolated with l -cysteine to release tannin in the buccal mucosa to treat sialorrhea. Thiolation of xanthan gum resulted in increased adhesion on the buccal mucosa when compared to native xanthan gum. In addition, xanthan gum thiolate has a higher uptake of saliva whereas tannic acid ad-string and dry the oral mucosa. In this way, this system would be an efficient way of reducing the salivary flow of patients with sialorrhea. Angiogenesis is an important feature in regeneration of soft tissues.
Huang et al. [ 126 ] prepared injectable hydrogels composed of aldehyde-modified xanthan and carboxymethyl-modified chitosan containing potent angiogenic factor (antivascular endothelial growth factor, VEGF) to improve abdominal wall reconstruction. The hydrogel presented release properties mainly in tissues like digestive tract and open wounds. The hydrogel containing VEGF was able to accelerate the angiogenesis process and rebuild the abdominal wall. Menzel et al. [ 127 ] studied a new excipient aiming the use as nasal release system. Xanthan gum was used as a major polymer in which the-((2-amino-2-carboxyethyl) disulfanyl) nicotinic acid (Cys-MNA) was coupled. Characteristics, such as amount of the associated binder, mucoadhesive properties and stability against degradation, were analyzed in the resulting conjugate. Each gram of polymer was ligated with 252.52 ± 20.54 μmol of the binder. The muco-adhesion of the grafted polymer was 1.7 fold greater than that of thiolated xanthan and 2.5 fold greater than, that of native xanthan. In addition, the frequency of ciliary beating of nasal epithelial cells was poorly affected and was reversible only upon the removal of the polymer from the mucosa.
Cellulose and its derivatives are extensively utilized in the drug delivery systems basically for modification of the solubility and gelation of the drugs that resulted in the control of the release profile of the same [ 128 ]. Elseoud et al. [ 129 ] investigated the utilization of cellulose nanocrystals and chitosan nanoparticles for the oral releasing of repaglinide (an anti-hyperglycemic—RPG). The chitosan nanoparticles showed a mean size distribution of 197 nm while the hybrid nanoparticles of chitosan and cellulose nanocrystals containing RPG. Chitosan hybrid nanoparticles and oxidized cellulose nanocrystals containing RPG had a mean diameter of 251–310 nm. The presence of the hydrogen bonds between the cellulose nanocrystals and the drug, resulted in sustained release of the same, and subsequently the nanoparticles made with oxidized cellulose nanocrystals presented lower release when compared to the nanoparticles produced with native cellulose nanocrystals.
Agarwal et al. [ 130 ] have developed a drug targeting mechanism which is based on the conjugation of calcium alginate beads with carboxymethylcellulose (CMC) loaded 5-fluoroacyl (5-FU) and is targeted to the colon. The beads with lower CMC proportions presented greater swelling and muco-adhesiveness in the simulated colonic environment. With existence of colonic enzymes there was a 90% release of 5-FU encapsulated in the beads. Hansen et al. [ 131 ] investigated four cellulose derivatives, including, meteylcellulose, hydroxypropyl methylcellulose, sodium carboxymethylcellulose and cationic hydroxyethyl cellulose for application in drug release into the nasal mucosa. The association of these cellulose derivatives with an additional excipient, was also evaluated. The drug model employed in this process was acyclovir. The viability of the polymers as excipients for nasal release applications was also scrutinized for its ciliary beat frequency (CBF) and its infusion through the tissue system of the nostril cavity. An increase in thermally induced viscosity was observed when the cellulose derivatives were mixed with polymer graft copolymer. Further an increased permeation of acyclovir into the nasal mucosa was detected when it was combined with cationic hydroxyethylcellulose. None of the cellulose derivatives caused negative effects on tissues and cells of the nasal mucosa, as assessed by CBF.
They were discovered by Alec Bangham in 1960. Liposomes are used in the pharmaceutical and cosmetics industry for the transportation of diverse molecules and are among the most studied carrier system for drug delivery. Liposomes are an engrained formulation strategy to improve the drug delivery. They are vesicles of spherical form composed of phospholipids and steroids usually in the 50–450 nm size range [ 132 ]. These are considered as a better drug delivery vehicles since their membrane structure is analogous to the cell membranes and because they facilitate incorporation of drugs in them [ 132 ]. It has also been proved that they make therapeutic compounds stable, improve their biodistribution, can be used with hydrophilic and hydrophobic drugs and are also biocompatible and biodegradable. Liposomes are divided into four types: (1) conventional type liposomes: these consists of a lipid bilayer which can make either anionic, cationic, or neutral cholesterol and phospholipids, which surrounds an aqueous core material. In this case, both the lipid bilayer and the aqueous space can be filled with hydrophobic or hydrophilic materials, respectively. (2) PEGylated types: polyethylene glycol (PEG) is incorporated to the surface of liposome to achieve steric equilibrium, (3) ligand-targeted type: ligands like antibodies, carbohydrates and peptides, are linked to the surface of the liposome or to the end of previously attached PEG chains and (4) theranostic liposome type: it is an amalgamation kind of the previous three types of liposomes and generally consists of a nanoparticle along with a targeting, imaging and a therapeutic element [ 133 ].
The typical synthesis procedure for liposomes are as follows, thin layer hydration, mechanical agitation, solvent evaporation, solvent injection and the surfactant solubilization [ 134 ]. One aspect to point out on liposomes is that the drugs that are trapped within them are not bioavailable until they are released. Therefore, their accumulation in particular sites is very important to increase drug bioavailability within the therapeutic window at the right rates and times. Drug loading in liposomes is attained by active (drug encapsulated after liposome formation) and passive (drug encapsulated during liposome formation) approaches [ 135 ]. Hydrophilic drugs such as ampicillin and, 5-fluoro-deoxyuridine are typically confined in the aqueous core of the liposome and thus, their encapsulation does not depend on any modification in the drug/lipid ratio. However, the hydrophobic ones such as Amphotericin B, Indomethacin were found in the acyl hydrocarbon chain of the liposome and thus their engulfing are subjected to the characteristics of the acyl chain [ 136 ]. Among the passive loading approaches the mechanical and the solvent dispersion method as well as the detergent removal method can be mentioned [ 135 ].
There are obstacles with the use of liposomes for drug delivery purposes in the form of the RES (reticuloendothelial system), opsonization and immunogenicity although there are factors like enhanced permeability and EPR (retention effect) that can be utilized in order to boost the drug delivery efficiency of the liposomes [ 133 , 135 ]. Once liposomes get into the body, they run into opsonins and high density lipoproteins (HDLs) and low density lipoproteins (LDLs) while circulating in the bloodstream by themselves. Opsonins (immunoglobulins and fibronectin, for example) assist RES on recognizing and eliminating liposomes. HDLs and LDLs have interactions with liposomes and decrease their stability. Liposomes tends to gather more in the sites like the liver and the spleen, this is an advantage because then a high concentration of liposomes can help treat pathogenic diseases, although in the case of cancers this can lead to a delay in the removal of lipophilic anticancer drugs. This is the reason why as mentioned at the beginning, different types of liposomes have been developed, in this case PEGylated ones. Dimov et al. [ 137 ] reported an incessant procedure of flow system for the synthesis, functionalization and cleansing of liposomes. This research consists of vesicles under 300 nm in a lab-on-chip that are useful and potential candidates for cost-intensive drugs or protein encapsulation development [ 137 ]. This is very important because costs of production also determine whether or not a specific drug can be commercialized. Liposome-based systems have now been permitted by the FDA [ 133 , 135 , 138 , 139 , 140 ].
Polymeric micelles
Polymeric micelles are nanostructures made of amphiphilic block copolymers that gather by itself to form a core shell structure in the aqueous solution. The hydrophobic core can be loaded with hydrophobic drugs (e.g. camptothecin, docetaxel, paclitaxel), at the same time the hydrophilic shell makes the whole system soluble in water and stabilizes the core. Polymeric micelles are under 100 nm in size and normally have a narrow distribution to avoid fast renal excretion, thus permitting their accumulation in tumor tissues through the EPR effect. In addition, their polymeric shell restrains nonspecific interactions with biological components. These nanostructures have a strong prospective for hydrophobic drug delivery since their interior core structure permits the assimilation of these kind of drugs resulting in enhancement of stability and bioavailability [ 141 , 142 ].
Polymeric micelles are synthesized by two approaches: (1) convenient solvent-based direct dissolution of polymer followed by dialysis process or (2) precipitation of one block by adding a solvent [ 142 , 143 ]. The factors like, hydrophobic chain size in the amphiphilic molecule, amphiphiles concentration, solvent system and temperature, affects the micelle formation [ 144 ]. The micelle assembly creation starts when minimum concentration known as the critical micelle concentration (CMC) is reached by the amphiphilic molecules [ 143 ]. At lower concentrations, the amphiphilic molecules are indeed small and occur independently [ 143 ]. Drugs are loaded within polymeric micelles by three common methodologies such as direct dissolution process, solvent evaporation process, and the dialysis process. As of the direct dissolution process, the copolymer and the drugs combine with each other by themselves in the water medium and forms a drug loaded with the micelles. While in the solvent evaporation process, the copolymer and the intended drug is dissolved using a volatile organic solvent and finally, in case of the dialysis process, both the drug in solution and the copolymer in the organic solvent are combined in the dialysis bag and then dialyzed with the formation of the micelle [ 145 ].
The targeting of the drugs using different polymeric micelles as established by various mechanism of action including the boosted penetrability and the holding effect stimuli; complexing of a definite aiming ligand molecule to the surface of the micelle; or by combination of the monoclonal antibodies to the micelle corona [ 146 ]. Polymeric micelles are reported to be applicable for both drug delivery against cancer [ 143 ] and also for ocular drug delivery [ 147 ] as shown in Fig. 3 in which a polymeric micelle is used for reaching the posterior ocular tissues [ 147 ]. In the work by Li et al. [ 148 ], dasatinib was encapsulated within nanoparticles prepared from micellation of PEG-b-PC, to treat proliferative vitreoretinopathy (PVR), their size was 55 nm with a narrow distribution and they turned out to be noncytotoxic to ARPE-19 cells. This micellar formulation ominously repressed the cell proliferation, attachment and relocation in comparison to the free drugs [ 148 ]. The polymeric micelles is habitually get into the rear eye tissues through the transcleral pathway after relevant applications (Fig. 3 ; [ 147 ]).
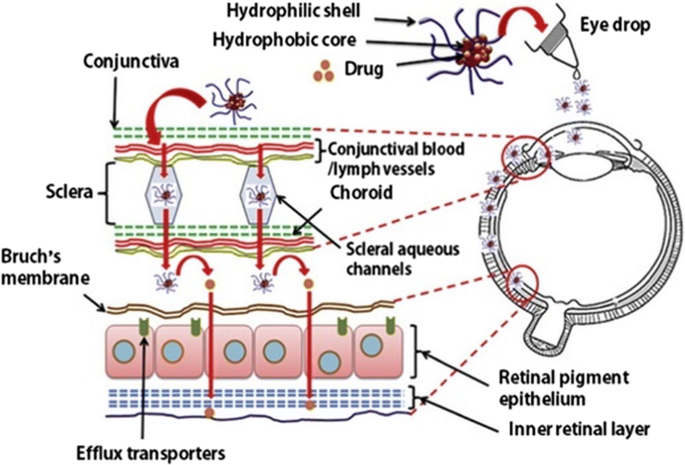
(the figure is reproduced from Mandal et al. [ 147 ] with required copyright permission)
Polymeric micelles used for reaching the posterior ocular tissues via the transcleral pathway after topical application
Dendrimers are highly bifurcated, monodisperse, well-defined and three-dimensional structures. They are globular-shaped and their surface is functionalized easily in a controlled way, which makes these structures excellent candidates as drug delivery agents [ 149 , 150 , 151 ]. Dendrimers can be synthesized by means of two approaches: The first one is the different route in which the dendrimer starts formation from its core and then it is extended outwards and the second is the convergent one, starts from the outside of the dendrimer [ 152 ]. Dendrimers are grouped into several kinds according to their functionalization moieties: PAMAM, PPI, liquid crystalline, core–shell, chiral, peptide, glycodendrimers and PAMAMOS, being PAMAM, the most studied for oral drug delivery because it is water soluble and it can pass through the epithelial tissue boosting their transfer via the paracellular pathway [ 153 ]. Dendrimers are limited in their clinical applications because of the presence of amine groups. These groups are positively charged or cationic which makes them toxic, hence dendrimers are usually modified in order to reduce this toxicity issue or to eliminate it. Drug loading in dendrimers is performed via the following mechanisms: Simple encapsulation, electrostatic interaction and covalent conjugation [ 154 ].
Drug is basically delivered by the dendrimers following two different paths, a) by the in vivo degradation of drug dendrimer’s covalent bonding on the basis of availability of suitable enzymes or favorable environment that could cleave the bonds and b) by discharge of the drug due to changes in the physical environment like pH, temperature etc., [ 154 ]. Dendrimers have been developed for transdermal, oral, ocular, pulmonary and in targeted drug delivery [ 155 ].
Jain et al. [ 156 ] have described the folate attached poly- l -lysine dendrimers (doxorubicin hydrochloride) as a capable cancer prevention drug carrier model for pH dependent drug discharge, target specificity, antiangiogenic and anticancer prospective, it was shown that doxorubicin-folate conjugated poly- l -lysine dendrimers increased the concentration of doxorubicin in the tumor by 121.5-fold after 24 h compared with free doxorubicin. Similarly, (Kaur et al. [ 157 ] developed folate-conjugated polypropylene imine dendrimers (FA-PPI) as a methotrexate (MTX) nanocarrier, for pH-sensitive drug release, selective targeting to cancer cells, and anticancer treatment. The in vitro studies on them showed sustained release, increased cell uptake and low cytotoxicity on MCF-7 cell lines [ 157 ]. Further, it has to be pointed out that the developed formulations, methotrexate (MTX)-loaded and folic acid-conjugated 5.0G PPI (MTX-FA-PPI), were selectively taken up by the tumor cells in comparison with the free drug, methotrexate (MTX).
Inorganic nanoparticles
Inorganic nanoparticles include silver, gold, iron oxide and silica nanoparticles are included. Studies focused on them are not as many as there are on other nanoparticle types discussed in this section although they show some potential applications. However, only few of the nanoparticles have been accepted for its clinical use, whereas the majority of them are still in the clinical trial stage. Metal nanoparticles, silver and gold, have particular properties like SPR (surface plasmon resonance), that liposomes, dendrimers, micelles do not possess. They showed several advantages such as good biocompatibility and versatility when it comes to surface functionalization.
Studies on their drug delivery-related activity have not been able to clear out whether the particulate or ionized form is actually related to their toxicity, and even though two mechanisms have been proposed, namely paracellular transport and transcytosis, there is not enough information about their in vivo transport and uptake mechanism [ 158 ]. Drugs can be conjugated to gold nanoparticles (AuNPs) surfaces via ionic or covalent bonding and physical absorption and they can deliver them and control their release through biological stimuli or light activation [ 159 ]. Silver nanoparticles exhibited antimicrobial activity, but as for drug delivery, very few studies have been carried out, for example, Prusty and Swain [ 160 ] synthesized an inter-linked and spongy polyacrylamide/dextran nano-hydrogels hybrid system with covalently attached silver nanoparticles for the release of ornidazole which turned out to have an in vitro release of 98.5% [ 160 ]. Similarly in another study, the iron oxide nanoparticles were synthesized using laser pyrolysis method and were covered with Violamycine B1, and antracyclinic antibiotics and tested against the MCF-7 cells for its cytotoxicity and the anti-proliferation properties along with its comparison with the commercially available iron oxide nanoparticles [ 161 ].
Nanocrystals
Nanocrystals are pure solid drug particles within 1000 nm range. These are 100% drug without any carriers molecule attached to it and are usually stabilized by using a polymeric steric stabilizers or surfactants. A nanocrystals suspension in a marginal liquid medium is normally alleviated by addition of a surfactant agent known as nano-suspension. In this case, the dispersing medium are mostly water or any aqueous or non-aqueous media including liquid polyethylene glycol and oils [ 162 , 163 ]. Nanocrystals possesses specific characters that permit them to overcome difficulties like increase saturation solubility, increased dissolution velocity and increased glueyness to surface/cell membranes. The process by which nanocrystals are synthesized are divided into top-down and bottom-up approaches. The top-down approach includes, sono-crystallization, precipitation, high gravity controlled precipitation technology, multi-inlet vortex mixing techniques and limited impinging liquid jet precipitation technique [ 162 ]. However, use of an organic solvent and its removal at the end makes this process quite expensive. The bottom-up approach involves, grinding procedures along with homogenization at higher pressure [ 162 ]. Among all of the methods, milling, high pressure homogenization, and precipitation are the most used methods for the production of nanocrystals. The mechanisms by which nanocrystals support the absorption of a drug to the system includes, enhancement of solubility, suspension rate and capacity to hold intestinal wall firmly [ 162 ]. Ni et al. [ 164 ] embedded cinaciguat nanocrystals in chitosan microparticles for pulmonary drug delivery of the hydrophobic drug. The nanoparticles were contrived for continuous release of the drug taking advantage of the swelling and muco-adhesive potential of the polymer. They found that inhalation efficacy might be conceded under the disease conditions, so more studies are needed to prove that this system has more potential [ 164 ].
Metallic nanoparticles
In recent years, the interest of using metallic nanoparticles has been growing in different medical applications, such as bioimaging, biosensors, target/sustained drug delivery, hyperthermia and photoablation therapy [ 35 , 165 ]. In addition, the modification and functionalization of these nanoparticles with specific functional groups allow them to bind to antibodies, drugs and other ligands, become these making these systems more promising in biomedical applications [ 166 ]. Although the most extensively studied, metallic nanoparticles are gold, silver, iron and copper, a crescent interest has been exploited regarding other kinds of metallic nanoparticles, such as, zinc oxide, titanium oxide, platinum, selenium, gadolinium, palladium, cerium dioxide among others [ 35 , 165 , 166 ].
Quantum dots
Quantum dots (QDs) are known as semiconductor nanocrystals with diameter range from 2 to 10 nm and their optical properties, such as absorbance and photoluminescence are size-dependent [ 167 ]. The QDs has gained great attention in the field of nanomedicine, since, unlike conventional organic dyes, the QDs presents emission in the near-infrared region (< 650 nm), a very desirable characteristic in the field of biomedical images, due to the low absorption by the tissues and reduction in the light scattering [ 167 , 168 ]. In addition, QDs with different sizes and/or compositions can be excited by the same light source resulting in separate emission colors over a wide spectral range [ 169 , 170 ]. In this sense, QDs are very appealing for multiplex imaging. In the medicine field QDs has been extensively studied as targeted drug delivery, sensors and bioimaging. A large number of studies regarding the applications of QDs as contrast agents for in vivo imaging is currently available in literature [ 168 , 171 , 172 , 173 ]. Han et al. [ 172 ] developed a novel fluorophore for intravital cytometric imaging based on QDs-antibodies conjugates coated with norbornene-displaying polyimidazole ligands. This fluorophore was used to label bone marrow cells in vivo. The authors found that the fluorophore was able to diffuse in the entire bone marrow and label rare populations of cells, such as hematopoietic stem and progenitor cells [ 172 ]. Shi et al. [ 171 ] developed a multifunctional biocompatible graphene oxide quantum dot covered with luminescent magnetic nanoplatform for recognize/diagnostic of a specific liver cancer tumor cells (glypican-3-expressing Hep G2). According to the authors the attachment of an anti-GPC3-antibody to the nanoplataform results in selective separation of Hep G2 hepatocellular carcinoma cells from infected blood samples [ 171 ]. QDs could also bring benefits in the sustained and/or controlled release of therapeutic molecules. Regarding the controlled release, this behavior can be achieved via external stimulation by light, heat, radio frequency or magnetic fields [ 170 , 174 , 175 ]. Olerile et al. [ 176 ] have developed a theranostic system based on co-loaded of QDs and anti-cancer drug in nanostructured lipid carriers as a parenteral multifunctional system. The nanoparticles were spherical with higher encapsulation efficiency of paclitaxel (80.7 ± 2.11%) and tumor growth inhibition rate of 77.85%. The authors also found that the system was able to specifically target and detect H22 tumor cells [ 176 ]. Cai et al. [ 177 ] have synthesized pH responsive quantum dots based on ZnO quantum dots decorated with PEG and hyaluronic acid for become stable in physiological conditions and for targeting specific cells with HA-receptor CD44, respectively. This nanocarrier was also evaluated for doxorubicin (DOX) sustained release. The nanocarrier was stable in physiological pH and DOX was loaded in the carrier by forming complex with Zn 2+ ions or conjugated to PEG. The DOX was released only in acidic intracellular conditions of tumor cells due to the disruption of ZnO QDs. The authors found that the anticancer activity was enhanced by the combination of DOX and ZnO QDs [ 177 ].
Protein and polysaccharides nanoparticles
Polysaccharides and proteins are collectively called as natural biopolymers and are extracted from biological sources such as plants, animals, microorganisms and marine sources [ 178 , 179 ]. Protein-based nanoparticles are generally decomposable, metabolizable, and are easy to functionalize for its attachment to specific drugs and other targeting ligands. They are normally produced by using two different systems, (a) from water-soluble proteins like bovine and human serum albumin and (b) from insoluble ones like zein and gliadin [ 180 ]. The usual methods to synthesize them are coacervation/desolvation, emulsion/solvent extraction, complex coacervation and electrospraying. The protein based nanoparticles are chemically altered in order to combine targeting ligands that identify exact cells and tissues to promote and augment their targeting mechanism [ 180 ]. Similarly, the polysaccharides are composed of sugar units (monosaccharides) linked through O-glycosidic bonds. The composition of these monomers as well as their biological source are able to confer to these polysaccharides, a series of specific physical–chemical properties [ 126 , 179 , 181 ]. One of the main drawback of the use of polysaccharides in the nanomedicine field is its degradation (oxidation) characteristics at high temperatures (above their melting point) which are often required in industrial processes. Besides, most of the polysaccharides are soluble in water, which limits their application in some fields of nanomedicine, such as tissue engineering [ 182 , 183 ]. However, techniques such as crosslinking of the polymer chains have been employed in order to guarantee stability of the polysaccharide chains, guaranteeing them stability in aqueous environments [ 182 , 183 ]. In Fig. 4 , examples of some polysaccharides used in nanomedicine obtained from different sources are summarized. The success of these biopolymers in nanomedicine and drug delivery is due to their versatility and specified properties such as since they can originate from soft gels, flexible fibers and hard shapes, so they can be porous or non-porous; they have great similarity with components of the extracellular matrix, which may be able to avoid immunological reactions [ 179 , 184 ].
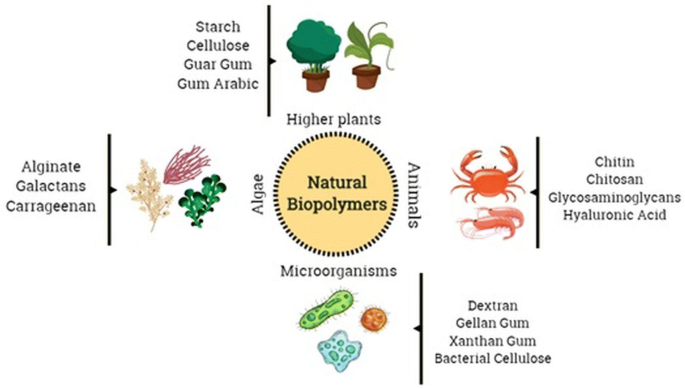
Different sources of natural biopolymers to be used in nanomedicine applications. Natural biopolymers could be obtained from higher plants, animals, microorganisms and algae
There is not much literature related to these kind of nanoparticles, however, since they are generated from biocompatible compounds they are excellent candidates for their further development as drug delivery systems. Yu et al. [ 185 ] synthesized Bovine serum albumin and tested its attachment and/or infiltration property through the opening of the cochlea and middle ear of guinea pigs. The nanoparticles considered as the drug transporters were tested for their loading capacity and release behaviors that could provide better bio-suitability, drug loading capacity, and well-ordered discharge mechanism [ 185 ].
Natural product-based nanotechnology and drug delivery
As per the World Health Organization (WHO) report, in developing countries, the basic health needs of approximately 80% of the population are met and/or complemented by traditional medicine [ 186 ]. Currently, the scientific community is focusing on the studies related to the bioactive compounds, its chemical composition and pharmacological potential of various plant species, to produce innovative active ingredients that present relatively minor side effects than existing molecules [ 5 , 187 ]. Plants are documented as a huge sources of natural compounds of medicinal importance since long time and still it holds ample of resources for the discovery of new and highly effective drugs. However, the discovery of active compounds through natural sources is associated with several issues because they originate from living beings whose metabolite composition changes in the presence of stress. In this sense, the pharmaceutical industries have chosen to combine their efforts in the development of synthetic compounds [ 187 , 188 , 189 ]. Nevertheless, the number of synthetic molecules that are actually marketed are going on decreasing day by day and thus research on the natural product based active compounds are again coming to the limelight in spite of its hurdles [ 189 , 190 ]. Most of the natural compounds of economic importance with medicinal potential that are already being marketed have been discovered in higher plants [ 187 , 191 ]. Several drugs that also possess natural therapeutic agents in their composition are already available commercially; their applications and names are as follows: malaria treatment (Artemotil ® derived from Artemisia annua L., a traditional Chinese medicine plant), Alzheimer’s disease treatment (Reminyl ® , an acetylcholinesterase inhibitor isolated from the Galanthus woronowii Losinsk), cancer treatment (Paclitaxel ® and its analogues derived from the Taxus brevifolia plant; vinblastine and vincristine extracted from Catharanthus roseus ; camptothecin and its analogs derived from Camptotheca acuminata Decne), liver disease treatment (silymarin from Silybum marianum ) [ 187 ].
The composition and activity of many natural compounds have already been studied and established. The alkaloids, flavonoids, tannins, terpenes, saponins, steroids, phenolic compounds, among others, are the bioactive molecules found in plants. However in most of the cases, these compounds have low absorption capacity due to the absence of the ability to cross the lipid membranes because of its high molecular sizes, and thus resulting in reduced bioavailability and efficacy [ 192 ]. These molecules also exhibit high systemic clearance, necessitating repeated applications and/or high doses, making the drug less effective for therapeutic use [ 189 ]. The scientific development of nanotechnology can revolutionize the development of formulations based on natural products, bringing tools capable of solving the problems mentioned above that limits the application of these compounds in large scale in the nanomedicine [ 7 , 189 ]. Utilization of nanotechnology techniques in the medical field has been extensively studied in the last few years [ 193 , 194 ]. Hence these can overcome these barriers and allow different compounds and mixtures to be used in the preparation of the same formulation. In addition, they can change the properties and behavior of a compound within the biological system [ 7 , 189 ]. Besides, bringing benefits to the compound relative to the solubility and stability of the compounds, release systems direct the compound to the specific site, increase bioavailability and extend compound action, and combine molecules with varying degrees of hydrophilicity/lipophilicity [ 7 ]. Also, there is evidence that the association of release systems with natural compounds may help to delay the development of drug resistance and therefore plays an important role in order to find new possibilities for the treatment of several diseases that have low response to treatment conventional approaches to modern medicine [ 7 , 189 ].
The natural product based materials are of two categories, (1) which are targeted to specific location and released in the specific sites to treat a number of diseases [ 43 , 195 ] and (2) which are mostly utilized in the synthesis process [ 196 ]. Most of the research is intended for treatment against the cancer disease, since it is the foremost reason of death worldwide nowadays [ 197 , 198 ]. In case of the cancer disease, different organs of the body are affected, and therefore the need for the development of an alternative medicine to target the cancerous cells is the utmost priority among the modern researchers, however, a number of applications of nanomedicine to other ailments is also being worked on [ 199 , 200 ]. These delivery systems are categorized in terms of their surface charge, particle size, size dispersion, shape, stability, encapsulation potential and biological action which are further utilized as per their requirements [ 33 ]. Some examples of biological compounds obtained from higher plants and their uses in the nanomedicine field are described in Fig. 5 . Pharmaceutical industries have continuously sought the development and application of new technologies for the advancement and design of modern drugs, as well as the enhancement of existing ones [ 71 , 201 ]. In this sense, the accelerated development of nanotechnology has driven the design of new formulations through different approaches, such as, driving the drug to the site of action (nanopharmaceutics); image and diagnosis (nanodiagnostic), medical implants (nanobiomaterials) and the combination diagnosis and treatment of diseases (nanotheranostics) [ 71 , 202 , 203 ].
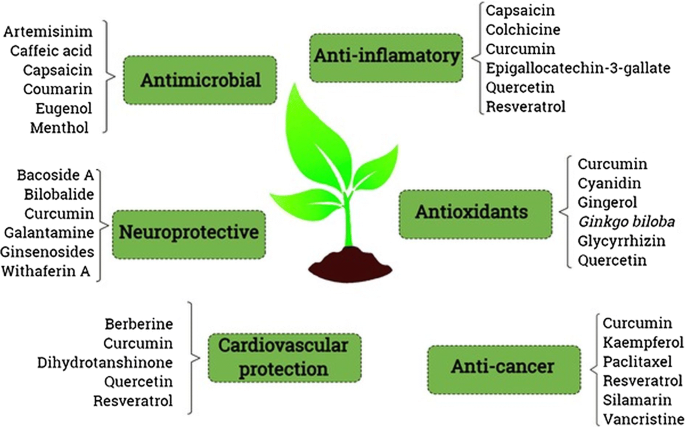
Examples of natural compounds extracted from higher plants used in nanomedicine aiming different approaches. Some of these extracts are already being marketed, others are in clinical trials and others are being extensively studied by the scientific community
Currently, many of the nanomedicines under development, are modified release systems for active ingredients (AI) that are already employed in the treatment of patients [ 203 , 204 ]. For this type of approach, it is evaluated whether the sustained release of these AIs modifies the pharmacokinetic profile and biodistribution. In this context, it can be ascertained that the nano-formulation offers advantages over the existing formulation if the AI is directed towards the target tissue shows increased uptake/absorption by the cells and lower toxicity profile for the organism [ 205 , 206 ]. This section is focused on berberine, curcumin, ellagic acid, resveratrol, curcumin and quercetin [ 8 ]. Some other compounds mentioned are doxorubicin, paclitaxel and vancomycin that also come from natural products.
Nanoparticles have been synthesized using natural products. For example, metallic, metal oxide and sulfides nanoparticles have been reported to be synthesized using various microorganisms including bacteria, fungi, algae, yeast and so on [ 207 ] or plant extracts [ 208 ]. For the first approach, the microorganism that aids the synthesis procedure is prepared in the adequate growth medium and then mixed with a metal precursor in solution and left for incubation to form the nanoparticles either intracellularly or extracellularly [ 209 , 210 , 211 ]. As for the second approach, the plant extract is prepared and mixed afterwards with the metal precursor in solution and incubated further at room temperature or boiling temperature for a definite time or exposed to light as an external stimulus to initiate the synthesis of nanoparticles [ 212 ].
Presently, these natural product based materials are considered as the key ingredients in the preparation and processing of new nano-formulations because they have interesting characteristics, such as being biodegradable, biocompatible, availability, being renewable and presenting low toxicity [ 178 , 179 , 213 ]. In addition to the aforementioned properties, biomaterials are, for the most part, capable of undergoing chemical modifications, guaranteeing them unique and desirable properties for is potential uses in the field of nanomedicine [ 45 , 214 ]. Gold, silver, cadmium sulfide and titanium dioxide of different morphological characteristics have been synthesized using a number of bacteria namely Escherichia coli , Pseudomonas aeruginosa , Bacillus subtilis and Klebsiella pneumoniae [ 211 ]. These nanoparticles, especially the silver nanoparticles have been abundantly studied in vitro for their antibacterial, antifungal, and cytotoxicity potential due to their higher potential among all metal nanoparticles [ 215 , 216 ]. In the event of microorganism mediated nanoparticle synthesis, maximum research is focused on the way that microorganisms reduce metal precursors and generate the nanoparticles. For instance, Rahimi et al. [ 217 ] synthesized silver nanoparticles using Candida albicans and studied their antibacterial activity against two pathogenic bacteria namely Staphylococcus aureus and E. coli. Similarly, Ali et al. [ 218 ] synthesized silver nanoparticles with the Artemisia absinthium aqueous extract and their antimicrobial activity was assessed versus Phytophthora parasitica and Phytophthora capsici [ 218 ]. Further, Malapermal et al. [ 219 ] used Ocimum basilicum and Ocimum sanctum extracts to synthesize nanoparticles and studied its antimicrobial potential against E. coli , Salmonella spp., S. aureus , and P. aeruginosa along with the antidiabetic potential. Likewise, Sankar et al. [ 220 ] also tested the effect of silver nanoparticles for both antibacterial and anticancer potential against human lung cancer cell line. Besides the use of microorganism, our group has synthesized silver, gold and iron oxide nanoparticles using various food waste materials such as extracts of Zea mays leaves [ 221 , 222 ], onion peel extract [ 223 ], silky hairs of Zea mays [ 224 ], outer peel of fruit of Cucumis melo and Prunus persica [ 225 ], outer peel of Prunus persica [ 226 ] and the rind extract of watermelon [ 227 ], etc. and have tested their potential antibacterial effects against various foodborne pathogenic bacteria, anticandidal activity against a number of pathogenic Candida spp., for their potential antioxidant activity and proteasome inhibitory effects.
For drug delivery purposes, the most commonly studied nanocarriers are crystal nanoparticles, liposomes, micelles, polymeric nanoparticles, solid lipid nanoparticles, superparamagnetic iron oxide nanoparticles and dendrimers [ 228 , 229 , 230 ]. All of these nanocarriers are formulated for natural product based drug delivery. For applications in cancer treatment, Gupta et al. [ 231 ] synthesized chitosan based nanoparticles loaded with Paclitaxel (Taxol) derived from Taxus brevifolia , and utilized them for treatment of different kinds of cancer. The authors concluded that the nanoparticle loaded drug exhibited better activity with sustained release, high cell uptake and reduced hemolytic toxicity compared with pure Paclitaxel [ 231 ]. Berberine is an alkaloid from the barberry plant. Chang et al. [ 232 ] created a heparin/berberine conjugate to increase the suppressive Helicobacter pylori growth and at the same time to reduce cytotoxic effects in infected cells [ 232 ] which is depicted in Fig. 6 .
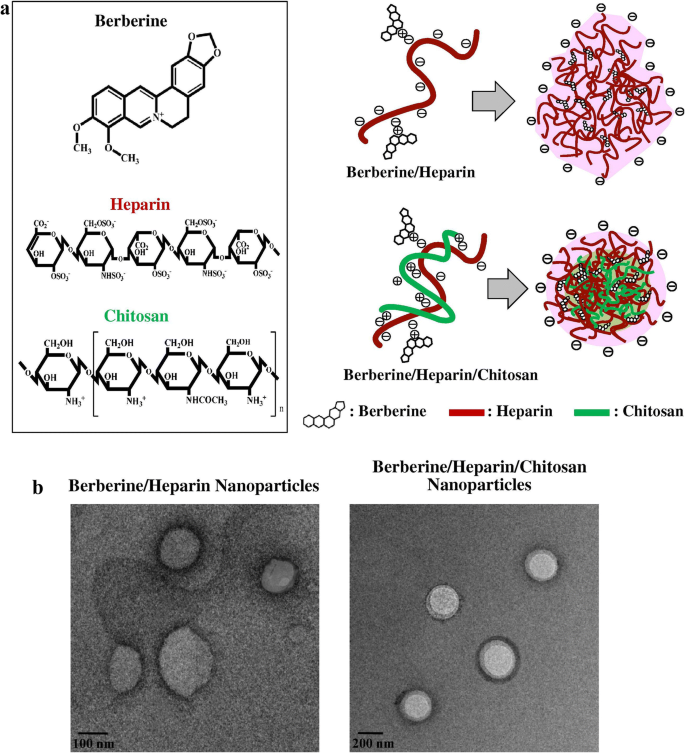
(the figure is reproduced from Chang et al. [ 232 ] with required copyright permission)
a Structure of berberine/heparin based nanoparticles and berberine/heparin/chitosan nanoparticles. b TEM images of the berberine/heparin nanoparticles and berberine/heparin/chitosan nanoparticles
Aldawsari and Hosny [ 233 ] synthesized ellagic acid-SLNs to encapsulate Vancomycin (a glycopeptide antibiotic produced in the cultures of Amycolatopsis orientalis ). Further, its in vivo tests were performed on rabbits and the results indicated that the ellagic acid prevented the formation of free oxygen radicals and their clearance radicals, thus preventing damages and promoting repair [ 233 ]. Quercetin is a polyphenol that belongs to the flavonoid group, it can be found in citrus fruits and vegetables and it has antioxidant properties. In a study by Dian et al. [ 234 ], polymeric micelles was used to deliver quercetin and the results showed that such micelles could provide continuous release for up to 10 days in vitro, with continuous plasma level and boosted complete accessibility of the drug under in vivo condition [ 234 ].
Daunorubicin is a natural product derived from a number of different wild type strains of Streptomyces , doxorubicin (DOX) is a hydrolated version of it used in chemotherapy [ 213 ]. Spillmann et al. [ 235 ] developed a multifunctional liquid crystal nanoparticle system for intracellular fluorescent imaging and for the delivery of doxorubicin in which the nanoparticles were functionalized with transferrin. Cellular uptake and sustained released were attained within endocytic vesicles in HEK 293T/17 cells. Perylene was used as a chromophore to track the particles and to encapsulate agents aimed for intracellular delivery [ 235 ]. Purama et al. [ 236 ] extracted dextran from two sucrose based lactic acid bacteria namely Streptococcus mutans and Leuconostoc mesenteroides . Agarwal et al. [ 237 ] formulated a dextran-based dendrimer formulation and evaluated its drug discharge capacity and haemolytic activity under in vitro condition. They concluded that the dendritic structure selectively enters the highly permeable portion of the affected cells without disturbing the healthy tissues thereby making more convenient for its application in the biomedical field [ 237 ]. Folate- functionalized superparamagnetic iron oxide nanoparticles developed previously for liver cancer cure are also been used for the delivery of Doxil (a form of doxorubicin which was the first FDA-approved nano-drug in 1995) [ 238 ]. The in vivo studies in rabbits and rats showed a two- and fourfold decrease compared with Doxil alone while folate aided and enhanced specific targeting [ 239 ]. Liposomes are the nanostructures that have been studied the most, and they have been used in several formulations for the delivery of natural products like resveratrol [ 240 ]. Curcumin, a polyphenolic compound obtained from turmeric, have been reported to be utilized in the cure of cancers including the breast, bone, cervices, liver, lung, and prostate [ 241 ]. Liposomal curcumin formulations have been developed for the treatment of cancer [ 242 , 243 ]. Cheng et al. [ 244 ] encapsulated curcumin in liposomes by different methods and compared the outcomes resulting that the one dependent on pH yielded stable products with good encapsulation efficiency and bio-accessibility with potential applications in cancer treatment [ 244 ].
Over all, it can be said that the sustained release systems of naturally occurring therapeutic compounds present themselves as a key tools for improving the biological activity of these compounds as well as minimizing their limitations by providing new alternatives for the cure of chronic and terminal diseases [ 8 , 245 ]. According to BBC Research, the global market for plant-derived pharmaceuticals will increase from $29.4 billion in 2017 to about $39.6 billion in 2022 with a compound annual growth rate (CAGR) of 6.15% in this period (BCC-RESEARCH). Some of nanostructure-based materials covered in this section have already been approved by the FDA. Bobo et al. [ 255 ] has provided the information on nanotechnology-based products already approved by the FDA (Table 1 ).
Regulation and reality: products now on the market
In the current medical nanotechnology scenario, there are 51 products based on this technology [ 204 , 246 , 247 , 248 ] which are currently being applied in clinical practice (Table 2 ). Notably, such nanomedicines are primarily developed for drugs, which have low aqueous solubility and high toxicity, and these nanoformulations are often capable of reducing the toxicity while increasing the pharmacokinetic properties of the drug in question.
According to a recent review by Caster et al. [ 249 ], although few nanomedicines have been regulated by the FDA there are many initiatives that are currently in progress in terms of clinical trials suggesting many nanotechnology-based new drugs will soon be able to reach the market. Among these nanomaterials that are in phase of study, 18 are directed to chemotherapeutics; 15 are intended for antimicrobial agents; 28 are for different medical applications and psychological diseases, autoimmune conditions and many others and 30 are aimed at nucleic acid based therapies [ 249 ]. The list of nanomedicine approved by FDA classified by type of carrier/material used in preparation of the formulation is shown in Table 2 .
Nanotechnology has dynamically developed in recent years, and all countries, whether developed or not, are increasing their investments in research and development in this field. However, researchers who work with practical applications of the nano-drugs deal with high levels of uncertainties, such as a framing a clear definition of these products; characterization of these nanomaterials in relation to safety and toxicity; and the lack of effective regulation. Although the list of approved nanomedicine is quite extensive, the insufficiency of specific regulatory guidelines for the development and characterization of these nanomaterials end up hampering its clinical potential [ 250 ]. The structure/function relationships of various nanomaterials, as well as their characteristics, composition and surface coating, interacts with the biological systems. In addition, it is important to evaluate the possibility of aggregate and agglomerate formation when these nanomedicines are introduced into biological systems, since they do not reflect the properties of the individual particle; this may generate different results and/or unexpected toxic effects depending on the nano-formulation [ 250 ].
The lack of standard protocols for nanomedicines characterization at physico-chemical and physiological/biological levels has often limited the efforts of many researchers to determine the toxic potential of nano-drugs in the early stages of testing, and that resulted in the failures in late-phase clinical trials. To simplify and/or shorten the approval process for nano based medicines/drugs, drug delivery system etc., a closer cooperation among regulatory agencies is warranted [ 204 , 251 ].
As a strategy for the lack of regulation of nanomedicines and nano drug delivery system; the safety assessment and the toxicity and compatibility of these are performed based on the regulations used by the FDA for conventional drugs. After gaining the status of a new research drug (Investigational New Drug, IND) by the FDA, nanomedicines, nano-drug delivery systems begin the clinical trials phase to investigate their safety and efficacy in humans. These clinical trials are divided into three phases: phase 1 (mainly assesses safety); phase 2 (mainly evaluates efficacy) and phase 3 (safety, efficacy and dosage are evaluated). After approval in these three phases the IND can be filed by the FDA to request endorsement of the new nanomedicine or nano drug delivery systems. However, this approach to nanomedicine regulation has been extensively questioned [ 204 , 246 , 252 ].
Due to the rapid development of nanotechnology as well as its potential use of nanomedicine, a reformed and more integrated regulatory approach is urgently required. In this regard, country governments must come together to develop new protocols that must be specific and sufficiently rigorous to address any safety concerns, thus ensuring the release of safe and beneficial nanomedicine for patients [ 204 , 252 , 253 ].
Future of nanomedicine and drug delivery system
The science of nanomedicine is currently among the most fascinating areas of research. A lot of research in this field in the last two decades has already led to the filling of 1500 patents and completion of several dozens of clinical trials [ 254 ]. As outlined in the various sections above, cancer appears to be the best example of diseases where both its diagnosis and therapy have benefited from nonmedical technologies. By using various types of nanoparticles for the delivery of the accurate amount of drug to the affected cells such as the cancer/tumour cells, without disturbing the physiology of the normal cells, the application of nanomedicine and nano-drug delivery system is certainly the trend that will remain to be the future arena of research and development for decades to come.
The examples of nanoparticles showed in this communications are not uniform in their size, with some truly measuring in nanometers while others are measured in sub-micrometers (over 100 nm). More research on materials with more consistent uniformity and drug loading and release capacity would be the further area of research. Considerable amount of progress in the use of metals-based nanoparticles for diagnostic purposes has also been addressed in this review. The application of these metals including gold and silver both in diagnosis and therapy is an area of research that could potentially lead to wider application of nanomedicines in the future. One major enthusiasm in this direction includes the gold-nanoparticles that appear to be well absorbed in soft tumour tissues and making the tumour susceptible to radiation (e.g., in the near infrared region) based heat therapy for selective elimination.
Despite the overwhelming understanding of the future prospect of nanomedicine and nano-drug delivery system, its real impact in healthcare system, even in cancer therapy/diagnosis, remains to be very limited. This attributes to the field being a new area of science with only two decades of real research on the subject and many key fundamental attributes still being unknown. The fundamental markers of diseased tissues including key biological markers that allow absolute targeting without altering the normal cellular process is one main future area of research. Ultimately, the application of nanomedicine will advance with our increasing knowledge of diseases at molecular level or that mirrors a nanomaterial-subcellular size comparable marker identification to open up avenues for new diagnosis/therapy. Hence, understanding the molecular signatures of disease in the future will lead to advances in nanomedicine applications. Beyond what we have outlined in this review using the known nanoprobes and nanotheragnostics products, further research would be key for the wider application of nanomedicine.
The concept of controlled release of specific drugs at the beleaguered sites, technology for the assessment of these events, drug’s effect in tissues/cellular level, as well as theoretical mathematical models of predication have not yet been perfected. Numerous studies in nanomedicine areas are centered in biomaterials and formulation studies that appear to be the initial stages of the biomedicine applications. Valuable data in potential application as drug therapeutic and diagnosis studies will come from animal studies and multidisciplinary researches that requires significant amount of time and research resources. With the growing global trend to look for more precise medicines and diagnosis, the future for a more intelligent and multi-centered approach of nanomedicine and nano-drug delivery technology looks bright.
There has been lots of enthusiasm with the simplistic view of development of nanorobots (and nanodevices) that function in tissue diagnosis and repair mechanism with full external control mechanism. This has not yet been a reality and remains a futuristic research that perhaps could be attained by mankind in the very near future. As with their benefits, however, the potential risk of nanomedicines both to humans and the environment at large require long term study too. Hence, proper impact analysis of the possible acute or chronic toxicity effects of new nanomaterials on humans and environment must be analyzed. As nanomedicines gain popularity, their affordability would be another area of research that needs more research input. Finally, the regulation of nanomedicines, as elaborated in the previous section will continue to evolve alongside the advances in nanomedicine applications.
The present review discusses the recent advances in nanomedicines, including technological progresses in the delivery of old and new drugs as well as novel diagnostic methodologies. A range of nano-dimensional materials, including nanorobots and nanosensors that are applicable to diagnose, precisely deliver to targets, sense or activate materials in live system have been outlined. Initially, the use of nanotechnology was largely based on enhancing the solubility, absorption, bioavailability, and controlled-release of drugs. Even though the discovery of nanodrugs deal with high levels of uncertainties, and the discovery of pharmacologically active compounds from natural sources is not a favored option today, as compared to some 50 years ago; hence enhancing the efficacy of known natural bioactive compounds through nanotechnology has become a common feature. Good examples are the therapeutic application of nanotechnology for berberine, curcumin, ellagic acid, resveratrol, curcumin and quercetin. The efficacy of these natural products has greatly improved through the use of nanocarriers formulated with gold, silver, cadmium sulphide, and titanium dioxide polymeric nanoparticles together with solid lipid nanoparticles, crystal nanoparticles, liposomes, micelles, superparamagnetic iron oxide nanoparticles and dendrimers.
There has been a continued demand for novel natural biomaterials for their quality of being biodegradable, biocompatible, readily availability, renewable and low toxicity. Beyond identifying such polysaccharides and proteins natural biopolymers, research on making them more stable under industrial processing environment and biological matrix through techniques such as crosslinking is among the most advanced research area nowadays. Polymeric nanoparticles (nanocapsules and nanospheres) synthesized through solvent evaporation, emulsion polymerization and surfactant-free emulsion polymerization have also been widely introduced. One of the great interest in the development of nanomedicine in recent years relates to the integration of therapy and diagnosis (theranostic) as exemplified by cancer as a disease model. Good examples have been encapsulated such as, oleic acid-coated iron oxide nanoparticles for diagnostic applications through near-infrared; photodynamic detection of colorectal cancer using alginate and folic acid based chitosan nanoparticles; utilization of cathepsin B as metastatic processes fluorogenic peptide probes conjugated to glycol chitosan nanoparticles; iron oxide coated hyaluronic acid as a biopolymeric material in cancer therapy; and dextran among others.
Since the 1990s, the list of FDA-approved nanotechnology-based products and clinical trials has staggeringly increased and include synthetic polymer particles; liposome formulations; micellar nanoparticles; protein nanoparticles; nanocrystals and many others often in combination with drugs or biologics. Even though regulatory mechanisms for nanomedicines along with safety/toxicity assessments will be the subject of further development in the future, nanomedicine has already revolutionized the way we discover and administer drugs in biological systems. Thanks to advances in nanomedicine, our ability to diagnose diseases and even combining diagnosis with therapy has also became a reality.
Abbreviations
Amaranth red
ciliary beat frequency
carbamazepine
colorectal cancer
carboxymethylcellulose
((2-amino-2-carboxyethyl) disulfanyl) nicotinic acid (Cys-MNA)
penetrability and holding
folic acid-conjugated dextran
Food and Drug Administration
ferrous oxide
hyaluronic acid
high density lipoproteins
hydroxypropylmethylcellulose
low density lipoproteins
magnetic resonance
near infrared
nanoparticle
perfluorohexane
repaglidine
antivascular endothelial growth factor
venlafaxine
xanthan gum
Swamy MK, Sinniah UR. Patchouli (Pogostemon cablin Benth.): botany, agrotechnology and biotechnological aspects. Ind Crops Prod. 2016;87:161–76.
Article CAS Google Scholar
Mohanty SK, Swamy MK, Sinniah UR, Anuradha M. Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): botanical, agronomical, phytochemical, pharmacological, and biotechnological aspects. Molecules. 1019;2017:22.
Google Scholar
Rodrigues T, Reker D, Schneider P, Schneider G. Counting on natural products for drug design. Nat Chem. 2016;8:531.
Article CAS PubMed Google Scholar
Siddiqui AA, Iram F, Siddiqui S, Sahu K. Role of natural products in drug discovery process. Int J Drug Dev Res. 2014;6(2):172–204.
CAS Google Scholar
Beutler JA. Natural products as a foundation for drug discovery. Curr Prot Pharmacol. 2009;46(1):9–11.
Thilakarathna SH, Rupasinghe H. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5:3367–87.
Article PubMed PubMed Central CAS Google Scholar
Bonifácio BV, da Silva PB, dos Santos Ramos MA, Negri KMS, Bauab TM, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomed. 2014;9:1.
Watkins R, Wu L, Zhang C, Davis RM, Xu B. Natural product-based nanomedicine: recent advances and issues. Int J Nanomed. 2015;10:6055.
Martinho N, Damgé C, Reis CP. Recent advances in drug delivery systems. J Biomater Nanobiotechnol. 2011;2:510.
Jahangirian H, Lemraski EG, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomed. 2017;12:2957.
Liu Z, Tabakman S, Welsher K, Dai H. Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009;2:85–120.
Article CAS PubMed PubMed Central Google Scholar
Orive G, Gascon AR, Hernández RM, Domı́nguez-Gil A, Pedraz JL. Techniques: new approaches to the delivery of biopharmaceuticals. Trends Pharmacol Sci. 2004;25:382–7.
Razzacki SZ, Thwar PK, Yang M, Ugaz VM, Burns MA. Integrated microsystems for controlled drug delivery. Adv Drug Deliv Rev. 2004;56:185–98.
Article PubMed CAS Google Scholar
Arayne MS, Sultana N, Qureshi F. nanoparticles in delivery of cardiovascular drugs. Pak J Pharm Sci. 2007;20:340–8.
CAS PubMed Google Scholar
Patra JK, Baek K-H. Green nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomater. 2014;2014:219.
Joseph RR, Venkatraman SS. Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine. 2017;12:683–702.
Mirza AZ, Siddiqui FA. Nanomedicine and drug delivery: a mini review. Int Nano Lett. 2014;4:94.
Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A. Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules. 2016;21:836.
Article CAS PubMed Central Google Scholar
Lam P-L, Wong W-Y, Bian Z, Chui C-H, Gambari R. Recent advances in green nanoparticulate systems for drug delivery: efficient delivery and safety concern. Nanomedicine. 2017;12:357–85.
Haba Y, Kojima C, Harada A, Ura T, Horinaka H, Kono K. Preparation of poly (ethylene glycol)-modified poly (amido amine) dendrimers encapsulating gold nanoparticles and their heat-generating ability. Langmuir. 2007;23:5243–6.
Shi X, Sun K, Baker JR Jr. Spontaneous formation of functionalized dendrimer-stabilized gold nanoparticles. J Phys Chem C. 2008;112:8251–8.
Park S-H, Oh S-G, Mun J-Y, Han S-S. Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Coll Surf B. 2006;48:112–8.
de Villiers MM, Aramwit P, Kwon GS. Nanotechnology in drug delivery. New York: Springer; 2008.
Kabanov AV, Lemieux P, Vinogradov S, Alakhov V. Pluronic ® block copolymers: novel functional molecules for gene therapy. Adv Drug Deliv Rev. 2002;54:223–33.
Wang N, Feng Y. Elaborating the role of natural products-induced autophagy in cancer treatment: achievements and artifacts in the state of the art. BioMed Res Int. 2015;2015:934207.
PubMed PubMed Central Google Scholar
Ouattara B, Simard RE, Holley RA. Piette GJ-P, Bégin A: Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int J Food Microbiol. 1997;37:155–62.
Sharma G, Raturi K, Dang S, Gupta S, Gabrani R. Combinatorial antimicrobial effect of curcumin with selected phytochemicals on Staphylococcus epidermidis . J Asian Nat Prod Res. 2014;16:535–41.
Abdelwahab SI, Sheikh BY, Taha MME, How CW, Abdullah R, Yagoub U, El-Sunousi R, Eid EE. Thymoquinone-loaded nanostructured lipid carriers: preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int J Nanomed. 2013;8:2163.
Krauel K, Pitaksuteepong T, Davies NM, Rades T. Entrapment of bioactive molecules in poly (alkylcyanoacrylate) nanoparticles. Am J Drug Deliv. 2004;2:251–9.
Tan Q, Liu W, Guo C, Zhai G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int J Nanomed. 2011;6:1621.
Sanna V, Roggio AM, Siliani S, Piccinini M, Marceddu S, Mariani A, Sechi M. Development of novel cationic chitosan-and anionic alginate–coated poly ( d, l- lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. Int J Nanomed. 2012;7:5501.
Casettari L, Illum L. Chitosan in nasal delivery systems for therapeutic drugs. J Control Release. 2014;190:189–200.
Obeid MA, Al Qaraghuli MM, Alsaadi M, Alzahrani AR, Niwasabutra K, Ferro VA. Delivering natural products and biotherapeutics to improve drug efficacy. Ther Deliv. 2017;8:947–56.
Miele E, Spinelli GP, Miele E, Di Fabrizio E, Ferretti E, Tomao S, Gulino A. Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. Int J Nanomed. 2012;7:3637.
McNamara K, Tofail SA. Nanosystems: the use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys Chem Chem Phys. 2015;17:27981–95.
Saadeh Y, Vyas D. Nanorobotic applications in medicine: current proposals and designs. Am J Robot Surg. 2014;1:4–11.
Article PubMed PubMed Central Google Scholar
Oliveira ON Jr, Iost RM, Siqueira JR Jr, Crespilho FN, Caseli L. Nanomaterials for diagnosis: challenges and applications in smart devices based on molecular recognition. ACS Appl Mater Interfaces. 2014;6:14745–66.
De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed. 2008;3:133.
Article Google Scholar
Holzinger M, Le Goff A, Cosnier S. Nanomaterials for biosensing applications: a review. Front Chem. 2014;2:63.
Golovin YI, Gribanovsky SL, Golovin DY, Klyachko NL, Majouga AG, Master AM, Sokolsky M, Kabanov AV. Towards nanomedicines of the future: remote magneto-mechanical actuation of nanomedicines by alternating magnetic fields. J Control Release. 2015;219:43–60.
Lu H, Wang J, Wang T, Zhong J, Bao Y, Hao H. Recent progress on nanostructures for drug delivery applications. J Nanomater. 2016;2016:20.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941.
Kumari A, Kumar V, Yadav S. Nanotechnology: a tool to enhance therapeutic values of natural plant products. Trends Med Res. 2012;7:34–42.
Chen F, Ehlerding EB, Cai W. Theranostic nanoparticles. J Nucl Med. 2014;55:1919–22.
Swierczewska M, Han H, Kim K, Park J, Lee S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv Drug Deliv Rev. 2016;99:70–84.
Chen K, Chen X. Design and development of molecular imaging probes. Curr Top Med Chem. 2010;10:1227–36.
Yhee JY, Son S, Kim SH, Park K, Choi K, Kwon IC. Self-assembled glycol chitosan nanoparticles for disease-specific theranostics. J Control Release. 2014;193:202–13.
Lee C-M, Jang D, Kim J, Cheong S-J, Kim E-M, Jeong M-H, Kim S-H, Kim DW, Lim ST, Sohn M-H, et al. Oleyl-Chitosan nanoparticles based on a dual probe for Optical/MR imaging in vivo. Bioconjug Chem. 2011;22:186–92.
Yang S-J, Lin F-H, Tsai H-M, Lin C-F, Chin H-C, Wong J-M, Shieh M-J. Alginate-folic acid-modified chitosan nanoparticles for photodynamic detection of intestinal neoplasms. Biomaterials. 2011;32:2174–82.
Ryu JH, Na JH, Ko HK, You DG, Park S, Jun E, Yeom HJ, Seo DH, Park JH, Jeong SY. Non-invasive optical imaging of cathepsin B with activatable fluorogenic nanoprobes in various metastatic models. Biomaterials. 2014;35:2302–11.
Lapčík L, Lapcik L, De Smedt S, Demeester J, Chabrecek P. Hyaluronan: preparation, structure, properties, and applications. Chem Rev. 1998;98:2663–84.
Article PubMed Google Scholar
Kim H, Kim Y, Kim I-H, Kim K, Choi Y. ROS-responsive activatable photosensitizing agent for imaging and photodynamic therapy of activated macrophages. Theranostics. 2014;4:1.
Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Jeong SY. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31:106–14.
Kamat M, El-Boubbou K, Zhu DC, Lansdell T, Lu X, Li W, Huang X. Hyaluronic acid immobilized magnetic nanoparticles for active targeting and imaging of macrophages. Bioconjug Chem. 2010;21:2128–35.
Arpicco S, Lerda C, Dalla Pozza E, Costanzo C, Tsapis N, Stella B, Donadelli M, Dando I, Fattal E, Cattel L. Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur J Pharm Biopharm. 2013;85:373–80.
Wang G, Gao S, Tian R, Miller-Kleinhenz J, Qin Z, Liu T, Li L, Zhang F, Ma Q, Zhu L. Theranostic hyaluronic acid-iron micellar nanoparticles for magnetic-field-enhanced in vivo cancer chemotherapy. ChemMedChem. 2018;13:78–86.
Choi KY, Jeon EJ, Yoon HY, Lee BS, Na JH, Min KH, Kim SY, Myung S-J, Lee S, Chen X. Theranostic nanoparticles based on PEGylated hyaluronic acid for the diagnosis, therapy and monitoring of colon cancer. Biomaterials. 2012;33:6186–93.
Gombotz WR, Wee S. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31:267–85.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–26.
Baghbani F, Moztarzadeh F, Mohandesi JA, Yazdian F, Mokhtari-Dizaji M. Novel alginate-stabilized doxorubicin-loaded nanodroplets for ultrasounic theranosis of breast cancer. Int J Biol Macromol. 2016;93:512–9.
Podgórna K, Szczepanowicz K, Piotrowski M, Gajdošová M, Štěpánek F, Warszyński P. Gadolinium alginate nanogels for theranostic applications. Coll Surf B. 2017;153:183–9.
Moscovici M. Present and future medical applications of microbial exopolysaccharides. Front Microbiol. 1012;2015:6.
Ding Z, Liu P, Hu D, Sheng Z, Yi H, Gao G, Wu Y, Zhang P, Ling S, Cai L. Redox-responsive dextran based theranostic nanoparticles for near-infrared/magnetic resonance imaging and magnetically targeted photodynamic therapy. Biomater Sci. 2017;5:762–71.
Hong S-P, Kang SH, Kim DK, Kang BS. Paramagnetic nanoparticle-based targeting theranostic agent for c6 rat glioma cell. J Nanomater. 2016; 2016:7617894. https://doi.org/10.1155/2016/7617894 .
Mignani S, El Kazzouli S, Bousmina M, Majoral JP. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: a concise overview. Adv Drug Deliv Rev. 2013;65:1316–30.
Lounnas V, Ritschel T, Kelder J, McGuire R, Bywater RP, Foloppe N. Current progress in structure-based rational drug design marks a new mindset in drug discovery. Comput Struc Biotechnol J. 2013;5:e201302011.
Mavromoustakos T, Durdagi S, Koukoulitsa C, Simcic M, Papadopoulos M, Hodoscek M, Golic Grdadolnik S. Strategies in the rational drug design. Curr Med Chem. 2011;18:2517–30.
Wong PT, Choi SK. Mechanisms of drug release in nanotherapeutic delivery systems. Chem Rev. 2015;115:3388–432.
Prachayasittikul V, Worachartcheewan A, Shoombuatong W, Songtawee N, Simeon S, Prachayasittikul V, Nantasenamat C. Computer-aided drug design of bioactive natural products. Curr Top Med Chem. 2015;15:1780–800.
Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116:2826–85.
Pelaz B, Alexiou C, Alvarez-Puebla RA, Alves F, Andrews AM, Ashraf S, Balogh LP, Ballerini L, Bestetti A, Brendel C, Bosi S. Diverse applications of nanomedicine. Acs Nano. 2017;11:2313–81.
Mattos BD, Rojas OJ, Magalhaes WLE. Biogenic silica nanoparticles loaded with neem bark extract as green, slow-release biocide. J Clean Prod. 2017;142:4206–13.
Kinnear C, Moore TL, Rodriguez-Lorenzo L, Rothen-Rutishauser B, Petri-Fink A. Form follows function: nanoparticle shape and its implications for nanomedicine. Chem Rev. 2017;117:11476–521.
Sethi M, Sukumar R, Karve S, Werner ME, Wang EC, Moore DT, Kowalczyk SR, Zhang L, Wang AZ. Effect of drug release kinetics on nanoparticle therapeutic efficacy and toxicity. Nanoscale. 2014;6:2321–7.
Mattos BD, Tardy BL, Magalhaes WLE, Rojas OJ. Controlled release for crop and wood protection: recent progress toward sustainable and safe nanostructured biocidal systems. J Control Release. 2017;262:139–50.
Siepmann F, Herrmann S, Winter G, Siepmann J. A novel mathematical model quantifying drug release from lipid implants. J Control Release. 2008;128:233–40.
Ding CZ, Li ZB. A review of drug release mechanisms from nanocarrier systems. Mater Sci Eng. 2017;76:1440–53.
Lee JH, Yeo Y. Controlled drug release from pharmaceutical nanocarriers. Chem Eng Sci. 2015;125:75–84.
Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116:2602–63.
Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2012;64:302–15.
Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernandez S, de la Fuente JM, Nienhaus GU, Parak WJ. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. Acs Nano. 2015;9:6996–7008.
Almalik A, Benabdelkamel H, Masood A, Alanazi IO, Alradwan I, Majrashi MA, Alfadda AA, Alghamdi WM, Alrabiah H, Tirelli N, Alhasan AH. Hyaluronic acid coated chitosan nanoparticles reduced the immunogenicity of the formed protein corona. Sci Rep. 2017;7:10542.
Martens TF, Remaut K, Deschout H, Engbersen JFJ, Hennink WE, van Steenbergen MJ, Demeester J, De Smedt SC, Braeckmans K. Coating nanocarriers with hyaluronic acid facilitates intravitreal drug delivery for retinal gene therapy. J Control Release. 2015;202:83–92.
Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc Natl Acad Sci USA. 2013;110:10753–8.
Gao WW, Zhang LF. Coating nanoparticles with cell membranes for targeted drug delivery. J Drug Target. 2015;23:619–26.
Muller J, Bauer KN, Prozeller D, Simon J, Mailander V, Wurm FR, Winzen S, Landfester K. Coating nanoparticles with tunable surfactants facilitates control over the protein corona. Biomaterials. 2017;115:1–8.
Gao H, Yang Z, Zhang S, Cao S, Shen S, Pang Z, Jiang X. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci Rep. 2013;3:2534.
Jain A, Jain SK. Ligand-appended BBB-targeted nanocarriers (LABTNs). Crit Rev Ther Drug Carrier Syst. 2015;32:149–80.
Shen HX, Shi SJ, Zhang ZR, Gong T, Sun X. Coating solid lipid nanoparticles with hyaluronic acid enhances antitumor activity against melanoma stem-like cells. Theranostics. 2015;5:755–71.
Gao X, Zhang J, Xu Q, Huang Z, Wang YY, Shen Q. Hyaluronic acid-coated cationic nanostructured lipid carriers for oral vincristine sulfate delivery. Drug Dev Ind Pharm. 2017;43:661–7.
Wang T, Hou JH, Su C, Zhao L, Shi YJ. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J Nanobiotechnol. 2017;15:7.
Muro S. Challenges in design and characterization of ligand-targeted drug delivery systems. J Control Release. 2012;164:125–37.
Kou L, Sun J, Zhai Y, He Z. The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J Pharm Sci. 2013;8:1–10.
Li Z, Zhang Y, Zhu D, Li S, Yu X, Zhao Y, Ouyang X, Xie Z, Li L. Transporting carriers for intracellular targeting delivery via non-endocytic uptake pathways. Drug delivery. 2017;24:45–55.
Salatin S, Yari Khosroushahi A. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J Cell Mol Med. 2017;21:1668–86.
Anirudhan TS, Nair AS. Temperature and ultrasound sensitive gatekeepers for the controlled release of chemotherapeutic drugs from mesoporous silica nanoparticles. J Mater Chem B. 2018;6:428–39.
Al-Ahmady Z, Kostarelos K. Chemical components for the design of temperature-responsive vesicles as cancer therapeutics. Chem Rev. 2016;116:3883–918.
Bai Y, Xie FY, Tian W. Controlled self-assembly of thermo-responsive amphiphilic h-shaped polymer for adjustable drug release. Chin J Polym Sci. 2018;36:406–16.
Zhang Z, Zhang D, Wei L, Wang X, Xu YL, Li HW, Ma M, Chen B, Xiao LH. Temperature responsive fluorescent polymer nanoparticles (TRFNPs) for cellular imaging and controlled releasing of drug to living cells. Coll Surf B. 2017;159:905–12.
Guo Y, Zhang Y, Ma J, Li Q, Li Y, Zhou X, Zhao D, Song H, Chen Q, Zhu X. Light/magnetic hyperthermia triggered drug released from multi-functional thermo-sensitive magnetoliposomes for precise cancer synergetic theranostics. J Control Release. 2017;272:145–58.
Hervault A, Thanh NT. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale. 2014;6:11553–73.
Mathiyazhakan M, Wiraja C, Xu CJ: A Concise Review of Gold Nanoparticles-Based Photo-Responsive Liposomes for Controlled Drug Delivery. Nano - Micro Letters 2018, 10.
Xu L, Qiu LZ, Sheng Y, Sun YX, Deng LH, Li XQ, Bradley M, Zhang R. Biodegradable pH-responsive hydrogels for controlled dual-drug release. J Mater Chem B. 2018;6:510–7.
Ma GL, Lin WF, Yuan ZF, Wu J, Qian HF, Xua LB, Chen SF. Development of ionic strength/pH/enzyme triple-responsive zwitterionic hydrogel of the mixed l -glutamic acid and l -lysine polypeptide for site-specific drug delivery. J Mater Chem B. 2017;5:935–43.
Grillo R, Gallo J, Stroppa DG, Carbo-Argibay E, Lima R, Fraceto LF, Banobre-Lopez M. Sub-micrometer magnetic nanocomposites: insights into the effect of magnetic nanoparticles interactions on the optimization of SAR and MRI performance. Acs Appl Mater Interfaces. 2016;8:25777–87.
Alonso J, Khurshid H, Devkota J, Nemati Z, Khadka NK, Srikanth H, Pan JJ, Phan MH. Superparamagnetic nanoparticles encapsulated in lipid vesicles for advanced magnetic hyperthermia and biodetection. J Appl Phys. 2016;119:083904.
Ulbrich K, Hola K, Subr V, Bakandritsos A, Tucek J, Zboril R. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem Rev. 2016;116:5338–431.
Chen CW, Syu WJ, Huang TC, Lee YC, Hsiao JK, Huang KY, Yu HP, Liao MY, Lai PS. Encapsulation of Au/Fe 3 O 4 nanoparticles into a polymer nanoarchitecture with combined near infrared-triggered chemo-photothermal therapy based on intracellular secondary protein understanding. J Mater Chem B. 2017;5:5774–82.
Portero A, Remunan-Lopez C, Criado M, Alonso M. Reacetylated chitosan microspheres for controlled delivery of anti-microbial agents to the gastric mucosa. J Microencapsul. 2002;19:797–809.
Artursson P, Lindmark T, Davis SS, Illum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm Res. 1994;11:1358–61.
Fernández-Urrusuno R, Calvo P, Remuñán-López C, Vila-Jato JL, Alonso MJ. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm Res. 1999;16:1576–81.
De Campos AM, Sánchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int J Pharm. 2001;224:159–68.
Al-Qadi S, Grenha A, Carrión-Recio D, Seijo B, Remuñán-López C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: in vivo evaluation of insulin-loaded formulations. J Control Release. 2012;157:383–90.
Silva MM, Calado R, Marto J, Bettencourt A, Almeida AJ, Gonçalves L. Chitosan Nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar Drugs. 2017;15:370.
Article PubMed Central Google Scholar
Pistone S, Goycoolea FM, Young A, Smistad G, Hiorth M. Formulation of polysaccharide-based nanoparticles for local administration into the oral cavity. Eur J Pharm Sci. 2017;96:381–9.
Liu S, Yang S, Ho PC. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J Pharm Sci. 2018;13:72–81.
Jain A, Jain SK. Optimization of chitosan nanoparticles for colon tumors using experimental design methodology. Artif Cells Nanomed Biotechnol. 2016;44:1917–26.
Sosnik A. Alginate particles as platform for drug delivery by the oral route: state-of-the-art. ISRN Pharm. 2014;2014:926157.
Patil NH, Devarajan PV. Insulin-loaded alginic acid nanoparticles for sublingual delivery. Drug Deliv. 2016;23:429–36.
Haque S, Md S, Sahni JK, Ali J, Baboota S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J Psychiatr Res. 2014;48:1–12.
Román JV, Galán MA, del Valle EMM. Preparation and preliminary evaluation of alginate crosslinked microcapsules as potential drug delivery system (DDS) for human lung cancer therapy. Biomed Phys Eng Expr. 2016;2:035015.
Garrait G, Beyssac E, Subirade M. Development of a novel drug delivery system: chitosan nanoparticles entrapped in alginate microparticles. J Microencapsul. 2014;31:363–72.
Costa J, Silva N, Sarmento B, Pintado M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur J Clin Microbiol Infect Dis. 2015;34:1255–62.
Goswami S, Naik S. Natural gums and its pharmaceutical application. J Sci Innovative Res. 2014;3:112–21.
Laffleur F, Michalek M. Modified xanthan gum for buccal delivery—a promising approach in treating sialorrhea. Int J Biol Macromol. 2017;102:1250–6.
Huang J, Deng Y, Ren J, Chen G, Wang G, Wang F, Wu X. Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr Polym. 2018;186:54–63.
Menzel C, Jelkmann M, Laffleur F, Bernkop-Schnürch A. Nasal drug delivery: design of a novel mucoadhesive and in situ gelling polymer. Int J Pharm. 2017;517:196–202.
Sun B, Zhang M, Shen J, He Z, Fatehi P, Ni Y. Applications of cellulose-based materials in sustained drug delivery systems. Curr Med Chem. 2017. https://doi.org/10.2174/0929867324666170705143308 .
Elseoud WSA, Hassan ML, Sabaa MW, Basha M, Hassan EA, Fadel SM. Chitosan nanoparticles/cellulose nanocrystals nanocomposites as a carrier system for the controlled release of repaglinide. Int J Biol Macromol. 2018;111:604–13.
Agarwal T, Narayana SGH, Pal K, Pramanik K, Giri S, Banerjee I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int J Biol Macromol. 2015;75:409–17.
Hansen K, Kim G, Desai KG, Patel H, Olsen KF, Curtis-Fisk J, Tocce E, Jordan S, Schwendeman SP. Feasibility investigation of cellulose polymers for mucoadhesive nasal drug delivery applications. Mol Pharm. 2015;12:2732–41.
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomed. 2015;10:975.
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharm. 2015;6:286.
Kotla NG, Chandrasekar B, Rooney P, Sivaraman G, Larrañaga A, Krishna KV, Pandit A, Rochev Y. Biomimetic lipid-based nanosystems for enhanced dermal delivery of drugs and bioactive agents. ACS Biomater Sci Eng. 2017;3:1262–72.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8:102.
Mohan A, Narayanan S, Sethuraman S, Krishnan UM. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. BioMed res int. 2014;2014:424239.
Dimov N, Kastner E, Hussain M, Perrie Y, Szita N. Formation and purification of tailored liposomes for drug delivery using a module-based micro continuous-flow system. Sci Rep. 2017;7:12045.
Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23:3319–29.
Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev. 2013;113:1904–2074.
Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–9.
Miyata K, Christie RJ, Kataoka K. Polymeric micelles for nano-scale drug delivery. React Funct Polym. 2011;71:227–34.
Xu W, Ling P, Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;2013:340315.
Kulthe SS, Choudhari YM, Inamdar NN, Mourya V. Polymeric micelles: authoritative aspects for drug delivery. Design Monomers Polym. 2012;15:465–521.
Devarajan PV, Jain S. Targeted drug delivery: concepts and design. Berlin: Springer; 2016.
Mourya V, Inamdar N, Nawale R, Kulthe S. Polymeric micelles: general considerations and their applications. Ind J Pharm Educ Res. 2011;45:128–38.
Wakaskar RR. Polymeric micelles for drug delivery. Int J Drug Dev Res. 2017;9:1–2.
Mandal A, Bisht R, Rupenthal ID, Mitra AK. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release. 2017;248:96–116.
Li Q, Lai KL, Chan PS, Leung SC, Li HY, Fang Y, To KK, Choi CHJ, Gao QY, Lee TW. Micellar delivery of dasatinib for the inhibition of pathologic cellular processes of the retinal pigment epithelium. Coll Surf B. 2016;140:278–86.
Kesharwani P, Xie L, Banerjee S, Mao G, Padhye S, Sarkar FH, Iyer AK. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3, 4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Coll Surf B. 2015;136:413–23.
Zhu J, Shi X. Dendrimer-based nanodevices for targeted drug delivery applications. J Mater Chem B. 2013;1:4199–211.
Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6:139.
Cheng Y, Xu Z, Ma M, Xu T. Dendrimers as drug carriers: applications in different routes of drug administration. J Pharm Sci. 2008;97:123–43.
Noriega-Luna B, Godínez LA, Rodríguez FJ, Rodríguez A, Larrea G, Sosa-Ferreyra C, Mercado-Curiel R, Manríquez J, Bustos E. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J Nanomater. 2014;2014:39.
Tripathy S, Das M. Dendrimers and their applications as novel drug delivery carriers. J Appl Pharm Sci. 2013;3:142–9.
Kesharwani P, Jain K, Jain NK. Dendrimer as nanocarrier for drug delivery. Progr Polym Sci. 2014;39:268–307.
Jain K, Gupta U, Jain NK. Dendronized nanoconjugates of lysine and folate for treatment of cancer. Eur J Pharm Biopharm. 2014;87:500–9.
Kaur A, Jain K, Mehra NK, Jain N. Development and characterization of surface engineered PPI dendrimers for targeted drug delivery. Artif Cells Nanomed Biotechnol. 2017;45:414–25.
Choi S-J, Lee JK, Jeong J, Choy J-H. Toxicity evaluation of inorganic nanoparticles: considerations and challenges. Mol Cell Toxicol. 2013;9:205–10.
Kong F-Y, Zhang J-W, Li R-F, Wang Z-X, Wang W-J, Wang W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22:1445.
Prusty K, Swain SK. Nano silver decorated polyacrylamide/dextran nanohydrogels hybrid composites for drug delivery applications. Mater Sci Eng. 2018;85:130–41.
Marcu A, Pop S, Dumitrache F, Mocanu M, Niculite C, Gherghiceanu M, Lungu C, Fleaca C, Ianchis R, Barbut A. Magnetic iron oxide nanoparticles as drug delivery system in breast cancer. Appl Surf Sci. 2013;281:60–5.
Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci. 2015;10:13–23.
Du J, Li X, Zhao H, Zhou Y, Wang L, Tian S, Wang Y. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int J Pharm. 2015;495:738–49.
Ni R, Zhao J, Liu Q, Liang Z, Muenster U, Mao S. Nanocrystals embedded in chitosan-based respirable swellable microparticles as dry powder for sustained pulmonary drug delivery. Eur J Pharm Sci. 2017;99:137–46.
McNamara K, Tofail SA. Nanoparticles in biomedical applications. Adv Phys. 2017;2:54–88.
Kudr J, Haddad Y, Richtera L, Heger Z, Cernak M, Adam V, Zitka O. Magnetic nanoparticles: from design and synthesis to real world applications. Nanomaterials. 2017;7:243.
Article PubMed Central CAS Google Scholar
Prasad PN. Nanophotonics. New York: Wiley; 2004.
Book Google Scholar
Volkov Y. Quantum dots in nanomedicine: recent trends, advances and unresolved issues. Biochem Biophys Res Commun. 2015;468:419–27.
Liu J, Lau SK, Varma VA, Moffitt RA, Caldwell M, Liu T, Young AN, Petros JA, Osunkoya AO, Krogstad T. Molecular mapping of tumor heterogeneity on clinical tissue specimens with multiplexed quantum dots. ACS Nano. 2010;4:2755–65.
Xu G, Zeng S, Zhang B, Swihart MT, Yong K-T, Prasad PN. New generation cadmium-free quantum dots for biophotonics and nanomedicine. Chem Rev. 2016;116:12234–327.
Shi Y, Pramanik A, Tchounwou C, Pedraza F, Crouch RA, Chavva SR, Vangara A, Sinha SS, Jones S, Sardar D. Multifunctional biocompatible graphene oxide quantum dots decorated magnetic nanoplatform for efficient capture and two-photon imaging of rare tumor cells. ACS Appl Mater Interfaces. 2015;7:10935–43.
Han H-S, Niemeyer E, Huang Y, Kamoun WS, Martin JD, Bhaumik J, Chen Y, Roberge S, Cui J, Martin MR. Quantum dot/antibody conjugates for in vivo cytometric imaging in mice. Proc Natl Acad Sci. 2015;112:1350–5.
So M-K, Xu C, Loening AM, Gambhir SS, Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol. 2006;24:339.
Zheng F-F, Zhang P-H, Xi Y, Chen J-J, Li L-L, Zhu J-J. Aptamer/graphene quantum dots nanocomposite capped fluorescent mesoporous silica nanoparticles for intracellular drug delivery and real-time monitoring of drug release. Anal Chem. 2015;87:11739–45.
Huang C-L, Huang C-C, Mai F-D, Yen C-L, Tzing S-H, Hsieh H-T, Ling Y-C, Chang J-Y. Application of paramagnetic graphene quantum dots as a platform for simultaneous dual-modality bioimaging and tumor-targeted drug delivery. J Mater Chem B. 2015;3:651–64.
Olerile LD, Liu Y, Zhang B, Wang T, Mu S, Zhang J, Selotlegeng L, Zhang N. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Coll Surf B. 2017;150:121–30.
Cai X, Luo Y, Zhang W, Du D, Lin Y. pH-Sensitive ZnO quantum dots–doxorubicin nanoparticles for lung cancer targeted drug delivery. ACS Appl Mater Interfaces. 2016;8:22442–50.
Balaji AB, Pakalapati H, Khalid M, Walvekar R, Siddiqui H. Natural and synthetic biocompatible and biodegradable polymers. In: Shimpi NG (ed) Biodegradable and biocompatible polymer composites: processing, properties and applications. Woodhead Publishing series in composites science and engineering. Duxford: Woodhead Publishing; 2017. p. 3–32.
Bassas-Galia M, Follonier S, Pusnik M, Zinn M. Natural polymers: a source of inspiration. In: Bioresorbable polymers for biomedical applications. New York: Elsevier; 2017. p. 31–64.
Chapter Google Scholar
Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res Int. 2014;2014:180549.
Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60:1650–62.
Poole-Warren L, Patton A. Introduction to biomedical polymers and biocompatibility. In: Biosynthetic polymers for medical applications. New York: Elsevier; 2016. p. 3–31.
Pertici G. Introduction to bioresorbable polymers for biomedical applications. In: Biosynthetic polymers for medical applications. New York: Elsevier; 2016. p. 3–29.
Cardoso MJ, Costa RR, Mano JF. Marine origin polysaccharides in drug delivery systems. Mar Drugs. 2016;14:34.
Yu Z, Yu M, Zhang Z, Hong G, Xiong Q. Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Res Lett. 2014;9:343.
Robinson M, Zhang X. The world medicines situation. Traditional medicines: global situation, issues and challenges. Geneva: World Health Organization; 2011. p. 1–12.
Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582–614.
David B, Wolfender J-L, Dias DA. The pharmaceutical industry and natural products: historical status and new trends. Phytochem Rev. 2015;14:299–315.
Namdari M, Eatemadi A, Soleimaninejad M, Hammed AT. A brief review on the application of nanoparticle enclosed herbal medicine for the treatment of infective endocarditis. Biomed Pharm. 2017;87:321–31.
Heinrich M. Ethnopharmacology in the 21st century-grand challenges. Front Pharm. 2010;1:8.
Kinghorn AD, Pan L, Fletcher JN, Chai H. The relevance of higher plants in lead compound discovery programs. J Nat Prod. 2011;74:1539–55.
Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559.
Patra JK, Das G, Baek K-H. Towards a greener environment: synthesis and applications of green nanoparticles. Pak J Agric Sci. 2016;53:59–79.
Duncan R, Gaspar R. Nanomedicine (s) under the microscope. Mol Pharm. 2011;8:2101–41.
Ramana KV, Singhal SS, Reddy AB. Therapeutic potential of natural pharmacological agents in the treatment of human diseases. BioMed Res Int. 2014;2014:573452.
Guo W. Green technology for nanoparticles in biomedical applications. In: Rai M, Posten C, editors. Green biosynthesis of nanoparticles: mechanisms and applications. Wallington: CABI; 2013.
Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–57.
Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–51.
Yohan D, Chithrani BD. Applications of nanoparticles in nanomedicine. J Biomed Nanotechnol. 2014;10:2371–92.
Ambesh P, Campia U, Obiagwu C, Bansal R, Shetty V, Hollander G, Shani J. Nanomedicine in coronary artery disease. Indian Heart J. 2017;69:244–51.
Grazu V, Moros M, Sánchez-Espinel C. Nanocarriers as nanomedicines: design concepts and recent advances. In: Frontiers of nanoscience. Vol. 4, New York: Elsevier; 2012. p. 337–440.
Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013;24:1159–66.
Devasena T. Diagnostic and therapeutic nanomaterials. In: Therapeutic and diagnostic nanomaterials. New York: Springer; 2017. p. 1–13.
Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. Pharm Ther. 2017;42:742.
Havel H, Finch G, Strode P, Wolfgang M, Zale S, Bobe I, Youssoufian H, Peterson M, Liu M. Nanomedicines: from bench to bedside and beyond. AAPS J. 2016;18:1373–8.
Kumar A, Chen F, Mozhi A, Zhang X, Zhao Y, Xue X, Hao Y, Zhang X, Wang PC, Liang X-J. Innovative pharmaceutical development based on unique properties of nanoscale delivery formulation. Nanoscale. 2013;5:8307–25.
Boroumand Moghaddam A, Namvar F, Moniri M, Md Tahir P, Azizi S, Mohamad R. Nanoparticles biosynthesized by fungi and yeast: a review of their preparation, properties, and medical applications. Molecules. 2015;20:16540–65.
Metz KM, Sanders SE, Pender JP, Dix MR, Hinds DT, Quinn SJ, Ward AD, Duffy P, Cullen RJ, Colavita PE. Green synthesis of metal nanoparticles via natural extracts: the biogenic nanoparticle corona and its effects on reactivity. ACS Sustain Chem Eng. 2015;3:1610–7.
Paul D, Sinha SN. Extracellular synthesis of silver nanoparticles using Pseudomonas aeruginosa KUPSB12 and its antibacterial activity. JJBS. 2014;7:245–50.
Kushwaha A, Singh VK, Bhartariya J, Singh P, Yasmeen K. Isolation and identification of E. coli bacteria for the synthesis of silver nanoparticles: characterization of the particles and study of antibacterial activity. Eur J Exp Biol. 2015;5:65–70.
Iravani S. Bacteria in nanoparticle synthesis: current status and future prospects. Int Sch Res Notices. 2014;2014:359316.
Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31:346–56.
Khan HA, Sakharkar MK, Nayak A, Kishore U, Khan A. 14-nanoparticles for biomedical applications: an overview. In: Narayan R, editor. Nanobiomaterials. Cambridge: Woodhead Publishing; 2018. p. 357–84.
Aravamudhan A, Ramos DM, Nada AA, Kumbar SG. Natural polymers: polysaccharides and their derivatives for biomedical applications. In: Natural and synthetic biomedical polymers. New York: Elsevier; 2014. p. 67–89.
Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20:8856–74.
Pajardi G, Rapisarda V, Somalvico F, Scotti A, Russo GL, Ciancio F, Sgrò A, Nebuloni M, Allevi R, Torre ML. Skin substitutes based on allogenic fibroblasts or keratinocytes for chronic wounds not responding to conventional therapy: a retrospective observational study. Int Wound J. 2016;13:44–52.
Rahimi G, Alizadeh F, Khodavandi A. Mycosynthesis of silver nanoparticles from Candida albicans and its antibacterial activity against Escherichia coli and Staphylococcus aureus . Trop J Pharm Res. 2016;15:371–5.
Ali M, Kim B, Belfield KD, Norman D, Brennan M, Ali GS. Inhibition of Phytophthora parasitica and P. capsici by silver nanoparticles synthesized using aqueous extract of Artemisia absinthium . Phytopathology. 2015;105:1183–90.
Malapermal V, Botha I, Krishna SBN, Mbatha JN. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum , and Ocimum sanctum (L.) using silver nanoparticles. Saudi J Biol Sci. 2017;24:1294–305.
Sankar R, Karthik A, Prabu A, Karthik S, Shivashangari KS, Ravikumar V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Coll Surf B. 2013;108:80–4.
Patra JK, Ali MS, Oh I-G, Baek K-H. Proteasome inhibitory, antioxidant, and synergistic antibacterial and anticandidal activity of green biosynthesized magnetic Fe3O4 nanoparticles using the aqueous extract of corn ( Zea mays L.) ear leaves. Artif Cells Nanomed Biotechnol. 2017;45:349–56.
Patra JK, Baek K-H. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front Microbiol. 2017;8:167.
Patra JK, Kwon Y, Baek K-H. Green biosynthesis of gold nanoparticles by onion peel extract: synthesis, characterization and biological activities. Adv Powder Technol. 2016;27:2204–13.
Patra JK, Baek K-H. Biosynthesis of silver nanoparticles using aqueous extract of silky hairs of corn and investigation of its antibacterial and anticandidal synergistic activity and antioxidant potential. IET Nanobiotechnol. 2016;10:326–33.
Patra JK, Baek K-H. Comparative study of proteasome inhibitory, synergistic antibacterial, synergistic anticandidal, and antioxidant activities of gold nanoparticles biosynthesized using fruit waste materials. Int J Nanomed. 2016;11:4691.
Patra JK, Baek K-H. Green synthesis of silver chloride nanoparticles using Prunus persica L. outer peel extract and investigation of antibacterial, anticandidal, antioxidant potential. Green Chem Lett Rev. 2016;9:132–42.
Patra JK, Das G, Baek K-H. Phyto-mediated biosynthesis of silver nanoparticles using the rind extract of watermelon ( Citrullus lanatus ) under photo-catalyzed condition and investigation of its antibacterial, anticandidal and antioxidant efficacy. J Photochem Photobiol B. 2016;161:200–10.
Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64:1020–37.
Zhu Z, Li Y, Yang X, Pan W, Pan H. The reversion of anti-cancer drug antagonism of tamoxifen and docetaxel by the hyaluronic acid-decorated polymeric nanoparticles. Pharmacol Res. 2017;126:84–96.
Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–36.
Gupta U, Sharma S, Khan I, Gothwal A, Sharma AK, Singh Y, Chourasia MK, Kumar V. Enhanced apoptotic and anticancer potential of paclitaxel loaded biodegradable nanoparticles based on chitosan. Int J Biol Macromol. 2017;98:810–9.
Chang C-H, Huang W-Y, Lai C-H, Hsu Y-M, Yao Y-H, Chen T-Y, Wu J-Y, Peng S-F, Lin Y-H. Development of novel nanoparticles shelled with heparin for berberine delivery to treat Helicobacter pylori. Acta Biomaterialia. 2011;7:593–603.
Aldawsari HM, Hosny KM. Solid lipid nanoparticles of Vancomycin loaded with Ellagic acid as a tool for overcoming nephrotoxic side effects: preparation, characterization, and nephrotoxicity evaluation. J Drug Deliv Sci Technol. 2018;45:76–80.
Dian L, Yu E, Chen X, Wen X, Zhang Z, Qin L, Wang Q, Li G, Wu C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res Lett. 2014;9:684.
Spillmann CM, Naciri J, Algar WR, Medintz IL, Delehanty JB. Multifunctional liquid crystal nanoparticles for intracellular fluorescent imaging and drug delivery. ACS Nano. 2014;8:6986–97.
Purama RK, Goswami P, Khan AT, Goyal A. Structural analysis and properties of dextran produced by Leuconostoc mesenteroides NRRL B-640. Carbohydr Polym. 2009;76:30–5.
Agarwal A, Gupta U, Asthana A, Jain NK. Dextran conjugated dendritic nanoconstructs as potential vectors for anti-cancer agent. Biomaterials. 2009;30:3588–96.
Barenholz YC. Doxil ® —the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34.
Maeng JH, Lee D-H, Jung KH, Bae Y-H, Park I-S, Jeong S, Jeon Y-S, Shim C-K, Kim W, Kim J. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials. 2010;31:4995–5006.
Bonechi C, Martini S, Ciani L, Lamponi S, Rebmann H, Rossi C, Ristori S. Using liposomes as carriers for polyphenolic compounds: the case of trans-resveratrol. PLoS ONE. 2012;7:e41438.
Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19:2032–46.
Wei X, Senanayake TH, Bohling A, Vinogradov SV. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: synthesis, pharmacokinetics, and tumor growth inhibition. Mol Pharm. 2014;11:3112–22.
Feng T, Wei Y, Lee RJ, Zhao L. Liposomal curcumin and its application in cancer. Int J Nanomed. 2017;12:6027.
Cheng C, Peng S, Li Z, Zou L, Liu W, Liu C. Improved bioavailability of curcumin in liposomes prepared using a pH-driven, organic solvent-free, easily scalable process. RSC Adv. 2017;7:25978–86.
Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC. Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Evid Based Complement Alternat Med. 2014;2014:651593.
Sainz V, Conniot J, Matos AI, Peres C, Zupanǒiǒ E, Moura L, Silva LC, Florindo HF, Gaspar RS. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468:504–10.
Hassan S, Prakash G, Ozturk AB, Saghazadeh S, Sohail MF, Seo J, Dokmeci MR, Zhang YS, Khademhosseini A. Evolution and clinical translation of drug delivery nanomaterials. Nano Today. 2017;15:91–106.
Agrahari V, Agrahari V. Facilitating the translation of nanomedicines to a clinical product: challenges and opportunities. Drug Discov Today. 2018;23(5):974–91.
Caster JM, Patel AN, Zhang T, Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev. 2016;2017:9.
Wacker MG, Proykova A, Santos GML. Dealing with nanosafety around the globe—regulation vs. innovation. Int J Pharm. 2016;509:95–106.
Lin P-C, Lin S, Wang PC, Sridhar R. Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv. 2014;32:711–26.
Grossman JH, Crist RM, Clogston JD. Early development challenges for drug products containing nanomaterials. AAPS J. 2017;19:92–102.
Tinkle S, McNeil SE, Mühlebach S, Bawa R, Borchard G, Barenholz YC, Tamarkin L, Desai N. Nanomedicines: addressing the scientific and regulatory gap. Ann NY Acad Sci. 2014;1313:35–56.
Pandit A, Zeugolis DI. Twenty-five years of nano-bio-materials: have we revolutionized healthcare? Fut Med. 2016;11(9):985–7.
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373–87.
Tran S, DeGiovanni P-J, Piel B, Rai P. Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Med. 2017;6:44.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1:10–29.
Grumezescu AM. Nanoscale fabrication, optimization, scale-up and biological aspects of pharmaceutical nanotechnology. New York: William Andrew; 2017.
Caster JM, Patel AN, Zhang T, Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev. 2017;9:e1416.
Drug approvals and databases. https://www.fda.gov/Drugs/InformationOnDrugs/default.htm . Accessed 16 Aug 2018.
D’Mello SR, Cruz CN, Chen M-L, Kapoor M, Lee SL, Tyner KM. The evolving landscape of drug products containing nanomaterials in the United States. Nat Nanotechnol. 2017;12:523.
Download references
Authors’ contributions
JKP, GD, LFF, EVRC, MDPRT, LSAT, LADT, RG, MKS, SS and SH wrote different sections of the manuscript. JKP, LFF, MDPRT, RG, SS, SH and HSS edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Jayanta Kumar Patra and Gitishree Das are grateful to Dongguk University, Republic of Korea for support. Leonardo Fernandes Fraceto and Estefânia V.R. Campos are grateful for the financial support provided by the São Paulo State Research Foundation (FAPESP) and National Council for Scientific and Technological Development (CNPQ). Maria del Pilar Rodriguez-Torres wishes to thank particularly DGAPA UNAM for the postdoctoral scholarship granted. Maria del Pilar Rodriguez-Torres, Laura Susana Acosta-Torres and Luis Armando Diaz-Torres thank Red de Farmoquimicos-CONACYT and DGAPA-UNAM PAPIIT-IN225516 project for support. Renato Grillo would like to thanks the São Paulo State Science Foundation (FAPESP, Grants #2015/26189-8). Han-Seung Shin thank Korea Environmental Industry & Technology Institute (A117-00197-0703-0) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agricultural-BioTechnology Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA)(710 003-07-7- SB120, 116075-3) for funding.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
We have included two figures (Figs. 3 and 6 ) and one table (Table 1 ) from previously published literature with required copyright permission from the copyright holder. We have mentioned this in the manuscript with proper citation.
Ethics approval and consent to participate
This work was supported by the Korea Environmental Industry & Technology Institute (A117-00197-0703-0) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agricultural-BioTechnology Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA)(710 003-07-7- SB120, 116075-3).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
Research Institute of Biotechnology & Medical Converged Science, Dongguk University-Seoul, Goyang-si, 10326, Republic of Korea
Jayanta Kumar Patra & Gitishree Das
Sao Paulo State University (UNESP), Institute of Science and Technology, Sorocaba, São Paulo, Zip Code 18087-180, Brazil
Leonardo Fernandes Fraceto & Estefania Vangelie Ramos Campos
Department of Biochemistry and Tissue Biology, Institute of Biology, State University of Campinas, Campinas, São Paulo, Zip code 13083-862, Brazil
Laboratorio de Investigación Interdisciplinaria, Área de Nanoestructuras y Biomateriales, Escuela Nacional de Estudios Superiores, Unidad Leon, Universidad Nacional Autonóma de México (UNAM), Boulevard UNAM No 2011. Predio El Saucillo y El Potrero, 37684, León, Guanajuato, Mexico
Maria del Pilar Rodriguez-Torres & Laura Susana Acosta-Torres
Centro de Investigaciones en Óptica, A.P. 1-948, C.P. 37000, León, Guanajuato, Mexico
Luis Armando Diaz-Torres
Department of Physics and Chemistry, School of Engineering, São Paulo State University (UNESP), Ilha Solteira, SP, 15385-000, Brazil
Renato Grillo
Department of Crop Science, Faculty of Agriculture, Universiti Putra Malaysia, 43400, Serdang, Selangor, Malaysia
Mallappa Kumara Swamy
Department of Biotechnology, Motilal Nehru National Institute of Technology Allahabad, Allahabad, Uttar Pradesh, 211004, India
Shivesh Sharma
Pharmacognosy Research Laboratories & Herbal Analysis Services UK, University of Greenwich, Medway Campus-Science, Grenville Building (G102/G107), Central Avenue, Chatham-Maritime, Kent, ME4 4TB, UK
Solomon Habtemariam
Department of Food Science and Biotechnology, Dongguk University, Ilsandong-gu, Goyang, Gyeonggi-do, 10326, Republic of Korea
Han-Seung Shin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Han-Seung Shin .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Patra, J.K., Das, G., Fraceto, L.F. et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16 , 71 (2018). https://doi.org/10.1186/s12951-018-0392-8
Download citation
Received : 19 July 2018
Accepted : 25 August 2018
Published : 19 September 2018
DOI : https://doi.org/10.1186/s12951-018-0392-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nanomedicine
- Nanomaterials
- Drug delivery
- Drug targeting
- Natural products
Journal of Nanobiotechnology
ISSN: 1477-3155
- Submission enquiries: [email protected]
- Utility Menu
Experimental Soft Condensed Matter Group
9 Oxford Street Cambridge, MA 02138

Advanced Drug Delivery and Encapsulation project awarded by Harvard Grid Accelerator! Congratulations, Kevin (project lead), Chenjing & Tiffany!
The Harvard Grid Accelerator announced awards to six interdisciplinary projects that have transformative potential in health, climate, and manufacturing. The Grid Accelerator identifies and supports the ‘de-risking’ of high-potential Harvard science and engineering lab-based projects with the goal of translating them into startups or other commercialization opportunities within a year. Advanced Drug Delivery and Encapsulation project in Weitz Group is selected and funded by Harvard Grid Accelerator! Congratulations, Kevin Jahnke (project lead) , Chenjing Yang , and Tiffany Chen !
“Research teams embarking on groundbreaking projects often find themselves at a critical juncture—the transition from visionary concepts to tangible commercial applications. Grid Accelerator funding and mentoring ensure that these transformative projects reach their full potential to address global challenges.”
—Isaac Kohlberg, Senior Associate Provost and Chief Technology Development Officer at Harvard University
Harvard Grid is a collaboration between Harvard’s Office of Technology Development (OTD) and the John A. Paulson School of Engineering and Applied Sciences (SEAS). The Grid partners with Harvard science and engineering researchers to help shape and advance their research from labs to markets for impact.
“Research teams embarking on groundbreaking projects often find themselves at a critical juncture—the transition from visionary concepts to tangible commercial applications,” Isaac Kohlberg, Senior Associate Provost and Chief Technology Development Officer at Harvard University. “Grid Accelerator funding and mentoring ensure that these transformative projects reach their full potential to address global challenges.”
“The Grid Accelerator brings critical elements to bear to take science from our labs out into the world to drive positive change and improve lives,” said David C. Parkes, John A. Paulson Dean and George F. Colony Professor of Computer Science at SEAS. “The projects funded by the Grid Accelerator awards are notable examples of the cross-disciplinary research conducted at SEAS.”
The six teams of Harvard researchers receiving the 2024 Harvard Grid Accelerator awards are:
- Methane Emissions Analytics: Leveraging satellite observations to measure and geospatially map methane emissions.
- Advanced Drug Delivery and Encapsulation: Developing asymmetric polysaccharide-coated lipid vesicles for improved drug delivery.
- AI-Driven Flexible Bioelectronics: Creating AI-driven flexible therapeutic solutions.
- Wearable Spasticity Management & Mitigation: Helping patients recover improved motility control and coordination.
- Navigation Aid for the Visually Impaired: Enabling visually impaired persons to navigate urban settings with independence and confidence.
- Non-Motorized Levitating Devices for Upper Atmosphere Sensing and Communication: Discovering climate data, communications pathways, and defense capabilities.
The Grid Accelerator builds upon a proven track record of the OTD Physical Sciences and Engineering Accelerator. Since 2013, projects advanced by Grid/OTD Accelerator support have culminated in 17 launched startups that have collectively raised $176 million, as well as technology licenses to established companies and sponsored research agreements.
“From a cast of several exciting possibilities, these six projects have a line-of-sight to launch, in some form, in about a year and impressed the selection committee with their potential to meaningfully elevate the human condition on a global scale,” Paul Hayre, Executive Director of the Harvard Grid, commented on the selection. “Our role now shifts from selection to support to see that they achieve their objectives.”
In addition to funding, the awardees gain access to educational programs and networks of industry, venture, product development, and investment experts.
For more information on these six projects and more, visit grid.harvard.edu .

Photo courtesy of John A. Paulson School of Engineering and Applied Sciences at Harvard
Extended project descriptions:
Methane emissions analytics.
Problem: There is growing demand from climate policy stakeholders to use satellite observations of greenhouse gases to quantify emissions.
Proposed Solution: A research team led by Daniel Jacob, Vasco McCoy Family Professor of Atmospheric Chemistry and Environmental Engineering and leader of the Atmospheric Chemistry Modeling Group at SEAS, is developing an Integrated Methane Inversion (IMI) cloud-based, open-source software tool. This tool leverages inverse methods to infer methane emissions from satellite data, enabling stakeholders to estimate emissions with high resolution for their domains and periods of interest, including continuous monitoring. The project will focus on the development of a Software as a Service (SaaS), automated reports and alerts, and the development of support and training materials.
Advanced Drug Delivery and Encapsulation
Problem: Current strategies to encapsulate drugs do not allow for the encapsulation of proteins and can lack tunability and cause low cellular uptake.
Proposed Solution: A research team led by David A. Weitz, Mallinckrodt Professor of Physics and Applied Physics at SEAS, proposes to engineer asymmetric polysaccharide-coated lipid vesicles to broaden the parameter space for engineering the mechanical and biochemical properties of lipid vesicles. If able to encapsulate and deliver any nucleic acid, protein, or small molecule into drug delivery vehicles, new treatment methods for gene and cancer therapy are possible. The team will use an inverted emulsion technique to form lipid vesicles with distinct leaflets, where each leaflet can be individually configured and modified. Additionally, they will functionalize the lipid vesicles with polysaccharides to enable cell-specific targeting. This will reduce the cost and side effects compared to current drug delivery vehicles and might lead to a completely new generation of vaccine technology and disease treatment.
AI-Driven Flexible Bioelectronics
Problem: The mismatch between traditional rigid electronics and the soft, dynamic nature of the human body limits the seamless integration of high-performance sensors, stimulators, and computational units with the human body.
Proposed Solution: A research team led by Jia Liu, Assistant Professor of Bioengineering at SEAS, is developing technology that converges flexible nanoelectronics, advanced surgical techniques, and sophisticated AI agents to create bioelectronic interfaces that can be precisely deployed for tissue modulation across various organs. These interfaces are designed for long-term stability and high-resolution recording and control, targeting specific tissue regions with minimized cross-stimulation for the treatment of conditions such as neurological disorders, cancers, and diabetes.
Wearable Spasticity Management and Mitigation
Problem: Spasticity causes involuntary muscle stiffness and contractions in over 12 million individuals worldwide, and is accompanied by a debilitating hindrance to movement, lack of independence, and chronic pain.
Proposed Solution: A research team led by Shriya Srinivasan, Assistant Professor of Bioengineering at SEAS, is developing a wearable stimulation system incorporating critical parameter optimization and user connectivity components necessary for large-scale clinical trials in patients with spasticity. Current approaches are invasive and bulky with modest to low efficacy. The research team aims to develop the Wearable Adaptive Vibrotactile Bracelet (WAVelet), a cost-effective and low-risk alternative.
Navigation Aid for the Visually Impaired
Problem: Existing navigation solutions for the more than 400 million people around the world who have impaired vision ranging from moderate impairment to blindness only partially address the navigation challenges identified by users.
Proposed Solution: A research team led by Patrick Slade, Assistant Professor of Bioengineering and leader of the Harvard Ability Lab at SEAS, developed a navigation system that combines novel computer vision techniques, sensor fusion, and personalized feedback to the user, requiring only a smartphone. The technology will provide the functionality of guide dogs at approximately 20 times lower cost, within the price range specified by the surveyed users, creating the potential to dramatically increase the number of people who can benefit from navigation assistance. Additionally, the technology adds essential independent navigation capabilities to help people travel to new destinations and provide environmental scene understanding.
Non-Motorized Levitating Devices for Upper Atmosphere Sensing and Communication
Problem: The mesosphere is an inaccessible region of the atmosphere (50-100 km altitudes) that is too high for conventional aircraft to generate lift, yet too low for satellites to maintain orbit. Flight in this near-space region would unlock a trove of climate data, communications pathways, and defense capabilities that no current hardware can achieve.
Proposed Solution: A research team led by Joost Vlassak, Abbott and James Lawrence Professor of Materials Engineering at SEAS, is developing an entirely new propulsion mechanism to enable mesospheric flight. The technology uses sunlight-induced thermal gradients to generate photophoretic forces, which propels lightweight devices upward without onboard power or fuel. The team previously modeled that a photophoretic force, thermal transpiration, could lift 100 mg-scale payloads onboard a 10 cm-wide device at an altitude of 70 km. They now aim to demonstrate that the devices can hold a variety of payloads and communicate while flying.
Tags: Grid Accelerator , SEAS , Accelerator , Funding , Startups
Press Contact: Kirsten Mabry | (617) 495-4157
Latest News

Sparking connections: Hosted Symposium at SEAS

David Weitz receives the 2024 Bower Award
The life and death of cracks - weitz lab researchers explore how fractures nucleate, propagate and stop, with papers published in nature physics and agu advances. congratulations, thomas, yiqiao, lizhi....

Weitz Lab Members Present Research at ASCB annual meeting in Boston
Filter news by month.
- March 2024 (2)
- February 2024 (1)
- January 2024 (1)
- December 2023 (2)
- November 2023 (1)
Squishy Physics
- Skip to content
- Skip to navigation
- Skip to footer
Developing the next generation of drug delivery technologies
Originally published: 23 december 2021.
Next generation therapeutics
Technologies
Medicines are changing. Across our laboratories, our ambition is to target any novel biology we uncover. To do this, we are developing an array of new modalities . These potential new therapies are different from the traditional small molecules and current large molecules which are often aimed at targets on the cell surface or delivered without a specific molecular-targeting strategy. As a result, we need advanced drug delivery systems for targeted and controlled release of our novel molecules in tissues and cells to optimise their potential benefits for patients. Our scientists are committed to breaking down the barriers between our most promising new drug candidates and their targets in tissues and cells. They are developing a broad range of nanoparticles that aim to deliver our new modalities to previously undruggable targets and precisely control their release in formulations that are easy to use and convenient for patients. They are also investigating innovative ways of getting oral formulations of biologic drugs across the intestinal wall – something which has eluded generations of drug designers. Ultimately, these next generation drug delivery technologies aim to translate the scientific progress underpinning our innovative new modalities into clinical benefits for patients.
Jump to section, cell-targeted delivery of therapies with lipid nanoparticles (lnps), tissue-targeted delivery to expand druggable targets, making treatment more convenient for patients with controlled release, alternative routes of administration – turning oral biologics from concept to reality.
Up to 80% of the targets we are seeking to reach are inside cells, making them challenging to access for large-charged molecules such as nucleotide-based therapies . Through a balanced approach of internally developed technologies and external collaborations, we are making progress to develop therapies and delivery agents that can reach these elusive intracellular targets.
Specifically, we are investigating lipid nanoparticles (LNPs) as a promising and innovative vehicle for intracellular delivery of nucleotide-based therapeutics for production of protein therapeutics in cells. LNPs have a successful track record of delivering nucleic acids and they are by far the most advanced approach for mRNA delivery. We have shown that LNPs can deliver mRNA into cells to initiate cellular protein production after intravenous, subcutaneous and pulmonary administration. We are now focusing our research on enhancing the efficacy and safety profile of this drug delivery system.
Hear more from Drug Delivery scientists about LNPs as a promising drug delivery technology

Our Advanced Drug Delivery teams have an ongoing collaboration with Fredrik Höök’s and Elin Esbjörner’s teams at Chalmers University of Technology, resulting in a publication in ACS Nanotechnology in 2022. Together, the team used surface-sensitive fluorescence microscopy to analyse single ionisable lipid-containing LNPs one at a time as they interacted with a synthetic membrane that mimicked the endosomal membrane environment. In a follow-up experiment, Audrey Gallud, Senior Scientist, Advanced Drug Delivery, Pharmaceutical Sciences, AstraZeneca and team incubated the LNPs in serum to simulate the effects of proteins binding to the surface as the LNPs travel through the body. This new technique provides a deeper understanding of how protein accumulation on the surface of the LNPs – called a protein corona – influences the way LNPs interact with endosomal membranes. 1
Additional research into the protein corona led by Kai Liu, Senior Scientist, Advanced Drug Delivery, Pharmaceutical Sciences and published in Nature Communications in 2023 showed that this surface accumulation of biomolecules affects the targeting, delivery, and ultimate destination of LNPs. Using high-throughput screening and proteomic analysis followed by in vivo studies, the team discovered that high-density lipoprotein (HDL) rich LNP coronas were most efficient for targeted delivery to the liver. Now, the team aims to use this as a framework to control corona composition, thereby improving LNP therapeutic efficacy. 2
Alongside research into LNP structure and function, we are collaborating with scientists at Gothenburg University with key contributions from Lennart Lindfors , Senior Principal Scientist, Advanced Drug Delivery, Pharmaceutical Sciences, AstraZeneca. The team aimed to determine how LNPs deliver mRNA into the cell and how it is then released. Research findings published in a 2019 publication in Nature Communications showed that cells exposed to LNPs secrete small vesicles (exosomes) containing the mRNA cargo. 3 More recent findings from the same collaboration published in Advanced Science in 2023 and led by Sepideh Hagvall, Associate Director Patient Safety Scientist, AstraZeneca add to this data and show that in heart cells and preclinical models, these mRNA containing exosomes travel to neighbouring cells resulting in local production of protein. 4
Learn more about a new approach to deliver RNA into cells in the animation below.

Our highly productive collaborations have been essential to our progress in developing next generation drug delivery systems. Our own drug delivery specialists come from all areas of academia and industry – attracted by our diverse, creative and rigorous research, and the knowledge that they are contributing to help target any novel biology we uncover – for the benefit of patients. Annette Bak Head of Advanced Drug Delivery, Pharmaceutical Sciences, AstraZeneca

By targeting delivery of a medicine precisely to the tissue where it is needed, we aim to achieve a therapeutic concentration while minimising the potential for off-target activity at other sites that could lead to side effects.
Nanoparticles are microscopic particles with at least one dimension less than 100 nm. Using nanoparticles to deliver drug candidates to their site of action has the potential to help us improve the therapeutic index of small molecules and new modalities. Combining drugs with carefully selected nanoparticles and functionalising the nanoparticles with targeting ligands has the potential to change their distribution in the body, target tissues of interest and control their release – increasing their concentration in diseased tissue relative to healthy tissue.
Discover how nanomedicines could help direct medicines to specific tissues in the body in the animation below

In preclinical and clinical programmes, we are currently focusing on a number of different nanoplatforms – polymeric nanoparticles, polymer conjugates and inorganic nanoparticles. In the case of polymeric nanoparticles, drug compounds are encapsulated in a polymer matrix, while in polymer conjugates they are chemically linked to branched polymers. The efficient “loading” of these nanoparticles with drugs and the control of their release rate in the body are key challenges that our teams are working to address. Ultimately, our goal is to fully understand the impact of the combination of these particles and our drugs in the body.
Ultra-small (<8 nm) silica particles are inorganic nanoparticles with a unique bio-distribution that are renally cleared. The nanoparticle can be linked to a drug molecule, imaging labels and an antibody to actively target tissues of interest. In collaboration with Memorial Sloan Kettering and Cornell University, we have shown that these nanoparticles containing attached engineered antibody fragments for imaging and detection of HER2-overexpressing breast cancer, penetrated the tumour, and showed significant accumulation within the tumour tissues. 5
Over the next few years, we hope to enhance the targeting and specificity of our novel nanoparticle delivery systems and expand our routes of administration – always with the potential patient benefit in mind. Marianne Ashford Senior Principal Scientist, Advanced Drug Delivery, Pharmaceutical Sciences, AstraZeneca

Many therapies require frequent dosing to maintain drug concentrations at therapeutic levels. By using controlled-release formulations to extend the half-life of our medicines inside the body, we aim to minimise dosing frequency and make treatments easier and more convenient for patients, especially when given by injection.
Polymeric particles and implants
Putting medicines into biodegradable polymeric particles, such as PLGA (poly lactic-co-glycolic acid) and polycaprolactone, enables us to control their release according to the diffusion and degradation characteristics of the particles. Biodegradable implants, which are larger (up to 1 mm in diameter) and often injected under the skin, work on the same principle – slowly releasing their contents and degrading over time thereby allowing for less frequent administration.
In preclinical studies, we have shown that using PGLA nanoparticles as carriers for anti-cancer drugs increased their anti-tumour activity, reduced their side effects and suggested that weekly therapy could be converted to monthly therapy. 6 More recently, a similar approach with block copolymers, has demonstrated more than five months of continuous release for a monoclonal antibody.
Silica particles
Silica particle-based controlled release, though at an earlier stage of development, is another attractive option, particularly for biological molecules because the body tolerates natural silicon which is widely found in tissues and fluids.
“Biologics are sensitive to changes in their environment and break down if they are exposed to harsh chemicals or solvents. To address this issue, we are combining biologics with silica particles, which are created using a water-based process. This process also allows creation of particles with specific sizes and shapes to influence their delivery characteristics. In preclinical experiments, we have already demonstrated the controlled release of an antibody from silica particles for up to two months after a single injection. 7 Furthermore, we have been able to reproduce a similar sustained release profile for a peptide,” explains Puneet Tyagi, Associate Director, BioPharmaceuticals Development, AstraZeneca
Atomic layer deposition (ALD) of metal oxides
ALD is a dry, metal oxide film deposition process which enables sustained release over long time periods following the formation of nanoshells on the surface of the particles. The nanoshells are produced directly on particles of an active ingredient. ALD has been extensively evaluated in preclinical development and demonstrated its potential to control drug release.
Development of oral formulations of biologics has been the ‘holy grail’ for pharmaceutical scientists since insulin became available in the 1920s. Many patients understandably prefer an oral formulation to an injection. Now, through a combination of advances in drug design and delivery technologies, oral biologics are becoming a reality.
Transient permeation enhancers
Transient permeation enhancers (TPEs) are excipients that can be co-formulated with drug modalities, such as peptides or antisense oligonucleotides , in a tablet to help promote the transport of these macromolecules across the natural barriers of the GI tract. TPEs work to briefly increase the fluidity of the cell membranes within the intestinal epithelium or open the tight junctions between cells to allow macromolecules to pass through. We are also able to design macromolecules so they are better suited for oral delivery when co-formulated with TPEs. This enables improved stability against intestinal enzymes as well as increased half-life to account for the expected variability in oral absorption.
We have recently reported on the potential of these approaches to enable oral delivery of both peptides as well as antisense oligonucleotides. 8,9 In addition, we have ongoing collaborations with the University of Uppsala, through the SweDeliver Forum, to better understand how TPEs can safely promote oral absorption of macromolecules as well as how to rationally select the best TPEs for a particular macromolecule. 10,11
In collaboration with Uppsala University through the SweDeliver Center, we now better understand how to safely and effectively use TPEs to enable oral absorption of peptides and other macromolecules. As a result, we are in a much better place to develop oral formulations for these types of molecules that are usually administered by injection. Nigel Davies Senior Principal Scientist, Advanced Drug Delivery, AstraZeneca
Ingestible injectables
Another emerging technology to enable oral delivery of biologics is “ingestible injectables”. As the name suggests, this aims to move the site of injection from subcutaneous to the gastrointestinal tract, taking advantage of the lack of pain receptors in the intestine. To work, the patient takes a pill which, after ingestion, activates to inject the biologic into the gastrointestinal mucosa using microneedles or liquid jets. The intention of ingestible injectables is to give similar bioavailability to a subcutaneous injection but in a pain-free manner. We are collaborating externally in this exciting space to bring about patient-centric delivery technology for biologics.
By advancing the science of drug delivery, we want to improve the way patients experience our medicines. With our next generation delivery technologies we are aiming to make medicines that are easier to use, require less frequent dosing and are more convenient – enabling patients to spend less time managing their disease. Shawn Davis Head of Drug Delivery, BioPharmaceuticals Development, R&D, AstraZeneca

Related stories
New insights to broaden therapeutic opportunities of oligonucleotides
MRC collaboration to drive innovation in cell and oligonucleotide therapies
Novel gene editing technology offers efficient route to future gene therapies
Get email alerts for new What Science Can Do stories
Sign up to email alerts
Your email address will only be used to provide you with services from AstraZeneca that you requested. You can opt out for email subscriptions at any time. Your personal information will not be provided to any third party without your permission.
You may also like

1. Aliakbarinodehi N, Gallud A, Mapar M, et al. Interaction Kinetics of Individual mRNA-Containing Lipid Nanoparticles with an Endosomal Membrane Mimic: Dependence on pH, Protein Corona Formation, and Lipoprotein Depletion. ACS Nano. 2022;16(12):20163-20173. doi:10.1021/acsnano.2c04829
2. Liu, K., Nilsson, R., Lázaro-Ibáñez, E. et al. Multiomics analysis of naturally efficacious lipid nanoparticle coronas reveals high-density lipoprotein is necessary for their function. Nat Commun 14, 4007 (2023). https://doi.org/10.1038/s41467-023-39768-9?
3. Maugeri M, Nawaz M, Papadimitriou A, et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat Commun. 2019;10(1):4333. doi:10.1038/s41467-019-12275-6
4. Nawez M, Hagvall S, Tanhruksa B, et al. Lipid Nanoparticles Deliver the Therapeutic VEGFA mRNA In Vitro and In Vivo and Transform Extracellular Vesicles for Their Functional Extensions. Adv Sci. 2023. https://doi.org/10.1002/advs.202206187
5. Zhang L, Aragon‐Sanabria V, Aditya A, Marelli M, et al. Engineered Ultrasmall Nanoparticle Drug‐Immune Conjugates with “Hit and Run” Tumor Delivery to Eradicate Gastric Cancer. Advanced Therapeutics. 2022 Nov 14:2200209.
6. Tyagi P, Pechenov S, RiosDoria J, et al. Evaluation of Pyrrolobenzodiazepine-Loaded Nanoparticles: A Targeted Drug Delivery Approach. J Pharm Sci. 2019;108(4):1590-1597. doi:10.1016/j.xphs.2018.11.023
7. Tyagi P, Koskinen M, Mikkola J, Leino L, Schwarz A. Silica microparticles for sustained zero-order release of an anti-CD40L antibody. Drug Deliv Transl Res. 2018;8(2):368-374. doi:10.1007/s13346-017-0408-1
8. Pechenov S, Revell J, Will S, et al. Development of an orally delivered GLP-1 receptor agonist through peptide engineering and drug delivery to treat chronic disease. Sci Rep. 2021;11(1):22521. Published 2021 Nov 18. doi:10.1038/s41598-021-01750-0
9. Gennemark P, Walter K, Clemmensen N, et al. An oral antisense oligonucleotide for PCSK9 inhibition. Sci Transl Med. 2021;13(593):eabe9117. doi:10.1126/scitranslmed.abe9117
10. Berg S, Suljovic D, Kärrberg L, et al. Intestinal Absorption of FITC-Dextrans and Macromolecular Model Drugs in the Rat Intestinal Instillation Model. Mol Pharm. 2022;19(7):2564-2572. doi:10.1021/acs.molpharmaceut.2c00261\
11. Berg S, Kärrberg L, Suljovic D, et al. Impact of Intestinal Concentration and Colloidal Structure on the Permeation-Enhancing Efficiency of Sodium Caprate in the Rat. Mol Pharm. 2022;19(1):200-212. doi:10.1021/acs.molpharmaceut.1c00724
Veeva ID: Z4-54753 Date of preparation: May 2023
You are now leaving AstraZeneca.com
You have selected a link that will take you to a site maintained by a third party who is solely responsible for its contents.
AstraZeneca provides this link as a service to website visitors. AstraZeneca is not responsible for the privacy policy of any third party websites. We encourage you to read the privacy policy of every website you visit.
Click ‘cancel’ to return to AstraZeneca’s site or ‘continue’ to proceed.
Important notice for users You are about to access AstraZeneca historic archive material. Any reference in these archives to AstraZeneca products or their uses may not reflect current medical knowledge and should not be used as a source of information on the present product label, efficacy data or safety data. Please refer to your approved national product label (SmPC) for current product information. I have read this warning and will not be using any of the contained product information for clinical purposes.
Parker H. Petit Institute for Bioengineering and Bioscience
Drug, design, development & delivery.

Pharmaceuticals save lives, alleviate suffering, and are highly cost-effective compared to other treatments. In the Petit Institute, researchers seek to further improve pharmaceuticals through research on drug design, drug development, and drug delivery. Our research on drug design emphasizes making new drugs to treat cancer, AIDS, bacterial infections, and other diseases. These new drugs are synthesized at Georgia Tech and are being tested for optimal activity. Drug development involves novel processes to manufacture drugs using cells, enzymes, and other biologically based approaches that increase purity and decrease cost. Much of this work occurs in collaboration with pharmaceutical companies in the context of a multi-university consortium lead by Georgia Tech. Drug delivery applies engineering technologies to make taking drugs easier, more effective, and less frequent for patients. A leading project in this area involves the use of painless microneedle patches that are being moved into clinical trials for influenza vaccination and insulin delivery to diabetics. Through this work, we will impact healthcare by making pharmaceuticals more effective, less expensive, and more convenient for patients. Petit Institute researchers have developed innovative educational programs for students interested in pharmaceuticals. Every year, undergraduate and graduate students from multiple departments in the sciences and engineering can take the class Drug Design, Development, and Delivery, which presents students with a holistic view of the field of pharmaceuticals, including technical and non-technical aspects, and features a series of four real-drug case studies led by the students. Associated with the class is an optional week-long trip to tour the pharmaceutical industry in Puerto Rico, which is one of the leading sites of drug manufacturing in the world. In addition to seminars, lunchtime roundtable discussions, and other activities, 10 – 15 doctoral students receive fellowships to support their pharmaceutical research.
Drug, Design, Development & Delivery Faculty

Alex Abramson

Amirali Aghazadeh

Julia Babensee

Pamela Bhatti

Andreas Bommarius


Blair Brettmann

Julie Champion

Marcus Cicerone

James Dahlman

J. Brandon Dixon

Simone Douglas-Green

Donald Doyle

Erik Dreaden

Stanislav Emelianov

Andrei Fedorov

Craig Forest

Stefan France

Michelle Gaines, Ph.D.

Andrés J. García

Eric Gaucher

Robert Guldberg

Nicholas V. Hud

Seung Soon Jang

Yonggang Ke

YongTae (Tony) Kim

Julia Kubanek

Raquel Lieberman

Brooks Lindsey

Nael McCarty

Alfred H. Merrill

Valeria Milam

Adegboyega “Yomi” Oyelere

Alyssa Panitch, Ph.D.

Mark Prausnitz

Felipe Garcia Quiroz

Krishnendu Roy

Philip J. Santangelo

A. Fatih Sarioglu

Francesca Storici

Mark Styczynski

Todd Sulchek

Johnna Temenoff

Susan Thomas

Peter Thule
Marvin Whiteley


Synbiotics in Human Health: Biology to Drug Delivery
- Reference work
- © 2024
- Kamal Dua 0
Discipline of Pharmacy, Graduate School of Health, University of Technology Sydney, Ultimo, Australia
You can also search for this editor in PubMed Google Scholar
- Reviews the role of synbiotics in the management of different disease states
- Explores the advanced drug delivery approaches for site-specific delivery of synbiotics
- Discusses the potential of nutraceuticals in mitigating health problems
- Table of contents
About this book
Editors and affiliations, about the editor, bibliographic information.
- Publish with us
This is a preview of subscription content, log in via an institution to check access.
Access this book
Other ways to access.
Licence this eBook for your library
Institutional subscriptions
Table of contents(35 entries)
Front matter, synbiotics in disease states, introduction to synbiotics.
- Komal Singh, Amanda Frank Mariki, Preet Amol Singh, Saahil Arora, Neha Bajwa
Role of Synbiotics on Modulation of Inflammation
- Bharti Verma, Sumel Ashique, Neeraj Mishra, Nitish Kumar, Nidhi Tyagi, Shubneesh Kumar et al.
Role of Synbiotics in Inflammatory Lung Diseases
- Sumel Ashique, Shubneesh Kumar, Aakash Upadhyay, Ashish Garg, Neeraj Mishra, Prashant Kumar et al.
Role of Synbiotics in Neurodegenerative Diseases
- Shvetank Bhatt, Rohini Pujari, Yuvraj Patil, Satish Shilpi, K. Anitha
Role of Synbiotics in Gastrointestinal Disorders
- Vijayaraj Surendran, Prathap Madeswaraguptha, K. S. Kokilambigai, Raghavendra Kumar Gunda
Role of Synbiotics in Cardiovascular Diseases
- Mahendra Saini, Santosh Kumar Singh, Hemant Kumar Yadav, Piyush Dave, Manish Gupta, Asif Ahmad Bhat et al.
Role of Synbiotics in Thrombotic Disorders
- Adeniyi S. Ohunayo, Olorunfemi R. Molehin, Oluwasegun S. Dauda
Role of Synbiotics in Reproductive Disorders
- Riya Thapa, Ritu M. Gilhotra, Asif Ahmad Bhat, Manish Purohit, Rashi Kulshrestha, Neelam Singla et al.
Role of Synbiotics in Metabolic Disorders
- Gurmeet Singh, Simran Deep Kaur, Sarmili Sahoo, Raj Kumar Narang, Neeraj Mishra, Amandeep Singh
Synbiotics for the Prevention and Treatment of Skin Disorders
- C. Sarath Chandran, Krishnameera Sajayan, Hafsa Mohammad, Shijina Kappally, Alan Raj, K. K. Swathy
Cellular and Molecular Mechanisms Involving Synbiotics in Various Disease State
- Nitin Verma, Komal Thapa, Neha Kanojia, Gagandeep Kaur, Parul Sood, Jatin Kumar et al.
Clinical Trial with Synbiotics in Various Disease State
- Satish Shilpi, Prinali Chimaniya, Khyati Saini, Hadiya Jan, Sandhya Chouhan, Jamal Basha Dudhekula et al.
Future Perspective and Safety Issues of Synbiotics in Different Diseases
- Shyam Sudhakar Gomte, Biswajit Rout, Tejas Girish Agnihotri, Vasu Peddinti, Aakanchha Jain
Advanced Drug Delivery Approaches Containing Synbiotics
Synbiotics and drug delivery: an introduction.
- Nikhil B. Khandale, Md Shahbaz Alam, Devendra S. Birla, Sukriti Vishwas, Monica Gulati, Molakpogu Ravindra Babu et al.
Emerging Era of “Biotics”: Prebiotics, Probiotics, and Synbiotics
- Rahul Nair, Priti Paul, Srushti Mahajan, Indrani Maji, Ujala Gupta, Sachin Kumar Singh et al.
Therapeutic Potential of Synbiotics in Management of Various Disorders
- Lovedeep Singh, Harpreet Kaur, Rajbir Bhatti
Synbiotics: Safety Assessment and Role in the Prevention of Diseases
- Lavanya Mude, Vyshnavi Tallapaneni, R. Kalaivanan, Veera Venkata Satyanarayana Reddy Karri
- Inflammation
- Drug Delivery
- Nutraceuticals
Book Title : Synbiotics in Human Health: Biology to Drug Delivery
Editors : Kamal Dua
DOI : https://doi.org/10.1007/978-981-99-5575-6
Publisher : Springer Singapore
eBook Packages : Medicine , Reference Module Medicine
Copyright Information : Springer Nature Singapore Pte Ltd. 2024
Hardcover ISBN : 978-981-99-5574-9 Due: 30 April 2024
eBook ISBN : 978-981-99-5575-6 Published: 29 March 2024
Edition Number : 1
Number of Pages : XXX, 718
Number of Illustrations : 154 b/w illustrations, 427 illustrations in colour
Topics : Pharmacology/Toxicology , Health Care Management , Biotechnology
Policies and ethics
- Find a journal
- Track your research
- skip to Cookie Notice
- skip to Main Navigation
- skip to Main Content
- skip to Footer
- Find a Doctor
- Find a Location
- Appointments & Referrals
- Patient Gateway
- Español
- Leadership Team
- Quality & Safety
- Equity & Inclusion
- Community Health
- Education & Training
- Centers & Departments
- Browse Treatments
- Browse Conditions A-Z
- View All Centers & Departments
- Clinical Trials
- Cancer Clinical Trials
- Cancer Center
- Digestive Healthcare Center
- Heart Center
- Mass General for Children
- Neuroscience
- Orthopaedic Surgery
- Information for Visitors
- Maps & Directions
- Parking & Shuttles
- Services & Amenities
- Accessibility
- Visiting Boston
- International Patients
- Medical Records
- Billing, Insurance & Financial Assistance
- Privacy & Security
- Patient Experience
- Explore Our Laboratories
- Industry Collaborations
- Research & Innovation News
- About the Research Institute
- Innovation Programs
- Education & Community Outreach
- Support Our Research
- Find a Researcher
- News & Events
- Ways to Give
- Patient Rights & Advocacy
- Website Terms of Use
- Apollo (Intranet)
- Like us on Facebook
- Follow us on Twitter
- See us on LinkedIn
- Print this page
Press Release Mar | 27 | 2024
A Combination of Approved Drugs Enhances the Delivery of Anti-Bacterial Medications to Treat Tuberculosis
Key takeaways.
- Researchers have found that approved drugs that were originally shown to normalize blood vessels surrounding tumors (to improve drug delivery to cancer cells) can enhance the delivery of anti-microbial medications to kill tuberculosis bacteria residing in the lungs
- These drugs (bevacizumab and losartan) alone and in combination with anti-microbial agents, promoted anti-bacterial host responses and improved health outcomes in lab models with tuberculosis
BOSTON – Tuberculosis (TB) is often overlooked in developed countries such as the United States, but this bacterial infection remains one of the deadliest diseases globally and results in millions of deaths annually.
Deaths can occur even with treatment, sometimes because of drug resistance in TB bacteria and other times due to poor delivery of TB-targeting drugs to patients’ infected lung tissue.
To address the latter challenge, a team led by researchers at Massachusetts General Hospital (MGH) in collaboration with scientists at the National Institute of Allergy and Infectious Disease (NIAID), the University of Notre Dame, and Hackensack Meridian School of Medicine , repurposed approved drugs that they originally tested to normalize blood vessels surrounding tumors to improve drug delivery to cancer cells.
In this latest research, which is published in the Proceedings of the National Academy of Sciences , these drugs effectively enhanced the delivery of anti-microbial medications to kill TB bacteria.
“Our team is interested in understanding and overcoming physiological barriers to drug delivery in pulmonary granulomas, the site of TB disease that manifests as abnormal lung masses. Even the most potent anti-bacterial drug will fail if it cannot reach the bacteria fueling the disease,” says senior and co–corresponding author Rakesh K. Jain, PhD , director of the E.L. Steele Laboratories for Tumor Biology at MGH and the Andrew Werk Cook Professor of Radiation Oncology at Harvard Medical School.
Poorly functioning blood vessels and an overabundant extracellular matrix (a network of proteins and other molecules that surround and give structure to tissues in the body) both reduce blood flow and drug delivery throughout granulomas, where TB bacteria reside and hide, evading attack by the body’s immune system.
“Our multidisciplinary team of engineers, cancer biologists, immunologists, microbiologists, and data analysts used a lab model of TB, which recapitulates human disease, to test what are called host-directed therapies—or HDTs—that ‘normalize’ the abnormal blood vessels and extracellular matrix in granulomas,” says Jain. These HDTs are bevacizumab, which acts on blood vessels, and losartan, which targets the extracellular matrix.
Jain and his colleagues previously showed that bevacizumab could improve drug delivery to TB granulomas. Now they’ve shown that combining bevacizumab and losartan in rabbits with TB enhances TB drug delivery, promotes anti-bacterial host responses, and improves health outcomes. Surprisingly, the HDTs themselves (without anti-bacterial agents) were able to reduce bacteria numbers in TB granulomas.
To identify the mechanisms involved, the investigators analyzed granuloma and lung tissues and found that the HDTs promoted inflammatory responses against TB bacteria, both in immune and non-immune cells in the lung.
“Because bevacizumab and losartan are approved, safe, and affordable, our preclinical study lays the groundwork for direct clinical translation to test these HDTs in patients with TB for the drugs’ ability to improve outcomes of anti-bacterial therapy,” says Jain.
Study co-authors include Meenal Datta (co–corresponding author), Laura E. Via (co–first author), Véronique Dartois (co–first author), Danielle M. Weiner, Matthew Zimmerman, Firat Kaya, April M. Walker, Joel D. Fleegle, Isaac D. Raplee, Colton McNinch, Maksym Zarodniuk1, Walid S. Kamoun, Changli Yue, Ashwin S. Kumar, Sonu Subudhi, Lei Xu, and Clifton E. Barry III (co–corresponding author).
This work was supported in part by grants from the Bill & Melinda Gates Foundation and the National Institutes of Health.
About the Massachusetts General Hospital
Massachusetts General Hospital, founded in 1811, is the original and largest teaching hospital of Harvard Medical School. The Mass General Research Institute conducts the largest hospital-based research program in the nation, with annual research operations of more than $1 billion and comprises more than 9,500 researchers working across more than 30 institutes, centers and departments. MGH is a founding member of the Mass General Brigham healthcare system.
- Press Release
Centers and Departments
- Research Institute
- Radiation Oncology
Check out the Mass General Research Institute blog
Bench Press highlights the groundbreaking research and boundary-pushing scientists working to improve human health and fight disease.
Support Research at Mass General
Your gift helps fund groundbreaking research aimed at understanding, treating and preventing human disease.
Find Info For
- Current Students
- Prospective Students
- Research and Partnerships
- Entrepreneurship and Commercialization
Quick Links
- Health and Life Sciences
- Info Security and AI
- Transformative Education
- Purdue Today
- Purdue Global
- Purdue in the News
March 27, 2024
Purdue researchers create biocompatible nanoparticles to enhance systemic delivery of cancer immunotherapy
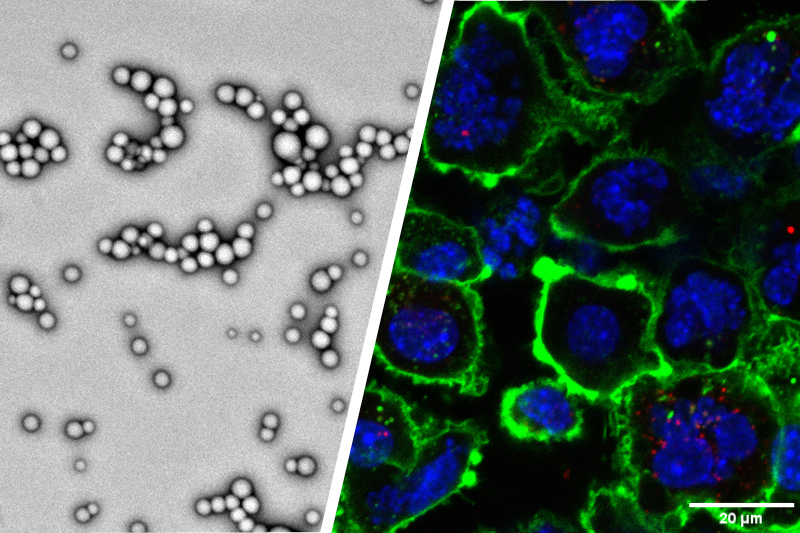
Purdue University researchers are developing and validating patent-pending nanoparticles (left) to enhance immunotherapy effects against tumors. The nanoparticles are modified with adenosine triphosphate, or ATP, to recruit dendritic cells (right), which are immune cells that recognize tumor antigens and bring specialized immune cells to fight off tumors. (Images provided by Yoon Yeo)
PLGA nanoparticles modified with ATP slowly release anti-cancer drugs and recruit immune cells to fight tumors
WEST LAFAYETTE, Ind. — Purdue University researchers are developing and validating patent-pending poly (lactic-co-glycolic acid), or PLGA, nanoparticles modified with adenosine triphosphate, or ATP, to enhance immunotherapy effects against malignant tumors.
The nanoparticles slowly release drugs that induce immunogenic cell death, or ICD, in tumors. ICD generates tumor antigens and other molecules to bring immune cells to a tumor’s microenvironment. The researchers have attached ATP to the nanoparticles, which also recruits immune cells to the tumor to initiate anti-tumor immune responses.
Yoon Yeo leads a team of researchers from the College of Pharmacy , the Metabolite Profiling Facility in the Bindley Bioscience Center , and the Purdue Institute for Cancer Research to develop the nanoparticles. Yeo is the associate department head and Lillian Barboul Thomas Professor of Industrial and Molecular Pharmaceutics and Biomedical Engineering; she is also a member of the Purdue Institute for Drug Discovery and the Purdue Institute for Cancer Research.
The researchers validated their work using paclitaxel, a chemotherapy drug used to treat several types of cancers. They found that tumors grew slower in mice treated with paclitaxel enclosed within ATP-modified nanoparticles than in mice treated with paclitaxel in non-modified nanoparticles.
“When combined with an existing immunotherapy drug, the ATP-modified, paclitaxel-loaded nanoparticles eliminated tumors in mice and protected them from rechallenge with tumor cells,” Yeo said.
The research has been published in the peer-reviewed journal ACS Nano .
Challenges to systemic immunotherapy delivery
Immunotherapy is a promising approach to fighting cancer, but Yeo said it does not benefit a large population of patients because they do not have the powerful immune cells needed to combat tumors.
“Pharmacological agents to activate immune cells can directly be given to tumors,” Yeo said. “Then the immune system can fight not only the treated tumors but also nontreated tumors in distant locations as the activated immune cells circulate in the bloodstream.”
However, Yeo said most tumors with poor prognoses are not always locatable or accessible. Therefore, they may not be effectively treated by local therapy. She and her team envisioned systemic delivery of immunotherapy, but there are challenges.
“For successful systemic administration, active ingredients that stimulate anti-tumor immune responses need to be simultaneously present in tumors to exert concerted effects on the target,” Yeo said. “The ingredients also must maintain their activity until they reach tumors, but not cause toxic off-target effects. Moreover, the carriers traditionally used in local drug delivery offer limited utility in systemic application because they may not be compatible with blood components.”
Yeo and her colleagues used biocompatible polymeric nanoparticles to deliver immunotherapy compounds and modified them to safely activate the immune system.
“We employed poly (lactic-co-glycolic acid), or PLGA, nanoparticles based on the strong track record of the polymer in FDA-approved products and its routine use in the systemic delivery of poorly water-soluble drugs,” Yeo said.
Tests verified the ATP-modified PLGA nanoparticles were well tolerated in mice upon multiple systemic injections. They were able to recruit dendritic cells, the immune cells that recognize tumor antigens and bring specialized immune cells to fight off tumors.
“Moreover, the nanoparticles were shown to control the release of paclitaxel to minimize its systemic toxicity,” Yeo said.
The next development steps
Yeo and her colleagues will continue their work on the ATP-modified nanoparticles.
“We are currently working on improving the delivery of the nanoparticles to tumors and combining them with other treatments that will circumvent the resistance to the nanoparticle-delivered immunotherapy,” Yeo said. “To finance these efforts, we will apply for continued support from the National Institutes of Health. We are also open to industry partnerships to take this technology to the clinic.”
Yeo disclosed the nanoparticles innovation to the Purdue Innovates Office of Technology Commercialization , which has applied for a patent from the U.S. Patent and Trademark Office to protect the intellectual property. Industry partners interested in developing the compound or commercializing it for the marketplace should contact Joe Kasper, assistant director of business development and licensing — life sciences, at [email protected] , about track code 69546 .
Yeo and the research team received funding from the National Institutes of Health, the National Center for Advancing Translational Sciences, the Indiana Clinical and Translational Sciences Institute, and the Purdue Institute for Cancer Research.
About Purdue University
Purdue University is a public research institution demonstrating excellence at scale. Ranked among top 10 public universities and with two colleges in the top four in the United States, Purdue discovers and disseminates knowledge with a quality and at a scale second to none. More than 105,000 students study at Purdue across modalities and locations, including nearly 50,000 in person on the West Lafayette campus. Committed to affordability and accessibility, Purdue’s main campus has frozen tuition 13 years in a row. See how Purdue never stops in the persistent pursuit of the next giant leap — including its first comprehensive urban campus in Indianapolis, the new Mitchell E. Daniels, Jr. School of Business, and Purdue Computes — at https://www.purdue.edu/president/strategic-initiatives .
About Purdue Innovates Office of Technology Commercialization
The Purdue Innovates Office of Technology Commercialization operates one of the most comprehensive technology transfer programs among leading research universities in the U.S. Services provided by this office support the economic development initiatives of Purdue University and benefit the university’s academic activities through commercializing, licensing and protecting Purdue intellectual property. In fiscal year 2023, the office reported 150 deals finalized with 203 technologies signed, 400 disclosures received and 218 issued U.S. patents. The office is managed by the Purdue Research Foundation, which received the 2019 Innovation & Economic Prosperity Universities Award for Place from the Association of Public and Land-grant Universities. In 2020, IPWatchdog Institute ranked Purdue third nationally in startup creation and in the top 20 for patents. The Purdue Research Foundation is a private, nonprofit foundation created to advance the mission of Purdue University. Contact [email protected] for more information.
Writer/Media contact: Steve Martin, [email protected]
Source: Yoon Yeo, [email protected]
Research Foundation News
Communication.
- OneCampus Portal
- Brightspace
- BoilerConnect
- Faculty and Staff
- Human Resources
- Colleges and Schools
Info for Staff
- Purdue Moves
- Board of Trustees
- University Senate
- Center for Healthy Living
- Information Technology
- Ethics & Compliance
- Campus Disruptions
Purdue University, 610 Purdue Mall, West Lafayette, IN 47907, (765) 494-4600
© 2015-24 Purdue University | An equal access/equal opportunity university | Copyright Complaints | Maintained by Office of Strategic Communications
Trouble with this page? Disability-related accessibility issue? Please contact News Service at [email protected] .
We've detected unusual activity from your computer network
To continue, please click the box below to let us know you're not a robot.
Why did this happen?
Please make sure your browser supports JavaScript and cookies and that you are not blocking them from loading. For more information you can review our Terms of Service and Cookie Policy .
For inquiries related to this message please contact our support team and provide the reference ID below.
More From Forbes
Onc invites public input on health it plan.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Group of people sitting on a seminar. They have their hand raised.
The Office of the National Coordinator for Health IT has published the 2024-2030 Federal Health IT Strategic Plan, providing a roadmap for the future. This plan envisions technology and health information significantly enhancing individual and community wellbeing. It aligns with healthcare CIOs' core mission to use technology to engage individuals, reduce costs, deliver high-quality care, and improve population health. The plan is structured around four main goals, setting a clear direction for future healthcare IT initiatives. Is this enough to make future progress for the industry?
Promoting Health and Wellness
The strategic plan strongly emphasizes empowering individuals to manage their health, enhancing the delivery and experience of care, and accelerating research and innovation. According to the latest report from the Office of the National Coordinator for Health Information Technology, most US provider organizations have an electronic medical record system, which is the starting point since they all have a patient portal allowing individuals to manage their health at their fingertips.
As the healthcare industry has moved towards digitalization, achieving interoperability among different systems continues to challenge us. EMR vendors have successfully ensured interoperability within organizations that use the same systems. However, this issue has consistently been a barrier in the last strategic plan.
Enhancing Care Delivery
Enhancing the delivery and experience of care is vital as provider organizations aim to facilitate easy access to care in the digital age. Patients now expect seamless and efficient interactions with their healthcare providers through technologies like virtual care, two-way messaging, and self-service scheduling features. We aim to surpass these expectations by deploying intuitive technologies that streamline patient and provider processes, thus enhancing outcomes and satisfaction.
The plan reaffirms the commitment to using IT to promote health and wellness, recognizing technology's role in empowering patients and communities. However, the digital divide and disparities in technology access and literacy hinder these efforts' reach and impact. Addressing these disparities is crucial for the equitable distribution of health IT benefits and ensuring everyone has the necessary tools and knowledge for health management. There's a significant need for health education, especially among those with poor health who require extensive care. Despite the importance of preventive care, which heavily relies on education, provider organizations currently receive limited reimbursement for such services.
This Popular Google App Will Stop Working In 3 Days—How To Migrate Your Data
Google suddenly reveals surprise android update that beats iphone, apple quietly adds free performance upgrade for all iphone 12 users, accelerating research and innovation.
The objective emphasizes that researchers and health IT users should actively access health data to foster improvements in individual and population health. Health IT prioritizes enhancing research and analysis at both personal and population levels. Notably, including data from underrepresented groups in research efforts is a crucial strategy to actively advance health equity. This objective underlines the goal of using health IT to achieve more inclusive health improvements and research outcomes, demonstrating a commitment to leveraging technology for the betterment of all.
Connecting Health Data
This goal highlights the importance of developing policies and technology components to meet the varied data needs of health IT users, aiming to ensure that regulatory constraints, privacy concerns, and the technological limitations of current health IT infrastructures do not hinder the pace of innovation.
The 2024-2030 federal health IT strategic draft plan envisions health IT as a catalyst for significant healthcare improvements, highlighting areas needing more focus. It underscores the importance of collaboration among federal agencies, healthcare providers, technology companies, and patient advocacy groups to address these challenges. With a 60-day comment period ending on May 28, 2024, there's an opportunity for feedback to enrich the plan with new initiatives and requirements, aiming to accelerate progress in health IT.

- Editorial Standards
- Reprints & Permissions
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 22 April 2005
The rise and rise of drug delivery
- Howard Rosen 1 &
- Thierry Abribat 2
Nature Reviews Drug Discovery volume 4 , pages 381–385 ( 2005 ) Cite this article
4694 Accesses
223 Citations
9 Altmetric
Metrics details
Drug delivery has typically focused on optimizing marketed compounds, improving their effectiveness or tolerability, and simplifying their administration. This role now includes the first biopharmaceuticals as well as more conventional drugs. As drug-delivery technologies come into play earlier in the development cycle, however, they can also enhance the screening and evaluation of new compounds and 'rescue' failed compounds, such as those with low solubility. In this article, we look back at how the burgeoning field of drug delivery came into being and describe approaches for future discovery and development.
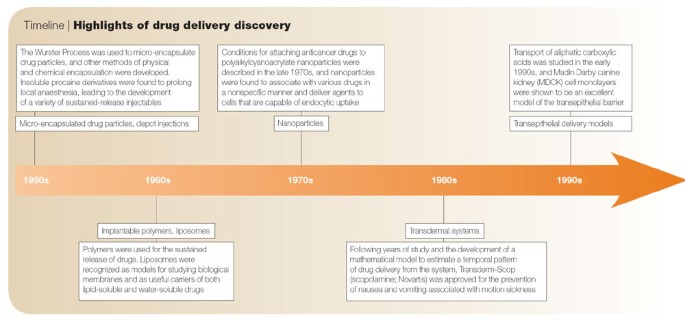
Timeline | Highlights of drug delivery discovery
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Levy, G. Pharmacokinetics of salicylate elimination in man. J. Pharm. Sci. 54 , 959–967 (1965).
Article CAS Google Scholar
Prisant, L. M. & Elliott, W. J. Drug delivery systems for treatment of systemic hypertension. Clin. Pharmacokinet. 42 , 931–940 (2003).
Maggi, L., Bruni, R. & Conte, U. High molecular weight polyethylene oxides (PEOs) as an alternative to HPMC in controlled release dosage forms. Int. J. Pharm. 195 , 229–238 (2000).
Theeuwes, F. Elementary osmotic pump. J. Pharm. Sci. 64 , 1987–1991 (1975).
Theeuwes, F. et al. Elementary osmotic pump for indomethacin. J. Pharm. Sci. 72 , 253–258 (1983).
Anderson, R. et al. Once-a-day controlled release versus immediate release oxybutynin chloride in the treatment of urinary urge incontinence. J. Urol. 161 , 1809–1812 (1999).
Versi, E., Appell, R., Mobley, D., Patton, W. & Saltzstein, D. Dry mouth with conventional and controlled-release oxybutynin in urinary incontinence. Obstet. Gynecol. 95 , 718–721 (2000).
CAS PubMed Google Scholar
Swanson, J. et al. Development of a new once-a-day formulation of methylphenidate for the treatmentof attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch. Gen. Psychiatry 60 , 204–211 (2003).
Columbo, P. et al. Drug release modulation by physical restrictions of matrix swelling. Int. J. Pharm. 63 , 43–48 (1990).
Article Google Scholar
Conte, U., Maggi, L., Colombo, P. & La Manna, A. Multi-layered hydrophilic matrices as constant release devices. J. Control. Release 26 , 39–47 (1993).
Conte, U., Colombo, P., Maggi, L. & La Manna, A. Compressed barrier layers for constant drug release in swellable matrix tablets. STP Pharm. Sci. 4 , 107–113 (1994).
CAS Google Scholar
Conte, U. & Maggi, L. Modulation of the dissolution profiles from Geomatrix multi-layer matrix tablets containing drugs on different solubility. Biomaterials 17 , 889–896 (1996).
Biederman, J. et al. Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatr Drugs. 5 , 833–841 (2003).
Semenchuk, M. R. Avinza élan. Curr. Opin. Investig. Drugs. 3 , 1369–1372 (2002).
Chourasia, M. K. & Jain, S. K. Design and development of multiparticulate system for targeted drug delivery to colon. Drug Deliv. 11 , 201–207 (2004).
Parojcic, J., Duric, Z., Jovanovic, M. & Ibric, S. An investigation into the factors influencing drug release from hydrophilic matrix tablets based on novel carbomer polymers. Drug Deliv. 11 , 59–65 (2004).
Viscusi, E. R., Reynolds, L., Chung, F., Atkinson, L. E. & Khanna, S. Patient-controlled transdermal fentanyl hydrochloride vs. intravenous morphine pump for postoperative pain. JAMA 291 , 1333–1341 (2004).
Prego, C., Garcia, M., Torres, D. & Alonso, M. J. Transmucosal macromolecular drug delivery. J. Control. Release 101 , 151–162 (2005).
Smith, J., Wood, E. & Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 21 , 43–49 (2004).
Eley, J. G., Pujari, V. D. & McLane, J. Poly (lactide-co-glycolide) nanoparticles containing coumarin-6 for suppository delivery: in vitro release profile and in vivo tissue distribution. Drug Deliv. 11 , 255–261 (2004).
Foran, T. M. New contraceptive choices across reproductive life. Med. J. Aust. 178 , 616–620 (2003).
PubMed Google Scholar
Toivonen, J. The levonorgestrel-releasing uterine device. Adv Contracept Deliv Syst. 10 , 191–198 (1994).
Jain, S. K., Chourasia, M. K., Jain, A. K., Jain, R. K. & Shrivastava, A. K. Development and characterization of mucoadhesive microspheres bearing salbutamol for nasal delivery. Drug Deliv. 11 , 113–122 (2004).
Casez, J. P. et al. Effects of nasal calcitonin on bone mineral density following parathyroidectomy in patients with primary hyperparathyroidism. Horm. Res. 59 , 263–269 (2003).
Ayuk, J., Stewart, S. E., Stewart, P. M., Sheppard, M. C., European Sandostatin LAR Group. Efficacy of Sandostatin LAR (long–acting somatostatin analogue) is similar in patients with untreated acromegaly and in those previously treated with surgery and/or radiotherapy. Clin. Endocrinol. (Oxf) 60 , 375–381 (2004).
Attanasio, R. et al. Lanreotide 60 mg, a new long-acting formulation: effectiveness in the chronic treatment of acromegaly. J. Clin. Endocrinol. Metab. 88 , 5258–5265 (2003).
Fowler, J. E. Jr, Viadur Study Group. Patient-reported experience with the Viadur 12-month leuprolide implant for prostate cancer. Urology 58 , 430–434 (2001).
Fowler, J. E. et al. Evaluation of an implant that delivers leuprolide for 1 year for the palliative treatment of prostate cancer. Urology 55 , 639–642 (2000).
Fowler, J. E. Jr, Gottesman, J. E., Reid, C. F., Andriole, G. L. Jr & Soloway, M. S. Safety and efficacy of an implantable leuprolide delivery system in patients with advanced prostate cancer. J. Urol. 164 , 730–734 (2000).
Tam, C. S., Heersche, J. N., Murray, T. M. & Parsons, J. A. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology 110 , 506–512 (1982).
Orskov, C., Wettergren, A. & Holst, J. J. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand. J. Gastroenterol. 31 , 665–670 (1996).
Barry, B. W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J. Pharm. Sci. 14 , 101–114 (2001).
Lee, W. R., Shen, S. C., Wang, K. H., Hu, C. H. & Fang, J. Y. The effect of laser treatment on skin to enhance and control transdermal delivery of 5-fluorouracil. J. Pharm. Sci. 91 , 1613–1626 (2002).
Shapiro, H., Harris, L., Hetzel, F. W. & Bar-Or, D. Laser assisted delivery of topical anesthesia for intramuscular needle insertion in adults. Lasers Surg. Med. 31 , 252–256 (2002).
Kaul, G. & Amiji, M. Long-circulating poly(ethylene glycol)-modified gelatin nanoparticles for intracellular delivery. Pharm. Res. 19 , 1061–1067 (2002).
Gabizon, A., Tzemach, D., Mak, L., Bronstein, M. & Horowitz, A. T. Dose dependency of pharmacokinetics and therapeutic efficacy of pegylated liposomal doxorubicin (DOXIL) in murine models. J. Drug Target 10 539–548 (2002).
Theodoulou, M. & Hudis, C. Cardiac profiles of liposomal anthracyclines: greater cardiac safety versus conventional doxorubicin? Cancer 100 , 2052–2063 (2004).
Abra, R. M. et al. The next generation of liposome delivery systems: recent experience with tumor-targeted, sterically-stabilized immunoliposomes and active-loading gradients. J. Liposome Res. 12 , 1–3 (2002).
Fricker, G. & Miller, D. S. Modulation of drug transporters at the blood–brain barrier. Pharmacology 10 , 169–176 (2004).
LaVan, D. A., McGuire, T. & Langer, R. Small-scale systems for in vivo drug delivery. Nature Biotechnol. 21 , 1184–1191 (2003).
Rabinow, B. E. Nanosuspensions in drug delivery. Nature Rev. Drug Discov. 3 , 785–796 (2004).
Business Wire, January 12, 2005. Elan Corp. Elan's proprietary NanoCrystal Technology is used by Johnson & Johnson Pharmaceutical Research & Development, L.L.C. (J&J PRD) in Phase III clinical trial of paliperidone palmitate [online], < http://www.elan.com/DrugDelivery/Announcements/12_01_2005.asp > Press Release 12 Jan (2005).
Download references
Acknowledgements
The authors wish to acknowledge J. Wright and D. Waxman for their help in preparing this manuscript.
Author information
Authors and affiliations.
Vice President Commercial Strategy at Gilead Sciences, Inc., 333 Lakeside Drive, Foster City, 94404, California
Howard Rosen
CEO and Vice President of R&D at OPi Pharmaceuticals, Les Jardins D'Ecole, 3 allée des Séquioas, Limonest, F-69760, Lyon, France
Thierry Abribat
You can also search for this author in PubMed Google Scholar
Ethics declarations
Competing interests.
H.B.R. is a former employee of ALZA Corp. and continues to be a shareholder of Johnson & Johnson. T.A. is a former employee of Theratechnologies and continues to be a shareholder.
Related links
Further information.
Theratechnologies
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Rosen, H., Abribat, T. The rise and rise of drug delivery. Nat Rev Drug Discov 4 , 381–385 (2005). https://doi.org/10.1038/nrd1721
Download citation
Published : 22 April 2005
Issue Date : 01 May 2005
DOI : https://doi.org/10.1038/nrd1721
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Using surface plasmon resonance, capillary electrophoresis and diffusion-ordered nmr spectroscopy to study drug release kinetics.
- Alena Libánská
- Tomáš Špringer
- Tomáš Etrych
Communications Chemistry (2023)
Coordination-driven self-assembly of metallo-nanodrugs for local inflammation alleviation
- Lijuan Tang
- Zhenghan Di
Nano Research (2023)
The evolution of commercial drug delivery technologies
- Ava M. Vargason
- Aaron C. Anselmo
- Samir Mitragotri
Nature Biomedical Engineering (2021)
Recent applications and strategies in nanotechnology for lung diseases
- Wenhao Zhong
- Xinyu Zhang
Nano Research (2021)
Separation of gold and other rare materials from an ensemble of heterogeneous particles using a NdFeB magnetic circuit
- Chiaki Uyeda
- Keiji Hisayoshi
- Kentaro Terada
Scientific Reports (2019)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

VIDEO
COMMENTS
Recent drug delivery systems (DDS) are formulated using advanced technology to accelerate systemic drug delivery to the specific target site, maximizing therapeutic efficacy and minimizing off-target accumulation in the body. As a result, they play an important role in disease management and treatment.
Abstract. Drug delivery technologies have enabled the development of many pharmaceutical products that improve patient health by enhancing the delivery of a therapeutic to its target site ...
Subsequently, nanoparticle delivery technology laid the foundation for all-mRNA vaccines, which addressed the global COVID-19 pandemic. Looking back, seven decades of effort have produced stunning drug delivery technologies to break down the barriers between new drug candidates and their targets in tissues and cells. Looking forward, the future ...
New methods of drug delivery ultimately aim for increased efficacy, as well as improving the experience for patients — from simplifying the method of taking a drug to improving its safety. The ...
The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on: • Controlled release. • Bioavailability and drug absorption. • Nanomedicines. • Gene delivery. • Tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.
Advances in nanoparticle design could make substantial contributions to personalized and non-personalized medicine. In this Review, Langer, Mitchell, Peppas and colleagues discuss advances in ...
Drug delivery is becoming a whole interdisciplinary and independent field of research and is gaining the attention of pharmaceutical makers, medical doctors and industry. A targeted and safe drug delivery could improve the performance of some classical medicines already on the market and, moreover, will have implications for the development and ...
Drug delivery systems describe technologies that carry drugs into or throughout the body. These technologies include the method of delivery, such as a pill that you swallow or a vaccine that is injected. Drug delivery systems can also describe the way that drugs are 'packaged'—like a micelle or a nanoparticle —that protects the drug ...
Drug Delivery publishes open access peer-reviewed research on the development and application principles of drug delivery and targeting at molecular, cellular, and higher levels.. Drug Delivery aims to serve both the academic and industrial communities and accepts research on the following topics:. All drug delivery systems, including oral, pulmonary, nasal, parenteral and transdermal delivery;
Research has shown that artificial nanocomposite teeth are more stable than acrylic teeth and composite microfill teeth and are more resistant to abrasion. ... Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology: efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in ...
Recently, there has been enormous developments in the field of delivery systems to provide therapeutic agents or natural based active compounds to its target location for treatment of various aliments [33, 34].There are a number of drug delivery systems successfully employed in the recent times, however there are still certain challenges that need to be addresses and an advanced technology ...
Artificial intelligence (AI) has emerged as a powerful tool that harnesses anthropomorphic knowledge and provides expedited solutions to complex challenges. Remarkable advancements in AI technology and machine learning present a transformative opportunity in the drug discovery, formulation, and testing of pharmaceutical dosage forms. By utilizing AI algorithms that analyze extensive biological ...
Drug delivery describes the method and approach to delivering drugs or pharmaceuticals and other xenobiotics to their site of action within an organism, with the goal of achieving a therapeutic ...
Advanced Drug Delivery and Encapsulation project in Weitz Group is selected and ... "Research teams embarking on groundbreaking projects often find themselves at a critical juncture—the transition from visionary concepts to tangible commercial applications. ... requiring only a smartphone. The technology will provide the functionality of ...
We are now focusing our research on enhancing the efficacy and safety profile of this drug delivery system. Hear more from Drug Delivery scientists about LNPs as a promising drug delivery technology Our Advanced Drug Delivery teams have an ongoing collaboration with Fredrik Höök's and Elin Esbjörner's teams at Chalmers University of ...
In the Petit Institute, researchers seek to further improve pharmaceuticals through research on drug design, drug development, and drug delivery. Our research on drug design emphasizes making new drugs to treat cancer, AIDS, bacterial infections, and other diseases. These new drugs are synthesized at Georgia Tech and are being tested for ...
Drug-eluting stents (DES) and balloons revolutionize atherosclerosis treatment by targeting hyperplastic tissue responses through effective local drug delivery strategies. This review examines approved and emerging endovascular devices, discussing drug release mechanisms and their impacts on arterial drug distribution. It emphasizes the crucial role of drug delivery in modern cardiovascular ...
Dr. Kamal Dua is a Senior Lecturer in the Discipline of Pharmacy at the Graduate School of Health, University of Technology Sydney (UTS), Australia. He has research experience of over 12 years in the field of drug delivery systems targeting inflammatory diseases. Dr. Dua is also a Node Leader of Drug Delivery Research in the Centre for Inflammation at Centenary Institute/UTS, where the targets ...
In this latest research, which is published in the Proceedings of the National Academy of Sciences, these drugs effectively enhanced the delivery of anti-microbial medications to kill TB bacteria. "Our team is interested in understanding and overcoming physiological barriers to drug delivery in pulmonary granulomas, the site of TB disease ...
Finally, we describe the path from preclinical drug delivery research to clinical approval, highlighting opportunities to improve the efficiency with which new drug delivery systems are discovered.
The research has been published in the peer-reviewed journal ACS Nano. ... based on the strong track record of the polymer in FDA-approved products and its routine use in the systemic delivery of poorly water-soluble drugs," Yeo said. ... We are also open to industry partnerships to take this technology to the clinic." ...
Listen. 1:07. Amazon.com Inc. is expanding same-day delivery of prescription medication to New York City and the greater Los Angeles area, allowing customers to receive drugs for the flu, diabetes ...
Promoting Health and Wellness. The strategic plan strongly emphasizes empowering individuals to manage their health, enhancing the delivery and experience of care, and accelerating research and ...
The recent history of drug-delivery technology ... Currently, insulin is a major focus of delivery research because of the need for lifelong treatment in diabetic patients. Oral, intrapulmonary ...