An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Dermatol
- v.61(2); Mar-Apr 2016

Methodology Series Module 2: Case-control Studies
Maninder singh setia.
Epidemiologist, MGM Institute of Health Sciences, Navi Mumbai, Maharashtra, India
Case-Control study design is a type of observational study. In this design, participants are selected for the study based on their outcome status. Thus, some participants have the outcome of interest (referred to as cases), whereas others do not have the outcome of interest (referred to as controls). The investigator then assesses the exposure in both these groups. The investigator should define the cases as specifically as possible. Sometimes, definition of a disease may be based on multiple criteria; thus, all these points should be explicitly stated in case definition. An important aspect of selecting a control is that they should be from the same ‘study base’ as that of the cases. We can select controls from a variety of groups. Some of them are: General population; relatives or friends; and hospital patients. Matching is often used in case-control control studies to ensure that the cases and controls are similar in certain characteristics, and it is a useful technique to increase the efficiency of the study. Case-Control studies can usually be conducted relatively faster and are inexpensive – particularly when compared with cohort studies (prospective). It is useful to study rare outcomes and outcomes with long latent periods. This design is not very useful to study rare exposures. Furthermore, they may also be prone to certain biases – selection bias and recall bias.
Introduction
Case-Control study design is a type of observational study design. In an observational study, the investigator does not alter the exposure status. The investigator measures the exposure and outcome in study participants, and studies their association.
In a case-control study, participants are selected for the study based on their outcome status. Thus, some participants have the outcome of interest (referred to as cases), whereas others do not have the outcome of interest (referred to as controls). The investigator then assesses the exposure in both these groups. Thus, by design, in a case-control study the outcome has to occur in some of the participants that have been included in the study.
As seen in Figure 1 , at the time of entry into the study (sampling of participants), some of the study participants have the outcome (cases) and others do not have the outcome (controls). During the study procedures, we will examine the exposure of interest in cases as well as controls. We will then study the association between the exposure and outcome in these study participants.

Example of a case-control study
Examples of Case-Control Studies
Smoking and lung cancer study.
In their landmark study, Doll and Hill (1950) evaluated the association between smoking and lung cancer. They included 709 patients of lung carcinoma (defined as cases). They also included 709 controls from general medical and surgical patients. The selected controls were similar to the cases with respect to age and sex. Thus, they included 649 males and 60 females in cases as well as controls.
They found that only 0.3% of males were non-smokers among cases. However, the proportion of non-smokers among controls was 4.2%; the different was statistically significant ( P = 0.00000064). Similarly they found that about 31.7% of the female were non-smokers in cases compared with 53.3% in controls; this difference was also statistically significant (0.01< p <0.02).
Melanoma and tanning (Lazovic et al ., 2010)
The authors conducted a case-control study to study the association between melanoma and tanning. The 1167 cases - individuals with invasive cutaneous melanoma – were selected from Minnesota Cancer Surveillance System. The 1101 controls were selected randomly from Minnesota State Driver's License list; they were matched for age (+/- 5 years) and sex.
The data were collected by self administered questionnaires and telephone interviews. The investigators assessed the use of tanning devices (using photographs), number of years, and frequency of use of these devices. They also collected information on other variables (such as sun exposure; presence of freckles and moles; and colour of skin, hair, among other exposures.
They found that melanoma was higher in individuals who used UVB enhances and primarily UVA-emitting devices. The risk of melanoma also increased with increase in years of use, hours of use, and sessions.
Risk factors for erysipelas (Pitché et al, 2015)
Pitché et al (2015) conducted a case-control study to assess the factors associated with leg erysipelas in sub-Saharan Africa. This was a multi-centre study; the cases and controls were recruited from eight countries in sub-Saharan Africa.
They recruited cases of acute leg cellulitis in these eight countries. They recruited two controls for each case; these were matched for age (+/- 5 years) and sex. Thus, the final study has 364 cases and 728 controls. They found that leg erysipelas was associated with obesity, lympoedema, neglected traumatic wound, toe-web intertrigo, and voluntary cosmetic depigmentation.
We have provided details of all the three studies in the bibliography. We strongly encourage the readers to read the papers to understand some practical aspects of case-control studies.
Selection of Cases and Controls
Selection of cases and controls is an important part of this design. Wacholder and colleagues (1992 a, b, and c) have published wonderful manuscripts on design and conduct of case-control of studies in the American Journal of Epidemiology. The discussion in the next few sections is based on these manuscripts.
Selection of case
The investigator should define the cases as specifically as possible. Sometimes, definition of a disease may be based on multiple criteria; thus, all these points should be explicitly stated in case definition.
For example, in the above mentioned Melanoma and Tanning study, the researchers defined their population as any histologic variety of invasive cutaneous melanoma. However, they added another important criterion – these individuals should have a driver's license or State identity card. This probably is not directly related to the clinic condition, so why did they add this criterion? We will discuss this in detail in the next few paragraphs.
Selection of a control
The next important point in designing a case-control study is the selection of control patients.
In fact, Wacholder and colleagues have extensively discussed aspects of design of case control studies and selection of controls in their article.
According to them, an important aspect of selecting a control is that they should be from the same ‘study base’ as that of the cases. Thus, the pool of population from which the cases and controls will be enrolled should be same. For instance, in the Tanning and Melanoma study, the researchers recruited cases from Minnesota Cancer Surveillance System; however, it was also required that these cases should either have a State identity card or Driver's license. This was important since controls were randomly selected from Minnesota State Driver's license list (this also included the list of individuals who have the State identity card).
Another important aspect of a case-control study is that we should measure the exposure similarly in cases and controls. For instance, if we design a research protocol to study the association between metabolic syndrome (exposure) and psoriasis (outcome), we should ensure that we use the same criteria (clinically and biochemically) for evaluating metabolic syndrome in cases and controls. If we use different criteria to measure the metabolic syndrome, then it may cause information bias.
Types of Controls
We can select controls from a variety of groups. Some of them are: General population; relatives or friends; or hospital patients.
Hospital controls
An important source of controls is patients attending the hospital for diseases other than the outcome of interest. These controls are easy to recruit and are more likely to have similar quality of medical records.
However, we have to be careful while recruiting these controls. In the above example of metabolic syndrome and psoriasis, we recruit psoriasis patients from the Dermatology department of the hospital as controls. We recruit patients who do not have psoriasis and present to the Dermatology as controls. Some of these individuals have presented to the Dermatology department with tinea pedis. Do we recruit these individuals as controls for the study? What is the problem if we recruit these patients? Some studies have suggested that diabetes mellitus and obesity are predisposing factors for tinea pedis. As we know, fasting plasma glucose of >100 mg/dl and raised trigylcerides (>=150 mg/dl) are criteria for diagnosis of metabolic syndrome. Thus, it is quite likely that if we recruit many of these tinea pedis patients, the exposure of interest may turn out to be similar in cases and controls; this exposure may not reflect the truth in the population.
Relative and friend controls
Relative controls are relatively easy to recruit. They can be particularly useful when we are interested in trying to ensure that some of the measurable and non-measurable confounders are relatively equally distributed in cases and controls (such as home environment, socio-economic status, or genetic factors).
Another source of controls is a list of friends referred by the cases. These controls are easy to recruit and they are also more likely to be similar to the cases in socio-economic status and other demographic factors. However, they are also more likely to have similar behaviours (alcohol use, smoking etc.); thus, it may not be prudent to use these as controls if we want to study the effect of these exposures on the outcome.
Population controls
These controls can be easily conducted the list of all individuals is available. For example, list from state identity cards, voter's registration list, etc., In the Tanning and melanoma study, the researchers used population controls. They were identified from Minnesota state driver's list.
We may have to use sampling methods (such as random digit dialing or multistage sampling methods) to recruit controls from the population. A main advantage is that these controls are likely to satisfy the ‘study-base’ principle (described above) as suggested by Wacholder and colleagues. However, they can be expensive and time consuming. Furthermore, many of these controls will not be inclined to participate in the study; thus, the response rate may be very low.
Matching in a Case-Control Study
Matching is often used in case-control control studies to ensure that the cases and controls are similar in certain characteristics. For example, in the smoking and lung cancer study, the authors selected controls that were similar in age and sex to carcinoma cases. Matching is a useful technique to increase the efficiency of study.
’Individual matching’ is one common technique used in case-control study. For example, in the above mentioned metabolic syndrome and psoriasis, we can decide that for each case enrolled in the study, we will enroll a control that is matched for sex and age (+/- 2 years). Thus, if 40 year male patient with psoriasis is enrolled for the study as a case, we will enroll a 38-42 year male patient without psoriasis (and who will not be excluded for other reason) as controls.
If the study has used ‘individual matching’ procedures, then the data should also reflect the same. For instance, if you have 45 males among cases, you should also have 45 males among controls. If you show 60 males among controls, you should explain the discrepancy.
Even though matching is used to increase the efficiency in case-control studies, it may have its own problems. It may be difficult to fine the exact matching control for the study; we may have to screen many potential enrollees before we are able to recruit one control for each case recruited. Thus, it may increase the time and cost of the study.
Nonetheless, matching may be useful to control for certain types of confounders. For instance, environment variables may be accounted for by matching controls for neighbourhood or area of residence. Household environment and genetic factors may be accounted for by enrolling siblings as controls.
If we use controls from the past (time period when cases did not occur), then the controls are sometimes referred to historic controls. Such controls may be recruited from past hospital records.
Strengths of a Case-Control Study
- Case-Control studies can usually be conducted relatively faster and are inexpensive – particularly when compared with cohort studies (prospective)
- It is useful to study rare outcomes and outcomes with long latent periods. For example, if we wish to study the factors associated with melanoma in India, it will be useful to conduct a case-control study. We will recruit cases of melanoma as cases in one study site or multiple study sites. If we were to conduct a cohort study for this research question, we may to have follow individuals (with the exposure under study) for many years before the occurrence of the outcome
- It is also useful to study multiple exposures in the same outcome. For example, in the metabolic syndrome and psoriasis study, we can study other factors such as Vitamin D levels or genetic markers
- Case-control studies are useful to study the association of risk factors and outcomes in outbreak investigations. For instance, Freeman and colleagues (2015) in a study published in 2015 conducted a case-control study to evaluate the role of proton pump inhibitors in an outbreak of non-typhoidal salmonellosis.
Limitations of a Case-control Study
- The design, in general, is not useful to study rare exposures. It may be prudent to conduct a cohort study for rare exposures
Since the investigator chooses the number of cases and controls, the proportion of cases may not be representative of the proportion in the population. For instance if we choose 50 cases of psoriasis and 50 controls, the prevalence of proportion of psoriasis cases in our study will be 50%. This is not true prevalence. If we had chosen 50 cases of psoriasis and 100 controls, then the proportion of the cases will be 33%.
- The design is not useful to study multiple outcomes. Since the cases are selected based on the outcome, we can only study the association between exposures and that particular outcome
- Sometimes the temporality of the exposure and outcome may not be clearly established in case-control studies
- The case-control studies are also prone to certain biases
If the cases and controls are not selected similarly from the study base, then it will lead to selection bias.
- Odds Ratio: We are able to calculate the odds ratios (OR) from a case-control study. Since we are not able to measure incidence data in case-control study, an odds ratio is a reasonable measure of the relative risk (under some assumptions). Additional details about OR will be discussed in the biostatistics section.
The OR in the above study is 3.5. Since the OR is greater than 1, the outcome is more likely in those exposed (those who are diagnosed with metabolic syndrome) compared with those who are not exposed (those who do are not diagnosed with metabolic syndrome). However, we will require confidence intervals to comment on further interpretation of the OR (This will be discussed in detail in the biostatistics section).
- Other analysis : We can use logistic regression models for multivariate analysis in case-control studies. It is important to note that conditional logistic regressions may be useful for matched case-control studies.
Calculating an Odds Ratio (OR)

Hypothetical study of metabolic syndrome and psoriasis

Additional Points in A Case-Control Study
How many controls can i have for each case.
The most optimum case-to-control ratio is 1:1. Jewell (2004) has suggested that for a fixed sample size, the chi square test for independence is most powerful if the number of cases is same as the number of controls. However, in many situations we may not be able recruit a large number of cases and it may be easier to recruit more controls for the study. It has been suggested that we can increase the number of controls to increase statistical power (if we have limited number of cases) of the study. If data are available at no extra cost, then we may recruit multiple controls for each case. However, if it is expensive to collect exposure and outcome information from cases and controls, then the optimal ratio is 4 controls: 1 case. It has been argued that the increase in statistical power may be limited with additional controls (greater than four) compared with the cost involved in recruiting them beyond this ratio.
I have conducted a randomised controlled trial. I have included a group which received the intervention and another group which did not receive the intervention. Can I call this a case-control study?
A randomised controlled trial is an experimental study. In contrast, case-control studies are observational studies. These are two different groups of studies. One should not use the word case-control study for a randomised controlled trial (even though you have a control group in the study). Every study with a control group is not a case-control study. For a study to be classified as a case-control study, the study should be an observational study and the participants should be recruited based on their outcome status (some have the disease and some do not).
Should I call case-control studies prospective or retrospective studies?
In ‘The Dictionary of Epidemiology’ by Porta (2014), the authors have suggested that even though the term ‘retrospective’ was used for case-control studies, the study participants are often recruited prospectively. In fact, the study on risk factors for erysipelas (Pitché et al ., 2015) was a prospective case case-control study. Thus, it is important to remember that the nature of the study (case-control or cohort) depends on the sampling method. If we sample the study participants based on exposure and move towards the outcome, it is a cohort study. However, if we sample the participants based on the outcome (some with outcome and some do not) and study the exposures in both these groups, it is a case-control study.
In case-control studies, participants are recruited on the basis of disease status. Thus, some of participants have the outcome of interest (referred to as cases), whereas others do not have the outcome of interest (referred to as controls). The investigator then assesses the exposure in both these groups. Case-control studies are less expensive and quicker to conduct (compared with prospective cohort studies at least). The measure of association in this type of study is an odds ratio. This type of design is useful for rare outcomes and those with long latent periods. However, they may also be prone to certain biases – selection bias and recall bias.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Bibliography
- En español – ExME
- Em português – EME
Case-control and Cohort studies: A brief overview
Posted on 6th December 2017 by Saul Crandon

Introduction
Case-control and cohort studies are observational studies that lie near the middle of the hierarchy of evidence . These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1). Although these studies are not ranked as highly as randomised controlled trials, they can provide strong evidence if designed appropriately.
Case-control studies
Case-control studies are retrospective. They clearly define two groups at the start: one with the outcome/disease and one without the outcome/disease. They look back to assess whether there is a statistically significant difference in the rates of exposure to a defined risk factor between the groups. See Figure 1 for a pictorial representation of a case-control study design. This can suggest associations between the risk factor and development of the disease in question, although no definitive causality can be drawn. The main outcome measure in case-control studies is odds ratio (OR) .
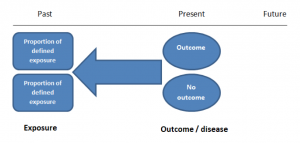
Figure 1. Case-control study design.
Cases should be selected based on objective inclusion and exclusion criteria from a reliable source such as a disease registry. An inherent issue with selecting cases is that a certain proportion of those with the disease would not have a formal diagnosis, may not present for medical care, may be misdiagnosed or may have died before getting a diagnosis. Regardless of how the cases are selected, they should be representative of the broader disease population that you are investigating to ensure generalisability.
Case-control studies should include two groups that are identical EXCEPT for their outcome / disease status.
As such, controls should also be selected carefully. It is possible to match controls to the cases selected on the basis of various factors (e.g. age, sex) to ensure these do not confound the study results. It may even increase statistical power and study precision by choosing up to three or four controls per case (2).
Case-controls can provide fast results and they are cheaper to perform than most other studies. The fact that the analysis is retrospective, allows rare diseases or diseases with long latency periods to be investigated. Furthermore, you can assess multiple exposures to get a better understanding of possible risk factors for the defined outcome / disease.
Nevertheless, as case-controls are retrospective, they are more prone to bias. One of the main examples is recall bias. Often case-control studies require the participants to self-report their exposure to a certain factor. Recall bias is the systematic difference in how the two groups may recall past events e.g. in a study investigating stillbirth, a mother who experienced this may recall the possible contributing factors a lot more vividly than a mother who had a healthy birth.
A summary of the pros and cons of case-control studies are provided in Table 1.
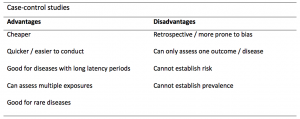
Table 1. Advantages and disadvantages of case-control studies.
Cohort studies
Cohort studies can be retrospective or prospective. Retrospective cohort studies are NOT the same as case-control studies.
In retrospective cohort studies, the exposure and outcomes have already happened. They are usually conducted on data that already exists (from prospective studies) and the exposures are defined before looking at the existing outcome data to see whether exposure to a risk factor is associated with a statistically significant difference in the outcome development rate.
Prospective cohort studies are more common. People are recruited into cohort studies regardless of their exposure or outcome status. This is one of their important strengths. People are often recruited because of their geographical area or occupation, for example, and researchers can then measure and analyse a range of exposures and outcomes.
The study then follows these participants for a defined period to assess the proportion that develop the outcome/disease of interest. See Figure 2 for a pictorial representation of a cohort study design. Therefore, cohort studies are good for assessing prognosis, risk factors and harm. The outcome measure in cohort studies is usually a risk ratio / relative risk (RR).
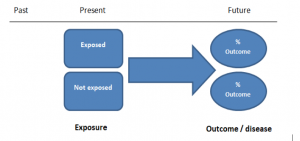
Figure 2. Cohort study design.
Cohort studies should include two groups that are identical EXCEPT for their exposure status.
As a result, both exposed and unexposed groups should be recruited from the same source population. Another important consideration is attrition. If a significant number of participants are not followed up (lost, death, dropped out) then this may impact the validity of the study. Not only does it decrease the study’s power, but there may be attrition bias – a significant difference between the groups of those that did not complete the study.
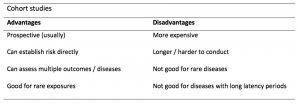
Cohort studies can assess a range of outcomes allowing an exposure to be rigorously assessed for its impact in developing disease. Additionally, they are good for rare exposures, e.g. contact with a chemical radiation blast.
Whilst cohort studies are useful, they can be expensive and time-consuming, especially if a long follow-up period is chosen or the disease itself is rare or has a long latency.
A summary of the pros and cons of cohort studies are provided in Table 2.
The Strengthening of Reporting of Observational Studies in Epidemiology Statement (STROBE)
STROBE provides a checklist of important steps for conducting these types of studies, as well as acting as best-practice reporting guidelines (3). Both case-control and cohort studies are observational, with varying advantages and disadvantages. However, the most important factor to the quality of evidence these studies provide, is their methodological quality.
- Song, J. and Chung, K. Observational Studies: Cohort and Case-Control Studies . Plastic and Reconstructive Surgery.  2010 Dec;126(6):2234-2242.
- Ury HK. Efficiency of case-control studies with multiple controls per case: Continuous or dichotomous data . Biometrics . 1975 Sep;31(3):643–649.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.  Lancet 2007 Oct;370(9596):1453-14577. PMID: 18064739.
Saul Crandon
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on Case-control and Cohort studies: A brief overview
Very well presented, excellent clarifications. Has put me right back into class, literally!
Very clear and informative! Thank you.
very informative article.
Thank you for the easy to understand blog in cohort studies. I want to follow a group of people with and without a disease to see what health outcomes occurs to them in future such as hospitalisations, diagnoses, procedures etc, as I have many health outcomes to consider, my questions is how to make sure these outcomes has not occurred before the “exposure disease”. As, in cohort studies we are looking at incidence (new) cases, so if an outcome have occurred before the exposure, I can leave them out of the analysis. But because I am not looking at a single outcome which can be checked easily and if happened before exposure can be left out. I have EHR data, so all the exposure and outcome have occurred. my aim is to check the rates of different health outcomes between the exposed)dementia) and unexposed(non-dementia) individuals.
Very helpful information
Thanks for making this subject student friendly and easier to understand. A great help.
Thanks a lot. It really helped me to understand the topic. I am taking epidemiology class this winter, and your paper really saved me.
Happy new year.
Wow its amazing n simple way of briefing ,which i was enjoyed to learn this.its very easy n quick to pick ideas .. Thanks n stay connected
Saul you absolute melt! Really good work man
am a student of public health. This information is simple and well presented to the point. Thank you so much.
very helpful information provided here
really thanks for wonderful information because i doing my bachelor degree research by survival model
Quite informative thank you so much for the info please continue posting. An mph student with Africa university Zimbabwe.
Thank you this was so helpful amazing
Apreciated the information provided above.
So clear and perfect. The language is simple and superb.I am recommending this to all budding epidemiology students. Thanks a lot.
Great to hear, thank you AJ!
I have recently completed an investigational study where evidence of phlebitis was determined in a control cohort by data mining from electronic medical records. We then introduced an intervention in an attempt to reduce incidence of phlebitis in a second cohort. Again, results were determined by data mining. This was an expedited study, so there subjects were enrolled in a specific cohort based on date(s) of the drug infused. How do I define this study? Thanks so much.
thanks for the information and knowledge about observational studies. am a masters student in public health/epidemilogy of the faculty of medicines and pharmaceutical sciences , University of Dschang. this information is very explicit and straight to the point
Very much helpful
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles
Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

An introduction to different types of study design
Conducting successful research requires choosing the appropriate study design. This article describes the most common types of designs conducted by researchers.
What Is A Case Control Study?
Julia Simkus
Editor at Simply Psychology
BA (Hons) Psychology, Princeton University
Julia Simkus is a graduate of Princeton University with a Bachelor of Arts in Psychology. She is currently studying for a Master's Degree in Counseling for Mental Health and Wellness in September 2023. Julia's research has been published in peer reviewed journals.
Learn about our Editorial Process
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
A case-control study is a research method where two groups of people are compared – those with the condition (cases) and those without (controls). By looking at their past, researchers try to identify what factors might have contributed to the condition in the ‘case’ group.
Explanation
A case-control study looks at people who already have a certain condition (cases) and people who don’t (controls). By comparing these two groups, researchers try to figure out what might have caused the condition. They look into the past to find clues, like habits or experiences, that are different between the two groups.
The “cases” are the individuals with the disease or condition under study, and the “controls” are similar individuals without the disease or condition of interest.
The controls should have similar characteristics (i.e., age, sex, demographic, health status) to the cases to mitigate the effects of confounding variables .
Case-control studies identify any associations between an exposure and an outcome and help researchers form hypotheses about a particular population.
Researchers will first identify the two groups, and then look back in time to investigate which subjects in each group were exposed to the condition.
If the exposure is found more commonly in the cases than the controls, the researcher can hypothesize that the exposure may be linked to the outcome of interest.
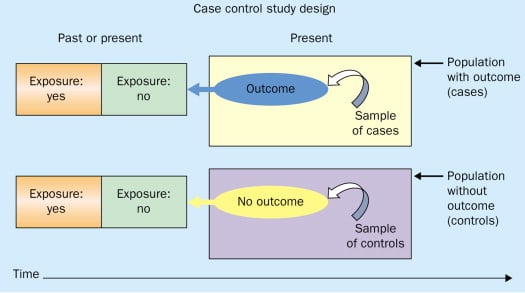
Figure: Schematic diagram of case-control study design. Kenneth F. Schulz and David A. Grimes (2002) Case-control studies: research in reverse . The Lancet Volume 359, Issue 9304, 431 – 434

Quick, inexpensive, and simple
Because these studies use already existing data and do not require any follow-up with subjects, they tend to be quicker and cheaper than other types of research. Case-control studies also do not require large sample sizes.
Beneficial for studying rare diseases
Researchers in case-control studies start with a population of people known to have the target disease instead of following a population and waiting to see who develops it. This enables researchers to identify current cases and enroll a sufficient number of patients with a particular rare disease.
Useful for preliminary research
Case-control studies are beneficial for an initial investigation of a suspected risk factor for a condition. The information obtained from cross-sectional studies then enables researchers to conduct further data analyses to explore any relationships in more depth.
Limitations
Subject to recall bias.
Participants might be unable to remember when they were exposed or omit other details that are important for the study. In addition, those with the outcome are more likely to recall and report exposures more clearly than those without the outcome.
Difficulty finding a suitable control group
It is important that the case group and the control group have almost the same characteristics, such as age, gender, demographics, and health status.
Forming an accurate control group can be challenging, so sometimes researchers enroll multiple control groups to bolster the strength of the case-control study.
Do not demonstrate causation
Case-control studies may prove an association between exposures and outcomes, but they can not demonstrate causation.
A case-control study is an observational study where researchers analyzed two groups of people (cases and controls) to look at factors associated with particular diseases or outcomes.
Below are some examples of case-control studies:
- Investigating the impact of exposure to daylight on the health of office workers (Boubekri et al., 2014).
- Comparing serum vitamin D levels in individuals who experience migraine headaches with their matched controls (Togha et al., 2018).
- Analyzing correlations between parental smoking and childhood asthma (Strachan and Cook, 1998).
- Studying the relationship between elevated concentrations of homocysteine and an increased risk of vascular diseases (Ford et al., 2002).
- Assessing the magnitude of the association between Helicobacter pylori and the incidence of gastric cancer (Helicobacter and Cancer Collaborative Group, 2001).
- Evaluating the association between breast cancer risk and saturated fat intake in postmenopausal women (Howe et al., 1990).
Frequently asked questions
1. what’s the difference between a case-control study and a cross-sectional study.
Case-control studies are different from cross-sectional studies in that case-control studies compare groups retrospectively while cross-sectional studies analyze information about a population at a specific point in time.
In cross-sectional studies , researchers are simply examining a group of participants and depicting what already exists in the population.
2. What’s the difference between a case-control study and a longitudinal study?
Case-control studies compare groups retrospectively, while longitudinal studies can compare groups either retrospectively or prospectively.
In a longitudinal study , researchers monitor a population over an extended period of time, and they can be used to study developmental shifts and understand how certain things change as we age.
In addition, case-control studies look at a single subject or a single case, whereas longitudinal studies can be conducted on a large group of subjects.
3. What’s the difference between a case-control study and a retrospective cohort study?
Case-control studies are retrospective as researchers begin with an outcome and trace backward to investigate exposure; however, they differ from retrospective cohort studies.
In a retrospective cohort study , researchers examine a group before any of the subjects have developed the disease, then examine any factors that differed between the individuals who developed the condition and those who did not.
Thus, the outcome is measured after exposure in retrospective cohort studies, whereas the outcome is measured before the exposure in case-control studies.
Boubekri, M., Cheung, I., Reid, K., Wang, C., & Zee, P. (2014). Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 10 (6), 603-611.
Ford, E. S., Smith, S. J., Stroup, D. F., Steinberg, K. K., Mueller, P. W., & Thacker, S. B. (2002). Homocyst (e) ine and cardiovascular disease: a systematic review of the evidence with special emphasis on case-control studies and nested case-control studies. International journal of epidemiology, 31 (1), 59-70.
Helicobacter and Cancer Collaborative Group. (2001). Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut, 49 (3), 347-353.
Howe, G. R., Hirohata, T., Hislop, T. G., Iscovich, J. M., Yuan, J. M., Katsouyanni, K., … & Shunzhang, Y. (1990). Dietary factors and risk of breast cancer: combined analysis of 12 case—control studies. JNCI: Journal of the National Cancer Institute, 82 (7), 561-569.
Lewallen, S., & Courtright, P. (1998). Epidemiology in practice: case-control studies. Community eye health, 11 (28), 57–58.
Strachan, D. P., & Cook, D. G. (1998). Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax, 53 (3), 204-212.
Tenny, S., Kerndt, C. C., & Hoffman, M. R. (2021). Case Control Studies. In StatPearls . StatPearls Publishing.
Togha, M., Razeghi Jahromi, S., Ghorbani, Z., Martami, F., & Seifishahpar, M. (2018). Serum Vitamin D Status in a Group of Migraine Patients Compared With Healthy Controls: A Case-Control Study. Headache, 58 (10), 1530-1540.
Further Information
- Schulz, K. F., & Grimes, D. A. (2002). Case-control studies: research in reverse. The Lancet, 359(9304), 431-434.
- What is a case-control study?
Leave a Comment Cancel reply
You must be logged in to post a comment.
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
The Case-Control Study : A Practical Review for the Clinician
From the Department of Pediatrics, University of Virginia Medical Center, Charlottesville (Dr Hayden), the Department of Pediatrics and Epidemiology, McGill University, Montreal (Dr Kramer), and the Department of Medicine, Yale University, New Haven, Conn (Dr Horwitz).
The retrospective case-control study is an important research strategy commonly encountered in the medical literature. A thoughtfully designed, carefully executed case-control study can be an invaluable source of clinical information, and physicians must often base important decisions about patient counseling and management on their interpretation of such studies. Unfortunately, the retrospective direction of case-control studies—looking "backwards" from an outcome event to an antecedent exposure—is accompanied by numerous methodological hazards. Careful attention must be paid to selection of appropriate study groups; definition and detection of the outcome event; definition and ascertainment of the exposure; assurance that the compared groups were equally susceptible to the outcome event at baseline; and careful statistical analysis. If systematic bias enters the research at any of these points, erroneous conclusions can result. Greater familiarity with the case-control method should enable clinicians to be more critically insightful when interpreting the results of published studies using this design format.
( JAMA 1982;247:326-331)
Hayden GF , Kramer MS , Horwitz RI. The Case-Control Study : A Practical Review for the Clinician . JAMA. 1982;247(3):326–331. doi:10.1001/jama.1982.03320280046028
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Study Design 101: Case Control Study
- Case Report
- Case Control Study
- Cohort Study
- Randomized Controlled Trial
- Practice Guideline
- Systematic Review
- Meta-Analysis
- Helpful Formulas
- Finding Specific Study Types
A study that compares patients who have a disease or outcome of interest (cases) with patients who do not have the disease or outcome (controls), and looks back retrospectively to compare how frequently the exposure to a risk factor is present in each group to determine the relationship between the risk factor and the disease.
Case control studies are observational because no intervention is attempted and no attempt is made to alter the course of the disease. The goal is to retrospectively determine the exposure to the risk factor of interest from each of the two groups of individuals: cases and controls. These studies are designed to estimate odds.
Case control studies are also known as "retrospective studies" and "case-referent studies."
- Good for studying rare conditions or diseases
- Less time needed to conduct the study because the condition or disease has already occurred
- Lets you simultaneously look at multiple risk factors
- Useful as initial studies to establish an association
- Can answer questions that could not be answered through other study designs
Disadvantages
- Retrospective studies have more problems with data quality because they rely on memory and people with a condition will be more motivated to recall risk factors (also called recall bias).
- Not good for evaluating diagnostic tests because it's already clear that the cases have the condition and the controls do not
- It can be difficult to find a suitable control group
Design pitfalls to look out for
Care should be taken to avoid confounding, which arises when an exposure and an outcome are both strongly associated with a third variable. Controls should be subjects who might have been cases in the study but are selected independent of the exposure. Cases and controls should also not be "over-matched."
Is the control group appropriate for the population? Does the study use matching or pairing appropriately to avoid the effects of a confounding variable? Does it use appropriate inclusion and exclusion criteria?
Fictitious Example
There is a suspicion that zinc oxide, the white non-absorbent sunscreen traditionally worn by lifeguards is more effective at preventing sunburns that lead to skin cancer than absorbent sunscreen lotions. A case-control study was conducted to investigate if exposure to zinc oxide is a more effective skin cancer prevention measure. The study involved comparing a group of former lifeguards that had developed cancer on their cheeks and noses (cases) to a group of lifeguards without this type of cancer (controls) and assess their prior exposure to zinc oxide or absorbent sunscreen lotions.
This study would be retrospective in that the former lifeguards would be asked to recall which type of sunscreen they used on their face and approximately how often. This could be either a matched or unmatched study, but efforts would need to be made to ensure that the former lifeguards are of the same average age, and lifeguarded for a similar number of seasons and amount of time per season.
Real-life Examples
Boubekri, M., Cheung, I., Reid, K., Wang, C., & Zee, P. (2014). Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine, 10 (6), 603-611. https://doi.org/10.5664/jcsm.3780
This pilot study explored the impact of exposure to daylight on the health of office workers (measuring well-being and sleep quality subjectively, and light exposure, activity level and sleep-wake patterns via actigraphy). Individuals with windows in their workplaces had more light exposure, longer sleep duration, and more physical activity. They also reported a better scores in the areas of vitality and role limitations due to physical problems, better sleep quality and less sleep disturbances.
Togha, M., Razeghi Jahromi, S., Ghorbani, Z., Martami, F., & Seifishahpar, M. (2018). Serum Vitamin D Status in a Group of Migraine Patients Compared With Healthy Controls: A Case-Control Study. Headache, 58 (10), 1530-1540. https://doi.org/10.1111/head.13423
This case-control study compared serum vitamin D levels in individuals who experience migraine headaches with their matched controls. Studied over a period of thirty days, individuals with higher levels of serum Vitamin D was associated with lower odds of migraine headache.
Related Formulas
- Odds ratio in an unmatched study
- Odds ratio in a matched study
Related Terms
A patient with the disease or outcome of interest.
Confounding
When an exposure and an outcome are both strongly associated with a third variable.
A patient who does not have the disease or outcome.
Matched Design
Each case is matched individually with a control according to certain characteristics such as age and gender. It is important to remember that the concordant pairs (pairs in which the case and control are either both exposed or both not exposed) tell us nothing about the risk of exposure separately for cases or controls.
Observed Assignment
The method of assignment of individuals to study and control groups in observational studies when the investigator does not intervene to perform the assignment.
Unmatched Design
The controls are a sample from a suitable non-affected population.
Now test yourself!
1. Case Control Studies are prospective in that they follow the cases and controls over time and observe what occurs.
a) True b) False
2. Which of the following is an advantage of Case Control Studies?
a) They can simultaneously look at multiple risk factors. b) They are useful to initially establish an association between a risk factor and a disease or outcome. c) They take less time to complete because the condition or disease has already occurred. d) b and c only e) a, b, and c
Evidence Pyramid - Navigation
- Meta- Analysis
- Case Reports
- << Previous: Case Report
- Next: Cohort Study >>

- Last Updated: Sep 25, 2023 10:59 AM
- URL: https://guides.himmelfarb.gwu.edu/studydesign101

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2850
- [email protected]
- https://himmelfarb.gwu.edu

Statistics and Research Methods for Acute Care and General Surgeons pp 125–137 Cite as
Randomized Trials and Case–Control Matching Techniques
- Emanuele Russo 34 ,
- Annalaura Montalti 35 ,
- Domenico Pietro Santonastaso 34 &
- Giuliano Bolondi 34
- First Online: 14 December 2022
307 Accesses
Part of the book series: Hot Topics in Acute Care Surgery and Trauma ((HTACST))
Randomized control trials (RCTs) are deemed to be among the most powerful and rigorous clinical research instruments. The main application is to evaluate the effectiveness and safety of new treatment or clinical approach. Researchers employ several strategies to reduce bias and increase the strength of results such as “blinding,” multicenter enrollment, and different randomization designs. Finding’s interpretation needs meticulous reporting of each phase of the trial. RCTs are not appropriate for the validation of screening tests and for the study of rare outcomes.
Case–control studies are a sub-type of retrospective observational studies. The main goal of case–control studies is to investigate the risk factors that led to the development of the disease. This type of design allows relative risk to be estimated by means of odds ratios and it is deemed to be an efficient means of studying rare diseases with a long-term latency period.
In case–control studies, matching techniques are often employed. Pairing techniques allow to control some confounding factors and increase statistical power in studies with small populations. Patient matching is increasingly performed by complex techniques such as propensity score and inverse probability.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Holy Bible Book of Daniel (1; 1–21).
Google Scholar
Amberson JB, McMahon BT, Pinner M. A clinical trial of sanocrysin in pulmonary tuberculosis. Am Rev Tuberc. 1931;24:401–35.
Streptomycin treatment of pulmonary tuberculosis. Br Med J. 1948;2(4582):769–82.
Article Google Scholar
Stolberg HO, Norman G, Trop I. Randomized controlled trials. Fundamentals of clinical research for radiologists. Am J Roentgenol. 2004;183:1539–44. https://doi.org/10.2214/ajr.183.6.01831539 .
De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351(12):1250–1.
Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21(19):2917–30. https://doi.org/10.1002/sim.1296 .
Horton R. From star signs to trial guidelines. Lancet. 2000;355(9209):1033–4. https://doi.org/10.1016/S0140-6736(00)02031-6 .
Article CAS Google Scholar
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. https://doi.org/10.1001/jama.2013.281053 .
Hannan EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv. 2008;1(3):211–7. https://doi.org/10.1016/j.jcin.2008.01.008 .
Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–86. https://doi.org/10.1056/NEJM200006223422506 .
Feys F, et al. Do randomized clinical trials with inadequate blinding report enhanced placebo effects for intervention groups and nocebo effects for placebo groups? Syst Rev. 2014;3:14.
Lee CS, Lee AY. How artificial intelligence can transform randomized controlled trials. Transl Vis Sci Technol. 2020;9(2):9. https://doi.org/10.1167/tvst.9.2.9 .
Banerjee A, Chitnis UB, Jadhav SL, Bhawalkar JS, Chaudhury S. Hypothesis testing, type I and type II errors. Ind Psychiatry J. 2009;18(2):127–31. https://doi.org/10.4103/0972-6748.62274 .
Moher D, Schulz KF, Altman DG, CONSORT GROUP (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134(8):657–62. https://doi.org/10.7326/0003-4819-134-8-200104170-00011 .
Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT Statement. JAMA. 1996;276(8):637–9. https://doi.org/10.1001/jama.276.8.637 .
Piaggio G, Elbourne DR, Altman DG, Pocock SJ, SJW E, CONSORT Group FT. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT Statement. JAMA. 2006;295(10):1152–60. https://doi.org/10.1001/jama.295.10.1152 .
Ioannidis JPA, Dixon DO, McIntosh M, Albert JM, Bozzette SA, Schnittman SN. Relationship between event rates and treatment effects in clinical site differences within multicenter trials: an example from primary Pneumocystis carinii Prophylaxi. Control Clin Trials. 1999;20:253–66.
CRASH-2 Collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto-Merino D, Woolley T. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377(9771):1096–101, 1101.e1–2. https://doi.org/10.1016/S0140-6736(11)60278-X .
Mitra B, Mazur S, Cameron PA, Bernard S, Burns B, Smith A, Rashford S, Fitzgerald M, Smith K, Gruen RL. Tranexamic acid for trauma: filling the GAP in evidence. Emerg Med Australas. 2014;26:194–7.
Hróbjartsson A, Boutron I. Blinding in randomized clinical trials: imposed impartiality. Clin Pharmacol Ther. 2011;90(5):732–6. https://doi.org/10.1038/clpt.2011.207 .
Karanicolas PJ, Farrokhyar F, Bhandari M. Practical tips for surgical research: blinding: who, what, when, why, how? Can J Surg. 2010;53(5):345–8.
Kao LS, Tyson JE, Blakely ML, Lally KP. Clinical research methodology I: introduction to randomized trials. J Am Coll Surg. 2008;206(2):361–9.
Suresh KP. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4:8–11.
Hopewell S, Dutton S, Yu LM, Chan AW, Altman DG. The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. BMJ. 2010;340:c723. https://doi.org/10.1136/bmj.c723 .
Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2–21. https://doi.org/10.1016/j.socscimed.2017.12.005 .
Sibbald B, Roland M. Why are randomized controlled trials important? BMJ. 1998;316:201.
Hein S, Weeland J. Introduction to the special issue. Randomized control trials (RCTs) in clinical and community settings: challenges, alternatives and supplementary designs. New Dir Child Adolesc Dev. 2019;2019(167):7–15. https://doi.org/10.1002/cad.20312 .
Thompson D. Understanding financial conflicts of interest. N Engl J Med. 1993;329:573–6.
Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289(4):454–65. https://doi.org/10.1001/jama.289.4.454 .
Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170(4):477–80.
Sason-Fisher RW, Bonevski B, Green LW, D’Este C. Limitations of the randomized controlled trial in evaluation population-based Health intervention. Am J Prev Med. 2007;33(2):155–61.
Kraemer HC, Robinson TN. Are certain multicenter randomized clinical trial structures misleading clinical and policy decisions? Contemp Clin Trials. 2005;26(5):518–29. https://doi.org/10.1016/j.cct.2005.05.002 .
Harris PNA, Tambyah PA, Lye DC, et al. MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). Effect of Piperacillin-Tazobactam vs Meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320(10):984–94. [Erratum in: JAMA. 2019 Jun 18;321(23):2370]. https://doi.org/10.1001/jama.2018.12163 .
Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Kahlmeter G. Antibiotics for ceftriaxone-resistant gram-negative bacterial bloodstream infections. JAMA. 2019;321(6):612–3. https://doi.org/10.1001/jama.2018.19345 .
Missing information on sample size. JAMA. 2019;321(23):2370. [Erratum for: JAMA. 2018;320(10):984–994]. https://doi.org/10.1001/jama.2019.6706 .
Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. https://doi.org/10.1136/bmj.i969 .
Wachoider S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. Am J Epidemiol. 1992;135(9):1042–50.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
Chesnaye NC, Stel VS, Tripepi G, Dekker FW, Fu EL, Zoccali C, Jager KJ. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2021;15(1):14–20. https://doi.org/10.1093/ckj/sfab158 .
Schulte PJ, Mascha EJ. Propensity score methods: theory and practice for anesthesia research. Anesth Analg. 2018;127(4):1074–84. https://doi.org/10.1213/ANE.0000000000002920 .
Rodríguez-Pardo J, Plaza Herráiz A, Lobato-Pérez L, Ramírez-Torres M, De Lorenzo I, Alonso de Leciñana M, Díez-Tejedor E, Fuentes B. Influence of oral anticoagulation on stroke severity and outcomes: a propensity score matching case-control study. J Neurol Sci. 2020;410:116685. https://doi.org/10.1016/j.jns.2020.116685 .
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79. https://doi.org/10.1002/sim.6607 .
Download references
Author information
Authors and affiliations.
Anesthesia and Intensive Care Unit, AUSL Romagna, Maurizio Bufalini Hospital, Cesena FC, Italy
Emanuele Russo, Domenico Pietro Santonastaso & Giuliano Bolondi
Risk and Compliance, Healthcare, KPMG Advisory S.p.A., Milan, Italy
Annalaura Montalti
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Emanuele Russo .
Editor information
Editors and affiliations.
General and Emergency Surgery Department, School of Medicine and Surgery, Milano-Bicocca University, Monza, Italy
Marco Ceresoli
Department of Surgery, College of Medicine and Health Science, United Arab Emirates University, Abu Dhabi, United Arab Emirates
Fikri M. Abu-Zidan
Department of Surgery, Stanford University, Stanford, CA, USA
Kristan L. Staudenmayer
General and Emergency Surgery Department, Bufalini Hospital, Cesena, Italy
Fausto Catena
Department of General, Emergency and Trauma Surgery, Pisa University Hospital, Pisa, Pisa, Italy
Federico Coccolini
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter.
Russo, E., Montalti, A., Santonastaso, D.P., Bolondi, G. (2022). Randomized Trials and Case–Control Matching Techniques. In: Ceresoli, M., Abu-Zidan, F.M., Staudenmayer, K.L., Catena, F., Coccolini, F. (eds) Statistics and Research Methods for Acute Care and General Surgeons. Hot Topics in Acute Care Surgery and Trauma. Springer, Cham. https://doi.org/10.1007/978-3-031-13818-8_10
Download citation
DOI : https://doi.org/10.1007/978-3-031-13818-8_10
Published : 14 December 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-13817-1
Online ISBN : 978-3-031-13818-8
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
A case-control study is a type of observational study commonly used to look at factors associated with diseases or outcomes.[1] The case-control study starts with a group of cases, which are the individuals who have the outcome of interest. The researcher then tries to construct a second group of individuals called the controls, who are similar to the case individuals but do not have the ...
Case-control studies are a type of observational study often used in fields like medical research, environmental health, or epidemiology. While most observational studies are qualitative in nature, case-control studies can also be quantitative, and they often are in healthcare settings. Case-control studies can be used for both exploratory and ...
Case-control studies are one of the major observational study designs for performing clinical research. The advantages of these study designs over other study designs are that they are relatively quick to perform, economical, and easy to design and implement. Case-control studies are particularly appropriate for studying disease outbreaks, rare diseases, or outcomes of interest.
A case-control study (also known as case-referent study) is a type of observational study in which two existing groups differing in outcome are identified and compared on the basis of some supposed causal attribute. Case-control studies are often used to identify factors that may contribute to a medical condition by comparing subjects who have the condition with patients who do not have ...
A case control study is a retrospective, observational study that compares two existing groups. Researchers form these groups based on the existence of a condition in the case group and the lack of that condition in the control group. They evaluate the differences in the histories between these two groups looking for factors that might cause a ...
Case-control studies are particularly useful for studying ... Hoes A. Clinical epidemiology. Principles, methods and applications for clinical research. ... Case-control studies: research in ...
Go to: Case-Control study design is a type of observational study. In this design, participants are selected for the study based on their outcome status. Thus, some participants have the outcome of interest (referred to as cases), whereas others do not have the outcome of interest (referred to as controls). The investigator then assesses the ...
A case-control study, like other medical research, can help scientists find new medications and treatments. ... Mann, C. J. (2003). Observational research methods. Research design II: cohort ...
Introduction. Case-control and cohort studies are observational studies that lie near the middle of the hierarchy of evidence. These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1). Although these studies are not ranked as highly as ...
A case-control study is a research method where two groups of people are compared - those with the condition (cases) and those without (controls). By looking at their past, researchers try to identify what factors might have contributed to the condition in the 'case' group.
Research, Methods, Statistics JAMA Guide to Statistics and Methods Guide to Statistics and Methods. Select Your Interests. ... Case-control studies are time-efficient and less costly than RCTs, particularly when the outcome of interest is rare or takes a long time to occur, because the cases are identified at study onset and the outcomes have ...
As an actual example of a case-control study, children with autism spectrum disorder (ASD) may be compared with normally developing children to determine whether a history of maternal antidepressant use during pregnancy is more frequent among cases than among controls; if it is, and if the association remains statistically significant after adjusting for confounding variables, one may ...
The retrospective case-control study is an important research strategy commonly encountered in the medical literature. A thoughtfully designed, carefully executed case-control study can be an invaluable source of clinical information, and physicians must often base important decisions about patient counseling and management on their interpretation of such studies.
A case-control study was conducted to investigate if exposure to zinc oxide is a more effective skin cancer prevention measure. The study involved comparing a group of former lifeguards that had developed cancer on their cheeks and noses (cases) to a group of lifeguards without this type of cancer (controls) and assess their prior exposure to ...
Case-control studies are one of the major observational study designs for performing clinical research. The advantages of these study designs over other study designs are that they are relatively quick to perform, economical, and easy to design and implement. Case-control studies are particularly appropriate for studying disease outbreaks, rare ...
Contrary to cohort research, case-control studies are less likely to have loss to follow-up. Before doing more extensive and expensive studies (such as cohort study), case-control studies are sometimes conducted as preliminary research to determine any potential correlations. ... Observational research methods. Research design II: cohort ...
Case-control studies are a sub-type of retrospective observational studies. The main goal of case-control studies is to investigate the risk factors that led to the development of the disease. ... M., Abu-Zidan, F.M., Staudenmayer, K.L., Catena, F., Coccolini, F. (eds) Statistics and Research Methods for Acute Care and General Surgeons. Hot ...