Upgrading medtech commercial operations in China
China’s burgeoning market for medtech, estimated at $70 billion in 2021, 1 Based on McKinsey analysis of National Health Commission (NHC) data and annual reports regarding revenue of more than 150 medtech companies and their market share. could more than double this decade if the government’s Healthy China 2030 plan stays on track. The plan projects 9 percent annual spending growth through this decade. 2 Based on McKinsey analysis of National Health Commission (NHC) data and annual reports regarding revenue of more than 150 medtech companies and their market share. This is significant. That growth would lift China’s healthcare spending as a percentage of GDP to almost equal footing with some developed economies in Europe and would lift China’s medtech market to about 20 percent of the global market by 2030.
China could remain the world’s most appealing growth market for medtech. The growth pace, however, has slowed, and uncertainties facing business leaders are likely to continue. While multinational medtech participants in China and domestic companies in this critical industry continue to deliver essential healthcare goods and services, they need to rethink—and, in most cases, restructure—their commercial operations in response to the new market dynamics.

The old model was geared for rapid (even sprawling) expansion
Many multinational companies (MNCs) and domestic companies in China’s once surging medtech market thrived under a commercial model that valued multiple layers in the sales force and sprawling networks of numerous distributors; financial rigor was secondary. The old model came into fashion when annual growth rates for the industry averaged 15 percent in the first decade of this century and more than 10 percent in the second decade (Exhibit 1).
This model needs to be reexamined. Medtech companies could look to redeploy resources with financial discipline in ways that sustain growth, improve efficiency, and deliver better products and services to customers and patients. They are operating in a new pricing and competitive landscape that has more diverse stakeholders and requires more discipline and innovation.
In some areas, domestic companies are markedly improving their performance across their operations. China’s 134 listed medtech companies generated $44 billion in 2021 revenues, an impressive CAGR of 36 percent since 2019—nearly triple the market’s overall rate of growth. More than five Chinese medtech companies have obtained the FDA’s breakthrough designation, which helps expedite the development, assessment and review of novel medical devices that can potentially provide more effective treatment or that diagnose life-threatening or irreversibly debilitating diseases or conditions.
Policy reforms have shifted the old model
Overall GDP growth in China slowed to 3.0 percent in 2022 and is expected to rise only modestly to 4.5 to 5.5 percent in 2023. 3 “The director of the National Bureau of Statistics answered reporters’ questions on the national economic operation in 2022,” National Bureau of Statistics, January 17, 2023. Baseline forecast is based on McKinsey analysis of data from several analyst houses, January 2, 2023. The government paid high prices for medtech equipment and services, which were a factor in attracting many of the world’s leading MNCs to China in the last two decades. However, after years of generous criteria for payments, recent reforms in the government’s procurement and medical insurance programs have shifted much of the old model. For example, the price for drug-eluting stents used in cardiac surgery was cut by more than 90 percent after reforms in 2021, decreasing to $100 from a previous range of $1,400 to $2,100. 4 “China’s high-value consumables industry has officially entered the era of volume-based procurement,” China Association for Medical Devices Industry, July 22, 2021. As a result, hospitals face significant cost constraints, especially for less differentiated, commoditized products.
Five levers for transforming the medtech commercial model in China
Multinational and domestic medtech companies could consider reevaluating their commercial model to sustain or increase growth in China, focusing on five interlinked functions in operations:
- channel restructuring to prepare for the price-sensitive environment while expanding coverage to sustain the growth
- commercial resources reassessment to adapt to evolving strategic priorities and outperform the market
- omnichannel customer engagement to improve sales efficiency, broaden reach, and enhance customer experience
- ecosystem partnerships to develop new innovation engines and deliver integrated solutions to a broader customer base
- a focus on the basics to cope with margin pressure and the rise of local players
Which of these levers is most difficult to pull? Which one can be a source of competitive advantage even for companies that have already started channel reforms or partnership scouting? There isn’t a singular answer. The optimal response will vary by segments and companies. Business leaders will need to explore suitable approaches with appropriate levels of emphasis and execution.
In our view, complex channel restructuring is most urgent for companies that relied heavily on high-markup distributors and for those whose portfolios were most affected by the government-mandated price drop (reforms known as volume-based procurement [VBP] 5 These tenders are for contracts issued through the government’s VBP reforms, which could be at national, provincial, or city levels across products. ). That said, VBP has created opportunities to serve broader markets. To tap into them, companies will also need effective ways to acquire new customers and retain existing ones via an omnichannel approach. Moreover, innovating through license-in and partnership agreements can help propel sustainable growth in the mid to long term. As VBP and trends in the macroeconomy ease the overall pace of revenue momentum in medtech, companies need to plan now to avoid having to continually put out fires in the near term.
Companies will also need to strengthen capabilities along the levers. For example, companies that have historically focused on consumables and that are now seeking to add equipment into their portfolio will need to recruit distributors with experience in engaging nonclinical stakeholders. They will also need to reassess their sales capabilities and resource allocation.
Great companies excel in this analysis. They can assess early the development needs for each lever, precisely identify the real source of advantage, efficiently transform commercial capabilities and models, and successfully outpace market growth.
Restructuring the channels for customer touch points
In a nation with more than 36,500 hospitals to care for 1.4 billion people, 6 “Number of medical and health institutions nationwide by the end of November 2021,” National Health Commission, January 17, 2022. the landscape of medtech companies and distributors has been fragmented. Distributors, together with the internal sales force of manufacturers, have largely acted as representatives to manufacturers’ customers, the hospitals, and clinics.
Two forces in the new economics of China’s healthcare system are upending these roles and responsibilities. First, VBP is sharply reducing margins left to distributors. Bidders are expected to cut price-to-hospital 7 Price-to-hospital is the price set by distributors when selling medtech products directly to hospitals. by more than 50 percent to win contracts. Second, as they pertain to medical insurance payments, reforms 8 Reforms in the government’s DRG/DIP will lower healthcare payments through 2025 in all Chinese cities. DRG/DIP reforms affect 70 percent of the government’s basic medical insurance funding, which covers medical expenses for 97 percent of the population. This is based on the Three-year action plan for DRG/DIP payment reform , issued by China’s National Healthcare Security Administration (NHSA) in December 2021. tied to diagnosis-related group or diagnosis intervention packet (DRG/DIP) have a compounding effect in reducing the profit margins of manufacturers and distributors. In certain product categories, smaller distributors that are unable to navigate these shifts profitably have already walked away. Distribution consolidation is evident. Sinopharm, for example, the largest healthcare distributor in China, reported a 21 percent increase to $17 billion for medtech revenues in 2021. Its expanding customer roster of manufacturers includes global leaders such as Medtronic, Johnson & Johnson, Danaher, Becton Dickinson, Stryker, and Smith+Nephew.
Medtech companies are responding to reforms by reevaluating their relationships with distributors. Roles and responsibilities are being reshaped as more companies are adapting to the new economics and sharply lowering payments they can offer distributors (Exhibit 2). Some have narrowed the tasks for platform distributors to only managing logistics and handling accounts receivable.
Since VBP reforms created the potential for larger orders and increased access to hospitals in the broader market, companies have reevaluated how to expand access. Some companies have expanded their distributor networks to create the potential for larger orders from hospitals or have invested in wider sales coverage to pursue and serve customers they previously could not reach. Others are bringing the distributor function in-house, consolidating provincial warehouse operations, and assessing distributors’ capabilities to define roles—followed by agreement on how tighter margins will be shared. Across the board, companies are more proactively managing their distributors and assessing the economics of that channel.
Reassessing and reallocating commercial resources
Despite its slowing growth pace, China’s market for medtech continues to offer huge potential. Companies that prove nimbler in redeploying management and sales resources to emerging product categories of high-growth innovation will likely outperform competitors that remain too focused on established categories.
In 2022, for example, Boston Scientific unveiled a series of innovative products, such as the Rezūm water vapor system for treating enlarged prostate glands. It also has put more resources into supporting new products. 9 “Two products of Boston Scientific have been approved for launch using Hainan Lecheng real-world data,” PEOPLE.CN, March 5, 2022. As another example, in response to the national VBP classifying coronary drug-eluting stents as a commodity, Abbott Laboratories pared sales staff supporting that product. Furthermore, the company’s focus on innovative continuous glucose monitors has increased annual sales for the monitors to more than $100 million. 10 “‘Disappearing’ medical device distributors,” MedTrend, QQ, February 18, 2022; Yang Xue, “The blood sugar market exploded, Abbott sold 20 billion RMB single product globally, and the domestic four companies are competing in the 10 billion market,” VBData, July 6, 2022.
Divesting commoditized assets or outsourcing them for a new company to distribute is also a potential path forward. In pharmaceuticals, as companies reduce costs, they can generate revenues by licensing out their less-differentiated products that have recorded declining profits. For example, Eli Lilly sold two commodity antibiotics and its corresponding Suzhou plant to Eddingpharm, a specialty pharmaceuticals manufacturer in Shanghai. In all cases, sales force synergy, partnership feasibility, and financial potential need to be carefully evaluated. Divesting or outsourcing assets might result in lower revenue in situations where manufacturers would need to share more margin with partners. In contrast, prospects for profits might be higher if those assets are retained under the existing model.
Overall, bottom-up sales and marketing analysis is essential to reassess commercial performance, understand cost-to-serve among portfolios, and strategize how to reallocate resources and investments. These steps will need to be considered with channel restructuring because adjustments will determine the workloads of the internal sales force.
Broadening omnichannel customer engagement
Better coordination of customer engagements across different channels, enabled by data and analytics, will help make sales operations more efficient in casting a wider net and enhancing customer experience. 11 “ Omnichannel engagement in medtech: The time is now ,” McKinsey, May 19, 2021.
Most medtech companies in China do connect with customers in multiple channels, both online and offline, but they rarely have coordinated engagement across channels. Efforts often are scattered among teams in marketing, sales, service, and distribution. Valuable insights created by each team from ongoing customer engagements are not promptly shared or made accessible.
To cite one common shortfall, marketing teams have made efforts in priority customer engagements involving online webinars and live streaming projects, but they often do not properly track, assess, and transfer all possible leads generated from digital marketing. Thus, they miss an opportunity to better equip frontline sales teams to follow up and be responsive to customer needs or questions.
Companies need to update and improve how their different departments communicate and work together. Crucial elements for sharpening omnichannel customer engagement include appropriately sharing data across functions and fostering collaboration among cross-functional teams to analyze the implications of that data. These changes can transform the fundamental ways an organization works.
Sales force capabilities and constraints are another challenge, especially when medtech companies expand into smaller (lower-tier) cities or markets for volume-based pricing products. Broadly speaking, companies in these circumstances need to take two important steps. First, they could learn from—and archive for easy access—intelligence from customer interactions across all the channels. Second, they could leverage data and analytics engines to better synchronize next-best actions with improved customer engagement effectiveness.
Partnering with other companies to expand possibilities
More partnerships, diverse stakeholders, and sources of solutions.
New alliances are helping traditional healthcare participants expand operations through various collaborations and, in many cases, build further momentum with domestic and multinational (MNC) medtech companies to pursue high-growth markets.
Ecosystem 1.0 – The Era of Expansion is a pragmatic alliance that involves traditional stakeholders, including government organizations, academic institutions, service providers, payers, investors, distributors, contractors, and other manufacturers.
As partnerships take shape, companies can expedite access to innovative offerings, broaden customer coverage with diverse commercial models, and achieve new options for manufacturing. In one case, Siemens Healthineers of Germany established a strategic collaboration with Shanghai Electric. They will jointly develop and localize medical equipment in China and accelerate penetration into grassroots healthcare institutions. In another case, Illumina, the global leader in groundbreaking sequencing and array technologies, partnered with Sequoia Capital to launch a genomics incubator in China.
A broader, more diverse set of stakeholders has coalesced and joined medtech companies in China to shape Ecosystem 2.0 – The Era of Transformation. China’s 14th Five-Year Plan articulates the urgency to spur partnerships by integrating global resources and pathbreaking digital technologies, including 5G, AI, and IoT.
Domestic companies and MNCs with domestic operations are embracing proven innovations by companies with headquarters beyond China’s borders to expand their offerings in high-growth product lines. Medtech licensing deals that were rarely observed three years ago have become more common.
New solutions are increasingly coming from collaborations with digital or AI companies and analytic integrators of data gathered from actual clinical outcomes. For example, Koninklijke Philips and Chinese online medical company Hongyun Rongtong agreed to jointly create a telemedicine platform to promote the adoption of high-quality medical resources by hospitals in smaller cities. Medtech companies can also learn from other industries, as innovators in many industries do. For example, an executive at Tencent, one of China’s most renowned internet and technology companies, said at a recent conference that its Intelligent Mobility unit benefits from “a rich auto service ecology” that encompasses about 40 auto manufacturers and the more than 150 models they offer.
Medtech companies in China have built partnerships and combined complementary strengths to capture value along the patient journey from prevention, diagnosis, and treatment to disease management in patient homes or outpatient clinics after treatment. Through these new relationships, manufacturers benefit from the network effect as escalating ranks of participants create the potential for more appealing opportunities (see sidebar, “More partnerships, diverse stakeholders, and sources of solutions”).
However, not all partnerships will prove to be sufficiently rewarded. How can companies best balance investment decisions and benefits returned? This remains an important question. A holistic assessment and the prioritization of ecosystem projects are essential to the success of medtech companies.
Staying true to the basics of commercial excellence
The basics in commercial excellence are becoming even more important as margin pressure grows and the quality of domestic companies improves.
Setting priorities for customer segments and allocating resources creatively and with discipline are essential to strengthening market positions. To be sure, changes in the landscape of healthcare providers in China have been dynamic. Yet, in some ways, the biggest factor—the expansion of large healthcare providers for patients—has not changed. More than 3,000 Class III hospitals (with 3.2 million beds)—those that offer comprehensive levels of care and sophisticated procedures—still contribute 56 percent of patient flow. 12 McKinsey analysis of data from the statistics center of the NHC. Recent government policies, such as tiered medical-system reforms and funding support, have diverted patient growth volume toward these large institutions in smaller cities.
Other priority basics include differentiating commercial models by product categories, optimizing sales responsibilities and coverage, and creating excellence in pricing. These fundamentals often do not get the attention they deserve. For example, capital equipment offerings should be linked to customer solutions. Leading medtech companies continue to benefit by providing educational programs with information that is valued by medical professionals, especially those featuring new products used in new procedures. Moreover, cost efficiencies are critical for products that have few differentiating elements. As mentioned above, these products often are candidates to be sold or discontinued after trade-offs are assessed.
Before starting reviews of these basics, leaders could consider a systematic value-oriented assessment: What do our distribution channels cost? Are we allocating resources in line with our strategic priorities?
Transforming the commercial model requires fundamental changes for medtech companies in China aspiring to win their share of the market’s alluring opportunities for growth. By embracing the five levers outlined in this article, they can strengthen a foundation for success in the new dynamics driving the nation’s evolving market for medtech.
Jody Tian is a partner in McKinsey’s Hong Kong office, Wei Wei is a senior expert in the Shanghai office, Kevin Y Wu is a partner in the Beijing office, and Delphine Zurkiya is a senior partner in the Boston office.
The authors wish to thank Franck Le Deu and Peter Pfeiffer for their contributions to this article.
Explore a career with us
Related articles.

AI for medtech commercial growth: Five missteps to avoid
Vision 2028: How China could impact the global biopharma industry
Accelerating growth in medtech: The next surge in portfolio moves
- Work & Careers
- Life & Arts
- Currently reading: China sets the pace in adoption of AI in healthcare technology
- The dark side of digital devices for children and how to beat it
- Africa’s health-tech start-ups find support from incubator cash
- Young people must be given a voice in crafting their digital health futures
- Pakistan start-up looks to break taboos around menstruation
- Mexican platform advances digital diagnostics in Latin America
- Kenyan mothers-to-be get the healthcare message
China sets the pace in adoption of AI in healthcare technology

- China sets the pace in adoption of AI in healthcare technology on x (opens in a new window)
- China sets the pace in adoption of AI in healthcare technology on facebook (opens in a new window)
- China sets the pace in adoption of AI in healthcare technology on linkedin (opens in a new window)
- China sets the pace in adoption of AI in healthcare technology on whatsapp (opens in a new window)
Eleanor Olcott
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
A live-stream video of a 76-year-old woman pottering about her kitchen plays on Li Hong’s phone. Li is in London, 8,700km from her mother in the Chinese city of Kunming.
Li has narrowed the distance between them by installing cameras in her mother’s apartment, where she lives alone. The system has built-in microphones and speakers, enabling the pair to discuss the latest readings from the blood pressure monitor of Li’s mother, who has a heart condition. “It’s like I am back in China with her. The technology is so convenient,” says Li.
China has been quick to deploy a range of new technologies to relieve the burden on hospitals, care systems and families caring for the sick and elderly. But it is in medical artificial intelligence that the country’s early adoption of new solutions has been particularly notable, says Eric Topol , a US doctor and author of Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again .
China has shifted faster than the US in medical AI from research to implementation, driven in part by the availability of high-quality data, says Topol. “China has a massive data advantage when it comes to medical AI research,” he says, explaining that Chinese researchers can train AI models on data sets covering entire provinces. In contrast, their US counterparts are restricted to working with information from single hospitals — largely operated by private businesses that keep records on internal servers.
AI is widely used in healthcare to help doctors analyse scans and images, improving the speed and accuracy of their diagnoses. Airdoc, a Beijing-based medical AI group, recently became the first company to gain regulatory approval for its retina-scanning software to be deployed in Chinese hospitals. “The eye is a window into the rest of the body,” says He Chao, Airdoc’s chief technology officer, noting that changes in the retina, including discolouration, can offer clues about conditions including high blood pressure and diabetes.
“In China, some of the early adoption of medical AI is also driven by need,” says Topol. “They don’t have enough radiologists and physicians to match up with the population.” Airdoc’s retina scanners have been deployed in rural hospitals that lack specialist ophthalmologists — China has 44,800 such practitioners to serve its rapidly ageing population of 1.4bn.
The success of companies such as Airdoc has relied on their access to vast amounts of diverse medical data from Chinese patients. This trove of information allows researchers to train algorithms that will eventually perform functions in clinical settings, such as diagnosing diseases from medical images and scans.
In the case of myopia (nearsightedness) — which afflicts 53 per cent of children and teenagers in China — Airdoc has developed a machine-learning model that measures the size and shape of the lens in a patient’s eye. Implantable collamer lens (ICL) surgery is an increasingly common procedure, in which an artificial lens is implanted between an eye’s natural lens and the iris to produce clearer vision.
The process is fraught with complexity, however, because of possible post-operative changes in the pupil and iris that could mean the lens does not fit correctly. A British Journal of Ophthalmology article describes how Airdoc’s machine-learning model offers 80 per cent-plus accuracy in predicting these changes and selecting the right size of ICL.
“Hospitals are motivated to pursue this digital transformation because China is facing a tough challenge in healthcare,” says Sally Ye, a Shanghai-based healthcare analyst at Omdia, a tech consultancy. “The medical infrastructure is insufficient, and AI digitalisation is a way to solve this problem.”

Ye says Chinese AI companies have an edge over those elsewhere as China has an abundance of the low-cost labour that is required to annotate medical data and standardise it for machine learning.
“China has a big workforce of data scientists, IT engineers and medical professionals that can work on these labour-intensive projects at a relatively low cost,” she says. Beijing’s policymakers have thrown their support behind medical AI companies that come up with technological innovations to ease the burden on the country’s hospital system. Medical and health tech forms a core pillar of the flagship “Healthy China 2030” policy.
Money flowed into medical AI after the policy’s publication in 2016, with large internet companies and start-ups battling to be the first to gain regulatory approval and be deployed in Chinese hospitals.
In 2020 alone, Chinese start-ups attracted $1.4bn of financing, compared with $2.4bn by their US counterparts, and the two nations accounted for 90 per cent of global investment into medical AI start-ups, according to Omdia research.
China has a massive data advantage when it comes to medical AI Eric Topol
But the race to get ahead has also encouraged some companies to obtain data through unregulated channels. CN-Healthcare, a Chinese medical media platform and consultancy, reported that, in 2017, third-party data brokers were selling medical records from hospitals to AI companies.
“Medical AI companies don’t have a strong understanding of data protection,” says Deng Yong, associate professor in medical and health law at Beijing University of Chinese Medicine, adding that they have tended to view data compliance as a hindrance.
Ensuring medical data is both anonymised and secure is expensive, and Deng says there has been a tendency to cut corners. A group of Chinese researchers last year found technical vulnerabilities in how mainland hospitals were processing patient data, which exposed the identities of individuals in a data breach.
Hackers have also been on the hunt for poorly secured medical records or data from wearable health devices, which can be sold on to other medical companies or to criminals, who use the information for blackmail or to make false medical claims.
In 2020, Cyble, a US cyber security group, identified a data hack on the Chinese healthcare AI company Huiying, a medical imaging devices manufacturer.
Beenu Arora, founder and chief executive of Cyble, says personal health records and Covid-19 test results were taken from the company’s servers and advertised for sale on the dark web. Huiying did not respond to a request for comment.
Arora says the digitalisation of healthcare, which has accelerated during the pandemic, has increased the intensity of cyber attacks on the medical, healthcare and pharmaceutical industries. “These breaches can lead to patients’ histories being used for potential abuse or criminal activities,” he says.
The vulnerabilities found on Huiying’s database are not unique to China. Tech blog The Verge reported in early December that, based on government data, the personal health information of more than 40m people in the US had been exposed in data breaches in 2021.

But, while healthcare organisations in the US must report medical and health data breaches when they affect 500 or more people, the same requirement does not exist in China. Nevertheless, a partner at a law firm in Shanghai says that although data breaches have occurred in China, none has been “very serious”, adding that “the overall trend in China is towards building a better privacy protection regime”.
At Airdoc, He Chao says the company has the “strictest procedures” and has invested in data protection both by anonymising the medical records that its algorithms are trained on and by inviting external cyber security companies to test vulnerabilities. “These costs are a necessity,” says Chao. “Our company relies on data.”

These security measures are becoming an industry standard after Beijing introduced the Personal Information Protection Law in November, designed to prevent data hacks and other nefarious uses of sensitive personal information.
Much like the EU’s General Data Protection Regulation, the PIPL stipulates that an individual’s explicit consent must be obtained before their medical health data is collected and places the burden on medical AI companies to ensure that data is secured.
China’s AI community is debating how to best secure data privacy, says Jeffrey Ding, a postdoctoral fellow at Stanford University’s Center for International Security and Cooperation in the US and author of the ChinAI newsletter.
“Federated learning is becoming more popular in China as a privacy tool,” says Ding, referring to the practice of spreading data sets across multiple servers to bolster security. “You can never guarantee privacy,” says Topol, “but AI is helping us get much better on this issue.”
Promoted Content
Explore the series.

Follow the topics in this article
- Technology sector Add to myFT
- Data protection Add to myFT
- Digital health Add to myFT
- Coronavirus Add to myFT
- Healthcare Add to myFT
International Edition
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- ADVERTISEMENT FEATURE Advertiser retains sole responsibility for the content of this article
China’s rising star of medical innovation
Produced by

Shengjing Hospital makes a conscious effort to bring new medical research into practice. Credit: nicolas_/E+/Getty
Northeast China’s biggest university-affiliated hospital is planning to establish the region’s largest centre for neurological diseases, and success hinges on an ambitious recruitment drive to bring in global researchers. The hospital is also seeking further collaborations with research teams overseas and elevating the role of research in the clinic, including in reproductive medicine and digestive diseases.
The growing centre for neurological diseases at Shengjing Hospital of China Medical University, in Shenyang, Liaoning, is set up to benefit patients and further research.
Take Anhua Wu, the hospital’s vice president and a neurosurgeon, who divides his time between the operating theatre and the laboratory to make it easier to put new medical research into practice.
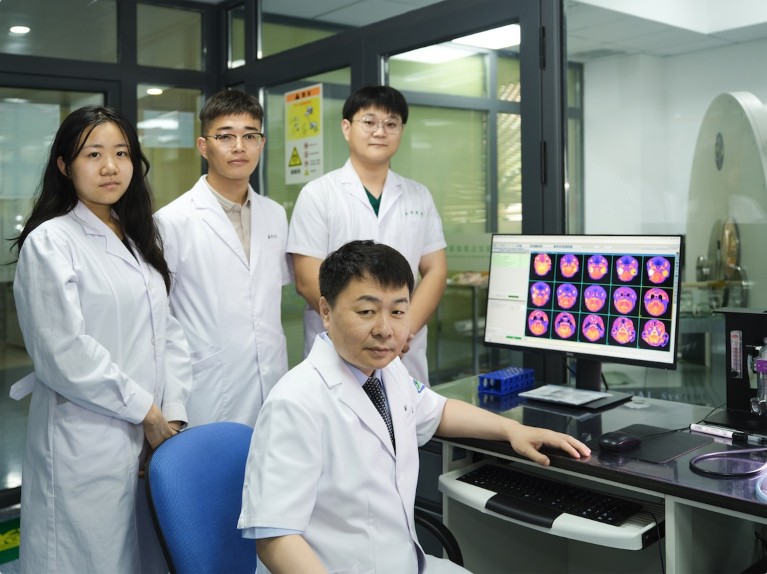
Anhua Wu(sitting) with his graduate students at the Shengjing Hospital of China Medical University. Credit: Shengjing Hospital of China Medical University
Wu’s team studies the role of a protein, TIM-3, in a particularly aggressive brain tumour, glioblastoma, which has limited treatment options and a median five-year survival rate of less than 10%.
TIM-3 is a checkpoint protein that normally ‘checks’ immune cells, preventing the immune response from becoming so strong that it kills healthy cells. When things go wrong, TIM-3 stops the immune cells from killing cancer cells.
Working in lab dishes, the Wu team identified a chemical feedback loop that involves TIM-3 in the crosstalk between glioblastoma cancer cells and immune cells.
New Strategy
“It suggests a potential immunotherapy strategy,” says Wu, whose results were published in iScience in 2022 1 .
Other neurology research underway at Shengjing Hospital includes investigations of the molecular and cellular mechanisms that underpin nerve damage repair, and how that could improve treatment of progressive neurodegenerative diseases such as Alzheimer’s or Parkinson’s.
Shengjing Hospital researchers also work on brain-computer interfaces (BCIs), which use brain activity to directly operate computers, or even robotic limbs to make human-computer interactions more efficient, or to help people who are paralysed to move.
Another area of research is neuromodulators, chemicals that change how fast nerve cells communicate with one another. They are used to treat pain and mental health disorders.
“These projects are supported by the hospital’s development goal of establishing the largest centre for neurological diseases in northeast China,” says Wu. “A centre that will ultimately improve treatments for patients,” he says.
The benefits to patients of having closer links between research and clinical practice, and across specialities, is also evident at the Shengjing Hospital’s Centre of Reproductive Medicine.
“Our experience treating infertile couples shows that issues raised in the clinic help design better in vitro fertilization strategies,” says Da Li, vice chair of the centre, whose team is using several specialities to understand mechanisms behind sperm quality. They include proteomics, the large-scale study of proteins and their fluctuations during biological processes, and modifier-omics, the large-scale study of chemicals that modify gene activity.
Across specialties

Da Li at the Shengjing Hospital of China Medical University. Credit: Shengjing Hospital of China Medical University
The Li team’s study of 528 couples and a further 25 men, describes fluctuations in 322 sperm proteins associated with factors like sperm motility after short ejaculatory abstinence. The results were published in the journal Molecular & Cellular Proteomics in 2019 2 .
The sharing of knowledge across specialities brings fresh perspectives needed to solve complex diseases, he says.
“Studies in metabolomics have given a new understanding of polycystic ovary syndrome (PCOS) — a very common hormone problem for women of childbearing age,” says Li, “Women with PCOS are also more likely to get gestational diabetes.”
Li’s team papers published in Nature Communications 3 and PNAS 4 describe a metabolic regulatory network in mice. The network centres on the TET3 protein, a member of the family of proteins that regulate gene activity. The paper suggests TET3, beyond its known role in stem cells and cancer development, also participates in the control of glucose production in the liver, the body’s glucose reservoir.
“Previous studies found a correlation between energy metabolism and fertility,” says Li. He hopes the understanding of TET3 could one day lead to new fertility treatments.
Global health
Shengjing Hospital is also striving to benefit patients around the world by promoting best medical practice and developing genetic databases to support the use of reproductive technology in Asian populations.
Siyu Sun is president of the hospital, head of its digestive disease department, and editor-in-chief of Endoscopic Ultrasound, a journal published by the hospital, and with an editorial board of more than 130 top experts from 30 countries.

Siyu Sun at the Shengjing Hospital of China Medical University. Credit: Shengjing Hospital of China Medical University
“Our hospital is helping researchers develop the next generation of ultrasound and endoscopy devices in China,” says Sun.
Sun’s team is also drafting guidelines on endoscopic ultrasound-guided bile duct and gallbladder drainage. Meanwhile, Li’s team is leading the development standards for non-invasive chromosome screening of embryos, which they hope will be adopted internationally.
All these initiatives are made possible, because Shengjing Hospital provides diverse opportunities and abundant funding for researchers to do cross-disciplinary research, and commercialize medical innovations, says Sun.
“We drive innovation and discovery by integrating clinical and fundamental research,” he says.
Calling for global talent
Shengjing Hospital of Chinese Medical University is committed to moving clinical research into new medical practice and technologies, with a cross-disciplinary mindset, world-class facilities and competitive renumeration.
Those are just some of the benefits the hospital hopes will attract top-notch researchers from around the globe. The hospital has recently launched a recruitment campaign to attract researchers, including clinical researchers, with backgrounds ranging from neurology to reproduction.
Recruits will join a hospital with a reputation for innovation. For example, Shengjing Hospital is known for its adoption of minimally invasive precision surgery, including for the treatment of malignant tumours in the female reproductive system. It is the first hospital in northeast China to use the robotic da Vinci Surgical System to remove bile duct cysts.
“Our global research recruits will join other top scientists in our hospital to drive to research quality and diversity,” says hospital president, Siyu Sun.
Application email: [email protected]
Please visit us: http://www.sj-hospital.org/
Guo, Q., et al. iScience 25 , 105329 (2022).
Article PubMed Google Scholar
Shen, Z., et al. Mol Cell Proteomics 18 , 109-117 (2019).
Article Google Scholar
Li, D., et al. Nat Commun 11 , 342 (2020).
Xie, D., et al. Proc Natl Acad Sci USA 119 , e2122217119 (2022).
Download references
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Internationalization of China’s medical device industry: a case study in Brazil
RAUSP Management Journal
ISSN : 2531-0488
Article publication date: 1 December 2021
Issue publication date: 19 April 2022
The singularity of being the first Chinese manufacturer of drug-eluting stents to arrive in Brazil and the country being selected as the company's first experience outside its home country motivated the interest in the study of this case, vis-à-vis with the characteristic of internationalization medical device companies according to the Uppsala model. Considering this context, the following research question was outlined: “How did Microport internationalize before the distribution of its stents product in Brazil?” The aim of the study is to investigate Microport's internationalization process for the distribution of its drug-eluting stents in Brazil.
Design/methodology/approach
Exploratory research under the qualitative method was adopted. It chose the single case study as a procedure for data collection, as it is a revealing, exemplary subject that offers opportunities for access to unusual research. The company MicroPort was chosen because in the period when Chinese medical device companies were focused on gaining market share in China, MicroPort began its international expansion, choosing Brazil as the first country to have its own subsidiary. It consists in the case of the internationalization of a high-tech EMNE in an emerging country that has institutional and cultural differences.
Taking advantage of new technology in highly internationalized environments favors its insertion; the internationalization of medical technology can expand according to the Uppsala Model, which does not explain internationalization, but rather its evolution. Cultural and behavioral issues reinforce that the development of the market for medical devices depends on local perspectives and values. The formation of an ecosystem in the local market for internationalization is observed. One implication of the study is that MicroPort's experience and the application of the Uppsala model for international expansion can serve as an important learning experience for Brazilian multinational companies.
Research limitations/implications
Empirical analysis carried out in the context of a single company. Although the results can be used as lessons learned from the application of the Uppsala model for international expansion of EMNE in an emerging market, caution should be exercised when generalizing its findings. Future studies could carry out comparative cases considering other emerging multinational companies, from the same sector or even from different industries, investing in other emerging markets. There is a limitation of the fact that the case studied does not explore the concepts of the later stages of the Uppsala model.
Practical implications
High-tech EMNEs internationalizing in other markets need to adopt aggressive strategies. The need to adopt different strategies for supply chain operations according to the specificities of the markets in which they operate. Important contributions to the Uppsala model, with regard to the process of passing stages, learning and networking. The findings of this study have similarities to the process described as a sequence of distinct phases of activities.
Social implications
A local top management team is essential to deal with institutional issues of government agencies when EMNE is internationalized in a culturally distant market. When there are major institutional differences between the country of origin and the host country, the autonomy in the management of the foreign subsidiary positively influences the acceleration of the internationalization process of companies in the high-tech sector. When there are major institutional differences between the country of origin and the country of destination, the use of local social networks positively influences the acceleration of the internationalization process of companies in the high-technology sector.
Originality/value
Regardless of these limitations, the study provided an exciting case of internationalization of a Chinese company in Brazil operating in a high-tech medical sector. The challenges for the internationalization of EMNEs continue, which makes it opportune for future studies to include more research in this area. The propositions suggested in the study may be the first step.
- Internationalization
- Supply chain strategies
- Medical devices
- Chinese multinational
Uppsala model
Pereira, G.M.d.C. and Ogasavara, M.H. (2022), "Internationalization of China’s medical device industry: a case study in Brazil", RAUSP Management Journal , Vol. 57 No. 2, pp. 199-212. https://doi.org/10.1108/RAUSP-03-2021-0046
Emerald Publishing Limited
Copyright © 2021, Gustavo Menoncin de Carvalho Pereira and Mario Henrique Ogasavara.
Published in RAUSP Management Journal . Published by Emerald Publishing Limited. This article is published under the Creative Commons Attribution (CC BY 4.0) licence. Anyone may reproduce, distribute, translate and create derivative works of this article (for both commercial and non-commercial purposes), subject to full attribution to the original publication and authors. The full terms of this licence maybe seen at http://creativecommons.org/licences/by/4.0/legalcode
Introduction
There is a strong interest in researching new international ventures, particularly from emerging markets ( Mikhailova & Olsen, 2016 ; Jiang, Kotabe, Zhang, Hao, Paul & Wang, 2020 ). In the case of multinational companies from emerging countries (EMNCs), there is much to learn by examining their foreign direct investment (FDI) and how these firms use their resources and capabilities in foreign markets ( Khan, Lew & Rao-Nicholson, 2020 ). Interest is even more significant on the movement of Chinese EMNCs, both in developed countries ( Buckley, 2019 ; Sutherland, Hennart & Anderson, 2019 ) and other emerging countries ( Fornes & Butt-Philip, 2014 ).
There is also an interest in the internationalization of high-tech industries ( Khan, Lew, & Rao-Nicholson, 2020 ), such as medical technology ( Laurell, Achtenhagen & Andersson, 2017 ). However, a critical factor for the success of medical devices’ internationalization is to develop distribution channels in the host country ( Dalenberg, 2020 ).
Medical device sales are estimated to reach US$800bn in 2030 ( KPMG, 2018 ), as diseases caused by lifestyle become more prevalent. In addition, there is an increase in heart risk factors following COVID-19 ( Cransac-Miet, Zeller, Chagué, Faure, Bichat, Danchin, Boulin & Cottin, 2021 ). Around 43% of the drug-eluting stent market will be concentrated in China, India and Brazil, with Brazil accounting for 3% of global sales (Global Data, 2014).
The medical device market in mature countries like the USA, Japan and the European Community is increasingly saturated ( Zelkha, 2012 ). This fact creates interest from multinational companies in new markets with high growth potential, especially in emerging economies ( Boyen & Ogasavara, 2013 ). Therefore, emerging markets are attractive and part of multinationals’ expansion strategies focuses on them (Abbot, 2019; Boston Scientific, 2020 ; Medtronic, 2020 ).
The internationalization of medical device companies, in which products are first developed locally for the national market and then for the international one ( Mikhailova & Olsen, 2016 ), is in line with Uppsala model’s proposal ( Johansson & Vahlne, 1977 ). This theoretical model understands that internationalization depends critically on learning processes and networking, which take considerable time and effort ( Johansson & Vahlne, 1977 , 2009). Therefore, it is a process-oriented view of internationalization associated with a gradual movement in which business relationships are channels for learning and aligning relevant information and capabilities.
In 2018, the largest medical device companies in the world, such as Abbott, Boston Scientific and Medtronic, already had their operations consolidated in Brazil, where they dominated the local market but with no manufacturing in the country ( ANVISA, 2018 ). In that period, Chinese companies had a solid performance in the domestic market, with the expansion of China’s health-care industry ( Euromonitor International, 2018 ). At the end of 2017, MicroPort, the largest Chinese company in the segment of drug-eluting stents ( Boston Healthcare, 2020 ), established its first foreign subsidiary in Brazil ( MicroPort, 2018 ).
The uniqueness of being the first Chinese drug-eluting stent manufacturer to arrive in Brazil, and the selection of the country to be the company’s first experience outside its headquarters motivated the interest for this case study, in addition to analyzing the internationalization of medical device companies according to the Uppsala model. Furthermore, it is the internationalization of an EMNC toward an emerging economy with significant institutional and cultural differences ( Thøgersen, Barcellos, Perin, & Zhou, 2015 ), making it an intriguing case for analysis.
The study contributes to the literature in two main points. First, understanding the internationalization of a high technology medical EMNC to an emerging market with significant institutional and cultural differences. Thus, it is essential to understand the context of the Chinese company MicroPort within the internationalization of the medical device industry, its entry into the Brazilian market and the evolution of the distribution channel strategy in the country. Second, by contributing to the Uppsala model, in aspects such as the learning process, sequence of steps, relationship with local networks and how these aspects apply to MicroPort’s presence in Brazil.
We organized the article into six sections. After the introduction, we present the literature review, followed by the methodological procedures, results, discussion and finally the conclusions, with the main contributions, limitations and suggestions for future research.
Literature review
The Uppsala model is one of the references for studying the internationalization process of companies ( Dow, Liesch, & Welch, 2017 ). Johansson and Vahlne (1977) developed the model by investigating the international expansion of Swedish companies. According to the authors, companies should learn from their experience of operations and activities in the foreign market. They build experience from their knowledge of the market where they operate, and this knowledge influences the decisions on the level of commitment and the activities that subsequently grow from it, leading to the next level of commitment, which requires more learning.
The model is dynamic and has four stages ( Figure 1 ). According to Hult, Gonzalez-Perez and Lagerström (2020) , the first step is exporting to another country occasionally. By gaining knowledge and confidence, the company begins to export regularly, using the services of a representative, thus moving on to the second stage. When establishing a foreign subsidiary to distribute its products in a target market, it becomes a multinational company and enters the third stage. The target market may grow enough to justify manufacturing in that country, making the company skip the sales subsidiary stage and move directly to local production, the fourth step ( Dow, Liesch, & Welch, 2017 ). Figueira-de-Lemos, Johanson and Vahlne (2012) observe that this is the typical internationalization path in a stable environment, where the company’s commitment increases with the accumulation of knowledge and vice-versa – the acquired knowledge makes the company decide to build a factory in that country.
The model proposed in 1977 was revised in 2009, by describing the business environment as a network of relationships without borders (Johansson and Vahlne, 2009). Although recognizing a global world, the authors confirmed the gradual process of internationalization. This means that Born Globals ( Knight & Cavusgil, 2004 ), with international activities since their inception, have actually a more regional scope and do not really cross the world significantly (Johansson & Vahlne, 2009, Lopez, Kundu, & Ciravegna, 2009 ).
For Hult, Gonzalez-Perez and Lagerström (2020) , the application of the model seeks to form an ecosystem composed of business organizations involved in commerce, production, commercial functions and cross-border trade. According to Vahlne (2020) , the Uppsala model explains the nature of the globalization process by sharing the characteristics of the internationalization process. However, it is difficult to adapt to the nature of activities and different national and organizational cultures, depending on knowledge development and several mutual commitments ( Vahlne et al. , 2012 ).
In the case of medical devices, Lee’s study (2018) similarly separates the internationalization process (in stages): access through regulation; expansion by registering more innovations; and increased infrastructure . The study approaches the Uppsala model on the importance of learning, especially in the case of medical devices, which need continuous cooperation for research and development.
Distribution channels
The choice of distribution channels is essential for the implementation of the company’s market strategy. These decisions start with collecting the correct information and establishing the goals ( Machková, 2006 ).
Distribution channels are the routes through which goods and services go from producers to customers. There are different types of distribution channels, depending on the number of intermediaries ( Kotler & Keller, 2015 ). The distribution channel is the set of interdependent organizations involved in making a product or service available for use or consumption; the strategy focused on managing distribution channels can bring significant and positive results for companies ( Coughlan, Anderson, Stern, & El-Ansary, 2012 ).
Kotler and Keller (2015) define four potential distribution channels ( Figure 2 ). In the direct distribution channel , there are no intermediate levels. In the indirect channel , there are three possible configurations:
two levels (wholesaler and retailer); and
three intermediate levels, in which the intermediary buys from large wholesalers and sells to small retailers.
Direct distribution channels are more suitable for producers with the ability to sell their products and services directly. The advantages include direct contact and communication with the consumer, effective and immediate feedback, and low costs due to the absence of intermediaries. On the other hand, indirect distribution channels have intermediaries for distribution, who play different roles and can be wholesalers, retailers and different types of agents ( Rolnicki, 1998 ). The advantages are that goods and services sales are more efficient, expenses are lower and the producer does not have to take care of all aspects of the sales process, as the intermediary plays this role.
The choice of distribution channels also depends on the product’s characteristics, durability, life cycle, technical level, after-sales services, production capacities and location. In addition, the opportunities for materials and personnel, availability of intermediaries for distribution, the level of services provided, social and economic conditions, financial services and legislation are also part of the process ( Machková, 2006 ).
Methodology
Context and research method.
The study’s problem regards investigating particularities and understanding details specific to the context. Therefore, we chose qualitative exploratory research.
Theory-building through case studies is a research strategy that involves the analysis of one or more cases to create theoretical constructs or propositions from the empirical evidence provided by the case ( Eisenhardt, 1989 ; Eisenhardt & Graebner, 2007 ). A single-case study can create more complicated theories than multiple cases because single-case researchers can adjust their theory precisely to the many details of a particular case. By contrast, multiple-case researchers retain only the relationships that are replicated in most or all cases ( Eisenhardt & Graebner, 2007 ).
Based on this assumption, as this is a revealing, exemplary subject matter that provides opportunities for access to unusual research ( Yin, 2006 ; Eisenhardt & Graebner, 2007 ), we chose the single-case study as the procedure for data collection. In addition, Mariotto, Zanni and Moraes (2014) argue that a single example can challenge a well-established proposition and generate approaches to initial studies like this one, as long as the goal is an empirical generalization.
We chose MicroPort because of some particularities. First, when Chinese medical device companies focused on gaining market share in China, on taking the lead from North American and European companies in their country, MicroPort began its international expansion, choosing Brazil as the first country to have a subsidiary outside China. Additionally, it is a case of internationalization of a (Chinese) high-tech (medical) EMNC in an emerging country (Brazil) that has significant institutional and cultural differences, meeting one of the contributions intended by this study.
MicroPort was founded in 1998, in an incubation center at Zhangjiang High-Technology Park, in Shanghai. In 1999, it launched its first product, a balloon catheter for coronary angioplasty. MicroPort’s first entry into the international market took place in 2003, with exports to Japan. In 2004, the European Community approved its conventional stent, and the firm developed its first drug-eluting stent in China. In 2010, MicroPort went public on the Hong Kong Stock Exchange. The approval of its products by the health surveillance regulatory authorities in China (CFDA) and Europe (EC) boosted its business expansion worldwide.
For this case study, we got general information on the company during a visit (SHG) in 2018/2019, and through interviews conducted with the president (E1CEI) and the vice-president of international business (E1VPI). From these first contacts, new interviews emerged with managers from the international areas of operations (E1OPI), regulation (E1RAI) and quality (E1QAI). In addition, we interviewed local managers of the Brazilian subsidiary, from the operations (E1OPB), regulation and quality (E1RQB), finance (E1FIB) and sales (E1VEB) areas. In the interviews, we identified critical events related to developing strategies for internationalization and distribution channels. The focus was on questions such as “what,” “why” and “how” about critical events, activities and choices that took place over time ( Pettigrew, 1997 ). This process interpretation provided important events, names and dates that served as a template for a deeper investigation.
After the interviews, we did an in loco study, with access to data from primary and secondary sources, such as meeting minutes (ATA1; ATA2; ATA3), seminars’ field notes (SEM1; SEM2) and short presentations at annual conferences (CONF1; CONF2), in addition to company’s yearbooks. These documents provided a triangulation between written documents and interviews to complement and minimize interviewees’ retrospective bias.
Data underwent graphical analysis, which allowed presenting information synthetically ( Miles & Huberman, 1994 ). Visual mapping was supported by further analysis involving a search for patterns or common sequences of events ( Buchanan & Bryman, 2009 ), to advance to mid-range theoretical explanations ( Miles & Huberman, 1994 ).
Based on both primary and secondary data, it was possible to understand MicroPort’s internationalization process. It began in 2013, with the development of an innovative product, the Firehawk drug-eluting stent . It is innovative because, unlike other similar stents, it releases the drug at the lesion site, achieving clinical results comparable to the reference stents in the market (Lansky, Wijns, Kelbæk, Roven, Zheng et al. , 2018). In 2014, overseas revenues from the cardiology segment represented 5% of total income. However, with the advance of the internationalization process, overseas revenue reached the level of 11% in 2020, and, specifically in Brazil, the market share was close to 5%.
Over the years, MicroPort’s strategy to go beyond China was to get approval for its products in different countries (E1CEI; E1VPI). Later, the firm looked for local representatives to register products in the target market (E1RAI) and import, receive, store and distribute MicroPort products (E1OPI). This strategy is consistent with the Uppsala model’s Steps 1 and 2 and focused on distributing its products without a subsidiary’s local and direct presence.
The firm replicated this strategy in Brazil by identifying an exclusive distributor in the country (E1CEI). This distributor met the necessary conditions required by the National Sanitary Surveillance Agency (Anvisa) for registering products in the country, with the required regulatory licenses for import, storage and distribution (E1RAI; E1QAI).
In this first stage, the firm exported products to the distributor (E1OPI). Then, it proceeded to prospect customers, getting the recommendation and experimentation of opinion formers (E1CEI; E1VPI). Thus, MicroPort began distributing its products in the Brazilian market.
MicroPort soon noticed the competitive market in Brazil, where the major market players had already established themselves in specific segments (Medtronic, Boston Scientific, Abbott, Terumo, Biotronik) (E1CEI; E1VPI). Because of increasing awareness in Brazil about the various heart diseases and the growing number of older people in the country, the company expected a significant increase over the following years (E1CEI; E1VPI). Despite being the largest market in Latin America, Brazil did not know MicroPort’s best performance. Countries like Argentina, for example, with the same distribution model, showed equal or better performance (E1CEI; E1VPI). Given the country’s characteristics and the unmet potential, MicroPort then decided to go beyond Step 2 of the Uppsala model and establish its first subsidiary outside China, with the primary goal of boosting its market share with the Firehawk drug-eluting stent as its leading product (E1CEI; E1VPI).
technical manager (Pharmacist);
operations manager; and
sales manager (E1CEI; E1VPI; E1OPI; E1RAI).
It also started transferring product registration to the subsidiary since the distributor has the record from previous sales (E1RQB; E1RAI; CONF1; ATA1).
Next, the process to get the operating permit (AFE) from Anvisa began (E1RQB; E1VPI; E1RAI; SEM2) and thus allowed import, store and distribution of products on its own (E1OPI; E1OPB). Finally, the company received authorization to start its activities in 2018, getting an unlimited import license (E1FIB; ATA2; CONF2). As a result, the first Firehawk import to Brazil was in September 2018 (ATA1).
The Brazilian subsidiary started to perform all supply chain activities locally, from planning to the arrival of products in the country (E1OPB; E1OPI; ATA3; SHG; SEM2). The activities include transferring products to a customs storage station (EADI) to await Anvisa inspection. After approval, the process proceeds to the Internal Revenue Service (E1RQB; E1OPB; E1FIB; E1OPI). Finally, the products are sent to the warehouse, where they are received, checked and labeled, thus being available for distribution (E1RQB; E1OPB; ATA3).
non-exclusive distributors;
direct sales, in which the company serves hospitals directly; and
commercial agents, who sell to hospitals (E1VEB; E1CEI; E1VPI).
In the first channel, the Brazilian subsidiary makes the products available to distributors, who resell them to hospitals; in the second, sales take place without intermediaries, with the company’s own sales force and distribution logistics; and, in the third, the logistics are on the subsidiary, but the sales force is commissioned (E1VEB; E1CEI; E1VPI; E1OPI; SEM2). Figure 3 shows the whole distribution chain, from China to end-patients, including the roles and responsibilities of each link of the chain.
MicroPort’s internationalization history shows that taking advantage of new technology in highly internationalized environments favors the insertion, as Mikhailova and Olsen (2016) observed. For example, in five years, the Firehawk product had achieved registration in 40 countries ( MicroPort, 2020 ).
This study confirms Johansson and Vahlne (1977 , 2009). Therefore, the internationalization of medical technology (E1CE1; E1VP1) can expand according to the Uppsala model, assuming that this process depends on the company’s learning capabilities and the constitution of a network.
As for Firehawk’s strategy of commercial insertion outside China, we can consider Vahlne’s thesis (2020) that the Uppsala model does not explain internationalization but rather its evolution. Step 1 of the model assumes occasional and irregular exports – which occur in the first movements (E1CEI; E1VPI; E1RAI). In Step 2, the expansion process takes place through the transfer of ownership, in this case, the registration of products, to exclusive distributors. Hånell et al. (2018) mention this as a practice in EMNCs. In our case, we can make a comparison between the evolution of MicroPort’s internationalization and the Uppsala model stages ( Table 1 ):
From our observations (SEM1; SEM2; SHG), we found that the dynamics of MicroPort’s internationalization model differs regionally, as confirmed by the corporation’s international leadership (E1CEI; E1VPI). In the case of Brazil, there was an evolution to Stage 3, which consists of setting up its own subsidiary (ATA2; ATA3; CONF2).
MicroPort’s movement in a market like Brazil suggests that cultural and behavioral issues ( Vahlne, 2020 ) strengthen that medical device market development depends on local perspectives and values. In other words, the subsidiary must have greater autonomy in the local operation by using a team of local managers that handle institutional specificities better.
In MicroPort’s operation in Brazil, we observed the formation of an ecosystem in the local market for internationalization, as proposed by Hult, Gonzalez-Perez and Lagerström (2020) ; interviews (E1CEI; E1VPI) and global corporate presentations (SHG; CONF1; CONF2) confirmed it. This ecosystem develops through the network of local relationships, such as commercial agents and distributors.
A managerial implication of the present study is that MicroPort’s experience and application of the Uppsala model to international expansion can serve as an essential learning experience for Brazilian multinational companies. MicroPort, like most Brazilian companies with global visibility, is an EMNC that faces challenges in the internationalization process ( Nolke, 2014 ). Unfortunately, studies related to the rise of EMNCs and the application of the Uppsala model are rare ( Meyer & Thaijongrak, 2013 ). However, Elango and Pattnaik (2011) show that knowledge, gradual learning and network relationships, which are fundamental assumptions of the Uppsala model, are essential resources to explain these companies’ internationalization.
Our study reinforces this assumption by showing the challenge and complexity of a high-tech Chinese company’s internationalization to an emerging country (Brazil) in the medical device sector. It also shows the influence of the institutional environment and the importance of local managers. This sector has its own features in a regulatory environment governed by Anvisa. With the experience acquired in the local market and the specificities of the industry, the Chinese company left out Stage 2 to enter Stage 3 of the Uppsala model. This action required transferring the registrations of its products and obtaining the regulatory and fiscal licenses to be able to import and sell in the country.
As the demand for medical devices originates in the patient, seeking a closer relationship between the manufacturer and the final consumer strengthens the concepts of distribution channels, as presented by Machková (2006) . The interactions between the chain links are represented by MicroPort’s branch in Brazil (E1VEB; E1OPB; CONF2), which supplies hospitals and clinics through direct sales and indirectly through distributors and commercial agents. Getting to the patients at the time and in the amount needed requires orchestrating activities between all the parties involved, which defines the relationship network’s success.
The purpose of this article was to understand how an EMNC from a high-tech sector internationalized to a country with significant institutional and cultural differences. To achieve this goal, we addressed MicroPort’s case regarding its drug-eluting stent product distribution in Brazil.
The Uppsala model refers to a gradual internationalization involving countries that are culturally closer and later to those more distant. In our case, MicroPort started exporting to Asian countries, such as Japan. Nevertheless, when it decided to enter a distant country (like Brazil), MicroPort used the Uppsala model regarding local learning and the entry stages in the target market, such as the definition of distribution channels.
The study provided essential contributions to the literature. First, it showed that high-tech EMNCs, when internationalizing to other markets, need aggressive strategies. MicroPort has a clear guideline to become a global company. With research and development (R&D) and launching an innovative product (Firehawk), it achieved registration and approval in more than 40 countries in just five years. MicroPort believes that companies’ globalization is necessary for business development efforts in the medical field ( Mikhailova & Olsen, 2016 ).
Second, it confirms the need to adopt different strategies for supply chain operations, according to the specificities of the markets where firms operate. Lee (2018) stress that the medical device distribution model is not simple and requires the technology holder to expand its control and influence over all actors in the new market chain. For example, in Brazil, the medical device distribution structure takes several forms.
Third, it adds essential contributions to the Uppsala model, concerning the processes of going through stages, learning and building a network relationship. Our findings are similar to the process described as a sequence of distinct phases of activities ( Evers, Andersson, & Hannibal, 2012 ) and resembles Mikhailova and Olsen’s (2016) results.
In the case of Brazil, moving from Step 2 to Step 3 of the Uppsala model required, among the first actions of MicroPort, hiring experienced Brazilian professionals to handle institutional issues related to government bodies (such as Anvisa, Internal Revenue Service) and access public notices to compete in biddings. Thus, we present here the first insight from this study: I-1 : The formation of a local top management team is essential to deal with institutional issues of government bodies when an EMNC internationalizes to a culturally distant market .
As far as learning is concerned, MicroPort has gone and is still going through a learning process in Brazil. Nevertheless, the findings showed that the company’s culture of respecting regionalism without imposing its headquarters’ management model accelerated the internationalization process in Brazil. Hence, we have a second insight from this study: I-2 : When there are significant institutional differences between the home country and the host country, autonomy in managing the foreign subsidiary positively affects the acceleration of the internationalization process of companies in the high tech sector .
Concerning the network of relationships, a relevant finding for the Uppsala model is that MicroPort’s building emerged from sales representatives rather than a direct approach to customers. This caused the brand to be introduced in the market not because of the reputation of the manufacturing country but because well-known representatives were offering its products. The company sought to sponsor agents with established networks with hospitals, and approach them gradually, which enabled a less imposing kind of learning. Therefore, we present a third insight of this study: I-3 : When there are significant institutional differences between the home country and the host country, the use of local relationship networks positively affects the acceleration of the internationalization process of companies in a high-tech sector.
The study findings show that MicroPort, despite being from a country with a culture and habits quite different from Brazil, respected Brazilian cultural issues, avoiding potential and expected cultural shock. In 2021, as part of its growth strategy in the country, the subsidiary hired an experienced Brazilian female executive to be the general manager of local operations ( Medicina S/A, 2021 ) to get closer to opinion-making doctors, thus achieving greater exhibition of its innovative product, the Firehawk. Additionally, actions in the media ( BandNews, 2021 ) and participation in regional conferences, such as the one organized by the Latin American Society of Interventional Cardiology ( MicroPort Brasil, 2021 ), are also the first step toward a more direct relationship with doctors.
We acknowledge the limitations of this study, which suggest future research. First, we carried out the empirical analysis with a single company. Although the results may be used as lessons learned from applying the Uppsala model to the international expansion of an EMNC in an emerging market, caution is necessary for generalizing our findings. Future studies should carry out comparative cases, considering other emerging multinational companies from the same sector or different industries investing in other emerging markets. Second, there is a limitation that the case studied did not explore the concepts of the later stages of the Uppsala model. We suggest further studies with companies that decided to produce in the host country after completing the first steps. Finally, despite collecting relevant information, we did not have access to more concrete data on the level of investment made in Brazil during the internationalization process. These are the company’s strategic data that will be hardly available, even for future studies.
Regardless of these limitations, the study provided an intriguing case of a Chinese company’s internationalization to Brazil, operating in a high-tech medical sector. The challenges for EMNCs’ internationalization continue, which makes more research in this area timely. However, our findings can be the first step.
The internationalization process proposed by the Uppsala model
Interaction between the links of the supply chain, from China to the patients
Comparison between stages: Uppsala model and MicroPort
Source: Based on primary data from interviews with top headquarters’ executives and local managers of the Brazilian operation, as well as secondary data obtained from minutes of meetings, reports and summaries of corporate presentations
ABBOTT. ( 2020 ). 2019 Annual report . Retrieved from: www.abbottinvestor.com/static-files/8829af8d-783a-42ef-9566-37a699e79188
ANVISA. ( 2018 ). Relatório de definição e análise do problema regulatório? Contribuições Para a revisão normativa da RDC n°185/2006 . Retrieved from: www.gov.br/anvisa/pt-br/assuntos/produtosparasaude/temas-em-destaque/arquivos/7263json-file-1
BandNews. ( 2021 ). Jornal BandNews TV 2 a Edição – Mirângela machado, diretora geral da MicroPort fala sobre novo stent . Retrieved from: www.youtube.com/watch?v=hedhTLvr5iw
Boston Healthcare. ( 2020 ). Analysis of China’s first national tender on coronary stents . Retrieved from: www.bostonhealthcare.com/analysis-of-chinas-first-national-tender-on-coronary-stents/
Boston Scientific. ( 2020 ). 2019 Annual report . Retrieved from: Retrieved from: www.bostonscientific.com/content/dam/bostonscientific/corporate/annual-report/2019_Boston_Scientific_Annual_Report.pdf
Boyen , M.H. , & Ogasavara , M.H. ( 2013 ). Internationalization patterns of multinational lodging firms in Brazil . Tourism and Hospitality Research , 13 ( 4 ), 181 – 200 .Retrieved from: doi: https://doi.org/10.1177/1467358414525623 .
Buchanan , D. , & Bryman , A. ( 2009 ). Studying processes in and around organizations . The SAGE handbook of organizational research methods , London : Sage Publishing .
Buckley , P. ( 2019 ). China goes global: provenance, projection, performance and policy . International Journal of Emerging Markets , 14 ( 1 ), 1 – 20 . Retrieved from doi: https://doi.org/10.1108/IJoEM-01-2017-0006 .
Coughlan , A.T. , Anderson , E. , Stern , L.W. , & El-Ansary , A.I. ( 2012 ). Canais de marketing e distribuição , São Paulo : Pearson .
Cransac-Miet , A. , Zeller , M. , Chagué , F. , Faure , A.S. , Bichat , F. , Danchin , N. , … Cottin , Y. ( 2021 ). Impact of COVID-19 lockdown on lifestyle adherence in stay-at-home patients with chronic coronary syndromes: towards a time-bomb . International Journal of Cardiology , 323 , 285 – 287 . Retrieved from: doi: https://doi.org/10.1016/j.ijcard.2020.08.094 .
Dalenberg , D. ( 2020 ). International medical device marketing . International Journal of Health and Economic Development , 6 ( 1 ), 15 – 26 .
Dow , D. , Liesch , P. , & Welch , L. ( 2017 ). Inertia and managerial intentionality: Extending the Uppsala model . Management International Review , 58 ( 3 ), 465 – 493 . Retrieved from: doi: https://doi.org/10.1007/s11575-017-0340-0 .
Eisenhardt , K.M. ( 1989 ). Building theories from case study research . The Academy of Management Review , 14 ( 4 ), 532 – 550 .Retrieved from: doi: https://doi.org/10.5465/amr.1989.4308385 .
Eisenhardt , K.M. , & Graebner , M. ( 2007 ). Theory building from cases: Opportunities and challenges academy of . Academy of Management Journal , 50 ( 1 ), 25 – 32 .Retrieved from: doi: https://doi.org/10.5465/amj.2007.24160888 .
Elango , B. , & Pattnaik , C. ( 2011 ). Learning before making the big leap: Acquisition strategies of emerging market firms . Management International Review , 51 ( 4 ), 451 – 481 .Retrieved from: doi: https://doi.org/10.1007/s11575-011-0085-0 .
Euromonitor International. ( 2018 ). Top countries to drive medical device manufacturing report , Euromonitor International .
Evers , N. , Andersson , S. , & Hannibal , M. ( 2012 ). Stakeholders and marketing capabilities in international new ventures: Evidence from Ireland, Sweden and Denmark . Journal of International Marketing , 20 ( 4 ), 46 – 71 . Retrieved from: doi: https://doi.org/10.1509%2Fjim.12.0077
Figueira-de-Lemos , F. , Johanson , J. , & Vahlne , J.-E. ( 2011 ). Risk management in the internationalization process of the firm: A note on the Uppsala model . Journal of World Business , 46 ( 2 ), 143 – 153 . Retrieved from: https://doi.org/10.1016/j.jwb.2010.05.008 .
Fornes , G. , & Butt-Philip , A. ( 2014 ). Chinese outward investments to emerging markets: Evidence from Latin America . European Business Review , 26 ( 6 ), 494 – 513 . Retrieved from: doi: https://doi.org/10.1108/EBR-03-2013-0059 .
Global Data. ( 2014 ). Coronary stents: Global analysis and market forecasts , GlobalData .
Hånell , S.M. , Nordman , E.R. , Tolstoy , D. & Sharma , D.D. (2018). Pursuing Innovation: An Investigation of the Foreign Business Relationships of Swedish SMEs* . British Journal of Management . doi: 10.1111/1467-8551.12315 .
Hult , G.T.M. , Gonzalez-Perez , M.A. , & Lagerström , K. ( 2020 ). The theoretical evolution and use of the Uppsala model of internationalization in the international business ecosystem . Journal of International Business Studies , 51 ( 1 ), 38 – 49 . Retrieved from: doi: https://doi.org/10.1057/s41267-019-00293-x .
Jiang , G. , Kotabe , M. , Zhang , F. , Hao , A.W. , Paul , J. , & Wang , C.L. ( 2020 ). The determinants and performance of early internationalizing firms: a literature review and research agenda . International Business Review , 29 ( 4 ), 101662 . Retrieved from: https://doi.org/10.1016/j.ibusrev.2019.101662 . doi: 10.1016/j.ibusrev.2019.101662 .
Johansson , J. , & Vahlne , J.-E. ( 1977 ). The internationalization process of the firm: A model of knowledge development and increasing foreign market commitments . Journal of International Business Studies , 8 ( 1 ), 23 – 32 . Retrieved from: doi: https://doi.org/10.1057/palgrave.jibs.8490676 .
Johanson , J. , & Vahlne , J.-E. ( 2009 ). The Uppsala internationalization process model revisited: From liability of foreignness to liability of outsidership . Journal of International Business Studies , 40 ( 9 ), 1411 – 1431 . Retrieved from: doi: https://doi.org/10.1057/jibs.2009.24 .
Khan , Z. , Lew , Y.K. , & Rao-Nicholson , R. ( 2020 ). The role of dynamic capabilities in global strategy of emerging economies’ multinationals . International Studies of Management & Organization , 50 ( 1 ), 1 – 4 . Retrieved from: doi: https://doi.org/10.1080/00208825.2019.1703375 .
Knight , G.A. , & Cavusgil , S.T. ( 2004 ). Innovation, organizational capabilities, and the born-global firm . Journal of International Business Studies , 35 ( 2 ), 124 – 141 . Retrieved from: doi: https://doi.org/10.1057/palgrave.jibs.8400071 .
Kotler , P. , & Keller , K.L. ( 2015 ). Marketing management , New York, NY : Pearson/Prentice Hall .
KPMG. ( 2018 ). Medical devices 2030 , KPMG International Cooperative .
Lansky , A. , Wijns , W. , Xu , B. , Kelbæk , H. , Van Royen , N. , Zheng , M. , et al. ( 2018 ). Targeted therapy with a localised abluminal groove, low-dose sirolimus-eluting, biodegradable polymer coronary stent (TARGET all comers): A multicentre, open-label, randomised non-inferiority trial . The Lancet , 392 ( 10153 ), 1117 – 1126 . Retrieved from: doi: https://doi.org/10.1016/S0140-6736(18)31649-0 .
Laurell , H. , Achtenhagen , L. , & Andersson , S. ( 2017 ). The changing role of network ties and critical capabilities in an international new venture’s early development . International Entrepreneurship and Management Journal , 13 ( 1 ), 113 – 140 . Retrieved from: doi: https://doi.org/10.1007/s11365-016-0398-3 .
Lee , M. ( 2018 ). Strategies for promoting the medical device industry in Korea: An analytical hierarchy process analysis . International Journal of Environmental Research and Public Health , 15 ( 12 ), 2659 Retrieved from: doi: https://doi.org/10.3390/ijerph15122659 .
Lopez , L.E. , Kundu , S.K. , & Ciravegna , L. ( 2009 ). Born global or born regional? Evidence from an exploratory study in the costa Rican software industry . Journal of International Business Studies , 40 ( 7 ), 1228 – 1238 . Retrieved from: doi: https://doi.org/10.1057/jibs.2008.69 .
Machková , H. ( 2006 ). Mezinárodní marketing , Prague : Grada Publishing House .
Mariotto , F.L. , Zanni , P.P. , & Moraes , G. H. S. M. ( 2014 ). What is the use of a single-case study in management research? RAE . Revista de Administração de Empresas , 54 ( 4 ), 358 – 369 . Retrieved from: doi: https://doi.org/10.1590/S0034-759020140402 .
Medicina S/A. ( 2021 ). Mirangela machado assume diretoria geral da MicroPort brasil . Retrieved from: https://medicinasa.com.br/mirangela-machado-microport/
Medtronic. ( 2020 ). 2018 Annual report . Retrieved from: https://investorrelations.medtronic.com/static-files/da4767cb-a6c9-4716-801e-48a0a3155e1f
Meyer , K.E. , & Thaijongrak , O. ( 2013 ). The dynamics of emerging economy MNEs: How the internationalization process model can guide future research . Asia Pacific Journal of Management , 30 ( 4 ), 1125 – 1153 . Retrieved from: https://doi.org/10.1007/s10490-012-9313-9 doi: 10.1007/s10490-012-9313-9 .
MicroPort. ( 2018 ). 2017 Annual report . Retrieved from: www.microport.com/assets/financial-reports/reports/en/2017-annual-en.pdf
MicroPort. ( 2020 ). MicroPort news . Retrieved from: www.microport.com/en/news/first-implant-of-firehawk-stent-successfully-completed-in-oman
MicroPort Brasil. ( 2021 ). Solaci 2021 . Retrieved from: www.linkedin.com/posts/microportbrasil_solacicaci2021-solaci-solacicaci-activity-6829570082692247552-q-fr
Mikhailova , O. , & Olsen , P.I. ( 2016 ). Internationalization of an academic invention through successive science-business networks: The case of TAVI . Journal of International Entrepreneurship , 14 ( 3 ), 441 – 471 . Retrieved from: https://doi.org/10.1007/s10843-016-0186-8 .
Miles , M.B. , & Huberman , A.M. ( 1994 ). Qualitative data analysis: an expanded sourcebook , Sage Publishing .
Nolke , A. ( 2014 ). Multinational corporations from emerging markets , Hampshire : Palgrave-Macmillan .
Pettigrew , A.M. ( 1997 ). What is a processual analysis? Scandinavian Journal of Management , 13 ( 4 ), 337 – 348 . Retrieved from: https://doi.org/10.1016/S0956-5221(97)00020-1 .
Rolnicki , K. ( 1998 ). Managing channels of distribution: the marketing executive’s complete guide , New York, NY : Amacom .
Sutherland , D. , Hennart , J.-F. , & Anderson , J.R. ( 2019 ). How does the routing of FDI to and via tax havens confound our understanding of Chinese MNE identity? A critical review of the empirical literature on Chinese MNEs . Asian Business & Management , 18 ( 5 ), 337 – 359 .Retrieved from: https://doi.org/10.1057/s41291-019-00058-2 .
Thøgersen , J. , Barcellos , M.D. , Perin , M.G. , & Zhou , Y. ( 2015 ). Consumer buying motives and attitudes towards organic food in two emerging markets: China and Brazil . International Marketing Review , 32 ( 3/4 ), 389 – 413 . Retrieved from: https://doi.org/10.1108/IMR-06-2013-0123 .
Vahlne , J.-E. ( 2020 ). Development of the Uppsala model of internationalization process: From internationalization to evolution . Global Strategy Journal , 10 ( 2 ), 239 – 250 . Retrieved from: https://doi.org/10.1002/gsj.1375 .
Vahlne , J.-E. , Ivarsoon , I. & Johanson , J. ( 2012 ). Overcoming the liability of outsidership – The challenge of HQ of the global firms . Journal of International Management , 18 ( 2012 ), 224 – 232 .
Yin , R. ( 2006 ). Case study research: Design and methods , CA : Sage Publishing .
Zelkha , S. ( 2012 ). Benchmarking of a medical device company’s product development process . Thesis Science Magister, Massachusetts Institute of Technology , Cambridge .
Author contribution: Gustavo Menoncin de Carvalho Pereira – Corresponding author, Conceptualization (Lead), Data curation (Equal), Investigation (Lead), Methodology (Lead), Resources (Equal), Supervision (Supporting), Validation (Lead), Writing-original draft (Lead), Writing-review & editing (Supporting). Mario Henrique Ogasavara – Conceptualization (Supporting), Formal analysis (Lead), Investigation (Supporting), Methodology (Supporting), Project administration (Lead), Resources (Equal), Supervision (Lead), Validation (Equal), Writing-original draft (Supporting), Writing-review & editing (Supporting).
Corresponding author
Related articles, we’re listening — tell us what you think, something didn’t work….
Report bugs here
All feedback is valuable
Please share your general feedback
Join us on our journey
Platform update page.
Visit emeraldpublishing.com/platformupdate to discover the latest news and updates
Questions & More Information
Answers to the most commonly asked questions here
- Open access
- Published: 16 January 2024
Mapping of health technology assessment in China: a comparative study between 2016 and 2021
- Shimeng Liu ORCID: orcid.org/0009-0007-6878-6272 1 , 2 na1 ,
- Yu Xia 1 , 2 na1 ,
- Yi Yang 1 , 2 ,
- Jian Ming 1 , 2 ,
- Hui Sun 1 , 2 , 3 ,
- Yan Wei 1 , 2 &
- Yingyao Chen ORCID: orcid.org/0000-0002-3470-0748 1 , 2
Global Health Research and Policy volume 9 , Article number: 4 ( 2024 ) Cite this article
541 Accesses
1 Altmetric
Metrics details
Health Technology Assessment (HTA) in China has recently expanded from purely academic research to include policy or decision-oriented practice, especially after HTA evidence was used to update the National Reimbursement Drug List for the first time in 2017. This study aims to identify the progress and challenges of HTA development from 2016 to 2021 and inform policies and decisions to promote further HTA development in China.
We conducted a cross-sectional web-based survey with policy makers, researchers and industry-providers in China in 2016 and 2021 respectively. The ‘Mapping of HTA Instrument’, was utilized to assess the HTA development across eight domains: Institutionalization, Identification, Priority setting, Assessment, Appraisal, Reporting, Dissemination of findings and conclusions, and Implementation in policy and practice. To reduce the influence of confounders and compare the mapping outcomes between the 2016 and 2021 groups, we conducted 1:1 Propensity Score Matching (PSM). Univariate analysis was conducted to compare the differences between the two groups. The overall results were further compared with those of a mapping study that included ten countries.
In total, 212 and 255 respondents completed the survey in 2016 and 2021, respectively. The total score of the HTA development level in China in 2021 was higher than that in 2016 before PSM (89.38 versus 83.96). Following PSM, 183 respondents from the 2016 and 2021 groups were matched. Overall, the mean scores for most indicators in the Institutionalization domain and Dissemination domain in 2021 were higher than those in 2016 ( P < 0.05). The Appraisal domain in 2021 was more explicit, transparent and replicable than that in 2016 ( t = −3.279, P < 0.05). However, the mean scores of most indicators in the Assessment domain were higher in 2016 than those in 2021 ( P < 0.05).
Conclusions
Our study suggest that the level of HTA development in China progressed significantly from 2016 to 2021. However, before engaging in HTA activities, further efforts are required to enhance the assessment process. For instance, it is important to establish a clear goal and scope for HTA; adapt standardized methodologies for evaluating the performance of systematic reviews or meta-analyses; and provide comprehensive descriptions of the safety, clinical effectiveness, cost, and cost-effectiveness of the assessed technologies, thus improving the development of HTA in China.
Health Technology Assessment (HTA) carefully assesses the costs and benefits of intervention to inform reimbursement and coverage decisions regarding how to allocate healthcare resources to different health technologies [ 1 , 2 ]. HTA has played an increasingly important role on healthcare decision-making in China with the growth of HTA agencies and larger HTA research output since its introduction in the 1990s [ 3 , 4 ]. However, the further development of HTA in China is confronted with potential challenges. Although it has been occasionally used by policy makers, it exists most often in the academic sector [ 5 , 6 , 7 , 8 , 9 ]. In 2016, Chen et al. conducted a study to understand the current development of HTA in China with the aim of identifying areas for improvement. This study identifies that overall HTA development in China is lower than that in developed countries [ 9 ].
From 2016 to 2021, HTA in China has gradually broadened from pure academic research to policy-or decision-oriented practices [ 6 , 10 , 11 ]. A milestone achievement for HTA in China, was the use of HTA evidence to update the National Reimbursement Drug List (NRDL) for the first time in 2017 [ 3 , 12 ]. This was followed by the inclusion of anti-cancer drugs in the NRDL in 2018 and decision to routinize the use of HTA evidence in review processes in 2019 and 2020 by the National Healthcare Security Administration (NHSA) [ 11 , 13 ]. In this period, however, some problems have become evident. For example, there is a lack of policy mechanisms for using HTA to support decision-making, insufficient HTA technical staff and expertise, and challenges regarding integrating multi-dimensional value assessment into evidence-informed deliberative processes [ 3 ]. In addition, researchers, and policy-maker’s understanding of HTA in China has gradually deepened, and the expectations for HTA development have increased.
Our primary aim is to determine what is the current development level of HTA in China, focusing on the NRDL process. Furthermore, how do the current progress and challenges compare to before 2017? To have a better understanding of the development level and potential challenges of HTA in China, we conducted this study to elicit diverse stakeholder views in China and compared it with the survey we conducted in 2016, thus informing policies and decisions to promote further development of HTA in China.
Study design and sample
This was a cross-sectional study in which an anonymous web-based survey was conducted using Sojump software from June to November, 2016, and February to April, 2021. The survey targeted policy makers, researchers and representatives of the pharmaceutical and medical device industry involved in HTA. Similar to the prior conducted 2016 survey, convenience sampling and snowball sampling were used to identify potential respondents. The process began by involving known HTA experts, who were then asked to recommend other stakeholders who met the above study criteria for participation. In addition, we searched the attendance lists of major HTA conferences in China and Chinese journals in the HTA field between 2013 and 2020.
Survey development
In the distributed questionnaire, we incorporated the ‘Mapping of HTA Instrument’, Which has been employed to gauge respondents' perspectives on HTA development across seven middle-income countries (Argentina, Brazil, India, Indonesia, Malaysia, Mexico, and Russia) and three high-income countries (Australia, Canada, and the United Kingdom) [ 14 ]. The Mapping method adopted in this study was the same as that used in 2016, which is based on multiple HTA guidelines and standards that have been adopted broadly as well as discussions among many international HTA experts [ 9 , 14 ]. The survey instrument was distinguished by two key elements: (i) the institutionalization of HTA and (ii) HTA process itself. Overall, the instrument comprises eight domains: (1) institutionalization of HTA; (2) identification; (3) priority setting; (4) assessment; (5) appraisal; (6) reporting; (7) dissemination of findings and conclusions; and (8) implementation in policy and practice.
For detailed information on the eight domains, their indicators, and the scoring methods of this instrument, please refer to Additional file 1 : Figure S1. Respondents provided answers in either a dichotomous (YES/NO) or Likert scale format (0–3) to represent their views on each domain of HTA development. The maximum score for the eight domains is 146.
The questionnaire comprises two parts: the Mapping of HTA and demographic information. The demographic information section included items regarding the participant’s gender, age, region, years of work experience, educational background, highest degree obtained, and profession.
Data collection
To reach a diverse range of participants, the survey link was shared with the target respondents through various channels, including email invitations, social media platforms such as WeChat (one of the most widely used Chinese social media platforms), and the professional network of the National Health Commission Key Laboratory of Health Technology Assessment [ 15 ]. Additionally, we distributed the link to stakeholders who were knowledgeable about HTA in China.
Before the main survey, cognitive pre-tests were conducted to ensure the face validity of the survey instrument and comprehensibility of the survey method. We conducted iterative offline pre-tests (n = 7) and after making the necessary revisions, we conducted additional online pre-tests (n = 29). During the pre-tests, the respondents were provided with test questions and encouraged to share feedback verbally.
Throughout the data collection process, the participants were explicitly informed about the voluntary nature of their involvement in the survey. They were given the freedom to decide whether to participate, and informed consent was obtained from all participants before completing the questionnaire. This ensured that their rights and privacy were respected throughout the research process. To ensure data quality, we cross-verified the dates provided by the participants with the actual survey dates to avoid inconsistencies or errors in the data.
Data analysis
To mitigate the impact of confounding factors that could lead to outcome bias in the nonrandom assignment and enable a potential unbiased comparison, the Propensity Score Matching (PSM) methodology was typically applied [ 16 , 17 ]. This method summarizes all relevant baseline characteristics into a single composite score, which can be used to determine whether there is sufficient overlap in characteristics between groups [ 18 ].
To minimize discrepancies and compare the mapping outcomes between the 2016 and 2021 groups, a 1:1 PSM was employed with a caliper size of 0.05 [ 16 ]. The matching algorithm included the following variables: years of work experience, profession, and educational background, which exhibited significant differences between the 2016 and 2021 groups in univariate analysis. Consequently, 183 cases from 2016 and 2021 were generated and used for further analyses. Descriptive analyses were conducted for all study variables.
Continuous variables were presented as means with standard deviations and were compared using Student t -test. Categorical variables were reported as frequencies and proportions and were compared using the chi-square test. Statistical significance was set at a two-sided P -value < 0.05. Statistical analyses were performed using R software (version 4.1.1) and Stata Statistics (version 14.2).
Baseline characteristics
In total, 212 and 255 complete responses were obtained in 2016 and 2021, respectively. Detailed characteristics of the respondents in both groups are presented in Table 1 . Prior to PSM, most respondents in 2016 were researchers (72.2%), resided in Eastern China (78.3%), and had educational backgrounds in medicine (51.9%). The most common highest degree obtained was a bachelor’s degree (47.2%). The average age of the respondents was 38.9 years (± 8.7 years), and the average years of work experience was 10.61 years (± 6.5 years).
Among the 255 respondents in the 2021 group, 52.9% were researchers. The majority of participants had an educational background in medicine (57.6%), and the most common highest degree obtained was a bachelor's degree (48.2%). Most respondents resided in Eastern China (86.3%). The average age of the respondents was 37.8 years (± 9.1 years), and the average years of work experience was 7.9 years (± 7.2 years). There were no significant differences regarding gender, age, or region between the two groups ( P > 0.05).
The PSM process yielded a matched sample of 183 respondents in both the 2016 and 2021 groups, using a 1:1 nearest neighbor matching algorithm. Following PSM adjustment, no significant differences were observed between the two matched groups regarding the propensity score (generated by the PSM method, as shown in Additional file 2 : Figure S2). This indicates that the baseline characteristics were effectively balanced between the two groups.
Total score in each domain before PSM
As indicated in Table 2 , the overall score for HTA development in China was higher in 2021 than in 2016 prior to applying PSM. Furthermore, the mean scores across various domains, including institutionalization, priority setting, assessment, appraisal, reporting, dissemination of findings and conclusions, and implementation in policy and practice., suggest notable progress in HTA development over time. However, the identification domain identified in 2021 had a relatively lower overall mean score than that in 2016.
The detailed score in each domain after PSM
Within the “Institionization” domain, most respondents (63.4% in 2016 and 71.0% in 2021) indicated that China lacked an HTA agency that met the specified survey criteria. These criteria encompass aspects such as reporting to a Minister of Health/human resources or other relevant authorities, generating and/or endorsing HTA reports, and informing decisions regarding the introduction, reimbursement, and disinvestment of health technologies. Additionally, the respondents assessed the presence of essential elements for establishing a formal HTA program in China (see Table 3 ).
The mean score for ‘interest in HTA expressed by government/policy makers which can be retrieved in official documents’ (item I.1, t = −4.455, P < 0.05), ‘commitment toward HTA from government/policy makers and it is expressed in official documents’ (item I.2, t = −2.134, P < 0.05), ‘support for HTA from various stakeholders’ (item I.5, t = −3.856, P < 0.05), and ‘the availability of human resource development’ (item I.8, t = −3.289, P < 0.05) were significantly higher in 2021 than in 2016.
The ‘Identification’ domain focuses on the implementation of emerging technologies in need of assessment or those identified in the early monitoring system(s). The mean score of the indicator related to the existence of monitoring system(s) for emerging technologies (item II.1) is significantly higher in 2016 (1.98 out of 3) than in 2021(1.51 out of 3), ( t = 4.237, P < 0.05). Regarding the performance of other activities involving identification (item II.2), the mean scores is 1.99 out of 3 in 2016 and 1.98 out of 3 in 2021.
Regarding the characteristics of China’s priority setting process, most indicators had a mean score of nearly 2 out of 3, demonstrating that these indicators were largely present. The indicator related to stakeholder involvement (item III.3) consistently received the highest score in both 2016 and 2021. However, respondents noted that the clarity of information on priorities (item III.4, t = 2.144, P < 0.05) and the extent of available literature (item III.5, t = 5.484, P < 0.05) were more explicit in 2016 than in 2021.
The ‘Assessment’ domain comprises 16 indicators categorized into five dimensions, inclusion of goal and scope, description of alternative technologies, aspects of assessment contents, standardized methods incorporation, and generalizability of the HTA scheme. The mean score of indicators concerning healthcare problems, patient population, and practitioners or users (item IV.1) was higher in 2016 than in 2021 ( P < 0.05).
Regarding describing the technical characteristics of health technologies under study and their alternatives (item IV.2), the mean score in 2021 was lower than that in 2016 ( t = 4.469, P < 0.05). Respondents reported that HTA activities in 2016 focused more on safety, clinical effectiveness, cost, and cost-effectiveness (item IV.3) than those in 2021 ( P < 0.05). However, the organizational analysis showed the opposite trend ( t = −2.408, P < 0.05).
Across all three indicators in the fourth dimension (Do HTAs incorporate standardized methods, item IV.4), the scores were below 2, indicating that this aspect of the HTA process was considered less developed by respondents. The indicator assessing whether HTA plans in China addressed generalizability and transferability (item IV.5) received the highest mean score among all 16 indicators in both 2016 (2.30 out of 3) and 2021 (2.23 out of 3).
The ‘Appraisal’ domain investigated whether a transparent and deliberative appraisal system, according to the participants, was in place. The respondents in 2021 believed that the appraisal process was more explicit, transparent, and replicable than those in 2016 (item V.1, t = −3.279, P < 0.05).
The ‘Reporting’ domain related to the utilization of the best practice guidelines in conducting and reporting HTA (item VI.1) received a score of 2.28 out of 3 in 2016 and 2.34 out of 3 in 2021, indicating that this aspect of HTA reporting was considered well developed in China over the five-year period.
Respondents reported a mean score of 2.93 out of 4 in 2016 and 2.70 out of 4 in 2021 for the number of HTA reports produced per year (item VI.2a, t = 2.564, P < 0.05), indicating a declining trend from 2016 to 2021. In contrast, the mean score for the number of HTA reports related to the NRDL per year (item VI.2b) was 3.09 out of 4 in both 2016 and 2021.
The ‘Dissemination of findings and conclusions’ domain related to the timeless of HTA report dissemination to decision makers and some dissemination strategies. It is worth noting that all indicators related to dissemination strategies (item VII.2) in 2021 showed significant improvement compared to those for 2016 ( P < 0.05).
The ‘Implementation in policy and practice’ domain is relevant to the policy and practice information provision and the HTA impact measurement. The mean scores for each indicator were above 2 out of 3, showing that these indicators were largely present. These findings suggest that HTA implementation in policy and practice is moderately well developed in China.
Overall, the level of HTA development in China was higher than that in middle-income countries (Argentina, Brazil, India, Indonesia, Malaysia, Mexico, and Russia) and lower than that in high-income countries (Australia, Canada, and the United Kingdom) in 2016 and 2021 (see Additional file 3 : Table S1). In our previous research conducted in 2016, we found that China scored lower than all ten countries regarding institutionalization level based on the views of survey respondents. However, the current results indicate that China has made significant advancements in institutionalization, although in 2021 the score for China is still lower than that for middle-income countries.
In this study, we employed HTA mapping instruments to gather diverse stakeholder perspectives in China during in 2016 and 2021. By comparing the mapping outcomes between these two groups using PSM, we gained valuable insights into the current state of HTA and the progress made in HTA development in China from 2016 to 2021. In addition, this study can also provide practical insights for the ‘selected countries’ [ 14 ], identifying the benefits of evaluating their HTA development after three to five years, especially in those countries that have announced healthcare reforms and changes to their HTA processes [ 19 , 20 , 21 ]. This evaluation can assist various stakeholders, including governments, HTA organizations, industry players, and other relevant actors in assessing HTA development at the country level. Furthermore, the study results provide valuable information to inform HTA strategies and justify investments in HTA.
Overall, the mean scores for most domains in 2021 were higher than those in 2016, indicating an improvement in HTA development. However, it is worth noting that the assessment and implementation domain have lower scores in 2021 than in 2016. Although China scored lower than the three developed countries, its overall HTA development score was comparable to those of the ten countries studied. This recognition signifies the significant achievements that China has made in HTA development since 2016.
Although there has been a significant improvement in China’s level of institutionalization in 2021 compared to 2016, our findings indicate that it still lags behind other countries. While HTA in China has recently expanded from pure academic research to include policy or decision-oriented practices, there is remains a lack of well-established systems for integrating HTA evidence into advisory, pricing, and reimbursement decision-making processes within health administration and payer organizations in China [ 11 , 13 , 22 , 23 ]. In addition to China, the process of HTA institutionalization in other low- and middle-income countries remains immature [ 24 , 25 ]. The insufficient institutionalization of HTA hinders its further development and utilization. It is crucial to prioritize national-level HTA institutionalization to promote the continued advancement. The key aspects of this institutionalization process include the identification and prioritization of HTA issues, adequate funding for HTA activities and human resources, establishment of implementation standards for various health technologies, development of HTA appraisal guidelines, and implementation of robust quality control mechanisms for HTA [ 6 , 11 , 19 ].
The scores for most indicators in the assessment domain were lower in 2021 than in 2016, potentially indicating the declining quality and validity of HTA evidence. Although HTA development in China has made significant strides in utilizing HTA evidence to inform policy decisions and successfully update the NRDL, it is important to acknowledge that most HTA studies in China are sponsored by manufacturers [ 11 , 26 ]. This raises concerns about potential conflicts of interest and uncertainties regarding the quality of the submitted cost and cost-effectiveness analysis (CEA) evidence. For example, Xie et al. have discovered that industry-sponsored CEAs are significantly more likely to report incremental cost-effectiveness ratios below commonly used thresholds than CEAs lacking such sponsorship [ 27 ]. To mitigate the potential influence of the utilization of pharmacoeconomic evidence provided by the industry, it is imperative for concerned institutions to establish and endorse standardized procedures for the disclosure of Financial Conflicts of Interest (FCoI) in pharmacoeconomic evaluations. This should encompass the comprehensive FCoI status disclosure of the input parameters’ sources. When submitting relevant studies, the mandatory inclusion of existing disclosure forms, such as the International Committee of Medical Journal Editors (ICMJE) disclosure form, should be enforced to enhance transparency.
Furthermore, even when relevant evidence is available, the adoption of appropriate methodologies is crucial for generating meaningful results that support decision making. Although global resources and best practices can be leveraged and used, China still faces challenges in adapting methodological approaches to meet national or local needs [ 11 ]. This is particularly true when applying standard HTA methodologies to assess the efficacy and cost-effectiveness of traditional Chinese medicine, which may require special considerations [ 11 , 26 ]. According to the top 10 challenges identified by The International Network of Agencies for Health Technology Assessment (INAHTA), there is a perception from INAHTA member agencies that the quality of evidence is declining, with fewer randomized trials being conducted and more observational and real-world data being used [ 24 ] .
The development score for an explicit, transparent, and replicable appraisal process improved in 2021 compared to that in 2016. Notably, in 2017, a significant change was implemented in the criteria for determining the inclusion of drugs in the NRDL, whereby both clinical and economic evaluations were stipulated as requirements for inclusion [ 3 , 12 ]. This shift from qualitative expert consensus to quantitative evidence has led to substantial progress in value-based price negotiations for price and reimbursement decisions [ 3 ]. Furthermore, the NHSA has provided guidelines and material requirements to pharmaceutical companies to ensure the provision of comprehensive dossiers supporting the inclusion of their products in the NRDL [ 28 , 29 , 30 ]. This measure enhances the explicit, transparent, and replicable appraisal processes.
This study had some limitations. First, the generalizability of the findings may be limited due to the convenience sampling approach. It was not possible to identify the statistics of the target population of HTA stakeholders in China. Therefore, the representativeness of our sample could not be fully assessed. Second, the questionnaire was adapted from an international study on the mapping levels of HTA development. There may have been imprecision and misinterpretation when translating the mapping instrument from English to Chinese. Third, within the policy-maker and researcher group, owing to their different levels of education, understanding, and application of HTA, the attached meaning to responses may vary across respondents.
From the perspective of multiple stakeholders, HTA development in China has made significant progress from 2016 to 2021, however, more efforts should be given to the assessment process ensuring a higher quality of HTA evidence before conducting HTA activity. Further qualitative research such as in-depth interviews and focus group discussions with multiple stakeholders, including HTA researchers and policy makers, is required to determine the specific reasons that influence the development level of HTA in China.
Availability of data and materials
The data will be shared on reasonable request to the corresponding author.
Abbreviations
Health Technology Assessment
National Reimbursement Drug List
National Healthcare Security Administration
Propensity Score Matching
Cost-Effectiveness Analysis
Financial Conflicts of Interest
International Committee of Medical Journal Editors
The International Network of Agencies for Health Technology Assessment
O’Rourke B, Oortwijn W, Schuller T. The new definition of health technology assessment: a milestone in international collaboration. Int J Technol Assess Health Care. 2020;36(3):187–90.
Article PubMed Google Scholar
Kristensen FB, Husereau D, Huić M, Drummond M, Berger ML, Bond K, et al. Identifying the Need for Good Practices in Health Technology Assessment: summary of the ISPOR HTA Council Working Group Report on Good Practices in HTA. Value Health. 2019;22(1):13–20.
Chen Y, He Y, Chi X, Wei Y, Shi L. Development of health technology assessment in China: new challenges. Biosci Trends. 2018;12(2):102–8.
Chen Y, Banta D, Tang Z. Health technology assessment development in China. Int J Technol Assess Health Care. 2009;25(Suppl 1):202–9.
Liu W, Shi L, Pong RW, Dong H, Mao Y, Tang M, et al. Differences in evaluating health technology assessment knowledge translation by researchers and policy makers in China. Int J Technol Assess Health Care. 2014;30(6):612–20.
Article CAS PubMed Google Scholar
Zhen X, Sun X, Dong H. Health technology assessment and its use in drug policies in China. Value Health Reg Issues. 2018;15:138–48.
Ming J, He Y, Yang Y, Hu M, Zhao X, Liu J, et al. Health technology assessment of medical devices: current landscape, challenges, and a way forward. Cost Eff Resour Alloc. 2022;20(1):54.
Article PubMed PubMed Central Google Scholar
Koh L, Glaetzer C, Chuen Li S, Zhang M. Health Technology assessment, international reference pricing, and budget control tools from China’s perspective: What Are the current developments and future considerations? Value Health Reg Issues. 2016;9:15–21.
Chen Y, Chi X, He Y, Wei Y, Oortwijn W, Shi L. Mapping of health technology assessment in China: situation analysis and international comparison. Int J Technol Assess Health Care. 2019;35(5):401–7.
Shi L, Mao Y, Tang M, Liu W, Guo Z, He L, et al. Health technology assessment in China: challenges and opportunities. Global Health J. 2017;1(1):11–20.
Article Google Scholar
Chen Y, Zhao K, Liu G, Chen W. Health technology assessment to inform decision making in China: progress, challenges, and sustainability. BMJ. 2023;381:e068910.
Li H, Liu GG, Wu J, Wu J-H, Dong C-H, Hu S-L. Recent pricing negotiations on innovative medicines pilot in China: experiences, implications, and suggestions. Value Health Reg Issues. 2018;15:133–7.
Chen Y, Dong H, Wei Y, Yang Y, Ming J, Yu H. Using health technology assessment to inform insurance reimbursement of high technology medicines in China: an example of cancer immunotherapy. BMJ. 2023;381:e069963.
Oortwijn W, Broos P, Vondeling H, Banta D, Todorova L. Mapping of health technology assessment in selected countries. Int J Technol Assess Health Care. 2013;29(4):424–34.
Key Lab of Health Technology Assesment, National Health Commission(Fudan University). The offical website of the National Health Commission Key Laboratory of Health Technology Assessment. 2015. https://chta.fudan.edu.cn/ . Accessed 28 June 2023.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424.
Walia M, Irani L, Chaudhuri I, Atmavilas Y, Saggurti N. Effect of sharing health messages on antenatal care behavior among women involved in microfinance-based self-help groups in Bihar India. Glob Health Res Policy. 2020;5:3.
Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–63.
Leelahavarong P, Doungthipsirikul S, Kumluang S, Poonchai A, Kittiratchakool N, Chinnacom D, et al. Health technology assessment in thailand: institutionalization and contribution to healthcare decision making: review of literature. Int J Technol Assess Health Care. 2019;35(6):467–73.
Downey LE, Mehndiratta A, Grover A, Gauba V, Sheikh K, Prinja S, et al. Institutionalising health technology assessment: establishing the Medical Technology Assessment Board in India. BMJ Glob Health. 2017;2(2):e000259.
Kim T, Sharma M, Teerawattananon Y, Oh C, Ong L, Hangoma P, et al. Addressing challenges in health technology assessment institutionalization for furtherance of universal health coverage through south-south knowledge exchange: lessons from Bhutan, Kenya, Thailand, and Zambia. Value Health Reg Issues. 2021;24:187–92.
Drummond MF, Augustovski F, Bhattacharyya D, Campbell J, Chaiyakanapruk N, Chen Y, et al. Challenges of health technology assessment in pluralistic healthcare systems: an ISPOR Council Report. Value Health. 2022;25(8):1257–67.
MacQuilkan K, Baker P, Downey L, Ruiz F, Chalkidou K, Prinja S, et al. Strengthening health technology assessment systems in the global south: a comparative analysis of the HTA journeys of China, India and South Africa. Glob Health Action. 2018;11(1):1527556.
O’Rourke B, Werkö SS, Merlin T, Huang LY, Schuller T. The “Top 10” challenges for health technology assessment: INAHTA viewpoint. Int J Technol Assess Health Care. 2020;36(1):1–4.
Kuchenbecker R, Polanczyk CA. Institutionalizing health technology assessment in Brazil: challenges ahead. Value Health Reg Issues. 2012;1(2):257–61.
Yue X, Li Y, Wu J, Guo JJ. Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China. Value In Health Reg Issues. 2021;24:1–5.
CAS Google Scholar
Xie F, Zhou T. Industry sponsorship bias in cost effectiveness analysis: registry based analysis. BMJ. 2022;22(377):e069573.
The National Healthcare Security Administration of China. Notice on the work plan for the adjustment of National Medical Insurance Drug List. 2019. http://www.nhsa.gov.cn/art/2019/4/17/art_37_1214.html . Accessed 16 June 2023.
National Healthcare Security Administration of China and Ministry of Human Resources and Social Security of China. The National Medical Insurance Drug List through Negotiation in 2019. 2019. http://www.nhsa.gov.cn/art/2019/11/28/art_14_2052.html . Accessed 16 June 2023.
National Healthcare Security Administration. Announcement about the work plan for the adjustment of the national reimbursement drug list 2021 and the application guide for the adjustment of the national reimbursement drug list 2021. 2021. http://www.nhsa.gov.cn/art/2021/6/30/art_109_6616.html . Accessed 17 June 2023.
Download references
Acknowledgements
We appreciate all the participants in this web-based survey for their cooperation. We also thank Prof. Wija Oortwijn for the great work of developing the instrument to map the level of HTA development at country level.
This study was supported by China Medical Board (CMB) under Grant 19-318, A Demonstration Program on Health Technology Assessment in China.
Author information
Shimeng Liu and Yu Xia co-first authors contributed equally to this paper.
Authors and Affiliations
School of Public Health, Fudan University, Shanghai, 200032, China
Shimeng Liu, Yu Xia, Yi Yang, Jian Ming, Hui Sun, Yan Wei & Yingyao Chen
National Health Commission Key Laboratory of Health Technology Assessment (Fudan University), Shanghai, 200032, China
Shanghai Health Development Research Center, Shanghai, 201199, China
You can also search for this author in PubMed Google Scholar
Contributions
YC, SL and YW contributed to the study conception and design. Material preparation and data collection were performed by SL and YC. YX, YY, JM, HS and YW also participated in data collection. Data analysis were performed by YX and SL. The manuscript was written by SL and YX. YC and YW conceived the perspective and contributed to the writing. YC made the critical revision of this manuscript. All authors commented on previous versions of the manuscript. All authors approved the final manuscript.
Corresponding authors
Correspondence to Yan Wei or Yingyao Chen .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was approved by the institutional review board, Public Health School of Fudan University (IRB00002408 & FWA00002399).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1: figure s1..
The Mapping of HTA instrument. Note This instrument was developed by Wija Oortwijn et al. This figure contains the detailed eight domains and their indicators as well as the scores (max. total score 146).
Additional file 2: Figure S2.
Common support test. Note After PSM adjustment, there were no significant differences between the two matched groups with regard to the propensity score, that is, baseline characteristics were balanced.
Additional file 3. Table S1.
Level of HTA development per domain in the selected middle-and high-income countries. Note Data of the seven middle-income and three high-income countries are from the research of Oortwijn et al. [ 14 ]. Middle-income countries include Argentina, Brazil, India, Indonesia, Malaysia, Mexico, and Russia, high-income countries include Australia, Canada, and the United Kingdom.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Liu, S., Xia, Y., Yang, Y. et al. Mapping of health technology assessment in China: a comparative study between 2016 and 2021. glob health res policy 9 , 4 (2024). https://doi.org/10.1186/s41256-023-00339-6
Download citation
Received : 18 July 2023
Accepted : 09 December 2023
Published : 16 January 2024
DOI : https://doi.org/10.1186/s41256-023-00339-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health technology assessment
- Development level
- Propensity score matching
Global Health Research and Policy
ISSN: 2397-0642
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
KPMG hit by Hong Kong High Court in $400 million China Medical fraud
Reporting by Matthew Miller; Editing by Jennifer Hughes and Christopher Cushing
Our Standards: The Thomson Reuters Trust Principles. , opens new tab

Woodside seeks better terms to develop Trinidad's Calypso gas project
Australian oil and gas producer Woodside Energy would like to see improved fiscal terms from the Trinidad and Tobago government before committing to develop a deepwater natural gas project there, CEO Meg O'Neill told Reuters this week.

Risk Factors of Enternal Nutrition Intolerance in Septic Patients: A Case-control Study
- Published: 22 March 2024
Cite this article
- Li-zhu Wang 1 ,
- Yan Xiang 1 ,
- Qian Li 1 ,
- Yi-rong Zhu 1 ,
- Jue Fang 1 ,
- Xiao-dan Lu 1 &
- Zhao-cai Zhang 2 , 3 , 4
Explore all metrics
This study aimed to investigate the incidence of enteral nutrition intolerance (ENI) in patients with sepsis and explore potential risk factors.
A case-control study was conducted in patients with sepsis who were receiving enteral nutrition (EN) at a tertiary hospital in China. The included patients were divided into the ENI group and the non-ENI group. Univariate and multivariate analyses were performed to identify the risk factors for ENI.
A total of 859 patients were included in the study. Among them, 288 (33.53%) patients experienced symptoms of ENI, including diarrhea, vomiting, bloating, and gastric retention. Logistic regression analysis revealed that the Acute Physiology and Chronic Health Evaluation H (APACHE H) score, thoracocentesis, and usage of cardiotonic drugs (namely, inotropes) were independent predictors of the ENI.
The incidence of ENI is relatively high in patients with sepsis, especially in those who have higher APACHE H scores, have undergone thoracocentesis, and have received inotropes.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis 3). Jama, 2016,315(8):775–787
Article PubMed PubMed Central Google Scholar
Fleischmann-Struzek C, Mellhammar L, Rose N, et al. Incidence and mortality of hospital-and ICU-treated sepsis: results from an updated and expanded systematic. review and meta-analysis. Intensive Care Med, 2020,46(8):1552–1562
Article CAS PubMed PubMed Central Google Scholar
McClave SA, Lowen CC, Martindale RG. The 2016 ESPEN Arvid Wretlind lecture: The gut in stress. Clin Nutr, 2018,37(2):19–36
Article PubMed Google Scholar
Rokyta R Jr, Matejovic M, Krouzecky A, et al. Postpyloric enteral nutrition in septic patients: Effects on hepato-splanchnic hemodynamics and energy status. Intensive Care Med, 2004,30(14):714–717
Gatt M, MacFie J, Anderson AD, et al. Changes in superior mesenteric artery bloodflow after oral, enteral, and parenteral feeding in humans. Crit Care Med, 2009,37(12):171–176
Evans L, Andrew R, Waleed A, et al. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med, 2021,49(11):1974–1982
Longhitano Y, Zanza C, Thangathurai D, et al. Gut alterations in septic patients: a biochemical literature reviews. Rev Recent Clin Trials, 2020,15(4):289–297
Article CAS PubMed Google Scholar
Blaser AR, Deane AM, Preiser JC, et al. Enteral feeding intolerance: updates in Definitions and Pathophysiology. Nutr Clin Pract, 2021,36(1):40–49
Article Google Scholar
Berk Takir H, Karakurt Z, Salturk C, et al. Does total parenteral nutrition increasethe mortality of patients with severe sepsis in the ICU? Turk Thorac J, 2015,16(2):53–58
Lavrentieva A, Kontakiotis T, Bitzani M. Enteral nutrition intolerance in criticallyill septic burn patients. Burn Care Res, 2014,5(12):313–318
Heyland DK, Ortiz A, Stoppe C, et al. Incidence, risk factors, and clinical consequence of enteral feeding intolerance in the mechanically ventilated critically ill: an analysis of a multicenter, multiyear database. Crit Care Med, 2021,49(1):49–59
Yu K, Guo N, Zhang D, et al. Prevalence and risk factors of enteral nutrition intolerance in intensive care unit patients: a retrospective study. Chin Med J (Engl), 2022,135(15):1814–1820
Paul S, Simon H, Marianne C, et al. Feeding the critically ill obese patient: a systematic review protocol. JBI Database Systematic Rev Implement Rep, 2015,13(10):95–109
Jenkins B, Calder PC, Marino LV. A systematic review of the definitions and prevalence of feeding intolerance in critically ill adults. Clin Nutr ESPEN, 2022,49:92–102
Xiao Y, Xu L. Prevalence and Risk Factors of Enteral Feeding Intolerance in Critically Ill Patients and the Effectiveness of Preventive Treatments: A Prospective Study. Saudi J Med Med Sci, 2023,11(2):135–142
Chen T, Wang T, Li Q, et al. Current status and influencing factors of enteral. feeding intolerance in patients with severe acute pancreatitis. Chin J Nurs, 2017,52(6):716–720
Google Scholar
Virani FR, Peery T, Rivas O. Incidence and Effects of Feeding Intolerance in Trauma Patients. JPEN J Parenter Enteral Nutr, 2019,43(6):742–749
Chen W, Wang H, Chen Y, et al. The independent risk factors of early diarrhea in enteral nutrition for ICU patients. J Int Med Res, 2019,47(10):4929–4939
Haussner F, Chakraborty S, Halbgebauer R, et al. Challenge to the intestinal mucosa during sepsis. Front Immunol, 2019,10:891–907
Bordejé ML, Montejo JC, Mateu ML, et al. Intra-Abdominal pressure as a marker of enteral nutrition intolerance in critically ill patients. the PIANE study. Nutrients, 2019,11(11):2616
Zhao HB, Jia L, Yan QQ, et al. Effect of Clostridium butyricum and Butyrate on Intestinal Barrier Functions: Study of a Rat Model of Severe Acute Pancreatitis with Intra-Abdominal Hypertension. Front Physiol, 2020,11:561061
Leng Y, Jiang C, Xing X, et al. Prevention of Severe Intestinal Barrier Dysfunction Through a Single-Species Probiotics is Associated with the Activation of Microbiome-Mediated Glutamate-Glutamine Biosynthesis. Shock, 2021,55(1):128–137
Regli A, Hockings LE, Musk GC, et al. Commonly applied positive end-expiratory pressures do not prevent functional residual capacity decline in the setting of intra-abdominal hypertension: a pig model. Crit Care, 2010,14(04):R128
Regli A, Mahendran R, Fysh ET, et al. Matching positive end-expiratory pressure to intra-abdominal pressure improves oxygenation in a porcine sick lung model of intra-abdominal hypertension. Crit Care, 2012,16(05):R208
Silva PL, Ball L, Rocco PRM, et al. Physiological and Pathophysiological. Consequences of Mechanical Ventilation. Semin Respir Crit Care Med, 2022,43(3):321–334
Santos CL, Moraes L, Santos RS, et al. Effects of different tidal volumes in pulmonary and extrapulmonary lung injury with or without intraabdominal hypertension. Intensive Care Med, 2012,38(03):499–508
Pelosi P, Luecke T, Rocco PR. Chest wall mechanics and abdominal pressure. during general anesthesia in normal and obese individuals and in acute lung injury. Curr Opin Crit Care, 2011,17(01):72–79
Gayen S. Malignant Pleural Effusion: Presentation, Diagnosis, and Management. Am J Med, 2022,135(10): 1188–1192
Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet, 2018,392(10141):75–87
Jacobi J. The pathophysiology of sepsis-2021 update: Part 2, organ dysfunction and assessment. Am J Health Syst Pharm, 2022,79(6):424–436
De Backer D, Arias Ortiz J, Levy B. The medical treatment of cardiogenic shock: cardiovascular drugs. Curr Opin Crit Care, 2021,27(4):426–432
Download references
Acknowledgments
We thank LetPub ( www.letpub.com ) for its linguistic assistance during the preparation of this manuscript and express our deep gratitude to Bin SHAO and Huang-kai SHOU for their valuable contributions to the data collection.
Author information
Authors and affiliations.
Department of Nursing, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310009, China
Li-zhu Wang, Yan Xiang, Qian Li, Yi-rong Zhu, Jue Fang & Xiao-dan Lu
Department of Critical Care Medicine, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310009, China
Zhao-cai Zhang
Key Laboratory of the Diagnosis and Treatment for Severe Trauma and Burn of Zhejiang Province, Hangzhou, 310009, China
Zhejiang Province Clinical Research Center for Emergency and Critical Care Medicine, Hangzhou, 310009, China
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Zhao-cai Zhang .
Ethics declarations
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
This work was supported by the National Key Research and Development Program of China (No. 2021YFC2501800), the National Natural Science Foundation of China (Nos. 82272182 and 82072202), the Zhejiang Provincial Natural Science Foundation of China (No. LHDMD22H02001) and the Zhejiang University Horizontal Program (No. K-Horizontal 20202295).
Rights and permissions
Reprints and permissions
About this article
Wang, Lz., Xiang, Y., Li, Q. et al. Risk Factors of Enternal Nutrition Intolerance in Septic Patients: A Case-control Study. CURR MED SCI (2024). https://doi.org/10.1007/s11596-024-2849-3
Download citation
Received : 28 October 2023
Accepted : 11 February 2024
Published : 22 March 2024
DOI : https://doi.org/10.1007/s11596-024-2849-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- enteral nutrition intolerance
- influencing factor
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cost Eff Resour Alloc

Health technology assessment of medical devices: current landscape, challenges, and a way forward
1 Real World Solutions, IQVIA, Shanghai, 200124 China
2 National Health Commission Key Laboratory of Health Technology Assessment, School of Public Health, Fudan University, Shanghai, 200032 China

Xinran Zhao
Yingyao chen, associated data.
The data used and/or analyzed during the study are available from the corresponding author on reasonable request.
Health Technology Assessment (HTA) has been widely recognized as informing healthcare decision-making, and interest in HTA of medical devices has been steadily increasing. How does the assessment of medical devices differ from that of drug therapies, and what innovations can be adopted to overcome the inherent challenges in medical device HTA?
HTA Accelerator Database was used to describe the landscape of HTA reports for medical devices from HTA bodies, and a literature search was conducted to understand the growth trend of relevant HTA publications in four case studies. Another literature review was conducted for a narrative synthesis of the characteristic differences and challenges of HTA in medical devices. We further conducted a focused Internet search of guidelines and a narrative review of methodologies specific to the HTA of medical devices.
The evidence of HTA reports and journal publications on medical devices around the world has been growing. The challenges in assessing medical devices include scarcity of well-designed randomized controlled trials, inconsistent real-world evidence data sources and methods, device-user interaction, short product lifecycles, inexplicit target population, and a lack of direct medical outcomes. Practical solutions in terms of methodological advancement of HTA for medical devices were also discussed in some HTA guidelines and literature.
To better conduct HTA on medical devices, we recommend considering multi-source evidence such as real-world evidence; standardizing HTA processes, methodologies, and criteria; and integrating HTA into decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12962-022-00389-6.
Introduction
Health Technology Assessment (HTA) is a multidisciplinary process that uses a number of methods to determine the value of health technologies at different stages of their life cycle. HTA aims to provide evidence for health policy decision-making and for establishing an equitable, efficient, and high-quality health system [ 1 ]. Since its first application in the United States in the 1970s, HTA has developed rapidly and has been applied globally, becoming the basis for health decisions such as pricing and reimbursement in many different countries and regions. However, more of the existing HTA research concerns medicines rather than medical devices. Medical devices differ considerably from drug therapies in terms of their product lifecycle, regulatory environment, diversity, user–device interaction, and so on [ 2 ]. Even within medical devices, there are significant differences between therapeutic, instrumental, and diagnostic products. Moreover, various studies have investigated how these differences have posed great challenges to the HTA of medical devices and have thus called for applying a more innovative approach to medical devices compared to drugs. However, few studies have offered practical or actionable solutions. There is still a lack of consensus on the HTA of medical devices with regard to dimensions, process, criteria, and methods.
This study aimed to (1) describe the current landscape of HTA activities specific to medical devices; (2) analyze the characteristics of medical devices and the resulting challenges in the HTA of medical devices compared to pharmaceuticals; (3) perform a focused search of websites of official HTA agencies to identify international HTA guidelines specific to medical devices, intending to summarize implementable solutions to the HTA of medical devices. In addition, we supplemented the analysis of HTA guidelines with a narrative review of existing studies discussing the challenges of, and potential suggestions for, the HTA of medical devices.
To understand the landscape for HTA conducted on medical devices, we performed a retrospective analysis using IQVIA’s HTA Accelerator Database ( www.iqvia.com/landing/hta-accelerator ). It contains over 33,000 HTA publications that cover 100 HTA bodies in 40 countries. The primary data source came from the HTA submissions that could be tracked by local language. Market access experts from IQVIA were responsible for regularly tracking and translating all newly published HTA reports. The database captured over 250 available data elements such as the general information in the HTA report, including publication country, agency, publication date, disease area, product types, comparators, recommendations, etc. In this study, we focused only on HTA reports specific to medical devices in the HTA Accelerator Database by selecting the product type as “medical device.” We limited the assessment type of HTA submissions to health technology assessment or rapid review (including the assessment of safety, efficacy, cost-effectiveness, etc.), while other submissions such as clinical guidelines and public health reviews were excluded. As the earliest reports dated back to the year 2000, we extracted HTA reports published from 2000 onwards.
To better demonstrate the current research progress on the HTA status of medical devices, we examined four case studies on medical devices, including (1) stents (2) hip and knee arthroplasty, (3) the da Vinci Surgical System, and (4) transcatheter aortic valve implantation (TAVI) and mitral valve repair (TMVR). We did not intend for the case studies to be representative of all medical devices as there is a great deal of diversity in medical devices beyond those four cases, such as diagnostic or instrumental devices. Instead, through our choice of target devices, we aimed to cover a range of heterogeneous cases in terms of disease epidemiology, procedure characteristics, technology maturity, and demographics. We used the number of HTA-related publications to measure the activity level of the current HTA research. We conducted a literature search and tracked the growth trend of relevant HTA publications on PubMed, Embase, and Web of Science. We included HTA studies and economic evaluations and excluded relevant systematic reviews or meta-analyses. The detailed search strategy in each database is listed in Additional file 1 : Table S1.
In addition, a narrative literature review was conducted for a synthesis of the characteristic differences and challenges of HTA in Medical Devices. The literature search was performed using PubMed, Embase, and Web of Science. We included relevant empirical studies or reviews discussing the use of HTA for medical devices. The detailed search strategy in each database is listed in Additional file 1 : Table S2.
Two reviewers (J.M. and Y.H.) independently assessed the titles and abstracts of all identified study and then reviewed full text to determine the potential eligibility for the above narrative literature review. Disagreements on whether a specific study should be considered were resolved by a third investigator (X.Z.).
To guide the efficient application of HTAs, we performed a gray literature search of official websites of major HTA agencies to identify HTA guidelines with respect to medical devices. As guidelines represent a consensus in the academic community, we believed that international HTA guidelines have reflected, to some extent, the current best possible practice. We complemented the search by reviewing the bibliographies of relevant literature identified through a target literature review of methodological publications on the HTA of medical devices. Only those (either guidelines or articles) that were specific to medical devices and elaborate economic evaluation, decision-analytic modeling, and/or HTA were included.
Current status of the HTA of medical devices
Published reports from hta bodies.
In total, around 2300 HTA reports from agencies across 30 countries or regions were identified. We presents the overall trend of HTA report submissions in Fig. 1 . Overall, the body of HTA reports for medical devices increased across the world. Before 2010, the number of HTA reports published for medical devices was limited, ranging from three in 2000 to 20 in 2009. Since 2011, the number of published HTA reports has increased rapidly to reach 340 reports in 2019. Within the last 20 years, there has been a 100-fold increase in the number of HTA reports for medical devices.

Number of Health Technology Assessment (HTA) reports for medical devices by country-year: 2000–2020
Journal publications on HTA of medical devices
Figure 2 shows the growth trend of HTA-related publications on the four selected devices, respectively. Overall, we observed a general upward trend in the four products, despite annual fluctuations, indicating that the HTA of medical devices has been growing rapidly. In addition, the level of development of HTA was also related to the characteristics of the medical device, such as technology maturity and disease epidemiology. We observed from Fig. 2 a that there was a larger body of publications on stents compared to other devices since the stent was a mature device with broader applicable patient populations, indications, and long years of availability. In a comparison, TAVI and TMVR, as a relatively new product, had fewer relevant HTA publications. Additionally, there was significant growth in HTA publications for all four devices since their first market launch. The overall trend in relevant publications suggested a progressive increase in the HTA publications and academic interest in medical devices.

Summary of HTA-related literature. a Annual number of HTA-related publications on stents; b annual number of HTA-related publications on hip and knee arthroplasty; c annual number of HTA-related publications on Da Vinci; d annual number of HTA-related publications on TAVI and TMVR
Narrative synthesis of characteristic differences and challenges of HTA of medical devices
After a literature search on journal publications discussing HTA of medical devices, a total of 1646 records were identified, and 26 publications were included in our review after title and abstract screening or full text review. The PRISMA flowchart of literature review is provided in Additional file 1 : Fig. S1.
The characteristic differences and challenges of HTA in medical devices are summarized in Table Table1. 1 . Overall, there were several key characteristic differences between drugs and medical devices, including the availability of treatment outcomes and other factors that may impact efficacy. First, the treatment outcome for medical devices was not as clear and straightforward as it would be with drugs, because an intervention with device involves the medical devices themselves as well as other subsequent treatments. Furthermore, devices usually had multiple applications, making it hard to assess each application in the same way that traditional drugs were assessed for an individual indication. Second, Randomized Controlled Trials (RCTs) for medical devices are rare compared to drugs, resulting in a lack of sufficient efficacy/effectiveness data and making it difficult for economic evaluation. Third, the product life cycle of medical devices was generally much shorter than that of drugs, which may result in multiple specifications within a single product class and unclear definition of standard of care. Additionally, the efficacy of medical device treatments depends on the medical devices themselves and their use.
Summary of characteristic differences and challenges of HTA of medical devices
Discussions on practical solutions for the challenges of HTA of medical devices
We obtained a total of eight HTA guidelines specific to medical devices issued by HTA agencies or research initiatives across six regions. The National Institute for Health and Care Excellence (NICE) in the United Kingdom issued an HTA methods guide for their Medical Technologies Evaluation Programme in 2011 [ 29 ]. Following the methods guide, NICE also issued the Diagnostics Assessment Programme manual specifically for diagnostic technologies demonstrating higher test accuracy, but at a greater cost compared to those in current use [ 30 ]. In Canada, Health Quality Ontario (HQO) released a method and process guide for HTA in 2018, with a scope spanning from medical devices, diagnostics, and surgical procedures to complex health system interventions [ 31 ]. In Australia, two HTA guidelines have been developed separately for therapeutic and diagnostic devices by the Medical Services Advisory Committee (MSAC) [ 32 , 33 ]. In the Asia–Pacific region, the Singapore Agency for Care Effectiveness (ACE) was the only national HTA organization that has released HTA guidelines on medical devices [ 34 ]. Apart from these official HTA agencies, an international collaborative network also contributed to the methodological advancement of HTA for medical devices. For example, the European Network for Health Technology Assessment (EUnetHTA), has launched a series of research initiatives to develop a methodological framework for HTA of therapeutic medical devices [ 35 ].
Available clinical evidence
Given that RCT evidence for medical devices was generally limited, an open-minded and flexible attitude to other forms of evidence e.g., case reports (series), cohort studies, case control studies, and real-world studies was highly recommended [ 29 , 34 , 35 ]. Both the UK and EUnetHTA guidelines have pointed out the high risk of bias in non-randomized controlled trials [ 30 , 35 ]. At the same time, several tools have been developed, although they may not be specific for medical devices. The Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies of Interventions (ACROBAT-NRSI) could be used to assess the risk of bias in non-randomized controlled studies [ 36 ]. In addition, the quality assessment for case reports (series) could refer to the checklist developed by the Canadian Institute of Health Economics [ 37 ].
The draft guidance released by the United States Food and Drug Administration (FDA) in 2016 has spurred a surge in the literature describing how real-world evidence (RWE) can be used to support regulatory approval for medical devices [ 38 ]. RWE refers to any evidence on healthcare generated from multiple sources outside clinical trial settings, which is usually in the form of electronic medical records (EMR), electronic health records (EHR), hospital databases, patient registries, claims data, etc. [ 39 ]. In addition to market authorization, RWE was also relevant in post-marketing surveillance, coverage decisions, outcome-based contracting, resource use, and treatment compliance [ 40 , 41 ]. Especially for medical device products for which the regulatory environment does not require RCTs, or in situations where RCTs traditionally have been lacking such as measuring disease burden and detecting new safety signals, RWE could offer unique perspectives.
Unlike randomized clinical trials, most RWE comes from observational studies and might have many drawbacks. While current medical device-specific HTA guidelines have underscored the potential bias associated with RWE and several tools may be available for assessment of bias for non-randomized studies, few guidelines have addressed other common issues including data quality, availability, standards, and privacy [ 29 , 30 , 32 , 33 , 35 , 42 ]. For example, a European study that mapped RWE studies of three medical device products has revealed that the accessibility of data sources for RWE varied greatly across European countries. The study also suggested the types and definitions of variables included in each data source were not consistent, making a comparison across databases impossible [ 43 ]. Therefore, there is a need for RWE guidance on medical devices which would not only provide overarching frameworks but also standardize methods and processes ranging from data storage, collection, and sharing to analytic approaches.
Device–user interaction
International medical device-specific HTA guidelines have emphasized the need to account for the learning curve effect in HTA. The EUnetHTA has suggested that it is necessary to establish a break-in period before the formal evaluation to ensure that users have sufficient time to adapt to the new technology. Also, various degrees of operator proficiency across different types of medical research centers (e.g., teaching hospitals and non-teaching hospitals) would lead to heterogeneity in HTA. Therefore, the EUnetHTA proposed a three-tiered approach to accounting for the learning curve in its HTA guidelines for therapeutic devices. Firstly, assessors should screen for studies that estimate an association between user proficiency or healthcare settings (e.g., teaching or non-teaching hospitals) and clinical outcomes. Secondly, if the effect of the learning curve was not reported in the RCT and relevant information could not be obtained by contacting the investigators, then other types of evidence such as non-randomized controlled and non-comparative effectiveness studies could also be considered in order to explore the association between operator proficiency, types of study centers, and clinical outcomes. Lastly, subgroup analyses could be applied where existing studies were divided into different subgroups based on the level of operator proficiency. Statistical methods such as meta-analysis could be used to estimate the difference in medical outcomes between these subgroups and hence quantify the effect of the learning curve [ 35 ]. The radiofrequency ablation (RFA) for liver tumors treatment serves as an example. In a systematic review, researchers divided 100 case reports into four subgroups according to the surgeons’ previous RFA experience (i.e., having done < 20, 21–50, 51–99, > 100 cases respectively). The results of the meta-analysis showed the tumor recurrence rate decreased (18%, 16%, 14%, and 10% respectively in the four subgroups) as surgeons accumulated experience [ 44 ].
Short product life cycle and quick upgrade
In practice, a Bayesian approach was recommended to account for the iterative nature of medical devices in HTA [ 35 ]. The Bayesian approach is a statistical method that infers the posterior distribution of unknown parameters according to Bayes’ theorem based on prior knowledge and sample data. Considering that medical devices are incrementally upgraded with minor modifications, clinical trials and/or early research data of the former version of the medical device product, sometimes even data of comparator products could be a source for prior information used in the Bayesian approach.
Inexplicit target population and lack of direct clinical outcomes
Given the lack of direct clinical outcomes for screening and diagnostic devices, the HQO allows the use of established surrogate endpoints or intermediate clinical indicators to predict patients’ final medical outcomes. For instance, the association between intermediate indicators (e.g., blood pressure) and cardiovascular-related deaths has already been established through statistical models [ 31 ]. In terms of evaluating screening or diagnostic technologies, NICE, MSAC, and EUnetHTA stress that product performance should be reflected in the entire care pathway. In this way, the HTA should not only evaluate the test accuracy, but also consider the impact of the diagnostic results (no matter how accurate they were) on subsequent treatment pathways and the final medical outcomes [ 30 , 32 , 35 ]. One particular technique described by international HTA guidelines is the linked analysis [ 30 , 32 ]. In its first step, a linked analysis collects comprehensive data on the test accuracy of diagnostic technologies and the effectiveness of subsequent clinical interventions following the diagnostic results. Then, these data are modeled to simulate the whole care pathway and to estimate the impact of the diagnostic device on the final medical outcome [ 30 ]. However, it is worth mentioning that there were two premises for conducting linked analysis: (1) the effectiveness of clinical interventions subsequent to the diagnostic results must be established by confirmatory trials and should be available; (2) Patients’ baseline characteristics in these confirmatory trials of the subsequent clinical interventions should resemble the population to which the diagnostic devices were applied.
Considering multi-source evidence such as real-world evidence
Most HTAs of pharmaceuticals have been performed using economic evaluations with parameters derived from RCTs. However, the market authorization for most medical devices does not require rigorous RCTs, leading to limited clinical evidence. The scarcity of clinical research has made RWE particularly important in generating clinical effectiveness and safety data for the HTA of medical devices. Unlike the ideal experimental environment of RCTs, the “real world” refers to actual clinical settings where patients have not been selected based on pre-specified criteria. Patients enrolled in RWE studies tend to cover different subgroups so that they are representative of the whole population. For this reason, RWE reflects the true effects of clinical interventions. Correspondingly, HTA based on RWE could provide healthcare decision-makers with insights that came from real-world settings. As the uptake of newly introduced medical devices often requires a break-in period, this creates the perfect timing to collect real-world data on products’ safety and effectiveness. In addition to RWE, HTA could also collect public opinions from multiple third parties (patients, manufacturers, health care providers) regarding current evidence, treatment pattern, and patient categories.
Standardization of tools and evaluation criteria for HTA of medical devices
Existing HTA guidelines mainly focus on drugs and cannot be applied directly to the HTA of medical devices even with adaptation. Therefore, we suggest that separate HTA guidelines for medical devices are needed to standardize the topic identification, selection of comparator, evaluation methods, cost measurement, effect/utility measurement, evidence synthesis, systemic review, and ethnic requirements. Moreover, the HTA report should follow a consistent reporting paradigm. We also recommend that decision-makers follow the same HTA guidelines to conduct HTA appraisals. The formulation of HTA guidelines should be transparent and publicly available. At the same time, regular updates are necessary to reflect the evolution of HTA methods, and international collaboration is needed in overcoming the inherent challenges in medical device HTA.
Intergration of HTA of medical devices into decision-making
As a bridge connecting scientific research and health decision-making, the development of HTA is closely interwoven with established mechanisms such that the results of HTA could be translated into real practice. HTA as well as value assessment methods have been adopted around the world in national coverage decisions for pharmaceuticals. Nevertheless, the application of HTA in medical devices decision-making is in an earlier stage with higher uncertainty. Therefore, it is essential to explore an effective mechanism that would enable the translation of the results of HTA of medical devices into decision-making. Specifically, the decision translation mechanism could take the form of regulatory authorization, market access and reimbursement, and price negotiations where HTA could be introduced. We believe that better integration of HTA into decision-making would further encourage evidence generation and the adoption of HTA standards and ultimately promote an evidence-based, decision-making culture.
The body of HTA reports and journal publications on medical devices around the world has been growing. Our analysis revealed that medical devices differ considerably from pharmaceuticals in many respects, which has made the HTA of medical devices quite challenging. These challenges include scarcity of well-designed RCTs, inconsistent RWE data sources and methods, device-user interaction, short product lifecycle, inexplicit target population, and lack of direct medical outcomes.
Practical solutions found in the HTA guidelines to account for these challenges include (1) adopting an open mind toward evidence other than that generated through an RCT, such as RWE, especially as newly introduced medical devices often require a break-in period; (2) accounting for the learning curve that impacts the device-user interaction through several means including subgroup analyses; (3) applying a Bayesian approach to account for the iterative nature of medical devices; and (4) ensuring that product performance is measured across the entire care pathway through techniques such as linked analyses.
Based on the results of the above analysis, we call on both academic communities and relevant agencies to standardize the process, methodologies, and criteria of HTAs on medical devices, particularly when an HTA has involved RWE studies. We also recommend that national authorities better integrate the HTA of medical devices into decision-making and promote a more evidence-based culture.
Acknowledgements
The authors would like to thank the following persons, who graciously contributed their valuable time, knowledge, and input over the course of this research: Yanfeng Ren (Fudan University), Xinyu Liang (University of Michigan), Guanqi Hong (employee of IQVIA), Xinyi Wang (employee of IQVIA), Yaping Ai (employee of IQVIA), Min Jin (employee of IQVIA).
Institutional review board statement
Not applicable.
Author contributions
Conceptualization, JM, YH, YW, YC and MH; methodology, JM, JL and YX; investigation, YH and YY; formal analysis, YH and JM; data curation, JM, YH, XZ and YY; writing—original draft preparation, YH and JM; writing—review and editing, YY, MH, XZ, YW and YC; supervision, JL, YX, MH and YC. All authors read and approved the final manuscript.
This work was supported by the National Key Research and Development Program of China (No. 2018YFC1312900).
Availability of data and materials
Declarations.
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jian Ming and Yunzhen He contributed equally to this work
Contributor Information
Yan Wei, Email: nc.ude.naduf@iewnay .
Yingyao Chen, Email: nc.ude.umhs@nehcyy .
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, carbon conduction effect and multi-scenario carbon emission responses of land use patterns transfer: a case study of the baiyangdian basin in china.

- 1 School of Public Administration, Hebei University of Economics and Business, Shijiazhuang, China
- 2 Hebei Collaborative Innovation Center for Urban-Rural Integrated Development, Hebei University of Economics and Business, Shijiazhuang, China
- 3 Center for Urban Sustainability and Innovation Development (CUSID), Hebei University of Economics and Business, Shijiazhuang, China
Carbon pooling and release occur all the time in all corners of the earth, where the land use factor is key to influencing the realization of carbon peaking and neutrality. Land use patterns and carbon emissions change under different scenarios and analyzing the correlation will help formulate scientific land use policies for the future. In this study, through remote sensing data, we investigated the changes in land use patterns and carbon emissions in the Baiyangdian basin in China from 2000 to 2020 and analyzed the carbon conduction effect with the help of a land transfer matrix. The geographical simulation and optimization system-future land use simulation (GeoSOS-FLUS) and Markov models were used to predict the land use changes and carbon emissions under the four different scenarios for the region in 2035. The results indicated that 1) the net land use carbon emissions increased from 52,163.03 × 10 3 to 260,754.91.28 × 10 3 t from 2000 to 2020, and the carbon source-sink ratio exhibited a general uptrend; 2) the net carbon emissions due to terrestrial transfers increased over time. The carbon conduction effects due to the transfer of forests, grasslands, water areas, and unused lands to built-up lands also showed a rising trend, albeit the latter two exhibited only small changes; 3) in 2035, the net carbon uptake under the four development scenarios was predicted to be 404,238.04 × 10 3 , 402,009.45 × 10 3 , 404,231.64 × 10 3 , and 404,202.87×10 3 t, respectively, with all values much higher than that of the study area in 2020. The maximum carbon sink capacity was 817.88 × 10 3 t under the double-carbon target scenario, and the maximum carbon source emission was 405,033.61 × 10 3 t under the natural development scenario. The above results provide an essential reference for low carbon-based urban land use regulations for the Baiyangdian basin and other similar projects in the future.
1 Introduction
Global climate change poses a significant threat to sustainable development and the survival of humans ( Rong et al., 2022 ). The terrestrial carbon system is an important component of the global carbon cycle, which plays a critical role in mitigating global warming by effectively regulating the regional climate through the absorption and release of greenhouse gases from the atmosphere ( Yu et al., 2022 ). Land use activities primarily affect the carbon cycle of the ecosystem ( Mendelsohn and Sohngen, 2019 ), with their carbon emissions being second only to the burning of fossil fuels ( Wang Z. et al., 2022 ). Thus, regulating land use activities to reduce carbon emissions is an important means of promoting carbon neutrality from a practical perspective ( Carpio et al., 2021 ). Therefore, several studies have aimed to demonstrate how carbon emissions from land use can help achieve a range of low carbon development goals, particularly carbon peak and neutrality ( Yang and Liu, 2022 ).
Most studies on land use carbon emissions focus on accounting, mechanisms and consequences, projections, and impact factors ( Le Quéré et al., 2012 ; Houghton and Nassikas, 2017 ; Yu et al., 2022 ). The accounting of land use carbon emissions mainly involves assessing the emissions by using bookkeeping, the Intergovernmental Panel on Climate Change (IPCC) inventory, the Carnegie-Ames-Stanford approach (CASA) model, the global production efficiency model (GLO-PEM), and the integrated valuation of ecosystem services and tradeoffs (InVEST) model ( Piao et al., 2022 ; Raihan et al., 2022 ; Walker et al., 2022 ). Houghton and Nassikas (2017) used a bookkeeping model and estimated the average global net carbon fluxes induced by land use and coverage change (LUCC) from 2006 to 2015 to be 1.11 ± 0.35 Pg C yr –1 ; Ghosh et al. (2022) proposed a method to establish a low-carbon city by extensively analyzing land use carbon emissions and sequestration potential using the InVEST model. Regarding the land use carbon emission effects, it primarily investigates the impact of vegetation and soil carbon storage, as well as the dynamic evolution characteristics ( Wang et al., 2020 ; Wolswijk et al., 2022 ). Affuso and Hite (2013) showed that participatory decision-making on land use can triple the net energy value of biofuels and reduce carbon emissions by 20%; Ghorbani et al. (2023) showed that soil carbon storage and atmospheric carbon dioxide (CO 2 ) emissions were directly affected by the changes in the soil characteristics and land use; rising pastures and forests increased the soil organic carbon and microbial biomass carbon in both topsoil and subsoil. For the prediction of land use carbon emissions, Cellular Automata-Markov (CA-Markov), Conversion of Land Use and its Effects at Small regional extent (CLUE-S), Future Land Use Simulation (FLUS), and Patch-generating Land Use Simulation (PLUS) models were used to predict the land use spatial layout for carbon emission analysis ( Wang H. et al., 2022 ; Wu et al., 2022 ). Liu et al. (2018) used a system dynamics approach to establish a multi-perspective integrated measurement model to quantitatively predict new towns on a sector-by-sector basis. They showed that cities need to rely on regional green spaces to mitigate carbon emissions; Yao et al. (2023) proposed a bottom-up cadastral land scale carbon emission prediction framework based on vector cellular automata. Although the aforementioned works serve as excellent examples for the study of land use carbon emissions, only a few studies have focused on carbon emission conduction due to the change in land type ( Li et al., 2023 ). Investigating the effects of land type changes on carbon emissions under various scenarios can provide new perspectives to formulate appropriate land regulation and carbon emission reduction policies ( Ke Y. et al., 2022 ). However, most of the existing research is based on past land use data, and there remains a lack of studies predicting changes in future land use patterns under multiple scenarios and the resultant carbon emissions ( Chuai et al., 2019 ).
Therefore, the objectives of this study were 1) Based on the land use data, combined with the carbon emission estimation model, obtain the carbon emission characteristics of the Baiyangdian basin from 2000 to 2020. 2) Use the land transfer matrix to analyze the carbon transfer effect caused by land use transfer in each period 3) Predict the land use pattern under four different development scenarios in 2035, as well as the resulting carbon emissions, to provide a reference for the city to assess the pressure of carbon emission reduction ( Harper et al., 2018 ).
The rest of the paper is as follows: Section 2 presents an overview of the study area and data sources, Section 3 describes the empirical methodology, Section 4 is the results and analyses section, and Section 5 provides the discussion and conclusions.
2 Study area overview and data sources
2.1 study area overview.
The Xiong’an New Area, China, as a hub to relieve Beijing of non-capital functions, is critical to accelerating the synergistic development of the Beijing-Tianjin-Hebei region, with its land use changes being typical of the current era ( Zhou et al., 2021 ). The Baiyangdian basin, as the ecological hinterland of the Xiong’an New Area, is a prime example of healthy synergies between the carbon system and the development of the city ( Li et al., 2008 ; Zhao et al., 2021 ; Xia et al., 2023 ). The study area is situated in the northern part of the North China Plain, between 113°45′–116°26′ eastern longitudes and 37°51′–40°39′ northern latitudes ( Figure 1 ), which belongs to a warm-temperate monsoon climate. The Baiyangdian basin in this study refers to the administrative area of Hebei Province flowing through nine branches such as the Zuma Long River, the Cao River, and the Zhao Wang Xin River, involving 35 counties (cities and districts) under the jurisdiction of Baoding City, Zhangjiakou City, Shijiazhuang City and Cangzhou City, with a total land area of 34,353.07 km 2 . The basin exhibits an intricate geography, with highlands in the west (mountains) and lowlands in the east (plains). The mountainous area mainly comprises forests and grasslands (17.79% and 19.69% of the total basin area, respectively), and the plains are primarily cultivable land (46.25% of the total basin area).
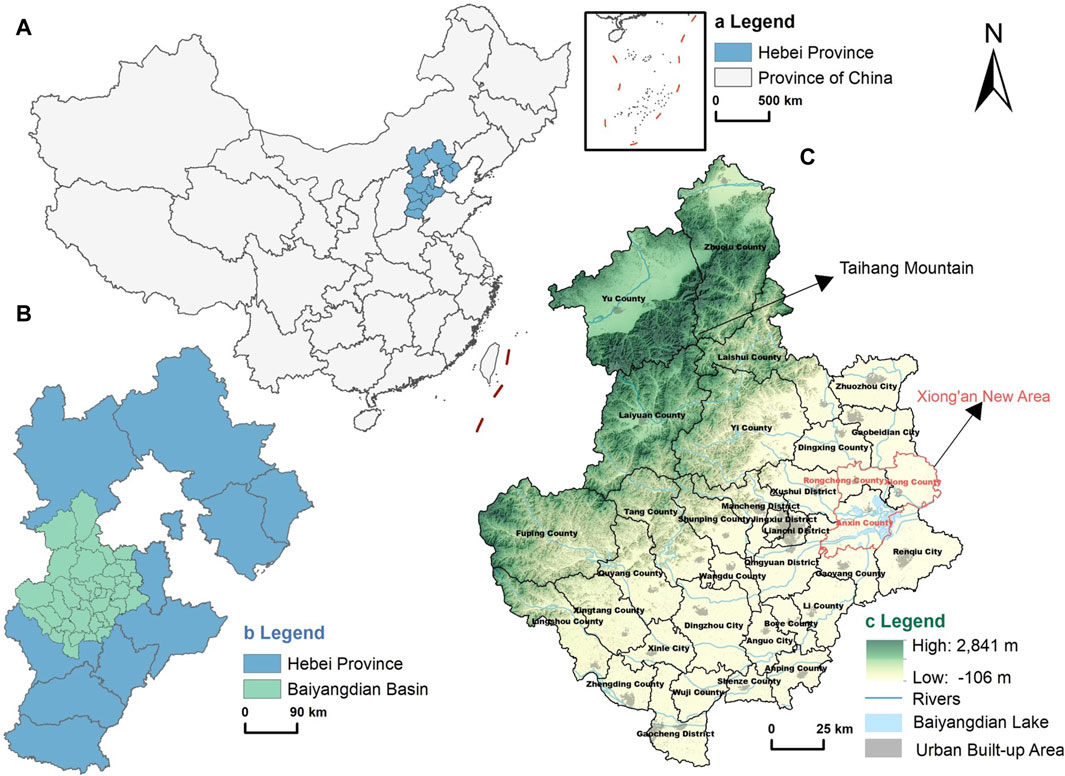
FIGURE 1 . Spatial location of the study area. (A) China scope; (B) Hebei Province; (C) Baiyangdian Basin.
2.2 Data sources
In this study, we employed remote sensing image data, Digital Elevation Model (DEM) data, slope data, meteorological data, and fossil energy data as follows:
(1) The Landsat4-5/Thematic Mapper (TM) and Landsat8/Operational Land Imager (OLI) remote sensing images used in this study were obtained from the Chinese Academy of Sciences Geospatial Data Cloud ( https://www.gscloud.cn/ ). The data identifiers and dates of the selected images are LT51230332000145BJC00 2000-05-24, LT51230322010156IKR00 2010-06-05, and LC81230332020120LGN00 2020-4-29, respectively. Concerning previous classification standards and combined with research needs, the land was divided into cultivable land, forest, grassland, built-up land, unused land, and water area, with a 30-m spatial resolution.
(2) The DEM data were obtained from the Chinese Academy of Sciences Geospatial Data Cloud ( https://www.gscloud.cn/ ), and further, the slope data was extracted from the DEM data, with an initial resolution of 30 m.
(3) Annual average precipitation and average temperature for the basin study area were collected from the China Meteorological Data Network ( https://data.cma.cn/ ), the spatial resolution is 0.5° × 0.5°.
(4) Data on the consumption of the eight main fossil energy sources used to indirectly estimate carbon emissions from built-up land were obtained from the statistical yearbooks of counties and cities and the National Bureau of Statistics ( http://www.stats.gov.cn/ ) for the years 2000–2020. The corresponding energy carbon emission coefficients were the missing values recommended by the Intergovernmental Panel on Climate Change (IPCC).
3 Research methods
3.1 land use carbon emission calculations.
Carbon sinks include grasslands, forests, unused lands, and water areas ( Guo and Fang, 2021 ). Cultivable land can act as both a carbon source and sink due to its different functions ( Ma and Wang, 2015 ). Therefore, this paper accurately measured the carbon emission values for these five land use types by the direct estimation method. Equation 1 is calculated as follows:
where i = 1, 2, 3, 4, and 5 represent cultivatable land, forest, grassland, water area, and unused land, respectively ( Yue et al., 2020 ); C i is i land type carbon emissions ; S i is i land type area; and V i is i land type carbon emission coefficient ( Table 1 ).
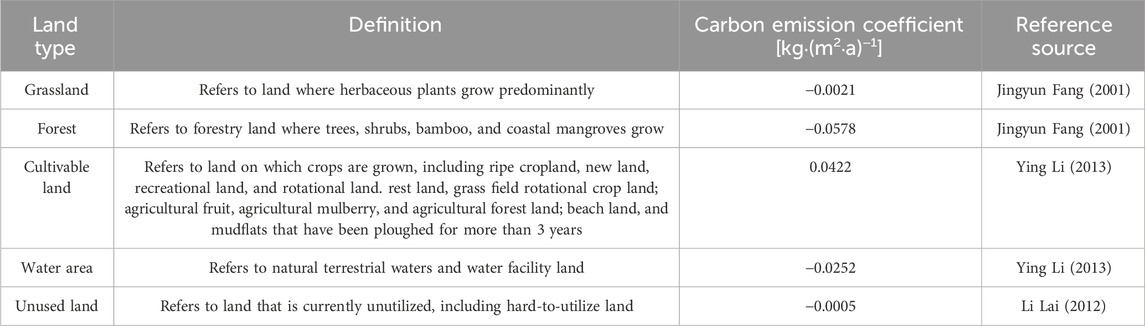
TABLE 1 . Land use carbon emission coefficients.
Carbon emissions from built-up land occur mainly from human activities and the energy production and industrial processes they host ( Zhang et al., 2021 ). This paper indirectly estimated the carbon emissions of the eight main fossil fuel sources through their consumption. Equation 2 is calculated as follows:
where E c is built − up land carbon emission , j is the energy source type; E j is the energy consumption; θ j is the energy to standard coal factor; and f j is the carbon emission ( Lu et al., 2022 ) ( Table 2 ).
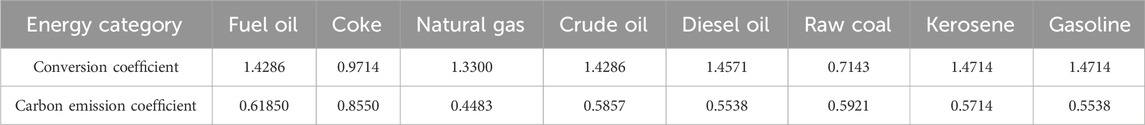
TABLE 2 . Standard coal conversion and carbon emission coefficients for the energy sources.
According to the principle of indicator system construction, this paper selects five indicators, including population, carbon emission intensity, gross domestic product (GDP), historical carbon emissions, and the proportion of the tertiary industry, to construct the Baiyangdian Basin Carbon Emission Indicator System from the perspective of fairness, efficiency, and feasibility ( Table 3 ). The entropy value technique was initially applied to calculate the weights of individual indicators. Subsequently, this method was supplemented by a total carbon emission measurement model to ultimately quantify the indirect carbon emissions originating from the different land types in the study region ( Tang et al., 2022 ).
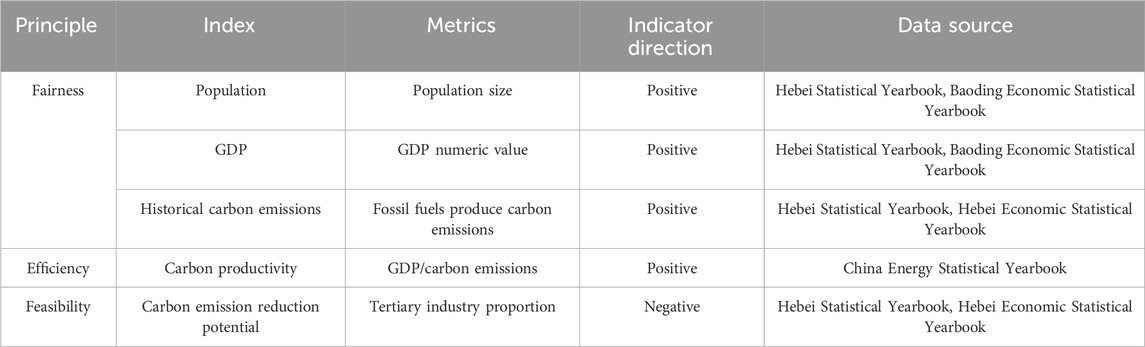
TABLE 3 . Indicator system for the allocation of carbon emissions.
3.2 Estimation of land transfer-based carbon emission conduction
Land use changes can cause carbon transfer, which is defined as the carbon conduction effect of land use carbon emissions. Two factors, the difference between the level of carbon sequestration and carbon emissions following a change in land class and the area of change, mainly determine the amount of carbon emissions they transmit ( Qiao et al., 2016 ). The area of land class conversion can be calculated from a land use transfer matrix indicating the amount of change and the direction of transfer, and its Eq. 3 is:
where N is the number of land use types; and S i j is the area transferred from land type i to land type j. The carbon transmission due to the interconversion between land categories other than built-up land ( C t ) can be estimated from the transfer matrix and the difference between the carbon emission coefficients ( δ i 1 − δ i 2 ) of each category using Eq. 4 :
This paper considers the built-up land within the Baiyangdian basin to be spatially homogeneous, neglecting the carbon emission differences that may arise in distinct spatial scenarios per unit area. Therefore, during the study period T1∼T2, the carbon emissions transferred from built-up land to the other types of land can be calculated by Eq. 5 :
In contrast, Eq. 6 is used to calculate the transfer of carbon emissions from other land types to built-up land:
Where C t is the Carbon emission transmission; δ i is the carbon emission coefficient ( δ i 1 ) or carbon absorption coefficient ( δ i 2 ) for land use types other than built-up land; δ T 1 and δ T 2 are the carbon emission generated on the unit area of built-up land in T1 and T2 years, respectively ( Zhang et al., 2014b ), and the unit is t·(km 2 ·a) −1 . E b 1 and E b 2 are the carbon emissions generated by built-up land in T1 and T2 years, respectively; S bi and S ib are the areas of built-up land in years T1 and T2; S bi and S ib are the areas of interconversion of built-up land and other land types, respectively ( Zhang et al., 2014a ).
3.3 GeoSOS-FLUS model
In this study, the GeoSOS-FLUS model was used to simulate future land use change in the Baiyangdian basin. The model has two main components, scenario setting and model building ( Sun et al., 2021 ). As a resource on which human activity depends, changes in land use and spatial distribution characteristics essentially depend on a tradeoff between economic development and ecological protection. Therefore, based on previous studies and specific planning policies of each city in the Baiyangdian basin, we established four development scenarios, namely, natural development, balanced development, cultivable land protection, and double-carbon target, and analyzed their effects ( Tao et al., 2015 ; Hong et al., 2021 ; Wang Z. et al., 2022 ). The different scenario settings and corresponding scenario descriptions are shown in Table 4 .

TABLE 4 . Land use change rules for different scenarios.
The GeoSOS-FLUS model includes the following two framework contents:
(1) The study identified six key factors, namely, elevation, slope, temperature, precipitation, distance to the road, and distance to the railway, responsible for driving land use changes in the Baiyangdian basin. To evaluate the probability of each land use type suitability, we employed an artificial neural network (ANN) algorithm ( Wang et al., 2019 ). To verify the accuracy of the calculations, we simulated a land use distribution map for 2020 using the land use types of the study region in 2010 and matched the findings with the land use distribution map for the same year. The Kappa coefficient is 0.7464 and has an overall accuracy of 84.20%, demonstrating good simulation results.
(2) The model sampled the first-stage land use distribution data and proposed an adaptive inertia competitive roulette mechanism to simulate the land use scenario distribution. A degree of uncertainty and complexity in land use conversion remained, influenced by a variety of factors. Due to the application of the sampling method and competitive mechanism, the proposed model could effectively avoid error transmission, along with the adverse effects of uncertainty and complexity. In other words, the GeoSOS-FLUS model exhibited good accuracy and enabled the simulation predictions to be consistent with the actual data.
3.4 Markov prediction
The Markov process can predict the possible state of an event at any particular instance in the future according to the current state of the event by following the “no aftereffect” principle ( Yang et al., 2020 ). In this study, the transfer probability matrix was solved by a Markov process according to the change relation of time series to make an energy knot prediction. The Markov model was employed to predict future land use patterns in the Baiyangdian basin energy structure based on historical energy data from 2000 to 2020. Let us assume that at time m , the state vector of the energy consumption structure in the basin can be expressed as Eq. 7 :
where S r ( m ), S c ( m ), S o ( m ), S g ( m ), S k ( m ), S d ( m ), S f ( m ), and S n ( m ) are the proportions of raw coal, coke, crude oil, gasoline, kerosene, diesel, fuel oil, and natural gas in energy consumption, respectively. The transition matrix at time m∼m+1 can be expressed as Eq. 8 :
where i and j are energy types; and P i − j m is the probability of energy i conversion to energy j . The model effect coefficient, w , was used as the criterion to judge the quality of prediction results, which can be computed as Eq. 9 :
where S r is the real value, S p is the predicted value, and S r ¯ is the average of the actual values. If the value is closer to 1, the prediction results are close to the actual values and the prediction accuracy is high.
4 Results and analysis
4.1 changes in land use carbon emissions.
Based on the calculations, Table 5 shows land use carbon emissions in the Baiyangdian basin for the years 2000, 2010, and 2020. The net land use carbon emissions in the basin were found to steadily increase over the past two decades, with a total amount of 208,591.88 × 10 3 t and an average annual increase of 10,429.59 × 10 3 t. Grasslands, forests, water areas, and unused lands acted as carbon sinks, increasing the total carbon absorption by 9.59 × 10 3 t. The combined carbon emissions from cultivable and built-up lands as carbon sources increased by 208,601.48 × 10 3 t. The carbon source emission to sink absorption ratio in the basin increased, especially reaching the highest value of 330.21 in 2020, 4.9 times higher than in 2000. This indicated that the carbon sources in the basin were continuously rising, and the carbon sink was continuously declining.
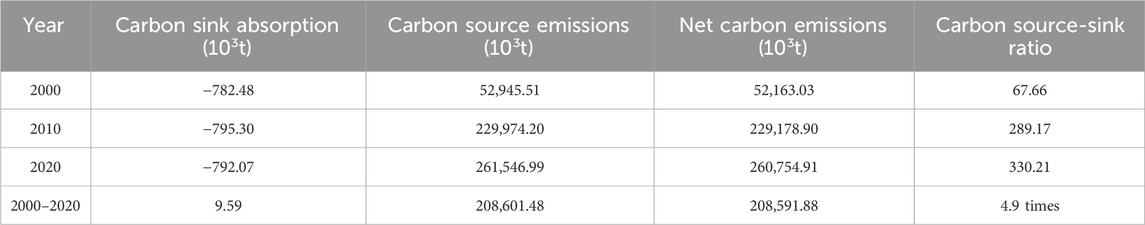
TABLE 5 . Changes in carbon source emissions, sink absorption, and its ratio in the Baiyangdian basin.
Specifically, carbon emissions from built-up lands were on an upward trend, along with carbon sequestration in forests and water areas were on an upward trend, other land types steadily declined as carbon sources or sinks ( Figure 2 ). In the case of building sites, from 2000 to 2010, rapid urbanization, enhanced land intensification, and a mass of cultivable lands, forests, and grasslands were transformed into built-up lands, resulting in an expansion trend of built-up land, which is manifested in the fact that the rate of transferring in is 18.5 times higher than the rate of transferring out. The land use dynamic attitude (k) reached 0.0087, with the land area expanding by 268.36 km 2 . This increase accounted for 94.52% of carbon emissions in 2000, rising to 98.72% in 2020, representing the largest contribution to carbon emissions ( Figure 2 ). Due to the minimal net conversion of land from other categories to built-up land within their respective land usage dynamics between 2000 and 2010, the proportion of carbon emissions attributed to built-up land was the lowest during the entire research period, in 2020 ( Table 6 ). The area of cultivated land has been decreasing from 2000 to 2010, with 637.45 km 2 of cultivated land being transferred out at a rate 12.67 times faster than the rate of transfer in, making it the land category with the largest reduction in area share of any land category.
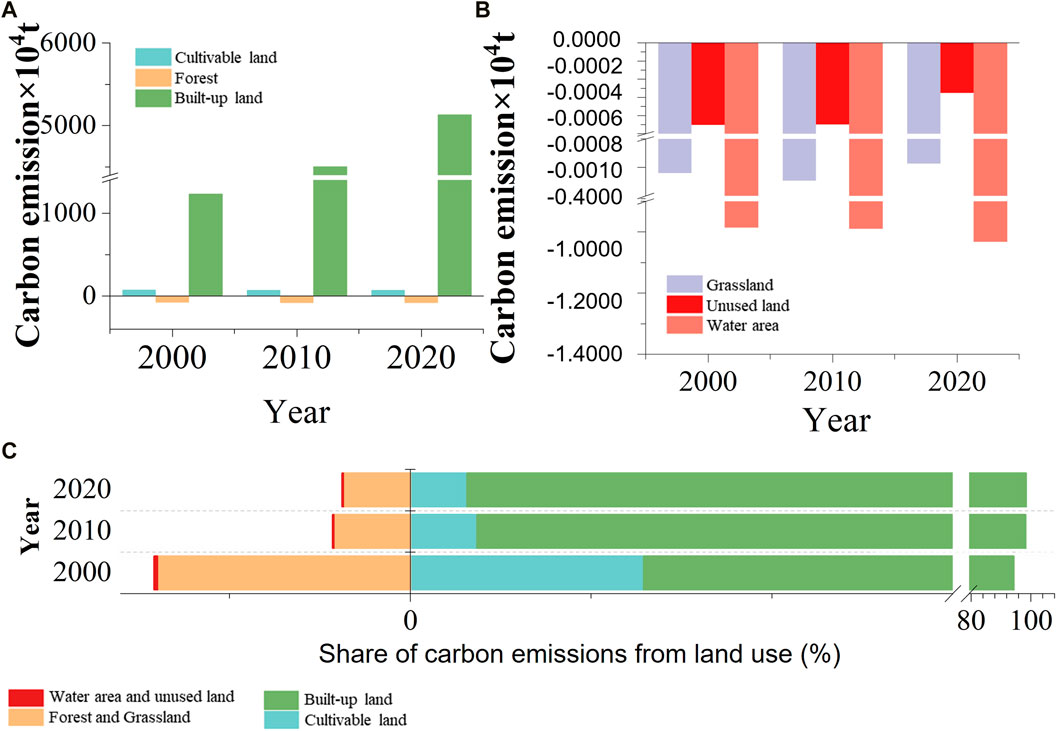
FIGURE 2 . Change and proportion of carbon emissions from land use. (A,B) Land use carbon emissions; (C) Proportion of carbon emissions.
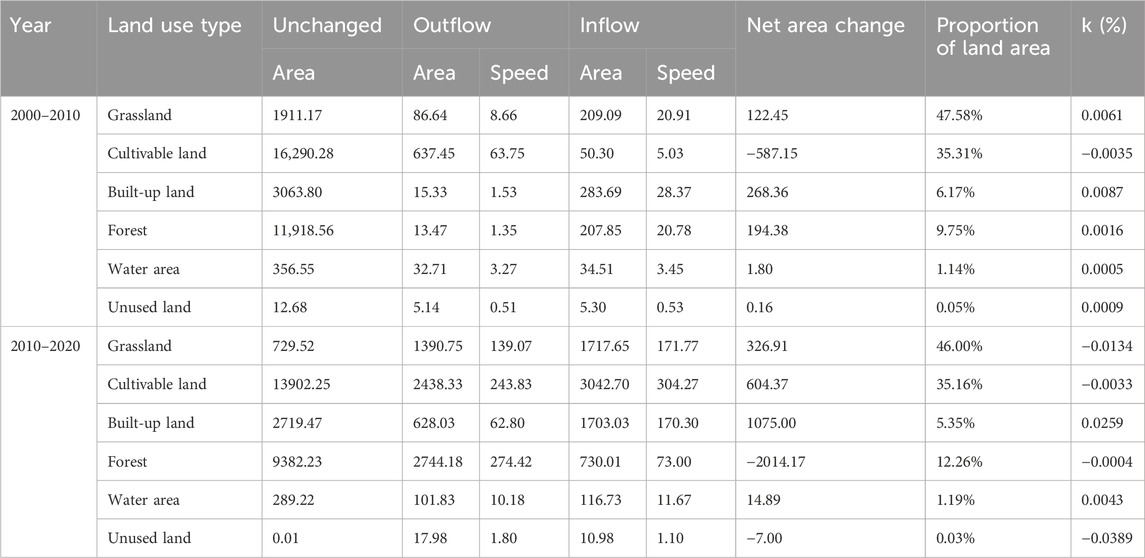
TABLE 6 . Land use changes in the Baiyangdian basin from 2000 to 2020.
Among the carbon sinks, grassland and unused land demonstrated a marginal reduction in carbon sequestration, with the corresponding proportion decreasing from 0.54% in 2000 to 0.49% in 2020 ( Figure 2B ). The fluctuating trends in the carbon uptake ratios of grasslands and unused lands could be attributed to their continued transfer to built-up and cultivable lands. In 2000–2010, 86.64 km 2 of grassland and 5.14 km 2 of unused land were transferred, and in 2010–2020, 1,390.75 km 2 of grassland and 17.98 km 2 of unused land were transferred.
4.2 Carbon conduction effects due to land type changes
To determine the carbon emissions after each stage of land type transfer, we combined the land use transfer matrix with the carbon source/sink capacity of the land type. The values with an asterisk in Table 7 indicate the net carbon emissions from land use transfer at each period of the study period and increased over time. Throughout the entire study period, carbon emissions were determined by the carbon source category. The key role of built-up land was highlighted by the study’s finding that built-up land accounted for most carbon emissions from total carbon sources. The transfer of cultivable lands and grasslands to built-up lands had the most significant effect on carbon transfer through the conversion of carbon sinks into sources. This impact is due to the release of carbon stored in the soil, ecosystem, and biomass. This transformation of cultivated land and grassland into built-up land accounted for 84.05% of the total carbon emissions ( Table 7 ). The carbon conduction from cultivable to built-up land was 1.02 × 10 3 t from 2000 to 2010, which increased to 42.44 × 10 3 t from 2010 to 2020. The carbon conduction from forests, and grasslands, to built-up land also showed an increasing trend. The carbon conduction effect caused by water area and unused land transfer is the same as that caused by grassland cultivation, which also shows an increasing trend.
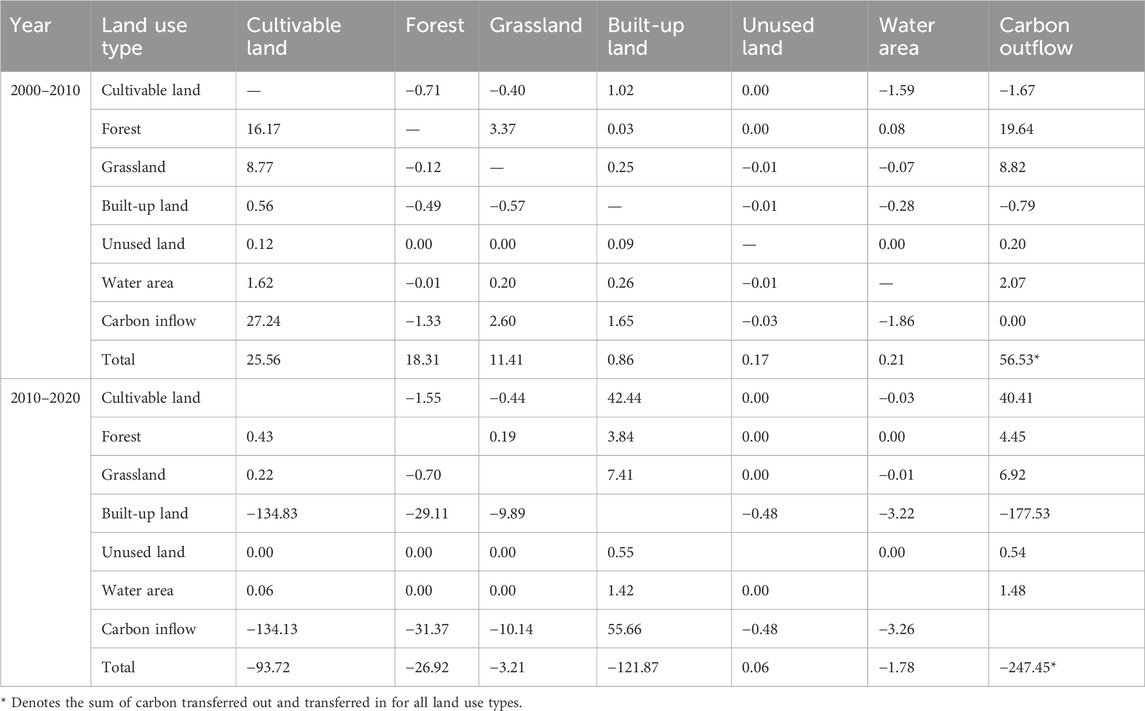
TABLE 7 . Carbon conduction effect of land type transfer in the Baiyangdian basin from 2000 to 2020 (10 3 t).
4.3 Multi-scenario simulation and prediction of land use structure
In this paper, four scenarios were simulated using the GeoSOS-FLUS model for the prediction of the Baiyangdian basin land use in 2035 ( Figure 3 ). The 2035 balanced development, cultivable land protection, and double-carbon target scenarios were largely consistent, but with differences in certain regions.
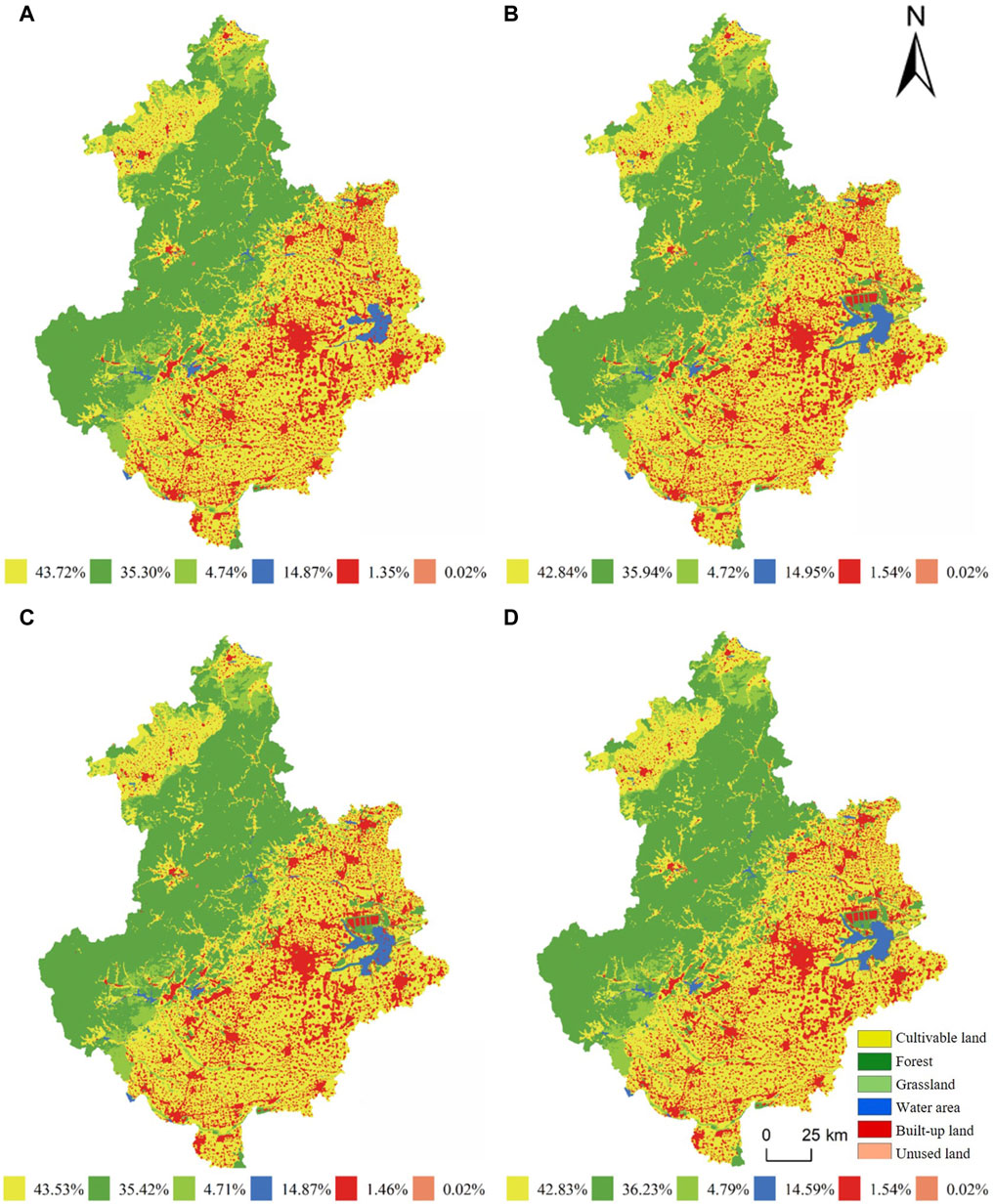
FIGURE 3 . Land use change simulation for the Baiyangdian basin in 2035 under different scenarios (The total land area is 34,353.07 km 2 ). (A) Natural development; (B) Balanced development; (C) Cultivable land protection; (D) double-carbon target.
In the natural development scenario, the area of forests, water area, grasslands, cultivable lands, and unused lands in 2035 was 12,118.94, 462.03, 1626.05, 15,009.93, and 10.98 km 2 , respectively. Compared to 2020, the water area increased by 54.17 km 2 at most; the forest area increased by 39.68 km 2 ; and the cultivable land reduced by 5.03%, with a reduction of 795 km 2 . In the balanced development scenario, compared to 2020, the area of built-up lands, forests, and water bodies increased by 918.30, 260.43, and 119.7 km 2 , respectively. Accordingly, the area of the cultivable lands and grasslands was reduced by 1096.82 km 2 and 218.22 km 2 , respectively. As urbanization converts large parts of farmlands, food security will be further threatened if the focus remains only on economic development. In the cultivable land protection scenario, the built-up land area was relatively small, close to 5104.76 km 2 . Compared with the natural development scenario, the area of forests and grasslands increased by 41.48 and 38.61 km 2 , respectively, while the area of cultivable lands and grassland decreased by 65.88 and 10.43 km 2 , respectively, indicating a severe deterioration of the Baiyangdian basin ecology. This also suggested that even in the cultivable land protection scenario, only a small portion of cultivable land, grassland, and water area were expanded. It also demonstrated the need to place a higher priority on environmental preservation, rather than economic development in the Baiyangdian basin. In the double-carbon target scenario, the area of cultivable lands, forests, grasslands, water area, unused lands, and built-up lands changed to 14705.15 km 2 , 12438.86 km 2 , 1615.62 km 2 , 527.85 km 2 , 6.17 km 2 , and 5007.5 km 2 , respectively. In comparison to 2020, the grassland area decreased by 190.96 km 2 and the carbon-emitting cultivable land decreased by 1099.78 km 2 . Moreover, the area of water area rose by 119.99 km 2 , while that of the forests expanded by 359.6 km 2 ( Table 8 ).
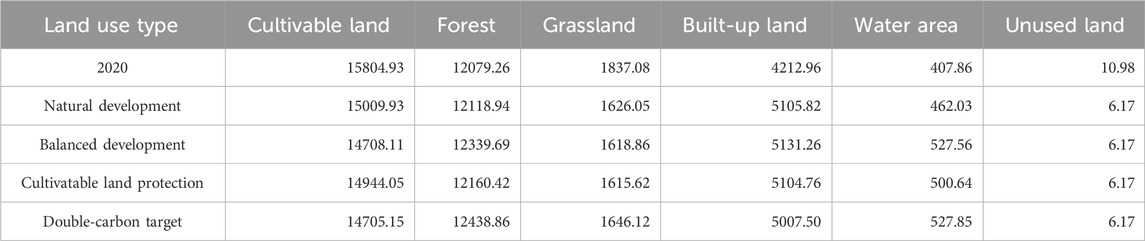
TABLE 8 . Area of each land use type under multi-scenario modeling (km 2 ).
4.4 Prediction of land use carbon emissions
4.4.1 direct land use carbon emissions.
The cultivable land carbon emissions in the Baiyangdian Basin in 2020 were calculated to be 666.97 × 10 3 t, compared to which the emissions in the four scenarios set in 2035 were predicted to decrease slightly ( Table 9 ). In particular, the cultivable land carbon emissions for the balanced development and double-carbon target scenarios were likely to decrease more about 7%. Regarding the carbon sinks, the maximum carbon uptake of the forests under the double-carbon target scenario was 801.26 × 10 3 t, an increase of 3.1% compared with that in 2020, followed by 794.68 × 10 3 t under the balanced development scenario. The carbon uptake of grasslands remained largely unchanged, with an average value was about 3.5 × 10 3 t. Water areas exhibited the largest carbon uptake in the double-carbon target scenario. Compared with 2020, the water area carbon emissions increased for all four scenarios, with a growth ratio of 23.71%. The unused land area was relatively small, which also remained largely unchanged. The predicted results for the different scenarios are shown in Table 9 .
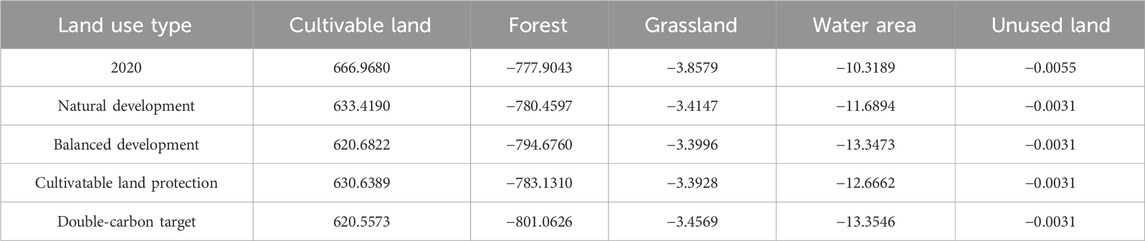
TABLE 9 . Prediction results of direct carbon emissions from land use under multi-scenario simulation (10 3 t).
4.4.2 Indirect land use carbon emissions
We chose the energy consumption data of the Baiyangdian basin in 2019 as the initial value and combined it with the average transfer probability matrix P obtained by Eq. 9 . We subsequently utilized the Markov model for forecasting the energy composition in 2020 and verified the accuracy by comparing the predictions with the actual data ( Figure 4 ).
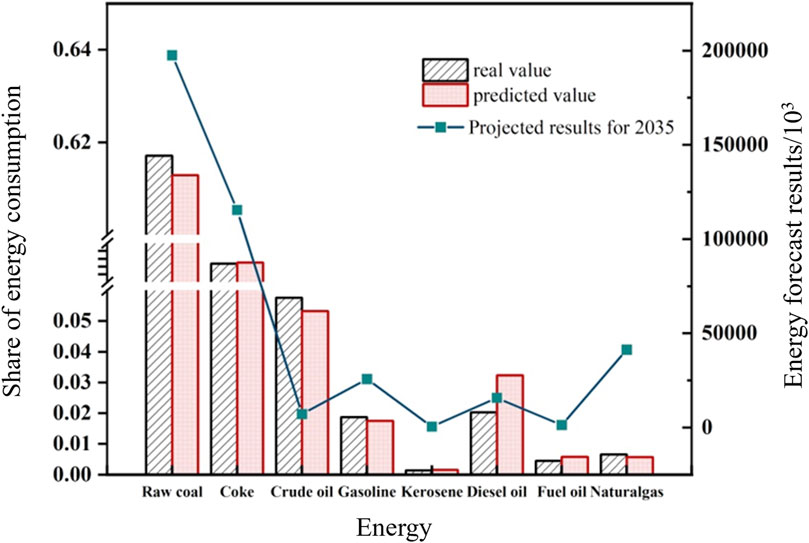
FIGURE 4 . Share of energy consumption in the Baiyangdian Basin by energy source and projections.
According to Eq. 9 , the model effect coefficient w is 0.999 close to 1, indicating a good prediction result, and hence, a reasonable and reliable prediction model. Therefore, the energy consumption in 2020 was selected as the initial vector and combined with the average transfer probability matrix, P , obtained from historical energy data from 2000 to 2020, and the energy consumption structure of the Baiyangdian basin in 2035 was predicted ( Figure 4 ).
Using the carbon emissions data from 2000 to 2020 for the Baiyangdian basin, we employed the grey prediction GM (1,1) model to forecast the 2035 emissions and assess the model precision. The carbon emissions due to built-up lands in the basin in 2035 were expected to reach 404,400.19 × 10 3 t.
4.4.3 Summary of land use carbon emissions in the Baiyangdian basin
In this study, we built a Markov model to forecast total terrestrial carbon emissions in the Baiyangdian basin ( Table 10 ). The land use carbon sinks were primarily related to the land type area, and the carbon sources were primarily related to the continuous growth of energy consumption. Compared with 2020, the net land use carbon emissions in the basin were predicted to increase in 2035 for the four scenarios by 143,483.14 × 10 3 , 143,454.55 × 10 3 , 143,476.74 × 10 3 , and 143,447.97 × 10 3 t, respectively. All values were much higher than the net carbon emissions of the study area in 2020. In the natural development scenario, the carbon source emission peaked at 405,033.61 × 10 3 t, increasing by 143,486.62 × 10 3 t, about 55.03%. The lowest carbon sink absorption out of the four scenarios was 795.57 × 10 3 t, with a slight increase of 3.48 × 10 3 t compared with 2020. In the balanced development and cultivable land protection scenarios, carbon emissions increased significantly, carbon sink absorptions increased by 19.34 × 10 3 t and 7.11 × 10 3 t, and carbon source emissions increased by 143,473.89 × 10 3 t and 143,483.84 × 10 3 t, respectively. In the double-carbon target scenario, the lowest carbon source emission was 405,020.75 × 10 3 t and the highest carbon sink absorption was 817.88 × 10 3 t in the fourth scenario, which has a significant increase compared to the other three scenarios. Generally, land use carbon emissions in the natural development scenarios were the highest, followed by the balanced development, cultivable land protection, and double-carbon target scenarios. Therefore, it is worth thinking about how to balance carbon emission and absorption in the Baiyangdian Basin to achieve healthy development ( Chuai et al., 2016 ).
TABLE 10 . Total land use carbon emissions prediction results under multi-scenario simulations (10 3 t).
5 Conclusion and discussion
5.1 discussion.
The macro-scale carbon sinks in the study area could be rapidly accounted for using the direct carbon emission coefficients of the different land types. The relevant national-scale or regional studies by Fang Jingyun and other scholars (2023) served as the basis for the direct carbon emission coefficients of land use employed in this study. The next step should be to improve the monitoring of ecosystem carbon fluxes across countries and the investigation of carbon density across the various land types to correct the coefficients in a localized manner. Additionally, the GeoSOS-PLUS model combines the transformation, as well as pattern analysis strategies, which can effectively uncover the causes of land type changes and compare the simulation results for various scenarios. This can offer guidance for decision-making and future policy planning. In contrast to Zhou et al. (2020) who used the conventional CA-Markov model to simulate the land use in the built-up land of the urban Shanghai area, our experimental results demonstrated that the GeoSOS-PLUS model significantly improved the simulation and predictions of land use patterns in the Baiyangdian basin, with an overall accuracy of 99%. This addressed the issue of the conventional CA model not adequately accounting for the connection between the influencing variables and spatial changes.
Based on the goal of carbon neutrality, this study makes the following suggestions:
a) The government should strictly regulate the unchecked growth of built-up in developing areas; moderately resume plowing in forests, lakes, and grasslands; and boost the capacity of forests as carbon sinks. They must realize the concept of increasing sinks and reducing sources through the creation of rational and scientific land use policies.
b) Improving the industrial and energy consumption structures; investing more in clean energy resources; and creating a green, diversified energy supply system should be prioritized.
c) When creating the national “dual carbon” roadmap, the unbalanced distribution of regional carbon sources and sinks must be considered and objectively examined for their growth potential.
It should be noted that this paper uses fixed coefficients to calculate carbon emissions, and the coefficients can be optimized by combining the localized measured data or by using multi-source remote sensing image data to improve the accuracy of the calculation. When analyzing the transmission effect of carbon emissions, this paper does not consider the difference in carbon emission coefficients of the same land use type in different counties and cities, and the same land type in different regions may have differences in carbon emission capacity due to factors such as the degree of land intensification. In the future, we will carry out in-depth research on the refinement of the carbon transmission effect due to the internal transformation of land use types. In addition, there is a long way to go to achieve the goal of “double carbon,” and the carbon emission accounting and prediction model established for the characteristics of China’s land use has clarified the important paths affecting China’s carbon emissions from land use and has been widely applied in cities in the central and eastern parts of China as well as in northern China. For regions outside of China, it is necessary to combine regional characteristics, add more factors describing land characteristics, and continuously improve the accuracy of the model, which can also be committed to the study of carbon emissions in other regions.
5.2 Conclusion
In this study, we first analyzed the land use carbon emissions and subsequent transmissions caused by land use changes in the Baiyangdian basin. We then simulated four scenarios based on the GeoSOS-FLUS model, namely, natural development, balanced development, cultivable land protection, and double-carbon target ( Yang et al., 2022 ), and finally predicted the land use carbon emissions in the Baiyangdian basin in 2035 using a Markov model. The double-carbon target scenario further illustrated the critical position of ecological conservation ( Ke N. et al., 2022 ). In general, from the new direction of carbon emission control, combined with regional land use, our study makes outstanding contributions to regional land rational planning and ecological protection ( Chen et al., 2022 ). The primary conclusions of the study are as follows:
1. The ratio of the Baiyangdian basin carbon source emission to sink absorption has been steadily increasing, especially rapidly in 2020, reaching a maximum of 330.21, 4.9 times higher than in 2000. This shows that the carbon sources (sinks) are consistently rising (declining). Over the past 20 years, the net land use carbon emissions in the basin increased by 208,591.88 × 10 3 t, with an average yearly rise of 10,429.59 × 10 3 t.
2. The net carbon emissions from land transfer in the basin exhibit a clear rising trend between 2000 and 2020. The carbon conduction effect due to forests and grasslands being converted to built-up land also shows an increasing trend, whereas the reverse transfer from built-up land to carbon sinks increases only slightly.
3. After simulating four scenarios in the Baiyangdian basin in 2035, it was found that the net land use carbon emissions under the natural development, balanced development, cultivable land protection, and double-carbon target scenarios are predicted to be 404,238.04 × 10 3 , 402,009.45 × 10 3 , 404,231.64 × 10 3 , and 404,202.87 × 10 3 t, respectively, much higher than the values in 2020. However, carbon emissions from cultivable lands show a decreasing trend; the rate of increase of carbon emissions from built-up lands slowed down, and the carbon absorption by forests and grasslands gradually increased. These trends establish the carbon source-sink ratio as a highly suitable parameter for the future planning of ecological vs economic development.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XG: Writing–original draft, Writing–review and editing. MiZ: Writing–original draft, Writing–review and editing. MnZ: Writing–original draft, Writing–original draft. ZG: Writing–review and editing. XL: Writing–review and editing. ZY: Writing–review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This project is supported by the National Natural Science Foundation of China (NSFC): Research on Safety Resilience Evaluation of Critical Infrastructure Systems in Urban Cities and Optimization of Operation (72374063); the National Natural Science Foundation of China (NSFC): Realization Mechanisms, Influencing Factors and Optimization of Urban Ecosystem Service Delivery: A Case Study of Beijing and its Surrounding Areas (42371279); the Social Science Foundation of Hebei Province: Research on Optimization of Ecological Spatial Pattern and Quality Improvement of Baiyangdian Basin Based on Multi-source Data (HB22GL030) Funding Support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Affuso, E., and Hite, D. (2013). A model for sustainable land use in biofuel production: an application to the state of Alabama. Energy Econ. 37, 29–39. doi:10.1016/j.eneco.2013.01.003
CrossRef Full Text | Google Scholar
Ali, G., Pumijumnong, N., and Cui, S. (2018). Valuation and validation of carbon sources and sinks through land cover/use change analysis: the case of Bangkok metropolitan area. Land use policy 70, 471–478. doi:10.1016/j.landusepol.2017.11.003
Carpio, A., Ponce-Lopez, R., and Lozano-García, D. F. (2021). Urban form, land use, and cover change and their impact on carbon emissions in the Monterrey Metropolitan area, Mexico. Urban Clim. 39, 100947. doi:10.1016/j.uclim.2021.100947
Chen, H., Qi, S., and Tan, X. (2022). Decomposition and prediction of China's carbon emission intensity towards carbon neutrality: from perspectives of national, regional and sectoral level. Sci. Total Environ. 825, 153839. doi:10.1016/j.scitotenv.2022.153839
PubMed Abstract | CrossRef Full Text | Google Scholar
Chuai, X., Huang, X., Qi, X., Li, J., Zuo, T., Lu, Q., et al. (2016). A preliminary study of the carbon emissions reduction effects of land use control. Sci. Rep. 6, 36901. doi:10.1038/srep36901
Chuai, X., Yuan, Y., Zhang, X., Guo, X., Zhang, X., Xie, F., et al. (2019). Multiangle land use-linked carbon balance examination in Nanjing City, China. Land Use Policy 84, 305–315. doi:10.1016/j.landusepol.2019.03.003
Chuai, X. W., Lai, L., Huang, X. J., Zhao, R., Wang, W., and Chen, Z. (2012). Temporospatial changes of carbon footprint based on energy consumption in China. J. Geogr. Sci. 22 (1), 110–124. (in Chinese). doi:10.1007/s11442-012-0915-4
Fang, J., Chen, A., Peng, C., Zhao, S., and Ci, L. (2001). Changes in forest biomass carbon storage in China between 1949 and 1998. Science 292, 2320–2322. doi:10.1126/science.1058629
Ghorbani, M., Amirahmadi, E., Konvalina, P., Moudry, J., Kopecky, M., and Hoang, T. N. (2023). Carbon pool dynamic and soil microbial respiration affected by land use alteration: a case study in humid subtropical area. Land 12, 459. doi:10.3390/land12020459
Ghosh, S., Dinda, S., Das Chatterjee, N., Dutta, S., and Bera, D. (2022). Spatial-explicit carbon emission-sequestration balance estimation and evaluation of emission susceptible zones in an Eastern Himalayan city using Pressure-Sensitivity-Resilience framework: an approach towards achieving low carbon cities. J. Clean. Prod. 336, 130417. doi:10.1016/j.jclepro.2022.130417
Guo, X., and Fang, C. (2021). Integrated land use change related carbon source/sink examination in jiangsu province. Land 10, 1310. doi:10.3390/land10121310
Harper, A. B., Powell, T., Cox, P. M., House, J., Huntingford, C., Lenton, T. M., et al. (2018). Land-use emissions play a critical role in land-based mitigation for Paris climate targets. Nat. Commun. 9, 2938. doi:10.1038/s41467-018-05340-z
Hong, C., Burney, J. A., Pongratz, J., Nabel, J. E., Mueller, N. D., Jackson, R. B., et al. (2021). Global and regional drivers of land-use emissions in 1961–2017. Nature 589, 554–561. doi:10.1038/s41586-020-03138-y
Houghton, R. A., and Nassikas, A. A. (2017). Global and regional fluxes of carbon from land use and land cover change 1850–2015. Glob. Biogeochem. Cycles 31, 456–472. doi:10.1002/2016gb005546
Ke, N., Lu, X., Zhang, X., Kuang, B., and Zhang, Y. (2022a). Urban land use carbon emission intensity in China under the “double carbon” targets: spatiotemporal patterns and evolution trend. Environ. Sci. Pollut. Res. 30 (7), 18213–18226. doi:10.1007/s11356-022-23294-0
Ke, Y., Xia, L., Huang, Y., Li, S., Zhang, Y., Liang, S., et al. (2022b). The carbon emissions related to the land-use changes from 2000 to 2015 in Shenzhen, China: implication for exploring low-carbon development in megacities. J. Environ. Manag. 319, 115660. doi:10.1016/j.jenvman.2022.115660
Le Quéré, C., Andres, R. J., Boden, T., Conway, T., Houghton, R. A., House, J. I., et al. (2012). The global carbon budget 1959–2011. Earth Syst. Sci. Data Discuss. 5, 165–185. doi:10.5194/essd-5-165-2013
Li, W., Chen, Z., Li, M., Zhang, H., Li, M., Qiu, X., et al. (2023). Carbon emission and economic development trade-offs for optimizing land-use allocation in the Yangtze River Delta, China. Ecol. Indic. 147, 109950. doi:10.1016/j.ecolind.2023.109950
Li, Y., Ge, Y. X., and Liang, Y. (2013). Relationship between agricultural carbon emissions and agricultural gross value of output. Chin. J. Agric. Resour. Regional Plan. 34, 60–65. doi:10.7621/cjarrp.1005-9121.20130310
Li, Y., Huang, X., Zhen, F., Zhang, H. L., Qi, Y. W., and Wang, T. (2008). Simple sequence repeat analysis of genetic diversity in primary core collection of peach (Prunus persica). Trans. Chin. Soc. Agric. Eng. 24 (2), 102–110. doi:10.1111/j.1744-7909.2007.00598.x
Liebmann, H., and Kuder, T. (2012). Pathways and strategies of urban regeneration-deindustrialized cities in eastern Germany. Eur. Plan. Stud. 20, 1155–1172. doi:10.1080/09654313.2012.674348
Liu, L. Y., Zheng, B. H., and Bedra, K. B. (2018). Quantitative analysis of carbon emissions for new town planning based on the system dynamics approach. Sustain. Cities Soc. 42, 538–546. doi:10.1016/j.scs.2018.08.006
Lu, X., Zhang, Y., Li, J., and Duan, K. (2022). Measuring the urban land use efficiency of three urban agglomerations in China under carbon emissions. Environ. Sci. Pollut. Res. 29, 36443–36474. doi:10.1007/s11356-021-18124-8
Ma, X., and Wang, Z. (2015). Progress in the study on the impact of land-use change on regional carbon sources and sinks. Acta Ecol. Sin. 35, 5898–5907. doi:10.5846/stxb201312112932
Mendelsohn, R., and Sohngen, B. (2019). The net carbon emissions from historic land use and land use change. J. For. Econ. 34, 263–283. doi:10.1561/112.00000505
Piao, S., He, Y., Wang, X., and Chen, F. (2022). Estimation of China’s terrestrial ecosystem carbon sink: methods, progress and prospects. Sci. China Earth Sci. 65, 641–651. doi:10.1007/s11430-021-9892-6
Qiao, W. F., Mao, G. X., Wang, Y. H., et al. (2016). Research on urban expansion and land use change in Nanjing over the past 32 years. Journal of Geo-information Science 18(2), 200–209. doi:10.3724/SP.J.1047.2016.00200
Raihan, A., Muhtasim, D. A., Farhana, S., Hasan, M. A. U., Pavel, M. I., Faruk, O., et al. (2022). Nexus between economic growth, energy use, urbanization, agricultural productivity, and carbon dioxide emissions: new insights from Bangladesh. Energy Nexus 8, 100144. doi:10.1016/j.nexus.2022.100144
Rong, T., Zhang, P., Zhu, H., Jiang, L., Li, Y., and Liu, Z. (2022). Spatial correlation evolution and prediction scenario of land use carbon emissions in China. Ecol. Inf. 71, 101802. doi:10.1016/j.ecoinf.2022.101802
Sun, Q., Qi, W., and Yu, X. (2021). Impacts of land use change on ecosystem services in the intensive agricultural area of North China based on Multi-scenario analysis. Alexandria Eng. J. 60, 1703–1716. doi:10.1016/j.aej.2020.11.020
Tang, W., Cui, L., Zheng, S., and Hu, W. (2022). Multi-scenario simulation of land use carbon emissions from energy consumption in shenzhen, China. Land 11, 1673. doi:10.3390/land11101673
Tao, Y., Li, F., Wang, R., and Zhao, D. (2015). Effects of land use and cover change on terrestrial carbon stocks in urbanized areas: a study from Changzhou, China. J. Clean. Prod. 103, 651–657. doi:10.1016/j.jclepro.2014.07.055
Walker, W. S., Gorelik, S. R., Cook-Patton, S. C., Baccini, A., Farina, M. K., Solvik, K. K., et al. (2022). The global potential for increased storage of carbon on land. Proc. Natl. Acad. Sci. 119 (23), e2111312119. doi:10.1073/pnas.2111312119
Wang, H., Jin, Y., Hong, X., Tian, F., Wu, J., and Nie, X. (2022a). Integrating IPAT and CLUMondo models to assess the impact of carbon peak on land use. Land 11, 573. doi:10.3390/land11040573
Wang, W., Wang, W., Xie, P., and Zhao, D. (2020). Spatial and temporal disparities of carbon emissions and interregional carbon compensation in major function-oriented zones: a case study of Guangdong province. J. Clean. Prod. 245, 118873. doi:10.1016/j.jclepro.2019.118873
Wang, Y., Shen, J., Yan, W., and Chen, C. (2019). Backcasting approach with multi-scenario simulation for assessing effects of land use policy using GeoSOS-FLUS software. MethodsX 6, 1384–1397. doi:10.1016/j.mex.2019.05.007
Wang, Z., Li, X., Mao, Y., Li, L., Wang, X., and Lin, Q. (2022b). Dynamic simulation of land use change and assessment of carbon storage based on climate change scenarios at the city level: a case study of Bortala, China. Ecol. Indic. 134, 108499. doi:10.1016/j.ecolind.2021.108499
Wolswijk, G., Barrios Trullols, A., Hugé, J., Otero, V., Satyanarayana, B., Lucas, R., et al. (2022). Can mangrove silviculture Be carbon neutral? Remote Sens. 14, 2920. doi:10.3390/rs14122920
Wu, Z., Zhou, L., and Wang, Y. (2022). Prediction of the spatial pattern of carbon emissions based on simulation of land use change under different scenarios. Land 11, 1788. doi:10.3390/land11101788
Xia, C., Zhang, J., Zhao, J., Xue, F., Li, Q., Fang, K., et al. (2023). Exploring potential of urban land-use management on carbon emissions-A case of Hangzhou, China. Ecol. Indic. 146, 109902. doi:10.1016/j.ecolind.2023.109902
Yang, B., Chen, X., Wang, Z., Li, W., Zhang, C., and Yao, X. (2020). Analyzing land use structure efficiency with carbon emissions: a case study in the Middle Reaches of the Yangtze River, China. J. Clean. Prod. 274, 123076. doi:10.1016/j.jclepro.2020.123076
Yang, S., Fu, W., Hu, S., and Ran, P. (2022). Watershed carbon compensation based on land use change: evidence from the Yangtze River Economic Belt. Habitat Int. 126, 102613. doi:10.1016/j.habitatint.2022.102613
Yang, X., and Liu, X. (2022). Carbon conduction effect and temporal-spatial difference caused by land type transfer in Chang-Zhu-Tan urban agglomeration from 1995 to 2018. Acta Ecol. Sin. 42, 338–347. doi:10.1016/j.chnaes.2022.02.004
Yang, X., and Liu, X. Z. (2023). Path analysis and mediating effects of influencing factors of land use carbon emissions in Chang-Zhu-Tan urban agglomeration. Technol. Forecast. Soc. Change 188, 122268. doi:10.1016/j.techfore.2022.122268
Yao, Y., Sun, Z. H., Li, L. L., Cheng, T., Chen, D. S., Zhou, G. X., et al. (2023). CarbonVCA: a cadastral parcel-scale carbon emission forecasting framework for peak carbon emissions. Cities 138, 104354. doi:10.1016/j.cities.2023.104354
Yu, Z., Chen, L., Tong, H., Chen, L., Zhang, T., Li, L., et al. (2022). Spatial correlations of land-use carbon emissions in the Yangtze River Delta region: a perspective from social network analysis. Ecol. Indic. 142, 109147. doi:10.1016/j.ecolind.2022.109147
Yue, C., Ciais, P., Houghton, R. A., and Nassikas, A. A. (2020). Contribution of land use to the interannual variability of the land carbon cycle. Nat. Commun. 11, 3170. doi:10.1038/s41467-020-16953-8
Zhang, D., Wang, Z., Li, S., and Zhang, H. (2021). Impact of land urbanization on carbon emissions in urban agglomerations of the middle reaches of the Yangtze River. Int. J. Environ. Res. Public Health 18, 1403. doi:10.3390/ijerph18041403
Zhang, J., Zhang, A., Dong, J., and Basin, E. (2014a). Carbon emission effect of land use and influencing factors decomposition of carbon emission in Wuhan urban agglomeration. Resour. Environ. Yangtze Basin 23, 595–602. doi:10.11870/cjlyzyyhj201405001
Zhang, M., Zhang, Z., Huang, X. J., et al. (2014b). Design and development of gis and rs-based land use carbon balance accounting module. Bulletin of Surveying and Mapping 2, 101–103. doi:10.13474/j.cnki.11-2246.2014.0065
Zhao, C., Gong, J., Zeng, Q., Yang, M., and Wang, Y. (2021). Landscape pattern evolution processes and the driving forces in the wetlands of lake Baiyangdian. Sustainability 13, 9747. doi:10.3390/su13179747
Zhou, L., Dang, X. W., Sun, Q. K., and Wang, S. H. (2020). multi-scenario simulation of urban land change in Shanghai by random forest and CA-Markov model. Sustain. Cities Soc. 55, 102045. doi:10.1016/j.scs.2020.102045
Zhou, Y., Chen, M., Tang, Z., and Mei, Z. (2021). Urbanization, land use change, and carbon emissions: quantitative assessments for city-level carbon emissions in Beijing-Tianjin-Hebei region. Sustain. Cities Soc. 66, 102701. doi:10.1016/j.scs.2020.102701
Keywords: land use, carbon source-sink ratio, carbon conduction effects, Markov prediction, Baiyangdian basin
Citation: Gao X, Zhao M, Zhang M, Guo Z, Liu X and Yuan Z (2024) Carbon conduction effect and multi-scenario carbon emission responses of land use patterns transfer: a case study of the Baiyangdian basin in China. Front. Environ. Sci. 12:1374383. doi: 10.3389/fenvs.2024.1374383
Received: 22 January 2024; Accepted: 05 March 2024; Published: 13 March 2024.
Reviewed by:
Copyright © 2024 Gao, Zhao, Zhang, Guo, Liu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meiran Zhao, [email protected]
† These authors have contributed equally to this work and share first authorship
This article is part of the Research Topic
Dynamics of Land Use and Carbon Emissions in the Context of Carbon Neutrality and Carbon Peaking
share this!
March 19, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Study reveals how China's local bureaucrats struggle for power through negative media coverage
by University of Michigan

Recent research on China's factional competition uncovers how local bureaucrats, who are connected to influential national leaders, strategically use the media to criticize members of rival factions, thus harming their promotion prospects and weakening their factions.
The work is published in the journal Political Science Research and Methods .
Led by Ji Yeon (Jean) Hong, associate professor of political science at the University of Michigan, the research analyzed millions of media reports in Chinese national and regional newspapers spanning from 2000-2014, along with information on the political networks of elite Chinese bureaucrats. It mapped out patterns of behavior among local bureaucrats in leveraging negative media coverage to attack political rivals.
Given the limited freedom of local media on criticizing local governments , provincial leaders linked to strong national leaders, such as Politburo Standing Committee members, encouraged local media to cover negative political incidents such as corruption investigation in other provinces.
More importantly, when reporting on others, provincial leaders are more likely to target provinces connected to weaker national political leaders. The bigger the power gap between the national leaders, the more frequent the negative reporting is.
"This suggests that factional competition encourages strong factions to attack weaker factions more frequently than the reverse," Hong said. "This often leads to power consolidation, strengthening strong factions and weakening weaker factions."
The research further explores the consequences of such negative news coverage on the promotion prospects of provincial leaders. It finds that these negative reports indeed harm the reported-on cadres and their factions.
Specifically, news reports on corruption substantially reduce the promotion chances of the reported-on province's party secretary, the top local political leader, who is likely to hold political responsibility and suffer a substantial disadvantage if more corrupt cadres are caught within his or her region, while leaders of provinces where the media reports on other provinces' corruption cases enjoy a higher probability of promotion.
Even though the Chinese personnel system--including promotion, demotion and allocation of party cadres--is not transparent, one of the factors that the Communist Party formally emphasizes in cadre evaluation is public perception . Without an electoral mechanism, it is not straightforward to elicit the public's evaluation of a cadre or its governance, but the media strongly affect public opinion in China. In such circumstances, the media role is critical.
The research covers the period ranging from the end of the Jiang Zemin administration to the beginning of Xi Jinping's regime. The Hu Jintao and Wen Jiabao administrations, which constitute the bulk of the period under analysis, are not typically framed as a period in which one faction or one top leader dominated others. Furthermore, during this period intellectuals within the party actively debated the possibility of intraparty democracy.
"This implies that the recent personalization of power in China's central politics might not be a unique feature of the current leadership, but an outcome of latent behavioral patterns in China's elite politics," Hong said.
Provided by University of Michigan
Explore further
Feedback to editors

NASA touts space research in anti-cancer fight
7 hours ago

Beyond cloning: Harnessing the power of virtual quantum broadcasting
15 hours ago

Prestigious journals make it hard for scientists who don't speak English to get published, study finds
Mar 23, 2024

Scientists develop ultra-thin semiconductor fibers that turn fabrics into wearable electronics
Saturday Citations: An anemic galaxy and a black hole with no influence. Also: A really cute bug

Research team proposes a novel type of acoustic crystal with smooth, continuous changes in elastic properties

New findings shed light on finding valuable 'green' metals

No 'human era' in Earth's geological history, scientists say
Mar 22, 2024

Research uncovers a rare resin fossil find: A spider that aspires to be an ant

Using physics principles to understand how cells self-sort in development
Relevant physicsforums posts, who should have been the 4th laureate in the nobel prize in physics.
10 hours ago
Interesting anecdotes in the history of physics?
Cover songs versus the original track, which ones are better, for ww2 buffs, who is your favorite jazz musician and what is your favorite song.
Mar 20, 2024
Biographies, history, personal accounts
Mar 19, 2024
More from Art, Music, History, and Linguistics
Related Stories

Negative media attention during election campaign does not harm party leaders: study
Jan 25, 2018

Political rage on social media is making us cynical: Study
Mar 11, 2024

Businesses benefiting from political connections harm China's economic growth
Apr 21, 2022

Research finds a corruption gender gap in bureaucracies
Mar 31, 2022

More than a tenth of the world's billionaires have held or sought political office, finds global analysis
Oct 30, 2023

Study: Exclusionary rhetoric use by any political party increases votes for far-right parties
Nov 9, 2023
Recommended for you

New study suggests that while social media changes over decades, conversation dynamics stay the same

World Happiness Report: Why we might be measuring happiness wrong

Advanced statistical analysis highlights the role of interaction between US Supreme Court judges

The majority of Americans do not support anti-democratic behavior, even when elected officials do: Study
Mar 18, 2024

Standing together against hate: A collective responsibility
Mar 6, 2024

Although trust in science remains high, the public questions scientists' adherence to science's norms
Mar 4, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
The numbers bear this out. After China's 12th Five-Year Plan started in 2011, the central government invested more than 10 billion yuan (US$1.5 billion) in 'health informatization' — the ...
Distortions in the medical technology sector flourish due to growing policy support The government is committed to advancing local industrial capabilities in this prominent, high-tech sector, which remains dominated by foreign suppliers. Consequently, high-end medical devices featured among the Made in China 2025 key industries released in 2015 .
China's burgeoning market for medtech, estimated at $70 billion in 2021, 1 Based on McKinsey analysis of National Health Commission (NHC) data and annual reports regarding revenue of more than 150 medtech companies and their market share. could more than double this decade if the government's Healthy China 2030 plan stays on track. The plan projects 9 percent annual spending growth through ...
This study applied a qualitative case study by identifying the medical device sector in China as a whole case. 23,24. Data collection. Data and materials were collected from multiple sources. First, official data and policy documents were collected from the China Food and Drug Administration (CFDA) and the General Administration of Customs ...
Medical technologies are a key focus of China's industrial policy strategy and central to Beijing's plans to move up the value chain. To spur on the development of local firms and promote self-reliance in this highly innovative sector, Chinese officials are steering research into key technologies and offering financial assistance to promising firms. In addition, biased procurement processes ...
Beijing's policymakers have thrown their support behind medical AI companies that come up with technological innovations to ease the burden on the country's hospital system. Medical and health ...
China wants to use and manufacture more of its own medical equipment. Credit: VCG via Getty Images. As a sinology student in the early 1990s at Nanjing University, Elisabeth Staudinger "got a ...
It is the first hospital in northeast China to use the robotic da Vinci Surgical System to remove bile duct cysts. "Our global research recruits will join other top scientists in our hospital to ...
1 Digital China Health Technologies Corporation Limited, Beijing, China 2 ... Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China ... the proposed studies still have a few limitations. First, the privacy risk that we estimated for the case study is based on the cancer patients' data without including patients ...
And the development and evolution on the policies and regulations of medical technology management in China can be mainly divided into four stages: before 2009, 2009-2015, 2015-2018, 2018-present. Before 2009, only a few individual medical technologies (such as the transplantation of hematopoietic stem cells, reproductive assistant ...
Regardless of these limitations, the study provided an intriguing case of a Chinese company's internationalization to Brazil, operating in a high-tech medical sector. The challenges for EMNCs' internationalization continue, which makes more research in this area timely. However, our findings can be the first step.
China is at the forefront of healthcare innovation, utilizing robots and machine technology. Since 2016, China has issued a series of relevant policies, including the "Internet+" Three-Year Action and Implementation Plan for Artificial Intelligence, the "Guiding Opinions on Promoting and Regulating the Application and Development of Big Data in Health and Medical Care" and the plan ...
He received his B.S. in Computer Science and Technology from Zhejiang University in 2005, and Ph.D. in Computer Science and Technology from Zhejiang University and Imperial College London in 2010. After Ph.D., he worked as a Research Associate and then Research Fellow in the Department of Computing, Imperial College London until 2017.
Health Technology Assessment (HTA) in China has recently expanded from purely academic research to include policy or decision-oriented practice, especially after HTA evidence was used to update the National Reimbursement Drug List for the first time in 2017. This study aims to identify the progress and challenges of HTA development from 2016 to 2021 and inform policies and decisions to promote ...
Originality/value Regardless of these limitations, the study provided an exciting case of internationalization of a Chinese company in Brazil operating in a high-tech medical sector. The ...
The development of medical device sector in China has attracted much attention from around the world due to the nation's huge market and rising research and development (R&D) capabilities. 1,2 The sales of medical devices in China totaled no more than CNY 14.5 billion in 2000, but increased to CNY 308 billion in 2015. Since 2013, China has been ranked as the second-largest medical device ...
The China Medical case is the most high-profile and closely watched contest in years over the production of Chinese audit work papers, an issue that has put Hong Kong and U.S. regulators at ...
A medical device manufacturer sharpens pricing and contracting strategies with analytics. ZS helped a multinational medical supplies manufacturer prioritize analytics in the P&C space and take advantage of deeper business insights, leading to more data-driven and effective commercial strategies to increase profitability. Read on.
China Medical Technologies, Inc. (CMED) was a Cayman Islands corporation based in China, currently in liquidation following fraud allegations. It purported to develop, manufacture, and market advanced in vitro diagnostic ("IVD") products using enhanced chemiluminescence ("ECLIA") technology, fluorescent in-situ hybridization ("FISH") technology, and surface plasmon resonance ("SPR ...
Environmental and economic assessments of industry-level medical waste disposal technologies - A case study of ten Chinese megacities. Author links open overlay panel Aimin Ji a 1, Jinghua Guan b 1 ... environment and economy assessment of medical waste disposal technologies in China. Sci. Total. Environ, 796 (2021), Article 148964, 10.1016/j ...
Application of Best Available Technologies on Medical Wastes Disposal/Treatment in China (with case study) Author links open overlay panel Chen Jiang, Zhiyuan Ren, Yajing Tian, Kaixiang Wang. Show more. Add to Mendeley. Share. ... The non-incineration technology of medical waste disposal was rapid developed within the country. Up to the end of ...
The success rate for translation of newly engineered medical technologies into clinical practice is low. Traversing the "translational valleys of death" requires a high level of knowledge of the complex landscape of technical, ethical, regulatory, and commercialization challenges along a multi-agency path of approvals.
Objective This study aimed to investigate the incidence of enteral nutrition intolerance (ENI) in patients with sepsis and explore potential risk factors. Methods A case-control study was conducted in patients with sepsis who were receiving enteral nutrition (EN) at a tertiary hospital in China. The included patients were divided into the ENI group and the non-ENI group. Univariate and ...
The concepts of healthy cities and smart cities are popular in emerging research in the 21st century. This study focuses on the existing interrelations between the two notions in terms of socio-spatial quality, technology, and innovation, particularly regarding industrial sites that no longer have a role and constitute 'urban voids' with high volumetric concentrations. The fast expansion ...
Case studies are important because they help make something being discussed more realistic for both teachers and learners. ... China Medical Technologies, Inc. (CMED) was a Cayman Islands corporation based in China, currently in liquidation following fraud allegations.... The China Medical case is the most high-profile and closely watched ...
The global urban population is expected to reach 68% by 2050 due to the acceleration of the urbanization process [].The construction industry is a rapidly growing sector, accounting for 40% of total global energy consumption [2,3].In 2020, China pledged to 'peak carbon emissions by 2030 and achieve carbon neutrality by 2060' [].China's construction sector alone emits close to 2 billion ...
Given that RCT evidence for medical devices was generally limited, an open-minded and flexible attitude to other forms of evidence e.g., case reports (series), cohort studies, case control studies, and real-world studies was highly recommended [29, 34, 35].
Carbon pooling and release occur all the time in all corners of the earth, where the land use factor is key to influencing the realization of carbon peaking and neutrality. Land use patterns and carbon emissions change under different scenarios and analyzing the correlation will help formulate scientific land use policies for the future. In this study, through remote sensing data, we ...
The study also highlights the urgent issue of water scarcity in China, with significant challenges in agriculture, industry, domestic water use, and ecological conservation. Despite efforts to ...
The work is published in the journal Political Science Research and Methods.. Led by Ji Yeon (Jean) Hong, associate professor of political science at the University of Michigan, the research ...