AIDS Research and Therapy
Call for papers, 20th anniversary collection, covid-19 and hiv: clinical presentation, outcomes and impact on clinical services, treatment outcomes and paving the way for an hiv cure in low and middle income countries, spatial inequality, infectious diseases and disease control.
We are excited to announce the 20th Anniversary issue of journal AIDS Research & Therapy. We invite manuscripts focused on latest research that may influence policy into curbing epidemic.
This collection is no longer accepting submissions

This new thematic series aims to explore the clinical presentation and outcomes of COVID-19 in people with HIV, and the impact of COVID-19 on services and treatment outcomes for people living with HIV, in both high- and low-resource settings.
Currently open for submissions - submit to the series here

Discussion of HIV treatment outcomes in LMICs has historically been neglected, even though it is in these countries that a cure is most crucial. This series covers the ways treatment outcomes can be improved along the way to a HIV cure in states defined as LMICs by the WHO.
Cross-journal collection
This collection focuses on emerging infectious diseases in humans and animals, including the impact of antimicrobial resistance, and brings together research that investigates the relationship between spatial inequalities of all kinds and the impact and prevalence of these infectious diseases. This collection also welcomes papers that seek solutions towards disease control across areas with particularly unequal distribution of resources and opportunities.
Currently open for submissions - submit to the series here

Featured Article: Standard of care in advanced HIV disease: review of HIV treatment guidelines in six sub-Saharan African countries
In this article the authors aimed to review national guidelines for AHD management across six selected countries in sub-Saharan Africa for benchmarking against the 2021 WHO recommendations.
- Most accessed
Efficacy and safety of switching to dolutegravir/lamivudine in virologically suppressed people with HIV-1 aged ≥ 50 years: week 48 pooled results from the TANGO and SALSA studies
Authors: Sharon Walmsley, Don E. Smith, Miguel Górgolas, Pedro E. Cahn, Thomas Lutz, Karine Lacombe, Princy N. Kumar, Brian Wynne, Richard Grove, Gilda Bontempo, Riya Moodley, Chinyere Okoli, Michelle Kisare, Bryn Jones, Andrew Clark and Mounir Ait-Khaled
Assessing high-risk sexual practices associated with human immunodeficiency virus infection among young female sex workers in Lubumbashi, Democratic Republic of the Congo: a cross-sectional study
Authors: Olivier Mukuku, Yannick Nkiambi Kiakuvue, Georges Yumba Numbi, Bienvenu Mukuku Ruhindiza, Christian Kakisingi, Claude Mulumba Mwamba and Joe Kabongo Katabwa
Factors influencing rapid antiretroviral therapy initiation in Jiulongpo, Chongqing, China: a retrospective cohort from 2018 to 2022
Authors: Cheng Chen, Hao Chen, Lingli Wu, Qin Gong and Jingchun He
Support, not blame: safe partner disclosure among women diagnosed with HIV late in pregnancy in South Africa and Uganda
Authors: Adelline Twimukye, Yussif Alhassan, Beate Ringwald, Thokozile Malaba, Landon Myer, Catriona Waitt, Mohammed Lamorde, Helen Reynolds, Saye Khoo and Miriam Taegtmeyer
Diffuse myocardial fibrosis is uncommon in people with perinatally acquired human immunodeficiency virus infection
Authors: Jason L. Williams, Frances Hung, Elizabeth Jenista, Piers Barker, Hrishikesh Chakraborty, Raymond Kim, Andrew W. McCrary, Svati H. Shah, Nathan Thielman and Gerald S. Bloomfield
Most recent articles RSS
View all articles
Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options
Authors: David M Murdoch, Willem DF Venter, Annelies Van Rie and Charles Feldman
The biology of how circumcision reduces HIV susceptibility: broader implications for the prevention field
Authors: Jessica L. Prodger and Rupert Kaul
Assessing the sensitivity and specificity of First Response HIV-1-2 test kit with whole blood and serum samples: a cross-sectional study
Authors: Raymond Boadu, George Darko, Priscilla Nortey, Patricia Akweongo and Bismark Sarfo
Repeat HIV testing of individuals with discrepant HIV self-test results in Central Uganda
Authors: Rose Kisa, Joseph K. B. Matovu, Esther Buregyeya, William Musoke, Caroline J. Vrana-Diaz, Jeffrey E. Korte and Rhoda K. Wanyenze
Women and HIV in Sub-Saharan Africa
Authors: Gita Ramjee and Brodie Daniels
Most accessed articles RSS
SARS-CoV-2 and COVID-19
Find a selection of articles published across Springer Nature, as well as additional commentary and books relevant to SARS-CoV-2 and COVID-19 research.
Aims and scope
Announcing the launch of in review.
AIDS Research and Therapy , in partnership with Research Square, is now offering In Review. Authors choosing this free optional service will be able to:
- Share their work with fellow researchers to read, comment on, and cite even before publication
- Showcase their work to funders and others with a citable DOI while it is still under review
- Track their manuscript - including seeing when reviewers are invited, and when reports are received
From the Blog
World aids day 2023: highlights from the bmc series.
01 December 2023
“Yathu Yathu”(For us, By us!): an innovative and co-designed intervention to deliver peer-led community-based sexual and reproductive health service to adolescents and young people in Lusaka, Zambia
22 November 2022
A Personalized Approach to HIV
14 January 2022

Dr Barbara Castelnuovo - Editor in Chief
Dr Barbara Castelnuovo is an Italian/Ugandan clinical researcher. She is a specialist in Infectious Diseases trained at the University of Milan, with a PhD in Medical Sciences from the University of Antwerp. She has worked as an HIV specialist at the Infectious Diseases Institute, Makerere University, Uganda since 2004 and she is currently the Head of the Research Department. Dr Castelnuovo has designed and implemented several clinical observational studies, and clinical trials. Her research interest are HIV long term outcomes, models of care and research capacity building.

Dr Patricia Price - Editor in Chief
A/Prof Patricia Price is an Adjunct Principal Research Fellow at Curtin University, coordinating research initiatives at Curtin University in Western Australia and at the University of Indonesia. She has published over 260 papers in international journals.
Current projects address how the varied manifestations and consequences of CMV infection arise from the infection of very few cells – evaluating the roles of immune activation, CMV burden and NK cells in healthy aging donors, HIV patients and renal transplant recipients. Recent studies in collaboration with Dr Silvia Lee address the potential of γδ T-cells to mediate the effects of CMV on cardiovascular and neurocognitive health. The group are now studying variations in HCMV-encoded homologues of immune-related genes in clinical samples.
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- SNAPP Editorial Login
- Contact Support for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
2022 Citation Impact 2.2 - 2-year Impact Factor 2.6 - 5-year Impact Factor 0.933 - SNIP (Source Normalized Impact per Paper) 0.740 - SJR (SCImago Journal Rank)
2023 Speed 9 days submission to first editorial decision for all manuscripts (Median) 117 days submission to accept (Median)
2023 Usage 682,500 downloads 671 Altmetric mentions
- More about our metrics
ISSN: 1742-6405
- Submission enquiries: [email protected]
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Research & training, advances in hiv/aids research.
For an update on what medical science is doing to fight the global HIV/AIDS pandemic, read a Parade article by NIH Director Francis S. Collins and NIAID Director Anthony S. Fauci, AIDS in 2010: How We're Living with HIV .
Over the past several decades, researchers have learned a lot about the human immunodeficiency virus (HIV) and the disease it causes, acquired immunodeficiency syndrome (AIDS). But still more research is needed to help the millions of people whose health continues to be threatened by the global HIV/AIDS pandemic.
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as well as to better understand the virus with the goal of finding a cure. For example, in recent months, NIAID and its partners made progress toward finding a vaccine to prevent HIV infection. Check out other promising areas of NIAID-funded research on HIV/AIDS at http://www.niaid.nih.gov/topics/hivaids/Pages/Default.aspx .
Other NIH institutes, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute on Alcohol Abuse and Alcoholism, also support research to better control and ultimately end the HIV/AIDS pandemic. Some of these researchers have found a simple, cost-effective way to cut HIV transmission from infected mothers to their breastfed infants. Others have developed an index to help measure the role of alcohol consumption in illness and death of people with HIV/AIDS.
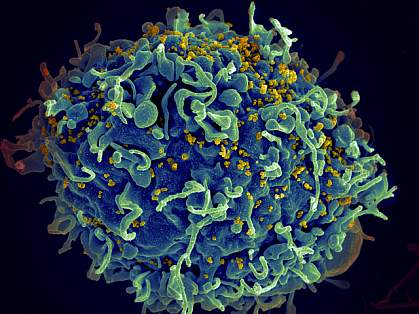
Find out more about these discoveries and what they mean for improving the health of people in the United States and all around the globe.
This page last reviewed on August 20, 2015
Connect with Us
- More Social Media from NIH
AIDS Research and Therapy
Call for papers, 20th anniversary collection, covid-19 and hiv: clinical presentation, outcomes and impact on clinical services, treatment outcomes and paving the way for an hiv cure in low and middle income countries, spatial inequality, infectious diseases and disease control.

We are excited to announce the 20th Anniversary issue of journal AIDS Research & Therapy. We invite manuscripts focused on latest research that may influence policy into curbing epidemic.
This collection is no longer accepting submissions

This new thematic series aims to explore the clinical presentation and outcomes of COVID-19 in people with HIV, and the impact of COVID-19 on services and treatment outcomes for people living with HIV, in both high- and low-resource settings.
Currently open for submissions - submit to the series here

Discussion of HIV treatment outcomes in LMICs has historically been neglected, even though it is in these countries that a cure is most crucial. This series covers the ways treatment outcomes can be improved along the way to a HIV cure in states defined as LMICs by the WHO.

Cross-journal collection
This collection focuses on emerging infectious diseases in humans and animals, including the impact of antimicrobial resistance, and brings together research that investigates the relationship between spatial inequalities of all kinds and the impact and prevalence of these infectious diseases. This collection also welcomes papers that seek solutions towards disease control across areas with particularly unequal distribution of resources and opportunities.
Currently open for submissions - submit to the series here
Featured Article: Standard of care in advanced HIV disease: review of HIV treatment guidelines in six sub-Saharan African countries
In this article the authors aimed to review national guidelines for AHD management across six selected countries in sub-Saharan Africa for benchmarking against the 2021 WHO recommendations.
- Most accessed
Efficacy and safety of switching to dolutegravir/lamivudine in virologically suppressed people with HIV-1 aged ≥ 50 years: week 48 pooled results from the TANGO and SALSA studies
Authors: Sharon Walmsley, Don E. Smith, Miguel Górgolas, Pedro E. Cahn, Thomas Lutz, Karine Lacombe, Princy N. Kumar, Brian Wynne, Richard Grove, Gilda Bontempo, Riya Moodley, Chinyere Okoli, Michelle Kisare, Bryn Jones, Andrew Clark and Mounir Ait-Khaled
Assessing high-risk sexual practices associated with human immunodeficiency virus infection among young female sex workers in Lubumbashi, Democratic Republic of the Congo: a cross-sectional study
Authors: Olivier Mukuku, Yannick Nkiambi Kiakuvue, Georges Yumba Numbi, Bienvenu Mukuku Ruhindiza, Christian Kakisingi, Claude Mulumba Mwamba and Joe Kabongo Katabwa
Factors influencing rapid antiretroviral therapy initiation in Jiulongpo, Chongqing, China: a retrospective cohort from 2018 to 2022
Authors: Cheng Chen, Hao Chen, Lingli Wu, Qin Gong and Jingchun He
Support, not blame: safe partner disclosure among women diagnosed with HIV late in pregnancy in South Africa and Uganda
Authors: Adelline Twimukye, Yussif Alhassan, Beate Ringwald, Thokozile Malaba, Landon Myer, Catriona Waitt, Mohammed Lamorde, Helen Reynolds, Saye Khoo and Miriam Taegtmeyer
Diffuse myocardial fibrosis is uncommon in people with perinatally acquired human immunodeficiency virus infection
Authors: Jason L. Williams, Frances Hung, Elizabeth Jenista, Piers Barker, Hrishikesh Chakraborty, Raymond Kim, Andrew W. McCrary, Svati H. Shah, Nathan Thielman and Gerald S. Bloomfield
Most recent articles RSS
View all articles
Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options
Authors: David M Murdoch, Willem DF Venter, Annelies Van Rie and Charles Feldman
The biology of how circumcision reduces HIV susceptibility: broader implications for the prevention field
Authors: Jessica L. Prodger and Rupert Kaul
Assessing the sensitivity and specificity of First Response HIV-1-2 test kit with whole blood and serum samples: a cross-sectional study
Authors: Raymond Boadu, George Darko, Priscilla Nortey, Patricia Akweongo and Bismark Sarfo
Repeat HIV testing of individuals with discrepant HIV self-test results in Central Uganda
Authors: Rose Kisa, Joseph K. B. Matovu, Esther Buregyeya, William Musoke, Caroline J. Vrana-Diaz, Jeffrey E. Korte and Rhoda K. Wanyenze
Women and HIV in Sub-Saharan Africa
Authors: Gita Ramjee and Brodie Daniels
Most accessed articles RSS

SARS-CoV-2 and COVID-19
Find a selection of articles published across Springer Nature, as well as additional commentary and books relevant to SARS-CoV-2 and COVID-19 research.
Aims and scope
Announcing the launch of in review.
AIDS Research and Therapy , in partnership with Research Square, is now offering In Review. Authors choosing this free optional service will be able to:
- Share their work with fellow researchers to read, comment on, and cite even before publication
- Showcase their work to funders and others with a citable DOI while it is still under review
- Track their manuscript - including seeing when reviewers are invited, and when reports are received
From the Blog
World aids day 2023: highlights from the bmc series.
01 December 2023
“Yathu Yathu”(For us, By us!): an innovative and co-designed intervention to deliver peer-led community-based sexual and reproductive health service to adolescents and young people in Lusaka, Zambia
22 November 2022
A Personalized Approach to HIV
14 January 2022

Dr Barbara Castelnuovo - Editor in Chief
Dr Barbara Castelnuovo is an Italian/Ugandan clinical researcher. She is a specialist in Infectious Diseases trained at the University of Milan, with a PhD in Medical Sciences from the University of Antwerp. She has worked as an HIV specialist at the Infectious Diseases Institute, Makerere University, Uganda since 2004 and she is currently the Head of the Research Department. Dr Castelnuovo has designed and implemented several clinical observational studies, and clinical trials. Her research interest are HIV long term outcomes, models of care and research capacity building.

Dr Patricia Price - Editor in Chief
A/Prof Patricia Price is an Adjunct Principal Research Fellow at Curtin University, coordinating research initiatives at Curtin University in Western Australia and at the University of Indonesia. She has published over 260 papers in international journals.
Current projects address how the varied manifestations and consequences of CMV infection arise from the infection of very few cells – evaluating the roles of immune activation, CMV burden and NK cells in healthy aging donors, HIV patients and renal transplant recipients. Recent studies in collaboration with Dr Silvia Lee address the potential of γδ T-cells to mediate the effects of CMV on cardiovascular and neurocognitive health. The group are now studying variations in HCMV-encoded homologues of immune-related genes in clinical samples.
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- SNAPP Editorial Login
- Contact Support for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
2022 Citation Impact 2.2 - 2-year Impact Factor 2.6 - 5-year Impact Factor 0.933 - SNIP (Source Normalized Impact per Paper) 0.740 - SJR (SCImago Journal Rank)
2023 Speed 9 days submission to first editorial decision for all manuscripts (Median) 117 days submission to accept (Median)
2023 Usage 682,500 downloads 671 Altmetric mentions
- More about our metrics
ISSN: 1742-6405
- Submission enquiries: [email protected]

Volume 18, issue 1, December 2021
100 articles in this issue
Impact of using creative arts programming to support HIV treatment in adolescents and young adults in Eswatini
Authors (first, second and last of 8).
- Tara E. Ness
- Vedika Agrawal
- Bhekumusa Lukhele
- Content type: Research
- Open Access
- Published: 20 December 2021
- Article: 100
A systematic review and meta-analysis of HIV associated neurocognitive disorders (HAND) among people with HIV in Ethiopia
Authors (first, second and last of 4).
- Yosef Zenebe
- Mogesie Necho
- Content type: Review
- Published: 19 December 2021
- Article: 99
Combination antiretroviral therapy is associated with reduction in liver fibrosis scores in patients with HIV and HBV co-infection
Authors (first, second and last of 5).
- Rongrong Yang
- Shicheng Gao
- Article: 98
Trends and correlates of HIV prevalence among adolescents in South Africa: evidence from the 2008, 2012 and 2017 South African National HIV Prevalence, Incidence and Behaviour surveys
Authors (first, second and last of 9).
- Musawenkosi Mabaso
- Goitseone Maseko
- Nompumelelo Zungu
- Published: 14 December 2021
- Article: 97
HIV/AIDS late presentation and its associated factors in China from 2010 to 2020: a systematic review and meta-analysis
- Chengqing Sun
- Gengfeng Fu
- Published: 11 December 2021
- Article: 96
- Spatial inequality, infectious diseases and disease control
The lived experience of HIV-infected patients in the face of a positive diagnosis of the disease: a phenomenological study
- Behzad Imani
- Shirdel Zandi
- Mohamad Mirzaei
- Published: 07 December 2021
- Article: 95
Renal function in Japanese HIV-1-positive patients who switch to tenofovir alafenamide fumarate after long-term tenofovir disoproxil fumarate: a single-center observational study
- Kensuke Abe
- Nobuyuki Takahashi
- Article: 94
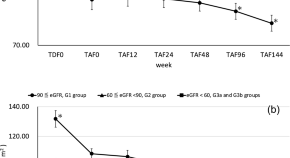
Time to initial highly active antiretroviral therapy discontinuation and its predictors among HIV patients in Felege Hiwot comprehensive specialized hospital: a retrospective cohort study
- Tewodros Getnet Amera
- Kassawmar Angaw Bogale
- Yibekal Manaye Tefera
- Published: 04 December 2021
- Article: 93
- Treatment outcomes and paving the way for an HIV cure in Low and Middle Income Countries
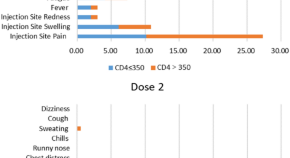
Adverse events of inactivated COVID-19 vaccine in HIV-infected adults
- Yubin Zhang
- Content type: Short Report
- Article: 92
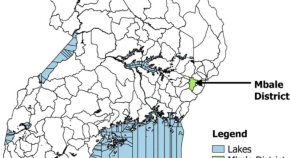
Determinants of viral load non-suppression among adolescents in Mbale District, Eastern Rural Uganda
- Aduragbemi Banke-Thomas
- Rita Nakalega
- Article: 91
Protein-losing enteropathy caused by disseminated Mycobacterium avium complex infection in a patient receiving antiretroviral therapy: an autopsy case report
Authors (first, second and last of 6).
- Keiji Konishi
- Hidenori Nakagawa
- Michinori Shirano
- Content type: Case Report
- Published: 29 November 2021
- Article: 90

Perceived satisfaction with HIV care and its association with adherence to antiretroviral therapy and viral suppression in the African Cohort Study
Authors (first, second and last of 17).
- Nicole Dear
- the AFRICOS Study Group
- Published: 25 November 2021
- Article: 89
Communication needs for improved uptake of PrEP and HIVST services among key populations in Nigeria: a mixed-method study
- Olawale Durosinmi-Etti
- Emmanuel Kelechi Nwala
- Abiye Kalaiwo
- Published: 20 November 2021
- Article: 88
Disability and self-care living strategies among adults living with HIV during the COVID-19 pandemic
Authors (first, second and last of 13).
- Kelly K. O’Brien
- Ahmed M. Bayoumi
- Steven E. Hanna
- Published: 19 November 2021
- Article: 87
- COVID-19 and HIV: clinical presentation, outcomes and impact on clinical services
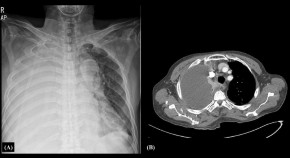
Actinomycosis presenting as an isolated pleural effusion in a patient with an HIV infection: a case report and literature review
- Jung Wan Park
- Yon Hee Kim
- Tae Hyong Kim
- Published: 17 November 2021
- Article: 86
Barriers to and enablers of uptake of antiretroviral therapy in integrated HIV and tuberculosis treatment programmes in sub-Saharan Africa: a systematic review and meta-analysis
- Benjamin Momo Kadia
- Christian Akem Dimala
- Adrian D. Smith
- Published: 16 November 2021
- Article: 85
Patient characteristics and determinants of CD4 at diagnosis of HIV in Mexico from 2008 to 2017: a 10-year population-based study
- Amilcar Azamar-Alonso
- Sergio A. Bautista-Arredondo
- Jean-Eric Tarride
- Published: 13 November 2021
- Article: 84
Cytomegalovirus may influence vascular endothelial health in Indonesian HIV-infected patients after 5 years on ART
Authors (first, second and last of 10).
- Ika Prasetya Wijaya
- Birry Karim
- Patricia Price
- Published: 11 November 2021
- Article: 83
Reconstruction and repair, using mini-plate and bone graft for persons living with HIV with giant cell tumor of long bone: retrospective analysis of a single-center experience
- Published: 02 November 2021
- Article: 82
Factors associated with antiretroviral therapy adherence among people living with HIV in Haiti: a cross-sectional study
- Ludentz Dorcélus
- Joseph Bernard Jr.
- Clerveau Vanessa
- Article: 81
Safety and effectiveness of switching to Abacavir/Lamivudine plus rilpivirine for maintenance therapy in virologically suppressed HIV-1 individuals in Singapore (SEALS)
- Published: 01 November 2021
- Article: 80
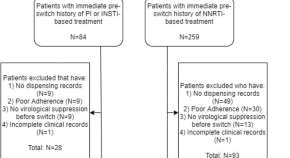
Factors associated with viremia in people living with HIV on antiretroviral therapy in Guatemala
Authors (first, second and last of 11).
- Dean W. Ortíz
- Olivia Roberts-Sano
- Jane A. O´Halloran
- Published: 27 October 2021
- Article: 79
Acute HIV infection syndrome mimicking COVID-19 vaccination side effects: a case report
- Julian Triebelhorn
- Stefanie Haschka
- Christoph D. Spinner
- Published: 26 October 2021
- Article: 78
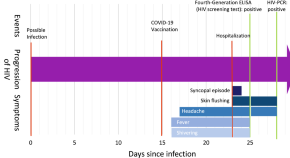
Ribonuclease zymogen induces cytotoxicity upon HIV-1 infection
- Ian W. Windsor
- Dawn M. Dudley
- Ronald T. Raines
- Article: 77
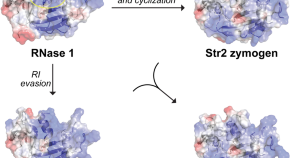
Long-term remission of AIDS-related primary central nervous system lymphoma in a patient under antiretroviral therapy: a case report and review of the literature
- Pieter-Jan Gijs
- Olivier Clerc
- Published: 19 October 2021
- Article: 76
Considerations for designing and implementing combination HIV cure trials: findings from a qualitative in-depth interview study in the United States
- Karine Dubé
- John Kanazawa
- Michael J. Peluso
- Published: 18 October 2021
- Article: 75
Acquired HIV drug resistance and virologic monitoring in a HIV hyper-endemic setting in KwaZulu-Natal Province, South Africa
Authors (first, second and last of 19).
- Benjamin Chimukangara
- Richard J. Lessells
- Kogieleum Naidoo
- Published: 16 October 2021
- Article: 74
Tocilizumab in HIV patient with severe COVID-19: case report
Authors (first, second and last of 7).
- Pedro Vieira Bertozzi
- Amanda de Oliveira Vicente
- Rodrigo Afonso da Silva Sardenberg
- Article: 73
Treatment outcomes among adults with HIV/non-communicable disease multimorbidity attending integrated care clubs in Cape Town, South Africa
- Blessings Gausi
- Natacha Berkowitz
- Published: 14 October 2021
- Article: 72
A retrospective study of survival and risk factors for mortality among people living with HIV who received antiretroviral treatment in a resource-limited setting
- Weerawat Manosuthi
- Lantharita Charoenpong
- Chalor Santiwarangkana
- Published: 12 October 2021
- Article: 71
HIV Care Coordination promotes care re-engagement and viral suppression among people who have been out of HIV medical care: an observational effectiveness study using a surveillance-based contemporaneous comparison group
- Mary K. Irvine
- McKaylee M. Robertson
- Bruce Levin
- Article: 70
Management of HIV-2 resistance to antiretroviral therapy in a HIV-1/HIV-2/HBV co-infected patient
- Margarida Cardoso
- Joana Vasconcelos
- Perpétua Gomes
- Article: 69
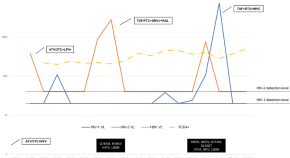
A systematic and meta-analysis of heterosexual behaviors and HIV prevalence among Chinese men who have sex with men
- Jiangping Zhang
- Published: 10 October 2021
- Article: 68
A realist evaluation of the continuum of HIV services for men who have sex with men
- Willy Dunbar
- Marie Colette Alcide Jean-Pierre
- Yves Coppieters
- Published: 09 October 2021
- Article: 67
Determinants of viral suppression among adolescents on antiretroviral treatment in Ehlanzeni district, South Africa: a cross-sectional analysis
- Emeka F. Okonji
- Brian van Wyk
- Gail D. Hughes
- Article: 66
MR spectroscopy in HIV associated neurocognitive disorder in the era of cART: a review
- Joga Chaganti
- Bruce James Brew
- Article: 65
Next generation sequencing based in-house HIV genotyping method: validation report
- Alisen Ayitewala
- Isaac Ssewanyana
- Charles Kiyaga
- Content type: Methodology
- Published: 02 October 2021
- Article: 64
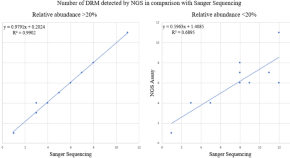
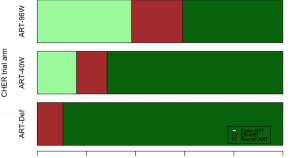
Early ART-initiation and longer ART duration reduces HIV-1 proviral DNA levels in children from the CHER trial
- Helen Payne
- Man K. Chan
- Nigel J. Klein
- Published: 29 September 2021
- Article: 63
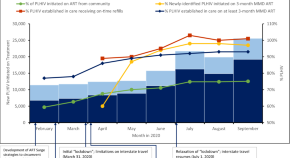
Expanding access to HIV services during the COVID-19 pandemic—Nigeria, 2020
Authors (first, second and last of 46).
- Andrew T. Boyd
- Ibrahim Jahun
- Mahesh Swaminathan
- Published: 19 September 2021
- Article: 62
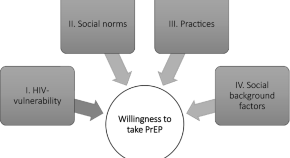
Underlying factors related to HIV/AIDS prevention: investigating the willingness to take pre-exposure prophylaxis among men-who-have-sex-with-men in Germany
- Michele Pazzini
- Zsófia S. Ignácz
- Julia Tuppat
- Published: 17 September 2021
- Article: 61
Associations between antiretroviral therapy-related experiences and mental health status among people living with HIV in China: a prospective observational cohort study
- Jinzhao Xie
- Published: 09 September 2021
- Article: 60
HIV diagnosis period influences ART initiation: findings from a prospective cohort study in China
- Tinglong Yang
- Xueying Yang
- Article: 59
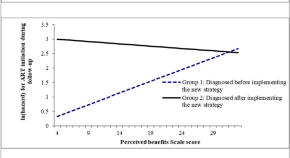
Virologic outcomes of switching to boosted darunavir plus dolutegravir with respect to history of drug resistance
Authors (first, second and last of 12).
- Christoph Boesecke
- the DUALIS Study Group
- Published: 08 September 2021
- Article: 58
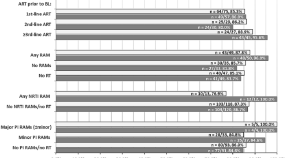
Acute myocardial infarction associated with abacavir and tenofovir based antiretroviral drug combinations in the United States
- Kunchok Dorjee
- Manisha Desai
- Arthur L. Reingold
- Published: 06 September 2021
- Article: 57
Lactic acidosis and hyperlactatemia associated with lamivudine accumulation and sepsis in a kidney transplant recipient—a case report and review of the literature
- Alexa Hollinger
- Nadine Cueni
- Anne Leuppi-Taegtmeyer
- Published: 04 September 2021
- Article: 56
Advanced baseline immunosuppression is associated with elevated levels of plasma markers of fungal translocation and inflammation in long-term treated HIV-infected Tanzanians
- Godfrey Barabona
- Macdonald Mahiti
- Takamasa Ueno
- Published: 26 August 2021
- Article: 55
Factors associated with North–South research collaboration focusing on HIV/AIDS: lessons from ClinicalTrials.gov
- Hesborn Wao
- Juliana A. Were
- Published: 25 August 2021
- Article: 54
Tenofovir alafenamide nephrotoxicity: a case report and literature review
- Thornthun Ueaphongsukkit
- Sivaporn Gatechompol
- Suwasin Udomkarnjananun
- Published: 21 August 2021
- Article: 53
Factors associated with testing for HIV and hepatitis C among behaviorally vulnerable men in Germany: a cross-sectional analysis upon enrollment into an observational cohort
- Trevor A. Crowell
- for the BRAHMS Study Team
- Published: 16 August 2021
- Article: 52
Closing gaps in histoplasmosis: clinical characteristics and factors associated with probable/histoplasmosis in HIV/AIDS hospitalized patients, a retrospective cross-sectional study in two tertiary centers in Pereira, Colombia
- Julián Andrés Hoyos Pulgarin
- John Alexander Alzate Piedrahita
- Deving Arias Ramos
- Published: 12 August 2021
- Article: 51
For authors
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 21 June 2023
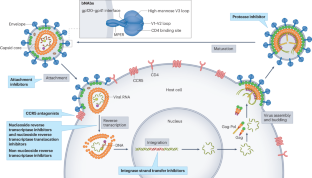
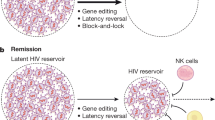
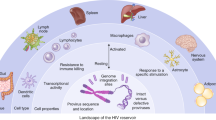
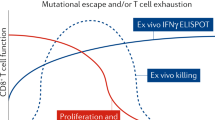
Prevention, treatment and cure of HIV infection
- Raphael J. Landovitz ORCID: orcid.org/0000-0002-1442-714X 1 ,
- Hyman Scott ORCID: orcid.org/0000-0001-8775-7214 2 , 3 &
- Steven G. Deeks ORCID: orcid.org/0000-0001-6371-747X 3
Nature Reviews Microbiology volume 21 , pages 657–670 ( 2023 ) Cite this article
8409 Accesses
15 Citations
199 Altmetric
Metrics details
- Antiviral agents
- HIV infections
The development of antiretroviral therapy for the prevention and treatment of HIV infection has been marked by a series of remarkable successes. However, the efforts to develop a vaccine have largely failed, and efforts to discover a cure are only now beginning to gain traction. In this Review, we describe recent progress on all fronts — pre-exposure prophylaxis, vaccines, treatment and cure — and we discuss the unmet needs, both current and in the coming years. We describe the emerging arsenal of drugs, biologics and strategies that will hopefully address these needs. Although HIV research has largely been siloed in the past, this is changing, as the emerging research agenda is marked by multiple cross-discipline synergies and collaborations. As the limitations of antiretroviral drugs as a means to truly end the epidemic are becoming more apparent, there is a great need for continued efforts to develop an effective preventative vaccine and a scalable cure, both of which remain formidable challenges.
This is a preview of subscription content, access via your institution

Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Why and where an HIV cure is needed and how it might be achieved
Thumbi Ndung’u, Joseph M. McCune & Steven G. Deeks
Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021
Steven G. Deeks, Nancie Archin, … The International AIDS Society (IAS) Global Scientific Strategy working group
CD8+ T cells in HIV control, cure and prevention
David R. Collins, Gaurav D. Gaiha & Bruce D. Walker
Landovitz, R. J. et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N. Engl. J. Med. 385 , 595–608 (2021).
CAS PubMed PubMed Central Google Scholar
Grant, R. M. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363 , 2587–2599 (2010).
Joint United Nations Programme on HIV/AIDS. Global HIV and AIDS statistics — 2022 fact sheet. UNAIDS https://www.unaids.org/en/resources/fact-sheet (2022).
Cohen, S. E. et al. Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report. Lancet HIV https://doi.org/10.1016/S2352-3018(18)30288-1 (2018).
Article PubMed PubMed Central Google Scholar
Cohen, S. M., Hu, X., Sweeney, P., Johnson, A. S. & Hall, H. I. HIV viral suppression among persons with varying levels of engagement in HIV medical care, 19 US jurisdictions. J. Acquir. Immune Defic. Syndr. 67 , 519–527 (2014).
PubMed Google Scholar
Havlir, D. V. et al. HIV testing and treatment with the use of a community health approach in rural Africa. N. Engl. J. Med. 381 , 219–229 (2019).
PubMed PubMed Central Google Scholar
Hayes, R. J. et al. Effect of universal testing and treatment on HIV incidence — HPTN 071 (PopART). N. Engl. J. Med. 381 , 207–218 (2019).
Makhema, J. et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N. Engl. J. Med. 381 , 230–242 (2019).
Cohen, M. S. et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 365 , 493–505 (2011).
Larmarange, J. et al. The impact of population dynamics on the population HIV care cascade: results from the ANRS 12249 Treatment as Prevention trial in rural KwaZulu-Natal (South Africa). J. Int. AIDS Soc. 21 , e25128 (2018).
Thigpen, M. C. et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med. 367 , 423–434 (2012).
CAS PubMed Google Scholar
Liu, A. Y. et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern. Med. 176 , 75–84 (2016).
Choopanya, K. et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 381 , 2083–2090 (2013).
Mayer, K. H. et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 396 , 239–254 (2020).
Molina, J. M. et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 Infection. N. Engl. J. Med. 373 , 2237–2246 (2015).
World Health Organization. Guidelines on Long-Acting Injectable Cabotegravir for HIV Prevention (WHO, 2022).
Gandhi, R. T. et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA panel. J. Am. Med. Assoc. 329 , 63–84 (2022).
Google Scholar
Van Damme, L. et al. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367 , 411–422 (2012).
Marrazzo, J. M. et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 372 , 509–518 (2015).
Abdool Karim, Q. et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329 , 1168–1174 (2010).
Baeten, J. M. et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367 , 399–410 (2012).
Baeten, J. M. et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N. Engl. J. Med. 375 , 2121–2132 (2016).
World Health Organization. WHO recommends the dapivirine vaginal ring as a new choice for HIV prevention for women at substantial risk of HIV infection. WHO https://www.who.int/news/item/26-01-2021-who-recommends-the-dapivirine-vaginal-ring-as-a-new-choice-for-hiv-prevention-for-women-at-substantial-risk-of-hiv-infection (2021).
Baeten, J. M., Hendrix, C. W. & Hillier, S. L. Topical microbicides in HIV prevention: state of the promise. Annu. Rev. Med. 71 , 361–377 (2020).
Delany-Moretlwe, S. et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet 399 , 1779–1789 (2022).
Marzinke, M. A. et al. Characterization of human immunodeficiency virus (HIV) infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J. Infect. Dis. 224 , 1581–1592 (2021).
Gulick, R. M. et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337 , 734–739 (1997).
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Clinicalinfo.HIV.gov https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new (2023).
Trickey, A. et al. Associations of modern initial antiretroviral drug regimens with all-cause mortality in adults with HIV in Europe and North America: a cohort study. Lancet HIV 9 , e404–e413 (2022).
Venter, W. D. F. et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N. Engl. J. Med. 381 , 803–815 (2019).
Editorial. PEPFAR looks to the future. Lancet HIV 9 , e367 (2022).
Orkin, C. et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 8 , e185–e196 (2021).
Christopoulos, K. A. et al. First demonstration project of long-acting injectable antiretroviral therapy for persons with and without detectable HIV viremia in an urban HIV clinic. Clin. Infect. Dis. 76 , e645–e651 (2022).
PubMed Central Google Scholar
Segal-Maurer, S. et al. Capsid Inhibition with lenacapavir in multidrug-resistant HIV-1 infection. N. Engl. J. Med. 386 , 1793–1803 (2022).
Benhabbour, S. R. et al. Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nat. Commun. 10 , 4324 (2019).
Kovarova, M. et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat. Commun. 9 , 4156 (2018).
Haynes, B. F., Burton, D. R. & Mascola, J. R. Multiple roles for HIV broadly neutralizing antibodies. Sci. Transl Med. 11 , eaaz2686 (2019).
Caskey, M., Klein, F. & Nussenzweig, M. C. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat. Med. 25 , 547–553 (2019).
Gaudinski, M. R. et al. Safety and pharmacokinetics of broadly neutralising human monoclonal antibody VRC07-523LS in healthy adults: a phase 1 dose-escalation clinical trial. Lancet HIV 6 , e667–e679 (2019).
Gaudinski, M. R. et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 15 , e1002493 (2018).
Corey, L. et al. Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition. N. Engl. J. Med. 384 , 1003–1014 (2021).
Sneller, M. C. et al. Combination anti-HIV antibodies provide sustained virological suppression. Nature 606 , 375–381 (2022).
Gaebler, C. et al. Prolonged viral suppression with anti-HIV-1 antibody therapy. Nature 606 , 368–374 (2022).
Pegu, A. et al. Potent anti-viral activity of a trispecific HIV neutralizing antibody in SHIV-infected monkeys. Cell Rep. 38 , 110199 (2022).
Collins, F. et al. The NIH-led research response to COVID-19. Science 379 , 441–444 (2023).
Buchbinder, S. P. et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372 , 1881–1893 (2008).
Rerks-Ngarm, S. et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361 , 2209–2220 (2009).
Haynes, B. F. et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366 , 1275–1286 (2012).
Gray, G. E. et al. Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa. Sci. Transl Med. 11 , eaax1880 (2019).
Moodie, Z. et al. Analysis of the HVTN 702 Phase 2b-3 HIV-1 vaccine trial in South Africa assessing RV144 antibody and T-cell correlates of HIV-1 acquisition risk. J. Infect. Dis. 226 , 246–257 (2022).
Gray, G. E. et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N. Engl. J. Med. 384 , 1089–1100 (2021).
Barouch, D. H. et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 392 , 232–243 (2018).
Hansen, S. G. et al. Immune clearance of highly pathogenic SIV infection. Nature 502 , 100–104 (2013).
Jiang, C. et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 585 , 261–267 (2020).
Turk, G. et al. A possible sterilizing cure of HIV-1 infection without stem cell transplantation. Ann. Intern. Med. 175 , 95–100 (2022).
Jardine, J. G. et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 351 , 1458–1463 (2016).
Haynes, B. F. et al. Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat. Rev. Immunol. 23 , 142–158 (2023).
Leggat, D. J. et al. Vaccination induces HIV broadly neutralizing antibody precursors in humans. Science 378 , eadd6502 (2022).
Sanders, R. W. et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 9 , e1003618 (2013).
Borst, A. J. et al. Germline VRC01 antibody recognition of a modified clade C HIV-1 envelope trimer and a glycosylated HIV-1 gp120 core. eLife 7 , e37688 (2018).
Arunachalam, P. S. et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med. 26 , 932–940 (2020).
McMahan, K. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590 , 630–634 (2021).
Ndung’u, T., McCune, J. M. & Deeks, S. G. Why and where an HIV cure is needed and how it might be achieved. Nature 576 , 397–405 (2019).
Deeks, S. G. et al. Research priorities for an HIV cure: International AIDS Society global scientific strategy 2021. Nat. Med. 27 , 2085–2098 (2021).
Dybul, M. et al. The case for an HIV cure and how to get there. Lancet HIV 8 , e51–e58 (2021).
Ho, Y. C. et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155 , 540–551 (2013).
Siliciano, J. D. et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4 + T cells. Nat. Med. 9 , 727–728 (2003).
Wagner, T. A. et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345 , 570–573 (2014).
Maldarelli, F. et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345 , 179–183 (2014).
Einkauf, K. B. et al. Parallel analysis of transcription, integration, and sequence of single HIV-1 proviruses. Cell 185 , 266–282.e15 (2022).
Cohn, L. B. et al. HIV-1 integration landscape during latent and active infection. Cell 160 , 420–432 (2015).
Nixon, C. C. et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 578 , 160–165 (2020).
Badley, A. D., Sainski, A., Wightman, F. & Lewin, S. R. Altering cell death pathways as an approach to cure HIV infection. Cell Death Dis. 4 , e718 (2013).
Yates, K. B. et al. Epigenetic scars of CD8 + T cell exhaustion persist after cure of chronic infection in humans. Nat. Immunol. 22 , 1020–1029 (2021).
Rutishauser, R. L. et al. TCF-1 regulates HIV-specific CD8 + T cell expansion capacity. JCI Insight 6 , e136648 (2021).
Mancuso, P. et al. CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat. Commun. 11 , 6065 (2020).
Kessing, C. F. et al. In vivo suppression of HIV rebound by didehydro-cortistatin A, a “block-and-lock” strategy for HIV-1 treatment. Cell Rep. 21 , 600–611 (2017).
Borducchi, E. N. et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540 , 284–287 (2016).
Bailon, L. et al. Safety, immunogenicity and effect on viral rebound of HTI vaccines in early treated HIV-1 infection: a randomized, placebo-controlled phase 1 trial. Nat. Med. 28 , 2611–2621 (2022).
Nishimura, Y. et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543 , 559–563 (2017).
Mendoza, P. et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561 , 479–484 (2018).
Borducchi, E. N. et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563 , 360–364 (2018).
Niessl, J. et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat. Med. 26 , 222–227 (2020).
Gunst, J. D. et al. Early intervention with 3BNC117 and romidepsin at antiretroviral treatment initiation in people with HIV-1: a phase 1b/2a, randomized trial. Nat. Med. 28 , 2424–2435 (2022).
Tebas, P. et al. CCR5-edited CD4 + T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. J. Clin. Invest. 131 , e144486 (2021).
Gardner, M. R. et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519 , 87–91 (2015).
Martinez-Navio, J. M. et al. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity 50 , 567–575.e5 (2019).
Casazza, J. P. et al. Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nat. Med. 28 , 1022–1030 (2022).
Hutter, G. et al. Long-term control of HIV by CCR5 delta32/delta32 stem-cell transplantation. N. Engl. J. Med. 360 , 692–698 (2009).
Gupta, R. K. et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 568 , 244–248 (2019).
Okoye, A. A. et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med. 24 , 1430–1440 (2018).
Henrich, T. J. et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: an observational study. PLoS Med. 14 , e1002417 (2017).
Mitchell, J. L. et al. Plasmacytoid dendritic cells sense HIV replication before detectable viremia following treatment interruption. J. Clin. Invest. 130 , 2845–2858 (2020).
Gondim, M. V. P. et al. Heightened resistance to host type 1 interferons characterizes HIV-1 at transmission and after antiretroviral therapy interruption. Sci. Transl Med. 13 , eabd8179 (2021).
Prator, C. A. et al. Circulating CD30 + CD4 + T cells increase before human immunodeficiency virus rebound after analytical antiretroviral treatment interruption. J. Infect. Dis. 221 , 1146–1155 (2020).
Landovitz, R. J. et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV 7 , e472–e481 (2020).
Eshleman, S. H. et al. HIV RNA screening reduces integrase strand transfer inhibitor resistance risk in persons receiving long-acting cabotegravir for HIV prevention. J. Infect. Dis. 226 , 2170–2180 (2022).
Beacroft, L. & Hallett, T. B. The potential impact of a “curative intervention” for HIV: a modelling study. Glob. Health Res. Policy 4 , 2 (2019).
Lehman, D. A. et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J. Infect. Dis. 211 , 1211–1218 (2015).
Dube, K. et al. Participant experiences using novel home-based blood collection device for viral load testing in the HIV cure trials with analytical treatment interruptions. HIV Res. Clin. Pract. 23 , 76–90 (2022).
Deeks, S. G. et al. Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral-treated individuals with drug-resistant human immunodeficiency virus type 1. J. Infect. Dis. 189 , 312–321 (2004).
Bertagnolli, L. N. et al. Autologous IgG antibodies block outgrowth of a substantial but variable fraction of viruses in the latent reservoir for HIV-1. Proc. Natl Acad. Sci. USA 117 , 32066–32077 (2020).
Blazkova, J. et al. Distinct mechanisms of long-term virologic control in two HIV-infected individuals after treatment interruption of anti-retroviral therapy. Nat. Med. 27 , 1893–1898 (2021).
Jones, R. B. & Walker, B. D. HIV-specific CD8 + T cells and HIV eradication. J. Clin. Invest. 126 , 455–463 (2016).
Collins, D. R., Gaiha, G. D. & Walker, B. D. CD8 + T cells in HIV control, cure and prevention. Nat. Rev. Immunol. 20 , 471–482 (2020).
Fukazawa, Y. et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 21 , 132–139 (2015).
Imamichi, H. et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl Acad. Sci. USA 117 , 3704–3710 (2020).
Pollack, R. A. et al. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe 21 , 494–506.e4 (2017).
Gaiha, G. D. et al. Structural topology defines protective CD8 + T cell epitopes in the HIV proteome. Science 364 , 480–484 (2019).
Mothe, B. et al. Therapeutic vaccination refocuses T-cell responses towards conserved regions of HIV-1 in early treated individuals (BCN 01 study). eClinicalMedicine 11 , 65–80 (2019).
Korber, B. & Fischer, W. T cell-based strategies for HIV-1 vaccines. Hum. Vaccin. Immunother. 16 , 713–722 (2020).
Stevenson, E. M. et al. HIV-specific T cell responses reflect substantive in vivo interactions with antigen despite long-term therapy. JCI Insight 6 , e142640 (2021).
Stevenson, E. M. et al. SARS CoV-2 mRNA vaccination exposes latent HIV to Nef-specific CD8 + T-cells. Nat. Commun. 13 , 4888 (2022).
Duette, G. et al. The HIV-1 proviral landscape reveals that Nef contributes to HIV-1 persistence in effector memory CD4 + T cells. J. Clin. Invest. 132 , e154422 (2022).
Collins, D. R. et al. Functional impairment of HIV-specific CD8 + T cells precedes aborted spontaneous control of viremia. Immunity 54 , 2372–2384.e7 (2021).
Lewin, S. R. et al. Multi-stakeholder consensus on a target product profile for an HIV cure. Lancet HIV 8 , e42–e50 (2021).
Colasanti, J. et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin. Infect. Dis. 62 , 648–654 (2016).
Joint United Nations Programme on HIV/AIDS. African leaders unite in pledge to end AIDS in children. UNAIDS https://www.unaids.org/en/keywords/children (2023).
Patel, P. et al. Pregnancy outcomes and pharmacokinetics in pregnant women living with HIV exposed to long-acting cabotegravir and rilpivirine in clinical trials. HIV Med. 24 , 568–579 (2022).
Penazzato, M. et al. Advancing the prevention and treatment of HIV in children: priorities for research and development. Lancet HIV 9 , e658–e666 (2022).
Persaud, D. et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med. 369 , 1828–1835 (2013).
Violari, A. et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat. Commun. 10 , 412 (2019).
Frange, P. et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 3 , e49–54 (2016).
Hartana, C. A. et al. Immune correlates of HIV-1 reservoir cell decline in early-treated infants. Cell Rep. 40 , 111126 (2022).
Uprety, P. et al. Human immunodeficiency virus type 1 DNA decay dynamics with early, long-term virologic control of perinatal infection. Clin. Infect. Dis. 64 , 1471–1478 (2017).
Capparelli, E. V. et al. Safety and pharmacokinetics of intravenous 10-1074 and VRC01LS in young children. J. Acquir. Immune Defic. Syndr. 91 , 182–188 (2022).
Bonacci, R. A., Smith, D. K. & Ojikutu, B. O. Toward greater pre-exposure prophylaxis equity: increasing provision and uptake for black and Hispanic/Latino individuals in the US. Am. J. Prev. Med. 61 , S60–S72 (2021).
Harris, N. S. et al. Vital signs: status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis — United States, 2013–2018. MMWR Morb. Mortal. Wkly. Rep. 68 , 1117–1123 (2019).
Xavier Hall, C. D., Feinstein, B. A., Rusie, L., Phillips Ii, G. & Beach, L. B. Race and sexual identity differences in PrEP continuum outcomes among Latino men in a large Chicago area healthcare network. AIDS Behav. 26 , 1943–1955 (2022).
Monroe, A. K. et al. Integrase inhibitor prescribing disparities in the DC and Johns Hopkins HIV cohorts. Open Forum Infect. Dis. 8 , ofab338 (2021).
Joint United Nations Programme on HIV/AIDS. In danger: UNAIDS global AIDS update 2022 (UNAIDS, 2022).
Yukl, S. A. et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin Patient. PLoS Pathog. 9 , e1003347 (2013).
Jensen, B.-E. O. et al. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat. Med. 29 , 583–587 (2023).
Hsu, J. et al. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 186 , 1115–1126.e8 (2023).
Mendoza, D. et al. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 119 , 4645–4655 (2012).
Saez-Cirion, A. et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9 , e1003211 (2013).
Download references
Author information
Authors and affiliations.
Center for Clinical AIDS Research and Education, David Geffen School of Medicine, University of California, Los Angeles, CA, USA
Raphael J. Landovitz
Bridge HIV, San Francisco Department of Public Health, San Francisco, CA, USA
Hyman Scott
Division of HIV, Infectious Diseases & Global Medicine, Department of Medicine, University of California, San Francisco, CA, USA
Hyman Scott & Steven G. Deeks
You can also search for this author in PubMed Google Scholar
Contributions
S.G.D. researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Steven G. Deeks .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Microbiology thanks Linda-Gail Bekker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Landovitz, R.J., Scott, H. & Deeks, S.G. Prevention, treatment and cure of HIV infection. Nat Rev Microbiol 21 , 657–670 (2023). https://doi.org/10.1038/s41579-023-00914-1
Download citation
Accepted : 12 May 2023
Published : 21 June 2023
Issue Date : October 2023
DOI : https://doi.org/10.1038/s41579-023-00914-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The possible mechanisms of cu and zn in the treatment and prevention of hiv and covid-19 viral infection.
- Shatha A Albalawi
- Raneem A Albalawi
- Mohamed S. Abdel-Maksoud
Biological Trace Element Research (2024)
Making genome editing a success story in Africa
- Hussein M. Abkallo
- Patrick Arbuthnot
- Charles Wondji
Nature Biotechnology (2024)
PrEPping the skin
- Andrea Du Toit
Nature Reviews Microbiology (2023)
AZD5582 plus SIV-specific antibodies reduce lymph node viral reservoirs in antiretroviral therapy-suppressed macaques
- Amir Dashti
- Sophia Sukkestad
- Ann Chahroudi
Nature Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
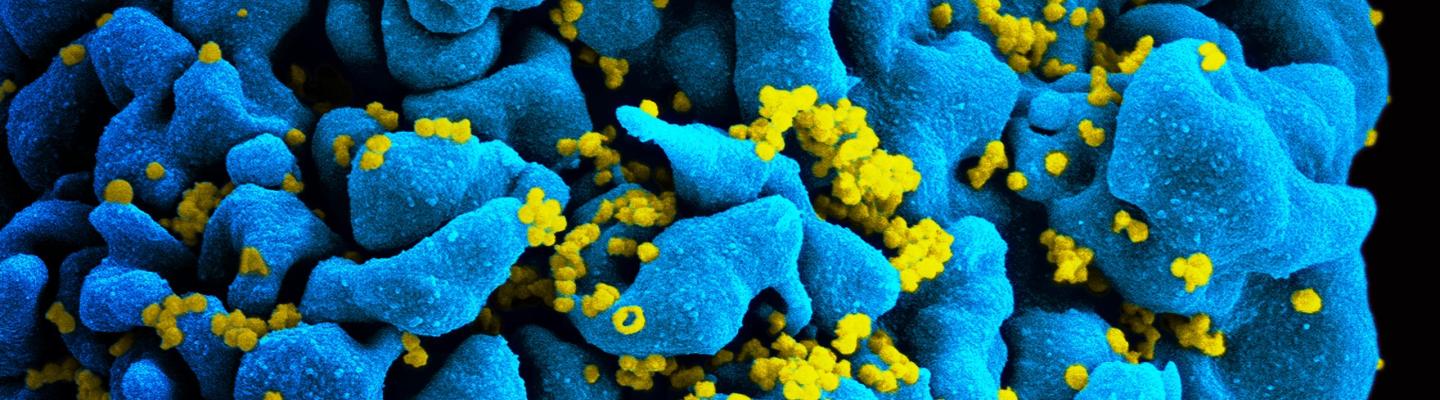
Future Directions for HIV Treatment Research
A major goal of NIAID-supported research on HIV treatment today is to develop long-acting therapies that—unlike current antiretrovirals, which require daily dosing—could be taken only once a week, once a month, or even less often. Such long-acting therapies might be easier for some people to stick to than daily pills, and might also be less toxic and more cost effective. The three types of agents under study are long-acting drugs, broadly neutralizing antibodies, and therapeutic vaccines.
Long-Acting Drugs
NIAID-supported scientists aim to develop a new array of drugs for HIV treatment that include longer-acting pills as well as alternative formulations such as injections, patches, and implants. The complexity of developing such products has led NIAID to create a consortium of experts who can facilitate relationships among the many types of researchers needed to translate an idea for a long-acting HIV drug into a workable solution. Called LEAP, for Long-Acting/Extended Release Antiretroviral Resource Program, the consortium includes scientists and clinicians from academia, industry, and government, as well as patient advocates. Read more about LEAP.
NIAID also will investigate the effectiveness of two investigational long-acting HIV drugs, rilpivirine LA and cabotegravir LA, in people for whom adhering to conventional antiretroviral therapy has been a challenge. Another study is planned to test whether the combination of monthly injections of cabotegravir LA and monthly infusions of an NIAID-discovered broadly neutralizing antibody called VRC01LS can keep HIV suppressed in people whose infection was previously controlled by antiretroviral therapy.
Broadly Neutralizing Antibodies
Scientists at the NIAID Vaccine Research Center (VRC) and NIAID-supported scientists at other institutions are developing and testing multiple antibodies for the treatment of HIV. Antibodies are good candidates for treatment because they have few side effects and can be modified to ensure they last a long time in the body, suggesting that dosing could be every other month or even less often. Importantly, the antibodies under investigation can powerfully stop a wide range of HIV strains from infecting human cells in the laboratory and thus are known as broadly neutralizing antibodies, or bNAbs.
In the context of treatment, bNAbs can potentially thwart HIV in three ways:
- By binding directly to the virus, preventing it from entering a cell and accelerating its elimination.
- By binding to an HIV-infected cell, recruiting immune-system components that facilitate cell killing.
- By binding to a key fragment of HIV, forming a complex that may lead to the stimulation of immune cells in a manner similar to a vaccine, thereby preparing the immune system for future encounters with the virus.
Clinical studies have established that giving infusions of certain bNAbs to people living with HIV can suppress the virus, albeit to a limited degree. Further studies have shown that treating people living with HIV with just one bNAb fosters the emergence of HIV strains that are resistant to the antibody. Thus, just as antiretroviral therapy requires a combination of drugs to effectively suppress HIV, it appears that antibody-based therapy will require a combination of either multiple bNAbs or bNAbs and long-acting drugs to suppress the virus. Studies in monkeys infected with a simian version of HIV have already demonstrated that combinations of complementary bNAbs powerfully suppress the virus for an extended period. NIAID is now funding and conducting clinical trials of this strategy for treating HIV in people.
In addition, scientists are engineering changes to known bNAbs to optimize them for HIV treatment and prevention applications. These changes are designed to increase the number of HIV strains an antibody can block, how long the antibody lasts in the body, how powerfully the antibody attaches to the virus, and how efficiently the antibody triggers the immune system to attack both the virus and HIV-infected cells.
Therapeutic HIV Vaccines
Perhaps the ideal treatment for HIV infection would be a therapeutic vaccine. Unlike a vaccine designed to prevent HIV infection, a therapeutic vaccine would be given to people already infected with the virus. Such a vaccine would stimulate the immune system to be ready to control any future emergence of HIV and thereby end the need for further therapy, perhaps save periodic booster shots. Such an approach could lead to sustained viral remission , meaning treatment or vaccination that would result in prolonged undetectable levels of HIV without regular antiretroviral therapy.
The presence of rare people living with HIV who can control the virus naturally either from the time of infection or after halting antiretroviral therapy is evidence that a therapeutic vaccine could theoretically alter the immune system to achieve long-term control of HIV. Nevertheless, attempts to create effective therapeutic HIV vaccines have so far been unsuccessful. To help improve results, NIAID is working to advance the underlying science—in particular, to improve understanding of immune responses that sustainably suppress HIV and to improve the potency of those responses.
Three of the NIAID-funded Martin Delaney Collaboratories are pursuing strategies that involve therapeutic vaccines to achieve long-term control of HIV or reduction of the reservoir of all virus-carrying cells. Read more about the Martin Delaney Collaboratories .
Future Directions for Developing Daily HIV Drugs
At the same time, NIAID continues to support research to develop new drugs with unique mechanisms of action for daily antiretroviral therapy. Such drugs likely would be effective against HIV strains with resistance to other drug types.
For example, basic NIAID-supported research contributed to development of the experimental drug islatravir (also known as EFdA or MK-8591), which belongs to a class of drugs known as nucleoside reverse transcriptase translocation inhibitors, or NRTTIs. NIAID research also contributed to the development of maturation inhibitors, investigational drugs that target the same stage of the HIV lifecycle as protease inhibitors but act by a different mechanism.
Researchers also are attempting to target other parts of the HIV lifecycle. For example, the experimental inhibitor fostemsavir blocks HIV from infecting immune cells by attaching to the gp120 protein on the virus’ surface. Another example is development of capsid assembly inhibitors, which halt construction of the viral capsid, the protein shell that encloses HIV’s genetic material.
For more information on investigational antiretroviral treatments, see the AIDS info Drug Database.

amfAR, The Foundation for AIDS Research, is one of the world’s leading nonprofit organizations dedicated to the support of AIDS research, HIV prevention, treatment education, and advocacy. Since 1985, amfAR has invested more than $635 million in its programs and has awarded more than 3,800 grants to research teams worldwide.

amfAR’s top research priority is the pursuit of a cure for HIV/AIDS. From 2015 to 2020, we invested close to $53 million in 109 cure-focused research projects in 15 countries. Grants and fellowships support teams of scientists pursuing innovative strategies including cell and gene therapy, shock and kill, and post-treatment control. Many of these are supported through the amfAR Research Consortium on HIV Eradication, or ARCHE, launched in 2010 to accelerate the pace of cure research. Additionally, amfAR fellowships allow talented young researchers to conduct original investigations under the guidance of experienced scientists, helping to ensure the long-term vitality of AIDS research.

Policy and Advocacy
Informed by thorough research and analysis, amfAR is a highly respected advocate of rational and compassionate HIV/AIDS-related public policy. The Foundation is engaged in efforts to secure necessary increases in funding for HIV/AIDS research and global HIV/AIDS programs, expand access to treatment and care for marginalized populations, advocate harm reduction policies aimed at reducing the spread of HIV and hepatitis C (HCV) among people who inject drugs, and protect the civil rights of all people affected by or vulnerable to HIV/AIDS.
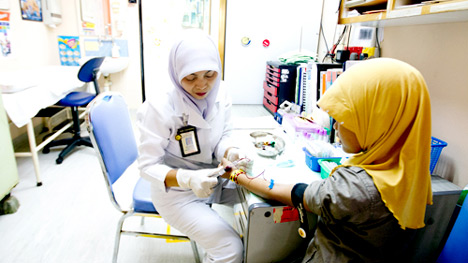
Established in 2001, amfAR’s TREAT Asia program (Therapeutics Research, Education, and AIDS Training in Asia) is a collaborative network of clinics, hospitals, research institutions, and civil society that is working to ensure the safe and effective delivery of HIV treatments to adults and children across the Asia-Pacific through research, education and advocacy. The network currently encompasses 21 pediatric and 21 adult sites throughout the region.

Impact Report 2022
This selection of major accomplishments shows the depth and breadth – and impact – of amfAR’s work.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- AIDS Res Ther


An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Office of AIDS Research
Provide input on the next nih strategic plan for hiv and hiv-related research.

This virtual workshop will review the state of science on HIV and women to inform the NIH research agenda

Explore NIH HIV Research Portfolio with New Data Tools

OAR releases FY 2025 NIH HIV/AIDS Professional Judgment Budget

NIH OAR: 35 Years of Advancing AIDS Research

- Get the latest public health information from CDC
- Get the latest research information from NIH | Español
Because of a lapse in government funding, the information on this website may not be up to date, transactions submitted via the website may not be processed, and the agency may not be able to respond to inquiries until appropriations are enacted.
The NIH Clinical Center (the research hospital of NIH) is open. For more details about its operating status, please visit cc.nih.gov .
Updates regarding government operating status and resumption of normal operations can be found at USA.gov .
Provide input on HIV research priorities until March 28, 2024: The NIH Office of AIDS Research is seeking input to inform development of the FY 2026‒2030 NIH Strategic Plan for HIV and HIV-Related Research. Learn more, and contribute by March 28, 2024.

Welcome to OAR
The Office of AIDS Research (OAR) coordinates HIV/AIDS research across the National Institutes of Health (NIH). The NIH provides the largest public investment in HIV/AIDS research globally.
As HIV crosses nearly every area of medicine and scientific investigation, the response to the HIV pandemic requires a multi-Institute, multidisciplinary, global research program. OAR provides scientific coordination and management of this research program.
OAR Spotlight

In her first blog as Acting OAR Director, Diana Finzi, Ph.D., discusses groundbreaking HIV research findings and perspectives from community members at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI).
This page last reviewed on September 19, 2023
Meet the Director

Recent Updates
Research news.

Researchers a step closer to a cure for HIV
By 2030, the World Health Organization (WHO), the Global Fund and UNAIDS are hoping to end the human immunodeficiency virus (HIV) and AIDS epidemic. An international team of researchers led by Eric Arts, professor at the Schulich School of Medicine & Dentistry, and Jamie Mann, senior lecturer at the University of Bristol (U.K.), has brought us another step closer to meeting this goal, by finding an effective and affordable targeted treatment strategy for an HIV cure.
In a first, the study published in the journal Emerging Microbes and Infections demonstrated the team's patented therapeutic candidate, an HIV-virus-like-particle (HLP), is 100 times more effective than other candidate HIV cure therapeutics for people living with chronic HIV on combined antiretroviral therapy (cART). If successful in clinical trials, HLP could be used by millions of people living around the world to free them of HIV. This study was done using blood samples from people living with chronic HIV.
HLPs are dead HIV particles hosting a comprehensive set of HIV proteins that increase immune responses without infecting a person. When compared with other potential cure approaches, HLP is an affordable biotherapeutic and can be administered by intramuscular injection -- similar to the seasonal flu vaccine.
"The development of this HIV cure was ten years in the making but with strong support from our collaborators in the U.S., Canada and Uganda, we have observed a striking ability of HLP to drive out the last remnants of HIV-1, which we hope will provide an affordable cure for all," said Arts, who is a Canada Research Chair in HIV Pathogenesis and Viral Control. "To live HIV-free is a goal for the 39 million infected. It is also the priority of the UN and WHO to end the HIV pandemic by 2030."
HIV is a retrovirus that attacks the body's immune system and if left untreated, can lead to acquired immunodeficiency syndrome (AIDS). The virus weakens a person's immune system by destroying CD4-T white blood cells, which are tasked with helping the immune system fight infections. Approximately 95 per cent of people living with HIV have chronic HIV -- where the virus is slowly causing a slow destruction of the patients' immune systems when they initiated lifelong cART.
While cART is effective at treating HIV, it has been unable to completely eliminate the virus from the body. This is because of the virus' ability to create a "latent reservoir" -- where it hides dormant inside of cells, safe from detection. Using blood samples from 32 participants living with chronic HIV from the U.S., Uganda and Canada, who were on stable cART for a median of approximately 13 years, researchers found that HLP was able to specifically target just the immune cells containing latent HIV reservoir and purge these cells of their HIV, a critical step towards an HIV-1 cure. An HIV cure is typically described as therapy and approach that eliminates all HIV without the need of continuous antiretroviral therapy.
"Over time, the virus grows more diverse within a single individual that is not on treatment which makes it more difficult to target," said co-lead author Ryan Ho, master's student in the department of microbiology and immunology. "This formulation we've crafted covers the theoretical diversity so it can reach the HIV-1 in all those people living with HIV."
Minh Ha Ngo, lead author and postdoctoral scholar in the department of microbiology and immunology, says one concern expressed among people living with HIV for years is that continued use of cART could lead to the virus becoming unreachable and unable to be eliminated. The results of this study, by contrast, demonstrate that combining HLP with cART is still able to trigger the latent reservoir, even in chronic cases. If these dormant latent reservoirs can be awakened, then they can be eliminated from the body.
"Owing to its high mutation rate, HIV exhibits remarkable genetic diversity, resulting in different viral subtypes; some of which predominate in particular regions of the globe," said Mann. "We were excited to see preliminary evidence that our HLP cure therapy reverses latency irrespective of the subtype of the individual's infection. Whilst this needs to be explored further, it hints at the global applicability of our approach."
In the future, researchers plan to test HLP on a larger representative HIV cohort with subtype C infections, which includes people living in South Africa, Ethiopia, Vietnam and India. This would help determine if the treatment strategy is effective for most people living with acute and chronic HIV.
Current studies involve confirming a lack of toxicity in preparation for human clinical trials.
These studies will be made possible with the advanced Pathogen Research Centre at Schulich Medicine & Dentistry. This study was conducted in collaboration with University of Bristol, University of Toronto, Case Western Reserve University, Rakai Health Sciences Program, Johns Hopkins University School of Medicine and the U.S. National Institutes of Health.
The study was funded by the American Foundation for AIDS Research, and by the Canadian Institutes of Health Research, U.S. National Institute of Allergy and Infectious Diseases; part of the National Institutes of Health, and the Canada Research Chairs Program.
- HIV and AIDS
- Infectious Diseases
- Sexual Health
- Diseases and Conditions
- Chronic Illness
- Immune System
- Antiretroviral drug
- Ionizing radiation
Story Source:
Materials provided by University of Western Ontario . Original written by Cynthia Fazio. Note: Content may be edited for style and length.
Journal Reference :
- Minh Ha Ngo, Joshua Pankrac, Ryan C. Y. Ho, Emmanuel Ndashimye, Rahul Pawa, Renata Ceccacci, Tsigereda Biru, Abayomi S. Olabode, Katja Klein, Yue Li, Colin Kovacs, Robert Assad, Jeffrey M. Jacobson, David H. Canaday, Stephen Tomusange, Samiri Jamiru, Aggrey Anok, Taddeo Kityamuweesi, Paul Buule, Ronald M. Galiwango, Steven J. Reynolds, Thomas C. Quinn, Andrew D. Redd, Jessica L. Prodger, Jamie F. S. Mann, Eric J. Arts. Effective and targeted latency reversal in CD4 + T cells from individuals on long term combined antiretroviral therapy initiated during chronic HIV-1 infection . Emerging Microbes & Infections , 2024; 13 (1) DOI: 10.1080/22221751.2024.2327371
Cite This Page :
Explore More
- New Artificial Reef Stands Up to Storms
- Persistent Hiccups in a Far-Off Galaxy
- Lighting Up the Future
- Sugarcane's Complex Genetic Code Cracked
- Microbiome of 4,000-Year-Old Teeth
- Magnetic Fields of Milky Way's Black Hole
- Neural Networks Think Alike
- Old Immune Systems Revitalized
- More Heat Likely to Reach Antarctica
- Could Tardigrade Proteins Slow Aging in Humans?
Trending Topics
Strange & offbeat.
Western News

By 2030, the World Health Organization (WHO), the Global Fund and UNAIDS are hoping to end the human immunodeficiency virus (HIV) and AIDS epidemic. An international team of researchers led by Eric Arts, professor at the Schulich School of Medicine & Dentistry , and Jamie Mann, senior lecturer at the University of Bristol (U.K.), has brought us another step closer to meeting this goal, by finding an effective and affordable targeted treatment strategy for an HIV cure.
In a first, the study published in the journal Emerging Microbes and Infections demonstrated the team’s patented therapeutic candidate, an HIV-virus-like-particle (HLP), is 100 times more effective than other candidate HIV cure therapeutics for people living with chronic HIV on combined antiretroviral therapy (cART). If successful in clinical trials, HLP could be used by millions of people living around the world to free them of HIV. This study was done using blood samples from people living with chronic HIV.
HLPs are dead HIV particles hosting a comprehensive set of HIV proteins that increase immune responses without infecting a person. When compared with other potential cure approaches, HLP is an affordable biotherapeutic and can be administered by intramuscular injection – similar to the seasonal flu vaccine.
“The development of this HIV cure was ten years in the making but with strong support from our collaborators in the U.S., Canada and Uganda, we have observed a striking ability of HLP to drive out the last remnants of HIV-1, which we hope will provide an affordable cure for all,” said Arts, who is a Canada Research Chair in HIV Pathogenesis and Viral Control. “To live HIV-free is a goal for the 39 million infected. It is also the priority of the UN and WHO to end the HIV pandemic by 2030.”
HIV is a retrovirus that attacks the body’s immune system and if left untreated, can lead to acquired immunodeficiency syndrome (AIDS). The virus weakens a person’s immune system by destroying CD4-T white blood cells, which are tasked with helping the immune system fight infections. Approximately 95 per cent of people living with HIV have chronic HIV – where the virus is slowly causing a slow destruction of the patients’ immune systems when they initiated lifelong cART.
Larger global studies planned in future

Postdoctoral scholar Minh Ha Ngo (Megan Morris/Schulich School of Medicine & Dentistry photo)
While cART is effective at treating HIV, it has been unable to completely eliminate the virus from the body. This is because of the virus’ ability to create a “latent reservoir” – where it hides dormant inside of cells, safe from detection. Using blood samples from 32 participants living with chronic HIV from the U.S., Uganda and Canada, who were on stable cART for a median of approximately 13 years, researchers found that HLP was able to specifically target just the immune cells containing latent HIV reservoir and purge these cells of their HIV, a critical step towards an HIV-1 cure. An HIV cure is typically described as therapy and approach that eliminates all HIV without the need of continuous antiretroviral therapy.
“ Over time, the virus grows more diverse within a single individual that is not on treatment which makes it more difficult to target, ” said co-lead author Ryan Ho, master’s student in the department of microbiology and immunology. “This formulation we’ve crafted covers the theoretical diversity so it can reach the HIV-1 in all those people living with HIV.”
Minh Ha Ngo, lead author and postdoctoral scholar in the department of microbiology and immunology, says one concern expressed among people living with HIV for years is that continued use of cART could lead to the virus becoming unreachable and unable to be eliminated. The results of this study, by contrast, demonstrate that combining HLP with cART is still able to trigger the latent reservoir, even in chronic cases. If these dormant latent reservoirs can be awakened, then they can be eliminated from the body.

Jamie Mann, senior lecturer at the University of Bristol (Jamie Mann photo)
“Owing to its high mutation rate, HIV exhibits remarkable genetic diversity, resulting in different viral subtypes; some of which predominate in particular regions of the globe,” said Mann. “We were excited to see preliminary evidence that our HLP cure therapy reverses latency irrespective of the subtype of the individual’s infection. Whilst this needs to be explored further, it hints at the global applicability of our approach.”
In the future, researchers plan to test HLP on a larger representative HIV cohort with subtype C infections, which includes people living in South Africa, Ethiopia, Vietnam and India. This would help determine if the treatment strategy is effective for most people living with acute and chronic HIV.
Current studies involve confirming a lack of toxicity in preparation for human clinical trials.
These studies will be made possible with the advanced Pathogen Research Centre at Schulich Medicine & Dentistry. This study was conducted in collaboration with University of Bristol, University of Toronto, Case Western Reserve University, Rakai Health Sciences Program, Johns Hopkins University School of Medicine and the U.S. National Institutes of Health.
The study was funded by the American Foundation for AIDS Research, and by the Canadian Institutes of Health Research, U.S. National Institute of Allergy and Infectious Diseases; part of the National Institutes of Health, and the Canada Research Chairs Program.
Topic Headline 2 Research
SHARE THIS STORY
Up next ….

Bioethicist Maxwell Smith named first Chair in Applied Public Health at Western
Headline 3 , Research
Leader in public health ethics receives national honour
Related Stories
Popular this week.
- Geoffrey Little named new vice-provost and chief librarian 835 views
- Researchers a step closer to HIV cure 583 views
- Bioethicist Maxwell Smith named first Chair in Applied Public Health at Western 152 views
Part 1. Overview Information
National Institutes of Health ( NIH )
P30 Center Core Grants
- August 31, 2022 - Implementation Changes for Genomic Data Sharing Plans Included with Applications Due on or after January 25, 2023. See Notice NOT-OD-22-198 .
- August 5, 2022 - Implementation Details for the NIH Data Management and Sharing Policy. See Notice NOT-OD-22-189 .
See Section III. 3. Additional Information on Eligibility .
The National Institute of Mental Health (NIMH) Division of AIDS Research (DAR) encourages applications for Center Core Grants (P30) to support HIV/AIDS Research Centers (ARCs). These Research Centers are intended to provide infrastructural support that facilitates the development of high impact science in HIV/AIDS relevant to the NIMH mission. The NIMH ARCs support innovative, interdisciplinary research in several areas, including basic, neuro-HIV, behavioral and social, biostatistics and data science, and integrated biobehavioral, clinical, translational, and implementation science.
30 days prior to the application due date
All applications are due by 5:00 PM local time of applicant organization.
Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
Not Applicable
It is critical that applicants follow the Multi-Project (M) Instructions in the How to Apply - Application Guide , except where instructed to do otherwise (in this NOFO or in a Notice from the NIH Guide for Grants and Contracts ). Conformance to all requirements (both in the How to Apply - Application Guide and the NOFO) is required and strictly enforced. Applicants must read and follow all application instructions in the How to Apply - Application Guide as well as any program-specific instructions noted in Section IV. When the program-specific instructions deviate from those in the How to Apply - Application Guide , follow the program-specific instructions. Applications that do not comply with these instructions may be delayed or not accepted for review.
There are several options available to submit your application through Grants.gov to NIH and Department of Health and Human Services partners. You must use one of these submission options to access the application forms for this opportunity.
- Use the NIH ASSIST system to prepare, submit and track your application online.
- Use an institutional system-to-system (S2S) solution to prepare and submit your application to Grants.gov and eRA Commons to track your application. Check with your institutional officials regarding availability.
Part 2. Full Text of Announcement
Section i. notice of funding opportunity description.
The National Institute of Mental Health (NIMH) Division of AIDS Research (DAR) encourages applications for Center Core Grants (P30) to support HIV/AIDS Research Centers (ARCs). These Research Centers are intended to provide infrastructural support that facilitates the development of high impact science in HIV/AIDS relevant to the NIMH mission. The NIMH ARCs support innovative, interdisciplinary research in several areas, including basic, neuro-HIV, behavioral and social, biostatistics and data science, integrated biobehavioral, clinical, translational, and implementation science.
NIMH ARCs align with the DAR scientific emphases by orienting toward a HIV behavioral science or neuro-HIV focus. The intent is to support research that addresses the most current research priorities in the field. The proposed Center priorities should align with the National HIV/AIDS Strategy , the NIH Office of AIDS Research (OAR) strategic plan , and the NIMH Strategic Plan with respect to HIV research. ARC awards permit the centralized coordination of affiliated research activities, foster the development of scientific innovations and new collaborations, encourage interdisciplinary research, and facilitate the dissemination of public health advances to implementing agencies, academia, affected communities, and policymakers. These Centers must have the potential to support research over a range of cross-cutting areas that may include biological, biomedical, behavioral, neuroscience, mental health, prevention, clinical sciences, biostatistics and data science, and implementation science research.
To recognize persistent and pervasive inequities in the care, treatment and prevention services for many who are living with HIV, Centers should also provide attention and leadership to address systemic factors that influence health disparities in all aspects of HIV treatment and prevention, such as structural racism, health and socio-economic inequities, social and structural determinants of health, intersectional stigma and discrimination, sexual orientation and gender discrimination, population mobility, and geographic context (e.g., state, region, city, jurisdiction, neighborhood). Centers are strongly encouraged to advance research that adopts an intersectionality approach and promotes integrated behavioral and biomedical science.
Potential research areas that may serve as organizing themes or key areas of interest for the overall research approach of the Center to address such multi-level factors include but are not limited to:
- Identification of mutable multi-level risk and resilience factors that are associated with acquiring HIV, lack of HIV testing, and poor HIV treatment outcomes. This includes individual, contextual, systemic, and structural factors which can facilitate or impede optimal access and use of effective HIV prevention and treatment options. Mental health, trauma and violence, stigma and discrimination, and human mobility are important subsets of this constellation of potential areas for inquiry that are high priorities for DAR. Onward efforts to develop or tailor interventions for populations challenged by intersectional stigma will benefit from clear understanding of the associated multi-level mechanisms, pathways and context. Lastly, researchers are encouraged to use a life course perspective, addressing the unique developmental factors that influence risk and resilience across the life span.
- Development, testing, and modeling feasible, scalable and cost-effective interventions to modify known risk and protective multi-level factors, joined with research to determine the most effective combination of interventions to promote HIV testing, prevention and care continuum outcomes.
- Development and testing of methods and strategies to assist people with HIV (PWH) and their families to cope with HIV, engage in HIV care and other services to enhance quality of life and improve health outcomes, improve the quality of patient-provider communication in care settings, prevent complications and reduce the impact of co-morbidities (especially mental disorders), and avoid new sexually transmitted infections and the onward transmission of HIV.
- Examination of multi-level factors influencing adoption, adherence and use of HIV prevention and treatment regimens, and development and testing of methods to improve sustained adherence to drug regimens, including novel long-acting HIV prevention and treatment modalities (e.g., vaginal rings, long-acting injections, implants, broadly neutralizing antibodies, and vaccines).
- Understand heterogeneous forms of population mobility and mobility’s impact on disparities in care, sexual behavior and sexual networks, HIV testing, (dis)engagement in HIV prevention and care, and HIV acquisition and transmission.
- Development, testing, and modeling strategies to translate effective prevention and HIV care continuum interventions into real world settings both domestically and internationally, and the use of communication, as well as dissemination and implementation science approaches to maximize uptake and achieve public health impact. Interventions are encouraged to target factors beyond those at the individual level in order to improve these health outcomes – including health care provider, clinic, and systems level factors.
- Pursuit of innovative data science approaches to promote HIV treatment and prevention delivery advances. This may include methods such as artificial intelligence, machine learning, and predictive analytics applied to large data sets such as electronic medical records, pharmacy claims data, and medical insurance data, as well as the integration of digital technology data (e.g., smartphones, wearables or other sensors) with other datasets to improve service delivery and impact.
- Development, testing, validating, and using innovative measurement approaches, digital health tools, or AI algorithms to assess mental health, neurodevelopment functioning, and social and structural determinants of health in PWH.
- Development of effective strategies to integrate behavioral science with biomedical advances in HIV prevention and treatment across the lifespan.
- Identification of the cellular, molecular, viral and host-genetic mechanisms underlying HIV-associated CNS dysfunction in the context of viral suppression.
- Seeking to understand the unique biological and behavioral mechanisms underlying mental health disorders in PWH.
- Development and testing of potential preclinical therapeutics to prevent/treat HIV associated CNS dysfunction and understand the adverse impact of HIV treatment on CNS outcomes.
- Identification of latent HIV/CNS reservoirs and testing of novel eradication strategies targeting the brain.
- Using biological measures such as imaging to assess CNS disease associated with HIV in the setting of neuroinflammation and CNS viral reservoirs.
AIDS Research Centers are expected to make several important contributions. First, Centers should demonstrate excellence and leadership in thematic areas that capitalize on the expertise of affiliated investigators. In this way, ARC-affiliated scientists serve as thought leaders who can guide the field in new directions in HIV prevention and treatment research based on the accumulating evidence. Second, given that ARCs provide infrastructural support, their investigators are expected to compete for research projects from NIMH, other components of NIH, and other funding sources that will address key scientific questions relevant to their thematic areas of interest. Third, ARCs are expected to provide mentoring for the next generation of HIV neuro and behavioral scientists, particularly for investigators from groups that are under-represented in health-related research.
Applicants are strongly encouraged to develop and maintain collaborations with industry, government, community, implementing agencies, and other scientific networks and institutions, to optimize the impact of Center-supported scientific advances and activities. In all potential thematic areas, Center programs are expected to address the key inequities that are evident in HIV prevention and treatment. For example, progress through the HIV care continuum is quite variable – and there are persistent racial/ethnic, age, and gender-based differences in the achievement of sustained viral suppression. To address this continuing research to service gaps, informed by local needs, there should be strong emphasis on potential collaborations with entities funded by the Centers for Disease Control and Prevention (CDC), the Human Resource Services Administration (HRSA), other implementing agencies, including local (e.g., city, state) health departments and community-based organizations.
For behaviorally themed Centers, the priorities and overall strategy should encourage and foster research that integrates and aligns with the priorities of the other federal agencies which provide HIV prevention and treatment service delivery to meet the goals of the United States National HIV/AIDS Strategy. The National HIV/AIDS Strategy focuses on maximizing the ease of obtaining HIV testing and diagnosis, ensuring initiation and sustainment of effective HIV treatment as early as possible, deploying PrEP and other effective prevention tools to those at highest risk for HIV, and responding and containing outbreaks of HIV infection when/where they occur. Success of these strategies depends on partnerships among local and state health departments, community-based organizations, service and care providers, and other groups funded by the CDC, the HIV/AIDS Bureau of HRSA, the Substance Abuse and Mental Health Services Administration (SAMHSA), the Indian Health Service (IHS), or the U.S. Department of Veterans Affairs (VA). Those ARCs with an intervention science emphasis are also expected to advance the science of dissemination and implementation of evidence-based interventions that have the potential to lower HIV incidence and prevalence and improve health outcomes when delivered as part of full-scale programs. In these ways, ARCs are expected to serve as local, regional, national, and global resources for rigorous HIV/AIDS prevention, treatment, and implementation science.
The ARCs focused on Neuro-HIV are encouraged to maintain collaborations with existing research resources such as National NeuroAIDS Tissue Consortium (NNTC ), CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER), the MACS-WIHS Combined Cohort Study , and the AIDS Clinical Trials Group (ACTG) to facilitate high priority research relating to HIV associated CNS dysfunction and HIV persistence in the CNS. They are expected to serve as local, regional, national, and global resources for rigorous Neuro-HIV research.
Applicants are strongly encouraged to consult with the Scientific/Research Contacts listed in Section VII for questions concerning research areas that are of highest priority.
All applicants are expected to collect Common Data Elements (CDEs) for HIV-related human subjects research funded by the NIMH consistent with the NIMH Common Data Elements for HIV-Funded Research Policy ( NOT-MH-23-105 ). The NIMH CDEs for HIV-funded research should be briefly described in the Data Management and Sharing Plan . All data, models, and related tools, software, and/or code, and findings from the awarded studies are expected to be shared using the NIMH Data Archives (NDA) consistent with the NIMH Data Management and Sharing Policy .
The NIMH has published updated policies and guidance for investigators regarding human research protection and clinical research data and safety monitoring ( NOT-MH-19-027 ). The application’s PHS Human Subjects and Clinical Trials Information, including the Data and Safety Monitoring Plan, should reflect the policies and guidance in this notice. Plans for the protection of research participants and data and safety monitoring will be reviewed by the NIMH for consistency with NIMH and NIH policies and federal regulations.
See Section VIII. Other Information for award authorities and regulations.
Investigators proposing NIH-defined clinical trials may refer to the Research Methods Resources website for information about developing statistical methods and study designs.
Section II. Award Information
Grant: A financial assistance mechanism providing money, property, or both to an eligible entity to carry out an approved project or activity.
The OER Glossary and the How to Apply - Application Guide provides details on these application types. Only those application types listed here are allowed for this NOFO.
Optional: Accepting applications that either propose or do not propose clinical trial(s).
The number of awards is contingent upon NIH appropriations and the submission of a sufficient number of meritorious applications.
Applicants may request up to $1,500,000 total costs per year.
An ARC may request a project period of up to five years.
NIH grants policies as described in the NIH Grants Policy Statement will apply to the applications submitted and awards made from this NOFO.
Section III. Eligibility Information
1. Eligible Applicants
Higher Education Institutions
- Public/State Controlled Institutions of Higher Education
- Private Institutions of Higher Education
The following types of Higher Education Institutions are always encouraged to apply for NIH support as Public or Private Institutions of Higher Education:
- Hispanic-serving Institutions
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
- Asian American Native American Pacific Islander Serving Institutions (AANAPISIs)
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
For-Profit Organizations
- Small Businesses
- For-Profit Organizations (Other than Small Businesses)
Local Governments
- State Governments
- County Governments
- City or Township Governments
- Special District Governments
- Indian/Native American Tribal Governments (Federally Recognized)
- Indian/Native American Tribal Governments (Other than Federally Recognized)
Federal Governments
- U.S. Territory or Possession
- Independent School Districts
- Public Housing Authorities/Indian Housing Authorities
- Native American Tribal Organizations (other than Federally recognized tribal governments)
- Faith-based or Community-based Organizations
- Regional Organizations
In order to demonstrate sufficient research capacity, it is expected that the applicant organization have a minimum of 4 active NIMH research project awards (R-series) and 2 additional NIH awardsards at the time of submission.
Non-domestic (non-U.S.) Entities (Foreign Organization) are not eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are not eligible to apply.
Foreign components, as defined in the NIH Grants Policy Statement , are not allowed.
Applicant organizations
Applicant organizations must complete and maintain the following registrations as described in the How to Apply- Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. Failure to complete registrations in advance of a due date is not a valid reason for a late submission, please reference NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications for additional information.
- NATO Commercial and Government Entity (NCAGE) Code – Foreign organizations must obtain an NCAGE code (in lieu of a CAGE code) in order to register in SAM.
- Unique Entity Identifier (UEI) - A UEI is issued as part of the SAM.gov registration process. The same UEI must be used for all registrations, as well as on the grant application.
- eRA Commons - Once the unique organization identifier is established, organizations can register with eRA Commons in tandem with completing their Grants.gov registration; all registrations must be in place by time of submission. eRA Commons requires organizations to identify at least one Signing Official (SO) and at least one Program Director/Principal Investigator (PD/PI) account in order to submit an application.
- Grants.gov – Applicants must have an active SAM registration in order to complete the Grants.gov registration.
Program Directors/Principal Investigators (PD(s)/PI(s))
All PD(s)/PI(s) must have an eRA Commons account. PD(s)/PI(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in eRA Commons. If the PD/PI is also the organizational Signing Official, they must have two distinct eRA Commons accounts, one for each role. Obtaining an eRA Commons account can take up to 2 weeks.
Any individual(s) with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) is invited to work with his/her organization to develop an application for support. Individuals from diverse backgrounds, including underrepresented racial and ethnic groups, individuals with disabilities, and women are always encouraged to apply for NIH support. See, Reminder: Notice of NIH's Encouragement of Applications Supporting Individuals from Underrepresented Ethnic and Racial Groups as well as Individuals with Disabilities, NOT-OD-22-019 .
For institutions/organizations proposing multiple PDs/PIs, visit the Multiple Program Director/Principal Investigator Policy and submission details in the Senior/Key Person Profile (Expanded) Component of the How to Apply - Application Guide .
2. Cost Sharing
This NOFO does not require cost sharing as defined in the NIH Grants Policy Statement Section 1.2- Definitions of Terms.
3. Additional Information on Eligibility
Number of Applications
Applicant organizations may submit more than one application, provided that each application is scientifically distinct.
The NIH will not accept duplicate or highly overlapping applications under review at the same time per NIH Grants Policy Statement Section 2.3.7.4 Submission of Resubmission Application . This means that the NIH will not accept:
- A new (A0) application that is submitted before issuance of the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application that is submitted before issuance of the summary statement from the review of the previous new (A0) application.
- An application that has substantial overlap with another application pending appeal of initial peer review (see NIH Grants Policy Statement 2.3.9.4 Similar, Essentially Identical, or Identical Applications ).
Section IV. Application and Submission Information
1. Requesting an Application Package
The application forms package specific to this opportunity must be accessed through ASSIST or an institutional system-to-system solution. A button to apply using ASSIST is available in Part 1 of this NOFO. See the administrative office for instructions if planning to use an institutional system-to-system solution.
2. Content and Form of Application Submission
It is critical that applicants follow the Multi-Project (M) Instructions in the How to Apply - Application Guide , except where instructed in this notice of funding opportunity to do otherwise and where instructions in the How to Apply - Application Guide are directly related to the Grants.gov downloadable forms currently used with most NIH opportunities. Conformance to the requirements in the How to Apply - Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
Letter of Intent
Although a letter of intent is not required, is not binding, and does not enter into the review of a subsequent application, the information that it contains allows IC staff to estimate the potential review workload and plan the review.
By the date listed in Part 1. Overview Information , prospective applicants are asked to submit a letter of intent that includes the following information:
- Descriptive title of proposed activity
- Name(s), address(es), and telephone number(s) of the PD(s)/PI(s)
- Names of other key personnel
- Participating institution(s)
- Number and title of this funding opportunity
The letter of intent should be sent to:
Page Limitations
All page limitations described in the How to Apply- Application Guide and the Table of Page Limits must be followed.
Instructions for the Submission of Multi-Component Applications
The following section supplements the instructions found in How to Apply- Application Guide and should be used for preparing a multi-component application.
Revision applications must include an Overall component and the components that are affected by the revision. Therefore, the component requirements listed below may not apply to the revision application.
The application should consist of the following components:
- Overall: required, maximum of 1
- Administrative Core: required; maximum of 1
- Developmental Core: required; maximum of 1
- Research Core: required; minimum of 3
Overall Component
When preparing the application, use Component Type ‘Overall’.
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions, as noted.
SF424(R&R) Cover (Overall)
Complete entire form.
PHS 398 Cover Page Supplement (Overall)
Note: Human Embryonic Stem Cell lines from other components should be repeated in cell line table in Overall component.
Research & Related Other Project Information (Overall)
Follow standard instructions.
Project/Performance Site Locations (Overall)
Enter primary site only.
A summary of Project/Performance Sites in the Overall section of the assembled application image in eRA Commons compiled from data collected in the other components will be generated upon submission.
Research and Related Senior/Key Person Profile (Overall)
Include only the Project Director/Principal Investigator (PD/PI) and any multi-PDs/PIs (if applicable to this NOFO) for the entire application.
In the Biosketch, provide information thatthe Center Director is an internationally recognized scientist and an accomplished administrator capable of leading a complex, interdisciplinary organization to achieve its key aims and objectives. He or she must be able to develop short- and long-term strategic objectives and plans for identifying and responding to emerging priorities and initiatives. Directors have final responsibility for the scientific, administrative, and operational aspects of their Centers
A summary of Senior/Key Persons followed by their Biographical Sketches in the Overall section of the assembled application image in eRA Commons will be generated upon submission.
Budget (Overall)
The only budget information included in the Overall component is the Estimated Project Funding section of the SF424 (R&R) Cover.
A budget summary in the Overall section of the assembled application image in eRA Commons compiled from detailed budget data collected in the other components will be generated upon submission.
PHS 398 Research Plan (Overall)
Introduction to Application: For Resubmission and Revision applications, an Introduction to Application is required in the Overall component.
Specific Aims: Describe thematic aims of the overall Center and outline how the different Cores will contribute to these aims.
Research Strategy:
The Research Strategy section of the application must include an overall description of the proposed Center, including objectives and integrating theme; justification of Center goals and proposed organization; and a diagram illustrating the organization and function of the programmatic and advisory structure of the Center.
Organize the Research Strategy into sections on Significance, Innovation, and Approach. The Overall Research Strategy should describe the impact that a Center could have to enhance HIV/AIDS research at the applicant institution(s) and exert a sustained influence on the HIV/AIDS research field. Use this section to describe the proposed Overall Research Strategy and the Strategic Plan of the Center and how it will contribute to meeting the goals and objectives of the Center Program. Also, explain the rationale for selection of the strategies, opportunities, action plans and outcome measurements proposed to accomplish the specific aims.
- Significance: Describe the effect that a Center award would have on Center investigators' HIV/AIDS research efforts. Summarize HIV/AIDS science at the institution that justifies the need for Center support. Describe how the proposed Center provides added value to HIV/AIDS research conducted at the institution. Demonstrate the Center's ability to support the research base, foster synergy, and enhance HIV/AIDS research collaborations.
- Innovation: Describe how Center resources will be utilized in unique ways to achieve the scientific goals of HIV/AIDS investigators at the institution. Provide the degree of variety across the disciplines involved in HIV/AIDS research projects within the Center structure. Illustrate unique ways to incorporate community involvement, collaboration, and outreach in the Center.
- Approach: Describe the Center scientific planning process. Describe the leadership plan for the Center. The roles and responsibilities of the Center Director and Core Directors should be described.
Applicants should list active NIMH research project awards (R-series) and additional NIH awards at the time of application.
A strategic plan must be outlined that identifies the immediate and long-term research goals of the Center. A process for implementing the activities to achieve the goals set by the Center should be clearly defined. Center applications should identify the highest priority goals with a detailed plan of activities to meet those goals for each year of the award. The application should define specific milestones that the Center expects to meet, and a process for evaluating their progress and making changes if course correction is needed. Goals for each successive year should build on those identified for the first year of funding.
Applicants should describe clearly how interactions and collaborations of key personnel will enhance and expand the development and productivity of Center research efforts, and how other investigators will benefit from shared resources, formal and informal planning activities, and developmental or pilot support provided by the Center award.
Letters of Support: The applicant must provide a letter(s) from the appropriate institutional official(s) (e.g., Dean, President, or Provost) that describes resource support for the Center that will be provided by the applicant institution.
Resource Sharing Plan : Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the How to Apply - Application Guide .
Other Plan(s):
All instructions in the How to Apply- Application Guide must be followed, with the following additional instructions:
- All applicants planning research (funded or conducted in whole or in part by NIH) that results in the generation of scientific data are required to comply with the instructions for the Data Management and Sharing Plan. All applications, regardless of the amount of direct costs requested for any one year, must address a Data Management and Sharing Plan.
To advance the goal of advancing research through widespread data sharing among researchers, investigators funded by NIMH under this NOFO are expected to share those data via the National Institute of Mental Health Data Archive (NDA; see NOT-MH-23-100 ). Established by the NIH, NDA is a secure informatics platform for scientific collaboration and data-sharing that enables the effective communication of detailed research data, tools, and supporting documentation. NDA links data across research projects through its Global Unique Identifier (GUID) and Data Dictionary technology. Investigators funded under this NOFO are expected to use these technologies to submit data to NDA.
To accomplish this objective, it will be important to formulate a) an enrollment strategy that will obtain the information necessary to generate a GUID for each participant, and b) a budget strategy that will cover the costs of data submission. The NDA website provides two tools to help investigators develop appropriate strategies: 1) t he NDA Data Submission Cost Model which offers a customizable Excel worksheet that includes tasks and hours for the Program Director/Principal Investigator and Data Manager to budget for data sharing; and 2) plain language text to be considered in your informed consent available from the NDA's Data Contribution page . Investigators are expected to certify the quality of all data generated by grants funded under this NOFO prior to submission to NDA and review their data for accuracy after submission. Submission of descriptive/raw data is expected semi-annually (every January 15 and July 15); submission of all other data is expected at the time of publication, or prior to the end of the grant, whichever occurs first (see NDA Sharing Regimen for more information); Investigators are expected to share results, positive and negative, specific to the cohorts and outcome measures studied.For more guidance on submitting data to NDA, refer to the NDA Data Management and Sharing Plan on the NDA website . NDA staff will work with investigators to help them submit data types not yet defined in the NDA Data Dictionary .
Only limited items are allowed in the Appendix. Follow all instructions for the Appendix as described in How to Apply- Application Guide ; any instructions provided here are in addition to the How to Apply - Application Guide instructions.
PHS Human Subjects and Clinical Trials Information (Overall)
When involving human subjects research, clinical research, and/or NIH-defined clinical trials follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the How to Apply - Application Guide , with the following additional instructions:
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, there must be at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or a Delayed Onset Study record within the application. The study record(s) must be included in the component(s) where the work is being done, unless the same study spans multiple components. To avoid the creation of duplicate study records, a single study record with sufficient information for all involved components must be included in the Overall component when the same study spans multiple components.
Study Record: PHS Human Subjects and Clinical Trials Information
All instructions in the How to Apply - Application Guide must be followed.
Delayed Onset Study
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start). All instructions in the How to Apply- Application Guide must be followed.
PHS Assignment Request Form (Overall)
All instructions in the How to Apply- Application Guide must be followed.
Administrative Core
When preparing your application, use Component Type ‘Admin Core.’
All instructions in the SF424 (R&R) Application Guide must be followed, with the following additional instructions, as noted.
SF424 (R&R) Cover (Administrative core)
Complete only the following fields:
- Applicant Information
- Type of Applicant (optional)
- Descriptive Title of Applicant’s Project
- Proposed Project Start/Ending Dates
PHS 398 Cover Page Supplement (Administrative Core
Enter Human Embryonic Stem Cells in each relevant component.
Research & Related Other Project Information (Administrative Core)
Human Subjects: Answer only the ‘Are Human Subjects Involved?’ and 'Is the Project Exempt from Federal regulations?’ questions.
Vertebrate Animals: Answer only the ‘Are Vertebrate Animals Used?’ question.
Project Narrative: Do not complete.
Project /Performance Site Location(s) (Administrative Core)
List all performance sites that apply to the specific component.
Note: The Project Performance Site form allows up to 300 sites, prior to using additional attachment for additional entries.
Research & Related Senior/Key Person Profile (Administrative Core)
- In the Project Director/Principal Investigator section of the form, use Project Role of ‘Other’ with Category of ‘Administrative Core Director and provide a valid eRA Commons ID in the Credential field.
- In the additional Senior/Key Profiles section, list Senior/Key persons that are working in the component.
- Include a single Biographical Sketch for each Senior/Key person listed in the application regardless of the number of components in which they participate. When a Senior/Key person is listed in multiple components, the Biographical Sketch can be included in any one component.
- If more than 100 Senior/Key persons are included in a component, the Additional Senior Key Person attachments should be used.
- The Administrative Core Director must be the Center Director.
Budget (Administrative Core)
Budget forms appropriate for the specific component will be included in the application package.
The Administrative Core Director is expected to devote a minimum of 3.6 person months to their role.
The Administrative Core may include a limited number of administrative and clerical personnel, with a detailed description of their responsibilities for the Center, only if all the following conditions are met: 1) the administrative or clerical services are integral to a project or activity; 2) individuals involved can be specifically identified with the project or activity; and 3) the costs are not also recovered as indirect costs. Rent may be allowed only in unusual and exceptional circumstances whereby the applicant project institution has a documented shortage of space on campus.
Travel expenses for key personnel to attend annual NIMH AIDS Research Center Directors meetings in Washington DC should be budgeted, including travel for community members who are active in Center input and activities. Limited support is available to cover travel of the Administrative Core Director and other investigators to scientific meetings that are justified as essential to the conduct of research supported by the Center. Travel of technical staff for training that is justified as essential to enhancing the quality of the research projects is also an allowable expense.
Note: The R&R Budget form included in many of the component types allows for up to 100 Senior/Key Persons in section A and 100 Equipment Items in section C prior to using attachments for additional entries. All other SF424 (R&R) instructions apply.
PHS 398 Research Plan (Administrative Core)
Introduction to Application: For Resubmission and Revision applications, an Introduction to Application is allowed for each component.
Specific Aims: All Centers must have an Administrative Core that provides scientific direction and administrative leadership, performs key decision-making functions, develops and implements strategic plans, monitors and evaluates progress toward Center goals and objectives, and ensures efficient fiscal operations. The Specific Aims should describe how the Core administration will coordinate and manage activities across the Center and have an impact on the research infrastructure and strategies.
Research Strategy: Organize the Research Strategy into sections on Significance, Innovation, and Approach.
Significance: Explain the role of the Administrative Core in the Center as a whole and the responsibility of the Core for the overall administration, coordination and management of the Center.
Innovation: Explain the unique utilization of Core resources to achieve the scientific goals of all HIV/AIDS investigators at the participating institutions.
Approach: A well-developed administrative plan is integral to the success of the Center and must be clearly defined in the application. The plan should include a discussion of the structure, referencing, as necessary, the roles of administrative staff, including the functions to be performed.
The Administrative Core should clearly identify personnel and resources needed to oversee the Center and ensure management of the Cores. The Core must provide a clear and explicit discussion of how fiscal and other resources will be prioritized, allocated, and managed.
The process for implementing the activities to achieve the goals set by the Center should be clearly defined. Mechanisms for internal review, decision-making, and priority-setting processes appropriate to planned activities must be defined. Appropriate criteria and review processes for evaluating affiliated investigators' ongoing participation in the Center should be established and include such factors as productivity, research direction, and overall contribution.
Centers should articulate a plan to measure progress toward strengthening the research capacity of the home institution. Evidence of such impacts may include broad dissemination of research products, citations by other published materials, or other indicators of scientific advancement.
The administrative structure must include an external Advisory Committee charged with providing objective advice and evaluation of Centers' strategic directions, research programs, community engagement, capacity building, and other key activities. A plan for meeting with the advisory committee should be outlined and a process for responding to its recommendations should be delineated. Information from this advisory committee meeting should become part of the annual progress report, including the date/s of the meeting; a brief, written report from the advisory committee; and the Administrative Core Director's written responses to the advisory committee's recommendations, if any. This should be included as an attachment to item G.1 of the RPPR.
The Administrative Core will also manage interactions with the Advisory Committee. Describe the plans and procedures for nominating and selecting members of this committee. Describe the expected composition of the Advisory Committee (number of members, types of expertise, etc.). Do NOT name the members of the committee who are not from the Center Institution in the application so as not to limit the pool of potential reviewers. Do not contact potential members until review is complete. The Advisory Committee should include both users of the Cores and investigators external to the Center who may have expertise in the design and management of other core facilities or expertise in the technologies, services, and/or resources offered by the Cores or expertise in the research fields covered by the research community.
Procedures should be outlined for a set of specific actions used to conduct the affairs of the Center, and how various tasks will be accomplished within the Center. Policies and procedures should describe, for example:
- The proposed roles of the Administrative Core Director, Core Directors, Center investigators, advisory boards, and institutional officials in the decision-making process.
- The annual strategic planning process
- How funds will be allocated to all types of Cores and activities
- Procedures for changes in support of any Cores
- How Center-sponsored conferences, seminars, workshops, and other activities will be identified
- How priorities for communication, outreach and additional Center activities will be established
- The authority and responsibility of internal and external advisory committees
Resource Sharing Plan: Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the SF424 (R&R) Application Guide, with the following modification:
Only limited items are allowed in the Appendix. Follow all instructions for the Appendix as described in the SF424 (R&R) Application Guide; any instructions provided here are in addition to the SF424 (R&R) Application Guide instructions.
PHS Human Subjects and Clinical Trials Information (Administrative Core)
When involving human subjects research, clinical research, and/or NIH-defined clinical trials follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the SF424 (R&R) Application Guide, with the following additional instructions:
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, you must include at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or a Delayed Onset Study record.
All instructions in the SF424 (R&R) Application Guide must be followed.
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start). All instructions in the SF424 (R&R) Application Guide must be followed.
Developmental Core
When preparing your application, use Component Type ‘Developmental Core.’
SF424 (R&R) Cover (Developmental Core)
PHS 398 Cover Page Supplement (Developmental Core)
Research & Related Other Project Information (Developmental Core)
Project Narrative: Do not complete.
Project /Performance Site Location(s) (Developmental Core)
Research & Related Senior/Key Person Profile (Developmental Core
- In the Project Director/Principal Investigator section of the form, use Project Role of ‘Other’ with Category of ‘Developmental Core Director and provide a valid eRA Commons ID in the Credential field.
Budget (Developmental Core)
Developmental Cores may use up to 10% of the annual P30 award to offer short-term funding support for preliminary studies that will inform the development of larger, peer-reviewed research applications that can compete successfully for NIH or other funding. These funds can only be used to support 1 – 2 year, formative or feasibility studies proposed by new or established investigators, including the research activities of newly recruited faculty.
PHS 398 Research Plan (Developmental Core)
Specific Aims: Describe briefly how the Core will expand and promote the research priorities of the Center and how it will support the next generation of HIV/AIDS researchers.
Research Strategy: Organize the Research Strategy into sections on Significance, Innovation, and Approach. Describe how the proposed activities will contribute to meeting the Center's goals and objectives and explain the rationale for selection of the general methods and approaches proposed to accomplish the specific aims. This section also should indicate the relevance of the Developmental Core to the Overall Research Strategy of the Center application.
Significance: Describe the effect that the Developmental Core would have on Center investigators' HIV/AIDS research efforts. Describe how the Core provides added value to HIV/AIDS research conducted at the institution and describe the Core's ability to support the next generation of HIV/AIDS researchers.
Innovation: Explain the unique utilization of Core resources to achieve the scientific goals of HIV/AIDS investigators at the institution.
Approach: The Developmental Core can serve a range of Center functions, such as capacity building, providing internal peer review and support services, funding pilot or preliminary research, facilitating science generation, education in grant and manuscript writing, organizing seminars and conferences, and establishing new interdisciplinary collaborations that address emerging scientific priorities.
A Developmental Core can support 1 – 2 year, formative or feasibility studies proposed by new or established investigators, including the research activities of newly recruited faculty. These pilot award projects are expected to be innovative, interdisciplinary research that addresses the highest priority HIV prevention and treatment science that will position the investigator(s) for subsequent research that aligns with NIMH DAR priorities.
A systematic approach for soliciting, reviewing, and selecting rigorous pilot studies should be described in this section of the application. All pilot projects must comply with applicable NIH policies and the evidence that proposed plans for protection of human subjects; inclusion of women, minorities, and individuals of all ages; and assurance of animal welfare must be submitted to the NIMH Program Official prior to study initiation.
Resource Sharing Plan: Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the SF424 (R&R) Application Guide, with the following modification:
- Only limited items are allowed in the Appendix. Follow all instructions for the Appendix as described in the SF424 (R&R) Application Guide.
PHS Human Subjects and Clinical Trials Information (Developmental Core)
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, you must include at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or a Delayed Onset Study record.
All instructions in the SF424 (R&R) Application Guide must be followed
Delayed Onset Study All instructions in the SF424 (R&R) Application Guide must be followed
Research Core
When preparing your application in ASSIST, use Component Type 'Research Core.'
SF424 (R&R) Cover (Research Core)
PHS 398 Cover Page Supplement (Research Core)
Research & Related Other Project Information (Research Core)
Project Narrative: Do not complete.
Project /Performance Site Location(s) (Research Core)
Research & Related Senior/Key Person Profile (Research Core)
- In the Project Director/Principal Investigator section of the form, use Project Role of ‘Other’ with Category of ‘Research Core Director' and provide a valid eRA Commons ID in the Credential field.
- Research Core Directors must be recognized scientists and skilled administrators who are well qualified to lead the Core to achieve its goals and objectives. Each Research Core Director must be experienced in the relevant scientific area and assume responsibility for the scientific, administrative, and operational aspects of the Core.
Budget (Research Core)
The time commitment of a Research Core Director should be based on the breadth and complexity of the Core and the effort needed to administer it but should not fall below 2.4 person months.
PHS 398 Research Plan (Research Core)
Specific Aims: Clearly state how the Core will contribute to the goals of the Center, how this Core will provide services to HIV/AIDS research beyond what is currently available, and/or increase effectiveness by sharing expertise or centralizing labor-intensive tasks, how it will encourage and facilitate collaborative work, or provide user training to new investigators.
Research Strategy: Organize the Research Strategy into sections on Significance, Innovation, and Approach. Describe how the proposed activities will contribute to meeting the Center's goals and objectives and explain the rationale for selection of the general methods and approaches proposed to accomplish the specific aims. This section should also indicate the relevance of the Core to the Overall Research Strategy of the Center application. Applicants should describe the operation of the Core.
Significance: Describe the effect that a Core would have on Center investigators' HIV/AIDS research efforts. Describe how the Core provides added value to HIV/AIDS research conducted at the institutions. Describe the strengths of the Core.
Innovation: Describe how Core resources are utilized in unique ways to achieve the scientific goals of HIV/AIDS investigators at the participating institution.
Approach: A Research Core can be developed around any research activity that can provide resources to Center investigators. A Research Core is expected to be used as a shared resource and service and is intended to provide access to knowledge and technology that enhances the research productivity of the Center, scientific interaction within the Center and consultation being provided by the Center. A Research Core also provides access to services that facilitate the research and strengthens the administrative and organizational cohesion of the Center. A Research Core should clearly describe a plan for identifying new or expanded services that it provides. In addition, a Research Core should clearly describe a plan for identifying potential users of the shared resources, and for providing the resources to investigators who may request them. The potential benefits of these resources and a mechanism to evaluate these benefits must be detailed in the description of a Research Core.
PHS Human Subjects and Clinical Trials Information (Research Core)
All instructions in the SF424 (R&R) Application Guide must be followed.
3. Unique Entity Identifier and System for Award Management (SAM)
See Part 2. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
4. Submission Dates and Times
Part I. contains information about Key Dates and times. Applicants are encouraged to submit applications before the due date to ensure they have time to make any application corrections that might be necessary for successful submission. When a submission date falls on a weekend or Federal holiday , the application deadline is automatically extended to the next business day.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies) using ASSIST or other electronic submission systems. Applicants must then complete the submission process by tracking the status of the application in the eRA Commons , NIH’s electronic system for grants administration. NIH and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Applications that miss the due date and time are subjected to the NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications .
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in How to Apply- Application Guide.
5. Intergovernmental Review (E.O. 12372)
This initiative is not subject to intergovernmental review .
6. Funding Restrictions
All NIH awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Pre-award costs are allowable only as described in the NIH Grants Policy Statement Section 7.9.1 Selected Items of Cost.
7. Other Submission Requirements and Information
Applications must be submitted electronically following the instructions described in the How to Apply - Application Guide . Paper applications will not be accepted.
For information on how applications will be automatically assembled for review and funding consideration after submission, refer to: http://grants.nih.gov/grants/ElectronicReceipt/files/Electronic_Multi-project_Application_Image_Assembly.pdf .
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit How to Apply - Application Guide . If you encounter a system issue beyond your control that threatens your ability to complete the submission process on-time, you must follow the Dealing with System Issues guidance. For assistance with application submission, contact the Application Submission Contacts in Section VII.
Important reminders:
All PD(s)/PI(s) and component Project Leads must include their eRA Commons ID in the Credential field of the Senior/Key Person Profile form . Failure to register in the Commons and to include a valid PD/PI Commons ID in the credential field will prevent the successful submission of an electronic application to NIH.
The applicant organization must ensure that the unique entity identifier provided on the application is the same identifier used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in How to Apply - Application Guide .
See more tips for avoiding common errors.
Upon receipt, applications will be evaluated for completeness and compliance with application instructions by the Center for Scientific Review, NIH. Applications that are incomplete or non-compliant will not be reviewed.
Use of Common Data Elements in NIH-funded Research
Many NIH ICs encourage the use of common data elements (CDEs) in basic, clinical, and applied research, patient registries, and other human subject research to facilitate broader and more effective use of data and advance research across studies. CDEs are data elements that have been identified and defined for use in multiple data sets across different studies. Use of CDEs can facilitate data sharing and standardization to improve data quality and enable data integration from multiple studies and sources, including electronic health records. NIH ICs have identified CDEs for many clinical domains (e.g., neurological disease), types of studies (e.g., genome-wide association studies (GWAS)), types of outcomes (e.g., patient-reported outcomes), and patient registries (e.g., the Global Rare Diseases Patient Registry and Data Repository). NIH has established a “Common Data Element (CDE) Resource Portal" ( http://cde.nih.gov/ ) to assist investigators in identifying NIH-supported CDEs when developing protocols, case report forms, and other instruments for data collection. The Portal provides guidance about and access to NIH-supported CDE initiatives and other tools and resources for the appropriate use of CDEs and data standards in NIH-funded research. Investigators are encouraged to consult the Portal and describe in their applications any use they will make of NIH-supported CDEs in their projects.
NIMH expects investigators for this funding announcement to collect Common Data Elements (CDEs) for HIV-related human subjects research funded by NIMH. Unless NIMH stipulates otherwise during the negotiation of the terms and conditions of a grant award, this Notice applies to all grant applications involving human research participants. The necessary funds for collecting and submitting these CDE data from all research participants to the NIMH Data Archive (NDA) should be included in the requested budget. A cost estimator ( https://nda.nih.gov/ndarpublicweb/Documents/NDA_Data_Submission_Costs.xlsx ) is available to facilitate the calculation of these costs. NIMH may seek further information regarding CDEs prior to award. Details for HIV-funded NIMH research can be found at NOT-MH-23-105 and the NIMH webpage on Data Management and Sharing for Applicants and Awardees .
Post Submission Materials
Applicants are required to follow the instructions for post-submission materials, as described in the policy .
Section V. Application Review Information
1. Criteria
Only the review criteria described below will be considered in the review process. Applications submitted to NIH in support of the NIH mission are evaluated for scientific and technical merit through the NIH peer review system.
A proposed Clinical Trial application may include study design, methods, and intervention that are not by themselves innovative but address important questions or unmet needs. Additionally, the results of the clinical trial may indicate that further clinical development of the intervention is unwarranted or lead to new avenues of scientific investigation.
Reviewers will provide an overall impact score to reflect their assessment of the likelihood for the project to exert a sustained, powerful influence on the research field(s) involved, in consideration of the following review criteria and additional review criteria (as applicable for the project proposed).
Reviewers will consider each of the review criteria below in the determination of scientific merit and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. For example, a project that by its nature is not innovative may be essential to advance a field.
Significance
Does the project address an important problem or a critical barrier to progress in the field? Is the prior research that serves as the key support for the proposed project rigorous? If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field?
Specific to this NOFO:
- How strong is the rationale that describes the benefit that the Center would have for Center investigators' HIV/AIDS research efforts?
- Are the proposed Center functions likely to provide added value to HIV/AIDS research conducted at the institution, and demonstrate the Center's ability to foster synergy and enhance HIV/AIDS research collaborations?
- To what extent does the thematic framework and research agenda focus on a significant public-health topic consistent with the HIV/AIDS research priorities of NIMH? Is there evidence that the Center will foster equitable partnerships of researchers with community?
- For renewal applications , is there evidence of prior success in terms of impact on the field of HIV more broadly, including but not limited to, peer-reviewed publications, new grant funding, mentorship, development of important collaborations or partnerships, changes in clinical practice, clinical guidelines, or policy?
In addition, for applications involving clinical trials
Are the scientific rationale and need for a clinical trial to test the proposed hypothesis or intervention well supported by preliminary data, clinical and/or preclinical studies, or information in the literature or knowledge of biological mechanisms? For trials focusing on clinical or public health endpoints, is this clinical trial necessary for testing the safety, efficacy or effectiveness of an intervention that could lead to a change in clinical practice, community behaviors or health care policy? For trials focusing on mechanistic, behavioral, physiological, biochemical, or other biomedical endpoints, is this trial needed to advance scientific understanding?
Investigator(s)
Are the PD(s)/PI(s), collaborators, and other researchers well suited to the project? If Early Stage Investigators or those in the early stages of independent careers, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative or multi-PD/PI, do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
With regard to the proposed leadership for the project, do the PD/PI(s) and key personnel have the expertise, experience, and ability to organize, manage and implement the proposed clinical trial and meet milestones and timelines? Do they have appropriate expertise in study coordination, data management and statistics? For a multicenter trial, is the organizational structure appropriate and does the application identify a core of potential center investigators and staffing for a coordinating center?
Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed?
Specific to this NOFO:
- To what extent does the application provide the degree of variety across the disciplines involved in HIV/AIDS research projects within the Center structure, and illustrate unique ways to incorporate community involvement, collaboration, and outreach in the Center?
Does the design/research plan include innovative elements, as appropriate, that enhance its sensitivity, potential for information or potential to advance scientific knowledge or clinical practice?
Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific aims of the project? Have the investigators included plans to address weaknesses in the rigor of prior research that serves as the key support for the proposed project? Have the investigators presented strategies to ensure a robust and unbiased approach, as appropriate for the work proposed? Are potential problems, alternative strategies, and benchmarks for success presented? If the project is in the early stages of development, will the strategy establish feasibility and will particularly risky aspects be managed? Have the investigators presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?
If the project involves human subjects and/or NIH-defined clinical research, are the plans to address:
1) the protection of human subjects from research risks, and 2) inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion or exclusion of individuals of all ages (including children and older adults), justified in terms of the scientific goals and research strategy proposed?
- Evaluate the strategic plan that is outlined which identifies the immediate and long-term goals of the Center. How well is the process defined in detail compelling that implementation of the activities will achieve the goals set by the Center?
- Evaluate the coordination among the operations and research. To what extent does the application describe each Core's essential function and aims, relevance to the Center's thematic area(s), and added-value to the HIV/AIDS science at the institution?
- Is there synergistic potential among the Center research components? How strong is the justification for each research Core and pilot/developmental studies in terms of the central theme and the overall research goals of the Center?
- Does the Center have the potential to achieve a whole greater than the sum of its parts? Are the plans for interactions with participating institutions and organizations adequately explained? Are the management plans and arrangements feasible?
Does the application adequately address the following, if applicable
Study Design
Is the study design justified and appropriate to address primary and secondary outcome variable(s)/endpoints that will be clear, informative and relevant to the hypothesis being tested? Is the scientific rationale/premise of the study based on previously well-designed preclinical and/or clinical research? Given the methods used to assign participants and deliver interventions, is the study design adequately powered to answer the research question(s), test the proposed hypothesis/hypotheses, and provide interpretable results? Is the trial appropriately designed to conduct the research efficiently? Are the study populations (size, gender, age, demographic group), proposed intervention arms/dose, and duration of the trial, appropriate and well justified?
Are potential ethical issues adequately addressed? Is the process for obtaining informed consent or assent appropriate? Is the eligible population available? Are the plans for recruitment outreach, enrollment, retention, handling dropouts, missed visits, and losses to follow-up appropriate to ensure robust data collection? Are the planned recruitment timelines feasible and is the plan to monitor accrual adequate? Has the need for randomization (or not), masking (if appropriate), controls, and inclusion/exclusion criteria been addressed? Are differences addressed, if applicable, in the intervention effect due to sex/gender and race/ethnicity?
Are the plans to standardize, assure quality of, and monitor adherence to, the trial protocol and data collection or distribution guidelines appropriate? Is there a plan to obtain required study agent(s)? Does the application propose to use existing available resources, as applicable?
Data Management and Statistical Analysis
Are planned analyses and statistical approach appropriate for the proposed study design and methods used to assign participants and deliver interventions? Are the procedures for data management and quality control of data adequate at clinical site(s) or at center laboratories, as applicable? Have the methods for standardization of procedures for data management to assess the effect of the intervention and quality control been addressed? Is there a plan to complete data analysis within the proposed period of the award?
Environment
Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
If proposed, are the administrative, data coordinating, enrollment and laboratory/testing centers, appropriate for the trial proposed?
Does the application adequately address the capability and ability to conduct the trial at the proposed site(s) or centers? Are the plans to add or drop enrollment centers, as needed, appropriate?
If international site(s) is/are proposed, does the application adequately address the complexity of executing the clinical trial?
If multi-sites/centers, is there evidence of the ability of the individual site or center to: (1) enroll the proposed numbers; (2) adhere to the protocol; (3) collect and transmit data in an accurate and timely fashion; and, (4) operate within the proposed organizational structure?
Additional Review Criteria - Overall
As applicable for the project proposed, reviewers will evaluate the following additional items while determining scientific and technical merit, and in providing an overall impact score, but will not give separate scores for these items.
- Is the Administrative Core Director an accomplished administrator capable of leading a complex, interdisciplinary organization to achieve its key aims and objectives?
- To what extent does the proposed Administrative Core have an appropriate and adequate administrative structure with an internal organization capable of planning, conducting, and evaluating Center activities?
- How well does the Core clearly delineate procedures and plans for Center administration, data management, and analysis?
- How clear are the mechanisms for internal review, decision-making, and priority-setting processes appropriate to conduct the activities of the Center?
- Is a standing outside advisory committee proposed or established that can provide appropriate and objective advice and evaluation regularly to the Administrative Core Director, and is an appropriate process well-detailed for responding to recommendations of the committee?
- Are the Developmental Core leadership and personnel well suited to the project? If Early Stage Investigators or those in the early stages of independent careers, how strong is the justification for their experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If there is Core co-leadership, do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
- To what extent is the Developmental Core Director scientifically qualified to direct the activities of the Core, experienced in the scientific areas in which the Core proposes to develop pilot projects, as well as in the scientific process of developing ideas into applications?
- Does the proposed Developmental Core Director demonstrate the ability to assume responsibility for the administrative and operational aspects of the Developmental Core?
- Is the plan to recruit innovative pilot projects by independent investigators well-detailed with the goal of providing support for short term scientific studies in order to develop preliminary data for peer-reviewed research applications?
- Is the mechanism for reviewing potential projects, making funding decisions and awards, and monitoring projects to ensure effective use of pilot project funds clearly described?
- How clear is the process for assuring that pilot projects conducted under the Center's auspices comply with applicable NIH policies?
- For renewal applications , what is the strength of the evidence of prior success in fostering the career development trainees and Early-Stage Investigators? This isncluding but not limited to, publications grant funding, mentoring from trainees/ESIs, leadership positions in public health, healthcare, or government service, or other demonstrations of impact on the field of HIV more broadly.
Research Cores
- What is the strength of the Research Cores leadership and personnel for leading the Research Core activities? If Early Stage Investigators or those in the early stages of independent careers, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If there is Core co-leadership, do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
- To what extent is the Research Core Director scientifically qualified to handle the nature and complexity of the research objectives of the Research Core, experienced in the scientific area in which the Core functions, and has demonstrated ability to assume responsibility for and manage the scientific, administrative, and operational aspects of the Research Core?
- Is the time commitment of the Research Core Director adequate based on the breadth and complexity of the Core and the effort needed to administer it?
- How well described is the Research Core activity, in terms of being structured around the proposed overall Center research activity and being capable of providing the necessary resources to meet the goals of the Center?
- Is the proposed Research Core adequately structured for use as shared resources and services intended to provide access to knowledge and technology to facilitate and enhance the research productivity of the Center? To what extent does the Core promote scientific interaction within the Center, and foster consultation with Center investigators?
- Is the research that is proposed to be conducted within the Research Core directed toward improving and expanding the resource, and how clearly defined is the plan for identifying new or expanded services that it could provide?
- Is there adequate justification for the shared resource? Are the potential benefits of these resources detailed, and is there a mechanism to evaluate these benefits?
- Is the research Core structured to reflect the overall level of funding requested for the Center?
Study Timeline
Specific to applications involving clinical trials
Is the study timeline described in detail, taking into account start-up activities, the anticipated rate of enrollment, and planned follow-up assessment? Is the projected timeline feasible and well justified? Does the project incorporate efficiencies and utilize existing resources (e.g., CTSAs, practice-based research networks, electronic medical records, administrative database, or patient registries) to increase the efficiency of participant enrollment and data collection, as appropriate?
Are potential challenges and corresponding solutions discussed (e.g., strategies that can be implemented in the event of enrollment shortfalls)?
Protections for Human Subjects
For research that involves human subjects but does not involve one of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials. For additional information on review of the Human Subjects section, please refer to the Guidelines for the Review of Human Subjects .
Inclusion of Women, Minorities, and Individuals Across the Lifespan
When the proposed project involves human subjects and/or NIH-defined clinical research, the committee will evaluate the proposed plans for the inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion (or exclusion) of individuals of all ages (including children and older adults) to determine if it is justified in terms of the scientific goals and research strategy proposed. For additional information on review of the Inclusion section, please refer to the Guidelines for the Review of Inclusion in Clinical Research .
Vertebrate Animals
The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following three points: (1) a complete description of all proposed procedures including the species, strains, ages, sex, and total numbers of animals to be used; (2) justifications that the species is appropriate for the proposed research and why the research goals cannot be accomplished using an alternative non-animal model; and (3) interventions including analgesia, anesthesia, sedation, palliative care, and humane endpoints that will be used to limit any unavoidable discomfort, distress, pain and injury in the conduct of scientifically valuable research. Methods of euthanasia and justification for selected methods, if NOT consistent with the AVMA Guidelines for the Euthanasia of Animals, is also required but is found in a separate section of the application. For additional information on review of the Vertebrate Animals Section, please refer to the Worksheet for Review of the Vertebrate Animals Section.
Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
Resubmissions
For Resubmissions, the committee will evaluate the application as now presented, taking into consideration the responses to comments from the previous scientific review group and changes made to the project.
For Renewals, the committee will consider the progress made in the last funding period.
For Revisions, the committee will consider the appropriateness of the proposed expansion of the scope of the project. If the Revision application relates to a specific line of investigation presented in the original application that was not recommended for approval by the committee, then the committee will consider whether the responses to comments from the previous scientific review group are adequate and whether substantial changes are clearly evident.
Additional Review Considerations - Overall
As applicable for the project proposed, reviewers will consider each of the following items, but will not give scores for these items, and should not consider them in providing an overall impact score.
Applications from Foreign Organizations
Select Agent Research
Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Resource Sharing Plans
Reviewers will comment on whether the Resource Sharing Plan(s) (e.g., Sharing Model Organisms ) or the rationale for not sharing the resources, is reasonable.
Authentication of Key Biological and/or Chemical Resources:
For projects involving key biological and/or chemical resources, reviewers will comment on the brief plans proposed for identifying and ensuring the validity of those resources.
Budget and Period of Support
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
2. Review and Selection Process
Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s), convened by NIMH in accordance with NIH peer review policy and procedures , using the stated review criteria. Assignment to a Scientific Review Group will be shown in the eRA Commons.
As part of the scientific peer review, all applications will receive a written critique.
Applications may undergo a selection process in which only those applications deemed to have the highest scientific and technical merit (generally the top half of applications under review) will be discussed and assigned an overall impact score.
Applications will be assigned to the appropriate NIH Institute or Center. Applications will compete for available funds with all other recommended applications. Following initial peer review, recommended applications will receive a second level of review by the appropriate national Advisory Council or Board. The following will be considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by scientific peer review.
- Availability of funds.
- Relevance of the proposed project to program priorities.
3. Anticipated Announcement and Award Dates
After the peer review of the application is completed, the PD/PI will be able to access their Summary Statement (written critique) via the eRA Commons . Refer to Part 1 for dates for peer review, advisory council review, and earliest start date.
Information regarding the disposition of applications is available in the NIH Grants Policy Statement Section 2.4.4 Disposition of Applications .
Section VI. Award Administration Information
1. award notices.
If the application is under consideration for funding, NIH will request "just-in-time" information from the applicant as described in the NIH Grants Policy Statement . This request is not a Notice of Award nor should it be construed to be an indicator of possible funding.
A formal notification in the form of a Notice of Award (NoA) will be provided to the applicant organization for successful applications. The NoA signed by the grants management officer is the authorizing document and will be sent via email to the recipient's business official.
Recipients must comply with any funding restrictions described in Section IV.6. Funding Restrictions. Selection of an application for award is not an authorization to begin performance. Any costs incurred before receipt of the NoA are at the recipient's risk. These costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this NOFO will be subject to terms and conditions found on the Award Conditions and Information for NIH Grants website. This includes any recent legislation and policy applicable to awards that is highlighted on this website.
Institutional Review Board or Independent Ethics Committee Approval: Recipient institutions must ensure that protocols are reviewed by their IRB or IEC. To help ensure the safety of participants enrolled in NIH-funded studies, the recipient must provide NIH copies of documents related to all major changes in the status of ongoing protocols.
The NIMH has published policies and guidance for investigators regarding human research protection, data and safety monitoring, Independent Safety Monitors and Data and Safety Monitoring Boards, reportable events, and participant recruitment monitoring ( NOT-MH-19-027 ). The application’s PHS Human Subjects and Clinical Trials Information should reflect the manner in which these policies will be implemented for each study record. These plans will be reviewed by the NIMH for consistency with NIMH and NIH policies and federal regulations. The NIMH will expect clinical trials to be conducted in accordance with these policies including, but not limited to: timely registration to ClinicalTrials.gov, submission of review determinations from the clinical trial’s data and safety monitoring entity (at least annually), timely submission of reportable events as prescribed, and establishment of recruitment milestones and progress reporting.
Individual awards are based on the application submitted to, and as approved by, the NIH and are subject to the IC-specific terms and conditions identified in the NoA.
ClinicalTrials.gov: If an award provides for one or more clinical trials. By law (Title VIII, Section 801 of Public Law 110-85), the "responsible party" must register and submit results information for certain “applicable clinical trials” on the ClinicalTrials.gov Protocol Registration and Results System Information Website ( https://register.clinicaltrials.gov ). NIH expects registration and results reporting of all trials whether required under the law or not. For more information, see https://grants.nih.gov/policy/clinical-trials/reporting/index.htm
Institutional Review Board or Independent Ethics Committee Approval: Grantee institutions must ensure that all protocols are reviewed by their IRB or IEC. To help ensure the safety of participants enrolled in NIH-funded studies, the recipient must provide NIH copies of documents related to all major changes in the status of ongoing protocols.
Data and Safety Monitoring Requirements: The NIH policy for data and safety monitoring requires oversight and monitoring of all NIH-conducted or -supported human biomedical and behavioral intervention studies (clinical trials) to ensure the safety of participants and the validity and integrity of the data. Further information concerning these requirements is found at http://grants.nih.gov/grants/policy/hs/data_safety.htm and in the application instructions (SF424 (R&R) and PHS 398).
Investigational New Drug or Investigational Device Exemption Requirements: Consistent with federal regulations, clinical research projects involving the use of investigational therapeutics, vaccines, or other medical interventions (including licensed products and devices for a purpose other than that for which they were licensed) in humans under a research protocol must be performed under a Food and Drug Administration (FDA) investigational new drug (IND) or investigational device exemption (IDE).
Prior Approval of Pilot Projects
Recipient-selected projects that involve {clinical trials or studies involving greater than minimal risk to human subjects} require prior approval by NIH prior to initiation.
- The recipient institution will comply with the NIH Guidance on Changes That Involve Human Subjects in Active Awards and That Will Require Prior NIH Approval .
- The recipient institution will provide NIH with specific plans for data and safety monitoring, and will notify the IRB and NIH of serious adverse events and unanticipated problems, consistent with NIH DSMP policies .
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the NoA. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Recipients, and Activities , including of note, but not limited to:
- Federal-wide Standard Terms and Conditions for Research Grants
- Prohibition on Certain Telecommunications and Video Surveillance Services or Equipment
- Acknowledgment of Federal Funding
If a recipient is successful and receives a Notice of Award, in accepting the award, the recipient agrees that any activities under the award are subject to all provisions currently in effect or implemented during the period of the award, other Department regulations and policies in effect at the time of the award, and applicable statutory provisions.
If a recipient receives and award, the recipient must follow all applicable nondiscrimination laws. The recipient agrees to this when registering in SAM.gov. The recipient must also submit an Assurance of Compliance ( HHS-690 ). To learn more, see the Laws and Regulations Enforced by the HHS Office for Civil Rights website .
HHS recognizes that NIH research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research. For additional guidance regarding how the provisions apply to NIH grant programs, please contact the Scientific/Research Contact that is identified in Section VII under Agency Contacts of this NOFO.
In accordance with the statutory provisions contained in Section 872 of the Duncan Hunter National Defense Authorization Act of Fiscal Year 2009 (Public Law 110-417), NIH awards will be subject to System for Award Management (SAM.gov) requirements. SAM.gov requires Federal agencies to review and consider information about an applicant in the designated integrity and performance system (currently SAM.gov) prior to making an award. An applicant can review and comment on any information in the responsibility/qualification records available in SAM.gov. NIH will consider any comments by the applicant, in addition to the information available in the responsibility/qualification records in SAM.gov, in making a judgement about the applicant’s integrity, business ethics, and record of performance under Federal awards when completing the review of risk posed by applicants as described in 2 CFR Part 200.206 “Federal awarding agency review of risk posed by applicants.” This provision will apply to all NIH grants and cooperative agreements except fellowships.
3. Data Management and Sharing
Consistent with the 2023 NIH Policy for Data Management and Sharing, when data management and sharing is applicable to the award, recipients will be required to adhere to the Data Management and Sharing requirements as outlined in the NIH Grants Policy Statement . Upon the approval of a Data Management and Sharing Plan, it is required for recipients to implement the plan as described.
When multiple years are involved, recipients will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the NIH Grants Policy Statement.
Progress reports should briefly describe status of pilot projects, including data and safety monitoring, and should notify NIH of serious adverse events and unanticipated problems.
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the NIH Grants Policy Statement . NIH NOFOs outline intended research goals and objectives. Post award, NIH will review and measure performance based on the details and outcomes that are shared within the RPPR, as described at 2 CFR Part 200.301.
The Federal Funding Accountability and Transparency Act of 2006 as amended (FFATA), includes a requirement for recipients of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All recipients of applicable NIH grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over the threshold. See the NIH Grants Policy Statement for additional information on this reporting requirement.
In accordance with the regulatory requirements provided at 2 CFR Part 200.113 and Appendix XII to 2 CFR Part 200, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (Responsibility/Qualification in SAM.gov, formerly FAPIIS). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 2 CFR Part 200 – Award Term and Condition for Recipient Integrity and Performance Matters.
Section VII. Agency Contacts
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants.
eRA Service Desk (Questions regarding ASSIST, eRA Commons, application errors and warnings, documenting system problems that threaten submission by the due date, and post-submission issues)
Finding Help Online: https://www.era.nih.gov/need-help (preferred method of contact) Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
General Grants Information (Questions regarding application instructions, application processes, and NIH grant resources) Email: [email protected] (preferred method of contact) Telephone: 301-637-3015
Grants.gov Customer Support (Questions regarding Grants.gov registration and Workspace) Contact Center Telephone: 800-518-4726 Email: [email protected]
Christopher Gordon, PhD National Institute of Mental Health (NIMH) Telephone: 240-627-3867 Email: [email protected]
Nicholas Gaiano, Ph.D. National Institute of Mental Health (NIMH) Telephone: 301-827-3420 Email: [email protected]
Rita Sisco National Institute of Mental Health (NIMH) Telephone: 301-443-2805 Email: [email protected]
Section VIII. Other Information
Recently issued trans-NIH policy notices may affect your application submission. A full list of policy notices published by NIH is provided in the NIH Guide for Grants and Contracts . All awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Awards are made under the authorization of Sections 301 and 405 of the Public Health Service Act as amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and 2 CFR Part 200.

Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files .
Study record managers: refer to the Data Element Definitions if submitting registration or results information.
Search for terms

- Advanced Search
- See Studies by Topic
- See Studies on Map
- How to Search
- How to Use Search Results
- How to Find Results of Studies
- How to Read a Study Record

- Learn About Studies
- Other Sites About Studies
- Glossary of Common Site Terms

- Submit Studies to ClinicalTrials.gov PRS
- Why Should I Register and Submit Results?
- FDAAA 801 and the Final Rule
- How to Apply for a PRS Account
- How to Register Your Study
- How to Edit Your Study Record
- How to Submit Your Results
- Frequently Asked Questions
- Support Materials
- Training Materials

- Selected Publications
- Clinical Alerts and Advisories
- Trends, Charts, and Maps
- Downloading Content for Analysis

- ClinicalTrials.gov Background
- About the Results Database
- History, Policies, and Laws
- ClinicalTrials.gov Modernization
- Media/Press Resources
- Linking to This Site
- Terms and Conditions
- Search Results
- Study Record Detail

An Open Comparative Study of the Effectiveness and Incomparable Study of the Immunogenicity and Safety of the Vaccine (CoviVac) for Adults Aged 60 Years and Older
- Study Details
- Tabular View
- No Results Posted

Inclusion Criteria:
Volunteers must meet the following inclusion criteria:
Type of participants
• Healthy volunteers or volunteers with a history of stable diseases that do not meet any of the criteria for non-inclusion in the study.
Other inclusion criteria
- Written informed consent of volunteers to participate in a clinical trial
- Volunteers who are able to fulfill the Protocol requirements (i.e., fill out a self-observation Diary, come to control visits).
Exclusion Criteria:
SARS-CoV-2 infection • A case of established COVID-19 disease confirmed by PCR and/or ELISA in the last 6 months.
Diseases or medical conditions
- Serious post-vaccination reaction (temperature above 40 C, hyperemia or edema more than 8 cm in diameter) or complication (collapse or shock-like condition that developed within 48 hours after vaccination; convulsions, accompanied or not accompanied by a feverish state) to any previous vaccination.
- Burdened allergic history (anaphylactic shock, Quincke's edema, polymorphic exudative eczema, serum sickness in the anamnesis, hypersensitivity or allergic reactions to the introduction of any vaccines in the anamnesis, known allergic reactions to vaccine components, etc.).
- Guillain-Barre syndrome (acute polyradiculitis) in the anamnesis.
- The axillary temperature at the time of vaccination is more than 37.0 ° C.
- Acute infectious diseases (recovery earlier than 4 weeks before vaccination) according to anamnesis.
- Donation of blood or plasma (in the amount of 450 ml or more) less than 2 months before inclusion in the study.
- Severe and/or uncontrolled diseases of the cardiovascular, bronchopulmonary, neuroendocrine systems, gastrointestinal tract, liver, kidneys, hematopoietic, immune systems.
- Is registered at the dispensary for tuberculosis, leukemia, oncological diseases, autoimmune diseases.
- Any confirmed or suspected immunosuppressive or immunodeficiency condition in the anamnesis.
- Splenectomy in the anamnesis.
- Neutropenia (decrease in the absolute number of neutrophils less than 1000/mm3), agranulocytosis, significant blood loss, severe anemia (hemoglobin less than 80 g/l) according to anamnesis.
- Anorexia according to anamnesis.
Prior or concomitant therapy
- Vaccination with any vaccine carried out within 30 days before vaccination / the first dose of the studied vaccine or planned administration within 30 days after vaccination / the last dose of the studied vaccine.
- Prior vaccination with an experimental or registered vaccine that may affect the interpretation of the study data (any coronavirus or SARS vaccines).
- Long-term use (more than 14 days) of immunosuppressants or other immunomodulatory drugs (immunoregulatory peptides, cytokines, interferons, immune system effector proteins (immunoglobulins), interferon inducers (cycloferon) during the six months preceding the study, according to anamnesis.
- Treatment with systemic glucocorticosteroids (≥ 20 mg of prednisone, or an analog, for more than 15 days during the last month).
- Volunteers who received immunoglobulin preparations or blood transfusion during the last 3 months prior to the start of the study according to anamnesis.
Other non-inclusion criteria
• Participation in any other clinical trial within the last 3 months.
Exclusion criteria:
- Withdrawal of Informed consent by a volunteer;
- The volunteer was included in violation of the inclusion/non-inclusion criteria of the Protocol;
- Any condition of a volunteer that requires, in the reasoned opinion of a medical researcher, the withdrawal of a volunteer from the study;
- Taking unauthorized medications (see section 6.2);
- The volunteer refuses to cooperate or is undisciplined (for example, failure to attend a scheduled visit without warning the researcher and/or loss of communication with the volunteer), or dropped out of observation;
- For administrative reasons (termination of the study by the Sponsor or regulatory authorities), as well as in case of gross violations of the Protocol that may affect the results of the study.
- For Patients and Families
- For Researchers
- For Study Record Managers
- Customer Support
- Accessibility
- Viewers and Players
- Freedom of Information Act
- HHS Vulnerability Disclosure
- U.S. National Library of Medicine
- U.S. National Institutes of Health
- U.S. Department of Health and Human Services

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- About the Director
- Advisory Boards and Groups
- Strategic Plan
- Offices and Divisions
- Careers at NIMH
- Staff Directories
- Getting to NIMH

Decoding the Mind: Basic Science Revolutionizes Treatment of Mental Illnesses
By Linda Brady, Margaret Grabb, Susan Koester, Yael Mandelblat-Cerf, David Panchision, Jonathan Pevsner, Ashlee Van’t-Veer, and Aleksandra Vicentic on behalf of the NIMH Division of Neuroscience and Basic Behavioral Science
March 21, 2024 • 75th Anniversary
Follow the NIMH Director on
For 75 years, NIMH has transformed the understanding and treatment of mental illnesses through basic and clinical research—bringing hope to millions of people. This Director’s Message, guest written by NIMH’s Division of Neuroscience and Basic Behavioral Science , is part of an anniversary series celebrating this momentous milestone.
The Division of Neuroscience and Basic Behavioral Science (DNBBS) at the National Institute of Mental Health (NIMH) supports research on basic neuroscience, genetics, and basic behavioral science. These are foundational pillars in the quest to decode the human mind and unravel the complexities of mental illnesses.
At NIMH, we are committed to supporting and conducting genomics research as a priority research area . As the institute celebrates its 75th Anniversary , we are spotlighting DNBBS-supported efforts connecting genes to cells to circuits to behavior that have led to a wealth of discoveries and knowledge that can improve the diagnosis, treatment, and prevention of mental illnesses.
Making gene discoveries
Medical conditions often run in families. For instance, if someone in your immediate family has high blood pressure, you are more likely to have it too. It is the same with mental disorders—often they run in families. NIMH is supporting research into human genetics to better understand why this occurs. This research has already led to the discovery of hundreds of gene variants that make us more or less likely to develop a mental disorder.
There are two types of genetic variation: common and rare. Common variation refers to DNA changes often seen in the general population, whereas rare variation is DNA changes found in only a small proportion of the population. Individually, most common gene variants have only a minor impact on the risk for a mental disorder. Instead, most disorders result from many common gene variants that, together, contribute to the risk for and severity of that disorder.
NIMH is committed to uncovering the role of genes in mental disorders with the aim of improving the lives of people who experience them. One of the many ways NIMH contributes to the discovery of common gene variants is by supporting the Psychiatric Genomics Consortium (PGC) . The consortium of almost 1,000 scientists across the globe, including ones in the NIMH Intramural Research Program and others conducting NIMH-supported research, is one of the largest and most innovative biological investigations in psychiatry.
Global collaborations such as the PGC are critical to amassing the immense sample sizes needed to identify common gene variants. Data from the consortium’s almost one million participants have already led to transformative insights about genetic contributors to mental illnesses and the genetic relationships of these illnesses to each other. To date, studies conducted as part of the consortium have uncovered common variation in over a dozen mental illnesses.
In contrast to common gene variants, rare gene variants are very uncommon in the general population. When they do occur, they often have a major impact on the occurrence of an illness, particularly when they disrupt gene function or regulation. Rare variants involving mutations in a single gene have been linked to several mental disorders, often through NIMH-supported research. For instance, a recent NIMH-funded study found that rare variation in 10 genes substantially increased the risk for schizophrenia. However, it is important to note that genetics is not destiny; even rare variants only raise the risk for mental disorders, but many other factors, including your environment and experiences, play important roles as well.
Because of the strong interest among researchers and the public in understanding how genes translate to changes in the brain and behavior, NIMH has developed a list of human genes associated with mental illnesses. These genes were identified through rare variation studies and are meant to serve as a resource for the research community. The list currently focuses on rare variants, but NIMH plans to continue expanding it as evidence accumulates for additional gene variants (rare or common).
Moreover, mental illnesses are a significant public health burden worldwide . For this reason, NIMH investments in genomics research extend across the globe. NIMH has established the Ancestral Populations Network (APN) to make genomics studies more diverse and shed light on how genetic variation contributes to mental disorders across populations. APN currently includes seven projects with more than 100 researchers across 25 sites worldwide.
Connecting biology to behavior
While hundreds of individual genes have been linked to mental illnesses, the function of most of these genes in the brain remains poorly understood. But high-tech advances and the increased availability of computational tools are enabling researchers to begin unraveling the intricate roles played by genes.
In addition to identifying genetic variation that raises the risk for mental illnesses, NIMH supports research that will help us understand how genes contribute to human behavior. This information is critical to discovering approaches to diagnose, treat, and ultimately prevent or cure mental illnesses.
An NIMH-funded project called the PsychENCODE consortium focuses on understanding how genes impact brain function. PsychENCODE is furthering knowledge of how gene risk maps onto brain function and dysfunction by cataloging genomic elements in the human brain and studying the actions of different cell types. The PsychENCODE dataset currently includes multidimensional genetic data from the postmortem brains of thousands of people with and without mental disorders.
Findings from the first phase of PsychENCODE were published as a series of 11 papers examining functional genomics in the developing and adult brains and in mental disorders. A second batch of PsychENCODE papers will be published later this year. These findings help clarify the complex relationships between gene variants and the biological processes they influence.
PsychENCODE and other NIMH-supported projects are committed to sharing biospecimens quickly and openly to help speed research and discovery.

Facilitating these efforts is the NIMH Repository and Genomics Resource (NRGR) , where samples are stored and shared. NRGR includes hundreds of thousands of samples, such as DNA, RNA, and cell lines, from people with and without mental disorders, along with demographic and diagnostic information.

Another NIMH initiative to connect risk genes to brain function is Scalable and Systematic Neurobiology of Psychiatric and Neurodevelopmental Disorder Risk Genes (SSPsyGene) . This initiative uses cutting-edge techniques to characterize the biological functions of 250 mental health risk genes—within the cells where they are expressed—to better understand how those genes contribute to mental illnesses. By systematically characterizing the biological functions of risk genes in cells, SSPsyGene will empower researchers to learn about biological pathways that may serve as new targets for treatment.
Genes also affect behavior by providing the blueprint for neurons, the basic units of the nervous system. Neurons communicate with each other via circuits in the brain, which enables us to process, integrate, and convey information. NIMH supports many initiatives to study the foundational role of neural networks and brain circuits in shaping diverse mental health-related behaviors like mood, learning, memory, and motivation.
For instance, studies supported through a basic-to-translational science initiative at NIMH focus on modifying neural activity to improve cognitive, emotional, and social processing . Similarly, another new funding opportunity encourages studies in humans and animals examining how emotional and social cues are represented across brain circuits to help address a core deficit in many mental disorders. These studies will increase understanding of the biological mechanisms that support behavior throughout life and offer interventions to improve these functions in healthy and clinical populations.
Developing treatments and therapeutics
The gene discovery and biology-to-behavior programs described here will lay the foundation for delivering novel therapeutics. To be prepared to rapidly implement findings from this research, NIMH supports several initiatives to identify behavioral and biological markers for use in clinical studies and increase our ability to translate research into practice.
Through its therapeutics discovery research programs , NIMH advances early stage discovery and development studies in humans and early efficacy trials for mental disorders. Taking these efforts a step further, NIMH supports the National Cooperative Drug Discovery/Development Groups for the Treatment of Mental Disorders , which encourage public–private partnerships to accelerate the discovery and development of novel therapeutics and new biomarkers for use in human trials. Moreover, NIMH is one of several institutes and centers in the NIH Blueprint Neurotherapeutics Network , launched to enable neuroscientists in academia and biotechnology companies to develop new drugs for nervous system disorders.
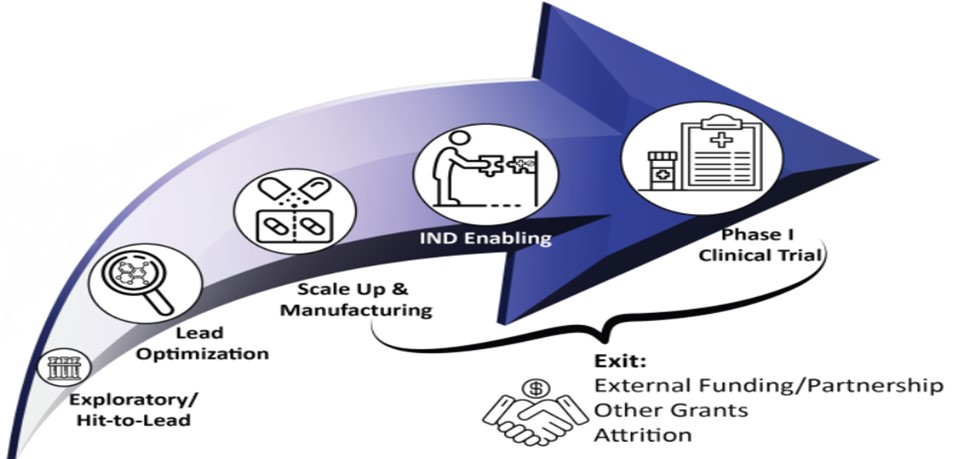
For the treatments of tomorrow, NIMH is building a new research program called Pre-Clinical Research on Gene Therapies for Rare Genetic Neurodevelopmental Disorders , which encourages early stage research to optimize gene therapies to treat disorders with prominent cognitive, social, or affective impairment. In parallel, NIMH’s Planning Grants for Natural History Studies of Rare Genetic Neurodevelopmental Disorders encourage the analysis of pre-existing data from people with rare disorders to learn about disease progression and enable future clinical trials with these populations.
NIMH's Division of Neuroscience and Basic Behavioral Science supports many different research projects that help us learn about genes and gene functions, how the brain develops and works, and impacts on behavior. By investing in basic neuroscience, genetics, and behavioral research, we're trying to find new targets for treatment and develop better therapies for mental disorders. We're hopeful these efforts will lead to new ways to treat and prevent mental illnesses in the near future and, ultimately, improve the lives of people in this country and across the globe.
This paper is in the following e-collection/theme issue:
Published on 27.3.2024 in Vol 13 (2024)
Patient Education and Decision Support for Long-Acting Injectable HIV Antiretroviral Therapy: Protocol for Tool Development and Pilot Testing with Ryan White HIV/AIDS Program Medical Case Management Programs in New York
Authors of this article:

- Mary Kathryn Irvine 1 , MPH, DPH ;
- Rebecca Zimba 2, 3 , MHS ;
- Tigran Avoundjian 1 , MPH, PhD ;
- Meghan Peterson 1 , MPH ;
- Connor Emmert 1 , MPH ;
- Sarah G Kulkarni 2 , MPH ;
- Morgan M Philbin 4 , MHS, PhD ;
- Elizabeth A Kelvin 2, 3 , MA, MPH, PhD ;
- Denis Nash 2, 3 , MPH, PhD
1 Bureau of Hepatitis, HIV and Sexually Transmitted Infections (BHHS), New York City Department of Health and Mental Hygiene, New York, NY, United States
2 Institute for Implementation Science in Population Health (ISPH), Graduate School of Public Health and Health Policy, City University of New York (CUNY), New York, NY, United States
3 Department of Epidemiology and Biostatistics, Graduate School of Public Health and Health Policy, City University of New York (CUNY), New York, NY, United States
4 Division of Vulnerable Populations, Department of Medicine, University of California San Francisco, San Francisco, CA, United States
Corresponding Author:
Mary Kathryn Irvine, MPH, DPH
Bureau of Hepatitis, HIV and Sexually Transmitted Infections (BHHS)
New York City Department of Health and Mental Hygiene
42-09 28th St.
New York, NY, 11101
United States
Phone: 1 347 396 7712
Fax:1 347 396 7559
Email: [email protected]
Background: Long-acting injectable (LAI) HIV antiretroviral therapy (ART) presents a major opportunity to facilitate and sustain HIV viral suppression, thus improving health and survival among people living with HIV and reducing the risk of onward transmission. However, realizing the public health potential of LAI ART requires reaching patients who face barriers to daily oral ART adherence and thus can clinically benefit from alternative treatment modalities. Ryan White HIV/AIDS Program Part A medical case management (MCM) programs provide an array of services to address barriers to HIV care and treatment among economically and socially marginalized people living with HIV. These programs have demonstrated effectiveness in improving engagement along the continuum of care, but findings of limited program impact on durable viral suppression highlight the need to further innovate and hone strategies to support long-term ART adherence.
Objective: This study aims to adapt and expand Ryan White MCM service strategies to integrate LAI ART regimen options, with the larger goal of improving health outcomes in the populations that could most benefit from alternatives to daily oral ART regimens.
Methods: In 3 phases of work involving patient and provider participants, this study uses role-specific focus groups to elicit perceptions of LAI versus daily oral ART; discrete choice experiment (DCE) surveys to quantify preferences for different ART delivery options and related supports; and a nonrandomized trial to assess the implementation and utility of newly developed tools at 6 partnering Ryan White HIV/AIDS Program Part A MCM programs based in urban, suburban, and semirural areas of New York. Findings from the focus groups and DCEs, as well as feedback from advisory board meetings, informed the design and selection of the tools: a patient-facing, 2-page fact sheet, including frequently asked questions and a side-by-side comparison of LAI with daily oral ART; a patient-facing informational video available on YouTube (Google Inc); and a patient-provider decision aid. Implementation outcomes, measured through provider interviews, surveys, and service reporting, will guide further specification of strategies to integrate LAI ART options into MCM program workflows.
Results: The study was funded in late April 2021 and received approval from the institutional review board in May 2021 under protocol 20-096. Focus groups were conducted in late 2021 (n=21), DCEs ran from June 2022 to January 2023 (n=378), and tools for piloting were developed by May 2023. The trial (May 2023 through January 2024) has enrolled >200 patients.
Conclusions: This study is designed to provide evidence regarding the acceptability, feasibility, appropriateness, and utility of a package of patient-oriented tools for comparing and deciding between LAI ART and daily oral ART options. Study strengths include formative work to guide tool development, a mixed methods approach, and the testing of tools in real-world safety-net service settings.
Trial Registration: Clinicaltrials.gov NCT05833542; https://clinicaltrials.gov/study/NCT05833542
International Registered Report Identifier (IRRID): DERR1-10.2196/56892
Introduction
Since 1990, the federal Ryan White HIV/AIDS Program (RWHAP) has funded cities and counties (via Part A) to provide HIV medical care and support services for those without alternative resources. The RWHAP is an essential platform for reducing HIV-related health disparities and scaling up evidence-based strategies to strengthen the HIV care continuum [ 1 , 2 ]. RWHAP service recipients account for more than half of the people living with HIV in the United States [ 1 , 3 - 6 ], and approximately 75% of people receiving HIV medical care in the United States attend facilities with RWHAP funding [ 2 ]. The central role of the RWHAP in the US response to HIV has been reaffirmed in the Ending the HIV Epidemic Plan [ 7 ]. In New York (NY), RWHAP Part A (RWPA) services primarily represent people who identify as Black (53%) or Latinx (37%), which are groups disproportionately affected by the US HIV epidemic [ 8 , 9 ] and less likely to be virally suppressed once diagnosed with HIV [ 10 ]. Among those retained in HIV care in 2022, people enrolled in RWPA services in New York City (NYC) were less likely to be virally suppressed (83% vs 93%) or experience durable viral suppression (defined as all viral loads <200 copies/mL) in 2022 (71% vs 86%) as compared with other people living with HIV in NYC. RWPA medical case management (MCM) programs are specifically designed to reduce barriers to HIV care and treatment through the coordination of medical and social services and the provision of antiretroviral therapy (ART) adherence support. The NYC Health Department oversees RWPA MCM services to >4000 people living with HIV per year in 29 programs in NYC and the suburban and semirural Tri-County area (Putnam, Rockland, and Westchester) north of NYC. Although these programs have shown effectiveness in engaging people in HIV care and treatment, substantial room for improvement in HIV viral suppression outcomes remains, highlighting the need to further innovate and develop strategies to increase long-term ART adherence [ 11 - 15 ].
Long-acting injectable (LAI) ART has been heralded as a major biomedical advance that could close gaps in the HIV care continuum, minimize HIV transmission risk, reduce racial and ethnic HIV outcome disparities, and accelerate the end of the HIV epidemic by making viral suppression attainable for those who struggle with daily oral ART adherence. After phase 3 clinical trials found a long-acting cabotegravir and rilpivirine injectable combination (CABENUVA) to be comparable in safety and efficacy and superior in patient satisfaction to daily oral ART [ 16 - 19 ], the US Food and Drug Administration (FDA) approved a monthly CABENUVA regimen in January 2021 [ 20 ] and a bimonthly regimen in February 2022 [ 21 ]. LAI ART addresses some important adherence barriers by eliminating the requirement for daily dosing, and patients have also noted that it can remove the daily reminder or stressor of HIV status and mitigate the risk of inadvertent HIV status disclosures [ 22 - 30 ]. However, there are challenges in LAI ART implementation that must be systematically addressed. Commonly reported barriers for patients include concerns over effectiveness [ 31 - 36 ], side effects [ 31 , 32 , 35 - 37 ], dosing frequency [ 33 , 35 , 36 ], getting to a clinic for each injection [ 34 , 38 , 39 ], and use of needles [ 32 , 37 ]. Providers have voiced concerns related to staffing resources, organizational logistics, patient readiness for LAI ART, adherence to injection appointments, and injection tolerability [ 35 , 40 , 41 ]. In the face of these challenges, uptake of LAI ART since its FDA approval has fallen short of expectations. As of the end of September 2023, approximately 20,000 people in the United States (including Puerto Rico) were on CABENUVA (Cinthya Avalos Meléndez of ViiV Healthcare, email to author, December 14, 2023). This represents <2% of the approximately 1.1 million people living with diagnosed HIV in the United States and its dependent areas [ 42 ]. Optimizing the public health impact of LAI ART will require implementation science to identify and tailor interventions to facilitate LAI uptake and engagement, particularly for the most marginalized patients. Without the necessary groundwork to assess and promote access, acceptability, and uptake in underserved populations, biomedical innovations tend to benefit those who are already relatively advantaged and can even exacerbate health disparities [ 43 - 45 ].
This paper describes the protocol for a mixed methods study designed to prepare RWPA MCM programs for extending LAI ART options to patients who have struggled to achieve or maintain viral suppression on daily oral ART and who have been underrepresented in phase 3 clinical trials. Specifically, we summarize the formative and survey research stages completed to guide the selection and development of LAI ART–related educational and decision-support tools, and we present the methods for a pilot test to evaluate and refine these tools for future scale-up.
Study Overview, Aims, and Partners
The Assessing Perceptions and Preferences Around Long-acting Injectables study is organized into 3 consecutive phases of work, each of which corresponds to a specific aim: (1) elicit perceptions, barriers, facilitators, and expectations of LAI versus daily oral ART delivery options in focus groups with RWPA MCM patients, core staff, and prescribing providers; (2) quantify preferences and drivers of engagement in ART delivery and support strategies, including options for LAI and daily oral ART, via discrete choice experiments (DCEs) with approximately 200 patients and 200 providers; and (3) select and pilot strategies to promote LAI ART uptake, adherence, and impact in real-world care settings. Each aim corresponds to distinct data collection activities, as summarized in Table 1 .
a MCM: medical case management.
b LAI: long-acting injectable.
c ART: antiretroviral therapy.
The study functions as an academic-government-provider collaboration, with a university-based lead agency working closely with a health department and 6 NY RWPA MCM service-provider agencies that were engaged as partners from the outset. The 6 provider agencies were purposively selected to represent the full range of NY RWPA MCM service settings (hospitals, community health centers, and social services community-based organizations) and the distinct geographic regions within the NY RWPA eligible metropolitan area. Three of the agencies have headquarters in NYC, which includes 5 counties: New York (Manhattan), Bronx, Kings (Brooklyn), Queens, and Richmond (Staten Island). The other 3 agencies have headquarters in the Tri-County area north of NYC: Westchester, Rockland, and Putnam. All 6 agencies had an annual caseload of at least 70 RWPA MCM patients at the time of the study proposal development. Although the FDA had not approved LAI ART until after the study proposal was submitted, LAI ART prescription was beginning when the project started and was being considered for some MCM enrollees at the partner agencies by the second year of the project in the months immediately preceding the pilot.
Completed Data Collection
Aim 1 focus groups.
The first study phase was directed at eliciting patient and provider perceptions and expectations of LAI versus daily oral ART, including barriers and facilitators to LAI ART use or delivery. The focus group guide was designed to explore potential influences (organized by domain from the Consolidated Framework for Implementation Research; CFIR [ 46 ]) on LAI ART implementation outcomes. Implementation outcomes were defined using RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) measures [ 47 ]. The primary purpose of the focus groups was to identify key intervention attributes (features of possible LAI ART implementation tools) to be included in the DCEs.
We set out to conduct focus groups of up to 9 participants each with NYC English-speaking patients, Tri-County English-speaking patients, Spanish-speaking patients, MCM administrators (who determine the use of program resources), MCM direct support service providers (who help develop and carry out patient care plans), and MCM program-affiliated primary care providers (who have the discretion to prescribe LAI ART). Patient focus group eligibility required being aged ≥18 years, currently enrolled in an RWPA MCM program at 1 of the 6 partnering agencies, comfortable conversing in Spanish or English, and virally unsuppressed (viral load ≥200 copies/mL) at most recent viral load test. Providers were eligible if they filled a core MCM role (administrator, support service provider, or prescribing provider) with any of the 6 partnering agencies. Provider focus group recruitment yielded 15 providers in 2 focus groups (administrators and support-service providers) plus 1 individual interview with a prescribing provider. Patient recruitment was more challenging and yielded 1 Tri-County participant and 4 NYC participants after no shows. Partner agencies had relatively few virally unsuppressed adults enrolled in MCM at the time of recruitment and noted that those patients were harder to engage in research. No Spanish-speaking patients were enrolled despite months of recruitment. Each participant received a US $25 gift card in appreciation of their time and input.
Focus group interview discussions were audio-recorded, transcribed, and coded in Dedoose using thematic analysis [ 48 ]. In a combined inductive-deductive approach, the qualitative analysts applied predefined codes derived from the CFIR and RE-AIM constructs and used grounded exploration to interpret category meanings [ 49 ] and attend to emergent themes salient to participants. Separately for patient and provider transcripts, initial codes were identified in an independent review of 1 focus group transcript by 2 study team members in dialogue with a senior investigator with extensive experience in conducting qualitative research. The draft (patient or provider) codebook was then independently applied to a second transcript, with discrepancies resolved in consensus sessions to optimize inter-rater reliability, yielding the final codebook that was applied to the entire data set. Findings were presented for study advisory board (AB) input and used for consensus- and evidence-based selection of 4 potential intervention attributes (with 3-4 levels each) for inclusion in the DCEs.
Patient and provider participants highlighted the potential for LAI ART options to reduce HIV stigma and adherence burden; providers noted that ART administration via injection by a health care worker offered protection against medication diversion. Both sets of participants mentioned COVID-19 vaccine-related attitudes and insurance preauthorization processes as barriers, and providers expressed concerns about the risks of missed injection appointments. Patients and providers desired more information and clearer messaging about LAI ART. The focus group participants also discussed MCM services that could be expanded to support LAI ART delivery: directly observed therapy, appointment transportation or accompaniment, reminder calls or texts, home visits, and financial incentives (eg, rewards for receiving an injection on time). These strategies, along with perceived LAI ART facilitators (less frequent injections, peer support, and postinjection follow-up communications from MCM staff) were integrated as treatment package features for the DCE surveys.
Aim 2 DCE Surveys
Dce methods.
DCEs offer an efficient means of assessing preferences and priorities for intervention-related attributes [ 50 ], which can guide intervention design and packaging to encourage uptake, engagement, and maintenance among the intended users. DCE participants are shown a series of choice sets juxtaposing ≥2 different hypothetical scenarios. Each scenario comprises intervention attributes, further defined by a number of levels, which are randomly combined to create hypothetical intervention alternatives. Participants are asked to choose the single preferred alternative for each choice set presented, recognizing the trade-offs between the desirable or undesirable characteristics of each. Through repetition of this process over several choice sets representing many possible combinations of attribute variations, investigators can identify which attributes and attribute levels participants value the most. Latent class multinomial logit regression is used to estimate utilities, which are measures of preference for levels within attributes. Positive utility values indicate greater preference.
DCE Survey Design
The aforementioned focus groups informed the team’s selection of four attributes: (1) type of ART, (2) service location or mode, (3) adherence support, and (4) rewards. The latter 3 were alternative-specific attributes defined according to the type of ART (daily oral or LAI). Focusing on 3 implementation outcomes identified by Proctor et al [ 51 ] as salient during the adoption phase of an innovation-decision process, we also included the brief (4-item) acceptability of intervention measure (AIM) on the provider and patient survey and the intervention appropriateness measure (IAM) and the feasibility of intervention measure (FIM) on the provider survey [ 52 ]. Contextual items at the end of the patient survey covered prior awareness of LAI ART and any experience with LAI ART, and contextual items at the end of the provider survey covered the length of time delivering MCM and vignettes about hypothetical patients as potential candidates for LAI ART.
Survey Recruitment
Eligibility for the patient DCE survey required being aged ≥18 years and enrolled in RWPA MCM services at any of the 6 partnering agencies. As with the focus groups, contact attempts for patient recruitment were conducted through MCM program staff at the partnering agencies. Eligibility for the provider DCE survey required having an RWPA MCM care team job role (ie, case manager or care coordinator, patient navigator or community health worker, program administrator with duties beyond clerical or data support, or prescribing provider). Given the target of 200 participants per survey (patient or provider) and the availability of fewer than 50 eligible providers at the 6 partner agencies, all 29 RWPA MCM programs were engaged for the provider survey. We offered the provider survey in English and the patient survey in English, Spanish, and Haitian Creole.
Patient Survey Participants and Preliminary Results
From June 2022 through January 2023, 201 NY RWPA MCM patients with a median age of 54 years (IQR 42-62) and a median MCM enrollment of 31 months (IQR 11-45) completed the DCE. Most patients (183/201, 91%) were Black or Latinx, and 39.3% (79/201) identified as women. Three-quarters (151/201, 75.1%) self-reported perfect adherence to daily oral ART. A two-group latent class analysis identified a smaller subset of patients with a strong preference for daily oral ART and a larger subset preferring LAI ART. Both groups preferred higher value monetary incentives and transportation to primary care or injection appointments over other rewards or supports for adherence. At the time of the survey, 85.6% (172/201) of the participants reported taking daily oral ART. About half of the participants (104/201) indicated that they had heard of LAI ART before taking the survey, but only 4.5% (9/201) reported having tried it.
Provider Survey Participants
From July 2022 through January 2023, 177 NY RWPA MCM staff members completed the DCE. Most provider participants identified as women (127/177, 71.8%), were aged 40 to 59 years (92/177, 52%), and had been providing RWPA MCM services for >2 years (124/177, 70.1%). The largest racial or ethnic group among the participating staff was Latinx or Hispanic (73/177, 41.2%), followed by non-Hispanic Black or African American (50/177, 28.2%), and non-Hispanic White (32/177, 18.1%). The most frequent job types were patient navigator (68/177, 38.4%), case manager or care coordinator (46/177, 26%), program director (33/177, 18.6%), and prescribing provider (21/177, 11.9%). As of the end of 2023, data from the provider DCE survey were in the early analysis stage.
Advisory Board
After receiving notice of the grant award (April 2021), the study team convened an AB for input in July 2021; February, May, and November 2022; and January, April, and August 2023. AB members, who consult on study tools, implementation, results interpretation, and dissemination, include clinical and nonclinical direct-service providers and administrators (from all 6 partnering provider agencies and a seventh interested MCM agency); Health Department staff; external researchers; and members of the HIV Health and Human Services Planning Council of NY (RWPA community planning body). In the January and April 2023 meetings, AB members provided critical input on the tools for inclusion in the pilot, including feedback on the content and visual design of draft versions.
Pilot Testing of ART Regimen Decision-Making Supports
Selection and design of tools to be piloted.
The third aim and phase of this study has focused on the synthesis and application of findings from aims 1 and 2 to develop LAI ART implementation tools and pilot test them with partnering agencies while gathering data to inform tool refinement for future research and scale-up. Tools were developed in consultation with the MCM program staff and other AB members familiar with MCM service settings, MCM patient needs, or decision-support processes. The selection and design of tools for the pilot study were guided by a combination of focus group findings, AB input, and preliminary patient DCE results. In addition to indirectly informing tool development by highlighting the attributes for inclusion in the DCEs, focus group findings and AB input directly influenced the study team’s decision to develop patient-facing educational materials. The latter decision stemmed from the focus group participants’ emphasis on the need for clearer messaging about LAI ART and AB members’ requests for materials that could be reviewed by patients on their own. AB members called attention to a general lack of patient-facing materials about LAI ART beyond the drug manufacturer’s consumer-directed materials, which partnering program staff viewed as unrelatable for many of their patients. Finally, the AB advocated for including a patient-facing video to present and compare ART regimen options in an engaging and entertaining medium while minimizing the time required during MCM visits for ART regimen-related education and decision-making processes.
The limited familiarity of patient DCE participants with LAI ART reinforced the need for clear, patient-facing educational materials describing LAI ART and directly comparing it with daily oral ART. Divergent patient preferences for daily oral versus LAI ART suggested a difference in perception between LAI ART phase 3 clinical trial participants and patients seen in our safety-net MCM program settings, which may reflect some of the patient-level factors limiting LAI ART uptake in practice. The mixed reception of LAI ART in the DCE reinforced the potential value of a decision-support tool to assist patients in weighing the advantages and disadvantages of available regimen types and assessing the fit of each option with their individual preferences, needs, challenges, and strengths as well as considering programmatic resources (such as transportation) that could reduce perceived barriers to treatment success. The study team developed a patient-provider ART regimen decision aid using the Ottawa Decision Support Framework [ 53 ] and tailored the tool to suit the RWPA MCM program context, the focus on ART regimen decisions, and the AB’s emphasis on brevity and accessibility.
Pilot Trial Design
Primary data collection for the pilot has entailed brief provider surveys and semistructured provider interviews drawing upon the CFIR to assess factors (barriers and facilitators) for pilot tool implementation. In addition, we intend to use the provider interviews and program reporting on pilot enrollment and service delivery to evaluate pilot implementation outcomes, drawing upon RE-AIM measures: reach (types and numbers of patients enrolled), effectiveness (preliminary effects on LAI ART uptake and concordance between treatment plan pursued and patient choice as recorded on the decision aid), adoption (documentation of specific tools’ use), implementation (alignment of reported tool use with study condition and guidance on tool use), and maintenance (ART adherence). This pilot study was designed as a nonrandomized, 2-arm trial of newly developed tools with ≥180 RWPA MCM patients. It was structured to allow comparison of an intervention arm including both patient education materials and the decision aid ( decision-aid arm ) with an intervention arm including only the patient education materials and usual care approaches (whatever agencies may already be doing) for ART regimen decision-making ( education-only arm ).
Study Setting and Participants
The pilot test was conducted at the 6 partnering RWPA MCM agencies. The characteristics and study arm assignments of the 6 agencies are summarized in Multimedia Appendix 1 . Cluster (agency-level) assignment was used for this study to minimize crossover between intervention conditions and avert the logistical and ethical dilemmas posed by assignment strategies that require providers to administer, maintain, and track different investigator-assigned intervention conditions within a single patient caseload [ 54 - 56 ]. Provider eligibility: Pilot-related primary data collection was limited to English-speaking adults responsible for overseeing or delivering RWPA MCM services or prescribing ART for MCM patients at a partnering agency. At each agency, 1 administrative staff member (eg, program director) and 1 direct-service provider (eg, case manager, patient navigator, or prescriber) was engaged to participate in implementation-related data collection through brief surveys and semistructured interviews. Patient eligibility: Patients were enrolled in a partnering NY RWPA MCM program, aged ≥18 years, and able to understand materials in English or Spanish. Patient eligibility for an LAI ART prescription could not be directly assessed by the study team and could be assessed differently by different providers or third-party payers; it could also change over time with FDA label indications. However, the study team communicated to partnering programs that patients should not be included in the pilot if they had known or suspected resistance to cabotegravir or rilpivirine, as this was a contraindication for LAI ART available as of the start of this pilot study (CABENUVA). On the basis of AB feedback that translating pilot materials to Spanish without translating to Haitian Creole would be viewed as inequitable, and given a lack of remaining time or resources to provide translations for tools (including audio for the video) in 3 languages, the pilot study was launched with only English-language tool versions.
Intervention Conditions
Education-only arm.
Participants received informational materials (including a fact sheet covering frequently asked questions [ Multimedia Appendix 2 ] and a short video [ Multimedia Appendix 3 ]) on their HIV treatment options and related support service options. These materials provide a comparison of the risks and benefits of LAI and oral ART regimens, set expectations about clinic visits, present information about side effects, and include additional resources to assist patients in preparing to discuss HIV treatment and support options with their care coordinator and prescribing provider. All materials are at or below an eighth-grade reading level, use graphics to bolster understanding for different levels of health literacy, and follow accessibility guidelines for font choice and size. Participants receiving this intervention were offered these materials by a care coordinator or patient navigator and were encouraged to review the materials on their own; they could also go over the materials with staff during an MCM program visit.
Decision-Aid Arm
Before or during an MCM visit, participants received informational materials (described in Education-Only Arm section) on HIV treatment types and related support service options. During an MCM visit, the participant and patient navigator or care coordinator reviewed the shared decision aid ( Multimedia Appendix 4 ) to weigh the participant’s treatment options and their fit to the participant’s interests, needs, assets, and constraints. The tool facilitates and records patient-provider agreement on a treatment plan to be integrated into the broader MCM care plan signed by both the patient and provider. Figure 1 illustrates the 2 intervention conditions and the workflow for each.

Nonrandom Assignment
We used purposive sampling to assign 3 of the 6 partnering RWPA MCM agencies to the decision-aid arm (which included the educational tools) and 3 to the education-only arm. Specifically, we ensured representation of at least 2 different agency types and MCM program sizes in each arm.
Owing to the need to engage service providers in the intervention through study team–led activities (eg, orientation to the project-specific educational materials or the decision aid), study arm assignments were transparent to agency staff and study team members (though not to patients).
Data Collection and Management
Aim 3 (pilot testing) involved repeated primary data collection with 2 providers at each of the 6 partnering agencies and use of secondary data on pilot participants, service delivery, and outcomes. In aim 3 pilot interviews with the implementing providers, oral informed consent was obtained in advance of the interview session by phone or videoconference. The interviews were audio-recorded and transcribed for coding in Dedoose. Aim 3 provider survey data were collected and managed using the NYC Health Department REDCap (Research Electronic Data Capture; Vanderbilt University) server, a web-based application for designing and managing surveys. The REDCap survey data will be analyzed using R (version 4.3.2; R Foundation for Statistical Computing).
Patient data for the pilot are being drawn from the local RWPA data system, the Electronic System for HIV/AIDS Research and Evaluation (eSHARE), in the form of provider-reported enrollments, services, patient characteristics, assessed needs, regimen types, and adherence data required for fee-for-service reimbursement in NY RWPA MCM contracts. eSHARE has been updated by the NYC Health Department to include pilot tools so that partnering MCM agencies could enter pilot activities along with (and as distinct from) other MCM services. No patients were enrolled in the RWPA MCM programs solely for the pilot; rather, patients already enrolled in MCM could be eligible to receive pilot-related services (ART regimen-related information and decision support with the tools developed for the project), along with the array of other MCM services. All patient- and service-level data are protected according to the Centers for Disease Control and Prevention’s physical and electronic data security and confidentiality policies [ 57 ]. Health Department staff extract and clean eSHARE data monthly, clean and freeze surveillance data sets quarterly, and conduct matches of the program to surveillance data semiannually. The deterministic matching algorithm has been described previously [ 58 ]. Through matching with surveillance data, patients are assigned a unique record number used to deduplicate data sets, which are stripped of personal identifiers before analysis.
Study Outcomes
Lai uptake (primary outcome).
The primary outcome is defined as the proportion of pilot participants who start LAI ART (whether initiating ART, transitioning from a prior regimen, or transitioning from a period of nonuse of ART). The denominator includes patients not already on LAI ART at the start of the pilot trial, and the numerator includes any of those in the denominator who began LAI ART during the trial. Using program-reported (eSHARE) data on regimen type, uptake will be measured continuously for up to 9 months (the duration of the pilot trial). Concordance: This outcome is defined as the proportion of participants whose intent (as documented via the decision aid) is carried through in terms of their subsequent treatment (self-administered daily oral ART, LAI ART, or some intermediate step such as directly observed therapy to meet the current FDA label requirement for viral suppression). Using eSHARE data on decision aid responses and subsequent regimen type or services related to ART adherence, concordance will be measured from the date of decision aid completion for up to 9 months. LAI maintenance: LAI maintenance is defined in terms of adherence to LAI ART, specifically the time from LAI initiation to first deviation from the injection schedule, among trial participants initiating LAI ART during the trial. A deviation includes any injection >1 week ahead of or 1 week after the treatment target date, where the injection target date depends on whether the prescribed regimen is monthly or bimonthly. Using eSHARE data on ART regimen type and adherence (as well as case closure owing to death), maintenance will be measured from the first report of LAI ART to the first reported deviation from the injection schedule or date of death from any cause, whichever comes first, assessed for up to 9 months from the first injection or up to 8 months of follow-up injections (counting from the first possible deviation from a scheduled follow-up injection).
Other Measures
To understand the influences on implementation, we assess provider-perceived acceptability, appropriateness, and feasibility of the tools at 3 points in the pilot: baseline, 4-5 months (midpoint), and 9 months (end of the pilot). Specifically, the provider survey includes the 4-item scales (AIM, IAM, and FIM) that were used in the provider DCE survey [ 52 ]. However, for the pilot survey, these 3 measures specifically refer to the pilot tools being implemented. The baseline and midpoint assessments also include a brief measure of organizational readiness for implementing change (ORIC), which captures commitment to change (5 items) and perceived efficacy to change (7 items) [ 59 ] based on the theory of organizational readiness for change [ 60 ]. As the ORIC measure is anticipatory, it is not part of the final assessment. The semistructured midpoint and end-of-pilot interviews elicit providers’ experiences of the piloted tools, including barriers, facilitators, and suggested refinements. Interviews will lend context to the ORIC, AIM, IAM, and FIM results and guide tool refinements.
Sample Size, Data Analysis, and Power Analysis
The original target was to enroll 180 patients or 30 (on average) per partner agency. As of mid-November 2023, 205 patients were enrolled. Using eSHARE data, we will describe trial participant demographics (eg, gender, age, race or ethnicity, income, and housing status) overall and by intervention arm. We will compare LAI ART uptake and, among those starting LAI ART, adherence by intervention arm, to assess the effects of the fuller package with the decision aid versus education alone. We will use log binomial regression to estimate risk ratios for uptake and adherence by intervention arm with generalized estimating equations and a small sample correction (owing to the small number of clusters) to maintain the type I error rate while accounting for clustering by site. To the extent possible given our sample size, we will adjust for potential confounders captured in eSHARE.
The brief provider survey measures will be analyzed through a comparison of responses on the same measures over time (eg, to assess change in perceived feasibility), as well as comparison of responses by intervention arm, agency type, and participant role. AIM, IAM, FIM, and ORIC scores will also be analyzed in relation to the levels of pilot service delivery as documented in eSHARE. To understand the potential for sustainability and scale-up, we will explore qualitative provider interview themes related to the acceptability and feasibility of the intervention. Qualitative and quantitative analyses will be integrated by examining thematic patterns reflecting RE-AIM and CFIR implementation constructs as they explain observed implementation outcomes [ 61 ].
Our pilot was designed to assess implementation of the newly developed tools and inform tool refinements. Data from the pilot will be used to generate estimates of LAI ART uptake that can, in turn, inform sample size and power calculations for a larger, controlled study of the refined tools’ implementation and treatment outcomes. Accordingly, data from the pilot study will be used to estimate the proportion of patients who start LAI ART and, among those initiating, the proportion who maintain LAI ART. With the original target of 180 patients (now exceeded), we will be able to estimate LAI uptake with precision (95% CI) with the levels of uptake described in Table 2 .
Assuming that half of the people who initiate LAI ART maintain it at 9 months, we will have the abovementioned precision estimates for the scenario of 50% LAI ART maintenance across a range of different levels of LAI ART uptake.
Ethical Considerations
This study was approved by the NYC Department of Health and Mental Hygiene IRB (protocol 20-096) and registered with ClinicalTrials.gov (Identifier NCT05833542, preresults). The pilot trial was granted a waiver of patient informed consent in accordance with the pre-2018 requirements set forth in 45 CFR 46.116(d) based on its reliance on secondary data analysis. Any changes to trial eligibility criteria, outcome measures, or analysis plans would be mutually agreed upon between the principal investigators, vetted with the AB, and submitted to the IRB as protocol modifications.
Informed consent was obtained from each participant before their participation in primary data collection: focus groups, DCE surveys, provider pilot interviews, or provider pilot surveys. No individual incentive was offered for provider pilot interviews or provider pilot surveys. Each focus group participant and each DCE survey participant received a US $25 gift card.
The primary data collected for this study are stored on secured servers without participant names or other personal identifiers. Study records can be linked to individual participants only by study team members, who retain a separate key linking study identifiers to other identifiers (eg, eSHARE identifier codes). Patient names are stored in the most secured environment, accessible only to NYC Health Department staff authorized to view names and only during a time-limited, secured session in Citrix, which prohibits access to the internet, email, portable media such as flash drives, or printers. All records used in secondary analyses are also stripped of personal identifiers before their inclusion in data sets for analysis.
The study was funded in late April 2021 and received official IRB approval in May 2021 under protocol 20-096. Focus groups were conducted in late 2021 and DCE data collection took place between June 2022 and January 2023. Patient education and patient-provider decision aid tools were developed by May 2023, and the trial continued through January 2024. Complete outcome data are expected by October 2024 and will be submitted for publication by December 2024. Study results will be reported in accordance with the CONSORT (Consolidated Standards of Reporting Trials) extension to cluster randomized trials and disseminated through scientific conferences, peer-reviewed publications, and meetings with local stakeholders. The investigators have also been sharing this work with colleagues in other jurisdictions and will disseminate the findings via the Ending the Epidemic Dashboard website.
As a biomedical advance that untethers HIV viral suppression from the requirement for daily medication adherence, LAI ART could greatly increase the opportunities for health, survival, transmission prevention, and health equity. However, realizing that potential will depend on reaching those who have difficulty achieving or maintaining viral suppression on daily oral ART regimens. In addition, at this relatively early stage of LAI ART availability through third-party payers, there is little information on how acceptable, appropriate, or feasible this treatment option may be for low-income, predominantly Black and Latinx patients and their HIV service providers. This study is designed to yield insights and field-tested strategies to help optimize the public health impact of LAI ART, with particular attention to the patient groups underrepresented in clinical trials, disproportionately burdened by HIV-related morbidity and mortality, and most able to benefit from gaining access to alternatives to daily oral ART. On the basis of the focus group participants’ and AB members’ calls for clear patient-facing information and findings of limited LAI ART familiarity and divergent ART regimen-type preferences among patient DCE participants, we prioritized the development and testing of a patient-facing fact sheet and educational video, along with a patient-provider decision aid to guide conversations about treatment options and related supports.
Limitations and Strengths
Agencies were purposively assigned to pilot intervention conditions (with the intent of balancing the characteristics of agencies represented in each condition) rather than through randomization. Additional limitations include a lack of blinding (of agencies, providers, or investigators) to the agencies’ assignments and a lack of control over agencies’, providers’, or patients’ exposure to other initiatives or interventions that may affect the outcome. We are aware of at least 1 other NY-based pilot test of LAI-related support strategies [ 62 ], as well as a NY State Department of Health clinical quality improvement committee focused on identifying and sharing LAI ART–related resources and best practices. However, the other NY-based pilot does not directly involve any of the agencies engaged in this trial. To our knowledge, our study is the first and only study to explicitly focus on the integration of LAI ART regimen options in RWPA or other RWHAP MCM service settings and the first to develop patient-directed LAI ART education materials or a patient-provider ART decision aid for use in an RWPA service population.
As with other HIV care quality improvement activities led by the NYC Health Department, agency participation is voluntary, meaning that agencies in the pilot could implement their assigned intervention condition incompletely or decline to implement. To address this limitation, we are tracking multiple implementation measures.
Conclusions
The United States National HIV/AIDS Strategy and National Institutes of Health have called for implementation science to produce evidence-based models of care [ 63 , 64 ]. Reviews of HIV care continuum research have also noted the need for practice-based evidence to inform program and policy development [ 65 - 67 ]. The implementation science design of this study has been selected to facilitate rapid research-to-practice translation of any LAI ART educational or decision-support tools that are found to be acceptable, feasible, and appropriate to these service settings and that show preliminary evidence of patient benefit. Through a robust academic-government-provider partnership, products from this study will be incorporated into local HIV services planning and delivery, as well as into future research.
Acknowledgments
This project was supported by the National Institutes of Health (NIH), grant number R34 MH 126809 (Project Officer Teri Senn), and by the Health Resources and Services Administration (HRSA), grant number H89HA00015 (Project Officer Axel Reyes). The authors would like to thank the 6 partnering provider agencies: State University of New York Downstate Health Sciences University (lead contacts: Lori Hurley and Antoinette Davis), La Casa de Salud (lead contacts: Mohammed Aldhuraibi and Pedro Gonzalez), Together Our Unity Can Health (lead contacts: Julian Palmer and Memunatu Attah-Issah), Open Door Family Medical Centers (lead contacts: Karin Palencia-Lua and José Miranda), Council on Adoptable Children (lead contacts: Denise Barrera and Katie Rivera), and Sun River Health (lead contacts: Aldonza Milian and Lisa Reid). This study also benefited from the support of other Ryan White Part A MCM service providers, Ryan White Part A participants, the HIV Health and Human Services Planning Council of New York, New York City Health Department HIV Care and Treatment Program leadership and quality management staff (Guadalupe Dominguez Plummer, Gina Gambone, Frances Silva, Grace Herndon, and Jacqueline Graham), and the Einstein-Rockefeller-CUNY Center for AIDS Research. The authors are indebted to Jennifer Carmona and Aimee Campbell, who provided early input critical to the grant proposal and the planning of the trial reported here. Finally, the authors would like to recognize the contributions of our other AB members and study team members (including Honoria Guarino, Will You, Chunki Fong, and Javier Lopez-Rios) to the larger Assessing Perceptions and Preferences Around Long-acting Injectables (APPLI) study and specifically the qualitative components and DCEs that guided the development of tools for pilot testing in the study’s clinical trial phase. Generative AI (eg, ChatGPT) was not used in the ideation or in the writing process of this protocol. The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the official views of the funding agencies supporting this project. The NIH and HRSA are not involved in the study design, conduct, or products, or in the decision to submit this work for publication. The cited personal communication from ViiV Healthcare, indicating approximately 20,000 people in the US (including Puerto Rico) on CABENUVA, is based on an estimate derived from the use of information under license from the following IQVIA information service: National Custom Patient with National Longitudinal Patient (LRx) for the period ending 2023-09-29. IQVIA expressly reserves all rights, including rights of copying, distribution, and republication.
Data Availability
The full institutional review board protocol and statistical code will be made available upon request. The NY State Public Health Law prohibits the study team from releasing a public use data set containing information tending to identify the participants in its research. The NYC Health Department staff retain sole custody of study data sets and designated staff are available to answer any inquiries. Any questions or requests for additional information can be emailed to the study contact person.
Authors' Contributions
MKI is the health department (NYC Department of Health and Mental Hygiene [DOHMH]) principal investigator and led the conception of the overall study and the writing of the R34 proposal. RZ (City University of New York [CUNY]) reviewed and edited all drafts of the grant proposal, led the analyses on aim 2 of the larger study, and contributed substantively to the preparation of the manuscript. TA (DOHMH) supervised the DOHMH analysts’ work on aims 1 and 3 of the larger study and contributed substantively to the study design and analysis plan. MP (DOHMH) and CE (DOHMH) conducted qualitative data collection for aim 1 and aim 3 and conducted qualitative analyses for aim 1. SGK (CUNY) and MMP (University of California, San Francisco [UCSF]) contributed substantively to the grant proposal and participated in ongoing planning. EAK (CUNY) contributed to the grant proposal and analysis plan and advised on study implementation. RZ (CUNY), TA (DOHMH), MP (DOHMH), and CE (DOHMH) prepared data, facilitated intervention implementation, participated in ongoing planning, and contributed to communication with service providers and other stakeholders while managing other aspects of the larger study. DN is the university-based, lead agency (CUNY) principal investigator and shares responsibility for the conceptualization and oversight of the overall study and this trial, serves as the primary contact with the grant funding agency, supervises CUNY team members on the grant, and guides dissemination. MKI drafted the manuscript, and all authors (MKI, RZ, TA, MP, CE, SGK, MMP, EAK, and DN) revised it for critically important intellectual content and provided the final approval of the manuscript.
Conflicts of Interest
The authors have reported only federal government grants to their institutions for this work. For work beyond this project, DN has received consulting fees from Gilead Sciences, Inc and Abbvie, Inc, and RZ, SGK, and DN have received research grant funding from Pfizer to their institution.
Partnering agency characteristics and intervention arm assignments for the pilot trial.
Long-acting injectable (LAI) antiretroviral therapy (ART) pilot fact sheet.
Long-acting injectable (LAI) antiretroviral therapy (ART) pilot video.
Long-acting injectable (LAI) antiretroviral therapy (ART) pilot decision aid.
- Mandsager P, Marier A, Cohen S, Fanning M, Hauck H, Cheever LW. Reducing HIV-related health disparities in the Health Resources and Services Administration's Ryan White HIV/AIDS Program. Am J Public Health. Nov 2018;108(S4):S246-S250. [ CrossRef ] [ Medline ]
- Weiser J, Beer L, Frazier EL, Patel R, Dempsey A, Hauck H, et al. Service delivery and patient outcomes in Ryan White HIV/AIDS program-funded and -nonfunded health care facilities in the United States. JAMA Intern Med. Oct 2015;175(10):1650-1659. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2015. HIV Surveillance Supplemental Report 2017. Jul 2017. URL: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-22-2.pdf [accessed 2024-02-23]
- Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep. Nov 28, 2014;63(47):1113-1117. [ FREE Full text ] [ Medline ]
- Doshi RK, Milberg J, Isenberg D, Matthews T, Malitz F, Matosky M, et al. High rates of retention and viral suppression in the US HIV safety net system: HIV care continuum in the Ryan White HIV/AIDS Program, 2011. Clin Infect Dis. Jan 01, 2015;60(1):117-125. [ CrossRef ] [ Medline ]
- Part A: grants to eligible metropolitan and transitional grant areas.. HRSA’s Ryan White HIV/AIDS Program. Sep 2023. URL: https://ryanwhite.hrsa.gov/sites/default/files/ryanwhite/resources/program-factsheet-part.pdf [accessed 2024-02-20]
- Ending the HIV epidemic in the U.S. Office of Infectious Disease and HIV/AIDS Policy, HHS. URL: https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview [accessed 2019-03-18]
- Sullivan PS, Satcher Johnson A, Pembleton ES, Stephenson R, Justice AC, Althoff KN, et al. Epidemiology of HIV in the USA: epidemic burden, inequities, contexts, and responses. Lancet. Mar 20, 2021;397(10279):1095-1106. [ CrossRef ] [ Medline ]
- Allgood KL, Hunt B, Rucker MG. Black: White disparities in HIV mortality in the United States: 1990-2009. J Racial Ethn Health Disparities. Mar 2016;3(1):168-175. [ CrossRef ] [ Medline ]
- Beer L, Bradley H, Mattson CL, Johnson CH, Hoots B, Shouse RL. Trends in racial and ethnic disparities in antiretroviral therapy prescription and viral suppression in the United States, 2009-2013. J Acquir Immune Defic Syndr. Dec 01, 2016;73(4):446-453. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Irvine MK, Chamberlin SA, Robbins RS, Myers JE, Braunstein SL, Mitts BJ, et al. Improvements in HIV care engagement and viral load suppression following enrollment in a comprehensive HIV care coordination program. Clin Infect Dis. Jan 15, 2015;60(2):298-310. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Irvine MK, Chamberlin SA, Robbins RS, Kulkarni SG, Robertson MM, Nash D. Come as you are: improving care engagement and viral load suppression among HIV care coordination clients with lower mental health functioning, unstable housing, and hard drug use. AIDS Behav. Jun 2017;21(6):1572-1579. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nash D, Robertson MM, Penrose K, Chamberlin S, Robbins RS, Braunstein SL, et al. Short-term effectiveness of HIV care coordination among persons with recent HIV diagnosis or history of poor HIV outcomes. PLoS One. Sep 24, 2018;13(9):e0204017. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Robertson MM, Penrose K, Nash D, Harriman G, Braunstein SL, Levin B, et al. Impact of an HIV care coordination program on the timeliness of viral suppression and immune recovery among clients newly diagnosed with HIV. AIDS Behav. Apr 2020;24(4):1237-1242. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Robertson MM, Penrose K, Irvine MK, Robbins RS, Kulkarni S, Braunstein SL, et al. Impact of an HIV care coordination program on durable viral suppression. J Acquir Immune Defic Syndr. Jan 01, 2019;80(1):46-55. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. Mar 19, 2020;382(12):1112-1123. [ CrossRef ] [ Medline ]
- Orkin C, Arasteh K, Górgolas Hernández-Mora M, Pokrovsky V, Overton ET, Girard PM, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. Mar 19, 2020;382(12):1124-1135. [ CrossRef ] [ Medline ]
- Orkin C, Oka S, Philibert P, Brinson C, Bassa AC, Gusev D, et al. Long-acting cabotegravir + rilpivirine for HIV treatment: FLAIR week 96 results. In: Proceedings of the Conference on Retroviruses and Opportunistic Infections. 2020. Presented at: CROI 2020; March 8-11 2020; Boston, MA. URL: https://www.croiconference.org/abstract/long-acting-cabotegravir-rilpivirine-for-hiv-treatment-flair-week-96-results/ [ CrossRef ]
- Overton ET, Richmond GJ, Rizzardini G, Jaeger H, Orrell C, Nagimova F, et al. Cabotegravir + rilpivirine every 2 months is noninferior to monthly: ATLAS-2M study. In: Proceedings of the Conference on Retroviruses and Opportunistic Infections. 2020. Presented at: CROI 2020; March 8-11, 2020; Boston, MA. URL: https://www.croiconference.org/abstract/cabotegravir-rilpivirine-every-2-months-is-noninferior-to-monthly-atlas-2m-study/ [ CrossRef ]
- FDA approves first extended-release, injectable drug regimen for adults living with HIV. U.S. Food & Drug Administration. Jan 21, 2021. URL: https://www.fda.gov/news-events/press-announcements/fda-approves-first-extended-release-injectable-drug-regimen-adults-living-hiv [accessed 2024-01-26]
- ViiV healthcare announces us FDA approval of Cabenuva (cabotegravir, rilpivirine) for use every two months, expanding the label of the first and only complete long-acting HIV treatment. ViiV Healthcare. Feb 1, 2022. URL: https://viivhealthcare.com/hiv-news-and-media/news/press-releases/2022/january/viiv-healthcare-announces-fda-approval-of-cabenuva-for-use-every-two-months/ [accessed 2024-01-26]
- Kilcrease C, Yusuf H, Park J, Powell A, Rn LJ, Rn JO, et al. Realizing the promise of long-acting antiretroviral treatment strategies for individuals with HIV and adherence challenges: an illustrative case series. AIDS Res Ther. Nov 26, 2022;19(1):56. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nachega JB, Scarsi KK, Gandhi M, Scott RK, Mofenson LM, Archary M, et al. Long-acting antiretrovirals and HIV treatment adherence. Lancet HIV. May 2023;10(5):e332-e342. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Palacios C, Wilpotte C, Adda A, Allaf S, Thibaut P, Chas J, et al. Expectations and acceptability of long-acting injectable antiretrovirals by patients living with HIV/AIDS. Infect Dis Now. Jun 2022;52(4):238-239. [ CrossRef ] [ Medline ]
- Mantsios A, Murray M, Karver TS, Davis W, Margolis D, Kumar P, et al. Efficacy and freedom: patient experiences with the transition from daily oral to long-acting injectable antiretroviral therapy to treat HIV in the context of phase 3 trials. AIDS Behav. Dec 2020;24(12):3473-3481. [ CrossRef ] [ Medline ]
- Harris S, Nikulina V, Gelpí-Acosta C, Morton C, Newsome V, Gunn A, et al. Prescription drug diversion: predictors of illicit acquisition and redistribution in three U.S. Metropolitan areas. AIMS Public Health. Dec 02, 2015;2(4):762-783. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Levi-Minzi MA, Surratt HL. HIV stigma among substance abusing people living with HIV/AIDS: implications for HIV treatment. AIDS Patient Care STDS. Aug 2014;28(8):442-451. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Surratt HL, O'Grady CL, Levi-Minzi MA, Kurtz SP. Medication adherence challenges among HIV positive substance abusers: the role of food and housing insecurity. AIDS Care. 2015;27(3):307-314. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kennedy CE, Zhao T, Vo AV, Nakubulwa R, Nabakka P, Jackson J, et al. High acceptability and perceived feasibility of long-acting injectable antiretroviral treatment among people living with HIV who are viremic and health workers in Uganda. AIDS Patient Care STDS. Jun 2023;37(6):316-322. [ CrossRef ] [ Medline ]
- Garris CP, Czarnogorski M, Dalessandro M, D'Amico R, Nwafor T, Williams W, et al. Perspectives of people living with HIV-1 on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings: results from the CUSTOMIZE hybrid III implementation-effectiveness study. J Int AIDS Soc. Sep 2022;25(9):e26006. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Simoni JM, Beima-Sofie K, Mohamed ZH, Christodoulou J, Tapia K, Graham SM, et al. Long-acting injectable antiretroviral treatment acceptability and preferences: a qualitative study among US providers, adults living with HIV, and parents of youth living with HIV. AIDS Patient Care STDS. Mar 2019;33(3):104-111. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Simoni JM, Tapia K, Lee SJ, Graham SM, Beima-Sofie K, Mohamed ZH, et al. A conjoint analysis of the acceptability of targeted long-acting injectable antiretroviral therapy among persons living with HIV in the U.S. AIDS Behav. Apr 2020;24(4):1226-1236. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Dandachi D, Dang BN, Lucari B, Swindells S, Giordano TP. Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care. Jun 2021;33(6):801-809. [ CrossRef ] [ Medline ]
- Carillon S, Gallardo L, Linard F, Chakvetadze C, Viard JP, Cros A, et al. Perspectives of injectable long acting antiretroviral therapies for HIV treatment or prevention: understanding potential users' ambivalences. AIDS Care. May 2020;32(sup2):155-161. [ CrossRef ] [ Medline ]
- Collins AB, Macon EC, Langdon K, Joseph R, Thomas A, Dogon C, et al. Perceptions of long-acting injectable antiretroviral therapy among people living with HIV who use drugs and service providers: a qualitative analysis in Rhode Island. J Urban Health. Oct 2023;100(5):1062-1073. [ CrossRef ] [ Medline ]
- Fletcher L, Burrowes SA, Khan GK, Sabin L, Johnson S, Kimmel SD, et al. Perspectives on long-acting injectable HIV antiretroviral therapy at an alternative care site: a qualitative study of people with HIV experiencing substance use and/or housing instability. Harm Reduct J. Jan 10, 2023;20(1):4. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Philbin MM, Parish C, Bergen S, Kerrigan D, Kinnard EN, Reed SE, et al. A qualitative exploration of women's interest in long-acting injectable antiretroviral therapy across six cities in the Women's Interagency HIV Study: intersections with current and past injectable medication and substance use. AIDS Patient Care STDS. Jan 2021;35(1):23-30. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Jolayemi O, Bogart LM, Storholm ED, Goodman-Meza D, Rosenberg-Carlson E, Cohen R, et al. Perspectives on preparing for long-acting injectable treatment for HIV among consumer, clinical and nonclinical stakeholders: a qualitative study exploring the anticipated challenges and opportunities for implementation in Los Angeles County. PLoS One. Feb 3, 2022;17(2):e0262926. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Philbin MM, Parish CL, Kinnard EN, Reed SE, Kerrigan D, Alcaide ML, et al. Multisite study of women living with HIV's perceived barriers to, and interest in, long-acting injectable antiretroviral therapy. J Acquir Immune Defic Syndr. Jul 01, 2020;84(3):263-270. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Collins LF, Corbin-Johnson D, Asrat M, Morton ZP, Dance K, Condra A, et al. Early experience implementing long-acting injectable cabotegravir/rilpivirine for human immunodeficiency virus-1 treatment at a Ryan White-funded clinic in the US South. Open Forum Infect Dis. Sep 2022;9(9):ofac455. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mantsios A, Murray M, Karver TS, Davis W, Galai N, Kumar P, et al. Multi-level considerations for optimal implementation of long-acting injectable antiretroviral therapy to treat people living with HIV: perspectives of health care providers participating in phase 3 trials. BMC Health Serv Res. Mar 20, 2021;21(1):255. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Diagnoses of HIV infection in the United States and dependent areas, 2021. Centers for Disease Control and Prevention. URL: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-34/index.html [accessed 2024-01-25]
- Singh GK, Azuine RE, Siahpush M. Widening socioeconomic, racial, and geographic disparities in HIV/AIDS mortality in the United States, 1987-2011. Adv Prev Med. 2013;2013:657961. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Havlir D, Gandhi M. Implementation challenges for long-acting antivirals as treatment. Curr Opin HIV AIDS. Jul 2015;10(4):282-289. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Meyers K, Golub SA. Planning ahead for implementation of long-acting HIV prevention: challenges and opportunities. Curr Opin HIV AIDS. Jul 2015;10(4):290-295. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. Aug 07, 2009;4:50. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. Sep 1999;89(9):1322-1327. [ CrossRef ] [ Medline ]
- Braun V, Clarke V. Thematic Analysis: A Practical Guide. Thousand Oaks, CA. SAGE Publications; 2021.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. Sep 18, 2013;13:117. [ FREE Full text ] [ CrossRef ] [ Medline ]
- de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. Feb 2012;21(2):145-172. [ CrossRef ] [ Medline ]
- Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. Mar 2011;38(2):65-76. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. Aug 29, 2017;12(1):108. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Stacey D, Légaré F, Boland L, Lewis KB, Loiselle MC, Hoefel L, et al. 20th anniversary Ottawa decision support framework: part 3 overview of systematic reviews and updated framework. Med Decis Making. Apr 2020;40(3):379-398. [ CrossRef ] [ Medline ]
- Featherstone K, Donovan JL. Random allocation or allocation at random? Patients' perspectives of participation in a randomised controlled trial. BMJ. Oct 31, 1998;317(7167):1177-1180. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Robinson EJ, Kerr C, Stevens A, Lilford R, Braunholtz D, Edwards S. Lay conceptions of the ethical and scientific justifications for random allocation in clinical trials. Soc Sci Med. Feb 2004;58(4):811-824. [ CrossRef ] [ Medline ]
- Heard K, O’Toole E, Naimpally R, Bressler L. Real-world challenges to randomization and their solutions. Poverty Action Lab. 2017. URL: https://www.povertyactionlab.org/sites/default/files/research-resources/2017.04.14-Real-World-Challenges-to-Randomization-and-Their-Solutions.pdf [accessed 2019-04-04]
- Data security and confidentiality guidelines for HIV, viral hepatitis, sexually transmitted disease, and tuberculosis programs: standards to facilitate sharing and use of surveillance data for public health action. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2011. URL: https://www.cdc.gov/nchhstp/programintegration/docs/PCSIDataSecurityGuidelines.pdf [accessed 2021-09-17]
- Drobnik A, Pinchoff J, Bushnell G, Ly S, Yuan J, Varma JK, et al. Matching HIV, tuberculosis, viral hepatitis, and sexually transmitted diseases surveillance data, 2000-2010: identification of infectious disease syndemics in New York City. J Public Health Manag Pract. 2014;20(5):506-512. [ CrossRef ] [ Medline ]
- Shea CM, Jacobs SR, Esserman DA, Bruce K, Weiner BJ. Organizational readiness for implementing change: a psychometric assessment of a new measure. Implement Sci. Jan 10, 2014;9:7. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Weiner BJ. A theory of organizational readiness for change. Implement Sci. Oct 19, 2009;4:67. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Creswell JW, Clark VL. Designing and Conducting Mixed Methods Research. Thousand Oaks, CA. SAGE Publications; 2006.
- The ALAI UP project. Columbia University. URL: https://www.alai-up.org/ [accessed 2023-11-17]
- National HIV/AIDS Strategy for the United States 2022–2025. The White House. 2021. URL: https://www.whitehouse.gov/wp-content/uploads/2021/11/National-HIV-AIDS-Strategy.pdf [accessed 2023-05-09]
- NIH Strategic Plan for HIV and HIV-related Research FY2021-2025. Office of AIDS Research, National Institutes of Health. URL: https://www.oar.nih.gov/sites/default/files/NIH_StrategicPlan_FY2021-2025.pdf [accessed 2023-05-09]
- Dombrowski JC, Irvine M, Nash D, Harriman G, Golden MR. Public health practice-driven research to improve HIV prevention in the United States. J Acquir Immune Defic Syndr. Dec 2019;82 Suppl 3(Suppl 3):S279-S285. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. Dec 2012;9(4):313-325. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Amico KR, Orrell C. Antiretroviral therapy adherence support: recommendations and future directions. J Int Assoc Provid AIDS Care. 2013;12(2):128-137. [ FREE Full text ] [ CrossRef ] [ Medline ]
Abbreviations
Edited by A Mavragani; The proposal for this study was peer reviewed by the National Institute of Mental Health.submitted 29.01.24; accepted 07.02.24; published 27.03.24.
©Mary Kathryn Irvine, Rebecca Zimba, Tigran Avoundjian, Meghan Peterson, Connor Emmert, Sarah G Kulkarni, Morgan M Philbin, Elizabeth A Kelvin, Denis Nash. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 27.03.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in JMIR Research Protocols, is properly cited. The complete bibliographic information, a link to the original publication on https://www.researchprotocols.org, as well as this copyright and license information must be included.
AIDS Walk NY
Join Trinity’s team for the annual AIDS Walk New York in support of AIDS treatment, research, and education. We'll meet for the 4-mile walk that begins and ends in Central Park. You can participate by walking with the team, and/or by donating or raising funds. The funds raised by AIDS Walk New York participants support much-needed HIV/AIDS services provided by GMHC and other HIV/AIDS service, social justice, and public health organizations in the tristate area. Visit aidswalkny.org for more information, or join Trinity’s team and donate at trinitywallstreet.org/AIDSwalk . Questions? Email Jayla Allen at [email protected] .
Share this event
Related articles, trinity’s team prepares for the aids walk 2023.
AIDS Walk: A Lifelong Commitment

IMAGES
VIDEO
COMMENTS
Aims and scope. AIDS Research and Therapy publishes studies of basic, translational, clinical, epidemiological, behavioral and educational science addressing treatment and prevention of HIV/AIDS and associated conditions. This includes studies of disease pathogenesis, novel treatment strategies and the impacts of established treatment regimans.
Advances in care over the last 30 years have helped transform HIV from a fatal disease into a chronic, manageable condition for many people. Innovations in treatment and access are at the core of ...
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as ...
AIDS Research and Therapy. Volumes and issues. Volume 19, issue 1. Search within journal. Search. Volume 19, issue 1, December 2022. 69 articles in this issue. Effects of syphilis infection among HIV-1-positive individuals on suppressive antiretroviral therapy Authors (first, second and last of 14)
We are excited to announce the 20th Anniversary issue of journal AIDS Research & Therapy. We invite manuscripts focused on latest research that may influence policy into curbing epidemic. Currently open for submissions. COVID-19 and HIV: clinical presentation, outcomes and impact on clinical services.
Find a journal Publish with us Track your research Search. Cart. AIDS Research and Therapy. Search within journal. Search. Volumes and issues. Volume 21 December 2024. December 2024, issue 1; Volume 20 December 2023. December 2023, issue 1; Volume 19 December 2022. December 2022, issue 1; Volume 18 December 2021 ...
AIDS Research and Therapy. Volumes and issues. Volume 18, issue 1. Search within journal. Search. Volume 18, issue 1, December 2021. 100 articles in this issue. Impact of using creative arts programming to support HIV treatment in adolescents and young adults in Eswatini Authors (first, second and last of 8)
The development of antiretroviral therapy for the prevention and treatment of HIV infection has been marked by a series of remarkable successes. ... Center for Clinical AIDS Research and Education ...
Leading the Way to a Cure. amfAR, The Foundation for AIDS Research, is one of the world's leading nonprofit organization dedicated to the support of AIDS research, HIV prevention, treatment education, and advocacy. Since 1985, amfAR has invested more than $635 million in its programs and has awarded more than 3,800 grants to research teams ...
The Long Acting Therapy to Improve Treatment Success in Daily Life study (LATITUDE/ ACTG A5359; NCT03635788), sponsored by the NIH Division of AIDS throughout the AIDS Clinical Trials Group (ACTG) is an ongoing Phase III, 4-step, 180-week open-label study which will compare treatment efficacy, safety, and durability of CAB LA + RPV LA Q4W to an ...
Scientists at the NIAID Vaccine Research Center (VRC) and NIAID-supported scientists at other institutions are developing and testing multiple antibodies for the treatment of HIV. Antibodies are good candidates for treatment because they have few side effects and can be modified to ensure they last a long time in the body, suggesting that ...
AIDS Research and Therapy. AIDS Research and Therapy is a peer-reviewed open access medical journal covering research on HIV/AIDS. It was established in 2004 and is published by BioMed Central. The editors-in-chief are Eric Arts ( University of Western Ontario) and Mark Boyd ( Kirby Institute ). According to the Journal Citation Reports, the ...
Journal profile. AIDS Research and Treatment publishes original research articles and review articles focused on all aspects of HIV and AIDS, from the molecular basis of the disease to translational and clinical research, prevention and education.
Research. amfAR's top research priority is the pursuit of a cure for HIV/AIDS. From 2015 to 2020, we invested close to $53 million in 109 cure-focused research projects in 15 countries. Grants and fellowships support teams of scientists pursuing innovative strategies including cell and gene therapy, shock and kill, and post-treatment control.
Articles from AIDS Research and Therapy are provided here courtesy of BMC. Follow NCBI. Connect with NLM. National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894. Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. NLM; NIH; HHS; USA.gov ...
Welcome to OAR. The Office of AIDS Research (OAR) coordinates HIV/AIDS research across the National Institutes of Health (NIH). The NIH provides the largest public investment in HIV/AIDS research globally. As HIV crosses nearly every area of medicine and scientific investigation, the response to the HIV pandemic requires a multi-Institute ...
Jan. 22, 2020 — Overcoming HIV latency -- induction of HIV in CD4+ T cells that lay dormant throughout the body - is a major step toward creating a cure for HIV. For the first time, scientists ...
By 2030, the World Health Organization (WHO), the Global Fund and UNAIDS are hoping to end the human immunodeficiency virus (HIV) and AIDS epidemic. An international team of researchers led by Eric Arts, professor at the Schulich School of Medicine & Dentistry, and Jamie Mann, senior lecturer at the University of Bristol (U.K.), has brought us another step closer to meeting this goal, by ...
Objective: To investigate the association of age at antiretroviral therapy (ART) initiation with CD4 +: CD8 + T-cell ratio in virally suppressed people with HIV on long-term ART, and to characterize potential CD4 +: CD8 + ratio recovery in this population by age.. Design: A longitudinal study of people attending an HIV clinic at the Royal Free Hospital NHS Trust, London, who initiated ART ...
If successful in clinical trials, Cummings celebrates the significance of the research and that the therapy could offer millions of people living with HIV a cure.
The ARCs focused on Neuro-HIV are encouraged to maintain collaborations with existing research resources such as National NeuroAIDS Tissue Consortium (NNTC), CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER), the MACS-WIHS Combined Cohort Study, and the AIDS Clinical Trials Group (ACTG) to facilitate high priority research relating to ...
HIV-infected individuals receiving regular antiretroviral therapy (ART) can present with a high viral load in cerebrospinal fluid (CSF) at times when it is suppressed in blood. This study presents data of HIV-infected patients who had undetectable or low plasma viral load in blood but presented with neurological signs and symptoms and were diagnosed to have CSF HIV viral escape. Records were ...
Treatment with systemic glucocorticosteroids (≥ 20 mg of prednisone, or an analog, for more than 15 days during the last month). Volunteers who received immunoglobulin preparations or blood transfusion during the last 3 months prior to the start of the study according to anamnesis. Other non-inclusion criteria
The Division of Neuroscience and Basic Behavioral Science (DNBBS) at the National Institute of Mental Health (NIMH) supports research on basic neuroscience, genetics, and basic behavioral science. These are foundational pillars in the quest to decode the human mind and unravel the complexities of mental illnesses. At NIMH, we are committed to supporting and conducting genomics research as a ...
Background: Long-acting injectable (LAI) HIV antiretroviral therapy (ART) presents a major opportunity to facilitate and sustain HIV viral suppression, thus improving health and survival among people living with HIV and reducing the risk of onward transmission. However, realizing the public health potential of LAI ART requires reaching patients who face barriers to daily oral ART adherence and ...
May 19, 2024, 9:00 am to 2:00 pm. Join Trinity's team for the annual AIDS Walk New York in support of AIDS treatment, research, and education. We'll meet for the 4-mile walk that begins and ends in Central Park. You can participate by walking with the team, and/or by donating or raising funds. The funds raised by AIDS Walk New York ...
Born of the Kaiser-Wilhelm Institute for Brain Research, who had been working on the biophysics of radiation. As for the Russian recruiters: at Leipzig there was a General "Katchkatchian" aided by a Major "Krassin"; a Colonel "K. K." Kikoin at Karlshorst had persuaded Hertz to go; and a Lt. Colonel "Kargin" had handled negotiations with Vollmer.