

Literature Reviews: Types of Clinical Study Designs
- Library Basics
- 1. Choose Your Topic
- How to Find Books
- Types of Clinical Study Designs
- Types of Literature
- 3. Search the Literature
- 4. Read & Analyze the Literature
- 5. Write the Review
- Keeping Track of Information
- Style Guides
- Books, Tutorials & Examples
Types of Study Designs
Meta-Analysis A way of combining data from many different research studies. A meta-analysis is a statistical process that combines the findings from individual studies. Example : Anxiety outcomes after physical activity interventions: meta-analysis findings . Conn V. Nurs Res . 2010 May-Jun;59(3):224-31.
Systematic Review A summary of the clinical literature. A systematic review is a critical assessment and evaluation of all research studies that address a particular clinical issue. The researchers use an organized method of locating, assembling, and evaluating a body of literature on a particular topic using a set of specific criteria. A systematic review typically includes a description of the findings of the collection of research studies. The systematic review may also include a quantitative pooling of data, called a meta-analysis. Example : Complementary and alternative medicine use among women with breast cancer: a systematic review. Wanchai A, Armer JM, Stewart BR. Clin J Oncol Nurs . 2010 Aug;14(4):E45-55.
Randomized Controlled Trial A controlled clinical trial that randomly (by chance) assigns participants to two or more groups. There are various methods to randomize study participants to their groups. Example : Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial . Barrett B, et al. Ann Fam Med . 2012 Jul-Aug;10(4):337-46.
Cohort Study (Prospective Observational Study) A clinical research study in which people who presently have a certain condition or receive a particular treatment are followed over time and compared with another group of people who are not affected by the condition. Example : Smokeless tobacco cessation in South Asian communities: a multi-centre prospective cohort study . Croucher R, et al. Addiction. 2012 Dec;107 Suppl 2:45-52.
Case-control Study Case-control studies begin with the outcomes and do not follow people over time. Researchers choose people with a particular result (the cases) and interview the groups or check their records to ascertain what different experiences they had. They compare the odds of having an experience with the outcome to the odds of having an experience without the outcome. Example : Non-use of bicycle helmets and risk of fatal head injury: a proportional mortality, case-control study . Persaud N, et al. CMAJ . 2012 Nov 20;184(17):E921-3.
Cross-sectional study The observation of a defined population at a single point in time or time interval. Exposure and outcome are determined simultaneously. Example : Fasting might not be necessary before lipid screening: a nationally representative cross-sectional study . Steiner MJ, et al. Pediatrics . 2011 Sep;128(3):463-70.
Case Reports and Series A report on a series of patients with an outcome of interest. No control group is involved. Example : Students mentoring students in a service-learning clinical supervision experience: an educational case report . Lattanzi JB, et al. Phys Ther . 2011 Oct;91(10):1513-24.
Ideas, Editorials, Opinions Put forth by experts in the field. Example : Health and health care for the 21st century: for all the people . Koop CE. Am J Public Health . 2006 Dec;96(12):2090-2.
Animal Research Studies Studies conducted using animal subjects. Example : Intranasal leptin reduces appetite and induces weight loss in rats with diet-induced obesity (DIO) . Schulz C, Paulus K, Jöhren O, Lehnert H. Endocrinology . 2012 Jan;153(1):143-53.
Test-tube Lab Research "Test tube" experiments conducted in a controlled laboratory setting.
Adapted from Study Designs. In NICHSR Introduction to Health Services Research: a Self-Study Course. http://www.nlm.nih.gov/nichsr/ihcm/06studies/studies03.html and Glossary of EBM Terms. http://www.cebm.utoronto.ca/glossary/index.htm#top
Study Design Terminology
Bias - Any deviation of results or inferences from the truth, or processes leading to such deviation. Bias can result from several sources: one-sided or systematic variations in measurement from the true value (systematic error); flaws in study design; deviation of inferences, interpretations, or analyses based on flawed data or data collection; etc. There is no sense of prejudice or subjectivity implied in the assessment of bias under these conditions.
Case Control Studies - Studies which start with the identification of persons with a disease of interest and a control (comparison, referent) group without the disease. The relationship of an attribute to the disease is examined by comparing diseased and non-diseased persons with regard to the frequency or levels of the attribute in each group.
Causality - The relating of causes to the effects they produce. Causes are termed necessary when they must always precede an effect and sufficient when they initiate or produce an effect. Any of several factors may be associated with the potential disease causation or outcome, including predisposing factors, enabling factors, precipitating factors, reinforcing factors, and risk factors.
Control Groups - Groups that serve as a standard for comparison in experimental studies. They are similar in relevant characteristics to the experimental group but do not receive the experimental intervention.
Controlled Clinical Trials - Clinical trials involving one or more test treatments, at least one control treatment, specified outcome measures for evaluating the studied intervention, and a bias-free method for assigning patients to the test treatment. The treatment may be drugs, devices, or procedures studied for diagnostic, therapeutic, or prophylactic effectiveness. Control measures include placebos, active medicines, no-treatment, dosage forms and regimens, historical comparisons, etc. When randomization using mathematical techniques, such as the use of a random numbers table, is employed to assign patients to test or control treatments, the trials are characterized as Randomized Controlled Trials.
Cost-Benefit Analysis - A method of comparing the cost of a program with its expected benefits in dollars (or other currency). The benefit-to-cost ratio is a measure of total return expected per unit of money spent. This analysis generally excludes consideration of factors that are not measured ultimately in economic terms. Cost effectiveness compares alternative ways to achieve a specific set of results.
Cross-Over Studies - Studies comparing two or more treatments or interventions in which the subjects or patients, upon completion of the course of one treatment, are switched to another. In the case of two treatments, A and B, half the subjects are randomly allocated to receive these in the order A, B and half to receive them in the order B, A. A criticism of this design is that effects of the first treatment may carry over into the period when the second is given.
Cross-Sectional Studies - Studies in which the presence or absence of disease or other health-related variables are determined in each member of the study population or in a representative sample at one particular time. This contrasts with LONGITUDINAL STUDIES which are followed over a period of time.
Double-Blind Method - A method of studying a drug or procedure in which both the subjects and investigators are kept unaware of who is actually getting which specific treatment.
Empirical Research - The study, based on direct observation, use of statistical records, interviews, or experimental methods, of actual practices or the actual impact of practices or policies.
Evaluation Studies - Works consisting of studies determining the effectiveness or utility of processes, personnel, and equipment.
Genome-Wide Association Study - An analysis comparing the allele frequencies of all available (or a whole genome representative set of) polymorphic markers in unrelated patients with a specific symptom or disease condition, and those of healthy controls to identify markers associated with a specific disease or condition.
Intention to Treat Analysis - Strategy for the analysis of Randomized Controlled Trial that compares patients in the groups to which they were originally randomly assigned.
Logistic Models - Statistical models which describe the relationship between a qualitative dependent variable (that is, one which can take only certain discrete values, such as the presence or absence of a disease) and an independent variable. A common application is in epidemiology for estimating an individual's risk (probability of a disease) as a function of a given risk factor.
Longitudinal Studies - Studies in which variables relating to an individual or group of individuals are assessed over a period of time.
Lost to Follow-Up - Study subjects in cohort studies whose outcomes are unknown e.g., because they could not or did not wish to attend follow-up visits.
Matched-Pair Analysis - A type of analysis in which subjects in a study group and a comparison group are made comparable with respect to extraneous factors by individually pairing study subjects with the comparison group subjects (e.g., age-matched controls).
Meta-Analysis - Works consisting of studies using a quantitative method of combining the results of independent studies (usually drawn from the published literature) and synthesizing summaries and conclusions which may be used to evaluate therapeutic effectiveness, plan new studies, etc. It is often an overview of clinical trials. It is usually called a meta-analysis by the author or sponsoring body and should be differentiated from reviews of literature.
Numbers Needed To Treat - Number of patients who need to be treated in order to prevent one additional bad outcome. It is the inverse of Absolute Risk Reduction.
Odds Ratio - The ratio of two odds. The exposure-odds ratio for case control data is the ratio of the odds in favor of exposure among cases to the odds in favor of exposure among noncases. The disease-odds ratio for a cohort or cross section is the ratio of the odds in favor of disease among the exposed to the odds in favor of disease among the unexposed. The prevalence-odds ratio refers to an odds ratio derived cross-sectionally from studies of prevalent cases.
Patient Selection - Criteria and standards used for the determination of the appropriateness of the inclusion of patients with specific conditions in proposed treatment plans and the criteria used for the inclusion of subjects in various clinical trials and other research protocols.
Predictive Value of Tests - In screening and diagnostic tests, the probability that a person with a positive test is a true positive (i.e., has the disease), is referred to as the predictive value of a positive test; whereas, the predictive value of a negative test is the probability that the person with a negative test does not have the disease. Predictive value is related to the sensitivity and specificity of the test.
Prospective Studies - Observation of a population for a sufficient number of persons over a sufficient number of years to generate incidence or mortality rates subsequent to the selection of the study group.
Qualitative Studies - Research that derives data from observation, interviews, or verbal interactions and focuses on the meanings and interpretations of the participants.
Quantitative Studies - Quantitative research is research that uses numerical analysis.
Random Allocation - A process involving chance used in therapeutic trials or other research endeavor for allocating experimental subjects, human or animal, between treatment and control groups, or among treatment groups. It may also apply to experiments on inanimate objects.
Randomized Controlled Trial - Clinical trials that involve at least one test treatment and one control treatment, concurrent enrollment and follow-up of the test- and control-treated groups, and in which the treatments to be administered are selected by a random process, such as the use of a random-numbers table.
Reproducibility of Results - The statistical reproducibility of measurements (often in a clinical context), including the testing of instrumentation or techniques to obtain reproducible results. The concept includes reproducibility of physiological measurements, which may be used to develop rules to assess probability or prognosis, or response to a stimulus; reproducibility of occurrence of a condition; and reproducibility of experimental results.
Retrospective Studies - Studies used to test etiologic hypotheses in which inferences about an exposure to putative causal factors are derived from data relating to characteristics of persons under study or to events or experiences in their past. The essential feature is that some of the persons under study have the disease or outcome of interest and their characteristics are compared with those of unaffected persons.
Sample Size - The number of units (persons, animals, patients, specified circumstances, etc.) in a population to be studied. The sample size should be big enough to have a high likelihood of detecting a true difference between two groups.
Sensitivity and Specificity - Binary classification measures to assess test results. Sensitivity or recall rate is the proportion of true positives. Specificity is the probability of correctly determining the absence of a condition.
Single-Blind Method - A method in which either the observer(s) or the subject(s) is kept ignorant of the group to which the subjects are assigned.
Time Factors - Elements of limited time intervals, contributing to particular results or situations.
Source: NLM MeSH Database
- << Previous: How to Find Books
- Next: Types of Literature >>
- Last Updated: Dec 29, 2023 11:41 AM
- URL: https://research.library.gsu.edu/litrev
A systematic literature review of clinical trials and therapeutic applications of ibogaine
Affiliations.
- 1 University of Basel Psychiatric Clinics, Wilhelm Klein-Strasse 27, 4002 Basel, Switzerland. Electronic address: [email protected].
- 2 University of Basel Psychiatric Clinics, Wilhelm Klein-Strasse 27, 4002 Basel, Switzerland.
- 3 University of Basel Psychiatric Clinics, Wilhelm Klein-Strasse 27, 4002 Basel, Switzerland; Department for Psychiatry, Psychotherapy and Psychosomatic, Psychiatric Hospital, University of Zurich, Zurich, Switzerland.
- PMID: 35012793
- DOI: 10.1016/j.jsat.2021.108717
Background: Iboga and its primary alkaloids, ibogaine and noribogaine, have been of interest to researchers and practitioners, mainly due to their putative efficacy in treating substance use disorders (SUDs). For many SUDs, still no effective pharmacotherapies exist. Distinct psychoactive and somatic effects of the iboga alkaloids set them apart from classic hallucinogens like LSD, mescaline, and psilocybin.
Aims: The study team performed this systematic review focusing on clinical data and therapeutic interventions involving ibogaine and noribogaine.
Methods: The team conducted a search for all publications up to December 7, 2020, using PubMed and Embase following PRISMA guidelines.
Results: In total, we identified 743 records. In this review, we consider 24 studies, which included 705 individuals receiving ibogaine or noribogaine. This review includes two randomized, double-blind, controlled clinical trials, one double-blind controlled clinical trial, 17 open-label studies or case series (including observational or retrospective studies), three case reports, and one retrospective survey. The published data suggest that ibogaine is an effective therapeutic intervention within the context of SUDs, reducing withdrawal symptoms and craving. Data also point toward a beneficial impact on depressive and trauma-related psychological symptoms. However, studies have reported severe medical complications and deaths, which seem to be associated with neuro- and cardiotoxic effects of ibogaine. Two of these fatalities were described in the 24 studies included in this review.
Conclusion: Treatment of SUDs and persisting comorbidities requires innovative treatment approaches. Rapid-onset therapies such as the application of ibogaine may offer novel treatment opportunities for specific individuals. Rigorous study designs within medical settings are necessary to warrant safe application, monitoring, and, possibly, medical intervention.
Keywords: Cocaine; Hallucinogen; Opioid; Psychedelic; Substance use disorder; Treatment.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Publication types
- Case Reports
- Systematic Review
- Alkaloids* / therapeutic use
- Hallucinogens* / adverse effects
- Ibogaine* / adverse effects
- Observational Studies as Topic
- Randomized Controlled Trials as Topic
- Retrospective Studies
- Substance Withdrawal Syndrome* / drug therapy
- Substance-Related Disorders* / drug therapy
- Hallucinogens
Methodological Approaches to Literature Review
- Living reference work entry
- First Online: 09 May 2023
- Cite this living reference work entry

- Dennis Thomas 2 ,
- Elida Zairina 3 &
- Johnson George 4
460 Accesses
The literature review can serve various functions in the contexts of education and research. It aids in identifying knowledge gaps, informing research methodology, and developing a theoretical framework during the planning stages of a research study or project, as well as reporting of review findings in the context of the existing literature. This chapter discusses the methodological approaches to conducting a literature review and offers an overview of different types of reviews. There are various types of reviews, including narrative reviews, scoping reviews, and systematic reviews with reporting strategies such as meta-analysis and meta-synthesis. Review authors should consider the scope of the literature review when selecting a type and method. Being focused is essential for a successful review; however, this must be balanced against the relevance of the review to a broad audience.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Akobeng AK. Principles of evidence based medicine. Arch Dis Child. 2005;90(8):837–40.
Article CAS PubMed PubMed Central Google Scholar
Alharbi A, Stevenson M. Refining Boolean queries to identify relevant studies for systematic review updates. J Am Med Inform Assoc. 2020;27(11):1658–66.
Article PubMed PubMed Central Google Scholar
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Article Google Scholar
Aromataris E MZE. JBI manual for evidence synthesis. 2020.
Google Scholar
Aromataris E, Pearson A. The systematic review: an overview. Am J Nurs. 2014;114(3):53–8.
Article PubMed Google Scholar
Aromataris E, Riitano D. Constructing a search strategy and searching for evidence. A guide to the literature search for a systematic review. Am J Nurs. 2014;114(5):49–56.
Babineau J. Product review: covidence (systematic review software). J Canad Health Libr Assoc Canada. 2014;35(2):68–71.
Baker JD. The purpose, process, and methods of writing a literature review. AORN J. 2016;103(3):265–9.
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326.
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6(1):1–12.
Brown D. A review of the PubMed PICO tool: using evidence-based practice in health education. Health Promot Pract. 2020;21(4):496–8.
Cargo M, Harris J, Pantoja T, et al. Cochrane qualitative and implementation methods group guidance series – paper 4: methods for assessing evidence on intervention implementation. J Clin Epidemiol. 2018;97:59–69.
Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126(5):376–80.
Article CAS PubMed Google Scholar
Counsell C. Formulating questions and locating primary studies for inclusion in systematic reviews. Ann Intern Med. 1997;127(5):380–7.
Cummings SR, Browner WS, Hulley SB. Conceiving the research question and developing the study plan. In: Cummings SR, Browner WS, Hulley SB, editors. Designing Clinical Research: An Epidemiological Approach. 4th ed. Philadelphia (PA): P Lippincott Williams & Wilkins; 2007. p. 14–22.
Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. JMLA. 2018;106(4):420.
Ferrari R. Writing narrative style literature reviews. Medical Writing. 2015;24(4):230–5.
Flemming K, Booth A, Hannes K, Cargo M, Noyes J. Cochrane qualitative and implementation methods group guidance series – paper 6: reporting guidelines for qualitative, implementation, and process evaluation evidence syntheses. J Clin Epidemiol. 2018;97:79–85.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009;26(2):91–108.
Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med. 2006;5(3):101–17.
Gregory AT, Denniss AR. An introduction to writing narrative and systematic reviews; tasks, tips and traps for aspiring authors. Heart Lung Circ. 2018;27(7):893–8.
Harden A, Thomas J, Cargo M, et al. Cochrane qualitative and implementation methods group guidance series – paper 5: methods for integrating qualitative and implementation evidence within intervention effectiveness reviews. J Clin Epidemiol. 2018;97:70–8.
Harris JL, Booth A, Cargo M, et al. Cochrane qualitative and implementation methods group guidance series – paper 2: methods for question formulation, searching, and protocol development for qualitative evidence synthesis. J Clin Epidemiol. 2018;97:39–48.
Higgins J, Thomas J. In: Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3, updated February 2022). Available from www.training.cochrane.org/handbook.: Cochrane; 2022.
International prospective register of systematic reviews (PROSPERO). Available from https://www.crd.york.ac.uk/prospero/ .
Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. J R Soc Med. 2003;96(3):118–21.
Landhuis E. Scientific literature: information overload. Nature. 2016;535(7612):457–8.
Lockwood C, Porritt K, Munn Z, Rittenmeyer L, Salmond S, Bjerrum M, Loveday H, Carrier J, Stannard D. Chapter 2: Systematic reviews of qualitative evidence. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. Available from https://synthesismanual.jbi.global . https://doi.org/10.46658/JBIMES-20-03 .
Chapter Google Scholar
Lorenzetti DL, Topfer L-A, Dennett L, Clement F. Value of databases other than medline for rapid health technology assessments. Int J Technol Assess Health Care. 2014;30(2):173–8.
Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for (SR) and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;6:264–9.
Mulrow CD. Systematic reviews: rationale for systematic reviews. BMJ. 1994;309(6954):597–9.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143.
Munthe-Kaas HM, Glenton C, Booth A, Noyes J, Lewin S. Systematic mapping of existing tools to appraise methodological strengths and limitations of qualitative research: first stage in the development of the CAMELOT tool. BMC Med Res Methodol. 2019;19(1):1–13.
Murphy CM. Writing an effective review article. J Med Toxicol. 2012;8(2):89–90.
NHMRC. Guidelines for guidelines: assessing risk of bias. Available at https://nhmrc.gov.au/guidelinesforguidelines/develop/assessing-risk-bias . Last published 29 August 2019. Accessed 29 Aug 2022.
Noyes J, Booth A, Cargo M, et al. Cochrane qualitative and implementation methods group guidance series – paper 1: introduction. J Clin Epidemiol. 2018b;97:35–8.
Noyes J, Booth A, Flemming K, et al. Cochrane qualitative and implementation methods group guidance series – paper 3: methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. J Clin Epidemiol. 2018a;97:49–58.
Noyes J, Booth A, Moore G, Flemming K, Tunçalp Ö, Shakibazadeh E. Synthesising quantitative and qualitative evidence to inform guidelines on complex interventions: clarifying the purposes, designs and outlining some methods. BMJ Glob Health. 2019;4(Suppl 1):e000893.
Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Healthcare. 2015;13(3):141–6.
Polanin JR, Pigott TD, Espelage DL, Grotpeter JK. Best practice guidelines for abstract screening large-evidence systematic reviews and meta-analyses. Res Synth Methods. 2019;10(3):330–42.
Article PubMed Central Google Scholar
Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):1–7.
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Brit Med J. 2017;358
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. 2016;355
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–12.
Tawfik GM, Dila KAS, Mohamed MYF, et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health. 2019;47(1):1–9.
The Critical Appraisal Program. Critical appraisal skills program. Available at https://casp-uk.net/ . 2022. Accessed 29 Aug 2022.
The University of Melbourne. Writing a literature review in Research Techniques 2022. Available at https://students.unimelb.edu.au/academic-skills/explore-our-resources/research-techniques/reviewing-the-literature . Accessed 29 Aug 2022.
The Writing Center University of Winconsin-Madison. Learn how to write a literature review in The Writer’s Handbook – Academic Professional Writing. 2022. Available at https://writing.wisc.edu/handbook/assignments/reviewofliterature/ . Accessed 29 Aug 2022.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708.
Tricco AC, Lillie E, Zarin W, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16(1):15.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Yoneoka D, Henmi M. Clinical heterogeneity in random-effect meta-analysis: between-study boundary estimate problem. Stat Med. 2019;38(21):4131–45.
Yuan Y, Hunt RH. Systematic reviews: the good, the bad, and the ugly. Am J Gastroenterol. 2009;104(5):1086–92.
Download references
Author information
Authors and affiliations.
Centre of Excellence in Treatable Traits, College of Health, Medicine and Wellbeing, University of Newcastle, Hunter Medical Research Institute Asthma and Breathing Programme, Newcastle, NSW, Australia
Dennis Thomas
Department of Pharmacy Practice, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia
Elida Zairina
Centre for Medicine Use and Safety, Monash Institute of Pharmaceutical Sciences, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, Parkville, VIC, Australia
Johnson George
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Johnson George .
Section Editor information
College of Pharmacy, Qatar University, Doha, Qatar
Derek Charles Stewart
Department of Pharmacy, University of Huddersfield, Huddersfield, United Kingdom
Zaheer-Ud-Din Babar
Rights and permissions
Reprints and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry.
Thomas, D., Zairina, E., George, J. (2023). Methodological Approaches to Literature Review. In: Encyclopedia of Evidence in Pharmaceutical Public Health and Health Services Research in Pharmacy. Springer, Cham. https://doi.org/10.1007/978-3-030-50247-8_57-1
Download citation
DOI : https://doi.org/10.1007/978-3-030-50247-8_57-1
Received : 22 February 2023
Accepted : 22 February 2023
Published : 09 May 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-50247-8
Online ISBN : 978-3-030-50247-8
eBook Packages : Springer Reference Biomedicine and Life Sciences Reference Module Biomedical and Life Sciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research

JAY SIWEK, M.D., MARGARET L. GOURLAY, M.D., DAVID C. SLAWSON, M.D., AND ALLEN F. SHAUGHNESSY, PHARM.D.
Am Fam Physician. 2002;65(2):251-258
Traditional clinical review articles, also known as updates, differ from systematic reviews and meta-analyses. Updates selectively review the medical literature while discussing a topic broadly. Nonquantitative systematic reviews comprehensively examine the medical literature, seeking to identify and synthesize all relevant information to formulate the best approach to diagnosis or treatment. Meta-analyses (quantitative systematic reviews) seek to answer a focused clinical question, using rigorous statistical analysis of pooled research studies. This article presents guidelines for writing an evidence-based clinical review article for American Family Physician . First, the topic should be of common interest and relevance to family practice. Include a table of the continuing medical education objectives of the review. State how the literature search was done and include several sources of evidence-based reviews, such as the Cochrane Collaboration, BMJ's Clinical Evidence , or the InfoRetriever Web site. Where possible, use evidence based on clinical outcomes relating to morbidity, mortality, or quality of life, and studies of primary care populations. In articles submitted to American Family Physician , rate the level of evidence for key recommendations according to the following scale: level A (randomized controlled trial [RCT], meta-analysis); level B (other evidence); level C (consensus/expert opinion). Finally, provide a table of key summary points.
American Family Physician is particularly interested in receiving clinical review articles that follow an evidence-based format. Clinical review articles, also known as updates, differ from systematic reviews and meta-analyses in important ways. 1 Updates selectively review the medical literature while discussing a topic broadly. An example of such a topic is, “The diagnosis and treatment of myocardial ischemia.” Systematic reviews comprehensively examine the medical literature, seeking to identify and synthesize all relevant information to formulate the best approach to diagnosis or treatment. Examples are many of the systematic reviews of the Cochrane Collaboration or BMJ's Clinical Evidence compendium. Meta-analyses are a special type of systematic review. They use quantitative methods to analyze the literature and seek to answer a focused clinical question, using rigorous statistical analysis of pooled research studies. An example is, “Do beta blockers reduce mortality following myocardial infarction?”
The best clinical review articles base the discussion on existing systematic reviews and meta-analyses, and incorporate all relevant research findings about the management of a given disorder. Such evidence-based updates provide readers with powerful summaries and sound clinical guidance.
In this article, we present guidelines for writing an evidence-based clinical review article, especially one designed for continuing medical education (CME) and incorporating CME objectives into its format. This article may be read as a companion piece to a previous article and accompanying editorial about reading and evaluating clinical review articles. 1 , 2 Some articles may not be appropriate for an evidence-based format because of the nature of the topic, the slant of the article, a lack of sufficient supporting evidence, or other factors. We encourage authors to review the literature and, wherever possible, rate key points of evidence. This process will help emphasize the summary points of the article and strengthen its teaching value.
Topic Selection
Choose a common clinical problem and avoid topics that are rarities or unusual manifestations of disease or that have curiosity value only. Whenever possible, choose common problems for which there is new information about diagnosis or treatment. Emphasize new information that, if valid, should prompt a change in clinical practice, such as the recent evidence that spironolactone therapy improves survival in patients who have severe congestive heart failure. 3 Similarly, new evidence showing that a standard treatment is no longer helpful, but may be harmful, would also be important to report. For example, patching most traumatic corneal abrasions may actually cause more symptoms and delay healing compared with no patching. 4
Searching the Literature
When searching the literature on your topic, please consult several sources of evidence-based reviews ( Table 1 ) . Look for pertinent guidelines on the diagnosis, treatment, or prevention of the disorder being discussed. Incorporate all high-quality recommendations that are relevant to the topic. When reviewing the first draft, look for all key recommendations about diagnosis and, especially, treatment. Try to ensure that all recommendations are based on the highest level of evidence available. If you are not sure about the source or strength of the recommendation, return to the literature, seeking out the basis for the recommendation.
In particular, try to find the answer in an authoritative compendium of evidence-based reviews, or at least try to find a meta-analysis or well-designed randomized controlled trial (RCT) to support it. If none appears to be available, try to cite an authoritative consensus statement or clinical guideline, such as a National Institutes of Health Consensus Development Conference statement or a clinical guideline published by a major medical organization. If no strong evidence exists to support the conventional approach to managing a given clinical situation, point this out in the text, especially for key recommendations. Keep in mind that much of traditional medical practice has not yet undergone rigorous scientific study, and high-quality evidence may not exist to support conventional knowledge or practice.
Patient-Oriented vs. Disease-Oriented Evidence
With regard to types of evidence, Shaughnessy and Slawson 5 – 7 developed the concept of Patient-Oriented Evidence that Matters (POEM), in distinction to Disease-Oriented Evidence (DOE). POEM deals with outcomes of importance to patients, such as changes in morbidity, mortality, or quality of life. DOE deals with surrogate end points, such as changes in laboratory values or other measures of response. Although the results of DOE sometimes parallel the results of POEM, they do not always correspond ( Table 2 ) . 2 When possible, use POEM-type evidence rather than DOE. When DOE is the only guidance available, indicate that key clinical recommendations lack the support of outcomes evidence. Here is an example of how the latter situation might appear in the text: “Although prostate-specific antigen (PSA) testing identifies prostate cancer at an early stage, it has not yet been proved that PSA screening improves patient survival.” (Note: PSA testing is an example of DOE, a surrogate marker for the true outcomes of importance—improved survival, decreased morbidity, and improved quality of life.)
Evaluating the Literature
Evaluate the strength and validity of the literature that supports the discussion (see the following section, Levels of Evidence). Look for meta-analyses, high-quality, randomized clinical trials with important outcomes (POEM), or well-designed, nonrandomized clinical trials, clinical cohort studies, or case-controlled studies with consistent findings. In some cases, high-quality, historical, uncontrolled studies are appropriate (e.g., the evidence supporting the efficacy of Papanicolaou smear screening). Avoid anecdotal reports or repeating the hearsay of conventional wisdom, which may not stand up to the scrutiny of scientific study (e.g., prescribing prolonged bed rest for low back pain).
Look for studies that describe patient populations that are likely to be seen in primary care rather than subspecialty referral populations. Shaughnessy and Slawson's guide for writers of clinical review articles includes a section on information and validity traps to avoid. 2
Levels of Evidence
Readers need to know the strength of the evidence supporting the key clinical recommendations on diagnosis and treatment. Many different rating systems of varying complexity and clinical relevance are described in the medical literature. Recently, the third U.S. Preventive Services Task Force (USPSTF) emphasized the importance of rating not only the study type (RCT, cohort study, case-control study, etc.), but also the study quality as measured by internal validity and the quality of the entire body of evidence on a topic. 8
While it is important to appreciate these evolving concepts, we find that a simplified grading system is more useful in AFP . We have adopted the following convention, using an ABC rating scale. Criteria for high-quality studies are discussed in several sources. 8 , 9 See the AFP Web site ( www.aafp.org/afp/authors ) for additional information about levels of evidence and see the accompanying editorial in this issue discussing the potential pitfalls and limitations of any rating system.
Level A (randomized controlled trial/meta-analysis): High-quality randomized controlled trial (RCT) that considers all important outcomes. High-quality meta-analysis (quantitative systematic review) using comprehensive search strategies.
Level B (other evidence): A well-designed, nonrandomized clinical trial. A nonquantitative systematic review with appropriate search strategies and well-substantiated conclusions. Includes lower quality RCTs, clinical cohort studies, and case-controlled studies with non-biased selection of study participants and consistent findings. Other evidence, such as high-quality, historical, uncontrolled studies, or well-designed epidemiologic studies with compelling findings, is also included.
Level C (consensus/expert opinion): Consensus viewpoint or expert opinion.
Each rating is applied to a single reference in the article, not to the entire body of evidence that exists on a topic. Each label should include the letter rating (A, B, C), followed by the specific type of study for that reference. For example, following a level B rating, include one of these descriptors: (1) nonrandomized clinical trial; (2) nonquantitative systematic review; (3) lower quality RCT; (4) clinical cohort study; (5) case-controlled study; (6) historical uncontrolled study; (7) epidemiologic study.
Here are some examples of the way evidence ratings should appear in the text:
“To improve morbidity and mortality, most patients in congestive heart failure should be treated with an angiotensin-converting enzyme inhibitor. [Evidence level A, RCT]”
“The USPSTF recommends that clinicians routinely screen asymptomatic pregnant women 25 years and younger for chlamydial infection. [Evidence level B, non-randomized clinical trial]”
“The American Diabetes Association recommends screening for diabetes every three years in all patients at high risk of the disease, including all adults 45 years and older. [Evidence level C, expert opinion]”
When scientifically strong evidence does not exist to support a given clinical recommendation, you can point this out in the following way:
“Physical therapy is traditionally prescribed for the treatment of adhesive capsulitis (frozen shoulder), although there are no randomized outcomes studies of this approach.”
Format of the Review
Introduction.
The introduction should define the topic and purpose of the review and describe its relevance to family practice. The traditional way of doing this is to discuss the epidemiology of the condition, stating how many people have it at one point in time (prevalence) or what percentage of the population is expected to develop it over a given period of time (incidence). A more engaging way of doing this is to indicate how often a typical family physician is likely to encounter this problem during a week, month, year, or career. Emphasize the key CME objectives of the review and summarize them in a separate table entitled “CME Objectives.”
The methods section should briefly indicate how the literature search was conducted and what major sources of evidence were used. Ideally, indicate what predetermined criteria were used to include or exclude studies (e.g., studies had to be independently rated as being high quality by an established evaluation process, such as the Cochrane Collaboration). Be comprehensive in trying to identify all major relevant research. Critically evaluate the quality of research reviewed. Avoid selective referencing of only information that supports your conclusions. If there is controversy on a topic, address the full scope of the controversy.
The discussion can then follow the typical format of a clinical review article. It should touch on one or more of the following subtopics: etiology, pathophysiology, clinical presentation (signs and symptoms), diagnostic evaluation (history, physical examination, laboratory evaluation, and diagnostic imaging), differential diagnosis, treatment (goals, medical/surgical therapy, laboratory testing, patient education, and follow-up), prognosis, prevention, and future directions.
The review will be comprehensive and balanced if it acknowledges controversies, unresolved questions, recent developments, other viewpoints, and any apparent conflicts of interest or instances of bias that might affect the strength of the evidence presented. Emphasize an evidence-supported approach or, where little evidence exists, a consensus viewpoint. In the absence of a consensus viewpoint, you may describe generally accepted practices or discuss one or more reasoned approaches, but acknowledge that solid support for these recommendations is lacking.
In some cases, cost-effectiveness analyses may be important in deciding how to implement health care services, especially preventive services. 10 When relevant, mention high-quality cost-effectiveness analyses to help clarify the costs and health benefits associated with alternative interventions to achieve a given health outcome. Highlight key points about diagnosis and treatment in the discussion and include a summary table of the key take-home points. These points are not necessarily the same as the key recommendations, whose level of evidence is rated, although some of them will be.
Use tables, figures, and illustrations to highlight key points, and present a step-wise, algorithmic approach to diagnosis or treatment when possible.
Rate the evidence for key statements, especially treatment recommendations. We expect that most articles will have at most two to four key statements; some will have none. Rate only those statements that have corresponding references and base the rating on the quality and level of evidence presented in the supporting citations. Use primary sources (original research, RCTs, meta-analyses, and systematic reviews) as the basis for determining the level of evidence. In other words, the supporting citation should be a primary research source of the information, not a secondary source (such as a nonsystematic review article or a textbook) that simply cites the original source. Systematic reviews that analyze multiple RCTs are good sources for determining ratings of evidence.
The references should include the most current and important sources of support for key statements (i.e., studies referred to, new information, controversial material, specific quantitative data, and information that would not usually be found in most general reference textbooks). Generally, these references will be key evidence-based recommendations, meta-analyses, or landmark articles. Although some journals publish exhaustive lists of reference citations, AFP prefers to include a succinct list of key references. (We will make more extensive reference lists available on our Web site or provide links to your personal reference list.)
You may use the following checklist to ensure the completeness of your evidence-based review article; use the source list of reviews to identify important sources of evidence-based medicine materials.
Checklist for an Evidence-Based Clinical Review Article
The topic is common in family practice, especially topics in which there is new, important information about diagnosis or treatment.
The introduction defines the topic and the purpose of the review, and describes its relevance to family practice.
A table of CME objectives for the review is included.
The review states how you did your literature search and indicates what sources you checked to ensure a comprehensive assessment of relevant studies (e.g., MEDLINE, the Cochrane Collaboration Database, the Center for Research Support, TRIP Database).
Several sources of evidence-based reviews on the topic are evaluated ( Table 1 ) .
Where possible, POEM (dealing with changes in morbidity, mortality, or quality of life) rather than DOE (dealing with mechanistic explanations or surrogate end points, such as changes in laboratory tests) is used to support key clinical recommendations ( Table 2 ) .
Studies of patients likely to be representative of those in primary care practices, rather than subspecialty referral centers, are emphasized.
Studies that are not only statistically significant but also clinically significant are emphasized; e.g., interventions with meaningful changes in absolute risk reduction and low numbers needed to treat. (See http://www.cebm.net/index.aspx?o=1116 .) 11
The level of evidence for key clinical recommendations is labeled using the following rating scale: level A (RCT/meta-analysis), level B (other evidence), and level C (consensus/expert opinion).
Acknowledge controversies, recent developments, other viewpoints, and any apparent conflicts of interest or instances of bias that might affect the strength of the evidence presented.
Highlight key points about diagnosis and treatment in the discussion and include a summary table of key take-home points.
Use tables, figures, and illustrations to highlight key points and present a step-wise, algorithmic approach to diagnosis or treatment when possible.
Emphasize evidence-based guidelines and primary research studies, rather than other review articles, unless they are systematic reviews.
The essential elements of this checklist are summarized in Table 3 .
Siwek J. Reading and evaluating clinical review articles. Am Fam Physician. 1997;55:2064-2069.
Shaughnessy AF, Slawson DC. Getting the most from review articles: a guide for readers and writers. Am Fam Physician. 1997;55:2155-60.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-17.
Flynn CA, D'Amico F, Smith G. Should we patch corneal abrasions? A meta-analysis. J Fam Pract. 1998;47:264-70.
Slawson DC, Shaughnessy AF, Bennett JH. Becoming a medical information master: feeling good about not knowing everything. J Fam Pract. 1994;38:505-13.
Shaughnessy AF, Slawson DC, Bennett JH. Becoming an information master: a guidebook to the medical information jungle. J Fam Pract. 1994;39:489-99.
Slawson DC, Shaughnessy AF. Becoming an information master: using POEMs to change practice with confidence. Patient-oriented evidence that matters. J Fam Pract. 2000;49:63-7.
Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Methods Work Group, Third U.S. Preventive Services Task Force. Current methods of the U.S. Preventive Services Task Force. A review of the process. Am J Prev Med. 2001;20(3 suppl):21-35.
CATbank topics: levels of evidence and grades of recommendations. Retrieved November 2001, from: http://www.cebm.net/ .
Saha S, Hoerger TJ, Pignone MP, Teutsch SM, Helfand M, Mandelblatt JS. for the Cost Work Group of the Third U.S. Preventive Services Task Force. The art and science of incorporating cost effectiveness into evidence-based recommendations for clinical preventive services. Am J Prev Med. 2001;20(3 suppl):36-43.
Evidence-based medicine glossary. Retrieved November 2001, from: http://www.cebm.net/index.aspx?o=1116 .
Continue Reading
More in afp, more in pubmed.
Copyright © 2002 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- Open access
- Published: 22 November 2017
A systematic literature review of evidence-based clinical practice for rare diseases: what are the perceived and real barriers for improving the evidence and how can they be overcome?
- Ana Rath 1 ,
- Valérie Salamon 1 ,
- Sandra Peixoto 1 ,
- Virginie Hivert 2 ,
- Martine Laville 3 ,
- Berenice Segrestin 3 ,
- Edmund A. M. Neugebauer 4 ,
- Michaela Eikermann 5 ,
- Vittorio Bertele 6 ,
- Silvio Garattini 6 ,
- Jørn Wetterslev 7 ,
- Rita Banzi 6 ,
- Janus C. Jakobsen 7 , 8 ,
- Snezana Djurisic ORCID: orcid.org/0000-0001-8259-8250 7 ,
- Christine Kubiak 9 ,
- Jacques Demotes-Mainard 9 &
- Christian Gluud 7
Trials volume 18 , Article number: 556 ( 2017 ) Cite this article
6327 Accesses
50 Citations
2 Altmetric
Metrics details
Evidence-based clinical practice is challenging in all fields, but poses special barriers in the field of rare diseases. The present paper summarises the main barriers faced by clinical research in rare diseases, and highlights opportunities for improvement.
Systematic literature searches without meta-analyses and internal European Clinical Research Infrastructure Network (ECRIN) communications during face-to-face meetings and telephone conferences from 2013 to 2017 within the context of the ECRIN Integrating Activity (ECRIN-IA) project.
Barriers specific to rare diseases comprise the difficulty to recruit participants because of rarity, scattering of patients, limited knowledge on natural history of diseases, difficulties to achieve accurate diagnosis and identify patients in health information systems, and difficulties choosing clinically relevant outcomes.
Conclusions
Evidence-based clinical practice for rare diseases should start by collecting clinical data in databases and registries; defining measurable patient-centred outcomes; and selecting appropriate study designs adapted to small study populations. Rare diseases constitute one of the most paradigmatic fields in which multi-stakeholder engagement, especially from patients, is needed for success. Clinical research infrastructures and expertise networks offer opportunities for establishing evidence-based clinical practice within rare diseases.
Peer Review reports
Clinical practice based on valid evidence is especially challenging in the field of rare diseases (RDs) [ 1 ], a group of diseases defined differently in several legislations. In Europe, diseases with prevalence equal to or lower that 5/10,000 inhabitants are considered rare [ 2 ]. In Asia, the definitions of RDs are < 1/10,000 inhabitants in Japan and Taiwan [ 3 ]. In the USA, a disease is considered rare if affecting fewer than 200,000 people, equivalent to about 6/10,000 inhabitants or less [ 4 ]. While some RDs are close to these prevalence thresholds, 10% to 20% are ultra-rare [ 5 , 6 ]. The distinction between rare and ultra-rare diseases is important because of its implication in the assessment of the value of orphan medicinal products [ 7 ].
There are roughly 6000 clinically different RDs spread in all medical specialties, the largest groups being developmental defects of genetic origin, cancers, neurological diseases, systemic and rheumatologic diseases, and inborn errors of metabolism [ 6 ]. RDs are, therefore, a heterogeneous field, mostly composed of chronic and life-threatening diseases, the only feature in common being the rarity which jeopardises the performance of the research and development process when compared to common diseases [ 8 ]. This is a matter of concern, as RDs are recognised as a major health issue, and are the target of an active European Union policy [ 9 , 10 , 11 , 12 ]. Moreover, incentives to the drug and device industries have been put into place to boost the development of therapies for RDs in the USA in 1983 [ 13 ] and in the European Union in 2000 [ 2 ].
Recently, the International Rare Diseases Research Consortium (IRDiRC) has challenged the international research community with two major objectives: to develop the capacity to diagnose most RDs, and to establish 200 new or repurposed therapies for RDs by 2020 [ 14 ]. As of December 2015, a total of 118 orphan medicinal products have reached the market in Europe intended for about 107 diseases [ 15 ] and 432 orphan medicinal products have reached the market in the USA [ 16 ]. These results are good, but are far from meeting the needs of RD patients [ 17 , 18 ]. Furthermore, the attrition of treatment options during the research and development process seems worse than with common diseases.
About 10% of the market authorisations for medicinal products for RDs are granted at a stage were the evidence is not yet firmly established through accelerated approval or conditional approval [ 19 , 20 ]. Without such approvals, there is a need for the monitoring of patients treated with the new interventions for many more years. The concept of adaptive pathways has been proposed, which aims to grant marketing authorisations based on a lower weight of evidence justified by the claim that patients will have earlier access to treatment [ 21 , 22 ]. Adaptive pathways are based on stepwise learning under conditions of acknowledged uncertainty, with iterative phases of data gathering and regulatory evaluations [ 23 ]. However, it has been criticised for lacking scientific support and ethical ground, and thus, for increasing uncertainty about the benefit-harm balance of new medicinal products [ 21 , 22 ].
There is a need to build the evidence from basic research to bedside, through rigorous clinical research adapted to the intrinsic complexity of RDs [ 1 ]. The European Clinical Research Infrastructure Network (ECRIN) Integrating Activity (ECRIN-IA) project Footnote 1 [ 24 ] has identified barriers for good clinical research within trials in general as well as regarding RDs, nutrition, and medical devices, and assessed how these barriers can be broken down in order to improve the production of evidence-based clinical research [ 25 , 26 , 27 ]. The aims of this paper are to summarise the main barriers faced by clinical research in the field of RDs and to highlight the opportunities for improvement at the European and international level (Table 1 ). These main barriers should be seen as additions to the barriers threatening all clinical trials, namely inadequate knowledge and understanding of clinical research and trial methodology; lack of funding; excessive monitoring; restrictive interpretation of privacy law and lack of transparency; overly complex or inadequate regulatory requirements; and inadequate clinical research infrastructures [ 25 ].
The present paper is based on personal ECRIN communications during four face-to-face meetings and six telephone conferences from 2013 to 2017, and systematic literature searches in May 2016 for appropriate articles using the following databases: The Cochrane Library (Wiley) (Issue 5 of 12, 2016) (including the Cochrane Database of Systematic Reviews (CDSR)), CENTRAL, National Health Service Economic Evaluation Database (NHSEED), and Database of Abstracts of Reviews of Effects (DARE, U.S. Library of Medicine); MEDLINE (Ovid SP) (1946 to May 2016); EMBASE (Ovid SP) (1974 to May 2016); and Science Citation Index Expanded (1900 to May 2016), using different terms covering barriers, evidence-based medicine, and RDs. No meta-analyses were performed. The exact search strategy is provided in Additional file 1 . A PRISMA flow diagram depicting the selection process and a PRISMA Checklist are provided in Fig. 1 and Additional file 2 . Articles obtained from the systematic literature search, which were relevant to the field of RDs, were included in Additional file 3 . Articles were selected and referenced in the review if they if they contributed to the discussion and conclusions drawn by the ECRIN expert panel, and included valid considerations on how barriers to the conduct of randomised clinical trials (RCTs) on RDs could affect their number, feasibility, and quality. The results are described narratively, which is a limitation of the data collected.
PRISMA 2009 flow diagram. PRISMA flow diagram depicting the selection process of relevant academic literature
Results and discussion
Search results.
The systematic searches identified a total of 148 references. The screening process narrowed the academic literature search down to 37 relevant references listed in Additional file 3 . Characteristics of included references: overviews and narrative reviews.
Main barriers related to clinical trials for rare diseases
Recruitment issues: a direct consequence from rarity.
Clinical trials on RDs are characterised by an intrinsic difficulty to identify patients. This problem resides in the difficulty to diagnose RDs, to record diseases, and to trace RD patients [ 28 ]. This is due in part to the scarce knowledge about these diseases, and to the fact that far from all countries have efficient processes for referral [ 29 ], resulting in significant delays in diagnosis. RD patients often remain undiagnosed even in the best conditions of expertise due to lack of knowledge about natural history or clinical signs and symptoms. However, the most recent technological developments such as lower-cost, next-generation gene sequencing is increasing the diagnostic capacity for monogenic diseases, thereby contributing to increased knowledge of potentially actionable ethiopathogenic mechanisms [ 30 ].
In all cases, RDs are poorly represented in medical nomenclatures used in health information systems [ 28 ], making it difficult to identify participants for clinical research from medical records. Most countries use the International Classification of Diseases (ICD-10) to record patients, where around 500 RDs have a specific code [ 31 ]. In countries using Systematized Nomenclature of Medicine (SNOMED), the situation is not much better because only around 40% of RDs are listed here (Ana Rath, personal communication on the Orphanet-SNOMED CT mapping exercise, August 2015).
Another source for identification of RD patients is disease-specific patient registries. There are 690 such registries in Europe, covering 984 RDs [ 32 ]. Most are national (482 registries), or regional (75 registries), with some being European (59 registries) or international (74 registries). However, quality, scope, and capacity of many registries are limited [ 28 ].
The geographical dispersion of patients requires multicentric, multinational collaboration, introducing additional regulatory and funding barriers. For severe RDs, travel to research centres may pose an insurmountable barrier to research participation. Solutions include the leveraging of technology to monitor patients remotely, and setting up community centres to better deliver these trials to patients who otherwise would be unable to access them [ 33 ]. Effective recruitment is also supported through partnership with patient organisations when they exist, but also with patient registries and centres of expertise.
These barriers hamper recruitment into clinical trials. In Europe, a voluntary policy has been undertaken in order to improve diagnostic rates, i.e. by enhancing the expertise of specialised centres, and to establish European Reference Networks (ERNs) expected to spread expertise and share best practice. ERNs are expected to catalyse the international cooperation and patient engagement needed for clinical research. In parallel, in order to increase the visibility of RD patients in health information systems, a specific standard nomenclature for RDs – the Orphanet nomenclature [ 34 ] – is promoted in the European member states [ 35 ]. The implementation of the Orphanet nomenclature of RDs (the Orphacode) which is linked to other nomenclatures and resources used both in the clinical setting (ICD-10, SNOMED Clinical Terms) and in the research setting (Online Mendelian Inheritance in Man (OMIM); Human Gene Nomenclature Committee (HGNC); Universal Protein Resource (UniProtKB); among others [ 31 ]) will make it possible to more easily identify patients from health records for clinical research. The Orphanet nomenclature should also enable data exploitation with the aim of improving knowledge of the natural history of RDs.
Limited knowledge on natural history of rare diseases
The natural history of most RDs is often difficult to document, yet it is a necessary step to inform to the trial design for the disease. Few relevant epidemiological studies are published due to the difficulty of identifying and documenting patients widely spread geographically, not always diagnosed properly, and rarely followed-up by academic centres in a systematic way. Most attempts to collect good quality data are supported by short-term grants, which do not allow continuity in the effort. The high cost of high-quality natural history studies has been a significant obstacle to their conduct. This is well identified as a barrier requiring solutions and has been the target of recommendations of the EU Committee of Experts on Rare Diseases (Commission Expert Groups on Rare Diseases (CEGRD), formerly EUCERD) [ 36 ] and of the International Rare Diseases Research Consortium [ 37 ]. The lack of natural history information provides little insight into how to choose outcomes or how to design and power a clinical trial. When disease-specific registries meet quality standards, their relevance for contributing to high-quality clinical trials is demonstrated [ 38 ]. Structure and design of natural history studies are pivotal to capture clinical information efficiently in order to be used in safety and efficacy determination. Knowledge of natural history is one of the first crucial steps for building evidence as it allows for a better choice of clinically relevant outcomes as well as of the duration needed to monitor for them to occur [ 33 ]. There is a need to capture clinical information more cost-efficiently and to help inform the optimal approach to treatment development. Data collection in the framework of European Reference Networks should be encouraged and facilitated by common interoperability standards and tools to address this issue.
Need for trial designs adapted to the small population size and clinical heterogeneity
RCTs are the goal standard for producing evidence on the efficacy of an intervention because they have a strong internal validity by minimising bias and confounder factors [ 1 , 39 ]. Systematic reviews of RCTs provide the highest level of evidence assessing the benefits and harms of interventions [ 1 ]. However, randomisation can prove to be difficult with RDs, mostly because of the small size of the patient population.
The European Medicines Agency (EMA), in a guideline on trials in RD populations, stated that there are no methods relevant for small trials that are not also applicable to large studies [ 40 ]. The problem for trials in RD populations is that the reverse would lead to requests for sample sizes that are not practicable, or simply impossible to reach.
The traditional RCT designs are difficult to conduct in small populations because it is very difficult to create homogeneous groups and to adequately assess changes between variable groups. Alternative methods have been proposed and could be applicable under certain conditions. We will briefly discuss some of them here. For in-depth analyses and comparisons between some of these different trial designs, see references [ 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 57 ].
The traditional fixed error rates (alpha = 0.05; beta = 0.20) cannot capture all desirable inferences in different clinical research settings. Therefore, Ioannidis and colleagues have developed models that optimise the selection of type I and type II errors according to available sample size and a plausible intervention effect [ 50 ].
Controlled rigorous designs that allow within-patient comparisons and treat all participants would assess therapies more accurately if feasible. Such study designs comprise n -of-1 designs and crossover trials. Both assess efficacy of a treatment based on short-term outcomes and mitigate the effects of clinical heterogeneity in a patient population [ 39 , 40 ].
Pragmatic RCTs could represent an alternative to early phase RCTs while keeping most of their methodological advantages. These pragmatic trials are intended to inform decisions in common practice, so eligibility criteria are more inclusive, comparisons are done against standards of care instead of placebo, and follow-up tends to evaluate longer-term effects than early phase RCTs [ 39 ].
Vickers and Scardino and Potter et al. argue for a wide adoption of what they call ‘clinically integrated’ or ‘hybrid design’ RCTs [ 39 , 51 ]. These trials incorporate aspects of observational and interventional trials (for instance, cohort multiple randomised controlled trials – cmRCTs), thus allowing for a more efficient knowledge transfer into real-world clinical practice. The designs promote longitudinal observational data collection (registries and cohorts). Such investment would be, in our view, the most efficient use of resources in the long term, as it allows for a better understanding of diseases and for the assessment of different interventions over time in a controlled way while knowledge progresses.
Other study designs more focused on proving the efficacy of interventions, more often drugs that are expected to transform the disease course, include adaptive designs. Response-adaptive methods change allocation ratios depending on which treatment appears to be best. Adaptive methods are defined by the EMA as a ‘statistical methodology (that) allows the modification of a design element (e.g. sample size, randomisation ratio, number of treatment arms) at an interim analysis with full control of type I error’ [ 52 ]. Adaptive trials are complex and need even stronger measures to prevent biasing adaptive decisions in the course of the trial [ 53 ]. Mauer and EORTC collaborators, for instance, point out the fact that regulatory and financial management need to be adaptive as well, so such trials increase the organisational and economical burdens [ 50 ]. Adaptive methods rely on real-time data, which may be easier in RD trials because recruitment tends to be slower. Some adaptive designs are now used for rare cancers [ 53 ]. Other sequential adaptive methods are proposed for testing different therapeutic possibilities in a small population [ 54 , 55 ]. Regulators accept or recommend some of these designs [ 56 ]. For an in extenso review of the different designs available and their acceptance by regulatory bodies (FDA, EMA) please see Billingham et al. [ 57 ].
The RD field needs the development of cost-efficient, novel, rigorous controlled trial designs and relevant analyses that are effective in studying efficacy in heterogeneous, small populations. Recently, the European Commission funded three projects in this area [ 58 , 59 , 60 ]. In addition, the IRDiRC consortium has established a task force to address the question and produce recommendations [ 61 ].
As for any other disease, the laws of probability and statistics apply to RDs. Therefore, valid evidence on interventions requires valid clinical research in the form of large, well-conducted RCTs [ 1 , 7 , 39 , 45 ].
Organisational challenges: a consequence from the need for multinational randomised clinical trials
Patients with most RDs are not so few as to prevent conducting large RCTs. In the EU, a prevalence of 1/100,000 with a RD (i.e. well below the threshold of 5/10,000) results in an availability of 5000 potential trial participants [ 62 ]. It requires, however, multinational cooperation, which introduces a new line of barriers in the form of comprehensive organisational, regulatory, and economical requirements. The identification of partners having both the expertise and the capacity to conduct international RCTs, the organisation of the collaboration, and also of the monitoring and follow-up are challenging. The collection and maintenance of high-quality data among all parties involved is a major issue, and specific measures should be put in place to ensure the best, easy-to-use quality. These challenges are greater in RDs, as they often need a multi-disciplinary management team as well as professionals from diverse hospital departments, which makes monitoring and organisation more complex.
Different legal frameworks in different countries contribute to the regulatory barriers of conducting multicentre international RCTs. Heterogeneity can involve all the following: ethics committee submission, patient information and consent, insurance acquisition, activation of the clinical centre, data protection rules, and investigator reimbursement. The need for harmonised procedures has been addressed by setting the Voluntary Harmonisation Procedure (VHP) in the European Union in order to organise the assessment of multinational RCTs, which is the responsibility of the Clinical Trials Facilitation Group of the Heads of Medicines Agencies (HMA) [ 63 ]. From 2009 to 2015, 22% of European clinical trials underwent that procedure, the numbers having increased impressively over time [ 64 ]. When the European clinical trials regulation is implemented in 2018 [ 65 ], the need for VHP is expected to decrease or completely disappear.
Need for more sensitive outcomes to quantify clinical benefit
Maybe more than in other fields, RDs are often characterised by important clinical variability, including age of onset, severity, speed of evolution, responsiveness to treatment, global impact in health status, and functional consequences. This situation leads to a very large range at baseline for many measures of efficacy, making it hard to detect important changes of an intervention. In fact, traditional RCTs assess average treatment impact in selected patients, and thus do not accommodate clinical heterogeneity very well [ 39 ]. Researchers often use surrogate outcomes to measure the effects of an intervention [ 66 ]. Such surrogates must be correlated to a clinically meaningful outcome. However, correlation alone does not make a surrogate valid [ 66 ]. Intensive analyses linking the intervention effect on the surrogate to patient-centred outcomes are needed [ 66 , 67 , 68 , 69 ]. Biomarker development is one source for potential surrogate outcomes. FDA and EMA orphan drug regulations contemplate approval of drugs for which the benefits for patients with unmet medical needs are based on reasonable evidence, often based on surrogate outcomes, that should demonstrate their clinical benefit during post-marketed studies [ 29 ]. Such ‘adaptive’ pathways and procedures seem to have their special problems making them less attractive or outright dangerous to patients [ 21 , 70 ]. Drug or medical device companies could base their marketing authorisation applications on uncontrolled or controlled observational studies rather than pivotal RCTs. Such applications could lead to marketing approval of interventions that are without effect or that are even harmful. Such interventions are difficult or impossible to remove from the market.
However, both patients and decision-makers will seek more patient-centred, clinically relevant benefits [ 39 ]. These patient-centred outcomes can be reported by clinicians (clinician-reported outcome measures) or other observers (observer-reported outcome measures), or by the patients themselves (patient-reported outcome measures, PROM) [ 71 ].
On the other hand, the frequent complexity of disease manifestations in multiple body systems may require more than one clinical outcome for one domain to adequately assess a clinically effective treatment. That puts extra burden on the statistical assessment of outcomes [ 72 ]. As single clinical outcomes may not adequately cover the multiple expression of a disease, novel approaches to combine independent clinical outcomes in multi-domain analyses could potentially help assessing the clinical efficacy of an intervention. However, such analyses are statistically complex, the weight of each clinical variable could not be adequately measured, and results could be difficult to compare from trial to trial [ 73 ]. Nevertheless, the development of multiple domain outcome strategies in smaller populations offers important advantages over single primary outcomes [ 74 ]. Well-chosen and designed multiple domain outcomes would capture broader therapeutic data, provide greater insight into overall treatment effects, and allow successful small trials with compelling new treatments when the benefit might be varying between individual patients.
The development of agreed standardised sets of outcomes, the core outcome sets (COS) would result in better comparability between clinical studies, by defining the minimum outcomes that should be assessed when evaluating a new intervention. Initiatives like COMET develop both COS and a consensus core outcome sets database in which several RDs are represented [ 75 ]. A task force on patient-centred outcome measures have been set up by the IRDiRC, and a first overview and recommendations document has been open to public consultation [ 67 ]. The landscape of initiatives on the matter, including those concerning RDs, is depicted [ 71 ]. The International Society for Pharmacoeconomics and Outcomes Research (ISPOR)[ 76 ] has set up a task force on Rare Disease Trials Clinical Outcome Assessment (COA) measurement [ 77 ]. This task force aims to provide recommendations on the development of patient-centred outcome measures (PCOMs) in conformity to the regulatory guidance for the evaluation and proof of treatment benefits for medicinal products approval. The recent recommendations of the ISPOR Pediatric PRO Task Force [ 78 ] provide good research practices in developing and implementing paediatric patient-reported outcomes instruments and, therefore, is of interest for RDs, because most of them are paediatric.
Understanding the clinical meaningfulness of clinical changes in a patient is difficult without significant prior clinical experience. A systematic approach using natural history and comparable disease information has to be developed. The construction of the future evidence starts by the collection of natural history data in a systematic way (registries, cohorts) and by the capture of data from clinical records in a structured way. Patient engagement should be sought and encouraged from this early stage.
Need for involvement of all the stakeholders in the study design and conduct
The design and specific methodological aspects of a clinical trial need to be carefully discussed with all relevant partners. As stated in Potter et al. [ 39 ] ‘ ideally, evaluative research should incorporate outcomes that are of greatest importance with respect to treatment goals, based on a consensus among patients, clinical providers, researchers, and policy decision-makers ’. The final goal is to translate knowledge into clinical practice, based on the best evidence. Doing this requires effective interaction among stakeholders from the earliest phase, i.e. data collection to increase the knowledge of each RD. Thus, databases and registries should incorporate patients’ views. Some experiences exist already in which patients contribute directly to data collection [ 79 ].
Usually, a significant proportion of those with an RD must be enrolled in trials to reach the required sample size. The relationship between the clinician and the patient needs to be based on mutual trust for the patients to agree to take part and once in the trial, to stay in and provide outcome data. These data must answer a question that is important for patients, clinicians, and policy-makers, and data must be collected in such a way that taking part in the trial leaves a participant willing to take part in more.
A trial involving a RD population must, in short, be compellingly efficient and involve all stakeholders, especially patients, in its design. The regulators as well should be included in the discussion about the most appropriate design for a specific trial, as early as possible in the research process. Protocol assistance and scientific advice from regulatory bodies have been shown to play a key role in guiding the conduct of studies to address the benefit/risk analysis for marketing authorisation and approval [ 80 ]. However, the scientific advice provided by regulatory authorities poses serious concerns about conflict of interests as it is delivered by the very same agency that will grant the marketing approval at a later time point.
Centres with expertise in RDs should play a major role in fostering clinical research networks and infrastructures and in disseminating and sharing study outcomes. Training of investigators and patients’ representatives will ensure a better understanding of regulatory, methodological, and ethical requirements. The development of European reference networks in the coming months offers an opportunity to put this statement into practice. In addition, support from organisations, such as ECRIN, can greatly enhance the organisation and management of multinational clinical trials on RDs. In effect, ECRIN brings together national networks of clinical trial units across Europe, making it possible to accelerate patient recruitment and trial implementation, while ensuring the appropriate management services for smooth trial conduct. By using ECRIN (or a similar infrastructure), there is an opportunity to develop common and harmonised practices for the submission, monitoring and reporting of multicentre and multinational RD clinical trials.
The main barriers described above for conducting RCTs in RD patients should be seen as additions to the barriers facing all clinical trials: inadequate knowledge and understanding of clinical research and trial methodology; lack of funding; excessive monitoring; restrictive interpretation of privacy law and lack of transparency; overly complex or inadequate regulatory requirements; and inadequate clinical research infrastructures [ 25 ].
The area of RDs is in particular need of a concerted approach of all interested parties as the challenges due to rarity are especially complex. There is a need for solid evidence before offering innovative treatments to patients [ 73 ]. The difficulties can only be overcome if a multi-stakeholder dialogue is going on, which is the recommendation of the EUCERD [ 81 ] and ICORD [ 82 ]. This multi-stakeholder approach is needed from the earliest stages of the construction of evidence, before any clinical research is commenced. Data production and collection in a way they can be shared, exploited, and re-used is a key issue to increase the knowledge of the natural history of each RD, thus identifying clinically relevant indicators for patient-centred care based on evidence [ 83 ]. Identification of optimal future outcomes is mandatory when designing future trials. Simulations may help to decide on the most appropriate study design [ 84 ]. Patient registries or systematic collections should take into consideration the views of patients. The establishment of multicentre databases/registries in a structured way, taking into account the requirements of high-quality clinical research and of regulatory exigencies, is mandatory to achieve good-quality clinical research [ 85 ]. The future European reference networks could provide a timely opportunity to work this way with the aim to conduct clinical ‘research done differently’ [ 86 ], meaning clinical effectiveness research (CER) with a clear focus on including stakeholders in the planning activities. They should also be the place for implementing educational training on clinical research for clinicians which is an already identified limitation in the conduct of multicentric RCTs [ 87 ].
However, clinical research for RDs poses specific problems that need to be addressed, and they have been roughly summarised in this paper. Multinational RCTs are needed in order to increase the size of the population studied despite the fact that this may create organisational, monitoring, and regulatory burdens. Tools and support provided by ECRIN are aimed to help overcome these barriers. Several multinational RCTs on RDs are already being conducted with the support of ECRIN [ 24 ].
If the data originate from small populations, there are special problems when two or more RCTs should be meta-analysed [ 88 ]. In these situations, special analytic methods need to be considered [ 88 ]. In these situations, using frequentist methods, it is also important to assess data with Trial Sequential Analysis to control random type I and type II errors due to sparse data and repetitive testing [ 1 , 89 , 90 ]. For a given required information size, a corresponding number of required trials exist. By increasing the number of trials, one can increase the power of a random-effects model meta-analysis [ 91 ].
Addressing the specific problems posed by RD clinical research is of paramount importance to provide best practice medical management to these patients. Improvements of the methodologies used for establishing evidence-based clinical practice within RD will also benefit clinical research on ‘personalised medicine’ for more common diseases. We need to make published clinical research become more valid, and to get all clinical research published, including research with negative results [ 1 , 83 , 92 , 93 , 94 , 95 , 96 , 97 , 98 ].
Funded by the European Union Framework Programme 7 (EU FP7; grant agreement no. 284395), ECRIN-IA involved 23 countries and brought together diverse stakeholders to overcome barriers to clinical research in three particularly difficult areas (rare diseases, medical devices, and nutrition). Specifically, the project aimed to develop tools, services, and infrastructure to facilitate multinational clinical research in Europe, and to support the development of pan-European disease networks to drive clinical projects. This in turn was intended to improve Europe’s attractiveness to industry, boost its scientific competitiveness, and result in better healthcare for European citizens. Originally planned for 4 years (2012 to 2015), the clinical trials work package was extended until 2017.
Abbreviations
Cochrane Database of Systematic Reviews
Commission Expert Groups on Rare Diseases
Clinical effectiveness research
Cohort multiple randomised controlled trial
Clinical outcome assessment
Core Outcome Measures in Effectiveness Trials
Core outcome sets
Database of Abstracts of Reviews of Effects
European Clinical Infrastructure Network
European Medicines Agency
European Organisation for Research and Treatment of Cancer
European Union Committee of Experts on Rare Diseases
Food and Drug Administration
Human Gene Nomenclature Committee
Head of Medicines Agencies
International Classification of Diseases
International Conference on Rare Diseases and Orphan Drugs
International Rare Diseases Research Consortium
International Society for Pharmacoeconomics and Outcomes Research
National Health Service Economic Evaluation Database
Online Mendelian Inheritance in Man
Patient-centred outcome measures
Patient-reported outcomes
Patient-reported outcome measures
Randomised clinical trial
- Rare diseases
Systematized Nomenclature of Medicine
Universal Protein Resource
Voluntary Harmonisation Procedure
Garattini S, Jakobsen JC, Wetterslev J, Bertele V, Banzi R, Rath A, et al. Evidence-based clinical practice: overview of threats to the validity of evidence and how to minimise them. Eur J Intern Med. 2016;32:13–21. Epub 11 May 2016.
Article PubMed Google Scholar
Regulation (EC) No 141/2000 of The European Parliament and of The Council of 16 December 1999 on orphan medicinal products. 2000. [cited 2017 November 14]; Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2000:018:0001:0005:en:PDF .
Song P, Gao J, Inagaki Y, Kokudo N, Tang W. Rare diseases, orphan drugs, and their regulation in Asia: current status and future perspectives. Intractable Rare Dis Res. 2012;1(1):3–9. Epub 1 Feb 2012.
PubMed PubMed Central Google Scholar
National Institute of Health. Public Law 107–280 107th Congress. Rare Diseases Act. 2002. [cited 2017 November 14]; Available from: https://history.nih.gov/research/downloads/PL107-280.pdf .
Rath AM, Nguengang Wakap S. Orphanet Report Series - Prevalence of rare diseases: Bibliographic data, Number 1. 2017. [cited 2017 November 14]; Available from: http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf .
Orphanet. 2017 [cited 2017 November 14]; Available from: http://www.orpha.net/consor/cgi-bin/index.php .
Schlander M, Garattini S, Holm S, Kolominsky-Rabas P, Nord E, Persson U, et al. Incremental cost per quality-adjusted life year gained? The need for alternative methods to evaluate medical interventions for ultra-rare disorders. J Comp Effectiveness Res. 2014;3(4):399–422. Epub 3 Oct 2014.
Article Google Scholar
Bell SA, Tudur Smith C. A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis. 2014;9:170. Epub 28 Nov 2014.
Article PubMed PubMed Central Google Scholar
European Union. Council Recommendation of 8 June 2009 on an action in the field of rare diseases. 2009. [cited 2017 November 14]; Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007:0010:EN:PDF .
Aymé S, Rodwell C. European Committee Expert Group on Rare Diseases. Report on the State of the Art of Rare Disease Activities in Europe. 2014. [cited 2017 November 14]; Available from: http://www.eucerd.eu/?page_id=15 .
Commission Of The European Communities. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of Regions on Rare Diseases: Europe’s challenge 2008. [cited 2017 November 14]; Available from: http://ec.europa.eu/health/ph_threats/non_com/docs/rare_com_en.pdf .
European Commission. Implementation Report on the Commission Communication on Rare Diseases: Europe’s challenges and council recommendation of 8 June 2009 on an action in the field of rare diseases. 2009. [cited 2017 November 14); Available from: https://ec.europa.eu/health//sites/health/files/rare_diseases/docs/2014_rarediseases_implementationreport_en.pdf .
U.S. Department of Health and Human Services. Federal Regulations. Title 21: Food and Drugs. PART 316—ORPHAN DRUGS. 2015. [cited 2017 November 14]; Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=316&showFR=1 .
International Rare Diseases Research Consortium. About. 2016. [cited 2017 November 14]; Available from: http://www.irdirc.org/ .
Rath AM, Salamon V. Orphanet Report Series - Lists of medicinal products for rare diseases in Europe. 2017. [cited 2017 November 14]; Available from: http://www.orpha.net/orphacom/cahiers/docs/GB/list_of_orphan_drugs_in_europe.pdf .
Food and Drug Administration. Rare Disease and Orphan Drug Designated Approvals. 2016. [cited 2017 November 14]; Available from: http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/drugandbiologicapprovalreports/ndaandblaapprovalreports/ucm373419.htm .
Joppi R, Bertele V, Garattini S. Orphan drugs, orphan diseases. The first decade of orphan drug legislation in the EU. Eur J Clin Pharmacol. 2013;69(4):1009–24. Epub 24 Oct 2012.
Westermark K, Holm BB, Soderholm M, Llinares-Garcia J, Riviere F, Aarum S, et al. European regulation on orphan medicinal products: 10 years of experience and future perspectives. Nat Rev Drug Discov. 2011;10(5):341–9. Epub 3 May 2011.
Food and Drug Administration. Novel Drugs Summary. 2015. [cited 2017 November 14]; Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm474696.htm .
European Medicines Agency. Latest news. 2016. [cited 2017 November 14]; Available from: http://www.ema.europa.eu/ .
Davis C, Lexchin J, Jefferson T, Gotzsche P, McKee M. ‘Adaptive pathways’ to drug authorisation: adapting to industry? BMJ. 2016;354:i4437. Epub 18 Aug 2016.
Health Action International, The International Society of Drug Bulletins, The Medicines in Europe Forum, The Mario Negri Institute for Pharmacological Research, Centre TNC. Joint Briefing Paper. ‘Adaptive licensing’ or ‘adaptive pathways’: Deregulation under the guise of earlier access. Brussels. 2015. [cited 2017 November 14]; Available from: http://www.isdbweb.org/en/publications/view/adaptive-licensing-or-adaptive-pathways-deregulation-under-the-guise-of-earlier-access .
Eichler HG, Oye K, Baird LG, Abadie E, Brown J, Drum CL, et al. Adaptive licensing: taking the next step in the evolution of drug approval. Clin Pharmacol Ther. 2012;91(3):426–37. Epub 18 Feb 2012.
Demotes-Mainard J, Kubiak C. A European perspective—the European clinical research infrastructures network. Ann Oncol. 2011;22 Suppl 7:vii44–9. Epub 9 Nov 2011.
Djurisic S, Rath A, Gaber S, Garattini S, Bertele V, Ngwabyt SN, et al. Barriers to the conduct of randomised clinical trials within all disease areas. Trials. 2017;18(1):360.
Laville M, Segrestin B, Masson Y, Ruano-Rodríguez C, Serra-Majem L, Hyesmaye M, et al. Evidence-based practice within nutrition: what are the barriers for improving the evidence and how can they be dealt with? Trials. 2017;18(1):425.
Neugebauer EAM, Rath A, Antoine S-L, Eikermann M, Seidel D, Koenen C, et al. Specific barriers to the conduct of randomised clinical trials on medical devices. Trials. 2017;18(1):427.
Valdez R, Ouyang L, Bolen J. Public health and rare diseases: oxymoron no more. Prev Chronic Dis. 2016;13:E05. Epub 15 Jan 2016.
Shash E, Negrouk A, Marreaud S, Golfinopoulos V, Lacombe D, Meunier F. International clinical trials setting for rare cancers: organisational and regulatory constraints-the EORTC perspective. Ecancermedicalscience. 2013;7:321. Epub 30 May 2013.
Boycott KM, Vanstone MR, Bulman DE, MacKenzie AE. Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat Rev Genet. 2013;14(10):681–91. Epub 4 Sep 2013.
Article CAS PubMed Google Scholar
Orphanet. Orphadata. 2016. [cited 2017 November 14]; Available from: http://www.orphadata.org/cgi-bin/inc/product1.inc.php .
Rath AM, Peixoto S. Disease registries in Europe. 2016. [cited 2017 November 14]; Available from: http://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf .
Augustine EF, Adams HR, Mink JW. Clinical trials in rare disease: challenges and opportunities. J Child Neurol. 2013;28(9):1142–50. Epub 10 Sep 2013.
Rath A, Olry A, Dhombres F, Brandt MM, Urbero B, Ayme S. Representation of rare diseases in health information systems: the Orphanet approach to serve a wide range of end users. Hum Mutat. 2012;33(5):803–8. Epub 17 Mar 2012.
Commission Expert Group for Rare Diseases. Recommendation on ways to improve codification for rare diseases. 2014. [cited 2017 November 14]; Available from: https://ec.europa.eu/health//sites/health/files/rare_diseases/docs/recommendation_coding_cegrd_en.pdf .
European Union Committee of Experts on Rare Diseases. EUCERD core recommendations on rare disease patient registration and data collection to the European Commission, Member States and all stakeholders. 2013. [cited 2017 November 14]; Available from: http://www.eucerd.eu/wp-content/uploads/2013/06/EUCERD_Recommendations_RDRegistryDataCollection_adopted.pdf .
International Rare Diseases Research Consortium. Policies and guidelines. 2013. [cited 2017 November 14); Available from: http://www.irdirc.org/wp-content/uploads/2013/06/IRDiRC_policies_24MayApr2013.pdf .
Rodger S, Lochmuller H, Tassoni A, Gramsch K, Konig K, Bushby K, et al. The TREAT-NMD care and trial site registry: an online registry to facilitate clinical research for neuromuscular diseases. Orphanet J Rare Dis. 2013;8:171. Epub 24 Oct 2013.
Potter BK, Khangura SD, Tingley K, Chakraborty P, Little J. Translating rare-disease therapies into improved care for patients and families: what are the right outcomes, designs, and engagement approaches in health-systems research? Genet Med. 2016;18(2):117–23. Epub 10 Apr 2015.
Tudur Smith C, Williamson PR, Beresford MW. Methodology of clinical trials for rare diseases. Best Pract Res Clin Rheumatol. 2014;28(2):247–62. Epub 30 Jun 2014.
Gupta S, Faughnan ME, Tomlinson GA, Bayoumi AM. A framework for applying unfamiliar trial designs in studies of rare diseases. J Clin Epidemiol. 2011;64(10):1085–94. Epub 3 May 2011.
Korn EL, McShane LM, Freidlin B. Statistical challenges in the evaluation of treatments for small patient populations. Sci Transl Med. 2013;5(178):178sr3. Epub 29 Mar 2013.
Cornu C, Kassai B, Fisch R, Chiron C, Alberti C, Guerrini R, et al. Experimental designs for small randomised clinical trials: an algorithm for choice. Orphanet J Rare Dis. 2013;8:48. Epub 28 Mar 2013.
Gagne JJ, Thompson L, O’Keefe K, Kesselheim AS. Innovative research methods for studying treatments for rare diseases: methodological review. BMJ. 2014;349:g6802. Epub 26 Nov 2014.
Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–76. Epub 11 Apr 2013.
Parmar MK, Sydes MR, Morris TP. How do you design randomised trials for smaller populations? A framework. BMC Med. 2016;14(1):183. Epub 26 Nov 2016.
Hilgers RD, Roes K, Stallard N. Directions for new developments on statistical design and analysis of small population group trials. Orphanet J Rare Dis. 2016;11(1):78. Epub 16 Jun 2016.
Roes KC. A framework: make it useful to guide and improve practice of clinical trial design in smaller populations. BMC Med. 2016;14(1):195. Epub 26 Nov 2016.
Nikolakopoulos S, Roes KC, van der Tweel I. Sequential designs with small samples: evaluation and recommendations for normal responses. Stat Methods Med Res. 2016. Epub 28 Jun 2016.
Ioannidis JP, Hozo I, Djulbegovic B. Optimal type I and type II error pairs when the available sample size is fixed. J Clin Epidemiol. 2013;66(8):903–10. e2 Epub 15 May 2013.
Vickers AJ, Scardino PT. The clinically-integrated randomized trial: proposed novel method for conducting large trials at low cost. Trials. 2009;10:14. Epub 7 Mar 2009.
European Medicines Agency. Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design. 2007. [cited 2017 November 14); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003616.pdf .
Mauer M, Collette L, Bogaerts J. Adaptive designs at European Organisation for Research and Treatment of Cancer (EORTC) with a focus on adaptive sample size re-estimation based on interim-effect size. Eur J Cancer. 2012;48(9):1386–91. Epub 28 Jan 2012.
Tamura RN, Krischer JP, Pagnoux C, Micheletti R, Grayson PC, Chen YF, et al. A small n sequential multiple assignment randomized trial design for use in rare disease research. Contemp Clin Trials. 2016;46:48–51. Epub 21 Nov 2015.
Hlavin G, Koenig F, Male C, Posch M, Bauer P. Evidence, eminence and extrapolation. Stat Med. 2016;35(13):2117–32. Epub 13 Jan 2016.
Guideline on clinical trials in small populations. Doc. Ref. CHMP/EWP/83561/2005, 2006. [cited 2017 November 14); Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003615.pdf .
Billingham L, Malottki K, Steven N. Research methods to change clinical practice for patients with rare cancers. Lancet Oncol. 2016;17(2):e70–80. Epub 13 Feb 2016.
Rheinisch-Westfälische Technische Hochschule Aachen University. Integrated DEsign and AnaLysis of small population group trials (IDEAL). 2013. [cited 2017 November 14); Available from: http://www.rwth-aachen.de/go/id/ehgl/?lidx=1 .
Warwick Medical School. Innovative methodology for small populations research (INSPIRE). 2016. [cited 2017 November 14); Available from: https://www2.warwick.ac.uk/fac/med/research/hscience/stats/currentprojects/inspire/ .
Community Research and Development Information Service. Advances in Small Trials dEsign for Regulatory Innovation and eXcellence (ASTERIX). 2016. [cited 2017 November 14); Available from: http://cordis.europa.eu/projects/rcn/110076_en.html .
International Rare Diseases Research Consortium. International Rare Diseases Research Consortium’s Task Force on Small Population Clinical Trials. 2016. [cited 2017 November 14); Available from: http://www.irdirc.org/activities/current-activities/tf-spct/ .
Joppi R, Bertele V, Garattini S. Orphan drug development is not taking off. Br J Clin Pharmacol. 2009;67(5):494–502. Epub 26 Jun 2009.
Article CAS PubMed PubMed Central Google Scholar
Heads of Medicines Agencies. Clinical trials facilitation group. 2016. [cited 2017 November 14); Available from: http://www.hma.eu/ctfg.html .
Heads of Medicines Agencies. Metrics on participation of National Competent Authorities. 2016. [cited 2017 November 14); Available from: http://www.hma.eu/fileadmin/dateien/Human_Medicines/01-About_HMA/Working_Groups/CTFG/2016_05_CTFG_Metrics_on_NCA_participation_Jan15_Jan16.pdf .
European Medicines Agency. Updates. 2017. [cited 2017 November 14]; Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000629.jsp&mid=WC0b01ac05808768df .
Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. J Hepatol. 2007;46(4):734–42. Epub 24 Feb 2007.
Molenberghs G, Geys H, Buyse M. Evaluation of surrogate endpoints in randomized experiments with mixed discrete and continuous outcomes. Stat Med. 2001;20(20):3023–38. Epub 9 Oct 2001.
Molenberghs G, Burzykowski T, Alonso A, Assam P, Tilahun A, Buyse M. A unified framework for the evaluation of surrogate endpoints in mental-health clinical trials. Stat Methods Med Res. 2010;19(3):205–36. Epub 18 Jul 2009.
Ensor H, Lee RJ, Sudlow C, Weir CJ. Statistical approaches for evaluating surrogate outcomes in clinical trials: a systematic review. J Biopharm Stat. 2015:1–21. Epub 24 Sep 2015.
Joppi R, Gerardi C, Bertele V, Garattini S. Letting post-marketing bridge the evidence gap: the case of orphan drugs. BMJ. 2016;353:i2978. Epub 24 Jun 2016.
International Rare Diseases Research Consortium. Patient-centered outcome measures initiatives in the field of rare diseases. 2016. [cited 2017 November 14]; Available from: http://www.irdirc.org/wp-content/uploads/2016/03/PCOM_Post-Workshop_Report_Final.pdf .
Jakobsen JC, Gluud C, Winkel P, Lange T, Wetterslev J. The thresholds for statistical and clinical significance—a five-step procedure for evaluation of intervention effects in randomised clinical trials. BMC Med Res Methodol. 2014;14:34. Epub 5 Mar 2014.
Perea-Milla E, Aycaguer LC, Cerda JC, Saiz FG, Rivas-Ruiz F, Danet A, et al. Efficacy of prescribed injectable diacetylmorphine in the Andalusian trial: Bayesian analysis of responders and non-responders according to a multi domain outcome index. Trials. 2009;10:70. Epub 18 Aug 2009.
Putzeist M, Mantel-Teeuwisse AK, Wied CC, Hoes AW, Leufkens HG, de Vrueh RL. Drug development for exceptionally rare metabolic diseases: challenging but not impossible. Orphanet J Rare Dis. 2013;8:179. Epub 19 Nov 2013.
Core Outcome Measures in Effectiveness Trials Initiative. Core Outcome Measures in Effectiveness Trials (COMET). 2016. [cited 2017 November 14]; Available from: http://www.comet-initiative.org .
International Society for Pharmacoeconomics and Outcomes Research. About. 2017. [cited 2017 November 14]; Available from: http://www.ispor.org .
International Society for Pharmacoeconomics and Outcomes Research. Clinical Outcome Assessment (COA) in Rare Disease Clinical Trials—Emerging Good Practices: Report of the ISPOR Rare Disease Trials COA Measurement Task Force. 2016. [cited 2017 November 14]; Available from: http://www.ispor.org/TaskForces/ClinicalOutcomesAssessment-RareDisease-ClinicalTrials.asp .
Matza LS, Patrick DL, Riley AW, Alexander JJ, Rajmil L, Pleil AM, et al. Pediatric patient-reported outcome instruments for research to support medical product labeling: report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value Health. 2013;16(4):461–79. Epub 26 Jun 2013.
LEUKOTREAT. Therapeutic strategies for leukodystrophy affected patients. LeukoDataBase & Ethics. 2015. [cited 2017 November 14]; Available from: http://www.leukotreat.eu/leukodatabase-ethics.php .
Tafuri G, Pagnini M, Moseley J, Massari M, Petavy F, Behring A, et al. How aligned are the perspectives of EU regulators and HTA bodies? A comparative analysis of regulatory-HTA parallel scientific advice. Br J Clin Pharmacol. 2016;82(4):965–73. Epub 2 Jun 2016.
European Union Committee of Experts on Rare Diseases. Recommendation to the European Commission and Member States on improving informed decisions based on the clinical added value of orphan medicinal products information flow. 2012. [cited 2017 November 14]; Available from: http://www.eucerd.eu/?post_type=document&p=1446 .
Forman J, Taruscio D, Llera VA, Barrera LA, Cote TR, Edfjall C, et al. The need for worldwide policy and action plans for rare diseases. Acta Paediatr. 2012;101(8):805–7. Epub 24 Apr 2012.
Skoog M, Saarimäki JM, Gluud C, Sheinin M, Erlendsson K, Aamdal S. Transparency and registration in clinical research in the Nordic countries (Report). Nordic Trial Alliance, NordForsk. Oslo. Norge. 2015:1–108. (cited 2017 November 14]; Available from: http://nta.nordforsk.org/projects/nta_transparency_report.pdf .
Bajard A, Chabaud S, Cornu C, Castellan AC, Malik S, Kurbatova P, et al. An in silico approach helped to identify the best experimental design, population, and outcome for future randomized clinical trials. J Clin Epidemiol. 2016;69:125–36. Epub 19 Jul 2015.
Bolignano D, Pisano A. Good-quality research in rare diseases: trials and tribulations. Pediatr Nephrol. 2016;31:217-23. Epub 29 Jan 2016.
Patient-Centered Outcome Measures Research Institute. About. 2017. [cited 2017 November 14]; Available from: http://www.pcori.org/about-us .
Ahmed R, Duerr U, Gavenis K, Hilgers R, Gross O. Challenges for academic investigator-initiated pediatric trials for rare diseases. Clin Ther. 2014;36(2):184–90. Epub 18 Feb 2014.
Friede T, Rover C, Wandel S, Neuenschwander B. Meta-analysis of few small studies in orphan diseases. Res Synth Methods. 2016;8:79-91. Epub 30 Jun 2016.
Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75. Epub 18 Dec 2007.
Imberger G, Thorlund K, Gluud C, Wetterslev J. False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis: an empirical review. BMJ Open. 2016;6(8):e011890. Epub 16 Aug 2016.
Kulinskaya E, Wood J. Trial sequential methods for meta-analysis. Res Synth Methods. 2014;5(3):212–20. Epub 9 Jun 2015.
Ioannidis JP. How to make more published research true. PLoS Med. 2014;11(10):e1001747. Epub 22 Oct 2014.
Alberts B, Kirschner MW, Tilghman S, Varmus H. Rescuing US biomedical research from its systemic flaws. Proc Natl Acad Sci U S A. 2014;111(16):5773–7. Epub 16 Apr 2014.
Ioannidis JP, Fanelli D, Dunne DD, Goodman SN. Meta-research: evaluation and improvement of research methods and practices. PLoS Biol. 2015;13(10):e1002264. Epub 3 Oct 2015.
Gotzsche PC. Blinding during data analysis and writing of manuscripts. Control Clin Trials. 1996;17(4):285–90. discussion 90–3 Epub 1 Aug 1996.
MacCoun R, Perlmutter S. Blind analysis: hide results to seek the truth. Nature. 2015;526(7572):187–9. Epub 10 Oct 2015.
The Academy of Medical Sciences Medical Research Council. Symposium Report. Reproducibility and reliability of biomedical research: improving research practice. Welcome Trust. 2015 [cited 2017 November 14]; Available from: http://www.acmedsci.ac.uk/policy/policy-projects/reproducibility-and-reliability-of-biomedical-research/ .
Jarvinen TL, Sihvonen R, Bhandari M, Sprague S, Malmivaara A, Paavola M, et al. Blinded interpretation of study results can feasibly and effectively diminish interpretation bias. J Clin Epidemiol. 2014;67(7):769–72. Epub 25 Feb 2014.
Download references
Acknowledgements
The EU FP7 grant supporting the ECRIN-IA (GA 284395) provided support for meetings and is acknowledged for making the present paper possible. The Mario Negri Institute is thanked for housing the ECRIN-IA meeting in February 2013. All participants of ECRIN-IA are acknowledged for participating in discussions identifying the barriers and threats to the conduct of RCTs. Sarah Louise Klingenberg, the Trial Search Coordinator of The Cochrane Hepato-Biliary Group at The Copenhagen Trial Unit, is thanked for conducting the academic literature searches.
The present review is founded by the European Commission through a grant awarded for the European Clinical Research Infrastructure Network-Integrated Activity project. Project reference: 284395. Funded under: FP7-INFRASTRUCTURES. The funding sources had no influence on data collection, design, analysis, interpretation, or any aspect pertinent to the study.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and affiliations.
Orphanet, Institut National de la Santé et de la Recherche Médicale (INSERM), Paris, France
Ana Rath, Valérie Salamon & Sandra Peixoto
EURORDIS – European Organisation for Rare Diseases, Paris, France
Virginie Hivert
Centre de Recherche en Nutrition Humaine Rhone-Alpes, Université de Lyon 1, Hospices Civils de Lyon, Groupement Hospitaler Sud, Pierre Benite, France
Martine Laville & Berenice Segrestin
Brandenburg Medical School, Neuruppin, and Witten/Herdecke University, Witten, Germany
Edmund A. M. Neugebauer
Institute for Research in Operative Medicine, Witten/Herdecke University, Witten and Brandenburg Medical School, Neuruppin, Germany
Michaela Eikermann
IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy
Vittorio Bertele, Silvio Garattini & Rita Banzi
Copenhagen Trial Unit, Centre for Clinical Intervention Research, Department 7812, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark
Jørn Wetterslev, Janus C. Jakobsen, Snezana Djurisic & Christian Gluud
Department of Cardiology, Holbæk Hospital, Holbæek, Denmark
Janus C. Jakobsen
European Clinical Research Infrastructure Network (ECRIN), Paris, France
Christine Kubiak & Jacques Demotes-Mainard
You can also search for this author in PubMed Google Scholar
Contributions
AR, SD, and CG drafted the manuscript. SD and CG performed systematic literature searches and selected the papers. VS, SP, VH, ML, BS, EAMN, ME, VB, SG, JW, RB, JCJ, CK, and JD critically revised the manuscript for important intellectual content. All authors had full access to all the data (including PDFs of the articles and the search results) and can take responsibility for the integrity of the data. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Snezana Djurisic or Christian Gluud .
Ethics declarations
Authors’ information.
Not applicable
Ethics approval and consent to participate
Consent for publication, competing interests.
All authors are involved in the ECRIN-IA project. AR, VS, SP, and VH work at Orphanet, a reference portal for information on rare diseases and orphan drugs for all audiences. Orphanet’s aim is to help improve the diagnosis, care, and treatment of patients with rare diseases. No additional conflicts are known.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:.
Academic literature search strategy. Exact search strategy applied for analyses. (DOCX 13 kb)
Additional file 2:
PRISMA 2009 Checklist. (DOC 63 kb)
Additional file 3:
Relevant references from academic literature search. Results listed from literature search in the form of relevant publications. (DOCX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Rath, A., Salamon, V., Peixoto, S. et al. A systematic literature review of evidence-based clinical practice for rare diseases: what are the perceived and real barriers for improving the evidence and how can they be overcome?. Trials 18 , 556 (2017). https://doi.org/10.1186/s13063-017-2287-7
Download citation
Received : 24 March 2017
Accepted : 05 October 2017
Published : 22 November 2017
DOI : https://doi.org/10.1186/s13063-017-2287-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Randomised clinical trials
- Evidence-based clinical practice
- Evidence-based medicine
- Specific barriers
- European Clinical Infrastructure Networks
ISSN: 1745-6215
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Library databases
- Library website
Evidence-Based Research: Levels of Evidence Pyramid
Introduction.
One way to organize the different types of evidence involved in evidence-based practice research is the levels of evidence pyramid. The pyramid includes a variety of evidence types and levels.
- systematic reviews
- critically-appraised topics
- critically-appraised individual articles
- randomized controlled trials
- cohort studies
- case-controlled studies, case series, and case reports
- Background information, expert opinion
Levels of evidence pyramid
The levels of evidence pyramid provides a way to visualize both the quality of evidence and the amount of evidence available. For example, systematic reviews are at the top of the pyramid, meaning they are both the highest level of evidence and the least common. As you go down the pyramid, the amount of evidence will increase as the quality of the evidence decreases.

Text alternative for Levels of Evidence Pyramid diagram
EBM Pyramid and EBM Page Generator, copyright 2006 Trustees of Dartmouth College and Yale University. All Rights Reserved. Produced by Jan Glover, David Izzo, Karen Odato and Lei Wang.
Filtered Resources
Filtered resources appraise the quality of studies and often make recommendations for practice. The main types of filtered resources in evidence-based practice are:
Scroll down the page to the Systematic reviews , Critically-appraised topics , and Critically-appraised individual articles sections for links to resources where you can find each of these types of filtered information.
Systematic reviews
Authors of a systematic review ask a specific clinical question, perform a comprehensive literature review, eliminate the poorly done studies, and attempt to make practice recommendations based on the well-done studies. Systematic reviews include only experimental, or quantitative, studies, and often include only randomized controlled trials.
You can find systematic reviews in these filtered databases :
- Cochrane Database of Systematic Reviews Cochrane systematic reviews are considered the gold standard for systematic reviews. This database contains both systematic reviews and review protocols. To find only systematic reviews, select Cochrane Reviews in the Document Type box.
- JBI EBP Database (formerly Joanna Briggs Institute EBP Database) This database includes systematic reviews, evidence summaries, and best practice information sheets. To find only systematic reviews, click on Limits and then select Systematic Reviews in the Publication Types box. To see how to use the limit and find full text, please see our Joanna Briggs Institute Search Help page .
You can also find systematic reviews in this unfiltered database :
To learn more about finding systematic reviews, please see our guide:
- Filtered Resources: Systematic Reviews
Critically-appraised topics
Authors of critically-appraised topics evaluate and synthesize multiple research studies. Critically-appraised topics are like short systematic reviews focused on a particular topic.
You can find critically-appraised topics in these resources:
- Annual Reviews This collection offers comprehensive, timely collections of critical reviews written by leading scientists. To find reviews on your topic, use the search box in the upper-right corner.
- Guideline Central This free database offers quick-reference guideline summaries organized by a new non-profit initiative which will aim to fill the gap left by the sudden closure of AHRQ’s National Guideline Clearinghouse (NGC).
- JBI EBP Database (formerly Joanna Briggs Institute EBP Database) To find critically-appraised topics in JBI, click on Limits and then select Evidence Summaries from the Publication Types box. To see how to use the limit and find full text, please see our Joanna Briggs Institute Search Help page .
- National Institute for Health and Care Excellence (NICE) Evidence-based recommendations for health and care in England.
- Filtered Resources: Critically-Appraised Topics
Critically-appraised individual articles
Authors of critically-appraised individual articles evaluate and synopsize individual research studies.
You can find critically-appraised individual articles in these resources:
- EvidenceAlerts Quality articles from over 120 clinical journals are selected by research staff and then rated for clinical relevance and interest by an international group of physicians. Note: You must create a free account to search EvidenceAlerts.
- ACP Journal Club This journal publishes reviews of research on the care of adults and adolescents. You can either browse this journal or use the Search within this publication feature.
- Evidence-Based Nursing This journal reviews research studies that are relevant to best nursing practice. You can either browse individual issues or use the search box in the upper-right corner.
To learn more about finding critically-appraised individual articles, please see our guide:
- Filtered Resources: Critically-Appraised Individual Articles
Unfiltered resources
You may not always be able to find information on your topic in the filtered literature. When this happens, you'll need to search the primary or unfiltered literature. Keep in mind that with unfiltered resources, you take on the role of reviewing what you find to make sure it is valid and reliable.
Note: You can also find systematic reviews and other filtered resources in these unfiltered databases.
The Levels of Evidence Pyramid includes unfiltered study types in this order of evidence from higher to lower:
You can search for each of these types of evidence in the following databases:
TRIP database
Background information & expert opinion.
Background information and expert opinions are not necessarily backed by research studies. They include point-of-care resources, textbooks, conference proceedings, etc.
- Family Physicians Inquiries Network: Clinical Inquiries Provide the ideal answers to clinical questions using a structured search, critical appraisal, authoritative recommendations, clinical perspective, and rigorous peer review. Clinical Inquiries deliver best evidence for point-of-care use.
- Harrison, T. R., & Fauci, A. S. (2009). Harrison's Manual of Medicine . New York: McGraw-Hill Professional. Contains the clinical portions of Harrison's Principles of Internal Medicine .
- Lippincott manual of nursing practice (8th ed.). (2006). Philadelphia, PA: Lippincott Williams & Wilkins. Provides background information on clinical nursing practice.
- Medscape: Drugs & Diseases An open-access, point-of-care medical reference that includes clinical information from top physicians and pharmacists in the United States and worldwide.
- Virginia Henderson Global Nursing e-Repository An open-access repository that contains works by nurses and is sponsored by Sigma Theta Tau International, the Honor Society of Nursing. Note: This resource contains both expert opinion and evidence-based practice articles.
- Previous Page: Phrasing Research Questions
- Next Page: Evidence Types
- Office of Student Disability Services
Walden Resources
Departments.
- Academic Residencies
- Academic Skills
- Career Planning and Development
- Customer Care Team
- Field Experience
- Military Services
- Student Success Advising
- Writing Skills
Centers and Offices
- Center for Social Change
- Office of Academic Support and Instructional Services
- Office of Degree Acceleration
- Office of Research and Doctoral Services
- Office of Student Affairs
Student Resources
- Doctoral Writing Assessment
- Form & Style Review
- Quick Answers
- ScholarWorks
- SKIL Courses and Workshops
- Walden Bookstore
- Walden Catalog & Student Handbook
- Student Safety/Title IX
- Legal & Consumer Information
- Website Terms and Conditions
- Cookie Policy
- Accessibility
- Accreditation
- State Authorization
- Net Price Calculator
- Contact Walden
Walden University is a member of Adtalem Global Education, Inc. www.adtalem.com Walden University is certified to operate by SCHEV © 2024 Walden University LLC. All rights reserved.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 February 2022
Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics
- Christoph U. Correll ORCID: orcid.org/0000-0002-7254-5646 1 , 2 , 3 ,
- Amber Martin 4 ,
- Charmi Patel 5 ,
- Carmela Benson 5 ,
- Rebecca Goulding 6 ,
- Jennifer Kern-Sliwa 5 ,
- Kruti Joshi 5 ,
- Emma Schiller 4 &
- Edward Kim ORCID: orcid.org/0000-0001-8247-6675 7
Schizophrenia volume 8 , Article number: 5 ( 2022 ) Cite this article
14k Accesses
46 Citations
14 Altmetric
Metrics details
- Schizophrenia
Clinical practice guidelines (CPGs) translate evidence into recommendations to improve patient care and outcomes. To provide an overview of schizophrenia CPGs, we conducted a systematic literature review of English-language CPGs and synthesized current recommendations for the acute and maintenance management with antipsychotics. Searches for schizophrenia CPGs were conducted in MEDLINE/Embase from 1/1/2004–12/19/2019 and in guideline websites until 06/01/2020. Of 19 CPGs, 17 (89.5%) commented on first-episode schizophrenia (FES), with all recommending antipsychotic monotherapy, but without agreement on preferred antipsychotic. Of 18 CPGs commenting on maintenance therapy, 10 (55.6%) made no recommendations on the appropriate maximum duration of maintenance therapy, noting instead individualization of care. Eighteen (94.7%) CPGs commented on long-acting injectable antipsychotics (LAIs), mainly in cases of nonadherence (77.8%), maintenance care (72.2%), or patient preference (66.7%), with 5 (27.8%) CPGs recommending LAIs for FES. For treatment-resistant schizophrenia, 15/15 CPGs recommended clozapine. Only 7/19 (38.8%) CPGs included a treatment algorithm.
Similar content being viewed by others

A meta-analysis of effectiveness of real-world studies of antipsychotics in schizophrenia: Are the results consistent with the findings of randomized controlled trials?
Lajos Katona, István Bitter & Pál Czobor

Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials
Hideaki Tani, Shotaro Takasu, … Hiroyoshi Takeuchi
The efficacy and heterogeneity of antipsychotic response in schizophrenia: A meta-analysis
Robert A. McCutcheon, Toby Pillinger, … Oliver D. Howes
Introduction
Schizophrenia is an often debilitating, chronic, and relapsing mental disorder with complex symptomology that manifests as a combination of positive, negative, and/or cognitive features 1 , 2 , 3 . Standard management of schizophrenia includes the use of antipsychotic medications to help control acute psychotic episodes 4 and prevent relapses 5 , 6 , whereas maintenance therapy is used in the long term after patients have been stabilized 7 , 8 , 9 . Two main classes of drugs—first- and second-generation antipsychotics (FGA and SGA)—are used to treat schizophrenia 10 . SGAs are favored due to the lower rates of adverse effects, such as extrapyramidal effects, tardive dyskinesia, and relapse 11 . However, pharmacologic treatment for schizophrenia is complicated because nonadherence is prevalent, and is a major risk factor for relapse 9 and poor overall outcomes 12 . The use of long-acting injectable (LAI) versions of antipsychotics aims to limit nonadherence-related relapses and poor outcomes 13 .
Patient treatment pathways and treatment choices are determined based on illness acuity/severity, past treatment response and tolerability, as well as balancing medication efficacy and adverse effect profiles in the context of patient preferences and adherence patterns 14 , 15 . Clinical practice guidelines (CPG) serve to inform clinicians with recommendations that reflect current evidence from meta-analyses of randomized controlled trials (RCTs), individual RCTs and, less so, epidemiologic studies, as well as clinical experience, with the goal of providing a framework and road-map for treatment decisions that will improve quality of care and achieve better patients outcomes. The use of clinical algorithms or other decision trees in CPGs may improve the ease of implementation of the evidence in clinical practice 16 . While CPGs are an important tool for mental health professionals, they have not been updated on a regular basis like they have been in other areas of medicine, such as in oncology. In the absence of current information, other governing bodies, healthcare systems, and hospitals have developed their own CPGs regarding the treatment of schizophrenia, and many of these have been recently updated 17 , 18 , 19 . As such, it is important to assess the latest guidelines to be aware of the changes resulting from consideration of updated evidence that informed the treatment recommendations. Since CPGs are comprehensive and include the diagnosis as well as the pharmacological and non-pharmacological management of individuals with schizophrenia, a detailed comparative review of all aspects of CPGs for schizophrenia would have been too broad a review topic. Further, despite ongoing efforts to broaden the pharmacologic tools for the treatment of schizophrenia 20 , antipsychotics remain the cornerstone of schizophrenia management 8 , 21 . Therefore, a focused review of guideline recommendations for the management of schizophrenia with antipsychotics would serve to provide clinicians with relevant information for treatment decisions.
To provide an updated overview of United States (US) national and English language international guidelines for the management of schizophrenia, we conducted a systematic literature review (SLR) to identify CPGs and synthesize current recommendations for pharmacological management with antipsychotics in the acute and maintenance phases of schizophrenia.
Systematic searches for the SLR yielded 1253 hits from the electronic literature databases. After removal of duplicate references, 1127 individual articles were screened at the title and abstract level. Of these, 58 publications were deemed eligible for screening at the full-text level, from which 19 were ultimately included in the SLR. Website searches of relevant organizations yielded 10 additional records, and an additional three records were identified by the state-by-state searches. Altogether, this process resulted in 32 records identified for inclusion in the SLR. Of the 32 sources, 19 primary CPGs, published/issued between 2004 and 2020, were selected for extraction, as illustrated in the PRISMA diagram (Fig. 1 ). While the most recent APA guideline was identified and available for download in 2020, the reference to cite in the document indicates a publication date of 2021.
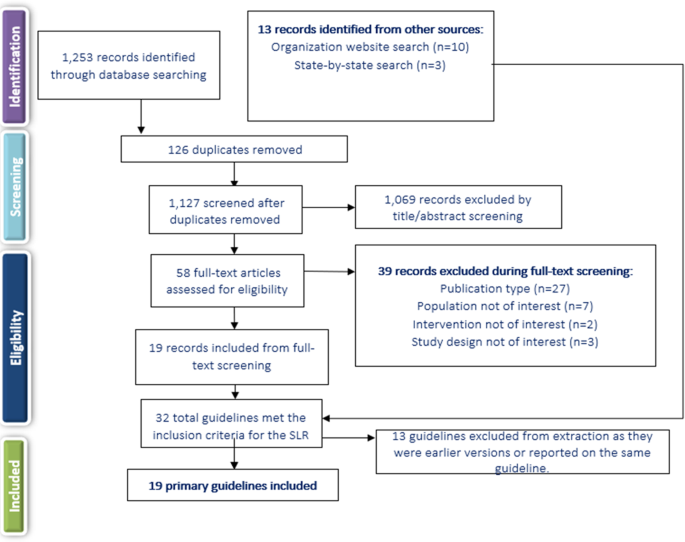
SLR systematic literature review.
Of the 19 included CPGs (Table 1 ), three had an international focus (from the following organizations: International College of Neuropsychopharmacology [CINP] 22 , United Nations High Commissioner for Refugees [UNHCR] 23 , and World Federation of Societies of Biological Psychiatry [WFSBP] 24 , 25 , 26 ); seven originated from the US; 17 , 18 , 19 , 27 , 28 , 29 , 30 , 31 , 32 three were from the United Kingdom (British Association for Psychopharmacology [BAP] 33 , the National Institute for Health and Care Excellence [NICE] 34 , and the Scottish Intercollegiate Guidelines Network [SIGN] 35 ); and one guideline each was from Singapore 36 , the Polish Psychiatric Association (PPA) 37 , 38 , the Canadian Psychiatric Association (CPA) 14 , the Royal Australia/New Zealand College of Psychiatrists (RANZCP) 39 , the Association Française de Psychiatrie Biologique et de Neuropsychopharmacologie (AFPBN) from France 40 , and Italy 41 . Fourteen CPGs (74%) recommended treatment with specific antipsychotics and 18 (95%) included recommendations for the use of LAIs, while just seven included a treatment algorithm Table 2 ). The AGREE II assessment resulted in the highest score across the CPGs domains for NICE 34 followed by the American Psychiatric Association (APA) guidelines 17 . The CPA 14 , BAP 33 , and SIGN 35 CPGs also scored well across domains.
Acute therapy
Seventeen CPGs (89.5%) provided treatment recommendations for patients experiencing a first schizophrenia episode 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 40 , 41 , but the depth and focus of the information varied greatly (Supplementary Table 1 ). In some CPGs, information on treatment of a first schizophrenia episode was limited or grouped with information on treating any acute episode, such as in the CPGs from CINP 22 , AFPBN 40 , New Jersey Division of Mental Health Services (NJDMHS) 32 , the APA 17 , and the PPA 37 , 38 , while the others provided more detailed information specific to patients experiencing a first schizophrenia episode 14 , 18 , 19 , 23 , 24 , 28 , 33 , 34 , 35 , 36 , 39 , 41 . The American Association of Community Psychiatrists (AACP) Clinical Tips did not provide any information on the treatment of schizophrenia patients with a first episode 29 .
There was little agreement among CPGs regarding the preferred antipsychotic for a first schizophrenia episode. However, there was strong consensus on antipsychotic monotherapy and that lower doses are generally recommended due to better treatment response and greater adverse effect sensitivity. Some guidelines recommended SGAs over FGAs when treating a first-episode schizophrenia patient (RANZCP 39 , Texas Medication Algorithm Project [TMAP] 28 , Oregon Health Authority 19 ), one recommended starting patients on an FGA (UNHCR 23 ), and others stated specifically that there was no evidence of any difference in efficacy between FGAs and SGAs (WFSBP 24 , CPA 14 , SIGN 35 , APA 17 , Singapore guidelines 36 ), or did not make any recommendation (CINP 22 , Italian guidelines 41 , NICE 34 , NJDMHS 32 , Schizophrenia Patient Outcomes Research Team [PORT] 30 , 31 ). The BAP 33 and WFBSP 24 noted that while there was probably no difference between FGAs and SGAs in efficacy, some SGAs (olanzapine, amisulpride, and risperidone) may perform better than some FGAs. The Schizophrenia PORT recommendations noted that while there seemed to be no differences between SGAs and FGAs in short-term studies (≤12 weeks), longer studies (one to two years) suggested that SGAs may provide benefits in terms of longer times to relapse and discontinuation rates 30 , 31 . The AFPBN guidelines 40 and Florida Medicaid Program guidelines 18 , which both focus on use of LAI antipsychotics, both recommended an SGA-LAI for patients experiencing a first schizophrenia episode. A caveat in most CPGs was that physicians and their patients should discuss decisions about the choice of antipsychotic and that the choice should consider individual patient factors/preferences, risk of adverse and metabolic effects, and symptom patterns 17 , 18 , 19 , 22 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 41 .
Most CPGs recommended switching to a different monotherapy if the initial antipsychotic was not effective or not well tolerated after an adequate antipsychotic trial at an appropriate dose 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 32 , 33 , 35 , 36 , 39 . For patients initially treated with an FGA, the UNHCR recommended switching to an SGA (olanzapine or risperidone) 23 . Guidance on response to treatment varied in the measures used but typically required at least a 20% improvement in symptoms (i.e. reduction in Positive and Negative Syndrome Scale or Brief Psychiatric Rating Scale scores) from pre-treatment levels.
Several CPGs contained recommendations on the duration of antipsychotic therapy after a first schizophrenia episode. The NJDMHS guidelines 32 recommended nine to 12 months; CINP 22 recommended at least one year; CPA 14 recommended at least 18 months; WFSBP 25 , the Italian guidelines 41 , and NICE 34 recommended 1 to 2 years; and the RANZCP 39 , BAP 33 , and SIGN 35 recommended at least 2 years. The APA 17 and TMAP 28 recommended continuing antipsychotic treatment after resolution of first-episode symptoms but did not recommend a specific length of therapy.
Twelve guidelines 14 , 18 , 22 , 24 , 28 , 30 , 31 , 33 , 34 , 35 , 36 , 39 , 40 (63.2%) discussed the treatment of subsequent/multiple episodes of schizophrenia (i.e., following relapse). These CPGs noted that the considerations guiding the choice of antipsychotic for subsequent/multiple episodes were similar to those for a first episode, factoring in prior patient treatment response, adverse effect patterns and adherence. The CPGs also noted that response to treatment may be lower and require higher doses to achieve a response than for first-episode schizophrenia, that a different antipsychotic than used to treat the first episode may be needed, and that a switch to an LAI is an option.
Several CPGs provided recommendations for patients with specific clinical features (Supplementary Table 1 ). The most frequently discussed group of clinical features was negative symptoms, with recommendations provided in the CINP 22 , UNHCR 23 , WFSBP 24 , AFPBN 40 , SIGN 35 , BAP 33 , APA 17 , and NJDMHS guidelines; 32 negative symptoms were the sole focus of the guidelines from the PPA 37 , 38 . The guidelines noted that due to limited evidence in patients with predominantly negative symptoms, there was no clear benefit for any strategy, but that options included SGAs (especially amisulpride) rather than FGAs (WFSBP 24 , CINP 22 , AFPBN 40 , SIGN 35 , NJDMHS 32 , PPA 37 , 38 ), and addition of an antidepressant (WFSBP 24 , UNHCR 23 , SIGN 35 , NJDMHS 32 ) or lamotrigine (SIGN 35 ), or switching to another SGA (NJDMHS 32 ) or clozapine (NJDMHS 32 ). The PPA guidelines 37 , 38 stated that the use of clozapine or adding an antidepressant or other medication class was not supported by evidence, but recommended the SGA cariprazine for patients with predominant and persistent negative symptoms, and other SGAs for those with full-spectrum negative symptoms. However, the BAP 33 stated that no recommendations can be made for any of these strategies because of the quality and paucity of the available evidence.
Some of the CPGs also discussed treatment of other clinical features to a limited degree, including depressive symptoms (CINP 22 , UNHCR 23 , CPA 14 , APA 17 , and NJDMHS 32 ), cognitive dysfunction (CINP 22 , UNHCR 23 , WFSBP 24 , AFPBN 40 , SIGN 35 , BAP 33 , and NJDMHS 32 ), persistent aggression (CINP 22 , WFSBP 24 , CPA 14 , AFPBN 40 , NICE 34 , SIGN 35 , BAP 33 , and NJDMHS 32 ), and comorbid psychiatric diagnoses (CINP 22 , RANZCP 39 , BAP 33 , APA 17 , and NJDMHS 32 ).
Fifteen CPGs (78.9%) discussed treatment-resistant schizophrenia (TRS); all defined it as persistent, predominantly positive symptoms after two adequate antipsychotic trials; clozapine was the unanimous first choice 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 . However, the UNHCR guidelines 23 , which included recommendations for treatment of refugees, noted that clozapine is only a reasonable choice in regions where white blood cell monitoring and specialist supervision are available, otherwise, risperidone or olanzapine are alternatives if they had not been used in the previous treatment regimen.
There were few options for patients who are resistant to clozapine therapy, and evidence supporting these options was limited. The CPA guidelines 14 therefore stated that no recommendation can be given due to inadequate evidence. Other CPGs discussed options (but noted there was limited supporting evidence), such as switching to olanzapine or risperidone (WFSBP 24 , TMAP 28 ), adding a second antipsychotic to clozapine (CINP 22 , NICE 34 , TMAP 28 , BAP 33 , Florida Medicaid Program 18 , Oregon Health Authority 19 , RANZCP 39 ), adding lamotrigine or topiramate to clozapine (CINP 22 , Florida Medicaid Program 18 ), combination therapy with two non-clozapine antipsychotics (Florida Medicaid Program 18 , NJDMHS 32 ), and high-dose non-clozapine antipsychotic therapy (BAP 33 , SIGN 35 ). Electroconvulsive therapy was noted as a last resort for patients who did not respond to any pharmacologic therapy, including clozapine, by 10 CPGs 17 , 18 , 19 , 22 , 24 , 28 , 32 , 35 , 36 , 39 .
Maintenance therapy
Fifteen CPGs (78.9%) discussed maintenance therapy to various degrees via dedicated sections or statements, while three others referred only to maintenance doses by antipsychotic agent 18 , 23 , 29 without accompanying recommendations (Supplementary Table 2 ). Only the Italian guideline provided no reference or comments on maintenance treatment. The CINP 22 , WFSBP 25 , RANZCP 39 , and Schizophrenia PORT 30 , 31 recommended keeping patients on the same antipsychotic and at the same dose on which they had achieved remission. Several CPGs recommended maintenance therapy at the lowest effective dose (NJDMHS 32 , APA 17 , Singapore guidelines 36 , and TMAP 28 ). The CPA 14 and SIGN 35 defined the lower dose as 300–400 mg chlorpromazine equivalents or 4–6 mg risperidone equivalents, and the Singapore guidelines 36 stated that the lower dose should not be less than half the original dose. TMAP 28 stated that given the relapsing nature of schizophrenia, the maintenance dose should often be close to the original dose. While SIGN 35 recommended that patients remain on the same antipsychotic that provided remission, these guidelines also stated that maintenance with amisulpride, olanzapine, or risperidone was preferred, and that chlorpromazine and other low-potency FGAs were also suitable. The BAP 33 recommended that the current regimen be optimized before any dose reduction or switch to another antipsychotic occurs. Several CPGs recommended LAIs as an option for maintenance therapy (see next section).
Altogether, 10/18 (55.5%) CPGs made no recommendations on the appropriate duration of maintenance therapy, noting instead that each patient should be considered individually. Other CPGs made specific recommendations: Both the Both BAP 33 and SIGN 35 guidelines suggested a minimum of 2 years, the NJDMHS guidelines 32 recommended 2–3 years; the WFSBP 25 recommended 2–5 years for patients who have had one relapse and more than 5 years for those who have had multiple relapses; the RANZCP 39 and the CPA 14 recommended 2–5 years; and the CINP 22 recommended that maintenance therapy last at least 6 years for patients who have had multiple episodes. The TMAP was the only CPG to recommend that maintenance therapy be continued indefinitely 28 .
Recommendations on the use of LAIs
All CPGs except the one from Italy (94.7%) discussed the use of LAIs for patients with schizophrenia to some extent. As shown in Table 3 , among the 18 CPGs, LAIs were primarily recommended in 14 CPGs (77.8%) for patients who are non-adherent to other antipsychotic administration routes (CINP 22 , UNHCR 23 , RANZCP 39 , PPA 37 , 38 , Singapore guidelines 36 , NICE 34 , SIGN 35 , BAP 33 , APA 17 , TMAP 28 , NJDMHS 32 , AACP 29 , Oregon Health Authority 19 , Florida Medicaid Program 18 ). Twelve CPGs (66.7%) also noted that LAIs should be prescribed based on patient preference (RANZCP 39 , CPA 14 , AFPBN 40 , Singapore guidelines 36 , NICE 34 , SIGN 35 , BAP 33 , APA 17 , Schizophrenia PORT 30 , 31 , AACP 29 , Oregon Health Authority 19 , Florida Medicaid Program 18 ).
Thirteen CPGs (72.2%) recommended LAIs as maintenance therapy 18 , 19 , 24 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 40 . While five CPGs (27.8%), i.e., AFPBN 40 , RANZCP 39 , TMAP 28 , NJDMHS 32 , and the Florida Medicaid Program 18 recommended LAIs specifically for patients experiencing a first episode. While the CPA 14 did not make any recommendations regarding when LAIs should be used, they discussed recent evidence supporting their use earlier in treatment. Five guidelines (27.8%, i.e., Singapore 36 , NICE 34 , SIGN 35 , BAP 33 , and Schizophrenia PORT 30 , 31 ) noted that evidence around LAIs was not sufficient to support recommending their use for first-episode patients. The AFPBN guidelines 40 also stated that LAIs (SGAs as first-line and FGAs as second-line treatment) should be more frequently considered for maintenance treatment of schizophrenia. Four CPGs (22.2%, i.e., CINP 22 , UNHCR 23 , Italian guidelines 41 , PPA guidelines 37 , 38 ) did not specify when LAIs should be used. The AACP guidelines 29 , which evaluated only LAIs, recommended expanding their use beyond treatment for nonadherence, suggesting that LAIs may offer a more convenient mode of administration or potentially address other clinical and social challenges, as well as provide more consistent plasma levels.
Treatment algorithms
Only Seven CPGs (36.8%) included an algorithm as part of the treatment recommendations. These included decision trees or flow diagrams that map out initial therapy, durations for assessing response, and treatment options in cases of non-response. However, none of these guidelines defined how to measure response, a theme that also extended to guidelines that did not include treatment algorithms. Four of the seven guidelines with algorithms recommended specific antipsychotic agents, while the remaining three referred only to the antipsychotic class.
LAIs were not consistently incorporated in treatment algorithms and in six CPGs were treated as a separate category of medicine reserved for patients with adherence issues or a preference for the route of administration. The only exception was the Florida Medicaid Program 18 , which recommended offering LAIs after oral antipsychotic stabilization even to patients who are at that point adherent to oral antipsychotics.
Benefits and harms
The need to balance the efficacy and safety of antipsychotics was mentioned by all CPGs as a basic treatment paradigm.
Ten CPGs provided conclusions on benefits of antipsychotic therapy. The APA 17 and the BAP 33 guidelines stated that antipsychotic treatment can improve the positive and negative symptoms of psychosis and leads to remission of symptoms. These CPGs 17 , 33 as well as those from NICE 34 and CPA 14 stated that these treatment effects can also lead to improvements in quality of life (including quality-adjusted life years), improved functioning, and reduction in disability. The CPA 14 and APA 17 guidelines noted decreases in hospitalizations with antipsychotic therapy, and the APA guidelines 17 stated that long-term antipsychotic treatment can also reduce mortality. The UNHCR 23 and the Italian 41 guidelines noted that early intervention increased positive outcomes. The WFSBP 24 , AFPBN 40 , CPA 14 , BAP 33 , APA 17 , and NJDMHS 32 affirmed that relapse prevention is a benefit of continued/maintenance treatment.
Some CPGs (WFSBP 24 , Italian 41 , CPA 14 , and SIGN 35 ) noted that reduced risk for extrapyramidal adverse effects and treatment discontinuation were potential benefits of SGAs vs. FGAs.
The risk of adverse effects (e.g., extrapyramidal, metabolic, cardiovascular, and hormonal adverse effects, sedation, and neuroleptic malignant syndrome) was noted by all CPGs as the major potential harm of antipsychotic therapy 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 37 , 39 , 40 , 41 , 42 . These adverse effects are known to limit long-term treatment and adherence 24 .
This SLR of CPGs for the treatment of schizophrenia yielded 19 most updated versions of individual CPGs, published/issued between 2004 and 2020. Structuring our comparative review according to illness phase, antipsychotic type and formulation, response to antipsychotic treatment as well as benefits and harms, several areas of consistent recommendations emerged from this review (e.g., balancing risk and benefits of antipsychotics, preferring antipsychotic monotherapy; using clozapine for treatment-resistant schizophrenia). On the other hand, other recommendations regarding other areas of antipsychotic treatment were mostly consistent (e.g., maintenance antipsychotic treatment for some time), somewhat inconsistent (e.g., differences in the management of first- vs multi-episode patients, type of antipsychotic, dose of antipsychotic maintenance treatment), or even contradictory (e.g., role of LAIs in first-episode schizophrenia patients).
Consistent with RCT evidence 43 , 44 , antipsychotic monotherapy was the treatment of choice for patients with first-episode schizophrenia in all CPGs, and all guidelines stated that a different single antipsychotic should be tried if the first is ineffective or intolerable. Recommendations were similar for multi-episode patients, but factored in prior patient treatment response, adverse effect patterns, and adherence. There was also broad consensus that the side-effect profile of antipsychotics is the most important consideration when making a decision on pharmacologic treatment, also reflecting meta-analytic evidence 4 , 5 , 10 . The risk of extrapyramidal symptoms (especially with FGAs) and metabolic effects (especially with SGAs) were noted as key considerations, which are also reflected in the literature as relevant concerns 4 , 45 , 46 , including for quality of life and treatment nonadherence 47 , 48 , 49 , 50 .
Largely consistent with the comparative meta-analytic evidence regarding the acute 4 , 51 , 52 and maintenance antipsychotic treatment 5 effects of schizophrenia, the majority of CPGs stated there was no difference in efficacy between SGAs and FGAs (WFSBP 24 , CPA 14 , SIGN 35 , APA 17 , and Singapore guidelines 36 ), or did not make any recommendations (CINP 22 , Italian guidelines 41 , NICE 34 , NJDMHS 32 , and Schizophrenia PORT 30 , 31 ); three CPGs (BAP 33 , WFBSP 24 , and Schizophrenia PORT 30 , 31 ) noted that SGAs may perform better than FGAs over the long term, consistent with a meta-analysis on this topic 53 .
The 12 CPGs that discussed treatment of subsequent/multiple episodes generally agreed on the factors guiding the choices of an antipsychotic, including that the decision may be more complicated and response may be lower than with a first episode, as described before 7 , 54 , 55 , 56 .
There was little consensus regarding maintenance therapy. Some CPGs recommended the same antipsychotic and dose that achieved remission (CINP 22 , WFSBP 25 , RANZCP 39 , and Schizophrenia PORT 30 , 31 ) and others recommended the lowest effective dose (NJDMHS 32 , APA 17 , Singapore guidelines 36 , TMAP 28 , CPA 14 , and SIGN 35 ). This inconsistency is likely based on insufficient data as well as conflicting results in existing meta-analyses on this topic 57 , 58 , 59 .
The 15 CPGs that discussed TRS all used the same definition for this condition, consistent with recent commendations 60 , and agreed that clozapine is the primary evidence-based treatment choice 14 , 17 , 18 , 19 , 22 , 23 , 24 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , reflecting the evidence base 61 , 62 , 63 . These CPGs also agreed that there are few options well supported by evidence for patients who do not respond to clozapine, with a recent meta-analysis of RCTs showing that electroconvulsive therapy augmentation may be the most evidence-based treatment option 64 .
One key gap in the treatment recommendations was how long patients should remain on antipsychotic therapy after a first episode or during maintenance therapy. While nine of the 17 CPGs discussing treatment of a first episode provided a recommended timeframe (varying from 1 to 2 years) 14 , 22 , 24 , 32 , 33 , 34 , 35 , 39 , 41 , the APA 17 and TMAP 28 recommended continuing antipsychotic treatment after resolution of first-episode symptoms but did not recommend a specific length of therapy. Similarly, six of the 18 CPGs discussing maintenance treatment recommended a specific duration of therapy (ranging from two to six years) 14 , 22 , 25 , 32 , 39 , while as many as 10 CPGs did not point to a firm end of the maintenance treatment, instead recommending individualized decisions. The CPGs not stating a definite endpoint or period of maintenance treatment after repeated schizophrenia episodes or even after a first episode of schizophrenia, reflects the different evidence types on which the recommendation is based. The RCT evidence ends after several years of maintenance treatment vs. discontinuation supporting ongoing antipsychotic treatment; however, naturalistic database studies do not indicate any time period after which one can safely discontinue maintenance antipsychotic care, even after a first schizophrenia episode 8 , 65 . In fact, stopping antipsychotics is associated not only with a substantially increased risk of hospitalization but also mortality 65 , 66 , 67 . In this sense, not stating an endpoint for antipsychotic maintenance therapy should not be taken as an implicit statement that antipsychotics should be discontinued at any time; data suggest the contrary.
A further gap exists regarding the most appropriate treatment of negative symptoms, such as anhedonia, amotivation, asociality, affective flattening, and alogia 1 , a long-standing challenge in the management of patients with schizophrenia. Negative symptoms often persist in patients after positive symptoms have resolved, or are the presenting feature in a substantial minority of patients 22 , 35 . Negative symptoms can also be secondary to pharmacotherapy 22 , 68 . Antipsychotics have been most successful in treating positive symptoms, and while eight of the CPGs provided some information on treatment of negative symptoms, the recommendations were generally limited 17 , 22 , 23 , 24 , 32 , 33 , 35 , 40 . Negative symptom management was a focus of the PPA guidelines, but the guidelines acknowledged that supporting evidence was limited, often due to the low number of patients with predominantly negative symptoms in clinical trials 37 , 38 . The Polish guidelines are also one of the more recently developed and included the newer antipsychotic cariprazine as a first-line option, which although being a point of differentiation from the other guidelines, this recommendation was based on RCT data 69 .
Another area in which more direction is needed is on the use of LAIs. While all but one of the 19 CPGs discussed this topic, the extent of information and recommendations for LAI use varied considerably. All CPGs categorized LAIs as an option to improve adherence to therapy or based on patient preference. However, 5/18 CPGs (27.8%) recommended the use of LAI early in treatment (at first episode: AFPBN 40 , RANZCP 39 , TMAP 28 , NJDMHS 32 , and Florida Medicaid Program 18 ) or across the entire illness course, while five others stated there was not sufficient evidence to recommend LAIs for these patients (Singapore 36 , NICE 34 , SIGN 35 , BAP 33 , and Schizophrenia PORT 30 , 31 ). The role of LAIs in first-episode schizophrenia was the only point where opposing recommendations were found across CPGs. This contradictory stance was not due to the incorporation of newer data suggesting benefits of LAIs in first episode and early-phase patients with schizophrenia 70 , 71 , 72 , 73 , 74 in the CPGs recommending LAI use in first-episode patients, as CPGs recommending LAI use were published between 2005 and 2020, while those opposing LAI use were published between 2011 and 2020. Only the Florida Medicaid CPG recommended LAIs as a first step equivalent to oral antipsychotics (OAP) after initial OAP response and tolerability, independent of nonadherence or other clinical variables. This guideline was also the only CPG to fully integrate LAI use in their clinical algorithm. The remaining six CPGs that included decision tress or treatment algorithms regarded LAIs as a separate paradigm of treatment reserved for nonadherence or patients preference rather than a routine treatment option to consider. While some CPGs provided fairly detailed information on the use of LAIs (AFPBN 40 , AACP 29 , Oregon Health Authority 19 , and Florida Medicaid Program 18 ), others mentioned them only in the context of adherence issues or patient preference. Notably, definitions of and means to determine nonadherence were not reported. One reason for this wide range of recommendations regarding the placement of LAIs in the treatment algorithm and clinical situations that prompt LAI use might be due to the fact that CPGs generally favor RCT evidence over evidence from other study designs. In the case of LAIs, there was a notable dissociation between consistent meta-analytic evidence of statistically significant superiority of LAIs vs OAPs in mirror-image 75 and cohort study designs 76 and non-significant advantages in RCTs 77 . Although patients in RCTs comparing LAIs vs OAPs were less severely ill and more adherent to OAPs 77 than in clinical care and although mirror-image and cohort studies arguably have greater external validity than RCTs 78 , CPGs generally disregard evidence from other study designs when RCT evidence exits. This narrow focus can lead to disregarding important additional data. Nevertheless, a most updated meta-analysis of all 3 study designs comparing LAIs with OAPs demonstrated consistent superiority of LAIs vs OAPs for hospitalization or relapse across all 3 designs 79 , which should lead to more uniform recommendations across CPGs in the future.
Only seven CPGs included treatment algorithms or flow charts to guide LAI treatment selection for patients with schizophrenia 17 , 18 , 19 , 24 , 29 , 35 , 40 . However, there was little commonality across algorithms beyond the guidance on LAIs mentioned above, as some listed specific treatments and conditions for antipsychotic switches, while others indicated that medication choice should be based on a patient’s preferences and responses, side effects, and in some cases, cost effectiveness. Since algorithms and flow charts facilitate the reception, adoption and implementation of guidelines, future CPGs should include them as dissemination tools, but they need to reflect the data and detailed text and be sufficiently specific to be actionable.
The systematic nature in the identification, summarization, and assessment of the CPGs is a strength of this review. This process removed any potential bias associated with subjective selection of evidence, which is not reproducible. However, only CPGs published in English were included and regardless of their quality and differing timeframes of development and publication, complicating a direct comparison of consensus and disagreement. Finally, based on the focus of this SLR, we only reviewed pharmacologic management with antipsychotics. Clearly, the assessment, other pharmacologic and, especially, psychosocial interventions are important in the management of individuals with schizophrenia, but these topics that were covered to varying degrees by the evaluated CPGs were outside of the scope of this review.
Numerous guidelines have recently updated their recommendations on the pharmacological treatment of patients with schizophrenia, which we have summarized in this review. Consistent recommendations were observed across CPGs in the areas of balancing risk and benefits of antipsychotics when selecting treatment, a preference for antipsychotic monotherapy, especially for patients with a first episode of schizophrenia, and the use of clozapine for treatment-resistant schizophrenia. By contrast, there were inconsistencies with regards to recommendations on maintenance antipsychotic treatment, with differences existing on type and dose of antipsychotic, as well as the duration of therapy. However, LAIs were consistently recommended, but mainly suggested in cases of nonadherence or patient preference, despite their established efficacy in broader patient populations and clinical scenarios in clinical trials. Guidelines were sometimes contradictory, with some recommending LAI use earlier in the disease course (e.g., first episode) and others suggesting they only be reserved for later in the disease. This inconsistency was not due to lack of evidence on the efficacy of LAIs in first-episode schizophrenia or the timing of the CPG, so that other reasons might be responsible, including possibly bias and stigma associated with this route of treatment administration. Lastly, gaps existed in the guidelines for recommendations on the duration of maintenance treatment, treatment of negative symptoms, and the development/use of treatment algorithms whenever evidence is sufficient to provide a simplified summary of the data and indicate their relevance for clinical decision making, all of which should be considered in future guideline development/revisions.
The SLR followed established best methods used in systematic review research to identify and assess the available CPGs for pharmacologic treatment of schizophrenia with antipsychotics in the acute and maintenance phases 80 , 81 . The SLR was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, including use of a prespecified protocol to outline methods for conducting the review. The protocol for this review was approved by all authors prior to implementation but was not submitted to an external registry.
Data sources and search algorithms
Searches were conducted by two independent investigators in the MEDLINE and Embase databases via OvidSP to identify CPGs published in English. Articles were identified using search algorithms that paired terms for schizophrenia with keywords for CPGs. Articles indexed as case reports, reviews, letters, or news were excluded from the searches. The database search was limited to CPGs published from January 1, 2004, through December 19, 2019, without limit to geographic location. In addition to the database sources, guideline body websites and state-level health departments from the US were also searched for relevant CPGs published through June 2020. A manual check of the references of recent (i.e., published in the past three years), relevant SLRs and relevant practice CPGs was conducted to supplement the above searches and ensure and the most complete CPG retrieval.
This study did not involve human subjects as only published evidence was included in the review; ethical approval from an institution was therefore not required.
Selection of CPGs for inclusion
Each title and abstract identified from the database searches was screened and selected for inclusion or exclusion in the SLR by two independent investigators based on the populations, interventions/comparators, outcomes, study design, time period, language, and geographic criteria shown in Table 4 . During both rounds of the screening process, discrepancies between the two independent reviewers were resolved through discussion, and a third investigator resolved any disagreement. Articles/documents identified by the manual search of organizational websites were screened using the same criteria. All accepted studies were required to meet all inclusion criteria and none of the exclusion criteria. Only the most recent version of organizational CPGs was included for data extraction.
Data extraction and synthesis
Information on the recommendations regarding the antipsychotic management in the acute and maintenance phases of schizophrenia and related benefits and harms was captured from the included CPGs. Each guideline was reviewed and extracted by a single researcher and the data were validated by a senior team member to ensure accuracy and completeness. Additionally, each included CPG was assessed using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) tool. Following extraction and validation, results were qualitatively summarized across CPGs.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of the SLR are available from the corresponding author upon request.
Correll, C. U. & Schooler, N. R. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr. Dis. Treat. 16 , 519–534 (2020).
Article PubMed PubMed Central Google Scholar
Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Prim. 1 , 15067 (2015).
Article PubMed Google Scholar
Millan, M. J. et al. Altering the course of schizophrenia: progress and perspectives. Nat. Rev. Drug Discov. 15 , 485–515 (2016).
Article CAS PubMed Google Scholar
Huhn, M. et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394 , 939–951 (2019).
Article CAS PubMed PubMed Central Google Scholar
Kishimoto, T., Hagi, K., Nitta, M., Kane, J. M. & Correll, C. U. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry 18 , 208–224 (2019).
Leucht, S. et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 379 , 2063–2071 (2012).
Carbon, M. & Correll, C. U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 16 , 505–524 (2014).
Correll, C. U., Rubio, J. M. & Kane, J. M. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry 17 , 149–160 (2018).
Emsley, R., Chiliza, B., Asmal, L. & Harvey, B. H. The nature of relapse in schizophrenia. BMC Psychiatry 13 , 50 (2013).
Correll, C. U. & Kane, J. M. Ranking antipsychotics for efficacy and safety in schizophrenia. JAMA Psychiatry 77 , 225–226 (2020).
Kane, J. M. & Correll, C. U. Pharmacologic treatment of schizophrenia. Dialogues Clin. Neurosci. 12 , 345–357 (2010).
Kane, J. M., Kishimoto, T. & Correll, C. U. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry 12 , 216–226 (2013).
Correll, C. U. et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J. Clin. Psychiatry 77 , 1–24 (2016).
Remington, G. et al. Guidelines for the pharmacotherapy of schizophrenia in adults. Can. J. Psychiatry 62 , 604–616 (2017).
American Psychiatric Association. Treatment recommendations for patients with schizophrenia. Am. J. Psychiatry 161 , 1–56 (2004).
Institute of Medicine (US) Committee to Advise the Public Health Service on Clinical Practice Guidelines, Field, M. J., & Lohr, K. N. (Eds.). Clinical Practice Guidelines: Directions for a New Program. (National Academies Press (US), 1990).
American Psychiatric Association. Practice Guideline for the Treatment of Patients With Schizophrenia , 3rd edn. (2021). https://doi.org/10.1176/appi.books.9780890424841 .
Florida Medicaid Drug Therapy Management Program. 2019–2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults . (2020). Available at: https://floridabhcenter.org/wp-content/uploads/2021/04/2019-Psychotherapeutic-Medication-Guidelines-for-Adults-with-References_06-04-20.pdf .
Mental Health Clinical Advisory Group, Oregon Health Authority. Mental Health Care Guide for Licensed Practitioners and Mental Health Professionals. (2019). Available at: https://sharedsystems.dhsoha.state.or.us/DHSForms/Served/le7548.pdf .
Krogmann, A. et al. Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr. 24 , 38–69 (2019).
Fountoulakis, K. N. et al. The report of the joint WPA/CINP workgroup on the use and usefulness of antipsychotic medication in the treatment of schizophrenia. CNS Spectr . https://doi.org/10.1017/S1092852920001546 (2020).
Leucht, S. et al. CINP Schizophrenia Guidelines . (CINP, 2013). Available at: https://www.cinp.org/resources/Documents/CINP-schizophrenia-guideline-24.5.2013-A-C-method.pdf .
Ostuzzi, G. et al. Mapping the evidence on pharmacological interventions for non-affective psychosis in humanitarian non-specialised settings: A UNHCR clinical guidance. BMC Med. 15 , 197 (2017).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J. Biol. Psychiatry 13 , 318–378 (2012).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 2: Update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J. Biol. Psychiatry 14 , 2–44 (2013).
Hasan, A. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia - a short version for primary care. Int. J. Psychiatry Clin. Pract. 21 , 82–90 (2017).
Moore, T. A. et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J. Clin. Psychiatry 68 , 1751–1762 (2007).
Texas Medication Algorithm Project (TMAP). Texas Medication Algorithm Project (TMAP) Procedural Manual . (2008). Available at: https://jpshealthnet.org/sites/default/files/inline-files/tmapalgorithmforschizophrenia.pdf .
American Association of Community Psychiatrists (AACP). Clinical Tips Series, Long Acting Antipsychotic Medications. (2017). Accessed at: https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view .
Buchanan, R. W. et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr. Bull. 36 , 71–93 (2010).
Kreyenbuhl, J. et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr. Bull. 36 , 94–103 (2010).
New Jersey Division of Mental Health Services. Pharmacological Practice Guidelines for the Treatment of Schizophrenia . (2005). Available at: https://www.state.nj.us/humanservices/dmhs_delete/consumer/NJDMHS_Pharmacological_Practice_Guidelines762005.pdf .
Barnes, T. R. et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 34 , 3–78 (2020).
National Institute for Health and Care Excellence (NICE). Psychosis and schizophrenia in adults: prevention and management. (2014). Available at: http://www.nice.org.uk/guidance/cg178 .
Scottish Intercollegiate Guidelines Network (SIGN). Management of Schizophrenia: A National Clinical Guideline . (2013). Available at: https://www.sign.ac.uk/assets/sign131.pdf .
Verma, S. et al. Ministry of Health Clinical Practice Guidelines: Schizophrenia. Singap. Med. J. 52 , 521–526 (2011).
CAS Google Scholar
Szulc, A. et al. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 1. Rekomendacje dotyczace leczenia schizofrenii z. objawami negatywnymi. Stand. farmakoterapii Polskiego Tow. Psychiatrycznego, czesc 1. 53 , 497–524 (2019).
Google Scholar
Szulc, A. et al. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 2. Rekomendacje dotyczace leczenia schizofrenii z. objawami negatywnymi. Stand. farmakoterapii Polskiego Tow. Psychiatrycznego, czesc 2. 53 , 525–540 (2019).
Galletly, C. et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust. N. Z. J. Psychiatry 50 , 410–472 (2016).
Llorca, P. M. et al. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry 13 , 340 (2013).
De Masi, S. et al. The Italian guidelines for early intervention in schizophrenia: development and conclusions. Early Intervention Psychiatry 2 , 291–302 (2008).
Article Google Scholar
Barnes, T. R. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 25 , 567–620 (2011).
Correll, C. U. et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 74 , 675–684 (2017).
Galling, B. et al. Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry 16 , 77–89 (2017).
Pillinger, T. et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry 7 , 64–77 (2020).
Rummel-Kluge, C. et al. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr. Bull. 38 , 167–177 (2012).
Angermeyer, M. C. & Matschinger, H. Attitude of family to neuroleptics. Psychiatr. Prax. 26 , 171–174 (1999).
CAS PubMed Google Scholar
Dibonaventura, M., Gabriel, S., Dupclay, L., Gupta, S. & Kim, E. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry 12 , 20 (2012).
McIntyre, R. S. Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: the UNITE global survey. J. Clin. Psychiatry 70 (Suppl 3), 5–11 (2009).
Tandon, R. et al. The impact on functioning of second-generation antipsychotic medication side effects for patients with schizophrenia: a worldwide, cross-sectional, web-based survey. Ann. Gen. Psychiatry 19 , 42 (2020).
Zhang, J. P. et al. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 16 , 1205–1218 (2013).
Zhu, Y. et al. Antipsychotic drugs for the acute treatment of patients with a first episode of schizophrenia: a systematic review with pairwise and network meta-analyses. Lancet Psychiatry 4 , 694–705 (2017).
Kishimoto, T. et al. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol. Psychiatry 18 , 53–66 (2013).
Leucht, S. et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 174 , 927–942 (2017).
Samara, M. T., Nikolakopoulou, A., Salanti, G. & Leucht, S. How many patients with schizophrenia do not respond to antipsychotic drugs in the short term? An analysis based on individual patient data from randomized controlled trials. Schizophr. Bull. 45 , 639–646 (2019).
Zhu, Y. et al. How well do patients with a first episode of schizophrenia respond to antipsychotics: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 27 , 835–844 (2017).
Bogers, J. P. A. M., Hambarian, G., Michiels, M., Vermeulen, J. & de Haan, L. Risk factors for psychotic relapse after dose reduction or discontinuation of antipsychotics in patients with chronic schizophrenia. A systematic review and meta-analysis. Schizophr. Bull. Open 46 , Suppl 1 S326 (2020).
Tani, H. et al. Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials. Neuropsychopharmacology 45 , 887–901 (2020).
Uchida, H., Suzuki, T., Takeuchi, H., Arenovich, T. & Mamo, D. C. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta-analysis. Schizophr. Bull. 37 , 788–799 (2011).
Howes, O. D. et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am. J. Psychiatry 174 , 216–229 (2017).
Masuda, T., Misawa, F., Takase, M., Kane, J. M. & Correll, C. U. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry 76 , 1052–1062 (2019).
Siskind, D., McCartney, L., Goldschlager, R. & Kisely, S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry 209 , 385–392 (2016).
Siskind, D., Siskind, V. & Kisely, S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can. J. Psychiatry 62 , 772–777 (2017).
Wang, G. et al. ECT augmentation of clozapine for clozapine-resistant schizophrenia: a meta-analysis of randomized controlled trials. J. Psychiatr. Res. 105 , 23–32 (2018).
Tiihonen, J., Tanskanen, A. & Taipale, H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am. J. Psychiatry 175 , 765–773 (2018).
Taipale, H. et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr. Res . 197 , 274–280 (2018).
Taipale, H. et al. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 19 , 61–68 (2020).
Carbon, M. & Correll, C. U. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 19 (Suppl 1), 38–52 (2014).
PubMed Google Scholar
Krause, M. et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 268 , 625–639 (2018).
Kane, J. M. et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry 77 , 1217–1224 (2020).
Schreiner, A. et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr. Res. 169 , 393–399 (2015).
Subotnik, K. L. et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry 72 , 822–829 (2015).
Tiihonen, J. et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am. J. Psychiatry 168 , 603–609 (2011).
Zhang, F. et al. Efficacy, safety, and impact on hospitalizations of paliperidone palmitate in recent-onset schizophrenia. Neuropsychiatr. Dis. Treat. 11 , 657–668 (2015).
Kishimoto, T., Nitta, M., Borenstein, M., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J. Clin. Psychiatry 74 , 957–965 (2013).
Kishimoto, T. et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr. Bull. 44 , 603–619 (2018).
Kishimoto, T. et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr. Bull. 40 , 192–213 (2014).
Kane, J. M., Kishimoto, T. & Correll, C. U. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J. Clin. Epidemiol. 66 , S37–41 (2013).
Kishimoto, T., Hagi, K., Kurokawa, S., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry 8 , 387–404 (2021).
Cook, D. J., Mulrow, C. D. & Haynes, R. B. Systematic reviews: synthesis of best evidence for clinical decisions. Ann. Intern. Med. 126 , 376–380 (1997).
Higgins, J. P. T. & Green, S. Cochrane Collaboration Handbook for Systematic Reviews of Interventions . (The Cochrane Collaboration and John Wiley & Sons, Ltd, 2008).
Download references
Acknowledgements
This study received financial funding from Janssen Scientific Affairs.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
The Zucker Hillside Hospital, Department of Psychiatry, Northwell Health, Glen Oaks, NY, USA
- Christoph U. Correll
Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Department of Psychiatry and Molecular Medicine, Hempstead, NY, USA
Charité Universitätsmedizin Berlin, Department of Child and Adolescent Psychiatry, Berlin, Germany
Evidera, Waltham, MA, USA
Amber Martin & Emma Schiller
Janssen Scientific Affairs, LLC, Titusville, NJ, USA
Charmi Patel, Carmela Benson, Jennifer Kern-Sliwa & Kruti Joshi
Goulding HEOR Consulting Inc., Vancouver, BC, Canada
Rebecca Goulding
Biohaven Pharmaceuticals, New Haven, CT, USA
You can also search for this author in PubMed Google Scholar
Contributions
C.C., A.M., R.G., C.P., C.B., K.J., J.K.S., E.S. and E.K. contributed to the conception and the design of the study. A.M., R.G. and E.S. conducted the literature review, including screening, and extraction of the included guidelines. All authors contributed to the interpretations of the results for the review; A.M. and C.C. drafted the manuscript and all authors revised it critically for intellectual content. All authors gave their final approval of the completed manuscript.
Corresponding author
Correspondence to Christoph U. Correll .
Ethics declarations
Competing interests.
C.C. has received personal fees from Alkermes plc, Allergan plc, Angelini Pharma, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Inc, Janssen Pharmaceutica/Johnson & Johnson, LB Pharma International BV, H Lundbeck A/S, MedAvante-ProPhase, Medscape, Neurocrine Biosciences, Noven Pharmaceuticals, Inc, Otsuka Pharmaceutical Co, Inc, Pfizer, Inc, Recordati, Rovi, Sumitomo Dainippon Pharma, Sunovion Pharmaceuticals, Inc, Supernus Pharmaceuticals, Inc, Takeda Pharmaceutical Company Limited, Teva Pharmaceuticals, Acadia Pharmaceuticals, Inc, Axsome Therapeutics, Inc, Indivior, Merck & Co, Mylan NV, MedInCell, and Karuna Therapeutics and grants from Janssen Pharmaceutica, Takeda Pharmaceutical Company Limited, Berlin Institute of Health, the National Institute of Mental Health, Patient Centered Outcomes Research Institute, and the Thrasher Foundation outside the submitted work; receiving royalties from UpToDate; and holding stock options in LB Pharma. A.M., R.G., and E.S. were all employees of Evidera at the time the study was conducted on which the manuscript was based. C.P., C.B., K.J., J.K.S., and E.K. were all employees of Janssen Scientific Affairs, who hold stock/shares, at the time the study was conducted.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Correll, C.U., Martin, A., Patel, C. et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophr 8 , 5 (2022). https://doi.org/10.1038/s41537-021-00192-x
Download citation
Received : 26 February 2021
Accepted : 02 November 2021
Published : 24 February 2022
DOI : https://doi.org/10.1038/s41537-021-00192-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Psychosis superspectrum ii: neurobiology, treatment, and implications.
- Roman Kotov
- William T. Carpenter
- Katherine G. Jonas
Molecular Psychiatry (2024)
Antipsychotic Discontinuation through the Lens of Epistemic Injustice
- Helene Speyer
- Lene Falgaard Eplov
Community Mental Health Journal (2024)
Delphi panel to obtain clinical consensus about using long-acting injectable antipsychotics to treat first-episode and early-phase schizophrenia: treatment goals and approaches to functional recovery
- Celso Arango
- Andrea Fagiolini
BMC Psychiatry (2023)
Machine learning methods to predict outcomes of pharmacological treatment in psychosis
- Lorenzo Del Fabro
- Elena Bondi
- Paolo Brambilla
Translational Psychiatry (2023)
Comparison of clinical outcomes in patients with schizophrenia following different long-acting injectable event-driven initiation strategies
- Carmela Benson
- Panagiotis Mavros
Schizophrenia (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Searching clinical...
Searching clinical trials registers: guide for systematic reviewers
Linked opinion.
Untapping the hidden value of clinical trial registries
- Related content
- Peer review
- Kylie E Hunter , senior evidence analyst 1 ,
- Angela C Webster , director 1 2 ,
- Matthew J Page , senior research fellow 3 ,
- Melina Willson , manager 1 ,
- Steve McDonald , senior research fellow 3 ,
- Slavica Berber , project officer 4 ,
- Peta Skeers , Cochrane information specialist 1 ,
- Ava G Tan-Koay , Cochrane information specialist 1 ,
- Anne Parkhill , information specialist 5 ,
- Anna Lene Seidler , senior research fellow 1
- 1 Evidence Integration, NHMRC Clinical Trials Centre, University of Sydney, Camperdown, NSW, Australia
- 2 School of Public Health, University of Sydney, Camperdown, NSW, Australia
- 3 Methods in Evidence Synthesis Unit, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia
- 4 Health Technology Assessment Team, NHMRC Clinical Trials Centre, University of Sydney, Camperdown, NSW, Australia
- 5 Centre for Health Communication and Participation, La Trobe University, Melbourne, VIC, Australia
- Correspondence to: K E Hunter [email protected]
- Accepted 25 February 2022
Systematic reviews should incorporate as much relevant evidence as possible to reduce bias and research waste and increase reliability of results. Clinical trials registers are a key resource for identifying potentially eligible studies, particularly those that are unpublished, and therefore searching these registers is mandated for best practice systematic reviews. However, the process of searching can be challenging and no clear and consistent guidance on how best to do this exists. This paper provides step-by-step guidance on how to conduct systematic searches for studies using clinical trials registers, with a case study to illustrate each step. The guidance encompasses where to search and how to formulate the search strategy, conduct the search, download results, screen records, obtain data, update searches, and report on these searches.
Summary points
Searching of clinical trials registers is strongly recommended for comprehensive systematic reviews and is mandatory for best practice Cochrane reviews, yet guidance is scarce on how to perform these searches and how to harvest information for identified studies from registers
This article provides 11 steps and several key recommendations on where to search, how to formulate search strategies, efficient screening methods, and reporting searches
This guidance can be used by researchers to identify additional eligible studies and obtain unpublished results for inclusion in systematic reviews, thereby reducing publication bias and research waste
Retrieval of study information from trial registers is also useful to identify research gaps and inform research prioritisation, to identify studies and potential investigators for collaborative methods such as a prospective meta-analysis, and to plan updates of traditional or living systematic reviews
Introduction
Systematic reviews are a cornerstone of evidence based medicine. 1 Positioned at the top of the evidence hierarchy, these reviews frequently underpin healthcare guidelines, policy, and practice. 2 Yet, their validity relies on identification and inclusion of all relevant and available evidence, both published and unpublished. Unpublished studies, however, are often difficult and time consuming to identify, resulting in suboptimal attempts at retrieval or even complete omission from systematic reviews. 3 4 5 6 7 This incomplete inclusion is problematic given that only about half of all biomedical studies ever publish their results, 8 and those that do, tend to yield more positive results and larger effect sizes than unpublished studies 9 (phenomena known as publication bias and selective outcome reporting). Substantial research waste 10 is the result, as is reliance on a biased subset of the evidence, which can lead to inappropriate recommendation of treatments that have lesser to no effect or could even be harmful. 11 For instance, more than 80 million patients had used the anti-inflammatory drug rofecoxib before it was withdrawn due to discovery of unpublished analyses showing that the drug increased the risk of myocardial infarction and stroke. 12
Best practice for systematic reviewers involves searching for unpublished studies to synthesise the totality of available evidence and to reduce bias, 13 and clinical trials registers represent a key resource for this search. Clinical trials registers are publicly available online registers of planned, ongoing, and completed clinical studies (primarily clinical trials but also some observational studies). 14 The registers include structured information on study design, conduct, and administration, and, more recently, have incorporated results reporting and investigator’s data sharing plans. Registers are becoming increasingly comprehensive since prospective registration (that is, registration before enrolment of the first participant) has been mandated by several regulatory, ethical, and legislative bodies, 15 16 17 and registration of observational studies is gaining support. 18 The World Health Organization’s Registry Network has 17 primary registries, which meet specific criteria for content, quality and validity, accessibility, unique identification, technical capacity, and administration. 17 These registries plus ClinicalTrials.gov ( https://www.clinicaltrials.gov ), which is provided by the US National Library of Medicine and is the largest clinical trials register (n=391 704 records on 11 October 2021), are all recognised by the International Committee of Medical Journal Editors (known as ICMJE), and all 18 registries provide data for WHO’s International Clinical Trials Registry Platform (ICTRP; https://trialsearch.who.int ), which on 31 October 2021 included more than 700 000 records of clinical trials. 14 Identification of unpublished studies from trial registers offers many advantages. For instance, reviewers can obtain unpublished results by direct communication with registrants or by direct extraction from registration records. Since WHO introduced results reporting requirements in 2015, 19 more registers have added results reporting functions, which has increased the opportunities for direct data extraction. In cases in which results cannot be retrieved, detection of unpublished studies is still useful to identify potential publication bias, by allowing an estimate of the amount of unavailable evidence that cannot be included in a review. In addition, identification of forthcoming evidence can inform decisions on whether additional studies on a topic are needed, when to plan an update of a traditional or living systematic review, and whether collaboration is possible via next generation systematic review methods. For instance, if several ongoing studies answering similar research questions are identified, researchers can agree to synthesise their results on completion using a prospective meta-analysis method. 20
Searching trial registers can be challenging because of the varied and relatively unsophisticated search interfaces compared with large bibliographical databases such as Medline. 21 22 This difficulty is not surprising given that registers were initially designed to deal with transparency and publication bias, 23 rather than specifically as a research resource for systematic reviews. Perhaps consequently, clear guidance on how to conduct register searches is lacking. The updated Cochrane Handbook, which is widely regarded as outlining the best practice method for conducting systematic reviews, mandates searching trial registers (via ClinicalTrials.gov and WHO ICTRP); however, a technical supplement that provides guidance on how to search these registers only contains a few sentences of advice. 24 This brief text contrasts with the extensive guidance dedicated to search strategies for bibliographical databases to identify published studies. 13 Therefore, to improve the validity and reliability of systematic reviews and reduce research waste and bias, consistent and clear guidance on how to search for registered studies is needed.
We present a step-by-step guide on how to search for registered studies for inclusion in systematic reviews ( fig 1 ). A registered study is defined as one that has met registration requirements of an ICMJE and WHO recognised registry, and that has been issued with a registration number. Although registers primarily include interventional trials, this guidance could be equally applied for identifying registered observational studies. Our advice is based on: a review of the literature; information available on registry websites; an online survey and consensus workshop on 18 June 2021 among coauthors (the steering group) who have extensive international experience as information specialists, trial registry staff, systematic reviewers, biostatisticians, methodologists, clinical trial experts, guideline developers, and clinicians; and an online survey of international experts drawn from Cochrane information specialists and health technology assessment reviewers open from 9 July to 2 August 2021 (n=14 respondents). Further details are available in the supplementary material: the methods used to develop our guidance (appendix 1), the author and steering group areas of expertise (appendix 2), the results of the steering group (appendix 3), and international surveys (appendix 4). We provide guidance on the methods, rationale, and challenges for each step, to increase search literacy, and to enable more reviewers to efficiently conduct effective and reproducible searches of trial registers.

Steps and recommendations to search for registered studies
- Download figure
- Open in new tab
- Download powerpoint
Case study—Transforming Obesity Prevention for CHILDren
We illustrate each step using the example of an ongoing systematic review and individual participant data meta-analysis of randomised controlled trials evaluating behavioural interventions for the early prevention of obesity in children (Transforming Obesity Prevention for CHILDren, TOPCHILD). 25 TOPCHILD searches are updated annually, and for this illustrative case study, we focus on the most recent search conducted on 18 March 2021 (ClinicalTrials.gov) and 22 March 2021 (ICTRP). To date, we have identified 71 eligible trials, of which 15 were identified only by searching trial registers, showing the importance of trial registers as a source of information for systematic reviews.
Step 0: Defining the research question and eligibility criteria
A first step in systematic reviews is to define the research question and eligibility criteria. Searches for registered studies should not commence until this preparatory step is undertaken.
Recommendation
Use an appropriate framework, such as the population, intervention, comparator, outcome (PICO) framework, to define research question and eligibility criteria.
Explanation —Clear prespecified eligibility criteria are crucial to derive an optimal search strategy. The Cochrane Handbook provides detailed guidance on this process. 26 27
Case study —TOPCHILD answers the primary research question 25 : compared with usual care, no intervention, or attentional control, what are the effects of behavioural obesity prevention interventions that are focused on the parent or caregiver and commence during pregnancy or infancy on child weight status at age 24 months? The PICO system 27 was applied to define the eligibility criteria in an iterative process through extensive consultation with experts in the specialty and consumers. The population is the parents or caregivers (including pregnant women) and their infants aged 0-12 months (at baseline); the intervention is the behavioural interventions targeting parents or caregivers, with the primary aim of preventing obesity in their children; the comparator is usual care, no intervention, or attentional control; the outcome is trials must collect at least one child weight related outcome post-intervention—for example, body mass index (BMI) or BMI z score, prevalence of overweight or obesity, and percentage fat content and adiposity; and the study type is randomised controlled trials (at individual level or by cluster).
Step 1: Determining where to search
With many different registry resources available for searching, researchers can find it challenging to decide where to search to maximise retrieval of relevant studies without imposing unnecessary duplication or burden.
As a minimum, search ClinicalTrials.gov and WHO ICTRP.
Explanation —ClinicalTrials.gov registry consistently scores higher than other registries in reviews of technical performance, functionality, and available features. 28 29 30 WHO ICTRP is a database or meta-register that includes data from 18 recognised registries globally. Although ICTRP includes data from ClinicalTrials.gov, Cochrane mandates searching both resources separately because unique records can be found from each and ClinicalTrials.gov has more search features and greater functionality. 13 24 31 Furthermore, ICTRP is not always accessible owing to technical reasons, 30 and it might not be as up to date—for example, on 11 October 2021, the ICTRP website indicated that the last ClinicalTrials.gov data file was imported on 5 July 2021.
Since 2019, the Cochrane Central Register of Controlled Trials (CENTRAL; https://www.cochranelibrary.com/central ) has included registration records sourced from ClinicalTrials.gov and ICTRP. However, searching CENTRAL alone is not supported by Cochrane guidance 13 24 and is insufficient to identify registered studies because of its low sensitivity. 32 This low sensitivity might be because register records as they appear in CENTRAL are less comprehensive than the original register entry, and thus are at a greater risk than other systems of being missed in a search. Regardless, CENTRAL will often form part of the search methods to identify published studies.
For some research questions, consider searching the European Union Clinical Trials Information System for drug trials (known as EU-CTIS, which replaced the European Union Clinical Trials Register (EU-CTR) on 31 January 2022) or regional registries (region specific research questions).
Explanation —Given ICTRP combines data from 18 recognised registries, it is generally not necessary to search other registries individually, and any additional yield might not be justified by the extra resources spent. In some instances, however, and if resources allow, other registries within the WHO Registry Network can be searched separately. For systematic reviews focusing on drug trials, reviewers could consider searching CTIS because this resource focuses on interventional clinical trials on medicines conducted in the EU. For geographically restricted research questions, region specific registries could be searched in addition to ClinicalTrials.gov and ICTRP. For instance, if the participants of interest are Indigenous Australians, we would recommend searching the Australian New Zealand Clinical Trials Registry (known as ANZCTR), and if the topic of interest is Chinese herbal medicine, a separate search of the Chinese Clinical Trials Registry might be prudent to maximise sensitivity.
Case study —For TOPCHILD, we searched ClinicalTrials.gov and ICTRP for registered trials. Given the interventions of interest were behavioural, searching of the drug focused EU-CTR (now CTIS) was not appropriate, and because obesity is a global health issue, regional searches were deemed unnecessary.
Step 2: Identifying key search concepts and deriving search terms
Search concepts describe the broad subject areas or topics of interest. They are used to derive search terms, which are specific words, phrases, and synonyms that reflect these concepts. These terms will be used to formulate a search strategy and determine the relevance of search results.
Identify one or two key concepts from the PICO (or other appropriate framework, step 0), typically population and intervention. For each concept, list synonyms or alternative terms expressing the same concept.
Explanation —Firstly, a mind map is useful for possible search terms for the key PICO elements—typically the population and intervention. Ideas can also be gathered from search strategies of systematic reviews on similar topics (if used substantively, these should be cited), 33 thesauruses, and Medical Subject Headings (MeSH) or other terms indexed for any known eligible studies. Both ClinicalTrials.gov and ICTRP also incorporate synonym searching using the Unified Medical Language System. For instance, if the term “obesity” is searched in ClinicalTrials.gov, the synonyms “obese” and “adiposity” are also automatically searched; but note that this function cannot be disabled in ClinicalTrials.gov if a search for a specific phrase only is preferred.
Case study —Before we searched the registers, we had formulated a complex search strategy for bibliographic databases (Medline, Embase, CENTRAL, CINAHL, Psycinfo) in consultation with a Cochrane information specialist. The strategy incorporated a wide range of concepts, including overweight and obesity; behavioural and lifestyle interventions; nutrition, diet, and feeding; physical activity; sedentary behaviours; sleep; health promotion and prevention; and children and families. For our register searches, we chose two main concepts: overweight/obesity and child. We decided to omit the intervention concepts because the diverse variety of eligible intervention types precluded reasonable specificity. Table 1 shows the synonyms or alternative search terms derived from our two chosen concepts. These terms were discussed among members of the research team and derived by consulting relevant Cochrane 34 and non-Cochrane 35 reviews, as well as from the ClinicalTrials.gov synonym function and MeSH trees.
Search concepts and corresponding synonyms or alternative terms
- View inline
Step 3: Formulating search strategies
This step aims to identify as many relevant records as possible to contribute to the review (that is, maximise sensitivity), while also balancing with reasonable specificity and precision so that screening is feasible. 24 A search strategy is the structured combination of concepts and terms used to search a database. Although bibliographical databases offer a broad suite of tools to formulate complex search strategies, the availability and functionality of similar tools on trial registries are limited and vary widely by trial registry ( table 2 ).
Key differences between searching Medline (via Ovid) and trial register resources (ClinicalTrials.gov and WHO ICTRP)
Focus search strategies on one or two key concepts identified in step 2 and aim to maximise sensitivity while balancing against reasonable specificity.
Explanation —Reviewers should start with a basic search that focuses on the single most specific concept, typically P (population or health condition) or I (intervention). 21 31 To enhance sensitivity, various synonyms and related terms should be combined for this concept using the Boolean operator “OR”—for example, “overweight OR obesity OR obese OR adiposity”. If the number of results retrieved is too high thus rendering screening infeasible, a second concept should be added to reduce the number of hits to only those where the two concepts overlap ( fig 2 ). 37 This overlap can be achieved using parentheses to group the terms for each concept and then combining concepts with the Boolean operator “AND”—for example, “(overweight OR obesity OR obese OR adiposity) AND (baby OR infant OR child OR toddler).” Recent updates to the ICTRP now allow use of parentheses and Boolean operators within the basic search; however, longer search strings can cause the system to time out or bring up error messages. If issues are experienced, we recommend conducting separate searches for each combination of concept terms—for example “overweight AND baby” then “overweight AND infant”. Following each search, the results would need to be downloaded, combined, and the duplicate records removed.

Combining concepts and search terms to adjust sensitivity and specificity
The advanced search interface of ICTRP should be used with caution because one study 31 found that the search can reduce sensitivity (often without improvements in specificity) compared with the basic interface. The same study found that the advanced search on ClinicalTrials.gov seems to increase precision while maintaining sensitivity and therefore might be appropriate when there are large numbers of search results.
Adjust search strategies according to the specific registry resource and become familiar with search tools and rules of each.
Explanation —Search interfaces on trial registries tend to be more simplistic and less sophisticated than those on large bibliographical databases, 21 22 due to limited funding and resources, their relatively small size, and the quality and structure of registration records compared with bibliographical records. Variation across registries also requires that search strategies are adjusted in each database. 21 22 Although ClinicalTrials.gov and ICTRP have some similarities in search functionality, many differences exist that need adjustments ( table 2 ). These limitations can make the process challenging and resource intensive, and consultation with an experienced librarian or information specialist might be useful to improve efficiency, if possible. 13
Test whether the search strategy retrieves pre-identified eligible studies (if possible).
Explanation —The combination of search terms should be trialled and revised in an iterative process. If the reviewer is already aware of studies that have been pre-identified as eligible, and knows that these studies are registered, we recommend testing the initial search strategy to see whether these are retrieved. Be prepared to experiment with search interfaces to become familiar with how they work. Try adding or removing search terms if the number of records retrieved seems too low to have captured everything, or too high for screening feasibility. The appropriate number of records retrieved should be determined on a case-by-case basis, in consideration of the specificity of the concept searched and previous availability of research knowledge. For instance, searches for a rare disease or a new drug would be expected to yield fewer and more precise results than they do for a common condition such as pregnancy or drug such as paracetamol (acetaminophen). Results from register searches might also be used to test the sensitivity of search strategies used for other databases, such as Medline. Specifically, for each relevant registration record identified, a targeted search should be conducted for matching publications regardless of the recruitment status listed. If a substantial number of relevant publications are identified that were missed during the initial database search, then reviewers should revise and repeat their database search strategy to improve sensitivity.
Apply filters (eg, by study type or participant age) only in exceptional circumstances.
Explanation —Most registries offer a range of filters that can be used to refine a search (eg, by study type or participant age). Although filters can be a powerful tool to increase the precision of a search, we recommend not to use them unless circumstances are exceptional—for example, when resources are extremely limited or only a rough search is required for scoping. The reasoning for this recommendation is that optimal use of filters relies on accurate data categorisation in the registry, which is not always achieved for registration records. For instance, while testing a search string with and without application of the ClinicalTrials.gov study type filter for “Interventional Studies (Clinical Trials),” we identified three (5%) of 57 records that were randomised controlled trials but had been incorrectly categorised as observational studies and, therefore, would have been wrongly excluded by the filter (Hunter, unpublished data, 2021).
Avoid limiting searches by recruitment status because this field might not be up to date and therefore eligible studies could be missed.
Explanation —Reviewers often limit register searches by “completed” recruitment status because these are the studies for which they logically expect results data to be available. 38 However, this approach should be avoided for two key reasons. Firstly, this limitation risks missing a substantial proportion of studies that are actually completed or have published results because recruitment status listed on trial registers is often out of date or inaccurate. 38 39 For instance, as of 23 December 2021, 46 406 (12%) of 399 046 records on ClinicalTrials.gov were labelled as having an “unknown” recruitment status, meaning that their last known status for “recruiting,” “not yet recruiting,” or “active, not recruiting” had not been updated or verified within the past two years despite having passed the completion date. This finding also underscores the need to conduct targeted searches for publications linked to studies identified by register searches, regardless of recruitment status. Secondly, filtering precludes the ability to identify ongoing trials and therefore negates many of the advantages of register searches described in this paper, such as the ability to assess the potential impact of publication bias on review findings.
Case study —We chose “overweight/obesity” as the primary concept of interest and avoided application of any filters or limits (eg, by start or end dates or recruitment status). When the output yielded too many irrelevant results, we added the concept “child” to enhance precision.
WHO ICTRP search strategy — Table 3 shows the TOPCHILD search strategy formulated for the ICTRP. We used the basic search interface to search for only the key concepts “overweight/obesity” and “child.” At the time of searching (22 March 2021), parentheses were not available for nested searching, only very short search strings worked, and synonym and truncation functions had issues. For instance, combining the search term “obesity” with variations of the term “infant” retrieved discrepant results (132 for “infants AND obesity”, 96 for “infant AND obesity”, 123 for “infant* AND obesity”), and the truncated term “obes*” retrieved fewer results than “obese” and “obesity”, indicating truncation was not functioning. 28 Because of these issues, we chose to conduct multiple separate searches combining varied terms for the two chosen concepts, then merge and remove duplicate records (this process is described further in step 4).
TOPCHILD (Transforming Obesity Prevention for CHILDren) search strategy for the World Health Organization’s International Clinical Trials Registry Platform basic interface
The ICTRP search function has since been updated (20 July 2021), and we re-ran our search strategy on 15 September 2021 to evaluate key changes ( table 3 ). Although we noted some apparent issues with synonyms, truncation, and duplication, sensitivity increased markedly from 0.18 to 0.78 (appendix 2), and we commend ICTRP’s efforts to improve the functionality of this important resource despite the limited funding and resources. These experiences from our case study highlight that reviewers should be cognisant of functionalities that might not always perform as intended, and therefore might require a flexible and adaptive approach.
ClinicalTrials.gov search strategy — Box 1 shows the ClinicalTrials.gov search strategy for TOPCHILD. We found the basic search interface to be sufficient for our requirements and were able to efficiently combine all terms for both concepts in a single search string. In the “Condition or disease” field, we linked several terms for the key concept “obesity” with the Boolean operator OR and this was combined with varied terms for the concept “child” in the “Other terms” field. The “Other terms” search encompasses many data fields, including study location and researcher affiliation. Thus, a few irrelevant studies were retrieved simply because they were conducted at an institution with “child” (or a synonym) in the name, eg, “Seattle Children’s Hospital”. Searching of fields that were further refined was not possible, a process that would be valuable to overcome this issue. Although ClinicalTrials.gov offers a filter for the word “child”, we chose not to use it to prevent erroneously excluding relevant records. On testing, we discovered that nine eligible trials enrolling mothers and infants (as dyads) only listed ages for the mother (>18 years) in the “Ages eligible for study” section, and thus, these trials would have been missed if the child filter was applied.
TOPCHILD (Transforming Obesity Prevention for CHILDren) search string for ClinicalTrials.gov advanced interface
Condition or disease: overweight OR obesity OR obese OR adiposity OR BMI OR weight gain
Other terms: baby OR infant OR child OR paediatric OR pediatric OR toddler OR offspring
2756 records were retrieved.
Step 4: Conducting the search, removing duplicate records, and preparing records for screening
Detailed records of all registry searches are important to keep for transparency, reproducibility, and efficiency. 28 This record keeping will also assist with completing the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram 40 (step 7) and reporting the search (step 10).
Keep detailed records of all register searches, including the exact date the search was conducted, names of registers searched, interfaces used (basic or advanced), full search strings, and number of records retrieved from each.
Explanation —Reviewers should document the date that each search is conducted and by whom; trial registers searched; interface used (basic or advanced); full search strings, including any limits or filters applied; and number of records retrieved from each. This documentation can be done in Microsoft Word or Excel or, as suggested in one study, 21 by screen capture or use of a note-taking software such as Evernote or OneNote. No options are currently available to save search strategies and history within ICTRP or ClinicalTrials.gov. However, ClinicalTrials.gov offers RSS (Really Simple Syndication) feeds and the option to save selected studies for easy retrieval, and if the search results page is bookmarked, updates will be automatic every time the page is opened.
Download search records into your preferred software and remove duplicates.
Explanation —Various methods are available for exporting and deduplicating search records in preparation for screening, and the optimal methods depend on the register searched and the reviewer’s preferred reference management or screening software (eg, Excel, Endnote, Refworks, Abstrackr, Mendeley, Covidence, DistillerSR, EPPI-Reviewer, Zotero, and Rayyan). 13 As each search string is run, the records retrieved should be downloaded by methods appropriate to the chosen software. For instance, to download results to Excel, use the CSV or TSV options on ICTRP and ClinicalTrials.gov, whereas for Endnote, download results in plain text format from ClinicalTrials.gov and in XML format from ICTRP, then import them using the appropriate Endnote filter ( https://endnote.com/downloads/filters/ ) . Note that the non-bibliographical nature of registration records makes mapping of data to Endnote fields difficult, and therefore much of the information only imports to the “Notes” field or is omitted. The Cochrane Collaboration’s preferred software, Covidence, 41 allows imports in EndNote XML format, and it is also compatible with Zotero, Refworks, Mendeley, or any tool that supports RIS, CSV, or PubMed XML formats.
Once all records are imported into the preferred software, duplicate records need to be identified and removed (a process often referred to as deduplication). We define duplicate records as records with the exact same registration number and title. Note that some researchers choose to register a single study on two different registers, resulting in two unique registration numbers. Although these records relate to the same study, they are not considered to be duplicates because they can contain different information, particularly if one is more up to date than the other. In this instance, both records should be kept but grouped as a single study.
Although the literature offers plenty of advice on deduplication for bibliographical databases, 42 43 many of these methods are not applicable for registration records because they rely on sorting by data fields that are not collected by registries—for example, author, journal, volume, or pages. Instead, we recommend use of unique trial registration numbers for deduplication in Excel, either by highlighting and manually deleting duplicates using the “Conditional formatting” function, or by use of the automatic “Remove duplicates” function if the number of records is large. Registration records sourced directly from a registry contain more detailed and up-to-date information than do those obtained from ICTRP, and therefore should be retained in case of duplicates.
Case study —For TOPCHILD, we recorded search dates, who conducted the searches, registries and interfaces searched, full search strings, and the number of records retrieved for each in a simple table in Microsoft Word. All records retrieved from ICTRP and ClinicalTrials.gov were downloaded into Excel using the TSV format and combined to one spreadsheet. ICTRP and ClinicalTrials.gov records are formatted differently so we needed to manually align key columns containing registration ID, title, and study type. Next, we deduplicated records using the registration ID column and “Remove duplicates” function in Excel.
Step 5: Title screening (optional)
The purpose of this step is to remove any obviously irrelevant records so that fewer records need to be screened in full, thus improving efficiency. In contrast with published studies, which generally have a structured abstract available for screening, the information available from downloaded registration records can range from only a title and web link to a full record of all available data fields. Depending on the amount of information available, the number of records, and personal preferences, some reviewers might choose to skip this step and screen all records in full.
If preliminary title screening is conducted, only exclude obviously irrelevant records.
Explanation —Screeners should review all study titles against eligibility criteria using their chosen software. Reviewers should be over-inclusive at this stage 13 and only exclude studies that are obviously irrelevant. In our experience, titles are sometimes not overly representative of the study content. Any uncertainties should be resolved through discussion.
Case study —Two reviewers independently screened all registration record titles in Excel using separate copies of the deduplicated spreadsheet created in step 4. We added a column adjacent to the titles and populated this with either “maybe” (proceed to full text screening) or “no” (exclude) for each record. Discrepancies were resolved by consensus or consultation with a third reviewer. We then sorted and copied all records marked “maybe” to a new spreadsheet. Examples of records excluded by title were those stating obviously irrelevant participants (eg, “Adolescents With Hepatosteatosis” and “Obese Adolescent Girls”), obviously irrelevant interventions (eg, “metformin” and “Setmelanotide”), or obviously irrelevant health conditions (eg, “Dengue fever”, “Endometrial Cancer”, and “vision impairment”).
Step 6: Full record screening
This step determines the final eligible studies to be included in the review and contribute to results.
Screen full registration records at the source registry website.
Explanation —Full registration records should be viewed at the source registry website by use of the link or registration ID downloaded with search results. This ensures access to the most detailed and recent information, which can be lost when downloading, importing, or uploading records to various software, especially since records are not in bibliographical format. For instance, registration records imported into Covidence from ClinicalTrials.gov often include only the title and record link, and records on ICTRP contain fewer data than does the source registry.
Screen all records in full at least once and consider an independent second reviewer if resources allow.
Explanation— Best practice for study selection in systematic reviews is independent double screening to ensure that no eligible studies are missed. 44 45 However, for rapid reviews or when resources are limited, one experienced reviewer is sufficient to screen registration records. A key factor to consider when deciding the number of screeners is the potential consequences of missing an eligible study. For instance, if a prospective meta-analysis is being conducted, identification of eligible studies is essential before results are known, and therefore, missing a potentially eligible study at the screening phase might be more consequential than for retrospective reviews and limit opportunities for harmonisation.
Screen records systematically with a hierarchical list of eligibility criteria, starting from the simplest (eg, study design, then population) and use the structured data fields on registers to expedite this process.
Explanation —Similar to screening full text publications, we recommend creating a simple hierarchy of reasons for exclusion, with study design first, then variables relating to participants, interventions, and outcomes. Downloaded registry records are generally displayed in a structured format in which each column represents a data field (eg, study design and eligibility criteria), and this structure should be leveraged to expedite screening. For example, registration records can be sorted by study design (interventional v observational) in Excel and this information can be quickly verified by viewing the full text record.
Once studies are determined to be eligible, reviewers should link corresponding registration records and publications, when applicable. As such, records are not counted as two separate studies, which could introduce bias. 13 Additionally, reviewers can check all identified data sources to extract the most comprehensive information. This process can lead to increased data availability, particularly for adverse events because evidence shows that these are more completely reported in ClinicalTrials.gov registration records than in linked publications. 46 Registration numbers are useful for linking records and publications, and other criteria to consider are detailed in the Cochrane Handbook. 13 Importantly, reviewers should consider and document how they will incorporate multiple data sources into a review (eg, registry data and published data), particularly if these sources contain conflicting information. 47
Case study —Two reviewers independently screened all records using a multi-step deductive process. Firstly, we sorted the Excel spreadsheet created in step 5 by the “Study type” column (interventional v observational) and checked entries labelled “observational” against the full record at the source registry’s website. If the “observational” categorisation was verified, we entered “no” (excluded) in an adjacent column because only randomised controlled trials are eligible for TOPCHILD. Interventional trials were labelled “yes” and copied to a new spreadsheet, where we created columns for key eligibility criteria to be checked hierarchically against the source registration record and marked yes or no: randomised controlled trials, intervention start was <1 year ago, lifestyle intervention, intervention continues after pregnancy, prevention focused, and infant weight related outcome. Once any category was marked with “no”, the record was moved to the “Excluded” category and a review of the remaining criteria was unnecessary. We also added a “Reason for exclude” column with the options: ineligible study design (not a randomised controlled trials), ineligible population (child aged >12 months), ineligible intervention (no lifestyle component, antenatal intervention only, not prevention focused), or no infant weight related outcome. Additionally, we added a “Notes” column for any comments (appendix 3).
Step 7: Completing a PRISMA flow diagram
This step transparently summarises the flow of information through the searching and screening stages of a review.
Complete a PRISMA flow diagram, which includes records retrieved from trial register searches.
Explanation —The PRISMA 2020 flow diagram 40 enables reviewers to report the number of records retrieved by searches (databases, registries, or other sources) and the number of studies screened, included, and excluded (with reasons).
Case study — Figure 3 is the PRISMA flow diagram for TOPCHILD. In summary, we identified 15 extra eligible trials by searching trial registers, in addition to 56 trials identified by database searches or other sources. These 15 trials included 8764 participants that met TOPCHILD eligibility criteria. Of these 15 trials, 13 were registered on ClinicalTrials.gov, one on the Chinese Clinical Trials Registry, and one on the Netherlands Trial Registry. Two trials were published but not identified in searches of bibliographical databases.

PRISMA flow diagram for Transforming Obesity Prevention for CHILDren (TOPCHILD) case study. Adapted with permission from Page et al 40
Step 8: Finalising eligible studies
Sometimes information is insufficient in a registration record to conclusively determine eligibility. For example, details of sequence generation might be lacking or participant eligibility criteria can be ambiguous.
If there are uncertainties about study eligibility, contact study registrants for clarification, if feasible.
Explanation —To facilitate communication with study investigators, registries display contact details that can be used to clarify eligibility queries. A particular issue with some prospectively registered studies is determining whether the study proceeded (eg, funding might have been withdrawn or never obtained), particularly if records are out of date and study investigators cannot be contacted. This situation can arise because some researchers think that registration of their study before submitting a grant application increases their chance of funding success. With low funding rates in many competitive schemes, this early registration can lead to many so-called zombie records of studies that did not start. In such cases, factors to consider are existence of ethical approval or related publications, whether the study is listed on an institutional or researcher webpage, and whether recruitment dates or other updates to the record were provided.
Case study —We emailed investigators from 19 trials for clarification of TOPCHILD eligibility. Most queries related to intervention timing or content, whether a child weight outcome was assessed after intervention and study design. We were informed that three trials never began due to withdrawal of funding.
Step 9: Obtaining data then synthesising as applicable
Trial registries are a useful resource for obtaining summary results. 48 49 Once eligible studies are determined, and a systematic review protocol has been published or is publicly available, attempts should be made to obtain data for inclusion in the review.
Attempt to obtain unpublished results data for eligible studies by checking registers and repositories and contacting study registrants.
Explanation —The process to obtain data depends on the type of review. For standard systematic reviews (retrospective reviews synthesising aggregate data), summary results might be available on registers, within other systematic reviews, or elsewhere, but often registrants need to be contacted for their data. For next generation systematic review approaches, such as individual participant data meta-analysis 50 and prospective meta-analysis, 20 study investigators should be invited to join a collaboration and share their raw data (individual participant data meta-analysis) or work together to harmonise outcomes to facilitate evidence synthesis (prospective meta-analysis or nested prospective meta-analysis), or both. 20 Obtaining individual participant data from study investigators can be challenging, despite strong support in principle for the concept of data sharing. 51 Barriers include concerns about participant consent, confidentiality, and data misuse; mechanisms to tackle these have been published. 51
Explore the potential effect of publication bias, selective outcome reporting, and data availability bias when results are missing.
Explanation —Often, aggregate data or individual participant data cannot be obtained for all eligible studies and for all outcomes. If non-reported results differ systematically from those that are reported, biases can be introduced, and it is important to explore the potential effect of this difference. For instance, the identification of additional unpublished studies or data via registries will give reviewers an idea of the extent of unpublished evidence that they could be missing from their review and thus allow for an assessment of risk of publication bias. Selective outcome reporting can also be detected by comparing outcomes documented in study registration records with those that are subsequently published. 48 For individual participant data meta-analyses, data availability bias should also be assessed because non-provision of data can be representative of poor study quality 52 or unfavourable results. 50 Extensive guidance on assessing risk of bias due to missing results is available in the Cochrane Handbook 53 and will be available in the forthcoming ROB-ME (Risk Of Bias due to Missing Evidence) tool. 54
Case study —The protocol for the TOPCHILD individual participant data meta-analysis is publicly available 25 and prespecifies methods to investigate potential bias arising from non-reporting of results. Representatives from 47 trials have joined the TOPCHILD Collaboration to date, and we have commenced data collection by direct communication with trial representatives and review of information available on registration records.
Step 10: Reporting the search
Clear and comprehensive reporting of search details is essential for transparency and reproducibility.
Report register searches in accordance with the PRISMA 2020 statement and PRISMA-Search.
Explanation —Items 6 and 7 of PRISMA 2020 40 are relevant to reporting register searches. Item 6 (“Information sources”) requires specification of all registers searched and the date each register was searched. Item 7 (“Search strategy”) requires presenting the full line-by-line search strategies used for each register, including any limits or filters applied (eg, date restrictions), as well as specifying whether the search strategy was validated, peer reviewed, or adapted or re-used from a previous review. Further guidance and examples for reporting register searches are available in PRISMA-Search, 33 which is a search specific extension to the PRISMA statement. PRISMA-Search also requires a description of the record deduplication process, including any software and processes used, and specifying the methods used for updating searches—for example, email alerts and re-running searches with date restrictions.
Case study —We will report TOPCHILD searches according to PRISMA 2020 and PRISMA-Search in upcoming publications. To enable reporting of each item, we have been recording relevant details, such as names of registers searched, search strategies and dates, and record management via a PRISMA flow chart.
Step 11: Updating searches
This step aims to identify any new eligible studies and to ensure reviews remain up to date.
Update searches at an appropriate frequency, depending on available resources, the research question (slow v fast-moving field) and type of review (eg, annually for standard reviews and monthly for living reviews).
Explanation —Typically, updating a register search involves repeating the initial search strategy, but restricting by registration date to avoid duplication of effort. Restricting by study start or completion dates should be avoided because a large proportion of trials are registered retrospectively (19% of trials on WHO ICTRP in 2020). 55 Both ClinicalTrials.gov and ICTRP have a function to limit searches by registration date in their advanced interface, labelled “First posted” in ClinicalTrials.gov and “Date of registration” in ICTRP. Alternatively, reviewers can sort retrieved records by date of registration, and select studies that have been registered since the last search. Regardless of the method used, reviewers should be conservative with date restrictions (that is, allow some overlap in search dates) because uploads could be delayed—for example, ICTRP uploads data from some registries weekly and from others every four weeks (last upload dates are listed on the ICTRP search page). The PRISMA flow diagram for updates 40 should be completed to summarise updated and cumulative searches.
Researchers can also decide to refine their search strategy for an update (beyond simply restricting dates) if they have identified shortcomings with their previous strategy—for example, eligible studies were missed. This stage is also a good opportunity to check for updates to registration records of studies already identified as eligible, such as to retrieve any recently linked publications or check if recruitment status has changed.
Case study —We updated TOPCHILD searches on 18 March 2021 (ClinicalTrials.gov) and 22 March 2021 (ICTRP), with minor refinements to resolve shortcomings in our initial March 2020 searches. We limited registration date from 1 January 2020 to the date of search (18 or 22 March 2021), allowing a few months overlap in search periods for delays in uploading records. For ClinicalTrials.gov, this date limit was applied by use of the advanced search field “First posted”. Date limits were not functioning on ICTRP, so we filtered records manually in Excel using the “Date of registration” field.
Although searching trial registers is often recommended for systematic reviews, generally little is understood about how best to conduct these searches, and their utility. Consequently, registry searches can be performed suboptimally (if at all) and can be considered an inconvenient afterthought to standard bibliographical searches. We address this gap by providing practical step-by-step guidance on how to search for trial registration records for inclusion in systematic reviews, and by highlighting the importance of these searches to mitigate bias and generate robust results based on the totality of evidence.
Although the focus of this paper is on searching for registration records, searching of other sources of unpublished studies should also be explored. The Cochrane Handbook offers helpful advice on this process, such as searching grey literature databases for reports, conference abstracts, and theses; contacting expert stakeholders; and creating a study website for outreach. 13
Opportunities for searching trial registers will change in line with innovative technological developments, such as text mining, artificial intelligence, machine learning, and big data. Trial registries can diversify to increase their value and utility, particularly in relation to results reporting, data sharing, and collaboration. Since WHO introduced results reporting requirements in 2015, 19 and other legislative, ethical, and regulatory levers followed, clinical trial registries have become an increasingly important hub for obtaining unpublished data, by providing direct access to summary results and providing links to preprints and publications. As of 11 October 2021, 51 372 studies registered on ClinicalTrials.gov had posted summary results. 56 In future, registries could be a gateway or link users to individual participant data sharing repositories. 51 In combination with results reporting, the need to publish all results of every study could be negated and the dissemination of evidence in clinical research could be transformed.
The use of trial registries can be greatly broadened in the context of emerging next generation systematic review methods. For instance, registries could have a pivotal role in enabling data sharing, which is typically a difficult and time-consuming process for those conducting individual participant data meta-analysis. 50 Since 2019, registries have collected information on data sharing plans for each study, which can inform on data availability for individual participant data meta-analysis. Searching registers also allows investigators to determine if any similar studies are planned or ongoing. This information enables researchers to avoid duplication (if emerging evidence is plentiful), or, for example, to align their research with other ongoing investigations by collecting the same core outcomes at the same timepoints. These efforts can involve the formation of a prospective meta-analysis. For prospective meta-analyses, eligible studies need to be identified for inclusion before their results are known. 20
In future, rather than relying solely on researchers to search for potential studies to collaborate with, registries could automatically link registrants planning similar studies at time of registration. This linkage would help to facilitate collaboration, enable prospective meta-analysis, maximise the value of research data, and avoid unnecessary duplication. 20 The potential benefits of such an approach became clear during the covid-19 pandemic, which resulted in an abundance of related trials rapidly launching. 57 Many of these trials have insufficient sample size alone to detect effects on important outcomes, such as mortality. Collaboration with similar trials is an efficient way to increase sample size and obtain sufficient statistical power to answer important research questions. One such example is an influential prospective meta-analysis, 58 which reported that corticosteroid treatment for covid-19 is associated with reduced 28 day all cause mortality compared with usual care or placebo.
To effectively achieve these goals, funding is required for technological innovation of trial registries, including increased automation and improved functionality of searching. In addition, further mechanisms should be introduced to promote and enforce prospective study registration and reporting of results, to maximise the retrieval of information via registry searches. Such innovations would position trial registries as the first place to search to access new evidence, ahead of searches for published studies, which can have a long lag time after study completion.
We hope that this step-by-step guidance on searching trial registers will facilitate identification of registered studies for inclusion in systematic reviews and encourage future innovative use of trial registries. These improvements would help to streamline evidence synthesis, reduce bias, and enhance the reliability and validity of systematic reviews, ultimately leading to better health outcomes.
Acknowledgments
We are grateful to Lisa Askie (methods scientist, WHO) and Lotty Hooft (director of Cochrane Netherlands, member of ICTRP Advisory Group) for their input at the conceptualisation phase of this study.
Contributors: KEH and ALS conceived the study. KEH designed the surveys and led the consensus workshop; all co-authors (ACW, MJP, MW, SM, SB, PS, AGT-K, AP, and ALS) are members of the steering group, completed the author survey, and actively participated in the workshop. KEH wrote the first draft of the manuscript, and all authors contributed to and revised the manuscript. KEH is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: None. KEH receives research funding support via two scholarships administered by the University of Sydney (Postgraduate Research Supplementary Scholarship in Methods Development (SC3504), and Research Training Program Stipend (SC3227)).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: KEH receives research funding support via two scholarships administered by the University of Sydney (Postgraduate Research Supplementary Scholarship in Methods Development), and Research Training Program Stipend). ALS is chair of the TOPCHILD Collaboration, of which KEH is a chief investigator, and ACW is a member of the Advisory Group. MW has been employed as manager of the Australian New Zealand Clinical Trials Registry (ANZCTR) from October 2020 to present, which involves overseeing the operational and some research activities of the ANZCTR. ALS, AGT-K, and PS work for the ANZCTR. KEH worked at the ANZCTR from 2009-20 in a project officer/research role; she remains peripherally involved in ANZCTR related research. ALS is convenor, and KEH and ACW are associate convenors, of the Cochrane Prospective Meta-analysis Methods Group. PS and AGT-K work as information specialists for the Cochrane Breast Cancer Group; this role involves the design and execution of search strategies for systematic reviews, as well as author support throughout the review process. MJP is lead author and SM is co-author of the PRISMA 2020 statement; MJP is also a co-author of the PRISMA-Search extension; they have no commercial interest in the use of these reporting guidelines.
Patient and public involvement: This guidance was tested by two first time registry users as well as by several experienced systematic reviewers.
Provenance and peer review: Not commissioned; externally peer reviewed.
- Tsafnat G ,
- Glasziou P ,
- Choong MK ,
- Galgani F ,
- ↵ National Health and Medical Research Council. NHMRC additional levels of evidence and grades for recommendations for developers of guidelines, 2009. https://www.nhmrc.gov.au/sites/default/files/images/NHMRC%20Levels%20and%20Grades%20(2009).pdf
- Weaver MA ,
- Platts-Mills TF
- Simpson A ,
- Johnson AL ,
- Bibens ME ,
- Greiner B ,
- Corcoran A ,
- Schmucker C ,
- Schell LK ,
- Portalupi S ,
- OPEN consortium
- Polanin JR ,
- Tanner-Smith EE ,
- Hennessy EA
- Vickers A ,
- ↵ Lefebvre C, Glanville J, Briscoe S, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.1 (updated September 2020): Cochrane. www.training.cochrane.org/handbook .
- ↵ World Health Organization. World Health Organization International Clinical Trials Registry Platform (ICTRP) search portal. https://trialsearch.who.int/ .
- DeAngelis CD ,
- Drazen JM ,
- Frizelle FA ,
- International Committee of Medical Journal Editors
- ↵ World Medical Assocation. Declaration of Helsinki: ethical principles for medical research involving human subjects. 2018. www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ .
- ↵ World Health Organization (WHO). WHO International Clinical Trials Registry Platform (ICTRP) registry criteria. 2009. Version 2.1. www.who.int/clinical-trials-registry-platform/network/registry-criteria (accessed 20 September 2021).
- ↵ World Health Organization. WHO statement on public disclosure of clinical trial results. 2015. www.who.int/ictrp/results/reporting .
- Seidler AL ,
- Hunter KE ,
- Berlin JA ,
- Isojarvi J ,
- Lefebvre C ,
- Glanville J
- Gregori D ,
- Berchialla P ,
- ↵ Lefebvre C, Glanville J, Briscoe S, et al. Technical supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT TJ, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA, ed. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.1 (updated September 2020): Cochrane. www.training.cochrane.org/handbook .
- Johnson BJ ,
- Transforming Obesity Prevention for CHILDren (TOPCHILD) Collaboration
- ↵ Lasserson TJ, Thomas J. Higgins JPT. Chapter 1: Starting a review. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021): Cochrane. www.training.cochrane.org/handbook .
- ↵ Thomas J, Kneale D, McKenzie JE, et al. Determining the scope of the review and the questions it will address. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook .
- Gusenbauer M ,
- Haddaway NR
- Venugopal N ,
- Schauberger U
- Glanville JM ,
- Tsujimoto Y ,
- Rethlefsen ML ,
- Kirtley S ,
- Waffenschmidt S ,
- PRISMA-S Group
- Moore THM ,
- Hennessy M ,
- Gonçalves RS ,
- Institute for Quality and Efficiency in Health Care (IQWiG)
- Safferman MR ,
- McKenzie JE ,
- Bossuyt PM ,
- ↵ Covidence systematic review software [program]: Veritas Health Innovation, Melbourne, Australia. www.covidence.org .
- Bramer WM ,
- Giustini D ,
- de Jonge GB ,
- Holland L ,
- McKeown S ,
- Pigott TD ,
- Espelage DL ,
- Knelangen M ,
- Riveros C ,
- Dechartres A ,
- Perrodeau E ,
- Boutron I ,
- Mayo-Wilson E ,
- Dickersin K ,
- MUDS investigators
- Le Cleach L ,
- Williams HC ,
- Trinquart L
- Baudard M ,
- Yavchitz A ,
- ↵ Tierney JF, Stewart LA, Clarke M. Chapter 26: Individual participant data. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021): Cochrane. www.training.cochrane.org/handbook .
- Bordewijk EM ,
- van Wely M ,
- ↵ Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021): Cochrane. www.training.cochrane.org/handbook .
- ↵ Page MJ, Sterne JAC, Boutron I, et al. Risk Of Bias due to Missing Evidence (ROB-ME): a new tool for assessing risk of non-reporting biases in evidence syntheses (Version 24 October 2020). https://sites.google.com/site/riskofbiastool//welcome/rob-me-tool .
- ↵ World Health Organisation. Percent of trials with enrollment year preceding registration year (1999-2020) (March 2021). www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/percent-of-trials-with-enrollment-year-preceding-registration-year (accessed 31 January, 2022).
- ↵ ClinicalTrials.gov. Number of registered studies with posted results over time 2021. https://clinicaltrials.gov/ct2/resources/trends#RegisteredStudiesOverTimePostedResults (accessed 10 September 2021).
- Petkova E ,
- Antman EM ,
- Sterne JAC ,
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group
- Search Menu
- Aesthetic Breast Reconstruction
- Aesthetic Breast Surgery
- Aesthetic Genital Plastic Surgery
- Body Contouring
- Breast Surgery
- Cosmetic Medicine
- Facial Surgery
- Oculoplastic Surgery
- Rhinoplasty
- SoMe and Behavioral Science
- Advance articles
- Editor's Choice
- Thematic Issues
- Supplements
- Highly Cited Collection
- Video Roundtables
- Author Guidelines
- Author Guidelines in Chinese
- Submission Site
- Permissions
- Open Access
- Advertising and Corporate Services
- Journals Career Network
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Aesthetic Surgery Journal
- Awards and Achievements
- The Aesthetic Society
- Editorial Board
- Self-Archiving Policy
- Instructions for reviewers
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Breast implant illness as a clinical entity: a systematic review of the literature.
- Article contents
- Figures & tables
- Supplementary Data
Raeesa Kabir, Eloise Stanton, Thomas J Sorenson, Kshipra Hemal, Carter J Boyd, Nolan S Karp, Mihye Choi, Breast Implant Illness as a Clinical Entity: A Systematic Review of the Literature, Aesthetic Surgery Journal , 2024;, sjae095, https://doi.org/10.1093/asj/sjae095
- Permissions Icon Permissions
Breast implant illness (BII) has become a contentious subject in recent years. While some studies have reported associations between breast implants and autoimmune diseases, others have failed to establish a definitive link.
The objective of this study is to provide a comprehensive, up-to-date evaluation of the literature surrounding BII, with an emphasis on identifying patient-related factors that may be associated with BII.
A systematic review was performed following PRISMA guidelines using Pubmed (MEDLINE), EMBASE, and Cochrane databases to search for relevant studies published in the last twenty years.
Thirty-one studies were included with a total of 39,505 implant patients and mean age of 44.2 ± 9.30 years. Fifteen studies reported implant explantation status with 72.4% patients choosing to remove their implants. Among these, nine studies reported symptom improvement in 83.5% patients. Fifty-three percent of patients undergoing explantation had total capsulectomy. Twenty-eight studies documented total numbers of patients experiencing symptoms related to BII, with 31.3% patients reporting such symptoms. Among these, sixteen studies of 4,109 BII patients distinguished whether the reason for implantation was cosmetic augmentation or reconstruction. When specified, more patients experiencing BII-related symptoms received implants for “cosmetic” versus “reconstructive” reasons (Cosmetic: 3,864/4,109; 94.0% vs. Reconstruction: 245/4,109; 5.96%, p < 0.001).
This review provides an overview of the current state of knowledge regarding BII. Our study highlights a potential relationship between BII and indication for implants (cosmetic vs. reconstructive) among other variables, offering valuable insight on factors associated with BII and directions for future research.
Supplementary data
Email alerts, citing articles via, looking for your next opportunity.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1527-330X
- Print ISSN 1090-820X
- Copyright © 2024 The Aesthetic Society
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wolters Kluwer - PMC COVID-19 Collection

A practical guide to preclinical systematic review and meta-analysis
1. introduction.
Preclinical systematic reviews (SRs) and meta-analyses (MAs) are important research activities to address the translational challenges of pain research. Systematic reviews provide empirical evidence to gain knowledge, inform future research agendas, and grant applications concurrent to developing researchers' professional skills.
Systematic reviews are an effective approach to consolidating high-volume, rapidly accruing, and often conflicting research on a specific topic. Designed to address a specific research question, SRs use predefined methods to identify, select, and critically appraise all available and relevant literature to answer that question in an unbiased manner. 18 This structured approach distinguishes SRs from narrative reviews. Where appropriate an MA can follow, whereby quantitative data are extracted and statistical techniques are used to summarise the outputs. Together, a SR and a MA can be conducted to assess the quality of experimental design, conduct, analysis and reporting and the reliability of the available and relevant data. 45
Through decades of innovation by the Cochrane Collaboration and others, SRs and MAs now lie at the centre of clinical evidence. The information provided has fundamentally revolutionised clinical medicine at all levels, from informing policy and funding decisions to determining optimal treatments for individual patients. Before a clinical research project or funding application, it is best practise to conduct an SR to ascertain what is already known and to identify knowledge gaps.
In the preclinical setting, SRs are relatively novel, partly because of inherent complexities and resource requirements for processing the large number and diverse preclinical publications, paradoxically a strong justification for SRs because they provide the means to synthesise evidence from heterogeneous studies. In some fields, they are gaining popularity (eg, stroke 37 ), and feasibility is improving with technical advances, for example, online review software, machine learning, and text mining. 3 However, it is important to highlight that not all SRs require machine learning expertise: research questions can be defined based upon capacity, SR software are free and widely accessible, and large-scale SRs constantly seek help from interested researchers who can learn as they participate.
The aim of this review is to highlight the exciting possibilities a preclinical SR can bring to your research toolkit, demonstrate the importance of preclinical SRs in generating empirical evidence to aid robust experimental design, inform research strategy, and support funding applications. We provide guidance and signpost resources to conduct a preclinical SR.
2. Importance of preclinical systematic reviews
Preclinical SRs offer a framework by which the range and quality of the evidence can be assessed, to improve study design, 1 rigour, 12 , 13 , 27 , 43 and reporting. 8 , 41 They summarise the knowledge into an easy-to-understand format in conjunction with identifying gaps in the knowledge base thereby providing the justification for raising funding for new studies. 6 , 9 , 36 , 42
To address translational challenges, SRs can inform robust experimental design. Experimental bias is a consequence of poor internal validity leading a researcher to incorrectly attribute an observed effect to an intervention. 26 Internal validity is comprised of mitigating a range of biases: selection, performance, detection, and attrition bias, 57 and quality assessments provide structured insight into whether the existing data are at risk of bias. 28 , 30 , 34 Concomitantly, SRs can also be used to inform study design, eg, optimal animal model and outcome measure. A MA can also be used to model the impact of publication bias (culture to publish novel, positive results, not neutral or negative data 52 ) and the consequential magnitude of overestimation of effects. 47
Systematic reviews make use of available data, prevent the unnecessary duplication of experiments, and offer the means to support scientific and technological developments that replace, reduce, or refine the use of animals in research (eg, as demonstrated by de Vries et al. 17 ).
Finally, SRs can be used to inform clinical trial design and establish whether there is evidence to justify a clinical trial. 29 , 46 , 58 Retrospective preclinical SRs for interventions that failed in clinical trials have demonstrated that prospective SRs of the animal literature would have concluded that there was insufficient evidence of effect to justify progressing into clinical development (reviewed by Pound and Ritskes-Hoitinga 40 ).
In summary, SRs provide the empirical evidence for improving study design, methods, and analysis to produce unbiased results and increase usability, accessibility, and reproducibility thereby increasing value and reducing research waste. 7 , 23 , 32 It is also important to note the challenges, for example, crediting research between primary researchers and research synthesisers, and the limitations that persist, for example, SRs and MAs cannot overcome deficiency in evidence, nor do they correct biases (reviewed by Gurevitch et al. 24 ).
3. The practical challenges of systematic reviews
There are several unique challenges for the conduct of preclinical SRs including, high-volume and rapidly accruing data and wide variation in the design, conduct, analysis, and reporting of preclinical studies. The methods for SRs are well developed, but are resource-intensive 55 and time-consuming. 49 This problem is exacerbated by the exponentially increasing number of publications. 4 In preclinical neuropathic pain research, the number of articles retrieved by a systematic search rose from 6506 in 2012 to 12,614 in 2015. 13 Comparatively, only 129 articles were identified in an SR of neuropathic pain clinical trials. 21
Widely accessible methods and resources to improve the feasibility of preclinical SRs and MAs have been developed by the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES), University of Edinburgh, United Kingdom, and the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE), Radboud University, NL. Both groups offer guidance and support to researchers.
The Systematic Review & Meta-analysis Facility (SyRF) is a free, fully integrated online platform for performing preclinical SRs. The SyRF includes a secure screening database, data repository, and analysis application. Educational resources are also available. In conjunction, Learn to SyRF is a platform researchers can use to create project-specific training courses enabling reviewers to learn, practice, and demonstrate reviewing skills before contributing to a review.
4. The review stages
Before starting a SR, we recommend engaging with training resources to familiarise with SR methodology. In addition to the learning resources available through the CAMARADES′ and SYRCLE's websites, there are several comprehensive reviews, 35 , 45 , 56 SYRCLE's starting guide, and a recent Pain Research Forum webinar. 50 Generic online courses include Systematic Review Methods (open source) and Cochrane Interactive Learning (varied subscriptions for access). You should also consider whether you have the necessary time and human resources available and how you will involve external experts including statisticians, librarians, and collaborators with existing SR experience.
The stages of an SR are described below (corresponding to Figs. Figs.1 1 and and2). 2 ). Decisions required at the screening, annotation, and outcome data extraction stages can be subjective, therefore, to minimise bias and human error should be performed by 2 independent reviewers and disagreements reconciled by a third independent reviewer.

“Getting Started” Infographic. This is a “how to guide” and describes each stage of the SR and MA processes and the resources for learning and development as well as performing a review. MAs, meta-analyses; SRs, systematic reviews.

An example of the systematic review workflow and the platforms that can be used at each stage.
4.1. Protocol development and registration (mandatory)
This is the most important step, and the preparatory time spent here will not be wasted. The protocol provides methodological transparency and reduces the risk of introducing bias. It defines the research question and the methods you plan to use including the search strategy; inclusion and exclusion criteria; data to be extracted; risk of bias/quality assessment, which based on reporting of methodological criteria allows reviewers to determine whether a study is at low, high, or unclear risk of bias, for example, CAMARADES checklist, 34 SYRCLE Risk of Bias Tool, 30 and GRADE adapted for preclinical SRs 28 ; data synthesis; and statistical analysis plan. 15 Other reporting biases including financial and academic conflicts of interest can also be assessed. Registering the protocol, for example, on the Open Science Framework Registries, PROSPERO or the SYRF Protocol Registry, allows others to locate reviews in progress and enables future replication. Some journals (eg, Pain Reports and BMJ Open Science) publish protocols and the associated peer review can significantly improve your SR. 51
4.2. Search strategy
The search strategy is informed by your research question. We recommend consulting a librarian or bibliographic database expert for help because this can be a complex task. An SR aims to capture all the relevant literature specific to your research question. Electronic databases of preclinical research include PubMed, Ovid Embase and Web of Science, and SYRCLE have developed animal search filters for the databases. 16 , 31 , 33 We do not recommend using Google Scholar because its algorithms are not transparent and searches are not easily reproduced. 25 It is necessary to construct individual search strategies for each database because databases differ in their coverage of journals and how articles are indexed. A narrow search strategy will risk missing relevant studies, too broad and you will add many irrelevant studies and consequently time to the screening process.
4.3. Study selection: screening for inclusion
This is the assessment of search results against your prespecified inclusion criteria. There are 2 phases: (1) title and abstract and (2) full-text screening. Full-text screening can be combined with the annotation and data extraction stage. A PRISMA flow diagram should be produced to report the number of records identified, included, and excluded, and the reasons for exclusions. 38
4.4. Annotation and data extraction
Annotation questions about study quality, risk of bias, and study design should be specific and objective, limiting the need for reviewer judgement. Avoid temptation to extract data not pertinent to the research question that will not be analysed. There are several tools to manually extract outcome data presented in graphs, eg, WebPlotDigitizer and the inbuilt Adobe measuring tool.
4.5. Analysis
The analysis of an SR can use qualitative 53 or quantitative, with MA 56 and without MA 5 or mixed techniques. A narrative summary can be used to synthesise study design and risk of bias information. Vesterinen et al. 56 provide comprehensive guidance for conducting a preclinical MA. A MA can be used to combine the outcome data of individual studies to estimate the overall intervention effect. A stratified MA or meta-regression can be used to investigate and quantify potential sources of heterogeneity, for example, study design characteristics and how they influence outcomes. The presence and magnitude of publication bias can also be estimated using statistical methods such as funnel plots; see Refs. 54 and 56 for further reading.
4.6. Reporting
Sena et al. 45 provide guidelines for the reporting of preclinical SRs. All aspects of the review process should be reported in adherence to the protocol with explanation for any deviations. It is also helpful to refer to the AMSTAR 2 critical appraisal tool for assessing the methodological quality of SRs. 48 Finally, to ensure transparency and sustainability of the SR, it is encouraged to make the data and analysis code available by uploading to a repository, eg, Open Science Framework or Figshare. In doing so, you are making it possible for others to perform secondary analyses thereby increasing the reach of your work.
5. Improving feasibility: the design of the review
Embarking on a preclinical SR can be daunting due to the complex, resource-intensive, and time-consuming processes. 13 , 20 , 51 Systematic reviews within the pain field to date have sought to answer broad research questions. 13 , 20 , 51 These large reviews have provided understanding of the range and quality of a field, however, there are more feasible possibilities ie, conducting smaller reviews, for time poor researchers and students.
5.1. Research question
The research question should be narrow, clearly defined, and answerable. Limiting the scope to a specific population, intervention, comparator, and/or outcome measure are ways to improve feasibility. Performing trial searches will assist you to determine possible workload and hone your research question. Other limitations may be added, for example, publication date; however, this adds a source of bias and must be justified.
5.2. Inclusion criteria for meta-analyses
A MA is not always appropriate; a systematic search, screen, and annotation (study characteristics and risk of bias) can inform a narrative summary and prospective animal studies. If outcome data are required, it is possible to reduce the data extraction burden by having inclusion criteria for the MA. For example, Federico et al. 20 calculated effect sizes based upon the availability of time-course data. Similarly, this could be achieved by only including studies at low risk of bias. Such decisions need to be justified and stated a priori within the protocol.
5.3. Efficient resource allocation
Multiple reviewers can be used to expedite the screening, annotation, and outcome data extraction stages (in parallel on SyRF). If you aim to conduct SRs for student projects, a single research question composed of several subquestions can be addressed. Students can collaborate during the protocol development, search, screen, and data extraction stages, thereby contributing to each stage of the project. Students will be able to demonstrate independent working by using the data pertinent to a subquestion and perform and report independent analyses in their examination submissions.
6. Improving feasibility: contribution to reviews
Systematic reviewers are regularly looking for contributors and it is worth contacting the authors of registered SRs to offer your assistance; it is a very efficient way to gain experience. Importantly, it provides collaborative opportunities for researchers with limited resources, eg, in low- and middle-income countries. As part of the recent IASP Cannabinoid Task Force, the preclinical SR recruited a crowd of reviewers to assist with the screening and data extraction phases. 51 CAMARADES are currently recruiting a crowd to help them build a systematic and continually updated summary of COVID19 evidence. 14 These reviews demonstrate it is possible to share the workload across a crowd, recruited locally or globally, although we recommend conducting training for reviewers to ensure quality.

7. Improving feasibility: automation tools for evidence synthesis
Several groups are taking advantage of emerging technologies to modernise the conventional review process and create living SRs. Systematic reviews are not often updated 22 and not incorporating the most recent data risks making an SR at risk of inaccuracy. 11 , 49 Living SRs are SRs that are continually updated, incorporating relevant new evidence as it becomes available. 19 Living SRs will ensure that decisions are dynamic and based upon the full body of evidence. Technological developments are continually being made to reduce the time and human effort required for SRs and automation tools can be used without making the review living (Fig. (Fig.3 3 ).

Automation technologies that are being developed for the different stages of the review process. Machine learning and text mining have improved the feasibility and efficiency of the early stages of the process 2 , 3 and tool development continues to ensure that the full potential of preclinical SRs are realised. Technological developments for the latter stages are in their infancy. However, we are currently developing a machine-assisted approach to extracting data from graphs that aims to reduce time and improve accuracy, 10 a feature that will soon be integrated into SyRF. SRs, systematic reviews; SyRF, Systematic Review & Meta-analysis Facility.
8. The future
Performing a SR enables researchers to hone their critical analysis skills and gain an in-depth understanding of the field. Like the vision proposed by Nakagawa et al., 39 we envisage a new community for pain research that comprises of primary researchers who perform research synthesis with support from systematic reviewers, librarians, and statisticians. Primary researchers will use SRs to generate hypothesis and inform future research design. Evidence synthesis will also be recognised as an end goal of the research process. Research will be designed, conducted, analysed, and reported accordingly (eg, for eligibility in prospective MAs as described by Seidler et al. 44 ), thereby mitigating biases and reducing research waste. Conducting SRs will lead to improvements in education, practice, and communication of pain research and improve the predictive validity of animal research, reduce research waste, and improve pain outcomes for patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgements
The authors thank Dr Emily Sena for her constructive feedback on the manuscript.
The work is funded by the BBSRC (grant number BB/M011178/1). J. Vollert and A.S.C. Rice are part of the European Quality In Preclinical Data (EQIPD) consortium. This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777364. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA.
Supplemental video content
A video abstract associated with this article can be found at http://links.lww.com/PAIN/B114 .
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
- Open access
- Published: 20 April 2024
The role of colchicine in the management of COVID-19: a Meta-analysis
- Kholoud Elshiwy 1 ,
- Ghada Essam El-Din Amin 1 , 2 ,
- Mohamed Nazmy Farres 3 ,
- Rasha Samir 3 &
- Mohamed Farouk Allam 1 , 4
BMC Pulmonary Medicine volume 24 , Article number: 190 ( 2024 ) Cite this article
Metrics details
The Coronavirus disease 2019 (COVID-19) pandemic has robustly affected the global healthcare and economic systems and it was caused by coronavirus-2 (SARS-CoV-2). The clinical presentation of the disease ranges from a flu-like illness to severe pneumonia and death. Till September 2022, the cumulative number of cases exceeded 600 million worldwide and deaths were more than 6 million. Colchicine is an alkaloid drug that is used in many autoinflammatory conditions e.g., gout, familial Mediterranean fever, and Behçet’s syndrome. Colchicine inhibits the production of superoxide and the release of interleukins that stimulate the inflammatory cascade. Colchicine decreases the differentiation of myofibroblast and the release of fibrotic mediators including transforming growth factor (TGF-β1) that are related to the fibrosis. Moreover, colchicine has been used to traet viral myocarditis caused by CMV or EBV, interstitial pneumonia, and pericarditis resulting from influenza B infection. Additionally, colchicine is considered safe and affordable with wide availability.
The aim of the current study was to assess the evidence of colchicine effectiveness in COVID-19 treatment.
A comprehensive review of the literature was done till May 2022 and yielded 814 articles after ranking the articles according to authors and year of publication. Only 8 clinical trials and cohort studies fulfilling the inclusion criteria were included for further steps of data collection, analysis, and reporting.
This meta-analysis involved 16,488 patients; 8146 patients in the treatment group and 8342 patients in the control group. The results showed that colchicine resulted in a significant reduction in the mortality rate among patients received colchicine in comparison with placebo or standard care (RR 0.35, 95%CI: 0.15–0.79). Colchicine resulted in a significant decrease in the need for O2 therapy in patients with COVID-19 (RR 0.07, 95%CI 0.02–0.27, P = 0.000024). However, colchicine had no significant effect on the following outcomes among COVID-19 patients: the need for hospitalization, ICU admission, artificial ventilation, and hospital discharge rate. Among the PCR confirmed COVID-19 patients, colchicine decreased the hospitalization rate (RR 0.75, 95%CI 0.57–0.99, P = 0.042). However, colchicine had no effect on mortality and the need for mechanical ventilation among this subgroup.
Colchicine caused a significant clinical improvement among COVID-19 patients as compared with the standard care or placebo, in terms of the need for O2, and mortality. This beneficial effect could play a role in the management of COVID-19 especially severe cases to decrease need for oxygen and to decrease mortality among these patients.
Peer Review reports
Introduction
The Coronavirus disease 2019 (COVID-19) that was caused by coronavirus − 2 (SARS-CoV-2) has significantly impacted the healthcare and economic systems worldwide. The disease first began in Wuhan, China at the end of 2019. Then, it spread worldwide and became a pandemic. The clinical picture of the disease ranges from a flu-like illness to a massive inflammatory response and death [ 1 ]. In 2002 and 2003, there were outbreaks of severe respiratory distress syndrome in China. They occurred by SARS-CoV, another member of the coronavirus family. In 2012, another outbreak was documented in the Middle East and was caused by Middle East respiratory syndrome coronavirus (MERS-CoV) [ 2 ]. The current coronavirus is characterized by higher infectivity and geographical spread in comparison with both SARS and MERS. Therefore, COVID-19 was considered a significant global health threat that required robust efforts to minimize the burden of this pandemic [ 3 ].
The World Health Organization (WHO) announced that COVID-19 is a pandemic on 11 March 2020 [ 4 ]. Since then, the number of COVID-19 patients significantly increased. Till September 2022, the cumulative number of cases exceeded 600 million worldwide and deaths were more than 6 million [ 5 ].
The clinical manifestations of COVID-19 encompass symptoms such as fever, cough, dyspnea, malaise, or anosmia or ageusia, which can aid in early detection of the disease [ 6 ]. The primary mode of COVID-19 transmission is predominantly through exposure to infectious respiratory droplets from close contact with either symptomatic patients or asymptomatic carriers, as well as through aerosol particles that can remain suspended in the air for extended periods [ 7 ]. Additionally, indirect transmission through contaminated fomites, fecal excretion, environmental contamination, and fluid pollution has been documented, with viral viability reaching up to 72 hours after infecting surfaces [ 7 , 8 ].
SARS-CoV-2 is a beta coronavirus that is a positive-stranded enveloped RNA virus. Similar to SARS-CoV and MERS-CoV, it is found in domestic and farm animals [ 9 , 10 ]. The SARS-CoV-2 is characterized by spike proteins called S proteins. These proteins facilitate the viral infection through binding the S proteins and the angiotensin-converting enzyme 2 receptors (ACE2). These receptors are found in many tissues such as pneumocytes, enterocytes, renal cells, and endothelial cells [ 11 ]. SARS-CoV-2 causes marked dysfunction of the epithelial barrier and the endothelial cells of the pulmonary capillaries which triggers the migration and accumulation of inflammatory cells. This initiates the inflammatory cascade by both innate and cell-mediated immunity which significantly influences the alveolar-capillary oxygen transmission and the oxygen diffusion capacity [ 12 ].
In severe cases of COVID-19, fulminant inflammation, stimulation of the coagulation pathways, and consumption of the clotting factors occur in the form of a “cytokine storm”. This happens under the effect of many inflammatory mediators including interleukins, tumor necrosis factor-α (TNF-α), and interferon (IFN-γ). In addition, vasodilators such as bradykinin increase vascular permeability and result in pulmonary edema [ 13 ].
These mechanisms of cell damage represent a target for already existing medications that modulate the immune response. Based on its anti-inflammatory effects, colchicine has gained attention to be utilized in the management of COVID-19 patients. Colchicine is an alkaloid drug that is formed from a plant called “ Colchicum autumnale ”, also named “autumn crocus”. Colchicine is used in many autoinflammatory conditions e.g., gout, familial Mediterranean fever, and Behçet’s syndrome. Colchicine has an anti-inflammatory effect that is mediated through its binding to the tubulins and inhibiting the polymerization of microtubules. Microtubules are a key component of the cytoskeleton and are composed of tubulin heterodimers. These structures are important in different cellular functions including intracellular trafficking, cell shape, cell migration, and division [ 14 ]..
Colchicine inhibits the production of superoxide and the release of interleukin 1β and IL-6. Colchicine also prevents the inflammatory cascade by decreasing the production of inflammasomes that stimulate caspase-1 activation and release of interleukins such as interlukin1β and interleukin IL18 [ 15 , 16 ]. Colchicine decreases the differentiation of myofibroblast and the release of fibrotic mediators including transforming growth factor (TGF-β1) [ 17 , 18 ]. Moreover, colchicine has been used in cardiac conditions caused by a viral infection like myocarditis caused by CMV or EBV, interstitial pneumonia, and pericarditis resulting from influenza B infection. These different mechanisms greatly decrease the inflammatory response that represents a cornerstone in the pathophysiologic process of COVID-19. Besides the aforementioned effects of colchicine, its usage is considered safe and affordable with wide availability [ 19 ].
The ongoing impact of COVID-19 on all life aspects, the scarcity of effective treatments and the emergence of new virus variants resulted in the urgent need to repurpose the already existing drugs and to invent new therapeutic agents. This raised concerns about the effectiveness of colchicine in COVID-19 treatment and the possibility of providing an improvement in the clinical course of the disease.
The aim of the current study was to evaluate the efficacy of colchicine on different clinical outcomes including mortality, duration of COVID-19 illness till recovery, need for hospitalization, need for O2 therapy, need for ICU admission, and need for artificial ventilation.
Methodology
Criteria for considering studies for this meta-analysis, types of studies.
The review was restricted to Clinical Trials and Cohort Studies, which investigated the Colchicine administration in COVID-19 patients, versus standard treatment/placebo.
Types of participants
Participants were adult patients with the diagnosis of COVID-19. Patients were considered to have a definite diagnosis of COVID-19 if they were laboratory-confirmed using reverse transcription polymerase chain reaction (RT-PCR) and/or high-resolution CT chest with CO-RADS 4 or 5. All healthcare settings (community/primary care, hospital outpatient, or long-stay institutional) were considered eligible.
Types of interventions
Clinical trials and Cohort Studies were included. Colchicine was administered in COVID-19 patients, versus standard treatment/placebo.
Types of outcome measures
At least one of these outcomes was considered; Mortality, Duration of COVID-19 illness till recovery, Need for hospitalization, Need for O2 therapy, Need for ICU admission, and Need for artificial ventilation.
Inclusion criteria
(i) Cohort studies. (ii) Randomized and non-randomized clinical trials. Studies conducted on adult human subjects. (iii) Studies conducted on patients diagnosed with COVID-19 confirmed with positive reverse transcription polymerase chain reaction (RT-PCR) and/or high-resolution CT chest with CO-RADS 4 or 5. (iv) Studies conducted in all healthcare settings (community/ primary care, hospital outpatient or long-stay institutional). Studies published in Arabic, English, French or Spanish languages.
Exclusion criteria
Review, opinion studies, Case series, Studies conducted on animals.
Search strategy for identification of studies
Published studies and abstracts on the role of colchicine in the management of COVID-19 were identified through a comprehensive search of electronic databases that included PubMed ( https://pubmed.ncbi.nlm.nih.gov/ ), ScienceDirect ( www.sciencedirect.com ), Scirus ( www.scirus.com/srsapp ), ISI Web of Knowledge ( http://www.isiwebofknowledge.com ), Google Scholar ( http://scholar.google.com ) and CENTRAL (Cochrane Central Register of Controlled Trials ( http://www.mrw.interscience.wiley.com/cochrane/cochrane_clcentral_articles_fs.htm ), using a combination of the following keywords: “Colchicine, COVID-19, Clinical Trail, Cohort Study”.
Methods of the meta-analysis
Locating and selecting studies.
Abstracts of articles identified using the search strategy above mentioned were viewed, and articles that appeared to fulfil the inclusion criteria were retrieved in full. Data on at least one of the outcome measures was included in the study. Each article identified was reviewed and categorized into one of the following groups: Included: Randomized and non-randomized clinical trials, and Cohort studies that met the described inclusion criteria and those where it was impossible to tell from the abstract, title or MESH headings. Excluded: review, opinion studies, case series, and studies conducted on animals. When there was a doubt, a second reviewer (MFA) assessed the article, and a consensus was reached. The literature was reviewed till May 31, 2022 and yielded 814 articles after ranking the articles according to authors and year of publication. Only articles fulfilling the inclusion criteria were included (total 8 articles) for further steps of data collection, analysis, and reporting. The studies that met our inclusion criteria were Deftereos et al., Tardif et al., RECOVERY Collaborative Group, Lopes et al., Sandhu et al., Mareev et al., Brunetti et al. and Scarsi et al. [ 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ]. All were in English and there were no available studies published in Arabic, French or Spanish language.
Data extraction
A copy of each identified paper was obtained, and relevant data was abstracted by the first reviewer for a quantitative overview. We extracted the following study data from full-text articles: first author name, year of publication, study design, study location, eligibility criteria, sample size, age, sex, description of intervention and control groups, primary and secondary outcomes. In case of discrepancies or when the information presented in a study was unclear, abstraction by a second reviewer (MFA) was sought to resolve the discrepancy.
Statistical considerations
Data were abstracted from every study in the form of a risk estimate and its 95% confidence interval. When a risk estimate and its 95% confidence interval were not available from the article, we calculated unadjusted values from the published data of the article, using the Epi Info 6 computer program version 6.04d.
Pooled estimates of relative risks were obtained by weighing each study by the inverse variance of the effect measure on a logarithmic scale. This approach to pool the results assumed that the study populations being compared were similar and hence corresponded to a fixed effect analysis. The validity of pooling the relative risks was tested (test of homogeneity) using chi square test.
A violation of this test suggested that the studies being pooled differed from one another. In the presence of significant heterogeneity of the effect measure among studies being compared, we performed a random effect analysis that was based on the method described by DerSimonian and Laird. The random effect analysis accounted for the interstudy variation. Because the test of homogeneity had low power, we reported the figures of the random effect analysis even with the absence of significant heterogeneity.
All statistical analyses for pooling the studies were performed on the MetaXL Software.
In 6 databases, we identified 814 articles; 499 duplicates were removed. Out of the remaining 315 abstracts, we excluded 298 after screening. Thus, 17 full-text studies were assessed for eligibility and 9 were excluded. Finally, eight studies were included for further qualitative and quantitative analyses (Fig. 1 ).
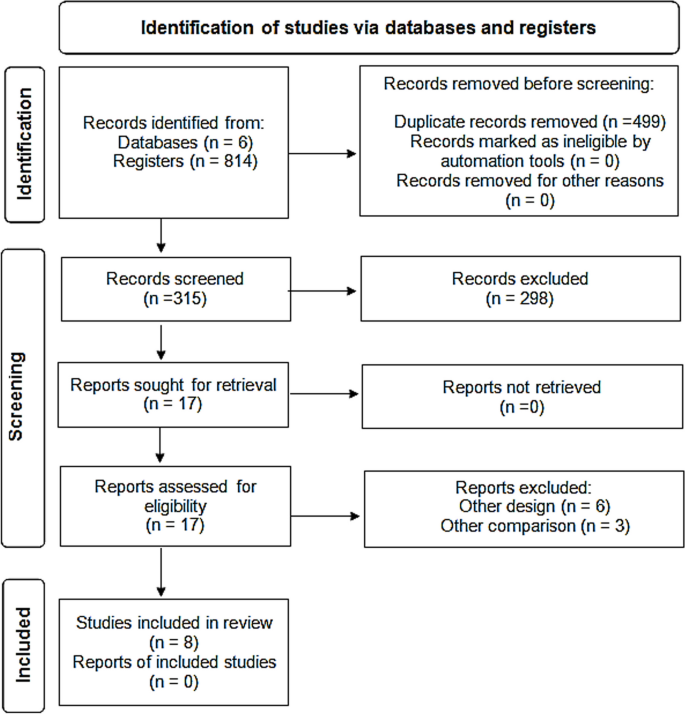
PRISMA flow diagram showing selection of studies. PRISMA; Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Characteristics of the included studies
Two studies were cohort (Brunetti et al. and Scarsi et al.) while the other studies were four randomized controlled clinical trials (Deftereos et al., RECOVERY Collaborative Group, Lopes et al., and Tardif et al.) and two non-randomized controlled clinical trials (Mareev et al., and Sandhu et al.).
Two studies were multicentre clinical trials (RECOVERY Collaborative Group, and Tardif et al.) . The other six studies were conducted in Greece (Deftereos et al.), Brazil (Lopes et al.), the USA (Brunetti et al. and Sandhu et al.), Russia (Mareev et al.), and Italy (Scarsi et al.) [ 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ].
The studies included both hospitalized and non-hospitalized COVID-19 patients, who were diagnosed either clinically or by laboratory diagnosis with PCR–RT testing and CT chest imaging (Table 1 ).
Table 2 and Fig. 2 showed that the meta-analysis of all included studies showed a significant difference in mortality between the treatment group with colchicine and the control group (RR 0.35, 95% CI: 0.15–0.79). There is significant heterogeneity among the studies (Homogeneity Test X2: 42.219, P -value < 0.000).
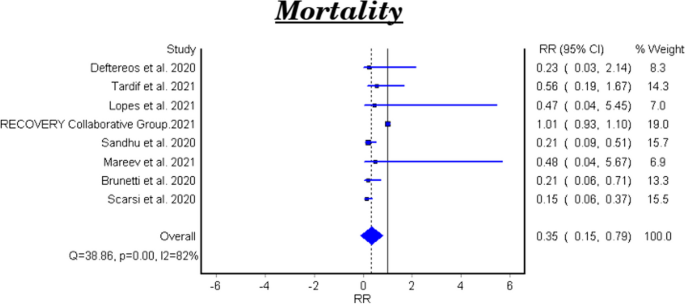
Forest plot for the efficacy of colchicine on mortality in patients with COVID-19
The meta-analytical result of the six clinical trials was insignificant between the treatment and control groups (RR 0.48, 95% CI 0.22–1.07). There is significant heterogeneity among the studies (Homogeneity Test X2: 11.562, P -value: 0.000). The meta-analytical result of the two cohort studies was significant between the treatment and control groups (RR 0.17, 95%CI 0.08–0.35).
Duration of COVID-19 illness till recovery
Table 3 shows the efficacy of colchicine on the duration of COVID-19 illness till recovery. Lopes et al. reported that the median duration of COVID-19 illness in the treatment group with colchicine was 7 days vs 9 days in the control group ( P -value =0.003) [ 25 ]. While Sandhu et al., and Mareev et al., demonstrated that colchicine had no significant effect on the illness duration [ 26 , 27 ]. (Table 3 ).
Need for hospitalization
Tardif et al., reported that colchicine did not show a significant effect on the COVID-19 patients’ need for hospitalization RR 0.79, 95% CI 0.60–1.03, P-value =0.081) [ 23 ].
Need for O2 therapy
Lopes et al., demonstrated that colchicine use resulted in a significant decrease in the need for O2 therapy in patients with COVID-19 (RR 0.07, 95% CI 0.02–0.27, P = 0.000024) [ 25 ].
Need for ICU admission
Table 4 and Fig. 3 show the efficacy of colchicine on need for ICU admission in patients with COVID-19. The meta-analytical result did not show a significant effect (RR 0.29, 95% CI: 0.07–1.17).
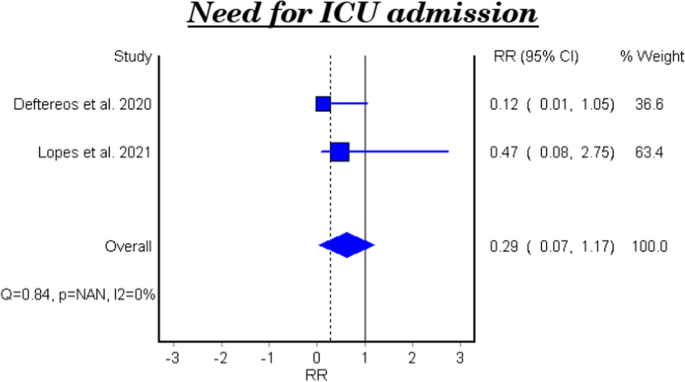
Forest plot for the efficacy of colchicine on need for ICU admission in patients with COVID-19
Need for artificial ventilation
Table 5 and Fig. 4 show the efficacy of colchicine on need for artificial ventilation in patients with COVID-19. The meta-analysis of four studies demonstrated that colchicine has no significant effect on the need for artificial ventilation (RR 0.40, 95% CI 0.14–1.13). There is significant heterogeneity among the studies (Homogeneity Test X2: 18.417, P -value: 0.000).
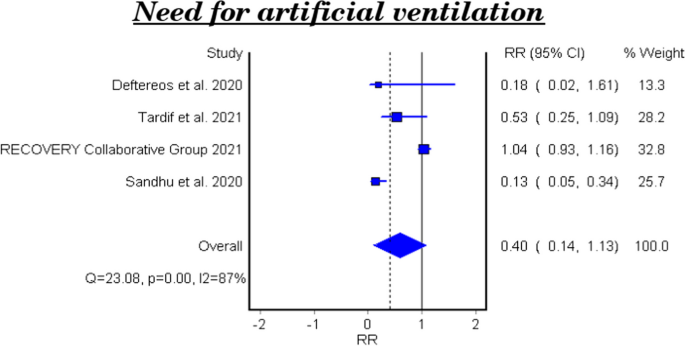
Forest plot for the efficacy of colchicine on need for artificial ventilation in patients with COVID-19
Hospital discharge rate
Table 6 and Fig. 5 show the efficacy of colchicine on hospital discharge rate in patients with COVID-19. The meta-analytical result of the three studies demonstrated that colchicine did not show a significant effect on the hospital discharge rate (RR 0.99, 95%CI 0.12–7.85).
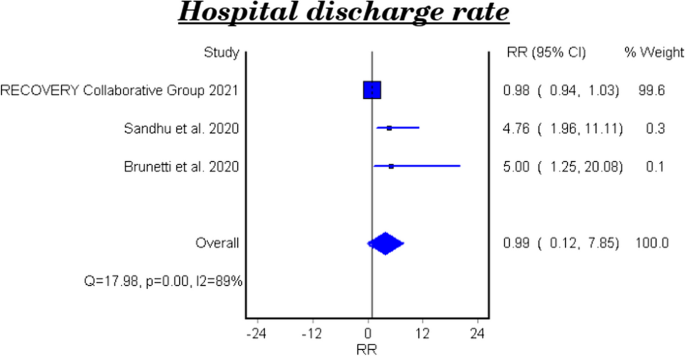
Forest plot for the efficacy of colchicine on hospital discharge rate in patients with COVID-19
The effect of colchicine on the hospital discharge rate in the clinical trials was not significant (RR 0.98, 95%CI 0.12–8.02), while a cohort study reported that colchicine showed a significant effect on the hospital discharge rate (RR 5.0, 95%CI 1.25–20.08, P-value 0.023) [ 28 ].
Subgroup analysis among PCR confirmed COVID-19 patients
Mortality among pcr confirmed covid-19 patients.
Table 7 and Fig. 6 show the efficacy of colchicine on mortality among PCR confirmed COVID-19 Patients. Colchicine did not show a significant effect on mortality among PCR confirmed COVID-19 patients (RR 1.02, 95% CI 0.74–1.41).
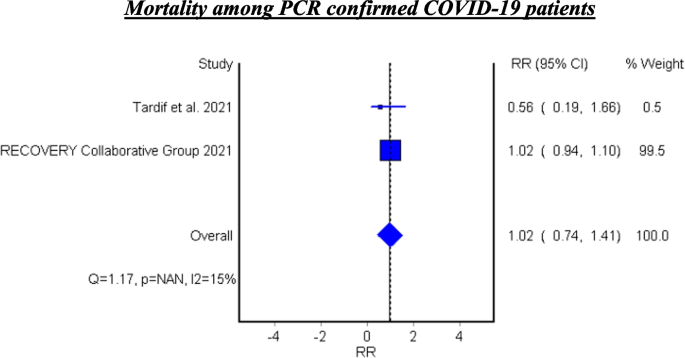
Forest plot for the efficacy of colchicine on mortality among PCR confirmed COVID-19 patients
See Fig. 6 .
Hospitalization among PCR confirmed COVID-19 patients
Tardif et al. assessed the efficacy of colchicine on hospitalization and reported that colchicine resulted in decreased hospitalization among the PCR confirmed COVID-19 patients (RR 0.75, 95%CI 0.57–0.99, P 0.042) [ 23 ].
Mechanical ventilation among PCR confirmed COVID-19 patients
Tardif et al. found that colchicine has no significant effect on mechanical ventilation among PCR confirmed COVID-19 Patients (RR 0.50, 95%CI 0.23–1.07, P 0.042) [ 23 ].
In this meta-analysis, the studies investigated the role of colchicine in the management of COVID-19 were reviewed.
After a comprehensive search, eight studies were identified. Two of them were cohort studies (Brunetti et al., and Scarsi et al.) while the other studies were four randomized control trials (Deftereos et al., Recovery Collaborative Group, Lopes et al., and Tardif et al.) and two non-randomized trials (Mareev et al., and Sandhu et al.). The current meta-analysis involved 16,488 patients; 8146 were in the treatment group who received colchicine and 8342 were in the control group who received a placebo or standard treatment [ 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ].
The efficacy of colchicine on mortality
The eight pooled studies evaluated the efficacy of colchicine on mortality among COVID-19 patients and showed a significant reduction in the mortality rate among patients received colchicine in comparison with placebo or standard care. This result coincides with the findings of a recent systematic review that reported a significant decrease in the all-cause mortality in three observational studies [ 28 ]. In addition, a recently published meta-analysis reported that colchicine resulted in decreased mortality among COVID-19 patients. This study pooled four randomized control trials and five observational studies and involved 5522 patients only [ 29 ].
On the other hand, Mehta, et al. and Toro-Huamanchumo, et al. documented that colchicine had no effect on the mortality rate among COVID-19 patients [ 30 , 31 ].
The heterogeneity test between the pooled studies showed a significant difference, which indicates interstudy variation. Pooling of these heterogeneous studies added more useful information.
According to our result, colchicine may have a beneficial effect to decrease mortality among COVID-19 patients. It was obvious that this effect occurred when colchicine was used within the early days of the disease. These findings can be explained by the anti-inflammatory role of colchicine that is mediated through the interaction between colchicine and microtubules which play an important role in cellular division, migration, and adhesion. This effect robustly influences the immune system response and reduces the inflammatory reaction. Also, colchicine decreases the release of cytokines and inflammatory mediators that stimulate the immune cells [ 32 ].
The subgroup analysis of the two cohort studies demonstrated a significant effect of colchicine on mortality among COVID-19 patients. However, the subgroup analysis for the six clinical trials showed that colchicine has no effect on mortality in the management of COVID-19. This result is consistent with the pooled analysis of a recent study where four clinical trials only were included [ 33 ]. This variation could be attributed to difference of the study design, variation in follow up duration and the colchicine regimen used in these studies.
The efficacy of colchicine on the duration of COVID-19 illness till recovery
The efficacy of colchicine on the duration of COVID-19 illness was assessed in three clinical trials. Lopes et al. found that hospitalized COVID-19 patients who received colchicine had a shorter duration of illness till recovery in comparison with the patients who received placebo [ 23 ]. This is similar to the result reported by a recent study [ 34 ]. This finding can be related to the anti-inflammatory and immune modulatory roles of colchicine in the management of COVID-19. On the other hand, two clinical trials reported that colchicine did not affect the duration of COVID-19 illness [ 23 , 25 ]. These findings agree with the results of a recently published study investigated the efficacy of colchicine on the duration of COVID-19 clinical course [ 31 ].
The efficacy of colchicine on need for hospitalization
Tardif et al., investigated the efficacy of colchicine among non-hospitalized COVID-19 patients vs placebo. They found that colchicine did not influence the need for hospitalization among the non-hospitalized patients [ 21 ]. A recent clinical trial was conducted to assess the effect of colchicine on the prognosis of non-hospitalized COVID-19 patients and the results showed no significant effect of colchicine on hospitalization rate of the patients [ 35 ].
The efficacy of colchicine on need for O2 therapy
Lopes et al., assessed the efficacy of colchicine on the need for O2 therapy and the results demonstrated that colchicine use resulted in a significant decrease in the need for O2 therapy in patients with COVID-19 [ 23 ]. This result can be understood based on the beneficial effect of colchicine on the inflammatory response.
The efficacy of colchicine on need for ICU admission
The pooled results of two clinical trials showed that colchicine did not improve the need of ICU admission compared to placebo or standard care. This finding is concomitant with a recent study that included six studies only [ 30 ].
The efficacy of colchicine on need for artificial ventilation
Four pooled studies evaluated the efficacy of colchicine on need for artificial ventilation and showed that colchicine did not decrease the need for artificial ventilation compared to placebo or standard care [ 20 , 21 , 22 , 24 ].
The heterogeneity test between the pooled studies regarding the need for artificial ventilation showed a significant difference, which indicates interstudy variation.
This can be attributed to the variation of duration and dose of colchicine regimens in these studies, and the severity of the disease. Tardif et al., included non-hospitalized COVID-19 patients while the other three studies involved hospitalized patients.
The efficacy of colchicine on hospital discharge rate
Three pooled studies evaluated the efficacy of colchicine on hospital discharge rate and showed that colchicine did not improve the hospital discharge rate in comparison with placebo or standard treatment [ 22 , 24 , 26 ].
Furthermore, the subgroup analysis of the pooled results included two clinical trials and showed that colchicine did not cause a significant improvement in the hospital discharge rate compared to placebo or standard treatment [ 22 , 24 ]. On the other hand, the cohort study demonstrated a beneficial effect of colchicine on the hospital discharge rate compared to standard care [ 26 ].
The variation of the results of the three studies could be attributed to the difference of study design, number of included patients, and the treatment regimens used.
Two pooled studies evaluated the efficacy of colchicine among PCR confirmed COVID-19 patients and showed that colchicine did not significantly decrease mortality among PCR confirmed patients [ 21 , 22 ].
In addition, Tardif et al. assessed the efficacy of colchicine on hospitalization rate among PCR confirmed COVID-19 patients and found that colchicine significantly decreased the hospitalization rate compared to placebo. Also, Tardif et al. evaluated the effectiveness of colchicine on mechanical ventilation rate among PCR confirmed COVID-19 patients and showed no beneficial effect of colchicine on mechanical ventilation in comparison with placebo [ 21 ].
The study demonstrates that colchicine administration leads to a notable reduction in mortality rates and a decrease in the necessity for oxygen therapy among individuals with COVID-19. Although its impact on broader outcomes like hospitalization rates, ICU admissions, and discharge rates remains minimal, there’s a significant finding regarding its efficacy in lowering hospitalizations specifically among PCR-confirmed COVID-19 patients. This detailed understanding highlights the potential of colchicine as a therapeutic intervention for COVID-19, particularly in mitigating mortality risks and oxygen therapy requirements. These results offer valuable insights for clinicians, highlighting the need to consider colchicine as a viable treatment option for COVID-19 patients, while also emphasizing the necessity for further exploration to optimize its clinical utility.
Availability of data and materials
Our study is a Systematic Review/Meta-analysis. The datasets analyzed during the current study are available in the published pooled study. Also, the datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Rahman MT, et al. Early prediction and HRCT evaluation of post covid-19 related lung fibrosis. Microbiol Insights. 2023;16:11786361231190334.
Article PubMed PubMed Central Google Scholar
Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–97.
Article CAS PubMed PubMed Central Google Scholar
Han Q, Lin Q, Jin S, You L. Coronavirus 2019-nCoV: a brief perspective from the front line. J Infect. 2020;80(4):373–7.
Hageman JR. The coronavirus disease 2019 (COVID-19). Pediatr Ann. 2020;49(3):e99–e100.
Article PubMed Google Scholar
WHO. World Health Organization. Coronavirus Disease (COVID-19) Dashboard With Vaccination Data. 2022. Available from: https://covid19.who.int/info/ .
Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, Hooft L, Emperador D, Domen J, Tans A, Janssens S, Wickramasinghe D, Lannoy V, Horn SRA, Van den Bruel A, Cochrane COVID-19 Diagnostic Test Accuracy Group. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst Rev. 2022;5(5):CD013665. https://doi.org/10.1002/14651858.CD013665.pub3 .
Mehraeen E, Salehi MA, Behnezhad F, Moghaddam HR, SeyedAlinaghi S. Transmission modes of COVID-19: a systematic review. Infect Disord Drug Targets. 2021;21(6):e170721187995.
Article CAS PubMed Google Scholar
van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–7.
Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74.
Pandit R, Matthews QL. A SARS-CoV-2: companion animal transmission and variants classification. Pathogens. 2023;12(6):775.
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.
Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.
Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7.
Bhattacharyya B, Panda D, Gupta S, et al. Anti-mitotic activity of colchicine and the structural basis for its interaction withTubulin. Med Res Rev. 2007;28(1):155–83.
Article Google Scholar
Cronstein BN, Esserman PR, Sunkureddi P. Mechanistic aspects of inflammation and clinical Management of Inflammation in acute gouty arthritis. J Clin Rheumatol. 2013;19(1):19–29.
Korkmaz S, Erturan I, NazIroǧlu M, et al. Colchicine modulates oxidative stress in serum and neutrophil of patients with Behçet disease through regulation of ca 2+ release and antioxidant system. J Membr Biol. 2011;244(3):113–20.
Bozkurt D, Bicak S, Sipahi S, Taskin H, Hur E, Ertilav M, Sen S, Duman S. The effects of colchicine on the progression and regression of encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28(5):53-57.
Lho Y, Do JY, Heo JY, Kim AY, Kim SW, Kang SH. Effects of TGF-β1 Receptor Inhibitor GW788388 on the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells. Int J Mol Sci. 2021;22(9):4739.
Schlesinger, N., Firestein, B. L., & Brunetti, L. Colchicine in COVID-19: an old drug, New Use In Current Pharmacology Reports 6(4): 137–145 (2020).
Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6)
Tardif JC, Bouabdallaoui N, L’Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9(8):924–32.
Group, R. C. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med. 2021;9(12):1419–26.
Lopes MI, Bonjorno LP, Giannini MC, et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7(1):1–8.
Sandhu T, Tieng A, Chilimuri S, Franchin G. A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe covid-19 infection. Can J Infect Dis Med Microbiol. 2020;2020:1–9.
Mareev VY, Orlova YA, Plisyk AG, et al. Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study. Kardiologiya. 2021;61(2):15–27.
Brunetti L, Diawara O, Tsai A, et al. Colchicine to weather the cytokine storm in hospitalized patients with COVID-19. J Clin Med. 2020;9(9):1–12.
Scarsi M, Piantoni S, Colombo E, et al. Association between treatment with colchicine and improved survival in a single-Centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79(10):1286–9.
Sanghavi D, Bansal P, Kaur IP, et al. Impact of colchicine on mortality and morbidity in COVID-19: a systematic review. Ann Med. 2022;54(1):775–89.
Elshafei MN, El-Bardissy A, Khalil A, et al. Colchicine use might be associated with lower mortality in COVID-19 patients: a meta-analysis. Eur J Clin Investig. 2021;51(9):1–5.
Mehta KG, Patel T, Chavda PD, et al. Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomised controlled trials. RMD Open. 2021;7(3):1–10.
Toro-Huamanchumo CJ, Benites-Meza JK, Mamani-García CS, et al. Efficacy of colchicine in the treatment of COVID-19 patients: a systematic review and Meta-analysis. J Clin Med. 2022;11(9)
Hariyanto TI, Halim DA, Jodhinata C, et al. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021;48(6):823–30.
Zein AFMZ, Raffaello WM. Effect of colchicine on mortality in patients with COVID-19 – a systematic review and meta-analysis. Diabet Metabol Syndrome: Clin Res Rev. 2022;16(2):102395.
Article CAS Google Scholar
Kow CS, Lee LH, Ramachandram DS, et al. The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID-19: a meta-analysis of randomized trials. Immun Inflamm Disease. 2022;10(2):255–64.
Eikelboom JW, Jolly SS, Belley-Cote EP, et al. Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial. Lancet Respir Med. 2022;19(22):1–9.
Google Scholar
Download references
Acknowledgements
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and affiliations.
Department of Family Medicine, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Kholoud Elshiwy, Ghada Essam El-Din Amin & Mohamed Farouk Allam
Department of Community, Environmental and Occupational Medicine, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Ghada Essam El-Din Amin
Department of Internal Medicine, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Mohamed Nazmy Farres & Rasha Samir
Department of Preventive Medicine and Public Health, Faculty of Medicine, University of Cordoba, 14004, Cordoba, Spain
Mohamed Farouk Allam
You can also search for this author in PubMed Google Scholar
Contributions
Kholoud Elshiwy: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Ghada Essam El-Din Amin: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Mohamed Nazmy: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Rasha Samir: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Mohamed Farouk Allam: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft.
Corresponding author
Correspondence to Kholoud Elshiwy .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Elshiwy, K., Amin, G.E.ED., Farres, M.N. et al. The role of colchicine in the management of COVID-19: a Meta-analysis. BMC Pulm Med 24 , 190 (2024). https://doi.org/10.1186/s12890-024-03001-0
Download citation
Received : 04 July 2023
Accepted : 08 April 2024
Published : 20 April 2024
DOI : https://doi.org/10.1186/s12890-024-03001-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Meta-analysis
- Ain Shams University
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 16 April 2024
Review on epidemiology, disease burden, and treatment patterns of IgA nephropathy in select APAC countries
- Omer Zaidi 1 ,
- Zhaoli Tang 2 ,
- Sandipan Bhattacharjee 3 &
- Kristin Pareja 3
BMC Nephrology volume 25 , Article number: 136 ( 2024 ) Cite this article
214 Accesses
Metrics details
Immunoglobulin type A (IgA) nephropathy is the most common primary glomerulonephritis (GN) worldwide with higher rates in East and Pacific Asia compared to North America and Europe. Despite high reported prevalence of IgAN in these countries, the overall disease prevalence across Asia is not available. Treatment patterns of IgAN patients across Asian countries have also not been summarized. The aim of this study was to review and summarize evidence on IgA nephropathy prevalence, treatment patterns, and humanistic and economic burden in mainland China, Taiwan, South Korea, Japan, and Australia.
A targeted literature review was conducted in PubMed and local databases in China (including Taiwan), South Korea, Japan, and Australia between January 2010-December 2021. Website literature searches were conducted using Google Scholar and Baidu.
Sixty-nine publications and 3 clinical guidelines were included. Incidence ranged from 0 to 10.7 per 100 000 people per year in Australia, Japan, and Taiwan, and ranged from 6.3 to 24.70% among patients who underwent renal biopsy in mainland China. Prevalence and diagnosis rates ranged from 0 to 72.1% in mainland China, South Korea, Taiwan, Japan, and Australia. Mortality rates in mainland China, South Korea, and Japan varied widely. The top 3 commonly used therapies were angiotensin-converting enzyme inhibitor/angiotensin receptor blockers (0.9-99.6%), corticosteroids (3.5-100%), and immunosuppressants (1.6-85.5%) in Japan, mainland China, and South Korea. Patient quality of life was measured by different tools, and annual hospitalization costs ranged from $1 284.73 to $2 252.12 (2015–2018) in China.
Conclusions
The prevalence of IgA nephropathy among the general population in select countries/regions is not commonly available, despite evidence from studies and clinical guidelines. In addition, it is observed across geographic regions that heterogeneity exists in prevalence rates, and large variations exist in treatment patterns. There is need to fill in these gaps to understand the contributing factors behind the differences through population-based, multi-center, and real-world studies.
Peer Review reports
Immunoglobulin type A nephropathy (IgAN), also known as Berger’s disease, is a kidney disease caused by kidney deposition of immunoglobulin type A (IgA) complexes involving galactose-deficient IgA [ 1 ] and resulting in inflammatory tissue damage [ 2 ]. IgAN affects the kidneys by attacking the glomeruli and is characterized by persistent urinary abnormalities including microscopic hematuria, gross hematuria, and/or proteinuria [ 2 , 3 ]. IgAN is the most common form of biopsy-proven primary glomerulonephritis (PGN) worldwide [ 3 ] and is one of the leading causes of chronic kidney disease (CKD) and end-stage renal disease (ESRD) [ 4 ].
Primary treatments for IgAN include angiotensin-converting enzyme inhibitor/angiotensin receptor blockers (ACEIs/ARBs), corticosteroids, and immunosuppressants [ 1 , 4 ]. These treatments aim to address symptoms and manifestations of IgAN but not the underlying cause. Nearly one-third of IgAN patients develop ESRD within 10 years [ 5 ]. On average, patients with IgAN die 6 years earlier than the general population [ 6 ]. In addition, patients’ quality of life (QoL) is greatly impacted due to pain, fatigue, and poor mental health [ 4 ], and indirect caregiver burden is high due to time spent caring for patients who progress to ESRD. Thus, caregivers’ QoL and psychological well-being can also be negatively impacted [ 7 ].
IgAN prevalence is highest in Asia, intermediate in Europe and the US, and lower in African countries [ 8 ]. The overall global incidence is approximately 2.5 per 100,000 people per year [ 2 ]. A higher prevalence of IgAN is seen in countries where routine screening is practiced [ 4 ]. While geographic variations of IgAN have been studied previously [ 3 , 9 ], few recent studies have focused on regional disease burden differences and treatment patterns in among IgAN patients across Asian countries/regions and Australia.
This review aimed to summarize the disease burden and treatment patterns of IgAN in select countries/regions in the Asia-Pacific region, specifically mainland China, Taiwan, South Korea, Japan, and Australia.
Data sources and search strategy
A targeted literature review (TLR) was conducted to identify relevant literature published from January 2010 to December 2021 for mainland China, Taiwan, South Korea, Australia, and Japan. The earliest year of publication was expanded from 2010 to 2001 to capture evidence more comprehensively on outcomes of interest. Medline and Embase were the primary databases for publications in English. For publications in local languages, WANFANG and China National Knowledge Infrastructure (CNKI) databases were searched for publications in Chinese, Korean Medical Database and Korean Information Service System (KISS) databases were searched for publications in Korean, and Scholarly and Academic Information Navigator (CiNii) was searched for publications in Japanese. Supplementary searches for clinical guidelines, conference proceedings, and websites of governmental and non-governmental organizations were conducted using Google, Baidu (for Chinese sources), and Naver (for Korean sources). Publications cited as references were also considered for screening.
Search terms included IgA nephropathy, Berger’s disease, incidence, prevalence, mortality, quality of life, cost, burden, and treatment. Observational studies, reviews, and registry studies were included in the search. Publications that reported prevalence, incidence, mortality, treatment patterns, guidelines, economic, and humanistic burden were included for data extraction. Search terms in English and local languages are listed in Supplementary Table S1 .
Study selection and data extraction
After the search was conducted and duplicates were removed, the title, abstract, and full texts of the remaining publications were screened. A second reviewer conducted the validation and finalization for publications to be included in the data extraction phase. During screening, the inclusion and exclusion criteria mainly focused on outcomes. Systematic reviews, observational studies including registry/database studies and other real-world studies, annual reports were considered for inclusion. Publications that reported evidence regarding epidemiology (incidence, prevalence, and mortality), humanistic and economic burden, and treatment patterns (treatment guidelines, duration, adherence, persistence, switching, and discontinuation) were included for data extraction. Studies that did not include outcomes of interest were excluded, as were studies with a small sample size (< 25). Strict predefined population, intervention, comparators, outcomes, and study design (PICOS) selection criteria and a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram were not used in this study. Study characteristics, patient characteristics, epidemiological outcomes, disease burden, and treatment patterns were extracted.
Study quality assessment
All eligible studies went through a quality assessment (QA) using a recommended checklist, according to the Center for Reviews and Dissemination Guidance for Undertaking Reviews in Health Care recommendations [ 10 ]. Quality assessment was performed for all eligible articles by two reviewers. The checklist consisted of 9 items excluding basic information for the included studies. Because all publications included in this study were observational studies or reviews, only the non-randomized clinical trial checklist was used for observational studies.
Sixty-nine publications were included for this review, among which 38 were from mainland China (2015–2021) [ 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ], 15 from Japan (2003–2021) [ 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 ], 10 from South Korea (2010–2020) [ 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 ], 3 from Taiwan (2014–2019) [ 74 , 75 , 76 ], and 3 from Australia (2001–2021) [ 77 , 78 , 79 ]; characteristics of the studies are shown in Supplementary Table S2 . Approximately 83% the publications reported a retrospective study design ( n = 57). For publications from mainland China, sample sizes ranged from 74 [ 37 ] to 4,367,829 [ 47 ], and male percentages ranged from 37.5% [ 17 ] to 97.3% [ 32 ]. For publications from Japan, sample sizes ranged from 52 [ 53 ] to 270,902 [ 63 ]; the male percentage ranged from 37.1% [ 58 ] to 56.96% [ 52 ]. For publications from South Korea, sample sizes ranged from 25 [ 64 ] to 5,114 [ 67 ]; the male percentage ranged from 36% [ 64 ] to 66.6% [ 73 ]. For publications from Taiwan, sample sizes ranged from 91 [ 75 ] to 7,073 [ 76 ]; the male percentage ranged from 45.9% [ 76 ] to 52.7% [ 75 ]. For publications from Australia, sample sizes ranged from 1,147 [ 78 ] to 2,457 [ 79 ]; the male percentage ranged from 60% [ 77 ] to 69.7% [ 79 ]. The Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline [ 1 ] and 2 country-specific guidelines [ 80 , 81 ] were also included for evidence on treatment patterns.
Sixty-eight journal articles were assessed for study quality (all details of the quality assessment are shown in Supplementary Table S3 ); one white paper was not included in the study quality assessment. Approximately 75% (51/68 articles) were deemed to be of good quality (i.e., without inherent flaws). Few studies reported the incidence/prevalence of IgAN directly and percentage of IgAN were extracted from included studies. The appropriateness of the statistical analysis conducted was not clear or not specified in 5 studies, as they did not define P values and the level of significance for all observations. Across studies, outcome measures were generally considered reliable. However, 33 articles stated that the results could be generalized to routine practice. In one case-control study, the similarity of both groups at the outset of the study was not clear.
Six publications provided evidence on IgAN incidence [ 30 , 61 , 63 , 74 , 77 , 78 ] in Australia ( n = 2), Japan ( n = 2), mainland China ( n = 1), and Taiwan ( n = 1). Most were cross-sectional observational studies ( n = 4), and sample sizes ranged from 156 [ 74 ] to 270,902 [ 63 ].
In Australia, IgAN incidence was estimated to be 1.41–10.5 per 100,000 people per year [ 77 , 78 ]. According to Briganti 2001 [ 78 ], IgAN incidence in Australia was lowest (0.0 per 100,000 per year) among male children and highest (10.7 per 100,000 per year) among male adults [ 78 ]. In Japan, only 2 studies reporting incidence data among children were identified. Utsunomiya 2003 [ 63 ] reported an incidence rate of 4.5 per 100,000 per year among 270,902 junior high and elementary school students; Kajiwara 2020 [ 61 ] reported a rate of 3.3 per 100,000 per year among 60,816 junior high and elementary school students. Both publications collected urine samples through a school urinary screening system in students 6 to 15 years old. In mainland China, the incidence rate of IgAN was estimated to be 6.3% among elderly patients who underwent renal biopsy and 24.7% among non-elderly patients who underwent renal biopsy [ 30 ]. In Taiwan, IgAN incidence was estimated to be 5.5 per million per year among the general population (around 23.5 million between 2014 and 2016), based on 1,445 renal biopsy records from a registry database [ 74 ]. In general, IgAN incidence was higher in males (5.7 per 100,000 per year) compared with females (2.9 per 100,000 per year) [ 78 ]. IgAN incidence was not reported in Korean populations.
Prevalence and diagnosis rate
IgAN prevalence among the general population was not reported in the included publications. But one cross-sectional study ( n = 3,623) reported an IgAN prevalence rate of 0.03% among the general Chinese pediatric population [ 34 ]. Thirty-five publications were identified with diagnosis rates among 2 populations: patients who received renal biopsies and PGN patients [ 13 , 14 , 17 , 18 , 19 , 21 , 22 , 24 , 30 , 31 , 33 , 34 , 35 , 36 , 39 , 40 , 43 , 44 , 45 , 46 , 47 , 48 , 52 , 59 , 67 , 68 , 69 , 70 , 71 , 72 , 74 , 75 , 76 , 79 ]. Twenty-one publications were from mainland China [ 13 , 14 , 17 , 18 , 19 , 21 , 24 , 30 , 31 , 33 , 34 , 35 , 36 , 39 , 40 , 43 , 44 , 45 , 46 , 47 , 48 ], 6 from South Korea [ 67 , 68 , 69 , 70 , 71 , 72 ], 3 from Taiwan [ 74 , 75 , 76 ], 3 from Japan [ 52 , 55 , 59 ], and 1 from Australia [ 79 ]. The majority (88%) were cohort studies ( n = 17) [ 13 , 21 , 31 , 33 , 35 , 36 , 39 , 40 , 43 , 44 , 45 , 46 , 52 , 68 , 69 , 70 , 71 ] and cross-sectional studies ( n = 13) [ 14 , 17 , 18 , 19 , 21 , 24 , 34 , 37 , 47 , 59 , 67 , 72 , 74 , 79 ], with the remainder being an annual report [ 76 ], a registry study [ 55 ] and a chart review [ 75 ]. Sample sizes ranged from 33 [ 70 ] to 43,67,829 [ 47 ].
In mainland China, the mean diagnosis rate of IgAN was estimated to be 24.1% among patients undergoing renal biopsies (median: 23.0%; range: 6.3-40.9%) [ 13 , 19 , 21 , 22 , 24 , 30 , 46 ] and 27.3% (median: 27.9%; range: 0-72.1%) [ 14 , 19 , 21 , 33 , 36 , 40 , 43 , 44 , 45 , 48 ] among PGN patients (Fig. 1 a); The mean IgAN diagnosis rate was estimated to be 21.7% (median: 17.5%; 17-30.4%) among children who underwent renal biopsy [ 17 , 18 , 35 ]. In Taiwan, the mean diagnosis rate of IgAN was 12.1% (median: 12.2%; range: 10.8-13.2%) among patients undergoing renal biopsies [ 74 , 75 ] and was reported similar (26%) among PGN patients [ 74 , 76 ] (Fig. 1 b). In South Korea, the mean diagnosis rate was 41% (median: 38.1%; range: 25.8-61.9%) among patients undergoing renal biopsies [ 67 , 69 , 71 , 72 ] and around 51.6% (average of 51.3% and 51.9%) among PGN patients [ 68 , 70 ] (Fig. 1 c). In Japan, Hattori 2016 reported a mean estimated IgAN diagnosis rate of 23% (median: 22.9%) among CKD patients [ 59 ]. In addition, the reported IgAN diagnosis rate among patients who underwent renal biopsy was 31%, with 6.9% in patients aged 65 to 80 years old and 10.5% in patients aged 80 years or older [ 52 , 55 ]. In Australia, Lee 2020 reported an IgAN diagnosis rate of 13% among patients undergoing renal biopsy [ 79 ].
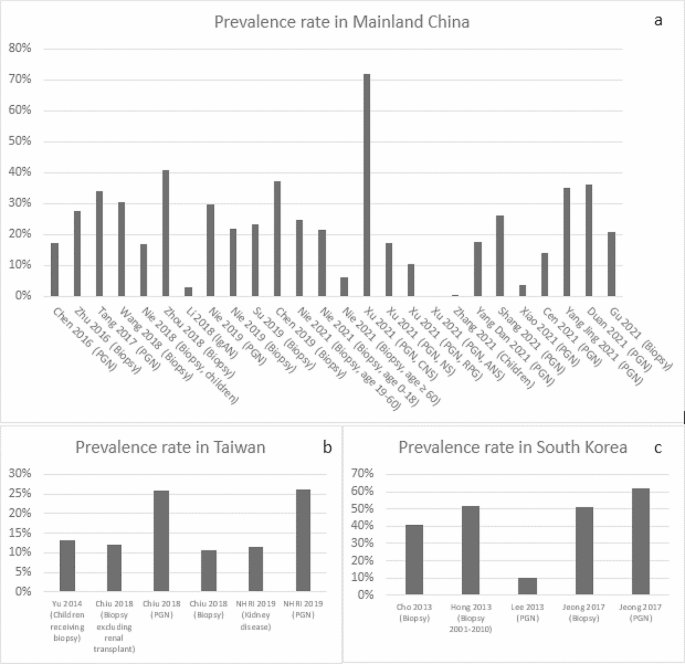
IgAN Prevalence in Mainland China, Taiwan and South Korea (Abbreviation: ANS, acute nephritic syndrome; CNS, chronic nephrotic syndrome; NHRI, National Health Research Institute & Taiwan Society of Nephrology; NS, nephritis syndrome(e; PGN, primary glomerulonephritis; RPG, rapidly progressive glomerulonephritis)
Disease progression and mortality
Among included studies, all-cause mortality was mainly reported as deaths due to ESRD. Seven publications from mainland China [ 23 , 26 , 27 , 28 , 29 , 41 , 42 ], 7 from Korea [ 64 , 65 , 66 , 68 , 70 , 71 , 73 ], 4 from Japan [ 50 , 51 , 57 , 62 ], and 1 from Taiwan [ 75 ] reported rate of progression to ESRD in IgAN. These studies varied in the definition of endpoint, patient characteristics, and follow-up duration. In China, the median rate of progression to ESRD was 4.1% [ 28 ] over 6 months, ranged from 1.3 to 15.8% (median: 1.3%) over 40–45 months [ 29 , 41 ], ranged from 6.6 to 15% (median: 8.3%) over 4–10 years [ 23 , 27 , 42 ], and 33% over 15 years [ 42 ]. In Korea, the median rate of progression to ESRD ranged from 2.5 to 39.7% (median: 19%) from 60 to 100 months [ 64 , 65 , 66 , 68 , 70 , 71 , 73 ].
Regarding direct reports on mortality, in mainland China, 0.7% of adult IgAN patients progressed to death according to 1 study of 944 patients from 2003 to 2014 with a median follow-up of 4.2 years [ 23 ]. In South Korea, the median death rate was 5.3% (range: 4.4-5.9%) [ 65 , 66 , 68 ] for 1,364 IgAN patients with a median follow-up of 100 months. In addition, 2 publications reported a standard mortality ratio (expressed as the ratio between the observed and the expected number of deaths in the general population) of 1.43 (95% confidence interval:1.04–1.92) among 1,364 IgAN patients in relation to the general population [ 65 , 68 ]. In Japan, IgAN mortality was estimated to be 0.3 per 100 person-years among non-smokers [ 51 ], 1.3 per 100 person-years among smokers [ 51 ] and 1.2 per 100 person-years among patients who received kidney replacement therapy [ 53 ] based on 2 retrospective studies [ 51 , 53 ]. No mortality data was found among IgAN patients in Taiwan or Australia.
- Treatment patterns
Twenty publications [ 1 , 11 , 15 , 26 , 27 , 29 , 42 , 49 , 50 , 54 , 56 , 57 , 58 , 60 , 62 , 64 , 68 , 71 , 73 , 81 ] and 3 clinical guidelines reported treatment patterns. Nine from mainland China [ 11 , 15 , 26 , 27 , 28 , 29 , 32 , 41 , 42 ], 8 from Japan [ 49 , 50 , 54 , 56 , 57 , 58 , 60 , 62 ], and 4 from South Korea [ 64 , 68 , 71 , 73 ]. 80% publications were retrospective studies ( n = 16) [ 11 , 15 , 26 , 27 , 29 , 42 , 49 , 56 , 57 , 58 , 60 , 62 , 64 , 68 , 71 , 73 ]. Sample sizes ranged from 25 [ 64 ] to 2,283 [ 50 ]. The KDIGO [ 1 ] and 2 country-specific treatment guidelines, 1 from mainland China [ 80 ] and 1 from Japan [ 81 ], were identified. No treatment guidelines were identified in Taiwan, South Korea, or Australia.
The KDIGO guidelines (2021 version) provide treatment recommendations for adults and children with IgAN [ 1 ]. The guidelines state that the management of IgAN should be multifaceted, optimized with supportive care, and include ACEIs/ARBs as tolerated or allowed, control blood pressure, minimize cardiovascular risk, and adherence to lifestyle changes including dietary counseling, smoking cessation, weight control, and exercise, as appropriate. The guidelines provide specific treatment recommendations according to the variant forms of IgAN, the level of proteinuria, and high-risk rate for progression after maximal supportive care. The main treatment regimens include ACEIs and ARBs, immunosuppressants, cyclophosphamide, tonsillectomy, and lifestyle modification [ 1 ]. Similar to the KDIGO guidelines, the primary treatment recommendations in the Chinese 2017 guidelines for children with IgAN were glucocorticoids, immunosuppressants, and ACEIs/ARBs [ 80 ]. Japanese 2020 guidelines covered children and adults, with different treatment recommendations based on symptoms and subtype of IgAN (the subgroup classification for adults was based on estimated glomerular filtration rate and proteinuria; symptoms among children were classified as mild or severe) [ 81 ].
In mainland China, 6 studies investigated adult populations [ 15 , 26 , 28 , 29 , 32 , 42 ] (Table 1 ) and 3 investigated pediatric populations [ 11 , 27 , 41 ] (Table 2 ). For drug usage among adult patients, ACEIs/ARBs had the largest median percentage at 66.7% (range: 38-90%) [ 15 , 26 , 28 , 29 , 32 , 42 ], followed by steroids, with median of 36% (corticosteroids/prednisone/intravenous methylprednisolone injection, range: 10-100%) [ 15 , 26 , 28 , 29 , 32 , 42 ] and immunosuppressants (including in combination with steroids), with median of 25.9% (cyclophosphamide, tacrolimus and tripterygium wilfordii, range: 1.6-72%) [ 15 , 26 , 28 , 29 , 32 , 42 ]. Among pediatric patients, immunosuppressants (cyclophosphamide/mycophenolate /Tripterygium wilfordii /leflunomide) were the common drugs recommended, with a median of 64% (range: 1.7–72.2%) [ 11 , 27 , 41 ], followed by ACEIs/ARBs, with a median of 49.5% (range: 2.5-70%) [ 11 , 27 , 41 ] and steroids with a median of 45% (range: 25.3-69.3% as sum of oral prednisone and intravenous methylprednisolone) [ 11 , 27 , 41 ].
In South Korea, 3 publications on adult IgAN patients [ 64 , 68 , 71 ] (Table 1 ) and 1 publication among pediatric patients [ 73 ] (Table 2 ) were identified. Among adults, ACEIs/ARBs were the most common treatments (27.7-83.4%) [ 68 , 71 , 73 ], followed by ACEIs/ARBs and corticosteroid combinations (33.9%) [ 64 ] and corticosteroids alone (12.4-28.8%) [ 68 , 71 , 73 ]. Among pediatric patients, the frequency of immunosuppressant use was 50.2% [ 73 ].
In Japan, 7 publications reported IgAN treatment patterns among adults [ 50 , 54 , 56 , 57 , 58 , 60 , 62 ] (Table 1 ) and 2 publications [ 49 , 54 ] among pediatric patients (Table 2 ). Among adults, ACEIs/ARBs were the most common treatment (25-99.6%) [ 50 , 54 , 56 , 57 , 58 , 60 , 62 ], followed by antiplatelet agents (58.1-96.8%) [ 54 ] and corticosteroid-immunosuppressant combination therapy (1.5-74%) [ 62 ]. Notably, the rate of administering steroid-immunosuppressant combination was only 1.5% in a retrospective cohort study that sampled 1,012 IgAN patients with a mean age of 32.96 ± 12 years [ 56 ]. Among pediatric patients, ACEIs/ARBs were the most frequently administered treatments (0.9-95.7%) [ 49 , 54 ], followed by antiplatelet agents (range: 1.2-82.6%) [ 49 , 54 ] and immunosuppressants (range: 4.6-68.5%) [ 49 ]. The frequency of administering treatments varied greatly across different subgroups. For example, the frequency of administering ACEIs/ARBs ranged from 0.9% for the diffuse mesangial proliferation subgroup ( n = 108) to 50.9% for the focal mesangial proliferation subgroup ( n = 173) in 1 retrospective study in Japanese children with IgAN from 1990 to 2004 [ 49 ]. Tonsillectomy or tonsillectomy combined with steroid was mostly reported in Japanese studies, with frequencies ranging from 1 to 66.2% across publications (Table 1 ). This is in accordance with the KDIGO 2021 guidelines’ evidence that supports the routine use of tonsillectomy in Japanese high-risk patients with IgAN [ 1 ]. No publications reporting IgAN treatment patterns were identified for Taiwan or Australia.
Humanistic burden
Four publications in China reported QoL, measured by the 36-Item Short Form Health Survey (SF-36) [ 16 , 25 ], Daily Living Ability Rating Scale (DLARS) [ 37 ], and QoL scale (QOLs) combined with Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) [ 38 ]. SF-36 scores reflect physical and mental health based on 8 health concepts, including physical and social functioning, role limitations due to physical and emotional problems, mental health, vitality, bodily pain, and general health (GH) perception [ 82 ]. Two publications evaluated the effects of individualized nursing intervention (INI, one improved nursing intervention which costs more time than routine nursing intervention [RNI]) on the psychological mood and QoL among IgAN patients [ 16 , 25 ]. There were two subgroups, the patients in the control group received RNI and patients in the intervention group received INI [ 16 , 25 ]. The mean GH score was 32.16 [ 16 ] among total IgAN patients ( n = 98; mean age: 32.74 years; male percentage: 50%) in 2017 and 80.15 increasing from 69.93 at baseline [ 25 ] after intervention among total IgAN patients ( n = 84; mean age: 33.57 years; male percentage: 60.7%) in 2019. In both publications, the intervention groups had higher mean GH scores than that in the control groups (39.47 vs. 24.84 [ 16 ] and 85.73 vs. 74.56 [ 25 ], respectively). Two other prospective studies assessed the effect of INI for IgAN patients [ 37 , 38 ]. Results showed that both mean DLARS and QOLs scores were higher among the intervention group compared to the control group (88.5 vs. 75.7 and 39.5 vs. 24.8, respectively) [ 37 , 38 ]. SAS and SDS scores were also evaluated by Qi 2021 [ 38 ], the mean SAS score decreased more in the intervention group (49.2 ± 6.3 decreased from 62.1 ± 5.8) than that in the control group (57 ± 4.9 decreased from 62.4 ± 6.1) from baseline. Similarly, the mean SDS score decreased more in the intervention group (43.3 ± 5.2 decreased from 56.2 ± 6) than in the control group (52.6 ± 6.4 decreased from 57 ± 6.2) from baseline [ 38 ].
Economic burden
No publications reported indirect costs, but 3 retrospective studies reported hospitalization costs for IgAN patients in China (see Supplementary Figure S1 ) [ 12 , 20 , 47 ]. Hospitalization cost per patient per year is ¥14,900 ($2,252.12; exchange rate of Chinese Yuan [CNY] and US dollar in 2018 was 6.616 [ 83 ]) as reported by Zheng 2018 [ 20 ], and between ¥9,618 ($1,532.26; exchange rate of CNY and US dollar in 2015 is 6.227 [ 83 ]) and ¥10,019 ($1,608.96) as reported by Peng 2015 [ 12 ]. One large database study covering 54.1% of tertiary hospitals in 31 Chinese provinces from 2010 to 2015 reported a hospitalization cost of ¥8,000/$1,284.73 (¥6,000-¥12,000) [ 47 ]. Drug costs accounted for 28.39% of total hospitalization costs, followed by diagnostic testing costs [ 12 ]. Length of stay per patient per year in China ranged from 10 to 14.3 days across 3 publications [ 12 , 20 , 47 ].
To our knowledge, this is the first TLR to summarize the evidence on IgAN disease burden and treatment patterns in mainland China, Taiwan, South Korea, Japan, and Australia. The findings of this review revealed evidence gaps in IgAN epidemiology and humanistic and economic burden. No incidence data was identified in South Korea; no mortality data was identified in Taiwan and Australia; no country/region-specific treatment guidelines were found for Taiwan, South Korea, or Australia; no evidence on treatment patterns from the publications was identified for Taiwan or Australia; and no humanistic burden or economic data was identified except for mainland China.
The IgAN incidence rates among Japanese, Taiwanese, and Australian populations ranged from 0 to 10.7 per 100,000 people per year, higher than the incidence rate reported in a recent systematic literature review (SLR) by Kwon 2021 [ 84 ] (1.29 per 100,000 people per year). Kwon 2021 [ 84 ] is an SLR focusing on US epidemiology, health-related QoL, and the economic burden of IgAN (the included studies were published from January 2010 to June 2020), similar to our study’s objective. Incidence rates among children and teenagers (0-4.5 per 100,000 per year) were similar to the incidence rate in Venezuela (0.03 per 100,000 per year) [ 85 ] and in Italy (0.31 per 100,000 per year) [ 86 ]. The overall prevalence and diagnosis rates of IgAN were similar across selected countries/regions. The diagnosis rates in this review differed from those found in PGN patients and patients who received renal biopsy in Kwon 2021 [ 84 ]; diagnosis rates of IgAN from our results were higher in PGN patients compared with patients who received renal biopsies since renal biopsies were often performed on PGN patients before diagnosis. This applied to both adult and pediatric populations. Compared to the US population in Kwon 2021 [ 84 ], the diagnosis rate among PGN populations in this review was higher (26-72.1% vs. 9.4-19.7%). The diagnosis rate among populations with renal biopsies was also higher (6.3-61.9% vs. 6.3-14.3%). Notably, though not covered by this review, the pathological profile such as Oxford Classification/MEST classification could also shed light upon disease burden, which could be further explored by future studies.
IgAN treatments primarily consisted of ACEIs/ARBs, and high utilization of steroids was found despite mixed evidence on their benefits and safety. There is limited data on IgAN treatment patterns from Taiwan and Australia. Among the publications that reported treatment patterns, few specified drugs’ generic names. The primary treatment patterns reported among select countries/regions in this study are similar to those in US as reported by Kwon 2021 (frequently used therapies were immunosuppressives, corticosteroids, and ACEIs/ARBs) [ 84 ]. Immunosuppressives were used more by children than adults based on data from mainland China, South Korea, and Japan. According to the KDIGO guideline regarding glomerular diseases, the immunosuppressive therapies including azathioprine, cyclophosphamide, calcineurin inhibitors, and rituximab are not recommended for treating IgAN. Mycophenolate mofetil is recommended in Chinese patients and tonsillectomy is recommended to be used in Japanese IgAN patients [ 1 ]. Only Chinese studies reporting SF-36 scores and other metrics were identified. Therefore, more studies on QoL in IgAN patients and caregivers in other regions are warranted.
Evidence of economic burden was identified only from studies in mainland China; Li 2018 was one retrospective national inpatient database study, which included the major hospitals that covers multiple geographic locations [ 47 ], other two studies used the data from one hospital. The mean cost per patient per year reported by Li 2018 is $1,284.73, while one Canadian retrospective study for costs and healthcare resource utilization reported a mean outpatient medication cost per patient per year of Canadian dollar (CAD) $221 in 2016 [ 87 ]. To control medical costs, hospitals in China are undergoing clinical pathway optimization programs [ 12 ].
Publications reported heterogeneous sample populations where IgAN prevalence/diagnosis rates were evaluated. Among 22 publications that reported IgAN prevalence/diagnosis rates, 15 measured IgAN prevalence for patients who underwent renal biopsy and 9 measured IgAN prevalence for patients diagnosed with PGN. Heterogeneity in IgAN prevalence/diagnosis rates may be attributed to differences in study years, patient race/ethnicity, patient age, treatment method, risk factors, diagnosis, and follow-up duration. Other study design–related factors that could introduce bias include sample size and gender composition.
Finally, differences in IgAN prevalence across regions should be noted. County/region-specific healthcare infrastructure and policies influence the epidemiological evidence of IgAN. systematic urine screening programs among individuals with asymptomatic, persistent microscopic hematuria with/without mild proteinuria are commonly implemented in certain countries/regions. These programs facilitate detection of IgAN patients who would otherwise receive a delayed diagnosis or none at all. Countries/regions where screening programs are performed may therefore have higher reported IgAN prevalence. Screening programs play a crucial role in early diagnosis and early treatment [ 88 ].
To our knowledge, this is the first TLR for IgAN in mainland China, Taiwan, South Korea, Japan, and Australia. However, several limitations should be noted. Due to the targeted nature of this review, the search focused on the most relevant literature, and the publications included in this study were prioritized, which potentially have led to an incomplete picture of IgAN-related epidemiology, treatment patterns and disease burden. Across included publications, the sample sizes varied widely and were not always reported. Additionally, this TLR did not weigh the data from included publications; therefore, biases should be considered when comparing outcomes. Studies came from primarily single institutions, and national-level data was not always available for the selected countries/regions. Moreover, this review only covered select Asia-Pacific countries/regions; future reviews and studies in other countries and regions within Asia-Pacific are therefore warranted. Despite these limitations, the evidence gathered in this literature review may help provide a preliminary understanding of the disease burden of IgAN in the Asia-Pacific region.
This TLR summarized evidence on Immunoglobulin type A nephropathy (IgAN) prevalence, treatment patterns, and humanistic and economic burden. Our results suggest that despite the overall scarcity of information in general, evidence on disease burden and treatment patterns has been reported by some studies and several clinical guidelines. The prevalence of IgAN among the general population is not commonly available, while that among patients receiving renal biopsies and diagnosed with PGN is more frequently reported. Heterogeneity in prevalence rates across geographic regions might be explained by differences in initial diagnosis in some regions due to variation in local screening policy and disease management. There is a need to understand how the disease progression differs by those practices. Treatment patterns have been reported mainly in studies from some Asia areas, but geographic variations are noticeable. There is also a need to generate more evidence to shed light upon the possible explanation to the differences in the treatment patterns across geographic regions. In sum, more real-world studies at national levels across select countries/regions are warranted to fill the evidence gaps, particularly regarding incidence, humanistic burden, and economic burden.
The prevalence of IgA nephropathy among the general population in select APAC countries/regions is not commonly available, despite evidence from studies and clinical guidelines. In addition, it is observed across geographic regions that heterogeneity exists in prevalence rates, and large variations exist in treatment patterns. Future studies are needed to fill in these gaps to understand the contributing factors behind the differences through population-based, multi-center, and real-world studies.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
Angiotensin-converting enzyme inhibitor
Acute nephritic syndrome
Asia Pacific
Angiotensin receptor blockers
Canadian dollar
Chronic kidney disease
China National Knowledge Infrastructure
Chronic nephrotic syndrome
Chinese Yuan
Cyclophosphamide
Daily Living Ability Rating Scale
Diffuse mesangial proliferation
Excerpta Medica Database
End-stage kidney failure
Focal mesangial proliferation
General health
Individualized nursing intervention
The Kidney Disease: Improving Global Outcomes
Korean Information Service System
Mycophenolate mofetil
Not reported
Nephritis syndrome
Primary glomerulonephritis
Population, intervention, comparators, outcomes, and study design
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Quality assessment
Routine nursing intervention
Rapidly progressive glomerulonephritis
Self-Rating Anxiety Scale
Standard deviation
Self-Rating Depression Scale
36-Item Short Form Health Survey
Systematic literature review
Targeted literature review
Taiwan Society of Nephrology
Working Group for National Survey on Status of Diagnosis and Treatment of Childhood Renal Diseases
Kidney Disease. Improving global outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–276.
Google Scholar
National Institute of Diabetes and Digestive and Kidney Diseases. IgA nephropathy [Available from: https://www.niddk.nih.gov/health-information/kidney-disease/iga-nephropathy .
Schena FP, Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol. 2018;38(5):435–42.
Article PubMed Google Scholar
Hassler JR. IgA nephropathy: a brief review. Semin Diagn Pathol. 2020;37(3):143–7.
Zhang H. KDIGO Zhinan Jiedu: IgA shenbing zhiliao[KDIGO guideline interpretation: treatment of IgA nephropathy. Chin J Practical Intern Medicine]. 2012;32(12):925–7.
CAS Google Scholar
Jarrick S, Lundberg S, Welander A, Carrero JJ, Hoijer J, Bottai M, et al. Mortality in IgA nephropathy: a nationwide population-based cohort study. J Am Soc Nephrol. 2019;30(5):866–76.
Article PubMed PubMed Central Google Scholar
Adejumo OA, Iyawe IO, Akinbodewa AA, Abolarin OS, Alli EO. Burden, psychological well-being and quality of life of caregivers of end stage renal disease patients. Ghana Med J. 2019;53(3):190–6.
Woo KT, Chan CM, Mooi CY, LC H, Tan HK, Foo M, et al. The changing pattern of primary glomerulonephritis in Singapore and other countries over the past 3 decades. Clin Nephrol. 2010;74(5):372–83.
Coppo R. Pediatric IgA Nephropathy in Europe. Kidney Dis (Basel). 2019;5(3):182–8.
Centre for Reviews and Dissemination. University of York. Systematic Reviews: CRD’s guidance for undertaking reviews in health care. 2009.
Working Group for National Survey on Status Diagnosis and Treatment of Childhood Renal Diseases. [Multicenter investigation of therapeutic status of children with IgA nephropathy in China]. Zhonghua Er Ke Za Zhi. 2013;51(7):486–90.
Peng Q, Xu G, Zhang C, Fang P. Wuhan Mou sanjia Yiyuan IgA shenbing Shen Chuanci huojian huanzhe linchuang lujing shishi xiaoguo pingjia[Evaluation of clinical pathway implementation effect in patients with IgA nephropathy renal puncture biopsy in a tertiary hospital in Wuhan]. Med Soc. 2015;28(10):18–20.
Zhu Z, Zou Q, Chen Y, Hu F, Bai J, Chao Q, et al. 224 Li Shen Huoti Zuzhi Jiancha De Linchuang lujing Yu Bingli fenxi[Analysis of the clinical pathway and pathologic features of 224 cases of renal biopsy]. Huaxi Med. 2016;31(5):845–9.
Tang L, Yao J, Kong X, Sun Q, Wang Z, Zhang Y, et al. Increasing prevalence of membranous nephropathy in patients with primary glomerular diseases: a cross-sectional study in China. Nephrol (Carlton). 2017;22(2):168–73.
Article Google Scholar
Zhou S, Fu J, Liu M, Yang S, Zhou Q, Yu X, et al. The prevalence and risk factors of abnormal circadian blood pressure in patients with IgA nephropathy. Clin Nephrol. 2017;88(12):344–53.
Article CAS PubMed Google Scholar
Lu H, Xiao L, Lu X, Liang J. Gexinghua huli moshi dui IgA shenbing huanzhe qingxu ji shenghuo zhiliang yingxiang de yanjiu [The effect of personalized nursing mode on the emotion and quality of life of patients with IgA nephropathy]. Contemp Med. 2017;23(5):30–3.
Wang N, Zhu T, Tao Y. Clinicopathological features of pediatric renal biopsies in the plateau regions of China. J Int Med Res. 2018;46(11):4539–46.
Nie S, He W, Huang T, Liu D, Wang G, Geng J, et al. The spectrum of biopsy-proven glomerular diseases among children in China: a national, cross-sectional survey. Clin J Am Soc Nephrol. 2018;13(7):1047–54.
Zhou Q, Yang X, Wang M, Wang H, Zhao J, Bi Y, et al. Changes in the diagnosis of glomerular diseases in east China: a 15-year renal biopsy study. Ren Fail. 2018;40(1):657–64.
Article CAS PubMed PubMed Central Google Scholar
Zheng X, Zhang J, Lu C. Xinjiang Weiwuerzu Zizhiqu renmin Yiyuan 2012 ~ 2017nian manxing shenzangbing huanzhe de jibing goucheng ji yiliao feiyong de hengduanmian diaocha [Disease composition and medical expenses of chronic kidney disease in people’s hospital of Xinjiang Uygur Autonomous Region from 2012 to 2017: a cross-sectional survey]. Chin J Evid-Based Med. 2018;18(9):903–6.
Nie P, Chen R, Luo M, Dong C, Chen L, Liu J et al. Clinical and pathological analysis of 4910 patients who received renal biopsies at a single center in Northeast China. Biomed Res Int 26 Mar 2019;2019:6869179.
Su S, Yu J, Wang Y, Wang Y, Li J, Xu Z. Clinicopathologic correlations of renal biopsy findings from northeast China: a 10-year retrospective study. Med (Baltim). 2019;98(23):e15880.
Cai Q, Shi S, Wang S, Ren Y, Hou W, Liu L, et al. Microangiopathic lesions in IgA nephropathy: a cohort study. Am J Kidney Dis. 2019;74(5):629–39.
Chen L, Luodelete M, Dong C, Li B, Zhang W, Nie P, et al. Pathological spectrum of glomerular disease in patients with renal insufficiency: a single-center study in northeastern China. Ren Fail. 2019;41(1):473–80.
Huang L. Tanjiu dui huanyou butong dengji IgAshenbing de huanzhe yuyi gexinghua huli ganyu duiyu xinli qingxu ji shenghuo zhiliang de yingxiang [Investigation of the effect of personalized nursing interventions on psychological, emotional and quality of life in patients with different grades of IgA nephropathy (IgAN)]. J Gen Pract Dentistry (Electronic Version). 2019;6(25):110–4.
Tian S, Yang X, Luo J, Guo H. Clinical and prognostic significance of C1q deposition in IgAN patients-a retrospective study. Int Immunopharmacol. 2020;88:106896.
Wu H, Xia Z, Gao C, Zhang P, Yang X, Wang R, et al. The correlation analysis between the Oxford classification of Chinese IgA nephropathy children and renal outcome– a retrospective cohort study. BMC Nephrol. 2020;21(1):247.
Liu Y, Wei W, Yu C, Xing L, Wang M, Liu R, et al. Epidemiology and risk factors for progression in Chinese patients with IgA nephropathy. Med Clin (Barc). 2021;157(6):267–73.
Wen D, Tang Y, Tan L, Tan J, Chen D, Zhang Y, et al. Sex disparities in IgA nephropathy: a retrospective study in Chinese patients. Int Urol Nephrol. 2021;53(2):315–23.
Nie P, Lou Y, Wang Y, Bai X, Zhang L, Jiang S, et al. Clinical and pathological analysis of renal biopsies of elderly patients in Northeast China: a single-center study. Ren Fail. 2021;43(1):851–9.
Feng S, Wang L, Liu X, Luo W, Xie M, Yang Q. 1002 li manxing shenzangbing huaner linchuang Ji Bingli fenxi[Clinical and pathological analysis of 1002 children with chronic kidney disease]. J Clin Pediatr. 2021;39(02):87–90.
Zhu L, Huang X, Zhang J, Li W, Chen E, Guo N. 102 Li Yizhishen IgA shenbing de huli tihui [Nursing experience of 102 cases of IgA nephropathy in transplanted kidneys]. Gen Pract Nurs. 2021;19(04):513–5.
Xu Z, Xiong Z. 3554 li shenzang bingli yu linchuang xiangguanxing fenxi [Analysis of renal pathology and clinical correlation in 3554 cases] [Shuoshi, https://doi.org/10.26921/d.cnki.ganyu.2021.001127] : M.S., Anhui Medical University; 2021.
Zhang P, Chen Z, Liu M. Huizhoushi dayawan diqu xuelingqian ertong niaoye shaicha fenxi [Analysis of urine screening in preschool children in Dayawan, Huizhou]. World’s Newest Med Inform Digest. 2021;21(84).
Yang D, Xie Y, He Z, Li Y, Li C. Qinhuangdaoshi 1459 Li xueling ertong shenzang jibing linchuang Yu Bingli fenxi [Clinical and pathological analysis of 1459 cases of renal disease in school-age children in Qinhuangdao]. Chin Healing Med. 2021;30(06):640–3.
Shang R, Zhu Y, Lin Z, Ma D, Ma Y, Ji M, et al. Yu Qiong liangdi yuanfaxing shenxiaoqiubing bingli leixing de bianqian duibi ji linchuang fenxi[Comparison and clinical analysis of pathological types of primary glomerular diseases in North Henan and Hainan]. J Clin Nephrol. 2021;21(2):111–8.
Lu X. Zhendui butong fenji IgA shenbing huanzhe kaizhan gexinghua huli moshi de linchuang xiaoguo guancha [Clinical effects of personalized care model for patients with different grades of IgA nephropathy]. Essent Health Readings. 2021;8:125.
Qi S. Zhendui butong fenji IgA shenbing kaizhan gexinghua huli moshi de linchuang xiaoguo guancha [Clinical effects of personalized care model for patients with different grades of IgA nephropathy]. Diet Health Care. 2021;8.
Pan Q, Ye Z, Zeng C, Ning W. Feishenbingxing tefaxing moxing shenbing Yu feishenbingxing IgA shenbing de linchuang tedian bijiao [Clinical comparative analysis of non-nephrotic idiopathic membranous nephropathy and non-nephrotic IgA nephropathy]. Anhui Med. 2021;25(2):268–70.
Xiao L, Wang J, Zhang M, He X, Gao J, Xi C. Yufangxing kangning zai budui guanbing shenbing zonghezheng zhiliao zhong de yingyong xiaoguo yanjiu [Study on the effect of preventive anticoagulation in the treatment of nephrotic syndrome in army officers and soldiers]. Northwest J De?F Med. 2021;42(01):30–6.
Zhao JL, Wang JJ, Huang GP, Feng CY. Primary IgA nephropathy with nephrotic-range proteinuria in Chinese children. Med (Baltim). 2021;100(21):e26050.
Article CAS Google Scholar
Le W, Liang S, Deng K, Hu Y, Zeng C, Liu D. 1126 li zhongguo hanzu chengren IgA shenbing huanzhe de changqi yuhou ji weixian yinsu fenxi [Long-term prognosis and risk factor analysis of 1126 Chinese Han adult patients with IgA nephropathy]. J Nephrol Dialysis Ren Transplantation. 2011;20(02):101–8.
Cen J, Hu H, Cheng Y, Liu Y, Wu S, Qin W, et al. Guangxi duominzu juju diqu dan zhongxin shenhuojian bingli ziliao ji minzu tedian fenxi [Pathological data of single-center kidney biopsy and analysis of ethnic characteristics in a multi-ethnic area of Guangxi]. J Chengdu Med Coll. 2021;16(04):482–5.
Yang J, Zhang L, Wang Y. Manxing Shenzangbing Shen Chuanci huojian bingli tezheng fenxi [Analysis of pathological features of renal puncture biopsy in chronic kidney disease]. Tibetan Med. 2021;42(05):49–51.
Duan Y, Lie C, Zhang L, AYiJiaKen K, Guo W, Li Y, et al. Xinjiang Weiwuer Zizhiqu 10 684 Li Shen huojian bingli ziliao Yu Liuxingbingxue tedian fenxi [Analysis of pathological data and epidemiological characteristics of 10 684 kidney biopsies in Xinjiang Uygur Autonomous Region]. Chin J Nephrol. 2021;37(06):490–8.
Gu C, Li Q, Liang W, Bi H, Xie M, Wu D. Guilin he jining liangsuo yiyuan 1370 Li Shen huojian jibing fenbu tezheng [Characteristics of disease distribution in 1370 kidney biopsies from two hospitals in Guilin and Jining]. J Cent South Univ (Medical Edition). 2021;46(09):974–82.
Li J, Cui Z, Long J, Huang W, Wang J, Zhang H, et al. Primary glomerular nephropathy among hospitalized patients in a national database in China. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association -. Eur Ren Association. 2018;33(12):2173–81.
Chen S, Tang Z, Xiang H, Li X, Chen H, Zhang H, et al. Etiology and outcome of crescentic glomerulonephritis from a single center in China: a 10-year review. Am J Kidney Dis. 2016;67(3):376–83.
Yata N, Nakanishi K, Shima Y, Togawa H, Obana M, Sako M, et al. Improved renal survival in Japanese children with IgA nephropathy. Pediatr Nephrol. 2008;23(6):905–12.
Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association -. Eur Ren Association. 2009;24(10):3068–74.
Yamamoto R, Nagasawa Y, Shoji T, Iwatani H, Hamano T, Kawada N, et al. Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis. 2010;56(2):313–24.
Yokoyama H, Sugiyama H, Sato H, Taguchi T, Nagata M, Matsuo S, et al. Renal disease in the elderly and the very elderly Japanese: analysis of the Japan Renal Biopsy Registry (J-RBR). Clin Exp Nephrol. 2012;16(6):903–20.
Komatsu H, Kikuchi M, Nakagawa H, Fukuda A, Iwakiri T, Toida T, et al. Long-term survival of patients with IgA nephropathy after dialysis therapy. Kidney Blood Press Res. 2013;37(6):649–56.
Matsuzaki K, Suzuki Y, Nakata J, Sakamoto N, Horikoshi S, Kawamura T, et al. Nationwide survey on current treatments for IgA nephropathy in Japan. Clin Exp Nephrol. 2013;17(6):827–33.
Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, et al. Japan Renal Biopsy Registry and Japan kidney Disease Registry: Committee Report for 2009 and 2010. Clin Exp Nephrol. 2013;17(2):155–73.
Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS ONE. 2014;9(3):e91756.
Sato R, Joh K, Komatsuda A, Ohtani H, Okuyama S, Togashi M, et al. Validation of the Japanese histologic classification 2013 of immunoglobulin A nephropathy for prediction of long-term prognosis in a Japanese single-center cohort. Clin Exp Nephrol. 2015;19(3):411–8.
Oshima Y, Moriyama T, Itabashi M, Takei T, Nitta K. Characteristics of IgA nephropathy in advanced-age patients. Int Urol Nephrol. 2015;47(1):137–45.
Hattori M, Iwano M, Sako M, Honda M, Okada H, Akioka Y, et al. Transition of adolescent and young adult patients with childhood-onset chronic kidney disease from pediatric to adult renal services: a nationwide survey in Japan. Clin Exp Nephrol. 2016;20(6):918–25.
Kaihan AB, Yasuda Y, Katsuno T, Kato S, Imaizumi T, Ozeki T, et al. The Japanese histologic classification and T-score in the Oxford classification system could predict renal outcome in Japanese IgA nephropathy patients. Clin Exp Nephrol. 2017;21(6):986–94.
Kajiwara N, Hayashi K, Fujiwara M, Nakayama H, Ozaki Y. Identification of children with chronic kidney disease through school urinary screening using urinary protein/creatinine ratio measurement: an observational study. Clin Exp Nephrol. 2020;24(5):450–7.
Miyabe Y, Karasawa K, Akiyama K, Ogura S, Takabe T, Sugiura N, et al. Grading system utilising the total score of Oxford classification for predicting renal prognosis in IgA nephropathy. Sci Rep. 2021;11(1):3584.
Utsunomiya Y, Koda T, Kado T, Okada S, Hayashi A, Kanzaki S, et al. Incidence of pediatric IgA nephropathy. Pediatr Nephrol. 2003;18(6):511–5.
Lee S, Choi S, Se-bin S, Kyunghwan J, Taewon L. Relative risk factors of prognosis in IgA nephropathy patients with depressed renal functions. Korean J Nephrol. 2010;29(2):198–207.
Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, et al. Mortality of IgA nephropathy patients: a single center experience over 30 years. PLoS ONE. 2012;7(12):e51225.
Lee Ha-Jung. Long-term patient and renal survivals and their predictable factor analyses in IgA nephropathy patients [Thesis]: College of Medicine, Seoul National University; 2012.
Cho BS, Hahn WH, Cheong HI, Lim I, Ko CW, Kim SY, et al. A nationwide study of mass urine screening tests on Korean school children and implications for chronic kidney disease management. Clin Exp Nephrol. 2013;17(2):205–10.
Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, et al. Mortality and renal outcome of primary glomerulonephritis in Korea: observation in 1,943 biopsied cases. Am J Nephrol. 2013;37(1):74–83.
Bae HJ, Moon KR, Kim YJ, Choi DE, Na KR, Lee KW, et al. Clinical and histopathological analysis of the kidney biopsies of 2,450 patients seen over 30 years at Chungnam National University Hospital. Korean J Med. 2015;84(3):379–88.
Jeong EG, Hyoun S, Lee SM, An WS, Kim SE, Son YK. Clinical outcomes of nephrotic syndrome in immunoglobulin a nephropathy. Saudi J Kidney Dis Transplantation. 2017;28(6):1314–20.
Kee YK, Yoon CY, Kim SJ, Moon SJ, Kim CH, Park JT, et al. Determination of the optimal target level of proteinuria in the management of patients with glomerular diseases by using different definitions of proteinuria. Med (Baltim). 2017;96(44):e8154.
Shin HS, Cho DH, Kang SK, Kim HJ, Kim SY, Yang JW, et al. Patterns of renal disease in South Korea: a 20-year review of a single-center renal biopsy database. Ren Fail. 2017;39(1):540–6.
Suh JS, Jang KM, Hyun H, Cho MH, Lee JH, Park YS, et al. Remission of proteinuria may protect against progression to chronic kidney disease in pediatric-onset IgA nephropathy. J Clin Med. 2020;9(7):2058.
Chiu HF, Chen HC, Lu KC, Shu KH. Taiwan Society of Nephrology. Distribution of glomerular diseases in Taiwan: preliminary report of National Renal Biopsy Registry-publication on behalf of Taiwan Society of Nephrology. BMC Nephrol. 2018;19(1):6.
Yu MC, Lee F, Huang WH, Hsueh S. Percutaneous ultrasound-guided renal biopsy in children: the need for renal biopsy in pediatric patients with persistent asymptomatic microscopic hematuria. Biomedical J. 2014;37(6):391–7.
National Health Research Institute & Taiwan Society of Nephrology. 2019 Annual Report on Kidney Disease in Taiwan.
Jegatheesan D, Nath K, Reyaldeen R, Sivasuthan G, John GT, Francis L, et al. Epidemiology of biopsy-proven glomerulonephritis in Queensland adults. Nephrol (Carlton). 2016;21(1):28–34.
Briganti EM, Dowling J, Finlay M, Hill PA, Jones CL, Kincaid-Smith PS, et al. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association. - Eur Ren Association. 2001;16(7):1364–7.
Lee AYS, Lin M-W. Do IgA nephropathy presentations display any seasonality? J Nephropathology. 2021;10(3):e33–e.
Subspecialty Group of Renal Diseases of the Society of Pediatrics Chinese Medical Association. [Evidence-based guidelines for diagnosis and treatment of primary IgA nephropathy (2016)]. Zhonghua Er Ke Za Zhi. 2017;55(9):643–6.
[Health, Labor and Welfare Scientific Research Grant Policy Research Project for Intractable Diseases. (Intractable Disease Policy Research Project)].[IgA Nephropathy 2020 Clinical Practice Guidelines].[Tokyo Igakusha]. 2021.
LoMartire R, Ang BO, Gerdle B, Vixner L. Psychometric properties of short Form-36 Health Survey, EuroQol 5-dimensions, and hospital anxiety and Depression Scale in patients with chronic pain. Pain. 2020;161(1):83–95.
OECD. Conversion rates - Exchange rates - OECD Data [Available from: http://data.oecd.org/conversion/exchange-rates.htm .
Kwon CS, Daniele P, Forsythe A, Ngai C. A systematic literature review of the epidemiology, health-related quality of life impact, and economic burden of immunoglobulin A nephropathy. J Health Econ Outcomes Res. 2021;8(2):36–45.
Orta-Sibu N, Lopez M, Moriyon JC, Chavez JB. Renal diseases in children in Venezuela, South America. Pediatr Nephrol. 2002;17(7):566–9.
Coppo R, Gianoglio B, Porcellini MG, Maringhini S. Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal biopsies in Children). Group of Renal Immunopathology of the Italian Society of Pediatric Nephrology and Group of Renal Immunopathology of the Italian Society of Nephrology. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association -. Eur Ren Association. 1998;13(2):293–7.
Barbour S, Lo C, Espino-Hernandez G, Sajjadi S, Feehally J, Klarenbach S, et al. The population-level costs of immunosuppression medications for the treatment of glomerulonephritis are increasing over time due to changing patterns of practice. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association -. Eur Ren Association. 2018;33(4):626–34.
Shen P, He L, Li Y, Wang Y, Chan M. Natural history and prognostic factors of IgA nephropathy presented with isolated microscopic hematuria in Chinese patients. Nephron Clin Pract. 2007;106(4):c157–61.
Download references
Acknowledgements
This work was presented as an abstract at the ISN World Congress of Nephrology 2022 meeting.
This work was supported by Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ.
Author information
Authors and affiliations.
OPEN Health, Boston, MA, USA
OPEN Health, Shanghai, China
Fen Du & Zhaoli Tang
Otsuka Pharmaceutical Development & Commercialization, Inc., NJ, Princeton, USA
Sandipan Bhattacharjee & Kristin Pareja
You can also search for this author in PubMed Google Scholar
Contributions
Research conception and/or design: Kristin Pareja, Sandipan Bhattacharjee, Omer Zaidi, Fen Du, and Zhaoli Tang; Literature searching strategy: Omer Zaidi, Fen Du, and Zhaoli Tang; literature screening and data extraction and analysis: Fen Du and Zhaoli Tang; All authors were involved in the drafting and /or substantial revision of manuscript; All authors accept accountability for their contributions and agree as a condition of authorship to ensure resolution of questions about the work. All authors approved the submitted version.
Corresponding author
Correspondence to Kristin Pareja .
Ethics declarations
Competing interests.
Kristin Pareja and Sandipan Bhattacharjee are employees of Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ, United States. Omer Zaidi, Fen Du, and Zhaoli Tang are employees of OPEN Health and were paid consultants by Otsuka.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zaidi, O., Du, F., Tang, Z. et al. Review on epidemiology, disease burden, and treatment patterns of IgA nephropathy in select APAC countries. BMC Nephrol 25 , 136 (2024). https://doi.org/10.1186/s12882-024-03555-5
Download citation
Received : 21 April 2023
Accepted : 21 March 2024
Published : 16 April 2024
DOI : https://doi.org/10.1186/s12882-024-03555-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- IgA nephropathy
- Epidemiology
- Disease burden
BMC Nephrology
ISSN: 1471-2369
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 16 April 2024
Approaches to locum physician recruitment and retention: a systematic review
- Nathan Ferreira 1 ,
- Odessa McKenna 1 ,
- Iain R. Lamb 2 ,
- Alanna Campbell 3 ,
- Lily DeMiglio 2 &
- Eliseo Orrantia ORCID: orcid.org/0000-0003-3023-8100 2
Human Resources for Health volume 22 , Article number: 24 ( 2024 ) Cite this article
197 Accesses
2 Altmetric
Metrics details
A robust workforce of locum tenens (LT) physicians is imperative for health service stability. A systematic review was conducted to synthesize current evidence on the strategies used to facilitate the recruitment and retention of LT physicians. English articles up to October 2023 across five databases were sourced. Original studies focusing on recruitment and retention of LT’s were included. An inductive content analysis was performed to identify strategies used to facilitate LT recruitment and retention. A separate grey literature review was conducted from June–July 2023. 12 studies were retained. Over half (58%) of studies were conducted in North America. Main strategies for facilitating LT recruitment and retention included financial incentives (83%), education and career factors (67%), personal facilitators (67%), clinical support and mentorship (33%), and familial considerations (25%). Identified subthemes were desire for flexible contracts (58%), increased income (33%), practice scouting (33%), and transitional employment needs (33%). Most (67%) studies reported deterrents to locum work, with professional isolation (42%) as the primary deterrent-related subtheme. Grey literature suggested national physician licensure could enhance license portability, thereby increasing the mobility of physicians across regions. Organizations employ five main LT recruitment facilitators and operationalize these in a variety of ways. Though these may be incumbent on local resources, the effectiveness of these approaches has not been evaluated. Consequently, future research should assess LT the efficacy of recruitment and retention facilitators. Notably, the majority of identified LT deterrents may be mitigated by modifying contextual factors such as improved onboarding practices.
Peer Review reports
Introduction
The shortage of a sustainable and robust physician workforce is a significant healthcare issue across most of the world [ 1 ]. In regions that face persistent challenges in physician availability, the continuity of the healthcare system heavily relies on locum tenens (LT) physicians, commonly referred to as “locums”. These healthcare providers work in a temporary capacity to fill vacancies or provide coverage for permanent physicians [ 2 , 3 ]. Their importance was highlighted during the COVID-19 pandemic as the lack of locums resulted in the suspension of hospital services and emergency department closures due to insufficient staffing [ 4 ].
Physician recruitment strategies primarily focus on filling permanent positions with minimal emphasis on attracting locum providers [ 5 ]. However, strategies aimed at facilitating the recruitment of permanent physicians may not effectively attract locums given fundamental distinctions in their employment preferences and priorities. LTs, for instance, are motivated by factors such as seeking greater autonomy, working part-time, transitioning into partial retirement, and supplementing income [ 6 , 7 ]. Their attraction to working as a locum may be due to advantages including reduced administrative burdens, lower workplace stress, and flexibility for maintaining a desired work–life balance [ 6 , 7 ]. Additional advantages include competitive salaries comparable to permanent positions without a long-term commitment, travel and accommodation stipends, subsidized malpractice insurance, and lower overhead expenses [ 6 , 7 ].
Governments and communities invest substantial financial resources to attract locums in order to sustain healthcare service delivery [ 6 , 8 ]. As such, existing research has investigated locum recruitment and retention factors [ 6 , 7 , 9 , 10 ]. Despite the important role that locums play in sustaining operational healthcare systems, particularly during periods of health human resources strain, there is a lack of consolidated of evidence on the recruitment of LT physicians. Consequently, there is a need for the synthesis of current research on facilitators used in the recruitment and retention of LTs. This will serve to better inform the development of comprehensive, evidence-based recruitment guidelines tailored specifically to LT physicians. Therefore, this study systematically reviewed existing literature to identify and synthesize the approaches used to recruit locum physicians. Ensuing results will provide valuable guidance to policymakers and healthcare organizations, aiding in the development of evidence-based recruitment policies and practices to address the unique needs of locum physicians.
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 11 ]. This research protocol was registered in the PROSPERO database (CRD42022339666).
Search strategy
Between April 26th and April 27th, 2022 we performed a systematic search of the electronic databases Ovid MEDLINE, Cochrane Database of Systematic Reviews, PsycINFO, CINAHL and Web of Science-Core Collection. Examples of the medical subject headings (MeSH) applied include “Contract Services”, “Career Choice”, “Personnel Staffing and Scheduling”, “Personnel Loyalty” and “Physician Incentive Plans”. This initial search has since been followed by an updated search in October of 2023 prior to submission for publication. Keywords were used to collect non-indexed material and those terms not captured by MeSH, such as “locum”. No limits were applied to the searches. Articles not available in English were excluded. Secondary research (e.g., meta‐analyses, dissertations, systematic reviews, case reports, commentaries, grey literature) were excluded from the scholarly search. Reference lists of included studies were searched for additional articles. Details of the scholarly search strategy appear in Additional file 1 : Appendix S1. This search strategy was developed in collaboration with a librarian and peer-reviewed by a second librarian.
Between June 12th and July 16th, 2023 we performed an iterative systematic hand-search of grey literature. This included public search engines (e.g., Google), grey literature repositories (e.g., OpenGrey), health care quality organizations, and data facilities across five countries, Canada, United States of America, United Kingdom, Australia, and India. Examples of the search terms and headings applied include “Locum”, “Contract”, “Temporary”, and “Locum Physician''. Search parameters were restricted to include only articles published in the year 1990 or later. For database searches information beyond the first 150 or 250 search results were not incorporated in the analysis. For full search histories please see Additional file 1 : Appendix S3.
Selection and screening process
A modified version of the PICO (population, intervention, comparison, outcomes) framework was used (Table 1 ) [ 12 ]. We included original qualitative, quantitative or mixed-methods studies focused on recruitment and retention initiatives specific to locums in any country across clinical settings. There was variability in how studies defined locum physicians (Table 2 ). Articles focusing on recruitment and retention of non-locum physicians and healthcare workers without an MD designation (with the exception of medical students training in a MD programme) were excluded, including articles that combined both populations in which individual data for locums could not be extracted. Articles that exclusively incorporated the recruitment and retention of locums in the interpretive context such that locum recruitment and retention initiatives were not prospectively mentioned in the study framework or methodology were excluded.
Retrieved articles were managed using Covidence online systematic review software (Veritas Health Innovation, Melbourne, Australia). Two reviewers independently performed title and abstract screening for relevance. Full texts were then reviewed against eligibility criteria (Table 1 ). In both stages of screening, discussion was used to resolve disagreements. Remaining discrepancies were resolved by a third reviewer.
Data extraction and synthesis
Data extraction took place within Covidence using two independent reviewers. A template was developed and piloted for two studies to ensure reviewer agreement prior to utilization. Outstanding conflicts were resolved by a third author. Extraction parameters included study design, participant characteristics, context of locum assignment, and strategies used to recruit and retain locums. Two authors (NF and OM) performed an inductive content analysis to characterize recurring patterns of the locum recruitment and retention strategies discussed in each paper included in the systematic analysis. Following the identification of these strategies, they were grouped into broader, overarching themes relevant to LT recruitment and retention. Methodologic quality of each study was assessed using the Mixed Methods Appraisal Tool (MMAT) [ 13 ]; two authors (NF and OM) conducted the appraisal independently and any discrepancies in appraisal were resolved by discussion with a third author (EO). Authors of included studies were contacted if data were missing.
Our initial search identified 5390 citations. After the removal of duplicates ( n = 812), 4578 studies’ titles and abstracts were screened. Following this stage, 242 articles were screened using full-text, and 230 were excluded from the review. Twelve studies [ 2 , 3 , 7 , 8 , 9 , 13 , 14 , 15 , 16 , 17 , 18 , 19 ] fulfilled inclusion criteria and were retained for data extraction. The PRISMA flow diagram detailing the screening procedure is displayed in Fig. 1 . Articles reporting data from the same participant population at separate time points are reported together.
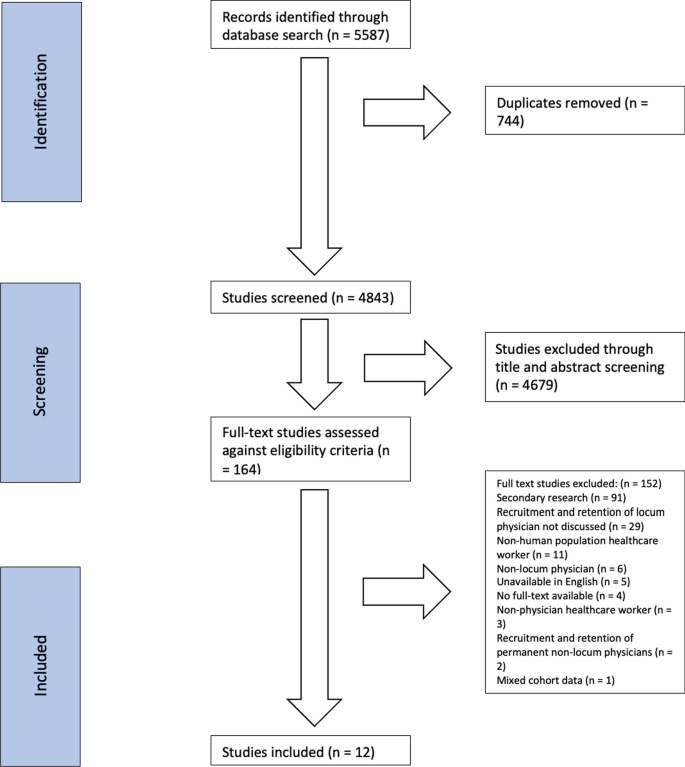
PRISMA flow diagram detailing the selection process
Study characteristics are summarized in Table 3 . Most ( n = 11, 92%) studies [ 2 , 3 , 7 , 8 , 9 , 13 , 14 , 15 , 17 , 18 , 19 ] were published within the last 20 years of our search. Four (33%) studies [ 7 , 9 , 13 , 19 ] were from the United States and four (33%) [ 8 , 14 , 15 , 16 ] were from the United Kingdom. A smaller portion ( n = 3, 25%) [ 2 , 3 , 17 ] originated in Canada. One (8%) study [ 18 ] was based in India. Quantitative studies [ 3 , 7 , 9 , 13 , 15 , 16 , 17 ] ( n = 7, 58%) were cross-sectional ( n = 6, 50%) [ 3 , 7 , 9 , 13 , 15 , 16 ] or pre–post study ( n = 1, 8%) [ 17 ] in design. Four (33%) studies [ 8 , 14 , 18 , 19 ] used a qualitative design, including semi-structured interviews ( n = 2, 17%) [ 18 , 19 ], focus groups ( n = 1, 8%) [ 14 ], and content analysis ( n = 1, 8%) [ 8 ]. One (8%) study [ 2 ] adopted a mixed-methods design.
The majority of studies [ 2 , 7 , 8 , 9 , 13 , 15 , 16 , 19 ] ( n = 8, 67%) specified clinical setting, but did not indicate whether it was rural or urban (Table 3 ). A variety of specialities were reported among locum populations. The majority ( n = 9, 75%) [ 2 , 3 , 7 , 13 , 14 , 15 , 16 , 18 , 19 ] included primary care physicians, and over half ( n = 5, 42%) [ 7 , 9 , 13 , 18 , 19 ] included specialists. Three (25%) studies [ 7 , 13 , 19 ] reported a subspecialist population. Two (17%) studies [ 2 , 17 ] included physicians in training, with one (8%) [ 2 ] involving resident physicians and another (8%) [ 17 ] medical students. One (8%) study [ 8 ] did not indicate the specialty of the physician population. A total of six (50%) studies [ 7 , 13 , 14 , 15 , 16 , 19 ] reported years of physician practice experience.
A diversity of locum recruitment approaches were reported across studies, with some ( n = 2, 17%) [ 7 , 8 ] using more than one method. Four (33%) studies [ 7 , 8 , 9 , 13 ] used a third-party recruitment agency, two (17%) [ 8 , 14 ] used a locum bank, word-of-mouth, or personal networks [ 16 ], informal means [ 7 ], and an unspecified novel recruitment software were each reported once [ 8 ] (8%). Four studies [ 2 , 15 , 18 , 19 ] (33%) did not report a specific method.
Quality assessment
The 2018 version of the MMAT was used to appraise the quality of retained articles [ 20 ]. Overall, nine (75%) of the articles [ 7 , 8 , 13 , 14 , 16 , 17 , 18 , 19 ] met 75–100% of the evaluated criteria, representing high quality. Three (25%) studies [ 2 , 9 , 15 ] met 50–75% of the evaluated criteria, representing moderate quality. Further details regarding the assessment of quality of retained articles appear in Additional file 1 : Appendix S2. Grey literature was assessed using the AACODS Checklist [ 21 ]. Additional information on the appraisal of grey literature can be found in Additional file 1 : Appendix S3 and in the supplemental content titled “Grey Literature Search Strategy, Data Extraction, and Evaluation”.
Facilitators of locum recruitment and retention
Six locum recruitment and retention themes were identified across retained studies (Table 4 ). Five overarching themes emerged for factors that facilitated LT recruitment and retention: financial incentives, familial considerations, educational or career-based factors, personal facilitators, and mentorship/clinical support. One theme focused on deterrents of locum work.
Ten (83%) studies [ 2 , 3 , 7 , 8 , 9 , 13 , 14 , 15 , 16 , 18 ] reported financial incentives with nine individual subthemes identified (Table 5 ). A significant portion ( n = 4, 33%) of studies’ [ 2 , 14 , 15 , 16 ] did not provide specific details about the nature of the financial incentives provided. Four (33%) of the studies’ [ 2 , 7 , 8 , 13 ] financial incentives referred to an increase in income. Reimbursement for locum travel and lodging was reported three times (25%) [ 3 , 7 , 9 ]. Reimbursement for medical licensure ( n = 2, 17%) and provision of malpractice insurance ( n = 2, 17%) were also reported [ 7 , 9 ]. Augmented pay for challenging work conditions [ 18 ], payment assistance for continuing medical education (CME) [ 18 ], supplementation of retirement income [ 13 ], and guaranteed income [ 3 ] were all reported once (8%) each.
Three (25%) studies [ 2 , 7 , 14 ] reported familial considerations as facilitators to recruitment and retention which included accommodating family (17%) [ 2 , 14 ], school accessibility (8%) [ 14 ], and unspecified (8%) [ 7 ]. Furthermore, eight (67%) studies [ 2 , 3 , 7 , 8 , 13 , 14 , 16 , 17 ] reported education or career-based incentives. A total of 13 subthemes related to educational and career-based factors facilitating recruitment and retention were reported (Table 5 ), which included pre-permanent practice scouting [ 2 , 7 , 13 , 17 ] and temporary or transition in employment [ 2 , 7 , 13 , 16 ] both reported four (33%) times. Freedom from administrative responsibilities and transitioning into retirement were reported three (25%) times [ 7 , 13 , 16 ]. Avoiding commitment [ 2 , 16 ], increasing skills and competencies [ 2 , 8 ], and a desire to take on part-time employment [ 7 , 13 ] was reported twice (17%). The remaining career-based facilitators to recruitment and retention were each reported once (8%), including acquiring cross-provincial locum medical licensure [ 3 ], facilitation of hospital credentialing and medical licensure [ 7 ], gaining exposure to running a medical practice [ 2 ], accessing novel CME opportunities [ 3 ], assistance with maintaining medical knowledge [ 14 ], and accessing peer-facilitated educational support [ 14 ].
A total of eight (67%) studies [ 2 , 7 , 8 , 13 , 14 , 15 , 16 , 17 ] reported using personal factors as facilitators of LT recruitment and retention. Within this category, five subthemes were identified (see Table 5 ). Seven (58%) reported using flexible contracts (e.g., suitable availability, work schedule flexibility, and work–life balance) [ 2 , 7 , 8 , 13 , 14 , 15 , 16 ]. Having the ability to travel and experience new communities (locum tourism) was reported five (42%) times [ 2 , 7 , 13 , 16 , 17 ]. Three (25%) studies [ 7 , 14 , 15 ] reported unspecified personal incentives including stress relief [ 14 ], structured support [ 14 ], facility amenities [ 7 ], working conditions [ 7 ], personal safety [ 15 ], and overall facility quality [ 7 ]. Compatibility with post and convenience of the assignment were reported once (8%) each [ 8 ].
Four (33%) studies [ 14 , 15 , 16 , 19 ] reported recruitment and retention facilitators involving mentorship and clinical support with four subthemes. Having a network of supportive colleagues [ 14 , 15 ] and a chance to become familiar with the practice before arrival [ 16 , 19 ] were reported twice (17%) each. Intentional relationship building, whereby the seasoned colleague met with the incoming locum to ensure comfort in the practice was reported once (8%) [ 19 ]. Availability of a back-up physician for support was reported once (8%) [ 19 ].
A total of eight (67%) studies [ 2 , 8 , 9 , 14 , 16 , 17 , 18 , 19 ] addressed deterrents of or barriers to locum work, encompassing a total of 19 reported subthemes. Professional isolation ( n = 5, 42%) [ 2 , 8 , 14 , 18 , 19 ] was reported most frequently followed by work unpredictability (33%) [ 2 , 8 , 16 , 19 ]. Insufficient patient continuity of care was reported three (25%) times [ 2 , 8 , 16 ]. The following deterrents/barriers were each reported twice (17%): inadequate employee onboarding and orientation [ 8 , 19 ], demanding locum work [ 2 , 17 ], poor job security [ 16 , 18 ], lack of information to make an informed decision about accepting the job post [ 8 , 9 ], and a lack of career advancement [ 16 , 18 ]. The following deterrents/barriers were reported just once (8%): excessive travelling [ 16 ], low patient volume [ 2 ], administrative burden [ 18 ], difficulty accessing time-off [ 18 ], inadequate housing [ 18 ], challenging working conditions [ 18 ], exclusion from pension plans [ 16 ], lack of equitable pay [ 18 ], low salary [ 18 ], feeling distanced from CME and limitations in staying up-to-date [ 14 ], and perceptions of inferior professional status by colleagues [ 16 ].
Facilitators of locum recruitment and retention within grey literature
Grey literature findings closely mirrored the facilitators and deterrents found in peer-reviewed literature. A notable exception captured in the ‘education and career’ theme involved the potential benefits of implementing a national physician licensure, which was absent in the primary literature but present in nearly a third ( n = 27, 26%) of the grey literature.
Interpretation
We identified 12 English language studies that explored the recruitment and retention of locums in Canada, USA, UK, and India over a 30-year period. Finance, education, and personal factors were the most used LT recruitment strategies while family considerations and clinical/mentorship support were less frequently cited. However, almost all studies [ 2 , 3 , 7 , 8 , 13 , 14 , 15 , 16 ] ( n = 8, 67%) reviewed reported using a combination of these recruitment approaches. While there is a paucity of evidence on whether employing multiple approaches leads to improved LT recruitment, utilizing a range of methods may still be a reasonable strategy. This approach prevents organizations from becoming overly reliant on a single approach and enables them to adapt their strategy more easily as required to maintain LT recruitment, retention and service. Further, as physicians choose locum positions based on different priorities, utilizing multiple strategies provides a range of incentives with wider appeal.
Across the five LT recruitment strategies, the diverse range of unique approaches used indicates there is no one-size-fits-all method. This suggests that organizations develop their own specific approach tailored to their available resources, location, and the anticipated needs of the LT physicians they aim to recruit. For instance, certain recruitment strategies incentives such as back-up availability, network of supportive colleagues and access to CME may not be feasible for some organizations given their size, location, and resource constraints. This may lead to the development of alternative recruitment approaches and/or increased emphasis on other strategies. Notably, we found that common recruitment and retention strategies used elsewhere, such as providing competitive salaries, were extensively used in the recruitment and retention of locums. However, approaches that seem to be specifically designed to address the unique requirements and preferences of locum physicians were also employed, such as offering reimbursements for travel and accommodation, providing support for family-related needs, offering flexible scheduling, and facilitating access to leisure activities. Although the effectiveness of these strategies is poorly defined, their implementation suggests that organizations recognize that conventional recruitment and retention approaches, effective in the broader health workforce, may not adequately address the unique aspects and challenges associated with the transient and temporary nature of locum work. For example, incentives like competitive compensation, while valued, might not be as appealing to those seeking the flexibility of short-term work assignments or lifestyle benefits. Therefore, acknowledging the appeal of locum work, creating incentives that emphasize these benefits, and addressing the related challenges are likely to enhance recruitment and retention efforts.
The finding that showed sites employed a wide range of recruitment and retention approaches highlights the complexity of this process. However, implementing such a wide range of strategies makes it challenging to identify the most effectives. Consequently, future work should identify optimal recruitment strategies within diverse health contexts and organizational structures. This would enable organizations to streamline their approach, maximizing recruitment success while efficiently utilizing their resources. This may be particularly valuable in resource poor healthcare environments where strategic asset allocation is essential.
Numerous factors were cited as deterrents of locum work, indicating that physicians’ decision to work as a LT is influenced by a variety of considerations. Although some of the cited deterrents were addressed by recruitment strategies, it is unclear whether these approaches were effective. In the studies reviewed, professional isolation and work assignment predictability were the two most cited deterrents to locum work appearing in 42% and 33% of studies, respectively. As temporary workers, there are inherent challenges in developing rapport with colleagues. Moreover, providing coverage introduces uncertainties regarding work schedules and conditions (e.g., hours worked, frequency and duration of assignments). Together these factors can contribute to lower job satisfaction, which may result in a decreased willingness to work as a locum. As a result, recruitment strategies should consider measures to address these deterrents. The wide range of deterrents emphasizes the importance for healthcare organizations to adopt comprehensive recruitment strategies that recognize and respond to the various unique needs of LT physicians. Further, many of these deterrents may be addressed by improving locum onboarding and job conditions, such as enhancing infrastructure quality and minimizing social isolation.
It is important to recognize information on locum recruitment and retention extends beyond peer-reviewed articles to include the grey literature. These non-academic resources contain potential insights into practical approaches for recruitment and retention, thus underscoring the need to evaluate the grey literature in this field. Interestingly, our review of the grey literature generally aligns with the facilitators and deterrents of locum work identified in this systematic review apart from support for a national physician license. Such a measure would enhance the portability of licensure, allowing improved mobility of physicians across regions, reducing administrative burdens and the time required for obtaining proper licensing, hospital privileges, and contractual agreements. This, in turn, may reduce barriers to locum recruitment and more effectively facilitate the transition of locums to their temporary place of practice. This finding, which was not identified in the systematic review, again reiterates the importance of assessing the grey literature to gain a comprehensive understanding on the current strategies being used for recruiting and retaining locum physicians.
Importantly, the success of LT physician recruitment relies on a collaborative effort that extends beyond responsibility of individual healthcare organizations. This is particularly important considering that facilitators of LT recruitment and retainment, such as remuneration, fall beyond the scope of health teams. Therefore, the various stakeholders in health human resources, including educational institutions, regulatory bodies, and professional associations, all play a role in LT recruitment efforts. Recognizing and embracing this shared responsibility will be crucial in fostering a robust and sustainable healthcare workforce that incorporates LT physicians.
Limitations
In the systematic and grey literature reviews, a comprehensive set of keywords related to locum recruitment and retention were used (as detailed in Additional file 1 : Appendices S1 and S3). However, some search terms, such as region-specific terminology used to describe locums, were not included. As a result, it is possible that relevant resources may have been missed during the literature search. However, the use of diverse keywords related to locum recruitment and retention would have captured relevant studies thus reducing the likelihood that relevant resources were missed. As described in the literature search strategies, date limitations were applied to both the systematic and grey literature searches, and only select databases were searched. Therefore, there is a possibility that relevant publications or grey literature produced outside of these date ranges or databases might have been missed. The quality of synthesized evidence was moderate as most of the retained quantitative studies were cross-sectional [ 3 , 7 , 9 , 13 , 15 , 16 ] (50%) or mixed-methods [ 2 ] (8%). Only one study adopted a pre–post study design [ 17 ], which is fraught with internal validity issues. Remaining studies [ 8 , 14 , 18 , 19 ] were qualitative and not inherently generalizable to broad populations. None of the studies were intervention-based, making it difficult to draw conclusions about the effectiveness of various recruitment and retention strategies. Inconsistent reporting on locum (LT) gender limited conclusions regarding differences in motivations for LT practice. Geographies of included studies reported were likely influenced by the methodological choice to include English only articles, limiting the generalizability of the presented findings to other regions. Further, the mix of qualitative and quantitative sources make it challenging to comprehend the cumulative size of the physician population raising each issue, and the relative significance of each issue compared to others.
Conclusions
This systematic review synthesized existing knowledge pertaining to international locum physician recruitment and retention strategies. Locum physicians are essential to the delivery of quality healthcare services across Canada and other parts of the world. We demonstrate that organizations employ five main LT recruitment strategies and deploy these in a variety of ways. Though these may be incumbent on local resources, more concerning is that the effectiveness of these approaches has not been tested. Given the present financial challenges within the global healthcare landscape there is a need to better understand recruitment and retention strategies of LTs so this limited resource can be used most effectively. Findings merit future research into the effectiveness of LT recruitment approaches via prospective methodologies.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Li JH, Scott A, McGrail M, Humphreys J, Witt J. Retaining rural doctors: doctors’ preferences for rural medical workforce incentives. Soc Sci Med. 2014;121:56–64.
Article PubMed Google Scholar
Myhre DL, Konkin J, Woloschuk W, Szafran O, Hansen C, Crutcher R. Locum practice by recent family medicine graduates. Can Fam Physician. 2010;56(5):E183–90.
PubMed PubMed Central Google Scholar
Rourke JTB, Incitti F, Rourke LL, Kennard MA. Keeping family physicians in rural practice—solutions favoured by rural physicians and family medicine residents. Can Fam Physician. 2003;49:1142–9.
Duong D. Why are emergency departments closing? Can Med Assoc J (CMAJ). 2022;194(33):E1138–9.
Article Google Scholar
Abelsen B, Strasser R, Heaney D, Berggren P, Sigurðsson S, Brandstorp H, et al. Plan, recruit, retain: a framework for local healthcare organizations to achieve a stable remote rural workforce. Hum Resour Health. 2020;18(1):63–63.
Article PubMed PubMed Central Google Scholar
Ferguson J, Tazzyman A, Walshe K, Bryce M, Boyd A, Archer J, et al. “You’re just a locum”: professional identity and temporary workers in the medical profession. Sociol Health Illn. 2021;43(1):149–66. https://doi.org/10.1111/1467-9566.13210 .
Alonzo AA, Simon AB. Have stethoscope, will travel: contingent employment among physician health care providers in the United States. Work Employment Soc. 2008;22(4):635–54.
Theodoulou I, Reddy AM, Wong J. Is innovative workforce planning software the solution to NHS staffing and cost crisis? An exploration of the locum industry. BMC Health Serv Res. 2018;18:1–13.
DiMeglio M, Furey W, Laudanski K. Content analysis of locum tenens recruitment emails for anesthesiologists. BMC Health Serv Res. 2018;18:1–7.
Waldie AC. Put out the welcome mat for locums. Can Med Assoc J (CMAJ). 1998;158(8):1009–1009.
Google Scholar
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906–105906.
Larkin J, Foley L, Smith SM, Harrington P, Clyne B. The experience of financial burden for people with multimorbidity: a systematic review of qualitative research. Health Expect. 2021;24(2):282–95.
Simon AB, Alonzo AA. The demography, career pattern, and motivation of Locum tenens physicians in the United States. J Healthc Manag. 2004;49(6):363–75.
PubMed Google Scholar
Jenson CM, Hutchins AJ, Rowlands G. Is small-group education the key to retention of sessional GPs? Educ Prim Care. 2006;17(3):218–26.
Jenson C, Reid F, Rowlands G. Locum and salaried general practitioners: an exploratory study of recruitment, morale, professional development and clinical governance. Educ Primary Care. 2008;19(3):285–302. https://doi.org/10.1080/14739879.2008.11493685 .
McKevitt C, Morgan M, Hudson M. Locum doctors in general practice: motivation and experiences. Br J Gen Pract. 1999;49(444):519–21.
CAS PubMed PubMed Central Google Scholar
Woloschuk W, Tarrant M. Does a rural educational experience influence students’ likelihood of rural practice? Impact of student background and gender. Med Educ. 2002;36(3):241–7.
Rajbangshi PR, Nambiar D, Choudhury N, Rao KD. Rural recruitment and retention of health workers across cadres and types of contract in north-east India: a qualitative study. WHO South East Asia J Public Health. 2017;6(2):51–9.
Lagoo J, Berry W, Henrich N, Gawande A, Sato L, Haas S. Safely practicing in a new environment: a qualitative study to inform physician onboarding practices. Jt Comm J Qual Patient Saf. 2020;46(6):314–20. https://doi.org/10.1016/j.jcjq.2020.03.002 .
Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed methods appraisal tool (MMAT), version 2018; 2018.
Tyndall J. AACODS checklist. Flinders University; 2010. https://www.library.sydney.edu.au/research/systematic-review/downloads/AACODS_Checklist.pdf
Download references
Acknowledgements
We gratefully acknowledge the help of Jennifer Dumond, Education Services Librarian at NOSM University, for peer-reviewing the search strategy.
Supported by Northern Ontario Academic Medicine Association (NOAMA) Academic Funding Plan (AFP) Innovation Fund Project #A-22-07.
Author information
Authors and affiliations.
Faculty of Medicine, University of Ottawa, Ottawa, ON, K1N 6N5, Canada
Nathan Ferreira & Odessa McKenna
Division of Clinical Sciences, Northern Ontario School of Medicine (NOSM) University, Marathon, ON, P0T 2E0, Canada
Iain R. Lamb, Lily DeMiglio & Eliseo Orrantia
Northern Ontario School of Medicine (NOSM) University, Sudbury, ON, P3E 2C6, Canada
Alanna Campbell
You can also search for this author in PubMed Google Scholar
Contributions
N.F and O.M were responsible for experimental design, the acquisition, analysis, interpretation of data and writing of the manuscript. A.C was responsible for acquisition, analysis, interpretation of data. I.R.L was responsible for interpretation of data, and the writing and revising of the manuscript. L.D and E.O were responsible for conception and experimental design, data analysis, data interpretation and revising the manuscript. All authors approve of the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Correspondence to Eliseo Orrantia .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. appendix s1.
: Search Strategy. Appendix S2 : Mixed-methods Appraisal Tool (MMAT) quality assessment of included studies. Appendix S3 : Grey Literature Search Strategy, Data Extraction, and Evaluation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ferreira, N., McKenna, O., Lamb, I.R. et al. Approaches to locum physician recruitment and retention: a systematic review. Hum Resour Health 22 , 24 (2024). https://doi.org/10.1186/s12960-024-00906-z
Download citation
Received : 29 November 2023
Accepted : 02 April 2024
Published : 16 April 2024
DOI : https://doi.org/10.1186/s12960-024-00906-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Workforce stability
- Health human resources
- Recruitment strategies
- Retention strategies
Human Resources for Health
ISSN: 1478-4491
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Online First
- Interventions to suppress puberty in adolescents experiencing gender dysphoria or incongruence: a systematic review
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-5898-0900 Jo Taylor ,
- Alex Mitchell ,
- Ruth Hall ,
- Claire Heathcote ,
- Trilby Langton ,
- Lorna Fraser ,
- http://orcid.org/0000-0002-0415-3536 Catherine Elizabeth Hewitt
- Department of Health Sciences , University of York , York , UK
- Correspondence to Dr Jo Taylor, Health Sciences, University of York, York, North Yorkshire, UK; dohs-gender-research{at}york.ac.uk
Background Treatment to suppress or lessen effects of puberty are outlined in clinical guidelines for adolescents experiencing gender dysphoria/incongruence. Robust evidence concerning risks and benefits is lacking and there is a need to aggregate evidence as new studies are published.
Aim To identify and synthesise studies assessing the outcomes of puberty suppression in adolescents experiencing gender dysphoria/incongruence.
Methods A systematic review and narrative synthesis. Database searches (Medline, Embase, CINAHL, PsycINFO, Web of Science) were performed in April 2022, with results assessed independently by two reviewers. An adapted version of the Newcastle-Ottawa Scale for cohort studies was used to appraise study quality. Only moderate-quality and high-quality studies were synthesised. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines were used.
Results 11 cohort, 8 cross-sectional and 31 pre-post studies were included (n=50). One cross-sectional study was high quality, 25 studies were moderate quality (including 5 cohort studies) and 24 were low quality. Synthesis of moderate-quality and high-quality studies showed consistent evidence demonstrating efficacy for suppressing puberty. Height increased in multiple studies, although not in line with expected growth. Multiple studies reported reductions in bone density during treatment. Limited and/or inconsistent evidence was found in relation to gender dysphoria, psychological and psychosocial health, body satisfaction, cardiometabolic risk, cognitive development and fertility.
Conclusions There is a lack of high-quality research assessing puberty suppression in adolescents experiencing gender dysphoria/incongruence. No conclusions can be drawn about the impact on gender dysphoria, mental and psychosocial health or cognitive development. Bone health and height may be compromised during treatment. More recent studies published since April 2022 until January 2024 also support the conclusions of this review.
PROSPERO registration number CRD42021289659.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/archdischild-2023-326669
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Increasing numbers of children and adolescents experiencing gender dysphoria/incongruence are being referred to specialist gender services.
National and international guidelines have changed over time and outline that medications to suppress puberty can be considered for adolescents experiencing gender dysphoria/incongruence.
Several systematic reviews report a limited evidence base for these treatments, and uncertainty about the benefits, risks and long-term effects.
WHAT THIS STUDY ADDS
No high-quality studies were identified that used an appropriate study design to assess the outcomes of puberty suppression in adolescents experiencing gender dysphoria/incongruence.
There is insufficient and/or inconsistent evidence about the effects of puberty suppression on gender-related outcomes, mental and psychosocial health, cognitive development, cardiometabolic risk, and fertility.
There is consistent moderate-quality evidence, although from mainly pre-post studies, that bone density and height may be compromised during treatment.
HOW THIS STUDY MIGHT AFFECT RESEARCH, POLICY OR PRACTICE
There is a lack of high-quality evidence to support the use of puberty suppression in adolescents experiencing gender dysphoria/incongruence, and large well-designed research is needed.
Introduction
Over the last 10-15 years, increasing numbers of children and adolescents experiencing gender dysphoria/incongruence are being referred to specialist paediatric gender services. 1 2
Gender dysphoria/incongruence in childhood is associated with high rates of co-occurring mental health and psychosocial difficulties, which can affect health and well-being. 3 Clinical guidelines recommend psychosocial care to alleviate gender-related distress and any co-occurring difficulties. For pubertal adolescents, medications to suppress or lessen effects of puberty are also outlined. Gonadotropin-releasing hormone analogues (GnRH-a) are used as first-line treatment, although other drugs with anti-androgenic properties including progestins and spironolactone are used in this population. 4 5 The effects differ depending on whether they are initiated in early puberty or mid-puberty, as well as the type of intervention used, with GnRH-a suppressing puberty when started early or suspending further progression when initiated in mid-puberty, and anti-androgens instead blocking specific downstream effects of sex hormones. 4
Rationales for puberty suppression in the Dutch treatment protocol, which has informed practice internationally, were to alleviate worsening gender dysphoria, allow time for gender exploration, and pause development of secondary sex characteristics to make passing in the desired gender role easier. 6 Practice guidelines propose other indications for puberty suppression, including allowing time and/or capacity for decision-making about masculinising or feminising hormone interventions, and improving quality of life. 4 7 8
Criteria in early treatment protocols for puberty suppression specified adolescents be at least age 12 years, at Tanner stage 2 in puberty, experienced gender dysphoria in childhood which persisted and intensified during puberty and met criteria for diagnosis of gender dysphoria. 6 It was also expected that any psychosocial difficulties that could interfere with treatment were managed. 6 The World Professional Association for Transgender Health standards of care 4 and other practice guidelines 5 8 9 have broadened these criteria, for example, removing minimum age. However, other recent guidelines have taken a more cautious approach and restricted inclusion criteria in response to uncertainties in the evidence base. 7 10
Systematic reviews have consistently found mainly low-quality evidence, limited data on key outcomes or long-term follow-up. 11–16 These reviews report that while puberty suppression may offer some benefit, there are concerns about the impact on bone health, and uncertainty regarding cognitive development, psychosocial outcomes and cardiometabolic health. They conclude there is insufficient evidence to support clinical recommendations.
The proliferation of research in this area and lack of evidence to support practice means there is an ongoing need to aggregate evidence. This systematic review aims to synthesise evidence published to April 2022 that reports outcomes of puberty suppression in adolescents experiencing gender dysphoria/incongruence.
The review forms part of a linked series examining the epidemiology, care pathways, outcomes and experiences for children and adolescents experiencing gender dysphoria/incongruence and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 17 The protocol was registered on PROSPERO (CRD42021289659. 18
Search strategy
A single search strategy was used to identify studies comprising two combined concepts: ‘children’, which included all terms for children and adolescents and ‘gender dysphoria’, which included associated terms such as gender-related distress and gender incongruence, and gender identity terms including transgender, gender diverse and non-binary.
MEDLINE ( online supplemental table S1 ), EMBASE and PsycINFO through OVID, CINAHL Complete through EBSCO, and Web of Science (Social Science Citation Index) were searched (13–23 May 2021 and updated on 27 April 2022).
Supplemental material
Reference lists of included studies and relevant systematic reviews were assessed for inclusion. 11–16 19 20
Inclusion criteria
The review included published research that reported outcomes of interventions used to suppress puberty for children and/or adolescents experiencing gender dysphoria/incongruence ( table 1 ).
- View inline
Inclusion and exclusion criteria
Selection process
The results of database and other searches were uploaded to Covidence 21 and screened independently by two reviewers. Full texts of potentially relevant articles were retrieved and reviewed against inclusion criteria by two reviewers independently. Disagreements were resolved through discussion and inclusion of a third reviewer.
Data extraction
Data on study characteristics, methods and reported outcomes were extracted into prepiloted data extraction templates by one reviewer and second-checked by another.
Study quality
Critical appraisal was undertaken by two reviewers independently, with consensus reached through discussion and involvement of a third reviewer where necessary.
Quality was assessed using a modified version ( online supplemental file 1 ) of the Newcastle-Ottawa Scale for cohort studies, a validated scale of eight items covering three domains: selection, comparability and outcome. 22 Scale modification included not scoring certain question(s) for cross-sectional and single-group designs, or particular outcomes; specification of key confounders to assess comparability of cohorts; guidance regarding sufficiency of follow-up and use of numerical scores for items and overall (maximum score 9 for cohorts, 8 for pre-post and cross-sectional studies with comparator). Total scores were calculated as percentages to account for different total scores (≤50% low quality, >50%–75% moderate quality, >75% high quality).
Narrative synthesis methods were used because of heterogeneity in study design, intervention, comparator, outcome and measurement. Due to high risk of bias in low-quality studies, these were excluded from the synthesis.
When synthesising results by outcome domains, care was taken to differentiate between different study designs, comparators and interventions. Where possible, potential differences in effects by birth-registered sex, treatment duration or treatment in early puberty versus late puberty were examined.
The database search yielded 28 147 records, 3181 of which were identified as potentially relevant for the linked systematic reviews and full texts reviewed. From these, 50 studies met inclusion criteria for this review ( figure 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Study flow diagram.
Study characteristics
Studies were published from 2006 to 2022 with the majority published in 2020–2022 (n=29). Studies were conducted in the Netherlands (n=17), 23–39 the US (n=15), 40–54 the UK (n=6), 55–60 Canada (n=4), 61–64 three in Belgium 65–67 and Israel 68–70 and one in Brazil 71 and Germany 72 ( online supplemental table S2 ).
The 50 studies included 11 cohorts comparing adolescents experiencing gender dysphoria/incongruence receiving puberty suppression with a comparator, 35 39–42 45 49 50 52 56 72 8 cross-sectional with a comparator 23 33 37 47 51 53 60 71 and 31 pre-post single group studies. 24–32 34 36 38 43 44 46 48 54 55 57–59 61–70 More than half of studies (n=29) used retrospective chart review.
All but 4 studies selected adolescents experiencing gender dysphoria/incongruence from specialist gender or endocrinology services: 43 from single services (in Belgium, Israel, the Netherlands and the UK these were large regional or national services) and 3 from multiple US services. 48–50 The other four included three US studies (national survey recruiting via community settings, 53 clinical and community settings, 51 US Military Healthcare Data Repository 54 ) and a study from Brazil recruiting via Facebook. 71
Overall, studies included 10 673 participants: 9404 were adolescents experiencing gender dysphoria/incongruence (4702 received puberty suppression, 4702 did not) and 1269 other comparators. Comparator groups included adolescents or adults experiencing gender dysphoria/incongruence who had not received puberty suppression, 35 39 40 42 51–53 60 71 72 untreated adolescents not experiencing gender dysphoria/incongruence, 36 47 50 both of these comparators 23 33 37 56 or adolescents receiving treatment for a different medical reason. 41 45 49
Most studies (n=39) assessed GnRH-a. In one, some participants received GnRH-a and some (birth-registered males) spironolactone. 62 In another, GnRH-a or progestins/anti-androgens were used but numbers taking each were not reported. 40 Among the other 11 studies, 5 assessed effects of progestins (cyproterone acetate, 66 67 lynestrenol, 65 66 medroxyprogesterone 44 and levonorgestrel-releasing intrauterine system 41 ) as alternatives to GnRH-a, 41 44 65–67 1 assessed bicalutamide 46 and 5 did not specify. 43 52–54 71
Of the 50 studies, 29 reported outcomes for feminising or masculinising hormones as well as for puberty suppression, either by including a mixed sample of those receiving the two different interventions or by assessing those who progressed to hormones following puberty suppression.
The most frequently measured outcomes were puberty suppression (n=30) and physical health outcomes (n=27) ( figure 2 , online supplemental table S3 ). Gender-related outcomes and body image were measured in five and four studies, respectively. Psychological health was measured in 13 studies, psychosocial in 9 studies and cognitive/neurodevelopmental outcomes in 3 studies. Side effects were reported in six, bone health in nine, and one study measured fertility.
Outcome categories by study quality and design.
One cross-sectional study was rated high quality, 37 25 moderate quality 23 24 29–32 34–36 39 48–51 54–59 64 65 67–69 and 24 low quality. 25–28 33 38 40–47 52 53 60–63 66 70–72 Of the 11 cohort studies, which were the only studies to include a comparator and assess outcomes over time, only 5 were rated moderate quality ( figure 2 , online supplemental table S4 ). 35 39 49 50 56
In most studies, there were concerns about sample representativeness due to single site recruitment, inclusion of a selected group and/or poor reporting of the eligible population. In studies including a comparator, most did not report or control for key differences between groups and only four used matched controls. 23 33 41 47 Most studies presented results for birth-registered males and females separately or controlled for this. Few studies controlled for age or Tanner stage or co-interventions that could influence outcomes.
Overall, studies used appropriate methods to ascertain exposure and assess outcomes. Adequacy of follow-up was evident in 18 studies, with multiple studies not reporting treatment duration, including participants receiving treatment at baseline, and not aligning follow-up with treatment initiation. Missing data at follow-up/analysis or poor reporting of this affected many studies.
Four studies did not report separate outcome data for adolescents receiving puberty suppression or masculinising/feminising hormones. 39 54 60 71 Two of these were of moderate quality and not included in the synthesis, 39 54 one of which was the only study to assess fertility outcomes. 39 One moderate-quality study assessed amplitude of click-evoked otoacoustic emissions. 23 This was excluded from the synthesis on the basis of not being clinically relevant.
Synthesis of outcomes
Gender dysphoria and body satisfaction.
Two pre-post studies measured gender dysphoria and body satisfaction (with primary and secondary sex or neutral body characteristics) and reported no change before and after receiving treatment 24 55 ( table 2 ).
Gender-related, body image, psychological, psychosocial, and cognitive/neurodevelopmental outcomes
Psychological health
One cross-sectional 37 and two pre-post studies 24 55 measured symptoms of depression (n=1), anxiety (n=1), anger (n=1), internalising and externalising symptoms (n=3), suicide and/or self-harm (n=2) and psychological functioning (n=2).
Three studies assessed internalising and externalising symptoms with one reporting improvements in both (pre-post 24 ), one improvement in internalising but not externalising symptoms when compared with adolescents under assessment by a gender service (cross-sectional 37 ) and one observed no change in either (pre-post). 55
For other psychological outcomes, there was either a single study, or two studies showing inconsistent results, with studies reporting either a small to moderate significant improvement or no change ( table 2 ).
Psychosocial outcomes
One cohort 56 and two pre-post 24 55 studies measured psychosocial functioning, one pre-post study assessed quality of life 55 and one cross-sectional study measured peer-relations ( table 2 ). 37
For psychosocial functioning, both pre-post studies reported no clinically significant change at follow-up. 24 55 The cohort study compared adolescents who were not immediately eligible for puberty suppression and received psychological support only, and adolescents who additionally received GnRH-a after 6 months. 56 Improvements were seen in both groups after 6 months of psychological support. This improvement was maintained over time for those receiving psychological support only. For those receiving GnRH-a, further improvements were observed at 12 and 18 months. At 18 months, psychosocial functioning in this group was considerably higher than in those still waiting for puberty suppression, and similar to adolescents not experiencing gender dysphoria/incongruence. However, there were considerably fewer participants included at final follow-up.
There was no change in quality of life pre-post, 55 and treated adolescents had better peer-relations compared with adolescents under assessment at a gender service but poorer peer-relations than adolescents not experiencing gender dysphoria/incongruence. 37
Cognitive/neurodevelopmental outcomes
One cross-sectional study measured executive functioning and found no difference between adolescents who were treated for <1 year compared with those not treated, but worse executive functioning in those treated for >1 year compared with those not treated. 51 A pre-post study found no differences in features typically associated with autism spectrum condition after treatment ( table 2 ). 59
Physical health outcomes
Bone health.
Five studies found decreases in bone mineral apparent density and z-scores pre-post treatment; however, absolute measures generally remained stable or increased/decreased slightly. 29 32 34 55 58 Results were similar across birth-registered males and females. 29 32 55 58 One study considered timing of treatment, and found similar decreases among those starting GnRH-a in early or late puberty ( table 3 ). 32
Physical health outcomes and side effects
Cardiometabolic health
Twelve pre-post studies measured body mass index (BMI), and in 10 studies there was no evidence of a clinically significant change in BMI and/or BMI SD score. 29 30 32 34 55 57 65 67–69 In one study, BMI increased for birth-registered males but not females. 58 Another study found BMI increased for birth-registered females who started GnRH-a in early puberty or mid-puberty, and birth-registered males in early puberty. 36
Three studies assessed cholesterol markers, one after GnRH-a (no changes), 34 one after cyproterone acetate (decrease in high-density lipoprotein (HDL) and triglycerides) 67 and one after lynestrenol (decrease in HDL, increase in low-density lipoprotein). 65 Three studies assessing GnRH-a reported blood pressure: two found similar systolic and diastolic blood pressure before and after treatment, 34 68 and one found a non-clinically significant increase in diastolic but not systolic blood pressure. 69 Two studies measured markers of diabetes (fasting glucose, HbA1c and/or insulin) and noted no changes. 65 67
Other physiological parameters
Five pre-post studies assessed other parameters from blood tests undertaken at baseline and follow-up, 30 31 34 65 67 three in those treated with GnRH-a, 30 31 34 one lynestrenol 65 and one cyproterone acetate. 67 Measurements included haemoglobin count (n=3), haematocrit percentage (n=3), creatinine (n=4), aspartate aminotransferase (n=3), alanine aminotransferase (n=3), γ-glutamyl transferase (n=1), alkaline phosphatase (n=2), prolactin (n=2), free thyroxin (n=3), thyroid-stimulating hormone (n=3), sex hormone binding globulin (n=3), vitamin D levels (n=1), dehydroepiandrosterone sulfate (n=3) and androstenedione (n=2). For most outcomes, no changes were reported. Where there were changes, these were not consistent in direction across studies.
One pre-post study assessing GnRH-a reported QTc prolongation, 64 and found no change in mean QTc, with no participants outside normal range.
Side effects
A cohort study of GnRH-a reported side effects including mild headaches or hot flushes (~20%) and moderate/severe headaches or hot flushes, mild fatigue, mood swings, weight gain and sleep problems (<10%) ( table 3 ). 55
Two studies assessed other medications and reported headaches and hot flushes as common and an increase in acne in a sample of birth-registered females receiving lynestrenol, 65 and complaints of fatigue in birth-registered males receiving cyproterone acetate. 67
Puberty suppression
Hormone levels.
Hormone levels were reported in nine studies of GnRH-a (two cohort, 49 50 seven pre-post 30 34 36 48 55 68 69 ), two in birth-registered females, 34 69 one in birth-registered males 68 and six including both ( table 4 ). 30 36 48–50 55
Puberty suppression outcomes
Five studies reported decreases in luteinising hormone, follicle-stimulating hormone, oestradiol and testosterone after receiving GnRH-a. 30 34 48 68 69 Another study, which reported luteinising and follicle-stimulating hormones, also found decreases in both pre-post. 55 One study reported that where baseline levels were high due to puberty starting, decreases were reported in testosterone and oestradiol. 36 One cohort study reporting pre-post data found smaller decreases in luteinising hormone, follicle-stimulating hormone, oestradiol and testosterone compared with other studies; however, it included a younger population, some of who were likely prepubertal. 50 The other cohort study included a comparator of adolescents with precocious puberty and found similar decreases in luteinising hormone and oestradiol. 49
One pre-post study of lynestrenol (birth-registered females) found a decrease in luteinising hormones but not follicle-stimulating hormone, oestradiol or testosterone. 65 One study of cyproterone acetate (birth-registered males) found no changes in luteinising hormone, follicle-stimulating hormone or oestradiol, but a decrease in total testosterone. 67
Pubertal progression
Puberty development was reported in four studies (two cohort, two pre-post). 30 35 49 67 One only included birth-registered males, 67 and three included both birth-registered males and females. 30 35 49
A cohort study assessing GnRH-a reported clinical pubertal escape in 2/21 adolescents treated for gender dysphoria/incongruence, in the form of breast enlargement or testicular enlargement together with deepening of voice, compared with no children treated for precocious puberty. 49 A pre-post study reported a decrease in testicular volume in birth-registered males, but unclear results with regard to breast development in birth-registered females (most started treatment at Tanner stage 4–5). 30 A pre-post study of birth-registered males using cyproterone acetate reported decreases in facial shaving and spontaneous erections. 67
A cohort study assessed whether secondary sex characteristics differed depending on receipt or timing of GnRH-a, and whether this affected which surgical interventions/techniques were later used. 35 The study found breast size was smallest in birth-registered females who received GnRH-a in Tanner stage 2/3 and largest in untreated participants. Those treated early in puberty were less likely to require a mastectomy and when surgery was required it was less burdensome. In birth-registered males, penile length was greater in those who received GnRH-a at Tanner stage 4/5 compared with Tanner stage 2/3, and greatest in untreated participants. 35 Those who received GnRH-a early required more invasive vaginoplasty techniques than those who received it later or not at all.
Menstrual suppression
Three studies (one cohort, two pre-post) measured menstrual suppression in birth-registered females, and found full suppression at follow-up, 30 49 55 which was similar to the effect seen in those with precocious puberty in the cohort study. 49
Height/Growth
Eleven studies (1 cohort, 50 10 pre-post 29 30 32 34 36 55 57 58 65 67 ) reported height, nine after GnRH-a, 29 30 32 34 36 50 55 57 58 one lynestrenol 65 and one cyproterone acetate. 67 The cohort study found a similar height velocity between the GnRH-a group and adolescent controls. 50 Six studies reported height Z or SD score 29 30 34 55 57 67 with two studies finding no change, 34 55 two a decrease for birth-registered males but not females, 29 57 one a decrease across birth-registered males and females 30 and one a decrease in birth-registered males with cyproterone acetate. 67 Absolute measures of height generally increased slightly or remained the same. 29 30 32 34 36 58 65 67
Body composition
Two studies reported changes in body composition pre-post, 30 57 reporting a significant decrease in lean mass SD score 57 and percentage 30 in males and females. One also measured body fat percentage and reported significant increases in both groups. 30
Bone geometry
One pre-post study measured the subperiosteal width and endocortical diameter of the hip bone and found that in birth-registered males these increased in those starting GnRH-a in early puberty and mid-puberty, but only in the early puberty group for birth-registered females. 36
This systematic review identified 50 studies reporting outcomes relating to puberty suppression in adolescents experiencing gender dysphoria/incongruence. No high-quality studies using an appropriate design were identified, and only four measured gender dysphoria as an outcome. Only 5 of the 11 cohort studies, which were the only studies to compare groups over time, were rated as moderate quality. 35 40 49 50 56
There was evidence from multiple mainly pre-post studies that puberty suppression exerts its expected physiological effect, as previously demonstrated in children with precocious puberty. 73 In adolescents experiencing gender dysphoria/incongruence, puberty suppression is initiated at different stages of puberty, 74 and two studies found that the effects on secondary sex characteristics may vary depending on whether treatment is initiated in early puberty versus mid-puberty, with potentially different outcomes for birth-registered males and females. 30 35 Multiple studies also found that bone density is compromised during puberty suppression, and gains in height may lag behind that seen in other adolescents. High-quality research is needed to confirm these findings; however, these potential risks should be explained to adolescents considering puberty suppression.
These findings add to other systematic reviews in concluding there is insufficient and/or inconsistent evidence about the effects of puberty suppression on gender dysphoria, body satisfaction, psychological and psychosocial health, cognitive development, cardiometabolic risk and fertility. 11–16 Regarding psychological health, one recent systematic review 14 reported some evidence of benefit while others have not. The results in this review found no consistent evidence of benefit. Inclusion of only moderate-quality to high-quality studies may explain this difference, as 8 of the 12 studies reporting psychological outcomes were rated as low-quality.
The lack of representativeness of samples and comparability of selected control groups were key concerns across studies. Only one study attempted to compare puberty suppression with psychosocial care, which is the only other treatment offered for gender dysphoria/incongruence in childhood, and this included a small sample, limited analyses, and little detail about the intervention. 56 Other studies lacked information about any psychological care provided to participants, and in studies that included a comparator there was limited information about any differences between groups. Large, well-designed studies with appropriate comparators that enable long-term outcomes of puberty suppression to be measured are needed.
Many studies reported effects of both puberty suppressants and hormones used in later adolescence for feminisation/masculinisation. In adolescents, GnRH-a often continues during hormone treatment, 74 or for adolescents who do not receive puberty suppression, GnRH-a or other anti-androgens may be offered at initiation of hormones. 66 This makes long-term follow-up of puberty suppression difficult to assess, including any differences between the types of interventions that are offered and when these are initiated, and the few studies reporting long-term outcomes either did not control for this or reported overall effects for both interventions. Although recent studies suggest nearly all adolescents who receive puberty suppression go on to feminising/masculinising hormones, 74–76 research is still needed to assess whether suppression will have any lasting effects for those who do not. Aggregation of studies reporting proportions of adolescents who progress to hormones and reasons for discontinuation would also offer useful insights.
Included studies assessed different outcomes across various outcome domains and employed multiple different measures. Agreement about the primary aim and related core outcomes of puberty suppression in this population would help to ensure studies measure key outcomes and facilitate future aggregation of evidence. Expert consensus recommendations to guide the methods and domains for assessing the neurodevelopmental effects of puberty suppression have been developed 77 ; however, there is currently no agreement across other outcome domains.
Strengths and limitations
Strengths include a published protocol with robust search strategies, use of PRISMA guidelines and comprehensive synthesis of moderate and high quality studies. Poor reporting across studies may have resulted in moderate-quality studies being rated low-quality and excluded from synthesis. As searches were conducted up to April 2022, this review does not include more recently published studies. However, the findings are in line with previous reviews despite the inclusion of numerous additional studies. In an update of the National Institute for Health and Care Excellence evidence review of GnRH-a performed in April 2023, 78 nine additional studies were identified, two of which they felt might materially affect their conclusions. 72 74 One was already included in this review, 72 and the other examined treatment trajectories which was not an outcome of interest. 74
Of other studies that we are aware have been published since April 2022 until January 2024, very few used a cohort design or an appropriate comparator and were of a similar low quality to moderate quality as the studies summarised in this review. Of those likely to contribute new data for synthesis, five examined physical growth and development, 79–83 one cardiometabolic health 84 and one psychological health. 85 The latter, a study from the US, found that adolescents who received puberty suppression before assessment for masculinising or feminising hormones had fewer symptoms of depression, anxiety, stress and suicidal thoughts compared with those who had not received puberty suppression. A sensitivity analysis found similar results, although no difference in suicidal thoughts. 85 Adding this study would provide no further clarity about whether puberty suppression improves psychological health due to the inconsistency of results between studies, and the limited high-quality research measuring these outcomes.
Two studies from the Netherlands found that height growth and bone maturation both decelerated during GnRH-a treatment, 80 81 and a third assessing only bone health found the same. 83 A Belgian study found stable height growth in birth-registered females but deceleration in birth-registered males. 82 These studies add strength to the conclusion that bone health and adult height may be compromised during GnRH-a, although like in previous studies the participants went on to receive masculinising or feminising hormones, and therefore the long-term outcomes of puberty suppression alone were not possible to determine.
Another new study, also from the Netherlands, assessed changes in body composition. 79 This found that in both birth-registered males and females lean mass z-scores decreased during puberty suppression and fat mass z-scores increased, although the rate and duration of change differed by birth-registered sex. These changes were also found in the two studies synthesised, 30 57 but as all three included no comparator uncertainty continues about the effect of puberty suppression on body composition.
A large study of adults in the US examined whether receipt of hormone interventions during adolescence was associated with cardiometabolic-related diagnoses, and for GnRH-a found no statistically significant differences for any diagnosis. 84 However, the study uses a retrospective cross-sectional design and is the only study to have examined cardiometabolic diagnoses, so no conclusions can be drawn about these outcomes.
To our knowledge, there are no additional moderate-quality or high-quality studies that have measured psychosocial or fertility outcomes, and only a single study assessing cognitive effects which measured a different outcome (white matter microstructure) to those included in this review. 86
Conclusions
There are no high-quality studies using an appropriate study design that assess outcomes of puberty suppression in adolescents experiencing gender dysphoria/incongruence. No conclusions can be drawn about the effect on gender-related outcomes, psychological and psychosocial health, cognitive development or fertility. Bone health and height may be compromised during treatment. High-quality research and agreement on the core outcomes of puberty suppression are needed.
Ethics statements
Patient consent for publication.
Not applicable.
- Thompson L ,
- Sarovic D ,
- Wilson P , et al
- Kaltiala R ,
- Bergman H ,
- Carmichael P , et al
- Coleman E ,
- Bouman WP , et al
- Hembree WC ,
- Cohen-Kettenis PT ,
- Gooren L , et al
- de Vries ALC ,
- Cohen-Kettenis PT
- The Swedish National Board of Health and Welfare
- Telfer MM ,
- Tollit MA ,
- Pace CC , et al
- University of California San Francisco Gender Affirming Health Program
- ↵ Medical treatment methods for Dysphoria associated with variations in gender identity in minors – recommendation . 2020 . Available : https://palveluvalikoima.fi/en/recommendations#genderidentity
- National Institute for Health and Care Excellence (NICE)
- Pasternack I ,
- Söderström I ,
- Saijonkari M , et al
- Ludvigsson JF ,
- Adolfsson J ,
- Höistad M , et al
- Wilson LM ,
- Sharma R , et al
- Anderson J ,
- Williams K , et al
- McKenzie JE ,
- Bossuyt PM , et al
- Taylor J , et al
- Ramos GGF ,
- Mengai ACS ,
- Daltro CAT , et al
- Veritas Health Innovation
- O’Connell D , et al
- van Heesewijk JO ,
- Menks WM , et al
- Steensma TD ,
- Doreleijers TAH , et al
- McGuire JK ,
- Steensma TD , et al
- Delemarre-van de Waal HA ,
- de Mutsert R ,
- Wiepjes CM , et al
- van der Loos MATC , et al
- Heijboer A , et al
- Schagen SEE ,
- Delemarre-van de Waal HA , et al
- Lustenhouwer P ,
- Cohen-Kettenis PT , et al
- Wouters FM ,
- Staphorsius AS ,
- Kreukels BPC ,
- Stoffers IE ,
- de Vries MC ,
- van de Grift TC ,
- van Gelder ZJ ,
- Mullender MG , et al
- van der Loos MA ,
- Hellinga I ,
- Vlot MC , et al
- van der Miesen AIR ,
- de Vries ALC , et al
- den Heijer M , et al
- Mulder CL ,
- Meißner A , et al
- Achille C ,
- Taggart T ,
- Eaton NR , et al
- Ford N , et al
- Grimstad FW ,
- Jacobson JD
- Stewart S ,
- Preston S , et al
- Khandheria MM ,
- Mejia-Otero JD ,
- Nokoff NJ ,
- Scarbro SL ,
- Moreau KL , et al
- Olson-Kennedy J ,
- Streeter LH ,
- Garofalo R , et al
- Pine-Twaddell E ,
- Newfield RS ,
- Marinkovic M
- Schulmeister C ,
- Millington K ,
- Kaufman M , et al
- Strang JF ,
- Nelson E , et al
- Tordoff DM ,
- Collin A , et al
- Turban JL ,
- Carswell JM , et al
- Hisle-Gorman E ,
- Schvey NA ,
- Adirim TA , et al
- Carmichael P ,
- Masic U , et al
- Dunsford M ,
- Skagerberg E , et al
- Ghelani R ,
- Brain C , et al
- Russell I ,
- Pearson B ,
- Arcelus J ,
- Witcomb GL , et al
- Chiniara LN ,
- Bonifacio HJ ,
- Khatchadourian K ,
- Khatchadourian K , et al
- Waldner RC ,
- Atallah J , et al
- Dhondt K , et al
- Lapauw B , et al
- Craen M , et al
- Elkon-Tamir E ,
- Segev-Becker A , et al
- Segev-Becker A ,
- Israeli G , et al
- Israeli G ,
- Elkon-Tamir E , et al
- Fontanari AMV ,
- Vilanova F ,
- Schneider MA , et al
- Becker-Hebly I ,
- Fahrenkrug S ,
- Campion F , et al
- Hou L , et al
- van der Loos MATC ,
- Hannema SE , et al
- Carruthers P , et al
- Hannema SE ,
- Klink DT , et al
- Kolbuck VD , et al
- NHS England
- Boogers LS ,
- Reijtenbagh SJP ,
- Wiepjes CM ,
- Willemsen LA ,
- Ciancia S ,
- Valentine A ,
- Furniss A , et al
- McGregor K ,
- McKenna JL ,
- Williams CR , et al
- van Heesewijk J ,
- Steenwijk MD ,
- Kreukels BPC , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
- Data supplement 3
- Data supplement 4
- Data supplement 5
Contributors LF, CEH, RH, TL and JT contributed to the conception of this review. RH, CEH, CH, AM and JT contributed to screening and selection. AM and JT completed data extraction. CEH, RH, AM and JT contributed to critical appraisal. CEH, AM and JT completed the synthesis and drafted the manuscript. All authors contributed to interpretation and reviewed and approved the manuscript prior to submission. CEH accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding This work was funded by NHS England to inform the Cass Review (Independent review of gender identity services for children and young people). The funder and Cass Review team had a role in commissioning the research programme but no role in the study conduct, interpretation or conclusion.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Linked Articles
- Original research Clinical guidelines for children and adolescents experiencing gender dysphoria or incongruence: a systematic review of guideline quality (part 1) Jo Taylor Ruth Hall Claire Heathcote Catherine Elizabeth Hewitt Trilby Langton Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326499
- Original research Care pathways of children and adolescents referred to specialist gender services: a systematic review Jo Taylor Ruth Hall Trilby Langton Lorna Fraser Catherine Elizabeth Hewitt Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326760
- Original research Psychosocial support interventions for children and adolescents experiencing gender dysphoria or incongruence: a systematic review Claire Heathcote Jo Taylor Ruth Hall Stuart William Jarvis Trilby Langton Catherine Elizabeth Hewitt Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326347
- Original research Gender services for children and adolescents across the EU-15+ countries: an online survey Ruth Hall Jo Taylor Claire Heathcote Trilby Langton Catherine Elizabeth Hewitt Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326348
- Original research Impact of social transition in relation to gender for children and adolescents: a systematic review Ruth Hall Jo Taylor Catherine Elizabeth Hewitt Claire Heathcote Stuart William Jarvis Trilby Langton Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326112
- Original research Characteristics of children and adolescents referred to specialist gender services: a systematic review Jo Taylor Ruth Hall Trilby Langton Lorna Fraser Catherine Elizabeth Hewitt Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326681
- Original research Clinical guidelines for children and adolescents experiencing gender dysphoria or incongruence: a systematic review of recommendations (part 2) Jo Taylor Ruth Hall Claire Heathcote Catherine Elizabeth Hewitt Trilby Langton Lorna Fraser Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326500
- Original research Masculinising and feminising hormone interventions for adolescents experiencing gender dysphoria or incongruence: a systematic review Jo Taylor Alex Mitchell Ruth Hall Trilby Langton Lorna Fraser Catherine Elizabeth Hewitt Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2023-326670
- Editorial Holistic approach to gender questioning children and young people Camilla C Kingdon Archives of Disease in Childhood 2024; - Published Online First: 09 Apr 2024. doi: 10.1136/archdischild-2024-327100
Read the full text or download the PDF:

IMAGES
VIDEO
COMMENTS
Systematic Review A summary of the clinical literature. A systematic review is a critical assessment and evaluation of all research studies that address a particular clinical issue. The researchers use an organized method of locating, assembling, and evaluating a body of literature on a particular topic using a set of specific criteria.
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study.
In this review, the authors highlight pivotal studies of historical interest and review contemporary randomized controlled trials (RCTs) that have impacted the clinical management of patients with resectable, non-metastatic colon cancer published since our institution's previous systematic literature review .
A literature review differs from a systematic review, which addresses a specific clinical question by combining the results of multiple clinical trials (an article on this topic will follow as part of this series of publications). A formal literature review is also an extension of the information gathering you might do to get a personal insight ...
The statistical methodology used to synthesize results in such a review is called 'meta-analysis'. There are five types of clinical systematic reviews described in this article (see Fig. . 1 ), including intervention, diagnostic test accuracy, prognostic, methodological and qualitative. This review will provide a very brief overview in a ...
For many SUDs, still no effective pharmacotherapies exist. Distinct psychoactive and somatic effects of the iboga alkaloids set them apart from classic hallucinogens like LSD, mescaline, and psilocybin. Aims: The study team performed this systematic review focusing on clinical data and therapeutic interventions involving ibogaine and noribogaine.
Literature reviews are most commonly performed to help answer a particular question. While you are at medical school, there will usually be some choice regarding the area you are going to review. Once you have identified a subject area for review, the next step is to formulate a specific research question. This is arguably the most important ...
ing. A literature review differs from a systematic review, which addresses a specific clinical question by combining the results of multiple clinical trials (an article on this topic will follow as part of this series of publications). A formal literature review is also an extension of the information
A literature review is defined as "a critical analysis of a segment of a published body of knowledge through summary, classification, and comparison of prior research studies, reviews of literature, and theoretical articles." (The Writing Center University of Winconsin-Madison 2022) A literature review is an integrated analysis, not just a summary of scholarly work on a specific topic.
The PICO model is widely used and taught in evidence-based health care as a strategy for formulating questions and search strategies and for characterizing clinical studies or meta-analyses . PICO stands for four different potential components of a clinical question: Patient, Population or Problem; Intervention; Comparison; Outcome.
Review. A clinical trial involves the study of t he effect of an investigational drug/any other intervention in a defined. population/participant. The clinica l research includes a treatment group ...
A literature review can broadly be described as a more or less systematic way of collecting and synthesizing previous research (Baumeister & Leary, 1997; Tranfield, Denyer, & Smart, ... Meta-analysis in clinical trials. Controlled Clinical Trials, 7 (1986), pp. 177-188, 10.1016/0197-2456(86)90046-2. View PDF View article Google Scholar. Edeling ...
Thus, in the past five years, the published records have almost tripled (743 records). Although most of the included studies lack rigorous clinical study designs (case reports, case series, retrospective surveys, observational studies), they suggest beneficial effects of ibogaine and noribogaine on OWS in patients who seek opioid abstinence.
Traditional clinical review articles, also known as updates, differ from systematic reviews and meta-analyses. Updates selectively review the medical literature while discussing a topic broadly ...
clinical trials registers represent a key resource for this search. Clinical trials registers are publicly available online registers of planned, ongoing, and completed ... Our advice is based on: a review of the literature; information available on registry websites; an online survey and consensus workshop on 18 June 2021 among coauthors (the ...
Background Evidence-based clinical practice is challenging in all fields, but poses special barriers in the field of rare diseases. The present paper summarises the main barriers faced by clinical research in rare diseases, and highlights opportunities for improvement. Methods Systematic literature searches without meta-analyses and internal European Clinical Research Infrastructure Network ...
Any non-randomized studies, including (but not limited to) parallel non-randomized clinical trials, single-arm clinical trials, case studies and reports, and any observational studies. Reviews, including systematic literature reviews. Editorials, letters, and commentaries. Others: Language: English. Publication years: 1990 to November 2, 2020
After careful review, a total of eighteen studies were excluded: four were excluded because they lacked guidance on review methodology; four were excluded because the methodology was irrelevant to urban planning (e.g., reviews of clinical trials); one was excluded because it was not written in English; six studies were excluded because they ...
Authors of a systematic review ask a specific clinical question, perform a comprehensive literature review, eliminate the poorly done studies, and attempt to make practice recommendations based on the well-done studies. Systematic reviews include only experimental, or quantitative, studies, and often include only randomized controlled trials.
Clinical practice guidelines (CPGs) translate evidence into recommendations to improve patient care and outcomes. To provide an overview of schizophrenia CPGs, we conducted a systematic literature ...
This scoping review aims to identify hindering and facilitating factors that impact the recruitment of HLAOA in clinical trials in the United States. Methods Two databases (PubMed, EMBASE) were searched for original research articles from inception until March 2022 reporting on factors that engaged HLAoa (≥65) in clinical trials.
Clinical trials registers are a key resource for identifying potentially eligible studies, particularly those that are unpublished, and therefore searching these registers is mandated for best practice systematic reviews. ... Our advice is based on: a review of the literature; information available on registry websites; an online survey and ...
This article provides a case series review of pediatric CdLS patients alongside a comprehensive literature review, exploring clinical variability and the relationship between genotypic changes and phenotypic outcomes. ... Wu, L.; Deng, F. Clinical study and genetic analysis of Cornelia de Lange syndrome caused by a novel MAU2 gene variant in a ...
A systematic review was performed following PRISMA guidelines using Pubmed (MEDLINE), EMBASE, and Cochrane databases to search for relevant studies published in the last twenty years. Results Thirty-one studies were included with a total of 39,505 implant patients and mean age of 44.2 ± 9.30 years.
Finally, SRs can be used to inform clinical trial design and establish whether there is evidence to justify a clinical trial. 29,46,58 Retrospective preclinical SRs for interventions that failed in clinical trials have demonstrated that prospective SRs of the animal literature would have concluded that there was insufficient evidence of effect ...
The last systematic literature review examining telehealth effectiveness was conducted in 2010. Given the increasing use of telehealth and technological developments in the field, a more contemporary review has been carried out. ... and highlights the importance of studies that match clinical practice. Apart from clinical effectiveness ...
A comprehensive review of the literature was done till May 2022 and yielded 814 articles after ranking the articles according to authors and year of publication. Only 8 clinical trials and cohort studies fulfilling the inclusion criteria were included for further steps of data collection, analysis, and reporting. This meta-analysis involved ...
Disease progression and mortality. Among included studies, all-cause mortality was mainly reported as deaths due to ESRD. Seven publications from mainland China [23, 26,27,28,29, 41, 42], 7 from Korea [64,65,66, 68, 70, 71, 73], 4 from Japan [50, 51, 57, 62], and 1 from Taiwan [] reported rate of progression to ESRD in IgAN.These studies varied in the definition of endpoint, patient ...
Original studies focusing on recruitment and retention of LT's were included. An inductive content analysis was performed to identify strategies used to facilitate LT recruitment and retention. A separate grey literature review was conducted from June-July 2023. 12 studies were retained. Over half (58%) of studies were conducted in North ...
Background Treatment to suppress or lessen effects of puberty are outlined in clinical guidelines for adolescents experiencing gender dysphoria/incongruence. Robust evidence concerning risks and benefits is lacking and there is a need to aggregate evidence as new studies are published. Aim To identify and synthesise studies assessing the outcomes of puberty suppression in adolescents ...