Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Electrophoresis articles from across Nature Portfolio
Electrophoresis is the process by which large charged molecules travel through a medium under a uniform electric field. The most common implementation, gel electrophoresis, is a method used to separate nucleic acids by length or proteins by size, conformation and charge based on their migration through a porous matrix.
Latest Research and Reviews
Valproate regulates inositol synthesis by reducing expression of myo -inositol-3-phosphate synthase
- Kendall C. Case
- Rachel J. Beltman
- Miriam L. Greenberg
Targeted profiling of human extrachromosomal DNA by CRISPR-CATCH
CRISPR-CATCH is used to isolate extrachromosomal DNA (ecDNA) molecules containing oncogenes from human cancer cells. CRISPR-CATCH followed by nanopore sequencing allows for methylation profiling, highlighting differences from the native chromosomal loci.
- King L. Hung
- Jens Luebeck
- Howard Y. Chang
Beovu, but not Lucentis impairs the function of the barrier formed by retinal endothelial cells in vitro
- Heidrun L. Deissler
- Catharina Busch
- Matus Rehak
Nematode CDC-37 and DNJ-13 form complexes and can interact with HSP-90
- Lukas Schmauder
- Eva Absmeier
- Klaus Richter
A new humanized antibody is effective against pathogenic fungi in vitro
- Tomas Di Mambro
- Tania Vanzolini
- Mauro Magnani
Benchmarking laboratory processes to characterise low-biomass respiratory microbiota
- Raiza Hasrat
- Jolanda Kool
- Thijs Bosch
News and Comment
Recovery of intact DNA nanostructures after agarose gel–based separation
- Gaëtan Bellot
- Mark A McClintock
- William M Shih
Guidelines for reporting the use of gel image informatics in proteomics
- Christine Hoogland
- Martin O'Gorman
- Andrew R Jones
Guidelines for reporting the use of capillary electrophoresis in proteomics
- Paula J Domann
- Satoko Akashi
- Chris F Taylor
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Recent (2018–2020) development in capillary electrophoresis
- Published: 22 March 2021
- Volume 414 , pages 115–130, ( 2022 )
Cite this article
- Ziting Gao 1 &
- Wenwan Zhong ORCID: orcid.org/0000-0002-3317-3464 1
11k Accesses
31 Citations
4 Altmetric
Explore all metrics
Development of new capillary electrophoresis (CE) methodology and instrumentation, as well as application of CE to solve new problems, remains an active research area because of the attractive features of CE compared to other separation techniques. In this review, we focus on the representative works about sample preconcentration, separation media or capillary coating development, detector construction, and multidimensional separation in CE, which are judiciously selected from the papers published in 2018–2020.
Similar content being viewed by others
Advances in capillary electrophoresis-based enzyme assays.
Gerhard K. E. Scriba & Fathalla Belal

The application of electrochemical detection in capillary electrophoresis
Sima Najafi Gamat, Lida Fotouhi & Zahra Talebpour
Capillary electrophoresis analysis based on crown ethers
Roya Mohammadzadeh kakhki & Hakimeh Assadi
Avoid common mistakes on your manuscript.
Introduction
Capillary electrophoresis (CE), even since its first introduction in 1981 [ 1 ], has been developed well into a mature and robust separation technique [ 2 ]. Compared to the traditional separation techniques like gel electrophoresis and liquid chromatography, CE carries the advantages of high simplicity in setup and miniaturization, fast separation with high resolution and efficiency, and low sample and solvent consumption. Thus, CE has been widely employed in biomedical research, forensic investigation, environmental monitoring, food quality examination [ 3 ], etc., to separate diverse analytes ranging from small ions, macromolecules, to microorganisms.
Continuous advancement of CE has been focused on overcoming the key obstacles in its operation, such as low concentration sensitivity and strong wall adsorption of compounds with large molecular mass or unfavorable charges [ 4 ], which could be particularly problematic when employed to analyze complex samples with high amounts of interfering compounds in the matrix. Modification of the separation condition with new column coating or buffer additives, fabrication of new separation setup to facilitate multidimensional separation, and development of preconcentration methods and detection systems have been actively explored [ 5 , 6 ]. Additionally, CE can be coupled with bioassays, such as enzyme assays [ 7 ] and systematic evolution of ligands by exponential enrichment (SELEX) [ 2 ], to expand its application scope. Herein, this review provides an overview of such developments in CE in the years of 2018–2020. For the works published before 2018, many elegant reviews can be found [ 2 , 4 – 6 ]. Notably, exciting CE applications in analysis of proteins, pharmaceuticals, nucleic acids, metabolites, and carbohydrates, have been thoroughly discussed in a nice review recently published [ 8 ], which also provides an outlook for future directions towards improving the analysis of single cells and bio-particles. Thus, in this review, we mainly covered emerging and evolving advances in the aspects of sample preconcentration, separation media or coating development, detector construction, and multidimensional separation.
Sample preconcentration
The small column dimension determines that standard CE can only take in a very small volume of the sample, which greatly limits the overall detection sensitivity. Therefore, many on-line and off-line sample preconcentration methods have been developed to utilize electric field, phase distribution, flow-gate, evaporation, etc., to improve analyte detectability [ 2 ].
Electrophoretic preconcentration
Electrophoretic preconcentration is the most common way for sample preconcentration in CE, because it is easy to be implemented without any change to the CE system. Primarily, it manipulates ion migration by controlling the direction of the electroosmotic flow (EOF) and analyte’s electrophoretic mobility, as well as the electric field strength difference between the sample plug and the background electrolytes. A few examples include field-amplified sample stacking/injection (FASS/FASI), large-volume sample stacking/injection (LVSS/LVSI), sweeping, electrokinetic supercharging (EKS), transient isotachophoresis (tITP), isoelectric focusing (IEF), dynamic pH junction, and micelle to sample stacking (MSS).
Still, it could be challenging to implement electrophoretic preconcentration in some operations. For example, interfacing EKS to CE-MS requires dedicated design, including volatile buffer selection, flow controlling, and careful interface assembly. A new approach of EKS-based CE-MS method was described by using low-pH BGE to suppress EOF and facilitate positively charged analyte ionization during ESI [ 9 ]. Fifty millimolar ammonium formate at pH 2.5 in the water/MeOH (60:40 v/v) was chosen for long injection with the aid of a leading electrolyte and terminating electrolyte. The separation sensitivity was enhanced by 5000-fold over conventional injection methods. This coupling was employed for separation of biogenic amines from rat brain stem and whole Drosophila tissue by CE-ESI-MS/MS. Alternatively, CE separation with dynamic pH barrage junction focusing is well compatible with mass spectrometric detection, offering low sample consumption and straightforward optimization. Such focusing is generally utilized for analysis of peptides and intact proteins by CE-MS, and achieved by generating a pH-induced electrophoretic mobility variation at the sample/pH barrage boundary. It has been successfully applied for the study of the MCF7 breast cancer cell proteome using the bottom-up approach [ 10 ], and for top-down proteomic analysis of the zebrafish brain cerebellum (Cb) and optic tectum (Teo) regions [ 11 ]. Also, quantitative analysis of bisphosphonate compounds [ 12 ] and digested monoclonal antibodies [ 13 ] was achieved by this technique.
In addition, electrophoretic focusing can be hyphenated with other preconcentration methods to further improve the enrichment effect. For instance, on-line hyphenation of FASI and tITP was developed by Xu et al. [ 14 ], and successful separation of sotalol and metoprolol in human urine was achieved within 10 min using phosphate as both the background and leading electrolyte, and glycine as the terminating electrolyte, with the limit of detection (LOD) and limit of quantitation (LOQ) for both compounds lower than 25 ng/mL. tITP was also coupled with LVSS by Kawai and co-authors to provide high salt tolerance to up to 10 mM NaCl in CE without losing resolution [ 15 ]. With the detection sensitivity enhanced by 2300 folds compared to the separation with normal injection, this method permitted profiling of the N-glycans in approximately 100 cells. Moreover, a dual stacking method coupling sweeping and MSS [ 16 ] achieved around 43–77 folds improvement in LOD for the organic anions in Angelica sinensis , with the recovery ranging from 94.4 to 108.4%.
New electrophoretic focusing techniques have been developed as well. To analyze bacteria in biological samples, Horká et al. etched a part of the fused silica capillary with supercritical water for static or dynamic adhesion of the bacteria cells [ 17 ]. The cells were then desorbed and preconcentrated by transient isotachophoretic stacking, subsequently separated by micelle electrokinetic chromatography (MEKC) with a non-inorganic surfactant. Such a design led to a high recovery of 83% that was independent of the sample matrix, and low LODs for bacteria in the physiological saline solution, bovine serum, or human blood, which were around 10 2 –10 3 cells mL −1 .
Chromatographic preconcentration
Chromatographic extraction, like solid-phase extraction (SPE), single-drop microextraction (SDME), three-phase microelectroextraction (3PEE), and electromembrane extraction (EME), can isolate and enrich the molecules having strong interaction with the extraction media from complex matrices, which can then be eluted for CE analysis.
New extraction materials have been developed to reduce sample consumption, decrease operating cost, and shorten analysis time. Wu et al. fabricated the magnetic particles coated by the copolymer consisting of poly(3,4-dihydroxyphenylalanine) and polyethyleneimine, and used them as the sorbents for off-line SPME of racemic catecholamines [ 18 ], which were then eluted and analyzed by FASI-CE. The magnetic particle–based SPME improved the detection sensitivity of the catecholamine enantiomers by 10 folds. Two novel periodic mesoporous organosilica materials have also been used as the sorbents for the extraction of 7 chiral drugs [ 19 ]. The good cleanup effect led to fast CE separation with high resolution reaching 2.4~8.5.
Off-line SPE systems contain no direct connection between the enrichment and separation devices, and are straightforward. To eliminate manual sample handling and increase reproducibility as well as sample recovery, on-line SPE-CE systems have been developed. Pont et al. investigated the performance of the gold nanoparticles coated by polymeric monoliths (AuNP@monoliths) as the microcartridges for protein analysis in SPE-CE-mass spectrometry (SPE-CE-MS) [ 20 ]. A 7-mm microcartridge was prepared by modifying the glycidyl methacrylate (GMA)–based monolithic capillary column with ammonia for attachment of AuNPs. The microcartridge can be simply used in a “plug-and-play” manner, providing high CE separating performance for analysis of human transthyretin with an LOD 50 times lower than that obtained by the traditional CE-MS method.
A promising extraction approach, hollow fiber liquid-phase microextraction (HF-LPME), was introduced in 1999 for preconcentration and extraction of pharmaceuticals prior to CE identification and determination [ 21 ]. It is a “green” microextraction technology that is highly compatible with CE because of its enhanced solvent stability, improved selectivity, and lack of carry-over and memory effects between each extraction [ 22 ]. Especially, hollow fiber liquid-liquid-liquid microextraction (HF-LLLME) has been reported to extract polar or ionizable compounds [ 23 ]. This method can also handle very complex matrices, as demonstrated by successful determination of basic drugs in physiological solutions, urine, and dried blood spot (DBS) samples [ 24 ].
Affinity-based extraction media have been employed to improve the specificity in target enrichment. Pero-Gascon et al. described an on-line aptamer-based affinity SPE as the pre-concentrator for CE-MS analysis of α-synuclein (α-syn) in blood (Fig. 1 ) [ 25 ]. Under the optimal separation conditions, an LOD of 0.2 μg·mL −1 was reached with excellent repeatability and microcartridge lifetime, and the method was proved to be effective for analysis of α-syn in the lysates of red blood cells collected from healthy controls and Parkinson’s disease (PD) patients.
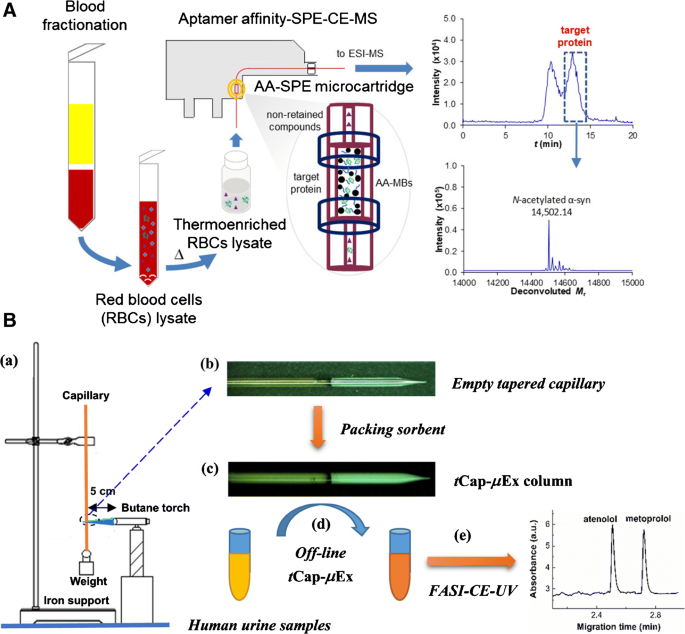
Schematic illustration of on-line aptamer affinity SPE-CE-mass spectrometry for the analysis of blood α-synuclein (A) and t Cap-μEx approach coupled with FASI-CE for quantification of two β-blockers in human urine samples (B). For more information, please see [ 25 , 27 ]
Coupling EME with CE is intuitive because it requires electric fields to assist analyte migration. To achieve a uniform three-dimensional (3D) electric field in EME, Tan and co-authors printed a 3D EME device using a ROVA 3D FDM printer [ 26 ]. The device consisted of a hemispherical electrode sample vial with a smaller hemispherical 3D-printed porous membrane sitting inside that allows for anions to migrate through. Its performance was evaluated for determination of anions in water, achieving low LODs around 0.16–0.18 μM for NO 3 − , ClO 4 − , and SO 4 2− .
Electrophoretic and chromatographic preconcentration can be combined to further enhance CE analysis of trace analytes in complex samples. Sun et al. coupled a novel microextraction method to FASI-CE for the enrichment and quantification of two beta-blockers in human urine (Fig. 1 ) [ 27 ]. They constructed a tapered SPE column by narrowing the diameter of a ~5-cm-long fused silica capillary from 530 to 20 μm, allowing for the packing of the 45-μm sorbent particles. This design is much smaller than the conventional SPE columns, does not require a frit to retain the sorbent particles, and can be reused for at least 8 times. Coupled with FASI-CE, such a column achieved about 21- and 19-fold sensitivity enhancement for atenolol and metoprolol, respectively, compared to the traditional CE method.
Extraction coupled with electrophoretic stacking can be used in non-aqueous CE as well, which is particularly advantageous in CE-MS because of the high volatility and low conductivity of the background electrolytes. For instance, SDME was combined with LVSS by Kim et al. for analysis of organic contaminants in soil sample [ 28 ]. After on-line SDME, the large volume of the pentanol extract was enriched by LVSS and further analyzed by non-aqueous CE/MS. This method achieved 600–1300 folds enrichment for the anionic analytes, including pesticide and herbicide compounds in soil samples, and provided impressive LODs in the range of 0.4–0.8 ppb.
Miscellaneous
Analyte cleanup and preconcentration designs not relying on electrophoresis and chromatography have been reported as well. For example, a membraneless evaporation process with diminished pressure was proposed by Paluch et al., allowing for sample preconcentration in a continuous mode [ 29 ]. This novel system could be used for preconcentration with different starting sample volumes, which was employed to determine the content of Zn in both drinking and waste water using CE-UV with an LOD of 0.025 mg/L.
Microdialysis (MD) was combined on-line with CE-C 4 D for analysis of saccharides in dairy products and honey by Tůma et al. [ 30 ] A flow-gating interface (FGI) made from a polydimethylsiloxane (PDMS) cross-connector was designed and inserted between the MD tubing and the inlet of CE, enabling the introduction of microdialysate to the FGI and then to the separation capillary. The method allowed rapid monitoring of food quality with minimum sample pretreatment and low LOQs in the range of 2.3–7.3 mg/L. In addition, to minimize dilution of the dialysate, a new type of FGI was developed, in which the sample was transferred into the air, not to the gating solution [ 31 ]. A drop of the dialysate was forced from the delivery capillary into the empty injection space by the air pump and hydrodynamically injected into the separation capillary. The design was successfully applied to determine the cations in yogurts by CE-C 4 D.
- Separation media and additives
CE separates analytes by their charge-to-size ratio, and successful separation is strongly dependent on the selection of the proper separation solution that can produce large enough difference in the electrophoretic mobility of the analytes and maintain the optimal and stable EOF. Thus, enormous efforts in CE method development have been devoted to finding the suitable separation media and buffer additives for analysis of diverse compounds.
Synthetic receptors as buffer additives
One big group of molecules used as the BGE additives in CE is synthetic receptors. They can interact with their ligands specifically, enhancing ligand resolution, and have lower molecular weights than proteins, more compatible as BGE additives. The biggest success of using synthetic receptors in CE separation is realized by the native and modified cyclodextrins (CDs), which excel at enantiomer separation [ 2 ].
Non-CD synthetic receptors have been employed as BGE additives in CE as well. Gamithromycin (Gam) was reported by Ren et al. to show good resolution of the chiral primary amines of primaquine (PMQ) and 1-aminoindan (AMI) [ 32 ], with N-methylformamide (NMF) as the non-aqueous solvent [ 33 ]. Our lab employed a group of non-CD receptors, tetrasulfonatocalix[4]arene (CX4), hexasulfonatocalix[6]arene (CX6), and cucurbit[7]uril (CB7), for analysis of methylation in peptides (Fig. 2 ) [ 34 ]. Peptides with different methylation levels at the same lysine position were separated using CX4 and CX6 in the BGE, which was further used to monitor the activity of the histone lysine demethylase JMJD2E. Later, this method was employed to analyze dual modifications, i.e., methylation and phosphorylation, on histone peptides using a linear polyacrylamide-coated capillary [ 35 ]. With the capability of separating the peptides carrying different modifications, the crosstalk between serine phosphorylation and lysine methylation was revealed.
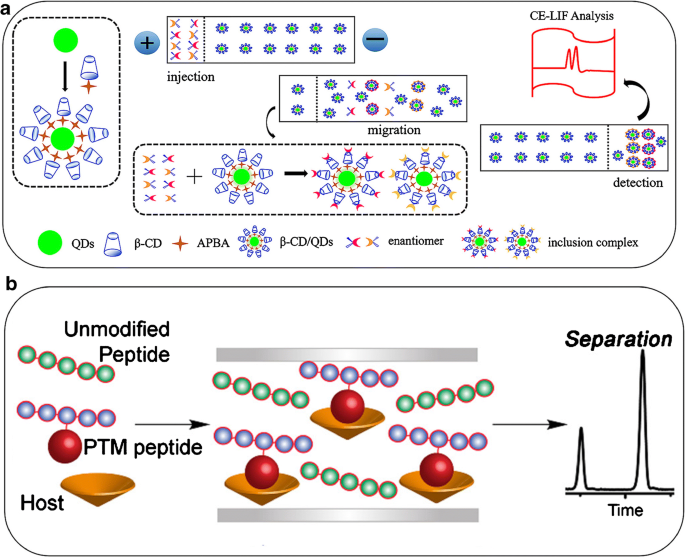
Schematic illustration of β-cyclodextrin–modified quantum dots as pseudo-stationary phase for enantioseparation (A) and the host-assisted CE process with minimized structures of the host complexes (B). For more information, please see [ 34 , 36 ]
Interestingly, synthetic receptors can be used to modify the surface of nanoparticles to enhance CE separation. CD-modified quantum dots (QDs) can act as both the pseudo-stationary phase to improve enantioseparation and the signaling tag for sensitive detection in CE-LIF without capillary pretreatment and analyte modification (Fig. 2 ) [ 36 ]. The RSDs of inter-day and intra-day repeatability varied in the range of 2.7–8.1% for the peak area, 0.7–3.9% for the migration time, and 1.5–3.8% for the resolution. In addition, Liu et al. investigated the streptomycin-modified gold nanoparticles (ST-AuNPs) as an active chiral selector for enantioseparation of adrenaline, noradrenaline, and isoprenaline [ 37 ]. Under the optimized conditions, the racemic mixtures of the respective adrenergic drugs were separated within 7 min with a resolution of 7.5 and good inter-day and intra-day repeatability.
Using the receptors as BGE additives can also help to evaluate receptor-ligand binding. Owing to its high versatility and fast separation speed, CE is a powerful tool for study of non-covalent interactions. However, analyte detectability is often an issue, since adding the receptors to the BGE can increase background UV absorbance and reduce the signals from the analytes. The impurities in the samples could interfere with the measurement as well. Ligand derivatives or multiple detectors can be considered to overcome these difficulties [ 38 ].
Ionic liquid as buffer additives
Ionic liquids, because of their unique ionic and solvation capability, can serve as buffer additives as well. They can be added to MEKC as either modifiers or surfactants for improved separation efficiency and selectivity. Non-chiral ILs can be also used for enantiomer separation, as done by Xue et al., in which several tetraalkylammonium amino acid ILs (TAA-AAILs) were tested for CE enantioseparation of free- or dansyl-amino acids [ 39 ]. TAA-AAILs were also introduced to NACE with β-CD as a chiral selector by Ren et al. [ 40 ] for separation of dansyl-amino acids (Dns-AA).
New chiral ILs have been developed in the past 2 years. For instance, a chiral IL, cholinium-clindamycin phosphate (Ch-CP), was developed and able to improve the separation of five racemic drugs, compared to clindamycin phosphate (CP) [ 41 ]. Noticeably, ILs can be mixed with other chiral selectors to produce a synergistic system for separation improvement. In one case, hydroxy acid–based chiral ionic liquids, namely tertramethylammonium- D -pantothenate (TMA- D -PAN), tertramethylammonium- D -quinate (TMA- D -QUI), was designed and applied to establish a synergistic system with maltodextrin for CE enantioseparation by Yang et al. [ 42 ], significantly improving enantioselectivity. The other case involved the synthesis of three amino alcohol–derived chiral ionic liquids, N,N,N- trimethyl- L -valinol-bis(trifluoromethanesulfon)imide, N,N- dimethyl- L -prolinol-bis(trifluoromethanesulfon)imide, and N,N,N- trimethyl- L -phenylalaninol-bis(trifluoromethanesulfon)imide), and their application for chiral separation when mixed with 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) [ 43 ]. This optimized synergistic system significantly improved the separation of six drugs.
Even though ILs have attractive features, there are some arguments that once ILs are dissolved, they are no longer ILs, but just cations and anions [ 44 ]. Thus, further studies are required to uncover the specific mechanism of ILs with excellent separation performance.
Organic solvents as separation media
Although water is the most common solvent used in CE, alternative solvents are needed to provide sufficient solubility for special analytes, and enhance compatibility with mass spectrometry (MS)–based detection. Organic solvents provide good solubility to hydrophobic analytes and offer low surface tension and high volatility, making non-aqueous capillary electrophoresis (NACE) more suitable than aqueous CE for being coupled with ESI-MS [ 45 ]. Single or dual solvents can be used. For example, acetonitrile containing 25 mM ammonium acetate and 100 mM trifluoroacetic acid was used for separation of nine benzodiazepines in serum with a capillary coated by multiple ionic polymers [ 46 ]. The separation was completed at a negative voltage within 12 min, with the LODs in MS reaching between 1.5 and 15.0 ng/mL. A mixture of 20% acetonitrile and 78% methanol with 2% formic acid and 20 mM ammonium formate was also employed for analysis of hydrophobic peptides [ 47 ], representing an effective alternative to the reversed-phase liquid chromatography (RPLC) for proteomic studies.
NACE offers several advantages over aqueous CE. For example, higher voltages can be used to provide fast analysis without increasing the risk of Joule heating. Additionally, by using different combinations of organic solvents or changing the proportion of the electrolytes, separation selectivity can be modified. Moreover, volatile solvents are more compatible with downstream MS detection. However, some concerns remain. There is lack of systematic investigations of solvent and electrolyte selection, and the correlation of the selection with separation selectivity. Besides, the presence of water in the electrophoresis medium may influence the separation efficiency.
Capillary coatings and surface modifications
When CE, especially CZE, is performed in a bare fused silica capillary, peak shape, resolution, and efficiency could be compromised by the interaction between samples and the inner wall of capillary. Analyte adsorption onto the capillary wall would change the wall conditions and thus affect the magnitude of EOF, leading to poor reproducibility in migration time. Analyte quantification would be strongly impacted by wall interaction as well, because the peak area would not be proportional to the injected amounts with some adsorbed on the wall. On the other hand, biological units can be immobilized on the capillary walls to conduct specific reactions prior to the separation, enabling investigations of enzymatic or cellular functions using CE. Thus, capillary coating and surface modification remain popular topics of CE research.
Non-covalent and dynamic modifications
Non-covalent, dynamic wall modification is typically the first choice researchers would explore to suppress analyte adsorption and modulate EOF, because it is simpler to be carried out and the coating material is easily exchangeable.
Jia et al. developed a dynamic modification method using the highly water-soluble methyl chitosan (MCh) polymer as the sieving matrix for protein separation. The charges of the amine groups could be tuned by adjusting the pH of BGE, providing high flexibility in modulating the charge of the wall and in turn the magnitude of EOF [ 48 ]. This method showed great potential for protein glycoform analysis.
Nanomaterials can be employed for dynamic coating of capillary in CE as well. Geng et al. proposed a low-cost and effective capillary coating using zeolites imidazolate framework-8 (ZIF-8) [ 49 ]. The large surface area and the accessible tunnels and cages of ZIF-8 can benefit protein and chiral compound separation. By electrostatic interaction, ZIF-8 nanocrystals coated the inner surface of silica capillary, providing fast baseline separation of Lys, CC, BSA, and RNase A in 10 min with good reproducibility and stability. Additionally, when L -glutamic acid was used as the selector ligand, D - and L -phenylalanine was successfully separated by the ZIF-8-coated capillary. In addition, carboxymethyl-β-cyclodextrin-functionalized magnetic microparticles (CD-MMPs) have been used to establish an open-tubular capillary electrochromatography system [ 50 ]. With an external magnetic field, coating by the CD-MMP was easy to realize, and the resultant capillary provided satisfactory repeatability and enantioselectivity for separation of propranolol, ofloxacin, amlodipine, chlortrimeton, tropicamide, and atenolol.
Covalent coatings
Compared to dynamic coating, covalently attaching the coating material to the capillary wall can provide enhanced coating stability and separation reproducibility. There have been substantial improvements achieved lately to simplify the coating procedure and reduce the technical demand, using small molecules, polymers, etc.
A new capillary coating was introduced by linking N-acryloylamido ethoxyethanol (AAEE) to the silica surface via Si-C bonds. The coated capillary was employed for CE-MS, and provided good pH stability and tolerance for harsh rinsing procedures (e.g., using strong acids, strong bases, and/or organic solvents for rinsing) [ 51 ]. With the optimized coating method, outstanding performance for each coated capillary was found, including long-term storage at ambient conditions. Besides, the coating gave reproducible results for small molecules, peptides, and proteins that could be easily adsorbed to capillary surfaces.
Compared to surface modification by small molecules, polymer coating could be more stable and provide higher surface coverage. One photosensitive coating agent, diazotized poly (vinyl alcohol- b -styrene) (diazo-P(VA- b -St)), was developed [ 52 ] and exhibited enhanced stability and outstanding repeatability for protein separation. This coating had the capability to suppress protein adsorption on the inner surface of capillary, leading to good CE separation performance and repeatability.
A mixed polymer coating method has been developed as well, using poly(2-methyl-2-oxazoline) (PMOXA) and poly(acrylic acid) (PAA). The PMOXA-NH 2 and PAA-SH were grafted on the bare fused silica capillary, and the coated capillary was used for protein adsorption and desorption under different separation conditions, which further applied for on-line extraction and preconcentration of lysozyme [ 53 ]. PMOXA/PAA-modified capillary exhibited an interesting behavior: surface adsorption was tunable by changing the pH and ionic strength of the running buffer. Lysozyme could be adsorbed onto the inner wall of capillary at pH 7 while desorbed at pH 3. The peak area of lysozyme was increased by 26 times compared to conventional CE separation, and the sensitivity was enhanced by 10 5 folds.
Nanomaterials can be covalently attached to the capillary wall as well. Zhao et al. used graphene oxide (GO) as the coating reagent and DR as the coupling reagent for capillary coating to inhibit protein adsorption, following the same layer-by-layer self-assembly strategy mentioned above for DR and PS-PEO [ 54 ]. The GO-coated capillary showed great separation performance for proteins with excellent stability and reproducibility.
In a brief summary, on-covalent/dynamic capillary coatings are easier to apply and replenish, while covalent coatings can lead to higher stability and eliminate the possibility of interfering the detection system. However, more diverse coating materials should be developed to simplify the coating procedure and provide more functionality of the coated capillary. Additionally, developing automatic capillary coating approaches in commercial CE instruments can help reduce the technical barrier in preparation of coated capillaries on demand, attracting increasing interests.
Wall immobilization of biological units
Other than EOF modulation and elimination of analyte-wall interaction, biological entities, like proteins and cells, can be immobilized on the capillary wall, producing on-line microreactors to enable in situ reaction monitoring by CE, which is highly useful in drug screening.

Enzyme immobilization
Enzymes can be immobilized onto the capillary wall by adsorption, cross-linking, encapsulation, and magnetic microparticles. The resultant immobilized enzyme microreactor (IMER) can realize analyte incubation, product separation, and detection in one capillary [ 55 ], providing a highly convenient system for rapid monitoring of enzyme activities. Various new immobilization methods have been reported in the past 2 years, aiming to ensure high enzyme activity and simplify immobilization process.
To enhance enzyme loading, nanomaterials can be used as the support, providing high relative surface areas for higher loading capacity of the enzyme molecules. One good example is to use the hydrophobic interaction and hydrogen bonding between GO and the negatively charged enzymes to facilitate CE-IMER [ 56 ]. In this method, poly (diallyldimethylammonium chloride) (PDDA) was firstly adsorbed onto the capillary wall to form a film and attract GO via electrostatic interaction. L -lactate dehydrogenase ( L -LDH) was then immobilized on GO through hydrophobic interaction. The CE-IMER method exhibited repeatability with inter-day and batch-to-batch RSG smaller than 3.49 and 6.37%, respectively.
Dopamine (DOPA) has been widely known for its unique feature in surface modification. It can self-assemble to form a polydopamine (PDA) coating which can be subsequently used for protein conjugation through non-covalent interactions, such as hydrophobic interaction and/or π-π stacking. The same strategy has been applied to immobilize beta-glucosidase (beta-Glu) on the capillary wall for CE-IMER [ 57 ]. The substrate of p-nitrophenyl beta- D -glucopyranoside and the inhibitor of castanospermine were used to demonstrate the system’s suitability for enzyme kinetic and inhibition studies. Immobilization on GO via PDA provided good enzyme stability and assay repeatability: 89.5% of the initial immobilized beta-Glu activity remained after 50 continuous runs.
One highly simple enzyme microreactor was constructed by Dadouch et al. [ 58 ]. They employed the strategy of transverse diffusion of laminar flow profiles (TDLFP) that relies on transverse diffusion to inject a plug of the reaction mixture and allow fast mixing inside the capillary and thus completion of the enzymatic reaction in a capillary. With the implementation of thermal control, fast enzymatic reaction and high-resolution separation were attained. Compared to off-line methodology, 1000-fold reduction of reactant consumption and 2-fold increase of the numbers of theoretical plates were observed.
Enzyme immobilization increases enzyme stability and reduces the experimental cost by avoiding the waste of enzyme. However, some concerns are associated with this approach. For example, when the incubation buffer and separation buffer are different, IMER is not suitable for enzymatic reactions. Moreover, immobilization operations could be complicated and hard to control, resulting in decreased enzyme activity [ 7 ].
Cell capillary electrophoresis
Cells are more suitable than enzymatic proteins to be used in drug screening and characterization by maintaining the natural conformation and bioactivity of proteins. Thus, immobilizing cells to the capillary wall and using them as the drug processor can benefit rapid drug screening and characterization [ 59 ]. A method called immobilized cell capillary electrophoresis (ICCE) has been introduced to screen the drugs targeting integrin macrophage antigen-1 (MAC-1, CD11b/CD18) under optimal physiological conditions [ 60 ]. HEK293 cells that have overexpression in MAC-1-, CD11b-, or CD18 were immobilized on the capillary wall, and used to study their interaction with dimethylsulfoxide (DMSO; negative control) and lactosyl derivative Gu-4 (antagonist of MAC-1). Twenty-nine phenylethanoid glycosides from Cistanches Herba were screened qualitatively and the binding parameters of active compounds were calculated quantitatively. Also, better pharmaceutical efficacy and lower toxic side effects of the identified compounds were confirmed by pharmaceutical efficacy assays, proving the feasibility of ICCE in high-throughput screening for drug discovery.
Similar to proteins, cells can also be adsorbed to the capillary wall without covalent linkage, in which the cells can be put under approximately physiological conditions and maintain their maximum activities. Such a method was employed to use tumor cells/tissues as the interaction phase to allow rapid screening of multi-target antitumor drugs (Fig. 3 ) [ 61 ]. Compared to cell cross-linking, this method can renew the cells every run. However, the stability of the whole cells and inter-day repeatability based on different cell physiological status still need further investigation.
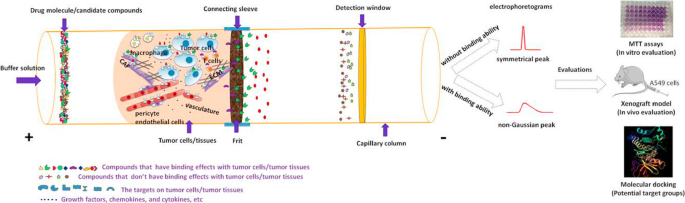
Schematic overflow of non-immobilized tumor cells/tissues CE for rapid screening of multi-target antitumor drugs. For more information, please see [ 61 ]
Multidimentional separations
For analysis of complex biological samples, one dimensional separation may not be sufficient. Thus, multidimensional CE has been developed to enhance resolution and separation performance.
One elegant work was conducted by Schlecht et al. to employ 2D CE for solving the issues of CE-MS coupling [ 62 ]. The first dimension using the mode of CZE, CIEF, MEKC, etc., was coupled with a UV detector and connected to CZE as the second dimension, which used volatile BGEs for high compatibility with MS. Successful separation and detection of intact monoclonal antibody (mAb) was achieved. Furthermore, their group developed MEKC-CZE-MS for analysis of protein impurities and fragments, and demonstrated the versatility of this system by analysis of the proteins extracted from soybeans [ 63 ].
However, coupling CE to other separation techniques is not entirely straightforward due to the ultralow sample volume and flow rate in CE, as well as the complicated design needed to connect the electric field–driven CE with the pressure-driven separation systems. Thus, off-line coupling is often employed. You et al. proposed the first example of off-line coupling of asymmetrical flow field flow fractionation (AF4) and capillary electrophoresis (CE) for separation of nanoparticles [ 64 ]. They studied the separation of a mixture of polystyrene nanoparticles (PS-NPs) that had different charge groups on the surface and comparable core sizes (20, 50, and 100 nm). With either method alone, low peak resolution and efficiency were observed, while the 2D off-line coupling of AF4 and CE system provided distinct regions for the mixtures having the core size of 20 and 50 nm, although separation of the 100-nm particles remained not successful.
Mass spectrometry (MS)
MS continues to be a powerful detector for CE. With the development of new and effective interfaces, CE can be coupled to many different MS systems to achieve high detection sensitivity.
Sasaki et al. used an ESI-MS adapter equipped with a grounded nebulizer for the regulation of electric currents at the interface between CE and the ESI source of HRMS [ 65 ]. This sheathless CE-MS coupling improved the detection sensitivity, and provided good repeatability and intermediate precision for analysis of 34 charged metabolites. Furthermore, they assessed their metabolome analysis platform using human-plasma extract and successfully detected 270 metabolites, proving the potential for large-scale screening of clinical samples for biomarker discovery.
A simple design was reported by Zhang et al. who placed a stainless steel needle at the capillary outlet for the nanoESI emitter and a reference electrode for the ESI spray to direct the liquid flow from the mobility capillary electrophoresis (MCE) separation channel to the MS (Fig. 4 ) [ 66 ]. In MCE, a constant liquid flow replaces EOF in separation, and can be used to determine the effective radius of an ion, and when coupled with MS, to reveal the size of globular biomolecules [ 67 ]. Under optimized conditions, this design enhanced the ion signal intensity by ~10 times, compared to a commercial nanoESI source, with the LODs for peptides reaching ~1 mg/mL to ~20 μg/mL. Similarly, a new medium throughput method was developed to couple MCE with native MS for protein analysis [ 68 ]. In this design, Tayler dispersion analysis (TDA) that provides the determination of the diffusion coefficient of analytes was integrated into MCE, allowing the measurement of ion separation, hydrodynamic radius, and effective charge in a single experiment (Fig. 4 ) [ 69 ]. In this work, the ion hydrodynamic radius and charge state distribution of 11 different globular proteins measured in the native MS were correlated to determine the shapes of these proteins, and monitor protein conformational changes, demonstrating feasibility in top-down proteomics and structural biology study regarding intact globular proteins and protein complexes.
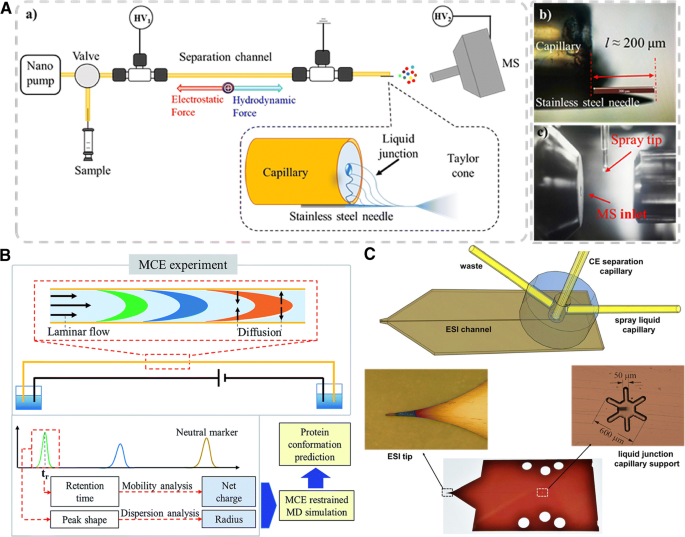
(A) Schematic diagram of a new sheathless ionization interface. (a) The instrument setup with a zoom in view of the straight nanoESI interface. (b) A zoom in photo of the straight nanoESI. (c) A photo of the straight nanoESI MS interface. (B) Schematic diagram of the MCE-based method for protein conformation and charge state analyses. (C) Scheme of the interface (top) and photographs of the liquid junction chip details. For more information, please see [ 66 , 69 , 72 ]
To improve the conventional interfaces between CE and ICP-MS, Holtkamp et al. designed a novel PTEE end-cap to convert a coaxial sheath-flow interface designed for coupling CE with ESI-MS to an effective interface between CE and ICP-MS [ 70 ]. After optimizing the position of the capillary retracted inside the sprayer needle, and the flow rates of the sheath liquid and the carrier gas, the LOD for 195 Pt could reach 0.08 μM with improved peak quality and sensitivity, compared to the commercially available CE-ICP-MS interface.
A mass spectrometry imaging (MSI) platform was built by coupling CE matrix-assisted laser desorption/ionization (MALDI) for improved coverage of neuropeptides [ 71 ]. In this method, the capillary was coated by polyethylenimine (PEI) to reduce peptide adsorption and LVSS was used to preconcentrate neuropeptides because of their low abundance in vivo. Such a continuous sample determination method detected a large number of neuropeptide with good reproducibility, demonstrating its ability to become an alternative for conventional LC-ESI-MS.
Computer modeling can help improve the interface design, as demonstrated by Krenkova et al. A hybrid liquid junction-based interface was designed, guided by computer modeling, to include a liquid junction, a short transport channel, and a pointed sprayer intruding to the ESI source, and can be prepared by microfabrication technologies using plastic materials (Fig. 4 ) [ 72 ]. This novel system was applied to analyze a wide range of small peptides, proteins, and labeled oligosaccharides with low band broadening, high sensitivity, and good separation reproducibility.
IEF-ESI-MS has become a powerful method for accurate identification and quantification of proteoforms. Xu et al. [ 73 ] developed the automatic, on-line IEF-MS/MS platform using an electrokinetically pumped sheath flow CE-MS interface. In this platform, the inlet of linear polyacrylamide (LPA)–coated capillary was inserted into an acidic anolyte solution while its outlet was positioned into the interface filled with an acidic sheath buffer. After focusing, the pH gradient was gradually disrupted with protons migrating from the acidic anolyte and anions from the sheath buffer. Quantitative analysis of protein expression in the brains of male and female zebrafish was achieved for understanding the cellular processes.
Also, CE-MS is well suited for metabolic profiling, as exhibited by using CE-time-of-flight MS for quantification of hydrophilic metabolites in 247 subjects with different cancer stages [ 74 ]. Another good example is to employ multisegment injection-CE-MS for high-throughput metabolomic analysis of the placental with sex-specific differences [ 75 ]. Besides, some preconcentration methods was coupled to the CE-MS platform to reduce sample volume and detection limit, such as LLE for improved analysis of the metabolome of mice plasma [ 76 ] and FASI for enhanced study of the metabolome in individual neurons [ 77 ].
Optical detection
The small dimension of the capillary column determines that only a very small volume of the sample can be injected, and the optical path length is small, both contributing to the poor concentration sensitivity of on-column detection for CE [ 78 ], in particular, when coupled with optical detection methods, such as ultraviolet/visible (UV-Vis), fluorescence, and luminescence detection. Analyte labels that can give out intense signals, or improved detector design, have been two popular solutions to the detection problem of CE.
Graphene quantum dots (GQDs) with relatively uniform sizes and excellent photoluminescence properties were employed to enhance the emission of a fluoroquinolone for fluorimetric detection of ofloxacin in milk samples [ 79 ]. GQDs were injected into the capillary prior to the sample to selectively interact with ofloxacin so that it can be detected by fluorescence. Under the optimal CE conditions, LOD and LQD could reach 10.7 and 35.5 ng/mL respectively with a commercial CE device equipped with a multi-wavelength photoluminescence detector.
CE with UV-excited fluorescence detection for sensitive chiral analysis of amino acids (AAs) was studied by Prior et al. [ 80 ], employing optical fibers to direct the UV light from a Xe-Hg lamp and the emitted fluorescence to a charge-coupled device (CCD). LODs for enantioseparation of D -AA were in the 10–100-nM range. Furthermore, the system was applied to analyze cerebrospinal fluid (CSF) with the assistance of an improved sample cleanup step. An enhanced fluorescence detection system was equipped with a concave silver mirror, greatly increasing the sensitivity of light-emitting diode–induced fluorescence (LEDIF) [ 81 ]. The concave silver mirror reflected the emission of the target molecule with enhanced convergence, which was then detected by the photomultiplier tube (PMT), resulting in 16-fold increase in detection sensitivity compare to that achieved without the concave mirror. Two FITC-labeled tumor markers, L -Leu and L -Val, were separated and detected in CE-LEDIF, with LODs reaching 150 nM. Additionally, an inexpensive and low-footprint silicon photomultiplier (SiPM) was used as a sensitive and affordable fluorescence detector [ 82 ]. SiPMs have a dynamic range that spans 5 orders of magnitude to detect down to 10 −12 M or 10 −21 mol of fluorescein. It was proved to be able to provide high sensitivity for the measurement of enzyme activity in single cells. A miniature, portable, and sensitive instrumentation could be achieved by introducing the commercially available SiPMs.
A novel 3D-printed LED-induced fluorescence detector was designed with an integrated excitation source and optical filters for on-line, dual-point capillary detection by Casto et al. (Fig. 5 ) [ 83 ]. This 3D detector provided LODs of 613 ± 13 pM for fluorescein and was able to continuously monitor the conjugation of bovine serum albumin by fluorescein isothiocyanate.
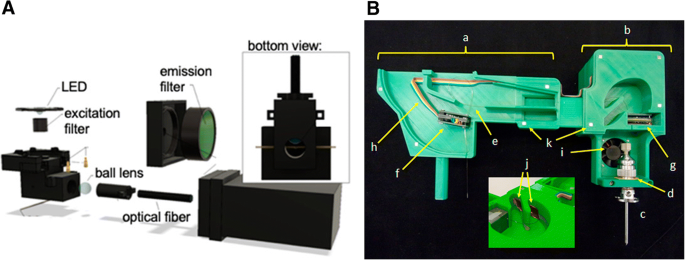
Schematic diagram of a miniature 3D-printed LED-induced fluorescence detector (A) and a new 3D-printed cartridge that is used to improve temperature control of the capillary (B). The 3D-printed cartridge was basically composed of a part that was accommodated in the CE instrument (a) and another one that approaches the ESI source (b), in which the ESI probe (c) was held using a flexible O-ring (d). The silica capillary (e) passed through the first (f) and the second C 4 D (g). The electrical wiring (h) was connected to the microcontroller on the back of b. In the same compartment, there was an electrical connection for the fan (i). The copper foils (j) covered the inner walls of the ducts. Four-millimeter neodymium magnets (k) were used to hold the covers of the compartments. For more information, please see [ 83 , 88 ]
In addition to fluorescence, chemiluminescence can be used for analyte detection in CE. A novel electrochemiluminescence (ECL) detector was designed to consist of silica sol-gel, zinc oxide nanoparticles (ZnO NPs), polyvinylpyrrolidone (PVP), and tris(2,2′-bipyridine) ruthenium (II) [ 84 ]. Under the optimized experimental conditions, LODs of quinapril hydrochloride (QHCl) and its metabolite quinaprilat hydrochloride (QTHCl) were 3.6 ng/mL and 3.9 ng/mL, respectively, with the BGE containing 14 mmol/L phosphate (pH 8.0) and 20% n-propyl alcohol.
Capacitively coupled contactless conductivity detection (C 4 D)
C 4 D has been widely used as a CE detector for quantification of both inorganic ions and organic compounds which could be applied in the field of forensic investigations and therapeutic developments.
To provide complementary information, C 4 D can be coupled with other detectors. For instance, it was coupled with UV detection to monitor meteorite bioleaching by Acidithiobacillus ferrooxidans [ 85 ] and thiol substrate hydrolysis during gold nanoparticle conjugation [ 86 ]. Coupled with MS and NACE separation, it was able to analyze food samples containing organic and inorganic compounds [ 87 ]. In this system, C 4 D could detect inorganic ions and MS had low detection limits for organic biomolecules.
When coupling the low-selectivity C 4 D with the powerful MS, how to improve the CE separation performance of the thermally unstable compounds and how to stabilize the temperature control are two main problems. A new cartridge was thus constructed by 3D printing to improve temperature control in CE (Fig. 5 ) [ 88 ]. Up to two C 4 D devices could be accommodated inside the cartridge to form CE-(C 4 D) 2 -ESI-MS/MS in an environment with proper dissipation of the thermal energy generated by Joule heating. Good peak shape and resolution, as well as reproducibility, were obtained for separation of monoethyl carbonate, a thermally unstable species.
Wang et al. optimized the structure of double-input (DI) C 4 D, correcting the distortion in the peak shape and applied it for inorganic ion analysis by CE [ 89 ]. Compared to traditional C 4 D, DIC 4 D exhibited lower baseline, higher sensitivity, lower noise, and good linear relationship.
Several new detection systems have also been developed during the period covered by this review. A remarkable one is to couple CE with surface plasmon resonance (SPR) through a microfluidic cell to achieve affinity assessment of antibody-antigen and enzyme-inhibitor binding in protein mixtures [ 90 ]. The SPR biosensor was used as the ground electrode.
When coupling CE with electrochemical detection, previous methods showed issues such as fragility or unavailability on the market. Hence, Kimlinger et al. presented a 3D-printed wall-jet electrode device to incorporate CE with electrochemical detection [ 91 ]. After separation, analytes were eluted by pressure, reducing the baseline noise compared to the traditional end-column detection. Separation of a mixture of catecholamines was realized by this system and the integration of a commercial CE device with the 3D electrode was demonstrated in a straightforward manner.
In addition, Nowak et al. demonstrated the proof of concept that microscale thermophoresis (MST) could be easily coupled to CE in an on-line flow system [ 92 ]. After electrophoretic separation, samples were directly introduced to the MST instrument in which they underwent thermophoretic analysis. During the measurement process, the EOF was temporally stopped. This system could be further applied to provide reliable data for measurement of ligand binding and molecular interactions, features unavailable for the current CE techniques. Furthermore, CE-MST coupled with MS is feasible by inserting the capillary outlet into the MS detector and using the MS-compatible buffers for thermophoretic analysis.
Conclusion and outlooks
CE, although a matured separation technique, continues to evolve to benefit diverse analytical and bioanalytical studies, because of its versatile separation platforms and easy accommodation with separation media and additives. By no means can this review include all of the CE-related works published between 2018 and 2020. Instead, we selectively highlighted some unique developments representative to the major trend of advancement in CE methods and instrumentation, hoping to provide an up-to-date overview of the current state-of-the-art development in the field.
With all these prior, elegant efforts to advance CE, we anticipate future works in CE methodology development will still be focused on improving analyte detection, tolerance to sample matrix, and analysis throughput in CE, because low tolerance for complex matrix components and low concentration sensitivity remain big challenges for CE applications. Thus, more convenient and effective sample cleanup, processing, and enrichment techniques are still in good demand. Methods and devices for enhancing CE coupling with the powerful and informative detectors, like MS, NMR, and even microscopy, will also be valuable in greatly expanding CE’s applicability and helping to improve our understanding of the functions of the targets in interest. During or even after the COVID-19 pandemic, great attentions have been paid to the analysis of viral particles, and the development of vaccines and anti-viral drugs. The strong capability of CE in characterization of biologics, vaccines, and native vesicles [ 93 ] provides good premise for CE to make substantial impacts to such developments. Newly discovered analyte-interacting reagents or solvents, such as synthetic receptors, affinity probes, and ionic liquids, have been broadening the scope of analytes addressable by CE. We anticipate more exploration of such reagents will be continued to develop CE solutions for challenging analytes [ 8 ].
Future development could further improve CE instrumentation as well to enhance the accessibility of CE platforms, and render cost-effective CE applications in resource-limited labs. For example, cheaper and purpose-made CE instruments have been developed for such purposes [ 94 95 ]. CZE with an automatic fraction collector which holds microtiter plates and even Petri dishes were fabricated for successful determination of bacteria from a complex microbiome by downstream culturing and sequencing [ 96 ], and isolation of intact sperm cells for quantitative PCR analysis of male DNA [ 97 ]. Simplified and portable CE devices will continue to be developed with the advancement in 3D printing and microfabrication.
CE analysis of biological molecules has been nicely reviewed in 2020 [ 8 ]. Some noticeable, very recent works in this area include identification and quantification of glycan/glycosylation accomplished through the development of new aminopyrene-based labeling tags [ 98 ]; new capillary coating materials like methyl chitosan [ 48 ]; and new preconcentration methods like on-line TiO 2 extraction and off-line Zr 4+ -modified monolith metal through ion affinity chromatography (IMAC) [ 99 100 ]. Single-cell analysis represents another important application area of CE, because the small dimension of capillary columns fit well for manipulation of individual cells. We discussed the impressive determination of single-cell metabolomics in individual neurons above [ 77 ]. Apart from this, CE-enabled quantification of signaling molecules was demonstrated by detecting intra- and extracellular nitric oxide with the integration of single-cell injection, on-column derivatization, and cell lysis [ 101 ]. Microfluidic CE also provides the miniaturization needed for monitoring the behaviors of single cells. For example, a microfluidic device modeling in vivo environments was reported to facilitate investigation of cell-cell interactions [ 8 ]. CE provides excellent performance in clinical analysis as well [ 102 ], because it can deliver high separation power, is highly versatile in its implementation, and consume small sample amounts. For example, a portable CE analyzer was employed for detection of abused drugs in oral fluid [ 103 ], and HF-LLME or microwave-assisted extraction (MAE) was coupled with CE for drug analysis in dried blood spot (DBS) samples [ 24 104 ]. No doubt CE will remain a highly effective tool for study of biomolecules in cells, tissues, or other biological samples, as well as for high-throughput analysis of drugs and their metabolites in body fluids. Worth noting, CE’s capability in rapid screening of nucleic acids and proteins, and its high separation power for modified biomolecules, such as DNA adducts, protein post-translational modification, and non-coding RNA alkylation, will certainly make it an important tool for analysis of epigenome and epitranscriptome, to gain better understanding of how biological processes are regulated by such factors.
In summary, as an established analytical tool, CE will continue to evolve into a more advanced analytical tool and be adopted by experts beyond the field of separation to help solve problems in diverse areas.
Jorgenson JW, Lukacs KD. Zone electrophoresis in open-tubular glass capillaries. Anal Chem. 1981;53(8):1298–302.
Article CAS Google Scholar
Harstad RK, Johnson AC, Weisenberger MM, Bowser MT. Capillary electrophoresis. Anal Chem. 2016;88(1):299–319.
Article CAS PubMed Google Scholar
Piñero M-Y, Bauza R, Arce L. Thirty years of capillary electrophoresis in food analysis laboratories: potential applications. Electrophoresis. 2011;32(11):1379–93.
Article PubMed Google Scholar
Timerbaev AR. Element speciation analysis using capillary electrophoresis: twenty years of development and applications. Chem Rev. 2013;113(1):778–812.
Ramos-Payán M, Ocaña-Gonzalez JA, Fernández-Torres RM, Llobera A, Bello-López MÁ. Recent trends in capillary electrophoresis for complex samples analysis: a review. Electrophoresis. 2018;39(1):111–25.
Voeten RLC, Ventouri IK, Haselberg R, Somsen GW. Capillary electrophoresis: trends and recent advances. Anal Chem. 2018;90(3):1464–81.
Article CAS PubMed PubMed Central Google Scholar
Cheng M, Chen Z. Recent advances in screening of enzymes inhibitors based on capillary electrophoresis. J Pharm Anal. 2018;8(4):226–33.
Article PubMed PubMed Central Google Scholar
Kristoff CJ, Bwanali L, Veltri LM, Gautam GP, Rutto PK, Newton EO, et al. Challenging bioanalyses with capillary electrophoresis. Anal Chem. 2020;92(1):49–66.
Wells SS, Dawod M, Kennedy RT. CE-MS with electrokinetic supercharging and application to determination of neurotransmitters. Electrophoresis. 2019;40(22):2946–53.
Yang Z, Shen X, Chen D, Sun L. Microscale reversed-phase liquid chromatography/capillary zone electrophoresis-tandem mass spectrometry for deep and highly sensitive bottom–up proteomics: identification of 7500 proteins with five micrograms of an MCF7 proteome digest. Anal Chem. 2018;90(17):10479–86.
Lubeckyj RA, Basharat AR, Shen X, Liu X, Sun L. Large-scale qualitative and quantitative top-down proteomics using capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry with nanograms of proteome samples. J Am Soc Mass Spectrom. 2019;30(8):1435–45.
Zhao T, Wang L, Li Y, Chen S, Wang R, Chen DDY. Quantification of the bisphosphonate alendronate using capillary electrophoresis mass spectrometry with dynamic pH barrage junction focusing. Electrophoresis. 2021;42(4):350–9.
Wang L, Cheng J, McNutt JE, Morin GB, Chen DDY. Dynamic pH barrage junction focusing of amino acids, peptides, and digested monoclonal antibodies in capillary electrophoresis–mass spectrometry. Electrophoresis. 2020;41(21–22):1832–42.
Xu L, Meng W, Lu J, Cui F, Gao L, Chen L, et al. Hyphenation of field-amplified sample injection and transient isotachophoresis in CE for the determination of sotalol and metoprolol in human urine samples. J Sep Sci. 2020;43(11):2193–200.
Kawai T, Ota N, Imasato A, Shirasaki Y, Otsuka K, Tanaka Y. Profiling of N-linked glycans from 100 cells by capillary electrophoresis with large-volume dual preconcentration by isotachophoresis and stacking. J Chromatogr A. 2018;1565:138–44.
Yang X, Hao L, Zhang S, Wang C, Wang Z. Sweeping-micelle to solvent stacking for the on-line preconcentration and determination of organic acids in Angelica sinensis by capillary electrophoresis. RSC Adv. 2018;8(15):7949–55.
Horká M, Šalplachta J, Karásek P, Ru̇žička F, Roth M. Online concentration of bacteria from tens of microliter sample volumes in roughened fused silica capillary with subsequent analysis by capillary electrophoresis and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. ACS Infectious Diseases. 2020;6(3):355–65.
Wu J, Li Z, Jia L. Solid phase extraction and capillary electrophoretic separation of racemic catecholamines by using magnetic particles coated with a copolymer prepared from poly(3,4-dihydroxyphenylalanine) and polyethyleneimine. Microchim Acta. 2019;186(9):627.
Article Google Scholar
Valimaña-Traverso J, Morante-Zarcero S, Pérez-Quintanilla D, García MÁ, Sierra I, Marina ML. Periodic mesoporous organosilica materials as sorbents for solid-phase extraction of drugs prior to simultaneous enantiomeric separation by capillary electrophoresis. J Chromatogr A. 2018;1566:135–45.
Pont L, Marin G, Vergara-Barberán M, Gagliardi LG, Sanz-Nebot V, Herrero-Martínez JM, et al. Polymeric monolithic microcartridges with gold nanoparticles for the analysis of protein biomarkers by on-line solid-phase extraction capillary electrophoresis-mass spectrometry. J Chromatogr A. 2020;461097.
Pedersen-Bjergaard S, Rasmussen KE. Liquid−liquid−liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal Chem. 1999;71(14):2650–6.
Filippou O, Bitas D, Samanidou V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J Chromatogr B. 2017;1043:44–62.
Kohler I, Schappler J, Rudaz S. Microextraction techniques combined with capillary electrophoresis in bioanalysis. Anal Bioanal Chem. 2013;405(1):125–41.
Miková B, Dvořák M, Ryšavá L, Kubáň P. Hollow fiber liquid-phase microextraction at-line coupled to capillary electrophoresis for direct analysis of human body fluids. Anal Chem. 2020;92(10):7171–8.
Pero-Gascon R, Benavente F, Minic Z, Berezovski MV, Sanz-Nebot V. On-line aptamer affinity solid-phase extraction capillary electrophoresis-mass spectrometry for the analysis of blood α-synuclein. Anal Chem. 2020;92(1):1525–33.
Tan ML, Zhang M, Li F, Maya F, Breadmore MC. A three-dimensional printed electromembrane extraction device for capillary electrophoresis. J Chromatogr A. 2019;1595:215–20.
Sun S, Wang Y, Liu X, Fu R, Yang L. Rapid and sensitive tapered-capillary microextraction combined to on-line sample stacking-capillary electrophoresis for extraction and quantification of two beta-blockers in human urine. Talanta. 2018;180:90–7.
Kim J, Choi K, Chung DS. Synergistic coupling of in-line single-drop microextraction and on-line large-volume sample stacking for capillary electrophoresis/mass spectrometry. Anal Bioanal Chem. 2019;411(5):1067–73.
Paluch J, Kozak J, Wieczorek M, Wozniakiewicz M, Golab M, Poltorak E, et al. Novel approach to sample preconcentration by solvent evaporation in flow analysis. Molecules. 2020;25(8).
Tůma P, Sommerová B, Daněček V. On-line coupling of capillary electrophoresis with microdialysis for determining saccharides in dairy products and honey. Food Chem. 2020;316:126362.
Opekar F, Hraníček J, Tůma P. Rapid determination of majority cations in yoghurts using on-line connection of capillary electrophoresis with mini-dialysis. Food Chem. 2020;308:125647.
Ren S, Zhang Q, Xue S, Liu S, Rui M. Use of gamithromycin as a chiral selector in capillary electrophoresis. J Chromatogr A. 1624;2020:461099.
Google Scholar
Yang X, Yan Z, Yu T, Du Y, Chen J, Liu Z, et al. Study of the enantioselectivity and recognition mechanism of chiral dual system based on chondroitin sulfate D in capillary electrophoresis. Anal Bioanal Chem. 2018;410(23):5889–98.
Lee J, Perez L, Liu Y, Wang H, Hooley RJ, Zhong W. Separation of methylated histone peptides via host-assisted capillary electrophoresis. Anal Chem. 2018;90(3):1881–8.
Lee J, Chen J, Sarkar P, Xue M, Hooley RJ, Zhong W. Monitoring the crosstalk between methylation and phosphorylation on histone peptides with host-assisted capillary electrophoresis. Anal Bioanal Chem. 2020;412(24):6189–98.
Wang T, Cheng Y, Zhang Y, Zha J, Ye J, Chu Q, et al. β-Cyclodextrin modified quantum dots as pseudo-stationary phase for direct enantioseparation based on capillary electrophoresis with laser-induced fluorescence detection. Talanta. 2020;210:120629.
Liu C, Zhang J, Zhang X, Zhao L, Li S. Enantiomeric separation of adrenaline, noradrenaline, and isoprenaline by capillary electrophoresis using streptomycin-modified gold nanoparticles. Microchim Acta. 2018;185(4):227.
Fanali S, Chankvetadze B. Some thoughts about enantioseparations in capillary electrophoresis. Electrophoresis. 2019;40(18–19):2420–37.
CAS PubMed Google Scholar
Xue S, Ren S, Wang L, Zhang Q. Evaluation of tetraalkylammonium amino acid ionic liquids as chiral ligands in ligand-exchange capillary electrophoresis. J Chromatogr A. 1611;2020:460579.
Ren S, Xue S, Sun X, Rui M, Wang L, Zhang Q. Investigation of the synergistic effect of chiral ionic liquids as additives in non-aqueous capillary electrophoresis for enantioseparation. J Chromatogr A. 1609;2020:460519.
Xu H, Du Y, Feng Z, Sun X, Liu J. Synthesis of a chiral ionic liquid, cholinium-clindamycin phosphate, as sole chiral selector in capillary electrophoresis. J Chromatogr A. 1615;2020:460721.
Yang X, Du Y, Feng Z, Liu Z, Li J. Establishment and molecular modeling study of maltodextrin-based synergistic enantioseparation systems with two new hydroxy acid chiral ionic liquids as additives in capillary electrophoresis. J Chromatogr A. 2018;1559:170–7.
Ma X, Du Y, Sun X, Liu J, Huang Z. Synthesis and application of amino alcohol-derived chiral ionic liquids, as additives for enantioseparation in capillary electrophoresis. J Chromatogr A. 1601;2019:340–9.
Zhang Q. Ionic liquids in capillary electrophoresis for enantioseparation. Trends Anal Chem. 2018;100:145–54.
Scriba GKE. Nonaqueous capillary electrophoresis–mass spectrometry. J Chromatogr A. 2007;1159(1):28–41.
Švidrnoch M, Boráňová B, Tomková J, Ondra P, Maier V. Simultaneous determination of designer benzodiazepines in human serum using non-aqueous capillary electrophoresis – tandem mass spectrometry with successive multiple ionic – polymer layer coated capillary. Talanta. 2018;176:69–76.
Cheng J, Chen DDY. Nonaqueous capillary electrophoresis mass spectrometry method for determining highly hydrophobic peptides. Electrophoresis. 2018;39(9–10):1216–21.
Jia Y, Cao J, Zhou J, Zhou P. Methyl chitosan coating for glycoform analysis of glycoproteins by capillary electrophoresis. Electrophoresis. 2020;41(9):729–34.
Geng Z, Song Q, Yu B, Cong H. Using ZIF-8 as stationary phase for capillary electrophoresis separation of proteins. Talanta. 2018;188:493–8.
Sun X, Guo J, Yu T, Du Y, Feng Z, Zhao S, et al. A novel coating method for CE capillary using carboxymethyl-β-cyclodextrin-modified magnetic microparticles as stationary for electrochromatography enantioseparation. Anal Bioanal Chem. 2019;411(6):1193–202.
Meixner M, Pattky M, Huhn C. Novel approach for the synthesis of a neutral and covalently bound capillary coating for capillary electrophoresis-mass spectrometry made from highly polar and pH-persistent N-acryloylamido ethoxyethanol. Anal Bioanal Chem. 2020;412(3):561–75.
Yu B, Peng Q, Usman M, Ahmed A, Chen Y, Chen X, et al. Preparation of photosensitive diazotized poly (vinyl alcohol-b-styrene) covalent capillary coatings for capillary electrophoresis separation of proteins. J Chromatogr A. 2019;1593:174–82.
Hou M, Zhang M, Chen L, Gong K, Pan C, Wang Y. Amplification of lysozyme signal detected in capillary electrophoresis using mixed polymer brushes coating with switchable properties. Talanta. 2019;202:426–35.
Zhao X-Y, Wang M-H, Liu X-F, Cong H-L, Shen Y-Q, Yu B. Preparation of diazoresin/graphene oxide covalent coated capillary for separation of proteins by capillary electrophoresis. Ferroelectrics. 2019;546:74–84.
Wang X, Li K, Adams E, Schepdael AV. Recent advances in CE-mediated microanalysis for enzyme study. Electrophoresis. 2014;35(1):119–27.
Li X, Yin Z, Cui X, Yang L. Capillary electrophoresis-integrated immobilized enzyme microreactor with graphene oxide as support: immobilization of negatively charged L-lactate dehydrogenase via hydrophobic interactions. Electrophoresis. 2020;41(3–4):175–82.
Wu Z-Y, Zhang H, Li Q-Q, Yang F-Q, Li D-Q. Capillary electrophoresis-based online immobilized enzyme reactor for beta-glucosidase kinetics assays and inhibitors screening. J Chromatogr B. 2019;1110–1111:67–73.
Dadouch M, Ladner Y, Bich C, Larroque M, Larroque C, Morel J, et al. An in-line enzymatic microreactor for the middle-up analysis of monoclonal antibodies by capillary electrophoresis. Analyst. 2020;145(5):1759–67.
Yu HM, Tseng MJ, Fang JM, Phutrakul S, Chen ST. Capillary electrophoresis using immobilized whole cells with overexpressed endothelin receptor for specific ligand screening. Electrophoresis. 2004;25(7–8):1034–41.
Wu R, Li C, Sun X, Zhang S, Liang C, Jiang Y, et al. Rapid screening of anti-tumor metastasis drugs targeting integrin macrophage antigen-1 using immobilized cell capillary electrophoresis. Analyst. 2018;143(20):4981–9.
Wu R, Li C, Li C, Ren J, Sun X, Zhang S, et al. Rapid screening of multi-target antitumor drugs by nonimmobilized tumor cells/tissues capillary electrophoresis. Anal Chim Acta. 2019;1045:152–61.
Schlecht J, Jooß K, Neusüß C. Two-dimensional capillary electrophoresis-mass spectrometry (CE-CE-MS): coupling MS-interfering capillary electromigration methods with mass spectrometry. Anal Bioanal Chem. 2018;410(25):6353–9.
Römer J, Montealegre C, Schlecht J, Kiessig S, Moritz B, Neusüß C. Online mass spectrometry of CE (SDS)-separated proteins by two-dimensional capillary electrophoresis. Anal Bioanal Chem. 2019;411(27):7197–206.
You Z, Jakubowski N, Panne U, Weidner SM. Separation of polystyrene nanoparticles with different coatings using two-dimensional off-line coupling of asymmetrical flow field flow fractionation and capillary electrophoresis. J Chromatogr A. 2019;1593:119–26.
Sasaki K, Sagawa H, Suzuki M, Yamamoto H, Tomita M, Soga T, et al. Metabolomics platform with capillary electrophoresis coupled with high-resolution mass spectrometry for plasma analysis. Anal Chem. 2019;91(2):1295–301.
Zhang F, Hong J, Xu W, Qu F. Straight nano-electrospray ionization and its coupling of mobility capillary electrophoresis to mass spectrometry. Talanta. 2020;206:120183.
He M, Luo P, Hong J, Wang X, Wu H, Zhang R, et al. Structural analysis of biomolecules through a combination of mobility capillary electrophoresis and mass spectrometry. ACS Omega. 2019;4(1):2377–86.
Wu H, Zhang R, Zhang W, Hong J, Xiang Y, Xu W. Rapid 3-dimensional shape determination of globular proteins by mobility capillary electrophoresis and native mass spectrometry. Chem Sci. 2020;11(18):4758–65.
Zhang W, Wu H, Zhang R, Fang X, Xu W. Structure and effective charge characterization of proteins by a mobility capillary electrophoresis based method. Chem Sci. 2019;10(33):7779–87.
Holtkamp HU, Morrow SJ, Kubanik M, Hartinger CG. Hyphenation of capillary electrophoresis to inductively coupled plasma mass spectrometry with a modified coaxial sheath-flow interface. J Chromatogr A. 2018;1561:76–82.
DeLaney K, Li L. Capillary electrophoresis coupled to MALDI mass spectrometry imaging with large volume sample stacking injection for improved coverage of C. borealis neuropeptidome. Analyst. 2020;145(1):61–9.
Krenkova J, Kleparnik K, Luksch J, Foret F. Microfabricated liquid junction hybrid capillary electrophoresis-mass spectrometry interface for fully automated operation. Electrophoresis. 2019;40(18–19):2263–70.
Xu T, Shen X, Yang Z, Chen D, Lubeckyj RA, McCool EN, et al. Automated Capillary Isoelectric Focusing-Tandem Mass Spectrometry for Qualitative and Quantitative Top-Down Proteomics. Anal Chem. 2020;92(24):15890–8.
Udo R, Katsumata K, Kuwabara H, Enomoto M, Ishizaki T, Sunamura M, et al. Urinary charged metabolite profiling of colorectal cancer using capillary electrophoresis-mass spectrometry. Sci Rep. 2020;10(1):21057.
Saoi M, Kennedy KM, Gohir W, Sloboda DM, Britz-McKibbin P. Placental metabolomics for assessment of sex-specific differences in fetal development during normal gestation. Sci Rep. 2020;10(1):9399.
Segers K, Zhang W, Aourz N, Bongaerts J, Declerck S, Mangelings D, et al. CE-MS metabolic profiling of volume-restricted plasma samples from an acute mouse model for epileptic seizures to discover potentially involved metabolomic features. Talanta. 2020;217:121107.
Liao H-W, Rubakhin SS, Philip MC, Sweedler JV. Enhanced single-cell metabolomics by capillary electrophoresis electrospray ionization-mass spectrometry with field amplified sample injection. Anal Chim Acta. 2020;1118:36–43.
Xu X, Li L, Weber SG. Electrochemical and optical detectors for capillary and chip separations. Trends Anal Chem. 2007;26(1):68–79.
Lahouidak S, Soriano ML, Salghi R, Zougagh M, Ríos Á. Graphene quantum dots for enhancement of fluorimetric detection coupled to capillary electrophoresis for detection of ofloxacin. Electrophoresis. 2019;40(18–19):2336–41.
Prior A, Coliva G, de Jong GJ, Somsen GW. Chiral capillary electrophoresis with UV-excited fluorescence detection for the enantioselective analysis of 9-fluorenylmethoxycarbonyl-derivatized amino acids. Anal Bioanal Chem. 2018;410(20):4979–90.
Huo F, Wan T, Wang Y, Liu Y, Karmaker PG, Yang X. Enhanced light-emitting diode induced fluorescence detection system with capillary electrophoresis. J Chromatogr A. 2020;1619:460935.
Petersen BV, Gallion L, Allbritton NL. Silicon photomultipliers as a low-cost fluorescence detector for capillary electrophoresis. Anal Chem. 2020;92(20):13683–7.
Casto LD, Do KB, Baker CA. A miniature 3D printed LED-induced fluorescence detector for capillary electrophoresis and dual-detector Taylor dispersion analysis. Anal Chem. 2019;91(15):9451–7.
Sun S, Wei Y, Wang H, Cao Y, Deng B. A novel electrochemiluminescence sensor coupled with capillary electrophoresis for simultaneous determination of quinapril hydrochloride and its metabolite quinaprilat hydrochloride in human plasma. Talanta. 2018;179:213–20.
Gonçalves Silva G, Yamassaki de Almeida E, Seber P, Henrique Settanni P, Pereira de Oliveira A, Ferreira Santos MS, et al. Application of capillary electrophoresis combined with conductometric and UV detection to monitor meteorite simulant bioleaching by Acidithiobacillus ferrooxidans. Electrophoresis. 2018;39(22):2898–2905.
Claude B, Cutolo G, Farhat A, Zarafu I, Ionita P, Schuler M, et al. Capillary electrophoresis with dual detection UV/C4D for monitoring myrosinase-mediated hydrolysis of thiol glucosinolate designed for gold nanoparticle conjugation. Anal Chim Acta. 2019;1085:117–25.
Beutner A, Scherer B, Matysik F-M. Dual detection for non-aqueous capillary electrophoresis combining contactless conductivity detection and mass spectrometry. Talanta. 2018;183:33–8.
Francisco KJM, do Lago CL. Improving thermal control of capillary electrophoresis with mass spectrometry and capacitively coupled contactless conductivity detection by using 3D printed cartridges. Talanta. 2018;185:37–41.
Wang C, Xing H, Zheng B, Yuan H, Xiao D. Simulation and experimental study on doubled-input capacitively coupled contactless conductivity detection of capillary electrophoresis. Sci Rep. 2020;10(1):7944.
Domínguez-Vega E, Haselberg R, Iperen Dv, Kool J, Somsen GW, de Jong GJ. Development of a surface plasmon resonance sensor for coupling to capillary electrophoresis allowing affinity assessment of protein mixture components. Sensors Actuators B Chem 2018;254:1040–1047.
Kimlinger MJ, Martin RS. The use of a 3D-printed microfluidic device and pressure mobilization for integrating capillary electrophoresis with electrochemical detection. Electroanalysis. 2018;30(10):2241–9.
Nowak PM, Wozniakiewicz M. On-line coupling between capillary electrophoresis and microscale thermophoresis (CE-MST); the proof-of-concept. Analyst. 2018;143(20):4854–9.
Morani M, Mai TD, Krupova Z, Defrenaix P, Multia E, Riekkola M-L, et al. Electrokinetic characterization of extracellular vesicles with capillary electrophoresis: a new tool for their identification and quantification. Anal Chim Acta. 2020;1128:42–51.
Furter JS, Boillat M-A, Hauser PC. Low-cost automated capillary electrophoresis instrument assembled from commercially available parts. Electrophoresis. 2020;41(24):2075–82.
Le TB, Hauser PC, Pham TNM, Kieu TLP, Le TPQ, Hoang QA, et al. Low-cost and versatile analytical tool with purpose-made capillary electrophoresis coupled to contactless conductivity detection: application to antibiotics quality control in Vietnam. Electrophoresis. 2020;41(23):1980–90.
Huge BJ, Champion MM, Dovichi NJ. Capillary zone electrophoresis with fraction collection for separation, culturing, and identification of bacteria from an environmental microbiome. Anal Chem. 2019;91(7):4649–55.
Wright SN, Huge BJ, Dovichi NJ. Capillary zone electrophoresis separation and collection of spermatozoa for the forensic analysis of sexual assault evidence. Electrophoresis. 2020;41(15):1344–53.
Krenkova J, Liskova M, Cmelik R, Vigh G, Foret F. Multi-cationic aminopyrene-based labeling tags for oligosaccharide analysis by capillary electrophoresis-mass spectrometry. Anal Chim Acta. 2020;1095:226–32.
Mancera-Arteu M, Lleshi N, Sanz-Nebot V, Giménez E, Benavente F. Analysis of glycopeptide biomarkers by on-line TiO2 solid-phase extraction capillary electrophoresis-mass spectrometry. Talanta. 2020;209:120563.
Shao H, Reider B, Jarvas G, Guttman A, Jiang Z, Tran NT, et al. On-line enrichment of N-glycans by immobilized metal-affinity monolith for capillary electrophoresis analysis. Anal Chim Acta. 2020;1134:1–9.
Yao H-W, Guo X-F, Wang H. Simultaneous quantitation of intra- and extracellular nitric oxide in single macrophage RAW 264.7 cells by capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 2020;92(17):11904–11.
Zhang C, Woolfork AG, Suh K, Ovbude S, Bi C, Elzoeiry M, et al. Clinical and pharmaceutical applications of affinity ligands in capillary electrophoresis: a review. J Pharm Biomed Anal. 2020;177:112882.
Saar-Reismaa P, Brilla C-A, Leiman K, Kaljurand M, Vaher M, Kulp M, et al. Use of a newly-developed portable capillary electrophoresis analyser to detect drugs of abuse in oral fluid: a case study. Talanta. 2020;211:120662.
Świądro M, Stelmaszczyk P, Wietecha-Posłuszny R, Dudek D. Development of a new method for drug detection based on a combination of the dried blood spot method and capillary electrophoresis. J Chromatogr B. 2020;1157:122339.
Download references
W. Zhong acknowledges the support from the National Science Foundation award (CHE-1707347).
Author information
Authors and affiliations.
Department of Chemistry, University of California-Riverside, 900 University Ave., Riverside, CA, 92521, USA
Ziting Gao & Wenwan Zhong
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Wenwan Zhong .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection celebrating ABCs 20th Anniversary .
Rights and permissions
Reprints and permissions
About this article
Gao, Z., Zhong, W. Recent (2018–2020) development in capillary electrophoresis. Anal Bioanal Chem 414 , 115–130 (2022). https://doi.org/10.1007/s00216-021-03290-y
Download citation
Received : 04 February 2021
Revised : 08 March 2021
Accepted : 11 March 2021
Published : 22 March 2021
Issue Date : January 2022
DOI : https://doi.org/10.1007/s00216-021-03290-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Capillary electrophoresis
- Preconcentration
- Capillary coatings
Advertisement
- Find a journal
- Publish with us
- Track your research
- Utility Menu
Whitesides Research Group

Capillary Electrophoresis
Sort by year.
- Aranson, I. S. (2)
- Archer, M.C. (1)
- Arias, F. (8)
- Arkan, N. (2)
- Armes, S. P. (1)
- Arthanari, H. (2)
- Ashcom, J. B. (1)
- Aspuru-Guzik, A. (3)
- Assi, F. (1)
- Atalay, A. (1)
- Affinity Capillary Electrophoresis (30)
- Protein Charge Ladders (20)
- Chiral Europium Shift Reagents (4)
- AMPs and MuPS (10)
- MagLev (16)
- Electrochemistry on Paper (16)
- Fuel Cells and Others (7)
- Hand-help Devices (10)
- Carbonic Anhydrase and Protein-Ligand Binding (59)
- Charge Regulation (13)
- Entrophy/Enthalpy Compensation (17)
- Hydrophobic Effect (35)
- Microbiology (5)
- Water in Biology (16)
- Co-factor Regeneration and Simple Transformation (78)
- Hybrids with Organometallics (6)
- Supporting Technology (61)
- Complex Synthesis: Carbohydrates (74)
- Patterning with SAMs: Proteins, Cells (59)
- Divalency and Trivalency (7)
- Polymeric Polyvalency, Influenza, Others (16)
- Thiolate-Disulfide Interchange (22)
- Cells in Gels in Paper (CiGiP) (10)
- Patterned Surfaces (66)
- PDMS Microdevices: Microfluidics (43)
- CHARGE TRANSPORT BY TUNNELING: THE "EGaln JUNCTION" (45)
- Complexity and Simplicity (6)
- Tribocharging (5)
- Vortex Structures (6)
- Molecular Information (2)
- Adaptive Reuse (7)
- Developing World: Paper (38)
- Developing World: PDMS (2)
- Developing World: String (2)
- Devices and Electrochemistry (24)
- MagLev (11)
- Other: Phage (3)
- PDMS-based (8)
- Electrets and Contact Electrification (25)
- Adaptive Materials (14)
- Silicon (61)
- Bioassays and Applications (61)
- Devices (139)
- Droplets and Bubbles (40)
- Optical Devices (37)
- Organisms: Bacteria and C. Elegans (23)
- AMPs and MuPS (7)
- Cross-linked High Carbon Solids (10)
- Proteins (42)
- Biopatterning (49)
- Devices and Structures (202)
- Electrochemistry (25)
- Microcontact Printing (152)
- Micromolding (129)
- Optical Systems (54)
- "Polyethylene Carboxylic Acid" (15)
- Buckling (8)
- Mechanism of Formation of RMgX (3)
- Mechanism of Reduction of L2PtR2 over Pt/H2 (1)
- SAMs, Wetting, Materials-by-Design (196)
- Indentation (7)
- Microlens Lithography (14)
- Micromolding in Capillaries (MIMIC) (35)
- Nanoreplication (11)
- Nanoskiving (19)
- Shadow-sphere Lithography (5)
- ORIGINS OF LIFE (6)
- Theoretical Methods (6)
- Chiral Europium Shift Reagents (2)
- Exchange Reactions and Rates (35)
- Other NMR (10)
- "Ate" Complexes of Copper and Silver (9)
- Other Organometallics (54)
- Copper and Silver Chemistry (28)
- Grignard Reactions and RLi Chemistry (39)
- Others (hg, Fe, Ti, ) (29)
- Platinum Chemistry (49)
- Editorial (33)
- Review Paper (97)
- Magnetic Levitation (10)
- Melamine-Cyanurate and Related: Cyrstal Engineering (23)
- Melamine-Cyanurate and Related: Molecular Aggregates (29)
- Meso-scale Self-Assembly: Capillary-based Structures (27)
- SOFT ROBOTICS (51)
Frontal analysis capillary electrophoresis: recent advances and future perspectives
Affiliations.
- 1 Department of Chemistry, Negros Oriental State University, Dumaguete City 6200, Philippines.
- 2 Australian Centre for Research on Separation Science, School of Natural Sciences-Chemistry, University of Tasmania, Hobart, Tasmania 7001, Australia.
- PMID: 30047805
- DOI: 10.4155/bio-2018-0051
The developments on frontal analysis capillary electrophoresis (FACE) from 2012 to 2017 to study interactions of simple and complex systems are reviewed. Most research papers focused on therapeutic drug-related studies; however, other studies include chemical sensing, drug delivery, inhibitor screening and capillary coating. New ligand-substrate systems such as template-molecularly imprinted polymer systems were reported. Comparison of FACE with other analytical techniques used to investigate binding interaction, and the determination of binding parameters using different isotherm models are also covered. In 2017, eight research papers were reported including new detection by ESI-MS. Future research direction of FACE may include high sensitivity detection and throughput screening of drugs, natural products and biomarkers for clinical diagnosis.
Keywords: binding parameters; capillary coating; frontal analysis capillary electrophoresis; ligand–substrate interaction; method of detection.
Publication types
- Electrophoresis, Capillary*
- Pharmaceutical Preparations / analysis*
- Spectrometry, Mass, Electrospray Ionization
- Pharmaceutical Preparations
This section contains information intended for healthcare professionals. Please certify that you are a healthcare professional.
Please indicate your country of residence:, what language is your preference.
- Multiple Myeloma
- Chronic Alcohol Abuse
- Hemoglobin Disorders
- Alpha-1 Antitrypsin Deficiency
- Central Nervous System Inflammatory Disease
- Capillary Electrophoresis
- Gel Electrophoresis
- Instruments
- Sebia Workflow Service
- Sebia Academy
- Company Overview
- Worldwide presence
- Research & Innovation
- Quality & Compliance
- Sustainability
CAPILLARYS 3 DBS
The high throughput solution for screening newborn hemoglobin disorders.

Key benefits
Main characteristics.
- Technologies
About CAPILLARYS 3 DBS
Capillary electrophoresis is a proven technology (1),(2),(3) screening for newborn hemoglobin disorders and provides high quality results. Sebia CAPILLARYS 3 DBS is the latest generation of instrument dedicated to newborn hemoglobin screening, offering a smooth workflow and excellent analytical features.
The CAPILLARYS 3 DBS instrument is an automated, multitasking capillary electrophoresis instrument using 12 capillaries simultaneously for hands-free electrophoretic separation at high throughput.
The CAPILLARYS 3 DBS enables the analysis of samples from microplate wells and provides fully automated electrophoresis separation, from the dried blood spots on filter paper (Guthrie card) through to the final electrophoretic pattern: sample identification, sample dilution, sample incubation, capillary washing, sample injection into the capillaries, migration, detection, processing of the results, and data transmission to LIS.
This instrument is an innovative alternative to commonly used technology such as IEF and HPLC for all the laboratories involved in newborn hemoglobin screening, bringing seamless sample traceability, high quality results, and high throughput while offering a reduced hands-on time.
High throughput instrument
Up to 70 samples/hour, enabling Newborn Screening laboratories to efficiently manage their workload and deliver results to pathologists and clinicians quickly.
Full traceability
From sample punch to result, there is no interruption in the traceability chain, preventing transcription mistakes (e.g. errors from manually entering the sample ID into the analysis software).
Walkaway Automation
Load up to 8, 96-well microplates with continuous loading. This saves hands on time and lets the lab staff focus on important tasks.
Automatic Reagent Control
RFID tags for all reagents and Hb AF Control ensure a smooth and efficient reagent management, reducing user intervention.
- Hb Neonat technique
Sample types: dried blood spot (3.2mm)
With 12 capillaries in parallel, the CAPILLARYS 3 DBS can process a large number of samples and can analyse up to eight 96-well microplates without reagent refill.
Throughput (tests/hour)
CAPI 3 NEONAT Hb 70
- Flexible positions for reagents allow for the optimization of on-board reagent management
- RFID identification for all reagents (reagent type, lot number, expiration date, volume) allows comprehensive traceability throughout workflow
- Embedded user interface with a touch screen
- Automatic start-up and shutdown maintenance
- Visual alarms (with instrument notifications)
- A dedicated workstation pilots the instrument and manages results
- Uses barcoded microplates (supplied with the CAPI 3 NEONAT Hb reagent kit – P/N:2506)
- Directly samples from the elution of dried blood spots on filter paper.
- Validated with automated PerkinElmer™ puncher instruments Panthera 9 and DBS models.
- Barcoded microplates enable full sample traceability: from sample card ID through to final result
- CAPILLARYS 3 DBS – P/N: 1244
Featured tests for CAPILLARYS 3 DBS
Newborn hemoglobin disorders screening.
The perfect match for screening hemoglobin disorders in newborns.
Related resources
Capillarys 3 dbs – introductory video.
The perfect match for screening hemoglobin disorders in newborns
Scientific publications
Newborn screening for sickle cell disease and other haemoglobinopathies – mdpi – 2019.
This special Issue related to newborn screening for Sickle Cell disease and other hemoglobinopathies tried to cover the most widely faced challenges in the field of newborn screening for SCD.
Learn more about our unique technologies
The worldwide leader in Capillary Electrophoresis with fully automated protein separation at high resolution.
Comprehensive menu with high-quality results on Agarose gel.
Interested in CAPILLARYS 3 DBS?
Get more information, request a demo.
(1) Hemoglobinopathies: Current: Practices for Screening, Confirmation and Follow-up – CDC – 2015 https://www.cdc.gov/ncbddd/sicklecell/documents/nbs_hemoglobinopathy-testing_122015.pdf
(2) NHS Sickle Cell and Thalassaemia Screening Programme- 2017 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/585126/NHS_SCT_Handbook_for_Newborn_Laboratories.pdf
(3) Newborn Screening for Sickle Cell Disease and Other Hemoglobinopathies: A Short Review on Classical Laboratory Methods—Isoelectric Focusing, HPLC, and Capillary Electrophoresis: https://www.mdpi.com/2409-515X/4/4/39
This section contains information intended for wide distribution and may therefore contain product details or information that is not available or valid in your country.
Please contact your local Sebia representative. Information intended for healthcare professionals. Carefully read the instructions in the reagent package inserts and instrument manuals.

IMAGES
VIDEO
COMMENTS
A capillary electrophoresis approach with capacitively coupled contactless cond. detection method has been developed for the detn. of inorg. metabolites (thiocyanate, nitrite and nitrate) in human saliva. Field amplified sample injection, as a simple sample stacking technique, was used in conjunction for online preconcn. of above inorg. anions.
Abstract. Development of new capillary electrophoresis (CE) methodology and instrumentation, as well as application of CE to solve new problems, remains an active research area because of the attractive features of CE compared to other separation techniques. In this review, we focus on the representative works about sample preconcentration ...
There are almost 50 thousand papers about capillary zone electrophoresis (CZE), about 20 thousand capillary isoelectric focusing (CIEF) and almost 400 thousand hits about CE with sodium dodecyl sulfate (CE-SDS). In contrast, a search for the same terms (but dating before 1998) would render only 130,000 hits on CE in general.
An online high-throughput microdialysis-capillary electrophoresis ... coating of the capillary wall is a common strategy which remains the subject of research. Poulsen et al. posed new capillary coating procedures using polyethylene ... Somsen is (co)author of over 150 peer-reviewed papers and is editor of Journal of Chromatography B. Author ...
Abstract. The purpose of this review is to highlight noteworthy advancements in the field of capillary gel electrophoresis for the separation and analysis of proteins from the period of 2015-2021. This review will provide an overview of the historical perspective and principles of the technique, introduce the challenges and limitations commonly ...
Abstract. This paper reviews the developments in capillary electrophoresis (CE) in 2020. A total of 222 research papers related to CE technology published in 2020 were retrieved from the ISI Web of Science. These papers were selected by using the keywords "capillary electrophoresis-mass spectrometry", "capillary isoelectric focusing", "micellar ...
Capillary electrophoresis (CE) modes has been largely applied for the separation of an extent variety of compounds due to its low reagent and sample requirements, versatility, comprehensiveness and simple operation, and the development of new methodologies and instrumentation is an active research field [2].
In this review, we confer the latest developments in capillary electrophoresis, used for the characterization of critical quality attributes of biopharmaceutical products covering the past 6 years (2015-2021). Monoclonal antibodies, due to their significant share in the market, have been given prioritized coverage.
Capillary Electrophoresis: A Three-Year Literature Review. Yueyang Li. Yueyang Li. Department of Chemistry, University of British Columbia, Vancouver, British Columbia V6T 1Z1, Canada. ... The Altmetric Attention Score is a quantitative measure of the attention that a research article has received online. Clicking on the donut icon will load a ...
An alkaline capillary-fed electrolysis cell of this type demonstrates water electrolysis performance exceeding commercial electrolysis cells, with a cell voltage at 0.5 A cm−2 and 85 °C of only ...
But others do reveal more recent avenues of research, for example in metabolomics. Finally, there are some special cases, such as "SARS-CoV" with an average publication year very close to 2020, suggesting papers involving capillary electrophoresis and SARS-CoV-2 vastly outnumber those published during the first SARS-CoV outbreak in 2002-2004.
Capillary electrophoresis (CE) interfaced to mass spectrometry (MS) with electrospray ionization typically incorporates acidic additives or organic solvents to assist in ionization. Vibrating sharp-edge spray ionization (VSSI) is a voltage-free method to interface CE and MS that does not require these additives, making it appealing for protein ...
The purpose of this review is to highlight noteworthy advancements in the field of capillary gel electrophoresis for the separation and analysis of proteins from the period of 2015-2021. This review will provide an overview of the historical perspective and principles of the technique, introduce the challenges and limitations commonly faced ...
Abstract. Since the introduction of modern capillary electrophoresis (CE) by Jorgenson and Lukacs in 1981, CE has evolved into a highly mature and versatile separation technique. After a first ...
A total of 291 research papers related to CE technology published in 2021 were retrieved from the ISI Web of Science using the keywords, "capillary electrophoresis-mass spectrometry" "capillary ...
Capillary Electrophoresis (CE) is particularly suitable for the analysis of diverse types of APIs covering cationic, anionic as well as neutral analytes as it offers a wide range of separation modes in combination with a wide variety of detection techniques. ... Papers dealing with various tools for effective and reproducible suppression of the ...
Electrophoresis is the process by which large charged molecules travel through a medium under a uniform electric field. The most common implementation, gel electrophoresis, is a method used to ...
A total of 291 research papers related to CE technology published in 2021 were retrieved from the ISI Web of Science using the keywords, "capillary electrophoresis-mass spectrometry" "capillary ...
Research Article Application of capillary electrophoresis-sodium dodecyl sulfate in assessing the purity of monoclonal antibody biopharmaceuticals Xiaoyue Song Department of Biologics and Biochemicals, Suzhou Institute for Drug Control, Suzhou, China View further author information
Development of new capillary electrophoresis (CE) methodology and instrumentation, as well as application of CE to solve new problems, remains an active research area because of the attractive features of CE compared to other separation techniques. In this review, we focus on the representative works about sample preconcentration, separation media or capillary coating development, detector ...
Based on IFs, this review introduces representative works on CE to facilitate readers' understanding of important research advances in CE technology over the last year. 该文为2022年毛细管电泳 (capillary electrophoresis, CE)技术年度回顾。. 归纳总结了以"capillary electrophoresis-mass spectrometry"或"capillary ...
Abstract. Development of new capillary electrophoresis (CE) methodology and instrumentation, as well as application of CE to solve new problems, remains an active research area because of the attractive features of CE compared to other separation techniques. In this review, we focus on the representative works about sample preconcentration ...
" Analysis by Capillary Electrophoresis of the Kinetics of Charge Ladder Formation for Bovine Carbonic Anhydrase." Analytical Chemistry, 74, Pp. 1870-1878. Analytical Chemistry, 74, Pp. 1870-1878.
The current protocol describes an alternative method for creatine quantification in biological tissue samples using capillary electrophoresis, with high separation efficiency that enables rapid analysis with low sample volumes. The protocol involves homogenization of snap-frozen tissue in phosphate buffer, followed by electrophoresis through a ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Vankova, L.; Vanek, D ...
ELECTROPHORESIS is an international separation science journal publishing advances in electrophoresis, ... In this paper, we review the basic concepts and latest advances in technology for ME coupled to MS during the period of 2016-2021, covering microchip materials, structures, fabrication techniques, and interfacing to electrospray ...
The developments on frontal analysis capillary electrophoresis (FACE) from 2012 to 2017 to study interactions of simple and complex systems are reviewed. Most research papers focused on therapeutic drug-related studies; however, other studies include chemical sensing, drug delivery, inhibitor screening and capillary coating.
Background. A new design of a flow-through coaxial electromembrane extraction (EME) probe that can be on-line coupled with CE instrument is described and tested. The supporting base of the probe is a PDMS microchip with T-shaped channels into which two coaxially arranged capillaries for inlet and outlet solutions are inserted. The extraction part of the probe is a porous polypropylene hollow ...
Capillary electrophoresis is a proven technology (1), (2), (3) screening for newborn hemoglobin disorders and provides high quality results. Sebia CAPILLARYS 3 DBS is the latest generation of instrument dedicated to newborn hemoglobin screening, offering a smooth workflow and excellent analytical features. The CAPILLARYS 3 DBS instrument is an ...