An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List


Treatments for Chronic Kidney Disease: A Systematic Literature Review of Randomized Controlled Trials
Juan jose garcia sanchez.
1 BioPharmaceuticals Medical, AstraZeneca, Academy House, 136 Hills Road, Cambridge, CB2 8PA UK
Juliette Thompson
2 Visible Analytics, Oxford, UK
David A. Scott
Rachel evans, elisabeth sörstadius.
3 BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden
Stephen Nolan
Eric t. wittbrodt.
4 BioPharmaceuticals Medical, AstraZeneca, Gaithersburg, MD USA
Alyshah Abdul Sultan
Bergur v. stefansson, dan jackson, keith r. abrams, associated data.
Delaying disease progression and reducing the risk of mortality are key goals in the treatment of chronic kidney disease (CKD). New drug classes to augment renin–angiotensin–aldosterone system (RAAS) inhibitors as the standard of care have scarcely met their primary endpoints until recently. This systematic literature review explored treatments evaluated in patients with CKD since 1990 to understand what contemporary data add to the treatment landscape. Eighty-nine clinical trials were identified that had enrolled patients with estimated glomerular filtration rate 13.9–102.8 mL/min/1.73 m 2 and urinary albumin-to-creatinine ratio (UACR) 29.9–2911.0 mg/g, with (75.5%) and without (20.6%) type 2 diabetes (T2D). Clinically objective outcomes of kidney failure and all-cause mortality (ACM) were reported in 32 and 64 trials, respectively. Significant reductions ( P < 0.05) in the risk of kidney failure were observed in seven trials: five small trials published before 2008 had evaluated the RAAS inhibitors losartan, benazepril, or ramipril in patients with ( n = 751) or without ( n = 84–436) T2D; two larger trials ( n = 2152–2202) published onwards of 2019 had evaluated the sodium-glucose co-transporter 2 (SGLT2) inhibitors canagliflozin (in patients with T2D and UACR > 300–5000 mg/g) and dapagliflozin (in patients with or without T2D and UACR 200–5000 mg/g) added to a background of RAAS inhibition. Significant reductions in ACM were observed with dapagliflozin in the DAPA-CKD trial. Contemporary data therefore suggest that augmenting RAAS inhibitors with new drug classes has the potential to improve clinical outcomes in a broad range of patients with CKD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-02006-z.
Key Summary Points
Introduction.
An estimated 840 million people worldwide have chronic kidney disease (CKD) [ 1 ], which was responsible for 1.2 million deaths and 35.8 million disability-adjusted life years in 2017 [ 2 ]. However, only 12% of sufferers are aware of their condition [ 3 ]. CKD is diagnosed when the estimated glomerular filtration rate (eGFR) declines below 60 mL/min/1.73 m 2 or the urinary albumin-to-creatinine ratio (UACR) equals or exceeds 30 mg/g for 3 months or longer [ 4 ]. As CKD progresses, healthcare costs increase and health-related quality of life (HRQoL) diminishes, with the greatest costs and HRQoL burden associated with kidney failure (eGFR < 15 mL/min/1.73 m 2 ) [ 5 , 6 ]. Adverse clinical outcomes, healthcare utilization and costs, and disease burden also increase as albuminuria worsens [ 7 – 9 ], and UACR 30–300 mg/g (moderately increased) and even > 300 mg/g (severely increased) are now considered important predictors of risk for CKD progression, cardiovascular events, and mortality [ 4 ]. Early identification and pharmacologic intervention could therefore delay or prevent CKD progression.
Current guidelines recommend using renin–angiotensin–aldosterone system (RAAS) inhibitors (either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker) to delay or prevent CKD progression [ 4 ]. Clinical trials of other drug classes to augment RAAS inhibitors, delay progression, and improve outcomes have scarcely met their primary endpoints [ 10 ], except for sodium-glucose co-transporter 2 (SGLT2) inhibitors. Initially developed as blood glucose-lowering agents, reports of renal and cardiovascular benefits in patients with type 2 diabetes (T2D) [ 11 – 14 ] as well as cardiovascular benefits in patients with heart failure (HF) [ 15 – 17 ] have prompted the evaluation of SGLT2 inhibitors in patients with CKD who are already receiving standard of care treatment with RAAS inhibitors.
This systematic literature review explored the treatments evaluated in patients with CKD since 1990 to allow an assessment of contemporary data relative to the overall treatment landscape.
This systematic literature review was conducted according to the recommendations of Cochrane [ 18 ], the Centre for Reviews and Dissemination [ 19 ], and the National Institute for Health and Care Excellence [ 20 ]. The protocol has been registered on PROSPERO (CRD42020190152).
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Sources and Searches
Using the terms listed in the Supplementary Material, we searched MEDLINE, Embase, and the Cochrane Library for peer-reviewed articles published between 1990 and November 2, 2020, that reported results from prospective, parallel-design randomized controlled trials that evaluated pharmacologic treatments for patients aged 18 years or more with CKD and albuminuria. Search filters for MEDLINE and Embase were obtained from the Scottish Intercollegiate Guideline Network [ 21 ], and adapted for Embase by Cochrane [ 22 ]. In line with guidelines for the inclusion of gray literature [ 18 – 20 , 23 ], the proceedings of key international conferences and trial registries were also searched (Supplementary Material).
Non-English-language publications, reviews, case studies, case reports, conference proceedings (other than those identified in the search described above), and animal studies were excluded.
Trial Selection
After removing duplicates from the combined search results, two independent reviewers screened the identified abstracts against predefined eligibility criteria (Table 1 ). Abstracts deemed eligible for inclusion were then compared and any discrepancies resolved mutually or by a third reviewer. This independent double-review process was repeated on the full-text articles to identify a final list of trials eligible for inclusion in this review.
Table 1
Eligibility criteria
CKD chronic kidney disease, HIV human immunodeficiency virus, RCT randomized controlled trial, T2D type 2 diabetes
a Including proxies: albumin-to-creatinine ratio, urinary protein-to-creatinine ratio, or reagent strip qualitative recording
b This was required to be reported in the trial eligibility criteria or as a baseline characteristic; trials were excluded if no information on albuminuria was reported or if patients with severely increased albuminuria were explicitly excluded from the trial
c Albuminuria could be reported using multiple methods
Data Extraction and Quality Assessment
Data were extracted by one reviewer and validated by a second, with disagreements resolved by a third (Supplementary Material). Binary variables included trial population, number or proportion of patients experiencing an event, and incidence rates per population or person-time. Continuous and time-to-event variables included hazard ratio (HR), odds ratio, relative risk, mean, median, standard deviation, standard error, range, 95% confidence interval (CI), interquartile range, and P value. Outcomes reported without P values or 95% CIs were assumed not to be statistically significant. Outcomes reported with P < 0.05 or with 95% CIs not crossing 1.0 for a HR or relative risk were assumed to be statistically significant.
Risk of bias and quality of reporting were assessed using eight questions from the PMG24 Company Evidence Submission Template (NICE single technology appraisal process) [ 24 ], developed based on previous recommendations [ 19 ]. Answers of “yes,” “no,” or “unclear due to inadequate reporting” were required. Depending on the question, answers of “yes” or “no” could indicate a higher or lower risk of bias (Supplementary Material).
Compliance with Ethics Guidelines
Search results.
Overall, 40,550 records were identified (Fig. 1 ). After removal of 20,773 duplicates, 19,777 abstracts were reviewed against eligibility criteria, and 19,557 were excluded. The full texts of 220 articles were reviewed, and 121 were excluded (Table S1). The addition of one more article, identified during a search of conference proceedings, resulted in 100 eligible articles providing data for 89 randomized controlled trials (Table 2 ).

Study selection PRISMA diagram
Table 2
Relevant characteristics of included trials
ACTRN Australian Clinical Trials Registration Number, ANZCTR Australian New Zealand Clinical Trials Registry, CKD chronic kidney disease, CRG Cochrane Renal Group, EAS erythropoiesis-stimulating agent, EudraCT European Union Drug Regulating Authorities Clinical Trials, ISRCTN International Standard Randomised Controlled Trials Number, JapicCTI Japan Pharmaceutical Information Center, NA not available, NCT national clinical trial, NR not reported, NSAID non-steroidal anti-inflammatory drug, PER protein excretion rate, SGLT2 sodium-glucose co-transporter 2, SOC standard of care , T2D type 2 diabetes, UMIN University Hospital Medical Information Network
a Primary/previous treatment class: Initially developed as blood glucose-lowering agents, observations of renal and cardiovascular benefits in patients with T2D [ 11 – 14 ] as well as cardiovascular benefits in patients with heart failure [ 15 – 17 ] has prompted the evaluation of SGLT2 inhibitors in patients with CKD
Trial Characteristics
Thirty-seven trials were multinational, 18 were conducted in Japan, and seven each were conducted in China and Italy, with the remaining trials conducted in a range of countries worldwide.
Sixty-six trials (74.2%) were published onwards of 2010, and 23 (25.8%) were published before 2010. Forty-three trials (48.3%) were phase 3 ( n = 29), phase 4 ( n = 10), phase 2/3 ( n = 3), or phase 3/4 ( n = 1), and most were double blind (61.8%) or open label (32.6%) (Fig. S1a, b). Forty-six trials (51.7%) did not report their trial phase.
Most trials enrolled 50–100 patients per arm, although 10 conducted onwards of 2004 enrolled more than 1000 patients per arm [ 25 – 27 , 34 , 47 , 60 , 73 – 75 , 93 ]. Forty-three trials (48.3%) enrolled patients with T2D, 29 enrolled patients with or without T2D (32.6%), and 17 enrolled patients without T2D (19.1%). Across all included trials, 75.5% of patients had T2D (Fig. S2a, b). All patients were followed for at least 12 weeks, although mean or median follow-up extended to at least 12 months in 60 trials (67.4%) and at least 24 months in 38 trials (42.7%).
Antihypertensive agents were the most common intervention assessed overall, but were approximately twice as common in trials of patients without T2D (88%) than trials of patients with (42%), or with or without (45%) T2D. Blood glucose-lowering agents were also common in trials of patients with T2D (37%). The most common comparators were placebo in trials of patients with T2D (53%) and active comparators in trials of patients without (53%), or with or without (38%) T2D. Placebo was also common in trials of patients without (35%), or with or without (34%) T2D (Fig. S3a, b).
Baseline Patient Characteristics
In more than 80% of trials, 50–100% of patients were male (Fig. S4). Mean age ranges were 51.0–72.1 years in trials of patients with or without T2D (except one trial with a mean age range of 34–35 years [ 82 ]), 53.8–70.2 years in trials of patients with T2D (except one trial with a mean age range of 34.0–35.0 years [ 66 ], and one trial with a median age of 33 years [ 65 ]), and 44.4–71.0 years in trials of patients without T2D.
While CKD etiologies other than diabetic nephropathy were infrequently reported in trials of patients with T2D, 13 trials (14.6%) of patients without T2D and 16 (18.0%) of patients with or without T2D reported glomerulonephritis as a key CKD etiology (Table S2a, b).
Mean eGFR ranged between 13.9 and 102.8 mL/min/1.73 m 2 , including two trials that enrolled patients with mean eGFR > 90 mL/min/1.73 m 2 (Table S3) [ 38 , 59 ]. Trials most commonly reported albuminuria as UACR (50.6%), with mean UACR ranging between 29.9 and 2911.0 mg/g. Other trials reported UACR via categorization into normo-, micro-, or macroalbuminuria (16.9%), albumin excretion rate (12.4%), protein excretion rate (20.2%), protein-to-creatinine ratio (18.0%), or urinary albumin value (13.5%) (Table S4a–f).
Thirty-one trials (34.8%) included patients with prior histories of cardiovascular disease, with the proportion of patients ranging from 1.7% to 92.0%, although cardiovascular disease history was either inconsistently defined or not defined at all (Table S5). Fourteen trials (15.7%) included patients with HF, with the proportion of patients ranging from 0.6% to 43.1% (Table S6). Eighty-two trials (92.1%) reported systolic and diastolic blood pressure (Table S7).
Composite Outcomes
Fifty-seven composite endpoints were identified, only 13 of which were used in more than one trial (Fig. S5a, b). Composite outcomes are summarized in Table S8.
Twelve trials (13.5%) reported significant reductions in the risks of composites comprising kidney failure plus one or more of doubling of serum creatinine, eGFR reduction (≥ 40% or ≥ 50%), mortality (all-cause, renal, or cardiovascular), myocardial infarction (MI), stroke, albuminuria progression, or other (Table 3 ). These included trials published before 2013 evaluating RAAS inhibitors losartan (RENAAL, ROAD) [ 61 , 112 ], ramipril (REIN-1, AASK) [ 115 , 123 ], irbesartan (IDNT) [ 62 ], valsartan (KVT) [ 83 ], and benazepril (ROAD, and an unnamed trial) [ 112 , 113 ] in patients with, without, or with or without T2D. Also included were trials published onwards of 2019 evaluating dipeptidyl peptidase 4 inhibitor linagliptin (CARMELINA) [ 34 ], endothelin A receptor antagonist atrasentan (SONAR) [ 47 ], and the non-steroidal mineralocorticoid receptor antagonist finerenone (FIDELIO-DKD) [ 26 ] in patients with T2D, as well as the SGLT2 inhibitor canagliflozin (CREDENCE) [ 27 ] in patients with T2D and UACR > 300–5000 mg/g. Another SGLT2 inhibitor, dapagliflozin, significantly reduced the risk of composite endpoints comprising kidney failure and at least 50% eGFR reduction plus cardiovascular and/or renal mortality in patients with or without T2D and UACR 200–5000 mg/g (DAPA-CKD) [ 25 ]. Kidney failure as an independent outcome is reported below.
Table 3
Composite endpoints with significant outcomes
CI confidence interval, eGFR estimated glomerular filtration rate, HF heart failure, HR hazard ratio, MI myocardial infarction, SOC standard of care
a Retinal photocoagulation, anti-vascular endothelial growth factor injection therapy for diabetic retinopathy, vitreous hemorrhage, and diabetes-related blindness
b Kidney failure not included as an endpoint
c Risk reduction
d Relative risk
f Unadjusted
g Uptitrated (optimal antiproteinuric) dose
h Conventional dose
i P value for noninferiority
Four trials (4.5%) reported significant reductions in the risks of composites comprising cardiovascular mortality without kidney failure, plus at least one of doubling serum creatinine, renal mortality, MI, stroke, hospitalization for HF, or hospitalization for HF or unstable angina (Table 3 ). These included the CARMELINA [ 34 ], FIDELIO-DKD [ 26 ], and CREDENCE [ 27 ] trials, as well as the DAPA-CKD trial of dapagliflozin, which significantly reduced the risk of a composite endpoint comprising cardiovascular mortality and hospitalization for HF [ 25 ]. Conversely, the risk of a composite endpoint comprising cardiovascular mortality and hospitalization for HF or unstable angina increased in the BEACON trial of bardoxolone methyl, a nuclear 1 factor (erythroid-derived 2)-related factor 2 activator, although patients in this trial had CKD stage 4, T2D, and median UACR 320 mg/g [ 73 ].
Renal Outcomes
Kidney failure.
Kidney failure (previously end-stage kidney disease or end-stage renal disease [ 124 ]) ensues when eGFR declines below 15 mL/min/1.73 m 2 (CKD stage 5) and the patient requires kidney replacement therapy (previously renal replacement therapy [ 124 ]) in the form of a transplant or dialysis [ 4 ].
Thirty-two trials (36.0%) reported numbers of patients progressing to kidney failure (Table S9). Significant risk reductions were observed in seven trials (7.9%): the RENAAL trial of losartan in patients with T2D and UACR ≥ 300 mg/g ( P = 0.002) [ 61 ], the ROAD trial of optimal antiproteinuric doses of losartan ( P = 0.046) and benazepril ( P = 0.042) in patients without T2D [ 112 ], an unnamed trial of conventionally dosed benazepril in patients without T2D ( P = 0.02) [ 113 ], the REIN-1 and AASK trials of ramipril in patients without T2D (both P = 0.01) [ 115 , 118 ], the CREDENCE trial of canagliflozin ( P = 0.002) [ 27 ], and the DAPA-CKD trial of dapagliflozin (HR 0.64; 95% CI 0.50–0.82) [ 25 ].
Dialysis and Transplantation
Dialysis, kidney transplantation, or both were reported in 17 (19.1%), seven (7.9%), and two trials (2.2%), respectively (Table S10). Significant outcomes were limited to three trials (3.4%). The lipid-lowering agent probucol lengthened mean time to starting dialysis in a trial of patients with T2D and UACR > 300 mg/g ( P = 0.009) [ 71 ], and the number of patients starting dialysis was significantly reduced in a trial of patients without T2D receiving the RAAS inhibitor captopril ( P < 0.005) [ 121 ], as well as patients receiving dapagliflozin in the DAPA-CKD trial (HR 0.66; 95% CI 0.48–0.90) [ 25 ].
Kidney Function Decline
Percentage eGFR declines, mean eGFR declines, and final eGFR measurements at end of follow-up were reported in 11 (12.4%), 30 (33.7%), and 25 (28.1%) trials, respectively (Table S11a–c).
The number of patients reaching an eGFR decline of 50% was significantly reduced in four trials (4.5%): the SONAR trial of atrasentan in patients with T2D and UACR 300–5000 mg/g ( P = 0.038) [ 47 ], the LORD trial of lipid-lowering agent atorvastatin in patients with or without T2D ( P = 0.023) [ 95 ], and the DAPA-CKD trial of dapagliflozin (HR 0.53; 95% CI 0.42–0.67) [ 25 ]. In the PREDICT trial of erythropoiesis-stimulating agent darbepoetin alfa, the number of patients without T2D reaching an eGFR decline of 50% was also significantly reduced among those targeting a higher (11–13 g/dL) versus lower (9–11 g/dL) hemoglobin level ( P = 0.008); however, targeting a higher hemoglobin level did not improve kidney outcomes overall [ 106 ]. The number of patients reaching an eGFR decline of at least 40% was significantly reduced in the FIDELIO-DKD trial of finerenone (HR 0.81; 95% CI 0.72–0.92) [ 26 ].
Twenty trials (22.5%) reported numbers of patients doubling their serum creatinine (Table S12). Significant risk reductions were observed in seven trials (7.9%): the SONAR trial of atrasentan ( P = 0.0055) [ 47 ], the FIDELIO-DKD trial of finerenone (HR 0.68; 95% CI 0.55–0.82) [ 26 ], the RENAAL trial of losartan ( P = 0.006) [ 61 ], the ROAD trial of optimal antiproteinuric doses of losartan ( P = 0.040) and benazepril ( P = 0.041) [ 112 ], an unnamed trial of conventional doses of benazepril ( P = 0.02) [ 113 ], the IDNT trial of irbesartan ( P < 0.001 vs amlodipine, P = 0.003 vs placebo) [ 62 ], and the CREDENCE trial of canagliflozin ( P < 0.001) [ 27 ].
Cardiovascular Outcomes
Heart failure.
Fourteen trials (15.7%) reported incidences of HF (Table S13), with significant reductions observed in two trials (2.2%): the ASCEND trial of endothelin type A receptor antagonist avosentan in patients with T2D ( P = 0.008 with a 25-mg dose, P = 0.05 with a 50-mg dose) [ 57 ] and the IDNT trial of irbesantan ( P = 0.004 vs amlodipine, P = 0.048 vs placebo) [ 64 ].
Hospitalization for HF or Unstable Angina
Hospitalization for HF and hospitalization for unstable angina were reported in 10 (11.2%) and two trials (2.2%), respectively (Table S14). Significant reductions in hospitalization for HF were observed in two trials (2.2%): the RENAAL trial of losartan ( P = 0.005) [ 61 ] and the CREDENCE trial of canagliflozin ( P < 0.001) [ 27 ]. Conversely, bardoxolone methyl significantly increased hospitalization for HF in the BEACON trial ( P < 0.001) [ 73 ].
MI and Stroke
Twenty-four trials (27.0%) reported acute, non-fatal, or fatal MI, and 25 trials (28.1%) reported non-fatal or fatal stroke (Tables S15 and S16). A significant reduction in MI was observed in patients receiving the calcium channel blocker amlodipine in the IDNT trial ( P = 0.021 vs placebo) [ 64 ]. A significant reduction in non-fatal stroke was observed in the SONAR trial of atrasentan ( P = 0.0021) [ 47 ], and significant reductions in ischemic ( P = 0.0073) or any stroke ( P = 0.01) were observed in the SHARP trial of a combination of lipid-lowering agents simvastatin and ezetimibe in patients with or without T2D [ 93 ]. Conversely, a significant increase in fatal or non-fatal stroke was observed in the TREAT trial of patients with CKD stages 3–4 and T2D receiving darbepoetin alfa ( P < 0.001) [ 75 ].
Mortality Outcomes
All-cause mortality.
Sixty-three trials (70.8%) reported all-cause mortality (ACM) (Table S17), with a significant reduction observed in the DAPA-CKD trial of dapagliflozin ( P = 0.004) [ 25 ].
Cardiovascular and Renal Mortality
Cardiovascular and renal mortality were reported in 18 (20.2%) and nine trials (10.1%), respectively, with no significant outcomes observed (Table S18).
Other Renal Outcomes
Egfr slopes.
eGFR slopes were reported in 15 trials (16.9%), with eGFR declines significantly reduced in three trials (3.4%): the RENAAL trial of losartan ( P = 0.01) [ 61 ], an unnamed trial of benazepril ( P = 0.006) [ 113 ], and the REIN-1 trial of ramipril ( P = 0.036) [ 118 ] (Table S19).
Albuminuria
UACR changes from baseline and final UACR measurements at end of follow-up were reported in 20 (22.5%) and 17 (19.1%) trials, respectively (Table S20a, b). Significant UACR decreases from baseline were observed in eight trials (9.0%): the GUARD, ASCEND, AWARD-7 and EMPA-REG-RENAL trials of dipeptidyl peptidase 4 inhibitor gemigliptin ( P < 0.001) [ 39 ], avosentan 25 or 50 mg ( P < 0.001) [ 57 ], glucagon-like peptide-1 receptor agonist dulaglutide 1.5 mg ( P = 0.0024) [ 37 ], and the SGLT2 inhibitor empagliflozin 25 mg ( P = 0.0257–0.0031) [ 42 ], respectively, in patients with T2D; unnamed trials of calcium channel blocker benidipine ( P < 0.0001 vs amlodipine) [ 84 ] and xanthine oxidase inhibitor topiroxostat ( P = 0.0092) [ 79 ] in patients with or without T2D; the ACCOMPLISH trial of a combination of benazepril and amlodipine ( P = 0.0001 vs benazepril combined with hydrochlorothiazide) in patients with or without T2D [ 85 ]; and the EVALUATE trial of selective aldosterone antagonist eplenerone in patients without T2D ( P = 0.0222) [ 107 ].
When final UACR measurements at end of follow-up were used, significant decreases in UACR from baseline were observed in four trials (4.5%): an unnamed trial of lipid-lowering agent rosuvastatin in patients with T2D ( P < 0.01 vs standard of care) [ 70 ], the AMADEO trial of RAAS inhibitors telmisartan and losartan in patients with T2D (both P < 0.0001) [ 58 ], the RENAAL trial of losartan ( P < 0.001) [ 61 ], and an unnamed trial of benidipine ( P < 0.01 vs amlodipine) in patients with or without T2D [ 84 ].
Health-Related Quality of Life
Five trials (5.6%) [ 75 , 97 , 99 , 100 ] reported HRQoL during treatment. In one trial (1.1%), Kidney Disease and Quality of Life physical function score improved significantly from baseline ( P < 0.0001) in patients with CKD and metabolic acidosis treated with veverimer, a first-in-class hydrochloric acid binder [ 104 ].
Early Trial Discontinuation
Ten trials (11.8%) were stopped early due to low recruitment or low event rates ( n = 2) [ 47 , 100 ], safety concerns ( n = 5) [ 53 , 57 , 73 , 74 , 115 ], negative results reported in a sister trial ( n = 1) [ 72 ], other reasons ( n = 1) [ 61 ], or for reasons not provided ( n = 1) [ 113 ]. On the advice of independent data monitoring committees, the CREDENCE [ 27 ] and DAPA-CKD [ 25 ] trials were stopped early after meeting prespecified efficacy criteria for early cessation and after demonstrating overwhelming efficacy, respectively.
Risk of Bias Assessment
For seven of eight questions, 65–100% of trials had a “lower” or “unclear” risk of bias, while 35% of trials were not double blind and therefore at a “higher” risk of bias. Potential conflicts of interest were identified in 57% of trials (Fig. S6a, b).
Key safety outcomes are provided in Table S21.
The highest overall incidence of treatment-related adverse events (AEs) was reported in a trial of phosphodiesterase type 5 inhibition for patients with diabetic nephropathy (active arm, 54.7%; placebo arm, 56.3%) [ 49 ]. In this trial, the most common treatment-related AEs occurred in the placebo arm, and included headache (7.8%), diarrhea (3.6%), dyspepsia (3.6%), and peripheral edema (1.6%) [ 49 ].
The highest overall incidence of serious AEs was reported in the TREAT trial of darbepoetin alfa (active arm, 61.6%; placebo arm, 60.4%), which was stopped early due to safety concerns [ 75 ]. The most common serious AE, reported in the placebo arm, was hypertension (24.5%) [ 75 ].
The 89 clinical trials identified by this systematic literature review included a broad range of patients with any stage of CKD (eGFR 13.9–102.8 mL/min/1.73 m 2 ) and albuminuria (UACR 29.9–2911.0 mg/g), with (75.5%) or without (20.6%) T2D.
Many trials evaluated the impact of treatment on one or more composite endpoints, and 16 trials reported significant reductions in risks of composites comprising kidney failure ( n = 12) or cardiovascular mortality without kidney failure ( n = 4) while evaluating RAAS inhibitors, SGLT2 inhibitors, finerenone, or other drug classes. However, these composites were diverse and assessed in a broad range of patients, hindering comparisons.
Clinically objective independent outcomes, such as kidney failure and ACM, were more consistently defined. Of 32 trials reporting incidences of kidney failure, seven observed significant risk reductions following treatment. These included a small trial of losartan ( n = 751) in patients with T2D [ 61 ] and four smaller trials of losartan, benazepril, and ramipril ( n = 84–436) in patients without T2D [ 112 , 113 , 115 , 118 ], all published before 2008. Consequently, RAAS inhibition became the standard of care for patients with CKD [ 4 ]. However, there had been a lack of success in developing new agents to augment RAAS inhibitors, delay progression, and improve outcomes, with trials of other drug classes scarcely meeting their primary endpoints until recently. Two large trials ( n = 2152 and 2202) published onwards of 2019 demonstrated significant reductions in the risk of kidney failure among patients with UACR ≥ 200 mg/g treated with SGLT2 inhibitors [ 25 , 27 ]. While the CREDENCE trial of canagliflozin only enrolled patients with T2D, the DAPA-CKD trial of dapagliflozin showed that kidney-protective effects from SGLT2 inhibition could be extended to patients with or without T2D [ 25 ]. A significant reduction in ACM observed in the same trial of dapagliflozin is the only example of a marked prolongation of survival reported to date in patients with CKD [ 25 ], and evidence from a recent systematic review confirms that well-designed clinical trials are required to optimize existing treatments to meet this unmet need [ 125 ].
Kidney failure and other clinical outcomes develop late in CKD, requiring trials with relatively long durations to enroll large patient populations [ 10 ]. Surrogate endpoints can be used to monitor disease progression and evaluate treatments in earlier stages of CKD [ 10 , 126 – 129 ]. However, this review identified a diverse range of surrogate endpoints, including specific eGFR changes from baseline (33.7%), final eGFR values at end of follow-up (28.1%), eGFR slopes (16.9%), and percentage eGFR declines from baseline (12.4%). Future clinical trials evaluating new treatments for patients in the earlier stages of CKD may therefore benefit from the standardization of surrogate endpoints.
While it has been shown elsewhere that HRQoL diminishes with progression of CKD [ 5 , 6 ], this review highlights the paucity of data showing that improvements with treatment are accompanied by improvements in HRQoL. Only five trials (5.6%) were identified that assessed HRQoL during treatment, with significant improvements limited to a trial of a hydrochloric acid binder for patients with metabolic acidosis [ 104 ]. Difficulties capturing changes in HRQoL, including the number of instruments used and differences in their sensitivities, have been highlighted recently [ 6 ].
This review has several limitations, including the exclusion of non-English-language publications and of trials enrolling patients without albuminuria. Phase was not reported in 51.7% of trials, and it is possible that some phase 2 trials were included against eligibility criteria. A “higher” risk of bias was identified for 35% of trials that were not double blind. Finally, eligibility criteria were broad and this review included patients with any stage of CKD, with or without T2D, and treated with any drug class since 1990. CKD etiologies differed markedly between patients with T2D and without T2D, and a diverse range of comparators was also identified. Surrogate and clinically objective measurements of declining kidney function and treatment efficacy have also evolved over time, and 57 different composite outcomes were identified. Given the breadth and diversity of the data acquired, the performance of a meta-analysis was considered to be infeasible.
Until recently, only RAAS inhibitors had shown that they could delay CKD progression and reduce the risk of kidney failure; however, this evidence was generated in just one small trial of patients with T2D and four smaller trials of patients without T2D. Contemporary data from the CREDENCE, DAPA-CKD, and FIDELIO-DKD trials suggest that adding an appropriate SGLT2 inhibitor or finerenone on top of standard of care RAAS inhibition can significantly improve a range of both kidney and cardiovascular outcomes in patients with or without T2D. Moreover, data from DAPA-CKD suggest that dapagliflozin added to standard of care RAAS inhibition can significantly decrease all-cause mortality in patients with or without T2D. Given the morbidity and mortality burden of CKD, the impact of CKD progression on HRQoL and healthcare costs, and the increasing prevalence of risk factors such as hypertension and diabetes in aging populations, these new drug classes potentially have an important role in the future treatment and management of CKD.
Below is the link to the electronic supplementary material.
Development of this manuscript and all associated publication costs, including the journal’s Rapid Service and Open Access Fees, were supported by AstraZeneca.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial support was provided by Matthew Young, DPhil, and Rachael Cazaly (Core, London, UK), supported by AstraZeneca according to Good Publication Practice guidelines ( https://www.acpjournals.org/doi/10.7326/M15-0288 ).
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the data interpretation, critically reviewed the manuscript, approved the final version, and accept accountability for the overall work. Study design was performed by Juan Jose Garcia Sanchez, Juliette Thompson, Glen James, Stephen Nolan, Naveen Rao, Bergur V. Stefansson, Alyshah Abdul Sultan, and Eric T. Wittbrodt. Data analyses were performed by Juan Jose Garcia Sanchez and Juliette Thompson. Ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Prior Presentation
Data presented in this article were also presented at the American Society of Nephrology Kidney Week meeting, October 22–25, 2020 (poster: PO0570).
Disclosures
Juan Jose Garcia Sanchez, Naveen Rao, Elisabeth Sörstadius, Glen James, Stephen Nolan, Eric T. Wittbrodt, Alyshah Abdul Sultan, Bergur V. Stefansson, and Dan Jackson are employees and shareholders of AstraZeneca. Juliette Thompson, David A. Scott, Rachel Evans, and Keith R. Abrams are partners/employees of Visible Analytics Ltd, which conducted this systematic review and received consultancy fees and expenses from AstraZeneca.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Global Prevalence of Chronic Kidney Disease – A Systematic Review and Meta-Analysis
* E-mail: [email protected]
Affiliation Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, United Kingdom
Affiliation Nuffield Department of Clinical Medicine, University of Oxford, Oxford, United Kingdom
- Nathan R. Hill,
- Samuel T. Fatoba,
- Jason L. Oke,
- Jennifer A. Hirst,
- Christopher A. O’Callaghan,
- Daniel S. Lasserson,
- F. D. Richard Hobbs

- Published: July 6, 2016
- https://doi.org/10.1371/journal.pone.0158765
- Reader Comments
Chronic kidney disease (CKD) is a global health burden with a high economic cost to health systems and is an independent risk factor for cardiovascular disease (CVD). All stages of CKD are associated with increased risks of cardiovascular morbidity, premature mortality, and/or decreased quality of life. CKD is usually asymptomatic until later stages and accurate prevalence data are lacking. Thus we sought to determine the prevalence of CKD globally, by stage, geographical location, gender and age. A systematic review and meta-analysis of observational studies estimating CKD prevalence in general populations was conducted through literature searches in 8 databases. We assessed pooled data using a random effects model. Of 5,842 potential articles, 100 studies of diverse quality were included, comprising 6,908,440 patients. Global mean(95%CI) CKD prevalence of 5 stages 13·4%(11·7–15·1%), and stages 3–5 was 10·6%(9·2–12·2%). Weighting by study quality did not affect prevalence estimates. CKD prevalence by stage was Stage-1 (eGFR>90+ACR>30): 3·5% (2·8–4·2%); Stage-2 (eGFR 60–89+ACR>30): 3·9% (2·7–5·3%); Stage-3 (eGFR 30–59): 7·6% (6·4–8·9%); Stage-4 = (eGFR 29–15): 0·4% (0·3–0·5%); and Stage-5 (eGFR<15): 0·1% (0·1–0·1%). CKD has a high global prevalence with a consistent estimated global CKD prevalence of between 11 to 13% with the majority stage 3. Future research should evaluate intervention strategies deliverable at scale to delay the progression of CKD and improve CVD outcomes.
Citation: Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. (2016) Global Prevalence of Chronic Kidney Disease – A Systematic Review and Meta-Analysis. PLoS ONE 11(7): e0158765. https://doi.org/10.1371/journal.pone.0158765
Editor: Giuseppe Remuzzi, Mario Negri Institute for Pharmacological Research and Azienda Ospedaliera Ospedali Riuniti di Bergamo, ITALY
Received: November 19, 2015; Accepted: June 21, 2016; Published: July 6, 2016
Copyright: © 2016 Hill et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data are from Dryad (datadryad.org); the DOI number is doi: 10.5061/dryad.3s7rd .
Funding: NH is funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford. FDRH is part funded as Director of the National Institute for Health Research (NIHR) School for Primary Care Research (SPCR), Theme Leader in the NIHR Oxford Biomedical Research Centre (BRC), and Director of the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Oxford. DSL is part funded by the NIHR Oxford Diagnostic Evidence Co-operative and NIHR Oxford BRC. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Chronic kidney disease (CKD) is associated with age-related renal function decline accelerated in hypertension, diabetes, obesity and primary renal disorders. [ 1 ] Cardiovascular disease (CVD) is the primary cause of morbidity and mortality where CKD is regarded as an accelerator of CVD risk and an independent risk factor for CVD events. [ 2 ] There is a graded inverse relationship between CVD risk and glomerular filtration rate (GFR) that is independent of age, sex and other risk factors. [ 3 – 6 ] Decreased renal function is a predictor of hospitalisation [ 1 , 2 ], cognitive dysfunction [ 7 ] and poor quality of life. [ 8 , 9 ] The healthcare burden is highest in early stages due to increased prevalence, affecting around 35% of those over 70 years. [ 10 ]
CKD is defined by indicators of kidney damage—imaging or proteinuria (commonly using albumin to creatinine ratio, ACR)—and decreased renal function (below thresholds of GFR estimated from serum creatinine concentration). [ 11 , 12 ] Current recommendations by Kidney Outcomes Quality Initiative (KDOQI) and National Institute for Health Excellence (NICE) [ 11 , 12 ] are to use serum creatinine concentration to estimate GFR (eGFR) and transform it using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. [ 13 ] CKD-EPI replaces the Modification of Diet in Renal Disease (MDRD) equation [ 14 ] as a more accurate predictor of clinical risk [ 15 ] and both these equations correct for selected non-renal influences (age, race, gender).
CKD can be classified into five stages using KDOQI [ 11 ] guidelines using thresholds of eGFR within the CKD range and/or evidence of structural renal changes e.g. proteinuria. NICE have suggested that stage 3 be subdivided into 3a and 3b reflecting increasing CVD risk. [ 12 ] The largest stage of CKD, with over 90% of cases, has been estimated from a UK retrospective lab audit study to be CKD stage 3 with 84% stage 3a (GFR of 45 to 59 ml/min/1·73m 2 ) and 16% stage 3b GFR of 30 to 44 ml/min/1·73m 2 . [ 16 ]
Changes over time in CKD prevalence are contentious. Data from the American National Health and Nutrition Examination Survey demonstrate that in the period 1999 to 2004 the prevalence of CKD stages 1 to 4 increased significantly when compared to the survey period 1988 to 1994 (13·1 versus 10·0%). [ 4 , 17 , 18 ] While this high (and rising 4 ,) prevalence is in part due to the ageing population, it is also associated with increases in hypertension and diabetes mellitus[ 1 ]. However, conversely a UK manuscript published in 2014 examined nationally representative cross-sectional studies within the UK and found that the prevalence estimates reported declined over time. [ 19 ]
CKD is recognised as having changed from a subspecialty issue to a global health concern. [ 20 ] The authors, therefore, sought to determine the global prevalence of CKD according to KDOQI criteria in published observational studies in the adult general population, by a systematic review and meta-analysis.
Materials and Methods
Search strategy and selection criteria.
The protocol has been published (PROSPERO: CRD42014009184) and conducted in accordance with the Meta-analysis Of Observational Studies in Epidemiology guidelines [ 21 ]. Search strategy was discussed with a librarian for optimum inclusion sensitivity. An early consensus panel on the search results expanded the criteria to include additional general populations not identified originally (e.g. laboratory based large population studies). The librarian performed iterative searches using the following repositories for published observational studies: Medline/PubMed, Embase, CINAHL, the Cochrane Register for Controlled Trials (CENTRAL), LILACS, SciELO, clinicaltrials.gov, WHO ICTRP. They used the Cochrane Collaboration’s Highly Sensitive Search Strategy to optimize results. [ 22 ] The search strategy for clinicaltrials.gov was Condition = (“kidney disease” OR “kidney failure” OR “kidney insufficiency” OR “kidney function” OR “kidney dysfunction” OR “renal disease” OR “renal failure” OR “renal insufficiency” OR “renal function” OR “renal dysfunction”) AND Outcome = prevalence. The reference lists of other systematic reviews on prevalence of CKD were searched for potentially relevant articles. All databases were searched from inception to the 1st September 2014.
Study selection and data extraction
Original peer-reviewed publications were selected by two authors (NH, SF) if they included: a >500 people, conducted from year 2000+, used MDRD/CKD-EPI formula, reported CKD prevalence using KDOQI criteria and were in the general population (even if limited—e.g. aged >65). Studies were excluded if they had no criteria for diagnosis of CKD, did not include prevalence, were in a specialist restricted population (e.g. acute hospital patient cohort, nursing home), were an audit of existing results already included or if there was a more recent updated study. Translations were sought for non-English articles.
Data extraction was with standardised forms by two independent reviewers (NH, SF) disagreement was resolved by adjudicator (DL). Data included quality assessment, prevalence of CKD, method used to calculate eGFR, study setting: year, country, the population, gender split, age, and so on. Authors of relevant articles were contacted to provide additional information whenever necessary and references of selected articles were hand searched for additional articles. The KDOQI definition of CKD stages was used [ 11 ] and the method, calibration and traceability of the creatinine assessment extracted.
Statistical analysis and quality assessment
CKD prevalence was defined by the studies as being calculated for Stages 1 to 5 (eGFR & ACR) or Stages 3 to 5 (eGFR alone). 95% confidence intervals (95%CI) were calculated for each prevalence value. Meta-analyses were performed in Stata version 14. A procedure for pooling proportions in the meta-analysis of multiple studies study was used and the results displayed in a forest plot. The 95%CI’s are based on score(Wilson) procedures [ 23 ]. Heterogeneity was quantified using the I-squared measure, The I 2 heterogeneity was categorised as follows: <25% low, 25 to 50% moderate and >50% high [ 24 ]. A Freeman-Tukey Double Arcsine Transformation [ 25 ] was used to stabilise the variance prior to calculation of the pooled estimates. Random effects models were selected for the meta-analyses with the assumption that CKD prevalence by country would be variable.
Subgroup analysis was undertaken by country, geographic region, age and gender. Geographic regions were defined based on the geographic proximity of the country the studies occurred in and the possible similarity in the ethnicity of the populations. Meta-regression was weighted by number of subjects unless otherwise specified [ 24 ]. Random effects meta-regressions using aggregate level data for CKD prevalence, study year, participant characteristics and co-morbidities were performed.
Methodological quality was assessed by one reviewer (NH) defined as adherence to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology Statement) recommendations. [ 26 ] The STROBE 22-point checklist was used to score each manuscript, items that had subdivision recommendations scored a point for each. Serum creatinine reporting quality was assessed by two reviews (NH & SF)—traceability of assay, number of measurements per patient, assay method used, and calibration of assay. A combined quality score was generated from methodological quality- as measured by STROBE adherence- and serum creatinine reporting quality. The weighting was arbitrarily chosen to be two-thirds STROBE adherence and one-third creatinine reporting. To assess bias, quality was used to weight CKD prevalence values in a meta-analysis.
Sensitivity analyses were undertaken to investigate the individual study influence and of limited populations (high altitude, single site of recruitment in rural area, single site of recruitment in urban area, laboratory audit, age by decile, or age restricted), studies that used age adjusted prevalence and using only high quality studies—quality score threshold of 56% (mean quality). Further sensitivity analyses were undertaken using studies that examined IDMS traceable creatinine only, studies that used double measuring of creatinine, studies that achieved two or more of the serum creatinine reporting quality items, and studies that used different eGFR equations (CKD-EPI or MDRD).
The search yielded 5,842 articles after duplicates had been removed and 143 articles were assessed relevant for the review by title and abstract. Forty-three were excluded on full manuscript assessment. A detailed review and data extraction was conducted on 100 manuscripts (covering 112 populations), Fig 1 . No additional studies were identified by examining reference lists. All studies that were included were published after the introduction of the KDOQI 2002 CKD definition guidelines [ 11 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
*112 Populations from 100 manuscripts as some manuscripts reported on more than one population or split their populations prior to analysis.
https://doi.org/10.1371/journal.pone.0158765.g001
China had the highest number of population samples with seventeen. [ 27 – 43 ] Numbers of participants ranged from 778 in a USA cohort [ 44 ] to 1,120,295 in a USA laboratory audit [ 2 ]. The S1 Appendix Study Table details the relevant details of all studies and populations.
The mean(95%CI) global prevalence of CKD was 13·4%(11·7–15·1%), I 2 = 99.9%, for the forty-four populations that measured prevalence by all 5 stages (1 to 5) [ 4 , 28 , 29 , 32 , 33 , 35 – 38 , 40 – 43 , 45 – 73 ], and 10·6%(9·2–12·2%),I 2 = 100%, in the sixty-eight populations [ 2 , 10 , 27 , 30 , 31 , 34 , 39 , 44 , 74 – 123 ] measuring Stages 3 to 5, Fig 2 .
Studies are ordered by number of participants and split by whether the report 3 stages of CKD (“Three”) or five stages of CKD (“Five”).
https://doi.org/10.1371/journal.pone.0158765.g002
CKD prevalence breakdown was provided in seventy-four populations. [ 2 , 4 , 10 , 27 – 29 , 32 , 35 , 37 – 43 , 46 , 47 , 50 – 56 , 60 , 63 , 65 – 71 , 73 – 84 , 86 – 91 , 98 – 102 , 106 – 111 , 113 , 116 , 118 , 121 , 122 ] The 1 to 5 stages mean CKD prevalence was higher (13·4% vs. 11·0%). The breakdown by stage using all available data was Stage-1 (eGFR>90+ACR>30): 3·5%(2·8–4·2%); Stage-2 (eGFR 60–89+ACR>30): 3·9%(2·7–5·3%); Stage-3 (eGFR 30–59): 7·6%(6·4–8·9%); Stage-4 = (eGFR 29–15): 0·4%(0·3–0·5%); and Stage-5 (eGFR<15): 0·1%(0·1–0·1%). Separate reporting of Stage 3a/3b was not possible due to lack of reporting. Sensitivity analyses determined that no individual study or group of studies (limited populations—i.e. laboratory audits, age restricted, single site recruitment—, age adjusted prevalence, etc.) were suspected of excess influence on the prevalence estimates. Further, there was no difference between studies that reported using the higher quality IDMS traceable assay and those that did not.
Effect of Age, Hypertension, BMI, Obesity, Diabetes, Smoking.
Univariate meta-regressions of CKD prevalence and covariates were undertaken. Mean population age, given in 94 of 112 populations, was significantly associated (β = 0·4%, p<0·001, R 2 = 25·5), as was prevalence of diabetes (n = 82, β = 0·16%, p = 0·006, R 2 = 8·0), prevalence of hypertension (n = 75, β = 0·15%, p = 0·002, R 2 = 11·4) but not average BMI or prevalence of obesity. Smoking (n = 60) was negatively associated with CKD prevalence (an increase of smoking status was associated with a decreased prevalence of CKD (β = -0·14 p = 0·07, R 2 = 4·2).
Prevalence of CKD increased with age, Fig 3 . To determine an estimated prevalence for each age the sample population was divided by mean age into deciles. Studies measuring 5 stages of CKD mean(95%CI) were—30s: 13·7%(10·8, 16·6%), 40s: 12·0%(9·9, 14·1%), 50s: 16·0%(13·5, 18·4%), 60s: 27·6%(26·7, 28·5%), 70s: 34·3%(31·9, 36·7%). Studies measuring stages 3 to 5–30s: 8·9%(4·7, 13·1%), 40s: 8·7%(6·9, 10·5%), 50s: 12·2%(9·8, 14·5%), 60s: 11·3%(8·1, 14·5%), 70s: 27·9%(16·40, 39·3%).
Each circle represents a study prevalence estimate with the size denoting the precision of the estimate.
https://doi.org/10.1371/journal.pone.0158765.g003
There were no significant differences in prevalence between groups of studies that adjusted for age compared to those that did not. Further, a sensitivity test found that older age restricted populations did not significantly change the estimated pooled prevalence for CKD, Stages 3 to 5 mean (95%CI) 10·2%(8·4–12·0%) vs. 10·6%(9·2–12·2%) and stages 1 to 5 mean (95%CI) 11·5%(9·3–13·9%) vs. 11·4%(9·4–13·1%). A sensitivity analysis examining glomerular filtration estimating equation was planned but only 12 studies used the CKD-EPI equation making the analysis unfeasible.
CKD prevalence by geographical grouping was examined, Table 1 . Geographical areas with more than one study were pooled using random effects models.
https://doi.org/10.1371/journal.pone.0158765.t001
Fifty-one studies reported sex-specific prevalence of CKD. [ 27 – 29 , 32 , 37 – 39 , 44 , 46 , 48 , 50 , 52 – 55 , 57 , 58 , 61 , 64 , 68 – 71 , 78 , 79 , 82 , 83 , 85 , 87 , 92 – 94 , 98 , 100 – 103 , 105 , 106 , 108 , 110 , 115 , 119 ] Male mean (95%CI) CKD prevalence, for studies that defined 5 stages of CKD, was 12·8%(10·8–11·9%) and for studies that used stages 3 to 5 it was 8·1%(6·3–10·2%). Female CKD prevalence for studies that defined CKD by stages 1 to 5 was 14·6%(12·7–16·7%) and for studies that used stages 3 to 5 it was 12·1%(10·6–13·8%). Thirty-eight studies [ 27 – 29 , 32 , 37 – 39 , 44 , 46 , 50 , 55 , 64 , 69 – 71 , 78 , 79 , 83 , 85 , 87 , 92 , 98 , 100 – 103 , 105 , 106 , 108 , 110 , 115 , 119 ] reported that CKD was more prevalent in women than in men with the pattern reversed in thirteen studies. [ 39 , 44 , 48 , 52 – 54 , 57 , 58 , 61 , 68 , 82 , 93 , 94 ]
The methodological quality of studies ranged from 32·1% [ 31 ] to 92·9%. [ 4 , 68 ] No study complied completely with the STROBE guidelines and the mean(SD) quality was 69·6(12·5)%.
Quality of serum creatinine measurement was assessed. Two studies scored 100%- four methods. [ 115 , 122 ] Thirty-six studies scored 0% [ 30 – 32 , 34 , 39 , 40 , 48 , 49 , 52 , 57 – 60 , 62 , 64 , 71 , 72 , 74 , 76 , 81 , 82 , 85 , 87 , 89 , 92 , 95 , 102 , 103 , 105 , 106 , 109 , 113 , 116 , 117 ], thirty-five studies scored 25%-one method-[ 2 , 28 , 29 , 33 , 35 – 38 , 41 , 43 , 47 , 51 , 53 , 61 , 65 , 67 – 70 , 75 , 77 – 79 , 83 , 84 , 97 – 100 , 108 , 110 , 114 , 121 ], twenty-seven scored 50%-two methods-[ 4 , 27 , 42 , 44 , 46 , 50 , 54 – 56 , 66 , 74 , 80 , 88 , 90 , 101 , 104 , 107 , 111 , 112 , 118 , 119 , 123 ] and ten scored 75%-three methods. [ 10 , 45 , 63 , 73 , 86 , 91 , 93 , 94 , 96 , 120 ]
Sensitivity analyses determined no difference in the prevalence estimate of CKD when using only high quality studies, studies that used double measures of creatinine only or studies that had two or more factors for the measurement of creatinine.
CKD prevalence Stages 1 to 5 was 13·4% and 10·6% in stages 3 to 5. This systematic review is the first meta-analysis of CKD prevalence globally and provides a comprehensive overview of the current literature. These estimates indicate that CKD may be more common than diabetes, which has an estimated prevalence of 8·2%. [ 124 ] However, the reported prevalence of CKD varied widely amongst the studies and had high heterogeneity.
CKD was more prevalent in women than in men. Two-thirds of studies -that reported gender-specific CKD prevalence- determined higher prevalence in women. Women, in general, have less muscle mass than men and muscle mass is a major determinant of serum creatinine concentration. However, the GFR estimation equations adjust for gender differences, using a correction factor for women. These findings add to the existing literature that recognise a gender-specific difference between CKD prevalence. [ 125 – 127 ]However, these data cannot answer why this may occur. We can speculate that this finding may be partially explained by selection bias inherent within the studies due to a different age demographic for the two sexes. Alternatively it may be due to complex factors in the disease pathology that are not captured within the studies. Or that there is in fact more renal disease in men but the eGFR equations preferentially identify renal disease in women in the stage 3 zones.
Studies that were outliers in terms of reported results were of interest. Smoking was found to be negatively associated with CKD prevalence but this finding was negated when a single outlier was removed. The outlier [ 120 ] was a study in which smoking was defined as >100 cigarettes ever and thus 69·1% were smokers. A Spanish study [ 106 ] (n: 7202, Quality: 52%, CKD: 21·3%) reported 66.7% hypertension prevalence within the population compared with a global mean (from all other studies) of 31·1%. Hypertension was not defined any differently. Further, 31.5% of their sample population had diabetes and 31.1% were obese. The population was reported as unrestricted older population but although it was older than other studies (mean age 60·6yrs) these rates of co-morbidity are unexpected and were not explained. A number of studies had very high prevalence of CKD (>30%) the highest of these was a Canadian study (n: 123,499, Quality: 52%, CKD: 36·4%), a laboratory audit of patients over 65 years. The prevalence observed may be due to selection bias as the mean age of this cohort was 74 years, with 23% diabetes in the sample population, two factors associated with renal decline.
The geographical stratification of results revealed that developed areas such as Europe, USA, Canada and Australia had higher rates of CKD prevalence in comparison to areas where economies are growing such as sub Saharan Africa, India etc. With the exception of Iran that had similar high level of CKD prevalence possibly due dietary risks, high BMI, high systolic BP and co-morbid conditions within the country [ 128 ]. Although percentage prevalence was higher in more developed areas projected worldwide population changes will increase the absolute numbers of people in developing countries where the populations of elderly are increasing. This increase will exacerbate the double burden of dealing with communicable and non communicable disease in a developing economy[ 129 ].
Serum creatinine measurement bias was inherent in the majority of the studies. Serum creatinine concentrations are highly variable within individuals, up to 21% within a 2-week period. [ 130 ] NICE guidelines advise two measures of eGFR 3-months apart and within the last 12-months to minimise intra-individual variation. Not all countries have such guidelines only 5 manuscripts reported this in study design. Jaffe creatinine assay was the main method used but it is known to systematically overestimate serum creatinine to varying degrees. [ 131 ] Thirty-seven of the studied populations reported that they calibrated directly to the laboratory to minimize assay bias effect and twenty-seven studies used a minimally biased traceably assay (IDMS). A comparison of these studies to the remainder found no significant difference in prevalence estimates. A third of the studies (n = 36) made no mention of measures, traceability, or calibrations. It is further known that the MDRD equation systematically overestimates CKD in the general population [ 13 ] and the prevalence rates calculated may be lower. Estimated GFR is accepted as the most useful index of kidney function in health and disease, but an uncorrected, untraceable single measure inherently introduces noise and outliers into the dataset. This latter point has been very recently clarified as an epidemiological study in Morocco found that up to 30% of patients initially classified as CKD 3a using the MDRD formula had improved renal function over 12 months and therefore would not have a CKD diagnosis[ 132 ].
Estimation of GFR from serum creatinine is the clinical standard worldwide and the CKD the KDOQI diagnostic criteria[ 11 ] guidelines emphasise the importance of estimation of GFR rather than use of serum creatinine concentration. However, the 2002 KDOQI guidelines that the included studies reference have stimulated controversies and questions. In particular, there have been concerns that use of its definition of CKD has caused excessive false identification of CKD and that its staging system was not sufficiently informative about prognosis. A new KDIGO guideline was published in 2013 [ 133 ] that sought to address this with the splitting of the stage 3 category to emphasise the risks of mortality and other outcomes vary greatly between these groups and have further and further sub-stratified by the inclusion of urinary albuminuria. There is a limitation in our study in that unfortunately the analysis of stages 3a and 3b was not possible due to lack of reporting and studies using the previous KDOQI guidelines so no conclusions about whether the patients really have ‘disease’ rather than normal variation due to aging could be drawn.
Observational studies are individually subject to bias and residual confounding from unspecified sources but it is difficult to quantify how much bias and/or confounding. One study may report an effect size adjusted for several possible confounders; others may report the crude prevalence. The authors have sought to address this limitation by using STROBE quality weighting and creatinine quality factors and participant per study-weighted rates. Ideally future research should report the crude and adjusted rates based on multiple measures over time.
This systematic review and meta-analysis significantly extends existing systematic reviews in a number of ways. The search strategy allowed the detection of a large number of additional studies that had not been considered in previous systematic reviews. It increased the number of reference databases searched. The reviewers undertook to screen non-English publications through the use of translations. The studies included used the same definitions of CKD and used broadly comparable definitions for severity markers or related conditions (albuminuria, hypertension, diabetes and obesity). However, there are limitations due to the heterogeneity that arises from differences in age and sex distributions, use of creatinine assays, different sampling frames, inclusion criteria of general population based studies, and time period of the study. A proportion of the variation across studies may not be due to real differences in CKD prevalence. However, the authors did seek to provide a robust assessment of the quality and use this to determine a weighted global prevalence of CKD in the meta-analysis. The prevalence rates calculated highlight the likely numbers of people with CKD that may be of relevance to health care providers and national health programs with finite resources with which to address this epidemic.
CKD constitutes a major cost burden to healthcare systems worldwide. The high prevalence and the extensive existing evidence that intervention is effective in reducing CVD events demonstrates a need for national initiatives that will slow the progression to end stage renal disease and reduce CVD-related events in CKD patients.
This comprehensive meta-analysis of observational studies confirms that CKD has a high prevalence. Using CKD prevalence weighted by quality, using ‘High’ quality studies only and using studies weighted by number of participants consistently estimated a global CKD prevalence of between 11 to 13%. Future research should evaluate intervention strategies deliverable at scale to delay the progression of CKD and improve CVD outcomes. Evaluation of the roles of these interventions and the associated costs needs to be undertaken. CKD prevalence studies should report more detail on disease definitions and population demographics and state unadjusted as well as adjusted findings.
Supporting Information
S1 appendix. study table—summary descriptions of included studies (n = 100) and the populations (n = 112) within those studies..
https://doi.org/10.1371/journal.pone.0158765.s001
S2 Appendix. PRISMA Checklist—Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.
https://doi.org/10.1371/journal.pone.0158765.s002
Acknowledgments
We wish to thank Ms. Nia Roberts for her extensive assistance in conducting the search and Dr Yaling Yang for her translation of a Chinese language manuscript. Systematic Review Registration: PROSPERO CRD42014009184.
Author Contributions
Conceived and designed the experiments: NH DL FDRH. Performed the experiments: SF NH DL COC FDRH. Analyzed the data: NH SF JO JH. Wrote the paper: NH SF COC JO JH DL FDRH.
- View Article
- PubMed/NCBI
- Google Scholar
- 11. KDOQI KDOQI. Chronic Kidney Disease: Evaluation, Classification, and Stratification2002. Available: http://www.kidney.org/professionals/kdoqi/guidelines_commentaries.cfm .
- 12. NICE NIfCE. CG73 Chronic kidney disease: full guideline: 2008; 2008 [6th June 2012]. The published full clinical guideline on Chronic kidney disease including recommendations and methods used.]. Available: http://guidance.nice.org.uk/CG73/Guidance/pdf/English .
- 22. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: Wiley-Blackwell; 2008.
- 124. IDF IDF. IDF Diabetes Atlas. 6th ed2013.
- 133. KDOQI KDOQI. Chronic Kidney Disease: Evaluation, Classification, and Stratification2012. Available: http://www.kidney.org/professionals/kdoqi/guidelines_commentaries.cfm .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 03 April 2024
Time to sound the alarm about the hidden epidemic of kidney disease
You have full access to this article via your institution.
Kidney disease is growing worldwide. The secretariat of the World Health Organization has welcomed the call to include it as a non-communicable disease that causes premature deaths. Credit: Vsevolod Zviryk/SPL
A quiet epidemic is building around the world. It is the third-fastest-growing cause of death globally. By 2040, it is expected to become the fifth-highest cause of years of life lost. Already, 850 million people are affected, and treating them is draining public-health coffers: the US government-funded health-care plan Medicare alone spends US$130 billion to do so each year. The culprit is kidney disease, a condition in which damage to the kidneys prevents them from filtering the blood.
And yet, in discussions of priorities for global public health, the words ‘kidney disease’ do not always feature. One reason for this is that kidney disease is not on the World Health Organization (WHO) list of priority non-communicable diseases (NCDs) that cause premature deaths. The roster of such NCDs includes heart disease, stroke, diabetes, cancer and chronic lung disease. With kidney disease missing, awareness of its growing impact remains low.
Chronic kidney disease and the global public health agenda: an international consensus
The authors of an article in Nature Reviews Nephrology this week want to change that ( A. Francis et al. Nature Rev. Nephrol . https://doi.org/10.1038/s41581-024-00820-6; 2024 ). They are led by the three largest professional organizations working in kidney health — the International Society of Nephrology, the American Society of Nephrology and the European Renal Association — and they’re urging the WHO to include kidney disease on the priority NCD list.
This will, the authors argue, bring attention to the growing threat, which is particularly dire for people in low- and lower-middle-income countries, who already bear two‑thirds of the world’s kidney-disease burden. Adding kidney disease to the list will also mean that reducing deaths from it could become more of a priority for the United Nations Sustainable Development Goals target to reduce premature deaths from NCDs by one-third by 2030.
As of now, rates of chronic kidney disease are likely to increase in low- and lower-middle-income countries as the proportion of older people in their populations increases. Inclusion on the WHO list could provide an incentive for health authorities to prioritize treatments, data collection and other research, along with funding, as with other NCDs.
Kidney disease often accompanies other conditions that do appear on the NCD list, such as heart disease, cancer and diabetes — indeed, kidney-disease deaths caused specifically by diabetes are on the list. But the article authors argue that “tackling diabetes and heart disease alone will not target the core drivers of a large proportion of kidney diseases”. Both acute and chronic kidney disease can have many causes. They can be caused by infection or exposure to toxic substances. Increasingly, the consequences of global climate change, including high temperatures and reduced availability of fresh water, are thought to be contributing to the global burden of kidney disease, as well.
The kidney glomerulus filters waste products from the blood. In people with damaged kidneys, this happens through dialysis. Credit: Ziad M. El-Zaatari/SPL
The WHO secretariat, which works closely with the nephrology community, welcomes the call to include kidney disease as an NCD that causes premature deaths, says Slim Slama, who heads the NCD unit at the secretariat in Geneva, Switzerland. The data support including kidney disease as an NCD driver of premature death, he adds.
The decision to include kidney disease along with other priority NCDs isn’t only down to the WHO, however. There must be conversations between the secretariat, WHO member states, the nephrology community, patient advocates and others. WHO member states need to instruct the agency to take the steps to make it happen, including providing appropriate funding for strategic and technical assistance.
Data and funding gaps
Three reports based on surveys by the International Society of Nephrology since 2016 highlight the scale of data gaps ( A. K. Bello et al. Lancet Glob. Health 12 , E382–E395; 2024 ). In many countries, screening for kidney disease is difficult to access and a large proportion of cases go undetected and therefore uncounted. For example, it is not known precisely how many people with kidney failure die each year because of lack of access to dialysis or transplantation: the numbers are somewhere between two million and seven million, according to the WHO. Advocates must push public-health officials in more countries to collect the data needed to monitor kidney disease and the impact of prevention and treatment efforts.
Even with better data, treatments for kidney disease are often prohibitively expensive. They include dialysis, an intervention to filter the blood when kidneys cannot. Dialysis is often required two or three times weekly for the remainder of the recipient’s life, or until they can receive a transplant, and it is notoriously costly. In Thailand, for example, it accounted for 3% of the country’s total health-care expenditures in 2022, according to the country’s parliamentary budget office.
End chronic kidney disease neglect
These costs could come down if people who have diabetes or high blood pressure, for example, could be routinely screened for impaired kidney function, because they are at high risk of developing chronic kidney disease. This would enable kidney damage to be detected early, before symptoms set in, opening the way for treatments that do not immediately require dialysis or transplant surgery.
New drugs that boost weight loss and treat type 2 diabetes could also help to prevent or reduce stress on the kidneys, but these, too, are too expensive for many people in need. That is why something needs to be done to make drugs more affordable. The pharmaceutical industry, which has become extremely profitable, has a crucial role. In Denmark, for example, the industry’s profits helped to tip the national economy from recession into growth in 2023, according to the public agency Statistics Denmark. The COVID-19 pandemic showed that making profits and making drugs available, and affordable, to a wide population need not be mutually exclusive. Similarly innovative thinking is now needed. “The whole world needs to reckon with this kidney problem,” says Valerie Luyckx, a biomedical ethicist at the University of Zurich in Switzerland.
The WHO adding kidney disease to its priority list could also attract funding for treatment, research and disease registries. That could jump-start the development of new treatments and help to make current treatments more affordable and accessible.
NCDs are responsible for 74% of deaths worldwide, but the world’s biggest donors to global health currently devote less than 2% of their budgets for international health assistance to NCD prevention and control, and not including kidney disease. Drawing more attention to the quiet rampage of kidney disease among some of the most vulnerable people would be one important step in turning these statistics around.
Nature 628 , 7-8 (2024)
doi: https://doi.org/10.1038/d41586-024-00961-5
Reprints and permissions
Related Articles
- Developing world
- Health care

AI can help to tailor drugs for Africa — but Africans should lead the way
Comment 09 APR 24

Rwanda 30 years on: understanding the horror of genocide
Editorial 09 APR 24

Africa’s postdoc workforce is on the rise — but at what cost?
Career Feature 02 APR 24

India is booming — but there are worries ahead for basic science
News 10 APR 24

Brazil budget cuts could leave science labs without power and water
News 08 APR 24

Will the Gates Foundation’s preprint-centric policy help open access?
News 04 APR 24

Bird flu outbreak in US cows: why scientists are concerned
News Explainer 08 APR 24
Adopt universal standards for study adaptation to boost health, education and social-science research
Correspondence 02 APR 24

Long COVID still has no cure — so these patients are turning to research
News Feature 02 APR 24
Postdoctoral Associate- Comparative Medicine
Houston, Texas (US)
Baylor College of Medicine (BCM)
Group Leader at Católica Biomedical Research Centre and Assistant or Associate Professor at Católica
Group Leader + Assistant/Associate Professor, tenure-track position in Biological and Biomedical Sciences, Data Science, Engineering, related fields.
Portugal (PT)
Católica Biomedical Research Centre
Faculty Positions at SUSTech Department of Biomedical Engineering
We seek outstanding applicants for full-time tenure-track/tenured faculty positions. Positions are available for both junior and senior-level.
Shenzhen, Guangdong, China
Southern University of Science and Technology (Biomedical Engineering)
Locum Associate or Senior Editor, Nature Cancer
To help us to build on the success of Nature Cancer we are seeking a motivated scientist with a strong background in any area of cancer research.
Berlin, Heidelberg or London - Hybrid working model
Springer Nature Ltd

Postdoctoral Research Fellows at Suzhou Institute of Systems Medicine (ISM)
ISM, based on this program, is implementing the reserve talent strategy with postdoctoral researchers.
Suzhou, Jiangsu, China
Suzhou Institute of Systems Medicine (ISM)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Reference Manager
- Simple TEXT file
People also looked at
Editorial article, editorial: cystic kidney diseases in children and adults: from diagnosis to etiology and back.

- 1 University of Zagreb School of Medicine, Zagreb, Croatia
- 2 Division of Nephrology, Dialysis and Transplantation, Department of Pediatrics, University Hospital Center Zagreb, Zagreb, Croatia
- 3 Department of Nephrology, Arterial Hypertension, Dialysis and Transplantation, University Hospital Center Zagreb, Zagreb, Croatia
- 4 Institute of Human Genetics, Center for Molecular Medicine Cologne, and Center for Rare and Hereditary Kidney Disease, Cologne, University Hospital of Cologne, Cologne, Germany
Editorial on the Research Topic Cystic kidney diseases in children and adults: from diagnosis to etiology and back
Renal cysts are often regarded as the most common abnormality associated with kidney disease ( 1 , 2 ). They are encountered in both adults and children, as isolated findings or as part of a more complex clinical condition ( 3 – 5 ). Isolated kidney cysts in adults sometimes require evaluation for kidney cancer or simple cysts may occur as a sign of age-related kidney tissue degeneration in the absence of any underlying specific kidney disease. Recent advances in understanding the underlying mechanisms have led to the concept of renal ciliopathies with more than 100 genes associated with ciliary dysfunction, resulting in conditions such as polycystic kidney disease (PKD), tuberous sclerosis complex (TSC) and nephronophthisis complex (NPHC), which may be associated with various extrarenal phenotypes ( Figure 1 ) ( 6 – 8 ). In addition to progressive CKD, these disorders are characterized by a variety of additional symptoms such as hepatic impairment, vision problems, developmental delays, intellectual disabilities, and skeletal abnormalities, which inconsistently present throughout the course of the disease ( 4 , 5 , 7 ). Furthermore, the significant phenotypic overlap makes it difficult to differentiate specific disorders, often necessitating genetic testing to reach a definite diagnosis ( 9 ). Despite a multitude of clinical and translational studies, in the majority of cases it is still challenging or even impossible to predict the individual clinical course, necessitating regular follow-up of the patients and a timely response in terms of treatment, which remains mostly symptomatic ( 10 ).
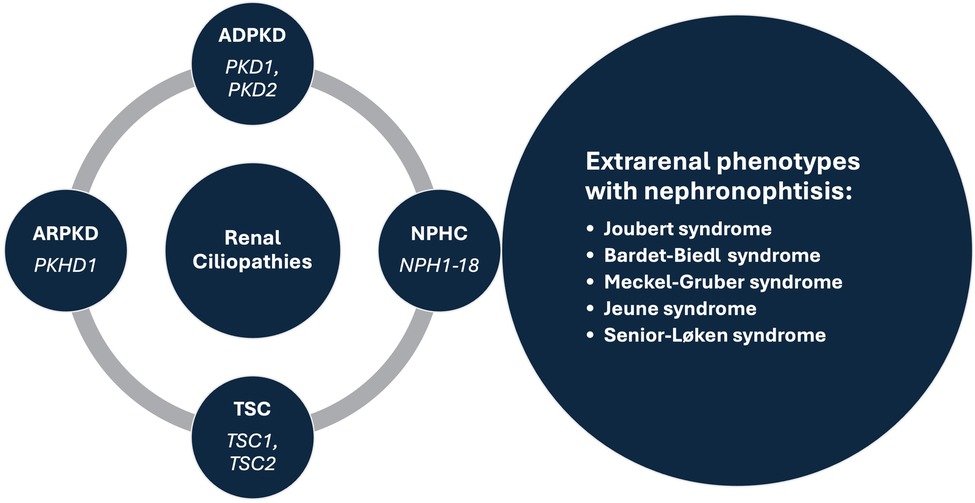
Figure 1 . Prominent syndromes and associated genes within the renal ciliopathies concept. ADPKD, autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease; NPHC, nephronophthisis complex; TSC, tuberous sclerosis complex.
The present special issue contains seven noteworthy articles describing engaging cases of children and adults with various disorders having a common denominator in the form of kidney cysts, systematically reviewing the current literature on the clinical characteristics of an HNF1B gene variant and biomarkers of kidney disease progression in autosomal dominant PKD (ADPKD), investigating the outcome of fetal renal cystic disease and exploring the utility of magnetic resonance imaging-based kidney volume assessment for risk stratification in children with ADPKD.
In more detail, Simičić Majce et al . describe a nonconsanguineous family with three members affected by BBS caused by compound heterozygous mutations in the BBS12 gene. Despite identical genotypes, the affected family members demonstrated significant diversity in clinical characteristics (different expressivity) of the BBS phenotype emphasizing the importance of genetic testing for the early diagnosis of this rare ciliopathy. Similarly, Fištrek Prlić et al. present two clinically distinct cases of autosomal dominant tubulointerstitial kidney disease (ADTKD) diagnosed only after genetic testing, along with an extensive review of the literature and a comprehensive overview of the condition. Both patients had uninformative renal ultrasound and urinalysis findings with only elevated serum creatinine levels indicating a kidney disease. An adult patient with a positive family history of CKD had no other symptoms, while an adolescent boy with an unremarkable family history had psychomotor impairment with epilepsy. After the testing they were diagnosed with MUC1 -related ADTKD and 17q12 microdeletion syndrome causing the loss of one copy of the transcription factor HNF1B and 14 additional genes, respectively, highlighting the importance of clinical awareness in diagnosing this syndrome. Finally, the third case report by Kasahara et al. advocates an interesting option to treat the chronic pain experienced by more than half of patients with ADPKD. They describe an adolescent girl with persistent pain associated with multiple renal cysts that prevented her from participating in daily activities. After being diagnosed with attention deficit disorder (ADHD) and appropriate treatment for this condition being initiated, she experienced significant pain relief and better control of her hypertension. Therefore, in patients with ADPKD it may be important to recognize concomitant ADHD and consider a trial of ADHD medications when chronic pain associated with ADPKD is present.
In line with the exploration of important associations between cystic kidney disease and other disorders a systematic review by Nittel et al. examined the prevalence of neurodevelopmental disorders (NDD) in patients with 17q12 microdeletions vs. HNF1B point mutations. The results of a diligent literature search revealed that NDDs are frequently observed in HNF1B -associated diseases, especially in the common 17q12 microdeletion, and should hence become a routine part of clinical care for patients with HNF1B -related diseases. On the other hand, a systematic review by Sorić Hosman et al. provided a critical overview of previously examined serum and urine biomarkers with a potential for predicting disease progression or response to therapy in patients with ADPKD. A comprehensive literature review identified several prognostic molecules that are involved in various processes central to the development of the disease, such as tubular injury, inflammation, metabolism, renin-angiotensin, or vasopressin system adjustments. Interestingly, the most accurate predictive models have been achieved when incorporating such serum and urine biomarkers with the Predicting Renal Outcome in Polycystic Kidney Disease (PROPKD) score which combines underlying genetic mutations and clinical risk factors, or with the Mayo Imaging Classification (MIC) which is based on age- and height-adjusted total kidney volume (TKV) measured by magnetic resonance imaging (MRI).
MRI-based kidney volume assessment was further investigated by Yilmaz et al. in a multicenter, cross-sectional, and case-controlled study involving 89 children and adolescents with a genetically confirmed and detailly characterized diagnosis of ADPKD. The study patients were stratified according to the innovative Leuven Imaging Classification (LIC) into different risk categories, with those in the highest risk category having an increased incidence of hypertension and a higher prevalence of PKD1 mutations. Therefore, the study advocates the use of MRI for the measurement of TKV in the pediatric population, in addition to the use of ambulatory blood pressure monitoring to recognize those with hypertension.
Finally, Botero-Calderon et al. presented a retrospective study evaluating clinical and imaging data, genetic testing results and postnatal follow-up outcomes of infants identified in utero with bilateral renal cystic disease at a single referral center over a period of 11 years. Among 17 patients with suspected renal ciliopathy, the most common diagnosis was autosomal recessive PKD (ARPKD, n = 4), followed by Bardet-Biedl syndrome (BBS, n = 3), autosomal dominant polycystic disease (ADPKD, n = 2), HNF1B-related disease ( n = 2), and Meckel-Gruber syndrome (MKS, n = 2), while four cases were not genetically resolved. In terms of postnatal management, the study revealed that the vast majority of neonatal survivors with renal ciliopathies are directed to the care of a pediatric nephrologist, while this proportion is much lower in those with genetically unresolved enlarged, echogenic kidneys, stressing the need for structured management programs for prenatally identified kidney disease.
In conclusion, our research topic provides a contemporary overview of current practices, unmet clinical needs and research gaps regarding the broad spectrum of renal ciliopathies that may be useful to a wide range of physicians and researchers dealing with these complex disorders.
Author contributions
LL: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. IV: Writing – review & editing, Conceptualization. MF: Writing – review & editing, Conceptualization. BB: Writing – review & editing, Conceptualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kwatra S, Krishnappa V, Mhanna C, Murray T, Novak R, Sethi SK, et al. Cystic diseases of childhood: a review. Urology . (2017) 110:184–91. doi: 10.1016/j.urology.2017.07.040
PubMed Abstract | Crossref Full Text | Google Scholar
2. De Groof J, Dachy A, Breysem L, Mekahli D. Cystic kidney diseases in children. Archives de Pédiatrie . (2023) 30(4):240–6. doi: 10.1016/j.arcped.2023.02.005
Crossref Full Text | Google Scholar
3. Mensel B, Kühn JP, Kracht F, Völzke H, Lieb W, Dabers T, et al. Prevalence of renal cysts and association with risk factors in a general population: an MRI-based study. Abdominal Radiology . (2018) 43(11):3068–74. doi: 10.1007/s00261-018-1565-5
4. McConnachie DJ, Stow JL, Mallett AJ. Ciliopathies and the kidney: a review. Am J Kidney Dis . (2021) 77(3):410–9. doi: 10.1053/j.ajkd.2020.08.012
5. Gambella A, Kalantari S, Cadamuro M, Quaglia M, Delvecchio M, Fabris L, et al. The landscape of HNF1B deficiency: a syndrome not yet fully explored. Cells . (2023) 12(2):307. doi: 10.3390/cells12020307
6. Modarage K, Malik SA, Goggolidou P. Molecular diagnostics of ciliopathies and insights into novel developments in diagnosing rare diseases. Br J Biomed Sci . (2022) 79. doi: 10.3389/bjbs.2021.10221
7. Devlin LA, Sayer JA. Renal ciliopathies. Curr Opin Genet Dev . (2019) 56:49–60. doi: 10.1016/j.gde.2019.07.005
8. Kurschat CE, Müller RU, Franke M, Maintz D, Schermer B, Benzing T. An approach to cystic kidney diseases: the clinician’s view. Nat Rev Nephrol . (2014) 10(12):687–99. doi: 10.1038/nrneph.2014.173
9. Lam BL, Leroy BP, Black G, Ong T, Yoon D, Trzupek K. Genetic testing and diagnosis of inherited retinal diseases. Orphanet J Rare Dis . (2021) 16(1):514. doi: 10.1186/s13023-021-02145-0
10. Yu ASL, Landsittel DP. Biomarkers in polycystic kidney disease: are we there? Adv Kidney Dis Health . (2023) 30(3):285–93. doi: 10.1053/j.akdh.2022.12.009
Keywords: cystic kidney disease, autosomal dominant polycystic kidney disease (ADPKD), autosomal recessive polycystic kidney disease (ARPKD), nephronophtisis complex (NPHC), Bardet Biedl syndrome (BBS)
Citation: Lamot L, Vuković Brinar I, Fištrek Prlić M and Beck B (2024) Editorial: Cystic kidney diseases in children and adults: from diagnosis to etiology and back. Front. Pediatr. 12:1401593. doi: 10.3389/fped.2024.1401593
Received: 15 March 2024; Accepted: 29 March 2024; Published: 10 April 2024.
Edited and Reviewed by: Michael L. Moritz , University of Pittsburgh, United States
© 2024 Lamot, Vuković Brinar, Fištrek Prlić and Beck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lovro Lamot [email protected]
This article is part of the Research Topic
Cystic Kidney Diseases in Children and Adults: From Diagnosis to Etiology and Back
A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives
- Open access
- Published: 05 November 2021
- Volume 39 , pages 33–43, ( 2022 )
Cite this article
You have full access to this open access article
- Marc Evans 1 ,
- Ruth D. Lewis 2 ,
- Angharad R. Morgan 2 ,
- Martin B. Whyte 3 ,
- Wasim Hanif 4 ,
- Stephen C. Bain 5 ,
- Sarah Davies 6 ,
- Umesh Dashora 7 ,
- Zaheer Yousef 8 ,
- Dipesh C. Patel 9 &
- W. David Strain 10
12k Accesses
56 Citations
11 Altmetric
Explore all metrics
Chronic kidney disease (CKD) is a complex disease which affects approximately 13% of the world’s population. Over time, CKD can cause renal dysfunction and progression to end-stage kidney disease and cardiovascular disease. Complications associated with CKD may contribute to the acceleration of disease progression and the risk of cardiovascular-related morbidities. Early CKD is asymptomatic, and symptoms only present at later stages when complications of the disease arise, such as a decline in kidney function and the presence of other comorbidities associated with the disease. In advanced stages of the disease, when kidney function is significantly impaired, patients can only be treated with dialysis or a transplant. With limited treatment options available, an increasing prevalence of both the elderly population and comorbidities associated with the disease, the prevalence of CKD is set to rise. This review discusses the current challenges and the unmet patient need in CKD.
Similar content being viewed by others

Association of systemic immune-inflammation index and systemic inflammation response index with chronic kidney disease: observational study of 40,937 adults
Peixian Huang, Yanpei Mai, … Yaqing Wen

Nephrotic syndrome: pathophysiology and consequences
Ponticelli Claudio & Moroni Gabriella

Management of Hypertension in Chronic Kidney Disease
Dan Pugh, Peter J. Gallacher & Neeraj Dhaun
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a complex and multifaceted disease, causing renal dysfunction and progression to end-stage kidney disease (ESKD) and cardiovascular disease. Complications associated with the disease contribute to the acceleration of CKD progression and risk of cardiovascular-related morbidities.
Despite its high prevalence and the clinical and economic burden of its associated complications, disease awareness remains profoundly low. Worldwide, only 6% of the general population and 10% of the high‐risk population are aware of their CKD statuses [ 1 ]. In addition, CKD recognition in primary care settings is also suboptimal, ranging from 6% to 50%, dependent upon primary care specialty, severity of disease, and experience. Awareness of CKD remains low in part because CKD is usually silent until its late stages. However, diagnosis of CKD during the later stages results in fewer opportunities to prevent adverse outcomes. Physician awareness of CKD is critical for the early implementation of evidence-based therapies that can slow progression of renal dysfunction, prevent metabolic complications, and reduce cardiovascular-related outcomes.
Currently CKD is not curable, and management of the disease relies on treatments which prevent CKD progression and cardiovascular disease. Despite available treatments, a residual risk of adverse events and CKD progression remains. This article reviews the challenges associated with CKD and the treatments available for patients, highlighting the unmet need for cardio-renal protection in patients with CKD.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
CKD Prevalence
CKD is a global health problem. A meta-analysis of observational studies estimating CKD prevalence showed that approximately 13.4% of the world’s population has CKD [ 2 ]. The majority, 79%, were at late stages of the disease (stage 3–5); however, the actual proportion of people with early CKD (stage 1 or 2) is likely to be much higher since early kidney disease is clinically silent [ 3 ].
Prevalence of CKD appears to be growing rapidly both in the UK and in the Western world. Based on the 2012 subnational population projections for England [ 4 ], the number of people with CKD stage 3–5 is projected to exceed 4 million by 2036 [ 5 ]. This rise in CKD prevalence is due to an increased aging population and prevalence of type 2 diabetes (T2DM), obesity, hypertension and cardiovascular disease that contribute to CKD [ 6 , 7 , 8 ].
The World Health Organization (WHO) estimated that the annual, global number of deaths caused directly by CKD is 5–10 million [ 9 ]. The presence of CKD advances mortality of comorbidities such as cardiovascular diseases, T2DM, hypertension, and infection with human immunodeficiency virus (HIV), malaria and Covid-19, thereby indirectly adding to CKD mortality [ 9 , 10 ]. A contributing cause of high morbidity and mortality associated with CKD is a lack of awareness of the disease, by both patients and providers [ 11 , 12 ]. Early stages of CKD are clinically silent and patients have no symptoms. Lack of treatment at this stage allows CKD to progress through to advanced stages of the disease, where patients may present complications and/or cardiovascular-related comorbidities, or ESKD. Raising awareness of CKD is therefore paramount to allow for early intervention and reduce the risk of comorbidities and mortality.
Classification of CKD
In order to better manage CKD and provide better care for patients, the classification of CKD was developed by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative [ 13 ] and the international guideline group Kidney Disease Improving Global Outcomes (KDIGO) [ 14 ]. CKD stratification is based upon the estimated glomerular filtration rate (eGFR) and albuminuria.
There are six eGFR categories. An eGFR of less than 60 mL/min per 1.73 m 2 for more than 3 months is indicative of impaired renal function and the severity of kidney damage increases with decreasing eGFR measurements. Patients with early onset of the disease, stage 1–2, have normal to mild decreased levels of eGFR (60 to ≥ 90 mL/min per 1.73 m 2 ). Patients with stage 3a–3b have mild to moderate decreased levels of eGFR (45–59 mL/min per 1.73 m 2 , respectively). Severely decreased levels of eGFR, stage 4–5 (15–29 to < 15 mL/min per 1.73 m 2 , respectively), are indicative of advanced stages of the disease and kidney failure.
Stratification also comprises three categories of albuminuria. Patients with an albumin to creatinine ratio (ACR) of 3 to at most 30 mg/mmol are classified as having microalbuminuria and at moderate risk of adverse outcomes. Those with ACR of greater than 30 mg/mmol are classified as having macroalbuminuria and being severely at risk of developing adverse events [ 15 ]. The eGFR and albuminuria categories independently predict adverse outcomes for patients with CKD, and the combination of both increases this risk further [ 16 ]. The CKD classification system aids clinicians in carrying out accurate assessments of CKD severity and other complications which helps to inform decisions associated with the management and monitoring of patients [ 3 , 17 , 18 ].
Clinical Burden of CKD
CKD is a complex disease, involving both non-modifiable (e.g. older age, family history and ethnicity) and modifiable risk factors (e.g. T2DM, hypertension and dyslipidaemia) which are responsible for the initiation of early CKD, CKD progression (stage 3–5) and ESKD.
In early stages of CKD (stage 1–2), factors such as hypertension, obesity and T2DM can trigger kidney function impairment. This causes glomerular/interstitial damage and results in impaired glomerular filtration, leading to decreased eGFR and increased albuminuria. At this stage, even though clinical symptoms do not present, the presence of additional risk factors, including hypertension, hyperglycaemia, smoking, obesity, dyslipidaemia and cardiovascular disease, may accelerate CKD progression and result in ESKD.
As the disease progresses, the clinical and economic burden of CKD increases (Fig. 1 ) as complications such as CKD mineral bone disorder, anaemia, hypertension and hyperkalaemia may occur and advanced stages of CKD, stage 4–5, ensue. Clinical symptoms, such as fatigue, itching of the skin, bone or joint pain, muscle cramps and swollen ankles, feet or hands, are often present at this stage [ 19 ]. Further deterioration of kidney function causes tubular and glomerular hypertrophy, sclerosis and fibrosis, leading to a significant reduction in eGFR, extreme albuminuria and kidney failure.
A schematic diagram showing the association between CKD progression and clinical and economic burden. Symptoms of CKD typically present during advanced stages of the disease where patients are at increased risk of cardiovascular disease and other comorbidities
Even though CKD progression may lead to kidney failure and renal death, patients with CKD are more likely to die from cardiovascular-related complications before reaching ESKD [ 20 ]. A study using data from a meta-analysis involving 1.4 million individuals found a significant increased risk of cardiovascular-related mortality, even in stage 2 of CKD (eGFR levels < 90 mL/min per 1.73 m 2 ) [ 16 , 21 , 22 ].
As the disease progresses, the risk of cardiovascular disease is markedly increased, such that 50% of patients with late-stage CKD, stage 4–5, have cardiovascular disease. The risk of atrial fibrillation (AF) and acute coronary syndrome (ACS) is doubled in patients with eGFR < 60 mL/min per 1.73 m 2 . AF is associated with a threefold higher risk of progression to ESKD. The incidence of heart failure (HF) is also threefold greater in patients with eGFR < 60 mL/min per 1.73 m 2 compared with > 90 mL/min per 1.73 m 2 and HF is associated with CKD progression, hospitalisation and death [ 23 ].
The increased risk of cardiovascular disease in patients with CKD is due in part to the traditional risk factors associated with cardiovascular disease such as hypertension, T2DM and dyslipidaemia. For instance, a large observational database linked study (Third National Health and Nutrition Examination Survey (NHANES) III) found a strong association between CKD and T2DM combined and an increased risk of mortality [ 24 ]. In this study, the authors observed a 31.1% mortality rate in patients with CKD and diabetes, compared to 11.5% in people with diabetes only. An observational study using both US and UK linked databases showed that the presence of both CKD and T2DM was related to increased risk of major adverse cardiac events (MACE), HF and arrhythmogenic cardiomyopathy (ACM) [ 25 ]. This risk was further elevated in older patients with atherosclerotic cardiovascular disease [ 25 ]. Similarly, the presence of both CKD and T2DM leads to a significant increased risk of all-cause and cardiovascular-related mortality versus T2DM alone [ 24 ].
The direct renal effect on cardiovascular disease is due to generalised inflammatory change, cardiac remodelling, narrowing of the arteries and vascular calcification, both contributing to the acceleration of vascular ageing and atherosclerotic processes, and leading to myocardial infarction, stroke and HF [ 26 ].
Together, these studies highlight the strong relationship which exists between CKD progression, number of comorbidities and heightened risk of cardiovascular disease and cardiovascular-related mortality.
Economic Burden of CKD
In addition to the clinical burden, management of CKD also requires significant healthcare resources and utilisation. In 2009–2010, the estimated cost of CKD to the National Health Service (NHS) in England was £1.45 billion [ 27 ]. Furthermore, in 2016, US Medicare combined expenditure for CKD and ESKD exceeded $114 billion (£86 billion) [ 28 ].
Although estimating the true cost of early CKD is difficult because of the lack of data available for unreported cases, CKD progression is associated with increased healthcare costs [ 29 , 30 ]. A study by Honeycutt et al. combined laboratory data from NHANES with expenditure data from Medicare and found that costs of CKD management increased with disease progression [ 29 ]. Estimated annual medical costs of CKD per person were not significant at stage 1, $1700 at stage 2, $3500 at stage 3 and $12,700 at stage 4.
Healthcare costs associated with early CKD are more likely to be from the sequalae of comorbid disease rather than kidney disease. Hence, patients with CKD stage 1 or 2 are at increased risk of hospitalisation if they also have T2DM (9%), cardiovascular disease (more than twofold), and both cardiovascular disease and T2DM (approximately fourfold) [ 31 ].
ESKD accounts for the largest proportion of CKD management costs. In 2009–2010, 50% of the overall CKD cost to NHS (England) was due to renal replacement therapy (RRT), which accounted for 2% of the CKD population [ 27 ]. The other 50% included renal primary care costs, such as treatment costs for hypertension and tests, consultation costs, non-renal care attributable to CKD and renal secondary care costs. Approximately £174 million was estimated for the annual cost of myocardial infarctions and strokes associated with CKD [ 27 ].
More recently, an economic analysis investigated the burden associated with the management of cardiovascular-related morbidity and mortality in CKD, according to the KDIGO categorisation of both eGFR and albuminuria [ 15 ]. Decreased eGFR levels increased both the risk of adverse clinical outcomes and economic costs, and albuminuria elevated this risk significantly. Furthermore, CKD progression correlated with increased CKD management costs and bed days. Stage 5 CKD (versus stage 1 (or without) CKD) per 1000 patient years was associated with £435,000 in additional costs and 1017 bed days.
The significant economic burden associated with CKD progression and ESKD highlights the importance of optimising CKD management and the unmet need for better treatment options in slowing disease progression in this patient population. Thus, early detection and intervention to slow the progression of the disease has the potential to make substantial savings in healthcare costs.
Current CKD Management Strategies
KDIGO and National Institute for Health and Care Excellence (NICE) have produced detailed guidelines for the evaluation and management of CKD [ 3 , 32 , 33 ]. Both recommend implementing strategies for early diagnosis of the disease in order to reduce the risk of cardiovascular disease, attenuate CKD progression and decrease the incidence of ESKD in this patient population. CKD is a complex disease and thus treatment requires a multifaceted approach utilising both non-pharmacological, e.g. diet and exercise regimes and pharmacological interventions such as antihypertensive and antihyperglycemic drugs [ 34 ]. There has, however, been no significant breakthrough in this area for over 2 decades.
The effect of lifestyle intervention on reducing disease progression is still unclear, although increased physical activity has been shown to slow the rate of eGFR decline [ 35 ] and ESKD progression [ 36 ], improve eGFR levels [ 35 ] and albuminuria [ 37 ], and reduce mortality in patients with CKD [ 35 , 38 , 39 , 40 ]. Similarly, diet regimes such as low-protein diet or Mediterranean diet reduce renal function decline and mortality rate in CKD [ 41 , 42 ]. Hence, dietary advice is recommended in accordance with CKD severity to control for daily calorie, salt, potassium, phosphate and protein intake [ 3 , 33 ]. However, patients with consistently elevated serum phosphate levels or metabolic acidosis [low serum bicarbonate levels (< 22 mmol/l)], associated with increased risk of CKD progression and death, may be treated with phosphate binding agents (e.g. aluminium hydroxide and calcium carbonate) or sodium bicarbonate, respectively [ 3 ].
To reduce the risk of cardiovascular disease, KDIGO and NICE recommend active lipid management and blood pressure control [ 33 , 43 , 44 ]. In early CKD stages 1 and 2, statins are recommended for all patients over 50 years of age, whilst in stage 3 and advanced stages of the disease, stage 4–5 (eGFR < 60 mL/min per 1.73 m 2 ), a combination of statins and ezetimibe is advised [ 43 ].
Management of hypertension includes a target blood pressure of less than 140/90 mmHg for patients with CKD and hypertension and less than 130/80 mmHg for patients with CKD and T2DM, and also in patients with albuminuria [ 3 , 32 ], alongside blood pressure lowering therapies and renin–angiotensin–aldosterone system (RAAS) blocking agents, such as angiotensin receptor blockers (ARB) or angiotensin-converting enzyme inhibitors (ACEi). As such, RAAS inhibitors (RAASi) are currently recommended to treat patients with diabetes, hypertension and albuminuria in CKD [ 45 ]. These RAAS blocking agents confer both renal and cardiovascular protection and are recommended as first-line treatment to treat hypertension in patients with CKD [ 34 , 46 ].
The clinical benefits of RAASi have been demonstrated in patients with CKD with and without diabetes [ 47 , 48 , 49 ]. These clinical benefits are in addition to their effects on reducing blood pressure and albuminuria, including a reduction in eGFR decline and a decreased risk of ESKD cardiac-related morbidity and all-cause mortality [ 47 , 48 , 49 ]. Nevertheless, despite their benefits, RAASi treatment can induce hyperkalaemia, and patients are often advised to reduce RAASi dosage or even discontinue their treatment, which prevents optimum clinical benefits of RAASi therapy being reached. In this instance, combination therapy with potassium binding agents, such as patiromer and sodium zirconium cyclosilicate, may be used alongside RAASi therapy to reduce RAASi-associated hyperkalaemia.
However long-term trials will be required to determine their effect on cardiovascular morbidity and mortality in CKD [ 50 , 51 , 52 ]. Despite these therapies being the mainstay of CKD management, there is still a residual risk of CKD progression and an unmet need for new treatments.
Novel/Emerging Treatments for CKD Management
Over the last 2 years, novel therapeutic approaches for CKD management have emerged, with particular attention on mineralocorticoid receptor antagonists (MRAs) and sodium–glucose co-transporter 2 (SGLT2) inhibitors. The clinical effectiveness of finerenone, a selective oral, non-steroidal MRA, has recently been demonstrated to lower risks of CKD progression and cardiovascular events in diabetic kidney disease (DKD) [ 53 ]. Finerenone is under review for approval by the European Medicines Agency (EMA) and US Food and Drug Administration (FDA).
Of these new and emerging therapies, SGTL2i offers the most clinical benefit with both cardiovascular and renal protective effects, independent of glucose lowering. Clinical trials of SGTL2 in T2DM with and without CKD overall showed a 14–31% reduction in cardiovascular endpoints including hospitalisation for HF and MACE and a 34–37% reduction in hard renal-specific clinical endpoints including a sustained reduction in eGFR, progression of albuminuria and progression to ESKD [ 54 , 55 , 56 , 57 , 58 ]. CREDENCE, was a double-blind, multicentre, randomised trial in diabetic patients with albuminuric CKD (eGFR 30 to < 90 mL/min per 1.73 m 2 and ACR ≥ 30 mg/mmol) [ 57 ]. In this trial, canagliflozin reduced the relative risk of the composite of ESKD, doubling of serum creatinine and renal-related mortality by 34%, relative risk of ESKD by 34% and risk of cardiovascular-related morbidity, including myocardial infarction and stroke, and mortality.
The SGTL2i dapagliflozin has proven its effectiveness in slowing CKD progression in addition to reducing cardiovascular risk in early stages of CKD. The DECLARE-TIMI58 trial involved 17,160 diabetic patients with established atherosclerotic cardiovascular disease and early-stage CKD (mean eGFR was 85.2 mL/min per 1.73 m 2 ) and were randomised to receive either dapagliflozin or placebo. Following a median follow-up of 4.2 years, there was a significant reduction in renal composite endpoints with dapagliflozin versus placebo, with a 46% reduction in sustained decline of at least 40% eGFR to less than 60 mL/min per 1.73 m 2 and a reduction in ESKD (defined as dialysis for at least 90 days, kidney transplantation, or confirmed sustained eGFR < 15 mL/min per 1.73 m 2 ) or renal death.
More recently, these cardiovascular and renal protective effects of SGTLT2i have also been demonstrated in a broad range of patients with more advanced stages of CKD (mean eGFR was 43.1 ± 12.4 mL/min per 1.73 m 2 ) without diabetes [ 58 , 59 ]. In the DAPA-CKD trial, many patients were without diabetes, including IgA nephropathy, ischemic/hypertension nephropathy and other glomerulonephritis [ 59 ]. Patients receiving dapagliflozin had a 39% relative risk reduction in the primary composite outcomes of a sustained decline in eGFR of at least 50%, ESKD and renal- or cardiovascular-related mortality and a 31% relative risk reduction of all-cause mortality compared to placebo [ 58 , 60 ]. Safety outcomes from clinical trials of dapagliflozin have also shown similar incidences of adverse events in both placebo and dapagliflozin arms [ 58 , 61 ].
The clinical benefits and safety outcomes from these trials highlight the potential use of SGTL2i in reducing cardiovascular burden and CKD progression in a broad range of CKD aetiologies at early and late stages where there is an unmet need. Currently, SGTL2i class drugs, including canagliflozin, dapagliflozin and empagliflozin, are approved by the US FDA for the treatment of T2DM and, more recently, dapagliflozin and canagliflozin for CKD and DKD respectively [ 62 , 63 ]. In addition, SGTL2i has been recommended for approval in the European Union (EU) by the Committee for Medicinal Products for Human Use (CHMP) of the EMA, for the treatment of CKD in adults with and without T2D [ 64 ]. Hence, there is now a need to raise awareness of the clinical applicability of these drugs in CKD to ensure full utilisation and maximum benefits are met, for both patients and providers.
This narrative review has summarised some of the key challenges associated with CKD. Early stages of the disease are clinically silent which prevents early intervention to slow the progression of the disease and allows progression of CKD and ESKD. At advanced stages of the disease, when clinical symptoms are present, patients with CKD are already at heightened risk of cardiovascular-related morbidity and mortality. Hence, advanced stages of CKD and ESKD are associated with poor outcomes and a significant clinical and economic burden.
At present, there are no treatments to cure CKD; as such, strategies for CKD management have been developed to target the modifiable risk factors in order to reduce cardiovascular disease morbidity in patients with CKD and slow the progression of CKD to ESKD. However, despite available treatment options, residual risk of adverse events and CKD progression remain; hence, an unmet need exists in CKD treatment. SGTL2i have the potential to fill this gap, with recent evidence from clinical trials showing a reduction in cardiovascular and renal adverse endpoints in a broad range of patients with CKD.
Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4(5):e307–19.
Article PubMed Google Scholar
Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS ONE. 2016;11(7):e0158765.
Article PubMed PubMed Central CAS Google Scholar
Kidney International Organisation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf . 2012. Accessed May 2021.
Office for National Statistics: Subnational population projections for England: 2012-based. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/subnationalpopulationprojectionsforengland/2014-05-29 . 2014. Accessed May 2021.
Public Health England: Chronic kidney disease prevalence model. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/612303/ChronickidneydiseaseCKDprevalencemodelbriefing.pdf . 2014. Accessed May 2021.
Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–72.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305.
Article CAS PubMed Google Scholar
Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Primary care Clin Office Pract. 2008;35(2):329–44.
Article Google Scholar
World Health Organization. The global burden of kidney disease and the sustainable development goals. https://www.who.int/bulletin/volumes/96/6/17-206441/en/ . 2018. Accessed Apr 2021.
Pecly IMD, Azevedo RB, Muxfeldt ES, et al. COVID-19 and chronic kidney disease: a comprehensive review. J Bras Nefrol. 2021;43(3):383–99.
Tuot DS, Wong KK, Velasquez A, et al. CKD awareness in the general population: performance of CKD-specific questions. Kidney Med. 2019;1(2):43–50.
Article PubMed PubMed Central Google Scholar
Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17(3):225–36.
Levey AS, Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266.
Google Scholar
Levey AS, Eckardt K-U, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–100.
Darlington O, Dickerson C, Evans M, et al. Costs and healthcare resource use associated with risk of cardiovascular morbidity in patients with chronic kidney Disease: evidence from a systematic literature review. Adv Ther. 2021;38(2):994–1010.
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52.
Tonelli M, Muntner P, Lloyd A, et al. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154(1):12–21.
Levey AS, Tangri N, Stevens LA. Classification of chronic kidney disease: a step forward. Ann Intern Med. 2011;154(1):65–7.
Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1057–64.
Tuegel C, Bansal N. Heart failure in patients with kidney disease. Heart. 2017;103(23):1848–53.
Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81.
Van Der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–52.
Article PubMed CAS Google Scholar
Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–15.
Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–8.
Article CAS PubMed PubMed Central Google Scholar
Cherney DZ, Repetto E, Wheeler DC, et al. Impact of cardio-renal-metabolic comorbidities on cardiovascular outcomes and mortality in type 2 diabetes mellitus. Am J Nephrol. 2020;51(1):74–82.
Stevens P, O’Donoghue D, De Lusignan S, Van Vlymen J, Klebe B, Middleton R, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72(1):92–9.
Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27(suppl_3):73–80.
Saran R, Robinson B, Abbott KC, et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3):A7.
Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24(9):1478–83.
Smith DH, Gullion CM, Nichols G, Keith DS, Brown JB. Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol. 2004;15(5):1300–6.
Wang V, Vilme H, Maciejewski ML, Boulware LE. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol. 2016;36:319–30.
National Institute for Health Care Excellence. Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182 . 2014. Accessed Apr 2021.
National Institute for Health and Care Excellence. Guideline chronic kidney disease. https://www.nice.org.uk/guidance/gid-ng10118/documents/draft-guideline . 2021. Accessed June 2021.
National Institute for Health Care Excellence: Management of chronic kidney disease. https://pathways.nice.org.uk/pathways/chronic-kidney-disease . Accessed Apr 2021.
Chen I-R, Wang S-M, Liang C-C, et al. Association of walking with survival and RRT among patients with CKD stages 3–5. Clin J Am Soc Nephrol. 2014;9(7):1183–9.
Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25(2):399–406.
Hellberg M, Höglund P, Svensson P, Clyne N. Randomized controlled trial of exercise in CKD—the RENEXC study. Kidney Int Rep. 2019;4(7):963–76.
MacKinnon HJ, Wilkinson TJ, Clarke AL, et al. The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review. Therap Adv Chronic Dis. 2018;9(11):209–26.
Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol. 2009;4(12):1901–6.
Clarke AL, Zaccardi F, Gould DW, et al. Association of self-reported physical function with survival in patients with chronic kidney disease. Clin Kidney J. 2019;12(1):122–8.
Chauveau P, Aparicio M, Bellizzi V, et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant. 2018;33(5):725–35.
Rhee CM, Ahmadi SF, Kovesdy CP, Kalantar-Zadeh K. Low-protein diet for conservative management of chronic kidney disease: a systematic review and meta-analysis of controlled trials. J Cachexia Sarcopenia Muscle. 2018;9(2):235–45.
Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85(6):1303–9.
National Institute for Health and Care Excellence. Cardiovasvcular disease: risk assessment and reduction, including lipid modification. https://www.nice.org.uk/guidance/cg181/resources/cardiovascular-disease-risk-assessment-and-reduction-including-lipid-modification-pdf-35109807660997 . 2014. Accessed Apr 2021.
de Boer IH, Caramori ML, Chan JC, et al. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4):S1–115.
National Collaborating Centre for Chronic Conditions. Chronic kidney disease: national clinical guideline for early identification and management in adults in primary and secondary care. London: Royal College of Physicians; 2008.
Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease: a meta-analysis of patient-level data. Ann Intern Med. 2001;135(2):73–87.
Evans M, Bain SC, Hogan S, Bilous RW. Irbesartan delays progression of nephropathy as measured by estimated glomerular filtration rate: post hoc analysis of the Irbesartan Diabetic Nephropathy Trial. Nephrol Dial Transplant. 2012;27(6):2255–63.
Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–41.
Wai H-T, Meah N, Katira R. Novel potassium binders: a clinical update. https://bjcardio.co.uk/2021/04/novel-potassium-binders-a-clinical-update-3/ . Accessed June 2021.
National Institute for Health and Care Excellence. Sodium zirconium cyclosilicate for treating hyperkalaemia. Technology appraisal guidance TA599. https://www.nice.org.uk/guidance/TA599 (2019). Accessed April 2021.
National Institute for Health and Care Excellence. Patiromer for treating hyperkalaemia. https://www.nice.org.uk/guidance/ta623/chapter/1-Recommendations . 2020. Accessed May 2021.
Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29.
Zinman B, Wanner C, Lachin J. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
Wheeler DC, Stefansson BV, Batiushin M, et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant. 2020;35(10):1700–11.
Heerspink HJ, Sjöström CD, Jongs N, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J. 2021;42(13):1216–27.
McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
U.S. Food & Drug Administration (FDA). FDA approves treatment for chronic kidney disease. https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease . 2021. Accessed May 2021.
Janssen Pharmaceutical of Johnson & Johnson. U.S. FDA approves INVOKANA® (canagliflozin) to treat diabetic kidney disease (DKD) and reduce the risk of hospitalization for heart failure in patients with type 2 diabetes (T2D) and DKD. https://www.prnewswire.com/news-releases/us-fda-approves-invokana-canagliflozin-to-treat-diabetic-kidney-disease-dkd-and-reduce-the-risk-of-hospitalization-for-heart-failure-in-patients-with-type-2-diabetes-t2d-and-dkd-300927348.html . 2019. Accessed May 2021.
European Medicines Agency. Forxiga: Summary of opinion (post authorisation). https://www.ema.europa.eu/en/medicines/human/EPAR/forxiga . 2021. Accessed May 2021.
Download references
Acknowledgements
This manuscript was supported by a grant from AstraZeneca UK Ltd. in respect of medical writing and publication costs (the journal’s Rapid Service and Open Access Fees). AstraZeneca has not influenced the content of the publication, and has reviewed this document for factual accuracy only.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
All authors have been involved in design of this review. Marc Evans, Ruth D. Lewis, and Angharad R. Morgan produced the primary manuscript. All authors have contributed to the drafting and revision of the manuscript and have approved the final version for publication. Marc Evans is responsible for the integrity of the work as a whole.
Disclosures
Marc Evans reports honoraria from AstraZeneca, Novo Nordisk, Takeda and NAPP, and research support from Novo Nordisk outside the submitted work. Ruth D. Lewis and Angharad R Morgan are employees of Health Economics and Outcomes Research Ltd., Cardiff, UK who received fees from AstraZeneca in relation to this study. Martin B. Whyte reports investigator-led research grants from Sanofi, Eli Lilly and AstraZeneca and personal fees from AstraZeneca, Boehringer Ingelheim and MSD outside the submitted work. Wasim Hanif reports grants and personal fees from AstraZeneca, grants and personal fees from Boerhinger Inglhiem, grants and personal fees from NAPP, from MSD, outside the submitted work. Stephen C. Bain reports personal fees and other from Abbott, personal fees and other from AstraZeneca, personal fees and other from Boehringer Ingelheim, personal fees and other from Eli Lilly, personal fees and other from Merck Sharp & Dohme, personal fees and other from Novo Nordisk, personal fees and other from Sanofi-aventis, other from Cardiff University, other from Doctors.net, other from Elsevier, other from Onmedica, other from Omnia-Med, other from Medscape, other from All-Wales Medicines Strategy Group, other from National Institute for Health and Care Excellence (NICE) UK, and other from Glycosmedia, outside the submitted work. PAK reports personal fees for lecturing from AstraZeneca, Boehringer Inglhiem, NAPP, MundiPharma and Novo Nordisk outside the submitted work. Sarah Davies has received honorarium from AstraZeneca, Boehringer Ingelheim, Lilly, Novo Nordisk, Takeda, MSD, NAPP, Bayer and Roche for attending and participating in educational events and advisory boards, outside the submitted work. Umesh Dashora reports personal fees from AstraZeneca, NAPP, Sanofi, Boehringer Inglhiem, Lilly and Novo Nordisk, outside the submitted work. Zaheer Yousef reports personal fees from AstraZeneca, personal fees from Lilly, personal fees from Boehringer Ingelheim and personal fees from Novartis outside the submitted work. Dipesh C. Patel reports personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, non-financial support from NAPP, personal fees from Novo Nordisk, personal fees from MSD, personal fees and non-financial support from Sanofi outside the submitted work. In addition, DCP is an executive committee member of the Association of British Clinical Diabetologists and member of the CaReMe UK group. W. David Strain holds research grants from Bayer, Novo Nordisk and Novartis and has received speaker honoraria from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, NAPP, Novartis, Novo Nordisk and Takeda. WDS is supported by the NIHR Exeter Clinical Research Facility and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and affiliations.
Diabetes Resource Centre, University Hospital Llandough, Penlan Road, Llandough, Cardiff, CF64 2XX, UK
Health Economics and Outcomes Research Ltd, Cardiff, UK
Ruth D. Lewis & Angharad R. Morgan
Faculty of Health and Medical Sciences, University of Surrey, Guildford, UK
Martin B. Whyte
University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK
Wasim Hanif
Diabetes Research Unit, Swansea University Medical School, Swansea, UK
Stephen C. Bain
Woodlands Medical Centre, Cardiff, UK
Sarah Davies
East Sussex Healthcare NHS Trust, Eastbourne, UK
Umesh Dashora
Department of Cardiology, University Hospital of Wales and Cardiff University, Cardiff, UK
Zaheer Yousef
Division of Medicine, Department of Diabetes, University College London, Royal Free Campus, London, UK
Dipesh C. Patel
Diabetes and Vascular Research Centre, University of Exeter Medical School, Exeter, UK
W. David Strain
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Marc Evans .
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Evans, M., Lewis, R.D., Morgan, A.R. et al. A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv Ther 39 , 33–43 (2022). https://doi.org/10.1007/s12325-021-01927-z
Download citation
Received : 24 August 2021
Accepted : 15 September 2021
Published : 05 November 2021
Issue Date : January 2022
DOI : https://doi.org/10.1007/s12325-021-01927-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cardiovascular disease
- Chronic kidney disease
- Diabetic kidney disease
- Sodium–glucose co-transporter 2 inhibitors
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 23 August 2022
Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review
- John R. Hurst 1 ,
- MeiLan K. Han 2 ,
- Barinder Singh 3 ,
- Sakshi Sharma 4 ,
- Gagandeep Kaur 3 ,
- Enrico de Nigris 5 ,
- Ulf Holmgren 6 &
- Mohd Kashif Siddiqui 3
Respiratory Research volume 23 , Article number: 213 ( 2022 ) Cite this article
6365 Accesses
20 Citations
42 Altmetric
Metrics details
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. COPD exacerbations are associated with a worsening of lung function, increased disease burden, and mortality, and, therefore, preventing their occurrence is an important goal of COPD management. This review was conducted to identify the evidence base regarding risk factors and predictors of moderate-to-severe exacerbations in patients with COPD.
A literature review was performed in Embase, MEDLINE, MEDLINE In-Process, and the Cochrane Central Register of Controlled Trials (CENTRAL). Searches were conducted from January 2015 to July 2019. Eligible publications were peer-reviewed journal articles, published in English, that reported risk factors or predictors for the occurrence of moderate-to-severe exacerbations in adults age ≥ 40 years with a diagnosis of COPD.
The literature review identified 5112 references, of which 113 publications (reporting results for 76 studies) met the eligibility criteria and were included in the review. Among the 76 studies included, 61 were observational and 15 were randomized controlled clinical trials. Exacerbation history was the strongest predictor of future exacerbations, with 34 studies reporting a significant association between history of exacerbations and risk of future moderate or severe exacerbations. Other significant risk factors identified in multiple studies included disease severity or bronchodilator reversibility (39 studies), comorbidities (34 studies), higher symptom burden (17 studies), and higher blood eosinophil count (16 studies).
Conclusions
This systematic literature review identified several demographic and clinical characteristics that predict the future risk of COPD exacerbations. Prior exacerbation history was confirmed as the most important predictor of future exacerbations. These prognostic factors may help clinicians identify patients at high risk of exacerbations, which are a major driver of the global burden of COPD, including morbidity and mortality.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide [ 1 ]. Based upon disability-adjusted life-years, COPD ranked sixth out of 369 causes of global disease burden in 2019 [ 2 ]. COPD exacerbations are associated with a worsening of lung function, and increased disease burden and mortality (of those patients hospitalized for the first time with an exacerbation, > 20% die within 1 year of being discharged) [ 3 ]. Furthermore, patients with COPD consider exacerbations or hospitalization due to exacerbations to be the most important disease outcome, having a large impact on their lives [ 4 ]. Therefore, reducing the future risk of COPD exacerbations is a key goal of COPD management [ 5 ].
Being able to predict the level of risk for each patient allows clinicians to adapt treatment and patients to adjust their lifestyle (e.g., through a smoking cessation program) to prevent exacerbations [ 3 ]. As such, identifying high-risk patients using measurable risk factors and predictors that correlate with exacerbations is critical to reduce the burden of disease and prevent a cycle of decline encompassing irreversible lung damage, worsening quality of life (QoL), increasing disease burden, high healthcare costs, and early death.
Prior history of exacerbations is generally thought to be the best predictor of future exacerbations; however, there is a growing body of evidence suggesting other demographic and clinical characteristics, including symptom burden, airflow obstruction, comorbidities, and inflammatory biomarkers, also influence risk [ 6 , 7 , 8 , 9 ]. For example, in the prospective ECLIPSE observational study, the likelihood of patients experiencing an exacerbation within 1 year of follow-up increased significantly depending upon several factors, including prior exacerbation history, forced expiratory volume in 1 s (FEV 1 ), St. George’s Respiratory Questionnaire (SGRQ) score, gastroesophageal reflux, and white blood cell count [ 9 ].
Many studies have assessed predictors of COPD exacerbations across a variety of countries and patient populations. This systematic literature review (SLR) was conducted to identify and compile the evidence base regarding risk factors and predictors of moderate-to-severe exacerbations in patients with COPD.
- Systematic literature review
A comprehensive search strategy was designed to identify English-language studies published in peer-reviewed journals providing data on risk factors or predictors of moderate or severe exacerbations in adults aged ≥ 40 years with a diagnosis of COPD (sample size ≥ 100). The protocol is summarized in Table 1 and the search strategy is listed in Additional file 1 : Table S1. Key biomedical electronic literature databases were searched from January 2015 until July 2019. Other sources were identified via bibliographic searching of relevant systematic reviews.
Study selection process
Implementation and reporting followed the recommendations and standards of the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement [ 10 ]. An independent reviewer conducted the first screening based on titles and abstracts, and a second reviewer performed a quality check of the excluded evidence. A single independent reviewer also conducted the second screening based on full-text articles, with a quality check of excluded evidence performed by a second reviewer. Likewise, data tables of the included studies were generated by one reviewer, and another reviewer performed a quality check of extracted data. Where more than one publication was identified describing a single study or trial, data were compiled into a single entry in the data-extraction table to avoid double counting of patients and studies. One publication was designated as the ‘primary publication’ for the purposes of the SLR, based on the following criteria: most recently published evidence and/or the article that presented the majority of data (e.g., journal articles were preferred over conference abstracts; articles that reported results for the full population were preferred over later articles providing results of subpopulations). Other publications reporting results from the same study were designated as ‘linked publications’; any additional data in the linked publications that were not included in the primary publication were captured in the SLR. Conference abstracts were excluded from the SLR unless they were a ‘linked publication.’
Included studies
A total of 5112 references (Fig. 1 ) were identified from the database searches. In total, 76 studies from 113 publications were included in the review. Primary publications and ‘linked publications’ for each study are detailed in Additional file 1 : Table S2, and study characteristics are shown in Additional file 1 : Table S3. The studies included clinical trials, registry studies, cross-sectional studies, cohort studies, database studies, and case–control studies. All 76 included studies were published in peer-reviewed journals. Regarding study design, 61 of the studies were observational (34 retrospective observational studies, 19 prospective observational studies, four cross-sectional studies, two studies with both retrospective and prospective cohort data, one case–control study, and one with cross-sectional and longitudinal data) and 15 were randomized controlled clinical trials.
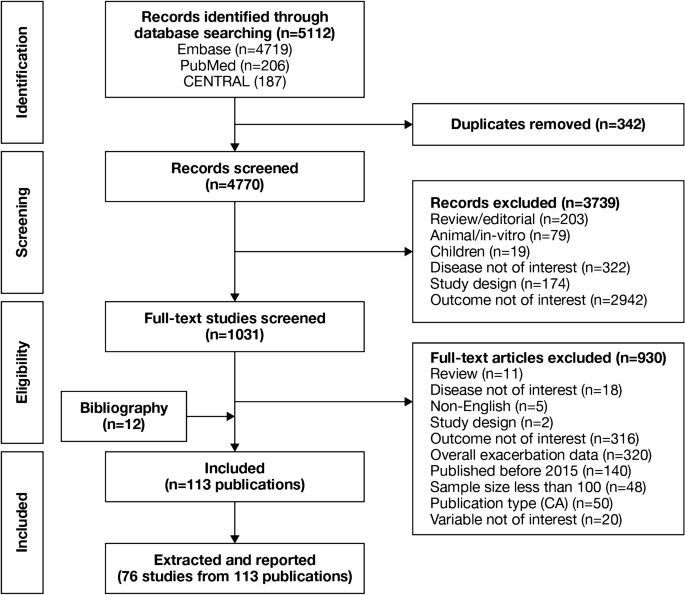
PRISMA flow diagram of studies through the systematic review process. CA conference abstract, CENTRAL Cochrane Central Register of Controlled Trials, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Of the 76 studies, 16 were conducted in North America (13 studies in the USA, two in Canada, and one in Mexico); 26 were conducted in Europe (seven studies in Spain, four in the UK, three in Denmark, two studies each in Bulgaria, the Netherlands, and Switzerland, and one study each in Sweden, Serbia, Portugal, Greece, Germany, and France) and 17 were conducted in Asia (six studies in South Korea, four in China, three in Taiwan, two in Japan, and one study each in Singapore and Israel). One study each was conducted in Turkey and Australia. Fifteen studies were conducted across multiple countries.
The majority of the studies (n = 54) were conducted in a multicenter setting, while 22 studies were conducted in a single-center setting. The sample size among the included studies varied from 118 to 339,389 patients.
Patient characteristics
A total of 75 studies reported patient characteristics (Additional file 1 : Table S4). The mean age was reported in 65 studies and ranged from 58.0 to 75.2 years. The proportion of male patients ranged from 39.7 to 97.6%. The majority of included studies (85.3%) had a higher proportion of males than females.
Exacerbation history (as defined per each study) was reported in 18 of 76 included studies. The proportion of patients with no prior exacerbation was reported in ten studies (range, 0.1–79.5% of patients), one or fewer prior exacerbation in ten studies (range, 46–100%), one or more prior exacerbation in eight studies (range, 18.4–100%), and two or more prior exacerbations in 12 studies (range, 6.1–55.0%).
Prognostic factors of exacerbations
A summary of the risk factors and predictors reported across the included studies is provided in Tables 2 and 3 . The overall findings of the SLR are summarized in Figs. 2 and 3 .
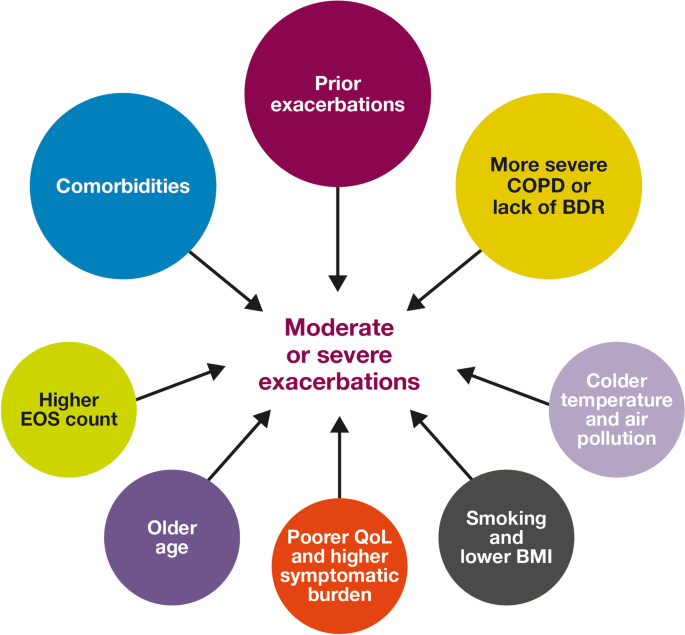
Risk factors for moderate-to-severe exacerbations in patients with COPD. Factors with > 30 supporting studies shown as large circles; factors with ≤ 30 supporting studies shown as small circles and should be interpreted cautiously. BDR bronchodilator reversibility, BMI body mass index, COPD chronic obstructive pulmonary disease, EOS eosinophil, QoL quality of life
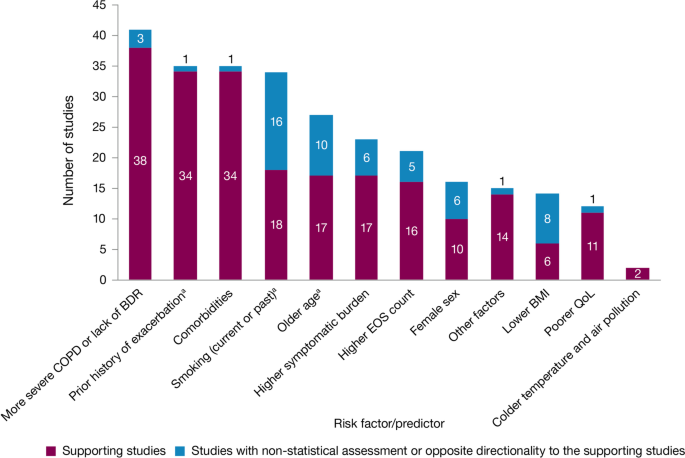
Summary of risk factors for exacerbation events. a Treatment impact studies removed. BDR bronchodilator reversibility, BMI body mass index, COPD chronic obstructive pulmonary disease, EOS eosinophil, QoL quality of life
Exacerbation history within the past 12 months was the strongest predictor of future exacerbations. Across the studies assessing this predictor, 34 out of 35 studies (97.1%) reported a significant association between history of exacerbations and risk of future moderate-to-severe exacerbations (Table 3 ). Specifically, two or more exacerbations in the previous year or at least one hospitalization for COPD in the previous year were identified as reliable predictors of future moderate or severe exacerbations. Even one moderate exacerbation increased the risk of a future exacerbation, with the risk increasing further with each subsequent exacerbation (Fig. 4 ). A severe exacerbation was also found to increase the risk of subsequent exacerbation and hospitalization (Fig. 5 ). Patients experiencing one or more severe exacerbations were more likely to experience further severe exacerbations than moderate exacerbations [ 11 , 12 ]. In contrast, patients with a history of one or more moderate exacerbations were more likely to experience further moderate exacerbations than severe exacerbations [ 11 , 12 ].
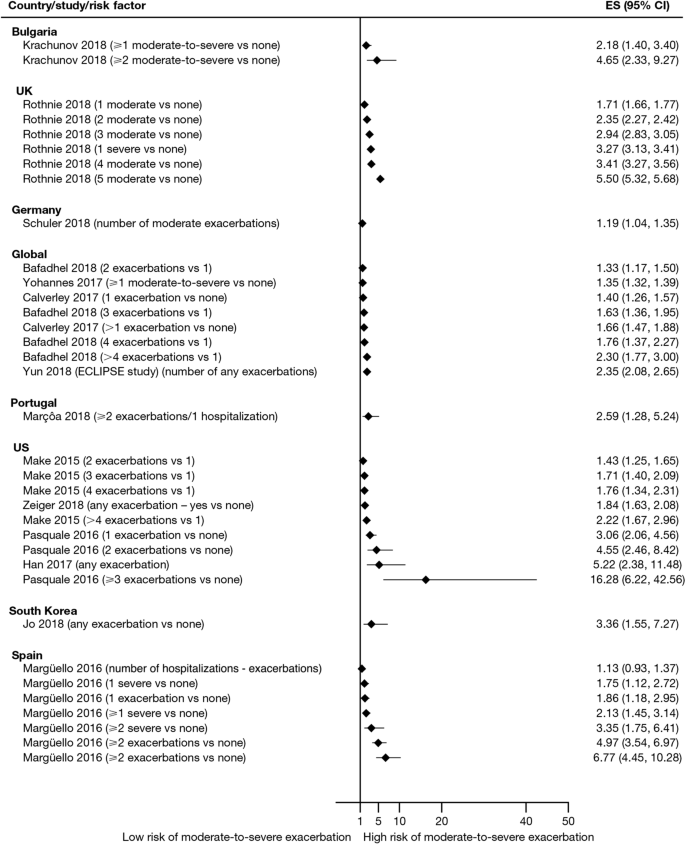
Exacerbation history as a risk factor for moderate-to-severe exacerbations. Yun 2018 included two studies; the study from which data were extracted (COPDGene or ECLIPSE) is listed in parentheses. CI confidence interval, ES effect size
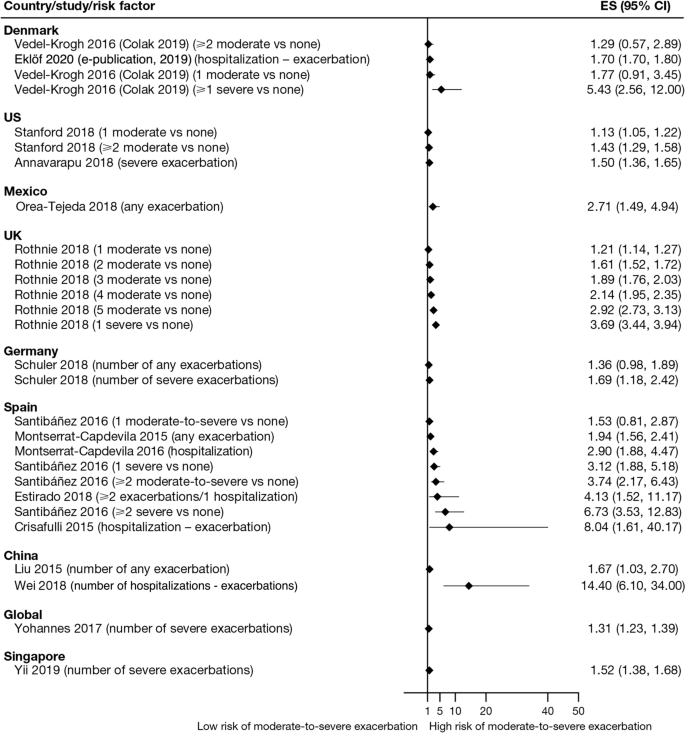
Exacerbation history as a risk factor for severe exacerbations. Where data have been extracted from a linked publication rather than the primary publication, the linked publication is listed in parentheses. CI confidence interval, ES , effect size
Overall, 35 studies assessed the association of comorbidities with the risk of exacerbation. All studies except one (97.1%) reported a positive association between comorbidities and the occurrence of moderate-to-severe exacerbations (Table 3 ). In addition to the presence of any comorbidity, specific comorbidities that were found to significantly increase the risk of moderate-to-severe exacerbations included anxiety and depression, cardiovascular comorbidities, gastroesophageal reflux disease/dyspepsia, and respiratory comorbidities (Fig. 6 ). Comorbidities that were significant risk factors for severe exacerbations included cardiovascular, musculoskeletal, and respiratory comorbidities, diabetes, and malignancy (Fig. 7 ). Overall, the strongest association between comorbidities and COPD readmissions in the emergency department was with cardiovascular disease. The degree of risk for both moderate-to-severe and severe exacerbations also increased with the number of comorbidities. A Dutch cohort study found that 88% of patients with COPD had at least one comorbidity, with hypertension (35%) and coronary heart disease (19%) being the most prevalent. In this cohort, the comorbidities with the greatest risk of frequent exacerbations were pulmonary cancer (odds ratio [OR] 1.85) and heart failure (OR 1.72) [ 7 ].
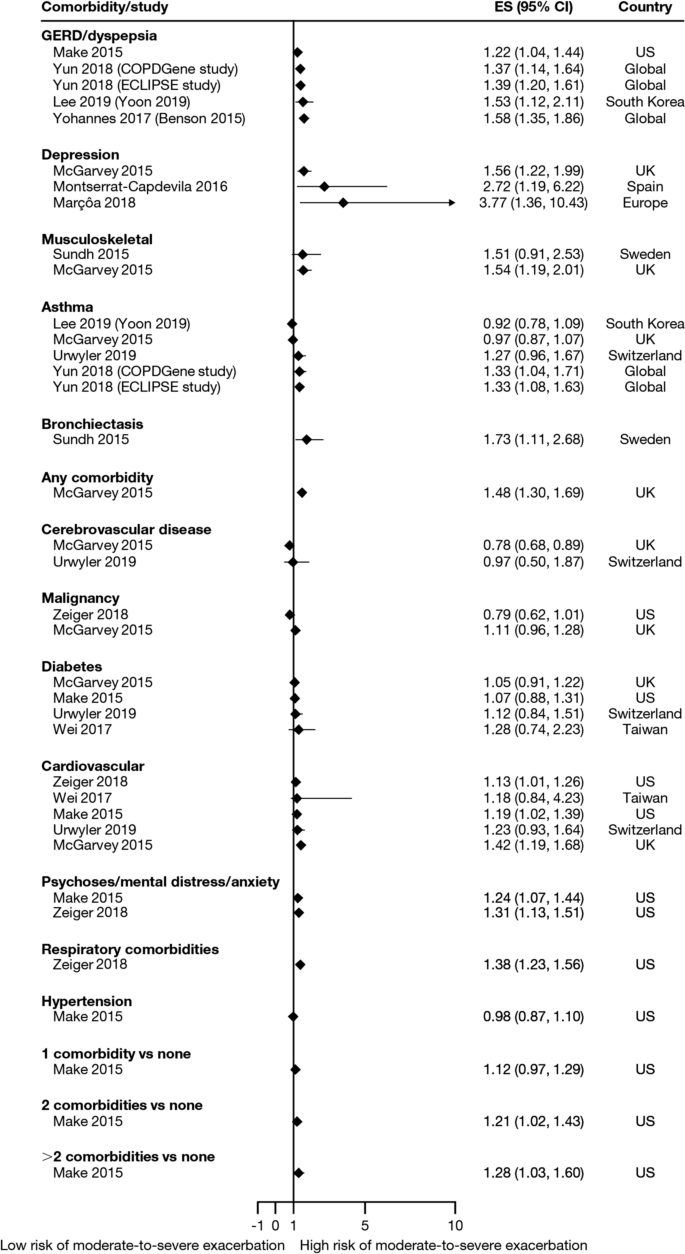
Comorbidities as risk factors for moderate-to-severe exacerbations. Yun 2018 included two studies; the study from which data were extracted (COPDGene or ECLIPSE) is listed in parentheses. Where data have been extracted from a linked publication rather than the primary publication, the linked publication is listed in parentheses. CI confidence interval, ES effect size, GERD gastroesophageal disease
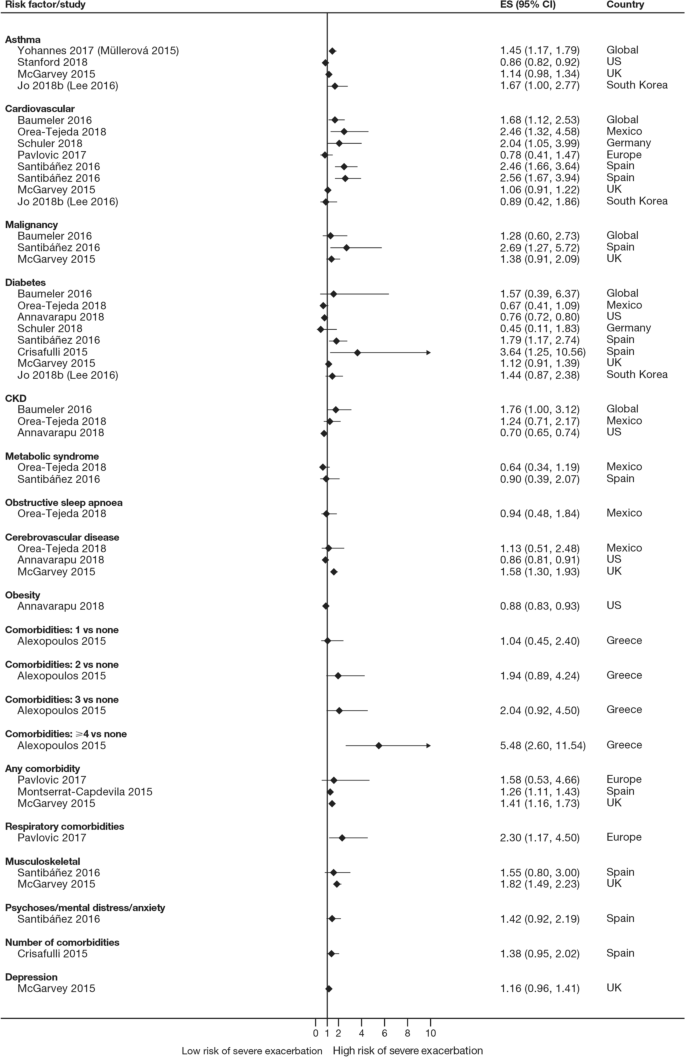
Comorbidities as risk factors for severe exacerbations. Where data have been extracted from a linked publication rather than the primary publication, the linked publication is listed in parentheses. CI confidence interval, CKD , chronic kidney disease, ES effect size
The majority of studies assessing disease severity or bronchodilator reversibility (39/41; 95.1%) indicated a significant positive relation between risk of future exacerbations and greater disease severity, as assessed by greater lung function impairment (in terms of lower FEV 1 , FEV 1 /forced vital capacity ratio, or forced expiratory flow [25–75]/forced vital capacity ratio) or more severe Global Initiative for Chronic Obstructive Lung Disease (GOLD) class A − D, and a positive relationship between risk of future exacerbations and lack of bronchodilator reversibility (Table 3 , Figs. 8 and 9 ).
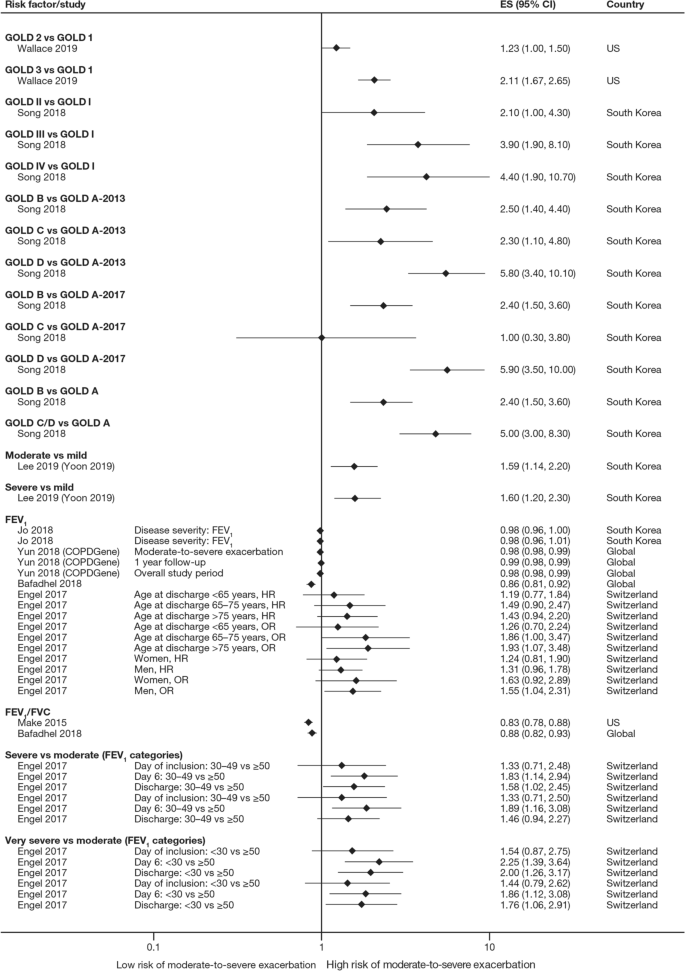
Disease severity as a risk factor for moderate-to-severe exacerbations. Yun 2018 included two studies; the study from which data were extracted (COPDGene or ECLIPSE) is listed in parentheses. Where data have been extracted from a linked publication rather than the primary publication, the linked publication is listed in parentheses. CI confidence interval, ES effect size, FEV 1 f orced expiratory volume in 1 s, FVC , forced vital capacity, GOLD Global Initiative for Obstructive Lung Disease, HR hazard ratio, OR odds ratio
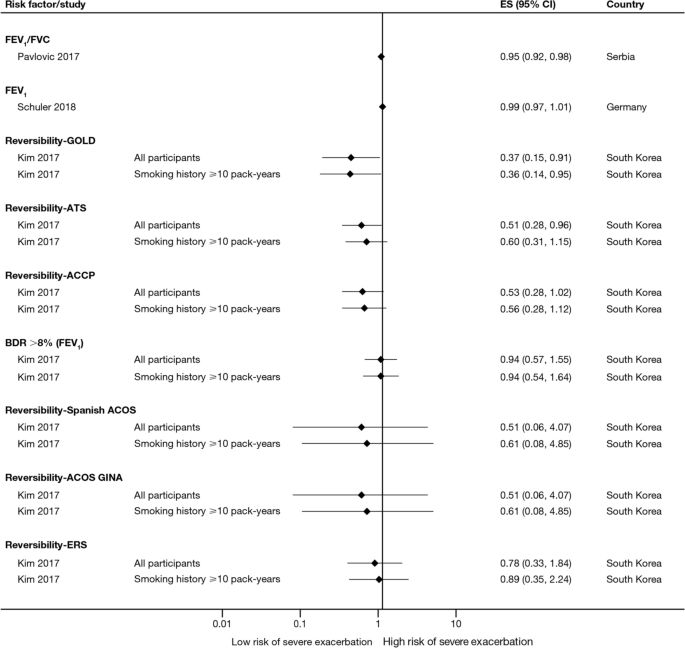
Disease severity and BDR as risk factors for severe exacerbations. ACCP American College of Chest Physicians, ACOS Asthma-COPD overlap syndrome, ATS American Thoracic Society, BDR bronchodilator reversibility, CI confidence interval, ERS European Respiratory Society, ES effect size, FEV 1 forced expiratory volume in 1 s, FVC forced vital capacity, GINA Global Initiative for Asthma, GOLD Global Initiative for Obstructive Lung Disease
Of 21 studies assessing the relationship between blood eosinophil count and exacerbations (Table 3 ), 16 reported estimates for the risk of moderate or severe exacerbations by eosinophil count. A positive association was observed between higher eosinophil count and a higher risk of moderate or severe exacerbations, particularly in patients not treated with an inhaled corticosteroid (ICS); however, five studies reported a significant positive association irrespective of intervention effects. The risk of moderate-to-severe exacerbations was observed to be positively associated with various definitions of higher eosinophil levels (absolute counts: ≥ 200, ≥ 300, ≥ 340, ≥ 400, and ≥ 500 cells/mm 3 ; % of blood eosinophil count: ≥ 2%, ≥ 3%, ≥ 4%, and ≥ 5%). Of note, one study found reduced efficacy of ICS in lowering moderate-to-severe exacerbation rates for current smokers versus former smokers at all eosinophil levels [ 13 ].
Of 12 studies assessing QoL scales, 11 (91.7%) studies reported a significant association between the worsening of QoL scores and the risk of future exacerbations (Table 3 ). Baseline SGRQ [ 14 , 15 ], Center for Epidemiologic Studies Depression Scale (for which increased scores may indicate impaired QoL) [ 16 ], and Clinical COPD Questionnaire [ 17 , 18 ] scores were found to be associated with future risk of moderate and/or severe COPD exacerbations. For symptom scores, six out of eight studies assessing the association between moderate-to-severe or severe exacerbations with COPD Assessment Test (CAT) scores reported a significant and positive relationship. Furthermore, the risk of moderate-to-severe exacerbations was found to be significantly higher in patients with higher CAT scores (≥ 10) [ 15 , 19 , 20 , 21 ], with one study demonstrating that a CAT score of 15 increased predictive ability for exacerbations compared with a score of 10 or more [ 18 ]. Among 15 studies that assessed the association of modified Medical Research Council (mMRC) scores with the risk of moderate-to-severe or severe exacerbation, 11 found that the risk of moderate-to-severe or severe exacerbations was significantly associated with higher mMRC scores (≥ 2) versus lower scores. Furthermore, morning and night symptoms (measured by Clinical COPD Questionnaire) were associated with poor health status and predicted future exacerbations [ 17 ].
Of 36 studies reporting the relationship between smoking status and moderate-to-severe or severe exacerbations, 22 studies (61.1%) reported a significant positive association (Table 3 ). Passive smoking was also significantly associated with an increased risk of severe exacerbations (OR 1.49) [ 20 ]. Of note, three studies reported a significantly lower rate of moderate-to-severe exacerbations in current smokers compared with former smokers [ 22 , 23 , 24 ].
A total of 14 studies assessed the association of body mass index (BMI) with the occurrence of frequent moderate-to-severe exacerbations in patients with COPD. Six out of 14 studies (42.9%) reported a significant negative association between exacerbations and BMI (Table 3 ). The risk of moderate and/or severe COPD exacerbations was highest among underweight patients compared with normal and overweight patients [ 23 , 25 , 26 , 27 , 28 ].
In the 29 studies reporting an association between age and moderate or severe exacerbations, more than half found an association of older age with an increased risk of moderate-to-severe exacerbations (58.6%; Table 3 ). Four of these studies noted a significant increase in the risk of moderate-to-severe or severe exacerbations for every 10-year increase in age [ 25 , 26 , 29 , 30 ]. However, 12 studies reported no significant association between age and moderate-to-severe or severe exacerbation risk.
Sixteen out of 33 studies investigating the impact of sex on exacerbation risk found a significant association (48.5%; Table 3 ). Among these, ten studies reported that female sex was associated with an increased risk of moderate-to-severe exacerbations, while six studies showed a higher exacerbation risk in males compared with females. There was some variation in findings by geographic location and exacerbation severity (Additional file 2 : Figs. S1 and S2). Notably, when assessing the risk of severe exacerbations, more studies found an association with male sex compared with female sex (6/13 studies vs 1/13 studies, respectively).
Both studies evaluating associations between exacerbations and environmental factors reported that colder temperature and exposure to major air pollution (NO 2 , O 3 , CO, and/or particulate matter ≤ 10 μm in diameter) increased hospital admissions due to severe exacerbations and moderate-to-severe exacerbation rates [ 31 , 32 ].
Four studies assessed the association of 6-min walk distance with the occurrence of frequent moderate-to-severe exacerbations (Table 3 ). One study (25.0%) found that shorter 6-min walk distance (representing low physical activity) was significantly associated with a shortened time to severe exacerbation, but the effect size was small (hazard ratio 0.99) [ 33 ].
Five out of six studies assessing the relationship between race or ethnicity and exacerbation risk reported significant associations (Table 3 ). Additionally, one study reported an association between geographic location in the US and exacerbations, with living in the Northeast region being the strongest predictor of severe COPD exacerbations versus living in the Midwest and South regions [ 34 ].
Overall, seven studies assessed the association of biomarkers with risk of future exacerbations (Table 3 ), with the majority identifying significant associations between inflammatory biomarkers and increased exacerbation risk, including higher C-reactive protein levels [ 8 , 35 ], fibrinogen levels [ 8 , 30 ], and white blood cell count [ 8 , 15 , 16 ].
This SLR has identified several demographic and clinical characteristics that predict the future risk of COPD exacerbations. Key factors associated with an increased risk of future moderate-to-severe exacerbations included a history of prior exacerbations, worse disease severity and bronchodilator reversibility, the presence of comorbidities, a higher eosinophil count, and older age (Fig. 2 ). These prognostic factors may help clinicians identify patients at high risk of exacerbations, which are a major driver of the burden of COPD, including morbidity and mortality [ 36 ].
Findings from this review summarize the existing evidence, validating the previously published literature [ 6 , 9 , 23 ] and suggesting that the best predictor of future exacerbations is a history of exacerbations in the prior year [ 8 , 11 , 12 , 13 , 14 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 26 , 29 , 34 , 35 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 ]. In addition, the effect size generally increased with the number of prior exacerbations, with a stronger effect observed with prior severe versus moderate exacerbations. This effect was observed across regions, including in Europe and North America, and in several global studies. This relationship represents a vicious circle, whereby one exacerbation predisposes a patient to experience future exacerbations and leading to an ever-increasing disease burden, and emphasizes the importance of preventing the first exacerbation event through early, proactive exacerbation prevention. The finding that prior exacerbations tended to be associated with future exacerbations of the same severity suggests that the severity of the underlying disease may influence exacerbation severity. However, the validity of the traditional classification of exacerbation severity has recently been challenged [ 61 ], and further work is required to understand relationships with objective assessments of exacerbation severity.
In addition to exacerbation history, disease severity and bronchodilator reversibility were also strong predictors for future exacerbations [ 8 , 14 , 16 , 18 , 19 , 20 , 22 , 23 , 24 , 26 , 28 , 29 , 33 , 37 , 40 , 43 , 44 , 45 , 46 , 48 , 50 , 51 , 52 , 56 , 59 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 ]. The association with disease severity was noted in studies that used GOLD disease stages 1–4 and those that used FEV 1 percent predicted and other lung function assessments as continuous variables. Again, this risk factor is self-perpetuating, as evidence shows that even a single moderate or severe exacerbation may almost double the rate of lung function decline [ 79 ]. Accordingly, disease severity and exacerbation history may be correlated. Margüello et al. concluded that the severity of COPD could be associated with a higher risk of exacerbations, but this effect was partly determined by the exacerbations suffered in the previous year [ 23 ]. It should be noted that FEV 1 is not recommended by GOLD for use as a predictor of exacerbation risk or mortality alone due to insufficient precision when used at the individual patient level [ 5 ].
Another factor that should be considered when assessing individual exacerbation risk is the presence of comorbidities [ 7 , 14 , 16 , 18 , 19 , 20 , 21 , 22 , 24 , 25 , 26 , 27 , 28 , 30 , 33 , 34 , 35 , 40 , 41 , 44 , 45 , 46 , 47 , 48 , 51 , 52 , 53 , 54 , 56 , 58 , 59 , 63 , 64 , 73 , 74 , 76 , 77 , 80 , 81 , 82 , 83 , 84 , 85 ]. Comorbidities are common in COPD, in part due to common risk factors (e.g., age, smoking, lifestyle factors) that also increase the risk of other chronic diseases [ 7 ]. Significant associations were observed between exacerbation risk and comorbidities, such as anxiety and depression, cardiovascular disease, diabetes, and respiratory comorbidities. As with prior exacerbations, the strength of the association increased with the number of comorbidities. Some comorbidities that were found to be associated with COPD exacerbations share a common biological mechanism of systemic inflammation, such as cardiovascular disease, diabetes, and depression [ 86 ]. Furthermore, other respiratory comorbidities, including asthma and bronchiectasis, involve inflammation of the airways [ 87 ]. In these patients, optimal management of comorbidities may reduce the risk of future COPD exacerbations (and improve QoL), although further research is needed to confirm the efficacy of this approach to exacerbation prevention. As cardiovascular conditions, including hypertension and coronary heart disease, are the most common comorbidities in people with COPD [ 7 ], reducing cardiovascular risk may be a key goal in reducing the occurrence of exacerbations. For other comorbidities, the mechanism for the association with exacerbation risk may be related to non-biological factors. For example, in depression, it has been suggested that the mechanism may relate to greater sensitivity to symptom changes or more frequent physician visits [ 88 ].
There is now a growing body of evidence reporting the relationship between blood eosinophil count and exacerbation risk [ 8 , 13 , 14 , 20 , 37 , 48 , 52 , 56 , 59 , 60 , 62 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 ]. Data from many large clinical trials (SUNSET [ 89 ], FLAME [ 96 ], WISDOM [ 98 ], IMPACT [ 13 ], TRISTAN [ 99 ], INSPIRE [ 99 ], KRONOS [ 91 ], TRIBUTE [ 48 ], TRILOGY [ 52 ], TRINITY [ 56 ]) have also shown relationships between treatment, eosinophil count, and exacerbation rates. Evidence shows that eosinophil count, along with other effect modifiers (e.g., exacerbation history), can be used to predict reductions in exacerbations with ICS treatment. Identifying patients most likely to respond to ICS should contribute to personalized medicine approaches to treat COPD. One challenge in drawing a strong conclusion from eosinophil counts is the choice of a cut-off value, with a variety of absolute and percentage values observed to be positively associated with the risk of moderate-to-severe exacerbations. The use of absolute counts may be more practical, as these are not affected by variations in other immune cell numbers; however, there is a lack of consensus on this point [ 100 ].
Across the studies examined, associations between sex and the risk of moderate and/or severe exacerbations were variable [ 14 , 16 , 18 , 20 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 37 , 40 , 42 , 44 , 45 , 46 , 47 , 48 , 51 , 52 , 56 , 58 , 59 , 63 , 73 , 74 , 77 , 80 , 83 , 84 , 85 ]. A greater number of studies showed an increased risk of exacerbations in females compared with males. In contrast, some studies failed to detect a relationship, suggesting that country-specific or cultural factors may play a role. A majority of the included studies evaluated more male patients than female patients; to further elucidate the relationship between sex and exacerbations, more studies in female patients are warranted. Over half of the studies that assessed the relationship between age and exacerbation risk found an association between increasing age and increasing risk of moderate-to-severe COPD exacerbations [ 14 , 16 , 18 , 20 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 33 , 40 , 42 , 44 , 45 , 47 , 51 , 52 , 54 , 56 , 63 , 73 , 74 , 77 , 80 , 83 , 85 ].
Our findings also suggested that patients with low BMI have greater risk of moderate and/or severe exacerbations. The mechanism underlying this increased risk in underweight patients is poorly understood; however, loss of lean body mass in patients with COPD may be related to ongoing systemic inflammation that impacts skeletal muscle mass [ 101 , 102 , 103 ].
A limitation of this SLR, that may have resulted in some studies with valid results being missed, was the exclusion of non-English-language studies and the limitation by date; however, the search strategy was otherwise broad, resulting in the review of a large number of studies. The majority of studies captured in this SLR were from Europe, North America, and Asia. The findings may therefore be less generalizable to patients in other regions, such as Africa or South America. Given that one study reported an association between geographic location within different regions of the US and exacerbations [ 34 ], it is plausible that risk of exacerbations may be impacted by global location. As no formal meta-analysis was planned, the assessments are based on a qualitative synthesis of studies. A majority of the included studies looked at exposures of certain factors (e.g., history of exacerbations) at baseline; however, some of these factors change over time, calling into question whether a more sophisticated statistical analysis should have been conducted in some cases to consider time-varying covariates. Our results can only inform on associations, not causation, and there are likely bidirectional relationships between many factors and exacerbation risk (e.g., health status). Finally, while our review of the literature captured a large number of prognostic factors, other variables such as genetic factors, lung microbiome composition, and changes in therapy over time have not been widely studied to date, but might also influence exacerbation frequency [ 104 ]. Further research is needed to assess the contribution of these factors to exacerbation risk.
This SLR captured publications up to July 2019. However, further studies have since been published that further support the prognostic factors identified here. For example, recent studies have reported an increased risk of exacerbations in patients with a history of exacerbations [ 105 ], comorbidities [ 106 ], poorer lung function (GOLD stage) [ 105 ], higher symptomatic burden [ 107 ], female sex [ 105 ], and lower BMI [ 106 , 108 ].
In summary, the literature assessing risk factors for moderate-to-severe COPD exacerbations shows that there are associations between several demographic and disease characteristics with COPD exacerbations, potentially allowing clinicians to identify patients most at risk of future exacerbations. Exacerbation history, comorbidities, and disease severity or bronchodilator reversibility were the factors most strongly associated with exacerbation risk, and should be considered in future research efforts to develop prognostic tools to estimate the likelihood of exacerbation occurrence. Importantly, many prognostic factors for exacerbations, such as symptom burden, QoL, and comorbidities, are modifiable with optimal pharmacologic and non-pharmacologic treatments or lifestyle modifications. Overall, the evidence suggests that, taken together, predicting and reducing exacerbation risk is an achievable goal in COPD.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Body mass index
COPD Assessment Test
Chronic obstructive pulmonary disease
Forced expiratory volume in 1 s
Global Initiative for Chronic Obstructive Lung Disease
Inhaled corticosteroid
Modified Medical Research Council
Quality of life
St. George’s Respiratory Questionnaire
World Health Organization. The top 10 causes of death. 2018. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death . Accessed 22 Jul 2020.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396:1204–22.
Article Google Scholar
Hurst JR, Skolnik N, Hansen GJ, Anzueto A, Donaldson GC, Dransfield MT, Varghese P. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Intern Med. 2020;73:1–6.
Article PubMed Google Scholar
Zhang Y, Morgan RL, Alonso-Coello P, Wiercioch W, Bała MM, Jaeschke RR, Styczeń K, Pardo-Hernandez H, Selva A, Ara Begum H, et al. A systematic review of how patients value COPD outcomes. Eur Respir J. 2018;52:1800222.
Global Initiative for Chronic Obstructive Lung Disease. 2022 GOLD Report. Global strategy for the diagnosis, management and prevention of COPD. 2022. https://goldcopd.org/2022-gold-reports-2/ . Accessed 02 Feb 2022.
Müllerová H, Shukla A, Hawkins A, Quint J. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4: e006171.
Article PubMed PubMed Central Google Scholar
Westerik JAM, Metting EI, van Boven JFM, Tiersma W, Kocks JWH, Schermer TR. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. 2017;18:31.
Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193:965–74.
Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerová H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38.
Article CAS PubMed Google Scholar
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Çolak Y, Afzal S, Marott JL, Nordestgaard BG, Vestbo J, Ingebrigtsen TS, Lange P. Prognosis of COPD depends on severity of exacerbation history: a population-based analysis. Respir Med. 2019;155:141–7.
Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198:464–71.
Pascoe S, Barnes N, Brusselle G, Compton C, Criner GJ, Dransfield MT, Halpin DMG, Han MK, Hartley B, Lange P, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7:745–56.
Yun JH, Lamb A, Chase R, Singh D, Parker MM, Saferali A, Vestbo J, Tal-Singer R, Castaldi PJ, Silverman EK, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10.
Yoon HY, Park SY, Lee CH, Byun MK, Na JO, Lee JS, Lee WY, Yoo KH, Jung KS, Lee JH. Prediction of first acute exacerbation using COPD subtypes identified by cluster analysis. Int J Chron Obstruct Pulmon Dis. 2019;14:1389–97.
Article CAS PubMed PubMed Central Google Scholar
Yohannes AM, Mulerova H, Lavoie K, Vestbo J, Rennard SI, Wouters E, Hanania NA. The association of depressive symptoms with rates of acute exacerbations in patients with COPD: results from a 3-year longitudinal follow-up of the ECLIPSE cohort. J Am Med Dir Assoc. 2017;18:955-959.e6.
Tsiligianni I, Metting E, van der Molen T, Chavannes N, Kocks J. Morning and night symptoms in primary care COPD patients: a cross-sectional and longitudinal study. An UNLOCK study from the IPCRG. NPJ Prim Care Respir Med. 2016;26:16040.
Jo YS, Yoon HI, Kim DK, Yoo CG, Lee CH. Comparison of COPD Assessment Test and Clinical COPD Questionnaire to predict the risk of exacerbation. Int J Chron Obstruct Pulmon Dis. 2018;13:101–7.
Marçôa R, Rodrigues DM, Dias M, Ladeira I, Vaz AP, Lima R, Guimarães M. Classification of Chronic Obstructive Pulmonary Disease (COPD) according to the new Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017: comparison with GOLD 2011. COPD. 2018;15:21–6.
Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, Cooper CB, Comellas A, Couper DJ, Curtis JL, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:619–26.
Yii ACA, Loh CH, Tiew PY, Xu H, Taha AAM, Koh J, Tan J, Lapperre TS, Anzueto A, Tee AKH. A clinical prediction model for hospitalized COPD exacerbations based on “treatable traits.” Int J Chron Obstruct Pulmon Dis. 2019;14:719–28.
McGarvey L, Lee AJ, Roberts J, Gruffydd-Jones K, McKnight E, Haughney J. Characterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med. 2015;109:228–37.
Margüello MS, Garrastazu R, Ruiz-Nuñez M, Helguera JM, Arenal S, Bonnardeux C, León C, Miravitlles M, García-Rivero JL. Independent effect of prior exacerbation frequency and disease severity on the risk of future exacerbations of COPD: a retrospective cohort study. NPJ Prim Care Respir Med. 2016;26:16046.
Engel B, Schindler C, Leuppi JD, Rutishauser J. Predictors of re-exacerbation after an index exacerbation of chronic obstructive pulmonary disease in the REDUCE randomised clinical trial. Swiss Med Wkly. 2017;147: w14439.
PubMed Google Scholar
Benson VS, Müllerová H, Vestbo J, Wedzicha JA, Patel A, Hurst JR. Evaluation of COPD longitudinally to identify predictive surrogate endpoints (ECLIPSE) investigators. Associations between gastro-oesophageal reflux, its management and exacerbations of chronic obstructive pulmonary disease. Respir Med. 2015;109:1147–54.
Santibáñez M, Garrastazu R, Ruiz-Nuñez M, Helguera JM, Arenal S, Bonnardeux C, León C, García-Rivero JL. Predictors of hospitalized exacerbations and mortality in chronic obstructive pulmonary disease. PLoS ONE. 2016;11: e0158727.
Article PubMed PubMed Central CAS Google Scholar
Jo YS, Kim YH, Lee JY, Kim K, Jung KS, Yoo KH, Rhee CK. Impact of BMI on exacerbation and medical care expenses in subjects with mild to moderate airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2018;13:2261–9.
Alexopoulos EC, Malli F, Mitsiki E, Bania EG, Varounis C, Gourgoulianis KI. Frequency and risk factors of COPD exacerbations and hospitalizations: a nationwide study in Greece (Greek Obstructive Lung Disease Epidemiology and health ecoNomics: GOLDEN study). Int J Chron Obstruct Pulmon Dis. 2015;10:2665–74.
PubMed PubMed Central Google Scholar
Liu D, Peng SH, Zhang J, Bai SH, Liu HX, Qu JM. Prediction of short term re-exacerbation in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1265–73.
Müllerová H, Maselli DJ, Locantore N, Vestbo J, Hurst JR, Wedzicha JA, Bakke P, Agusti A, Anzueto A. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147:999–1007.
de Miguel-Díez J, Hernández-Vázquez J, López-de-Andrés A, Álvaro-Meca A, Hernández-Barrera V, Jiménez-García R. Analysis of environmental risk factors for chronic obstructive pulmonary disease exacerbation: a case-crossover study (2004–2013). PLoS ONE. 2019;14: e0217143.
Krachunov II, Kyuchukov NH, Ivanova ZI, Yanev NA, Hristova PA, Borisova ED, Popova TP, Pavlov PS, Nikolova PT, Ivanov YY. Impact of air pollution and outdoor temperature on the rate of chronic obstructive pulmonary disease exacerbations. Folia Med (Plovdiv). 2017;59:423–9.
Article CAS Google Scholar
Baumeler L, Papakonstantinou E, Milenkovic B, Lacoma A, Louis R, Aerts JG, Welte T, Kostikas K, Blasi F, Boersma W, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology. 2016;21:883–90.
Annavarapu S, Goldfarb S, Gelb M, Moretz C, Renda A, Kaila S. Development and validation of a predictive model to identify patients at risk of severe COPD exacerbations using administrative claims data. Int J Chron Obstruct Pulmon Dis. 2018;13:2121–30.
Crisafulli E, Torres A, Huerta A, Méndez R, Guerrero M, Martinez R, Liapikou A, Soler N, Sethi S, Menéndez R. C-reactive protein at discharge, diabetes mellitus and ≥1 hospitalization during previous year predict early readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. COPD. 2015;12:311–20.
Bollmeier SG, Hartmann AP. Management of chronic obstructive pulmonary disease: a review focusing on exacerbations. Am J Health Syst Pharm. 2020;77:259–68.
Bafadhel M, Peterson S, De Blas MA, Calverley PM, Rennard SI, Richter K, Fagerås M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6:117–26.
Calverley PM, Anzueto AR, Dusser D, Mueller A, Metzdorf N, Wise RA. Treatment of exacerbations as a predictor of subsequent outcomes in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1297–308.
Calverley PM, Tetzlaff K, Dusser D, Wise RA, Mueller A, Metzdorf N, Anzueto A. Determinants of exacerbation risk in patients with COPD in the TIOSPIR study. Int J Chron Obstruct Pulmon Dis. 2017;12:3391–405.
Eklöf J, Sørensen R, Ingebrigtsen TS, Sivapalan P, Achir I, Boel JB, Bangsborg J, Ostergaard C, Dessau RB, Jensen US, et al. Pseudomonas aeruginosa and risk of death and exacerbations in patients with chronic obstructive pulmonary disease: an observational cohort study of 22 053 patients. Clin Microbiol Infect. 2020;26:227–34.
Estirado C, Ceccato A, Guerrero M, Huerta A, Cilloniz C, Vilaró O, Gabarrús A, Gea J, Crisafulli E, Soler N, Torres A. Microorganisms resistant to conventional antimicrobials in acute exacerbations of chronic obstructive pulmonary disease. Respir Res. 2018;19:119.
Fuhrman C, Moutengou E, Roche N, Delmas MC. Prognostic factors after hospitalization for COPD exacerbation. Rev Mal Respir. 2017;34:1–18.
Krachunov I, Kyuchukov N, Ivanova Z, Yanev NA, Hristova PA, Pavlov P, Glogovska P, Popova T, Ivanov YY. Stability of frequent exacerbator phenotype in patients with chronic obstructive pulmonary disease. Folia Med (Plovdiv). 2018;60:536–45.
Make BJ, Eriksson G, Calverley PM, Jenkins CR, Postma DS, Peterson S, Östlund O, Anzueto A. A score to predict short-term risk of COPD exacerbations (SCOPEX). Int J Chron Obstruct Pulmon Dis. 2015;10:201–9.
Montserrat-Capdevila J, Godoy P, Marsal JR, Barbé F. Predictive model of hospital admission for COPD exacerbation. Respir Care. 2015;60:1288–94.
Montserrat-Capdevila J, Godoy P, Marsal JR, Barbé F, Galván L. Risk factors for exacerbation in chronic obstructive pulmonary disease: a prospective study. Int J Tuberc Lung Dis. 2016;20:389–95.
Orea-Tejeda A, Navarrete-Peñaloza AG, Verdeja-Vendrell L, Jiménez-Cepeda A, González-Islas DG, Hernández-Zenteno R, Keirns-Davis C, Sánchez-Santillán R, Velazquez-Montero A, Puentes RG. Right heart failure as a risk factor for severe exacerbation in patients with chronic obstructive pulmonary disease: prospective cohort study. Clin Respir J. 2018;12:2635–41.
Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, Guasconi A, Montagna I, Vezzoli S, Petruzzelli S, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–84.
Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–80.
Pasquale MK, Xu Y, Baker CL, Zou KH, Teeter JG, Renda AM, Davis CC, Lee TC, Bobula J. COPD exacerbations associated with the modified Medical Research Council scale and COPD assessment test among Humana Medicare members. Int J Chron Obstruct Pulmon Dis. 2016;11:111–21.
Schuler M, Wittmann M, Faller H, Schultz K. Including changes in dyspnea after inpatient rehabilitation improves prediction models of exacerbations in COPD. Respir Med. 2018;141:87–93.
Singh D, Papi A, Corradi M, Pavlišová I, Montagna I, Francisco C, Cohuet G, Vezzoli S, Scuri M, Vestbo J. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β 2 -agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388:963–73.
Søgaard M, Madsen M, Løkke A, Hilberg O, Sørensen HT, Thomsen RW. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int J Chron Obstruct Pulmon Dis. 2016;11:455–65.
Stanford RH, Nag A, Mapel DW, Lee TA, Rosiello R, Schatz M, Vekeman F, Gauthier-Loiselle M, Merrigan JFP, Duh MS. Claims-based risk model for first severe COPD exacerbation. Am J Manag Care. 2018;24:e45–53.
Stanford RH, Lau MS, Li Y, Stemkowski S. External validation of a COPD risk measure in a commercial and medicare population: the COPD treatment ratio. J Manag Care Spec Pharm. 2019;25:58–69.
Vestbo J, Papi A, Corradi M, Blazhko V, Montagna I, Francisco C, Cohuet G, Vezzoli S, Scuri M, Singh D. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919–29.
Wei X, Ma Z, Yu N, Ren J, Jin C, Mi J, Shi M, Tian L, Gao Y, Guo Y. Risk factors predict frequent hospitalization in patients with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:121–9.
Whalley D, Svedsater H, Doward L, Crawford R, Leather D, Lay-Flurrie J, Bosanquet N. Follow-up interviews from The Salford Lung Study (COPD) and analyses per treatment and exacerbations. NPJ Prim Care Respir Med. 2019;29:20.
Zeiger RS, Tran TN, Butler RK, Schatz M, Li Q, Khatry DB, Martin U, Kawatkar AA, Chen W. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract. 2018;6:944-954.e945.
Vogelmeier CF, Kostikas K, Fang J, Tian H, Jones B, Morgan CL, Fogel R, Gutzwiller FS, Cao H. Evaluation of exacerbations and blood eosinophils in UK and US COPD populations. Respir Res. 2019;20:178.
Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook R, Criner GJ, Franssen FME, Humbert M, Hurst JR, O’Donnell D, et al. An updated definition and severity classification of COPD exacerbations: the Rome proposal. Am J Respir Crit Care Med. 2021;204:1251–8.
Adir Y, Hakrush O, Shteinberg M, Schneer S, Agusti A. Circulating eosinophil levels do not predict severe exacerbations in COPD: a retrospective study. ERJ Open Research. 2018;4:00022–2018.
Bartels W, Adamson S, Leung L, Sin DD, van Eeden SF. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease: factors predicting readmission. Int J Chron Obstruct Pulmon Dis. 2018;13:1647–54.
Kim V, Zhao H, Regan E, Han MK, Make BJ, Crapo JD, Jones PW, Curtis JL, Silverman EK, Criner GJ, COPDGene Investigators. The St. George’s Respiratory Questionnaire definition of chronic bronchitis may be a better predictor of COPD exacerbations compared with the classic definition. Chest. 2019;156:685–95.
Abston E, Comellas A, Reed RM, Kim V, Wise RA, Brower R, Fortis S, Beichel R, Bhatt S, Zabner J, et al. Higher BMI is associated with higher expiratory airflow normalised for lung volume (FEF25-75/FVC) in COPD. BMJ Open Respir Res. 2017;4: e000231.
Emura I, Usuda H, Satou K. Appearance of large scavenger receptor A-positive cells in peripheral blood: a potential risk factor for severe exacerbation of chronic obstructive pulmonary disease. Pathol Int. 2019;69:187–92.
Erol S, Sen E, Gizem Kilic Y, Yousif A, Akkoca Yildiz O, Acican T, Saryal S. Does the 2017 revision improve the ability of GOLD to predict risk of future moderate and severe exacerbation? Clin Respir J. 2018;12:2354–60.
Han MZ, Hsiue TR, Tsai SH, Huang TH, Liao XM, Chen CZ. Validation of the GOLD 2017 and new 16 subgroups (1A–4D) classifications in predicting exacerbation and mortality in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3425–33.
Huang TH, Hsiue TR, Lin SH, Liao XM, Su PL, Chen CZ. Comparison of different staging methods for COPD in predicting outcomes. Eur Resp J. 2018;51:1700577.
Jung YH, Lee DY, Kim DW, Park SS, Heo EY, Chung HS, Kim DK. Clinical significance of laryngopharyngeal reflux in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1343–51.
CAS PubMed PubMed Central Google Scholar
Kim J, Kim WJ, Lee CH, Lee SH, Lee MG, Shin KC, Yoo KH, Lee JH, Lim SY, Na JO, et al. Which bronchodilator reversibility criteria can predict severe acute exacerbation in chronic obstructive pulmonary disease patients? Respir Res. 2017;18:107.
Kobayashi S, Hanagama M, Ishida M, Sato H, Ono M, Yamanda S, Yamada M, Aizawa H, Yanai M. Clinical characteristics and outcomes in Japanese patients with COPD according to the 2017 GOLD classification: the Ishinomaki COPD Network Registry. Int J Chron Obstruct Pulmon Dis. 2018;13:3947–55.
Lee SH, Lee JH, Yoon HI, Park HY, Kim TH, Yoo KH, Oh YM, Jung KS, Lee SD, Lee SW. Change in inhaled corticosteroid treatment and COPD exacerbations: an analysis of real-world data from the KOLD/KOCOSS cohorts. Respir Res. 2019;20:62.
Pavlovic R, Stefanovic S, Lazic Z, Jankovic S. Factors associated with the rate of COPD exacerbations that require hospitalization. Turk J Med Sci. 2017;47:134–41.
Song JH, Lee CH, Um SJ, Park YB, Yoo KH, Jung KS, Lee SD, Oh YM, Lee JH, Kim EK, Kim DK. Clinical impacts of the classification by 2017 GOLD guideline comparing previous ones on outcomes of COPD in real-world cohorts. Int J Chron Obstruct Pulmon Dis. 2018;13:3473–84.
Sundh J, Johansson G, Larsson K, Lindén A, Löfdahl CG, Sandström T, Janson C. The phenotype of concurrent chronic bronchitis and frequent exacerbations in patients with severe COPD attending Swedish secondary care units. Int J Chron Obstruct Pulmon Dis. 2015;10:2327–34.
Urwyler P, Hussein NA, Bridevaux PO, Chhajed PN, Geiser T, Grendelmeier P, Zellweger LJ, Kohler M, Maier S, Miedinger D, et al. Predictive factors for exacerbation and reexacerbation in chronic obstructive pulmonary disease: an extension of the Cox model to analyze data from the Swiss COPD cohort. Multidiscip Respir Med. 2019;14:7.
Wallace AE, Kaila S, Bayer V, Shaikh A, Shinde MU, Willey VJ, Napier MB, Singer JR. Health care resource utilization and exacerbation rates in patients with COPD stratified by disease severity in a commercially insured population. J Manag Care Spec Pharm. 2019;25:205–17.
Halpin DMG, Decramer M, Celli BR, Mueller A, Metzdorf N, Tashkin DP. Effect of a single exacerbation on decline in lung function in COPD. Respir Med. 2017;128:85–91.
Bade BC, DeRycke EC, Ramsey C, Skanderson M, Crothers K, Haskell S, Bean-Mayberry B, Brandt C, Bastian LA, Akgün KM. Sex differences in veterans admitted to the hospital for chronic obstructive pulmonary disease exacerbation. Ann Am Thorac Soc. 2019;16:707–14.
Iyer AS, Bhatt SP, Dransfield M, Kinney G, Holm K, Wamboldt FS, Hanania N, Martinez C, Regan E, Foreman MG, et al. Psychological distress prospectively predicts severe exacerbations in smokers with and without airflow limitation—a longitudinal follow-up study of the COPDGene cohort [abstract]. Am J Respir Crit Care Med. 2017. https://doi.org/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A4709 .
Diamond M, Zhao H, Armstrong HF, Morrison M, Bailey KL, Carretta EE, Criner GJ, Han MK, Bleeker E, Cooper CB, et al. Anxiety and depression, either alone or in combination, are associated with respiratory exacerbations in smokers with and without COPD [abstract]. Am J Respir Crit Care Med. 2017;195:1615–31.
Google Scholar
Lau CS, Siracuse BL, Chamberlain RS. Readmission After COPD Exacerbation Scale: determining 30-day readmission risk for COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:1891–902.
Pikoula M, Quint JK, Nissen F, Hemingway H, Smeeth L, Denaxas S. Identifying clinically important COPD sub-types using data-driven approaches in primary care population based electronic health records. BMC Med Inform Decis Mak. 2019;19:86.
Wei YF, Tsai YH, Wang CC, Kuo PH. Impact of overweight and obesity on acute exacerbations of COPD—subgroup analysis of the Taiwan Obstructive Lung Disease cohort. Int J Chron Obstruct Pulmon Dis. 2017;12:2723–9.
Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Resp J. 2009;33:1165–85.
Polverino E, Dimakou K, Hurst J, Martinez-Garcia MA, Miravitlles M, Paggiaro P, Shteinberg M, Aliberti S, Chalmers JD. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J. 2018;52:1800328.
Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Platt RW, Wang C, Bourbeau J. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care Med. 2008;178:913–20.
Chapman KR, Hurst JR, Frent SM, Larbig M, Fogel R, Guerin T, Banerji D, Patalano F, Goyal P, Pfister P, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198:329–39.
Couillard S, Larivée P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151:366–73.
Ferguson GT, Rabe KF, Martinez FJ, Fabbri LM, Wang C, Ichinose M, Bourne E, Ballal S, Darken P, DeAngelis K, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6:747–58.
Ko FWS, Chan KP, Ngai J, Ng SS, Yip WH, Ip A, Chan TO, Hui DSC. Blood eosinophil count as a predictor of hospital length of stay in COPD exacerbations. Respirology. 2019;25:259–66.
MacDonald MI, Osadnik CR, Bulfin L, Hamza K, Leong P, Wong A, King PT, Bardin PG. Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest. 2019;156:92–100.
Müllerová H, Hahn B, Simard EP, Mu G, Hatipoğlu U. Exacerbations and health care resource use among patients with COPD in relation to blood eosinophil counts. Int J Chron Obstruct Pulmon Dis. 2019;14:683–92.
Bafadhel M, Greening NJ, Harvey-Dunstan TC, Williams JEA, Morgan MD, Brightling CE, Hussain SF, Pavord ID, Singh SJ, Steiner MC. Blood eosinophils and outcomes in severe hospitalised exacerbations of COPD. Chest. 2016;150:320–8.
Roche N, Chapman KR, Vogelmeier CF, Herth FJF, Thach C, Fogel R, Olsson P, Patalano F, Banerji D, Wedzicha JA. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med. 2017;195:1189–97.
Vestbo J, Vogelmeier CF, Small M, Siddall J, Fogel R, Kostikas K. Inhaled corticosteroid use by exacerbations and eosinophils: a real-world COPD population. Int J Chron Obstruct Pulmon Dis. 2019;14:853–61.
Watz H, Tetzlaff K, Wouters EFM, Kirsten A, Magnussen H, Rodriguez-Roisin R, Vogelmeier C, Fabbri LM, Chanez P, Dahl R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4:390–8.
Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, Barnes NC. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax. 2016;71:118–25.
Singh D. Predicting corticosteroid response in chronic obstructive pulmonary disease. Blood eosinophils gain momentum. Am J Respir Crit Care Med. 2017;196:1098–100.
Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sørensen TIA, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83.
Agustí AGN, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347–60.
Agustí AGN, Sauleda J, Miralles C, Gomez C, Togores B, Sala E, Batle S, Busquets X. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:485–9.
Labaki WW, Martinez FJ. Time to understand the infrequency of the frequent exacerbator phenotype in COPD. Chest. 2018;153:1087–8.
Hartley BF, Barnes NC, Lettis S, Compton CH, Papi A, Jones P. Risk factors for exacerbations and pneumonia in patients with chronic obstructive pulmonary disease: a pooled analysis. Respir Res. 2020;21:5.
Kim Y, Kim YJ, Kang YM, Cho WK. Exploring the impact of number and type of comorbidities on the risk of severe COPD exacerbations in Korean Population: a Nationwide Cohort Study. BMC Pulm Med. 2021;21:151.
Mackay AJ, Kostikas K, Roche N, Frent SM, Olsson P, Pfister P, Gupta P, Patalano F, Banerji D, Wedzicha JA. Impact of baseline symptoms and health status on COPD exacerbations in the FLAME study. Respir Res. 2020;21:93.
Smulders L, van der Aalst A, Neuhaus EDET, Polman S, Franssen FME, van Vliet M, de Kruif MD. Decreased risk of COPD exacerbations in obese patients. COPD. 2020;17:485–91.
Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, Citrome L, Gurr JA, Mooney LA, Moore BJ, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163:461–4.
Putcha N, Barr RG, Han M, Woodruff PG, Bleecker ER, Kanner RE, Martinez FJ, Tashkin DP, Rennard SI, Breysse P, et al. Understanding the impact of passive smoke exposure on outcomes in COPD [abstract]. Am J Respir Crit Care Med. 2015;191:411–20.
Wu Z, Yang D, Ge Z, Yan M, Wu N, Liu Y. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: a retrospective real world research. J Thorac Dis. 2018;10:5086–99.
Download references
Acknowledgements
Medical writing support, under the direction of the authors, was provided by Julia King, PhD, and Sarah Piggott, MChem, CMC Connect, McCann Health Medical Communications, funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines [ 109 ].
This study was supported by AstraZeneca.
Author information
Authors and affiliations.
UCL Respiratory, University College London, London, WC1E 6BT, UK
John R. Hurst
Division of Pulmonary and Critical Care, University of Michigan, Ann Arbor, MI, USA
MeiLan K. Han
Formerly of Parexel International, Mohali, India
Barinder Singh, Gagandeep Kaur & Mohd Kashif Siddiqui
Parexel International, Mohali, India
Sakshi Sharma
Formerly of AstraZeneca, Cambridge, UK
Enrico de Nigris
AstraZeneca, Gothenburg, Sweden
Ulf Holmgren
You can also search for this author in PubMed Google Scholar
Contributions
The authors have made the following declaration about their contributions. JRH and MKH made substantial contributions to the interpretation of data; BS, SS, GK, and MKS made substantial contributions to the acquisition, analysis, and interpretation of data; EdN and UH made substantial contributions to the conception and design of the work and the interpretation of data. All authors contributed to drafting or critically revising the article, have approved the submitted version, and agree to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Correspondence to John R. Hurst .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
JRH reports consulting fees from AstraZeneca; speaker fees from AstraZeneca, Chiesi, Pfizer, and Takeda; and travel support from GlaxoSmithKline and AstraZeneca. MKH reports assistance with conduction of this research and publication from AstraZeneca; personal fees from Aerogen, Altesa Biopharma, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, DevPro, GlaxoSmithKline, Integrity, Medscape, Merck, Mylan, NACE, Novartis, Polarean, Pulmonx, Regeneron, Sanofi, Teva, Verona, United Therapeutics, and UpToDate; either in kind research support or funds paid to the institution from the American Lung Association, AstraZeneca, Biodesix, Boehringer Ingelheim, the COPD Foundation, Gala Therapeutics, the NIH, Novartis, Nuvaira, Sanofi, and Sunovion; participation in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution; and stock options from Altesa Biopharma and Meissa Vaccines. BS, GK, and MKS are former employees of Parexel International. SS is an employee of Parexel International, which was funded by AstraZeneca to conduct this analysis. EdN is a former employee of AstraZeneca and previously held stock and/or stock options in the company. UH is an employee of AstraZeneca and holds stock and/or stock options in the company.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1: table s1..
Search strategies. Table S2. List of included studies with linked publications. Table S3. Study characteristics across the 76 included studies. Table S4. Clinical characteristics of the patients assessed across the included studies.
Additional file 2: Fig. S1.
Sex (male vs female) as a risk factor for moderate-to-severe exacerbations. Fig. S2. Sex (male vs female) as a risk factor for severe exacerbations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hurst, J.R., Han, M.K., Singh, B. et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res 23 , 213 (2022). https://doi.org/10.1186/s12931-022-02123-5
Download citation
Received : 02 March 2022
Accepted : 20 July 2022
Published : 23 August 2022
DOI : https://doi.org/10.1186/s12931-022-02123-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Exacerbations
- Comorbidities
- Hospitalization
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
Chronic kidney disease in inflammatory bowel disease, a systematic review and meta-analysis
Affiliations.
- 1 Nephrology and Renal Transplantation Research Group, KULeuven, Belgium.
- 2 Translational Research in GastroIntestinal Disorders, KULeuven, Leuven, Belgium.
- 3 Department of Experimental and Clinical Biomedical Sciences, University of Florence, Italy.
- 4 Department of Gastroenterology and Hepatology, University Hospitals Leuven, KULeuven , Leuven, Belgium.
- PMID: 38584452
- DOI: 10.1093/ecco-jcc/jjae049
Inflammatory bowel disease (IBD) is associated with various immune mediated disorders including spondylarthritis, pyoderma gangrenosum, primary sclerosing cholangitis and uveitis. Chronic kidney disease (CKD) is defined by a reduction in kidney function (eGFR less than 60ml/min/1.73m2) and/ or damage markers that are present for at least three months, regardless of the aetiology. Case reports and cohort studies suggest that IBD is associated with CKD. The extent and magnitude of a potential association is unknown. A comprehensive search was conducted in EMBASE, MEDLINE, Web of Science, the Cochrane database, and SCOPUS. Two separate reviewers were involved in the process of article selection and evaluation. Odds ratios were calculated in those papers with a comparison between an IBD population and a non-IBD control population, the Mantel Haenszel test was employed, utilizing a random effect model. The systematic review was registered in PROSPERO (RD42023381927). Fifty-four articles were included in the systematic review. Of these, eight articles included data on prevalence of CKD in IBD patients (n = 102,230) vs. healthy populations (n = 762,430). Of these, diagnosis of CKD was based on ICD codes in five studies vs. on eGFR in three studies. The overall odds ratio of developing CKD in the IBD population is 1.59 (95%CI 1.31-1.93), without any difference between studies using diagnostic coding (OR 1.70 95%CI 1.33-2.19) vs. diagnosis based on eGFR (OR 1.36 95%CI 1.33-1.64). IBD is associated with a clinically meaningful increased CKD prevalence. We provide recommendations on diagnostic evaluation, as well as suggestions for future research.
Keywords: 5-ASA; CKD; IBD; epidemiology; systematic review.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation. All rights reserved. For commercial re-use, please contact [email protected] for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact [email protected].

IMAGES
VIDEO
COMMENTS
Introduction. An estimated 840 million people worldwide have chronic kidney disease (CKD) [], which was responsible for 1.2 million deaths and 35.8 million disability-adjusted life years in 2017 [].However, only 12% of sufferers are aware of their condition [].CKD is diagnosed when the estimated glomerular filtration rate (eGFR) declines below 60 mL/min/1.73 m 2 or the urinary albumin-to ...
Delaying disease progression and reducing the risk of mortality are key goals in the treatment of chronic kidney disease (CKD). New drug classes to augment renin-angiotensin-aldosterone system (RAAS) inhibitors as the standard of care have scarcely met their primary endpoints until recently. This sy …
Chronic kidney disease is a progressive disease with no cure and high morbidity and mortality that occurs commonly in the general adult population, especially in people with diabetes and hypertension. Preservation of kidney function can improve outcomes and can be achieved through non-pharmacological strategies (eg, dietary and lifestyle adjustments) and chronic kidney disease-targeted and ...
Global prevalence of chronic kidney disease — a systematic review and meta-analysis. PLoS ONE 11, e0158765 (2016). Google Scholar Zhang, L. et al. Prevalence of chronic kidney disease in China ...
Chronic kidney disease (CKD) is a global public health problem affecting more than 697 million people 1.The prevalence of CKD has been increasing worldwide, with an annual increase of 8 to 16% ...
Objectives In this systematic review we aimed at assessing how artificial intelligence (AI), including machine learning (ML) techniques have been deployed to predict, diagnose, and treat chronic kidney disease (CKD). We systematically reviewed the available evidence on these innovative techniques to improve CKD diagnosis and patient management. Methods We included English language studies ...
Chronic kidney disease (CKD) is a complex disease which affects approximately 13% of the world's population. Over time, CKD can cause renal dysfunction and progression to end-stage kidney disease and cardiovascular disease. ... A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives Adv Ther ...
Abstract. Importance: Chronic kidney disease (CKD) is the 16th leading cause of years of life lost worldwide. Appropriate screening, diagnosis, and management by primary care clinicians are necessary to prevent adverse CKD-associated outcomes, including cardiovascular disease, end-stage kidney disease, and death.
Patient awareness of disease is the first step toward effective management and disease control. Awareness of chronic kidney disease (CKD) has consistently been shown to be low, but studies estimating patient awareness of CKD have used different methods. We sought to determine whether the estimated prevalence of CKD awareness differed by the wording used to ascertain awareness or by setting ...
Delaying disease progression and reducing the risk of mortality are key goals in the treatment of chronic kidney disease (CKD). New drug classes to augment renin-angiotensin-aldosterone system (RAAS) inhibitors as the standard of care have scarcely met their primary endpoints until recently. This systematic literature review explored treatments evaluated in patients with CKD since 1990 to ...
Chronic kidney disease (CKD) is a global health burden with a high economic cost to health systems and is an independent risk factor for cardiovascular disease (CVD). All stages of CKD are associated with increased risks of cardiovascular morbidity, premature mortality, and/or decreased quality of life. CKD is usually asymptomatic until later stages and accurate prevalence data are lacking ...
of chronic kidney disease (CKD). New drug classes to augment renin-angiotensin-aldos-terone system (RAAS) inhibitors as the standard of care have scarcely met their primary end-points until recently. This systematic literature review explored treatments evaluated in patients with CKD since 1990 to understand what contemporary data add to ...
We assigned 71 practices (enrolling 5690 patients) to the intervention group and 70 practices (enrolling 5492 patients) to the usual-care group. The hospitalization rate at 1 year was 20.7% (95% ...
This systematic literature review explored treatments evaluated in patients with CKD since 1990 to understand what contemporary data add to the treatment landscape.
The roster of such NCDs includes heart disease, stroke, diabetes, cancer and chronic lung disease. With kidney disease missing, awareness of its growing impact remains low.
The present special issue contains seven noteworthy articles describing engaging cases of children and adults with various disorders having a common denominator in form of kidney cysts, systematically reviewing the current literature regarding the clinical characteristics of a HNF1B gene variant and biomarkers of kidney disease progression in autosomal dominant PKD (ADPKD), investigating the ...
Chronic kidney disease (CKD) is a serious comorbidity affecting liver transplant recipients (LTRs). Calcineurin inhibitor dosing minimization protocols and everolimus use purportedly increased from 2010, potentially impacting CKD development. This systematic literature review was designed to identify CKD incidence in adult LTRs, focusing on ...
Chronic kidney disease (CKD) is a complex disease which affects approximately 13% of the world's population. Over time, CKD can cause renal dysfunction and progression to end-stage kidney disease and cardiovascular disease. Complications associated with CKD may contribute to the acceleration of disease progression and the risk of cardiovascular-related morbidities. Early CKD is asymptomatic ...
Background Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. COPD exacerbations are associated with a worsening of lung function, increased disease burden, and mortality, and, therefore, preventing their occurrence is an important goal of COPD management. This review was conducted to identify the evidence base regarding risk factors and ...
Background: Chronic kidney disease (CKD) is a progressive condition that leads to irreversible damage to the kidneys and is associated with an increased incidence of cardiovascular events and mortality. As novel interventions become available, estimates of economic and clinical outcomes are needed to guide payer reimbursement decisions.
Inflammatory bowel disease (IBD) is associated with various immune mediated disorders including spondylarthritis, pyoderma gangrenosum, primary sclerosing cholangitis and uveitis. Chronic kidney disease (CKD) is defined by a reduction in kidney function (eGFR less than 60ml/min/1.73m2) and/ or damage markers that are present for at least three ...