Stem Cell Research & Therapy
Thematic series - open for submissions, stromal cells and progenitor cells for osteoarticular regeneration.

Organoids and Tissue/Organ Chips
Sergey Nivens / stock.adobe.com
Stem cell therapy of COVID-19 and other respiratory diseases
koolsabuy / stock.adobe.com
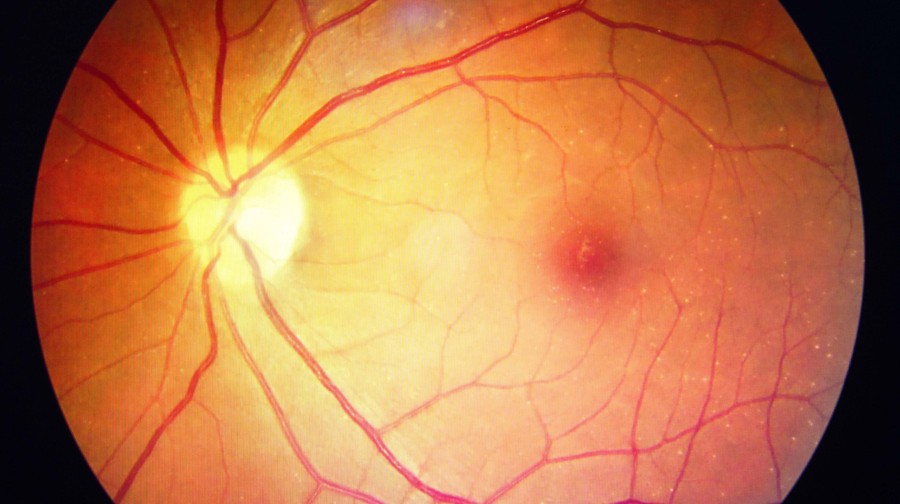
Adult stem cells in retinal diseases: where do we go from here?
Featured articles.

Vascular organoids: unveiling advantages, applications, challenges, and disease modelling strategies
Biodistribution of mesenchymal stromal cell-derived extracellular vesicles administered during acute lung injury
- Most accessed
- Collections
Improved therapeutic consistency and efficacy of CD317 + MSCs through stabilizing TSG6 by PTX3
Authors: Shaoquan Shi, Si Chen, Bowei Liang, Yumeng Li, Qi Ma, Meiqi Li, Jingting Zhang, Lan Yao and Jianyong Xu
Adipose-derived stem cell-based optimization strategies for musculoskeletal regeneration: recent advances and perspectives
Authors: Chenrui Yuan, Wei Song, Xiping Jiang, Yifei Wang, Chenkai Li, Weilin Yu and Yaohua He
The remodeling of ovarian function: targeted delivery strategies for mesenchymal stem cells and their derived extracellular vesicles
Authors: Yinhua Song, Jiachen Wu, Yang Liu, Na Xu, Hualin Bai, Lingjuan Wang, Jihui Ai and Kezhen Li
Matrix-free human pluripotent stem cell manufacturing by seed train approach and intermediate cryopreservation
Authors: Kevin Ullmann, Felix Manstein, Wiebke Triebert, Nils Kriedemann, Annika Franke, Jana Teske, Mira Mertens, Victoria Lupanow, Gudrun Göhring, Alexandra Haase, Ulrich Martin and Robert Zweigerdt
Response to comment on “Effectiveness and safety of stem cell therapy for diabetic foot: a meta-analysis update”
Authors: Yuming Sun, Jinhong Zhao, Lifang Zhang, Zhexuan Li and Shaorong Lei
The original article was published in Stem Cell Research & Therapy 2024 15 :85
The Research to this article has been published in Stem Cell Research & Therapy 2022 13 :416
Most recent articles RSS
View all articles
Stem cells: past, present, and future
Authors: Wojciech Zakrzewski, Maciej Dobrzyński, Maria Szymonowicz and Zbigniew Rybak
Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors
Authors: Paola Romina Amable, Rosana Bizon Vieira Carias, Marcus Vinicius Telles Teixeira, Ítalo da Cruz Pacheco, Ronaldo José Farias Corrêa do Amaral, José Mauro Granjeiro and Radovan Borojevic
CAR T cells in solid tumors: challenges and opportunities
Authors: Faroogh Marofi, Roza Motavalli, Vladimir A. Safonov, Lakshmi Thangavelu, Alexei Valerievich Yumashev, Markov Alexander, Navid Shomali, Max Stanley Chartrand, Yashwant Pathak, Mostafa Jarahian, Sepideh Izadi, Ali Hassanzadeh, Naghmeh Shirafkan, Safa Tahmasebi and Farhad Motavalli Khiavi
Stem cells: a potential treatment option for kidney diseases
Authors: Dongwei Liu, Fei Cheng, Shaokang Pan and Zhangsuo Liu
Most accessed articles RSS
Topical collection Stromal cells and progenitor cells for osteoarticular regeneration Edited by Christian Jorgensen Topical collection Organoids and Tissue/Organ Chips Edited by Albert J Banes and Rajashekhar Gangaraju
Thematic series Adult stem cells in retinal diseases: where do we go from here? Edited by Rajashekhar Gangaraju
Thematic series Stem cell therapy of COVID-19 and other respiratory diseases Edited by Hong-Long James Ji, Michael A. Matthay and Yuanlin Song
Thematic series NK cells as the next option for cancer treatment Edited by Michael O'Dwyer
Cross-journal collection Coronavirus research highlights
Thematic series Stem cells and gene editing Edited by Stephen H. Tsang
Cross-journal collection Pluripotent Stem Cells
Thematic series Regenerative neurology Edited by Simon Koblar and Anne Hamilton-Bruce
Thematic series Extracellular vesicles and regenerative medicine Edited by Jeffrey Karp, Kelvin Ng and Armand Keating
Cross-journal collection Mesenchymal stem/stromal cells – an update Edited by Richard Schäfer and Selim Kuci
Cross-journal collection Biology and clinical applications of stem cells for autoimmune and musculoskeletal disorders Edited by Christian Jorgensen and Anthony Hollander
Thematic series Functional imaging in regenerative medicine Edited by Timothy O'Brien and Rocky Tuan
Thematic series Emerging investigators Edited by Timothy O'Brien and Rocky Tuan
Thematic series Stem cells and genitourinary regeneration Edited by John D Jackson
Thematic series Cardiovascular regeneration Edited by Ronald Li
Thematic series Physical influences on stem cells Edited by Gordana Vunjak-Novakovic
Thematic series Stem cell research in the Asia-Pacific Edited by Oscar Lee, Songtao Shi, Yufang Shi and Ying Jin
Thematic series Clinical applications of stem cells Edited by Mahendra Rao
Review series Immunology and stem cells Edited by Christian Jorgensen
Review series Epigenetics and regulation
Review series Stem cell niche
Review series Induced pluripotent stem cells

Trending articles in Stem Cell Research & Therapy
Click here to view which Articles have been shared the most in the last quarter!
Stem Cell Research & Therapy: 10th Anniversary
To mark the 10th year anniversary, we have reviewed the milestone achievements and highlighted some of the best content selected by our Editors-in-Chief and Associate Editors. Read more here .
Editors-in-Chief
Rocky S Tuan, The Chinese University of Hong Kong, Hong Kong SAR, China Timothy O'Brien, University of Galway, Ireland
Journal submission information
Before you submit your manuscript to Stem Cell Research & Therapy , please ensure you have read and noted the below:
- Authors are requested to provide institutional email addresses. At the minimum, at least the corresponding author and submitting author should provide an institutional email address.
- Image integrity and standards: Cropped gels and blots can be included in the main text if it improves the clarity and conciseness of the presentation. In such cases, the cropping of the blot must be clearly evident and must be mentioned in the figure legend. Corresponding uncropped full-length gels and blot must be included in the supplementary files. These uncropped images should indicate where they were cropped, be labelled as in the main text and placed in a single supplementary figure. The manuscript's figure legends should state that 'Full-length blots/gels are presented in Supplementary Figure X'. Further information can be found under 'Digital image integrity' which are detailed on our Standards of Reporting page.
- Authors should clearly declare in the Acknowledgement Section if there is any work that was outsourced or data not collected by authors.
- Authors might be asked to provide original source data (raw data / original data / individual data points) if the reviewers or editors have any question.
- Authors should provide these information in the ethics declaration if the study involve human participants, human material, human data, or animals: (1) Title of the approved project; (2) Name of the institutional approval committee or unit; (3) Approval number; (4) Date of approval. Authors might be asked to provide ethics approval documents if the reviewers or editors have any question.
Aims and scope
Stem Cell Research & Therapy is the major forum for translational research into stem cell therapies. An international peer-reviewed journal, it publishes high-quality open access research articles with a special emphasis on basic, translational and clinical research into stem cell therapeutics and regenerative therapies, including animal models and clinical trials. The journal also provides reviews, viewpoints, commentaries, reports and methods.
Topics covered include, but are not limited to:
- Adult stem cells
- Animal models
- Biophysics and mechanobiology
- Cancer stem cells
- Cell culture and manufacture
- Clinical studies
- Disease modeling and drug screening
- Embryonic stem cells
- Ethical, legal and social aspects
- Genetics and epigenetics
- Genomics/proteomics/metabolomics
- Induced pluripotent stem cells
- Regenerative medicine
- Stem cell differentiation, proliferation and migration
- Stem cell therapy/transplantation
- Technologies
- Tissue engineering and biomaterials
Announcing the launch of In Review
Stem Cell Research & Therapy , in partnership with Research Square, is now offering In Review. Authors choosing this free optional service will be able to:
- Share their work with fellow researchers to read, comment on, and cite even before publication
- Showcase their work to funders and others with a citable DOI while it is still under review
- Track their manuscript - including seeing when reviewers are invited, and when reports are received
Editors' quotes

Study at Cambridge
About the university, research at cambridge.
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges and Departments
- Email and phone search
- Give to Cambridge
- Museums and collections
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Fees and funding
- Postgraduate events
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
Early-stage stem cell therapy trial shows promise for treating progressive MS
- Research home
- About research overview
- Animal research overview
- Overseeing animal research overview
- The Animal Welfare and Ethical Review Body
- Animal welfare and ethics
- Report on the allegations and matters raised in the BUAV report
- What types of animal do we use? overview
- Guinea pigs
- Naked mole-rats
- Non-human primates (marmosets)
- Other birds
- Non-technical summaries
- Animal Welfare Policy
- Alternatives to animal use
- Further information
- Funding Agency Committee Members
- Research integrity
- Horizons magazine
- Strategic Initiatives & Networks
- Nobel Prize
- Interdisciplinary Research Centres
- Open access
- Energy sector partnerships
- Podcasts overview
- S2 ep1: What is the future?
- S2 ep2: What did the future look like in the past?
- S2 ep3: What is the future of wellbeing?
- S2 ep4 What would a more just future look like?
- Research impact
An international team has shown that the injection of a type of stem cell into the brains of patients living with progressive multiple sclerosis (MS) is safe, well tolerated and has a long-lasting effect that appears to protect the brain from further damage.
I am cautiously very excited about our findings, which are a step towards developing a cell therapy for treating MS Stefano Pluchino
The study, led by scientists at the University of Cambridge, University of Milan Bicocca and Hospital Casa Sollievo della Sofferenza (Italy), is a step towards developing an advanced cell therapy treatment for progressive MS.
Over 2 million people live with MS worldwide, and while treatments exist that can reduce the severity and frequency of relapses, two-thirds of MS patients still transition into a debilitating secondary progressive phase of disease within 25-30 years of diagnosis, where disability grows steadily worse.
In MS, the body’s own immune system attacks and damages myelin, the protective sheath around nerve fibres, causing disruption to messages sent around the brain and spinal cord.
Key immune cells involved in this process are macrophages (literally ‘big eaters’), which ordinarily attack and rid the body of unwanted intruders. A particular type of macrophage known as a microglial cell is found throughout the brain and spinal cord. In progressive forms of MS, they attack the central nervous system (CNS), causing chronic inflammation and damage to nerve cells.
Recent advances have raised expectations that stem cell therapies might help ameliorate this damage. These involve the transplantation of stem cells, the body’s ‘master cells’, which can be programmed to develop into almost any type of cell within the body.
Previous work from the Cambridge team has shown in mice that skin cells re-programmed into brain stem cells, transplanted into the central nervous system, can help reduce inflammation and may be able to help repair damage caused by MS.
Now, in research published in the Cell Stem Cell , scientists have completed a first-in-human, early-stage clinical trial that involved injecting neural stem cells directly into the brains of 15 patients with secondary MS recruited from two hospitals in Italy. The trial was conducted by teams at the University of Cambridge, Milan Bicocca and the Hospitals Casa Sollievo della Sofferenza and S. Maria Terni (IT) and Ente Ospedaliero Cantonale (Lugano, Switzerland) and the University of Colorado (USA).
The stem cells were derived from cells taken from brain tissue from a single, miscarried foetal donor. The Italian team had previously shown that it would be possible to produce a virtually limitless supply of these stem cells from a single donor – and in future it may be possible to derive these cells directly from the patient – helping to overcome practical problems associated with the use of allogeneic foetal tissue.
The team followed the patients over 12 months, during which time they observed no treatment-related deaths or serious adverse events. While some side-effects were observed, all were either temporary or reversible.
All the patients showed high levels of disability at the start of the trial – most required a wheelchair, for example – but during the 12 month follow up period none showed any increase in disability or a worsening of symptoms. None of the patients reported symptoms that suggested a relapse and nor did their cognitive function worsen significantly during the study. Overall, say the researchers, this points to a substantial stability of the disease, without signs of progression, though the high levels of disability at the start of the trial make this difficult to confirm.
The researchers assessed a subgroup of patients for changes in the volume of brain tissue associated with disease progression. They found that the larger the dose of injected stem cells, the smaller the reduction in this brain volume over time. They speculate that this may be because the stem cell transplant dampened inflammation.
The team also looked for signs that the stem cells were having a neuroprotective effect – that is, protecting nerve cells from further damage. Their previous work showed how tweaking metabolism – how the body produces energy – can in turn reprogram microglia from ‘bad’ to ‘good’. In this new study, they looked at how the brain's metabolism changes after the treatment. They measured changes in the fluid around the brain and in the blood over time and found certain signs that are linked to how the brain processes fatty acids. These signs were connected to how well the treatment works and how the disease develops. The higher the dose of stem cells, the greater the levels of fatty acids, which also persisted over the 12-month period.
Professor Stefano Pluchino from the University of Cambridge, who co-led the study, said: “We desperately need to develop new treatments for secondary progressive MS, and I am cautiously very excited about our findings, which are a step towards developing a cell therapy for treating MS.
“We recognise that our study has limitations – it was only a small study and there may have been confounding effects from the immunosuppressant drugs, for example – but the fact that our treatment was safe and that its effects lasted over the 12 months of the trial means that we can proceed to the next stage of clinical trials.”
Co-leader Professor Angelo Vescovi from the University of Milano-Bicocca said: “It has taken nearly three decades to translate the discovery of brain stem cells into this experimental therapeutic treatment This study will add to the increasing excitement in this field and pave the way to broader efficacy studies, soon to come.”
Caitlin Astbury, Research Communications Manager at the MS Society, says: “This is a really exciting study which builds on previous research funded by us. These results show that special stem cells injected into the brain were safe and well-tolerated by people with secondary progressive MS. They also suggest this treatment approach might even stabilise disability progression. We’ve known for some time that this method has the potential to help protect the brain from progression in MS.
“This was a very small, early-stage study and we need further clinical trials to find out if this treatment has a beneficial effect on the condition. But this is an encouraging step towards a new way of treating some people with MS.”
Reference Leone, MA, Gelati, M & Profico, DC et al. Intracerebroventricular Transplantation of Foetal Allogeneic Neural Stem Cells in Patients with Secondary Progressive Multiple Sclerosis (hNSC-SPMS): a phase I dose escalation clinical trial. Cell Stem Cell; 27 Nov 2023; DOI: 10.1016/j.stem.2023.11.001

Read this next

Cambridge ReseARch Trail

‘Mini-placentas’ help scientists understand the causes of pre-eclampsia and pregnancy disorders

Ancient DNA reveals reason for high MS and Alzheimer's rates in Europe

Lab-grown ‘small blood vessels’ point to potential treatment for major cause of stroke and vascular dementia
Media enquiries.
Mature Adult Female with Disability
Credit: eyecrave productions (Getty Images)
Search research
Sign up to receive our weekly research email.
Our selection of the week's biggest Cambridge research news and features sent directly to your inbox. Enter your email address, confirm you're happy to receive our emails and then select 'Subscribe'.
I wish to receive a weekly Cambridge research news summary by email.
The University of Cambridge will use your email address to send you our weekly research news email. We are committed to protecting your personal information and being transparent about what information we hold. Please read our email privacy notice for details.
- multiple sclerosis (MS)
- spotlight on future therapeutics
- Stefano Pluchino
- School of Clinical Medicine
- Department of Clinical Neurosciences
Connect with us

© 2024 University of Cambridge
- Contact the University
- Accessibility statement
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- Terms and conditions
- University A-Z
- Undergraduate
- Postgraduate
- Cambridge University Press & Assessment
- Research news
- About research at Cambridge
- Spotlight on...

- See All Locations
- Primary Care
- Urgent Care Facilities
- Emergency Rooms
- Surgery Centers
- Medical Offices
- Imaging Facilities
- Browse All Specialties
- Diabetes & Endocrinology
- Digestive & Liver Diseases
- Ear, Nose & Throat
- General Surgery
- Neurology & Neurosurgery
- Obstetrics & Gynecology
- Orthopaedics
- Pain Medicine
- Pediatrics at Guerin Children’s
- Urgent Care
- Medical Records Request
- Insurance & Billing
- Pay Your Bill
- Advanced Healthcare Directive
- Initiate a Request
- Help Paying Your Bill
.cls-1{fill:#00a6d4;}.cls-2{fill:#fff;} discoveries magazine Discoveries
Regenerative medicine: a new path for als treatment.
Nov 17, 2023 Cassie Tomlin
A first-of-its-kind stem cell therapy for ALS passes a critical safety benchmark, advancing the search to slow down, reverse and prevent the disease. In a parallel study, investigators are growing patient-derived stem cells to model ALS, hoping to uncover its mechanisms and classify it with more specificity. Can the cure to this degenerative condition lie in the endlessly regenerative power of stem cells?

First, her hands stiffened. Then she developed a limp. Ashley Fisher was 48 when she was diagnosed with amyotrophic lateral sclerosis (ALS) . During the following three years, as the unstoppable disease took hold of her body, she lost the ability to work, hike by the beach or shop swap meets on Saturday mornings. So, she sought out what she could do with the time she had left: She enrolled in a clinical trial.
Even after paralysis stole her speech, Ashley consented to a five-hour spine operation, took immunosuppressive drugs for a year and underwent extensive testing at Cedars-Sinai ’s ALS Clinic , where a team monitored the impact of the procedure on her body—specifically, one of her legs. Their goal: to test the safety of a combination stem cell/gene therapy to treat the rare neurodegenerative disease, caused by the unexplained, unstoppable death of motor neurons in the brain and spinal cord.
Moments after Ashley’s death, in May 2021 at a hospital in Oregon, her daughter, Courtney Fisher Olsen, relayed to a nurse the urgent instructions impressed upon her in the previous months: Call Cedars-Sinai and ask them to collect Ashley’s spinal tissue.
“She made this research a priority, and she was really proud of it,” Courtney says. “She would do anything to be part of finding answers.”
Ashley, along with the 17 others in the study, gave investigators their only opportunity to make a critical advance: The trial proved, for the first time, the safety of the implantation into the lumbar spinal cord of specialized stem cells—neural progenitors—engineered to express a powerful growth factor known to protect neurons. The findings, published in September 2022 in Nature Medicine , cleared investigators to study the therapy’s efficacy and continue refining the approach they hope that, ultimately, will slow or stop the disease.
These patients are the heroes of this research. They knew we weren’t going to cure their disease, only pursue whether the cells and this surgical approach were safe. We are encouraged enough by this approach to proceed in an attempt to slow disease progression.”
Richard Lewis, MD

Dr. Richard Lewis is principal investigator of a stem cell trial at the ALS Clinic.
“These patients are the heroes of this research,” says Richard Lewis, MD, director of the Electromyography Lab and principal investigator of the study at the ALS Clinic. “They knew we weren’t going to cure their disease, only pursue whether the cells and this surgical approach were safe. We are encouraged enough with the results to proceed to more patients and attempt to slow disease progression.”
Until now, ALS has frustrated researchers with a notoriously impenetrable monolith. Only 5% to 10% of patients carry genes known to cause the disease. Without the ability to biopsy brain and spinal tissue, little is understood about its mechanisms. In the absence of biomarkers, physicians can only diagnose ALS after it has already taken hold, and the three Food and Drug Administration approved treatments do little to slow its progression. The highly specialized, resourceful clinicians at Cedars-Sinai ’s ALS Clinic, an ALS Association Certified Treatment Center of Excellence, can only leverage tools and technologies to support their chief goal: to preserve quality of life as patients become paralyzed and die.
Buoyed by breakthroughs in the study of stem cells, Cedars-Sinai investigators are challenging assumptions and evolving their questions about ALS. Because fresh progress in the disease is fueled by the body’s cells at their most naive state, the ALS Clinic team has embarked on a new clinical trial to test the safety of stem cell implantation directly into the cerebral cortices of ALS patients. Scientists are growing cells from ALS patients in petri dishes to model the disease. Having built the largest library of hyper-specific disease data, the team is reconsidering whether ALS is not one disease but a collection of conditions. They aim to differentiate between genetic and sporadic forms of ALS, and scour the models for the earliest signs of cellular decline. Every approach takes square aim at the ultimate questions: Why do patients develop ALS, and how can we stop the suffering?
A STARRING ROLE FOR STEM CELLS
For nearly 20 years, Clive Svendsen, PhD , executive director of the Board of Governors Regenerative Medicine Institute and the Kerry and Simone Vickar Family Foundation Distinguished Chair in Regenerative Medicine, has cultivated a multipronged sneak attack against neurogenerative disease. The approach aims to replace diseased astrocytes, star-shaped cells that support motor neurons. In ALS, diseased astrocytes play a role in motor neuron death, which causes paralysis. Glial cell line-derived neurotrophic factor (GDNF) is a potent growth factor that can protect motor neurons, but delivery to patients is difficult since GDNF is too large to cross the blood-brain barrier. So, years ago, Dr. Svendsen generated a line of human neural progenitors that can become astrocytes, and genetically engineered them to release GDNF.
In 2007, he and colleagues at the University of Wisconsin published a paper in PLOS One demonstrating that, when implanted into the lumbar spinal cords of rat models of ALS, the neural progenitors became astrocytes and released GDNF. After Dr. Svendsen joined Cedars-Sinai , further success showing the function and safety of the cells in animal studies earned his group an $8 million grant from the California Institute for Regenerative Medicine (CIRM). The team also was granted FDA approval, in 2016, to begin the 18-patient trial in which Ashley Fisher participated.
“Proving the safety and getting the cells to survive was a huge lift,” Dr. Svendsen says. “Drug development can stop at any point, so to have 18 patients and no safety issues—we’re very excited to move forward.”
IN HIS OWN WORDS: CLIVE SVENDSEN, PHD, ON THE PATIENT WHO INSPIRED HIS WORK

Clive Svendsen, PhD
“I had worked on Parkinson’s disease for years—but in 2002, in Wisconsin, I met Jeff Kaufman , a young patient with ALS. I was astounded at the horror of this disease—he had been an athletic man, a wonderful lawyer in his 30s with a lovely wife and four beautiful kids, when he was diagnosed. When I met him, he could only use his eyes to communicate through a computer. His first words to me were “Can stem cells help me?”
At the time, I was founder and co-director of the Stem Cell and Regenerative Medicine Center at the University of Wisconsin. After encountering Jeff, I dove into the literature and started asking questions. I realized that the approach we’d been studying in other neurodegenerative diseases—using astrocytes and GDNF as a protective strategy—could also protect motor neurons that die in ALS. So I switched my focus, applied for funding from the ALS Association and it’s all cumulated into the work we do now.
When I met Jeff, he had been fighting the disease for 10 years. He died at 54 in March 2010, and I’ve now been fighting for 20 years. While we don’t have the knockout blow yet, we have landed some punches, and I’m not going to stop.”
The Phase I/IIa study was overseen by Pablo Avalos, MD, associate director of Translational Medicine at the Board of Governors Regenerative Medicine Institute. A neurosurgical team, led by J. Patrick Johnson, MD, co-medical director of the Cedars-Sinai Spine Center and vice chair of the Department of Neurosurgery, injected the cells into the exact part of the spinal cord that controls movement in either the right or left leg. The procedure was found to be safe for all patients. Investigators also observed, as a secondary measure, that the cells slowed disease progression in the treated leg in some patients, though this did not reach overall statistical significance. ALS typically causes decline in both sides of the body at the same rate. Since patients received cells in only one side of the spinal cord, their own untreated leg acted as an “internal control” for comparison.
Postmortem spinal tissue revealed that the stem cells were still alive and producing GDNF in the treated side of the spine for up to three-and-a-half years after transplantation. Investigators continue to study postmortem spinal cord and brain tissue, searching for differences in dysfunction between lower motor neurons located in the spinal cord and upper motor neurons found in the motor cortex of the brain. While clinicians can detect ALS with electromyography and strength assessments that indicate lower motor neuron function, they have no measurable disease markers for upper motor neuron changes.
Frank Diaz, MD, PhD , a neuromuscular medicine specialist and assistant professor of Neurology, who diagnoses and treats patients in the ALS Clinic, is employing novel MRI techniques to identify markers of upper motor neuron dysfunction in postmortem brains and in living patients. He hopes to detect signal abnormalities in upper motor neurons. When paired with corresponding clinical data, this could support the development of more specific diagnostic tools, he says.
“Some patients do not have obvious clinical evidence of upper motor neuron dysfunction early on, which leads to delays in diagnosis and treatment, and even precludes their participation in clinical trials,” Dr. Diaz says. “Identifying markers of dysfunction will help us tremendously in understanding how the disease starts in the first place.”
Read: ALS and Genetics: What Do We Know?
MODELING DISEASE ORIGINS
In tandem with clinical trials, Cedars-Sinai investigators are leveraging patient-derived stem cells to model the disease in the lab and in animals.
Ritchie Ho, PhD , assistant professor of Neurology and Biomedical Sciences, who runs a lab at the Cedars-Sinai Board of Governors Regenerative Medicine Institute, is investigating motor neurons made from induced pluripotent stem cells (iPSCs) of ALS patients to identify the earliest signatures of disease and elucidate how disease pathways differ among patients based on their genes.
Dr. Ho’s research trajectory syncs with the groundbreaking discovery, in 2006, of iPSC technology. His PhD thesis focused on the mechanisms of stem cell reprogramming: the complex process of turning blood or skin cells back into a blank slate, so they can be coaxed, by the addition of specific protein growth factors, to become any type of cell in the body.
In a 2021 paper published in Cell Systems , Dr. Ho uncovered molecular differences between iPSC models of patients with sporadic and familial ALS. Though sporadic and familial disease lead to the same outcome, for the 90% of ALS patients without an identified gene associated with the disease, there is no explanation for its development.
“The hope is that, for those patients, we can use their own stem cells to analyze their genetic background,” Dr. Ho says. “Maybe the cause of their disease is genetic—there could be an undiscovered constellation of gene mutations. Maybe it’s not just one gene that causes ALS, but a series of unfortunate events, a critical mass gone wrong.”
This leads to questions about whether investigators can stratify ALS and its pathological processes to segment the disease more specifically based on whether the cells experience problems clearing proteins, processing energy or conducting other activities.
“ALS is potentially an umbrella disease—we don’t know, out of 1,000 patients, if there could be 100 different groups,” Dr. Ho says. “We’re coming to the point where the spheres of medicine and basic research are at a conjunction. We solve these unknowns through integrating patients’ genetics with how their cells behave in the petri dish and which RNA and proteins they express. Then we can connect these data patterns back to what’s going on in patients’ bodies as observed in the ALS Clinic and eventually develop truly personalized medicine. We hope to predict how a person will develop ALS and treat their cells before they degenerate.”
Read: ALS Patient Matt Ashley Shares Learnings From 'Heartbreaking' Disease
BUILDING THE FUTURE OF ALS RESEARCH
At the Cedars-Sinai Biomanufacturing Center , Dr. Svendsen’s group, in collaboration with Dhruv Sareen, PhD, the center’s executive director, is building the largest-ever collection of iPSCs from ALS patients as part of the nationwide Answer ALS initiative. The stem cell lines are paired with an open-source repository of corresponding clinical, genetic, molecular and biochemical information, amounting to the most comprehensive collection of ALS data in history.
The project is groundbreaking in scale and specificity, and intended to encourage and enable researchers everywhere to leverage the data to develop deeper questions—and pursue answers. Earlier this year, Dr. Svendsen’s group and collaborators published a paper in Nature Neuroscience utilizing Answer ALS data to uncover disease subtypes.
Though stem cells have vastly expanded our insights into ALS, the grim reality of the disease and the lack of treatments are never far from mind for devoted investigators.
“This is how depressing it is—we started collecting the lines four years ago, and 700 of those 1,000 patients have died,” Dr. Svendsen says. “That’s why we’re determined to do these big projects to understand more. We’re just starting to crack the surface in uncovering clusters of patients with certain characteristics, and we’ve started breaking down questions about what causes it. You can’t design a drug treatment for a disease if you don’t know its cause.”
Challenges in finding a cause remain: No model of any disease is perfect. What might get lost or introduced into iPSCs during “reprogramming” to make them less reliable imitators of how the disease behaves in the body? Investigators hope to address this open question to improve the best tools we have.
In the Newsroom: Engineering the Next Generation of Cell and Gene Therapies
STEM CELLS CONTINUE TO SHINE
Last year, with an additional $12 million from CIRM, Cedars-Sinai investigators launched another first-ever, 16-patient safety trial, transplanting the GDNF-producing stem cells into the brain, in a region of the motor cortex that controls hand movement. The research team hopes the operation will leave patients with nothing worse than a scar under their hairline, and that they’ll see a positive effect on hand use, which the team will monitor in the ALS Clinic.
Clinical trials proceed slowly, and participant selection is a frustrating paradox: Patients must show early stages of paralysis to qualify, by which time they likely cannot regain long-term function of their bodies. But investigators hope that the process of proving safety will ultimately lead to an efficacy trial combining delivery to both the spinal cord and motor cortex.
“This is a protective strategy and it’s definitely about timing,” Dr. Svendsen says. “If all the motor neurons are dead, no matter how much progenitor cells and GDNF we deliver, they’ve got nothing to act on. In the future, we can intervene earlier in the disease, and that’s when we might start slowing the progression.”
Cell-based regenerative medicine isn’t the only approach that offers hope for ALS patients. Scientists at Houston Methodist are focused on immunotherapy: A 2022 study published in Neurology proved the safety of the intravenous application of T cells meant to slow ALS progression. Another trial, completed at six sites including Cedars-Sinai , met safety benchmarks for the infusion of ALS patients’ own bone marrow stem cells into their spinal fluid; but a Phase III study, published in a 2022 Muscle Nerve paper, did not meet its efficacy endpoint.
Dr. Lewis remains focused on stem cells’ potential for progress, and hopes that continued clinical trials will validate the approach.
“We are not going to be able to convert this into a therapy for people who currently have the disease,” he says. “But the scientific question is an important one, and the fact that the stem cells survive is crucial—it’s a start.”
TRICKING TIME IN A DISEASE OF AGING
Descriptions of major disorders like cancer, lung disease and heart disease date to at least 1500 B.C.—but ALS wasn’t identified until 1869, and wasn’t widely known until 1939, when baseball player Lou Gehrig was diagnosed.
Because ALS typically presents between the ages of 55 and 75, perhaps a shift in life expectancy—from under 40 in premodern times to a turn of the 20th century increase—could explain why the neurodegenerative disease was undocumented before the modern era. But if ALS is a consequence of our expanded longevity, what explains why it strikes in old age? What are the hazards in aging?
After discovering, as outlined in a 2016 Nature Neuroscience study, that motor neurons derived from iPSCs of ALS patients more closely resemble fetal cells than adult cells, Ritchie Ho, PhD, is attempting to “age” them in the lab to more faithfully model late-onset disease. He hopes to accelerate iPSC maturation—to synthesize a lifespan—and search for signs of pre-programmed cellular “events” that instigate the onset of ALS.
“If we can activate the aging program, we can start to see the neurons become diseased and die, and how,” Dr. Ho says. “We’re trying to look at the very early origins of ALS, assuming there’s something in the neuron that sets it up to die, and trying to understand what that is in order to intervene before it manifests in paralysis.”
To try to trick time, Dr. Ho is measuring gene expressions of iPSC-built motor neurons against gene expressions in tissue from ALS patients and healthy patients. He’s attempting to adjust the manufactured cells to better mimic adult cells by adding small molecules known to switch genes on and off.
In theory, the artificial, expedited aging of iPSCs can help identify what triggers disease onset, Dr. Ho says. “If we can see the full cycle of ALS from start to finish—how the cellular-energy expenditure is different, how proteins accumulate and, at the last stage, how cells are dying—we can pick it apart and see where along a lifespan the disease processes happen.”
Blog & Magazines
Popular topics, make an appointment, call us 7 days a week, 6 am - 9 pm pt, support cedars-sinai.
Appointments at Mayo Clinic
Stem cells: what they are and what they do.
Stem cells offer promise for new medical treatments. Learn about stem cell types, current and possible uses, and the state of research and practice.
You've heard about stem cells in the news, and perhaps you've wondered if they might help you or a loved one with a serious disease. Here are some answers to frequently asked questions about stem cells.
What are stem cells?
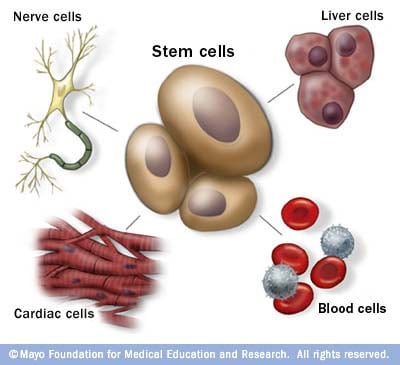
Stem cells: The body's master cells
Stem cells are the body's master cells. All other cells arise from stem cells, including blood cells, nerve cells and other cells.
Stem cells are a special type of cells that have two important properties. They are able to make more cells like themselves. That is, they self-renew. And they can become other cells that do different things in a process known as differentiation. Stem cells are found in almost all tissues of the body. And they are needed for the maintenance of tissue as well as for repair after injury.
Depending on where the stem cells are, they can develop into different tissues. For example, hematopoietic stem cells reside in the bone marrow and can produce all the cells that function in the blood. Stem cells also can become brain cells, heart muscle cells, bone cells or other cell types.
There are various types of stem cells. Embryonic stem cells are the most versatile since they can develop into all the cells of the developing fetus. The majority of stem cells in the body have fewer abilities to give rise to cells and may only help maintain and repair the tissues and organs in which they reside.
No other cell in the body has the natural ability to generate new cell types.
Why is there such an interest in stem cells?
Researchers are studying stem cells to see if they can help to:
- Increase understanding of how diseases occur. By watching stem cells mature into cells in bones, heart muscle, nerves, and other organs and tissue, researchers may better understand how diseases and conditions develop.
Generate healthy cells to replace cells affected by disease (regenerative medicine). Stem cells can be guided into becoming specific cells that can be used in people to regenerate and repair tissues that have been damaged or affected by disease.
People who might benefit from stem cell therapies include those with leukemia, Hodgkin disease, non-Hodgkin lymphoma and some solid tumor cancers. Stem cell therapies also might benefit people who have aplastic anemia, immunodeficiencies and inherited conditions of metabolism.
Stem cells are being studied to treat type 1 diabetes, Parkinson's disease, amyotrophic lateral sclerosis, heart failure, osteoarthritis and other conditions.
Stem cells may have the potential to be grown to become new tissue for use in transplant and regenerative medicine. Researchers continue to advance the knowledge on stem cells and their applications in transplant and regenerative medicine.
Test new drugs for safety and effectiveness. Before giving drugs in development to people, researchers can use some types of stem cells to test the drugs for safety and quality. This type of testing may help assess drugs in development for toxicity to the heart.
New areas of study include the effectiveness of using human stem cells that have been programmed into tissue-specific cells to test new drugs. For the testing of new drugs to be accurate, the cells must be programmed to acquire properties of the type of cells targeted by the drug. Techniques to program cells into specific cells are under study.
Where do stem cells come from?
There are several sources of stem cells:
Embryonic stem cells. These stem cells come from embryos that are 3 to 5 days old. At this stage, an embryo is called a blastocyst and has about 150 cells.
These are pluripotent (ploo-RIP-uh-tunt) stem cells, meaning they can divide into more stem cells or can become any type of cell in the body. This allows embryonic stem cells to be used to regenerate or repair diseased tissue and organs.
- Adult stem cells. These stem cells are found in small numbers in most adult tissues, such as bone marrow or fat. Compared with embryonic stem cells, adult stem cells have a more limited ability to give rise to various cells of the body.
Adult cells altered to have properties of embryonic stem cells. Scientists have transformed regular adult cells into stem cells using genetic reprogramming. By altering the genes in the adult cells, researchers can make the cells act similarly to embryonic stem cells. These cells are called induced pluripotent stem cells (iPSCs).
This new technique may allow use of reprogrammed cells instead of embryonic stem cells and prevent immune system rejection of the new stem cells. However, scientists don't yet know whether using altered adult cells will cause adverse effects in humans.
Researchers have been able to take regular connective tissue cells and reprogram them to become functional heart cells. In studies, animals with heart failure that were injected with new heart cells had better heart function and survival time.
Perinatal stem cells. Researchers have discovered stem cells in amniotic fluid as well as umbilical cord blood. These stem cells can change into specialized cells.
Amniotic fluid fills the sac that surrounds and protects a developing fetus in the uterus. Researchers have identified stem cells in samples of amniotic fluid drawn from pregnant women for testing or treatment — a procedure called amniocentesis.
Why is there controversy about using embryonic stem cells?
The National Institutes of Health created guidelines for human stem cell research in 2009. The guidelines define embryonic stem cells and how they may be used in research and include recommendations for the donation of embryonic stem cells. Also, the guidelines state that embryonic stem cells from embryos created by in vitro fertilization can be used only when the embryo is no longer needed.
Where do these embryos come from?
The embryos being used in embryonic stem cell research come from eggs that were fertilized at in vitro fertilization clinics but never implanted in women's uteruses. The stem cells are donated with informed consent from donors. The stem cells can live and grow in special solutions in test tubes or petri dishes in laboratories.
Why can't researchers use adult stem cells instead?
Progress in cell reprogramming and the formation of iPSCs has greatly enhanced research in this field. However, reprogramming is an inefficient process. When possible, iPSCs are used instead of embryonic stem cells since this avoids the ethical issues about use of embryonic stem cells that may be morally objectionable for some people.
Although research into adult stem cells is promising, adult stem cells may not be as versatile and durable as are embryonic stem cells. Adult stem cells may not be able to be manipulated to produce all cell types, which limits how adult stem cells can be used to treat diseases.
Adult stem cells are also more likely to contain irregularities due to environmental hazards, such as toxins, or from errors acquired by the cells during replication. However, researchers have found that adult stem cells are more adaptable than was first thought.
What are stem cell lines, and why do researchers want to use them?
A stem cell line is a group of cells that all descend from a single original stem cell and are grown in a lab. Cells in a stem cell line keep growing but don't become specialized cells. Ideally, they remain free of genetic defects and continue to create more stem cells. Clusters of cells can be taken from a stem cell line and frozen for storage or shared with other researchers.
What is stem cell therapy (regenerative medicine), and how does it work?
Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. It is the next chapter in organ transplantation and uses cells instead of donor organs, which are limited in supply.
Researchers grow stem cells in a lab. These stem cells are manipulated to specialize into specific types of cells, such as heart muscle cells, blood cells or nerve cells.
The specialized cells can then be implanted into a person. For example, if the person has heart disease, the cells could be injected into the heart muscle. The healthy transplanted heart muscle cells could then contribute to repairing the injured heart muscle.
Researchers have already shown that adult bone marrow cells guided to become heart-like cells can repair heart tissue in people, and more research is ongoing.
Have stem cells already been used to treat diseases?
Yes. Doctors have performed stem cell transplants, also known as bone marrow transplants, for many decades. In hematopoietic stem cell transplants, stem cells replace cells damaged by chemotherapy or disease or serve as a way for the donor's immune system to fight some types of cancer and blood-related diseases. Leukemia, lymphoma, neuroblastoma and multiple myeloma often are treated this way. These transplants use adult stem cells or umbilical cord blood.
Researchers are testing adult stem cells to treat other conditions, including some degenerative diseases such as heart failure.
What are the potential problems with using embryonic stem cells in humans?
For embryonic stem cells to be useful, researchers must be certain that the stem cells will differentiate into the specific cell types desired.
Researchers have discovered ways to direct stem cells to become specific types of cells, such as directing embryonic stem cells to become heart cells. Research is ongoing in this area.
Embryonic stem cells also can grow irregularly or specialize in different cell types spontaneously. Researchers are studying how to control the growth and development of embryonic stem cells.
Embryonic stem cells also might trigger an immune response in which the recipient's body attacks the stem cells as foreign invaders, or the stem cells might simply fail to function as expected, with unknown consequences. Researchers continue to study how to avoid these possible complications.
What is therapeutic cloning, and what benefits might it offer?
Therapeutic cloning, also called somatic cell nuclear transfer, is a way to create versatile stem cells independent of fertilized eggs. In this technique, the nucleus is removed from an unfertilized egg. This nucleus contains the genetic material. The nucleus also is removed from the cell of a donor.
This donor nucleus is then injected into the egg, replacing the nucleus that was removed, in a process called nuclear transfer. The egg is allowed to divide and soon forms a blastocyst. This process creates a line of stem cells that is genetically identical to the donor's cells — in essence, a clone.
Some researchers believe that stem cells derived from therapeutic cloning may offer benefits over those from fertilized eggs because cloned cells are less likely to be rejected once transplanted back into the donor. And it may allow researchers to see exactly how a disease develops.
Has therapeutic cloning in people been successful?
No. Researchers haven't been able to successfully perform therapeutic cloning with humans despite success in a number of other species.
Researchers continue to study the potential of therapeutic cloning in people.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Stem cell basics. National Institutes of Health. https://stemcells.nih.gov/info/basics/stc-basics/#stc-I. Accessed March 21, 2024.
- Lovell-Badge R, et al. ISSCR guidelines for stem cell research and clinical translation: The 2021 update. Stem Cell Reports. 2021; doi:10.1016/j.stemcr.2021.05.012.
- AskMayoExpert. Hematopoietic stem cell transplant. Mayo Clinic; 2024.
- Stem cell transplants in cancer treatment. National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/types/stem-cell-transplant/. Accessed March 21, 2024.
- Townsend CM Jr, et al. Regenerative medicine. In: Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 21st ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed March 21, 2024.
- Kumar D, et al. Stem cell based preclinical drug development and toxicity prediction. Current Pharmaceutical Design. 2021; doi:10.2174/1381612826666201019104712.
- NIH guidelines for human stem cell research. National Institutes of Health. https://stemcells.nih.gov/research-policy/guidelines-for-human-stem-cell-research. Accessed March 21, 2024.
- De la Torre P, et al. Current status and future prospects of perinatal stem cells. Genes. 2020; doi:10.3390/genes12010006.
- Yen Ling Wang A. Human induced pluripotent stem cell-derived exosomes as a new therapeutic strategy for various diseases. International Journal of Molecular Sciences. 2021; doi:10.3390/ijms22041769.
- Alessandrini M, et al. Stem cell therapy for neurological disorders. South African Medical Journal. 2019; doi:10.7196/SAMJ.2019.v109i8b.14009.
- Goldenberg D, et al. Regenerative engineering: Current applications and future perspectives. Frontiers in Surgery. 2021; doi:10.3389/fsurg.2021.731031.
- Brown MA, et al. Update on stem cell technologies in congenital heart disease. Journal of Cardiac Surgery. 2020; doi:10.1111/jocs.14312.
- Li M, et al. Brachyury engineers cardiac repair competent stem cells. Stem Cells Translational Medicine. 2021; doi:10.1002/sctm.20-0193.
- Augustine R, et al. Stem cell-based approaches in cardiac tissue engineering: Controlling the microenvironment for autologous cells. Biomedical Pharmacotherapy. 2021; doi:10.1016/j.biopha.2021.111425.
- Cloning fact sheet. National Human Genome Research Institute. https://www.genome.gov/about-genomics/fact-sheets/Cloning-Fact-Sheet. Accessed March 21, 2024.
- Dingli D (expert opinion). Mayo Clinic. Nov. 17, 2023.
Products and Services
- Sign up for Email: Get Your Free Resource – Coping with Cancer
- A Book: Living Medicine
- Give today to find cancer cures for tomorrow
- Acute lymphocytic leukemia
- Acute myelogenous leukemia
- Adjuvant therapy for cancer
- Amyloidosis
- Aplastic anemia
- Atypical cells: Are they cancer?
- Biopsy procedures
- Blood Cancers and Disorders
- Bone marrow transplant
- Cancer blood tests
- Myths about cancer causes
- Infographic: Cancer Clinical Trials Offer Many Benefits
- Cancer diagnosis: 11 tips for coping
- Cancer-related fatigue
- Cancer pain: Relief is possible
- Cancer risk: What the numbers mean
- Cancer surgery
- Cancer survival rate
- Cancer survivors: Care for your body after treatment
- Cancer survivors: Late effects of cancer treatment
- Cancer survivors: Managing your emotions after cancer treatment
- Cancer treatment myths
- Chemotherapy side effects: A cause of heart disease?
- Chronic lymphocytic leukemia
- Chronic myelogenous leukemia
- Curcumin: Can it slow cancer growth?
- What is type 1 diabetes? A Mayo Clinic expert explains
- Type 1 diabetes FAQs
- Cancer-related diarrhea
- DiGeorge syndrome (22q11.2 deletion syndrome)
- Eating during cancer treatment: Tips to make food tastier
- Epidermolysis bullosa
- Gaucher disease
- Heart cancer: Is there such a thing?
- High-dose vitamin C: Can it kill cancer cells?
- Hodgkin's lymphoma (Hodgkin's disease)
- Hodgkin's vs. non-Hodgkin's lymphoma: What's the difference?
- Low blood counts
- Measles Virus as a Cancer Fighter
- Monoclonal antibody drugs
- Mort Crim and Cancer
- Mouth sores caused by cancer treatment: How to cope
- Multiple myeloma
- Infographic: Multiple Myeloma
- Myelofibrosis
- Neuroblastoma
- No appetite? How to get nutrition during cancer treatment
- Non-Hodgkin's lymphoma
- Scleroderma
- Self-Image During Cancer
- Sickle cell anemia
- Sisters' Bone Marrow Transplant
- Small cell, large cell cancer: What this means
- Stem Cells 101
- Thalassemia
- Tumor vs. cyst: What's the difference?
- Type 1 diabetes
- Stem cell transplant
- How cancer spreads
- PICC line placement
- When cancer returns: How to cope with cancer recurrence
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Stem cells What they are and what they do
Let’s celebrate our doctors!
Join us in celebrating and honoring Mayo Clinic physicians on March 30th for National Doctor’s Day.

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic


Multiple myeloma: Its evolution, treatment and the quest to catch it early
Share this:.
By Nicole Brudos Ferrara
Multiple myeloma is a cancer of a type of white blood cell called a plasma cell in the bone marrow. When multiple myeloma develops in the bone marrow, cancerous plasma cells multiply, crowding out healthy cells.
"Over time, people develop abnormalities or mutations in their plasma cells. Those mutations cause plasma cells to become cancerous," says Joselle Cook, M.B.B.S., a Mayo Clinic hematologist specializing in multiple myeloma and other plasma cell disorders. "Older age is a risk factor. Multiple myeloma is commonly diagnosed in people in their 60s and 70s. We also know that Black people develop myeloma about 10 years earlier than white people, and two to three times more frequently."
Having a family history of multiple myeloma may also increase the risk of the disease.
An estimated 35,780 new cases of multiple myeloma will be diagnosed in the United States in 2024. While multiple myeloma is a serious condition, people with the disease are living longer because treatments have advanced. "The prognosis has changed remarkably over the last few years," says Dr. Cook.
Read on for an overview of how multiple myeloma evolves, how healthcare professionals treat it, and the quest to find a screening test to diagnose the disease before it can damage the body.
The evolution of multiple myeloma
Cancerous plasma cells — myeloma cells — make proteins that cause the symptoms and complications of multiple myeloma. "When the plasma cells develop mutations and produce a monoclonal protein, this group of conditions is called monoclonal gammopathies," says Dr. Cook.
The earliest phase of monoclonal gammopathies is monoclonal gammopathy of undetermined significance (MGUS) , which doesn't cause symptoms. When a person has MGUS, monoclonal protein, or M-protein, is found in their blood at a level too low to damage the body. "If we detect M-protein in a person's blood, and we aren't concerned about organ damage, we monitor them," says Dr. Cook.
If cancerous plasma cells continue to multiply and produce M-proteins, MGUS evolves into smoldering multiple myeloma. People still don't have symptoms at this phase but have a higher M-protein level in their blood and urine.
People with smoldering multiple myeloma are classified based on their risk of progressing to multiple myeloma: low risk, intermediate risk or high risk. "We may treat some people with high-risk smoldering multiple myeloma, typically as part of a clinical trial, but for the most part, we actively monitor people without treating them," says Dr. Cook.
Multiple myeloma might be suspected when blood tests conducted for another reason raise red flags. "We may see that the protein is quite elevated or that a patient has a lower blood count than usual or an abnormality in their kidney numbers," she says. This prompts a care team to order blood tests to detect M-protein and assess blood chemistry and kidney function, urine tests to detect proteins, imaging tests to identify bone problems, and a bone marrow biopsy to look for myeloma cells.
People with multiple myeloma can experience a variety of symptoms or none. This can make diagnosing the disease a challenge. When a healthcare professional suspects multiple myeloma, they frequently check for specific signs and symptoms. "People with multiple myeloma may have anemia . If the M-proteins deposit in the kidneys and cause them to fail, they will have kidney abnormalities. They may have bone pain and high blood calcium levels caused by bone destruction," says Dr. Cook.
Multiple myeloma treatment options
Treatment for multiple myeloma typically starts with a combination of medications called induction chemotherapy. "Treatment involves plasma-cell directed therapy," says Dr. Cook. "It's usually a combination of three or four drugs: A steroid, an immunomodulating agent (a drug that stimulates the immune system to fight cancer), an antibody ( anti-CD38 ) that targets a surface marker on the cancerous plasma cell, and a proteasome inhibitor that targets the cell's protein manufacturing."
Your care team will also decide if you are a candidate for a bone marrow transplant. "This decision depends on factors like fitness and age — it's a soft cut-off, but generally, we don't transplant patients over 75. There are exceptions, though," says Dr. Cook.
The drugs your care team uses in your induction chemotherapy will depend on your overall health and whether you are a candidate for a bone marrow transplant.
A bone marrow transplant — a stem cell transplant — is a procedure that infuses healthy blood-forming stem cells into your body to regenerate the bone marrow's ability to produce blood cells. Stem cell transplants can pose risks, and some people can have serious complications.
If eligible, people with multiple myeloma typically have a stem cell transplant after about four to six months of induction chemotherapy. Before the transplant, they receive a high dose of a different type of chemotherapy called conditioning chemotherapy.
Dr. Cook uses a garden metaphor to explain how conditioning and stem cell transplants work to treat multiple myeloma. "In myeloma, your bone marrow is akin to a garden overgrown with weeds (the myeloma). You use strong weed killers (the conditioning chemotherapy) to eliminate those weeds, but then the garden is barren. You need to plant seeds to allow the garden to grow. That's what a stem cell transplant does. The reinfusion of stem cells is like planting seeds so your bone marrow can recover faster."
Dr. Cook says other promising treatment options exist if you cannot undergo a bone marrow transplant. "For people who relapse after several types of treatment, CAR-T cell therapy , where people's T cells are engineered to recognize and kill a myeloma cell, offers great response rates and good survival. And we've seen success with new drugs called bispecific antibodies — specially designed antibodies that redirect a patient's T cells (immune cells) to kill myeloma cells," she says.
Radiation therapy may also be an option to treat areas of the body affected by myeloma that are painful or causing other problems, says Dr. Cook.
Research aimed at catching multiple myeloma early
"Being diagnosed early is important because you want to avoid organ damage, renal impairment and bone destruction. If we can detect and diagnose multiple myeloma early, we can prevent that damage," says Dr. Cook.
"MGUS and multiple myeloma are detected most often. It's less common to detect smoldering multiple myeloma," says Dr. Cook. She says researchers are exploring screening options — primarily blood tests — to identify MGUS and smoldering myeloma.
Dr. Cook is working with colleagues on a clinical trial in Rochester, Minnesota, to screen people of East African descent. Other clinical trials are studying people with MGUS and those in higher-risk populations. "There are screening studies focused on Black people and those who have first-degree relatives with myeloma or MGUS," she says.
Dr. Cook is confident that the outlook for people diagnosed with multiple myeloma will continue to improve. "There are so many treatment options being developed," she says. "The field is just forging ahead."
Learn more about multiple myeloma and find a clinical trial at Mayo Clinic.
Join the Blood Cancers and Disorders Support Group on Mayo Clinic Connect , an online community moderated by Mayo Clinic for patients and caregivers.
Also, read these articles:
- " CAR-T cell researchers at Mayo Clinic optimistic about future of treating blood cancers ."
- " Monoclonal antibody drugs for cancer: How they work "
- " Advances in treating multiple myeloma help extend quality of life for patients "
- " Investigating dual CAR-T cell therapy for multiple myeloma "
- " Is a cancer clinical trial right for me? "
- " Multiple myeloma: New, better treatments are improving outcomes "
- " What is multiple myeloma? "
Related Posts

After receiving CAR-T cell therapy at Mayo Clinic, Welsh-born John Cadwallader achieved remission and found new hope. He now receives care in the U.K. and is monitored by Mayo in the U.S. and London.

Mayo Clinic's Advanced Care at Home program helped David Elder recover at home from a bone marrow transplant to treat his multiple myeloma.

Learn about CAR-T cell therapy and research at Mayo Clinic to reduce its side effects and expand its use beyond blood cancers.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

‘Harvard Thinking’: Facing death with dignity

Study of 4.3M pregnant women clarifies safety of antiseizure meds
Aspirin cuts liver fat in trial

Members of the Mass General Cancer Center team Elizabeth Gerstner, (from left), William Curry, Marcela Maus, Bryan Choi, Kathleen Gallagher, and Matthew Frigault.
Novel teamwork, promising results for glioblastoma treatment
Researchers cite ‘academic medicine’ as key factor in success
MGH Communications
It’s fitting that the investigators who developed a new CAR T-based treatment for glioblastoma called their treatment platform “CAR-TEAM” cells, as it was a true team effort to bring their concept from the lab to the clinic.
The team, led by Marcela Maus and Bryan Choi recently published a study detailing the promising preliminary results of a first-in-human trial for the new therapy, which was designed to target two different sites on glioblastoma tumors.
All three patients in the initial trial showed dramatic but transient reductions in tumor size in imaging scans taken after their treatments.
The team is now exploring ways to improve the longevity of the treatment, either by delivering multiple doses over time or by pairing the treatment with chemotherapy.
While the encouraging results of the trial are the biggest takeaway, just as inspiring is the team effort that went into taking a promising finding from mice in 2019 all the way to a first-in-human trial in 2023.
“That is not something that’s been done a lot historically in an academic medicine setting — basic laboratory research going all the way to clinical trials in patients,” said Maus. “But we’re doing it, and I think it’s really important what you can achieve as a field when you take that approach.”
From concept to preclinical studies
The concept for the trial started with Maus, the director of Cellular Immunotherapy Program at the Mass General Cancer Center and an associate professor of medicine at Harvard Medical School. Choi, a brain tumor neurosurgeon , associate director of the Center for Brain Tumor Immunology and Immunotherapy at the Mass General Cancer Center, and assistant professor at HMS, was conducting a two-year research fellowship in her lab at the time.
“I had this idea for how to make CAR T cells work for a brain tumor based on previous work we’ve done and previous work in the field, and Dr. Choi was a force of nature in making it happen,” Maus recalled.
“I feel fortunate and grateful to have been able to participate in this trial over the past nine months. It has given me hope .” Tom Fraser, diagnosed with glioblastoma in 2021
Typically, CAR T cells target a single antigen or single tumor marker protein on the tumor cells. This treatment strategy has been highly effective in some forms of blood cancers that have a common antigen across tumor cells, such as lymphoma, but it has yet to be translated to solid tumors, which have a more diverse population of cells.
To address this challenge, Maus and Choi developed a second-generation treatment known as CAR-TEAM cells (T Cell Engager Antibody Molecules), which have a dual targeting mechanism that enables them to target two different proteins expressed by glioblastoma cells.
The first target was a common cancer mutation known as EGRFvlll. The second target was wild-type EGFR, a protein that is not detected in normal brain tissue but is expressed in more than 80 percent of cases of glioblastoma.
After successfully testing the treatment in mice in 2019 , the team effort involved work with internal stakeholders to secure the necessary institutional and FDA approvals needed to translate the strategy to human patients.
Key collaborators in this process included Mass General’s Elizabeth Gerstner , a neuro-oncologist, Will Curry, director of neurosurgical oncology, Matthew Frigault, administrative director of the cell therapy service, and Kathleen Gallagher, director of the immune monitoring laboratory , along with dozens of experts across fields ranging from cell therapy to patient care.
“To accomplish this trial, we needed experts in cell therapy — people who are administering it routinely, who understand how it works in the body, how people respond to it, and we have a wholesale cell therapy service — directed by my friend and former co-fellow Dr. Matthew Frigault — dedicated to that,” says Choi.
Choi helped in setting everything up for the trial and facilitating the treatment itself, which was administered directly to the tumor site. “As a neurosurgeon for several of the patients, I’m involved in performing surgeries to resect tumors and to place the catheter that we were approved to administer CAR T cells through,” he said.
The team effort also included physician assistants and nurses at Mass General who had to familiarize themselves with study protocol, be on the lookout for potential toxicities after the treatment was administered, and to record the clinical data in a useful way to inform future work, said Choi.
“None of this was done in a vacuum,” added Maus. “We had a larger sphere of collaborators, including a data safety monitoring board, scientific review committee, institutional review board and the FDA.
“We’ve had graduate students and postdoctoral fellows in the lab, combinations of physicians, Ph.D. scientists, technicians, clinical investigators, regulatory affairs, manufacturing people.
“When you think about all the people involved in the study, it’s probably 80 to 100.”
Making a lasting impact
“Right now, this is still an early phase study,” said Maus. “We want to be able to make a very significant long-term impact in patients with glioblastoma, so the next arms of the study will take on new ways of sort of enhancing that response to the tumor.
“All of our members have been just incredible in their dedication, their thoughtfulness and their integrity. We’re all just rooting for the patients and for this to make a difference.”
Share this article
You might like.
In podcast episode, a chaplain, a bioethicist, and a doctor talk about end-of-life care

Using 20 years of data, prenatal exposure to topiramate may not increase children’s risk of autism spectrum disorder
10 percent reduction seen in small study of disease that affects up to a third of U.S. adults
Maria Ressa named 2024 Commencement speaker
Nobel Prize-winning defender of press freedom will deliver principal address
Parkinson’s warning in skin biopsy
Medical office procedure identifies key biomarker that may lead to more reliable diagnosis of neurodegenerative disorders
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Medicine (Baltimore)
- v.99(11); 2020 Mar
Clinical efficacy and safety of stem cell therapy for knee osteoarthritis
a Department of neurosurgery, The Second Hospital of Jilin University
b Department of pediatrics, The First Hospital of Jilin University
c Department of hand surgery, The Second Hospital of Jilin University
d College of Mathematics, Jilin University
e Orthopaedic Medical Center, The Second Hospital of Jilin University, Changchun, China.
Background:
We performed a meta-analysis of the efficacy and safety of stem cell therapy as a clinical treatment of knee osteoarthritis. This meta-analysis is expected to provide evidence of the efficacy of stem cell therapy, which is currently controversial, as a conservative treatment for knee osteoarthritis.
An online search for relevant articles was conducted in the PubMed, EMBASE, and Cochrane Library databases. The search terms were “stem cells” and “osteoarthritis.” We conducted a quality assessment of the included articles and extracted the following indicators: Visual Analogue Scale (VAS) score, Subjective International Knee Documentation Committee (IKDC) score, Western Ontario and McMaster Universities (WOMAC) subscales, and adverse events. The RevMan5.3 software was used for determining effect sizes.
Nine randomized controlled trials involving 339 patients were included. VAS score and IKDC score from baseline to 24 months were improved in the stem cell therapy group compared to those in the control group. However, no significant difference was observed between the 2 groups in IKDC score changes from baseline to 6 and 12 months, as well as in WOMAC-Pain, WOMAC-Stiffness, and WOMAC-Physical Function score changes at each visit point.
Conclusion:
Stem cell therapy is certainly superior to traditional treatments in the conservative treatment of KOA; it considerably reduces pain with no obvious additional side effects.
1. Introduction
Knee osteoarthritis is a chronic degenerative bone metabolic disease that commonly occurs in middle-aged and older adults; it affects patients’ daily activities and even causes disability. [ 1 , 2 ] Its clinical features mainly include cartilage degenerative lesions, with clinical manifestations such as joint swelling, pain, and deformity. Thus, the main therapeutic purposes of knee osteoarthritis are to reduce or eliminate pain, correct joint deformities, and improve joint function through cartilage repair. [ 3 ]
In recent years, replacement of damaged articular cartilage by chondrocytes or cartilage tissue has been considered a potential approach for treating knee osteoarthritis. Studies have shown that it is feasible to induce human pluripotent stem cells to differentiate into chondrocytes; therefore, stem cell therapy has become a new method for local treatment of knee osteoarthritis. For example, mesenchymal stem cells (MSCs) have multi-directional differentiation potential and can be differentiated into osteoblasts and chondrocytes under specific induction conditions in vitro and in vivo, thereby repairing bone and articular cartilage. [ 4 , 5 ] However, there is still a dispute on the clinical effects of stem cells, [ 6 – 8 ] for which a multitude of clinical trials and meta-analyses have been conducted. [ 9 , 10 ]
We herein present a meta-analysis of the controversial efficacy and safety of stem cell therapy as a clinical treatment of knee osteoarthritis. This study is markedly distinguished from previous meta-analyses [ 9 , 10 ] because it focused on bone marrow MSCs, peripheral blood stem cells, and amniotic fluid free stem cells. In addition, we used updated data from several latest high-level randomized controlled trials (RCTs). [ 11 , 12 ] This meta-analysis is expected to provide an evidence of the efficacy of stem cell therapy as a conservative treatment of knee osteoarthritis.
All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
2.1. Study selection
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement, [ 13 ] 2 researchers independently screened the literature, as well as extracted and cross-checked the relevant data. If disagreements occurred, a decision regarding data extraction was made by a third researcher.
2.2. Search strategy
We conducted the search in PubMed (1970-May 2019), Embase (1970-May 2019), and The Cochrane Library (1970-May 2019) databases for relevant articles, with “stem cells” and “osteoarthritis” as search terms. We also manually screened relevant Chinese and English language journals and reference lists to include potential studies. The search strategy for PubMed is detailed herein as an example: (((“Stem Cells”[Mesh]) OR ((((((((((((((Cell, Stem[Title/Abstract]) OR Stem Cell[Title/Abstract]) OR Progenitor Cells[Title/Abstract]) OR Cell, Progenitor[Title/Abstract]) OR Cells, Progenitor[Title/Abstract]) OR Progenitor Cell[Title/Abstract]) OR Mother Cells[Title/Abstract]) OR Cell, Mother[Title/Abstract]) OR Cells, Mother[Title/Abstract]) OR Mother Cell[Title/Abstract]) OR Colony-Forming Unit[Title/Abstract]) OR Colony Forming Unit[Title/Abstract]) OR Colony-Forming Units[Title/Abstract]) OR Colony Forming Units[Title/Abstract]))) AND ((((((((((((((Osteoarthritides[Title/Abstract]) OR Osteoarthrosis[Title/Abstract]) OR Osteoarthroses[Title/Abstract]) OR Arthritis, Degenerative[Title/Abstract]) OR Arthritides, Degenerative[Title/Abstract]) OR Degenerative Arthritides[Title/Abstract]) OR Degenerative Arthritis[Title/Abstract]) OR Osteoarthrosis Deformans[Title/Abstract]) OR Polyarthritides[Title/Abstract]) OR Arthritides[Title/Abstract]) OR Polyarthritis[Title/Abstract]) OR Arthritis[Title/Abstract])) OR “Osteoarthritis”[Mesh]).
2.3. Eligibility criteria
The study inclusion criteria included:
- (1) studies involving patients with knee osteoarthritis;
- (2) studies including stem cell therapy as the test group, as well as placebo, hyaluronic acid, and steroid treatments as the control groups;
- (4) studies that used at least one of the following indicators: Visual Analogue Scale (VAS) score, Western Ontario and McMaster Universities (WOMAC) subscale, International Knee Documentation Committee (IKDC) score, and incidence of adverse events.
Studies were ineligible if they met any of the following conditions:
- (1) studies that used animals or cadavers as research objects;
- (2) studies that were unable to extract or convert valid data;
- (3) retrospective studies, literature reviews, or conference papers with no full text.
2.4. Data extraction
Data were extracted independently by 2 researchers using a predesigned data sheet. Valid data were converted as per the Cochrane Handbook for Systematic Reviews of Interventions, [ 14 ] in the case where standard deviation could not be acquired. If disagreements occurred, the decision regarding data extraction was done by the third reviewer. Each RCT was concurrently assessed with risk of bias.
2.5. Outcome measures
- VAS is a scoring scale that intuitively quantifies the intensity of pain in the knee. A lower score indicates milder pain.
- The WOMAC subscale is a rating scale that assesses the structure, stiffness, and function of the knee in pain A lower score indicates better knee condition.
- IKDC is a subjective scale for assessing the knee joint. A higher score indicates better symptoms, functions, and physical activities of the knee joint.
- Adverse events refer to treatment-related adverse reactions, including joint effusion, stiffness, and pain.
2.6. Statistical analysis
Statistical analysis was conducted using the RevMan 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The chi-square test was used to assess inter-study heterogeneity. I 2 > 50% indicated heterogeneity. A random effects model was used; otherwise, a fixed effects model was used. Relative risk and standardized mean difference were used for assessing binary variables and continuous variables, respectively. The 95% confidence interval estimates and hypothesis testing results for each variable were listed in a forest plot. For each endpoint with high heterogeneity, a sensitivity analysis, in which the included studies were removed one at a time, was conducted to screen the source of heterogeneity. A publication bias assessment using a funnel plot was performed if there were no less than 10 studies included.
3.1. Literature search
We retrieved 7054 relevant articles, and ultimately included 9 RCTs [ 11 , 12 , 15 – 21 ] involving 399 patients (Fig. (Fig.1). 1 ). In the studies by Kuah, [ 12 ] Lamo-Espinosa, [ 16 ] and Thomas Vangsness et al, [ 19 ] there were 2 parallel test groups, namely the high- and low-dose groups, in comparison with the control group. Therefore, for each study mentioned above, we conducted statistical analyses in 2 RCTs: high-dose vs control and low-dose vs control.

Flowchart of literature retrieval.
3.2. Study characteristics
There were 203 patients in the stem cell therapy group and 196 patients in the control group. The specific features and Jadad scores of the patients [ 22 , 23 ] are listed in Table Table1. 1 . The Jadad scale is a 7-point scale that includes random sequence generation, randomized hiding, blind method, withdrawal, and dropout.
Main characteristics of all the eligible studies included in the analysis.

3.3. Clinical outcomes
From baseline to 3 months, 4 studies [ 12 , 15 , 16 , 20 ] were included, involving 6 RCTs with 87 patients in the stem cell group and 79 patients in the control group Fig. Fig.2. 2 . There was no heterogeneity (I 2 = 0%) between the studies; thus, the fixed effects model was used for the analysis. According to Figure Figure2, 2 , SMD (standardized mean difference) = −0.36, 95% CI (confidence interval)[−0.67, −0.05], and P = .02. The VAS score in the stem cell group was significantly lower than that in the control group.

Forest plot of the change of VAS score. VAS = visual analogue scale score.
From baseline to 6 months, 4 studies [ 12 , 15 , 16 , 20 ] were included, involving 6 RCTs with 87 patients in the stem cell group and 79 patients in the control group. Because there was a high heterogeneity (I 2 = 86%) between the studies, the study by Bhattacharya et al [ 20 ] was removed from the sensitivity analysis, and the I 2 value was reduced to 0%. The fixed effects model was used. According to Figure Figure2, 2 , SMD = −0.86, 95% CI [−1.21, −0.52], and P < .00001. The VAS score in the stem cell group was significantly lower than that in the control group.
From baseline to 12 months, 3 studies [ 12 , 16 , 21 ] were included, involving 5 RCTs with 51 patients in the stem cell group and 43 patients in the control group. There was a low heterogeneity (I 2 = 8%) between the studies, and thus the fixed effects model was used. According to Figure Figure2, 2 , SMD = −0.86, 95% CI [−1.30, −0.43], and P = 0.0001. The VAS score in the stem cell group was significantly lower than that in the control group.
3.3.2. WOMAC-Pain
From baseline to 3 months, 2 studies [ 12 , 16 ] were included, involving four RCTs with 36 patients in the stem cell group and 28 patients in the control group Fig. Fig.3. 3 . There was a low heterogeneity (I 2 = 40%) between the studies, and thus the fixed effects model was used. According to Figure Figure3, 3 , SMD = −0.22, 95% CI [−0.73, 0.30], and P = .41. There was no significant difference in WOMAC-Pain score between the groups.

Forest plot of the change of WOMAC-Pain score. WOMAC = Western Ontario and McMaster Universities subscore.
From baseline to 6 months, 2 studies [ 12 , 16 ] were included, involving four RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was a low heterogeneity (I 2 = 48%) between the studies, and thus the fixed effects model was used. According to Figure Figure3, 3 , SMD = −0.08, 95% CI [−0.59, 0.44], and P = .77. There was no significant difference in WOMAC-Pain score between the groups.
From baseline to 12 months, 3 studies [ 12 , 16 ] were included, involving 4 RCTs with 43 patients in the stem cell group and 39 patients in the control group. There was no heterogeneity (I 2 = 0%) between the studies, and thus the fixed effects model was used. According to Figure Figure3, 3 , SMD = −0.09, 95% CI [−0.53, 0.36], and P = .70. There was no significant difference in WOMAC-Pain score between the groups.
3.3.3. WOMAC-Stiffness
From baseline to 3 months, 2 studies [ 12 , 16 ] were included, involving 4 RCTs with 36 patients in the stem cell group and 28 patients in the control group Fig. Fig.4. 4 . There was no heterogeneity (I 2 = 0%) between the studies, and the fixed effects model was used. According to Figure Figure4, 4 , SMD = −0.51, 95% CI [−1.02, 0.01], and P = .05. There was no significant difference in WOMAC-Stiffness score between the groups.

Forest plot of the change of WOMAC-Stiffness score. WOMAC = Western Ontario and McMaster Universities subscore.
From baseline to 6 months, 2 studies [ 12 , 16 ] were included, involving four RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was a low heterogeneity (I 2 = 36%) between the studies, and the fixed effects model was used. According to Figure Figure4, 4 , SMD = −0.25, 95% CI [−0.76, 0.27], and P = .35. There was no significant difference in WOMAC-Stiffness score between the groups.
From baseline to 12 months, 2 studies [ 12 , 16 ] were included, involving 4 RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was a low heterogeneity (I 2 = 9%) between the studies, and the fixed effects model was used. According to Figure Figure4, 4 , SMD = −0.46, 95% CI [−0.98, 0.05], and P = .08. There was no significant difference in WOMAC-Stiffness score between the groups.
3.3.4. WOMAC-Function
From baseline to 3 months, 2 studies [ 12 , 16 ] were included, involving 4 RCTs with 36 patients in the stem cell group and 28 patients in the control group Fig. Fig.5. 5 . There was no heterogeneity (I 2 = 0%) between the studies, and the fixed effects model was used. According to Figure Figure5, 5 , SMD = 0.15, 95% CI [−0.35, 0.66], and P = .55. There was no significant difference in WOMAC-Function score between the groups.

Forest plot of the change of WOMAC-Function. WOMAC = Western Ontario and McMaster Universities subscore.
From baseline to 6 months, 2 studies [ 12 , 16 ] were included, involving four RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was a low heterogeneity (I 2 = 29%) between the studies, and the fixed effects model was used. According to Figure Figure5, 5 , SMD = 0.43, 95% CI [−0.09, 0.95], and P = .1. There was no significant difference in WOMAC-Function score between the groups.
From baseline to 12 months, 2 studies [ 12 , 16 ] were included, involving 4 RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was no heterogeneity (I 2 = 0%) between the studies, and the fixed effects model was used. According to Figure Figure5, 5 , SMD = 0.18, 95% CI [−0.33, 0.68], and P = .49. There was no significant difference in WOMAC-Function score between the groups.
3.3.5. IKDC
From baseline to 6 months, 2 studies [ 17 , 18 ] were included, involving 2 RCTs with 53 patients in the stem cell group and 52 patients in the control group Fig. Fig.6. 6 . There was a high heterogeneity (I 2 = 51%) between the studies, and the random effects model was used. According to Figure Figure6, 6 , SMD = 0.16, 95% CI [−0.39,0.72], and P = .56. There was no significant difference in IKDC score between the groups.

Forest plot of the change of IKDC score. IKDC = International Knee Documentation Committee.
From baseline to 12 months, 2 studies [ 17 , 18 ] were included, involving 2 RCTs with 53 patients in the stem cell group and 52 patients in the control group. There was a high heterogeneity (I 2 = 83%) between the studies, and the random effects model was used. According to Figure Figure6, 6 , SMD = 0.36, 95% CI [−0.58, 1.31], and P = .45. There was no significant difference in IKDC score between the groups.
From baseline to 24 months, 2 studies [ 17 , 18 ] were included, involving 2 RCTs with 53 patients in the stem cell group and 52 patients in the control group. There was a high heterogeneity (I 2 = 75%) between the studies, and the random effects model was used. According to Figure Figure6, 6 , SMD = 0.53, 95% CI [−0.25, 1.32], and P = .18. There was no significant difference in IKDC score between the groups.
3.3.6. Adverse events
There were 2 included studies, [ 11 , 12 ] involving three RCTs with 28 patients in the stem cell group and 20 patients in the control group Fig. Fig.7. 7 . There was a high heterogeneity (I 2 = 73%) between the studies, and thus the random effects model was used. According to Figure Figure7, 7 , SMD = 1.54, 95% CI [0.57, 4.19], and P = .40. There was no significant difference in incidence of adverse events between the groups.

Forest plot of the change of adverse events.
4. Discussion
4.1. key findings.
Changes in VAS and IKDC scores from baseline to 24 months were superior in the stem cell group than in the control group, whereas there were no statistical differences in the changing trend of other indicators between the 2 groups, including the changes in IKDC scores at 6 months, IKDC score at 12 months, WOMAC-Pain score, WOMAC-Stiffness score, WOMAC-Function score, WOMAC-Pain score, and incidence of adverse events.
Pain relief is key to treating knee osteoarthritis, with VAS scores as an important endpoint for pain assessment. Kuah et al [ 12 ] found that relative to placebo, stem cell therapy considerably relieved pain at 3, 6, and 12 months after treatment. This conclusion has been confirmed in our meta-analysis. We found that the VAS scores in the stem cell group were significantly reduced at each visit point. Inflammatory response is known as one of the causes of pain. MSCs can release anti-inflammatory factors, thereby relieving pain. Lamo-Espinosa et al [ 16 ] believed that stem cells have a paracrine function and their anti-inflammatory properties contribute to pain relief. In addition, studies have found that in an acute renal failure model, MSCs can promote recovery of renal function by releasing anti-inflammatory factors and inhibiting production of pro-inflammatory cytokines, such as interleukin-1β, tumor necrosis factor, and interferon-γ. [ 24 ] Similar findings were observed in a pulmonary fibrosis model, in which MSCs release IL-1 receptor antagonist (IL-1RA) to inhibit interleukin-1α-producing T cells and TNF-producing macrophages, indicating that MSCs have anti-inflammatory properties. [ 25 ] WOMAC-Pain scores showed no statistical difference between the 2 groups at each visit point, but these data regarding WOMAC-Pain score were obtained only from 3 studies; [ 12 , 16 , 21 ] therefore, further studies with larger sample sizes are warranted to verify these findings.
Functional improvement of the knee joint is one of the ultimate purposes of knee osteoarthritis treatment. In this study, we used WOMAC-Stiffness, WOMAC-Function, and IKDC scores to comprehensively assess knee joint function. Statistical analysis results showed that there was no significant difference between the 2 groups in IKDC scores at 6 and 12 months, as well as in WOMAC-Stiffness and WOMAC-Function scores at each visit point. Studies have found that mesenchymal stem cell implantation achieves better outcomes in patients with grade 3 knee osteoarthritis than those in patients with grade 4 knee osteoarthritis. [ 26 ] We thus concluded that treatment with MSCs are effective in preventing or limiting the progression of knee osteoarthritis at the early stage. In the studies by Kuah [ 12 ] and Lamo-Espinosa et al, [ 16 ] patients with grade 3 osteoarthritis or higher accounted for 75% and over 80% of the total patients, respectively. Most patients developed knee osteoarthritis at the middle and late stages, for whom treatment with MSCs had no significant efficacy and was not conducive to functional recovery. Moreover, in most tissue engineering methods, MSCs are combined with cell scaffolds containing chondrogenic growth factors to form fully functional hyaline cartilage. Such a regimen is commonly used in small-animal models of surgically induced cartilage or osteochondral defects, but cannot be used for repairing large-area cartilage defects associated with knee osteoarthritis. [ 27 ] In addition, Centeno, [ 28 ] Emadedin, [ 29 ] and Vangsness et al [ 19 ] pointed out that treatment with approximately 2 × 10 7 stem cells can afford good clinical results. Kuah [ 12 ] and Lamo-Espinosa et al [ 16 ] reported that a stem cell dose of lower or higher than 2 × 10 7 may also impact the therapeutic efficacy of stem cells. In addition, the change in IKDC score at 24 months was higher in the stem cell group than in the control group; however, these data were extracted from only 2 studies. Therefore, further investigation with a larger sample size is warranted.
For adverse events, we sent an e-mail to the authors of the relevant research [ 18 , 19 , 21 ] to obtain data on the number of patients who experienced treatment-related adverse events, including arthralgia, joint effusion, and joint stiffness, in both the stem cell and control groups. Because of the lack of response from the other studies, only 2 studies [ 11 , 12 ] were included, involving 3 RCTs. There were no statistical differences in adverse events between the 2 groups, indicating that stem cell treatment has no obvious side effects. A study addressing 87 patients with lupus erythematosus [ 30 ] showed no adverse events associated with transplantation after 4 years of follow-up. Similarly, no graft-related adverse events occurred in many patients undergoing stem cell therapy for other diseases. [ 31 – 35 ] These findings indicated that the human body has good tolerance to MSCs, and that stem cell treatment has no significant side effects.
4.2. Limitations
Differences in the original RCT protocols led to insufficient representation of some outcome indicators. Thus, high-quality, large-scale RCTs are required to verify our findings. In addition, there was no uniform standard in the preparation and use of stem cells, which may cause certain heterogeneity.
5. Conclusion
Compared to traditional methods, stem cell treatment has a certain superiority as a conservative treatment of knee osteoarthritis, in terms of markedly reducing pain without inducing side effects.
Author contributions
Conceptualization: Rui Huang.
Data curation: Rui Huang, Wei Li, Ying Zhao.
Formal analysis: Rui Huang, Wei Li, Ying Zhao, Fan Yang.
Investigation: Rui Huang, Wei Li, Ying Zhao.
Methodology: Rui Huang, Wei Li, Fan Yang.
Project administration: Meng Xu.
Software: Rui Huang, Wei Li, Ying Zhao, Fan Yang.
Supervision: Rui Huang, Wei Li, Ying Zhao, Meng Xu.
Validation: Ying Zhao, Fan Yang, Meng Xu.
Visualization: Rui Huang, Wei Li, Fan Yang.
Writing – original draft: Rui Huang, Wei Li.
Writing – review & editing: Ying Zhao, Meng Xu.
Abbreviations: AD-MSCs = adipose-derived mesenchymal stem cells, BMAC = bone marrow aspirate concentrate, BM-MSCs = bone marrow mesenchymal stromal cells, Cl = confidence interval, HA = hyaluronic acid, HTO = high tibial osteotomy, IKDC = International Knee Documentation Committee, IL-1RA = IL-1 receptor antagonist, MSCs = mesenchymal stem cells, PBSC = peripheral blood stem cells, PRG = progenza, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis, PRP = platelet-poor plasma, RCTs = randomized controlled trials, SMD = standardized mean difference, VAS = Visual Analogue Scale, WOMAC = Western Ontario and McMaster Universities.
How to cite this article: Huang R, Li W, Zhao Y, Yang F, Xu M. Clinical efficacy and safety of stem cell therapy for knee osteoarthritis: A meta-analysis. Medicine . 2020;99:11(e19434).
The authors have no conflicts of interests to disclose.
OPINION article
New from old: recycling differentiated cells into stem cells using traditional chinese medicine. a tribute to professor rongxiang xu provisionally accepted.

- 1 University of California, Los Angeles, United States
- 2 University of Edinburgh, United Kingdom
The final, formatted version of the article will be published soon.
New hopes in cell therapies have steadily arisen with ongoing progress in stem cell research, and innumerable conditions could be now treated and cured, from musculoskeletal injuries to diabetes to cardiac failure, to cite but a few, with the appropriate stem cells available. Arguably, the ideal therapeutic stem cells are those that naturally heal, repair, and replenish the target tissue in life, and research from the last decades has uncovered the presence of such committed, specialized stem cells in most organs. However, these cell lineage-specific regenerative cells are rare, difficult to identify and purify, and virtually impossible to culture for amplification as functionally intact, undifferentiated units. For this reason, and with the notable exception of hematopoietic stem cells, these are not presently amenable to clinical utilization. From the 1980's, Prof. Rongxiang Xu developed the theory that some of the pluripotent stem cells that build the embryo in early development also establish through adult life a minor subset of cells which, although seemingly terminally differentiated, retain strong multi-lineage developmental potential. He called these "potential regenerative cells" (PRCs), proposing these can be recruited into tissue repair and regeneration when no other progenitor cells are available (1). Although this hypothesis could not be fully tested experimentally at the time, it later turned out as strikingly visionary: on the one hand, it was demonstrated that mature cells can be reprogrammed into stem cells in culture, a discovery rewarded by attribution of the Nobel Prize in Medicine or Physiology in 2012 (2). Hence, transition from a functional, specialized cell back into a naïve, unbiased stem cell is, biologically, feasible and might explain the persistence of Rongxiang Xu's PRCs in adult organs. Another example in support of PRC existence refers to mesenchymal stem cells. MSCs are multipotent cells that can differentiate in culture into bone, cartilage, fat, tendon, muscle, and indirectly support, via growth factor secretion, the regeneration of multiple other tissues (3). This extraordinary potential has stimulated the use of MSCs in over 2000 clinical trials in attempts to treat multiple conditions in cardiology, orthopaedics, cancerology, nephrology, and many other specialties including medical immunology, since MSCs are also immunosuppressive (see clinicaltrials.gov). Despite such a popularity, progress in MSC science has long been hindered by the unknown identity of native, tissueresident mesenchymal stem cells, since these cells appear and expand in extended cultures of total, unselected cell populations dissociated from bone marrow, adipose tissue, umbilical cord, or other organs. Put in other words, MSCs are normally produced in vitro from elusive, rare ancestor cells that have long resisted description. This changed when multicolour, stringent flow cytometry cell sorting and sensitive differentiation assays were used for MSC prospective identification. In full support of the PRC concept proposed by Rongxiang Xu, innate tissue resident mesenchymal stem cells turned out to be differentiated perivascular cells of documented function. Pericytes, which enwrap capillaries and microvessels, regulate blood pressure and control angiogenesis. Equivalent cells populate the tunica adventitia at the periphery of larger blood vessels. Both purified pericytes and adventicytes give rise to bona fide mesenchymal stem cells when cultured in vitro (4,5). The use of transgenic reporter mice, in which cell lineages can be tracked dynamically, has confirmed the progenitor cell potential of perivascular cells in vivo (6). In aggregate, all these results have confirmed that differentiated cells can be reprogrammed into regenerative cells in culture and in the living organism, thus supporting Dr Xu's hypothesis on the existence of PRCs in adult organs. Professor Rongxiang Xu contributed to the development of formulations, mostly inspired by traditional Chinese medicine, for stem cell (PRC) stimulation in situ and in culture. Such a supplement, for instance, named GIC, was claimed to regenerate the gastrointestinal mucosa, as well as nerves, in culture. More recently, the LifeRegen Inc. company developed a line of products inspired by Dr Xu's research and patents were issued for a proprietary blend of natural ingredients produced via an original manufacturing process. All LifeRegen protocols rely on the infusion of combined black sesame oil, skullcap root, and beeswax. These products are considered GRAS (generally recognized as safe) by the FDA, and represent a scientific breakthrough merging ancient Chinese medicine recipes and Rongxiang Xu's groundbreaking results that are, moreover, being supported and extended by ongoing current research. More precisely, GI Balance and Juvenate Skincare are LifeRegen's proprietary formulations developed to nurture and renew the gut lining and create a cellular, rejuvenation promoting protective barrier for the skin, respectively. The insightful Prof. Rongxiang Xu predicted that some of the cells that constitute developed organs retain stem potential, and hence can be qualified as "potentially regenerative cells", and devised formulations that can stimulate such cells into tissue regeneration. He left us a rich legacy of centers at prestigious institutions, such as the Rongxiang Xu Center for Regenerative Therapeutics at Beth Israel Deaconess Medical Center in Boston, the Rongxiang Xu Center for Regenerative Life Science at the University of Southern California, and the Rongxiang Xu College of Health and Human Services at Cal state LA.The LifeRegen company has resumed and considerably amplified and diversified Dr Xu's approach to regenerative medicine, and is now sponsoring clinical studies for the GI Balance supplement that in previous published research enabled regeneration and rejuvenation of the entire gut mucosa within 6 months. In a more fundamental investigation perspective, PRCs have been prospectively identified and characterized in depth, for instance as pericytes and other perivascular elements, and can be purified to homogeneity and in large numbers from multiple organs (3). This opens unforeseen possibilities in regenerative medicine research whereby such well-characterized potential regenerative cells can be selected from the bone marrow, pancreas, skin, adipose tissue and other organs and treated with natural supplements for enhanced proliferation, migration, and differentiation. Such protocols should be initialized in culture and further developed in vivo by transplantation into relevant animal hosts. In perspective are novel biomolecular manoeuvres to drive the improved healing and replenishment of, for instance but not exclusively, cardiovascular, musculoskeletal, and epithelial tissues.
Keywords: cell reprogramming, cell therapy, Chinese traditional medicine, Skin Regeneration, gut regeneration
Received: 16 Jan 2024; Accepted: 25 Mar 2024.
Copyright: © 2024 Peault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Mx. Bruno Peault, University of California, Los Angeles, Los Angeles, United States
People also looked at
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Stem cells articles from across Nature Portfolio
Stem cells are cells that have the capacity to self-renew by dividing and to develop into more mature, specialised cells. Stem cells can be unipotent, multipotent, pluripotent or totipotent, depending on the number of cell types to which they can give rise.
Exit from totipotency is controlled by DUXBL in mice
In mice, zygotic genome activation occurs at onset of the two-cell stage in embryonic development and coincides with the exit from totipotency. Our work shows that the transcription factor DUXBL participates in silencing part of the stage-specific two-cell-associated transcriptional program and is required for development to proceed.
Mapping human hematopoiesis
Understanding normal hematopoiesis is critical to understanding disease. Technological advances are driving insight into human hematopoiesis at unprecedented resolution. Integrating ‘-omics’ datasets with machine learning has yielded a high-resolution map of primary human bone marrow hematopoietic progenitor cells that supports the study of immune cell development, as well as the origins of disease.
- Kathrin M. Bernt
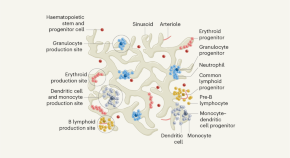
Powerful microscopy reveals blood-cell production in bone marrow
A method for imaging the production of blood cells in the bones of mice has revealed the organization of cell lineages, both in a steady state and in response to stressors, such as bleeding and infection.
- M. Carolina Florian
Related Subjects
- Adult stem cells
- Cancer stem cells
- Embryonic germ cells
- Embryonic stem cells
- Epigenetic memory
- Haematopoietic stem cells
- Heart stem cells
- Intestinal stem cells
- Mammary stem cells
- Mesenchymal stem cells
- Multipotent stem cells
- Muscle stem cells
- Neural stem cells
- Pluripotent stem cells
- Regeneration
- Reprogramming
- Self-renewal
- Skin stem cells
- Stem-cell differentiation
- Stem-cell niche
- Totipotent stem cells
- Transdifferentiation
Latest Research and Reviews

Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity
Antibody-mediated depletion of myeloid-biased haematopoietic stem cells in aged mice restores characteristic features of a more youthful immune system.
- Jason B. Ross
- Lara M. Myers
- Irving L. Weissman
Detection of biomagnetic signals from induced pluripotent stem cell-derived cardiomyocytes using deep learning with simulation data
- Takeshi Yamaguchi
- Yoshiaki Adachi
- Masaki Tanaka

Acellular porcine Achilles tendon patch encapsulating tendon-derived stem cells for rotator cuff repair in a rabbit model
- Yushun Fang
Cell-fate conversion of intestinal cells in adult Drosophila midgut by depleting a single transcription factor
The mechanisms underlying cell plasticity remain poorly understood. Here, Guo et.al discover that intestinal cells in the fly gut can alter their fates through the loss of a single gene, and identify several molecular barriers to cell reprogramming.
- Xingting Guo
- Chenhui Wang
Serotonin effects on human iPSC-derived neural cell functions: from mitochondria to depression
- Iseline Cardon
- Sonja Grobecker
- Christian H. Wetzel
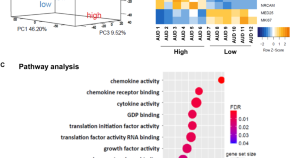
Molecular mechanisms involved in alcohol craving, IRF3, and endoplasmic reticulum stress: a multi-omics study
- Ming-Fen Ho
- Cheng Zhang
- Richard Weinshilboum
News and Comment
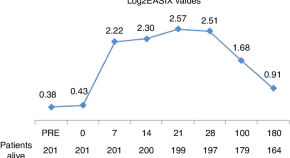
Improving the EASIX’ predictive power for NRM in adults undergoing allogeneic hematopoietic cell transplantation
- Silvia Escribano-Serrat
- Luis Gerardo Rodríguez-Lobato
- María Queralt Salas
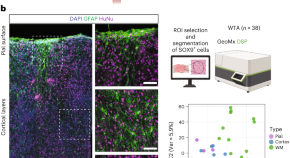
Glia-enriched cortical organoids implanted in mice capture astrocyte diversity
The underrepresentation of functional glial cells is a major challenge in brain organoid models. We developed an astroglia-enriched cortical organoid model that allows efficient generation of functional astrocytes and enables the formation of astroglial morphological subclasses with layer-specific gene expression profiles upon transplantation into the mouse brain.
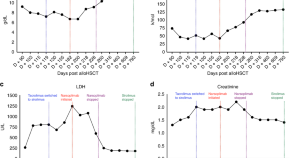
Successful use of narsoplimab to treat allogeneic transplant-associated thrombotic microangiopathy while maintaining sirolimus
- Mohammad Alhomoud
- Michael Scordo
- Miguel-Angel Perales
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Accelerating CAR T cell therapy: Lipid nanoparticles speed up manufacturing
Penn Engineers have developed a novel method for manufacturing CAR T cells, one that takes just 24 hours and requires only one step, thanks to the use of lipid nanoparticles (LNPs), the potent delivery vehicles that played a critical role in the Moderna and Pfizer-BioNTech COVID-19 vaccines.
For patients with certain types of cancer, CAR T cell therapy has been nothing short of life changing. Developed in part by Carl June, Richard W. Vague Professor at Penn Medicine, and approved by the Food and Drug Administration (FDA) in 2017, CAR T cell therapy mobilizes patients' own immune systems to fight lymphoma and leukemia, among other cancers.
However, the process for manufacturing CAR T cells themselves is time-consuming and costly, requiring multiple steps across days. The state of the art involves extracting patients' T cells, then activating them with tiny magnetic beads, before giving the T cells genetic instructions to make chimeric antigen receptors (CARs), the specialized receptors that help T cells eliminate cancer cells.
Now, Penn Engineers have developed a novel method for manufacturing CAR T cells, one that takes just 24 hours and requires only one step, thanks to the use of lipid nanoparticles (LNPs), the potent delivery vehicles that played a critical role in the Moderna and Pfizer-BioNTech COVID-19 vaccines.
In a new paper in Advanced Materials , Michael J. Mitchell, Associate Professor in Bioengineering, describes the creation of "activating lipid nanoparticles" (aLNPs), which can activate T cells and deliver the genetic instructions for CARs in a single step, greatly simplifying the CAR T cell manufacturing process. "We wanted to combine these two extremely promising areas of research," says Ann Metzloff, a doctoral student and NSF Graduate Research Fellow in the Mitchell lab and the paper's lead author. "How could we apply lipid nanoparticles to CAR T cell therapy?"
In some ways, T cells function like a military reserve unit: in times of health, they remain inactive, but when they detect pathogens, they mobilize, rapidly expanding their numbers before turning to face the threat. Cancer poses a unique challenge to this defense strategy. Since cancer cells are the body's own, T cells don't automatically treat cancer as dangerous, hence the need to first "activate" T cells and deliver cancer-detecting CARs in CAR T cell therapy.
Until now, the most efficient means of activating T cells has been to extract them from a patient's bloodstream and then mix those cells with magnetic beads attached to specific antibodies -- molecules that provoke an immune response. "The beads are expensive," says Metzloff. "They also need to be removed with a magnet before you can clinically administer the T cells. However, in doing so, you actually lose a lot of the T cells, too."
Made primarily of lipids, the same water-repellent molecules that constitute household cooking fats like butter and olive oil, lipid nanoparticles have proven tremendously effective at delivering delicate molecular payloads. Their capsule-like shape can enclose and protect mRNA, which provides instructions for cells to manufacture proteins. Due to the widespread use of the COVID-19 vaccines, says Metzloff, "The safety and efficacy of lipid nanoparticles has been shown in billions of people around the world."
To incorporate LNPs into the production of CAR T cells, Metzloff and Mitchell wondered if it might be possible to attach the activating antibodies used on the magnetic beads directly to the surface of the LNPs. Employing LNPs this way, they thought, might make it possible to eliminate the need for activating beads in the production process altogether. "This is novel," says Metzloff, "because we're using lipid nanoparticles not just to deliver mRNA encoding CARs, but also to initiate an advantageous activation state."
Over the course of two years, Metzloff carefully optimized the design of the aLNPs. One of the primary challenges was to find the right ratio of one antibody to another. "There were a lot of choices to make," Metzloff recalls, "since this hadn't been done before."
By attaching the antibodies directly to LNPs, the researchers were able to reduce the number of steps involved in the process of manufacturing CAR T cells from three to one, and to halve the time required, from 48 hours to just 24 hours. "This will hopefully have a transformative effect on the process for manufacturing CAR T cells," says Mitchell. "It currently takes so much time to make them, and thus they are not accessible to many patients around the world who need them."
CAR T cells manufactured using aLNPs have yet to be tested in humans, but in mouse models, CAR T cells created using the process described in the paper had a significant effect on leukemia, reducing the size of tumors, thereby demonstrating the feasibility of the technology.
Metzloff also sees additional potential for aLNPs. "I think aLNPs could be explored more broadly as a platform to deliver other cargoes to T cells," she says. "We demonstrated in this paper one specific clinical application, but lipid nanoparticles can be used to encapsulate lots of different things: proteins, different types of mRNA. The aLNPs have broad potential utility for T cell cancer therapy as a whole, beyond this one mRNA CAR T cell application that we've shown here."
This study was conducted at the University of Pennsylvania School of Engineering and Applied Science and is supported by the U.S. National Institutes of Health Director's New Innovator Award (DP2 TR002776), a U.S. National Science Foundation CAREER Award (CBET-2145491), an American Cancer Society Research Scholar Grant (RSG-22-122-01-ET), and a Burroughs Wellcome Fund Career Award at the Scientific Interface. Further support for this paper and the researchers involved came from the Emerson Collective, U.S. National Science Foundation Graduate Research Fellowships, a U.S. National Institutes of Health Ruth L. Kirschstein National Research Service Award (F31CA260922), the National Institute of Dental and Craniofacial Research of the US National Institutes of Health (T90DE030854), the University of Pennsylvania Fontaine Fellowship, the Norman and Selma Kron Research Fellowship, and the Robert Wood Johnson Foundation Health Policy Research Scholars Program. The researchers thank the Human Immunology Core at the University of Pennsylvania (RRID: SCR_022380) for assistance with primary human T cell procurement. The HIC is supported in part by NIH P30 AI045008 and P30 CA016520.
- Immune System
- Prostate Cancer
- Brain Tumor
- Skin Cancer
- Lung Cancer
- Car accident
- Cholesterol
Story Source:
Materials provided by University of Pennsylvania School of Engineering and Applied Science . Original written by Ian Scheffler. Note: Content may be edited for style and length.
Journal Reference :
- Ann E. Metzloff, Marshall S. Padilla, Ningqiang Gong, Margaret M. Billingsley, Xuexiang Han, Maria Merolle, David Mai, Christian G. Figueroa‐Espada, Ajay S. Thatte, Rebecca M. Haley, Alvin J. Mukalel, Alex G. Hamilton, Mohamad‐Gabriel Alameh, Drew Weissman, Neil C. Sheppard, Carl H. June, Michael J. Mitchell. Antigen Presenting Cell Mimetic Lipid Nanoparticles for Rapid mRNA CAR T Cell Cancer Immunotherapy . Advanced Materials , 2024; DOI: 10.1002/adma.202313226
Cite This Page :
Explore More
- Want to Feel Young? Protect Your Sleep
- Century-Old Powdered Milk in Antarctica
- New Artificial Reef Stands Up to Storms
- Persistent Hiccups in a Far-Off Galaxy
- Lighting Up the Future
- Sugarcane's Complex Genetic Code Cracked
- Microbiome of 4,000-Year-Old Teeth
- Magnetic Fields of Milky Way's Black Hole
- Neural Networks Think Alike
- Old Immune Systems Revitalized
Trending Topics
Strange & offbeat.

COMMENTS
Stem cell-based therapy: the history and cell source. a The timeline of major discoveries and advances in basic research and clinical applications of stem cell-based therapy. The term "stem ...
Stem cell-based therapies. Stem cell-based therapies are defined as any treatment for a disease or a medical condition that fundamentally involves the use of any type of viable human stem cells including embryonic stem cells (ESCs), iPSCs and adult stem cells for autologous and allogeneic therapies ().Stem cells offer the perfect solution when there is a need for tissue and organ ...
Stem-cell therapies articles from across Nature Portfolio. Atom; RSS Feed; Definition. Stem cell therapies are a type of cell therapy in which the cells used are either stem cells (as in the case ...
Dec. 7, 2023 — New research has found evidence that a novel stem cell treatment, using mRNA technology encapsulated into nanoparticles (LNP) that was successfully used to produce the COVID-19 ...
Stem cell therapy has continued to advance, bringing hope to cure diseases that were once considered incurable. The concepts underlying the use of stem cells in therapy depend on their inherent capacity for regenerating the original tissues of the body. ... There is renewed research effort to identify new, more effective treatment methods for ...
Stem cell therapies are being explored for the treatment of various diseases, and stem cell-derived exosomes may provide similar clinical benefits without the biosafety concerns. However, large ...
N Engl J Med 2023;389:1310-1319. Stem cells can renew themselves without differentiating. Aging, inflammation, and environmental exposures may drive phenotypic changes in tissue stem cells that ...
Stem Cell Research & Therapy is the major forum for translational research into stem cell therapies. An international peer-reviewed journal, it publishes high-quality open access research articles with a special emphasis on basic, translational and clinical research into stem cell therapeutics and regenerative therapies, including animal models and clinical trials.
Promising early results show that longstanding Harvard Stem Cell Institute (HSCI) research may have paved the way for a breakthrough treatment of Type 1 diabetes. Utilizing research from the Melton Lab, Vertex Pharmaceuticals has developed VX-880, an investigational stem cell-derived, fully differentiated pancreatic islet cell replacement therapy for people with type 1 diabetes (T1D). In ...
Now, in research published in the Cell Stem Cell, scientists have completed a first-in-human, early-stage clinical trial that involved injecting neural stem cells directly into the brains of 15 patients with secondary MS recruited from two hospitals in Italy. The trial was conducted by teams at the University of Cambridge, Milan Bicocca and the ...
Regenerative Medicine: A New Path for ALS Treatment. Nov 17, 2023 Cassie Tomlin. A first-of-its-kind stem cell therapy for ALS passes a critical safety benchmark, advancing the search to slow down, reverse and prevent the disease. In a parallel study, investigators are growing patient-derived stem cells to model ALS, hoping to uncover its ...
New research has found evidence that a novel stem cell treatment, using mRNA technology encapsulated into nanoparticles (LNP) that was successfully used to produce the COVID-19 vaccines, may boost ...
Browse open Calls for Papers beta. Read the latest articles of Current Stem Cell Research & Therapy at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Stem cells offer promise for new medical treatments. Learn about stem cell types, current and possible uses, and the state of research and practice. ... The embryos being used in embryonic stem cell research come from eggs that were fertilized at in vitro fertilization clinics but never implanted in women's uteruses. The stem cells are donated ...
Cell therapy, involving adult stem cells from bone marrow, has been shown to reduce the risk of heart attack and stroke in severe heart failure patients, according to a new study.
Recent advancements in stem cell technology open a new door for patients suffering from diseases and disorders that have yet to be treated. Stem cell-based therapy, including human pluripotent stem cells (hPSCs) and multipotent mesenchymal stem cells (MSCs), has recently emerged as a key player in regenerative medicine. hPSCs are defined as self-renewable cell types conferring the ability to ...
A cell therapy is a medicinal product containing cells, and is typically injected into a patient. ... Stem-cell therapies; Latest Research and Reviews. Autologous cell transplantation for ...
Stem cell therapy has continued to advance, bringing hope to cure diseases that were once considered incurable. The concepts underlying the use of stem cells in therapy depend on their inherent capacity for regenerating the original tissues of the body. Additionally, stem cells can be altered to provide powerful drugs or nanomaterials and have an ability to modulate the immune system. Moreover ...
Meagan Raeke. C: 267-693-6224. [email protected]. For Patients and the General Public: 1-800-789-7366. For Media Queries & Requests (24/7): 215-662-2560. Early Phase I clinical trial results from 6 patients with recurrent glioblastoma show that "dual-target" CAR T cell therapy shrinks brain tumors.
New study calls into question the superiority of stem cell therapy for treating knee pain. ScienceDaily . Retrieved March 21, 2024 from www.sciencedaily.com / releases / 2023 / 11 / 231102162631.htm
Stock image of a uterus and ovaries model. The California Institute for Regenerative Medicine (CIRM) has awarded a $5.3 million to Karen Aboody, MD of City of Hope for late-stage preclinical research to develop a neural stem cell mediated treatment for a chemo-resistant, metastatic ovarian cancer. Approximately 22,000 women are diagnosed with ovarian cancer—the most…
Type of disease: Stem-cell-based therapy is a new approach for the treatment of various diseases in different clinical trial studies. ... In the last 50 years, more than 40,000 research papers have focused on stem-cell-based therapies. In this review study, we present a general overview of the translation of stem cell therapy from scientific ...
One theory is that it all starts in the bone marrow, where a primitive reserve of stem cells replenishes the ranks of blood and immune cells to the tune of about 500 billion new cells every day.
A bone marrow transplant — a stem cell transplant — is a procedure that infuses healthy blood-forming stem cells into your body to regenerate the bone marrow's ability to produce blood cells. Stem cell transplants can pose risks, and some people can have serious complications. ... time and new options after CAR-T cell therapy for multiple ...
Stem-cell research articles from across Nature Portfolio. Stem-cell research is the area of research that studies the properties of stem cells and their potential use in medicine. As stem cells ...
The team, led by Marcela Maus and Bryan Choi recently published a study detailing the promising preliminary results of a first-in-human trial for the new therapy, which was designed to target two different sites on glioblastoma tumors. All three patients in the initial trial showed dramatic but transient reductions in tumor size in imaging ...
Kuah et al found that relative to placebo, stem cell therapy considerably relieved pain at 3, 6, and 12 months after treatment. This conclusion has been confirmed in our meta-analysis. We found that the VAS scores in the stem cell group were significantly reduced at each visit point. Inflammatory response is known as one of the causes of pain.
New hopes in cell therapies have steadily arisen with ongoing progress in stem cell research, and innumerable conditions could be now treated and cured, from musculoskeletal injuries to diabetes to cardiac failure, to cite but a few, with the appropriate stem cells available. Arguably, the ideal therapeutic stem cells are those that naturally heal, repair, and replenish the target tissue in ...
Stem cells articles from across Nature Portfolio. Atom. RSS Feed. Stem cells are cells that have the capacity to self-renew by dividing and to develop into more mature, specialised cells. Stem ...
For patients with certain types of cancer, CAR T cell therapy has been nothing short of life changing. Developed in part by Carl June, Richard W. Vague Professor at Penn Medicine, and approved by ...