- Food & Dining
- Coronavirus
- Real Estate
- Seattle History
- PNW Politics

Thesis Statements vs. Main Ideas
Related articles, how to write a unified essay, what is a circular narrative style, what narrative point of view is used in "and then there were none".
- Teacher Tips: How to Write Thesis Statements for High School Papers
- Explanation on Theme in Literature for Students
The thesis statement is a one-sentence statement that expresses the main idea of the essay. The thesis statement is an arguable statement that communicates the author’s stance on a topic to the reader. In order to better understand the differences between a thesis statement and main idea, it's important to understand the components of an essay. Essentially, it's not a “versus” situation because the thesis statement and main idea cannot exist without the other.
Purpose of the Thesis Statement
The thesis statement is the foundation of the essay because it tells the reader what the essay will be about and gives the essay direction. The goals of the thesis statement are to organize and development your argument and provide your reader with a road map or guide to your argument. The thesis statement should answer a question about the topic you are exploring as a writer. A good thesis statement will express only one main idea and will assert your conclusion about a topic.
Identifying the Main Idea
If you are given a topic to write about, it's important to first do your research and identify some main ideas associated with that topic. If you are writing about a piece of literature or specific work, you'll still need to do research but you will also need to read for key concepts or key ideas within that piece of writing first. Consider rereading the piece of literature to locate the main idea of the writer. Once you have located the main idea, you can then generate an opinion about the idea and create your thesis statement.
Developing the Main Idea
The topic of an essay is the subject, or what the essay is all about. An idea is what the writer says about the topic. The ideas include the main idea, which is then expressed in the form of a thesis statement. The main idea is not arguable like the thesis statement should be; it's simply an idea. Before writing, you should first develop the main idea because you cannot generate an arguable thesis statement about a topic until you have identified what you think of the topic.
Purpose of the Topic Sentence
The main idea of an essay is conveyed through the thesis statement and carried out through the topic sentences. An idea cannot be a statement, but a statement conveys an idea, hence the purpose of a thesis statement to state the point of an idea to convey to the reader in the development of an essay. Topic sentences also express the main idea. It's the first sentence of each subsequent paragraph in the essay and should be followed by supporting details, all supporting the topic sentence, which should support the main idea expressed in the thesis statement.
- Teacher Vision: Establishing the Main Idea
- Indiana University Bloomington: How to Write a Thesis Statement
Alyssa Sellors has been in the field of education for five years, teaching English and journalism at the high school level. In addition to teaching, she has also advised the school newspaper and currently advises the yearbook. As a yearbook adviser, she speaks at national conventions hosted by Journalism Educator’s Association and the National Scholastic Press Association.
What Is a Multi-Paragraph Essay?
How to write a college expository essay, how to start an introduction when writing an essay about poetry, what is the central conflict in "beowulf", what is the difference between an elizabethan & petrarchan sonnet, the importance of writing an effective thesis statement, what are the four tips for writing a good thesis statement for an expository essay, how to write an in-class essay, how to write a thesis statement for an autobiographical essay, most popular.
- 1 What Is a Multi-Paragraph Essay?
- 2 How to Write a College Expository Essay
- 3 How to Start an Introduction When Writing an Essay About Poetry
- 4 What Is the Central Conflict in "Beowulf"?

Thesis Statements
What this handout is about.
This handout describes what a thesis statement is, how thesis statements work in your writing, and how you can craft or refine one for your draft.
Introduction
Writing in college often takes the form of persuasion—convincing others that you have an interesting, logical point of view on the subject you are studying. Persuasion is a skill you practice regularly in your daily life. You persuade your roommate to clean up, your parents to let you borrow the car, your friend to vote for your favorite candidate or policy. In college, course assignments often ask you to make a persuasive case in writing. You are asked to convince your reader of your point of view. This form of persuasion, often called academic argument, follows a predictable pattern in writing. After a brief introduction of your topic, you state your point of view on the topic directly and often in one sentence. This sentence is the thesis statement, and it serves as a summary of the argument you’ll make in the rest of your paper.
What is a thesis statement?
A thesis statement:
- tells the reader how you will interpret the significance of the subject matter under discussion.
- is a road map for the paper; in other words, it tells the reader what to expect from the rest of the paper.
- directly answers the question asked of you. A thesis is an interpretation of a question or subject, not the subject itself. The subject, or topic, of an essay might be World War II or Moby Dick; a thesis must then offer a way to understand the war or the novel.
- makes a claim that others might dispute.
- is usually a single sentence near the beginning of your paper (most often, at the end of the first paragraph) that presents your argument to the reader. The rest of the paper, the body of the essay, gathers and organizes evidence that will persuade the reader of the logic of your interpretation.
If your assignment asks you to take a position or develop a claim about a subject, you may need to convey that position or claim in a thesis statement near the beginning of your draft. The assignment may not explicitly state that you need a thesis statement because your instructor may assume you will include one. When in doubt, ask your instructor if the assignment requires a thesis statement. When an assignment asks you to analyze, to interpret, to compare and contrast, to demonstrate cause and effect, or to take a stand on an issue, it is likely that you are being asked to develop a thesis and to support it persuasively. (Check out our handout on understanding assignments for more information.)
How do I create a thesis?
A thesis is the result of a lengthy thinking process. Formulating a thesis is not the first thing you do after reading an essay assignment. Before you develop an argument on any topic, you have to collect and organize evidence, look for possible relationships between known facts (such as surprising contrasts or similarities), and think about the significance of these relationships. Once you do this thinking, you will probably have a “working thesis” that presents a basic or main idea and an argument that you think you can support with evidence. Both the argument and your thesis are likely to need adjustment along the way.
Writers use all kinds of techniques to stimulate their thinking and to help them clarify relationships or comprehend the broader significance of a topic and arrive at a thesis statement. For more ideas on how to get started, see our handout on brainstorming .
How do I know if my thesis is strong?
If there’s time, run it by your instructor or make an appointment at the Writing Center to get some feedback. Even if you do not have time to get advice elsewhere, you can do some thesis evaluation of your own. When reviewing your first draft and its working thesis, ask yourself the following :
- Do I answer the question? Re-reading the question prompt after constructing a working thesis can help you fix an argument that misses the focus of the question. If the prompt isn’t phrased as a question, try to rephrase it. For example, “Discuss the effect of X on Y” can be rephrased as “What is the effect of X on Y?”
- Have I taken a position that others might challenge or oppose? If your thesis simply states facts that no one would, or even could, disagree with, it’s possible that you are simply providing a summary, rather than making an argument.
- Is my thesis statement specific enough? Thesis statements that are too vague often do not have a strong argument. If your thesis contains words like “good” or “successful,” see if you could be more specific: why is something “good”; what specifically makes something “successful”?
- Does my thesis pass the “So what?” test? If a reader’s first response is likely to be “So what?” then you need to clarify, to forge a relationship, or to connect to a larger issue.
- Does my essay support my thesis specifically and without wandering? If your thesis and the body of your essay do not seem to go together, one of them has to change. It’s okay to change your working thesis to reflect things you have figured out in the course of writing your paper. Remember, always reassess and revise your writing as necessary.
- Does my thesis pass the “how and why?” test? If a reader’s first response is “how?” or “why?” your thesis may be too open-ended and lack guidance for the reader. See what you can add to give the reader a better take on your position right from the beginning.
Suppose you are taking a course on contemporary communication, and the instructor hands out the following essay assignment: “Discuss the impact of social media on public awareness.” Looking back at your notes, you might start with this working thesis:
Social media impacts public awareness in both positive and negative ways.
You can use the questions above to help you revise this general statement into a stronger thesis.
- Do I answer the question? You can analyze this if you rephrase “discuss the impact” as “what is the impact?” This way, you can see that you’ve answered the question only very generally with the vague “positive and negative ways.”
- Have I taken a position that others might challenge or oppose? Not likely. Only people who maintain that social media has a solely positive or solely negative impact could disagree.
- Is my thesis statement specific enough? No. What are the positive effects? What are the negative effects?
- Does my thesis pass the “how and why?” test? No. Why are they positive? How are they positive? What are their causes? Why are they negative? How are they negative? What are their causes?
- Does my thesis pass the “So what?” test? No. Why should anyone care about the positive and/or negative impact of social media?
After thinking about your answers to these questions, you decide to focus on the one impact you feel strongly about and have strong evidence for:
Because not every voice on social media is reliable, people have become much more critical consumers of information, and thus, more informed voters.
This version is a much stronger thesis! It answers the question, takes a specific position that others can challenge, and it gives a sense of why it matters.
Let’s try another. Suppose your literature professor hands out the following assignment in a class on the American novel: Write an analysis of some aspect of Mark Twain’s novel Huckleberry Finn. “This will be easy,” you think. “I loved Huckleberry Finn!” You grab a pad of paper and write:
Mark Twain’s Huckleberry Finn is a great American novel.
You begin to analyze your thesis:
- Do I answer the question? No. The prompt asks you to analyze some aspect of the novel. Your working thesis is a statement of general appreciation for the entire novel.
Think about aspects of the novel that are important to its structure or meaning—for example, the role of storytelling, the contrasting scenes between the shore and the river, or the relationships between adults and children. Now you write:
In Huckleberry Finn, Mark Twain develops a contrast between life on the river and life on the shore.
- Do I answer the question? Yes!
- Have I taken a position that others might challenge or oppose? Not really. This contrast is well-known and accepted.
- Is my thesis statement specific enough? It’s getting there–you have highlighted an important aspect of the novel for investigation. However, it’s still not clear what your analysis will reveal.
- Does my thesis pass the “how and why?” test? Not yet. Compare scenes from the book and see what you discover. Free write, make lists, jot down Huck’s actions and reactions and anything else that seems interesting.
- Does my thesis pass the “So what?” test? What’s the point of this contrast? What does it signify?”
After examining the evidence and considering your own insights, you write:
Through its contrasting river and shore scenes, Twain’s Huckleberry Finn suggests that to find the true expression of American democratic ideals, one must leave “civilized” society and go back to nature.
This final thesis statement presents an interpretation of a literary work based on an analysis of its content. Of course, for the essay itself to be successful, you must now present evidence from the novel that will convince the reader of your interpretation.
Works consulted
We consulted these works while writing this handout. This is not a comprehensive list of resources on the handout’s topic, and we encourage you to do your own research to find additional publications. Please do not use this list as a model for the format of your own reference list, as it may not match the citation style you are using. For guidance on formatting citations, please see the UNC Libraries citation tutorial . We revise these tips periodically and welcome feedback.
Anson, Chris M., and Robert A. Schwegler. 2010. The Longman Handbook for Writers and Readers , 6th ed. New York: Longman.
Lunsford, Andrea A. 2015. The St. Martin’s Handbook , 8th ed. Boston: Bedford/St Martin’s.
Ramage, John D., John C. Bean, and June Johnson. 2018. The Allyn & Bacon Guide to Writing , 8th ed. New York: Pearson.
Ruszkiewicz, John J., Christy Friend, Daniel Seward, and Maxine Hairston. 2010. The Scott, Foresman Handbook for Writers , 9th ed. Boston: Pearson Education.
You may reproduce it for non-commercial use if you use the entire handout and attribute the source: The Writing Center, University of North Carolina at Chapel Hill
Make a Gift
- U.S. Locations
- UMGC Europe
- Learn Online
- Find Answers
- 855-655-8682
- Current Students
Online Guide to Writing and Research
The writing process, explore more of umgc.
- Online Guide to Writing
Thesis Statement and Controlling Idea
So what? This is the question you will get asked if your thesis statement, or main idea, is not obvious in your paper. Your thesis statement is the most important part of your writing; without it, your paper doesn’t have a main point or stance. A thesis statement states the purpose and topic of your writing, and the controlling idea indicates the direction and, often, the writing strategy you will adopt.

Generally, your thesis is placed at the end of your introduction and is a concise and simple sentence that combines your topic and your position on the topic. Like a road map, your thesis lets your readers know what to expect from the rest of your paper. Your body paragraphs support it, and your essay lacks direction without it.
It is important to keep in mind that this early in your writing, your thesis statement is really a working thesis that you use to begin thinking about your topic. You may revise this thesis many times before you are finished thinking and ready to write your final draft. Below are some sample thesis statements.
YOUR TOPIC + POSITION ON TOPIC = THESIS STATEMENT
Thesis statement do's and don'ts.
Present an argument, stance, or claim. Can your audience argue with it?
Provide a key to the organization of your paper. Can you construct body paragraphs that support it?
Mirror the assignment prompt. Are you following what is expected of you?
Present the thesis at the end of the introduction.
Answer the question: “so what?”
Present an argument that can be supported by reputable research. Is your argument logical?
Embrace the “how” and “why” elements. It’s a great strategy to present the problem, examine why it’s a problem, and show how it can be fixed.
Include announcement style language like “this paper will discuss” or “this will be shown in this essay.”
Be informative only with no argument or stance, such as, “Some high school seniors decide to take a gap year.”
Include overly broad or generalized statements like, “Kids of this generation are lazy.”
Force the reader to guess what the paper will prove or discuss
Be questions.
Key Takeaways
Your thesis is one statement at the end of your introduction and should be clear, concise, and arguable.
Without a thesis, your paper lacks direction and purpose.
Mailing Address: 3501 University Blvd. East, Adelphi, MD 20783 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License . © 2022 UMGC. All links to external sites were verified at the time of publication. UMGC is not responsible for the validity or integrity of information located at external sites.
Table of Contents: Online Guide to Writing
Chapter 1: College Writing
How Does College Writing Differ from Workplace Writing?
What Is College Writing?
Why So Much Emphasis on Writing?
Chapter 2: The Writing Process
Doing Exploratory Research
Getting from Notes to Your Draft
Introduction
Prewriting - Techniques to Get Started - Mining Your Intuition
Prewriting: Targeting Your Audience
Prewriting: Techniques to Get Started
Prewriting: Understanding Your Assignment
Rewriting: Being Your Own Critic
Rewriting: Creating a Revision Strategy
Rewriting: Getting Feedback
Rewriting: The Final Draft
Techniques to Get Started - Outlining
Techniques to Get Started - Using Systematic Techniques
Writing: Getting from Notes to Your Draft - Freewriting
Writing: Getting from Notes to Your Draft - Summarizing Your Ideas
Writing: Outlining What You Will Write
Chapter 3: Thinking Strategies
A Word About Style, Voice, and Tone
A Word About Style, Voice, and Tone: Style Through Vocabulary and Diction
Critical Strategies and Writing
Critical Strategies and Writing: Analysis
Critical Strategies and Writing: Evaluation
Critical Strategies and Writing: Persuasion
Critical Strategies and Writing: Synthesis
Developing a Paper Using Strategies
Kinds of Assignments You Will Write
Patterns for Presenting Information
Patterns for Presenting Information: Critiques
Patterns for Presenting Information: Discussing Raw Data
Patterns for Presenting Information: General-to-Specific Pattern
Patterns for Presenting Information: Problem-Cause-Solution Pattern
Patterns for Presenting Information: Specific-to-General Pattern
Patterns for Presenting Information: Summaries and Abstracts
Supporting with Research and Examples
Writing Essay Examinations
Writing Essay Examinations: Make Your Answer Relevant and Complete
Writing Essay Examinations: Organize Thinking Before Writing
Writing Essay Examinations: Read and Understand the Question
Chapter 4: The Research Process
Planning and Writing a Research Paper
Planning and Writing a Research Paper: Ask a Research Question
Planning and Writing a Research Paper: Cite Sources
Planning and Writing a Research Paper: Collect Evidence
Planning and Writing a Research Paper: Decide Your Point of View, or Role, for Your Research
Planning and Writing a Research Paper: Draw Conclusions
Planning and Writing a Research Paper: Find a Topic and Get an Overview
Planning and Writing a Research Paper: Manage Your Resources
Planning and Writing a Research Paper: Outline
Planning and Writing a Research Paper: Survey the Literature
Planning and Writing a Research Paper: Work Your Sources into Your Research Writing
Research Resources: Where Are Research Resources Found? - Human Resources
Research Resources: What Are Research Resources?
Research Resources: Where Are Research Resources Found?
Research Resources: Where Are Research Resources Found? - Electronic Resources
Research Resources: Where Are Research Resources Found? - Print Resources
Structuring the Research Paper: Formal Research Structure
Structuring the Research Paper: Informal Research Structure
The Nature of Research
The Research Assignment: How Should Research Sources Be Evaluated?
The Research Assignment: When Is Research Needed?
The Research Assignment: Why Perform Research?
Chapter 5: Academic Integrity
Academic Integrity
Giving Credit to Sources
Giving Credit to Sources: Copyright Laws
Giving Credit to Sources: Documentation
Giving Credit to Sources: Style Guides
Integrating Sources
Practicing Academic Integrity
Practicing Academic Integrity: Keeping Accurate Records
Practicing Academic Integrity: Managing Source Material
Practicing Academic Integrity: Managing Source Material - Paraphrasing Your Source
Practicing Academic Integrity: Managing Source Material - Quoting Your Source
Practicing Academic Integrity: Managing Source Material - Summarizing Your Sources
Types of Documentation
Types of Documentation: Bibliographies and Source Lists
Types of Documentation: Citing World Wide Web Sources
Types of Documentation: In-Text or Parenthetical Citations
Types of Documentation: In-Text or Parenthetical Citations - APA Style
Types of Documentation: In-Text or Parenthetical Citations - CSE/CBE Style
Types of Documentation: In-Text or Parenthetical Citations - Chicago Style
Types of Documentation: In-Text or Parenthetical Citations - MLA Style
Types of Documentation: Note Citations
Chapter 6: Using Library Resources
Finding Library Resources
Chapter 7: Assessing Your Writing
How Is Writing Graded?
How Is Writing Graded?: A General Assessment Tool
The Draft Stage
The Draft Stage: The First Draft
The Draft Stage: The Revision Process and the Final Draft
The Draft Stage: Using Feedback
The Research Stage
Using Assessment to Improve Your Writing
Chapter 8: Other Frequently Assigned Papers
Reviews and Reaction Papers: Article and Book Reviews
Reviews and Reaction Papers: Reaction Papers
Writing Arguments
Writing Arguments: Adapting the Argument Structure
Writing Arguments: Purposes of Argument
Writing Arguments: References to Consult for Writing Arguments
Writing Arguments: Steps to Writing an Argument - Anticipate Active Opposition
Writing Arguments: Steps to Writing an Argument - Determine Your Organization
Writing Arguments: Steps to Writing an Argument - Develop Your Argument
Writing Arguments: Steps to Writing an Argument - Introduce Your Argument
Writing Arguments: Steps to Writing an Argument - State Your Thesis or Proposition
Writing Arguments: Steps to Writing an Argument - Write Your Conclusion
Writing Arguments: Types of Argument
Appendix A: Books to Help Improve Your Writing
Dictionaries
General Style Manuals
Researching on the Internet
Special Style Manuals
Writing Handbooks
Appendix B: Collaborative Writing and Peer Reviewing
Collaborative Writing: Assignments to Accompany the Group Project
Collaborative Writing: Informal Progress Report
Collaborative Writing: Issues to Resolve
Collaborative Writing: Methodology
Collaborative Writing: Peer Evaluation
Collaborative Writing: Tasks of Collaborative Writing Group Members
Collaborative Writing: Writing Plan
General Introduction
Peer Reviewing
Appendix C: Developing an Improvement Plan
Working with Your Instructor’s Comments and Grades
Appendix D: Writing Plan and Project Schedule
Devising a Writing Project Plan and Schedule
Reviewing Your Plan with Others
By using our website you agree to our use of cookies. Learn more about how we use cookies by reading our Privacy Policy .
You can turn a subject into a central idea by focusing. Begin by reviewing what you know about your subject or by looking over notes you have made about it through listing, brainstorming, clustering, freewriting, or other prewriting activities.
With these details fresh in your mind, ask yourself:
What is my purpose in writing about this topic? What main point do I want to make about the topic?
WHAT IS MY PURPOSE?
Let's say you decide to write about high school. You might tell a story about your history class, compare two schools you attended, or argue that high schools should require foreign-language study.
If you want to compare the two high schools you attended, you can include details about their academic programs, athletic teams, students, or teachers. But you probably wouldn't argue that high schools should stay open in summer because doing so would take you outside your declared purpose.
WHAT IS MY MAIN POINT?
The next step in focusing is to decide what to say about your subject. What is the most interesting or important point you want to make about the schools you are comparing? The answer will be your main point, which ties all the details of the essay together.
Again, you turn an abstract subject into a central idea by stating a main point about that subject. If your main point is that entering a new school improved your attitude about education, your central idea might read:
Changing high schools made me a more serious student.
MAKING A POINT ABOUT A SUBJECT
In the box below, main points have been added to subjects to form working topic sentences or thesis statements.
Back to Top
CHECK YOUR WORKING CENTRAL IDEA
After writing a working central idea, check it for qualities that will make it effective as the basis of a paragraph or essay. Ask yourself:
Is my central idea expressed in a complete thought? Is it specific? Does it express an idea that is worth developing in a full-length paragraph or essay? Is it limited enough to discuss in a short piece of writing?
Never confuse a central idea with a simple subject. Central ideas are expressed in complete sentences; subjects are words or phrases. Take these subjects:
The city zoo. Professional athletes. Majoring in foreign languages.
Can you write a paragraph or essay on one of these subjects? Only if you decide on the main point you want to make about it. Try these as working central ideas:
The city zoo is in great need of repairs. Professional athletes are overpaid. Studying foreign languages leads to many career choices.
A CENTRAL IDEA IS SPECIFIC
Make your central idea specific. The key to this step is to focus your main point as precisely as you can. That will give you a clear direction to follow as you develop an essay or paragraph. Take this central idea:
Jogging isn't for everybody.
It is correct, but it leaves questions unanswered. For example, what kind of people should not jog? What ill effects might jogging cause them? Now, try this:
Jogging can be harmful to people who suffer from heart, back, or joint problems.
A CENTRAL IDEA CONTAINS A MAIN POINT THAT IS WORTH DEVELOPING
Make sure your main point is an idea-not just a fact-that is worth developing in a full-length paragraph or essay. Read these two sentences:
The War Memorial is in Ottawa. The War Memorial has been severely vandalized.
The first sentence is a statement of fact; it does not call for discussion. The second lends itself to discussion. For example, you might describe what the vandals did, explain how much repairs will cost, or discuss ways to prevent future problems.
A CENTRAL IDEA IS LIMITED
Essays that beginning college or university students write usually contain approximately five to seven paragraphs of about 50 to 100 words. Therefore, you should limit your working topic sentence or thesis, making it as specific as you can. Otherwise, you won't be able to make your point clearly and completely.
LIMIT THE DISCUSSION TO A MANAGEABLE LENGTH
Let's say you want to convince someone to stop smoking. You might limit yourself to three reasons to stop smoking: the health risks, the costs, and its effects on others.
Here's your working thesis:
Break the habit: otherwise, it will ruin your health, empty your wallet, and annoy your friends.
Your working topic sentences, which will control the three body paragraphs, could be as follows:
Smoking causes cancer, emphysema, and heart disease. You can save hundreds or even thousands of dollars a year by quitting. Smoking is offensive to friends and family.
LIMITING YOUR CENTRAL IDEA FURTHER
You begin a rough draft by discussing illnesses caused by smoking. However, you soon realize that you can't cover all three reasons for quitting and still keep the essay short. So you limit yourself to the issue of health risks.
Your thesis statement becomes:
Break the habit: smoking causes heart disease, emphysema, and cancer.
Your topic sentences become:
Smoking weakens the heart and impairs circulation. Smoking is a major cause of emphysema. Smoking has been linked directly to cancer of the mouth and the esophagus.
DIFFERENCES BETWEEN A TOPIC SENTENCE AND A THESIS
A topic sentence is the sentence that expresses the central idea of a paragraph. A thesis statement is a sentence that expresses the central idea of an essay.
It's a good idea to decide the topic sentence of a paragraph after writing the working version of an essay's thesis. A topic sentence explains one aspect or point in the thesis and, therefore, should always be more specific and limited than a thesis.
REVISE AND REFINE THE CENTRAL IDEA AS YOU WORK
You can revise a central idea whenever you need to. The working version of a topic sentence or thesis statement provides only a starting point and a sense of direction. Don't be afraid to look back to your central ideas and rewrite them often. As a matter of fact, focusing is something you should do throughout the writing process.

The Roadrunner's Guide to English: Thesis/Topic/Main Idea
- Planning to Read or Write
- Editing and Revising
- Proofreading
- Unstated Main Idea
- Thesis/Topic/Main Idea
- Examples/Supporting Ideas
- Modes of Organization
- Sentence Formation (Type 1 Errors)
- Mechanics (Type 2 Errors)
- Proper Word Usage (Type 3 Errors)
- Vocabulary, Context Clues, and Acquiring a Word
- Practice Games
- Style, Tone, and Inference
- Writing in Class
- English Language Learners
- Comma Splice
- Fact & Opinion
- Fused Sentence
- Identifying Dependent and Independent Clauses
- Interactive Semicolon
- Logical Fallacy
- Parts of Speech
- Pronoun/Antecedent Agreement
- Subject Identification
- Subject-Verb Agreement
- Supporting Details
Main Idea/ Thesis Statement
Author: Lydia Postell
The main idea , as we call it in reading class, is the same as the topic sentence in English class. The main idea, like the topic sentence, simply states what the reading passage is about in one sentence. There are several ways to find the main idea that you can apply to topic sentences as well.
1. Find the topic of the selection. In order to find the topic, ask yourself who or what the selection is about. When you answer either one of those questions, you will have found the topic. Here are a couple more hints. The topic will often be a word that is repeated throughout the selection. For instance, if you were reading a paragraph about dinosaurs and how they became extinct, you would see the reason they became extinct repeated several times throughout the selection, many times using different words.
2. Another important concept about the topic is that it is always written as a few words or even as one word. Sometimes the title of the selection is the topic.
3. A third way to look for a main idea is to consider the wording. When you see words that suggest you could find the information by creating a list, then you have found the main idea sentence. Examples of these word groups are several kinds , three disadvantages o f, several reasons for , three causes of , and others along these lines. These types of sentences announce the points the reading will deal with.
A couple of ideas to keep in mind: Main idea sentences take in everything in the reading passage, so when you look for a main idea sentence, be sure to look for the sentence that can take in all the points the paragraph or reading makes. Another important idea to keep in mind is that the main idea, like the topic sentence, may appear in other locations besides the beginning of an essay. While it may appear in the first two or three sentences, it may also appear in the middle or at the end of a reading selection. Don’t limit yourself by looking only at the beginning! Look for the most general sentence, and then ask yourself who or what the selection is about. If you are struggling at that point, you might wish to look at the wording of the sentences to see if one suggests that a list might be coming. If so, then that is your main idea sentence.
Thesis Statement
Author: Jenny Crisp
In writing, a thesis statement is the most important sentence you will write in an essay. For this class, and likely for most of your college courses, it should be the last sentence – or sometimes the last two sentences – in your introduction paragraph.
The thesis statement will say something about your topic, but doesn't just state your topic. For example, you would not want to write
[Bad thesis]: This is an essay about how everyone should own a dog.
Instead, your thesis statement should say something that is debatable – that is, it should have some attitude . If no one could possibly argue with your thesis statement, what would be the point of writing at all? So, let's work on that thesis statement from before:
How could any reader argue with that? It's your essay – you know what it's going to be about. No one reading your essay knows what it's about yet – otherwise why read it? So, to give it some attitude, let's quit talking about the fact that it’s an essay:
[Decent thesis]: Everyone should own a dog.
OK. That's better – that thesis statement has an attitude. But, if I'm reading your essay, I still don't really know where you're going with this, do I? That's where the other part of the thesis statement comes in: the essay map . An essay map gives your reader some idea of what's coming. What would you talk about in trying to convince your friends that everyone should own a dog? Those are the topics that you will explain in your body paragraphs, and they are the things you should list in your thesis statement’s essay map. Here's an example:
Good thesis: Everyone should own a dog because dogs provide companionship, provide protection, and provide great entertainment.
Now, there is a thesis statement! That one has attitude – it takes a side on the topic. It also provides a map of where the essay is going. With a thesis statement like this, readers know to expect a body paragraph about companionship, then one about protection, and then one about entertainment. Sounds more interesting than just saying “this essay will be about dogs,” huh?
Main Idea - Practice Exercise1
Select the stated main idea in the following passages.
Many people claim that they do not have time to work exercise into their daily schedules. However, exercise can be easily worked one's daily routine. For instance, one can do stretches while taking a shower. While standing in line at the grocery store, one can flex the abs or clench the rear. One can even do calf raises while talking on the phone. More ideas: try walking around the building during lunch breaks and always take the stairs.
Many parents tend to think of day-care centers as breeding grounds for colds. But new research suggests that children in day-care centers appear to develop immunity to many of the viruses responsible for the common cold. An article published in the Archives of Pediatrics and Adolescent Medicine found that children ages 6 to 11 who had been enrolled at large day-care centers as toddlers had about one-third as many colds as children who had stayed home as toddlers. Dr. Thomas Ball, one of the authors of the study, says that when children have colds as toddlers, their immune systems are learning from these experiences, and this learning will come back to protect children later in life. Such news should be reassuring to parents whose preschool children are in daycare.
- Main Idea - Practice 1 Answer Key
Main Idea - Practice Exercise 2
One sign of pregnancy is nausea upon awakening. Other signs are increase in size and tenderness of the breasts. Still other signs include increase in the frequency of urination and an increase in the size of the abdomen. Thus, aside from pregnancy tests, a woman can sometimes recognize the early signs and symptoms of pregnancy.
Is this year's holiday season making you tired? You can easily perk up by following a few easy tips. First, get plenty of rest. Second, snack wisely. Third, keep fit. Exercise is very important during the holidays--and not just for its weight benefits. Fourth, take a relaxing bath, and finally try sharing with others.
Are you confused by your holiday leftovers? Well, don't save any food that has been sitting around on your dining room table or counters for more than two hours after cooking. Do place the leftovers in the refrigerator while they are still warm. Don't waste those turkey scraps: add them to a salad or make a delicious soup. There are many strategies that you should use when dealing with holiday leftovers. You can even pool your leftovers with friends and neighbors by having an after-holiday potluck dinner.
Yesterday's storm did considerable damage to our neighborhood. Many stately oaks were uprooted, and several large old pine trees crushed the roofs of at least five houses. The hail that accompanied the storm damaged all of the cars that were not under shelter, and my neighbor's home was completely demolished. And my own "detached" garage was certainly detached from its foundation. Clearly, yesterday's storm caused much destruction.
Some folks think that pets are trustworthy and harmless creatures. However, it's surprising what little thieves these creatures can be. My daughter's ferret has stolen my checkbook, my calculator, my wallet, and my change purse. My officemate's dog stole a neighbor's T-bone steak right off the grill. My old dog Moonbear was known to steal freshly baked cherry pies and peanut butter cookies, while the dog that lived below us stole his master's roast one day.
Lara is quite different from her sister Lisa. Lara's hair is jet black and curly, while Lisa's is blond and straight. Lara stands 5 feet 10 inches in her stocking feet, while Lisa is a mere 5 feet 2 inches (in heels!). Furthermore, Lara's complexion is olive, quite unlike Lisa's rosy hue.
Many people are not good listeners. They may not even realize that they lack this skill. But almost anyone can become a better listener by being aware of certain negative listening habits. One such habit is jumping to conclusions before hearing the entire message. Another bad habit is to nod off when someone is speaking in a monotone. Turning off to speakers who are not necessarily experts is also a negative listening habit. Yet another bad habit to avoid is the habit of reacting emotionally to certain words.
- Main Idea - Practice 2 Answer Key
Thesis Statement Handout
- Thesis Statement Handout handout created by UNC Writing Center
- << Previous: Essay Structure
- Next: Paragraphs >>
- Last Updated: Sep 5, 2023 11:00 AM
- URL: https://libguides.daltonstate.edu/ENGL0098
Reading to Understand
- Reading to Understand (8 minutes)
- Working with Context Clues (5 minutes)
- The Main Idea
Steps for Identifying the Main Idea
- Knowledge Check
- Academic Reading Challenges (7 minutes)
The main idea is the point or message - what an author presents and what a reader takes from a text.
Searching for that main idea is a very important activity in understanding a text. It is usually found in the opening paragraph when the author is setting up the topic and expressing the thesis.
However, the location can vary according to the type of reading. For example, a research article's main idea is toward the end, whereas a persuasive essay's main idea is conveyed at the beginning.

Pre-read to Determine the Overall Topic
Examine the title and then skim the text to determine who or what the reading is about. If you see the same word repeated you know that it is likely the topic or at least an important element of the topic. The topic should be a noun or a noun phrase such as "online education." The topic itself does not convey any meeting us you must read on to determine the main idea.
Ask yourself questions about the text as you read in-depth. Pay close attention to the introduction, the first sentence of body paragraphs, and the conclusion. In these places, the author typically states and supports the main idea.
Questions to Ask Yourself While Reading :
- What elements make up this topic?
- What is the author saying about this topic?
- What does the author want me to know or believe about this topic?
Reflect on what you have read. If the main idea is not immediately apparent to you review the introduction and conclusion. The main idea should be a complete thought such as "because of its flexibility, comfort, and lower-cost online education is increasing in popularity for younger generations."
Questions to Ask Yourself While Reflecting:
- What is the message I take away from this reading?
- What point does the information add up to?
- What idea does the author reinforce in the conclusion?
- What is the final impression I have about this topic?
Finding the Main Idea
Once you believe you have found the main idea, check that each body paragraph relates to that main idea. The body paragraph should include supporting ideas that reinforce and provide greater detail about the main idea.
Some students find it beneficial to sketch the main idea and supporting ideas in their notes as a concept map.

- Previous Page: Working with Context Clues (5 minutes)
- Next Page: Academic Reading Challenges (7 minutes)
- Office of Student Disability Services
Walden Resources
Departments.
- Academic Residencies
- Academic Skills
- Career Planning and Development
- Customer Care Team
- Field Experience
- Military Services
- Student Success Advising
- Writing Skills
Centers and Offices
- Center for Social Change
- Office of Academic Support and Instructional Services
- Office of Degree Acceleration
- Office of Research and Doctoral Services
- Office of Student Affairs
Student Resources
- Doctoral Writing Assessment
- Form & Style Review
- Quick Answers
- ScholarWorks
- SKIL Courses and Workshops
- Walden Bookstore
- Walden Catalog & Student Handbook
- Student Safety/Title IX
- Legal & Consumer Information
- Website Terms and Conditions
- Cookie Policy
- Accessibility
- Accreditation
- State Authorization
- Net Price Calculator
- Contact Walden
Walden University is a member of Adtalem Global Education, Inc. www.adtalem.com Walden University is certified to operate by SCHEV © 2024 Walden University LLC. All rights reserved.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

6.1: The Topic, General Purpose, Specific Purpose, and Thesis
- Last updated
- Save as PDF
- Page ID 54935
Before any work can be done on crafting the body of your speech or presentation, you must first do some prep work—selecting a topic, formulating a general purpose, a specific purpose statement, and crafting a central idea, or thesis statement. In doing so, you lay the foundation for your speech by making important decisions about what you will speak about and for what purpose you will speak. These decisions will influence and guide the entire speechwriting process, so it is wise to think carefully and critically during these beginning stages.
Selecting a Topic
Generally, speakers focus on one or more interrelated topics—relatively broad concepts, ideas, or problems that are relevant for particular audiences. The most common way that speakers discover topics is by simply observing what is happening around them—at their school, in their local government, or around the world. Student government leaders, for example, speak or write to other students when their campus is facing tuition or fee increases, or when students have achieved something spectacular, like lobbying campus administrators for lower student fees and succeeding. In either case, it is the situation that makes their speeches appropriate and useful for their audience of students and university employees. More importantly, they speak when there is an opportunity to change a university policy or to alter the way students think or behave in relation to a particular event on campus.
But you need not run for president or student government in order to give a meaningful speech. On the contrary, opportunities abound for those interested in engaging speech as a tool for change. Perhaps the simplest way to find a topic is to ask yourself a few questions. See the textbox entitled “Questions for Selecting a Topic” for a few questions that will help you choose a topic.
Students speak about what is interesting to them and their audiences. What topics do you think are relevant today? There are other questions you might ask yourself, too, but these should lead you to at least a few topical choices. The most important work that these questions do is to locate topics within your pre-existing sphere of knowledge and interest. David Zarefsky (2010) also identifies brainstorming as a way to develop speech topics, a strategy that can be helpful if the questions listed in the textbox did not yield an appropriate or interesting topic. Starting with a topic you are already interested in will likely make writing and presenting your speech a more enjoyable and meaningful experience. It means that your entire speechwriting process will focus on something you find important and that you can present this information to people who stand to benefit from your speech.
Questions for Selecting a Topic
- What important events are occurring locally, nationally and internationally?
- What do I care about most?
- Is there someone or something I can advocate for?
- What makes me angry/happy?
- What beliefs/attitudes do I want to share?
- Is there some information the audience needs to know?
Once you have answered these questions and narrowed your responses, you are still not done selecting your topic. For instance, you might have decided that you really care about breeds of dogs. This is a very broad topic and could easily lead to a dozen different speeches. To resolve this problem, speakers must also consider the audience to whom they will speak, the scope of their presentation, and the outcome they wish to achieve.
Formulating the Purpose Statements
By honing in on a very specific topic, you begin the work of formulating your purpose statement. In short, a purpose statement clearly states what it is you would like to achieve. Purpose statements are especially helpful for guiding you as you prepare your speech. When deciding which main points, facts, and examples to include, you should simply ask yourself whether they are relevant not only to the topic you have selected, but also whether they support the goal you outlined in your purpose statement. The general purpose statement of a speech may be to inform, to persuade, to celebrate, or to entertain. Thus, it is common to frame a specific purpose statement around one of these goals. According to O’Hair, Stewart, and Rubenstein, a specific purpose statement “expresses both the topic and the general speech purpose in action form and in terms of the specific objectives you hope to achieve (2004). For instance, the home design enthusiast might write the following specific purpose statement: At the end of my speech, the audience will learn the pro’s and con’s of flipping houses . In short, the general purpose statement lays out the broader goal of the speech while the specific purpose statement describes precisely what the speech is intended to do. Some of your professors may ask that you include the general purpose and add the specific purpose.
Writing the Thesis Statement
The specific purpose statement is a tool that you will use as you write your talk, but it is unlikely that it will appear verbatim in your speech. Instead, you will want to convert the specific purpose statement into a central idea, or thesis statement that you will share with your audience.
Depending on your instructor’s approach, a thesis statement may be written two different ways. A thesis statement may encapsulate the main points of a speech in just a sentence or two, and be designed to give audiences a quick preview of what the entire speech will be about. The thesis statement for a speech, like the thesis of a research-based essay, should be easily identifiable and ought to very succinctly sum up the main points you will present. Some instructors prefer that your thesis, or central idea, be a single, declarative statement providing the audience with an overall statement that provides the essence of the speech, followed by a separate preview statement.
If you are a Harry Potter enthusiast, you may write a thesis statement (central idea) the following way using the above approach: J.K. Rowling is a renowned author of the Harry Potter series with a Cinderella like story having gone from relatively humble beginnings, through personal struggles, and finally success and fame .

Writing the Preview Statement
However, some instructors prefer that you separate your thesis from your preview statement. A preview statement (or series of statements) is a guide to your speech. This is the part of the speech that literally tells the audience exactly what main points you will cover. If you were to open your Waze app, it would tell you exactly how to get there. Best of all, you would know what to look for! So, if we take our J.K Rowling example, let’s rewrite that using this approach separating out the thesis and preview:
J.K. Rowling is a renowned author of the Harry Potter series with a Cinderella like rags to riches story. First, I will tell you about J.K. Rowling’s humble beginnings. Then, I will describe her personal struggles as a single mom. Finally, I will explain how she overcame adversity and became one of the richest women in the United Kingdom.
There is no best way to approach this. This is up to your instructor.
Writing the Body of Your Speech
Once you have finished the important work of deciding what your speech will be about, as well as formulating the purpose statement and crafting the thesis, you should turn your attention to writing the body of your speech. All of your main points are contained in the body, and normally this section is prepared well before you ever write the introduction or conclusion. The body of your speech will consume the largest amount of time to present; and it is the opportunity for you to elaborate on facts, evidence, examples, and opinions that support your thesis statement and do the work you have outlined in the specific purpose statement. Combining these various elements into a cohesive and compelling speech, however, is not without its difficulties, the first of which is deciding which elements to include and how they ought to be organized to best suit your purpose.
Good design is making something intelligible and memorable. Great design is making something memorable and meaningful.
~ Dieter Rams
Contributors and Attributions
- Template:ContribCCComm105

- Selected Reading
- UPSC IAS Exams Notes
- Developer's Best Practices
- Questions and Answers
- Effective Resume Writing
- HR Interview Questions
- Computer Glossary
Writing: Main Idea, Thesis Statement & Topic Sentences
Introduction.
While writing it is often noticed that convey of thoughts in proper organisation of language is not an easy task. Additionally, it is equally necessary for the readers to effectively understand what the writer wants to convey. The main idea concerns the summing up of the central idea that is catered to all throughout the writing. However, in this, both the thesis statement and topic sentences play a vital role in linking up the idea that is discussed in the writing with the help of supporting sentences.
Main Idea in Writings
In writing, the main idea is defined as the concepts that are to be dealt with all throughout the essay. Besides, this can be stated as the gist of the discussed topic. However, it is considered the effective main idea of the topic, when it states details, arguments as well as analysis of the topic or the idea that has been dealt with.
Therefore, in order to write an effective main idea, one needs to consider the topic thereby stating what are the things, that are to be mentioned specifically, concerning the topic. However, one needs to carefully narrow down the concept associated with the topic of the essay.
What is a Thesis sentence?
The notion of thesis sentence or statement is considered the central idea statement that supports in providing an explanation of the analysis or arguments that main idea that is associated with the essay. Moreover, this supports readers to effectively understand, what the essay is all about.
In order to write a thesis statement or sentence, the writer needs to create a purpose statement. However, this is stated as the controlling idea that provides an arguable assertion, of both the opinion and the position of the writer.
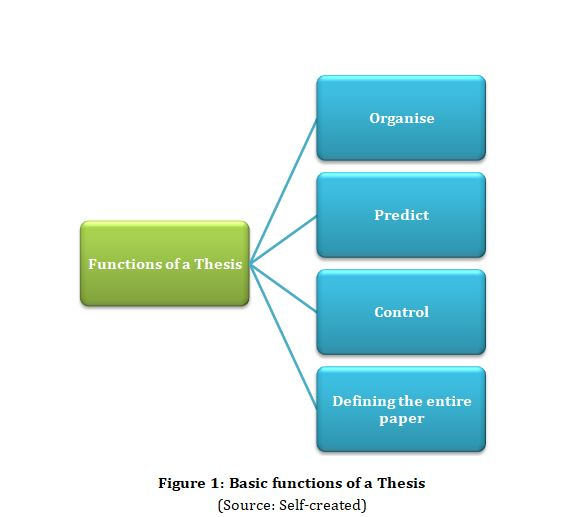
Significance of a Thesis Sentence
The thesis is considered to be equally important to the writer as well as the reader as it performs the basic functionalities. This includes organisation, control, prediction as well as defining the paper. The thesis is said to be present at the end of the introduction. This sentence provides facts and expresses the complete idea concerning the topic.
What is a Topic Sentence?
It can be said that the thesis statement and topic sentences are analogous in nature. This in simple terms means, the topic statement is the paragraph that talks about the thesis concerning the idea. This statement provides unity as well as coherence to the paragraph. The topic statement is considered integral to the organisation of the stated essay.
Purpose of a Topic sentence
The purpose of the Topic sentence is to provide unity to the paragraph that details the thesis. This also helps to develop the controlling idea that is associated with the thesis. The dominating idea needs to be expressed by the paragraph of the topic sentence and it appears to be the first sentence resembling the entire paragraph. This statement supports displaying the idea that can be either judgemental or descriptive or argumentative associated with the topic. This can as well be considered the declarative statement.
Ways to Begin a Topic Sentence
In order to start with a topic sentence one needs to consider certain steps. The first one is starting with a controversial statement that is followed by a quotation that has been taken from a noteworthy source. This furthermore is followed by referencing the current events. This may be followed by establishing the proof of the authority of the writer thereafter contributing to a rhetorical question. This is followed by the use of statistics and lastly, ended with a short statement that is dramatic in nature.
Difference between a Thesis and a Topic Sentence
Although, the differences are noticed between thesis statement and topic sentences, however they are analogous to each other. The topic sentences refers to the central or the main idea of the paragraph, whereas, the thesis statement is defined as conveying the central or the main idea of the complete essay concerning the topic that is discussed.
In this tutorial, extensive focus has been given to exploring the concepts that are linked with the writing of the main idea together with thesis statement and topic sentences. However, in order to write, an individual need to get acquainted with the main idea that is linked with the topic, as well as the thesis statement and topic sentences. All these are necessary for conveying a thought process together with an effective acknowledgement of the writer.
Q1. Why thesis is considered one of the best strategies for a writer and a reader?
Ans. The thesis is considered one of the best strategies for a writer and a reader, as for a writer it acts as a tool for planning and a sentence summary for the opinion of the writer. Together with this, it determines the real focus of the writer and an organisational framework associated with the topic sentence. On the other hand, for readers, the thesis provides contact with the writer thereby engaging and helping readers to follow the paper. This supports readers to identify the main ideas and how the topic sentence is linked with the thesis.
Q2. What is defined as the purpose of the thesis?
Ans. The purpose of the thesis is stated to be narrow that supports in full exploration of the issues. The nature of the paper whether persuasive or informative in nature is stated by the thesis.
Q3. What are the things to be done in order to write a topic sentence?
Ans. The things to be done in order to write a topic sentence involves, such as directly stating the details and facts, needs to have an effective introduction, rejecting high sounding truisms and many more.

Related Articles
- What is a Thesis Statement?
- How to Write a Thesis Statement?
- “Forests are our lifeline.” Write five sentences on this topic.
- return statement vs exit() in main() C++
- Return Statement vs Exit() in Main() using C++
- Developing the Essay Topic
- Writing a MongoDB insert statement for multiple insert at a time
- 15 Best Digital Marketing Thesis Topics
- Fashion As an Interdisciplinary Topic
- What is The Church-Turing Thesis in TOC?
- The Idea of Time
- How does IDEA Encryption work?
- Difference Between ACL and IDEA
- All the needs of animals living in a forest are fulfilled. Justify this statement in a few sentences.
- Synonymous Sentences in C++
Kickstart Your Career
Get certified by completing the course

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.2: Identifying Thesis Statements and Topic Sentences
- Last updated
- Save as PDF
- Page ID 25748
Being able to identify the purpose and thesis of a text, as you’re reading it, takes practice. This section will offer you that practice.
One fun strategy for developing a deeper understanding the material you’re reading is to make a visual “map” of the ideas. Mind maps, whether hand-drawn or done through computer programs, can be fun to make, and help put all the ideas of an essay you’re reading in one easy-to-read format.
Your understanding of what the “central” element of the mind map is might change as you read and re-read. Developing the central idea of your mind map is a great way to help you determine the reading’s thesis.
Figure 2.5. 1
- Hand-drawn Mind Map
Locating Explicit and Implicit Thesis Statements
In academic writing, the thesis is often explicit : it is included as a sentence as part of the text. It might be near the beginning of the work, but not always–some types of academic writing leave the thesis until the conclusion.
Journalism and reporting also rely on explicit thesis statements that appear very early in the piece–the first paragraph or even the first sentence.
Works of literature, on the other hand, usually do not contain a specific sentence that sums up the core concept of the writing. However, readers should finish the piece with a good understanding of what the work was trying to convey. This is what’s called an implicit thesis statement: the primary point of the reading is conveyed indirectly, in multiple locations throughout the work. (In literature, this is also referred to as the theme of the work.)
Academic writing sometimes relies on implicit thesis statements, as well.
This video offers excellent guidance in identifying the thesis statement of a work, no matter if it’s explicit or implicit.
Topic Sentences
We’ve learned that a thesis statement conveys the primary message of an entire piece of text. Now, let’s look at the next level of important sentences in a piece of text: topic sentences in each paragraph.
A useful metaphor would be to think of the thesis statement of a text as a general: it controls all the major decisions of the writing. There is only one thesis statement in a text. Topic sentences, in this relationship, serve as captains: they organize and sub-divide the overall goals of a writing into individual components. Each paragraph will have a topic sentence.
Figure 2.5. 2
It might be helpful to think of a topic sentence as working in two directions simultaneously. It relates the paragraph to the essay’s thesis, and thereby acts as a signpost for the argument of the paper as a whole, but it also defines the scope of the paragraph itself. For example, consider the following topic sentence:
Many characters in Lorraine Hansberry’s play A Raisin in the Sun have one particular dream in which they are following, though the character Walter pursues his most aggressively.
If this sentence controls the paragraph that follows, then all sentences in the paragraph must relate in some way to Walter and the pursuit of his dream.
Topic sentences often act like tiny thesis statements. Like a thesis statement, a topic sentence makes a claim of some sort. As the thesis statement is the unifying force in the essay, so the topic sentence must be the unifying force in the paragraph. Further, as is the case with the thesis statement, when the topic sentence makes a claim, the paragraph which follows must expand, describe, or prove it in some way. Topic sentences make a point and give reasons or examples to support it.
The following diagram illustrates how a topic sentence can provide more focus to the general topic at hand.
Placement of Topic Sentences
What if I told you that the topic sentence doesn’t necessarily need to be at the beginning? This might be contrary to what you’ve learned in previous English or writing classes, and that’s okay. Certainly, when authors announce a topic clearly and early on in a paragraph, their readers are likely to grasp their idea and to make the connections that they want them to make.
However, when authors are writing for a more sophisticated academic audience—that is an audience of college-educated readers—they will often use more sophisticated organizational strategies to build and reveal ideas in their writing. One way to think about a topic sentence, is that it presents the broadest view of what authors want their readers to understand. This is to say that they’re providing a broad statement that either announces or brings into focus the purpose or the meaning for the details of the paragraph. If the topic sentence is seen as the broadest view, then every supporting detail will bring a narrower—or more specific—view of the same topic.
With this in mind, take some time to contemplate the diagrams in the figure below. The widest point of each diagram (the bases of the triangles) represents the topic sentence of the paragraph. As details are presented, the topic becomes narrower and more focused. The topic can precede the details, it can follow them, it can both precede and follow them, or the details can surround the topic. There are surely more alternatives than those that are presented here, but this gives you an idea of some of the possible paragraph structures and possible placements for the topic sentence of a paragraph.
Consider some of the following examples of different topic sentence placements in a paragraph from a review essay of the beloved children’s book, The Cat in the Hat , by Dr. Seuss. Paragraph structures are labeled according to the diagrams presented above, and topic sentences are identified by red text.
Topic Sentence-Details-Topic Sentence
A good children’s book requires an exciting plot and a problem with which children can sympathize. In The Cat in the Hat there is plenty of action, depicted in the wild antics of the cat, and later in the amazing but dangerous and messy tricks of Thing 1 and Thing 2. All this excitement and action naturally draws children into the story and keeps the plot moving forward at a pace that maintains their interest. There is also tension to be resolved. The fish senses danger and constantly warns the children not to participate in the cat’s perilous stunts. And later, as the mother’s return becomes more imminent, the children begin to heed the fish’s warning and finally wish to contain the chaos and clean up the mess, but how? While this plot is fantastic enough to fuel any child’s imagination, it also contains a problem with which any child can relate: a mess and the threat of a parent’s disapproval. The careful balance of action, tension, and relatability is what makes this book an enduring childhood favorite.
Topic Sentence-Details
The careful balance of action, tension, and relatability is what makes Dr. Seuss’s The Cat in the Hat an enduring childhood favorite. In The Cat in the Hat there is plenty of action, depicted in the wild antics of the cat, and later in the amazing but dangerous and messy tricks of Thing 1 and Thing 2. All this excitement and action naturally draws children into the story and keeps the plot moving forward at a pace that maintains their interest. There is also tension to be resolved. The fish senses danger and constantly warns the children not to participate in the cat’s perilous stunts. And later, as the mother’s return becomes more imminent, the children begin to heed the fish’s warning and finally wish to contain the chaos and clean up the mess, but how? While this plot is fantastic enough to fuel any child’s imagination, it also contains a problem with which any child can relate: a mess and the threat of a parent’s disapproval.
You can relocate the topic sentence to the end here, and you’ll have an example of the Details-Topic Sentence method of organizing the paragraph.
Details-Topic Sentence-Details
In The Cat in the Hat there is plenty of action, depicted in the wild antics of the cat, and later in the amazing but dangerous and messy tricks of Thing 1 and Thing 2. All this excitement and action naturally draws children into the story and keeps the plot moving forward at a pace that maintains their interest. The careful balance of action, tension, and relatability is what makes Dr. Seuss’s The Cat in the Hat an enduring childhood favorite. There is definitely tension to be resolved here. The fish senses danger and constantly warns the children not to participate in the cat’s perilous stunts. And later, as the mother’s return becomes more imminent, the children begin to heed the fish’s warning and finally wish to contain the chaos and clean up the mess, but how? While this plot is fantastic enough to fuel any child’s imagination, it also contains a problem with which any child can relate: a mess and the threat of a parent’s disapproval.
Explicit and Implicit Topic Sentences
Similar to thesis statements, topic sentences may be explicit or implicit.
Consider the following paragraph from an essay titled “The Bothersome Beauty of Pigeons,” by author and Boise State writing professor, Bruce Ballenger. It’s important to note that this is a personal narrative essay rather than a more traditional academic essay, but the paragraph provides a good example of an implied topic. In this essay Ballenger takes the time to consider the beauty of pigeons, a bird that’s usually thought of as nothing more than a nuisance. Just prior to this paragraph, Ballenger talks about how he used a fake owl to scare away pigeons on his property. He goes on to explain,
My pigeons moved next door where an elderly couple feed them bird seed and have the time and willingness to clean up after their new charges; so it seems, in this case, things have worked out for everyone. But the large flocks still haunt the piazzas in Florence and Venice, the squares in London, and similar places in nearly every city across the globe. Despite their ability to distinguish between a Van Gogh and a Chagall, pigeons still deposit droppings that deface the great marble statues and facades–the works of art and architecture that are part of our human heritage–and yet people still buy bags of seed for about a dollar and pose for photographs, drenched in doves. Meanwhile, officials in these cities continue, sometimes quietly, to wage war against the birds (“Introduction”).
Here, Ballenger seems to be saying that in spite of the attempts of so many to rid themselves of the pigeons, others are still drawn to them and will feed them and encourage them to come back. His main idea seems to be that the battle against pigeons is a losing proposition, but he doesn’t come out and say so. His message in this paragraph is implied. Do you think this paragraph would be improved with an explicit topic sentence?
EXERCISE 1: Identify the Topic and Focus
Choose a piece of writing, perhaps an essay or some news articles found online, and for each paragraph identify (1) the topic and (2) the more focused idea. Remember, the topic sentence applies more focus to the broader topic to help narrow the scope of the paragraph. For example, the topic of a paragraph might be school lunches. The more focused idea of that same paragraph might be the idea of having students plant school gardens as a way to help incorporate more fresh produce in the menu.
License and Attributions:
CC licensed content, Previously shared:
Basic Reading and Writing. Authored by: Lumen. Located at: https://human.libretexts.org/Bookshelves/Composition/Book:_Basic_Reading_and_Writing_(Lumen)/ Module_2:_Critical_Reading/2.05:_Identifying_Thesis_Statements License: CC BY: Attribution.
Adaptions: Reformatted, some content removed to fit a broader audience.
Research Tutorial
- Library Research Tutorial
What Is a Thesis Statement?
- Topic Development
- Improve Your Research Question
- Good and Bad Research Questions
- Video Review
- Sources for Background Reading
- What about Wikipedia?
- Related Terms
- Subject Terms
- Boolean Searching
- Advanced Searching Techniques
- Definition of "Scholarly"
- Subject Guides
- Individual Databases
- Open Access Resources
- Google Scholar
- Library Catalog
- Evaluation of Sources
- Academic Writing
- Writing Resources
- Citing Sources
- Citation Formats
- Citation Resources
- Academic Integrity
- Research on the Job
Your instructor may ask you to provide a thesis statement, rather than a research question. The main difference between a thesis statement and a research question is that a thesis statement makes a claim upfront that you will attempt to validate in your paper. A thesis statement:
- States your position on a topic
- Is not always required when writing a research paper
- Is often your research question reworded as a statement with a position
For more information on writing thesis statements, see UMGC's Online Guide to Writing and Research: Thesis Statement and Controlling Idea .
- << Previous: 1. Research Question
- Next: Topic Development >>
- Last Updated: Nov 9, 2023 10:44 AM
- URL: https://libguides.umgc.edu/research-tutorial

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Identifying Thesis Statements, Claims, and Evidence
Thesis statements, claims, and evidence, introduction.
The three important parts of an argumentative essay are:
- A thesis statement is a sentence, usually in the first paragraph of an article, that expresses the article’s main point. It is not a fact; it’s a statement that you could disagree with. Therefore, the author has to convince you that the statement is correct.
- Claims are statements that support the thesis statement, but like the thesis statement, are not facts. Because a claim is not a fact, it requires supporting evidence.
- Evidence is factual information that shows a claim is true. Usually, writers have to conduct their own research to find evidence that supports their ideas. The evidence may include statistical (numerical) information, the opinions of experts, studies, personal experience, scholarly articles, or reports.
Each paragraph in the article is numbered at the beginning of the first sentence.
Paragraphs 1-7
Identifying the Thesis Statement. Paragraph 2 ends with this thesis statement: “People’s prior convictions should not be held against them in their pursuit of higher learning.” It is a thesis statement for three reasons:
- It is the article’s main argument.
- It is not a fact. Someone could think that peoples’ prior convictions should affect their access to higher education.
- It requires evidence to show that it is true.
Finding Claims. A claim is statement that supports a thesis statement. Like a thesis, it is not a fact so it needs to be supported by evidence.
You have already identified the article’s thesis statement: “People’s prior convictions should not be held against them in their pursuit of higher learning.”
Like the thesis, a claim be an idea that the author believes to be true, but others may not agree. For this reason, a claim needs support.
- Question 1. Can you find a claim in paragraph 3? Look for a statement that might be true, but needs to be supported by evidence.
Finding Evidence.
Paragraphs 5-7 offer one type of evidence to support the claim you identified in the last question. Reread paragraphs 5-7.
- Question 2. Which word best describes the kind of evidence included in those paragraphs: A report, a study, personal experience of the author, statistics, or the opinion of an expert?
Paragraphs 8-10
Finding Claims
Paragraph 8 makes two claims:
- “The United States needs to have more of this transformative power of education.”
- “The country [the United States] incarcerates more people and at a higher rate than any other nation in the world.”
Finding Evidence
Paragraphs 8 and 9 include these statistics as evidence:
- “The U.S. accounts for less than 5 percent of the world population but nearly 25 percent of the incarcerated population around the globe.”
- “Roughly 2.2 million people in the United States are essentially locked away in cages. About 1 in 5 of those people are locked up for drug offenses.”
Question 3. Does this evidence support claim 1 from paragraph 8 (about the transformative power of education) or claim 2 (about the U.S.’s high incarceration rate)?
Question 4. Which word best describes this kind of evidence: A report, a study, personal experience of the author, statistics, or the opinion of an expert?
Paragraphs 11-13
Remember that in paragraph 2, Andrisse writes that:
- “People’s prior convictions should not be held against them in their pursuit of higher learning.” (Thesis statement)
- “More must be done to remove the various barriers that exist between formerly incarcerated individuals such as myself and higher education.” (Claim)
Now, review paragraphs 11-13 (Early life of crime). In these paragraphs, Andrisse shares more of his personal story.
Question 5. Do you think his personal story is evidence for statement 1 above, statement 2, both, or neither one?
Question 6. Is yes, which one(s)?
Question 7. Do you think his personal story is good evidence? Does it persuade you to agree with him?
Paragraphs 14-16
Listed below are some claims that Andrisse makes in paragraph 14. Below each claim, please write the supporting evidence from paragraphs 15 and 16. If you can’t find any evidence, write “none.”
Claim: The more education a person has, the higher their income.
Claim: Similarly, the more education a person has, the less likely they are to return to prison.
Paragraphs 17-19
Evaluating Evidence
In these paragraphs, Andrisse returns to his personal story. He explains how his father’s illness inspired him to become a doctor and shares that he was accepted to only one of six biomedical graduate programs.
Do you think that this part of Andrisse’s story serves as evidence (support) for any claims that you’ve identified so far? Or does it support his general thesis that “people’s prior convictions should not be held against them in pursuit of higher learning?” Please explain your answer.
Paragraphs 20-23
Andrisse uses his personal experience to repeat a claim he makes in paragraph 3, that “more must be done to remove the various barriers that exist between formerly incarcerated individuals such as myself and higher education.”
To support this statement, he has to show that barriers exist. One barrier he identifies is the cost of college. He then explains the advantages of offering Pell grants to incarcerated people.
What evidence in paragraphs 21-23 support his claim about the success of Pell grants?
Paragraphs 24-28 (Remove questions about drug crimes from federal aid forms)
In this section, Andrisse argues that federal aid forms should not ask students about prior drug convictions. To support that claim, he includes a statistic about students who had to answer a similar question on their college application.
What statistic does he include?
In paragraph 25, he assumes that if a question about drug convictions discourages students from applying to college, it will probably also discourage them from applying for federal aid.
What do you think about this assumption? Do you think it’s reasonable or do you think Andrisse needs stronger evidence to show that federal aid forms should not ask students about prior drug convictions?
Supporting English Language Learners in First-Year College Composition Copyright © by Breana Bayraktar is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book

Are the main idea and the thesis statement the same thing?
2 answers by expert tutors.

Howard S. answered • 07/23/19
From Sage confidently learn to read for meaning and much much more
Main idea and thesis come down to almost but not totally the same thing.
Yes, the thesis definitely embodies a main idea.
At the same time, a thesis should exude more energy and focus than a main idea.
What does a thesis have that a main idea may not have?
A thesis has an extremely pointed, sharp, emphatic position.
A thesis should contain energetic, vibrant words, especially verbs.
A thesis should act as a frequent emphatic reminder throughout an
entire essay of the main position.
A thesis should emerge extremely concise: no wasted, extra ("fat")

Kathryn E. answered • 07/23/19
Language and communication, natural and easy.
The thesis statement expresses the main idea as a bus carries its passengers. It is the vehicle. The thesis statement is the sentence by which the author makes the reader aware of the main thing his or her writing is meant to prove or describe or inform about or discuss.
Still looking for help? Get the right answer, fast.
Get a free answer to a quick problem. Most questions answered within 4 hours.
Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.
RELATED TOPICS
Related questions, during “leiningen versus the ants,” leiningen’s attitude toward the ants changes from.
Answers · 1
Should I read the Hunger Games?
Answers · 22
Is the Great Gatsby still relevant?
Answers · 18
Is Ender's Game worth reading?
Answers · 5
Why is To Kill a Mockingbird so influential?
Answers · 16
RECOMMENDED TUTORS

find an online tutor
- Reading tutors
- Speed Reading tutors
- SAT Reading tutors
- Writing tutors
- SAT Writing tutors
- Reading Comprehension tutors
- Vocabulary tutors
- Dissertation Writing tutors
related lessons
- Reading Points on a Graph Worksheet
- Reading Quiz: At the Bus Depot
- Reading a Menu
- Reading A/B Stories
- Newsweek Reading and Vocabulary Exercise
- SAT Reading Test Format and Strategies
- ACT Reading Test Format and Strategies
- Telling Time and Reading Clock Hands

IMAGES
VIDEO
COMMENTS
The thesis statement is a one-sentence statement that expresses the main idea of the essay. The thesis statement is an arguable statement that communicates the author's stance on a topic to the reader. In order to better understand the differences between a thesis statement and main idea, it's important to understand the components of an essay.
The main idea, thesis statement, and topic sentences all provide structure to an essay. It is important for both readers and writers to understand the roles of each of these in order to maintain ...
Step 2: Write your initial answer. After some initial research, you can formulate a tentative answer to this question. At this stage it can be simple, and it should guide the research process and writing process. The internet has had more of a positive than a negative effect on education.
A thesis statement . . . Makes an argumentative assertion about a topic; it states the conclusions that you have reached about your topic. Makes a promise to the reader about the scope, purpose, and direction of your paper. Is focused and specific enough to be "proven" within the boundaries of your paper. Is generally located near the end ...
A thesis statement: tells the reader how you will interpret the significance of the subject matter under discussion. is a road map for the paper; in other words, it tells the reader what to expect from the rest of the paper. directly answers the question asked of you. A thesis is an interpretation of a question or subject, not the subject itself.
Exercise 3.8.1 3.8. 1. Read the following paragraph and then decide what the main idea is. One myth about exercise is that if a woman lifts weights, she will develop muscles as large as a man's. Without male hormones, however, a woman cannot increase her muscle bulk as much as a man's.
Your thesis statement is the most important part of your writing; without it, your paper doesn't have a main point or stance. A thesis statement states the purpose and topic of your writing, and the controlling idea indicates the direction and, often, the writing strategy you will adopt. Your thesis is like a road map, guiding your readers so ...
A thesis statement is a very common component of an essay, particularly in the humanities. It usually comprises 1 or 2 sentences in the introduction of your essay, and should clearly and concisely summarize the central points of your academic essay. A thesis is a long-form piece of academic writing, often taking more than a full semester to ...
Thesis Statements. A thesis is the main claim you are making in an argument, similar to the hypothesis in a scientific experiment. It is what you are trying to prove or persuade your audience to believe or do. It's helpful to develop a working thesis to guide your composition process. "Working" is the operative word here; your ideas are ...
is a complete sentence that expresses an opinion or an idea about a topic that can be supported or more fully developed in the body of the essay. summarizes the whole essay in one sentence and promises or reflects the essay's main purpose or "so what". provides the structure or unifying framework for the scope, focus, and direction of the ...
graph's main idea will be. •Topic sentences should: » relate back to the argument of the thesis; » concisely summarize the key idea of the paragraph; » can even contain key words from the thesis statement. ToPic SenTenceS and comPrehen Sion •opic sentences can help you with paragraph cohesion. Each paragraph should have one uniqueT ...
A thesis statement is a sentence that expresses the central idea of an essay. It's a good idea to decide the topic sentence of a paragraph after writing the working version of an essay's thesis. A topic sentence explains one aspect or point in the thesis and, therefore, should always be more specific and limited than a thesis.
Main ideas and supporting details have a pretty simple relationship. The main idea is the center of attention, and the supporting details function to support the main idea. If these were two people, this would be a pretty unfair relationship, but in writing or speaking, it is entirely acceptable. After all, only one idea can be the 'focus.'.
The main idea, as we call it in reading class, is the same as the topic sentence in English class. The main idea, like the topic sentence, simply states what the reading passage is about in one sentence. There are several ways to find the main idea that you can apply to topic sentences as well. 1. Find the topic of the selection.
Steps for Identifying the Main Idea. Step 1. Pre-read to Determine the Overall Topic. Examine the title and then skim the text to determine who or what the reading is about. If you see the same word repeated you know that it is likely the topic or at least an important element of the topic. The topic should be a noun or a noun phrase such as ...
support a thesis. A . thesis statement (the main point of a whole essay) is usually found at the . end of an introduction. A . topic sentence (the main point of a paragraph) is usually at the . beginning of a paragraph. Thesis statements and topic sentences are similar in some ways: − They are . full sentences. that communicate a full idea ...
The thesis statement for a speech, like the thesis of a research-based essay, should be easily identifiable and ought to very succinctly sum up the main points you will present. Some instructors prefer that your thesis, or central idea, be a single, declarative statement providing the audience with an overall statement that provides the essence ...
Although, the differences are noticed between thesis statement and topic sentences, however they are analogous to each other. The topic sentences refers to the central or the main idea of the paragraph, whereas, the thesis statement is defined as conveying the central or the main idea of the complete essay concerning the topic that is discussed.
Adaptions: Reformatted, some content removed to fit a broader audience. 5.2: Identifying Thesis Statements and Topic Sentences is shared under a CC BY-NC-ND license and was authored, remixed, and/or curated by LibreTexts. Topic sentences and thesis statements are similar to main ideas. This section discusses those similarities and the ...
The main difference between a thesis statement and a research question is that a thesis statement makes a claim upfront that you will attempt to validate in your paper. A thesis statement: ... Thesis Statement and Controlling Idea. Example thesis statement: Female managers encounter a glass ceiling in upper-level management positions in Fortune ...
Thesis Statements, Claims, and Evidence Introduction. The three important parts of an argumentative essay are: A thesis statement is a sentence, usually in the first paragraph of an article, that expresses the article's main point. It is not a fact; it's a statement that you could disagree with.
The thesis statement expresses the main idea as a bus carries its passengers. It is the vehicle. The thesis statement is the sentence by which the author makes the reader aware of the main thing his or her writing is meant to prove or describe or inform about or discuss.