

Class 9 Science Case Study Questions Chapter 4 Structure of Atom
- Post author: studyrate
- Post published:
- Post category: class 9th
- Post comments: 0 Comments
Case study Questions on Class 9 Science Chapter 4 are very important to solve for your exam. Class 9 Science Chapter 4 Class 9 Science Case Study Questions have been prepared for the latest exam pattern. You can check your knowledge by solving case study-based questions for Class 9 Science Chapter 4 Structure of Atom
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Structure of Atom Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 4 Structure of Atom
Case Study/Passage-Based Questions
Case Study 1: The maximum number of electrons that are permitted to be assigned to an energy shell of an atom is called the electron capacity of that shell. The distribution of electrons in different orbits or shells is governed by a scheme known as the Bohr-Bury scheme. According to this scheme : (i) The maximum number of electrons that can be present in any shell is given by the formula 2n2 where n is the number of energy levels. (ii) The maximum number of electrons that can be accommodated in the outermost shell is 8. Electrons are filled in the shells in a stepwise manner in increasing the order of energy of the energy shell.
What is the maximum electron capacity of the N shell? (a) 24 (b) 8 (c) 18 (d) 32
Answer: (d) 32
Identify the element with the configuration K-2, L-8, M-3. (a) Aluminium (b) Magnesium (c) Sodium (d) Beryllium
Answer: (a) Aluminium
Which of the following configuration represent sodium? (a) 2, 8, 4 (b) 2, 8, 5 (c) 2, 3 (d) 2, 8, 1
Answer: (d) 2, 8, 1
Case Study/Passage Based Questions
The given diagrams show the atomic structures of elements X and Y.
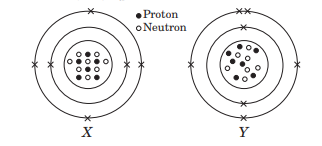
Element X and Y could be _ and _ respectively. (a) Be and B (b) C and O (c) F and N (d) C and N
Answer: (d) C and N
Valency of elements X and Y are respectively, (a) 4 and 3 (b) 2 and 5 (c) 1 and 4 (d) 3 and 4
Answer: (a) 4 and 3
Elements X and Y are (a) isotopes (b) isoelectronic (c) isobars (d) isomers.
Answer: (c) isobars
Case Study 2: The mass of an atom is due to the masses of protons and neutrons in the nucleus. The relative masses of protons and neutrons are almost equal to one. Therefore, the atomic mass of an element should be nearly a whole number. But in many cases the atomic masses are fractional. The main reason for these fractional atomic masses is that these elements occur in nature as a mixture of several isotopes. The atomic mass of an element is the average of the atomic masses of these isotopes in the ratio of their proportion of occurrence.
Chlorine occurs in nature in the form of two isotopes with atomic masses 35 u and 37 u in the ratio of 3 : 1 respectively. The atomic mass of chlorine is (a) 35.5 u (b) 34.5 u (c) 35 u (d) 36 u
Answer: (a) 35.5 u
An element occurs in two isotopic forms with atomic masses 10 and 11. What is the percentage abundance of two isotopes in the sample having an atomic mass of 10.80? (a) 20, 80 (b) 50, 50 (c) 25, 70 (d) 60, 40
Answer: (a) 20, 80
The fractional atomic masses of elements are due to the existence of (a) isotopes having different masses (b) diagonal relationship (c) equal number of electrons and protons (d) none of these.
Answer: (a) isotopes having different masses
Case Study 3: The structure of an atom consists of subatomic particles, mainly protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, which is the central region of an atom. Protons carry a positive charge, while neutrons are electrically neutral. Electrons, on the other hand, are negatively charged particles that orbit around the nucleus in energy levels or shells. The number of protons in an atom determines its atomic number, which is unique to each element. The mass number of an atom is the sum of its protons and neutrons. The atomic mass of an element is the weighted average mass of all its naturally occurring isotopes. Isotopes are atoms of the same element that have different numbers of neutrons. The arrangement and distribution of these subatomic particles determine the overall stability and properties of an atom.
Which subatomic particles are mainly located in the nucleus of an atom? a) Protons and electrons b) Neutrons and electrons c) Protons and neutrons d) Protons, neutrons, and electrons Answer: c) Protons and neutrons
What is the charge of protons? a) Positive b) Negative c) Neutral d) Variable Answer: a) Positive
Where are electrons located in an atom? a) Nucleus b) Energy levels or shells c) Protons d) Neutrons Answer: b) Energy levels or shells
What determines the atomic number of an atom? a) Number of electrons b) Number of protons c) Number of neutrons d) Sum of protons and neutrons Answer: b) Number of protons
What are atoms of the same element with different numbers of neutrons called? a) Isotopes b) Ions c) Elements d) Electrons Answer: a) Isotopes
Hope the information shed above regarding Case Study and Passage Based Questions for Class 9 Science Chapter 4 Structure of Atom with Answers Pdf free download has been useful to an extent. If you have any other queries about CBSE Class 9 Science Structure of Atom Case Study and Passage Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible By Team Study Rate
You Might Also Like
Class 9 mcq questions for chapter 11 work and energy with answers.

1569+ Class 9 Science MCQ Questions with Answers PDF Download
Rs aggarwal class 9 pdf with solutions, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom
- 23rd December 2023
NCERT Solutions for Class 9 Science (Chemistry) Chapter 4 Structure of the Atom provides detailed answers for all in-text and exercise Questions. These solutions contain an in-depth explanation of each topic involved in the chapter. All these solutions are prepared by expert teachers and updated for the current academic session.
NCERT Solutions for Class 9 Science (Chemistry) Chapter 4 Structure of the Atom Pure help students to understand the fundamental concepts given in class 9 Science textbook. We have prepared the answers to all the questions in an easy and well-structured manner. It helps students to grasp the chapter easily.
CBSE Class 9 Science Structure of the Atom Intext Questions (Solved)
PAGE NO – 39
Question 1: What are canal rays?
Answer: Canal rays are positively charged radiations. These rays consist of positively charged particles known as protons. They were discovered by Goldstein in 1886.
Question 2: If an atom contains one electron and one proton, will it carry any charge or not?
Answer: If an atom contains one electron and one proton, it will not carry any net charge. This is because a proton has a positive charge and an electron has a negative charge, and these two charges are equal in magnitude but opposite in sign. In such an atom, the positive charge of the proton and the negative charge of the electron will cancel each other out, resulting in a neutral overall charge. An example of such an atom is hydrogen, which typically has one proton and one electron in its most common form.
PAGE NO. 41
Question 1: On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer: According to Thomson’s model of the atom, an atom consists of both negatively and positively charged particles. The negatively charged particles are embedded in the positively charged sphere. These negative and positive charges are equal in magnitude. Thus, by counterbalancing each other’s effect, they make an atom neutral.
According to Thomson’s model, an atom is neutral because it contains an equal amount of positive and negative charges that balance each other out. In this model, the atom is like a positively charged sphere with negatively charged electrons embedded in it. The positive charge is spread out over the entire sphere, and the electrons are placed within this sphere. Since the number of negative charges (electrons) equals the number of positive charges in the sphere, the overall charge of the atom is neutral. This balance of equal and opposite charges ensures that the atom does not have a net charge.
Question 2: On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Answer: On the basis of Rutherford’s model of an atom, protons (positively-charged particles) are present in the nucleus of an atom.
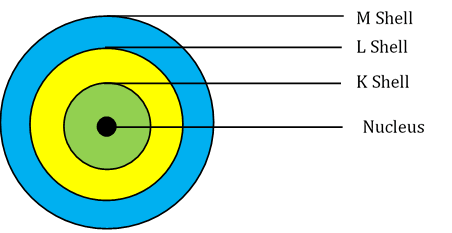
Question 3: Draw a sketch of Bohr’s model of an atom with three shells.
Question 4: What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of a metal other than gold?
Answer: If the α-scattering experiment is carried out using a foil of metal rather than gold, there would be no change in the observation. In the α-scattering experiment, a gold foil was taken because gold is malleable and a thin foil of gold can be easily made. It is difficult to make such foils from other metals.
Question 1: Name the three sub-atomic particles of an atom.
Answer: The three sub-atomic particles of an atom are:
- Protons
- Electrons, and
Question 2: Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer: Helium atom has two neutrons. The mass of an atom is the sum of the masses of protons and neutrons present in its nucleus.
Since helium atom has two protons, mass contributed by the two protons is (2 × 1) u = 2 u. Then, the remaining mass (4 − 2) u = 2 u is contributed by 2u /1u = 2 neutrons.
PAGE NO. 42
Question 1: Write the distribution of electrons in carbon and sodium atoms?
Answer: The total number of electrons in a carbon atom is 6. The distribution of electrons in carbon atom is given by:
Or, we can write the distribution of electrons in a carbon atom as 2, 4. The total number of electrons in a sodium atom is 11. The distribution of electrons in sodium atom is given by:
Or, we can write distribution of electrons in a sodium atom as 2, 8, 1.
Question 2: If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer: The maximum number of electrons that can occupy K and L-shells of an atom are 2 and 8 respectively. Therefore, if K and L-shells of an atom are full, then the total number of electrons in the atom would be (2 + 8) = 10 electrons.
PAGE NO. 43
Question 1: How will you find the valency of chlorine, sulphur and magnesium?
Answer: If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell.
On the other hand, if the number of electrons in the outermost shell of the atom of an element is greater than 4, then the valency of that element is determined by subtracting the number of electrons in the outermost shell from 8.
The distribution of electrons in chlorine, sulphur, and magnesium atoms are 2, 8, 7; 2, 8, 6 and 2, 8, 2 respectively. Therefore, the number of electrons in the outermost shell of chlorine, sulphur, and magnesium atoms are 7, 6, and 2 respectively.
Thus, The valency of chlorine = 8 −7 = 1 The valency of sulphur = 8 − 6 = 2 The valency of magnesium = 2
Question 1: If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom and (ii) what is the charge on the atom?
Answer: (i) The atomic number is equal to the number of protons. Therefore, the atomic number of the atom is 8.
(ii) Since the number of both electrons and protons is equal, therefore, the charge on the atom is 0.
Question 2: With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Answer: Mass number of oxygen = Number of protons + Number of neutrons = 8 + 8 = 16
Mass number of sulphur = Number of protons + Number of neutrons = 16 +16 = 32
PAGE NO. 45
Question 1: For the symbol H, D and T tabulate three sub – atomic particles found each of them.
Question 2: Write the electronic configuration of any pair of isotopes and isobars.
Answer: Isotopes: Isotopes are atoms which have the same number of protons but the number of neutrons differs. This leads to the variation in mass number too.
Two isotopes of carbon are 6 𝐶 12 and 6 𝐶 14 The electronic configuration of 6 𝐶 12 is 2, 4. The electronic configuration of 6 𝐶 14 is 2, 4. [Isotopes have same electronic configuration]
Isobars: Isobars are atoms which have the same mass number but differ in the atomic number. Electronic configuration of an isobar pair is as follows,
29 Ca 40 and 8 𝐴𝑟 40 are a pair of isobars of calcium. The electronic configuration of 29 Ca 40 is 2, 8, 8, 2 The electronic configuration of 18 𝐴𝑟 40 is 2, 8, 8. [Isobars have different electronic configuration]
NCERT Class 9 Science Structure of the Atom Exercise Questions (Solved)
Question 1: Compare the properties of electrons, protons and neutrons.
Question 2: What are the limitations of J.J. Thomson’s model of the atom?
Answer: J.J. Thomson’s model of the atom, known as the “plum pudding model,” had a few key limitations:
1. According to J.J. Thomson’s model of an atom, an atom consists of a positively charged sphere with electrons embedded in it. However, later Rutherford’s gold foil experiment showed that the positively charged particles reside at the center of the atom called the nucleus, and the electrons revolve around the nucleus.
2. Thomson’s model was unable to explain many chemical properties of elements, such as why atoms combine in fixed ratios to form compounds.
3. It didn’t clearly distinguish between the nucleus and the rest of the atom, and couldn’t explain why atoms are stable.
Question 3: What are the limitations of Rutherford’s model of the atom?
Answer: According to Rutherford’s model of an atom, electrons revolve around the nucleus in fixed orbits. But, an electron revolving in circular orbits will not be stable because, during the revolution, it will experience acceleration. Due to acceleration, the electrons will lose energy in the form of radiation and fall into the nucleus. In such a case, the atom would be highly unstable and collapse.
Question 4: Describe Bohr’s model of the atom.
Answer: Bohr’s model of the atom Niels Bohr proposed the following postulates regarding the model of the atom.
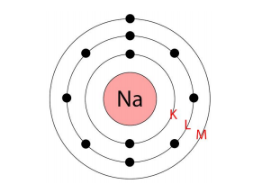
- Only certain orbits known as discrete orbits of electrons are allowed inside the atom.
- While revolving in these discrete orbits, the electrons do not radiate energy. These discrete orbits or shells are shown in the following diagram.
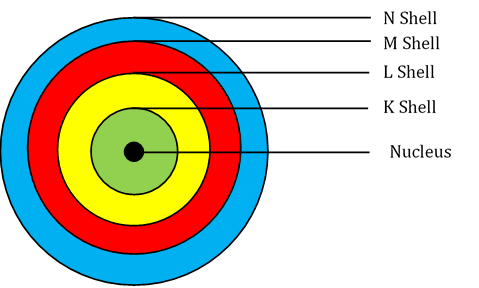
The first orbit (i.e., for n = 1) is represented by letter K. Similarly, for n = 2, it is L − shell, for n = 3, it is M − shell and for n = 4, it is N − shell. These orbits or shells are also called energy levels.
Question 5: Compare all the proposed models of an atom given in this chapter.
Question 6: Summarize the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Answer: The rules for writing of the distribution of electrons in various shells for the first eighteen elements are given below:
(i) The maximum number of electrons that a shell can accommodate is given by the formula ‘2n 2 ‘, where ‘n’ is the orbit number or energy level index (n = 1, 2, 3…). Now, Maximum number of electrons in different shells are: K shell n = 1: 2n 2 = 2(1) 2 = 2 L shell n = 2: 2n 2 = 2(2) 2 = 8 M shell n = 3: 2n 2 = 2(3) 2 = 18 N shell n = 4: 2n 2 = 2(4) 2 = 32
(ii) The outermost orbit can be accommodated by a maximum number of 8 electrons.
(iii) Shells are filled with electrons in a step-wise manner i.e., the outer shell is not occupied with electrons unless the inner shells are completely filled with electrons.
Question 7: Define valency by taking examples of silicon and oxygen.
Answer: Valency is the measure of the ability of an atom to combine with other atoms. It is determined by the number of electrons an atom can lose, gain, or share to become stable.
- Example with Silicon : Silicon has 4 electrons in its outermost shell. It can share these 4 electrons with other atoms to complete its outer shell, typically holding 8 electrons. So, the valency of silicon is 4, as it can form bonds by sharing 4 electrons.
- Example with Oxygen : Oxygen has 6 electrons in its outer shell. It needs 2 more electrons to complete its shell. So, it can either gain 2 electrons or share 2 electrons with other atoms. Therefore, the valency of oxygen is 2.
Question 8. Explain with examples: (i) Atomic number (ii) Mass number, (iii) Isotopes and (iv) Isobars. Give any two uses of isotopes.
(i) Atomic number: The atomic number of an element is the total number of protons present in the atom of that element. For example, nitrogen has 7 protons in its atom. Thus, the atomic number of nitrogen is 7.
(ii) Mass number: The mass number of an element is the sum of the number of protons and neutrons present in the atom of that element. For example, the atom of boron has 5 protons and 6 neutrons. So, the mass number of boron is 5 + 6 = 11.
(iii) Isotopes: They are atoms of the same element and have same atomic number but different mass number/atomic mass. For example: C 12 and 6 C 14
(iv) Isobars: They are atoms of different elements having same mass number but different atomic number. For example calcium, atomic number 20 and argon, atomic number 18. The number of electrons in these atoms is different, but the mass number of both these elements is 40. That is, the total number of neutrons is the same in the atoms of this pair of elements.
Two uses of isotopes are as follows:
- An isotope of uranium is used as a fuel in nuclear reactors.
- An isotope of cobalt is used in the treatment of cancer.
Question 9: Na has completely filled K and L shells. Explain.
Answer: Na has atomic number 11, so its electronic configuration is = 2, 8, 1 Sodium atom (Na) looses 1 electron to become stable and form Na + ion. so its electronic configuration is = 10 = 2, 8. The above configuration indicates completely filled K, L shells.
Question 10: If bromine atom is available in the form of, say, two isotopes 35 𝐵𝑟 79 (49.7%) and 35 𝐵𝑟 81 (50.3%), calculate the average atomic mass of bromine atom.
Answer: The average atomic mass of bromine
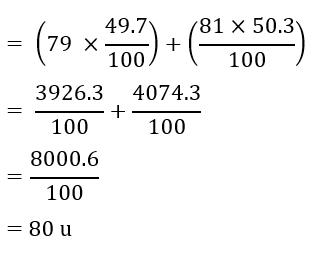
Question 11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 16 8 X and 18 8 X in the sample?
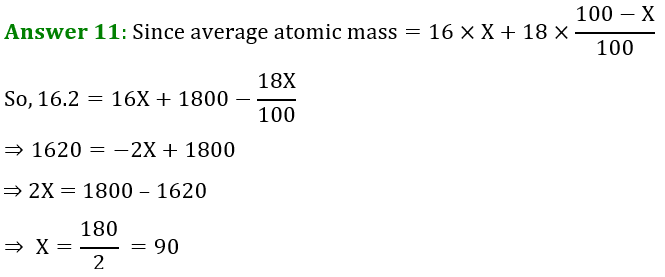
Question 12: When 90% is the X-16 sample so for X-18 sample % = 100-90=10% If Z = 3, what would be the valency of the element? Also, name the element.
Answer: By Z = 3, we mean that the atomic number of the element is 3. Its electronic configuration is 2, 1. Hence, the valency of the element is 1 (since the outermost shell has only one electron). Therefore, the element with Z = 3 is lithium.
Question 13: Composition of the nuclei of two atomic species X and Y are given as under
Give the mass numbers of X and Y. What is the relation between the two species?
Answer: Mass number of X = Number of protons + Number of neutrons = 6 + 6 = 12
Mass number of Y = Number of protons + Number of neutrons = 6 + 8 = 14
These two atomic species X and Y have the same atomic number, but different mass numbers. Hence, they are isotopes.
Question 14. For the following statements, write T for true and F for false. (a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons. (b) A neutron is formed by an electron and a proton combining together. Therefore it is neutral. (c) The mass of an electron is about 1/2000 times that of proton. (d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Solution: (a) False (b) False (c) True (d) False
Put a tick(✓) against correct choice and cross(x) against wrong choice in questions 15, 16 and 17.
Question 15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of (a) Atomic nucleus (b) Electron (c) Proton (d) Neutron
Answer: (a) Atomic nucleus
Question 16. Isotopes of an element have (a) The same physical properties (b) Different chemical properties (c) Different number of neutrons (d) Different atomic numbers.
Answer: (c) Different number of neutrons
Question 17. Number of valence electrons in Cl − ion are: (a) 16 (b) 8 (c) 17 (d) 18
Answer: (b) 8
Electronic distribution of Cl is K-2, L-8, M-7. Valence electrons are 7, hence chlorine gains one electron for the formation of Cl – . Therefore, its valency is 8.
Question 18. Which one of the following is a correct electronic configuration of Sodium? (a) 2, 8 (b) 8, 2, 1 (c) 2, 1, 8 (d) 2, 8, 1
Answer: (d) 2, 8, 1
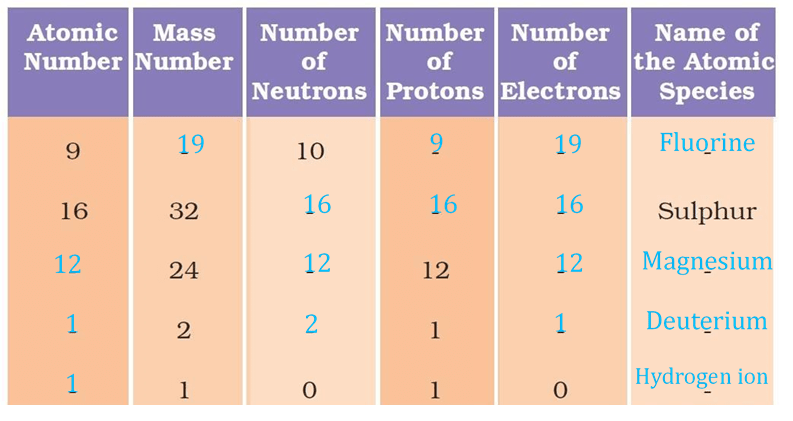
Question 19: Complete the following table.
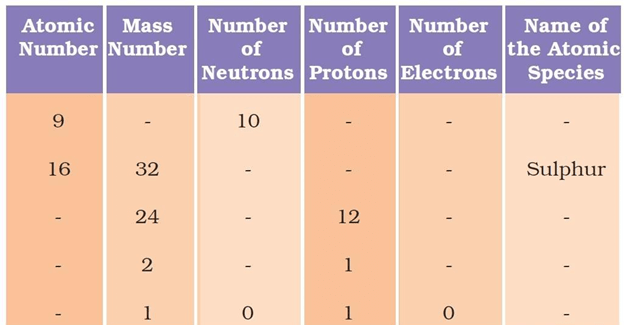
Answer: The following table depicts the missing data: Atomic number(Z) =Number of protons Mass number = Number of neutrons + atomic number (or) Mass number(A) = Number of neutrons + number of neutrons
Leave a Reply Cancel Reply
Your email address will not be published. Required fields are marked *
Name *
Email *
Add Comment *
Post Comment
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom in Hindi and English Medium prepared for new session 2024-25. Class 9 Science chapter 4 is modified and revised as per the new rationalised NCERT textbooks issued for academic year 2024-25.
Class 9 Science Chapter 4 Question Answers
- Class 9 Science Chapter 4 Exercises
- Class 9 Science Chapter 4 Intext Questions
- Class 9 Science Chapter 4 Extra Questions
- Class 9 Science Chapter 4 Hindi Medium
- Class 9 Science Chapter 4 Notes in English
- Class 9 Science Chapter 4 Notes in Hindi
- Class 9 Science Chapter 4 NCERT Book
- Class 9 Science NCERT Solutions
- Class 9 all Subjects NCERT Solutions
NCERT Class 9 Science textbook, Chapter 4 Structure of the Atom delves into the structure and components of atoms, providing insight into the fundamental building blocks of matter. The main topics covered in Chapter 4 are the concept of the atom as the basic unit of matter, Dalton’s Atomic Theory that is a brief overview of Dalton’s atomic theory and its postulates. Here we will study about Charged Particles in Matter which provides the explanation of charged particles in matter, including electrons, protons, and neutrons.
Distribution of Electrons in Different Orbits (Shells) Class 9 students learn in chapter 4 about explanation of electron distribution in shells or energy levels and introduction to the K, L, M, and N shells. It also explains the definition of valency and its significance, determining valency based on electron distribution. Here, we learn about Atomic Number and Mass Number with explanation of atomic number and its significance, definition of mass number and how it is determined. Class 9 science chapter 4 explores about the definition of isotopes and examples, explanation of isotopic notation.
A recap of the main concepts and ideas presented in Chapter 4 Structure of the Atom, provides a foundational understanding of the structure and components of atoms. It introduces students to the historical development of atomic models and sets the stage for further exploration of atomic and molecular structures in subsequent chapters and classes.
Preparing for NCERT Class 9 Science Chapter 4 Structure of the Atom, requires a proper approach to understanding the fundamental concepts related to atomic structure. Here are some steps and planning strategies for students to prepare this chapter 4 of 9th science effectively. First of all, read the Chapter Thoroughly, begin by reading the chapter carefully from the NCERT textbook. Pay attention to definitions, examples, and key points. While reading, take detailed notes on important concepts, definitions, and key terms.
To make science easy, summarize the main ideas of each section. Understand the historical development of atomic models, including Dalton’s atomic theory, J.J. Thomson’s experiments, Rutherford’s gold foil experiment, and Bohr’s model. Know the key contributions of these scientists. Study the components of an atom, including electrons, protons, and neutrons. Understand their properties, locations, and charges. Learn about Bohr’s model of the hydrogen atom, including the concept of energy levels or orbits and how it explains spectral lines.
Class IX Science chapter 4 Exercise and Intext question answers in PDF format to free download. Class 9 UP Board students also get here UP Board Solutions. Class 9 Science chapter 4 Intext questions given on Page 43 or Page 49 or Page 50 or Page 52 or Page 53 and Exercises in English Medium are available.
You can download these solutions from Page 53 ke Uttar or Page 56 ke Uttar or Page 57 ke Uttar or Page 58 ke uttar or Page 59 ke Uttar or Page 60 ke Uttar and Abhyaas ke Uttar to study online or in PDF file format. NCERT Solutions and Offline Apps for all other subjects based on latest CBSE Syllabus are also available to download.
Electron Distribution and Valency Understand how electrons are distributed in different shells or energy levels (K, L, M, N). Learn the maximum number of electrons each shell can hold. Know the definition of valency and how it relates to electron distribution. Practice determining the valency of elements based on their electron configuration. Learn how to calculate the number of protons, electrons, and neutrons in an atom. Isotopes: Understand isotopic notation.
Practice solving numerical problems related to atomic structure, atomic number, mass number, and electron distribution. Work through exercises and examples in the textbook. Utilize diagrams and visual aids in the chapter to help understand atomic structures and models. Supplement your learning with online resources, videos, and interactive simulations related to atomic structure. These can provide additional clarity. Group study can offer different perspectives and help reinforce your understanding.
Regularly revise your notes and the key points of the chapter 4 in 9th science. Repetition is key to retaining information. Closer to your exams, practice solving sample papers and previous year’s question papers to familiarize yourself with the exam pattern and practice time management. If you have doubts or find certain topics challenging, don’t hesitate to seek help from your teacher or classmates. Clarify your doubts as soon as possible. By following these planning strategies and staying organized, you can effectively prepare for NCERT Class 9 Science Chapter 4 and build a strong foundation in atomic structure and chemistry concepts.
Extra Questions on 9th Science Chapter 4
Is atomic number of an atom always equal to the number of electrons.
No, it is the case when the atom has no charge. In case of cation (positively charged), atomic number is more than the number of electrons and in case of anion (negatively charged), it is less than the number of electrons.
Nucleus of an atom has positive charge on it. Establish.
This can be established on the basis of Rutherford scattering experiment. Since some alpha particles were repelled by the nucleus of the atom, it is expected to have the same charge as on alpha particles. Therefore, nucleus of an atom has positive charge.
Were neutrons known at the time Rutherford performed the scattering experiment?
No, these were discovered later on by Chadwick in 1931 whereas scattering experiment was performed by Rutherford in 1911.
Why are isotopes of an element chemically similar?
Isotopes of an elements have same number of electrons and therefore, same value shell electronic distribution. Since the chemical properties of the atoms are related to valence shell configurations, the isotopes are chemically similar.
Why do elements which exist as isotopes have fractional atomic masses?
The different isotopes of an element differ in their mass number as well as atomic masses. In order to represent the atomic mass of the element, we have to consider average of the atomic masses of the different isotopes and also the ratio in which these are present. In most of the cases, the average comes out to be in fraction. Therefore, these elements have fractional atomic masses.
Why is a proton not a universal particle like electron?
A proton is the positively charged residue left when hydrogen gas is enclosed in the in the discharge tube. For the other gases, the positive residues formed contain different number of protons. Therefore, proton is not a universal particle like electron.
Tiwari Academy plays a significant role in helping students prepare for NCERT Class 9 Science Chapter 4. Here’s how Tiwari Academy can assist students in their preparation for this chapter. It provides detailed solutions to all the questions and exercises in the NCERT Class 9 Science textbook for Chapter 4. These solutions can help students understand how to approach and solve different types of problems related to the structure of atoms.
Important Questions on 9th Science Chapter 4
What are the limitations of j.j. thomson’s model of the atom.
According to J.J. Thomson’s model of an atom, an atom consists of a positively charged sphere with electrons embedded in it. However, it was later found that the positively charged particles reside at the center of the atom called the nucleus, and the electrons revolve around the nucleus.
What are the limitations of Rutherford’s model of the atom?
According to Rutherford’s model of an atom, electrons revolve around the nucleus in fixed orbits. But, an electron revolving in circular orbits will not be stable because during revolution, it will experience acceleration. Due to acceleration, the electrons will lose energy in the form of radiation and fall into the nucleus. In such a case, the atom would be highly unstable and collapse.
Define valency by taking examples of silicon and oxygen.
The valency of an element is the combining capacity of that element. The valency of an element is determined by the number of valence electrons present in the atom of that element. If the number of valence electrons of the atom of an element is less than or equal to four, then the valency of that element is equal to the number of valence electrons. For example, the atom of silicon has four valence electrons. Thus, the valency of silicon is four. On the other hand, if the number of valence electrons of the atom of an element is greater than four, then the valency of that element is obtained by subtracting the number of valence electrons from eight. For example, the atom of oxygen has six valence electrons. Thus, the valency of oxygen is (8 − 6) i.e., two.
Explain Atomic number with examples.
Atomic number: The atomic number of an element is the total number of protons present in the atom of that element. For example, nitrogen has 7 protons in its atom. Thus, the atomic number of nitrogen is 7.
What do you understand by isobars?
Isobars: They are atoms of different elements having same mass number but different atomic number. For example calcium, atomic number 20 and argon, atomic number 18. The number of electrons in these atoms is different, but the mass number of both these elements is 40. That is, the total number of neutrons is the same in the atoms of this pair of elements.
If Z = 3, what would be the valency of the element? Also, name the element.
By Z = 3, we mean that the atomic number of the element is 3. Its electronic configuration is 2, 1. Hence, the valency of the element is 1 (since the outermost shell has only one electron). Therefore, the element with Z = 3 is lithium.
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Intext Questions and chapter end exercise question answers are given below to free download. All the solutions are free to download without any registrations or login. We have updated all the contents on the basis of user’s suggestions and Feedback received from last academic session.
The Tiwari Academy website and apps offer explanations and videos that break down complex concepts in the chapter. Visual aids and clear explanations can help students grasp difficult topics more easily. It also provide historical context and explanations for the development of atomic models, making it easier for students to understand the evolution of atomic theory. The platform offer visual representations and interactive simulations of atomic structures, which can enhance students’ understanding of the arrangement of electrons, protons, and neutrons within an atom.
Questions related to class 9 science chapter 4, atomic structure and atomic models are frequently included in Class 9 Science examinations. Students can expect to see questions about atomic components, electron distribution, atomic number, and mass number in their exams. The chapter covers the historical development of atomic models, including Dalton’s atomic theory, J.J. Thomson’s experiments, Rutherford’s gold foil experiment, and Bohr’s model. Understanding the history of atomic theory is often tested in exams.
Question 1: An electron is regarded as a universal particle. Explain. Answer 1: The value of charge (e) and mass (m) of the electron always remain the same whatever may be the source of their emission. In the discharge tube, the electrons may be emitted either from the cathode or form the gas enclosed in the discharge tube. Whatever may be the metal which forms the cathode or the gas present in the discharge tube, these values remain the same. Therefore, electron is regarded as a universal particle.
Question 2: Why do the element helium, neon and argon have zero valency? Answer 2: Helium has two electrons in its only energy shell (K-shell). The other two elements have eight electrons in their valence shells. Since these are the maximum number of electrons which the atoms of these elements can have therefore, they do not have any urge or desire to take part in chemical combination. These elements are known as zero valent elements. They have therefore, valency equal to zero.
Class 9 Science chapter 4 bridges the gap between chemistry and physics by introducing concepts related to atomic structure. It sets the stage for students to explore topics like the periodic table, chemical bonding, and atomic physics in later classes. Knowledge of atomic structure is relevant to various scientific fields, including chemistry, physics, and materials science. Exams include questions that require students to apply their understanding of atomic structure to real-world scenarios. A strong foundation in Chapter 4 is, therefore, essential for success in future science studies.
Finally, the NCERT Class 9 Science Chapter 4, Structure of the Atom, is important not only for scoring well in exams but also for laying the groundwork for a deeper understanding of chemistry and physics concepts. Students should dedicate time and effort to comprehensively learn the concepts presented in this chapter to ensure success in their science studies.
« NCERT Solutions Class 9 Science Chapter 3
Ncert solutions class 9 science chapter 5 ».
Copyright 2024 by Tiwari Academy | A step towards Free Education

Talk to our experts
1800-120-456-456
- NCERT Solutions for Class 9 Science Chapter 4 - Structure Of The Atom
- NCERT Solutions

Download NCERT Solutions for Class 9 Science Chapter 4 - free PDF
Class 9 Science Chapter 4 describes the structure of the atom. In this chapter, students will discover novel ideas and hypotheses that have been developed and validated by several scientists. Applying the newly taught ideas would be much simpler if you use the Vedantu-prepared NCERT Solutions for Class 9 Science Chapter 4.
For CBSE Class 9 Chapter 4, Vedantu offers NCERT answers that have been created by subject-matter experts. To succeed in your studies, get the NCERT answer that our professionals have offered. The answers to the NCERT practice questions are provided in a step-by-step manner using the simple-to-understand format of pdf. The solutions to every question in the Class 9 curriculum are included in the NCERT answers pdf. Begin your study process under the direction of the Vedantu experts and ace your tests.
List of Topics Covered Under NCERT Solutions for Class 9 Science Chapter 4
A glance about the topic .
Atoms contains positively charged Protons, negatively charged electrons and the neutral charged Neutrons
Thomson model, Rutherford model and Bhor’s model attempted various experiments for finding the structure of atoms and position of electrons, protons and neutrons.
The total number of protons present in the atom is known as the atomic number.
The total number of protons and neutrons in the atom is known as the mass number.
An atom with the same atomic number and different mass number is known as Isotopes.
An atom with a different atomic number and the same mass number is known as Isobars.

Access NCERT Solutions for Science Class 9 Chapter 4 – Structure of the Atom
Intext Questions:
1. What are canal rays?
Ans: Canal rays are beams of positively charged ions. It was discovered in 1886 by Goldstein. Canal rays are also known as anode rays.
2. If an atom contains one electron and one proton, will it carry any charge or not?
Ans: An atom carrying one electron and one proton will carry no charge as the negatively charged particle will combine with the positively charged particles and the overall magnitude of the atom will be zero.
3. On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Ans: According to Thomson’s model of the atom:
1. An atom consists of both negatively and positively charged particles.
2. The negatively charged particles are embedded in the positively charged sphere.
3. These negative and positive charges are equal in magnitude.
4. They counterbalance each other’s effect and make an atom neutral.
4. On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Ans: According to Rutherford’s model of an atom, protons are present in the nucleus of an atom.
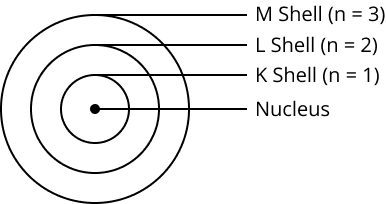
5. Draw a sketch of Bohr’s model of an atom with three shells.
6. Name the three subatomic particles of an atom.
Ans: Three subatomic particles of an atom are as follows:
ii. Electron
iii. Neutron.
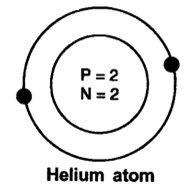
7. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Ans: Number of protons in Helium Atom = \[2\]
Atomic Mass = Number of Protons + Number of Neutrons
$4=2+\text{ Number of Neutrons}$
$ \text{Number of Neutrons = 4 - 2 = 2}$
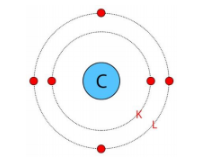
8. Write the distribution of electrons in carbon and sodium atoms?
Ans: Atomic number of carbon = \[6\] = Number of protons = Number of electrons
The distribution of electrons in carbon atom is given by:
First orbit or K-shell = $2$ electrons
Second orbit or L-shell = $4$ electrons
or, we can write the distribution of electrons in a carbon atom as $2,4$
Atomic number of sodium = $11$= Number of protons = Number of electrons
The distribution of electrons in sodium atom is given by:
Second orbit or L-shell = $8$ electrons
Third orbit or M-shell = $1$ electron
Or, we can write the distribution of electrons in a sodium atom as \[2,\text{ }8,1\].
9. If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Ans: Maximum number of electron in K-shell = $2$
Maximum number of electron in L-shell = $8$
If K and L-shells of an atom are full,
then the total number of electrons in the atom would be \[\left( 2\text{ }+\text{ }8 \right)\text{ }=\text{ }10\]electrons.
10. How will you find the valency of chlorine, sulphur and magnesium?
If the number of electrons in the outermost shell is less than $4$ then,
Valency of an atom = number of electrons in the outermost shell of the atom.
In the case of magnesium,
Thus, the valency of magnesium = $2$
If the number of electrons in the outermost shell is less than 4 then,
Valency of an atom = 8 – Number of electrons in the outermost shell.
In case of sulphur,
The valency of sulphur = 8 − 6 = 2
In case of chlorine,
The valency of chlorine = 8 −7 = 1
11. If the number of electrons in an atom is 8 and the number of protons are also 8, then
(i) What is the atomic number of the atom and (ii) What is the charge on the atom?
1. The atomic number = number of protons.
Hence, the atomic number of the atom is $8$.
2. Since the number of both electrons and protons is equal, therefore, the charge on the atom is $0$ i.e., it is a neutral atom.
12. With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Ans: Mass number = Number of protons + Number of neutrons
Mass number of $\mathrm{O}_{2}=8+8=16$
Mass number of $\mathrm{S}=16+16=32$
13. For the symbols H, D and T tabulate three sub-atomic particles found in each of them.
14. Write the electronic configuration of any one pair of isotopes and isobars.
Two isotopes of carbon are :
${ }_{6}^{12} \mathrm{C}$
${ }_{6}^{14} \mathrm{C}$
The electronic configuration of ${ }_{6}^{12} \mathrm{C}$ is $2,4$
The electronic configuration of ${ }_{6}^{14} \mathrm{C}$ is $2,4$
Two isobars of carbon are :
${ }_{20}^{40} \mathrm{Ca}$
${ }_{18}^{40} \mathrm{Ar}$
The electronic configuration of ${ }_{20}^{40} \mathrm{Ca}$ is \[\text{2, 8, 8, 2}\text{.}\]
The electronic configuration of ${ }_{18}^{40} \mathrm{Ar}$ is \[\text{2, 8, 8}\text{.}\]
Exercise Questions:
1. Compare the properties of electrons, protons and neutrons.
Ans: The difference between electron, proton and neutrons are as follows:
2. What are the limitations of J.J. Thomson’s model of the atom?
Ans: Limitations of J.J. Thomson’s model of the atom.
It fails to explain the stability of an atom.
It doesn’t talk about the nucleus of an atom.
It failed to explain the reason for positive and negative charges binding together.
It also doesn’t explain Rutherford’s model.
3. What are the limitations of Rutherford’s model of the atom?
Ans: Rutherford’s model of the atom fails to explain the stability of an atom. He argued that electrons move in a circular path called the orbit. The Revolution of electrons in the orbit will radiate energy which will make the atom unstable and electrons will fall inside the nucleus. But in reality this is not the case and Rutherford’s model fails to explain the reason for the same.
4. Describe Bohr’s model of the atom.
5. Compare all the proposed models of an atom given in this chapter.
Ans : Comparison of all models of an atom are given below:
6. Summarize the rules for the writing of the distribution of electrons in various shells for the first eighteen elements.
Ans: For the first eighteen elements, the rules for writing the distribution of electrons in various shells are as follows:
1. The maximum number of electrons that a shell can accommodate is determined by the formula ‘$\text{2}{{\text{n}}^{\text{2}}}$’, where ‘$\text{n}$’ is the orbit number \[\left( n\text{ }=\text{ }1,\text{ }2,\text{ }3\ldots \right).\] The maximum number of electrons present in an orbit of $\text{n = 1}$ is given by $\text{2}{{\text{n}}^{\text{2}}}=2{{(1)}^{2}}=2$
Again for second orbit, it is $\text{2}{{\text{n}}^{\text{2}}}=2{{(2)}^{2}}=2\times 4=8$
For third orbit, it is $\text{2}{{\text{n}}^{\text{2}}}=2{{(3)}^{2}}=2\times 9=18$
2. The outermost orbit can hold a maximum of eight electrons.
3. Shells are filled with electrons in a stepwise manner, with the inner shells being filled first, followed by the outer shells.
7. Define valency by taking examples of silicon and oxygen.
Ans: Valency of an element is defined as the number of electrons in its outermost shell.
In the case of silicon,
Outermost shell electron = $4$
If the number of electrons in the outermost shell of the atom of an element is less than or equal to $4$,
Valency of an atom = number of electrons in the outermost shell
Thus, the valency of silicon = $4$.
In the case of oxygen,
Outermost shell electron = \[6\]
If the number of electrons in the outermost shell of the atom of an element is greater than $4$,
Then, the valency of an atom = $8$ – Number of electrons in the outermost shell.
Thus, the valency of oxygen is \[\left( 8\text{ }-\text{ }6 \right)\text{ }=\text{ }2\].
8. Explain with examples.
i. Atomic number
ii. Mass number,
iii. Isotopes
iv. Isobars.
Give any two uses of isotopes.
i. Atomic Number: Total number of protons present in the atom of an element is called the atomic number of that element.
Example: Oxygen has $8$ protons. Thus, the atomic number of Oxygen is $8$.
ii. Mass Number: The sum of the number of protons and neutrons present in the atom of an element is called the mass number.
Example: Oxygen has $8$ protons and $8$ neutrons.
Therefore the mass number of oxygen is $8+8=16$
iii. Isotopes: Isotopes are atoms of the same element having the same atomic number, but different mass numbers.
Example : Three isotopes of Hydrogen are:
1) Protium $\left({ }^{1} \mathrm{H}\right)$
2) Deuterium $\left({ }_{1}^{2} \mathrm{H}\right)$
3) Tritium $\left({ }_{1}^{3} \mathrm{H}\right)$
(iv). Isobars: Isobars are atoms with the same mass number but different atomic numbers, i.e., isobars are atoms with the same mass number from different elements.
Example: ${ }_{20}^{40} \mathrm{Ca} \text { and }{ }_{18}^{40} \mathrm{Ar} \text { are two isobars. }$
9. Na+ has completely filled K and L shells. Explain.
Ans: Atomic number of Na = 11 = Total number of electrons
The electronic configuration of Na = 2, 8, 1.
The electronic configuration of Na+ ion = 2 (K-shell), 8 (L-shell)
Thereby Na+ ion has completely filling K and L shells.
10. If bromine atom is available in the form of, say, two isotopes ${ }_{35}^{79} \mathrm{Br}(49.7 \%) \text { and }{ }_{35}^{81} \mathrm{Br}(50.3 \%)$ calculate the average atomic mass of bromine atom.
Ans : Average atomic mass of Bromine atom
= \[\dfrac{\left[ 79\times 49.7~+~81\times 50.3 \right]}{100}\]
$ =\dfrac{3926.3+4074.3}{100}$
$ =\dfrac{8000.6}{100}$
=80.006
$\approx 80\text{u}$
11. The average atomic mass of a sample of an element X is \[16.2\text{ u}\]. What are the percentages of isotopes ${ }_{8}^{16} \mathrm{X}$ and ${ }_{8}^{18} \mathrm{X}$ in the sample?
Ans: Average atomic mass of an element X = \[16.2\text{ u}\]
Let percentage of isotope ${ }_{8}^{18} \mathrm{X}$ is $y%$
Thus, the percentage of isotope ${ }_{8}^{18} \mathrm{X}$ is $(100-y)%$
Average atomic mass of element X= [Atomic mass of ${ }_{8}^{18} \mathrm{X}$ $\times $ percentage + Atomic mass of ${ }_{8}^{16} \mathrm{X}$ $\times $ percentage ]
$16.2=[18 \times y \%+16 \times(100-y) \%]$
$16.2=\left[18 \times \dfrac{y}{100}+16 \times \dfrac{(100-y)}{100}\right]$
$16.2 \times 100=[18 y+1600-16 y]$
$1620=2 y+1600$
$1620-1600=2 y$
$y=\dfrac{20}{2}$
Thus, percentage of ${ }_{8}^{16} \mathrm{X}$ is $10%$
Percentage of ${ }_{8}^{16} \mathrm{X}$ is $(100-10)=90%$
12. If Z = 3, what would be the valency of the element? Also, name the element.
Ans: Atomic number \[=\text{ }Z\text{ }=\text{ }3.\]
Its electronic configuration is 2, 1.
Hence, the valency of the element is 1
(Since the outermost shell has only one electron).
Therefore, the element with Z = 3 is lithium (Li).
13. Composition of the nuclei of two atomic species X and Y are given as under X Y
Give the mass numbers of X and Y. What is the relation between the two species?
Mass number of \[X\text{ }=\text{ }6\text{ }+\text{ }6\text{ }=\text{ }12\]
Mass number = Number of protons + Number of neutrons
Mass number of \[Y\text{ }=\text{ }6\text{ }+\text{ }8\text{ }=\text{ }14\]
Atomic number = Number of protons
The atomic number of X= 6 = Atomic number of Y
These two atomic species X and Y have the same atomic number, but different mass numbers. Hence, they are isotopes.
14. For the following statements, write T for ‘True’ and F for ‘False’.
a). J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
Ans: False
b). A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
c). The mass of an electron is about 1/2000 times that of the proton.
Ans: True
d). An isotope of iodine is used for making tincture iodine, which is used as a medicine.
15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
Atomic nucleus
Ans: a) Atomic Nucleus
16. Isotopes of an element have
The same physical properties
Different chemical properties
Different number of neutrons
Different atomic numbers
Ans: c) Different numbers of neutrons.
Isotopes are atoms of the same element having the same atomic number, but different mass numbers.
17. Number of valence electrons in $\mathrm{Cl}^{-1}$ ion is:
Ans: Atomic number of $\mathrm{Cl}$ = $17$
Electronic configuration of $\mathrm{Cl}$ = \[2,\text{ }8,\text{ }7\]
Electronic configuration of $\mathrm{Cl}^{-1}$ ion \[=\text{ }2,\text{ }8,\text{ }8\]
Thus, the number of valence electrons in $\mathrm{Cl}^{-1}$ ion = $8$
18. Which one of the following is a correct electronic configuration of sodium?
Ans: Atomic number of sodium = = Number of electrons
So, electronic configuration of sodium = 2, 8, 1.
19. Complete the following table.
NCERT Solutions for Class 9 Science Chapter 4 - Structure of The Atom
You can opt for Chapter 4 - Structure of Atom NCERT Solutions for Class 9 Science PDF for Upcoming Exams and also You can Find the Solutions of All the Maths Chapters below.
Summary of Structure of the Atom Class 9
The 4 th chapter will deliver a constructed concept of how an atom is subdivided into subatomic particles. It will also tell you how scientists proved the existence of subatomic particles within an atom. The consecutive development of theories will make you understand how an atom stabilizes its charges. It is a fascinating chapter to learn and prepare a foundation to rely on. You will need these new concepts to study more advanced chapters in the future. To make it easier, you need to follow the Structure of The Atom Class 9 NCERT Solutions available here to find the right answers to all the questions.
Study the chapter well and focus on what the units are telling us to follow. Develop your concepts accordingly and find out new scientific facts about an atom and its subatomic particles. Proceed with proper attention. Once you finish a unit, solve the exercise, and judge your understanding of the concepts. You can also use NCERT Solutions for Class 9 Science Chemistry Chapter 4 as a platform to judge your base. Follow the best answers given in the NCERT Solutions for Class 9 Science Structure of The Atom to discover how to frame the perfect solution and score well in an exam.
How Should you Proceed with CBSE Class 9 Science Chapter 4 Solutions?
Here is what you need to do to study the new concepts of Class 9 Science Chapter 4.
1. Read the Chapter Thoroughly
The first step is to read the units of Ch 4 Science Class 9 properly and understand the new concepts. You must only proceed to the next unit when you are done with the previous one.
2. Answers the Exercise Questions
The next step is to find the questions in the unit exercise. Answer the questions on your own first. Seek the right answers from the NCERT Solutions Class 9 Science Structure of The Atom so that you can find out whether your answers were to the point or not.
3. Follow the format of NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom
When you are satisfied with your concept development, you need to follow the format used by the experts in NCERT Solutions for Class 9th Science Chapter 4 Structure of The Atom. The answers are based on the CBSE format. They are precise and easy to understand.
4. Focus on the Concept Delivery in NCERT Solutions for Class 9 Science Chapter Structure of The Atom
You will be able to understand how the concepts are focused and used to answer the questions in the NCERT Solutions Class 9 Science Chapter 4 Structure of The Atom. Just follow it and impress the examiner to score better.
Why Use the NCERT solution for Class 9 Science Chapter 4 from Vedantu?
Download Structure of The Atom Class 9 NCERT Solutions PDF for free from the portal. You can use it offline to study the different conceptual answers framed for the respective exercises. Vedantu experts have formed these answers for your convenience. You can save your precious time and concentrate on learning this chapter properly.
Advantages of NCERT Solutions for Class 9 Science Chapter 4
The following are some of the key benefits of practising the NCERT Solutions for Class 9 Science Chapter 4 “Structure of Atom.”
Expert Curated: Revision notes crafted by subject experts, ensuring coverage of all crucial topics for comprehensive understanding.
Simple and Precise Format: Notes presented in a straightforward, precise, and easy-to-understand format, promoting clarity and efficient revision.
Inclusion of Important Diagrams: The revision notes feature a list of key diagrams, enhancing conceptual understanding and aiding visual learners.
Illustrative Examples: Concepts are reinforced with examples, providing practical applications to enhance comprehension of the underlying theories.
NCERT Solutions for Class 9 Science Chapter 4, "Structure of the Atom," comprehensively addresses all exercises, providing a detailed understanding of crucial topics. The solutions cover fundamental concepts such as the discovery of electrons, protons, and neutrons, Bohr's model, and the modern atomic model. By offering step-by-step explanations and illustrations, this resource ensures clarity in comprehension. For further assistance, the downloadable NCERT solutions PDF serves as a valuable aid in exam preparation. We trust that this discussion has effectively addressed your queries, offering a strong foundation to master the aspects of atomic structure.
We wish you all the very best for the upcoming examinations!
NCERT Solutions for Class 9 Science
Chapter 1 - Matter in Our Surroundings
Chapter 2 - Is Matter Around us Pure
Chapter 3 - Atoms and Molecules
Chapter 4 - Structure of Atom
Chapter 5 - The Fundamental Unit of Life
Chapter 6 - Tissues
Chapter 7 - Diversity in Living Organisms
Chapter 8 - Motion
Chapter 9 - Force and Laws of Motion
Chapter 10 - Gravitation
Chapter 11 - Work and Energy
Chapter 12 - Sound
Chapter 13 - Why do We Fall ill
Chapter 14 - Natural Resources
Chapter 15 - Improvement in Food Resources
Along with this, students can also view additional study materials provided by Vedantu, for Class 9
NCERT Solutions For Class 9
Revision Notes for Class 9
CBSE Class 9 Syllabus

FAQs on NCERT Solutions for Class 9 Science Chapter 4 - Structure Of The Atom
1. Why use Solutions for Class 9 Science Chapter 4 PDF?
You can start studying Chapter 4 beforehand and prepare the syllabus for an exam efficiently. Rely on the precise answers and build your concept to stay ahead of the class. Download the NCERT Solutions for Class 9 Science Chapter 4 PDF for free and start your preparation today.
2. Why Should you Prefer the Answers in NCERT Solutions for Class 9 Science Ch 4?
These answers are prepared by the experienced teachers following the latest CBSE guidelines so that you can score well in the exam.
3. How NCERT Solutions for Class 9 Chemistry Chapter 4 can Help You?
The format used in the answers in Structure of The Atom Class 9 NCERT Solutions will help you follow the guidelines. You will be able to give the right answers by maintaining the word count and score better.
4. What is the structure of an atom?
The structure of an atom consists of three main particles - protons, electrons, and neutrons. The centre of an atom is called the nucleus, which consists of the positively charged protons and the no-charge neutrons. Outside the nucleus, there are electron shells that contain negatively charged electrons. Based on the number of protons and electrons, each atom shows its properties. For similar solutions, download the NCERT Solutions Class 9 Science Chapter 4 available on Vedantu website (vedantu.com) and the app.
5. Who discovered atom according to Chapter 4 of Class 9 Science?
It was John Dalton who gave us the first modern theory of an atom and its structure. According to Dalton, atoms are indivisible and indestructible units that form matter. Atoms present in the same element are similar or identical. Atoms of different elements can vary in mass and size. Compounds are created by combining different atoms. And, the rearrangement of atoms in the presence of a reactant can cause a chemical reaction and produce a chemical product.
6. Write the names of three subatomic parts of the atom.
The smallest unit present in any matter is an atom. It contains protons, electrons, and neutrons inside it and these are the three subatomic parts of an atom. While protons are positively charged, electrons are negatively charged and neutrons are neutral or no charge. To know more about the topic in detail, you can visit Vedantu website and mobile app.
7. What is Bohr's model according to Chapter 4 of Class 9 Science?
According to Bohr’s Model of Atom, an atom consists of a nucleus that is heavily charged (positive) due to the presence of protons. All the mass of an atom is constituted in the nucleus. Outside the nucleus, there are definiṯe orbits or energy levels that are circular - these consist of electrons. Every orbit has an exact energy amount. Whenever an electron jumps from one orbit to another, it causes a change in energy. To study more about atoms, students can download the NCERT solutions free of cost from the Vedantu website.
8. What is Thomson’s model of an atom?
According to J.J Thompson, the model of an atom is like a Christmas pudding. It has a positive sphere, in which, embedded are the electron particles like currants on a Christmas pudding. He believed that the positive and negative charges in an atom were equal and therefore, an atom was electrically neutral. But, we know that with experiments conducted by scientists later it was proved that JJ Thompson’s model of an atom was not correct.
NCERT Solutions for Class 9
myCBSEguide
- Class 9 Science Case...
Class 9 Science Case Study Questions
Table of Contents

myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
If you are wondering how to solve class 9 science case study questions, then myCBSEguide is the best platform to choose. With the help of our well-trained and experienced faculty, we provide solved examples and detailed explanations for the recently added Class 9 Science case study questions.
You can find a wide range of solved case studies on myCBSEguide, covering various topics and concepts. Class 9 Science case studies are designed to help you understand the application of various concepts in real-life situations.
The rationale behind Science
Science is crucial for Class 9 students’ cognitive, emotional, and psychomotor development. It encourages curiosity, inventiveness, objectivity, and aesthetic sense.
In the upper primary stage, students should be given a variety of opportunities to engage with scientific processes such as observing, recording observations, drawing, tabulating, plotting graphs, and so on, whereas in the secondary stage, abstraction and quantitative reasoning should take a more prominent role in science teaching and learning. As a result, the concept of atoms and molecules as matter’s building units, as well as Newton’s law of gravitation, emerges.
Science is important because it allows Class 9 Science students to understand the world around us. It helps to find out how things work and to find solutions to problems at the Class 9 Science level. Science is also a source of enjoyment for many people. It can be a hobby, a career, or a source of intellectual stimulation.
Case study questions in Class 9 Science
The inclusion of case study questions in Class 9 science CBSE is a great way to engage students in critical thinking and problem-solving. By working through real-world scenarios, Class 9 Science students will be better prepared to tackle challenges they may face in their future studies and careers. Class 9 Science Case study questions also promote higher-order thinking skills, such as analysis and synthesis. In addition, case study questions can help to foster creativity and innovation in students. As per the recent pattern of the Class 9 Science examination, a few questions based on case studies/passages will be included in the CBSE Class 9 Science Paper. There will be a paragraph presented, followed by questions based on it.
Examples of Class 9 science class case study questions
Class 9 science case study questions have been prepared by myCBSEguide’s qualified teachers. Class 9 case study questions are meant to evaluate students’ knowledge and comprehension of the material. They are not intended to be difficult, but they will require you to think critically about the material. We hope you find Class 9 science case study questions beneficial and that they assist you in your exam preparation.
The following are a few examples of Class 9 science case study questions.
Class 9 science case study question 1
- due to its high compressibility
- large volumes of a gas can be compressed into a small cylinder
- transported easily
- all of these
- shape, volume
- volume, shape
- shape, size
- size, shape
- the presence of dissolved carbon dioxide in water
- the presence of dissolved oxygen in the water
- the presence of dissolved Nitrogen in the water
- liquid particles move freely
- liquid have greater space between each other
- both (a) and (b)
- none of these
- Only gases behave like fluids
- Gases and solids behave like fluids
- Gases and liquids behave like fluids
- Only liquids are fluids
Answer Key:
- (d) all of these
- (a) shape, volume
- (b) the presence of dissolved oxygen in the water
- (c) both (a) and (b)
- (c) Gases and liquids behave like fluids
Class 9 science case study question 2
- 12/32 times
- 18 g of O 2
- 18 g of CO 2
- 18 g of CH 4
- 1 g of CO 2
- 1 g of CH 4 CH 4
- 2 moles of H2O
- 20 moles of water
- 6.022 × 1023 molecules of water
- 1.2044 × 1025 molecules of water
- (I) and (IV)
- (II) and (III)
- (II) and (IV)
- Sulphate molecule
- Ozone molecule
- Phosphorus molecule
- Methane molecule
- (c) 8/3 times
- (d) 18g of CH 4
- (c) 1g of H 2
- (d) (II) and (IV)
- (c) phosphorus molecule
Class 9 science case study question 3
- collenchyma
- chlorenchyma
- It performs photosynthesis
- It helps the aquatic plant to float
- It provides mechanical support
- Sclerenchyma
- Collenchyma
- Epithelial tissue
- Parenchyma tissues have intercellular spaces.
- Collenchymatous tissues are irregularly thickened at corners.
- Apical and intercalary meristems are permanent tissues.
- Meristematic tissues, in its early stage, lack vacuoles, muscles
- (I) and (II)
- (III) and (I)
- Transpiration
- Provides mechanical support
- Provides strength to the plant parts
- None of these
- (a) Collenchyma
- (b) help aquatic plant to float
- (b) Sclerenchyma
- (d) Only (III)
- (c) provide strength to plant parts
Cracking Class 9 Science Case Study Questions
There is no one definitive answer to Class 9 Science case study questions. Every case study is unique and will necessitate a unique strategy. There are, nevertheless, certain general guidelines to follow while answering case study questions.
- To begin, double-check that you understand the Class 9 science case study questions. Make sure you understand what is being asked by reading it carefully. If you’re unclear, seek clarification from your teacher or tutor.
- It’s critical to read the Class 9 Science case study material thoroughly once you’ve grasped the question. This will provide you with a thorough understanding of the problem as well as the various potential solutions.
- Brainstorming potential solutions with classmates or other students might also be beneficial. This might provide you with multiple viewpoints on the situation and assist you in determining the best solution.
- Finally, make sure your answer is presented simply and concisely. Make sure you clarify your rationale and back up your claim with evidence.
A look at the Class 9 Science Syllabus
The CBSE class 9 science syllabus provides a strong foundation for students who want to pursue a career in science. The topics are chosen in such a way that they build on the concepts learned in the previous classes and provide a strong foundation for further studies in science. The table below lists the topics covered in the Class 9 Science syllabus of the Central Board of Secondary Education (CBSE). As can be seen, the Class 9 science syllabus is divided into three sections: Physics, Chemistry and Biology. Each section contains a number of topics that Class 9 science students must study during the course.
CBSE Class 9 Science (Code No. 086)
Theme: Materials Unit I: Matter-Nature and Behaviour Definition of matter; solid, liquid and gas; characteristics – shape, volume, density; change of state-melting (absorption of heat), freezing, evaporation (cooling by evaporation), condensation, sublimation. Nature of matter: Elements, compounds and mixtures. Heterogeneous and homogenous mixtures, colloids and suspensions. Particle nature and their basic units: Atoms and molecules, Law of constant proportions, Atomic and molecular masses. Mole concept: Relationship of mole to mass of the particles and numbers. Structure of atoms: Electrons, protons and neutrons, valency, the chemical formula of common compounds. Isotopes and Isobars.
Theme: The World of the Living Unit II: Organization in the Living World Cell – Basic Unit of life: Cell as a basic unit of life; prokaryotic and eukaryotic cells, multicellular organisms; cell membrane and cell wall, cell organelles and cell inclusions; chloroplast, mitochondria, vacuoles, endoplasmic reticulum, Golgi apparatus; nucleus, chromosomes – basic structure, number. Tissues, Organs, Organ System, Organism: Structure and functions of animal and plant tissues (only four types of tissues in animals; Meristematic and Permanent tissues in plants).
Theme: Moving Things, People and Ideas Unit III: Motion, Force and Work Motion: Distance and displacement, velocity; uniform and non-uniform motion along a straight line; acceleration, distance-time and velocity-time graphs for uniform motion and uniformly accelerated motion, derivation of equations of motion by graphical method; elementary idea of uniform circular motion. Force and Newton’s laws: Force and Motion, Newton’s Laws of Motion, Action and Reaction forces, Inertia of a body, Inertia and mass, Momentum, Force and Acceleration. Elementary idea of conservation of Momentum. Gravitation: Gravitation; Universal Law of Gravitation, Force of Gravitation of the earth (gravity), Acceleration due to Gravity; Mass and Weight; Free fall. Floatation: Thrust and Pressure. Archimedes’ Principle; Buoyancy. Work, energy and power: Work done by a Force, Energy, power; Kinetic and Potential energy; Law of conservation of energy. Sound: Nature of sound and its propagation in various media, speed of sound, range of hearing in humans; ultrasound; reflection of sound; echo.
Theme: Food Unit IV: Food Production Plant and animal breeding and selection for quality improvement and management; Use of fertilizers and manures; Protection from pests and diseases; Organic farming.
PRESCRIBED BOOKS:
- Science-Textbook for class IX-NCERT Publication
- Assessment of Practical Skills in Science-Class IX – CBSE Publication
- Laboratory Manual-Science-Class IX, NCERT Publication
- Exemplar Problems Class IX – NCERT Publication
myCBSEguide: A true helper
There are numerous advantages to using myCBSEguide to achieve the highest results in Class 9 Science.
- myCBSEguide offers high-quality study materials that cover all of the topics in the Class 9 Science curriculum.
- myCBSEguide provides practice questions and mock examinations to assist students in the best possible preparation for their exams.
- On our myCBSEguide app, you’ll find a variety of solved Class 9 Science case study questions covering a variety of topics and concepts. These case studies are intended to help you understand how certain principles are applied in real-world settings
- myCBSEguide is that the study material and practice problems are developed by a team of specialists who are always accessible to assist students with any questions they may have. As a result, students may be confident that they will receive the finest possible assistance and support when studying for their exams.
So, if you’re seeking the most effective strategy to study for your Class 9 Science examinations, myCBSEguide is the place to go!
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
- Class 11 Biology Case Study Questions
- Class 12 Physical Education Case Study Questions
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.

NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom

Chapter 4 Structure of Atom NCERT Solutions for Class 9 Science
Contact form.
Extra Questions for Class 9 Science Chapter 4 Structure of the Atom
Extra questions for Class 9 Science Chapter 4 Structure of the Atom with answers is given below. Our subject expert prepared these solutions as per the latest NCERT textbook. These questions will be helpful to revise the all topics and concepts. CBSE Class 9 extra questions are the most simple and conceptual questions that are prepared by subject experts for the students to study well for the final exams. By solving these extra questions, students can be very efficient in their exam preparations.
Structure of the Atom Class 9 Science Extra Questions and Answers
Very short answer questions.
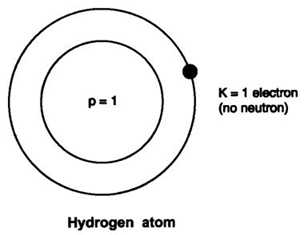
1: Draw the atomic structure of hydrogen atom. Answer:
2: Why are some elements chemically inert? Answer: Because their outermost shell is completely filled.
3: Why is atom electrically neutral? Answer: It has same number of protons and electrons, (positive charge = negative charge).
4: What is the charge and mass of a-particles? Answer: Charge is + 2 Mass is 4 a.m.u.
5: What are valence electrons? Answer: Electrons present in the outermost shell of an atom are called valence electrons.
6: An atom has atomic number 12, what is its valency and name the element? Answer: Atomic number = 12 ∴ Protons = Electrons = 12 Electrons Configuration = K L M -2 8 2 ∴ Valency = 2 Element is magnesium.
7: Find the number of neutrons in 27 13 X. Answer: Mass number = 27 ∴ p + n = 27 p = 13, (Atomic No. = Number of protons) ∴ 13 + n = 27 ∴ n = 14 ∴ Neutron =14
8: Where is the mass of atom is concentrated? Answer: Mass of an atom is concentrated in nucleus.
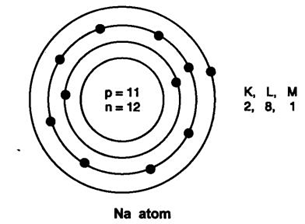
9: Name two elements with same number of protons and neutrons? Answer: Carbon (Protons = Neutrons = 6) Oxygen (Protons = Neutrons = 8)
10: Draw the atomic structure of sodium atom. Answer:
11: Name the isotope used for treatment of cancer. Answer: Isotope of cobalt. 12: AZX What does this symbol represent? Answer: X → Symbol of element A → Mass number Z → Atomic number
13: Can the value of ‘Z’ be same for two different atoms? Answer: No, (Z = atomic number), two different atoms cannot have same atomic number.
14: Can the value of A’ be same for two different atom? Answer: Yes, it can be e.g. Ca and Ar has A-40 (i.e., mass number).
Short Answer Type Questions
1: Name the scientist who discovered protons and neutrons in an atoms. Answer: Protons were discovered by E. Goldstein in 1866 and neutrons were discovered by J, Chadwick in 1932.
2: What is the contribution of Bohr and Bury together in the structure of atom’s explanation? Answer: Both Bohr and Bury gave the distribution of electrons into different atoms by giving the formula 2n 2 , where n = shell number.
3: Draw the atomic structure of (i) an atom with same number of sub-atomic particles, (ii) an atom with same number of electrons in L and M shell.
Answer: (i) An atom with same number of sub-atomic particles is He No. of protons = 2 No. of electrons = 2 No. of neutrons = 2
(ii) An atom with L and M shell filled → K L M- 2 8 8
4: What is an octate? Why would atoms want to complete their octate?
Answer: When the outermost shell of an atom i.e., L, M or N are completely filled with 8 electrons in the shell, it is said an octate. Atoms would want to complete their octate because they want to become stable.
5: Find the valency of 14 7 N and 35 17 Cl.
Answer: The atomic number of nitrogen = 7, No. of protons = 7, No. of electrons = 7 Electronic configuration = K L M = 2 5 – Valency = 3 Because either it will gain three electrons or share 3 electrons to complete its octate. The atomic number of chlorine = 17, p = 17, e=17 Electronic configuration = K L M= 2 8 7 Valency = 1 Because it will gain 1 electron to complete its octate.
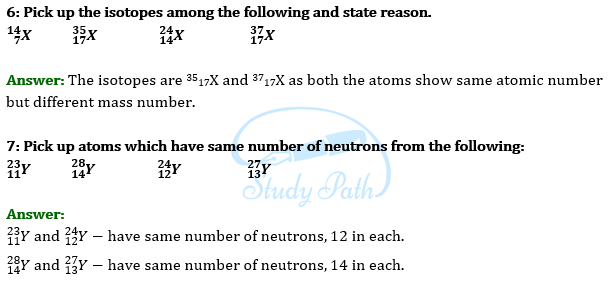
8: What are nucleons? What is the name given to those atoms which have same number of nucleons in it?
Answer: Protons and neutrons present in the nucleus are called nucleons Isobaric elements have same number of nucleons in it.
9: Give the difference between three sub-atomic particles.
Answer: Three sub-atomic particles are electron, proton and neutron

10. Give the names of three atomic species of hydrogen. Answer: Three atomic species of hydrogen are:
11: Atomic Mass exists as whole number, why do we write the atomic mass of chlorine as 35.5 u.
Answer: Chlorine has two isotopes and the mass of an atom is taken as the average mass of all the naturally occurring atoms of that element. This is obtained by knowing the percentage of each isotopic from and then the average mass is calculated Cl = 35 – 75% and Cl = 37 – 25% = 35.5 u
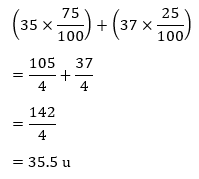
12: Give difference between isotopes, and isobars. Answer:
13: Number of protons and electrons are same in an atom. Then why is it wrong to say that atomic number of an atom is equal to its number of electrons.
Answer: Atomic number ≠ Number of electrons, although number of protons = number of electrons because the electron’s number can change in an atom by loss, or gain of it. But the proton’s number remain constant (as it does not take part in loss or gain). 14: An atom is electrically neutral, on loss or gain of electrons why does it become charged?
Answer: An atom is electrically neutral because of same number of protons and electrons. But it becomes charged, to become stable atom, loses or gains electrons. Hence, Number of protons ≠ Number of electrons If it loses electrons p > e; hence +ve charge is obtained. If it gains electrons e > p; hence -ve charge is obtained. 15: What is valency? Explain different types of valencies. Answer: The combining capacity of an atom is called its valency. There are 2 types of valencies.
Some atoms also show zero valency when there outermost shell is completely filled. 16: With the help of an activity in daily life, how can you prove that atoms are divisible.
Answer: Activity
- Take a scale, rub it on hair, try to attract a small bit of paper.
- Now divide the bit of paper further into smaller pieces
- Again bring the charged scale near to this pieces of papers.
- You will observe that the bits of paper still get attracted.
Conclusion: This activity shows that atom contains charges and these charges are opposite in nature which shows the attraction. Hence here scale and paper both are oppositely charged and hence attract each other. Also, every atom has at least one sub-atomic particle.
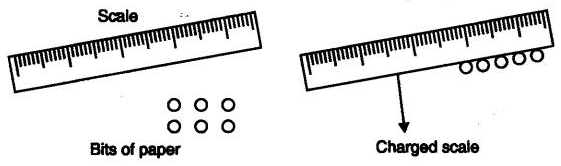
17: In the structure of an atom why are protons present in the centre and are not pulled outside by the electrons as both are oppositely charged with same unit of charge?
Answer: Protons are heavy with mass 1 unit and hence are concentrated in the centre of the atom. The mass of electrons is negligible i.e.1/1800 times less than that of protons. Hence are not able to attract the protons and pull them out of the nucleus, although their charge is of same value.
18: According to you, among the structure of atom studied which model is correct and why?
Answer: Bohr’s model of an atom is the best model and is correct because it gives the explanation of nucleons (protons and neutrons) in the centre and how electrons revolve around the nucleons in their discrete, special orbits, so electrons don’t loose/radiate energy and remain bonded in their shell.
Long Answer Type Questions
1. Give an activity to understand the implications of Rutherford’s a scattering experiment by a gold foil.
Answer: To understand the implications of Rutherford’s a-particle scattering experiment: Activity: Let a child stand in front of a wall with his eyes closed. Let him throw stones at the wall from a distance. He will hear sound for each strike of stone on the wall. This is like a nucleus of the atom. But if a blind-folded child has to throw stones at a barbed-wire fence, most of the stones would not hit the fencing and no sound would be heard. This is because there are lots of gap in the fence which allows the stone to pass through them. This is like empty space in an atom through which a-particles will pass through. Based on the above activity and similar reasoning Rutherford concluded the a-particle scattering experiment as: (1) Most of the space inside the atom is empty as a-particles passed through the foil. (2) Very few particles deflected from their path, this show that positive charge occupies less space. (3) A very small fraction of a-particles are deflected by 180°, this shows that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
2: What are isotopes? State its characteristics, give uses of isotopes?
Answer: Atoms of same element with same atomic number but different mass number are isotopes. Characteristics: (1) Physical properties of the isotopes are different e.g. mass, density. (2) Chemical properties of the isotopes are same due to same number of electrons. Uses: (1) Uranium isotope is used as a fuel in nuclear reactor (U-235). (2) Cobalt isotope is used for treatment of cancer (Co-60). (3) Iodine isotope is used in the treatment of goitre.
3: Explain Rutherford’s α-particle scattering experiment and give its observation and conclusion drawn.
Answer: Rutherford’s α-particle scattering experiment: Fast moving α-particles were made to fall on a thin gold foil. Particles have + 2 charge and 4u mass, and considerable amount of energy.
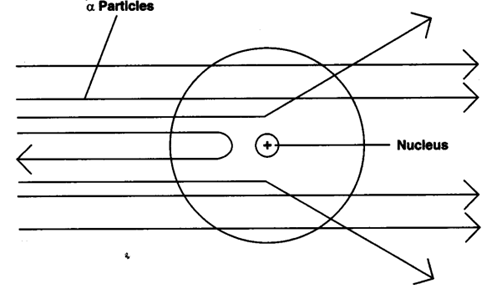
Observations: (1) Most of the α-particles passed straight through the foil. (2) Some of the α-particles were deflected by small angles by the foil. (3) One out of every 12000 particles rebounded.
Conclusion from observation: (1) Most of the space inside the foil is empty. (2) Positive charge of atom occupies very less space. (3) Mass of the atom is concentrated in the centre with all positive charge concentrated in small volume within the atom.
4: Establish the relationship between atomic number, mass number, isotopes, isobars and valency of an atom.
Answer: Atomic number — Gives the number of protons (Z) Mass number — Gives the number of protons and neutrons (A) Isotopes — When atoms of same element have same number of protons (Z) but different number of a neutrons (s) such atoms are called isotopes. Isobars — When atom of different element have same mass number (A) but different atomic number (Z) such atoms are called isobars. Valency — It is the combining capacity of an atom.
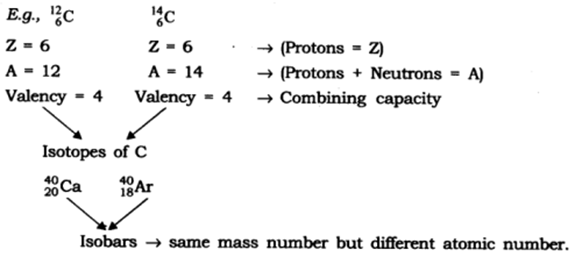
Value Based Questions
1: Aryan could not solve the following question in the group; his group mate explained him and solved his difficulty. The question was as follows: What information do you get from the given figure about the atomic number, mass number and valency of the given atom X’. (a) What is the answer for-the above question? (b) Name the element X’. (c) What value of Aryan’s friend is reflected in this behaviour?
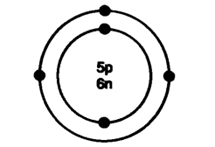
Answer: (a) The atomic number is 5. The mass number is 11. The valency is 3. (b) The element X’ is boron. (c) Aryan’s friend showed the value of helping and caring nature.
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom
These NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Questions and Answers are prepared by our highly skilled subject experts to help students while preparing for their exams.
Structure of the Atom NCERT Solutions for Class 9 Science Chapter 4
Class 9 science chapter 4 structure of the atom intext questions and answers.
Question 1. What are canal rays? Answer: Canal rays are positively charged radiations. These rays consist of positively charged particles known as protons. They were discovered by Goldstein in 1886.
Question 2. If an atom contains one electron and one proton, will it cany any charge or not? Answer: An electron is a negatively charged; particle, whereas a proton is a positively charged; particle. The magnitude of their charges is equal. Therefore, an atom containing one electron and one proton will not carry any charge. Thus, it will be a neutral atom.
Question 3. Name the three sub-atomic particles of an atom. Answer: The three sub-atomic particles of an atom are:
Question 4. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have? Answer: A helium atom has two neutrons. The mass of an atom is the sum of the masses of protons and neutrons present in its nucleus. Since helium atom has two protons, mass contributed by the two protons is (2 × 1) u = 2 u. Then, the remaining mass (4 – 2) u = 2 u is contributed \(\frac{2 \mathrm{u}}{1 \mathrm{u}}\) = 2 by neutrons.

Question 6. What do you think would be the observation if the a-particle scattering experiment is carried out using a foil of a metal other than gold? Answer: If the a-scattering experiment is carried out using a foil of a metal rather than gold, there would be no change in the observation. In the a- scattering experiment, a gold foil was taken because gold is malleable and a thin foil of gold can be easily made. It is difficult to make such foils from other metals.
Question 7. Write the distribution of electrons in carbon and sodium atoms? Answer: The total number of electrons in a carbon atom is 6. The distribution of electrons in carbon atom is given by: First orbit or K-shell = 2 electrons Second orbit or L-shell = 4 electrons Or, we can write the distribution of electrons in a carbon atom as 2, 4.
The total number of electrons in a sodium atom is 11. The distribution of electrons in sodium atom is given by: First orbit or K-shell = 2 electrons Second orbit or L-shell = 8 electrons Third orbit or M-shell = 1 electron Or, we can write the distribution of electrons in a sodium atom as 2, 8, 1.
Question 8. If K and L shells of an atom are full, then what would be the total number of electrons in the atom? Answer: The maximum number of electrons that can occupy K and L-shells of an atom are 2 and 8 respectively. Therefore, if K and L-shells of an atom are full, then the total number of electrons in the atom would be (2 + 8) = 10 electrons.
Question 9. If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom and (ii) what is the charge on the atom? Answer: (i) The atomic number is equal to the number of protons. Therefore, the atomic number of the atom is 8. (ii) Since the number of both electrons and protons is equal, therefore, the charge on the atom is 0.
Question 10. With the help of Table 4.1, find out the mass number of oxygen and sulphur atom. Answer: Mass number of oxygen = Number of protons + Number of neutrons = 8 + 8 = 16 Mass number of sulphur=Number of protons + Number of neutrons = 16 + 16 = 32
Question 11. For the symbol H, D and T tabulate three sub-atomic particles found in each of them. Answer:
Question 12. Write the electronic configuration of any one pair of isotopes and isobars. Answer: Two isoto of carbon are \(\frac {12}{6}\)C and \(\frac {14}{6}\)C. The electronic configuration of \(\frac {12}{6}\)C is 2, 4. The electronic configuration of \(\frac {14}{6}\)C is 2, 4. [Isotopes have the same electronic configuration] \(\frac {40}{20}\)Ca and \(\frac {40}{18}\)Ar are a pair of isobars The electronic configuration of \(\frac {40}{20}\)Ca is 2, 8, 8, 2. The electronic configuration of \(\frac {40}{18}\)Ar is 2, 8, 8.
Class 9 Science Chapter 4 Structure of the Atom Textbook Questions and Answers
Question 1. Compare properties of electrons, protons and neutrons. Answer:
Question 2. What are the limitations of J.J. Thomson’s model of the atom? Answer: According to J.J. Thomson’s model of an atom, an atom consists of a positively charged sphere with electrons embedded in it. However, it was later found that the positively charged particles reside at the centre of the atom called the nucleus, and the electrons revolve around the nucleus.
Question 3. What are the limitations of Rutherford’s model of the atom? Answer: According to Rutherford’s model of an atom, electrons revolve around the nucleus in fixed orbits. But, an electron revolving in circular orbits will not be stable because during revolution, it will experience acceleration. Due to acceleration, the electrons will lose energy in the form of radiation and fall into the nucleus. In such a case, the atom would be highly unstable and collapse.
Question 4. Describe Bohr’s model of the atom. Answer: Bohr’s model of the atom: Niels Bohr proposed the following postulates regarding the model of the atom.
- Only certain orbits known as discrete orbits of electrons are allowed inside the atom.
- While revolving in these discrete orbits, the electrons do not radiate energy.

Question 5. Summarize the rules for writing of distribution of electrons in various shells for the first eighteen elements. Answer: The rules for writing of the distribution of electrons in various shells for the first eighteen elements are given below. (i) The maximum number of electrons that a shell can accommodate is given by the formula ‘2n 2 ‘, where ‘n’ is the orbit number or energy level index (n = 1, 2,3..).
The maximum number of electrons present in an orbit of n = 1 is given by 2n 2 = 2 × 12 = 2 Similarly, for second orbit, it is 2n 2 = 2 × 2 2 = 8 For third orbit, it is 2n 2 = 2 × 3 2 = 18 And so on …… (ii) The outermost orbit can be accommodated by a maximum number of 8 electrons. (iii) Shells are filled with electrons in a stepwise manner i.e., the outer shell is not occupied with electrons unless the inner shells are completely filled with electrons.
Question 6. Define valency by taking examples of silicon and oxygen. Answer: The valency of an element is the combining capacity of that element. The valency of an element is determined by the number of valence electrons present in the atom of that element.
If the number of valence electrons of the atom of an element is less than or equal to four, then the valency of that element is equal to the number of valence electrons. For example, the atom of silicon has four valence electrons. Thus, the valency of silicon is four.
On the other hand, if the number of valence electrons of the atom of an element is greater than four, then the valency of that element is obtained by subtracting the number of valence electrons from eight. For example, the atom of oxygen has six valence electrons. Thus, tire valency of oxygen is(8 – 6) i.e., two.
Question 7. Explain with examples (i) Atomic number (ii) Mass number (iii) Isotopes and (iv) Isobars. Give any two uses of isotopes. Answer: (i) Atomic number: The atomic number of an element is the total number of protons present in the atom of that element. For example, nitrogen has 7 protons in its atom. Thus, the atomic number of nitrogen is 7.
(ii) Mass number: The mass number of an element is the sum of the number of protons and neutrons present in the atom of that element. For example, tire atom of boron has 5 protons and 6 neutrons. So, the mass number of boron is 5 + 6 = 11.
(iii) Isotopes: Isotopes are atoms of the same element having the same atomic number, but different mass numbers. For example, hydrogen has three isotopes. They are protium \(\left({ }_{1}^{1} \mathrm{H}\right)\), deuterium \(\left({ }_{1}^{2} \mathrm{H}\right)\), and tritium \(\left({ }_{1}^{3} \mathrm{H}\right)\).
(iv) Isobars: Isobars are atoms having the same mass number, but different atomic numbers i.e., isobars are atoms of different elements having the same mass number. For example, \({ }_{20}^{40} \mathrm{Ca}\) and \({ }_{18}^{40} \mathrm{Ar}\) andare isobars.
Two uses of isotopes are:
- One isotope of uranium is used as a fuel in nuclear reactors.
- One isotope of cobalt is used in the treatment of cancer.
Question 8. Na + has completely filled K and L shells. Explain. Answer: An atom of Na has a total of 11 electrons. Its electronic configuration is 2,8,1. But, Na + ion has one electron less than Na atom i.e., it has 10 electrons. Therefore, 2 electrons go to K-shell and 8 electrons go to L-shell, thereby completely filling K and L shells.

Question 10. The average atomic mass of a sample of an clement X is 16.2 u. What are the percentages of isotopes \({ }_{8}^{16} \mathrm{X}\) and \({ }_{8}^{18} \mathrm{X}\) in the sample? Answer: It is given that the average atomic mass of the sample of element X is 16.2 u. Let the percentage of isotope \({ }_{8}^{18} \mathrm{X}\) be y%. Thus, the percentage of isotope \({ }_{8}^{16} \mathrm{X}\) will be (100 – y) %.

Question 11. If Z = 3, what would be the valency of the element? Also, name the element Answer: By Z = 3, we mean that the atomic number of the element is 3. Its electronic configuration is 2, 1. Hence, the valency of the element is 1 (since the outermost shell has only one electron).
Therefore, the element with Z = 3 is lithium.

Question 13. For the following statements, write T for ‘True’ and F for ‘False’. (a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons. (b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral. (c) The mass of an electron is about \(\frac{1}{2000}\) times that of proton. (d) An isotope of iodine is used for making tincture iodine, which is used as a medicine. Answer: (a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons: (F) (b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral (F). (c) The mass of an electron is about \(\frac{1}{2000}\) times that of proton. (T) (d) An isotope of iodine is used for making tincture iodine, which is used as a medicine. (F)
Question 14. Put tick (√) against correct choice and cross (×) against wrong choice in the following question: Rutherford’s alpha-particle scattering experiment was responsible for the discovery of (a) Atomic nucleus (b) Electron (c) Proton (d) Neutron Answer: Rutherford’s alpha-particle scattering experiment was responsible for the discovery of (a) Atomic nucleus (√) (b) Electron (c) Proton (d) Neutron
Question 15. Put tick (√) against correct choice and cross (×) against wrong choice in the following question: Isotopes of an element have (a) the same physical properties (b) different chemical properties (c) different number of neutrons (d) different atomic numbers Answer: Isotopes of an element have (a) the same physical properties (b) different chemical properties (c) different number of neutrons (√) (d) different atomic numbers
Question 16. Put tick (√) against correct choice and cross (×) against wrong choice in the following question: Number of valence electrons in Cl- ion are: (a) 16 (b) 8 (c) 17 (d) 18 Answer: Number of valence electrons in Cl- ion are: (a) 16 (b) 8(√) (c) 17 (d) 18
Question 17. Which one of the following is a correct electronic configuration of sodium? (a) 2, 8 (b) 8, 2, 1 (c) 2, 1, 8 (d) 2, 8, 1 Answer: (d) The correct electronic configuration of sodium is 2, 8, 1.
Class 9 Science Chapter 4 Structure of the Atom Additional Important Questions and Answers
Very Short Answer Questions
Question 1. What are nucleons? Answer: The subatomic particles present in the nucleus of an atom are called nucleons. These include protons and neutrons.
Question 2. Which element does not contain any neutron? Answer: Hydrogen
Question 3. Who discovered the presence of the protons in an atom? Answer: Goldstein after his discovery of anode rays.
Question 4. What did Rutherford discover from his alpha particle scattering experiment? Answer: Rutherford from alpha particle scattering experiment had discovered the nucleus of an atom.
Question 5. Which subatomic particles were not known when Rutherford performed his experiment? Answer: Neutrons
Question 6. What is the number of protons and neutrons present in a helium atom? Answer: In a helium atom the number of both protons and neutrons is two.
Question 7. What is common in isotopes of an element? Answer: Atomic number
Question 8. What was the major drawback of Rutherford’s model of atom? Answer: Rutherford had failed to describe the stability of an atom.
Question 9. What are the fundamental building blocks of matter? Answer: Atoms and molecules
Question 10. What is the mass and charge of a proton? Answer: The mass of a .proton is taken as one unit and so also its charge is taken as one positive unit.
Question 11. What is the charge on the nucleus of an atom? Answer: The nucleus of an atom is positively charged.
Question 12. What are valence electrons? Answer: The electrons present in the outermost shell of an atom are called valence electrons.
Question 13. If an atom has atomic number-16, what are the number of valence electrons and what is its valency? Answer: Electronic configuration: 2, 8, 6. Valence electrons = 6 Valency = 2
Question 14. Which isotope of uranium is used as nuclear fuel in nuclear reactor? Answer: U-235.
Short Answer Questions
Question 1. How will you prove that matter is electrical in nature but neutral? Answer: When a glass rod rubbed with silk cloth is brought near an inflated balloon, the balloon gets attracted to the rod but when a glass rod without being rubbed to silk cloth is brought close to an inflated balloon nothing happens shows that matter is electrical in nature but neutral.
Question 2. What made the scientists to realize that atom is divisible after Dalton proposed his theory of atom in which he described atom as indivisible? Answer: The attraction of bits of paper by a comb after combing dry hair or gluing of a balloon to a wall after rubbing made the scientists think that atom is divisble.
Question 3. What is the charge and mass of an electron and a proton? Answer: Charge on an electron = -1.6 x 10 -19 C Mass of an electron = 1/1840 times of a proton Charge on a proton = + 1.6 x 10 -19 C Mass of an electron = mass of a hydrogen atom

Question 5. (i) What is the nucleus of atom? (ii) What charge does the nucleus of an atom carry? Answer: (i) Nucleus is a small dense region present in the centre of an atom. It is 10s times smaller than an atom. It is like a football placed in the centre of football field, (ii) It contains positively charged protons and neutral neutrons, therefore it is positively charged.

- An atom consists of a positively charged into it.
- The negative and positive charges are equal in magnitude and hence, an atom is electrically neutral.
Question 7. If in an element K shell is completely filled then what will be the (a) number of valence electrons (b) valence and (c) name of element. Answer: (a) Number of valence electrons = 2 (b) Valence = Zero (c) Name of element = Helium

Question 10. What are isotopes? State their uses. Answer: Isotopes of an element are the atoms which have the same atomic number: different atomic mass number because of the presence of the different number neutrons. These isotopes are used in
- Generation of electricity as fuels in nuclear power stations such as uranium-235.
- In chemotherapy such as Co-60 is used in treatment of cancer.
- Isotope of iodine is used in treatment of goitre.
- Research works to find the metabolic pathways.
Question 11. How is an isotope different from an isobar? Answer: Isotopes are the atoms of an element which have same atomic number – differ in the atomic mass number because they carry different number of neutrons but Isobars are the atoms of different elements which have same atomic mass number but they differ in their atomic number.
Question 12. Which of the following are the isotopes and isobars? Why? 18 X 8 , 18 Y 6 , 16 X 8 Answer: 18 X 8 , and 18 Y 6 are isobars because they have different atomic number but same atomic mass number. 18 X 8 , and 16 X 8 are isotopes because they have same atomic number but different atomic masses.
Question 13. How is an alpha particle different from an atom of helium? Answer: Alpha particle is the nucleus of helium represented by the formula without any electron but an atom of helium consists of 2 electrons along nucleus having two protons and neutrons.
Question 14. Calculate the number of electrons, protons and neutrons in the following 40 X 20 , 14 Y 6 , 238 Z 92 Answer: 40 X 20 – Number of protons = 20, Number of electrons = 20 Number of neutrons = 20 14 Y 6 – Number of protons = 6, Number of electrons = 6 Number of neutrons = 8 238 Z 92 – Number of protons = 92, Number of electrons = 92 Number of neutrons = 146
Question 15. Why the valence electrons are considered responsible for the chemical activity of an element? Answer: Valence electrons are the electrons present in the outermost shell of an atom. The number of these electrons determines that how many electrons an atom need to gain or lose, to gain stability. The lesser is the number of electrons required to be lost or gained, the reactive is the element.
Question 16. How is a discharge tube different from an ordinary tube? Answer: A discharge is a tube with the gas under very low pressure i.e. 1 atmospheric pressure where it is possible to generate the electric discharge but in an ordinary tube has average atmospheric pressure in which an electrical discharge cannot be produced in any condition.
Long Answer Questions

Question 2. What are anode rays? Describe their nature. Answer: Anode rays are the positively charged rays produced in a discharge tube having a perforated cathode. These rays consist of positively charge particles called protons. These rays get deflected towards the negatively charged electrode when passing through an electric field.
The charge on a proton is equal to the charge on an electron, 1.6 × 10 -19 C but is resite in sign being positively charged.
Question 3. What observations did Rutherford made in his alpha particles scattering experiment? What did he conclude from his observations? Answer: Rutherford in his alpha particle experiment when he made the alpha particles incident on the surface of a very thin and fine gold foil observed that
- Most of the fast moving alpha particles passed through the gold foil.
- Some of the alpha particles underwent deflection in their path.

- Very few i.e. one out of 12,000 alpha particle bounced back at 180°. From his observations Rutherford concluded and proposed that
- There is a positively charged centre in an atom. He called this positively charged centre as nucleus.
- The size of the nucleus is very small as compared to the size of an atom.
- The negatively charged electrons are present outside the nucleus revolving around it.

- Most of the α-particles passed straight through the foil.
- Some Of the α-particles were deflected by small angles by the foil.
- One out of every 12000 particles rebounded.
Conclusion from observation:
- Most of the space inside the foil is empty.
- Positive charge of atom occupies very less space.
- Mass of the atom is concentrated in the centre with all positive charge concentrated in small volume within the atom.

- Book Solutions
- State Boards
Case Study Questions Class 9 Science Matter in our Surroundings
Case study questions class 9 science chapter 1 matter in our surroundings.
CBSE Class 9 Case Study Questions Science Matter in our Surroundings. Important Case Study Questions for Class 9 Exam. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Matter in our Surroundings.
At Case Study Questions there will given a Paragraph. In where some Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks or 4 marks.
CBSE Case Study Questions Class 9 Science – Matter in our Surroundings
Case study 1:.
1.) A matter is anything that has mass and occupies space. Pen, paper, clips, sand, air, ice, etc. are different forms of matter. Every matter is made up of small particles. These particles are so tiny that they can’t be seen with naked eyes. Let’s see about the different characteristics of particles of matter.
- All matter is made up of very small particles.
- .Particles of matter has spaces between them.
- Particles of matter are continuously moving.
- Particles of matter attract each other.
Answer the following questions by referring above paragraph.
i.) Which of following is not matter?
c.) smell of perfume
d.) None of these
ii.) Thoughts coming in our mind are example of matter. True or false
c.) None of these
iii.) Which of the following is true about particles of matter?
a.) Particles of matter has spaces between them
b.) Particles of matter are continuously moving
c.) Particles of matter attract each other
d.) All of these
iv.) Give 5 examples of matter in our surroundings
v.) Enlist all properties of particles of matter
Answer key-1
iv.) pen, pencil, notebook, ice and water
v.) Different characteristics of particles of matter are
Case Study 2:
2.) There are three states of matter – solid, liquid and gas.
Solids have a definite shape, distinct boundaries and fixed volumes, that is, have negligible compressibility. Solids have a tendency to maintain their shape when subjected to outside force. Solids may break under force but it is difficult to change their shape, so they are rigid.
Liquids have no fixed shape but have a fixed volume. They take up the shape of the container in which they are kept. Liquids flow and change shape, so they are not rigid but can be called fluid.
Gas as has indefinite shape, no fixed volume. Gas gets the shape and volume of container.
Gas has very low density hence are light. Gas can flow easily and hence are called fluid.
i.) Which of the following state of matter takes shape of container in which it is filled?
d.) Both b and c
ii.) Distance between particles of matter least in
iii.) Compressibility is least in case of
iv.) Give properties of solids.
v.) Give properties of Gases.
Answer key-2
iv.) properties of solid are given below
- Solid has fixed volume.
- Solid has fixed shape.
- Solid has high density.
- Solids are heavy.
- Solid does not flow.
v.) Properties of gases are
- Gas has indefinite shape
- Gas has no fixed volume.
- Gas gets the shape and volume of container.
- Gas fills the container completely.
- Gas has very low density.
- Because of low density gas are light.
- Gas can flow easily and hence are called fluid.
Case Study 3:
3.) What happens inside the matter during change of state? On increasing the temperature of solids, the kinetic energy of the particles increases. Due to the increase in kinetic energy, the
Particles start vibrating with greater speed. The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid. The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
The temperature of the system does not change after the melting point is reached, till all the ice melts. This happens even though we continue to heat the beaker, that is, we continue to supply heat. This heat gets used up in changing the state by overcoming the forces of attraction between the particles. The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion. So, particles in water at 0 0 C (273 K) have more energy as compared to particles in ice at the same temperature.
The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. Boiling is a bulk phenomenon. Particles from the bulk of the liquid gain enough energy to change into the vapour state. A change of state directly from solid to gas without changing into liquid state is called sublimation and the direct change of gas to solid without changing into liquid is called deposition.
i.) A change of state directly from solid to gas without changing into liquid state is called
a.) Sublimation
b.) Deposition
c.) Boiling point
ii.) The direct change of gas to solid without changing into liquid is called
iii.) The energy supplied by heat to solid is used to overcome the forces of attraction between the particles. True or false
iv.) Define melting point and boiling point
v.) Define latent heat of fusion
Answer key-3
iv.) The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
v.) The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion.
Case Study 4:
4 .) Do we always need to heat or change pressure for changing the state of matter? Can you quote some examples from everyday life where change of state from liquid to vapour takes place without the liquid reaching the boiling point? In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour. This phenomenon of change of a liquid into vapors at any temperature below its boiling point is called evaporation.
i.) Evaporation of liquid takes place at
a.) Boiling point
b.) Above boiling point
c.) Below boiling point
ii.) Evaporation takes place at surface of liquid because
a.) They are heavy as compare to other particles
b.) They have sufficient kinetic energy to break the force
c.) They are light weight as compare to other particles
iii.) During evaporation particles of liquid change into vapour
a.) From the surface
b.) From the bottom
c.) From all over the liquid
iv.) Define evaporation.
v.) Explain process of evaporation
Answer key-4
iv.) The phenomenon of change of a liquid into vapors at any temperature below its boiling point is called evaporation.
v.) In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour. This phenomenon of change of a liquid into vapors at any temperature below its boiling point is called evaporation.
Case Study 5:
5.) You must have observed that the rate of evaporation increases with–
- an increase of surface area:
- We know that evaporation is a surface phenomenon. If the surface area is increased, the rate of evaporation increases. For example, while putting clothes for drying up we spread them out.
- an increase of temperature:
With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
In an open vessel, the liquid keeps on evaporating. The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation. This absorption of energy from the surroundings makes the surroundings cold. What happens when you pour some acetone (nail polish remover) on your palm? The particles gain energy from your palm or surroundings and evaporate causing the palm to feel cool. After a hot sunny day, people sprinkle water on the roof or open ground because the large latent heat of vaporization of water helps to cool the hot surface.
i.) Evaporation is surface phenomenon. True or false
ii.) As temperature increases the rate of evaporation is
a.) increases
b.) decreases
c.) remains constant
iii.) The rate of evaporation increases with
a.) Increase in wind speed
b.) Decrease in wind speed
c.) Does not have any effect from wind speed
iv.) What happens when you pour some acetone (nail polish remover) on your palm?
v.) We are able to sip hot tea from saucer than from cup. Why?
Answer key-5
iv.) The particles gain energy from your palm or surroundings and evaporate causing the palm to feel cool.
v.) We are able to sip hot tea from saucer than from cup. This is because saucer has large surface area, due to large surface area as compare to cut area tea evaporates at faster rate.
Thank you It helped me a lot
Why smell of Perfume is not a matter?
Because there is no particle
Because their are perfume particles suspended in air
These all case study questions are really helpful . Thanks
This is my first I was so nervous but these questions help me alot thank you
Smell of perfume is a matter because it have gas particles means perfume particles
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
West bengal class 10 history চতুর্থ অধ্যায়: সংঘবদ্ধতার গোড়ার কথা: বৈশিষ্ট্য ও বিশ্লেষণ question answer notes, essay on i learn things when i play, bse odisha 8th english questions answer solution, odisha board class 8 english face masks fool the bengal tigers question answers solution.
Sign in to your account
Username or Email Address
Remember Me
- Class 9 Chemistry Mcqs
- Class 9 Chemistry Mcqs Chapter 4 Structure of the Atom
Class 9 Chemistry Chapter 4 Structure of the Atom MCQs
Class 9 chemistry MCQs with answers are provided here for chapter 4 Structure of the Atom. These MCQs are based on the CBSE board curriculum and correspond to the most recent Class 9 chemistry syllabus. By practicing these Class 9 Multiple choice questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 9 Annual examinations as well as other entrance exams such as CTET and KVS.
Download Chapter 4 Structure of the Atom MCQs PDF by clicking on the button below. Download PDF
Recommended Videos
Structure of atom in one-shot – full chapter.

Class 9 Structure of the Atom MCQs
1. Atomic number (Z) is equal to ————–
(a) Number of protons in the nucleus of an atom.
(b) Number of electrons in a neutral atom
(c) Both (a) and (b)
(d) None of the above
Solution: Atomic number (Z) is equal to the number of protons in the nucleus.
2. Two atoms are said to be Isobars if ————–
(a) They have same atomic number but different mass number
(b) They have same number of electrons but different number of neutrons
(c) They have the same number of neutrons but different numbers of electrons.
Solution: Two atoms are said to be Isobars if they have the same mass number but different atomic numbers.
3.Mass of proton is ————
(a) 1.000 amu (b) 0.9073 amu
(c) 1.0073 amu (d) 5.486 x 10 -4 amu
Solution: Mass of the proton is 1.0073 amu
4.The mass number of the element is —————
(a) the sum of the number of electrons and protons
(b) the sum of the number of protons and neutrons
(c) the number of neutrons
(d) the number of protons
Solution: The mass number of the element is the sum of the number of protons and neutrons.
5.The atomic number of an element is equal to ————
(a) number of neutrons
(b) number of electrons
(c) number of protons
(d) number of neutrons + number of protons
Solution: The atomic number of an element is equal to number of protons.
6.An alpha particle is also known as ————–
(a) subatomic particle
(b) an unionised helium atom
(c) a neutral particle
(d) a doubly-charged helium ion
Solution: An alpha particle is a doubly-charged helium ion i.e He 2+
7.Which of the following statements about the electron is incorrect?
(a) It is a negatively charged particles
(b) The mass of the electron is equal to the mass of the neutron
(c) It is a basic constituent of all atom
(d) It is a constituent of cathode rays
Solution: the mass of an electron is equal to 1/1836 of the mass of a proton or neutron.
8.How many electrons are occupied in the M shell?
Solution: The electrons are occupy in the shell by using the 2n 2 rule. For M shell n=3 , so total 2 x 3 2 = 18 electrons.
9.Who discovered the electron?
(a) Goldstein
(b) J.J Thomson
(c) Chadwick
(d) Eugen Goldstein
Solution: J.J Thomson discovered the electron.
10. 7 N 15 and 8 O 16 are pair of ———-
(a) Isotopes
(b) Isobars
(c) Isotones
(d) none of these
Solution: 7 N 15 and 8 O 16 are pairs of isotones. Isotones are atomic species that share the same number of neutrons and differ in the number of protons. In case of 7 N 15 (number of proton = 7, number of neutron =8) 8 O 16 (number of proton = 8, number of neutron =8)
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


COMMENTS
Case Study/Passage Based Questions. The given diagrams show the atomic structures of elements X and Y. Element X and Y could be _ and _ respectively. (a) Be and B (b) C and O. (c) F and N (d) C and N. Show Answer. Valency of elements X and Y are respectively, (a) 4 and 3 (b) 2 and 5. (c) 1 and 4 (d) 3 and 4.
Statement 1 - Dalton's atomic theory suggested that the atom was indivisible and indestructible. Statement 2 - Electrons and protons are present inside the atom. Statement 3 - J.J. Thomson was the first one to propose a model for the structure of an atom. Statement 4 - Protons are positively charged particle.
Case Study Questions for Class 9 Science Chapter 4 Structure of Atom In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then questions based on it … Continue reading Case Study and Passage Based Questions for Class 9 ...
By practising these Class 9 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 9 Annual examinations. Download Class 9 Chemistry Chapter 4 - Structure of the Atom Important Questions with Answers PDF by clicking on the button below. Download PDF. Recommended Video
23rd December 2023. NCERT Solutions for Class 9 Science (Chemistry) Chapter 4 Structure of the Atom provides detailed answers for all in-text and exercise Questions. These solutions contain an in-depth explanation of each topic involved in the chapter. All these solutions are prepared by expert teachers and updated for the current academic session.
3. _______ is the radioactive element used in the treatment of cancer. (a) Iodine-131. (b) Uranium-234. (c) Plutonium-239. (d) Cobalt-60. Important Questions For Class 9 Science Chapter 4 Structure Of The Atom are provided here. Practice these questions to develop problem-solving skills & excel in the exams easily.
Answer: (i) Atomic number: The atomic number of an element is equal to the number of protons in the nucleus of its atom. e.g., Oxygen has 6 protons hence atomic no. = 6. (ii) Mass number: The mass number of an atom is equal to the number of protons and neutrons in its nucleus.
Class IX Science chapter 4 Exercise and Intext question answers in PDF format to free download. Class 9 UP Board students also get here UP Board Solutions. Class 9 Science chapter 4 Intext questions given on Page 43 or Page 49 or Page 50 or Page 52 or Page 53 and Exercises in English Medium are available.
Download NCERT Solutions for Class 9 Science Chapter 4 - free PDF. Class 9 Science Chapter 4 describes the structure of the atom. In this chapter, students will discover novel ideas and hypotheses that have been developed and validated by several scientists. Applying the newly taught ideas would be much simpler if you use the Vedantu-prepared ...
Answer: (i) The atomic number is equal to the number of protons. Therefore, the atomic number of the atom is 8. (ii) Since the number of both electrons and protons is equal, therefore, the charge on the atom is 0. Question 2: With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Case study questions in Class 9 Science. The inclusion of case study questions in Class 9 science CBSE is a great way to engage students in critical thinking and problem-solving. By working through real-world scenarios, Class 9 Science students will be better prepared to tackle challenges they may face in their future studies and careers.
Answer. (i) The atomic number is equal to the number of protons. Therefore, the atomic number of the atom is 8. (ii) Since the number of both electrons and protons is equal, therefore, the charge on the atom is 0. 2. With the help of Table 4.1, find out the mass number of oxygen and sulphur atom. Answer.
An atom 'M' of an element reacts with oxygen to form M 2 O 3. Calculate the valency of the element 'M'. Answer: Two atoms of element 'M' combine with 3 atoms of oxygen. ∴ Number of oxygen atoms combining with one atom of element 'M' = 3 2. Therefore, the valency of element 'M' = 3 2 × 2 = 3. Question 4.
Important Questions for Class 9 Science Chapter 4 Structure of the Atom. Get important questions for Class 9 Science Chapter 4 Structure of the Atom with PDF. Our subject expert prepared these important questions and answers as per the latest NCERT textbook. These important questions will be helpful to revise the important topics and concepts.
Answer: Mass number of helium is equal to its atomic mass but has no units. ∴ Mass number (A) of helium = 4. No. of protons in the nucleus = 2 Atomic number (Z) of the element = 2. No. of neutrons (n) = A - Z = 4 - 2 = 2. Question 9. Write the distribution of electrons in carbon and sodium atoms.
The exercises in Chapter 4 of NCERT Solutions for Class 9 Science are. Number 4.1 - Charged particles in matter 2 Questions ( 2 short) Number 4.2 - The structure of an atom 4 Questions ( 4 short) Number 4.2.4 - Neutrons 2 Questions ( 2 short) Number 4.3 - How are electrons distributed.
Answer: (a) The atomic number is 5. The mass number is 11. The valency is 3. (b) The element X' is boron. (c) Aryan's friend showed the value of helping and caring nature. Extra questions for Class 9 Science Chapter 4 Structure of the Atom with answers is given below. Our subject expert prepared these solutions as per the latest.
Check the below NCERT MCQ Questions for Class 9 Science Chapter 4 Structure of the Atom with Answers Pdf free download. MCQ Questions for Class 9 Science with Answers were prepared based on the latest exam pattern. We have Provided Structure of the Atom Class 9 Science MCQs Questions with Answers to help students understand the concept very well.
Answer: (i) The atomic number is equal to the number of protons. Therefore, the atomic number of the atom is 8. (ii) Since the number of both electrons and protons is equal, therefore, the charge on the atom is 0. Question 10. With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
At Case Study Questions there will given a Paragraph. In where some Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks or 4 marks. CBSE Case Study Questions Class 9 Science - Matter in our Surroundings Case Study 1: 1.) A matter is anything that has mass and occupies ...
The protons and neutrons are located in a small nucleus at the centre of the atom. 2. The electrons revolve rapidly around the nucleus at the centre of the atom. 3. There is a limit to the number of electrons that each energy level can hold. 4. Each energy level is associated with a fixed amount of energy.
Class 9 Chemistry Chapter 4 Structure of the Atom MCQs are provided here to help students prepare for the final exams. ... By practicing these Class 9 Multiple choice questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 9 Annual examinations as well as other entrance exams such as ...
2024 AP Exam Dates. The 2024 AP Exams will be administered in schools over two weeks in May: May 6-10 and May 13-17. AP coordinators are responsible for notifying students when and where to report for the exams. Early testing or testing at times other than those published by College Board is not permitted under any circumstances.