- Reference Manager
- Simple TEXT file

People also looked at
Hypothesis and theory article, type 2 diabetes mellitus: a pathophysiologic perspective.

- Department of Medicine, Duke University, Durham, NC, United States
Type 2 Diabetes Mellitus (T2DM) is characterized by chronically elevated blood glucose (hyperglycemia) and elevated blood insulin (hyperinsulinemia). When the blood glucose concentration is 100 milligrams/deciliter the bloodstream of an average adult contains about 5–10 grams of glucose. Carbohydrate-restricted diets have been used effectively to treat obesity and T2DM for over 100 years, and their effectiveness may simply be due to lowering the dietary contribution to glucose and insulin levels, which then leads to improvements in hyperglycemia and hyperinsulinemia. Treatments for T2DM that lead to improvements in glycemic control and reductions in blood insulin levels are sensible based on this pathophysiologic perspective. In this article, a pathophysiological argument for using carbohydrate restriction to treat T2DM will be made.
Introduction
Type 2 Diabetes Mellitus (T2DM) is characterized by a persistently elevated blood glucose, or an elevation of blood glucose after a meal containing carbohydrate ( 1 ) ( Table 1 ). Unlike Type 1 Diabetes which is characterized by a deficiency of insulin, most individuals affected by T2DM have elevated insulin levels (fasting and/or post glucose ingestion), unless there has been beta cell failure ( 2 , 3 ). The term “insulin resistance” (IR) has been used to explain why the glucose levels remain elevated even though there is no deficiency of insulin ( 3 , 4 ). Attempts to determine the etiology of IR have involved detailed examinations of molecular and intracellular pathways, with attribution of cause to fatty acid flux, but the root cause has been elusive to experts ( 5 – 7 ).
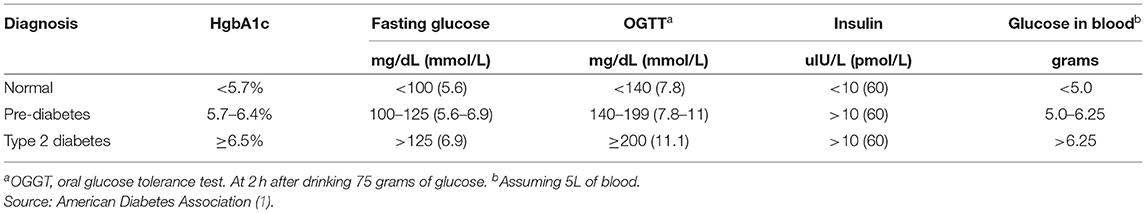
Table 1 . Definition of type 2 diabetes mellitus.
How Much Glucose Is in the Blood?
Keeping in mind that T2DM involves an elevation of blood glucose, it is important to understand how much glucose is in the blood stream to begin with, and then the factors that influence the blood glucose—both exogenous and endogenous factors. The amount of glucose in the bloodstream is carefully controlled—approximately 5–10 grams in the bloodstream at any given moment, depending upon the size of the person. To calculate this, multiply 100 milligrams/deciliter × 1 gram/1,000 milligrams × 10 deciliters/1 liter × 5 liters of blood. The “zeros cancel” and you are left with 5 grams of glucose if the individual has 5 liters of blood. Since red blood cells represent about 40% of the blood volume, and the glucose is in equilibrium, there may be an extra 40% glucose because of the red blood cell reserve ( 8 ). Adding the glucose from the serum and red blood cells totals about 5–10 grams of glucose in the entire bloodstream.
Major Exogenous Factors That Raise the Blood Glucose
Dietary carbohydrate is the major exogenous factor that raises the blood glucose. When one considers that it is common for an American in 2021 to consume 200–300 grams of carbohydrate daily, and most of this carbohydrate is digested and absorbed as glucose, the body absorbs and delivers this glucose via the bloodstream to the cells while attempting to maintain a normal blood glucose level. Thinking of it in this way, if 200–300 grams of carbohydrates is consumed in a day, the bloodstream that holds 5–10 grams of glucose and has a concentration of 100 milligrams/deciliter, is the conduit through which 200,000–300,000 milligrams (200 grams = 200,000 milligrams) passes over the course of a day.
Major Endogenous Factors That Raise the Blood Glucose
There are many endogenous contributors that raise the blood glucose. There are at least 3 different hormones that increase glucose levels: glucagon, epinephrine, and cortisol. These hormones increase glucose levels by increasing glycogenolysis and gluconeogenesis ( 9 ). Without any dietary carbohydrate, the normal human body can generate sufficient glucose though the mechanism of glucagon secretion, gluconeogenesis, glycogen storage and glycogenolysis ( 10 ).
Major Exogenous Factors That Lower the Blood Glucose
A reduction in dietary carbohydrate intake can lower the blood glucose. An increase in activity or exercise usually lowers the blood glucose ( 11 ). There are many different medications, employing many mechanisms to lower the blood glucose. Medications can delay sucrose and starch absorption (alpha-glucosidase inhibitors), slow gastric emptying (GLP-1 agonists, DPP-4 inhibitors) enhance insulin secretion (sulfonylureas, meglitinides, GLP-1 agonists, DPP-4 inhibitors), reduce gluconeogenesis (biguanides), reduce insulin resistance (biguanides, thiazolidinediones), and increase urinary glucose excretion (SGLT-2 inhibitors). The use of medications will also have possible side effects.
Major Endogenous Factors That Lower the Blood Glucose
The major endogenous mechanism to lower the blood glucose is to deliver glucose into the cells (all cells can use glucose). If the blood glucose exceeds about 180 milligrams/deciliter, then loss of glucose into the urine can occur. The blood glucose is reduced by cellular uptake using glut transporters ( 12 ). Some cells have transporters that are responsive to the presence of insulin to activate (glut4), others have transporters that do not require insulin for activation. Insulin-responsive glucose transporters in muscle cells and adipose cells lead to a reduction in glucose levels—especially after carbohydrate-containing meals ( 13 ). Exercise can increase the glucose utilization in muscle, which then increases glucose cellular uptake and reduce the blood glucose levels. During exercise, when the metabolic demands of skeletal muscle can increase more than 100-fold, and during the absorptive period (after a meal), the insulin-responsive glut4 transporters facilitate the rapid entry of glucose into muscle and adipose tissue, thereby preventing large fluctuations in blood glucose levels ( 13 ).
Which Cells Use Glucose?
Glucose can used by all cells. A limited number of cells can only use glucose, and are “glucose-dependent.” It is generally accepted that the glucose-dependent cells include red blood cells, white blood cells, and cells of the renal papilla. Red blood cells have no mitochondria for beta-oxidation, so they are dependent upon glucose and glycolysis. White blood cells require glucose for the respiratory burst when fighting infections. The cells of the inner renal medulla (papilla) are under very low oxygen tension, so therefore must predominantly use glucose and glycolysis. The low oxygen tension is a result of the countercurrent mechanism of urinary concentration ( 14 ). These glucose-dependent cells have glut transporters that do not require insulin for activation—i.e., they do not need insulin to get glucose into the cells. Some cells can use glucose and ketones, but not fatty acids. The central nervous system is believed to be able to use glucose and ketones for fuel ( 15 ). Other cells can use glucose, ketones, and fatty acids for fuel. Muscle, even cardiac muscle, functions well on fatty acids and ketones ( 16 ). Muscle cells have both non-insulin-responsive and insulin-responsive (glut4) transporters ( 12 ).
Possible Dual Role of an Insulin-Dependent Glucose-Transporter (glut4)
A common metaphor is to think of the insulin/glut transporter system as a key/lock mechanism. Common wisdom states that the purpose of insulin-responsive glut4 transporters is to facilitate glucose uptake when blood insulin levels are elevated. But, a lock serves two purposes: to let someone in and/or to keep someone out . So, one of the consequences of the insulin-responsive glut4 transporter is to keep glucose out of the muscle and adipose cells, too, when insulin levels are low. The cells that require glucose (“glucose-dependent”) do not need insulin to facilitate glucose entry into the cell (non-insulin-responsive transporters). In a teleological way, it would “make no sense” for cells that require glucose to have insulin-responsive glut4 transporters. Cells that require glucose have glut1, glut2, glut3, glut5 transporters—none of which are insulin-responsive (Back to the key/lock metaphor, it makes no sense to have a lock on a door that you want people to go through). At basal (low insulin) conditions, most glucose is used by the brain and transported by non-insulin-responsive glut1 and glut3. So, perhaps one of the functions of the insulin-responsive glucose uptake in muscle and adipose to keep glucose OUT of the these cells at basal (low insulin) conditions, so that the glucose supply can be reserved for the tissue that is glucose-dependent (blood cells, renal medulla).
What Causes IR and T2DM?
The current commonly espoused view is that “Type 2 diabetes develops when beta-cells fail to secrete sufficient insulin to keep up with demand, usually in the context of increased insulin resistance.” ( 17 ). Somehow, the beta cells have failed in the face of insulin resistance. But what causes insulin resistance? When including the possibility that the environment may be part of the problem, is it possible that IR is an adaptive (protective) response to excess glucose availability? From the perspective that carbohydrate is not an essential nutrient and the change in foods in recent years has increased the consumption of refined sugar and flour, maybe hyperinsulinemia is the cause of IR and T2DM, as cells protect themselves from excessive glucose and insulin levels.
Insulin Is Already Elevated in IR and T2DM
Clinical experience of most physicians using insulin to treat T2DM over time informs us that an escalation of insulin dose is commonly needed to achieve glycemic control (when carbohydrate is consumed). When more insulin is given to someone with IR, the IR seems to get worse and higher levels of insulin are needed. I have the clinical experience of treating many individuals affected by T2DM and de-prescribing insulin as it is no longer needed after consuming a diet without carbohydrate ( 18 ).
Diets Without Carbohydrate Reverse IR and T2DM
When dietary manipulation was the only therapy for T2DM, before medications were available, a carbohydrate-restricted diet was used to treat T2DM ( 19 – 21 ). Clinical experience of obesity medicine physicians and a growing number of recent studies have demonstrated that carbohydrate-restricted diets reverse IR and T2DM ( 18 , 22 , 23 ). Other methods to achieve caloric restriction also have these effects, like calorie-restricted diets and bariatric surgery ( 24 , 25 ). There may be many mechanisms by which these approaches may work: a reduction in glucose, a reduction in insulin, nutritional ketosis, a reduction in metabolic syndrome, or a reduction in inflammation ( 26 ). Though there may be many possible mechanisms, let's focus on an obvious one: a reduction in blood glucose. Let's assume for a moment that the excessive glucose and insulin leads to hyperinsulinemia and this is the cause of IR. On a carbohydrate-restricted diet, the reduction in blood glucose leads to a reduction in insulin. The reduction in insulin leads to a reduction in insulin resistance. The reduction in insulin leads to lipolysis. The resulting lowering of blood glucose, insulin and body weight reverses IR, T2DM, AND obesity. These clinical observations strongly suggest that hyperinsulinemia is a cause of IR and T2DM—not the other way around.
What Causes Atherosclerosis?
For many years, the metabolic syndrome has been described as a possible cause of atherosclerosis, but there are no RCTs directly targeting metabolic syndrome, and the current drug treatment focuses on LDL reduction, so its importance remains controversial. A recent paper compared the relative importance of many risk factors in the prediction of the first cardiac event in women, and the most powerful predictors were diabetes, metabolic syndrome, smoking, hypertension and BMI ( 27 ). The connection between dietary carbohydrate and fatty liver is well-described ( 28 ). The connection between fatty liver and atherosclerosis is well-described ( 29 ). It is very possible that the transport of excess glucose to the adipose tissue via lipoproteins creates the particles that cause the atherosclerotic damage (small LDL) ( Figure 1 ) ( 30 – 32 ). This entire process of dietary carbohydrate leading to fatty liver, leading to small LDL, is reversed by a diet without carbohydrate ( 26 , 33 , 34 ).
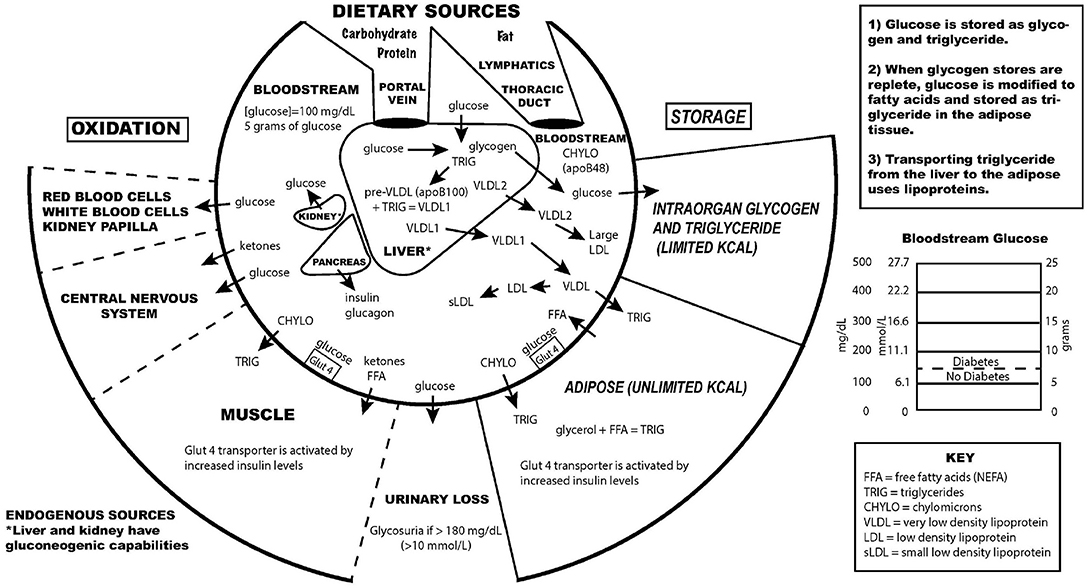
Figure 1 . Key aspects of the interconnection between glucose and lipoprotein metabolism.
Reducing dietary carbohydrate in the context of a low carbohydrate, ketogenic diet reduces hyperglycemia and hyperinsulinemia, IR and T2DM. In the evaluation of an individual for a glucose abnormality, measure the blood glucose and insulin levels. If the insulin level (fasting or after a glucose-containing meal) is high, do not give MORE insulin—instead, use an intervention to lower the insulin levels. Effective ways to reduce insulin resistance include lifestyle, medication, and surgical therapies ( 23 , 35 ).
The search for a single cause of a complex problem is fraught with difficulty and controversy. I am not hypothesizing that excessive dietary carbohydrate is the only cause of IR and T2DM, but that it is a cause, and quite possibly the major cause. How did such a simple explanation get overlooked? I believe it is very possible that the reductionistic search for intracellular molecular mechanisms of IR and T2DM, the emphasis on finding pharmaceutical (rather than lifestyle) treatments, the emphasis on the treatment of high total and LDL cholesterol, and the fear of eating saturated fat may have misguided a generation of researchers and clinicians from the simple answer that dietary carbohydrate, when consumed chronically in amounts that exceeds an individual's ability to metabolize them, is the most common cause of IR, T2DM and perhaps even atherosclerosis.
While there has historically been a concern about the role of saturated fat in the diet as a cause of heart disease, most nutritional experts now cite the lack of evidence implicating dietary saturated fat as the reason for lack of concern of it in the diet ( 36 ).
The concept of comparing medications that treat IR by insulin-sensitizers or by providing insulin itself was tested in the Bari-2D study ( 37 ). Presumably in the context of consuming a standard American diet, this study found no significant difference in death rates or major cardiovascular events between strategies of insulin sensitization or insulin provision.
While lifestyle modification may be ideal to prevent or cure IR and T2DM, for many people these changes are difficult to learn and/or maintain. Future research should be directed toward improving adherence to all effective lifestyle or medication treatments. Future research is also needed to assess the effect of carbohydrate restriction on primary or secondary prevention of outcomes of cardiovascular disease.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
EW receives royalties from popular diet books and is founder of a company based on low-carbohydrate diet principles (Adapt Your Life, Inc.).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care . (2016) 39 (Suppl. 1):S13–22. doi: 10.2337/dc16-S005
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. (1984) 74:1238–46. doi: 10.1172/JCI111533
3. Reaven GM. Compensatory hyperinsulinemia and the development of an atherogenic lipoprotein profile: the price paid to maintain glucose homeostasis in insulin-resistant individuals. Endocrinol Metab Clin North Am. (2005) 34:49–62. doi: 10.1016/j.ecl.2004.12.001
4. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. (1991) 14:173–94. doi: 10.2337/diacare.14.3.173
5. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. (2005) 365:1415–28. doi: 10.1016/S0140-6736(05)66378-7
6. Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. (2019) 234:8152–61. doi: 10.1002/jcp.27603
7. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. (2000) 106:171–6. doi: 10.1172/JCI10583
8. Guizouarn H, Allegrini B. Erythroid glucose transport in health and disease. Pflugers Arch. (2020) 472:1371–83. doi: 10.1007/s00424-020-02406-0
9. Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. (2017) 13:572–87. doi: 10.1038/nrendo.2017.80
10. Tondt J, Yancy WS, Westman EC. Application of nutrient essentiality criteria to dietary carbohydrates. Nutr Res Rev. (2020) 33:260–70. doi: 10.1017/S0954422420000050
11. Colberg SR, Hernandez MJ, Shahzad F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care. (2013) 36:e177. doi: 10.2337/dc13-0965
12. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. (2013) 34:121–38. doi: 10.1016/j.mam.2012.07.001
13. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. (2002) 3:267–77. doi: 10.1038/nrm782
14. Epstein FH. Oxygen and renal metabolism. Kidney Int. (1997) 51:381–5. doi: 10.1038/ki.1997.50
15. Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. (2006) 26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258
16. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. (2020) 370:364–8. doi: 10.1126/science.abc8861
17. Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. (2017) 66:241–55. doi: 10.2337/db16-0806
18. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab. (2008) 5:36. doi: 10.1186/1743-7075-5-36
CrossRef Full Text | Google Scholar
19. Allen F. The treatment of diabetes. Boston Med Surg J. (1915) 172:241–7. doi: 10.1056/NEJM191502181720702
20. Osler W, McCrae T. The Principles and Practice of Medicine . 9th ed. New York and London: Appleton & Company (1923).
21. Lennerz BS, Koutnik AP, Azova S, Wolfsdorf JI, Ludwig DS. Carbohydrate restriction for diabetes: rediscovering centuries-old wisdom. J Clin Invest. (2021) 131:e142246. doi: 10.1172/JCI142246
22. Steelman GM, Westman EC. Obesity: Evaluation and Treatment Essentials . 2nd ed. Boca Raton: CRC Press, Taylor & Francis Group (2016). 340 p.
23. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. (2019) 10:348. doi: 10.3389/fendo.2019.00348
24. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. (2011) 54:2506–14. doi: 10.1007/s00125-011-2204-7
25. Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. (2010) 33:1438–42. doi: 10.2337/dc09-2107
26. Bhanpuri NH, Hallberg SJ, Williams PT, McKenzie AL, Ballard KD, Campbell WW, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc Diabetol. (2018) 17:56. doi: 10.1186/s12933-018-0698-8
27. Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh-Ali AA, Ridker PM, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. (2021) 6:437–47. doi: 10.1001/jamacardio.2020.7073
28. Duwaerts CC, Maher JJ. Macronutrients and the adipose-liver axis in obesity and fatty liver. Cell Mol Gastroenterol Hepatol. (2019) 7:749–61. doi: 10.1016/j.jcmgh.2019.02.001
29. Zhang L, She Z-G, Li H, Zhang X-J. Non-alcoholic fatty liver disease: a metabolic burden promoting atherosclerosis. Clin Sci Lond Engl. (1979) 134:1775–99. doi: 10.1042/CS20200446
30. Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. (1995) 62:19–29. doi: 10.1093/ajcn/62.1.19
31. Packard C, Caslake M, Shepherd J. The role of small, dense low density lipoprotein (LDL): a new look. Int J Cardiol. (2000) 74 (Suppl. 1):S17–22. doi: 10.1016/S0167-5273(99)00107-2
32. Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2020) 41:2313–30. doi: 10.1093/eurheartj/ehz962
33. Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. (2004) 140:769. doi: 10.7326/0003-4819-140-10-200405180-00006
34. Tendler D, Lin S, Yancy WS, Mavropoulos J, Sylvestre P, Rockey DC, et al. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. (2007) 52:589–93. doi: 10.1007/s10620-006-9433-5
35. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. (1995) 222:339–50. doi: 10.1097/00000658-199509000-00011
36. Astrup A, Magkos F, Bier DM, Brenna JT, de Oliveira Otto MC, Hill JO, et al. Saturated fats and health: a reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76:844–57. doi: 10.1016/j.jacc.2020.05.077
37. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med . (2009) 360:2503–15. doi: 10.1056/NEJMoa0805796
Keywords: type 2 diabetes, insulin resistance, pre-diabetes, carbohydrate-restricted diets, hyperinsulinemia, hyperglycemia
Citation: Westman EC (2021) Type 2 Diabetes Mellitus: A Pathophysiologic Perspective. Front. Nutr. 8:707371. doi: 10.3389/fnut.2021.707371
Received: 09 May 2021; Accepted: 20 July 2021; Published: 10 August 2021.
Reviewed by:
Copyright © 2021 Westman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric C. Westman, ewestman@duke.edu
This article is part of the Research Topic
Carbohydrate-restricted Nutrition and Diabetes Mellitus
Type 2 Diabetes Research At-a-Glance
The ADA is committed to continuing progress in the fight against type 2 diabetes by funding research, including support for potential new treatments, a better understating of genetic factors, addressing disparities, and more. For specific examples of projects currently funded by the ADA, see below.
Greg J. Morton, PhD
University of Washington
Project: Neurocircuits regulating glucose homeostasis
“The health consequences of diabetes can be devastating, and new treatments and therapies are needed. My research career has focused on understanding how blood sugar levels are regulated and what contributes to the development of diabetes. This research will provide insights into the role of the brain in the control of blood sugar levels and has potential to facilitate the development of novel approaches to diabetes treatment.”
The problem: Type 2 diabetes (T2D) is among the most pressing and costly medical challenges confronting modern society. Even with currently available therapies, the control and management of blood sugar levels remains a challenge in T2D patients and can thereby increase the risk of diabetes-related complications. Continued progress with newer, better therapies is needed to help people with T2D.
The project: Humans have special cells, called brown fat cells, which generate heat to maintain optimal body temperature. Dr. Morton has found that these cells use large amounts of glucose to drive this heat production, thus serving as a potential way to lower blood sugar, a key goal for any diabetes treatment. Dr. Morton is working to understand what role the brain plays in turning these brown fat cells on and off.
The potential outcome: This work has the potential to fundamentally advance our understanding of how the brain regulates blood sugar levels and to identify novel targets for the treatment of T2D.
Tracey Lynn McLaughlin, MD
Stanford University
Project: Role of altered nutrient transit and incretin hormones in glucose lowering after Roux-en-Y gastric bypass surgery
“This award is very important to me personally not only because the enteroinsular axis (gut-insulin-glucose metabolism) is a new kid on the block that requires rigorous physiologic studies in humans to better understand how it contributes to glucose metabolism, but also because the subjects who develop severe hypoglycemia after gastric bypass are largely ignored in society and there is no treatment for this devastating and very dangerous condition.”
The problem: Roux-en-Y gastric bypass (RYGB) surgery is the single-most effective treatment for type 2 diabetes, with persistent remission in 85% of cases. However, the underlying ways by which the surgery improves glucose control is not yet understood, limiting the ability to potentially mimic the surgery in a non-invasive way. Furthermore, a minority of RYGB patients develop severe, disabling, and life-threatening low-blood sugar, for which there is no current treatment.
The project: Utilizing a unique and rigorous human experimental model, the proposed research will attempt to gain a better understanding on how RYGB surgery improves glucose control. Dr. McLaughlin will also test a hypothesis which she believes could play an important role in the persistent low-blood sugar that is observed in some patients post-surgery.
The potential outcome: This research has the potential to identify novel molecules that could represent targets for new antidiabetic therapies. It is also an important step to identifying people at risk for low-blood sugar following RYGB and to develop postsurgical treatment strategies.
Rebekah J. Walker, PhD
Medical College of Wisconsin
Project: Lowering the impact of food insecurity in African Americans with type 2 diabetes
“I became interested in diabetes research during my doctoral training, and since that time have become passionate about addressing social determinants of health and health disparities, specifically in individuals with diabetes. Living in one of the most racially segregated cities in the nation, the burden to address the needs of individuals at particularly high risk of poor outcomes has become important to me both personally and professionally.”
The problem: Food insecurity is defined as the inability to or limitation in accessing nutritionally adequate food and may be one way to address increased diabetes risk in high-risk populations. Food insecure individuals with diabetes have worse diabetes outcomes and have more difficulty following a healthy diet compared to those who are not food insecure.
The project: Dr. Walker’s study will gather information to improve and then will test an intervention to improve blood sugar control, dietary intake, self-care management, and quality of life in food insecure African Americans with diabetes. The intervention will include weekly culturally appropriate food boxes mailed to the participants and telephone-delivered diabetes education and skills training. It will be one of the first studies focused on the unique needs of food insecure African American populations with diabetes using culturally tailored strategies.
The potential outcome: This study has the potential to guide and improve policies impacting low-income minorities with diabetes. In addition, Dr. Walker’s study will help determine if food supplementation is important in improving diabetes outcomes beyond diabetes education alone.
Donate Today and Change Lives!
Newer Pharmacologic Treatments in Adults With Type 2 Diabetes: A Clinical Guideline From the American College of Physicians
Affiliations.
- 1 American College of Physicians, Philadelphia, Pennsylvania (A.Q., T.S., C.H.S.).
- 2 Portland Veterans Affairs Medical Center and Oregon Health & Science University, Portland, Oregon (A.J.O.).
- 3 Centers for Disease Control and Prevention, Atlanta, Georgia (L.A.H.).
- 4 David Geffen School of Medicine at the University of California, Los Angeles, Los Angeles, California (C.J.C.).
- PMID: 38639546
- DOI: 10.7326/M23-2788
Description: The American College of Physicians (ACP) developed this clinical guideline to update recommendations on newer pharmacologic treatments of type 2 diabetes. This clinical guideline is based on the best available evidence for effectiveness, comparative benefits and harms, consideration of patients' values and preferences, and costs.
Methods: This clinical guideline is based on a systematic review of the effectiveness and harms of newer pharmacologic treatments of type 2 diabetes, including glucagon-like peptide-1 (GLP-1) agonists, a GLP-1 agonist and glucose-dependent insulinotropic polypeptide agonist, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, and long-acting insulins, used either as monotherapy or in combination with other medications. The Clinical Guidelines Committee prioritized the following outcomes, which were evaluated using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach: all-cause mortality, major adverse cardiovascular events, myocardial infarction, stroke, hospitalization for congestive heart failure, progression of chronic kidney disease, serious adverse events, and severe hypoglycemia. Weight loss, as measured by percentage of participants who achieved at least 10% total body weight loss, was a prioritized outcome, but data were insufficient for network meta-analysis and were not rated with GRADE.
Audience and patient population: The audience for this clinical guideline is physicians and other clinicians. The population is nonpregnant adults with type 2 diabetes.
Recommendation 1: ACP recommends adding a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide-1 (GLP-1) agonist to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control (strong recommendation; high-certainty evidence).
• Use an SGLT-2 inhibitor to reduce the risk for all-cause mortality, major adverse cardiovascular events, progression of chronic kidney disease, and hospitalization due to congestive heart failure.
• Use a GLP-1 agonist to reduce the risk for all-cause mortality, major adverse cardiovascular events, and stroke.
Recommendation 2: ACP recommends against adding a dipeptidyl peptidase-4 (DPP-4) inhibitor to metformin and lifestyle modifications in adults with type 2 diabetes and inadequate glycemic control to reduce morbidity and all-cause mortality (strong recommendation; high-certainty evidence).
- Introduction
- Conclusions
- Article Information
D, Evidence-based therapy composite score was scored as 0, no evidence-based therapies; 1, 1 evidence-based therapy; 2, 2 evidence-based therapies; and 3, 3 evidence-based therapies.
eTable 1. Datamarts and Respective Health System Participants
eTable 2. Code Lists for Comorbidities and Qualifying ASCVD
- Trends in the Association Between Diabetes and Cardiovascular Events, 1994-2019 JAMA Research Letter November 8, 2022 This study uses administrative health care data from Ontario, Canada, to assess whether changes in diabetes management practices have affected trends in the association between diabetes vs prior cardiovascular disease and risk of cardiovascular events from 1994 to 2019 among adults aged 20 to 84 years. Calvin Ke, MD, PhD; Lorraine L. Lipscombe, MD, MSc; Alanna Weisman, MD, PhD; Limei Zhou, PhD; Peter C. Austin, PhD; Baiju R. Shah, MD, PhD; Gillian L. Booth, MD, MSc
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Nelson AJ , O’Brien EC , Kaltenbach LA, et al. Use of Lipid-, Blood Pressure–, and Glucose-Lowering Pharmacotherapy in Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease. JAMA Netw Open. 2022;5(2):e2148030. doi:10.1001/jamanetworkopen.2021.48030
Manage citations:
© 2024
- Permissions
Use of Lipid-, Blood Pressure–, and Glucose-Lowering Pharmacotherapy in Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease
- 1 Duke Clinical Research Institute, Durham, North Carolina
- 2 Wake Forest School of Medicine, Winston-Salem, North Carolina
- 3 Brigham and Women’s Hospital, Boston, Massachusetts
- 4 University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
- 5 Boehringer Ingelheim Pharmaceuticals, Ridgefield, Connecticut
- 6 University of Texas Southwestern Medical Center, Dallas
- 7 St Luke’s Health System, Kansas City, Missouri
- 8 Parkland Health and Hospital System, Dallas, Texas
- 9 University of Michigan, Ann Arbor
- 10 University of Michigan Medical School, Ann Arbor
- 11 Eli Lilly and Company, Indianapolis, Indiana
- Research Letter Trends in the Association Between Diabetes and Cardiovascular Events, 1994-2019 Calvin Ke, MD, PhD; Lorraine L. Lipscombe, MD, MSc; Alanna Weisman, MD, PhD; Limei Zhou, PhD; Peter C. Austin, PhD; Baiju R. Shah, MD, PhD; Gillian L. Booth, MD, MSc JAMA
Question What is the contemporary pattern of evidence-based pharmacotherapy use among a real-world population of US patients with type 2 diabetes and atherosclerotic cardiovascular disease?
Findings In this cohort study of 324 706 patients from health systems across the US, 58.6% of patients were receiving a statin (and a total of 26.8% of patients were receiving a high-intensity formation), 45.5% of patients were receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, 3.9% of patients were prescribed a glucagon-like peptide-1 receptor agonist, and 2.8% of patients were receiving a sodium glucose cotransporter-2 inhibitors.
Meaning These findings suggest that multifaceted interventions are needed to overcome the large gaps in evidence-based pharmacotherapy use among this increasing population of patients at high risk of adverse outcomes.
Importance Based on contemporary estimates in the US, evidence-based therapies for cardiovascular risk reduction are generally underused among patients with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD).
Objective To determine the use of evidence-based cardiovascular preventive therapies in a broad US population with diabetes and ASCVD.
Design, Setting, and Participants This multicenter cohort study used health system–level aggregated data within the National Patient-Centered Clinical Research Network, including 12 health systems. Participants included patients with diabetes and established ASCVD (ie, coronary artery disease, cerebrovascular disease, and peripheral artery disease) between January 1 and December 31, 2018. Data were analyzed from September 2020 until January 2021.
Exposures One or more health care encounters in 2018.
Main Outcomes and Measures Patient characteristics by prescription of any of the following key evidence-based therapies: high-intensity statin, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin-receptor blocker (ARB) and sodium glucose cotransporter-2 inhibitors (SGLT2I) or glucagon-like peptide-1 receptor agonist (GLP-1RA).
Results The overall cohort included 324 706 patients, with a mean (SD) age of 68.1 (12.2) years and 144 169 (44.4%) women and 180 537 (55.6%) men. A total of 59 124 patients (18.2% ) were Black, and 41 470 patients (12.8%) were Latinx. Among 205 885 patients with specialized visit data from the prior year, 17 971 patients (8.7%) visited an endocrinologist, 54 330 patients (26.4%) visited a cardiologist, and 154 078 patients (74.8%) visited a primary care physician. Overall, 190 277 patients (58.6%) were prescribed a statin, but only 88 426 patients (26.8%) were prescribed a high-intensity statin; 147 762 patients (45.5%) were prescribed an ACEI or ARB, 12 724 patients (3.9%) were prescribed a GLP-1RA, and 8989 patients (2.8%) were prescribed an SGLT2I. Overall, 14 918 patients (4.6%) were prescribed all 3 classes of therapies, and 138 173 patients (42.6%) were prescribed none. Patients who were prescribed a high-intensity statin were more likely to be men (59.9% [95% CI, 59.6%-60.3%] of patients vs 55.6% [95% CI, 55.4%-55.8%] of patients), have coronary atherosclerotic disease (79.9% [95% CI, 79.7%-80.2%] of patients vs 73.0% [95% CI, 72.8%-73.3%] of patients) and more likely to have seen a cardiologist (40.0% [95% CI, 39.6%-40.4%] of patients vs 26.4% [95% CI, 26.2%-26.6%] of patients).
Conclusions and Relevance In this large cohort of US patients with diabetes and ASCVD, fewer than 1 in 20 patients were prescribed all 3 evidence-based therapies, defined as a high-intensity statin, either an ACEI or ARB, and either an SGLT2I and/or a GLP-1RA. These findings suggest that multifaceted interventions are needed to overcome barriers to the implementation of evidence-based therapies and facilitate their optimal use.
Up to two-thirds of individuals with type 2 diabetes will develop atherosclerotic cardiovascular disease (ASCVD) in their lifetimes. 1 - 3 In individuals with diabetes, ASCVD is more extensive, less amenable to treatment, and associated with worse outcomes compared with the general population. 4 Fortunately, a number of secondary prevention therapies have been shown to reduce the morbidity and mortality of ASCVD in individuals with diabetes; yet, for a variety of reasons, they are underused in clinical practice. 5
Estimates from studies evaluating individuals with diabetes and ASCVD demonstrate a wide variation in the use of key preventive pharmacotherapies, namely high-intensity statins (from 24.7%-45.4%), angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-II receptor blockers (ARBs) (from 53.1%-72.0%), and antihyperglycemic agents with proven cardiovascular benefit, such as sodium glucose cotransporter-2 inhibitors (SGLT2I) and glucagon-like peptide-1 receptor agonists (GLP-1RA) (from 2.5% to 17.6%). 6 - 13 While these data consistently demonstrate concerning gaps in the use of evidence-based therapy, there is significant variation in the magnitude of these estimates owing to differences in data source (eg, registries vs trials vs single-site studies), evolving prescribing trends, and selected patient characteristics. Thus, there is considerable uncertainty as to how representative these findings are for most individuals with diabetes and ASCVD, and we hypothesize that a real-world estimate of evidence-based therapy use will be considerably lower.
The objective of this study was to describe the contemporary use of lipid-, blood pressure–, and glucose-lowering pharmacotherapy among a large, national and representative cohort of individuals with diabetes and ASCVD in the US. Accurately determining the patterns and gaps in evidence-based therapy in this high-risk and increasing population will more precisely inform ongoing implementation programs aimed to increase adoption and improve outcomes.
For this cohort study, participating sites obtained formal determination from their local institutional review board that this study was not human participants research and thus waiver for informed consent was provided. Because of specifications in the data-use agreements that prohibit release of health system–level data, it is not possible to provide data generated from sites participating in this study. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
This was a multicenter cohort study performed using data from the US National Patient-Centered Clinical Research Network (PCORnet). PCORnet is composed of multiple clinical data research networks (CDRNs), which in turn comprise 1 or more datamarts (eTable 1 in the Supplement ). Datamarts are collections of data that participating health systems generate and store using the PCORnet Common Data Model (CDM) version 5.1 and include demographics, vital signs, diagnoses, encounters, clinician types, procedures, prescription orders, and laboratory results. 14 These datamarts enable multisite research by housing individual patient-level data that can be queried and analyzed by site and then returned in aggregate and standardized form.
A study period from January 1, 2018, until December 31, 2018, was defined, with an eligible patient’s most recent inpatient or outpatient encounter during this period considered their index date. As previously described, 15 International Classification of Diseases, Ninth Revision ( ICD-9 ), International Statistical Classification of Diseases, Tenth Revision, Clinical Modification ( ICD-10-CM ), and Current Procedural Terminology codes were used to select patients aged 18 years and older with evidence of diabetes and ASCVD during the 5-year period preceding the index date (ie, January 1, 2014, to December 31, 2018). All code lists are available in the eTable 2 in the Supplement . Briefly, ASCVD was defined as patients with coronary artery disease (eg, obstructive coronary atherosclerosis, prior myocardial infarction, prior percutaneous coronary intervention, or coronary artery bypass grafts), peripheral arterial disease (eg, vascular claudication, prior peripheral percutaneous or open revascularization, or amputation from poor circulation), or cerebrovascular disease (eg, carotid atherosclerosis, ischemic stroke, or prior percutaneous or open cerebrovascular revascularization).
Demographic information was obtained from the CDM demographic and location tables. We used existing information on race and ethnicity, which was documented in the medical record based on self-report and/or clinician observation. Race and ethnicity data were included in this analysis because of prior work suggesting associations between race and ethnicity and clinical care patterns. Comorbid conditions defined by ICD-9 and ICD-10-CM codes were extracted from the CDM diagnosis table, active smoking status from the CDM vital table, and laboratory results (ie, lipid profile, estimated glomerular filtration rate [eGFR] and hemoglobin A 1c [HbA 1c ]) from the investigations table. Lower- and upper-bound truncation points for biologically plausible measurement ranges were based on a test query to improve data quality prior to aggregation. Medication prescriptions were defined using RxNorm concept unique identifiers from the CDM prescribing table. Patients were considered to be using a medication of interest if it was listed in the prescribing table at any time in the 12 months prior to their index date. Health care resource utilization was obtained from the CDM encounter and diagnosis tables over a 12-month period prior to index date. Clinicians of interest were endocrinologists, cardiologists, and primary care physicians.
Evidence-based therapy was defined as the use of a high-intensity statin (atorvastatin 40-80 mg or rosuvastatin 20-40 mg), an ACEI or ARB (or angiotensin-II receptor/neprilysin inhibitor [ARNI]), and either an SGLT2I and/or GLP-1RA. Although simvastatin 80 mg is considered a high-intensity formulation, it is not recommended by the US Food and Drug Administration and does not appear in the American College of Cardiology guidelines; thus, it was not considered an evidence-based therapy. Patients in this cohort were considered to have indications for all 3 components and given a composite score of 0 to 3 reflecting the number of evidence-based therapies prescribed. Patients with HbA 1c less than 7% (to convert to proportion of total hemoglobin, multiply by 0.01) with or without metformin were ascribed 1 point for the SGLT2I and/or GLP-1RA domain in the 3-point composite score. Of note, it is now recognized that metformin monotherapy is no longer adequate for these patients with high risk, and while this is reflected in the current guidelines, it was not contemporary guidance during the study period. Although some heterogeneity with regard to effects on specific cardiovascular (CV) and kidney outcomes has been found within the SGLT2I and GLP-1RA classes, for the purposes of the analyses and their interpretation, these medications were considered to exhibit class effects. 16 , 17
Site-level aggregate data were summarized using weighted summary measures to account for sample size from each site. Categorical variables are presented as frequencies (percentages) by summing numerators and denominators for each site. Missing data for categorical variables are presented for each variable as frequencies (percentages) of the expected column total. Continuous variables are presented as pooled means; variances across sites were tested for homogeneity according to Hartley test, 18 and pooled SDs are presented. To understand patient and health care characteristics associated with the prescription of the evidence-based therapies of interest, the cohort was dichotomized into low (ie, use of 0 or 1 evidence-based therapy) and high (ie, use of 2 or 3 evidence-based therapies) score groups. Statistical comparison between treatment groups prescribed each medication of interest (ie, high-intensity statin, ACEI or ARB, and SGLT2I and/or GLP-1RA) was not possible, given the lack of mutual exclusivity; however, descriptive comparisons were made on the basis of clinically relevant differences and narrow 95% CIs, generated using pooled SDs for continuous variables and assumed binomial proportions for categorical variables.
All analyses were performed using SAS statistical software version 9.1 (SAS Institute). Data were analyzed from September 2020 to January 2021.
Twelve geographically diverse health systems contributing to 5 CDRNs and 16 datamarts responded within the required timeframe and were able to distribute the query (eTable 1 in the Supplement ). Within these participating datamarts, there were 561 259 eligible patients, of whom 324 706 patients (57.9%) had complete medication tables, and among these, 205 885 patients (63.4%) had data on encounters by clinician type, 188 662 patients (58.1%) had laboratory results, and 161 874 patients (49.9%) reported insurance status ( Figure 1 ).
Among 324 706 patients included in analysis, the overall mean (SD) age was 68.1 (12.2) years, 144 169 (44.4%) were women and 180 537 (55.6%) were men. A total of 9282 patients (2.8%) were Asian, 59 124 patients (18.2%) were Black, 41 470 patients (12.3%) were Latinx, and 207 846 patients (64.0%) were White. Coronary artery disease was present in 237 012 patients (73.0%), 60 125 patients (18.5%) had cerebrovascular disease, and 151 709 patients (46.7%) had peripheral arterial disease. Baseline characteristics are presented overall and by evidence-based therapy in Table 1 .
Use of lipid-, blood pressure–, and glucose-lowering therapies are presented in Figure 2 . Overall, 190 346 patients (58.6%) were prescribed a statin, but only 87 160 patients (26.8%) were prescribed a high-intensity statin. Use of nonstatin low-density cholesterol–lowering therapies was low, with 8161 patients (2.5%) prescribed ezetimibe and 1055 patients (0.3%) prescribed a PCSK9 inhibitor. ACEIs or ARBs were prescribed in 147 762 patients (45.5%). Of the antihyperglycemic medications, metformin was prescribed in 120 821 patients (37.2%), sulfonylureas in 42 027 patients (12.9%), and insulin in 118 508 patients (36.5%). Use of glucose-lowering drugs with proven CV benefit was low, with 12 724 patients (3.9%) of patients prescribed a GLP-1RA and 8989 patients (2.8%) prescribed an SGLT2I.
Compared with the overall cohort, patients prescribed a high-intensity statin were more likely to be men (59.9% [95% CI, 59.6%-60.3%] of patients vs 55.6% [95% CI, 55.4%-55.8%] of patients), more likely to have coronary (79.9% [95% CI, 79.7%-80.2%] of patients vs 73.0% [95% CI, 72.8%-73.3%] of patients) or cerebrovascular (23.5% [95% CI, 23.2%-23.8%] of patients vs 18.5% [95% CI, 18.4%-18.7%] of patients) disease, and more likely to have seen a cardiologist (40.0% [95% CI, 39.6%-40.4%] of patients vs 26.4% [95% CI, 26.2%-26.6%] of patients). Patients prescribed a high-intensity statin, compared with the overall cohort, had a greater burden of heart failure (38.8% [95% CI, 38.5%-39.2%] of patients vs 32.1% [95% CI, 31.9%-32.3%] of patients), cigarette smoking (15.1% [95% CI, 14.8%-15.3%] of patients vs 11.6% [95% CI, 11.5%-11.7%] of patients) and dyslipidemia (90.3% [95% CI, 90.1%-90.5%] of patients vs 82.9% [95% CI, 82.8%-83.1%] of patients).
The demographics of participants prescribed an ACEI or ARB did not differ significantly from the overall cohort. However, patients receiving an ACEI or ARB, compared with the overall cohort, were more likely to have peripheral artery disease (50.1% [95% CI, 49.9%-50.4%] of patients vs 46.7% [95% CI, 46.6%-46.9%] of patients), hypertension (96.9% [95% CI, 96.8%-97.0%] of patients vs 92.1% [95% CI, 92.0%-92.2%] of patients), and dyslipidemia (87.6% [95% CI, 87.5%-87.8%] of patients vs 82.9% [95% CI, 82.8%-83.1%] of patients). Those prescribed an ACEI or ARB were also more likely to have seen a primary care physician (82.1% [95% CI, 81.9%-82.4%] of patients vs 74.8% [95% CI, 74.7%-75.0%] of patients) or a cardiologist (34.1% [95% CI, 33.8%-34.4%] of patients vs 26.4% [95% CI, 26.6%-26.6%] of patients) in the prior 12 months ( Table 1 ).
Patients prescribed an SGLT2I or GLP-1RA, compared with the overall cohort, were younger (mean age: SGLT2I, 63.2 [95% CI, 63.0-63.4] years; GLP-1RA: 62.9 [95% CI, 62.7-63.1] years; overall: 68.1 [95% CI, 68.0-68.1] years), were more likely to have private insurance (SGLT2I: 17.1% [95% CI, 16.0%-18.2%] of patients; GLP-1RA: 15.5% [95% CI, 14.5%-16.5%] of patients; overall: 12.0% [95% CI, 11.9%-12.2%] of patients), and had fewer medical comorbidities (mean Charlson comorbidity index score: SGLT2I: 3.3 [95% CI, 3.2-3.3]; GLP-1RA: 3.8 [95% CI, 3.8-3.9]; overall: 4.1 [95% CI, 4.1-4.1]), and lower prevalence of heart failure (SGLT2I: 21.3% [95% CI, 20.5%-22.2%] of patients; GLP-1RA: 26.3% [95% CI, 25.5%-27.1%] of patients; overall: 32.1% [95% CI, 31.9%-32.3%] of patients) and atrial fibrillation (SGLT2I: 13.8% [95% CI, 13.1%-14.5%] of patients; GLP-1RA: 14.8% [95% CI, 14.2%-15.4%] of patients; overall: 21.3% [95% CI, 21.2%-21.5%] of patients). Patients prescribed either an SGLT2I or a GLP-1RA had similar rates of end-organ diabetes complications yet were more likely to have visited an endocrinologist in the prior 12 months compared with the overall population (SGLT2I: 28.4% [95% CI, 27.2%-29.7%] of patients; GLP-1RA: 30.5% [95% CI, 29.5%-31.6%] of patients; overall: 8.7% [95% CI, 8.6%-8.9%] of patients). Patients prescribed an SGLT2I, compared with those prescribed a GLP-1RA, were less likely to be women (36.5% [95% CI, 35.5%-37.5%] of patients vs 46.7% [95% CI, 45.8%-47.5%] of patients) and less likely to be Black (13.4% [95% CI, 12.7%-14.1%] of patients vs 17.5% [95% CI, 16.8%-18.1%] of patients). Patients prescribed an SGLT2I had fewer diabetes end-organ complications compared with those prescribed a GLP-1RA (neuropathy: 31.0% [95% CI, 30.1%-32.0%] of patients vs 40.1% [95% CI, 39.3%-41.0%] of patients; retinopathy: 11.6% [95% CI, 11.2%-11.6%] of patients vs 17.0% [95% CI, 16.4%-17.7%] of patients; DKA: 1.6% [95% CI, 1.3%-1.9%] of patients vs 2.4% [95% CI, 2.1%-2.7%] of patients; diabetic foot: 3.8% [95% CI, 3.4%-4.2%] of patients vs 6.1% [95% CI, 5.7%-6.5%] of patients). Of note, heart failure (26.3% [95% CI, 25.5%-27.1%] of patients vs 21.3% [95% CI, 20.5%-22.2%] of patients) and mild kidney dysfunction (eGFR 30-59 mL/min/m 2 : 32.9% [95% CI, 31.9%-33.9%] of patients vs 26.1% [95% CI, 25.0%-27.3%] of patients) were more common among those prescribed a GLP-1RA than those prescribed an SGLT2I.
Overall, 138 173 patients (42.6%) were prescribed no evidence-based CV-risk mitigating medications, 103 420 patients (31.9%) were prescribed 1 medication, 68 195 patients (21.0%) were prescribed 2 medications, and only 14 918 patients (4.6%) were prescribed 3 evidence-based medications.
Groups of patients with low (0 or 1 points) and high (2 or 3 points) composite medication scores are presented in Table 2 . Patients with high evidence-based therapy scores, compared with those with low scores, had similar racial and ethnic characteristics but were less likely to be women (41.5% [95% CI, 41.2%-41.8%] of patients vs 45.4% [95% CI, 45.2%-45.6%] of patients). Patients with high composite scores, compared with those with low scores, had a higher burden of hypertension (96.4% [95% CI, 96.2%-96.5%] of patients vs 90.7% [95% CI, 90.6%-90.8%] of patients) and dyslipidemia (90.6% [95% CI, 90.4%-90.8%] of patients vs 80.3% [95% CI, 80.2%-80.3%] of patients) and a higher prevalence of end-organ diabetes complications (neuropathy: 31.3% [95% CI, 30.9%-31.6%] of patients vs 24.6% [95% CI, 24.4%-24.8%] of patients; retinopathy: 12.4% [95% CI, 12.2%-12.6%] of patients vs 8.3% [95% CI, 8.2%-8.5%] of patients; DKA: 2.1% [95% CI, 2.1%-2.2%] of patients vs 1.6% [95% CI, 1.5%-1.6%] of patients). Regional and payer variation in care was observed, with lower scores more common than high scores among patients in the Northeast (48.3% [95% CI, 48.1%-48.6%] of patients vs 40.7% [95% CI, 40.3%-41.1%] of patients) and patients with Medicare coverage (49.5% [95% CI, 49.2%-49.8%] of patients vs 35.7% [95% CI, 35.2%-36.2%] of patients). Patients with high scores, compared with those with low scores, were more likely to have seen a cardiologist (39.2% [95% CI, 38.8%-39.6%] of patients vs 22.3% [95% CI, 22.1%-22.5%] of patients) or primary care physician (83.4% [95% CI, 83.1%-83.7%] of patients vs 72.1% [95% CI, 71.9%-72.3%] of patients) in the prior 12 months.
This cohort study including more than 300 000 patients across multiple health systems represents a large contemporary landscape evaluation of the real-world cardiometabolic care patterns of patients in the US with both diabetes and ASCVD. The study has a number of important findings. First, more than one-third of patients were receiving none of the 3 key evidence-based therapies associated with significant CV benefit, and fewer than 1 in 20 patients were receiving all 3. Second, more than one-quarter of patients were prescribed a guideline-recommended dose of statin, less than half were prescribed an ACEI or ARB, and fewer than 1 in 15 patients were prescribed an antihyperglycemic agent with CV benefit. Third, while endocrinologist encounters were more common among those receiving either an SGLT2I or GLP-1RA, they were infrequent care episodes and reinforce the need for other physicians, such as cardiologists and primary care physicians, to assist with adoption of these agents.
These data suggest that previously described gaps in the use of evidence-based therapies for individuals with diabetes and ASCVD in selected environments extend to this large, distributed network of health systems across the US. The finding that only 58.6% of patients in this study were prescribed a statin is considerably lower than a recently published estimate of 74.6% from a database of commercially insured patients in the US. 9 Notably, the rate of overall statin use in this study was similar to findings from a comparable Medical Expenditure Panel Survey population from 2013, which reported that only 52.7% of patients with diabetes and ASCVD were receiving a statin. 19 In this context, these new data raise concerns that despite strengthening of guideline recommendations in the years prior to our study window, 20 there has been minimal progress in increasing the use of these widely available, cost-effective, safe, and proven medications in the general population.
A plethora of data support the role of ACEIs and ARBs in diabetes with and without chronic kidney disease, 21 - 24 ASCVD with or without diabetes 25 - 27 and as first-line agents in hypertension with or without diabetes. 28 Thus, our cohort had at least 1 indication for either an ACEI or an ARB, and yet only 45% of patients were prescribed one. This estimate is lower than other current national estimates from survey (55% 29 ) and health system (IQR, 51%-69% 30 ) data evaluating similar populations and lower than that reported in contemporary registry (72% 6 ) and clinical trial (80% 31 ) cohorts. While higher use may be observed among registry and trial cohorts owing to their enrichment for patients whose medical histories are less complex and who are more adherent with medical instruction, there were no significant differences in the prevalence of chronic kidney disease, CV and non-CV comorbidity, or diabetes complications among patients receiving ACEIs or ARBs vs those not receiving either drug in this cohort, making the risk-treatment paradox a less obvious explanation in this study. Given their potential role in mitigating the increasing prevalence of chronic kidney disease 32 among patients with diabetes and that treatment benefits from established and emerging diabetes therapies (eg, SGLT2I, 33 GLP-1RA, 34 fineronone 35 ) were observed at high levels of background ACEI or ARB therapy, further enquiry into the barriers preventing the use of these inexpensive and well-tolerated medications is urgently required.
More than one-third of patients were receiving none of the evidence-based therapies, with just one-quarter achieving a higher composite score of 2 or more evidence-based therapies. While interpretation is limited by univariable comparison, there was no obvious evidence of risk-treatment paradox, 36 , 37 with patients with higher composite evidence-based therapy scores having similar comorbidity scores and risk profiles. Furthermore while other studies have described marked disparity in preventive care patterns by race and sex, 38 - 41 these were less apparent in our cohort, with only a modest difference in high-intensity statin use favoring men compared with women. Given that our cohort was restricted to those with a recent encounter, patients with less access to care would have been more likely to be excluded; as Black and Latinx patients continue to suffer from inequitable health system access, 42 , 43 disparate patterns among these groups may have been attenuated and deserve further, dedicated enquiry.
Only 6.7% of patients in the cohort were prescribed either an SGLT2I or GLP-1RA, which is considerably lower than other contemporary estimates of 9.9% from an insured population 9 and 17% from a diabetes registry. 6 Some potential barriers to the optimal use of SGLT2I and GLP-1RA are cost and insurance formulary preferences, as evidenced by the greater proportion of patients receiving these drugs in this study having private insurance. Since the acquisition of these data, a number of consensus documents and guidelines have emerged calling on cardiologists to embrace these agents as key tenets of cardiovascular risk reduction 44 - 49 ; the impact of these publications on adoption of SGLT2I and GLP-1RA remains to be seen.
There are several limitations to this study. The use of aggregate data meant that multivariable analyses for factors associated with individual therapy prescription or high vs low composite score were not possible. Furthermore, a granular understanding of the contributors to missingness of relevant data are also not possible with an aggregated data set. In this context, there was an obligate loss in sample size, as data completeness in the CDM varied among datamarts, particularly with respect to insurance status and laboratory values. Medication use was discerned from prescribing information available in PCORnet, and actual use or dispensing was not observed. A more nuanced analysis comparing medication prescription, dispensing, and actual use is not currently possible in the PCORnet environment and would require linkage with individual pharmacy dispensing records and claims data. Prescriptions administered outside of the PCORnet health system are also not captured. In contrast, patients who abandon their prescription or discontinue without informing their physician would be considered as prescribed and thus our estimate may overall be optimistic. While these functions of the data set contribute to variations, definitions, and estimates of clinical use (ie, a composite of prescription, dispensing, adherence, and continuation) the presence of a medication in the electronic health record represents a real-world assessment of treatment status. Our assessment of evidence-based therapy prescription must only be considered an estimate, as access to patient-level data was not available and thus it was not possible to consider the impact of relative or absolute contraindications. The intent was to generate an inclusive cohort of patients without removing any from the denominator; thus, while every patient had a potential evidence-based therapy score of 3, this is a broad generalization, since a number of patients could never be prescribed all 3 medications owing to contraindications, allergies, or intolerance (eg, dialysis, prior rhabdomyolysis). However, there are significant strengths of this type of analysis; namely, the large and unselected nature of this data set encompasses not only geographic variation but also patient, physician, and practice diversity, which strengthen the generalizability of the present findings.
In this cohort study of more than 300 000 patients with diabetes and ASCVD in contemporary clinical practice from the US, more than one-third of patients were not receiving any guideline-directed, evidence-based, CV risk–mitigating therapies (or doses), and fewer than 1 in 20 patients were receiving all 3 therapies. It is particularly concerning that only one-quarter were prescribed a high-intensity statin and less than half an ACE or ARB, treatments that are inexpensive and well tolerated. These estimates of evidence-based therapy prescription are considerably lower than those observed in other recent analyses in selected populations. These findings amplify the need to close these critical gaps between evidence generation and clinical practice for most patients in the US with diabetes and ASCVD.
Accepted for Publication: December 19, 2021.
Published: February 17, 2022. doi:10.1001/jamanetworkopen.2021.48030
Open Access: This is an open access article distributed under the terms of the CC-BY-NC-ND License . © 2022 Nelson AJ et al. JAMA Network Open .
Corresponding Author: Christopher B. Granger, MD, Duke Clinical Research Institute, 200 Morris St, Durham, NC 27701 ( [email protected] ).
Author Contributions: Drs Al-Khalidi and Kaltenbach had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Nelson, O’Brien, Kaltenbach, Lopes, Cavender, Gaynor, Kirk, Magwire, McGuire, Pak, Pop-Busui, Richardson, Senyucel, Pagidipati, Granger.
Acquisition, analysis, or interpretation of data: Nelson, Kaltenbach, Green, Morse, Al-Khalidi, Aroda, Lingvay, Pop-Busui, Kelsey, Pagidipati, Granger.
Drafting of the manuscript: Nelson, Kaltenbach.
Critical revision of the manuscript for important intellectual content: O’Brien, Green, Lopes, Morse, Al-Khalidi, Aroda, Cavender, Gaynor, Kirk, Lingvay, Magwire, McGuire, Pak, Pop-Busui, Richardson, Senyucel, Kelsey, Pagidipati, Granger.
Statistical analysis: Nelson, Kaltenbach, Al-Khalidi.
Obtained funding: Nelson, Gaynor, Pak, Pagidipati, Granger.
Administrative, technical, or material support: Morse, Cavender, Gaynor, Pak.
Supervision: O’Brien, Green, Lopes, Cavender, Richardson, Pagidipati.
Conflict of Interest Disclosures: Dr Nelson reported receiving grants from Diabetes Australia and the Royal Australasian College of Physicians. Dr O’Brien reported receiving grants from Novartis, Glaxo Smith Kline, and Bristol Myer Squib outside the submitted work. Dr Green reported receiving grants and personal fees from Boehringer Ingelheim/Lilly Alliance, Sanofi/Lexicon, Glaxo Smith Kline, and AstraZeneca; personal fees from Novo Nordisk, Hawthorne Effect, Pfizer, Regeneron Pharmaceuticals, and Bayer; and grants from Merck, GlaxoSmithKline, and Roche outside the submitted work. Dr Lopes reported receiving personal fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Merck, Portola; grants from Amgen; and grants and personal fees from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi outside the submitted work. Dr Aroda reported receiving grants from Applied Therapeutics, Novo Nordisk, Sanofi, Eli Lilly and Company, and Fractyl; personal fees from Duke University, Liberum, Novo Nordisk, and Pfizer; and that her spouse is employed by Janssen and Merck outside the submitted work. Dr Cavender reported receiving grants and personal fees from Amgen, Boehringer Ingelheim, and Novo Nordisk; grants from AstraZeneca and Novartis; and personal fees from Merck and Edwards Lifesciences outside the submitted work. Dr Lingvay reported receiving personal fees from Duke Clinical Research Institute during the conduct of the study and personal fees from Novo Nordisk, Eli Lilly and Company, Merck, Janssen, Sanofi, Boehringer Ingelheim, Intarcia, Bayer, AstraZeneca, Target RWE, Mannkind, Valerita, AstraZeneca, and DataRevive and grants from Novo Nordisk, Sanofi, Merck, Pfizer, and Mylan outside the submitted work. Dr Magwire reported receiving personal fees from Novo Nordisk and Boehringer Ingelheim outside the submitted work. Dr McGuire reported receiving personal fees from Boehringer Ingelheim, Janssen, Sanofi, AstraZeneca, Merck, Pfizer, Novo Nordisk, Esperion, Lilly, Lexicon Pharmaceuticals, CSL Behring, Applied Therapeutics, Metavant, Afimmune, GlaxoSmithKline, Eisai, and Bayer outside the submitted work. Dr Pop-Busui reported receiving personal fees from Boehringer Ingelheim, Novo Nordisk, Bayer, and Averitas, grants from AstraZeneca and the National Institutes of Health, and serving as an associated editor for Diabetes outside the submitted work. Dr Senyucel reported owning stock in Eli Lilly and Company outside the submitted work. Dr Kelsey reported receiving grants from the National Institutes of Health during the conduct of the study. Dr Pagidipati reported receiving personal fees and grants from Boehringer Ingelheim, Eli Lilly and Company, and AstraZeneca and grants from Amgen, Novo Nordisk, Novartis, Regeneron, Sanofi, and Verily Life Sciences outside the submitted work. Dr Granger reported receiving grants and personal fees from Boehringer Ingelheim, Bristol Myer Squib, Janssen, Pfizer, and Medtronic; grants from Akros Pharma, Apple, AstraZeneca, Daichi-Sankyo, and Novartis; and personal fees from AbbVie, Bayer, Boston Scientific, CeleCor, Correvio, Espero, Merck, Novo Nordisk, Rhoshan Pharmaceuticals, and Roche Diagnostics outside the submitted work. No other disclosures were reported.
Funding/Support: This study was funded by Boehringer Ingelheim and Eli Lilly and Company. The research reported in this publication was conducted using the National Patient-Centered Clinical Research Network, developed with funding from the Patient-Centered Outcomes Research Institute.
Role of the Funder/Sponsor: The funders were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The views presented in this publication are solely the responsibility of the authors and do not necessarily represent the views of organizations participating in, collaborating with, or funding National Patient-Centered Clinical Research Network or of the Patient-Centered Outcomes Research Institute.
Additional Contributions: Gretchen Sanders, MSN, provided operational support; Mary Williams, BSc, and Stephanie Poley, PhD, assisted in design and data collection; ; Yinghong Zhang, BA, assisted in design, data collection, and interpretation; and Vladimir Demyanenko, MS, assisted in data collection and interpretation. They are employees at Duke Clinical Research Institute and were not compensated outside of their normal salaries.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 17 April 2024
Navigating outpatient care of patients with type 2 diabetes after hospital discharge - a qualitative longitudinal study
- Léa Solh Dost 1 , 2 ,
- Giacomo Gastaldi 3 ,
- Marcelo Dos Santos Mamed 4 , 5 &
- Marie P. Schneider 1 , 2
BMC Health Services Research volume 24 , Article number: 476 ( 2024 ) Cite this article
281 Accesses
Metrics details
The transition from hospital to outpatient care is a particularly vulnerable period for patients as they move from regular health monitoring to self-management. This study aimed to map and investigate the journey of patients with polymorbidities, including type 2 diabetes (T2D), in the 2 months following hospital discharge and examine patients’ encounters with healthcare professionals (HCPs).
Patients discharged with T2D and at least two other comorbidities were recruited during hospitalization. This qualitative longitudinal study consisted of four semi-structured interviews per participant conducted from discharge up to 2 months after discharge. The interviews were based on a guide, transcribed verbatim, and thematically analyzed. Patient journeys through the healthcare system were represented using the patient journey mapping methodology.
Seventy-five interviews with 21 participants were conducted from October 2020 to July 2021. The participants had a median of 11 encounters (min–max: 6–28) with HCPs. The patient journey was categorized into six key steps: hospitalization, discharge, dispensing prescribed medications by the community pharmacist, follow-up calls, the first medical appointment, and outpatient care.
Conclusions
The outpatient journey in the 2 months following discharge is a complex and adaptive process. Despite the active role of numerous HCPs, navigation in outpatient care after discharge relies heavily on the involvement and responsibilities of patients. Preparation for discharge, post-hospitalization follow-up, and the first visit to the pharmacy and general practitioner are key moments for carefully considering patient care. Our findings underline the need for clarified roles and a standardized approach to discharge planning and post-discharge care in partnership with patients, family caregivers, and all stakeholders involved.
Peer Review reports
Care transition is defined as “the movement patients make between healthcare practitioners and settings as their condition and care needs change in the course of a chronic or acute illness” [ 1 ]. The transition from hospital to outpatient care is a particularly vulnerable period for patients as they move from a medical environment with regular health monitoring to self-management, where they must implement a large amount of information received during their hospital stay [ 2 , 3 , 4 , 5 , 6 ]. This transition period can be defined as “the post-hospital syndrome,” which corresponds to a transient period of vulnerability (e.g., 30 days) for various health problems, such as stress, immobility, confusion, and even cognitive decline in older adults, leading to complications [ 7 ]. Furthermore, discharged patients may experience a lack of care coordination, receive incomplete information, and inadequate follow-ups, leading to potential adverse events and hospital readmissions [ 8 , 9 , 10 ].
People with type 2 diabetes mellitus (T2D) represent a high proportion of hospitalized patients, and their condition and medications are associated with a higher rate of hospital readmission [ 11 , 12 , 13 ]. Moreover, T2D is generally associated with multiple comorbidities. This complex disease requires time-consuming self-management tasks such as polypharmacy, adaptations of medication dosages, diet, exercise, and medical follow-up, especially during care transition [ 14 , 15 , 16 ].
Various interventions and practices, such as enhanced patient education, discharge counseling, and timely follow-up, have been studied to improve care transition for patients with chronic diseases; however, they have shown mixed results in reducing costs and rehospitalization [ 17 , 18 , 19 , 20 ]. In addition, patient perspectives and patient-reported outcomes are rarely considered; however, their involvement and monitoring are essential for seamless and integrated care [ 21 , 22 ]. Care integration, an approach to strengthening healthcare systems in partnership with people, focuses on patient health needs, the quality of professional services, and interprofessional collaboration. This approach prevents care fragmentation for patients with complex needs [ 23 , 24 ]. Therefore, knowledge of healthcare system practices is essential to ensure integrated, coordinated, and high-quality care. Patient perspectives are critical, considering the lack of literature on how patients perceive their transition from hospital to autonomous care management [ 25 , 26 ].
Patients’ journeys during hospitalization have been described in the literature using various methods such as shadowing, personal diaries, and interviews; however, patients’ experiences after hospital discharge are rarely described [ 26 , 27 ]. Jackson et al. described the complexity of patient journeys in outpatient care after discharge using a multiple case study method to follow three patients with chronic obstructive pulmonary disease from hospitalization to 3 months post-discharge [ 26 ]. The literature does not provide an in-depth understanding of the experiences of patients with comorbidities during care transition upon hospital discharge. The assumption about the patient journey after discharge is that multiple and multi-professional encounters will ensure the transition of care from hospitalization to self-management, but often without care coordination.
This study aimed to investigate the healthcare trajectories of patients with comorbidities, including T2D, during the 2 months following hospital discharge and to examine patients’ encounters with healthcare professionals (HCPs).
While this article focuses on patients’ journeys to outpatient care, another article describes and analyzes patients’ medication management, knowledge, and adherence [ 28 ]. This study followed the Consolidated Criteria for Reporting Qualitative Research (COREQ).
Study design and population
A qualitative longitudinal research approach was adopted, with four individual semi-structured interviews over 2 months after discharge (approximately 3, 10, 30, and 60 days after discharge) that took place at home, by telephone, secured video call, or at the university at the participant’s convenience. Participants were recruited during hospitalization. The inclusion criteria were patients with T2D, with at least two other comorbidities, at least one medication change during hospitalization, hospitalization duration of at least 3 days, and those who returned home after discharge and self-managed their medications. A family caregiver could also participate in the interviews alongside to participants.
Researcher characteristics
All the researchers were trained in qualitative studies. The ward diabetologist and researcher (GG) who enrolled the patients in the study participated in most participants’ care during hospitalization. LS (Ph.D. student and community pharmacist) was unknown to participants and presented herself during hospitalization as a “researcher” rather than a pharmacist to avoid any risk of influencing participants’ answers. MS is a professor in pharmacy, whose research focuses on medication adherence in chronic diseases and aims at better understanding this behavior and its consequences for patients and the healthcare system. MDS is a researcher, linguist, and clinical psychologist, with a particular interest in patients living with chronic conditions such as diabetes and a strong experience in qualitative methodology and verbal data analysis.
Data collection
The interviews were based on four semi-structured interview guides based on existing frameworks and theories: the World Health Organization’s five dimensions for adherence, the Information-Motivation-Behavioral Skills model, and the Social Cognitive Theory [ 29 , 30 , 31 ]. For in-depth documentation of participants’ itinerary in the healthcare system, the interview guides included questions on the type, reason, and moment of the HCP’s encounters and patient relationships with HCPs. Interview guides are available in Supplementary File 1 . During the development phase of the study, the interview guides were reviewed for clarity and validity and adapted by two patient partners from the Geneva University Hospitals’ Patient Partner Platform for Research and Patient and Public Involvement. Thematic saturation was considered reached when no new code or theme emerged and new data repeated previously coded information [ 32 ]. Sociodemographic and clinical data were collected from hospital databases and patient questionnaires. The interviews were audio-recorded, anonymized, and transcribed verbatim.
Data analysis
The sociodemographic and clinical characteristics were descriptively analyzed. Transcriptions were double-coded until similar codes were obtained, and thematic analysis, as described by Braun and Clarke [ 33 , 34 ], was used in a systematic, iterative, and comparative manner. A patient journey mapping methodology was used to illustrate the trajectories of each participant and provide a comprehensive understanding of their experiences. Patient journey mapping is a visual method adapted from the marketing industry that is increasingly used in various health settings and contexts to illustrate and evaluate healthcare services and patient experiences [ 35 ]. In this analysis, we used the term “healthcare professionals” when more than one profession could be involved in participants’ healthcare. Otherwise, when a specific HCP was involved, we used the designated profession (e.g. physicians, pharmacists).
A. Participants description
Twenty-one participants were interviewed between October 2020 and September 2021, generating 75 interviews. All participants took part in Interview 1, 19 participants in Interview 2, 16 participants in Interview 3 and 19 participants in Interview 4, with a median duration of 41 minutes (IQR: 34-49) per interview. Interviews 1,2,3 and 4 took place respectively 5 days (IQR: 4-7), 14 days (13-20), 35 days (33-38), and 63 days (61-68) after discharge. Nine patients were newly diagnosed with T2D, and 12 had a previous diagnosis of T2D, two of whom were untreated. Further information on participants is described in Table 1 . The median number of comorbidities was six (range: 3–11), and participants newly diagnosed with diabetes tended to have fewer comorbidities (median: 4; range: 3–8). More detailed information regarding sociodemographic characteristics and medications has been published previously [ 28 ].
B. Journey mappings
Generic patient journey mapping, presented in Fig. 1 , summarizes the main and usual encounters participants had with their HCPs during the study period. Generic mapping results from all individual patient journey mappings from discharge to 2 months after discharge are available in Supplementary File 2 .
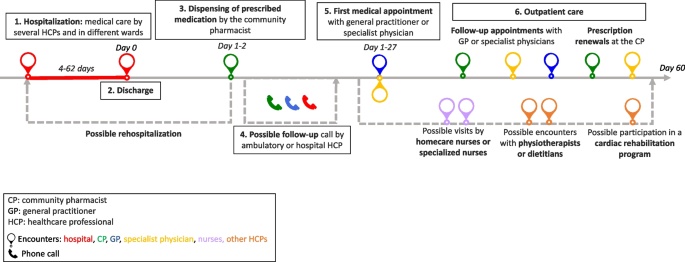
Generic patient journey mapping from hospitalization to two months after discharge
During the 2 months following discharge, the participants had a median number of 10 (range: 6–28) encounters with HCPs. The HCPs met by participants are represented in Fig. 2 . All participants visited their pharmacists at least once, and 16 of the 21 participants met their general practitioners (GPs) at least once. Five participants received home care assistance, four went to an outpatient cardiac rehabilitation program, and five were readmitted during the study period.
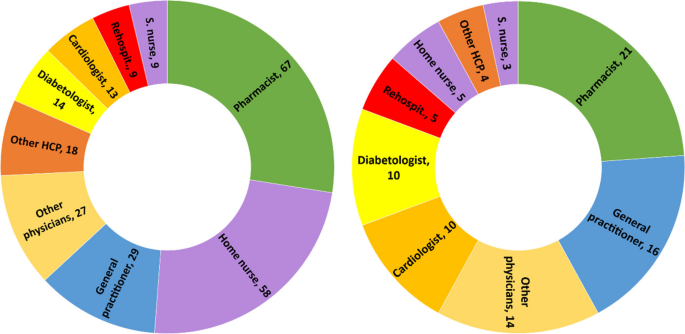
Healthcare professionals seen by participants during the study period. left: n=cumulative encounters; right: n=encountered at least once. Abbreviation: S.nurse: specialized nurse; Other physicians: ophthalmologists, neurologists, hematologists, immunologists, addictologists; other HCP: physiotherapists, dietitians, massage therapist
The first HCP encountered was at the community pharmacy on the same day or day after discharge, except for one participant who did not pick up her medication. The first medical appointment with a physician occurred between days 1 and 27 after discharge (median: 8; IQR: 6-14).
Participants newly diagnosed with diabetes had a closer follow-up after discharge than participants with a former diagnosis of T2D (median: 7; IQR: 6–10 vs median: 9; IQR: 5–19), fewer encounters with HCPs (median: 8; IQR: 7–10 vs. 11; IQR: 8–17), and fewer comorbidities (median: 4; IQR: 4–7 vs. 7; IQR: 5–9). Most participants newly diagnosed with T2D or receiving insulin treatment benefited from either a follow-up call, home visit by a nurse, or diabetes care appointment.
C. Qualitative analysis
Transcripts were analyzed longitudinally and categorized into six key steps based on the verbal data. These key steps, shown in Fig. 1 , represent the identified thematic categories and refer to the following elements: 1. Hospitalization, 2. Discharge, 3. Dispensing of prescribed medications at the pharmacy, 4. Possible follow-up call, 5. First medical appointment, and 6. Outpatient care.
Hospitalization: hospital constraints and care organization
Most participants thought they had benefited from adequate medical care by committed and attentive HCPs but highlighted different constraints and gaps. Some participants noted constraints related to the hospital environment, such as loss of autonomy during their stay, lack of privacy, and the large number of hospital staff encountered. This resulted in participants repeating the same information several times, causing frustration, misunderstanding and a lack of coordination for some participants:
“Twenty or thirty staff members come in during the day! So, it's hard to keep track of [what] is bein g said or done. The best thing for me [...] would be to have clear information from just one person.” Participant 8; interview 1 (P18.1)
Participants had different opinions on the hospital’s care organization. Some participants found that care coordination between the wards was well-organized. In contrast, others highlighted poor coordination and communication between the hospital wards, resulting in long waiting times, care fragmentation, and contradictory or unclear information. Some participants felt that they did not benefit from comprehensive and integrated care and that the hospital staff focused on the cause of their hospitalization, neglecting other comorbidities:
“They were not interested [in my diabetes and my sight]. I was there for the heart and that was where [my care] stopped.” P17.1
Patients’ involvement in decision-making regarding medical care varied. Some participants were involved in their care and took part in medical decisions. Written information, adequate communication, and health professionals’ interest in patients were highlighted by some participants:
“They took the information sheet and they explained everything to me. They didn't just come once; they came several times to explain everything to me.” P5.1
Other participants found the information difficult to understand, particularly because of their fatigue and because the information was provided orally.
Discharge: an unclear process
The discharge process was unclear for patients who could not identify a specific related outpatient medical visit or a key step that summarized their hospital stay and prepared them for discharge:
“Well, there's no real preparation [for discharge]. I was waiting for them to give me the go-ahead so I could go home, that’s all...” P7.4
For some participants, outpatient care follow-up was organized before discharge by the hospital team (generally by making an appointment with the patient’s GP before discharge), whereas others had no post-discharge follow-up scheduled during their hospitalization. Approximately half of the participants refused follow-ups during their hospitalization, such as home care services provided by a nurse, or a rehabilitation hospital stay. The main reason for this refusal was that patients did not perceive the need for follow-up:
“It's true that I was offered a lot of services, which I turned down because I didn't realize how I would manage back at home.” P22.2
Dispensing prescribed medications by the community pharmacist: the first HCP seen after discharge
On behalf of half the participants, a family caregiver went to the usual community or hospital outpatient pharmacy to pick up the medications. The main reasons for delegation were tiredness or difficulty moving. In some cases, this missed encounter would have allowed participants to discuss newly prescribed medications with the pharmacist:
“[My husband] went to get the medication. And I thought afterward, […] that I could have asked [the pharmacist]: “But listen, what is this medication for?” I would have asked questions” P2.3
Participants who met their pharmacist after hospital discharge reported a range of pharmaceutical practices, such as checking the prescribed medication against medication history, providing information and explanations, and offering services such as the preparation of pillboxes. For some, the pharmacists’ work at discharge did not differ from regular prescriptions, whereas others found that they received further support and explanations:
“She took the prescription […] checked thoroughly everything and then she wrote how, when, and how much to take on each medication box. She managed it very well and I had good explanations.” P20.3
Some participants experienced problems with generic substitution, the unavailability of medications, or dispensing errors, complicating their journey through the healthcare system.
Possible follow-up call by HCP: an unsystematic practice
Some participants received a call from their GP or hospital physician a few days after discharge to check their health or answer questions. These calls reassured participants and their caregivers, who knew they had a point of contact in case of difficulty. Occasionally, participants received calls from their community pharmacists to ensure proper understanding and validate medication changes issued during hospitalization. Some participants did not receive any calls and were disappointed by the lack of follow-up:
“There is no follow-up! Nobody called me from the hospital to see how I was doing […]” P8.2
First medical appointment: a key step in the transition of care
The first medical appointment was made in advance by the hospital staff or the patient after discharge. For some participants, this first appointment did not differ from usual care. For most, it was a crucial appointment that allowed them to discuss their hospitalization and new medications and organize their follow-up care. Being cared for by a trusted HCP enabled some patients to feel safe, relieved, and well-cared for, as illustrated by the exchange between a patient and her daughter:
Daughter: When [my mom] came back from the GP, she felt much better [...] It was as if a cork had popped. Was it psychological? Patient: Maybe… I just felt better. D: Do you think it was the fact that she paid attention to you as a doctor? P: She took care of me. She did it in a delicate way. [silence] - P23.2
Some participants complained that their physicians did not receive the hospital discharge letter, making it difficult to discuss hospitalization and sometimes resulting in delayed care.
Outpatient care: a multifaceted experience
During the 2 months after hospital discharge, participants visited several physicians (Fig. 2 ), such as their GP and specialist physicians, for follow-ups, routine check-ups, medical examinations, and new prescriptions. Most participants went to their regular pharmacies to renew their prescriptions, for additional medication information, or for health advice.
Some participants had home care nurses providing various services, such as toileting, care, checks on vital functions, or preparing weekly pill boxes. While some participants were satisfied with this service, others complained that home nurses were unreliable about appointment times or that this service was unnecessary. Some participants were reluctant to use these services:
“The [homecare nurse] makes you feel like you're sick... It's a bit humiliating.” P22.2
Specialized nurses, mostly in diabetology, were appreciated by patients who had dedicated time to talk about different issues concerning diabetes and medication and adapted explanations to the patient’s knowledge. Participants who participated in cardiac rehabilitation said that being in a group and talking to people with the same health problems motivated them to undertake lifestyle and dietary changes:
“In the rehabilitation program, I’m part of a team [of healthcare professionals and patients], I have companions who have gone through the same thing as me, so I’m not by myself. That's better for motivation.” P16.2
Navigating the outpatient healthcare system: the central role of patients
Managing medical appointments is time-consuming and complex for many participants. Some had difficulty knowing with whom to discuss and monitor their health problems. Others had difficulty scheduling medical appointments, especially with specialist physicians or during holidays. A few participants did not attend some of their appointments because of physical or mental vulnerabilities. Restrictions linked to the type of health insurance coverage made navigating the healthcare system difficult for some participants:
“Some medications weren't prescribed by my GP [...] but by the cardiologist. So, I must ask my GP for a delegation to see the cardiologist. And I have to do this for three or four specialists... Well, it’s a bit of a hassle […] it's not always easy or straightforward”. P11.2
Some participants had financial difficulties or constraints, such as expenses from their hospitalization, ambulance transportation, and medications not covered by their health insurance plans. This led to misunderstandings, stress, and anxiety, especially because some participants could not return to work or, to a lesser extent, because of their medical condition.
To ensure continuity of care, some participants were proactive in their case management, for example, by calling to confirm or obtain further information on an appointment or to ensure information transfer. Written convocations for upcoming medical appointments and tailored explanations helped the participants organize their care. Family caregivers were also key in taking participants to various consultations, reminding them, and managing their medical appointments.
Information transfer: incomplete and missing information
Information transfer between and within settings was occasionally lacking. Even weeks after hospitalization, some documents were not transmitted to outpatient physicians, sometimes delaying medical care. Some participants reported receiving incomplete, unclear, or contradictory information from different HCPs, sometimes leading to doubts, seeking a second medical opinion, or personal searches for information. A few proactive participants ensured good information transmission by making a copy of the prescription or sending copies of their documents to physicians:
“My GP hasn't received anything from the hospital yet. I’ve sent him the PDF with the medication I take before our appointment […] Yes, It’s the patient that does all the job.” P10.3
Interprofessional work: a practice highlighted by some participants
Several participants highlighted the interprofessional work they observed in the outpatient setting, especially because they had several comorbidities; therefore, several physicians followed their care:
“My case is very complex! For example, between the cardiologist and the diabetologist, they need to communicate closely because there could be consequences or interactions with the medications I take [for my heart and my diabetes].” P4.2
Health professionals referred their patients to the most appropriate provider for better follow-up (e.g., a nurse specializing in addictology referred a patient to a nurse specializing in diabetology for questions and follow-up on blood sugar levels). Interprofessional collaboration between physicians and pharmacists was noted by some participants, especially for prescription refills or ordering medications.
Patient-HCPs relationships: the importance of trust
Trust in the care relationship was discussed by the participants regarding different HCPs, especially GPs and community pharmacists. Most participants highlighted the communication skills and active listening of healthcare providers. Knowing an HCP for several years helped build trust and ensure an updated medical history:
“I've trusted this pharmacist for 20 years. I can phone her or go to the pharmacy to ask any question[...] I feel supported.” P3.2
Some participants experienced poor encounters owing to a lack of attentive listening or adapted communication, especially when delivering bad news (new diagnoses or deterioration of health status). Professional competencies were an important aspect of the patient-HCP relationship, and some participants lost confidence in their physician or pharmacist because of inadequate medical or pharmaceutical care management or errors, such as the physician prescribing the wrong medication dosage, the pharmacist delivering the wrong pillbox or the general practitioner refusing to see a patient:
“I think I'll find another doctor… In fact, the day I was hospitalized, I called before to make an appointment with her and she refused to see me […] because I had a fever, and I hadn’t done a [COVID] test.” P6.2
Most participants underlined the importance of their GP because they were available, attentive to their health issues, and had a comprehensive view of their medications and health, especially after hospitalization:
“Fortunately, there are general practitioners, who know everything. With some specialists, the body is fragmented, but my GP knows the whole body.” P14.1
After hospitalization, the GP’s role changed for some participants who saw their GP infrequently but now played a central role.
Community pharmacist: an indistinct role
Pharmacists and their teams were appreciated by most participants for their interpersonal competencies, such as kindness, availability, professional flexibility, and adaptability to patients’ needs to ensure medication continuity (e.g., extension of the prescription, home delivery, or extending time to pay for medications). The role of community pharmacists varied according to the participants. Some viewed pharmacists as simple salespeople:
“It's like a grocery store. [...] I go there, it's ordered, I take my medication, I pay and I leave.” P23.3
For others, the pharmacist provided medication and advice and was a timely source of information but did not play a central role in their care. For others, the pharmacist’s role is essential for medication monitoring and safety:
“I always go to the same pharmacy […] because I know I have protection: when [the pharmacist] enters the medications in his computer, if two medications are incompatible, he can verify. [...] There is this follow-up that I will not have if I go each time somewhere else.” P10.4
The patient journey mapping methodology, coupled with qualitative thematic analysis, enabled us to understand and shed light on the intricacies of the journey of polypharmacy patients with T2Din the healthcare system after discharge. This provided valuable insights into their experiences, challenges, and opportunities for improvement.
This study highlights the complex pathways of patients with comorbidities by considering the population of patients with T2D as an example. Our population included a wide variety of patients, both newly diagnosed and with known diabetes, hospitalized for T2D or other reasons. Navigating the healthcare system was influenced by the reason for hospitalization and diagnosis. For example, newly diagnosed participants with T2D had a closer follow-up after discharge, participants were more likely to undergo cardiac rehabilitation after infarction, and participants with a former T2D diagnosis were more complex, with more comorbidities and more HCP encounters. Our aim was not to compare these populations but to highlight particularities and differences in their health care and these qualitative data reveal the need for further studies to improve diabetes management during inpatient to outpatient care transition.
The variability in discharge practices and coordination with outpatient care highlights the lack of standardization during and after hospital discharge. Some participants had a planned appointment with their GP before discharge, others had a telephone call with a hospital or ambulatory physician, and some had no planned follow-up, causing confusion and stress. Although various local or national guidelines exist for managing patients discharged from the hospital [ 36 , 37 , 38 , 39 ], there are no standard practices regarding care coordination implemented in the setting of this study. The lack of local coordination has also been mentioned in other studies [ 5 , 40 , 41 ].
Our results also raise questions about the responsibility gap in the transition of care. Once discharged from the hospital, who is responsible for the patient until their first medical appointment? This responsibility is not clearly defined among hospital and outpatient care providers, with more than 25% of internal medicine residents indicating their responsibility for patients ending at discharge [ 42 , 43 ]. Importance should be given to clarifying when and who will take over the responsibility of guaranteeing patient safety and continuity of care and avoiding rehospitalization [ 44 ].
The first visit with the community pharmacist after discharge and the referring physician were the key encounters. While the role of the GP at hospital discharge is well-defined, the community pharmacist’s role lacks clarity, even though they are the first HCP encountered upon hospital discharge. A meta-analysis showed the added value of community pharmacists and how their active participation during care transition can reduce readmission [ 18 ]. A better definition of the pharmacist’s role and integration into care coordination could benefit patient safety during the transition and should be assessed in future studies.
Our findings showed that the time elapsed between discharge and the first medical appointment varied widely (from 1 to 27 days), correlating with findings in the literature showing that more than 80% of patients see their GP within 30 days [ 45 ]. Despite the first medical appointment being within the first month after discharge, some patients in our study reported a lack of support and follow-up during the first few days after discharge. Care coordination at discharge is critical, as close outpatient follow-up within the first 7–10 days can reduce hospital readmission rates [ 46 , 47 ]. Furthermore, trust and communication skills are fundamental components of the patient-HCP relationship, underlined in our results, particularly during the first medical appointment. Relational continuity, especially with a particular HCP who has comprehensive patient knowledge, is crucial when patients interact with multiple clinicians and navigate various settings [ 48 , 49 ].
Navigating the outpatient healthcare system after discharge was complex for most participants and relied heavily on patient involvement and responsibility. While some participants who received clear information felt more empowered and engaged in their care, others highlighted the difficulty in organizing their care during this vulnerable period. Such difficulties in case management have been described previously [ 50 , 51 ]. Moreover, services proposed by HCPs (e.g., home assistance) do not always correspond to patient needs and are sometimes refused. This highlights the tension between HCPs’ medical recommendations, priorities, and patient expectations. This tension between medical priorities and patient needs was felt during hospitalization and shaped the 2 months following discharge. HCPs need to assess patient needs and preferences during hospitalization and transition for follow-up services. They must also ensure that the offered services meet at least the most relevant of patients’ perceived needs to improve seamless care and patient safety [ 52 , 53 ].
Examples of a lack of communication and information transfer were described in our results at different levels among HCPs, between participants or family caregivers, and HCPs, and these findings correlate with the literature [ 3 , 54 , 55 , 56 ]. Although family caregivers play an important role in supporting patients in the healthcare system, they are also additional interlocutors, leading to missed opportunities for patient-pharmacist interactions when dispensing discharged medication. Therefore, it is paramount to integrate and involve family caregivers in shared decision-making and communicate with patients remotely when they are not present [ 57 ].
Opportunities to improve the discharge of patients returning home after discharge without home care are highlighted in this article. Our insights can serve as a valuable foundation for healthcare providers and policymakers seeking to optimize patient experience and quality of care in the post-discharge phase. Different professionals should be integrated into standard practice through guidelines to ensure improved collaboration from hospital discharge to outpatient care. During hospitalization:
an appointment should be scheduled with the referring physician shortly after discharge to guarantee continuity of care
a hospital discharge interview should be conducted in a systematic way to summarize and securely close the hospitalization
the community pharmacist should be informed before the patient’s discharge to prepare and reconcile medications before and after hospitalization
In outpatient care:
an in-person or phone encounter with the pharmacy team should be scheduled for the patient and/or caregivers at discharge
a contact point (phone number, email, or virtual chat assistant) or scheduled follow-up should be implemented to answer questions and redirect patients before they can meet with the referring physician
a long-term and active communication channel between HCPs should be established.
In other countries, several outpatient services are already available for patients discharged home to enhance continuity of care and patient safety after discharge. The telehealth-based Transitional Care Management Programme, a local initiative in a New York hospital, involves contacting discharged patients 24 to 48 hours after discharge to support understanding of discharge instructions, medication access, follow-up appointments, and social needs [ 58 ]. The Australian Government has introduced the Transition Care Program that provides short-term care for older people, including social work, nursing support, personal care, and allied health care [ 59 ]. In England, the NHS has introduced the Discharge Medicines Service (DMS) in community pharmacies, which aims to improve communication between hospitals and community pharmacies and to ensure that patients understand changes to their medications [ 60 ].
Limitations
This study has several limitations. First, the accuracy of the encounter dates with HCPs, as described by the participants, could not be verified using a second data source (e.g., medical or pharmacy records). Additionally, recall biases cannot be excluded, especially during interviews 3 and 4, which took place at longer intervals (20 days between interviews 2 and 3 and 30 days between interviews 3 and 4). Nevertheless, our findings express a patient's representation of their healthcare system navigation experience. Secondly, these results may not be generalizable to populations with other long-term diseases, even though we recruited patients with different reasons for hospitalization, including age, sex, and comorbidities. In addition, the study region is predominantly an urban area with a high density of HCPs, which may influence patient journeys in the healthcare system. Finally, we excluded patients whose medications were managed by HCPs because these patients might have had different experiences, difficulties, and needs. This exclusion criterion was chosen because our objective was to investigate patients’ medication self-management, as described in another article [ 28 ].
A patient’s journey in the 2 months following discharge is unique for each individual and constitutes a complex and adaptive process. Despite the active role of numerous HCPs, navigation in outpatient care after discharge relies heavily on the involvement and responsibilities of polypharmacy. The findings of this study highlight the need to standardize the approach for discharge planning and post-discharge care in partnership with patients and caregivers. Preparation for discharge, the first visit to the pharmacy, and the first appointment with the GP are key moments for all patients, along with the involvement of other medical and nurse specialists, as needed. Standardizing practices, clarifying responsibilities, integrating community pharmacists during the transition, empowering patients, and enhancing interprofessional communication and collaboration should be explored and implemented to achieve better patient outcomes and a more seamless healthcare journey for individuals transitioning from the hospital to the community.
Availability of data and materials
The qualitative codes in French and anonymized patient datasets are available from the corresponding author on reasonable request. Individual patient journeys are provided in the Supplementary Files.
Abbreviations
General practitioner
Healthcare professional
type 2 diabetes mellitus
Coleman EA, Boult C. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–7.
Article PubMed Google Scholar
Allen J, Hutchinson AM, Brown R, Livingston PM. User experience and care for older people transitioning from hospital to home: Patients’ and carers’ perspectives. Health Expect. 2018;21(2):518–27.
Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. Jama. 2007;297(8):831–41.
Article CAS PubMed Google Scholar
Allen J, Hutchinson AM, Brown R, Livingston PM. Quality care outcomes following transitional care interventions for older people from hospital to home: a systematic review. BMC Health Serv Res. 2014;14:346.
Article PubMed PubMed Central Google Scholar
Hesselink G, Flink M, Olsson M, Barach P, Dudzik-Urbaniak E, Orrego C, et al. Are patients discharged with care? A qualitative study of perceptions and experiences of patients, family members and care providers. BMJ Qual Saf. 2012;21(Suppl 1):i39-49.
World Health Organization (WHO). Transitions of Care. 2016.
Google Scholar
Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–2.
Article CAS PubMed PubMed Central Google Scholar
Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–28.
Banholzer S, Dunkelmann L, Haschke M, Derungs A, Exadaktylos A, Krähenbühl S, et al. Retrospective analysis of adverse drug reactions leading to short-term emergency hospital readmission. Swiss Med Wkly. 2021;151:w20400.
World Health Organization (WHO). Medication Safety in Transitions of Care. 2019.
Müller-Wieland D, Merkel M, Hamann A, Siegel E, Ottillinger B, Woker R, et al. Survey to estimate the prevalence of type 2 diabetes mellitus in hospital patients in Germany by systematic HbA1c measurement upon admission. Int J Clin Pract. 2018;72(12):e13273.
Blanc AL, Fumeaux T, Stirnemann J, Dupuis Lozeron E, Ourhamoune A, Desmeules J, et al. Development of a predictive score for potentially avoidable hospital readmissions for general internal medicine patients. PLoS One. 2019;14(7):e0219348.
Hansen LO, Greenwald JL, Budnitz T, Howell E, Halasyamani L, Maynard G, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421–7.
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10(1):107–11.
Iglay K, Hannachi H, Joseph Howie P, Xu J, Li X, Engel SS, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243–52.
Russell LB, Suh DC, Safford MA. Time requirements for diabetes self-management: too much for many? J Fam Pract. 2005;54(1):52–6.
PubMed Google Scholar
Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–8.
Lussier ME, Evans HJ, Wright EA, Gionfriddo MR. The impact of community pharmacist involvement on transitions of care: a systematic review and meta-analysis. J Am Pharm Assoc. 2020;60(1):153.
Article Google Scholar
Donzé J, John G, Genné D, Mancinetti M, Gouveia A, Méan M, et al. Effects of a Multimodal Transitional Care Intervention in Patients at High Risk of Readmission: The TARGET-READ Randomized Clinical Trial. JAMA Intern Med. 2023.
Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–107.
Noonan VK, Lyddiatt A, Ware P, Jaglal SB, Riopelle RJ, Bingham CO 3rd, et al. Montreal Accord on Patient-Reported Outcomes (PROs) use series - Paper 3: patient-reported outcomes can facilitate shared decision-making and guide self-management. J Clin Epidemiol. 2017;89:125–35.
Hesselink G, Schoonhoven L, Barach P, Spijker A, Gademan P, Kalkman C, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417–28.
(WHO) WHO. Systems in the who European region: framework for action on integrated health services delivery. Copenhagen: WHO; 2016.
Damery S, Flanagan S, Combes G. The effectiveness of interventions to achieve co-ordinated multidisciplinary care and reduce hospital use for people with chronic diseases: study protocol for a systematic review of reviews. Syst Revi. 2015;4(1):64.
Noor F, Gulis G, Karlsson LE. Exploration of understanding of integrated care from a public health perspective: a scoping review. J Public Health Res. 2023;12(3):22799036231181210.
Jackson K, Oelke ND, Besner J, Harrison A. Patient journey: implications for improving and integrating care for older adults with chronic obstructive pulmonary disease. Can J Aging. 2012;31(2):223–33.
Gualandi R, Masella C, Viglione D, Tartaglini D. Exploring the hospital patient journey: what does the patient experience? PLoS One. 2019;14(12):e0224899.
Solh Dost L, Gastaldi G, Schneider M. Patient medication management, understanding and adherence during the transition from hospital to ambulatory care – a qualitative longitudinal study in polymorbid type 2 diabetes patients. BMC Health Services Research, in press
World Health Organization (WHO). Adherence to long-term therapies: Evidence for action. 2003.
Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–73.
Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health. 1998;13(4):623–49.
Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: how many interviews are enough? Qual Health Res. 2016;27(4):591–608.
Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qual Res Sport Exercise Health. 2019;11(4):589–97.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Davies EL, Bulto LN, Walsh A, Pollock D, Langton VM, Laing RE, et al. Reporting and conducting patient journey mapping research in healthcare: a scoping review. J Adv Nurs. 2023;79(1):83–100.
California pharmacists association. Transitions of Care Resource Guide https://cdn.ymaws.com/www.cshp.org/resource/resmgr/Files/Practice-Policy/For_Pharmacists/transitions_of_care_final_10.pdf . Accessed 20 Nov 2023.
National Health Service (NHS). Guidance: Hospital discharge and community support guidance. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1087354/Hospital-Discharge-and-Community-Support-Guidance-2022-v2.pdf . Accessed 01 Apr 2024.
Winnipeg Regional Health Authority. Safe Patient Discahrge Guideline. 2017. https://wrha.mb.ca/files/guideline-safe-discharge.pdf . Accessed 01 Apr 2024.
Haute Autorité de Santé, France. Check-List de Sortie d'hospitalisation supérieure à 24 heures https://www.has-sante.fr/jcms/c_2035081/fr/check-list-de-sortie-d-hospitalisation-superieure-a-24h . Accessed 04 Apr 2024.
Wong ELY, Yam CHK, Cheung AWL, Leung MCM, Chan FWK, Wong FYY, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242.
Urban R, Paloumpi E, Rana N, Morgan J. Communicating medication changes to community pharmacy post-discharge: the good, the bad, and the improvements. Int J Clin Pharm. 2013;35(5):813–20.
Young E, Stickrath C, McNulty MC, Calderon AJ, Chapman E, Gonzalo JD, et al. Internal medicine residents’ perceived responsibility for patients at hospital discharge: a national survey. J Gen Intern Med. 2016;31(12):1490–5.
Jones CD, Vu MB, O’Donnell CM, Anderson ME, Patel S, Wald HL, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417–24.
Watts R, Pierson J, Gardner H. Co-ordination of the discharge planning process in critical care. J Clin Nurs. 2007;16(1):194–202.
Roughead EE, Kalisch LM, Ramsay EN, Ryan P, Gilbert AL. Continuity of care: when do patients visit community healthcare providers after leaving hospital? Intern Med J. 2011;41(9):662–7.
Riverin BD, Strumpf EC, Naimi AI, Li P. Optimal timing of physician visits after hospital discharge to reduce readmission. Health Serv Res. 2018;53(6):4682–703.
Coppa K, Kim EJ, Oppenheim MI, Bock KR, Conigliaro J, Hirsch JS. Examination of post-discharge follow-up appointment status and 30-day readmission. J Gen Intern Med. 2021;36(5):1214–21.
Haggerty JL, Roberge D, Freeman GK, Beaulieu C. Experienced continuity of care when patients see multiple clinicians: a qualitative metasummary. Ann Fam Med. 2013;11(3):262–71.
Baker R, Freeman G, Boulton M, Windridge K, Tarrant C, Low J, et al. Continuity of care: patients’ and carers’ views and choices in their use of primary care services. Report for the national co-ordinating center for NHS Service Delivery and Organisation R & D (NCCSDO). 2006.
Arora VM, Prochaska ML, Farnan JM, D’Arcy MJt, Schwanz KJ, Vinci LM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–91.
Allen J, Hutchinson AM, Brown R, Livingston PM. User experience and care integration in transitional care for older people from hospital to home: a meta-synthesis. Qual Health Res. 2016;27(1):24–36.
Krook M, Iwarzon M, Siouta E. The discharge process-from a patient’s perspective. SAGE Open Nurs. 2020;6:2377960819900707.
PubMed PubMed Central Google Scholar
Huber DL, McClelland E. Patient preferences and discharge planning transitions. J Prof Nurs. 2003;19(4):204–10.
Ravenscroft E. Navigating the health care system: insights from consumers with multimorbidity. J Nurs Healthcare Chronic Illness. 2010;2:215–24.
Ancker JS, Witteman HO, Hafeez B, Provencher T, Van de Graaf M, Wei E. The invisible work of personal health information management among people with multiple chronic conditions: qualitative interview study among patients and providers. J Med Internet Res. 2015;17(6):e137.
Manias E, Gerdtz M, Williams A, McGuiness J, Dooley M. Communicating about the management of medications as patients move across transition points of care: an observation and interview study. J Eval Clin Pract. 2016;22(5):635–43.
Mackie BR, Mitchell M, Marshall AP. Patient and family members’ perceptions of family participation in care on acute care wards. Scandinavian J Caring Sci. 2019;33(2):359–70.
Michelle E, Rachel CSF, Jonathan S, Priscilla H, Farrukh NJ. Telehealth-based transitional care management programme to improve access to care. BMJ Open Qual. 2023;12(4):e002495.
Department of Health and Aged Care, Australia. Transition Care Programme. https://www.health.gov.au/our-work/transition-care-programme . Accessed 03 Apr 2024
National Health Service (NHS) Discharge Medicines Service. 2021. https://www.england.nhs.uk/primary-care/pharmacy/pharmacy-services/nhs-discharge-medicines-service/ . Accessed 03 Apr 2024
Download references
Acknowledgments
The authors would like to thank all the patients who took part in this study. We would also like to thank the Geneva University Hospitals Patients Partners +3P platform as well as Mrs Tourane Corbière and Mr Joël Mermoud, patient partners, who reviewed interview guides for clarity and significance.
Open access funding provided by University of Geneva This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
School of Pharmaceutical Sciences, University of Geneva, Geneva, Switzerland
Léa Solh Dost & Marie P. Schneider
Institute of Pharmaceutical Sciences of Western Switzerland, University of Geneva, Geneva, Switzerland
Division of Endocrinology, Diabetes, Hypertension and Nutrition, Department of Medicine, Geneva University Hospitals, Geneva, Switzerland
Giacomo Gastaldi
Institute of Psychology and Education, University of Neuchatel, Neuchâtel, Switzerland
Marcelo Dos Santos Mamed
Institute of Psychology, University of Lausanne, Lausanne, Switzerland
You can also search for this author in PubMed Google Scholar
Contributions
LS, GG, and MS conceptualized and designed the study. LS and GG screened and recruited participants. LS conducted the interviews. LS, GG, and MS performed data analysis and interpretation. LS drafted the manuscript and LS and MS worked on the different versions. MDS contributed its expertise and external opinion as a clinical psychologist and linguist. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Léa Solh Dost or Marie P. Schneider .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval was sought and granted by the Cantonal Research Ethics Commission, Geneva (CCER) (2020-01779). Informed consent to participate was obtained for all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Interview guides.
Additional file 2.
Individual patient journey mappings from discharge to 2 months after discharge.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Solh Dost, L., Gastaldi, G., Dos Santos Mamed, M. et al. Navigating outpatient care of patients with type 2 diabetes after hospital discharge - a qualitative longitudinal study. BMC Health Serv Res 24 , 476 (2024). https://doi.org/10.1186/s12913-024-10959-4
Download citation
Received : 17 January 2024
Accepted : 07 April 2024
Published : 17 April 2024
DOI : https://doi.org/10.1186/s12913-024-10959-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 2 diabetes mellitus
- Outpatient care
- Hospital discharge
- Qualitative research
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Hosted content
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 73, Issue 5
- Glucagon-like peptide-1 receptor agonists and risk of major adverse liver outcomes in patients with chronic liver disease and type 2 diabetes
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-3634-6591 Axel Wester 1 ,
- http://orcid.org/0000-0002-1496-1799 Ying Shang 1 ,
- Emilie Toresson Grip 1 , 2 ,
- Anthony A Matthews 3 ,
- http://orcid.org/0000-0002-8474-1759 Hannes Hagström 1 , 4
- 1 Department of Medicine Huddinge , Karolinska Institutet , Stockholm , Sweden
- 2 Quantify Research , Stockholm , Sweden
- 3 Unit of Epidemiology, Institute of Environmental Medicine , Karolinska Institutet , Stockholm , Sweden
- 4 Division of Hepatology, Department of Upper GI , Karolinska University Hospital , Stockholm , Sweden
- Correspondence to Dr Axel Wester, Department of Medicine Huddinge, Karolinska Institutet, Stockholm, Sweden; axel.wester{at}ki.se
Objective Phase II trials suggest glucagon-like peptide-1 receptor (GLP1) agonists resolve metabolic dysfunction-associated steatohepatitis but do not affect fibrosis regression. We aimed to determine the long-term causal effect of GLP1 agonists on the risk of major adverse liver outcomes (MALO) in patients with any chronic liver disease and type 2 diabetes.
Design We used observational data from Swedish healthcare registers 2010–2020 to emulate a target trial of GLP1 agonists in eligible patients with chronic liver disease and type 2 diabetes. We used an inverse-probability weighted marginal structural model to compare parametric estimates of 10-year MALO risk (decompensated cirrhosis, hepatocellular carcinoma, liver transplantation or MALO-related death) in initiators of GLP1 agonists with non-initiators. We randomly sampled 5% of the non-initiators to increase computational efficiency.
Results GLP1 agonist initiators had a 10-year risk of MALO at 13.3% (42/1026) vs 14.6% in non-initiators (1079/15 633) in intention-to-treat analysis (risk ratio (RR)=0.91, 95% CI=0.50 to 1.32). The corresponding 10-year per-protocol risk estimates were 7.4% (22/1026) and 14.4% (1079/15 633), respectively (RR=0.51, 95% CI=0.14 to 0.88). The per-protocol risk estimates at 6 years were 5.4% (21/1026) vs 9.0% (933/15 633) (RR=0.60, 95% CI=0.29 to 0.90) and at 8 years 7.2% (22/1026) vs 11.7% (1036/15 633) (RR=0.61, 95% CI=0.21 to 1.01).
Conclusion In patients with chronic liver disease and type 2 diabetes who adhered to therapy over time, GLP1 agonists may result in lower risk of MALO. This suggests that GLP1 agonists are promising agents to reduce risk of chronic liver disease progression in patients with concurrent type 2 diabetes, although this needs to be corroborated in randomised trials.
- PHARMACOTHERAPY
- FATTY LIVER
- EPIDEMIOLOGY
- DIABETES MELLITUS
Data availability statement
No data are available. No data are available due to Swedish regulations.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/gutjnl-2023-330962
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Disclaimer: this video summarises a scientific article published by BMJ Publishing Group Limited (BMJ). The content of this video has not been peer-reviewed and does not constitute medical advice. Any opinions expressed are solely those of the contributors. Viewers should be aware that professionals in the field may have different opinions. BMJ does not endorse any opinions expressed or recommendations discussed. Viewers should not use the content of the video as the basis for any medical treatment. BMJ disclaims all liability and responsibility arising from any reliance placed on the content.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Glucagon-like peptide-1 receptor (GLP1) agonists might resolve metabolic dysfunction-associated steatohepatitis, but their effect on hard clinical outcomes in patients with chronic liver diseases of any aetiology and concurrent type 2 diabetes is unknown.
WHAT THIS STUDY ADDS
Using Swedish register data, we emulated a target trial of GLP1 agonists in patients with chronic liver disease and type 2 diabetes and fitted an inverse-probability weighted marginal structural model to estimate 10-year risks of major adverse liver outcomes (MALO). The risk of MALO was 49% lower in initiators of GLP1 agonists in the per-protocol analysis, but our data were not compatible with a protective intention-to-treat effect.

HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
GLP1 agonists might be a treatment option to reduce MALO risk in patients with type 2 diabetes and any chronic liver disease who adhere to the treatment over time, although this would need to be corroborated by randomised clinical trials.
Introduction
Chronic liver diseases are highly prevalent and can progress to decompensated cirrhosis, hepatocellular carcinoma (HCC) and liver-related death. 1–5 Type 2 diabetes strongly predicts presence and severity of metabolic dysfunction-associated steatotic liver disease (MASLD) and is also a major risk factor for disease progression in other liver diseases, likely due to interaction of hepatic steatosis and steatohepatitis with other liver diseases. 6–9
Currently, no approved pharmacotherapy exists for MASLD, but one promising drug class is glucagon-like peptide-1 receptor (GLP1) agonists, which are currently approved in patients with type 2 diabetes or obesity to achieve weight loss and control blood glucose. 10–12 Importantly, phase II trials indicate that GLP1 agonists resolve metabolic dysfunction-associated steatohepatitis (MASH) in patients with non-cirrhotic MASLD but do not cause fibrosis regression. 13 14 Large phase III trials that aim to estimate the effect of GLP1 agonists on resolving MASH or reducing hepatic fibrosis are, however, many years from completion. 15
Although achieving surrogate histopathological endpoints (eg, fibrosis regression) is considered by regulatory agencies to likely translate to improved prognosis, robust evidence is needed to understand if GLP1 agonists reduce the risk of long-term clinical outcomes, such as liver decompensation or HCC. 16 17 Given that the metabolic syndrome is a major driver of liver-related outcomes both in patients with MASLD and other chronic liver diseases such as alcohol-related liver disease or viral hepatitis C, there could be a similar effect of GLP1 agonists in patients with chronic liver diseases of any aetiology with concomitant metabolic traits, such as type 2 diabetes. 18–20 For instance, insulin resistance is the strongest predictor of liver fibrosis in patients with alcohol-related liver disease. 21 Therefore, we designed a target trial that would estimate the long-term causal effect of GLP1 agonists on major adverse liver outcomes (MALO) in patients with any chronic liver disease and type 2 diabetes, and then emulated it using observational data from Swedish healthcare registers.
Data sources
The Decoding the Epidemiology of LIVER disease in Sweden (DELIVER) cohort includes data from Swedish national healthcare registers on all patients with any chronic liver disease in Sweden 1964–2020. 22 The data include all International Classification of Diseases (ICD) codes from inpatient and specialised outpatient care, dates and causes of death, and automatically recorded information on filled prescriptions from any pharmacy in Sweden. 23–29 The positive predictive value is 96% for MASLD with comorbid type 2 diabetes, and >90% for most diagnoses related to cirrhosis. 30 31 A detailed overview of the registers is provided in online supplemental methods .
Supplemental material
Target trial specification and emulation.
A causal question is best answered by data from randomised trials, but when unavailable, researchers often resort to observational data from existing databases. To avoid common methodological pitfalls in observational studies, causal inference from such data can be viewed as an attempt to emulate a hypothetical pragmatic randomised trial—a target trial. 32 An overview of the target trial emulation concept is provided in reference. 33 After specifying the target trial protocol, it is emulated using the available observational data and appropriate methodology. For this observational study, we first specified the protocol of a target trial that would estimate the effect of GLP1 agonists on MALO risk in patients with chronic liver disease and type 2 diabetes. We then emulated the target trial using data from DELIVER. Table 1 summarises all protocol components from the target trial and its emulation, which we describe in detail below. All diagnoses and medications were defined by the ICD or Anatomical Therapeutic Chemical (ATC) codes in online supplemental tables 1–3 . Diagnoses were identified from January 1997 and forward (when ICD-10 was introduced in Sweden), and drugs from July 2005 and forward (when the Swedish Prescribed Drug Register was initiated).
- View inline
Protocol of a target trial and an emulated trial using observational data
Eligibility criteria
All Swedish residents ≥18 years of age between January 2010, when uptake of GLP1 agonists increased in Sweden, and November 2020 with any chronic liver disease and type 2 diabetes were identified. 22 To avoid structural positivity issues (ie, patients with zero probability of initiating a GLP1 agonist at baseline), patients were required to have filled at least one prescription of metformin within a year before baseline corresponding to a daily dose of ≥1 g (ie, patients potentially eligible for second-line treatment with a GLP1 agonist). Patients were excluded if they previously filled prescriptions of GLP1 agonists, had a history of a contraindication to GLP1 agonists (defined as pancreatitis, inflammatory bowel disease or severe chronic kidney disease) or prior MALO (defined below). As the non-initiator population was large, we randomly sampled 5% of this group to increase computational efficiency ( figure 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Flow chart of the study population. Numbers represent study participants (initiators or non-initiators), while numbers in parentheses represent the corresponding number of unique patients. Note that the numbers of excluded study participants represent the total number of times that unique patients were non-eligible for any of the emulated target trials. If somebody was non-eligible for all the 131 emulated target trials, they would contribute with an addition of 131 to this total number.
Treatment strategies
We compared two treatment strategies: initiation of any GLP1 agonist (ATC code A10BJ) at baseline and continuation of treatment over follow-up unless a contraindication was diagnosed after baseline; and no initiation of a GLP1 agonist at baseline and continuation of no GLP1 agonist treatment during follow-up, unless indicated as deemed by the treating physician. Since we lacked data to specifically determine who were indicated for GLP1 agonists, we assumed that it was indicated in all non-initiators who started the drug during follow-up. We assessed drug continuation by summing the number of months of filled prescriptions. A gap between two successive prescriptions was allowed if it was less than twice the time intended for the most recently filled prescription. For example, an initiator who filled a prescription for 3 months treatment was considered to have stopped the treatment after 6 months, unless the prescription was refilled before that.
Treatment assignment
Patients were classified into two groups according to the strategy their data were compatible with at baseline, that is, GLP1 agonist initiators and non-initiators. We assumed groups were exchangeable at baseline conditional on baseline covariates (similar probability of initiating the drug in both arms, within levels of the covariates): age, sex, education (<10, 10–12 and >12 years), diabetes duration, liver disease aetiology, compensated cirrhosis, and a range of comorbidities and medications: obesity, cardiovascular disease, microvascular complications to diabetes, chronic obstructive pulmonary disease, alcohol use disorder, mental health disorder, the use of antidiabetic medications except metformin or GLP1 agonists and direct-acting antivirals in patients with viral hepatitis. As the relationship between age and the probability of initiating a GLP1 agonist might not be linear, we modelled age using linear, quadratic and cubic terms. If patients had coding for more than one liver disease aetiology, they were classified according to a predefined hierarchy ( online supplemental methods ).
The outcome of interest was the first MALO during follow-up, defined as decompensated cirrhosis (variceal bleeding, ascites, portal hypertension or hepatorenal syndrome), HCC, liver transplantation or MALO-related death. MALO was defined by ICD codes in the National Patient Register (main or secondary diagnosis), the Cancer Register or the Cause of Death Register (main or contributing cause) ( online supplemental table 3 ). These outcomes have been validated and found to have positive predictive values >90% ( online supplemental table 4 ). 31
Everyone was followed from baseline to the earliest of MALO, emigration from Sweden, 10 years of follow-up or December 2020. The follow-up was measured in calendar months.
Causal contrasts
We estimated observational analogues of the intention-to-treat and per-protocol effects.
Statistical analyses
We sequentially emulated the target trial as a series of separate target trials starting in each 131 calendar months between January 2010 and November 2020, meaning that patients could enter multiple target trials if eligible. To avoid immortal time bias, the baseline is best defined as the time when eligible patients initiate a treatment strategy. 32 The GLP agonist initiators naturally have one such time point. The definition of the baseline is, however, more challenging in the non-initiators since the same individual can be eligible at multiple times. One solution that avoids immortal time bias is to emulate a target trial that uses all those eligibility times as the baseline and consider each individual at each of those times as different individuals. 34 For example, a patient who fulfilled all eligibility criteria in our study in January 2010 and did not initiate a GLP1 agonist in that month would enter the target trial that started in January 2010 as a non-initiator. If still eligible in February 2010, this patient would also enter the target trial that started in this month as a non-initiator. If the same patient initiated a GLP1 agonist in March 2010 and still fulfilled all eligibility criteria, the patient would enter this target trial as an initiator and be non-eligible for all subsequent target trials as previous use of GLP1 agonists was an exclusion criterion. Target trials with only initiators or only non-initiators were excluded. Allowing repeated eligibility is statistically more efficient than choosing only 1 month as baseline and accounts for the fact that patients can be eligible in several different months during the study period. 35 36
We fitted a marginal structural model using parametric pooled logistic regression with an indicator for treatment group, a flexible time-varying intercept (linear and quadratic terms), product terms between treatment group and time, and a target trial indicator to pool data for all the emulated trials and estimate intention-to-treat and per-protocol effects. For estimation of the intention-to-treat effect, we weighted the model using inverse-probability of treatment weights (IPTW). The IPTW models included all baseline covariates, and the weights were stabilised. The balance between treatment groups was assessed using standardised mean differences (SMD) and inspection of kernel density plots. An SMD<0.1 is generally regarded to indicate good balance. 37 For estimation of the per-protocol effect, the same marginal structural model as above was used, but patients were additionally censored when deviating from their assigned treatment strategy and stabilised inverse-probability of censoring weights (IPCWs) were applied to adjust for baseline and time-varying covariates associated with adherence. 34 38 The marginal structural model was weighted using the product of the IPTWs and the IPCWs. The IPCW models included age, sex, education, diabetes duration and liver disease aetiology at baseline, and the following time-varying covariates: compensated cirrhosis and the same range of comorbidities and medications as described above. If patients stopped GLP1 agonist treatment because of a contraindication during follow-up (eg, an episode of pancreatitis), or started GLP1 agonist treatment, their censoring weights remained constant from that date forward. The weights are described in online supplemental table 5 .
The average 10-year absolute risks under each strategy were estimated using the predicted values from the marginal structural models, then resulting risk differences (RDs) and risk ratios (RRs) were calculated. Non-parametric bootstrapping with 500 replications was used to estimate 95% CIs.
We examined the intention-to-treat effect in subgroups according to liver disease aetiology (MASLD, other than MASLD), and liver disease severity at baseline (compensated cirrhosis, no cirrhosis). Several sensitivity analyses were done to assess the robustness of our results. First, we updated the eligibility criteria to include those with a lower daily dose of metformin (0.5 g instead of 1 g). Second, the time gap between two successive prescriptions was restricted to ≤30 days. Third, we censored non-initiators if they initiated a GLP1 agonist during follow-up, regardless of whether it was indicated, and IPCWs were applied as described above. Fourth, the inverse-probability weights were truncated at the 1st and 99th percentile before being applied to the marginal structural model, to avoid the impact of extreme values on the risk estimates. Fifth, we used standardisation to adjust for confounding at baseline, rather than IPTW. 39 In this analysis, the IPCWs were used in the per-protocol analysis as described above. Sixth, we estimated intention-to-treat and per-protocol point estimates including all non-initiators (without sampling 5%), to assess whether the sampling affected our risk estimates. Finally, to estimate how strongly an unmeasured confounder would need to be associated with both the exposure and outcome to fully explain any differences in risk between treatment groups, we calculated the E-value. 40
We additionally calculated the intention-to-treat and per-protocol effects at 2, 4, 6 and 8 years of follow-up. Because 2 years is a more plausible duration of a future randomised trial of GLP1 agonists in patients with chronic liver disease than 10 years, we computed the minimum sample size required in a clinical trial of GLP1 agonists to demonstrate an effect of equal strength as our estimated 2-year RR, using a 5% alpha and 80% power.
Analyses were done in Stata V.17.0 (StataCorp).
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
We included 1026 initiators of GLP1 agonists and 15 633 non-initiators, who participated in up to 123 target trials. The patient characteristics at baseline before and after weighting are summarised in table 2 . After IPTW, all baseline characteristics were well balanced (SMD<0.1). Kernel density plots also indicated good balance ( online supplemental figure 1 ). Of the initiators, 635 (61.9%) started treatment with liraglutide, 231 (22.5%) semaglutide, 120 (11.7%) dulaglutide, 25 (2.4%) exenatide and 15 (1.5%) lixisenatide at baseline.
Patient characteristics at baseline before and after inverse-probability of treatment weighting
In the intention-to-treat analysis, initiators and non-initiators were followed for a median (p25–p75) of 64 (36–96) and 76 months (50–100), respectively. 350 of 605 (57.9%) initiators still at risk at 2 years were continuous users at this time. The corresponding numbers at 4, 6, 8 and 10 years were 143 of 331 (43.2%), 64 of 188 (34.0%), 24 of 98 (24.5%) and 5 of 19 (26.3%), respectively. In the per-protocol analysis, where the patients were censored if they deviated from their assigned treatment strategy, the median follow-up was 43 (21–75) and 76 months (50–100), respectively. After a median of 14 months (8–26) follow-up, 517 (50.4%) initiators stopped the treatment; 21 (2.1%) initiators stopped the treatment after developing a contraindication, and 496 (48.3%) stopped the treatment without one of the prespecified contraindications. Of the 1026 initiators, 361 (35.2%) were censored in the per-protocol analysis because they stopped the treatment with a prespecified contraindication the first 2 years, another 92 (9.0%) between 2 and 4 years, 28 (2.7%) between 4 and 6 years, 11 (1.1%) between 6 and 8 years, and 4 (0.4%) between 8 and 10 years. Among the non-initiators, 2357 (15.1%) started treatment with a GLP1 agonist after a median follow-up of 31 months (14–52).
In the intention-to-treat analysis, MALO occurred in 42 initiators and 1079 non-initiators. The events in non-initiators corresponded to 486 distinct events (since participation in multiple target trials was allowed, some contributed with events to more than one target trial). The 10-year risk of MALO was 13.3% (95% CI=7.4% to 19.2%) in the initiators and 14.6% (95% CI=13.1% to 16.1%) in the non-initiators (RD=−1.3, 95% CI=−7.2 to 4.6, RR=0.91, 95% CI=0.50 to 1.32) ( table 3 , figure 2A ).
Inverse-probability weighted risk curves of major adverse liver outcomes comparing initiators of glucagon-like peptide-1 receptor (GLP1) agonists with non-initiators. (A) intention-to-treat effect, (B) per-protocol effect.
Risk of major adverse liver outcomes according to intention-to-treat and per-protocol analyses
In per-protocol analysis, we observed 22 events of MALO in the initiators and 1079 in the non-initiators. The 10-year MALO risk was 7.4% (95% CI=2.1% to 12.6%) in the initiators and 14.4% (95% CI=12.9% to 15.9%) in the non-initiators (RD=−7.1, 95% CI=−12.5 to –1.6; RR=0.51, 95% CI=0.14 to 0.88) ( table 3 , figure 2B ). This corresponds to a number needed to treat (initiate and continue with the GLP1 agonist treatment strategy) to avoid one event of MALO over the course of 10 years of 14 (95% CI=8 to 63).
Results from the intention-to-treat subgroup analyses are summarised in table 4 . In patients with MASLD, the 10-year risk of MALO was 15.8% in the initiators and 11.2% in the non-initiators (RR=1.41, 95% CI=0.53 to 2.30). The 10-year risk in patients with compensated cirrhosis was 36.5% and 34.6% in initiators and non-initiators, respectively (RR=1.05, 95% CI=0.20 to 1.91).
Risk of major adverse liver outcomes at 10 years according to intention-to-treat analyses in subgroups
The risk estimates from a sensitivity analysis that allowed patients to have a lower dose of metformin at baseline were similar to the main analyses (intention-to-treat RR=0.94, 95% CI=0.58 to 1.30; per-protocol RR=0.63, 95% CI=0.27 to 0.99) ( online supplemental table 6 ). The per-protocol estimates were also similar when restricting the gap between two successive prescriptions to ≤30 days (RR=0.48, 95% CI=0.11 to 0.85) ( online supplemental table 7 ). In addition, similar per-protocol estimates were found when the non-initiators were censored if they initiated a GLP1 agonist during follow-up (RR=0.55, 95% CI=0.18 to 0.92) ( online supplemental table 8 ). Both the intention-to-treat and per-protocol estimates were similar to the main analyses when the weights were truncated (intention-to-treat RR=0.84, 95% CI=0.48 to 1.20; per-protocol RR=0.55, 95% CI=0.16 to 0.93) ( online supplemental table 9 ). Moreover, results were similar to the main analysis when using standardisation to adjust for confounders at baseline (intention-to-treat RR=0.87, 95% CI=0.53 to 1.22; per-protocol RR=0.58, 95% CI=0.19 to 0.97) ( online supplemental table 10 ). Point estimates were similar to the main analysis when including all non-initiators (n=312 661) (intention-to-treat RR=1.01, per-protocol RR=0.53) ( online supplemental table 11 ). The E-value for the RRs in the main per-protocol analysis was 3.33 for the point estimate and 1.53 for the 95% CI, suggesting how strong an unmeasured confounder needs to be to fully explain the estimated per-protocol effect and shift the 95% CI to include the null.
Risk estimates for the intention-to-treat and per-protocol analyses at 2, 4, 6 and 8 years are presented in table 3 . At 2 years follow-up, a more plausible duration of a clinical trial than 10 years, the risk of MALO was 2.3% in the initiators and 3.2% in the non-initiators (overall event probability of 2.7%) when analysed by the intention-to-treat principle (RD=−0.9, 95% CI=−2.2 to 0.4; RR=0.72, 95% CI=0.31 to 1.13). A clinical trial of GLP1 agonists on the risk of MALO with 2 years of follow-up would need enrolment of at least 10 776 patients per arm to provide evidence for such effect.
We emulated a nationwide target trial to answer the question whether GLP1 agonists can prevent development of MALO in patients with chronic liver diseases and type 2 diabetes. The main finding was that the 10-year risk of MALO was 49% lower in patients who initiated and adhered to GLP1 agonists compared with non-initiators in the per-protocol analysis, but the estimates from the intention-to-treat analysis were imprecise with a 95% CI for the RR ranging from 0.50 to 1.32.
A placebo-controlled randomised phase II trial of 320 patients with MASH reported that the proportion achieving MASH resolution without worsening fibrosis after 72 weeks was more than tripled in the arm receiving 0.4 mg of the GLP1 agonist semaglutide compared with placebo (59% vs 17%). 14 We followed patients with any chronic liver disease and type 2 diabetes for up to 10 years and found a similarly strong effect of GLP1 agonists on the risk of MALO in per-protocol analysis, but an imprecise intention-to-treat effect. However, MALO risk was similar in the initiator arm when restricting the analysis to patients with MASLD, although this should be interpreted cautiously as we noted wide CIs for these estimates. The phase II trial only included patients with fibrosis stages F2–F3 in the primary outcome analysis, whereas we included patients of any fibrosis stage including compensated cirrhosis. 14 Our study did not provide support for a protective effect of GLP1 agonists in the subgroup with compensated cirrhosis, which is in line with a recent phase II trial in patients with MASH-related compensated cirrhosis. 41
Based on the observed probability of MALO, our data indicate that a clinical trial of GLP1 agonists in patients with any chronic liver disease and type 2 diabetes using 2-year MALO risk as the outcome would demand an immense number of patients, at least 10 776 patients per arm. This can be contrasted to the ongoing phase III trial of semaglutide looking at both histopathological endpoints (after 1.5 years) and MALO (after 4.5 years) that is planning to recruit 1200 patients with MASH (identifier NCT04822181 ). The emulation of a target trial is an appealing option to give timely answers to key research questions when data from large clinical trials are currently unavailable. 32 The difference between our intention-to-treat and per-protocol estimates is that many initiators stopped treatment without one of our prespecified contraindications. Patients who ended their treatment might have done so for good reasons, for example, severe gastrointestinal symptoms, but we were unable to capture this in our data. Moreover, since we lacked data to specifically determine whether GLP1 agonists were indicated for the non-initiators during follow-up, we assumed that it was indicated for all non-initiators that started treatment. Therefore, none of the non-initiators were censored for not adhering to protocol, whereas many initiators stopped treatment and were then censored in the per-protocol analysis. Large observational studies including detailed data with relevance for the choice of pharmacological treatment in type 2 diabetes, such as glycated haemoglobin, are warranted.
The main strength of our study was the use of an emulated target trial design to overcome common biases in observational analyses, including immortal time bias. 34 In fact, bias in observational studies often arise predominantly due to poor study design, rather than confounding due to lack of randomisation, and estimates from carefully emulated target trials closely resemble those from randomised trials. 36 There were two reasons why we designed a target trial that compared GLP1 agonists to non-initiators rather than an active comparator, another common approach. 42–44 First, it would have asked a different research question, that is, whether MALO risk differs from the active comparator. An ideal active comparator has an identical indication as the drug of interest and no effect on the outcome, but the effect of other antidiabetic medications on MALO risk is mostly unknown. Second, the average treatment effect in the whole study population is not identifiable when comparing to an active comparator. 45 We additionally fitted an inverse-probability weighted marginal structural model to account for possible time-varying confounders associated with adherence when estimating the per-protocol effect. Whereas the intention-to-treat effect is the most used causal contrast in clinical trials, the per-protocol analysis indicates what the effect of a treatment strategy would be if adhered to, which is of great importance in the real-world when physicians and patients decide on an appropriate treatment strategy. 38 When there is feedback between time-varying confounders and treatments, then standard regression models (eg, Cox) will produce biased estimates. 39 The family of g methods has been developed for this purpose (including inverse-probability weighted marginal structural models and standardisation). 39 In addition, we used the validated population-based Swedish national healthcare registers to minimise selection bias and estimate the long-term effect of GLP1 agonists. 23–25 29–31
Some limitations should be acknowledged. Despite a median follow-up of 5–6 years, few initiators experienced MALO, yielding estimates with low precision and preventing estimation of per-protocol effects in subgroups. The low number of events in initiators during late follow-up (only one event in the last 4 years and none in the last 2 years) could possibly explain part of the per-protocol effect, however, the RRs were stable across time from year 6 and forward. Additionally, we lacked data on some important covariates. First, we had no data on fibrosis stage, beyond classifying patients as cirrhotic (F4) or non-cirrhotic (F0–F3). Trials in MASLD are usually confined to patients with F2–F3, or cirrhosis, but we likely included some patients with F0–F1 where MALO is unlikely to occur. Second, we had no laboratory data, such as glycated haemoglobin, to assess diabetes severity and need for escalating to second-line treatment with GLP1 agonists. Diabetes duration, microvascular complications and other antidiabetic medications were, however, used as proxies for diabetes severity. The E-value suggested that an unmeasured confounder would need to increase the probability of both initiating a GLP1 agonist and experiencing MALO more than threefold to fully explain the estimated per-protocol effect. 40 This suggests that the observed RR in the per-protocol analysis might be explained by residual confounding (eg, fibrosis stage). For example, a study of patients with biopsy-proven MASLD found that patients with F3 had a fourfold higher hazard of MALO than patients with F0. 17 However, to fully explain the estimated effect, an unmeasured confounder would also need to be three times more likely to occur in either group. The presence of compensated cirrhosis was balanced between groups and other parameters associated with fibrosis such as age and cardiovascular disease were also well balanced and thus suggests that large differences in fibrosis are unlikely. Additionally, we sampled 5% of non-initiators to increase computational efficiency. Point estimates were, however, similar when including all non-initiators.
In conclusion, the risk of MALO in patients with chronic liver diseases and type 2 diabetes was lower if they initiated a GLP1 agonist and adhered to this treatment over time. The data were, however, not compatible with a protective intention-to-treat effect. Randomised trials using MALO as an outcome might be unfeasible, motivating further large observational studies using appropriate methodology to further delineate the effect of GLP1 agonists on the risk of MALO, complementing future phase III trials of GLP1 agonists.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
This study involves human participants and the Regional Ethics Board of Stockholm approved the study and waived informed consent (protocol number 2017/1019-31/1).
Acknowledgments
AW was supported by the Syskonen Svensson foundation for medical research (2021-00284), Mag-tarmfonden, the Bengt Ihre foundation (SLS-973809), Professor Nanna Svartz foundation (2022-00448) and the Stockholm County Council (FoUI-985859). AAM was supported by the Strategic Research Program in Epidemiology at Karolinska Institutet, Forte (2020-00029), and the Swedish Research Council (2021-02236). HH was supported by the Stockholm County Council (FoUI-960537), the Swedish Research Council (2021-01293) and the Swedish Cancer Society (22210 Pj01H).
- Collaborators GBDC
- Charette JH , et al
- Younossi ZM ,
- de Avila L , et al
- Koenig AB ,
- Abdelatif D , et al
- Anstee QM ,
- Arias-Loste MT , et al
- Tesfai K , et al
- Björkström K ,
- Franzén S ,
- Eliasson B , et al
- Elkrief L ,
- Rautou PE ,
- Sarin S , et al
- Rao Kondapally Seshasai S ,
- Kaptoge S ,
- Thompson A , et al
- Wilding JPH ,
- Batterham RL ,
- Calanna S , et al
- Andreadis P ,
- Karagiannis T ,
- Malandris K , et al
- Konerman MA ,
- Harrison SA
- Armstrong MJ ,
- Aithal GP , et al
- Newsome PN ,
- Buchholtz K ,
- Cusi K , et al
- Dufour J-F ,
- Bugianesi E , et al
- Patel J , et al
- Hagström H ,
- Ekstedt M , et al
- Pirola CJ , et al
- Israelsen M ,
- Detlefsen S , et al
- Wester A , et al
- Westergren K ,
- Holmberg L , et al
- Ludvigsson JF ,
- Almqvist C ,
- Bonamy A-KE , et al
- Andersson E ,
- Ekbom A , et al
- Otterblad-Olausson P ,
- Pettersson BU , et al
- Svedberg P ,
- Olén O , et al
- Wettermark B ,
- Fored CM , et al
- Brooke HL ,
- Talbäck M ,
- Hörnblad J , et al
- Bengtsson B ,
- Askling J ,
- Ludvigsson JF , et al
- Hernán MA ,
- Matthews AA ,
- Islam N , et al
- Hernández-Díaz S , et al
- García-Albéniz X ,
- Dickerman BA ,
- Logan RW , et al
- VanderWeele TJ ,
- Abdelmalek MF ,
- Armstrong MJ , et al
- Abrahami D ,
- McDonald EG ,
- Schnitzer ME , et al
- Huitfeldt A ,
- Hernan MA ,
- Kalager M , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
AAM and HH are joint senior authors.
X @wester_axel, @YingShang1, @hanneshagstrom
Correction notice This article has been corrected since it published Online First. The senior author statement has been added.
Contributors Contributor and guarantor information:
Study conception and design: All
Acquisition of data: HH
Statistical analysis: AW and AAM.
Analysis and interpretation of data: All
Drafting of manuscript: AW.
Critical revision: All
Guarantors of article: AW and HH.
Funding AW was supported by the Syskonen Svensson foundation for medical research (2021-00284), Mag-tarmfonden, the Bengt Ihre foundation (SLS-973809), Professor Nanna Svartz foundation (2022-00448), and the Stockholm County Council (FoUI-985859). AAM was supported by the Strategic Research Program in Epidemiology at Karolinska Institutet, Forte (2020-00029), and the Swedish Research Council (2021-02236). HH was supported by the Stockholm County Council (FoUI-960537), the Swedish Research Council (2021-01293), and the Swedish Cancer Society (2 2210 Pj 01 H). The funders had no role in the conduct of this study.
Disclaimer The funders had no role in the conduct of this study.
Competing interests HH: institution has received research grants from Astra Zeneca, EchoSens, Gilead, Intercept, MSD, Novo Nordisk and Pfizer, all outside the current study. The other authors declare no conflicts.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Linked Articles
- Commentary Glucagon-like peptide-1 receptor agonists to treat chronic liver disease: real-world evidence or ambiguity? Samy Suissa Ruben Hernaez Gut 2024; 73 721-724 Published Online First: 18 Mar 2024. doi: 10.1136/gutjnl-2024-331976
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Diabetes articles from across Nature Portfolio
Diabetes describes a group of metabolic diseases characterized by high blood sugar levels. Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes) or by insulin resistance (cells do not respond to insulin; type 2 diabetes).
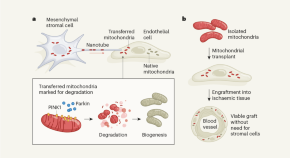
Cells destroy donated mitochondria to build blood vessels
Organelles called mitochondria are transferred to blood-vessel-forming cells by support cells. Unexpectedly, these mitochondria are degraded, kick-starting the production of new ones and boosting vessel formation.
- Chantell S. Evans
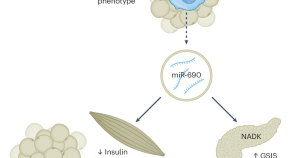
Macrophage vesicles in antidiabetic drug action
Thiazolidinediones (TZDs) are potent insulin-sensitizing drugs, but their use is accompanied by adverse side-effects. Rohm et al. now report that TZD-stimulated macrophages release miR-690-containing vesicles that improve insulin sensitization and bypass unwanted side-effects.
- Rinke Stienstra
- Eric Kalkhoven
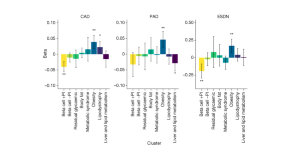
Genetic risk variants lead to type 2 diabetes development through different pathways
The largest genome-wide association study for type 2 diabetes so far, which included several ancestry groups, led to the identification of eight clusters of genetic risk variants. The clusters capture different biological pathways that contribute to the disease, and some clusters are associated with vascular complications.
Related Subjects
- Diabetes complications
- Diabetes insipidus
- Gestational diabetes
- Type 1 diabetes
- Type 2 diabetes
Latest Research and Reviews
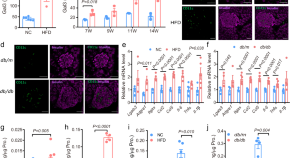
Galectin-3 impairs calcium transients and β-cell function
Galectin-3, mainly produced and secreted by macrophages, is elevated in diabetes. Here, the authors show that galectin-3 directly interacts with voltage-gated channel auxiliary subunit gamma 1 (CACNG1) and blocks calcium transients and subsequent insulin secretion.
- Pingping Li
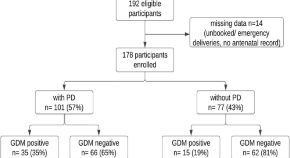
Association of periodontal disease with gestational diabetes mellitus among postpartum women at a private tertiary care hospital of Karachi, Pakistan: a cross-sectional study
- Wafa Zehra Jamal
- Farhan Raza Khan
- Shafquat Rozi
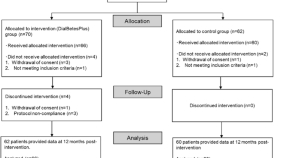
Effectiveness of DialBetesPlus, a self-management support system for diabetic kidney disease: Randomized controlled trial
- Mitsuhiko Nara
- Kazuhiko Ohe
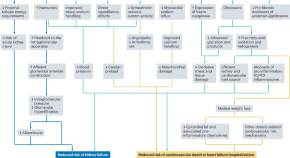
Applications of SGLT2 inhibitors beyond glycaemic control
Here, the authors discuss the beneficial effects of sodium–glucose cotransporter 2 (SGLT2) inhibitors for a range of clinical outcomes beyond glucose lowering, including kidney and cardiovascular protection. They also discuss the need for implementation and adherence initiatives to help translate the benefits of these agents into real-world clinical outcomes.
- Daniel V. O’Hara
- Carolyn S. P. Lam
- Meg J. Jardine
Novel PLGA-encapsulated-nanopiperine promotes synergistic interaction of p53/PARP-1/Hsp90 axis to combat ALX-induced-hyperglycemia
- Rishita Dey
- Sudatta Dey
- Asmita Samadder
Butyrate and iso-butyrate: a new perspective on nutrition prevention of gestational diabetes mellitus
- Weiling Han
- Guanghui Li
News and Comment
Repurposing a diabetes drug to treat Parkinson’s disease
In a multicenter clinical trial, patients with early-stage Parkinson’s disease treated with lixisenatide, a drug currently used for the treatment of diabetes, showed improvement in their motor scores compared with those on placebo.
- Sonia Muliyil
A novel system for non-invasive measurement of blood levels of glucose
- Olivia Tysoe
Diabetes drug slows development of Parkinson’s disease
The drug, which is in the same family as blockbuster weight-loss drugs such as Wegovy, slowed development of symptoms by a small but statistically significant amount.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 10, Issue 3
- Thrice daily consumption of a novel, premeal shot containing a low dose of whey protein increases time in euglycemia during 7 days of free-living in individuals with type 2 diabetes
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-7275-4379 Kieran Smith 1 ,
- http://orcid.org/0000-0002-5207-1498 Guy S Taylor 1 ,
- Lise H Brunsgaard 2 ,
- Mark Walker 3 ,
- Kelly A Bowden Davies 1 , 4 ,
- Emma J Stevenson 1 ,
- http://orcid.org/0000-0003-2246-4925 Daniel J West 1
- 1 Population Health Sciences Institute , Newcastle University , Newcastle upon Tyne , UK
- 2 Health and Performance Nutrition , Arla Foods Ingredients Group P/S , Viby J , Denmark
- 3 Biosciences Institute , Newcastle University , Newcastle upon Tyne , UK
- 4 Sport and Exercise Sciences , Manchester Metropolitan University , Manchester , UK
- Correspondence to Dr Daniel J West; daniel.west{at}newcastle.ac.uk
Introduction During acute feeding trials, consuming a large dose of whey protein (WP) before meals improves postprandial glucose regulation in people with type 2 diabetes. It is unclear if the reported benefits of premeal WP supplementation are translatable to everyday care or are associated with clinically meaningful, real-world glycemic outcomes. This study examined the application of a novel, premeal shot containing a low dose of WP on parameters of free-living glycemic control in people with type 2 diabetes.
Research design and methods In a randomized, placebo-controlled, single-blind crossover design, 18 insulin naive individuals with type 2 diabetes ((mean±SD) age, 50±6 years; HbA 1c (glycated hemoglobin), 7.4%±0.8%; duration of diabetes, 6±5 years) consumed a ready-to-drink WP shot (15 g of protein) or a nutrient-depleted placebo beverage 10 min before breakfast, lunch, and dinner over a 7-day free-living period. Free-living glucose control was measured by blinded continuous glucose monitoring and determined by the percentage of time spent above range (>10 mmol/L), in euglycemic range (3.9–10.0 mmol/L), below range (<3.9 mmol/L) and mean glucose concentrations.
Results Mealtime WP supplementation reduced the prevalence of daily hyperglycemia by 8%±19% (30%±25% vs 38%±28%, p<0.05), thereby enabling a 9%±19% (~2 hours/day) increase in the time spent in euglycemia (p<0.05). Mean 24-hour blood glucose concentrations were 0.6±1.2 mmol/L lower during WP compared with placebo (p<0.05). Similar improvements in glycemic control were observed during the waken period with premeal WP supplementation (p<0.05), whereas nocturnal glycemic control was unaffected (p>0.05). Supplemental compliance/acceptance was high (>98%), and no adverse events were reported.
Conclusions Consuming a novel premeal WP shot containing 15 g of protein before each main meal reduces the prevalence of daily hyperglycemia, thereby enabling a greater amount of time spent in euglycemic range per day over 7 days of free-living in people with type 2 diabetes.
Trial registration number ISRCTN17563146 ; www.isrctn.com/ISRCTN17563146
- Diabetes Mellitus, Type 2
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjdrc-2022-002820
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Postprandial glycemic excursions are predominant contributors to overall glycemic control. During laboratory-based feeding trials, the consumption of whey protein prior to a meal reduces postprandial glycemia in individuals with type 2 diabetes.
It is unclear if the reported benefits of premeal whey protein supplementation are translatable to everyday care or are associated with clinically meaningful glycemic outcomes.
WHAT THIS STUDY ADDS
This is the first study to investigate the application of a bespoke-made, premeal shot containing a low dose of whey protein hydrolyzate on parameters of day-to-day glycemic control in free-living people with type 2 diabetes.
Our study shows that consuming a small amount of whey protein before each main meal reduced the daily time spent in hyperglycemia in adults with type 2 diabetes, compared with the ingestion of placebo treatment.
The reduction in hyperglycemia enabled a 2-hour increase in time spent within euglycemia per day and reduced mean daily glucose concentrations without compromising the risk of hypoglycemia.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
The prevalence of hyperglycemia remains an underappreciated problem for people with controlled type 2 diabetes treated with available therapies.
Given the financial implications associated with antihyperglycemic pharmacotherapies, the provision of premeal whey protein supplementation may offer an effective non-pharmaceutical approach to regulate glycemia.
Introduction
For people with controlled type 2 diabetes (T2D), the regulation of postprandial glycemia (PPG) is essential to achieving optimal glycemic control, 1 2 which may reduce the risk of complications associated with hyperglycemia. 3 4 Indeed, PPG excursions are predominant contributors to overall glycemic control, as measured by glycated hemoglobin (HbA 1c ). 1 2 However, there is growing recognition that PPG are not only determinants of HbA 1c but are also an independent cardiovascular risk factor, 5 6 thus supporting the development of strategies that limit PPG excursions. Given the reluctance from patients to initiate new antihyperglycemic medications or to intensify existing treatment regimens, 7 the use of non-pharmaceutical approaches to regulate PPG is desirable.
Nutrition plays an integral role in the management of T2D 8 and represents an opportunity to optimize glycemic control in a cost-effective manner. 9 We 10 and others 11–13 have demonstrated that consuming whey protein (WP) at a fixed interval before a main meal (preload) effectively reduces PPG excursions in individuals with T2D. The consumption of a WP preload stimulates the early and sustained release of insulin and several gut peptides, including glucagon-like peptide 1 (GLP-1), and delays gastric emptying, thereby reducing the glycemic response to a meal. However, despite promising acute evidence, the application of mealtime WP on key clinical outcomes is unclear. For instance, although one study reported a statistically significant, but clinically modest, reduction in HbA 1c following the chronic application of premeal WP, 14 HbA 1c is unable to provide insight into day-to-day glycemic variability or the frequency of hyperglycemic events. 15 There is also a wide range of possible mean glucose values and the time spent within desirable glycemic ranges with a given HbA 1c that limits the precision by which it can be used to detect changes in glycemic control at the individual level. 15 16 Accordingly, the utility of mealtime WP supplementation on day-to-day glucose control remains to be established.
It must be recognized that achieving meaningful and sustainable improvements in glycemic control requires approaches that are applicable and deliverable within the real world. 17 To date, WP preloads have been presented as unpalatable, powdered supplements that require dilution and mixing with flavoring immediately prior to their consumption. 10–13 This often produces solutions that are large in volume and cumbersome in their delivery. 18 While such approaches have proven effective under tightly controlled clinical trials, the degree to which these results can be extrapolated into everyday care is uncertain. 19 Indeed, there is a general unwillingness to consume powdered protein supplements publicly 20 with patient embarrassment and challenging social conditions highlighted as key deterrents to adherence to diabetes treatments. 21 To maximize the real-world application of mealtime WP supplementation, premeal interventions need to be translatable into treatments that are compatible with contemporary living.
In the present study, we examined the application of a novel, ready-to-drink WP preload that was created specifically for free-living glucose management over 7 days of free-living in individuals with T2D. Given PPG excursions are predominant contributors to overall glycemic control, 1 2 we hypothesized that thrice daily mealtime WP supplementation would reduce daily hyperglycemic exposure, thereby increasing the time spent in euglycemia, as measured by continuous glucose monitoring (CGM).
Research design and methods
Participants.
Patients, recruited by study advertisements, were aged 30–60 years with a duration of diabetes of ≥1 year, treated with lifestyle and/or oral antihyperglycemic medications, which were stable for ≥3 months preceding study enrollment. All participants were required to have an HbA 1c of <80 mmol/mol (9.5%) and be of stable body mass and with a body mass index of ≤40 kg/m 2 . Individuals treated with injectable therapies (exogenous insulin and GLP-1 receptor agonists) and those with a history of gastrointestinal disease or a requirement for medications known to affect gastrointestinal function or appetite were excluded. Respondents who met our inclusion criteria were invited to attend our Newcastle National Institute for Health Research Clinical Research Facility (Newcastle upon Tyne Hospitals, Newcastle upon Tyne, UK) for a screening visit. All participants provided their written informed consent prior to enrollment into the study in accordance with Good Clinical Practice. Participant recruitment and testing were conducted between March 2019 and September 2021.
Study design
Patients entered into a single-blind, randomized, placebo-controlled, crossover design assessing the influence of premeal WP consumption on free-living glycemic control. In a counterbalanced order, patients randomly consumed a WP-rich, ready-to-drink shot (WP containing 15.6 g of dietary protein) or a protein-depleted placebo (PLA) shot 10 min before each of their main meals over a 7-day free-living period. Treatment sequences were determined by an online randomizer ( www.randomization.com ) that randomly assigned trial order in a balanced permutation. Free-living glycemic control was measured by a blinded CGM (Dexcom G6; Dexcom, San Diego, California, USA) that was implanted into subcutaneous tissue of the anterior-medial aspect of the lower abdomen. This device has a reported mean absolute relative difference of ~10% compared with a reference instrument and demonstrates consistent accuracy throughout its 10-day lifespan. 22 Dexcom CGMs were fitted ~48 hours prior to the start of the trial and were removed in-clinic on completion of each free-living week. To account for compression artifacts, the anatomical location of CGM placement was chosen on the participants’ non-dominant sleeping side; this anatomical site was kept consistent throughout. Physical activity patterns were quantified by a wrist-worn activity monitor (GeneActiv, ActivInsights, UK). An ~14-day washout period separated each free-living phase. All medications were kept stable and unaltered throughout the study.
Throughout the duration of the intervention, participants were instructed to make no changes to their habitual dietary or physical activity patterns. Although the consumption of alcohol was permitted during this study, participants were asked to refrain from consuming excessive amounts of alcoholic beverages, which was defined as consuming alcohol (>3 alcoholic beverages per day) >3 days per week. The duration of the study was scheduled to coincide with periods representing participant’s normal daily life (ie, there were no planned activities, vacations, or unusual bouts of strenuous activity).
Free-living dietary intake was assessed by completion of an online, multipass 24-hour dietary recall system ( https://intake24.co.uk/ ). Dietary recalls were completed daily with participants submitting a log of all foods and drinks consumed from the previous 24 hours. All foods within the Intake24 system are linked to the UK National Diet and Nutrition Survey database. Participants were given detailed instructions on how to use this application prior to study enrollment. Paper supplement logs were also completed to document the timings of both supplement consumption and the commencement of the main meals. This paper log was used to identify self-reported postprandial events and to cross-reference with the timing of meals submitted by the Intake24 application; the latter was used to measure supplement adherence. Participants did not include the WP or PLA shots into their dietary recalls.
Intervention
Patients were instructed to consume a WP or PLA preload shot 10 min prior to each of their main meals (breakfast, lunch, and dinner) over a 7-day free-living period. The premeal WP and PLA shots were produced by Arla Foods Ingredients Group P/S (Viby J, Denmark) specifically for free-living glucose management. Both treatments were stable at both temperate and chilled environmental conditions and had a shelf-life of 6 months. The premeal drinks were presented in a contemporary, ready-to-drink format as a 100 mL beverage ‘shot’ and were of similar viscosity and texture. To account for any subtle differences in mouthfeel and to maintain supplement blinding, the premeal supplements were presented as two different flavors: WP, cocoa-cappuccino; PLA, strawberry. The premeal WP treatment used a hydrolyzed WP ingredient (Lacprodan DI-6820; Arla Food Ingredients Group P/S) to produce a palatable, ready-to-drink beverage. Each WP shot (418 kJ) contained 15.6 g of dietary protein, and a small amount of dietary carbohydrates (3 g) and fat (2.3 g) from 100 mL of low-viscosity liquid. Further detailed information regarding the product development of the novel WP shot has been published elsewhere. 23 The PLA shot (<142 kJ) contained small amounts of dietary carbohydrate (3.9 g) and fat (2.2 g), with negligible protein content (<0.1 g).
Interstitial glucose concentrations were measured every fifth minute (288 values per day) over a period of 7 days. CGM data were accepted if >90% of the available daily data and >70% of the available weekly data were collected. 24 Data from the Dexcom CGMs were stored using the Dexcom Clarity Professional web software (Dexcom, USA). Physical activity data were converted into 15 s epoch files using the GENEActiv PC software V.3.2, which were subsequently analyzed using Microsoft Excel Macro files provided by the manufacturer. Energy and macronutrient intake collected by the Intake24 dietary recall were exported as a Microsoft Excel spreadsheet.
The primary outcome of this study was the time spent in hyperglycemia (>10 mmol/L) during a 7-day free-living period. In addition to the primary outcome, secondary outcomes included the time spent in euglycemic range (TIR), time below range, time above range, PPG events (incremental area under the curve (iAUC) and the peak incremental change in glucose (∆PPG)), measures of intraday (coefficent of variance (%CV), mean amplitude of glycemic excursions (MAGE)) and interday (mean of daily differences (MODD)) glycemic variability, and indices of hypoglycemic and hyperglycemic risk (low blood glucose index and high blood glucose index, respectively). Glucose management indicator (GMI), which gives an approximation of HbA 1c based on the average glucose levels collected by CGM, was also calculated. 25
Glycemic ranges were defined as <3.0 mmol/L (time below range level 1), 3.0–3.8 mmol/L (time below range level 2), 3.9–7.8 mmol/L (time in tight euglycemic range), 3.9–10.0 mmol/L (TIR), 10.0–13.9 mmol/L (time above range level 1), and >13.9 mmol/L (time above range level 2). 24 26 %CV was calculated as the SD divided by mean glucose multiplied by 100. GMI (mmol/mol) was calculated using the formula proposed by Bergenstal et al . 25 Risk indices for high and low blood glucose, MAGE, and MODD were computed using an automated software package (EasyGV V.9.0R2; University of Oxford, UK). Daytime and nocturnal glycemia were defined as 06:00–23:55 hours and 24:00–05:55 hours, respectively. 26 The iAUC for cumulative PPG events was calculated using the trapezoidal rule, depicting the area above baseline concentrations, which was accepted as the reported timing of consumption of the preload. PPG excursions were accepted if there were no self-reported eating occassions within 120 min following the commencement of the meal. Where the time of meal commencement could not be established, the data were excluded from analysis. PPG events were identified and averaged per individual: that is, if 10 iAUC events were identified, the iAUC was calculated per event and then averaged per 10 to give a single iAUC event.
Sample size
A sample size estimate was made using interstitial glucose collected during a 6-hour laboratory visit from preliminary data. 10 To detect a difference of at least 10% in time spent above 10 mmol/L in the postprandial period, 18 participants were required to fully complete two trials (WP vs PLA), to test the null hypothesis that the population means are equal across trials with a probability of 0.8 and a type 1 error of 0.05. Sample size calculation was completed using Stata, with PLA mean time above 10 mmol/L at 63.0% vs 51.5% following WP (SD of the mean differences of 16%).
Statistical analysis
All data were assessed for normal distribution by a Shapiro-Wilks test. Non-parametric data were logarithmically transformed and reassessed for distribution. Where transformation failed, data were assessed non-parametrically. Paired sampled t tests or Wilcoxon signed-rank test were used to explore treatment differences on variables that displayed normal or non-normal distribution, respectively. Inferential statistics were conducted using the software package IBM SPSS Statistics (V.27; IBM, USA). Significance was set at alpha p<0.05. Treatment differences in the time spent in glycemic ranges are expressed as the absolute percentage point change. Data are presented as means±SD unless stated otherwise.
The Consolidated Standards of Reporting Trials flow diagram is shown in online supplemental figure 1 . A total of 26 participants were recruited for this study. From this cohort, eight participants were withdrawn for the following reasons: one participant withdrew their consent prior to randomization; three participants were withdrawn due abnormal laboratory findings (laboratory measured HbA 1c >80 mmol/mol (9.5%)); two participants were withdrawn due to the COVID-19 pandemic and the cessation of research activities during March–September 2020; one due to a change in glucose-lowering medication during the trial; and one due to non-adherence. Therefore, data are analyzed and presented on n=18. Patient characteristics are presented in table 1 . In brief, participants had a mean HbA 1c of 57.4±9.2 mmol/mol (7.4%±0.8%) and a self-reported diabetes duration of 6.2±4.9 years. All participants were of white Europid descent. The most common antihyperglycemic treatments were either metformin monotherapy (n=5, 28%) or the combination of metformin and a sulfonylurea (n=6, 33%). Hypertensive and statin therapies were prescribed for 78% (n=14) and 56% (n=10) of participants, respectively. Five of the 18 participants were women (28%).
Supplemental material
- View inline
Patient characteristics
Glycemic control
Mean 24-hour glucose control.
Key glycemic parameters during 7 days of free-living are presented in figure 1 and table 2 . During the PLA free-living week, patients spent 38.1%±28.4% of the 24- hour period at blood glucose >10.0 mmol/L. The prevalence of daily hyperglycemia (>10.0 mmol/L) was reduced by −8.3%±19.3% following treatment with mealtime WP supplementation ( figure 1A ), resulting in −117±276 min less per 24 hours spent in hyperglycemia (p=0.024). The subsequent reduction in hyperglycemic exposure enabled patients to achieve an increase in TIR of +8.7%± 19.0%, compared with PLA (p=0.035; figure 1B ). Within this euglycemic range, premeal WP supplementation increased the time spent between glucose concentrations of 3.9–7.8 mmol/L by +8.8%±14.7% (p=0.022), corresponding to an average increase of +127±210 min per day ( figure 1C ). The time spent within hyperglycemic levels 1 and 2 were numerically, but not statistically (p=0.089 and p=0.352, respectively), lower following the WP preload ( table 2 ).
- Download figure
- Open in new tab
- Download powerpoint
The mean±SD percentage of time spent per day in (A) hyperglycemia (>10.0 mmol/L), (B) euglycemia (3.9–10.0 mmol/L), and (C) normoglycemia (3.9–7.8 mmol/L) during 7 days of free-living with premeal supplementation of a whey protein (WP) (blue circles) or placebo (PLA) (red circles) preload. Panel (D) depicts the per patient delta change in the percentage of time spent >10.0 mmol/L with premeal WP supplementation relative to PLA. *Denotes a statistical treatment effect as measured by a paired samples t test or Wilcoxon signed rank test (p<0.05).
Free-living glycemic control parameters
Patient’s GMI, which is an estimate of overall glycemic control, was reduced by −2.9±5.6 mmol/mol during the WP treatment arm compared with PLA (p=0.045), indicative of improved glycemic control and a reduction in hyperglycemia ( table 2 ). Indeed, relative to PLA, mean 24-hour glucose concentrations were −0.6±1.2 mmol/L lower following the premeal WP treatment (p=0.045). Despite a reduction in mean 24-hour glucose concentrations and time spent >10.0 mmol/L, this was not accompanied by an increase in time below range or low blood glucose index (all p>0.317). Markers of glycemic variability were similar between treatments ( table 2 ).
Diurnal and nocturnal glucose control
Mean glucose concentrations during the waken hours (06:00–23:55 hours) were −0.7±1.2 mmol/L lower during the WP week compared with PLA (p=0.023). Similarly, TIR was +8.7%±20.5% greater after treatment with premeal WP supplementation compared with PLA (p=0.048), and correspondingly, less time was spent in hyperglycemia (−8.9%±20.9%; p=0.048). On the other hand, parameters of glycemic control during the nocturnal period (24:00 –05:55 hours) were comparable between treatments ( online supplemental table 1 ).
Postprandial glucose control
A total of n=652 postprandial events were identified and analyzed. The number of postprandial events were similar between treatments (WP, n=321; PLA, n=331). Relative to PLA, PPG iAUC 0–120min were −24%±29% lower during the WP free-living week (p=0.003). On average, ∆PPG was reduced by −0.7±0.9 mmol/L (p=0.007). The within-subject timing of meals were similar between free-living weeks (mean difference, 27±24 min), as shown in online supplemental figure 2 .
Energy intake
There was a modest reduction in self-reported energy intake during the WP week compared with PLA (−631±1314 kJ per day), although this was statistically insignificant (p=0.057). When accounting for the energy content associated with the preloads, cumulative energy intake was similar between free-living weeks (p=0.635).
Physical activity
There were no differences in the time spent at differing physical activity levels between the WP and PLA weeks (p>0.605).
All participants tolerated the PLA and WP treatments well and reported no gastrointestinal side effects. Supplemental compliance during the intervention weeks was exemplary (WP, 97.5%±2.4%; PLA, 99.3%±2.4%).
This study examined the application of premeal WP supplementation on daily glycemic control over 1 week in people with T2D. For the first time, we demonstrate that daily hyperglycemia can be significantly reduced by the provision of a low dose of WP (15 g) ingested prior to each main meal over 7 days of free-living. This enabled patients to achieve 2 hours more per day spent within euglycemia, without increasing the risk of hypoglycemia. These results occurred without a change in patient medication, dietary intake, or physical activity levels, thereby demonstrating the utility of premeal WP supplementation for the management of hyperglycemia.
The present study extends from previous acute laboratory work highlighting the PPG-lowering efficacy of premeal WP for people with T2D. 10–13 However, for the first time, we report the translatability and reproducibility of these findings under real-world and free-living conditions. It was found that premeal ingestion of our novel WP shot effectively mitigated free-living PPG excursions, thereby reducing the prevalence of hyperglycemia by ~2 hours per day, compared with PLA. This subsequently enabled an absolute daily increase in TIR of 8.7%, the magnitude of which is substantial. Indeed, an increase in TIR of ≥5% is considered clinically significant 27 and may be associated with a reduced risk of developing vascular complications. 28 29 For instance, for every 10% reduction in TIR (roughly equivalent to the increase in TIR in the present study with mealtime WP supplementation), the risk of developing retinopathy or microalbuminuria was increased by 64% and 40%, respectively, in the Diabetes Control and Complications Trial cohort. 29 A similar 10% reduction in TIR has also been associated with a 13% and 25% increased risk of developing cardiovascular autonomic neuropathy and peripheral neuropathy, respectively, in people with long-standing T2D. 30 31 Promisingly, the preload treatments were well accepted among participants, as demonstrated by their exemplary compliance, and no adverse side effects were reported supporting the application of this novel mealtime therapy.
The findings presented herein are of interest given our data demonstrate that the intervention of available oral antihyperglycemic medications are insufficient in the protection against hyperglycemia. Current recommendations state that people with T2D should strive for <6 hours per day at glucose concentrations >10.0 mmol/L. 24 However, despite the continued use of patient’s antihyperglycemic agents, our cohort spent up to 9 hours (38%) of the day at glucose concentrations >10.0 mmol/L during the PLA free-living week. This observation is similar to what has been reported by others 32 and underscores the need for further strategies designed to reduce the prevalence of hyperglycemia in people with T2D.
Our primary finding that premeal WP supplementation reduces the prevalence of hyperglycemia corroborates with data demonstrating a reduction in overall hyperglycemia following the application of PPG-lowering therapies. 33 Indeed, our observed reduction in time spent >10.0 mmol/L derived from the waken/feeding period, as shown by the reduction in diurnal but not nocturnal hyperglycemia. Although the free-living nature of our study makes it difficuilt to discern mechanisms associated with our findings, prior literature has demonstrated that a WP preload of similar amounts (15–20 g) elevates GLP-1 above preprandial concentrations for ~180 min following ingestion of a meal. 12 13 23 The ingestion of WP also modestly stimulates the secretion of glucose-dependent insulinotropic polypeptide (GIP) 12 ; though the relevance of this to the observed improvement in glycemic control is likely minimal since endogenous GIP has little to no effect on PPG in individuals with T2D. 34 Herein, it is possible that diurnal increases in GLP-1 secretion from thrice daily WP supplementation may have enhanced β-cell glucose sensitivity and delayed the rate of gastric emptying, thereby slowing the systemic appearance of meal-derived glucose and augmenting an efficient islet response. 35 36 These effects are consistent with what was previously observed following adminstration of a protein preload given prior to an oral glucose load, 37 supporting this assertion. Nonetheless, literature examining the glucoregulatory effects of premeal WP have been conducted solely following an overnight fast. Considering the regulation of PPG displays a clear circadian pattern, 38 whether a low dose of mealtime WP supplementation is sufficient to augmenting a PPG-lowering milieu to meals consumed later in the day is unclear and requires future study.
The present analyses demonstrates that the addition of premeal WP to patient care has the potential to reduce daily hyperglycemia and increase TIR without increasing the risk of hypoglycemia, as shown by the low blood glucose index and time spent below range. An increase in TIR is also suggestive that the frequency of erratic glycemic swings were reduced with mealtime WP. 39 Fluctuations in glycemia are posited to be implicated in the pathogenesis of diabetes-related vascular complications, supporting the development of strategies designed to reduce glycemic variability. 40 Although there were no changes in amplitude markers of glycemic variability with WP supplementation (ie, %CV), this likely reflects the relative glycemic stability of the cohort studied since all patients had a %CV <36%. 24 Nevertheless, the risk of microvascular complications has recently been shown to be inversely related to TIR, independent of %CV. 28 Therefore, although there were no changes in amplitude markers of glycemic variability with the WP treatment, this is unlikely to affect the clinical value of our results.
To the best of the authors’ knowledge, this is the first study designed and powered to examine the use of premeal WP on free-living glycemic management captured by masked CGM. Our study is rendered timely as its design benefits from incorporating current recommendations that endorse the use of CGM when assessing glycemic control at the individual level. 26 Offering ecological validity to our findings, patient’s GMI during PLA was identical to their laboratory-measured HbA 1c . This suggests that our reported data reflects a true change from participant’s habitual glycemic control. 25 In this regard, an increase in TIR of ~9% with premeal WP supplementation is projected to confer a ~5–7 mmol/mol (0.6%) reduction in HbA 1c . 16 27 This assertion is supported by our findings of a reduction in mean daily glucose concentrations (−0.6 mmol/L). In the context of available treatments for T2D, the magnitude of this projected reduction in HbA 1c is akin to what would be expected from the adminstration of thiazolidinediones, sodium–glucose cotransporter 2 inhibitors and dipeptidyl peptidase-IV inhibitors. 41 Considering adherence to pharmacological agents can be poor, 7 the data presented herein may hold important implications for the management of hyperglycemia.
When accounting for the energy associated with the preloads, daily energy intake was similar between treatments. This was despite patients consuming an additional ~836 kJ/day when adherent to the WP shot, compared with PLA. Therefore, patients may modestly adjust their energy intake to account for the caloric load associated with a small WP preload. This is in line with previous observations that reported no change in body mass following the long-term ingestion of mealtime WP supplementation (~753 kJ/day) in people with T2D. 14 These collective findings are appealing and suggest that the adherence to a low dose of premeal WP is unlikely to compromise weight management in obese and dysglycemic populations.
There are several strengths associated with our study including our randomized, placebo-controlled, crossover design, and the counterbalanced administration of treatments to minimize treatment order effects. Furthermore, and unique to this study, patients were provided with premeal WP and PLA shots created specifically for the real-world application of mealtime WP supplementation. Nonetheless, our study is not without its limitations. First, our analyses were conducted on people of white, Europid descent; thus, the applicability of these results to other ethnic groups and races is unclear. Second, although our study was powered to test the primary outcome, we acknowledge that our analyses are conducted on a small sample of people with T2D (n=18). Since our patients were of relatively controlled diabetes and treated with oral therapies, our findings cannot be extrapolated to the wider T2D population. Finally, although our findings indicate an improvement in glycemic control with mealtime WP supplementation, the long-term evidence supporting our data is lacking with only one study to date reporting a modest improvement in HbA 1c with premeal WP supplementation. 14 Whether the results presented herein are sustainable longer term or are associated with improvements in clinically relevant end points cannot be inferred and require further investigation. Importantly, however, the glucose-lowering mechanisms by which premeal WP supplementation regulates PPG remain functionable after its chronic application 14 supporting these findings.
In summary, we show that thrice daily consumption of a novel preload shot containing a low dose of WP reduces daily hyperglycemia, increasing the time spent in euglycamia by ~2 hours per day during 7 days of free-living. This is of importance given our analysis clearly demonstrates that the prevelance of hyperglycemia is an underappreciated problem for people with controlled T2D treated with available oral medications. The provision of a contemporary WP preload shot may represent an effective sole or adjunctive therapy for the treatment of hyperglycemia, which could also have important financial implications at a time where public health budgets are constrained.
Ethics statements
Patient consent for publication.
Not required.
Ethics approval
This randomized controlled, crossover trial was conducted in line with Good Clinical Practice and the revised 1983 Declaration of Helsinki. This study involves human participants. Ethical approval was obtained from the North East–Newcastle & North Tyneside 1 Research Ethics Committee (reference: 18/NE/0372). Ethics granted: January 16, 2019. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank the study participants for their time, effort, and commitment, as well as the research teams at the Newcastle National Institute for Health Research Clinical Research Facility, Newcastle upon Tyne, for their assistance with data collection.
- Monnier L ,
- Lapinski H ,
- Woerle HJ ,
- Neumann C ,
- Zschau S , et al
- Holman RR ,
- Bethel MA , et al
- Esposito K ,
- Ciotola M ,
- Carleo D , et al
- Cavalot F ,
- Pagliarino A ,
- Valle M , et al
- Nathan DM ,
- D'Agostino RB , et al
- Polonsky WH ,
- Dennison M ,
- Gardner CD , et al
- Briggs A , et al
- Campbell MD , et al
- Stevens JE ,
- Cukier K , et al
- Little TJ ,
- Bound MJ , et al
- Watson LE ,
- Phillips LK ,
- Wu T , et al
- Connor CG ,
- Mullen DM , et al
- Bergenstal RM ,
- Cheng P , et al
- Edelman SV ,
- Polonsky WH
- Bowden Davies KA ,
- Stevenson EJ , et al
- Tan R-D , et al
- MacDonald A ,
- Lilburn M ,
- Davies P , et al
- Davies MJ ,
- Gagliardino JJ ,
- Gray LJ , et al
- Puhr SA , et al
- Taylor GS ,
- Allerton DM , et al
- Battelino T ,
- Bergenstal RM , et al
- Close KL , et al
- Battelino T , et al
- Vigersky RA ,
- Zhou J , et al
- Riddlesworth TD , et al
- Park JY , et al
- Ahmad I , et al
- van Dijk J-W ,
- Manders RJF ,
- Hartgens F , et al
- Rayner CK ,
- Phillips LK , et al
- Stensen S ,
- Gasbjerg LS ,
- Krogh LL , et al
- Vølund A , et al
- Nguyen NQ ,
- Stevens JE , et al
- Tulipani A , et al
- Lindgren O ,
- Deacon CF , et al
- Kovatchev B ,
- Ceriello A ,
- Davidson MB , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Contributors KS, EJS, and DJW designed the research. KS conducted the research, analyzed the data, and wrote the manuscript. DJW analyzed the data and wrote the manuscript. GST, MW, KABD, LHB and EJS reviewed and edited the manuscript. DJW is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of data analysis.
Funding This work was supported by a grant awarded to DJW and EJS (grant no: BH172513) from Arla Foods Ingredients Group P/S (Viby J, Denmark). Arla Foods Ingredients Group P/S produced whey protein and placebo treatments. Arla Foods Ingredients Group P/S had no role in the collection, analysis, or interpretation of data. CGM equipment were provided by an equipment award to DJW from Dexcom (San Diego, California, USA).
Competing interests DJW and EJS have received research funding, travel expenses, and consultancy fees from Arla Foods Ingredients Group P/S. EJS has received research funding from The Dairy Council. LHB is an employee of Arla Foods Ingredients Group P/S.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:

IMAGES
VIDEO
COMMENTS
Introduction. Insulin resistance and β-cell dysfunction are the 2 major hallmarks of type 2 diabetes mellitus (T2DM) that appear as the result of disturbed homeostasis [].Failure of β-cells (∼80% of their β-cell function) and insulin resistance in muscles and the liver is a vicious triumvirate responsible for the core physiological defects.
However, type 2 diabetes makes up more than 90% of diagnosed diabetes cases in the United States. 35 Thus, our findings largely reflect risk-factor treatment and control in those with type 2 diabetes.
Type 2 diabetes accounts for nearly 90% of the approximately 537 million cases of diabetes worldwide. The number affected is increasing rapidly with alarming trends in children and young adults (up to age 40 years). Early detection and proactive management are crucial for prevention and mitigation of microvascular and macrovascular complications and mortality burden. Access to novel therapies ...
Type 2 diabetes articles from across Nature Portfolio. Type 2 diabetes mellitus, the most frequent subtype of diabetes, is a disease characterized by high levels of blood glucose (hyperglycaemia ...
Type 2 Diabetes Mellitus (T2DM) is characterized by chronically elevated blood glucose (hyperglycemia) and elevated blood insulin (hyperinsulinemia). When the blood glucose concentration is 100 milligrams/deciliter the bloodstream of an average adult contains about 5-10 grams of glucose. Carbohydrate-restricted diets have been used effectively to treat obesity and T2DM for over 100 years ...
The worldwide prevalence of type 2 diabetes mellitus (T2DM) in adults has increased from ~150 million affected people in 2000 to >450 million in 2019 and is projected to rise further to ~700 ...
Despite the successful development of new therapies for the treatment of type 2 diabetes, such as glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 inhibitors, the search for novel treatment options that can provide better glycaemic control and at reduce complications is a continuous effort. The present Review aims to present an overview of novel targets and ...
In 2021, type 2 diabetes accounted for 90% of all diabetes prevalence. Most of this burden is attributable to social risk factors—such as high BMI, dietary risks, environmental and occupational risks, tobacco use, alcohol use, and low physical activity—that thrive on the obesogenic way our environments are designed and the inequitable way ...
Chatterjee, S. et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes ...
Type 2 diabetes (T2D) affects an estimated 10.5% of adults in the US, 1 with increasing prevalence in younger age groups 2,3 and approximately 21% of those individuals with diabetes being undiagnosed. 1 Without adequate treatment and management, the condition can result in blindness, kidney disease, cardiovascular diseases including atherosclerosis and heart failure, and other comorbidities ...
Type 2 diabetes accounts for nearly 90% of the approximately 537 million cases of diabetes worldwide. The number affected is increasing rapidly with alarming trends in children and young adults (up to age 40 years). ... 4 Diabetes Research Centre, University of Leicester and the Leicester NIHR Biomedical Research Centre, Leicester General ...
Prevention of Cardiovascular Disease in Type 1 Diabetes. C. Manrique-Acevedo, I.B. Hirsch, and R.H. EckelN Engl J Med 2024;390:1207-1217. More than half of newly diagnosed cases of type 1 diabetes ...
This review explores the current conventional drugs used in the treatment of type 2 DM, the associated limitations related to their usage and the cutting edge novel nanoformulations that are under continual research for circumventing the stated drawbacks of the conventional drug use. 2. Pathophysiology of diabetes.
The ADA is committed to continuing progress in the fight against type 2 diabetes by funding research, including support for potential new treatments, a better understating of genetic factors, addressing disparities, and more. For specific examples of projects currently funded by the ADA, see below. Greg J. Morton, PhD.
Description: The American College of Physicians (ACP) developed this clinical guideline to update recommendations on newer pharmacologic treatments of type 2 diabetes. This clinical guideline is based on the best available evidence for effectiveness, comparative benefits and harms, consideration of patients' values and preferences, and costs.
415 million people live with diabetes worldwide, and an estimated 193 million people have undiagnosed diabetes. Type 2 diabetes accounts for more than 90% of patients with diabetes and leads to microvascular and macrovascular complications that cause profound psychological and physical distress to both patients and carers and put a huge burden on health-care systems.
AbstractBackground:. The literature on the association between diabetes severity and cancer risk is limited and inconclusive. The study aimed to evaluate the association between the adapted Diabetes Complications Severity Index (aDCSI) and the duration of type 2 diabetes and cancer risk.Methods:. Patients ages 20 years or older with newly diagnosed type 2 diabetes between January 1, 2007, and ...
Key Points. Question What is the contemporary pattern of evidence-based pharmacotherapy use among a real-world population of US patients with type 2 diabetes and atherosclerotic cardiovascular disease?. Findings In this cohort study of 324 706 patients from health systems across the US, 58.6% of patients were receiving a statin (and a total of 26.8% of patients were receiving a high-intensity ...
The rising burden of type 2 diabetes mellitus (T2DM) is a substantial concern for health-care systems worldwide, with 1 in 11 people globally currently diagnosed with diabetes mellitus, ~90% of ...
The transition from hospital to outpatient care is a particularly vulnerable period for patients as they move from regular health monitoring to self-management. This study aimed to map and investigate the journey of patients with polymorbidities, including type 2 diabetes (T2D), in the 2 months following hospital discharge and examine patients' encounters with healthcare professionals (HCPs).
Evidence on the economic burden of type 2 diabetes has increased significantly. Studies have emerged documenting the economic costs of type 2 diabetes in low- and middle-income countries (LMICs), providing insights into its impact in underdeveloped nations. However, there is a lack of research on the labour market effects of diabetes in LMICs.
Design We used observational data from Swedish healthcare registers 2010-2020 to emulate a target trial of GLP1 agonists in eligible patients with chronic liver disease and type 2 diabetes. We used an inverse-probability weighted marginal structural model to compare parametric estimates of 10-year MALO risk (decompensated cirrhosis, hepatocellular carcinoma, liver transplantation or MALO ...
The largest genome-wide association study for type 2 diabetes so far, which included several ancestry groups, led to the identification of eight clusters of genetic risk variants.
This study examined the application of a novel, premeal shot containing a low dose of WP on parameters of free-living glycemic control in people with type 2 diabetes. Research design and methods In a randomized, placebo-controlled, single-blind crossover design, 18 insulin naive individuals with type 2 diabetes ((mean±SD) age, 50±6 years ...