

IEEE Technical Community Spotlight
Ieee accepting articles for open access journals.

These journals target a rapid publishing schedule and are fully compliant with funder mandates, including Plan S as all articles will be published under the Creative Commons Attribution License (CC-BY) enabling authors to retain copyright.
The open access journals follow IEEE’s established high standard of peer review, providing fully open access publications for high-quality, cutting-edge scientific and technical content accessible to researchers around the globe. Independent editorial boards are led by an accomplished expert as editor-in-chief. The call for submissions presents a unique opportunity for authors to benefit from the visibility each journal’s launch will generate and their published papers will be exposed to more than 5 million unique monthly users of the IEEE Xplore ® Digital Library.
IEEE’s gold fully open access journals include:
- IEEE Open Journal of Antennas and Propagation
- IEEE Open Journal of Circuits and Systems
- IEEE Open Journal of the Communications Society
- IEEE Open Journal of the Computer Society
- IEEE Open Journal of Engineering in Medicine and Biology
- IEEE Open Journal of Industry Applications
- IEEE Open Journal of the Industrial Electronics Society
- IEEE Open Journal of Intelligent Transportation Systems
- IEEE Open Journal of Nanotechnology
- IEEE Open Journal of Power Electronics
- IEEE Open Journal of Signal Processing
- IEEE Open Journal of Solid-State Circuits
- IEEE Open Journal of Vehicular Technology
- IEEE Journal of the Electron Devices Society
- IEEE Journal of Exploratory Solid-State Computational Devices and Circuits
- IEEE Photonics Journal
- IEEE Open Access Journal of Power and Energy
- IEEE Journal of Selected Topics in Applied Earth Observations and Remote Sensing
- IEEE Transactions on Quantum Engineering
Coming Soon
- IEEE Open Journal of Ultrasonics, Ferroelectrics, and Frequency Control
- IEEE Transactions on Neural Systems and Rehabilitation Engineering
- IEEE Journal of Microwaves
The Article Processing Charge (APC) is US$1750 with discounts or special offers available for IEEE and IEEE Society members. For more information on of IEEE’s gold fully open access journals, editorial boards, submission dates, and links to submit a paper, please visit http://open.ieee.org
IEEE Access, IEEE’s broad scope open access journal, is also accepting papers for six discipline-specific Sections aligned with the following technical communities: IEEE Broadcast Technology Society, IEEE Electronics Packaging Society, IEEE Photonics Society, IEEE Reliability Society, IEEE Engineering in Medicine and Biology Society, and IEEE Power & Energy Society. Additional discipline-specific Sections will be coming in the coming months.
To learn more about the IEEE Access Sections, please visit: https://ieeeaccess.ieee.org/special-sections/society-council-sections/
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

is Mainsite

- Search all IEEE websites
- Mission and vision
- IEEE at a glance
- IEEE Strategic Plan
- Organization of IEEE
- Diversity, Equity, & Inclusion
- Organizational Ethics
- Annual Report
- History of IEEE
- Volunteer resources
- IEEE Corporate Awards Program
- Financials and Statistics
- IEEE Future Directions
- IEEE for Industry (Corporations, Government, Individuals)
- IEEE Climate Change
- Humanitarian and Philanthropic Opportunities
- Select an option
- Get the latest news
- Access volunteer resources (Code of Ethics, financial forms, tools and templates, and more)
- Find IEEE locations
- Get help from the IEEE Support Center
- Recover your IEEE Account username and password
- Learn about the IEEE Awards program and submit nomination
- View IEEE's organizational structure and leadership
- Apply for jobs at IEEE
- See the history of IEEE
- Learn more about Diversity, Equity & Inclusion at IEEE
- Join an IEEE Society
- Renew your membership
- Member benefits
- IEEE Contact Center
- Connect locally
- Memberships and Subscriptions Catalog
- Member insurance and discounts
- Member Grade Elevation
- Get your company engaged
- Access your Account
- Learn about membership dues
- Learn about Women in Engineering (WIE)
- Access IEEE member email
- Find information on IEEE Fellows
- Access the IEEE member directory
- Learn about the Member-Get-a-Member program
- Learn about IEEE Potentials magazine
- Learn about Student membership
- Affinity groups
- IEEE Societies
- Technical Councils
- Technical Communities
- Geographic Activities
- Working groups
- IEEE Regions
- IEEE Collabratec®
- IEEE Resource Centers
- IEEE DataPort
- See the IEEE Regions
- View the MGA Operations Manual
- Find information on IEEE Technical Activities
- Get IEEE Chapter resources
- Find IEEE Sections, Chapters, Student Branches, and other communities
- Learn how to create an IEEE Student Chapter
- Upcoming conferences
- IEEE Meetings, Conferences & Events (MCE)
- IEEE Conference Application
- IEEE Conference Organizer Education Program
- See benefits of authoring a conference paper
- Search for 2025 conferences
- Search for 2024 conferences
- Find conference organizer resources
- Register a conference
- Publish conference papers
- Manage conference finances
- Learn about IEEE Meetings, Conferences & Events (MCE)
- Visit the IEEE SA site
- Become a member of the IEEE SA
- Find information on the IEEE Registration Authority
- Obtain a MAC, OUI, or Ethernet address
- Access the IEEE 802.11™ WLAN standard
- Purchase standards
- Get free select IEEE standards
- Purchase standards subscriptions on IEEE Xplore®
- Get involved with standards development
- Find a working group
- Find information on IEEE 802.11™
- Access the National Electrical Safety Code® (NESC®)
- Find MAC, OUI, and Ethernet addresses from Registration Authority (regauth)
- Get free IEEE standards
- Learn more about the IEEE Standards Association
- View Software and Systems Engineering Standards
- IEEE Xplore® Digital Library
- Subscription options
- IEEE Spectrum
- The Institute
- Proceedings of the IEEE
- IEEE Access®
- Author resources
- Get an IEEE Xplore Digital Library trial for IEEE members
- Review impact factors of IEEE journals
- Request access to the IEEE Thesaurus and Taxonomy
- Access the IEEE copyright form
- Find article templates in Word and LaTeX formats
- Get author education resources
- Visit the IEEE Xplore digital library
- Find Author Digital Tools for IEEE paper submission
- Review the IEEE plagiarism policy
- Get information about all stages of publishing with IEEE
- IEEE Learning Network (ILN)
- IEEE Credentialing Program
- Pre-university
- IEEE-Eta Kappa Nu
- Accreditation
- Access continuing education courses on the IEEE Learning Network
- Find STEM education resources on TryEngineering.org
- Learn about the TryEngineering Summer Institute for high school students
- Explore university education program resources
- Access pre-university STEM education resources
- Learn about IEEE certificates and how to offer them
- Find information about the IEEE-Eta Kappa Nu honor society
- Learn about resources for final-year engineering projects
- Access career resources
IEEE Reaches a Transformative Open Access Read and Publish Agreement with Finnish Consortium FinELib
Piscataway, N.J. – 24 August 2021 – IEEE , the world’s largest technical professional organization dedicated to advancing technology for humanity, and FinELib, a consortium of Finnish universities, universities of applied sciences, research institutes, and public libraries, have entered an Open Access Read and Publish agreement.
With this new agreement, researchers and other users at the participating institutions will be able to access IEEE journals, conferences, and standards via the IEEE Xplore Digital Library. The agreement also grants affiliated corresponding authors the opportunity to publish their work open access. Under the terms of the three-year agreement, the costs of accessing subscription content and the article processing charges (APCs) required to publish open access are covered by the license fees paid by consortium members, making the process easier and more convenient for authors.
Participating members of the FinELib consortium will have:
- Open access publishing rights in over 160 hybrid IEEE journals and all IEEE fully open access journals, making articles instantly available and free to read by the general public
- Publication of all open access IEEE journal articles with a Creative Commons Attribution (CC-BY) license unless otherwise requested by the author
- Read access rights to peer-reviewed journals, access to approximately 200,000 new conference papers added each year, as well as IEEE standards (totaling more than 4.8 million articles overall, including nearly 275,000 new articles in 2020 alone)
Aria Tuuliniemi, Head of Services at FinELib, says “We are pleased that FinELib can now offer researchers at the participating institutions the option to publish open access with IEEE at no additional charge and in compliance with Plan S and Finnish national OA policies. We look forward to collaborating with IEEE to provide smoother processes to our members and authors.”
“IEEE is proud to announce this new pilot OA model agreement with FinELib,” said Karen Hawkins, IEEE Chief Marketing Officer. “With this agreement, Finnish researchers now have a sustainable means to publish the results of their research in IEEE's leading open access and hybrid publications while maintaining access to over five million documents of published research in IEEE Xplore . By offering this pilot agreement, IEEE has an opportunity to test a model that we can offer more broadly if it proves to successfully and sustainably meet the needs of our readers and authors. IEEE is committed to exposing vital research to the global community and to making the publication experience for the research community in Finland and across the globe as easy as possible in the journals on which the scientific and engineering community relies.”
To learn more about the IEEE open access options for authors and institutions, please visit open.ieee.org .
A not-for-profit organization, IEEE is the world’s largest technical professional organization dedicated to advancing technology for the benefit of humanity. Through its highly cited publications, conferences, technology standards, professional and educational activities, IEEE is the trusted voice in a wide variety of areas ranging from electrical engineering, aerospace systems, telecommunications and computer science to biomedical engineering, artificial intelligence, and consumer electronics. IEEE has expanded its open access program and launched many new fully open access titles in fields such as computing, telecommunications, biomedical engineering, nanotechnology and more. Learn more .
About FinELib
FinELib is a consortium of Finnish higher education institutions, research institutes, and public libraries. We negotiate e-resource license agreements for the Finnish scholarly community and are committed to achieving full and immediate open access in line with Finland’s OA policy. We currently have multiple transformative agreements in place, allowing Finnish authors to publish their research OA in thousands of journals. More information: https://finelib.fi/ .
Media contacts:
Monika Stickel +1 732 562 6027 [email protected] Francine Tardo +1 732 465 5865 [email protected]
IEEE.org | IEEE Xplore Digital Library | IEEE Standards | IEEE Spectrum | More Sites

Committees & Communities
IEEE PES Awards & Scholarships
Every year we are proud to recognize leading engineers with distinct awards and honors and to offer scholarships to cultivate the next generation of power and energy professionals.
View All Awards >
About Membership
Membership programs, member support tools.
Become A PES Member
You’re invited to join the leading global network of +42k power and energy students and professionals.
Get Started Today
Technical Activities
Technical reports, trending technologies.
Latest Posts:
Nuclear Power and Flexible Grid Solutions
Codes and Standards for Advanced Reactors
Software Bill of Materials (SBoM) for Electric Power Systems (EPS)
Upcoming Committee Meetings
Visit our calendar for information on upcoming technical committee meetings.
Calendar of Events >
About Conferences & Meetings
Papers, participation & proposals.
Conference Sponsorship Opportunities
With over 30 PES global conferences annually, we offer many sponsorship opportunities.
Support IEEE PES >
About Publications
Journals & transactions, education overview & resources, about pes university.
Visit the Resource Center
The largest digital library dedicated to the power and energy industry, find the resource you’ve been looking for!
New! Interactive Online Courses
A new series of interactive continuing education courses for those non-engineers and adjacent professionals working in the power industry.
View All >
Preparation and Submission of Papers for the IEEE Open Access Journal of Power and Energy
Home / Publications / PES Author’s Kit / Preparation and Submission of Papers for the IEEE Open Access Journal of Power and Energy
Part 11: Preparation and Submission of Papers for the IEEE Open Access Journal of Power and Energy
(Part 11 last revised January 2024)
The IEEE Power and Energy Technology Systems Journal is now the IEEE Open Access Journal of Power and Energy . Submissions can be either practice or research oriented.
Authors are responsible for complying with the guidelines in effect at the time of submission, so you should check for the most current submission requirements as often as needed.
Introduction
The IEEE Open Access Journal of Power and Energy is intended to be a technical journal focusing on the development, planning, design, construction, maintenance, installation and operation of equipment, structures, materials and power systems for the safe, sustainable, economic, and reliable conversion, generation, transmission, distribution, storage, and usage of electric energy, including its measurement and control. It is a fully electronic, open access publication. Publication is by volume only (one volume and single issue a year).
Open access provides unrestricted access to peer-reviewed journal articles via the Internet. Hence, members and non-members can access content without restriction. In lieu of paid subscriptions, authors are required to pay a publication fee after their paper has been accepted ($1,995 per manuscript up to a maximum of 11.5 pages in the final accepted version effective 1 January 2024; $1,950 for papers submitted in 2023).
To be accepted and published in the IEEE Open Access Journal of Power and Energy , a paper must be of unquestionably high quality. The Editorial Board has the sole responsibility and authority for judging all submissions for acceptance, revision and resubmission, or rejection. The required abstract (150–200 words) and up to 10 keywords will be reviewed in conjunction with the paper.
Papers may not be submitted for a specific PES meeting and, therefore, may be submitted at any time.
After publication, papers are available on IEEE Xplore ® and in the PES Resource Center .
Guidelines for Authors, Reviewers, and Editors
For authors, for reviewers, for editors, technical areas and associated topics.
The technical areas covered by the IEEE Open Access Journal of Power and Energy are enumerated below.
Power System Planning and Forecast
- Integrated resource planning including conventional, renewable, storage device, demand-side, and distributed resources
- Transmission planning
- Distribution planning
- Power system expansion planning
- Energy forecast
- Power system reliability and resiliency
Power System Operation
- Power system stability and control
- Power system security
- State estimation and energy management systems
- Unit commitment and economic dispatch
- Power system market operation
- Synchrophasor technology
- Power system restoration
- Situational awareness and visualization
Power System Analysis and Modeling
- Power system computational methods
- Artificial intelligence in power system analysis
- Power system optimization and economics
- Probabilistic methods and uncertainty
- Power system dynamic modeling
- Power system fault analysis
Renewable and Conventional Power Generation
- Bulk renewable generation and integration
- Distributed renewable generation and integration
- Integrated multiple energy systems
- Solar power
- Marine power
- Other inverter-based power generation
Transmission Systems
- High voltage AC transmission systems
- High voltage DC transmission systems
- FACTS devices
- Superconducting technologies
Active Distribution Networks
- Planning, operation and control of active distribution networks
- Distributed generations
- Demand side management, demand response, and transactive energy
- Energy efficiency
- Smart home, building, community, and city
- Integration of electrical vehicles and distributed energy storage
- DC distribution networks
- Power quality and harmonics
- Planning, operation and control of microgrids
- Islanding and protection of microgrids
- Networked microgrids
Communication and Cyber-physical Security
- Power system communication
- Cyber-physical security
- Big data and its applications in power systems
- Internet of Things in power systems
Power System Equipment, Protection and Relay
- Equipment for power delivery
- Power system protection and relay
- Intelligent monitoring and smart sensors
Electric Machinery
- Design of high performance electric machines
- Motor drives
- Efficient energy conversion
- Wind/solar power generation technique
- Electromagnetic compatibility
Emerging Technologies
- Power system education
- Power system testbeds
- Utility application of power electronics
- Emission reduction
- Application of cloud computing and technology
- Application of block-chain technology
- Data center and power grid
- High voltage engineering
- Wireless charging and applications in electric power
- Community electrification
- Other emerging technologies
Paper Preparation
Compose your paper using word processing software. (Please check that the “Track Changes” feature has been turned off and that the filename does not exceed 50 characters including the extension.) Then convert your word processing file to PDF format.
We encourage you to use LaTeX to produce your paper if it contains many math equations. An IEEE Publications Department template is available on the web at ieeeauthorcenter.ieee.org/create-your-ieee-article/use-authoring-tools-and-ieee-article-templates/ieee-article-templates/templates-for-transactions/ . Please use bare_jrnl.tex in the WIN or MAC LaTeX2e Transactions Style File. This template will produce a paper that satisfies PES formatting requirements. The stylistic differences from the Word templates are acceptable.
Journal papers are limited to 10 pages at submission including any references, attributions, biographies, etc. Papers exceeding 10 pages will be returned without review.
The name and affiliation (including city and country) of each author must appear on the paper.
Guidance for IEEE Publications Regarding AI-Generated Text
The use of artificial intelligence (AI)–generated text in an article shall be disclosed in the acknowledgements section of any paper submitted to an IEEE Conference or Periodical. The sections of the paper that use AI-generated text shall have a citation to the AI system used to generate the text.
Writing/Preparing Abstracts
The abstract is what users and researchers will read when deciding whether your article pertains to their interests and needs. For this reason, your abstract is an extremely important and powerful representation of your article. As an author, you should spend time ensuring that it is readable and that it contains a complete description of your research.
In approximately 150–200 words, you will need to summarize your findings, and describe the implications of those findings.
The abstract must be an accurate reflection of what is in your article as follows.
- Your abstract must be self-contained, without abbreviations, footnotes, or references. It should be a microcosm of the full article.
- Your abstract must be written as one paragraph, and should not contain displayed mathematical equations or tabular material.
- Your abstract should include three or four different keywords or phrases, as this will help readers to find it. (It is important to avoid over-repetition of such phrases as this can result in a page being rejected by search engines.)
- Ensure that your abstract reads well and is grammatically correct.
When submitting an abstract for your original research, it is important to state the primary objective and offer any tested hypotheses. Describe your research design and methodology and accurately state the following: the methods and procedures you employed, the main outcomes and results , and the conclusions that might be drawn from these data and results. Include any implications for further research or application/practice.
Graphical Abstract
This journal accepts graphical abstracts and they must be peer reviewed. For more information about graphical abstracts and their specifications, please visit the following link: ieeeauthorcenter.ieee.org/create-your-ieee-article/prepare-supplementary-materials-for-your-article/developing-a-graphical-abstract/
Please note that the graphical abstract provided by the author will need to follow a standard naming convention. An author providing a graphical abstract should identify it as follows:
- For an image-only graphical abstract, the author should label it: gagraphic
- For a video graphical abstract, the author needs to also include a cover image for the video to display as a still frame (or static image) in front of the video: gacovergraphic gavideo
- For an audio graphical abstract, the author needs to also include a cover image for the audio file to display as a static image for the audio: gacovergraphic gaaudio
Paper Submission Information
Ieee policy regarding plagiarism.
Authors are expected to comply with the IEEE policy regarding plagiarism as stated below:
8.2 Publication Guidelines
8.2.1 Publication Principles
B. RESPONSIBILITIES OF AUTHORS OF ARTICLES PUBLISHED BY IEEE
7. IEEE defines plagiarism as the use of someone else’s prior ideas, processes, results, or words without explicitly acknowledging the original author and source. Plagiarism in any form is unacceptable and is considered a serious breach of professional conduct, with potentially severe ethical and legal consequences. Section 8.2.4.D provides detailed guidelines for a) handling allegations of plagiarism, b) applying appropriate corrective actions when findings of plagiarism have been reached, and c) referencing previously published material.
9. Except as indicated in IEEE Policies, Section 6.4 (Multiple Publication of Original Technical Material in IEEE Periodicals) and Section 8.1.7 of this Manual, authors should only submit original work that has neither appeared elsewhere for publication, nor which is under review for another publication. If authors have used their own previously published work(s) as a basis for a new submission, they are required to cite the previous work(s) and very briefly indicate how the new submission offers substantive novel contributions beyond those of the previously published work(s). Section 8.2.4.G provides guidelines for handling instances of inappropriate multiple submission and prior publication.
In addition to the sanctions applied by IEEE, if an author is found to have committed plagiarism PES imposes the following sanction:
Individuals whose names are placed on the IEEE Prohibited Author List will not be allowed to organize or lead any PES sponsored activities such as invited conference sessions, distinguished lectureship, editorship of PES publications, paper reviewer for PES, etc.
PES Policy Regarding Bibliography Papers
Because of the electronic search capabilities available through the IEEE and other technical societies, bibliography papers merely listing publications are not of interest. Even if such papers include brief summaries of the listed papers, such information cannot be considered as an original contribution of archival value which is a prerequisite for publication in the Journal. Authors should note that such manuscripts will be rejected without review.
Authors who developed bibliography papers may contact the IEEE Technical Committees for their interest in placing the papers on their web sites.
Paper Submission Procedures
An author submits a manuscript by uploading a PDF file of the paper directly onto ScholarOne Manuscripts , a site on the World Wide Web where the paper can be accessed by the Journal Editor-in-Chief, Associate Editors, and Reviewers. (It is recommended that the PDF file be under 1 MB. Papers exceeding this file size may require a longer review time.) Upon acceptance of a paper for publication, the author will be required to upload final files to ScholarOne Manuscripts . In addition, the author will be required to submit a completed IEEE Copyright Form electronically via ScholarOne Manuscripts . Any paper received for initial submission at the Executive Office will be returned so that the author can upload it.
Please note that the same paper cannot be submitted to more than one publication.
PES policy allows papers presented at PES conferences to be submitted for its journals after upgrading with new and additional content. The policy requires that for a PES conference paper to be considered for a journal publication it must have at least 60% new content reflecting new data, experimental results, analysis, conclusions, etc. They are expected to comply with the IEEE policy regarding plagiarism as stated above.
Uploading Instructions
In order for a paper to be submitted and reviewed, it must first be uploaded by the author onto ScholarOne Manuscripts . If you are uploading a revised manuscript in response to a “Revise and Resubmit” decision, please see the next section for instructions.
Navigate to the PES portal page for ScholarOne Manuscripts or to the specific ScholarOne Manuscripts site for the Journal:
- PES portal page: http://mc.manuscriptcentral.com/pes-ieee
- IEEE Open Access Journal of Power and Energy: https://mc.manuscriptcentral.com/oajpe
Follow the directions on the review site and enter all required information. Your entries will appear on the final screen. If there are errors, you can return to earlier pages and correct them.
- Upload the PDF file of your manuscript and check the proof carefully. Look at all special characters, mathematical symbols, Greek letters, equations, and tables. Check your images for clarity and legibility.
Note that the author who logged in to upload the manuscript is automatically designated the “Corresponding Author.” If you wish, you may designate a co-author as the “Corresponding Author” and all future communication regarding the paper will be through that individual.
You must supply the names of all of your co-authors and their contact information. Please do so in the same order they are listed on the manuscript. Though there is room for multiple affiliations for each author, we request you fill in only the first as that is the only one that will be referenced.
Submit the Manuscript
You may leave your manuscript in the Unsubmitted and Manuscripts in Draft area in your Author Center and return later to review it, but please understand that until you click the “Submit” button, the manuscript will not enter the review process. Once you submit your manuscript you will not be able to make any changes to it unless requested to do so by the Editor-in-Chief as a result of the review process.
Once you submit your manuscript you will get an immediate submission confirmation that provides you with a manuscript ID. Be sure to include it in any correspondence regarding your manuscript.
Preliminary Check for Reviewability
After your paper has been submitted to ScholarOne Manuscripts , the PDF file will be given a preliminary check to ensure that it is reviewable—e.g., that the text and graphics are clear and legible, all graphics are present, and the format is appropriate. (Please see Part 4 of the Author’s Kit for specifics.) If your paper is reviewable, it will be released for review as soon as it is checked. If the manuscript is not reviewable, it will be unsubmitted from ScholarOne Manuscripts and you will be sent an e-mail message requesting that you correct the problems and upload a corrected manuscript onto ScholarOne Manuscripts . The paper will not be reviewed until the manuscript is replaced with one that is reviewable.
Peer Review
The articles in this journal are peer reviewed in accordance with the requirements set forth in the IEEE Publication Services and Products Board Operations Manual ( https://pspb.ieee.org/images/files/files/opsmanual.pdf ). Each published article was reviewed by a minimum of two independent reviewers using a single-anonymous peer review process, where the identities of the reviewers are not known to the authors, but the reviewers know the identities of the authors. Articles will be screened for plagiarism before acceptance.
Review Time
"revise and resubmit" manuscript uploads.
If you are uploading a revised manuscript in response to a “Revise and Resubmit” decision by the Editor-in-Chief, DO NOT UPLOAD YOUR MANUSCRIPT AS A NEW PAPER! Submitting a revised manuscript differs from submitting a manuscript for the first time. Here is some information that should clarify the process. You will find more detailed instructions on the ScholarOne Manuscripts site. (Please check that the “Track Changes” feature has been turned off in your word processing file before converting it to PDF format and uploading it.)
- You must have been the Submitting Author when the original manuscript was first submitted in order to submit a manuscript in response to a “Revise and Resubmit” decision.
- As a Submitting Author, you already have an account on ScholarOne Manuscripts . Do not create a new account! See directions above if you do not remember your login ID or password.
- Log in to the review site: https://mc.manuscriptcentral.com/oajpe
- If the paper title is in the “Manuscripts with Decisions” area, click on the “Create a Revision” button. A new manuscript record will be created with the same Manuscript ID and “.R1” (for revision 1). This record “shell” contains all key information previously entered with the exception of the text of your manuscript. Check the existing data about the paper. Make all necessary revisions and supply any missing information.
- Respond to the decision letter and reviewer comments by entering your text in the Response to Decision Letter field.
- You will be asked to enter (or confirm, if information is already provided on the screen) the title, the abstract, the keywords, and additional comments pertaining to the paper. Use the Special Characters Palette if necessary. After each entry or confirmation, click “Save and Continue” once you are satisfied. (It is important that you check the abstract and revise it if necessary.)
- Your manuscript is not entered into the review process until you click “Submit.” You should receive an automated confirmation of the submission almost immediately.
Upon Acceptance of the Paper
Upon acceptance of a paper for publication, the author is required to upload the following final files to ScholarOne Manuscripts :
- LaTeX or Microsoft Word source file of the paper
- PDF file of the paper
- Separate graphics files in Word, eps, ps, tiff, ppt, or Excel format if the graphics are not embedded in the source file
- Biosketches and photos of all authors.
In addition, the author will be required to submit a completed IEEE Copyright Form electronically via ScholarOne Manuscripts .
A proof of the paper will be sent to the corresponding author for review and approval.
Note: PES policy does not allow the author line to be changed once a paper has been accepted for publication.
Publication Information
Posting on ieee xplore ®.
After a paper is accepted and the final files are uploaded by the author to ScholarOne Manuscripts , the author-supplied PDF file is posted on IEEE Xplore ® as an Early Access article. Failure to provide a PDF file as one of the final files will delay the posting on IEEE Xplore ® .
Proof of the Paper
Your manuscript will be edited by the IEEE Publications Department. The process of preparing a manuscript for publication can, on occasion, change, delete, or modify characters and equations. A proof of the manuscript will be sent to the corresponding author for review and approval. It is essential that the proof be checked as thoroughly as possible since the responsibility for the final text is the author’s. Please note when checking your proof:
- Check all mathematics and equations very carefully. It is the author’s responsibility to verify that they have converted properly. (Note: TeX and LaTeX documents are more likely to be converted properly with the rest of the text. Equations in other formats generally must be re-keyed.)
- Check all figures and tables and verify that they are numbered correctly.
- Biographies and photos, if included, should be verified. If photos are submitted at the proof stage, be sure they are appropriately identified.
- If any authors are members of the IEEE, please provide their membership grade and years of grade.
Failure to return the corrected proof in the allotted time frame will delay publication.
It is the policy of the IEEE to own the copyright to the technical contributions it publishes on behalf of the interests of the IEEE, its authors, and their employers, and to facilitate the appropriate reuse of this material by others. To comply with United States copyright law, authors are required to sign and submit a completed IEEE Copyright Form. This form returns to authors and their employers full rights to reuse their material for their own purposes.
Color Figures
Any color figures submitted will be automatically processed by IEEE for online color free of charge.
Presentation at a PES General Meeting
An author who is interested in presenting an accepted Journal paper at a PES General Meeting must upload the paper’s abstract and the full paper (as a supporting document) to the meeting’s submission and review site. They will be sent directly to the appropriate Technical Committee Program Chair (TCPC) based on the committee selection and that TCPC will determine if the topic of the paper is suited for presentation in one of his or her technical sessions. The author will be notified of the decision by e-mail. See the PES web site for details for specific meetings. (Please note: The author should follow the steps on the review site and upload the full paper in addition to the abstract. This is for the TCPC’s use only. The full paper will not be published in the Proceedings. Do not upload the full paper as its own submission. It must be uploaded along with the abstract so there will be only one conference paper number.)
Accepted Journal papers that will be presented at a PES General Meeting (in a poster session, or if invited by a Technical Committee, in a session of another format) will have their abstracts published in the Conference Proceedings for that meeting. At least one author of the Journal paper must register for the meeting and pay the appropriate registration fee! Papers will not be scheduled for presentation nor abstracts of the papers published in the Proceedings unless the fee is paid. Registration forms will be made available on the PES web site.
Questions, Comments, and the Future
Changes in the use of electronics in publishing are dynamic, and we recognize that the instructions given in this document will need to be revised. We also know that you, the author, are in a unique position of knowing both your capabilities and your needs. We welcome your suggestions regarding this document.
All comments, questions, and suggestions about this document will be forwarded to the correct person if you send them to:
IEEE PES Executive Office
Author’s kit.
The author’s kit is available to offer guidelines for the submission for all technical work for review.
LEARN MORE
Advertise In IEEE Magazines
Editorial and digital ad opportunities are available in our leading IEEE magazines.
Open Calls For Transactions
IEEE PES Transactions distribute the latest findings to researchers, engineers, and educators in the power and energy field.
© Copyright 2024 IEEE — All rights reserved. A not-for-profit organization, IEEE is the world’s largest technical professional organization dedicated to advancing technology for the benefit of humanity.
Toward Integrating Intelligence and Programmability in Open Radio Access Networks: A Comprehensive Survey
Ieee account.
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
Open Access Rights Management
All open access articles will be published under either the Creative Commons Attribution License (CC BY) or the Creative Commons Attribution, NonCommercial, No Derivatives License (CC BY-NC-ND).
CC BY and CC BY-NC-ND allow authors to retain copyright of their article and permit a very broad range of reuse. Under CC BY, reuse for commercial purposes or to create derivative works is permitted, whereas under CC BY-NC-ND, reuse cannot be for commercial purposes or change the work in any way. Under the CC BY licenses, authors are responsible for protecting their content from possible abuses such as infringement and plagiarism.
Note that publishing open access requires payment of an open access article processing charge.
Open Access Article Processing Charges
IEEE offers three options for open access (OA) journal publishing.
- Hybrid journals are traditional subscription journals that accept OA content. Over 100 IEEE Transactions, Journals, and Letters offer hybrid publication. The article processing charge for publishing in a hybrid journal can be found by referring to the current IEEE Article Processing Charges List for the most up-to-date prices . Some hybrid journals may charge additional fees such as overlength fees; see the author instructions of the individual journal for specific details.
- Topical journals are fully open access journals that are dedicated to a specific subject area. Article processing charges for publishing in a topical journal can be found by referring to the current IEEE Article Processing Charges List for the most up-to-date prices . Some topical journals may charge additional fees such as overlength fees; see the author instructions of the individual journal for specific details.
- IEEE Access is a multidisciplinary, fully open access journal that covers all of IEEE’s fields of interest. The article processing charge for publishing in IEEE Access can be found by referring to the current IEEE Article Processing Charges List for the most up-to-date prices.

IEEE Low and Lower-Middle Income Country Open Access Discount Program
All IEEE gold open access journals participate in the low and lower-middle income country discount program. Authors located in lower-middle-income countries are eligible for a discount ranging from 25% to 50%, while authors located in low-income countries are eligible for a 100% waiver. Application of the discounts is subject to acceptance of the specified article, which is not guaranteed. Visit the IEEE Open site for details about these discounts.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 May 2024
Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial
- Donna H. Ryan 1 ,
- Ildiko Lingvay ORCID: orcid.org/0000-0001-7006-7401 2 ,
- John Deanfield 3 ,
- Steven E. Kahn 4 ,
- Eric Barros ORCID: orcid.org/0000-0001-6613-4181 5 ,
- Bartolome Burguera 6 ,
- Helen M. Colhoun ORCID: orcid.org/0000-0002-8345-3288 7 ,
- Cintia Cercato ORCID: orcid.org/0000-0002-6181-4951 8 ,
- Dror Dicker 9 ,
- Deborah B. Horn 10 ,
- G. Kees Hovingh 5 ,
- Ole Kleist Jeppesen 5 ,
- Alexander Kokkinos 11 ,
- A. Michael Lincoff ORCID: orcid.org/0000-0001-8175-2121 12 ,
- Sebastian M. Meyhöfer 13 ,
- Tugce Kalayci Oral 5 ,
- Jorge Plutzky ORCID: orcid.org/0000-0002-7194-9876 14 ,
- André P. van Beek ORCID: orcid.org/0000-0002-0335-8177 15 ,
- John P. H. Wilding ORCID: orcid.org/0000-0003-2839-8404 16 &
- Robert F. Kushner 17
Nature Medicine ( 2024 ) Cite this article
13k Accesses
2260 Altmetric
Metrics details
- Health care
- Medical research
In the SELECT cardiovascular outcomes trial, semaglutide showed a 20% reduction in major adverse cardiovascular events in 17,604 adults with preexisting cardiovascular disease, overweight or obesity, without diabetes. Here in this prespecified analysis, we examined effects of semaglutide on weight and anthropometric outcomes, safety and tolerability by baseline body mass index (BMI). In patients treated with semaglutide, weight loss continued over 65 weeks and was sustained for up to 4 years. At 208 weeks, semaglutide was associated with mean reduction in weight (−10.2%), waist circumference (−7.7 cm) and waist-to-height ratio (−6.9%) versus placebo (−1.5%, −1.3 cm and −1.0%, respectively; P < 0.0001 for all comparisons versus placebo). Clinically meaningful weight loss occurred in both sexes and all races, body sizes and regions. Semaglutide was associated with fewer serious adverse events. For each BMI category (<30, 30 to <35, 35 to <40 and ≥40 kg m − 2 ) there were lower rates (events per 100 years of observation) of serious adverse events with semaglutide (43.23, 43.54, 51.07 and 47.06 for semaglutide and 50.48, 49.66, 52.73 and 60.85 for placebo). Semaglutide was associated with increased rates of trial product discontinuation. Discontinuations increased as BMI class decreased. In SELECT, at 208 weeks, semaglutide produced clinically significant weight loss and improvements in anthropometric measurements versus placebo. Weight loss was sustained over 4 years. ClinicalTrials.gov identifier: NCT03574597 .
Similar content being viewed by others

Effects of a personalized nutrition program on cardiometabolic health: a randomized controlled trial

Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial

What is the pipeline for future medications for obesity?
The worldwide obesity prevalence, defined by body mass index (BMI) ≥30 kg m − 2 , has nearly tripled since 1975 (ref. 1 ). BMI is a good surveillance measure for population changes over time, given its strong correlation with body fat amount on a population level, but it may not accurately indicate the amount or location of body fat at the individual level 2 . In fact, the World Health Organization defines clinical obesity as ‘abnormal or excessive fat accumulation that may impair health’ 1 . Excess abnormal body fat, especially visceral adiposity and ectopic fat, is a driver of cardiovascular (CV) disease (CVD) 3 , 4 , 5 , and contributes to the global chronic disease burden of diabetes, chronic kidney disease, cancer and other chronic conditions 6 , 7 .
Remediating the adverse health effects of excess abnormal body fat through weight loss is a priority in addressing the global chronic disease burden. Improvements in CV risk factors, glycemia and quality-of-life measures including personal well-being and physical functioning generally begin with modest weight loss of 5%, whereas greater weight loss is associated with more improvement in these measures 8 , 9 , 10 . Producing and sustaining durable and clinically significant weight loss with lifestyle intervention alone has been challenging 11 . However, weight-management medications that modify appetite can make attaining and sustaining clinically meaningful weight loss of ≥10% more likely 12 . Recently, weight-management medications, particularly those comprising glucagon-like peptide-1 receptor agonists, that help people achieve greater and more sustainable weight loss have been developed 13 . Once-weekly subcutaneous semaglutide 2.4 mg, a glucagon-like peptide-1 receptor agonist, is approved for chronic weight management 14 , 15 , 16 and at doses of up to 2.0 mg is approved for type 2 diabetes treatment 17 , 18 , 19 . In patients with type 2 diabetes and high CV risk, semaglutide at doses of 0.5 mg and 1.0 mg has been shown to significantly lower the risk of CV events 20 . The SELECT trial (Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity) studied patients with established CVD and overweight or obesity but without diabetes. In SELECT, semaglutide was associated with a 20% reduction in major adverse CV events (hazard ratio 0.80, 95% confidence interval (CI) 0.72 to 0.90; P < 0.001) 21 . Data derived from the SELECT trial offer the opportunity to evaluate the weight loss efficacy, in a geographically and racially diverse population, of semaglutide compared with placebo over 208 weeks when both are given in addition to standard-of-care recommendations for secondary CVD prevention (but without a focus on targeting weight loss). Furthermore, the data allow examination of changes in anthropometric measures such as BMI, waist circumference (WC) and waist-to-height ratio (WHtR) as surrogates for body fat amount and location 22 , 23 . The diverse population can also be evaluated for changes in sex- and race-specific ‘cutoff points’ for BMI and WC, which have been identified as anthropometric measures that predict cardiometabolic risk 8 , 22 , 23 .
This prespecified analysis of the SELECT trial investigated weight loss and changes in anthropometric indices in patients with established CVD and overweight or obesity without diabetes, who met inclusion and exclusion criteria, within a range of baseline categories for glycemia, renal function and body anthropometric measures.
Study population
The SELECT study enrolled 17,604 patients (72.3% male) from 41 countries between October 2018 and March 2021, with a mean (s.d.) age of 61.6 (8.9) years and BMI of 33.3 (5.0) kg m − 2 (ref. 21 ). The baseline characteristics of the population have been reported 24 . Supplementary Table 1 outlines SELECT patients according to baseline BMI categories. Of note, in the lower BMI categories (<30 kg m − 2 (overweight) and 30 to <35 kg m − 2 (class I obesity)), the proportion of Asian individuals was higher (14.5% and 7.4%, respectively) compared with the proportion of Asian individuals in the higher BMI categories (BMI 35 to <40 kg m − 2 (class II obesity; 3.8%) and ≥40 kg m − 2 (class III obesity; 2.2%), respectively). As the BMI categories increased, the proportion of women was higher: in the class III BMI category, 45.5% were female, compared with 20.8%, 25.7% and 33.0% in the overweight, class I and class II categories, respectively. Lower BMI categories were associated with a higher proportion of patients with normoglycemia and glycated hemoglobin <5.7%. Although the proportions of patients with high cholesterol and history of smoking were similar across BMI categories, the proportion of patients with high-sensitivity C-reactive protein ≥2.0 mg dl −1 increased as the BMI category increased. A high-sensitivity C-reactive protein >2.0 mg dl −1 was present in 36.4% of patients in the overweight BMI category, with a progressive increase to 43.3%, 57.3% and 72.0% for patients in the class I, II and III obesity categories, respectively.
Weight and anthropometric outcomes
Percentage weight loss.
The average percentage weight-loss trajectories with semaglutide and placebo over 4 years of observation are shown in Fig. 1a (ref. 21 ). For those in the semaglutide group, the weight-loss trajectory continued to week 65 and then was sustained for the study period through week 208 (−10.2% for the semaglutide group, −1.5% for the placebo group; treatment difference −8.7%; 95% CI −9.42 to −7.88; P < 0.0001). To estimate the treatment effect while on medication, we performed a first on-treatment analysis (observation period until the first time being off treatment for >35 days). At week 208, mean weight loss in the semaglutide group analyzed as first on-treatment was −11.7% compared with −1.5% for the placebo group (Fig. 1b ; treatment difference −10.2%; 95% CI −11.0 to −9.42; P < 0.0001).
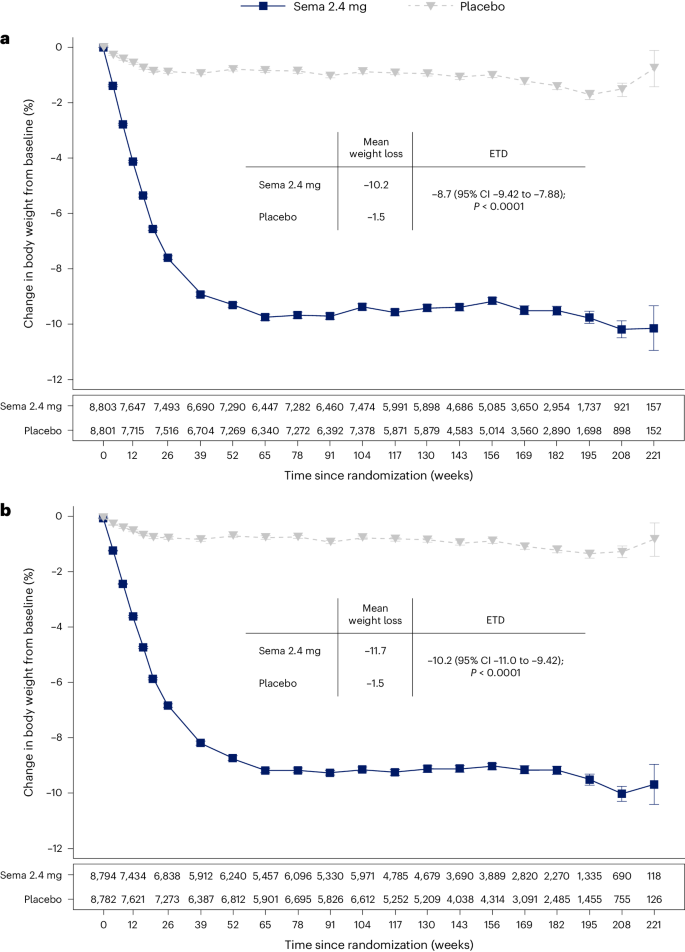
a , b , Observed data from the in-trial period ( a ) and first on-treatment ( b ). The symbols are the observed means, and error bars are ±s.e.m. Numbers shown below each panel represent the number of patients contributing to the means. Analysis of covariance with treatment and baseline values was used to estimate the treatment difference. Exact P values are 1.323762 × 10 −94 and 9.80035 × 10 −100 for a and b , respectively. P values are two-sided and are not adjusted for multiplicity. ETD, estimated treatment difference; sema, semaglutide.
Categorical weight loss and individual body weight change
Among in-trial (intention-to-treat principle) patients at week 104, weight loss of ≥5%, ≥10%, ≥15%, ≥20% and ≥25% was achieved by 67.8%, 44.2%, 22.9%, 11.0% and 4.9%, respectively, of those treated with semaglutide compared with 21.3%, 6.9%, 1.7%, 0.6% and 0.1% of those receiving placebo (Fig. 2a ). Individual weight changes at 104 weeks for the in-trial populations for semaglutide and placebo are depicted in Fig. 2b and Fig. 2c , respectively. These waterfall plots show the variation in weight-loss response that occurs with semaglutide and placebo and show that weight loss is more prominent with semaglutide than placebo.
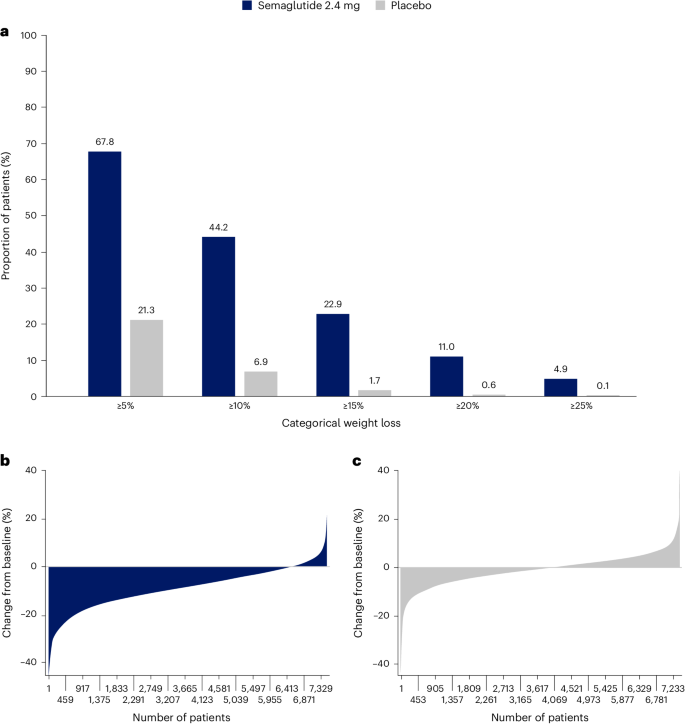
a , Categorical weight loss from baseline at week 104 for semaglutide and placebo. Data from the in-trial period. Bars depict the proportion (%) of patients receiving semaglutide or placebo who achieved ≥5%, ≥10%, ≥15%, ≥20% and ≥25% weight loss. b , c , Percentage change in body weight for individual patients from baseline to week 104 for semaglutide ( b ) and placebo ( c ). Each patient’s percentage change in body weight is plotted as a single bar.
Change in WC
WC change from baseline to 104 weeks has been reported previously in the primary outcome paper 21 . The trajectory of WC change mirrored that of the change in body weight. At week 208, average reduction in WC was −7.7 cm with semaglutide versus −1.3 cm with placebo, with a treatment difference of −6.4 cm (95% CI −7.18 to −5.61; P < 0.0001) 21 .
WC cutoff points
We analyzed achievement of sex- and race-specific cutoff points for WC by BMI <35 kg m − 2 or ≥35 kg m − 2 , because for BMI >35 kg m − 2 , WC is more difficult technically and, thus, less accurate as a risk predictor 4 , 25 , 26 . Within the SELECT population with BMI <35 kg m − 2 at baseline, 15.0% and 14.3% of the semaglutide and placebo groups, respectively, were below the sex- and race-specific WC cutoff points. At week 104, 41.2% fell below the sex- and race-specific cutoff points for the semaglutide group, compared with only 18.0% for the placebo group (Fig. 3 ).
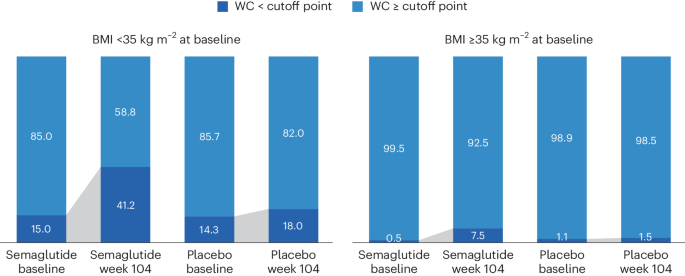
WC cutoff points; Asian women <80 cm, non-Asian women <88 cm, Asian men <88 cm, non-Asian men <102 cm.
Waist-to-height ratio
At baseline, mean WHtR was 0.66 for the study population. The lowest tertile of the SELECT population at baseline had a mean WHtR <0.62, which is higher than the cutoff point of 0.5 used to indicate increased cardiometabolic risk 27 , suggesting that the trial population had high WCs. At week 208, in the group randomized to semaglutide, there was a relative reduction of 6.9% in WHtR compared with 1.0% in placebo (treatment difference −5.87% points; 95% CI −6.56 to −5.17; P < 0.0001).
BMI category change
At week 104, 52.4% of patients treated with semaglutide achieved improvement in BMI category compared with 15.7% of those receiving placebo. Proportions of patients in the BMI categories at baseline and week 104 are shown in Fig. 4 , which depicts in-trial patients receiving semaglutide and placebo. The BMI category change reflects the superior weight loss with semaglutide, which resulted in fewer patients being in the higher BMI categories after 104 weeks. In the semaglutide group, 12.0% of patients achieved a BMI <25 kg m − 2 , which is considered the healthy BMI category, compared with 1.2% for placebo; per study inclusion criteria, no patients were in this category at baseline. The proportion of patients with obesity (BMI ≥30 kg m − 2 ) fell from 71.0% to 43.3% in the semaglutide group versus 71.9% to 67.9% in the placebo group.
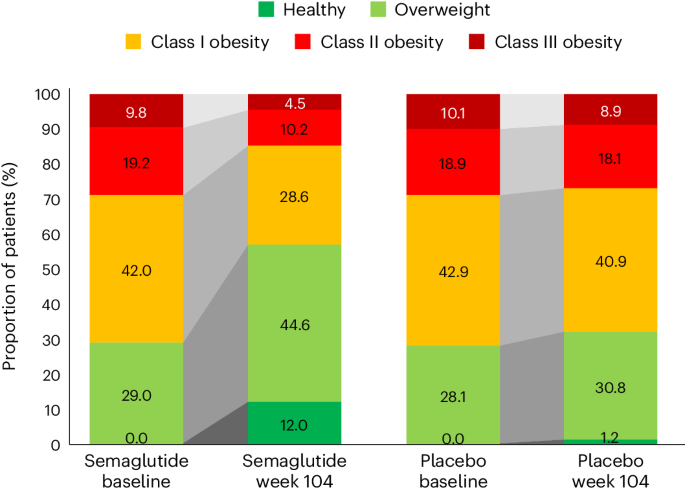
In the semaglutide group, 12.0% of patients achieved normal weight status at week 104 (from 0% at baseline), compared with 1.2% (from 0% at baseline) for placebo. BMI classes: healthy (BMI <25 kg m − 2 ), overweight (25 to <30 kg m − 2 ), class I obesity (30 to <35 kg m − 2 ), class II obesity (35 to <40 kg m − 2 ) and class III obesity (BMI ≥40 kg m − 2 ).
Weight and anthropometric outcomes by subgroups
The forest plot illustrated in Fig. 5 displays mean body weight percentage change from baseline to week 104 for semaglutide relative to placebo in prespecified subgroups. Similar relationships are depicted for WC changes in prespecified subgroups shown in Extended Data Fig. 1 . The effect of semaglutide (versus placebo) on mean percentage body weight loss as well as reduction in WC was found to be heterogeneous across several population subgroups. Women had a greater difference in mean weight loss with semaglutide versus placebo (−11.1% (95% CI −11.56 to −10.66) versus −7.5% in men (95% CI −7.78 to −7.23); P < 0.0001). There was a linear relationship between age category and degree of mean weight loss, with younger age being associated with progressively greater mean weight loss, but the actual mean difference by age group is small. Similarly, BMI category had small, although statistically significant, associations. Those with WHtR less than the median experienced slightly lower mean body weight change than those above the median, with estimated treatment differences −8.04% (95% CI −8.37 to −7.70) and −8.99% (95% CI −9.33 to −8.65), respectively ( P < 0.0001). Patients from Asia and of Asian race experienced slightly lower mean weight loss (estimated treatment difference with semaglutide for Asian race −7.27% (95% CI −8.09 to −6.46; P = 0.0147) and for Asia −7.30 (95% CI −7.97 to −6.62; P = 0.0016)). There was no difference in weight loss with semaglutide associated with ethnicity (estimated treatment difference for Hispanic −8.53% (95% CI −9.28 to −7.76) or non-Hispanic −8.52% (95% CI −8.77 to 8.26); P = 0.9769), glycemic status (estimated treatment difference for prediabetes −8.53% (95% CI −8.83 to −8.24) or normoglycemia −8.48% (95% CI −8.88 to −8.07; P = 0.8188) or renal function (estimated treatment difference for estimated glomerular filtration rate (eGFR) <60 or ≥60 ml min −1 1.73 m − 2 being −8.50% (95% CI −9.23 to −7.76) and −8.52% (95% CI −8.77 to −8.26), respectively ( P = 0.9519)).
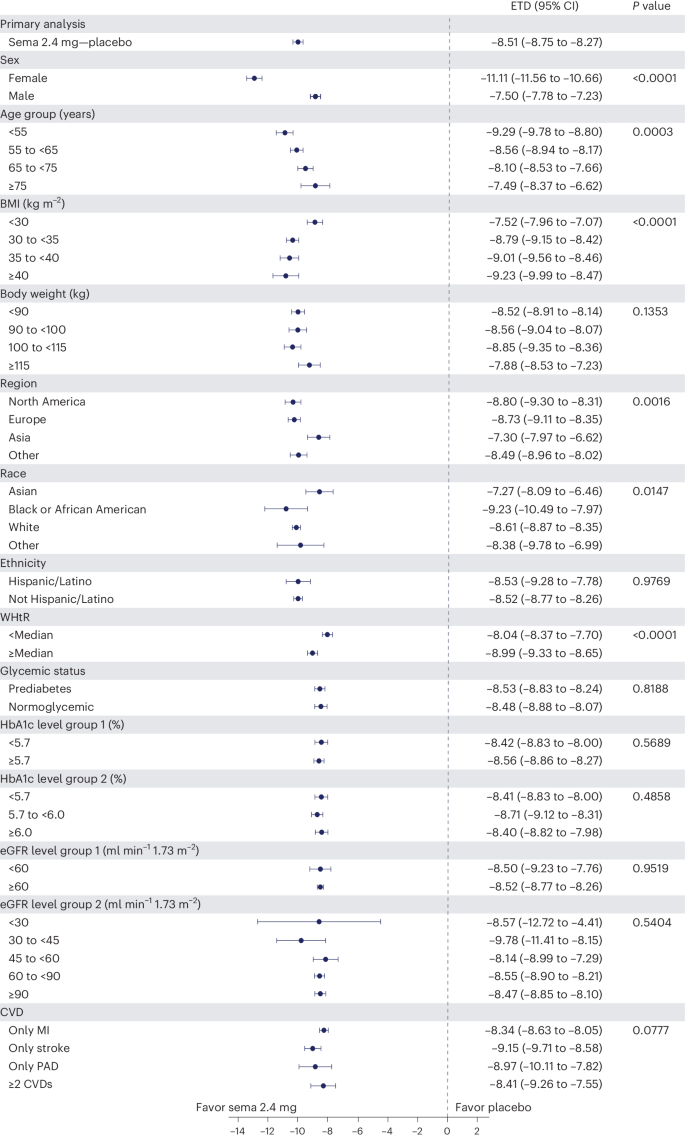
Data from the in-trial period. N = 17,604. P values represent test of no interaction effect. P values are two-sided and are not adjusted for multiplicity. The dots show estimated treatment differences, and the error bars show 95% CIs. Details of the statistical models are available in Methods . ETD, estimated treatment difference; HbA1c, glycated hemoglobin; MI, myocardial infarction; PAD, peripheral artery disease; sema, semaglutide.
Safety and tolerability according to baseline BMI category
We reported in the primary outcome of the SELECT trial that adverse events (AEs) leading to permanent discontinuation of the trial product occurred in 1,461 patients (16.6%) in the semaglutide group and 718 patients (8.2%) in the placebo group ( P < 0.001) 21 . For this analysis, we evaluated the cumulative incidence of AEs leading to trial product discontinuation by treatment assignment and by BMI category (Fig. 6 ). For this analysis, with death modeled as a competing risk, we tracked the proportion of in-trial patients for whom drug was withdrawn or interrupted for the first time (Fig. 6 , left) or cumulative discontinuations (Fig. 6 , right). Both panels of Fig. 6 depict a graded increase in the proportion discontinuing semaglutide, but not placebo. For lower BMI classes, discontinuation rates are higher in the semaglutide group but not the placebo group.
Data are in-trial from the full analysis set. sema, semaglutide.
We reported in the primary SELECT analysis that serious adverse events (SAEs) were reported by 2,941 patients (33.4%) in the semaglutide arm and by 3,204 patients (36.4%) in the placebo arm ( P < 0.001) 21 . For this study, we analyzed SAE rates by person-years of treatment exposure for BMI classes (<30 kg m − 2 , 30 to <35 kg m − 2 , 35 to <40 kg m − 2 , and ≥40 kg m − 2 ) and provide these data in Supplementary Table 2 . We also provide an analysis of the most common categories of SAEs. Semaglutide was associated with lower SAEs, primarily driven by CV event and infections. Within each obesity class (<30 kg m − 2 , 30 to <35 kg m − 2 , 35 to <40 kg m − 2 , and ≥40 kg m − 2 ), there were fewer SAEs in the group receiving semaglutide compared with placebo. Rates (events per 100 years of observation) of SAEs were 43.23, 43.54, 51.07 and 47.06 for semaglutide and 50.48, 49.66, 52.73 and 60.85 for placebo, with no evidence of heterogeneity. There was no detectable difference in hepatobiliary or gastrointestinal SAEs comparing semaglutide with placebo in any of the four BMI classes we evaluated.
The analyses of weight effects of the SELECT study presented here reveal that patients assigned to once-weekly subcutaneous semaglutide 2.4 mg lost significantly more weight than those receiving placebo. The weight-loss trajectory with semaglutide occurred over 65 weeks and was sustained up to 4 years. Likewise, there were similar improvements in the semaglutide group for anthropometrics (WC and WHtR). The weight loss was associated with a greater proportion of patients receiving semaglutide achieving improvement in BMI category, healthy BMI (<25 kg m − 2 ) and falling below the WC cutoff point above which increased cardiometabolic risk for the sex and race is greater 22 , 23 . Furthermore, both sexes, all races, all body sizes and those from all geographic regions were able to achieve clinically meaningful weight loss. There was no evidence of increased SAEs based on BMI categories, although lower BMI category was associated with increased rates of trial product discontinuation, probably reflecting exposure to a higher level of drug in lower BMI categories. These data, representing the longest clinical trial of the effects of semaglutide versus placebo on weight, establish the safety and durability of semaglutide effects on weight loss and maintenance in a geographically and racially diverse population of adult men and women with overweight and obesity but not diabetes. The implications of weight loss of this degree in such a diverse population suggests that it may be possible to impact the public health burden of the multiple morbidities associated with obesity. Although our trial focused on CV events, many chronic diseases would benefit from effective weight management 28 .
There were variations in the weight-loss response. Individual changes in body weight with semaglutide and placebo were striking; still, 67.8% achieved 5% or more weight loss and 44.2% achieved 10% weight loss with semaglutide at 2 years, compared with 21.3% and 6.9%, respectively, for those receiving placebo. Our first on-treatment analysis demonstrated that those on-drug lost more weight than those in-trial, confirming the effect of drug exposure. With semaglutide, lower BMI was associated with less percentage weight loss, and women lost more weight on average than men (−11.1% versus −7.5% treatment difference from placebo); however, in all cases, clinically meaningful mean weight loss was achieved. Although Asian patients lost less weight on average than patients of other races (−7.3% more than placebo), Asian patients were more likely to be in the lowest BMI category (<30 kg m − 2 ), which is known to be associated with less weight loss, as discussed below. Clinically meaningful weight loss was evident in the semaglutide group within a broad range of baseline categories for glycemia and body anthropometrics. Interestingly, at 2 years, a significant proportion of the semaglutide-treated group fell below the sex- and race-specific WC cutoff points, especially in those with BMI <35 kg m − 2 , and a notable proportion (12.0%) fell below the BMI cutoff point of 25 kg m − 2 , which is deemed a healthy BMI in those without unintentional weight loss. As more robust weight loss is possible with newer medications, achieving and maintaining these cutoff point targets may become important benchmarks for tracking responses.
The overall safety profile did not reveal any new signals from prior studies, and there were no BMI category-related associations with AE reporting. The analysis did reveal that tolerability may differ among specific BMI classes, since more discontinuations occurred with semaglutide among lower BMI classes. Potential contributors may include a possibility of higher drug exposure in lower BMI classes, although other explanations, including differences in motivation and cultural mores regarding body size, cannot be excluded.
Is the weight loss in SELECT less than expected based on prior studies with the drug? In STEP 1, a large phase 3 study of once-weekly subcutaneous semaglutide 2.4 mg in individuals without diabetes but with BMI >30 kg m − 2 or 27 kg m − 2 with at least one obesity-related comorbidity, the mean weight loss was −14.9% at week 68, compared with −2.4% with placebo 14 . Several reasons may explain the observation that the mean treatment difference was −12.5% in STEP 1 and −8.7% in SELECT. First, SELECT was designed as a CV outcomes trial and not a weight-loss trial, and weight loss was only a supportive secondary endpoint in the trial design. Patients in STEP 1 were desirous of weight loss as a reason for study participation and received structured lifestyle intervention (which included a −500 kcal per day diet with 150 min per week of physical activity). In the SELECT trial, patients did not enroll for the specific purpose of weight loss and received standard of care covering management of CV risk factors, including medical treatment and healthy lifestyle counseling, but without a specific focus on weight loss. Second, the respective study populations were quite different, with STEP 1 including a younger, healthier population with more women (73.1% of the semaglutide arm in STEP 1 versus 27.7% in SELECT) and higher mean BMI (37.8 kg m − 2 versus 33.3 kg m − 2 , respectively) 14 , 21 . Third, major differences existed between the respective trial protocols. Patients in the semaglutide treatment arm of STEP 1 were more likely to be exposed to the medication at the full dose of 2.4 mg than those in SELECT. In SELECT, investigators were allowed to slow, decrease or pause treatment. By 104 weeks, approximately 77% of SELECT patients on dose were receiving the target semaglutide 2.4 mg weekly dose, which is lower than the corresponding proportion of patients in STEP 1 (89.6% were receiving the target dose at week 68) 14 , 21 . Indeed, in our first on-treatment analysis at week 208, weight loss was greater (−11.7% for semaglutide) compared with the in-trial analysis (−10.2% for semaglutide). Taken together, all these issues make less weight loss an expected finding in SELECT, compared with STEP 1.
The SELECT study has some limitations. First, SELECT was not a primary prevention trial, and the data should not be extrapolated to all individuals with overweight and obesity to prevent major adverse CV events. Although the data set is rich in numbers and diversity, it does not have the numbers of individuals in racial subgroups that may have revealed potential differential effects. SELECT also did not include individuals who have excess abnormal body fat but a BMI <27 kg m − 2 . Not all individuals with increased CV risk have BMI ≥27 kg m − 2 . Thus, the study did not include Asian patients who qualify for treatment with obesity medications at lower BMI and WC cutoff points according to guidelines in their countries 29 . We observed that Asian patients were less likely to be in the higher BMI categories of SELECT and that the population of those with BMI <30 kg m − 2 had a higher percentage of Asian race. Asian individuals would probably benefit from weight loss and medication approaches undertaken at lower BMI levels in the secondary prevention of CVD. Future studies should evaluate CV risk reduction in Asian individuals with high CV risk and BMI <27 kg m − 2 . Another limitation is the lack of information on body composition, beyond the anthropometric measures we used. It would be meaningful to have quantitation of fat mass, lean mass and muscle mass, especially given the wide range of body size in the SELECT population.
An interesting observation from this SELECT weight loss data is that when BMI is ≤30 kg m − 2 , weight loss on a percentage basis is less than that observed across higher classes of BMI severity. Furthermore, as BMI exceeds 30 kg m − 2 , weight loss amounts are more similar for class I, II and III obesity. This was also observed in Look AHEAD, a lifestyle intervention study for weight loss 30 . The proportion (percentage) of weight loss seems to be less, on average, in the BMI <30 kg m − 2 category relative to higher BMI categories, despite their receiving of the same treatment and even potentially higher exposure to the drug for weight loss 30 . Weight loss cannot continue indefinitely. There is a plateau of weight that occurs after weight loss with all treatments for weight management. This plateau has been termed the ‘set point’ or ‘settling point’, a body weight that is in harmony with the genetic and environmental determinants of body weight and adiposity 31 . Perhaps persons with BMI <30 kg m − 2 are closer to their settling point and have less weight to lose to reach it. Furthermore, the cardiometabolic benefits of weight loss are driven by reduction in the abnormal ectopic and visceral depots of fat, not by reduction of subcutaneous fat stores in the hips and thighs. The phenotype of cardiometabolic disease but lower BMI (<30 kg m − 2 ) may be one where reduction of excess abnormal and dysfunctional body fat does not require as much body mass reduction to achieve health improvement. We suspect this may be the case and suggest further studies to explore this aspect of weight-loss physiology.
In conclusion, this analysis of the SELECT study supports the broad use of once-weekly subcutaneous semaglutide 2.4 mg as an aid to CV event reduction in individuals with overweight or obesity without diabetes but with preexisting CVD. Semaglutide 2.4 mg safely and effectively produced clinically significant weight loss in all subgroups based on age, sex, race, glycemia, renal function and anthropometric categories. Furthermore, the weight loss was sustained over 4 years during the trial.
Trial design and participants
The current work complies with all relevant ethical regulations and reports a prespecified analysis of the randomized, double-blind, placebo-controlled SELECT trial ( NCT03574597 ), details of which have been reported in papers describing study design and rationale 32 , baseline characteristics 24 and the primary outcome 21 . SELECT evaluated once-weekly subcutaneous semaglutide 2.4 mg versus placebo to reduce the risk of major adverse cardiac events (a composite endpoint comprising CV death, nonfatal myocardial infarction or nonfatal stroke) in individuals with established CVD and overweight or obesity, without diabetes. The protocol for SELECT was approved by national and institutional regulatory and ethical authorities in each participating country. All patients provided written informed consent before beginning any trial-specific activity. Eligible patients were aged ≥45 years, with a BMI of ≥27 kg m − 2 and established CVD defined as at least one of the following: prior myocardial infarction, prior ischemic or hemorrhagic stroke, or symptomatic peripheral artery disease. Additional inclusion and exclusion criteria can be found elsewhere 32 .
Human participants research
The trial protocol was designed by the trial sponsor, Novo Nordisk, and the academic Steering Committee. A global expert panel of physician leaders in participating countries advised on regional operational issues. National and institutional regulatory and ethical authorities approved the protocol, and all patients provided written informed consent.
Study intervention and patient management
Patients were randomly assigned in a double-blind manner and 1:1 ratio to receive once-weekly subcutaneous semaglutide 2.4 mg or placebo. The starting dose was 0.24 mg once weekly, with dose increases every 4 weeks (to doses of 0.5, 1.0, 1.7 and 2.4 mg per week) until the target dose of 2.4 mg was reached after 16 weeks. Patients who were unable to tolerate dose escalation due to AEs could be managed by extension of dose-escalation intervals, treatment pauses or maintenance at doses below the 2.4 mg per week target dose. Investigators were allowed to reduce the dose of study product if tolerability issues arose. Investigators were provided with guidelines for, and encouraged to follow, evidence-based recommendations for medical treatment and lifestyle counseling to optimize management of underlying CVD as part of the standard of care. The lifestyle counseling was not targeted at weight loss. Additional intervention descriptions are available 32 .
Sex, race, body weight, height and WC measurements
Sex and race were self-reported. Body weight was measured without shoes and only wearing light clothing; it was measured on a digital scale and recorded in kilograms or pounds (one decimal with a precision of 0.1 kg or lb), with preference for using the same scale throughout the trial. The scale was calibrated yearly as a minimum unless the manufacturer certified that calibration of the weight scales was valid for the lifetime of the scale. Height was measured without shoes in centimeters or inches (one decimal with a precision of 0.1 cm or inches). At screening, BMI was calculated by the electronic case report form. WC was defined as the abdominal circumference located midway between the lower rib margin and the iliac crest. Measures were obtained in a standing position with a nonstretchable measuring tape and to the nearest centimeter or inch. The patient was asked to breathe normally. The tape touched the skin but did not compress soft tissue, and twists in the tape were avoided.
The following endpoints relevant to this paper were assessed at randomization (week 0) to years 2, 3 and 4: change in body weight (%); proportion achieving weight loss ≥5%, ≥10%, ≥15% and ≥20%; change in WC (cm); and percentage change in WHtR (cm cm −1 ). Improvement in BMI category (defined as being in a lower BMI class) was assessed at week 104 compared with baseline according to BMI classes: healthy (BMI <25 kg m − 2 ), overweight (25 to <30 kg m − 2 ), class I obesity (30 to <35 kg m − 2 ), class II obesity (35 to <40 kg m − 2 ) and class III obesity (≥40 kg m − 2 ). The proportions of individuals with BMI <35 or ≥35 kg m − 2 who achieved sex- and race-specific cutoff points for WC (indicating increased metabolic risk) were evaluated at week 104. The WC cutoff points were as follows: Asian women <80 cm, non-Asian women <88 cm, Asian men <88 cm and non-Asian men <102 cm.
Overall, 97.1% of the semaglutide group and 96.8% of the placebo group completed the trial. During the study, 30.6% of those assigned to semaglutide did not complete drug treatment, compared with 27.0% for placebo.
Statistical analysis
The statistical analyses for the in-trial period were based on the intention-to-treat principle and included all randomized patients irrespective of adherence to semaglutide or placebo or changes to background medications. Continuous endpoints were analyzed using an analysis of covariance model with treatment as a fixed factor and baseline value of the endpoint as a covariate. Missing data at the landmark visit, for example, week 104, were imputed using a multiple imputation model and done separately for each treatment arm and included baseline value as a covariate and fit to patients having an observed data point (irrespective of adherence to randomized treatment) at week 104. The fit model is used to impute values for all patients with missing data at week 104 to create 500 complete data sets. Rubin’s rules were used to combine the results. Estimated means are provided with s.e.m., and estimated treatment differences are provided with 95% CI. Binary endpoints were analyzed using logistic regression with treatment and baseline value as a covariate, where missing data were imputed by first using multiple imputation as described above and then categorizing the imputed data according to the endpoint, for example, body weight percentage change at week 104 of <0%. Subgroup analyses for continuous and binary endpoints also included the subgroup and interaction between treatment and subgroup as fixed factors. Because some patients in both arms continued to be followed but were off treatment, we also analyzed weight loss by first on-treatment group (observation period until first time being off treatment for >35 days) to assess a more realistic picture of weight loss in those adhering to treatment. CIs were not adjusted for multiplicity and should therefore not be used to infer definitive treatment effects. All statistical analyses were performed with SAS software, version 9.4 TS1M5 (SAS Institute).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data will be shared with bona fide researchers who submit a research proposal approved by the independent review board. Individual patient data will be shared in data sets in a deidentified and anonymized format. Information about data access request proposals can be found at https://www.novonordisk-trials.com/ .
Obesity and overweight. World Health Organization https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2021).
Cornier, M. A. et al. Assessing adiposity: a scientific statement from the American Heart Association. Circulation 124 , 1996–2019 (2011).
Article PubMed Google Scholar
Afshin, A. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377 , 13–27 (2017).
Jensen, M. D. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 63 , 2985–3023 (2014).
Poirier, P. et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113 , 898–918 (2006).
Dai, H. et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. PLoS Med. 17 , e1003198 (2020).
Article PubMed PubMed Central Google Scholar
Ndumele, C. E. et al. Cardiovascular–kidney–metabolic health: a presidential advisory from the American Heart Association. Circulation 148 , 1606–1635 (2023).
Garvey, W. T. et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pr. 22 , 1–203 (2016).
Article Google Scholar
Ryan, D. H. & Yockey, S. R. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr. Obes. Rep. 6 , 187–194 (2017).
Wing, R. R. et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 34 , 1481–1486 (2011).
Article CAS PubMed PubMed Central Google Scholar
Wadden, T. A., Tronieri, J. S. & Butryn, M. L. Lifestyle modification approaches for the treatment of obesity in adults. Am. Psychol. 75 , 235–251 (2020).
Tchang, B. G. et al. Pharmacologic treatment of overweight and obesity in adults. in (eds. Feingold, K. R. et al.) Endotext https://www.ncbi.nlm.nih.gov/books/NBK279038/ (MDText.com, 2000).
Müller, T. D., Blüher, M., Tschöp, M. H. & DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 21 , 201–223 (2022).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384 , 989–1002 (2021).
Article CAS PubMed Google Scholar
Wegovy (semaglutide) summary of product characteristics. European Medicines Agency https://www.ema.europa.eu/en/documents/product-information/wegovy-epar-product-information_en.pdf (2023).
WEGOVY (semaglutide) prescribing information. Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215256s007lbl.pdf (2023).
Sorli, C. et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 5 , 251–260 (2017).
Ozempic (semaglutide) summary of product characteristics. European Medicines Agency https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf (2023).
OZEMPIC (semaglutide) prescribing information. Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf (2017).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375 , 1834–1844 (2016).
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 389 , 2221–2232 (2023).
Ross, R. et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 16 , 177–189 (2020).
Snijder, M. B., van Dam, R. M., Visser, M. & Seidell, J. C. What aspects of body fat are particularly hazardous and how do we measure them? Int. J. Epidemiol. 35 , 83–92 (2006).
Lingvay, I. et al. Semaglutide for cardiovascular event reduction in people with overweight or obesity: SELECT study baseline characteristics. Obesity 31 , 111–122 (2023).
Basset, J. The Asia-Pacific perspective: redefining obesity and its treatment. International Diabetes Institute, World Health Organization Regional Office for the Western Pacific, International Association for the Study of Obesity & International Obesity Task Force https://www.vepachedu.org/TSJ/BMI-Guidelines.pdf (2000).
Hu, F. in Obesity Epidemiology (ed. Hu, F.) 53–83 (Oxford University Press, 2008).
Browning, L. M., Hsieh, S. D. & Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr. Res. Rev. 23 , 247–269 (2010).
Sattar, N. et al. Treating chronic diseases without tackling excess adiposity promotes multimorbidity. Lancet Diabetes Endocrinol. 11 , 58–62 (2023).
Obesity classification. World Obesity https://www.worldobesity.org/about/about-obesity/obesity-classification (2022).
Unick, J. L. et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care 34 , 2152–2157 (2011).
Speakman, J. R. et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis. Model. Mech. 4 , 733–745 (2011).
Ryan, D. H. et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am. Heart J. 229 , 61–69 (2020).
Download references
Acknowledgements
Editorial support was provided by Richard Ogilvy-Stewart of Apollo, OPEN Health Communications, and funded by Novo Nordisk A/S, in accordance with Good Publication Practice guidelines ( www.ismpp.org/gpp-2022 ).
Author information
Authors and affiliations.
Pennington Biomedical Research Center, Baton Rouge, LA, USA
Donna H. Ryan
Department of Internal Medicine/Endocrinology and Peter O’ Donnell Jr. School of Public Health, University of Texas Southwestern Medical Center, Dallas, TX, USA
Ildiko Lingvay
Institute of Cardiovascular Science, University College London, London, UK
John Deanfield
VA Puget Sound Health Care System and University of Washington, Seattle, WA, USA
Steven E. Kahn
Novo Nordisk A/S, Søborg, Denmark
Eric Barros, G. Kees Hovingh, Ole Kleist Jeppesen & Tugce Kalayci Oral
Endocrinology and Metabolism Institute, Cleveland Clinic, Cleveland, OH, USA
Bartolome Burguera
Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK
Helen M. Colhoun
Obesity Unit, Department of Endocrinology, Hospital das Clínicas, University of São Paulo, São Paulo, Brazil
Cintia Cercato
Internal Medicine Department D, Hasharon Hospital-Rabin Medical Center, Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
Dror Dicker
Center for Obesity Medicine and Metabolic Performance, Department of Surgery, University of Texas McGovern Medical School, Houston, TX, USA
Deborah B. Horn
First Department of Propaedeutic Internal Medicine, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
Alexander Kokkinos
Department of Cardiovascular Medicine, Cleveland Clinic, and Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH, USA
A. Michael Lincoff
Institute of Endocrinology & Diabetes, University of Lübeck, Lübeck, Germany
Sebastian M. Meyhöfer
Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Jorge Plutzky
University of Groningen, University Medical Center Groningen, Department of Endocrinology, Groningen, the Netherlands
André P. van Beek
Department of Cardiovascular and Metabolic Medicine, University of Liverpool, Liverpool, UK
John P. H. Wilding
Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Robert F. Kushner
You can also search for this author in PubMed Google Scholar
Contributions
D.H.R., I.L. and S.E.K. contributed to the study design. D.B.H., I.L., D.D., A.K., S.M.M., A.P.v.B., C.C. and J.P.H.W. were study investigators. D.B.H., I.L., D.D., A.K., S.M.M., A.P.v.B., C.C. and J.P.H.W. enrolled patients. D.H.R. was responsible for data analysis and manuscript preparation. All authors contributed to data interpretation, review, revisions and final approval of the manuscript.
Corresponding author
Correspondence to Donna H. Ryan .
Ethics declarations
Competing interests.
D.H.R. declares having received consulting honoraria from Altimmune, Amgen, Biohaven, Boehringer Ingelheim, Calibrate, Carmot Therapeutics, CinRx, Eli Lilly, Epitomee, Gila Therapeutics, IFA Celtics, Novo Nordisk, Pfizer, Rhythm, Scientific Intake, Wondr Health and Zealand Pharma; she declares she received stock options from Calibrate, Epitomee, Scientific Intake and Xeno Bioscience. I.L. declares having received research funding (paid to institution) from Novo Nordisk, Sanofi, Mylan and Boehringer Ingelheim. I.L. received advisory/consulting fees and/or other support from Altimmune, AstraZeneca, Bayer, Biomea, Boehringer Ingelheim, Carmot Therapeutics, Cytoki Pharma, Eli Lilly, Intercept, Janssen/Johnson & Johnson, Mannkind, Mediflix, Merck, Metsera, Novo Nordisk, Pharmaventures, Pfizer, Regeneron, Sanofi, Shionogi, Structure Therapeutics, Target RWE, Terns Pharmaceuticals, The Comm Group, Valeritas, WebMD and Zealand Pharma. J.D. declares having received consulting honoraria from Amgen, Boehringer Ingelheim, Merck, Pfizer, Aegerion, Novartis, Sanofi, Takeda, Novo Nordisk and Bayer, and research grants from British Heart Foundation, MRC (UK), NIHR, PHE, MSD, Pfizer, Aegerion, Colgate and Roche. S.E.K. declares having received consulting honoraria from ANI Pharmaceuticals, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk and Oramed, and stock options from AltPep. B.B. declares having received honoraria related to participation on this trial and has no financial conflicts related to this publication. H.M.C. declares being a stockholder and serving on an advisory panel for Bayer; receiving research grants from Chief Scientist Office, Diabetes UK, European Commission, IQVIA, Juvenile Diabetes Research Foundation and Medical Research Council; serving on an advisory board and speaker’s bureau for Novo Nordisk; and holding stock in Roche Pharmaceuticals. C.C. declares having received consulting honoraria from Novo Nordisk, Eli Lilly, Merck, Brace Pharma and Eurofarma. D.D. declares having received consulting honoraria from Novo Nordisk, Eli Lilly, Boehringer Ingelheim and AstraZeneca, and received research grants through his affiliation from Novo Nordisk, Eli Lilly, Boehringer Ingelheim and Rhythm. D.B.H. declares having received research grants through her academic affiliation from Novo Nordisk and Eli Lilly, and advisory/consulting honoraria from Novo Nordisk, Eli Lilly and Gelesis. A.K. declares having received research grants through his affiliation from Novo Nordisk and Pharmaserve Lilly, and consulting honoraria from Pharmaserve Lilly, Sanofi-Aventis, Novo Nordisk, MSD, AstraZeneca, ELPEN Pharma, Boehringer Ingelheim, Galenica Pharma, Epsilon Health and WinMedica. A.M.L. declares having received honoraria from Novo Nordisk, Eli Lilly, Akebia Therapeutics, Ardelyx, Becton Dickinson, Endologix, FibroGen, GSK, Medtronic, Neovasc, Provention Bio, ReCor, BrainStorm Cell Therapeutics, Alnylam and Intarcia for consulting activities, and research funding to his institution from AbbVie, Esperion, AstraZeneca, CSL Behring, Novartis and Eli Lilly. S.M.M. declares having received consulting honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daichii-Sankyo, esanum, Gilead, Ipsen, Eli Lilly, Novartis, Novo Nordisk, Sandoz and Sanofi; he declares he received research grants from AstraZeneca, Eli Lilly and Novo Nordisk. J.P. declares having received consulting honoraria from Altimmune, Amgen, Esperion, Merck, MJH Life Sciences, Novartis and Novo Nordisk; he has received a grant, paid to his institution, from Boehringer Ingelheim and holds the position of Director, Preventive Cardiology, at Brigham and Women’s Hospital. A.P.v.B. is contracted via the University of Groningen (no personal payment) to undertake consultancy for Novo Nordisk, Eli Lilly and Boehringer Ingelheim. J.P.H.W. is contracted via the University of Liverpool (no personal payment) to undertake consultancy for Altimmune, AstraZeneca, Boehringer Ingelheim, Cytoki, Eli Lilly, Napp, Novo Nordisk, Menarini, Pfizer, Rhythm Pharmaceuticals, Sanofi, Saniona, Tern Pharmaceuticals, Shionogi and Ysopia. J.P.H.W. also declares personal honoraria/lecture fees from AstraZeneca, Boehringer Ingelheim, Medscape, Napp, Menarini, Novo Nordisk and Rhythm. R.F.K. declares having received consulting honoraria from Novo Nordisk, Weight Watchers, Eli Lilly, Boehringer Ingelheim, Pfizer, Structure and Altimmune. E.B., G.K.H., O.K.J. and T.K.O. are employees of Novo Nordisk A/S.
Peer review
Peer review information.
Nature Medicine thanks Christiana Kartsonaki, Peter Rossing, Naveed Sattar and Vikas Sridhar for their contribution to the peer review of this work. Primary Handling Editor: Sonia Muliyil, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 effect of semaglutide treatment or placebo on waist circumference from baseline to week 104 by subgroups..
Data from the in-trial period. N = 17,604. P values represent test of no interaction effect. P values are two-sided and not adjusted for multiplicity. The dots show estimated treatment differences and the error bars show 95% confidence intervals. Details of the statistical models are available in Methods . BMI, body mass index; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ETD, estimated treatment difference; HbA1c, glycated hemoglobin; MI, myocardial infarction; PAD, peripheral artery disease; sema, semaglutide.
Supplementary information
Reporting summary, supplementary tables 1 and 2.
Supplementary Table 1. Baseline characteristics by BMI class. Data are represented as number and percentage of patients. Renal function categories were based on the eGFR as per Chronic Kidney Disease Epidemiology Collaboration. Albuminuria categories were based on UACR. Smoking was defined as smoking at least one cigarette or equivalent daily. The category ‘Other’ for CV inclusion criteria includes patients where it is unknown if the patient fulfilled only one or several criteria and patients who were randomized in error and did not fulfill any criteria. Supplementary Table 2. SAEs according to baseline BMI category. P value: two-sided P value from Fisher’s exact test for test of no difference.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ryan, D.H., Lingvay, I., Deanfield, J. et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat Med (2024). https://doi.org/10.1038/s41591-024-02996-7
Download citation
Received : 01 March 2024
Accepted : 12 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1038/s41591-024-02996-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Help | Advanced Search
Computer Science > Computer Vision and Pattern Recognition
Title: sakuga-42m dataset: scaling up cartoon research.
Abstract: Hand-drawn cartoon animation employs sketches and flat-color segments to create the illusion of motion. While recent advancements like CLIP, SVD, and Sora show impressive results in understanding and generating natural video by scaling large models with extensive datasets, they are not as effective for cartoons. Through our empirical experiments, we argue that this ineffectiveness stems from a notable bias in hand-drawn cartoons that diverges from the distribution of natural videos. Can we harness the success of the scaling paradigm to benefit cartoon research? Unfortunately, until now, there has not been a sizable cartoon dataset available for exploration. In this research, we propose the Sakuga-42M Dataset, the first large-scale cartoon animation dataset. Sakuga-42M comprises 42 million keyframes covering various artistic styles, regions, and years, with comprehensive semantic annotations including video-text description pairs, anime tags, content taxonomies, etc. We pioneer the benefits of such a large-scale cartoon dataset on comprehension and generation tasks by finetuning contemporary foundation models like Video CLIP, Video Mamba, and SVD, achieving outstanding performance on cartoon-related tasks. Our motivation is to introduce large-scaling to cartoon research and foster generalization and robustness in future cartoon applications. Dataset, Code, and Pretrained Models will be publicly available.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
Share this page:
Submission Guidelines
Submission checklist.
Carefully review each item listed below before submitting your article. Article submissions that do not follow the guidelines below will be returned to draft or immediately rejected.
- Manuscript should be prepared in a double column, single-spaced format using a required IEEE Access template. A Word or LaTex file and a PDF file are both required upon submission. Content on each file must match exactly. File sizes should not exceed 40MB. Download IEEE Access Templates for Microsoft Word and LaTex.
- The use of artificial intelligence (AI)–generated text in an article shall be disclosed in the acknowledgements section of any paper submitted to an IEEE Conference or Periodical. The sections of the paper that use AI-generated text shall have a citation to the AI system used to generate the text. For more information please click here.
- Author lists should be carefully considered before submission. For more information on what constitutes an author, please click here. Contributors who do not meet IEEE’s definition of authorship should be included in the Acknowledgment section of the article. Omitting an author who contributed to your article or including a person who did not fulfill authorship requirements is considered a breach of publishing ethics. Once the list and order of authors has been established, the list and order of authors should not be altered without permission of all living authors of that article, and the decision to allow such changes rests with the Editor.
- Corresponding authors are required to have an ORCID ID associated with their account. For more information on ORCID, click here.
- Short biographies are required for ALL authors on an article submission. As indicated in the required IEEE Access templates, biographies of all authors should be included directly within the article below the references section.
- All authors should be listed on both the source file (Word of LaTex file) and the manuscript PDF file. When submitting your files ensure the submission system successfully extracts the full author list from your files.
- The article should be thoroughly reviewed for proper grammar before being submitted. Articles with poor grammar will be immediately rejected. If needed, IEEE Access offers Paperpal Preflight to assist authors in checking their manuscript for grammar issues prior to submission. Check your manuscript on Paperpal Preflight by clicking here .
- All research works should be carefully referenced. More information to avoid plagiarism is listed below.
- The article should not be submitted elsewhere at the same time. More information about duplicate submission can be found here .
- Supplementary material for review (if any)
- Manuscript keywords (minimum of 3 and maximum of 10). Please carefully select the keywords as this is how we select a relevant Associate Editor to manage the peer review of your article.
- You will be required to select a manuscript type upon submission. A list and description of manuscript types are listed below. If you are unsure, please leave it as “Research Article”. *Please note that should your article be accepted, the manuscript type will be published on the article.
- Opposed reviewers (if any)
- Video (if any, maximum file size is 100MB)
- If the article was previously rejected after peer review with encouragement to update and resubmit, then a complete “list of updates” must be included in a separate document. The list of updates should have the following regarding each comment: 1) reviewers’ concerns, 2) authors’ response to the concerns, 3) actual changes implemented.
- IEEE Access does not have a page limit; however, we strongly recommend keeping the page count under 50 pages for ease of readability. Longer articles may result in longer peer review times. Additional content can be added as supplementary material.
- In alignment with the IEEE Code of Ethics and with respect to the wishes of the subject of the image, IEEE will no longer accept submitted papers which include the ‘Lena image’. For more information please click here .
Manuscript Types Acceptable for Peer Review
At a glance.
- Journal: IEEE Access
- Format: Open Access
- Frequency: Continuous
- Submission to Publication: 4-6 weeks (typical)
- Topics: All topics in IEEE
- Average Acceptance Rate: 27%
- Model: Binary Peer Review
- Article Processing Charge: US $1,995
Featured Articles
Effect of Data Characteristics Inconsistency on Medium and Long-Term Runoff Forecasting by Machine Learning
View in IEEE Xplore
Reducing Losses and Energy Storage Requirements of Modular Multilevel Converters With Optimal Harmonic Injection
Enhancing Accuracy in Actigraphic Measurements: A Lightweight Calibration Method for Triaxial Accelerometers
© 2024 IEEE - All rights reserved. Use of this website signifies your agreement to the IEEE TERMS AND CONDITIONS.
A not-for-profit organization, IEEE is the world’s largest technical professional organization dedicated to advancing technology for the benefit of humanity.
AWARD RULES:
NO PURCHASE NECESSARY TO ENTER OR WIN. A PURCHASE WILL NOT INCREASE YOUR CHANCES OF WINNING.
These rules apply to the “2024 IEEE Access Best Video Award Part 1″ (the “Award”).
- Sponsor: The Sponsor of the Award is The Institute of Electrical and Electronics Engineers, Incorporated (“IEEE”) on behalf of IEEE Access , 445 Hoes Lane, Piscataway, NJ 08854-4141 USA (“Sponsor”).
- Eligibility: Award is open to residents of the United States of America and other countries, where permitted by local law, who are the age of eighteen (18) and older. Employees of Sponsor, its agents, affiliates and their immediate families are not eligible to enter Award. The Award is subject to all applicable state, local, federal and national laws and regulations. Entrants may be subject to rules imposed by their institution or employer relative to their participation in Awards and should check with their institution or employer for any relevant policies. Void in locations and countries where prohibited by law.
- Agreement to Official Rules : By participating in this Award, entrants agree to abide by the terms and conditions thereof as established by Sponsor. Sponsor reserves the right to alter any of these Official Rules at any time and for any reason. All decisions made by Sponsor concerning the Award including, but not limited to the cancellation of the Award, shall be final and at its sole discretion.
- How to Enter: This Award opens on January 1, 2024 at 12:00 AM ET and all entries must be received by 11:59 PM ET on June 30, 2024 (“Promotional Period”).
Entrant must submit a video with an article submission to IEEE Access . The video submission must clearly be relevant to the submitted manuscript. Only videos that accompany an article that is accepted for publication in IEEE Access will qualify. The video may be simulations, demonstrations, or interviews with other experts, for example. Your video file should not exceed 100 MB.
Entrants can enter the Award during Promotional Period through the following method:
- The IEEE Author Portal : Entrants can upload their video entries while submitting their article through the IEEE Author Portal submission site .
- Review and Complete the Terms and Conditions: After submitting your manuscript and video through the IEEE Author Portal, entrants should then review and sign the Terms and Conditions .
Entrants who have already submitted a manuscript to IEEE Access without a video can still submit a video for inclusion in this Award so long as the video is submitted within 7 days of the article submission date. The video can be submitted via email to the article administrator. All videos must undergo peer review and be accepted along with the article submission. Videos may not be submitted after an article has already been accepted for publication.
The criteria for an article to be accepted for publication in IEEE Access are:
- The article must be original writing that enhances the existing body of knowledge in the given subject area. Original review articles and surveys are acceptable even if new data/concepts are not presented.
- Results reported must not have been submitted or published elsewhere (although expanded versions of conference publications are eligible for submission).
- Experiments, statistics, and other analyses must be performed to a high technical standard and are described in sufficient detail.
- Conclusions must be presented in an appropriate fashion and are supported by the data.
- The article must be written in standard English with correct grammar.
- Appropriate references to related prior published works must be included.
- The article must fall within the scope of IEEE Access
- Must be in compliance with the IEEE PSPB Operations Manual.
- Completion of the required IEEE intellectual property documents for publication.
- At the discretion of the IEEE Access Editor-in-Chief.
- Disqualification: The following items will disqualify a video from being considered a valid submission:
- The video is not original work.
- A video that is not accompanied with an article submission.
- The article and/or video is rejected during the peer review process.
- The article and/or video topic does not fit into the scope of IEEE Access .
- The article and/or do not follow the criteria for publication in IEEE Access .
- Videos posted in a comment on IEEE Xplore .
- Content is off-topic, offensive, obscene, indecent, abusive or threatening to others.
- Infringes the copyright, trademark or other right of any third party.
- Uploads viruses or other contaminating or destructive features.
- Is in violation of any applicable laws or regulations.
- Is not in English.
- Is not provided within the designated submission time.
- Entrant does not agree and sign the Terms and Conditions document.
Entries must be original. Entries that copy other entries, or the intellectual property of anyone other than the Entrant, may be removed by Sponsor and the Entrant may be disqualified. Sponsor reserves the right to remove any entry and disqualify any Entrant if the entry is deemed, in Sponsor’s sole discretion, to be inappropriate.
- Entrant’s Warranty and Authorization to Sponsor: By entering the Award, entrants warrant and represent that the Award Entry has been created and submitted by the Entrant. Entrant certifies that they have the ability to use any image, text, video, or other intellectual property they may upload and that Entrant has obtained all necessary permissions. IEEE shall not indemnify Entrant for any infringement, violation of publicity rights, or other civil or criminal violations. Entrant agrees to hold IEEE harmless for all actions related to the submission of an Entry. Entrants further represent and warrant, if they reside outside of the United States of America, that their participation in this Award and acceptance of a prize will not violate their local laws.
- Intellectual Property Rights: Entrant grants Sponsor an irrevocable, worldwide, royalty free license to use, reproduce, distribute, and display the Entry for any lawful purpose in all media whether now known or hereinafter created. This may include, but is not limited to, the IEEE A ccess website, the IEEE Access YouTube channel, the IEEE Access IEEE TV channel, IEEE Access social media sites (LinkedIn, Facebook, Twitter, IEEE Access Collabratec Community), and the IEEE Access Xplore page. Facebook/Twitter/Microsite usernames will not be used in any promotional and advertising materials without the Entrants’ expressed approval.
- Number of Prizes Available, Prizes, Approximate Retail Value and Odds of winning Prizes: Two (2) promotional prizes of $350 USD Amazon gift cards. One (1) grand prize of a $500 USD Amazon gift card. Prizes will be distributed to the winners after the selection of winners is announced. Odds of winning a prize depend on the number of eligible entries received during the Promotional Period. Only the corresponding author of the submitted manuscript will receive the prize.
The grand prize winner may, at Sponsor’ discretion, have his/her article and video highlighted in media such as the IEEE Access Xplore page and the IEEE Access social media sites.
The prize(s) for the Award are being sponsored by IEEE. No cash in lieu of prize or substitution of prize permitted, except that Sponsor reserves the right to substitute a prize or prize component of equal or greater value in its sole discretion for any reason at time of award. Sponsor shall not be responsible for service obligations or warranty (if any) in relation to the prize(s). Prize may not be transferred prior to award. All other expenses associated with use of the prize, including, but not limited to local, state, or federal taxes on the Prize, are the sole responsibility of the winner. Winner(s) understand that delivery of a prize may be void where prohibited by law and agrees that Sponsor shall have no obligation to substitute an alternate prize when so prohibited. Amazon is not a sponsor or affiliated with this Award.
- Selection of Winners: Promotional prize winners will be selected based on entries received during the Promotional Period. The sponsor will utilize an Editorial Panel to vote on the best video submissions. Editorial Panel members are not eligible to participate in the Award. Entries will be ranked based on three (3) criteria:
- Presentation of Technical Content
- Quality of Video
Upon selecting a winner, the Sponsor will notify the winner via email. All potential winners will be notified via their email provided to the sponsor. Potential winners will have five (5) business days to respond after receiving initial prize notification or the prize may be forfeited and awarded to an alternate winner. Potential winners may be required to sign an affidavit of eligibility, a liability release, and a publicity release. If requested, these documents must be completed, signed, and returned within ten (10) business days from the date of issuance or the prize will be forfeited and may be awarded to an alternate winner. If prize or prize notification is returned as undeliverable or in the event of noncompliance with these Official Rules, prize will be forfeited and may be awarded to an alternate winner.
- General Prize Restrictions: No prize substitutions or transfer of prize permitted, except by the Sponsor. Import/Export taxes, VAT and country taxes on prizes are the sole responsibility of winners. Acceptance of a prize constitutes permission for the Sponsor and its designees to use winner’s name and likeness for advertising, promotional and other purposes in any and all media now and hereafter known without additional compensation unless prohibited by law. Winner acknowledges that neither Sponsor, Award Entities nor their directors, employees, or agents, have made nor are in any manner responsible or liable for any warranty, representation, or guarantee, express or implied, in fact or in law, relative to any prize, including but not limited to its quality, mechanical condition or fitness for a particular purpose. Any and all warranties and/or guarantees on a prize (if any) are subject to the respective manufacturers’ terms therefor, and winners agree to look solely to such manufacturers for any such warranty and/or guarantee.
11.Release, Publicity, and Privacy : By receipt of the Prize and/or, if requested, by signing an affidavit of eligibility and liability/publicity release, the Prize Winner consents to the use of his or her name, likeness, business name and address by Sponsor for advertising and promotional purposes, including but not limited to on Sponsor’s social media pages, without any additional compensation, except where prohibited. No entries will be returned. All entries become the property of Sponsor. The Prize Winner agrees to release and hold harmless Sponsor and its officers, directors, employees, affiliated companies, agents, successors and assigns from and against any claim or cause of action arising out of participation in the Award.
Sponsor assumes no responsibility for computer system, hardware, software or program malfunctions or other errors, failures, delayed computer transactions or network connections that are human or technical in nature, or for damaged, lost, late, illegible or misdirected entries; technical, hardware, software, electronic or telephone failures of any kind; lost or unavailable network connections; fraudulent, incomplete, garbled or delayed computer transmissions whether caused by Sponsor, the users, or by any of the equipment or programming associated with or utilized in this Award; or by any technical or human error that may occur in the processing of submissions or downloading, that may limit, delay or prevent an entrant’s ability to participate in the Award.
Sponsor reserves the right, in its sole discretion, to cancel or suspend this Award and award a prize from entries received up to the time of termination or suspension should virus, bugs or other causes beyond Sponsor’s control, unauthorized human intervention, malfunction, computer problems, phone line or network hardware or software malfunction, which, in the sole opinion of Sponsor, corrupt, compromise or materially affect the administration, fairness, security or proper play of the Award or proper submission of entries. Sponsor is not liable for any loss, injury or damage caused, whether directly or indirectly, in whole or in part, from downloading data or otherwise participating in this Award.
Representations and Warranties Regarding Entries: By submitting an Entry, you represent and warrant that your Entry does not and shall not comprise, contain, or describe, as determined in Sponsor’s sole discretion: (A) false statements or any misrepresentations of your affiliation with a person or entity; (B) personally identifying information about you or any other person; (C) statements or other content that is false, deceptive, misleading, scandalous, indecent, obscene, unlawful, defamatory, libelous, fraudulent, tortious, threatening, harassing, hateful, degrading, intimidating, or racially or ethnically offensive; (D) conduct that could be considered a criminal offense, could give rise to criminal or civil liability, or could violate any law; (E) any advertising, promotion or other solicitation, or any third party brand name or trademark; or (F) any virus, worm, Trojan horse, or other harmful code or component. By submitting an Entry, you represent and warrant that you own the full rights to the Entry and have obtained any and all necessary consents, permissions, approvals and licenses to submit the Entry and comply with all of these Official Rules, and that the submitted Entry is your sole original work, has not been previously published, released or distributed, and does not infringe any third-party rights or violate any laws or regulations.
12.Disputes: EACH ENTRANT AGREES THAT: (1) ANY AND ALL DISPUTES, CLAIMS, AND CAUSES OF ACTION ARISING OUT OF OR IN CONNECTION WITH THIS AWARD, OR ANY PRIZES AWARDED, SHALL BE RESOLVED INDIVIDUALLY, WITHOUT RESORTING TO ANY FORM OF CLASS ACTION, PURSUANT TO ARBITRATION CONDUCTED UNDER THE COMMERCIAL ARBITRATION RULES OF THE AMERICAN ARBITRATION ASSOCIATION THEN IN EFFECT, (2) ANY AND ALL CLAIMS, JUDGMENTS AND AWARDS SHALL BE LIMITED TO ACTUAL OUT-OF-POCKET COSTS INCURRED, INCLUDING COSTS ASSOCIATED WITH ENTERING THIS AWARD, BUT IN NO EVENT ATTORNEYS’ FEES; AND (3) UNDER NO CIRCUMSTANCES WILL ANY ENTRANT BE PERMITTED TO OBTAIN AWARDS FOR, AND ENTRANT HEREBY WAIVES ALL RIGHTS TO CLAIM, PUNITIVE, INCIDENTAL, AND CONSEQUENTIAL DAMAGES, AND ANY OTHER DAMAGES, OTHER THAN FOR ACTUAL OUT-OF-POCKET EXPENSES, AND ANY AND ALL RIGHTS TO HAVE DAMAGES MULTIPLIED OR OTHERWISE INCREASED. ALL ISSUES AND QUESTIONS CONCERNING THE CONSTRUCTION, VALIDITY, INTERPRETATION AND ENFORCEABILITY OF THESE OFFICIAL RULES, OR THE RIGHTS AND OBLIGATIONS OF ENTRANT AND SPONSOR IN CONNECTION WITH THE AWARD, SHALL BE GOVERNED BY, AND CONSTRUED IN ACCORDANCE WITH, THE LAWS OF THE STATE OF NEW JERSEY, WITHOUT GIVING EFFECT TO ANY CHOICE OF LAW OR CONFLICT OF LAW, RULES OR PROVISIONS (WHETHER OF THE STATE OF NEW JERSEY OR ANY OTHER JURISDICTION) THAT WOULD CAUSE THE APPLICATION OF THE LAWS OF ANY JURISDICTION OTHER THAN THE STATE OF NEW JERSEY. SPONSOR IS NOT RESPONSIBLE FOR ANY TYPOGRAPHICAL OR OTHER ERROR IN THE PRINTING OF THE OFFER OR ADMINISTRATION OF THE AWARD OR IN THE ANNOUNCEMENT OF THE PRIZES.
- Limitation of Liability: The Sponsor, Award Entities and their respective parents, affiliates, divisions, licensees, subsidiaries, and advertising and promotion agencies, and each of the foregoing entities’ respective employees, officers, directors, shareholders and agents (the “Released Parties”) are not responsible for incorrect or inaccurate transfer of entry information, human error, technical malfunction, lost/delayed data transmissions, omission, interruption, deletion, defect, line failures of any telephone network, computer equipment, software or any combination thereof, inability to access web sites, damage to a user’s computer system (hardware and/or software) due to participation in this Award or any other problem or error that may occur. By entering, participants agree to release and hold harmless the Released Parties from and against any and all claims, actions and/or liability for injuries, loss or damage of any kind arising from or in connection with participation in and/or liability for injuries, loss or damage of any kind, to person or property, arising from or in connection with participation in and/or entry into this Award, participation is any Award-related activity or use of any prize won. Entry materials that have been tampered with or altered are void. If for any reason this Award is not capable of running as planned, or if this Award or any website associated therewith (or any portion thereof) becomes corrupted or does not allow the proper playing of this Award and processing of entries per these rules, or if infection by computer virus, bugs, tampering, unauthorized intervention, affect the administration, security, fairness, integrity, or proper conduct of this Award, Sponsor reserves the right, at its sole discretion, to disqualify any individual implicated in such action, and/or to cancel, terminate, modify or suspend this Award or any portion thereof, or to amend these rules without notice. In the event of a dispute as to who submitted an online entry, the entry will be deemed submitted by the authorized account holder the email address submitted at the time of entry. “Authorized Account Holder” is defined as the person assigned to an email address by an Internet access provider, online service provider or other organization responsible for assigning email addresses for the domain associated with the email address in question. Any attempt by an entrant or any other individual to deliberately damage any web site or undermine the legitimate operation of the Award is a violation of criminal and civil laws and should such an attempt be made, the Sponsor reserves the right to seek damages and other remedies from any such person to the fullest extent permitted by law. This Award is governed by the laws of the State of New Jersey and all entrants hereby submit to the exclusive jurisdiction of federal or state courts located in the State of New Jersey for the resolution of all claims and disputes. Facebook, LinkedIn, Twitter, G+, YouTube, IEEE Xplore , and IEEE TV are not sponsors nor affiliated with this Award.
- Award Results and Official Rules: To obtain the identity of the prize winner and/or a copy of these Official Rules, send a self-addressed stamped envelope to Kimberly Rybczynski, IEEE, 445 Hoes Lane, Piscataway, NJ 08854-4141 USA.

IMAGES
VIDEO
COMMENTS
IEEE Access, a Multidisciplinary, Open Access Journal. IEEE Access is a multidisciplinary, online-only, gold fully open access journal, continuously presenting the results of original research or development across all IEEE fields of interest. Supported by article processing charges (APCs), its hallmarks are rapid peer review, a submission-to ...
Enter the Best Video Award for the Opportunity to Win $500. IEEE 10 Year Anniversary Twitter Giveaway. IEEE Author Portal Saves IEEE Authors Time and Effort. Reviewer Best Practices. IEEE Access is an award-winning, multidisciplinary, all-electronic archival journal, continuously presenting the results of original research.
IEEE Access is a multidisciplinary, online-only, gold fully open access journal, continuously presenting the results of original research or development across all IEEE fields of interest. Supported by article processing charges (APCs), its hallmarks are rapid peer review, a submission-to-publication time of 4 to 6 weeks, and articles that are freely available to all readers.
Open Access Choices for IEEE Authors. Open access (OA) in IEEE publications marks a significant step outside of IEEE's traditional publication model. For many years, IEEE required authors to transfer their copyright to IEEE at manuscript submission, thereby giving IEEE the ability to: Protect the published content against various forms of ...
An author may choose to publish in a traditional journal or in a fully open access journal. There are many benefits of publishing scholarly research. Some reasons why some authors may choose to publish their research through open access depend on the unique circumstances and goals of the individual author. These reasons could include:
IEEE Access is an award-winning, multidisciplinary, all-electronic archival journal continuously presenting the results of original research and development across all of IEEE s fields of interest. With open access to all readers and a rapid peer review and publication process, it has an impact factor of ï X ï ò ó.*.
IEEE Access is a multidisciplinary, open access (OA), applications-oriented, online-only archival journal that continuously presents the results of original research or development across all IEEE fields of interest.. IEEE Access will publish articles that are of high interest to readers, original, technically correct, and clearly presented. Supported by article processing charges (APCs), its ...
June 29, 2020. IEEE is accepting paper submissions for its gold fully open access journals spanning a wide range of technologies including telecommunications, biomedical engineering, automotive technology, signal processing, industrial applications, power and energy, and more. These journals target a rapid publishing schedule and are fully ...
Digital Twin technology is an emerging concept that has become the centre of attention for industry and, in more recent years, academia. The advancements in industry 4.0 concepts have facilitated its growth, particularly in the manufacturing industry. The Digital Twin is defined extensively but is best described as the effortless integration of data between a physical and virtual machine in ...
Piscataway, N.J. - 24 August 2021 - IEEE, the world's largest technical professional organization dedicated to advancing technology for humanity, and FinELib, a consortium of Finnish universities, universities of applied sciences, research institutes, and public libraries, have entered an Open Access Read and Publish agreement.. With this new agreement, researchers and other users at the ...
Top 10 Published Articles of IEEE Access Over the past ten years, IEEE Access has published some of the most groundbreaking research in electrical and electronics engineering and computer science. In celebration of the 10 Year Publishing Anniversary, our Editors have selected the Top 10 articles published in IEEE Access over the last decade based on downloads, citations, and overall impact in ...
IEEE Xplore, delivering full text access to the world's highest quality technical literature in engineering and technology. | IEEE Xplore. IEEE Account. Change Username/Password ... CALL FOR PAPERS. ABOUT. 20-24 OCT 2024 | Kobe, Japan . SEE ALL UPCOMING. IEEE Personal Account. Change username/password; Purchase Details. Payment Options;
(Part 11 last revised January 2024) The IEEE Power and Energy Technology Systems Journal is now the IEEE Open Access Journal of Power and Energy.Submissions can be either practice or research oriented. Authors are responsible for complying with the guidelines in effect at the time of submission, so you should check for the most current submission requirements as often as needed.
This paper divides the concepts and essential techniques necessary for realizing the Metaverse into three components (i.e., hardware, software, and contents) and three approaches (i.e., user interaction, implementation, and application) rather than marketing or hardware approach to conduct a comprehensive analysis.
Open RAN is an emerging vision and an advancement of the Radio Access Network (RAN). Its purpose is to implement a vendor and network-generation agnostic RAN, provide networking solutions across all service requests, and implement artificial intelligence solutions in different stages of an end-to-end communication path. The 5th Generation (5G) and beyond the 5th Generation (B5G) of networking ...
All IEEE gold open access journals participate in the low and lower-middle income country discount program. Authors located in lower-middle-income countries are eligible for a discount ranging from 25% to 50%, while authors located in low-income countries are eligible for a 100% waiver. Application of the discounts is subject to acceptance of ...
Learn More About IEEE Access A Multidisciplinary Open Access Journal. IEEE Access is a multidisciplinary, online-only, gold fully open access journal, continuously presenting the results of original research or development across all IEEE fields of interest. Supported by article processing charges (APCs), its hallmarks are rapid peer review, a submission-to-publication time of 4 to 6 weeks ...
Mamba, an architecture with RNN-like token mixer of state space model (SSM), was recently introduced to address the quadratic complexity of the attention mechanism and subsequently applied to vision tasks. Nevertheless, the performance of Mamba for vision is often underwhelming when compared with convolutional and attention-based models. In this paper, we delve into the essence of Mamba, and ...
In this paper, an intelligent identification method for rail vehicle running state is proposed based on Tiny Machine Learning (TinyML) technology, and an IoT system is developed with small size and low energy consumption. The system uses a Micro-Electro-Mechanical System (MEMS) sensor to collect acceleration data for machine learning training.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format ...
K. G. Shanthi received her BE degree in Electronics and Communication Engineering from Madras University and ME degree in VLSI Design from Anna University, India, in 1996 and 2005 respectively. She rec ineived PhD degree in Information and Communication from Anna University, India, in 2015. She is in the teaching profession for the past 22 years and is currently associated with R.M.K college ...
Sakuga-42M Dataset: Scaling Up Cartoon Research. Zhenglin Pan, Yu Zhu, Yuxuan Mu. Hand-drawn cartoon animation employs sketches and flat-color segments to create the illusion of motion. While recent advancements like CLIP, SVD, and Sora show impressive results in understanding and generating natural video by scaling large models with extensive ...
In this double issue of the Journal of New Music Research, we have gathered together seven papers on a broad range of topics, including music information retrieval, instrumental acoustics, instrument design and luthiery, sound synthesis, music and language, scales and modes, and aesthetics.This range of subject areas nicely illustrates the uniquely broad, multi- and trans-disciplinarity of the ...
AWARD RULES: NO PURCHASE NECESSARY TO ENTER OR WIN. A PURCHASE WILL NOT INCREASE YOUR CHANCES OF WINNING. These rules apply to the "2024 IEEE Access Best Video Award Part 1″ (the "Award").. Sponsor: The Sponsor of the Award is The Institute of Electrical and Electronics Engineers, Incorporated ("IEEE") on behalf of IEEE Access, 445 Hoes Lane, Piscataway, NJ 08854-4141 USA ...