How to Write a Bibliography for a Research Paper

Do not try to “wow” your instructor with a long bibliography when your instructor requests only a works cited page. It is tempting, after doing a lot of work to research a paper, to try to include summaries on each source as you write your paper so that your instructor appreciates how much work you did. That is a trap you want to avoid. MLA style, the one that is most commonly followed in high schools and university writing courses, dictates that you include only the works you actually cited in your paper—not all those that you used.

Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% off with 24start discount code, assembling bibliographies and works cited.
- If your assignment calls for a bibliography, list all the sources you consulted in your research.
- If your assignment calls for a works cited or references page, include only the sources you quote, summarize, paraphrase, or mention in your paper.
- If your works cited page includes a source that you did not cite in your paper, delete it.
- All in-text citations that you used at the end of quotations, summaries, and paraphrases to credit others for their ideas,words, and work must be accompanied by a cited reference in the bibliography or works cited. These references must include specific information about the source so that your readers can identify precisely where the information came from.The citation entries on a works cited page typically include the author’s name, the name of the article, the name of the publication, the name of the publisher (for books), where it was published (for books), and when it was published.
The good news is that you do not have to memorize all the many ways the works cited entries should be written. Numerous helpful style guides are available to show you the information that should be included, in what order it should appear, and how to format it. The format often differs according to the style guide you are using. The Modern Language Association (MLA) follows a particular style that is a bit different from APA (American Psychological Association) style, and both are somewhat different from the Chicago Manual of Style (CMS). Always ask your teacher which style you should use.
A bibliography usually appears at the end of a paper on its own separate page. All bibliography entries—books, periodicals, Web sites, and nontext sources such radio broadcasts—are listed together in alphabetical order. Books and articles are alphabetized by the author’s last name.
Most teachers suggest that you follow a standard style for listing different types of sources. If your teacher asks you to use a different form, however, follow his or her instructions. Take pride in your bibliography. It represents some of the most important work you’ve done for your research paper—and using proper form shows that you are a serious and careful researcher.
Bibliography Entry for a Book
A bibliography entry for a book begins with the author’s name, which is written in this order: last name, comma, first name, period. After the author’s name comes the title of the book. If you are handwriting your bibliography, underline each title. If you are working on a computer, put the book title in italicized type. Be sure to capitalize the words in the title correctly, exactly as they are written in the book itself. Following the title is the city where the book was published, followed by a colon, the name of the publisher, a comma, the date published, and a period. Here is an example:
Format : Author’s last name, first name. Book Title. Place of publication: publisher, date of publication.
- A book with one author : Hartz, Paula. Abortion: A Doctor’s Perspective, a Woman’s Dilemma . New York: Donald I. Fine, Inc., 1992.
- A book with two or more authors : Landis, Jean M. and Rita J. Simon. Intelligence: Nature or Nurture? New York: HarperCollins, 1998.
Bibliography Entry for a Periodical
A bibliography entry for a periodical differs slightly in form from a bibliography entry for a book. For a magazine article, start with the author’s last name first, followed by a comma, then the first name and a period. Next, write the title of the article in quotation marks, and include a period (or other closing punctuation) inside the closing quotation mark. The title of the magazine is next, underlined or in italic type, depending on whether you are handwriting or using a computer, followed by a period. The date and year, followed by a colon and the pages on which the article appeared, come last. Here is an example:
Format: Author’s last name, first name. “Title of the Article.” Magazine. Month and year of publication: page numbers.
- Article in a monthly magazine : Crowley, J.E.,T.E. Levitan and R.P. Quinn.“Seven Deadly Half-Truths About Women.” Psychology Today March 1978: 94–106.
- Article in a weekly magazine : Schwartz, Felice N.“Management,Women, and the New Facts of Life.” Newsweek 20 July 2006: 21–22.
- Signed newspaper article : Ferraro, Susan. “In-law and Order: Finding Relative Calm.” The Daily News 30 June 1998: 73.
- Unsigned newspaper article : “Beanie Babies May Be a Rotten Nest Egg.” Chicago Tribune 21 June 2004: 12.
Bibliography Entry for a Web Site
For sources such as Web sites include the information a reader needs to find the source or to know where and when you found it. Always begin with the last name of the author, broadcaster, person you interviewed, and so on. Here is an example of a bibliography for a Web site:
Format : Author.“Document Title.” Publication or Web site title. Date of publication. Date of access.
Example : Dodman, Dr. Nicholas. “Dog-Human Communication.” Pet Place . 10 November 2006. 23 January 2014 < http://www.petplace.com/dogs/dog-human-communication-2/page1.aspx >
After completing the bibliography you can breathe a huge sigh of relief and pat yourself on the back. You probably plan to turn in your work in printed or handwritten form, but you also may be making an oral presentation. However you plan to present your paper, do your best to show it in its best light. You’ve put a great deal of work and thought into this assignment, so you want your paper to look and sound its best. You’ve completed your research paper!
Back to How To Write A Research Paper .
ORDER HIGH QUALITY CUSTOM PAPER

- Try for free
How to Write a Bibliography (MLA, APA Examples)

Learn how to easily write a bibliography by following the format outlined in this article.
This resource will help your students properly cite different resources in the bibliography of a research paper, and how to format those citations, for books, encyclopedias, films, websites, and people.
What is a bibliography?
According to Infoplease.com, A bibliography is a list of the types of sources you used to get information for your report. It is included at the end of your report, on the last page (or last few pages).
What are the types of bibliography styles (MLA, APA, etc.)?
The 3 most common bibliography/citation styles are:
- MLA Style: The Modern Language Association works cited page style
- APA Style: The American Psychological Association style
- Chicago Style: The bibliography style defined by the Chicago Manual of Style
We’ll give examples of how to create bibliography entries in various styles further down in this article.
What sources do you put in a bibliography?
An annotated bibliography should include a reference list of any sources you use in writing a research paper. Any printed sources from which you use a text citation, including books, websites, newspaper articles, journal articles, academic writing, online sources (such as PDFs), and magazines should be included in a reference list. In some cases, you may need or want to cite conversations or interviews, works of art, visual works such as movies, television shows, or documentaries - these (and many others) can also be included in a reference list.
How to get started writing your bibliography
You will find it easier to prepare your MLA, APA, or Chicago annotated bibliography if you keep track of each book, encyclopedia, journal article, webpage or online source you use as you are reading and taking notes. Start a preliminary, or draft, bibliography by listing on a separate sheet of paper all your sources. Note down the full title, author’s last name, place of publication, web address, publisher, and date of publication for each source.
Haven't started your paper yet and need an outline? These sample essay outlines include a research paper outline from an actual student paper.
How to write a bibliography step-by-step (with examples)
General Format: Author (last name first). Title of the book. Publisher, Date of publication.
MLA Style: Sibley, David Allen. What It’s Like to Be a Bird. From Flying to Nesting, Eating to Singing, What Birds Are Doing, and Why. Alfred A. Knopf, 2020.
APA Style: Sibley, D.A. (2020). What It’s Like to Be a Bird. From Flying to Nesting, Eating to Singing, What Birds Are Doing, and Why . Alfred A. Knopf.
Notes: Use periods, not commas, to separate the data in the entry. Use a hanging indent if the entry is longer than one line. For APA style, do not use the full author’s first name.
Websites or webpages:
MLA Style: The SB Nation Family of Sites. Pension Plan Puppets: A Toronto Maple Leafs Blog, 2022, www.pensionplanpuppets.com. Accessed 15 Feb. 2022.
APA Style: American Heart Association. (2022, April 11). How to keep your dog’s heart healthy. https://www.heart.org/en/news/2022/04/11/how-to-keep-your-dogs-heart-healthy
Online news article from a newspaper site:
APA Style: Duehren, A. (2022, April 9). Janet Yellen faces challenge to keep pressure on Russia. Wall Street Journal. https://www.wsj.com/articles/janet-yellen-faces-challenge-to-keep-pressure-on-russia-while-addressing-global-consequences-11650366000
Print journal articles:
MLA Style: Booch, Grady. "Patterns in Object-Oriented Design." IEEE Software Engineering, vol. 6, no. 6, 2006, pp. 31-50.
APA Style: Booch, G. (2006). Patterns in object-oriented design. IEEE Software Engineering, 6(6), 31–50.
Note: It is suggested that you include a DOI and a webpage address when referencing either a printed journal article, and electronic journal article, or an journal article that appears in both formats.
MLA Style: Gamma, Eric, and Peter A. Coad. “Exceptions to the Unified Modeling Language in Python Patterns.” IEEE Software Engineering, vol. 2, no. 6, 8 Mar. 2006, pp. 190-194. O’Reilly Software Engineering Library, https://doi.org/10.1006/se.20061. Accessed 26 May 2009.
APA Style: Masters, H., Barron, J., & Chanda, L. (2017). Motivational interviewing techniques for adolescent populations in substance abuse counseling. NAADAC Notes, 7(8), 7–13. https://www.naadac.com/notes/adolescent-techniques
ML:A Style: @Grady_Booch. “That’s a bold leap over plain old battery power cars.” Twitter, 13 Mar. 2013, 12:06 p.m., https://twitter.com/Grady_Booch/status/1516379006727188483.
APA Style: Westborough Library [@WestboroughLib]. (2022, April 12). Calling all 3rd through 5th grade kids! Join us for the Epic Writing Showdown! Winner receives a prize! Space is limited so register, today. loom.ly/ypaTG9Q [Tweet; thumbnail link to article]. Twitter. https://twitter.com/WestboroughLib/status/1516373550415896588.
Print magazine articles:
General format: Author (last name first), "Article Title." Name of magazine. Volume number, (Date): page numbers.
MLA Style: Stiteler, Sharon. "Tracking Red-Breasted Grosbeak Migration." Minnesota Bird Journal, 7 Sept. 2019, pp. 7-11.
APA Style: Jordan, Jennifer, "Filming at the Top of the World." Museum of Science Magazine. Volume 47, No. 1, (Winter 1998): p. 11.
Print newspaper articles:
General format: Author (last name first), "Article Title." Name of newspaper, city, state of publication. (date): edition if available, section, page number(s).
MLA Style: Adelman, Martin. "Augustus Announces Departure from City Manager Post." New York Times, late ed., 15 February 2020, p. A1
APA Style: Adelman, M. (2020, February 15). Augustus announced departure from city manager post. New York Times, A1.
Encyclopedias:
General Format: Encyclopedia Title, Edition Date. Volume Number, "Article Title," page numbers.
MLA Style: “Gorillas.” The Encyclopedia Brittanica. 15th ed. 2010.
APA Style: Encyclopedia Brittanica, Inc. (1997.) Gorillas. In The Encyclopedia Brittanica (15th ed., pp. 50-51). Encyclopedia Brittanica, Inc.
Personal interviews:
General format: Full name (last name first). Personal Interview. (Occupation.) Date of interview.
MLA Style: Smithfield, Joseph. Personal interview. 19 May 2014.
APA Style: APA does not require a formal citation for a personal interview. Published interviews from other sources should be cited accordingly.
Films and movies:
General format: Title, Director, Distributor, Year.
MLA Style: Fury. Directed by David Ayer, performances by Brad Pitt, Shia LaBeouf, Jon Bernthal, Sony Pictures, 2014.
APA Style: Ayer, D. (Director). (2014). Fury [Film]. Sony Pictures.
Featured High School Resources

Related Resources

About the author

TeacherVision Editorial Staff
The TeacherVision editorial team is comprised of teachers, experts, and content professionals dedicated to bringing you the most accurate and relevant information in the teaching space.

- Grades 6-12
- School Leaders
FREE Poetry Worksheet Bundle! Perfect for National Poetry Month.
How To Write a Bibliography (Three Styles, Plus Examples)
Give credit where credit is due.

Writing a research paper involves a lot of work. Students need to consult a variety of sources to gather reliable information and ensure their points are well supported. Research papers include a bibliography, which can be a little tricky for students. Learn how to write a bibliography in multiple styles and find basic examples below.
IMPORTANT: Each style guide has its own very specific rules, and they often conflict with one another. Additionally, each type of reference material has many possible formats, depending on a variety of factors. The overviews shown here are meant to guide students in writing basic bibliographies, but this information is by no means complete. Students should always refer directly to the preferred style guide to ensure they’re using the most up-to-date formats and styles.
What is a bibliography?
When you’re researching a paper, you’ll likely consult a wide variety of sources. You may quote some of these directly in your work, summarize some of the points they make, or simply use them to further the knowledge you need to write your paper. Since these ideas are not your own, it’s vital to give credit to the authors who originally wrote them. This list of sources, organized alphabetically, is called a bibliography.
A bibliography should include all the materials you consulted in your research, even if you don’t quote directly from them in your paper. These resources could include (but aren’t limited to):
- Books and e-books
- Periodicals like magazines or newspapers
- Online articles or websites
- Primary source documents like letters or official records
Bibliography vs. References
These two terms are sometimes used interchangeably, but they actually have different meanings. As noted above, a bibliography includes all the materials you used while researching your paper, whether or not you quote from them or refer to them directly in your writing.
A list of references only includes the materials you cite throughout your work. You might use direct quotes or summarize the information for the reader. Either way, you must ensure you give credit to the original author or document. This section can be titled “List of Works Cited” or simply “References.”
Your teacher may specify whether you should include a bibliography or a reference list. If they don’t, consider choosing a bibliography, to show all the works you used in researching your paper. This can help the reader see that your points are well supported, and allow them to do further reading on their own if they’re interested.
Bibliography vs. Citations
Citations refer to direct quotations from a text, woven into your own writing. There are a variety of ways to write citations, including footnotes and endnotes. These are generally shorter than the entries in a reference list or bibliography. Learn more about writing citations here.
What does a bibliography entry include?
Depending on the reference material, bibliography entries include a variety of information intended to help a reader locate the material if they want to refer to it themselves. These entries are listed in alphabetical order, and may include:
- Author/s or creator/s
- Publication date
- Volume and issue numbers
- Publisher and publication city
- Website URL
These entries don’t generally need to include specific page numbers or locations within the work (except for print magazine or journal articles). That type of information is usually only needed in a footnote or endnote citation.
What are the different bibliography styles?
In most cases, writers use one of three major style guides: APA (American Psychological Association), MLA (Modern Language Association), or The Chicago Manual of Style . There are many others as well, but these three are the most common choices for K–12 students.
Many teachers will state their preference for one style guide over another. If they don’t, you can choose your own preferred style. However, you should also use that guide for your entire paper, following their recommendations for punctuation, grammar, and more. This will ensure you are consistent throughout.
Below, you’ll learn how to write a simple bibliography using each of the three major style guides. We’ve included details for books and e-books, periodicals, and electronic sources like websites and videos. If the reference material type you need to include isn’t shown here, refer directly to the style guide you’re using.
APA Style Bibliography and Examples
Source: Verywell Mind
Technically, APA style calls for a list of references instead of a bibliography. If your teacher requires you to use the APA style guide , you can limit your reference list only to items you cite throughout your work.
How To Write a Bibliography (References) Using APA Style
Here are some general notes on writing an APA reference list:
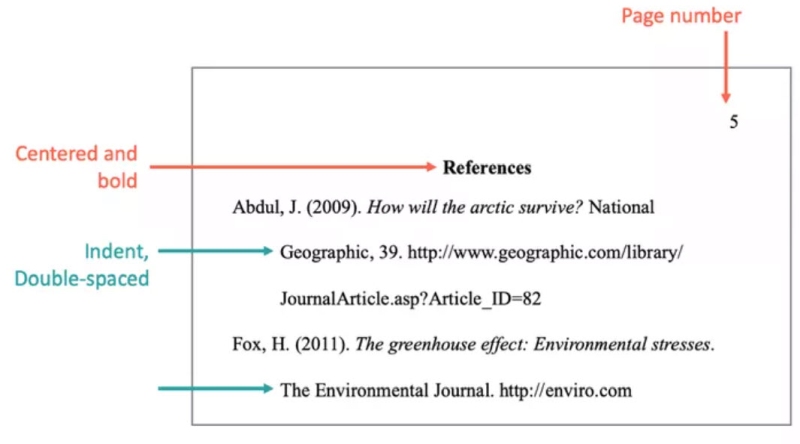
- Title your bibliography section “References” and center the title on the top line of the page.
- Do not center your references; they should be left-aligned. For longer items, subsequent lines should use a hanging indent of 1/2 inch.
- Include all types of resources in the same list.
- Alphabetize your list by author or creator, last name first.
- Do not spell out the author/creator’s first or middle name; only use their initials.
- If there are multiple authors/creators, use an ampersand (&) before the final author/creator.
- Place the date in parentheses.
- Capitalize only the first word of the title and subtitle, unless the word would otherwise be capitalized (proper names, etc.).
- Italicize the titles of books, periodicals, or videos.
- For websites, include the full site information, including the http:// or https:// at the beginning.
Books and E-Books APA Bibliography Examples
For books, APA reference list entries use this format (only include the publisher’s website for e-books).
Last Name, First Initial. Middle Initial. (Publication date). Title with only first word capitalized . Publisher. Publisher’s website
- Wynn, S. (2020). City of London at war 1939–45 . Pen & Sword Military. https://www.pen-and-sword.co.uk/City-of-London-at-War-193945-Paperback/p/17299
Periodical APA Bibliography Examples
For journal or magazine articles, use this format. If you viewed the article online, include the URL at the end of the citation.
Last Name, First Initial. Middle Initial. (Publication date). Title of article. Magazine or Journal Title (Volume number) Issue number, page numbers. URL
- Bell, A. (2009). Landscapes of fear: Wartime London, 1939–1945. Journal of British Studies (48) 1, 153–175. https://www.jstor.org/stable/25482966
Here’s the format for newspapers. For print editions, include the page number/s. For online articles, include the full URL.
Last Name, First Initial. Middle Initial. (Year, Month Date) Title of article. Newspaper title. Page number/s. URL
- Blakemore, E. (2022, November 12) Researchers track down two copies of fossil destroyed by the Nazis. The Washington Post. https://www.washingtonpost.com/science/2022/11/12/ichthyosaur-fossil-images-discovered/
Electronic APA Bibliography Examples
For articles with a specific author on a website, use this format.
Last Name, First Initial. Middle Initial. (Year, Month Date). Title . Site name. URL
- Wukovits, J. (2023, January 30). A World War II survivor recalls the London Blitz . British Heritage . https://britishheritage.com/history/world-war-ii-survivor-london-blitz
When an online article doesn’t include a specific author or date, list it like this:
Title . (Year, Month Date). Site name. Retrieved Month Date, Year, from URL
- Growing up in the Second World War . (n.d.). Imperial War Museums. Retrieved May 12, 2023, from https://www.iwm.org.uk/history/growing-up-in-the-second-world-war
When you need to list a YouTube video, use the name of the account that uploaded the video, and format it like this:
Name of Account. (Upload year, month day). Title [Video]. YouTube. URL
- War Stories. (2023, January 15). How did London survive the Blitz during WW2? | Cities at war: London | War stories [Video]. YouTube. https://youtu.be/uwY6JlCvbxc
For more information on writing APA bibliographies, see the APA Style Guide website.
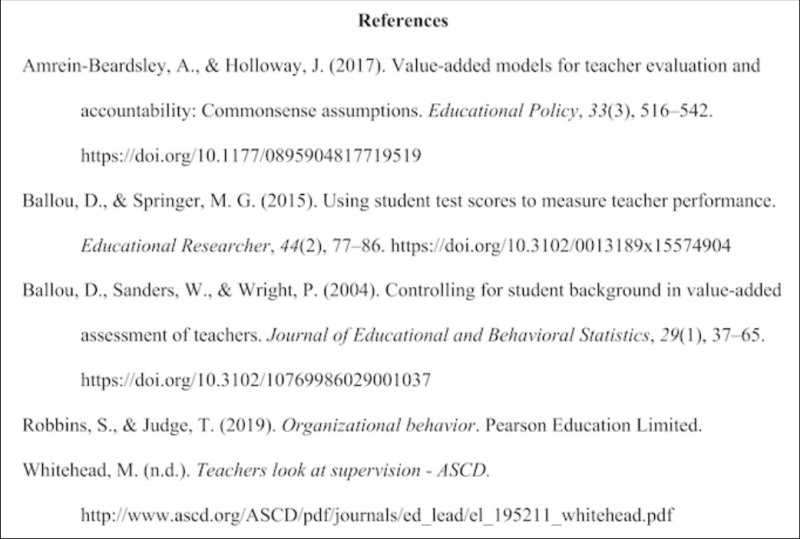
APA Bibliography (Reference List) Example Pages
Source: Simply Psychology
More APA example pages:
- Western Australia Library Services APA References Example Page
- Ancilla College APA References Page Example
- Scribbr APA References Page Example
MLA Style Bibliography Examples

Source: PressBooks
MLA style calls for a Works Cited section, which includes all materials quoted or referred to in your paper. You may also include a Works Consulted section, including other reference sources you reviewed but didn’t directly cite. Together, these constitute a bibliography. If your teacher requests an MLA Style Guide bibliography, ask if you should include Works Consulted as well as Works Cited.
How To Write a Bibliography (Works Cited and Works Consulted) in MLA Style
For both MLA Works Cited and Works Consulted sections, use these general guidelines:
- Start your Works Cited list on a new page. If you include a Works Consulted list, start that on its own new page after the Works Cited section.
- Center the title (Works Cited or Works Consulted) in the middle of the line at the top of the page.
- Align the start of each source to the left margin, and use a hanging indent (1/2 inch) for the following lines of each source.
- Alphabetize your sources using the first word of the citation, usually the author’s last name.
- Include the author’s full name as listed, last name first.
- Capitalize titles using the standard MLA format.
- Leave off the http:// or https:// at the beginning of a URL.
Books and E-Books MLA Bibliography Examples
For books, MLA reference list entries use this format. Add the URL at the end for e-books.
Last Name, First Name Middle Name. Title . Publisher, Date. URL
- Wynn, Stephen. City of London at War 1939–45 . Pen & Sword Military, 2020. www.pen-and-sword.co.uk/City-of-London-at-War-193945-Paperback/p/17299
Periodical MLA Bibliography Examples
Here’s the style format for magazines, journals, and newspapers. For online articles, add the URL at the end of the listing.
For magazines and journals:
Last Name, First Name. “Title: Subtitle.” Name of Journal , volume number, issue number, Date of Publication, First Page Number–Last Page Number.
- Bell, Amy. “Landscapes of Fear: Wartime London, 1939–1945.” Journal of British Studies , vol. 48, no. 1, pp. 153–175. www.jstor.org/stable/25482966
When citing newspapers, include the page number/s for print editions or the URL for online articles.
Last Name, First Name. “Title of article.” Newspaper title. Page number/s. Year, month day. Page number or URL
- Blakemore, Erin. “Researchers Track Down Two Copies of Fossil Destroyed by the Nazis.” The Washington Post. 2022, Nov. 12. www.washingtonpost.com/science/2022/11/12/ichthyosaur-fossil-images-discovered/
Electronic MLA Bibliography Examples
Last Name, First Name. Year. “Title.” Month Day, Year published. URL
- Wukovits, John. 2023. “A World War II Survivor Recalls the London Blitz.” January 30, 2023. https://britishheritage.com/history/world-war-ii-survivor-london-blitz
Website. n.d. “Title.” Accessed Day Month Year. URL.
- Imperial War Museum. n.d. “Growing Up in the Second World War.” Accessed May 9, 2023. https://www.iwm.org.uk/history/growing-up-in-the-second-world-war.
Here’s how to list YouTube and other online videos.
Creator, if available. “Title of Video.” Website. Uploaded by Username, Day Month Year. URL.
- “How did London survive the Blitz during WW2? | Cities at war: London | War stories.” YouTube . Uploaded by War Stories, 15 Jan. 2023. youtu.be/uwY6JlCvbxc.
For more information on writing MLA style bibliographies, see the MLA Style website.
MLA Bibliography (Works Cited) Example Pages

Source: The Visual Communication Guy
More MLA example pages:
- Writing Commons Sample Works Cited Page
- Scribbr MLA Works Cited Sample Page
- Montana State University MLA Works Cited Page
Chicago Manual of Style Bibliography Examples
The Chicago Manual of Style (sometimes called “Turabian”) actually has two options for citing reference material : Notes and Bibliography and Author-Date. Regardless of which you use, you’ll need a complete detailed list of reference items at the end of your paper. The examples below demonstrate how to write that list.
How To Write a Bibliography Using The Chicago Manual of Style

Source: South Texas College
Here are some general notes on writing a Chicago -style bibliography:
- You may title it “Bibliography” or “References.” Center this title at the top of the page and add two blank lines before the first entry.
- Left-align each entry, with a hanging half-inch indent for subsequent lines of each entry.
- Single-space each entry, with a blank line between entries.
- Include the “http://” or “https://” at the beginning of URLs.
Books and E-Books Chicago Manual of Style Bibliography Examples
For books, Chicago -style reference list entries use this format. (For print books, leave off the information about how the book was accessed.)
Last Name, First Name Middle Name. Title . City of Publication: Publisher, Date. How e-book was accessed.
- Wynn, Stephen. City of London at War 1939–45 . Yorkshire: Pen & Sword Military, 2020. Kindle edition.
Periodical Chicago Manual of Style Bibliography Examples
For journal and magazine articles, use this format.
Last Name, First Name. Year of Publication. “Title: Subtitle.” Name of Journal , Volume Number, issue number, First Page Number–Last Page Number. URL.
- Bell, Amy. 2009. “Landscapes of Fear: Wartime London, 1939–1945.” Journal of British Studies, 48 no. 1, 153–175. https://www.jstor.org/stable/25482966.
When citing newspapers, include the URL for online articles.
Last Name, First Name. Year of Publication. “Title: Subtitle.” Name of Newspaper , Month day, year. URL.
- Blakemore, Erin. 2022. “Researchers Track Down Two Copies of Fossil Destroyed by the Nazis.” The Washington Post , November 12, 2022. https://www.washingtonpost.com/science/2022/11/12/ichthyosaur-fossil-images-discovered/.
Electronic Chicago Manual of Style Bibliography Examples
Last Name, First Name Middle Name. “Title.” Site Name . Year, Month Day. URL.
- Wukovits, John. “A World War II Survivor Recalls the London Blitz.” British Heritage. 2023, Jan. 30. britishheritage.com/history/world-war-ii-survivor-london-blitz.
“Title.” Site Name . URL. Accessed Day Month Year.
- “Growing Up in the Second World War.” Imperial War Museums . www.iwm.org.uk/history/growing-up-in-the-second-world-war. Accessed May 9, 2023.
Creator or Username. “Title of Video.” Website video, length. Month Day, Year. URL.
- War Stories. “How Did London Survive the Blitz During WW2? | Cities at War: London | War Stories.” YouTube video, 51:25. January 15, 2023. https://youtu.be/uwY6JlCvbxc.
For more information on writing Chicago -style bibliographies, see the Chicago Manual of Style website.
Chicago Manual of Style Bibliography Example Pages

Source: Chicago Manual of Style
More Chicago example pages:
- Scribbr Chicago Style Bibliography Example
- Purdue Online Writing Lab CMOS Bibliography Page
- Bibcitation Sample Chicago Bibliography
Now that you know how to write a bibliography, take a look at the Best Websites for Teaching & Learning Writing .
Plus, get all the latest teaching tips and ideas when you sign up for our free newsletters .

You Might Also Like

Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Referencing
A Quick Guide to Harvard Referencing | Citation Examples
Published on 14 February 2020 by Jack Caulfield . Revised on 15 September 2023.
Referencing is an important part of academic writing. It tells your readers what sources you’ve used and how to find them.
Harvard is the most common referencing style used in UK universities. In Harvard style, the author and year are cited in-text, and full details of the source are given in a reference list .
Harvard Reference Generator
Instantly correct all language mistakes in your text
Be assured that you'll submit flawless writing. Upload your document to correct all your mistakes.

Table of contents
Harvard in-text citation, creating a harvard reference list, harvard referencing examples, referencing sources with no author or date, frequently asked questions about harvard referencing.
A Harvard in-text citation appears in brackets beside any quotation or paraphrase of a source. It gives the last name of the author(s) and the year of publication, as well as a page number or range locating the passage referenced, if applicable:
Note that ‘p.’ is used for a single page, ‘pp.’ for multiple pages (e.g. ‘pp. 1–5’).
An in-text citation usually appears immediately after the quotation or paraphrase in question. It may also appear at the end of the relevant sentence, as long as it’s clear what it refers to.
When your sentence already mentions the name of the author, it should not be repeated in the citation:
Sources with multiple authors
When you cite a source with up to three authors, cite all authors’ names. For four or more authors, list only the first name, followed by ‘ et al. ’:
Sources with no page numbers
Some sources, such as websites , often don’t have page numbers. If the source is a short text, you can simply leave out the page number. With longer sources, you can use an alternate locator such as a subheading or paragraph number if you need to specify where to find the quote:
Multiple citations at the same point
When you need multiple citations to appear at the same point in your text – for example, when you refer to several sources with one phrase – you can present them in the same set of brackets, separated by semicolons. List them in order of publication date:
Multiple sources with the same author and date
If you cite multiple sources by the same author which were published in the same year, it’s important to distinguish between them in your citations. To do this, insert an ‘a’ after the year in the first one you reference, a ‘b’ in the second, and so on:
Prevent plagiarism, run a free check.
A bibliography or reference list appears at the end of your text. It lists all your sources in alphabetical order by the author’s last name, giving complete information so that the reader can look them up if necessary.
The reference entry starts with the author’s last name followed by initial(s). Only the first word of the title is capitalised (as well as any proper nouns).

Sources with multiple authors in the reference list
As with in-text citations, up to three authors should be listed; when there are four or more, list only the first author followed by ‘ et al. ’:
Reference list entries vary according to source type, since different information is relevant for different sources. Formats and examples for the most commonly used source types are given below.
- Entire book
- Book chapter
- Translated book
- Edition of a book
Journal articles
- Print journal
- Online-only journal with DOI
- Online-only journal with no DOI
- General web page
- Online article or blog
- Social media post
Sometimes you won’t have all the information you need for a reference. This section covers what to do when a source lacks a publication date or named author.
No publication date
When a source doesn’t have a clear publication date – for example, a constantly updated reference source like Wikipedia or an obscure historical document which can’t be accurately dated – you can replace it with the words ‘no date’:
Note that when you do this with an online source, you should still include an access date, as in the example.
When a source lacks a clearly identified author, there’s often an appropriate corporate source – the organisation responsible for the source – whom you can credit as author instead, as in the Google and Wikipedia examples above.
When that’s not the case, you can just replace it with the title of the source in both the in-text citation and the reference list:
Harvard referencing uses an author–date system. Sources are cited by the author’s last name and the publication year in brackets. Each Harvard in-text citation corresponds to an entry in the alphabetised reference list at the end of the paper.
Vancouver referencing uses a numerical system. Sources are cited by a number in parentheses or superscript. Each number corresponds to a full reference at the end of the paper.
A Harvard in-text citation should appear in brackets every time you quote, paraphrase, or refer to information from a source.
The citation can appear immediately after the quotation or paraphrase, or at the end of the sentence. If you’re quoting, place the citation outside of the quotation marks but before any other punctuation like a comma or full stop.
In Harvard referencing, up to three author names are included in an in-text citation or reference list entry. When there are four or more authors, include only the first, followed by ‘ et al. ’
Though the terms are sometimes used interchangeably, there is a difference in meaning:
- A reference list only includes sources cited in the text – every entry corresponds to an in-text citation .
- A bibliography also includes other sources which were consulted during the research but not cited.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Caulfield, J. (2023, September 15). A Quick Guide to Harvard Referencing | Citation Examples. Scribbr. Retrieved 9 April 2024, from https://www.scribbr.co.uk/referencing/harvard-style/
Is this article helpful?

Jack Caulfield
Other students also liked, harvard in-text citation | a complete guide & examples, harvard style bibliography | format & examples, referencing books in harvard style | templates & examples, scribbr apa citation checker.
An innovative new tool that checks your APA citations with AI software. Say goodbye to inaccurate citations!

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
31 Bibliography
Annotated bibliography.
A bibliography is an alphabetized list of sources showing the author, date, and publication information for each source.
An annotation is like a note; it’s a brief paragraph that explains what the writer learned from the source.
Annotated bibliographies combine bibliographies and brief notes about the sources.
Writers often create annotated bibliographies as a part of a research project, as a means of recording their thoughts and deciding which sources to actually use to support the purpose of their research. Some writers include annotated bibliographies at the end of a research paper as a way of offering their insights about the source’s usability to their readers.
Instructors in college often assign annotated bibliographies as a means of helping students think through their source’s quality and appropriateness to their research question or topic. (23)
Formatting the Annotated Bibliography
The citations (bibliographic information – title, date, author, publisher, etc.) in the annotated bibliography are formatted using the particular style manual (APA, MLA, Chicago, etc.) that your discipline requires.
Annotations are written in paragraph form, usually 3-7 sentences (or 80-200 words). Depending on your assignment your annotations will generally include the following:
- Summary: Summarize the information given in the source. Note the intended audience. What are the main arguments? What is the point of this book or article? What topics are covered? If someone asked what this article/book is about, what would you say?
- Evaluate/Assess: Is this source credible? Who wrote it? What are their credentials? Who is the publisher? Is it a useful source? How does it compare with other sources in your bibliography? Is the information reliable? Is this source biased or objective? What is the goal of this source?
- Reflect/React: Once you’ve summarized and assessed a source, you need to ask how it fits into your research. State your reaction and any additional questions you have about the information in your source. Was this source helpful to you? How does it help you shape your argument? How can you use this source in your research project? Has it changed how you think about your topic? Compare each source to other sources in your annotated bibliography in terms of its usefulness and thoroughness in helping answer your research question. (24)
Annotated Bibliography Examples
In the following examples, the bold font indicates the reflection component of the annotation that is sometimes required in an assignment.
APA style 6 th edition for the journal citation:
Waite, L. J., Goldschneider, F. K., & Witsberger, C. (1986). Nonfamily living and the erosion of traditional family orientations among young adults. American Sociological Review , 51, 541-554.
The authors, researchers at the Rand Corporation and Brown University, use data from the National Longitudinal Surveys of Young Women and Young Men to test their hypothesis that nonfamily living by young adults alters their attitudes, values, plans, and expectations, moving them away from their belief in traditional sex roles. They find their hypothesis strongly supported in young females, while the effects were fewer in studies of young males. Increasing the time away from parents before marrying increased individualism, self-sufficiency, and changes in attitudes about families. In contrast, an earlier study by Williams cited below shows no significant gender differences in sex role attitudes as a result of nonfamily living. (25)
MLA 8 style for a website citation:
Anderson, L.V. “Can You Libel Someone on Twitter?” Slate.com, The Slate Group, A Graham Holdings. Company, 26 Nov. 2012, http://www.slate.com/articles/technology/explainer/2012/11/libel_on_twitter_you_can_be_sued_for_libel_for_what_you_write_on_facebook.html . Accessed 2 Apr. 2018.
This article provides an overview of defamation law in the United States compared to the United Kingdom, in layman’s terms. It also explains how defamation law applies to social media platforms and individuals who use social media. Libelous comments posted on social media can be subject to lawsuit, depending on the content of the statement, and whether the person is a public or private figure. The article is found on the website, Slate.com, which is a web-based daily magazine that focuses on general interest topics. While the writer’s credentials are unavailable, she does thank Sandra S. Baron, Executive Director of the Media Law Resource Center and Jeff Hermes, director of the Digital Media Law Project for providing information. She also links to the United States laws that she cites. I would use the article to compare United States law to United Kingdom law and for background information. (1)
Information creation is a process. Scholars produce information in the forms of peer-reviewed journal articles, books, and conference presentations, to name a few. As a student researcher, you will be expected to create research projects such as essays, reports, visual presentations, and annotated bibliographies. Most scholarly writing makes an argument—whether it is to persuade your readers that your claim is true or to act on it. In order to create a sound argument, you must gather sources that will argue and counter-argue your claims.
When creating an argument, the researcher typically organizes their report or presentation with the claim/thesis at the beginning, which answers their research question. Then they provide reasons and supporting evidence to validate their claim. They acknowledge and respond to counter-arguments by citing sources that disagree with them, and refuting or conceding those counter-claims. Their conclusion restates their thesis and discusses why their research is important to the scholarly conversation, as well as potential areas for further research.
A Roman numeral outline is one way to organize your argument before you begin writing. It helps to identify sources for each section of your outline, so you know if you need further research to support your argument.
An annotated bibliography is one way to present research, and can be used as a cumulative assignment, or a precursor to your actual research paper. A good annotated bibliography will provide a variety of sources that met all your research needs—background, evidence, argument, and method. In other words, you should be able to take your annotated bibliography and write a complete research report based on those sources. (1)
Introduction to College Research Copyright © by Lumen Learning is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book

The Quintessence of Basic and Clinical Research and Scientific Publishing pp 657–665 Cite as
How to Create a Bibliography
- Rohan Reddy 4 ,
- Samuel Sorkhi 4 ,
- Saager Chawla 4 &
- Mahadevan Raj Rajasekaran 5
- First Online: 01 October 2023
618 Accesses
This chapter describes the fundamental principles and practices of referencing sources in scientific writing and publishing. Understanding plagiarism and improper referencing of the source material is paramount to producing original work that contains an authentic voice. Citing references helps authors to avoid plagiarism, give credit to the original author, and allow potential readers to refer to the legitimate sources and learn more information. Furthermore, quality references serve as an invaluable resource that can enlighten future research in a field. This chapter outlines fundamental aspects of referencing as well as how these sources are formatted as per recommended citation styles. Appropriate referencing is an important tool that can be utilized to develop the credibility of the author and the arguments presented. Additionally, online software can be useful in helping the author organize their sources and promote proper collaboration in scientific writing.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
AWELU (2022) The functions of references. Lund University. https://www.awelu.lu.se/referencing/the-functions-of-references/ . Accessed 28 Dec 2022
Masic I (2013) The importance of proper citation of references in biomedical articles. Acta Inform Med 21(3):148–155
Article PubMed PubMed Central Google Scholar
Neville C (2012) Referencing: principles, practice and problems. RGUHS J Pharm Sci 2(2):1–8
Google Scholar
Horkoff T (2015) Writing for success 1st Canadian edition: BCcampus. Chapter 7. Sources: choosing the right ones. https://opentextbc.ca/writingforsuccess/chapter/chapter-7-sources-choosing-the-right-ones/ . Accessed 28 Dec 2022
A guide to database and catalog searching: bibliographic elements. Northwestern State University. https://libguides.nsula.edu/howtosearch/bibelements . Updated 5 Aug 2021; Accessed 28 Dec 2022
Research process: step 7: citing and keeping track of sources. University of Rio Grande. https://libguides.rio.edu/c.php?g=620382&p=4320145 . Updated 12 Dec 2022; Accessed 28 Dec 2022
Pears R, Shields G (2022) Cite them right. Bloomsbury Publishing
Harvard system (1999) Bournemouth University. http://ibse.hk/Harvard_System.pdf . Accessed 28 Dec 2022
Gitanjali B (2004) Reference styles and common problems with referencing: Medknow. https://www.jpgmonline.com/documents/author/24/11_Gitanjali_3.pdf . Accessed 28 Dec 2022
Williams K, Spiro J, Swarbrick N (2008) How to reference Harvard referencing for Westminster Institute students. Westminster Institute of Education Oxford Brookes University
Harvard: reference list and bibliography: University of Birmingham. 2022. https://intranet.birmingham.ac.uk/as/libraryservices/library/referencing/icite/harvard/referencelist.aspx . Accessed 28 Dec 2022
Limited SN (2017) Responsible referencing. Nat Methods 14(3):209
Article Google Scholar
Hensley MK (2011) Citation management software: features and futures. Ref User Serv Q 50(3):204–208
Ramesh A, Rajasekaran MR (2014) Zotero: open-source bibliography management software. RGUHS J Pharm Sci 4:3–6
Shapland M (2000) Evaluation of reference management software on NT (comparing Papyrus with Procite, Reference Manager, Endnote, Citation, GetARef, Biblioscape, Library Master, Bibliographica, Scribe, Refs). University of Bristol
EndNote 20. Clarivate. 2022. https://endnote.com/product-details . Accessed 28 Dec 2022
Angélil-Carter S (2014) Stolen language? Plagiarism in writing. Routledge
Book Google Scholar
Howard RM (1995) Plagiarisms, authorships, and the academic death penalty. Coll Engl 57(7):788–806
Barrett R, Malcolm J (2006) Embedding plagiarism education in the assessment process. Int J Educ Integr 2(1):38–45
Introna L, Hayes N, Blair L, Wood E (2003) Cultural attitudes towards plagiarism. University of Lancaster, Lancaster, pp 1–57
Neville C (2016) The complete guide to referencing and avoiding plagiarism. McGraw-Hill Education (UK), London, p 43
Campion EW, Anderson KR, Drazen JM (2001) Internet-only publication. N Engl J Med 345(5):365
Li X, Crane N (1996) Electronic styles: a handbook for citing electronic information. Information Today, Inc.
APA (2005) Concise rules of APA style. APA, Washington, DC
Snyder PJ, Peterson A (2002) The referencing of internet web sites in medical and scientific publications. Brain Cogn 50(2):335–337
Article PubMed Google Scholar
Barroga EF (2014) Reference accuracy: authors’, reviewers’, editors’, and publishers’ contributions. J Korean Med Sci 29(12):1587–1589
Standardization IOf (1997) Information and documentation—bibliographic references—part 2: electronic documents or parts thereof, ISO 690-2: 1997. ISO
Download references
Conflict of Interest
None declared.
Author information
Authors and affiliations.
Department of Urology, University of California at San Diego, San Diego, CA, USA
Rohan Reddy, Samuel Sorkhi & Saager Chawla
Department of Urology, University of California and VA San Diego Healthcare System, San Diego, CA, USA
Mahadevan Raj Rajasekaran
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mahadevan Raj Rajasekaran .
Editor information
Editors and affiliations.
Retired Senior Expert Pharmacologist at the Office of Cardiology, Hematology, Endocrinology, and Nephrology, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, MD, USA
Gowraganahalli Jagadeesh
Professor & Director, Research Training and Publications, The Office of Research and Development, Periyar Maniammai Institute of Science & Technology (Deemed to be University), Vallam, Tamil Nadu, India
Pitchai Balakumar
Division Cardiology & Nephrology, Office of Cardiology, Hematology, Endocrinology and Nephrology, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, MD, USA
Fortunato Senatore
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter.
Reddy, R., Sorkhi, S., Chawla, S., Rajasekaran, M.R. (2023). How to Create a Bibliography. In: Jagadeesh, G., Balakumar, P., Senatore, F. (eds) The Quintessence of Basic and Clinical Research and Scientific Publishing. Springer, Singapore. https://doi.org/10.1007/978-981-99-1284-1_39
Download citation
DOI : https://doi.org/10.1007/978-981-99-1284-1_39
Published : 01 October 2023
Publisher Name : Springer, Singapore
Print ISBN : 978-981-99-1283-4
Online ISBN : 978-981-99-1284-1
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Utility Menu
fa3d988da6f218669ec27d6b6019a0cd
A publication of the harvard college writing program.
Harvard Guide to Using Sources
- The Honor Code
- Sample Bibliography
Below you’ll find a Bibliography adapted from a research paper written by Aishani Aatresh for her Technology, Environment, and Society course.
- Citation Management Tools
- In-Text Citations
- Bibliography
- Examples of Commonly Cited Sources
- Frequently Asked Questions about Citing Sources in Chicago Format
PDFs for This Section
- Citing Sources
- Online Library and Citation Tools
APA Citation Style
Citation examples.
- Paper Format
- Style and Grammar Guidelines
- Citation Management Tools
- What's New in the 7th Edition?
- APA Style References Guidelines from the American Psychological Association
- APA Style (OWL - Online Writing Lab, Purdue University)
- Common Reference Examples Handout
- Journal Article
- Magazine Article
- Newspaper Article
- Edited Book Chapter
- Dictionary Entry
- Government Report
- YouTube Video
- Facebook Post
- Webpage on a Website
- Supplemental Reference Examples
- Archival Documents and Collections
Parenthetical citations: (Grady et al., 2019; Jerrentrup et al., 2018)
Narrative citations: Grady et al. (2019) and Jerrentrup et al. (2018)
- If a journal article has a DOI, include the DOI in the reference.
- If the journal article does not have a DOI and is from an academic research database, end the reference after the page range (for an explanation of why, see the database information page). The reference in this case is the same as for a print journal article.
- Do not include database information in the reference unless the journal article comes from a database that publishes original, proprietary content, such as UpToDate (see an example on the database information page).
- If the journal article does not have a DOI but does have a URL that will resolve for readers (e.g., it is from an online journal that is not part of a database), include the URL of the article at the end of the reference.
- If the journal article has an article number instead of a page range, include the article number instead of the page range (as shown in the Jerrentrup et al. example).
Parenthetical citations: (Rabinowitz, 2019; Sapolsky, 2017)
Narrative citations: Rabinowitz (2019) and Sapolsky (2017)
- If the book includes a DOI, include the DOI in the reference after the publisher name.
- Do not include the publisher location.
- If the book does not have a DOI and comes from an academic research database, end the book reference after the publisher name. Do not include database information in the reference. The reference in this case is the same as for a print book.
Parenthetical citations: (Schaefer & Shapiro, 2019; Schulman, 2019)
Narrative citations: Schaefer and Shapiro (2019) and Schulman (2019)
- If a magazine article has a DOI, include the DOI in the reference.
- If the magazine article does not have a DOI and is from an academic research database, end the reference after the page range. Do not include database information in the reference. The reference in this case is the same as for a print magazine article.
- If the magazine article does not have a DOI but does have a URL that will resolve for readers (e.g., it is from an online magazine that is not part of a database), include the URL of the article at the end of the reference.
- If the magazine article does not have volume, issue, and/or page numbers (e.g., because it is from an online magazine), omit the missing elements from the reference (as in the Schulman example).
Parenthetical citation: (Carey, 2019)
Narrative citation: Carey (2019)
- If the newspaper article is from an academic research database, end the reference after the page range. Do not include database information in the reference. The reference in this case is the same as for a print newspaper article.
- If the newspaper article has a URL that will resolve for readers (e.g., it is from an online newspaper), include the URL of the article at the end of the reference.
- If the newspaper article does not have volume, issue, and/or page numbers (e.g., because it is from an online newspaper), omit the missing elements from the reference, as shown in the example.
- If the article is from a news website (e.g., CNN, HuffPost)—one that does not have an associated daily or weekly newspaper—use the format for a webpage on a website instead.
Parenthetical citation: (Aron et al., 2019)
Narrative citation: Aron et al. (2019)
- If the edited book chapter includes a DOI, include the chapter DOI in the reference after the publisher name.
- If the edited book chapter does not have a DOI and comes from an academic research database, end the edited book chapter reference after the publisher name. Do not include database information in the reference. The reference in this case is the same as for a print edited book chapter.
- Do not create references for chapters of authored books. Instead, write a reference for the whole book and cite the chapter in the text if desired (e.g., Kumar, 2017, Chapter 2).
Parenthetical citation: (Merriam-Webster, n.d.)
Narrative citation: Merriam-Webster (n.d.)
- Because entries in Merriam-Webster’s Dictionary are updated over time and are not archived, include a retrieval date in the reference.
- Merriam-Webster is both the author and the publisher, so the name appears in the author element only to avoid repetition.
- To quote a dictionary definition, view the pages on quotations and how to quote works without page numbers for guidance. Additionally, here is an example: Culture refers to the “customary beliefs, social forms, and material traits of a racial, religious, or social group” (Merriam-Webster, n.d., Definition 1a).
Parenthetical citation: (National Cancer Institute, 2019)
Narrative citation: National Cancer Institute (2019)
The specific agency responsible for the report appears as the author. The names of parent agencies not present in the group author name appear in the source element as the publisher. This creates concise in-text citations and complete reference list entries.
Parenthetical citation: (Harvard University, 2019)
Narrative citation: Harvard University (2019)
- Use the name of the account that uploaded the video as the author.
- If the account did not actually create the work, explain this in the text if it is important for readers to know. However, if that would mean citing a source that appears unauthoritative, you might also look for the author’s YouTube channel, official website, or other social media to see whether the same video is available elsewhere.
Parenthetical citations: (APA Databases, 2019; Gates, 2019)
Narrative citations: APA Databases (2019) and Gates (2019)
- Present the name of the individual or group author the same as you would for any other reference. Then provide the Twitter handle (beginning with the @ sign) in square brackets, followed by a period.
- Provide the first 20 words of the tweet as the title. Count a URL, a hashtag, or an emoji as one word each, and include them in the reference if they fall within the first 20 words.
- If the tweet includes an image, a video, a poll, or a thumbnail image with a link, indicate that in brackets after the title: [Image attached], [Video attached], [Thumbnail with link attached].
- The same format used for Twitter is also used for Instagram.
Parenthetical citation: (News From Science, 2019)
Narrative citation: News From Science (2019)
- Provide the first 20 words of the Facebook post as the title. Count a URL or other link, a hashtag, or an emoji as one word each, and include them in the reference if they fall within the first 20 words.
- If a status update includes images, videos, thumbnail links to outside sources, or content from another Facebook post (such as when sharing a link), indicate that in square brackets.
Parenthetical citations: (Fagan, 2019; National Institute of Mental Health, 2018; Woodyatt, 2019; World Health Organization, 2018)
Narrative citations: Fagan (2019), National Institute of Mental Health (2018), Woodyatt (2019), and World Health Organization (2018)
- Provide as specific a date as is available on the webpage. This might be a year only; a year and month; or a year, month, and day.
- Italicize the title of a webpage.
- When the author of the webpage and the publisher of the website are the same, omit the publisher name to avoid repetition (as in the World Health Organization example).
- When contents of a page are meant to be updated over time but are not archived, include a retrieval date in the reference (as in the Fagan example).
- Use the webpage on a website format for articles from news websites such as CNN and HuffPost (these sites do not have associated daily or weekly newspapers). Use the newspaper article category for articles from newspaper websites such as The New York Times or The Washington Post .
- Create a reference to an open educational resources (OER) page only when the materials are available for download directly (i.e., the materials are on the page and/or can be downloaded as PDFs or other files). If you are directed to another website, create a reference to the specific webpage on that website where the materials can be retrieved. Use this format for material in any OER repository, such as OER Commons, OASIS, or MERLOT.
- Do not create a reference or in-text citation for a whole website. To mention a website in general, and not any particular information on that site, provide the name of the website in the text and include the URL in parentheses. For example, you might mention that you used a website to create a survey.
The following supplemental example references are mention in the Publication Manual:
- retracted journal or magazine article
- edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM)
- edition of the International Statistical Classification of Diseases and Related Health Problems (ICD)
- religious work
- annotated religious work
Archival document and collections are not presented in the APA Publication Manual, Seventh Edition . This content is available only on the APA Style website . This guidance has been expanded from the 6th edition.
Archival sources include letters, unpublished manuscripts, limited-circulation brochures and pamphlets, in-house institutional and corporate documents, clippings, and other documents, as well as such nontextual materials as photographs and apparatus, that are in the personal possession of an author, form part of an institutional collection, or are stored in an archive such as the Archives of the History of American Psychology at the University of Akron or the APA Archives. For any documents like these that are available on the open web or via a database (subscription or nonsubscription), follow the reference templates shown in Chapter 10 of the Publication Manual.
The general format for the reference for an archival work includes the author, date, title, and source. The reference examples shown on this page may be modified for collections requiring more or less specific information to locate materials, for different types of collections, or for additional descriptive information (e.g., a translation of a letter). Authors may choose to list correspondence from their own personal collections, but correspondence from other private collections should be listed only with the permission of the collector.
Keep in mind the following principles when creating references to archival documents and collections:
- As with any reference, the purpose is to direct readers to the source, despite the fact that only a single copy of the document may be available and readers may have some difficulty actually seeing a copy.
- Include as much information as is needed to help locate the item with reasonable ease within the repository. For items from collections with detailed finding aids, the name of the collection may be sufficient; for items from collections without finding aids, more information (e.g., call number, box number, file name or number) may be necessary to help locate the item.
- If several letters are cited from the same collection, list the collection as a reference and provide specific identifying information (author, recipient, and date) for each letter in the in-text citations (see Example 3).
- Use square brackets to indicate information that does not appear on the document.
- Use “ca.” (circa) to indicate an estimated date (see Example 5).
- Use italics for titles of archival documents and collections; if the work does not have a title, provide a description in square brackets without italics.
- Separate elements of the source (e.g., the name of a repository, library, university or archive, and the location of the university or archive) with commas. End the source with a period.
- If a publication of limited circulation is available in libraries, the reference may be formatted as usual for published material, without the archival source.
- Note that private letters (vs. those in an archive or repository) are considered personal communications and cited in the text only.
1. Letter from a repository
Frank, L. K. (1935, February 4). [Letter to Robert M. Ogden]. Rockefeller Archive Center (GEB Series 1.3, Box 371, Folder 3877), Tarrytown, NY, United States.
- Parenthetical citation: (Frank, 1935)
- Narrative citation: Frank (1935)
- Because the letter does not have a title, provide a description in square brackets.
2. Letter from a private collection
Zacharius, G. P. (1953, August 15). [Letter to William Rickel (W. Rickel, Trans.)]. Copy in possession of Hendrika Vande Kemp.
- Parenthetical citation: (Zacharius, 1953)
- Narrative citation: Zacharius (1953)
- In this example, Hendrika Vande Kemp is either the author of the paper or the author of the paper has received permission from Hendrika Vande Kemp to cite a letter in Vande Kemp’s private collection in this way. Otherwise, cite a private letter as a personal communication .
3. Collection of letters from an archive
Allport, G. W. (1930–1967). Correspondence. Gordon W. Allport Papers (HUG 4118.10), Harvard University Archives, Cambridge, MA, United States.
- Parenthetical citation: (Allport, 1930–1967)
- Narrative citation: Allport (1930–1967)
To cite specific letters in the text, provide the author and range of years as shown in the reference list entry, plus details about who wrote the specific letter to whom and when the specific letter was written.
- Parenthetical citation: (Allport, 1930–1967, G. Boring to Allport, December 26, 1937)
- Narrative citation: Allport (1930–1967, Allport to G. Boring, March 1, 1939)
- Use the parenthetical citation format to cite a letter that E. G. Boring wrote to Allport because Allport is the author in the reference. Use either the parenthetical or narrative citation format to cite letters that Allport wrote.
4. Unpublished papers, lectures from an archive or personal collection
Berliner, A. (1959). Notes for a lecture on reminiscences of Wundt and Leipzig. Anna Berliner Memoirs (Box M50), Archives of the History of American Psychology, University of Akron, Akron, OH, United States.
- Parenthetical citation: (Berliner, 1959)
- Narrative citation: Berliner (1959)
5. Archival/historical source for which the author and/or date is known or is reasonably certain but not stated on the document
Allport, A. (presumed). (ca. 1937). Marion Taylor today—by the biographer [Unpublished manuscript]. Marion Taylor Papers, Schlesinger Library, Radcliffe College, Cambridge, MA, United States.
- Parenthetical citation: (Allport, ca. 1937)
- Narrative citation: Allport (ca. 1937)
- Because the author is reasonably certain but not stated on the document, place the word “presumed” in parentheses after the name, followed by a period.
- Because the date is reasonably certain but not stated on the document, the abbreviation “ca.” (which stands for “circa”) appears before the year in parentheses.
6. Archival source with group author
Subcommittee on Mental Hygiene Personnel in School Programs. (1949, November 5–6). Meeting of Subcommittee on Mental Hygiene Personnel in School Programs. David Shakow Papers (M1360), Archives of the History of American Psychology, University of Akron, Akron, OH, United States.
- Parenthetical citation: (Subcommittee on Mental Hygiene Personnel in School Programs, 1949)
- Narrative citation: Subcommittee on Mental Hygiene Personnel in School Programs (1949)
7. Interview recorded and available in an archive
Smith, M. B. (1989, August 12). Interview by C. A. Kiesler [Tape recording]. President’s Oral History Project, American Psychological Association, APA Archives, Washington, DC, United States.
- Parenthetical citation: (Smith, 1989)
- Narrative citation: Smith (1989)
- For interviews and oral histories recorded in an archive, list the interviewee as the author. Include the interviewer’s name in the description.
8. Transcription of a recorded interview, no recording available
Sparkman, C. F. (1973). An oral history with Dr. Colley F. Sparkman/Interviewer: Orley B. Caudill. Mississippi Oral History Program (Vol. 289), University of Southern Mississippi, Hattiesburg, MS, United States.
- Parenthetical citation: (Sparkman, 1973)
- Narrative citation: Sparkman (1973)
9. Newspaper article clipping, historical, in personal collection
Psychoanalysis institute to open. (1948, September 18). [Clipping from an unidentified Dayton, OH, United States, newspaper]. Copy in possession of author.
- Parenthetical citation: (“Psychoanalysis Institute to Open,” 1948)
- Narrative citation: “Psychoanalysis Institute to Open” (1948)
- Use this format only if you are the person who is in possession of the newspaper clipping.
10. Historical publication of limited circulation
Sci-Art Publishers. (1935). Sci-Art publications [Brochure]. Roback Papers (HUGFP 104.50, Box 2, Folder “Miscellaneous Psychological Materials”), Harvard University Archives, Cambridge, MA, United States.
- Parenthetical citation: (Sci-Art Publishers, 1935)
- Narrative citation: Sci-Art Publishers (1935)
11. Archived photographs, no author and no title
[Photographs of Robert M. Yerkes]. (ca. 1917–1954). Robert Mearns Yerkes Papers (Box 137, Folder 2292), Manuscripts and Archives, Yale University Library, New Haven, CT, United States.
- Parenthetical citation: ([Photographs of Robert M. Yerkes], ca. 1917–1954)
- Narrative citation: [Photographs of Robert M. Yerkes] (ca. 1917–1954)
- Because the archived photographs do not have a title, provide a bracketed description instead.
- Because the archived photographs do not have an author, move the bracketed description to the author position of the reference.
12. Microfilm
U.S. Census Bureau. (1880). 1880 U.S. census: Defective, dependent, and delinquent classes schedule: Virginia [Microfilm]. NARA Microfilm Publication T1132 (Rolls 33–34), National Archives and Records Administration, Washington, DC, United States.
- Parenthetical citation: (U.S. Census Bureau, 1880)
- Narrative citation: U.S. Census Bureau (1880)
Read the full APA guidelines on citing ChatGPT
OpenAI. (2023). ChatGPT (Mar 14 version) [Large language model]. https://chat.openai.com/chat
- Parenthetical citation: (OpenAI, 2023)
- Narrative citation: OpenAI (2023)
Author: The author of the model is OpenAI.
Date: The date is the year of the version you used. Following the template in Section 10.10, you need to include only the year, not the exact date. The version number provides the specific date information a reader might need.
Title: The name of the model is “ChatGPT,” so that serves as the title and is italicized in your reference, as shown in the template. Although OpenAI labels unique iterations (i.e., ChatGPT-3, ChatGPT-4), they are using “ChatGPT” as the general name of the model, with updates identified with version numbers.
The version number is included after the title in parentheses. The format for the version number in ChatGPT references includes the date because that is how OpenAI is labeling the versions. Different large language models or software might use different version numbering; use the version number in the format the author or publisher provides, which may be a numbering system (e.g., Version 2.0) or other methods.
Bracketed text is used in references for additional descriptions when they are needed to help a reader understand what’s being cited. References for a number of common sources, such as journal articles and books, do not include bracketed descriptions, but things outside of the typical peer-reviewed system often do. In the case of a reference for ChatGPT, provide the descriptor “Large language model” in square brackets. OpenAI describes ChatGPT-4 as a “large multimodal model,” so that description may be provided instead if you are using ChatGPT-4. Later versions and software or models from other companies may need different descriptions, based on how the publishers describe the model. The goal of the bracketed text is to briefly describe the kind of model to your reader.
Source: When the publisher name and the author name are the same, do not repeat the publisher name in the source element of the reference, and move directly to the URL. This is the case for ChatGPT. The URL for ChatGPT is https://chat.openai.com/chat . For other models or products for which you may create a reference, use the URL that links as directly as possible to the source (i.e., the page where you can access the model, not the publisher’s homepage).
What to include and what to exclude
Works included in a reference list.
The reference list provides a reliable way for readers to identify and locate the works cited in a paper. APA Style papers generally include reference lists, not bibliographies.
In general, each work cited in the text must appear in the reference list, and each work in the reference list must be cited in the text. Check your work carefully before submitting your manuscript or course assignment to ensure no works cited in the text are missing from the reference list and vice versa, with only the following exceptions.
Works Excluded From a Reference List
There are a few kinds of works that are not included in a reference list. Usually a work is not included because readers cannot recover it or because the mention is so broad that readers do not need a reference list entry to understand the use.
Information on works included in a reference list is covered in Sections 2.12 and 8.4 of the APA Publication Manual, Seventh Edition
*This guidance has been expanded from the 6th edition.*
- Personal communications such as emails, phone calls, or text messages are cited in the text only, not in the reference list, because readers cannot retrieve personal communications.
- General mentions of whole websites, whole periodicals, and common software and apps in the text do not require in-text citations or reference list entries because the use is broad and the source is familiar.
- The source of an epigraph does not usually appear in the reference list unless the work is a scholarly book or journal. For example, if you open the paper with an inspirational quotation by a famous person, the source of the quotation does not appear in the reference list because the quotation is meant to set the stage for the work, not substantiate a key point.
- Quotations from research participants in a study you conducted can be presented and discussed in the text but do not need citations or reference list entries. Citations and reference list entries are not necessary because the quotations are part of your original research. They could also compromise participants’ confidentiality, which is an ethical violation.
- References included in a meta-analysis, which are marked with an asterisk in the reference list, may be cited in the text (or not) at the author’s discretion. This exception is relevant only to authors who are conducting a meta-analysis.
DOIs and URLs
The DOI or URL is the final component of a reference list entry. Because so much scholarship is available and/or retrieved online, most reference list entries end with either a DOI or a URL.
- A DOI is a unique alphanumeric string that identifies content and provides a persistent link to its location on the internet. DOIs can be found in database records and the reference lists of published works.
- A URL specifies the location of digital information on the internet and can be found in the address bar of your internet browser. URLs in references should link directly to the cited work when possible.
Follow these guidelines for including DOIs and URLs in references:
- Include a DOI for all works that have a DOI, regardless of whether you used the online version or the print version.
- If a print work does not have a DOI, do not include any DOI or URL in the reference.
- If an online work has both a DOI and a URL, include only the DOI.
- For works without DOIs from websites (not including academic research databases), provide a URL in the reference (as long as the URL will work for readers).
- For works without DOIs from most academic research databases , do not include a URL or database information in the reference because these works are widely available. The reference should be the same as the reference for a print version of the work.
- For works from databases that publish original, proprietary material available only in that database (such as the UpToDate database) or for works of limited circulation in databases (such as monographs in the ERIC database), include the name of the database or archive and the URL of the work. If the URL requires a login or is session-specific (meaning it will not resolve for readers), provide the URL of the database or archive home page or login page instead of the URL for the work. See the page on including database information in references for more information.
- If the URL is no longer working or no longer provides readers access to the content you intend to cite, follow the guidance for works with no source .
- Other alphanumeric identifiers such as the International Standard Book Number (ISBN) and the International Standard Serial Number (ISSN) are not included in APA Style references.
Follow these guidelines to format DOIs and URLs:
- Present both DOIs and URLs as hyperlinks (i.e., beginning with “http:” or “https:”).
- Because a hyperlink leads readers directly to the content, it is not necessary to include the words “Retrieved from” or “Accessed from” before a DOI or URL.
- It is acceptable to use either the default display settings for hyperlinks in your word-processing program (e.g., usually blue font, underlined) or plain text that is not underlined.
- Leave links live if the work is to be published or read online.
- Follow the current recommendations of the International DOI Foundation to format DOIs in the reference list, which as of this publication is as follows:
https://doi.org/ xxxxx
- The string “https://doi.org/” is a way of presenting a DOI as a link, and “xxxxx” refers to the DOI number.
- The preferred format of the DOI has changed over time. Although older works use previous formats (e.g., “http:/dx.doi.org/” or “doi:” or “DOI:” before the DOI number), in your reference list, standardize DOIs into the current preferred format for all entries. For example, use https://doi.org/10.1037/a0040251 in your reference even though that article, published in 2016, presented the number in an older format.
- Copy and paste the DOI or URL from your web browser directly into your reference list to avoid transcription errors. Do not change the capitalization or punctuation of the DOI or URL. Do not add line breaks manually to the hyperlink; it is acceptable if your word-processing program automatically adds a break or moves the hyperlink to its own line.
- Do not add a period after the DOI or URL because this may interfere with link functionality.
When a DOI or URL is long or complex, you may use shortDOIs or shortened URLs if desired.
- Use the shortDOI service provided by the International DOI Foundation to create shortDOIs. A work can have only one DOI and only one shortDOI; the shortDOI service will either produce a new shortDOI for a work that has never had one or retrieve an existing shortDOI.
- Some websites provide their own branded shortened URLs, and independent URL shortening services are available as well. Any shortened URL is acceptable in a reference as long as you check the link to ensure that it takes you to the correct location.
- << Previous: Home
- Next: Paper Format >>
- Last Updated: Jan 24, 2024 12:02 PM
- URL: https://guides.lib.udel.edu/apa
Bibliography In Research Paper: Know Everything About It
Learn about what is a bibliography in a research paper and how to write a bibliography that makes your research paper undeniably perfect.
Imagine yourself as a research student and you have to submit a thesis for which you collected all the information and wrote your thesis but forgot to add a bibliography to it. Now your professor rejects your thesis for that one mistake. How would you feel? Wouldn’t it be disappointing?
It indeed is disappointing but do you really think a bibliography is that important? In fact, have you ever wondered what a bibliography is? You must’ve seen it at the end of every book or article that you read but you wouldn’t have paid much attention to it. Read the article to know the importance of a bibliography in a research paper and how to write it according to the guidelines given.
What is a Bibliography?
A bibliography is a list of sources or materials that you might have referred to while writing an article or a research paper. If the reader wants to explore more about the content apart from what you’ve written, they would refer to the sources that you’ve mentioned and a bibliography can be helpful.
For example, if you’re reading a fiction novel about the culture of a European country in the 18th- century and you wish to learn more about it, what would you do? Here comes the importance of a bibliography. The author might have mentioned his research works – from where he collected information regarding European culture and he might’ve cited all those previous works in the form of a bibliography at the end of the book. This could help readers like you to explore more about the topic.
Bibliography vs References
Bibliography and references appear synonymous and thus, most of us try to use them interchangeably. But did you know this isn’t right? While both of them appear similar, there is this small difference that actually makes a lot of sense if you get to know it.
If we see the definition of reference, is the citation of all the works/sources that one used “ within the body of the paper”. This is the most important key difference. While references refer to the work that is present within the paper, a bibliography refers to the works which are “ not specifically referred to within the body of the paper” that is, it needn’t be necessary for a bibliography to have the exact sources on its body and you can cite a source even if you just used it to refer something regarding the topic.
Steps To Write A Bibliography In Research Paper
- The initial and foremost step to be followed before writing a bibliography is to make a note of all the books/sources/works you read when you’re doing your background research for a paper.
- Once you have a list of the books or sources, you need to check for the following information in the source :
If it is printed, check out for
- Authors’ name
- Title of publication
- Date of publication
- The publishing company of a book
- Page number(s)
If it is from a website, check out for
- Author and editor name
- Title of the webpage
- The company that posted that webpage
- URL of that webpage
- Before writing out your bibliography, you should know that there are two major guidelines for writing a bibliography (MLA Format and APA Format), and use them according to the need of your paper.
- Once you’re done writing a bibliography, make sure that you cited all the sources that you mentioned in the paper and whether you cited them in the correct format or not.
- If there are any mistakes or necessary changes, make them before you submit your paper.
Writing a bibliography might be a tiresome process as there’s a huge list of sources that need to be mentioned but adding a bibliography gives your research paper a more professional touch and sets your paper apart from the rest of the crowd.
Over 65,000 accurate scientific figures to boost your impact
We know! It’s hard to believe but like most scientific facts, it’s the truth. Mind the Graph has over 65,000 scientific figures that boost the impact of your research paper and helps your readers to connect with your work and understand the concepts better.

Subscribe to our newsletter
Exclusive high quality content about effective visual communication in science.
About Sowjanya Pedada
Sowjanya is a passionate writer and an avid reader. She holds MBA in Agribusiness Management and now is working as a content writer. She loves to play with words and hopes to make a difference in the world through her writings. Apart from writing, she is interested in reading fiction novels and doing craftwork. She also loves to travel and explore different cuisines and spend time with her family and friends.
Content tags
- Privacy Policy
Buy Me a Coffee

Home » Research Report – Example, Writing Guide and Types
Research Report – Example, Writing Guide and Types
Table of Contents

Research Report
Definition:
Research Report is a written document that presents the results of a research project or study, including the research question, methodology, results, and conclusions, in a clear and objective manner.
The purpose of a research report is to communicate the findings of the research to the intended audience, which could be other researchers, stakeholders, or the general public.
Components of Research Report
Components of Research Report are as follows:
Introduction
The introduction sets the stage for the research report and provides a brief overview of the research question or problem being investigated. It should include a clear statement of the purpose of the study and its significance or relevance to the field of research. It may also provide background information or a literature review to help contextualize the research.
Literature Review
The literature review provides a critical analysis and synthesis of the existing research and scholarship relevant to the research question or problem. It should identify the gaps, inconsistencies, and contradictions in the literature and show how the current study addresses these issues. The literature review also establishes the theoretical framework or conceptual model that guides the research.
Methodology
The methodology section describes the research design, methods, and procedures used to collect and analyze data. It should include information on the sample or participants, data collection instruments, data collection procedures, and data analysis techniques. The methodology should be clear and detailed enough to allow other researchers to replicate the study.
The results section presents the findings of the study in a clear and objective manner. It should provide a detailed description of the data and statistics used to answer the research question or test the hypothesis. Tables, graphs, and figures may be included to help visualize the data and illustrate the key findings.
The discussion section interprets the results of the study and explains their significance or relevance to the research question or problem. It should also compare the current findings with those of previous studies and identify the implications for future research or practice. The discussion should be based on the results presented in the previous section and should avoid speculation or unfounded conclusions.
The conclusion summarizes the key findings of the study and restates the main argument or thesis presented in the introduction. It should also provide a brief overview of the contributions of the study to the field of research and the implications for practice or policy.
The references section lists all the sources cited in the research report, following a specific citation style, such as APA or MLA.
The appendices section includes any additional material, such as data tables, figures, or instruments used in the study, that could not be included in the main text due to space limitations.
Types of Research Report
Types of Research Report are as follows:
Thesis is a type of research report. A thesis is a long-form research document that presents the findings and conclusions of an original research study conducted by a student as part of a graduate or postgraduate program. It is typically written by a student pursuing a higher degree, such as a Master’s or Doctoral degree, although it can also be written by researchers or scholars in other fields.
Research Paper
Research paper is a type of research report. A research paper is a document that presents the results of a research study or investigation. Research papers can be written in a variety of fields, including science, social science, humanities, and business. They typically follow a standard format that includes an introduction, literature review, methodology, results, discussion, and conclusion sections.
Technical Report
A technical report is a detailed report that provides information about a specific technical or scientific problem or project. Technical reports are often used in engineering, science, and other technical fields to document research and development work.
Progress Report
A progress report provides an update on the progress of a research project or program over a specific period of time. Progress reports are typically used to communicate the status of a project to stakeholders, funders, or project managers.
Feasibility Report
A feasibility report assesses the feasibility of a proposed project or plan, providing an analysis of the potential risks, benefits, and costs associated with the project. Feasibility reports are often used in business, engineering, and other fields to determine the viability of a project before it is undertaken.
Field Report
A field report documents observations and findings from fieldwork, which is research conducted in the natural environment or setting. Field reports are often used in anthropology, ecology, and other social and natural sciences.
Experimental Report
An experimental report documents the results of a scientific experiment, including the hypothesis, methods, results, and conclusions. Experimental reports are often used in biology, chemistry, and other sciences to communicate the results of laboratory experiments.
Case Study Report
A case study report provides an in-depth analysis of a specific case or situation, often used in psychology, social work, and other fields to document and understand complex cases or phenomena.
Literature Review Report
A literature review report synthesizes and summarizes existing research on a specific topic, providing an overview of the current state of knowledge on the subject. Literature review reports are often used in social sciences, education, and other fields to identify gaps in the literature and guide future research.
Research Report Example
Following is a Research Report Example sample for Students:
Title: The Impact of Social Media on Academic Performance among High School Students
This study aims to investigate the relationship between social media use and academic performance among high school students. The study utilized a quantitative research design, which involved a survey questionnaire administered to a sample of 200 high school students. The findings indicate that there is a negative correlation between social media use and academic performance, suggesting that excessive social media use can lead to poor academic performance among high school students. The results of this study have important implications for educators, parents, and policymakers, as they highlight the need for strategies that can help students balance their social media use and academic responsibilities.
Introduction:
Social media has become an integral part of the lives of high school students. With the widespread use of social media platforms such as Facebook, Twitter, Instagram, and Snapchat, students can connect with friends, share photos and videos, and engage in discussions on a range of topics. While social media offers many benefits, concerns have been raised about its impact on academic performance. Many studies have found a negative correlation between social media use and academic performance among high school students (Kirschner & Karpinski, 2010; Paul, Baker, & Cochran, 2012).
Given the growing importance of social media in the lives of high school students, it is important to investigate its impact on academic performance. This study aims to address this gap by examining the relationship between social media use and academic performance among high school students.
Methodology:
The study utilized a quantitative research design, which involved a survey questionnaire administered to a sample of 200 high school students. The questionnaire was developed based on previous studies and was designed to measure the frequency and duration of social media use, as well as academic performance.
The participants were selected using a convenience sampling technique, and the survey questionnaire was distributed in the classroom during regular school hours. The data collected were analyzed using descriptive statistics and correlation analysis.
The findings indicate that the majority of high school students use social media platforms on a daily basis, with Facebook being the most popular platform. The results also show a negative correlation between social media use and academic performance, suggesting that excessive social media use can lead to poor academic performance among high school students.
Discussion:
The results of this study have important implications for educators, parents, and policymakers. The negative correlation between social media use and academic performance suggests that strategies should be put in place to help students balance their social media use and academic responsibilities. For example, educators could incorporate social media into their teaching strategies to engage students and enhance learning. Parents could limit their children’s social media use and encourage them to prioritize their academic responsibilities. Policymakers could develop guidelines and policies to regulate social media use among high school students.
Conclusion:
In conclusion, this study provides evidence of the negative impact of social media on academic performance among high school students. The findings highlight the need for strategies that can help students balance their social media use and academic responsibilities. Further research is needed to explore the specific mechanisms by which social media use affects academic performance and to develop effective strategies for addressing this issue.
Limitations:
One limitation of this study is the use of convenience sampling, which limits the generalizability of the findings to other populations. Future studies should use random sampling techniques to increase the representativeness of the sample. Another limitation is the use of self-reported measures, which may be subject to social desirability bias. Future studies could use objective measures of social media use and academic performance, such as tracking software and school records.
Implications:
The findings of this study have important implications for educators, parents, and policymakers. Educators could incorporate social media into their teaching strategies to engage students and enhance learning. For example, teachers could use social media platforms to share relevant educational resources and facilitate online discussions. Parents could limit their children’s social media use and encourage them to prioritize their academic responsibilities. They could also engage in open communication with their children to understand their social media use and its impact on their academic performance. Policymakers could develop guidelines and policies to regulate social media use among high school students. For example, schools could implement social media policies that restrict access during class time and encourage responsible use.
References:
- Kirschner, P. A., & Karpinski, A. C. (2010). Facebook® and academic performance. Computers in Human Behavior, 26(6), 1237-1245.
- Paul, J. A., Baker, H. M., & Cochran, J. D. (2012). Effect of online social networking on student academic performance. Journal of the Research Center for Educational Technology, 8(1), 1-19.
- Pantic, I. (2014). Online social networking and mental health. Cyberpsychology, Behavior, and Social Networking, 17(10), 652-657.
- Rosen, L. D., Carrier, L. M., & Cheever, N. A. (2013). Facebook and texting made me do it: Media-induced task-switching while studying. Computers in Human Behavior, 29(3), 948-958.
Note*: Above mention, Example is just a sample for the students’ guide. Do not directly copy and paste as your College or University assignment. Kindly do some research and Write your own.
Applications of Research Report
Research reports have many applications, including:
- Communicating research findings: The primary application of a research report is to communicate the results of a study to other researchers, stakeholders, or the general public. The report serves as a way to share new knowledge, insights, and discoveries with others in the field.
- Informing policy and practice : Research reports can inform policy and practice by providing evidence-based recommendations for decision-makers. For example, a research report on the effectiveness of a new drug could inform regulatory agencies in their decision-making process.
- Supporting further research: Research reports can provide a foundation for further research in a particular area. Other researchers may use the findings and methodology of a report to develop new research questions or to build on existing research.
- Evaluating programs and interventions : Research reports can be used to evaluate the effectiveness of programs and interventions in achieving their intended outcomes. For example, a research report on a new educational program could provide evidence of its impact on student performance.
- Demonstrating impact : Research reports can be used to demonstrate the impact of research funding or to evaluate the success of research projects. By presenting the findings and outcomes of a study, research reports can show the value of research to funders and stakeholders.
- Enhancing professional development : Research reports can be used to enhance professional development by providing a source of information and learning for researchers and practitioners in a particular field. For example, a research report on a new teaching methodology could provide insights and ideas for educators to incorporate into their own practice.
How to write Research Report
Here are some steps you can follow to write a research report:
- Identify the research question: The first step in writing a research report is to identify your research question. This will help you focus your research and organize your findings.
- Conduct research : Once you have identified your research question, you will need to conduct research to gather relevant data and information. This can involve conducting experiments, reviewing literature, or analyzing data.
- Organize your findings: Once you have gathered all of your data, you will need to organize your findings in a way that is clear and understandable. This can involve creating tables, graphs, or charts to illustrate your results.
- Write the report: Once you have organized your findings, you can begin writing the report. Start with an introduction that provides background information and explains the purpose of your research. Next, provide a detailed description of your research methods and findings. Finally, summarize your results and draw conclusions based on your findings.
- Proofread and edit: After you have written your report, be sure to proofread and edit it carefully. Check for grammar and spelling errors, and make sure that your report is well-organized and easy to read.
- Include a reference list: Be sure to include a list of references that you used in your research. This will give credit to your sources and allow readers to further explore the topic if they choose.
- Format your report: Finally, format your report according to the guidelines provided by your instructor or organization. This may include formatting requirements for headings, margins, fonts, and spacing.
Purpose of Research Report
The purpose of a research report is to communicate the results of a research study to a specific audience, such as peers in the same field, stakeholders, or the general public. The report provides a detailed description of the research methods, findings, and conclusions.
Some common purposes of a research report include:
- Sharing knowledge: A research report allows researchers to share their findings and knowledge with others in their field. This helps to advance the field and improve the understanding of a particular topic.
- Identifying trends: A research report can identify trends and patterns in data, which can help guide future research and inform decision-making.
- Addressing problems: A research report can provide insights into problems or issues and suggest solutions or recommendations for addressing them.
- Evaluating programs or interventions : A research report can evaluate the effectiveness of programs or interventions, which can inform decision-making about whether to continue, modify, or discontinue them.
- Meeting regulatory requirements: In some fields, research reports are required to meet regulatory requirements, such as in the case of drug trials or environmental impact studies.
When to Write Research Report
A research report should be written after completing the research study. This includes collecting data, analyzing the results, and drawing conclusions based on the findings. Once the research is complete, the report should be written in a timely manner while the information is still fresh in the researcher’s mind.
In academic settings, research reports are often required as part of coursework or as part of a thesis or dissertation. In this case, the report should be written according to the guidelines provided by the instructor or institution.
In other settings, such as in industry or government, research reports may be required to inform decision-making or to comply with regulatory requirements. In these cases, the report should be written as soon as possible after the research is completed in order to inform decision-making in a timely manner.
Overall, the timing of when to write a research report depends on the purpose of the research, the expectations of the audience, and any regulatory requirements that need to be met. However, it is important to complete the report in a timely manner while the information is still fresh in the researcher’s mind.
Characteristics of Research Report
There are several characteristics of a research report that distinguish it from other types of writing. These characteristics include:
- Objective: A research report should be written in an objective and unbiased manner. It should present the facts and findings of the research study without any personal opinions or biases.
- Systematic: A research report should be written in a systematic manner. It should follow a clear and logical structure, and the information should be presented in a way that is easy to understand and follow.
- Detailed: A research report should be detailed and comprehensive. It should provide a thorough description of the research methods, results, and conclusions.
- Accurate : A research report should be accurate and based on sound research methods. The findings and conclusions should be supported by data and evidence.
- Organized: A research report should be well-organized. It should include headings and subheadings to help the reader navigate the report and understand the main points.
- Clear and concise: A research report should be written in clear and concise language. The information should be presented in a way that is easy to understand, and unnecessary jargon should be avoided.
- Citations and references: A research report should include citations and references to support the findings and conclusions. This helps to give credit to other researchers and to provide readers with the opportunity to further explore the topic.
Advantages of Research Report
Research reports have several advantages, including:
- Communicating research findings: Research reports allow researchers to communicate their findings to a wider audience, including other researchers, stakeholders, and the general public. This helps to disseminate knowledge and advance the understanding of a particular topic.
- Providing evidence for decision-making : Research reports can provide evidence to inform decision-making, such as in the case of policy-making, program planning, or product development. The findings and conclusions can help guide decisions and improve outcomes.
- Supporting further research: Research reports can provide a foundation for further research on a particular topic. Other researchers can build on the findings and conclusions of the report, which can lead to further discoveries and advancements in the field.
- Demonstrating expertise: Research reports can demonstrate the expertise of the researchers and their ability to conduct rigorous and high-quality research. This can be important for securing funding, promotions, and other professional opportunities.
- Meeting regulatory requirements: In some fields, research reports are required to meet regulatory requirements, such as in the case of drug trials or environmental impact studies. Producing a high-quality research report can help ensure compliance with these requirements.
Limitations of Research Report
Despite their advantages, research reports also have some limitations, including:
- Time-consuming: Conducting research and writing a report can be a time-consuming process, particularly for large-scale studies. This can limit the frequency and speed of producing research reports.
- Expensive: Conducting research and producing a report can be expensive, particularly for studies that require specialized equipment, personnel, or data. This can limit the scope and feasibility of some research studies.
- Limited generalizability: Research studies often focus on a specific population or context, which can limit the generalizability of the findings to other populations or contexts.
- Potential bias : Researchers may have biases or conflicts of interest that can influence the findings and conclusions of the research study. Additionally, participants may also have biases or may not be representative of the larger population, which can limit the validity and reliability of the findings.
- Accessibility: Research reports may be written in technical or academic language, which can limit their accessibility to a wider audience. Additionally, some research may be behind paywalls or require specialized access, which can limit the ability of others to read and use the findings.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

Institutional Review Board – Application Sample...

Evaluating Research – Process, Examples and...
Bibliography Examples: How to Cite Sources Correctly
A bibliography is a list of sources that you consulted while writing your paper. It includes all the books, articles, and other sources you used to gather information for your research. A bibliography is an important part of academic writing, and it helps readers to locate the sources you used.
There are different styles of bibliographies, including MLA, APA, and Chicago, and each style has its own set of rules for formatting and citing sources. MLA style is commonly used in the humanities, while APA style is used in the social sciences. Chicago style is used in history and some social sciences. Each style has its own set of rules for formatting and citing sources, so it is important to follow the guidelines for the style you are using.
In this article, we will provide bibliography examples in different styles. We will also explain how to format your bibliography and how to cite sources correctly. Whether you are a student or a researcher, this article will help you create a bibliography that is accurate and complete.
Bibliography Examples

Understanding Bibliography
Bibliography is a list of sources that you have used in your research or writing. The sources can be books, articles, websites, or any other material that you have consulted while working on your project. A bibliography provides detailed information about each source, such as the author’s name, the title of the work, the publication date, and more. It is usually included at the end of a research paper, essay, or book.
The purpose of a bibliography is to give credit to the authors whose work you have used in your research. It also allows your readers to find the sources that you have used and to verify the accuracy of your information. A bibliography is an important part of any research paper or essay because it demonstrates the depth and breadth of your research. It also shows that you have taken the time to read and understand the work of other scholars in your field.
When creating a bibliography, it is important to follow the guidelines of the citation style that you are using. There are many different citation styles, such as APA, MLA, Chicago, and more. Each style has its own rules for formatting the bibliography, so it is important to consult a style guide or manual to ensure that your bibliography is accurate and complete. (ĐÃ DÙNG Ở INTRO)
In addition to the basic information about each source, a bibliography may also include annotations that provide a brief summary or evaluation of the work. An annotated bibliography can be a useful tool for researchers because it allows them to quickly evaluate the relevance and quality of a source.
Types of Bibliographies
When it comes to writing a bibliography, there are three main types that you should be aware of: enumerative, analytical, and annotated. Each type has its own unique characteristics and purposes.
Enumerative Bibliography
An enumerative bibliography is a list of works that are cited in a particular document. It is the most common type of bibliography and is used to provide readers with a comprehensive list of sources that were used in the creation of the document. This type of bibliography is typically organized alphabetically by the author’s last name and includes basic information such as the title, author, publisher, and date of publication.
Analytical Bibliography
An analytical bibliography is a more in-depth type of bibliography that provides detailed information about the physical characteristics of each cited source. This includes information such as the number of pages, type of binding used, and illustrations. Analytical bibliographies are often used in the study of rare books and manuscripts, as they provide valuable information about the history and evolution of a particular work.
Annotated Bibliography
An annotated bibliography is a type of bibliography that includes a brief summary or evaluation of each cited source. This type of bibliography is often used in academic research to provide readers with a deeper understanding of the sources used in a particular document. Annotations can include information such as the author’s credentials, the main arguments of the work, and its relevance to the research topic.
Formatting Styles
APA style is commonly used in the social sciences and is known for its emphasis on author and date information. When creating an APA-style bibliography, you should include the following information for each source:
- Author’s last name and initials
- Year of publication
- Title of the article or book
- Title of the journal or publisher
- Volume and issue number (if applicable)
- Page numbers
APA style also requires that you use hanging indents for each entry, meaning that the first line of each entry should be flush with the left margin, while subsequent lines should be indented.
MLA style is commonly used in the humanities and is known for its emphasis on author and page number information. When creating an MLA-style bibliography, you should include the following information for each source:
- Author’s last name and first name
- Medium of publication (e.g., print, web, etc.)
MLA style requires that you use a hanging indent for each entry, just like APA style.
Chicago Style
Chicago style is commonly used in history and other humanities fields. When creating a Chicago-style bibliography, you should include the following information for each source:
Unlike APA and MLA styles, Chicago style requires that you use footnotes or endnotes to cite your sources. These notes should be numbered and correspond to a superscript number in the text.
Book Example
When citing a book, the following information should be included in the bibliography:
- Author’s last name, first name.
- Title of the book.
- Publisher’s name.
- Year of publication.
Here is an example of how to format a book citation in the bibliography:
Smith, John. The History of America . Random House , 2020.
Journal Article Example
When citing a journal article, the following information should be included in the bibliography:
- Title of the article.
- Title of the journal.
- Volume and issue number.
- Page numbers.
Here is an example of how to format a journal article citation in the bibliography:
Johnson, Emily. “The Effects of Social Media on Mental Health.” Journal of Psychology , vol. 25, no. 3, 2022, pp. 45-60.
Website Example
When citing a website, the following information should be included in the bibliography:
- Title of the webpage.
- Title of the website.
- Publisher or sponsor of the website.
- Date of publication or last update.
Here is an example of how to format a website citation in the bibliography:
Doe, Jane. “The Benefits of Yoga.” Yoga Journal , Yoga Journal, 2021, www.yogajournal.com/benefits-of-yoga/.
Film Example
When citing a film, the following information should be included in the bibliography:
- Director’s last name, first name.
- Title of the film.
- Production company.
- Year of release.
Here is an example of how to format a film citation in the bibliography:
Spielberg, Steven. Jurassic Park . Universal Pictures, 1993.
Common Mistakes in Bibliographies
When creating a bibliography, it is important to avoid common mistakes that can lead to lower grades or even accusations of plagiarism. Here are some common mistakes to watch out for:
1. Missing References or Citations
One of the most common mistakes is missing references or citations. Remember that citations and references go hand in hand. A citation within the text needs a reference in your reference page or bibliography and vice versa.
2. Incomplete or Incorrect Information
Make sure that all of the information you include in your bibliography is complete and correct. This includes the author’s name, the title of the work, the publication date, and the publisher. Incomplete or incorrect information can make it difficult for others to find and use your sources.
3. Falsified Information
Falsifying information in your bibliography is a serious offense that can lead to accusations of plagiarism. Make sure that all of the information you include is accurate and truthful.
4. Improper Formatting
Proper formatting is essential for a well-organized and easy-to-read bibliography. Make sure that you follow the formatting guidelines provided by your instructor or use a standard formatting style such as MLA or APA.
5. Using Outdated or Inappropriate Sources
Using outdated or inappropriate sources can weaken the credibility of your work. Make sure that you use current and relevant sources that are appropriate for your topic.
By avoiding these common mistakes, you can create a well-organized and accurate bibliography that will enhance the credibility of your work.
Tips for Creating an Effective Bibliography
1. Know the Formatting Style
Before you begin creating your bibliography, make sure you understand the formatting style required by your instructor or publisher. Common styles include MLA, APA, and Chicago. Each style has its own rules for formatting citations, so it is important to be familiar with the specific requirements of the style you are using.
2. Use Reliable Sources
Your bibliography should only include sources that are reliable and relevant to your topic. Be sure to verify the credibility of your sources before including them in your bibliography. This can be done by checking the author’s credentials, the publisher, and the date of publication.
3. Organize Your Sources
When creating your bibliography, organize your sources in alphabetical order by the author’s last name. If the author’s name is not available, use the title of the source instead. Be consistent with your formatting throughout your bibliography.
4. Include All Necessary Information
Each source in your bibliography should include all necessary information, such as the author’s name, title of the source, publication date, and publisher. For online sources, include the URL or DOI.
5. Proofread Your Bibliography
Before submitting your bibliography, take the time to proofread it for errors and inconsistencies. Check for spelling and grammatical errors, as well as formatting errors. A well-organized and error-free bibliography can enhance the credibility of your research.
By following these tips, you can create an effective bibliography that accurately reflects the sources you used in your research.
Frequently Asked Questions
What are some commonly used bibliography formats?
There are several commonly used bibliography formats, including MLA, APA, Chicago, and Harvard. Each format has its own set of guidelines for citing sources, so it’s important to choose the appropriate format for your project.
How can I write a bibliography for a school project?
To write a bibliography for a school project, you should first gather all of the sources you used in your research. Then, follow the guidelines for the appropriate bibliography format to create a citation for each source. Be sure to include all of the necessary information, such as the author’s name, title of the source, publication date, and publisher.
What is the Harvard style of bibliography and can you provide an example?
The Harvard style of bibliography is a commonly used format that is known for its simplicity and flexibility. In this format, sources are listed alphabetically by the author’s last name, and each citation includes the author’s name, publication date, and title of the source. Here’s an example of a Harvard-style bibliography entry for a book:
Smith, John. (2010). The History of Modern Art. New York: Penguin Books.
Can you suggest a reliable bibliography generator?
There are several reliable bibliography generators available online, including EasyBib, Citation Machine, and BibMe. These tools can help you create citations in the appropriate format quickly and easily.
What is the difference between a bibliography and references?
A bibliography is a list of all the sources you used in your research, while references are a list of the sources you cited in your paper. In other words, a bibliography includes all the sources you consulted, even if you didn’t use them in your paper, while references only include the sources you actually cited.
Can you provide an example of a simple bibliography?
Yes, here’s an example of a simple bibliography in MLA format:
Smith, John. The History of Modern Art. Penguin Books, 2010.
Jones, Sarah. “The Role of Women in the Renaissance.” Renaissance Quarterly, vol. 63, no. 2, 2010, pp. 345-362.
Brown, David. “The Impact of Technology on Society.” Scientific American, vol. 303, no. 3, 2010, pp. 48-55.
Note that each citation includes the author’s name, title of the source, publication date, and publisher (if applicable). The sources are listed in alphabetical order by the author’s last name.
Last Updated on September 5, 2023

Leave a Comment Cancel reply
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Citing sources
Citation Styles Guide | Examples for All Major Styles
Published on June 24, 2022 by Jack Caulfield . Revised on November 7, 2022.
A citation style is a set of guidelines on how to cite sources in your academic writing . You always need a citation whenever you quote , paraphrase , or summarize a source to avoid plagiarism . How you present these citations depends on the style you follow. Scribbr’s citation generator can help!
Different styles are set by different universities, academic associations, and publishers, often published in an official handbook with in-depth instructions and examples.
There are many different citation styles, but they typically use one of three basic approaches: parenthetical citations , numerical citations, or note citations.
Parenthetical citations
- Chicago (Turabian) author-date
CSE name-year
Numerical citations
CSE citation-name or citation-sequence
Note citations
- Chicago (Turabian) notes and bibliography
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
Types of citation: parenthetical, note, numerical, which citation style should i use, parenthetical citation styles, numerical citation styles, note citation styles, frequently asked questions about citation styles.
The clearest identifying characteristic of any citation style is how the citations in the text are presented. There are three main approaches:
- Parenthetical citations: You include identifying details of the source in parentheses in the text—usually the author’s last name and the publication date, plus a page number if relevant ( author-date ). Sometimes the publication date is omitted ( author-page ).
- Numerical citations: You include a number in brackets or in superscript, which corresponds to an entry in your numbered reference list.
- Note citations: You include a full citation in a footnote or endnote, which is indicated in the text with a superscript number or symbol.
Citation styles also differ in terms of how you format the reference list or bibliography entries themselves (e.g., capitalization, order of information, use of italics). And many style guides also provide guidance on more general issues like text formatting, punctuation, and numbers.
Prevent plagiarism. Run a free check.
In most cases, your university, department, or instructor will tell you which citation style you need to follow in your writing. If you’re not sure, it’s best to consult your institution’s guidelines or ask someone. If you’re submitting to a journal, they will usually require a specific style.
Sometimes, the choice of citation style may be left up to you. In those cases, you can base your decision on which citation styles are commonly used in your field. Try reading other articles from your discipline to see how they cite their sources, or consult the table below.
The American Anthropological Association (AAA) recommends citing your sources using Chicago author-date style . AAA style doesn’t have its own separate rules. This style is used in the field of anthropology.
APA Style is defined by the 7th edition of the Publication Manual of the American Psychological Association . It was designed for use in psychology, but today it’s widely used across various disciplines, especially in the social sciences.
Generate accurate APA citations with Scribbr
The citation style of the American Political Science Association (APSA) is used mainly in the field of political science.
The citation style of the American Sociological Association (ASA) is used primarily in the discipline of sociology.
Chicago author-date
Chicago author-date style is one of the two citation styles presented in the Chicago Manual of Style (17th edition). It’s used mainly in the sciences and social sciences.
The citation style of the Council of Science Editors (CSE) is used in various scientific disciplines. It includes multiple options for citing your sources, including the name-year system.
Harvard style is often used in the field of economics. It is also very widely used across disciplines in UK universities. There are various versions of Harvard style defined by different universities—it’s not a style with one definitive style guide.
Check out Scribbr’s Harvard Reference Generator
MLA style is the official style of the Modern Language Association, defined in the MLA Handbook (9th edition). It’s widely used across various humanities disciplines. Unlike most parenthetical citation styles, it’s author-page rather than author-date.
Generate accurate MLA citations with Scribbr
The American Chemical Society (ACS) provides guidelines for a citation style using numbers in superscript or italics in the text, corresponding to entries in a numbered reference list at the end. It is used in chemistry.
The American Medical Association ( AMA ) provides guidelines for a numerical citation style using superscript numbers in the text, which correspond to entries in a numbered reference list. It is used in the field of medicine.
CSE style includes multiple options for citing your sources, including the citation-name and citation-sequence systems. Your references are listed alphabetically in the citation-name system; in the citation-sequence system, they appear in the order in which you cited them.
The Institute of Electrical and Electronics Engineers ( IEEE ) provides guidelines for citing your sources with IEEE in-text citations that consist of numbers enclosed in brackets, corresponding to entries in a numbered reference list. This style is used in various engineering and IT disciplines.
The National Library of Medicine (NLM) citation style is defined in Citing Medicine: The NLM Style Guide for Authors, Editors, and Publishers (2nd edition).
Vancouver style is also used in various medical disciplines. As with Harvard style, a lot of institutions and publications have their own versions of Vancouver—it doesn’t have one fixed style guide.
The only proofreading tool specialized in correcting academic writing - try for free!
The academic proofreading tool has been trained on 1000s of academic texts and by native English editors. Making it the most accurate and reliable proofreading tool for students.
Try for free
The Bluebook: A Uniform System of Citation is the main style guide for legal citations in the US. It’s widely used in law, and also when legal materials need to be cited in other disciplines.
Chicago notes and bibliography
Chicago notes and bibliography is one of the two citation styles presented in the Chicago Manual of Style (17th edition). It’s used mainly in the humanities.
The Oxford University Standard for the Citation of Legal Authorities ( OSCOLA ) is the main legal citation style in the UK (similar to Bluebook for the US).
There are many different citation styles used across different academic disciplines, but they fall into three basic approaches to citation:
- Parenthetical citations : Including identifying details of the source in parentheses —usually the author’s last name and the publication date, plus a page number if available ( author-date ). The publication date is occasionally omitted ( author-page ).
- Numerical citations: Including a number in brackets or superscript, corresponding to an entry in your numbered reference list.
- Note citations: Including a full citation in a footnote or endnote , which is indicated in the text with a superscript number or symbol.
Check if your university or course guidelines specify which citation style to use. If the choice is left up to you, consider which style is most commonly used in your field.
- APA Style is the most popular citation style, widely used in the social and behavioral sciences.
- MLA style is the second most popular, used mainly in the humanities.
- Chicago notes and bibliography style is also popular in the humanities, especially history.
- Chicago author-date style tends to be used in the sciences.
Other more specialized styles exist for certain fields, such as Bluebook and OSCOLA for law.
The most important thing is to choose one style and use it consistently throughout your text.
A scientific citation style is a system of source citation that is used in scientific disciplines. Some commonly used scientific citation styles are:
- Chicago author-date , CSE , and Harvard , used across various sciences
- ACS , used in chemistry
- AMA , NLM , and Vancouver , used in medicine and related disciplines
- AAA , APA , and ASA , commonly used in the social sciences
APA format is widely used by professionals, researchers, and students in the social and behavioral sciences, including fields like education, psychology, and business.
Be sure to check the guidelines of your university or the journal you want to be published in to double-check which style you should be using.
MLA Style is the second most used citation style (after APA ). It is mainly used by students and researchers in humanities fields such as literature, languages, and philosophy.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Caulfield, J. (2022, November 07). Citation Styles Guide | Examples for All Major Styles. Scribbr. Retrieved April 9, 2024, from https://www.scribbr.com/citing-sources/citation-styles/
Is this article helpful?

Jack Caulfield
Other students also liked, apa vs. mla | the key differences in format & citation, the basics of in-text citation | apa & mla examples, how to avoid plagiarism | tips on citing sources, scribbr apa citation checker.
An innovative new tool that checks your APA citations with AI software. Say goodbye to inaccurate citations!

This paper is in the following e-collection/theme issue:
Published on 11.4.2024 in Vol 26 (2024)
This is a member publication of Imperial College London (Jisc)
Regulatory Standards and Guidance for the Use of Health Apps for Self-Management in Sub-Saharan Africa: Scoping Review
Authors of this article:

- Benard Ayaka Bene 1, 2 , MBBS, MPH ;
- Sunny Ibeneme 3 , MD, PhD ;
- Kayode Philip Fadahunsi 1 , MBBS, MPH ;
- Bala Isa Harri 4 , MBBS, MPH, MSc ;
- Nkiruka Ukor 5 , MSc ;
- Nikolaos Mastellos 1 , BSc, PhD ;
- Azeem Majeed 1 , MD ;
- Josip Car 1, 6 , MSc, MD, PhD
1 Department of Primary Care and Public Health, School of Public Health, Imperial College London, London, United Kingdom
2 Department of Public Health, Federal Ministry of Health, Abuja, Nigeria
3 Digital Health Specialist, UNICEF East Asia Pacific Regional Office, Bangkok, Thailand
4 Department of Health Planning, Research and Statistics, Federal Ministry of Health, Abuja, Nigeria
5 Strategic Health Information Cluster, World Health Organization, Abuja, Nigeria
6 School of Life Course & Population Sciences, King’s College London, London, United Kingdom
Corresponding Author:
Benard Ayaka Bene, MBBS, MPH
Department of Primary Care and Public Health
School of Public Health
Imperial College London
The Reynolds Building
St Dunstan’s Road
London, W6 8RP
United Kingdom
Phone: 44 7598439185
Email: [email protected]
Background: Health apps are increasingly recognized as crucial tools for enhancing health care delivery. Many countries, particularly those in sub-Saharan Africa, can substantially benefit from using health apps to support self-management and thus help to achieve universal health coverage and the third sustainable development goal. However, most health apps published in app stores are of unknown or poor quality, which poses a risk to patient safety. Regulatory standards and guidance can help address this risk and promote patient safety.
Objective: This review aims to assess the regulatory standards and guidance for health apps supporting evidence-based best practices in sub-Saharan Africa with a focus on self-management.
Methods: A methodological framework for scoping reviews was applied. A search strategy was built and applied across the following databases, gray literature sources, and institutional websites: PubMed, Scopus, World Health Organization (WHO) African Index Medicus, OpenGrey, WHO Regional Office for Africa Library, ICTworks, WHO Directory of eHealth policies, HIS Strengthening Resource Center, International Telecommunication Union, Ministry of Health websites, and Google. The search covered the period between January 2005 and January 2024. The findings were analyzed using a deductive descriptive content analysis. The policy analysis framework was adapted and used to organize the findings. The Reporting Items for Stakeholder Analysis tool guided the identification and mapping of key stakeholders based on their roles in regulating health apps for self-management.
Results: The study included 49 documents from 31 sub-Saharan African countries. While all the documents were relevant for stakeholder identification and mapping, only 3 regulatory standards and guidance contained relevant information on regulation of health apps. These standards and guidance primarily aimed to build mutual trust; promote integration, inclusion, and equitable access to services; and address implementation issues and poor coordination. They provided guidance on systems quality, software acquisition and maintenance, security measures, data exchange, interoperability and integration, involvement of relevant stakeholders, and equitable access to services. To enhance implementation, the standards highlight that legal authority, coordination of activities, building capacity, and monitoring and evaluation are required. A number of stakeholders, including governments, regulatory bodies, funders, intergovernmental and nongovernmental organizations, academia, and the health care community, were identified to play key roles in regulating health apps.
Conclusions: Health apps have huge potential to support self-management in sub-Saharan Africa, but the lack of regulatory standards and guidance constitutes a major barrier. Hence, for these apps to be safely and effectively integrated into health care, more attention should be given to regulation. Learning from countries with effective regulations can help sub-Saharan Africa build a more robust and responsive regulatory system, ensuring the safe and beneficial use of health apps across the region.
International Registered Report Identifier (IRRID): RR2-10.1136/bmjopen-2018-025714
Introduction
Health apps are the most widely used digital health products globally [ 1 , 2 ]. Harnessing the potential of health apps creates a huge opportunity in providing support for health care delivery, including patient communication, patient education, and decision support for self-management [ 3 - 8 ]. Health apps can be an effective tool to strengthen health systems worldwide, especially in low- and middle-income countries including those in sub-Saharan Africa [ 4 , 5 , 9 ]. As a result, the attainment of universal health coverage (UHC) and sustainable development goal (SDG) 3, good health and well-being, can be accelerated [ 8 , 10 ].
Many health apps fall below the expected quality threshold [ 11 ]. Several studies have found that widely used health apps are often technically unreliable and clinically unsafe [ 12 - 14 ] and do not comply with ethical standards and the principles of confidentiality of information and data privacy [ 15 , 16 ]. In addition, many commercially available health apps were not developed using interoperability standards that are widely accepted in sub-Saharan Africa (eg, Fast Healthcare Interoperability Resources [FHIR]) [ 17 - 20 ]. Consequently, it becomes difficult to integrate these apps into a clinical workflow.
Hence, regulation through robust mechanisms is crucial to enhance the development, implementation, and adoption of health apps. Regulatory standards and guidance are essential for the safety of patients as they ensure quality assurance of any new technology in health care and contribute to building mutual trust while promoting the optimal use of the technology [ 21 - 23 ]. Therefore, to ensure that health apps that are used to support the self-management of patients are technically reliable and clinically safe, interoperable across systems, and compliant with the principles of confidentiality of information and data privacy, there is a need for effective regulatory standards. Furthermore, effective regulation can help ensure that health apps for self-management are culturally functional and competent and are accessible to those who need them regardless of gender, ethnicity, geographical location, or financial status [ 24 - 31 ].
Since 2005, there have been ongoing efforts to strengthen digital health governance at both the national and international levels [ 32 , 33 ]. In 2018, the World Health Organization (WHO) member states renewed their commitment to using digital health technologies (DHTs) to advance UHC and SDG 3 [ 33 ]. However, to date, the extent to which the use of health apps for self-management is regulated across countries within the WHO African Region (also known as sub-Saharan Africa) remains unclear. Therefore, this review was conducted to identify available regulatory standards and guidance and assess the extent to which they regulate health apps for self-management in sub-Saharan Africa. The review also mapped out the key stakeholders and their roles in regulating health apps for self-management across sub-Saharan Africa.
Review Questions
The review attempted to answer the following questions: (1) What regulatory standards and guidance are available for regulating health apps for self-management across sub-Saharan Africa? (2) To what extent do regulatory standards and guidance regulate health apps for self-management in terms of what aspects are regulated; why, how, and for whom; and what aspects are not regulated? (3) Who are the key stakeholders and what are their roles in regulating health apps for self-management?
Study Design
The process of this scoping review followed the methodological framework for conducting a scoping study originally described by Arksey and O’Malley [ 34 ] and the updated methodological guidance for conducting a Joanna Briggs Institute scoping review [ 34 - 37 ]. The reporting of the review was guided by the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist [ 38 ]. A completed PRISMA-ScR checklist is provided in Multimedia Appendix 1 . The protocol of this scoping review was published in BMJ Open [ 30 ].
Identifying Relevant Documents
Two reviewers (BAB and SI) developed the search strategy with the assistance of a librarian and in consultation with other research team members (KPF, BIH, NU, NM, AM, and JC). The following key terms were included: policy, legislation, strategy, regulation, standard, criterion, framework, guidance, guideline, digital health, eHealth, app, WHO African Region, and sub-Saharan Africa, and the names of all sub-Saharan African countries.
Owing to the absence of regulatory standards and guidance in scientific databases, the search focus was narrowed down to gray literature sources and institutional websites, including OpenGrey, WHO Regional Office for Africa (AFRO) Library, repositories for digital health policies (ICTworks, WHO’s Directory of eHealth Policies, and Health Information System Strengthening Resource Center), as well as the websites of WHO, International Telecommunication Union (ITU), and Ministries of Health (MOHs). The only scientific databases searched were PubMed, Scopus, and WHO AIM. PubMed was not included in the protocol. We also conducted a systematic search on Google. We used truncation to increase the yield of the results. The search strategy was then applied across PubMed, Scopus, and WHO AIM databases using Boolean terms (mainly OR and AND ) to combine search results. Gray literature sources and institutional websites were searched using phrases containing ≥2 keywords such as “eHealth regulation,” “digital health regulatory standard,” “eHealth regulatory standard,” “digital health regulation,” “digital health policy,” “eHealth policy,” “digital health strategy,” and “eHealth strategy.” For Google search, we added the names of the country to the phrases (eg, “digital health regulation Nigeria”). The reference lists of the included documents were also searched, and key individuals at the MOHs, WHO Country Offices, and the WHO AFRO were contacted for related documents. When our search was conducted, the WHO Directory of eHealth policies website was unavailable, and the WHO AFRO Library was undergoing reconstruction. The search strategies for PubMed, Scopus, and WHO AIM are provided in Multimedia Appendix 2 . The search was conducted between 2005 and January 2024.
Study Selection
The search results obtained from PubMed, Scopus, and WHO AIM were imported into Mendeley (Elsevier) [ 39 ] to remove duplicates. The search conducted on OpenGrey did not yield any results, whereas relevant records obtained from institutional websites, repositories, and Google were downloaded as PDF copies and uploaded to Mendeley. After removing duplicates, the remaining results were imported into Covidence (Veritas Health Innovation) [ 40 ] for screening. Two reviewers (BAB and SI) applied the predefined eligibility criteria ( Textbox 1 ) to screen the documents in 2 stages (title and abstract or executive summary). All discrepancies were discussed until the reviewers reached agreement.
Inclusion criteria
- Type of document: Regulatory standards, guidance, policies, strategies, and committee or government reports that address regulatory issues related to the use of health apps for self-management
- Location: Documents developed and implemented in countries within sub-Saharan Africa
- Date of publication: Documents developed since 2005; the global efforts toward promoting standards to minimize variability and potential harms that could arise from poorly regulated use of digital health began in 2005 [ 33 ]
- Language: Documents written in English language and other official languages of sub-Saharan African countries (Portuguese and French)
Exclusion criteria
- Type of document: Standards, guidance, policies, strategies, and reports not related to regulation of health apps
- Location: Documents from countries outside sub-Saharan Africa
- Date of publication: Documents developed before 2005
- Language: None
Data Charting (Extraction)
Two reviewers (BAB and SI), in consultation with the other members of the research team, developed the data extraction forms using an iterative process that included piloting data extraction and refinement until a consensus was reached.
We proposed in the study protocol [ 30 ] that data extraction would be conducted by the 2 reviewers independently. However, owing to the approach adopted for data extraction (deductive qualitative content analysis), 1 reviewer, rather than 2, initially extracted data from the included documents, and any concerns were discussed with a second reviewer [ 41 ]. Unresolved issues were then discussed and resolved with a third reviewer in a steering group meeting.
Collating, Summarizing, and Reporting Results
To address the research questions (particularly question 2), we adopted a deductive descriptive qualitative content analysis method to analyze and report the key findings. The policy analysis framework by Walt and Gilson [ 42 ] was adapted and applied to ensure that there was a consistent way of organizing the key findings: (1) Content (which aspects are regulated and which aspects are not?)—these are the components that directly or indirectly address regulatory issues related to the use of health apps for self-management, including areas that have not been addressed. (2) Context (why are those aspects regulated?)—this characterizes the rationale indicated for addressing regulatory issues related to the use of health apps for self-management. (3) Process (how are the regulatory standards developed and implemented?)—this describes the methods or approaches used to develop and implement regulatory standards. (4) Actors (who are the regulatory standards targeted toward?)—these are the key actors targeted by the standards.
Using a deductive descriptive qualitative content analysis approach, we examined each included document to systematically identify texts for concepts, patterns, and other relevant information. We then categorized them under content, context, process, or actors in relation to regulating health apps for self-management. The findings under content and context were further organized based on 4 predefined regulatory categories or themes as documented in the literature, namely (1) technical and clinical safety [ 12 - 14 ], (2) data protection and security [ 15 , 16 ], (3) standards and interoperability [ 28 , 31 ], and (4) inclusion and equitable access [ 24 - 29 ].
To address the third research question, the Reporting Items for Stakeholder Analysis (RISA) tool [ 41 ] was used as a guide to group key stakeholders based on role categorization as recognized globally by the WHO, the ITU, and UNESCO [ 32 , 33 , 43 ].
Ethical Considerations
Primary data were not collected in this study. Therefore, no ethics approval was required.
Search Results
A total of 2900 records were obtained after removing duplicates. Although the literature search was conducted in English, the search also yielded documents written in French and Portuguese from the ICTworks repository [ 44 ]. Following the initial screening of the title and abstract (or executive summaries), 73 documents were retrieved for full-text assessment. After applying the inclusion criteria for the full-text assessment, 49 documents were found eligible for inclusion in the review.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram [ 45 ] showing the study selection process is presented in Figure 1 .

Types of Documents
On the basis of the inclusion criteria, 3 categories of documents were considered for this review, namely “stand-alone regulatory standards and guidance that potentially regulate health apps for self-management,” “national policies and strategies on digital health,” and “other national documents that relate to the regulation of health apps for self-management.” Table 1 presents the types of documents obtained for each country within sub-Saharan Africa.
Characteristics of the Included Documents
Stand-alone regulatory standards and guidance.
We identified and included 6 stand-alone regulatory standards [ 18 , 19 , 46 - 49 ] from 3 countries (Ethiopia, Kenya, and Nigeria). All 6 documents were written in English. The years of development ranged between 2013 and 2021, as indicated in Multimedia Appendix 3 . The years of implementation were not specifically stated.
Although none of the included regulatory standards were exclusively developed to regulate health apps for self-management, 3 of them (Kenya Standards and Guidelines for mHealth Systems [ 18 ], Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ], and Health Sector Information and Communications Technology Standards and Guidelines [ 48 ]) provided concept and information relevant to the regulation of health apps and were included in the qualitative content analysis. The Kenya Standards and Guidelines for mHealth Systems [ 18 ] provides standards and guidelines on the design, development, and implementation of mobile health (mHealth) solutions to ensure they are interoperable, scalable, and sustainable. The Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] outlines the principles, requirements, and standards for eHealth systems interoperability in Kenya. The Health Sector Information and Communications Technology Standards and Guidelines [ 48 ] provide guidance and a consistent approach across the health sector in Kenya for establishing, acquiring, and maintaining current and future information systems and information and communications technology (ICT) infrastructure that foster interoperability across systems. These 3 documents are a good combination of regulatory standards and guidance that provide content and context relevant to the regulation of health apps in sub-Saharan Africa.
The remaining 3 standards (standard for electronic health record [EHR] system in Ethiopia [ 19 ], standards and guidelines for electronic medical record systems in Kenya [ 46 ], and the health information exchange standard operating procedure and guideline [ 49 ]) were exclusively developed for EHRs or electronic medical records. However, they contain information relevant for mapping stakeholders with potential roles in regulating health apps for supporting self-management.
National Policies and Strategies on Digital Health
This review includes 35 national policies and strategies that are related to digital health (potentially covering health apps) [ 50 - 84 ] from 31 countries written in English, French, and Portuguese (Benin, Botswana, Burkina Faso, Burundi, Cameroon, Comoros, Côte d’Ivoire [Ivory Coast], Democratic Republic of the Congo, Eswatini, Ethiopia, Gabon, Ghana, Kenya, Liberia, Madagascar, Malawi, Mali, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, South Africa, Tanzania, Togo, Uganda, Zambia, and Zimbabwe). Although the literature search was conducted in English, it also yielded documents written in French and Portuguese from the ICTworks repository. The years of development and implementation range between 2005 and 2030. Policies and strategies written in French and Portuguese were translated into English using Google Translate. Documents labeled as national development plans, strategic plans, and strategic development plans were considered as national strategies.
National policies and strategies do not offer specific standards or guidance, but rather outline the country’s vision, policy directions, and strategies for using digital technologies in health care. They provide useful information for identifying digital health stakeholders who can play a role in regulating health apps for self-management. For example, Nigeria has a separate National Digital Health Policy [ 72 ] and a National Digital Health Strategy [ 71 ]. Both documents were developed by building on the lessons learned from the end-term evaluation of the previous National Health ICT Strategic Framework [ 85 ]. They describe Nigeria’s renewed vision, mission, goals, objectives, and strategies for the development and implementation of digital health with the aim to improve the quality, efficiency, and effectiveness of health service delivery and health outcomes.
It is worth noting that for countries with >1 policy or strategy, we included only the most recent versions. For instance, as mentioned earlier, Nigeria now has both a national digital health policy and a national digital health strategy. These 2 documents supersede and thus replace the old National Health ICT Strategic Framework [ 86 ]. Details of included documents are presented in Multimedia Appendix 3 .
Other Related National Documents
We included 8 other documents [ 20 , 85 , 87 - 92 ] from 6 countries (Ethiopia, Kenya, Liberia, Nigeria, South Africa, and Tanzania) that did not fall under either stand-alone regulatory standards and guidance or national policies and strategies. These were mostly frameworks, road maps, and reports that potentially provide information relevant to the use of health apps. The years of development and implementation range from 2016 to 2025. These documents do not provide standards or guidance, but they contain information that can help map the digital health stakeholders that potentially play a role in regulating health apps for self-management. When multiple versions of a document exist, only the latest version was taken into consideration. Multimedia Appendix 3 provides details of the included documents.
Content: Aspects That Are Regulated and Aspects That Are Not
Technical and clinical safety.
Technical and clinical safety standards are required to prevent or minimize the harm that may arise from the use of the health ICT systems (including mHealth systems) as well as to improve the health outcomes and user satisfaction. As shown in Figure 2 , two subthemes were generated from included standards [ 18 , 47 , 48 ] as content under technical and clinical safety: v(1) guidance on system quality and (2) guidance on software or app development, acquisition, support, and maintenance.

Notably, 2 of the included standards [ 18 , 47 ] provide guidance on system quality to ensure the quality, security, reliability, performance, and maintenance of eHealth and mHealth systems. The Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] recommend the implementation of a data quality protocol to ensure that the data collection, collation, analysis, interpretation, dissemination, and use are managed in accordance with the quality standards. Similarly, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] recommends the inclusion of the following requirements in the technical manual: (1) minimum hardware requirements that should incorporate the preferred hardware architecture, (2) minimum software requirements that should include the minimum version of the underlying operating system as well as acceptable versions of related software, and (3) a detailed list of software dependencies (external libraries) necessary for the system to function properly.
The included standards [ 18 , 48 ] cover guidance on software or app development, acquisition, support, and maintenance, which aim to ensure the efficiency and effectiveness of eHealth and mHealth systems. The Kenya Standards and Guidelines for mHealth Systems [ 18 ] recommends a technical manual to provide a detailed description of the system’s installation and maintenance processes for system administrators and implementers; a developer’s guide for software developers and programmers to provide them with an overview of the system, description of the software design methodologies, description of the system architecture, and technical design diagrams; and a user manual to aid users in understanding how the system works and how each feature operates; in addition, the technical manual contains instructions for operating the software; entering and updating data; and generating, saving, and printing reports.
Although the contents generated here provide guidance that is relevant to health apps, they are not specific to health apps. Moreover, there are no clear measures to enable individuals or organizations that use health apps to manage clinical risk appropriately.
Data Protection and Security
Data protection and security are crucial aspects of managing patient information, thus ensuring the confidentiality, integrity, and availability of data as well as the rights and interests of the patient. Two subthemes related to data protection and security are (1) security measures for adequate protection of patients’ digital records and (2) guidance on data exchange.
The included standards [ 18 , 48 ] provide security measures for eHealth or mHealth systems to ensure the adequate protection of digitally accessible patient records. These measures include authentication, accountability, identification, authorization, integrity, confidentiality, availability, security, administration, and audit. This will help to achieve confidentiality, integrity, availability, and nonrepudiation of patient data or health records. Additional levels of security such as data encryption are required when there is a need to store sensitive information on removable devices or media or outside the MOH premises.
The Kenya Standards and Guidelines for mHealth Systems [ 18 ] provide the following guidance on data exchange to ensure privacy: (1) anonymize client data as much as possible before they can be shared; (2) where possible, use pseudonyms for the client data before they can be shared; (3) aggregate client data before they can be shared to reduce possibilities of tracing the data back to the client; and (4) minimize data so that access is available only to the data set required for that particular use. With regard to privacy rules, the Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] propose that a notice of privacy practices should be given to patients describing how their information may be used or shared while also specifying their legal rights.
Standards and Interoperability
Standards and interoperability are essential concepts in the field of IT, especially for systems that need to communicate and exchange data, as seen in the use of health apps for self-management. Two subthemes related to standards and interoperability are (1) interoperability as a basic requirement and (2) minimum standards to enable integration.
All the regulatory standards [ 18 , 47 , 48 ] highlight the importance of having interoperability as a basic requirement when selecting software products or services for use within the health system. This facilitates interaction across systems. For instance, to facilitate seamless interaction between mHealth systems and primary information systems for data capture, reporting, and decision support in various domains of the health system, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] recommends the incorporation of at least 3 types of interoperability, namely, technical interoperability, semantic interoperability, and process interoperability.
Furthermore, 2 regulatory standards [ 18 , 47 ] proposed minimum interoperability standards to enable the integration of services and data exchange between various systems in health care. For instance, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] suggests standards (for interoperability) for mHealth systems that are consistent with the recommendations in internationally accepted standards. They include the following: (1) clinical messaging—ensuring mHealth systems conform to Health Level 7 (HL7) version 3 standards and corresponding implementation guideline; (2) clinical terminology—ensuring terminologies and classifications for clinical concepts (eg, International Classification of Diseases, tenth revision—for diseases; Systemized Nomenclature of Medicine—for clinical data coding; Logical Observation Identifiers Names and Codes—for laboratories; and RxNorm—for Pharmacies); (3) the mHealth system must use the latest versions of international standards, such as HL7 Clinical Document Architecture for electronic sharing of clinical documents; (4) concepts—mHealth systems will use the idea of “concepts” so that information can be transmitted between systems without losing meaning or context, and HL7 Reference Implementation Model or other appropriate standards are recommended for implementing concepts; (5) architecture—to develop mHealth systems, developers should define the system architecture that should include data elements and business logic. Furthermore, to define how mHealth systems interact with other systems, developers of mHealth solutions must provide application programming interfaces. FHIR is the preferred application programming interface interoperability standard.
Inclusion and Equitable Access
Inclusion and equitable access are essential principles to ensure that health apps are culturally appropriate and relevant and accessible to everyone, regardless of gender, ethnicity, location, or economic status.
All the included regulatory standards [ 18 , 47 , 48 ] indicate that they were developed based on a combination of participatory and consultative approaches involving multiple actors or stakeholders, thus promoting inclusion. However, there are no specific measures or guidance to ensure adequate engagement and representation of all the relevant stakeholders and to sustain that engagement.
The Kenya Standards and Guidelines for mHealth Systems [ 18 ] proposes the following systems attributes to ensure equitable access to mHealth services at all times and from anywhere: (1) allocation of adequate storage and bandwidth capacity; (2) fast response time; (3) fast recovery capabilities; (4) performance monitoring; (5) business continuity processes, for example, backups; and (6) redundant sites and links. Furthermore, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] prescribes the following metrics for measuring system availability: (1) downtime per year, (2) mean time between failure, (3) mean time to repair, and (4) failure in time.
Although the abovementioned systems attributes and metrics for measuring system availability are important, the included standards do not offer any concrete guidance or model for achieving a sustainable funding mechanism for health apps to ensure that they are readily available and accessible to those who need them.
Context: Reasons Why Those Aspects Are Regulated
The 3 standards [ 18 , 47 , 48 ] were developed to address unsafe, isolated, and inconsistent implementation. The Health Sector ICT Standards and Guidelines [ 48 ] suggest that although there has been a lot of ICT investment in the health sector leading to improvement in service delivery and information exchange, there remains the challenge of inconsistency in ICT implementation and harmonization of the health sector system requirements. Hence, there is a need to adopt global best practices for software development, acquisition, support, and maintenance by the MOH. In addition, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] indicates that standards and guidelines are necessary to ensure a consistent approach to the development of ICT systems. Similarly, the Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] recognize the need to ensure that the processes of collecting, collating, analyzing, interpreting, disseminating, and using data are consistent with data quality standards.
To build mutual trust and maximize the benefits of eHealth information exchange, the Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] reiterate that as health data are constantly being exchanged across health information systems, robust security standards are required to maintain their integrity and confidentiality. This will build the trust of service users and consequently help to maximize the benefits of eHealth information exchange such as in self-management.
Two of the included regulatory standards [ 47 , 48 ] indicate that the context for standards and interoperability was (1) to address poor coordination, duplication of efforts, and inefficient use of resources and (2) to promote the integration of ICT systems.
The Kenya Standards and Guidelines for E-Health Systems Interoperability [ 47 ] acknowledge that the absence of interoperability standards over the years has led to the duplication of efforts and the inefficient use of ICT resources in health care. Now that ICT has become increasingly relevant in improving efficiency in health service delivery, the Kenya MOH recognizes the need to adopt a standardized approach, hence the development of interoperability standards for eHealth systems. In addition, the Health Sector ICT Standards and Guidelines [ 48 ] emphasize the relevance of interoperability as a requirement for addressing the inconsistency in implementing ICT in the health sector.
The Health Sector ICT Standards and Guidelines [ 48 ] consider “integration of ICT systems” as one of its key guiding principles, acknowledging the lack of information systems integration as a challenge experienced by ICT services across Kenya.
The contexts for inclusion and equitable access as generated from included standards [ 18 , 47 , 48 ] were (1) to promote inclusion and (2) to promote equitable access to services.
To promote inclusion, the standards [ 18 , 47 , 48 ] highlight the importance of involving and engaging multiple actors and stakeholders during the development process. However, no emphasis was placed on the need to sustain stakeholder engagement during the implementation process.
Pertaining to equitable access, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] acknowledges that the public health care system is largely unavailable to most of the population in many developing countries because of geographical location, resource constraints, inefficiencies, and lack of awareness. Hence, it recognizes the importance of ensuring that mHealth services are always accessible by users and from anywhere as well as the need to put in place mechanisms to make this happen.
Process: How the Regulations Are Developed and Implemented
Two themes were generated from the included standards: development and implementation processes [ 18 , 47 , 48 ].
Development Process
All the included standards [ 18 , 47 , 48 ] indicate that they were developed through a participatory process and in consultation with a range of subject experts and interest groups. In addition, the standards [ 18 , 47 , 48 ] adopted a multisectoral approach to engage health-related stakeholders from government ministries or agencies and development partners and a range of subject experts and interest groups. It has also been reported that these standards [ 18 , 47 , 48 ] were developed based on international best practices and with reference to international standards. However, there is no indication that a stakeholder engagement strategy was adopted to sustain the engagement of stakeholders through the entire development and implementation process.
Implementation Process
The 3 regulatory standards [ 18 , 47 , 48 ] identify the key requirements to ensure effective implementation of IT services in the health sector. These are (1) legal authority, (2) coordination, (3) building capacity, and (4) monitoring and evaluation.
The included standards [ 18 , 47 , 48 ] were established based on the legal provisions enshrined in the health and other related acts and laws of Kenya as well as the relevant policies and strategies. Hence, it is expected that their implementation will comply with and be backed by those legal provisions. For example, the Health Sector ICT Standards and Guidelines [ 48 ] indicate that its implementation will be supported by the authority from the Kenya Communications Act 2009, E-Government Strategy, and National ICT Policy. Similarly, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] asserts that it will be implemented by complying with existing and relevant national policies, legal frameworks, strategies, and standards, including the Health Information Policy, ICT Standards, and System Interoperability Principles.
The included standards [ 18 , 47 , 48 ] report that the implementation of regulations will require robust coordination mechanisms. For instance, the Health Sector ICT Standards and Guidelines [ 48 ] indicate that, as the Ministry’s ICT resource manager, the principal secretary (also the head of ICT), in collaboration with the ICT Governance Committee, is responsible for coordinating the implementation of the standard. The ICT Governance Committee comprises representatives from the heads of departments and ICT development partners in the health sector. The committee’s responsibilities include overseeing, enforcing, and reviewing standards as well as initiating ICT projects.
The Health Sector ICT Standards and Guidelines [ 48 ] highlight the need for capacity building or training of the MOH staff and stakeholders who are the primary users of the Ministry’s ICT services. This will enhance their capacity to implement the guidelines provided in the document in line with the ministry’s human resource development policies, regulations, and rules. However, it is acknowledged that building capacity for health ICT is a challenge given that there is low adoption of ICT among health providers, and ICT is not routinely included in the course content of most training programs. The Kenya Standards and Guidelines for mHealth Systems [ 18 ] listed the “number of mHealth practitioners trained on the standards and guidelines” as one of the indicators for monitoring and evaluating mHealth interventions.
The Health Sector ICT Standards and Guidelines [ 48 ] assert that monitoring and evaluation is an essential role of the MOH to ensure efficiency, accountability, and transparency throughout the implementation period. It further stresses that all those who use the Ministry’s ICT services are required to adhere to the provisions in the standard as the MOH will carry out quarterly monitoring exercises on the use of the standard to ensure compliance based on clear indicators. Furthermore, the ICT Governance Committee will periodically review and amend the standard to keep it relevant and effective. Similarly, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] establishes the following key indicators for effectively monitoring and evaluating the implementation of the standards and guidelines: (1) the number of counties in which the MOH has disseminated the standards and guidelines, (2) the number of counties successfully implementing the standards and guidelines, (3) the number of mHealth practitioners trained on the standards and guidelines, (4) the number of mHealth practitioners accessing the standards and guidelines, (5) the number of mHealth practitioners who correctly understand the standards and guidelines, (6) the number of stakeholders who adhere to the standards and guidelines, (7) the number of mHealth systems that follow the required development steps, and (8) the number of mHealth practitioners who have implemented their systems by using the standards and guidelines. In addition, the Kenya Standards and Guidelines for mHealth Systems [ 18 ] indicates that the outlined standards will be reviewed every 3 years to ensure they are up to date with new changes including the changes in policies and systems upgrades.
Although all the abovementioned indicators are relevant, the implementation process is not explicit on the approach for regulating health apps and ensuring compliance with regulatory standards and guidance.
Actors: Those the Regulations Are Targeted at
The included standards [ 18 , 47 , 48 ] identified 2 main groups of actors for whom the regulations and guidance were targeted. They included (1) those who provide digital health services and (2) those who use the ICT infrastructure of the MOH.
Two of the standards [ 47 , 48 ] indicated that the regulations should be implemented by all individuals and organizations that provide ICT-related health care services to the public. Similarly, the Health Sector ICT Standards and Guidelines [ 48 ] state that all those who access or use the MOH ICT infrastructure are expected to adhere to the guidelines outlined in the document.
Mapping of Stakeholders
To address the third research question, we conducted a stakeholder mapping guided by the RISA tool [ 41 ].
A total of 11 categories of key stakeholders were identified from all 49 included documents (6 stand-alone regulatory standards and guidance, 35 national policies or strategies, and 8 other related documents). These categories are consistent with the digital health stakeholders recognized by the WHO, ITU, and UNESCO [ 32 , 33 , 43 ]. Table 2 presents the mapping of stakeholders according to their role categorization. A more detailed table with a potential role description with regard to regulating health apps for self-management is presented in Multimedia Appendix 4 .
a WHO: World Health Organization.
This paper presents the findings of a scoping review of regulatory standards and guidance for the use of health apps for self-management in sub-Saharan Africa. To the best of our knowledge, this is the first study that attempted to identify and assess the extent to which regulatory standards and guidance regulate and guide the use of health apps for self-management in sub-Saharan Africa as well as map out the key stakeholders and their potential roles.
Our findings reveal that only 1 country (Kenya) in sub-Saharan Africa currently has national regulatory standards that could potentially regulate the use of health apps for self-management. The included standards failed to adequately address adequate attention to inclusion and equitable access. This is concerning given the growing need to promote the adoption of culturally appropriate and relevant health apps and to ensure that they are available to those who need them regardless of gender, ethnicity, geographical location, or financial status [ 24 - 29 ]. Consequently, this review provides insights into the regulation of health apps for self-management in sub-Saharan Africa, which needs to be given more attention if the potential of these apps is to be harnessed in the region.
Principal Findings
We identified 49 documents from 31 countries in sub-Saharan Africa. Although none of the included standards provided a specific set of regulations on health apps for self-management, we identified 3 standards [ 18 , 47 , 48 ] that provided relevant information regarding the regulation of health apps. The included national policies and strategies, in contrast, only outline the goals and commitments made by national governments to promote the adoption of digital technologies in the health sector and the plans and paths set forth to achieve these goals. However, the information they provided was relevant for identifying and mapping digital health stakeholders who potentially have vital roles in regulating the use of health apps for self-management.
The policy analysis framework (content, context, process, and actors) [ 42 ] was adapted and applied to organize the key findings. The content covered the following areas: guidance on systems quality; guidance on software and app development, acquisition, support, and maintenance; security measures for adequate protection of patients’ digital records; guidance on data exchange; interoperability as a basic requirement; minimum standards to enable integration; involvement and engagement of relevant stakeholders; and system attributes for equitable access to services. Meanwhile, the context was to address unsafe, isolated, and inconsistent implementation; to build mutual trust and maximize the benefits of eHealth information exchange; to address poor coordination, duplication of efforts, and inefficient use of resources; to promote the integration of ICT systems; and to promote inclusion and equitable access to services. The process involved the development process (which covers participatory and consultative processes and multisectoral approach, with reference to international standards and best practices) and the implementation process (which covers legal authority, coordination, capacity building, and monitoring and evaluation). The targeted actors were those who provided digital health services and those who used the ICT infrastructure of the MOH.
Furthermore, key stakeholders with potential roles in regulating health apps for self-management were identified. They include the government, regulatory bodies, funders, intergovernmental and nongovernmental organizations, academia, and the health care community.
Implications of the Study Findings for Practice
Regulatory standards and guidance act as a bridge between technological innovation and its safe and effective use in health care. They ensure that while technology continues to advance, the safety and trust of patients are never compromised. Among the plethora of health apps on the market, the over-the-counter, nonregulated apps such as wellness and fitness apps are the most mainstream [ 93 - 95 ]. On the other side of the spectrum, there are regulated health apps that are classified under medical devices or software as medical device products [ 94 , 95 ]. Some of these are prescription-only apps, such as digital therapeutics (DTx) apps for managing substance dependence [ 95 , 96 ].
Although some high-income countries have made significant strides in ensuring the safety, effectiveness, and accessibility of health apps, the journey has indeed not been without challenges and hurdles. Sub-Saharan Africa, although dealing with its own unique set of challenges, has the opportunity to learn from the experiences of these high-income countries. This could potentially allow the region to bypass some of the hurdles encountered by high-income countries in their journeys.
Technical and clinical safety are essential requirements that health apps must meet before they can be considered for use for self-management to minimize the risk of harm to patients. It is well documented that health apps that function poorly pose a serious threat to the safety of patients. An example illustrating how health apps used for self-management can threaten patient safety is evident in a study [ 12 ]. This study [ 12 ] revealed that widely used health apps designed to calculate and estimate insulin doses could endanger patients by providing incorrect or inappropriate dose recommendations. Similarly, 2 successive studies that assessed the contents and tools of apps for asthma discovered that none of the apps in the first study offered comprehensive information or adequate tools for asthma self-management, whereas the follow-up study, which was conducted 2 years later, showed a 2-fold increase in the number of asthma apps, yet there was no improvement in the content and tools offered by the newer apps. In fact, many apps recommended self-management procedures that were not supported by evidence [ 13 , 14 ]. Accordingly, some health apps that support the self-management of long-term conditions do not adhere to evidence-based guidelines and are unresponsive to the evolving health needs of patients.
Although the context of included regulatory standards with regard to technical and clinical safety was to address unsafe, isolated, and inconsistent implementation, the guidance provided by these regulatory standards is not specific to health apps, and they do not provide appropriate guidance and standards for health organizations and other key stakeholders to establish a framework for managing the clinical risks associated with deploying and implementing self-management health apps. Considering the rapid advancements in digital health (including artificial intelligence [AI] or machine learning and big data), health apps will increasingly play a crucial role in supporting self-management through digitally enabled care pathways that will improve personalized care and health outcomes [ 97 , 98 ]. Therefore, it is imperative to ensure the technical reliability and clinical safety of health apps for self-management through robust regulatory standards and guidance. For instance, a guide on the criteria for health app assessment, developed by the UK government, includes technical stability and clinical safety as criteria for deciding whether health apps should be considered for use in the National Health Service (NHS) [ 99 ]. In addition, medical device apps are required to conform to the NHS clinical risk management standards as part of the clinical safety requirements [ 99 , 100 ]. In the event of any concerns regarding the safety of a medical device app, the Yellow Card reporting system can be used by a responsible clinical safety officer or any other individual to notify the Medicines and Healthcare products Regulatory Agency (MHRA) [ 101 , 102 ].
To adequately manage patient information when health apps are used for self-management, data protection and security standards and guidance are required. They guarantee that data are kept and handled safely and responsibly within the provisions of the law and that patients’ rights and interests are respected.
There have been ongoing concerns about compliance with ethical standards, the principles of confidentiality of information, and data privacy. For example, an assessment of apps that had previously been endorsed by the former UK NHS Apps Library revealed substantial gaps in compliance with data protection principles regarding the collection, storage, and transmission of personal information. This has raised a fundamental concern about the credibility of developer disclosures and whether these disclosures can be trusted by certification programs [ 15 ]. A study assessed the privacy practices of the 36 most popular apps for depression and smoking cessation for Android and iOS in the United States and Australia [ 16 ]. The findings revealed that although only 69% (25/36) of the apps included a privacy policy, 92% (33/36) of the apps shared data with a third party, and only 92% (23/25 with privacy policy) of the apps disclosed sharing data with a third party in their policy. Although 81% (29/36) of the apps shared data with Google and Facebook for the purposes of advertising, marketing, or analytics, only 43% (12/28) of the apps that shared data with Google and 50% (6/12) of the apps that shared data with Facebook disclosed this in their policy [ 16 ].
In this regard, health app developers and providers in the United Kingdom are required to conduct a data protection risk assessment before they launch or update their apps to ensure compliance with the United Kingdom General Data Protection Regulation (GDPR) and other relevant regulations, including the Data Protection Act 2018 [ 103 ]. By conducting a data protection risk assessment, health app developers and providers can demonstrate that they are accountable; they respect the privacy and dignity of their users; and that they deliver safe, effective, and ethical solutions [ 104 ].
Health apps are expected to play an increasingly important role in supporting self-management. However, this ambition can only be achieved if citizens trust that these apps are collecting and analyzing data safely and in accordance with robust regulatory standards and guidance. It is also crucial that these apps provide reliable information that clinicians can act on [ 98 ]. The context of the standards included in this study regarding data protection and security was to build mutual trust and maximize the benefits of eHealth information exchange. Trust is a key factor in the successful adoption and use of health apps, and transparency in data handling and clinical decision-making is essential to build and maintain that trust. This is also paramount for the widespread acceptance and impact of health apps on health care outcomes in sub-Saharan Africa.
We acknowledge the existence of numerous national laws related to data protection and security outside the health sector. Hence, guidelines that link these legislations together must be provided to ensure compliance with all relevant laws and guidance when using patient data. An example of how to achieve this is the United Kingdome’s guide to good practice for digital and data-driven health technologies that provides guidelines on how to abide by the laws and principles that govern data security and protection in the United Kingdom, including the GDPR, Data Protection Act 2018, and Caldicott Principles [ 105 ].
Standards and interoperability are essential for effectively developing, deploying, and implementing health apps to support self-management in sub-Saharan Africa. Interoperability is the ability of different systems, devices, or applications to communicate and exchange data with each other in a coordinated manner, thus providing timely and seamless portable information across organizational, regional, and national boundaries and optimizing both individual and population health [ 106 ]. In the same vein, standards enable interoperability between systems or devices through a common language and a common set of expectations [ 106 ].
Interoperability is crucial in improving the quality, safety, and efficiency of care delivery as well as empowering patients and providers with access to relevant and timely information [ 99 ]. One of the most widely used and accepted interoperability standards for health care data exchange is FHIR [ 106 , 107 ]. FHIR is a global industry standard developed by HL7 International. FHIR is designed to be quick to learn and implement and to support a variety of use cases, including self-management [ 108 ]. By using apps that are based on an FHIR standard, patients can benefit from data analytics that show how their health data relate to their chronic conditions or wellness goals [ 109 ]. They could also access all their health information from one place, even if they visit different health professionals who use different electronic medical records or EHR, thus promoting integrated care [ 28 , 31 , 33 , 109 - 115 ]. As a result, patient care can easily be coordinated.
The context of the included regulatory standards with regard to standards and interoperability was to address poor coordination, duplication of efforts, and inefficient use of resources and to promote the integration of ICT systems. However, in sub-Saharan Africa, there are many challenges and barriers to the adoption and implementation of interoperability standards, such as the lack of awareness or knowledge of the benefits and requirements of interoperability standards among stakeholders; lack of incentives or regulations to encourage or enforce the adoption of interoperability standards by app developers and vendors; lack of resources or capacity to implement interoperability standards, including technical expertise, infrastructure, funding, or governance; and lack of alignment or coordination among the different actors and initiatives involved in developing, deploying, and implementing the digital health interventions [ 30 , 116 - 119 ]. To address these challenges, some possible solutions may include raising awareness and education on the importance and value of interoperability standards for health apps among all relevant actors; developing and implementing policies and guidelines that promote or mandate the use of interoperability standards by app developers and vendors; providing technical assistance and support for app developers and vendors to adopt and implement interoperability standards, such as tools, frameworks, testing, certification, or accreditation; and establishing and strengthening collaboration and coordination among the different stakeholders and initiatives involved in health app development, deployment, and implementation in sub-Saharan Africa. In addition, the Digital Health Platform Handbook, a toolkit developed by the collaborative efforts of the WHO and ITU [ 120 ], can help countries in sub-Saharan Africa to develop and implement digital health platforms as the underlying infrastructure for interoperable and integrated national digital health systems. The digital health platform is a system-wide approach to developing digital health solutions with the aim to overcome the problems of siloed, vertical, and isolated applications and systems that hamper data management, innovation, efficiency, and impact in the health sector.
Inclusion and equitable access are crucial to ensuring that health apps and related services are culturally appropriate and relevant as well as accessible to all who need them, regardless of gender, ethnicity, geographical location, ability, or financial status [ 24 - 29 ]. This is the key to promoting a “sense of belonging” and “ownership” and thus underscoring the importance of stakeholder mapping and involvement or engagement through the development and implementation process [ 22 ].
In this study, the included regulatory standards demonstrate the importance of inclusion by adopting both a participatory and consultative approach involving multiple stakeholders from different sectors. However, the standards do not provide clear guidance to ensure the adequate participation and sustained engagement of all relevant stakeholders. The lack of concise guidance to ensure the adequate participation and engagement of all relevant stakeholders, especially the susceptible and disadvantaged groups, can increase the risk of tokenistic tendencies, which can undermine the cultural appropriateness of health apps [ 25 , 121 ]. Some susceptible groups, such as women and people with low socioeconomic status, may face additional barriers to accessing and using health apps, such as lack of digital literacy, privacy concerns, cultural norms, or stigma [ 25 ]. Similarly, the cost of developing, maintaining, and updating health apps may not be covered by public or private health insurance schemes, which could limit their affordability and availability for low-income or uninsured populations [ 95 ]. However, there is no specific guidance or model for an effective funding mechanism for health apps in the included regulatory standards.
To address these challenges and ensure equitable access to health apps for self-management in sub-Saharan Africa, possible measures may include developing policies and regulations that support integrating health app interventions into existing health systems and financing mechanisms and engaging with stakeholders from different sectors and backgrounds (including health professionals, patients, communities, governments, civil society, academia, and industry) to co-develop and co-implement frameworks or models that promote the use of health apps for self-management in ways that are responsive to the local context and needs. Moreover, establishing regulations that provide appropriate financing or reimbursement options will reduce the risk of developers of good quality health apps turning to data mining for revenue, thus increasing privacy concerns [ 95 ]. For instance, in Germany, the reimbursement of health apps classified as medical devices (Digitale Gesundheitsanwendungen) was introduced in 2021 under the statutory health insurance [ 122 , 123 ]. When a medical device is prescribed by a physician or a physiotherapist, the manufacturer must submit an application to the German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte) for approval [ 123 ]. The Federal Association of the Statutory Health Insurance Funds (Spitzenverband Bund der Krankenkassen) determines and negotiates the reimbursement thresholds following approval. However, the manufacturer must demonstrate that the app is safe, functional, and of good quality; complies with data protection requirements; and benefits patient care [ 123 ].
The process of regulating health apps essentially involves the development and implementation of regulatory standards and guidance. According to our study, the development process comprises a participatory and consultative process, a multisectoral approach, and a reference to international standards and best practices. In contrast, the implementation process is ongoing and requires appropriate legal authority, coordination, capacity building, and monitoring and evaluation.
We recognize that health apps can be accessed and used by patients from different parts of the world, and this means that countries need to carefully consider whether health apps that are accessed and used by their citizens meet the national or regional legal and ethical requirements, including their cultural and linguistic needs [ 23 ]. For countries in sub-Saharan Africa, a cross-border or regional collaboration between national legal authorities through the coordination of agencies such as the African Medicines Regulatory Harmonization (AMRH) may help to ensure that health apps built for the region are safe, effective, and user-friendly for everyone, considering the contextual differences of the countries [ 23 ]. For instance, all medical device companies that want to sell their products in the European market must obtain a Conformité Européenne (CE) mark for their devices, which indicates that they meet the legal requirements and can be freely circulated within the European Union [ 124 ]. Although the European Union member states regulate medical devices, the European Medicines Agency is involved in the regulatory process.
The regulation of health apps is extremely complex and involves a wide range of stakeholders. Therefore, a robust coordination mechanism is essential to reduce the risk of fragmentation and duplication of efforts and to promote the efficient use of resources. Most countries in sub-Saharan Africa have units in health ministries that coordinate and oversee the regulation of medical products. These units should be autonomous, full-fledged departments with legal authority (boards or commissions) to ensure independent, transparent, and accountable decision-making, but this is often not the case [ 125 ]. These units are recognized by the national authorities as regulators (eg, the National Medicines Regulatory Authority [NMRA]) [ 126 ]. Such organizational structures hinder the effectiveness of the national regulatory authorities in fulfilling their mandate and prevent the establishment of quality management systems to ensure transparent and accountable decision-making [ 125 ].
Furthermore, Essén et al [ 23 ] analyzed health app policy or regulation in 9 high-income countries (Sweden, Norway, Denmark, Netherlands, Belgium, Germany, England, the United States, and Singapore) and found that most of these countries adopted centralized approaches to app evaluation. Although centralized approaches might have advantages over self-evaluation, they may create bottlenecks and limit the availability of high-quality health apps for users. As suggested by Essén et al [ 23 ], a decentralized approach, such as the accreditation of evaluation agencies, maybe a worthwhile solution. However, this will require adequate coordination to ensure the consistency and reliability of the evaluation criteria and methods across different agencies as well as the transparency and accountability of the accreditation process. A possible way to achieve this is to adopt a common framework that can guide the evaluation and accreditation of health apps.
Similarly, the postmarket surveillance (PMS) system, which is a new regulation for medical devices in Europe, is a process of collecting and analyzing data on medical devices after they have been launched into the market to ensure their safety and performance and to identify any problems or need for improvements [ 127 , 128 ]. The PMS system is important because premarket data, which are obtained from testing a medical device before it is launched, have limitations in capturing the long-term performance and risks of the device [ 128 ]. Currently, the PMS system does not cover fitness and wellness apps, which are commonly used in self-management. Hence, Yu [ 93 ] proposed that the PMS system should also be applied to DHTs, such as fitness and wellness apps. They argue that the postmarket data would help regulators periodically review and adjust the regulatory standards for these groups of health apps based on their risks and benefits.
Drawing on the experience of the United Kingdom, it can be clearly demonstrated that the regulation of health apps is a complex, a multifaceted, and an evolving process that involves different regulators and criteria depending on the nature and function of the app. For instance, a centralized NHS Apps Library was launched as a beta site in April 2017 to provide patients with a collection of trusted and easy-to-use digital health tools [ 129 ]. The library provided access to a range of health apps that were reviewed and approved by the NHS, including apps that could help patients manage conditions such as diabetes, mental health, and chronic obstructive pulmonary disease [ 130 ]. However, the library was closed in December 2021 [ 131 ]. Although no reason for the closure was provided on the website, it is likely because of persistent concerns regarding the safety of patients and data privacy involving multiple apps including those listed in the library [ 12 , 14 - 16 , 131 , 132 ]. The NHS App was introduced in January 2019 before the closure of the NHS Apps Library to serve as the gateway for accessing NHS services including ordering repeat prescriptions and booking or managing appointments [ 133 ].
Furthermore, the United Kingdom Health Security Agency, formerly known as Public Health England, issued a guidance on criteria for health app assessment in October 2017 [ 99 ]. The purpose of this guidance was to ensure that all health apps built for the UK population work well and provide clear information about their functions, benefits, and intended outcomes for patients and health care professionals. On the basis of this guidance, those intending to build an app are required to conform to certain regulations before being considered for the app assessment process. The 2 main regulations are the medical device regulation and the Care Quality Commission (CQC) registration. Apps that are considered as medical devices must register with the MHRA and have a CE mark. Apps providing health or social care that fit into 1 of 14 regulated activities are required to register with the CQC before they can be assessed [ 134 ]. CQC is an independent regulator of health and social care services in England.
Similarly, the Organisation for the Review of Care and Health Apps (ORCHA) is a UK-based organization that independently evaluates and distributes health apps. It provides services such as app review, accreditation, curation, and recommendation within the United Kingdom and across the world [ 135 ]. ORCHA also enables organizations (including the NHS) to build a decentralized web-based digital health library of consumer-friendly over-the-counter apps [ 135 - 137 ]. These apps are continuously assessed by ORCHA against the standards and regulations in clinical and professional assurance, data quality and privacy, and usability and accessibility [ 137 ].
In addition, the Digital Technology Assessment Criteria (DTAC) were introduced in beta in October 2020, and its first official version was subsequently launched in February 2021 [ 138 ]. The DTAC plays a crucial role in ensuring that digital health tools meet the necessary standards in areas such as clinical safety, data protection, technical security, interoperability, usability, and accessibility. By serving as the national baseline criteria for DHTs in the NHS and social care, it provides a valuable framework for health care organizations during procurement. It also offers guidance for developers on the expectations for their digital technologies within the NHS and social care. This is an example of how a harmonized framework can help ensure the quality and safety of DHTs, including health apps.
In addition, the National Institute for Health and Care Excellence Evidence Standards Framework is a set of evidence standards for a wide range of DHTs designed to help evaluators and decision makers in the health care system to consistently identify DHTs that are likely to offer benefits to the users and the health care system [ 139 ]. The Evidence Standards Framework was first published in March 2019 and is ideally used before DHTs (including health apps) are considered for commissioning or procurement by the NHS [ 140 ]. It is a crucial tool for ensuring that DHTs are clinically effective and offer value to the health and care system in the United Kingdom. In August 2022, the framework was updated to include AI and data-driven technologies with adaptive algorithms [ 140 ].
Furthermore, DTx apps, which are a type of medical device, are not allowed into the UK market unless they comply with the UK GDPR and meet the requirements of DTAC. In addition, they must bear the CE or UK Conformity Assessed marks [ 141 ]. This means that DTx apps must demonstrate their safety and efficacy through clinical trials and comply with the relevant regulations for data protection and quality standards as regulated by the MHRA. DTx products are also recognized as DHTs under the National Institute for Health and Care Excellence Evidence Standards Framework [ 142 ]. DTx incorporates software to treat, prevent, or manage specific diseases or conditions [ 143 , 144 ]. The fact that DTx products typically focus on a narrow clinical indication and generate evidence of clinical efficacy underscores their potential to make a substantial contribution to self-management and health care delivery in general. The increasing recognition of the role of DTx in patient care by regulators is also noteworthy, and the creation of regulatory and reimbursement pathways for approved apps further enables DTx products to continue to play an important role in impacting health care delivery [ 1 , 143 ]. This is a testament to the potential of regulated health apps to revolutionize health care and improve patient outcomes.
Among the many lessons to learn from the experience of the United Kingdom is that the regulation of health apps must evolve to keep pace with advances in DHTs and adapt to the changing needs and demands of digital health. Moreover, efforts are being made to streamline the multifaceted approaches to simplify app regulation and access in the United Kingdom [ 23 ]. Therefore, a robust and dynamic coordination mechanism, along with political will, skilled personnel, reliable funding, and a robust framework for monitoring and evaluating progress and aligning key performance indicators, is essential for countries in sub-Saharan Africa to keep pace with the advancement in the regulation of health apps. There is also a need to strengthen collaboration and ensure regulatory harmonization among national regulatory authorities and continental bodies such as the regional economic communities, AMRH, and the WHO AFRO [ 126 ].
Capacity building and monitoring and evaluation are important factors for ensuring effective regulation of health apps given the complex nature of the process. The regulation of medical products (including health apps) in sub-Saharan Africa generally includes licensing and accreditation, evaluation, inspection, quality control, information dissemination and promotion, and monitoring of adverse events [ 125 ]. Therefore, high-level skills as well as effective monitoring and evaluation will be required to ensure the success of the process. For most countries in sub-Saharan Africa, the NMRA is responsible for coordinating and overseeing the regulatory system of medical products [ 125 , 126 ]. However, in most cases, NMRAs are unable to perform the core regulatory functions expected of them [ 145 ]. More than 90% of African countries have limited or no capacity to regulate medical products, with only 7% having moderately developed capabilities [ 145 ]. The lack of effective NMRAs in Africa exposes the citizens to potential harm by allowing unsafe, low-quality, and fake medical products to circulate and be used [ 145 ].
Although it is the responsibility of governments to establish functional regulatory systems and ensure effective monitoring and evaluation of the regulatory process, the involvement of international and continental organizations to support sub-Saharan African countries improve the regulatory capacity of their national regulatory agencies would be extremely beneficial. For instance, the African Medicines Agency (AMA) was established in November 2019 as a treaty adopted by the African Union Member States to help address the concerns arising from weak regulatory systems on the continent. At present, 37 countries have signed the AMA treaty, including 26 countries that have ratified it [ 146 ]. The main objective of the AMA is to enhance the capacity of States Parties and regional economic communities to regulate medical products to improve the quality, safety, and efficacy of medical products on the continent [ 147 ]. The AMA, in collaboration with other existing capacity building initiatives or organizations, such as the WHO Global Initiative on Digital Health, ITU, AMRH, WHO AFRO, and United Nations Children’s Fund, can assist sub-Saharan African countries in aligning their regulatory requirements with available resources and support them to acquire the necessary tools and skills to build effective and sustainable regulatory systems for health apps. This can be achieved by adopting a decentralized approach to engage a network of technical experts across the African Union similar to the model of the European Medicines Agency [ 148 ].
Actors or Stakeholders
The regulation of health apps often requires working with a wide range of actors or stakeholders. However, in this review, we identified only 2 main actor groups (those who provide digital health services and those who use the ICT infrastructure of the health ministry). These are the groups that are targeted by the included regulatory standards.
From a broader perspective, 12 categories of stakeholders according to their potential role in regulating health apps for the self-management were mapped in this study. The potential contribution of these stakeholders to the regulation of health apps for self-management in sub-Saharan Africa not only depends on their roles and responsibilities but also on their interests, needs, expectations, and influence [ 41 , 149 - 151 ]. Thus, a robust stakeholder analysis is paramount as it can help define the scope of the regulatory process, prioritize the requirements, manage the expectations, and ensure the engagement and participation of stakeholders throughout the regulatory process [ 41 , 152 - 156 ]. Our stakeholder mapping, as presented in Table 2 (refer to Multimedia Appendix 4 for more details), lays the foundation for national governments to conduct a robust stakeholder analysis and to adopt an all-inclusive stakeholder engagement strategy to manage and sustain the engagement and participation of all relevant stakeholders [ 157 , 158 ].
Recommendations
Our review found that the regulation of health apps in sub-Saharan Africa is especially poor and almost nonexistent, as only Kenya has national standards that could address some of the regulatory issues related to health apps. Therefore, we recommend the following actions to help sub-Saharan African countries improve the regulation of health apps to support self-management:
- Establish a clear and consistent definition of what constitutes a health app (considering AI or machine learning) and what level of regulation is required for different types of apps.
- Develop and implement criteria and guidelines that ensure the quality, safety, and usability of health apps.
- Engage with independent app evaluators, such as ORCHA, to adopt a common framework that can guide the evaluation and accreditation of health apps and use the framework to create and maintain decentralized and transparent platforms that showcase and evaluate health apps for users and health care professionals.
- Develop and implement policies and regulations that enable sustainable funding for health apps such as integrating the use of health apps for self-management into existing health systems and financing pathways or mechanisms.
- Support and facilitate innovation and collaboration across the sub-Saharan Africa region, especially in areas including but not limited to data security and privacy, interoperability standards, usability, accessibility, funding, capacity building, and monitoring and evaluation of the regulatory process.
- Manage and sustain the engagement, involvement, and participation of all relevant stakeholders in the regulatory process by conducting a robust stakeholder analysis and adopting an all-inclusive stakeholder engagement strategy.
Strengths and Limitations of the Study
This study has several strengths, which include an extensive search of gray literature and repositories, contact with key individuals, and the use of a systematic approach. Given that regulatory standards and guidance are unavailable in scientific databases, a wide range of gray literature and repositories were searched. In addition, contact was made with key staff members to obtain relevant documents, including those at the MOHs, the WHO country offices, and the WHO AFRO. Second, to enhance the strength of the study, a policy analysis framework was adapted and used to systematically organize the key study findings, whereas a deductive descriptive qualitative content analysis approach was used to identify and analyze texts that contained relevant concepts and other related information based on the 4 predefined themes. Third, the RISA tool was used to guide the mapping of key stakeholders. This has further increased the robustness of the study findings.
The limitations of this study include the fact that our literature search was conducted in English. Although the literature search was conducted in English, it yielded documents written in French and Portuguese from the ICTworks repository. Second, regulatory standards and guidance are not readily available on scientific databases; hence, it is possible that some relevant documents might have been missed. However, efforts were made to obtain these documents by contacting key stakeholders including key contact persons at the WHO AFRO, WHO country offices, and MOHs. In addition, contacting key individuals only for the purposes of requesting documents rather than conducting direct interviews was one of the limitations of this study. Interviewing key contact persons and stakeholders to obtain additional information could have strengthened the review; however, we did not interview any key individuals or stakeholders because it was beyond the scope of this review. Nonetheless, we recommend that future studies consider incorporating interviews to explore the perspectives of key stakeholders.
Conclusions
Health apps are increasingly being used by patients to manage their health, and sub-Saharan African countries can leverage these apps to advance their progress toward achieving SDG 3 (good health and well-being) and UHC, especially given the rapid advancement of AI and big data. However, our study has established that the regulation of health apps in sub-Saharan Africa is inadequate to ensure that health apps are technically reliable and clinically safe; interoperable across systems; compliant with the principles of confidentiality of information and data privacy; culturally appropriate and relevant; and accessible to everyone regardless of gender, ethnicity, location, or income. Therefore, the region can learn from the experiences of some high-income countries such as the United Kingdom and Germany to develop and implement a robust and responsive regulatory system that supports the widespread adoption of safe, effective, and beneficial health apps for its population.
Following the publication of this review, a summary of the findings will be disseminated to the relevant organizations. In addition, the key findings will be summarized and presented at national, regional, and international conferences.
Acknowledgments
The authors would like to thank Rebecca Jones, the Library Manager and Liaison Librarian at Charing Cross Library, who advised and assisted with the search strategy for this study. This work is part of the PhD research of BAB, which is sponsored by the government of Nigeria. AM and JC were supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration Northwest London (NIHR200180). The views expressed in this publication are those of the authors and not necessarily those of the government of Nigeria or the NIHR or the Department of Health and Social Care. In the Results and Discussion sections, Microsoft Copilot in Bing [ 159 ] was used to help summarize and modify a few texts as well as suggest some citations.
Data Availability
The search strategy for PubMed, Scopus, and the World Health Organization AIM is presented in Multimedia Appendix 1 . All data generated or analyzed during this study are included in this published article (and its supplementary information files). The documents analyzed are available directly from the relevant institutional websites, ICTworks repository [ 44 ] or upon request from the relevant government departments in each country. Additionally, documents in the list of references that are not accessible on the web can be solicited from the corresponding author on reasonable request.
Authors' Contributions
BAB and JC conceived the study. BAB designed the study with contributions from JC and NM. BAB drafted the manuscript, and JC, NM, AM, SI, KPF, BIH, and NU read and contributed to it. AM was the clinical lead, and JC acted as a guarantor for this study. The final manuscript was read and approved by all the authors.
Conflicts of Interest
None declared.
PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist.
Database search strategy.
Details of included documents.
Mapping of the stakeholders according to their potential role in regulating health apps for self-management.
- Aitken M, Nass D. Digital health trends 2021: innovation, evidence, regulation, and adoption. IQVIA Institute for Human Data Science. 2021. URL: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/digital-health-trends-2021/iqvia-institute-digital-health-trends-2021.pdf?&_=1669449368070 [accessed 2022-11-26]
- Mobile app threat landscape report. RiskIQ. 2020. URL: https://www.riskiq.com/2020-mobile-threat-landscape-report-thank-you/ [accessed 2021-07-19]
- El-Sappagh S, Ali F, Hendawi A, Jang JH, Kwak KS. A mobile health monitoring-and-treatment system based on integration of the SSN sensor ontology and the HL7 FHIR standard. BMC Med Inform Decis Mak. May 10, 2019;19(1):97. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Labrique AB, Vasudevan L, Kochi E, Fabricant R, Mehl G. mHealth innovations as health system strengthening tools: 12 common applications and a visual framework. Glob Health Sci Pract. Aug 06, 2013;1(2):160-171. [ FREE Full text ] [ CrossRef ]
- Adepoju IOO, Albersen BJA, De Brouwere V, van Roosmalen J, Zweekhorst M. mHealth for clinical decision-making in sub-Saharan Africa: a scoping review. JMIR Mhealth Uhealth. Mar 23, 2017;5(3):e38. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Vegesna A, Tran M, Angelaccio M, Arcona S. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health. Jan 2017;23(1):3-17. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Use of appropriate digital technologies for public health: Report by the Director-General. World Health Organization. 2016. URL: https://iris.who.int/handle/10665/274134 [accessed 2023-05-06]
- El-Osta A, Rowe C, Majeed A. Developing a shared definition of self-driven healthcare to enhance the current healthcare delivery paradigm. J R Soc Med. Nov 2022;115(11):424-428. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hussein R. A review of realizing the universal health coverage (UHC) goals by 2030: part 2- what is the role of eHealth and technology? J Med Syst. Jul 2015;39(7):72. [ CrossRef ] [ Medline ]
- Sustainable development goal 3: Ensure healthy lives and promote well-being for all at all ages. United Nations. URL: https://sdgs.un.org/goals/goal3 [accessed 2023-05-07]
- Coronavirus: apps to help the elderly. Organisation for the Review of Care and Health Apps. 2020. URL: https://orchahealth.com/coronavirus-apps-to-help-the-elderly/ [accessed 2021-07-19]
- Huckvale K, Adomaviciute S, Prieto JT, Leow MKS, Car J. Smartphone apps for calculating insulin dose: a systematic assessment. BMC Med. May 06, 2015;13:106. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Huckvale K, Car M, Morrison C, Car J. Apps for asthma self-management: a systematic assessment of content and tools. BMC Med. Nov 22, 2012;10:144. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Huckvale K, Morrison C, Ouyang J, Ghaghda A, Car J. The evolution of mobile apps for asthma: an updated systematic assessment of content and tools. BMC Med. Mar 23, 2015;13:58. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Huckvale K, Prieto JT, Tilney M, Benghozi PJ, Car J. Unaddressed privacy risks in accredited health and wellness apps: a cross-sectional systematic assessment. BMC Med. Sep 07, 2015;13:214. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Huckvale K, Torous J, Larsen ME. Assessment of the data sharing and privacy practices of smartphone apps for depression and smoking cessation. JAMA Netw Open. Apr 05, 2019;2(4):e192542. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ndlovu K, Mars M, Scott RE. Interoperability frameworks linking mHealth applications to electronic record systems. BMC Health Serv Res. May 13, 2021;21(1):459. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kenya standards and guidelines for mHealth systems. Kenya Ministry of Health. 2017. URL: https://www.health.go.ke/wp-content/uploads/2020/02/Revised-Guidelines-For-Mhealth-Systems-May-Version.pdf [accessed 2023-03-21]
- Standard for electronic health record system (EHRs) in Ethiopia. Ethiopia Minister of Health. 2021. URL: https://registry.betterehealth.eu/ehealth-policies/standard-electronic-health-record-system-ehrs-ethiopia [accessed 2023-04-21]
- National health normative standards framework for digital health interoperability in South Africa. South Africa Department of Health. 2021. URL: https://www.health.gov.za/wp-content/uploads/2022/10/HNSF_Gazette_21_October_2022.pdf [accessed 2023-05-15]
- Ferretti A, Ronchi E, Vayena E. From principles to practice: benchmarking government guidance on health apps. Lancet Digit Health. Jun 2019;1(2):e55-e57. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Diao JA, Venkatesh KP, Raza MM, Kvedar JC. Multinational landscape of health app policy: toward regulatory consensus on digital health. NPJ Digit Med. May 11, 2022;5(1):61. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Essén A, Stern AD, Haase CB, Car J, Greaves F, Paparova D, et al. Health app policy: international comparison of nine countries' approaches. NPJ Digit Med. Mar 18, 2022;5(1):31. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative. Diabetes Care. Feb 2002;25(2):259-268. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chaney SC, Mechael P. Self-Care Trailblazer Group. 2020. URL: https://media.psi.org/wp-content/uploads/2020/09/31000510/Digital-Self-Care-Final.pdf [accessed 2021-05-20]
- Kanzaveli T. Healthcare: shiftingfrom “one size fits all” to “one size fits one”. Medium. 2017. URL: https://tkanzaveli.medium.com/healthcare-shifting-from-one-size-fits-all-to-one-size-fits-one-d56136ded705 [accessed 2022-03-04]
- Myth 1 – one app will fit all!. Organisation for the Review of Care and Health Apps. URL: https://orchahealth.com/myth-1-one-app-will-fit-all/ [accessed 2022-03-04]
- Aitken M, Lyle J. Patient adoption of mHealth: use, evidence and remaining barriers to mainstream acceptance. IQVIA Institute for Human Data Science. Sep 2015. URL: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/patient-adoption-of-mhealth.pdf [accessed 2021-05-21]
- Mechael P, Batavia H, Kaonga N. Barriers and gaps affecting mhealth in low and middle income countries: policy white paper. Center for Global Health and Economic Development Earth Institute, Columbia University. 2010. URL: http://www.globalproblems-globalsolutions-files.org/pdfs/mHealth_Barriers_White_Paper.pdf [accessed 2021-03-24]
- Bene BA, Ibeneme S, Fadahunsi KP, Harri BI, Ukor N, Mastellos N, et al. Regulatory standards and guidance for the use of health applications for self-management in Africa: scoping review protocol. BMJ Open. Feb 11, 2022;12(2):e058067. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Aitken M, Gauntlett C. Patient apps for improved healthcare: from novelty to mainstream. IMS Institute for Healthcare Informatics. 2013. URL: https://ignacioriesgo.es/wp-content/uploads/2014/03/iihi_patient_apps_report_editora_39_2_1.pdf [accessed 2024-03-10]
- National eHealth Strategy Toolkit. World Health Organization, International Telecommunication Union. 2012. URL: https://www.itu.int/pub/D-STR-E_HEALTH.05-2012 [accessed 2021-06-28]
- Global strategy on digital health 2020-2025. World Health Organization. 2021. URL: https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf [accessed 2021-06-23]
- Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. Feb 2005;8(1):19-32. [ FREE Full text ] [ CrossRef ]
- Anderson S, Allen P, Peckham S, Goodwin N. Asking the right questions: scoping studies in the commissioning of research on the organisation and delivery of health services. Health Res Policy Syst. Jul 09, 2008;6:7. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. Sep 20, 2010;5:69. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. Oct 2020;18(10):2119-2126. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. Oct 02, 2018;169(7):467-473. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Leitner C, Potenziani D. Mendeley reference manager. Mendeley. 2022. URL: https://www.mendeley.com/reference-management/reference-manager [accessed 2022-08-03]
- Better systematic review management. Covidence. URL: https://www.covidence.org/ [accessed 2023-02-13]
- Franco-Trigo L, Fernandez-Llimos F, Martínez-Martínez F, Benrimoj SI, Sabater-Hernández D. Stakeholder analysis in health innovation planning processes: A systematic scoping review. Health Policy. Oct 2020;124(10):1083-1099. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Walt G, Gilson L. Reforming the health sector in developing countries: the central role of policy analysis. Health Policy Plan. Dec 1994;9(4):353-370. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Digital health: a call for government leadership and cooperation between ICT and health. Broadband Commission. 2017. URL: https://broadbandcommission.org/wp-content/uploads/2021/09/WGHealth_Report2017-.pdf [accessed 2021-06-28]
- Vota W. Every African country’s national eHealth strategy or digital health policy. ICT works. 2019. URL: https://www.ictworks.org/african-national-ehealth-strategy-policy/ [accessed 2023-12-10]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. Jul 21, 2009;6(7):e1000097. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Standards and guidelines for electronic medical record systems in Kenya. Kenya Ministry of Medical Services, Kenya Ministry of Public Health and Sanitation. 2010. URL: http://guidelines.health.go.ke:8000/media/Standards_and_Guidelines_for_EMR_Systems.pdf [accessed 2023-04-21]
- Kenya standards and guidelines for E-health systems interoperability. Kenya Ministry of Health, AfyaInfo Project. 2014. URL: https://pdf.usaid.gov/pdf_docs/PA00TB2K.pdf [accessed 2023-03-21]
- Health sector ICT standards and guidelines. Kenya Ministry of Health. 2013. URL: https://www.medbox.org/pdf/5e148832db60a2044c2d2895 [accessed 2023-03-21]
- Health information exchange standard operating procedure (SOP) and guideline. Nigeria Federal Ministry of Health. Jul 2020.
- National eHealth strategy 2018-2022. Benin Ministry of Health. 2017.
- The eHealth strategy of botswana 2020-2024. Botswana Ministry of Health. URL: https://ehealth.ub.bw/bhdc/Docs/MOH%20ehealth%20Strategy%20Book%20A4.pdf [accessed 2023-04-22]
- Health sector digital strategy 2016-2020. Burkina Faso Ministry of Health.
- National health informatics development plan of Burundi. Burundi Ministry of Public Health. 2015.
- The 2020-2024 national digital health strategic plan. Cameroon Ministry of Public Health. 2020.
- National eHealth strategy 2017-2021. Comoros Ministry of Health. 2016.
- eHealth strategic plan. Cote d’Ivoire Minister of Health and Public Hygiene. 2011.
- National development plan for health informatics. Democratic Republic of Congo Ministry of Public Health. 2014.
- Kingdom of Swaziland eHealth strategy 2016 - 2020. Kingdom of Swaziland Ministry Of Health. 2016.
- Information revolution strategic plan (2018-2025). Ethiopia Ministry of Health. 2018.
- Strategic master plan of the health information system of the Gabon. Gabon Ministry of Public Health and Population. 2017.
- National e-Health strategy. Ghana Ministry of Health. 2010.
- Kenya national e-Health strategy. Kenya Ministry of Medical Services, Kenya Ministry of Public Health & Sanitation. 2011.
- Kenya national eHealth policy 2016-2030. Kenya Ministry of Health. 2016.
- National strategy - Liberia - 2016-2021. Liberia Ministry of Health. 2016.
- Strategic plan for strengthening the health information system of Madagascar 2018–2022. Madagascar Ministry of Public Health. 2017.
- National digital health strategy 2020-2025. Malawi Ministry of Health. 2020.
- National eHealth policy in Mali. Mali Ministry of Health and Public Hygiene. 2013.
- Health 2015: seamless continuity of care. Mauritius Ministry of Health and Quality of Life. 2015.
- Strategic plan of information system for health 2009-2014. Mozambique Ministry of Health. 2009.
- National eHealth strategy 2019-2023. Niger Ministry of Public Health. 2018.
- National digital health strategy 2021-2025. Nigeria Federal Ministry of Health. 2021.
- National digital health policy. Nigeria Federal Ministry of Health. 2021.
- National digital health strategic plan 2018-2023. Rwanda Ministry of Health. 2018. URL: https://elearning.helinanet.org/mod/resource/view.php?id=890 [accessed 2023-05-09]
- Strategic plan for digital health 2018-2023. Senegal Ministry of Health and Social Action. 2018.
- National digital health strategy 2018-2023. Sierra Leone Ministry of Health and Sanitation, Sierra Leone Ministry of Information and Communication. 2018.
- The national digital health strategy 2019 – 2024. Tanzania Ministry of Health, Community Development, Gender, Elderly and Children. 2019.
- National digital health strategy for South Africa 2019 - 2024. South Africa Department of Health. 2019.
- Strategic plan for the development of eHealth in Togo 2013-2015. Togo Ministry of Health. 2012.
- Uganda national eHealth policy. Uganda Ministry of Health. 2016.
- Uganda national eHealth strategy 2017 - 2021. Uganda Ministry of Health. URL: https://health.go.ug/sites/default/files/National%20e_Health%20Strategy_0.pdf [accessed 2023-05-16]
- National eHealth strategy 2017-2021. Zambia Ministry of Health. 2017.
- Zimbabwe’s E-Health strategy 2012-2017. Ministry of Health and Child Welfare. 2012.
- National eHealth strategy 2021-2025. Namibia Ministry of Health & Social Services. 2021. URL: https://www.scribd.com/document/639371316/eHealth-Strategy-Namibia-2021# [accessed 2023-05-13]
- Health sector ICT policy and strategy. Ghana Ministry of Health. 2005. URL: https://www.moh.gov.gh/wp-content/uploads/2016/02/Health-Sector-ICT-Policy-and-Strategy.pdf [accessed 2023-05-08]
- Adebola OJ. Beyond national digital health strategy: final report of end term evaluation for the National Health ICT Strategic Framework 2015-2020. Nigeria Federal Ministry of Health. May 2021.
- National Health ICT Strategic Framework 2015 - 2020. Nigeria Federal Ministry of Health. 2016. URL: https://www.health.gov.ng/doc/HealthICTStrategicFramework.pdf [accessed 2023-05-16]
- Digital health blueprint. Ethiopia Ministry of Health. 2021. URL: http://repository.iifphc.org/bitstream/handle/123456789/1658/Ethiopian-Digital-Health-Blueprint.pdf?sequence=1&isAllowed=y [accessed 2023-05-16]
- Kenya health information systems interoperability framework. Kenya Ministry of Health. 2020. URL: https://www.data4sdgs.org/sites/default/files/services_files/Kenya%20Health%20Information%20Systems%20Interoperability%20Framework.pdf [accessed 2023-05-16]
- National community health digitization strategy 2020-2025. Kenya Ministry of Health, Division of Community Health Services. 2021. URL: https://www.eahealth.org/sites/www.eahealth.org/files/content/attachments/2021-08-02/eCHIS-Strategy-2020-2025.pdf [accessed 2023-05-16]
- Leitner C, Potenziani D. Health information systems interoperability in Liberia. IntraHealth International. 2016. URL: https://elearning.helinanet.org/mod/resource/view.php?id=938 [accessed 2023-05-16]
- Narrative for 2022 national digital health annual operational plan (AOP). Nigeria Federal Ministry of Health. 2022.
- Tanzania digital health investment road map 2017-2023. Tanzania Ministry of Health, Community Development, Gender, Elderly and Children, President’s Office Regional Administration and Local Government. 2017.
- Yu H. Regulation of digital health technologies in the European Union: intended versus actual use. In: Cohen GI, Minssen T, Price II NW, Robertson C, Shachar C, editors. The Future of Medical Device Regulation: Innovation and Protection. Cambridge. Cambridge University Press; Mar 31, 2022;103-114.
- Policy for device software functions and mobile medical applications: guidance for industry and Food and Drug Administration staff. U.S. Food and Drug Administration. 2022. URL: https://www.fda.gov/media/80958/download [accessed 2023-10-10]
- Gordon WJ, Landman A, Zhang H, Bates DW. Beyond validation: getting health apps into clinical practice. NPJ Digit Med. 2020;3:14. [ FREE Full text ] [ CrossRef ] [ Medline ]
- FDA clears mobile medical app to help those with opioid use disorder stay in recovery programs. U.S. Food and Drug Administration. 2018. URL: https://www.fda.gov/news-events/press-announcements/fda-clears-mobile-medical-app-help-those-opioid-use-disorder-stay-recovery-programs [accessed 2021-01-27]
- Digital maturity model: achieving digital maturity to drive growth. Deloitte. 2018. URL: https://www2.deloitte.com/content/dam/Deloitte/global/Documents/Technology-Media-Telecommunications/deloitte-digital-maturity-model.pdf [accessed 2021-10-20]
- May E. How digital health apps are empowering patients. Deloitte. 2021. URL: https://www2.deloitte.com/us/en/blog/health-care-blog/2021/how-digital-health-apps-are-empowering-patients.html [accessed 2023-10-06]
- Guidance: criteria for health app assessment. Public Health England. 2017. URL: https://www.gov.uk/government/publications/health-app-assessment-criteria/criteria-for-health-app-assessment [accessed 2023-10-16]
- Clinical risk management standards. National Health Service Digital. 2020. URL: https://digital.nhs.uk/services/clinical-safety/clinical-risk-management-standards [accessed 2023-10-28]
- Report a problem with a medicine or medical device. Gov.uk. URL: https://www.gov.uk/report-problem-medicine-medical-device [accessed 2023-11-07]
- Digital technology assessment criteria for health and social care (DTAC) - Version 1.0. National Health Service X. 2021. URL: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Ftransform.england.nhs.uk%2Fmedia%2Fdocuments%2FDTAC_version_1.0_FINAL_updated_16.04.odt&wdOrigin=BROWSELINK [accessed 2023-11-07]
- Data protection impact assessment: NHS login - formerly Citizen Identity. National Health Service Digital. 2022. URL: https://digital.nhs.uk/services/nhs-login/data-protection-impact-assessment [accessed 2023-11-07]
- Risks and data protection impact assessments (DPIAs). Information Commissioner’s Office. URL: https://ico.org.uk/for-organisations/uk-gdpr-guidance-and-resources/accountability-and-governance/accountability-framework/risks-and-data-protection-impact-assessments-dpias/ [accessed 2023-11-07]
- A guide to good practice for digital and data-driven health technologies. Department of Health and Social Care. 2021. URL: https://www.gov.uk/government/publications/code-of-conduct-for-data-driven-health-and-care-technology/initial-code-of-conduct-for-data-driven-health-and-care-technology [accessed 2023-10-30]
- Interoperability in healthcare. Healthcare Information and Management Systems Society (HIMSS). 2023. URL: https://www.himss.org/resources/interoperability-healthcare [accessed 2023-10-17]
- DAPB4020: UK core Fast Healthcare Interoperability Resources (FHIR) release 4 (R4) governance. National Health Service Digital. 2022. URL: https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/dapb4020-uk-core-fhir-r4-governance [accessed 2023-10-17]
- Fast Healthcare Interoperability Resources (FHIR). National Health Service Digital. 2022. URL: https://digital.nhs.uk/services/fhir-apis [accessed 2023-10-17]
- FHIR Interoperability Basics: 4 things to know. Health IT Analytics. 2022. URL: https://healthitanalytics.com/news/4-basics-to-know-about-the-role-of-fhir-in-interoperability [accessed 2023-11-07]
- Giordanengo A, Bradway M, Pedersen R, Grøttland A, Hartvigsen G, Årsand E. Integrating data from apps, wearables and personal electronic health record (pEHR) systems with clinicians’ electronic health records (EHR) systems. Int J Integr Care. Nov 09, 2016;16(5):16. [ FREE Full text ] [ CrossRef ]
- A plan for digital health and social care. Department of Health & Social Care. 2022. URL: https://www.gov.uk/government/publications/a-plan-for-digital-health-and-social-care/a-plan-for-digital-health-and-social-care [accessed 2022-12-01]
- Ryu B, Kim N, Heo E, Yoo S, Lee K, Hwang H, et al. Impact of an electronic health record-integrated personal health record on patient participation in health care: development and randomized controlled trial of MyHealthKeeper. J Med Internet Res. Dec 07, 2017;19(12):e401. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Winter A, Takabayashi K, Jahn F, Kimura E, Engelbrecht R, Haux R, et al. Quality requirements for electronic health record systems*. A Japanese-German information management perspective. Methods Inf Med. Aug 07, 2017;56(7):e92-e104. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wachter RM. Making IT work: harnessing the power of health information technology to improve care in England. National Advisory Group on Health Information Technology. 2016. URL: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/550866/Wachter_Review_Accessible.pdf [accessed 2021-07-22]
- Framework on integrated people-centred health services (IPCHS). World Health Organisation. 2023. URL: https://www.who.int/teams/integrated-health-services/clinical-services-and-systems/service-organizations-and-integration [accessed 2023-06-05]
- Ibeneme S, Karamagi H, Muneene D, Goswami K, Chisaka N, Okeibunor J. Strengthening health systems using innovative digital health technologies in Africa. Front Digit Health. 2022;4:854339. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ibeneme S, Ukor N, Ongom M, Dasa T, Muneene D, Okeibunor J. Strengthening capacities among digital health leaders for the development and implementation of national digital health programs in Nigeria. BMC Proc. 2020;14(Suppl 10):9. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Delivering safe digital health. Organisation for the Review of Care and Health Apps. URL: https://orchahealth.com/ [accessed 2023-10-22]
- Mamuye AL, Yilma TM, Abdulwahab A, Broomhead S, Zondo P, Kyeng M, et al. Health information exchange policy and standards for digital health systems in Africa: a systematic review. PLOS Digit Health. Oct 2022;1(10):e0000118. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Digital health platform handbook: Building a digital information infrastructure (infostructure) for health. World Health Organization, International Telecommunication Union. 2022. URL: https://www.itu.int/dms_pub/itu-d/opb/str/D-STR-E_HEALTH.10-2020-PDF-E.pdf [accessed 2021-05-22]
- Framework for involving patients in patient safety 2021. National Health Service England. 2021. URL: https://www.england.nhs.uk/patient-safety/framework-for-involving-patients-in-patient-safety/ [accessed 2023-03-23]
- Olesch A. Towards harmonised EU landscape for digital health: summary of the roundtable discussions in selected EIT Health InnoStars countries. EIT Health InnoStars. Jan 2023. URL: https://eithealth.eu/wp-content/uploads/2023/02/EIT_Health_DiGA_report_Jan2023.pdf [accessed 2023-10-10]
- Grieb J, Tschammler D, Färber C, Woitz S. Digital health laws and regulations germany. Global Legal Group. 2023. URL: https://iclg.com/practice-areas/digital-health-laws-and-regulations/germany [accessed 2023-11-03]
- Human regulatory: medical devices. European Medicines Agency. URL: https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices [accessed 2023-10-12]
- Strengthening the capacity for regulation of medical products in the African region. World Health Organization Regional Office for Africa. 2013. URL: https://iris.who.int/bitstream/handle/10665/94308/AFR_RC63_7.pdf?sequence=1 [accessed 2023-10-17]
- Ncube BM, Dube A, Ward K. Establishment of the African Medicines Agency: progress, challenges and regulatory readiness. J Pharm Policy Pract. Mar 08, 2021;14(1):29. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Post market surveillance system. European Union Medical Device Regulation. 2023. URL: https://eumdr.com/post-market-surveillance-system/ [accessed 2023-10-31]
- Dayal R. Effective post-market surveillance for medical devices: An essential part of medical devices regulation (MDR). Capgemini. 2020. URL: https://www.capgemini.com/insights/expert-perspectives/effective-post-market-surveillance-for-medical-devices-an-essential-part-of-mdr/ [accessed 2023-10-31]
- NHS app library reaches 70 apps in honour of the NHS birthday. Northampton General Hospital NHS Trust. 2018. URL: https://www.northamptongeneral.nhs.uk/News/News-Archive/2018/NHS-App-Library-reaches-70-apps-in-honour-of-the-NHS-birthday.aspx [accessed 2023-09-21]
- Developers invited to add to NHS apps library. National Health Service Digital. 2018. URL: https://digital.nhs.uk/news/2018/developers-invited-to-add-to-nhs-apps-library [accessed 2023-09-22]
- The NHS apps library has closed. National Health Service Digital. 2021. URL: https://digital.nhs.uk/services/nhs-apps-library#:~:text=The%20NHS%20Apps%20Library%20was%20decommissioned%20in%20December%202021.&text=Further%20information%20can%20be%20found%20on%20the%20NHS.UK%20website [accessed 2023-09-22]
- Larsen ME, Huckvale K, Nicholas J, Torous J, Birrell L, Li E, et al. Using science to sell apps: evaluation of mental health app store quality claims. NPJ Digit Med. 2019;2:18. [ FREE Full text ] [ CrossRef ] [ Medline ]
- About the NHS app. National Health Service. Dec 4, 2023. URL: https://www.nhs.uk/nhs-app/about-the-nhs-app/ [accessed 2023-09-22]
- Scope of registration: regulated activities. Care Quality Commission. 2022. URL: https://www.cqc.org.uk/guidance-providers/scope-registration-regulated-activities [accessed 2023-11-05]
- Distributing great apps into health and care services across the world. Organisation for the Review of Care and Health Apps. 2020. URL: https://orchahealth.com/wp-content/uploads/2020/12/Health-and-Care-1.pdf [accessed 2023-10-09]
- Our founder, our story and our values: we exist to make digital health healthy. Organisation for the Review of Care and Health Apps. URL: https://orchahealth.com/about-us/ [accessed 2023-10-09]
- Health app library: empower your community with safe access to health apps and digital health products. Organisation for the Review of Care and Health Apps. URL: https://orchahealth.com/our-products/health-app-library/#:~:text=A%20Health%20App%20Library%20is,on%20the%20Health%20App%20Library [accessed 2023-10-09]
- Digital technology assessment criteria (DTAC). National Health Service X. URL: https://www.nhsx.nhs.uk/key-tools-and-info/digital-technology-assessment-criteria-dtac/ [accessed 2023-10-09]
- Evidence standards framework (ESF) for digital health technologies. National Institute for Health and Care Excellence. 2023. URL: https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies [accessed 2023-10-08]
- Tsang L, Kerr-Peterson H. UK NICE updates its evidence standards framework for data-driven digital health technologies. Ropes & Gray. 2022. URL: https://www.ropesgray.com/en/insights/alerts/2022/10/uk-nice-updates-its-evidence-standards-framework-for-data-driven-digital-health-technologies [accessed 2023-10-09]
- Guidance: medical device stand-alone software including apps (including IVDMDs). Medicines and healthcare products regulatory agency. 2023. URL: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1168485/Medical_device_stand-alone_software_including_apps__including_IVDMDs_.pdf [accessed 2023-10-09]
- Digital therapeutics in the United Kingdom. Digital Therapeutics Alliance. 2021. URL: https://dtxalliance.org/wp-content/uploads/2021/06/DTA_DTx-Overview_UK.pdf [accessed 2023-10-09]
- Transforming global healthcare by advancing digital therapeutics. Digital Therapeutics Alliance. 2023. URL: https://dtxalliance.org/ [accessed 2023-10-10]
- International Organization for Standardization (ISO) digital therapeutic definition. Digital Therapeutic Alliance. Jun 2023. URL: https://dtxalliance.org/wp-content/uploads/2023/06/DTA_FS_ISO-Definition.pdf [accessed 2023-10-09]
- Ndomondo-Sigonda M, Miot J, Naidoo S, Dodoo A, Kaale E. Medicines regulation in Africa: current state and opportunities. Pharmaceut Med. 2017;31(6):383-397. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Chinele J. East Africa shows solid support for African Medicines Agency treaty. Health Policy Watch. Aug 16, 2023. URL: https://healthpolicy-watch.news/east-africa-shows-solid-support-for-african-medicines-agency-treaty/ [accessed 2023-10-09]
- Treaty for the establishment of the African Medicines Agency 2019. African Union. 2019. URL: https://au.int/sites/default/files/treaties/36892-treaty-0069_-_ama_treaty_e.pdf [accessed 2023-10-17]
- European Medicines Agency: about us. European Medicines Agency. Mar 1, 2023. URL: https://www.ema.europa.eu/en/documents/other/about-us-european-medicines-agency-ema_en.pdf [accessed 2023-10-18]
- Bryson JM. What to do when stakeholders matter. Public Adm Rev. Mar 2004;6(1):21-53. [ FREE Full text ] [ CrossRef ]
- Iyawa G, Herselman M, Botha A. Potential stakeholders and perceived benefits of a digital health innovation ecosystem for the Namibian context. Procedia Computer Science. 2017;121:431-438. [ CrossRef ]
- Ferretti V. From stakeholders analysis to cognitive mapping and multi-attribute value theory: an integrated approach for policy support. European Journal of Operational Research. Sep 2016;253(2):524-541. [ CrossRef ]
- Brugha R, Varvasovszky Z. Stakeholder analysis: a review. Health Policy Plan. Sep 2000;15(3):239-246. [ CrossRef ] [ Medline ]
- Schmeer K. Guidelines for conducting a stakeholder analysis 1999. Partnerships for Health Reform, Abt Associates. 1999. URL: https://www.ktecop.ca/wordpress/wp-content/uploads/guidelines-stakeholder-analysis-PHR-1999.pdf [accessed 2023-10-17]
- Gilmour J, Beilin R. Stakeholder mapping for effective risk assessment and communication. Australian Centre of Excellence for Risk Analysis, University of Melbourne. Apr 2007. URL: https://cebra.unimelb.edu.au/__data/assets/pdf_file/0006/2220990/gilmour0609.pdf [accessed 2023-10-17]
- Quality, service improvement and redesign tools: stakeholder analysis. National Health Service England, National Health Service Improvement. 2022. URL: https://www.england.nhs.uk/wp-content/uploads/2022/02/qsir-stakeholder-analysis.pdf [accessed 2023-10-20]
- Craven MP, Lang AR, Martin JL. Developing mHealth apps with researchers: multi-stakeholder design considerations. Springer; 2014. Presented at: Third International Conference, DUXU 2014, held as a part of HCI International; June 22-27, 2014;15-24; Heraklion, Greece. URL: https://doi.org/10.1007/978-3-319-07635-5_2 [ CrossRef ]
- How to encourage stakeholder participation. SustaiNet Software International. URL: https://sustainet.com/how-to-encourage-stakeholder-participation/ [accessed 2023-10-20]
- Stakeholder engagement. Organisation for Economic Cooperation and Development. URL: https://www.oecd.org/governance/better-international-rulemaking/compendium/keyprinciples/stakeholderengagement.htm [accessed 2023-10-20]
- Microsoft Copilot in Bing. Microsoft. URL: https://www.bing.com/chat?form=NTPCHB [accessed 2023-03-15]
Abbreviations
Edited by A Mavragani; submitted 19.05.23; peer-reviewed by N O'Brien, A Essén; comments to author 07.09.23; revised version received 08.12.23; accepted 23.02.24; published 11.04.24.
©Benard Ayaka Bene, Sunny Ibeneme, Kayode Philip Fadahunsi, Bala Isa Harri, Nkiruka Ukor, Nikolaos Mastellos, Azeem Majeed, Josip Car. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 11.04.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.

IMAGES
VIDEO
COMMENTS
Bibliography Entry for a Book. A bibliography entry for a book begins with the author's name, which is written in this order: last name, comma, first name, period. After the author's name comes the title of the book. If you are handwriting your bibliography, underline each title. If you are working on a computer, put the book title in ...
An annotated bibliography should include a reference list of any sources you use in writing a research paper. Any printed sources from which you use a text citation, including books, websites, newspaper articles, journal articles, academic writing, online sources (such as PDFs), and magazines should be included in a reference list.
The heading Bibliography is bolded and centred at the top of the page. Unlike the rest of a Chicago format paper, the bibliography is not double-spaced. However, add a single line space between entries. If a bibliography entry extends onto more than one line, subsequent lines should be indented (hanging indent), as seen in the example below ...
Step 3: Create Your Entries. If you created a preliminary bibliography, then you need to alphabetize your entries. The entries will be alphabetized by the author's last name, corporation, or title of the work. All styles will require a ½ inch hanging indent after the first line of the bibliographical citation.
Writing a research paper involves a lot of work. Students need to consult a variety of sources to gather reliable information and ensure their points are well supported. Research papers include a bibliography, which can be a little tricky for students. Learn how to write a bibliography in multiple styles and find basic examples below.
When it is time to turn in your Bibliography, type all of your sources into a list. Use the examples in MLA Format Examples or APA Format Examples as a template to insure that each source is formatted correctly. List the sources in alphabetical order using the author's last name.
Formatting a Harvard style bibliography. Sources are alphabetised by author last name. The heading 'Reference list' or 'Bibliography' appears at the top. Each new source appears on a new line, and when an entry for a single source extends onto a second line, a hanging indent is used: Harvard bibliography example.
For bibliography entries, you list the sources alphabetically by last name, so you will list the last name of the author or creator first in each entry. You should single-space within a bibliography entry and double-space between them. When an entry goes longer than one line, use a hanging indent of .5 inches for subsequent lines.
When you cite a source with up to three authors, cite all authors' names. For four or more authors, list only the first name, followed by ' et al. ': Number of authors. In-text citation example. 1 author. (Davis, 2019) 2 authors. (Davis and Barrett, 2019) 3 authors.
A bibliography is an alphabetized list of sources showing the author, date, and publication information for each source. An annotation is like a note; it's a brief paragraph that explains what the writer learned from the source. Annotated bibliographies combine bibliographies and brief notes about the sources.
To produce a high-quality bibliography, choose reliable and credible sources, categorize them as primary or secondary sources, and understand the different types of sources that are available. By following these principles, researchers can create a bibliography that enhances the credibility and rigor of their research paper.
Below you'll find a Bibliography adapted from a research paper written by Aishani Aatresh for her Technology, Environment, and Society course. Barnard, Anne, and Grace Ashford. "Can New York Really Get to 100% Clean Energy by 2040?". New York Times, November 29, 2021, sec.
Works Included in a Reference List. The reference list provides a reliable way for readers to identify and locate the works cited in a paper. APA Style papers generally include reference lists, not bibliographies. In general, each work cited in the text must appear in the reference list, and each work in the reference list must be cited in the ...
Reference in research papers: A reference is a detailed description of the source of information that you want to give credit to via a citation. The references in research papers are usually in the form of a list at the end of the paper. The essential difference between citations and references is that citations lead a reader to the source of ...
A bibliography is a list of sources or materials that you might have referred to while writing an article or a research paper. If the reader wants to explore more about the content apart from what you've written, they would refer to the sources that you've mentioned and a bibliography can be helpful. For example, if you're reading a ...
Bibliography Examples In MLA, APA and Chicago. When it comes to examples of bibliographies, it can get confusing. This is because the word "bibliography" can have a double meaning when it comes to writing styles. "Bibliography" can be a catch-all word to mean all source lists in all writing styles. It is also the title of the Chicago ...
Citation Examples | Books, Articles, Websites & More. Published on April 9, 2021 by Jack Caulfield . Revised on January 17, 2024. The most common citation styles are APA and MLA. To cite a source in these styles, you need a brief in-text citation and a full reference. Use the interactive tool to understand how a citation is structured and see ...
Example: Parenthetical citation (APA) Evolution is a gradual process that "can act only by very short and slow steps" (Darwin, 1859, p. 510). An alternative to this type of in-text citation is the system used in numerical citation styles , where a number is inserted into the text, corresponding to an entry in a numbered reference list.
Thesis. Thesis is a type of research report. A thesis is a long-form research document that presents the findings and conclusions of an original research study conducted by a student as part of a graduate or postgraduate program. It is typically written by a student pursuing a higher degree, such as a Master's or Doctoral degree, although it ...
A bibliography provides detailed information about each source, such as the author's name, the title of the work, the publication date, and more. It is usually included at the end of a research paper, essay, or book. Purpose. The purpose of a bibliography is to give credit to the authors whose work you have used in your research.
The Bluebook: A Uniform System of Citation is the main style guide for legal citations in the US. It's widely used in law, and also when legal materials need to be cited in other disciplines. Bluebook footnote citation. 1 David E. Pozen, Freedom of Information Beyond the Freedom of Information Act, 165, U. P🇦 . L.
A research report comprises several sections such as The Title, The Abstract, Literature Review, Research Design, Data Analysis, Conclusion, Bibliography, etc. Bibliography: It is generally presented at the end of one's work. It's a list of sources including books, articles, or journals the researcher has referred to while preparing their work.
Background: Health apps are increasingly recognized as crucial tools for enhancing health care delivery. Many countries, particularly those in sub-Saharan Africa, can substantially benefit from using health apps to support self-management and thus help to achieve universal health coverage and the third sustainable development goal. However, most health apps published in app stores are of ...