Memory Stages: Encoding Storage and Retrieval
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
“Memory is the process of maintaining information over time.” (Matlin, 2005) “Memory is the means by which we draw on our past experiences in order to use this information in the present’ (Sternberg, 1999).
Memory is the term given to the structures and processes involved in the storage and subsequent retrieval of information.
Memory is essential to all our lives. Without a memory of the past, we cannot operate in the present or think about the future. We would not be able to remember what we did yesterday, what we have done today, or what we plan to do tomorrow. Without memory, we could not learn anything.
Memory is involved in processing vast amounts of information. This information takes many different forms, e.g., images, sounds, or meaning.
For psychologists, the term memory covers three important aspects of information processing :


Memory Encoding
When information comes into our memory system (from sensory input), it needs to be changed into a form that the system can cope with so that it can be stored.
Think of this as similar to changing your money into a different currency when you travel from one country to another. For example, a word that is seen (in a book) may be stored if it is changed (encoded) into a sound or a meaning (i.e., semantic processing).
There are three main ways in which information can be encoded (changed):
1. Visual (picture) 2. Acoustic (sound) 3. Semantic (meaning)
For example, how do you remember a telephone number you have looked up in the phone book? If you can see it, then you are using visual coding, but if you are repeating it to yourself, you are using acoustic coding (by sound).
Evidence suggests that this is the principle coding system in short-term memory (STM) is acoustic coding. When a person is presented with a list of numbers and letters, they will try to hold them in STM by rehearsing them (verbally).
Rehearsal is a verbal process regardless of whether the list of items is presented acoustically (someone reads them out), or visually (on a sheet of paper).
The principle encoding system in long-term memory (LTM) appears to be semantic coding (by meaning). However, information in LTM can also be coded both visually and acoustically.
Memory Storage
This concerns the nature of memory stores, i.e., where the information is stored, how long the memory lasts (duration), how much can be stored at any time (capacity) and what kind of information is held.
The way we store information affects the way we retrieve it. There has been a significant amount of research regarding the differences between Short Term Memory (STM ) and Long Term Memory (LTM).
Most adults can store between 5 and 9 items in their short-term memory. Miller (1956) put this idea forward, and he called it the magic number 7. He thought that short-term memory capacity was 7 (plus or minus 2) items because it only had a certain number of “slots” in which items could be stored.
However, Miller didn’t specify the amount of information that can be held in each slot. Indeed, if we can “chunk” information together, we can store a lot more information in our short-term memory. In contrast, the capacity of LTM is thought to be unlimited.
Information can only be stored for a brief duration in STM (0-30 seconds), but LTM can last a lifetime.
Memory Retrieval
This refers to getting information out of storage. If we can’t remember something, it may be because we are unable to retrieve it. When we are asked to retrieve something from memory, the differences between STM and LTM become very clear.
STM is stored and retrieved sequentially. For example, if a group of participants is given a list of words to remember and then asked to recall the fourth word on the list, participants go through the list in the order they heard it in order to retrieve the information.
LTM is stored and retrieved by association. This is why you can remember what you went upstairs for if you go back to the room where you first thought about it.
Organizing information can help aid retrieval. You can organize information in sequences (such as alphabetically, by size, or by time). Imagine a patient being discharged from a hospital whose treatment involved taking various pills at various times, changing their dressing, and doing exercises.
If the doctor gives these instructions in the order that they must be carried out throughout the day (i.e., in the sequence of time), this will help the patient remember them.
Criticisms of Memory Experiments
A large part of the research on memory is based on experiments conducted in laboratories. Those who take part in the experiments – the participants – are asked to perform tasks such as recalling lists of words and numbers.
Both the setting – the laboratory – and the tasks are a long way from everyday life. In many cases, the setting is artificial, and the tasks are fairly meaningless. Does this matter?
Psychologists use the term ecological validity to refer to the extent to which the findings of research studies can be generalized to other settings. An experiment has high ecological validity if its findings can be generalized, that is, applied or extended to settings outside the laboratory.
It is often assumed that if an experiment is realistic or true-to-life, then there is a greater likelihood that its findings can be generalized. If it is not realistic (if the laboratory setting and the tasks are artificial) then there is less likelihood that the findings can be generalized. In this case, the experiment will have low ecological validity.
Many experiments designed to investigate memory have been criticized for having low ecological validity. First, the laboratory is an artificial situation. People are removed from their normal social settings and asked to take part in a psychological experiment.
They are directed by an “experimenter” and may be placed in the company of complete strangers. For many people, this is a brand new experience, far removed from their everyday lives. Will this setting affect their actions? Will they behave normally?
He was especially interested in the characteristics of people whom he considered to have achieved their potential as individuals.
Often, the tasks participants are asked to perform can appear artificial and meaningless. Few, if any, people would attempt to memorize and recall a list of unconnected words in their daily lives. And it is not clear how tasks such as this relate to the use of memory in everyday life.
The artificiality of many experiments has led some researchers to question whether their findings can be generalized to real life. As a result, many memory experiments have been criticized for having low ecological validity.
Matlin, M. W. (2005). Cognition . Crawfordsville: John Wiley & Sons, Inc.
Miller, G. A. (1956). The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Review , 63 (2): 81–97.
Sternberg, R. J. (1999). Cognitive psychology (2 nd ed.) . Fort Worth, TX: Harcourt Brace College Publishers.
- Utility Menu
GA4 Tracking Code
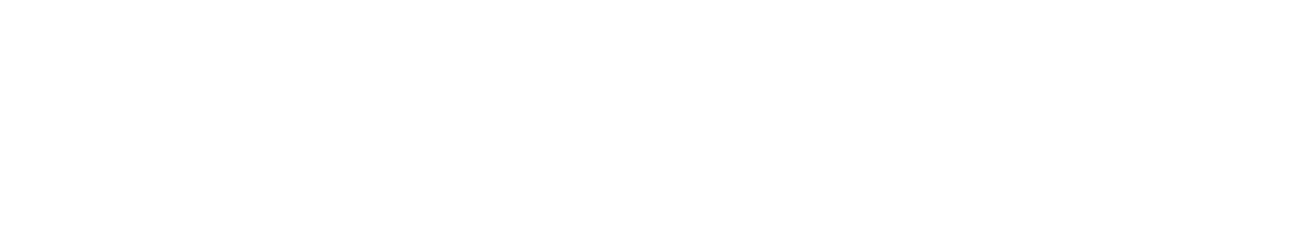
fa51e2b1dc8cca8f7467da564e77b5ea
- Make a Gift
- Join Our Email List
- How Memory Works
Memory is the ongoing process of information retention over time. Because it makes up the very framework through which we make sense of and take action within the present, its importance goes without saying. But how exactly does it work? And how can teachers apply a better understanding of its inner workings to their own teaching? In light of current research in cognitive science, the very, very short answer to these questions is that memory operates according to a "dual-process," where more unconscious, more routine thought processes (known as "System 1") interact with more conscious, more problem-based thought processes (known as "System 2"). At each of these two levels, in turn, there are the processes through which we "get information in" (encoding), how we hold on to it (storage), and and how we "get it back out" (retrieval or recall). With a basic understanding of how these elements of memory work together, teachers can maximize student learning by knowing how much new information to introduce, when to introduce it, and how to sequence assignments that will both reinforce the retention of facts (System 1) and build toward critical, creative thinking (System 2).
Dual-Process Theory
Think back to a time when you learned a new skill, such as driving a car, riding a bicycle, or reading. When you first learned this skill, performing it was an active process in which you analyzed and were acutely aware of every movement you made. Part of this analytical process also meant that you thought carefully about why you were doing what you were doing, to understand how these individual steps fit together as a comprehensive whole. However, as your ability improved, performing the skill stopped being a cognitively-demanding process, instead becoming more intuitive. As you continue to master the skill, you can perform other, at times more intellectually-demanding, tasks simultaneously. Due to your knowledge of this skill or process being unconscious, you could, for example, solve an unrelated complex problem or make an analytical decision while completing it.
In its simplest form, the scenario above is an example of what psychologists call dual-process theory. The term “dual-process” refers to the idea that some behaviors and cognitive processes (such as decision-making) are the products of two distinct cognitive processes, often called System 1 and System 2 (Kaufmann, 2011:443-445). While System 1 is characterized by automatic, unconscious thought, System 2 is characterized by effortful, analytical, intentional thought (Osman, 2004:989).

Dual-Process Theories and Learning
How do System 1 and System 2 thinking relate to teaching and learning? In an educational context, System 1 is associated with memorization and recall of information, while System 2 describes more analytical or critical thinking. Memory and recall, as a part of System 1 cognition, are focused on in the rest of these notes.
As mentioned above, System 1 is characterized by its fast, unconscious recall of previously-memorized information. Classroom activities that would draw heavily on System 1 include memorized multiplication tables, as well as multiple-choice exam questions that only need exact regurgitation from a source such as a textbook. These kinds of tasks do not require students to actively analyze what is being asked of them beyond reiterating memorized material. System 2 thinking becomes necessary when students are presented with activities and assignments that require them to provide a novel solution to a problem, engage in critical thinking, or apply a concept outside of the domain in which it was originally presented.
It may be tempting to think of learning beyond the primary school level as being all about System 2, all the time. However, it’s important to keep in mind that successful System 2 thinking depends on a lot of System 1 thinking to operate. In other words, critical thinking requires a lot of memorized knowledge and intuitive, automatic judgments to be performed quickly and accurately.
How does Memory Work?
In its simplest form, memory refers to the continued process of information retention over time. It is an integral part of human cognition, since it allows individuals to recall and draw upon past events to frame their understanding of and behavior within the present. Memory also gives individuals a framework through which to make sense of the present and future. As such, memory plays a crucial role in teaching and learning. There are three main processes that characterize how memory works. These processes are encoding, storage, and retrieval (or recall).
- Encoding . Encoding refers to the process through which information is learned. That is, how information is taken in, understood, and altered to better support storage (which you will look at in Section 3.1.2). Information is usually encoded through one (or more) of four methods: (1) Visual encoding (how something looks); (2) acoustic encoding (how something sounds); (3) semantic encoding (what something means); and (4) tactile encoding (how something feels). While information typically enters the memory system through one of these modes, the form in which this information is stored may differ from its original, encoded form (Brown, Roediger, & McDaniel, 2014).

- Retrieval . As indicated above, retrieval is the process through which individuals access stored information. Due to their differences, information stored in STM and LTM are retrieved differently. While STM is retrieved in the order in which it is stored (for example, a sequential list of numbers), LTM is retrieved through association (for example, remembering where you parked your car by returning to the entrance through which you accessed a shopping mall) (Roediger & McDermott, 1995).
Improving Recall
Retrieval is subject to error, because it can reflect a reconstruction of memory. This reconstruction becomes necessary when stored information is lost over time due to decayed retention. In 1885, Hermann Ebbinghaus conducted an experiment in which he tested how well individuals remembered a list of nonsense syllables over increasingly longer periods of time. Using the results of his experiment, he created what is now known as the “Ebbinghaus Forgetting Curve” (Schaefer, 2015).

Through his research, Ebbinghaus concluded that the rate at which your memory (of recently learned information) decays depends both on the time that has elapsed following your learning experience as well as how strong your memory is. Some degree of memory decay is inevitable, so, as an educator, how do you reduce the scope of this memory loss? The following sections answer this question by looking at how to improve recall within a learning environment, through various teaching and learning techniques.
As a teacher, it is important to be aware of techniques that you can use to promote better retention and recall among your students. Three such techniques are the testing effect, spacing, and interleaving.
- The testing effect . In most traditional educational settings, tests are normally considered to be a method of periodic but infrequent assessment that can help a teacher understand how well their students have learned the material at hand. However, modern research in psychology suggests that frequent, small tests are also one of the best ways to learn in the first place. The testing effect refers to the process of actively and frequently testing memory retention when learning new information. By encouraging students to regularly recall information they have recently learned, you are helping them to retain that information in long-term memory, which they can draw upon at a later stage of the learning experience (Brown, Roediger, & McDaniel, 2014). As secondary benefits, frequent testing allows both the teacher and the student to keep track of what a student has learned about a topic, and what they need to revise for retention purposes. Frequent testing can occur at any point in the learning process. For example, at the end of a lecture or seminar, you could give your students a brief, low-stakes quiz or free-response question asking them to remember what they learned that day, or the day before. This kind of quiz will not just tell you what your students are retaining, but will help them remember more than they would have otherwise.
- Spacing. According to the spacing effect, when a student repeatedly learns and recalls information over a prolonged time span, they are more likely to retain that information. This is compared to learning (and attempting to retain) information in a short time span (for example, studying the day before an exam). As a teacher, you can foster this approach to studying in your students by structuring your learning experiences in the same way. For example, instead of introducing a new topic and its related concepts to students in one go, you can cover the topic in segments over multiple lessons (Brown, Roediger, & McDaniel, 2014).
- Interleaving. The interleaving technique is another teaching and learning approach that was introduced as an alternative to a technique known as “blocking”. Blocking refers to when a student practices one skill or one topic at a time. Interleaving, on the other hand, is when students practice multiple related skills in the same session. This technique has proven to be more successful than the traditional blocking technique in various fields (Brown, Roediger, & McDaniel, 2014).
As useful as it is to know which techniques you can use, as a teacher, to improve student recall of information, it is also crucial for students to be aware of techniques they can use to improve their own recall. This section looks at four of these techniques: state-dependent memory, schemas, chunking, and deliberate practice.
- State-dependent memory . State-dependent memory refers to the idea that being in the same state in which you first learned information enables you to better remember said information. In this instance, “state” refers to an individual’s surroundings, as well as their mental and physical state at the time of learning (Weissenborn & Duka, 2000).
- Schemas. Schemas refer to the mental frameworks an individual creates to help them understand and organize new information. Schemas act as a cognitive “shortcut” in that they allow individuals to interpret new information quicker than when not using schemas. However, schemas may also prevent individuals from learning pertinent information that falls outside the scope of the schema that has been created. It is because of this that students should be encouraged to alter or reanalyze their schemas, when necessary, when they learn important information that may not confirm or align with their existing beliefs and conceptions of a topic.
- Chunking. Chunking is the process of grouping pieces of information together to better facilitate retention. Instead of recalling each piece individually, individuals recall the entire group, and then can retrieve each item from that group more easily (Gobet et al., 2001).
- Deliberate practice. The final technique that students can use to improve recall is deliberate practice. Simply put, deliberate practice refers to the act of deliberately and actively practicing a skill with the intention of improving understanding of and performance in said skill. By encouraging students to practice a skill continually and deliberately (for example, writing a well-structured essay), you will ensure better retention of that skill (Brown et al., 2014).
For more information...
Brown, P.C., Roediger, H.L. & McDaniel, M.A. 2014. Make it stick: The science of successful learning . Cambridge, MA: Harvard University Press.
Gobet, F., Lane, P.C., Croker, S., Cheng, P.C., Jones, G., Oliver, I. & Pine, J.M. 2001. Chunking mechanisms in human learning. Trends in Cognitive Sciences . 5(6):236-243.
Kaufman, S.B. 2011. Intelligence and the cognitive unconscious. In The Cambridge handbook of intelligence . R.J. Sternberg & S.B. Kaufman, Eds. New York, NY: Cambridge University Press.
Osman, M. 2004. An evaluation of dual-process theories of reasoning. Psychonomic Bulletin & Review . 11(6):988-1010.
Roediger, H.L. & McDermott, K.B. 1995. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, and Cognition . 21(4):803.
Schaefer, P. 2015. Why Google has forever changed the forgetting curve at work.
Weissenborn, R. & Duka, T. 2000. State-dependent effects of alcohol on explicit memory: The role of semantic associations. Psychopharmacology . 149(1):98-106.
- Designing Your Course
- In the Classroom
- Getting Feedback
- Equitable & Inclusive Teaching
- Advising and Mentoring
- Teaching and Your Career
- Teaching Remotely
- Tools and Platforms
- Comprehending and Communicating Knowledge
- Motivation and Metacognition
- Promoting Engagement
- Bok Publications
- Other Resources Around Campus
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 24 September 2019
The neurobiological foundation of memory retrieval
- Paul W. Frankland ORCID: orcid.org/0000-0002-1395-3586 1 , 2 , 3 , 4 , 5 ,
- Sheena A. Josselyn ORCID: orcid.org/0000-0001-5451-489X 1 , 2 , 3 , 4 , 6 &
- Stefan Köhler ORCID: orcid.org/0000-0003-1905-6453 7 , 8
Nature Neuroscience volume 22 , pages 1576–1585 ( 2019 ) Cite this article
20k Accesses
98 Citations
129 Altmetric
Metrics details
- Classical conditioning
- Hippocampus
- Learning and memory
- Optogenetics
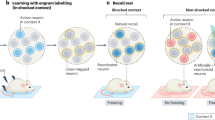
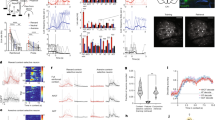
Memory retrieval involves the interaction between external sensory or internally generated cues and stored memory traces (or engrams) in a process termed ‘ecphory’. While ecphory has been examined in human cognitive neuroscience research, its neurobiological foundation is less understood. To the extent that ecphory involves ‘reawakening’ of engrams, leveraging recently developed technologies that can identify and manipulate engrams in rodents provides a fertile avenue for examining retrieval at the level of neuronal ensembles. Here we evaluate emerging neuroscientific research of this type, using cognitive theory as a guiding principle to organize and interpret initial findings. Our Review highlights the critical interaction between engrams and retrieval cues (environmental or artificial) for memory accessibility and retrieval success. These findings also highlight the intimate relationship between the mechanisms important in forming engrams and those important in their recovery, as captured in the cognitive notion of ‘encoding specificity’. Finally, we identify several questions that currently remain unanswered.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Engram neurons: Encoding, consolidation, retrieval, and forgetting of memory
Engram mechanisms of memory linking and identity
Prefrontal feature representations drive memory recall
Tulving, E. & Pearlstone, Z. Availability versus accessibility of information in memory for words. J. Verbal Learn. Verbal Behav. 5 , 381–391 (1966).
Article Google Scholar
Tulving, E. Ecphoric processes in episodic memory. Philos. Trans. R. Soc. Lond. B 302 , 361–370 (1983).
Tulving, E. Elements of Episodic Memory . (Oxford University Press, 1983).
Semon, R. Die Mneme . (W. Engelmann, 1904).
Josselyn, S. A., Köhler, S. & Frankland, P. W. Heroes of the engram. J. Neurosci. 37 , 4647–4657 (2017).
Article CAS PubMed PubMed Central Google Scholar
Tulving, E. Episodic memory: from mind to brain. Annu. Rev. Psychol. 53 , 1–25 (2002).
Article PubMed Google Scholar
Eichenbaum, H. Still searching for the engram. Learn. Behav. 44 , 209–222 (2016).
Article PubMed PubMed Central Google Scholar
Josselyn, S. A., Köhler, S. & Frankland, P. W. Finding the engram. Nat. Rev. Neurosci. 16 , 521–534 (2015).
Article CAS PubMed Google Scholar
Tonegawa, S., Liu, X., Ramirez, S. & Redondo, R. Memory engram cells have come of age. Neuron 87 , 918–931 (2015).
Tonegawa, S., Pignatelli, M., Roy, D. S. & Ryan, T. J. Memory engram storage and retrieval. Curr. Opin. Neurobiol. 35 , 101–109 (2015).
DeNardo, L. A. et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 22 , 460–469 (2019).
Denny, C. A. et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83 , 189–201 (2014).
Reijmers, L. G., Perkins, B. L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317 , 1230–1233 (2007).
Sørensen, A. T. et al. A robust activity marking system for exploring active neuronal ensembles. eLife 5 , e13918 (2016).
Josselyn, S. A. & Frankland, P. W. Memory allocation: mechanisms and function. Annu. Rev. Neurosci. 41 , 389–413 (2018).
Ben-Yakov, A., Dudai, Y. & Mayford, M. R. Memory retrieval in mice and men. Cold Spring Harb. Perspect. Biol. 7 , a021790 (2015).
Robins, S. K. Memory and optogenetic intervention: separating the engram from the ecphory. Philos. Sci. 85 , 1078–1089 (2018).
Denny, C. A., Lebois, E. & Ramirez, S. From engrams to pathologies of the brain. Front. Neural Circuits 11 , 23 (2017).
Article PubMed PubMed Central CAS Google Scholar
Tanaka, K. Z. et al. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84 , 347–354 (2014).
Han, J. H. et al. Selective erasure of a fear memory. Science 323 , 1492–1496 (2009).
Rashid, A. J. et al. Competition between engrams influences fear memory formation and recall. Science 353 , 383–387 (2016).
Zhou, Y. et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 12 , 1438–1443 (2009).
Sano, Y. et al. CREB regulates memory allocation in the insular cortex. Curr. Biol. 24 , 2833–2837 (2014).
Hsiang, H. L. et al. Manipulating a “cocaine engram” in mice. J. Neurosci. 34 , 14115–14127 (2014).
Lacagnina, A. F. et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci. 22 , 753–761 (2019).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484 , 381–385 (2012).
Yiu, A. P. et al. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron 83 , 722–735 (2014).
Kim, J., Kwon, J. T., Kim, H. S., Josselyn, S. A. & Han, J. H. Memory recall and modifications by activating neurons with elevated CREB. Nat. Neurosci. 17 , 65–72 (2014).
Abdou, K. et al. Synapse-specific representation of the identity of overlapping memory engrams. Science 360 , 1227–1231 (2018).
Ryan, T. J., Roy, D. S., Pignatelli, M., Arons, A. & Tonegawa, S. Memory. Engram cells retain memory under retrograde amnesia. Science 348 , 1007–1013 (2015).
Redondo, R. L. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513 , 426–430 (2014).
Cowansage, K. K. et al. Direct reactivation of a coherent neocortical memory of context. Neuron 84 , 432–441 (2014).
Vetere, G. et al. Memory formation in the absence of experience. Nat. Neurosci. 22 , 933–940 (2019).
Roy, D. S., Muralidhar, S., Smith, L. M. & Tonegawa, S. Silent memory engrams as the basis for retrograde amnesia. Proc. Natl. Acad. Sci. USA 114 , E9972–E9979 (2017).
Nader, K., Schafe, G. E. & Le Doux, J. E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406 , 722–726 (2000).
Suzuki, A. et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24 , 4787–4795 (2004).
Roy, D. S. et al. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531 , 508–512 (2016).
Perusini, J. N. et al. Optogenetic stimulation of dentate gyrus engrams restores memory in Alzheimer’s disease mice. Hippocampus 27 , 1110–1122 (2017).
Guskjolen, A. et al. Recovery of “lost” infant memories in mice. Curr. Biol. 28 , 2283–2290.e3 (2018).
Okuyama, T., Kitamura, T., Roy, D. S., Itohara, S. & Tonegawa, S. Ventral CA1 neurons store social memory. Science 353 , 1536–1541 (2016).
Choi, J. H. et al. Interregional synaptic maps among engram cells underlie memory formation. Science 360 , 430–435 (2018).
Gouty-Colomer, L. A. et al. Arc expression identifies the lateral amygdala fear memory trace. Mol. Psychiatry 21 , 364–375 (2016).
Nabavi, S. et al. Engineering a memory with LTD and LTP. Nature 511 , 348–352 (2014).
Schacter, D.L. Searching for Memory: The Brain, the Mind, and the Past . (Basic Books, 1996).
Roy, D. S., Okuyama, T. & Tonegawa, S. Tagging activated neurons with light. Nat. Biotechnol. 35 , 827–828 (2017).
Davis, R. L. & Zhong, Y. The biology of forgetting—a perspective. Neuron 95 , 490–503 (2017).
Frankland, P. W., Köhler, S. & Josselyn, S. A. Hippocampal neurogenesis and forgetting. Trends Neurosci. 36 , 497–503 (2013).
Hardt, O., Nader, K. & Nadel, L. Decay happens: the role of active forgetting in memory. Trends Cogn. Sci. 17 , 111–120 (2013).
Tulving, E. & Thomson, D. M. Encoding specificity and retrieval processes in episodic memory. Psychol. Rev. 80 , 352–373 (1973).
Eich, E. Mood as a mediator of place dependent memory. J. Exp. Psychol. Gen. 124 , 293–308 (1995).
Godden, D. R. & Baddeley, A. D. Context‐dependent memory in two natural environments: On land and underwater. Br. J. Psychol. 66 , 325–331 (1975).
Smith, S. M. & Vela, E. Environmental context-dependent memory: a review and meta-analysis. Psychon. Bull. Rev. 8 , 203–220 (2001).
Bouton, M. E. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 114 , 80–99 (1993).
Maren, S., Phan, K. L. & Liberzon, I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14 , 417–428 (2013).
Jafarpour, A., Fuentemilla, L., Horner, A. J., Penny, W. & Duzel, E. Replay of very early encoding representations during recollection. J. Neurosci. 34 , 242–248 (2014).
Johnson, J. D., McDuff, S. G., Rugg, M. D. & Norman, K. A. Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron 63 , 697–708 (2009).
Manning, J. R., Polyn, S. M., Baltuch, G. H., Litt, B. & Kahana, M. J. Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. Proc. Natl. Acad. Sci. USA 108 , 12893–12897 (2011).
Polyn, S. M., Natu, V. S., Cohen, J. D. & Norman, K. A. Category-specific cortical activity precedes retrieval during memory search. Science 310 , 1963–1966 (2005).
Ritchey, M., Wing, E. A., LaBar, K. S. & Cabeza, R. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb. Cortex 23 , 2818–2828 (2013).
Staresina, B. P., Henson, R. N., Kriegeskorte, N. & Alink, A. Episodic reinstatement in the medial temporal lobe. J. Neurosci. 32 , 18150–18156 (2012).
Staresina, B. P. et al. Hippocampal pattern completion is linked to gamma power increases and alpha power decreases during recollection. eLife 5 , e17397 (2016).
Yaffe, R. B. et al. Reinstatement of distributed cortical oscillations occurs with precise spatiotemporal dynamics during successful memory retrieval. Proc. Natl. Acad. Sci. USA 111 , 18727–18732 (2014).
St-Laurent, M., Abdi, H. & Buchsbaum, B. R. Distributed patterns of reactivation predict vividness of recollection. J. Cogn. Neurosci. 27 , 2000–2018 (2015).
Danker, J. F., Tompary, A. & Davachi, L. Trial-by-trial hippocampal encoding activation predicts the fidelity of cortical reinstatement during subsequent retrieval. Cereb. Cortex 27 , 3515–3524 (2017).
PubMed Google Scholar
Horner, A. J., Bisby, J. A., Bush, D., Lin, W. J. & Burgess, N. Evidence for holistic episodic recollection via hippocampal pattern completion. Nat. Commun. 6 , 7462 (2015).
Staresina, B. P., Cooper, E. & Henson, R. N. Reversible information flow across the medial temporal lobe: the hippocampus links cortical modules during memory retrieval. J. Neurosci. 33 , 14184–14192 (2013).
Staudigl, T., Vollmar, C., Noachtar, S. & Hanslmayr, S. Temporal-pattern similarity analysis reveals the beneficial and detrimental effects of context reinstatement on human memory. J. Neurosci. 35 , 5373–5384 (2015).
Guzowski, J. F., McNaughton, B. L., Barnes, C. A. & Worley, P. F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2 , 1120–1124 (1999).
Deng, W., Mayford, M. & Gage, F. H. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife 2 , e00312 (2013).
Khalaf, O. et al. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 360 , 1239–1242 (2018).
Ramirez, S. et al. Creating a false memory in the hippocampus. Science 341 , 387–391 (2013).
Tayler, K. K., Tanaka, K. Z., Reijmers, L. G. & Wiltgen, B. J. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol. 23 , 99–106 (2013).
Richards, B. A. & Frankland, P. W. The conjunctive trace. Hippocampus 23 , 207–212 (2013).
Orsini, C. A., Yan, C. & Maren, S. Ensemble coding of context-dependent fear memory in the amygdala. Front. Behav. Neurosci. 7 , 199 (2013).
Emiliani, V., Cohen, A. E., Deisseroth, K. & Häusser, M. All-optical interrogation of neural circuits. J. Neurosci. 35 , 13917–13926 (2015).
Yang, S. J. et al. Extended field-of-view and increased-signal 3D holographic illumination with time-division multiplexing. Opt. Express 23 , 32573–32581 (2015).
Yang, W. & Yuste, R. Holographic imaging and photostimulation of neural activity. Curr. Opin. Neurobiol. 50 , 211–221 (2018).
Carrillo-Reid, L., Han, S., Yang, W., Akrouh, A. & Yuste, R. Controlling visually guided behavior by holographic recalling of cortical ensembles. Cell 178 , 447–457.e5 (2019).
Marshel, J. H. et al. Cortical layer-specific critical dynamics triggering perception. Science 365 , eaaw5202 (2019).
Squire, L. R. & Alvarez, P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr. Opin. Neurobiol. 5 , 169–177 (1995).
Teyler, T. J. & DiScenna, P. The hippocampal memory indexing theory. Behav. Neurosci. 100 , 147–154 (1986).
McClelland, J. L., McNaughton, B. L. & O’Reilly, R. C. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102 , 419–457 (1995).
Norman, K. A. & O’Reilly, R. C. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol. Rev. 110 , 611–646 (2003).
Josselyn, S. A. & Frankland, P. W. Infantile amnesia: a neurogenic hypothesis. Learn. Mem. 19 , 423–433 (2012).
Wheeler, A. L. et al. Identification of a functional connectome for long-term fear memory in mice. PLOS Comput. Biol. 9 , e1002853 (2013).
Vetere, G. et al. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron 94 , 363–374.e4 (2017).
Moscovitch, M., Cabeza, R., Winocur, G. & Nadel, L. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu. Rev. Psychol. 67 , 105–134 (2016).
Frankland, P. W. & Bontempi, B. The organization of recent and remote memories. Nat. Rev. Neurosci. 6 , 119–130 (2005).
Rescorla, R.A. Pavlovian Second-order Conditioning (Psychology Revivals): Studies in Associative Learning . (Psychology Press, 2014).
Ohkawa, N. et al. Artificial association of pre-stored information to generate a qualitatively new memory. Cell Rep. 11 , 261–269 (2015).
Chen, B. K. et al. Artificially enhancing and suppressing hippocampus-mediated memories. Curr. Biol. 29 , 1885–1894.e4 (2019).
Anderson, M. C. Rethinking interference theory: Executive control and the mechanisms of forgetting. J. Mem. Lang. 49 , 415–445 (2003).
Garner, A. R. et al. Generation of a synthetic memory trace. Science 335 , 1513–1516 (2012).
Sekeres, M. J. et al. Recovering and preventing loss of detailed memory: differential rates of forgetting for detail types in episodic memory. Learn. Mem. 23 , 72–82 (2016).
Richards, B. A. & Frankland, P. W. The persistence and transience of memory. Neuron 94 , 1071–1084 (2017).
Nadel, L. & Moscovitch, M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 7 , 217–227 (1997).
Kitamura, T. et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356 , 73–78 (2017).
Tonegawa, S., Morrissey, M. D. & Kitamura, T. The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci. 19 , 485–498 (2018).
Schapiro, A. C., Turk-Browne, N. B., Botvinick, M. M. & Norman, K. A. Complementary learning systems within the hippocampus: a neural network modelling approach to reconciling episodic memory with statistical learning. Philos. Trans. R. Soc. Lond. B 372 , 20160049 (2017).
Cai, D. J. et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534 , 115–118 (2016).
Yokose, J. et al. Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science 355 , 398–403 (2017).
Gilboa, A. & Marlatte, H. Neurobiology of schemas and schema-mediated memory. Trends Cogn. Sci. 21 , 618–631 (2017).
Richards, B. A. et al. Patterns across multiple memories are identified over time. Nat. Neurosci. 17 , 981–986 (2014).
Tse, D. et al. Schema-dependent gene activation and memory encoding in neocortex. Science 333 , 891–895 (2011).
Barry, D. N. & Maguire, E. A. Remote memory and the hippocampus: a constructive critique. Trends Cogn. Sci. 23 , 128–142 (2019).
Barry, D. N. & Maguire, E. A. Consolidating the case for transient hippocampal memory traces. Trends Cogn. Sci. 23 , 635–636 (2019).
Moscovitch, M. & Nadel, L. Sculpting remote memory: enduring hippocampal traces and vmPFC reconstructive processes. Trends Cogn. Sci. 23 , 634–635 (2019).
Han, J. H. et al. Neuronal competition and selection during memory formation. Science 316 , 457–460 (2007).
Rogerson, T. et al. Molecular and cellular mechanisms for trapping and activating emotional memories. PLoS One 11 , e0161655 (2016).
Kawashima, T. et al. Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nat. Methods 10 , 889–895 (2013).
Guenthner, C. J., Miyamichi, K., Yang, H. H., Heller, H. C. & Luo, L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78 , 773–784 (2013).
Download references
Acknowledgements
We thank A.Ramsaran and A.Park for drawing the figures, and we thank T. Ryan for comments on an earlier draft of this manuscript. This work was supported by Canadian Institutes of Health Research grants to P.W.F. (FDN-143227) and S.A.J. (FDN-388455) and a Natural Sciences and Engineering Research Council Discovery grant to S.K. (RGPIN-5770).
Author information
Authors and affiliations.
Program in Neurosciences & Mental Health, Hospital for Sick Children, Toronto, Ontario, Canada
Paul W. Frankland & Sheena A. Josselyn
Institute of Medical Sciences, University of Toronto, Toronto, Ontario, Canada
Department of Psychology, University of Toronto, Toronto, Ontario, Canada
Department of Physiology, University of Toronto, Toronto, Ontario, Canada
Child & Brain Development Program, Canadian Institute for Advanced Research, Toronto, Ontario, Canada
- Paul W. Frankland
Brain, Mind & Consciousness Program, Canadian Institute for Advanced Research, Toronto, Ontario, Canada
Sheena A. Josselyn
Department of Psychology, University of Western Ontario, London, Ontario, Canada
Stefan Köhler
The Brain and Mind Institute, University of Western Ontario, London, Ontario, Canada
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Paul W. Frankland or Stefan Köhler .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Stephen Maren and Steve Ramirez for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Frankland, P.W., Josselyn, S.A. & Köhler, S. The neurobiological foundation of memory retrieval. Nat Neurosci 22 , 1576–1585 (2019). https://doi.org/10.1038/s41593-019-0493-1
Download citation
Received : 21 May 2019
Accepted : 08 August 2019
Published : 24 September 2019
Issue Date : October 2019
DOI : https://doi.org/10.1038/s41593-019-0493-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Active forgetting and neuropsychiatric diseases.
- Jacob A. Berry
- Dana C. Guhle
- Ronald L. Davis
Molecular Psychiatry (2024)
Neurogenesis-dependent remodeling of hippocampal circuits reduces PTSD-like behaviors in adult mice
- Risako Fujikawa
- Adam I. Ramsaran
Memory persistence: from fundamental mechanisms to translational opportunities
- Santiago Abel Merlo
- Mariano Andrés Belluscio
- Emiliano Merlo
Translational Psychiatry (2024)
The cognitive impact of light: illuminating ipRGC circuit mechanisms
- Heather L. Mahoney
- Tiffany M. Schmidt
Nature Reviews Neuroscience (2024)
Perceived authenticity across three forms of educational simulations—the role of interactant representation, task alignment, and continuity of simulation
- Caroline Corves
- Matthias Stadler
- Martin R. Fischer
European Journal of Psychology of Education (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Learning Objectives
- Explain retrieval cues and the three types of retrieval (recall, recognition, and relearning)
So you have worked hard to encode (via effortful processing) and store some important information for your upcoming final exam. How do you get that information back out of storage when you need it? The act of getting information out of memory storage and back into conscious awareness is known as retrieval . This would be similar to finding and opening a paper you had previously saved on your computer’s hard drive. Now it’s back on your desktop, and you can work with it again. Our ability to retrieve information from long-term memory is vital to our everyday functioning. You must be able to retrieve information from memory in order to do everything from knowing how to brush your hair and teeth, to driving to work, to knowing how to perform your job once you get there.

Memory Cues
What factors determine what information can be retrieved from memory? One critical factor is the type of hints, or cues , in the environment. You may hear a song on the radio that suddenly evokes memories of an earlier time in your life, even if you were not trying to remember it when the song came on. Nevertheless, the song is closely associated with that time, so it brings the experience to mind.
The general principle that underlies the effectiveness of retrieval cues is the encoding specificity principle (Tulving & Thomson, 1973): when people encode information, they do so in specific ways. For example, take the song on the radio: perhaps you heard it while you were at a terrific party, having a great, philosophical conversation with a friend. Thus, the song became part of that whole complex experience. Years later, even though you haven’t thought about that party in ages, when you hear the song on the radio, the whole experience rushes back to you. In general, the encoding specificity principle states that, to the extent a retrieval cue (the song) matches or overlaps the memory trace of an experience (the party, the conversation), it will be effective in evoking the memory. A classic experiment on the encoding specificity principle had participants memorize a set of words in a unique setting. Later, the participants were tested on the word sets, either in the same location they learned the words or a different one. As a result of encoding specificity, the students who took the test in the same place they learned the words were actually able to recall more words (Godden & Baddeley, 1975) than the students who took the test in a new setting. In this instance, the physical context itself provided cues for retrieval. This is why it’s good to study for midterms and finals in the same room you’ll be taking them in.
One caution with this principle, though, is that, for the cue to work, it can’t match too many other experiences (Nairne, 2002; Watkins, 1975). Consider a lab experiment. Suppose you study 100 items; 99 are words, and one is a picture—of a penguin, item 50 in the list. Afterwards, the cue “recall the picture” would evoke “penguin” perfectly. No one would miss it. However, if the word “penguin” were placed in the same spot among the other 99 words, its memorability would be exceptionally worse. This outcome shows the power of distinctiveness : one picture is perfectly recalled from among 99 words because it stands out. Now consider what would happen if the experiment were repeated, but there were 25 pictures distributed within the 100-item list. Although the picture of the penguin would still be there, the probability that the cue “recall the picture” (at item 50) would be useful for the penguin would drop correspondingly. Watkins (1975) referred to this outcome as demonstrating the cue overload principle . That is, to be effective, a retrieval cue cannot be overloaded with too many memories. For the cue “recall the picture” to be effective, it should only match one item in the target set (as in the one-picture, 99-word case).
To sum up how memory cues function: for a retrieval cue to be effective, a match must exist between the cue and the desired target memory; furthermore, to produce the best retrieval, the cue-target relationship should be distinctive.
Types of Retrieval
There are three ways you can retrieve information out of your long-term memory storage system: recall, recognition, and relearning. Recall is what we most often think about when we talk about memory retrieval: it means you can access information without cues. For example, you would use recall for an essay test. Recognition happens when you identify information that you have previously learned after encountering it again. It involves a process of comparison. When you take a multiple-choice test, you are relying on recognition to help you choose the correct answer. Here is another example. Let’s say you graduated from high school 10 years ago, and you have returned to your hometown for your 10-year reunion. You may not be able to recall all of your classmates, but you recognize many of them based on their yearbook photos.
The third form of retrieval is relearning , and it’s just what it sounds like. It involves learning information that you previously learned. Whitney took Spanish in high school, but after high school she did not have the opportunity to speak Spanish. Whitney is now 31, and her company has offered her an opportunity to work in their Mexico City office. In order to prepare herself, she enrolls in a Spanish course at the local community center. She’s surprised at how quickly she’s able to pick up the language after not speaking it for 13 years; this is an example of relearning.
Recall and Recognition
Psychologists measure memory performance by using production tests (involving recall) or recognition tests (involving the selection of correct from incorrect information, e.g., a multiple-choice test). For example, with our list of 100 words, one group of people might be asked to recall the list in any order (a free recall test), while a different group might be asked to circle the 100 studied words out of a mix with another 100, unstudied words (a recognition test). In this situation, the recognition test would likely produce better performance from participants than the recall test.
We usually think of recognition tests as being quite easy, because the cue for retrieval is a copy of the actual event that was presented for study. After all, what could be a better cue than the exact target (memory) the person is trying to access? In most cases, this line of reasoning is true; nevertheless, recognition tests do not provide perfect indexes of what is stored in memory. That is, you can fail to recognize a target staring you right in the face, yet be able to recall it later with a different set of cues (Watkins & Tulving, 1975). For example, suppose you had the task of recognizing the surnames of famous authors. At first, you might think that being given the actual last name would always be the best cue. However, research has shown this not necessarily to be true (Muter, 1984). When given names such as Tolstoy, Shaw, Shakespeare, and Lee, subjects might well say that Tolstoy and Shakespeare are famous authors, whereas Shaw and Lee are not. But, when given a cued recall test using first names, people often recall items (produce them) that they had failed to recognize before.
For example, in this instance, a cue like George Bernard ________ often leads to a recall of “Shaw,” even though people initially failed to recognize Shaw as a famous author’s name. Yet, when given the cue “William,” people may not come up with Shakespeare, because William is a common name that matches many people (the cue overload principle at work). This strange fact—that recall can sometimes lead to better performance than recognition—can be explained by the encoding specificity principle. As a cue, George Bernard _________ matches the way the famous writer is stored in memory better than does his surname, Shaw, does (even though it is the target). Further, the match is quite distinctive with George Bernard ___________, but the cue William _________________ is much more overloaded (Prince William, William Yeats, William Faulkner, will.i.am).
The phenomenon we have been describing is called the recognition failure of recallable words , which highlights the point that a cue will be most effective depending on how the information has been encoded (Tulving & Thomson, 1973). The point is, the cues that work best to evoke retrieval are those that recreate the event or name to be remembered, whereas sometimes even the target itself, such as Shaw in the above example, is not the best cue. Which cue will be most effective depends on how the information has been encoded.
Retrieval and Reconstruction
Whenever we think about our past, we engage in the act of retrieval. We usually think that retrieval is an objective act because we tend to imagine that retrieving a memory is like pulling a book from a shelf, and after we are done with it, we return the book to the shelf just as it was. However, research shows this assumption to be false; far from being a static repository of data, the memory is constantly changing. In fact, every time we retrieve a memory, it is altered. For example, the act of retrieval itself (of a fact, concept, or event) makes the retrieved memory much more likely to be retrieved again, a phenomenon called the testing effect or the retrieval practice effect (Pyc & Rawson, 2009; Roediger & Karpicke, 2006). However, retrieving some information can actually cause us to forget other information related to it, a phenomenon called retrieval-induced forgetting (Anderson, Bjork, & Bjork, 1994). Thus the act of retrieval can be a double-edged sword—strengthening the memory just retrieved (usually by a large amount) but harming related information (though this effect is often relatively small).
Retrieval of distant memories is reconstructive. We weave the concrete bits and pieces of events in with assumptions and preferences to form a coherent story (Bartlett, 1932). For example, if during your 10th birthday, your dog got to your cake before you did, you would likely tell that story for years afterward. Say, then, in later years you misremember where the dog actually found the cake, but repeat that error over and over during subsequent retellings of the story. Over time, that inaccuracy would become a basic fact of the event in your mind. Just as retrieval practice (repetition) enhances accurate memories, so will it strengthen errors or false memories (McDermott, 2006). Sometimes memories can even be manufactured just from hearing a vivid story. Consider the following episode, recounted by Jean Piaget, the famous developmental psychologist, from his childhood:
One of my first memories would date, if it were true, from my second year. I can still see, most clearly, the following scene, in which I believed until I was about 15. I was sitting in my pram . . . when a man tried to kidnap me. I was held in by the strap fastened round me while my nurse bravely tried to stand between me and the thief. She received various scratches, and I can still vaguely see those on her face. . . . When I was about 15, my parents received a letter from my former nurse saying that she had been converted to the Salvation Army. She wanted to confess her past faults, and in particular to return the watch she had been given as a reward on this occasion. She had made up the whole story, faking the scratches. I therefore must have heard, as a child, this story, which my parents believed, and projected it into the past in the form of a visual memory. . . . Many real memories are doubtless of the same order. (Norman & Schacter, 1997, pp. 187–188)
Piaget’s vivid account represents a case of a pure reconstructive memory. He heard the tale told repeatedly, and doubtless told it (and thought about it) himself. The repeated telling cemented the events as though they had really happened, just as we are all open to the possibility of having “many real memories … of the same order.” The fact that one can remember precise details (the location, the scratches) does not necessarily indicate that the memory is true, a point that has been confirmed in laboratory studies, too (e.g., Norman & Schacter, 1997).
Review the concepts from this section on encoding, storage, and retrieval in the following CrashCourse video:
You can view the transcript for “How We Make Memories: Crash Course Psychology #13” here (opens in new window) .
CC licensed content, Original
- How Memory Functions. Authored by : OpenStax College. Located at : https://openstax.org/books/psychology-2e/pages/8-1-how-memory-functions . License : CC BY: Attribution . License Terms : Download for free at https://openstax.org/books/psychology-2e/pages/1-introduction
- Modification, adaptation, and original content. Provided by : Lumen Learning. License : CC BY: Attribution
CC licensed content, Shared previously
- Memory (Encoding, Storage, Retrieval). Authored by : Kathleen B. McDermott and Henry L. Roediger III . Provided by : Washington University in St. Louis. Located at : http://nobaproject.com/textbooks/wendy-king-introduction-to-psychology-the-full-noba-collection/modules/memory-encoding-storage-retrieval . Project : The Noba Project. License : CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
All rights reserved content
- How We Make Memories – Crash Course Psychology #13. Provided by : CrashCourse. Located at : https://youtu.be/bSycdIx-C48?list=PL8dPuuaLjXtOPRKzVLY0jJY-uHOH9KVU6 . License : Other . License Terms : Standard YouTube License
creation of a permanent record of information
act of getting information out of long-term memory storage and back into conscious awareness
continuous storage of information
the hypothesis that a retrieval cue will be effective to the extent that information encoded from the cue overlaps or matches information in the engram or memory trace.
accessing information without cues
identifying previously learned information after encountering it again, usually in response to a cue
learning information that was previously learned
General Psychology Copyright © by OpenStax and Lumen Learning is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book
How Memory Works
Reviewed by Psychology Today Staff
Memory is a continually unfolding process. Initial details of an experience take shape in memory; the brain’s representation of that information then changes over time. With subsequent reactivations, the memory grows stronger or fainter and takes on different characteristics. Memories reflect real-world experience, but with varying levels of fidelity to that original experience.
The degree to which the memories we form are accurate or easily recalled depends on a variety of factors, from the psychological conditions in which information is first translated into memory to the manner in which we seek—or are unwittingly prompted—to conjure details from the past.
On This Page
- How Memories Are Made
- How Memories Are Stored in the Brain
- How We Recall Memories
- False and Distorted Memories
The creation of a memory requires a conversion of a select amount of the information one perceives into more permanent form. A subset of that memory will be secured in long-term storage, accessible for future use. Many factors during and after the creation of a memory influence what (and how much) gets preserved.
Memory serves many purposes, from allowing us to revisit and learn from past experiences to storing knowledge about the world and how things work. More broadly, a major function of memory in humans and other animals is to help ensure that our behavior fits the present situation and that we can adjust it based on experience.
Encoding is the first stage of memory. It is the process by which the details of a person’s experience are converted into a form that can be stored in the brain. People are more likely to encode details of what they are paying attention to and details that are personally significant.
Retention, or storage, is the stage in which information is preserved in memory following its initial encoding. These stored memories are incomplete : Some of the information that is encoded during an experience fades during retention, sometimes quickly, while other details remain. A related term, memory consolidation , refers to the neurobiological process of long-term memory formation.
Sleep facilitates the retention of memories, though why exactly this is the case is not fully understood. Research has found that people tend to show better memory performance if they sleep after a phase of studying rather than staying awake. Researchers have proposed that sleep supports memory consolidation in the brain, though other explanations include tha t sleep aids retention by eliminating interference from memories that would be formed while awake.
While memories are usually described in terms of mental concepts, such as single packages of personal experience or specific facts, they are ultimately reducible to the workings and characteristics of the ever-firing cells of the brain. Scientists have narrowed down regions of the brain that are key to memory and developed an increasingly detailed understanding of the material form of these mental phenomena.
The hippocampus and other parts of the medial temporal lobe are critical for many forms of memory, though various other parts of the brain play roles as well. These include areas of the more recently evolved cerebral cortex, the outermost layer of the brain, as well as deep-seated structures such as the basal ganglia. The amygdala is important for memory as well, including the integration of emotional responses into memory. The extent to which different brain regions are involved in memory depends on the type of memory.
Memory involves changes to the brain’s neural networks. Neurons in the brain are connected by synapses, which are bound together by chemical messengers (neurotransmitters) to form larger networks. Memory storage is thought to involve changes in the strength of these connections in the areas of the brain that have been linked to memory.
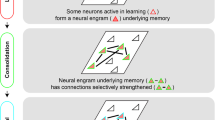
A memory engram , or memory trace, is a term for the set of changes in the brain on which a memory is based. These are thought to include changes at the level of the synapses that connect brain cells. Research suggests an engram is not located in one specific location in the brain, but in multiple, interconnected locations. Engram cells are groups of cells that support a memory: They are activated and altered during learning and reactivated during remembering.
After memories are stored in the brain, they must be retrieved in order to be useful. While we may or may not be consciously aware that information is being summoned from storage at any given moment, this stage of memory is constantly unfolding—and the very act of remembering changes how memories are subsequently filed away.
Retrieval is the stage of memory in which the information saved in memory is recalled, whether consciously or unconsciously. It follows the stages of encoding and storage. Retrieval includes both intentional remembering, as when one thinks back to a previous experience or tries to put a name to a face, and more passive recall, as when the meanings of well-known words or the notes of a song come effortlessly to mind.
A retrieval cue is a stimulus that initiates remembering. Retrieval cues can be external, such as an image, text, a scent, or some other stimulus that relates to the memory. They can also be internal, such as a thought or sensation that is relevant to the memory. Cues can be encountered inadvertently or deliberately sought in the process of deliberately trying to remember something.
Multiple factors influence why we remember what we do. Emotionally charged memories tend to be relatively easy to recall. So is information that has been retrieved from memory many times, through studying, carrying out a routine, or some other form of repetition. And the “encoding specificity principle” holds that one is more likely to recall a memory when there is greater similarity between a retrieval cue (such as an image or sound in the present) and the conditions in which the memory was initially formed.
After a memory is retrieved, it is thought to undergo a process called reconsolidation , during which its representation in the brain can change based on input at the time of remembering. This capacity for memories to be reformed after retrieval has been explored as a potential element of psychotherapeutic interventions (for dampening the intensity of threatening memories, for example).
“Flashbulb memories” are what psychologists have called memories of one’s personal experience of significant and emotionally intense events, such as the 9/11 attacks and other highly distinctive occurrences. These memories may seem especially vivid and reliable even if the accuracy of the remembered details diminishes over time.
Priming is what happens when being exposed to one stimulus (such as a word) affects how a person responds to another, related one. For example, if someone is shown a list of words that includes nurse , he may be more likely to subsequently fill out the word stem nu____ with that word. Measures of priming can be used to demonstrate implicit memory, or memory that does not involve conscious recollection.
Memories have to be reconstructed in order to be used, and the piecing-together of details leaves plenty of room for inaccuracies—and even outright falsehoods—to contaminate the record. These errors reflect a memory system that is built to craft a useful account of past experience, not a perfect one. (For more, see False Memories .)
Memories may be rendered less accurate based on conditions when they are first formed, such as how much attention is paid during the experience. And the malleability of memories over time means internal and external factors can introduce errors. These may include a person’s knowledge and expectations about the world (used to fill in the blanks of a memory) and misleading suggestions by other people about what occurred.
False memories can be as simple as concluding that you were shown a word that you actually weren’t , but it may also include believing you experienced a dramatic event that you didn’t. People may produce such false recollections by unwittingly drawing on the details of actual, related experiences, or in some cases, as a response to another person’s detailed suggestions (perhaps involving some true details) about an imaginary event that is purported to be real.
It probably depends on the kind of memory. Minor manipulations like convincing people they saw a word that they did not see seem to be fairly easy to do. Getting people to conclude they had an experience (like spilling punch at a wedding) that was in fact made up seems to require more work—including, in one study, a couple of conversations and encouragement to think more about the “memory”—and may fully succeed only for a minority of people. Still, researchers who have investigated the implanting of false memories argue that in some cases, enough outside suggestion could result in the creation of false or distorted memories that have serious legal consequences.
Déjà vu, a French phrase that translates to “already seen,” is the sense of having seen or experienced something before, even though one is in fact encountering it for the first time. While the cause is not fully understood, one explanation for why déjà vu happens is that there is some resemblance between a current experience and a previous one, but the previous experience is not readily identified in the moment. Others have suggested that déjà vu may result from new information somehow being passed straight to long-term memory, or from the spontaneous activation of a part of the brain called the rhinal cortex, involved in the sense of familiarity.

To save your brain from neurobiological boredom, stand up and be squatted every now and then, and start to think of sitting as part of your interruption of standing.

Are studies of music therapy’s impact reliable?

Whether or not you remember, you dream several times a night. Your dreams are crucial in enhancing memory, integrating new skills, regulating emotions and healing trauma.

We encounter failure in our daily lives. How we interpret the meaning of failure and, in turn, how we remember and react to failure, can be influenced by our cultural upbringing.

Are the dreams we have of lost loved ones meaningful?

Could a blue dye help with depression?
Being persuasive relies on critical thinking, but critical thinking itself relies on the ability to remember information.

Early detection of cognitive decline is vital to optimizing treatment, quality of life, and hope. Neuropsychological assessment is a top diagnostic tool for evaluating cognition.

They're neither uninformed nor always cranky.

The "mind's eye" gets an assist from our physical eyes in remembering our past and imagining our future.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

Sticking up for yourself is no easy task. But there are concrete skills you can use to hone your assertiveness and advocate for yourself.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction
The significance of forgetting
- Executive attention
- Patterns of acquisition in working memory
- Mnemonic systems
- Physiological aspects of long-term memory
- Autobiographical memory
- Eyewitness memory
- Interference
- Challenges to interference theory
Our editors will review what you’ve submitted and determine whether to revise the article.
- LiveScience - Memory Definition and Types of Memory
- Healthline - 14 Natural Ways to Improve Your Memory
- Stanford Encyclopedia of Philosophy - Memory
- Engineering LibreTexts - Memory
- Psychology Today - Memory
- National Center for Biotechnology Information - PubMed Central - Memory: An Extended Definition
- Frontiers - Memory: An Extended Definition
- Verywell Mind - What is Memory?
- University of Minnesota Libraries - Open Textbooks - Memories as Types and Stages
- BCcampus Open Publishing - Memories as Types and Stages
- memory - Children's Encyclopedia (Ages 8-11)
- memory - Student Encyclopedia (Ages 11 and up)
- Table Of Contents
memory , the encoding, storage, and retrieval in the human mind of past experiences.
That experiences influence subsequent behavior is evidence of an obvious but nevertheless remarkable activity called remembering. Memory is both a result of and an influence on perception , attention , and learning . The basic pattern of remembering consists of attention to an event followed by the representation of that event in the brain . Repeated attention, or practice , results in a cumulative effect on memory and enables activities such as a skillful performance on a musical instrument , the recitation of a poem, and reading and understanding words on a page.
Learning could not occur without the function of memory. So-called intelligent behavior demands memory, remembering being prerequisite to reasoning . The ability to solve any problem or even to recognize that a problem exists depends on memory. Routine action, such as the decision to cross a street, is based on remembering numerous earlier experiences. The act of remembering an experience and bringing it to consciousness at a later time requires an association, which is formed from the experience, and a “retrieval cue,” which elicits the memory of the experience.
Practice (or review) tends to build and maintain memory for a task or for any learned material. During a period without practice, what has been learned tends to be forgotten . Although the adaptive value of forgetting may not be obvious, dramatic instances of sudden forgetting (as in amnesia ) can be seen to be adaptive. In this sense, the ability to forget can be interpreted as having been naturally selected in animals . Indeed, when one’s memory of an emotionally painful experience leads to severe anxiety , forgetting may produce relief. Nevertheless, an evolutionary interpretation might make it difficult to understand how the commonly gradual process of forgetting was selected for.

In speculating about the evolution of memory, it is helpful to consider what would happen if memories failed to fade. Forgetting clearly aids orientation in time; since old memories weaken and new ones tend to be vivid, clues are provided for inferring duration. Without forgetting, adaptive ability would suffer; for example, learned behavior that might have been correct a decade ago may no longer be appropriate or safe. Indeed, cases are recorded of people who (by ordinary standards) forget so little that their everyday activities are full of confusion. Thus, forgetting seems to serve the survival not only of the individual but of the entire human species.
Additional speculation posits a memory-storage system of limited capacity that provides adaptive flexibility specifically through forgetting. According to this view, continual adjustments are made between learning or memory storage (input) and forgetting (output). There is evidence in fact that the rate at which individuals forget is directly related to how much they have learned. Such data offer gross support for models of memory that assume an input-output balance.
Whatever its origins, forgetting has attracted considerable investigative attention. Much of this research has been aimed at discovering those factors that change the rate of forgetting. Efforts are made to study how information may be stored, or encoded in the human brain. Remembered experiences may be said to consist of encoded collections of interacting information, and interaction seems to be a prime factor in forgetting.
Memory researchers have generally supposed that anything that influences the behavior of an organism endowed with a central nervous system leaves—somewhere in that system—a “trace” or group of traces. So long as these traces endure, they can, in theory, be restimulated, causing the event or experience that established them to be remembered.
Time-dependent aspects of memory
Research by the American psychologist and philosopher William James (1842–1910) led him to distinguish two types of memory: primary, for handling immediate concerns, and secondary, for managing a storehouse of information accumulated over time. Memory researchers have since used the term short-term memory to refer to the primary or short-lived memory functions identified by James. Long-term memory refers to the relatively permanent information that is stored in and retrieved from the brain.
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Sweepstakes
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
The Psychology of Forgetting and Why Memory Fails
- Time's Role in Forgetting
How Forgetting is Measured
Theories about forgetting, frequently asked questions.
Forgetting is an all too common part of daily life. Sometimes these memory slips are simple and fairly innocuous, such as forgetting to return a phone call. Other times, forgetting can be much more dire and even have serious consequences, such as an eyewitness forgetting important details about a crime.
Memory failures are an almost daily occurrence. Forgetting is so common that you probably rely on numerous methods to help you remember important information, such as jotting down notes in a daily planner or scheduling important events on your phone's calendar.
As you are frantically searching for your missing car keys, it may seem that the information about where you left them is permanently gone from your memory. However, forgetting is generally not about actually losing or erasing this information from your long-term memory.
Forgetting typically involves a failure in memory retrieval . While the information is somewhere in your long-term memory, you are not able to actually retrieve and remember it.
Why Time Plays a Key Role in Forgetting
Psychologist Hermann Ebbinghaus was one of the first to scientifically study forgetting. In experiments where he used himself as the subject, Ebbinghaus tested his memory using three-letter nonsense syllables. He relied on such nonsense words because using previously known words would have involved drawing on his existing knowledge and associations in his memory.
In order to test for new information, Ebbinghaus tested his memory for periods of time ranging from 20 minutes to 31 days. He then published his findings in 1885 in Memory: A Contribution to Experimental Psychology.
His results, plotted in what is known as the Ebbinghaus forgetting curve, revealed a relationship between forgetting and time. Initially, information is often lost very quickly after it is learned. Factors such as how the information was learned and how frequently it was rehearsed play a role in how quickly these memories are lost. Information stored in long-term memory is surprisingly stable.
The forgetting curve also showed that forgetting does not continue to decline until all of the information is lost. At a certain point, the amount of forgetting levels off.
Sometimes it might seem that information has been forgotten, but even a subtle cue can help trigger the memory. Imagine the last time you took an exam for school. While you might have initially felt forgetful and unprepared, seeing the information presented on the test probably helped cue the retrieval of information you might not have known you even remembered.
So how do we know when something has been forgotten? There are a few different ways to measure this:
- Recall : People who have been asked to memorize something, such as a list of terms, might be asked to recall the list from memory. By seeing how many items are remembered, researchers are able to identify how much information has been forgotten. This method might involve the use of free recall (recalling items without hints) or prompted recall (utilizing hints to trigger memories).
- Recognition : This method involves identifying information that was previously learned. On a test, for example, students might have to recognize which terms they learned about in a chapter of their assigned reading.
Of course, many factors can contribute to forgetting. Sometimes you might be distracted when you learn new information, which might mean that you never truly retain the information long enough to remember it later. Well-known memory researcher Elizabeth Loftus has proposed four key explanations for why forgetting occurs . These have led to some major theories of forgetting.
Interference Theory
What did you have for dinner Tuesday night of last week? Is that difficult to recall? If someone had asked you that question Wednesday morning, you probably would have had no problem recalling what you had for dinner the night before.
But as intervening days pass, the memories of all the other meals you have eaten since then start to interfere with your memory of that one particular meal. This is a good example of what psychologists call the interference theory of forgetting.
According to interference theory, forgetting is the result of different memories interfering with one another. The more similar two or more events are to one another, the more likely interference will occur.
It is difficult to remember what happened on an average school day two months ago because so many other days have occurred since then. Unique and distinctive events, however, are less likely to suffer from interference. Your high school graduation, wedding, and the birth of your first child are much more likely to be recalled because they are singular events—days like no other.
Interference also plays a role in what is known as the serial position effect , or the tendency to recall the first and last items of a list. For example, imagine that you wrote down a shopping list but forgot to take it with you to the store. In all likelihood, you will probably be able to easily recall the first and last items on your list, but you might forget many of the items that were in the middle.
The first thing you wrote down and the last thing you wrote down stand out as being more distinct, while the fourth item and seventh item might seem so similar that they interfere with each other. There are two basic types of interference that can occur:
- Retroactive interference happens when newly acquired information interferes with old memories. For example, a teacher learning the names of her new class of students at the start of a school year might find it more difficult to recall the names of the students in her class last year. The new information interferes with the old information.
- Proactive interference occurs when previously learned information makes it more difficult to form new memories. Learning a new phone number or locker combination might be more difficult, for example, because your memories of your old phone number and combination interfere with the new information.
Eliminating interference altogether is impossible, but there are a few things you can do to minimize its effects. One of the best things you can do is rehearse new information in order to better commit it to memory. In fact, many experts recommend overlearning important information, which involves rehearsing the material over and over again until it can be reproduced perfectly with no errors.
Another tactic to fight interference is to switch up your routine and avoid studying similar material back to back. For example, don't try to study vocabulary terms for your Spanish language class right after studying terms for your German class. Break up the material and switch to a completely different subject each study session.
Sleep also plays an essential role in memory formation. Researchers suggest that sleeping after you learn something new is one of the best ways to turn new memories into lasting ones.
Decay Theory of Forgetting
According to the trace theory of memory, physical and chemical changes in the brain results in a memory "trace." Information in short-term memory lasts several seconds and if it is not rehearsed, the neurochemical memory trace quickly fades. According to the trace decay theory of forgetting, the events that happen between the formation of a memory and the recall of the memory have no impact on recall.
Trace theory proposes that the length of time between the memory and recalling that information determines whether the information will be retained or forgotten. If the time interval is short, more information will be recalled. If a longer period of time passes, more information will be forgotten and memory will be poorer.
The idea that memories fade over time is hardly new. The Greek philosopher Plato suggested such a thing more than 2,500 years ago. Later, experimental research by psychologists such as Ebbinghaus bolstered this theory.
One of the problems with this theory is that it is difficult to demonstrate that time alone is responsible for declines in recall. In real-world situations, many things happen between the formation of a memory and the recall of that information. A student who learns something in class, for example, might have hundreds of unique and individual experiences between learning that information and having to recall it on an exam.
Was forgetting the date that the American Revolutionary War began due to the length of time between learning the date in your American History class and being tested on it? Or did the multitude of information acquired during that interval of time play a role? Testing this can be exceedingly difficult. It is nearly impossible to eliminate all the information that might have an influence on the creation of the memory and the recall of the memory.
Another problem with decay theory is it does not account for why some memories fade quickly while others linger. Novelty is one factor that plays a role. For example, you are more likely to remember your very first day of college than all of the intervening days between it and graduation. That first day was new and exciting, but all the following days probably seem quite similar to each other.

Retrieval Failure Theory
Sometimes the memories are there, but we just can't seem to access them. Two of the basic reasons for this failure in memory retrieval are related to encoding failures and lack of retrieval cues.
A common reason why we don't remember information is because it never made it into long-term memory in the first place.
Try this well-known demonstration first used by researchers Nickerson and Adams. From memory, try to draw the back side of a penny. Once you are done, compare your drawing to an actual penny.
Are you surprised by how poorly you recalled what the back of a penny looks like? While you probably had a good idea about the overall shape and color, the actual details were probably pretty fuzzy. Why?
Since you don't actually need to know what the back of a penny looks like to differentiate it from other coins, you only really focus on the information you do need—the overall size, shape, and color of the coin. You aren't able to recall what the back of a penny really looks like because that information was never really encoded into memory in the first place.
Cue-Dependent Theory of Forgetting
Other researchers have suggested that sometimes information is actually present in memory, but that it cannot be recalled unless retrieval cues are present. These cues are elements that were present at the time that the actual memory was encoded.
For example, remembering the details of your first date with your spouse might be easier if you smell the same scent that your partner was wearing on that first date. The retrieval cue (the scent) was present when that memory was created, so smelling it again can trigger the retrieval of those memories.
A Word From Verywell
Forgetting is simply a part of life. Numerous theories explain how and why we forget. In many situations, several of these explanations might account for why we cannot remember. The passage of time can make memories more difficult to access, while the abundance of information vying for our attention can create competition between old and new memories. Still, we can work to become better at recalling information .
In addition to experiencing some type of memory retrieval failure, forgetting can also be caused by trauma to the head, the use of alcohol or drugs, diseases such as dementia or multiple sclerosis , stroke, and more.
According to these psychological theories, the four types of forgetting are interference, decay, retrieval failure, and cue dependence.
While a normal part of aging, forgetting can be a symptom of depression, Alzheimer's disease, or some type of infection. If you're concerned about your forgetfulness, your healthcare provider can perform tests to determine its cause.
Psychology professionals sometimes refer to forgetting as amnesia, memory loss, or disremembering.
Aronowitz S. Retrieval is central to the distinctive function of episodic memory . Behav Brain Sci . 2018;41:e2. doi:10.1017/S0140525X17001248
Murre JM, Dros J. Replication and analysis of Ebbinghaus' forgetting curve . PLoS One . 2015;10(7):e0120644. doi:10.1371/journal.pone.0120644
Chubala CM, Neath I, Surprenant AM. A comparison of immediate serial recall and immediate serial recognition . Can J Exp Psychol . 2019;73(1):5–27. doi:10.1037/cep0000158
Darby KP, Sloutsky VM. The cost of learning: Interference effects in memory development . J Exp Psychol. 2015;144(2):410–431. doi:10.1037/xge0000051
Troyer A.K. Serial position effect . In: Kreutzer JS, DeLuca J, Caplan B, eds. Encyclopedia of Clinical Neuropsychology . Springer, 2011. doi:10.1007/978-0-387-79948-3
Shibata K, Sasaki Y, Bang J. et al . Overlearning hyperstabilizes a skill by rapidly making neurochemical processing inhibitory-dominant . Nat Neurosci. 2017 ; 20:470-475. doi:10.1038/nn.4490
Rasch B, Born J. About sleep's role in memory . Physiol Rev . 2013;93(2):681-766. doi:10.1152/physrev.00032.2012
McKeown D, Mercer T, Bugajska K, Duffy P, Barker E. The visual nonverbal memory trace is fragile when actively maintained, but endures passively for tens of seconds . Mem Cognit . 2019. doi:10.3758/s13421-019-01003-6
Ricker TJ, Vergauwe E, Cowan N. Decay theory of immediate memory: From Brown (1958) to today (2014) . Q J Exp Psychol (Hove) . 2016;69(10):1969-1995. doi:10.1080/17470218.2014.914546
Nickerson RS, Adams MJ. Long-term memory for a common object . Cog Psychol. 1979 ;11 (3), 287–307. doi:10.1016/0010-0285(79)90013-6
Jonker TR, Seli P, Macleod CM. Less we forget: retrieval cues and release from retrieval-induced forgetting . Mem Cognit . 2012;40(8):1236–1245. doi:10.3758/s13421-012-0224-2
Penn Medicine. Memory loss .
National Institute on Aging. Memory, forgetfulness, and aging: What's normal and what's not?
Hunt RR, Worthen JB. Distinctiveness and Memory . Oxford University Press; 2006.
Tulving E. Cue-dependent forgetting . American Scientist. 1974;62(1):74-82.
Willingham DT. Cognition: The Thinking Animal (3rd ed.). Pearson/Prentice Hall; 2007.
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Psychol
The Influences of Emotion on Learning and Memory
Emotion has a substantial influence on the cognitive processes in humans, including perception, attention, learning, memory, reasoning, and problem solving. Emotion has a particularly strong influence on attention, especially modulating the selectivity of attention as well as motivating action and behavior. This attentional and executive control is intimately linked to learning processes, as intrinsically limited attentional capacities are better focused on relevant information. Emotion also facilitates encoding and helps retrieval of information efficiently. However, the effects of emotion on learning and memory are not always univalent, as studies have reported that emotion either enhances or impairs learning and long-term memory (LTM) retention, depending on a range of factors. Recent neuroimaging findings have indicated that the amygdala and prefrontal cortex cooperate with the medial temporal lobe in an integrated manner that affords (i) the amygdala modulating memory consolidation; (ii) the prefrontal cortex mediating memory encoding and formation; and (iii) the hippocampus for successful learning and LTM retention. We also review the nested hierarchies of circular emotional control and cognitive regulation (bottom-up and top-down influences) within the brain to achieve optimal integration of emotional and cognitive processing. This review highlights a basic evolutionary approach to emotion to understand the effects of emotion on learning and memory and the functional roles played by various brain regions and their mutual interactions in relation to emotional processing. We also summarize the current state of knowledge on the impact of emotion on memory and map implications for educational settings. In addition to elucidating the memory-enhancing effects of emotion, neuroimaging findings extend our understanding of emotional influences on learning and memory processes; this knowledge may be useful for the design of effective educational curricula to provide a conducive learning environment for both traditional “live” learning in classrooms and “virtual” learning through online-based educational technologies.
Introduction
Emotional experiences are ubiquitous in nature and important and perhaps even critical in academic settings, as emotion modulates virtually every aspect of cognition. Tests, examinations, homework, and deadlines are associated with different emotional states that encompass frustration, anxiety, and boredom. Even subject matter influences emotions that affect one’s ability to learn and remember. The usage of computer-based multimedia educational technologies, such as intelligent tutoring systems (ITSs) and massive open online courses (MOOCs), which are gradually replacing traditional face-to-face learning environments, is increasing. This may induce various emotional experiences in learners. Hence, emotional influences should be carefully considered in educational courses design to maximize learner engagement as well as improve learning and long-term retention of the material ( Shen et al., 2009 ). Numerous studies have reported that human cognitive processes are affected by emotions, including attention ( Vuilleumier, 2005 ), learning and memory ( Phelps, 2004 ; Um et al., 2012 ), reasoning ( Jung et al., 2014 ), and problem-solving ( Isen et al., 1987 ). These factors are critical in educational domains because when students face such difficulties, it defeats the purpose of schooling and can potentially render it meaningless. Most importantly, emotional stimuli appear to consume more attentional resources than non-emotional stimuli ( Schupp et al., 2007 ). Moreover, attentional and motivational components of emotion have been linked to heightened learning and memory ( Pekrun, 1992 ; Seli et al., 2016 ). Hence, emotional experiences/stimuli appear to be remembered vividly and accurately, with great resilience over time.
Recent studies using functional neuroimaging techniques detect and recognize human emotional states and have become a topic of increasing research in cognitive neuroscience, affective neuroscience, and educational psychology to optimize learning and memory outcomes ( Carew and Magsamen, 2010 ; Um et al., 2012 ). Human emotions comprise complex interactions of subjective feelings as well as physiological and behavioral responses that are especially triggered by external stimuli, which are subjectively perceived as “personally significant.” Three different approaches are used to monitor the changes in emotional states: (1) subjective approaches that assess subjective feelings and experiences; (2) behavioral investigations of facial expressions ( Jack and Schyns, 2015 ), vocal expressions ( Russell et al., 2003 ), and gestural changes ( Dael et al., 2012 ); and (3) objective approaches via physiological responses that include electrical and hemodynamic of the central nervous system (CNS) activities ( Vytal and Hamann, 2010 ) in addition to autonomic nervous system (ANS) responses such as heart rate, respiratory volume/rate, skin temperature, skin conductance and blood volume pulses ( Li and Chen, 2006 ). The CNS and ANS physiological responses (brain vs. body organs) can be objectively measured via neuroimaging and biosensors and are more difficult to consciously conceal or manipulate compared to subjective and behavioral responses. Although functional neuroimaging enables us to identify brain regions of interest for cognitive and emotional processing, it is difficult to comprehend emotional influences on learning and memory retrieval without a fundamental understanding of the brain’s inherent emotional operating systems.
The aim of this current article was to highlight an evolutionary approach to emotion, which may facilitate understanding of the effects of emotion on learning and memory. We initially present the terminology used in affective neuroscience studies, describe the roles of emotion and motivation in learning and memory, and outline the evolutionary framework and the seven primary emotional system. This is followed by the emotional-cognitive interactions in the various brain regions that are intimately involved in emotion and memory systems. This is performed to define the congruent interactions in these regions are associated with long-term memory (LTM) retention. We then discuss the emerging studies that further our understanding of emotional effects deriving from different modalities of emotional content. This is followed by a discussion of four major functional neuroimaging techniques, including functional magnetic resonance imaging (fMRI), positron emission tomography (PET), electroencephalography (EEG), and functional near-infrared spectroscopy (fNIRS). We then present the important factors for consideration in experimental design, followed by a description of psychiatric disorders, such as depression and anxiety, which are emotionally charged dysfunctions that are strongly detrimental to cognitive performance. Our review ends with concluding remarks on the current issues and future research possibilities with respect to the efficient enhancement of educational practices and technologies.
Emotions, Moods, Feelings, Affects and Drives
Subjective terms used in affective neuroscience include emotions, moods, feelings, affects and drives. Although emotion has long been studied, it bears no single definition. A review of 92 putative definitions and nine skeptical statements ( Kleinginna and Kleinginna, 1981 ) suggests a definition with a rather broad consensus:
- simple Emotions describe a complex set of interactions between subjective and objective variables that are mediated by neural and hormonal systems, which can (a) give rise to affective experiences of emotional valence (pleasure-displeasure) and emotional arousal (high-low activation/calming-arousing); (b) generate cognitive processes such as emotionally relevant perceptual affect, appraisals, labeling processes; (c) activate widespread psychological and physiological changes to the arousing conditions; and (d) motivate behavior that is often but not always expressive, goal-directed and adaptive.
Although this definition may be adequate for everyday purposes, it does not encompass some important aspects of emotional systems such as how emotions operate to create subjectively experienced feelings and how they control personality dimensions. Accordingly, Panksepp (1998) suggested the following:
- simple Emotions are the psychoneural processes that are influential in controlling the vigor and patterning of actions in the dynamic flow of intense behavioral interchanges between animals as well as with certain objects that are important for survival. Hence, each emotion has a characteristic “feeling tone” that is especially important in encoding the intrinsic values of these interactions, depending on their likelihood of either promoting or hindering survival (both in the immediate “personal” and long-term “reproductive” sense). Subjective experiential-feelings arise from the interactions of various emotional systems with the fundamental brain substrates of “the self,” that is important in encoding new information as well as retrieving information on subsequent events and allowing individuals efficiently to generalize new events and make decisions.
He went further to propose seven primary emotional systems/prototype emotional states, namely SEEKING, RAGE, FEAR, LUST, CARE, PANIC/GRIEF, and PLAY that represent basic foundations for living and learning.
Moods last longer than emotions, which are also characterized by positive and negative moods. In contrast, feelings refer to mental experiences that are necessarily valence, either good or bad as well as accompanied by internal physiological changes in the body, specifically the viscera, including the heart, lungs, and gut, for maintaining or restoring homeostatic balances. Feelings are not commonly caused emotions. Because the generation of emotional feelings requires a neural re-mapping of different features of the body state in the CNS, resulting from cognitive “appraisal” where the anterior insular cortex plays a key integrative role ( Craig and Craig, 2009 ; Damasio and Carvalho, 2013 ). Nonetheless, Panksepp (2005) has defended the view that emotional operating systems (caudal and medial subcortical brain regions) appeared to generate emotional experiences via localized electrical stimulation of the brain stimulation (ESB) rather dependent on changes of the external environment or bodily states. Affects are subjective experienced emotional feelings that are difficult to describe, but have been linked to bodily states such as homeostatic drives (hunger and thirst) and external stimuli (visual, auditory, taste, touch, smell) ( Panksepp, 2005 ). The latter are sometimes called “core affect,” which refers to consciously accessible elemental processes involving pleasure and arousal that span bipolar dimensions ( Russell and Barrett, 1999 ). In addition, a “drive” is an inherent action program that is responsible for the satisfaction of basic and instinctual (biologically pre-set) physiological needs, e.g., hunger, thirst, libido, exploration, play, and attachment to mates ( Panksepp, 1998 ); this is sometimes called “homeostatic drive.” In brief, a crucial characteristic shared by emotion, mood, feeling, affect and drive is their intrinsic valence, which lies on the spectrum of positive and negative valence (pleasure-displeasure/goodness-badness). The term emotion exemplifies the “umbrella” concept that includes affective, cognitive, behavioral, expressive and physiological changes; emotion is triggered by external stimuli and associated with the combination of feeling and motivation.
Recent Evidence Regarding the Role of Emotion in Learning and Memory
The impact of emotion on learning processes is the focus of many current studies. Although it is well established that emotions influence memory retention and recall, in terms of learning, the question of emotional impacts remains questionable. Some studies report that positive emotions facilitate learning and contribute to academic achievement, being mediated by the levels of self-motivation and satisfaction with learning materials ( Um et al., 2012 ). Conversely, a recent study reported that negative learning-centered state (confusion) improve learning because of an increased focus of attention on learning material that leads to higher performances on post tests and transfer tests ( D’Mello et al., 2014 ). Confusion is not an emotion but a cognitive disequilibrium state induced by contradictory data. A confused student might be frustrated with their poor understanding of subject matter, and this is related to both the SEEKING and RAGE systems, with a low-level of activation of rage or irritation, and amplification of SEEKING. Hence, motivated students who respond to their confusion seek new understanding by doing additional cognitive work. Further clarification of this enhances learning. Moreover, stress, a negative emotional state, has also been reported to facilitate and/or impair both learning and memory, depending on intensity and duration ( Vogel and Schwabe, 2016 ). More specifically, mild and acute stress facilitates learning and cognitive performance, while excess and chronic stress impairs learning and is detrimental to memory performance. Many other negative consequences attend owing to overactivity of the hypothalamic-pituitary-adrenal (HPA) axis, which results in both impaired synaptic plasticity and learning ability ( Joëls et al., 2004 ). Nonetheless, confounding influences of emotions on learning and memory can be explained in terms of attentional and motivational components. Attentional components enhance perceptual processing, which then helps to select and organize salient information via a “bottom-up” approach to higher brain functions and awareness ( Vuilleumier, 2005 ). Motivational components induce curiosity, which is a state associated with psychological interest in novel and/or surprising activities (stimuli). A curiosity state encourages further exploration and apparently prepares the brain to learn and remember in both children and adults ( Oudeyer et al., 2016 ). The term “surprising” might be conceptualized as an incongruous situation (expectancy violation) refers to a discrepancy between prior expectations and the new information; it may drive a cognitive reset for “learned content” that draws one’s attention.
Similarly, emotionally enhanced memory functions have been reported in relation to selective attention elicited by emotionally salient stimuli ( Vuilleumier, 2005 ; Schupp et al., 2007 ). During the initial perceptual stage, attention is biased toward emotionally salient information that supports detection by the salient input. Thus, stimulating selective attention increases the likelihood for emotional information to become encoded in LTM storage associated with a top-down control in sensory pathways that are modulated by the frontal and parietal cortices. This is an example of an indirect influence on perception and attention that regulates selective sensory processing and behavioral determination ( Vuilleumier, 2005 ). Because the human sensory systems have no capacity to simultaneously process everything at once, which necessitates attentional mechanisms. Top-down attentional processing obtains adequate attentional resource allocation to process emotional valence information for encoding and retrieval via cooperation with the brain regions such as the ventromedial prefrontal cortex and superior temporal sulcus, along with the primary visual cortex (helps to realize both emotion and conceptualization). Similarly, experimental studies have examined the phenomenon by using various attentional tasks, including filtering (dichotic listening and Stroop task), search (visual search), cuing (attentional probe, spatial cuing) and attentional blink [rapid serial visual presentation (RSVP)] paradigms ( Yiend, 2010 ). These investigations demonstrated biased attentional processing toward emotionally stimulating material content attended by increased sensory responses. One study reported that emotional stimuli induce a “pop-out” effect that leads to the attentional capture and privileged processing ( Öhman et al., 2001 ). Moreover, a study using the RSVP paradigm compared healthy subjects with a group of patients with bilateral amygdala damage. The results revealed that healthy subjects exhibited increased perception and attention toward emotional words compared to patients, indicating that the amygdala plays a crucial role in emotional processing ( Anderson and Phelps, 2001 ). In addition, functional neuroimaging showed that the insular cortex, the secondary somatosensory cortex, the cingulate cortex and nuclei in the tegmentum and hypothalamus are the brain regions that regulate attentional focus by integrating external and internal inputs to create emotional feeling states, thus modulating a motivational state that obtains homeostasis ( Damasio et al., 2000 ). All emotional systems associated with strong motivational components such as psychological salient bodily need states operate through the SEEKING system that motivates appetitive/exploratory behavior to acquire resources needed for survival ( Montag and Panksepp, 2017 ).
The distinction between emotion and homeostasis, is the process of regulation for continuously changing internal states via appropriate corrective responses that respond to both internal and external environmental conditions to maintain an optimal physiological state in the body. Homeostatic affects , such as hunger and thirst, are not considered prototype emotional states. Because homeostatic affects have never been mapped using ESB that arouse basic emotional responses ( Panksepp, 2005 , 2007 ). However, emotional prototypes can be thought of as evolutionary extensions/predictions of impending homeostatic threats; for example, SEEKING might be an evolutionary extension of intense hunger and thirst (the major sources of suffering that signal energy depletion to search for food and water intake) ( Watt, 2012 ). Homeostatic imbalances engage the mesolimbic motivational system via hypothalamic interactions with the extended trajectory of the SEEKING system [centrally including the lateral hypothalamus, ventral basal ganglia, and ventral tegmental area (VTA)]. It is the distributed functional network that serves the general function of finding resources for survival that gets hungry animals to food, thirsty animals to water, cold animals to warmer environments, etc. ( Panksepp, 1998 ). To summarize, both emotion and motivation are crucial for the maintenance of psychological and physiological homeostasis, while emotional roles are particularly important in the process of encoding new information containing emotional components. The latter increases attention toward salient new information by selectively enhancing detection, evaluation, and extraction of data for memorization. In addition, motivational components promote learning and enhance subsequent memory retrieval while generalizing new events consequent to adaptive physiological changes.
The Evolutionary Framework of Emotion and The Seven Primary Emotional Systems
Evolution built our higher minds (the faculty of consciousness and thoughts) on a foundation of primary-process of emotional mechanism that preprogrammed executive action systems (the prototype emotions) rely on cognitive processing (interpretation) and appraisal in the organisms attempt to decipher the type of situation they might be in; in other words, how to deal with emotionally challenging situations, whether it is a play situation or a threat situation (where RAGE and FEAR might be the appropriate system to recruit). Emotion offers preprogrammed but partially modifiable (under the secondary process of learning and memory) behavioral routines in the service of the solution of prototypical adaptive challenges, particularly in dealing with friend vs. foe; these routines are evolutionary extensions of homeostasis and embed a prediction beyond the current situation to a potentially future homeostatic benefit or threat. Thus, evolution uses whatever sources for survival and procreative success. According to Panksepp and Solms (2012) , key CNS emotional-affective processes are (1) Primary-process emotions; (2) Secondary-process learning and memory; and (3) Tertiary-process higher cognitive functions. Fundamentally, primary emotional processes regulate unconditioned emotional actions that anticipate survival needs and consequently guide secondary process via associative learning mechanisms (classical/Pavlovian and instrumental/operant conditioning). Subsequently, learning process sends relevant information to higher brain regions such as the prefrontal cortex to perform tertiary cognition process that allows planning for future based on past experiences, stored in LTM. In other words, the brain’s neurodevelopment trajectory and “wiring up” activations show that there is a genetically coded aversion to situations that generate RAGE, FEAR and other negative states for minimizing painful things and maximizing pleasurable kinds of stimulation. These are not learned- all learning (secondary-process) is piggybacked on top of the “primary-process emotions” that are governed by “Law of Affect” (see Figure Figure1 1 ). What now follows is an explanation of these CNS emotional-affective processing sub-levels and their inter-relationships.

Shows the nested hierarchies of circular emotional control and cognitive regulation for “bottom-up” influences and “top-down” regulations. The schematic shows conceptual relationships between primary processes of emotional system (lower brain function), as well as secondary processes of cognitive system and tertiary processing (higher brain function). Primary emotional processing for homeostatic, sensory and emotional affects facilitate secondary learning and memory processing via the “SEEKING” system that promotes survival and reproductive success (bottom-up instinctual influences). As secondary processes are continually integrated with primary emotional processing, they mature to higher brain cognitive faculties to generate effective solutions for living and subsequently exert top-down regulatory control over behavior. The primary emotional processing is mediated by complex unconditioned emotional responses (evolutionary “memories”) through “Law of Affect”; sometimes called “reinforcement principle” that explains how the brain emotional networks control learning. This bi-circular causation for higher brain functionality is coordinated by lower brain functions [adapted from ( Panksepp and Solms, 2012 )].
Primary-Process Emotions (Prototype Emotional States)
The emotional operating system is an inherited and genetically encoded circuitry that anticipates key survival and homeostatic needs. Thus, animals and humans share primary emotional network at the subcortical level, which includes the midbrain’s periaqueductal grey (PAG) and VTA, basal ganglia (amygdala and nucleus accumbens), and insula, as well as diencephalon (the cingulate and medial frontal cortices through the lateral and medial hypothalamus and medial thalamus). Subcortical brain regions are involved in three sub-components of affects: (1) core emotional feelings (fear, anger, joy and various forms of distress); (2) homeostatic drives/motivational experiences (hunger and thirst); and (3) sensory affects (pain, taste, temperature and disgust). Primary-process emotions are not unconscious. Strong emotion is intrinsically conscious at least in the sense that it is experienced even if we might mislabel it, or animal clearly is not able to attach a semantic label-these are simply not realistic standards for determining whether something is conscious or not conscious. Nonetheless, the emotional experiences guide behavior to promote survival and procreative success as well as mediate learning (‘ rewarding ’ and ‘ punishing ’ learning effects) and thinking at secondary and tertiary levels.
Secondary-Process Emotions (Learning and Memory)
Primary emotional systems guide associative learning and memory (classical/operant conditioning and emotional habit) processes via the mediation of emotional networks. This includes the basal ganglia (basolateral and central amygdala, nucleus accumbens, thalamus and dorsal striatum), and the medial temporal lobe (MTL) including hippocampus as well as the entorhinal cortex, perirhinal cortex, and parahippocampal cortices that responsible for declarative memories. Thus, secondary processes of learning and memory scrutinize and regulate emotional feelings in relation to environmental events that subsequently refine effective solutions to living.
Tertiary-Process Emotions (Higher Cognitive Functions)
Higher cognitive functions operate within the cortical regions, including the frontal cortex for awareness and consciousness functions such as thinking, planning, emotional regulation and free-will (intention-to-act), which mediate emotional feelings. Hence, cognition is an extension of emotion (just as emotion is an extension of homeostasis aforementioned). Tertiary processes are continually integrated with the secondary processes and reach a mature level (higher brain functions) to better anticipating key survival issues, thus yielding cognitive control of emotion via “top-down” regulation. In other words, brain-mind evolution enables human to reason but also regulate our emotions.
Psychologist Neisser (1963) suggested that cognition serves emotion and homeostatic needs where environmental information is evaluated in terms of its ability to satisfy or frustrate needs. In other words, cognition is in the service of satisfying emotional and homeostatic needs. This infers that cognition modulates, activates and inhibits emotion. Hence, emotion is not a simple linear event but rather a feedback process that autonomously restores an individual’s state of equilibrium. More specifically stated, emotion regulates the allocation of processing resources and determines our behavior by tuning us to the world in certain biased ways, thus steering us toward things that “feel good” while avoiding things that “feel bad.” This indicates that emotion guides and motivates cognition that promotes survival by guiding behavior and desires according to unique goal orientation ( Northoff et al., 2006 ). Therefore, the CNS maintains complex processes by continually monitoring internal and external environments. For example, changes in internal environments (contraction of visceral muscles, heart rate, etc.) are sensed by an interoceptive system (afferent peripheral nerves) that signals the sensory cortex (primary, secondary and somatosensory) for integration and processing. Thus, from an evolutionary perspective, human mental activity is driven by the ancient emotional and motivational brain systems shared by cross-mammalians that encode life-sustaining and life-detracting features to promote adaptive instinctual responses. Moreover, emotional and homeostasis mechanisms are characterized by intrinsic valence processing that is either a positive/pleasure or negative/displeasure bias. Homeostasis imbalance is universally experienced as negative emotional feelings and only becomes positively valenced when rectified. Hence, individuals sustain bodily changes that underlie psychological (emotional) and biological (homeostatic) influences on two sides, i.e., one side is oriented toward the survival and reproductive success that is associated with positively valenced emotional and physiologic homeostasis (anticipatory response) and the other responds to survival and reproductive failure associated with negatively valenced emotional and physiologic homeostasis (reactive response). Consequently, cognition modulates both emotional and homeostatic states by enhancing survival and maximizing rewards while minimizing risk and punishments. Thus, this evolutionary consideration suggests the brain as a ‘predictive engine’ to make it adaptive in a particular environment. Figure Figure2 2 demonstrates this cyclic homeostatic regulation.

Conceptually maps the homeostatic regulation of internal and external inputs that affect cognition, emotion, feeling, and drive: Inputs → Homeostasis ↔ Emotion ∗ ↔ Cognition. This lead to the experience of one’s self via overt behavior that is biased by a specific emotion stimulated by bodily changes that underlie psychological/physiological states. ∗ Represents emotion associated with a combination of feeling and motivation/drive; ↔ indicates a bi-directional interaction; and → indicates a one-directional relationship. Adapted from Damasio and Carvalho (2013) .
Panksepp (1998) identified seven primary emotional systems that govern mammalian brains as follows: SEEKING, RAGE, FEAR, LUST, CARE, PANIC/GRIEF, and PLAY. Here, we use UPPERCASE letters to denote unconditional emotional responses (emotional primes). These primary emotional neural networks are situated in the subcortical regions; moreover, the evidence demonstrates that decortication leaves primary emotional systems intact ( Panksepp et al., 1994 ). Hence, cortical regions are non-essential for the generation of prototype emotional states but are responsible for their modulation and regulation. The present article emphasizes SEEKING because it is the most fundamental of the primary emotional systems and is crucial for learning and memory. The SEEKING system facilitates learning because when fully aroused, it fills the mind with interest that then motivates the individual to search out and learn things that they need, crave and desire. Accordingly, SEEKING generates and sustains curiosity’s engagement for a particular purpose while also promoting learning via its mediation of anticipatory eagerness ( Oudeyer et al., 2016 ). In other words, the SEEKING system has been designed to automatically learn by exploring anything that results in acquired behavioral manifestations for survival operations, all the way from the mesolimbic-mesocortical dopamine system through to the prefrontal cortex (PFC); thus, it is intimately linked with LTM formation ( Blumenfeld and Ranganath, 2007 ). Consequently, it is the foundation of secondary learning and higher cognitive processes when compared with the remaining six emotional systems. However, this system is less activated during chronic stress, sickness, and depression, all of which are likely to impair learning and various higher cognitions. On the other hand, overactivity of this system promotes excessively impulsive behaviors attended by manic thoughts and psychotic delusions. Moreover, massive lesion of SEEKING’s neural network (midline subcortical regions-the PAG, VTA, nucleus accumbens (NAc), medial forebrain and anterior cingulate) lead to consciousness disorder, specifically akinetic mutism (AKM) syndrome that the patient appears wakeful, attentive but motionless ( Schiff and Plum, 2000 ; Watt and Pincus, 2004 ). In brief, the SEEKING system holds a critical position that optimizes the performance of emotion, motivation, and cognition processes by generating positive subjective emotional states-positive expectancy, enthusiastic exploration, and hopefulness. Because the seven primary emotional systems and their associated key neuroanatomical and key neurochemical features have been reviewed elsewhere ( Panksepp, 2011a , b ), they are not covered in this review.
Emotion–Cognition Interactions and its Impacts on Learning and Memory
Studies in psychology ( Metcalfe and Mischel, 1999 ) and neuroscience ( Dolcos et al., 2011 ) proposed that cognition and emotion processes are operated at two separate but interacting systems: (i) the “cool cognitive system” is hippocampus-based that is associated with emotionally neutral cognitive functions as well as cognitive controls; and (ii) the “hot emotional system” is amygdala-based that responsible for emotional processing and responses toward unconditioned emotional stimuli such as appetitive and fear-evoking conditions. In addition, an early view of a dorsal/ventral stream distinction was commonly reported between both systems. The dorsal stream encompasses the dorsolateral prefrontal cortex (DLPFC) and lateral parietal cortex, which are involved in the cool system for active maintenance of controlled processes such as cognitive performance and the pursuit of goal-relevant information in working memory (WM) amidst interference. In contrast, the hot system involves the ventral neural system, including the amygdala, ventrolateral prefrontal cortex (VLPFC) and medial prefrontal cortex (mPFC) as well as orbitofrontal (OFC) and occipito-temporal cortex (OTC), all of which encompass emotional processing systems ( Dolcos et al., 2011 ). Nonetheless, recent investigations claim that distinct cognitive and emotional neural systems are not separated but are deeply integrated and contain evidence of mediation and modulation ( Dolcos et al., 2011 ; Okon-Singer et al., 2015 ). Consequently, emotions are now thought to influence the formation of a hippocampal-dependent memory system ( Pessoa, 2008 ), exerting a long-term impact on learning and memory. In other words, although cognitive and affective processes can be independently conceptualized, it is not surprising that emotions powerfully modify cognitive appraisals and memory processes and vice versa. The innate emotional systems interact with higher brain systems and probably no an emotional state that is free of cognitive ramifications. If cortical functions were evolutionarily built upon the pre-existing subcortical foundations, it provides behavioral flexibility ( Panksepp, 1998 ).
The hippocampus is located in the MTL and is thought to be responsible for the potentiation and consolidation of declarative memory before newly formed memories are distributed and stored in cortical regions ( Squire, 1992 ). Moreover, evidence indicates that the hippocampus functions as a hub for brain network communications-a type of continuous exchange of information center that establishes LTM dominated by theta wave oscillations ( Battaglia et al., 2011 ) that are correlated with learning and memory ( Rutishauser et al., 2010 ). In other words, hippocampus plays a crucial role in hippocampal-dependent learning and declarative memories. Numerous studies have reported that the amygdala and hippocampus are synergistically activated during memory encoding to form a LTM of emotional information, that is associated with better retention ( McGaugh et al., 1996 ; Richter-Levin and Akirav, 2000 ; Richardson et al., 2004 ). More importantly, these studies (fear-related learning) strongly suggest that the amygdala’s involvement in emotional processing strengthens the memory network by modulating memory consolidation; thus, emotional content is remembered better than neutral content.
In addition to amygdala-hippocampus interactions, one study reported that the PFC participates in emotional valence (pleasant vs. unpleasant) processing during WM ( Perlstein et al., 2002 ). Simons and Spiers (2003) also reviewed studies of interactions between the PFC and MTL during the memory encoding and retrieval processes underlying successful LTM. They demonstrated that the PFC is crucial for LTM because it engages with the active maintenance of information linked to the cognitive control of selection, engagement, monitoring, and inhibition. Hence, it detects relevant data that appears worthwhile, which is then referred for encoding, thus leading to successful LTM ( Simons and Spiers, 2003 ). Consistent findings were reported for recognition tasks investigated by fMRI where the left PFC-hippocampal network appeared to support successful memory encoding for neutral and negative non-arousing words. Simultaneously, amygdala-hippocampus activation was observed during the memory encoding of negative arousing words ( Kensinger and Corkin, 2004 ). Moreover, Mega et al. (1996) proposed two divisions for the limbic system: (i) the paleocortex division (the amygdala, orbitofrontal cortex, temporal polar and anterior insula), and (ii) the archicortical division (the hippocampus and anterior cingulate cortex). The first component is responsible for the implicit integration of affects, drives and object associations; the second deals with explicit sensory processing, encoding, and attentional control. Although divided into two sub-divisions, the paleocortex and archicortical cortex remain integrated during learning. Here, the paleocortex appears to manage the internal environment for implicit learning while integrating affects, drives, and emotions. Simultaneously, the archicortical division appears to manage external environment input for explicit learning by facilitating attention selection with attendant implicit encoding. To some extent, the paleocortex system might come to exercise a supervisory role and link the ancient affective systems to the newer cognitive systems.
Amygdala–Hippocampus Interactions
The findings of previous studies suggest that the amygdala is involved in emotional arousal processing and modulation of the memory processes (encoding and storage) that contribute to the emotional enhancement of memory ( McGaugh et al., 1996 ; Richter-Levin and Akirav, 2000 ). Activation of the amygdala during the encoding of emotionally arousing information (both pleasant/unpleasant) has been reported that correlates with subsequent recall. Because of the interaction between basolateral complex of the amygdala (BLA) with other brain regions that are involved in consolidating memories, including the hippocampus, caudate nucleus, NAc, and other cortical regions. Thus, BLA activation results from emotionally arousing events, which appear to modulate memory storage-related regions that influence long-term memories ( McGaugh, 2004 ). Memory consolidation is a part of the encoding and retention processes where labile memories of newly learned information become stabilized and are strengthened to form long-lasting memories ( McGaugh, 2000 ). Moreover, the amygdala transmits direct feedback/projection along the entire rostral-caudal cortices to the visual cortex of the ventral stream system, including primary visual (V1) and temporal cortices ( Amaral et al., 2003 ); furthermore, the amygdala activates the frontal and parietal regions during negative emotion processing that are involved in attention control. Consequently, during emotional processing, direct projections from the amygdala to sensory cortices enhance attentional mechanism might also allow the parallel processing of the attentional (fronto-parietal) system ( Vuilleumier, 2005 ). This suggests that amygdala activation is associated with enhanced attention and is a part of how salience enhances information retention.
In addition to attentional biases toward emotional content during memory encoding, emotionally arousing experiences have been found to induce the release of adrenal stress hormones, followed by the activation of β-noradrenergic receptors in the BLA, which then release epinephrine and glucocorticoids in the BLA, while enhancing memory consolidation of emotional experiences ( McGaugh and Roozendaal, 2002 ). Thus, there is evidence that the consolidation of new memory that is stimulated by emotionally arousing experiences can be enhanced through the modulating effects of the release of stress hormones and stress-activated neurotransmitters associated with amygdala activation. The BLA comprises the basal amygdala (BA) and lateral amygdala (LA), which project to numerous brain regions involved in learning and memory, including the hippocampus and PFC ( Cahill and McGaugh, 1998 ; Sharot and Phelps, 2004 ; McGaugh, 2006 ). However, stress and emotion do not always induce strong memories of new information. Indeed, they have also been reported to inhibit WM and LTM under certain conditions related to mood and chronic stress ( Schwabe and Wolf, 2010 ). Consequently, understanding, managing, and regulating emotion is critical to the development of enhanced learning programs informed by the significant impacts of learning and memory under different types of stress ( Vogel and Schwabe, 2016 ).
Prefrontal Cortex–Hippocampus Interaction
The PFC is located in the foremost anterior region of the frontal lobe and is associated with higher-order cognitive functions such as prediction and planning of/for the future ( Barbey et al., 2009 ). Moreover, it is thought to act as a control center for selective attention ( Squire et al., 2013 ), and also plays a critical role in WM as well as semantic processing, cognitive control, problem-solving, reasoning and emotional processing ( Miller and Cohen, 2001 ; Yamasaki et al., 2002 ). The PFC is connected to sub-cortical regions in the limbic system, including the amygdala and various parts of the MTL ( Simons and Spiers, 2003 ). Its involvement in WM and emotional processing are intimately connected with the MTL structures that decisively affect LTM encoding and retrieval ( Blumenfeld and Ranganath, 2007 ) in addition to self-referential processing ( Northoff et al., 2006 ). Structurally, the PFC is divided into five sub-regions: anterior (BA 10), dorsolateral (BA 9 and 46), ventrolateral (BA 44, 45, and 47), medial (BA 25 and 32) and orbitofrontal (BA 11, 12, and 14) ( Simons and Spiers, 2003 ).
The mPFC has been associated with anticipatory responses that reflect cognitive expectations for pleasant/unpleasant experiences (appraising rewarding/aversive stimuli to generate emotional responses) ( Ochsner et al., 2002 ; Ochsner and Gross, 2005 ). Specifically, increased mPFC activation has been noted during reappraisal and is associated with the suppressed subjective experience of negative emotions. Furthermore, an fMRI study revealed concurrent activation levels of the dorsomedial prefrontal cortex (dmPFC) with emotional valence when processing emotional stimuli: (i) activation was associated with positive valence, and (ii) deactivation was associated with negative valence ( Heinzel et al., 2005 ). Similarly, emotional and non-emotional judgment task using the International Affective Pictures System (IAPS) demonstrated increased activation of the mPFC, specifically both ventromedial prefrontal cortex (vmPFC) and dmPFC during emotional judgment when compared with non-emotional judgment. However, an inverse relationship was observed in the lateral prefrontal cortex (VLPFC and DLPFC) during non-emotional judgment ( Northoff et al., 2004 ). These findings suggested reciprocal interactions between cognitive and emotional processing between dorsal and lateral neural systems when processing emotional and cognitive tasking demands ( Bartolic et al., 1999 ).
Other studies reported strong cognition-emotion interactions in the lateral prefrontal cortex with increased activity in the DLPFC, which plays a key role in top-down modulation of emotional processing ( Northoff et al., 2004 ; Comte et al., 2014 ). This indicates increased attentional control of regulatory mechanisms that process emotional content. For instance, one study reported that cognitive task appeared to require active retention in WM, noting that the process was influenced by emotional stimuli when subjects were instructed to remember emotional valence information over a delay period ( Perlstein et al., 2002 ). Their findings revealed increased activation in the right DLPFC in response to pleasant IAPS pictures, but with an opposite effect in response to unpleasant pictures (decreased activity in the right DLPFC). This could be interpreted as increased WM-related activity when processing positive emotional stimuli, thus leading to positive emotion maintenance of stimulus representation in WM. Furthermore, they observed that the DLPFC contributed to increased LTM performance linked to stronger item associations and greater organization of information in WM during pleasant compared to unpleasant emotion ( Blumenfeld and Ranganath, 2006 ).
Another study investigated the PFC’s role in emotional mediation, reporting that the right VLPFC provided cognitive resources for both emotional reappraisal and learning processes via two separate subcortical pathways: (i) a path through NAc appeared to greater reappraisal success (suppress negative emotion) and (ii) another path through the ventral amygdala appeared to reduced reappraisal success (boost negative experience). This result indicates the VLPFC’s role in the regulation of emotional responses (reducing negative appraisal and generating positive appraisal) by retrieving appropriate information from memory ( Wager et al., 2008 ). Certain characteristics of emotional content were found to mediate the encoding and retrieval of selective information by leading high levels of attention, distinctiveness, and information organization that enhanced recall for emotional aspects of complex events ( Talmi, 2013 ). Hence, this direction of additional attention to emotional information appears to enhance LTM with the pronounced effects deriving from positive emotions compared with negative emotions. Effects of emotion on memory was also investigated using immediate (after 20 s) and delayed (after 50 min) testing paradigm, has shown that better recall for emotionally negative stimuli during immediate test compared to delayed test because of attentional allocation for encoding while the delayed test demonstrated that the role of amygdala in modulating memory consolidation of emotional stimuli. Because selective attention drives priority assignment for emotional material ( Talmi et al., 2007 ). Meanwhile, the distinctiveness and organization of information can improve memory because unique attributes and inter-item elaboration during encoding serve as retrieval cues, which then lead to high possibilities for correct recall ( Erk et al., 2003 ). Consistent findings were also reported by ( Dolcos et al., 2004 ), who suggested an emotional mediation effect deriving from PFC activity in relation to cognitive functions such as strategic memory, semantic memory, and WM, which subsequently enhanced memory formation. Table Table1 1 summarizes cognitive-emotional functions associated with each sub-region of the PFC and corresponding Brodmann areas. Taken together, these findings indicate that the PFC is a key component in both cognitive and emotional processing for successful LTM formation and retrieval.
The prefrontal cortex (PFC) sub-regions, corresponding Brodmann areas, and associated cognitive-emotional functions.
| PFC region | BA | Functions | |
|---|---|---|---|
| Cognitive | Emotional | ||
| aPFC | 10 | Engaged in higher-level cognitive functions (i.e., problem solving, planning and reasoning) and executive processes including WM ( ). | Controls social-emotional interaction to coordinate rapid action selection processes, detection of emotional conflicts and inhibition of emotionally driven responses. Disruption leads to loss of control over automatic emotional tendencies and more errors in rule-driven responses ( ). |
| The pursuit of higher behavioral goals, with specialized roles in the explicit processing of internal mental states in WM, relational integration, and memory retrieval ( ). | |||
| DLPFC | 9, 46 | Left DLPFC manipulates information in WM while right DLPFC manipulates information in reasoning processes ( ; ). | Active maintenance of valence information in WM with increased WM-related activity in response to positive emotion (specifically in the right DLPFC) which leads to PFC-mediated cognitive functions in WM (i.e., increased cognitive flexibility and problem solving) ( ). |
| Left DLPFC is associated with encoding and organization of material to be remembered; Right DLPFC is associated with memory retrieval ( ). | Reward processing ( ). Emotion regulation ( ). | ||
| VLPFC | 44, 45, 47 | Left VLPFC supports mnemonic control (i.e., task switching, WM and semantic retrieval), and supports access to stored conceptual representations ( ). | Emotion regulation ( ). |
| Left VLPFC is involved in elaborative (semantic/phonological) encoding of information into episodic memory, the specification of retrieval cues and the maintenance of LTM retrieval ( ; ). Right VLPFC supports memory encoding and retrieval of visuospatial stimuli, action imitation and motor inhibition ( ). | Inhibition of distracting emotions (right VLPFC for inhibition of negative emotions) ( ). | ||
| mPFC | 25, 32 | Learning, memory, and decision-making ( ; ). | Dorsal-caudal mPFC involved in appraisal-expression of negative emotion; ventral-rostral PFC generates emotional regulation-responses ( ). |
| OFC | 11, 12, 14 | Decision making ( ). | Emotional processing and responses ( ), social and emotional judgment ( ), facilitation of regret ( ). |
| Reward processing and reinforcement learning ( ). | |||
Effects Deriving From Different Modalities of Emotional Stimuli on Learning and Memory
As discussed above, evidence indicates the neural mechanisms underlying the emotional processing of valence and arousal involve the amygdala and PFC, where the amygdala responds to emotionally arousing stimuli and the PFC responds to the emotional valence of non-arousing stimuli. We have thus far primarily discussed studies examining neural mechanisms underlying the processing of emotional images. However, recent neuroimaging studies have investigated a wider range of visual emotional stimuli. These include words ( Sharot et al., 2004 ), pictures ( Dolcos et al., 2005 ; Weymar et al., 2011 ), film clips ( Cahill et al., 1996 ), and faces ( González-Roldan et al., 2011 ), to investigate neural correlates of emotional processing and the impact of emotion on subsequent memory. These studies provided useful supplemental information for future research on emotional effects of educational multimedia content (combination of words and pictures), an increasingly widespread channel for teaching and learning.
An event-related fMRI study examined the neural correlates of responses to emotional pictures and words in which both were manipulated in terms of positive and negative valence, and where neutral emotional content served as a baseline (“conditioned stimuli”/no activating emotion with valence rating of 5 that spans between 1/negative valence-9/positive valence), even though all stimuli were consistent in terms of arousal levels ( Kensinger and Schacter, 2006 ). Subjects were instructed to rate each stimulus as animate or inanimate and common or uncommon . The results revealed the activation of the amygdala in response to positive and negative valence (valence-independent) for pictures and words. A lateralization effect was observed in the amygdala when processing different emotional stimuli types. The left amygdala responded to words while either the right and/or bilateral amygdala activation regions responded to pictures. In addition, participants were more sensitive to emotional pictures than to emotional words. The mPFC responded more rigorously during the processing of positive than to that of negative stimuli, while the VLPFC responded more to negative stimuli. The researchers concluded that arousal-related responses occur in the amygdala, dmPFC, vmPFC, anterior temporal lobe and temporo-occipital junction, whereas valence-dependent responses were associated with the lateral PFC for negative stimuli and the mPFC for positive stimuli. The lateralization of the amygdala’s activation was consistent with that in other studies that also showed left-lateralized amygdala responses for words ( Hamann and Mao, 2002 ) vs. right-lateralized amygdala responses for images ( Pegna et al., 2005 ). However, a wide range of studies suggest that lateralization likely differs with sex ( Hamann, 2005 ), individual personality ( Hamann and Canli, 2004 ), mood ( Rusting, 1998 ), age ( Allard and Kensinger, 2014 ), sleep ( Walker, 2009 ), subject’s awareness of stimuli ( Morris et al., 1998 ), stress ( Payne et al., 2007 ) and other variables. Hence, these factors should be considered in future studies.
Event-related potentials (ERPs) were used to investigate the modality effects deriving from emotional words and facial expressions as stimuli in healthy, native German speakers ( Schacht and Sommer, 2009a ). German verbs or pseudo-words associated with positive, negative or neutral emotions were used, in addition to happy vs. angry faces, as well as neutral and slightly distorted faces. The results revealed that negative posterior ERPs were evoked in the temporo-parieto-occipital regions, while enhanced positive ERPs were evoked in the fronto-central regions (positive verbs and happy faces) when compared with neutral and negative stimuli. These findings were in agreement with the previous findings ( Schupp et al., 2003 ; Schacht and Sommer, 2009b ). While the same neuronal mechanisms appear to be involved in response to both emotional stimuli types, latency differences were also reported with faster responses to facial stimuli than to words, likely owing to more direct access to neural circuits-approximately 130 ms for happy faces compared to 380 ms for positive verbs ( Schacht and Sommer, 2009a ). Moreover, augmented responses observed in the later positive complex (LPP), i.e., larger late positive waves in response to emotional verbs (both positive and negative) and angry faces, all associated with the increased motivational significance of emotional stimuli ( Schupp et al., 2000 ) and increased selective attention to pictures ( Kok, 2000 ).
Khairudin et al. (2011) investigated effects of emotional content on explicit memory with two standardized stimuli: emotional words from the Affective Norms for English Words (ANEW) and emotional pictures from the IAPS. All stimuli were categorized as positive, negative or neutral, and displayed in two different trials. Results revealed that better memory for emotional images than for emotional words. Moreover, a recognition test demonstrated that positive emotional content was remembered better than negative emotional content. Researchers concluded that emotional valence significantly impacts memory and that negative valence suppressed the explicit memory. Another study by Khairudin et al. (2012) investigated the effects of emotional content on explicit verbal memory by assessing recall and recognition for emotionally positive, negative and neutral words. The results revealed that emotion substantially influences memory performance and that both positive and negative words were remembered more effectively than neutral words. Moreover, emotional words were remembered better in recognition vs. recall test.
Another group studied the impacts of emotion on memory using emotional film clips that varied in emotion with neutral, positive, negative and arousing contents ( Anderson and Shimamura, 2005 ). A subjective experiment for word recall and context recognition revealed that memory, for words associated with emotionally negative film clips, was lower than emotionally neutral, positive and arousing films. Moreover, emotionally arousing film clips were associated with enhanced context recognition memory but not during a free word recall test. Therefore, clarifying whether emotional stimuli enhance recognition memory or recall memory requires further investigation, as it appears that emotional information was better remembered for recognition compared to recall. In brief, greater attentional resource toward emotional pictures with large late positive waves of LPP in the posterior region, the amygdala responds to emotional stimuli (both words and pictures) independent on its valence, leading to enhanced memory. Table Table2 2 summarizes studies on the brain regions that respond to standardized stimuli as cited above, and also for pictures of emotional facial expression or Pictures of Facial Affect (POFA), Affective Norms for English Words (ANEW) for emotional words, as well as for the International Affective Digitized Sound System (IDAS) for emotional sounds.
Comparison of different emotional stimulus categories.
| Study | Stimulus types | Emotion categories | Investigation | Brain imaging modality | Brain regions of interest | Findings | Subjects | Status | Age |
|---|---|---|---|---|---|---|---|---|---|
| Pictures (IAPS) and words (ANEW) | Positive, negative, and neutral | Brain responses to emotionally positive, negative, and arousing words | Event-related fMRI | Amygdala, PFC, anterior temporal lobe, and temporooccipital junction | ∙ Amygdala, dmPFC, and vmPFC responded equally to both pictures and words regardless of valence. ∙ mPFC was more activated for positive content. ∙ VLPFC was more activated for negative content. ∙ Greater sensitivity for emotional pictures than words. | 21 adults (10 Female, 11 Male) | Healthy | 18–35 years | |
| Words (ANEW) | High-arousal positive, high-arousal negative, and neutral | Brain responses to positive and negative emotionally arousing words | Event-related fMRI | Amygdala, vmPFC | ∙ Left amygdala activated for both positive and negative words. ∙ No activation observed in the vmPFC in response to positive or negative words. | 14 adults (All) | Healthy | 20–31 years | |
| Faces | Positive, negative, and neutral | Responses to emotional face expression without primary visual areas | Event-related fMRI | Amygdala | ∙ Right amygdala activated for all emotional faces (anger, happiness, and fear). | 1 Male | Blind sight patient | 52 years | |
| Pictures (IAPS) | Negative and neutral | Amygdala response to emotional experience during study and LTM | Event-related fMRI | Amygdala | ∙ Left amygdala activation during encoding was a predictor of subsequent recognition memory for pictures with high emotional intensity ratings. | 10 Female | Healthy | – | |
| Film clips | Aggressive, sad, and neutral | Responses of EEG frequency bands on the emotional film content | EEG | Occipital (Posterior), central and frontal (anterior) | ∙ EEG theta (4–6 Hz) was more synchronized in occipital and frontal regions for the aggressive films compared with neutral films. ∙ EEG theta (4–6 Hz) respond specifically to visual emotional stimulus. ∙ EEG alpha is associated with attention and habituation. | 18 adults (All Female) | Healthy | 20–33 years | |
| Pictures (IAPS) | Pleasant, neutral, and unpleasant | Brain responses to emotional pictures | ERP | Midline (Fz, Cz, and Pz) | ∙ More positivity for pleasant and unpleasant pictures than neutral pictures in the posterior regions. ∙ An indication of selective emotional processing (resulted from the motivational relevance of emotional pictures compared to neutral ones). | 14 Female | – | 18–24 years | |
| Words (Spanish nouns) Pictures (IAPS) | Negative, positive, neutral, and relaxing | Processing of emotional information in words and pictures | ERP | Frontal and parieto-occipital Centro-parietal and frontal regions | ∙ Both emotional words and pictures were associated with an early posterior negativity and LPC. ∙ Emotional pictures elicited greater amplitude of early posterior negativity after stimulus presentation at the frontal and parieto-occipital regions. ∙ Positive pictures were associated with enhanced early posterior negativity amplitude in the right parieto-occipital regions. ∙ An arousal-dependent effect was observed in the left parieto-occipital regions for both positive and negative stimuli. | 21 volunteers (19 Female, 2 Male) 28 volunteers (21 Female, 7 Male) | Healthy Healthy | 19–27 years 19–29 years | |
| Facial expression (POFA) | Fearful vs. neutral | Spatial attention effects on emotional face processing. | ERP | Frontal, central and posterior regions | Faces enhanced N170 amplitude reflecting that spatial attention modulates face encoding at lateral posterior electrodes. However, N170 was insensitive to emotional expression. | 20 subjects (11 Female, 7 Male, 2 excluded due to excess artifacts) | Healthy | 18–32 years | |
| Sentence | Negative/high arousal and Neutral/ low arousal | Impact of emotional verb processing in short sentences (Reading) | ERP | Centro-parietal regions | Effect on LPC of negative and high-arousal words, while LPC was not affected by arousal-related words alone. Reported the importance of valence and arousal in emotion-related ERP effects. | 21 participants (11 Female, 10 Male) | Healthy | – | |
| Sound (IADS) | Pleasant, unpleasant, and neutral | Auditory cortex response to emotional stimuli | fNIRS | Auditory cortex | Both pleasant and unpleasant sounds led to greater activation in the left and right auditory cortex compared with neutral sound. | 17 participants (10 Female, 7 Male) | Healthy | – |
Neuroimaging Techniques for the Investigation of Emotional-Cognitive Interactions
The brain regions associated with cognitive-emotional interactions can be studied with different functional neuroimaging techniques (fMRI, PET, and fNIRS) to examine hemodynamic responses (indirect measurement). EEG is used to measure brain electrical dynamics (direct measurement) associated with responses to cognitive and emotional tasks. Each technique has particular strengths and weaknesses, as described below.
Functional Magnetic Resonance Imaging (fMRI)
Functional magnetic resonance imaging is a widely used functional neuroimaging tool for mapping of brain activation as it provides a high spatial resolution (a few millimeters). fMRI is an indirect measure of hemodynamic response by measuring changes in local ratios of oxy-hemoglobin vs. deoxy-hemoglobin, typically known as a blood oxygenation level dependent (BOLD) signal ( Cabeza and Nyberg, 2000 ). Dolcos et al. (2005) examined the effects of emotional content on memory enhancement during retrieval process using event-related fMRI to measure retrieval-related activity after a retention interval of 1 year. The researchers concluded that successful retrieval of emotional pictures involved greater activation of the amygdala as well as the entorhinal cortex and hippocampus than that of neutral pictures. Both the amygdala and hippocampus were rigorously activated during recollection compared to familiarity recognition, whereas no differences were found in the entorhinal cortex for either recollection or familiarity recognition. Moreover, a study investigates motivation effect (low vs. high monetary reward) on episodic retrieval by manipulating task difficulty, fMRI data reports that increased activation in the substantia nigra/VTA, MTL, dmPFC, and DLPFC when successful memory retrieval with high difficulty than with low difficulty. Moreover, reward-related of functional connectivities between the (i) SN/VTA–MTL and (ii) SN/VTA–dmPFC appear to increases significantly with increases retrieval accuracy and subjective motivation. Thus, Shigemune et al. (2017) suggest that reward/motivation-related memory enhancement modulated by networking between the SN/VTA (reward-related), dmPFC (motivation-related) and MTL (memory-related) network as well as DLPFC (cognitive controls) with high task difficulty.
Taken together, these findings indicate that the amygdala and MTL have important roles in the recollection of emotional and motivational memory. Another fMRI study reported that greater success for emotional retrieval (emotional hits > misses ) was associated with neural activation of the bilateral amygdala, hippocampus, and parahippocampus, whereas a higher success rate for neutral retrieval is associated with a greater activity in right posterior parahippocampus regions ( Shafer and Dolcos, 2014 ). Hence, fMRI has clearly revealed interactions between cognitive and emotional neural networks during information processing, particularly in response to emotion-related content. Such interactions appear to modulate memory consolidation while also mediating encoding and retrieval processes that underlie successful LTM formation and memory recall. More specifically, it appears that amygdala activation modulates both the hippocampus and visual cortex during visual perception and enhances the selection and organization of salient information via the “bottom-up” approach to higher cognitive functions directed at awareness. Although fMRI is widely used, it poses several limitations such as poor temporal resolution, expensive setup costs, plus the difficulty of having a subject hold still during the procedure in an electromagnetically shielded room (immobility). Furthermore, fMRI is slightly more metabolically sluggish, as BOLD signal exhibits an initial dip, where the increase of subsequent signal is delayed by 2–3 s and it takes approximately 6–12 s to reach to a peak value that reflects the neural responses elicited by a stimulus ( Logothetis et al., 2001 ). This means that fMRI having a coarse temporal resolution (several seconds) when compared with electrophysiological techniques (a few milliseconds) and also not a great technique for visualizing subcortical regions (mesencephalon and brainstem) due to metabolically sluggish compared to PET.
Positron Emission Tomography (PET)
Positron emission tomography is another functional neuroimaging tool that maps CNS physiology and neural activation by measuring glucose metabolism or regional cerebral blood flow (rCBF). PET uses positron-emitting radionuclides such as 18 F-fluorodeoxyglucose (FDG) and positron-emitting-oxygen isotope tagged with water ([ 15 O] H 2 O), etc. This technique identifies different neural networks involving pleasant, unpleasant and neutral emotions ( Lane et al., 1997 ). It thus far appears that increased rCBF in the mPFC, thalamus, hypothalamus, and midbrain associated with pleasant and unpleasant emotional processing, while unpleasant emotions are more specifically associated with the bilateral OTC, cerebellum, left parahippocampal gyrus, hippocampus, and amygdala; moreover, the caudate nucleus is associated with pleasant emotions.
Using PET scanning demonstrated that emotional information enhances visual memory recognition via interactions between perception and memory systems, specifically with greater activation of the lingual gyrus for visual stimuli ( Taylor et al., 1998 ). The results also showed that strong negative emotional valence appeared to enhance the processing of early sensory input. Moreover, differences in neural activation appeared in the left amygdaloid complex (AC) during encoding, while the right PFC and mPFC responded during recognition memory. Similarly, Tataranni et al. (1999) identified CNS regions associated with appetitive states (hunger and satiation) ( Tataranni et al., 1999 ). Hunger stimulated increased rCBF uptake in multiple regions including the hypothalamus, insular cortex, limbic and paralimbic regions (anterior cingulate cortex, parahippocampal and hippocampal formation, the anterior temporal and posterior orbitofrontal cortex), as well as the thalamus, caudate, precuneus, putamen, and cerebellum. Satiation was associated with increased rCBF uptake in the bilateral vmPFC, the DLPFC, and the inferior parietal lobule. These results imply that (i) subcortical regions associated with emotion/motivation involved in hunger that signals distressing feeling (discomfort, pain and anxiety) for the regulation of food intake; and (ii) the PFC associated with inhibition of inappropriate behavioral response involved in satiation that signals excessive food consumption for a termination of meal.
In a study of emotional self-generation using PET noted that the insular cortex, secondary somatosensory cortex, and hypothalamus, as well as the cingulate cortex and nuclei in the brainstem’s tegmentum, including PAG, parabrachial nucleus, and substantia nigra maintained current homeostasis by generating regulatory signals ( Damasio et al., 2000 ). PET scanning has also been used for neuroanatomical mapping of emotions ( Davidson and Irwin, 1999 ), emotional processing ( Choudhary et al., 2015 ), and cognitive functions ( Cabeza and Nyberg, 2000 ). Although PET scanning has a relatively good spatial resolution for both the brain and bodily functions, it is costly and yields lower temporal resolution than does EEG and is invasive as opposed to fMRI. Moreover, PET tends to show better activation of more ancient brain regions in the mesencephalon and brainstem when compared to fMRI. Hence, it is generally reserved for the clinical diagnoses of cancers, neurological diseases processes (e.g., epilepsy and Alzheimer’s disease), and heart diseases.
Electroencephalography (EEG)
Electroencephalography obtains high temporal resolution in milliseconds, portable, less expensive, and non-invasive techniques by attaching scalp electrodes to record brain electrical activity. Moreover, numerous studies reported that EEG is useful in mapping CNS cognitive and emotional processing. The technique offers a comprehensive range of feature extraction and analysis methods, including power spectral analysis, EEG coherence, phase delay, and cross-power analysis. One study examined changes in EEG oscillations in the amygdala during the consolidation of emotionally aroused memory processing that exhibited theta (4–8 Hz) activity ( Paré et al., 2002 ), indicating the facilitation of memory consolidation, improved retention of emotional content, and enhanced memory recall. This finding was later supported by the revelation of increased theta activity in the right frontal ( Friese et al., 2013 ) and right temporal cortices ( Sederberg et al., 2003 ) and consequently associated with the successful encoding of new information. Another study ( Buzsáki, 2002 ) revealed that theta oscillations were positively related to the activation of the hippocampus represent the active brain state during sensory, motor and memory-related processing. The theta waves are generated through an interaction between the entorhinal cortex, the Schaffer collateral (CA3 region) and the pyramidal cell dendrites (both CA3 and CA1 regions) that result in a synaptic modification underlie learning and memory. Thus, theta oscillation is thought to be associated with the encoding of new memories.

Increased gamma oscillation in the neocortex and right amygdala have been reported in response to emotionally arousing pictures during learning and memory tasks undertaken by 148 right-handed female participants ( Headley and Paré, 2013 ). A more detailed study by Müller et al. (1999) reported increased gamma potentials in the left frontal and temporal regions in response to images having a negative valence, whereas increased gamma-bands in the right frontal regions were observed in responses to images with positive valence for 11 right-handed male participants. During an emotionally positive experience, another study reported significantly increased EEG theta-alpha coherence between prefrontal and posterior parietal regions ( Aftanas and Golocheikine, 2001 ). They concluded the change was associated with heightened attention in association with improved performance in memory and emotional processing. Thus, we have a number of EEG investigations of left and right hemispheric activity while processing positive (pleasant) and negative (unpleasant) stimuli that revealed differences in regional electrophysiological activation. Nonetheless, EEG exhibits a relatively poor spatial resolution approximately 5 to 9 cm compared with fMRI and PET ( Babiloni et al., 2001 ). Thus, scalp EEG unable to measure activation much below cortex owing to the distortion of scalp potentials where different volume conduction effects of the cortex, dura mater, skull, and scalp resulting in imprecise localization of the electromagnetic field patterns associated with neural current flow. Subsequent studies have demonstrated that the EEG spatial resolution can be improved using high-resolution EEG (high-density electrode arrays to increase spatial sampling) with surface Laplacian estimation and cortical imaging (details discussion of this area is beyond the scope of this review, see ( Nunez et al., 1994 ) for theoretical and experimental study) or integrating multiple imaging modalities that provide complement information, for instance EEG-fMRI and EEG-fNIRS ( Dale and Halgren, 2001 ).
Functional Near-Infrared Spectroscopy (fNIRS)
Functional near-infrared spectroscopy is an emerging and relatively low-cost imaging technique that is also portable and non-invasive. It can be used to map the hemodynamic responses associated with brain activation. This technology measures cerebral changes in the concentration of oxygenated hemoglobin (oxy-Hb) vs. deoxygenated hemoglobin (deoxy-Hb) using optodes (light emitters and detectors) placed on the scalp ( Villringer et al., 1993 ). It is limited to visualizations of cortical activity compared to the subcortical regions, and findings only imply increased brain activity associated with increased glucose and oxygen consumption. Elevations in cerebral blood flow and oxygen delivery exceed quo oxygen consumption, thereby enabling changes in local cerebral blood oxygenation to be measured by optic penetration.
The number of studies that have implemented this investigative technique are associated with task performance ( Villringer et al., 1993 ), including exercise ( Perrey, 2008 ), cognitive workload ( Durantin et al., 2014 ), psychiatric disorders ( Ehlis et al., 2014 ), emotional processing ( Bendall et al., 2016 ), and aging ( Hock et al., 1995 ). One study used fNIRS to examine the relationship between subjective happiness and emotional changes ( Oonishi et al., 2014 ). The results revealed that the level of subjective happiness influenced the pattern of left-right PFC activation during the emotion-related task, showing increased oxy-Hb in the left PFC when viewing pleasant pictures, and increased oxy-Hb in the right PFC when viewing unpleasant pictures. Viewing unpleasant emotional stimuli accompanied increased in oxy-Hb levels in the bilateral VLPFC while also activating several regions in both the right VLPFC (BA45/47) and left VLPFC (BA10/45/46/47). However, another fNIRS study reported that viewing pleasant emotional stimuli was associated with decreased oxy-Hb in the left DLPFC (BA46/10) when affective images were presented for 6 s ( Hoshi et al., 2011 ). Thus, this study found an opposite pattern indicating left hemisphere involvement in positive/approach processing and right hemisphere involvement in negative/withdrawal processing ( Davidson, 1992 ; Davidson and Irwin, 1999 ). This inconsistent finding of frontal hemispheric asymmetric might result from the comparison of state-related changes rather than baseline levels of asymmetric. Thus, several issues should take into consideration: (i) methodological issues to assess hemispheric asymmetry, including requires repeat measures of anterior asymmetry for at least two sessions, stimulus content should comprise both positive valence and negative valence while maintaining at a similar level of arousal and with a baseline resting condition, appropriate selection of reference electrode and individual differences, etc; and (ii) conceptual issues is related to the fact that prefrontal cortex is an anatomically and functionally heterogeneous and complex region interacts with other cortical and subcortical structures during emotional processing ( Davidson, 2004 ). Another fNIRS study examined the relationship between PFC function and cognitive control of emotion ( Ozawa et al., 2014 ). This was done by presenting emotional IAPS pictures for 5.2 s, followed by the n -back task. The results revealed a significantly greater increase in oxy-HB in the mPFC and left superior frontal gyrus in response to negative pictures compared with neutral pictures. Meanwhile, no significant hemodynamic changes were observed during image presentation and the n -back task, indicating the need for further investigation.
Factors Affecting the Effect of Emotion on Learning and Memory
The preceding section described neuroimaging techniques used to examine brain responses to emotional stimuli during WM processing leading to LTM. This section presents six key factors that are recommended for consideration in the experimental design and appropriate protocol.
Individual Differences
A number of studies have reported numerous influences in addition to a range of individual differences in emotional processing. These include personality traits ( Montag and Panksepp, 2017 ), intellectual ability ( Brackett et al., 2004 ), and sex ( Cahill, 2003 ). Moreover, sex hormones and personality traits (e.g., extraversion and neuroticism) appear to influence individual responses to emotional stimuli as well as modulate emotional processing. Appropriate screening with psychological testing as well as balancing experimental cohorts in terms of sex can help reduce spurious results owing to individual differences.
Age-Related Differences
Studies have also shown that older adults are associated with the greater familiarity with psychological stress and emotional experiences, thus causing positivity biases in emotional processing and better emotional control than in younger adults ( Urry and Gross, 2010 ; Allard and Kensinger, 2014 ). Consequently, the age of participants in a sample population should be considered for both cognitive and emotional studies.
Emotional Stimulus Selection
The selection of emotional stimuli for experimental studies is generally divided into two streams: (1) discrete emotional, and (2) dimensional emotions of valence, arousal, dominance and familiarity ( Russell, 1980 ; Barrett, 1998 ). The latter include pictures from the IAPS database and words from the ANEW database, which are both available for non-commercial research. Appropriate selection of emotional stimuli is another important consideration that ensures experimental tasks are suitable for the investigation of emotional processing in learning and memory. Furthermore, the type of stimulus determines stimulus presentation duration, especially for experimental tasks involving the induction of emotions.
Self-assessment Techniques
There are numerous self-assessment techniques used to measure individual emotional states ( Bradley and Lang, 1994 ). The most widely used techniques are the Self-Assessment Manikin (SAM), the Semantic Differential (SD) scale, and the Likert scale. The SAM is a non-verbal pictorial assessment technique directly measures emotional responses to emotional stimuli for valence, arousal, and dominance. The SD scale consists of a set of bipolar adjective pairs for the subjective rating of image stimuli. The Likert’s “ x -point” scale allows participants to rate their own emotional responses. If a study does not seek to assess distinct emotional states but rather involves the assessment of two primary dimensions of emotion (positive and negative valence), then the Positive and Negative Affect Schedule (PANAS) is a recommended method ( Watson et al., 1988 ). Thus, selection of the most appropriate self-assessment technique is an important part of the experimental design but can also become an overwhelming task.
Selection of Brain Imaging Techniques
As mentioned above, the two major types of brain imaging techniques EEG (direct) and fMRI/PET/fNIRS (indirect) have respective advantages and disadvantages. To overcome these limitations, simultaneous or combined dual-modality imaging (EEG-fMRI or EEG-fNIRS) can now be implemented for complementary data collection. Although functional neuroimaging works to identify the neural correlates of emotional states, technologies such as deep brain stimulation (DBS) and connectivity maps might provide new opportunities to seek understanding of emotions and its corresponding psychological responses.
Neurocognitive Research Design
The neuroscience of cognition and emotion requires appropriate task designs to accomplish specific study objectives ( Amin and Malik, 2013 ). Environmental factors, ethical issues, memory paradigms, cognitive task difficulty, and emotional induction task intensity must be considered for this.
Numerous neuroimaging studies cited thus far have indicated that emotions influence memory processes, to include memory encoding, memory consolidation, and memory retrieval. Emotional attentional and motivational components might explain why emotional content exhibits privileged information processing. Emotion has a “pop-out” effect that increases attention and promotes bottom-up instinctual impact that enhances awareness. Significant emotional modulation affects memory consolidation in the amygdala, and emotional content also appears to mediate memory encoding and retrieval in the PFC, leading to slow rates of memory lapse accompanied by the accurate recall. Moreover, cognitive and emotional interactions also appear to modulate additional memory-related CNS regions, such as the frontal, posterior parietal and visual cortices. The latter are involved in attentional control, association information, and the processing of visual information, respectively. Therefore, higher-level cognitive functions such as learning and memory, appear to be generally guided by emotion, as outlined in the Panksepp’s framework of brain processing ( Panksepp, 1998 ).
Neuroimaging findings also indicate the involvement of the PFC in emotional processing by indirectly influencing WM and semantic memory ( Kensinger and Corkin, 2003 ). This is reflected by the involvement of the DLPFC in WM and the role played by VLPFC in semantic processing, both of which have been found to enhance or impair semantic encoding task performance when emotion is involved. Various parts of the lateral PFC (ventrolateral, dorsolateral and medial prefrontal cortical regions) are suspected of having key roles that support memory retrieval ( Simons and Spiers, 2003 ). All of these findings suggest that PFC-MTL interactions underlie effective semantic memory encoding and thus strategically mediate information processing with increased transfer to the hippocampus, consequently enhancing memory retrieval. Accordingly, learning strategies that emphasize emotional factors are more likely to result in long-term knowledge retention. This consideration is potentially useful in the design of educational materials for academic settings and informed intelligent tutoring systems.
Based on numerous previous findings, future research might take emotional factors more seriously and more explicitly in terms of their potential impact on learning. By monitoring the emotional state of students, the utilization of scientifically derived knowledge of stimulus selection can be particularly useful in the identification of emotional states that advance learning performance and outcomes in educational settings. Moreover, functional neuroimaging investigations now include single and/or combined modalities that obtain complementary datasets that inform a more comprehensive overview of neuronal activity in its entirety. For example, curiosity and motivation promote learning, as it appears cognitive network become energized by the mesolimbic-mesocortical dopamine system (generalized motivational arousal/SEEKING system). In addition, the identification of emotional impact on learning and memory potentially has direct implications for healthy individuals as well as patients with psychiatric disorders such as depression, anxiety, schizophrenia, autism, mania, obsessive-compulsive disorder and post-traumatic stress disorder (PTSD) ( Panksepp, 2011a ). To emphasize, depression and anxiety are the two most commonly diagnosed psychiatric disorders associated with learning/memory impairment and pose negative consequences that (i) limit the total amount of information that can otherwise be learned, and (ii) inhibit immediate recall as well as memory retention and retrieval of newly learned information. Depression and anxiety are also associated with negative emotions such as hopelessness, anxiety, apathy, attention deficit, lack of motivation, and motor and mental insufficiencies. Likewise, neuroscience studies report that decreased activation of the dorsal limbic (the anterior and posterior cingulate) as well as in the prefrontal, premotor and parietal cortices causes attentional disturbance, while increased neural activation in the ventral paralimbic region (the subgenual cingulate, anterior insula, hypothalamus and caudate) is associated with emotional and motivational disorders ( Mayberg, 1997 ).
Concluding Remarks, Open Questions, and Future Directions
Substantial evidence has established that emotional events are remembered more clearly, accurately and for longer periods of time than are neutral events. Emotional memory enhancement appears to involve the integration of cognitive and emotional neural networks, in which activation of the amygdala enhances the processing of emotionally arousing stimuli while also modulating enhanced memory consolidation along with other memory-related brain regions, particularly the amygdala, hippocampus, MTL, as well as the visual, frontal and parietal cortices. Similarly, activation of the PFC enhances cognitive functions, such as strategic and semantic processing that affect WM and also promote the establishment of LTM. Previous studies have primarily used standardized emotional visual, or auditory stimuli such as pictures, words, facial expression, and film clips, often based on the IAPS, ANEW, and POFA databases for emotional pictures, words and facial expressions, respectively. Further studies have typically focused on the way individuals memorize (intentional or incidental episodic memory paradigm) emotional stimuli in controlled laboratory settings. To our knowledge, there are few objective studies that employed brain-mapping techniques to examine semantic memory of learning materials (using subject matter) in the education context. Furthermore, influences derived from emotional factors in human learning and memory remains unclear as to whether positive emotions facilitate learning or negative emotions impair learning and vice versa. Thus, several remaining questions should be addressed in future studies, including (i) the impact of emotion on semantic knowledge encoding and retrieval, (ii) psychological and physiological changes associated with semantic learning and memory, and (iii) the development of methods that incorporate emotional and motivational aspects that improve educational praxes, outcomes, and instruments. The results of studies on emotion using educational learning materials can indeed provide beneficial information for informed designs of new educational courses that obtain more effective teaching and help establish better informed learning environments. Hence, to understand how emotion influence learning and memory requires understanding of an evolutionary consideration of the nested hierarchies of CNS emotional-affective processes as well as a large-scale network, including the midbrain’s PAG and VTA, basal ganglia (amygdala and NAc), and insula, as well as diencephalon (the cingulate and medial frontal cortices through the lateral and medial hypothalamus and medial thalamus) together with the MTL, including the hippocampus as well as the entorhinal cortex, perirhinal cortex, and parahippocampal cortices that responsible for declarative memories. Moreover, the SEEKING system generates positive subjective emotional states-positive expectancy, enthusiastic exploration, and hopefulness, apparently, initiates learning and memory in the brain. All cognitive activity is motivated from ‘underneath’ by basic emotional and homeostatic needs (motivational drives) that explore environmental events for survival while facilitating secondary processes of learning and memory.
Author Contributions
CMT drafted this manuscript. CMT, HUA, MNMS, and ASM revised this draft. All authors reviewed and approved this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Ministry of Education (MOE), Malaysia for the financial support. We gratefully thank Frontiers in Psychology, Specialty Section Emotion Sciences reviewers and the journal Associate Editor, for their helpful input and feedback on the content of this manuscript.
Funding. This research work was supported by the HiCoE grant for CISIR (Ref No. 0153CA-002), Ministry of Education (MOE), Malaysia.
- Aftanas L., Golocheikine S. (2001). Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci. Lett. 310 57–60. 10.1016/S0304-3940(01)02094-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Allard E. S., Kensinger E. A. (2014). Age-related differences in neural recruitment during the use of cognitive reappraisal and selective attention as emotion regulation strategies. Front. Psychol. 5 : 296 10.3389/fpsyg.2014.00296 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Amaral D. G., Behniea H., Kelly J. (2003). Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience 118 1099–1120. 10.1016/S0306-4522(02)01001-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Amin H., Malik A. S. (2013). Human memory retention and recall processes. Neurosciences 18 330–344. [ PubMed ] [ Google Scholar ]
- Anderson A. K., Phelps E. A. (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411 305–309. 10.1038/35077083 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anderson L., Shimamura A. P. (2005). Influences of emotion on context memory while viewing film clips. Am. J. Psychol. 118 323–337. [ PubMed ] [ Google Scholar ]
- Ashby F. G., Isen A. M. (1999). A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev. 106 529–550. 10.1037/0033-295X.106.3.529 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Babiloni F., Cincotti F., Carducci F., Rossini P. M., Babiloni C. (2001). Spatial enhancement of EEG data by surface Laplacian estimation: the use of magnetic resonance imaging-based head models. Clin. Neurophysiol. 112 724–727 10.1016/S1388-2457(01)00494-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Badre D., Wagner A. D. (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45 2883–2901. 10.1016/j.neuropsychologia.2007.06.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barbey A. K., Koenigs M., Grafman J. (2013). Dorsolateral prefrontal contributions to human working memory. Cortex 49 1195–1205. 10.1016/j.cortex.2012.05.022 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barbey A. K., Krueger F., Grafman J. (2009). Structured event complexes in the medial prefrontal cortex support counterfactual representations for future planning. Philos. Trans. R. Soc. B Biol. Sci. 364 1291–1300. 10.1098/rstb.2008.0315 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barrett L. F. (1998). Discrete emotions or dimensions? The role of valence focus and arousal focus. Cogn. Emot. 12 579–599. 10.1080/026999398379574 [ CrossRef ] [ Google Scholar ]
- Bartolic E., Basso M., Schefft B., Glauser T., Titanic-Schefft M. (1999). Effects of experimentally-induced emotional states on frontal lobe cognitive task performance. Neuropsychologia 37 677–683. 10.1016/S0028-3932(98)00123-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Battaglia F. P., Benchenane K., Sirota A., Pennartz C. M., Wiener S. I. (2011). The hippocampus: hub of brain network communication for memory. Trends Cogn. Sci. 15 310–318. 10.1016/j.tics.2011.05.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bayer M., Sommer W., Schacht A. (2010). Reading emotional words within sentences: the impact of arousal and valence on event-related potentials. Int. J. Psychophysiol. 78 299–307. 10.1016/j.ijpsycho.2010.09.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bechara A., Damasio H., Damasio A. R. (2000). Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex 10 295–307. 10.1093/cercor/10.3.295 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bendall R. C., Eachus P., Thompson C. (2016). A brief review of research using near-infrared spectroscopy to measure activation of the prefrontal cortex during emotional processing: the importance of experimental design. Front. Hum. Neurosci. 10 : 529 10.3389/fnhum.2016.00529 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blumenfeld R. S., Ranganath C. (2006). Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J. Neurosci. 26 916–925. 10.1523/JNEUROSCI.2353-05.2006 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blumenfeld R. S., Ranganath C. (2007). Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13 280–291. 10.1177/1073858407299290 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brackett M. A., Mayer J. D., Warner R. M. (2004). Emotional intelligence and its relation to everyday behaviour. Pers. Individ. Dif. 36 1387–1402. 10.1016/S0191-8869(03)00236-8 [ CrossRef ] [ Google Scholar ]
- Bradley M. M., Lang P. J. (1994). Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 25 49–59. 10.1016/0005-7916(94)90063-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brod G., Werkle-Bergner M., Shing Y. L. (2013). The influence of prior knowledge on memory: a developmental cognitive neuroscience perspective. Front. Behav. Neurosci. 7 : 139 10.3389/fnbeh.2013.00139 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Buzsáki G. (2002). Theta oscillations in the hippocampus. Neuron 33 325–340. 10.1016/S0896-6273(02)00586-X [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cabeza R., Nyberg L. (2000). Imaging cognition II: an empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 12 1–47. 10.1162/08989290051137585 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cahill L. (2003). Sex-and hemisphere-related influences on the neurobiology of emotionally influenced memory. Prog. Neuro Psychopharmacol. Biol. Psychiatry 27 1235–1241. 10.1016/j.pnpbp.2003.09.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cahill L., Haier R. J., Fallon J., Alkire M. T., Tang C., Keator D., et al. (1996). Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc. Natl. Acad. Sci. U.S.A. 93 8016–8021. 10.1073/pnas.93.15.8016 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cahill L., McGaugh J. L. (1998). Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 21 294–299. 10.1016/S0166-2236(97)01214-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Camille N., Coricelli G., Sallet J., Pradat-Diehl P., Duhamel J. -R., Sirigu A. (2004). The involvement of the orbitofrontal cortex in the experience of regret. Science 304 1167–1170. 10.1126/science.1094550 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Canli T., Zhao Z., Brewer J., Gabrieli J. D., Cahill L. (2000). Event-related activation in the human amygdala associates with later memory for individual emotional experience. J. Neurosci. 20 : RC99 . [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carew T. J., Magsamen S. H. (2010). Neuroscience and education: an ideal partnership for producing evidence-based solutions to guide 21 st century learning. Neuron 67 685–688. 10.1016/j.neuron.2010.08.028 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Choudhary M., Kumar A., Tripathi M., Bhatia T., Shivakumar V., Beniwal R. P., et al. (2015). F-18 fluorodeoxyglucose positron emission tomography study of impaired emotion processing in first episode schizophrenia. Schizophr. Res. 162 103–107. 10.1016/j.schres.2015.01.028 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Comte M., Schön D., Coull J. T., Reynaud E., Khalfa S., Belzeaux R., et al. (2014). Dissociating bottom-up and top-down mechanisms in the cortico-limbic system during emotion processing. Cereb. Cortex 26 144–155. 10.1093/cercor/bhu185 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Craig A. D., Craig A. (2009). How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10 59–70. 10.1038/nrn2555 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Curtis C. E., D’Esposito M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 7 415–423. 10.1016/S1364-6613(03)00197-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cuthbert B. N., Schupp H. T., Bradley M. M., Birbaumer N., Lang P. J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 52 95–111. 10.1016/S0301-0511(99)00044-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- D’Mello S., Lehman B., Pekrun R., Graesser A. (2014). Confusion can be beneficial for learning. Learn. Instr. 29 153–170. 10.1016/j.learninstruc.2012.05.003 [ CrossRef ] [ Google Scholar ]
- Dael N., Mortillaro M., Scherer K. R. (2012). Emotion expression in body action and posture. Emotion 12 1085–1101. 10.1037/a0025737 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dale A. M., Halgren E. (2001). Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Curr. Opin. Neurobiol. 11 202–208. 10.1016/S0959-4388(00)00197-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Damasio A., Carvalho G. B. (2013). The nature of feelings: evolutionary and neurobiological origins. Nat. Rev. Neurosci. 14 143–152. 10.1038/nrn3403 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Damasio A. R., Grabowski T. J., Bechara A., Damasio H., Ponto L. L., Parvizi J., et al. (2000). Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 3 1049–1056. 10.1038/79871 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davidson R. J. (1988). EEG measures of cerebral asymmetry: conceptual and methodological issues. Int. J. Neurosci. 39 71–89. 10.3109/00207458808985694 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davidson R. J. (1992). Emotion and affective style: hemispheric substrates. Psychol. Sci. 3 39–43. 10.1111/j.1467-9280.1992.tb00254.x [ CrossRef ] [ Google Scholar ]
- Davidson R. J. (2004). What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biol. Psychol. 67 219–234. 10.1016/j.biopsycho.2004.03.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davidson R. J., Irwin W. (1999). The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 3 11–21. 10.1016/S1364-6613(98)01265-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dobbins I. G., Foley H., Schacter D. L., Wagner A. D. (2002). Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron 35 989–996. 10.1016/S0896-6273(02)00858-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dolcos F., Iordan A. D., Dolcos S. (2011). Neural correlates of emotion–cognition interactions: a review of evidence from brain imaging investigations. J. Cogn. Psychol. 23 669–694. 10.1080/20445911.2011.594433 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dolcos F., LaBar K. S., Cabeza R. (2004). Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage 23 64–74. 10.1016/j.neuroimage.2004.05.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dolcos F., LaBar K. S., Cabeza R. (2005). Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc. Natl. Acad. Sci. U.S.A. 102 2626–2631. 10.1073/pnas.0409848102 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dolcos F., McCarthy G. (2006). Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 26 2072–2079. 10.1523/JNEUROSCI.5042-05.2006 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Durantin G., Gagnon J.-F., Tremblay S., Dehais F. (2014). Using near infrared spectroscopy and heart rate variability to detect mental overload. Behav. Brain Res. 259 16–23. 10.1016/j.bbr.2013.10.042 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ehlis A.-C., Schneider S., Dresler T., Fallgatter A. J. (2014). Application of functional near-infrared spectroscopy in psychiatry. Neuroimage 85 478–488. 10.1016/j.neuroimage.2013.03.067 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Erk S., Kiefer M., Grothe J., Wunderlich A. P., Spitzer M., Walter H. (2003). Emotional context modulates subsequent memory effect. Neuroimage 18 439–447. 10.1016/S1053-8119(02)00015-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15 85–93. 10.1016/j.tics.2010.11.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Euston D. R., Gruber A. J., McNaughton B. L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron 76 1057–1070. 10.1016/j.neuron.2012.12.002 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Friese U., Köster M., Hassler U., Martens U., Trujillo-Barreto N., Gruber T. (2013). Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. Neuroimage 66 642–647. 10.1016/j.neuroimage.2012.11.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- González-Roldan A. M., Martínez-Jauand M., Muñoz-García M. A., Sitges C., Cifre I., Montoya P. (2011). Temporal dissociation in the brain processing of pain and anger faces with different intensities of emotional expression. Pain 152 853–859. 10.1016/j.pain.2010.12.037 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grimshaw G. M., Carmel D. (2014). An asymmetric inhibition model of hemispheric differences in emotional processing. Front. Psychol. 5 : 489 10.3389/fpsyg.2014.00489 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Haber S. N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35 4–26. 10.1038/npp.2009.129 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hamann S. (2005). Sex differences in the responses of the human amygdala. Neuroscientist 11 288–293. 10.1177/1073858404271981 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hamann S., Canli T. (2004). Individual differences in emotion processing. Curr. Opin. Neurobiol. 14 233–238. 10.1016/j.conb.2004.03.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hamann S., Mao H. (2002). Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport 13 15–19. 10.1097/00001756-200201210-00008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harmon-Jones E., Gable P. A., Peterson C. K. (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol. Psychol. 84 451–462. 10.1016/j.biopsycho.2009.08.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harmon-Jones E., Gable P. A. (2017). On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: an updated review of the evidence. Psychophysiology 10.1111/psyp.12879 [Epub ahead of print]. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Headley D. B., Paré D. (2013). In sync: gamma oscillations and emotional memory. Front. Behav. Neurosci. 7 : 170 10.3389/fnbeh.2013.00170 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Heinzel A., Bermpohl F., Niese R., Pfennig A., Pascual-Leone A., Schlaug G., et al. (2005). How do we modulate our emotions? Parametric fMRI reveals cortical midline structures as regions specifically involved in the processing of emotional valences. Cogn. Brain Res. 25 348–358. 10.1016/j.cogbrainres.2005.06.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hinojosa J. A., Carretié L., Valcárcel M. A., Méndez-Bértolo C., Pozo M. A. (2009). Electrophysiological differences in the processing of affective information in words and pictures. Cogn. Affect. Behav. Neurosci. 9 173–189. 10.3758/CABN.9.2.173 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hock C., Mueller-Spahn F., Schuh-Hofer S., Hofmann M., Dirnagl U., Villringer A. (1995). Age dependency of changes in cerebral hemoglobin oxygenation during brain activation: a near-infrared spectroscopy study. J. Cereb. Blood Flow Metab. 15 1103–1108. 10.1038/jcbfm.1995.137 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Holmes A., Vuilleumier P., Eimer M. (2003). The processing of emotional facial expression is gated by spatial attention: evidence from event-related brain potentials. Cogn. Brain Res. 16 174–184. 10.1016/S0926-6410(02)00268-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoshi Y., Huang J., Kohri S., Iguchi Y., Naya M., Okamoto T., et al. (2011). Recognition of human emotions from cerebral blood flow changes in the frontal region: a study with event-related near-infrared spectroscopy. J. Neuroimaging 21 e94–e101. 10.1111/j.1552-6569.2009.00454.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Isen A. M., Daubman K. A., Nowicki G. P. (1987). Positive affect facilitates creative problem solving. J. Pers. Soc. Psychol. 52 1122–1131. 10.1037/0022-3514.52.6.1122 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jack R. E., Schyns P. G. (2015). The human face as a dynamic tool for social communication. Curr. Biol. 25 R621–R634. 10.1016/j.cub.2015.05.052 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Joëls M., Karst H., Alfarez D., Heine V. M., Qin Y., Riel E. V., et al. (2004). Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress 7 221–231. 10.1080/10253890500070005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jung N., Wranke C., Hamburger K., Knauff M. (2014). How emotions affect logical reasoning: evidence from experiments with mood-manipulated participants, spider phobics, and people with exam anxiety. Front. Psychol. 5 : 570 10.3389/fpsyg.2014.00570 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kensinger E. A., Corkin S. (2003). Effect of negative emotional content on working memory and long-term memory. Emotion 3 378–393. 10.1037/1528-3542.3.4.378 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kensinger E. A., Corkin S. (2004). Two routes to emotional memory: distinct neural processes for valence and arousal. Proc. Natl. Acad. Sci. U.S.A. 101 3310–3315. 10.1073/pnas.0306408101 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kensinger E. A., Schacter D. L. (2006). Processing emotional pictures and words: effects of valence and arousal. Cogn. Affect. Behav. Neurosci. 6 110–126. 10.3758/CABN.6.2.110 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khairudin R., Givi M. V., Shahrazad W. W., Nasir R., Halim F. (2011). Effects of emotional contents on explicit memory process. Pertanika J. Soc. Sci. Humanit. 19 17–26. [ Google Scholar ]
- Khairudin R., Nasir R., Halim F., Zainah A., WS W. S., Ismail K., et al. (2012). Emotion and explicit verbal memory: evidence using Malay Lexicon. Asian Soc. Sci. 8 38 . [ Google Scholar ]
- Kleinginna P. R., Jr., Kleinginna A. M. (1981). A categorized list of emotion definitions, with suggestions for a consensual definition. Motiv. Emot. 5 345–379. 10.1007/BF00992553 [ CrossRef ] [ Google Scholar ]
- Koechlin E., Basso G., Pietrini P., Panzer S., Grafman J. (1999). The role of the anterior prefrontal cortex in human cognition. Nature 399 148–151. 10.1038/20178 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kok A. (2000). Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP). Biol. Psychol. 54 107–143. 10.1016/S0301-0511(00)00054-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Krause C. M., Viemerö V., Rosenqvist A., Sillanmäki L., Åström T. (2000). Relative electroencephalographic desynchronization and synchronization in humans to emotional film content: an analysis of the 4–6, 6–8, 8–10 and 10–12 Hz frequency bands. Neurosci. Lett. 286 9–12. 10.1016/S0304-3940(00)01092-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lane R. D., Reiman E. M., Bradley M. M., Lang P. J., Ahern G. L., Davidson R. J., et al. (1997). Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35 1437–1444. 10.1016/S0028-3932(97)00070-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levy B. J., Wagner A. D. (2011). Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann. N. Y. Acad. Sci. 1224 40–62. 10.1111/j.1749-6632.2011.05958.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li L., Chen J.-H. (2006). Emotion Recognition Using Physiological Signals Advances in Artificial Reality and Tele-Existence. Berlin: Springer; 437–446. 10.1007/11941354_44 [ CrossRef ] [ Google Scholar ]
- Logothetis N. K., Pauls J., Augath M., Trinath T., Oeltermann A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature 412 150–157. 10.1038/35084005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mayberg H. (1997). Limbic-Cortical Dysregulation. The Neuropsychiatry of Limbic and Subcortical Disorders. Washington, DC: American Psychiatric Press; 167–178. [ Google Scholar ]
- McGaugh J. L. (2000). Memory–a century of consolidation. Science 287 248–251. 10.1126/science.287.5451.248 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McGaugh J. L. (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 27 1–28. 10.1146/annurev.neuro.27.070203.144157 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McGaugh J. L. (2006). Make mild moments memorable: add a little arousal. Trends Cogn. Sci. 10 345–347. 10.1016/j.tics.2006.06.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McGaugh J. L., Cahill L., Roozendaal B. (1996). Involvement of the amygdala in memory storage: interaction with other brain systems. Proc. Natl. Acad. Sci. U.S.A. 93 13508–13514. 10.1073/pnas.93.24.13508 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McGaugh J. L., Roozendaal B. (2002). Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 12 205–210. 10.1016/S0959-4388(02)00306-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mega M. S., Cummings J. L., Salloway S., Malloy P. (1996). The limbic system: an anatomic, phylogenetic, and clinical perspective. J. Neuropsychiatry Clin. Neurosci. 9 315–330. [ PubMed ] [ Google Scholar ]
- Metcalfe J., Mischel W. (1999). A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol. Rev. 106 3–19. 10.1037/0033-295X.106.1.3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller E. K., Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24 167–202. 10.1146/annurev.neuro.24.1.167 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moll J., de Oliveira-Souza R., Bramati I. E., Grafman J. (2002). Functional networks in emotional moral and nonmoral social judgments. Neuroimage 16 696–703. 10.1006/nimg.2002.1118 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Montag C., Panksepp J. (2017). Primary emotional systems and personality: an evolutionary perspective. Front. Psychol. 8 : 464 10.3389/fpsyg.2017.00464 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morris J. S., Öhman A., Dolan R. J. (1998). Conscious and unconscious emotional learning in the human amygdala. Nature 393 467–470. 10.1038/30976 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Müller M. M., Keil A., Gruber T., Elbert T. (1999). Processing of affective pictures modulates right-hemispheric gamma band EEG activity. Clin. Neurophysiol. 110 1913–1920. 10.1016/S1388-2457(99)00151-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Neisser U. (1963). The imitation of man by machine. Science 139 193–197. 10.1126/science.139.3551.193 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Northoff G., Heinzel A., Bermpohl F., Niese R., Pfennig A., Pascual-Leone A., et al. (2004). Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional–cognitive interaction. Hum. Brain Mapp. 21 202–212. 10.1002/hbm.20002 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Northoff G., Heinzel A., De Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage 31 440–457. 10.1016/j.neuroimage.2005.12.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Northoff G., Richter A., Gessner M., Schlagenhauf F., Fell J., Baumgart F., et al. (2000). Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: a combined fMRI/MEG study. Cereb. Cortex 10 93–107. 10.1093/cercor/10.1.93 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nunez P., Silberstein R., Cadusch P., Wijesinghe R., Westdorp A., Srinivasan R. (1994). A theoretical and experimental study of high resolution EEG based on surface Laplacians and cortical imaging. Electroencephalogr. Clin. Neurophysiol. 90 40–57. 10.1016/0013-4694(94)90112-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ochsner K. N., Bunge S. A., Gross J. J., Gabrieli J. D. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 14 1215–1229. 10.1162/089892902760807212 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ochsner K. N., Gross J. J. (2005). The cognitive control of emotion. Trends Cogn. Sci. 9 242–249. 10.1016/j.tics.2005.03.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Öhman A., Flykt A., Esteves F. (2001). Emotion drives attention: detecting the snake in the grass. J. Exp. Psychol. 130 466–478. 10.1037/0096-3445.130.3.466 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Okon-Singer H., Hendler T., Pessoa L., Shackman A. J. (2015). The neurobiology of emotion–cognition interactions: fundamental questions and strategies for future research. Front. Hum. Neurosci. 9 : 58 10.3389/fnhum.2015.00058 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oonishi S., Hori S., Hoshi Y., Seiyama A. (2014). Influence of Subjective Happiness on the Prefrontal Brain Activity: An fNIRS Study Oxygen Transport to Tissue XXXVI. Berlin: Springer; 287–293. [ PubMed ] [ Google Scholar ]
- Opialla S., Lutz J., Scherpiet S., Hittmeyer A., Jäncke L., Rufer M., et al. (2015). Neural circuits of emotion regulation: a comparison of mindfulness-based and cognitive reappraisal strategies. Eur. Arch. Psychiatry Clin. Neurosci. 265 45–55. 10.1007/s00406-014-0510-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oudeyer P.-Y., Gottlieb J., Lopes M. (2016). Intrinsic motivation, curiosity, and learning: theory and applications in educational technologies. Prog. Brain Res. 229 257–284. 10.1016/bs.pbr.2016.05.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ozawa S., Matsuda G., Hiraki K. (2014). Negative emotion modulates prefrontal cortex activity during a working memory task: a NIRS study. Front. Hum. Neurosci. 8 : 46 10.3389/fnhum.2014.00046 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Panksepp J. (1998). Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford: Oxford university press. [ Google Scholar ]
- Panksepp J. (2005). Affective consciousness: core emotional feelings in animals and humans. Conscious. Cogn. 14 30–80. 10.1016/j.concog.2004.10.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Panksepp J. (2007). Criteria for basic emotions: is DISGUST a primary “emotion”? Cogn. Emot . 21 1819–1828. 10.1080/02699930701334302 [ CrossRef ] [ Google Scholar ]
- Panksepp J. (2011a). The basic emotional circuits of mammalian brains: do animals have affective lives? Neurosci. Biobehav. Rev . 35 1791–1804. 10.1016/j.neubiorev.2011.08.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Panksepp J. (2011b). Cross-species affective neuroscience decoding of the primal affective experiences of humans and related animals. PLoS ONE 6 : e21236 10.1371/journal.pone.0021236 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Panksepp J., Normansell L., Cox J. F., Siviy S. M. (1994). Effects of neonatal decortication on the social play of juvenile rats. Physiol. Behav. 56 429–443. 10.1016/0031-9384(94)90285-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Panksepp J., Solms M. (2012). What is neuropsychoanalysis? Clinically relevant studies of the minded brain. Trends Cogn. Sci. 16 6–8. 10.1016/j.tics.2011.11.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Papousek I., Weiss E. M., Schulter G., Fink A., Reiser E. M., Lackner H. K. (2014). Prefrontal EEG alpha asymmetry changes while observing disaster happening to other people: cardiac correlates and prediction of emotional impact. Biol. Psychol. 103 184–194. 10.1016/j.biopsycho.2014.09.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Paré D., Collins D. R., Pelletier J. G. (2002). Amygdala oscillations and the consolidation of emotional memories. Trends Cogn. Sci. 6 306–314. 10.1016/S1364-6613(02)01924-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Payne J. D., Jackson E. D., Hoscheidt S., Ryan L., Jacobs W. J., Nadel L. (2007). Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn. Mem. 14 861–868. 10.1101/lm.743507 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pegna A. J., Khateb A., Lazeyras F., Seghier M. L. (2005). Discriminating emotional faces without primary visual cortices involves the right amygdala. Nat. Neurosci. 8 24–25. 10.1038/nn1364 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pekrun R. (1992). The impact of emotions on learning and achievement: towards a theory of cognitive/motivational mediators. Appl. Psychol. 41 359–376. 10.1111/j.1464-0597.1992.tb00712.x [ CrossRef ] [ Google Scholar ]
- Perlstein W. M., Elbert T., Stenger V. A. (2002). Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proc. Natl. Acad. Sci. U.S.A. 99 1736–1741. 10.1073/pnas.241650598 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Perrey S. (2008). Non-invasive NIR spectroscopy of human brain function during exercise. Methods 45 289–299. 10.1016/j.ymeth.2008.04.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pessoa L. (2008). On the relationship between emotion and cognition. Nat. Rev. Neurosci. 9 148–158. 10.1038/nrn2317 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Phelps E. A. (2004). Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 14 198–202. 10.1016/j.conb.2004.03.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Plichta M. M., Gerdes A. B., Alpers G., Harnisch W., Brill S., Wieser M., et al. (2011). Auditory cortex activation is modulated by emotion: a functional near-infrared spectroscopy (fNIRS) study. Neuroimage 55 1200–1207. 10.1016/j.neuroimage.2011.01.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Poldrack R. A., Wagner A. D., Prull M. W., Desmond J. E., Glover G. H., Gabrieli J. D. (1999). Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10 15–35. 10.1006/nimg.1999.0441 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ramnani N., Owen A. M. (2004). Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 5 184–194. 10.1038/nrn1343 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Richardson M. P., Strange B. A., Dolan R. J. (2004). Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat. Neurosci. 7 278–285. 10.1038/nn1190 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Richter-Levin G., Akirav I. (2000). Amygdala-hippocampus dynamic interaction in relation to memory. Mol. Neurobiol. 22 11–20. 10.1385/MN:22:1-3:011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rolls E. T. (2000). The orbitofrontal cortex and reward. Cereb. Cortex 10 284–294. 10.1093/cercor/10.3.284 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Russell J. A. (1980). A circumplex model of affect. J. Pers. Soc. Psychol. 39 1161–1178. 10.1037/h0077714 [ CrossRef ] [ Google Scholar ]
- Russell J. A., Bachorowski J.-A., Fernández-Dols J.-M. (2003). Facial and vocal expressions of emotion. Annu. Rev. Psychol. 54 329–349. 10.1146/annurev.psych.54.101601.145102 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Russell J. A., Barrett L. F. (1999). Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J. Pers. Soc. Psychol. 76 805–819. 10.1037/0022-3514.76.5.805 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rusting C. L. (1998). Personality, mood, and cognitive processing of emotional information: three conceptual frameworks. Psychol. Bull. 124 165–196. 10.1037/0033-2909.124.2.165 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rutishauser U., Ross I. B., Mamelak A. N., Schuman E. M. (2010). Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464 903–907. 10.1038/nature08860 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schacht A., Sommer W. (2009a). Emotions in word and face processing: early and late cortical responses. Brain Cogn. 69 538–550. 10.1016/j.bandc.2008.11.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schacht A., Sommer W. (2009b). Time course and task dependence of emotion effects in word processing. Cogn. Affect. Behav. Neurosci. 9 28–43. 10.3758/CABN.9.1.28 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schiff N. D., Plum F. (2000). The role of arousal and “gating” systems in the neurology of impaired consciousness. J. Clin. Neurophysiol. 17 438–452. 10.1097/00004691-200009000-00002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schupp H. T., Cuthbert B. N., Bradley M. M., Cacioppo J. T., Ito T., Lang P. J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology 37 257–261. 10.1111/1469-8986.3720257 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schupp H. T., Markus J., Weike A. I., Hamm A. O. (2003). Emotional facilitation of sensory processing in the visual cortex. Psychol. Sci. 14 7–13. 10.1111/1467-9280.01411 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schupp H. T., Stockburger J., Codispoti M., Junghöfer M., Weike A. I., Hamm A. O. (2007). Selective visual attention to emotion. J. Neurosci. 27 1082–1089. 10.1523/JNEUROSCI.3223-06.2007 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schwabe L., Wolf O. T. (2010). Learning under stress impairs memory formation. Neurobiol. Learn. Mem. 93 183–188. 10.1016/j.nlm.2009.09.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sederberg P. B., Kahana M. J., Howard M. W., Donner E. J., Madsen J. R. (2003). Theta and gamma oscillations during encoding predict subsequent recall. J. Neurosci. 23 10809–10814. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Seli P., Wammes J. D., Risko E. F., Smilek D. (2016). On the relation between motivation and retention in educational contexts: the role of intentional and unintentional mind wandering. Psychon. Bull. Rev. 23 1280–1287. 10.3758/s13423-015-0979-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shafer A. T., Dolcos F. (2014). Dissociating retrieval success from incidental encoding activity during emotional memory retrieval, in the medial temporal lobe. Front. Behav. Neurosci. 8 : 177 10.3389/fnbeh.2014.00177 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharot T., Delgado M. R., Phelps E. A. (2004). How emotion enhances the feeling of remembering. Nat. Neurosci. 7 1376–1380. 10.1038/nn1353 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharot T., Phelps E. A. (2004). How arousal modulates memory: disentangling the effects of attention and retention. Cogn. Affect. Behav. Neurosci. 4 294–306. 10.3758/CABN.4.3.294 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shen L., Wang M., Shen R. (2009). Affective e-learning: using” Emotional” data to improve learning in pervasive learning environment. Educ. Technol. Soc. 12 176–189. [ Google Scholar ]
- Shigemune Y., Tsukiura T., Nouchi R., Kambara T., Kawashima R. (2017). Neural mechanisms underlying the reward-related enhancement of motivation when remembering episodic memories with high difficulty. Hum. Brain Mapp. 10.1002/hbm.23599 [Epub ahead of print]. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Simons J. S., Spiers H. J. (2003). Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 4 637–648. 10.1038/nrn1178 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Squire L. R. (1992). Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99 195–231. 10.1037/0033-295X.99.2.195 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Squire R. F., Noudoost B., Schafer R. J., Moore T. (2013). Prefrontal contributions to visual selective attention. Annu. Rev. Neurosci. 36 451–466. 10.1146/annurev-neuro-062111-150439 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Talmi D. (2013). Enhanced emotional memory cognitive and neural mechanisms. Curr. Dir. Psychol. Sci. 22 430–436. 10.1177/0963721413498893 [ CrossRef ] [ Google Scholar ]
- Talmi D., Schimmack U., Paterson T., Moscovitch M. (2007). The role of attention and relatedness in emotionally enhanced memory. Emotion 7 89–102. 10.1037/1528-3542.7.1.89 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tataranni P. A., Gautier J.-F., Chen K., Uecker A., Bandy D., Salbe A. D., et al. (1999). Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl. Acad. Sci. U.S.A. 96 4569–4574. 10.1073/pnas.96.8.4569 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taylor S. F., Liberzon I., Fig L. M., Decker L. R., Minoshima S., Koeppe R. A. (1998). The effect of emotional content on visual recognition memory: a PET activation study. Neuroimage 8 188–197. 10.1006/nimg.1998.0356 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Um E., Plass J. L., Hayward E. O., Homer B. D. (2012). Emotional design in multimedia learning. J. Educ. Psychol. 104 485–498. 10.1037/a0026609 [ CrossRef ] [ Google Scholar ]
- Urry H. L., Gross J. J. (2010). Emotion regulation in older age. Curr. Dir. Psychol. Sci. 19 352–357. 10.1177/0963721410388395 [ CrossRef ] [ Google Scholar ]
- Villringer A., Planck J., Hock C., Schleinkofer L., Dirnagl U. (1993). Near infrared spectroscopy (NIRS): a new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci. Lett. 154 101–104. 10.1016/0304-3940(93)90181-J [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vogel S., Schwabe L. (2016). Learning and memory under stress: implications for the classroom. Sci. Learn. 1 1–10. 10.1038/npjscilearn.2016.11 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Volman I., Roelofs K., Koch S., Verhagen L., Toni I. (2011). Anterior prefrontal cortex inhibition impairs control over social emotional actions. Curr. Biol. 21 1766–1770. 10.1016/j.cub.2011.08.050 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 9 585–594. 10.1016/j.tics.2005.10.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vytal K., Hamann S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J. Cogn. Neurosci. 22 2864–2885. 10.1162/jocn.2009.21366 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wager T. D., Davidson M. L., Hughes B. L., Lindquist M. A., Ochsner K. N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59 1037–1050. 10.1016/j.neuron.2008.09.006 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wagner A. D., Maril A., Bjork R. A., Schacter D. L. (2001). Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage 14 1337–1347. 10.1006/nimg.2001.0936 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Walker M. P. (2009). The role of sleep in cognition and emotion. Ann. N. Y. Acad. Sci. 1156 168–197. 10.1111/j.1749-6632.2009.04416.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Watson D., Clark L. A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54 1063–1070. 10.1037/0022-3514.54.6.1063 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Watt D. F. (2012). “Theoretical challenges in the conceptualization of motivation in neuroscience: Implications for the bridging of neuroscience and psychoanalysis,” in From the Couch to the Lab: Trends in Psychodynamic Neuroscience eds Fotopoulou A., Pfaff D., Conway M. A. (Oxford: Oxford University Press; ). [ Google Scholar ]
- Watt D. F., Pincus D. I. (2004). “Neural substrates of consciousness: implications for clinical psychiatry,”in Textbook of Biological Psychiatry ed. Panksepp J. (Hoboken, NJ: Wiley; ) 75–110. [ Google Scholar ]
- Weymar M., Löw A., Hamm A. O. (2011). Emotional memories are resilient to time: evidence from the parietal ERP old/new effect. Hum. Brain Mapp. 32 632–640. 10.1002/hbm.21051 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yamasaki H., LaBar K. S., McCarthy G. (2002). Dissociable prefrontal brain systems for attention and emotion. Proc. Natl. Acad. Sci. U.S.A. 99 11447–11451. 10.1073/pnas.182176499 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yiend J. (2010). The effects of emotion on attention: a review of attentional processing of emotional information. Cogn. Emot. 24 3–47. 10.1080/02699930903205698 [ CrossRef ] [ Google Scholar ]
- Artificial Intelligence
What is Retrieval Augmented Generation (RAG)?
In the AI space, where technological development is happening at a rapid pace, Retrieval Augmented Generation, or RAG, is a game-changer. But what is RAG, and why does it hold such importance in the present AI and natural language processing (NLP) world?
Before answering that question, let's briefly talk about Large Language Models (LLMs) . LLMs, like GPT-3, are AI bots that can generate coherent and relevant text. They learn from the massive amount of text data they read. We all know the ultimate chatbot, ChatGPT, which we have all used to send a mail or two. RAG enhances LLMs by making them more accurate and relevant. RAG steps up the game for LLMs by adding a retrieval step. The easiest way to think of it is like having both a very large library and a very skillful writer in your hands. You interact with RAG by asking it a question; it then utilizes its access to a rich database to mine relevant information and pieces together a coherent and detailed answer with this information. Overall, you get a two-in-one response because it contains both correct data and is full of details. What makes RAG unique? By combining retrieval and generation, RAG models significantly improve the quality of answers AI can provide in many disciplines. Here are some examples:
- Customer Support : Ever been frustrated with a chatbot that gives vague answers? RAG can provide precise and context-aware responses, making customer interactions smoother and more satisfying.
- Healthcare : Think of a doctor accessing up-to-date medical literature in seconds. RAG can quickly retrieve and summarize relevant research, aiding in better medical decisions.
- Insurance : Processing claims can be complex and time-consuming. RAG can swiftly gather and analyze necessary documents and information, streamlining claims processing and improving accuracy
These examples highlight how RAG is transforming industries by enhancing the accuracy and relevance of AI-generated content.
In this blog, we'll dive deeper into the workings of RAG, explore its benefits, and look at real-world applications. We’ll also discuss the challenges it faces and potential areas for future development. By the end, you'll have a solid understanding of Retrieval-Augmented Generation and its transformative potential in the world of AI and NLP. Let's get started!
Looking to build a RAG app tailored to your needs? We've implemented solutions for our customers and can do the same for you. Book a call with us today!
Understanding Retrieval-Augmented Generation
Retrieval-Augmented Generation (RAG) is a smart approach in AI to improve the accuracy and credibility of Generative AI and LLM models by bringing together two key techniques: retrieving information and generating text. Let’s break down how this works and why it’s so valuable.
What is RAG and How Does It Work?
Think of RAG as your personal research assistant. Imagine you’re writing an essay and need to include accurate, up-to-date information. Instead of relying on your memory alone, you use a tool that first looks up the latest facts from a huge library of sources and then writes a detailed answer based on that information. This is what RAG does—it finds the most relevant information and uses it to create well-informed responses.
How Retrieval and Generation Work Together
- Retrieval : First, RAG searches through a vast amount of data to find pieces of information that are most relevant to the question or topic. For example, if you ask about the latest smartphone features, RAG will pull in the most recent articles and reviews about smartphones. This retrieval process often utilizes embeddings and vector databases. Embeddings are numerical representations of data that capture semantic meanings, making it easier to compare and retrieve relevant information from large datasets. Vector databases store these embeddings, allowing the system to efficiently search through vast amounts of information and find the most relevant pieces based on similarity.
- Generation : After retrieving this information, RAG uses a text generation model that relies on deep learning techniques to create a response. The generative model takes the retrieved data and crafts a response that is easy to understand and relevant. So, if you’re looking for information on new phone features, RAG will not only pull the latest data but also explain it in a clear and concise manner.
You might have some questions about how the retrieval step operates and its implications for the overall system. Let’s address a few common doubts:
- Is the Data Static or Dynamic? The data that RAG retrieves can be either static or dynamic. Static data sources remain unchanged over time, while dynamic sources are frequently updated. Understanding the nature of your data sources helps in configuring the retrieval system to ensure it provides the most relevant information. For dynamic data, embeddings and vector databases are regularly updated to reflect new information and trends.
- Who Decides What Data to Retrieve? The retrieval process is configured by developers and data scientists. They select the data sources and define the retrieval mechanisms based on the needs of the application. This configuration determines how the system searches and ranks the information. Developers may also use open-source tools and frameworks to enhance retrieval capabilities, leveraging community-driven improvements and innovations.
- How Is Static Data Kept Up-to-Date? Although static data doesn’t change frequently, it still requires periodic updates. This can be done through re-indexing the data or manual updates to ensure that the retrieved information remains relevant and accurate. Regular re-indexing can involve updating embeddings in the vector database to reflect any changes or additions to the static dataset.
- How Does Static Data Differ from Training Data? Static data used in retrieval is separate from the training data. While training data helps the model learn and generate responses, static data enhances these responses with up-to-date information during the retrieval phase. Training data helps the model learn how to generate clear and relevant responses, while static data keeps the information up-to-date and accurate.
It’s like having a knowledgeable friend who’s always up-to-date and knows how to explain things in a way that makes sense.
What problems does RAG solve
RAG represents a significant leap forward in AI for several reasons. Before RAG, Generative AI models generated responses based on the data they had seen during their training phase. It was like having a friend who was really good at trivia but only knew facts from a few years ago. If you asked them about the latest trends or recent news, they might give you outdated or incomplete information. For example, if you needed information about the latest smartphone release, they could only tell you about phones from previous years, missing out on the newest features and specs.
RAG changes the game by combining the best of both worlds—retrieving up-to-date information and generating responses based on that information. This way, you get answers that are not only accurate but also current and relevant. Let’s talk about why RAG is a big deal in the AI world:
- Enhanced Accuracy : RAG improves the accuracy of AI-generated responses by pulling in specific, up-to-date information before generating text. This reduces errors and ensures that the information provided is precise and reliable.
- Increased Relevance : By using the latest information from its retrieval component, RAG ensures that the responses are relevant and timely. This is particularly important in fast-moving fields like technology and finance, where staying current is crucial.
- Better Context Understanding : RAG can generate responses that make sense in the given context by utilizing relevant data. For example, it can tailor explanations to fit the needs of a student asking about a specific homework problem.
- Reducing AI Hallucinations : AI hallucinations occur when models generate content that sounds plausible but is factually incorrect or nonsensical. Since RAG relies on retrieving factual information from a database, it helps mitigate this problem, leading to more reliable and accurate responses.
Here’s a simple comparison to show how RAG stands out from traditional generative models:
| Feature | Traditional Generative Models | Retrieval-Augmented Generation (RAG) |
|---|---|---|
| Information Source | Generates text based on training data alone | Retrieves up-to-date information from a large database |
| Accuracy | May produce errors or outdated info | Provides precise and current information |
| Relevance | Depends on the model's training | Uses relevant data to ensure answers are timely and useful |
| Context Understanding | May lack context-specific details | Uses retrieved data to generate context-aware responses |
| Handling AI Hallucinations | Prone to generating incorrect or nonsensical content | Reduces errors by using factual information from retrieval |
In summary, RAG combines retrieval and generation to create AI responses that are accurate, relevant, and contextually appropriate, while also reducing the likelihood of generating incorrect information. Think of it as having a super-smart friend who’s always up-to-date and can explain things clearly. Really convenient, right?
Technical Overview of Retrieval-Augmented Generation (RAG)
In this section, we’ll be diving into the technical aspects of RAG, focusing on its core components, architecture, and implementation.
Key Components of RAG
- BM25 : This model improves the effectiveness of search by ranking documents based on term frequency and document length, making it a powerful tool for retrieving relevant information from large datasets.
- Dense Retrieval : Uses advanced neural network and deep learning techniques to understand and retrieve information based on semantic meaning rather than just keywords. This approach, powered by models like BERT, enhances the relevance of the retrieved content.
- GPT-3 : Known for its ability to produce highly coherent and contextually appropriate text. It generates responses based on the input it receives, leveraging its extensive training data.
- T5 : Converts various NLP tasks into a text-to-text format, which allows it to handle a broad range of text generation tasks effectively.
There are other such models that are available which offer unique strengths and are also widely used in various applications.
How RAG Works: Step-by-Step Flow
- User Input : The process begins when a user submits a query or request.
- Search : The retrieval model (e.g., BM25 or Dense Retrieval) searches through a large dataset to find documents relevant to the query.
- Selection : The most pertinent documents are selected from the search results.
- Input Processing : The selected documents are passed to the generative model (e.g., GPT-3 or T5).
- Response Generation : The generative model creates a coherent response based on the retrieved information and the user’s query.
- Output : The final response is delivered to the user, combining the retrieved data with the generative model’s capabilities.
RAG Architecture
Data flows from the input query to the retrieval component, which extracts relevant information. This data is then passed to the generation component, which creates the final output, ensuring that the response is both accurate and contextually relevant.
Implementing RAG
For practical implementation:
- Hugging Face Transformers : A robust library that simplifies the use of pre-trained models for both retrieval and generation tasks. It provides user-friendly tools and APIs to build and integrate RAG systems efficiently. Additionally, you can find various repositories and resources related to RAG on platforms like GitHub for further customization and implementation guidance.
- LangChain : Another valuable tool for implementing RAG systems. LangChain provides an easy way to manage the interactions between retrieval and generation components, enabling more seamless integration and enhanced functionality for applications utilizing RAG. For more information on LangChain and how it can support your RAG projects, check out our detailed blog post here .
For a comprehensive guide on setting up your own RAG system, check out our blog, "Building a Retrieval-Augmented Generation (RAG) App: A Step-by-Step Tutorial", which offers detailed instructions and example code.
Applications of Retrieval-Augmented Generation (RAG)
Retrieval-Augmented Generation (RAG) isn’t just a fancy term—it’s a transformative technology with practical applications across various fields. Let’s dive into how RAG is making a difference in different industries and some real-world examples that showcase its potential and AI applications.
Industry-Specific Applications
Customer Support Imagine chatting with a support bot that actually understands your problem and gives you spot-on answers. RAG enhances customer support by pulling in precise information from vast databases, allowing chatbots to provide more accurate and contextually relevant responses. No more vague answers or repeated searches; just quick, helpful solutions.
Content Creation Content creators know the struggle of finding just the right information quickly. RAG helps by generating content that is not only contextually accurate but also relevant to current trends. Whether it’s drafting blog posts, creating marketing copy, or writing reports, RAG assists in producing high-quality, targeted content efficiently.
Healthcare In healthcare, timely and accurate information can be a game-changer. RAG can assist doctors and medical professionals by retrieving and summarizing the latest research and treatment guidelines. . This makes RAG highly effective in domain-specific fields like medicine, where staying updated with the latest advancements is crucial.
Education Think of RAG as a supercharged tutor. It can tailor educational content to each student’s needs by retrieving relevant information and generating explanations that match their learning style. From personalized tutoring sessions to interactive learning materials, RAG makes education more engaging and effective.
Implementing a RAG App is one option. Another is getting on a call with us so we can help create a tailored solution for your RAG needs. Discover how Nanonets can automate customer support workflows using custom AI and RAG models.
Automate your customer support using Nanonets' RAG models
Automated FAQ Generation Ever visited a website with a comprehensive FAQ section that seemed to answer every possible question? RAG can automate the creation of these FAQs by analyzing a knowledge base and generating accurate responses to common questions. This saves time and ensures that users get consistent, reliable information.
Document Management Managing a vast array of documents within an enterprise can be daunting. RAG systems can automatically categorize, summarize, and tag documents, making it easier for employees to find and utilize the information they need. This enhances productivity and ensures that critical documents are accessible when needed.
Financial Data Analysis In the financial sector, RAG can be used to sift through financial reports, market analyses, and economic data. It can generate summaries and insights that help financial analysts and advisors make informed investment decisions and provide accurate recommendations to clients.
Research Assistance Researchers often spend hours sifting through data to find relevant information. RAG can streamline this process by retrieving and summarizing research papers and articles, helping researchers quickly gather insights and stay focused on their core work.
Best Practices and Challenges in Implementing RAG
In this final section, we’ll look at the best practices for implementing Retrieval-Augmented Generation (RAG) effectively and discuss some of the challenges you might face.
Best Practices
- Data Quality Ensuring high-quality data for retrieval is crucial. Poor-quality data leads to poor-quality responses. Always use clean, well-organized data to feed into your retrieval models. Think of it as cooking—you can’t make a great dish with bad ingredients.
- Model Training Training your retrieval and generative models effectively is key to getting the best results. Use a diverse and extensive dataset to train your models so they can handle a wide range of queries. Regularly update the training data to keep the models current.
- Evaluation and Fine-Tuning Regularly evaluate the performance of your RAG models and fine-tune them as necessary. Use metrics like precision, recall, and F1 score to gauge accuracy and relevance. Fine-tuning helps in ironing out any inconsistencies and improving overall performance.
- Handling Large Datasets Managing and retrieving data from large datasets can be challenging. Efficient indexing and retrieval techniques are essential to ensure quick and accurate responses. An analogy here can be finding a book in a massive library—you need a good catalog system.
- Contextual Relevance Ensuring that the generated responses are contextually relevant and accurate is another challenge. Sometimes, the models might generate responses that are off the mark. Continuous monitoring and tweaking are necessary to maintain relevance.
- Computational Resources RAG models, especially those utilizing deep learning, require significant computational resources, which can be expensive and demanding. Efficient resource management and optimization techniques are essential to keep the system running smoothly without breaking the bank.
Recap of Key Points: We’ve explored the fundamentals of RAG, its technical overview, applications, and best practices and challenges in implementation. RAG’s ability to combine retrieval and generation makes it a powerful tool in enhancing the accuracy and relevance of AI-generated content.
The future of RAG is bright, with ongoing research and development promising even more advanced models and techniques. As RAG continues to evolve, we can expect even more accurate and contextually aware AI systems.
Found the blog informative? Have a specific use case for building a RAG solution? Our experts at Nanonets can help you craft a tailored and efficient solution. Schedule a call with us today to get started!
Related content


IMAGES
COMMENTS
Abstract. Memory retrieval involves the interaction between external sensory or internally generated cues and stored memory traces (or engrams) in a process termed 'ecphory'. While ecphory has been examined in human cognitive neuroscience research, its neurobiological foundation is less understood. To the extent that ecphory involves ...
Recall: This type of memory retrieval involves being able to access the information without being cued. Answering a question on a fill-in-the-blank test is a good example of recall. Recollection: This type of memory retrieval involves reconstructing memory, often utilizing logical structures, partial memories, narratives, or clues. For example, writing an answer on an essay exam often involves ...
Nature Reviews Psychology (2023) Accurately retrieving information from memory boosts later retrieval. However, retrieving memories can also open a window to errors when erroneous information is ...
Memory is the term given to the structures and processes involved in the storage and subsequent retrieval of information. Memory is essential to all our lives. Without a memory of the past, we cannot operate in the present or think about the future. We would not be able to remember what we did yesterday, what we have done today, or what we plan ...
This paper explores memory from a cognitive neuroscience perspective and examines associated neural mechanisms. It examines the different types of memory: working, declarative, and non-declarative, and the brain regions involved in each type. The paper highlights the role of different brain regions, such as the prefrontal cortex in working ...
Memories are stored in long-term memory and retrieved through activation by retrieval cues. Retrieval fails for several reasons, like context mismatch, overgrown pathways, and interference. We can ...
There are three main processes that characterize how memory works. These processes are encoding, storage, and retrieval (or recall). Encoding . Encoding refers to the process through which information is learned. That is, how information is taken in, understood, and altered to better support storage (which you will look at in Section 3.1.2).
Retrieving a memory seems to involve a set of distinct processes, with different neural substrates, that are coordinated to orchestrate the act of remembering. Recent advances in cognitive science ...
Abstract. Memory retrieval involves the interaction between external sensory or internally generated cues and stored memory traces (or engrams) in a process termed 'ecphory'. While ecphory has ...
Memory is the process of retaining of knowledge over a period for the function of affecting future actions. It can be divided into declarative and procedural types. The process of memory consolidation is done in the hippocampus. The long-term memories are spread among various areas of the cerebrum depending on the different perceptual ...
Spontaneous Retrieval in Prospective Memory. In J. S. Nairne (Ed.), The foundations of remembering: Essays in honor of Henry L. Roediger, III (pp. 225-240). Psychology Press. Abstract. This chapter focuses on episodic memory. The authors take a broad view that episodic memory also allows people to mentally place themselves forward in time.
There are three ways you can retrieve information out of your long-term memory storage system: recall, recognition, and relearning. Recall is what we most often think about when we talk about memory retrieval: it means you can access information without cues. For example, you would use recall for an essay test.
After the encoding process information is stored so that it can be retrieved when needed (Brem, Ran & Pascual-leone, 2013). This essay will explore the three different types of memory mentioned earlier and how the encoding, storage and retrieval model explains the process of forming, maintaining and recalling memories.
Retrieval cues can be external, such as an image, text, a scent, or some other stimulus that relates to the memory. They can also be internal, such as a thought or sensation that is relevant to ...
We outline a theoretical framework encompassing the relationship between encoding and retrieval processes in episodic memory. There are no cortical regions or networks that are specialized for encoding; rather, successful encoding depends on the same regions that are engaged during on-line processing.
Memory is the encoding, storage, and retrieval in the human mind of past experiences. The basic pattern of remembering involves attention to an event followed by representation of that event in the brain. Repeated attention, or practice, enables activities such as playing a musical instrument or recitation of a poem.
Abstract. One of the key goals of memory research is to develop a basic understanding of the nature and characteristics of memory processes and systems. Another important goal is to develop useful applications of basic research to everyday life. This editorial considers two lines of work that illustrate some of the prospects for applying memory ...
Retrieval practice is a learning technique that repeatedly has been shown to enhance long-term retention when compared to other methods of learning, such as re-reading (Roediger & Karpicke, 2006a; Wiklund-Hörnqvist et al., 2014), group discussions (Stenlund et al., 2017), and concept mapping (Karpicke & Blunt, 2011).This retrieval-based benefit on long-term learning is commonly denoted as the ...
Decay Theory of Forgetting. According to the trace theory of memory, physical and chemical changes in the brain results in a memory "trace." Information in short-term memory lasts several seconds and if it is not rehearsed, the neurochemical memory trace quickly fades. According to the trace decay theory of forgetting, the events that happen ...
Memory of episodes (events) is at the core of psychological research on memory. This type of memory encompasses events that an individual has experienced and thus is a collection of experiences that occurred at a specific time and place (Tulving, Citation 1972, Citation 1983, Citation 2002).The retrieval of information is typically different for verbally encoded events versus those encoded in ...
Decent Essays. 686 Words. 3 Pages. Open Document. Memory retrieval is likely to be good after repeated testing of that material. In fact, practising retrieval has a larger effect on memory than revising the information (Hockley, 2009). Another factor that influences the quality of retrieved information is the way one studied the material.
c. Psychologists describe memory as. Multiple choice question. the process of focusing on some stimuli while ignoring others. the retention of information or experience over time. the process by which information comes out of storage. the process by which information gets into storage. b.
Right VLPFC supports memory encoding and retrieval of visuospatial stimuli, action imitation and motor inhibition (Levy and Wagner, 2011). Inhibition of distracting emotions (right VLPFC for inhibition of negative emotions) (Dolcos and McCarthy, 2006). mPFC: 25, 32: Learning, memory, and decision-making (Euston et al., 2012; Brod et al., 2013).
Retrieval-Augmented Generation (RAG) is a smart approach in AI to improve the accuracy and credibility of Generative AI and LLM models. ... Imagine you're writing an essay and need to include accurate, up-to-date information. Instead of relying on your memory alone, you use a tool that first looks up the latest facts from a huge library of ...