- Open access
- Published: 06 September 2022

Browning of the white adipose tissue regulation: new insights into nutritional and metabolic relevance in health and diseases
- Sabrina Azevedo Machado 1 na1 ,
- Gabriel Pasquarelli-do-Nascimento 1 na1 ,
- Debora Santos da Silva 1 ,
- Gabriel Ribeiro Farias 1 ,
- Igor de Oliveira Santos 1 ,
- Luana Borges Baptista 1 &
- Kelly Grace Magalhães ORCID: orcid.org/0000-0002-7435-5272 1
Nutrition & Metabolism volume 19 , Article number: 61 ( 2022 ) Cite this article
18k Accesses
52 Citations
46 Altmetric
Metrics details
Adipose tissues are dynamic tissues that play crucial physiological roles in maintaining health and homeostasis. Although white adipose tissue and brown adipose tissue are currently considered key endocrine organs, they differ functionally and morphologically. The existence of the beige or brite adipocytes, cells displaying intermediary characteristics between white and brown adipocytes, illustrates the plastic nature of the adipose tissue. These cells are generated through white adipose tissue browning, a process associated with augmented non-shivering thermogenesis and metabolic capacity. This process involves the upregulation of the uncoupling protein 1, a molecule that uncouples the respiratory chain from Adenosine triphosphate synthesis, producing heat. β-3 adrenergic receptor system is one important mediator of white adipose tissue browning, during cold exposure. Surprisingly, hyperthermia may also induce beige activation and white adipose tissue beiging. Physical exercising copes with increased levels of specific molecules, including Beta-Aminoisobutyric acid, irisin, and Fibroblast growth factor 21 (FGF21), which induce adipose tissue browning. FGF21 is a stress-responsive hormone that interacts with beta-klotho. The central roles played by hormones in the browning process highlight the relevance of the individual lifestyle, including circadian rhythm and diet. Circadian rhythm involves the sleep–wake cycle and is regulated by melatonin, a hormone associated with UCP1 level upregulation. In contrast to the pro-inflammatory and adipose tissue disrupting effects of the western diet, specific food items, including capsaicin and n-3 polyunsaturated fatty acids, and dietary interventions such as calorie restriction and intermittent fasting, favor white adipose tissue browning and metabolic efficiency. The intestinal microbiome has also been pictured as a key factor in regulating white tissue browning, as it modulates bile acid levels, important molecules for the thermogenic program activation. During embryogenesis, in which adipose tissue formation is affected by Bone morphogenetic proteins that regulate gene expression, the stimuli herein discussed influence an orchestra of gene expression regulators, including a plethora of transcription factors, and chromatin remodeling enzymes, and non-coding RNAs. Considering the detrimental effects of adipose tissue browning and the disparities between adipose tissue characteristics in mice and humans, further efforts will benefit a better understanding of adipose tissue plasticity biology and its applicability to managing the overwhelming burden of several chronic diseases.
The adipose tissues (ATs) are endocrine and dynamic organs that display high morphological and functional plasticity. White AT (WAT) was named that way because it presents white adipocytes in its composition. In contrast, brown AT (BAT) has as its main integrant the brown adipocytes [ 1 ]. Both ATs play various physiological roles, including energy storage, endocrine regulation, and thermogenesis. As a means of adapting, mammalians developed a mechanism to maintain their body temperatures under unfavorable climates [ 2 ]. This process, called adaptative thermogenesis, occurs due to the elevated plasticity of ATs, which allows reversible changes in their morphology and functions [ 3 ]. Studies have shown the capacity of progenitor cells as well as mature adipocytes to differentiate into a model that presents similarities with the brown profile, called brite or beige AT [ 4 , 5 , 6 ]. When this phenomenon occurs in mature adipocytes it is called browning of WAT [ 4 ].
During the browning process, WAT show increased mitochondrial number, augmented energy expenditure, fat multilocularization, and thermogenic genes expressions, such as Peroxisome proliferator-activated receptor-a (PPARα) and PPARγ, PR domain containing 16 (PRDM16), peroxisome proliferator-activated receptor-gamma coactivator 1 a (PGC1-α), cell death-inducing DNA fragmentation factor-like effector A (CIDEA), and Uncoupling Protein 1 (UCP1) [ 7 , 8 , 9 ]. WAT browning occurs in specific conditions through exposure to certain stimuli such as cold, thyroid hormones, diet, natriuretic peptides, medication, and exercise [ 10 , 11 , 12 , 13 , 14 ] (Fig. 1 ).
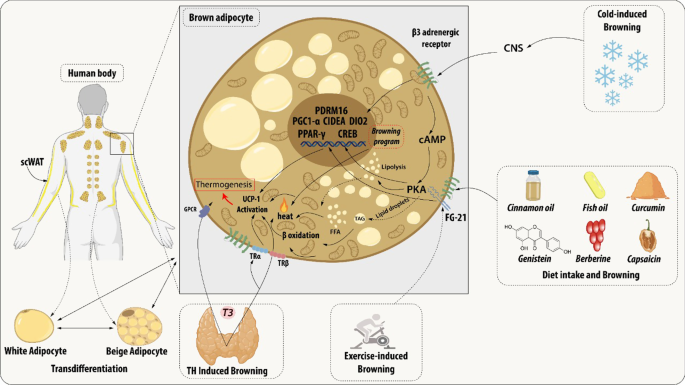
Brown adipocyte regulation by exogenous agents. Thyroid hormones act on thermogenesis through interaction with their Thyroid receptors (TR) and the G-protein-coupled receptor (GPCR). TRα promotes an increase in adrenergic signaling while TRβ acts to stimulate uncoupling protein 1 (UCP1). β3 receptor (β3-AR) is expressed constitutively on the surface of the adipocyte and acts to regulate the transcription and activation of genes related to mitochondrial biogenesis, brown adipocyte differentiation, and lipid storage. This receptor can be activated through cold, which is the main mechanism for activating browning, agonist drugs, or diet. Chronic exposure to cold and food intake, such as curcumin and fish oil, promotes thermogenesis by releasing catecholamines from the central nervous system (CNS) that bind to β-AR, thus initiating a signaling cascade. An increase in the concentration of cAMP is elicited which consequently leads to the enhanced activity of protein kinase A (PKA) which promotes the cAMP-response element-binding protein (CREB). This pathway is related to the transcription of thermogenic genes such as peroxisome proliferator-activated receptor γ (PPARγ), Type II iodothyronine deiodinase (DIO2), PR domain containing 16 (PRDM16), Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and Cell death-inducing DNA fragmentation factor-like effector A (CIDEA). The other dietary components can participate in the induction of browning through the modulation of the gut microbiota promoting the increase of the expression of thermogenic genes. The release of growth factor 21 (FGF21) is mediated by the practice of physical exercises and physiological changes. FGF21 will interact with its FGFR receptor and that activation induces a self-phosphorylation of the FGFR that mediates the activation of pathways related to increased expression of UCP1
The increase in energy expenditure can act as a therapeutic approach to metabolic syndrome, and also can be associated with poor prognosis of diseases associated with hypermetabolism [ 15 , 16 , 17 , 18 ]. For this reason, efforts have been employed to identify the key participants in the regulation of browning. This review aims to present and describe the current studies related to both endogenous and exogenous most relevant agents and their biological mechanisms at biochemical and molecular levels.
Introduction
Originally cited as energy storage organs, exclusively, ATs are currently known to express and secrete a variety of bioactive peptides, the adipokines, including leptin, resistin, vaspin, visfatin, hepcidin, adiponectin, and inflammatory cytokines. These bioactive secreted factors act both locally and systematically, modulating different biological processes and consequently influencing the metabolism of various organs, such as the liver, muscle, pancreas, and brain via endocrine mechanisms [ 19 , 20 ]. Besides adipocytes, AT contains an extracellular matrix, nerve tissue, stromovascular cells, and immune cells, which together act as an integrated unit [ 21 ].
Presently, two main subtypes of ATs have been described: WAT and BAT. Brown and white adipocytes have widely different morphologies, not only in terms of composition but also in the form of lipid storage (number and size of lipid droplets) and the disposition and number of mitochondria. These differences correspond to distinct functional roles, diverging in energy metabolism, storage, and distribution [ 19 , 22 ].
WAT, the most abundant AT in the body, contains the white adipocytes, which present unilocular lipid droplets, scarce mitochondria, and lipid storage capacity [ 23 ]. Since the discovery of the adipokines, WAT is also recognized as an important endocrine organ, actively participating in the regulation of physiologic and pathologic processes, including immunity and inflammation [ 24 , 25 ]. Widely distributed throughout the body, there are two main representative types of WATs, the visceral WAT (vWAT) and the subcutaneous WAT (scWAT). While one is distributed around organs and provides protective padding, the other is located under the skin and provides insulation against heat or cold, respectively [ 26 ].
In contrast, brown adipocytes display multilocular lipid droplets, a large number of mitochondria, and thermogenic capacity due to elevated uncoupling protein 1 (UCP1) amounts anchored in its mitochondrial inner membrane [ 27 ]. The BAT utilizes this high mitochondrial content and elevated UCP1 amounts to uncouple oxidative phosphorylation from adenosine triphosphate (ATP) synthesis to dissipate chemical energy as heat [ 28 ]. Thus, BAT affects the metabolism of the entire body, being able to alter insulin sensitivity and modify the susceptibility to increase weight. For a long time, BAT was only considered an energy-producing organ in rodents and newborns, undergoing involution with age. However, BAT has also been identified in human adults near the aorta and within the supraclavicular region of the neck. Nevertheless, the origin of BAT is still under debate [ 26 , 29 ].
Recently, a type of AT showing intermediary characteristics between that of white and brown adipocytes, which has mixed structural features of both, was identified as beige AT [ 29 ]. This type of AT was reported as a set of adipocytes in WAT that might acquire a thermogenic phenotype with higher UCP1 expression, similar to brown adipocytes after enough stimulus [ 29 , 30 ].
There are two major mechanisms described related to beige cells arising: d e novo differentiation which occurs from a progenitor resident cell and transdifferentiation which consist of differentiation of a mature white adipocyte through a molecular mechanism. The first theory is based on that beige adipocytes come from progenitor cells differentiation induced by adipogenic stimulation such as cold exposure, adrenergic signaling, exercise, natriuretic peptides, thyroid hormones, diets, and food components [ 31 , 32 ]. Currently, several specific cell markers were identified in various types of progenitor cells such as in smooth muscle-like cells (Myh11 + ), preadipocytes (Pdgfrb + , SMA + ), adipocytes progenitor cells (Sca-1 + Pdgfra + CD81 + ) [ 33 , 34 , 35 ]. These adipogenic stimulation actives transcriptional machinery of browning that is characterized by the expression of Ucp1, Prdm16, Zfp516, and Pgc1a genes that will promote a beige differentiation [ 36 ].
On the other hand, the transdifferentiation hypothesis proposes beige cells arise from mature white adipocytes, in a reversible process, after adipogenic stimulus without the participation of a progenitor-like state of cells [ 37 ]. The underlying molecular mechanisms for transdifferentiation are under intensive research, but some studies already show that this plasticity process occurs mainly in scWAT depots [ 29 ]. Known as browning, this process has gained increasing attention in the research area as an alternative method of energy stimulation. UCP1 expression can be stimulated when white adipocytes are exposed to stimuli, previously referred as to adipogenic stimulus, [ 20 , 27 , 29 ], driven by a set of molecules known as browning markers.
The uncoupling protein 1 (UCP-1)
The non-shivering thermogenesis is a phenomenon that occurs in brown and beige ATs due mostly to the action of UCP1 [ 38 ]. UCPs are transmembrane proteins that belong to the mitochondrial anion carrier family (MACF), i.e., mediate specific metabolite exchanges between the cell cytoplasm and the mitochondrial matrix and thus enable the activation of essential biochemical pathways [ 39 , 40 ]. The UCPs exhibit 5 isoforms, ranging from UCP1 to UCP5 are present in several tissues [ 41 , 42 ]. UCP1 is the main isoform associated with thermogenesis, it is widely and selectively expressed in the inner mitochondrial membrane of the adipocyte, representing about 10% of the total mitochondrial protein in human epicardial AT [ 43 , 44 , 45 , 46 ].
UCP1 protein is described as participating in thermogenesis by interfering in proton leakage within the chemiosmotic gradient during the mitochondrial oxidative phosphorylation by the translocating fatty acids (FAs). This gradient is obtained from the oxidation of substrates and provides the required force to induce the respiratory machinery to produce ATP. Once UCP1 promotes proton leakage, the energy obtained cannot be stored in the form of ATP and is alternatively dissipated as heat [ 47 , 48 ]. Thus, it is evident that direct regulation of UCP1 protein activity is one of the means of regulating thermogenesis, and that occurs in opposite ways by cytosolic purine nucleotides and long-chain fatty acids (LCFA), promoting inhibition or activation of UCP1, respectively [ 49 ].
The other form of regulating UCP1 is at the transcriptional level. UCP1 gene is transcribed only in brown and beige adipocytes, associates with the differentiation state of these cells, and is quantitatively regulated in response to many physiological signals [ 9 ]. These characteristics are consequences of the transcriptional control mediated by trans-acting factors on regulatory regions found in the 5’ non-coding region of the UCP1 gene. The proximal regulatory region, which is found immediately upstream of the transcription start site, contains cAMP response element-binding protein (CREB) [ 50 , 51 ] and CCAAT-enhancer-binding protein (C/EBP) [ 52 ] binding sites. Also in the proximity of the site of transcription start, activating transcription factor-2 (ATF2)-binding site interacts with transcriptional coregulators, such as PGC-1α, impacting UCP1 gene transcription [ 9 ]. In opposition to these proximal regulatory sites, a strong enhancer region is placed more than 2 kb upstream of the transcription initiation site [ 50 , 53 ] and contains a cluster of response elements for nuclear hormone receptors [ 7 , 54 ].
UCP1 gene activation and repression depend on which trans-acting factors bind to the regulatory region. For example, CREB binding sites mediate a positive transcriptional response to cAMP [ 50 ] and a negative response to AP2 (c-Jun/c- Fos) complexes [ 51 ]. Another example is the PPARγ binding site found in the distal enhancer region, which associates with gene activation after binding to its main ligand but represses UCP1 transcription when interacting with liver X receptor (LXR) and its corepressor receptor-interacting protein 140 (RIP140) [ 55 ]. RIP140 inhibits UCP1 gene transcription by enabling the assembly of DNA and histone methyltransferases on the UCP1 gene, altering the methylation status of CpG islands in the promoter region and histones, impacting gene expression through transcription machinery accessibility [ 56 ].
Although some epigenetic modifications are associated with repressed UCP1 gene expression, as in H3K9 demethylation marks, chromatin modifications indicative of activation of this gene also occur, such as in the case of H3K4 trimethylated marks, which are enriched in BAT [ 57 ]. Also participating in fine-tuning of gene expression, microRNAs (miRs) are characterized to be a group of short non-coding RNAs (ncRNAs) generated by the sequential processing of longer ribonucleic acid molecules [ 58 ]. While miR-328 [ 59 ] and miR-455 [ 60 ] are described to be activators of UCP1 gene expression, miR-27 [ 61 , 62 ], and miR-133 [ 63 ] display UCP1 gene transcription inhibitory activity .
The roles of WAT and BAT in metabolic syndrome is well characterized, but the physiological and biochemical modulations of BAT remain unclear [ 64 , 65 , 66 ]. Several studies showed that UCP1-dependent BAT activity was mostly found to be beneficial in decreasing inflammation, and improving cardiometabolic homeostasis [ 67 , 68 , 69 ]. However, this tissue has a lower activity in obese in comparison to healthy individuals [ 70 ].
It is well established that the deficiency of the UCP1 gene is not enough to protect against diet-induced obesity (DIO), but can modulate important physiological and metabolic parameters in mice [ 64 , 65 ]. The food intake-induced browning is inhibited in the absence of UCP1, demonstrating the intimate relationship between this differentiation process and UCP1 [ 71 , 72 ]. More than that, UCP1 -/- female mice fed with a western diet displayed increased whitening of BAT, as well as metabolic disruption indicated by glucose intolerance, upregulation of genes related to inflammation, liver steatosis, immune cell infiltration, and endoplasmic reticulum (ER)/oxidative stress [ 73 ]. Accordingly, even during standard diet under cold exposure, UCP1−/−, male mice showed BAT immune cell infiltration and ER stress profile [ 74 ]. The lack of UCP1 promotes de novo lipogenesis and hyperplasia of inguinal WAT, leading to an increase in FA trafficking to the liver [ 75 ].
In contrast, the upregulation of UCP1 or even only its activation can perform a paradoxical role in hypermetabolic scenarios and associate with a worse prognosis [ 76 , 77 ]. It is proven that diet-induced whitening is related to the upregulation of this gene. A greater expression of browning markers (e.g., UCP1, PGC-1α, TBX1) was found in obese human patients mainly in vWAT [ 78 ] Besides that, mice affected by cancer-associated cachexia tend to show increased thermogenesis gene expression and BAT activation [ 79 ].
The browning process is spontaneously induced by tumor-secreted factors and IL-6 during cachexia development, which can lead to full depletion of AT [ 80 , 81 ]. Interestingly, the elicitation of browning after burn injury is associated with the hypermetabolic response, as well as an increase in lipolysis and free fatty acid efflux that can outcome in liver steatosis [ 82 , 83 ]. In addition to the therapeutic impact of the browning process in obesity and metabolic diseases, recent discoveries regarding the impact of UCP1-dependent BAT activity in hypermetabolism conditions should be further investigated in the context of UCP1 to appropriately regulate browning for application in different situations.
Beta 3 adrenergic receptor activation
Increasing energy expenditure through activation of BAT shows potential for treating metabolic diseases, and that is the reason this approach has been deeply investigated [ 84 ]. The white/brown plasticity of ATs and tissue thermogenesis appear to be activated by a β-3 adrenergic receptor (β-3AR) system [ 4 , 30 , 85 ]. β3-ARs are expressed predominantly on white and brown adipocytes [ 86 ]. Murine WAT expresses β3-AR transcripts in a greater proportion compared to other β-ARs, similar to BAT [ 87 ]. Although β3-AR mRNA levels are lower in humans than in rodent AT, its roles seem to be fundamental in the regulation of energy balance and glucose homeostasis [ 88 ].
Browning of WAT occurs mainly by noradrenaline and adrenaline stimulation, which influence lipolysis after binding to different adrenoceptor subtypes on the cell-surface membrane of fat cells. The interaction with β3-AR initiates a cascade of signal transduction that ends with the overexpression of thermogenic proteins, such as UCP-1 [ 88 , 89 ]. The adaptive thermogenic response is initiated by the central (CNS) and sympathetic (SNS) nervous systems with the release of norepinephrine (NE) and stimulation of β3-AR, through the G protein-coupled receptor Gs, which in turn activates the adenylyl cyclase (AC), stimulating the production of cyclic adenosine monophosphate (cAMP), and activating the protein kinase A (PKA) pathway. Then, these signals from the cAMP pathway, finally, upregulate UCP-1 and lipolysis [ 88 , 89 , 90 , 91 , 92 ].
A distinguishing feature of the β3-AR, already seen in past studies, is that it appears to be relatively resistant to desensitization and down-regulation, leading to the hypothesis that one of its functions might be to maintain signaling during periods of sustained sympathetic stimulation, as in diet-associated β3-AR activation or cold exposure [ 87 ]. Cold temperature exposure elicits a coordinated physiological response aimed at maintaining their body temperature. This response activates the mentioned cascade and generates heat in beige adipocytes within scWAT and BAT [ 85 ]. Thus, it was seen that mice with a combined target disruption of the three β1, β2, and β3 adrenergic receptors (TKO mice) have increased susceptibility to cold-induced hypothermia as well as diet-induced obesity [ 91 ]. Thereby, mice β3-AR activation started to be studied, effectively mimicking cold exposure effects [ 84 , 91 ].
Initial studies demonstrated that WAT UCP1 mRNA and protein levels are strongly decreased in β3-AR knockout (KO) mice [ 30 , 93 ]. In addition, β3-AR agonists are well-known for inducing ectopic UCP1 expression in WAT coupled with a significant mitochondrial enhancement in rodents, and for augmenting glucose homeostatic activity of their BAT [ 84 , 94 ]. On the other hand, in humans, early efforts to increase browning activation with the use of β3-adrenoreceptor agonists have failed in clinical trials because of their β1- and β2-AR-mediated cardiovascular effects [ 13 , 84 , 94 ]. However, a recent study showed that mirabegron, a selective β3-agonist previously developed for the treatment of overactive bladder, was shown to increase BAT activity as compared to placebo. This study used an oral dose of 200 mg in healthy male subjects, and despite not having severe cardiovascular side effects, they have been shown to increase heart rate and systolic blood pressure [ 84 ]. That is the reason long-term studies are warranted to investigate the effectiveness and cardiovascular safety of this type of treatment to induce weight loss and metabolic health improvements.
Genetic factors must be considered in influencing adipocyte lipolysis regulation. Genetic variance in β3-AR and its specific G-coupling protein has functional effects on lipolysis. Polymorphism in the G-β3 gene, for example, influences catecholamine-induced lipolysis in human fat cells by altering the coupling of β3-AR to G-proteins [ 88 ]. This proves once again the importance of the β3-AR presence for the thermogenic process.
Temperature-induced browning
It is well established that temperature can modulate biochemical, inflammatory, and immunological processes systemically, displaying relevant physiological impact [ 95 , 96 ]. Despite this, the influence of warmer temperatures is better described compared to cold conditions due to their immediate danger. Fever, triggered by infectious and inflammatory processes, was associated with a worse prognosis in the past centuries, demanding greater medical attention for a long time [ 97 ]. However, currently, it is recognized that both hyper and hypothermia, in properly regulated circumstances, are beneficial response mechanisms to infection in mild and severe profiles, respectively [ 98 ].
Hypothermia is also associated with an advantageous mechanism against severe systemic inflammation. In experimental studies, the infectious or aseptic systemic inflammation process is elicited by the intravenous administration of bacterial lipopolysaccharide (LPS) in mice [ 99 , 100 , 101 ]. The variances of body temperature are modulated by the environmental temperature and concentration of LPS introduced [ 102 ]. Animals housed in hyperthermal conditions or exposed to lower LPS concentrations displayed polyphasic fever. In thermoneutral conditions, the fever was also usually elicited to induce the immunological response. However, if the mice were housed in cooler acclimation or administered with higher LPS concentrations, the effect elicited was hypothermia, which associates with arterial hypotension aimed to avoid infection spread, followed by polyphasic fever.
The ideal body temperature is obtained by the modulation of blood vessel tension degree, and heat production by thermogenesis. Cutaneous vasoconstriction and thermogenesis processes occur to increase body temperature and avoid heat loss. Conversely, the opposite effect, skin vasodilatation and thermogenesis inhibition, is stimulated to induce hypothermia [ 102 , 103 , 104 , 105 ].
Temperature is a paradoxical agent with important roles not only in biological events but also in the development of several diseases [ 106 , 107 , 108 , 109 , 110 ]. Hypothermia, specifically, displays a typical profile, in which the energy is preserved. The decrease in body temperature also favors the development of an anti-inflammatory profile and immunosuppression, which can act as a double-edged sword depending on the condition [ 108 , 111 , 112 , 113 ]. In the tumoral context, hypothermia provides an immunosuppressive, hence, pro-tumoral microenvironmental due to impaired CD8+ T cell function, an increase of the regulatory and Th2 cells, and higher levels of the cytokines IL-4 and IL-10, which increase cancer progression and metastasis [ 114 , 115 , 116 ].On the other hand, hyperthermia is recognized to elicit a more robust immune response against infection, injury, and cancer [ 117 ]. Several studies reported an increase in IFN-γ and IL-2 secretion from peripheric T cells, enhancement of cytotoxicity, DC maturation, and increase of tumor-specific CD8+ T cells [ 118 , 119 , 120 , 121 ].
In inflammatory conditions, such as neurological damage, atherosclerosis, systemic inflammation, and hypothermia (cryotherapy) can be beneficial [ 122 , 123 , 124 , 125 , 126 ]. Cryotherapy benefits are illustrated by an experimental approach that submitted healthy and physical activity practitioners men to intense exercises aimed to induce muscle injury [ 127 ]. It was observed that cryotherapy mediated the increase of IL-10, reduction of pro-inflammatory cytokine IL-1, reduction of muscle damage and blood cholesterol, decrease oxidative stress and improve the lipid profile not only in healthy patients but also in patients with active-phase ankylosing spondylitis [ 124 , 128 , 129 ]. Cryotherapy also shows to be neuroprotective capacity, alleviating sequelae from ischemic or hemorrhage stroke, cardiac arrest, intracranial pressure elevation, and traumatic brain injury [ 130 ].
In the same line, recent research evaluated the impact of spontaneous body hyperthermia after brain injury. Metabolic modulations were observed as the diminishment of both cerebral and arterial glucose levels and increase of lactate-pyruvate ratio. However, these changes were not associated with a worse prognostic [ 131 ]. Additionally, induced hyperthermia in healthy men promoted an increase in cerebral metabolic rate of oxygen (CMRO 2 ), also increase IL-6 and myeloperoxidase (MPO) systemically, but did not promote the same inflammatory and oxidative phenomenon in the brain [ 132 ]. In peripheral organs such as the liver, hyperthermia is associated with an increase in oxidative metabolism, vasodilation, and an increase of heat shock proteins (HSP) expression. HSP displays an important role in metabolism such as modulation of both glucose and lipid metabolism in the liver and improving the mitochondrial skeletal muscle functionality [ 133 ].
In addition, temperature modulates directly the shivering and non-shivering thermogenesis processes. The occurrence of these events maintains proper body temperature under adverse thermal acclimation. Once shivering thermogenesis is decreased in cold acclimation (around 4 °C), non-shivering thermogenesis is the major way to produce heat in this context [ 2 , 4 ]. The detection of the thermal changes begins with the capture of sensory stimuli by cutaneous thermoreceptors, which promote the sensitization of afferent nerves. The stimuli are directed to the CNS, which then induces thermoregulatory responses, including vasoconstriction and catecholamines secretion. These catecholamines, mainly NE, increase BAT activation, hence heat production through a UCP1-dependent manner [ 134 , 135 ].
BAT is a highly innervated and vascularized organ that displays considerable amounts of β3-ARs, which is also expressed in WAT, though at a lower level. NE binding to β3-ARs promotes systemic adrenergic activation, which induces a signal cascade culminating in the accumulation of adipokines, such as Zinc-α2-glycoprotein (ZAG), increase in thermogenesis-related gene expression, as UCP1, thus enabling mobilization and oxidation of free fatty acids (FFAs) in both tissues, increasing BAT activity and promoting browning in WAT. [ 84 , 136 , 137 ].
It is known that the results observed in humans do not always represent the same effects previously described in mice or even contradictory results can be obtained under similar conditions for the same species [ 138 ]. Unfortunately, this premise can also be applied to cold-induced browning. Leitner and colleagues showed that in human fewer than half of the BAT deposits is stimulated by cold exposure, hence, the thermogenic function was lower than expected [ 44 ].
Brychta and others demonstrated that the profile of men with obesity was associated with a reduced tolerance limit to chill temperatures, suggesting that thermogenesis was diminished in these individuals, as well as energy expenditure [ 139 ]. Blauw et al. [ 140 ] demonstrated a surprising association between the impairment of glycemic homeostasis, diabetes, and obesity in humans housed in the United States of America (USA) at warmer temperatures and associated these results with a decrease in BAT activity. Taking the assessment on a global scale, Kanazawa evaluated the parallel between higher temperatures, weight gain, and obesity. The data analyzed allowed to predict that global warming could be responsible for an increase of more than 10% in the obesity rates in 120 years, counting from 1961 [ 141 ].
Notably, the use of cold as a browning inducer has been carefully applied not only because of the side effects that can be displayed at the whole-body level but also due to the contradictory effect observed in humans. If on one hand, the anti-inflammatory and immunosuppressive role mediated by cold and cold-induced browning is beneficial in healthy individuals, for ill people these same effects may become harmful. In contrast, intriguing research has brought a new perspective on temperature-based browning. Li and colleagues discovered that a hydrogel-based photothermal therapy leads to a successful increase of beige activation in both mice and humans. The therapy consists of increasing the local temperature, around 41˚C, without evident stress on skin or adjacent tissues [ 142 ]. This promising study succeeds previous findings that pointed to the occurrence of WAT browning after burn injury [ 143 , 144 ]. The characterization of possible inducers of browning is a strongly growing field since the applicability of these inducers as therapy in humans has proven to be a major clinical hurdle. However, even under promising advances is clear that further investigation regarding the mechanisms triggered by this stimulus pathway should be conducted.
Exercise-induced browning
Physical exercising is already associated with improvements in several processes related to the cardiovascular system, skeletal muscle, and ATs [ 193 ]. Following this, several studies show that physical activity provides better quality of life [ 194 ] and helps in the treatment of several metabolic diseases and obesity [ 195 ] through increasing AT lipolysis, vascularization, blood flow, and promoting the secretion of hormones and adipokines [ 196 ]. Among the main adipokines, FGF21 (Fibroblast growth factor 21) and leptin stand out, which act in an autocrine/paracrine manner, regulating WAT browning process [ 197 ]. After physical activities, the adipokine leptin stimulates activity in the sympathetic nerve and together with insulin act synergistically in different neuronal subsets of proopiomelanocortin (POMC) inducing browning of WAT through decreased hypothalamic inflammation caused by exercise [ 198 ]. During exercise, the increase in glucagon, which already has thermogenic potential [ 199 ], and the decrease in insulin in the liver lead to FGF21 secretion [ 200 ].
The exercise induces pleiotropic effects in the liver, AT, immune system, and skeletal muscle by enabling myokine secretion upon contraction [ 201 ]. After activities, muscle cells increase the expression of PGC-1α, inducing BAT thermogenesis and mitochondrial biogenesis. Among the myokines involved in the browning process, interleukin-6 (IL-6) is a modulatory cytokine secreted by several tissues, including skeletal muscle and AT. A study showed that mouse AT when treated with IL-6 for 6 h induces the expression of PGC-1α and mitochondrial enzymes [ 202 ]. In addition, analyses showed that IL-6 is involved in the increase of UCP1 mRNA in inguinal WAT (igWAT) stimulated by physical activity [ 203 ]. Another relevant myokine is Irisin; Once exercising increases the expression of PGC-1α, it induces increased levels of the fibronectin domain-containing protein 5 (FNDC5) protein, which, after being cleaved, is released as the hormone irisin [ 204 ]. Irisin was shown to stimulate UCP1 expression and thermogenic differentiation of white fat precursor cells in vitro and in vivo [ 145 ].
The myokine myostatin (Mstn), a growth factor that limits muscle growth and development, is negatively involved in the WAT browning process, as Mstn-deficient mice showed high expression of genes associated with FA oxidation, mitochondrial biogenesis, lipid transport together with the positive regulation of PGC-1α and UCP1, this mechanism occurs through the phosphorylation of AMPK, necessary for the activation of PGC1α and FNDC5 [ 199 ]. Metrnl, the gene encoding for Meteorin-like protein, is a myokine known to be induced by resistance exercise dependently on PGC-1α4. Metrnl regulates genes involved in thermogenesis, as it is capable of promoting the activation of M2 macrophages by inciting the expression of IL-4 and thus triggering the production of catecholamines [ 206 ], responsible for favoring thermogenesis in AT [ 207 ]. Beta-Aminoisobutyric acid is another myokine that has increased levels during exercise and can induce the brown adipocyte phenotype in human-induced pluripotent stem cells during differentiation to mature white adipocytes [ 146 ].
Intense physical activity causes increased heart rate and stretching of cardiomyocytes, which cause the secretion of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), molecules that stimulate lipolysis, UCP1 expression, and mitochondrial biogenesis [ 208 ]. It also induces an increase in lactate, which binds to receptor GRP81 on adipocytes, leads to an increase in P38 phosphorylation, and thus mediates the browning of WAT by activating the PGC-1a, PPAR, γ, and Ucp1 genes [ 147 ]. During lipolysis, FAs are not only used as an energy source but also undergo the re-esterification process where they are converted into triglycerides in AT. This re-esterification consumes ATP generating AMP. AMP in turn can activate AMPK, which then induces greater expression of PGC-1α and mitochondrial biogenesis [ 210 ].Another the important effect induced by exercise that plays an important role in the browning of WAT is oxidative stress in skeletal muscle, whish it responsible for the increase in H 2 O 2 through the reduction of glutathione levels, a molecule capable of supplying electrons to glutathione peroxidase, thus increasing H 2 O 2 levels. And also by increasing the activity of superoxide dismutase 2 (SOD2), which reduces ROS to H 2 O 2 . When H 2 O 2 enters the circulation, it is directed to WAT and subsequently induces the expression of thermogenic genes [ 148 ]. Exercise also increases the level of succinate, resulting in augmented levels of mitochondrial reactive oxygen species, which in turn promotes the sulphenylation of Cys253 to increase UCP1 activity [ 149 ].
Although the studies conducted in mice seem promising, the effect of exercise on WAT browning in humans has proven to be controversial. A survey conducted with sedentary subjects participating in a 12-week bicycle-training program showed scWAT increased expression of UCP1, carnitine palmitoyltransferase 1B (CPT1B), TBX1 [ 15 ]. However, other studies have not achieved similar effects. Tsiloulis and colleagues collected scWAT of obese men after 6 weeks of physical training and the mRNA levels of UCP1, CD137, CITED, TBX1, LHX8, and TCF21 were not altered [ 211 ]. Many factors may be involved in this diversity of results since the duration, frequency, and degree of intensity are associated with these effects. Thus, more human studies need to be conducted as many questions still need to be clarified.
Fibroblast growth factor 21
The fibroblast growth factor family (FGF) performs a range of cellular metabolic and physiological responses to maintain overall homeostasis. FGF 21 was first identified in mice and humans in 2,000 by Nishimura and colleagues through cDNA identification in different organs [ 150 ]. While the gene in mice is located in chromosome 7 and encodes a preprotein of 210 amino acids (aa), in humans it is found in chromosome 19 and encodes a preprotein of 209 aa. Proteins belonging to the FGF family exert a wide range of functions, from promoting cell proliferation and differentiation to systemic effects [ 151 , 152 ], acting as autocrine/paracrine/endocrine factors [ 153 ]. Most FGF family members have a high affinity to heparin sulfate, except for the endocrine FGF (FGF) subgroup, which consists of FGF 19 (FGF 15 in rodents), FGF 21 e FGF 23 in humans [ 151 ]. FGF molecules lack an extracellular heparin-binding domain and thus can enter the blood system [ 154 ].
FGF 21 binds to a fibroblast growth factor tyrosine kinase receptor (FGFR), which can be found in seven isoforms: 1b, 1c, 2b, 2c, 3b, 3c, and 4. The FGF 21 requires its dimerization with a klotho protein, called beta-klotho (KLB). Thus, the FGFR-KLB receptors lead to the intracellular cascade that goes through the phosphorylation of FGFR substrate 2α (FRS2α) and the activation of Ras-MAPKs and PI3K-Akt kinases [ 154 , 155 , 156 ]. Once FGF21 signaling requires KLB to activate FGFRs, the co-expression of these two receptors determines the sensitivity of a tissue or organ to FGF21 [ 157 ]. FGF 21 is defined as a stress-responsive hormone [ 152 ], which effect is subtle in physiological conditions but significantly exacerbated under nutritional, metabolic, oxidative, hormonal, or environmental challenges. Consequently, starvation and overfeeding, ketogenic and high-carbohydrate diets, physical exercises, protein restriction [ 158 ], type 2 diabetes (T2D), obesity [ 153 ], and nonalcoholic fatty liver disease (NAFLD) [ 159 ] can induce the expression or/and signaling of FGF 21 [ 158 ].
FGF 21 is synthesized mainly in the liver and thymus but is also detected in skeletal muscle, pancreas, intestine, heart, β cells, and WAT and BAT [ 160 ]. As an important metabolic regulator, acting mostly in glucose and lipid homeostasis, FGF 21 triggers lipolysis and FFAs released in circulation from WAT during prolonged fasting or starvation [ 29 , 160 ]. PPAR-α is activated in the presence of FFA and improves FFA oxidation and ketone bodies formation for acting as energy sources during prolonged fasting. The interaction between FFA/PPAR-α/retinoid X receptor (RXR) has a PPAR-α response element, which activates FGF 21 promoter [ 161 , 162 ]. Thus, when PPAR-α activity increases, the production of FGF 21 in the liver also augments, leading to energy production, increased ketogenesis, gluconeogenesis, appetite, and systemic glucose uptake as adaptive responses to starvation [ 163 ]. The activity of FGF 21 is not limited to starvation conditions, but it is also increased in adaptation to high-fat (HF) intake [ 164 ].
Human studies inform that FGF21 production is stimulated in situations of decreased thermogenesis, reduction in adiponectin levels, and tissue breakdown markers, such as transaminases elevation mare than changes in levels of FFAs [ 165 ]. Another means of increasing FGF21 levels, through PPAR-α activity, is through intense physical activity, growth hormone therapy, lactation, and milk ingestion in neonates [ 163 , 166 ]. Macronutrients such as proteins also regulate FGF 21 production through amino acid restriction [ 167 ]. This process starts when the general control non-derepressible 2 (GCN2)-eukaryotic initiation factor 2 (eIF2) α pathway is activated inducing the binding of activating transcription factor 4 (ATF4) to PGC-1 α [ 168 , 169 ].
After being secreted, its most important target is WAT, where FGF21 improves insulin sensitivity [ 153 , 160 ] and increments GLUT1 expression and consequently glucose uptake, as shown by in vitro 3T3-L1 adipocyte analyses [ 153 , 170 ]. The response element-binding protein (ChREBP) is sensitive to carbohydrates in the liver and ChREBP interaction with PPAR-γ in adipocytes modulates the expression of FGF21. In other words, the upregulation of ChREBP may induce the expression of this FGF [ 160 ]. Another example of FGF 21 influence on carbohydrate metabolism is through the suppression of hepatic pyruvate dehydrogenase (PD) complex through PD kinase 4 activity [ 171 ]. Additional transcription factors, such as retinoic acid (RA) receptor β (RARβ), TRβ, cyclic AMP response element-binding protein H (CREBH), RA receptor-related orphan receptor α (RORα), respond to determinants in the liver and regulates FGF 21 production [ 151 ].
WAT is not only a target of FGF21, but it is the major mediator of its effects. The processes of glucose- and insulin-sensitive responses depend on adiponectin production and secretion by this tissue [ 172 ]. Adiponectin also reduces the levels of sphingolipid ceramides in obese animals, which have been associated with lipotoxicity [ 173 ]. The action of FGF21 in WAT includes paracrine and autocrine actions and is mediated through the induction of PGC-1α protein in cold and through the enhanced levels of the thermogenic protein UCP1, which is a key protein for heat production [ 174 ]. BAT requires the FGFR1/KLB complex to respond to FGF21, which induces glucose uptake and thermogenesis through the induction of UCP1, in response to its autocrine and paracrine production. FGF 21 impact derives from increased PGC-1α levels and, consequently, expression of UCP1 [ 174 ]. In conclusion, FGF 21 is involved in glucose uptake, lipogenesis, and lipolysis, depending on the metabolic state of the adipocytes. This dual phenomenon may depend on nutritional condition, FGF21 concentrations reached between pharmacological administration and physiological secretion [ 175 ].
Thyroid-hormone-induced browning
Thyroid Hormone (TH) is essential for metabolism in mammals and associates with many processes, including organism development, metabolic regulation, neural differentiation, and growth [ 176 ]. Many genes are regulated after its conversion from the prohormone thyroxine (T4) to the activated form triiodothyronine (T3) [ 177 ] by 5′-deiodinase type 2 (D2), enzyme known to be expressed in the hypothalamus, WAT, BAT, and skeletal muscle, and to be required for adaptive thermogenesis [ 178 ]. TH is produced in the follicles of the thyroid gland and is synthesized through iodination of tyrosine residues in the glycoprotein thyroglobulin [ 179 , 180 ]. The main means of regulator its production is through thyroid-stimulating hormone (TSH), which binds to the TSH receptor (TSH-R) expressed in the thyroid follicular cell basolateral membrane and is released by the anterior pituitary in response to a circulating TH [ 181 ].
The biological response of TH is complex and highly regulated. It is mediated by thyroid hormone nuclear receptors (TRs). The TR genes produce two main types of receptors, α and β, and their isoforms α1, α2, α3, β1, β2, and β3, but only α1, β1, β2, and β3 are T3-binding receptors, which are differentially expressed in tissues and have distinct roles in TH signaling [ 178 , 182 ]. TH enters the cell through membrane proteins monocarboxylate transporter 8 (MCT8) and solute carrier organic anion transporter family member 1C1 9 (OATP1C1), then interacts with TR in the nucleus, which binds to the genomic thyroid-hormone responsive elements (TREs) and other nuclear proteins, including corepressors, coactivators, and cointegrators, leading to chromatin remodeling and the regulation of the UCP1 gene transcription [ 183 , 184 ].
This hormone is correlated with weight and energy expenditure. Thus, hypothyroidism, characterized by diminished TH levels, leads to hypometabolism, a condition associated with reduced resting energy expenditure, weight gain, high cholesterol levels, reduced lipolysis, and gluconeogenesis. On the other hand, hyperthyroidism, and elevated TH levels, induce a hypermetabolic state, characterized by increased resting energy expenditure, lower cholesterol levels, increased lipolysis and gluconeogenesis, and weight loss. Consequently, TH controls energy balance by regulating energy storage and expenditure regulating key metabolic pathways [ 178 ].
TH regulates basal metabolic rate (BMR) through ATP production, used for metabolic processes, and by generating and maintaining ion gradient [ 185 , 186 , 187 ]. BMR is induced by the stimulation of two main gradients, the Na+/K+ gradient across the cell membrane and the Ca 2+ gradient between the cytoplasm and sarcoplasmic reticulum and produces heat during ATP hydrolysis [ 188 ]. TH maintains the BMR levels through the uncoupling oxidative phosphorylation in the mitochondria. When ATP production is compromised in skeletal muscle, TH increases the leak of protons through the mitochondrial inner membrane, stimulating more oxidation to maintain ATP synthesis [ 189 ].
TH regulates metabolism primarily through actions in the brain, WAT, BAT, skeletal muscle, liver, and pancreas [ 178 ]. This action, as already said, is through TH receptors (TR) isoforms, WAT has the adrenergic signaling increased by TRα [ 190 ], otherwise BAT expresses TR α and β, as it needs TRα for adrenergic stimulation and TRβ for stimulating of UCP1, both for thermogenesis [ 176 ]. In humans, T3 administration induced UCP1 expression, dependent on the presence of TRβ, which induces “browning” [ 191 ]. TH regulates several aspects of lipid metabolism and human BAT from lipogenesis to lipoprotein signaling [ 192 ]. Rats administrated with T3 showed how the central nervous system is important to the activation of BAT by TH through inhibition of hypothalamic AMP-activated protein kinase (AMPK). Stimulation of sympathetic nervous system (SNS) activity leads to thermogenic gene expression in BAT [ 193 ].
As discussed previously, β-AR is stimulated by NE in response to SNS [ 1 ]. The expression of UCP1, required for BAT thermogenesis, is regulated by NE and T3 synergistically, once the induction in separate is twofold, while combined is 20 -fold [ 194 ]. Another way that UCP1 expression and thermogenesis are induced is through bile acid stimulation. G protein-coupled membrane bile acid receptor (TGR5) is stimulated in BAT and results in D2 stimulation and local T3 production [ 178 ].
In conclusion, several mechanisms have been proposed for the TH influence in the browning process, including cold exposure, adrenergic activation [ 167 ], and bile acid signal [ 178 ]. Thus, the stimulation of BAT activation and WAT browning increase the energy expenditure, loss of weight [ 195 ], D2 activation, UCP1 level increase, and consequent thermogenesis [ 192 ].
Circadian rhythm and browning
As previously discussed here, several exogenous factors are able to elicit browning of WAT and BAT activation. However, endogenous factors also play an important role in regulating the phenotype and physiology of these tissues. One of the most important endogenous factors that are related to the regulation of AT is the circadian rhythm, which is a refined system that acts as a master biological clock synchronizing daily and seasonal variations with the behavioral, cellular and tissue-autonomous clock, as well as several biological processes that include sleep–wake cycle, hormone secretion, lipid and glucose homeostasis, energy balance and body temperature [ 196 ]. The circadian rhythm is controlled by melatonin synthesis, which can occur both in the CNS, more specifically in the pineal gland being regulated by light/dark stimulus via the suprachiasmatic nucleus of the hypothalamus (SCN), and in peripheral tissues where its regulation remains unclear [ 197 ].
Disruption of circadian rhythm caused by aging, shift-work, irregular sleep, insomnia, or long exposure to light during the night is associated with sleep and metabolic disorders such as cardiovascular diseases, diabetes type 2 and obesity. Regarding metabolic diseases, AT plays a central role in metabolic and whole-body energy homeostasis, once its secretes several adipokines that regulate diverse processes in CNS and peripheral tissues. Leptin, a hormone mainly produced by adipocytes, is released into the circulation where it crosses the blood–brain barrier (BBB), through a saturable system, and interact with its receptor in the hypothalamus LepRb [ 198 , 199 ]. Hsuchou and colleagues demonstrated that leptin signaling disruption through a pan-leptin receptor knockout (POKO) in mice was able to dysregulate feeding behavior, metabolic and circadian rhythm profile and thus promote an accentuating of obesity [ 200 ]. Beyond control of feeding and metabolic processes, leptin also displays a role in energy balance through the increase of AT thermogenesis in BAT by sympathetic activation [ 201 , 202 ].
Recent studies have proposed that diurnal rhythm promotes differential modulation in activity, thermogenesis and fat oxidation in BAT. It was observed that plasmatic lipid metabolism was improved during daytime with a higher expression of lipoprotein lipase, FA uptake, and modulates lipid plasmatic concentration in BAT [ 203 ]. In the same line, Matsushita and colleagues, assessed forty-four healthy men who received diet-induced thermogenesis (DIT) under room temperature (27 °C) and cold (19 °C) in the morning and in the evening by using 18 F-fluoro-2-deoxy-D-glucose positron emission tomography. It was observed that thermogenic parameters presented better performance during the morning [ 204 ].
Moreover, several studies have established that melatonin directly impacts BAT morphology and function, also, in a mechanism dependent on adrenergic activation mediated by NE release. Melatonin is related to an increase of BAT volume, and thermogenic capacity, associated with the increase of UCP1 mRNA expression and mitochondrial mass and functionality, as well as seric lipid concentration. These profiles are significatively impaired under melatonin deficiency but reverse with oral melatonin replacement [ 205 , 206 , 207 ]. Growing evidence confirms the intimate relationship between circadian rhythm and AT, with emphasis on metabolic homeostasis and modulation of BAT activity. The characterization of how this process happens emerges as a strong diagnostic tool as well as a therapeutic approach concerning sleep disorders and metabolic diseases.
Food-intake and browning
Several studies suggest that food items can affect AT function. Among them curcumin, present in saffron, proved to be involved in the browning process, as mice treated with 50 or 100 mg/kg/day of this compound increased inguinal WAT expression of several browning-associated genes, such as Ucp1, Pgc1a, Prdm16, Dio2, PPARα, and CIDEA, and displayed mitochondrial biogenesis in this tissue. Curcumin stimulation was unable to induce the same effects in the epididymal WAT, though. This process was mediated by the NE-β3-AR pathway since the levels of NE and β3-AR were elevated in the inguinal WAT [ 208 ]. Although studies are scarce regarding the impact of thyme in the WAT browning process, it was observed that 20 µM of thymol, a substance present in the essential oils of thyme, in the complete medium when placed in contact with 3T3-LI preadipocytes for 6–8 days was able to induce an increased gene and protein expression of the PGC-1α, PPARγ, and UCP1. Such increases were related to the activation of β3-AR, AMPK, PKA, and Mitogen-activated protein kinase (p38 MAPK) being accompanied by an increase in mitochondrial biogenesis [ 209 ].
Cinnamon oil contains trans-cinnamic acid, which exposure to 3T3-L1 white adipocytes at 100 µM high gene expression of Lhx8, Ppargc1, Prdm16, Ucp1, and Zic1 and markers of UCP1, PRDM16, and PGC-1α, indicating WAT browning [ 210 ]. Quercetin, a flavonoid present in the onion, also proved to be efficient in the browning process since mice fed for 8 days with 0.5% onion peel extract (OPE) during a high-fat diet (HFD) exhibited increased expression levels OF UCP-1, PRMD16, AND PGC1-α in retroperitoneal white adipose tissue (rWAT) [ 211 ]. Just as the combination of quercetin and resveratrol also induces the WAT browning phenotype [ 212 ]. The resveratrol, present in the bark of grapes and other plants, also increases the expression of UCP-1, PRDM16, and PPARγ, suggesting that resveratrol induces the formation of beige adipocytes through the phosphorylation of AMPK, once treatment coupled with inhibition or the deletion of AMPK did not produce the same effects [ 208 ]. The same was observed in the substances found in the mushroom and honey, which induced increased expression of brown fat markers via AMPK and PGC-1α [ 213 ].
The peppers have capsaicin, an active compound responsible for the burning sensation that is also involved in the browning of WAT. One study used wild-type (WT) and TRPV1 −/− mice fed with HFD containing 0.01% capsaicin. The WT animals showed an increase in the expression of Ucp-1, Pgc-1α, Sirt-1, Prdm16, and exhibited browning of WAT via activation of the transient receptor potential vanilloid 1(TRPV1), which is related to the synthesis of catecholamine or sirtuin 1 (SIRT1)-mediated deacetylation of PPARγ, facilitating PPARγ-PRDM-16 interaction. The same did not happen in animals TRPV1 −/− , demonstrating that capsaicin depends on the role of TRPV1 in the browning process [ 214 ]. Menthol, an organic compound extracted from Mentha piperite oil, was shown to activate Transient Receptor Potential Cation Channel Subfamily M Member 8 (TRPM8), and this matched the increase in the thermogenic Ucp1 gene and the expression of Pgc-1α through PKA phosphorylation induced by free intracellular Ca 2+ in adipocytes treated with menthol for 8 h [ 215 ]. Other substances, such as carotenoids, are involved in the WAT browning process. Fucoxanthin, β-carotene, and citrus fruits are efficient in modulating the Ucp1 expression ( 216 , 217 , 218 ).
Another food component that is involved in the browning process of WAT is berberine, a molecule derived from the plants Coptis chinensis and Hydrastis canadensis . Obese male C57BLKS/J-Leprdb/Leprdb mice (db/db) were injected for 4 weeks with berberine (5 mg/kg/day). The group discovered berberine promotes BAT thermogenesis and WAT browning, since the igWAT, but not the epididymal, showed high levels of mRNA and UCP1 protein expression and increased mitochondrial biogenesis after injections. The brown adipocyte markers PGC-1α, CIDEA, Cox8b, and lsdp5 were also elevated and AMPK and PGC-1α are involved [ 219 ]. In another study, the polyphenols from tea extracts (0.5%) present in the high-fat diet for 8 weeks reduced the size of adipocytes and induced browning markers in WAT, and the size of lipid droplets and whitening markers were reduced in the BAT [ 220 ]. Another analysis with the extract induced 77.5 or 155 mg/kg/day for 8 days, in which there was an increase in UCP-1 and PPARγ [ 221 ].
Genistein, present in soy, indirectly induces browning since it is capable of increasing irisin levels, through PGC-1α / FNDC5, which increases Ucp1 and Tmem26 expression ( 222 ). In Magnolia Officinalis, two magnolol compounds (20 µM) and Honokiol (1–20 µM) when used to stimulate 3T3-L1 adipocytes increased protein levels of PGC-1α, PRDM16, and UCP-1 [ 223 ]. Honokiol also increased protein expression levels of CIDEA, COX8, FGF21, PGC-1α, and UCP1 [ 224 ]. The herb panax ginseng contains ginsenoside Rg1 (10 μM of ginsenoside Rb1), which is capable of considerably increasing the mRNA expression of UCP1, PGC-1α, and PRDM16 in mature 3T3-L1 adipocytes via PPARγ [ 225 ], as well as activating the AMP-activated protein kinase pathway [ 226 ].
The fish oil is rich in n-3 polyunsaturated fatty acids (PUFAs), components that are associated with the formation of beige adipocytes, among them is eicosapentaenoic acid (EPA). Mice fed different diets, including with EPA, for 8 weeks showed increased expression of β3-AR, PGC-1α, and UCP1 and exhibited high expression of PPAR [ 227 ], though this effect is controversial since another animal study investigating a diet containing pure EPA (3.6% as EPA ethyl ester) did not show the expression of beige adipocyte marker genes of inguinal and visceral WAT, but only in BAT [ 228 ]. Docosahexaenoic acid (DHA) (1.2%) together with EPA (2.4%) increased oxygen consumption and rectal temperature, as well as UCP1 and β3AR levels via the central nervous system. However, knockout mice for TRPV1 did not achieve the same effect, showing that such events were mediated by SNS, TRPV1, and catecholamines [ 229 ]. Conjugated linoleic acids (CLAs) also showed potential to induce browning process in the WAT [ 230 ].
Once the overwhelming impact of infectious diseases has been alleviated by the development of efficient therapeutics, life expectancy has been continuously increasing (World Health Organization, 2019). Age-associated diseases, including type 2 diabetes (T2D), cardiovascular diseases (CVDs), neurodegenerative pathologies, and obesity statistics are alarming and correlates with changes in the lifestyle of individuals throughout the world, including the diet, and impair the health spam rise. Western diets (WDs) are composed by food items enriched in processed sugar, white flour and salt and poor in fibers, vitamins and minerals [ 231 ]. At the same time, the diet may be the remedy against the burden caused by these chronic diseases. While overnutrition often correlates with inflammatory and metabolic detrimental effects at molecular level, undernutrition without starvation presents many benefits. Calorie restriction (CR) and intermittent fasting (IF) are promising interventions against the overweight and obesity numbers, climbing specially in Western countries [ 232 ].
CR, defined as reduced calorie consumption without malnutrition, is the best studied dietary intervention that increase health spam in experimental models. A plethora of human studies place CR as beneficial for expanding the health spam [ 232 ]. These studies proceeded Weindruch and Sohal positive correlations between CR and health spam [ 233 ] Click or tap here to enter text.. AT plasticity is one of the connections between CR and health benefits. Fabbiano and colleagues analyzed mice under CR and described that this regimen induces functional beige fat development in WAT, phenomenon that occur via enhanced type 2 immune response and SIRT1 expression in AT macrophages [ 234 ].
The stress resistance provided by the IF practice places this regimen as a feasible dietary intervention against various devastating complex pathologies. Differently from CR, intermittent fasting (IF) does not influence the meal size, but decrease the number of meals in a given period [ 232 ]. The fasting state leads to a metabolic switch, which increases the usage of free fatty acid (FFA) as energy source in comparison to glucose. In addition, IF favors the synthesis of ketone bodies (KBs) by the liver, molecules that act as an energy source during nutrient deprivation and induce a plethora of beneficial effects on the organism by acting upon the muscle, liver, heart, brain, intestine and AT [ 235 , 236 , 237 ]. Moreover, during prolonged fasting periods, the levels of the bioenergetic sensors NADH, ATP, and acetyl-CoA decrease and the amounts of NAD+, AMP, CoA rise, molecules that act as epigenetic cofactors and lead to the activation of stress resistance mediators, as sirtuins (SIRTs), NRF2 (nuclear factor erythroid 2–related factor 2), and AMPK (AMP-activated protein kinase). IF also impacts positively on AT remodeling. A DIO animal model submitted to repetitive fasting cycles displayed increased glucose tolerance, and diminished adipocyte hypertrophy and tissue inflammation [ 238 ]. Mouse studies show that IF induces WAT mass decrease, elevation of AT UCP1 expression and thermogenic capacity [ 239 , 240 ], and augmented beige pre-adipocytes recruitment to WAT [ 241 , 242 , 243 ] (Fig. 2 ).
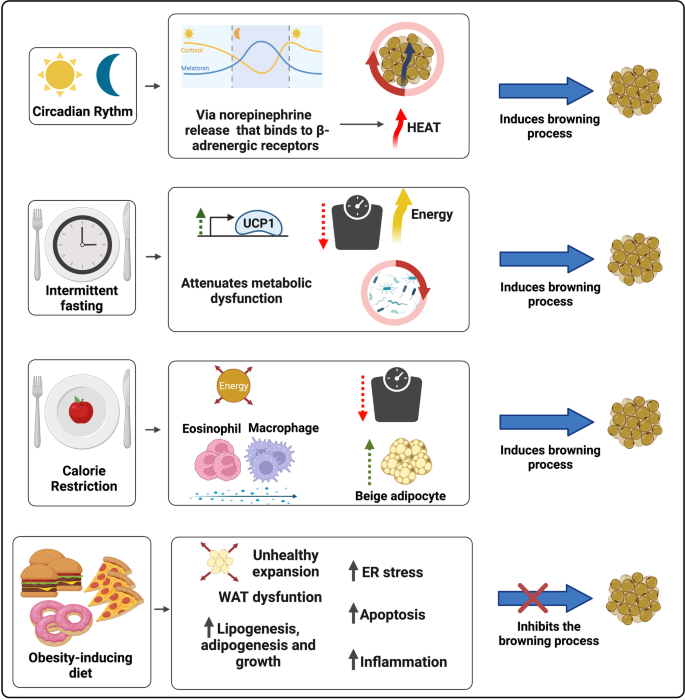
The impact of circadian rhythm and different diets on the WAT browning modulation. The secretion of melatonin, a circadian rhythm regulating neurohormone, is mediated by the release of Norepinephrine (NE), which binds to β-adrenergic receptors. Adrenergic activation is one of the main mechanisms of WAT browning induction and BAT activation. Intermittent fasting (IF) associates with weight reduction, improved metabolic status due to increased glycemic tolerance, decreased white adipocyte hypertrophy and AT inflammation, and augmented expression of thermogenic genes (such as UCP1) and recruitment of beige adipocytes. IF is also modulates the intestinal microbiome composition and diversity, a shift closely related to the induction of browning in the WAT. Caloric restriction (CR) is also associated with weight loss, promotes greater recruitment of beige adipocytes through the participation of M2 macrophage and eosinophil infiltration and in WAT. Finally, obesity-inducing diets correlate with increased lipid accumulation, WAT unhealthy expansion and dysregulation. Abnormal expansion of WAT promotes ER stress, greater induction of adipose cell apoptosis and inflammation through NF-κB transcription factor activation and increased pro-inflammatory cytokines secretion
An elegant study conducted by Li and colleagues informed that mice under IF cycles display an intestinal microbiome composition shift associated with increased levels of the fermentation products lactate and acetate. They also show that the modulation of the gut microbiota by IF is crucial for its browning effect, as microbiota-depleted mice present impaired IF-induced AT beiging and fecal microbiota transfer from these mice to antibiotics treated animals display increased browning of WAT [ 244 ] (Fig. 2 ). Unexpectedly, a human study conducted by von Schwartzenberg and colleagues showed that CR may diminish bacterial abundance, deeply change gut microbiome composition and diversity, impair nutrient absorption, and favor the outgrowth of the pathobiont ( Clostridioides difficile ). This diet also led to a decrease in bile acid (BA) levels [ 245 ].
BA, nonesterified fatty acids, are synthesized during the browning of WAT, a phenomena associated with the potentiation of the lipolytic machinery [ 246 ]. These fatty acids can not only activate UCP-1 allosterically, but also serve as fuel for oxidative phosphorylation and consequently heat generation in BAT [ 1 ]. Furthermore, in the liver they are used for the generation of acylcarnitines and VLDL which is used as source for thermogenesis [ 247 ]. Moreover, studies show that the increase in brite and brown adipocytes in WAT leads to an elevation in lipoprotein lipase (LPL) activity and subsequently an increase in circulating lipids available for BAT through intravascular hydrolysis of chylomicron triglycerides [ 248 ]. Consequently, these mechanisms result in the generation of cholesterol-enriched lipoprotein remnants, which upon activation of BAT accelerates the flow of cholesterol to the liver [ 249 ].
BA are steroid acids derived from dietary cholesterol catabolism. These acids are synthesized in the liver and act to aid digestion and absorption of fat in the intestine, in addition to playing an essential role in lipid metabolism. BA act in other tissues, such as AT, as signaling molecules through interaction with the nuclear Farnesoid X receptor (FXR) and the G protein-coupled membrane receptor (TGR5) [ 250 ]. Recent studies have shown that BA play a relevant role in BAT activation and increased thermogenesis in adipocytes. In rodents, the activation of BAT by BA is dependent on its interaction with the TGR5 receptor and expression of the enzyme type 2 iodothyronine deiodinase (DIO2). Additionally, experiments with oral supplementation of BA in humans indicated increased BAT activity in humans [ 251 , 252 ]. Another experiment performed under thermoneutrality, demonstrated an improvement in glycemic metabolism and lipogenesis in the liver and fat accumulation in the TA and also induced an improvement in thermogenic parameters and mitigation of the impact of diet-induced obesity after feeding mice with HFD associated with BA [ 253 ].
Moreover, BAT activation also promotes liver protection. In a study performed with animals under alcohol-induced hepatic steatosis or liver injury, activation of the TGR5 receptor induced improvement of clinical condition. The increase in thermogenesis in BAT promotes an increase in lipid metabolism with lower availability of circulating FFA and, consequently, lower absorption of these molecules by the liver [ 254 ]. However, if on the one hand BAs have been shown to be effective in inducing browning, on the other hand the excess of these acids is capable of promoting an antagonistic effect, such as mitochondrial dysfunction and expression of genes associated with cellular senescence in adipose cells [ 255 ].
Transcriptional regulation of WAT browning
ATs are embryologically distinct from other tissues and are formed according to specific stimuli during embryo development, including Bone morphogenetic proteins (BMPs), pleiotropic molecules that interact with type I and type II BMP receptors and influence embryogenesis [ 256 , 257 ]. BMPs interact with type I and type II BMP receptors serine/threonine kinase activity and influence lineage determination [ 258 ]. Tang and colleagues showed that transfection of C3H10T1/2 cells with BMPs coped with phenotypes: while BMP2 associated with the osteogenic lineage, BMP4 led to adipogenic differentiation [ 259 ].Noteworthy, BMP4 overexpression was found to increase UCP1 and other beiging markers, as Hoxc9, Tbx1, and Tbx15 [ 260 ]. These different phenotypes induced by the BMPs, including the induced beige adipocytes, highlight how relevant transcriptional regulation is for determining the cells functions and characteristics. The main proteins that regulate gene expression are the transcriptional factors (TFs), DNA binding proteins that modulate gene transcription by interacting with the gene promoter or cis-regulatory elements, such as enhancers and silencers, and include PPAR proteins, PGC-1α, and PRDM16 [ 261 , 262 ].
In addition to its roles in ATs development [ 263 ], PPARγ is a central TF for adipogenesis and lipid storage regulation, influences cell thermogenic capacity, and impacts lipid metabolism and insulin sensitivity [ 264 ]. This TF is expressed in elevated levels in ATs [ 265 ], and upon ligand binding PPARγ recruits different cofactor sets for controlling the expression of specific genes. PPARγ cooperates with the basic leucine-zipper factor C/EBPα and interacts with the majority of adipocyte-selective genes [ 266 , 267 ]. The use of PPARγ full agonists is associated with improved insulin sensitivity and induces WAT browning, but can cause detrimental effects, such as undesirable weight gain and augmented visceral adiposity [ 264 ].
Although PPARγ is sufficient for converting white adipocytes into cells that display a brown-like phenotype in vitro [ 268 ], PPAR-α and PPAR-β/δ also influence the browning process. PPAR-β/δ agonist enhances beta-oxidation and improves glucose tolerance, key characteristics of the white-to-beige plasticity [ 269 ]. PPARα acts synergistically with PPARγ in inducing robust WAT browning in vivo [ 268 ], and is currently considered a prominent target for treating metabolic disorders [ 269 ].
A way that PPAR agonists provoke WAT browning is by stabilizing PRDM16 [ 270 ], a protein that activates a complete set of thermogenic genes in WAT [ 271 ]. PRDM16 is essential for browning particularly in scWAT, once its induction in visceral depots does not correlate with thermogenesis [ 272 ]. Mice lacking Prdm16 in scWAT are unable to induce browning within subcutaneous depots after stimuli [ 273 ]. Ectopic PRDM16 expression induce thermogenic genes in several cell types [ 274 ]. PRDM16 AT overexpression in rodents copes with augmented energy expenditure and DIO resistance [ 272 ] PRDM16 acts by binding to specific regulatory sequences in DNA and by interacting with other proteins [ 271 ], such as PGC-1α [ 275 ].
PGC-1α plays a key role in the adapting thermogenesis. First described in cold-induced adaptive thermogenesis analyses [ 276 ], this transcriptional coactivator participates in the regulation of a plethora of cellular functions, including mitochondrial biogenesis, oxidative phosphorylation, and gluconeogenesis [ 277 , 278 ]. Once PGC-1α influences genes related to energy metabolism, it is expressed mostly in tissues that require an elevated amount of energy, like AT, liver, skeletal muscle, and brain [ 279 ]. Pgc1α is activated by the action of the cAMP-PKA-p38/MAPK signaling pathway and physically interacts with Nuclear Respiratory Factors 1 and 2 (NRF1 and NRF2) and co-activates PPARγ, PPARα, and ERRα/β/γ [ 280 ]. When overexpressed, Pgc1α induces mitochondrial biogenesis [ 281 ].
Another key regulator of the browning process is CIDEA. Initially described as a mitochondrial protein, CIDEA was further discovered to be associated with cell lipid droplets (LD) [ 282 , 283 , 284 ]. This molecule leads to the occurrence of browning by inhibiting the suppression of UCP1 gene expression mediated by liver-X receptors (LXRs) and increasing PPARγ binding strength to the UCP1 enhancer [ 285 ]. As detailed in this review, UCP1 is found in the inner mitochondrial membrane and acts by uncoupling the electron transport chain and oxidative phosphorylation, releasing energy as heat [ 1 ]. The existence of molecular markers for the browning process can be useful for investigating AT plasticity status and correlate with health and disease.
Zfp516 is a TF that directly binds to the UCP1 and PGC1α promoters and induce WAT browning upon cold exposure. Zfp516 overexpression copes with augmented multilocular lipid droplets (LDs) biogenesis and increased oxygen consumption and UCP1 levels [ 286 ]. HSF1 was described to cooperate with PGC1α in igWAT, favoring the induction of the thermogenic and mitochondrial gene programs, which leads to augmented energy consumption. HSF1-deficient mice are cold intolerant due to decreased β oxidation and UCP1 expression. HSF1 activation associates with scWAT browning, non-shivering thermogenesis and energy consumption [ 287 , 288 ]. IRF4 also cooperates with PGC1α and was described to inhibit lipogenesis in adipocytes [ 289 ]. While IRF4 overexpression favors beiging in epididymal WAT, the absence of this factor is linked to diminished energy expenditure and increased risk to hypothermia [ 290 ]. The TF NFIA presents a crucial role in the initial steps of thermogenic gene regulation, as its increased levels in thermogenic adipocytes precedes PPARγ upregulation in these cells [ 291 ].
Members of “Early B-Cell Factor” (EBF) protein family play key roles in the regulation of thermogenesis. The TF EBF2 uncouples adipocyte mitochondrial respiration and is sufficient for WAT browning. Increased EBF2 levels in WAT leads to activation of the thermogenic program, favoring increased oxygen consumption and resistance for weight gain. EBF2-KO mice show impaired WAT browning and ablates the brown fat-specific characteristics of BAT [ 292 , 293 , 294 ]. EBF2 activity is regulated by the action of ZFP423, which binds to EBF2 and recruits NuRD (nucleosome remodeling deacetylase) corepressor complex to suppress EBF2 activity in thermogenic genes regulatory sequences [ 295 ]. Absence of ZFP423 associates with PPARγ binding to thermogenic gene enhancers, WAT browning and non-shivering thermogenesis [ 296 ]. The protein transducin-like enhancer of split 3 (TLE3) was first described by Villanueva and colleagues to increase PPARγ adipogenic activity [ 297 ]. Deletion of TLE3 copes with increased energy expenditure and mitochondrial oxidative metabolism in adipocytes, characteristics associated with the browning process [ 298 ] KLF11, TAF7L, ZBTB16, EWS, PLAC8, ERRα, ΕRRγ and other TFs that were described to promote WAT browning. In contrast, FOXO1, TWIST1, p107, LXRα, pRB, RIP140, REVERBα acting repressing AT beiging by impacting on the activity of EBF2, PRDM16, PGC1α and other activating TF [ 299 , 300 , 301 ].
In order the transcription to occur, chromatin needs to be accessible to polymerases and TFs. Remodeling enzymes, which are classified in covalent histone modifiers and ATP-dependent chromatin remodeling complexes, actively modify chromatin status in response to environmental cues [ 302 ]. Histone covalent modifiers are enzymes that chemically modify positively-charged amino acids (mainly lysine) present in these proteins. Histone acetyltransferases (HATs) and histone deacetylases (HDACs) catalyze the removal or insertion of acetyl group, respectively, influencing histone acetylation pattern [ 303 ]. Acetylation decreases histones’ positive charge, leading to histone-DNA looser interaction, chromatin decompaction and gene activation. These epigenetic elements classified into three major families based on the acetyl group transfer mechanisms: the CREB binding protein (CBP)/P300 family (CBP, P300), the GCN5-related N-acetyltransferases (GNAT) family (GCN5, PCAF, and Hat1), and the MYST family (MYST1, MYST2, TIP60) [ 304 ]. The HAT CBP was shown to inhibit the browning process, once it is key in white adipocyte differentiation [ 305 ]. Histone acetylation pattern has been described to present a critical role in adipocyte identity, as cold exposure leads beige adipocytes to show histone acetylation pattern associated with brown phenotype [ 306 ]. The HATs GCN5/PCAF were also shown to participate in the browning process [ 307 ].
HDACs catalyze the deacetylation of amino acid residues in histones, reactions favor gene repression. These enzymes are categorized into four groups, namely class I (HDAC1–3 and 8), class IIa (HDAC4, 5, 7, and 9), class IIb (HDAC6 and 10), class III (SIRT1–7), and class IV (HDAC11) [ 304 ]. HDAC1 expression is augmented in WAT and copes with decreased levels of proteins associated with non-shivering thermogenesis, as UCP1 and PPARγ [ 308 ]. HDAC3 levels also suppress WAT browning, as absence in WAT correlates with H3K27ac on enhancers of Ucp1 and Ppar g , signature associated with tissue improved oxidative capacity, mitochondrial biogenesis, and thermogenesis [ 309 ]. Experiments involving the deletion of HDAC9 shows that this enzyme also contributes for the metabolic dysfunction characteristic of HFD-fed rodents [ 310 ]. HDAC11 is another element that impairs WAT beiging, once its removal favors at thermogenesis in diet-induced obese (DIO) mice [ 311 ]. Other key HDAC is the SIRT family (SIRT1, SIRT2, SIRT3, SIRT5, and SIRT6). SIRT1 deletion in HFD-treated mice is associated with diminished amounts of PGC-1α, FGF21, and UCP1 in epididymal WAT (eWAT) [ 312 ].
Histones can also be post-translationally modified by histone methyltransferases (HMTs) and histone demethylases, which influence gene activation or suppression depending on the modified residue position and valency [ 313 ]. There are two categories of HMTs: lysine methyltransferases (KMTs) and arginine methyltransferases (RMTs). Rodent studies show that inactivation of the KMT MLL3 favors improved insulin sensitivity and augmented energy expenditure [ 314 ]. Absence of another KMT, the EHMT1, was shown to impair the thermogenic program in WAT [ 315 ]. Histone demethylases (HDM) catalyze histone demethylation processes and are also classified according to the characteristics of the modified amino acid. The KDM LSD1 expression, which was found to be induced by chronic cold exposure and β3-adrenergic stimulation, leads to increased mitochondrial activity in WAT by cooperating with NRF1 [ 316 , 317 , 318 ]. In addition, rodents presenting increased LSD1 levels are associated with igWAT browning in lean animals and with lower weight gain in the context of DIO [ 317 ] Also induced by β3-adrenergic stimulation, the KDM3A JMJD1A directly regulates ppar a and ucp1 genes and was found to be crucial for WAT browning [ 319 ]. Specific DNA sequences called CpG islands can also be methylated for gene transcription regulation by DNA methyltransferases (DNMTs), which switches off gene expression. Ten-eleven translocation (TET) family enzymes switch on the gene expression by demethylation [ 320 ].
Gene transcription can also be impacted by the action of ATP-dependent chromatin remodeling complexes, which, differently from the covalent histone modifiers, alter the interaction between the DNA and the nucleosome non-covalently using ATP hydrolysis as an energy source. The chromatin remodelers are categorized in INO80, CHD, ISWI, and SWI/SNF [ 321 ]. The mammalian SWI/SNF (mSWI/SNF or BAF) complex can act with either Brahma homolog (BRM) or BRM-related gene 1 (BRG1) ATPases [ 322 ]. Abe and colleagues suggested that BRG1 is necessary for thermogenesis induction [ 319 ].
Other key actors in gene transcription regulation are the microRNAs (MiR), small non-coding regulatory ribonucleic acids (maximum 200 nucleotides) that influence gene expression post transcriptionally. These elements regulate gene expression by several mechanisms, including binding to mRNA strands to repress protein translation and to favor decapping, deadenylation and ultimately degradation of target mRNA in P-bodies, and direct translation inhibition. However, many studies suggest that miRNAs are also capable of activating transcription protein translation, fact that highlights miRNAs as central participants in the fine regulation of gene expression in response to specific stimuli [ 323 ]. Raymond and others described that miR-32 inhibition copes with impaired WAT browning [ 324 ]. MiR-181, which is induced by tryptophan‐derived metabolites produced by the intestinal microbiota, was also connected with WAT physiology, once it promotes energy expenditure and insulin sensitivity in rodent models and its removal may cope with the development of obesity in these animals [ 325 ]. Another miRNA that influences WAT homeostasis is miR-26, which deletion leads to WAT enlargement and overexpression inhibits the progression of obesity in the DIO in mice [ 326 ]. miR-196a, miR-455, miR-30b/c are other examples of miRNAs that are induced by browning inducers and promote this WAT plasticity process [ 327 , 328 ].
In contrast, miR-27, miR-133, and miR-150 inhibit TFs associated with the browning process [ 61 , 63 , 329 ]. Fu and colleagues showed that miR-34a inhibits WAT beiging via FGF21 [ 330 ], miRNA-155, miRNA Let-7i-5p and miR-125b- 5p are molecules that impair WAT browning process, which inhibition promotes beiging [ 331 , 332 , 333 ] (Fig. 3 ). Other small non-coding RNAs (sncRNAs) impact on gene expression and are extensively reviewed elsewhere.
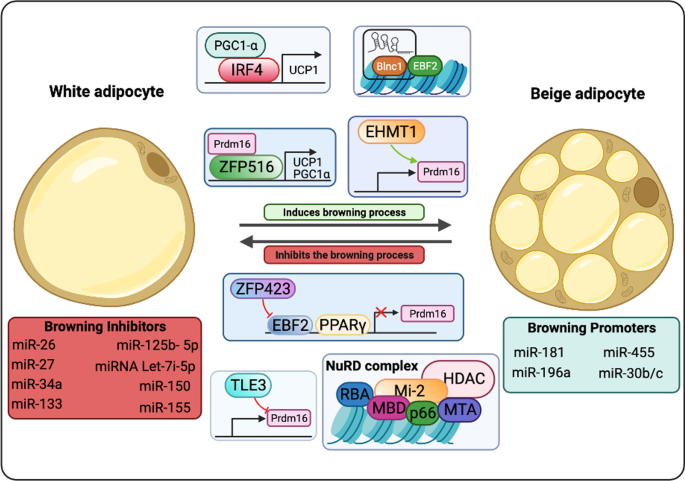
WAT browning transcriptional regulation. The transcriptional regulation of the WAT beiging process involves the action of a plethora of specific proteins and nucleic acids. This orchestra of trans-acting factors modulates genes associated with oxidative capacity, mitochondrial biogenesis and non-shivering thermogenesis. While the action of some factors, such as PGC-1α, IRF4, PRDM16, ZFP516, EHMT1 and RNAs, copes with WAT browning, TLE3, ZFP423, and the NuRD complex, another set of RNAs e others inhibit this process, favoring adipogenesis and white adipocyte differentiation
Non-coding RNAs that present average length longer than 200 nucleotides are called LncRNAs [ 302 , 334 ] These nucleic acids have been described to influence gene transcription through several mechanisms, including the induction of more efficient protein translation by binding to an internal ribosome entry site (IRES), increase of mRNAs stability, polyubiquitination process inhibition thus increasing protein stability, binding to specific proteins in the cytoplasm, and acting as a sponge sequestering miRNAs [ 335 , 336 , 337 , 338 , 339 ] . Zhao and collaborators identified lncRNA 1 (Blnc1) as a driver for beige adipocyte differentiation and WAT browning [ 293 ].
All the regulatory elements discussed here cooperate for generating a phenotype in response to environmental stimuli, such as physical exercising, intermittent fasting, calorie restriction, thyroid hormones, microbiota-associated metabolites, thyroid hormones and others [ 299 ] The lncRNA Blnc1 forms a Blnc1/hnRNP-U/EBF2 ribonucleoprotein complex and is required for EBF2 transcriptional activity [ 292 , 340 ]. The TF ZBTB7B also interacts with this lncRNA through the hnRNP-U/Blnc1 for beige adipocyte differentiation and thermogenesis [ 341 ]. LncRNAs also interact with other TFs, such as PGC-1α, ZFP516, and PRDM16 for full transcription [ 274 , 280 , 286 ]. PRDM16, a coregulator of PPAR, cooperates with EHMT1 for WAT browning induction. As EHMT1, other covalent histone modifiers form complexes with ATP-dependent chromatin-remodelers [ 342 ]. The chromatin-remodelers BRG1 and the BAF also interact with the TF EBF2 for gene transcription [ 292 , 293 ]. PGC1α favors increased transcription by recruiting HATs, as CBP/p300 and GCN5 [ 343 ].
Noteworthy, AT functional and morphological plasticity is represented by the extreme dynamic beige adipocyte chromatin state. Beige adipocytes show a chromatin signature similar to the pattern presented by brown adipocytes after cold exposure and display a chromatin signature associated with white adipocytes after re-warming to 30 °C. However, if the beige adipocytes were cold-induced, these cells displayed epigenetic marks that favored rapid thermogenic genes expression upon β-adrenergic stimulation, even after temperature rise, suggesting the occurrence of epigenomic memory [ 306 , 344 ].
WAT browning, health impact and applications
For the past decades, the browning process has been deeply explored for managing both metabolic syndromes, and hypermetabolic diseases. Proposing treatment, the cold was the pioneer mechanism associated with a great elicitation of this event in mice. However, the applicability and outcomes of this approach in humans are far from exciting. Technological advances provide efficient mechanisms that can be employed to improve a biological system. Recent studies applied bioengineers to achieve the improvement of browning in adipose tissue. The injection of M2 AT macrophages (ATMs) from transgenic mwRIP140KD mice, a specific knockdown of RIP140 that is related to activation of M1 polarization, in HFD-fed obese wild type mice could recover the disruption induced by obesity through browning induction [ 345 ]. WAT-derived stem cells (ASCs) from humans and rats could be differentiated into BAT under browning conditions in three-dimensional (3D) polyethylene glycol (PEG) hydrogel [ 346 ].
In addition to bioengineering, a field that has been explored is the transplantation or re-implantation of BAT, also termed ex-BAT, to increase this endogenous tissue. The harvested scWAT is differentiated into brite and then is re-implanted in the same area promoting the local increase of BAT, displaying great outcomes [ 347 ]. Aiming for a less invasive procedure, the use of a microneedle patch to address the browning agents directly to the scWAT for inducing browning of this tissue is another strategy investigated presently [ 348 ]. These strategies aim to promote the increase of endogenous BAT, as well as its activity with an applicable method, reducing the degree of invasiveness and risks of rejection by the body.
Several studies have targeted increasing BAT mass and activity, but when the aim is inhibiting the browning process to avoid a worsening prognosis, what is proposed? Expanding knowledge of the pathways involved is the main way of inhibiting that event. It is well established the role of parathyroid hormone (PTH) in the elicitation of browning. In the tumor context, it was identified as a peptide-derived tumor, parathyroid hormone-related protein (PTHrP) that favors browning and cachexia [ 16 ]. Blocking PTHR specifically in ATs inhibits browning and wasting of this tissue, as well preserves muscle integrity, and ameliorates muscle-related strength, protecting mice from cachexia [ 349 ].
Currently, some pharmacological approaches have displayed alternative and prominent ways to restrain browning. Metformin demonstrated to be efficient to prevent the browning of the scWAT, as well as the inhibition of mevalonate pathways by the use of Statin or Fluvastatin, which implicates in the disruption of browning [ 350 , 351 ]. However, further studies should be conducted to promote a broader and more detailed debate on the topic.
Given the data presented, it is evident that the benefits and dangers associated with activation or inhibition of the browning process are closely linked to the type of disorders displayed in the individual's body at the metabolic, cellular, or physiological level. Taking as criteria patients with metabolic syndromes and obesity, browning process emerges as a promising therapeutic approach, mainly due to its several benefits, such as improvement of clinical conditions associated with lower side effects, and the multi-stimulatory character, which allows numerous safe and feasible ways of induction. In contrast, the ways and means of inhibiting browning to prevent the development of comorbidities in cases of chronic or acute hypermetabolism are still poorly explored. In this way, browning process needs to be deeply explored and can be used as a key factor in different therapeutical approaches in health and diseases. Emerging insights into the metabolic and immunological role of browning of the white adipose tissue are also discussed, along with the developments that can be expected from these promising targets for therapy of metabolic and chronic disease in the forthcoming future.
Mouse and human AT research
A major discussion pointed by the scientific community about the investigation of AT in mice is how much the results obtained in the animal model would reproduce the anatomorphophysiologies of these tissues in humans, since, unlike humans, mice have a significant amount of BAT during embryonic and adult, as well as they differ in several other factors, such as expression of molecular markers, activation profile and location. Human BAT was discovered to be more similar to beige compared to classical BAT markers [ 352 , 353 ]. Another relevant point concerns the fact that the BAT deposits most used for research purposes are the BAT located in the interscapular region (iBAT), while in humans there is a greater abundance of this tissue in the clavicular and neck regions, presenting compositional differences that represent obstacles in the overlapping of scientific findings. In this sense, Mo and colleagues identified an analogous deposit of BAT in mouse embryos, which is maintained during adulthood, and in humans called supraclavicular BAT (scBAT). The scBAT shows similarities to scBAT in humans in terms of location, morphology and thermogenic capacity [ 354 ].
Another alternative developed in order to attenuate the anatomical, physiological and molecular differences between the organisms was to submit mice under conditions called “physiologically humanized” in which middle-aged animals under thermoneutrality (30˚C) and diets aligned with the dietary pattern of human beings are used. humans. In which the authors observed a greater similarity between several cellular and molecular parameters between the BAT of humans and mice [ 355 ]. The technique generated controversies that were discussed by Kajimura and Spiegelman and replicated by the authors of the original article ( 356 , 357 ).
Conclusions and perspectives
Current research place WAT browning as an extremely dynamic process that is influenced by several factors , including temperature, physical exercising, thyroid hormones, circardian rhythm, food components and dietary regimens. The participation of AT plasticity on the organism metabolic health and inflammatory status spot this process as a promising therapeutic target for decreasing the risk associated with many chronic diseases. Further efforts in investigating AT plasticity must alleviate the burden of these devastating life-style associated pathologies.
Availability of data and materials
Not applicable.
Abbreviations
5′-Deiodinase type 2
Amino acids
Adenylyl cyclase
AMP-activated protein kinase
Atrial natriuretic peptide
Adipose stromal cells
Adipose tissue
Activating transcription factor
Adipose tissue macrophages
Adenosine triphosphate
Brown adipose tissue
Blood–brain barrier
Bone morphogenetic proteins
Basal metabolic rate
Brain natriuretic peptide
BRM-related gene 1
Brahma homolog
CCAAT-enhancer-binding protein
Calcium ion
Cyclic adenosine monophosphate
CREB binding protein
Response element-binding protein
Cell death-inducing DNA fragmentation factor-like effector A
Conjugated linoleic acids
Central nervous systems
Carnitine palmitoyltransferase 1B
Calorie restriction
CAMP response element-binding protein
Cyclic AMP response element-binding protein H
Docosahexaenoic acid
Diet-induced obese
2 Iodothyronine deiodinase
Diet-induced thermogenesis
DNA methyltransferases
Epicardial AT
Early B-cell factor
Endocrine FGF
Eukaryotic initiation factor
Eicosapentaenoic acid
Epididymal WAT
Fatty acids
Free fatty acids
Fibroblast growth factor family
Fibroblast growth factor tyrosine kinase receptor
Fibronectin domain-containing protein 5
FGFR substrate 2α
Farnesoid X receptor
Non-derepressible 2
Glucose transporter
GCN5-related N-acetyltransferases
Histone acetyltransferase
Histone deacetylase
Histone demethylase
High-fat diet
Histone methytransferases
Interscapular BAT
Intermittent fasting
Interleukin
Internal ribosome entry site
Beta-Klotho
Lysine methyltransferases
Long-chain fatty acids
Lipid droplet
Long non-coding RNA
Lipopolysaccharide
Liver X receptor
Mitochondrial anion carrier family
Membrane proteins monocarboxylate transporter 8
Messenger RNA
Myogenic factor 5
Nonalcoholic fatty liver disease
Non-coding RNAs
Norepinephrine
Nuclear factor erythroid-related factor 2
Nuclear respiratory factor 1
Nuclear respiratory factor 2
Nucleosome remodeling deacetylase
Organic anion transporter family member 1C1 9
Onion peel extract
Mitogen-activated protein kinase
Pyruvate dehydrogenase
Polyethylene glycol
Peroxisome proliferator-activated receptor-gamma coactivator 1 α
Protein kinase A
Proopiomelanocortin
Peroxisome proliferator-activated receptor
PR domain containing 16
Parathyroid hormone
Parathyroid hormone receptor
Parathyroid hormone-related protein
Polyunsaturated fatty acids
Retinoic acid
Retinoic acid receptor
Receptor-interacting protein 140
Arginine methyltransferases
Retinoic acid receptor-related orphan receptor α
Retroperitoneal white adipose tissue
Retinoid X receptor
Supraclavicular BAT
Suprachiasmatic nucleus
Subcutaneous WAT
Small non-coding RNA
Sympathetic nervous system
Type 2 diabetes
Triiodothyronine
T-box transcription factor TBX1
Ten-eleven translocation
Transcription factor
Transforming growth fator beta
G protein-coupled membrane bile acid receptor
Thyroid Hormone
T help 2 cells
Transducin-like enhancer of split 3
Thyroid-hormone responsive elements
Transient receptor potential cation channel subfamily M member 8
Transient receptor potential vanilloid 1
Thyroid hormone nuclear receptors
Thyroid stimulating hormone
Thyroid stimulating hormone receptor
Uncoupling protein 1
Visceral WAT
- White adipose tissue
Zinc-α2-glycoprotein
β-3 Adrenergic receptor
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359.
Article CAS PubMed Google Scholar
Moriya K, Arnold J, LeBlanc J. Shivering and nonshivering thermogenesis in exercised cold-deacclimated rats. Eur J Appl Physiol Occup Physiol. 1988;57(4):467–73.
Prunet-Marcassus B, Cousin B, Caton D, André M, Pénicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006;312(6):727–36.
Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Metab. 2010;298(6):E1244–53.
CAS Google Scholar
Suárez-Zamorano N, Fabbiano S, Chevalier C, Stojanović O, Colin DJ, Stevanović A, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21(12):1497–501.
Article PubMed PubMed Central CAS Google Scholar
Johann K, Cremer AL, Fischer AW, Heine M, Pensado ER, Resch J, et al. Thyroid-hormone-induced browning of white adipose tissue does not contribute to thermogenesis and glucose consumption. Cell Rep. 2019;27(11):3385-3400.e3.
Barberá MJ, Schlüter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor α activates transcription of the brown fat uncoupling protein-1 gene: a link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276(2):1486–93.
Article PubMed Google Scholar
Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108(1):143–8.
Villarroya F, Peyrou M, Giralt M. Transcriptional regulation of the uncoupling protein-1 gene. Biochimie. 2017;134:86–92.
Kalinovich AV, de Jong JMA, Cannon B, Nedergaard J. UCP1 in adipose tissues: two steps to full browning. Biochimie. 2017;134:127–37.
Yau WW, Singh BK, Lesmana R, Zhou J, Sinha RA, Wong KA, et al. Thyroid hormone (T 3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy. 2019;15(1):131–50.
Pérez-Martí A, Garcia-Guasch M, Tresserra-Rimbau A, Carrilho-Do-Rosário A, Estruch R, Salas-Salvadó J, et al. A low-protein diet induces body weight loss and browning of subcutaneous white adipose tissue through enhanced expression of hepatic fibroblast growth factor 21 (FGF21). Mol Nutr Food Res. 2017;61(8):1–32.
Article CAS Google Scholar
Finlin BS, Memetimin H, Confides AL, Kasza I, Zhu B, Vekaria HJ, et al. Human adipose beiging in response to cold and mirabegron. JCI Insight. 2018;3(15):e121510.
Article PubMed Central Google Scholar
Otero-Díaz B, Rodríguez-Flores M, Sánchez-Muñoz V, Monraz-Preciado F, Ordoñez-Ortega S, Becerril-Elias V, et al. Exercise induces white adipose tissue browning across the weight spectrum in humans. Front Physiol. 2018;9:1781.
Article PubMed PubMed Central Google Scholar
Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63(10):3253–65.
Article PubMed CAS Google Scholar
Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–4.
Article CAS PubMed PubMed Central Google Scholar
Abdullahi A, Samadi O, Auger C, Kanagalingam T, Boehning D, Bi S, et al. Browning of white adipose tissue after a burn injury promotes hepatic steatosis and dysfunction. Cell Death Dis. 2019;10(12):870.
Lucchini FC, Wueest S, Challa TD, Item F, Modica S, Borsigova M, et al. ASK1 inhibits browning of white adipose tissue in obesity. Nat Commun. 2020;11(1):1642.
Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11(4):253–6.
Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol. 2016;231:R77-99.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–56.
Andrade-Oliveira V, Câmara NOS, Moraes-Vieira PM. Adipokines as drug targets in diabetes and underlying disturbances. J Diabetes Res. 2015;2015:1.
Article Google Scholar
Jokinen R, Pirnes-karhu S, Pietiläinen KH, Pirinen E. Adipose tissue NAD+-homeostasis, sirtuins and poly(ADP-ribose) polymerases—important players in mitochondrial metabolism and metabolic health. Redox Biol. 2017;12(February):246–63.
Parimisetty A, Dorsemans AC, Awada R, Ravanan P, Diotel N, Lefebvre C. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J Neuroinflam. 2016;13:1.
Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–43.
Park A. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J Stem Cells. 2014;6(1):33.
Dalal S. Lipid metabolism in cancer cachexia. Ann Palliat Med. 2019;8(1):13–23.
Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–76.
Cuevas-Ramos D, Mehta R, Aguilar-Salinas CA. Fibroblast growth factor 21 and browning of white adipose tissue. Front Physiol. 2019;10:37.
Bargut TCL, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA. Browning of white adipose tissue: lessons from experimental models. Horm Mol Biol Clin Investig. 2017. https://doi.org/10.1515/hmbci-2016-0051 .
Shao M, Wang QA, Song A, Vishvanath L, Busbuso NC, Scherer PE, et al. Cellular origins of beige fat cells revisited. Diabetes. 2019;68(10):1874–85.
Vargas-Castillo A, Fuentes-Romero R, Rodriguez-Lopez LA, Torres N, Tovar AR. Understanding the biology of thermogenic fat: is browning a new approach to the treatment of obesity? Arch Med Res. 2017;48(5):401–13.
Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19(5):810–20.
Shao M, Vishvanath L, Busbuso NC, Hepler C, Shan B, Sharma AX, et al. De novo adipocyte differentiation from Pdgfrβ+ preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat Commun. 2018;9(1):1–16.
Oguri Y, Shinoda K, Kim H, Alba DL, Bolus WR, Wang Q, et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell. 2020;182(3):563-577.e20.
Tabuchi C, Sul HS. Signaling pathways regulating thermogenesis. Front Endocrinol (Lausanne). 2021;26(12):243.
Google Scholar
Himms-hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670.
Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol - Endocrinol Metab. 2006;291(2):350–7.
Schenk H, Franke H, Heim T. Structure and function of brown adipose tissue. Acta Histochem. 1977;59:195–208.
Klingenberg M, Echtay KS, Bienengraeber M, Winkler E, Huang SG. Structure-function relationship in UCP1. Int J Obes. 1999;23:S24–9.
Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3 StUCP and AtUCP. Biochem J. 2000;345(2):161–79.
Echtay KS. Mitochondrial uncoupling proteins—What is their physiological role? Free Radic Biol Med. 2007;43(10):1351–71.
Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112(Pt 1):35–9.
CAS PubMed PubMed Central Google Scholar
Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci USA. 2017;114(32):8649–54.
Klingenberg M, Echtay KS. Uncoupling proteins: the issues from a biochemist point of view. Biochim Biophys Acta Bioenerg. 2001;1504(1):128–43.
Chechi K, Grundberg E, Richard D, Chechi K, Vijay J, Voisine P, et al. UCP1 expression—associated gene signatures of human epicardial adipose tissue Find the latest version : UCP1 expression—associated gene signatures of human epicardial adipose tissue. JCI Insight. 2019;4(8):e123618.
Hoang T, Smith MD, Jelokhani-Niaraki M. Expression, folding, and proton transport activity of human uncoupling protein-1 (ucp1) in lipid membranes. J Biol Chem. 2013;288(51):36244–58.
Ježek P, Jabůrek M, Porter RK. Uncoupling mechanism and redox regulation of mitochondrial uncoupling protein 1 (UCP1). Biochim Biophys Acta Bioenerg. 2019;1860(3):259–69.
Macher G, Koehler M, Rupprecht A, Kreiter J, Hinterdorfer P, Pohl EE. Inhibition of mitochondrial UCP1 and UCP3 by purine nucleotides and phosphate. Biochim Biophys Acta Biomembr. 2018;1860(3):664–72.
Kozak UC, Kopecky J, Teisinger J, Enerbäck S, Boyer B, Kozak LP. An upstream enhancer regulating brown-fat-specific expression of the mitochondrial uncoupling protein gene. Mol Cell Biol. 1994;14(1):59–67.
Yubero P, Barbera MJ, Alvarez R, Vinas O, Mampel T, Iglesias R, et al. Dominant negative regulation by c-Jun of transcription of the uncoupling protein-1 gene through a proximal cAMP-regulatory element: a mechanism for repressing basal and norepinephrine-induced expression of the gene before brown adipocyte differentiation. Mol Endocrinol. 1998;12(7):1023–37.
Manchado C, Yubero P, Vinas O, Iglesias R, Villarroya F, Mampel T, et al. CCAAT/enhancer-binding proteins α and β in brown adipose tissue: Evidence for a tissue-specific pattern of expression during development. Biochem J. 1994;302(3):695–700.
Cassard-Doulcier AM, Gelly C, Bouillaud F, Ricquier D. A 211-bp enhancer of the rat uncoupling protein-1 (UCP-1) gene controls specific and regulated expression in brown adipose tissue. Biochem J. 1998;333(2):243–6.
Sears IB, MacGinnitie MA, Kovacs LG, Graves RA. Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 1996;16(7):3410–9.
Wang H, Zhang Y, Yehuda-Shnaidman E, Medvedev AV, Kumar N, Daniel KW, et al. Liver X receptor α is a transcriptional repressor of the uncoupling protein 1 gene and the brown fat phenotype. Mol Cell Biol. 2008;28(7):2187–200.
Kiskinis E, Hallberg M, Christian M, Olofsson M, Dilworth SM, White R, et al. RIP140 directs histone and DNA methylation to silence Ucp1 expression in white adipocytes. EMBO J. 2007;26(23):4831–40.
Shore A, Karamitri A, Kemp P, Speakman JR, Lomax MA. Role of Ucp1 enhancer methylation and chromatin remodelling in the control of Ucp1 expression in murine adipose tissue. Diabetologia. 2010;53(6):1164–73.
Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37.
Oliverio M, Schmidt E, Mauer J, Baitzel C, Hansmeier N, Khani S, et al. Dicer1-miR-328-Bace1 signalling controls brown adipose tissue differentiation and function. Nat Cell Biol. 2016;18(3):328–36.
Zhang H, Guan M, Townsend KL, Huang TL, An D, Yan X, et al. Micro RNA -455 regulates brown adipogenesis via a novel HIF 1an- AMPK - PGC 1α signaling network. EMBO Rep. 2015;16(10):1378–93.
Chen SZ, Xu X, Ning LF, Jiang WY, Xing C, Tang QQ, et al. MiR-27 impairs the adipogenic lineage commitment via targeting lysyl oxidase. Obesity. 2015;23(12):2445–53.
Kang T, Lu W, Xu W, Anderson L, Bacanamwo M, Thompson W, et al. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells. J Biol Chem. 2013;288(48):34394–402.
Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol. 2012;14(12):1330–5.
Enerbäck S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90.
Liu X, Rossmeisl M, McClaine J, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111(3):399–407.
Hankir MK, Seyfried F. Do bariatric surgeries enhance brown/beige adipose tissue thermogenesis? Front Endocrinol (Lausanne). 2020;11(April):1–11.
Omran F, Christian M. Inflammatory signaling and brown fat activity. Front Endocrinol (Lausanne). 2020;11(March):1–16.
Reynés B, van Schothorst EM, Keijer J, Ceresi E, Oliver P, Palou A. Cold induced depot-specific browning in ferret aortic perivascular adipose tissue. Front Physiol. 2019;10(September):1–13.
Shankar K, Kumar D, Gupta S, Varshney S, Rajan S, Srivastava A, et al. Role of brown adipose tissue in modulating adipose tissue inflammation and insulin resistance in high-fat diet fed mice. Eur J Pharmacol. 2019;854(February):354–64.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–8.
Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–9.
Ohtomo T, Ino K, Miyashita R, Chigira M, Nakamura M, Someya K, et al. Chronic high-fat feeding impairs adaptive induction of mitochondrial fatty acid combustion-associated proteins in brown adipose tissue of mice. Biochem Biophys Rep. 2017;10:32–8.
PubMed PubMed Central Google Scholar
Winn NC, Vieira-Potter VJ, Gastecki ML, Welly RJ, Scroggins RJ, Zidon TM, et al. Loss of UCP1 exacerbates western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R74-84.
Bond LM, Burhans MS, Ntambi JM. Uncoupling protein-1 deficiency promotes brown adipose tissue inflammation and ER stress. PLoS ONE. 2018;13(11):1–11.
Bond LM, Ntambi JM. UCP1 deficiency increases adipose tissue monounsaturated fatty acid synthesis and trafficking to the liver. J Lipid Res. 2018;59(2):224–36.
Alcalá M, Calderon-Dominguez M, Bustos E, Ramos P, Casals N, Serra D, et al. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci Rep. 2017;7(1):1–12.
Tamucci KA, Namwanje M, Fan L, Qiang L. The dark side of browning. Protein Cell. 2018;9(2):152–63.
Lim J, Park HS, Kim J, Jang YJ, Kim J-H, Lee Y, et al. Depot-specific UCP1 expression in human white adipose tissue and its association with obesity-related markers. Int J Obes. 2020;44(3):697–706.
Han J, Meng Q, Shen L, Wu G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018;17(1):1–8.
Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20(3):433–47.
Li L, Li B, Li M, Speakman JR. Switching on the furnace: Regulation of heat production in brown adipose tissue. Mol Asp Med. 2019;68(August):60–73.
Jeschke MG. The hepatic response to thermal injury: Is the liver important for postburn outcomes? Mol Med. 2009;15(9–10):337–51.
Cuyàs E, Verdura S, Micol V, Joven J, Bosch-Barrera J, Encinar JA, et al. Revisiting silibinin as a novobiocin-like Hsp90 C-terminal inhibitor: computational modeling and experimental validation. Food Chem Toxicol. 2019;132:110645.
Cypess AM, Weiner LS, Roberts-Toler C, Elía EF, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21(1):33–8.
Lim S, Park J, Um JY. Ginsenoside Rb1 induces beta 3 adrenergic receptor–dependent lipolysis and thermogenesis in 3T3-L1 adipocytes and db/db mice. Front Pharmacol. 2019;10:1154.
Grujic D, Susulic VS, Harper M-E, Himms-Hagen J, Cunningham BA, Corkey BE, et al. 3-adrenergic receptors on white and brown adipocytes mediate 3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake: a study using transgenic and gene knockout mice*. J Biol Chem. 1997;272:17689.
Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper M-E, et al. Targeted disruption of the 3-adrenergic receptor gene*. J Biol Chem. 1995;270:29483.
Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471–82.
Concha F, Prado G, Quezada J, Ramirez A, Bravo N, Flores C, et al. Nutritional and non-nutritional agents that stimulate white adipose tissue browning. Rev Endocr Metab Disord. 2019;20:161–71.
Seki T, Hosaka K, Lim S, Fischer C, Honek J, Yang Y, et al. Endothelial PDGF-CC regulates angiogenesis-dependent thermogenesis in beige fat. Nat Commun. 2016;7:1.
Guilherme A, Yenilmez B, Bedard AH, Henriques F, Liu D, Lee A, et al. Control of adipocyte thermogenesis and lipogenesis through β3-adrenergic and thyroid hormone signal integration. Cell Rep. 2020;31(5):107598.
Alcalá M, Calderon-Dominguez M, Serra D, Herrero L, Viana M. Mechanisms of impaired brown adipose tissue recruitment in obesity. Front Physiol. 2019;10:94.
Jimenez M, Barbatelli G, Allevi R, Cinti S, Seydoux J, Giacobino JP, et al. β3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur J Biochem. 2003;270(4):699–705.
Herz CT, Kiefer FW. Adipose tissue browning in mice and humans. J Endocrinol. 2019;241(3):R97-109.
Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15(6):335–49.
Castellani JW, Young AJ. Human physiological responses to cold exposure: acute responses and acclimatization to prolonged exposure. Auton Neurosci. 2016;196:63–74.
Gensini GF, Conti AA. The evolution of the concept of “fever” in the history of medicine: from pathological picture per se to clinical epiphenomenon (and vice versa). J Infect. 2004;49(2):85–7.
Garami A, Steiner AA, Romanovsky AA. Fever and hypothermia in systemic inflammation. Handb Clin Neurol. 2018;157(February):565–97.
Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64(3):769–74.
Copeland S, Shaw Warren H, Lowry SF, Galvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12(1):60–7.
Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9(1):5790.
Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289:1244–52.
Corrigan JJ, Fonseca MT, Flatow EA, Lewis K, Steiner AA. Hypometabolism and hypothermia in the rat model of endotoxic shock: Independence of circulatory hypoxia. J Physiol. 2014;592(17):3901–16.
Wanner SP, Yoshida K, Kulchitsky VA, Ivanov AI, Kanosue K, Romanovsky AA. Lipopolysaccharide-induced neuronal activation in the paraventricular and dorsomedial hypothalamus depends on ambient temperature. PLoS ONE. 2013;8(9):4–9.
Osaka T. Lipopolysaccharide-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. J Therm Biol. 2006;31:229–34.
Tham S, Thompson R, Landeg O, Murray KA, Waite T. Indoor temperature and health: a global systematic review. Public Health. 2020;179:9–17.
Cheshire WP. Thermoregulatory disorders and illness related to heat and cold stress. Auton Neurosci. 2016;196:91–104.
Rumbus Z, Matics R, Hegyi P, Zsiboras C, Szabo I, Illes A, et al. Fever is associated with reduced, hypothermia with increased mortality in septic patients: a meta-analysis of clinical trials. PLoS ONE. 2017;12(1):1–15.
Fu SH, Gasparrini A, Rodriguez PS, Jha P. Mortality attributable to hot and cold ambient temperatures in India: a nationally representative case-crossover study. PLoS Med. 2018;15(7):1–17.
Chen R, Yin P, Wang L, Liu C, Niu Y, Wang W, et al. Association between ambient temperature and mortality risk and burden: time series study in 272 main Chinese cities. BMJ. 2018;363:k4306.
Lee SL, Battistella FD, Go K. Hypothermia induces T-cell production of immunosuppressive cytokines. J Surg Res. 2001;100(2):150–3.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334(19):1209–15.
Hylander BL, Repasky EA. Thermoneutrality, mice, and cancer: a heated opinion. Trends Cancer. 2016;2(4):166–75.
Mace TA, Zhong L, Kilpatrick C, Zynda E, Lee C-T, Capitano M, et al. Differentiation of CD8 + T cells into effector cells is enhanced by physiological range hyperthermia. J Leukoc Biol. 2011;90(5):951–62.
Kokolus KM, Capitano ML, Lee CT, Eng JWL, Waight JD, Hylander BL, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA. 2013;110(50):20176–81.
Du G, Liu Y, Li J, Liu W, Wang Y, Li H. Hypothermic microenvironment plays a key role in tumor immune subversion. Int Immunopharmacol. 2013;17(2):245–53.
Dieing A, Ahlers O, Hildebrandt B, Kerner T, Tamm I, Possinger K, et al. The effect of induced hyperthermia on the immune system. Prog Brain Res. 2007;162:137–52.
Kobayashi Y, Ito Y, Ostapenko VV, Sakai M, Matsushita N, Imai K, et al. Fever-range whole-body heat treatment stimulates antigen-specific T-cell responses in humans. Immunol Lett. 2014;162(1):256–61.
Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007;82(5):1322–31.
Umar D, Das A, Gupta S, Chattopadhyay S, Sarkar D, Mirji G, et al. Febrile temperature change modulates CD4 T cell differentiation via a TRPV channel-regulated Notch-dependent pathway. Proc Natl Acad Sci USA. 2020;117(36):22357–66.
Matsumoto K, Yamamoto N, Hagiwara S, Saito M, Furue H, Shigetomi T, et al. Optimization of hyperthermia and dendritic cell immunotherapy for squamous cell carcinoma. Oncol Rep. 2011;25(6):1525–32.
PubMed Google Scholar
Jackson TC, Kochanek PM. A new vision for therapeutic hypothermia in the era of targeted temperature management: a speculative synthesis. Ther Hypotherm Temp Manag. 2019;9(1):13–47.
Gu LJ, Xiong XX, Ito T, Lee J, Xu BH, Krams S, et al. Moderate hypothermia inhibits brain inflammation and attenuates stroke-induced immunodepression in rats. CNS Neurosci Ther. 2014;20(1):67–75.
Stanek A, Cholewka A, Wielkoszyński T, Romuk E, Sieroń A. Whole-body cryotherapy decreases the levels of inflammatory, oxidative stress, and atherosclerosis plaque markers in male patients with active-phase ankylosing spondylitis in the absence of classical cardiovascular risk factors. Mediators Inflamm. 2018;2018:1.
Lee JH, Wei ZZ, Cao W, Won S, Gu X, Winter M, et al. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol Dis. 2016;96:248–60.
Wilson LJ, Dimitriou L, Hills FA, Gondek MB, Cockburn E. Whole body cryotherapy, cold water immersion, or a placebo following resistance exercise: a case of mind over matter? Eur J Appl Physiol. 2019;119(1):135–47.
Ziemann E, Olek RA, Grzywacz T, Kaczor JJ, Antosiewicz J, Skrobot W, et al. Whole-body cryostimulation as an effective way of reducing exercise-induced inflammation and blood cholesterol in young men. Eur Cytokine Netw. 2014;25(1):14–23.
Stanek A, Romuk E, Wielkoszyński T, Bartuś S, Cieślar G, Cholewka A. Decreased lipid profile and oxidative stress in healthy subjects who underwent whole-body cryotherapy in closed cryochamber with subsequent kinesiotherapy. Oxid Med Cell Longev. 2019;2019:1.
Coolbaugh CL, Damon BM, Bush EC, Welch EB, Towse TF. Cold exposure induces dynamic, heterogeneous alterations in human brown adipose tissue lipid content. Sci Rep. 2019;9(1):13600.
Sun YJ, Zhang ZY, Fan B, Li GY. Neuroprotection by therapeutic hypothermia. Front Neurosci. 2019;13:1–11.
Svedung Wettervik TM, Engquist H, Lenell S, Howells T, Hillered L, Rostami E, et al. Systemic hyperthermia in traumatic brain injury—relation to intracranial pressure dynamics, cerebral energy metabolism, and clinical outcome. J Neurosurg Anesthesiol. 2021;33(4):329–36.
Bain AR, Hoiland RL, Donnelly J, Nowak-Flück D, Sekhon M, Tymko MM, et al. Cerebral metabolism, oxidation and inflammation in severe passive hyperthermia with and without respiratory alkalosis. J Physiol. 2020;598(5):943–54.
Thorne AM, Ubbink R, Bruggenwirth IMA, Nijsten MW, Porte RJ, de Meijer VE. Hyperthermia-induced changes in liver physiology and metabolism: a rationale for hyperthermic machine perfusion. Am J Physiol Gastrointest Liver Physiol. 2020;319(1):G43-50.
Clapham JC. Central control of thermogenesis. Neuropharmacology. 2012;63(1):111–23.
Morrison SF. Central neural control of thermoregulation and brown adipose tissue. Auton Neurosci. 2016;196:14–24.
Fan G, Dang X, Li Y, Chen J, Zhao R, Yang X. Zinc-α2-glycoprotein promotes browning of white adipose tissue in cold-exposed male mice. Mol Cell Endocrinol. 2020;501:110669.
Luo X, Jia R, Zhang Q, Sun B, Yan J. Cold-Induced browning dynamically alters the expression profiles of inflammatory adipokines with tissue specificity in mice. Int J Mol Sci. 2016;17(5):795.
Article PubMed Central CAS Google Scholar
Reitman ML. Of mice and men—environmental temperature, body temperature, and treatment of obesity. FEBS Lett. 2018;592(12):2098–107.
Brychta RJ, Huang S, Wang J, Leitner BP, Hattenbach JD, Bell SL, et al. Quantification of the capacity for cold-induced thermogenesis in young men with and without obesity. J Clin Endocrinol Metab. 2019;104(10):4865–78.
Blauw LL, Aziz NA, Tannemaat MR, Blauw CA, de Craen AJ, Pijl H, et al. Diabetes incidence and glucose intolerance prevalence increase with higher outdoor temperature. BMJ Open Diabetes Res Care. 2017;5(1):e000317.
Kanazawa S. Does global warming contribute to the obesity epidemic? Environ Res. 2020;182:108962.
Li Y, Wang D, Ping X, Zhang Y, Zhang T, Wang L, et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell. 2022;185(6):949-966.e19.
Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, et al. Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Rep. 2015;13(8):1538–44.
Vinaik R, Barayan D, Abdullahi A, Jeschke MG. NLRP3 inflammasome mediates white adipose tissue browning after burn. Am J Physiol Endocrinol Metab. 2019;317(5):E751–9.
Böstrom P, Wu JJM, et al. A PGC1- a -dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19(1):96–108.
Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, et al. p38 Mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24(7):3057–67.
Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt H. Exercise-induced oxidative stress: friend or foe? J Sport Heal Sci. 2020;9(5):415–25.
Reddy A, Bozi LHM, Yaghi OK, Mills EL, Xiao H, Nicholson HE, et al. pH-gated succinate secretion regulates muscle remodeling in response to exercise. Cell. 2020;183(1):62-75.e17.
Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta Gene Struct Expr. 2000;1492(1):203–6.
Giralt M, Gavaldà-Navarro A, Villarroya F. Fibroblast growth factor-21, energy balance and obesity. Mol Cell Endocrinol. 2015;418:66–73.
Tacer KF, Bookout AL, Ding X, Kurosu H, John GB, Wang L, et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24(10):2050–64.
Kharitonenkov A, Jaskunas SR, Shanafelt AB, Shiyanova TL, Koester A, Ford AM, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627.
Luo Y, Ye S, Li X, Lu W. Emerging structure-function paradigm of endocrine FGFs in metabolic diseases. Trends Pharmacol Sci. 2019;40:142–53.
Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27(9):3417–28.
Yang C, Jin C, Li X, Wang F, McKeehan WL, Luo Y. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One. 2012;7(3):e33870.
Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78(1):223–41.
Martínez-Garza Ú, Torres-Oteros D, Yarritu-Gallego A, Marrero PF, Haro D, Relat J. Fibroblast growth factor 21 and the adaptive response to nutritional challenges. Int J Mol Sci. 2019;20:4692.
Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139(2):456–63.
Cuevas-Ramos DASCA. Modulation of energy balance by fibroblast growth factor 21. Hormone Mol Biol Clin Investig. 2016. https://doi.org/10.1515/hmbci-2016-0023 .
Oishi K, Uchida D, Ishida N. Circadian expression of FGF21 is induced by PPARα activation in the mouse liver. FEBS Lett. 2008;582(25–26):3639–42.
Lundåsen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SEH, et al. PPARα is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360(2):437–40.
Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab. 2008;8(2):169–74.
Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250–9.
Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, Zhang Y, et al. FGF21 and the late adaptive response to starvation in humans. J Clin Invest. 2015;125(12):4601–11.
Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5(6):426–37.
Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–22.
De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem J. 2012;443(1):165–71.
De Sousa-Coelho AL, Relat J, Hondares E, Pérez-Martí A, Ribas F, Villarroya F, et al. FGF21 mediates the lipid metabolism response to amino acid starvation. J Lipid Res. 2013;54(7):1786–97.
Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor γ and altered metabolic states. Mol Pharmacol. 2008;74(2):403–12.
Samms RJ, Murphy M, Fowler MJ, Cooper S, Emmerson P, Coskun T, et al. Dual effects of fibroblast growth factor 21 on hepatic energy metabolism. J Endocrinol. 2015;227(1):37–47.
Lin Z, Tian H, Lam KSL, Lin S, Hoo RCL, Konishi M, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17(5):779–89.
Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17(5):790–7.
Fisher FF, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–81.
Pérez-Martí A, Sandoval V, Marrero PF, Haro D, Relat J. Nutritional regulation of fibroblast growth factor 21: from macronutrients to bioactive dietary compounds. Hormone Mol Biol Clin Investig. 2017. https://doi.org/10.1515/hmbci-2016-0034 .
Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122:3035–43.
Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938.
Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–82.
Rubio IG, Medeiros-Neto G. Mutations of the thyroglobulin gene and its relevance to thyroid disorders. Curr Opin Endocrinol Diabetes Obes. 2009;16(5):373–8.
Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376–408.
Carvalho DP, Dupuy C. Thyroid hormone biosynthesis and release. Mol Cell Endocrinol. 2017;458:6–15.
Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–70.
Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol. 2014;10:582–91.
Lindsey RC, Mohan S. Thyroid hormone acting via TRβ induces expression of browning genes in mouse bone marrow adipose tissue. Endocrine. 2017;56(1):109–20.
Haber RS, Loeb JN. Stimulation of potassium efflux in rat liver by a low dose of thyroid hormone: evidence for enhanced cation permeability in the absence of Na. K-ATPase Induct Endocrinol. 1986;118(1):207–11.
Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–64.
Freake HC, Schwartz HL, Oppenheimer JH. The regulation of lipogenesis by thyroid hormone and its contribution to thermogenesis. Endocrinology. 1989;125(6):2868–74.
Clausen T, Van Hardeveld C, Everts ME. Significance of cation transport in control of energy metabolism and thermogenesis. Physiol Rev. 1991;71:733–74.
Barbe P, Larrouy D, Boulanger C, Chevillotte E, Viguerie N, Thalamas C, et al. Triiodothyronine-mediated upregulation of UCP2 and UCP3 mRNA expression in human skeletal muscle without coordinated induction of mitochondrial respiratory chain genes. FASEB J. 2001;15(1):13–5.
Liu YY, Schultz JJ, Brent GA. A thyroid hormone receptor α gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem. 2003;278(40):38913–20.
Lee JY, Takahashi N, Yasubuchi M, Kim Y, Hashizaki H, Kim MJ, et al. Triiodothyronine induces ucp-1 expression and mitochondrial biogenesis in human adipocytes. Am J Physiol Cell Physiol. 2012;302(2):c463.
Weiner J, Hankir M, Heiker JT, Fenske W, Krause K. Thyroid hormones and browning of adipose tissue. Mol Cell Endocrinol. 2017;458:156–9.
López M, Varela L, Vázquez MJ, Rodríguez-Cuenca S, González CR, Velagapudi VR, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16(9):1001–8.
Bianco AC, Sheng X, Silva JE. Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis*. J Biol Chem. 1988;263:18168.
Martínez-Sánchez N, Moreno-Navarrete JM, Contreras C, Rial-Pensado E, Fernø J, Nogueiras R, et al. Thyroid hormones induce browning of white fat. J Endocrinol. 2017;232(2):351–62.
Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56(4):371–81.
Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, et al. Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol (Lausanne). 2019;17(10):249.
Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–47.
Hsuchou H, Kastin AJ, Tu H, Markadakis EN, Stone KP, Wang Y, et al. Effects of cell-type specific leptin receptor mutation on leptin transport across the BBB. Peptides. 2011;32(7):1392–9.
Hsuchou H, Wang Y, Cornelissen-Guillaume GG, Kastin AJ, Jang E, Halberg F, et al. Diminished leptin signaling can alter circadian rhythm of metabolic activity and feeding. J Appl Physiol. 2013;115(7):995–1003.
Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW, et al. Direct effects of leptin on brown and white adipose tissue. J Clin Invest. 1997;100(11):2858.
Hoffmann A, Ebert T, Hankir MK, Flehmig G, Klöting N, Jessnitzer B, et al. Leptin improves parameters of brown adipose tissue thermogenesis in lipodystrophic mice. Nutrients. 2021;13(8):1–10.
Van Den Berg R, Kooijman S, Noordam R, Van Heemst D, Biermasz NR, Rensen PCN. A diurnal rhythm in brown adipose tissue causes rapid clearance and combustion of plasma lipids at wakening. Cell Rep. 2018;22:3521–33.
Matsushita M, Nirengi S, Hibi M, Wakabayashi H, Lee S, Domichi M, et al. Diurnal variations of brown fat thermogenesis and fat oxidation in humans. Int J Obes. 2021;45(11):2499–505.
Halpern B, Mancini MC, Bueno C, Barcelos IP, De Melo ME, Lima MS, et al. Melatonin increases brown adipose tissue volume and activity in patients with melatonin deficiency: a proof-of-concept study. Diabetes. 2019;68(5):947–52.
Halpern B, Mancini MC, Mendes C, MacHado CML, Prando S, Sapienza MT, et al. Melatonin deficiency decreases brown adipose tissue acute thermogenic capacity of in rats measured by 18F-FDG PET. Diabetol Metab Syndr. 2020;12(1):1–7.
Agil A, Navarro-Alarcon M, Ali FAZ, Albrakati A, Salagre D, Campoy C, et al. Melatonin enhances the mitochondrial functionality of brown adipose tissue in obese-diabetic rats. Antioxidants (Basel, Switzerland). 2021;10(9):1482.
Wang S, Liang X, Yang Q, et al. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int J Obes. 2015;39(6):967–76.
Choi JH, Kim SW, Yu R, Yun JW. Monoterpene phenolic compound thymol promotes browning of 3T3 - L1 adipocytes. Eur J Nutr. 2016;56(7):2329–41.
Kang N, Mukherjee S, Yun J. Trans-cinnamic acid stimulates white fat browning. Nutrients. 2019;11(3):577.
Article CAS PubMed Central Google Scholar
Lee SG, Parks JS, Kang HW. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J Nutr Biochem. 2017;2016(46):62–71.
Arias N, Picó C, Teresa Macarulla M, Oliver P, Miranda J, Palou A, et al. A combination of resveratrol and quercetin induces browning in white adipose tissue of rats fed an obesogenic diet. Obes (Silver Spring). 2016;25:111–21.
Choi JH, Yun JW. Chrysin induces brown fat-like phenotype and enhances lipid metabolism in 3T3-L1 adipocytes. Nutrition. 2016;32:1002–10.
Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173(15):2369–89.
Jiang C, Zhai M, Yan D, Li D, Li C, Zhang Y. Dietary menthol-induced TRPM8 activation enhances WAT “ browning ” and ameliorates diet-induced obesity. Oncotarget. 2017;8(43):75114–26.
Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun. 2005;332:392–7.
Serra F, Bonet ML, Puigserver P, Oliver J, Palou A. Stimulation of uncoupling protein 1 expression in brown adipocytes by naturally occurring carotenoids. Int J Obes Relat Metab Disord. 1999;23:650–6.
Chou Y, Ho C, Pan M. Immature Citrus reticulata extract promotes browning of beige adipocytes in high-fat diet-induced C57BL / 6 mice. Food Chem. 2018;66:9769–9703.
Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y, et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun. 2014;5(1):1.
Neyrinck AM, Bindels LB, Geurts L, Van M, Cani PD, Delzenne NM. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J Nutr Biochem. 2017;49:15–21.
Chen L, Chien Y, Liang C, Chan C, Huang H, Chien Y, et al. Green tea extract induces genes related to browning of white adipose tissue and limits weight-gain in high energy diet-fed rat. Food Nutr Res. 2017;61(1):1347480.
Palacios-gonzález B, Vargas-castillo A, Velázquez-villegas LA, Vasquez-reyes S, López P, Noriega LG, et al. ScienceDirect genistein increases the thermogenic program of subcutaneous WAT and increases energy expenditure in mice*. J Nutr Biochem. 2019;68:59–68.
Parray HA, Lone J, Park JP, Choi JW, Yun J. Magnolol promotes thermogenesis and attenuates oxidative stress in 3T3-L1 adipocytes. Nutrition. 2018;50:82–90.
Lone J, Yun JW. Honokiol exerts dual effects on browning and apoptosis of adipocytes. Pharmacol Reports. 2017;69(6):1357–65.
Mu Q, Fang X, Li X, Zhao D, Mo F, Jiang G, et al. Ginsenoside Rb1 promotes browning through regulation of PPAR g in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2015;466(3):530–5.
Lee K, Seo Y, Song J, Lee B. Ginsenoside Rg1 promotes browning by inducing UCP1 expression and mitochondrial activity in 3T3-L1 and subcutaneous white adipocytes. J Ginseng Res. 2018;43(4):589–99.
Bargut TC, Silva-e-Silva AC, Souza-Mello V, Mandarim-de-Lacerda CAAM, Mandarim A. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur J Nutr. 2016;55(1):159–69.
Pahlavani M, Razafimanjato F, Ramalingam L, Nishan S. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J Nutr Biochem. 2016;39:101–9.
Kim M, Goto T, Yu R, Uchida K, Tominaga M, Kano Y. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci Rep. 2015;5:1–12.
Shen W, Chuang C, Martinez K, Reid T, Brown JM, Xi L, et al. Conjugated linoleic acid reduces adiposity and increases markers of browning and infl ammation in white adipose tissue of mice. J Lipid Res. 2013;54(4):909–22.
Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity. Circulation. 2016;133(2):187–225.
Hofer SJ, Carmona-Gutierrez D, Mueller MI, Madeo F. The ups and downs of caloric restriction and fasting: from molecular effects to clinical application. EMBO Mol Med. 2022;14(1):e14418.
Weindruch R, Sohal RS. Caloric intake and aging. N Engl J Med. 1997;337(14):986–94.
Fabbiano S, Suárez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ, et al. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab. 2016;24(3):434–46.
Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. 2018;26(2):254–68.
Patel S, Alvarez-Guaita A, Melvin A, Rimmington D, Dattilo A, Miedzybrodzka EL, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019;29(3):707-718.e8.
de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–51.
Liu B, Hutchison AT, Thompson CH, Lange K, Heilbronn LK. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes Res Clin Pract. 2019;13(4):408–15.
Liu B, Page AJ, Hutchison AT, Wittert GA, Heilbronn LK. Intermittent fasting increases energy expenditure and promotes adipose tissue browning in mice. Nutrition. 2019;1(66):38–43.
de Marinho TS, Ornellas F, Aguila MB, Mandarim-de-Lacerda CA. Browning of the subcutaneous adipocytes in diet-induced obese mouse submitted to intermittent fasting. Mol Cell Endocrinol. 2020;513:110872.
Shinoda K, Luijten IHN, Hasegawa Y, Hong H, Sonne SB, Kim M, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015;21(4):389–94.
Xue R, Lynes MD, Dreyfuss JM, Shamsi F, Schulz TJ, Zhang H, et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat Med. 2015;21(7):760–8.
Zuriaga MA, Fuster JJ, Gokce N, Walsh K. Humans and mice display opposing patterns of “browning” gene expression in visceral and subcutaneous white adipose tissue depots. Front Cardiovasc Med. 2017;5:4.
Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26(4):672-685.e4.
von Schwartzenberg RJ, Bisanz JE, Lyalina S, Spanogiannopoulos P, Ang QY, Cai J, et al. Caloric restriction disrupts the microbiota and colonization resistance. Nat. 2021;595(7866):272–7.
Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, Bartness TJ. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metab. 2016;5(8):626–34.
Simcox J, Geoghegan G, Maschek JA, Bensard CL, Pasquali M, Miao R, et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 2017;26(3):509-522.e6.
Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, Nilsson SK, et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Atherosclerosis. 2016;1(252):e239–40.
Berbeé JFP, Boon MR, Khedoe PPSJ, Bartelt A, Schlein C, Worthmann A, et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun. 2015;6(1):1–11.
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–91.
Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nat. 2006;439(7075):484–9.
Broeders EPM, Nascimento EBM, Havekes B, Brans B, Roumans KHM, Tailleux A, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. 2015;22(3):418–26.
Zietak M, Kozak LP. Bile acids induce uncoupling protein 1-dependent thermogenesis and stimulate energy expenditure at thermoneutrality in mice. Am J Physiol Endocrinol Metab. 2016;310(5):E346–54.
Fan M, Wang Y, Jin L, Fang Z, Peng J, Tu J, et al. Bile acid-mediated activation of brown fat protects from alcohol-induced steatosis and liver injury in mice. Cell Mol Gastroenterol Hepatol. 2022;13(3):809–26.
Zhou W, VanDuyne P, Zhang C, Riessen R, Barragan M, Rowitz BM, et al. Bile acid excess impairs thermogenic function in brown adipose tissue. bioRxiv. 2020;155:213.
Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347(1):11–20.
Schmierer B, Hill CS. TGFβ–SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8(12):970–82.
Qian S, Tang Y, Tang Q-Q. Adipose tissue plasticity and the pleiotropic roles of BMP signaling. J Biol Chem. 2021;1(296):100678.
Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2004;101(26):9607–11.
Qian S-W, Tang Y, Li X, Liu Y, Zhang Y-Y, Huang H-Y, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci USA. 2013;110(9):E798-807.
Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, et al. Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Hum Mol Genet. 2014;23(22):5866–78.
Ong CT, Corces VG. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12(4):283–93.
Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, et al. Initiation of myoblast to brown fat switch by a PRDM16–C/EBP-β transcriptional complex. Nature. 2009;460(7259):1154–8.
Ma X, Wang D, Zhao W, Xu L. Deciphering the roles of PPARγ in adipocytes via dynamic change of transcription complex. Front Endocrinol (Lausanne). 2018;9:1–10.
Lehrke M, Lazar MA. The many faces of PPARγ. Cell. 2005;123(6):993–9.
Madsen MS, Siersbæk R, Boergesen M, Nielsen R, Mandrup S. Peroxisome proliferator-activated receptor γ and C/EBPα synergistically activate key metabolic adipocyte genes by assisted loading. Mol Cell Biol. 2014;34(6):939–54.
Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22(21):2941–52.
Kroon T, Harms M, Maurer S, Bonnet L, Alexandersson I, Lindblom A, et al. PPARγ and PPARα synergize to induce robust browning of white fat in vivo. Mol Metab. 2020;36(February):1–14.
Rachid TL, Silva-Veiga FM, Graus-Nunes F, Bringhenti I, Mandarim-de-Lacerda CA, Souza-Mello V. Differential actions of PPAR-α and PPAR-β/δ on beige adipocyte formation: a study in the subcutaneous white adipose tissue of obese male mice. PLoS ONE. 2018;13(1):e0191365.
Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPAR agonists Induce a White-to-Brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15(3):395–404.
Ishibashi J, Seale P. Functions of Prdm16 in thermogenic fat cells. Temperature. 2015;2(1):65–72.
Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121(1):96–105.
Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156(1–2):304–16.
Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, et al. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014;19(4):593–604.
Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6(1):38–54.
Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Am J Physiol Adv Physiol Educ. 2006;30(4):145–51.
Austin S, St-Pierre J. PGC1α and mitochondrial metabolism - emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125(21):4963–71.
Uguccioni G, Hood DA. The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am J Physiol Endocrinol Metab. 2011;300(2):361–71.
Correia JC, Ferreira DMS, Ruas JL. Intercellular: Local and systemic actions of skeletal muscle PGC-1s. Trends Endocrinol Metab. 2015;26(6):305–14.
Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–70.
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–24.
Christianson JL, Boutet E, Puri V, Chawla A, Czech MP. Identification of the lipid droplet targeting domain of the Cidea protein. J Lipid Res. 2010;51(12):3455–62.
Puri V, Ranjit S, Konda S, Nicoloro SMC, Straubhaar J, Chawla A, et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci USA. 2008;105(22):7833–8.
Reynolds TH, Banerjee S, Sharma VM, Donohue J, Couldwell S, Sosinsky A, et al. Effects of a high fat diet and voluntary wheel running exercise on cidea and cidec expression in liver and adipose tissue of mice. PLoS One. 2015;10(7):e0130259.
Jash S, Banerjee S, Lee MJ, Farmer SR, Puri V. CIDEA transcriptionally regulates UCP1 for britening and thermogenesis in human fat cells. iScience. 2019;20:73–89.
Dempersmier J, Sambeat A, Gulyaeva O, Paul SM, Hudak CSS, Raposo HF, et al. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol Cell. 2015;57(2):235–46.
Ma X, Xu L, Alberobello AT, Gavrilova O, Bagattin A, Skarulis M, et al. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1α transcriptional axis. Cell Metab. 2015;22(4):695–708.
Liu J, Lee J, Hernandez MAS, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161(5):999–1011.
Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13(3):249–59.
Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, et al. IRF4 is a key thermogenic transcriptional partner of PGC-1α. Cell. 2014;158(1):69–83.
Hiraike Y, Waki H, Yu J, Nakamura M, Miyake K, Nagano G, et al. NFIA co-localizes with PPARγ and transcriptionally controls the brown fat gene program. Nat Cell Biol. 2017;19(9):1081–92.
Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013;17(4):562–74.
Zhao XY, Li S, Wang GX, Yu Q, Lin JD. A long noncoding RNA transcriptional regulatory circuit drives thermogenic adipocyte differentiation. Mol Cell. 2014;55(3):372–82.
Stine RR, Shapira SN, Lim HW, Ishibashi J, Harms M, Won KJ, et al. EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol Metab. 2015;5(1):57–65.
Shao M, Ishibashi J, Kusminski CM, Wang QA, Hepler C, Vishvanath L, et al. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab. 2016;23(6):1167–84.
Shao M, Zhang Q, Truong A, Shan B, Vishvanath L, Li L, et al. ZFP423 controls EBF2 coactivator recruitment and PPARγ occupancy to determine the thermogenic plasticity of adipocytes. Genes Dev. 2021;35(21–22):1461–74.
Villanueva CJ, Waki H, Godio C, Nielsen R, Chou WL, Vargas L, et al. TLE3 is a dual-function transcriptional coregulator of adipogenesis. Cell Metab. 2011;13(4):413–27.
Pearson S, Loft A, Rajbhandari P, Simcox J, Lee S, Tontonoz P, et al. Loss of TLE3 promotes the mitochondrial program in beige adipocytes and improves glucose metabolism. Genes Dev. 2019;33(13–14):747–62.
Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol. 2016;17(8):480–95.
Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016;17(11):691–702.
Loft A, Forss I, Mandrup S. Genome-wide insights into the development and function of thermogenic adipocytes. Trends Endocrinol Metab. 2017;28(2):104–20.
Squillaro T, Peluso G, Galderisi U, Di Bernardo G. Long non-coding RNAs in regulation of adipogenesis and adipose tissue function. Elife. 2020;1(9):1–15.
Nanduri R. Epigenetic regulators of white adipocyte browning. Epigenomes. 2021;5(1):3.
Ong BX, Brunmeir R, Zhang Q, Peng X, Idris M, Liu C, et al. Regulation of thermogenic adipocyte differentiation and adaptive thermogenesis through histone acetylation. Front Endocrinol (Lausanne). 2020;27(11):95.
Yamauchi T, Oike Y, Kamon J, Waki H, Komeda K, Tsuchida A, et al. Increased insulin sensitivity despite lipodystrophy in Crebbp heterozygous mice. Nat Genet. 2002;30(2):221–6.
Roh HC, Tsai LTY, Shao M, Tenen D, Shen Y, Kumari M, et al. Warming induces significant reprogramming of beige, but not brown. Adipocyte Cell Identity Cell Metab. 2018;27(5):1121-1137.e5.
CAS PubMed Google Scholar
Jin Q, Wang C, Kuang X, Feng X, Sartorelli V, Ying H, et al. Gcn5 and PCAF regulate PPARγ and Prdm16 expression to facilitate brown adipogenesis. Mol Cell Biol. 2014;34(19):3746–53.
Li F, Wu R, Cui X, Zha L, Yu L, Shi H, et al. Histone deacetylase 1 (HDAC1) negatively regulates thermogenic program in brown adipocytes via coordinated regulation of histone H3 lysine 27 (H3K27) deacetylation and methylation. J Biol Chem. 2016;291(9):4523–36.
Ferrari A, Longo R, Fiorino E, Silva R, Mitro N, Cermenati G, et al. HDAC3 is a molecular brake of the metabolic switch supporting white adipose tissue browning. Nat Commun. 2017;8(1):1.
Chatterjee TK, Basford JE, Knoll E, Tong WS, Blanco V, Blomkalns AL, et al. HDAC9 knockout mice are protected from adipose tissue dysfunction and systemic metabolic disease during high-fat feeding. Diabetes. 2014;63(1):176–87.
Bagchi RA, Ferguson BS, Stratton MS, Hu T, Cavasin MA, Sun L, et al. HDAC11 suppresses the thermogenic program of adipose tissue via BRD2. JCI Insight. 2018;3(15):120159–120159.
Mayoral R, Osborn O, McNelis J, Johnson AM, Oh DY, Izquierdo CL, et al. Adipocyte SIRT1 knockout promotes PPARγ activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity. Mol Metab. 2015;4(5):378–91.
Thakore PI, Black JB, Hilton IB, Gersbach CA. Editing the epigenome: technologies for programmable transcription and epigenetic modulation. Nat Methods. 2016;13(2):127–37.
Lee J, Saha PK, Yang QH, Lee S, Jung YP, Suh Y, et al. Targeted inactivation of MLL3 histone H3-Lys-4 methyltransferase activity in the mouse reveals vital roles for MLL3 in adipogenesis. Proc Natl Acad Sci USA. 2008;105(49):19229–34.
Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nat. 2013;504(7478):163–7.
Duteil D, Tosic M, Willmann D, Georgiadi A, Kanouni T, Schüle R. Lsd1 prevents age-programed loss of beige adipocytes. Proc Natl Acad Sci USA. 2017;114(20):5265–70.
Duteil D, Metzger E, Willmann D, Karagianni P, Friedrichs N, Greschik H, et al. LSD1 promotes oxidative metabolism of white adipose tissue. Nat Commun. 2014;5(1):1–14.
Zeng X, Jedrychowski MP, Chen Y, Serag S, Lavery GG, Gygi SP, et al. Lysine-specific demethylase 1 promotes brown adipose tissue thermogenesis via repressing glucocorticoid activation. Genes Dev. 2016;30(16):1822–36.
Abe Y, Rozqie R, Matsumura Y, Kawamura T, Nakaki R, Tsurutani Y, et al. JMJD1A is a signal-sensing scaffold that regulates acute chromatin dynamics via SWI/SNF association for thermogenesis. Nat Commun. 2015;6(1):1–14.
Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25(10):1010–22.
Shapira SN, Seale P. Transcriptional control of brown and beige fat development and function. Obesity (Silver Spring). 2019;27(1):13–21.
Kadam S, Emerson EBM. Each of these complexes contains one of two ATPases, BRG1 or BRM, and a variable subunit composition of BRG1-associated factors (BAFs). In Mol Cell. 1996;2003(11):377–89.
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402.
Ng R, Hussain NA, Zhang Q, Chang C, Li H, Fu Y, et al. miRNA-32 drives brown fat thermogenesis and trans-activates subcutaneous white fat browning in mice. Cell Rep. 2017;19(6):1229–46.
Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med. 2019;11(496):eaav1892–eaav1892.
Acharya A, Berry DC, Zhang H, Jiang Y, Jones BT, Hammer RE, et al. MiR-26 suppresses adipocyte progenitor differentiation and fat production by targeting Fbxl19. Genes Dev. 2019;33(19–20):1367–80.
Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLOS Biol. 2012;10(4):e1001314.
Hu F, Wang M, Xiao T, Yin B, He L, Meng W, et al. miR-30 promotes thermogenesis and the development of beige fat by targeting RIP140. Diabetes. 2015;64(6):2056–68.
Chou CF, Lin YY, Wang HK, Zhu X, Giovarelli M, Briata P, et al. KSRP ablation enhances brown fat gene program in white adipose tissue through reduced mir-150 expression. Diabetes. 2014;63(9):2949–61.
Fu T, Seok S, Choi S, Huang Z, Suino-Powell K, Xu HE, et al. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol Cell Biol. 2014;34(22):4130–42.
Chen Y, Siegel F, Kipschull S, Haas B, Fröhlich H, Meister G, et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun. 2013;4(1):1–13.
Giroud M, Karbiener M, Pisani DF, Ghandour RA, Beranger GE, Niemi T, et al. Let-7i-5p represses brite adipocyte function in mice and humans. Sci Rep. 2016;6(1):1–11.
Giroud M, Pisani DF, Karbiener M, Barquissau V, Ghandour RA, Tews D, et al. miR-125b affects mitochondrial biogenesis and impairs brite adipocyte formation and function. Mol Metab. 2016;5(8):615.
West KA, Lagos D. Long non-coding RNA function in CD4+ T cells: what we know and what next? Non-Coding RNA. 2019;5(3):43.
De Jesus BB, Marinho SP, Arros S, Sousa-Franco A, Alves-Vale C, Carvalho T, et al. Silencing of the lncRNA Zeb2-NAT facilitates reprogramming of aged fibroblasts and safeguards stem cell pluripotency. Nat Commun. 2018;9(1):1–11.
Faghihi MA, Zhang M, Huang J, Modarresi F, Van der Brug MP, Nalls MA, et al. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 2010;11(5):1–13.
Zhang A, Zhao JC, Kim J, Fong KW, Yang YA, Chakravarti D, et al. LncRNA HOTAIR enhances the androgen-receptor-mediated transcriptional program and drives castration-resistant prostate cancer. Cell Rep. 2015;13(1):209–21.
Hu WL, Jin L, Xu A, Wang YF, Thorne RF, Zhang XD, et al. GUARDIN is a p53-responsive long non-coding RNA that is essential for genomic stability. Nat Cell Biol. 2018;20(4):492–502.
Lee S, Kopp F, Chang TC, Sataluri A, Chen B, Sivakumar S, et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164(1–2):69–80.
Shapira SN, Lim HW, Rajakumari S, Sakers AP, Ishibashi J, Harms MJ, et al. EBF2 transcriptionally regulates brown adipogenesis via the histone reader DPF3 and the BAF chromatin remodeling complex. Genes Dev. 2017;31(7):660–73.
Li S, Mi L, Yu L, Yu Q, Liu T, Wang GX, et al. Zbtb7b engages the long noncoding RNA Blnc1 to drive brown and beige fat development and thermogenesis. Proc Natl Acad Sci USA. 2017;114(34):E7111–20.
Clapier CR, Iwasa J, Cairns BR, Peterson CL. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat Rev Mol Cell Biol. 2017;18(7):407–22.
Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, et al. Activation of PPARγ coactivator-1 through transcription factor docking. Science (80-). 1999;286(5443):1368–71.
Roh HC, Tsai LTY, Lyubetskaya A, Tenen D, Kumari M, Rosen ED. Simultaneous transcriptional and epigenomic profiling from specific cell types within heterogeneous tissues in vivo. Cell Rep. 2017;18(4):1048–61.
Liu PS, Lin YW, Burton FH, Wei LN. Injecting engineered anti-inflammatory macrophages therapeutically induces white adipose tissue browning and improves diet-induced insulin resistance. Adipocyte. 2015;4(2):123–8.
Yang JP, Anderson AE, McCartney A, Ory X, Ma G, Pappalardo E, et al. Metabolically active three-dimensional brown adipose tissue engineered from white adipose-derived stem cells. Tissue Eng Part A. 2017;23(7–8):253–62.
Blumenfeld NR, Kang HJ, Fenzl A, Song Z, Chung JJ, Singh R, et al. A direct tissue-grafting approach to increasing endogenous brown fat. Sci Rep. 2018;8(1):7957.
Zhang Y, Liu Q, Yu J, Yu S, Wang J, Qiang L, et al. Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano. 2017;11(9):9223–30.
Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B, et al. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab. 2016;23(2):315–23.
Auger C, Knuth CM, Abdullahi A, Samadi O, Parousis A, Jeschke MG. Metformin prevents the pathological browning of subcutaneous white adipose tissue. Mol Metab. 2019;29(August):12–23.
Balaz M, Becker AS, Balazova L, Straub L, Müller J, Gashi G, et al. Inhibition of mevalonate pathway prevents adipocyte browning in mice and men by affecting protein prenylation. Cell Metab. 2019;29(4):901-916.e8.
Lee P, Swarbrick MM, Ho KKY. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev. 2013;34(3):413–38.
Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE. 2012;7(11):e49452.
Mo Q, Salley J, Roshan T, Baer LA, May FJ, Jaehnig EJ, et al. Identification and characterization of a supraclavicular brown adipose tissue in mice. JCI Insight. 2017;2(11):e93166.
de Jong JMA, Sun W, Pires ND, Frontini A, Balaz M, Jespersen NZ, et al. Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Nat Metab. 2019;1(8):830–43.
Kajimura S, Spiegelman BM. Confounding issues in the ‘humanized’ BAT of mice. Nat Metab. 2020;2(4):303–4.
de Jong JMA, Cannon B, Nedergaard J, Wolfrum C, Petrovic N. Reply to ‘confounding issues in the “humanized” brown fat of mice.’ Nat Metab. 2020;2(4):305–6.
Download references
Author information
Sabrina Azevedo Machado and Gabriel Pasquarelli-do-Nascimento have contributed equally to this work.
Authors and Affiliations
Laboratory of Immunology and Inflammation, Department of Cell Biology, University of Brasilia, Brasília, DF, Brazil
Sabrina Azevedo Machado, Gabriel Pasquarelli-do-Nascimento, Debora Santos da Silva, Gabriel Ribeiro Farias, Igor de Oliveira Santos, Luana Borges Baptista & Kelly Grace Magalhães
You can also search for this author in PubMed Google Scholar
Contributions
SAM, GP, GFR, LBB, DSS, and IOS wrote different sections of the manuscript. IOS prepared the figure. KGM prepared, revised, and wrote the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Kelly Grace Magalhães .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Machado, S.A., Pasquarelli-do-Nascimento, G., da Silva, D. et al. Browning of the white adipose tissue regulation: new insights into nutritional and metabolic relevance in health and diseases. Nutr Metab (Lond) 19 , 61 (2022). https://doi.org/10.1186/s12986-022-00694-0
Download citation
Received : 19 May 2022
Accepted : 19 August 2022
Published : 06 September 2022
DOI : https://doi.org/10.1186/s12986-022-00694-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Nutrition & Metabolism
ISSN: 1743-7075
- General enquiries: [email protected]
Protocol for the in vitro isolation and culture of mature adipocytes and white adipose tissue explants from humans and mice
Affiliations.
- 1 Department of Endocrinology and Nutrition, Research Unit, Institut d'Investigació Sanitària Pere Virgili (IISPV), Hospital Universitari de Tarragona Joan XXIII, 43005 Tarragona, Spain; CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, 28029 Madrid, Spain; Department of Medicine and Surgery, Universitat Rovira i Virgili (URV), 43201 Reus, Spain.
- 2 Department of Endocrinology and Nutrition, Research Unit, Institut d'Investigació Sanitària Pere Virgili (IISPV), Hospital Universitari de Tarragona Joan XXIII, 43005 Tarragona, Spain; CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, 28029 Madrid, Spain.
- 3 Department of Endocrinology and Nutrition, Research Unit, Institut d'Investigació Sanitària Pere Virgili (IISPV), Hospital Universitari de Tarragona Joan XXIII, 43005 Tarragona, Spain; CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, 28029 Madrid, Spain. Electronic address: [email protected].
- PMID: 37924518
- PMCID: PMC10656257
- DOI: 10.1016/j.xpro.2023.102693
White adipose tissue (WAT) explants culture allows the study of this tissue ex vivo, maintaining its structure and properties. Concurrently, isolating mature adipocytes facilitates research into fat cell metabolism and hormonal regulation. Here, we present a protocol for obtaining, isolating, and processing mature adipocytes, alongside the cultivation of WAT explants from humans and mice. We describe steps for WAT retrieval, culturing of WAT explants, WAT digestion, and adipocytes separation. We then detail procedures for culturing isolated mature adipocytes. For complete details on the use and execution of this protocol, please refer to Villanueva-Carmona et al. (2023). 1 .
Keywords: Cell Culture; Cell Isolation; Metabolism; Model Organisms.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
- Adipocytes*
- Adipose Tissue, White*
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 04 January 2019
Adipogenesis and metabolic health
- Alexandra L. Ghaben 1 &
- Philipp E. Scherer ORCID: orcid.org/0000-0003-0680-3392 1
Nature Reviews Molecular Cell Biology volume 20 , pages 242–258 ( 2019 ) Cite this article
42k Accesses
792 Citations
50 Altmetric
Metrics details
- Fat metabolism
- Mesenchymal stem cells
Obesity is characterized by increased adipose tissue mass and has been associated with a strong predisposition towards metabolic diseases and cancer. Thus, it constitutes a public health issue of major proportion. The expansion of adipose depots can be driven either by the increase in adipocyte size (hypertrophy) or by the formation of new adipocytes from precursor differentiation in the process of adipogenesis (hyperplasia). Notably, adipocyte expansion through adipogenesis can offset the negative metabolic effects of obesity, and the mechanisms and regulators of this adaptive process are now emerging. Over the past several years, we have learned a considerable amount about how adipocyte fate is determined and how adipogenesis is regulated by signalling and systemic factors. We have also gained appreciation that the adipogenic niche can influence tissue adipogenic capability. Approaches aimed at increasing adipogenesis over adipocyte hypertrophy can now be explored as a means to treat metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
White adipocyte dysfunction and obesity-associated pathologies in humans
The endocrine function of adipose tissues in health and cardiometabolic disease
Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics
Spalding, K. L. et al. Dynamics of fat cell turnover in humans. Nature 453 , 783–787 (2008).
Article CAS PubMed Google Scholar
Hirsch, J. & Han, P. W. Cellularity of rat adipose tissue: effects of growth, starvation, and obesity. J. Lipid Res. 10 , 77–82 (1969).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19 , 1338–1344 (2013).
Article PubMed PubMed Central CAS Google Scholar
Vishvanath, L. et al. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 23 , 350–359 (2016).
Tang, W. et al. White fat progenitor cells reside in the adipose vasculature. Science 322 , 583–586 (2008). This foundational study is the first explicit in vivo lineage tracing to identify early molecular markers of preadipocytes and localize these cells to the vasculature.
Article CAS PubMed PubMed Central Google Scholar
Gupta, R. K. et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 15 , 230–239 (2012).
Salans, L. B., Knittle, J. L. & Hirsch, J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J. Clin. Invest. 47 , 153–165 (1968).
Krotkiewski, M., Björntorp, P., Sjöström, L. & Smith, U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Invest. 72 , 1150–1162 (1983).
McLaughlin, T. et al. Enhanced proportion of small adipose cells in insulin-resistant versus insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 50 , 1707–1715 (2007).
Lundgren, M. et al. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia 50 , 625–633 (2007).
Yang, J., Eliasson, B., Smith, U., Cushman, S. W. & Sherman, A. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetics. Obesity 20 , 932–938 (2012).
Lönn, M., Mehlig, K., Bengtsson, C. & Lissner, L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 24 , 326–331 (2010).
Article PubMed CAS Google Scholar
Halberg, N. et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 29 , 4467–4483 (2009).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29 , 1575–1591 (2009).
Laurencikiene, J. et al. Regulation of lipolysis in small and large fat cells of the same subject. J. Clin. Endocrinol. Metab. 96 , E2045–E2049 (2011).
Skurk, T., Alberti-Huber, C., Herder, C. & Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 92 , 1023–1033 (2007).
Meyer, L. K., Ciaraldi, T. P., Henry, R. R., Wittgrove, A. C. & Phillips, S. A. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte 2 , 217–226 (2013).
Bambace, C. et al. Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc. Pathol. 20 , e153–e156 (2011).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19 , 1252 (2013).
Kajimura, S., Spiegelman, B. M. & Seale, P. Brown and beige fat: physiological roles beyond heat-generation. Cell Metab. 22 , 546–559 (2015).
Wang, W. & Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17 , 691–702 (2016).
Inagaki, T., Sakai, J. & Kajimura, S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol. 17 , 480 (2016).
Stern, J. H., Rutkowski, J. M. & Scherer, P. E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 23 , 770–784 (2016).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156 , 20–44 (2014).
Scherer, P. E. The multifaceted roles of adipose tissue-therapeutic targets for diabetes and beyond: the 2015 Banting lecture. Diabetes 65 , 1452–1461 (2016).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372 , 425 (1994).
Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. A. Novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270 , 26746–26749 (1995).
Zhu, Q. & Scherer, P. E. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat. Rev. Nephrol. 14 , 105 (2017).
Fasshauer, M. & Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 36 , 461–470 (2015).
Pflimlin, E. et al. Acute and repeated treatment with 5-PAHSA or 9-PAHSA isomers does not improve glucose control in mice. Cell Metab. 28 , 217–227 (2018).
Yore, M. M. et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159 , 318–332 (2014).
Cao, H. et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134 , 933–944 (2008).
Dobson, D. E. et al. 1-Butyryl-glycerol: a novel angiogenesis factor secreted by differentiating adipocytes. Cell 61 , 223–230 (1990).
Holland, W. L. et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5 , 167–179 (2007).
Xia, J. Y. et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22 , 266–278 (2015).
Sun, K. et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl Acad. Sci. USA 109 , 5874–5879 (2012).
Ye, R. et al. Impact of tamoxifen on adipocyte lineage tracing: inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol. Metab. 4 , 771–778 (2015). This study highlights some potential drawbacks to using tamoxifen-inducible genetic recombination systems to study adipogenesis. In particular, tamoxifen administration may itself induce adipogenesis, and has a lengthy wash-out period.
Farmer, S. R. Transcriptional control of adipocyte formation. Cell Metab. 4 , 263–273 (2006).
Cristancho, A. G. & Lazar, M. A. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 12 , 722–734 (2011).
Cawthorn, W. P., Scheller, E. L. & MacDougald, O. A. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J. Lipid Res. 53 , 227–246 (2012).
Wang, E. A., Israel, D. I., Kelly, S. & Luxenberg, D. P. Bone morphogenetic protein-2 causes commitment and differentiation in C3Hl0T1/2 and 3T3 cells. Growth Factors 9 , 57–71 (1993). This early study identifies extracellular BMP signalling as a strong commitment stimulus in cultured preadipocyte cell lines.
Huang, H. et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl Acad. Sci. USA 106 , 12670–12675 (2009).
Bowers, R. R., Kim, J. W., Otto, T. C. & Lane, M. D. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc. Natl Acad. Sci. USA 103 , 13022–13027 (2006).
Gupta, R. K. et al. Transcriptional control of preadipocyte determination by Zfp423. Nature 464 , 619–623 (2010). This study identifies the intracellular transcription factor ZFP423 as a commitment factor to the adipocyte lineage.
Shao, M. et al. Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Mol. Metab. 6 , 111–124 (2017).
Hepler, C. et al. Directing visceral white adipocyte precursors to a thermogenic adipocyte fate improves insulin sensitivity in obese mice. eLife 6 , e27669 (2017).
Article PubMed PubMed Central Google Scholar
Quach, J. M. et al. Zinc finger protein 467 is a novel regulator of osteoblast and adipocyte commitment. J. Biol. Chem. 286 , 4186–4198 (2011).
Oishi, Y. et al. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 1 , 27–39 (2005).
Tong, Q. et al. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science 290 , 134–138 (2000).
Boyle, K. B. et al. The transcription factors Egr1 and Egr2 have opposing influences on adipocyte differentiation. Cell Death Differ. 16 , 782–789 (2009).
Tontonoz, P., Hu, E. & Spiegelman, B. M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79 , 1147–1156 (1994). This study identifies PPARγ expression as sufficient to drive adipocyte differentiation in cell culture.
Barak, Y. et al. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4 , 585–595 (1999).
Rosen, E. D. et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4 , 611–617 (1999).
Wang, F., Mullican, S. E., DiSpirito, J. R., Peed, L. C. & Lazar, M. A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc. Natl Acad. Sci. USA 110 , 18656–18661 (2013).
Schupp, M. & Lazar, M. A. Endogenous ligands for nuclear receptors: digging deeper. J. Biol. Chem. 285 , 40409–40415 (2010).
Lago, R. M., Singh, P. P. & Nesto, R. W. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 370 , 1129–1136 (2007).
Nissen, S. E. & Wolski, K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch. Intern. Med. 170 , 1191–1201 (2010).
Mahaffey, K. W. et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am. Heart J. 166 , 240–249 (2013).
Article PubMed Google Scholar
Wu, Z. et al. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 3 , 151–158 (1999).
Freytag, S. O., Paielli, D. L. & Gilbert, J. D. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 8 , 1654–1663 (1994).
Wu, Z., Bucher, N. L. & Farmer, S. R. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol. Cell. Biol. 16 , 4128–4136 (1996).
Lefterova, M. I. et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 22 , 2941–2952 (2008). Comprehensive mechanistic study describing the adipogenic transcriptional cooperation between PPARγ and C/EBP transcription factors on a genome-wide scale.
Wang, Q. A. et al. Distinct regulatory mechanisms govern embryonic versus adult adipocyte maturation. Nat. Cell Biol. 17 , 1099–1111 (2015).
Plikus, M. V. et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 355 , 748–752 (2017). This study describes in vivo regeneration of adipocytes from fibroblast-like cells during wound healing.
Marangoni, R. G. et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 67 , 1062–1073 (2015).
Zoico, E. et al. Adipocytes WNT5a mediated dedifferentiation: a possible target in pancreatic cancer microenvironment. Oncotarget 7 , 20223–20235 (2016).
Bi, P. et al. Notch activation drives adipocyte dedifferentiation and tumorigenic transformation in mice. J. Exp. Med. 213 , 2019–2037 (2016).
Wang, Q. A. et al. Reversible de-differentiation of mature white adipocytes into preadipocyte-like precursors during lactation. Cell Metab. 28 , 282–288 (2018). This study uses in vivo lineage tracing to demonstrate that adipocytes may reversibly differentiate and de-differentiate in the mouse mammary gland.
Löfgren, P. et al. Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. J. Clin. Endocrinol. Metab. 90 , 6207–6213 (2005).
Tseng, W., Somaiah, N., Lazar, A., Lev, D. & Pollock, R. Novel systemic therapies in advanced liposarcoma: a review of recent clinical trial results. Cancers 5 , 529 (2013).
Sheybani, E. F., Eutsler, E. P. & Navarro, O. M. Fat-containing soft-tissue masses in children. Pediatr. Radiol. 46 , 1760–1773 (2016).
Karastergiou, K. & Fried, S. K. Multiple adipose depots increase cardiovascular risk via local and systemic effects. Curr. Atheroscler. Rep. 15 , 361–361 (2013).
Poissonnet, C. M., Burdi, A. R. & Garn, S. M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 10 , 1–11 (1984).
Jiang, Y., Berry, Daniel, C., Tang, W., Graff & Jonathan, M. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 9 , 1007–1022 (2014).
Hong, K. Y. et al. Perilipin + embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development 142 , 2623–2632 (2015).
Kang, S., Kong, X. & Rosen, E. D. in Methods in Enzymology Vol. 537 (ed. MacDougald, O. A.) 1–16 (Academic Press, 2014).
Jeffery, E., Church, C. D., Holtrup, B., Colman, L. & Rodeheffer, M. S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol. 17 , 376–385 (2015). This study demonstrates that high-fat feeding activates preadipocyte proliferation specifically in visceral depots of mice.
Kim, S. M. et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 20 , 1049–1058 (2014).
Macotela, Y. et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 61 , 1691–1699 (2012).
McLaughlin, T. M. et al. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity 18 , 926–931 (2010).
Fujiwara, T., Yoshioka, S., Yoshioka, T., Ushiyama, I. & Horikoshi, H. Characterization of new oral antidiabetic agent CS-045: studies in KK and ob/ob mice and Zucker fatty rats. Diabetes 37 , 1549–1558 (1988).
Combs, T. P. et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145 , 367–383 (2004).
Tchoukalova, Y. D. et al. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc. Natl Acad. Sci. USA 107 , 18226–18231 (2010).
van Harmelen, V., Röhrig, K. & Hauner, H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism 53 , 632–637 (2004).
Kruglikov, I. L. & Scherer, P. E. Dermal adipocytes: from irrelevance to metabolic targets? Trends Endocrinol. Metab. 27 , 1–10 (2016).
Kasza, I. et al. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLOS Genet. 10 , e1004514 (2014).
Schmidt, B. & Horsley, V. Unraveling hair follicle-adipocyte communication. Exp. Dermatol. 21 , 827–830 (2012).
Festa, E. et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146 , 761–771 (2011).
Schmidt, B. A. & Horsley, V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 140 , 1517–1527 (2013).
Owczarczyk-Saczonek, A. et al. The use of adipose-derived stem cells in selected skin diseases (vitiligo, alopecia, and nonhealing wounds). Stem Cells Int. 2017 , 11 (2017).
Article CAS Google Scholar
Na, Y. K., Ban, J.-J., Lee, M., Im, W. & Kim, M. Wound healing potential of adipose tissue stem cell extract. Biochem. Biophys. Res. Commun. 485 , 30–34 (2017).
Marino, G. et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J. Surg. Res. 185 , 36–44 (2013).
Holm, J. S., Toyserkani, N. M. & Sorensen, J. A. Adipose-derived stem cells for treatment of chronic ulcers: current status. Stem Cell Res. Ther. 9 , 142–142 (2018).
Hamrick, M. W., McGee-Lawrence, M. E. & Frechette, D. M. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front. Endocrinol. 7 , 69 (2016).
Article Google Scholar
Uezumi, A., Fukada, S.-i, Yamamoto, N., Takeda, Si & Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12 , 143–152 (2010).
Kopinke, D., Roberson, E. C. & Reiter, J. F. Ciliary Hedgehog signaling restricts injury-induced adipogenesis. Cell 170 , 340–351 (2017).
Horowitz, M. C. et al. Bone marrow adipocytes. Adipocyte 6 , 193–204 (2017).
Zhou, B. O. et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 19 , 891–903 (2017).
Yokota, T. et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96 , 1723–1732 (2000).
Bilwani, F. A. & Knight, K. L. Adipocyte-derived soluble factor(s) inhibits early stages of B lymphopoiesis. J. Immunol. 189 , 4379–4386 (2012).
Kelly, K. A., Tanaka, S., Baron, R. & Gimble, J. M. Murine bone marrow stromally derived BMS2 adipocytes support differentiation and function of osteoclast-like cells in vitro. Endocrinology 139 , 2092–2101 (1998).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. & Morrison, S. J. Leptin receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15 , 154–168 (2014).
Yue, R., Zhou, B. O., Shimada, I. S., Zhao, Z. & Morrison, S. J. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell 18 , 782–796 (2016).
Naveiras, O. et al. Bone marrow adipocytes as negative regulators of the hematopoietic microenvironment. Nature 460 , 259–263 (2009).
Ambrosi, T. H. et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20 , 771–784 (2017).
Jeffery, E. et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 24 , 142–150 (2016).
Lee, Y. H., Petkova, A. P., Mottillo, E. P. & Granneman, J. G. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15 , 480–491 (2012).
Shao, M. et al. De novo adipocyte differentiation from Pdgfrβ(+) preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat. Commun. 9 , 890 (2018).
Burl, R. B. et al. Deconstructing adipogenesis induced by β3-adrenergic receptor activation with single-cell expression profiling. Cell Metab. 28 , 300–309 (2018).
Schwalie, P. C. et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559 , 103–108 (2018). A study using single-cell sequencing to identify subpopulations of fibroblasts from subcutaneous adipose tissue in mice. The authors identify an anti-adipogenic population that may inhibit adipocyte differentiation in vitro.
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454 , 961–967 (2008).
Cohen, P. et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156 , 304–316 (2014).
Sanchez-Gurmaches, J. et al. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 16 , 348–362 (2012).
Haeusler, R. A., McGraw, T. E. & Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 19 , 31 (2017).
Accili, D. & Taylor, S. I. Targeted inactivation of the insulin receptor gene in mouse 3T3-L1 fibroblasts via homologous recombination. Proc. Natl Acad. Sci. USA 88 , 4708–4712 (1991). A study identifying the requirement for insulin signalling in adipocyte differentiation.
Chaika, O. V. et al. CSF-1 receptor/insulin receptor chimera permits CSF-1-dependent differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 272 , 11968–11974 (1997).
Laustsen, P. G. et al. Lipoatrophic diabetes in Irs1(−/−)/Irs3(−/−) double knockout mice. Genes Dev. 16 , 3213–3222 (2002).
Tseng, Y.-H., Kriauciunas, K. M., Kokkotou, E. & Kahn, C. R. Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol. Cell. Biol. 24 , 1918–1929 (2004).
Tomiyama, K. et al. Wortmannin, a specific phosphatidylinositol 3-kinase inhibitor, inhibits adipocytic differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 212 , 263–269 (1995).
Solheim, M. H. et al. Mice carrying a dominant-negative human PI3K mutation are protected from obesity and hepatic steatosis but not diabetes. Diabetes 67 , 1297–1309 (2018).
Article PubMed CAS PubMed Central Google Scholar
Park, J.-Y., Kim, Y., Im, J. A., You, S. & Lee, H. Inhibition of adipogenesis by oligonol through Akt-mTOR inhibition in 3T3-L1 adipocytes. Evid. Based Complement. Alternat. Med. 2014 , 895272 (2014).
PubMed PubMed Central Google Scholar
Xu, J. & Liao, K. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J. Biol. Chem. 279 , 35914–35922 (2004).
George, S. et al. A family with severe insulin resistance and diabetes mellitus due to a missense mutation in AKT2. Science 304 , 1325–1328 (2004).
Yeh, W. C., Bierer, B. E. & McKnight, S. L. Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3-L1 cells. Proc. Natl Acad. Sci. USA 92 , 11086–11090 (1995).
Martin, S. K. et al. Brief report: the differential roles of mTORC1 and mTORC2 in mesenchymal stem cell differentiation. Stem Cells 33 , 1359–1365 (2015).
Nakae, J. et al. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell 4 , 119–129 (2003).
Blüher, M. et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell 3 , 25–38 (2002).
Bäck, K. & Arnqvist, H. J. Changes in insulin and IGF-I receptor expression during differentiation of human preadipocytes. Growth Horm. IGF Res. 19 , 101–111 (2009).
Weitzman, E. D. et al. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J. Clin. Endocrinol. Metab. 33 , 14–22 (1971).
Hackett, R. A., Steptoe, A. & Kumari, M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J. Clin. Endocrinol. Metab. 99 , 4625–4631 (2014).
Bruehl, H., Wolf, O. T. & Convit, A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology 34 , 815–821 (2009).
Chapman, A. B., Knight, D. M. & Ringold, G. M. Glucocorticoid regulation of adipocyte differentiation: hormonal triggering of the developmental program and induction of a differentiation-dependent gene. J. Cell Biol. 101 , 1227–1235 (1985).
Hauner, H., Schmid, P. & Pfeiffer, E. F. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J. Clin. Endocrinol. Metab. 64 , 832–835 (1987).
Shugart, E. & Umek, R. Dexamethasone signaling is required to establish the postmitotic state of adipocyte development. Cell Growth Differ. 8 , 1091–1098 (1997).
CAS PubMed Google Scholar
Wiper-Bergeron, N., Salem, H. A., Tomlinson, J. J., Wu, D. & Haché, R. J. G. Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPβ by GCN5. Proc. Natl Acad. Sci. USA 104 , 2703–2708 (2007).
Tomlinson, J. J., Boudreau, A., Wu, D., Atlas, E. & Haché, R. J. Modulation of early human preadipocyte differentiation by glucocorticoids. Endocrinology 147 , 5284–5293 (2006).
Tomlinson, J. J. et al. Insulin sensitization of human preadipocytes through glucocorticoid hormone induction of forkhead transcription factors. Mol. Endocrinol. 24 , 104–113 (2010).
Tseng, Y.-H. et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454 , 1000–1004 (2008).
Modica, S. et al. Bmp4 promotes a brown to white-like adipocyte shift. Cell Rep. 16 , 2243–2258 (2016).
Son, J.-W. et al. Association of serum bone morphogenetic protein 4 levels with obesity and metabolic syndrome in non-diabetic individuals. Endocr. J. 58 , 39–46 (2011).
Ross, S. E. et al. Inhibition of adipogenesis by Wnt signaling. Science 289 , 950–953 (2000).
Cawthorn, W. P. et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 50 , 477–489 (2012).
Isakson, P., Hammarstedt, A., Gustafson, B. & Smith, U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 58 , 1550–1557 (2009).
Park, Y.-K. et al. Hypoxia-inducible factor-2α-dependent hypoxic induction of Wnt10b expression in adipogenic cells. J. Biol. Chem. 288 , 26311–26322 (2013).
Nakamura, Y. et al. Repression of adipogenesis through promotion of Wnt/β-catenin signaling by TIS7 up-regulated in adipocytes under hypoxia. Biochim. Biophys. Acta 1832 , 1117–1128 (2013).
Rajashekhar, G. et al. IFATS collection: adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells 26 , 2674–2681 (2008).
Zehentner, B. K., Leser, U. & Burtscher, H. BMP-2 and Sonic Hedgehog have contrary effects on adipocyte-like differentiation of C3H10T1/2 cells. DNA Cell Biol. 19 , 275–281 (2000).
Fontaine, C., Cousin, W., Plaisant, M., Dani, C. & Peraldi, P. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells 26 , 1037–1046 (2008).
Suh, J. M. et al. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 3 , 25–34 (2006).
Gustafson, B. & Smith, U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J. Biol. Chem. 281 , 9507–9516 (2006).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112 , 1796–1808 (2003).
Cancello, R. et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 55 , 1554–1561 (2006).
Samad, F., Yamamoto, K., Pandey, M. & Loskutoff, D. J. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol. Med. 3 , 37–48 (1997).
Alessi, M. C. et al. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 49 , 1374–1380 (2000).
Ignotz, R. A. & Massagué, J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc. Natl Acad. Sci. USA 82 , 8530–8534 (1985).
Clouthier, D. E., Comerford, S. A. & Hammer, R. E. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. J. Clin. Invest. 100 , 2697–2713 (1997).
Torti, F., Dieckmann, B., Beutler, B., Cerami, A. & Ringold, G. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science 229 , 867–869 (1985).
Charrière, G. et al. Preadipocyte conversion to macrophage: evidence of plasticity. J. Biol. Chem. 278 , 9850–9855 (2003).
Marcelin, G. et al. A PDGFRα-mediated switch toward CD9 high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab. 25 , 673–685 (2017).
Hepler, C. et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7 , e39636 (2018). Study using single-cell sequencing to characterize functionally distinct populations of PDGFRβ + fibroblasts in visceral mouse adipose tissue. The authors identify a population primed for adipogenesis in vitro as well as a population that inhibits adipogenesis in vitro .
Qiu, Y. et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157 , 1292–1308 (2014).
Lee, M.-W. et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160 , 74–87 (2015).
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of adipose and limit obesity. Nature 519 , 242–246 (2015).
Wernstedt, A. I. et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20 , 103–118 (2014).
Sun, M. et al. Meta-analysis on shift work and risks of specific obesity types. Obes. Rev. 19 , 28–40 (2018).
Yang, X. et al. Nuclear receptor expression links the circadian clock to metabolism. Cell 126 , 801–810 (2006).
Aggarwal, A. et al. The circadian clock regulates adipogenesis by a Per3 crosstalk pathway to Klf15. Cell Rep. 21 , 2367–2375 (2017).
Grimaldi, B. et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 12 , 509–520 (2010).
Costa, M. J. et al. Circadian rhythm gene period 3 Is an inhibitor of the adipocyte cell fate. J. Biol. Chem. 286 , 9063–9070 (2011).
Shimba, S. et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl Acad. Sci. USA 102 , 12071–12076 (2005).
Kawai, M. et al. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-γ nuclear translocation. Proc. Natl Acad. Sci. USA 107 , 10508–10513 (2010).
Bahrami-Nejad, Z. et al. A transcriptional circuit filters oscillating circadian hormonal inputs to regulate fat cell differentiation. Cell Metab. 27 , 854–868 (2018). This study identifies how disruption of circadian glucocorticoid signalling leads to increased preadipocyte differentiation in 3T3-L1 cell lines.
Rolo, A. P., Teodoro, J. S. & Palmeira, C. M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 52 , 59–69 (2012).
Carrière, A., Fernandez, Y., Rigoulet, M., Pénicaud, L. & Casteilla, L. Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Lett. 550 , 163–167 (2003).
Lee, H., Lee, Y. J., Choi, H., Ko, E. H. & Kim, J.-W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 284 , 10601–10609 (2009).
Tormos, K. V. et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 14 , 537–544 (2011).
Calzadilla, P. et al. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 12 , 6936–6951 (2011).
Kanda, Y., Hinata, T., Kang, S. W. & Watanabe, Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 89 , 250–258 (2011).
Vigilanza, P., Aquilano, K., Baldelli, S., Rotilio, G. & Ciriolo Maria, R. Modulation of intracellular glutathione affects adipogenesis in 3T3-L1 cells. J. Cell. Physiol. 226 , 2016–2024 (2010).
Mahadev, K. et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H 2 O 2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 24 , 1844–1854 (2004).
Mahadev, K. et al. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J. Biol. Chem. 276 , 48662–48669 (2001).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114 , 1752–1761 (2017).
Houstis, N., Rosen, E. D. & Lander, E. S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440 , 944–948 (2006).
Anderson, E. J. et al. Mitochondrial H 2 O 2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119 , 573–581 (2009).
Agarwal, A. K. et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat. Genet. 31 , 21–23 (2002).
Magré, J. et al. Identification of the gene altered in Berardinelli–Seip congenital lipodystrophy on chromosome 11q13. Nat. Genet. 28 , 365–370 (2001).
Kim, C. A. et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J. Clin. Endocrinol. Metab. 93 , 1129–1134 (2008).
Hayashi, Y. K. et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J. Clin. Invest. 119 , 2623–2633 (2009).
Bi, J. et al. Seipin promotes adipose tissue fat storage through the ER Ca 2+ -ATPase SERCA. Cell Metab. 19 , 861–871 (2014).
Gale, S. E. et al. A regulatory role for 1-acylglycerol-3-phosphate-O-acyltransferase 2 in adipocyte differentiation. J. Biol. Chem. 281 , 11082–11089 (2006).
Chen, W. et al. The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology 150 , 4552–4561 (2009).
Payne, V. A. et al. The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes 57 , 2055–2060 (2008).
Fan, J. Y. et al. Morphological changes of the 3T3-L1 fibroblast plasma membrane upon differentiation to the adipocyte form. J. Cell Sci. 61 , 219–230 (1983).
Razani, B. et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 277 , 8635–8647 (2002).
Nystrom, F. H., Chen, H., Cong, L.-N., Li, Y. & Quon, M. J. Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in transfected COS-7 cells and rat adipose cells. Mol. Endocrinol. 13 , 2013–2024 (1999).
Hill, M. M. et al. PTRF-cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132 , 113–124 (2008).
Crewe, C. et al. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 175 , 695–708 (2018).
Conlan, R. S., Pisano, S., Oliveira, M. I., Ferrari, M. & Mendes Pinto, I. Exosomes as reconfigurable therapeutic systems. Trends Mol. Med. 23 , 636–650 (2017).
Oral, E. A. et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 346 , 570–578 (2002).
Shimomura, I., Hammer, R. E., Ikemoto, S., Brown, M. S. & Goldstein, J. L. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401 , 73 (1999).
Pajvani, U. B. et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 11 , 797–803 (2005).
Hussain, I. & Garg, A. Lipodystrophy syndromes. Endocrinol. Metab. Clin. North Am. 45 , 783–797 (2016). Recent review on the pathophysiology of human lipodystrophies and treatment options.
Wojtanik, K. M. et al. The role of LMNA in adipose: a novel mouse model of lipodystrophy based on the Dunnigan-type familial partial lipodystrophy mutation. J. Lipid Res. 50 , 1068–1079 (2009).
Arioglu, E. et al. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann. Intern. Med. 133 , 263–274 (2000).
Owen, K. R., Donohoe, M., Ellard, S. & Hattersley, A. T. Response to treatment with rosiglitazone in familial partial lipodystrophy due to a mutation in the LMNA gene. Diabet. Med. 20 , 823–827 (2003).
Lüdtke, A. et al. Long-term treatment experience in a subject with Dunnigan-type familial partial lipodystrophy: efficacy of rosiglitazone. Diabet. Med. 22 , 1611–1613 (2005).
Palmer, A. K. & Kirkland, J. L. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp. Gerontol. 86 , 97–105 (2016).
Karagiannides, I. et al. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280 , R1772–R1780 (2001).
Caso, G. et al. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism 62 , 337–340 (2013).
Xu, M. et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 4 , e12997 (2015). This study demonstrates that removal of senescent cells from aged adipose tissue enhances adipogenesis in vivo. Interestingly, the authors demonstrate that FDA-approved JAK1/JAK2 inhibitors may be effective at reducing ageing phenotypes in vivo.
Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA 112 , E6301–E6310 (2015).
Baker, D. J. et al. Clearance of p16 Ink4a -positive senescent cells delays ageing-associated disorders. Nature 479 , 232–236 (2011).
Berry, D. C. et al. Cellular aging contributes to failure of cold-induced beige adipocyte formation in old mice and humans. Cell Metab. 25 , 166–181 (2017).
van Heemst, D. Insulin, IGF-1 and longevity. Aging Dis. 1 , 147–157 (2010).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1 , e00065 (2012).
Dutchak, P. A. et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148 , 556–567 (2012).
Lotta, L. A. et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 49 , 17–26 (2016).
Chu, A. Y. et al. Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat. Genet. 49 , 125–130 (2017).
Kusminski, C. M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18 , 1539–1549 (2012).
Senol-Cosar, O. et al. Tenomodulin promotes human adipocyte differentiation and beneficial visceral adipose tissue expansion. Nat. Commun. 7 , 10686 (2016).
Morley, T. S., Xia, J. Y. & Scherer, P. E. Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements. Nat. Commun. 6 , 7906 (2015).
Kim, J.-Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117 , 2621–2637 (2007).
Shepherd, P. R. et al. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J. Biol. Chem. 268 , 22243–22246 (1993).
Okuno, A. et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Invest. 101 , 1354–1361 (1998).
Adams, M. et al. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J. Clin. Invest. 100 , 3149–3153 (1997).
Saiki, A. et al. Tenomodulin is highly expressed in adipose tissue, increased in obesity, and down-regulated during diet-induced weight loss. J. Clin. Endocrinol. Metab. 94 , 3987–3994 (2009).
Hammarstedt, A., Graham, T. E. & Kahn, B. B. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol. Metab. Syndr. 4 , 42 (2012). This is a human correlational study establishing a link between increased fat cell size and reduced insulin sensitivity in non-obese individuals.
Arner, E. et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 59 , 105–109 (2010).
Acosta, J. R. et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 59 , 560–570 (2016).
Fall, C. H. et al. Fetal and infant growth and cardiovascular risk factors in women. BMJ 310 , 428–432 (1995).
Hales, C. N. et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303 , 1019–1022 (1991).
Ravelli, G.-P., Stein, Z. A. & Susser, M. W. Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 295 , 349–353 (1976).
Yajnik, C. S. et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int. J. Obes. Relat. Metab. Disord. 27 , 173–180 (2003).
Krishnaveni, G. et al. Truncal adiposity is present at birth and in early childhood in South Indian children. Indian Pediatr. 42 , 527–538 (2005).
Wang, W. et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl Acad. Sci. USA 111 , 14466–14471 (2014).
Rajakumari, S. et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 17 , 562–574 (2013).
Shao, M. et al. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab. 23 , 1167–1184 (2016).
Stine, R. R. et al. EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol. Metab. 5 , 57–65 (2016).
Khanh, V. C. et al. Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of Sirtuin 1. Biochem. Biophys. Res. Commun. 500 , 682–690 (2018).
Berry, R. & Rodeheffer, M. S. Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15 , 302–308 (2013).
Hepler, C., Vishvanath, L. & Gupta, R. K. Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev. 31 , 127–140 (2017).
Download references
Acknowledgements
The authors apologize to their colleagues for not being able to include all their important work related to adipogenesis and metabolic health. The authors also thank members of the Touchstone Diabetes Center for their helpful critical reading of this manuscript. P.E.S. is supported by US National Institutes of Health (NIH) grants R01-DK55758, P01-DK088761, R01-DK099110 and P01-AG051459 and by an unrestricted grant from the Novo Nordisk Research Foundation. A.L.G. is supported by the NIH, National Institute of General Medical Sciences Training Grant T32-GM008203.
Reviewer information
Nature Reviews Molecular Cell Biology thanks K. Spalding and other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and affiliations.
Touchstone Diabetes Center, Department of Internal Medicine, The University of Texas Southwestern Medical Center, Dallas, TX, USA
Alexandra L. Ghaben & Philipp E. Scherer
You can also search for this author in PubMed Google Scholar
Contributions
Both authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Philipp E. Scherer .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary box 1.
A pathological condition in which the systemic and cellular response to insulin action is impaired. At the cellular level, insulin resistance can be defined as reduced signal transduction along the PI3K–AKT–mTOR pathway per unit of insulin, resulting in decreased glucose uptake per unit of insulin in adipocytes and skeletal muscle or decreased suppression of hepatic gluconeogenesis. Systemically, compared with healthy individuals, individuals with insulin resistance have elevated plasma insulin levels, elevated plasma glucose levels and impaired glucose clearance in response to the same bolus of insulin.
A cellular dysfunction arising from accumulation of lipid intermediates in cells other than adipocytes. In liver, accumulation of lipids contributes to the pathogenesis of non-alcoholic fatty liver disease and to insulin resistance. Lipid accumulation in skeletal muscle can contribute to insulin resistance, whereas in cardiac muscle, it can cause apoptotic cell death. Pancreatic lipid accumulation can lead to dysregulation of β-cell insulin secretion, and ultimately to apoptotic cell death.
Signalling molecules (proteins or lipids) secreted from adipose tissue.
A term referring to an animal that maintains a constant body temperature.
The brown adipose tissue depot found between the scapulae in rodents and infant humans.
The thermogenic adipose tissue depot found above the clavicles in adult humans and mice, which in humans contains the highest proportion, by volume, of total brown adipose tissue.
The sympathetic nervous system is a system of peripheral nerves that signal primarily with the neurotransmitter noradrenaline through activation of adrenergic receptors. Sympathetic output refers to central signals that increase the activity of these nerves.
The generation of glucose from non-carbohydrate carbon sources (glycerol, odd-chain fatty acids, lactate or certain amino acids). In mammals, the liver, kidney, intestine and skeletal muscle are the only organs capable of gluconeogenesis.
A class of sphingolipids extensively reported to contribute to insulin resistance. The anti-diabetic adipokine adiponectin exhibits effects partially through receptor-associated ceramidase activity.
A stromal, fibroblast-like cell found throughout the body that is capable of differentiating to form an adipocyte, chondrocyte or osteoblast.
A class of nuclear receptors capable of directly binding to both DNA and a steroid or other endocrine hormone. Nuclear hormone receptors are able to directly regulate transcription upon ligand binding.
Straight-chain, 20-carbon polyunsaturated fatty acids that contain an oxygen moiety. Characteristically, eicosanoids are signalling lipids.
A subclass of eicosanoids derived from arachidonic acid with a characteristic five-carbon ring. The signalling actions of prostaglandins are highly tissue-specific.
A representative, commonly studied human subcutaneous adipose depot. This depot is anatomically located subcutaneously, along the hips, buttocks and thighs.
One of the commonly studied human visceral adipose depots. The omental adipose depot is inside the peritoneum, starts near the stomach and spleen and extends deep into the abdomen.
Striated muscle anatomically located within or just beneath the superficial fascia of the dermis that controls local movement of the skin. It is present in many lower mammals but absent in humans.
The age-related loss of muscle mass.
A combination of five metabolic risk factors: high blood pressure, elevated fasting blood glucose, hypertriglyceridaemia, decreased serum high-density lipoprotein and increased abdominal adiposity, which collectively increase the risk of heart disease, diabetes and stroke.
Small, flask-like invaginations of plasma membrane that are abundant in many mammalian cell types including adipocytes. They have been implicated in various processes, including endocytosis, signalling, lipid regulation and mechanosensing.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Ghaben, A.L., Scherer, P.E. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol 20 , 242–258 (2019). https://doi.org/10.1038/s41580-018-0093-z
Download citation
Published : 04 January 2019
Issue Date : April 2019
DOI : https://doi.org/10.1038/s41580-018-0093-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative

This article is cited by
Effects of mifepristone on adipocyte differentiation in mouse 3t3-l1 cells.
- Takeshi Hashimoto
- Katsuya Hirano
Cellular & Molecular Biology Letters (2024)
PDGFRβ + cell HIF2α is dispensable for white adipose tissue metabolic remodeling and hepatic lipid accumulation in obese mice
Lipids in Health and Disease (2024)
WRN loss accelerates abnormal adipocyte metabolism in Werner syndrome
- Sofie Lautrup
- Wai-Yee Chan
Cell & Bioscience (2024)
- Carolina E. Hagberg
- Kirsty L. Spalding
Nature Reviews Molecular Cell Biology (2024)
Diabetes mellitus in breast cancer survivors: metabolic effects of endocrine therapy
- Nisha S. Thomas
- Rebecca L. Scalzo
- Elizabeth A. Wellberg
Nature Reviews Endocrinology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- April 27, 2024 | Revolutionizing Spinal Injury Treatment: Common Drug Found To Prevent Damage to Fat Tissue
- April 27, 2024 | Unusual New Species of Giant Kangaroo Discovered in Australia and New Guinea
- April 27, 2024 | Interstellar Peptides Point to Extraterrestrial Origin of Life’s Building Blocks
- April 27, 2024 | These Incredibly Popular Drugs Have Been Linked to Migraines
- April 27, 2024 | Breaking the Oil Habit: How Synthetic Bacteria Could Revolutionize Chemical Production
Revolutionizing Spinal Injury Treatment: Common Drug Found To Prevent Damage to Fat Tissue
By Ohio State University April 27, 2024
Recent research reveals that gabapentin can prevent metabolic dysfunction caused by spinal cord injuries by inhibiting overactive neural proteins, offering a potential early treatment strategy to mitigate related cardiometabolic diseases.
Study in mice finds that a widely-used medication prevents damage to fat tissue from injuries
A new animal study suggests that conditions like diabetes, heart attacks, and vascular diseases, commonly diagnosed in individuals with spinal cord injuries, may be linked to unusual neuronal activity following the injury. This activity can cause abdominal fat tissue compounds to leak and pool in the liver and other organs, a new animal study has found.
After discovering the connection between dysregulated neuron function and the breakdown of triglycerides in fat tissue in mice, researchers found that a short course of the drug gabapentin , commonly prescribed for nerve pain, prevented the damaging metabolic effects of the spinal cord injury.
Gabapentin inhibits a neural protein that, after the nervous system is damaged, becomes overactive and causes communication problems – in this case, affecting sensory neurons and the abdominal fat tissue to which they’re sending signals.
Mechanism of Action
“We believe there is maladaptive reorganization of the sensory system that causes the fat to undergo changes, initiating a chain of reactions – triglycerides start breaking down into glycerol and free fatty acids that are released in circulation and taken up by the liver, the heart, the muscles, and accumulating, setting up conditions for insulin resistance,” said senior author Andrea Tedeschi, assistant professor of neuroscience in The Ohio State University College of Medicine .
“Through administration of gabapentin, we were able to normalize metabolic function.”
The study was recently published in the journal Cell Reports Medicine .
Previous research has found that cardiometabolic diseases are among the leading causes of death in people who have experienced a spinal cord injury. These often chronic disorders can be related to dysfunction in visceral white fat (or adipose tissue), which has a complex metabolic role of storing energy and releasing fatty acids as needed for fuel, but also helping keep blood sugar levels at an even keel.
Earlier investigations of these diseases in people with neuronal damage have focused on adipose tissue function and the role of the sympathetic nervous system – nerve activity known for its “fight or flight” response, but also a regulator of adipose tissue that surrounds the abdominal organs.
Instead, Debasish Roy – a postdoctoral researcher in the Tedeschi lab and first author on the paper – decided to focus on sensory neurons in this context. Tedeschi and colleagues have previously shown that a neuronal receptor protein called alpha2delta1 is overexpressed after spinal cord injury, and its increased activation interferes with post-injury function of axons, the long, slender extensions of nerve cell bodies that transmit messages.
Observations from Experiments
In this new work, researchers first observed how sensory neurons connect to adipose tissue under healthy conditions, and created a spinal cord injury mouse model that affected only those neurons – without interrupting the sympathetic nervous system.
Experiments revealed a cascade of abnormal activity within seven days after the injury in neurons – though only in their communication function, not their regrowth or structure – and in visceral fat tissue. Expression of the alpha2delta1 receptor in sensory neurons increased as they over-secreted a neuropeptide called CGRP, all while communicating through synaptic transmission to the fat tissue – which, in a state of dysregulation, drove up levels of a receptor protein that engaged with the CGRP.
“These are quite rapid changes. As soon as we disrupt sensory processing as a result of spinal cord injury, we see changes in the fat,” Tedeschi said. “A vicious cycle is established – it’s almost like you’re pressing the gas pedal so your car can run out of gas but someone else continues to refill the tank, so it never runs out.”
The result is the spillover of free fatty acids and glycerol from fat tissue, a process called lipolysis, that has gone out of control. Results also showed an increase in blood flow in fat tissue and recruitment of immune cells to the environment.
“The fat is responding to the presence of CGRP, and it’s activating lipolysis,” Tedeschi said. “CGRP is also a potent vasodilator, and we saw increased vascularization of the fat – new blood vessels forming as a result of the spinal cord injury. And the recruitment of monocytes can help set up a chronic pro-inflammatory state.”
Silencing the genes that encode the alpha2delta1 receptor restored the fat tissue to normal function, indicating that gabapentin – which targets alpha2delta1 and its partner, alpha2delta2 – was a good treatment candidate. Tedeschi’s lab has previously shown in animal studies that gabapentin helped restore limb function after spinal cord injury and boosted functional recovery after stroke .
But in these experiments, Roy discovered something tricky about gabapentin: The drug prevented changes in abdominal fat tissue and lowered CGRP in the blood – and in turn prevented spillover of fatty acids into the liver a month later, establishing normal metabolic conditions. But paradoxically, the mice developed insulin resistance – a known side effect of gabapentin.
The team changed drug delivery tactics, starting with a high dose and tapering off – and stopping after four weeks.
“This way, we were able to normalize metabolism to a condition much more similar to control mice,” Roy said. “This suggests that as we discontinue administration of the drug, we retain beneficial action and prevent spillover of lipids in the liver. That was really exciting.”
Finally, researchers examined how genes known to regulate white fat tissue were affected by targeting alpha2delta1 genetically or with gabapentin, and found both of these interventions after spinal cord injury suppress genes responsible for disrupting metabolic functions.
Tedeschi said the combined findings suggest starting gabapentin treatment early after a spinal cord injury may protect against detrimental conditions involving fat tissue that lead to cardiometabolic disease – and could enable discontinuing the drug while retaining its benefits and lowering the risk for side effects.
Reference: “α2δ1-mediated maladaptive sensory plasticity disrupts adipose tissue homeostasis following spinal cord injury” by Debasish Roy, Elliot Dion, Jesse A. Sepeda, Juan Peng, Sai Rishik Lingam, Kristy Townsend, Andrew Sas, Wenjing Sun and Andrea Tedeschi, 24 April 2024, Cell Reports Medicine . DOI: 10.1016/j.xcrm.2024.101525
This work was supported by grants from the National Institute of Neurological Disorders and Stroke and the National Institutes of Health , and by the Chronic Brain Injury program at Ohio State.
More on SciTechDaily

NASA Astronauts Gear Up for Boeing Starliner’s Groundbreaking Test Flight
Hubble space telescope is back online – partially.
Startling Findings: Midwest and Southeast Experience Dramatic Increase in Anal Cancer

A Radical New Approach in Synthetic Chemistry
Rethinking Neural Intelligence: Scientists Uncover Surprising Memory Capabilities of the Spinal Cord

Don’t Miss: A “Ring of Fire” in the Sky
Scientists Uncover Missing Link Between Poor Diet and Cancer

The Wild Weather of Hot Jupiters
Be the first to comment on "revolutionizing spinal injury treatment: common drug found to prevent damage to fat tissue", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 1, 2024 report
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study finds little gender difference in the perception of ambient room temperature
by Bob Yirka , Medical Xpress

A large team of medical researchers at the U.S. National Institutes of Health, has found very slight gender differences in temperature perception of a room at ambient conditions and very few gender differences in physiological response to a perceived chill.
In their paper published in Proceedings of the National Academy of Sciences , the group describes experiments they conducted with both male and female volunteers in a climate-controlled room.
Prior studies and anecdotal evidence have suggested that women, on average, feel colder when living or working in a room at typical ambient temperatures than men. But as the researchers involved in this new study note, very little research has been done to find the basis for such claims.
Therefore the research team conducted controlled experiments with healthy, lean young adults of both genders sitting in a climate-controlled room to learn more about the conditions that might lead to such claims.
The research team asked 16 women and 12 men to first undergo an analysis of several of their metabolic parameters, such as the proportion of brown adipose tissue in their body, and skin and core body temperature. Next each was asked to sit in a room for five hours as the researchers changed the temperature over short intervals of time.
All the volunteers wore clothing given to them by the researchers to make sure what they were wearing was not affecting temperature perception. Temperatures were varied from 17°C (63°F) to 31°C (88°F). At each temperature change, the volunteers were asked whether they were feeling chilly or not—they were also monitored for shivering.
In looking at their data, the research team found very little in the way of gender differences —most of the volunteers reported feeling chilly at nearly the same temperature. They also found no temperature differences for the onset of shivering.
They did find one small physiological difference, however. Women, they noted, had what they describe as "a cooler lower temperature," than men, which meant that the women began to make metabolic changes sooner than men as temperatures dropped, helping them to stay warm. This also resulted in a slightly higher core body temperature when exposed to colder temperatures.
The researchers conclude by suggesting that body size and composition were more likely than gender to influence the perception of chilliness.
© 2024 Science X Network
Explore further
Feedback to editors

AI experts explore ethical use of video technology to support patients at risk of falls
14 minutes ago

Two-punch treatment delivers blood cancer knockout: Study shows drug combo eradicates cancer cells in lab-based tests
22 minutes ago
Study sheds light on the debate around two types of shoulder replacement surgery for osteoarthritis
30 minutes ago
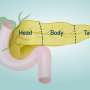
Researchers find difference in pancreatic cancer cells, offering new hope for immunotherapy effectiveness
38 minutes ago

Identifying risks of human flea infestations in plague-endemic areas of Madagascar
42 minutes ago

Closing the US/Mexico border during COVID-19 increased HIV transmission, study finds
58 minutes ago

Cardiologists train large AI model to assess heart structure, function

Deep-learning decoding for a noninvasive brain-computer interface

Study finds network of inflammatory molecules may act as biomarker for risk of future cerebrovascular disease
5 hours ago

Brief anger may impair blood vessel function, says new research
Related stories.
Macrophages produce heat from brown adipose tissue in response to cold, study reveals
Mar 26, 2024

Yes, women might 'feel the cold' more than men. Here's why
Jun 7, 2022

Cool room temperature inhibited cancer growth in mice
Aug 3, 2022

Hot flashes in cold weather: Study suggests brown adipose tissue activity may explain why
Sep 27, 2023

We are more sensitive to temperature than we thought, study reveals
Jan 11, 2024

Mushrooms found to keep themselves cooler than their surroundings
May 8, 2023
Recommended for you

An electrifying discovery may help doctors deliver more effective gene therapies
17 hours ago

Cranberry extracts could boost microbiota and counter cardiometabolic diseases
22 hours ago
New regulator of eating behavior identified
Apr 30, 2024
New drug candidate reverses obesity in mice
23 hours ago
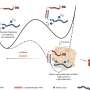
Tsetse fly protein provides anticoagulant with its own on-off switch
X-chromosome inactivation may reduce autism risk, study in mice suggests
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Types of Adipose Tissue. Adipose tissue can be classified by morphology into white, brown, or beige subsets. In addition, white adipose tissue (WAT) can be broadly classified by location, largely defined as subcutaneous (located under the skin) and visceral/omental (located intra-abdominally, adjacent to internal organs).Adipose tissue is comprised of many different cell types, which ...
An alternative strategy involves single-nucleus sequencing (sNuc-seq), which can capture adipocytes, and has been used to describe mouse white 8, 9 and human brown adipose tissue 10. To compare ...
Adipose tissues are dynamic tissues that play crucial physiological roles in maintaining health and homeostasis. Although white adipose tissue and brown adipose tissue are currently considered key endocrine organs, they differ functionally and morphologically. The existence of the beige or brite adipocytes, cells displaying intermediary characteristics between white and brown adipocytes ...
White adipose tissue serves a plethora of physiological functions, which are compromised in obesity. The mechanisms through which obese white adipose tissue contributes to pathologies including ...
Subcutaneous adipose tissue (SAT) accounts for 80-90 % of total body fat, and its main depots are the abdominal, subscapular (on the upper back), gluteal, and femoral areas. Alternatively, visceral adipose tissue (VAT) is mainly located inside the intraabdominal cavity and accounts for up to 10-20 % of total body fat [4].
White adipose tissue, once regarded as morphologically and functionally bland, is now recognized to be dynamic, plastic and heterogenous, and is involved in a wide array of biological processes including energy homeostasis, glucose and lipid handling, blood pressure control and host defence 1.High-fat feeding and other metabolic stressors cause marked changes in adipose morphology, physiology ...
INTRODUCTION. White adipose tissue (WAT) displays a remarkable capacity for undergoing changes in size and morphology. The close relationship between WAT mass and common cardiometabolic disorders such as insulin resistance, type 2 diabetes, and hypertension has prompted intense research into the mechanisms driving WAT plasticity and phenotype.
Anatomy. White adipose tissue (WAT) is widely dispersed in humans. Major depots reside in subcutaneous region in the upper (deep and superficial abdominal) and lower body (gluteal-femoral), as ...
The immune and endocrine dysfunctions of white adipose tissue are a hallmark of metabolic disorders such as obesity and type 2 diabetes. In humans, white adipose tissue comprises distinct depots broadly distributed under the skin (hypodermis) and as internal depots (visceral). Depot-specific ASCs could account for visceral and subcutaneous adipose tissue properties, by regulating adipogenesis ...
Obesity, characterized by the excessive accumulation of adipose tissue, has emerged as a major public health concern worldwide. To develop effective strategies for treating obesity, it is essential to comprehend the biological properties of different adipose tissue types and their respective roles in maintaining energy balance. Adipose tissue serves as a crucial organ for energy storage and ...
Abstract. Adipose, or fat, tissue (AT) was once considered an inert tissue that primarily existed to store lipids, and was not historically recognized as an important organ in the regulation and maintenance of health. With the rise of obesity and more rigorous research, AT is now recognized as a highly complex metabolic organ involved in a host ...
Abstract. Adipose, or fat, tissue (AT) was once considered an inert tissue that primarily existed to store lipids, and was not historically recognized as an important organ in the regulation and maintenance of health. With the rise of obesity and more rigorous research, AT is now recognized as a highly complex metabolic organ involved in a host ...
The adipose organ adapts and responds to internal and environmental stimuli by remodeling both its cellular and extracellular components. Under conditions of energy surplus, the subcutaneous white adipose tissue (WAT) is capable of expanding through the enlargement of existing adipocytes (hypertrophy), followed by de novo adipogenesis (hyperplasia), which is impaired in hypertrophic obesity.
White adipose tissue (WAT) explants culture allows the study of this tissue ex vivo, maintaining its structure and properties. Concurrently, isolating mature adipocytes facilitates research into fat cell metabolism and hormonal regulation. Here, we present a protocol for obtaining, isolating, and pr …
Abstract Background and Purpose White adipose tissue (WAT) is involved in rheumatoid arthritis (RA). ... Xin'an Medicine Research Center, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital), Wuhu, China ... Investigation (lead) Search for more papers by this author. Qi-Hai Wang, Qi-Hai Wang. School of Pharmacy, Anhui ...
White adipose tissue (WAT) was long considered an inactive tissue that primarily served a thermal insulation purpose 1.In the mid-twentieth century, it became increasingly apparent that WAT is ...
Dysfunction of adipose tissue could lead to insulin resistance, obesity and type 2 diabetes. Thus, our present study aimed to investigate metabolites alterations in white adipose tissue (WAT) of diabetic GK rats after IT surgery. Ten-week-old male diabetic GK rats were randomly subjected to IT and Sham-IT surgery.
White adipose tissue (AT) dysfunction plays an important role in the development of cardiometabolic alterations associated with obesity. AT dysfunction is characterized by the loss of the expansion capacity of the AT, an increment in adipocyte hypertrophy, and changes in the secretion profile of adipose cells, associated with accumulation of macrophages and inflammation.
Several lines of preclinical and clinical research have confirmed that chronic low-grade inflammation of adipose tissue is mechanistically linked to metabolic disease and organ tissue complications in the overweight and obese organism. Despite this widely confirmed paradigm, numerous open questions and knowledge gaps remain to be investigated. This is mainly due to the intricately intertwined ...
An increase in white adipose tissue is associated with obesity and reduced metabolic function. Interestingly, however, adipose tissue expansion through the generation of new adipocytes ...
A more comprehensive understanding of the changes in adipose tissue thermogenesis under physiological and pathological conditions, as well as the underlying regulatory mechanisms, is essential for the development of novel interventions to improve health and prevent disease. PURPOSE OF REVIEW By providing a concise overview of adipose tissue types, elucidating the regulation of adipose ...
Previous research has found that cardiometabolic diseases are among the leading causes of death in people who have experienced a spinal cord injury. These often chronic disorders can be related to dysfunction in visceral white fat (or adipose tissue), which has a complex metabolic role of storing energy and releasing fatty acids as needed for ...
The research team asked 16 women and 12 men to first undergo an analysis of several of their metabolic parameters, such as the proportion of brown adipose tissue in their body, and skin and core ...