Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 June 2022

Investigating the association between infertility and psychological distress using Australian Longitudinal Study on Women's Health (ALSWH)
- Tanmay Bagade ORCID: orcid.org/0000-0003-2536-2537 1 ,
- Kailash Thapaliya ORCID: orcid.org/0000-0002-2897-6844 1 , 3 ,
- Erica Breuer ORCID: orcid.org/0000-0003-0952-6650 1 ,
- Rashmi Kamath ORCID: orcid.org/0000-0002-4350-3586 2 ,
- Zhuoyang Li ORCID: orcid.org/0000-0002-7622-150X 1 ,
- Elizabeth Sullivan ORCID: orcid.org/0000-0002-8718-2753 1 &
- Tazeen Majeed ORCID: orcid.org/0000-0002-8512-3901 1
Scientific Reports volume 12 , Article number: 10808 ( 2022 ) Cite this article
4689 Accesses
4 Citations
28 Altmetric
Metrics details
- Epidemiology
- Health care
- Risk factors
Infertility affects millions of people globally. Although an estimated 1 in 6 couples in Australia are unable to conceive without medical intervention, little is known about the mental health impacts of infertility. This study investigated how infertility impacts the mental health of women. The study used nationally representative Australian Longitudinal Study on Women's Health (ALSWH) data. We analysed data from survey periods 2–8 conducted every three years between 2000 and 2018 for 6582 women born in 1973–78. We used a Generalised Equation Modelling (GEE) method to investigate the association of primary, secondary and resolved fertility status and psychological distress over time. Multiple measures were used to measure psychological distress: the (1) the mental health index subscale of the 36-item short form survey (SF-36), (2) the Center for Epidemiological Studies Depression Scale (CESD-10), (3) the Goldberg Anxiety and Depression Scale (GADanx) anxiety subscale; and a (4) composite psychological distress variable. About a third (30%) of women reported infertility at any of the survey rounds; a steady increase over 18 years from 1.7% at round 2 to 19.3% at round 8. Half of the women reporting primary or secondary infertility reported psychological distress, with the odds of having psychological distress was higher in women reporting primary (odds ratio (OR) 1.24, 95% confidence interval (CI) 1.06–1.45), secondary (OR 1.27, 95% CI 1.10–1.46) or resolved infertility (OR 1.15, 95% CI 1.05–1.26) compared to women reporting normal fertility status. Women with partners, underweight or higher BMI, smoking, and high-risk alcohol use had higher odds of psychological distress, whereas women in paid work had significantly lower odds of psychological distress ( p < 0.001). Diabetes, high blood pressure, asthma, and other chronic physical illness were independently associated with higher odds of psychological distress. Infertility has a significant impact on mental health even after it is resolved. Frequent mental health assessment and a holistic approach to address the lifestyle factors should be undertaken during the treatment of infertility.
Similar content being viewed by others

Depressive symptoms, anxiety, and quality of life of Japanese women at initiation of ART treatment
The associations between infertility-related stress, family adaptability and family cohesion in infertile couples.
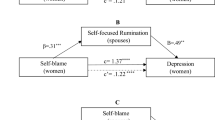
Spouse’s coping strategies mediate the relationship between women’s coping strategies and their psychological health among infertile couples
Introduction.
In 2018, an estimated 49–180 million couples globally were suffering from infertility 1 , defined by the World Health Organisation as a disease that affects the couple's inability to achieve conception despite regular and unprotected intercourse for over 12 months 2 . The estimated Global Burden of Disease due to female and male infertility has increased since 1990 3 . The global age-standardised Disability Adjusted Life Years due to female infertility increased by 15.83% from 1990 to 2017, compared to an 8.84% increase in male infertility 3 . In developed countries, infertility prevalence is 15% 4 , whereas, in Australia, the prevalence is estimated to be 17% for women aged between 28 and 33 years 5 . While the prevalence is unknown in the First Nations people of Australia, Gilbert et al. noted a higher incidence of risk factors associated with infertility 6 . Despite having a higher total fertility rate, the First Nations people in Australia may be disproportionately affected by infertility 6 .
Infertility is defined as primary when a pregnancy is never achieved or secondary when a minimum of one pregnancy is attained, but the individuals have difficulties achieving another conception 2 . It may take several years for couples with infertility to achieve a healthy full-term pregnancy, and some do not achieve this even with intervention 7 . Individuals often only seek infertility-related services when they strongly feel that embracing parenthood is a required social role for them at that stage of their life; therefore, the individual's definition of infertility might be different from what is understood in the literature 8 .
The process of conceiving and having a healthy child can be a challenging and stressful journey, which can further make the individuals feel powerless 9 . A systematic review on quality of life in infertility patients revealed that individuals could spend an average of 8.22 years with infertility 10 . Individuals scored significantly lower on mental health, social functioning and emotional behaviour and failed treatment was associated with lower quality of life 10 . Jacob et al. reported that women seeking infertility treatment show a 16% higher level of psychological distress than those without infertility 11 . Likewise, several other studies conducted in the USA, Finland, Norway have shown an association between infertility and adverse mental health outcomes 12 , 13 , 14 . Berg and Wilson summarised the cluster of anxiety, irritability, depression, blaming the self, lethargy, loneliness, and vulnerability as common infertility-related mental health symptoms 15 . The psychological effects of infertility are not limited to short-term impacts but can affect the long-term mental health and wellbeing of couples. Although infertility can also result in poor mental health outcomes among men 16 , women often experience more psychological distress over time 17 . Women who elect not to have children or are childless due to fertility issues reported poorer social wellbeing and emotional health than the overall female population of Australia 18 , 19 , 20 .
The literature on infertility is disproportionately skewed towards clinical research related to causes of infertility, diagnosis, or Artificial Reproductive Techniques (ART), and lesser on the anthropological, population and public health aspects 21 . Previous studies (nationally and internationally) have looked at cross-sectional data to assess the association between infertility and mental health 17 , 22 , 23 , 24 . However, the study findings of previous studies are either qualitative or limited to a specific facility or have smaller homogenous sample sizes. Furthermore, due to the lengthy duration of infertility diagnosis and treatment, the association of fertility status with mental health outcomes cannot be studied through short-term cross-sectional studies. In a longitudinal analysis, Herbert and colleagues found that depression was a crucial hurdle for women with fertility issues to seek medical advice 25 . Apart from Herbert and colleagues' study, few longitudinal studies have incorporated the impacts of sociodemographic factors such as income, geographical location, marital status, and lifestyle factors such as tobacco and alcohol intake and Body Mass Index (BMI) on fertility status and mental health.
Therefore, this study aims to fill this knowledge gap by presenting a longitudinal analysis of the association of infertility and mental health in Australian women, taking into account sociodemographic and lifestyle factors. From a broader needs perspective, our study is in alignment with one of the five priority areas (maternal, sexual, and reproductive health) of the recently launched National Women's Health Strategy 2020 to 2030 by the Department of Health, Australia 26 , which highlights the growing importance of this issue and the need for this project.
Data source
This study used data over 18 years from survey 2 (22–27 years of age in 2000) to survey 8 (40–45 years of age in 2018) of the 1973–78 birth cohort of the Australian Longitudinal Study on Women's Health (ALSWH) 27 .
Data collection for the study's survey 1 commenced in 1996 when women were aged 18–23 years old. The participants were randomly selected from the national health insurer's database (Medicare Australia) and were broadly representative of women of a similar age in the Australian population 27 . As data on fertility status was only collected from Survey 2 (1996) onwards, Survey 1 has been excluded from this study (details below). Questionnaires, reports and other research outcomes are available on the ALSWH website ( http://www.alswh.org.au ), and more details have been published elsewhere 27 . All methods were performed in accordance with the relevant guidelines and regulations.
Data sampling
The flow chart in Fig. 1 displays the sampling procedure, along with the inclusion and exclusion criteria. We included data from 6582 of the 14,247 women who had completed at least one survey from Survey 2 to Survey 8. We excluded data from women (1) who had not completed Survey 2, which was considered the baseline for this analysis (n = 4559); (2) those with three or more missing surveys (n = 3018); and (3) those with missing information about fertility status on three or more surveys (n = 88) (discussed further below).

Proportion of 6580 women reporting poor mental health, anxiety, depression and any psychological distress at each survey, according to the fertility status.
Defining 'fertility status' using multiple variables approach
We used the following variables related to 'infertility' and 'child status' to determine the women's 'fertility status' overtime:
Infertility
From Survey 2 onwards, women were asked if they and their partner (current or previous) ever had problems with infertility, defined as having tried unsuccessfully to get pregnant for 12 months or more. Women selected one of the four response options: never tried to get pregnant; no problem with infertility; yes, but have not sought help/treatment; and yes, and have sought help/treatment.
Number and timing of child(ren)
We matched the data from eligible participants with an additional ALSWH data set of "child data" including the number and the presence or absence of children.
Fertility status
We used the above variables to determine the women's fertility status overtime in four mutually exclusive categories:
No infertility issues/voluntarily childfree : no infertility problems reported on any survey and no reported births in the 'child data'/ pregnancies
Primary infertility : infertility problems reported at one or more surveys and no children at all surveys
Secondary infertility : infertility problems reported at one or more surveys with child(ren) born before reported infertility
Resolved infertility : infertility problems at one or more surveys but the child(ren) born after they reported infertility
In further analysis, the category of 'No infertility/ voluntarily child free' was chosen as the reference category.
Measurement of mental health
Presence and levels of reported anxiety and depressive symptoms were assessed by the three validated measures described below:
Current mental health
The SF-36 Mental Health Index (SF-36 MHI) is a five-item subscale of the SF-36 quality of life measure 28 which was collected at all survey rounds. The five items were used to generate a score of 0 to 100 with higher scores indicating better mental health 29 . We applied the commonly used cut-point of ≤ 52 to categorise women as reporting psychological distress at each survey 30 , 31 . Previous research has established this cut point to be conservatively within the bounds of a clinically meaningful indicator of psychological distress 29 .
Current depressive symptoms
The 10-item version of the Center for Epidemiological Studies Depression Scale (CESD-10) scores (ranging from 0 to 30) were used to measure current depressive symptoms at each survey 28 . A cut-point of ≥ 10 was indicative of a potential clinical diagnosis of depression 32 .
Current anxiety symptoms
The Goldberg Anxiety and Depression Scale (GADanx) anxiety subscale was included in questionnaires from Survey 3 onwards and was used to measure current symptoms indicative of an anxiety disorder. A score greater than five indicates a potential anxiety disorder 33 .
Additionally, we created a composite variable for 'any psychological distress'. Women were identified as having 'any psychological distress' if in any survey, they (1) self-reported anxiety or depression in the last 3 years or (2) their scores on any of the above three validated measures were above or below the respective cut-points described above.
Explanatory variables
We included sociodemographic, chronic health and behavioural factors in the analysis to check and adjust for potential confounding in the association between infertility and mental health.
Demographic factors: the highest level of qualification (no education, school certificate, trade/certificate/diploma and higher education), marital status (partnered, not partnered), area of residence as per the Accessibility/remoteness Index of Australia (ARIA) Plus classification system 34 ; paid work status (not in paid work/in paid work); self-reported general health (excellent/good, fair/poor);
Chronic health issues including diabetes, heart disease, high blood pressure, asthma, cancer and other major physical illness and smoking status (non-smoker, ex-smoker, current smoker); and
Health behavior factors: alcohol consumption (non-drinker, low-risk drinker, high-risk drinker) and BMI (< 18.5, 18.5–24.9, 25–29.9, ≥ 30)
Missing at random data were filled using the 'last observation carried forward (LOCF)' approach to maintain the sample size and reduce the bias caused by the attrition of participants in the study.
Statistical analysis
We used descriptive statistics to analyse baseline demographics and describe psychological distress. We used longitudinal, repeated measures models utilising the generalised estimating equations (GEE) method in parameter estimation for both univariate and multivariate data modelling to test for the presence of an association between ‘Fertility Status and Psychological distress’ (composite variable). GEE analysis requires some key assumptions to be maintained, such as dependent variable linearly related to the predicters, high number of clusters, and observations independent of each other 35 . ALSWH data fulfilled these assumptions, and, therefore, GEE was considered the analysis of choice. Furthermore, GEE enables us to conduct longitudinal data analysis with dichotomous, categorical, nominal or ordinal variables 35 and, therefore, was effective for examining the ALSWH data. Each GEE model (described below) was adjusted for time (six survey rounds) and 'fertility status' with 'psychological distress' as the dependent variable. Variables with a p value of less than 0.25 in the initial univariate approach were selected and entered into the multivariate models as described below 36 .
Model 1: Adjusted for time and fertility status
Model 2: Adjusted for time and fertility status + demographic factors
Model 3: Adjusted for time and fertility status + demographic factors + common chronic health conditions
Model 4: Fully adjusted for time and fertility status + demographic factors + common chronic health conditions + health behaviour factors.
For health conditions (Model 3 and Model 4), the status of 'No' was the reference category. The exchangeable correlation structure approach was utilised for the multivariate modelling based on a smaller QIC (Quasilikelihood under the Independence model Criterion) statistic. The results were reported using odds ratios and corresponding 95% confidence intervals. SAS 9.4 was used for all analyses.
Ethics approval
This project was approved by the Human Research Ethics Committees (HREC) of the University of Newcastle and the University of Queensland (The University of Newcastle HREC EC00144 , ratified by The University of Queensland HREC EC00456/7 ). The ALSWH survey program has ongoing ethical approval from the Human Research Ethics Committees (HRECs) of the Universities of Newcastle and Queensland (approval numbers H-076-0795 and 2004000224, respectively, for the 1973–78 cohort). Informed consent was obtained from all participants. All participants consented to joining the study and were free to withdraw or suspend their participation at any time with no need to provide a reason.
A total of 6582 women were eligible to be included in the analysis. There was a steady increase in the proportion of women reporting problems with infertility over time. The proportion of women reporting having infertility problems and seeking treatment increased from 1.7% at survey 2 to 19.3% at survey 8, which shows a massive increase in reported infertility (see Table 1 ).
Using the definition for ‘fertility status over time’, the four mutually exclusive categories were created (from the original variable). As seen in Table 2 , 69.2% of women had no reported infertility problem or were voluntarily childfree. Among the women with some reported infertility problems, the majority (18.11%) had resolved infertility.
Table 3 compares each fertility status category's demographic, health, and health behaviour factors at baseline survey 2. The majority of the women in all the categories of fertility status had higher education, were not partnered, were working in paid jobs and living in urban areas. Women reported their general health to be excellent or good. Chronic health conditions such as diabetes, heart disease, high blood pressure, asthma and other physical illnesses were reported higher in women with primary infertility as compared to women reporting secondary infertility, resolved infertility or no infertility. Most women were non-smokers, engaged in low-risk alcohol use, and had an acceptable BMI (Table 3 ).
Figure 1 presents the proportions of the reported psychological distress variables—SF-36 MHI, CESD10 and Goldberg anxiety and depression scales, along with the psychological distress variable over time. The proportion of women with no infertility reporting poor mental health on the SF-36 MHI decreased and stabilised over time; however, the proportion of women reporting poor mental health with primary infertility gradually increased. Fluctuation in reporting poor mental health was seen among women with secondary and resolved infertility; however, a considerable proportion reported poor mental health by survey 8 (women aged 42–45 years). Considerable fluctuation in the proportion of women reporting current depressive symptoms (CESD10) was seen over time. Compared to Survey 2 and Survey 3, few women reported current anxiety symptoms over time. Nearly 50% of women with primary and secondary infertility met the criteria for any psychological distress using the composite psychological distress variable. Women without infertility issues and resolved infertility also had a high proportion of women (40% and 45% respectively) reporting any psychological distress.
The results of the study showed that the odds of reporting psychological distress significantly increased in all models with time (see Table 4 ). The effect of fertility status on the psychological distress was significant for women with primary and secondary infertility ( p < 0.001) and for women with resolved infertility ( p < 0.001), as compared to women without infertility problems or voluntarily child free. In the fully adjusted GEE model, many covariates made significant independent contributions to psychological distress. For example, women who were partnered, reported having diabetes, high blood pressure, asthma, major physical illness, were currently smoking or were ex-smoker, were high-risk drinkers, and reported either with BMI < 18.5 ≥ 30 had higher odds of reported psychological distress over time ( p < 0.05), compared to women in reference categories. Women who were in paid work were less likely to report psychological distress over time ( p < 0.05). Few variables such as area of residence, heart disease, cancer was not statistically significant in the adjusted models (Table 4 ).
This research project assessed the longitudinal associations between fertility status and psychological distress over time among Australian women. To the best of our knowledge, this study is one of the first in Australia to explore these longitudinal associations over 18 years of data. The findings of the longitudinal analysis show that fertility status is an enduring condition that has a significant association with mental health outcomes among our sample of Australian women of reproductive age followed for 18 years from their 20 s to their early 40 s. The study also indicated that mental health impact remains highly substantial among the women who reported primary or secondary infertility and those who reported ‘resolved infertility’. This finding reveals the long-term impact of fertility issues and problems on women.
These findings are corroborated by previous research, which showed that infertility impacts women’s overall self-esteem, confidence, and performance 37 . A previous longitudinal study by Herbert et al. on Australian women’s health data found that out of 5936 women, 1031 women with infertility reported higher odds of self-reported depression than 4905 women who were not suffering from infertility 25 . Herbert et al. also indicated that women with depression and depressive symptoms were less likely to utilise healthcare to treat infertility, which may not resolve infertility 25 . Similarly, other researchers have demonstrated unresolved infertility's adverse long-term mental health impact through several longitudinal studies in Italy, Canada, Denmark, Australia, and Germany 7 , 16 , 38 , 39 .
The desire to have a child is common for many couples and individuals and is emphasised by continued cultural and societal norms 9 , 40 . In several pronatalist cultures, childlessness is associated with the stigma of disgrace, shame, and societal shunning, in addition to marital discord 41 , 42 . In many countries, cultural and societal pressure demands women to have at least one biological child or face discrimination, stigmatisation, and ostracism 42 , 43 . Infertility/subfertility is also associated with higher intimate partner violence 44 . Couples, therefore, choose to remain silent and avoid the anxiety associated with the stigma of infertility and its treatment 45 . Galhardo et al. recorded higher levels of depression and a sense of shame in couples diagnosed with infertility compared to those with no known diagnosis of infertility and adoption-ready couples with a diagnosis of infertility 46 . The researchers also found that couples with the diagnosis of infertility resorted to negative coping mechanisms (avoidant) in comparison to the rational styles (acceptance) of coping reported by adoption-ready couples 46 .
The impact of infertility on mental health may be due to various intersecting reasons. These include the slow and unpredictable success rates of infertility treatment (Chambers, Sullivan, & Ho, 2006), leading to added stress and anxiety, particularly in socioeconomically disadvantaged groups 47 . In many countries, assisted reproductive technology (ART) is expensive, and ART services are either partially included or not included under the government primary healthcare package, nor is it entirely covered by private health insurance 1 . Therefore, the financial barrier to access ART services adds to the infertility treatment-related stress 1 .
In this study, partnered women reported more psychological distress than non-partnered women. This difference may be linked to the socio-cultural aspects of infertility, where in some cultures, childbearing is considered an essential component of married life and is viewed as a symbol of social status 21 , 22 . Alternatively, it may be explained by understanding how couples cope with infertility compared to non-partnered individuals. Two longitudinal studies have highlighted that couples with infertility that use active and passive coping strategies (such as avoidance) have higher psychological distress compared to couples engaged in meaning-based coping strategies (problem-focused strategies, motivation, and optimism) 45 , 48 .
In this study, women in paid employment reported significantly lower psychological distress (Chambers et al., 2013). This finding is not surprising since paid employment gives financial stability and helps couples with higher socioeconomic status have more resources to seek and utilise infertility treatment 47 .
We found that having an underweight BMI or being obese was significantly associated with psychological distress. Scott et al. (2008) analysed 62,277 people in the world mental health surveys and reported that high BMI was modestly associated with mental health disorders in women 49 . Previous studies have highlighted that obesity or being underweight, both extremes can adversely affect fertility 50 , 51 , 52 . Esmaeilzadeh et al. (2013) have also found that women with infertility experience had a 4.8-fold increased risk of obesity compared to women without infertility 53 . Therefore, both high or extremely low BMI should be considered risk factors during mental health and infertility treatment.
Further, the analysis of behavioural health factors such as the prevalence of smoking and alcohol were significantly higher in women reporting psychological distress. Apart from anxiety, there was a significant prevalence of certain chronic illnesses such as diabetes, high blood pressure, asthma, and other major physical illnesses in women reporting psychological distress. Herbert et al. have also reported similar findings (2010). Although our study did not find a significant association between fertility status and psychological distress in women with cancers, previous studies have highlighted that fertility-related psychological distress is prevalent in cancer patients and survivors 54 . In another extensive epidemiological review that analysed 82 articles, Direkvand-M et al. iterated that modifying lifestyle factors such as smoking, alcohol and physical activity, and early diagnosis and management of chronic diseases can significantly help to improve the fertility status in women 55 . Lifestyle risk factors such as smoking, alcohol, unhealthy dietary habits and physical inactivity are responsible for several chronic diseases. The significant findings regarding the lifestyle factors call for a holistic approach during infertility treatment, including awareness and promoting behavioural changes in diet, physical activity, smoking, and alcohol use.
Our study had a few expected limitations. Firstly, the gender differences could not be analysed. Although men with infertility also suffer from poor mental health outcomes 16 , 56 , nationally representative men-only longitudinal data on infertility is unavailable in Australia. Future researchers would focus on assessing gender differences longitudinally. Secondly, we assumed that data was ‘missing at random’, and these missing values were filled in using the last observation carried forward approach that may have caused analytic bias; however, we tried to minimise this possibility by only imputing missing at random observations and by excluding women with three or more missing surveys. Despite a rigorous participant recruitment strategy, the ALSWH study has lower representation from minority groups, Indigenous population, refugees, and non-English speaking migrants. This low representation from diverse community groups is an issue that needs to be addressed at multiple levels starting from the national policy and is, therefore, beyond the scope of the study. We were also unable to include infertility treatments in our assessments as these are not covered by the Medicare (healthcare access scheme for Australians and some visa holders) and hence not a part of the survey and/or linked administrative data. The other limitation of the study was that we could not include clinical conditions related to infertility, such as, polycystic ovarian syndrome (PCOS) or endometriosis. Apart from affecting fertility, PCOS is significantly associated with severe mental health distress, body dissatisfaction and eating disorders 57 , 58 . Further studies should be conducted to understand the relationship of clinical conditions that affect fertility status such as PCOS on mental health distress.
However, the study has several conceptual, methodological, and analytical strengths that have helped us understand the importance of using an integrated approach to treat patients with infertility. We conducted an extensive literature review to understand the gaps in the literature and identify the strengths of different longitudinal studies. ALSWH is the largest and longest-running survey and provides an in-depth insight into various aspects of women’s health in Australia. The longitudinal approach gives an opportunity to follow up the trends of fertility status and mental health in women’s health for 18 years. We independently assessed three validated measures of psychological distress and created a composite score to define psychological distress at each survey; defined fertility status in four mutually exclusive categories by using survey and child and birth data to capture all the relevant details. With these rich data over seven surveys and covering almost 18 years, Generalised Estimating Equation was a robust technique to assess the longitudinal associations.
This study highlighted that infertility is a multidimensional stressor causing anxiety, stress, and depression with long-lasting mental health consequences and makes a strong case for infertility as a strategic public health priority. The study has important implications for implementing the current Australian National women’s health strategy 2020–2030. The implication includes improving the assessment of infertility issues as well as equitable access and affordability of infertility treatment for all groups of population. The assessment of infertility should consist of a comprehensive approach beyond clinically focused management and plans for risky lifestyle behaviours such as smoking and alcohol use. Regular mental health screening should be conducted for all patients, especially in women with primary, secondary, or resolved infertility with easy and accessible access to mental health support to protect women from long-term mental health impacts.
Infertility is rapidly emerging as a significant global health issue. Human overpopulation is causing over consumption of natural resources and causing climate change. The global warming crisis and environmental pollutants are increasing the burden of diseases and a rise in fertility issues. Environmental health affects human health and therefore, we should also draw attention to on the broader issues such as climate change and overpopulation.
Data availability
The Australian Government Department of Health owns ALSWH survey data and due to the personal nature of the data collected, release by ALSWH is subject to strict contractual and ethical restrictions. Ethical review of ALSWH is by the Human Research Ethics Committees at The University of Queensland and The University of Newcastle. De-identified data are available to collaborating researchers where a formal request to use the material has been approved by the ALSWH Data Access Committee. The committee is receptive of requests for datasets required to replicate results. Information on applying for ALSWH data is available from https://alswh.org.au/for-data-users/applying-for-data .
Starrs, A. M. et al. Accelerate progress-sexual and reproductive health and rights for all: Report of the Guttmacher-Lancet Commission. Lancet 391 , 2642–2692. https://doi.org/10.1016/s0140-6736(18)30293-9 (2018).
Article PubMed Google Scholar
WHO. WHO fact sheet on infertility. Glob. Reprod. Health 6 , e52. https://doi.org/10.1097/grh.0000000000000052 (2021).
Article Google Scholar
Sun, H. et al. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging (Albany NY) 11 , 10952–10991. https://doi.org/10.18632/aging.102497 (2019).
Evers, J. L. H. Female subfertility. Lancet 360 , 151–159. https://doi.org/10.1016/S0140-6736(02)09417-5 (2002).
Herbert, D. L., Lucke, J. C. & Dobson, A. J. Infertility, medical advice and treatment with fertility hormones and/or in vitro fertilisation: A population perspective from the Australian Longitudinal Study on Women’s Health. Aust. N. Z. J. Public Health 33 , 358–364. https://doi.org/10.1111/j.1753-6405.2009.00408.x (2009).
Gilbert, E. W., Boyle, J. A., Campbell, S. K. & Rumbold, A. R. Infertility in Aboriginal and Torres Strait Islander people: A cause for concern?. Aust. N. Z. J. Obstet. Gynaecol. 60 , 479–481. https://doi.org/10.1111/ajo.13158 (2020).
Schanz, S. et al. Long-term life and partnership satisfaction in infertile patients: A 5-year longitudinal study. Fertil. Steril. 96 , 416–421. https://doi.org/10.1016/j.fertnstert.2011.05.064 (2011).
Greil, A. L., Slauson-Blevins, K. & McQuillan, J. The experience of infertility: A review of recent literature. Sociol. Health Illn. 32 , 140–162. https://doi.org/10.1111/j.1467-9566.2009.01213.x (2010).
Gonzalez, L. O. Infertility as a transformational process: A framework for psychotherapeutic support of infertile women. Issues Ment. Health Nurs. 21 , 619–633. https://doi.org/10.1080/01612840050110317 (2000).
Article CAS PubMed Google Scholar
Chachamovich, J. R. et al. Investigating quality of life and health-related quality of life in infertility: A systematic review. J. Psychosom. Obstet. Gynecol. 31 , 101–110. https://doi.org/10.3109/0167482X.2010.481337 (2010).
Jacob, M. C., McQuillan, J. & Greil, A. L. Psychological distress by type of fertility barrier. Hum. Reprod. 22 , 885–894. https://doi.org/10.1093/humrep/del452 (2007).
Klemetti, R., Raitanen, J., Sihvo, S., Saarni, S. & Koponen, P. Infertility, mental disorders and well-being—A nationwide survey. Acta Obstet. Gynecol. Scand. 89 , 677–682. https://doi.org/10.3109/00016341003623746 (2010).
Biringer, E., Howard, L. M., Kessler, U., Stewart, R. & Mykletun, A. Is infertility really associated with higher levels of mental distress in the female population? Results from the North-Trøndelag Health Study and the Medical Birth Registry of Norway. J. Psychosom. Obstet. Gynecol. 36 , 38–45 (2015).
McQuillan, J., Greil, A. L., White, L. & Jacob, M. C. Frustrated fertility: Infertility and psychological distress among women. J. Marriage Fam. 65 , 1007–1018 (2003).
Berg, B. J. & Wilson, J. F. Psychiatric morbidity in the infertile population: A reconceptualization. Fertil. Steril. 53 , 654–661 (1990).
Article CAS Google Scholar
Fisher, J. R. W., Baker, G. H. W. & Hammarberg, K. Long-term health, well-being, life satisfaction, and attitudes toward parenthood in men diagnosed as infertile: Challenges to gender stereotypes and implications for practice. Fertil. Steril. 94 , 574–580. https://doi.org/10.1016/j.fertnstert.2009.01.165 (2010).
Oddens, B. J., den Tonkelaar, I. & Nieuwenhuyse, H. Psychosocial experiences in women facing fertility problems—A comparative survey. Hum. Reprod. 14 , 255–261. https://doi.org/10.1093/humrep/14.1.255 (1999).
Graham, M. L., Hill, E., Shelley, J. M. & Taket, A. R. An examination of the health and wellbeing of childless women: A cross-sectional exploratory study in Victoria, Australia. BMC Womens Health 11 , 1–7. https://doi.org/10.1186/1472-6874-11-47 (2011).
Maximova, K. & Quesnel-Vallée, A. Mental health consequences of unintended childlessness and unplanned births: Gender differences and life course dynamics. Soc. Sci. Med. 68 , 850–857. https://doi.org/10.1016/j.socscimed.2008.11.012 (2009).
Holton, S., Fisher, J. & Rowe, H. Motherhood: Is it good for women’s mental health?. J. Reprod. Infant Psychol. 28 , 223–239. https://doi.org/10.1080/02646830903487359 (2010).
Widge, A. Sociocultural attitudes towards infertility and assisted reproduction in India. Curr. Pract. Controv. Assist. Reprod. 60 , 74 (2002).
Google Scholar
Khan, A. R., Iqbal, N. & Afzal, A. Impact of Infertility on mental health of women. Int. J. Indian Psychol. 7 (1), 804–809. https://doi.org/10.25215/0701.089 (2019).
Ramezanzadeh, F. et al. A survey of relationship between anxiety, depression and duration of infertility. BMC Womens Health 4 , 9. https://doi.org/10.1186/1472-6874-4-9 (2004).
Article PubMed PubMed Central Google Scholar
Hasanpoor-Azghdy, S. B., Simbar, M. & Vedadhir, A. The emotional-psychological consequences of infertility among infertile women seeking treatment: Results of a qualitative study. Iran. J. Reprod. Med. 12 , 131 (2014).
PubMed PubMed Central Google Scholar
Herbert, D. L., Lucke, J. C. & Dobson, A. J. Depression: An emotional obstacle to seeking medical advice for infertility. Fertil. Steril. 94 , 1817–1821. https://doi.org/10.1016/j.fertnstert.2009.10.062 (2010).
Mishra, G. D., Chung, H.-F. & Dobson, A. J. The current state of women’s health in Australia: Informing the development of the National Women’s Health Strategy 2020–2030. (Canberra, Australia, 2018).
Lee, C. et al. Cohort profile: The Australian Longitudinal Study on women’s health. Int. J. Epidemiol. 34 , 987–991. https://doi.org/10.1093/ije/dyi098 (2005).
Holden, L. et al. Mental Health: Findings from the Australian Longitudinal Study on Women’s Health (Australian Government Department of Health and Ageing, 2013).
Rumpf, H.-J., Meyer, C., Hapke, U. & John, U. Screening for mental health: Validity of the MHI-5 using DSM-IV Axis I psychiatric disorders as gold standard. Psychiatry Res. 105 , 243–253 (2001).
Holmes, W. C. A short, psychiatric, case-finding measure for HIV seropositive outpatients: Performance characteristics of the 5-item mental health subscale of the SF-20 in a male, seropositive sample. Med. Care 36 , 237–243. https://doi.org/10.1097/00005650-199802000-00012 (1998).
Silveira, E. et al. Performance of the SF-36 health survey in screening for depressive and anxiety disorders in an elderly female Swedish population. Qual. Life Res. 14 , 1263–1274. https://doi.org/10.1007/s11136-004-7753-5 (2005).
Andresen, E. M., Malmgren, J. A., Carter, W. B. & Patrick, D. L. Screening for depression in well older adults: Evaluation of a short form of the CES-D. Am. J. Prev. Med. 10 , 77–84 (1994).
Goldberg, D., Bridges, K., Duncan-Jones, P. & Grayson, D. Detecting anxiety and depression in general medical settings. BMJ 297 , 897–899. https://doi.org/10.1136/bmj.297.6653.897 (1988).
Article CAS PubMed PubMed Central Google Scholar
Remoteness, M. Accessibility/Remoteness Index of Australia (ARIA) (Commonwealth Department of Health and Aged Care 2, I, 2001).
Ghisletta, P. & Spini, D. An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals. J. Educ. Behav. Stat. 29 , 421–437. https://doi.org/10.3102/10769986029004421 (2004).
Costanza, M. C. & Afifi, A. Comparison of stopping rules in forward stepwise discriminant analysis. J. Am. Stat. Assoc. 74 , 777–785 (1979).
Ezzell, W. The impact of infertility on women’s mental health. N. C. Med. J. 77 , 427. https://doi.org/10.18043/ncm.77.6.427 (2016).
Agostini, F. et al. Assisted reproductive technology treatments and quality of life: A longitudinal study among subfertile women and men. J. Assist. Reprod. Genet. 34 , 1307–1315. https://doi.org/10.1007/s10815-017-1000-9 (2017).
Daniluk, J. C. Reconstructing their lives: A longitudinal, qualitative analysis of the transition to biological childlessness for infertile couples. J. Couns. Dev. 79 , 439–449. https://doi.org/10.1002/j.1556-6676.2001.tb01991.x (2001).
Riggs, D. W. & Bartholomaeus, C. The desire for a child among a sample of heterosexual Australian couples. J. Reprod. Infant Psychol. 34 , 442–450. https://doi.org/10.1080/02646838.2016.1222070 (2016).
Wilkes, S. & Fankam, F. Review of the management of female infertility: A primary care perspective. Clin. Med. Rev. Women’s Health 2010 , 11–23 (2010).
Tabong, P.T.-N. & Adongo, P. B. Infertility and childlessness: a qualitative study of the experiences of infertile couples in Northern Ghana. BMC Pregnancy Childbirth 13 , 72. https://doi.org/10.1186/1471-2393-13-72 (2013).
Sembuya, R. Mother or nothing: the agony of infertility. Bull. World Health Organ. 88 , 881–882 (2010).
Stellar, C., Garcia-Moreno, C., Temmerman, M. & van der Poel, S. A systematic review and narrative report of the relationship between infertility, subfertility, and intimate partner violence. Int. J. Gynecol. Obstet. 133 , 3–8. https://doi.org/10.1016/j.ijgo.2015.08.012 (2016).
Schmidt, L., Christensen, U. & Holstein, B. The social epidemiology of coping with infertility. Hum. Reprod. 20 , 1044–1052. https://doi.org/10.1093/humrep/deh687 (2005).
Galhardo, A., Cunha, M. & Pinto-Gouveia, J. Psychological aspects in couples with infertility. Sexologies 20 , 224–228 (2011).
Chambers, G. M., Hoang, V. & Illingworth, P. J. Socioeconomic disparities in access to ART treatment and the differential impact of a policy that increased consumer costs. Hum. Reprod. 28 , 3111–3117. https://doi.org/10.1093/humrep/det302 (2013).
Peterson, B. D. et al. The longitudinal impact of partner coping in couples following 5 years of unsuccessful fertility treatments. Hum. Reprod. 24 , 1656–1664. https://doi.org/10.1093/humrep/dep061 (2009).
Scott, K. M. et al. Obesity and mental disorders in the general population: Results from the world mental health surveys. Int. J. Obes. (Lond.) 32 , 192–200. https://doi.org/10.1038/sj.ijo.0803701 (2008).
Boutari, C. et al. The effect of underweight on female and male reproduction. Metabolism 107 , 154229. https://doi.org/10.1016/j.metabol.2020.154229 (2020).
Talmor, A. & Dunphy, B. Female obesity and infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 29 , 498–506. https://doi.org/10.1016/j.bpobgyn.2014.10.014 (2015).
Sharma, R., Biedenharn, K. R., Fedor, J. M. & Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 11 , 66. https://doi.org/10.1186/1477-7827-11-66 (2013).
Esmaeilzadeh, S., Delavar, M. A., Basirat, Z. & Shafi, H. Physical activity and body mass index among women who have experienced infertility. Arch. Med. Sci. 9 , 499–505. https://doi.org/10.5114/aoms.2013.35342 (2013).
Logan, S., Perz, J., Ussher, J. M., Peate, M. & Anazodo, A. Systematic review of fertility-related psychological distress in cancer patients: Informing on an improved model of care. Psychooncology 28 , 22–30. https://doi.org/10.1002/pon.4927 (2019).
Direkvand-Moghadam, A., Delpisheh, A. & Khosravi, A. Epidemiology of female infertility; A review of literature. Biosci. Biotechnol. Res. Asia 10 , 559–567. https://doi.org/10.13005/bbra/1165 (2013).
Ying, L. Y., Wu, L. H. & Loke, A. Y. Gender differences in experiences with and adjustments to infertility: A literature review. Int. J. Nurs. Stud. 52 , 1640–1652. https://doi.org/10.1016/j.ijnurstu.2015.05.004 (2015).
Himelein, M. J. & Thatcher, S. S. Polycystic ovary syndrome and mental health: A review. Obstet. Gynecol. Surv. 61 , 723–732. https://doi.org/10.1097/01.ogx.0000243772.33357.84 (2006).
Brutocao, C. et al. Psychiatric disorders in women with polycystic ovary syndrome: A systematic review and meta-analysis. Endocrine 62 , 318–325. https://doi.org/10.1007/s12020-018-1692-3 (2018).
Download references
Acknowledgements
A research team conducts the Australian Longitudinal Study on Women’s Health at the University of Newcastle and the University of Queensland. We are grateful to the women who participated in the study and the Australian Government Department of Health and Ageing for funding this extraordinary initiative. We acknowledge Hunter Medical Research Institute, Australia, for funding the statistical support of this research project.
The study received a grant from Hunter Medical Research Institute, Australia, for statistical support (Grant Number: G2100167).
Author information
Authors and affiliations.
College of Health, Medicine and Wellbeing, The University of Newcastle (UON), University Drive, Callaghan, NSW, 2308, Australia
Tanmay Bagade, Kailash Thapaliya, Erica Breuer, Zhuoyang Li, Elizabeth Sullivan & Tazeen Majeed
Samatva Wellness Center, Noida, India
Rashmi Kamath
Registry of Senior Australians (ROSA), South Australian Health and Medical Research Institute (SAHMRI), North Terrace, Adelaide, SA, 5000, Australia
Kailash Thapaliya
You can also search for this author in PubMed Google Scholar
Contributions
T.B., T.M., and E.B. designed the key concept and the project with expert input from E.S. and Z.L. T.B and R.K. wrote the introduction, T.M. and Z.L. oversaw the statistical plan and K.T. was involved in data cleaning and some descriptive analysis, while T.M. conducted the complete analysis, T.M. and K.T. wrote the methods and T.M. write the results section of the manuscript with inputs from E.B. and Z.L. T.B. wrote the discussion, with inputs from T.M., E.B., ES and Z.L. on content and structure. All the authors – T.B., K.T., E.B., R.K., Z.L., E.S. and T.M. reviewed the manuscript multiple times and provided input to the subsequent drafts. The corresponding author (T.B.) and the authors had full access to information used in this study and had the final responsibility for the decision to submit for publication.
Corresponding author
Correspondence to Tanmay Bagade .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bagade, T., Thapaliya, K., Breuer, E. et al. Investigating the association between infertility and psychological distress using Australian Longitudinal Study on Women's Health (ALSWH). Sci Rep 12 , 10808 (2022). https://doi.org/10.1038/s41598-022-15064-2
Download citation
Received : 02 March 2022
Accepted : 17 June 2022
Published : 25 June 2022
DOI : https://doi.org/10.1038/s41598-022-15064-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The social determinants of mental health disorders among women with infertility: a systematic review.
- Tanmay Bagade
- Amanual Getnet Mersha
- Tazeen Majeed
BMC Women's Health (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Search Menu
- Advance articles
- Editor's Choice
- ESHRE Pages
- Mini-reviews
- Author Guidelines
- Submission Site
- Reasons to Publish
- Open Access
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Human Reproduction
- About the European Society of Human Reproduction and Embryology
- Editorial Board
- Self-Archiving Policy
- Dispatch Dates
- Contact ESHRE
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, materials and methods, supplementary data, acknowledgments.
- < Previous
Top 10 priorities for future infertility research: an international consensus development study † ‡
Members of the Priority Setting Partnership for Infertility are listed in the Appendix.
- Article contents
- Figures & tables
- Supplementary Data
J M N Duffy, G D Adamson, E Benson, S Bhattacharya, S Bhattacharya, M Bofill, K Brian, B Collura, C Curtis, J L H Evers, R G Farquharson, A Fincham, S Franik, L C Giudice, E Glanville, M Hickey, A W Horne, M L Hull, N P Johnson, V Jordan, Y Khalaf, J M L Knijnenburg, R S Legro, S Lensen, J MacKenzie, D Mavrelos, B W Mol, D E Morbeck, H Nagels, E H Y Ng, C Niederberger, A S Otter, L Puscasiu, S Rautakallio-Hokkanen, L Sadler, I Sarris, M Showell, J Stewart, A Strandell, C Strawbridge, A Vail, M van Wely, M Vercoe, N L Vuong, A Y Wang, R Wang, J Wilkinson, K Wong, T Y Wong, C M Farquhar, Priority Setting Partnership for Infertility , Top 10 priorities for future infertility research: an international consensus development study , Human Reproduction , Volume 35, Issue 12, December 2020, Pages 2715–2724, https://doi.org/10.1093/humrep/deaa242
- Permissions Icon Permissions
Can the priorities for future research in infertility be identified?
The top 10 research priorities for the four areas of male infertility, female and unexplained infertility, medically assisted reproduction and ethics, access and organization of care for people with fertility problems were identified.
Many fundamental questions regarding the prevention, management and consequences of infertility remain unanswered. This is a barrier to improving the care received by those people with fertility problems.
Potential research questions were collated from an initial international survey, a systematic review of clinical practice guidelines and Cochrane systematic reviews. A rationalized list of confirmed research uncertainties was prioritized in an interim international survey. Prioritized research uncertainties were discussed during a consensus development meeting. Using a formal consensus development method, the modified nominal group technique, diverse stakeholders identified the top 10 research priorities for each of the categories male infertility, female and unexplained infertility, medically assisted reproduction and ethics, access and organization of care.
Healthcare professionals, people with fertility problems and others (healthcare funders, healthcare providers, healthcare regulators, research funding bodies and researchers) were brought together in an open and transparent process using formal consensus methods advocated by the James Lind Alliance.
The initial survey was completed by 388 participants from 40 countries, and 423 potential research questions were submitted. Fourteen clinical practice guidelines and 162 Cochrane systematic reviews identified a further 236 potential research questions. A rationalized list of 231 confirmed research uncertainties was entered into an interim prioritization survey completed by 317 respondents from 43 countries. The top 10 research priorities for each of the four categories male infertility, female and unexplained infertility (including age-related infertility, ovarian cysts, uterine cavity abnormalities and tubal factor infertility), medically assisted reproduction (including ovarian stimulation, IUI and IVF) and ethics, access and organization of care were identified during a consensus development meeting involving 41 participants from 11 countries. These research priorities were diverse and seek answers to questions regarding prevention, treatment and the longer-term impact of infertility. They highlight the importance of pursuing research which has often been overlooked, including addressing the emotional and psychological impact of infertility, improving access to fertility treatment, particularly in lower resource settings and securing appropriate regulation. Addressing these priorities will require diverse research methodologies, including laboratory-based science, qualitative and quantitative research and population science.
We used consensus development methods, which have inherent limitations, including the representativeness of the participant sample, methodological decisions informed by professional judgment and arbitrary consensus definitions.
We anticipate that identified research priorities, developed to specifically highlight the most pressing clinical needs as perceived by healthcare professionals, people with fertility problems and others, will help research funding organizations and researchers to develop their future research agenda.
The study was funded by the Auckland Medical Research Foundation, Catalyst Fund, Royal Society of New Zealand and Maurice and Phyllis Paykel Trust. G.D.A. reports research sponsorship from Abbott, personal fees from Abbott and LabCorp, a financial interest in Advanced Reproductive Care, committee membership of the FIGO Committee on Reproductive Medicine, International Committee for Monitoring Assisted Reproductive Technologies, International Federation of Fertility Societies and World Endometriosis Research Foundation, and research sponsorship of the International Committee for Monitoring Assisted Reproductive Technologies from Abbott and Ferring. Siladitya Bhattacharya reports being the Editor-in-Chief of Human Reproduction Open and editor for the Cochrane Gynaecology and Fertility Group. J.L.H.E. reports being the Editor Emeritus of Human Reproduction . A.W.H. reports research sponsorship from the Chief Scientist’s Office, Ferring, Medical Research Council, National Institute for Health Research and Wellbeing of Women and consultancy fees from AbbVie, Ferring, Nordic Pharma and Roche Diagnostics. M.L.H. reports grants from Merck, grants from Myovant, grants from Bayer, outside the submitted work and ownership in Embrace Fertility, a private fertility company. N.P.J. reports research sponsorship from AbbVie and Myovant Sciences and consultancy fees from Guerbet, Myovant Sciences, Roche Diagnostics and Vifor Pharma. J.M.L.K. reports research sponsorship from Ferring and Theramex. R.S.L. reports consultancy fees from AbbVie, Bayer, Ferring, Fractyl, Insud Pharma and Kindex and research sponsorship from Guerbet and Hass Avocado Board. B.W.M. reports consultancy fees from Guerbet, iGenomix, Merck, Merck KGaA and ObsEva. E.H.Y.N. reports research sponsorship from Merck. C.N. reports being the Co Editor-in-Chief of Fertility and Sterility and Section Editor of the Journal of Urology , research sponsorship from Ferring and retains a financial interest in NexHand. J.S. reports being employed by a National Health Service fertility clinic, consultancy fees from Merck for educational events, sponsorship to attend a fertility conference from Ferring and being a clinical subeditor of Human Fertility . A.S. reports consultancy fees from Guerbet. J.W. reports being a statistical editor for the Cochrane Gynaecology and Fertility Group. A.V. reports that he is a Statistical Editor of the Cochrane Gynaecology & Fertility Review Group and the journal Reproduction . His employing institution has received payment from Human Fertilisation and Embryology Authority for his advice on review of research evidence to inform their ‘traffic light’ system for infertility treatment ‘add-ons’. N.L.V. reports consultancy and conference fees from Ferring, Merck and Merck Sharp and Dohme. The remaining authors declare no competing interests in relation to the present work. All authors have completed the disclosure form.
The ultimate aim of infertility research is to improve clinical practice and optimize the chances of people with fertility problems achieving parenthood. For this to be possible, research needs to address questions that are pertinent to people with infertility, be conducted using appropriate methods, and be reported in a comprehensive, transparent and accessible manner ( Duffy et al. , 2017 ). The first step in research production is to identify appropriate questions. Traditionally, research funding organizations and researchers have identified, refined and prioritized their own research agenda. It is unlikely that such prioritization has used formal consensus methods, engaged wider stakeholders, including people with fertility problems, and was independent of commercial interests. There has been modest improvement in some countries, including the Netherlands, the UK and the USA, which has emphasized the importance of including patients and the public in developing research priorities ( Graham et al. , 2020 ).
Sir Iain Chalmers, founder of the Cochrane Collaboration, has advocated for research priorities to be jointly identified by healthcare professionals, patients and communities ( Chalmers and Glasziou, 2009 ). He established the James Lind Alliance, which brings together healthcare professionals, patients and others, in priority setting partnerships. Using formal consensus methods, each priority setting partnership engages in an open and transparent process to identify and prioritize unanswered research questions, known as research uncertainties, in a particular area of health care ( James Lind Alliance, 2018 ). The expectation is that prioritized research uncertainties will establish the future research agenda of funding organizations and researchers. As a result, it is hoped that the gap will close between what research is needed and what research is pursued ( Wilkinson et al. , 2019a ).
An international collaboration has brought healthcare professionals, people with fertility problems and others together within a Priority Setting Partnership for Infertility to develop future research priorities for male infertility, female and unexplained infertility, medically assisted reproduction and ethics, access and organization of care.
An international multidisciplinary steering group, including healthcare professionals, people with fertility problems and researchers, was established to provide a diverse range of perspectives to inform key methodological decisions. The steering group was convened during the development of the study protocol, before the launch of the initial survey and interim prioritization survey, and before the consensus development meeting. A systematic review of registered, progressing and completed priority setting research settings was completed to assist with the planning and delivery of the study ( Graham et al. , 2020 ).
Research uncertainties related to infertility associated with endometriosis, miscarriage and polycystic ovary syndrome were not considered because of other current or completed research prioritization initiatives ( Horne et al. , 2017 ; Prior et al. , 2017 ).
Research priorities were developed in a three-stage process using consensus methods advocated by the James Lind Alliance (2018) . Potential research uncertainties were gathered through an online survey of healthcare professionals, people with fertility problems and others. Healthcare professionals, including embryologists, fertility specialists and gynecologists, were recruited through the British Fertility Society, Core Outcomes in Women’s Health (CROWN) initiative, Cochrane Gynaecology and Fertility Group, Fertility and Sterility Forum, Reproductive Medicine Clinical Study Group and Royal College of Obstetricians and Gynecologists. People with fertility problems were recruited through Fertility Europe, an umbrella organization of more than 20 European patient organizations, including Fertility Network UK and Freya, Fertility New Zealand, RESOLVE: The National Infertility Association, and the Women’s Voices Involvement Panel hosted by the Royal College of Obstetricians and Gynecologists. Other people could register to participate, including healthcare funders, healthcare regulators and researchers. Recruitment was supported by an active social media campaign. Potential participants received an explanatory video abstract, a plain-language summary and survey instructions. Before completing the survey, participants provided demographic details, including age, gender and geographical location, and information pertaining to their professional or personal experience of infertility. Participants were invited to suggest up to five research questions related to infertility that they considered unanswered.
After the survey had closed, the survey responses were examined in detail within an iterative process. Individual responses were reviewed by at least two members of the steering group. Responses were excluded if they included questions that did not fit the scope of the study, were not answerable by research, related to a specific person or situation or were ambiguous. Incomplete responses were also excluded. The remaining responses were formatted into appropriate research questions.
In addition, research recommendations were identified from a systematic review of clinical practice guidelines and Cochrane systematic reviews. Clinical practice guidelines relevant to infertility were identified by searching bibliographical databases, including Embase, International Guideline Library and MEDLINE, from 2007 to July 2017. Research recommendations were extracted verbatim from clinical practice guidelines. Using a data extraction tool available to the Cochrane Gynaecology and Fertility Group, research recommendations were extracted from individual Cochrane reviews evaluating potential fertility treatments. Research recommendations from clinical practice guidelines and Cochrane systematic reviews were reviewed by two members of the steering group and formatted into appropriate research questions. Differences in opinion were resolved by discussion with the steering group.
The long list of potential research questions was organized by allocating individual research questions in four categories: male infertility; female and unexplained infertility, including age-related infertility, ovarian cysts, uterine cavity abnormalities and tubal factor infertility; medically assisted reproduction including ovarian stimulation, IUI and IVF; and ethics, access and organization of care. These categories were identified in consultation with the steering group. Duplicate research questions were removed. Research questions were checked against the published research evidence, including clinical practice guidelines, Cochrane systematic reviews and randomized trials, and those questions considered to be already answered were removed.
The long list of confirmed research uncertainties was entered into an interim prioritization survey. Initial survey participants were invited to participate in the survey. In addition, healthcare professionals, people with fertility problems and others were recruited using the same methods as the initial survey. Before completing the survey, participants provided demographic details, including age, gender and geographical location, and information pertaining to their professional or personal experience of infertility. Participants were invited to select the research uncertainties they considered most important. After the survey had closed, questions were ranked based on the frequency they had been chosen by participants.
The top 15 research uncertainties in each category were discussed during a consensus development meeting (data are presented in the Supplementary Table S1 ). A formal consensus development method, the modified nominal group technique, was used to identify the top 10 research uncertainties for each category ( James Lind Alliance, 2018 ). Healthcare professionals, people with fertility problems and others who had completed the initial or interim prioritization survey were invited to participate. The modified nominal group technique does not depend on statistical power. In consultation with the steering group, the aim was to recruit between 15 and 30 participants, as this number has yielded sufficient results and assured validity in other settings ( Murphy et al. , 1998 ).
Before the consensus development meeting, participants provided demographic details, including age, gender and geographical location, and information pertaining to their professional or personal experience of infertility. Following an introductory session, participants were assigned to one of two groups, each with a facilitator, to discuss the ranking of prioritized research uncertainties. The assignments were pre-specified to ensure a mixture of healthcare professionals, people with fertility problems and others. The groups were provided with a set of cards with an individual research uncertainty printed on each. Each participant was asked to contribute their opinions on the research uncertainties they felt most and least strongly about. Following this initial discussion, participants were invited to discuss the ordering of the research uncertainties. By the end of the session, the research uncertainties were placed in ranked order. The rankings from the two groups were aggregated into a single ranking order and presented to the entire group. Participants were invited to discuss the ordering of the research uncertainties. By the end of the discussion, the research uncertainties were placed in a final ranked order.
The National Research Ethics Service, UK, advised the study did not require formal review.
The initial survey was completed by 179 healthcare professionals (46%), 153 people with fertility problems (39%) and 56 others (14%), from 40 countries ( Table I ). Four hundred and twenty-three responses were submitted ( Fig. 1 ). Following review, 136 responses (32%) were excluded. Clinical practice guidelines relevant to infertility were identified by searching bibliographical databases; the search strategy identified 3680 records. After excluding 731 duplicate records, 2949 titles and abstracts were screened. Thirty-two potentially relevant clinical practice guidelines were evaluated. Fourteen clinical practice guidelines met the inclusion criteria, including two guidelines related to infertility in general ( Loh et al. , 2014 ; National Institute for Health and Care Excellence, 2017 ), five guidelines related to male infertility (American Urological Association, 2010; Jarvi et al. , 2010 ; Jungwirth et al. , 2018 ), five guidelines related to uterine anomalies ( Kroon et al. , 2011 ; American Association of Gynecologic Laparoscopists, 2012 ; Carranza-Mamane et al. , 2015 ; Practice Committee of the American Society for Reproductive Medicine, 2016 a, 2017 ) and two guidelines related to medically assisted reproduction ( Practice Committee of the American Society for Reproductive Medicine, 2016b ; Penzias et al. , 2017 ). Thirteen research recommendations were extracted from the clinical practice guidelines. The Cochrane Gynaecology and Fertility Group provided research recommendations from 162 Cochrane systematic reviews. Two hundred and twenty-three potential research questions were extracted from these research recommendations. A long list of 533 potential research uncertainties was reviewed, 241 duplicate research uncertainties were removed and 51 research uncertainties which had been answered by research were also removed.
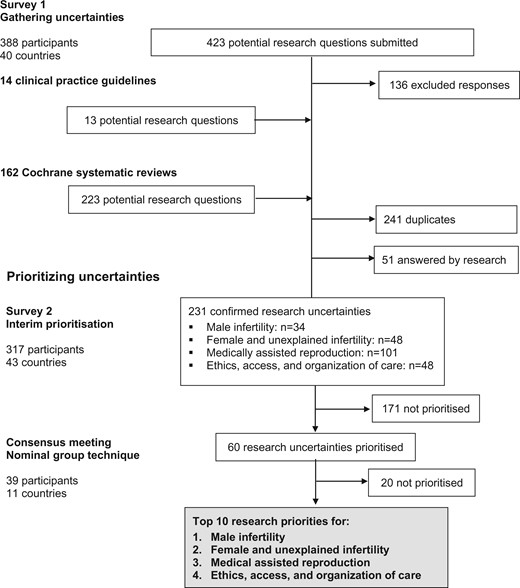
Overview of the process of identifying research uncertainties .
Characteristics of the participants in a survey to identify the priorities for future infertility research.
A rationalized list of 231 confirmed research uncertainties was developed, which included 34 research uncertainties related to male infertility, 48 research uncertainties related to female and unexplained infertility, 101 research uncertainties related to medically assisted reproduction and 48 research uncertainties related to ethics, access and organization of care. These confirmed research uncertainties were entered into an interim prioritization survey, which was completed by 143 healthcare professionals, 119 people with fertility problems and 55 others, from 43 countries.
Nineteen healthcare professionals, 14 people with personal experience of infertility and 8 others, from 11 countries, participated in the consensus development meeting. The modified nominal group technique was used to prioritize the top 10 research uncertainties for male infertility, female and unexplained infertility, medically assisted reproduction and ethics, access and organization of care. Fifteen highly prioritized research uncertainties for each category were discussed during the consensus development meeting ( Supplementary Table SI ). The 15 highly prioritized research uncertainties were initially discussed by two separate groups and at the end of the discussion, they ranked the research uncertainties. The first-round ranking is presented in Supplementary Table SI . The rankings from the two groups were aggregated into a single ranking order and discussed by the entire group ( Supplementary Table SI ). Participants were encouraged to discuss and finalize the rank order of the research priorities. The top 10 research priorities are presented in Fig. 2 .

The top 10 priorities for future infertility research in each of the four categories .
The Priority Setting Partnership for infertility has brought together healthcare professionals, people with fertility problems and others to identify the top 10 research priorities for future infertility research. These research priorities are diverse and seek answers to questions regarding prevention, treatment and the longer-term impact, as well as wider contextual issues related to access and public health policy. They highlight the importance of pursuing research which has often been overlooked, including addressing the emotional and psychological impact of infertility, improving access to fertility treatment, particularly in lower resource settings, and securing appropriate regulation. Addressing these priorities will require diverse research methodologies, including laboratory-based science, qualitative and quantitative research and population science.
Strengths and limitations
The James Lind Alliance (2018) has published guidance to inform the design of research priority setting studies. This study has followed this guidance to ensure the research priorities were developed using a clear and transparent process using formal consensus development methods. The study design, development and delivery were also informed by a systematic review of research priority setting studies relevant to women’s health ( Graham et al. , 2020 ). With 388 respondents from 40 countries participating in the initial survey, 317 respondents from 43 countries participating in the interim prioritization survey, and 41 participants from 11 countries included in the consensus development meeting, the global participation achieved in this study should secure the generalizability of the results within an international context. The study included people with fertility problems and they were able to suggest potential research uncertainties during the initial survey, share their views regarding the importance of research uncertainties during the interim prioritization survey and participate fully in the consensus development meeting which prioritized the final research priorities.
This consensus study is not without limitations. Consideration should be given to the representativeness of the study’s participants. For example, when considering the initial survey, there was a higher response from participants who identified as living in Europe (115 participants; 30%). To participate in the initial survey and interim prioritization survey, English proficiency and literacy, a computer and internet access were required. We appreciate that limitations in the representativeness of the sample could impact upon the research uncertainties suggested and prioritized. There is uncertainty regarding the optimal consensus development method to prioritize research uncertainties, and methodological research is required to evaluate different approaches to priority setting and the use of different consensus methods. Further contextual information, including the number of people the research priority impacts upon, the feasibility of answering the research priority, and the resources required to address the research uncertainty could have assisted participants to prioritize research uncertainties. Future methodological research should evaluate the use of contextual information in research priority studies.
Reflections on the research priorities
Reproductive medical care for men has lagged behind that for women. Setting impactful and tractable priorities for male reproduction is consequently a critically important task. For diagnosis, the variation in morphology is extraordinary and counting sperm is challenging, severely limiting our ability to make predictions of male reproductive potential from the standard semen analysis, and begging the question: are there other, better tests of sperm? We need to explore how overall health affects male fertility and whether treating other diseases improves it. Because a man does not live in a vacuum, we need to understand how the environment affects male reproduction. When considering the treatment of male infertility, men often ask what they can do to improve their fertility, and well-conducted studies into diet and nutraceuticals are essential. The endocrine system drives the making of sperm and further evidence is required to understand if hormonal therapy could improve the production of sperm and improve live birth rates.
The priorities for unexplained infertility seek answers to several challenging and long-standing questions, including the prevention of age-related infertility and exploring the role of fibroids, polyps, intrauterine adhesions and uterine septa in unexplained infertility. It is also surprising that it remains unclear what the first-line treatment is for couples with unexplained infertility, IVF or IUI, and the timing of the superior treatment for that couple.
When considering medically assisted reproduction, new large prospective cohorts that consider all variables and use advance methodology will be required to address casual relationships related to implantation failure. Similar complexity will exist when studying oocyte yield and quality over subsequent IVF cycles, even though similar stimulation protocols have been used. The three research priorities concerning the effectiveness of IVF are seeking to identify optimal ovarian stimulation protocols in poor responders, sperm selection techniques and embryo selection. These contrast with the research priorities which explore if, when, and how IUI should be used. To answer these effectiveness questions, well-designed randomized controlled trials will be required ( Wilkinson et al. , 2019b ). The psychological impact of fertility treatment is brought into sharper focus with research priorities related to the emotional and psychological impact of repeated fertility treatment failure and in children following gamete donation. Strong involvement of patient representatives, psychologists and behavioral scientists will be required to establish the appropriate qualitative and quantitative studies to address these important priorities.
The research priorities for ethics, access and organization of care broadly fall into two overarching themes: access and infertility as a public health issue. When considering access, cost is a major barrier to appropriate care, which is reflected in the research priorities aiming to explore interventions to reduce the cost of fertility treatment and increase the availability of fertility treatment in lower-resources settings. Turning to infertility as a public health issue, prevention of infertility should be a key priority for public health initiatives. We need to determine the minimum standard of care that people with fertility problems should expect, especially if we are seeking reimbursements for this care.
Wider context
A prioritized list of research uncertainties, developed to specifically highlight the most pressing clinical needs as perceived by healthcare professionals, people with fertility problems and others, should help funding organizations and researchers to set their future research agenda. The selected list of research uncertainties should serve to focus a discussion regarding the allocation of limited resources.
Many of the research priorities will require national and international collaboration. Several countries, including China, the Netherlands, the UK and the USA, have developed national networks to undertake infertility research ( Devall et al. , 2020 ). Further development of national infrastructure is required. Collaboration should spread beyond national boundaries and develop within an international context. It is hoped the development of a prioritized research agenda could be an important enabler to deepen international collaboration. Development of generic infrastructure could help foster collaboration, including the use of minimum data sets, known as core outcome sets, low-cost data repositories and standardized approaches to the reporting of research. A core outcome set has recently been developed for future infertility trials ( Duffy et al. , 2018 ). Over 400 healthcare professionals, researchers and patients, from 40 countries, have used formal consensus development methods to identify a core outcome set for infertility ( Duffy et al. , 2020a ). Consensus definitions have also been agreed for individual core outcomes ( Duffy et al. , 2020b ). It is hoped the core outcome set will provide generic tools to collect outcomes during research, provide concise guidance regarding statistical analysis and standardize the approach to research reporting ( Duffy et al. , 2019 ).
Research priorities identified in this study correspond with research priorities identified by the Priority Setting Partnership for Miscarriage, including determining the emotional and psychological impact of miscarriage, investigating the modifiable risk factors which cause miscarriage and identifying specific comorbidities which cause miscarriage ( Prior et al. , 2017 ). Other similarities exist when considering the research uncertainties prioritized by the Priority Setting Partnership for Endometriosis and International Polycystic Ovary Syndrome Network ( Horne et al. , 2017 ).
Answering the prioritized research questions would represent a significant step forward for our specialty. The steering group recognizes the important role of research which stems from the intellectual curiosity of individuals, fundamental research which does not have an immediate clinical application and research which is funded by special interest groups raising funding for the topic of their particular interest. A blended research strategy should offer the optimal pathway to improving clinical care and patient outcomes.
Perhaps the most important part of this process has been the strengthening of relationships between partner organizations, healthcare professionals and people with lived experience of infertility. The prioritized list of uncertainties that require research should help funding organizations and researchers to set their future research agenda. Our approach should ensure that future research has the necessary reach and relevance to inform clinical practice and to improve patient outcomes.
Supplementary data are available at Human Reproduction online.
We would like to thank the initial survey, interim prioritization survey and consensus development meeting participants, and colleagues at the Cochrane Gynaecology and Fertility Group, University of Auckland, New Zealand.
Authors’ roles
Study concept and design: J.M.N.D., S.B., B.C., C.C., J.L.H.E., R.G.F., S.F., L.C.G., A.W.H., N.P.J., Y.K., J.M.L.K., R.S.L., S.L., B.W.M., H.N., E.H.Y.N., C.N., A.S.O., L.P., S.R.H., M.S., J.S., A.S., C.S., A.V., M.v.W., M.A.V., N.L.V., A.Y.W., R.W., J.W. and C.M.F. Acquisition of data: J.M.N.D., S.B., K.B., C.B., C.C., J.L.H.E., R.G.F., A.F., S.F., L.C.G., A.W.H., N.P.J., Y.K., J.M.L.K., R.S.L., S.L., B.W.M., E.H.Y.N., C.N., A.S.O., L.P., S.R.H., M.S., J.S., A.S., C.S., A.V., M.v.W., M.A.V., N.L.V., A.Y.W., R.W., J.W. and C.M.F. Analysis and interpretation of data: J.M.D., G.D.A., E.B., S.B., S.B., M.B., K.B., B.C., C.C., J.L.H.E., R.G.F., A.F., S.F., L.C.G., E.G., M.H., A.W.H., M.L.H., N.P.J., V.J., Y.K., J.M.L.K., R.S.L., S.L., J.M., D.M., B.W.M., D.E.M., H.N., E.H.Y.N., C.N., A.S.O., L.P., S.R.H., L.S., I.S., M.S., J.S., A.S., C.S., A.V., M.v.W., M.V., N.L.V., A.Y.W., R.W., J.W., K.W., T.W. and C.M.F. Drafting of the manuscript: J.M.D., B.C., S.L., H.N., C.N., M.S., M.v.W., M.V., R.W., J.W. and C.M.F. Critical revision of the manuscript for important intellectual content: G.D.A., E.B., S.B., S.B., M.B., B.K., C.C., J.L.H.E., R.G.F., A.F., S.F., L.C.G., E.G., M.H., A.W.H., M.L.H., N.P.J., V.J., Y.K., J.M.L.K., R.S.L., J.M., M.M., D.M., B.W.M., D.M., E.H.Y.N., A.S.O., L.P., S.R.H., L.S., I.S., J.S., A.S., C.S., A.V., N.L.V., A.Y.W., K.W. and T.W. Statistical analysis: J.M.D., J.W. and A.V. Study supervision: C.M.F.
This research was funded by the Catalyst Fund, Royal Society of New Zealand, Auckland Medical Research Foundation and Maurice and Phyllis Paykel Trust. The funders had no role in the design and conduct of the study, the collection, management, analysis or interpretation of data or manuscript preparation. B.W.M. is supported by a National Health and Medical Research Council Practitioner Fellowship (GNT1082548). Siladitya Bhattacharya was supported by the Auckland Foundation Seelye Travelling Fellowship.
Conflict of interest
G.D.A. reports research sponsorship from Abbott, personal fees from Abbott and LabCorp, a financial interest in Advanced Reproductive Care, committee membership of the FIGO Committee on Reproductive Medicine, International Committee for Monitoring Assisted Reproductive Technologies, International Federation of Fertility Societies and World Endometriosis Research Foundation, and research sponsorship of the International Committee for Monitoring Assisted Reproductive Technologies from Abbott and Ferring. Siladitya Bhattacharya reports being the Editor-in-Chief of Human Reproduction Open and editor for the Cochrane Gynaecology and Fertility Group. J.H.L.E. reports being the Editor Emeritus of Human Reproduction . A.W.H. reports research sponsorship from the Chief Scientist’s Office, Ferring, Medical Research Council, National Institute for Health Research and Wellbeing of Women and consultancy fees from AbbVie, Ferring, Nordic Pharma and Roche Diagnostics. M.L.H. reports grants from Merck, grants from Myovant, grants from Bayer, outside the submitted work and ownership in Embrace Fertility, a private fertility company. N.P.J. reports research sponsorship from AbbVie and Myovant Sciences and consultancy fees from Guerbet, Myovant Sciences, Roche Diagnostics and Vifor Pharma. J.M.L.K. reports research sponsorship from Ferring and Theramex. R.S.L. reports consultancy fees from AbbVie, Bayer, Ferring, Fractyl, Insud Pharma and Kindex and research sponsorship from Guerbet and Hass Avocado Board. B.W.M. reports consultancy fees from Guerbet, iGenomix, Merck, Merck KGaA and ObsEva. E.H.Y.N. reports research sponsorship from Merck. C.N. reports being the Co Editor-in-Chief of Fertility and Sterility and Section Editor of the Journal of Urology , research sponsorship from Ferring and retains a financial interest in NexHand. J.S. reports being employed by a National Health Service fertility clinic, consultancy fees from Merck for educational events, sponsorship to attend a fertility conference from Ferring and being a clinical subeditor of Human Fertility . A.S. reports consultancy fees from Guerbet. J.W. reports being a statistical editor for the Cochrane Gynaecology and Fertility Group. A.V. reports that he is a Statistical Editor of the Cochrane Gynaecology & Fertility Review Group and the journal Reproduction . His employing institution has received payment from Human Fertilisation and Embryology Authority for his advice on review of research evidence to inform their ‘traffic light’ system for infertility treatment ‘add-ons’. N.L.V. reports consultancy and conference fees from Ferring, Merck and Merck Sharp and Dohme. The remaining authors declare no competing interests in relation to the present work. All authors have completed the disclosure form.
Priority Setting Partnership for Infertility
Dr Hisham AlAhwany, University of Nottingham, UK; Ofra Balaban, CHEN: Patient Fertility Association, Israel; Faith Barton, UK; Dr Yusuf Beebeejaun, King’s Fertility, Fetal Medicine Research Institute, UK; Professor Jacky Boivin, Cardiff University, UK; Professor Jan J. A. Bosteels, Imelda Hospital, Belgium; Professor Carlos Calhaz-Jorge, Faculdade de Medicina da Universidade de Lisboa, Portugal; Dr Arianna D’Angelo, Wales Fertility Institute, UK; Dr Leona F. Dann, Health Quality and Safety Commission, New Zealand; Professor Christopher J. De Jonge, University of Minnesota Medical Center, United States; Elyce du Mez, University of Auckland, New Zealand; Professor Rui A. Ferriani, University of Sao Paulo, Brazil; Dr Marie-Odile Gerval, Chelsea and Westminster Hospital NHS Foundation Trust, UK; Lynda J. Gingel, UK; Dr Ellen M. Greenblatt, Mount Sinai Fertility, University of Toronto, Toronto; Professor Geraldine Hartshorne, University of Warwick, UK; Charlie Helliwell, New Zealand; Charlotte Helliwell, New Zealand; Lynda J. Hughes, The Fertility Clinic, London Health Sciences Centre, Canada; Dr Junyoung Jo, Conmaul Hospital of Korean Medicine, Republic of Korea; Jelena Jovanović, Serbia; Professor Ludwig Kiesel, University of Münster, Germany; Dr Chumnan Kietpeerakool, Khon Kaen University, Thailand; Dr Elena Kostova, Cochrane Gynaecology and Fertility, New Zealand; Professor Tansu Kucuk, Acibadem Maslak Hospital, Turkey; Rajesh Kumar, National Foundation for the Deaf, New Zealand; Robyn L. Lawrence, The Liggins Institute, The University of Auckland, New Zealand; Nicole Lee, Canada; Katy E. Lindemann, UK; Professor Olabisi M. Loto, Obafemi Awolowo University, Nigeria; Associate Professor Peter J. Lutjen, Monash University, Australia; Michelle MacKinven, Fertility New Zealand; New Zealand; Dr Mariano Mascarenhas, Leeds Teaching Hospital NHS Trust, UK; Helen McLaughlin, Endometriosis UK, UK; David J. Mills, UK; Dr Selma M. Mourad, Isala Hospital Zwolle, The Netherlands; Linh K. Nguyen, Vietnam; Professor Robert J. Norman, Robinson Research Institute, University of Adelaide, Adelaide; Maja Olic, NGO Counselling Center for In Vitro Fertilisation, Serbia; Kristine L. Overfield, NISIG: National Infertility Support and Information Group, Ireland; Maria Parker-Harris, UK; David G. Ramos, Spain; Aleksandra Rendulic, Serbia; Sjoerd Repping, Amsterdam University Medical Centres, The Netherlands; Professor Roberta Rizzo, University of Ferrara, Italy; Professor Pietro Salacone, Italy; Catherine H. Saunders, The Dartmouth Institute for Health Policy and Clinical Practice, USA; Dr Rinku Sengupta, UK; Dr Ioannis A. Sfontouris, Eugonia: Assisted Reproduction Unit, Greece; Natalie R. Silverman, The Fertility Podcast, UK; Dr Helen L. Torrance, University Medical Center Utrecht, The Netherlands; Dr Eleonora P. Uphoff, UK; Dr Sarah A. Wakeman, Fertility Associates, New Zealand; Professor Tewes Wischmann, Heidelberg University, Germany; Dr Bryan J. Woodward, UK; and Mohamed A. Youssef, Cairo University, Egypt.
This article has not been externally peer reviewed.
This article has been published simultaneously in Fertility and Sterility.
American Association of Gynecologic Laparoscopists . Practice guidelines for the diagnosis and management of submucous leiomyomas . J Minim Invasive Gynecol 2012 ; 19 : 152 – 171 .
American Urological Association. The Management of Obstructive Azoospermia . Linthicum, MD: American Urological Association , 2010 .
Carranza-Mamane B Havelock J Hemmings R Cheung A Sierra S Carranza-Mamane B Case A Cathie D Graham J Havelock J et al. The management of uterine fibroids in women with otherwise unexplained infertility . J Obstet Gynaecol Can 2015 ; 37 : 277 – 285 .
Google Scholar
Chalmers I Glasziou P. Avoidable waste in the production and reporting of research evidence . Lancet 2009 ; 374 : 86 – 89 .
Devall AJ Out JH Mol BWJ Duffy JMN Collura B Dyer S. Coordination and planning of clinical research on a national and global level . Fertil Steril 2020 ; 113 : 1100 – 1106 .
Duffy JMN AlAhwany H Bhattacharya S Collura B Curtis C Evers JLH Farquharson RG Franik S Giudice LC Khalaf Y et al. Developing a core outcome set for future infertility research: an international consensus development study . Hum Reprod 2020 a; 35 : 2725–2734.
Duffy JMN Bhattacharya S Bhattacharya S Bofill M Collura B Curtis C Evers JLH Giudice LC Farquharson RG Franik S et al. Standardizing definitions for the infertility core outcome set: an international consensus development study . Hum Reprod 2020 b; 35 :2735–2745.
Duffy JMN Bhattacharya S Curtis C Evers JLH Farquharson RG Franik S Khalaf Y Legro RS Lensen S Mol BW , COMMIT: Core Outcomes Measures for Infertility Trials et al. A protocol developing, disseminating and implementing a core outcome set for infertility . Hum Reprod Open 2018 ; 2018 : hoy007 .
Duffy JMN Bhattacharya S Herman M Mol B Vail A Wilkinson J Farquhar C , the Cochrane Gynaecology and Fertility Group . Reducing research waste in benign gynaecology and fertility research . BJOG: Int J Obstet Gy 2017 ; 124 : 366 – 369 .
Duffy JMN Ziebland S von Dadelszen P McManus RJ. Tackling poorly selected, collected, and reported outcomes in obstetrics and gynecology research . Am J Obstet Gynecol 2019 ; 220 : 71.e71 – 71.e74 .
Graham L Illingworth B Showell M Vercoe M Crosbie E Gingel L Farquhar C Horne A Prior M Stephenson J et al. Research priority setting in women’s health: a systematic review . BJOG: Int J Obstet Gy 2020 ; 127 : 694 – 700 .
Horne AW Saunders PTK Abokhrais IM Hogg L. Top ten endometriosis research priorities in the UK and Ireland . Lancet 2017 ; 389 : 2191 – 2192 .
James Lind Alliance . The James Lind Alliance Guidebook . Southampton, UK : National Institute for Health Research Evaluation, Trials and Studies Coordinating Centre , 2018 .
Google Preview
Jarvi K Lo K Fischer A Grantmyre J Zini A Chow V Mak VC. Guideline the workup of azoospermic males . Can Urol Assoc J 2010 ; 4 : 163 – 167 .
Jungwirth A Diemer T Kopa Z Krausz C Minhas S Tournaye H. EAU Guidelines on Male Infertility . Arnhem, The Netherlands : European Association of Urology , 2018 .
Kroon B Johnson N Chapman M Yazdani A Hart R , on behalf of the Australasian CREI Consensus Expert Panel on Trial evidence (ACCEPT) group. Fibroids in infertility–consensus statement from ACCEPT (Australasian CREI Consensus Expert Panel on Trial evidence) . Aust N Z J Obstet Gynaecol 2011 ; 51 : 289 – 295 .
Loh SF Agarwal R Chan JK Chia SJ Cho LW Lim LH Lau MSK Loh SKE Hendricks MS Nair S et al. Academy of Medicine-Ministry of Health Clinical Practice Guidelines: assessment and management of infertility at primary healthcare level . Singapore Med J 2014 ; 55 – 58 ; quiz 66.
Murphy MK Black NA Lamping DL McKee CM Sanderson CF Askham J Marteau T. Consensus development methods, and their use in clinical guideline development . Health Technol Assess 1998 ; 2 : 1 – 88 , i-iv,
National Institute for Health and Care Excellence . Fertility Problems: Assessment and Treatment . London, UK : National Institute for Health and Care Excellence , 2017
Penzias A Bendikson K Butts S Coutifaris C Falcone T Fossum G Gitlin S Gracia C Hansen K La Barbera A et al. Performing the embryo transfer: a guideline . Fertil Steril 2017 ; 107 : 882 – 896 .
Practice Committee of the American Society for Reproductive Medicine . Uterine septum: a guideline . Fertil Steril 2016 a; 106 : 530 – 540 .
Practice Committee of the American Society for Reproductive Medicine . Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline . Fertil Steril 2016 b; 106 : 1634 – 1647 .
Practice Committee of the American Society for Reproductive Medicine . Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline . Fertil Steril 2017 ; 108 : 416 – 425 .
Prior M Bagness C Brewin J Coomarasamy A Easthope L Hepworth-Jones B Hinshaw K O'Toole E Orford J Regan L et al. Priorities for research in miscarriage: a priority setting partnership between people affected by miscarriage and professionals following the James Lind Alliance methodology . BMJ Open 2017 ; 7 : e016571 .
Wilkinson J Bhattacharya S Duffy JMN Kamath MS Marjoribanks J Repping S Vail A Wely M Farquhar CM. Reproductive medicine: still more ART than science? BJOG: Int J Obstet Gy 2019 a; 126 : 138 – 141 .
Wilkinson J Brison DR Duffy JMN Farquhar CM Lensen S Mastenbroek S van Wely M Vail A. Don’t abandon RCTs in IVF. We don’t even understand them . Hum Reprod 2019 b; 34 : 2093 – 2098 .
Author notes
Members of the Priority Setting Partnership for Infertility are listed in the Appendix .
Email alerts
Companion article.
- Standardizing definitions and reporting guidelines for the infertility core outcome set: an international consensus development study † ‡
Citing articles via
- Recommend to your Library
Affiliations
- Online ISSN 1460-2350
- Copyright © 2024 European Society of Human Reproduction and Embryology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
REVIEW article
Psychological assessment in infertility: a systematic review and meta-analysis.

- 1 Student Research Committee, Kashan University of Medical Sciences, Kashan, Iran
- 2 Cellular, Molecular and Genetics Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 3 Research Center for Biochemistry and Nutrition in Metabolic Diseases, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran
Infertility is a prevalent worldwide health issue and is defined by the World Health Organization (WHO) as a global health problem. Considering the importance of the psychological dimensions of infertility, various measurement tools have been used to measure the variables involved in infertility, of which the most widely used are the following: the Symptom Checklist 90 (SCL90), the Brief Symptom Inventory (BSI), the State-Trait Anxiety Inventory Form (STAI), and the Depression Anxiety Stress Scale (DASS). Therefore, given the problems of infertile people in terms of psychological dimensions, the aim of this meta-analysis was to assess the psychological assessment score in infertility. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol, we applied an online database with no time restriction. Data were gathered using a random-effect model to estimate the standard mean difference (SMD) for the evaluation of the strength of association analyses. Our data demonstrated a significant higher SCL90 score ( CI SCL 90 : 0.96, 0.34–1.57, heterogeneity: 94%, p heterogeneity < 0.001), and a non-significant higher DASS score ( CI Anxiety : 0.82, -0.14 to 1.79; CI Depression : 0.8, -0.28 to 1.87; and CI Stress : 0.82, -0.24 to 1.88). It is essential to seek for strategies to help infertile patients overcome their infertility-related psychological problems.
Introduction
Infertility is a widespread, serious health problem that is defined as a global public health problem by the World Health Organization (WHO) ( Agarwal et al., 2015 ; Cong et al., 2016 ). Infertility is considered as the failed achievement of a viable pregnancy following 1 year of unprotected intercourse, and it is estimated that more than 186 million people suffer from it worldwide ( Inhorn and Patrizio, 2015 ). This period for women over 35 years of age, however, has been considered 6 months ( Practice Committee of the American Society for Reproductive Medicine, 2013 ). The overall estimated burden of subfertility/infertility is announced to be high. Based on the WHO reports, infertility affects over 10% of women ( Boivin et al., 2011 ; Kalkhoran et al., 2011 ; Direkvand Moghaddam et al., 2016 ; Sarkar and Gupta, 2016 ) and 7% of men ( Krausz and Riera-Escamilla, 2018 ). Indeed, the prevalence of secondary infertility is reported to be about 4.9% ( Akhondi et al., 2019 ).
The reasons for infertility might be categorized into four major groups: (1) male factors, (2) female factors, (3) both male and female factors, and (4) unknown etiology ( Speroff and Fritz, 2005 ; Blundell, 2007 ; Krausz, 2011 ). Currently, three major therapeutic strategies, such as surgical therapy (especially endoscopic techniques), pharmacological therapy, and assisted reproductive technology (ART), are available to treat male infertility. ART encompasses all interventional measures, including in vitro handling of both human sperm and oocytes and of embryos for reproduction issues. ART includes in vitro fertilization (IVF) and embryo transfer, gamete and embryo cryopreservation, embryo biopsy, intracytoplasmic sperm injection (ICSI), and preimplantation genetic testing ( Dyer et al., 2016 ).
Infertility affects different life aspects, such as social, mental, and physical ones ( Tanha et al., 2014 ). It also can lead to the development of stigma, shame, depression, anxiety, guilt ( Figure 1 ), and low self-esteem (2019). It is one of the personal and social issues affecting the whole couple’s life and family function, leading to the development of psychological stress and or psychiatric disorders ( Boivin, 2003 ). Infertility can also be considered as one of the most stressful life events. Infertility is believed to be associated with health issues, feeling of grievance, stressful experiences, depression, lack of self-confidence, disappointment, threat, sin, and marital problems ( Noorbala et al., 2008 ). Infertility can generate stress in the family ( Dyer et al., 2005 ; Ozkan and Baysal, 2006 ; Sadeghian et al., 2006 ; Peyvandi et al., 2011 ; Xu et al., 2017 ). It is speculated that psychological factors rather than biological ones might be the primary cause of infertility. This can be an important field of interest for psychologists ( Podolska, 2011 ).
Figure 1. Infertility is a real challenge as a couple disorder and sexual dysfunction is an emerging paradigm. A couple-centered approach for the treatment for infertility is mandatory requiring a multidisciplinary team for the comprehensive management of ISS including the whole sexual, rational, and emotional aspects of infertile couples. ART assisted reproductive technology, ED erectile dysfunction, HSDD hypoactive sexual desire disorder, and ISS Inferto-Sex Syndrome. This figure is adapted from Luca et al. (2021) .
Due to the importance of the psychological dimension of infertility, various psychological treatments for infertility have been used so far ( Domar et al., 1992 ; McQueeney et al., 1997 ; Hosaka et al., 2002 ; McNaughton-Cassill et al., 2002 ; Cousineau et al., 2008 ; Faramarzi et al., 2008 ; Lancastle and Boivin, 2008 ; Panagopoulou et al., 2009 ; Hämmerli et al., 2010 ; Hughes and da Silva, 2011 ; Vizheh et al., 2013 ; Frederiksen et al., 2015 ). Due to the importance of the psychological dimensions of infertility, various measurement tools have been used to assess the variables involved in infertility. The most widely used factors are the following: the Symptom Checklist 90 (SCL90) ( Derogatis et al., 1977 ), the Brief Symptom Inventory (BSI) ( Derogatis and Spencer, 1993 ), the Depression Anxiety Stress Scale (DASS) ( Parkitny and McAuley, 2010a ), and the State-Trait Anxiety Inventory Form (STAI) ( Spielberger, 1983 ).
Given the importance of psychological issues and problems in the quality of life and the quality of physical therapy for infertile people, paying attention to the psychological dimensions of this problem is strongly required. On the other hand, due to the wide spectrum of mental problems, and in other words, the subjectivity of psychological problems, it is necessary to be able to measure the severity of psychological problems with small tools and break them down into smaller components so that we can study them in the form of scientific problems. One of the important tools to achieve this goal is the use of self-reported psychometric tools, which, despite the problems that this type of measurement has, can partially explain the total dimensions of the problem. Despite the number of studies investigating the association between psychological assessment scores and infertility, there are still some contradictory results. Thus, we aimed to evaluate the association between the psychological assessment score and infertility patients through a meta-analysis approach.
Materials and methods
Search strategy.
This study has been done according to the PRISMA protocol ( Moher et al., 2009 ). A systematic search was performed by two independent researchers (SAT and AHM) from the online database to find relevant publications until September 2021. The keywords used in our search strategy were ((”Infertility”) AND (”Psychological assessment” OR “Symptom Checklist-90” OR “SCL90” OR “Brief Symptom Inventory” OR “BSI” OR “Depression Anxiety Stress Scale” OR “DASS” OR “State-Trait Anxiety Inventory Form” OR “STAI”)). No restrictions were considered for the time and language of publications.
Inclusion and exclusion criteria
Inclusion criteria include cases defined according to infertility diagnosis; all studies evaluating the SCL90, BSI, DASS, and STAI scores of patients with infertility; studies that reported the SCL90, BSI, DASS, and STAI scores at baseline through clinical trials on infertile patients vs. healthy controls; and those that reported mean ± standard deviation (SD). In the case of the same dataset with more than one publication, only articles with more complete findings were included in our study. On the other hand, comments, short letters, clinical trials without a healthy control group, communications, reviews, meta-analyses, case reports, and animal studies were excluded from the meta-analysis.
Data extraction
Two independent researchers performed data extraction and study selection. The reported mean ± SD for the SCL90, BSI, DASS, and STAI scores were extracted from patients compared with the control group. In addition, we extracted the information from each study ( Tables 1 – 5 ).
Table 1. SCL90 assessment studies in infertility.
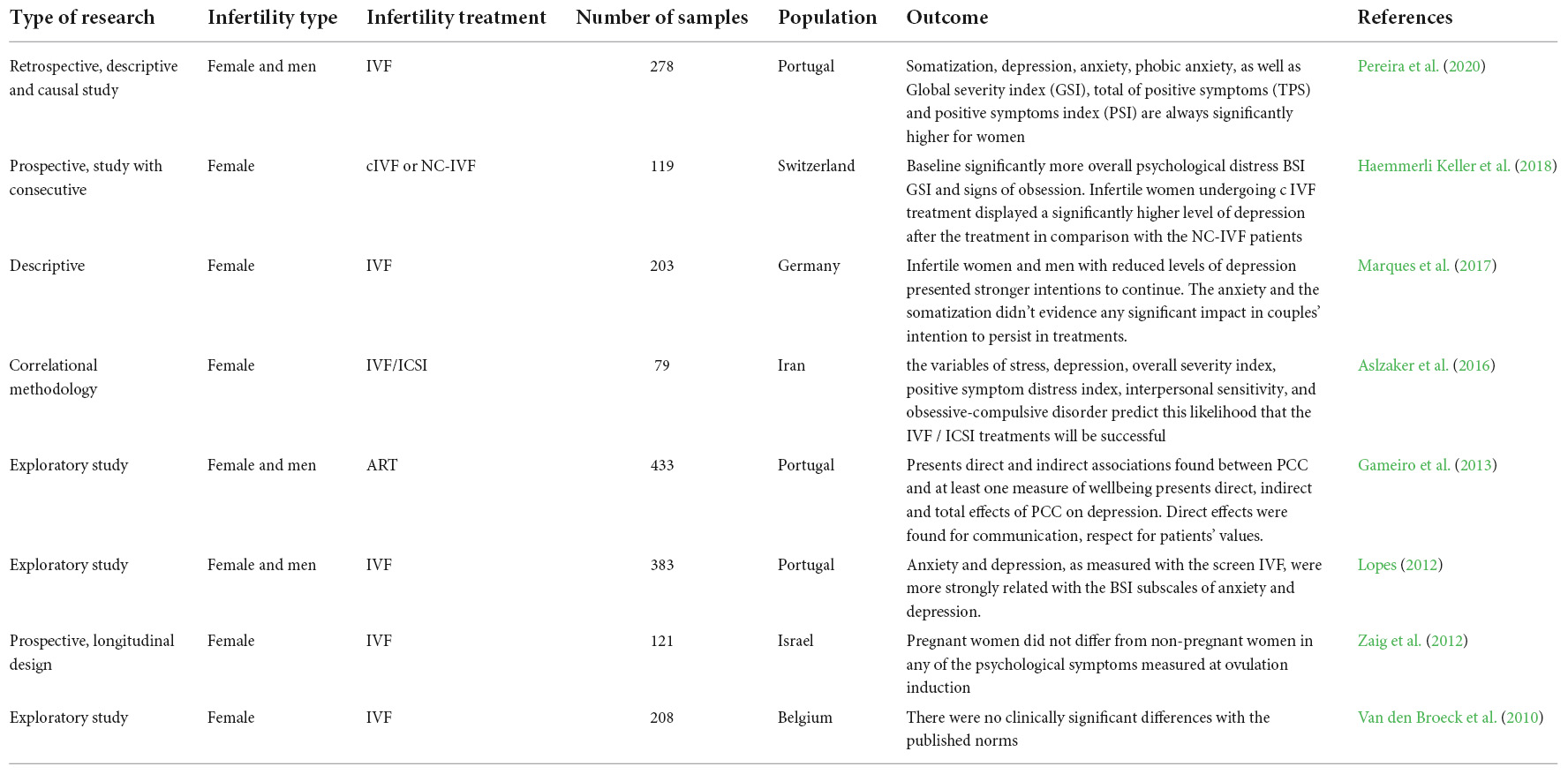
Table 2. BSI assessment studies in infertility.
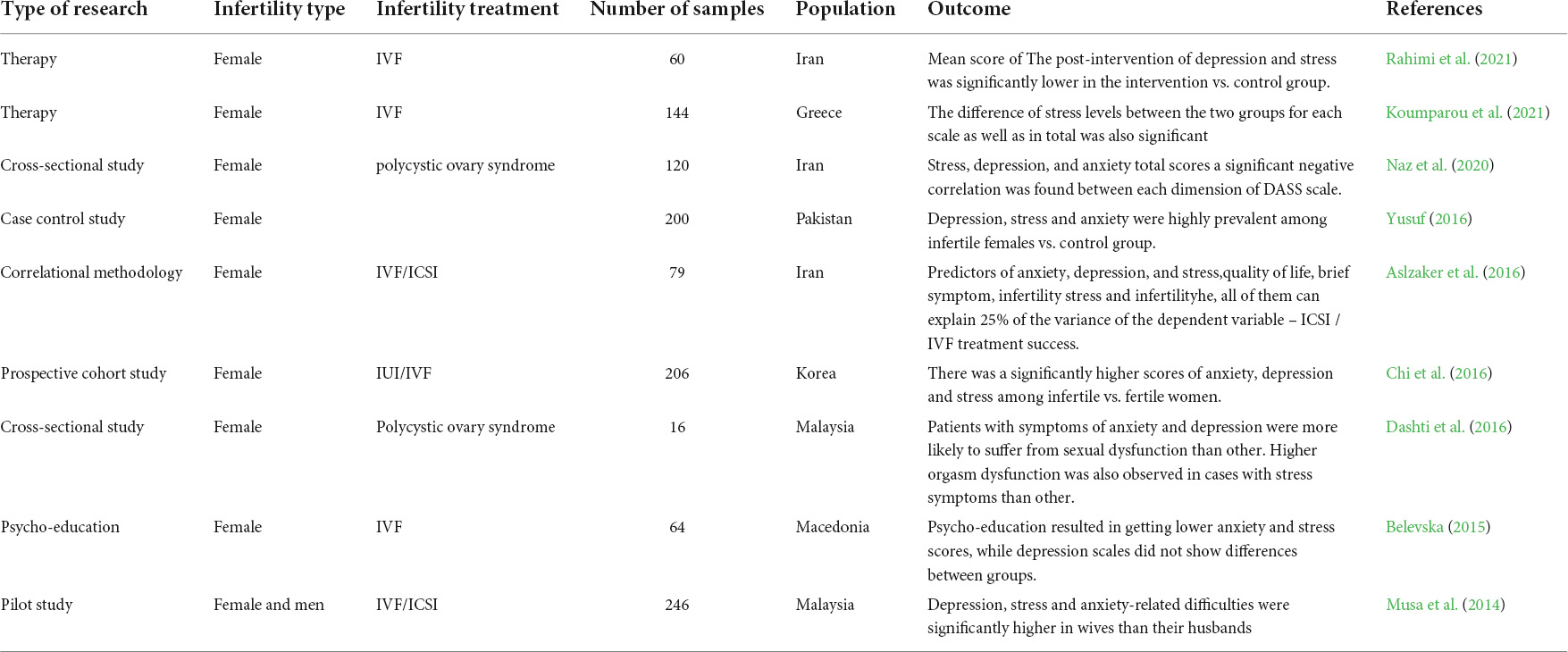
Table 3. DASS assessment studies in infertility.
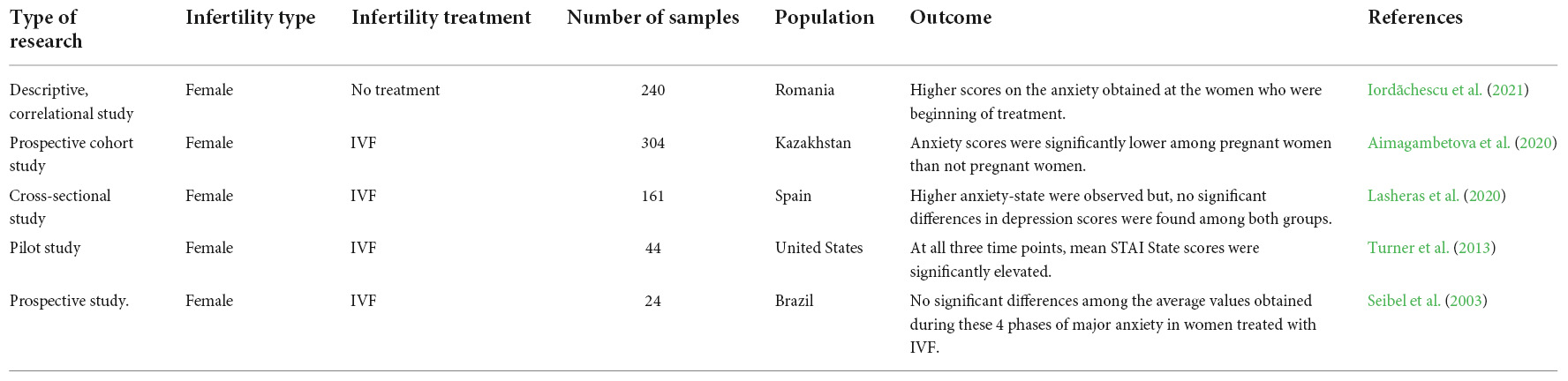
Table 4. STAI-S assessment studies in infertility.
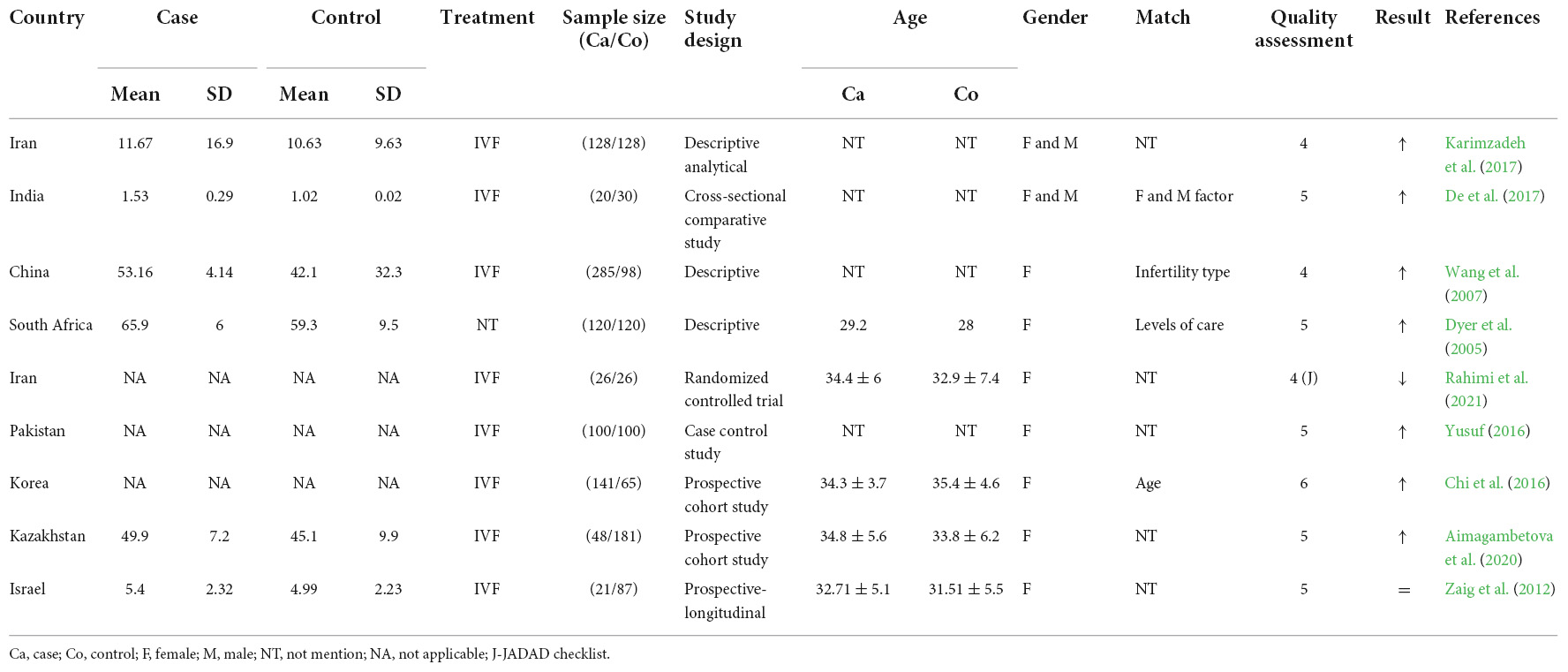
Table 5. General characteristics of included studies in the meta-analysis.
Quality assessment
A form of the Newcastle Ottawa scale (NOS) was designed for observational studies and was used to assess the quality of selected studies. The NOS considers a maximum of ten points for each study. The studies that had an NOS score of 5 or more were considered high quality publications ( Peterson et al., 2011 ). We used the JADAD checklist for evaluation of the quality of interventional studies ( Jadad et al., 1996 ). Two independent reviewers filled out the scores for each eligible study.
Statistical analysis
For both the infertility and control groups, the SCL90, BSI, DASS, and STAI scores were reported using mean ± SD and 95% confidence interval ( CI ). The overall mean ± SD data were calculated using a random-effect model and/or a fixed-effect model. The SMD and CI were considered the overall if there was true heterogeneity between included studies, we employed a random-effect model; otherwise, a fixed-effect model was used. To assess between-study heterogeneity, Cochran’s Q -test and I 2 were used. A random-effect model was used if p heterogeneity was less than 0.1 ( Higgins et al., 2011 ). Statistical analyses were carried out using review manager software (STATA version 14, StataCorp).
We identified 32 studies in our systematic review. The last remaining nine articles were included in the meta-analysis, and the characteristics of these studies are illustrated in Figure 2 . The results of the overall and stratified analyses are summarized in Figure 3 . Regarding SCL90 and DASS, there was a statistically significant association between higher SCL90 scores and infertility. However, there was no significant difference between the case and control groups regarding the higher score of DASS. Indeed, true heterogeneities were observed across studies for the two abovementioned parameters.
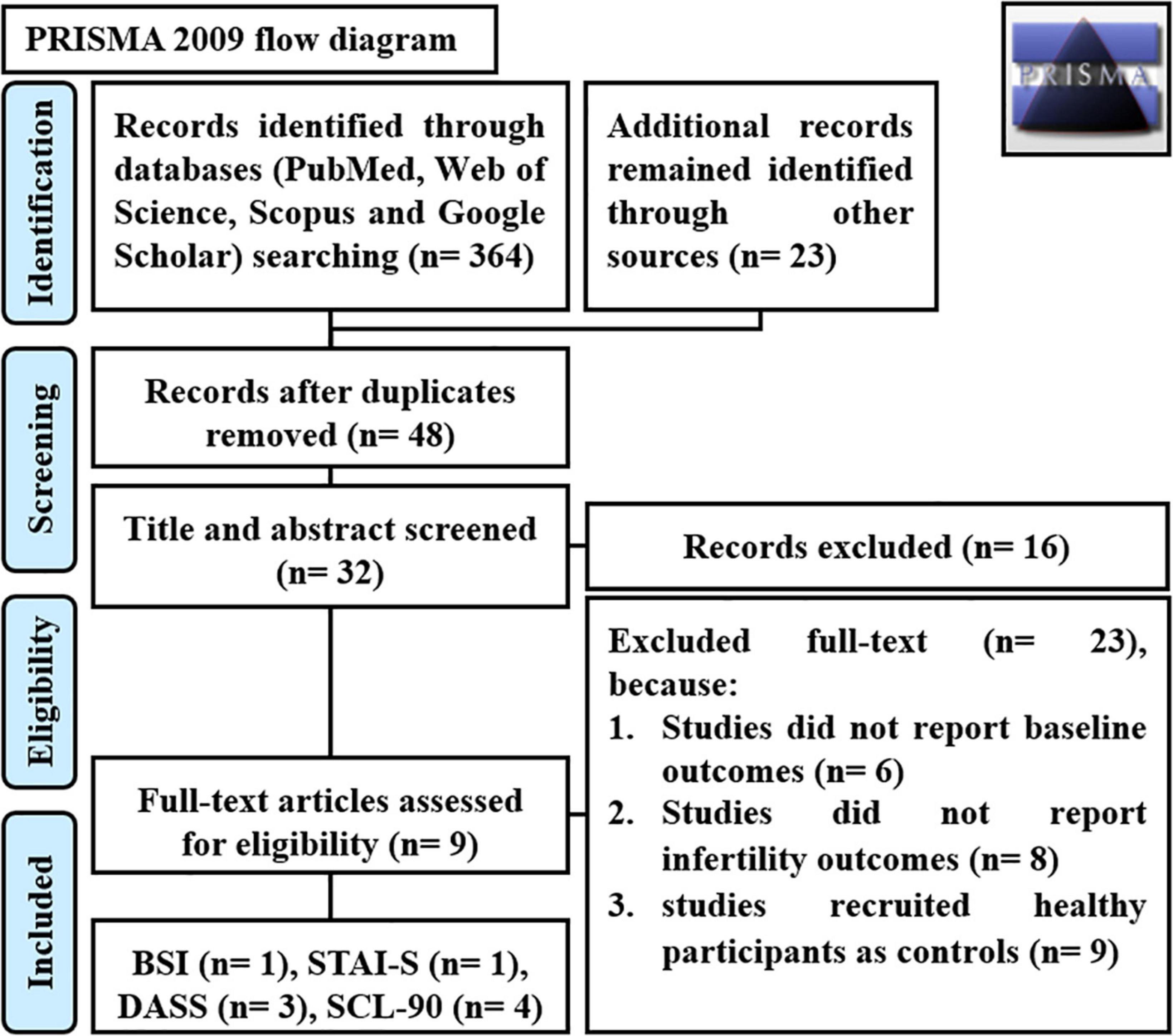
Figure 2. PRISMA flow diagram. SCL90 Symptom Checklist 90, BSI Brief Symptom Inventory, STAI State-Trait Anxiety Inventory Form, and DASS Depression Anxiety Stress Scale.
Figure 3. Forest plot mean difference and 95% confidence intervals (CI) of the meta-analysis on psychological assessment score and association with infertility: Depression Anxiety Stress Scale (DASS) (A–C) (Anxiety, Depression, and Stress respectively); Symptom Checklist 90 (SCL90) (D) . Horizontal lines represent 95% CI.
In our meta-analysis, we found a statistically significant higher score of SCL90 but there was not a statistically significant association between a higher score of DASS and infertility. Only one study was performed regarding the relationship between BSI and STAI in association with infertility. Therefore, further study is needed to investigate the association between infertility and BSI and STAI. In line with our findings, Petersen et al. in gynecologic cancer ( Petersen et al., 2005 ), Shi et al. in polycystic ovarian syndrome (PCOS) ( Shi et al., 2011 ), and Pan et al. (2013) in breast cancer reported higher SCL90 scores in subjects compared with the control group. Similar investigations were also performed. Barber and Steadman demonstrated a non-significantly different DASS score in pregnant vs. non-pregnant women ( Barber and Steadman, 2018 ). In contrast, Rothemund et al. observed no significant difference regarding the SCL90 score in women at high risk for breast cancer ( Rothemund et al., 2001 ). Furthermore, Ali et al., Jamshidian-QalehShahi et al., and Ho et al. reported higher DASS scores in subjects with infertility ( Ali et al., 2011 ; Jamshidian-QalehShahi et al., 2017 ; Ho et al., 2020 ).
In comparison with the DASS, the SCL90 is used to measure more general psychological symptoms ( Eivors et al., 2008 ). Additionally, it examines more general and complete psychological distress among people compared with the DASS score ( Modabernia et al., 2010 ). Indeed, the physicalization and obsessive-compulsive scales, which are higher in infertile cases, are included in the SCL90 while they are not measured by the DASS score ( Conrad et al., 2003 ; Burns, 2007 ). One of the scales which is examined more completely in the SCL90 test than the DASS score and is also observed in infertile people is interpersonal sensitivity, i.e., infertile people usually have a higher score on this scale due to the psychological nature of infertility ( Noorbala et al., 2009 ). Furthermore, one of the scales that exists meaningfully in infertile people and is measured by the SCL90 test is paranoid ideations due to the psychological burden of bereavement in infertility ( Sadeghian et al., 2006 ). In general, the SCL90 test examines the psychological symptoms caused by infertility in a wider way than the DASS test, and it is a more reliable scale for measuring the overall symptoms. However, the DASS test, which is applied for the measurement of depression, stress, and anxiety, is more specialized than the SCL90 since it examines these three scales, and if the goal is only to examine these three scales, the DASS test will be more specialized ( Parkitny and McAuley, 2010b ).
The components of anxiety and depression exist in infertile patients. Since the nature of infertility is felt by infertile people, such as experiencing grief and experiencing a feeling of deficiency in the body, the intensity of these components will increase psychologically in these people. Generally, some aspects of depression are caused by the feeling of impaired efficiency ( Lapane et al., 1995 ). In addition, biological data indicate decreased serotonin and norepinephrine levels in these people following the emergence of depression problems ( Shi et al., 2011 ). Studies demonstrate the negative impacts of these two factors on fertility treatment ( Szkodziak et al., 2020 ).
A study on a rat sample of depression and menopause illustrated significantly higher levels of follicle-stimulating hormone (FSH), Luteinizing hormone (LH), cortisone, and Adrenocorticotropic hormone (ACTH) in groups exposed to mild stress factors vs. those without such an exposure. On the other hand, dopamine levels were lower in the stress groups ( Gu et al., 2018 ).
Men and women equally suffer from obsessive-compulsive disorder (OCD) that most often develops in early life. Infertile cases with a history of OCD might be focused on contamination obsessions and cleaning rituals associated with infertility treatments due to their assumption regarding sterile techniques associated with injections as a part of various treatments for infertility ( Williams and Koran, 1997 ) that can enhance their exclusive compulsive ritualistic behaviors and or trigger their newly emerged rituals. Treatment compliance can be impaired due to the existing needle and blood injury phobias and their associated unmanageable anxiety state. Caregivers might be required to adapt therapeutic protocols or request psychological or psychiatric care before going on treatment ( Williams and Zappert, 2006 ). Cognitive behavioral therapies and behavioral strategies should also be encouraged ( Williams and Zappert, 2006 ). A close association was observed between hypothalamus-pituitary disorders and psychological scores in PCOS. In the current study, we observed a significant positive correlation between serum concentration of 3-methoxy-4-hydroxyphenylglycol and phobic subscale scores of SCL90 in total samples. Hostility subscale scores demonstrated a significant negative correlation with serum concentration of Dihydroxyphenylacetic acid of PCOS cases, however, no significant correlation was observed between other monoamine neurotransmitters and their metabolites and other subscales of the SCL90 score ( Shi et al., 2011 ).
Several lines of research support the positive impacts of harp therapy, group psychotherapy, cognitive behavioral therapy, and mind-body intervention (Eastern body-mind-spirit, Integrative body-mind-spirit, and mind-body intervention) on the pregnancy rate, anxiety level, or marital function of infertile couples ( Ying et al., 2016 ). A complex intervention should be developed based on sound evidence to target both men and women in infertile couples who have undergone IVF treatment, especially during the stressful interval waiting for the results of pregnancy tests following failed cycles.
This meta-analysis has several limitations. First, the main limitation is the insufficient number of eligible performed studies containing the subgroup analysis. Second, psychological symptoms are non-specific, and they can be affected by various factors. Although some of these factors, such as age and gender, were considered in included studies, not all of them have been considered in the total associated factors. For a better understanding of the association between psychological status and infertility, it is advisable to consider all of the abovementioned factors. Additionally, the included studies derived from different sample sources and diverse applied methodologies, will naturally increase the data heterogeneity. Last, the low number of eligible articles, the moderate quality (mean of 5 in the NOS), and the high heterogeneity of effect sizes among the included studies in each independent meta-analysis are factors that affect reliability and are better avoided. Some other confounding factors, such as disease phase (acute or remission phase), the age of the disease onset, and the presence of concomitant psychopharmacological treatments of patients, were not considered in the meta-analysis.
In conclusion, we found evidence supporting an increased significant SCL90 score and a non-significant higher DASS score in infertility patients. The prevalence of psychological problems in infertile cases seems to be increasing in many countries. These findings suggest that psychological treatment might have some beneficial impacts on infertility cases. Further research is needed to confirm this association.
Author contributions
SAT, AHM, and AM designed the study. SAT, AHM, and AM collected data. SAT, AHM, AM, and MB wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abedinia, N., Hamad, A. H., and Ali, S. K. (2013). Psychology and Personality Types of Infertile and Fertile Women. Iraqi Nat. J. Nurs. Special. 26, 63–70.
Google Scholar
Adib-Rad, H., Basirat, Z., Faramarzi, M., Mostafazadeh, A., and Bijani, A. (2019). Psychological distress in women with recurrent spontaneous abortion: A case-control study. Turk. J. Obstetr. Gynecol. 16:151. doi: 10.4274/tjod.galenos.2019.88899
PubMed Abstract | CrossRef Full Text | Google Scholar
Agarwal, A., Mulgund, A., Hamada, A., and Chyatte, M. R. (2015). A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 13, 1–9. doi: 10.1186/s12958-015-0032-1
Aimagambetova, G., Issanov, A., Terzic, S., Bapayeva, G., Ukybassova, T., Baikoshkarova, S., et al. (2020). The effect of psychological distress on IVF outcomes: Reality or speculations? PLoS One 15:e0242024. doi: 10.1371/journal.pone.0242024
Akhondi, M. M., Ranjbar, F., Shirzad, M., Ardakani, Z. B., Kamali, K., and Mohammad, K. (2019). Practical difficulties in estimating the prevalence of primary infertility in Iran. Int. J. F. S. 13:113.
Ali, S., Sabih, F., Rashid, F., Jehan, S., and Anwar, M. (2011). Psychological morbidity amongst infertile couples. J. Islamic Intl. Med. College 6, 3–7.
Aslzaker, M., Pourshahbaz, A., Bagheri Lankarani, N., Mohammadkhani, P., and Geranmayepour, S. (2016). Effects of infertility stress, psychological symptoms, and quality of life on predicting success rate of IVF/ICSI treatment in infertile women. Practice Clin. Psychol. 4, 275–281. doi: 10.18869/acadpub.jpcp.4.4.275
Barber, C. C., and Steadman, J. (2018). Distress levels in pregnant and matched non-pregnant women. Austr. New Zealand J. Obstetr. Gynaecol. 58, 128–131. doi: 10.1111/ajo.12712
Belevska, J. (2015). The impact of psycho-education on in vitro fertilisation treatment efficiency. Prilozi 36, 211–216. doi: 10.1515/prilozi-2015-0069
Blundell, R. (2007). Causes of infertility. Int. J. Mol. Med. Adv. Sci. 3, 63–65.
Boivin, J. (2003). A review of psychosocial interventions in infertility. Soc. Sci. Med. 57, 2325–2341. doi: 10.1016/S0277-9536(03)00138-2
CrossRef Full Text | Google Scholar
Boivin, J., Griffiths, E., and Venetis, C. A. (2011). Emotional distress in infertile women and failure of assisted reproductive technologies: Meta-analysis of prospective psychosocial studies. BMJ 342:d223. doi: 10.1136/bmj.d223
Burns, L. H. (2007). Psychiatric aspects of infertility and infertility treatments. Psychiatr. Clin. North Am. 30, 689–716. doi: 10.1016/j.psc.2007.08.001
Chen, D., Yu, J., Wang, W.-h, and He, C.-y (2008). Mental status of infertile women treated with IVF-ET and effects of outcome of their treatment. China J. Modern Med. 12.
Chi, H.-J., Park, I.-H., Sun, H.-G., Kim, J.-W., and Lee, K.-H. (2016). Psychological distress and fertility quality of life (FertiQoL) in infertile Korean women: The first validation study of Korean FertiQoL. Clin. Exp. Reprod. Med. 43:174. doi: 10.5653/cerm.2016.43.3.174
Cong, J., Li, P., Zheng, L., and Tan, J. (2016). Prevalence and risk factors of infertility at a rural site of Northern China. PLoS One 11:e0155563. doi: 10.1371/journal.pone.0155563
Conrad, R., Schilling, G., Hagemann, T., Haidl, G., and Liedtke, R. (2003). Somatization and alexithymia in male infertility. A replication study. Hautarzt 54, 530–535. doi: 10.1007/s00105-002-0469-y
Cousineau, T. M., Green, T. C., Corsini, E., Seibring, A., Showstack, M. T., Applegarth, L., et al. (2008). Online psychoeducational support for infertile women: A randomized controlled trial. Hum. Reprod. 23, 554–566. doi: 10.1093/humrep/dem306
Dashti, S., Latiff, L. A., Hamid, H. A., Sani, S. M., Akhtari-Zavare, M., Abu Bakar, A. S., et al. (2016). Sexual dysfunction in patients with polycystic ovary syndrome in Malaysia. Asian Pac. J. Cancer Prev. 17, 3747–3751.
De, D., Roy, P. K., and Sarkhel, S. (2017). A psychological study of male, female related and unexplained infertility in Indian urban couples. J. Reprod. Infant Psychol. 35, 353–364. doi: 10.1080/02646838.2017.1315632
Derogatis, L. R., and Spencer, P. (1993). Brief symptom inventory: BSI , Vol. 18. Pearson Upper Saddle River, NJ: Pearson education.
Derogatis, L. R., Lipman, R., and Covi, L. (1977). SCL-90. Administration, scoring and procedures manual-I for the R (revised) version and other instruments of the psychopathology rating scales series. Chicago: Johns Hopkins University School of Medicine.
Direkvand Moghaddam, A., Delpisheh, A., and Sayehmiri, K. (2016). An investigation of the worldwide prevalence of infertility as a systematic review. Qom Univ. Med. Sci. J. 10, 76–87.
Domar, A. D., Zuttermeister, P. C., Seibel, M., and Benson, H. (1992). Psychological improvement in infertile women after behavioral treatment: A replication. F. S. 58, 144–147. doi: 10.1016/S0015-0282(16)55151-6
Dyer, S. J., Abrahams, N., Mokoena, N., Lombard, C. J., and van der Spuy, Z. M. (2005). Psychological distress among women suffering from couple infertility in South Africa: A quantitative assessment. Hum. Reprod. 20, 1938–1943. doi: 10.1093/humrep/deh845
Dyer, S., Chambers, G. M., de Mouzon, J., Nygren, K.-G., Zegers-Hochschild, F., Mansour, R., et al. (2016). International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted reproductive technology 2008, 2009 and 2010. Hum. Reprod. 31, 1588–1609. doi: 10.1093/humrep/dew082
Eivors, A., Button, E., Warner, S., Turner, K., Fassino, S., Abbate-Daga, G., et al. (2008). Scl-90: An outpatient psychiatric rating. Hillsdale, NJ: Erlbaum.
Faramarzi, M., Kheirkhah, F., Esmaelzadeh, S., Alipour, A., Hjiahmadi, M., and Rahnama, J. (2008). Is psychotherapy a reliable alternative to pharmacotherapy to promote the mental health of infertile women? A randomized clinical trial. Eur. J. Obstetr. Gynecol. Reprod. Biol. 141, 49–53. doi: 10.1016/j.ejogrb.2008.07.012
Frederiksen, Y., Farver-Vestergaard, I., Skovgård, N. G., Ingerslev, H. J., and Zachariae, R. (2015). Efficacy of psychosocial interventions for psychological and pregnancy outcomes in infertile women and men: A systematic review and meta-analysis. BMJ Open 5:e006592. doi: 10.1136/bmjopen-2014-006592
Gameiro, S., Canavarro, M. C., and Boivin, J. (2013). Patient centred care in infertility health care: Direct and indirect associations with wellbeing during treatment. Patient Educ. Counsel. 93, 646–654. doi: 10.1016/j.pec.2013.08.015
Gu, S., Jing, L., Li, Y., Huang, J. H., and Wang, F. (2018). Stress induced hormone and neuromodulator changes in menopausal depressive rats. Front. Psychiatry 9:253. doi: 10.3389/fpsyt.2018.00253
Haemmerli Keller, K., Alder, G., Loewer, L., Faeh, M., Rohner, S., and von Wolff, M. (2018). Treatment-related psychological stress in different in vitro fertilization therapies with and without gonadotropin stimulation. Acta Obstetr. Gynecol. Scand. 97, 269–276. doi: 10.1111/aogs.13281
Hamdouna, O. S. (2014). The psychological consequences of sterility among a sample of infertile women in Gaza City. J. Al Quds Open Univ. Educ. Psychol. Res. Stud. 2:2.
Hämmerli, K., Znoj, H., and Berger, T. (2010). Internet-based support for infertile patients: A randomized controlled study. J. Behav. Med. 33, 135–146. doi: 10.1007/s10865-009-9243-2
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi: 10.1136/bmj.d5928
Ho, T. T. T., Le, M. T., Truong, Q. V., Nguyen, V. Q. H., and Cao, N. T. (2020). Psychological burden in couples with infertility and its association with sexual dysfunction. Sex. Disabil. 38, 123–133. doi: 10.1007/s11195-019-09612-4
Hosaka, T., Matsubayashi, H., Sugiyama, Y., Izumi, S.-i, and Makino, T. (2002). Effect of psychiatric group intervention on natural-killer cell activity and pregnancy rate. Gen. Hosp. Psychiatry 24, 353–356. doi: 10.1016/S0163-8343(02)00194-9
Hughes, E. G., and da Silva, A. M. (2011). A pilot study assessing art therapy as a mental health intervention for subfertile women. Hum. Reprod. 26, 611–615. doi: 10.1093/humrep/deq385
Inhorn, M. C., and Patrizio, P. (2015). Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 21, 411–426. doi: 10.1093/humupd/dmv016
Iord ǎ chescu, D. A., Paica, C. I., Boca, A. E., Gic ǎ , C., Panaitescu, A. M., Peltecu, G., et al. (2021). Anxiety, difficulties, and coping of infertile women. Healthcare 9:466. doi: 10.3390/healthcare9040466
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J. M., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 17, 1–12. doi: 10.1016/0197-2456(95)00134-4
Jamshidian-QalehShahi, P., Aghaei, A., and Golparvar, M. (2017). Investigating the effectiveness of iranian–islamic positive therapy on depression. anxiety, and stress of infertile women. J. Isfahan Med. Sch. 35, 70–76.
Kalkhoran, L. F., Bahrami, H., Farrokhi, N. A., Zeraati, H., and Tarahomi, M. (2011). Comparing anxiety, depression and sexual life satisfaction in two groups of fertile and infertile women in Tehran. J. Reprod. Infertil. 12, 157–162.
Karimzadeh, M., Salsabili, N., Asbagh, F. A., Teymouri, R., Pourmand, G., and Naeini, T. S. (2017). Psychological disorders among Iranian infertile couples undergoing assisted reproductive technology (ART). Iranian J. Public Health 46:333.
Koumparou, M., Bakas, P., Pantos, K., Economou, M., and Chrousos, G. (2021). Stress management and In Vitro Fertilization (IVF): A pilot randomized controlled trial. Psychiatriki 32, 290–299. doi: 10.22365/jpsych.2021.029
Krausz, C. (2011). Male infertility: Pathogenesis and clinical diagnosis. Best Practice Res. Clin. Endocrinol. Metab. 25, 271–285. doi: 10.1016/j.beem.2010.08.006
Krausz, C., and Riera-Escamilla, A. (2018). Genetics of male infertility. Nat. Rev. Urol. 15, 369–384. doi: 10.1038/s41585-018-0003-3
Lancastle, D., and Boivin, J. (2008). A feasibility study of a brief coping intervention (PRCI) for the waiting period before a pregnancy test during fertility treatment. Hum. Reprod. 23, 2299–2307. doi: 10.1093/humrep/den257
Lapane, K. L., Zierler, S., Lasater, T. M., Stein, M., Barbour, M. M., and Hume, A. L. (1995). Is a history of depressive symptoms associated with an increased risk of infertility in women? Psychos. Med. 57, 509–513. doi: 10.1097/00006842-199511000-00001
Lasheras, G., Mestre-Bach, G., Clua, E., Rodríguez, I., and Farré-Sender, B. (2020). Cross-border reproductive care: Psychological distress in a sample of women undergoing in vitro fertilization treatment with and without oocyte donation. Int. J. F. S. 14:130.
Lopes, V. G. B. (2012). Are patients at risk for emotional maladjustment to fertility treatmant less willing to comply with treatment?: Results from the validation of the portuguese version of rhew SCREENIVF. Hum. Reprod. 29, 293–302. doi: 10.1093/humrep/det418
Luca, G., Parrettini, S., Sansone, A., Calafiore, R., and Jannini, E. (2021). The Inferto-Sex Syndrome (ISS): Sexual dysfunction in fertility care setting and assisted reproduction. J. Endocrinol. Invest. 44, 2071–2102. doi: 10.1007/s40618-021-01581-w
Marques, I., Balreira, M. L., and Temótio, J. (2017). How does depressive and anxious symptomatology influence the intention of infertile couples to persist in fertility treatments? Berlin: XVII world congress of psychiatry.
McNaughton-Cassill, M. E., Bostwick, J. M., Arthur, N. J., Robinson, R. D., and Neal, G. S. (2002). Efficacy of brief couples support groups developed to manage the stress of in vitro fertilization treatment. Mayo Clin. Proc. 77, 1060–1066. doi: 10.4065/77.10.1060
McQueeney, D. A., Stanton, A. L., and Sigmon, S. (1997). Efficacy of emotion-focused and problem-focused group therapies for women with fertility problems. J. Behav.Med. 20, 313–331. doi: 10.1023/A:1025560912766
Modabernia, M., Shojaie, T. H., Falahi, M., and Faghirpour, M. (2010). Normalizing SCL-90-R inventory in Guilan high-school students. J. Guilan Univ. Med. Sci. 19, 58–65.
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 62, 1006–1012. doi: 10.1016/j.jclinepi.2009.06.005
Musa, R., Ramli, R., Yazmie, A. W. A., Khadijah, M. B. S., Hayati, M. Y., Midin, M., et al. (2014). A preliminary study of the psychological differences in infertile couples and their relation to the coping styles. Compr. Psychiatry 55, S65–S69. doi: 10.1016/j.comppsych.2013.01.001
Naz, M. S. G., Tehrani, F. R., Lak, T. B., Mohammadzadeh, F., Nasiri, M., Badr, F. K., et al. (2020). Quality of life and emotional states of depression, anxiety and stress in adolescents with polycystic ovary syndrome: A cross-sectional study. Psychol. Res. Behav. Manag. 13:203. doi: 10.2147/PRBM.S241192
Noorbala, A. A., Ramazanzadeh, F., Malekafzali, H., Abedinia, N., Forooshani, A. R., Shariat, M., et al. (2008). Effects of a psychological intervention on depression in infertile couples. Int. J. Gynecol. Obstetr. 101, 248–252. doi: 10.1016/j.ijgo.2007.12.010
Noorbala, A. A., Ramezanzadeh, F., Abedinia, N., and Naghizadeh, M. M. (2009). Psychiatric disorders among infertile and fertile women. Soc. Psychiatry Psychiatr. Epidemiol. 44, 587–591. doi: 10.1007/s00127-008-0467-1
Noorbala, A. A., Ramezanzadeh, F., Abedinia, N., Yazdi, S. A. B., and Jafarabadi, M. (2007). Study of psychiatric disorders among fertile and infertile women and some predisposing factors. J. Fam. Reprod. Health 6–11.
Ozkan, M., and Baysal, B. (2006). Emotional distress of infertile women in Turkey. Clin. Exp. Obstetr. Gynecol. 33, 44–46.
Pan, X.-F., Fei, M.-D., Zhang, K. Y., Fan, Z.-L., Fu, F.-H., and Fan, J.-H. (2013). Psychopathological profile of women with breast cancer based on the symptom checklist-90-R. Asian Pac. J. Cancer Prev. 14, 6579–6584. doi: 10.7314/APJCP.2013.14.11.6579
Panagopoulou, E., Montgomery, A., and Tarlatzis, B. (2009). Experimental emotional disclosure in women undergoing infertility treatment: Are drop outs better off? Soc. Sci. Med. 69, 678–681. doi: 10.1016/j.socscimed.2009.06.031
Parkitny, L., and McAuley, J. (2010a). The depression anxiety stress scale (DASS). J. Physiother. 56:204.
Parkitny, L., and McAuley, J. (2010b). The depression anxiety stress scale (DASS). J. Physiother. 56:204.
Pereira, B. S., Martins, R. S., Brito, C. B., and Pereira, P. A. (2020). Influence of psychosocial factors on the effectiveness of infertility treatments. Acta Obstétr. Ginecol. Portuguesa 14, 208–216.
Petersen, R. W., Graham, G., and Quinlivan, J. A. (2005). Psychologic changes after a gynecologic cancer. J. Obstetr. Gynaecol. Res. 31, 152–157. doi: 10.1111/j.1341-8076.2005.00263.x
Peterson, J., Welch, V., Losos, M., and Tugwell, P. (2011). The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute.
Peyvandi, S., Hosseini, S. H., Daneshpour, M., Mohammadpour, R., and Qolami, N. (2011). The prevalence of depression, anxiety and marital satisfaction and related factors in infertile women referred to infertility clinics of Sari city in 2008. J. Mazandaran Univ. Med. Sci. 20, 26–32.
Podolska, M. (2011). Bidzan M. Niepłodność jako problem psychologiczny. Ginekol. Pol. 82, 44–49.
Practice Committee of the American Society for Reproductive Medicine (2013). Definitions of infertility and recurrent pregnancy loss: A committee opinion. F. S. 99:63. doi: 10.1016/j.fertnstert.2012.09.023
Rahimi, R., Hasanpour, S., Mirghafourvand, M., and Esmaeilpour, K. (2021). Effect of Hope-oriented group counseling on mental health of infertile women with failed IVF cycles: A randomized controlled trial. BMC Psychiatry 21:286. doi: 10.1186/s12888-021-03280-5
Rothemund, Y., Paepke, S., and Flor, H. (2001). Perception of risk, anxiety, and health behaviors in women at high risk for breast cancer. Int. J. Behav. Med. 8, 230–239. doi: 10.1207/S15327558IJBM0803_5
Sadeghian, E., Heydarianpour, A., and Abed, P. (2006). Comparison of psychiatric problems in infertile men and women referring to infertility clinic of Hamadan Fatemyeh Hospital. Arak Med. Univ. J. 9, 31–39.
Santa-Cruz, D. C., Caparros-Gonzalez, R. A., Romero-Gonzalez, B., Peralta-Ramirez, M. I., Gonzalez-Perez, R., and García-Velasco, J. A. (2020). Hair cortisol concentrations as a biomarker to predict a clinical pregnancy outcome after an IVF cycle: A pilot feasibility study. Int. J. Environ. Res. Public Health 17:3020. doi: 10.3390/ijerph17093020
Sarkar, S., and Gupta, P. (2016). Socio-demographic correlates of women’s infertility and treatment seeking behavior in India. J. Reprod. Infertil. 17:123.
Seibel, D., Lobo, D. S., Motta, E. L., Kotecki, J. A., Fuentes, D., and Serafini, P. C. (2003). Assessment of anxiety in women undergoing in vitro fertilization (IVF) by the state-trait anxiety inventory (STAI). F. S. 80:242. doi: 10.1016/S0015-0282(03)01591-7
Shi, X., Zhang, L., Fu, S., and Li, N. (2011). Co-involvement of psychological and neurological abnormalities in infertility with polycystic ovarian syndrome. Arch. Gynecol. Obstetr. 284, 773–778. doi: 10.1007/s00404-011-1947-1
Speroff, L., and Fritz, M. A. (2005). Clinical gynecologic endocrinology and infertility. Philadelphia: lippincott Williams & wilkins.
Spielberger, C. D. (1983). State-trait anxiety inventory for adults. Washington, D.C: American Psychological Association. doi: 10.1037/t06496-000
Szkodziak, F., Krzyżanowski, J., and Szkodziak, P. (2020). Psychological aspects of infertility. A systematic review. J. Int. Med. Res. 48:0300060520932403. doi: 10.1177/0300060520932403
Tanha, F. D., Mohseni, M., and Ghajarzadeh, M. (2014). Sexual function in women with primary and secondary infertility in comparison with controls. Int. J. Impotence Res. 26, 132–134. doi: 10.1038/ijir.2013.51
Turner, K., Reynolds-May, M. F., Zitek, E. M., Tisdale, R. L., Carlisle, A. B., and Westphal, L. M. (2013). Stress and anxiety scores in first and repeat IVF cycles: A pilot study. PLoS One 8:e63743. doi: 10.1371/journal.pone.0063743
Van den Broeck, U., D’Hooghe, T., Enzlin, P., and Demyttenaere, K. (2010). Predictors of psychological distress in patients starting IVF treatment: Infertility-specific versus general psychological characteristics. Hum. Reprod. 25, 1471–1480. doi: 10.1093/humrep/deq030
Vikström, J., Josefsson, A., Bladh, M., and Sydsjö, G. (2015). Mental health in women 20–23 years after IVF treatment: A Swedish cross-sectional study. BMJ Open 5:e009426. doi: 10.1136/bmjopen-2015-009426
Vizheh, M., Pakgohar, M., Babaei, G., and Ramezanzadeh, F. (2013). Effect of counseling on quality of marital relationship of infertile couples: A randomized, controlled trial (RCT) study. Arch. Gynecol. Obstetr. 287, 583–589. doi: 10.1007/s00404-012-2595-9
Wang, K., Li, J., Zhang, J. X., Zhang, L., Yu, J., and Jiang, P. (2007). Psychological characteristics and marital quality of infertile women registered for in vitro fertilization-intracytoplasmic sperm injection in China. F. S. 87, 792–798. doi: 10.1016/j.fertnstert.2006.07.1534
Williams, K. E., and Koran, L. M. (1997). Obsessive-compulsive disorder in pregnancy, the puerperium, and the premenstruum. J. Clin. Psychiatry 58:13029. doi: 10.4088/JCP.v58n0709
Williams, K. E., and Zappert, L. N. (2006). “Psychopathology and psychopharmacology in the infertile patient,” in Infertility counseling: A comprehensive handbook for clinicians , eds S. Covington and L. Burns (Cambridge: Cambridge University Press), 97–116. doi: 10.1017/CBO9780511547263.008
Xu, H., Ouyang, N., Li, R., Tuo, P., Mai, M., and Wang, W. (2017). The effects of anxiety and depression on in vitro fertilisation outcomes of infertile Chinese women. Psychol. Health. Med. 22, 37–43. doi: 10.1080/13548506.2016.1218031
Ying, L., Wu, L. H., and Loke, A. Y. (2016). The effects of psychosocial interventions on the mental health, pregnancy rates, and marital function of infertile couples undergoing in vitro fertilization: A systematic review. J. Assisted Reprod. Genet. 33, 689–701. doi: 10.1007/s10815-016-0690-8
Yusuf, L. (2016). Depression, anxiety and stress among female patients of infertility. A case control study. Pakistan J. Med. Sci. 32:1340. doi: 10.12669/pjms.326.10828
Zaig, I., Azem, F., Schreiber, S., Gottlieb-Litvin, Y., Meiboom, H., and Bloch, M. (2012). Women’s psychological profile and psychiatric diagnoses and the outcome of in vitro fertilization: Is there an association? Arch. Womens Men. Health 15, 353–359. doi: 10.1007/s00737-012-0293-z
Keywords : infertility, psychological assessment, SCL90, BSI, DASS, STAI
Citation: Tavousi SA, Behjati M, Milajerdi A and Mohammadi AH (2022) Psychological assessment in infertility: A systematic review and meta-analysis. Front. Psychol. 13:961722. doi: 10.3389/fpsyg.2022.961722
Received: 05 June 2022; Accepted: 03 October 2022; Published: 28 October 2022.
Reviewed by:
Copyright © 2022 Tavousi, Behjati, Milajerdi and Mohammadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir Hossein Mohammadi, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
New reproductive technologies in the treatment of human infertility and genetic disease
Affiliation.
- 1 Department of Biology, Princeton University, NJ 08544-1014.
- PMID: 2203176
- DOI: 10.1007/BF00489454
In this paper I will discuss three areas in which advances in human reproductive technology could occur, their uses and abuses, and their effects on society. First is the potential to drastically increase the success rate and availability of in vitro fertilization and embryo freezing. Second is the ability to perform biopsies on embryos prior to the onset of pregnancy. Finally, I will consider the adding or altering of genes in embryos, commonly referred to as "genetic engineering". As new reproductive technologies pass from experimental models into the potential for medical utilization, I believe that it will be important for lawmakers everywhere to avoid the impulse to outlaw procedures that a society believes to be 'unnatural' at a first glance. Rather, I would hope that they can respond thoughtfully with legislation that serves two purposes--to protect the rights of couples to overcome infertility or to reduce the risk of genetic disease in their children-to-be, and more importantly, to protect children-to-be from the abuses that could result from some of the practices that I will discuss.
Publication types
- Research Support, U.S. Gov't, P.H.S.
- Cryopreservation
- Ethics, Medical
- Fertilization in Vitro
- Genetic Diseases, Inborn / prevention & control*
- Genetic Engineering
- Infertility / therapy*
- Reproductive Techniques*
- Risk Assessment
- Twinning, Monozygotic
- United States
Grants and funding
- HD20275/HD/NICHD NIH HHS/United States
- HD24383/HD/NICHD NIH HHS/United States
- Open access
- Published: 08 September 2014
Infertility care and the introduction of new reproductive technologies in poor resource settings
- Luis Bahamondes 1 &
- Maria Y Makuch 1
Reproductive Biology and Endocrinology volume 12 , Article number: 87 ( 2014 ) Cite this article
7377 Accesses
27 Citations
19 Altmetric
Metrics details
The overall prevalence of infertility was estimated to be 3.5–16.7% in developing countries and 6.9–9.3% in developed countries. Furthermore, according to reports from some regions of sub-Saharan Africa, the prevalence rate is 30–40%. The consequences of infertility and how it affects the lives of women in poor-resource settings, particularly in developing countries, has become an important issue to be discussed in reproductive health. In some societies, the inability to fulfill the desire to have children makes life difficult for the infertile couple. In many regions, infertility is considered a tragedy that affects not only the infertile couple or woman, but the entire family.
This is a position paper which encompasses a review of the needs of low-income infertile couples, mainly those living in developing countries, regarding access to infertility care, including ART and initiatives to provide ART at low or affordable cost. Information was gathered from the databases MEDLINE, CENTRAL, POPLINE, EMBASE, LILACS, and ICTRP with the key words: infertility, low income, assisted reproductive technologies, affordable cost, low cost.
There are few initiatives geared toward implementing ART procedures at low cost or at least at affordable cost in low-income populations. Nevertheless, from recent studies, possibilities have emerged for new low-cost initiatives that can help millions of couples to achieve the desire of having a biological child.
Conclusions
It is necessary for healthcare professionals and policymakers to take into account these new initiatives in order to implement ART in resource-constrained settings.
The prevalence of infertility has been calculated as ranging from 4 to 14% worldwide, and the international consensus is that 8–10% of cohabiting couples are infertile, with variations in this percentage according to the region considered [ 1 – 4 ]. In 2002, in an analysis of data from demographic and health surveys in developing countries (excluding China), it was estimated that more than 186 million women who were married or in a stable union and of reproductive age presented primary or secondary infertility problems [ 5 ]. An analysis of 25 population-based surveys estimated the overall prevalence of infertility to be 3.5–16.7% in developing countries and 6.9–9.3% in developed countries [ 6 , 7 ]. Furthermore, according to reports from some regions of sub-Saharan Africa, the prevalence rate is 30–40% [ 8 ].
It has also been estimated that around 5% of the world population decides voluntarily to be childless and does not choose the parental role as part of personal and adult development. Therefore, it may be legitimate to think that parenthood is socially expected and continues to be an important objective of the life project, considered part of personal development and adult life among most men and women in the world [ 9 – 16 ].
In a predominantly fertile world, it is expected that pregnancy will occur when men and women decide to become parents, and it is surprising when this does not happen. For most men and women, infertility is a disruption of the life project and a social situation that differs from the majority. Involuntary childlessness is considered a major life issue associated with psychological suffering and long-term consequences. Women who desire a child and cannot achieve pregnancy perceive themselves as different from women who are fertile, and feel they are losing something important in their lives [ 17 – 20 ]. The diagnosis of infertility may become a life crisis, and most couples need to develop mechanisms to cope with the temporary or permanent loss of fertility and the possibility of having a biological child [ 17 , 21 , 22 ]. After diagnosis, men and women have feelings of loss of control over their life project, and feel they are unable to achieve their life expectations [ 17 , 18 , 23 – 25 ].
Infertile couples may have difficulty communicating their feelings to family and friends with children because they do not perceive a comprehensive social environment to understand their situation [ 13 , 26 – 28 ]. Frequently, they feel alone and without support to deal with the experience of infertility, and a need for sharing experience with other infertile couples, guidance through the treatment process, and written information about practical and emotional aspects of treatment to help them through the experience of infertility [ 29 ].
Over the last decades, many women have married or established stable relationships after achieving other life goals such as a career and financial stability, postponing the desire to have children. The mean age of women at the time of their first pregnancy has increased in recent years, and in the last decade was 28–29 years old in most of western hemisphere countries. In this scenario, many women try to become pregnant with their first child when fertility starts to decline and the risk of infertility is higher [ 30 ].
The consequences of infertility and how it affects the lives of women in poor-resource settings, particularly in developing countries, has become an important issue to be discussed in reproductive health. In some societies, the inability to fulfill the desire to have children, together with the social stigma associated with infertility, makes life difficult for the infertile couple. In many regions, infertility is considered a tragedy that affects not only the infertile couple or woman, but the entire family [ 13 , 31 , 32 ].
Many societies still view infertility as affecting only the women, and those who are childless are frequently neglected or exposed to humiliation and domestic violence [ 33 – 38 ]. Furthermore, this situation and their own suffering because of the impossibility of conceiving a child may lead to diverse psychological problems, such as distress, anxiety, depression, low self-esteem, feelings of blame and guilt, and reduced sexual interest [ 33 , 37 , 39 – 41 ].
Seeking for solutions, when there are no services available in the public sector or at an affordable cost, women may be induced to seek ineffective therapies [ 37 , 39 , 41 ]. Consequently, access to the diagnosis and treatment of infertility, including assisted reproductive technologies (ART), contributes to resolving social inequities and emotional difficulties. Infertility is more than a health problem; it is a social issue and a public health matter [ 42 ].
The treatment of infertility in low-resource settings is a challenge for policymakers and the health system. However, it is mainly a human rights issue: All men and women who desire to have children, to have a family, and not to be different from most of the individuals of their social environment should have the opportunity to solve this problem. The poorest sector of the populations of developing countries are probably those more prone to infertility due to poverty, poor education, early sexual debut, no access to services for counseling and treatment when necessary, unsafe abortion [ 37 ], among other factors. Probably, they represent the population most in need of ART [ 43 – 47 ].
More than 30 years have elapsed since the first publication reporting the birth of a child after in vitro fertilization (IVF) [ 48 ], much research has been done, and other techniques like intracytoplasmic sperm injection (ICSI) have emerged. ART has brought great hope to many couples, who have referred to this technique as their last chance of having a child that is biologically related to them [ 49 ].
Although ART is a common treatment for infertile couples worldwide, the availability of such procedures is lacking in developing countries; even in developed countries, low-income couples have great difficulties of access due to the high cost charged by private clinics and the lack of services offered by the public sector [ 45 , 47 , 50 ].
Infertility: medical causes
Approximately one-third of infertility cases are due to the male factor, one-third to the female factor and the remaining third to a combination of male and female factors or to unidentified causes [ 51 ]. In settings with poor access to health services, common causes of infertility are post-partum and post-abortion infections, tuberculosis, and sexually transmitted infections (STIs) [ 52 ]. Infertility can also be a consequence of infections after female genital circumcision [ 53 ].
In some developing countries, cohabitation is a common practice and adolescent pregnancy rates are high. This early age of sexual debut may be related to a high prevalence of reproductive tract infection (RTI), and consequently both male and female infertility [ 2 , 3 , 6 ]. The prevalence of male and female RTI, and the infertility which may consequently occur, is high in developing countries [ 53 , 54 ], and the best way to reduce this incidence is through prevention and RH education, an important task for governments; however, this represents a challenge [ 45 – 47 ], because education is a long-term process and individuals who are infertile because they do not use means of prevention need to be treated. It is well-known that tubal obstruction was the first diagnosis for which IVF was developed and indicated, and RTI often provokes tubal obstruction. The fact that this cause of infertility is more common in developing areas and among low-income populations is an indicator of the necessity of implementing ART in low-income populations.
Access to infertility health care
As part of the United Nations (UN) Program of Action, consensus was reached on a comprehensive concept of RH that includes the right of men and women to choose the number, timing, and spacing of the children they desire to have. This Program of Action includes the need to incorporate family planning programs, the prevention and treatment of RTI, and the prevention and treatment of infertility as part of RH services [ 55 ]. Furthermore, the Millennium Developments Goals of the UN 2000 established universal access to RH as one of the targets to be achieved by 2015 [ 56 ]. Infertility is one of the neglected aspects of RH care, particularly in developing countries. In many developing countries, infertile couples with limited resources are confronted with difficulties and very restricted possibilities of gaining access to infertility services within the public health sector [ 26 , 45 – 47 ].
The urgent need for many women to resolve their childlessness is a situation that increases the demand from poor couples in developing countries for good quality infertility care [ 33 ]. Delays in gaining access to the diagnosis of infertility, to infertility services and to services that offer ART may negatively affect the possibility and the success of treatment for many couples. Access to diagnosis and treatment for infertility, including ART procedures, contributes to diminishing inequities, and may reduce suffering related to the difficulty of access and emotional suffering due to infertility. This represents a step forward in guaranteeing the right of women and men to decide when they desire to have children and to help infertile couples to have at least one biological child [ 55 , 56 ].
More than a decade ago, the World Health Organization (WHO) recommended that infertility must be considered a global health problem and also recommended the development of initiatives to improve access to infertility services and the care of infertile couples, including the development of low-cost ART [ 57 ]. Although this is still not sufficient and is far from representing a solution, some initiatives have been implemented to reduce the burden of infertility [ 7 , 33 , 57 – 60 ]. In this context, the European Society for Human Reproduction and Embryology (ESHRE) created a Task Force on Infertility and Developing Countries with the objective of explore new approaches to ART that will be useful in developing settings [ 61 ].
The nonexistence of infertility care in resource-constrained settings has been justified many times by the fact that other important and life-threatening health issues are a priority in the health sector, including maternal morbidity and mortality, vaccination, malaria, dengue fever, yellow fever, and the drugs required for people living with HIV and AIDS [ 54 , 62 , 63 ]. The implementation of infertility care, including affordable ART treatment, frequently represents a challenge; an understanding of the impact infertility has on the life of men, and particularly women, not only as a health problem but an emotional and social problem, as well as strong political commitment, is needed for action. According to the WHO [ 54 ], ‘relatively few of the world’s infertile men and women can be said to have complete and equitable access to the complete range of infertility treatments at affordable levels’.
Simplified procedures for ART
Many new initiatives to reduce the cost of ART procedures without hampering the results in terms of pregnancy rates and number of babies taken home have been developed over the last 10 years. However, despite the efforts to make these procedures available at affordable cost, efforts are still scarce; most of the procedures come from developed countries, and are still not translate into actions in many settings.
Many professionals working with ART procedures use controlled ovarian stimulation and follicle development with gonadotropin-releasing hormone (Gn-RH) analogues or antagonist and with gonadotropins in order to develop many ovarian follicles to obtain more embryos, and consequently have the possibility to transfer more than one embryo to maximize the possibility of pregnancy. However, in recent years, there have been many reports presenting reliable data on low-cost ART with acceptable pregnancy rates, and in some cases, rates that are similar to those obtained with high-cost procedures [ 64 , 65 ].
Low cost does not necessarily jeopardize the quality of the procedures. Low-cost ART is based on the use of affordable stimulation protocols, clinical judgment rather than sophisticated laboratory testing, reduction or elimination of all superfluous pre-procedure investigations, careful use of disposable materials, and well-established protocols for laboratory routines. It has to be taken into account that the use of low-cost protocols does not avoid the necessity of the infrastructure of a good laboratory.
The use of the natural cycle or simplified ovarian stimulation ART treatment can reduce the cost of drugs, as well as the possibility of multiple pregnancies. However, to the best of our knowledge, there are no randomized clinical trials comparing natural cycle ART with standard ART. A recent review [ 66 ] showed that there is no evidence that clomiphene citrate (CC) versus gonadotropins and Gn-RH analogue or antagonists are equivalent in terms of follicular development. Nevertheless, CC is still the drug used in many protocols to reduce cost. It is well established that CC mimics the effects of Gn-RH analogue and prevents the luteinizing hormone (LH) surge [ 64 ].
A group of researchers [ 67 ] used CC (50 mg daily) with intermittent doses of human menopausal gonadotropin (hMG) 150 IU on alternate days from the 5th day onwards; follicular development was monitored only with pelvic ultrasound. The researchers performed embryo transfer for more than two-thirds of the women and showed that with this protocol, birth and clinical pregnancy rates per embryo transfer were similar to those expected with high-cost procedures. The average direct cost per cycle was US$ 675 for IVF and US$ 725 for an ICSI cycle.
Whether the use of low dose of CC in ART reduces premature ovulation rate and increases the transfer rate was also evaluated [ 68 ]. Women who underwent one natural-IVF cycle with human chorionic gonadotropin to induce ovulation were compared with women who underwent one natural-IVF cycle with 25 mg/day CC for almost 7 days. Women who used CC presented a significantly lower premature ovulation rate in comparison to those who did not use it and the transfer rate was higher among the CC group in comparison to women who did not use CC. Clinical pregnancy rates were not significantly different between groups.
Another low-cost strategy is the transfer of a single embryo. A Japanese-based study [ 69 ] assessed a cohort of more than 7,000 women who received a single-embryo transfer according to age (≤29, 30–34, 35–39, 40–44, and ≥ 45 years) who performed 20,244 cycles with a CC stimulation or natural-IVF cycle. Fertilization (80.3%) and cleavage (91.1%) rates were not significantly different among different age groups; however, overall live birth rate decreased as age increased, and was no higher than 1% in women aged 45 years or older. The results showed that single embryo transfer could be a strategy to reduce the cost of ART cycles.
Lopez-Regalado and co-workers [ 70 ] evaluated the pregnancy rate with single embryo transfer versus double embryo transfer among women under 38 years old. The cumulative live birth delivery rate in the single embryo transfer group was similar to the women who received double embryo transfer. Additionally, multiple gestations were significantly lower in the group who was treated with single embryo transfer than the other group (0% vs. 26.4%; P < .05). Rate of implantation, cumulative pregnancy rates per transfer, and cumulative live birth delivery were similar among both groups. Similar results were obtained in other settings [ 71 , 72 ]. In a recent Cochrane review [ 73 ], the policy of single embryo transfer versus two embryo transfer was evaluated regarding pregnancy rates. The authors concluded that if a single fresh embryo is transferred, it is associated with a lower live birth rate than double embryo transfer. Nevertheless, they also observed no significant differences when single and double embryo transfer re compared regarding cumulative the live birth rate with repeated single embryo transfer, involving either two cycles of fresh single versus one cycle of fresh single embryo followed by one frozen single embryo in a natural or hormone-stimulated cycle. Furthermore, it was observed that single embryo transfer was linked to lower rates of multiple pregnancies which it is important in baby survival and cost of use of the intensive care unit. However, the evidence is related to young women without a poor prognosis.
Some years ago, a very low-cost ART procedure was reported in which the gametes and embryos were incubated in a capsule in the woman’s vagina, thereby avoiding the use of expensive and complex laboratory. This procedure resulted in adequate pregnancy rates of 19% per cycle [ 74 ].
In this vein, a recent case-series report [ 75 ] described the results of a pilot trial that involved a simplified laboratory method for human IVF. The described system reproduces the atmospheric and culture conditions for fertilization and pre-implantation embryogenesis, with no need for a culture chamber with gases. Using the described culture system, 8 out of 23 embryos implanted, one miscarried at eight weeks of gestation, and seven babies were born.
This simplified system could be incorporated worldwide to improve the capacity of offering ART at affordable cost in low-resource settings. There is no doubt that this strategy does not resolve all the issues involved in low-cost ART; however, it is a big step forward to help services to implement ART at affordable cost in low-income settings. The authors estimated that the first cost of a single IVF cycle using these methodologies and protocols must be less than 200 € [ 76 ].
In developing countries and poor-resource settings, infertility care and treatment that includes access to ART at low cost or at least at affordable costs for the underprivileged segments of society is a neglected RH issue in public health in most countries. Services are mainly available in the private sector at a high cost for the majority of the population [ 33 , 50 , 61 ]. Frequently, health authorities and governments justify this neglected health issue and the lack of infertility services based on the fact that there are other urgent, life-threatening health problems to consider [ 55 ]. This suggests that many policymakers are not aware of the profound implications of infertility in the lives of individuals, couples, and families. Furthermore, in some settings, when unattended, this health issue may become a source of discrimination, abandonment, and violence, particularly for women.
Even in some settings in developed countries, for low-income populations like Latinos and African-American descendants in the United States (US) [ 77 ] or migrants in the European Union [ 78 ], infertility diagnosis and treatment including ART is a neglected RH issue. As an example of this inequity, a US survey [ 79 , 80 ] evaluated more than 4,000 couples to assess the likelihood of seeking an infertility evaluation and infertility treatment. Among those seeking an evaluation, only 50% reported they had undergone any treatment. Low income, employment status, and non-white ethnicity were strongly correlated with the possibility of not seeking treatment; among those who received treatment, only a small proportion was treated with ART.
ART is an excellent solution for many infertile couples who cannot conceive the desired child by other treatments. High-quality ART procedures at no cost for patients or at an affordable cost to the underprivileged segment of the society is a moral and social obligation of governments that have promised to improve and provide RH services that are accessible to most sectors of the population [ 54 ]. Twenty years have elapsed from United Nations International Conference on Population and Development [ 56 ] and it is necessary to take initiatives to offer treatments to infertile couples that include ART procedures, improved access to the underprivileged population, and the guarantee of well-equipped and competently staffed infertility centers.
The lack of ART services at low or affordable cost increases the practice of couples seeking these procedures outside their country of residence. A new phenomenon of cross-border reproductive care can be observed worldwide, and many questions are emerging, mainly regarding whether patients traveling abroad for ART procedures are at any risk and if there is a really cost-benefit result. In addition, it is not clear whether the healthcare professionals (HCPs) who offer this kind of service advise potential patients of the real pregnancy rates, complications, offspring risks, or of the availability of gamete donors and surrogate mothers, among other thing [ 81 ].
The fact that there is a respectable body of research showing the effectiveness of low-cost technology with the aim of facilitating the inclusion of ART in restricted-resource settings does not guarantee the implementation of these strategies in infertility services. There seems to be a gap between the development of low-cost ART technology and the use of these strategies in a clinical context. The results of recent research on the new low-cost and simplified ART system for culturing gametes without the needs of a sophisticated laboratory [ 76 ] are encouraging, because this work addresses a fundamental obstacle for millions of couples worldwide, namely access to ART at very low or at least affordable cost [ 77 , 82 ]. It is obvious that further studies are needed to validate these early results and to replicate them in different settings. As stated, it is necessary to evaluate the real cost, mainly in relation to hidden costs (personnel and infrastructure) [ 82 ]; however, any reduction in cost will be a great step for many couples.
The introduction of these technologies requires a specialized, organized medical and paramedical staff; a minimum of infrastructure within the health system—regarding the supply of materials and improvement in existing services—is necessary. Moreover, patients and staff need to interact with a cooperative understanding. HCPs need to explain clearly the specific characteristics of the treatment, the real possibilities of pregnancy, and potential risks to their patients, and must respect cultural and religious beliefs. Patients, on the other hand, need to follow the medication scheme and the intricate rules of these procedures carefully.
Given the magnitude of the problem—the number of people all over the world who suffer from infertility, the impact infertility has on people’s lives, and that ART is presently out of the reach for the majority of those who need it—it is legitimate to question the extent to which initiatives have to be carried out for these procedures to become part of national infertility policies. This is an issue for each country to resolve. However, as Fathalla et al. [ 83 ] stated almost a decade ago, it is time to cross the boundary from talking and writing to taking action.
Greenhall E, Vessey M: The prevalence of subfertility: a review of the current confusion and a report of two new studies. Fertil Steril. 1990, 54 (6): 978-983.
CAS PubMed Google Scholar
World Health Organization: Infertility: A Tabulation of Available Data on Prevalence of Primary and Secondary Infertility. Geneva Programme on Maternal and Child Health and Family Planning. Division of Family Health. 1991, Geneva: World Health Organization
Google Scholar
Lunenfeld B, Van Steirteghem A: Infertility in the third millennium: implications for the individual, family and society: condensed meeting report from the Bertarelli Foundation’s second global conference. Hum Reprod Update. 2004, 10 (4): 317-326. 10.1093/humupd/dmh028.
Article CAS PubMed Google Scholar
Larsen U: Research on infertility: which definition should we use?. Fertil Steril. 2005, 83 (4): 846-852. 10.1016/j.fertnstert.2004.11.033.
Article PubMed Google Scholar
Rutstein SO, Iqbal HS: Infecundity, Infertility, and Childlessness in Developing Countries. 2004, Geneve, Switzerland: DHS Comparative Reports, WHO
Boivin J, Bunting L, Collins AJ, Nygren GK: International estimates of infertility prevalence and infertility treatment seeking: potential need and demand for infertility medical care. Hum Reprod. 2007, 22 (6): 1506-1512. 10.1093/humrep/dem046.
Ombelet W: Reproductive healthcare systems should include accessible infertility diagnosis and treatment: an important challenge for resource poor countries. Int J Gynaecol Obstet. 2009, 106 (2): 168-171. 10.1016/j.ijgo.2009.03.033.
Leke RJ, Oduma JA, Bassol-Mayagoitia S, Bacha AM, Grigor KM: Regional and geographical variations in infertility: effects of environmental, cultural, and socioeconomic factors. Environ Health Perspect. 1993, 101 (Suppl 2): 73-80. 10.1289/ehp.93101s273.
Article PubMed Central PubMed Google Scholar
Daniluk J: infertility: intrapersonal and interpersonal impact. Fertil Steril. 1988, 49 (6): 982-990.
Daniels K: Management of the psychosocial aspects of infertility. Aust NZJ Obstet Gynecol. 1992, 32 (1): 57-61. 10.1111/j.1479-828X.1992.tb01902.x.
Article CAS Google Scholar
Langridge P: Children are not just miniature adults. Nurs Times. 2005, 101 (10): 47-
PubMed Google Scholar
Lampic C, Svanberg AS, Karlström P, Tydén T: Fertility awareness, intentions concerning childbearing, and attitudes towards parenthood among female and male academics. Hum Reprod. 2006, 21 (2): 558-564.
Dyer SJ: The value of children in African countries: insights from studies on infertility. J Psychosom Obstet Gynaecol. 2007, 28 (2): 69-77. 10.1080/01674820701409959.
Holton S, Fisher J, Rowe H: To have or not to have? Australian women’s childbearing desires, expectations and outcomes. J Pop Res. 2011, 28 (4): 353-379. 10.1007/s12546-011-9072-3.
Article Google Scholar
Roberts E, Metcalfe A, Jack M, Tough SC: Factors that influence the childbearing intentions of Canadian men. Hum Reprod. 2011, 26 (5): 1202-1208. 10.1093/humrep/der007.
Article PubMed Central CAS PubMed Google Scholar
Peterson BD, Pirritano M, Tucker L, Lampic C: Fertility awareness and parenting attitudes among American male and female undergraduate university students. Hum Reprod. 2012, 27 (5): 1375-1382. 10.1093/humrep/des011.
Menning B: The emotional needs of infertile couples. Fertil Steril. 1980, 34 (4): 313-319.
Shapiro C: Infertility and Pregnancy Loss. 1993, San Francisco: Jossey-Bass
Sandelowski M: Without a child: the world of infertile women. Health Care Women Int. 1988, 9 (3): 147-161. 10.1080/07399338809515814.
Kraft AD, Palombo J, Mitchell D, Dean C, Meyers S, Schmidt AW: The psychological dimensions of infertility. Am J Orthopsychiatry. 1980, 50 (4): 618-628.
Seibel MM, Taymor ML: Emotional aspects of infertility. Fertil Steril. 1982, 37 (2): 137-145.
Platt JJ, Ficher I, Silver MJ: Infertile couples: personality traits and self-ideal concept discrepancies. Fertil Steril. 1973, 24 (12): 972-976.
Elstein M: Effect of infertility on psychosexual function. Br Med J. 1975, 3 (5978): 296-299. 10.1136/bmj.3.5978.296.
Vassard D, Lund R, Pinborg A, Boivin J, Schmidt L: The impact of social relations among men and women in fertility treatment on the decision to terminate treatment. Hum Reprod. 2012, 27 (12): 3502-3512. 10.1093/humrep/des353.
Van Balen F, Trimbos-Kemper T, Verdurmen J: Perception of diagnosis and openness of patients about infertility. Patient Educ Couns. 1996, 28 (3): 247-252. 10.1016/0738-3991(95)00852-7.
Greil AL: Infertility and psychological distress: a critical review of the literature. Soc Sci Med. 1997, 11 (11): 1506-1512.
Schmidt L: Social and psychological consequences of infertility and assisted reproduction—what are the research priorities?. Hum Fertil (Camb). 2009, 12 (1): 14-20. 10.1080/14647270802331487.
Read SC, Carrier ME, Boucher ME, Whitley R, Bond S, Zelkowitz P: Psychosocial services for couples in infertility treatment: what do couples really want?. Patient Educ Couns. 2014, 94 (3): 390-395. 10.1016/j.pec.2013.10.025.
The ESHRE Capri Workshop Group: Europe the continent with the lowest fertility. Hum Reprod Updated. 2010, 16 (6): 590-602.
Daar AS, Merali Z: Infertility and social suffering: the case of ART in developing countries. Current Practices and Controversies in Assisted Reproduction. Edited by: Vayena E, Rowe PJ, Griffin PD. 2002, Geneva, Switzerland: World Health Organization, 15-21.
Dyer SJ, Abrahams N, Hoffman M, van der Spuy ZM: ‘Men leave me as I cannot have children’: women’s experiences with involuntary childlessness. Hum Reprod. 2002, 17 (6): 1663-1668. 10.1093/humrep/17.6.1663.
Van Balen F, Gerrits T: Quality of infertility care in poor-resource areas and the introduction of new reproductive technologies. Hum Reprod. 2001, 16 (2): 215-219. 10.1093/humrep/16.2.215.
Van Balen F: The psychologization of infertility. Infertility Around the Globe. Edited by: Inhorn MC, van Balen F. 2002, London: University of California Press, 79-98.
Widge A: Sociocultural attitudes towards infertility and assisted reproduction in India. Current Practices and Controversies in Assisted Reproduction. Edited by: Vayena E, Rowe PJ, Griffin PD. 2002, Geneva, Switzerland: World Health Organization, 60-74.
Araoye MO: Epidemiology of infertility: social problems of the infertile couple. West Afr J Med. 2003, 22 (2): 190-196.
Ombelet W, Cooke I, Dyer S, Serour G, Devroey P: Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008, 14 (6): 605-621. 10.1093/humupd/dmn042.
Dhont N, van de Wijgert J, Coene G, Gasarabwe A, Temmerman M: ‘Mama and papa nothing’: living with infertility among an urban population in Kigali, Rwanda. Hum Reprod. 2011, 26 (3): 623-629. 10.1093/humrep/deq373.
Brkovich AM, Fisher WA: Psychological distress and infertility: forty years of research. J Psychosom Obstet Gynaecol. 1998, 19 (4): 218-228. 10.3109/01674829809025700.
Inhorn MC: Right to assisted reproductive technology: overcoming infertility in low-resource countries. Int J Gynaecol Obstet. 2009, 106 (2): 172-174. 10.1016/j.ijgo.2009.03.034.
Van Balen F, Bos HMW: The social and cultural consequences of being childless in poor-resources areas. Facts Views Vis Obgyn. 2009, 1 (2): 106-121.
PubMed Central CAS PubMed Google Scholar
Hammarberg K, Kirkman M: Infertility in resource-constrained settings: moving towards amelioration. Reprod Biomed Online. 2013, 26 (2): 189-195. 10.1016/j.rbmo.2012.11.009.
Blackwell RE, Hammond KR, Steinkampf MP: A one year experience with a capitated health care plan for infertility. Fertil Steril. 2001, 75 (4): 749-753. 10.1016/S0015-0282(01)01673-9.
Luna F: Assisted reproductive technology in Latin America: some ethical and sociocultural issues. Current Practices and Controversies in Assisted Reproduction. Edited by: Vayena E, Rowe PJ, Griffin PD. 2002, Geneva, Switzerland: World Health Organization, 31-40.
Makuch MY, Petta CA, Osis MJ, Bahamondes L: Low priority level for infertility services within the public health sector: a Brazilian case study. Hum Reprod. 2010, 25 (2): 430-435. 10.1093/humrep/dep405.
Makuch MY, SimôniadePadua K, Petta CA, Duarte Osis MJ, Bahamondes L: Inequitable access to assisted reproductive technology for the low-income Brazilian population: a qualitative study. Hum Reprod. 2011, 26 (8): 2054-2060. 10.1093/humrep/der158.
Makuch MY, Bahamondes L: Barriers to access to infertility care and assisted reproductive technology within the public health sector in Brazil. Facts Views Vis Obgyn. 2012, 4 (4): 221-226.
Steptoe PC, Edwards RG: Birth after the reimplantation of a human embryo. Lancet. 1978, 2 (8085): 366-
Vayena E, Peterson HB, Adamson D, Nygren KG: Assisted reproductive technologies in developing countries: are we caring yet?. Fertil Steril. 2009, 92 (2): 413-416. 10.1016/j.fertnstert.2009.02.011.
Soullier N, Bouyer J, Pouly JL, Guibert J, Rochebrochard E: Estimating the success of an in vitro fertilization programme using multiple imputation. Hum Reprod. 2008, 23 (1): 187-192.
Johnson MH, Everitt BJ: Essential Reproduction. 2000, Oxford: Blackwell Sciences Ltd.
Serour GI: Reproductive and sexual health rights: 15 years after the International Conference on Population and Development. Int J Gynaecol Obstet. 2009, 106 (2): 99-101. 10.1016/j.ijgo.2009.03.041.
Obermeyer CM: The consequences of female circumcision for health and sexuality: an update on the evidence. Cult Health Sex. 2005, 7 (5): 443-461. 10.1080/14789940500181495.
World Health Organization: Infections, pregnancies and infertility: perspectives on prevention. Fertil Steril. 1987, 47 (6): 944-949.
Nachtigall RD: International disparities in access to infertility services. Fertil Steril. 2006, 85 (4): 871-875. 10.1016/j.fertnstert.2005.08.066.
United Nations: Report of the International Conference on Population and Development, Cairo, Egypt, 5–13 September 1994. 1995, New York: United Nations Population Fund, E.95.XIII.18
Nations U: The Millennium Development Goals. 2000, New York: United Nations
Vayena E, Rowe PJ, Griffin PD: Current practices and controversies in assisted reproduction. Report of a Meeting. 2002, Geneva, Switzerland: World Health Organization, 383-385.
Hovatta O, Cooke I: Cost-effective approaches to in vitro fertilization: means to improve access. Int J Gynaecol Obstet. 2006, 94 (3): 287-291. 10.1016/j.ijgo.2006.04.012.
Ombelet W, Campo R: Affordable IVF for developing countries. Reprod Biomed Online. 2007, 15 (3): 267-265.
Gerrits T: Biomedical infertility care in low resource countries: barriers and access. Facts Views Vis Obgyn Monog. 2012, 2: 1-6.
European Society for Human Reproduction (ESHRE): Task Force Developing Countries and Infertility. http://www.eshre.eu/Specialty-groups/Task-Forces/Task-Force-Developing-Countries-and-Infertility . (10 May 2014, date last accessed)
Okonofua FE: The case against new reproductive technologies in developing countries. Br J Obstet Gynaecol. 1996, 103 (10): 957-962. 10.1111/j.1471-0528.1996.tb09542.x.
Hamberger L, Janson PO: Global importance of infertility and its treatment: role of fertility technologies. Int J Gynaecol Obstet. 1997, 58 (1): 149-158. 10.1016/S0020-7292(97)00286-5.
Kawachiya S, Segawa T, Kato K, Takehara Y, Teramoto S, Kato O: The effectiveness of clomiphene citrate in suppressing the LH surge in the minimal stimulation IVF protocol. Fertil Steril. 2006, 86 (Suppl. 2): S412-
Teramoto S, Kato O: Minimal ovarian stimulation with clomiphene citrate: a large-scale retrospective study. Reprod Biomed Online. 2007, 15 (2): 134-148. 10.1016/S1472-6483(10)60701-8.
Allersma T, Farquhar C, Cantineau AE: Natural cycle in vitro fertilisation (IVF) for subfertile couples. Cochrane Database Syst Rev. 2013, 8: CD010550
Aleyamma TK, Kamath MS, Muthukumar K, Mangalaraj AM, George K: Affordable ART: a different perspective. Hum Reprod. 2011, 26 (12): 3312-3318. 10.1093/humrep/der323.
von Wolff M, Nitzschke M, Stute P, Bitterlich N, Rohner S: Low-dosage clomiphene reduces premature ovulation rates and increases transfer rates in natural-cycle IVF. Reprod Biomed Online. 2014, 29 (2): 209-215. 10.1016/j.rbmo.2014.04.013.
Kato K, Takehara Y, Segawa T, Kawachiya S, Okuno T, Kobayashi T, Bodri D, Kato O: Minimal ovarian stimulation combined with elective single embryo transfer policy: age-specific results of a large, single-centre, Japanese cohort. Reprod Biol Endocrinol. 2012, 10: 35-10.1186/1477-7827-10-35.
López-Regalado ML, Clavero A, Gonzalvo MC, Serrano M, Martínez L, Mozas J, Rodríguez-Serrano F, Fontes J, Castilla JA: Randomised clinical trial comparing elective single-embryo transfer followed by single-embryo cryotransfer versus double embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2014, 178 (7): 192-198.
Ercan CM, Kerimoglu OS, Sakinci M, Korkmaz C, Duru NK, Ergun A: Pregnancy outcomes in a university hospital after legal requirement for single-embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2014, 175 (4): 163-166.
Chai J, Yeung TW, Lee VC, Li RH, Lau EY, Yeung WS, Ho PC, Ng EH: Live birth rate, multiple pregnancy rate, and obstetric outcomes of elective single and double embryo transfers: Hong Kong experience. Hong Kong Med J. 2014, 20 (2): 102-106.
Pandian Z, Marjoribanks J, Ozturk O, Serour G, Bhattacharya S: Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev. 2013, 7: CD003416
Frydman R, Ranoux C: INVO: a simple, low cost effective assisted reproductive technology. Hum Reprod ESHRE Monographs. 2008, 1: 85-89.
Van Blerkom J, Ombelet W, Klerkx E, Janssen M, Dhont N, Nargund G, Campo R: First births with a simplified culture system for clinical IVF and embryo transfer. Reprod Biomed Online. 2014, 28 (3): 310-320. 10.1016/j.rbmo.2013.11.012.
Ombelet W: The Walking Egg Project: Universal access to infertility care - from dream to reality. Facts Views Vis Obgyn. 2013, 5 (2): 161-175.
Henne MB, Bundorf MK: Insurance mandates and trends in infertility treatments. Fertil Steril. 2008, 89 (1): 66-73. 10.1016/j.fertnstert.2007.01.167.
van Rooij FB, van Balen F, Hermanns JM: Migrants and the meaning of parenthood: involuntary childless Turkish migrants in The Netherlands. Hum Reprod. 2006, 21 (7): 1832-1838. 10.1093/humrep/del046.
Kessler LM, Craig BM, Plosker SM, Reed DR, Quinn GP: Infertility evaluation and treatment among women in the United States. Fertil Steril. 2013, 100 (4): 1025-1032. 10.1016/j.fertnstert.2013.05.040.
Ethics Committee of American Society for Reproductive Medicine: Cross-border reproductive care: a committee opinion. Fertil Steril. 2013, 100 (3): 645-650.
Ombelet W: Is global access to infertility care realistic? The Walking Egg Project. Reprod Biomed Online. 2014, 28 (3): 267-272. 10.1016/j.rbmo.2013.11.013.
Johnson MH, Cohen J, Grudzinskas G: Accessible and affordable IVF: is Bob Edwards’ dream about to become reality?. Reprod BioMed Online. 2014, 28 (3): 265-266. 10.1016/j.rbmo.2014.01.001.
Fathalla MF, Sinding SW, Rosenfield A, Fathalla MMF: Sexual and reproductive health for all: a call for action. Lancet. 2006, 368 (9552): 2095-2100. 10.1016/S0140-6736(06)69483-X.
Download references
Author information
Authors and affiliations.
Department of Obstetrics and Gynecology, School of Medical Sciences, University of Campinas (UNICAMP),, Campinas,, SP,, Brazil
Luis Bahamondes & Maria Y Makuch
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Luis Bahamondes .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
LB and MYM participated in the design and writing of the manuscript and also approved the final manuscript. Both authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Bahamondes, L., Makuch, M.Y. Infertility care and the introduction of new reproductive technologies in poor resource settings. Reprod Biol Endocrinol 12 , 87 (2014). https://doi.org/10.1186/1477-7827-12-87
Download citation
Received : 07 July 2014
Accepted : 01 September 2014
Published : 08 September 2014
DOI : https://doi.org/10.1186/1477-7827-12-87
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Infertility
- Assisted reproductive technologies

Reproductive Biology and Endocrinology
ISSN: 1477-7827
- General enquiries: [email protected]
Articles in the field of infertility (2013–2022): a bibliometric analysis
- Assisted Reproduction Technologies
- Published: 05 October 2023
- Volume 40 , pages 2871–2877, ( 2023 )
Cite this article

- Ying Sun ORCID: orcid.org/0009-0006-9575-0542 1
355 Accesses
1 Altmetric
Explore all metrics
To carry out an in-depth analysis of the scientific research on infertility, we performed the first bibliometric analysis focusing on studies involving global literature on infertility during the period 2013–2022. Analysis of 33239 articles in the field of infertility showed a significant increasing trend in the number of publications over the period 2013–2022, with authors mainly from the USA and China. Shanghai Jiao Tong University published the most articles. This study is the first to analyze the global field of infertility (2013–2022) from multiple indicators by bibliometrics, thus providing new insights into the research hotspots and development trends in the field of infertility.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
A scientometric analysis of reproductive medicine.
Bibliometrics and visualization analysis of literature on male hypogonadism from 2000 to 2023: research focus and frontiers
Ireland’s contribution to urology and nephrology research in the new millennium: a bibliometric analysis, data availability.
The authors will supply the relevant data in response to reasonable requests.
Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, Panner Selvam MK, Shah R. Male infertility. Lancet. 2021;397(10271):319–33.
Article PubMed Google Scholar
Sharma A, Minhas S, Dhillo WS, Jayasena CN. Male infertility due to testicular disorders. J Clin Endocrinol Metab. 2021;106(2):e442–59.
Buckett W, Sierra S. The management of unexplained infertility: an evidence-based guideline from the Canadian Fertility and Andrology Society. Reprod Biomed Online. 2019;39(4):633–40.
Yao DF, Mills JN. Male infertility: lifestyle factors and holistic, complementary, and alternative therapies. Asian J Androl. 2016;18(3):410–8.
Article CAS PubMed PubMed Central Google Scholar
Sharma R, Biedenharn KR, Fedor JM, Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11:66.
Article PubMed PubMed Central Google Scholar
Bala R, Singh V, Rajender S, Singh K. Environment, Lifestyle, and Female Infertility. Reprod Sci. 2021;28(3):617–38.
Özen Çınar İ. Bibliometric analysis of breast cancer research in the period 2009–2018. Int J Nurs Pract. 2020;26(3):e12845.
Shuaib W, Khan MS, Shahid H, Valdes EA, Alweis R. Bibliometric analysis of the top 100 cited cardiovascular articles. Am J Cardiol. 2015;115(7):972–81.
Salvati M, Frati A, Russo N, Caroli E, Polli FM, Minniti G, Delfini R. Radiation-induced gliomas: report of 10 cases and review of the literature. Surg Neurol. 2003;60(1):60–67; discussion 67.
Ugolini D, Neri M, Casilli C, Ceppi M, Canessa PA, Ivaldi GP, Paganuzzi M, Bonassi S. A bibliometric analysis of scientific production in mesothelioma research. Lung Cancer. 2010;70(2):129–35.
van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38.
Zeb A, Liu W, Wu J, Lian J, Lian Y. Knowledge domain and emerging trends in nanoparticles and plants interaction research: A scientometric analysis. NanoImpact. 2021;21:100278.
Article CAS PubMed Google Scholar
Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12(5):593–608.
Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000–2014). Expert Opin Biol Ther. 2014;14(9):1295–317.
Qin Y, Zhang Q, Liu Y. Analysis of knowledge bases and research focuses of cerebral ischemia-reperfusion from the perspective of mapping knowledge domain. Brain Res Bull. 2020;156:15–24.
Xiao F, Li C, Sun J, Zhang L. Knowledge Domain and Emerging Trends in Organic Photovoltaic Technology: A Scientometric Review Based on CiteSpace Analysis. Front Chem. 2017;5:67.
Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS ONE. 2019;14(10):e0223994.
Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sørensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22(6):1634–7.
van der Steeg JW, Steures P, Eijkemans MJ, Habbema JD, Hompes PG, Burggraaff JM, Oosterhuis GJ, Bossuyt PM, van der Veen F, Mol BW. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324–8.
Bellver J, Ayllón Y, Ferrando M, Melo M, Goyri E, Pellicer A, Remohí J, Meseguer M. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril. 2010;93(2):447–54.
Starosta A, Gordon CE, Hornstein MD. Predictive factors for intrauterine insemination outcomes: a review. Fertil Res Pract. 2020;6(1):23.
Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19(3):221–31.
Guo D, Wu W, Tang Q, Qiao S, Chen Y, Chen M, Teng M, Lu C, Ding H, Xia Y, et al. The impact of BMI on sperm parameters and the metabolite changes of seminal plasma concomitantly. Oncotarget. 2017;8(30):48619–34.
Palomba S, Daolio J, Romeo S, Battaglia FA, Marci R, La Sala GB. Lifestyle and fertility: the influence of stress and quality of life on female fertility. Reprod Biol Endocrinol. 2018;16(1):113.
Benatta M, Kettache R, Buchholz N, Trinchieri A. The impact of nutrition and lifestyle on male fertility. Arch Ital Urol Androl. 2020;92(2). https://doi.org/10.4081/aiua.2020.2.121 .
Zhang J, Song L, Xu L, Fan Y, Wang T, Tian W, Ju J, Xu H. Knowledge Domain and Emerging Trends in Ferroptosis Research: A Bibliometric and Knowledge-Map Analysis. Front Oncol. 2021;11:686726.
Xiao GY, Liu IH, Cheng CC, Chang CC, Lee YH, Cheng WT, Wu SC. Amniotic fluid stem cells prevent follicle atresia and rescue fertility of mice with premature ovarian failure induced by chemotherapy. PLoS ONE. 2014;9(9):e106538.
Lee HJ, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, Tilly JL. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25(22):3198–204.
Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339–47.
Sun M, Wang S, Li Y, Yu L, Gu F, Wang C, Yao Y. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4(4):80.
Liu T, Huang Y, Zhang J, Qin W, Chi H, Chen J, Yu Z, Chen C. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 2014;23(13):1548–57.
Zhankina R, Baghban N, Askarov M, Saipiyeva D, Ibragimov A, Kadirova B, Khoradmehr A, Nabipour I, Shirazi R, Zhanbyrbekuly U, et al. Mesenchymal stromal/stem cells and their exosomes for restoration of spermatogenesis in non-obstructive azoospermia: a systemic review. Stem Cell Res Ther. 2021;12(1):229.
Gauthier-Fisher A, Kauffman A, Librach CL. Potential use of stem cells for fertility preservation. Andrology. 2020;8(4):862–78.
Mohammed SS, Mansour MF, Salem NA. Therapeutic Effect of Stem Cells on Male Infertility in a Rat Model: Histological, Molecular, Biochemical, and Functional Study. Stem Cells Int. 2021;2021:8450721.
Sánchez-Migallón MC, Valiente-Soriano FJ, Nadal-Nicolás FM, Di Pierdomenico J, Vidal-Sanz M, Agudo-Barriuso M. Corrigendum to survival of melanopsin expressing retinal ganglion cells long term after optic nerve trauma in mice. Exp Eye Res. 2018;174:93–7. https://doi.org/10.1016/j.exer.2018.05.029 .
Monsefi M, Fereydouni B, Rohani L, Talaei T. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iran J Reprod Med. 2013;11(7):537–44.
PubMed PubMed Central Google Scholar
Download references
Author information
Authors and affiliations.
Zhengzhou KingMed Center for Clinical Laboratory Co. Ltd., Henan Province, Zhengzhou City, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ying Sun .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 16 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Sun, Y. Articles in the field of infertility (2013–2022): a bibliometric analysis. J Assist Reprod Genet 40 , 2871–2877 (2023). https://doi.org/10.1007/s10815-023-02960-3
Download citation
Received : 24 May 2023
Accepted : 27 September 2023
Published : 05 October 2023
Issue Date : December 2023
DOI : https://doi.org/10.1007/s10815-023-02960-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Infertility
- Bibliometric analysis
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 27 April 2024
Impact of primary care posttraumatic stress disorder (PC-PTSD) on fertility problem of Iranian women with infertility during the COVID-19 pandemic
- Mahbobeh Faramarzi ORCID: orcid.org/0000-0002-3568-7039 1 ,
- Shiva Shafierizi ORCID: orcid.org/0000-0001-7993-7397 2 ,
- Hajar Pasha ORCID: orcid.org/0000-0003-2663-1604 1 , 3 ,
- Zahra Basirat ORCID: orcid.org/0000-0002-3191-1355 1 ,
- Fatemeh Nasiri‑ Amiri ORCID: orcid.org/0000-0001-6794-7627 1 &
- Farzan Kheirkhah ORCID: orcid.org/0000-0003-1420-5480 3
BMC Women's Health volume 24 , Article number: 261 ( 2024 ) Cite this article
Metrics details
Infertility continued to be a major stressor among women with infertility during COVID-19pandemic. This study aimed to evaluate the impact of primary care posttraumatic stress disorder (PC-PTSD) on fertility problem of Iranian women with infertility during COVID-19 pandemic.
In this cross-sectional study, 386 women with infertility completed the questionnaires of PC-PTSD-5 and Fertility Problem Inventory (FPI) at an infertility center between 2020 and 2022.
The mean of fertility problems was 145.20 (± 32.31). In terms of FPI subscales, the means were as follows: Sexual concern 21.80 (± 7.58), social concern 26.53 (± 8.94), relationship concern 26.02 (± 9.18), need for parenthood concern 40.88 (± 8.98), and rejection of childfree lifestyle 29.96 (± 7.69). The highest mean of FPI subscales was related to the need for parenthood concern in women with infertility. The strongest correlation was found between the subscales of sexual concern and social concern followed by sexual concern and relationship concern. The variables of PC-PTSD were a predictor of fertility problems (β = 0.203, P < .0001). Additionally, the variables of PC-PTSDwere a predictor of sexual concern (β = 0.248, P < .0001), social concern (β = 0.237, P < .0001), relationship concern (β = 0.143, P < .020), and need for parenthood concern (β = 0.101, P < .010). After adjusting for demographic characteristics, there was a significant relationship between FPI with job (β=-0.118, P < .031), education (β=-0.130, P < .023), living place (β = 0.115, P < .035), smoking (β = 0.113, P < .036), relationship with husband (β = 0.118, P < .027), and PC-PTSD symptom (β = 0.158, P < .0001). In addition, the multivariate linear regression showed a significant association between sexual concern and education (β=-0.152, P < .008), smoking (β = 0.129, P < .018), PC-PTSD symptom (β = 0.207, P < .0001); social concern and job (β=-0.119, P < .033), PC-PTSD symptom (β = 0.205, P < .0001); relationship concern and education (β=-0.121, P < .033), living place (β = 0.183, P < .001), relationship with husband (β = 0.219, P < .0001); and rejection of childfree lifestyle and job (β=-0.154, P < .007).
Systematic PTSD screening during COVID-19 pandemic by healthcare providers can be uniquely used to identify, evaluate, and treat trauma-related health conditions in infertility settings, which can link women with infertility to mental health services. This can be novel and useful for future policymakers and practitioners in the infertility field.
Peer Review reports
Introduction
Posttraumatic Stress Disorder (PTSD) is a chronic disorder that occurs after exposure to traumatic events [ 1 ], when a person experiences severe emotional stress such as war, natural disasters, and life-threatening illnesses [ 2 ] such as COVID-19. PTSD is a psychiatric condition related to high levels of functional impairment and poor quality of life [ 3 ] Generally, PTSD is diagnosed based on several clusters of symptoms that appear after exposure to extreme stressors [ 4 ]. The fifth edition of the Diagnostic and Statistical Manual (DSM-5) pays more attention to behavioral symptoms associated with PTSD and proposes four distinct diagnostic clusters, including re-experiencing, avoidance, negative cognitions and mood, and arousal [ 1 ]. PTSD is diagnosed when the DSM-5 symptom criteria algorithm is met and the symptoms cause significant distress and/or impairment. PTSD is now in a new trauma- and stressor-related disorders category, which indicates the cognizance alternation of diseases [ 5 ].
The COVID-19 pandemic led to emerging and unprecedented mental health challenges. Millions of people lost their loved ones during the COVID-19 pandemic, which can lead to grief, namely denial, guilt, anger, and PTSD. Trauma exposure and PTSD have been linked with numerous adverse health conditions. PTSD and a peak in the prevalence of mental health problems such as unresolved bereavement and depression occurred during the COVID-19 pandemic [ 6 ]. This disease can cause disturbance in individual and family functioning, leading to significant medical, social, financial, and physical health problems [ 5 ]. During the COVID-19 pandemic, 25–35% of people suffered from mental stress and anxiety [ 7 , 8 ]. In particular, with the spread of COVID-19, a number of factors increased people’s stress and anxiety, including the fear of contracting the disease, the spread of false news and rumors, fear of death, prohibitions or restrictions on movement, interference in daily activities, financial and occupational problems, and the reduction of social interactions with colleagues, friends, and family [ 9 , 10 ].
PTSD can be a risk factor reducing fecundity, increasing the search for infertility treatment and testing among women [ 11 ]. Furthermore, infertile persons are at high risk for experiencing trauma and its associated adverse mental health consequences. Infertility as an important problem has a significant negative social impact on infertile couples [ 12 ]. It is associated with psychological problems such as anxiety, depression, low self-esteem, sexual distress, stress, and so forth [ 13 ]. Women with infertility can experience infertility-related stress that affects their life and infertility treatment approaches [ 12 ]. Both infertility and its treatment are associated with stressful experiences and perceived as a stressful and uncontrollable event [ 14 ]. Couples with infertility consider it as the most difficult challenge they must overcome. Additionally, women report more anxiety, stress, and depression than men do [ 15 ].
Simultaneously, stress-related disorders increase in individuals and societies affected with large disasters such as the global COVID-19 pandemic, especially in women with infertility [ 16 ]. According to the literature, COVID-19 affects the reproductive system and response to fertility treatment [ 17 ]. Infection with COVID-19 alters the normal immune response with local and systemic injury to organs and tissues. Genital failure can occur after the virus enters the body in both males and females. The possible problem of the male reproductive system is due to rising number of angiotensin-converting enzyme 2 (ACE2) receptors in the testes, Sertoli cells, seminiferous tubule cells, and spermatogonia. Moreover, COVID-19 can also disrupt the function of female reproductive system and affect women’s fertility. The virus may be transmitted sexually and cause infertility or testicular damage [ 18 ].
The infertility experience and its treatment are associated with the symptoms of PTSD. A previous study showed that after the appearance of COVID-19, infertility treatment was delayed for several months [ 12 ]. This could be a major stressor during COVID-19 pandemic among women with infertility [ 19 ].
Nowadays, there is scarce data regarding the impact of COVID-19 pandemic on infertility-related stress. In addition, PTSD demands healthcare service in infertile populations due to the increase in medical complications [ 5 ]. The COVID-19 pandemic influenced all areas of life, including infertility and assisted reproductive techniques [ 20 ] on the other hand; such psychological problems can affect infertility outcomes. Therefore, it is important to determine infertility-related stress and its related factors in infertile patients before beginning infertility treatment [ 13 ]. Although a wide range of psychological consequences can result from trauma exposure, the present study focuses on PC-PTSD symptoms due to their association with poor reproductive health outcomes and the relative infrequency of universal PTSD screening in health infertility settings. Therefore, this research was designed to evaluate the impact of primary care posttraumatic stress disorder on fertility problem of Iranian women with infertility during the COVID-19 pandemic.
Methods and materials
Study design and the participants.
The present cross-sectional study was performed on women with infertility who enrolled in the two infertility centers in Babol City, Mazandaran Province, Iran, from September 2020 to January 2022 during the COVID-19 pandemic, when the COVID-19 cases were at their peak. The inclusion criteria were as follows: Age ≥ 18 years of age, a minimum of a primary school education, access to the Internet, not currently receiving psychotherapy, not pregnant, and willingness to enter the study, not taking psychiatric drugs in three last months, not report of mental retardation, not self-reporting severe psychiatric disorders, and not substance abuse. The following volunteers were excluded from the study: Incomplete answers to the questionnaires withdrew from further cooperation in research, and experienced stressful life events six months ago.
Sample size determination
The sample size was determined to be 386 subjects based on pilot data obtained before the study ( p = .49, α = 0.05, and d = 0.5).
Data collection
Informed consent.
was obtained from all subjects after the research project and objectives were explained. Women with infertility who agreed to participate in the study were instructed to complete the questionnaires. Subjects were given the option of completing paper questionnaires inside the clinic or receiving a questionnaire link (via the DigiSurvey® platform) via Telegram® or WhatsApp® to fill out at home, for a week or less. A total of 460 individuals were invited to enter the research, of which 60 were deemed ineligible. A total of 400 women with infertility were enrolled in the study and completed questionnaires. Among the respondents, 151 responded to online questionnaires and 235 responded to paper questionnaires. Fourteen questionnaires were excluded due to inaccuracies in question completion, leaving 386 for data analysis.
Measurements
Fertility problem inventory (fpi).
FPI was used to assess infertility stress and problems. This scale was developed by Newton (1999). The FPI includes 46-item tools and sub-scales, namely sexual concern, social concern, relationship concern, need for parenthood, and rejection of childfree lifestyles. Answers are assigned from 1 (strongly disagree) to 6 (strongly agree). The total score range is from 46 to 276. The higher scores showed higher levels of stress [ 21 ]. A cut-off score of FPI scores is considered by, a raw score of 167 or above as assessed in females and of 147 or above as assessed in males [ 22 ]. The validity and reliability of the Persian version were evaluated by Samani et al. (2017). The overall integrity was 0.87. Cronbach’s alpha for all sub-scales was more than 0.7 [ 23 ].
Primary care posttraumatic stress disorder (PC‑PTSD‑5)
This scale evaluates posttraumatic stress symptoms, which is a five-question self-report screening. Scores are ranged on a binary scale of 0 to 1 (0 = no; 1 = yes). The total score consists of adding the score of five questions. A cut-off score of 3 is considered for PTSD symptoms [ 5 ]. Higher scores show increased posttraumatic stress symptoms. The Cronbach’s alpha for the Iranian version of PC-PTSD was 0.7. In this study, a validated Persian version was done [ 24 ].
Statistical analysis
We used the mean and standard deviation for describing infertile women’s characteristics. Simple and multivariate linear regression models analyzed the predictors for fertility problem symptoms and their subscales. Age, Husband age, job, education level, living place, smoking, relationship with husband, history of assisted reproductive technology failure, pc-PTSD score as independent variables, fertility problem symptoms, and its subscales score as dependent variables were considered. For data analysis, IBM SPSS Statistics Version 22 was used. P-value < 0.05 was the significance level.
The age range of most women with infertility was 25–35 years (58.5%) and their husbands were aged 30–40 years (67.5%). Most women with infertility held high school diploma (50.4%), lived in rural areas (58.2%), were housewives (74.25%), and their husbands were self-employed (63.2%). The majority of women with infertility were satisfied with their husbands (96.7%). The infertility duration in the majority of participants was less than 10 years (82.6%). The mean duration of Marriage (years) was 7.6 ± 4.68. The demographic characteristics of the research population are summarized in Table 1 .
The mean of fertility problems was 145.20 (± 32.31). In terms of fertility problem subscales, the means were as follows: Sexual concern 21.80 (± 7.58), social concern 26.53(± 8.94), relationship concern 26.02(± 9.18), need for parenthood concern 40.88(± 8.98), and rejection of childfree lifestyle 29.96(± 7.69). The highest mean of FPI domains was related to the need for parenthood concern in women with infertility. There was a significant correlation between the domains of fertility problems ( P < .0001). The strongest correlation was observed between the domains of sexual concern and social concern, followed by sexual concern and relationship concern. (Table 2 ).
There was a significant association between PC-PTSD with all the domains of FPI except for the rejection of childfree lifestyle items. The results of this study on predictors of fertility problems on concerning PC-PTSD of women with infertility revealed a significant positive association between fertility problems and PC-PTSD. Based on the results of linear regression analysis, the variable of PC-PTSD (β = 0.203, P < .0001) was a predictor of fertility problems. PC-PTSD factors explained 4.1% of fertility problem variance. Furthermore, there was a significant positive association between fertility problem subscales and PC-PTSD. According to linear regression analysis, the variables of PC-PTSD were a predictor of sexual concern (β = 0.248, P < .0001), social concern (β = 0.237, P < .0001), relationship concern (β = 0.143, P < .020), and need for parenthood concern (β = 0.101, P < .010). In other words, more PC-PTSD symptom is associated with more frequent fertility problems and their subcomponents except for the rejection of a childfree lifestyle in women in infertility. The regression results revealed that 6.1% and 5.6% of sexual and social concerns in women with infertility could be explained based on Pc-PTSD symptoms, respectively (Table 3 ).
After adjusting the variables of demographic characteristics by applying multiple linear regression, there was significant relationship between fertility problem and job (β=-0.118, P < .031), education (β=-0.130, P < .023), living place (β = 0.115, P < .035), smoking (β = 0.113, P < .036), relationship with husband (β = 0.118, P < .027), and PC-PTSD symptom (β = 0.158, P < .0001). Moreover, the multivariate linear regression showed a significant association between sexual concern and education (β=-0.152, P < .008), smoking(β = 0.129, P < .018), PC-PTSD symptom (β = 0.207, P < .0001); social concern and job (β=-0.119, P < .033), PC-PTSD symptom (β = 0.205, P < .0001); relationship concern and education (β=-0.121, P < .033), living place (β = 0.183, P < .001), relationship with husband (β = 0.219, P < .0001); and rejection of childfree lifestyle and job (β=-0.154, P < .007)(Table 4 ).
This research focused on the impact of primary care post-traumatic stress disorder on fertility problem of Iranian women with infertility from September 2020 to January 2022 during the COVID-19 pandemic.
The present study indicated that PTSD and fertility problems are associated with each other. A history of PTSD can increase infertility-related stress. In agreement with our study, Roozitalab et al. (2021) demonstrated a direct significant relationship between posttraumatic stress disorder and level of stress in women with infertility [ 25 ]. While Azad et al. (2022) reported that COVID-19 pandemic did not affect infertility-related stress in women with infertility [ 12 ]. Nearly half of the women with infertility met the criteria for any psychological distress. Fertility status is an enduring condition that has a significant association with mental health outcomes. Many covariates make significant independent contributions to psychological distress. The COVID‑19 pandemic hurts the quality of life and the mental health of women seeking fertility services [ 26 ]. Furthermore, the review of literature highlighted that the couples with infertility also confronted the psychological effect of COVID‑19 pandemic due to quarantine, social distancing, travel restrictions, and cancellation of treatment [ 27 ]. Therefore, appropriate and timely psychological counseling along with organizational and social support are essential for coping with negative impacts of COVID‑19 pandemic on women with infertility [ 26 ].
The findings of our study after adjusting for other variables indicated that PC-PTSD was a significant risk factor for sexual concern subscales of fertility problems among women with infertility. The participants presenting with more PC-PTSD symptoms had higher sexual concerns than those who had fewer symptoms of PC-PTSD. A previous study revealed that women reported higher stress levels in subscales of sexual concern and need for parenthood [ 28 ]. Pasha (2020) reported that one of the important concerns of couples is sexual relationships during a pandemic and that COVID-19 gives rise to public health concerns over sexual health [ 29 ]. A review of literature showed that infertile women were unsatisfied with marriage as their ultimate dream of marriage was to give birth to children. Their dissatisfaction also had a direct effect on sexual life and they had a reduced desire to have sex. Although procreation is the driving force for sexual intercourse among couples, consistent failure of attempts to have children can decrease sexual activity with husbands [ 30 ].
The gathered data revealed that PC-PTSD was a significant risk factor for the social concern subscale of fertility problems. In other words, the subjects with more PC-PTSD symptoms had higher social concerns than those who had fewer symptoms of PC-PTSD, which was in line with the study of Awtani et al. (2017) [ 28 ]. Additionally, Arba˘g et al. (2023) reported a decrease in social relations among women with infertility due to the COVID-19 pandemic [ 20 ]. Studies revealed that social support protected people against the adverse health effects of accepting infertility during the COVID-19 pandemic and that more social support was related to less emotional distress [ 31 , 32 ].
It seems that women with infertility used to be exposed to uncomfortable questions raised by their friends and relatives even before COVID-19. This pandemic prompted them to refuse contact or hide from others and socially isolat themselves [ 33 ]. A review of literature revealed that women with infertility experienced social isolation by staying at home, avoiding meeting their friends, and not speaking about receiving infertility treatment [ 34 ]. The decrease in social relationships during COVID-19 pandemic can reduce not only exposure to questions about infertility and social gatherings one attends but also decrease the social gatherings where they feel comfortable with each other. Also, infertile women stated that the inactivity of infertility clinics during the pandemic had a negative effect on their lives. They experienced sadness, hopelessness, exhaustion, unreliability, and anger from waiting [ 20 ], which increased infertility stress. Discontinuation of fertility care during COVID-19 pandemic had a significant psychological impact on women with an increase in stress and anxiety [ 35 ]. In this regard, Tokgoz et al. (2022) indicated that the level of fear and anxiety was found to be higher in women with infertility whose ART cycles were postponed due to COVID-19 outbreak [ 36 ]. There was higher anxiety and distress among women with infertility with postponed or interrupted treatment strategies during the COVID-19 pandemic. The COVID-19 pandemic and delayed infertility treatment was important stressors among women with infertility [ 37 ]. Therefore, psychological intervention and collecting accurate information about resolving sexual and social concerns should be attempted, especially during this difficult period [ 35 ].
The finding of the present study showed that participants with unsatisfied relationships with their spouses had higher stress levels associated with fertility problems as well as relationship concerns domain compared to subjects whose were satisfied relationships with their husbands. The review of literature showed that infertility can increase marital conflicts and problems, which is associated with serious consequences for social well-being. Poor marital quality can be linked with many family and community problems. Infertility is considered a social concern decreasing the emotional bonds of couples and deteriorating the quality of married life [ 38 , 39 ]. This can be difficult as the marital relationship is considered the most important factor of support in relation with infertility treatment strategy. This finding highlights the need for early detection of marital conflicts in couples with infertility and marriage enrichment intervention, which creates positive changes as the couple practices healthy interaction skills.
The results of the present study revealed that higher stress levels in women with infertility were in the domains of need for parenthood followed by rejection of a childfree lifestyle, which was in line with the findings of Shayesteh-Parto et al. (2023) study [ 38 ]. In its explanation, it can be stated that in underdeveloped countries, people without children are often considered to not have children as those who voluntarily select not to have children, while in developed societies, childfree is voluntary and is an appropriate option for growth and development [ 12 ]. For most women, giving birth is an important part of their life, and all societies place great value on having children [ 40 ]. Tabong et al. (2013) reported that couples with infertility suffer from social effects of childlessness. Couples without children are socially stigmatized and denied leadership roles in their communities. Both men and women with infertility are deprived of membership in the ancestral world, thereby losing the opportunity to live again. It seems that health policymakers should be more concerned about infertility and provide optimal solutions in this field [ 30 ].
The finding of this study also demonstrated that the fertility problem was significantly associated with some socio-demographic data, such as job, educational status, and living place. The infertility problem, social concern, and rejection of a childfree lifestyle were lower in employed women than in unemployed women. In contrast with our study, Azad et al. (2023) showed that total infertility stress was significantly associated with employment [ 12 ]. In the interpretation of this finding, it can be acknowledged that employed women experience less stress due to their educational background, financial position, professional career, and high socioeconomic status, which can lead to a more powerful position in their community. In contrast, homemakers from a lower socio-economic background are more likely to be challenged with the social stigma of infertility. In a similar investigation, Esra Arba˘ et al. (2023) stated that the fertility treatment protocol affects women with infertility not only psychologically and physiologically but also economically. The women with infertility stated that the costs of treatment were superimposed on their pandemic-related financial difficulty [ 20 ]. It seems that unemployed women suffer more from these difficult financial conditions, which can exacerbate their stress.
Our study also indicated a significant negative relationship between educational status level of subjects with two FPI subscales of sexual concerns and relationship concerns. Participants with a university education had fewer fertility problems, sexual concerns, and relationship concerns than those with a high school diploma who experienced higher fertility problems. A review of literature showed that total infertility stress was significantly associated with educational status. Educational levels of subjects revealed a significant adverse correlation with some FPI subscales [ 12 ]. In another study, there was a significant association between FPI scores and educational status in couples with infertility [ 41 ], While Teklemicheal et al. (2022) revealed that fertility problems are not related to educational status [ 42 ].
According to demographic characteristics of participants, higher stress levels of fertility problems and the relationship concerns domain were observed among women with infertility that lived in urban areas than those who lived in rural parts. In agreement with the present study, Dierickx et al. (2018) revealed a strong social pressure on women living in cities with infertility [ 43 ]. Besides, Kucuk et al. (2022) stated that cultural and socio-demographic features influence the level of stigmatization of women with infertility; the higher the level of stigma, the more difficult for women with infertility to cope with infertility stress [ 44 ].
Current data indicate that women with infertility who smoked had more infertility-related stress as well as sexual concerns domains than those who were non- smokers. Stress can be considered a significant risk factor for cigarette smokers [ 45 ]. A review of literature has reported that women are prone to increased stress due to infertility [ 46 ]. Drug addiction and alcohol are prevalent in individuals with infertility [ 47 ]. Moreover, PTSD is associated with increased health-compromising behaviors such as tobacco use [ 5 ]. There are several opinions on the impact of stress and smoking behaviors. Smokers often use cigarettes to alleviate stress [ 45 ]. However, previous studies have reported that while smoking may provisionally decrease stress, it may give rise to or worsen negative emotional conditions and increase negative coping strategies leading to overall higher stress levels in individuals [ 48 ]. Therefore, more attention on the part of health policymakers is essential for healthy or unhealthy coping strategies in populations with infertility.
It is suggested to use screening approaches for mental health that help healthcare professionals determine potential stressors for fertility problems during COVID-19 pandemic, which can improve fertility problems. Identifying women with infertility, who have active PTSD symptoms related with the infertility treatment due to high rates of undetected PTSD and low rates of mental health services is especially important.
Data availability
The data supported during the present study are available from the corresponding author upon reasonable request.
American Psychiatric Association. Posttraumatic Stress Disorder 2013. https://www.psychiatry.org/file%20library/psychiatrists/practice/dsm/apa_dsm-5-ptsd.pdf .
Awakh Kismi F, Noorbala AA, Khoshdel AR, Taghva A, Ebrahimi MR. Psychometric properties of the posttraumatic stress disorder screening test for primary care. Contemp Psychol. 2017;12(1):18–22. https://civilica.com/doc/732619/ .
Google Scholar
Schnurr PP, Lunney CA. Symptom change and quality of life in PTSD. Depress Anxiety. 2016;33(3):247–55. https://doi.org/10.1002/da.22477 .
Article PubMed Google Scholar
Miao XR, Chen QB, Wei K, Tao KM, Lu ZJ. Posttraumatic stress disorder: from diagnosis to prevention. Military Med Res. 2018;5(32). https://doi.org/10.1186/s40779-018-0179-0 .
Prins A, Bovin MJ, Smolenski DJ, Marx BP, Kimerling R, Jenkins-Guarnieri MA, et al. The primary care PTSD screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206–11. https://doi.org/10.1007/s11606-016-3703-5 .
Article PubMed PubMed Central Google Scholar
Mortazavi SS, Assari S, Alimohamadi A, Rafiee M, Shati M, Fear. Loss, social isolation, and incomplete grief due to COVID-19: a recipe for a psychiatric pandemic. Basic Clin Neurosci. 2020;11(2):225–32. https://doi.org/10.32598/bcn.11.covid19.2549.1 .
Article CAS PubMed PubMed Central Google Scholar
Cao W, Fang Z, Hou G, Han M, Xu X, Dong J et al. The psychological impact of the COVID-19 epidemic on college students in China. Psychiatry Res. 2020:112934.
Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17(5):1729.
Asmundson GJ, Taylor S. How health anxiety influences responses to viral outbreaks like COVID-19: what all decision-makers, health authorities, and health care professionals need to know. J Anxiety Disord. 2020;71:102211.
Jungmann SM, Witthöft M. Health anxiety, cyberchondria, and coping in the current COVID-19 pandemic: which factors are related to coronavirus anxiety? J Anxiety Disord, 2020:102239.
Wamser-Nanney R. Trauma exposure, PTSD and indices of fertility. J Psychosom Obstet Gynaecol. 2020;41(2):116–21. https://doi.org/10.1080/0167482X.2019.1619691 .
Azad N, Azargoon A, Yousefi B, Hemmatian N, Ziari A, Naderi Eram M. Impact of COVID-19 pandemic on infertility-related stress in women undergoing intrauterine insemination cycle. Novel Biomed. 2023;11(1):23–9.
Maroufizadeh S, Karimi E, Vesali S, Samani RO. Anxiety and depression after failure of assisted reproductive treatment among patients experiencing infertility. Int J Gynecol Obstet. 2015;130(3):253–6.
Article Google Scholar
Boivin J, Harrison C, Mathur R, Burns G, Pericleous-Smith A, Gameiro S. Patient experiences of fertility clinic closure during the COVID-19 pandemic: appraisals, coping and emotions. Hum Reprod. 2020;218. https://doi.org/10.1093/humrep/deaa218 .
Pasch LA, Sullivan KT. Stress and coping in couples facing infertility. Current opinion in psychology. 2017; 13; 131–5. https://doi.org/10.1016/j.copsyc.2016.07.004 .
Cosic K, Popovic S, Sarlija M, Kesedzic I. Impact of human disasters and COVID-19 pandemic on mental health: potential of digital psychiatry. Psychiatria Danubina. 2020;32:25–31.
Lee W, Mok A, Chung JP. Potential effects of COVID-19 on reproductive systems and fertility; assisted reproductive technology guidelines and considerations: a review. Hong Kong Med J. 2021;27(2):118.
CAS PubMed Google Scholar
Săndulescu MS, Văduva CC, Siminel MA, Dijmărescu AL, Vrabie SC, Camen IV, et al. Impact of COVID-19 on fertility and assisted reproductive technology (ART): a systematic review. Rom J Morphol Embryol. 2022;63(3):503–10. https://doi.org/10.47162/RJME.63.3.04 .
Vaughan DA, Shah JS, Penzias AS, Domar AD, Toth TL. Infertility remains a top stressor despite the COVID-19 pandemic. Reprod Biomed Online. 2020;41(3):425–7.
Arba˘g E, Tokat MA, ¨Oz¨oztürk S. Emotions, thoughts, and coping strategies of women with infertility problems on changes in treatment during Covid-19 pandemic: a qualitative study. Women’s Stud Int Forum. 2023;102735. https://doi.org/10.1016/j.wsif.2023.102735 .
Newton CR, Sherrard W, Glavac I. The fertility problem inventory: measuring perceived infertility-related stress. Fertil Steril. 1999;72(1):54–62.
Article CAS PubMed Google Scholar
Patel A, Nair BVS, Das SK, Kumar P, Sharma PSVN. Re-examining Psychometric properties of Fertility Problem Inventory: a clinic-based study from India. J Hum Reprod Sci. 2022;15(2):177–86. https://doi.org/10.4103/jhrs.jhrs_154_21 .
Samani RO, Almasi-Hashiani A, Shokri F, Maroufizadeh S, Vesali S, Sepidarkish M. Validation study of the Fertility Problem Inventory in Iranian infertile patients. Middle East Fertil Soc J. 2017;22(1):48–53.
Sadeghi M, Taghva A, Goudarzi N, Rahnejat A. Validity and reliability of Persian version of post-traumatic stress disorder scale in war veterans. Iran J War Public Health. 2016;8:243–9.
- Roozitalab S, Rahimzadeh M, Mirmajidi SR, Ataee M, Esmaelzadeh Saeieh S. The relationship between infertility, stress, and quality of life with Posttraumatic Stress Disorder in Infertile Women. J Reprod Infertil. 2021;22(4):282–88. https://doi.org/10.18502/jri.v22i4.7654 .
Bagade T, Thapaliya K, Breuer E, Kamath R, Li Z, Sullivan E, et al. Investigating the association between infertility and psychological distress using the Australian longitudinal study on women’s Health (ALSWH). Sci Rep. 2022;12(1):10808. https://doi.org/10.1038/s41598-022-15064-2 .
Torales J, O’Higgins M, Castaldelli–Maia JM, Ventriglio A. The outbreak of COVID–19 coronavirus and its impact on global mental health. Int J Soc Psychiatry. 2020;66:317–20.
Awtani M, Mathur K, Shah S, Banker M. Infertility stress in couples undergoing intrauterine insemination and in vitro fertilization treatments. J Hum Reprode Sci. 2017;10(3):221–25. https://doi.org/10.4103/jhrs.JHRS_39_17 .
Pasha H. Novel coronavirus and sexual challenge during the pandemic. Mal J Med Health Sci. 2020;16(4):1–8.
Tabong PTN, Adongo PB. Infertility and childlessness: a qualitative study of the experiences of infertile couples in Northern Ghana. BMC Pregnancy Childbirth. 2013;13:72. https://doi.org/10.1186/1471-2393-13-72 .
Ben-Kimhy R, Youngster M, Medina-Artom TR, Avraham S, Gat I, Marom Haham LM, et al. Fertility patients under COVID-19: attitudes, perceptions, and psychological reactions. Hum Reprod. 2020;3591227774–83. https://doi.org/10.1093/humrep/deaa248 .
Gordon JL, Balsom AA. The psychological impact of fertility treatment suspensions during the COVID-19 pandemic. PLoS ONE. 2020;15(9):e0239253. https://doi.org/10.1371/journal.pone.023925 .
Sezgin H, Hocaoglu Ç. Infertilitenin Psikiyatrik Y¨onü/Psychiatric aspects of infertility. Psikiyatride Guncel Yaklasimlar. 2014;6(2):165–84. https://doi.org/10.5455/cap.20131001091415 .
Karaca A, Unsal G. Psychosocial problems and coping strategies among Turkish women with infertility. Asian Nurs Res. 2015;9(3):243–50.
Lablanchea O, Salle B, Perie MA, Labrune E, Langlois-Jacques C, Fraison E. Psychological effect of COVID-19 pandemic among women undergoing infertility care, a French cohort – PsyCovART psychological effect of COVID-19: PsyCovART. J Gynecol Obstet Hum Reprod. 2022;51:102251. https://doi.org/10.1016/j.jogoh.2021.102251 .
Tokgoz VY, Kaya Y, Tekin AB. The level of anxiety in infertile women whose ART cycles are postponed due to the COVID-19 outbreak. J Psychosom Obstet Gynaecol. 2022;43(2):114–21. https://doi.org/10.1080/0167482X.2020.1806819 .
Barra F, La Rosa VL, Vitale SG, Commodari E, Altieri M, Scala C, et al. Psychological status of infertile patients who had in vitro fertilization treatment interrupted or postponed due to COVID-19 pandemic: a cross-sectional study. J Psychosom Obstet Gynecol. 2022;43(2):145–52.
Shayesteh-Parto F, Hasanpoor-Azghady SB, Arefi S, Amiri-Farahani L. Infertility-related stress and its relationship with emotional divorce among Iranian infertile people. BMC Psychiatry. 2023;23:666. https://doi.org/10.1186/s12888-023-05159-z .
Hasanpoor-Azghady SB, Simbar M, Vedadhir AA, Azin SA, Amiri-Farahani L. The social construction of infertility among Iranian infertile women: a qualitative study. J Reprod Infertil. 2019;20(3):178.
PubMed PubMed Central Google Scholar
Henny MW, Bos, Floor B, Van Rooij. The influence of social and cultural factors on infertility and new reproductive technologies. J Psychosom Obstet Gynecol. 2007;28(2):65–8. https://doi.org/10.1080/01674820701447439 .
Sepidarkish M, Almasi-Hashiani A, Shokri F, Vesali S, Karimi E, Omani Samani R. Prevalence of infertility problems among Iranian infertile patients referred to Royan Institute. Int J Fertil Steril. 2016;10(3):278–82.
Teklemicheal AG, Kassa EM, Weldetensaye EK. Prevalence and correlates of infertility related psychological stress in women with infertility: a cross-sectional hospital-based survey. BMC Psychol. 2022;10(1):91.
Dierickx S, Rahbari l, Longman CH, Jaiteh F, Coene G. I am always crying on the inside: a qualitative study on the implications of infertility on women’s lives in urban Gambia. Reproductive Health. 2018;15:151. https://doi.org/10.1186/s12978-018-0596 .
Kucuk S, Koruk F. Being an infertile woman in a highly fertile region of Turkey: Stigmatisation and coping experiences. Electron J Gen Med. 2022;19(2):em346. https://doi.org/10.29333/ejgm/11545 .
Lawless MH, Harrison KA, Grandits GA, Eberly LE, Allen SS. Perceived stress and smoking-related behaviors and symptomatology in male and female smokers. Addict Behav. 2015;51:80–3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4558262/ .
Kirca N, Pasinoglu T. Psychosocial problems during infertility treatment. Curr App Psychiatry. 2013;5:162–78.
King RB. Subfecundity and anxiety in a nationally representative sample. Social Sci Med. 2003;56:739–51.
Hajek P, Taylor T, McRobbie H. The effect of stopping smoking on perceived stress levels. Addiction. 2010;105(8):1466–71. https://doi.org/10.1111/j.1360-0443.2010.02979.x .
Download references
Acknowledgements
The authors thank women with infertility involved in the study.
Not applicable.
Author information
Authors and affiliations.
Infertility and Reproductive Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran
Mahbobeh Faramarzi, Hajar Pasha, Zahra Basirat & Fatemeh Nasiri‑ Amiri
Student Research Committee, Babol University of Medical Sciences, Babol, Iran
Shiva Shafierizi
Social Determinants of Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran
Hajar Pasha & Farzan Kheirkhah
You can also search for this author in PubMed Google Scholar
Contributions
MF, SSH and HP were all involved in the design and implementation of this study. MF, SSH, and ZB conceptualized the analysis of the data. MF and HP analyzed the data.HP drafted the maniscript. All authors read and provided input to the final draft maniscript.
Corresponding author
Correspondence to Hajar Pasha .
Ethics declarations
Ethical approval and consent to participate.
This study was confirmed by the ethics committee of the Babol University of Medical Sciences with the ethical code MUBABOL. HRI.REC.1399.105. Informed consent was obtained from all women with infertility before study initiation. The study was conducted by the Declaration of Helsinki.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Faramarzi, M., Shafierizi, S., Pasha, H. et al. Impact of primary care posttraumatic stress disorder (PC-PTSD) on fertility problem of Iranian women with infertility during the COVID-19 pandemic. BMC Women's Health 24 , 261 (2024). https://doi.org/10.1186/s12905-024-03102-2
Download citation
Received : 28 October 2023
Accepted : 18 April 2024
Published : 27 April 2024
DOI : https://doi.org/10.1186/s12905-024-03102-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Infertility
- Infertility-related stress
- Need for parenthood
- Relationship concern
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2023 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
Navigating the Impact of Infertility on Relationships
Be kind to yourself
Wendy Wisner is a health and parenting writer, lactation consultant (IBCLC), and mom to two awesome sons.
:max_bytes(150000):strip_icc():format(webp)/28685632_10156312296594515_9028950978590849197_n-815354ed2f8e44be900fda9060a465f5.jpg)
Dr. Sabrina Romanoff, PsyD, is a licensed clinical psychologist and a professor at Yeshiva University’s clinical psychology doctoral program.
:max_bytes(150000):strip_icc():format(webp)/SabrinaRomanoffPhoto2-7320d6c6ffcc48ba87e1bad8cae3f79b.jpg)
Verywell Mind / Getty
Emotional and Psychological Effects
What is the link between infertility and relationship stress, what the stress of infertility looks like, strategies and coping mechanisms, resources and help for couples facing infertility, the bottom line.
Having kids is a big decision, but once you finally make that choice with confidence there's nothing worse than feeling like you can't bring that dream to fruition—or feel like your body isn't doing what it was built to do.
Infertility, which is defined as an inability to get pregnant after 12 months of actively trying, is more common than you might realize, affecting about 1 in 8 couples. Anyone who’s experienced it will tell you that infertility is so much more than a medical issue. It has profound impacts on a person’s mental health and identity . Living with infertility can lead to strong feelings of loss, grief, shame, and depression.
But infertility doesn’t just affect folks on an individual level. It touches both members of the couple. Many couples feel isolated from other couples and experience collective feelings of shame and stigma about their inability to conceive. This can lead to considerable strain within the relationships and can impact communication, trust, resilience , and more.
It's bad enough that infertility itself can be so painful, but when your relationship becomes strained as well, it can be extremely distressing. We get it and are here to help. We reached out to two mental health professionals who work with expectant parents to discuss the impacts of infertility on couples and how to cope.
Before we look at the profound impacts infertility can have on relationships, it’s important to consider how infertility affects each member of the couple. These mental health impacts tend to spill over into the relationship itself.
“The emotional and psychological effects of infertility are deep and often misunderstood by anyone who has not experienced navigating infertility,” says Rachel Goldberg, LMFT, a therapist who specializes in infertility and founder of Rachel Goldberg Therapy . “There is a range of emotions that individuals may experience, including grief, sadness, anger, guilt, shame, and anxiety.”
Common Feelings Caused By Infertility
- Constant thoughts about getting pregnant
- Decreased self-esteem
- Identity issues
- Feeling like your life is out of your control
- Feelings of inadequacy
- Feelings of failure
Besides these challenging and charged emotions, people who are experiencing infertility may be especially prone to mental health issues. For example, research has found that about 40% of women experiencing infertility have clinical depression or anxiety. There are fewer studies out there about how infertility affects men’s mental health but a study from 2023 found that 14% to 23% of infertile men experience depression.
While not every couple experiences significant strain while dealing with infertility—and sometimes infertility can actually bring couples together—it’s quite common for infertility to strain relationships.
The link between infertility and relationship stress is noteworthy, says Becca Reed , LCSW, PMH-C, perinatal mental health and trauma therapist. “Infertility often ushers in a profound emotional journey that is marked by feelings of loss, inadequacy, and isolation,” she says. “These experiences can strain even the strongest relationships, affecting communication and emotional intimacy.”
One reason for this is that couples may cope quite differently with their feelings about the fertility issues they are experiencing, says Goldberg. “For instance, one partner may have the attitude of whatever it takes, even if that means very costly IVF cycles over and over, while the other partner may feel that putting their life on pause for possibly years for an unknown outcome isn’t worth it,” she describes.
Becca Reed, LCSW
Infertility often ushers in a profound emotional journey that is marked by feelings of loss, inadequacy, and isolation. These experiences can strain even the strongest relationships, affecting communication and emotional intimacy.
These types of differences can lead to misaligned expectations and immense pressure between the two members of the couple, Goldberg says. In addition, the member of the couple who is found to be the source of the infertility may experience fears of abandonment and concerns about feeling less desired.
“Overall, the emotional toll of infertility can lead to increased conflict, decreased intimacy, and feelings of isolation within the relationship,” says Goldberg.
There are several ways that infertility can strain relationships. Here’s what our experts said:
Communication Becomes Tense
Often, when a couple is experiencing infertility, communication becomes strained. At times, communication may even shut down altogether as the couple moves through difficult feelings. There’s also often a sense that the other partner doesn’t understand your feelings, says Goldberg.
Sometimes, there’s truth to the idea that a partner simply can’t empathize with what you are going through, Goldberg notes. “For example, if the female partner is undergoing all the very invasive treatments (which is the case in the majority of instances), then even with a partner who genuinely desires to understand, they likely cannot fully empathize as they are not the one on the table with their legs open repeatedly, getting pricked and prodded,” she describes.
Misunderstandings
Misunderstandings are common as couples navigate fertility issues. “ Misunderstandings can result from one partner feeling blamed or perceived as less invested,” Goldberg says.
The person undergoing more fertility testing and treatment might feel like their partner is less invested in the outcome than they are, or vice versa. The person who is diagnosed with a fertility problem may feel like their partner is blaming them for what they are going through together as a couple.
Decision Fatigue
Another factor that can put a huge strain on relationships is decision fatigue . There are just so many decisions to make when it comes to fertility treatments and couples may argue about them. They may also just feel exhausted and burned out from making so many difficult decisions.
“Decision-making regarding fertility treatments, especially given the very hefty price tag that insurance rarely covers, is an added stress that can seep into relationship difficulties,” Goldberg remarks.
No Room For Other Experiences
Dealing with infertility can become completely all-encompassing, says Goldberg. It can leave “little room for other aspects of the relationship, creating disconnection,” she describes.
Other aspects of your relationship you used to enjoy, such as sex or spending time out socially, may be impacted, or may diminish altogether.
Too Much Focus on Fertility
It’s not just what you do together as a couple that can change, even the types of conversations you have can change too. “The challenges related to infertility can feel like a wall between partners,” Reed says. “You might find all your talks circling back to fertility treatments leaving little room for the laughs and shared moments that used to lighten your days.”
Dealing with infertility as a couple is hard, period. But there is hope. Simply recognizing how you are feeling, individually and as a couple, is a great first step.
“Couples can work through and cope with infertility challenges by acknowledging that infertility can be extremely disheartening, it’s normal for conflicts to arise, and committing to open communication or even scheduling time to discuss their feelings,” says Goldberg. “It's important for each partner to validate each other's feelings and experiences even if they disagree or don’t fully understand.”
Tips For Nurturing Your Relationships During Infertility
Goldberg shared her top practical tips for nurturing your relationship as you navigate infertility:
- Try to schedule regular “feelings” check-ins related to fertility concerns and goals
- Make an effect to find ways to connect privately, that don’t involve baby making (e.g., doing a challenging puzzle together, cooking an intricate meal together)
- Practice active listening together
- Practice learning to empathize with your spouse’s perspective
- Plan joyful activities on days you are expecting news about your journey (e.g., a special breakfast after a scheduled ultrasound to see follicle growth; a weekend getaway after an IVF treatment is over)
Here’s maybe the most important advice: You don’t have to do this alone. There is support out there for both individuals and couples dealing with the stress of infertility.
Support Groups
Joining an infertility support group for couples can be hugely helpful. You may be able to find one through your local fertility clinic, or through a therapist who specializes in infertility and holds support groups for couples.
“One of the most helpful resources for showing grace to oneself is joining a support group of others experiencing infertility,” says Goldberg. It can also be helpful to find a friend or two who has struggled similarly and connect with them, she adds.
“Having someone who can truly understand the emotions and challenges involved can really help an individual to feel less like something is wrong with them and less isolated,” Goldberg describes. “It’s also helpful to allow oneself to grieve the losses associated with infertility while also feeling emboldened by the courage and strength to put themselves through all the appointments, treatments and angst that come with that.”
Therapy is one of the most helpful ways to manage the strain that infertility can place on couples, Reed says. “It offers a neutral and welcoming space to unload some of those heavy feelings and fears,” she explains. “It’s a place to learn new ways to communicate and support each other while also finding strategies to cope with the ups and downs of fertility treatments.”
There are several different individual therapy types that can help the emotional upheaval that comes from dealing with infertility. Reed recommends Eye Movement Desensitization and Reprocessing (EMDR) therapy and Brainspotting, both of which can help you work through the traumas sometimes experienced during infertility. Research has found that Cognitive Behavioral Therapy (CBT) is effective at managing the psychological effects of infertility.
Partners may find it helpful to enter couples therapy to work on communication and manage the ways that infertility has affected their relationship. “A therapist specializing in infertility can help normalize all the feelings each of them is experiencing and help educate them on things they may not know yet,” Goldberg describes. “It is a place where couples can process grief, resentments , and find constructive ways to support each other.”
Without a doubt, infertility is challenging—not just on each person, but on the couple who is going through it together. You can’t underestimate the impact it might have on your relationships. If you are experiencing some heavy impacts as a couple, you are far from alone. This stuff is hard!
You can get through this—we promise. But you don’t have to go through this on your own. Please reach out for support—from your physician, therapist, or a trusted friend—to help you navigate the chopping waters of infertility as a couple.
American Psychiatric Association. Infertility: The Impact of Stress and Mental Health .
Sharma A, Shrivastava D. Psychological Problems Related to Infertility . Cureus . 2022;14(10):e30320. doi:10.7759/cureus.30320
Kiani Z, Fakari FR, Hakimzadeh A, et al. Prevalence of depression in infertile men: a systematic review and meta-analysis . BMC Public Health. 2023;23:1972. doi:10.1186/s12889-023-16865-4
Bal Z, Uçar T. The effect of cognitive behavioural therapy and eye movement desensitization and reprocessing techniques on infertile women: a randomized controlled trial . Reprod Biomed Online . 2024;48(2):103612.
Hildebrand A, Grand D, Stemmler M. Brainspotting – the efficacy of a new therapy approach for the treatment of Posttraumatic Stress Disorder in comparison to Eye Movement Desensitization and Reprocessing . Mediterr J Clin Psychol . 2017;5(1).
By Wendy Wisner Wendy Wisner is a health and parenting writer, lactation consultant (IBCLC), and mom to two awesome sons.
Maintenance work is planned for Wednesday 1st May 2024 from 9:00am to 11:00am (BST).
During this time, the performance of our website may be affected - searches may run slowly and some pages may be temporarily unavailable. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.

Environmental Science: Water Research & Technology
Harnessing exoelectrogens in a novel microbial desalination cell: a study on the impact of salinity on sago effluent treatment and power generation.

* Corresponding authors
a Department of Chemical Engineering, National Institute of Technology, Tiruchirappalli, Tamilnadu, India E-mail: [email protected]
Microbial desalination cell (MDC) provides integral solutions for addressing water scarcity and environmental challenges. This research paper investigates a novel MDC with two distinct exoelectrogens, Shewanella putrefaciens MTCC 8104 (MDC – 1) and mixed culture (MDC – 2) at three different NaCl concentrations (10 g L −1 , 20 g L −1 and 30 g L −1 ) and brackish water in the desalination chamber utilizing sago effluent as an anolyte. The maximum chemical oxygen demand (COD) removal and desalination efficiency of 95.1 ± 2% and 13.2 ± 2% were observed for 30 g L −1 NaCl for MDC – 1. Furthermore, the power density obtained at 30 g L −1 NaCl concentration for MDC – 1 was 60.22 ± 0.2 mW m −2 and 43.09 ± 0.2 mW m −2 for MDC – 2. The internal resistance of the Shewanella putrefaciens inoculated MDC – 1 was very low compared to MDC – 2. However, the dynamics changed in brackish water treatment, where MDC – 1 faced challenges due to the diffusion of ions other than Na+ and Cl − , leading to increased internal resistance and reduced power output. In contrast, the mixed culture in MDC – 2 adapted well to the brackish water ions, showcasing higher oxidation–reduction potential, increased power, and low internal resistance. These findings underscore the superior performance of Shewanella putrefaciens in NaCl desalination, while a mixed culture proves more adaptable and effective in real-time brackish water treatment. As conductivity increases, internal resistance diminishes, suggesting the potential future application of MDC in treating real seawater and brackish water by optimizing volume ratios, biofilm performance and preventing membrane fouling.
Article information
Download citation, permissions.
S. Prakash, S. Naina Mohamed and K. Ponnusamy, Environ. Sci.: Water Res. Technol. , 2024, Advance Article , DOI: 10.1039/D4EW00081A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
Automation, Inequality, and Productivity
Working Papers
The Simple Macroeconomics of AI
Economic Policy
This paper evaluates claims about the large macroeconomic implications of new advances in AI. It starts from a task-based model of AI’s effects, working through automation and task complementarities. It establishes that, so long as AI’s microeconomic effects are driven by cost savings/productivity improvements at the task level, its macroeconomic consequences will be given by a version of Hulten’s theorem: GDP and aggregate productivity gains can be estimated by what fraction of tasks are impacted and average task-level cost savings. Using existing estimates on exposure to AI and productivity improvements at the task level, these macroeconomic effects appear nontrivial but modest|no more than a 0:71% increase in total factor productivity over 10 years. The paper then argues that even these estimates could be exaggerated, because early evidence is from easy-to-learn tasks, whereas some of the future effects will come from hard-to-learn tasks, where there are many context-dependent factors affecting decision-making and no objective outcome measures from which to learn successful performance. Consequently, predicted TFP gains over the next 10 years are even more modest and are predicted to be less than 0:55%. Acemoglu also explores AI’s wage and inequality effects. The paper shows theoretically that even when AI improves the productivity of low-skill workers in certain tasks (without creating new tasks for them), this may increase rather than reduce inequality. Empirically, the paper finds that AI advances are unlikely to increase inequality as much as previous automation technologies because their impact is more equally distributed across demographic groups, but there is also no evidence that AI will reduce labor income inequality. AI is also predicted to widen the gap between capital and labor income. Finally, some of the new tasks created by AI may have negative social value (such as design of algorithms for online manipulation), and Acemoglu discusses how to incorporate the macroeconomic effects of new tasks that may have negative social value.
Relevant News
Generative ai: the likely effects on productivity, growth, wages and inequality.
Apr 4, 2024
Event Recording
Get the Latest Research
Subscribe to our newsletter to receive the latest news and research in your inbox.
" * " indicates required fields
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
How Pew Research Center will report on generations moving forward
Journalists, researchers and the public often look at society through the lens of generation, using terms like Millennial or Gen Z to describe groups of similarly aged people. This approach can help readers see themselves in the data and assess where we are and where we’re headed as a country.
Pew Research Center has been at the forefront of generational research over the years, telling the story of Millennials as they came of age politically and as they moved more firmly into adult life . In recent years, we’ve also been eager to learn about Gen Z as the leading edge of this generation moves into adulthood.
But generational research has become a crowded arena. The field has been flooded with content that’s often sold as research but is more like clickbait or marketing mythology. There’s also been a growing chorus of criticism about generational research and generational labels in particular.
Recently, as we were preparing to embark on a major research project related to Gen Z, we decided to take a step back and consider how we can study generations in a way that aligns with our values of accuracy, rigor and providing a foundation of facts that enriches the public dialogue.
A typical generation spans 15 to 18 years. As many critics of generational research point out, there is great diversity of thought, experience and behavior within generations.
We set out on a yearlong process of assessing the landscape of generational research. We spoke with experts from outside Pew Research Center, including those who have been publicly critical of our generational analysis, to get their take on the pros and cons of this type of work. We invested in methodological testing to determine whether we could compare findings from our earlier telephone surveys to the online ones we’re conducting now. And we experimented with higher-level statistical analyses that would allow us to isolate the effect of generation.
What emerged from this process was a set of clear guidelines that will help frame our approach going forward. Many of these are principles we’ve always adhered to , but others will require us to change the way we’ve been doing things in recent years.
Here’s a short overview of how we’ll approach generational research in the future:
We’ll only do generational analysis when we have historical data that allows us to compare generations at similar stages of life. When comparing generations, it’s crucial to control for age. In other words, researchers need to look at each generation or age cohort at a similar point in the life cycle. (“Age cohort” is a fancy way of referring to a group of people who were born around the same time.)
When doing this kind of research, the question isn’t whether young adults today are different from middle-aged or older adults today. The question is whether young adults today are different from young adults at some specific point in the past.
To answer this question, it’s necessary to have data that’s been collected over a considerable amount of time – think decades. Standard surveys don’t allow for this type of analysis. We can look at differences across age groups, but we can’t compare age groups over time.
Another complication is that the surveys we conducted 20 or 30 years ago aren’t usually comparable enough to the surveys we’re doing today. Our earlier surveys were done over the phone, and we’ve since transitioned to our nationally representative online survey panel , the American Trends Panel . Our internal testing showed that on many topics, respondents answer questions differently depending on the way they’re being interviewed. So we can’t use most of our surveys from the late 1980s and early 2000s to compare Gen Z with Millennials and Gen Xers at a similar stage of life.
This means that most generational analysis we do will use datasets that have employed similar methodologies over a long period of time, such as surveys from the U.S. Census Bureau. A good example is our 2020 report on Millennial families , which used census data going back to the late 1960s. The report showed that Millennials are marrying and forming families at a much different pace than the generations that came before them.
Even when we have historical data, we will attempt to control for other factors beyond age in making generational comparisons. If we accept that there are real differences across generations, we’re basically saying that people who were born around the same time share certain attitudes or beliefs – and that their views have been influenced by external forces that uniquely shaped them during their formative years. Those forces may have been social changes, economic circumstances, technological advances or political movements.
When we see that younger adults have different views than their older counterparts, it may be driven by their demographic traits rather than the fact that they belong to a particular generation.
The tricky part is isolating those forces from events or circumstances that have affected all age groups, not just one generation. These are often called “period effects.” An example of a period effect is the Watergate scandal, which drove down trust in government among all age groups. Differences in trust across age groups in the wake of Watergate shouldn’t be attributed to the outsize impact that event had on one age group or another, because the change occurred across the board.
Changing demographics also may play a role in patterns that might at first seem like generational differences. We know that the United States has become more racially and ethnically diverse in recent decades, and that race and ethnicity are linked with certain key social and political views. When we see that younger adults have different views than their older counterparts, it may be driven by their demographic traits rather than the fact that they belong to a particular generation.
Controlling for these factors can involve complicated statistical analysis that helps determine whether the differences we see across age groups are indeed due to generation or not. This additional step adds rigor to the process. Unfortunately, it’s often absent from current discussions about Gen Z, Millennials and other generations.
When we can’t do generational analysis, we still see value in looking at differences by age and will do so where it makes sense. Age is one of the most common predictors of differences in attitudes and behaviors. And even if age gaps aren’t rooted in generational differences, they can still be illuminating. They help us understand how people across the age spectrum are responding to key trends, technological breakthroughs and historical events.
Each stage of life comes with a unique set of experiences. Young adults are often at the leading edge of changing attitudes on emerging social trends. Take views on same-sex marriage , for example, or attitudes about gender identity .
Many middle-aged adults, in turn, face the challenge of raising children while also providing care and support to their aging parents. And older adults have their own obstacles and opportunities. All of these stories – rooted in the life cycle, not in generations – are important and compelling, and we can tell them by analyzing our surveys at any given point in time.
When we do have the data to study groups of similarly aged people over time, we won’t always default to using the standard generational definitions and labels. While generational labels are simple and catchy, there are other ways to analyze age cohorts. For example, some observers have suggested grouping people by the decade in which they were born. This would create narrower cohorts in which the members may share more in common. People could also be grouped relative to their age during key historical events (such as the Great Recession or the COVID-19 pandemic) or technological innovations (like the invention of the iPhone).
By choosing not to use the standard generational labels when they’re not appropriate, we can avoid reinforcing harmful stereotypes or oversimplifying people’s complex lived experiences.
Existing generational definitions also may be too broad and arbitrary to capture differences that exist among narrower cohorts. A typical generation spans 15 to 18 years. As many critics of generational research point out, there is great diversity of thought, experience and behavior within generations. The key is to pick a lens that’s most appropriate for the research question that’s being studied. If we’re looking at political views and how they’ve shifted over time, for example, we might group people together according to the first presidential election in which they were eligible to vote.
With these considerations in mind, our audiences should not expect to see a lot of new research coming out of Pew Research Center that uses the generational lens. We’ll only talk about generations when it adds value, advances important national debates and highlights meaningful societal trends.
- Age & Generations
- Demographic Research
- Generation X
- Generation Z
- Generations
- Greatest Generation
- Methodological Research
- Millennials
- Silent Generation

Kim Parker is director of social trends research at Pew Research Center
How Teens and Parents Approach Screen Time
Who are you the art and science of measuring identity, u.s. centenarian population is projected to quadruple over the next 30 years, older workers are growing in number and earning higher wages, teens, social media and technology 2023, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Dialogues Clin Neurosci
- v.20(1); 2018 Mar
Language: English | Spanish | French
The relationship between stress and infertility
La relación entre estrés e infertilidad, relation entre stress et infertilité, kristin l. rooney.
Boston IVF, Waltham, Massachusetts USA
Alice D. Domar
Boston IVF, Waltham, Massachusetts USA; Department of Obstetrics, Gynecology and Reproductive Biology, Harvard Medical School, Boston, Massachusetts, USA; Department of Obstetrics and Gynecology, Beth Israel Deaconess Medical Center, Boston Massachusetts, USA
The relationship between stress and infertility has been debated for years. Women with infertility report elevated levels of anxiety and depression, so it is clear that infertility causes stress. What is less clear, however, is whether or not stress causes infertility. The impact of distress on treatment outcome is difficult to investigate for a number of factors, including inaccurate self-report measures and feelings of increased optimism at treatment onset. However, the most recent research has documented the efficacy of psychological interventions in lowering psychological distress as well as being associated with significant increases in pregnancy rates. A cognitive-behavioral group approach may be the most efficient way to achieve both goals. Given the distress levels reported by many infertile women, it is vital to expand the availability of these programs.
Por años ha sido debatida la relación entre estrés e infertilidad. En las mujeres con infertilidad se encuentran puntuaciones elevadas de ansiedad y depresión, por lo que está claro que la infertilidad causa estrés. Sin embargo, lo que está menos claro es si el estrés causa o no infertilidad. Por numerosos factores, como las inexactas mediciones de auto-reporte y los sentimientos de aumentado optimismo al comienzo de los tratamientos es difícil investigar el impacto del distrés en el resultado terapéutico. Ahora bien, la investigación más reciente ha documentado la eficacia de las intervenciones psicológicas en la reducción del distrés psicológico, además de asociarse con aumentos significativos en la frecuencia de embarazos. Una aproximación grupal cognitivo conductual puede ser la forma más eficiente para alcanzar ambos objetivos. Es vital expandir la disponibilidad de estos programas, dado los niveles de distrés reportados por muchas mujeres infértiles.
La relation entre le stress et l'infertilité est débattue depuis des années. Les niveaux d'anxiété et de dépression des femmes infertiles sont élevés, il est donc clair que l'infertilité provoque du stress. Ce qui est néanmoins moins clair c'est de savoir si le stress entraîne, ou pas, de l'infertilité. De nombreux facteurs rendent difficile la recherche sur l'effet de l'anxiété sur les résultats thérapeutiques, comme les auto-mesures imprécises, et les sentiments d'optimisme accru au début du traitement. Cependant, d'après les recherches les plus récentes, la prise en charge psychologique est efficace pour diminuer l'anxiété et elle s'associe aussi à des taux de grossesses significativement augmentés. C'est l'approche cognitivo-comportementale de groupe qui semble la plus efficace pour atteindre ces deux buts. Il est vital d'élargir la disponibilité de ces programmes compte tenu des niveaux d'anxiété rapportés par de nombreuses femmes infertiles.
Introduction
Infertility is often a silent struggle. Patients who are struggling to conceive report feelings of depression, anxiety, isolation, and loss of control. Depression levels in patients with infertility have been compared with patients who have been diagnosed with cancer. 1 It is estimated that 1 in 8 couples (or 12% of married women) have trouble getting pregnant or sustaining a pregnancy. 2 Despite the prevalence of infertility, the majority of infertile women do not share their story with family or friends, thus increasing their psychological vulnerability. The inability to reproduce naturally can cause feelings of shame, guilt, and low self-esteem. These negative feelings may lead to varying degrees of depression, anxiety, distress, and a poor quality of life.
Patients who undergo assisted reproductive treatment (ART) are at significant risk of experiencing psychiatric disorders and it is important to recognize, acknowledge, and assist these patients as they cope with their infertility diagnosis and treatment.
Infertility is a life crisis affecting patients from all around the world. Infertile patients experience a tremendous amount of emotional turmoil as the result of their diagnosis. The risk of depression, anxiety, and distress is high for infertile patients.
It has been hypothesized since biblical times that stress can hamper fertility. This raises one of the most compelling mind/body questions: does infertility cause stress or does stress cause infertility? The answer thus far is not clear; the relationship between distress and infertility may not have a clear cause and effect direction. It is definitive that infertility leads to significant distress and that psychological interventions are likely to be associated with decreases in depression and increases in pregnancy rates. However, the impact of distress on treatment outcome is less definitive.
This article will review the psychiatric disorders associated with infertility treatment and the potential impact of those symptoms on reproductive treatment outcome, as well as the efficacy of psychological interventions on both distress and pregnancy rates.
The psychological impact of infertility: depression, anxiety, and distress
One of the main challenges in assessing the distress levels in women with infertility is the accuracy of self-report measures. It is possible that women “fake good” in order to appear mentally healthier than they are. It is also possible that women feel a sense of hopefulness/increased optimism prior to initiating infertility treatment, which is when most assessments of distress are collected. Some early studies concluded that infertile women did not report any significant differences in symptoms of anxiety and depression than fertile women. However, a 2004 study 3 utilized a structured psychiatric interview. A total of 122 women were interviewed prior to their first infertility clinic visit and the results were striking; 40% of women were diagnosed as having anxiety, depression, or both. Subsequent research has supported these findings. Volgsten and colleagues 4 reported a 31% prevalence of psychiatric symptoms, the most common of which was major depression. In a large Danish study of 42 000 women 5 who underwent ART treatment and were screened for depression prior to treatment, 35% screened positive. In another recent study of 174 women undergoing infertility treatment, 39% met the criteria for major depressive disorder. 6 In one of the largest studies to date, 7 352 women and 274 men were assessed in infertility clinics in northern California. It was determined that 56% of the women and 32% of the men reported significant symptoms of depression and 76% of the women and 61% of the men scored reported significant symptoms of anxiety. Not surprisingly, recent research documents that infertility patients consistently report significantly more symptoms of anxiety and depression than fertile individuals. 8 Finally, in a recent concerning study on suicidality in 106 women with infertility, 9.4% of the women reported having suicidal thoughts or attempts. 9
A recent literature review on the prevalence of psychological symptoms in infertility concluded that 25% to 60% of infertile individuals report psychiatric symptoms and that their levels of anxiety and depression are significantly higher than in fertile controls. 10
The medications used to treat infertility, including clomiphene, leuprolide, and gonadotropins, are associated with psychological symptoms such as anxiety, depression, and irritability. Thus, when assessing symptoms of women mid-treatment, it is difficult to differentiate between the psychological impact of infertility versus the side effects of the medication. Thus, studies which included measures of these symptoms prior to beginning medication, or after going off it, may be more accurate than those done only on women as they cycle.
The further into treatment a patient goes, the more often they display symptoms of depression and anxiety. Patients with one treatment failure had significantly higher levels of anxiety, and patients with two failures experienced more depression when compared with those without a history of treatment. 11 However, it has also been shown that the more depressed the infertile woman, the less likely she is to start infertility treatment and the more likely she is to drop out after only one cycle. 12 Researchers have also shown that despite a good prognosis and having the finances available to pay for treatment, discontinuation is most often due to psychological reasons. 13 - 15
The impact of stress on treatment outcome
One of the most controversial areas in the field of reproductive medicine is the potential impact of psychological factors on pregnancy rates. Although there are a variety of old wives' tales which support the notion that stress hampers reproduction function, this theory has been challenging to confirm. There have been dozens of studies which have investigated the relationship between psychological symptoms prior to and during ART cycles and subsequent pregnancy rates, with conflicting results. Some have shown that the more distressed the women prior to and during treatment, the lower the pregnancy rates, 16 - 19 while other studies have not. 20 - 21
There are several possible explanations for these discrepancies. One is that individuals may not accurately report their level of distress when completing psychological questionnaires. Research supports this theory. In a study of fecundity in 339 women in the United Kingdom trying to conceive, 22 self-reported symptoms of depression, anxiety, and stress were not significantly associated with time to pregnancy. However, in a similar study on 501 women in the United States, levels of salivary α-amylase, a biomarker of stress, were significantly correlated with time to pregnancy. 23 Women in the highest quartile of α-amylase levels at baseline were twice as likely to subsequently experience infertility. Finally, in a recent study in 135 IVF patients, cortisol was measured through samplings of hair, which measures levels from the prior 3 to 6 months. 24 The hair Cortisol levels were significantly correlated to pregnancy rates (P= 0.017). These findings match what most infertility patients believe; that psychological symptoms have a negative impact on fertility. 25
Miscarriage
According to the American College of Obstetricians and Gynecologists (ACOG), studies reveal that anywhere from 10% to 25% of all clinically recognized pregnancies will end in miscarriage. 26 Pregnancy loss occurs for many reasons, one of the leading being the chromosomal abnormality of the fetus. Patients who experience a pregnancy loss have met the criteria for post-traumatic stress disorder; the majority of women report suffering from anxiety and depression. 27
Many patients undergoing ART are taking advantage of a relatively new scientific advancement known as preimplantation genetic screening (PGS). PGS allows scientists to identity chromosomal defects through the biopsy of a blastocyst and thus can allow the transfer of only normal blastocysts. Patients who take advantage of this testing may increase their chance of pregnancy by eliminating the embryos which would likely result in a miscarriage. PGS is gaining in popularity, with some ART centers only transferring one PGS normal blastocyst per cycle.
However, there are disadvantages of this new science for patients: the cost of PGS can add thousands of dollars to an already expensive treatment cycle, some embryos don't survive to their fifth day, which is when the biopsy must be performed, and some patients will find that there are no chromosomally normal blastocysts to transfer, which can be emotionally devastating. In addition, because the blastocyst is biopsied around day 5 of development and it takes up to 2 weeks to get the biopsy results, all blastocysts are frozen after biopsy and if any are later determined to be normal, the patient must wait a minimum of a month before she can undergo a thaw cycle to transfer the biopsied blastocyst. So PGS adds another waiting period. Instead of the wait between transfer and pregnancy test, there are two waits: waiting for the biopsy results, and then waiting between transfer and pregnancy test.
Repeat failure
Some patients will get pregnant quite easily from ART, conceiving on their first cycle. However, that is the exception; for many it may take years, or not happen at all. The cause of infertility is not always clear; it may be an underlying health condition such as polycystic ovarian syndrome (PCOS), endometriosis, or male factor infertility, or the frustrating diagnosis of unexplained infertility. Knowing the root cause of an infertility diagnosis can reduce the burden for patients as they understand why this may be happening to them; while still heart-broken, they can place blame on “something.” Patients with unexplained infertility do not know why they cannot get pregnant. They may become obsessed with this diagnosis. In fact, infertile women may display a high prevalence of obsession. 28 Changes to lifestyle, such as exercise, diet, caffeine intake, and sleep may be altered as an attempt to reverse the diagnosis. For some, these changes paired with ART treatment may lead to a pregnancy; for others, it sadly may not.
Psychosocial interventions for women with infertility
There have been dozens of studies on the efficacy of psychological interventions on women with infertility, with outcomes including pregnancy rates/live birth rates as well as multiple measures of psychological distress. Unfortunately, the various meta-analyses performed in the past 14 years fail to agree on the results.
Boivin 29 included 25 studies in her meta-analysis.
The conclusions on efficacy were:
- Interventions were more impactful on reducing negative affect than interpersonal functioning,
- There were no significant differences in pregnancy rates,
- Group interventions which included actual skills acquisition were more effective than counseling ones,
- Men and women benefitted equally.
Hammerli et al 30 included 21 controlled studies in their meta-analysis and concluded that psychological interventions were not associated with any significant changes in psychological status and that non-ART patients experienced significantly higher pregnancy rates. They also concluded that interventions of six or more sessions were more impactful than shorter ones.
Ying et al 31 only included 20 randomized studies in their systematic review. They concluded that there were methodological issues with the studies which reported significant results for both pregnancy rates and psychological distress and recommended that more rigorous research needs to be conducted, especially on the most stressful time for infertility patients: waiting for the results of the pregnancy test.
Frederiksen et al 32 included 39 studies and reported on both pregnancy rates and psychological symptoms. They concluded that there were statistically significant and robust overall effects of psychosocial interventions... “on both pregnancy rates and a variety of different psychological symptoms.” The conclusions also were that effect sizes were greater for women than for men and higher pregnancy rates were associated with greater decreases in anxiety.
Another 2016 systematic analysis, a Cochrane review, also included 39 studies 33 but the authors stated that the quality of the included studies did not warrant any conclusions. Finally, the third 2016 review 34 included only 12 studies of which seven were intervention designs. The conclusions based upon these seven studies were that psychological interventions are associated with less psychological distress, higher pregnancy rates, and improved marital satisfaction.
The mind/body program for infertility
It is evident that infertility patients experience distress, depression, anxiety, and decreased quality of life. It is important for infertility providers and counselors to offer assistance to these patients by way of psychological interventions and emotional support.
The Mind/Body Program for Infertility was created and launched in September 1987. Because psychological interventions for infertile patients can improve psychological outcomes and marital relationships 34 as well as increase patient retention and improve pregnancy rates, 25 it was hypothesized that a research-based clinical program had the potential to accomplish all of these goals. The program has ten sessions, is a group model, and the partners of participants attend three of these sessions. Mind/Body therapy has been proven a successful way to reduce stress and increase pregnancy rates 35 and provides patients with skills in cognitive behavior therapy, relaxation training, lifestyle changes, journaling, self-awareness, and social support components.
The Mind/Body program includes two sessions of cognitive behavioral therapy (CBT) which is a form of psychotherapy that emphasizes the important role of thinking in how we feel and what we do. Participants challenge automatic thought patterns, such as “I will never have a baby,” “the infertility is all my fault,” or “my husband is going to leave me for a fertile woman.”
Relaxation techniques have been widely shown to reduce negative emotions in a range of medical patients, 36 more specifically, they have been shown to significantly reduce anxiety scores in women undergoing infertility treatment. 37 Patients learn a different technique each week, including progressive muscle relaxation, hatha yoga, meditation, imagery, etc, and are encouraged to try each one and then practice the one(s) which are most effective for them.
A study of both male and female infertility patients explored the benefit of expressive writing. The authors found that both partners exhibited decreased depressive symptoms. 38 Participants in the mind/body program do a journaling exercise during the seventh session of the program and are encouraged to continue if they found it helpful. They also are encouraged to maintain a daily gratitude diary.
Mindfulness is commonly used as a coping strategy for infertility patients and is introduced early in the program. A study of first time IVF patients randomized to a mindfulness-based intervention versus control found that women who attended the intervention revealed a significant increase in mindfulness, self-compassion, meaning-based coping strategies, and most importantly had higher pregnancy rates. 39
There have been a number of RCTs on the efficacy of the mind/body program. 35 , 37 , 40 Participants experience significantly lower levels of distress as well as a higher pregnancy rate than the control subjects.
Self-administered interventions
Psychological interventions do not necessarily need to be administered by a clinician; there are self-administered options available as well. A randomized controlled prospective study of 166 first-time IVF patients evaluated the use of a self-administered cognitive coping and relaxation intervention (CCRI).The findings suggested that patients utilizing the CCRI displayed more positive reappraisal coping, improved QoL and reported less anxiety. 41 In addition, the intervention participants had a 67% lower dropout rate than the controls.
The 2-week waiting period between embryo transfer and the pregnancy test has been recognized as a very stressful time during IVF treatment. Another self-administered tool is the Positive Reappraisal Coping Intervention (PRCI). The PCRI encourages a form of coping that helps people take account of positive aspects of stressful situations; a strategy particularly useful for unpredictable and uncontrollable stressors such as the 2-week waiting period. 42 Research on this tool has found it beneficial to utilize during the 2-week waiting period. 43
A recent randomized controlled prospective pilot study included an online version of the mind/body program. 44 Women who were randomized to the intervention group experienced significant decreases in anxiety and depression and a higher pregnancy rate.
A diagnosis of infertility can be a tremendous burden for patients. The pain and suffering of infertility patients is a major problem. Patients must be counseled and supported as they go through treatment. Although neither the American Society for Reproductive Medicine nor the European Society for Human Reproduction and Embryology have formal requirements for psychological counseling for infertility patients, there is acknowledgement that incorporating psychological interventions into routine practice at ART clinics is beneficial. It has been well documented that infertility causes stress. The impact of stress on ART outcome is still somewhat controversial. However, it is clear that psychological interventions for women with infertility have the potential to decrease anxiety and depression and may well lead to significantly higher pregnancy rates.
Acknowledgments
The authors do not have any acknowledgements to report. The authors have nothing to disclose.
Selected abbreviations and acronyms

COMMENTS
Increased demand for in vitro fertilization (IVF) due to socio-demographic trends, and supply facilitated by new technologies, converged to transform the way a substantial proportion of humans reproduce. The purpose of this article is to describe the societal and demographic trends driving increased worldwide demand for IVF, as well as to provide an overview of emerging technologies that ...
These and subsequent advances in the rapidly developing field of assisted reproductive technology (ART) have provided hope for people struggling with the medical, emotional, and financial effects of infertility and for those wanting to conceive where there are risks of the recurrence of monogenic disorders. 4 , 5 As of 2020, an estimated 8 ...
Despite growing concerns around links between social media use and mental health (Igor, 2014), public health research has focused more on self-tracking apps designed to manage mental health (Donker et al., 2013) than the emotional processes and effects of using them - a gap that we believe warrants further attention. The pressures and ...
According to a 2019 CDC survey, 12.7% of women between the ages of 15 and 49 received infertility services, most women sought medical help to become pregnant (9.5%), 6.7% sought basic medical advice, 5.8% of the population was only tested for infertility, and assisted reproductive technology (ART) was the least sought service (0.6%) .
Infertility is the inability to conceive a child after a certain period of time the length of which varies by country. The term can also refer to women who are unable to carry a pregnancy to term ...
These tools aim to harness the power of information and technology to improve care, facilitate communication and support patients through stressful treatment cycles. To warrant patient engagement, digital support tools must be perceived as useful. This review identifies and narratively analyses tools developed for fertility patients to date ...
Infertility is a global health issue affecting women and men of reproductive age with increasing incidence worldwide, in part due to greater awareness and better diagnosis. Assisted reproduction technologies (ART) are considered the ultimate step in the treatment of infertility. Recently, artificial intelligence (AI) has been progressively used in the many fields of medicine, integrating ...
The literature on infertility is disproportionately skewed towards clinical research related to causes of infertility, diagnosis, or Artificial Reproductive Techniques (ART), and lesser on the ...
20 Department of Obstetrics and Gynaecology, Penn State College of Medicine, Pennsylvania. 21 Fertility Plus, Auckland, New Zealand. 22 Reproductive Medicine Unit, University College Hospital, London, UK. 23 Department of Obstetrics and Gynaecology, Monash University, Melbourne, Australia.
Recently, infertility has been affecting a large number of women than men. It is the male or female reproductive system's disease. Consequently, after 12 months or more of usual insecure sexual intercourse, if they fail to attain pregnancy then that failure is defined as infertility. As per the National Institute of Child Health and Human Development, people in the USA have infertility ...
Infertility is a common, yet often misunderstood, experience. Infertility is an important topic for family scientists because of its effects on families; its relevance to research in related areas, such as fertility trends and reproductive health; and its implications for practitioners who work with individuals and couples experiencing infertility.
Assisted reproductive technology (ART) is increasingly influencing the fertility trends of high-income countries characterized by a pattern of delayed childbearing. However, research on the impact of ART on completed fertility is limited and the extent to which delayed births are realized later in life through ART is not well understood. This study uses data from Australian fertility clinics ...
The top 10 research priorities for each of the four categories male infertility, female and unexplained infertility (including age-related infertility, ovarian cysts, uterine cavity abnormalities and tubal factor infertility), medically assisted reproduction (including ovarian stimulation, IUI and IVF) and ethics, access and organization of ...
Introduction. Infertility is a widespread, serious health problem that is defined as a global public health problem by the World Health Organization (WHO) (Agarwal et al., 2015; Cong et al., 2016).Infertility is considered as the failed achievement of a viable pregnancy following 1 year of unprotected intercourse, and it is estimated that more than 186 million people suffer from it worldwide ...
Abstract. In this paper I will discuss three areas in which advances in human reproductive technology could occur, their uses and abuses, and their effects on society. First is the potential to drastically increase the success rate and availability of in vitro fertilization and embryo freezing. Second is the ability to perform biopsies on ...
Background The overall prevalence of infertility was estimated to be 3.5-16.7% in developing countries and 6.9-9.3% in developed countries. Furthermore, according to reports from some regions of sub-Saharan Africa, the prevalence rate is 30-40%. The consequences of infertility and how it affects the lives of women in poor-resource settings, particularly in developing countries, has ...
Background Human instinctively desire to have offspring. Infertility can cause painful emotional experiences throughout the life mainly known as quality of life impairment. This study aimed to investigate the impact of infertility on a woman's quality of life. Methods A number of 180 infertile and 540 fertile women participated in this matched case-control study. The cases were selected ...
Infertility is characterized by failure to establish a clinical pregnancy after 12 months of regular and unprotected sexual intercourse. Infertility concerns an estimated 8-12% of the global population, and is associated with factors including time of unwanted non-conception, age of female partner and number of diseases impacting fertility.
To carry out an in-depth analysis of the scientific research on infertility, we performed the first bibliometric analysis focusing on studies involving global literature on infertility during the period 2013-2022. Analysis of 33239 articles in the field of infertility showed a significant increasing trend in the number of publications over the period 2013-2022, with authors mainly from the ...
Technology has developed into one of the most useful tools to treat infertility. The ability to track ovulation, freeze eggs for future use, perform genetic screenings, and undergo uterine transplants or assisted reproductive procedures has opened doors that used to be welded shut. Technology has changed the future of infertility.
Infertility continued to be a major stressor among women with infertility during COVID-19pandemic. This study aimed to evaluate the impact of primary care posttraumatic stress disorder (PC-PTSD) on fertility problem of Iranian women with infertility during COVID-19 pandemic. In this cross-sectional study, 386 women with infertility completed the questionnaires of PC-PTSD-5 and Fertility ...
It has profound impacts on a person's mental health and identity. Living with infertility can lead to strong feelings of loss, grief, shame, and depression. But infertility doesn't just affect folks on an individual level. It touches both members of the couple. Many couples feel isolated from other couples and experience collective feelings ...
Microbial desalination cell (MDC) provides integral solutions for addressing water scarcity and environmental challenges. This research paper investigates a novel MDC with two distinct exoelectrogens, Shewanella putrefaciens MTCC 8104 (MDC - 1) and mixed culture (MDC - 2) at three different NaCl concentrations (10 g L −1, 20 g L −1 and 30 g L −1) and brackish water in the ...
About 10 years ago Greil published a review and critique of the literature on the socio-psychological impact of infertility. He found at the time that most scholars treated infertility as a medical condition with psychological consequences rather than as a socially constructed reality. This article examines research published since the last review.
April 2024. Read the paper. This paper evaluates claims about the large macroeconomic implications of new advances in AI. It starts from a task-based model of AI's effects, working through automation and task complementarities. It establishes that, so long as AI's microeconomic effects are driven by cost savings/productivity improvements at ...
Introduction and background. In simple language, infertility means the inability to procreate. Infertility is a disease of the male or female reproductive system defined by the failure to achieve a pregnancy after 12 months or more of regular unprotected sexual intercourse [].Infertility affects millions of people of reproductive age worldwide - and has an impact on their families and communities.
How Pew Research Center will report on generations moving forward. Journalists, researchers and the public often look at society through the lens of generation, using terms like Millennial or Gen Z to describe groups of similarly aged people. This approach can help readers see themselves in the data and assess where we are and where we're ...
The relationship between stress and infertility has been debated for years. Women with infertility report elevated levels of anxiety and depression, so it is clear that infertility causes stress. What is less clear, however, is whether or not stress causes infertility. The impact of distress on treatment outcome is difficult to investigate for ...