Masks Strongly Recommended but Not Required in Maryland
Respiratory viruses continue to circulate in Maryland, so masking remains strongly recommended when you visit Johns Hopkins Medicine clinical locations in Maryland. To protect your loved one, please do not visit if you are sick or have a COVID-19 positive test result. Get more resources on masking and COVID-19 precautions .
- Vaccines
- Masking Guidelines
- Visitor Guidelines


Pancreatic Cancer: Experts Answer 10 Commonly Asked Questions
Featured Expert:

Jin He, M.D., Ph.D.
Pancreatic cancer is the fourth most common cause of cancer death in the United States. While great strides are being made in the search and treatment of pancreatic cancer, there are still many questions about the complex disease. Jin He, M.D., Ph.D. , surgical oncologist, provided responses to frequently asked questions about pancreatic cancer:
Can pancreatic cancer be prevented?
A: Unfortunately, most pancreatic cancer cannot be prevented, but you can reduce your risk by maintaining a healthy weight, stopping smoking and limiting your alcohol intake. Other risk factors include chronic pancreatitis and family history. Occasionally, precancerous lesions can be identified and, if removed early, can prevent pancreatic cancer from developing.
What are the symptoms of pancreatic cancer?
A: There are no specific symptoms for early-stage pancreatic cancer, but if you notice unintentional weight loss, jaundice (yellowing of the skin) and stomach pain, we recommend that you see your primary care physician.
What tests are available to detect pancreatic cancer?
A: There are currently no simple tests for pancreatic cancer. Most cases are found when symptoms develop or an imaging study, such as a CT or MRI scan , is done for another reason. There is active research at Johns Hopkins that is aimed at developing a test for pancreatic cancer in the blood, urine and stool.
Why is pancreatic cancer usually found in the later stages?
A: Pancreatic cancer usually does not cause symptoms, so approximately 50 percent of pancreatic cancers will not be identified until they have already metastasized (spread to other parts of the body).
Is there a direct correlation between breast cancer and pancreatic cancer?
A: There is a relationship between BRCA mutations (breast and ovarian cancer) and pancreatic cancer. A BRCA mutation approximately doubles the lifetime risk of developing pancreatic cancer. Around 5 percent of people with pancreatic cancer have a BRCA mutation. However, breast cancer is very common, so not all patients with breast cancer are thought to have an increased risk of pancreatic cancer.
Is pancreatic cancer a genetic disease?
A: Pancreatic cancer can be genetic, but the vast majority of pancreatic cancer is sporadic. Many genes play a role in the growth of pancreatic cancer. The four major driver genes include KRAS, P53, P16 and SMAD4.
If you have a strong family history of pancreatic cancer, you should contact a genetic counseling and screening program. Familial pancreatic cancer is defined as having two or more first-degree relatives with pancreatic cancer. Johns Hopkins has a familial pancreatic cancer registry for surveillance of such patients.
Can pancreatitis be a precursor for pancreatic cancer?
A: Yes, it can be. However, most cases of pancreatitis are unrelated to pancreatic cancer.
What are the chances of a pancreatic cyst becoming cancerous?
A: Pancreatic cysts are very common, and the majority are not cancerous, but some may be, and others may be precancerous. There are several different types of pancreatic cysts ranging from benign to malignant. The key is to determine the malignant risk of your specific cyst.
Is it possible to have full recovery from pancreatic cancer?
A: Yes, it is possible to have a full recovery from pancreatic cancer.
Can you live without a pancreas?
A: Yes, you can live without a pancreas, but you will be diabetic, which means you will have to take insulin regularly. You will also need to take enzyme pills to help with the digestion of food.
Pancreatic Cancer Prevention | Lana's Story

Lana Brandt had a strong family history of pancreatic cancer. After experiencing several bouts of pancreatitis, she learned that she carried a genetic mutation that increased her risk of pancreatic cancer. As a means of pancreatic cancer prevention, experts at Johns Hopkins removed her pancreas and spleen using a minimally invasive technique. They then performed an autoislet cell transplant to help her body continue to produce insulin after surgery.
Find a Doctor
Specializing In:
- Laparoscopic Pancreas Surgery
- Neuroendocrine Tumors
- Robotic Pancreas Surgery
- Whipple procedure
- Pancreaticoduodenectomy
- Pancreatic Surgery
- Pancreatic Cancer
Find a Treatment Center
- Hepato-Pancreato-Biliary Surgery
- Skip Viragh Center for Pancreas Cancer
Find Additional Treatment Centers at:
- Howard County Medical Center
- Sibley Memorial Hospital
- Suburban Hospital
Request an Appointment

Pancreatic Cancer Surgery
Chemotherapy for Pancreatic Cancer

Pancreatic Cancer Vaccine: What to Know
Related Topics
- Pancreatic Cancer Treatment
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Advances in the...
Advances in the management of pancreatic cancer
- Related content
- Peer review
- Marco Del Chiaro , professor, division chief , clinical director 1 2 ,
- Toshitaka Sugawara , assistant clinical professor 1 3 ,
- Sana D Karam , professor 2 4 ,
- Wells A Messersmith , professor , division head, , associate director 2 5
- 1 Division of Surgical Oncology, Department of Surgery, University of Colorado School of Medicine, Aurora, CO, USA
- 2 University of Colorado Cancer Center, University of Colorado School of Medicine, Aurora, CO, USA
- 3 Department of Hepatobiliary and Pancreatic Surgery, Graduate School of Medicine, Tokyo Medical and Dental University, Tokyo, Japan
- 4 Department of Radiation Oncology, University of Colorado School of Medicine, Aurora, CO, USA
- 5 Division of Medical Oncology, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, USA
- Corresponding Author: M Del Chiaro marco.delchiaro{at}cuanschutz.edu
Pancreatic cancer remains among the malignancies with the worst outcomes. Survival has been improving, but at a slower rate than other cancers. Multimodal treatment, including chemotherapy, surgical resection, and radiotherapy, has been under investigation for many years. Because of the anatomical characteristics of the pancreas, more emphasis on treatment selection has been placed on local extension into major vessels. Recently, the development of more effective treatment regimens has opened up new treatment strategies, but urgent research questions have also become apparent. This review outlines the current management of pancreatic cancer, and the recent advances in its treatment. The review discusses future treatment pathways aimed at integrating novel findings of translational and clinical research.
Introduction
Pancreatic cancer has been considered a deadly disease with a very small probability of long term survival. 1 Despite slow progress, long term survival rates have greatly improved, especially for resected patients. From 1975 to 2011, the five year survival for resected pancreatic cancer improved from 1.5% to 17.4%. 2 However, more recent data show that five year survival for all pancreatic cancers between 2012-18 reached only 11.5% in the United States. 3
As a systemic disease, the changes in the survival of patients with pancreatic cancer have been affected most by the improvements in systemic treatments. 4 5 Consequently, the anatomical factors influencing the resectability of pancreatic cancer, which are defined in the National Comprehensive Cancer Network (NCCN) clinical practice guidelines, 6 have diminished in importance owing to better local and systemic control with higher response rates.
This review summarizes and contextualizes recent studies on the management of pancreatic cancer, and discusses potential treatments that are on the horizon. A detailed discussion of the preclinical or translational studies of diagnosis tools, drugs, and procedures is outside the scope of this review.
Sources and selection criteria
We searched Pubmed, the Cochrane database, and the Central Register of Controlled Trials (clinicaltrials.gov) between January 2000 and December 2022 for English language literature. We used the following keywords and keywords combinations: “pancreatic cancer”, “molecular characteristics”, “biology”, “resectability”, “metastatic”, “treatment”, “surgery”, “chemotherapy”, “radiation therapy”, “immunotherapy”, “prevention”, “precursor”, and “risk factor”. We also included the NCCN clinical practice guidelines, 6 the European Society for Medical Oncology (ESMO) clinical practice guidelines, 7 and the clinical practice guidelines from the Japan Pancreas Society. 8 We included studies based on the level of evidence; randomized controlled trials, meta-analyses, systematic reviews, and large retrospective cohort studies were prioritized. Meta-analyses included retrospective and prospective studies unless otherwise specified. We prioritized the most recent studies and excluded narrative reviews, case series, and case reports. We included additional landmark studies published before January 2000, as well as after December 2022.
Epidemiology
Pancreatic cancer is reported to account for 495 773 new cases and 466 003 deaths worldwide as of 2020, with the incidence and mortality rates stable or slightly increased in many countries. 9 In the US, the estimated incidence of pancreatic cancer is increasing, with more than 50 000 new cases in 2020. Mortality rates have also increased moderately in men, to 12.7 per 100 000 men in 2020; but have remained stable in women, ranging from 9.3 to 9.6 per 100 000 women. Accordingly, pancreatic cancer is the third most common cause of cancer related death in 2023, and is predicted to become the second leading cause of cancer mortality by 2040. 3 10
Clinical presentation/features
Symptoms of pancreatic cancer are mostly non-specific, and generally manifest after the tumor has grown and metastasized. In a multicenter prospective study of 391 patients who were referred for suspicion of pancreatic cancer (119 had pancreatic cancer), the most common initial symptoms were decreased appetite (28%), indigestion (27%), and change in bowel habits (27%). 11 The initial symptoms were similar between the pancreatic cancer group and the non-cancer group, though several subsequent symptoms were associated with pancreatic cancer: jaundice (49% v 12%), fatigue (51% v 26%), decreased appetite (48% v 26%), weight loss (55% v 22%), and change in bowel habits (41% v 16%).
Risk factors
Box 1 summarizes the risk factors for pancreatic cancer. Research is continuing into subtypes and modifiers of familial syndromes.
Factors that increase the risk of pancreatic cancer
Family history.
Up to 10% of all pancreatic cancers are estimated to be familial (meaning that at least two first degree relatives have pancreatic cancer)
Patients who have two first degree relatives with pancreatic cancer have a standardized incidence ratio of 6.4 (lifetime risk 8-12%) 12
Patients who have three first degree relatives with pancreatic cancer have a standardized incidence ratio of 32.0 (lifetime risk 40%) 12
Approximately 20% of these families have a germline mutation that is already reported and known
Germline mutation and hereditary syndrome
LKB1/STK11: Peutz-Jeghers syndrome; relative risk 132 13
CDKN2A/p16: familial atypical multiple mole melanoma syndrome; relative risk 13-22 14
PRSS1/CPA1/CTRC/SPINK1: hereditary pancreatitis; relative risk 53-87 15
BRCA1 and BRCA2: hereditary breast ovarian cancer syndrome; relative risk 2 and 10, respectively 16
MLH1/MSH2/MSH6/PMS2: Lynch syndrome; relative risk up to 8.6 17
PALB2/ATM: relative risk unknown
Lifestyle factors
Smoking: current smoker relative risk 1.8; former smoker relative risk 1.2 18
Obesity: five unit increment in body mass index relative risk 1.10 19
Diabetes mellitus * : relative risk 1.94 20
Chronic pancreatitis: relative risk 16.16 21
*Diabetes mellitus is also a symptom of pancreatic cancer; new onset diabetes in older people could be an early sign of pancreatic cancer and can lead to early diagnosis. 22 The association between diabetes and pancreatic cancer is currently undergoing further research. 23
Precancerous lesion
Molecular research has proposed two evolutionary models of pancreatic cancer: the classic “stepwise” model, with gradual accumulation of driver gene mutations, and the novel “punctuated” model, 24 in which driver gene mutation occurs simultaneously by chromosomal rearrangements. The stepwise model is characterized by tumor evolution from a precancerous lesion (low grade or high grade dysplasia) to invasive cancer, and is believed to be the main evolutionary pattern. Pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasms (IPMNs) are well known precancerous lesions. By contrast to PanIN, which is a microscopic neoplastic lesion, IPMNs can be detected and followed by imaging studies. Consequently, extensive studies have been conducted to evaluate the association between imaging findings and pathological findings of IPMNs. Branch duct IPMNs have been reported to have a low malignant nature (1.0% patient years), 25 but harbor a risk of concomitant pancreatic cancer (0.8%). 26 Main duct IPMNs have been reported to be a high risk factor for pancreatic cancer (odds ratio 5.66). 27
Screening and early detection
Because early stage (ie, stage I, T1N0M0) disease or precancerous lesions are more likely to be curable, the goal of screening or surveillance for pancreatic cancer is to detect lesions of 2 cm or smaller, or patients with high grade dysplasia. 28 Several studies have estimated an interval of several years between a high grade dysplasia lesion (high grade PanIN and IPMN) and invasive cancer, which can give opportunities for early detection and intervention: 2.3-11 years for high grade PanIN, 29 30 and more than three years for high grade IPMN. 31 The International Cancer of the Pancreas Screening (CAPS) consortium has published consensus guidelines about screening for high risk patients who have high risk germline mutations or relatives with pancreatic cancer, or both. 28 A recent prospective cohort study (CAPS5) from the CAPS group including 1461 high risk patients showed positive results of surveillance 25 ; seven of nine patients (77.8%) who developed pancreatic cancer had stage I cancer. However, only three of the eight patients (37.5%) who had IPMNs with worrisome features had high grade dysplasia (five had low grade dysplasia). A multicenter retrospective study (n=2552) of the CAPS consortium showed that 13 of the 28 patients (46.4%) who developed high grade dysplasia or cancer developed the new lesion during the scheduled examination interval. 32 Regarding IPMNs in the general population, a recent retrospective study showed that only 177 of 1439 patients with resected IPMN (12.3%) had high grade dysplasia, and 497 (34.5%) had a diagnosis of invasive cancer. 33 These results suggest that a novel strategy distinct from current guidelines 34 35 is needed for IPMN lesions, and new diagnostic tests are needed to detect tiny tumors.
The United States Preventive Services Task Force (USPSTF) 36 recommends avoiding pancreatic cancer screening in asymptomatic adults with average risk, considering the relatively low prevalence (estimated 64 050 new cases in 2023). 36 However, the USPSTF does not discuss screening in patients with risk factors of age and lifestyle, and neither do the consensus guidelines of the CAPS consortium. A risk assessment model including all known risk factors ( box 1 ) could help to identify good candidates for pancreatic cancer screening.
Carbohydrate antigen 19-9 (CA19-9) is a cell surface tetrasaccharide often elevated in pancreatic cancer, as well as in other cancers and some benign diseases. Historically, CA19-9 has not been used for early detection, owing to its insufficient sensitivity for early stage pancreatic cancer. 37 We also know that 5-10% of the population does not synthesize CA19-9, owing to a deficiency of a fucosyltransferase enzyme. However, a recent large retrospective cohort study showed that CA19-9 levels increase from two years before diagnosis of pancreatic cancer, with a sensitivity of 50% and specificity of 99% within 0-6 months before diagnosis in early stage disease. In addition, in cases with CA19-9 levels below the cut-off value, the combination of LRG1 and TIMP1 could complement CA19-9, leading to the identification of cases missed by CA19-9 alone. 38 Novel tests (ie, cytology 39 and DNA alterations 40 ) using pancreatic juice and cystic fluid have been reported to play a promising role in identifying high grade dysplasia and invasive cancer with high specificity. However, the sensitivity of these tests is low (˂50%). Extensive studies have investigated the role of liquid biopsy in pancreatic cancer: circulating tumor cells, 41 circulating tumor DNA, 42 43 microRNA, 44 exosomes, 45 and methylation signatures of cell free DNA. 46 Although these new biomarkers show promise, many problems remain unsolved with regard to standardization of testing techniques and cut-off values ( table 1 ). However, advances in this field could increase survival drastically.
Summary of novel techniques for diagnosis and early detection of pancreatic cancer
- View inline
Diagnosis and evaluation
The performance of diagnosis tools is summarized in box 2 .
Imaging study and biomarker for diagnosis of pancreatic cancer
CT (computed tomography) is the standard modality; accuracy 89% (95% confidence interval 85 to 93) 47
MRI (magnetic resonance imaging) has a similar performance to CT; accuracy 90% (95% confidence interval 86 to 94) 47
PET (positron emission tomography) has a worse performance; accuracy 84% (95% confidence interval 79 to 89) 47
Endoscopic ultrasound has a similar performance to CT; accuracy 89% (95% confidence interval 87 to 92) 47
Endoscopic ultrasound can identify masses that are indeterminate by CT; accuracy 75% (95% confidence interval 67 to 82) 48
CA19-9 is the most widely used and validated biomarker; area under curve 0.83-0.91 49
Imaging study for evaluation
CT (computed tomography) is the standard tool to evaluate the extent of the primary tumor and determine its anatomical resectability. Two meta-analyses showed similar performance of CT (sensitivity 70%, specificity 95%) and MRI (magnetic resonance imaging) (sensitivity 65%, specificity 95%) in the diagnosis of vascular involvement. 50 51 A meta-analysis showed that endoscopic ultrasound performed similarly to CT in evaluating vascular invasion. 52 A multimodal approach (ie, CT plus MRI plus endoscopic ultrasound) provides a better assessment of resectability. Several studies have attempted to evaluate the response to chemotherapy with imaging studies to determine the course of treatment (ie, proceeding to surgery or continuing chemotherapy). However, the currently used response evaluation criteria in solid tumors (RECIST) are not sufficient to reassess local response after chemotherapy in pancreatic cancer, especially regarding the involvement of vessels. Distinguishing scar areas with fibrosis that occur with treatment from cancer cell death from viable tumor associated desmoplasia is challenging; both are common in pancreatic cancer. A meta-analysis including six studies with 217 patients showed the difficulty of using CT scans to predict margin negative resection after preoperative treatment; the sensitivity was 81% and specificity was as low as 42%. 53 MRI 54 55 and fluorodeoxyglucose PET (positron emission tomography)/CT or PET/MRI 56 have been reported to be associated with the pathological response to preoperative treatment, though the ability to evaluate the vessel involvement and resectability is unclear. However, it should also be noted that even in the setting of histological response assessment, moderate inter-rater reliability differences have been reported between pathologists. 57
Biomarker for evaluation
CA19-9 has been used to assess response to treatment and predict prognosis. A meta-analysis showed that CA19-9 was associated with the effect of preoperative treatment, and suggested that either normalization of CA19-9 or a decrease of more than 50% from the baseline level are positive predictors of survival. 58 A recent retrospective study analyzing the combination of CT and CA19-9 showed a good predictive performance of survival after chemoradiotherapy. 59 However, the optimal evaluation of response to treatment remains unclear. The ability of liquid biopsy ( table 1 ) to detect minimal residual disease following all planned treatment could identify a new subset of patients who require further treatment, and would lead to a true precision medicine approach, as has been achieved with other cancer types. 60
Cancer cell intrinsic and tumor microenvironment factor
Transcriptional studies have proposed several classifications of pancreatic cancer. A recent bioinformatic study 61 from The Cancer Genome Atlas research network supported the two subgroup model 62 : the basal-like subtype, which has low levels of GATA6 expression, and the classic subtype. In a prospective translational trial, the basal-like subtype was reported to be associated with a poor response to chemotherapy with FOLFIRINOX (combined leucovorin calcium (folinic acid), fluorouracil, irinotecan, and oxaliplatin) for patients with advanced cancer. 63 However, a more recent study using single cell analysis suggested that pancreatic cancer consists of a mixture of tumor cells with both molecular subtypes, and the composition is plastic and unstable. 64
In addition to the cancer cells themselves, the tumor microenvironment has been identified as being an essential factor associated with tumor progressions and tumor immunity. Pancreatic cancer is notorious for poor tumor cellularity and an abundant, fibrotic extracellular matrix. Although the dense extracellular matrix has been known to impair drug delivery and immune cell migration, it appears to have an essential role in maintaining the tumor microenvironment and supporting the progression of tumor cells. 65 Therefore, the efficacy of controlling the extracellular matrix by targeting its components (ie, collagen, cancer associated fibroblasts, and hyaluronan) and cytokines (ie, transforming growth factor β and sonic hedgehog) has been evaluated.
Figure 1 outlines the current management for pancreatic cancer based on the anatomic resectability of the tumor, with the first consensus statement defined in 2009, 66 before the advent of more effective systemic treatments. In primary resectable disease, upfront surgery followed by adjuvant chemotherapy has been considered the standard of care. By contrast, for borderline resectable and locally advanced diseases, preoperative treatment is generally proposed, because of the high likelihood of micrometastasis and the low likelihood of margin negative resection in these tumors. 67 However, the improvement of medical treatment is challenging this concept; neoadjuvant treatment for resectable diseases is under investigation. At present, the recommendation is that the decision for treatment should be made at a multidisciplinary conference at a high volume center.

Current management for pancreatic cancer. CA19-9=carbohydrate antigen 19-9
- Download figure
- Open in new tab
- Download powerpoint
Systemic treatment
The standard drug treatment for systemic treatment is still cytotoxic chemotherapy, and the efficacy of targeted treatment or immunotherapy remains unproven. Table 2 summarizes the clinical trials of medical treatment.
Summary of key studies of medical treatment of pancreatic cancer
Systemic treatment for metastatic disease
Gemcitabine became the standard chemotherapy drug for pancreatic cancer more than 20 years ago. In 1997, gemcitabine showed clinical benefit and marginally improved overall survival compared with fluorouracil (median survival 5.65 v 4.41 months) in a small randomized controlled trial that included 63 patients in each arm. 68 Consequently, several trials were performed investigating combinations with gemcitabine. 69 70 71 However, most studies did not show a significant improvement in overall survival; the combinations tested included fluorouracil, 72 irinotecan, 73 oxaliplatin, 74 75 cisplatin, 76 77 and capecitabine. 78 79 80 Unfortunately, the addition of targeted treatment to gemcitabine based chemotherapy also did not show a survival benefit, with any of tipifarnib, 81 cetuximab, 82 bevacizumab, 83 84 axitinib, 85 and vandetanib. 86 In 2011, a landmark randomized phase 2/3 trial (PRODIGE 4/ACCORD 11) defined a new standard chemotherapy for metastatic pancreatic cancer. 71 This multicenter trial enrolled 171 patients in each arm and showed a significant improvement in survival, with a median overall survival of 11.1 months in the FOLFIRINOX group, compared with 6.8 months in the gemcitabine group (hazard ratio 0.57; 95% confidence interval 0.45 to 0.73). FOLFIRINOX also had a higher response rate (31.6%) than gemcitabine (9.4%). Subsequently, the MPACT trial, a large randomized phase 3 study, showed another cytotoxic combination option (gemcitabine/nab-paclitaxel) for metastatic pancreatic cancer. 87 This study included 861 patients, and showed that gemcitabine/nab-paclitaxel improved survival compared with gemcitabine alone (median survival 8.5 v 6.7 months; hazard ratio 0.72; 95% confidence interval 0.62 to 0.83). FOLFIRINOX and gemcitabine/nab-paclitaxel have formed the cytotoxic “backbones” for multiple clinical trials.
Nanoliposomal irinotecan is a drug encapsulating irinotecan sucrosofate salt payload in tiny pegylated liposomal particles, which theoretically can enhance the exposure of irinotecan to tumor cells. A recent randomized phase 3 trial (NAPOLI-3) enrolled 770 patients with metastatic pancreatic cancer and compared NALIRIFOX (combined liposomal irinotecan, fluorouracil, folinic acid, and oxaliplatin) (n=383) to gemcitabine/nab-paclitaxel (n=387) as the first line treatment. 88 Preliminary results showed an improved overall survival (median 11.1 v 9.2 months; hazard ratio 0.84; 95% confidence interval 0.71 to 0.99), which was the primary endpoint, and an improved progression free survival (7.4 v 5.6 months; 0.70; 0.59 to 0.84). For Asian populations, S-1 (an oral fluoropyrimidine derivative) is another treatment option, after it showed non-inferiority to gemcitabine for advanced pancreatic cancer in a randomized phase 3 study (GEST). 89
Second line systemic treatment for advanced disease
Second line regimens after gemcitabine based chemotherapy for advanced pancreatic cancer have been studied in several trials. The CONKO-003 randomized phase 3 trial showed that the addition of oxaliplatin to folinic acid and fluorouracil (5FU/LV) significantly improved overall survival (median 5.9 v 3.3 months, hazard ratio 0.66; 95% confidence interval 0.48 to 0.91). 90 By contrast, another randomized phase 3 trial (PANCREOX) found a deleterious effect on survival of oxaliplatin (mFOLFOX6) over infusional fluorouracil/leucovorin (hazard ratio 1.78; 95% confidence interval 1.08 to 2.93) in the second line setting. 91
A large randomized phase 3 trial (NAPOLI-1) investigated the efficacy of nanoliposomal irinotecan to 5FU/LV for metastatic disease after gemcitabine based treatment. 92 The results showed that nanoliposomal irinotecan plus 5FU/LV incrementally improved survival compared with 5FU/LV (6.1 v 4.2 months, hazard ratio 0.67; 95% confidence interval 0.49 to 0.92). Patients who received nanoliposomal irinotecan monotherapy, however, had similar survival to those who received 5FU/LV (4.9 v 4.2 months, 0.99; 0.77 to 1.28). Further studies on second line regimens after FOLFIRINOX or gemcitabine/nab-paclitaxel are warranted.
Maintenance systemic treatment for advanced disease
A poly adenosine diphosphate ribose polymerase (PARP) inhibitor was investigated as the maintenance treatment in patients who had germline loss-of-function mutations in BRCA1 or BRCA2, and platinum sensitive advanced disease. A randomized double blind phase 3 trial (POLO) showed no survival benefit of olaparib (n=62) compared with placebo (n=92) (median overall survival 19.0 v 19.2 months; hazard ratio 0.83; 95% confidence interval 0.56 to 1.22), but did show an improvement in progression free survival, which resulted in US Food and Drug Administration approval. 93 94 Another PARP inhibitor, niraparib, combined with an anti-CTLA-4 (ipilimumab) drug, showed a median overall survival of 17.3 months (95% confidence interval 12.8 to 21.9 months) in a phase 1b/2 trial. 95 Maintenance treatment for non-BRCA mutated patients with metastatic diseases following FOLFIRINOX was evaluated in the PANOPTIMOX-PRODIGE 35 phase 2 trial. 96 This study randomly assigned 273 patients to six month FOLFIRINOX (n=91), four month FOLFIRINOX followed by leucovorin/5-FU maintenance (n=92), or a sequential treatment alternating gemcitabine and FOLFIRI.3 every two months (n=90). The results showed a comparable six month progression free survival rate and median progression free survival in the maintenance arm eliminating oxaliplatin (44%, 5.7 months), and the worst survival in the gemcitabine/FOLFIRI approach (34%, 4.5 months) compared with the six month FOLFIRINOX arm (47%, 6.3 months).
Adjuvant systemic treatment
Adjuvant systemic treatment is recommended for all eligible resected patients. The first large randomized phase 3 trial that showed the survival benefit of adjuvant chemotherapy was the ESPAC-1 trial, which assigned resected patients (n=289) to 5-FU/LV versus control. 4 97 Adjuvant chemotherapy prolonged the median overall survival by 4.6 months (hazard ratio 0.71; 95% confidence interval 0.55 to 0.92). 4 The CONKO-001 randomized phase 3 trial showed that adjuvant gemcitabine (n=179) improved overall survival compared with observation (n=172) (median 22.8 v 20.2 months; hazard ratio 0.76; 95% confidence interval 0.61 to 0.95). 98 When 5FU/LV and gemcitabine were compared head-to-head, no difference in overall survival was found, but gemcitabine had less toxicity in the ESPAC-3 randomized phase 3 trial. 99 Subsequently, multiple trials tried to find a new effective combination treatment with gemcitabine. A randomized phase 3 trial combining erlotinib with gemcitabine was negative, 100 but the addition of capecitabine had a survival benefit over gemcitabine alone (28.0 v 25.5 months; 0.82; 0.68 to 0.98) in the ESPAC-4 phase 3 trial. 101 However, this combination treatment was short lived; FOLFIRINOX drastically changed the survival of patients and became the new standard regimen for adjuvant treatment. The PRODIGE 24/CCTG PA6 phase 3 trial randomly assigned 493 resected patients to receive adjuvant modified (dose reduced) FOLFIRINOX (mFOLFIRINOX) or gemcitabine for 24 weeks. The mFOLFIRINOX group (n=247) showed a significantly improved median overall survival (53.5 v 35.5 months; 0.68; 0.54 to 0.85). 5 102 By contrast, gemcitabine/nab-paclitaxel failed to show a survival benefit over gemcitabine alone in a randomized phase 3 trial (APACT). 103 It did not meet the primary endpoint of disease free survival by central review, 103 although overall survival improved marginally in the gemcitabine/nab-paclitaxel group (40.5 v 36.2 months; 0.82; 0.680 to 0.996). In Asia, S-1 is the standard regimen, based on the results of a randomized phase 3 trial. 104 The role of adjuvant treatment after neoadjuvant chemotherapy and surgical resection is still debatable. A recent large retrospective study showed a potential benefit in survival for patients able to receive adjuvant chemotherapy after neoadjuvant and surgery. 105
Neoadjuvant systemic treatment
One of the underpinnings of neoadjuvant treatment is that 36% of patients with pancreatic cancer are unable to receive adjuvant chemotherapy after resection, 106 and surgical resection alone does not achieve long term survival for most patients. The rationale for neoadjuvant treatment is to increase the dose intensity and tolerance of planned systemic treatment before patients are weakened by surgery, and to avoid delayed treatment of micrometastatic disease, which is the main cause of mortality. 107 Two prospective single arm phase 2 studies showed the safety of neoadjuvant gemcitabine plus a platinum based drug. 108 109
The only published phase 3 trial of neoadjuvant systemic treatment (PREOPANC-1) randomly assigned 246 patients with resectable (54.1%) or borderline resectable disease (45.9%) to neoadjuvant chemoradiotherapy (n=119) or upfront surgery (n=127). 110 111 The neoadjuvant chemoradiotherapy arm received three cycles of neoadjuvant gemcitabine with 36 Gy radiotherapy in 15 fractions and four cycles of adjuvant gemcitabine, whereas the upfront surgery arm received six cycles of adjuvant gemcitabine. Long term results showed a consistent survival benefit of neoadjuvant treatment regardless of the resectability of the primary tumors, for borderline resectable diseases (hazard ratio 0.67; 95% confidence interval 0.45 to 0.99) and resectable diseases (0.79; 0.54 to 1.16). However, the chemotherapy regimen (gemcitabine alone) was outdated. The recent ESPAC-5 phase 2 trial 112 randomly assigned 90 patients with borderline resectable diseases to neoadjuvant treatment (n=56), which included multiagent neoadjuvant chemotherapy and single agent chemoradiotherapy, or upfront surgery (n=33). It showed a better one year overall survival in the neoadjuvant treatment groups compared with the upfront surgery group (76% v 39%; hazard ratio 0.29; 95% confidence interval 0.14 to 0.60), although it did not provide evidence of the optimal regimen owing to the small sample size.
Regarding resectable diseases, one concern of neoadjuvant treatment is the possibility of disease progression during neoadjuvant treatment, which could cause patients to miss the opportunity for surgical resection. Indeed, the role of neoadjuvant treatment for resectable disease is still under investigation. A randomized phase 2 trial (PACT-15) showed that neoadjuvant chemotherapy with the PEFG regimen (cisplatin, epirubicin, fluorouracil, and gemcitabine) improved overall survival compared with adjuvant gemcitabine and adjuvant PEFG regimen for resectable disease. 113 The Prep-02/JSAP-05 phase 2/3 trial randomly assigned patients with resectable (about 80%) or borderline resectable diseases to one month neoadjuvant gemcitabine plus S-1 (n=182), or upfront surgery (n=180). Both arms received six month S-1 as the adjuvant treatment. 114 The results showed improved overall survival in the neoadjuvant chemotherapy arm (36.7 v 26.6 months; hazard ratio 0.72; 95% confidence interval 0.55 to 0.94). Conversely, studies of FOLFIRINOX have not shown positive results. 115 116 The SWOG S1505 phase 2 study showed equivalent efficacy of neoadjuvant mFOLFIRINOX versus nab-paclitaxel/gemcitabine for three months for resectable disease. 115 The median overall survival in both arms (23.2 and 23.6 months) did not show improvement compared with previous trials of adjuvant treatment.
A recent phase 2 trial (NORPACT-1) randomly assigned 140 patients with resectable diseases to the neoadjuvant FOLFIRINOX arm (n=77) or the upfront surgery arm (n=63), and found no survival benefit of neoadjuvant FOLFIRINOX. However, the results have several problems. While not significant, the median survival was 13.4 months shorter (25.1 v 38.5 months) in the neoadjuvant FOLFIRINOX arm, despite the higher rates of node negative (N0) and margin negative (R0) resection in that arm. Given the high resection rate (n=63, 82%) despite the low completion rate of neoadjuvant chemotherapy (n=40, 52%), and the high rate of adjuvant chemotherapy other than FOLFIRINOX (75%) in the neoadjuvant group, it seems that the neoadjuvant group did not receive sufficient FOLFIRINOX chemotherapy. In addition, whether two months is sufficient for neoadjuvant FOLFIRINOX is unclear. Three ongoing large randomized phase 3 trials might provide some insight into the optimal sequence and the number of cycles of FOLFIRINOX; two are recruiting patients (ALLIANCE-A021806 and PREOPANC-3), and one recently opened ( NCT05529940 ). The first two trials plan to enrol more than 300 patients with resectable disease to assess the overall survival of perioperative FOLFIRINOX (eight cycles of neoadjuvant and four cycles of adjuvant) compared with adjuvant FOLFIRINOX (12 cycles). The NCT05529940 trial plans to enrol more than 600 patients and evaluate the two year survival of perioperative FOLFIRINOX (six cycles of neoadjuvant and six cycles of adjuvant) compared with adjuvant FOLFIRINOX (12 cycles).
Systemic treatment for locally advanced disease
After the positive results of FOLFIRINOX and gemcitabine/nab-paclitaxel for metastatic disease, several studies have investigated its efficacy in locally advanced diseases. A systematic review that analyzed 315 patients with locally advanced diseases from 11 studies between 1994 and 2015 showed that FOLFIRINOX was associated with a longer median overall survival of 24.2 months (95% confidence interval 21.7 to 26.8 months). 117 The proportion of patients who underwent surgical resection after FOLFIRINOX ranged from 0-43%. A phase 2 study (LAPACT) investigated gemcitabine/nab-paclitaxel for 106 patients 118 ; the median overall survival was 18.8 months (90% confidence interval 15.0 to 24.0 months). In total, 62 patients (58%) completed induction gemcitabine/nab-paclitaxel, and 17 patients (16%) underwent surgical resection. Another randomized phase 2 study (NEOLAP-AIO-PAK-0113) showed high surgical conversion rates of gemcitabine/nab-paclitaxel (23/64, 35.9%) and gemcitabine/nab-paclitaxel followed by FOLFIRINOX (29/66, 43.9%). 119 No survival differences were observed between the two arms (hazard ratio 0.86; 95% confidence interval 0.55 to 1.36). These results suggest a new potential treatment strategy for surgical conversion of locally advanced disease, which could achieve longer survival in selected patients.
Surgical treatment
Pancreatectomy, especially pancreaticoduodenectomy, has been considered a high risk surgery. The centralization of pancreatectomy has played an essential role in the improvement of perioperative outcomes. The 90 day mortality is reported to be under 5-10% in experienced high volume centers. 120 121 A recent meta-analysis including 46 retrospective studies (2015-2021) showed a significantly lower postoperative morbidity rate in high volume centers compared with low volume centers (47.1% v 56.2%; odds ratio 0.75; 95% confidence interval 0.65 to 0.88). 121
Surgery for locally advanced and borderline disease
Some experts have pushed for more aggressive operations for patients with borderline resectable and locally advanced diseases with the advent of more effective systemic drugs. Resection after neoadjuvant treatment was reported to have similar short term outcomes compared with upfront resection in a meta-analysis 122 including randomized controlled trials and a subgroup report of a randomized phase 3 trial. 123 However, data on arterial resection and reconstruction are more controversial, and depend on the resected artery and the technical approach; the mortality rates were reported as 5.7% for resection of the superior mesenteric artery, 124 and 1.7% for resection of the celiac axis. 125 More recently, arterial divestment has been proposed as an alternative to arterial resection in selected patients. A retrospective study of a high volume center reported a mortality rate of 7.0% for arterial resections and 2.3% for arterial divestment from 2015 to 2019, although the breakdown of resected arteries was not shown by periods. 126 To be clear, these aggressive procedures should be performed only when long term survival is expected. A previous meta-analysis including 13 studies (2005-2015) with 355 locally advanced tumors showed no significant association between the resection rate after chemotherapy and overall survival. 117 However, large, retrospective studies recently showed that conversion surgery for locally advanced diseases after FOLFIRINOX was associated with improved survival in a selected subgroup. 127 128 Further studies are expected.
Surgery for patients with metastatic disease
Macroscopic distant metastasis is a contraindication to surgical resection in general. However, several studies have reported a potential role of resection in highly selected patients with limited metastatic diseases. A meta-analysis including three retrospective studies (2016-2019) showed a longer overall survival (23-56 months v 11-16 months) in patients with synchronous liver metastasis who underwent resection after chemotherapy (n=44) compared with those who did not (n=166). 129 In another review, lung metastasectomy was associated with a longer survival with a median overall survival after resection ranging from 18.6 to 38.3 months. 130 A large retrospective study suggested that only patients who achieved a complete pathological response of metastasis could derive a survival benefit from resection. 131 Further studies are expected to provide data on patient selection criteria and metastatic sites. A single arm phase 2 study ( NCT04617457 ) and a randomized phase 3 trial ( NCT03398291 ) are recruiting patients with oligometastasis in liver from pancreatic cancer to evaluate the efficacy of resection after chemotherapy.
Minimally invasive surgery
Minimally invasive surgery for pancreatic cancer had until recently been lagging behind that for other cancers. Results of a recent randomized trial 132 (n=656) and a meta-analysis of three randomized controlled trials 133 (n=224) showed that laparoscopic pancreatoduodenectomy was associated with a shorter hospital stay, but a similar postoperative morbidity rate. Box 3 summarizes the studies comparing robotic pancreatoduodenectomy with open or laparoscopic pancreatoduodenectomy. Notably, all studies on pancreatoduodenectomy to date have included patients with diseases other than pancreatic cancer. Another meta-analysis including 12 randomized or matched studies (n=4346) showed a similar morbidity rate, but a higher margin negative resection rate (odds ratio 1.46) and shorter time to adjuvant treatment, in the laparoscopic distal pancreatectomy group. 139 Most recently, an international randomized trial (DIPLOMA) 140 including 117 patients with resectable pancreatic cancer in the minimally invasive distal pancreatectomy group and 114 patients in the open distal pancreatectomy group showed the non-inferiority of the oncological safety of minimally invasive distal pancreatectomy: a higher margin negative resection rate (73% v 69%) and comparable lymph node yield and intraperitoneal recurrence.
Comparison between robotic pancreaticoduodenectomy and open pancreatoduodenectomy or laparoscopic pancreatoduodenectomy
Robotic pancreatoduodenectomy ( v open pancreatoduodenectomy ) 134 135 136 | robotic pancreatoduodenectomy ( v laparoscopic pancreatoduodenectomy ) 135 137 138.
R0 resection: Comparable 135 136 or higher 134 | Comparable 135
Lymph nodes harvested: Comparable 135 136 or more 134 | Comparable 135 or more 137 138
Operating time: Longer 134 135 136 | Comparable 135 137 138
Estimated blood loss: Less 134 135 136 | Comparable 138 or less 135 137
Conversion rate: Not applicable | Lower 137 138
Overall mortality rate: Comparable 134 or lower 136 | Comparable 138
Overall morbidity rate: Comparable 134 135 or lower 136 | Comparable 135 137 138
Surgical site infection: Less 134 135 | Comparable 135 138
Pancreatic fistula: Comparable 134 135 or less 136 | Comparable 135 137 138
Hemorrhage: Comparable 135 | Comparable 135
Delayed gastric emptying: Comparable 134 136 or less 135 | Comparable 135 137 138
Length of stay: Comparable 134 135 or longer 136 | Comparable 135 137 or shorter 138
Radiotherapy
Radiotherapy is used as a part of local treatment for pancreatic cancer, generally combined with chemotherapy. Since the gold standard for this disease remains surgical resection, 67 the role of radiotherapy has been logically examined in both the adjuvant setting and in locally advanced inoperable patients. The neoadjuvant application of radiotherapy has also been investigated in several studies. In this setting, however, high level evidence comparing the role of radiotherapy in a head-to-head design to neoadjuvant chemotherapy is lacking. The true efficacy of radiotherapy on long term survival remains unclear, especially when combined with modern multiagent systemic treatments and surgical resection. Another concern in many radiotherapy studies is the heterogeneity of the treatment technique and dose used. For example, the techniques have evolved from conventional treatments to intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT), more ablative approaches with adaptive planning platforms. Box 4 summarizes the characteristics of radiotherapy by types and doses. Table 3 summarizes the clinical studies of radiotherapy.
Characteristics of radiotherapy for pancreatic cancer by types and doses
3 dimensional conformal radiation therapy (3d-crt).
Using multiple beams shaped to conform to a tumor that is identified its size, shape, and location by 3D imaging (ie, CT, MRI)
Generally used dose:* 45.0-56.0 Gy in 1.75-2.20 Gy fractions
Image guided radiation therapy (IGRT)
An adjunctive technique to adjust the tumor location difference by using 3D imaging (ie, CT, MRI) performed immediately before each radiation treatment
Intensity modulated radiation therapy (IMRT)
Possible to adjust the irradiation intensity within a target volume
Possible to deliver a concentrated dose to a tumor and better spare the normal tissue
Stereotactic body radiotherapy (SBRT)
Accurately irradiate a tumor with high dose radiation in three dimensions from multiple directions
High local control rate, comparable toxicity 141
Generally used dose:* 30.0-40.0 Gy in 6.00-8.00 Gy fractions
*Based on the ASTRO clinical practice guideline. 142
Summary of key studies of radiotherapy for pancreatic cancer
Adjuvant radiotherapy
In theory, the purpose of adjuvant radiotherapy is to reduce the risk of local recurrence. NCCN guidelines recommend considering adjuvant chemoradiation treatment for patients with positive surgical margins. 67 However, prospective studies that support adjuvant radiotherapy are lacking, regardless of the surgical margin status. The aforementioned large randomized phase 3 trial (ESPAC-1) included 289 resected patients: 51 (17.6%) had positive resection margins. The results showed worse survival in the chemoradiotherapy arm (n=145) than in the non-radiotherapy arm (n=144) (median overall survival 15.9 v 17.9 months; hazard ratio 1.28; 95% confidence interval 0.99 to 1.66). 4 This study has discouraged further studies of adjuvant radiotherapy in Europe. However, the study had two major drawbacks. Firstly, the chemotherapy regimen was different in the chemotherapy arm (fluorouracil/leucovorin) and the chemoradiotherapy arm (fluorouracil). Secondly, the total dose of radiotherapy (20 Gy) did not reach 45 Gy, which represents the treatment dose of conventional, fractionated external beam radiotherapy. 67 Two randomized phase 2 studies investigated adjuvant gemcitabine plus radiotherapy for patients with negative resection margins. The first study administered 50.4 Gy in 28 fractions of radiotherapy, and found a lower local alone recurrence rate (11% v 24%), but did not show a difference in overall survival or disease free survival between the two arms (45 patients each). 143 The other small study (n=38) used a modern SBRT technique (25 Gy in five fractions), but showed no difference in any of the survival endpoints (recurrence free survival, locoregional recurrence free survival, or overall survival), even in the node positive subgroup. 144 An older systematic review included five randomized controlled trials (1985-2005) of adjuvant chemoradiotherapy consisting of fluorouracil based chemotherapy plus conventional radiotherapy, and showed no survival benefit of chemoradiation (pooled hazard ratio 1.09; 95% confidence interval 0.89 to 1.32). 145 The subgroup analysis in this study showed a possible efficacy of adjuvant chemoradiotherapy in patients with positive resection margins.
The RTOG0848 trial is a randomized phase 2/3 study that enrolled 322 resected patients. The ongoing phase 3 of this trial assesses the survival benefit of added radiotherapy (50.4 Gy) after six cycles of adjuvant gemcitabine based chemotherapy. However, because the standard of care regimen of adjuvant chemotherapy has changed to FOLFIRINOX, the results of this study might have a limited impact on clinical practice. Ultimately, the role of adjuvant radiotherapy is still ambiguous.
Neoadjuvant radiotherapy
One of the primary goals of neoadjuvant radiotherapy is to reduce the rate of positive margin resection, which is a risk factor for local recurrence. Two single arm phase 2 studies showed the tolerability and feasibility of concurrent radiotherapy combined with fluorouracil plus cisplatin 146 (n=41) and gemcitabine (n=41). 147 However, the evidence on the efficacy of neoadjuvant radiotherapy is inconsistent. A meta-analysis of three randomized controlled trials (n=189) that investigated chemoradiotherapy (fluorouracil based chemotherapy with a radiotherapy dose of 45-50.4 Gy) did not show any difference in overall survival between neoadjuvant chemoradiotherapy and adjuvant chemoradiotherapy (hazard ratio 0.93; 95% confidence interval 0.69 to 1.25). 148 The aforementioned PREOPANC-1 phase 3 trial, which showed a survival benefit of neoadjuvant chemoradiation for borderline resectable disease, did not evaluate effects with and without radiation. 110 Regarding neoadjuvant chemoradiotherapy with FOLFIRINOX, a phase 2 trial (n=48) showed that neoadjuvant FOLFIRINOX plus chemoradiotherapy in borderline resectable disease showed a high rate of margin negative resection, and prolonged median progression free survival and even median overall survival. 149 By contrast, a randomized phase 2 trial (ALLIANCE-A021501) showed worse survival in the patients with borderline resectable disease who were allocated to the neoadjuvant mFOLFIRINOX plus radiotherapy (SBRT or hypofractionated image guided radiotherapy) arm (n=56) compared with those who allocated to the neoadjuvant mFOLFIRINOX alone arm (n=70) (median overall survival 17.1 v 29.8 months; median event free survival 10.2 v 15.0 months). 150 However, the number of patients was modest, and the dropout rates were high in both the chemotherapy arm (71.4%) and the chemoradiation arm (82.1%). A meta-analysis comprising 15 studies (512 patients) of neoadjuvant FOLFIRINOX with or without radiotherapy for resectable and borderline resectable disease showed a better rate of margin negative resection in the chemoradiotherapy group (97.6% v 88.0%). 151 No differences were observed in resection rate, overall survival, or pathological outcomes. The PANDAS-PRODIGE 44 study, a randomized phase 2 study, assigned 130 patients with borderline resectable diseases to mFOLFIRINOX or mFOLFIRINOX plus conformal external radiation (50.4 Gy). This ongoing study aims to evaluate the histological negative margin resection rate as the primary endpoint.
Radiation for locally advanced disease
For locally advanced pancreatic cancer, radiation is used as the primary modality for local control and, on rare occasions, to facilitate margin negative resection in select patients who achieve good responses to treatment. 67 Trials for locally advanced diseases have reported various levels of efficacy. A randomized trial assigned 37 patients to receive chemoradiotherapy (gemcitabine, 50.4 Gy) and 34 patients to receive gemcitabine alone. The trial showed improved overall survival in the chemoradiotherapy group (11.1 v 9.2 months). 152 Progression free survival was not different, but the sample size was notably small. The LAP07 trial was a large randomized phase 3 trial that aimed to investigate the survival benefit of adding radiotherapy to chemotherapy (54 Gy plus capecitabine) compared with chemotherapy (gemcitabine or gemcitabine plus erlotinib) after four months of gemcitabine based induction chemotherapy. 153 The results showed no differences in overall (median 15.2 v 16.5 months; hazard ratio 1.03; 95% confidence interval 0.79 to 1.34) or progression free survival (9.9 v 8.4 months; 0.78; 0.61 to 1.01) between the chemoradiotherapy group (n=133) and the chemotherapy group (n=136). An older randomized phase 3 trial (2000-01 FFCD/SFRO) also compared gemcitabine chemotherapy (n=60) to chemoradiotherapy with fluorouracil and cisplatin (60 Gy) (n=59), 154 and showed worse overall survival (median 8.6 v 13.0 months) and progression free survival in the chemoradiotherapy group. 154 The study, however, suffered from major inconsistencies in the proportion of patients who received at least 75% of the planned dose of induction chemotherapy, being only 42.4% in the chemoradiotherapy group compared with 73.3% in the chemotherapy group.
Given that conventional fractionated radiotherapy techniques combined with gemcitabine based chemotherapy have failed to show a significant survival advantage, the focus of research has moved to SBRT and FOLFIRINOX. A meta-analysis of 1147 patients from 21 studies including randomized controlled trials (2002-2014) compared conventional external beam techniques to SBRT. 141 The estimated two year overall survival was higher in the SBRT group (26.9% v 13.7%), with less acute grade 3/4 toxicity (5.6% v 37.7%) and similar late grade 3/4 toxicity (9.0% v 10.1%). A phase 2 trial (LAPC-1) enrolled 50 patients to receive eight cycles of FOLFIRINOX followed by SBRT (40 Gy in five fractions). 155 In total, 39 patients underwent SBRT (78.0%) and seven (14.0%) patients underwent surgical resection; all had negative margins and pathological N0 stage. The overall survival in the resected patients was longer than in the unresected patients (median 24 v 15 months; three year survival rate 43% v 6.5%). A systematic review including 2446 patients from 28 phase 2/3 studies also showed a similar resection rate of 12.1% (95% confidence interval 10.0% to 14.5%). Therefore, this newest chemoradiotherapy approach could give the best chance of curative intent surgery, and achieve long term survival in a highly selected subgroup of patients.
Four phase 2/3 trials are ongoing. The CONKO-007 trial is a large randomized phase 3 trial enrolling 525 patients to evaluate chemoradiotherapy (50.4 Gy with gemcitabine) after induction chemotherapy with FOLFIRINOX (n=402) or gemcitabine (n=93) for three months; the primary endpoint was margin negative resection rate. The first results came out in 2022, and showed a higher rate of margin negative resection (resection and circumferential resection margin) (9.0% v 19.6%) in the chemoradiation arm (n=168, 61 underwent surgery) compared with the chemotherapy arm, which was continuing FOLFIRINOX or gemcitabine (n=167, 60 underwent surgery). 156 However, the total surgical resection margin negativity rate and survival did not reach statistical significance. The publication is pending. The other three trials are phase 2 trials and are still recruiting patients (SCALOP-2, 157 MASTERPLAN, 158 and GABRINOX-ART 159 ). These studies could provide more data about gemcitabine/nab-paclitaxel and SBRT for locally advanced pancreatic cancer. However, we are unable to draw a conclusion without well designed phase 3 trials using the latest technology and chemotherapy regimen.
Supportive care and palliative care
Weight loss is seen in more than half of patients at diagnosis of pancreatic cancer 11 ; as a result, the rates of malnutrition 160 161 (33.7-70.6%) and sarcopenia 162 (up to 74%) are high. Malnutrition and sarcopenia have been reported to be associated with poor outcomes of surgical resection and chemotherapy. 163 Given that the majority of patients suffer from metastatic diseases, palliative care, including pain management and nutrition support, is essential to their quality of life, and even prognosis. Table 4 highlights major studies on these topics.
Summary of studies on supportive/palliative care for pancreatic cancer
Emerging diagnostic tools and treatments
Diagnostic tools.
Fibrosis, both chemoradiotherapy induced and cancer associated, has been reported to be associated with overall survival. An MRI probe targeting chemoradiotherapy induced collagen (type I collagen) can detect this change in fibrosis. 170 Radiolabeled fibroblast activation protein inhibitors (FAPI) can target the expression of fibroblast activation protein in cancer associated fibroblasts, which is abundant in pancreatic cancer. 171 A meta-analysis showed superior performance of FAPI PET over FDG PET/CT/MRI for the determination of tumor, node, metastases (TNM) classification and peritoneal carcinomatosis. 172 A phase 2 trial is recruiting patients to evaluate the efficacy of FAPI PET/CT in patients with locally advanced disease ( NCT05518903 ).
Radiomics using machine learning or deep learning (artificial intelligence) is a new field of research, driven by advances in computer systems. Theoretically, a computer can learn and identify features and differences that a human cannot. A systematic review showed that radiomics models of the primary tumors had good performance in predicting patient prognosis. 173 Further studies with larger sample sizes for training and validating models with risk factors, images, and biomarkers will yield more conclusive results in this regard.
In the era of neoadjuvant chemotherapy, a new question has emerged of how to manage patients who have tumor progression during neoadjuvant treatment. A phase 2 trial ( NCT03322995 ) is recruiting patients (n=125) with resectable and borderline resectable disease to evaluate the efficacy of adaptive modification of neoadjuvant treatment (four months). Based on the results of restaging after four cycles of FOLFIRINOX, a decision will be made to either continue the same regimen, or switch to a gemcitabine based regimen and chemoradiotherapy. For locally advanced pancreatic cancer, the NEOPAN phase 3 trial successfully enrolled 171 patients with locally advanced pancreatic cancer to compare the progression free survival of FOLFIRINOX (12 cycles) with gemcitabine (four cycles), with preliminary results expected soon. Few data exist on the comparison of FOLFIRINOX with gemcitabine/nab-paclitaxel for both localized and advanced cancer. A randomized phase 2 study (PASS-01) is recruiting patients (planned n=150) with metastatic disease to investigate the difference in progression free survival between the two regimens. Moreover, genomic factors and putative biomarkers will be explored using whole genome sequencing and RNA sequencing, and patient derived organoids.
Immunotherapy has been largely ineffective in pancreatic cancer, potentially owing to both tumor cell intrinsic and tumor microenvironment factors. Recent trials have taken a combined approach. CISPD3, a randomized phase 3 trial (n=110), showed an improved objective response rate (50.0% v 23.9%; P=0.010) by adding sintilimab (a monoclonal antibody against programmed cell death protein 1) to FOLFIRINOX for metastatic patients, 174 albeit without superior overall survival and progression free survival. The same group is conducting a phase 3 trial to evaluate the same regimen in patients with borderline resectable and locally advanced diseases ( NCT03983057 ). Given the results of basic research in pancreatic cancer showing that the extracellular matrix plays an essential role in the tumor microenvironment and progression, several new agents have been introduced. A phase 2 trial ( NCT03336216 ) combining an immune checkpoint inhibitor with chemotherapy (FOLFIRINOX or gemcitabine based regimen) and cabiralizumab (a colony stimulating factor 1 receptor inhibitor that suppresses the activities of tumor associated macrophages) has been conducted. However, a 2020 press release 175 176 announced that this study missed the primary endpoint of progression free survival.
Pamrevlumab, a recombinant human monoclonal antibody against connective tissue growth factor, has been investigated in a randomized phase 3 trial (LAPIS) combined with FOLFIRINOX or gemcitabine/nab-paclitaxel (up to six cycles) for locally advanced tumors. The study has completed recruitment (n=284) and is continuing to evaluate the primary endpoint of overall survival. Most recently, a phase 1 trial proposed a notable approach to stimulating cancer immunity in pancreatic cancer, with promising results. 177 The study adopted the messenger RNA (mRNA) vaccine technique to make a personalized mRNA vaccine encoding five or more neoantigens, which were bioinformatically predicted from the resected primary tumor. This adjuvant treatment consisted of one dose of atezolizumab (anti-PDL1 (programmed death ligand 1) antibody) and eight doses (one week) of mRNA neoantigen vaccines, followed by 12 cycles of mFOLFIRINOX. In total, 16 of 28 resected patients received personalized vaccines, and eight patients responded to the vaccines, with no recurrence among responders after a median follow-up of 18.0 months. Larger studies will help establish whether this is a breakthrough in immunotherapy for pancreatic cancer.
Treatment strategies targeting specific genomic alterations have been explored in various molecularly defined patient subsets. Given the positive results for metastatic cancer in the POLO trial, 93 olaparib is being studied in a randomized phase 2 trial (APOLLO) to evaluate the additional benefit of one year of treatment on recurrence free survival in patients with a pathogenic BRCA1, BRCA2, or PALB2 mutation, who have received at least three months of multi-agent chemotherapy after curative resection. KRAS is an attractive target owing to its high rate of mutation (90%) in pancreatic cancer. 178 Although KRAS G12C mutations are rare (1.6% of pancreatic ductal adenocarcinoma (PDAC) cases), the ability to create covalent G12C inhibitors led to FDA approval in non-small cell lung cancer, and promising initial results in PDAC. Sotorasib, a KRAS G12C inhibitor, showed a median progression free survival of four months, and an objective response rate of 21% in metastatic patients with KRAS G12C mutations who received at least two lines of chemotherapy in a phase 1/2 trial. 179 Another KRAS G12C inhibitor, adagrasib, showed a median progression free survival of 6.6 months, and an objective response rate of 50% (5/10 patients), in patients with advanced pancreatic cancer in a phase 1/2 trial (KRYSTAL). 180
By contrast to the low mutation rate in BRCA1 (1.08%), BRCA2 (1.48%), PALB2 (0.54%), and KRAS G12C (1-2%), other KRAS mutations are quite common, and pan-KRAS inhibitors are under investigation. Two phase 1 studies of pan-RAS inhibitors are recruiting patients ( NCT04678648 and NCT05379985 ). Further studies on other KRAS targeting approaches are expected.
Radiotherapy has been suggested to have synergistic effects on local and even distant tumors when combined with immunotherapy. 181 182 A large randomized phase 3 trial showed that chemoradiotherapy followed by durvalumab (PDL1 inhibitor) had significantly longer overall survival than placebo in locally advanced, non-small cell lung cancer. 183 Recently, a phase 2 trial (CheckPAC) assigned 84 patients with refractory metastatic pancreatic cancer to receive SBRT/nivolumab (n=41) or SBRT/nivolumab/ipilimumab (n=43). 184 The SBRT/nivolumab/ipilimumab arm had a higher disease control rate (37.2% v 17.1%). Further studies with an immunotherapy–SBRT backbone are anticipated in locally advanced and metastatic disease settings.
Figure 2 summarizes all the data discussed above, and gives perspective on the future of the management of pancreatic cancer.

Precision medicine for pancreatic cancer. PanIN=pancreatic intraepithelial neoplasm
Several national and international guidelines for the management of pancreatic cancer have been published. Recommendations of those guidelines are proposed considering the evidence and the healthcare system of each country. We reviewed two major guidelines of the US and Europe, and also included the recent 2022 updated Japanese guideline. 6 7 8 All recommendations of these guidelines are made based on the metastatic status and the anatomical resectability of the primary tumors. Regarding treatments for resectable diseases, the NCCN guidelines list neoadjuvant chemotherapy as an option for high risk patients, and the Japan Pancreas Society guidelines recommend neoadjuvant for all patients. The ESMO guidelines recommend only upfront surgery. The NCCN and ESMO guidelines recommend mFOLFIRINOX as the first option of adjuvant chemotherapy, although S-1 monotherapy is recommended by the Japan Pancreas Society guidelines. Conversion surgery for locally advanced disease is an option in the NCCN and Japan Pancreas Society guidelines. No recommendation is made for conversion surgery for metastatic disease in any of the three sets of guidelines. Radiotherapy is listed as an option for non-metastatic diseases in the NCCN guidelines, while the other guidelines do not recommend it for resectable diseases. The NCCN guidelines recommend genetic testing of inherited mutations for all patients with pancreatic cancer, but no clear recommendations are made in the other sets of guidelines.
Conclusions
In the US and Europe, the incidence of pancreatic cancer has been increasing consistently, and this trend is estimated to continue for several decades. Advances in the combination of cytotoxic drugs have resulted in improvements in survival for all stages of the disease, and are changing treatment algorithms. Further investigation into the role of immuno-oncology agents and radiation could help a subset of patients. In addition, extensive efforts need to focus on risk assessment, screening, and early detection.
Research questions
・In patients treated with upfront systemic treatment, what is the optimal duration of systemic treatment and patient selection for surgical resection?
・How can immuno-oncology and targeted treatment be made effective?
・What is the optimal combination and sequence of radiotherapy, and who are the ideal targets?
・What is the specific population that needs routine screening and what is an effective combination of tests to detect precancerous lesions?
Glossary of abbreviations
NCCN: National Comprehensive Cancer Network
ESMO: European Society for Medical Oncology
PanIN: pancreatic intraepithelial neoplasia
IPMN: intraductal papillary mucinous neoplasm
CAPS: International Cancer of the Pancreas Screening
USPSTF: United States Preventive Services Task Force
CA19-9: carbohydrate antigen 19-9
RECIST: response evaluation criteria in solid tumors
FOLFIRINOX: combined leucovorin calcium (folinic acid), fluorouracil, irinotecan, and oxaliplatin
NALIRIFOX: combined liposomal irinotecan, fluorouracil, folinic acid, and oxaliplatin
mFOLFIRINOX: modified FOLFIRINOX
PEFG regimen: cisplatin, epirubicin, fluorouracil, and gemcitabine
IMRT: intensity modulated radiation therapy
SBRT: stereotactic body radiation therapy
3D-CRT: 3 dimensional conformal radiation therapy
IGRT: image guided radiation therapy
FAPI: fibroblast activation protein inhibitors
PDAC: pancreatic ductal adenocarcinoma
PDL1: programmed death ligand 1
State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors.
Competing interests : We have read and understood the BMJ policy on declaration of interests and declare the following interests: MDC receives grants from Haemonetics, and is the primary investigator of a Boston Scientific sponsored study. TS does not have conflicts of interest to declare. SDK receives investigator initiated clinical trial funding from Genentech and AstraZeneca. She also receives preclinical research support from Roche and Amgen. WAM receives institutional clinical trial funding from Genentech, Beigene, Pfizer, NGM, Gossamer, ALX, Exelixis, EDDC/D3, Mirati, RasCal Therapeutics, and CanBAS. He is also a Data and Safety Monitoring Board member of QED, Amgen, and Zymeworks.
Funding: This study is supported by funding sources: R01 DE028529-01 (SDK), R01 DE028282-01 (SDK), 1R01CA284651-01 (SDK), 1P50CA261605-01 (SDK), and the V Foundation Translational Research Award. The funders had no role in considering the study design or in the collection, interpretation of data, writing of the manuscript, or decision to submit the article for publication.
Contributors: Authors MDC and TS are joint first authors. The design, literature search, review, and writing of this manuscript was led by MDC and TS, and supported by SDK and WAM. MDC is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
We thank Hiroyuki Ishida for his drawings in figure 2 , and Michael J Kirsch for his assistance in proofreading this manuscript.
Patient involvement: No patients were involved in the writing of this review.
Provenance and peer review: commissioned; externally peer reviewed.
- Schwartz LM ,
- Bengtsson A ,
- Andersson R ,
- Siegel RL ,
- Miller KD ,
- Neoptolemos JP ,
- Stocken DD ,
- European Study Group for Pancreatic Cancer
- Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group
- ↵ National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 2.2022)
- Ducreux M ,
- Caramella C ,
- ESMO Guidelines Committee
- Okusaka T ,
- Nakamura M ,
- Yoshida M ,
- Committee for Revision of Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society
- Wehner MR ,
- Matrisian LM ,
- Walter FM ,
- Mendonça SC ,
- Petersen GM ,
- Giardiello FM ,
- Brensinger JD ,
- Tersmette AC ,
- Frants RR ,
- van Der Velden PA ,
- Rebours V ,
- Boutron-Ruault MC ,
- Deters CA ,
- Snyder CL ,
- Kastrinos F ,
- Mukherjee B ,
- Bosetti C ,
- Greenwood DC ,
- Kirkegård J ,
- Mortensen FV ,
- Cronin-Fenton D
- Pannala R ,
- Early Detection Initiative Consortium
- Chan-Seng-Yue M ,
- Katona BW ,
- Balduzzi A ,
- Marchegiani G ,
- Pollini T ,
- Goggins M ,
- Overbeek KA ,
- International Cancer of the Pancreas Screening (CAPS) consortium
- Yachida S ,
- Gerstung M ,
- Leshchiner I ,
- PCAWG Evolution & Heterogeneity Working Group ,
- PCAWG Consortium
- Niknafs N ,
- Fischer CG ,
- Goggins MG ,
- International Cancer of the Pancreas Screening Consortium
- Sandini M ,
- Mihaljevic AL ,
- Fernández-Del Castillo C ,
- Kamisawa T ,
- European Study Group on Cystic Tumours of the Pancreas
- Davidson KW ,
- US Preventive Services Task Force
- Steinberg WM ,
- Gelfand R ,
- Anderson KK ,
- Fahrmann JF ,
- Schmidt CM ,
- Visser IJ ,
- Levink IJM ,
- Peppelenbosch MP ,
- Fuhler GM ,
- Becker TM ,
- Yildirim HC ,
- Aktepe OH ,
- Pietrasz D ,
- Hadden WJ ,
- Laurence JM ,
- Krishna SG ,
- Ugbarugba E ,
- Leeflang MMG ,
- Treadwell JR ,
- Mitchell MD ,
- Teitelbaum U ,
- Erickson B ,
- Evangelista L ,
- Zucchetta P ,
- Moletta L ,
- Janssen BV ,
- van Roessel S ,
- van Dieren S ,
- International Study Group of Pancreatic Pathologists (ISGPP)
- Lahouel K ,
- DYNAMIC Investigators
- Raphael BJ ,
- Hruban RH ,
- Aguirre AJ ,
- Cancer Genome Atlas Research Network. Electronic address: [email protected] ,
- Cancer Genome Atlas Research Network
- Moffitt RA ,
- Marayati R ,
- Fischer SE ,
- Denroche RE ,
- Wilson GW ,
- Callery MP ,
- Fishman EK ,
- Talamonti MS ,
- William Traverso L ,
- ↵ National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 2.2022). https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- Burris HA 3rd . ,
- Andersen J ,
- Goldstein D ,
- National Cancer Institute of Canada Clinical Trials Group
- Milandri C ,
- Desseigne F ,
- Groupe Tumeurs Digestives of Unicancer ,
- PRODIGE Intergroup
- Berlin JD ,
- Catalano P ,
- Thomas JP ,
- Kugler JW ,
- Haller DG ,
- Benson AB 3rd .
- Rocha Lima CM ,
- Labianca R ,
- Heinemann V ,
- Quietzsch D ,
- Gieseler F ,
- Colucci G ,
- Di Costanzo F ,
- Gruppo Oncologico Italia Meridionale (GOIM) ,
- Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD) ,
- Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC)
- Herrmann R ,
- Ruhstaller T ,
- Swiss Group for Clinical Cancer Research ,
- Central European Cooperative Oncology Group
- Bernhard J ,
- Dietrich D ,
- Scheithauer W ,
- Cunningham D ,
- Van Cutsem E ,
- van de Velde H ,
- Karasek P ,
- Cascinu S ,
- Berardi R ,
- Italian Group for the Study of Digestive Tract Cancer (GISCAD)
- Vervenne WL ,
- Bennouna J ,
- Kindler HL ,
- Niedzwiecki D ,
- Richel DJ ,
- Middleton G ,
- Palmer DH ,
- Greenhalf W ,
- Von Hoff DD ,
- Wainberg ZA ,
- Macarulla T ,
- Stieler JM ,
- Wang-Gillam A ,
- NAPOLI-1 Study Group
- Williet N ,
- Le Malicot K ,
- PRODIGE 35 Investigators/Collaborators
- Neuhaus P ,
- Hochhaus A ,
- Liersch T ,
- Tempero MA ,
- O’Reilly EM ,
- APACT Investigators
- Fukutomi A ,
- JASPAC 01 Study Group
- Sugawara T ,
- Rodriguez Franco S ,
- Sherman S ,
- Nassour I ,
- Chabot JA ,
- Heinrich S ,
- Pestalozzi BC ,
- Schäfer M ,
- OʼReilly EM ,
- Perelshteyn A ,
- Jarnagin WR ,
- Versteijne E ,
- Groothuis K ,
- Dutch Pancreatic Cancer Group
- van Dam JL ,
- Cicconi S ,
- Balzano G ,
- Sohal DPS ,
- Labori KJ ,
- Bratlie SO ,
- Biörserud C ,
- Beumer BR ,
- Philip PA ,
- Portales F ,
- Kunzmann V ,
- Siveke JT ,
- German Pancreatic Cancer Working Group (AIO-PAK) and NEOLAP investigators
- Cameron JL ,
- Coleman J ,
- Ratnayake B ,
- Pendharkar SA ,
- van Dongen JC ,
- Wismans LV ,
- Suurmeijer JA ,
- Bachellier P ,
- Petrucciani N ,
- Belloni E ,
- Klaiber U ,
- Gemenetzis G ,
- De Simoni O ,
- Tonello M ,
- Sakaguchi T ,
- Valente R ,
- Del Chiaro M
- Minimally Invasive Treatment Group in the Pancreatic Disease Branch of China’s International Exchange and Promotion Association for Medicine and Healthcare (MITG-P-CPAM)
- Vissers FL ,
- van Hilst J ,
- International Minimally Invasive Pancreatic Resection Trialists Group
- Da Dong X ,
- Felsenreich DM ,
- Kamarajah SK ,
- Bundred J ,
- Cucchetti A ,
- Bocchino A ,
- Abu Hilal M ,
- Tchelebi LT ,
- Lehrer EJ ,
- Trifiletti DM ,
- Godfrey D ,
- Goodman KA ,
- Van Laethem J-L ,
- Büchler MW ,
- Dervenis C ,
- Pancreatic Cancer Meta-analysis Group
- Scoazec JY ,
- Small W Jr . ,
- Freedman GM ,
- Murphy JE ,
- Janssen QP ,
- Kivits IG ,
- Loehrer PJ Sr . ,
- Cardenes H ,
- van Laethem J-L ,
- LAP07 Trial Group
- Chauffert B ,
- Bonnetain F ,
- Nuyttens JJ ,
- Eskens FALM ,
- Fietkau R ,
- Ghadimi M ,
- Grützmann R ,
- Strauss VY ,
- Virdee PS ,
- Samalin E ,
- Álvaro Sanz E ,
- Garrido Siles M ,
- Rey Fernández L ,
- Villatoro Roldán R ,
- Rueda Domínguez A ,
- Griffin OM ,
- O’Connor D ,
- Ozola Zalite I ,
- Francisco Gonzalez M ,
- Heckler M ,
- Hüttner FJ ,
- Hamauchi S ,
- Abernethy AP ,
- Currow DC ,
- Solheim TS ,
- Laird BJA ,
- Balstad TR ,
- Koulouris AI ,
- Alexandre L ,
- Daeninck PJ ,
- Erstad DJ ,
- Sojoodi M ,
- Taylor MS ,
- Spielman B ,
- Veldhuijzen van Zanten SEM ,
- Pieterman KJ ,
- Wijnhoven BPL ,
- ↵ Columbus G. Nivolumab/cabiralizumab combo misses PFS endpoint in pancreatic cancer. 2020. https://www.onclive.com/view/nivolumabcabiralizumab-combo-misses-pfs-endpoint-in-pancreatic-cancer
- Bendell JC ,
- Soares KC ,
- Strickler JH ,
- George TJ ,
- Bekaii-Saab TS ,
- Sharabi AB ,
- DeWeese TL ,
- Twyman-Saint Victor C ,
- Antonia SJ ,
- Villegas A ,
- PACIFIC Investigators
- Johansen JS ,

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

People with pancreatic cancer are living longer, thanks to improved approaches
Share this:.
By Jessica Saenz
A diagnosis of pancreatic cancer is almost synonymous with hopelessness. As the least survivable type of cancer, the perception is understandable. "As soon as patients were diagnosed, they were often told by their physician to start making arrangements," says Mark Truty, M.D. , a surgical oncologist at Mayo Clinic who specializes in pancreatic surgery.
But the tides are turning, thanks to new and improved treatment methods that are helping people with pancreatic cancer live longer. Dr. Truty and Robert McWilliams, M.D. , a medical oncologist at Mayo Clinic, talk about Mayo Clinic's approach to pancreatic cancer care , and how it's leading to improved survival and quality of life.
Capturing the full picture from the time of diagnosis and beyond
Before moving forward with treatment, Dr. Truty says it's critical to understand as much about each person's cancer as possible. "When a patient is first diagnosed, they need really good imaging and molecular testing to see, not just where the tumor is, but if there's any evidence of spread. We do a lot of tests at the beginning and throughout to make sure that the cancer is truly localized and has not spread."
In most instances, a CT scan or MRI scan is used to identify the location of the cancer and possible spread, but Dr. Truty says standard scans are just one piece of the puzzle. "Historically, patients have gotten a scan where the tumor appears to be localized, and then they underwent surgery. But that paradigm has not resulted in the outcomes we wanted."
This is where PET scans and additional molecular testing play an important role.
Dr. Truty says that PET scans and newer genetic testing are key to staging the cancer and assessing its behavior accurately. They can help determine if treatment is working effectively to shrink the tumor, whereas traditional CT scans have distinct limitations in assessing response in pancreatic primary tumors. "If we see a response we’re anticipating on the PET scan, those are the patients that do very well. If we're not seeing a response, then we have to pivot and switch their therapy to see if we can achieve a better outcome," he says. "We've also been using novel genetic testing developed at Mayo Clinic to test the blood of patients, as well as the fluid of the abdomen through laparoscopy , to see if we can pick up some cancer DNA."
This method is helping cancer experts at Mayo Clinic determine who might be at risk for pancreatic cancer recurrence and individualize their treatment to reduce the risk of the cancer returning. "We're the first center to do this routinely for every single patient we see," Dr. Truty says.
Tailoring testing and treatment for each person
Initial testing and staging of pancreatic cancer can help uncover weaknesses or potential threats for each unique pancreatic cancer case. "As we've learned more about the genetics of pancreatic cancer — and how to find patients who can benefit — we've been able to tailor therapies according to the patient's genetics and their DNA, or the DNA changes that are specific to the cancer itself," says Dr. McWilliams.
In a study led by Mayo Clinic Center for Individualized Medicine , researchers found that nearly 1 in 6 people diagnosed with pancreatic cancer had an inherited cancer-related gene mutation that may have predisposed them to pancreatic cancer. The most common genetic mutation in those patients was the BRCA2 gene, which is linked to breast cancer.
Niloy Jewel Samadder, M.D. , a Mayo Clinic gastroenterologist and hepatologist, and the study's senior author, said that patients with mutations had a 50% longer survival. Data from this study and others have led to recent changes in guidelines that advocate for genetic testing for all pancreatic cancer patients, regardless of their cancer stage or family history of cancer.
Though the majority of people with pancreatic cancer do not have a germline mutation, Dr. McWilliams says it's important to use all the tools available for each patient. While it may not achieve a cure, it can help select therapies to improve quality of life so patients can live longer and more comfortably.
"There's a national trial, called the POLO Trial , which showed that patients on chemotherapy with BRCA1 or BRCA2 mutations are eligible for a maintenance therapy with just a pill, rather than IV chemotherapy, which is really good from a side effects standpoint," says Dr. McWilliams.
Redefining what is considered inoperable
Dr. Truty says patients who are able to have surgery to remove their pancreatic cancer can live significantly longer, but in cases where the tumor has grown outside of the pancreas to encase critical blood vessels, pancreatic cancer has been considered inoperable.
About one-third of pancreatic cancer tumors grow to surround blood vessels outside the pancreas. "Those patients have historically not been considered for surgery," he says. "Theoretically, 50% of patients with diagnosed pancreatic cancer have the potential to undergo an operation. The question is: How do we get them to surgery? And how do we optimize their outcomes to make sure that they live as long as they possibly can?"
Drs. Truty, McWilliams and pancreatic cancer experts at Mayo Clinic use an approach called neoadjuvant therapy, which delivers chemotherapy — or a combination of chemotherapy and radiation — to destroy microscopic cancer cells in the body before surgery. By combining this method with personalized surgery for each patient's anatomy, they can remove tumors entirely and reconstruct blood vessels as needed. This has resulted in the ability to operate on patients who previously did not have that option, leading to better results than ever before.
"We're creating custom surgeries for each patient that aren't being done anywhere else on the planet. That's why so many people come to us after they've been told their tumors are inoperable," says Dr. Truty.
Though surgery can lead to the best outcomes in many cases, Dr. Truty emphasizes that the goal of pancreatic cancer treatment is not surgery. "The goal for anyone with cancer is to extend their life and maintain a reasonable quality of life. Sometimes an operation is necessary to achieve this, and sometimes it will decrease the likelihood of one or the other, or both. That's why before we even consider an operation, we have to make sure that operation has the highest probability that we'll achieve both of those goals."
Pancreatic cancer continues to have the highest mortality rate, but Dr. McWilliams says there's plenty of reason for patients to be hopeful. "It's a very serious cancer. It's something that is life-threatening for a lot of people, but it's not necessarily a death sentence," he says. "It's something that we have treatments for, and our treatments are only getting better."
And this progress, he says, is driven by clinical trials. "Clinical trials are how we advance the science. For patients who are looking for the latest and greatest, and want to help advance the options for their cancer, participation in clinical trials is crucial."
Dr. Truty says he hopes more people with pancreatic cancer seek out second opinions from cancer centers who are leveraging new approaches and providing patients more options. "Historically, it's been such a nihilistic disease, but things have really changed. We have not settled for the standard of care — this results in standard outcomes which have not been good. We have to treat patients differently — starting from the beginning," he says. "And if you can do that all the way through treatment, then those patients really do have exceptional outcomes."
Learn more about panc r eatic cancer and find a pancreatic cancer clinical trial at Mayo Clinic.
Read these articles:
- " 5 things to know about pancreatic cancer "
- " PET/MRI biomarkers guide personalized treatment for people with pancreatic cancer, study finds "
- " Identifying inherited gene mutations in pancreatic cancer can lead to targeted therapies, better survival "
- " Aggressive Approach to Pancreatic Cancer Yields Outstanding Outcome "
Also watch this video: " Mayo Clinic Minute: Advances in pancreatic cancer treatment extending lives
Related Posts

New Mayo Clinic research represents a promising step toward identifying biological signals or biomarkers that may aid in early detection.

After a pancreatic cancer diagnosis and multiple cardiac arrests during treatment, "Tiger" Dick Whetstone finds help and hope at Mayo Clinic.

Dr. Maria Linnaus discusses the link between obesity and cancer risk and how bariatric surgery may reduce that risk.
Advertisement

- Previous Article
- Next Article
Cancer-Associated Fibroblasts: From Spectators to Protagonists in Pancreatic Cancer Progression
Cancer Res 2024;84:2938–40
G. Mucciolo and W. Li contributed equally to this article.
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Get Permissions
- Cite Icon Cite
- Search Site
- Version of Record September 16 2024
Gianluca Mucciolo , Wenlong Li , Giulia Biffi; Cancer-Associated Fibroblasts: From Spectators to Protagonists in Pancreatic Cancer Progression. Cancer Res 15 September 2024; 84 (18): 2938–2940. https://doi.org/10.1158/0008-5472.CAN-24-2448
Download citation file:
- Ris (Zotero)
- Reference Manager
Our knowledge of the origins, heterogeneity, and functions of cancer-associated fibroblasts (CAF) in pancreatic ductal adenocarcinoma (PDAC) has exponentially increased over the last two decades. This has been facilitated by the implementation of new models and single-cell technologies. However, a few key studies preceded the current exciting times in CAF research and were fundamental in initiating the investigation of CAFs and of their roles in PDAC. With their study published in Cancer Research in 2008, Hwang and colleagues have been first to successfully isolate and immortalize human pancreatic stellate cells (HPSC) from PDAC tissues. This new tool allowed them to probe the roles of CAFs in PDAC as never done before. By performing complementary in vitro and in vivo analyses, the authors demonstrated the involvement of HPSCs in PDAC malignant cell proliferation, invasion, and therapy resistance. Here, we leverage that seminal study as a framework to discuss the advances made over the last 16 years in understanding the complexity and central roles of CAFs in PDAC progression.
See related article by Hwang and colleagues, Cancer Res 2008;68:918–26
Client Account
Citing articles via, email alerts.
- Online First
- Collections
- Online ISSN 1538-7445
- Print ISSN 0008-5472

AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Information on Advertising & Reprints
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Ready to start planning your care? Call us at 800-525-2225 to make an appointment.
Pancreatic Cancer
- Pancreatic Cancer Screening and Risk Evaluation Program
- Pancreatic Cancer Stages 0, 1, 2, 3 & 4
- Pancreatic Cancer Surgery
- Chemotherapy for Pancreatic Cancer
- Radiation Therapy for Pancreatic Cancer
- Pancreatic Tumor Registry

Jeffrey Drebin, Chair of the Department of Surgery, is one of MSK’s many experts in diagnosing and treating pancreatic cancer.
This guide from Memorial Sloan Kettering (MSK) can help answer your questions about pancreatic cancer. It’s based on MSK’s strong expertise in treating pancreatic cancer and our credentials as a National Pancreas Foundation Center of Excellence .
You may have just learned you have pancreatic cancer, or are worried you have it. It’s common to have questions. How do you know if you have pancreatic cancer? What causes pancreatic cancer? What’s the best hospital for treating pancreatic cancer?
Request an Appointment
- Make an appointment
About MSK’s guide to pancreatic cancer
This guide can support you and your loved ones as you learn more about this disease. We share expert information about pancreatic cancer symptoms and the latest treatments. We have information about pancreatic cancer research studies, also known as clinical trials , that you may be able to join.
Who is this disease guide for?
- You’re waiting to learn if you have cancer This guide gives you information about pancreatic cancer so you’re better prepared. If you want to know right away if you have cancer, we have information about MSK’s rapid diagnosis program .
- You want a second opinion This guide explains new treatments for pancreatic cancer. Learning about them can help you decide if you want a second opinion . MSK’s experts offer second opinions about both diagnosis and treatment options, no matter where you live.
- You’re worried about your current treatment plan This guide can help you learn about other treatment options. MSK experts only use the latest treatments for pancreatic cancer, some only offered at MSK and very few other hospitals.
- You’re worried about your genetic risk for cancer This guide can help you learn about your risk for pancreatic cancer. We offer cancer genetic risk assessments to see if you’re at higher risk for some cancers. About 10 out of every 100 cases of pancreatic cancer are from inherited genes passed on from parents to children.
- You’re a caregiver to someone who has cancer We have information about how to support a loved one who has cancer , even if they’re not an MSK patient. At MSK, supporting caregivers is as important as caring for people with cancer.
Where is the pancreas and what does it do?
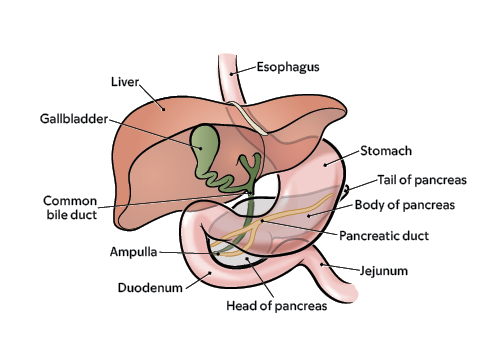
What the pancreas looks like and its anatomy in your body
The pancreas is a small gland. It’s located in your abdomen (belly) between your stomach and intestines.
The main job of the pancreas is to make enzymes, a kind of protein that helps you digest food. These enzymes are made by a type of cell called an exocrine cell. Most of your pancreas is made up of exocrine cells.
A very small part of the pancreas is made of endocrine cells. These cells make hormones, including insulin, that control the level of sugar in your blood.
Pancreatic tumors start in either exocrine or endocrine cells. Most pancreatic cancers are exocrine tumors, not neuroendocrine tumors.
Types of pancreatic tumors
There are about 20 different types of tumors that can grow in the pancreas. Exocrine pancreatic cancer tumors are the most common. More than 9 out of every 10 cases of pancreatic cancer are exocrine tumors.
The most common kind is adenocarcinoma. Others are:
- Acinar cell carcinoma
- Intraductal papillary-mucinous neoplasm (IPMN)
- Mucinous cystic neoplasm
Neuroendocrine pancreatic cancer tumors are less common. Fewer than 1 out of every 10 cases of pancreatic cancer are neuroendocrine tumors. They include pancreatic neuroendocrine (islet cell) tumors .

What makes pancreatic cancer so hard to treat and deadly?
How pancreatic cancer spreads .
The pancreas is deep in the body. That makes it harder for your healthcare provider to see or feel a tumor during regular exams.
There often are no symptoms of early pancreatic cancer. Tumors can grow large and spread to healthy organs before there are signs of the disease.
As a pancreatic tumor grows, it can spread to nearby organs, such as the bile duct, intestines, or stomach. It also can spread to nearby blood vessels.
Pancreatic tumor cells also can break away. These cells can spread to the lymph nodes, liver, or somewhere else in the abdomen (belly).
Pancreatic cancer is not the most common cancer, but it’s deadly
Pancreatic cancer is not that common, with about 64,000 cases diagnosed each year in the United States. The number of new cases is rising.
There are no recommended screening tests for people at average risk for pancreatic cancer. That makes it harder to catch this cancer early, before it spreads.
Pancreatic cancer is hard to cure.
The 5-year survival rate for pancreatic cancer has improved in recent years because of better treatments and diagnosis. But it’s still low at around 12%. That means out of every 100 people who get pancreatic cancer, only 12 will be alive 5 years later.
The disease rarely causes symptoms in its early stages. It’s often diagnosed only after it metastasized (spread) from the pancreas to other parts of the body. After it spreads it’s much harder to treat.
Why should I choose Memorial Sloan Kettering to treat pancreatic cancer?
Msk’s team of pancreatic cancer experts .
MSK’s team of pancreatic cancer experts is among the nation’s largest. We’re a leading hospital for pancreatic cancer care and research.
Our surgeons operate on about 350 people with pancreatic cancer a year. Our oncologists (cancer doctors) and other subspecialists treat about 850 people a year. That’s among the highest volumes of pancreatic cancer cases in both New York City and the country.
MSK is home to the David M. Rubenstein Center for Pancreatic Cancer Research (CPCR). Its mission is to offer the latest pancreatic cancer treatments and resources while supporting your physical and emotional well-being.
Research excellence
The David M. Rubenstein Center of Pancreatic Research is finding new ways to treat pancreatic cancer through research studies, also known as clinical trials . We led the first clinical trial to test mRNA vaccines as a possible new treatment for pancreatic cancer. The vaccine trains the body to protect itself against its own cancer cells.
National center of excellence
As a National Pancreas Foundation Centers of Excellence , MSK offers the best possible treatment results to give you a better quality of life.
Being named a NPF Center of Excellence means MSK:
- Passed a very strict review of our doctors.
- Offers many programs to support people who have pancreatic cancer. This includes pain management and symptom control, counseling , nutrition, integrative medicine , rehabilitation , pre-habilitation, and other services.
- Shows excellence in our clinical trials as we test new treatments for pancreatic cancer.
Pancreatic cyst surveillance
MSK’s Pancreatic Cyst Surveillance Program is one of the largest in the country . It monitors pancreatic cysts and has provided treatment for pancreatic cysts for more than 5,000 people. Each year, MSK sees more than 300 new patients who have cysts in their pancreas.
Related topics:


- Questions To Ask
What Questions Should I Ask My Doctor?
If you are in the midst of dealing with pancreatic cancer, you have a lot on your mind and you may have difficulty knowing where to start. Since every patient has a unique case, your doctors are your best source of information and you have every right to ask them questions.
Heather Sentkoski, a clinical social worker who was here at Johns Hopkins, compiled the following list of suggestions as a guideline.
If you are meeting with a surgeon or oncologist for the first time
Do not be afraid to ask:
- Have you ever treated a pancreatic cancer patient before?
- How many surgeries have you performed on pancreatic cancer patients?
- What has the general outcome of those patients been?
- Where were you trained? (medical school, residency)
- Which surgeons did you study under?
At any point in the relationship with your physican, you have the right to ask
- What is the diagnosis?
- What treatments are recommended?
- Are there other treatment options available that you do not provide (i.e. clinical trials, drugs available elsewhere that you do not have access to)?
- What are the likely benefits of each treatment?
- What are the side effects of each treatment?
- What are the medications being prescribed? What are they for? What are their side effects?
- Are there any clinical drug trials I can participate in?
- How should I expect to feel during the treatment(s)?
- What are the risks of the treatment(s)?
- Will my diet need to be changed or modified?
- Will I need to take enzymes, vitamins, etc?
- Are there other things I can do to improve the quality of my life?
Do not forget to ask about the things that are most important to you
- How will this affect my ability to work?
- Can this treatment be done as an outpatient so that I can spend more time at home with family?
- Will I have any physical limitations?
- How will my current lifestyle be changed?
- Do you have suggestions on what I should tell my family?
Finally - and most importantly - ask these questions of YOURSELF
- Does my doctor appear interested in answering my questions?
- Or, does my doctor look annoyed when I ask questions, like I'm doubting their expertise or I am holding them up?
- Do I feel that my doctor cares about my medical outcome?
- Have I told my family everything that I should? Remember Ira Byock's The Four Things That Matter Most. The four things we should tell our loved ones: “Please forgive me,” “I forgive you,” “Thank you,” and “I love you”
If you are uncomfortable with the results of some of these questions, you may want to re-evaluate your choice of physician or get a second opinion.
An inherited genetic variation in DNA that you are born with
Third Degree Relatives - First cousins, great-aunts and uncles
Second Degree Relatives - Aunts, uncles, grandparents, nieces and nephews
First Degree relatives - Blood relatives in your immediate family: parents, children, and siblings
Also known as a pancreatoduodenectomy, the Whipple procedure is the surgery typically performed to remove cancers of the head of the pancreas (the part of the pancreas on the right side of your body). It typically involves the surgical removal of the head of the pancreas, a portion of the duodenum and a portion of the bile ducts.
This is an experimental type of treatment. It is a medication made of killed or weakened cells, organisms or manufactured materials, which is used to boost the body's immune system. Ideally, this will allow the body to fight and kill the cancer cells more effectively. Vaccines include whole killed cancer cells or specific proteins from the cancer.
Unable to be surgically removed. This usually means that the cancer has spread beyond the areas that can be removed surgically.
The part of the pancreas that bends backwards, hooking around two very important blood vessels, the superior mesenteric artery and vein. The word "uncinate" comes from the word uncus which means "hook."
A painless procedure in which high frequency sound waves are used to generate pictures of the inside of the body. An ultrasound devise can be placed at the end of a scope, and the scope inserted into the duodenum, providing very detailed pictures of the pancreas. This is called "endoscopic ultrasound."
This term simply refers to a "mass" or neoplasm. For example, a collection of pus is a tumor. This is a general term that can refer to either benign or malignant growths.
A clot within the blood vessels. It may occlude (block) the vessel or may be attached to the wall of the vessel without blocking the blood flow.
An inflammation of the veins accompanied by thrombus formation. It is sometimes referred to as Trousseau's sign.
The long thin part of gland in the left part of abdomen, near the spleen.
A slender hollow tube inserted into the body to relieve a blockage. For example, pancreas cancers often grow into the bile duct as the bile duct passes through the pancreas. This can block the flow of bile and cause the patient to become jaundiced. In these cases the flow of bile can be reestablished by placing a stent into the bile duct, through the area of blockage.
Excessive amounts of fat in the stool. Sometimes this can appear as an oil slick on top of the toilet water after the patient has had a bowel movement. It can be a sign that the pancreas isn't functioning well.
A classification system that is used to describe the extent of disease. Clinicians use it to predict the likely survival of a patient.
A flat, scale-like cell. Although most pancreatic cancers look like ducts under the microscope, a small fraction look like squamous cells.
A maroon colored, rounded organ in the upper left part of the abdomen, near the tail of the pancreas. This organ is part of your immune system and filters the lymph and blood in your body. It is often removed during the distal pancreatectomy surgical procedure.
A long (20 foot) tube that stretches from the stomach to the large intestine. It helps absorb nutrients from food as the food is transported to the large intestine. There are three sections: the duodenum, the jejunum and the ileum. Due to its proximity to the pancreas, the duodenum is the section of the small intestine most often affected by pancreatic cancer.
An infection of the blood. This can be life-threatening and is often treated with antibiotics.
A malignant tumor that looks like connective tissues (bone, cartilage, muscle)under the microscope. Sarcomas are extremely rare in the pancreas.
Able to be removed surgically. Usually this means that the cancer is confined to areas typically removed surgically.
The use of high-energy waves similar to x-rays to treat a cancer. Radiation therapy is usually used to treat a local area of disease and often is given in combination with chemotherapy.
A thick ring of muscle (a sphincter) between the stomach and duodenum. This sphincter helps control the release of the stomach contents into the small intestine.
A forecast for the probable outcome of a disease based on the experience of large numbers of other patients with similar stage disease. Importantly, making a prognosis is not an exact science. Some patients with poor prognosis beat the odds and live longer than anyone would have predicted. Steve Dunn's Cancer Guide has an excellent article on statistics and prognoses and stories of other cancer patients.
A cancer in the organ where it started in. A primary cancer of the pancreas is one that started in the pancreas as opposed to a cancer that started somewhere else and only later spread to the pancreas.
The biochemical study of plants; concerned with the identification, biosynthesis, metabolism of chemical constituents of plants; especially in regards to natural products.
Around the ampulla of Vater in the duodenum. The peri-ampullary region is comprised of 4 structures; the ampulla, the duodenum, the bile duct and the head of the pancreas. It is sometimes difficult to tell which structure a tumor originated in. In such cases the diagnosis will be a peri-ampullary tumor.
A medical doctor specially trained to study disease processes. Pathologists make the microscopic diagnosis that is used to establish the diagnosis of cancer.
A term used to describe certain tumors which grow in finger-like projections. Pathologists use this term to describe some precancerous lesions in the pancreas (intraductal papillary mucinous neoplasm).
Any treatment that reduces the severity of a disease or its symptoms. Palliative care is often a part of the treatment plan for patients with advanced pancreatic cancer.
An oblong organ located between the stomach and the spine. The pancreas secretes enzymes needed for the digestion of food and it produces hormones such as insulin and glucagon which help control blood sugar.
A surgically created opening in an organ that can also be referred to as an anastamosis. Sometimes when surgeons remove a segment of bowel they create an ostomy to allow for the bowel contents to exit the body.
A medical doctor who specializes in the treatment of tumors. Oncologists often treat patients with pancreatic cancer with chemotherapy.
An abnormal new growth of tissue that grows more rapidly than normal cells and will continue to grow if not treated. These growths will compete with normal cells for nutrients. This is a general term that can refer to benign or malignant growths. It is a synonym for the word tumor.
Chemotherapy and radiation therapy that is given to patients before surgery. Some centers feel that the use of neoadjuvant therapy improves local and regional control of disease and that it may make more patients surgical candidates.
The thin section of the pancreas between the head and the body of the gland.
An alteration in the DNA of a cell. Think of it as a typographically error in the DNA code.
A cancer that has spread from one organ to another. Pancreas cancer most frequently metastasizes to the liver. In general, cancers that have metastasized are generally not treated surgically, but instead are treated with chemotherapy and/or radiation therapy.
A cancer that has the potential of invading nearby tissues, spreading to other organs (metastasizing) and possibly leading to the patient's death.
A painless method for visualizing internal organs. A tube-like machine with a powerful magnet generates images of the inside of the body. It does not involve the use of Xrays.
Normal, round, raisin to grape-sized collections of lymphocytes (white blood cells) found throughout the body. Lymph nodes are connected to each other by lymphatic vessels. They normally help fight infection, but also are one of the first sites to which cancers spread. In general, the spread of cancer to lymph nodes portends a worse prognosis for the patient. There are exceptions to this.
A primary pancreatic cancer that has spread to regional lymph nodes and/or resectable (removable) tissues. Removable tissues include some lymph nodes and parts of the duodenum and stomach that are routinely removed in some surgical treatments for pancreatic cancer.
The largest organ in the body, located in the right upper part of the abdomen. It performs many life-maintaining functions including the production of bile. The liver detoxifies the blood of drugs, alcohol and other harmful chemicals. It processes nutrients absorbed by the intestine and stores essential nutrients, vitamins and minerals. Bilirubin is a chemical produced when old or damaged blood cells breakdown. The liver chemically process the bilirubin so that it can dissolve in water and be excreted through the urine. When this process is disrupted, jaundice can develop.
A technique that surgeons can use to visualize and even biopsy (take tissue samples of) organs inside of the abdomen without making large incisions. Very small incisions are made in the belly and small tubes (called trocars) are then inserted. Gas is pumped in through one of the tubes to create enough space to work in. The surgeon inserts a small camera through one of the tubes and examines the lining and contents of the abdominal cavity by looking at the projected image on the television screen. With specially designed laparascopic instruments, biopsies and fluid samples can be taken for examination. Some surgeons feel that this technique can help "stage" a patient less invasively than with open surgery.
Yellowing of the skin or yellowing of the whites of the eyes caused by the accumulation of bile pigments (usually due to an obstruction of the bile ducts).
A hormone produced by the endocrine cells of the islets of Langerhans cells of the pancreas. Insulin acts to lower blood sugar levels.
A term used to indicate that cancerous cells are present in the duct but have not yet invaded deeper tissues.
The widest part of the pancreas. It is found in the right part of abdomen, nestled in the curve of the duodenum, which forms an impression in the side of the pancreas.
A hormone produced by the endocrine (islets of Langerhans) cells of the pancreas. When blood sugar levels are low, glucagon acts to raise blood sugar levels.
Gemzar is the trade name for the chemotherapy drug gemcitabine. It is frequently used to treat pancreatic cancer. It has been shown, in controlled clinical trials, to improve quality of life.
A green pear-shaped organ located on the right side of the abdomen just under the liver. The gallbladder is essentially a reservoir for holding bile.
A chemotherapeutic drug commonly used to treat pancreatic cancer.
The exocrine cells (acinar cells) of the pancreas produce and transport chemicals that will exit the body through the digestive system. The chemicals that the exocrine cells produce are called enzymes. They are secreted in the duodenum where they assist in the digestion of food.
A test used to visualize and examine the pancreas and bile ducts. A tube is inserted through a patient's nose (or throat), down through the esophagus and stomach then into the small intestine (duodenum). There, a small probe is inserted into the ampulla of Vater. A dye is injected through the probe and into the pancreatic and bile ducts. X-rays are then taken to visualize the pancreatic and bile ducts. these ducts can be seen as white structures (this is because the injected dye is opaque). Because pancreas cancers often block the pancreatic and/or bile ducts, this technique can be useful in establishing a diagnosis of pancreas cancer.
A chemical that causes a reaction in other substances, in this case as a part of the digestive process.
A medical doctor who specializes in the treatment of hormonal abnormalities.
These are specialized cells that produce hormones released into the bloodstream . For example, the islets of Langerhans are endocrine cells in the pancreas that produce the hormone insulin. This hormone helps control blood sugar(glucose) levels. Some rare tumors of the pancreas, the endocrine (Islet Cell) tumors, can produce these same hormones. It is very important that these rare tumors be properly diagnosed because it will determine the treatment and prognosis.
Surgical removal of a structure or part of a structure. For example, pancreat ectomy is the surgical removal of the pancreas (or a portion of it).
The first portion of the small intestine. It is about 1 foot long. It is the part of the intestinal track that comes after the stomach.
A small anatomic structure. This is essentially a tube that carries various bodily fluids. The pancreatic duct runs the full length of the pancreas and drains into the duodenum.
The chemical in every cell that carries genetic information.
A dome shaped muscle that separates the lungs and heart from the abdomen. This muscle assists in breathing.
The disease in which the body is unable to appropriately control blood sugar (glucose) levels. This may be caused by failure of the pancreas to produce adequate amounts of insulin.
A fluid filled sac. Some tumors of the pancreas, including the serous cystadenomas and intraductal papillary mucinous neoplasms, form cysts. Cysts have a distinct appearance in CT scans. They are important to recognize because the treatment of cystic tumors can differ from that for solid tumors.
A dye, taken by mouth or injected, that is sometimes used during x-ray examinations to highlight areas that otherwise might not be seen.
A way to image internal organs. A series of x-ray pictures taken by a machine that encircles the body like a giant tube. Computers are then used to generate cross-sectional images of the inside of the body.
The treatment of a cancer by chemicals. For pancreatic cancer these include: Gemzar (Gemcitabine), 5-flurouracil, leukovorin, taxol, and others.
A small, flexible tube inserted into the body to inject or suck out fluids.
A cancer-causing chemical. Cigarette smoke contains a number of carcinogens.
A malignant tumor. It has the potential of invading into the adjacent tissues, spreading to other organs and may eventually lead to the patient's death.
A dramatic weight loss and general wasting that occurs during chronic disease.
A blood marker for pancreas cancer. It is not a good screening test for diagnosing possible pancreas cancers in individuals without symptoms. Instead, it can be useful in following the progress of patients known to have a cancer by measuring how their cancer is responding to treatment.
The middle part of the pancreas between the neck and the tail. The superior mesenteric blood vessels run behind this part of the gland.
The removal and microscopic examination of a small tissue sample.
A duct that carries bile from the liver to the intestine. This term may refer to the hepatic, cystic or common bile duct.
A green fluid produced by the liver that helps digest fats. It is transported from the liver to the duodenum by the bile duct. When the flow of bile is blocked, patients may become jaundiced (yellow skinned).
Tumors which are non-cancerous. These generally grow slowly and do not invade adjacent organs or spread (metastasize) beyond the pancreas.
The collection of excess amounts of fluid in the abdominal cavity (belly). It often is a sign that the cancer has spread to either the liver or to the portal vein that goes to the liver, or that the cancer involves the internal lining of the abdomen. If normal liver function is affected, a complex set of biochemical checks and balances is disrupted and abnormal amounts of fluid are retained.
The large artery that carries oxygen-rich blood from the heart. From the heart it arches backwards and descends into the abdomen where it gives off many branches to supply the organs. The superior mesenteric artery is a major branch of the aorta that can be involved by pancreatic cancer.
A radiographic technique used to visualize blood vessels. A contrast medium (a dye) is usually injected into the vessels to make them appear white on the x-rays.
A condition marked by a diminished apetite and aversion to food. Often results in physical signs of wasting.
A condition characterized by a deficiency in red blood cells. This can lead to fatigue among other symptoms.
A surgical joining of two hollow structures. It is similar to attaching two ends of a garden hose. For example, a gastrojejunostomy is a surgical procedure that connects the stomach and the jejunum (small intestine.)
This widening of the pancreatic duct as it reaches the duodenum is an landmark for physicians. It is where the bile duct and pancreatic duct join before draining into the duodenum (small intestine). Tumors in the head of the pancreas may squeeze this duct partially or completely closed. This can lead to problems with digestion and jaundice.
Chemotherapy given to patients after their cancers have been surgically removed. It is a secondary treatment given to supplement surgical treatment. (see Neoadjuvant chemotherapy which is chemotherapy given before surgery)
A benign (non-cancerous) tumor made up of cells that form glands (collections of cells surrounding an empty space).
The form of cancer that most people are talking about when they refer to "cancer of the pancreas." These tumors account for 75% of all pancreas cancers. Microscopically, adenocarcinomas form glands. These tumors can grow large enough to invade nerves which can cause back pain. They also frequently spread (metastasize) to the liver or lymph nodes. If this happens the tumor may be considered unresectable.
A pus-filled cavity. Usually caused by an infection.
The portion of the body between the diaphragm and the pelvis.
- Patient Care & Health Information
- Diseases & Conditions
- Pancreatic cancer
- What is pancreatic cancer? A Mayo Clinic expert explains
Learn more about pancreatic cancer from Mayo Clinic surgical oncologist Chee-Chee Stucky, M.D.
Hi. I'm Dr. Chee-Chee Stucky, a surgical oncologist at Mayo Clinic. In this video, we'll cover the basics of pancreatic cancer: What is it? Who gets it? What are the symptoms, diagnosis and treatment? Whether you're looking for answers for yourself or someone you love, we're here to give you the best information available. Understanding pancreatic cancer starts with understanding the pancreas. This small, fish-shaped organ sits behind the stomach, producing enzymes that aid digestion and hormones that regulate blood sugar. Pancreatic cancer typically starts in the ducts of the pancreas. Small changes in the cellular DNA result in uncontrolled multiplication and accumulation of cells in clusters called tumors. If untreated, these cancer cells can spread outside of the pancreas to other parts of the body.
While anyone can get pancreatic cancer, there are certain risk factors to be aware of. Most pancreatic cancer is diagnosed after age 65. Smoking, diabetes, chronic pancreatitis or inflammation of the pancreas, family history of pancreatic cancer, and certain genetic syndromes are all known risk factors. Carrying extra weight that is unhealthy for your body may also be a contributing factor. New research has found that the specific combination of smoking, diabetes and poor diet increases the risk of pancreatic cancer the most beyond any one factor alone.
Unfortunately, we don't usually see the signs of pancreatic cancer until it's in more advanced stages. When present, symptoms may include: Abdominal pain that radiates to the back. A loss of appetite or unintentional weight loss. Jaundice, which is the yellowing of your skin or eyes. Light colored stools. Dark colored urine. Particularly itchy skin. Diabetes that's becoming unusually difficult to control. Blood clots or fatigue.
If your doctors think you may have pancreatic cancer, they may recommend one or more diagnostic tests. For instance, imaging tests like an ultrasound, CT scan, MRI, or PET scan, can help your doctor see a clearer picture of your internal organs. An endoscopic ultrasound, or EUS, is when the doctor passes a tiny camera down the esophagus and into the stomach to get a close-up view of the pancreas. During the EUS, the doctor might collect a biopsy of the tissue for further testing. Sometimes pancreatic cancer can shed specific proteins called tumor markers in your blood. So your doctors may request blood tests to identify elevation of these markers, one of which is called CA 19-9. If a diagnosis is confirmed, the next step is to determine the extent or stage of the cancer. The stages are numbered one through four and may need to be determined by additional testing. Feel free to ask lots of questions during this process. Or get a second opinion to feel the most confident and empowered moving into treatment.
When recommending treatment for pancreatic cancer, your doctor is considering many factors, including your overall health and personal preferences. They may recommend one or a combination of the following treatments: Chemotherapy uses drugs that release chemicals that enter the body and kill cancerous cells that may be throughout. Radiation, similarly kills the cancer cells, but with high-energy beams directed at the tumor. Surgery is used to physically remove the cancer and the immediate surrounding area. Ask your doctor if you qualify for clinical trials that test new treatments. And lastly, there is palliative care. This care is provided by a team of doctors, nurses, social workers, and other trained professionals who specialize in providing much needed relief from the pain and unpleasant symptoms of a serious illness.
Getting diagnosed with a life-threatening illness can be devastating to both the patient and their loved ones. But we have some of the following suggestions that may help patients cope: Learn about your condition. Knowledge is power and information can make you feel more confident in your treatment decisions. Find support. This can mean a support system of family and friends, a cancer support group of people going through the same experience, or qualified counselor like your therapist or religious leader. Lean on those around you when you're feeling helpless, overwhelmed, or uncertain. You may want to consider hospice care, which provides comfort and support to terminally ill patients and their loved ones. If you'd like to learn even more about pancreatic cancer, watch our other related videos or visit mayoclinic.org. We wish you well.
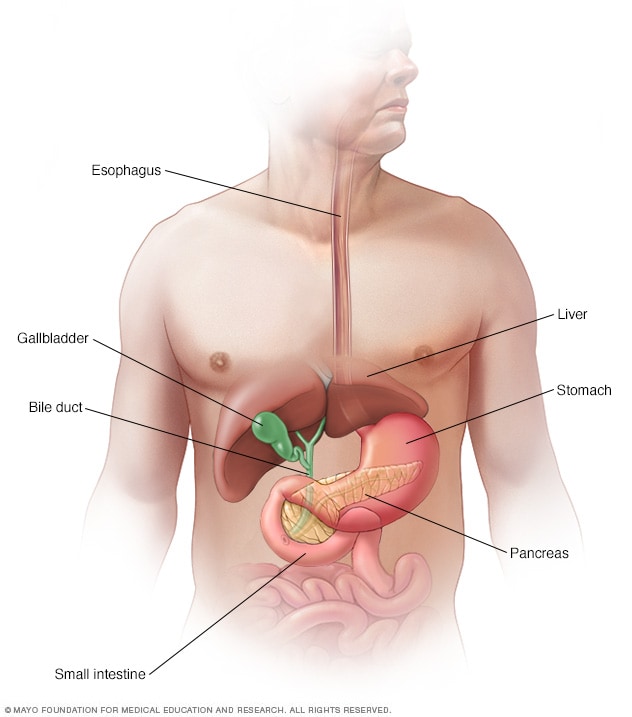
The pancreas in the digestive system
The pancreas is a long, flat gland that lies horizontally behind your stomach. It has a role in digestion and in regulating the level of sugar in your blood.
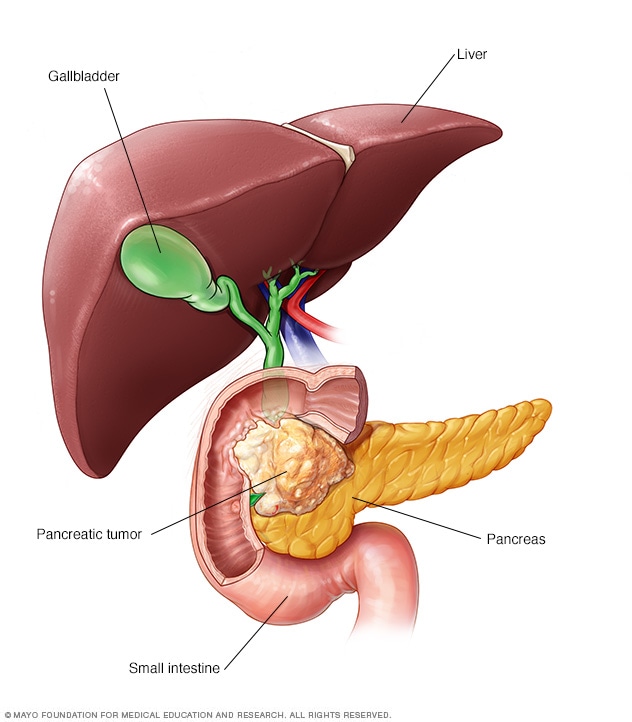
Pancreatic cancer is cancer that forms in the cells of the pancreas.
Pancreatic cancer is a type of cancer that begins as a growth of cells in the pancreas. The pancreas lies behind the lower part of the stomach. It makes enzymes that help digest food and hormones that help manage blood sugar.
The most common type of pancreatic cancer is pancreatic ductal adenocarcinoma. This type begins in the cells that line the ducts that carry digestive enzymes out of the pancreas.
Pancreatic cancer rarely is found at its early stages when the chance of curing it is greatest. This is because it often doesn't cause symptoms until after it has spread to other organs.
Your health care team considers the extent of your pancreatic cancer when creating your treatment plan. Treatment options may include surgery, chemotherapy, radiation therapy or a mix of these.
Products & Services
- A Book: Mayo Clinic Family Health Book
- A Book: Mayo Clinic on Digestive Health
- Assortment of Pill Aids from Mayo Clinic Store
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Pancreatic cancer often doesn't cause symptoms until the disease is advanced. When they happen, signs and symptoms of pancreatic cancer may include:
- Belly pain that spreads to the sides or back.
- Loss of appetite.
- Weight loss.
- Yellowing of the skin and the whites of the eyes, called jaundice.
- Light-colored or floating stools.
- Dark-colored urine.
- New diagnosis of diabetes or diabetes that's getting harder to control.
- Pain and swelling in an arm or leg, which might be caused by a blood clot.
- Tiredness or weakness.
When to see a doctor
Make an appointment with a health care professional if you have symptoms that worry you.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get Mayo Clinic cancer expertise delivered to your inbox.
Subscribe for free and receive an in-depth guide to coping with cancer, plus helpful information on how to get a second opinion. You can unsubscribe at any time. Click here for an email preview.
Error Select a topic
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth coping with cancer guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest about cancer news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
It's not clear what causes pancreatic cancer. Doctors have found some factors that might raise the risk of this type of cancer. These include smoking and having a family history of pancreatic cancer.
Understanding the pancreas
The pancreas is about 6 inches (15 centimeters) long and looks something like a pear lying on its side. It releases hormones, including insulin. These hormones help the body process the sugar in the foods you eat. The pancreas also makes digestive juices to help the body digest food and take in nutrients.
How pancreatic cancer forms
Pancreatic cancer happens when cells in the pancreas develop changes in their DNA. A cell's DNA holds the instructions that tell a cell what to do. In healthy cells, the instructions tell the cells to grow and multiply at a set rate. The cells die at a set time. In cancer cells, the changes give different instructions. The changes tell the cancer cells to make many more cells quickly. Cancer cells can keep living when healthy cells would die. This causes there to be too many cells.
The cancer cells might form a mass called a tumor. The tumor can grow to invade and destroy healthy body tissue. In time, cancer cells can break away and spread to other parts of the body.
Most pancreatic cancer begins in the cells that line the ducts of the pancreas. This type of cancer is called pancreatic ductal adenocarcinoma or pancreatic exocrine cancer. Less often, cancer can form in the hormone-producing cells or the neuroendocrine cells of the pancreas. These types of cancer are called pancreatic neuroendocrine tumors or pancreatic endocrine cancer.
Risk factors
Factors that might raise the risk of pancreatic cancer include:
- Type 2 diabetes.
- Chronic inflammation of the pancreas, called pancreatitis.
- Family history of DNA changes that can increase cancer risk. These include changes in the BRCA2 gene, Lynch syndrome and familial atypical multiple mole melanoma (FAMMM) syndrome.
- Family history of pancreatic cancer.
- Older age. Most people with pancreatic cancer are over 65.
- Drinking a lot of alcohol.
Complications
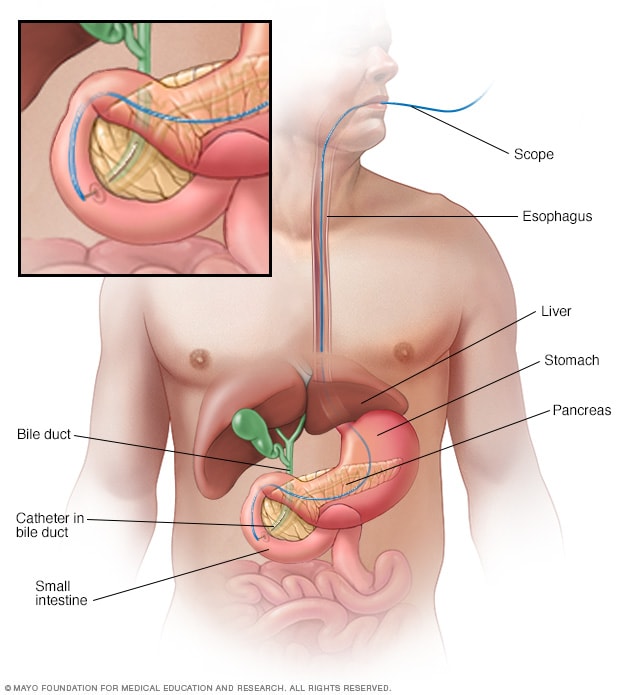
Endoscopic retrograde cholangiopancreatography
Endoscopic retrograde cholangiopancreatography (ERCP) uses a dye to highlight the bile ducts and pancreatic duct on X-ray images. A thin, flexible tube with a camera on the end, called an endoscope, is passed down your throat and into your small intestine. The dye enters the ducts through a small hollow tube, called a catheter, passed through the endoscope. Tiny tools passed through the catheter also can be used to remove gallstones.
As pancreatic cancer progresses, it can cause complications such as:
- Weight loss. People with pancreatic cancer might lose weight as the cancer uses more of the body's energy. Nausea and vomiting caused by cancer treatments or a cancer pressing on the stomach might make it hard to eat. Sometimes the body has trouble getting nutrients from food because the pancreas isn't making enough digestive juices.
Jaundice. Pancreatic cancer that blocks the liver's bile duct can cause jaundice. Signs include yellowing of the skin and the whites of the eyes. Jaundice can cause dark-colored urine and pale-colored stools. Jaundice often occurs without belly pain.
If the bile duct is blocked, a plastic or metal tube called a stent can be put inside it. The stent helps hold the bile duct open. This is done using a procedure called endoscopic retrograde cholangiopancreatography, also called ERCP.
During ERCP , a health care professional puts a long tube with a tiny camera, called an endoscope, down the throat. The tube goes through the stomach and into the upper part of the small intestine. The health professional puts a dye into the pancreatic ducts and bile ducts through a small tube that fits through the endoscope. The dye helps the ducts show up on imaging tests. The health professional uses those images to place a stent at the right spot in the duct to help hold it open.
Pain. A growing tumor may press on nerves in your abdomen, causing pain that can become severe. Pain medications can help you feel more comfortable. Treatments, such as radiation and chemotherapy, might help slow tumor growth and provide some pain relief.
When medicines aren't helping, a health care professional might suggest a celiac plexus block. This procedure uses a needle to put alcohol into the nerves that control pain in the belly. The alcohol stops the nerves from sending pain signals to the brain.
Bowel blockage. Pancreatic cancer can grow into or press on the first part of the small intestine, called the duodenum. This can block the flow of digested food from the stomach into the intestines.
A health care professional might suggest putting a tube called a stent in the small intestine to hold it open. Sometimes, it might help to have surgery to place a feeding tube. Or surgery can attach the stomach to a lower part of the intestines where the cancer isn't causing a blockage.
Screening for people with a high risk of pancreatic cancer
Screening uses tests to look for signs of pancreatic cancer in people who don't have symptoms. It might be an option if you have a very high risk of pancreatic cancer. Your risk might be high if you have a strong family history of pancreatic cancer or if you have an inherited DNA change that increases the risk of cancer.
Pancreatic cancer screening might involve imaging tests, such as MRI and ultrasound. These tests are generally repeated every year.
The goal of screening is to find pancreatic cancer when it's small and most likely to be cured. Research is ongoing, so it's not yet clear whether screening can lower the risk of dying of pancreatic cancer. There are risks to screening. This includes the chance of finding something that requires surgery but later turns out to not be cancer.
Talk about the benefits and risks of pancreatic cancer screening with your health care team. Together you can decide whether screening is right for you.
Genetic testing for cancer risk
If you have a family history of pancreatic cancer, discuss it with a health care professional. The health professional can review your family history and help you understand whether genetic testing might be right for you.
Genetic testing can find DNA changes that run in families and increase the risk of cancer. If you're interested in genetic testing, you might be referred to a genetic counselor or other health care professional trained in genetics.
Ways to lower risk
You might reduce your risk of pancreatic cancer if you:
- Stop smoking. If you smoke, talk to a member of your health care team about ways to help you stop. These might include support groups, medicines and nicotine replacement therapy.
- Maintain a healthy weight. If you are at a healthy weight, work to maintain it. If you need to lose weight, aim for a slow, steady weight loss of 1 to 2 pounds (0.5 to 1 kilogram) a week. To help you lose weight, exercise most days of the week. Slowly increase the amount of exercise you get. Choose a diet rich in vegetables, fruit and whole grains with smaller portions.
Pancreatic cancer care at Mayo Clinic
- AskMayoExpert. Pancreatic cancer (adult). Mayo Clinic; 2022.
- Groggins M, et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancrease Screening (CAPS) Consortium. Gut. 2020; doi:10.1136/gutjnl- 2019- 319352.
- Debouk M, et al. The Multicenter Cancer of Pancreas screening study: Impact on stage and survival. Journal of Clinical Oncology. 2022; doi:10.1200/JCO.22.00298.
- Pancreatic adenocarcinoma. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455. Accessed April 27, 2023.
- De la Fuente J, et al. How I approach screening for pancreatic cancer. American Journal of Gastroenterology. 2021; doi:10.14309/ajg.0000000000001305.
- Fernandez-del Castillo C. Clinical manifestations, diagnosis and staging of exocrine pancreatic cancer. https://www.uptodate.com/contents/search. Accessed April 26, 2023.
- Pancreatic cancer treatment (PDQ) — Health professional version. National Cancer Institute. https://www.cancer.gov/types/pancreatic/hp/pancreatic-treatment-pdq. Accessed April 26, 2023.
- Distress management. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455 Accessed April 27, 2023.
- Ryan DP. Initial systemic chemotherapy for metastatic exocrine pancreatic cancer. https://www.uptodate.com/contents/search. Accessed April 26, 2023.
- AskMayoExpert. Pancreas ductal adenocarcinoma: Diagnosis and treatment (adult). Mayo Clinic; 2022.
- Fernandez-del Castillo C, et al. Overview of surgery in the treatment of exocrine pancreatic cancer and prognosis. https://www.uptodate.com/contents/search. Accessed April 26, 2023.
- NPF Centers of Excellence. National Pancreas Foundation. https://pancreasfoundation.org/patient-resources/npf-centers-of-excellence/. Accessed April 27, 2023.
- Niederhuber JE, et al., eds. Carcinoma of the pancreas. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. http://www.clinicalkey.com. Accessed April 28, 2023.
- Sugumar A, et al. Distinguishing pancreatic cancer from autoimmune pancreatitis. Current Gastroenterology Reports. 2010;12:91.
- Ami T. Allscripts EPSi. Mayo Clinic. Feb. 28, 2022.
- Pancreatic Cancer Genetic Epidemiology (PACGENE) Study. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00526578. Accessed April 26, 2023.
- Thind K et al. Immunotherapy in pancreatic cancer treatment: A new frontier. Therapeutic Advances in Gastroenterology. 2017;10:168.
- Tee MC, et al. Laparoscopic pancreaticoduodenectomy: Is it an effective procedure for pancreatic ductal adenocarcinoma? Advances in Surgery. 2015;49:143.
- Merrell KW, et al. Predictors of locoregional failure and impact on overall survival in patients with resected exocrine pancreatic cancer. International Journal of Radiation Oncology. 2016;doi:10.1016/j.ijrobp.2015.11.003.
- Moris M, et al. Risk factors for malignant progression of intraductal papillary mucinous neoplasms. Digestive and Liver Disease. 2015;47:495.
- Pancreatic cancer: Symptoms and signs. Cancer.Net. https://www.cancer.net/cancer-types/pancreatic-cancer/symtpms-and-signs. Accessed May 2, 2023.
- Loncle C, et al. The pancreatitis-associated protein VMP1, a key regulator of inducible autophagy, promotes KRAS-G12D-mediated pancreatic cancer initiation. Cell Death and Disease. 2016;7:32295.
- Thiels CA, et al. Outcomes of pancreaticoduodenectomy for pancreatic neuroendocrine tumors: Are combined procedures justified? Journal of Gastrointestinal Surgery. 2016;20:891.
- Dr. Mark Truty (surgery, MN) better outcomes with chemo
- Dr. Wallace Video
- Infographic: Pancreatic Cancer: Minimally Invasive Surgery
- Infographic: Pancreatic Cancers-Whipple
- Pancreatic Cancer Survivor
- Pancreatic ultrasound
- Whipple procedure
Associated Procedures
- Chemotherapy
- Endoscopic ultrasound
- Integrative medicine
- Needle biopsy
- Palliative care
- Positron emission tomography scan
- Proton therapy
- Radiation therapy
News from Mayo Clinic
- Teamwork and a new nickname inspire patient through pancreatic cancer treatment April 19, 2024, 03:07 p.m. CDT
- Researchers make unexpected discovery in how pancreatic cancer spreads Dec. 15, 2023, 03:00 p.m. CDT
- Mayo Clinic's AI innovation inspires hope in early detection of pancreatic cancer Oct. 02, 2023, 03:25 p.m. CDT
- Long-term pancreatic cancer survivors report excellent post-surgery quality of life Aug. 28, 2023, 01:30 p.m. CDT
- Staging pancreatic cancer early with minimally invasive surgery shows positive results in patient prognosis, Mayo Clinic study finds June 29, 2023, 05:16 p.m. CDT
- Mayo Clinic to lead new radiotracer trial for detecting pancreatic cancer Jan. 30, 2023, 05:55 p.m. CDT
- Helpful guidelines if you test positive or negative for COVID-19 Nov. 30, 2022, 05:00 p.m. CDT
- Identifying inherited gene mutations in pancreatic cancer can lead to targeted therapies, better survival Nov. 24, 2022, 03:30 p.m. CDT
- 5 things to know about pancreatic cancer Nov. 18, 2022, 05:00 p.m. CDT
- Mayo Clinic Q and A: Pancreatic cancer risk, symptoms and treatment Nov. 10, 2022, 04:00 p.m. CDT
- Advances in treating pancreatic cancer mean options and hope--2022 Nov. 01, 2022, 04:30 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
5X Challenge
Thanks to generous benefactors, your gift today can have 5X the impact to advance AI innovation at Mayo Clinic.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Year in Review
- Published: 16 December 2022
Pancreatic cancer in 2022
The war on pancreatic cancer: progress and promise
- Christine A. Iacobuzio-Donahue ORCID: orcid.org/0000-0002-4672-3023 1 , 2
Nature Reviews Gastroenterology & Hepatology volume 20 , pages 75–76 ( 2023 ) Cite this article
1869 Accesses
4 Citations
17 Altmetric
Metrics details
The year 2022 was notable for substantial research progress related to pancreatic ductal adenocarcinoma (PDAC). The first single-cell and spatial transcriptomic atlases of PDAC were reported, a mechanism for how Schwann cells promote perineural invasion was explored, and, finally, the role of exercise in abrogating immunosuppression was shown.
Key advances
Spatial transcriptomics and other single-cell technologies reveal distinct transitional populations linking acinar-to-ductal metaplasia to pancreatic intraepithelial neoplasia and enrichment of metallothionein-expressing inflammatory cancer-associated fibroblasts in chemoresistant pancreatic cancer 3 .
Schwann cells within the tumour microenvironment organize into tumour-activated Schwann cell tracts that promote migration along nerves via activation of JUN 6 .
Aerobic exercise restrains pancreatic cancer growth in mice through IL-15–IL-15RA-mediated activation of CD8 + T cells, and evidence for this relationship was found in humans 7 .
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
Placental growth factor promotes neural invasion and predicts disease prognosis in resectable pancreatic cancer
- Andreas Göhrig
- , Georg Hilfenhaus
- … Christian Fischer
Journal of Experimental & Clinical Cancer Research Open Access 30 May 2024
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Park, W., Chawla, A. & O’Reilly, E. M. Pancreatic cancer: a review. JAMA 326 , 851–862 (2021).
Article CAS Google Scholar
Kindler, H. L. et al. Overall survival results from the POLO trial: a phase III study of active maintenance olaparib versus placebo for germline BRCA-mutated metastatic pancreatic cancer. J. Clin. Oncol. https://doi.org/10.1200/JCO.21.01604 (2022).
Article Google Scholar
Cui Zhou, D. et al. Spatially restricted drivers and transitional cell populations cooperate with the microenvironment in untreated and chemo-resistant pancreatic cancer. Nat. Genet. 54 , 1390–1405 (2022).
Grippo, P. J. & Tuveson, D. A. Deploying mouse models of pancreatic cancer for chemoprevention studies. Cancer Prev. Res. 3 , 1382–1387 (2010).
Schlesinger, Y. et al. Single-cell transcriptomes of pancreatic preinvasive lesions and cancer reveal acinar metaplastic cells’ heterogeneity. Nat. Commun. 11 , 4516 (2020).
Deborde, S. et al. Reprogrammed Schwann cells organize into dynamic tracks that promote pancreatic cancer invasion. Cancer Discov. 12 , 2454–2473 (2022).
Kurz, E. et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell 40 , 720–737.e5 (2022).
Liu, J. et al. Blood metallothionein transcript as a biomarker for metal sensitivity: low blood metallothionein transcripts in arsenicosis patients from Guizhou, China. Environ. Health Perspect. 115 , 1101–1106 (2007).
Li, G. et al. Recent advances in c-Jun N-terminal kinase (JNK) inhibitors. Curr. Med. Chem. 28 , 607–627 (2021).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52 , 17–35 (2020).
Download references
Author information
Authors and affiliations.
Department of Pathology, David. M. Rubenstein Center for Pancreatic Cancer Research, Human Oncology and Pathogenesis Program, New York, NY, USA
Christine A. Iacobuzio-Donahue
Memorial Sloan Kettering Cancer Center, New York, NY, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christine A. Iacobuzio-Donahue .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Iacobuzio-Donahue, C.A. The war on pancreatic cancer: progress and promise. Nat Rev Gastroenterol Hepatol 20 , 75–76 (2023). https://doi.org/10.1038/s41575-022-00728-1
Download citation
Published : 16 December 2022
Issue Date : February 2023
DOI : https://doi.org/10.1038/s41575-022-00728-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Georg Hilfenhaus
- Christian Fischer
Journal of Experimental & Clinical Cancer Research (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Get Our Latest News!
- Join Our Community

Pancreatic Cancer Questions to Ask the Healthcare Team
Home Facing Pancreatic Cancer Pancreatic Cancer Diagnosis Choosing Your Healthcare Team for Pancreatic Cancer Pancreatic Cancer Questions to Ask the Healthcare Team
On This Page:

Questions About a Doctor’s Experience
Questions about your diagnosis, questions about treatment, questions about surgery, questions about side effects, questions about diet, questions about social concerns, questions to ask yourself.
The amount of information received from healthcare professionals after diagnosis may be overwhelming. Preparing in advance of meetings can help you gather useful information and better understand your diagnosis as well as treatment options.
It can be very helpful to bring another person along to medical appointments. A trusted friend or family member can be supportive, provide an extra set of ears and ensure all questions are answered. Recording the meeting is a good way to avoid missing important information. Always ask the doctor for permission before recording.
You should feel comfortable and supported by your healthcare team. The Pancreatic Cancer Action Network strongly recommends seeking a healthcare team that suits all of your physical, mental and emotional needs.
- How many people with pancreatic cancer do you care for each year?
- What have been the results for these patients? Did they have a similar diagnosis?
- Do you work with a team of expert physicians to manage care? Who are they and what are their specialties?
- What is my diagnosis? What type of pancreatic cancer do I have?
- What is the stage of my cancer? What does this mean?
- What are the symptoms that I may experience from the cancer?
- What treatment(s) do you recommend? Why?
- Are there any clinical trials available to me at this hospital? At other local hospitals?
- Do you provide molecular profiling or refer patients to the Pancreatic Cancer Action Network’s Know Your Tumor® precision medicine service to help determine treatment options?
- What are the potential benefits and risks of each of my treatment options?
- Explain the medications being prescribed for me. What does each one do?
- What type of blood tests, scans or other tests will I need during my treatment? How often?
- Can my tumor be removed through surgery ? Why or why not?
- How many pancreatic surgeries have you performed? How many in the past year?
- How many pancreatic surgeries are performed at your hospital every year?
- What are the possible complications of pancreatic surgery?
- How long should I expect to be in the hospital recovering after pancreatic surgery?
- Would you be able to recommend another experienced surgeon for a second opinion?
- What are the potential side effects of my treatment options? How likely are they to occur?
- How can I expect to feel during the treatment?
- What medication(s) will be prescribed to help manage my side effects? Do these medications have additional side effects?
- How can I contact you in case of an emergency or if I have further concerns?
- Do I need to change or modify my diet?
- Do you have a dietitian that you recommend?
- Will I need to take pancreatic enzymes or vitamins? If so, how often?
- Will my ability to work, travel or drive be affected?
- Will I need to spend time in the hospital?
- Will I have physical limitations?
- Are there any lifestyle changes I should make?
- What support programs are available for me and my family?
- Who can I speak with about my financial and/or insurance concerns?
- Who can help me navigate the medical system? Is there an oncology social worker or patient navigator available at this hospital?
- Does the doctor seem interested in my questions? Is the communication easy?
- Did I get enough time with the doctor to answer all of my questions?
- Do I feel comfortable with the doctor and his/her recommendations?
- Will I be able to reach him/her if I have any questions or concerns while being treated?
- Is the doctor open to me seeking a second opinion?
Even if you feel comfortable with the answers a doctor provides, it might be beneficial to seek a second opinion. You have a right to seek a second opinion. The Pancreatic Cancer Action Network strongly recommends seeking a second opinion, as needed, at any point in your diagnosis. Second opinions can be extremely valuable when making treatment decisions. They can help provide additional information about treatment options as well as more confidence in the treatment plan. Many doctors welcome hearing the opinions of their colleagues. To receive the names of doctors who specialize in treating pancreatic cancer, contact PanCAN Patient Services.
Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Pancreatic Cancer
- What Is Pancreatic Cancer?
- Key Statistics for Pancreatic Cancer
- What’s New in Pancreatic Cancer Research?
- Pancreatic Cancer Risk Factors
- What Causes Pancreatic Cancer?
- Can Pancreatic Cancer Be Prevented?
- Can Pancreatic Cancer Be Found Early?
- Signs and Symptoms of Pancreatic Cancer
- Tests for Pancreatic Cancer
- Pancreatic Cancer Stages
- Survival Rates for Pancreatic Cancer
Questions to Ask About Pancreatic Cancer
- Surgery for Pancreatic Cancer
- Ablation or Embolization Treatments for Pancreatic Cancer
- Radiation Therapy for Pancreatic Cancer
- Chemotherapy for Pancreatic Cancer
- Targeted Therapy for Pancreatic Cancer
- Immunotherapy for Pancreatic Cancer
- Pain Control for Pancreatic Cancer
- Treating Pancreatic Cancer, Based on Extent of the Cancer
- Living as a Pancreatic Cancer Survivor
- Second Cancers After Pancreatic Cancer
- lf You Have Pancreatic Cancer
It’s important to have honest, open discussions with your cancer care team. They want to answer all your questions, so that you can make informed treatment and life decisions. Here are some questions to consider.
When you’re told you have pancreatic cancer
When deciding on a treatment plan, during treatment, after treatment.
- What kind of pancreatic cancer do I have?
- Has my cancer spread beyond the pancreas?
- Is the tumor resectable (removable by surgery)?
- Will I need any other tests before we can decide on treatment?
- Do I need to see any other doctors or health professionals?
- If I’m concerned about the costs and insurance coverage for my diagnosis and treatment, who can help me?
- What are my treatment options ?
- What do you recommend and why?
- How quickly do we need to decide on treatment?
- How much experience do you have treating this type of cancer?
- What is the goal of each treatment?
- Should I get a second opinion ? How do I do that? Can you recommend a doctor or cancer center?
- Should I think about taking part in a clinical trial ?
- What should I do to be ready for treatment?
- How long will treatment last? What will it be like? Where will it be done?
- What risks or side effects should I expect? How long are they likely to last?
- Will treatment affect my daily activities?
Once treatment begins, you’ll need to know what to expect and what to look for. Not all of these questions may apply to you, but asking the ones that do may be helpful.
- How will we know if the treatment is working?
- Is there anything I can do to help manage side effects ?
- What symptoms or side effects should I tell you about right away?
- How can I reach you on nights, holidays, or weekends?
- Do I need to change what I eat during treatment?
- Are there any limits on what I can do?
- Can I exercise during treatment? If so, what kind should I do, and how often?
- Can you suggest a mental health professional I can see if I start to feel overwhelmed, depressed, or distressed ?
- What if I need social support during treatment because my family lives far away?
- What type of follow-up will I need after treatment?
- Do I need a special diet after treatment?
- How much and what of exercise should I do?
- How will we know if the cancer has come back? What should I watch for?
- What will my options be if the cancer comes back?
Along with these sample questions, be sure to write down some of your own. For instance, you might want more information about recovery times. You may also want to ask about clinical trials for which you may qualify.
Keep in mind that doctors aren’t the only ones who can give you information. Other health care professionals, such as nurses and social workers, can answer some of your questions. To find out more about speaking with your health care team, see The Doctor-Patient Relationship .

The American Cancer Society medical and editorial content team
Our team is made up of doctors and oncology certified nurses with deep knowledge of cancer care as well as editors and translators with extensive experience in medical writing.
Last Revised: February 5, 2024
American Cancer Society medical information is copyrighted material. For reprint requests, please see our Content Usage Policy .
American Cancer Society Emails
Sign up to stay up-to-date with news, valuable information, and ways to get involved with the American Cancer Society.
More in Pancreatic Cancer
- About Pancreatic Cancer
- Causes, Risk Factors, and Prevention
- Early Detection, Diagnosis, and Staging
- After Treatment
Help us end cancer as we know it, for everyone.

If this was helpful, donate to help fund patient support services, research, and cancer content updates.
You need to enable JavaScript to run this app.
- Gift Baskets
- Children's Books
- The Grief Library
- The Hope Library
- Hope & Teenagers
- Eulogy Examples
- The Hope Kit Podcast
- The Hope Kit Mission, Vision, & Values.
- My Life - Jason Clawson
- instagram Instagram

Navigating Life After a Pancreatic Cancer Diagnosis: Emotional and Practical Steps
Receiving a diagnosis of pancreatic cancer can be overwhelming, leaving you grappling with a whirlwind of emotions, questions, and practical concerns. it’s important to remember that while this journey is undoubtedly challenging, there are steps you can take—both emotionally and practically—to navigate the road ahead with resilience and hope., understanding your emotions.
Emotional Reactions: Frustration and Anger
It’s natural to experience a spectrum of emotions, including frustration and anger. These feelings may arise from a sense of helplessness or fear about the future. Allow yourself to feel these emotions without judgment; they are a normal part of processing your diagnosis. Consider these strategies to help manage these feelings:
- Express Yourself : Journaling or speaking with trusted friends or family can help you articulate your feelings. This act of expression can be cathartic and provide clarity.
- Find a Support Group : Connecting with others who are experiencing similar challenges can offer comfort and understanding. Look for local or online support groups specifically for pancreatic cancer patients.
Seeking Professional Help: Therapists and Counselors
Professional guidance can be invaluable during this time. Therapists and counselors trained in oncology can provide you with coping strategies tailored to your situation. Here’s how to find the right support:
- Look for Specialization : Seek out therapists who have experience working with cancer patients. They can help you navigate the emotional landscape and develop coping mechanisms.
- Consider Family Counseling : Cancer affects not just the individual, but also family dynamics. Family counseling can help loved ones process their emotions and communicate effectively.
Preparing for Surgery: Practical Steps with Optimism
If surgery is part of your treatment plan, preparation is key. Here are practical steps to help you approach this with a sense of optimism:
- Educate Yourself : Understanding your surgical procedure can reduce anxiety. Ask your oncologist detailed questions about what to expect before, during, and after surgery.
- Plan Your Recovery : Preparing your home for post-op care can ease the transition. Arrange for assistance with daily tasks, stock up on necessary supplies, and create a comfortable recovery space.
- Visualize Success : Embrace a positive mindset by visualizing a successful surgery and recovery. Techniques such as guided imagery can help foster a sense of control and optimism.
Post-Operative Care: Nurturing Your Well-Being
The period following surgery is crucial for your healing journey. Here are some practical tips to support your recovery:
- Follow Medical Advice : Adhere to your medical team’s guidelines regarding diet, activity levels, and medication. Your doctors are there to support your recovery.
- Prioritize Self-Care : Engage in activities that bring you joy and comfort, whether it’s reading, listening to music, or spending time with loved ones. Allow yourself the time and space to heal.
- Stay Connected : Maintain open communication with your healthcare team. Don’t hesitate to reach out if you have questions or concerns about your recovery.
Embracing a Supportive Community
Remember, you don’t have to navigate this journey alone. Lean on your support network of friends, family, and healthcare professionals. Surround yourself with positivity, and allow the love and care of those around you to uplift you.
In the face of uncertainty, cultivating a spirit of hope and resilience can empower you. Each step you take, whether emotional or practical, contributes to your healing journey. Embrace the support available to you, cherish the moments of joy, and approach the future with optimism. You are not defined by your diagnosis; you are a courageous individual navigating a challenging path, and there is strength in seeking help and support.
You may also like

IMAGES
VIDEO
COMMENTS
By Alison Lee Satake How cancer spreads or metastasizes is a big question for cancer researchers and patients. Mayo Clinic researchers studying pancreatic cancer — the third deadliest form of cancer in the U.S. — recently made a discovery that advances knowledge of how metastasis unfolds.
Pancreatic cancer is the fourth most common cause of cancer death in the United States. While great strides are being made in the search and treatment of pancreatic cancer, there are still many questions about the complex disease. Jin He, M.D., Ph.D., surgical oncologist, provided responses to frequently asked questions about pancreatic cancer.
Find what's new in pancreatic cancer research, including current progress in treatment with stroma modifying drugs and immunotherapy. Selected NCI-supported programs that address pancreatic cancer are also described.
Recently, the development of more effective treatment regimens has opened up new treatment strategies, but urgent research questions have also become apparent. This review outlines the current management of pancreatic cancer, and the recent advances in its treatment.
Find research articles on pancreatic cancer, which may include news stories, clinical trials, blog posts, and descriptions of active studies.
Core Tip: Pancreatic cancer is still a highly lethal gastrointestinal cancer with a low 5-year survival rate and difficulty in early detection. A comprehensive understanding of the risk factors for pancreatic cancer is of great clinical significance for effective prevention of pancreatic cancer.
This review aims to summarize the latest knowledge on factors, diagnosis, and treatment of pancreatic cancer, and aims to promote further research on this under-studied malignant tumor. At present, we urgently need to identify high-risk patients with ...
Research: Increase levels of research and funding for pancreatic cancer There are still many unanswered questions about the biology and aetiology of pancreatic cancer; in particular, the discovery of biomarkers that will aid earlier detection of pancreatic cancer and a more precise definition/understanding of "high-risk populations."
Pancreatic cancer is a disease in which malignant cells originate in the pancreatic tissue. Cancer of the exocrine component of the pancreas (adenocarcinomas) represents the majority of pancreatic ...
Finally, we discuss the future of pancreatic cancer research and areas for improvement in the next decade. The incidence of pancreatic ductal adenocarcinoma (PDAC) is rising.
Treatment A lot of research is focused on finding better treatments for pancreatic cancer. Improving surgery and radiation therapy are major goals, as is determining the best combination of treatments for people with certain stages of cancer.
For the first time in more than 10 years, the FDA has approved a new first-line treatment, called NALIRIFOX, for patients with metastatic pancreatic cancer.
We passionately pursue pancreatic cancer research from every angle. The unifying hypothesis that forms the basis for our research at Johns Hopkins is that pancreatic cancer is fundamentally a disease of mutations in cancer-associated genes. Our efforts began in earnest in 1991 when a surgeon (Charles Yeo) teamed with a pathologist (Ralph Hruban ...
But the tides are turning, thanks to new and improved treatment methods that are helping people with pancreatic cancer live longer. Dr. Truty and Robert McWilliams, M.D., a medical oncologist at Mayo Clinic, talk about Mayo Clinic's approach to pancreatic cancer care, and how it's leading to improved survival and quality of life.
Pancreatic cancer is a notoriously lethal condition characterised by aggressive malignancy and dismal outcomes. However, translational advances are showing us that hope is on the horizon.
What is research? Research is the process of investigating to solve a problem, increase knowledge or create something new. Medical research examines the causes, diagnosis, and treatment of disease. In the case of pancreatic cancer research, it aims to discover why the disease happens and how to prevent it, the signs and symptoms, best treatments and how to improve quality of life for patients ...
10 Questions to Ask About Clinical Trials. by Jennifer Kennedy — Jan 23, 2017. Pancreatic cancer patients who participate in clinical research have better outcomes. Every treatment available today was approved through a clinical trial. The Pancreatic Cancer Action Network strongly recommends clinical trials at diagnosis and during every ...
Despite progress significant advances in immunotherapy for some solid tumors, pancreatic ductal adenocarcinoma (PDAC) remains unresponsive poorly responsive to such interventions, largely due to its highly immunosuppressive tumor microenvironment (TME) with limited CD8 + T cell infiltration. This study explores the role of the epigenetic factor Sin3B in the PDAC TME.
Abstract. Our knowledge of the origins, heterogeneity, and functions of cancer-associated fibroblasts (CAF) in pancreatic ductal adenocarcinoma (PDAC) has exponentially increased over the last two decades. This has been facilitated by the implementation of new models and single-cell technologies. However, a few key studies preceded the current exciting times in CAF research and were ...
This guide from Memorial Sloan Kettering (MSK) can help answer your questions about pancreatic cancer. It's based on MSK's strong expertise in treating pancreatic cancer and our credentials as a National Pancreas Foundation Center of Excellence.
What Questions Should I Ask My Doctor? If you are in the midst of dealing with pancreatic cancer, you have a lot on your mind and you may have difficulty knowing where to start. Since every patient has a unique case, your doctors are your best source of information and you have every right to ask them questions.
Pancreatic cancer — Overview covers symptoms, risk factors, prevention, diagnosis, surgery, chemotherapy and other treatment for cancer of the pancreas.
The year 2022 was notable for substantial research progress related to pancreatic ductal adenocarcinoma (PDAC). The first single-cell and spatial transcriptomic atlases of PDAC were reported, a ...
A pancreatic cancer diagnosis can be overwhelming. See what you should ask your doctor so you can have the information you need to make the best decisions.
Questions to Ask About Pancreatic Cancer It's important to have honest, open discussions with your cancer care team. They want to answer all your questions, so that you can make informed treatment and life decisions. Here are some questions to consider.
City of Hope's original campus in Duarte took care of entrepreneur and philanthropist Emmet Stephenson Jr.'s wife Toni when she was diagnosed with lymphoma in 2013.The cancer treatment and research center helped cure her before they were blindsided by pancreatic cancer, an even deadlier form of cancer, seven years later.
Have other known active cancer(s) likely to require treatment in the next 2 years; Had prior radiotherapy or systemic treatment for PDAC; Active, uncontrolled bacterial, viral, or fungal infection(s) requiring systemic antiinfective therapy that has been administered less than 2 weeks prior to the first dose of BNT321
Receiving a diagnosis of pancreatic cancer can be overwhelming, leaving you grappling with a whirlwind of emotions, questions, and practical concerns. It's important to remember that while this journey is undoubtedly challenging, there are steps you can take—both emotionally and practically—to navigate the road ahead with resilience and hope. Understanding Your Emotions Emotional ...
A roundup of notable gifts compiled by the Chronicle:. City of Hope. Emmet Stephenson Jr. and his daughter, Tessa Stephenson Brand, pledged $150 million to establish the Stephenson Prize, a $1 million annual award to a scientist or team of scientists making the most promising advancements in pancreatic cancer research and treatments. The prize is open to researchers around the world; the first ...