

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
| 2023-07-14 | |
| 2020-01-17 | |
| 2020-01-17 | |
| 2020-01-17 | |
| 2023-04-10 | |
| 2023-06-27 | |
| 2020-10-26 | |
| 2023-04-10 | |
| The following documents are samples. IRBIS does NOT generate these documents with application-specific information. | |
| 2017-10-30 | |
| 2013-03-14 | |
| 2017-04-17 | |
| 2018-04-19 | |
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)
- Introduction to Genomics
- Educational Resources
- Policy Issues in Genomics
- The Human Genome Project
- Funding Opportunities
- Funded Programs & Projects
- Division and Program Directors
- Scientific Program Analysts
- Contact by Research Area
- News & Events
- Research Areas
- Research investigators
- Research Projects
- Clinical Research
- Data Tools & Resources
- Genomics & Medicine
- Family Health History
- For Patients & Families
- For Health Professionals
- Jobs at NHGRI
- Training at NHGRI
- Funding for Research Training
- Professional Development Programs
- NHGRI Culture
- Social Media
- Broadcast Media
- Image Gallery
- Press Resources
- Organization
- NHGRI Director
- Mission & Vision
- Policies & Guidance
- Institute Advisors
- Strategic Vision
- Leadership Initiatives
- Diversity, Equity, and Inclusion
- Partner with NHGRI
- Staff Search
Sample Consent Forms
These consent forms were used in various NIH-funded research projects and have been approved by the relevant local IRBs. These completed forms are meant to complement the sample language found in the other sections. They are not provided as guidance or templates promoted by NHGRI, but as references to inform investigators and IRBs considering these issues. Consent forms should be tailored to each individual study.
| Date | Name | Study Feature(s) |
|---|---|---|
| July 2014 | Natural History Study of the Clinical and Molecular Manifestations of Smith-Magenis Syndrome | |
| NA | Natural History Study of the Clinical and Molecular Manifestations of Smith-Magenis Syndrome | |
| December 2009 | ||
| NA | Genetics of Obesity, Diabetes, and Heart Disease in African Diaspora Populations | Return of Research Results |
| December 2014 | Incorporation of Genomic Sequencing into Pediatric Cancer Care | Return of Research Results |
| December 2014 | Incorporación de la secuenciación genómica al tratamiento pediátrico contra el cáncer | Return of Research Results |
| NA | Authorization for Tissue Donation in National Institutes of Health Research Project | Open Access Data |
| May 2008 | Developing a research resource for studies of human genetic variation | Open Access Data |
| NA | Building a comprehensive public resource to study tissue-specific gene expression and regulation | |
| NA | Building a comprehensive public resource to study tissue-specific gene expression and regulation | |
| NA | Consent Process Building a diverse database that can inform thousands of studies on a variety of health conditions | eConsent Return of Research Results |
| March 2021 | Research Program (PDF) | Return of Research Results |
| June 2018 | Building a central knowledgebase that defines the clinical relevance of genes and variants to improve patient care | Consent in Clinical Setting |
Additional Resources
- The Global Alliance for Genomics and Health maintains sample consent clauses relevant to genomics research projects that may be helpful when developing consent forms.
- The NIH Office of Human Subjects Research Protections has created a useful list of consent templates for NIH Intramural Researchers . These include consent templates that have been updated to comply with the Revised Common Rule, as well as older forms that might be useful in crafting consent language. Note: these templates are not specifically for genomics projects .
Related Content

Last updated: March 16, 2022
- IRB-SBS Home
- Contact IRB-SBS
- IRB SBS Staff Directory
- IRB SBS Board Members
- About the IRB-SBS
- CITI Training
- Education Events
- Virginia IRB Consortium
- IRB-SBS Learning Shots
- HRPP Education & Training
- Student Support
- Access iProtocol
- Getting Started
- iProtocol Question Guide
- iProtocol Management
- Protocol Review Process
- Certificate of Confidentiality
- Deception and/or Withholding Information from a Participant
- Ethnographic Research
- IRB-SBS 101
- IRB-SBS Glossary
- Participant Pools
- Paying Participants
- Research in an Educational Setting
- Research in an International Setting and/or Location
- Risk-Sensitive Populations
- Student Researchers and Faculty Sponsors
- Study Funding and the IRB
- Understanding Risk in Research
- Vulnerable Participants
- IRB-SBS PAM & Ed
- Federal Regulations
- Ethical Principals
- Partner Offices
- Determining Human Subjects Research
- Determining HSR or SBS

Consent Templates
The templates below were created to help you create the documents you will need to communicate to participants what they will do in the study. The documents you provide participants will range from recruitment materials to post-debrief consent forms, and you need to submit everything that you provide to a participant to our Board for review. For more information about the consent process see Consent .
- General Consent Template : This form covers all of the basic elements that are required for a consent document. Even if you don't plan to use this exact document, refer to it to ensure that you have all of the appropriate elements in place in your consent procedure.
- Electronic Consent Template : This form is modeled after the General Consent Template with some modifications that make it more appropriate for an online format. For more information about this template, see Electronic Consent .
- Parent Consent : If you are including minors in your study, you will need to provide a consent form for parents and an age appropriate assent form for minors. This form is a guide for creating a parent informed consent document. This form can also be used as a guide for surrogate consent procedures.
- Minor Assent (for ages 13-17) : This template provides the basic elements required for older minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity.
- Minor Assent (for ages 7-12) : This template provides the basic elements required for younger minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity. For children younger than 7, assent forms are not required but include information in the consent section regarding what you will say to them about the study (where appropriate).
- Capacity to Consent Template : For some participant populations, it may be necessary to determine if a participant is able to provide consent; if not, a surrogate can be used (you will also need a surrogate consent form and participant assent form, similar to the parent/child consent/assent forms).
- GDPR Informed Consent Addendum : If you are collecting data from citizens of the European Union or the United Kingdom, you will need to provide additional information to your participants, per the GDPR. For more information, see the Research in an International Setting and/or Location and International Research Data Source .
- Study Information Sheet : While many studies do not require researchers to collected signed consent forms, we generally require that participants receive a Study Information Sheet to provide them with information about the study. This information can be provided as a paper document at the beginning of a survey.
- Electronic Study Information Page : This template is similar to the Study Information Sheet with modification for an electronic delivery. For more information about this template, see Electronic Consent .
- Parent Notification Template : Typically used for studies in an educational setting (particularly where the study is exempt but parent notification is still required), this template is a guide for creating a notification letter to send home to parents.
- Oral Consent Card : Typically used in anthropology studies where the participant may be uncomfortable with a form and/or unable to use it, the Oral Consent Card provides all of the elements required for consent in a bullet format so that the researcher can refer to each point as he or she is obtaining consent from the participant.
- Oral Consent Template : This form is also used in situations where the participant is uncomfortable with a form and/or unable to use it. It is more suited to non-anthropology research (though anthropologists are welcome to refer to it as well).
- Sample Debriefing Form : A debriefing form is a summary of the study given to a participant in a deception study and/or a study that includes students from a participant pool. The purpose is to educate participants about the study and to provide them with resources, particularly if the study is upsetting.
- Advertising Flyer Template : Recruitment materials are part of the consent process and it is important that participants are accurately informed about the study throughout the process. You are not required to use this flyer template (it is a model appropriate for a flyer posted around campus), but it is important that you follow the guide provided in Recruitment .
- ResearchMatch Advertising Template : The NIH funds a free and secure recruitment tool called ResearchMatch that helps to connect researchers with volunteers that are interested in participating in studies. If you are interested in using ResearchMatch to help advertise for your study, complete this ResearchMatch Advertising Template and upload.
- Materials Release Form : The data you collect from your participants may be useful in other spheres, such as an educational tool and/or library archive. Using data in this manner is beyond the scope of the study and you should seek additional permission to use the participant's data in this way. This form allows a participant to declare how they would like their materials to be used by the researcher if the researcher wants to use the materials in situations beyond the study.
- Data Release Form : This form is similar to the Post-Debrief Consent Form; it is used when a participant has been recorded or photographed without their knowledge.
- Post-Debrief Consent Form : This form is used in a deception study after the deception is revealed to the participant. The participant is given an opportunity to decide if they still want to participate after the true purpose of the study is revealed.
- The title of protocol must match the title on all consent forms. The title must be relevant, appropriate, and easy to understand. Include the project title on all pages of the consent form.
- List the page numbers on all pages of the consent form in the standard format: Page 1.
- Delete all colored text from the final copy of your form. The colored text is for explanation purposes only.
- Make sure that the form matches the descriptions in the protocol and vice versa.
- Include all relevant information in the consent form rather than referring to previous verbal explanations. The consent form should provide a complete explanation of what the participant is agreeing to do in the study.
- Be aware of the needs of the participant. Avoid using jargon and acronyms that the participant may not understand; make sure the reading comprehension level is appropriate.
- Do not use statements that make implicit demands on participants to participate , e.g., "You will enjoy and benefit from participating in this study."
- Prepare the consent forms in the standard format provided in the template, with all headings addressed. Use the standard language provided on the template where appropriate.
- Please proofread the consent forms for grammar and spelling errors.
- Do not use language that revokes a participant’s legal rights . A consent form is not a legal document.
- Do not require the participants to sign consent to long statements written in first person , e.g., “I agree to participate in this research study. I understand that the risks are minimal and that I will receive no benefits. I know how to withdraw from this study. I will receive $X in payment for participating. I understand that if I withdraw from the study before my participation is complete, I will receive prorated payment according to the following schedule . . . I agree not to hold the researchers liable for any injuries resulting from participation in this study. . .” DO ask participants to sign consent to a simple agreement statement at the end of the consent form: “I agree to participate in the research study described above.”
Human Subjects Division
- [email protected]
- 206.543.0098
Consent Examples
About this page.
To assist UW researchers with designing subject-focused consent, the UW IRB provides example consent forms. Many of these examples are actual UW IRB approved consent forms designed by UW researchers. Some of the examples were created using one of our consent templates . The use of our template is not required and some of the examples deviate significantly from our templates.
We encourages researchers to use the Designing the Consent Process guidance and the examples below to create consent forms and processes that: (1) are written from the perspective of the subject population being enrolled, emphasizing the Key Information that is mostly likely to assist those subjects with deciding whether to enroll; and (2) are designed and presented in a way that facilitates comprehension and understanding.
- Exempt Research Example Consents
- Expedited and Full Board Research Example Consents
- Key Information Examples
University of Washington Office of Research
Or support offices.
- Human Subjects Division (HSD)
- Office of Animal Welfare (OAW)
- Office of Research (OR)
- Office of Research Information Services (ORIS)
- Office of Sponsored Programs (OSP)
OR Research Units
- Applied Physics Laboratory (APL-UW)
- WA National Primate Research Center (WaNPRC)
Research Partner Offices
- Corporate and Foundation Relations (CFR)
- Enivronmental Health and Safety (EH&S)
- Grant and Contract Accounting (GCA)
- Institute of Translational Health Sciences (ITHS)
- Management Accounting and Analysis (MAA)
- Post Award Fiscal Compliance (PAFC)
Collaboration
- Centers and Institutes
- Collaborative Proposal Development Resources
- Research Fact Sheet
- Research Annual Report
- Stats and Rankings
- Honors and Awards
- Office of Research
© 2024 University of Washington | Seattle, WA
- Privacy Policy

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________

Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Research Problem – Examples, Types and Guide

Conceptual Framework – Types, Methodology and...

Dissertation Methodology – Structure, Example...

APA Research Paper Format – Example, Sample and...

Institutional Review Board – Application Sample...

Evaluating Research – Process, Examples and...
Research Informed Consent Form

Thank you for downloading!
How would you rate your free form.
Updated June 23, 2023
A research informed consent form is used for the purpose of freeing students/faculty of any liability while performing a research study with human participants. Not only does the consent form liberate the researchers of accountability, it briefs the participants of how the research will be conducted, presented and reported. The participants must be fully aware of any risks or potential discomfort that may arise during the study. It should also be made known that participation is voluntary and that the participants can withdraw from the study. A step-by-step guide to filling out a general research informed consent form can be found below.
Instructions – Use to fill in the blank template.
How to Write
Step 1 – Download in PDF , Microsoft Word (.docx) , or Open Document Text (.odt) .
Step 2 – The title of the research study being conducted must be included at the top of the consent form.

Step 3 – Enter the following information related to the primary researcher in the fields provided:
- Phone number
- Email address
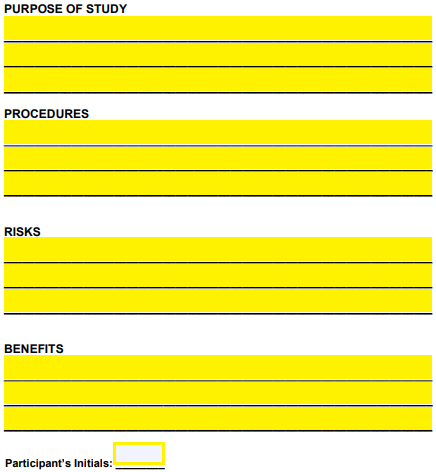
Step 4 – The purpose of the study, the procedures, the risks, and the benefits should be listed under each appropriate corresponding category with the participant’s initials included at the bottom of the page.
Step 5 – Any compensation that is to be provided to the participant(s) should be included under the “Compensation” section on page 2.

Step 6 – A telephone number and email address should be supplied under the “Contact Information” section in case the participant does not want to contact the primary researcher directly. The participant must initial the bottom of the second page.

Step 7 – Signatures must be produced by the participant and the researcher, with the date on which the form was signed next to each signature.


UCL Research Ethics
- Advice on writing an information sheet and consent form

Writing a Participant Information Sheet and Consent Form
Recruitment documents help people make informed choices about whether to participate in a research study. Find out how to write a Participant Information Sheet, example forms and further guidance.
Writing a Participant Information Sheet
Participant Information Sheets must be designed to assist participants to make informed choices. Potential recruits must be given sufficient information to allow them to decide whether or not they want to take part. The process of obtaining consent and the accompanying documentation must be approved by a research ethics committee and, where only verbal consent to research is contemplated include consideration of an appropriate process for witnessing the consent.
Researchers must take the steps necessary to ensure that all participants in the research understand the process in which they are to be engaged, including why their participation is necessary, how it will be used, and how and to whom it will be reported so that the prospective participant can make an informed decision about whether they really do want to take part.
It is highly recommended that the information provided is presented on headed paper and is accurate, clear and simple so that someone with a reading age of 8 would understand the contents (use short words, sentences and paragraphs). The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon and abbreviations, bias, coercion or any inappropriate inducements.
What should the Participant Information Sheet include?
- A friendly invitation to participate.
- A brief and simple explanation of the purposes of the research and a statement explaining how the participant was chosen and how many other participants will be involved in the study.
- A statement that participation is voluntary; refusal to participate will involve no penalty or loss of benefits to which the participant is otherwise entitled; and the participant may discontinue participation at any time without penalty or loss of benefits.
- A thorough explanation of the expected duration of participation in the research and the procedures to be followed.
- A description of any reasonably foreseeable risks or discomforts and any benefits to the participant. For research involving more than minimal risk, an explanation as to whether any compensation or any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
- A statement describing the extent, if any, to which confidentiality of records identifying the participant will be maintained.
- It is considered good practice for researchers to debrief participants at the conclusion of the research and to provide them with copies of any reports or other publications arising from their participation.
- If appropriate, a statement indicating that the data might be used for additional or subsequent research.
- An explanation of who to contact for answers to pertinent questions about the research and the rights of the participant and who to contact in the event of a research-related injury to the participant.
- If applicable, a statement declaring that each researcher who may have access to children (aged under 18) or vulnerable adults has undergone a satisfactory criminal records check.
- Remember to thank your participant for considering taking part in the study and include a statement indicating that the research study has been approved by the UCL Research Ethics Committee.
Language and layout
It is highly recommended that the information provided is presented on headed paper and is accurate, clear, and simple. The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon, and abbreviations, bias, coercion, or any inappropriate inducements.
The following points should be considered when writing an information sheet:
- Use clear, non-technical language. We recommend that you refer to the Plain English Campaign
- Use appropriate language for the target audience. For example, consider the different ways needed to communicate with primary school children as opposed to their teachers, or people with expertise in the area of study as opposed to people with no such knowledge
- Divide the text into paragraphs for ease of reading
- Consider using sub-headings for clarity, such as questions and answers
- Make sure the font and font size are legible.
Ask someone else to review your information sheet before it is circulated.
Further guidance
- Guidance on obtaining consent from research participants online (for online and in-person study designs) , Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL
- Recording & Obtaining Consent , UCL Research Ethics Committee Guidance Note 2: Extract from Nuffield Council on Bioethics website
Example forms (annotated)
- Template Participant Information Sheet (Word)
- Template Consent Form (Word)
Page last updated: June 2024
Medical Research: Forms & Consent Templates
Main navigation.
This section contains all of the forms and consent templates that apply to investigators from: • School of Medicine (SoM) • Veteran's Affairs (VA) Hospital
*Please note that when creating a protocol for IRB submission, these investigators need to select the Medical eProtocol Application category.
If you have questions or are having trouble accessing these forms, please contact IRB Education ( email or call 650-724-7141).
The consent/assent form should be in a language that is understandable to someone without a medical or scientific background. Please use the Microsoft Readability Statistics tool as needed when writing your consent form.
See consent template updates for recent changes.
|
|
| ||
| Regular | For greater than minimal risk research | [03/2024] | [03/2024] | [01/2024] |
| Regular without HIPAA | Use VA Form 10-0493 | N/A | N/A | [01/2024] |
| Expedited | For minimal risk research (e.g., blood draws, non-invasive specimen collection, interviews, surveys, behavioral interventions, etc.) | [03/2024] | [03/2024] | [01/2024] |
| Expedited without HIPAA | Use VA Form 10-0493 | N/A | N/A | [01/2024] |
| Somatic cell donation | For stem cell research | [09/2023] | [09/2023] | N/A |
| Adults (18+) unable to provide consent | Use legally authorized representative (LAR) consent for adults (18+) | [01/2023] | [01/2023] | N/A |
| Children, age 13-17 years | Use for children age 13-17 | [04/2024] | [04/2024] | N/A |
| Children, age 7-12 years | Use for children age 7-12 | [04/2024] | [04/2024] | N/A |
| Expedited research information sheet | Use for waiver of documentation (i.e., waiver of signature) for minimal risk procedures | [09/2023] | [09/2023] | N/A |
| Exempt research information sheet | For exempt research | [09/2023] | [09/2023] | N/A |
| Screening script, level 1a | Use for waiver of documentation for screening | [01/2023] | [01/2023] | N/A |
| Screening script, level 1b | Use for waiver of documentation for screening and retaining contact information for future research | [01/2023] | [01/2023] | N/A |
| VA HIPAA Authorization (VA Form 10-0493) | Use regular or expedited consent without HIPAA | N/A | N/A | [09/2015] |
| Schedule of Procedures table | Use or insert into consent when standard of care procedures overlap with research procedures | [10/2017] | [10/2017] | N/A |
| General Data Protection Regulation (GDPR) consent form language | Insert into consent when study takes place in the European Union/European Economic Area (EU/EEA) | [01/2023] | [01/2023] | N/A |
Other Forms:
| | | |
| SIR Self-Assessment Checklist - IND/IDE | |
eProtocol Forms:
| New, Modification, Continuing Review (These are for informational purposes only)
| • Medical: - - - - - - • • • • Non-Medical: - - - - | |
| is used to submit: • applications - see examples above. - Complete and attach supplemental required attachments as needed (see above) • to approved protocols (need prior IRB approval to implement). • applications. • unanticipated problems, events/information requiring prompt reporting. • (not required for Exempt research). | ||
Personal data in research: how to register your project with Sikt and store your data
Do you plan to use human participants or personal data in your research project? Learn about Sikt's notification form and get advice on consent forms, security measures and data storage.

The course will consist of two parts. We highly recommend participating in both parts if you plan to submit a notification form to Sikt in the near future.
It is possible to only participate in part 2 if you already have submitted your form or just want to learn more about how to store and share your research data at UiO.
1. Personal data and a practical guide to the Sikt notification form
- Time: 12:30 – 13:30
- Facilitator: Lisa Nordick, Senior advisor and data protection officer at HF
Research projects can encounter personal data in many contexts, for example through interviews, questionnaires, social media posts or restricted archival materials.
To ensure that the planned processing is in accordance with data protection legislation, all research projects that process personal data must send a notification form to Sikt, the Norwegian Agency for Shared Services in Education and Research (former NSD). We go through the different steps of the form and give hands-on advice.
2. Data storage and sharing at UiO
- Time: 13:30 – 14:30
- Facilitator: Anne Schad Bergsaker, Section Manager for Advanced User Contact at UiOs IT Department.
Have you heard about TSD, Educloud, OneDrive or ‘storage hotel’? Do you plan on sharing your research data with your supervisor, using UiOs Dictaphone app, or use and encrypted flash drive?
All research data, like interview recordings, questionnaire data or drafts of your paper have to be stored somewhere. We present the storage solutions and sharing available at UiO, how to access them and which are suitable for what kind of project.
Introductory course to GDPR
We highly recommend that you complete UiO's course on protection of personal data before participating. The digital introductory course provides basic knowledge about data protection, information security and UiO's routines.
E-learning course in protection of personal data (hf.uio.no)
Participation
The course is open to all students, PhD candidates, postdocs and other researchers who need an introduction to Sikt's services including the notification form and data storage and sharing at UiO. The course language is English. Please register to share which data you plan to work with and what kinds of storage and sharing solutions you are most interested in. Drop- in participation without registration is possible.
Sign up (nettskjema.no)
- Share on Facebook
- Share on Twitter
Human Subjects Office
Medical terms in lay language.
Please use these descriptions in place of medical jargon in consent documents, recruitment materials and other study documents. Note: These terms are not the only acceptable plain language alternatives for these vocabulary words.
This glossary of terms is derived from a list copyrighted by the University of Kentucky, Office of Research Integrity (1990).
For clinical research-specific definitions, see also the Clinical Research Glossary developed by the Multi-Regional Clinical Trials (MRCT) Center of Brigham and Women’s Hospital and Harvard and the Clinical Data Interchange Standards Consortium (CDISC) .
Alternative Lay Language for Medical Terms for use in Informed Consent Documents
A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
ABDOMEN/ABDOMINAL body cavity below diaphragm that contains stomach, intestines, liver and other organs ABSORB take up fluids, take in ACIDOSIS condition when blood contains more acid than normal ACUITY clearness, keenness, esp. of vision and airways ACUTE new, recent, sudden, urgent ADENOPATHY swollen lymph nodes (glands) ADJUVANT helpful, assisting, aiding, supportive ADJUVANT TREATMENT added treatment (usually to a standard treatment) ANTIBIOTIC drug that kills bacteria and other germs ANTIMICROBIAL drug that kills bacteria and other germs ANTIRETROVIRAL drug that works against the growth of certain viruses ADVERSE EFFECT side effect, bad reaction, unwanted response ALLERGIC REACTION rash, hives, swelling, trouble breathing AMBULATE/AMBULATION/AMBULATORY walk, able to walk ANAPHYLAXIS serious, potentially life-threatening allergic reaction ANEMIA decreased red blood cells; low red cell blood count ANESTHETIC a drug or agent used to decrease the feeling of pain, or eliminate the feeling of pain by putting you to sleep ANGINA pain resulting from not enough blood flowing to the heart ANGINA PECTORIS pain resulting from not enough blood flowing to the heart ANOREXIA disorder in which person will not eat; lack of appetite ANTECUBITAL related to the inner side of the forearm ANTIBODY protein made in the body in response to foreign substance ANTICONVULSANT drug used to prevent seizures ANTILIPEMIC a drug that lowers fat levels in the blood ANTITUSSIVE a drug used to relieve coughing ARRHYTHMIA abnormal heartbeat; any change from the normal heartbeat ASPIRATION fluid entering the lungs, such as after vomiting ASSAY lab test ASSESS to learn about, measure, evaluate, look at ASTHMA lung disease associated with tightening of air passages, making breathing difficult ASYMPTOMATIC without symptoms AXILLA armpit
BENIGN not malignant, without serious consequences BID twice a day BINDING/BOUND carried by, to make stick together, transported BIOAVAILABILITY the extent to which a drug or other substance becomes available to the body BLOOD PROFILE series of blood tests BOLUS a large amount given all at once BONE MASS the amount of calcium and other minerals in a given amount of bone BRADYARRHYTHMIAS slow, irregular heartbeats BRADYCARDIA slow heartbeat BRONCHOSPASM breathing distress caused by narrowing of the airways
CARCINOGENIC cancer-causing CARCINOMA type of cancer CARDIAC related to the heart CARDIOVERSION return to normal heartbeat by electric shock CATHETER a tube for withdrawing or giving fluids CATHETER a tube placed near the spinal cord and used for anesthesia (indwelling epidural) during surgery CENTRAL NERVOUS SYSTEM (CNS) brain and spinal cord CEREBRAL TRAUMA damage to the brain CESSATION stopping CHD coronary heart disease CHEMOTHERAPY treatment of disease, usually cancer, by chemical agents CHRONIC continuing for a long time, ongoing CLINICAL pertaining to medical care CLINICAL TRIAL an experiment involving human subjects COMA unconscious state COMPLETE RESPONSE total disappearance of disease CONGENITAL present before birth CONJUNCTIVITIS redness and irritation of the thin membrane that covers the eye CONSOLIDATION PHASE treatment phase intended to make a remission permanent (follows induction phase) CONTROLLED TRIAL research study in which the experimental treatment or procedure is compared to a standard (control) treatment or procedure COOPERATIVE GROUP association of multiple institutions to perform clinical trials CORONARY related to the blood vessels that supply the heart, or to the heart itself CT SCAN (CAT) computerized series of x-rays (computerized tomography) CULTURE test for infection, or for organisms that could cause infection CUMULATIVE added together from the beginning CUTANEOUS relating to the skin CVA stroke (cerebrovascular accident)
DERMATOLOGIC pertaining to the skin DIASTOLIC lower number in a blood pressure reading DISTAL toward the end, away from the center of the body DIURETIC "water pill" or drug that causes increase in urination DOPPLER device using sound waves to diagnose or test DOUBLE BLIND study in which neither investigators nor subjects know what drug or treatment the subject is receiving DYSFUNCTION state of improper function DYSPLASIA abnormal cells
ECHOCARDIOGRAM sound wave test of the heart EDEMA excess fluid collecting in tissue EEG electric brain wave tracing (electroencephalogram) EFFICACY effectiveness ELECTROCARDIOGRAM electrical tracing of the heartbeat (ECG or EKG) ELECTROLYTE IMBALANCE an imbalance of minerals in the blood EMESIS vomiting EMPIRIC based on experience ENDOSCOPIC EXAMINATION viewing an internal part of the body with a lighted tube ENTERAL by way of the intestines EPIDURAL outside the spinal cord ERADICATE get rid of (such as disease) Page 2 of 7 EVALUATED, ASSESSED examined for a medical condition EXPEDITED REVIEW rapid review of a protocol by the IRB Chair without full committee approval, permitted with certain low-risk research studies EXTERNAL outside the body EXTRAVASATE to leak outside of a planned area, such as out of a blood vessel
FDA U.S. Food and Drug Administration, the branch of federal government that approves new drugs FIBROUS having many fibers, such as scar tissue FIBRILLATION irregular beat of the heart or other muscle
GENERAL ANESTHESIA pain prevention by giving drugs to cause loss of consciousness, as during surgery GESTATIONAL pertaining to pregnancy
HEMATOCRIT amount of red blood cells in the blood HEMATOMA a bruise, a black and blue mark HEMODYNAMIC MEASURING blood flow HEMOLYSIS breakdown in red blood cells HEPARIN LOCK needle placed in the arm with blood thinner to keep the blood from clotting HEPATOMA cancer or tumor of the liver HERITABLE DISEASE can be transmitted to one’s offspring, resulting in damage to future children HISTOPATHOLOGIC pertaining to the disease status of body tissues or cells HOLTER MONITOR a portable machine for recording heart beats HYPERCALCEMIA high blood calcium level HYPERKALEMIA high blood potassium level HYPERNATREMIA high blood sodium level HYPERTENSION high blood pressure HYPOCALCEMIA low blood calcium level HYPOKALEMIA low blood potassium level HYPONATREMIA low blood sodium level HYPOTENSION low blood pressure HYPOXEMIA a decrease of oxygen in the blood HYPOXIA a decrease of oxygen reaching body tissues HYSTERECTOMY surgical removal of the uterus, ovaries (female sex glands), or both uterus and ovaries
IATROGENIC caused by a physician or by treatment IDE investigational device exemption, the license to test an unapproved new medical device IDIOPATHIC of unknown cause IMMUNITY defense against, protection from IMMUNOGLOBIN a protein that makes antibodies IMMUNOSUPPRESSIVE drug which works against the body's immune (protective) response, often used in transplantation and diseases caused by immune system malfunction IMMUNOTHERAPY giving of drugs to help the body's immune (protective) system; usually used to destroy cancer cells IMPAIRED FUNCTION abnormal function IMPLANTED placed in the body IND investigational new drug, the license to test an unapproved new drug INDUCTION PHASE beginning phase or stage of a treatment INDURATION hardening INDWELLING remaining in a given location, such as a catheter INFARCT death of tissue due to lack of blood supply INFECTIOUS DISEASE transmitted from one person to the next INFLAMMATION swelling that is generally painful, red, and warm INFUSION slow injection of a substance into the body, usually into the blood by means of a catheter INGESTION eating; taking by mouth INTERFERON drug which acts against viruses; antiviral agent INTERMITTENT occurring (regularly or irregularly) between two time points; repeatedly stopping, then starting again INTERNAL within the body INTERIOR inside of the body INTRAMUSCULAR into the muscle; within the muscle INTRAPERITONEAL into the abdominal cavity INTRATHECAL into the spinal fluid INTRAVENOUS (IV) through the vein INTRAVESICAL in the bladder INTUBATE the placement of a tube into the airway INVASIVE PROCEDURE puncturing, opening, or cutting the skin INVESTIGATIONAL NEW DRUG (IND) a new drug that has not been approved by the FDA INVESTIGATIONAL METHOD a treatment method which has not been proven to be beneficial or has not been accepted as standard care ISCHEMIA decreased oxygen in a tissue (usually because of decreased blood flow)
LAPAROTOMY surgical procedure in which an incision is made in the abdominal wall to enable a doctor to look at the organs inside LESION wound or injury; a diseased patch of skin LETHARGY sleepiness, tiredness LEUKOPENIA low white blood cell count LIPID fat LIPID CONTENT fat content in the blood LIPID PROFILE (PANEL) fat and cholesterol levels in the blood LOCAL ANESTHESIA creation of insensitivity to pain in a small, local area of the body, usually by injection of numbing drugs LOCALIZED restricted to one area, limited to one area LUMEN the cavity of an organ or tube (e.g., blood vessel) LYMPHANGIOGRAPHY an x-ray of the lymph nodes or tissues after injecting dye into lymph vessels (e.g., in feet) LYMPHOCYTE a type of white blood cell important in immunity (protection) against infection LYMPHOMA a cancer of the lymph nodes (or tissues)
MALAISE a vague feeling of bodily discomfort, feeling badly MALFUNCTION condition in which something is not functioning properly MALIGNANCY cancer or other progressively enlarging and spreading tumor, usually fatal if not successfully treated MEDULLABLASTOMA a type of brain tumor MEGALOBLASTOSIS change in red blood cells METABOLIZE process of breaking down substances in the cells to obtain energy METASTASIS spread of cancer cells from one part of the body to another METRONIDAZOLE drug used to treat infections caused by parasites (invading organisms that take up living in the body) or other causes of anaerobic infection (not requiring oxygen to survive) MI myocardial infarction, heart attack MINIMAL slight MINIMIZE reduce as much as possible Page 4 of 7 MONITOR check on; keep track of; watch carefully MOBILITY ease of movement MORBIDITY undesired result or complication MORTALITY death MOTILITY the ability to move MRI magnetic resonance imaging, diagnostic pictures of the inside of the body, created using magnetic rather than x-ray energy MUCOSA, MUCOUS MEMBRANE moist lining of digestive, respiratory, reproductive, and urinary tracts MYALGIA muscle aches MYOCARDIAL pertaining to the heart muscle MYOCARDIAL INFARCTION heart attack
NASOGASTRIC TUBE placed in the nose, reaching to the stomach NCI the National Cancer Institute NECROSIS death of tissue NEOPLASIA/NEOPLASM tumor, may be benign or malignant NEUROBLASTOMA a cancer of nerve tissue NEUROLOGICAL pertaining to the nervous system NEUTROPENIA decrease in the main part of the white blood cells NIH the National Institutes of Health NONINVASIVE not breaking, cutting, or entering the skin NOSOCOMIAL acquired in the hospital
OCCLUSION closing; blockage; obstruction ONCOLOGY the study of tumors or cancer OPHTHALMIC pertaining to the eye OPTIMAL best, most favorable or desirable ORAL ADMINISTRATION by mouth ORTHOPEDIC pertaining to the bones OSTEOPETROSIS rare bone disorder characterized by dense bone OSTEOPOROSIS softening of the bones OVARIES female sex glands
PARENTERAL given by injection PATENCY condition of being open PATHOGENESIS development of a disease or unhealthy condition PERCUTANEOUS through the skin PERIPHERAL not central PER OS (PO) by mouth PHARMACOKINETICS the study of the way the body absorbs, distributes, and gets rid of a drug PHASE I first phase of study of a new drug in humans to determine action, safety, and proper dosing PHASE II second phase of study of a new drug in humans, intended to gather information about safety and effectiveness of the drug for certain uses PHASE III large-scale studies to confirm and expand information on safety and effectiveness of new drug for certain uses, and to study common side effects PHASE IV studies done after the drug is approved by the FDA, especially to compare it to standard care or to try it for new uses PHLEBITIS irritation or inflammation of the vein PLACEBO an inactive substance; a pill/liquid that contains no medicine PLACEBO EFFECT improvement seen with giving subjects a placebo, though it contains no active drug/treatment PLATELETS small particles in the blood that help with clotting POTENTIAL possible POTENTIATE increase or multiply the effect of a drug or toxin (poison) by giving another drug or toxin at the same time (sometimes an unintentional result) POTENTIATOR an agent that helps another agent work better PRENATAL before birth PROPHYLAXIS a drug given to prevent disease or infection PER OS (PO) by mouth PRN as needed PROGNOSIS outlook, probable outcomes PRONE lying on the stomach PROSPECTIVE STUDY following patients forward in time PROSTHESIS artificial part, most often limbs, such as arms or legs PROTOCOL plan of study PROXIMAL closer to the center of the body, away from the end PULMONARY pertaining to the lungs
QD every day; daily QID four times a day
RADIATION THERAPY x-ray or cobalt treatment RANDOM by chance (like the flip of a coin) RANDOMIZATION chance selection RBC red blood cell RECOMBINANT formation of new combinations of genes RECONSTITUTION putting back together the original parts or elements RECUR happen again REFRACTORY not responding to treatment REGENERATION re-growth of a structure or of lost tissue REGIMEN pattern of giving treatment RELAPSE the return of a disease REMISSION disappearance of evidence of cancer or other disease RENAL pertaining to the kidneys REPLICABLE possible to duplicate RESECT remove or cut out surgically RETROSPECTIVE STUDY looking back over past experience
SARCOMA a type of cancer SEDATIVE a drug to calm or make less anxious SEMINOMA a type of testicular cancer (found in the male sex glands) SEQUENTIALLY in a row, in order SOMNOLENCE sleepiness SPIROMETER an instrument to measure the amount of air taken into and exhaled from the lungs STAGING an evaluation of the extent of the disease STANDARD OF CARE a treatment plan that the majority of the medical community would accept as appropriate STENOSIS narrowing of a duct, tube, or one of the blood vessels in the heart STOMATITIS mouth sores, inflammation of the mouth STRATIFY arrange in groups for analysis of results (e.g., stratify by age, sex, etc.) STUPOR stunned state in which it is difficult to get a response or the attention of the subject SUBCLAVIAN under the collarbone SUBCUTANEOUS under the skin SUPINE lying on the back SUPPORTIVE CARE general medical care aimed at symptoms, not intended to improve or cure underlying disease SYMPTOMATIC having symptoms SYNDROME a condition characterized by a set of symptoms SYSTOLIC top number in blood pressure; pressure during active contraction of the heart
TERATOGENIC capable of causing malformations in a fetus (developing baby still inside the mother’s body) TESTES/TESTICLES male sex glands THROMBOSIS clotting THROMBUS blood clot TID three times a day TITRATION a method for deciding on the strength of a drug or solution; gradually increasing the dose T-LYMPHOCYTES type of white blood cells TOPICAL on the surface TOPICAL ANESTHETIC applied to a certain area of the skin and reducing pain only in the area to which applied TOXICITY side effects or undesirable effects of a drug or treatment TRANSDERMAL through the skin TRANSIENTLY temporarily TRAUMA injury; wound TREADMILL walking machine used to test heart function
UPTAKE absorbing and taking in of a substance by living tissue
VALVULOPLASTY plastic repair of a valve, especially a heart valve VARICES enlarged veins VASOSPASM narrowing of the blood vessels VECTOR a carrier that can transmit disease-causing microorganisms (germs and viruses) VENIPUNCTURE needle stick, blood draw, entering the skin with a needle VERTICAL TRANSMISSION spread of disease
WBC white blood cell

IMAGES
VIDEO
COMMENTS
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. General Consent Form Templates Social and Behavioral Research Projects (last updated 03/16/2023)
se this template if your research is NOT. derally-sponsore. A. D participants are adults.Avoid Common Problems with Consent Forms. Read these tips!1. ustomize this template to reflect the specifics of your study and participan. population.Text in [brackets] represents study-specific information that must be added.A ba.
Sample Informed Consent Form - ©NCPI. The following is a sample consent form for a research project. It is a research project on faculty life on campus, carried out by the principle investigator (PI) of this project from the fake-named Century University. The interviewer (the investigator) should have the interviewee read this form carefully ...
The forms should be provided to participants in addition to the main study consent form. The language in these forms can also be adapted and added to consent forms for studies in which COVID-19 screening and testing procedures are being done for study purposes, i.e., the results of the screening and/or testing will be used as study data. To do ...
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
Consent Template Exempt Research This consent form is an example, designed specifically for Exempt survey research and is provided purely as a service by the IRB. The IRB does not review or approve the content of exempt consent forms. The consent form should not include any mention of IRB approval and it should not include the standard IRB ...
The UW IRB provides the UW research community with a variety of consent templates that align with regulatory and policy requirements and best practices as described in our main Consent guidance and guidance on Designing the Consent Process. The first two templates, marked with an asterisk, are the templates most non-exempt studies will choose from.
Sample Consent Forms. These consent forms were used in various NIH-funded research projects and have been approved by the relevant local IRBs. These completed forms are meant to complement the sample language found in the other sections. They are not provided as guidance or templates promoted by NHGRI, but as references to inform investigators ...
Include the project title on all pages of the consent form. List the page numbers on all pages of the consent form in the standard format: Page 1. Delete all colored text from the final copy of your form. The colored text is for explanation purposes only. Make sure that the form matches the descriptions in the protocol and vice versa.
A statement that this is a Bucknell University research project. 2. An explanation of the purposes of the research. 3. A detailed description of the procedures to be followed. ... Example Consent Forms Some examples involving different types of studies are provided below. ese are meant to serve as general guides, not strict templates.
The consent form must include their name, title(s), phone number and/or email. Question about participant's rights or research-related injury should be directed to the Institutional Review Board (IRB): Crown Family School of Social Work, Policy, and Practice University of Chicago 969 East 60th Street Chicago, Illinois 60637 Telephone: 773-834 ...
Many of these examples are actual UW IRB approved consent forms designed by UW researchers. Some of the examples were created using one of our consent templates. The use of our template is not required and some of the examples deviate significantly from our templates. We encourages researchers to use the Designing the Consent Process guidance ...
RESEARCH PARTICIPANT CONSENT FORM [insert . title of project - consent form title should match grant/contract title] [insert. Principal Investigator's name] ... See Examples of Informed Consent Text Provided by Institutional Review Boards at U.S. Medical Schools to view examples of commonly used consent form language organized by readability ...
Your specific consent form should include information pertinent to your specific research project, and may need to be considerably different from this sample. The highlighted sections are to be filled in by you.) My name is (name of person doing project), and I am a (student/professor, etc.) at Union College. I am inviting you to participate in ...
Informed Consent Templates in Research. Here is an example of an informed consent template that can be used in research studies: Title of Study: [Insert Title of Study] Investigator (s): [Insert Name (s) of Investigator (s)] Introduction. You are being invited to participate in a research study.
A Consent Form is read by the participant, signed and handed back to the researcher and should include the following features: 1. Use University of Wollongong/AHS letterhead. 2. Provide the title of the research project, the researcher(s) name, supervisor's name (for student research), the Unit in which the researcher is based and the name of ...
How to Write. Step 1 - Download in PDF, Microsoft Word (.docx), or Open Document Text (.odt). Step 2 - The title of the research study being conducted must be included at the top of the consent form. Step 3 - Enter the following information related to the primary researcher in the fields provided: Step 4 - The purpose of the study, the ...
Informed consents should include theBelow is an example of an. ev. ew Board Hofstra University Office of. Research and Sponsored Programs 516-463-50541. Introduction and Purpose of the StudyInclude a brief overview. of the study on a level of understanding for the person who will be signing the form. Remember.
An example of an online informed consent form is posted at the end of this document. Telephone: Typically, these components will be written as the beginning of the "script" and verbal consent will be requested before beginning the telephone survey. A copy of the script must be provided to the IRB. Paper Survey: Typically, these components ...
Researchers must take the steps necessary to ensure that all participants in the research understand the process in which they are to be engaged, including why their participation is necessary, how it will be used, and how and to whom it will be reported so that the prospective participant can make an informed decision about whether they really do want to take part.
If you have questions or are having trouble accessing these forms, please contact IRB Education ( email or call 650-724-7141). The consent/assent form should be in a language that is understandable to someone without a medical or scientific background. Please use the Microsoft Readability Statistics tool as needed when writing your consent form.
1. Personal data and a practical guide to the Sikt notification form. Time: 12:30 - 13:30; Facilitator: Lisa Nordick, Senior advisor and data protection officer at HF; Research projects can encounter personal data in many contexts, for example through interviews, questionnaires, social media posts or restricted archival materials.
Human Subjects Office / IRB Hardin Library, Suite 105A 600 Newton Rd Iowa City, IA 52242-1098. Voice: 319-335-6564 Fax: 319-335-7310
A Consent Form is read by the participant, signed and handed back to the researcher and should include the following features: 1. Use University of Wollongong/AHS letterhead. 2. Provide the title of the research project, the researcher(s) name, supervisor's name (for student research), the Unit in which the researcher is based and the name of ...