Selecting a Research Topic: Overview
- Refine your topic
- Background information & facts
- Writing help
Here are some resources to refer to when selecting a topic and preparing to write a paper:
- MIT Writing and Communication Center "Providing free professional advice about all types of writing and speaking to all members of the MIT community."
- Search Our Collections Find books about writing. Search by subject for: english language grammar; report writing handbooks; technical writing handbooks
- Blue Book of Grammar and Punctuation Online version of the book that provides examples and tips on grammar, punctuation, capitalization, and other writing rules.
- Select a topic
Choosing an interesting research topic is your first challenge. Here are some tips:
- Choose a topic that you are interested in! The research process is more relevant if you care about your topic.
- If your topic is too broad, you will find too much information and not be able to focus.
- Background reading can help you choose and limit the scope of your topic.
- Review the guidelines on topic selection outlined in your assignment. Ask your professor or TA for suggestions.
- Refer to lecture notes and required texts to refresh your knowledge of the course and assignment.
- Talk about research ideas with a friend. S/he may be able to help focus your topic by discussing issues that didn't occur to you at first.
- WHY did you choose the topic? What interests you about it? Do you have an opinion about the issues involved?
- WHO are the information providers on this topic? Who might publish information about it? Who is affected by the topic? Do you know of organizations or institutions affiliated with the topic?
- WHAT are the major questions for this topic? Is there a debate about the topic? Are there a range of issues and viewpoints to consider?
- WHERE is your topic important: at the local, national or international level? Are there specific places affected by the topic?
- WHEN is/was your topic important? Is it a current event or an historical issue? Do you want to compare your topic by time periods?

Table of contents
- Broaden your topic
- Information Navigator home
- Sources for facts - general
- Sources for facts - specific subjects
Start here for help
Ask Us Ask a question, make an appointment, give feedback, or visit us.
- Next: Refine your topic >>
- Last Updated: Jul 30, 2021 2:50 PM
- URL: https://libguides.mit.edu/select-topic
Speech Ch 6

Get better grades with Learn
82% of students achieve A’s after using Learn

Vocabulary for Achievement: Third Course

The Art of Public Speaking
If you are trying to reach FDAnews.com, you’re in the right place.
We regret to inform you the production of FDAnews publications and databases has come to an end and we are closing our doors. Thank you for your support and readership over the years.
If you have any questions specific to legacy FDAnews publications, please email [email protected] .
If you are looking for clinical research information and insights, or information about clinical trials, we invite you to visit:
- Avoca Quality Consortium (AQC) a pre-competitive collaborative consortium of 200+ companies dedicated to elevating clinical trial quality and unlocking resources to improve clinical research.
- WCG Insights to access some of the latest thought leadership relevant to the clinical research industry.
- Clinical Trial Listing Service to find details about current clinical trial opportunities.

About the Avoca Quality Consortium
The Avoca Quality Consortium is a pre-competitive collaborative of sponsors, providers, CROs, and sites who share the objective of elevating clinical trial quality and building efficiencies into clinical trial execution, while bringing key stakeholders into greater alignment. Gain access to a comprehensive library of leading practices and resources across the entire clinical trial ecosystem, including medical device, inspection readiness, provider qualification/oversight, QbD/RBQM, and more.
MAGI@home 2024
MAGI@home brings you and your team best-in-class, accredited training and education from the comfort of your home or office. Event Details
American Speech-Language-Hearing Association
- Certification
- Publications
- Continuing Education
- Practice Management
- Audiologists
- Speech-Language Pathologists
- Academic & Faculty
- Audiology & SLP Assistants
Assessment and Evaluation of Speech-Language Disorders in Schools
This is a guide to ASHA documents and references to consider when conducting comprehensive speech-language assessments. Speech-language assessment is a complex process. Assessing, describing, and interpreting an individual's communication ability requires the integration of a variety of information gathered in the evaluation process. ASHA's Preferred Practice Patterns for the Professions of Speech-Language Pathology (2004) indicates that comprehensive speech-language pathology assessment includes these components:
- Case history, including medical status, education, socioeconomic, cultural, and linguistic backgrounds and information from teachers and other related service providers
- Patient/client/student and family interview
- Review of auditory, visual, motor, and cognitive status
- Standardized and/or non-standardized measures of specific aspects of speech, spoken and non-spoken language, cognitive-communication, and swallowing function, including observations and analysis of work samples
- Identification of potential for effective intervention strategies and compensations
- Selection of standardized measures for speech, language, cognitive-communication, and/or swallowing assessment with consideration for documented ecological validity and cultural sensitivity
- Follow-up services to monitor communication and swallowing status and ensure appropriate intervention and support for individuals with identified speech, language, cognitive-communication, and/or swallowing disorders
The Individuals with Disabilities Education Act (IDEA, 2004) has specific provisions concerning the assessment of students (Sections 300.301-300.305) in schools. In addition, SLPs need to follow state and local requirements for the assessments of students.
It is important to note the distinctions between the terms evaluation and assessment according to IDEA Part C Guidelines. Evaluation means the "procedures used by qualified personnel to determine a child's initial and continuing eligibility..." IDEA (2004), Part B requires that an evaluation be comprehensive and assess all areas of suspected disability. It is important for the clinician to involve other assessment staff as part of the multidisciplinary evaluation team to address educational and/or behavioral concerns for students who are not meeting the grade-level expectations (IDEA, 2004, Section 34 CFR 300.304).
Assessment means "the ongoing procedures used by qualified personnel to identify the child's unique strengths and needs and the early intervention services appropriate to meet those needs throughout the period of the child's eligibility...and includes the assessment of the child...and the assessment of the child's family..." (IDEA, Part C, Section 303.321)
ASHA Practice Policy Documents
- Scope of Practice
- Rights and Responsibilities of Test Takers
- Issues in Learning Disabilities: Assessment and Diagnosis
Research and Evidence Based Practice
- ASHA's Evidence Maps
- ASHA's Practice Portal
Language/Speech Sampling
- Comprehensive Assessment for Disorders of Reading and Writing: Typical Components
- Myths and Realities of Language Sample Analysis (2010)
- Pediatric Stuttering Assessment Template
- Synthesizing Information from Language Samples and Standardized Tests in School-Age Bilingual Assessment 2017
- The Clinical Utility of Language Samples (2010)
- What? You Want Me to Do A Language Sample? (2008)
- Using Language Sample Databases (2010)
- Using Language Sample Analysis to Assess Spoken Language Production in Adolescents (2016)
Pediatric Swallowing and Feeding Templates
- Pediatric Clinical Swallowing Evaluation Template [PDF]
- Pediatric Videofluoroscopic Swallow Study (VFSS) Template [PDF]
Multicultural/Multilingual Assessment
- Alternative Assessment of Language and Literacy in Culturally and Linguistically Diverse Populations (2003)
- Dynamic Assessment Multimedia Guide
- Phonemic Inventories Across Languages
- Working with Internationally Adopted Children
- Assessment, Evaluation, Test FAQs for Parents
- Best Assessment Ever? (2016)
- Evaluating Children in U.S. Public Schools With Speech Sound Disorders (2020)
- Glossary of Assessment Terms
- IDEA Part C: Evaluation and Assessment Definitions
- Screening Evaluation, and Assessment Procedures from Center for Parent Information and Resources
- School-Age Hearing Screening
- Understanding the Difference Between Auditory Processing, Speech and Language Disorders, and Reading Disorders [PDF]

Information For
- Academic Programs & Faculty
- Advertising Disclaimer
- Advertise with us
ASHA Corporate Partners
- Become A Corporate Partner

The American Speech-Language-Hearing Association (ASHA) is the national professional, scientific, and credentialing association for 234,000 members, certificate holders, and affiliates who are audiologists; speech-language pathologists; speech, language, and hearing scientists; audiology and speech-language pathology assistants; and students.
- All ASHA Websites
- Work at ASHA
- Marketing Solutions
Get Involved
- ASHA Community
- Become a Mentor
- Become a Volunteer
- Special Interest Groups (SIGs)
Connect With ASHA
American Speech-Language-Hearing Association 2200 Research Blvd., Rockville, MD 20850 Members: 800-498-2071 Non-Member: 800-638-8255
MORE WAYS TO CONNECT
Media Resources
- Press Queries
Site Help | A–Z Topic Index | Privacy Statement | Terms of Use © 1997- American Speech-Language-Hearing Association
The independent source for health policy research, polling, and news.
A Current Snapshot of the Medicare Part D Prescription Drug Benefit
Juliette Cubanski Published: Oct 09, 2024
Medicare Part D is a voluntary outpatient prescription drug benefit for people with Medicare provided through private plans that contract with the federal government. Beneficiaries can choose to enroll in either a stand-alone prescription drug plan (PDP) to supplement traditional Medicare or a Medicare Advantage plan, mainly HMOs and PPOs, that provides all Medicare-covered benefits, including prescription drugs (MA-PD). This brief provides an overview of the Medicare Part D program, plan availability, enrollment, and spending and financing, based on KFF analysis of data from the Centers for Medicare & Medicaid Services (CMS), the Congressional Budget Office (CBO), and other sources. It also provides an overview of changes to the Part D benefit based on provisions in the Inflation Reduction Act . (A separate KFF brief provides more detail about Part D plan availability, premiums, and cost sharing.)
Key Takeaways
- In 2025, 524 PDPs will be offered across the 34 PDP regions nationwide (excluding the territories), a 26% decrease from 2024. Despite the overall reduction, beneficiaries in each state will have a choice of at least a dozen stand-alone plans, plus many Medicare Advantage drug plans.
- Compared to 2024, fewer plans will be available for enrollment of Part D Low-Income Subsidy (LIS) beneficiaries for no premium (“benchmark” plans) in 2025 – 115 plans, a 9% reduction compared to 2024. The number of benchmark plans will vary from 2 to 6 across states.
- Changes to the Medicare Part D benefit under the Inflation Reduction Act are taking effect in 2025, including a new $2,000 out-of-pocket cap, an increase in the share of drug costs above the cap paid for by Part D plans and drug manufacturers, and a reduction in Medicare’s share of these costs.
- In 2024, 53 million of the 67 million Medicare beneficiaries are enrolled in Medicare Part D plans, including employer-only group plans; of the total, 57% are enrolled in MA-PDs and 43% are enrolled in stand-alone PDPs. As of June 2024, 3 million Part D enrollees receive premium and cost-sharing assistance through the LIS program.
- The Congressional Budget Office (CBO) estimates that spending on Part D benefits will total $137 billion in 2025 , representing 15% of net total Medicare spending. Funding for Part D comes from general revenues (75%), beneficiary premiums (15%), and state contributions (13%).
- Medicare’s aggregate reinsurance payments to Part D plans are projected to account for 17% of total Part D spending in 2025, a substantial reduction from 2024. This change reflects the reduction in Medicare’s liability for catastrophic drug costs from 80% in 2024 to 20% for brands and 40% for generics in 2025.
Medicare Prescription Drug Plan Availability in 2025
In 2025, 524 PDPs will be offered across the 34 PDP regions nationwide (excluding the territories), a 26% decrease from 2024 and the lowest number of PDPs available since the Part D program’s beginning in 2006 (Figure 1). While the availability of stand-alone PDPs has been trending downward over time, along with a decline in PDP enrollment, the availability of Medicare Advantage drug plans has expanded in recent years, and more people in Medicare are now getting Part D drug coverage through Medicare Advantage plans .
Despite the overall reduction in the number of PDPs for 2025, beneficiaries in each state will have a choice of at least a dozen stand-alone PDPs, ranging from 12 PDPs in Alaska, Hawaii, and New York to 18 PDPs in California (Figure 2). In addition, beneficiaries will be able to choose from among many MA-PDs available at the local level.
Low-Income Subsidy Plan Availability in 2025
Beneficiaries with low incomes and modest assets are eligible for assistance with Part D plan premiums and cost sharing. Through the Part D Low-Income Subsidy (LIS) program, additional premium and cost-sharing assistance is available for Part D enrollees with low incomes (less than 150% of poverty, or $22,590 for individuals/$30,660 for married couples in 2024) and modest assets (up to $17,220 for individuals/$34,360 for couples in 2024). As of 2024, anyone who qualifies for the LIS program receives full benefits, under a provision of the Inflation Reduction Act, meaning they pay only modest copayments for prescription drugs and are eligible for a full premium subsidy; in previous years, people with incomes between 135% and 150% of poverty received partial LIS benefits.
In 2025, fewer plans will be available for enrollment of LIS beneficiaries for no premium (“benchmark” plans) compared to 2024 – 115 plans, a 9% reduction, and the lowest number of benchmark plans available since Part D started (Figure 3). Just over one-fifth (22%) of PDPs in 2025 are benchmark plans.
Some enrollees have fewer benchmark plan options than others because benchmark plan availability varies at the Part D region level. The number of premium-free PDPs in 2023 ranges across states from 2 plans in 9 states (Alaska, Delaware, Florida, Illinois, Maryland, Nevada, Ohio, South Carolina, and Texas) and the District of Columbia to 6 plans in 1 state (Wisconsin) (Figure 4). LIS enrollees can select any plan offered in their area, but if they are enrolled in a non-benchmark plan, they may be required to pay some portion of their plan’s monthly premium.
Changes to Part D Under the Inflation Reduction Act
The Inflation Reduction Act contained several provisions to lower prescription drug spending by Medicare and beneficiaries, including major changes to the Medicare Part D program, which started to take effect in 2023. These changes were designed to address several concerns, including the lack of a hard cap on out-of-pocket spending for Part D enrollees; the inability of the federal government to negotiate drug prices with manufacturers; a significant increase in Medicare “reinsurance” spending for Part D enrollees with high drug costs; prices for many Part D covered drugs rising faster than the rate of inflation; and the relatively weak financial incentives faced by Part D plan sponsors to control high drug costs. Provisions in the law include:
- Limiting the price of insulin products to no more than $35 per month in all Part D plans and makes adult vaccines covered under Part D available for free, as of 2023.
- Requiring drug manufacturers to pay a rebate to the federal government if prices for drugs covered under Part D and Part B increase faster than the rate of inflation, with the initial period for measuring Part D drug price increases running from October 2022-September 2023.
- Expanding eligibility for full benefits under the Part D Low-Income Subsidy program in 2024.
- Adding a hard cap on out-of-pocket drug spending under Part D by eliminating the 5% coinsurance requirement for catastrophic coverage in 2024 and capping out-of-pocket spending at $2,000 in 2025.
- Shifting more of the responsibility for catastrophic coverage costs to Part D plans and drug manufacturers, starting in 2025.
- Authorizing the Secretary of the Department of Health and Human Services to negotiate the price of some drugs covered under Medicare , with negotiated prices first available for 10 Part D drugs in 2026.
Part D Plan Premiums and Benefits in 2025
The 2025 Part D base beneficiary premium – which is based on bids submitted by both PDPs and MA-PDs and is not weighted by enrollment – is $36.78 , a 6% increase from 2024. Annual growth in the base beneficiary premium is capped at 6% due to a provision in the Inflation Reduction Act. A new Part D premium stabilization demonstration for PDPs is also helping to moderate premium increases that Part D enrollees might otherwise have faced in 2025, as insurers adjust to higher costs associated with the new $2,000 out-of-pocket spending cap and increased liability for drug costs above the cap. The demonstration limits monthly PDP premium increases to $35 between 2024 and 2025.
The monthly amount that Part D enrollees pay for individual Part D plans is different from the base beneficiary premium, and enrollees may see their premium increase by more than 6% (or less, or even decrease) if they stay in the same plan for 2025. Actual monthly premiums paid by Part D enrollees in 2025 will vary considerably, ranging from $0 to $100 or more in most regions. In addition to the monthly premium, Part D enrollees with higher incomes ($103,000/individual; $206,000/couple) pay an income-related premium surcharge , ranging from $12.90 to $81.00 per month in 2024 (depending on income).
Most MA-PD enrollees pay no premium beyond the monthly Part B premium (although high-income MA enrollees are required to pay a premium surcharge). MA-PD sponsors can use rebate dollars from Medicare payments to lower or eliminate their Part D premiums , so the average premium for drug coverage in MA-PDs is heavily weighted by zero-premium plans. In 2024, the enrollment-weighted average monthly portion of the premium for drug coverage in MA-PDs is substantially lower than the average monthly PDP premium ($9 versus $43) .
The Part D defined standard benefit is changing for 2025 and will include a new $2,000 cap on out-of-pocket drug spending. The benefit will have three phases, including a deductible, an initial coverage phase, and catastrophic coverage. For 2025, under the standard benefit, Part D enrollees will pay a deductible of $590 (up from $545 in 2024), and will then pay 25% of their drug costs in the initial coverage phase until their out-of-pocket spending totals $2,000. At that point, they will qualify for catastrophic coverage and will pay no additional out-of-pocket costs.
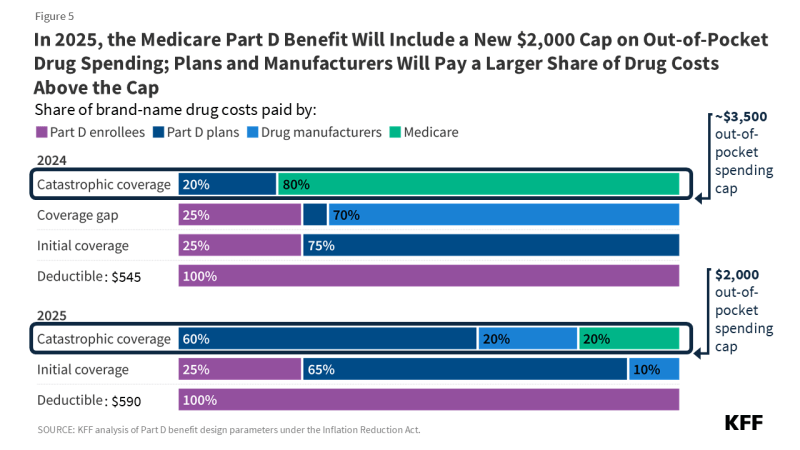
Part D plans must offer either the defined standard benefit or an alternative equal in value (“actuarially equivalent”) and can also provide enhanced benefits. Both basic and enhanced benefit plans vary in terms of their specific benefit design, coverage, and costs, including deductibles, cost-sharing amounts, utilization management tools (i.e., prior authorization, quantity limits, and step therapy), and which drugs are covered on their formularies. Plan formularies must include drug classes covering all disease states, and a minimum of two chemically distinct drugs in each class. Part D plans are required to cover all drugs in six “protected” classes: immunosuppressants, antidepressants, antipsychotics, anticonvulsants, antiretrovirals, and antineoplastics.
Part D and Low-Income Subsidy Enrollment in PDPs and MA-PDs
Enrollment in Medicare Part D plans is voluntary, except for beneficiaries who are eligible for both Medicare and Medicaid and certain other low-income beneficiaries who are automatically enrolled in a PDP if they do not choose a plan on their own. However, beneficiaries face a penalty equal to 1% of the national average premium for each month they delay enrollment unless they have drug coverage from another source that is at least as good as standard Part D coverage (“creditable coverage”).
In 2024, 53 million Medicare beneficiaries are enrolled in Medicare Part D plans, including employer-only group plans; of the total, 57% are enrolled in MA-PDs and 43% are enrolled in stand-alone PDPs (Figure 6). Another 0.8 million beneficiaries are estimated to have drug coverage through employer-sponsored retiree plans where the employer receives a subsidy from the federal government equal to 28% of drug expenses between $590 and $12,150 per retiree in 2025. Several million beneficiaries are estimated to have other sources of drug coverage, including employer plans for active workers, FEHBP, TRICARE, and Veterans Affairs (VA). Around 11% of people with Medicare are estimated to lack creditable drug coverage.
Recent years have seen a growing divide in the Part D plan market between stand-alone PDPs, where the number of plans has generally been trending downward over time in conjunction with a reduction in PDP enrollment, and MA-PDs, where plan availability and enrollment have grown steadily in recent years. The widespread availability of low or zero-premium MA-PDs, while PDPs charge substantially higher premiums, could tilt enrollment even more towards Medicare Advantage plans in the future.
As of June 2024, 14.3 million Part D enrollees receive premium and cost-sharing assistance through the LIS program. As with overall Part D enrollment, more people receiving LIS are enrolled in MA-PDs than PDPs . Beneficiaries who are dual-eligible individuals, those enrolled in Medicare Savings Programs (QMBs, SLMBs, Qis), and those who receive Supplemental Security Income payments from Social Security automatically qualify for the additional assistance, and Medicare automatically enrolls them into PDPs with premiums at or below the regional average (the Low-Income Subsidy benchmark) if they do not choose a plan on their own. Other beneficiaries are subject to both an income and asset test and need to apply for the Low-Income Subsidy through either the Social Security Administration or Medicaid.
Part D Spending and Financing
Part d spending.
In its June 2024 Medicare baseline projections, the Congressional Budget Office (CBO) estimated that spending on Part D benefits would total $137 billion in 2025 , representing 15% of total Medicare outlays (net of offsetting receipts from premiums and state transfers). However, based on actual bid data submitted by Part D plans for coverage in 2025, CBO estimates higher federal spending on Part D of between $10 billion and $20 billion relative to its initial projections for 2025. CBO also estimates that Medicare will spend an additional $5 billion in 2025 on subsidies to plans that are participating in the Part D premium stabilization demonstration.
In general, Part D spending depends on several factors, including the total number of people enrolled in Part D, their health status and the quantity and type of drugs used, the number of people with high drug costs (above the catastrophic threshold), the number of people receiving the Low-Income Subsidy, the price of drugs covered by Part D and the ability of plan sponsors to negotiate discounts (rebates) with drug companies and preferred pricing arrangements with pharmacies, and to manage use (e.g., promoting use of generic drugs, prior authorization, step therapy, quantity limits, and mail order).
Part D Financing
Financing for Part D comes from general revenues (75%), beneficiary premiums (15%), and state contributions (13%). The monthly premium paid by Part D enrollees was initially set to cover 25.5% of the cost of standard drug coverage, but with the Inflation Reduction Act’s 6% premium stabilization provision and the new Part D premium stabilization program in effect, enrollees are paying a lower share of costs overall. Medicare subsidizes the remainder, based on bids submitted by plans for their expected benefit payments, and taking into account the additional payments that insurers participating in the Part D premium stabilization demonstration are receiving. Higher-income Part D enrollees pay a larger share of standard Part D costs, ranging from 35% to 85%, depending on income.
Payments to Plans
For 2025, Medicare’s actuaries estimate that Part D plans will receive direct subsidy payments averaging $1,417 per enrollee overall, $1,504 for enrollees receiving the LIS, and $445 in reinsurance payments for high-cost enrollees; employers are expected to receive, on average, $640 for retirees in employer-subsidy plans. Part D plans also receive additional risk-adjusted payments based on the health status of their enrollees, and plans’ potential total losses or gains are limited by risk-sharing arrangements with the federal government (“risk corridors”).
As of 2025, Medicare’s reinsurance payments to plans for total spending incurred by Part D enrollees above the catastrophic coverage threshold will subsidize 20% of brand-name drug spending and 40% of generic drug spending, down from 80% in previous years, due to a provision in the Inflation Reduction Act. With this change in effect, Medicare’s aggregate reinsurance payments to Part D plans are projected to account for 17% of total Part D spending in 2025, based on KFF analysis of data from the 2024 Medicare Trustees report . This is a substantial reduction from 2024, when reinsurance spending had grown to account for close to half of total Part D spending (46%) (Figure 7). Moving forward, the largest portion of total Part D spending will be accounted for by direct subsidy payments to plans (54% of total spending in 2025).
- Medicare Part D
- Prescription Drugs
- Medicare Advantage
Also of Interest
- Medicare Part D Premiums Are Increasing for Many But Not All Stand-Alone Plans in 2025, Reflecting Effects of New Premium Stabilization Demonstration
- What to Know About Medicare Part D Premiums
- Key Facts About Medicare Part D Enrollment, Premiums, and Cost Sharing in 2024


COMMENTS
Select a topic. Choosing an interesting research topic is your first challenge. Here are some tips: Choose a topic that you are interested in! The research process is more relevant if you care about your topic. Narrow your topic to something manageable. If your topic is too broad, you will find too much information and not be able to focus.
Choosing a research topic; Finding inspiration; Preliminary research helps you learn more about your possible topic; Finding out more about your topic can help inspire your idea; Topic scope is crucial to a successful research paper; Techniques for narrowing a topic; 5W Example;
Study with Quizlet and memorize flashcards containing terms like All of the following are criteria for choosing good speech topics except, What is the problem with trying to give a five minute presentation on the ecology?, What does the speaker do in the the discovery phase of selecting a topic? and more.
Sample flowchart representing a decision process when confronted with a lamp that fails to light. In psychology, decision-making (also spelled decision making and decisionmaking) is regarded as the cognitive process resulting in the selection of a belief or a course of action among several possible alternative options. It could be either rational or irrational.
The scientific method is an empirical method for acquiring knowledge that has characterized the development of science since at least the 17th century. The scientific method involves careful observation coupled with rigorous scepticism, because cognitive assumptions can distort the interpretation of the observation.Scientific inquiry includes creating a hypothesis through inductive reasoning ...
Psychology is the scientific study of mind and behavior. [1] [2] Its subject matter includes the behavior of humans and nonhumans, both conscious and unconscious phenomena, and mental processes such as thoughts, feelings, and motives.Psychology is an academic discipline of immense scope, crossing the boundaries between the natural and social sciences. ...
If you are looking for clinical research information and insights, or information about clinical trials, we invite you to visit: Avoca Quality Consortium (AQC) a pre-competitive collaborative consortium of 200+ companies dedicated to elevating clinical trial quality and unlocking resources to improve clinical research.
Keep in mind that if you're alluding to a source's ideas or words to frame your own point, you'll still need to apply the guidelines above to avoid plagiarizing. If you're writing on the same topic for multiple assignments, it can be tempting to recycle some of your previous words—this is called "self-plagiarism".
The American Speech-Language-Hearing Association (ASHA) is the national professional, scientific, and credentialing association for 234,000 members, certificate holders, and affiliates who are audiologists; speech-language pathologists; speech, language, and hearing scientists; audiology and speech-language pathology assistants; and students.
Beneficiaries can choose to enroll in either a stand-alone prescription drug plan (PDP) to supplement traditional Medicare or a Medicare Advantage plan, mainly HMOs and PPOs, that provides all ...
Wikipedia [c] is a free content online encyclopedia written and maintained by a community of volunteers, known as Wikipedians, through open collaboration and the wiki software MediaWiki.Wikipedia is the largest and most-read reference work in history, [3] [4] and is consistently ranked among the ten most visited websites; as of August 2024, it was ranked fourth by Semrush, [5] and seventh by ...
Research shows that healthy caregivers lead to better outcomes for the person being cared for. Adult day centers benefit by reducing time consuming paperwork, minimizing lengthy phone calls, and having more engaged caregivers.
Environmental, social, and governance (ESG) is shorthand for an investing principle that prioritizes environmental issues, social issues, and corporate governance. [1] Investing with ESG considerations is sometimes referred to as responsible investing or, in more proactive cases, impact investing. [1]The term ESG first came to prominence in a 2004 report titled "Who Cares Wins", which was a ...
octagon.lhohq.info
The abortion debate most commonly relates to the induced abortion of a pregnancy, which is also how the term "abortion" is used in a legal sense. [nb 1] The terms "elective abortion" and "voluntary abortion" refer to the interruption of pregnancy, before viability, at the request of the woman but not for medical reasons. [34]In medical parlance, "abortion" can refer to a spontaneous ...