- The Open University
- Guest user / Sign out
- Study with The Open University

My OpenLearn Profile
Personalise your OpenLearn profile, save your favourite content and get recognition for your learning

Addressing ethical issues in your research proposal
This article explores the ethical issues that may arise in your proposed study during your doctoral research degree.
What ethical principles apply when planning and conducting research?
Research ethics are the moral principles that govern how researchers conduct their studies (Wellcome Trust, 2014). As there are elements of uncertainty and risk involved in any study, every researcher has to consider how they can uphold these ethical principles and conduct the research in a way that protects the interests and welfare of participants and other stakeholders (such as organisations).
You will need to consider the ethical issues that might arise in your proposed study. Consideration of the fundamental ethical principles that underpin all research will help you to identify the key issues and how these could be addressed. As you are probably a practitioner who wants to undertake research within your workplace, consider how your role as an ‘insider’ influences how you will conduct your study. Think about the ethical issues that might arise when you become an insider researcher (for example, relating to trust, confidentiality and anonymity).
What key ethical principles do you think will be important when planning or conducting your research, particularly as an insider? Principles that come to mind might include autonomy, respect, dignity, privacy, informed consent and confidentiality. You may also have identified principles such as competence, integrity, wellbeing, justice and non-discrimination.
Key ethical issues that you will address as an insider researcher include:
- Gaining trust
- Avoiding coercion when recruiting colleagues or other participants (such as students or service users)
- Practical challenges relating to ensuring the confidentiality and anonymity of organisations and staff or other participants.
(Heslop et al, 2018)
A fuller discussion of ethical principles is available from the British Psychological Society’s Code of Human Research Ethics (BPS, 2021).
You can also refer to guidance from the British Educational Research Association and the British Association for Applied Linguistics .

Ethical principles are essential for protecting the interests of research participants, including maximising the benefits and minimising any risks associated with taking part in a study. These principles describe ethical conduct which reflects the integrity of the researcher, promotes the wellbeing of participants and ensures high-quality research is conducted (Health Research Authority, 2022).
Research ethics is therefore not simply about gaining ethical approval for your study to be conducted. Research ethics relates to your moral conduct as a doctoral researcher and will apply throughout your study from design to dissemination (British Psychological Society, 2021). When you apply to undertake a doctorate, you will need to clearly indicate in your proposal that you understand these ethical principles and are committed to upholding them.
Where can I find ethical guidance and resources?
Professional bodies, learned societies, health and social care authorities, academic publications, Research Ethics Committees and research organisations provide a range of ethical guidance and resources. International codes such as the Universal Declaration of Human Rights underpin ethical frameworks (United Nations, 1948).
You may be aware of key legislation in your own country or the country where you plan to undertake the research, including laws relating to consent, data protection and decision-making capacity, for example, the Data Protection Act, 2018 (UK). If you want to find out more about becoming an ethical researcher, check out this Open University short course: Becoming an ethical researcher: Introduction and guidance: What is a badged course? - OpenLearn - Open University
You should be able to justify the research decisions you make. Utilising these resources will guide your ethical judgements when writing your proposal and ultimately when designing and conducting your research study. The Ethical Guidelines for Educational Research (British Educational Research Association, 2018) identifies the key responsibilities you will have when you conduct your research, including the range of stakeholders that you will have responsibilities to, as follows:
- to your participants (e.g. to appropriately inform them, facilitate their participation and support them)
- clients, stakeholders and sponsors
- the community of educational or health and social care researchers
- for publication and dissemination
- your wellbeing and development
The National Institute for Health and Care Research (no date) has emphasised the need to promote equality, diversity and inclusion when undertaking research, particularly to address long-standing social and health inequalities. Research should be informed by the diversity of people’s experiences and insights, so that it will lead to the development of practice that addresses genuine need. A commitment to equality, diversity and inclusion aims to eradicate prejudice and discrimination on the basis of an individual or group of individuals' protected characteristics such as sex (gender), disability, race, sexual orientation, in line with the Equality Act 2010.
The NIHR has produced guidance for enhancing the inclusion of ‘under-served groups’ when designing a research study (2020). Although the guidance refers to clinical research it is relevant to research more broadly.
You should consider how you will promote equality and diversity in your planned study, including through aspects such as your research topic or question, the methodology you will use, the participants you plan to recruit and how you will analyse and interpret your data.
What ethical issues do I need to consider when writing my research proposal?

You might be planning to undertake research in a health, social care, educational or other setting, including observations and interviews. The following prompts should help you to identify key ethical issues that you need to bear in mind when undertaking research in such settings.
1. Imagine you are a potential participant. Think about the questions and concerns that you might have:
- How would you feel if a researcher sat in your space and took notes, completed a checklist, or made an audio or film recording?
- What harm might a researcher cause by observing or interviewing you and others?
- What would you want to know about the researcher and ask them about the study before giving consent?
- When imagining you are the participant, how could the researcher make you feel more comfortable to be observed or interviewed?
2. Having considered the perspective of your potential participant, how would you take account of concerns such as privacy, consent, wellbeing and power in your research proposal?
[Adapted from OpenLearn course: Becoming an ethical researcher, Week 2 Activity 3: Becoming an ethical researcher - OpenLearn - Open University ]
The ethical issues to be considered will vary depending on your organisational context/role, the types of participants you plan to recruit (for example, children, adults with mental health problems), the research methods you will use, and the types of data you will collect. You will need to decide how to recruit your participants so you do not inappropriately exclude anyone. Consider what methods may be necessary to facilitate their voice and how you can obtain their consent to taking part or ensure that consent is obtained from someone else as necessary, for example, a parent in the case of a child.
You should also think about how to avoid imposing an unnecessary burden or costs on your participants. For example, by minimising the length of time they will have to commit to the study and by providing travel or other expenses. Identify the measures that you will take to store your participants’ data safely and maintain their confidentiality and anonymity when you report your findings. You could do this by storing interview and video recordings in a secure server and anonymising their names and those of their organisations using pseudonyms.
Professional codes such as the Code of Human Research Ethics (BPS, 2021) provide guidance on undertaking research with children. Being an ‘insider’ researching within your own organisation has advantages. However, you should also consider how this might impact on your research, such as power dynamics, consent, potential bias and any conflict of interest between your professional and researcher roles (Sapiro and Matthews, 2020).
How have other researchers addressed any ethical challenges?
The literature provides researchers’ accounts explaining how they addressed ethical challenges when undertaking studies. For example, Turcotte-Tremblay and McSween-Cadieux (2018) discuss strategies for protecting participants’ confidentiality when disseminating findings locally, such as undertaking fieldwork in multiple sites and providing findings in a generalised form. In addition, professional guidance includes case studies illustrating how ethical issues can be addressed, including when researching online forums (British Sociological Association, no date).
Watch the videos below and consider what insights the postgraduate researcher and supervisor provide regarding issues such as being an ‘insider researcher’, power relations, avoiding intrusion, maintaining participant anonymity and complying with research ethics and professional standards. How might their experiences inform the design and conduct of your own study?
Postgraduate researcher and supervisor talk about ethical considerations
Your thoughtful consideration of the ethical issues that might arise and how you would address these should enable you to propose an ethically informed study and conduct it in a responsible, fair and sensitive manner.
British Educational Research Association (2018) Ethical Guidelines for Educational Research. Available at: https://www.bera.ac.uk/publication/ethical-guidelines-for-educational-research-2018 (Accessed: 9 June 2023).
British Psychological Society (2021) Code of Human Research Ethics . Available at: https://cms.bps.org.uk/sites/default/files/2022-06/BPS%20Code%20of%20Human%20Research%20Ethics%20%281%29.pdf (Accessed: 9 June 2023).
British Sociological Association (2016) Researching online forums . Available at: https://www.britsoc.co.uk/media/24834/j000208_researching_online_forums_-cs1-_v3.pdf (Accessed: 9 June 2023).
Health Research Authority (2022) UK Policy Framework for Health and Social Care Research . Available at: https://www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/uk-policy-framework-health-social-care-research/uk-policy-framework-health-and-social-care-research/#chiefinvestigators (Accessed: 9 June 2023).
Heslop, C., Burns, S., Lobo, R. (2018) ‘Managing qualitative research as insider-research in small rural communities’, Rural and Remote Health , 18: pp. 4576.
Equality Act 2010, c. 15. Available at: https://www.legislation.gov.uk/ukpga/2010/15/introduction (Accessed: 9 June 2023).
National Institute for Health and Care Research (no date) Equality, Diversity and Inclusion (EDI) . Available at: https://arc-kss.nihr.ac.uk/public-and-community-involvement/pcie-guide/how-to-do-pcie/equality-diversity-and-inclusion-edi (Accessed: 9 June 2023).
National Institute for Health and Care Research (2020) Improving inclusion of under-served groups in clinical research: Guidance from INCLUDE project. Available at: https://www.nihr.ac.uk/documents/improving-inclusion-of-under-served-groups-in-clinical-research-guidance-from-include-project/25435 (Accessed: 9 June 2023).
Sapiro, B. and Matthews, E. (2020) ‘Both Insider and Outsider. On Conducting Social Work Research in Mental Health Settings’, Advances in Social Work , 20(3). Available at: https://doi.org/10.18060/23926
Turcotte-Tremblay, A. and McSween-Cadieux, E. (2018) ‘A reflection on the challenge of protecting confidentiality of participants when disseminating research results locally’, BMC Medical Ethics, 19(supplement 1), no. 45. Available at: https://bmcmedethics.biomedcentral.com/articles/10.1186/s12910-018-0279-0
United Nations General Assembly (1948) The Universal Declaration of Human Rights . Resolution A/RES/217/A. Available at: https://www.un.org/en/about-us/universal-declaration-of-human-rights#:~:text=Drafted%20by%20representatives%20with%20different,all%20peoples%20and%20all%20nations . (Accessed: 9 June 2023).
Wellcome Trust (2014) Ensuring your research is ethical: A guide for Extended Project Qualification students . Available at: https://wellcome.org/sites/default/files/wtp057673_0.pdf (Accessed: 9 June 2023).
More articles from the research proposal collection

Writing your research proposal
A doctoral research degree is the highest academic qualification that a student can achieve. The guidance provided in these articles will help you apply for one of the two main types of research degree offered by The Open University.
Level: 1 Introductory
Defining your research methodology
Your research methodology is the approach you will take to guide your research process and explain why you use particular methods. This article explains more.

Writing your proposal and preparing for your interview
The final article looks at writing your research proposal - from the introduction through to citations and referencing - as well as preparing for your interview.
Free courses on postgraduate study

Are you ready for postgraduate study?
This free course, Are you ready for postgraduate study, will help you to become familiar with the requirements and demands of postgraduate study and ensure you are ready to develop the skills and confidence to pursue your learning further.

Succeeding in postgraduate study
This free course, Succeeding in postgraduate study, will help you to become familiar with the requirements and demands of postgraduate study and to develop the skills and confidence to pursue your learning further.

Applying to study for a PhD in psychology
This free OpenLearn course is for psychology students and graduates who are interested in PhD study at some future point. Even if you have met PhD students and heard about their projects, it is likely that you have only a vague idea of what PhD study entails. This course is intended to give you more information.
Become an OU student
Ratings & comments, share this free course, copyright information, publication details.
- Originally published: Tuesday, 27 June 2023
- Body text - Creative Commons BY-NC-SA 4.0 : The Open University
- Image 'Pebbles balance on a stone see-saw' - Copyright: Photo 51106733 / Balance © Anatoli Styf | Dreamstime.com
- Image 'Camera equipment set up filming a man talking' - Copyright: Photo 42631221 © Gabriel Robledo | Dreamstime.com
- Image 'Applying to study for a PhD in psychology' - Copyright free
- Image 'Succeeding in postgraduate study' - Copyright: © Everste/Getty Images
- Image 'Addressing ethical issues in your research proposal' - Copyright: Photo 50384175 / Children Playing © Lenutaidi | Dreamstime.com
- Image 'Writing your proposal and preparing for your interview' - Copyright: Photo 133038259 / Black Student © Fizkes | Dreamstime.com
- Image 'Defining your research methodology' - Copyright free
- Image 'Writing your research proposal' - Copyright free
- Image 'Are you ready for postgraduate study?' - Copyright free
Rate and Review
Rate this article, review this article.
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews
For further information, take a look at our frequently asked questions which may give you the support you need.
- Technical Support
- Find My Rep
You are here
100 Questions (and Answers) About Research Ethics
- Emily E. Anderson - Loyola University Chicago, USA
- Amy Corneli - Duke University School of Medicine, USA
- Description
100 Questions (and Answers) About Research Ethics is an essential guide for graduate students and researchers in the social and behavioral sciences. It identifies ethical issues that individuals must consider when planning research studies as well as provides guidance on how to address ethical issues that might arise during research implementation. Questions such as assessing risks, to protecting privacy and vulnerable populations, obtaining informed consent, using technology including social media, negotiating the IRB process, and handling data ethically are covered.
Acting as a resource for students developing their thesis and dissertation proposals and for junior faculty designing research, this book reflects the latest U.S. federal research regulations to take effect mostly in January 2018.
See what’s new to this edition by selecting the Features tab on this page. Should you need additional information or have questions regarding the HEOA information provided for this title, including what is new to this edition, please email [email protected] . Please include your name, contact information, and the name of the title for which you would like more information. For information on the HEOA, please go to http://ed.gov/policy/highered/leg/hea08/index.html .
For assistance with your order: Please email us at [email protected] or connect with your SAGE representative.
SAGE 2455 Teller Road Thousand Oaks, CA 91320 www.sagepub.com
“The book is extremely informative, well-organized, and an invaluable tool for students, faculty, and literally all researchers across disciplines. I have taught research methods courses for many years, and the ethics and responsible conduct of research for several years, and found this text to be loaded with easily accessible information I find incredibly useful for teaching these courses. The text answers many questions that students typically ask that take considerable effort to answer and eliminates my concern that I am not always providing the most useful or accurate responses. It is extremely informative and well done and is a must for researchers and practitioners engaged in the research enterprise. Well done in all respects! A+.”
“The approach to the book makes it easy to incorporate in a class setting; it is an ideal reference and quick-check for students, but there is still a great deal of substantive material. The questions can provide a ready jumping off point for class discussions and can easily serve as the foundation for class topics and projects.”
“This book will be helpful to both professional and student researchers. As Chair of my institution's IRB, I will be recommending it to my IRB members, and also to my colleagues who are planning to submit protocols for review.”
KEY FEATURES:
- Emphasizes holistic, broader ethical thinking within the regulatory/compliance framework rather than focusing narrowly on compliance with guidance provided by IRBs and federal regulations.
- Offers guidance for helping potential participants make an informed decision about study participation so that researchers ensure that volunteers understand what it entails and give their informed consent without any coercion.
- Offers guidance for preparing an IRB application, a process that is challenging for even the most seasoned researchers.
Sample Materials & Chapters
Part 3 Protecting Privacy and Confidentiality
Part 6 Designing Ethical Research
For instructors
Select a purchasing option, related products.

This title is also available on SAGE Research Methods , the ultimate digital methods library. If your library doesn’t have access, ask your librarian to start a trial .
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
First do no harm: An exploration of researchers’ ethics of conduct in Big Data behavioral studies
Roles Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft
* E-mail: [email protected]
Affiliation Institute for Biomedical Ethics, University of Basel, Basel, Switzerland
Roles Formal analysis, Methodology, Supervision, Validation, Writing – original draft
Roles Validation, Writing – review & editing
Affiliation Division of Clinical Psychology and Psychotherapy, Faculty of Psychology, University of Basel, Basel, Switzerland
Roles Funding acquisition, Supervision, Validation, Writing – review & editing
- Maddalena Favaretto,
- Eva De Clercq,
- Jens Gaab,
- Bernice Simone Elger

- Published: November 5, 2020
- https://doi.org/10.1371/journal.pone.0241865
- Reader Comments
Research ethics has traditionally been guided by well-established documents such as the Belmont Report and the Declaration of Helsinki. At the same time, the introduction of Big Data methods, that is having a great impact in behavioral research, is raising complex ethical issues that make protection of research participants an increasingly difficult challenge. By conducting 39 semi-structured interviews with academic scholars in both Switzerland and United States, our research aims at exploring the code of ethics and research practices of academic scholars involved in Big Data studies in the fields of psychology and sociology to understand if the principles set by the Belmont Report are still considered relevant in Big Data research. Our study shows how scholars generally find traditional principles to be a suitable guide to perform ethical data research but, at the same time, they recognized and elaborated on the challenges embedded in their practical application. In addition, due to the growing introduction of new actors in scholarly research, such as data holders and owners, it was also questioned whether responsibility to protect research participants should fall solely on investigators. In order to appropriately address ethics issues in Big Data research projects, education in ethics, exchange and dialogue between research teams and scholars from different disciplines should be enhanced. In addition, models of consultancy and shared responsibility between investigators, data owners and review boards should be implemented in order to ensure better protection of research participants.
Citation: Favaretto M, De Clercq E, Gaab J, Elger BS (2020) First do no harm: An exploration of researchers’ ethics of conduct in Big Data behavioral studies. PLoS ONE 15(11): e0241865. https://doi.org/10.1371/journal.pone.0241865
Editor: Daniel Jeremiah Hurst, Rowan University School of Osteopathic Medicine, UNITED STATES
Received: July 22, 2020; Accepted: October 21, 2020; Published: November 5, 2020
Copyright: © 2020 Favaretto et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The raw data and the transcripts related to the project cannot be openly released due to ethical constraints (such as easy re-identification of the participants and the sensitive nature of parts of the interviews). The main points of contact for fielding data access requests for this manuscript are: the Head of the Institute for Biomedical Ethics (Bernice Elger: [email protected] ), the corresponding author (Maddalena Favaretto: [email protected] ), and Anne-Christine Loschnigg ( [email protected] ). Data sharing is contingent on the data being handled appropriately by the data requester and in accordance with all applicable local requirements. Upon request, a data sharing agreement will be stipulated between the Institute for Biomedical Ethics and the one requesting the data that will state that: 1) The shared data must be deleted by the end of 2023 as stipulated in the recruitment email sent to the study participants designed in accordance to the project proposal of the NRP 75 sent to the Ethics Committee northwest/central Switzerland (EKNZ); 2) The people requesting the data agree to ensure its confidentiality, they should not attempt to re-identify the participants and the data should not be shared with any further third stakeholder not involved in the data sharing agreement signed between the Institute for Biomedical Ethics and those requesting the data; 3) The data will be shared only after the Institute for Biomedical Ethics has received specific written consent for data sharing from the study participants.
Funding: The funding for this study was provided by the Swiss National Science Foundation in the framework of the National Research Program “Big Data”, NRP 75 (Grant-No: 407540_167211, recipient: Prof. Bernice Simone Elger). We confirm that the Swiss National Science Foundation had no involvement in the study design, collection, analysis, and interpretation of data, the writing of the manuscript and the decision to submit the paper for publication.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Big Data methods have a great impact in behavioral sciences [ 1 – 3 ], but challenge the traditional interpretation and validity of research principles in psychology and sociology by raising new and unpredictable ethical concerns. Traditionally, research ethics have been guided by well-established reports and declarations such as the Belmont Report and the Declaration of Helsinki [ 4 – 6 ]. At the core of these documents are three fundamental principles– respect for persons , beneficence , and justice –and their related interpretations and practices, such as the acknowledgment of participants’ autonomous participation and the need to obtain informed consent, minimization of harm, risk benefit assessment, fairness in distribution and dissemination of research outcomes, and fair participant selection (e.g. to avoid additional burden to vulnerable populations) [ 7 ].
As data stemming from human interactions is more and more available to scholars, thanks to a) the increased distribution of technological devices, b) the growing use of digital services, and c) the implementation of new digital technologies [ 8 , 9 ], researchers and institutional bodies are confronted with novel ethical questions. These encompass harm, that might be caused by the linkage of publicly available datasets on research participants [ 10 ], the level of privacy users expect in digital platforms such as social media [ 11 ], the level of protection that investigators should ensure for the anonymity of their participants in research using sensing devices and tracking technologies [ 12 ], and the role of individuals in consenting in participating in large scale data studies [ 13 ].
Consent is one of the most challenged practices in data research. In this context subjects are often unaware of the fact that their data is collected and analyzed and lack the appropriate control over their data, preventing them the possibility to withdraw from a study, that allows for autonomous participation [ 14 , 15 ]. When it comes to the principle of beneficence , Big Data brings about issues with regard to the appropriate risk-benefit ratio for participants as it becomes more difficult for researchers to anticipate unintended harmful consequences [ 8 ]. For example, it is increasingly complicated to ensure anonymity of the participant as risks of re-identification abound in Big Data practices [ 12 ]. Finally, interventions and knowledge developed from Big Data research might benefit only part of the population thus creating issues of justice and fairness [ 10 ]; this is mainly due to the deepening of the digital divide between people who have access to digital resources and those who do not, on the basis of a significant number of demographic variables such as income, ethnicity, age, skills, geographical location and gender [ 10 , 16 ].
There is evidence that researchers and regulatory bodies are struggling to appropriately address these novel ethical questions raised by Big Data. For instance, a group of researchers based at Queen’s Mary University in the UK used a model of geographic profiling on a series of publicly available datasets in order to reveal the identity of famous British artist Banksy [ 17 ]. The study was criticized by scholars for being disrespectful of the privacy of a private citizen and their family and a deliberate violation of the artist’s right of and preference for remaining anonymous [ 18 ]. Another example is the now infamous case of the Emotional Contagion study. Using a specific software, a research team manipulated the News Feeds of 689,003 Facebook users in order investigate how “emotional states can be transferred to others via emotional contagion, leading people to experience the same emotions without their awareness” [ 19 ]. Ethics scholars and the public criticized this study because it was performed without obtaining the appropriate consent from Facebook users and it could have cause psychological harm by showing participants only negative feeds on their homepage [ 20 , 21 ].
Given these substantial challenges, it is legitimate to ask whether the principles set by the Belmont Report are still relevant for digital research practices. Scholars advocate for the construction of flexible guidelines and for the need to revise, reshape and update the guiding principles of research ethics in order to overcome the challenges raised in data research and provide adequate assistance to investigators [ 22 – 24 ].
As ethics governance of Big Data research is currently at debate, researchers’ own ethical attitudes influence significantly how ethical issues are presently dealt with. As researchers are experts on the technical details of their own research, it is also useful for research ethicists and members of ethical committees and Institutional Review Boards (IRB) to be knowledgeable of these attitudes. Therefore, this paper aims to explore the code of ethics and research practices of behavioral scientists involved in Big Data studies in the behavioral sciences in order to investigate perceived strategies to promote ethical and responsible conduct of Big Data research. We have conducted interviews with researchers in the fields of sociology and psychology from eminent universities both in Switzerland and the United States, where we asked them to share details about the type of strategies they develop to protect research participants in their projects; what ethical principles they apply to their projects; their opinion on how Big Data research should ideally be conducted and what ethical challenges they have faced in their research. The present study aims to contribute to the existing literature on the code of conduct of researchers involved in digital research in different countries and the value of traditional ethical principles [ 14 , 22 , 23 ] in order to contribute to the discussion around the construction of harmonized and applicable principles for Big Data studies. This manuscript aims at investigating the following research questions: 1) what are the ethical principles that can still be considered relevant for Big Data research in the behavioral sciences; 2) what are the challenges that Big data methods are posing to traditional ethical principles; 3) what are the investigators’ responsibilities and roles in reflecting upon strategies to protect research participants.
Material and methods
This study is part of a larger research project that investigated the ethical and regulatory challenges of Big Data research. We decided to focus on behavioral sciences, specifically phycology and sociology, for two main reasons. First, the larger research project aimed at investigating the challenges introduced by Big Data methods for regulatory bodies such as Research Ethics Committees (RECs) and Institutional Review Boards (IRBs) [ 25 ]. Both in Switzerland and the United States, Big Data research methods in these two fields are questioning the concept of human research subject–due to the increased distance and detachment between research subjects and investigators brought by digitalized means for data collection (e.g. social media profiles, data networks, transaction logs etc.) and analysis [ 18 ]. As a consequence current legislation in charge of regulating academic research, such as the Human Research Act (HRA) [ 26 ], the Federal Act of Data Protection [ 27 ] and the Common Rule [ 18 ], is being increasingly challenged. Second, especially in Switzerland, behavioral studies using Big Data methods are at the moment among the most underregulated types of research projects [ 26 , 28 , 29 ]. In fact, the current definition of human subject leaves many Big Data projects out of the scope of regulatory overview despite the possible ethical challenges they pose. For instance, according to the HRA research that involves anonymized data from research participants does not need ethics approval [ 26 ].
In addition, we selected Switzerland and the United States to recruit participants: Switzerland, where Big Data research is a quite recent phenomenon, was chosen because the study was designed, funded and conducted there. The United States were selected as a as a comparative sample, where advanced Big Data research has been taking place for several years in the academic environment, as evidenced by the numerous grants placed for Big Data research projects by federal institutions, such as the National Science Foundation (NSF) [ 30 , 31 ] and the National Institute of Health (NIH) [ 32 ].
For the purpose of our study we defined Big Data as an overarching umbrella term that designates a set of advanced digital techniques (e.g. data mining, neural networks, deep learning, artificial intelligence, natural language processing, profiling, scoring systems) that are increasingly used in research to analyze large datasets with the aim of revealing patterns, trends and associations about individuals, groups and society in general [ 33 ]. Within this definition we selected participants that conducted heterogeneous Big Data research projects: from internet-based research and social media studies, to aggregate analysis of corporate datasets, to behavioral research using sensing devices. Participant selection was based on their involvement in Big Data research and was conducted systematically by browsing the professional pages of all professors affiliated to the departments of psychology and sociology of all twelve Swiss Universities and the top ten American Universities according to the Times Higher Education University Ranking 2018. Other candidates were identified through snowballing. Through our systematic selection we also identified a consistent number of researchers with a background in data science that were involved in research projects in behavioral sciences (in sociology, psychology and similar fields) during the time of their interview. Since their profile matched the selection criteria, we included them in our sample.
We conducted 39 semi structured interviews with academic scholars involved in research projects that adopt Big Data methodologies. Twenty participants were from Swiss universities and 29 came from American institutions. They comprised of a majority of professors (n = 34) and a few senior researchers or postdocs (n = 5). Ethics approval was sought from the Ethics Committee northwest/central Switzerland (EKNZ) who deemed our study exempt. Oral informed consent was sought prior the start of each interview. Interviews were administered using a semi-structured interview guide developed, through consensus and discussion, after the research team had the time to familiarize with the literature and studies on Big Data research and data ethics. The questions explored topics like: ethical issues related to Big Data studies in the behavioral sciences; ethics of conduct with regards to Big Data research project; institutional regulatory practices; definition and understanding of the term Big Data; and opinions towards data driven studies ( Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0241865.t001
Interviews were tape recorded and transcribed ad-verbatim. We subsequently transferred the transcripts into the qualitative software MAXQDA (version 2018) to support with data management and the analytic process [ 34 ]. Analysis of the dataset was done using thematic analysis [ 35 ]. The first four interviews were independently read and coded by two members of the research team in order to explore the thematic elements of the interviews. To ensure consistency during the analysis process, the two researchers subsequently confronted the preliminary open-ended coding and they developed an expanded coding scheme that was used for all of the remaining transcripts. Several themes relevant for this study were agreed upon during the coding sessions such as: a) responsibility and the role of the researcher in Big Data research; b) research standards for Big Data studies; c) attitudes towards the use of publicly available data; d) emerging ethical issues from Big Data studies. Since part of the data has already been published, we refer to a previous publication [ 33 ] for additional information on methodology, project design, data collection and data analysis.
Researcher’s code of ethics for Big Data studies was chosen as a topic to explore since participants, by identifying several ethical challenges related to Big Data, expressed concerns regarding the protection of the human subject in digital research and expressed shared strategies and opinions on how to ethically conduct Big Data studies. Consequently, all the interviews that were coded within the aforementioned topics were read again, analyzed and sorted into sub-topics. This phase was performed by the first author while the second author supervised this phase by checking for consistency and accuracy.
For this study we conducted 39 interviews with respectively 21 sociologists (9 from CH and 12 from the US), 11 psychologists (6 from CH and 5 from the US), and 7 data scientists (5 from CH and 2 from the US). Among them, 27 scholars (12 from CH and 21 from US) stated that they were working on Big Data research projects or on projects that involve Big Data methodologies, four participants (all from CH) noted that they were not involved in Big Data research and eight (7 from CH and one from the US) were unsure whether their research could be described or considered as Big Data research ( Table 2 ).
https://doi.org/10.1371/journal.pone.0241865.t002
Respondents, while discussing codes of ethics and ethical practices for Big Data research, both a) shared their personal strategies that they implemented in their own research projects to protect research subjects, and b) generally discussed the appropriate research practices to be implemented in Big Data research. Table 3 illustrates the type of Big Data our participants were working with at the time of the interview.
https://doi.org/10.1371/journal.pone.0241865.t003
Our analysis identified several themes and subthemes. They were then divided and analyzed within three major thematic clusters: a) ethical principles for Big Data research; b) challenges that Big Data is introducing for research principles; c) ethical reflection and responsibility in research. Table 4 reports the themes and subthemes that emerged from the interviews and their occurrence in the dataset. Representative anonymized quotes were taken from the interviews to further illustrate the reported results.
https://doi.org/10.1371/journal.pone.0241865.t004
Ethical principles for digital research
Belmont principles, beneficence and avoiding harm..
First, many of the respondents shared their opinions on what ethical guidelines and principles they consider important to conduct ethical research in the digital era. Table 5 illustrates the number of researchers that mentioned a specific ethical principle or research practice as relevant for Big Data research.
https://doi.org/10.1371/journal.pone.0241865.t005
Three of our participants, generally referred to the principles stated in the Belmont Report and the ones related to the Declaration of Helsinki.
I think the Belmont Report principles. The starting point so. . . .you know beneficence, respect for the individuals, justice… and applying those and they would take some work for how to apply those exactly or what it would mean translating to this context but that would be the starting point (P18, US–data science).
A common concern was minimization of harm for research participants and the importance of beneficence as prominent components of scholarly research.
And…on an ethical point of view… and I guess we should be careful that experiment doesn’t harm people or not offend people for example if it's about religion or something like that it can be tricky (P25, CH–psychology).
Beneficence, in the context of digital Big Data research, was sometimes associated with the possibility of giving back to the community as a sort of tradeoff for the inconvenience that research might cause to research participants. On this, P9, an American sociologist, shared:
I mean it's interesting that the ethical challenges that I faced… (pause) had more to do with whether I feel, for instance in working in the developing world…is it really beneficial to the people that I'm working with, I mean what I'm doing. You know I make heavy demands on these people so one of the ethical challenges that I face is, am I giving back enough to the community.
While another American scholar, a psychologist, was concerned about how to define acceptable risks in digital research and finding the right balance between benefit and risks for research projects.
P17: Expecting benefit from a study that should outweigh the respective risks. I mean, I think that's a pretty clear one. This is something I definitely I don't know the answer to and I'm curious about how much other people have thought about it. Because like what is an acceptable sort of variation in expected benefits and risks. Like, you could potentially say “on average my study is expected to deliver higher benefits than risks”… there's an open question of like, … some individuals might regardless suffer under your research or be hurt. Even if some others are benefitting in some sense.
For two researchers, respect for the participant and their personhood was deemed particularly important irrespective of the type of research conducted. P19, an American sociologist, commented:
What I would like to see is integrity and personhood of every single individual who is researched, whether they are dead or alive, that that be respected in a very fundamental way. And that is the case whether it's Big Data, and whether is interviews, archival, ethnographic, textual or what have you. And I think this is a permanent really deep tension in wissenshaftlich ( scientific research ) activities because we are treating the people as data. And that's a fundamental tension. And I think it would be deeply important to explicitly sanitize that tension from the get-go and to hang on to that personhood and the respect for that personhood.
Informed consent and transparency.
Consent was by far the most prominent practice that emerged from the interviews as three quarters of our participants mentioned it, equally distributed among American and Swiss researchers. Numerous scholars emphasized how informed consent is at the foundation of appropriate research practices. P2, a Swiss psychologist, noted:
But of course it's pretty clear to me informed consent is very important and it’s crucial that people know what it is what kind of data is collected and when they would have the possibility of saying no and so on. I think that’s pretty standard for any type of data. (…) I mean it all goes down to informed consent.
For a few of our participants, in the era of Big Data, it becomes not really a matter of consent but a matter of awareness. Since research with Big Data could theoretically be performed without the knowledge of the participant, research subjects at least have to be made aware that they are part of a research project as claimed by P38 a Swiss sociologist who said:
I think that everything comes down to the awareness of the subject about what is collected about them. I mean, we have collected data for ages, right? And I mean, before it was using pen and paper questionnaires, phone interviews or…there’s been data collection about private life of people for, I mean, since social science exists. So, I think the only difference now is the awareness.
Another practice that was considered fundamental by our participants was the right of participants to withdraw from a research study that, in turn, was translated in giving the participants more control over their data in the context of Big Data research. For example, while describing their study with social media, a Swiss sociologist (P38) explained that”the condition was that everybody who participated was actually able to look at his own data and decide to drop from the survey any time”. Another Swiss sociologist (P37), when describing a study design in which they asked participants to install an add-on on their browser to collect data on their Facebook interactions, underlined the importance of giving participants control over their data and to teach them how to manage them, in order to create a trust based exchange between them and the investigators:
And there you'd have to be sure that people…it's not just anonymizing them, people also need to have a control over their data, that's kind of very important because you need kind of an established trust between the research and its subjects as it were. So they would have the opportunity of uninstall the…if they're willing to take part, that's kind of the first step, and they would need to download that add-on and they'd also be instructed on how to uninstall the add-on at any point in time. They'd be also instructed on how to pause the gathering of their data at any point in time and then again also delete data that well…at first I thought it was a great study now I'm not so sure about, I want to delete everything I've ever collected.
The same researcher suggested to create regulations that ensure ownership of research data to participants in order to allow them to have actual power over their participation past the point of initial consent.
And legal parameters then should be constructed as such that it has to be transparent, that it guards the rights of the individual (…) in terms of having ownership of their data. Particularly if it's private data they agree to give away. And they become part of a research process that only ends where their say. And they can always withdraw the data at any point in time and not just at the beginning with agreeing or not agreeing to taking part in that. But also at different other points in time. So that i think the…you have to include them more throughout your research process. Which is more of a hassle, costs more money and more time, but in the end you kind of. . . .it makes it more transparent and perhaps it makes it more interesting for them as well and that would have kind of beneficial effects for the larger public I suppose.
In addition, transparency of motives and practices was also considered a fundamental principle for digital research. For instance, transparency was seen as a way for research participants to be fully informed about the research procedures and methods used by investigators. According to a few participants transparency is key to guarantee people’s trust the research system and to minimize their worry and reservations about participating in research studies. On this P14, an American psychologist, noted:
I think we need to have greater transparency and more. . . . You know our system, we have in the United States is that…well not a crisis, the problem that we face in the United States which you also face I'm sure, is that…you know, people have to believe that this is good stuff to do (participating in a study). And if they don't believe that this is good stuff to do then it's a problem. And so. . . .so I think that that. . . .and I think that the consent process is part of it but I think that the other part of it is that the investigators and the researchers, the investigators and the institutions, you know, need to be more transparent and more accountable and make the case that this is something worth doing and that they're being responsible about it.
A Swiss sociologist, P38, who described how they implemented transparency in their research project by giving control to participants over the data they were collecting on them, highlighted that the fear individuals might have towards digital and Big Data research might come from lack of information and understanding about what data investigators are collecting on them and how they are using it. In this sense transparency of practices not only ensures that more individuals trust the research systems, but it will also assist them in making a truly informed decision about their participation in a study.
And if I remember correctly the conditions were: transparency, so every subject had to have access to the full data that we were collecting. They had also the possibility to erase everything if they wanted to and to drop from the campaign. I guess it's about transparency. (…) So, I think this is key, so you need to be transparent about what kind of data you collect and why and maybe what will happen to the data. Because people are afraid of things they don't understand so the better they understand what's happening the more they would be actually. . . . not only they will be willing to participate but also the more they will put the line in the right place. So, this I agree, this I don't agree. But the less you understand the further away you put the line and you just want to be on the safe side. So, the better they understand the better they can draw the line at the right place, and say ok: this is not your business, this I'm willing to share with you.
In addition, one of our participants considered transparency to be an important value also between scholars from different research teams. According to this participant, open and transparent communication and exchange between research would help implement appropriate ethical norms for digital research. They shared:
But I think part of it is just having more transparency among researchers themselves. I think you need to have like more discussions like: here's what I'm doing…here's what I'm doing…just more sharing in general, I think, and more discussion. (…) People being more transparent on how they're doing their work would just create more norms around it. Because I think in many cases people don't know what other people have been doing. And that's part of the issues that, you know, it's like how do I apply these abstract standards to this case, I mean that can be though. But if you know what everybody is doing it makes a little bit easier. (P3-US, Sociologist)
On the other hand, however, a sociologist from Switzerland (P37), noted that the drive towards research transparency might become problematic for ensuring the anonymity of research participants as more information you share about research practices and methods the more possibilities of backtracking and re-identifying the participants to the study.
It’s problematic also because modern social science, or science anyway, has a strong and very good drive towards transparency. But transparency also means, that the more we become transparent the less we can guarantee anonymity (…) If you say: "well, we did a crawl study", people will ask "well, where are you starting, what are your seeds for the crawler?". And it's important to, you know, to be transparent in that respect.
Privacy and anonymity.
Respect for the privacy of research participants, and protection from possible identification, usually achieved through anonymization of data, were the second most mentioned standards to be considered while conducting Big Data research. P33, a Swiss sociologist, underlined how “If ever, then privacy has…like it’s never been more important than now”, since information about individuals is becoming increasingly available thanks to digital technologies, and how institutions now have a responsibility to ensure that such privacy is respected. A Swiss data scientist, P29, described the privacy aspect embedded in their research with social media and how their team is constantly developing strategies to ensure anonymity of research subjects. They told:
Yeah, there is a privacy aspect of course, that's the main concern, that you basically…if you’re able to reconstruct like the name of the person and then the age of the person, the address of the person, of course you can link it then to the partner of the person, right? If she or he has, they're sharing the same address. And then you can easily create the story out of that, right? And then this could be an issue but…again, like we try to reapply some kind of anonymization techniques. We have some people working mostly on that. There is a postdoc in our group who is working on anonymization techniques.
Similarly, an American researcher, P6 Sociologist, underlined how it should become a routine practice for every research project to consider and implement practices to protect human participants from possible re-identification:
In the social science world people have to be at least sensitive to the fact that they could be collecting data that allows for the deductive identification of individuals. And that probably…that should be a key focus of every proposal of how do you protect against that.
Challenges introduced by Big Data to research ethics and ethical principles
A consistent number of our researcher, on the other hand, recognized how Big Data research and methods are introducing numerous challenges related to the principles and practices they consider fundamental for ethical research and reflected upon the limits of the traditional ethical principles.
When discussing informed consent, participants noted that that it might not be the main standard to refer to when creating ethical frameworks for research practices as it cannot be ensured anymore in much digital research. For instance, P14, an American psychologist noted:
I think that that the kind of informed consent that we, you know, when we sign on to Facebook or Reddit or Twitter or whatever, you know, people have no idea of what that means and they don't have any idea of what they're agreeing to. And so, you know the idea that that can bear the entire weight of all this research is, I think…I think notification is really important, you can ask for consent but the idea that that can bear the whole weight for allowing people to do whatever/ researchers to do whatever they want, I think it's misguided.
Similarly, P18, an American scholar with a background in data science, felt that although there is still a place for informed consent in the digital era, this practice should be appropriately revisited and reconsidered as it cannot be applied anymore in the stricter sense, for instance when analyzing aggregated databases where personal identifiers are removed and it would be impossible to trace back the individual to ask them for consent. Data aggregation is the process of gathering data from multiple sources and presenting it in a summarized format. Through the process of data aggregation, data can be stripped from personal identifiers thus ensuring anonymization of the dataset and analyzing aggregate data should, theoretically not reveal personal information about the user. The participant shared:
Certainly, I think there is [space for informed consent in digital research]. And like I said I think we should require people to have informed consent about their data being used in aggregate analysis. And I think right now we do not have informed consent. (…) So, I think again, under the strictest interpretation even to consent to have one’s data involved in an aggregate analysis should involve that. But I don't know, short of that, what would be an acceptable tradeoff or level of treatment. Whether simply aggregating the analysis is good enough and if so what level of aggregation is necessary.
As for consent, many of our participants while recognizing the importance of privacy and anonymity, also reflected on some of the challenges that Big Data and digitalization of research are creating for these research standards. First, a few respondents highlighted how in digital research the risk of identification of participants is quite high as anonymized datasets could almost always be de-anonymized, especially if data is not adequately secured. On this, P1, an American sociologist explained:
I understand and recognize that there are limits to anonymization. And that under certain circumstances almost every anonymized dataset can be de-anonymized. That's what the research that shows us. I mean sometimes that requires significant effort and then you ask yourself would someone really invest like, you know, supercomputers to solve this problem to de-anonymize…
A Swiss sociologist (P38) described how anonymization practices towards the protection of the privacy of the research participant could, on the other hand, diminish the value of the data for research as anonymization would destroy some of the information the researcher is actually interested in.
You know, we cannot do much about it. So… there is a tendency now to anonymize the data but basically ehm…anonymization means destruction of information in the data. And sometimes the information that is destroyed is really the information we need…
Moreover, it was also claimed how digital practices in research are currently blurring the line between private and public spaces creating additional challenges for the protection of the privacy of the research participant and practices of informed consent. A few of our researchers highlighted how research subjects might have an expectation of privacy even in public digital spaces such as social media and public records. In this context, an American sociologist, P9, noted how participants could have a problem in allowing researchers to link together publicly available datasets as they would prefer information stemming from this linkage to remain private:
P9USR: Well because the question is…even if you have no expectation of privacy in your Twitter account, you know Twitter is public. And even if you have no expectation of privacy in terms of whether you voted or not, I don't know, in Italy maybe it's a public record whether if you show up at the pool or not. Right? I can go to the city government and see who voted in the last elections right? (…) So…who voted is listed or what political party they're member of is listed, is public information. But you might have expectation of privacy when it comes to linking those data. So even though you don't expect privacy in Twitter and you don't expect privacy in your voting records, maybe you don't like it when someone links those things together.
In addition, a sociologist, P19 from the US, noted how even with just linking information of some publicly available data, research subjects could be easily identified.
However, when one goes to the trouble of linking up some of the aspects of these publicly available sets it may make some individuals identifiable in a way that they haven't been before. Even though one is purely using publicly available data. So, you might say that it kind of falls into an intermediate zone. And raises practical and ethical questions on protection when working with publicly available data. I don't know how many other people you have interviewed who are working in this particular grey zone.
Two of our participants while describing personal strategies to handle matters of expectation of privacy and consent, discussed the increased blur between private and public spaces and how it is becoming increasingly contextual to adequately handle matters of privacy on social media.
P2USR: So, for example when I study journalists, I assume that their Tweets are public data just because Twitter is the main platform for journalists to kind of present their public and professional accomplishments and so I feel fine kind of using their tweets, like in the context of my research. I will say the same thing, about Facebook data for example. So, some of the journalists kind of… that I interviewed are… are not on Facebook anymore, but at the time we became friends on Facebook and there were postings and I… I wouldn't feel as comfortable, I wouldn't use their Facebook data. I just think that somehow besides the norms of the Facebook platform is that it's more private data, from…especially when it's not a public page so… But it's like… it's fuzzy.
Responsibility and ethical reflection in research
Due to the challenges introduced by digital methods, some of our participants elaborated on their opinions regarding the role of ethical reflection and their responsibility in addressing such challenges in order to ensure the protection of research participants.
Among them, some researches emphasized the importance for investigators to apply ethical standards to appropriately perform their research projects. However, a couple of them recognized how not all researchers might have the background and expertise to acknowledge the ethical issues stemming from their research projects or to be adequately familiar with ethical frameworks. On this, P12, an American sociologist, highlighted the importance of education in ethics for research practitioners:
I also want to re-emphasize that I think that as researchers in this field we need to have training in ethics because a lot of the work that we're doing (pause) you know can be on the border of infringing on people’s privacy.
In addition, self-reflection, ethical interrogation and evaluation about the appropriateness of certain research practices was a theme that emerged quite often during our interviews. For an American psychologist, P4, concerned about issues of consent in digital research, it is paramount that investigator begin to interrogate themselves upon what type of analysis would be ethically appropriate without explicit consent of participants.
And it is interesting by the way around Big Data because in many cases those data were generated by people who didn't sign any consent form. And they have their data used for research. Even (for the) secondary analysis of our own data the question is: what can you do without consent?
Similarly, P26, a sociologist from Switzerland, reflected upon the difficulties that researchers might encounter in evaluating what type of data investigators can consider unproblematic to collect and analyze even in digital public spaces, like social media:
Even though again, it's often not as clear cut, but I think if people make information public that is slightly different from when you are posting privately within a network and assume that the only people really seeing that are your friends. I see that this has its own limits as well because certain things…well A: something like a profile image I think is always by default public on Facebook…so… there you don't really have a choice to post it privately. I guess your only choice is not to change it ever. And then the other thing is that…I know it because I study (…) internet skills, I know a lot of people are not very skilled. So, there are a lot of instances where people don't realize they're posting publicly. So even if something is public you can't assume people had meant it to be public.
Moreover, reflection and evaluation of the intent behind a research study was considered important by P31, a Swiss data scientist, for ethical research in Big Data. The researcher recognized that this is difficult to put into practice as investigators with ill intent might lie about their motivations and you could have negative consequences even with the noblest of intents.
I find it really difficult to answer that. I would say, the first thing that comes to my mind is the evaluation of intent… rather than other technicality. And I think that's a lacking point. But also the reason why I don't give that answer immediately is like…intent is really difficult to probe… and it's probably for some people quite easy to know what is the accepted intent. And then I can of course give you a story that is quite acceptable to you. And also with good intent you can do evil things. So, it's difficult but I would say that discussion about the intent is very important. So that would be maybe for me a minimal requirement. At least in the discussions.
In this context, some scholars also discussed their perception regarding responsibility of protecting research participants in digital studies and the role investigators play in overcoming ethical issues.
For a few of them it was clear that the responsibility of protecting the data subjects should fall on the investigators themselves. For instance, an American scholar, P22 sociologist, while discussing the importance of creating an ethical framework for digital research that uses publicly available data of citizens shared:
So, I do think (the responsibility) it's on researchers (…) and I get frustrated sometimes when people say "well it's not up to us, if they post it there then it's public". It's like well it is up to us, it's literally our job, we do it all day, try to decide, you know, what people want known about them and what people don't. So, we should apply those same metrics here.
However, other researchers also pointed out how the introduction of digital technologies and digital methods for behavioral research is currently shifting the perceived responsibility scholars have. P16, an American sociologist, shared some concerns regarding the use of sensor devices for behavioral research and reflected on how much responsibility they, as investigators, have in assuring data protection of their research subjects since the data they work with is owned by the company that provided the device for data collection:
There's still seems to be this question about…whether. . . .what the Fitbit corporation is doing with those data and whether we as researchers should be concerned about that. We're asking people to wear Fitbits for a study. Or whether that's just a separate issue. And I don't know what the answer to that is, I just know that it seems like the type of question that it's going to come up over and over and over again.
One a similar note, P14, an American psychologist, noted that while researchers actually have a responsibility of preventing harm that might derive from data research, it should be a responsibility in part shared with data holders. They claimed:
Do I think that the holders of data have a responsibility to try to you know, try to prevent misuse of data? Yeah, I think they probably do. (…) I think there is a notion of stewardship there. Then I think that investigators also have an independent obligation to make sure to think about the data they're analyzing and trying to get and think about what they're using it for. So not to use data in order to harm other people or those kinds of things.
Finally, a few participants hinted at the fact that research ethics boards like Institutional Review Boards (IRBs) and Ethics Committees (ECs) should play a bigger role of responsibility in ensuring that investigators actually perform their research ethically. For instance, P16, an American sociologist, complained that IRBs do not provide adequate follow-up to researchers to ensure that they are appropriately following the approved research protocols.
There does seem to be kind of a big gap even in the existing system. Which is that a researcher proposes a project, the IRB hopefully works with the researcher and the project gets approved and there's very little follow-up and very little support for sort of making sure that the things that are laid out at the IRB actually in the proposal and the project protocol actually happen. And not that I don't believe that most researchers have good intensions to follow the rules and all of that but there are so many of kind of different projects and different pressures that things can slip by and there's… there's nobody.
As Big Data methodologies are becoming widespread in research, it is important to reach international consensus on whether and how traditional principles for research ethics, such as the ones described in the Belmont Report, are still relevant for the new ethical questions introduced by Big Data and internet research [ 22 , 23 ]. Our study offers a relevant contribution to this debate as it investigated the methodological strategies and code of ethics researchers from different jurisdictions—Swiss and American investigators—apply in their Big Data research projects. It is interesting to notice how, despite regional difference, participants shared very similar ethical priorities. This might be due to the international nature of academic research, where scholars share similar codes of ethics and apply similar strategies for the protection of research participants.
Our results point out that in their code of conduct, researchers mainly referred to the traditional ethical principles enshrined in the Belmont report and the Declaration of Helsinki, like respect for persons in the practice of informed consent, beneficence, minimization of harm through protection of privacy and anonymization, and justice. This finding shows that such principles are still considered relevant in behavioral sciences to address the ethical issues of Big Data research, despite the critique of some that rules designed for medical research cannot be applied in sociological research [ 36 ]. Even before the advent of Big Data, the practical implementation of the Belmont Report principles has never been an easy endeavor as they were originally conceived to be flexible to accommodate a wide range of different research settings and methods. However it has been argued that exactly this flexibility makes them the perfect framework in which investigators can “clarify trade-offs, suggest improvements to research designs, and enable researchers to explain their reasoning to each other and the public” in digital behavioral research [ 2 ].
Our study shows how scholars still place great importance on the practice of informed consent. They considered crucial that participants are appropriately notified of their research participation, are adequately informed about at least some of the details and procedures of the study, and are given the possibility to withdraw at any point in time. A recent study, however, has highlighted that there is currently no consensus among investigators on how to collect meaningful informed consent among participants in digital research [ 37 ]. Similarly, a few researchers from our study recognized that consent, although preferable in theory, might not be the most adequate practice to refer to when designing ethical frameworks. In the era of Big Data behavioral research, informed consent becomes an extremely complex practice that is intrinsically dependent on the context of the study and the type of Big Data used. For instance, in certain behavioral studies that analyze track data from devices related to a limited number of participants, it would be feasible to ask for consent prior to beginning of the study. However, recombination and reanalysis of the data, possibly across ecosystems far removed from the original source of the data, makes it very difficult to fully inform participants about the range of uses to which their data would be put through, the type of information that could emerge from the analysis of the data, and the unforeseeable harms that the disclosure of such information could cause [ 38 ]. In online studies and internet-mediated research, consent often amounts to an agreement to unread terms of service or a vague privacy policy provided by digital platforms [ 18 ]. Sometimes valid informed consent is not even required by official guidelines when the analyzed data can be considered ‘in the public domain’ [ 39 ], leaving participants unaware that research is performed on their data. It has been argued however that researchers should not just assume that public information is freely accessible for collection and research just because it is public. Researchers should take into consideration what the subject might have intended or desired regarding the possibility for their data to be used for research purposes [ 40 ]. At the same level, we can also argue that even when information is harvested with consent, the subject might a) not wish for their data to be analyzed or reused outside of the purview of the original research purpose and b) fail to understand what is the extent of the information that the analysis of the dataset might reveal about them.
Matzner and Ochs argue that practices of informed consent “are widely accepted since they cohere with notions of the individual that we have been trained to adopt for several centuries” [ 41 ], however they also emphasize how such notions are being altered and challenged by the openness and transience of data-analytics that prevent us from continuing to consider the subject and the researcher within a self-contained dynamic. Since respect for persons , in the form of informed consent, is just one of the principles that needs to be balanced when considering research ethics [ 42 ], it becomes of outmost importance to find the right balance between the perceived necessity of still ensuring consent from participants and the reality that such consent is sometimes impossible to obtain properly. Salganik [ 2 ], for instance, suggests that in the context of digital behavioral research rather than “informed consent for everything”, researchers should follow a more complex rule: “some form of consent for most things”. This means that, assuming informed consent is required, it should be evaluated on a case by case basis whether consent is a) practically feasible and b) actually necessary. This practice might however leave too much space to the discretion of the investigator who might not have the skills to appropriately evaluate the ethical facets of their research projects [ 43 ].
Next to consent, participants from our study also argued in favor of ensuring more control to participants over their own data. In the past years, in fact, it has been argued that individuals often lack the control to manage, protect and delete their data [ 20 , 28 ]. Strategies of dynamic consent could be considered a potential tool to address ethical issues related to consent in Big Data behavioral research. Dynamic consent, a model where online tools are developed to have individuals engage in decisions about how their personal information should be used and which allows them some degree of control over the use of their data, are currently mainly developed for biomedical Big Data research [ 44 , 45 ]. Additional research could be performed to investigate if such models can be translated and applied also for behavioral digital research.
Strictly linked to consent is the matter of privacy. Many researchers underlined the importance of respecting the privacy and anonymity of research participants to protect them from possible harm. At the same time, they also recognized the many challenges related to such practice. They highlighted the difficulty of ensuring complete anonymity of the data and prevent re-identification of participants in Big Data research, especially since high level of anonymization could cause the loss of essential information for the research project. The appropriate trade-off between ensuring maximum anonymization for participants while maintaining quality of the dataset is still hotly debated [ 12 ]. Growing research in data science strives towards developing data models to ensure maximum protection for participants [ 46 ]. On the other hand, our participants also referred to the current debate surrounding the private nature of personal data as opposed to publicly available data and how Big Data and digital technologies are blurring the line between private and public spheres. Some respondents expressed concern or reservation towards the analysis of publicly available data–especially without informed consent–as it could still be considered an infringement of the privacy of research participants and also cause them harm. This shows how researchers are well aware of the problems of considering privacy a binary concept (private vs public data) and that they are also willing to reflect upon strategies to protect the identity of participants even when handling publicly available data. According to Zook et al. [ 47 ], breaches of privacy are the main means by which Big Data can do harm as it might reveal sensitive information about people. Besides the already mentioned “Tagging Banksy” project [ 17 ], another distressing example is what happened in 2013, after the New York City Taxi & Limousine Commission released an anonymized dataset of 173 million individual cab rides–including the pickup and drop-off times, locations, fare and tip amount. Many researchers who freely accessed this database showed how easy it was to elaborate the dataset so that it revealed private information about the taxi-drivers, such as their religious belief, average income and even an estimation of their home address [ 48 ]. It becomes therefore increasingly crucial that investigators in the behavioral sciences recognize how privacy is contextual, situational and changes over time as it depends on multiple factors such as the context in which the data were created and obtained, and the expectations of those whose data is used [ 2 , 47 , 49 , 50 ]. For instance, as reported by one of our respondents, users might not have expectations of privacy on some publicly available information when taken singularly or separately–e.g. social media and voter data, but they might have privacy concerns on the information that the linkage of this data might reveal–e.g. who they voted for. This difficulty, if not impossibility, of defining a widespread single norm or rule for protecting privacy, shows again the intrinsic context dependency of Big Data studies, and highlights how researchers are increasingly called to critically evaluate their decisions on a case by case basis rather than by blindingly applying a common rule.
As new methods of data collection and analysis in behavioral sciences create controversy and appropriately balancing and evaluating ethical principles is becoming a source of difficult decisions for researchers [ 2 ], our participants underlined the importance of ethical reflection and education towards the appropriate development of research projects. They also recognized how investigators are called to critically reflect about the design of their studies and the consequences they might have for research participants [ 51 ]. However, as claimed by one of our participants, not all researchers, especially those coming from more technical disciplines like data science, might have the expertise and tools to proactively think about ethical issues when designing a research project [ 22 ] and might need additional guidance. We therefore argue that education in ethics, exchange and dialogue between research teams and scholars from different disciplines must be implemented. As suggested by Zook et al. [ 47 ] discussion and debate of ethical issues are an essential part of establishing a community of ethical practitioners and integrating ethical reflection into coursework and training can enable a bigger number of scholars to raise appropriate ethical questions when reviewing or developing a project.
Within the current discussion, we have seen how context-dependency, although never spelled out explicitly by our participants, becomes a major theme in the debate over ethical practices in Big Data studies. Our results have in fact highlighted that a one-size fits all approach to research ethics, or a definite overarching set of norms or rules to protect research participants, is not opportune to appropriately handle the multifaceted ethical issues of Big Data. The context-dependent nature of some of the ethical challenges of Big Data studies, such as consent and privacy, might require a higher level of flexibility together with a more situational and dialogic approach to research ethics [ 23 ]. For instance, the Association of Internet Researchers (AoIR) in the development of their Ethical Guidelines for Internet research agrees that the adequate process approach for ethical internet research is one that is reflective and dialogical “as it begins with reflection on own research practices and associated risks and is continuously discussed against the accumulated experience and ethical reflections of researchers in the field and existing studies carried out” [ 52 ]. As a consequence we argue that applying context specific assessments increases the chances of solving ethical issues and appropriately protecting research participants [ 53 ]. Many authors in the field are thus promoting methodological approaches that focus on contextually-driven decision-making for Big Data research. Zimmer, for example, suggests the application of contextual integrity’s decision heuristic on different research studies to appropriately assess the ethical impact of the study on the privacy of its participants and consequently overcome the conceptual gaps left by the Belmont Report for Big Data research ethics [ 50 ]. Similarly, Steinmann et al. [ 53 ] provide an heuristic tool in the form of a “privacy matrix” to assist researchers in the contextual assessment of their research projects.
But what should drive investigators’ ethical reflection and decision making? Despite the multifaceted challenges introduced by Big Data and digital research, we argue that the principles stated in the Belmont Report can still be considered a valuable guidance for academic investigators. As argued by Rothstein [ 28 ], we believe Big Data exceptionalism is no viable option and new challenges should not serve as a catalyst for abandoning foundational principles of research ethics. This is in line with the current best practices suggested by institutional bodies like the American Psychological Association (APA), that claim that the core ethical principles set by the Belmont report should be expanded to address the risks and benefits of today’s data [ 6 ]. Numerous research groups are striving towards the design of ethical frameworks in Big Data research that stay true to the foundational principles of research ethics, but at the same time accommodate the needs and changes introduced by Big Data methods. Steinmann et al. [ 53 ], for instance, suggest to consider five principles (non-maleficence, beneficence, justice, autonomy, and trust) as a well-defined pluralism of values that, by having clear and direct utility in designating practical strategies for protecting privacy, should guide researchers in the evaluation of their research projects. Xafis et al. [ 38 ], in the development of an ethical framework for Biomedical Big Data research, provide a set of 16 values relevant for many Big Data uses divided in substantive values (such as justice, public benefit, solidarity or minimization of harm) and procedural values (accountability, consistency, transparency and trustworthiness) that should be used by investigators to identify and solve ethical issues within their research project. Vitak et al. [ 22 ] recommend the implementation of the principle of transparency, intended as a flexible principle that finds application in different ethical components related both to intent of research (what you are doing with data and why) and practice (how you’re getting the data–informed consent (disclosing purpose and potential use) and how you are processing the data–data anonymity). Also, according to some of our participants, enhancement of transparency in research practices would be positive on different levels. First, it would assist participants in trusting the research system and minimize their worry about participating in research studies; in addition, enhanced transparency between research teams would assist in building up the knowledge to face the ethical issues that emerge in heterogeneous research projects. Although the principle of transparency is becoming increasingly embedded in research practices as something highly recommended, there is still some uncertainty regarding how this principle would actually translate in practice, in order to overcome challenges posed to ethical practices like consent. At the moment much of the debate on transparency mainly focuses on the implementation of algorithmic transparency with Big Data [ 54 ], more research should focus on how put research transparency in practice
Finally, a very relevant theme that our participants reflected upon, that it is rarely addressed by the current literature on Big Data studies, was the topic of responsibility. Some of our respondents in fact interrogated themselves whether the introduction of digital technologies and methods implies a shift of responsibility in protecting research participants. Although all those who discussed responsibility admitted that at least part of it should definitely fall on investigators themselves, some pointed that also other actors involved in Big Data research could share some of this responsibility such as data holders, data owners–in case of the use of corporate data. Digital research has in fact changed the traditional research subject/investigator dynamic [ 18 ] by introducing other factors/actors in the process (social media platforms, private firms etc.) and therefore raises ethical challenges for which researchers do not always have the necessary skills to either anticipate or face [ 25 , 43 ]. To the best of our knowledge, it seems that this aspect of responsibility has not yet entered the ethics debate. This might be due to the practical difficulties that such a debate would necessarily imply such as communication, coordination and compromise between stakeholders with very different goals and interests at stake [ 55 , 56 ]. However, our results show that there are relevant questions and issues that should be further addressed such as: who should bear the responsibility of protecting the research subject in Big Data studies? How much should data owners, data holders, ethics committees and even users be involved in sharing such responsibility? We believe that academic investigators should not bear all the responsibility of the ethical design of research projects alone, or singularly confront themselves with the ethical implications of digital research [ 57 ]. At the moment, models of consultancy between ethics committees and researchers are advocated to assist investigators foresee ethical issues [ 25 , 43 ]. These models, together with the implementation of sustainable and transparent collaboration/partnership with data holders and owners [ 58 ], could assist the creation of appropriate paradigms of shared responsibility that could definitely play a significant role in the development of ethically sound research projects.
Limitations
First, since our respondents were mainly from the fields of psychology and sociology, the study might have overlooked the perspectives of other relevant fields for human subject research that make use of Big Data methodologies (e.g., medicine, nursing sciences, geography, urban planning, computer science, linguistics, etc.). In addition, the findings of this study are based on a small sample of researchers from only two countries that share similar ethical norms and values. For these reasons, the findings from this analysis are not generalizable globally. Future research that takes into account additional disciplines and different countries might contribute to delivering a more comprehensive understanding of the opinions and attitudes of researchers. Finally, a limitation must be acknowledged regarding the definition of Big Data used for this study. Using the term Big Data as an umbrella term prevented us from undertaking a more nuanced analysis of the different types of data used by our participants and their specific characteristics (for instance the different ethical challenges posed by online social media data as compared to sensor data obtained with the consent of the participants). In our discussion we referred to the contextual dependency of the ethical issues of Big Data and the necessity of a continuous ethical reflection that assesses the specific nuances of the different types of Big Data in heterogeneous research projects. However we already recognized the risks of conceptualizing Big Data as a broad overarching concept [ 33 ]. As a consequence, we believe that future research on Big Data ethics will benefit from a deconstruction of the term into its different constituents in order to provide a more nuanced analysis of the topic.
This study investigated the code of ethics and the research strategies that researchers apply when performing Big Data research in the behavioral sciences and it also illustrates some of the challenges scholars encounter in practically applying ethical principles and practices. Our results point out how researchers find the traditional principles of the Belmont Report to be a suitable guide to perform ethical data research. At the same time, they also recognized how Big Data methods and practices are increasingly challenging such principles. Consent and protection of privacy were considered still paramount practices in research. However, they were also considered the most challenged practices since digitalization of research has blurred the boundary between “public and private” and made obtaining consent from participants impossible in certain cases.
Based the results and discussion of our study, we suggest three key items that future research and policymaking should focus on:
- Development of research ethics frameworks that stay true to the principles of the Belmont Report but also accommodate the context dependent nature of the ethical issues of Big Data research;
- Implementation of education in ethical reasoning and training in ethics for investigators from diversified curricula: from social science and psychology to more technical fields such as data science and informatics;
- Design of models of consultancy and shared responsibility between the different stakeholders involved in the research endeavor (e.g. investigators, data owners and review boards) in order to enhance protection of research participants.
Supporting information
S1 file. interview guide..
Semi structured interview guide that illustrates the main questions and themes that the researchers asked to the participants (questions relevant for this study are highlighted in yellow).
https://doi.org/10.1371/journal.pone.0241865.s001
- View Article
- Google Scholar
- 2. Salganik MJ. Bit by bit: Social research in the digital age. Princeton: Princeton University Press; 2019. https://doi.org/10.1002/sim.7973 pmid:30259528
- PubMed/NCBI
- 6. Paxton A. The Belmont Report in the Age of Big Data: Ethics at the Intersection of Psychological Science and Data Science. In: Sang Eun Woo LT, and Proctor Robert W., editor. Big data methods for psychological research: New horizons and challenges: American Psychological Association; 2020.
- 16. Hargittai E, editor Whose data traces, whose voices? Inequality in online participation and why it matters for recommendation systems research. Proceedings of the 13th ACM Conference on Recommender Systems; 2019.
- 22. Vitak J, Shilton K, Ashktorab Z, editors. Beyond the Belmont principles: Ethical challenges, practices, and beliefs in the online data research community. Proceedings of the 19th ACM Conference on Computer-Supported Cooperative Work & Social Computing; 2016.
- 24. Markham A, Buchanan E. Ethical decision-making and internet research: Version 2.0. recommendations from the AoIR ethics working committee. Available online: aoir org/reports/ethics2 pdf. 2012.
- 26. Research with human subjects. A manual for practitioners. Bern: Swiss Academy of Medical Sciences (SAMS); 2015.
- 30. National Science Foundation. Core Techniques and Technologies for Advancing Big Data Science & Engineering (BIGDATA) (NSF-12-499) 2012. Available from: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=nsf12499 (Accessed July 2019).
- 31. National Science Foundation. Critical Techniques and Technologies for Advancing Big Data Science & Engineering (BIGDATA) (NSF-14-543) 2014. Available from: https://www.nsf.gov/publications/pub_summ.jsp?ods_key=nsf14543&org=NSF (Accessed July 2019).
- 32. National Institute of Health. Big Data to Knowledge 2019. Available from: https://commonfund.nih.gov/bd2k (Accessed November 19, 2019).
- 34. Guest G, MacQueen KM, Namey EE. Applied thematic analysis: Sage Publications; 2011.
- 37. Shilton K, Sayles S, editors. " We Aren't All Going to Be on the Same Page about Ethics": Ethical Practices and Challenges in Research on Digital and Social Media. 2016 49th Hawaii International Conference on System Sciences (HICSS); 2016: IEEE.
- 39. British Psychological Society. Ethics Guidelines for Internet-mediated Research 2017. Available from: www.bps.org.uk/publications/policy-and-guidelines/research-guidelines-policy-documents/research-guidelines-poli (Accessed September 2020).
- 41. Matzner T, Ochs C. Sorting Things Out Ethically, Privacy as a Research Issue beyond the Individual In: Zimmer M, Kinder-Kurlanda K, editors. Internet Research Ethics for the Social Age. Oxford: Peter Lang; 2017.
- 48. Franceschi-Bicchierai L. Redditor cracks anonymous data trove to pinpoint Muslim cab drivers 2015. Available from: https://mashable.com/2015/01/28/redditor-muslim-cab-drivers/#0_uMsT8dnPqP (Accessed June 2020).
- 49. Nissenbaum H. Privacy in context: Technology, policy, and the integrity of social life: Stanford University Press; 2009. https://doi.org/10.1007/s00259-009-1337-0 pmid:20033153
- 51. Goel V. As Data Overflows Online, Researchers Grapple With Ethics 2014. Available from: https://www.nytimes.com/2014/08/13/technology/the-boon-of-online-data-puts-social-science-in-a-quandary.html (Accessed May 2020).
- 52. Franzke AS, Bechmann A, Zimmer M, Ess C, Researchers" AoI. Internet Research: Ethical Guidelines 3.0 2019. Available from: https://aoir.org/reports/ethics3.pdf (Accessed July 2020).
- 53. Steinmann M, Matei SA, Collmann J. A theoretical framework for ethical reflection in big data research. In: Collmann J, Matei SA, editors. Ethical Reasoning in Big Data. Switzerland: Springer; 2016. p. 11–27.
- 54. Rader E, Cotter K, Cho J, editors. Explanations as mechanisms for supporting algorithmic transparency. Proceedings of the 2018 CHI conference on human factors in computing systems; 2018.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Published: 22 May 2023
Addressing the ethical and societal challenges posed by genome-wide association studies of behavioral and brain-related traits
- Matthieu C. de Hemptinne 1 &
- Danielle Posthuma ORCID: orcid.org/0000-0001-7582-2365 1
Nature Neuroscience volume 26 , pages 932–941 ( 2023 ) Cite this article
4098 Accesses
3 Citations
47 Altmetric
Metrics details
Genome-wide association studies
Genome-wide association studies have led to the identification of robust statistical associations of genetic variants with numerous brain-related traits, including neurological and psychiatric conditions, and psychological and behavioral measures. These results may provide insight into the biology underlying these traits and may facilitate clinically useful predictions. However, these results also carry the risk of harm, including possible negative effects of inaccurate predictions, violations of privacy, stigma and genomic discrimination, raising serious ethical and legal implications. Here, we discuss ethical concerns surrounding the results of genome-wide association studies for individuals, society and researchers. Given the success of genome-wide association studies and the increasing availability of nonclinical genomic prediction technologies, better laws and guidelines are urgently needed to regulate the storage, processing and responsible use of genetic data. Also, researchers should be aware of possible misuse of their results, and we provide guidance to help avoid such negative impacts on individuals and society.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

The serotonin theory of depression: a systematic umbrella review of the evidence

Leveraging functional genomic annotations and genome coverage to improve polygenic prediction of complex traits within and between ancestries
Uffelmann, E. et al. Genome-wide association studies. Nat. Rev. Methods Primers. 1 , 59 (2021).
Article CAS Google Scholar
Yengo, L. et al. A saturated map of common genetic variants associated with human height from 5.4 million individuals of diverse ancestries. Nature 610 , 704–712 (2022).
Jansen, P. R. et al. Genome-wide meta-analysis of brain volume identifies genomic loci and genes shared with intelligence. Nat. Commun. 11 , 5606 (2020).
Article CAS PubMed PubMed Central Google Scholar
Klein, R. J. et al. Complement factor H polymorphism in age-related macular degeneration. Science 308 , 385–389 (2005).
Choi, S. W., Mak, T. S. H. & O’Reilly, P. F. A guide to performing Polygenic Risk Score analyses. Nat. Protoc. 15 , 2759–2772 (2020).
Khera, A. V. et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 50 , 1219–1224 (2018).
Landi, I. et al. Prognostic value of polygenic risk scores for adults with psychosis. Nat. Med. 27 , 1576–1581 (2021).
Adeyemo, A. et al. Responsible use of polygenic risk scores in the clinic: potential benefits, risks and gaps. Nat. Med. 27 , 1876–1884 (2021). This is a comprehensive summary of the state of polygenic score research, and includes thoughtful discussion on the needs and challenges as PRSs move closer to widespread use in the clinic .
Article Google Scholar
Rossello, X. et al. Risk prediction tools in cardiovascular disease prevention: a report from the ESC Prevention of CVD Programme led by the European Association of Preventive Cardiology (EAPC) in collaboration with the Acute Cardiovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP). Eur. Heart J. Acute Cardiovasc. Care 9 , 522–532 (2020).
Article PubMed Google Scholar
European Commission. Joint Research Centre. Genome-wide association studies, polygenic scores and social science genetics: overview and policy implications (Publications Office, 2019).
Wray, N. R., Yang, J., Goddard, M. E. & Visscher, P. M. The genetic interpretation of area under the ROC curve in genomic profiling. PLoS Genet. 6 , e1000864 (2010).
Article PubMed PubMed Central Google Scholar
Chatterjee, N., Shi, J. & García-Closas, M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat. Rev. Genet. 17 , 392–406 (2016).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604 , 502–508 (2022).
Savage, J. E. et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 50 , 912–919 (2018).
Martin, A. R. et al. Current clinical use of polygenic scores will risk exacerbating health disparities. Nat. Genet. 51 , 584–591 (2019).
Mostafavi, H. et al. Variable prediction accuracy of polygenic scores within an ancestry group. eLife 9 , e48376 (2020).
Kong, A. et al. The nature of nurture: effects of parental genotypes. Science 359 , 424–428 (2018).
Article CAS PubMed Google Scholar
Office for Civil Rights: Health Information Privacy (US HHS, accessed 1 April 2023); https://www.hhs.gov/hipaa/index.html
Phillips, A. M. Reading the fine print when buying your genetic self online: direct-to-consumer genetic testing terms and conditions. New Genet. Soc. 36 , 273–295 (2017).
Peck, L., Borle, K., Folkersen, L. & Austin, J. Why do people seek out polygenic risk scores for complex disorders, and how do they understand and react to results? Eur. J. Hum. Genet. 30 , 81–87 (2022).
Shoenbill, K., Fost, N., Tachinardi, U. & Mendonca, E. A. Genetic data and electronic health records: a discussion of ethical, logistical and technological considerations. J. Am. Med. Inform. Assoc. 21 , 171–180 (2014).
Putt, S. et al. Exploration of experiences with and understanding of polygenic risk scores for bipolar disorder. J. Affect. Disord. 265 , 342–350 (2020).
Lewis, A. C. F. & Green, R. C. Polygenic risk scores in the clinic: new perspectives needed on familiar ethical issues. Genome Med. 13 , 14 (2021). This paper provides a good overview of the different ethical issues related to the use of polygenic risk scores in clinical practice .
Green, R. C., Lautenbach, D. & McGuire, A. L. GINA, genetic discrimination, and genomic medicine. N. Engl. J. Med. 372 , 397–399 (2015).
Fost, N. Ethical issues in genetics. Pediatr. Clin. North Am. 39 , 79–89 (1992).
Joly, Y. et al. The Genetic Discrimination Observatory: confronting novel issues in genetic discrimination. Trends Genet. 37 , 951–954 (2021).
Tiller, J. et al. Genetic discrimination by Australian insurance companies: a survey of consumer experiences. Eur. J. Hum. Genet. 28 , 108–113 (2020).
Clayton, E. W., Evans, B. J., Hazel, J. W. & Rothstein, M. A. The law of genetic privacy: applications, implications, and limitations. J. Law Biosci. 6 , 1–36 (2019).
Phillips, A. et al. Informing relatives of their genetic risk: an examination of the Belgian legal context. Eur. J. Hum. Genet . https://doi.org/10.1038/s41431-021-01016-3 (2022).
Kraft, S. A., Duenas, D., Wilfond, B. S. & Goddard, K. A. B. The evolving landscape of expanded carrier screening: challenges and opportunities. Genet. Med. 21 , 790–797 (2019).
Turley, P. et al. Problems with using polygenic scores to select embryos. N. Engl. J. Med. 385 , 78–86 (2021). In this paper, the ethical and legal aspect of embryo selection using polygenic risk scores is discussed as well as the gains and the risks on society.
Kostick, K., Brannan, C., Pereira, S. & Lázaro‐Muñoz, G. Psychiatric genetics researchers’ views on offering return of results to individual participants. Am. J. Med. Genet. B Neuropsychiatr. Genet. 180 , 589–600 (2018).
Karavani, E. et al. Screening human embryos for polygenic traits has limited utility. Cell 179 , 1424–1435 (2019). In this paper, theory, simulations and real data are used to assess the impact of embryo selection based on polygenic risk scores. It is shown that such predictions have wide confidence intervals and in practice are hardly useful.
Ball, P. Polygenic screening of embryos is here, but is it ethical? (The Observer, 2021).
Baruch, S., Kaufman, D. & Hudson, K. L. Genetic testing of embryos: practices and perspectives of US in vitro fertilization clinics. Fertil. Steril. 89 , 1053–1058 (2008).
Al-Khelaifi, F. et al. Genome-wide association study reveals a novel association between MYBPC3 gene polymorphism, endurance athlete status, aerobic capacity and steroid metabolism. Front. Genet. 11 , 595 (2020).
Morgan, M. D. et al. Genome-wide study of hair colour in UK Biobank explains most of the SNP heritability. Nat. Commun. 9 , 5271 (2018).
Dondorp, W. & de Wert, G. Refining the ethics of preimplantation genetic diagnosis: a plea for contextualized proportionality. Bioethics 33 , 294–301 (2019).
Bayefsky, M. J. Comparative preimplantation genetic diagnosis policy in Europe and the USA and its implications for reproductive tourism. Reprod. Biomed. Soc. Online 3 , 41–47 (2016).
Corveleyn, A., Morris, M., Zika, E., & Institute for Prospective Technological Studies. Preimplantation genetic diagnosis in Europe (Publications Office, 2007).
Bayefsky, M. Who should regulate preimplantation genetic diagnosis in the United States? AMA J. Ethics 20 , 1160–1167 (2018).
Rodriguez, L. L., Brooks, L. D., Greenberg, J. H. & Green, E. D. The complexities of genomic identifiability. Science 339 , 275–276 (2013).
Homer, N. et al. Resolving individuals contributing trace amounts of DNA to highly complex mixtures using high-density SNP genotyping microarrays. PLoS Genet. 4 , e1000167 (2008).
D. Conley. What’s your polygenic score? Scientific American (13 March 2017).
Shabani, M. & Marelli, L. Re‐identifiability of genomic data and the GDPR. EMBO Rep. 20 , e48316 (2019).
Hansson, M. G. et al. The risk of re-identification versus the need to identify individuals in rare disease research. Eur. J. Hum. Genet. 24 , 1553–1558 (2016).
Beil, A. et al. Disclosure of clinically actionable genetic variants to thoracic aortic dissection biobank participants. BMC Med. Genomics 14 , 66 (2021).
Wolf, S. M. Return of individual research results and incidental findings: facing the challenges of translational science. Annu. Rev. Genomics Hum. Genet. 14 , 557–577 (2013).
Wolf, S. M. The challenge of incidental findings. J. Law. Med. Ethics 36 , 216–218 (2008).
De Clercq, E., Kaye, J., Wolf, S. M., Koenig, B. A. & Elger, B. S. Returning results in biobank research: global trends and solutions. Genet. Test. Mol. Biomark. 21 , 128–131 (2017).
Bredenoord, A. L., Onland-Moret, N. C. & Van Delden, J. J. M. Feedback of individual genetic results to research participants: in favor of a qualified disclosure policy. Hum. Mutat. 32 , 861–867 (2011). This article provides a very thoughtful overview of ethical issues concerning whether and when researchers have a moral obligation to return genetic research results to participants. It also includes clear suggestions on a qualified disclosure policy .
Jarvik, G. P. et al. Return of genomic results to research participants: the floor, the ceiling, and the choices in between. Am. J. Hum. Genet. 94 , 818–826 (2014).
Dashti, H. S. et al. Genome-wide association study of breakfast skipping links clock regulation with food timing. Am. J. Clin. Nutr. 110 , 473–484 (2019).
van de Vegte, Y. J., Said, M. A., Rienstra, M., van der Harst, P. & Verweij, N. Genome-wide association studies and Mendelian randomization analyses for leisure sedentary behaviours. Nat. Commun. 11 , 1770 (2020).
Coffee and Caffeine Genetics Consortium. et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol. Psychiatry 20 , 647–656 (2015).
Gerard, R. W. The role of pure science. Science 88 , 361–368 (1938).
Carlson, J. & Harris, K. Quantifying and contextualizing the impact of bioRxiv preprints through automated social media audience segmentation. PLoS Biol. 18 , e3000860 (2020). This paper introduces a method to assess the impact of preprints, which can aid in tracking online (mis)appropriation of GWAS results .
Winkelman, W. D., Missmer, S. A., Myers, D. & Ginsburg, E. S. Public perspectives on the use of preimplantation genetic diagnosis. J. Assist. Reprod. Genet. 32 , 665–675 (2015).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8 , 2281–2308 (2013).
Xu, M. CCR5-Δ32 biology, gene editing, and warnings for the future of CRISPR–Cas9 as a human and humane gene editing tool. Cell Biosci. 10 , 48 (2020).
van Beers, B. C. Rewriting the human genome, rewriting human rights law? Human rights, human dignity, and human germline modification in the CRISPR era. J. Law Biosci. 7 , lsaa006 (2020).
Coller, B. S. Ethics of human genome editing. Annu. Rev. Med. 70 , 289–305 (2019).
Read the Affordable Care Act, Health Care Law (HealthCare, accessed 1 April 2023); https://www.healthcare.gov/where-can-i-read-the-affordable-care-act/1
EU Law - EUR-Lex (accessed 1 April 2023, EUR-Lex); https://eur-lex.europa.eu/homepage.html?locale=en
Taylor, M. J., Wallace, S. E. & Prictor, M. United Kingdom: transfers of genomic data to third countries. Hum. Genet. 137 , 637–645 (2018).
Wertz, D. C. & Knoppers, B. M. Serious genetic disorders: can or should they be defined? Am. J. Med. Genet. 108 , 29–35 (2002).
Botkin, J. R. Fetal privacy and confidentiality. Hastings Cent. Rep. 25 , 32–39 (1995).
Lázaro-Muñoz, G., Pereira, S., Carmi, S. & Lencz, T. Screening embryos for polygenic conditions and traits: ethical considerations for an emerging technology. Genet. Med. 23 , 432–434 (2021). This is a very clear commentary on ethical issues related to polygenic embryonic screening .
Ganna, A. et al. Large-scale GWAS reveals insights into the genetic architecture of same-sex sexual behavior. Science 365 , eaat7693 (2019).
Dawood, K., Bailey, J. M. & Martin, N. G. Genetic and Environmental Influences on Sexual Orientation. in Handbook of Behavior Genetics (ed. Kim, Y. K.) 11 (Springer, 2009).
Check Hayden, E. Ethics: taboo genetics. Nature 502 , 26–28 (2013).
Sniekers, S. et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat. Genet. 49 , 1107–1112 (2017).
Hsu, S. Super-intelligent humans are coming. Nautilus (2 October 2014).
Bulik-Sullivan, B. K. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47 , 291–295 (2015).
Watanabe, K. et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat. Genet. 51 , 1339–1348 (2019).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50 , 693–698 (2018).
Brainstorm Consortium. et al. Analysis of shared heritability in common disorders of the brain. Science 360 , eaap8757 (2018).
Hill, W. D., Harris, S. E. & Deary, I. J. What genome-wide association studies reveal about the association between intelligence and mental health. Curr. Opin. Psychol. 27 , 25–30 (2019).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50 , 1112–1121 (2018).
Lencz, T. et al. Concerns about the use of polygenic embryo screening for psychiatric and cognitive traits. Lancet Psychiatry https://doi.org/10.1016/S2215-0366(22)00157-2 (2022).
Wedow, R., Martschenko, D. O. & Trejo, S. Scientists must consider the risk of racist misappropriation of research (Scientific American, 2022).
Molteni, M. Buffalo shooting ignites a debate over the role of genetics researchers in white supremacist ideology. STAT (23 May 2022).
Wills, M. Are clusters races? A discussion of the rhetorical appropriation of Rosenberg et al.’s ‘Genetic structure of human populations’. Philos. Theor. Pract. Biol . 9 , 12 (2017).
Lewis, A. C. F. et al. Getting genetic ancestry right for science and society. Science 376 , 250–252 (2022).
Piffer, D. New genes, same results: group-level genotypic intelligence for 26 and 52 populations. topseudoscience (2 June 2017).
McKinley, J., Traub, A. & Closson, T. Gunman kills 10 at Buffalo supermarket in racist attack (The New York Times, 2022).
Jones, D. What is the ‘great replacement’ and how is it tied to the Buffalo shooting suspect? (NPR, 2022).
Download references
Acknowledgements
This work is supported by the Netherlands Organization for Scientific Research—Gravitation project ‘BRAINSCAPES: a Roadmap from Neurogenetics to Neurobiology’ (024.004.012) and the European Research Council advanced grant ‘From GWAS to Function’ (ERC-2018-ADG 834057). We thank E. Uffelmann and P. Jansen for critical reading and fruitful discussions on earlier versions of this paper, and E. Bunnik for critically reading a pre-final version from a bioethical point of view.
Author information
Authors and affiliations.
Department of Complex Trait Genetics, Center for Neurogenomics and Cognitive Research, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands
Matthieu C. de Hemptinne & Danielle Posthuma
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Danielle Posthuma .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Neuroscience thanks Andrew McIntosh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
de Hemptinne, M.C., Posthuma, D. Addressing the ethical and societal challenges posed by genome-wide association studies of behavioral and brain-related traits. Nat Neurosci 26 , 932–941 (2023). https://doi.org/10.1038/s41593-023-01333-4
Download citation
Received : 29 April 2022
Accepted : 14 April 2023
Published : 22 May 2023
Issue Date : June 2023
DOI : https://doi.org/10.1038/s41593-023-01333-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Let’s talk about diversity in human neuroscience.
Nature Methods (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Original article
- Open access
- Published: 13 July 2021
Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures
- Shivadas Sivasubramaniam 1 ,
- Dita Henek Dlabolová 2 ,
- Veronika Kralikova 3 &
- Zeenath Reza Khan 3
International Journal for Educational Integrity volume 17 , Article number: 14 ( 2021 ) Cite this article
17k Accesses
12 Citations
4 Altmetric
Metrics details
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own committees to focus on or approve activities that have ethical impact. In contrast, lesser-developed countries (worldwide) are trying to set up these committees to govern their academia and research. As the first European consortium established to assist academic integrity, European Network for Academic Integrity (ENAI), we felt the importance of guiding those institutions and communities that are trying to conduct research with ethical principles. We have established an ethical advisory working group within ENAI with the aim to promote ethics within curriculum, research and institutional policies. We are constantly researching available data on this subject and committed to help the academia to convey and conduct ethical behaviour. Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications of research projects among peers. Therefore, this short paper preliminarily aims to critically review the available information on ethics, the history behind establishing ethical principles and its international guidelines to govern research.
The paper is based on the workshop conducted in the 5th International conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019. During the workshop, we have detailed a) basic needs of an ethical committee within an institution; b) a typical ethical approval process (with examples from three different universities); and c) the ways to obtain informed consent with some examples. These are summarised in this paper with some example comparisons of ethical approval processes from different universities. We believe this paper will provide guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Introduction
Ethics and ethical behaviour (often linked to “responsible practice”) are the fundamental pillars of a civilised society. Ethical behaviour with integrity is important to maintain academic and research activities. It affects everything we do, and gets precedence with anything that would include/affect, transform, or impact upon individuals, communities or any living creatures. In other words, ethics would help us improve our living standards (LaFollette, 2007 ). The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law, but is also gaining recognition in all disciplines engaged in research. Therefore, institutions are expected to develop ethical guidelines in research to maintain quality, initiate/own integrity and above all be transparent to be successful by limiting any allegation of misconduct (Flite and Harman, 2013 ). This is especially true for higher education organisations that promote research and scholarly activities. Many European institutions have developed their own regulations for ethics by incorporating international codes (Getz, 1990 ). The lesser developed countries are trying to set up these committees to govern their academia and research. World Health Organization has stated that adhering to “ ethical principles … [is central and important]... in order to protect the dignity, rights and welfare of research participants ” (WHO, 2021 ). Ethical guidelines taught to students can help develop ethical researchers and members of society who uphold values of ethical principles in practice.
As the first European-wide consortium established to assist academic integrity (European Network for Academic Integrity – ENAI), we felt the importance of guiding those institutions and communities that are trying to teach, research, and include ethical principles by providing overarching understanding of ethical guidelines that may influence policy. Therefore, we set up an advisory working group within ENAI in 2018 to support matters related to ethics, ethical committees and assisting on ethics related teaching activities.
Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications among peers. This became the premise for this research paper. We first carried out a literature survey to review and summarise existing ethical governance (with historical perspectives) and procedures that are already in place to guide researchers in different discipline areas. By doing so, we attempted to consolidate, document and provide important steps in a typical ethical application process with example procedures from different universities. Finally, we attempted to provide insights and findings from practical workshops carried out at the 5th International Conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019, focussing on:
• highlighting the basic needs of an ethical committee within an institution,
• discussing and sharing examples of a typical ethical approval process,
• providing guidelines on the ways to teach research ethics with some examples.
We believe this paper provides guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Background literature survey
Responsible research practice (RRP) is scrutinised by the aspects of ethical principles and professional standards (WHO’s Code of Conduct for responsible Research, 2017). The Singapore statement on research integrity (The Singapore Statement on Research integrity, 2010) has provided an internationally acceptable guidance for RRP. The statement is based on maintaining honesty, accountability, professional courtesy in all aspects of research and maintaining fairness during collaborations. In other words, it does not simply focus on the procedural part of the research, instead covers wider aspects of “integrity” beyond the operational aspects (Israel and Drenth, 2016 ).
Institutions should focus on providing ethical guidance based on principles and values reflecting upon all aspects/stages of research (from the funding application/project development stage upto or beyond project closing stage). Figure 1 summarizes the different aspects/stages of a typical research and highlights the needs of RRP in compliance with ethical governance at each stage with examples (the figure is based on Resnik, 2020 ; Žukauskas et al., 2018 ; Anderson, 2011 ; Fouka and Mantzorou, 2011 ).
Summary of the enabling ethical governance at different stages of research. Note that it is imperative for researchers to proactively consider the ethical implications before, during and after the actual research process. The summary shows that RRP should be in line with ethical considerations even long before the ethical approval stage
Individual responsibilities to enhance RRP
As explained in Fig. 1 , a successfully governed research should consider ethics at the planning stages prior to research. Many international guidance are compatible in enforcing/recommending 14 different “responsibilities” that were first highlighted in the Singapore Statement (2010) for researchers to follow and achieve competency in RRP. In order to understand the purpose and the expectation of these ethical guidelines, we have carried out an initial literature survey on expected individual responsibilities. These are summarised in Table 1 .
By following these directives, researchers can carry out accountable research by maximising ethical self-governance whilst minimising misconducts. In our own experiences of working with many researchers, their focus usually revolves around ethical “clearance” rather than behaviour. In other words, they perceive this as a paper exercise rather than trying to “own” ethical behaviour in everything they do. Although the ethical principles and responsibilities are explicitly highlighted in the majority of international guidelines [such as UK’s Research Governance Policy (NICE, 2018 ), Australian Government’s National Statement on Ethical Conduct in Human Research (Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR), 2018 ), the Singapore Statement (2010) etc.]; and the importance of holistic approach has been argued in ethical decision making, many researchers and/or institutions only focus on ethics linked to the procedural aspects.
Studies in the past have also highlighted inconsistencies in institutional guidelines pointing to the fact that these inconsistencies may hinder the predicted research progress (Desmond & Dierickx 2021 ; Alba et al., 2020 ; Dellaportas et al., 2014 ; Speight 2016 ). It may also be possible that these were and still are linked to the institutional perceptions/expectations or the pre-empting contextual conditions that are imposed by individual countries. In fact, it is interesting to note many research organisations and HE institutions establish their own policies based on these directives.
Research governance - origins, expectations and practices
Ethical governance in clinical medicine helps us by providing a structure for analysis and decision-making. By providing workable definitions of benefits and risks as well as the guidance for evaluating/balancing benefits over risks, it supports the researchers to protect the participants and the general population.
According to the definition given by National Institute of Clinical care Excellence, UK (NICE 2018 ), “ research governance can be defined as the broad range of regulations, principles and standards of good practice that ensure high quality research ”. As stated above, our literature-based research survey showed that most of the ethical definitions are basically evolved from the medical field and other disciplines have utilised these principles to develop their own ethical guidance. Interestingly, historical data show that the medical research has been “self-governed” or in other words implicated by the moral behaviour of individual researchers (Fox 2017 ; Shaw et al., 2005 ; Getz, 1990 ). For example, early human vaccination trials conducted in 1700s used the immediate family members as test subjects (Fox, 2017 ). Here the moral justification might have been the fact that the subjects who would have been at risk were either the scientists themselves or their immediate families but those who would reap the benefits from the vaccination were the general public/wider communities. However, according to the current ethical principles, this assumption is entirely not acceptable.
Historically, ambiguous decision-making and resultant incidences of research misconduct have led to the need for ethical research governance in as early as the 1940’s. For instance, the importance of an international governance was realised only after the World War II, when people were astonished to note the unethical research practices carried out by Nazi scientists. As a result of this, in 1947 the Nuremberg code was published. The code mainly focussed on the following:
Informed consent and further insisted the research involving humans should be based on prior animal work,
The anticipated benefits should outweigh the risk,
Research should be carried out only by qualified scientists must conduct research,
Avoiding physical and mental suffering and.
Avoiding human research that would result in which death or disability.
(Weindling, 2001 ).
Unfortunately, it was reported that many researchers in the USA and elsewhere considered the Nuremberg code as a document condemning the Nazi atrocities, rather than a code for ethical governance and therefore ignored these directives (Ghooi, 2011 ). It was only in 1964 that the World Medical Association published the Helsinki Declaration, which set the stage for ethical governance and the implementation of the Institutional Review Board (IRB) process (Shamoo and Irving, 1993 ). This declaration was based on Nuremberg code. In addition, the declaration also paved the way for enforcing research being conducted in accordance with these guidelines.
Incidentally, the focus on research/ethical governance gained its momentum in 1974. As a result of this, a report on ethical principles and guidelines for the protection of human subjects of research was published in 1979 (The Belmont Report, 1979 ). This report paved the way to the current forms of ethical governance in biomedical and behavioural research by providing guidance.
Since 1994, the WHO itself has been providing several guidance to health care policy-makers, researchers and other stakeholders detailing the key concepts in medical ethics. These are specific to applying ethical principles in global public health.
Likewise, World Organization for Animal Health (WOAH), and International Convention for the Protection of Animals (ICPA) provide guidance on animal welfare in research. Due to this continuous guidance, together with accepted practices, there are internationally established ethical guidelines to carry out medical research. Our literature survey further identified freely available guidance from independent organisations such as COPE (Committee of Publication Ethics) and ALLEA (All European Academics) which provide support for maintaining research ethics in other fields such as education, sociology, psychology etc. In reality, ethical governance is practiced differently in different countries. In the UK, there is a clinical excellence research governance, which oversees all NHS related medical research (Mulholland and Bell, 2005 ). Although, the governance in other disciplines is not entirely centralised, many research funding councils and organisations [such as UKRI (UK-Research and Innovation; BBSC (Biotechnology and Biological Sciences Research Council; MRC (Medical Research Council); EPSRC (Economic and Social Research Council)] provide ethical governance and expect institutional adherence and monitoring. They expect local institutional (i.e. university/institutional) research governance for day-to-day monitoring of the research conducted within the organisation and report back to these funding bodies, monthly or annually (Department of Health, 2005). Likewise, there are nationally coordinated/regulated ethics governing bodies such as the US Office for Human Research Protections (US-OHRP), National Institute of Health (NIH) and the Canadian Institutes for Health Research (CIHR) in the USA and Canada respectively (Mulholland and Bell, 2005 ). The OHRP in the USA formally reviews all research activities involving human subjects. On the other hand, in Canada, CIHR works with the Natural Sciences and Engineering Research Council (NSERC), and the Social Sciences and Humanities Research Council (SSHRC). They together have produced a Tri-Council Policy Statement (TCPS) (Stephenson et al., 2020 ) as ethical governance. All Canadian institutions are expected to adhere to this policy for conducting research. As for Australia, the research is governed by the Australian code for the responsible conduct of research (2008). It identifies the responsibilities of institutions and researchers in all areas of research. The code has been jointly developed by the National Health and Medical Research Council (NHMRC), the Australian Research Council (ARC) and Universities Australia (UA). This information is summarized in Table 2 .
Basic structure of an institutional ethical advisory committee (EAC)
The WHO published an article defining the basic concepts of an ethical advisory committee in 2009 (WHO, 2009 - see above). According to this, many countries have established research governance and monitor the ethical practice in research via national and/or regional review committees. The main aims of research ethics committees include reviewing the study proposals, trying to understand the justifications for human/animal use, weighing the merits and demerits of the usage (linking to risks vs. potential benefits) and ensuring the local, ethical guidelines are followed Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research, 2020 ; Guide for Research Ethics - Council of Europe, 2014 ). Once the research has started, the committee needs to carry out periodic surveillance to ensure the institutional ethical norms are followed during and beyond the study. They may also be involved in setting up and/or reviewing the institutional policies.
For these aspects, IRB (or institutional ethical advisory committee - IEAC) is essential for local governance to enhance best practices. The advantage of an IRB/EEAC is that they understand the institutional conditions and can closely monitor the ongoing research, including any changes in research directions. On the other hand, the IRB may be overly supportive to accept applications, influenced by the local agenda for achieving research excellence, disregarding ethical issues (Kotecha et al., 2011 ; Kayser-Jones, 2003 ) or, they may be influenced by the financial interests in attracting external funding. In this respect, regional and national ethics committees are advantageous to ensure ethical practice. Due to their impartiality, they would provide greater consistency and legitimacy to the research (WHO, 2009 ). However, the ethical approval process of regional and national ethics committees would be time consuming, as they do not have the local knowledge.
As for membership in the IRBs, most of the guidelines [WHO, NICE, Council of Europe, (2012), European Commission - Facilitating Research Excellence in FP7 ( 2013 ) and OHRP] insist on having a variety of representations including experts in different fields of research, and non-experts with the understanding of local, national/international conflicts of interest. The former would be able to understand/clarify the procedural elements of the research in different fields; whilst the latter would help to make neutral and impartial decisions. These non-experts are usually not affiliated to the institution and consist of individuals representing the broader community (particularly those related to social, legal or cultural considerations). IRBs consisting of these varieties of representation would not only be in a position to understand the study procedures and their potential direct or indirect consequences for participants, but also be able to identify any community, cultural or religious implications of the study.
Understanding the subtle differences between ethics and morals
Interestingly, many ethical guidelines are based on society’s moral “beliefs” in such a way that the words “ethics”‘and “morals” are reciprocally used to define each other. However, there are several subtle differences between them and we have attempted to compare and contrast them herein. In the past, many authors have interchangeably used the words “morals”‘and “ethics”‘(Warwick, 2003 ; Kant, 2018 ; Hazard, GC (Jr)., 1994 , Larry, 1982 ). However, ethics is linked to rules governed by an external source such as codes of conduct in workplaces (Kuyare et al., 2014 ). In contrast, morals refer to an individual’s own principles regarding right and wrong. Quinn ( 2011 ) defines morality as “ rules of conduct describing what people ought and ought not to do in various situations … ” while ethics is “... the philosophical study of morality, a rational examination into people’s moral beliefs and behaviours ”. For instance, in a case of parents demanding that schools overturn a ban on use of corporal punishment of children by schools and teachers (Children’s Rights Alliance for England, 2005 ), the parents believed that teachers should assume the role of parent in schools and use corporal or physical punishment for children who misbehaved. This stemmed from their beliefs and what they felt were motivated by “beliefs of individuals or groups”. For example, recent media highlights about some parents opposing LGBT (Lesbian, Gay, Bisexual, and Transgender) education to their children (BBC News, 2019 ). One parent argued, “Teaching young children about LGBT at a very early stage is ‘morally’ wrong”. She argued “let them learn by themselves as they grow”. This behaviour is linked to and governed by the morals of an ethnic community. Thus, morals are linked to the “beliefs of individuals or group”. However, when it comes to the LGBT rights these are based on ethical principles of that society and governed by law of the land. However, the rights of children to be protected from “inhuman and degrading” treatment is based on the ethical principles of the society and governed by law of the land. Individuals, especially those who are working in medical or judicial professions have to follow an ethical code laid down by their profession, regardless of their own feelings, time or preferences. For instance, a lawyer is expected to follow the professional ethics and represent a defendant, despite the fact that his morals indicate the defendant is guilty.
In fact, we as a group could not find many scholarly articles clearly comparing or contrasting ethics with morals. However, a table presented by Surbhi ( 2015 ) (Difn website c ) tries to differentiate these two terms (see Table 3 ).
Although Table 3 gives some insight on the differences between these two terms, in practice many use these terms as loosely as possible mainly because of their ambiguity. As a group focussed on the application of these principles, we would recommend to use the term “ethics” and avoid “morals” in research and academia.
Based on the literature survey carried out, we were able to identify the following gaps:
there is some disparity in existing literature on the importance of ethical guidelines in research
there is a lack of consensus on what code of conduct should be followed, where it should be derived from and how it should be implemented
The mission of ENAI’s ethical advisory working group
The Ethical Advisory Working Group of ENAI was established in 2018 to promote ethical code of conduct/practice amongst higher educational organisations within Europe and beyond (European Network for Academic Integrity, 2018 ). We aim to provide unbiased advice and consultancy on embedding ethical principles within all types of academic, research and public engagement activities. Our main objective is to promote ethical principles and share good practice in this field. This advisory group aims to standardise ethical norms and to offer strategic support to activities including (but not exclusive to):
● rendering advice and assistance to develop institutional ethical committees and their regulations in member institutions,
● sharing good practice in research and academic ethics,
● acting as a critical guide to institutional review processes, assisting them to maintain/achieve ethical standards,
● collaborating with similar bodies in establishing collegiate partnerships to enhance awareness and practice in this field,
● providing support within and outside ENAI to develop materials to enhance teaching activities in this field,
● organising training for students and early-career researchers about ethical behaviours in form of lectures, seminars, debates and webinars,
● enhancing research and dissemination of the findings in matters and topics related to ethics.
The following sections focus on our suggestions based on collective experiences, review of literature provided in earlier sections and workshop feedback collected:
a) basic needs of an ethical committee within an institution;
b) a typical ethical approval process (with examples from three different universities); and
c) the ways to obtain informed consent with some examples. This would give advice on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Setting up an institutional ethical committee (ECs)
Institutional Ethical Committees (ECs) are essential to govern every aspect of the activities undertaken by that institute. With regards to higher educational organisations, this is vital to establish ethical behaviour for students and staff to impart research, education and scholarly activities (or everything) they do. These committees should be knowledgeable about international laws relating to different fields of studies (such as science, medicine, business, finance, law, and social sciences). The advantages and disadvantages of institutional, subject specific or common (statutory) ECs are summarised in Fig. 2 . Some institutions have developed individual ECs linked to specific fields (or subject areas) whilst others have one institutional committee that overlooks the entire ethical behaviour and approval process. There is no clear preference between the two as both have their own advantages and disadvantages (see Fig. 2 ). Subject specific ECs are attractive to medical, law and business provisions, as it is perceived the members within respective committees would be able to understand the subject and therefore comprehend the need of the proposed research/activity (Kadam, 2012 ; Schnyder et al., 2018 ). However, others argue, due to this “ specificity ”, the committee would fail to forecast the wider implications of that application. On the other hand, university-wide ECs would look into the wider implications. Yet they find it difficult to understand the purpose and the specific applications of that research. Not everyone understands dynamics of all types of research methodologies, data collection, etc., and therefore there might be a chance of a proposal being rejected merely because the EC could not understand the research applications (Getz, 1990 ).
Summary of advantages and disadvantages of three different forms of ethical committees
[N/B for Fig. 2 : Examples of different types of ethical application procedures and forms used were discussed with the workshop attendees to enhance their understanding of the differences. GDPR = General Data Protection Regulation].
Although we recommend a designated EC with relevant professional, academic and ethical expertise to deal with particular types of applications, the membership (of any EC) should include some non-experts who would represent the wider community (see above). Having some non-experts in EC would not only help the researchers to consider explaining their research in layperson’s terms (by thinking outside the box) but also would ensure efficiency without compromising participants/animal safety. They may even help to address the common ethical issues outside research culture. Some UK universities usually offer this membership to a clergy, councillor or a parliamentarian who does not have any links to the institutions. Most importantly, it is vital for any EC members to undertake further training in addition to previous experience in the relevant field of research ethics.
Another issue that raises concerns is multi-centre research, involving several institutions, where institutionalised ethical approvals are needed from each partner. In some cases, such as clinical research within the UK, a common statutory EC called National Health Services (NHS) Research Ethics Committee (NREC) is in place to cover research ethics involving all partner institutions (NHS, 2018 ). The process of obtaining approval from this type of EC takes time, therefore advanced planning is needed.
Ethics approval forms and process
During the workshop, we discussed some anonymised application forms obtained from open-access sources for qualitative and quantitative research as examples. Considering research ethics, for the purpose of understanding, we arbitrarily divided this in two categories; research based on (a) quantitative and (b) qualitative methodologies. As their name suggests their research approach is extremely different from each other. The discussion elicited how ECs devise different types of ethical application form/questions. As for qualitative research, these are often conducted as “face-to-face” interviews, which would have implications on volunteer anonymity.
Furthermore, discussions posited when the interviews are replaced by on-line surveys, they have to be administered through registered university staff to maintain confidentiality. This becomes difficult when the research is a multi-centre study. These types of issues are also common in medical research regarding participants’ anonymity, confidentially, and above all their right to withdraw consent to be involved in research.
Storing and protecting data collected in the process of the study is also a point of consideration when applying for approval.
Finally, the ethical processes of invasive (involving human/animals) and non-invasive research (questionnaire based) may slightly differ from one another. Following research areas are considered as investigations that need ethical approval:
research that involves human participants (see below)
use of the ‘products’ of human participants (see below)
work that potentially impacts on humans (see below)
research that involves animals
In addition, it is important to provide a disclaimer even if an ethical approval is deemed unnecessary. Following word cloud (Fig. 3 ) shows the important variables that need to be considered at the brainstorming stage before an ethical application. It is worth noting the importance of proactive planning predicting the “unexpected” during different phases of a research project (such as planning, execution, publication, and future directions). Some applications (such as working with vulnerable individuals or children) will require safety protection clearance (such as DBS - Disclosure and Barring Service, commonly obtained from the local police). Please see section on Research involving Humans - Informed consents for further discussions.
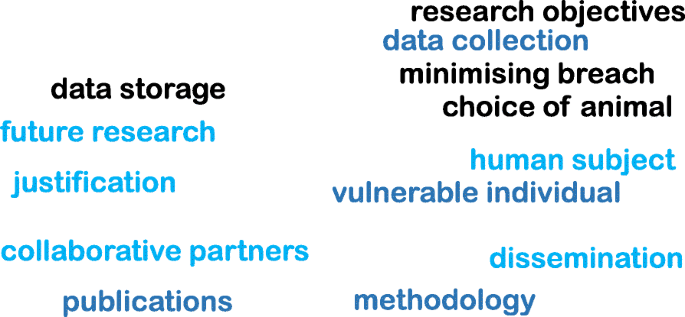
Examples of important variables that need to be considered for an ethical approval
It is also imperative to report or re-apply for ethical approval for any minor or major post-approval changes to original proposals made. In case of methodological changes, evidence of risk assessments for changes and/or COSHH (Control of Substances Hazardous to Health Regulations) should also be given. Likewise, any new collaborative partners or removal of researchers should also be notified to the IEAC.
Other findings include:
in case of complete changes in the project, the research must be stopped and new approval should be seeked,
in case of noticing any adverse effects to project participants (human or non-human), these should also be notified to the committee for appropriate clearance to continue the work, and
the completion of the project must also be notified with the indication whether the researchers may restart the project at a later stage.
Research involving humans - informed consents
While discussing research involving humans and based on literature review, findings highlight the human subjects/volunteers must willingly participate in research after being adequately informed about the project. Therefore, research involving humans and animals takes precedence in obtaining ethical clearance and its strict adherence, one of which is providing a participant information sheet/leaflet. This sheet should contain a full explanation about the research that is being carried out and be given out in lay-person’s terms in writing (Manti and Licari 2018 ; Hardicre 2014 ). Measures should also be in place to explain and clarify any doubts from the participants. In addition, there should be a clear statement on how the participants’ anonymity is protected. We provide below some example questions below to help the researchers to write this participant information sheet:
What is the purpose of the study?
Why have they been chosen?
What will happen if they take part?
What do they have to do?
What happens when the research stops?
What if something goes wrong?
What will happen to the results of the research study?
Will taking part be kept confidential?
How to handle “vulnerable” participants?
How to mitigate risks to participants?
Many institutional ethics committees expect the researchers to produce a FAQ (frequently asked questions) in addition to the information about research. Most importantly, the researchers also need to provide an informed consent form, which should be signed by each human participant. The five elements identified that are needed to be considered for an informed consent statement are summarized in Fig. 4 below (slightly modified from the Federal Policy for the Protection of Human Subjects ( 2018 ) - Diffn website c ).
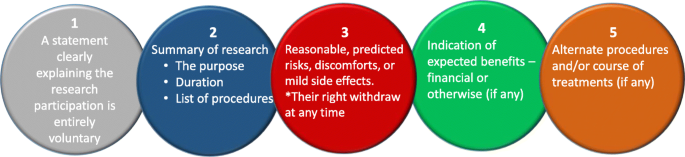
Five basic elements to consider for an informed consent [figure adapted from Diffn website c ]
The informed consent form should always contain a clause for the participant to withdraw their consent at any time. Should this happen all the data from that participant should be eliminated from the study without affecting their anonymity.
Typical research ethics approval process
In this section, we provide an example flow chart explaining how researchers may choose the appropriate application and process, as highlighted in Fig. 5 . However, it is imperative to note here that these are examples only and some institutions may have one unified application with separate sections to demarcate qualitative and quantitative research criteria.
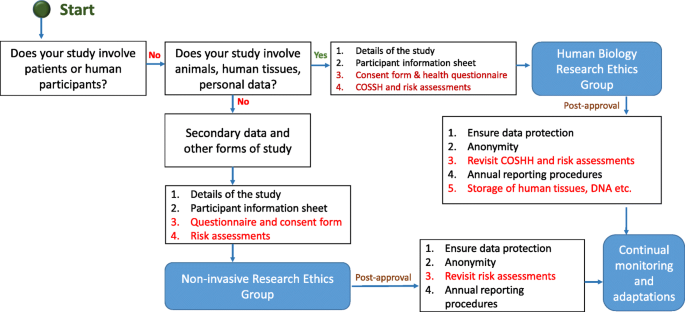
Typical ethical approval processes for quantitative and qualitative research. [N/B for Fig. 5 - This simplified flow chart shows that fundamental process for invasive and non-invasive EC application is same, the routes and the requirements for additional information are slightly different]
Once the ethical application is submitted, the EC should ensure a clear approval procedure with distinctly defined timeline. An example flow chart showing the procedure for an ethical approval was obtained from University of Leicester as open-access. This is presented in Fig. 6 . Further examples of the ethical approval process and governance were discussed in the workshop.
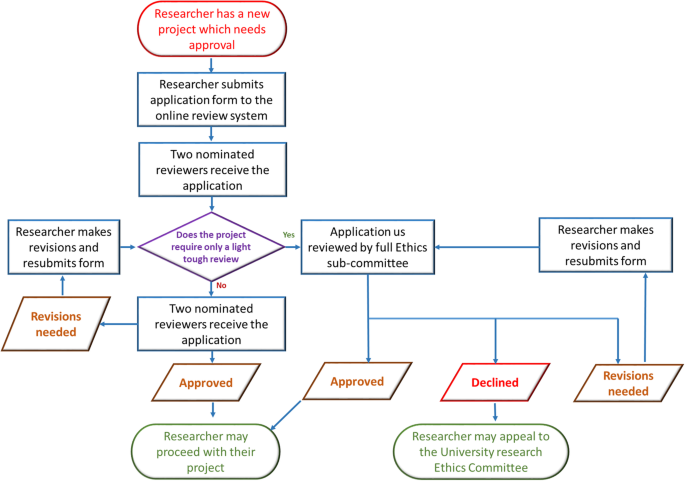
An example ethical approval procedures conducted within University of Leicester (Figure obtained from the University of Leicester research pages - Difn website d - open access)
Strategies for ethics educations for students
Student education on the importance of ethics and ethical behaviour in research and scholarly activities is extremely essential. Literature posits in the area of medical research that many universities are incorporating ethics in post-graduate degrees but when it comes to undergraduate degrees, there is less appetite to deliver modules or even lectures focussing on research ethics (Seymour et al., 2004 ; Willison and O’Regan, 2007 ). This may be due to the fact that undergraduate degree structure does not really focus on research (DePasse et al., 2016 ). However, as Orr ( 2018 ) suggested, institutions should focus more on educating all students about ethics/ethical behaviour and their importance in research, than enforcing punitive measures for unethical behaviour. Therefore, as an advisory committee, and based on our preliminary literature survey and workshop results, we strongly recommend incorporating ethical education within undergraduate curriculum. Looking at those institutions which focus on ethical education for both under-and postgraduate courses, their approaches are either (a) a lecture-based delivery, (b) case study based approach or (c) a combined delivery starting with a lecture on basic principles of ethics followed by generating a debate based discussion using interesting case studies. The combined method seems much more effective than the other two as per our findings as explained next.
As many academics who have been involved in teaching ethics and/or research ethics agree, the underlying principles of ethics is often perceived as a boring subject. Therefore, lecture-based delivery may not be suitable. On the other hand, a debate based approach, though attractive and instantly generates student interest, cannot be effective without students understanding the underlying basic principles. In addition, when selecting case studies, it would be advisable to choose cases addressing all different types of ethical dilemmas. As an advisory group within ENAI, we are in the process of collating supporting materials to help to develop institutional policies, creating advisory documents to help in obtaining ethical approvals, and teaching materials to enhance debate-based lesson plans that can be used by the member and other institutions.
Concluding remarks
In summary, our literature survey and workshop findings highlight that researchers should accept that ethics underpins everything we do, especially in research. Although ethical approval is tedious, it is an imperative process in which proactive thinking is essential to identify ethical issues that might affect the project. Our findings further lead us to state that the ethical approval process differs from institution to institution and we strongly recommend the researchers to follow the institutional guidelines and their underlying ethical principles. The ENAI workshop in Vilnius highlighted the importance of ethical governance by establishing ECs, discussed different types of ECs and procedures with some examples and highlighted the importance of student education to impart ethical culture within research communities, an area that needs further study as future scope.
Declarations
The manuscript was entirely written by the corresponding author with contributions from co-authors who have also taken part in the delivery of the workshop. Authors confirm that the data supporting the findings of this study are available within the article. We can also confirm that there are no potential competing interests with other organisations.
Availability of data and materials
Authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
ALL European academics
Australian research council
Biotechnology and biological sciences research council
Canadian institutes for health research
Committee of publication ethics
Ethical committee
European network of academic integrity
Economic and social research council
International convention for the protection of animals
institutional ethical advisory committee
Institutional review board
Immaculata university of Pennsylvania
Lesbian, gay, bisexual, and transgender
Medical research council)
National health services
National health services nih national institute of health (NIH)
National institute of clinical care excellence
National health and medical research council
Natural sciences and engineering research council
National research ethics committee
National statement on ethical conduct in human research
Responsible research practice
Social sciences and humanities research council
Tri-council policy statement
World Organization for animal health
Universities Australia
UK-research and innovation
US office for human research protections
Alba S, Lenglet A, Verdonck K, Roth J, Patil R, Mendoza W, Juvekar S, Rumisha SF (2020) Bridging research integrity and global health epidemiology (BRIDGE) guidelines: explanation and elaboration. BMJ Glob Health 5(10):e003237. https://doi.org/10.1136/bmjgh-2020-003237
Article Google Scholar
Anderson MS (2011) Research misconduct and misbehaviour. In: Bertram Gallant T (ed) Creating the ethical academy: a systems approach to understanding misconduct and empowering change in higher education. Routledge, pp 83–96
BBC News. (2019). Birmingham school LGBT LESSONS PROTEST investigated. March 8, 2019. Retrieved February 14, 2021, available online. URL: https://www.bbc.com/news/uk-england-birmingham-47498446
Children’s Rights Alliance for England. (2005). R (Williamson and others) v Secretary of State for Education and Employment. Session 2004–05. [2005] UKHL 15. Available Online. URL: http://www.crae.org.uk/media/33624/R-Williamson-and-others-v-Secretary-of-State-for-Education-and-Employment.pdf
Council of Europe. (2014). Texts of the Council of Europe on bioethical matters. Available Online. https://www.coe.int/t/dg3/healthbioethic/Texts_and_documents/INF_2014_5_vol_II_textes_%20CoE_%20bio%C3%A9thique_E%20(2).pdf
Dellaportas S, Kanapathippillai S, Khan, A and Leung, P. (2014). Ethics education in the Australian accounting curriculum: a longitudinal study examining barriers and enablers. 362–382. Available Online. URL: https://doi.org/10.1080/09639284.2014.930694 , 23, 4, 362, 382
DePasse JM, Palumbo MA, Eberson CP, Daniels AH (2016) Academic characteristics of orthopaedic surgery residency applicants from 2007 to 2014. JBJS 98(9):788–795. https://doi.org/10.2106/JBJS.15.00222
Desmond H, Dierickx K (2021) Research integrity codes of conduct in Europe: understanding the divergences. https://doi.org/10.1111/bioe.12851
Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR). (2018). Available Online. URL: https://www.nhmrc.gov.au/about-us/publications/australian-code-responsible-conduct-research-2018
Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research (2020, October 26). Available online. URL: https://www.enago.com/academy/importance-of-ethics-committees-in-scholarly-research/
Difn website c - Ethics vs Morals - Difference and Comparison. Retrieved July 14, 2020. Available online. URL: https://www.diffen.com/difference/Ethics_vs_Morals
Difn website d - University of Leicester. (2015). Staff ethics approval flowchart. May 1, 2015. Retrieved July 14, 2020. Available Online. URL: https://www2.le.ac.uk/institution/ethics/images/ethics-approval-flowchart/view
European Commission - Facilitating Research Excellence in FP7 (2013) https://ec.europa.eu/research/participants/data/ref/fp7/89888/ethics-for-researchers_en.pdf
European Network for Academic Integrity. (2018). Ethical advisory group. Retrieved February 14, 2021. Available online. URL: http://www.academicintegrity.eu/wp/wg-ethical/
Federal Policy for the Protection of Human Subjects. (2018). Retrieved February 14, 2021. Available Online. URL: https://www.federalregister.gov/documents/2017/01/19/2017-01058/federal-policy-for-the-protection-of-human-subjects#p-855
Flite, CA and Harman, LB. (2013). Code of ethics: principles for ethical leadership Perspect Health Inf Mana; 10(winter): 1d. PMID: 23346028
Fouka G, Mantzorou M (2011) What are the major ethical issues in conducting research? Is there a conflict between the research ethics and the nature of nursing. Health Sci J 5(1) Available Online. URL: https://www.hsj.gr/medicine/what-are-the-major-ethical-issues-in-conducting-research-is-there-a-conflict-between-the-research-ethics-and-the-nature-of-nursing.php?aid=3485
Fox G (2017) History and ethical principles. The University of Miami and the Collaborative Institutional Training Initiative (CITI) Program URL https://silo.tips/download/chapter-1-history-and-ethical-principles # (Available Online)
Getz KA (1990) International codes of conduct: An analysis of ethical reasoning. J Bus Ethics 9(7):567–577
Ghooi RB (2011) The nuremberg code–a critique. Perspect Clin Res 2(2):72–76. https://doi.org/10.4103/2229-3485.80371
Hardicre, J. (2014) Valid informed consent in research: an introduction Br J Nurs 23(11). https://doi.org/10.12968/bjon.2014.23.11.564 , 567
Hazard, GC (Jr). (1994). Law, morals, and ethics. Yale law school legal scholarship repository. Faculty Scholarship Series. Yale University. Available Online. URL: https://digitalcommons.law.yale.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3322&context=fss_papers
Israel, M., & Drenth, P. (2016). Research integrity: perspectives from Australia and Netherlands. In T. Bretag (Ed.), Handbook of academic integrity (pp. 789–808). Springer, Singapore. https://doi.org/10.1007/978-981-287-098-8_64
Kadam R (2012) Proactive role for ethics committees. Indian J Med Ethics 9(3):216. https://doi.org/10.20529/IJME.2012.072
Kant I (2018) The metaphysics of morals. Cambridge University Press, UK https://doi.org/10.1017/9781316091388
Kayser-Jones J (2003) Continuing to conduct research in nursing homes despite controversial findings: reflections by a research scientist. Qual Health Res 13(1):114–128. https://doi.org/10.1177/1049732302239414
Kotecha JA, Manca D, Lambert-Lanning A, Keshavjee K, Drummond N, Godwin M, Greiver M, Putnam W, Lussier M-T, Birtwhistle R (2011) Ethics and privacy issues of a practice-based surveillance system: need for a national-level institutional research ethics board and consent standards. Can Fam physician 57(10):1165–1173. https://europepmc.org/article/pmc/pmc3192088
Kuyare, MS., Taur, SR., Thatte, U. (2014). Establishing institutional ethics committees: challenges and solutions–a review of the literature. Indian J Med Ethics. https://doi.org/10.20529/IJME.2014.047
LaFollette, H. (2007). Ethics in practice (3rd edition). Blackwell
Larry RC (1982) The teaching of ethics and moral values in teaching. J High Educ 53(3):296–306. https://doi.org/10.1080/00221546.1982.11780455
Manti S, Licari A (2018) How to obtain informed consent for research. Breathe (Sheff) 14(2):145–152. https://doi.org/10.1183/20734735.001918
Mulholland MW, Bell J (2005) Research Governance and Research Funding in the USA: What the academic surgeon needs to know. J R Soc Med 98(11):496–502. https://doi.org/10.1258/jrsm.98.11.496
National Institute of Health (NIH) Ethics in Clinical Research. n.d. Available Online. URL: https://clinicalcenter.nih.gov/recruit/ethics.html
NHS (2018) Flagged Research Ethics Committees. Retrieved February 14, 2021. Available online. URL: https://www.hra.nhs.uk/about-us/committees-and-services/res-and-recs/flagged-research-ethics-committees/
NICE (2018) Research governance policy. Retrieved February 14, 2021. Available online. URL: https://www.nice.org.uk/Media/Default/About/what-we-do/science-policy-and-research/research-governance-policy.pdf
Orr, J. (2018). Developing a campus academic integrity education seminar. J Acad Ethics 16(3), 195–209. https://doi.org/10.1007/s10805-018-9304-7
Quinn, M. (2011). Introduction to Ethics. Ethics for an Information Age. 4th Ed. Ch 2. 53–108. Pearson. UK
Resnik. (2020). What is ethics in Research & why is it Important? Available Online. URL: https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
Schnyder S, Starring H, Fury M, Mora A, Leonardi C, Dasa V (2018) The formation of a medical student research committee and its impact on involvement in departmental research. Med Educ Online 23(1):1. https://doi.org/10.1080/10872981.2018.1424449
Seymour E, Hunter AB, Laursen SL, DeAntoni T (2004) Establishing the benefits of research experiences for undergraduates in the sciences: first findings from a three-year study. Sci Educ 88(4):493–534. https://doi.org/10.1002/sce.10131
Shamoo AE, Irving DN (1993) Accountability in research using persons with mental illness. Account Res 3(1):1–17. https://doi.org/10.1080/08989629308573826
Shaw, S., Boynton, PM., and Greenhalgh, T. (2005). Research governance: where did it come from, what does it mean? Research governance framework for health and social care, 2nd ed. London: Department of Health. https://doi.org/10.1258/jrsm.98.11.496 , 98, 11, 496, 502
Book Google Scholar
Speight, JG. (2016) Ethics in the university |DOI: https://doi.org/10.1002/9781119346449 scrivener publishing LLC
Stephenson GK, Jones GA, Fick E, Begin-Caouette O, Taiyeb A, Metcalfe A (2020) What’s the protocol? Canadian university research ethics boards and variations in implementing tri-Council policy. Can J Higher Educ 50(1)1): 68–81
Surbhi, S. (2015). Difference between morals and ethics [weblog]. March 25, 2015. Retrieved February 14, 2021. Available Online. URL: http://keydifferences.com/difference-between-morals-and-ethics.html
The Belmont Report (1979). Ethical Principles and Guidelines for the Protection of Human Subjects of Research. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Retrieved February 14, 2021. Available online. URL: https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf
The Singapore Statement on Research Integrity. (2020). Nicholas Steneck and Tony Mayer, Co-chairs, 2nd World Conference on Research Integrity; Melissa Anderson, Chair, Organizing Committee, 3rd World Conference on Research Integrity. Retrieved February 14, 2021. Available online. URL: https://wcrif.org/documents/327-singapore-statement-a4size/file
Warwick K (2003) Cyborg morals, cyborg values, cyborg ethics. Ethics Inf Technol 5(3):131–137. https://doi.org/10.1023/B:ETIN.0000006870.65865.cf
Weindling P (2001) The origins of informed consent: the international scientific commission on medical war crimes, and the Nuremberg code. Bull Hist Med 75(1):37–71. https://doi.org/10.1353/bhm.2001.0049
WHO. (2009). Research ethics committees Basic concepts for capacity-building. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/Ethics_basic_concepts_ENG.pdf
WHO. (2021). Chronological list of publications. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/publications/year/en/
Willison, J. and O’Regan, K. (2007). Commonly known, commonly not known, totally unknown: a framework for students becoming researchers. High Educ Res Dev 26(4). 393–409. https://doi.org/10.1080/07294360701658609
Žukauskas P, Vveinhardt J, and Andriukaitienė R. (2018). Research Ethics In book: Management Culture and Corporate Social Responsibility Eds Jolita Vveinhardt IntechOpenEditors DOI: https://doi.org/10.5772/intechopen.70629 , 2018
Download references
Acknowledgements
Authors wish to thank the organising committee of the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania for accepting this paper to be presented in the conference.
Not applicable as this is an independent study, which is not funded by any internal or external bodies.
Author information
Authors and affiliations.
School of Human Sciences, University of Derby, DE22 1, Derby, GB, UK
Shivadas Sivasubramaniam
Department of Informatics, Mendel University in Brno, Zemědělská, 1665, Brno, Czechia
Dita Henek Dlabolová
Centre for Academic Integrity in the UAE, Faculty of Engineering & Information Sciences, University of Wollongong in Dubai, Dubai, UAE
Veronika Kralikova & Zeenath Reza Khan
You can also search for this author in PubMed Google Scholar

Contributions
The manuscript was entirely written by the corresponding author with contributions from co-authors who have equally contributed to presentation of this paper in the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania. Authors have equally contributed for the information collection, which were then summarised as narrative explanations by the Corresponding author and Dr. Zeenath Reza Khan. Then checked and verified by Dr. Dlabolova and Ms. Králíková. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Shivadas Sivasubramaniam .
Ethics declarations
Competing interests.
We can also confirm that there are no potential competing interest with other organisations.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sivasubramaniam, S., Dlabolová, D.H., Kralikova, V. et al. Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures. Int J Educ Integr 17 , 14 (2021). https://doi.org/10.1007/s40979-021-00078-6
Download citation
Received : 17 July 2020
Accepted : 25 April 2021
Published : 13 July 2021
DOI : https://doi.org/10.1007/s40979-021-00078-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Higher education
- Ethical codes
- Ethics committee
- Post-secondary education
- Institutional policies
- Research ethics
International Journal for Educational Integrity
ISSN: 1833-2595
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- Ethical Considerations in Research | Types & Examples
Ethical Considerations in Research | Types & Examples
Published on 7 May 2022 by Pritha Bhandari .
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people.
The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating behaviours, and improving lives in other ways. What you decide to research and how you conduct that research involve key ethical considerations.
These considerations work to:
- Protect the rights of research participants
- Enhance research validity
- Maintain scientific integrity
Table of contents
Why do research ethics matter, getting ethical approval for your study, types of ethical issues, voluntary participation, informed consent, confidentiality, potential for harm, results communication, examples of ethical failures, frequently asked questions about research ethics.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe for research subjects.
You’ll balance pursuing important research aims with using ethical research methods and procedures. It’s always necessary to prevent permanent or excessive harm to participants, whether inadvertent or not.
Defying research ethics will also lower the credibility of your research because it’s hard for others to trust your data if your methods are morally questionable.
Even if a research idea is valuable to society, it doesn’t justify violating the human rights or dignity of your study participants.
Prevent plagiarism, run a free check.
Before you start any study involving data collection with people, you’ll submit your research proposal to an institutional review board (IRB) .
An IRB is a committee that checks whether your research aims and research design are ethically acceptable and follow your institution’s code of conduct. They check that your research materials and procedures are up to code.
If successful, you’ll receive IRB approval, and you can begin collecting data according to the approved procedures. If you want to make any changes to your procedures or materials, you’ll need to submit a modification application to the IRB for approval.
If unsuccessful, you may be asked to re-submit with modifications or your research proposal may receive a rejection. To get IRB approval, it’s important to explicitly note how you’ll tackle each of the ethical issues that may arise in your study.
There are several ethical issues you should always pay attention to in your research design, and these issues can overlap with each other.
You’ll usually outline ways you’ll deal with each issue in your research proposal if you plan to collect data from participants.
Voluntary participation means that all research subjects are free to choose to participate without any pressure or coercion.
All participants are able to withdraw from, or leave, the study at any point without feeling an obligation to continue. Your participants don’t need to provide a reason for leaving the study.
It’s important to make it clear to participants that there are no negative consequences or repercussions to their refusal to participate. After all, they’re taking the time to help you in the research process, so you should respect their decisions without trying to change their minds.
Voluntary participation is an ethical principle protected by international law and many scientific codes of conduct.
Take special care to ensure there’s no pressure on participants when you’re working with vulnerable groups of people who may find it hard to stop the study even when they want to.
Informed consent refers to a situation in which all potential participants receive and understand all the information they need to decide whether they want to participate. This includes information about the study’s benefits, risks, funding, and institutional approval.
- What the study is about
- The risks and benefits of taking part
- How long the study will take
- Your supervisor’s contact information and the institution’s approval number
Usually, you’ll provide participants with a text for them to read and ask them if they have any questions. If they agree to participate, they can sign or initial the consent form. Note that this may not be sufficient for informed consent when you work with particularly vulnerable groups of people.
If you’re collecting data from people with low literacy, make sure to verbally explain the consent form to them before they agree to participate.
For participants with very limited English proficiency, you should always translate the study materials or work with an interpreter so they have all the information in their first language.
In research with children, you’ll often need informed permission for their participation from their parents or guardians. Although children cannot give informed consent, it’s best to also ask for their assent (agreement) to participate, depending on their age and maturity level.
Anonymity means that you don’t know who the participants are and you can’t link any individual participant to their data.
You can only guarantee anonymity by not collecting any personally identifying information – for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, and videos.
In many cases, it may be impossible to truly anonymise data collection. For example, data collected in person or by phone cannot be considered fully anonymous because some personal identifiers (demographic information or phone numbers) are impossible to hide.
You’ll also need to collect some identifying information if you give your participants the option to withdraw their data at a later stage.
Data pseudonymisation is an alternative method where you replace identifying information about participants with pseudonymous, or fake, identifiers. The data can still be linked to participants, but it’s harder to do so because you separate personal information from the study data.
Confidentiality means that you know who the participants are, but you remove all identifying information from your report.
All participants have a right to privacy, so you should protect their personal data for as long as you store or use it. Even when you can’t collect data anonymously, you should secure confidentiality whenever you can.
Some research designs aren’t conducive to confidentiality, but it’s important to make all attempts and inform participants of the risks involved.
As a researcher, you have to consider all possible sources of harm to participants. Harm can come in many different forms.
- Psychological harm: Sensitive questions or tasks may trigger negative emotions such as shame or anxiety.
- Social harm: Participation can involve social risks, public embarrassment, or stigma.
- Physical harm: Pain or injury can result from the study procedures.
- Legal harm: Reporting sensitive data could lead to legal risks or a breach of privacy.
It’s best to consider every possible source of harm in your study, as well as concrete ways to mitigate them. Involve your supervisor to discuss steps for harm reduction.
Make sure to disclose all possible risks of harm to participants before the study to get informed consent. If there is a risk of harm, prepare to provide participants with resources, counselling, or medical services if needed.
Some of these questions may bring up negative emotions, so you inform participants about the sensitive nature of the survey and assure them that their responses will be confidential.
The way you communicate your research results can sometimes involve ethical issues. Good science communication is honest, reliable, and credible. It’s best to make your results as transparent as possible.
Take steps to actively avoid plagiarism and research misconduct wherever possible.
Plagiarism means submitting others’ works as your own. Although it can be unintentional, copying someone else’s work without proper credit amounts to stealing. It’s an ethical problem in research communication because you may benefit by harming other researchers.
Self-plagiarism is when you republish or re-submit parts of your own papers or reports without properly citing your original work.
This is problematic because you may benefit from presenting your ideas as new and original even though they’ve already been published elsewhere in the past. You may also be infringing on your previous publisher’s copyright, violating an ethical code, or wasting time and resources by doing so.
In extreme cases of self-plagiarism, entire datasets or papers are sometimes duplicated. These are major ethical violations because they can skew research findings if taken as original data.
You notice that two published studies have similar characteristics even though they are from different years. Their sample sizes, locations, treatments, and results are highly similar, and the studies share one author in common.
Research misconduct
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement about data analyses.
Research misconduct is a serious ethical issue because it can undermine scientific integrity and institutional credibility. It leads to a waste of funding and resources that could have been used for alternative research.
Later investigations revealed that they fabricated and manipulated their data to show a nonexistent link between vaccines and autism. Wakefield also neglected to disclose important conflicts of interest, and his medical license was taken away.
This fraudulent work sparked vaccine hesitancy among parents and caregivers. The rate of MMR vaccinations in children fell sharply, and measles outbreaks became more common due to a lack of herd immunity.
Research scandals with ethical failures are littered throughout history, but some took place not that long ago.
Some scientists in positions of power have historically mistreated or even abused research participants to investigate research problems at any cost. These participants were prisoners, under their care, or otherwise trusted them to treat them with dignity.
To demonstrate the importance of research ethics, we’ll briefly review two research studies that violated human rights in modern history.
These experiments were inhumane and resulted in trauma, permanent disabilities, or death in many cases.
After some Nazi doctors were put on trial for their crimes, the Nuremberg Code of research ethics for human experimentation was developed in 1947 to establish a new standard for human experimentation in medical research.
In reality, the actual goal was to study the effects of the disease when left untreated, and the researchers never informed participants about their diagnoses or the research aims.
Although participants experienced severe health problems, including blindness and other complications, the researchers only pretended to provide medical care.
When treatment became possible in 1943, 11 years after the study began, none of the participants were offered it, despite their health conditions and high risk of death.
Ethical failures like these resulted in severe harm to participants, wasted resources, and lower trust in science and scientists. This is why all research institutions have strict ethical guidelines for performing research.
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information – for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Bhandari, P. (2022, May 07). Ethical Considerations in Research | Types & Examples. Scribbr. Retrieved 13 May 2024, from https://www.scribbr.co.uk/research-methods/ethical-considerations/
Is this article helpful?

Pritha Bhandari
Other students also liked, a quick guide to experimental design | 5 steps & examples, data collection methods | step-by-step guide & examples, how to avoid plagiarism | tips on citing sources.
- News stories
- Blog articles
- NSPCC Learning podcast
- Why language matters
- Sign up to newsletters
- Safeguarding in Education Update
- CASPAR email alert
- Key topics home
- Safeguarding and child protection
- Child abuse and neglect
- Child health and development
- Safer recruitment
- Case reviews
- Online safety
- Research and resources home
- NSPCC research
- Safeguarding resources
- How Safe conference
- Self-assessment tool
- Schools and colleges
- Training home
- Basic safeguarding courses
- Advanced training
- Elearning courses
- Designated person training
- Schools and education courses
- Services home
- Direct work: children and families
- Talk Relationships
- Consultancy
- Library and Information Service
- Support for local communities
- NSPCC Helpline
- Speak out Stay safe schools service
- My learning
- Self-assessment
- /g,'').replace(/ /g,'')" v-html="suggestion">
Research with children: ethics, safety and promoting inclusion
Guidance about managing the risk of harm, obtaining informed consent and what researchers should do if they have concerns about a child.
Conducting research with children can help us understand what they think about the issues that affect them. But any research involving children must balance the aims of the research, the safety and wellbeing of the children and their rights to participate in research that impacts on them.
By providing the right support and knowing when to take appropriate action, researchers can ensure that children feel respected and can participate safely.
Types of research methods involving children
There are several ways to gather information about children and their lives, including:
- asking children about their feelings, opinions and experiences through face-to-face interviews, in focus groups with children or by questionnaire.
- observing children's behaviour using monitored experiments and activities or in an uncontrolled environment to see how they react during specific situations.
- analysing information contained in files about children, for example reviewing information held in social care case records, case reviews or school files.
Identifying and managing risk
Research for the sake of research isn’t ethically sound. You must assess the justification for conducting research with children, and there must be clear benefits. Ensure there is a balance between the needs of the research, the need to hear children's voices and the need to minimise the risk of harm.
Consider whether the research poses any risk of harm to the children or researchers involved. Research shouldn’t involve any greater stress than is commonly experienced in day-to-day life.
In research about children’s experiences, the main risk is the potential to trigger or cause emotional or psychological distress. For example:
- children may find participating in research stressful
- the research may reawaken, trigger or uncover feelings or memories
- children may be worried about how researchers will respond to the information they have shared.
The impact of these issues can be affected by a range of factors, including:
- the age, and potential vulnerability, of the children involved
- the sensitivity of the research topic
- the way the research is collected.
Once you have identified potential risks, you should establish how you can avoid or minimise them.
Explain research confidentiality and obtain consent
Before taking part, children should understand the nature of the research and what will be expected of them.
It is important to explain confidentiality to the child before they give their consent and at the beginning of the research process. Explain:
- how you will use what they tell you
- how you will keep that confidential
- who else will see their data
- when you will have to break confidentiality and the process that you will follow.
> Find out more about sharing information
Adapt the collection methodology
The way information is collected can minimise distress caused to children. For example:
- position sensitive questions in the middle of an interview or focus group session to allow the children to warm up and cool down from these topics
- take breaks during data collection
- conduct the research in a friendly and comfortable environment
- avoid times of day when support services aren’t available.
Respond to distress and disclosures
It’s important to have a clear plan for responding to children’s distress or disclosures of harm to themselves or someone else.
You should have a process in place for referring child protection concerns. You should also provide information or encourage participants to seek help when an unmet need, such as a mental health concern, is disclosed.
Debriefing children at the end of the research can help identify any needs. This also provides another opportunity to refer participants to appropriate help or address any concerns.
Signpost to sources of further support
It is good practice to give children information about sources of support and remind them of these throughout the process.
A thank you leaflet containing information and contact details is particularly useful and should be given to all participants.
Childline provides confidential help and advice to children or young people. Calls to 0800 1111 are free and children can also contact Childline online . You can also download or order Childline posters and wallet cards .
Assess and mitigate risks to researchers
You should also consider the potential impact of research on the researchers.
If researchers feel affected by what is discussed during the sessions, they should seek support through supervision with their manager. It may also be helpful to direct them to the NSPCC helpline to talk about anything that is concerning them.
Research supervisors should ensure that proper attention is paid to the researcher’s safety, particularly in lone working situations.
> Take a look at our guidance on lone working
Set out and approve your research plan
You should produce a research plan which clearly outlines, assesses and provides mitigation for potential risks.
Where possible, we recommend that plans for research with children are approved through a rigorous ethics review process by an appropriate ethics body.
> Read more about how the NSPCC approaches research governance
Participation and inclusive research
Consider who you need to seek data from as part of the research. Not every study can include a complete cross-section of society, but you should aim to be as inclusive as possible.
Children can face different types of discrimination which could act as barriers to their participation in research. By addressing these barriers, research can be opened up to wider social and cultural groups and this will improve the quality of the study.
To facilitate participation with children, you could:
- adapt research tools to meet their needs and abilities
- translate supporting documents into other languages
- hold the research at a time and place which is easy for children to access
- consider whether the language or design of the research excludes any groups of children, or whether any specific recruitment strategies are needed to make sure there is a representative sample of children
- consider if children would prefer to have an interviewer of a specific gender, ethnic background or age.
Sharing the results of the research with the children who take part is important in creating a positive and inclusive experience. You may want to create a version of your report, a presentation, or some other creative output specifically for children.
Informed consent
When you ask for permission for children to be involved in your research, ensure that children and their parents or carers (if needed) fully understand:
- what the research is about
- what is expected of participants
- what the risks and benefits of participating in the research are
- that participating in the research is voluntary.
This is known as informed consent.
Make sure you consider children’s capacity to consent to involvement in the research. This will depend on their level of understanding of the potential risks and benefits of taking part.
When considering whether a child is mature enough to make decisions about things that directly affect them, professionals often talk about whether the child is ‘Gillick competent’. Gillick competency means a young person is mature enough to fully understand what they are agreeing to.
> Learn more about Gillick competency and applying the guidelines
If a child isn’t fully able to understand the consequences of taking part, but is able to express willingness to participate and understand what is expected of them, it may be appropriate to seek their ‘assent’. In such cases consent should always be sought from their parent or carer.
Consent should also be obtained from the parent, carer or other appropriate adult for all children under the age of 8, and in most cases for children aged 8-15. For young people aged 16 and 17, you should carefully consider whether parental consent is appropriate.
If you don’t seek parental consent, you should justify your approach and consider whether parents should be informed of the research.
If a child doesn’t consent or assent to participate in research, this overrides the consent from the parent, guardian, carer, or other appropriate adult with a duty of care.
It must be made clear that there are no negative consequences to refusing to take part in research and that children or their parent or carer can also withdraw consent at any time. You should regularly check with young people and their families during the research that they are still happy to take part.
Requesting and recording consent
You should have a formal record that consent has been given before the research starts. Using a consent form enables children to confirm they understand what the research will involve and are happy to take part. See our research ethics principles for an example of good practice of guidelines for seeking consent from young people and their parents.
Storing personal information
You should keep all participants’ personal information confidential and comply with the Data Protection Act 2018.
> Find out more about children and data protection on our children and the law pages
Complaints procedure
A complaints procedure should be in place when conducting any research. It should include a way to talk to someone not connected with the research, information on how children can make a complaint and the measures in place to ensure their interests are adequately represented. You should make the complaints procedure available when obtaining consent and promote it again during and after the research.
What to do if you think a child has experienced abuse
If you are concerned that a child might be at risk of harm, the child’s welfare should take priority over the research.
If you are concerned about a child
If you notice patterns of behaviour which worry you, you must share your concerns.
- Follow the child protection procedures set out as part of your research plan . This should include information on how to respond to safeguarding concerns.
- Contact the NSPCC Helpline on 0808 800 5000 or email [email protected] . Our child protection specialists will talk through your concerns with you and give you expert advice.
- Contact your local child protection services . Their contact details can be found on the website for the local authority the child lives in.
- Contact the police .
> Find out more about recognising and responding to abuse
If a child tells you about abuse
If a child discloses abuse to you, it's really important to:
- listen carefully to what they're saying
- let them know they've done the right thing by telling you
- tell them it's not their fault
- say you will take them seriously
- explain what you'll do next
- follow the instructions above to report what the child has told you as soon as possible.
Don’t confront the alleged abuser but follow the instructions above to report what the child has told you as soon as possible.
> See our information about responding to disclosures of abuse for more tips
Further information and resources
For further reading about research and ethics when working with young people, search the NSPCC Library catalogue using the keyword "research methods" and "ethics".

The NSPCC Library and Information Service helps professionals access the latest child protection research, policy and practice resources and can answers your safeguarding questions and enquiries.

Find out more about how our research activities are designed to improve our knowledge about how to prevent child abuse, stop it when it happens and lessen the impact on children.

Subscribe to our safeguarding awareness newsletter and receive free weekly email alerts to keep you up-to-date with all the latest safeguarding and child protection news.

After Her Death, Scientists Made This Woman Immortal. But She Never Agreed to That.
Her genetic material rewrote the future of medicine—all without her consent.
Gear-obsessed editors choose every product we review. We may earn commission if you buy from a link. Why Trust Us?
In March 2024, Tuskegee University unveiled a monument commemorating Henrietta Lacks and her contributions to the fight against polio. Just days later, submissions closed for the Henrietta Lacks Hometown Initiative’s contest to design a memorial in her native Halifax County, Virginia.
It’s not surprising that Lacks, whose “immortal” HeLa cells were pivotal in developing treatments for diseases such as polio, HIV/AIDS, and COVID-19 , continues to be honored more than 70 years after her death. But Lacks’ legacy is complicated due to the ethical concerns surrounding the use of her special cells.
Lacks, who died of cancer at age 31 in 1951, was never aware that her cells led to significant medical advancements—or that they were taken without her consent. And even now, her strange case raises questions about the the morally dubious methods through which we achieved unquestionably positive breakthroughs in medicine.
The Immortal Life of Henrietta Lacks
Though her cells live on in labs across the world, Henrietta Lacks’ life was short and full of challenges, and she remained largely unrecognized during her lifetime. It wasn’t until author Rebecca Skloot investigated the origins of the famous HeLa cells that Lacks’ story gained widespread attention with the bestselling 2010 book, T he Immortal Life of Henrietta Lacks .

Thanks to Skloot and the descendants of Lacks, who have worked to retell her story through initiatives like HeLa100 , we now know more about the life of the woman often referred to as “the mother of modern medicine .”
On August 1, 1920, Lacks was born in Roanoke, Virginia. According to Biography , following her mother’s death in 1924, Henrietta moved to her grandfather’s log cabin, a former slave quarters on a plantation. There, she lived with her cousin, David “Day” Lacks. At 14, she gave birth to their first child, Lawrence, and the couple married in 1941. Before moving to Maryland, they had a second child, Elsie, in 1939, and later expanded their family with three more children: David Jr., Deborah, and Joe.
Henrietta had been experiencing “abnormal pain and bleeding in her abdomen” when she visited The Johns Hopkins Hospital in Baltimore on February 1, 1951. Lacks had to travel to Hopkins for treatment because, as Hopkins Medicine’s own website notes , at the time, the hospital “was one of only a few hospitals to treat poor African-Americans.”
Lacks was quickly diagnosed with cervical cancer by physician Howard Jones. She received radium treatments there, and without her consent, doctors extracted two cervical samples. Despite treatment, her condition worsened, leading to her readmission to the hospital on August 8. Lacks passed away on October 4, 1951, and was laid to rest in an unmarked grave in Clover, Virginia, where she spent her early years.
But unbeknownst to Lacks, or her family, the cervical samples taken without her consent revealed something extraordinary.
Lacks’ tumor cells were sent to the lab of Dr. George Otto Gey, a prominent cancer researcher. Gey collected cells “from all patients—regardless of their race or socioeconomic status—who came to The Johns Hopkins Hospital with cervical cancer,” according to the hospital’s website. But unlike previous samples that died within a day or two, Lacks’ cells amazingly doubled in number every 20 to 24 hours.
That discovery led to a medical revolution. As Biography summarizes:
“Gey isolated and multiplied a specific cell, creating a cell line. He dubbed the resulting sample HeLa, derived from the name Henrietta Lacks. The HeLa strain revolutionized medical research. Jonas Salk used the HeLa strain to develop the polio vaccine, sparking mass interest in the cells. As demand grew, scientists cloned the cells in 1955. Since that time, over 10,000 patents involving HeLa cells have been registered. Researchers have used the cells to study disease and to test human sensitivity to new products and substances. More recently, the cells enabled the development of COVID-19 vaccines.”
It took nearly two decades for the Lacks family to learn about their relation to the immortal HeLa cells. During that time, the cells’ origin was mistakenly attributed in the media to fictitious names such as “Helen Lane” or “Helen Larson.”

In February 2010, Johns Hopkins addressed ethical concerns about the acquisition of the initial HeLa cells in a statement:
“It’s important to note that at the time the cells were taken from Mrs. Lacks’ tissue, the practice of obtaining informed consent from cell or tissue donors was essentially unknown among academic medical centers. Sixty years ago, there was no established practice of seeking permission to take tissue for scientific research purposes.”
But that’s not quite the full story. Skloot’s research in The Immortal Life of Henrietta Lacks revealed that additional cell samples were taken from Lacks beyond the initial two.
Lacks’ cells were special, but by the time Gey hoped to collect more samples, she had died. “Though no law or code of ethics required doctors to ask permission before taking tissue from a living patient,” Skloot wrote, “the law made it very clear that performing an autopsy or removing tissue from the dead without permission was illegal.”
After Lacks’ death, doctors sought consent from her husband, Day, to perform an autopsy, but he initially refused. When they approached Day a second time, suggesting that the autopsy could yield test results that might benefit their children in the future, Day relented and gave his permission. Unfortunately, the promised test results were never provided to the Lacks family.
Johns Hopkins maintains that the obfuscation wasn’t for financial gain. The university says, “Johns Hopkins has never sold or profited from the discovery or distribution of HeLa cells and does not own the rights to the HeLa cell line. Rather, Johns Hopkins offered HeLa cells freely and widely for scientific research.”
But other companies have financially benefited from the HeLa cells. Notably, Thermo Fisher Scientific reached a settlement with the Lacks family in 2023 over products related to the HeLa cell line, as reported by NPR . This settlement acknowledges the commercial use and financial gains derived from Lacks’ cells.
Dr. Gey’s actions, which were typical of an era when the potential benefits to many patients took precedence over the rights of individual medical research subjects, could have been driven by the belief that prioritizing consent might impede scientific progress. It wasn’t until 15 years after Lacks’ cells were harvested that a landmark research paper changed that belief.
The Evolution of Medical Ethics in America
Ethical standards in American medical science have dramatically evolved over the past century, notably in the attitude toward eugenics, which is the science of promoting desirable qualities in the human race, usually through some kind of controlled breeding.

“When most people think of eugenics, they think of the unspeakable acts of Adolf Hitler and Dr. Josef Mengele ,” Dr. Marilyn M. Singleton wrote in an article for the Journal of American Physicians and Surgeons . “ But history tells us that some of America’s best and brightest promoted eugenics as settled science and necessary for the preservation of society.” (For example, Harvard professor W.E.B. DuBois supported selective breeding within the Black community, birth control pioneer Margaret Sanger advocated for “negative eugenics,” and even the NAACP ran “Better Baby” contests to fund its anti-lynching efforts.)
After World War II, the revelation of Nazi medical crimes during the Doctors’ Trials at Nuremberg, including the experiments by Mengele, radically changed American views on eugenics and led to the Nuremberg Code, which prioritized voluntary consent in medical research. But the U.S. didn’t legally adopt the Nuremberg Code, nor immediately recognize the similarities between Nazi human experiments and its own practices, such as collecting Lacks’ cells without her consent.

In 1966, two decades after the start of the Doctors’ Trial, and 15 years after the death of Henrietta Lacks, Dr. Henry K. Beecher published a paper in The New England Journal of Medicine titled, “ Ethics and Clinical Research .” It’s also known within medical circles today colloquially as “ Beecher’s Bombshell .”
The paper criticized the use of unwitting patients in experiments that offered no benefit and often harmed them, detailing 22 shocking cases including soldiers given placebos for rheumatic fever based solely on their serial number, and patients being injected with live cancer cells without their informed consent.
The experiments chronicled in Beecher’s report rocked the medical world, since these were conducted not by German men on trial, but by respected institutions, prestigious scientists, and the U.S. military. Years later, the Office of Human Research Protections stated that this paper contributed to “the impetus for the first [National Institutes of Health] and [Food and Drug Administration] regulations.”
Beecher’s paper strongly advocated for informed consent, challenging the prevailing notion among some circles that ethical scrutiny could hinder scientific progress.
How We Remember Henrietta Lacks
The story of Henrietta Lacks is often framed by the medical breakthroughs that her immortal cells facilitated, with a focus on the collective benefit rather than the ethical missteps that scientists took, and her family’s ignorance and lack of compensation. But decades before Lacks’ story became widely known, Beecher already argued against this idea:
“An experiment is ethical or not at its inception," Beecher concludes, “it does not become ethical post hoc—ends do not justify means. There is no ethical distinction between ends and means.”

It’s impossible to say whether Henrietta Lacks would have consented to the use of her cells, especially with the knowledge of the remarkable medical achievements they’d unlock—achievements even the doctors who collected the cells couldn’t have predicted.
We know now that those HeLa cells changed medicine forever, and though some tried to obscure their origins, Henrietta Lacks is immortalized in medical history. But we also know now that, regardless of the laws at the time, it should have been Lacks’ choice whether she was immortalized at all. If only someone had thought to ask.
Michael Natale is the news editor for Best Products , covering a wide range of topics like gifting, lifestyle, pop culture, and more. He has covered pop culture and commerce professionally for over a decade. His past journalistic writing can be found on sites such as Yahoo! and Comic Book Resources , his podcast appearances can be found wherever you get your podcasts, and his fiction can’t be found anywhere, because it’s not particularly good.

.css-cuqpxl:before{padding-right:0.3125rem;content:'//';display:inline;} Pop Mech Pro .css-xtujxj:before{padding-left:0.3125rem;content:'//';display:inline;}

Can the Army’s New Rifle Take on Russian Armor?

Overhaul Your Shop

The Source of All Consciousness May Be Black Holes

F-22s are Flocking to This Island in the Pacific

Special Ops Gunship Shreds “Fishing Boat”

Russia's Unprecedented Nuclear Drills

Ukraine Is Using an Ancient Weapon on Russia

Inside the CIA’s Quest to Steal a Soviet Sub

Jumpstart Your Car With a Cordless Tool Battery

Lost Villa of Rome’s Augustus Potentially Found
Could Freezing Your Brain Help You Live Forever?
- Philanthropy News Digest

American Psychological Foundation invites applications for research in ethics and risk management
The American Psychological Foundation invites applications for the Trust Grant in Honor of Eric A. Harris, EdD, JD.
A grant of $5,000 will be awarded through the program in support of an early career psychologist or graduate student working on research or projects in the area of ethics and risk management.
To be eligible, applicants must be a graduate student or early career psychologist (no more than 10 years postdoctoral) and be affiliated with a nonprofit charitable, educational, or scientific institution, or a governmental entity operating exclusively for charitable and educational purposes.
For complete program guidelines and application instructions, see the American Psychological Foundation website.
View another RFP related to medical research
Search rfps.

Not the right RFP?
See who will support your mission with Foundation Directory Online.
Foundation Directory Online allows you to search all 100,000 U.S foundations at once. Target your search by field of interest, type of support, geographic focus, and more.
Find the plan that's right for you
The nonprofit news you need
Get the latest nonprofit news, funding opportunities, job openings, and more delivered to your inbox with Philanthropy News Digest newsletters.
Candid gets you the information you need to do good.
To subscribe, select any of the newsletters listed below.
- RFP Bulletin
- Job Bulletin
- PND Connections

Ethical Issues in Community and Patient Stakeholder–Engaged Health Research
- © 2023
- Emily E. Anderson ORCID: https://orcid.org/0000-0003-2197-1239 0
Neiswanger Institute for Bioethics, Stritch School of Medicine, Loyola University Chicago, Maywood, USA
You can also search for this editor in PubMed Google Scholar
- First book to cover the full range of ethical issues related to a variety of different forms of stakeholder engagement
- Contains case studies highlighting unique issues that arise in stakeholder engagement relevant to different populations
- Synthesizes current conversations, best practices, and future directions in stakeholder–engaged health research
Part of the book series: Philosophy and Medicine (PHME, volume 146)
2848 Accesses
1 Citations
22 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
Table of contents (20 chapters)
Front matter, introduction: what we talk about when we talk about the ethics of engaging patient and community stakeholders in health research.
Emily E. Anderson
Theorizing Participatory Research
- Andrew Evans, Angela Potochnik
An Evolving Ethical Framework for Patient and Community-Engaged Research
- Lisa Mikesell
Beyond Good Intentions: Principles for Anti-racist Community-Engaged Research
- Alexis Grant, Andrea L. DaViera
Demystifying How Academic-Community Partnerships Use Reflexivity and Praxis to Promote Participatory Research Principles of Equity and Justice
- Jeni Hebert-Beirne, Sylvia Gonzalez, Melissa Chrusfield, Adlaide Holloway, Jennifer Plascencia Lopez, Dolores Castañeda
Reflexivity: A Strategy for Ethics- and Values-Driven Community Partnerships in Mental Health
- Tommy Chou, Stacy L. Frazier
In the Field
How do you define community and why is it important.
- Laurene Tumiel-Berhalter, Linda Kahn
Community Researchers and Ethical Considerations: Burdens in the Field
- Maghboeba Mosavel, Briona Phillips
Community-Engaged Research with People with Developmental Disabilities: A Conversation with Community Researchers about their Ethical Inclusion
- Brendan Durkin, Micah Fialka-Feldman, Jacob Myers, Ariel Schwartz, Katherine McDonald
Community Conversations about Culturally Responsive Health Research for African American Communities
- Adina Black, Millicent Robinson, Paige Castro-Reyes, Al Richmond, Latajah Lassus, Nancy Shore
The Power of Personal Narrative: My Experience with SACHRP, the FDA, and Bioethics
- Gianna McMillan
Unique Ethical Considerations
Patient advocacy organizations and conflicts of interest in research.
- Lisa Parker, Barbara Mintzes
Representing and Protecting: Gatekeepers in Community-Engaged Research
- Ryan Spellecy
Paying Research Participants and Community and Patient Research Partners: An Engaging Ethical Issue
- Lisa Ballance, Elizabeth Ripley
Rethinking Risks and Benefits in Stakeholder-Engaged Research: Lessons from HIV, Substance Use, and Sexual Health Research with Marginalized Communities
- Adrian Guta, Peter A. Newman, Adam Bourne
Data Decisions and Ethics: The Case of Stakeholder-Engaged Research
- Melody S. Goodman, Kristyn A. Pierce, James M. DuBois, Vetta Sanders Thompson
- Community-engaged Research
- history of codes of ethics
- epistemology and stakeholder engagement
- the Belmont principle
- CIOMS Ethical guidelines
- philosophy of patient engagement
- promoting equitable collaboration
- stakeholder voices in healthcare
- human research protections
About this book
This book provides in-depth analyses of a wide range of topics surrounding ethical issues in community and patient stakeholder–engaged health research, and highlights where consensus exists, is emerging, or remains elusive. Topics in this book cover the history of stakeholder engagement in health research; how codes of ethics and regulations have (or have not) addressed stakeholder engagement; how to promote equitable collaboration; the ethical perspectives of different stakeholders; and the unique challenges posed by stakeholder- engaged research to the protection of human research participants and the research ethics review process. The book includes discussion of unique issues that arise in stakeholder engagement relevant to different populations, settings, and research designs. This book is relevant for anyone with a role or interest in stakeholder-engaged research, including patient and community research partners; academic researchers; research ethics scholars and educators; and funders.
Editors and Affiliations
About the editor.
Emily E. Anderson, PhD, MPH, is Professor in the Neiswanger Institute for Bioethics and Healthcare Leadership at Loyola University Chicago’s Stritch School of Medicine. Dr. Anderson holds a PhD in health care ethics from Saint Louis University and an MPH from University of Illinois Chicago. She teaches courses on research ethics and responsible conduct of research to bioethics and biomedical sciences graduate students as well as medical students. In addition to the ethics of stakeholder engagement in research, her areas of interest and expertise include researcher and physician professionalism and misconduct; ethical issues in research with vulnerable populations; informed consent; institutional review board (IRB) policy; and the application of qualitative research techniques to the study of research ethics. She has published over 50 articles in top journals including the American Journal of Bioethics, Journal of Law, Medicine, and Ethics, Academic Medicine, and IRB: Ethics & Human Research. Dr. Anderson currently serves as associate editor of Narrative Inquiry in Bioethics . Dr. Anderson has been a co-investigator on several federally-funded research and educational projects and recently co-authored the book 100 Questions and Answers about Research Ethics (Sage Publications, 2018) with Dr. Amy Corneli (at Duke University).
Bibliographic Information
Book Title : Ethical Issues in Community and Patient Stakeholder–Engaged Health Research
Editors : Emily E. Anderson
Series Title : Philosophy and Medicine
DOI : https://doi.org/10.1007/978-3-031-40379-8
Publisher : Springer Cham
eBook Packages : Religion and Philosophy , Philosophy and Religion (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2023
Hardcover ISBN : 978-3-031-40378-1 Published: 02 October 2023
Softcover ISBN : 978-3-031-40381-1 Due: 31 October 2023
eBook ISBN : 978-3-031-40379-8 Published: 29 September 2023
Series ISSN : 0376-7418
Series E-ISSN : 2215-0080
Edition Number : 1
Number of Pages : XVI, 327
Number of Illustrations : 1 b/w illustrations
Topics : Ethics , Medicine/Public Health, general , Public Health , Community and Environmental Psychology
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- MyU : For Students, Faculty, and Staff
Catherine Qi Zhao Earns 2024 George W. Taylor Award for Distinguished Research

Department of Computer Science & Engineering’s (CS&E) Associate Professor Catherine Qi Zhao earned the 2024 George W. Taylor Award for Distinguished Research from the University of Minnesota’s College of Science and Engineering (CSE). Established in 1982, the Taylor Award for Distinguished Research recognizes younger faculty members who have shown outstanding ability in research. The award includes a $5,000 grant for professional development in teaching and research.
“This award is an incredible honor,” Zhao said. “I am deeply grateful for the continous support from the department and college at the University of Minnesota, without which our achievements would not have been possible. I also want to extend my sincere appreciation to all of the members of our lab, collaborators, and colleagues for their invaluable contributions. They are a huge player in this award.”
Zhao earned the award for her contributions to research in the areas of artificial intelligence (AI), computer vision, machine learning, and AI for humans. She started her journey in AI as a computer scientist developing intelligent visual systems at the University of California. During her time as a postdoctoral associate at the California Institute of Technology, she pioneered work on visual attention which culminated in a project named, “SALICON”. Their AI models were capable of understanding and predicting human attention. That breakthrough paved the way for a widespread adoption of the SALICON dataset and the application of their model across industry and academia.
Building upon that success, Zhao’s work shifted towards understanding atypical patterns in clinical populations, particularly in individuals with neurodevelopmental issues such as autism spectrum disorder. Her team created the first AI-based autism screening model, which garnered significant media attention. With the recent boom in AI technologies, Zhao’s work recently focused on trustworthy AI. This January, she was appointed a Dean’s Fellow in the College of Science and Engineering , focusing on exploring the ethical dimensions of AI and its role in university education and research.
“You can see the trajectory of my work moving towards AI for social good, especially today with the wide capabilities of AI,” Zhao said. “Looking ahead, I want to continue pushing the boundaries of AI research, exploring its applications and ensuring responsible deployment for the benefit of humanity. I am eager to collaborate with colleagues and partners at the University and community to maximize our social impact.”
Zhao is the fifth CS&E faculty member to receive the George W. Taylor Award for Distinguished Research, joining Chad Myers , Arindam Banerjee, Tian He, and Zhi-Li Zhang . The complete list of previous winners can be found on the CSE website .
Related news releases
- Chad Myers Named Distinguished McKnight University Professor
- Chad Myers Earns Award for Outstanding Contributions to Graduate and Professional Education
- CS&E’s Yao-Yi Chiang and Jina Kim Named to First DEI Committee for ACM SIGSPATIAL
- Catherine Zhao appointed Dean’s Fellow for College’s AI strategy
- Shana Watters Earns the Women’s Center Charlotte Striebel Equity Award
- Future undergraduate students
- Future transfer students
- Future graduate students
- Future international students
- Diversity and Inclusion Opportunities
- Learn abroad
- Living Learning Communities
- Mentor programs
- Programs for women
- Student groups
- Visit, Apply & Next Steps
- Information for current students
- Departments and majors overview
- Departments
- Undergraduate majors
- Graduate programs
- Integrated Degree Programs
- Additional degree-granting programs
- Online learning
- Academic Advising overview
- Academic Advising FAQ
- Academic Advising Blog
- Appointments and drop-ins
- Academic support
- Commencement
- Four-year plans
- Honors advising
- Policies, procedures, and forms
- Career Services overview
- Resumes and cover letters
- Jobs and internships
- Interviews and job offers
- CSE Career Fair
- Major and career exploration
- Graduate school
- Collegiate Life overview
- Scholarships
- Diversity & Inclusivity Alliance
- Anderson Student Innovation Labs
- Information for alumni
- Get engaged with CSE
- Upcoming events
- CSE Alumni Society Board
- Alumni volunteer interest form
- Golden Medallion Society Reunion
- 50-Year Reunion
- Alumni honors and awards
- Outstanding Achievement
- Alumni Service
- Distinguished Leadership
- Honorary Doctorate Degrees
- Nobel Laureates
- Alumni resources
- Alumni career resources
- Alumni news outlets
- CSE branded clothing
- International alumni resources
- Inventing Tomorrow magazine
- Update your info
- CSE giving overview
- Why give to CSE?
- College priorities
- Give online now
- External relations
- Giving priorities
- Donor stories
- Impact of giving
- Ways to give to CSE
- Matching gifts
- CSE directories
- Invest in your company and the future
- Recruit our students
- Connect with researchers
- K-12 initiatives
- Diversity initiatives
- Research news
- Give to CSE
- CSE priorities
- Corporate relations
- Information for faculty and staff
- Administrative offices overview
- Office of the Dean
- Academic affairs
- Finance and Operations
- Communications
- Human resources
- Undergraduate programs and student services
- CSE Committees
- CSE policies overview
- Academic policies
- Faculty hiring and tenure policies
- Finance policies and information
- Graduate education policies
- Human resources policies
- Research policies
- Research overview
- Research centers and facilities
- Research proposal submission process
- Research safety
- Award-winning CSE faculty
- National academies
- University awards
- Honorary professorships
- Collegiate awards
- Other CSE honors and awards
- Staff awards
- Performance Management Process
- Work. With Flexibility in CSE
- K-12 outreach overview
- Summer camps
- Outreach events
- Enrichment programs
- Field trips and tours
- CSE K-12 Virtual Classroom Resources
- Educator development
- Sponsor an event

IMAGES
VIDEO
COMMENTS
According to Sieber , ethical issues in research can be classified into five categories, related to: (a) communication with participants and the community, (b) acquisition and use of research data, (c) external influence on research, (d) risks and benefits of the research, and (e) selection and use of research theories and methods. Many of ...
Research ethics are a set of principles that guide your research designs and practices in both quantitative and qualitative research. In this article, you will learn about the types and examples of ethical considerations in research, such as informed consent, confidentiality, and avoiding plagiarism. You will also find out how to apply ethical principles to your own research projects with ...
To highlight ethical issues in research with people who use the mental health services: Authors' experience in mental health: ... Case studies—projects submitted to ethics boards: Research ethics boards review process may threaten core foundational principles. It is necessary a proportionate representation of qualitative researchers on ...
Principles that come to mind might include autonomy, respect, dignity, privacy, informed consent and confidentiality. You may also have identified principles such as competence, integrity, wellbeing, justice and non-discrimination. Key ethical issues that you will address as an insider researcher include: Gaining trust.
Moreover, while researchers may make every effort to foreground and account for ethical considerations when planning a project to achieve ethical clearance, when it comes to home-based research, there are often unanticipated ethical dilemmas that emerge as one embarks, moves through, and engages in research, much of which cannot be predicted or ...
Definition. Ethics is a set of standards, a code, or value system, worked out from human reason and experience, by which free human actions are determined as ultimately right or wrong, good, or evil. If acting agrees with these standards, it is ethical, otherwise unethical. Scientific research refers to a persistent exercise towards producing ...
Preview. 100 Questions (and Answers) About Research Ethics is an essential guide for graduate students and researchers in the social and behavioral sciences. It identifies ethical issues that individuals must consider when planning research studies as well as provides guidance on how to address ethical issues that might arise during research ...
It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants. As such, all research involving human beings should be reviewed by an ethics committee to ensure that the appropriate ethical standards are being upheld. Discussion of the ethical principles of beneficence, justice and ...
Research ethics has traditionally been guided by well-established documents such as the Belmont Report and the Declaration of Helsinki. At the same time, the introduction of Big Data methods, that is having a great impact in behavioral research, is raising complex ethical issues that make protection of research participants an increasingly difficult challenge. By conducting 39 semi-structured ...
Ethical issues in research projects are overseen by IRBs, which mandate that a full research proposal must be submitted, evaluated and approved before the start of any project. Such a research ...
Principle 14: Research projects should include appropriate mechanisms and procedures for reporting on ethical aspects of the research and complying with these guidelines. Although the AIATSIS and similar codes typically address the same issues as mentioned in the ethical research principles presented in this paper, ...
What are the ethical issues in such studies, and how can we navigate them? Find examples and guidance in this collection of open-access articles. Hannes, K., & Parylo, O. (2014). Let's Play it Safe: Ethical Considerations from Participants in a Photovoice Research Project. International Journal of Qualitative Methods, 13(1), 255-274.
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own ...
3) of institutional research ethics and led him to embrace an "ethics of care" (Robinson, 2020, p. 12). Research ethics can be fully grasped only in practice. Yet, the review alerted him to issues that he needed to explore in his relationship with TfT. Daryl explained a few of these moral dilemmas and how he attempted to handle them.
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating ...
Ethics or moral philosophy is a branch of philos-. ophy with standards or codes or value systems. and involves defending, systematizing, recommending concepts of right, and minimizing. wrong ...
During the implementation of a research project, it is important to ensure and document that the work is carried out in an ethically sound manner. Two aspects of this are discussed in this chapter. One is honesty, openness and accountability towards colleagues, managers, partners and funding bodies. This is especially about reporting any ...
Follow the child protection procedures set out as part of your research plan. This should include information on how to respond to safeguarding concerns. Contact the NSPCC Helpline on 0808 800 5000 or email [email protected]. Our child protection specialists will talk through your concerns with you and give you expert advice.
Best ethical practices. 4.3.1. Research design. Research ethics starts with a sound research design. Knight, Chidlow and Minbaeva assert that the research design "sets out the research plan for empirically addressing a research question (s) that aims to develop theory in a feasible way" (2022: 45).
On August 1, 1920, Lacks was born in Roanoke, Virginia. According to Biography, following her mother's death in 1924, Henrietta moved to her grandfather's log cabin, a former slave quarters on ...
The American Psychological Foundation invites applications for the Trust Grant in Honor of Eric A. Harris, EdD, JD.. A grant of $5,000 will be awarded through the program in support of an early career psychologist or graduate student working on research or projects in the area of ethics and risk management.
This book provides in-depth analyses of a wide range of topics surrounding ethical issues in community and patient stakeholder-engaged health research, and highlights where consensus exists, is emerging, or remains elusive. Topics in this book cover the history of stakeholder engagement in health research; how codes of ethics and regulations ...
The ethical concerns are likely to get still more heated when the value of expensive biotech treatments for chronic illnesses is debated. After all, a needed pill for cholesterol might cost $3 daily, which amounts to nearly $1,100 per year. Compare that to a biologic that carries a $20,000-per-year price tag — or something even more costly.
Ethical issues are a consideration within general PPI research, especially when working with more vulnerable populations, such as children or adults living with a disability. It can also be the case that traditional PPI methods lack generalisability, as people who volunteer to be part of such a group are more likely be older, middle class and ...
Intergenerational trauma research typically focuses on parent survivors. No specific guidelines are available for conducting research with parent survivors despite potentially unique risks. To investigate research safety with parent survivors, we conducted an online survey of 38 researchers regarding experiences of parent survivors in their research, precautions taken, ethical review ...
Department of Computer Science & Engineering's (CS&E) Associate Professor Catherine Qi Zhao earned the 2024 George W. Taylor Award for Distinguished Research from the University of Minnesota's College of Science and Engineering (CSE). Established in 1982, the Taylor Award for Distinguished Research recognizes younger faculty members who have shown outstanding ability in research.