Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 24 August 2020

Immunotherapy for advanced thyroid cancers — rationale, current advances and future strategies
- Jena D. French ORCID: orcid.org/0000-0002-8881-6543 1 , 2
Nature Reviews Endocrinology volume 16 , pages 629–641 ( 2020 ) Cite this article
2983 Accesses
48 Citations
25 Altmetric
Metrics details
- Immunotherapy
- Thyroid cancer
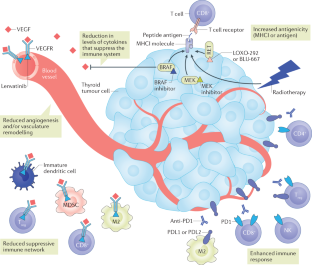
In the past decade, the field of cancer immunotherapy has been revolutionized by immune checkpoint blockade (ICB) technologies. Success across a broad spectrum of cancers has led to a paradigm shift in therapy for patients with advanced cancer. Early data are now accumulating in progressive thyroid cancers treated with single-agent ICB therapies and combination approaches that incorporate ICB technologies. This Review discusses our current knowledge of the immune response in thyroid cancers, the latest and ongoing immune-based approaches, and the future of immunotherapies in thyroid cancer. Physiologically relevant preclinical mouse models and human correlative research studies will inform development of the next stage of immune-based therapies for patients with advanced thyroid cancer.
Despite advances in treatment strategies, patients with advanced thyroid cancers would benefit from novel therapies.
Advanced thyroid cancers are commonly infiltrated by immune cells, including CD8 + T cells, but little is known about the tumour-specific T cell response.
A subset of patients has received clinical benefit from immune checkpoint blockade therapies; however, the majority achieve only a partial response.
Novel combination therapies that target both the tumour and the immune response are under investigation.
Additional studies are necessary to better understand the potential of the antitumour immune response and T cell-based therapies for patients with advanced thyroid cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Immunotherapy in hematologic malignancies: achievements, challenges and future prospects
Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response
Immunotherapy in breast cancer: an overview of current strategies and perspectives
Howlader, N., et al. (eds) SEER Cancer Statistics Review, 1975–2013. National Cancer Institute: Surveillance Epidemiology, and End Results Program http://seer.cancer.gov/csr/1975_2013/ (2016).
Lim, H., Devesa, S. S., Sosa, J. A., Check, D. & Kitahara, C. M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317 , 1338–1348 (2017).
PubMed PubMed Central Google Scholar
Mazzaferri, E. L. & Kloos, R. T. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 86 , 1447–1463 (2001).
CAS PubMed Google Scholar
Elisei, R. & Pinchera, A. Advances in the follow-up of differentiated or medullary thyroid cancer. Nat. Rev. Endocrinol. 8 , 466–475 (2012).
Franc, S. et al. Complete surgical lymph node resection does not prevent authentic recurrences of medullary thyroid carcinoma. Clin. Endocrinol. 55 , 403–409 (2001).
CAS Google Scholar
Lin, B. et al. The incidence and survival analysis for anaplastic thyroid cancer: a SEER database analysis. Am. J. Transl Res. 11 , 5888–5896 (2019).
Busaidy, N. L. & Cabanillas, M. E. Differentiated thyroid cancer: management of patients with radioiodine nonresponsive disease. J. Thyroid Res. 2012 , 618985 (2012).
Durante, C. et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 91 , 2892–2899 (2006).
Schlumberger, M. et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2 , 356–358 (2014).
PubMed Google Scholar
Haugen, B. R. et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26 , 1–133 (2016).
Smallridge, R. C. et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 22 , 1104–1139 (2012).
Wells, S. A. Jr. et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25 , 567–610 (2015).
Albero, A., Lopez, J. E., Torres, A., de la Cruz, L. & Martin, T. Effectiveness of chemotherapy in advanced differentiated thyroid cancer: a systematic review. Endocr. Relat. Cancer 23 , R71–R84 (2016).
Harris, P. J. & Bible, K. C. Emerging therapeutics for advanced thyroid malignancies: rationale and targeted approaches. Expert. Opin. Investig. Drugs 20 , 1357–1375 (2011).
CAS PubMed PubMed Central Google Scholar
Brose, M. S. et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384 , 319–328 (2014).
Elisei, R. et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 31 , 3639–3646 (2013).
Schlumberger, M. et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372 , 621–630 (2015).
Wells, S. A. Jr. et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30 , 134–141 (2012).
Subbiah, V. et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol. 36 , 7–13 (2018).
Pozdeyev, N. et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin. Cancer Res. 24 , 3059–3068 (2018).
Adnane, L., Trail, P. A., Taylor, I. & Wilhelm, S. M. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 407 , 597–612 (2006).
Okamoto, K. et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 340 , 97–103 (2013).
Tohyama, O. et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J. Thyroid Res. 2014 , 638747 (2014).
Yamamoto, Y. et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc. Cell 6 , 18 (2014).
Bible, K. C. et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 97 , 3179–3184 (2012).
Savvides, P. et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 23 , 600–604 (2013).
Sosa, J. A. et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid 24 , 232–240 (2014).
Takahashi, S. et al. A phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 15 , 717–726 (2019).
Romei, C., Ciampi, R. & Elisei, R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat. Rev. Endocrinol. 12 , 192–202 (2016).
Subbiah, V., Yang, D., Velcheti, V., Drilon, A. & Meric-Bernstam, F. State-of-the-art strategies for targeting RET-dependent cancers. J. Clin. Oncol. 38 , 1209–1221 (2020).
Moura, M. M. et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br. J. Cancer 100 , 1777–1783 (2009).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12 , 252–264 (2012).
Nguyen, L. T. & Ohashi, P. S. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat. Rev. Immunol. 15 , 45–56 (2015).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27 , 670–684 (2007).
Baitsch, L. et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS ONE 7 , e30852 (2012).
Fife, B. T. et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 10 , 1185–1192 (2009).
Legat, A., Speiser, D. E., Pircher, H., Zehn, D. & Fuertes Marraco, S. A. Inhibitory receptor expression depends more dominantly on differentiation and activation than “exhaustion” of human CD8 T cells. Front. Immunol. 4 , 455 (2013).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192 , 1027–1034 (2000).
Latchman, Y. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2 , 261–268 (2001).
Gong, J., Chehrazi-Raffle, A., Reddi, S. & Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J. Immunother. Cancer 6 , 8 (2018).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359 , 1350–1355 (2018).
Taube, J. M. et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 20 , 5064–5074 (2014).
Chen, P. L. et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 6 , 827–837 (2016).
Kamphorst, A. O. et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl Acad. Sci. USA 114 , 4993–4998 (2017).
Kelderman, S., Schumacher, T. N. & Kvistborg, P. Mismatch repair-deficient cancers are targets for anti-PD-1 therapy. Cancer Cell 28 , 11–13 (2015).
Riaz, N. et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171 , 934–949 (2017).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348 , 124–128 (2015).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515 , 568–571 (2014).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127 , 2930–2940 (2017).
Daud, A. I. et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Invest. 126 , 3447–3452 (2016).
Huang, A. C. et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545 , 60–65 (2017).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357 , 409–413 (2017).
Meyer, C. et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 63 , 247–257 (2014).
Tarhini, A. A. et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE 9 , e87705 (2014).
Weber, R. et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front. Immunol. 9 , 1310 (2018).
Neubert, N. J. et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci. Transl Med. 10 , eaan3311 (2018).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351 , 1463–1469 (2016).
Gajewski, T. F. et al. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Adv. Exp. Med. Biol. 1036 , 19–31 (2017).
Sautes-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19 , 307–325 (2019).
Senbabaoglu, Y. et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol. 17 , 231 (2016).
French, J. D., Bible, K., Spitzweg, C., Haugen, B. R. & Ryder, M. Leveraging the immune system to treat advanced thyroid cancers. Lancet Diabetes Endocrinol. 5 , 469–481 (2016).
French, J. D. et al. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J. Clin. Endocrinol. Metab. 95 , 2325–2333 (2010).
Bastman, J. J. et al. Tumor-infiltrating T cells and the PD-1 checkpoint pathway in advanced differentiated and anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 101 , 2863–2873 (2016).
Cunha, L. L. et al. Infiltration of a mixture of different immune cells may be related to molecular profile of differentiated thyroid cancer. Endocr. Relat. Cancer 19 , L31–L36 (2012).
Rosenbaum, M. W. et al. PD-L1 and IDO1 are expressed in poorly differentiated thyroid carcinoma. Endocr. Pathol. 29 , 59–67 (2018).
Bongiovanni, M. et al. Very low expression of PD-L1 in medullary thyroid carcinoma. Endocr. Relat. Cancer 24 , L35–L38 (2017).
Dadu, R. et al. Immune markers in medullary thyroid cancer (MTC) and their clinical significance [abstract 491]. Thyroid 25 (Suppl. 1), A-195–A-196 (2015).
Google Scholar
Pozdeyev, N. et al. Comprehensive immune profiling of medullary thyroid cancer. Thyroid https://doi.org/10.1089/thy.2019.0604 (2020).
Article PubMed PubMed Central Google Scholar
Cristescu, R. et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362 , eaar3593 (2018).
Giannini, R. et al. Immune profiling of thyroid carcinomas suggests the existence of two major phenotypes: an ATC-like and a PDTC-like. J. Clin. Endocrinol. Metab. 104 , 3557–3575 (2019).
Rashidian, M. et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med. 214 , 2243–2255 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04029181 (2019).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499 , 214–218 (2013).
Sharkey, M. S., Lizee, G., Gonzales, M. I., Patel, S. & Topalian, S. L. CD4(+) T-cell recognition of mutated B-RAF in melanoma patients harboring the V599E mutation. Cancer Res. 64 , 1595–1599 (2004).
Veatch, J. R. et al. Tumor-infiltrating BRAFV600E-specific CD4+ T cells correlated with complete clinical response in melanoma. J. Clin. Invest. 128 , 1563–1568 (2018).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344 , 641–645 (2014).
Kahles, A. et al. Comprehensive analysis of alternative splicing across tumors from 8,705 patients. Cancer Cell 34 , 211–224 (2018).
Eustatia-Rutten, C. F. et al. Diagnostic value of serum thyroglobulin measurements in the follow-up of differentiated thyroid carcinoma, a structured meta-analysis. Clin. Endocrinol. 61 , 61–74 (2004).
Robbins, R. J. et al. Factors influencing the basal and recombinant human thyrotropin-stimulated serum thyroglobulin in patients with metastatic thyroid carcinoma. J. Clin. Endocrinol. Metab. 89 , 6010–6016 (2004).
Caballero, Y. et al. The value of thyroperoxidase as a prognostic factor for differentiated thyroid cancer – a long-term follow-up study. Thyroid. Res. 8 , 12 (2015).
Fabbro, D. et al. Expression of thyroid-specific transcription factors TTF-1 and PAX-8 in human thyroid neoplasms. Cancer Res. 54 , 4744–4749 (1994).
Ehlers, M. et al. Epitope-specific antitumor immunity suppresses tumor spread in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 102 , 2154–2161 (2016).
Rocklin, R. E., Gagel, R., Feldman, Z. & Tashjian, A. H. Jr. Cellular immune responses in familial medullary thyroid carcinoma. N. Engl. J. Med. 296 , 835–838 (1977).
George, J. M., Williams, M. A., Almoney, R. & Sizemore, G. Medullary carcinoma of the thyroid. Cellular immune response to tumor antigen in a heritable human cancer. Cancer 36 , 1658–1661 (1975).
Bachleitner-Hofmann, T. et al. Pilot trial of autologous dendritic cells loaded with tumor lysate(s) from allogeneic tumor cell lines in patients with metastatic medullary thyroid carcinoma. Oncol. Rep. 21 , 1585–1592 (2009).
Schott, M. et al. Calcitonin-specific antitumor immunity in medullary thyroid carcinoma following dendritic cell vaccination. Cancer Immunol. Immunother. 51 , 663–668 (2002).
Schott, M. et al. Immunotherapy for medullary thyroid carcinoma by dendritic cell vaccination. J. Clin. Endocrinol. Metab. 86 , 4965–4969 (2001).
Nikiforova, M. N., Wald, A. I., Roy, S., Durso, M. B. & Nikiforov, Y. E. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J. Clin. Endocrinol. Metab. 98 , E1852–E1860 (2013).
Garrido, F., Ruiz-Cabello, F. & Aptsiauri, N. Rejection versus escape: the tumor MHC dilemma. Cancer Immunol. Immunother. 66 , 259–271 (2017).
Carretero, R. et al. Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes. Int. J. Cancer 131 , 387–395 (2012).
Garrido, F., Cabrera, T. & Aptsiauri, N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int. J. Cancer 127 , 249–256 (2010).
Angell, T. E., Lechner, M. G., Jang, J. K., LoPresti, J. S. & Epstein, A. L. MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. Clin. Cancer Res. 20 , 6034–6044 (2014).
Severson, J. J. et al. PD-1+Tim-3+ CD8+ T lymphocytes display varied degrees of functional exhaustion in patients with regionally metastatic differentiated thyroid cancer. Cancer Immunol. Res. 3 , 620–630 (2015).
Shi, X. et al. Association between programmed death-ligand 1 expression and clinicopathological characteristics, structural recurrence, and biochemical recurrence/persistent disease in medullary thyroid carcinoma. Thyroid 29 , 1269–1278 (2019).
Chowdhury, S. et al. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 7 , 32318–32328 (2016).
Angell, T. E. et al. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 24 , 1385–1393 (2014).
Chintakuntlawar, A. V. et al. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J. Clin. Endocrinol. Metab. 102 , 1943–1950 (2017).
Taube, J. M. et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl Med. 4 , 127ra37 (2012).
Feng, D. et al. BRAF(V600E)-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene 38 , 6752–6766 (2019).
Marzec, M. et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl Acad. Sci. USA 105 , 20852–20857 (2008).
Parsa, A. T. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13 , 84–88 (2007).
Gogali, F. et al. Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. J. Clin. Endocrinol. Metab. 97 , 1474–1482 (2012).
French, J. D. et al. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 97 , E934–E943 (2012).
Qing, W. et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid 22 , 905–910 (2012).
Ryder, M., Ghossein, R. A., Ricarte-Filho, J. C., Knauf, J. A. & Fagin, J. A. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr. Relat. Cancer 15 , 1069–1074 (2008).
Caillou, B. et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS ONE 6 , e22567 (2011).
Marvel, D. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Invest. 125 , 3356–3364 (2015).
Damuzzo, V. et al. Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry B Clin. Cytom. 88 , 77–91 (2015).
Bronte, V. et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7 , 12150 (2016).
Angell, T. E. et al. Circulating myeloid-derived suppressor cells predict differentiated thyroid cancer diagnosis and extent. Thyroid 26 , 381–389 (2016).
Suzuki, S. et al. Immunosuppression involving increased myeloid-derived suppressor cell levels, systemic inflammation and hypoalbuminemia are present in patients with anaplastic thyroid cancer. Mol. Clin. Oncol. 1 , 959–964 (2013).
Mehnert, J. M. et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer 19 , 196 (2019).
Capdevila, J. et al. PD-1 blockade in anaplastic thyroid carcinoma. J. Clin. Oncol. https://doi.org/10.1200/JCO.19.02727 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03246958 (2019).
Kollipara, R., Schneider, B., Radovich, M., Babu, S. & Kiel, P. J. Exceptional response with immunotherapy in a patient with anaplastic thyroid cancer. Oncologist 22 , 1149–1151 (2017).
Iyer, P. C. et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J. Immunother. Cancer 6 , 68 (2018).
Wang, J. R. et al. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAF(V600E)-mutated anaplastic thyroid carcinoma. Thyroid 29 , 1036–1043 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04061980 (2020).
Bradley, S. D. et al. BRAFV600E co-opts a conserved MHC class I internalization pathway to diminish antigen presentation and CD8+ T-cell recognition of melanoma. Cancer Immunol. Res. 3 , 602–609 (2015).
Frederick, D. T. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19 , 1225–1231 (2013).
Sumimoto, H., Imabayashi, F., Iwata, T. & Kawakami, Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 203 , 1651–1656 (2006).
Chintakuntlawar, A. et al. A phase 2 study of pembrolizumab combined with chemoradiotherapy as initial treatment for anaplastic thyroid cancer. Thyroid 29 , 1615–1622 (2019).
Sherman, E. J. et al. Pilot study combining PD-L1 antibody durvalumab (D) with CTLA-4 antibody tremelimumab (T) and stereotactic body radiotherapy (SBRT) to treat metastatic anaplastic thyroid cancer (ATC) [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 6088 (2019).
Wang, Y. et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin. Cancer Res. 25 , 1709–1717 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03122496 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03211117 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03215095 (2020).
Voron, T. et al. Control of the immune response by pro-angiogenic factors. Front. Oncol. 4 , 70 (2014).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15 , 325–340 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02973997 (2020).
Dierks, C., Miething, C., Thomusch, O., von Bubnoff, N. & Duyster, J. Lenvatinib and pembrolizumab as save and effective combination treatment in 8 patients with metastasized anaplastic (ATC) or poorly differentiated thyroid carcinoma (PDTC) [abstract 1824P]. Ann. Oncol. 29 (Suppl. 8), viii646 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04171622 (2020).
Makker, V. et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 20 , 711–718 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03820986 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03829332 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02811861 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04199104 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03884101 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03713593 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03937219 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03914300 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03181100 (2020).
Oh, C. Y. et al. ALK and RET inhibitors promote HLA class I antigen presentation and unmask new antigens within the tumor immunopeptidome. Cancer Immunol. Res. 7 , 1984–1997 (2019).
Franco, A. T. et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc. Natl Acad. Sci. USA 108 , 1615–1620 (2011).
Jolly, L. A., Massoll, N. & Franco, A. T. Immune suppression mediated by myeloid and lymphoid derived immune cells in the tumor microenvironment facilitates progression of thyroid cancers driven by HrasG12V and Pten loss. J. Clin. Cell Immunol. 7 , 451 (2016).
Jolly, L. A. et al. Fibroblast-mediated collagen remodeling within the tumor microenvironment facilitates progression of thyroid cancers driven by BrafV600E and Pten loss. Cancer Res. 76 , 1804–1813 (2016).
Knauf, J. A. et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 65 , 4238–4245 (2005).
McFadden, D. G. et al. p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc. Natl Acad. Sci. USA 111 , E1600–E1609 (2014).
Vanden Borre, P. et al. Combined BRAF(V600E)- and SRC-inhibition induces apoptosis, evokes an immune response and reduces tumor growth in an immunocompetent orthotopic mouse model of anaplastic thyroid cancer. Oncotarget 5 , 3996–4010 (2014).
Vanden Borre, P. et al. The next generation of orthotopic thyroid cancer models: immunocompetent orthotopic mouse models of BRAF V600E-positive papillary and anaplastic thyroid carcinoma. Thyroid 24 , 705–714 (2014).
Olson, B., Li, Y., Lin, Y., Liu, E. T. & Patnaik, A. Mouse models for cancer immunotherapy research. Cancer Discov. 8 , 1358–1365 (2018).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331 , 1565–1570 (2011).
Ryder, M. et al. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS ONE 8 , e54302 (2013).
Knauf, J. A. et al. Hgf/Met activation mediates resistance to BRAF inhibition in murine anaplastic thyroid cancers. J. Clin. Invest. 128 , 4086–4097 (2018).
Brauner, E. et al. Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti tumor immunity in an immunocompetent murine model of anaplastic thyroid cancer. Oncotarget 7 , 17194–17211 (2016).
Gunda, V. et al. Anti-PD-1/PD-L1 therapy augments lenvatinib’s efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int. J. Cancer 144 , 2266–2278 (2019).
Gunda, V. et al. Combinations of BRAF inhibitor and anti-PD-1/PD-L1 antibody improve survival and tumour immunity in an immunocompetent model of orthotopic murine anaplastic thyroid cancer. Br. J. Cancer 119 , 1223–1232 (2018).
Larkin, J., Hodi, F. S. & Wolchok, J. D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373 , 1270–1271 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02834013 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01968109 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03744468 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT02179918 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT02904226 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT02628574 (2019).
Tolcher, A. W. et al. Phase Ib study of utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin. Cancer Res. 23 , 5349–5357 (2017).
Lenzo, J. C. et al. Control of macrophage lineage populations by CSF-1 receptor and GM-CSF in homeostasis and inflammation. Immunol. Cell Biol. 90 , 429–440 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT02718911 (2019).
Wiehagen, K. R. et al. Combination of CD40 agonism and CSF-1R blockade reconditions tumor-associated macrophages and drives potent antitumor immunity. Cancer Immunol. Res. 5 , 1109–1121 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT03502330 (2019).
Long, G. V. et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet. Oncol. 20 , 1083–1097 (2019).
Zhang, W. et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front. Immunol. 11 , 18 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT04097769 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT03957096 (2020).
Schurch, C. M. et al. Targeting CD47 in anaplastic thyroid carcinoma enhances tumor phagocytosis by macrophages and is a promising therapeutic strategy. Thyroid 29 , 979–992 (2019).
Bilusic, M. et al. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol. Immunother. 63 , 225–234 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT01856920 (2019).
Gerard, A. C. et al. Correlation between the loss of thyroglobulin iodination and the expression of thyroid-specific proteins involved in iodine metabolism in thyroid carcinomas. J. Clin. Endocrinol. Metab. 88 , 4977–4983 (2003).
Hoang-Vu, C. et al. Gene expression of differentiation- and dedifferentiation markers in normal and malignant human thyroid tissues. Exp. Clin. Endocrinol. 100 , 51–56 (1992).
Bhoj, V. G. et al. GDNF family receptor alpha 4 (GFRa4)-targeted adoptive T-cell immunotherapy for medullary thyroid carcinoma [abstract]. Cancer Res. 76 (Suppl. 14), 2295 (2016).
Min, I. M. et al. CAR T therapy targeting ICAM-1 eliminates advanced human thyroid tumors. Clin. Cancer Res. 23 , 7569–7583 (2017).
Park, S. et al. Micromolar affinity CAR T cells to ICAM-1 achieves rapid tumor elimination while avoiding systemic toxicity. Sci. Rep. 7 , 14366 (2017).
Vedvyas, Y. et al. Manufacturing and preclinical validation of CAR T cells targeting ICAM-1 for advanced thyroid cancer therapy. Sci. Rep. 9 , 10634 (2019).
Holzinger, A. & Abken, H. CAR T cells targeting solid tumors: carcinoembryonic antigen (CEA) proves to be a safe target. Cancer Immunol. Immunother. 66 , 1505–1507 (2017).
Parkhurst, M. R. et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 19 , 620–626 (2011).
Newick, K., O’Brien, S., Moon, E. & Albelda, S. M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 68 , 139–152 (2017).
Chacon, J. A. et al. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin. Cancer Res. 21 , 611–621 (2015).
Harao, M. et al. 4-1BB-enhanced expansion of CD8(+) TIL from triple-negative breast cancer unveils mutation-specific CD8(+) T cells. Cancer Immunol. Res. 5 , 439–445 (2017).
Yossef, R. et al. Enhanced detection of neoantigen-reactive T cells targeting unique and shared oncogenes for personalized cancer immunotherapy. JCI Insight 3 , e122467 (2018).
PubMed Central Google Scholar
Zacharakis, N. et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 24 , 724–730 (2018).
Frey, N. & Porter, D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol. Blood Marrow Transpl. 25 , e123–e127 (2019).
Postow, M. A. & Hellmann, M. D. Adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378 , 1163–1165 (2018).
Calabrese, L. & Mariette, X. The evolving role of the rheumatologist in the management of immune-related adverse events (irAEs) caused by cancer immunotherapy. Ann. Rheum. Dis. 77 , 162–164 (2018).
Ascierto, P. A. et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat. Med. 25 , 941–946 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03072160 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03215810 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04165967 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03638375 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03610490 (2020).
Download references
Author information
Authors and affiliations.
Department of Medicine, Division of Endocrinology, Metabolism, and Diabetes, University of Colorado Denver, Aurora, CO, USA
Jena D. French
University of Colorado Cancer Center, University of Colorado Denver, Aurora, CO, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jena D. French .
Ethics declarations
Competing interests.
J.D.F. is involved in a clinical trial funded by Merck and Eisai.
Additional information
Peer review information.
Nature Reviews Endocrinology thanks L. Bastholt, J. Hadoux, M. Schlumberger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
American Cancer Society: www.cancer.org
Rights and permissions
Reprints and permissions
About this article
Cite this article.
French, J.D. Immunotherapy for advanced thyroid cancers — rationale, current advances and future strategies. Nat Rev Endocrinol 16 , 629–641 (2020). https://doi.org/10.1038/s41574-020-0398-9
Download citation
Accepted : 15 July 2020
Published : 24 August 2020
Issue Date : November 2020
DOI : https://doi.org/10.1038/s41574-020-0398-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Tiparp as a prognostic biomarker and potential immunotherapeutic target in male papillary thyroid carcinoma.
- Jianlin Zhang
Cancer Cell International (2024)
Genomic alterations in thyroid cancer: biological and clinical insights
- Iñigo Landa
- Maria E. Cabanillas
Nature Reviews Endocrinology (2024)
Multi-omics profiling of papillary thyroid microcarcinoma reveals different somatic mutations and a unique transcriptomic signature
- Tienan Feng
Journal of Translational Medicine (2023)
High expression of IL4I1 is correlated with poor prognosis and immune infiltration in thyroid cancer
BMC Endocrine Disorders (2023)
Photothermal therapy of papillary thyroid cancer tumor xenografts with targeted thyroid stimulating hormone receptor antibody functionalized multiwalled carbon nanotubes
- Seung Soo Lee
- Fatma Oudjedi
- Mark A. Trifiro
Cancer Nanotechnology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Unleashing CAR-T cell therapy to destroy solid tumors in thyroid cancer
Share this:.
By Susan Buckles
Mayo Clinic researchers will jump four hurdles to apply chimeric antigen receptor-T cell therapy (CAR-T cell therapy) to solid tumors in thyroid cancer. This regenerative immunotherapy has shown promising results in blood cancers, and new research is focused on using this treatment on more types of malignancies.
"CAR-T cell therapy is unlike other therapeutics," says Saad Kenderian, M.B., Ch.B. , a hematologist and cancer researcher. "Other therapies may slow down cancer. CAR-T cell therapy has shown great promise in stopping B-cell lymphomas and leukemias. Some of my patients have gone into complete remission that has lasted for years after just one treatment."
CAR-T cell therapy harnesses the power of the body's immune system to kill tumors. T cells are taken from a patient, then genetically modified and returned to the body to act as guardians against cancer.
In a team science approach, Dr. Kenderian and John Copland III, Ph.D. , are collaborating as principal investigators on research to develop CAR-T technology for solid tumors in thyroid cancer. However, to do so they must address four challenges.
1. There are no clear targets in solid tumors
CAR-T cells are engineered to target specific proteins and antigens that are on the surface of cancer cells. Antigens are substances that activate the body's immune system. CARs are genetically programmed to trigger an immune response and destroy cancer cells.
"However, there are few clear targets on solid tumors like we have in liquid tumors," says Dr. Kenderian. "For CAR-T cell therapy to be successful, the first thing that we need to do is identify a protein to target that is unique to cancer cells that is not also expressed on normal tissue."
The teams of Dr. Kenderian and Dr. Copland believe they have identified such a target in the thyroid stimulating hormone receptor (TSHR) which is uniquely found on thyroid cancer cells in the thyroid gland. They are engineering THSR targeting into CAR-T cell therapy for thyroid cancer. This is known as TSHR CAR-T.
2. One treatment type doesn't fit all solid tumors
Solid cancers are comprised of many cell subsets. As a result, a tumor may have genetic mutations in some cells that aren't present in others. This is known as tumor heterogeneity, and it makes it difficult to treat these cancers with a single therapy.
"To overcome the tumor heterogeneity, we are using a strategy to combine TSHR CAR-T cell therapy with small molecules to block cancer cells from growing and metastasizing," says Dr. Copland. "We are studying whether loading CAR-T cells with this synthetic receptor in combination will trigger a cancer fighting response."
3. Solid tumors may be resistant to CAR-T cell therapy
Unlike blood cancers, solid tumors exist in a microenvironment that suppresses the immune system. In addition, dense clusters of malignant cells may create a barrier that blocks the CARs from bringing their cancer fighting mechanisms into cells.
"One strategy for overcoming this challenge is to target cells that are contributing to tumor aggressiveness," says Dr. Copland. "Another is to develop technology known as dual CARs that recognize two different targets. We are studying whether that will overcome tumor resistance."
4. Treating solid tumors may cause side effects
CAR-T cell therapy could mistakenly attack nearby healthy tissue, triggering what's known as "off-target effects." The result might be adverse side effects that are hard for the patient to tolerate. TSHR is a unique target that is expressed only on thyroid cancer cells and not on normal tissues, which minimizes the risk.
On-site biomanufacturing is a bridge to clinical trials
Dr. Kenderian and Dr. Copland are refining the TSHR CAR-T cell technology in the lab and in preclinical models. Biomanufacturing at Mayo Clinic will provide a bridge to accelerate this technology from the lab to early-stage clinical trials.
The process development team within Mayo Clinic's Center for Regenerative Biotherapeutics is now conducting test runs and establishing standard operating procedures in preparation for biomanufacturing this technology at Mayo Clinic.
"On-site biomanufacturing is critical to preserving the integrity of the cells during manufacturing," says Dr. Kenderian. "This is a very complex technology with many components that would make it difficult, if not impossible, to ship to an outside manufacturer."
Dr. Kenderian and Dr. Copland are driven by a passion to provide new therapies for patients who have few or no therapeutic options. Their goal is to advance CAR-T cell therapy for thyroid cancer to a first-in-human clinical trial by early 2025 .
Learn more about CAR-T cell therapy and thyroid cancer .
Also, read these articles:
- CAR-T cell therapy helps man continue community advocacy
- Hope, time and new options after CAR-T cell therapy for multiple myeloma
- CAR-T cell therapy restores hope for leukemia patient
- CAR-T cell researchers at Mayo Clinic optimistic about future of treating blood cancers
A version of this article was originally published on the Mayo Clinic News Network .
Related Posts

Nurse practitioner Katherine Prinsen shares what she discusses with her thyroid cancer patients when planning for the future.

Dr. Trenton Foster explains why it's important to have thyroid nodules evaluated by a health care professional to rule out thyroid cancer.

Awareness of thyroid cancer symptoms and risk factors can help adolescents and young adults recognize early warning signs and find prompt treatment.
Clinical Trials
Thyroid cancer.
Displaying 34 studies
The purpose of this study is to determine the optimal approach to the diagnosis and treatment of patients with thyroid disorders.
The purpose of this study is to explore how using different terms (with or without the word cancer) to identify papillary thyroid cancer might affect the decisions patients would make about treatment.
The purpose of this study is to field test and update an existing conversation aid prototype designed to support shared decision making in the diagnosis and treatment of thyroid cancer, and to conduct a pilot randomized multi-center trial to test the refined conversation aid.
The objective of this project is to evaluate a treatment decision aid for patients with low risk thyroid cancer.
The purpose of this study is to evaluate microRNA biomarkers in the blood and fine-needle aspirate biopsies of thyroid nodules to determine their usefullness in pre-operative diagnosis, in particular to distinguish benign from cancerous thyroid nodules.
This randomized phase II trial is studying the side effects and how well giving intensity-modulated radiation therapy (IMRT) and paclitaxel together with or without pazopanib hydrochloride works in treating patients with anaplastic thyroid cancer. Specialized radiation therapy that delivers a high dose of radiation directly to the tumor may kill more tumor cells and cause less damage to normal tissue. Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. Pazopanib hydrochloride may stop the growth of tumor cells by blocking ...
The purpose of this study is to assess the use of lenvatinib to treat anaplastic thyroid cancer.
The purpose of this study is to evaluate the safety and effectiveness of Selpercatinib, compared to a standard treatment, in participants with rearranged during transfection (RET)-mutant medullary thyroid cancer (MTC) that cannot be removed by surgery or has spread to other parts of the body. Participants who are assigned to the standard treatment and discontinue due to progressive disease have the option to potentially crossover to selpercatinib.
The purpose of this study is to evaluate response, survival, safety, and tolerability of treatment with lenvatinib for patients who have anaplastic thyroid cancer.
The purpose of this study is to examine the safety and evaluate the response of VB-111 on DTC.
The objective of this study is to determine intensity of I-123 uptake in follicular thyroid lesions before surgery and correlate with final pathology findings.
The purpose of this study is to evaluate the efficiency of Radiofrequency ablation (RFA) therapy to treat papillary thyroid carcinoma.
The purpose of this first-in-human study is designed to evaluate the safety, tolerability, pharmacokinetics (PK) and preliminary anti-tumor activity of LOXO-292 administered orally to patients with advanced solid tumors, including RET-fusion-positive solid tumors, medullary thyroid cancer (MTC) and other tumors with RET activation.
The purpose of this study is to investigate radiotracer 18F-tetrafluoroborate (18F-TFB) for imaging of patients with differentiated thyroid cancer
The purpose of this study is to determine whether an exercise program reduces fatigue and improves physical activity in thyroid cancer patients, and to determine the effect of a home-based program compared to a center- based program.
The purpose of this pilot study is to evaluate the effects of Radiofrequency Ablation (RFA) on thyroid hormones to treat thyroide nodules.
Radiofrequency ablation (RFA) of thyroid nodules would locally destroy follicular thyroid cells and could possibly impart conformational changes to the chemistry of thyroid hormones, thus altering their bioactive profiles. To evaluate this phenomenon, in vitro investigations to characterize qualitative and quantitve Mass Spectrometric chromatographic profile for thyroid hormones will be performed before and after RFA from patients undergoing ablation for thyroid nodules. {If there is a blood draw for clinical tests, we would request left over specimen from clinical ...
This randomized phase II trial studies how well iodine I-131works with or without selumetinib in treating patients with thyroid cancer that has returned or has spread from where it started to other places in the body. Many thyroid cancers absorb iodine. Because of this, doctors often give radioactive iodine (iodine I-131) alone to treat thyroid cancer as part of standard practice. It is thought that the more thyroid tumors are able to absorb radioactive iodine, the more likely it is that the radioactive iodine will cause those tumors to shrink. Selumetinib may help radioactive iodine work better in patients whose ...
This phase II trial studies how well pembrolizumab, chemotherapy, and radiation therapy work with or without surgery in treating patients with anaplastic thyroid cancer. Monoclonal antibodies, such as pembrolizumab, may interfere with the ability of tumor cells to grow and spread. Drugs used in chemotherapy, such as docetaxel and doxorubicin hydrochloride, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Radiation therapy uses high-energy x-rays to kill tumor cells and shrink tumors. Giving pembrolizumab, chemotherapy, and radiation therapy with or without surgery ...
The purpose of this study is to perform a comprehensive immunophenotypic analysis of peripheral bloods samples from patients with benign and malignant thyroid disease. This data will be used to determine whether patients with advanced thyroid cancers have significantly altered numbers and/or subtypes of circulating immune cells, in particular immunosuppressive monocytes.
This phase II trial studies how well inolitazone dihydrochloride (efatutazone dihydrochloride) and paclitaxel work in treating patients with anaplastic thyroid cancer that has spread to other places in the body and usually cannot be cured or controlled with treatment (advanced). Drugs used in chemotherapy, such as efatutazone dihydrochloride and paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading.
This phase II trial studies the effect of pembrolizumab, dabrafenib, and trametinib before surgery in treating patients with BRAF V600E-mutated anaplastic thyroid cancer. BRAF V600E is a specific mutation (change) in the BRAF gene, which makes a protein that is involved in sending signals in cells and in cell growth. It may increase the growth and spread of tumor cells. Dabrafenib and trametinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Immunotherapy with monoclonal antibodies, such as pembrolizumab, may help the body's immune system attack the cancer, and may interfere with ...
The intent is to collect relevant clinical data on patients exposed to Agent Orange plus assessment of the tissue for genetic mutations known to be associated with growth of thyroid cancer and pituitary tumors and report our findings as a descriptive case series.
The purpose of this study is to establish a human biobank of thyroid-derived tissues and blood samples from patients with thyroid disorders at Mayo Clinic in Rochester.
This phase II trial studies how well cabozantinib-s-malate works in treating patients with thyroid cancer that does not respond to treatment. Cabozantinib-s-malate may stop the growth of thyroid cancer by blocking some of the enzymes needed for cell growth. Cabozantinib-s-malate may also stop the growth of thyroid cancer by blocking blood flow to the tumor.
The purpose of this study is to determine how well cabozantinib, nivolumab, and ipilimumab work in treating patients with differentiated thyroid cancer that does not respond to radioactive iodine and that worsened after treatment with a drug targeting the vascular endothelial growth factor receptor (VEGFR), a protein needed to form blood vessels. Cabozantinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Immunotherapy with monoclonal antibodies, such as nivolumab and ipilimumab, may help the body's immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread. ...
The purpose of this study is to evaluate the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary antineoplastic activity of BLU-667 administered orally in patients with medullary thyroid cancer, RET-altered NSCLC and other RET-altered solid tumors.
This phase II trial studies how well cabozantinib-s-malate works in treating younger patients with sarcomas, Wilms tumor, or other rare tumors that have come back, do not respond to therapy, or are newly diagnosed. Cabozantinib-s-malate may stop the growth of tumor cells by blocking some of the enzymes needed for tumor growth and tumor blood vessel growth.
The purpose of this study is to find out more about the side effects of rovalpituzumab tesirine (SC16LD6.5) and what doses of rovalpituzumab tesirine (SC16LD6.5) are safe for people with specific delta-like protein 3-expressing cancers.
The purpose of this study is to assess the safety/tolerability profile of E7386 as a single agent administered orally in participants with selected advanced or recurrent neoplasms and to determine the maximum tolerated dose (MTD) and/or recommended Phase 2 dose (RP2D) of E7386.
The purpose of this multicenter prospective observational case-control study is to train and validate Adela’s cfMeDIP-seq based methylome profiling platform to detect and differentiate multiple cancer subtypes. In addition, this study includes longitudinal follow-up for a subset of participants to train and validate the methylome profiling platform to detect minimal residual disease and recurrence.
The purpose of this study is to determine the prevalence of genetic mutations in cancer patients from various ethnic populations seeking care at Mayo Clinic cancer clinics.
The purpose of this study is to evaluate the challenges, behavioral patterns, and preferences of minority patient participation in clinical trials. Also, to develop and validate a personalized clinical trial educational platform to boost participation among underserved cancer patients.
The purpose of this study is to collect blood and tissue samples from patients with and without cancer to evaluate laboratory tests for early cancer detection which may help researchers develop tests for the early detection of cancers.
GRAIL is using deep sequencing of circulating cell-free nucleic acids (cfNAs) to develop assays to detect cancer early in blood. The purpose of this study is to collect biological samples from donors with a new diagnosis of cancer (blood and tumor tissue) and from donors who do not have a diagnosis of cancer (blood) in order to characterize the population heterogeneity in cancer and non-cancer subjects and to develop models for distinguishing cancer from non-cancer.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.

- Events & Education
- ATA Publications
- ATA Guidelines & Statements
- Research Grants
- Thyroid Cancer Patient Information
- Trainees Corner
- Corporate Leadership Council
- ATA Career Center
- Laboratory Services Library
- Scientific & Professional Interest
- Thyroid Cancer Staging Calculator
- (CEA) Doubling Time Calculator
- Change In Thyroid Nodule Volume Calculator
- Thyroid Patient Information
- Find an Endocrinology – Thyroid Specialist
- Patient Support Links
- Clinical Thyroidology for the Public
- Friends of the ATA Newsletter
- ATA Practice Guidelines
- Clinical Trials
- ATA Research Accomplishments
- Member Benefits
- Become an ATA Member
- Renew Your Membership
- Member Guidelines & Categories
- Society Committees
- Member Directory
- Trainee Membership
- Meet our Members
- Women in Thyroidology
- Thyroid Online Access
- Clinical Thyroidology Online
- Video Endocrinology
- Leadership & Staff
- Committees & Workgroups
- Diversity, Equity, Inclusion
- Awards & Recognition
- Our History
- Give Online
- Valerie Anne Galton Fund
- Samuel Refetoff Fund
- Ridgway Legacy Fund
- Memorial or Tribute Gift Donation
- Workplace Giving
- Estate and Planned Giving
- Donate by Mail/Fax/Phone
- Research Accomplishments
Home » Thyroid Cancer
Leer en Español
Thyroid cancer is relatively uncommon compared to other cancers. In the United States, it is estimated that in 2021 approximately 44,000 people will receive a new diagnosis of thyroid cancer, compared to over 280,000 with breast cancer and over 150,000 with colon cancer. However, despite this, approximately 2,000 patients die of thyroid cancer each year. In 2018, the last year for which statistics are available, almost 900,000 patients were living with thyroid cancer in the United States. Thyroid cancer is usually very treatable and is often cured with surgery (see Thyroid Surgery brochure ) and, if indicated, radioactive iodine (see Radioactive Iodine brochure ). Even when thyroid cancer is more advanced, effective treatment is available for the most common forms of thyroid cancer. Even though the diagnosis of cancer is terrifying, the prognosis for most patients with papillary and follicular thyroid cancer is excellent.
Thyroid Cancer FAQs
What is the thyroid gland.
The thyroid gland is a butterfly-shaped endocrine gland that is normally located in the lower front of the neck. The thyroid’s job is to make thyroid hormones, which are secreted into the blood and then carried to every tissue in the body. Thyroid hormone helps the body use energy, stay warm and keep the brain, heart, muscles, and other organs working as they should.
WHAT ARE THE TYPES OF THYROID CANCER?
PAPILLARY THYROID CANCER. Papillary thyroid cancer is the most common type, making up about 70% to 80% of all thyroid cancers. Papillary thyroid cancer can occur at any age. It tends to grow slowly and often spreads to lymph nodes in the neck. Papillary cancer has a generally excellent outlook, even if there is spread to the lymph nodes.
FOLLICULAR THYROID CANCER. Follicular thyroid cancer makes up about 10% to 15% of all thyroid cancers in the United States. Follicular cancer can spread through the blood to distant organs, particularly the lungs and bones.
Papillary and follicular thyroid cancers are also known as well–Differentiated Thyroid Cancers (DTC). The information in this brochure refers to these differentiated thyroid cancers. The other types of thyroid cancer listed below will be covered in other brochures.
MEDULLARY THYROID CANCER. Medullary thyroid cancer (MTC), accounts for approximately 2% of all thyroid cancers. Approximately 25% of all MTC runs in families and is associated with other endocrine tumors (see Medullary Thyroid Cancer brochure ). In family members of an affected person, a test for a genetic mutation in the RET proto-oncogene can lead to an early diagnosis of medullary thyroid cancer and, as a result, to curative surgery. 75% of patients with Medullary thyroid cancer do not have a hereditary form.
ANAPLASTIC THYROID CANCER. Anaplastic thyroid cancer is the most advanced and aggressive thyroid cancer and the least likely to respond to treatment. Anaplastic thyroid cancer is very rare and is found in less than 2% of patients with thyroid cancer (See Anaplastic Thyroid Cancer brochure ).
WHAT ARE THE SYMPTOMS OF THYROID CANCER?
Thyroid cancer often presents as a lump or nodule in the thyroid and usually does not cause any other symptoms (see Thyroid Nodule brochure ). Blood tests generally do not help to find thyroid cancer and thyroid blood tests such as TSH are usually normal, even when a cancer is present. Neck examination by your doctor is a common way in which thyroid nodules and thyroid cancer are found. Often, thyroid nodules are discovered incidentally on imaging tests like CT scans and neck ultrasounds done for completely unrelated reasons. You may have found a thyroid nodule by noticing a lump in your neck while looking in a mirror, buttoning your collar, or fastening a necklace. Rarely, thyroid cancers and nodules may cause symptoms. You may complain of pain in the neck, jaw, or ear. If a nodule is large enough to compress your windpipe or esophagus, it may cause difficulty with breathing, swallowing, or cause a “tickle in the throat” sensation. Even less commonly, you may develop hoarseness if a thyroid cancer invades the nerve that controls your vocal cords.
Cancers arising in thyroid nodules generally do not cause symptoms, and thyroid function tests are typically normal even when you have cancer. The best way to find a thyroid nodule is to make sure that your doctor examines your neck as part of your periodic check-up.
WHAT CAUSES THYROID CANCER?
Thyroid cancer is more common in people who have a history of exposure to high doses of radiation, have a family history of thyroid cancer, and are older than 40 years of age. However, for most people, we don’t know why thyroid cancer develops.
High dose radiation exposure, especially during childhood, increases the risk of developing thyroid cancer. Radiation therapy used to treat cancers such as Hodgkin’s disease (cancer of the lymph nodes) or breast cancer has been associated with an increased risk for developing thyroid cancer if the treatment included exposure to the head, neck or chest. Routine X-ray exposure such as dental X-rays, chest X-rays and mammograms are not associated with a high risk of thyroid cancer. As always, you should minimize radiation exposure by only having tests which are medically necessary.
Exposure to radioactivity released during nuclear disasters (1986 accident at the Chernobyl power plant in Russia or the 2011 nuclear disaster in Fukushima, Japan) has also been associated with an increased risk of developing thyroid cancer, particularly in exposed children, and thyroid cancers can be seen in exposed individuals as many as 40 years after exposure.
You can be protected from developing thyroid cancer in the event of a nuclear disaster by taking potassium iodide (see Nuclear Radiation and the Thyroid brochure ). This prevents the absorption of radioactive iodine and has been shown to reduce the risk of thyroid cancer. The American Thyroid Association recommends that anyone living within 200 miles of a nuclear facility be given potassium iodide to take if a nuclear accident occurs. If you live near a nuclear reactor and want more information about the role of potassium iodide, check the recommendations from your state at the following link: www.thyroid.org/web-links-for-important- documentsabout- potassium-iodide/ .
HOW IS THYROID CANCER DIAGNOSED?
If your doctor suspects from your physical exam and ultrasound that you may have cancer, you will need to have a fine needle aspiration biopsy. The results of the biopsy can be highly suggestive of thyroid cancer and will prompt surgical treatment. Thyroid cancer can only be diagnosed with certainty after the nodule is removed surgically (see Thyroid Nodule brochure ). Thyroid nodules are very common, but less than 1 in 10 will be a thyroid cancer.
WHAT IS THE TREATMENT FOR THYROID CANCER?
Surgery. The first step in treatment for all types of thyroid cancer is surgery (see Thyroid Surgery brochure ). The extent of surgery for differentiated thyroid cancers may be removing only the lobe involved with the cancer, called a lobectomy, or removing the entire thyroid, called a total thyroidectomy. The extent of surgery will depend on the size of the tumor and whether or not the tumor has spread beyond the thyroid gland. If your tumor involves both lobes of the thyroid gland or it is found on testing to have spread beyond the gland, a total thyroidectomy will be recommended. If you have thyroid cancer present in the lymph nodes of the neck (lymph node metastases), these lymph nodes can be removed at the time of the initial thyroid surgery or sometimes, as a second procedure. However, if your cancer is small, only in one lobe of the gland and if it has not spread to lymph nodes, a lobectomy may be a good option. Recent studies even suggest that if you have a small tumor measuring less than 1cm across, called papillary thyroid microcarcinoma, you may be observed very safely without surgery. If you have a total thyroidectomy, you will need to take thyroid hormone medication for the rest of your life (see Thyroid Hormone Treatment brochure ). However, if you have a lobectomy, you may not need to take thyroid hormone replacement. Thyroid cancer is often cured by surgery alone, especially if the cancer is small. If your cancer is larger, if it has spread to lymph nodes, or if your doctor feels that you are at high risk for recurrent cancer, radioactive iodine may be used after the thyroid gland is removed.
Radioactive iodine therapy (Also referred to as I-131 therapy). Thyroid cells and most differentiated thyroid cancers absorb iodine so radioactive iodine can be used to eliminate all remaining normal thyroid tissue and potentially destroy residual cancerous thyroid tissue after thyroidectomy (see Radioactive Iodine brochure ). The procedure to eliminate residual thyroid tissue is called radioactive iodine ablation. Since most other tissues in the body do not efficiently absorb or concentrate iodine, radioactive iodine used during the ablation procedure usually has little or no effect on tissues outside of the thyroid. However, in some patients who receive larger doses of radioactive iodine for treatment of thyroid cancer metastases, radioactive iodine can affect the glands that produce saliva and result in a dry mouth. If higher doses of radioactive iodine are necessary, there may also be a small risk of developing other cancers later in life. This risk is very small, and increases as the dose of radioactive iodine increases. The potential risks of treatment can be minimized by using the smallest dose possible. Balancing potential risks against the benefits of radioactive iodine therapy is an important discussion that you should have with your doctor if radioactive iodine therapy is recommended.
If your doctor recommends radioactive iodine therapy, your TSH level will need to be elevated prior to the treatment. This can be done in one of two ways.
The first is by stopping thyroid hormone pills (levothyroxine) for 3-6 weeks. This causes high levels of TSH to be produced by your body naturally. This results in hypothyroidism, which may involve symptoms such as fatigue, cold intolerance and others, that can be significant. To minimize the symptoms of hypothyroidism your doctor may prescribe T3 (Cytomel®, liothyronine) which is a short acting form of thyroid hormone that is usually taken after the levothyroxine is stopped until 2 weeks before the radioactive iodine treatment.
Alternatively, TSH can be increased sufficiently without stopping thyroid hormone medication by injecting a synthetic form of TSH into your body. Recombinant human TSH (rhTSH, Thyrogen®) can be given as two injections in the days prior to radioactive iodine treatment. The benefit of this approach is that you can continue taking the thyroid hormone medication and avoid possible symptoms related to hypothyroidism.
Regardless of whether you become hypothyroid (stop thyroid hormone) or use recombinant TSH therapy, you may also be asked to go on a low iodine diet for 1 to 2 weeks prior to treatment (see Low Iodine Diet FAQ ), which will result in improved absorption of radioactive iodine, maximizing the treatment effect.
TREATMENT OF ADVANCED THYROID CANCER.
Thyroid cancer that spreads (metastasizes) outside the neck area is rare but can be a serious problem. Surgery and radioactive iodine remain the best way to treat such cancers as long as these treatments continue to work. However, for more advanced cancers, or when radioactive iodine therapy is no longer effective, other forms of treatment are needed.
Medications have now been approved for the treatment of advanced thyroid cancer. These drugs rarely cure advanced cancers that have spread widely throughout the body, but they can slow down or partially reverse the growth of the cancer. These treatments are usually given by an oncologist (cancer specialist) and often require care at a regional or university medical center. These agents can also be used to change a tumor that stopped responding to radioactive iodine to respond to this treatment again. This is called redifferentiation therapy.
External beam radiation directs precisely focused X-rays to areas that need to be treated. This may be tumor that has recurred locally in the neck or spread to bones or other organs. This can kill or slow the growth of those tumors.
WHAT IS THE FOLLOW-UP FOR PATIENTS WITH THYROID CANCER?
Periodic follow-up examinations are essential for all patients with thyroid cancer, because the thyroid cancer can return—sometimes several years after successful initial treatment. These follow-up visits include a careful history and physical examination, with particular attention to the neck area. Neck ultrasound is an important tool to view the neck and look for nodules, lumps or cancerous lymph nodes that might indicate the cancer has returned. Blood tests are also important for thyroid cancer patients. Most patients who have had a thyroidectomy for cancer require thyroid hormone replacement with levothyroxine once the thyroid is removed (see Thyroid Hormone Treatment brochure ). The dose of levothyroxine prescribed by your doctor will in part be determined by the initial extent of your thyroid cancer. More advanced cancers usually require higher doses of levothyroxine to suppress TSH (lower the TSH below the low end of the normal range). In cases of minimal or very low risk thyroid cancer, it is typically recommended to keep TSH in the normal range. The TSH level is a good indicator of whether the levothyroxine dose is correct and should be followed periodically by your doctor.
Another important blood test is measurement of thyroglobulin (Tg). Thyroglobulin is a protein produced by normal thyroid tissue and differentiated thyroid cancer cells. The test is useful if you have had a thyroidectomy and radioactive iodine ablation, when the thyroglobulin levels usually become very low or undetectable. If your level is low and then starts to rise, it is concerning for possible cancer recurrence. If you have thyroglobulin antibodies (TgAb) the Tg blood test can be more difficult to interpret.
In addition to routine blood tests, your doctor may want to check a whole-body iodine scan to determine if any thyroid cancer cells remain. These scans are only done for high risk patients and have been largely replaced by routine neck ultrasound and thyroglobulin measurements that are more accurate to detect cancer recurrence, especially when done together.
WHAT IS THE PROGNOSIS OF THYROID CANCER?
Overall, your prognosis with differentiated thyroid cancer is excellent, especially if you are younger than 55 years of age and have a small cancer. If your papillary thyroid cancer has not spread beyond the thyroid gland, patients like you rarely if ever die from thyroid cancer. If you are older than 55 years of age, or have a larger or more aggressive tumor, your prognosis remains very good, but the risk of cancer recurrence is higher. The prognosis may not be quite as good if your cancer is more advanced and cannot be completely removed with surgery or destroyed with radioactive iodine treatment. Nonetheless, even if this is your situation, you will likely be able to live a long time and feel well, despite the fact that you are living with cancer. It is important to talk to your doctor about your individual profile of cancer and expected prognosis. It will be necessary to have lifelong monitoring, even after successful treatment.

Adult Thyroid Information
Pediatric thyroid information, printable brochures.
Thyroid Cancer (Papillary and Follicular) Brochure PDF
Thyroid Cancer FAQs PDF
El folleto de Cáncer De Tiroides (de tipo Papilar y folicular)
Is removal of thyroid tissue through incisions made in the mouth safe and effective for treating thyroid cancer? – Clinical Thyroidology® for the Public
Editorial collaboration medscape & american thyroid association®, clinical thyroidology ® for the public – highlighted article, further information.
For information on thyroid patient support organizations, please visit the Patient Support Links section on the ATA website at www.thyroid.org
THYROID NEWS

Publications
About the ata.
© 2024 American Thyroid Association.
We would value your opinion on our patient brochures, if you would like to provide your feedback, please click this link , or Click to dismiss
- Patient Care & Health Information
- Diseases & Conditions
- Thyroid cancer
- What is thyroid cancer? A Mayo Clinic expert explains
Learn more about thyroid cancer from endocrinologist Mabel Ryder, M.D.
I'm Dr. Mabel Ryder, an endocrinologist at Mayo Clinic. In this video, we'll cover the basics of thyroid cancer: What is it? Who gets it? The symptoms. Diagnosis and treatment. Whether you're looking for answers for yourself or someone you love, we're here to give you the best information available. What is the thyroid? This is a butterfly shaped gland that sits at the base of your neck. It's an important gland responsible for producing hormones that control a lot of vital functions in your body, such as your heart and your heart rate, your blood pressure, your body temperature, and your weight. When thyroid cells mutate, changes to their DNA cause them to grow and multiply. Where healthy cells typically die, these abnormal cells grow and grow and eventually form a tumor. Sometimes these cells invade nearby tissue, and can spread or metastasize to other parts of the body. There are several different kinds of thyroid cancer. Some grow slowly. Others can be more aggressive. Because we're able to detect small thyroid cancers with new technology, the rate of thyroid cancer incidence has gone up. However, most cancers are very treatable and the prognosis for most patients with thyroid cancer is excellent.
There are other things that can increase your chances of developing thyroid cancer. Women are three times more likely to develop thyroid cancer. And exposure to high levels of radiation, for instance, radiation therapy to the head or neck for other cancers, can increase your risk. Certain hereditary genetic syndromes may also play a role. Different types of thyroid cancer are more likely to affect different age groups. Papillary thyroid cancer is the most common form of thyroid cancer. And although it can occur at any age, it generally affects people ages 30 to 50. Follicular thyroid cancer usually affects people older than age 50. Anaplastic thyroid cancer is a very rare type of cancer that typically occurs in adults 60 and older. And medullary thyroid cancer. Although uncommon, up to 30 percent of patients with medullary thyroid cancer are associated with genetic syndromes that can increase your risk for other tumors as well.
Typically, thyroid cancer doesn't trigger any signs or symptoms in its early stages. As it grows, you may notice a lump that can be felt through the skin in your neck. You may notice changes to your voice, including hoarseness of your voice, or difficulty swallowing. Some may develop pain in their neck or throat. Or you may develop swollen lymph nodes in your neck. If you're experiencing any of these issues and are concerned, make an appointment with your doctor.
Most often, diagnosing thyroid cancer starts with the physical exam. Your doctor will feel for physical changes in your neck and the thyroid. This usually is followed by blood tests and ultrasound imaging. Armed with this information, doctors may decide to do a biopsy to remove a small sample of tissue from your thyroid. In some cases, genetic testing may be done to help determine any associated hereditary causes. If diagnosed with thyroid cancer, several other tests may be done to help your doctor determine whether your cancer has spread beyond the thyroid and outside of the neck. These tests may include blood tests to check tumor markers and imaging tests, such as CT scans, MRI, or nuclear imaging tests, such as a radioiodine whole-body scan.
Fortunately, most thyroid cancers can be beaten with treatments. Very small cancers - under 1 centimeter - have a low risk of growing or spreading and, thus, might not need treatment right away. Instead, your doctor may recommend observation with blood tests, an ultrasound, and a physical exam once or twice per year. In many people, this small cancer - under 1 centimeter - might never grow and may never require surgery. In cases where further treatment is necessary, surgery is common. Depending on your cancer, your doctor may remove just a portion of the thyroid - a procedure known as thyroidectomy. Or your doctor may remove all of the thyroid. Other treatments may include thyroid hormone therapy, alcohol ablation, radioactive iodine, targeted drug therapy, external radiation therapy, and chemotherapy, in some. Ultimately, what your treatment looks like will depend on the stage of your cancer and the type of thyroid cancer you have.
If you've been diagnosed with thyroid cancer, you might feel as if you aren't sure what to do next. And that's normal, everyone eventually find their own way of coping with a cancer diagnosis. But until you find what works for you, try the following. Learn all you can to help you make decisions about your care. Connect with other survivors. Talking to people who share your situation can be incredibly helpful. And control what you can about your health. Take steps to keep your body healthy during and after treatment. Eat a healthy diet full of a variety of fruits and vegetables. Get enough rest. And try to incorporate physical activity when you can. Being diagnosed with cancer can be frightening, but take comfort in the fact that most cases of this cancer are treatable. If you'd like to learn even more about thyroid cancer, watch our other related videos or visit mayoclinic.org. We wish you well.
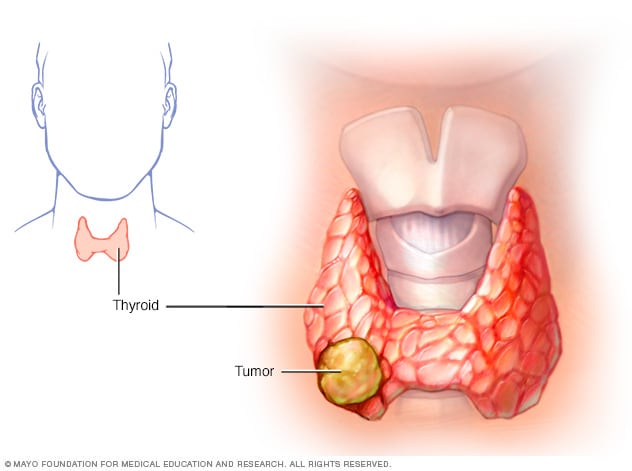
Thyroid cancer occurs in the cells of the thyroid.
Thyroid cancer is a growth of cells that starts in the thyroid. The thyroid is a butterfly-shaped gland located at the base of the neck, just below the Adam's apple. The thyroid produces hormones that regulate heart rate, blood pressure, body temperature and weight.
Thyroid cancer might not cause any symptoms at first. But as it grows, it can cause signs and symptoms, such as swelling in your neck, voice changes and difficulty swallowing.
Several types of thyroid cancer exist. Most types grow slowly, though some types can be very aggressive. Most thyroid cancers can be cured with treatment.
Thyroid cancer rates seem to be increasing. The increase may be caused by improved imaging technology that allows health care providers to find small thyroid cancers on CT and MRI scans done for other conditions (incidental thyroid cancers). Thyroid cancers found in this way are usually small cancers that respond well to treatments.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Thyroid gland
The thyroid gland is located at the base of the neck, just below the Adam's apple.
Most thyroid cancers don't cause any signs or symptoms early in the disease. As thyroid cancer grows, it may cause:
- A lump (nodule) that can be felt through the skin on your neck
- A feeling that close-fitting shirt collars are becoming too tight
- Changes to your voice, including increasing hoarseness
- Difficulty swallowing
- Swollen lymph nodes in your neck
- Pain in your neck and throat
When to see a doctor
If you experience any signs or symptoms that worry you, make an appointment with your health care provider.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get Mayo Clinic cancer expertise delivered to your inbox.
Subscribe for free and receive an in-depth guide to coping with cancer, plus helpful information on how to get a second opinion. You can unsubscribe at any time. Click here for an email preview.
Error Select a topic
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth coping with cancer guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest about cancer news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Thyroid cancer happens when cells in the thyroid develop changes in their DNA. A cell's DNA contains the instructions that tell the cell what to do. The changes, which doctors call mutations, tell the cells to grow and multiply rapidly. The cells go on living when healthy cells would naturally die. The accumulating cells form a mass called a tumor.
The tumor can grow to invade nearby tissue and can spread (metastasize) to the lymph nodes in the neck. Sometimes the cancer cells can spread beyond the neck to the lungs, bones and other parts of the body.
For most thyroid cancers, it's not clear what causes the DNA changes that cause the cancer.
Types of thyroid cancer
Thyroid cancer is classified into types based on the kinds of cells found in the tumor. Your type is determined when a sample of tissue from your cancer is examined under a microscope. The type of thyroid cancer is considered in determining your treatment and prognosis.
Types of thyroid cancer include:
- Papillary thyroid cancer. This is the most common type of thyroid cancer. It can happen at any age, but it most often affects people ages 30 to 50. Most papillary thyroid cancers are small and respond well to treatment, even if the cancer cells spread to the lymph nodes in the neck. A small portion of papillary thyroid cancers are aggressive and may grow to involve structures in the neck or spread to other areas of the body.
- Follicular thyroid cancer. This rare type of thyroid cancer usually affects people older than 50. Follicular thyroid cancer cells don't often spread to the lymph nodes in the neck. But some large and aggressive cancers may spread to other parts of the body. Follicular thyroid cancer most often spreads to the lungs and bones.
- Hurthle cell thyroid cancer. This rare type of thyroid cancer was once considered a type of follicular thyroid cancer. Now it is considered its own type because the cancer cells behave differently and respond to different treatments. Hurthle cell thyroid cancers are aggressive and can grow to involve structures in the neck and spread to other parts of the body.
- Poorly differentiated thyroid cancer. This rare type of thyroid cancer is more aggressive than other differentiated thyroid cancers and often doesn't respond to the usual treatments.
- Anaplastic thyroid cancer. This rare type of thyroid cancer grows quickly and can be difficult to treat. However, treatments can help slow the progression of the disease. Anaplastic thyroid cancer tends to occur in people older than 60. It can cause severe signs and symptoms, such as neck swelling that worsens very quickly and may lead to difficulty breathing and swallowing.
- Medullary thyroid cancer. This rare type of thyroid cancer begins in thyroid cells called C cells, which produce the hormone calcitonin. Elevated levels of calcitonin in the blood can indicate medullary thyroid cancer at a very early stage. Some medullary thyroid cancers are caused by a gene called RET that's passed from parents to children. Changes in the RET gene can cause familial medullary thyroid cancer and multiple endocrine neoplasia, type 2. Familial medullary thyroid cancer increases the risk of thyroid cancer. Multiple endocrine neoplasia, type 2, increases the risk of thyroid cancer, adrenal gland cancer and other types of cancers.
- Other rare types. Other very rare types of cancer can start in the thyroid. These include thyroid lymphoma, which begins in the immune system cells of the thyroid, and thyroid sarcoma, which begins in the connective tissue cells of the thyroid.
Risk factors
Factors that may increase the risk of thyroid cancer include:
- Female sex. Thyroid cancer occurs more often in women than in men. Experts think it may be related to the hormone estrogen. People who are assigned female sex at birth generally have higher levels of estrogen in their bodies.
- Exposure to high levels of radiation. Radiation therapy treatments to the head and neck increase the risk of thyroid cancer.
- Certain inherited genetic syndromes. Genetic syndromes that increase the risk of thyroid cancer include familial medullary thyroid cancer, multiple endocrine neoplasia, Cowden syndrome and familial adenomatous polyposis. Types of thyroid cancer that sometimes run in families include medullary thyroid cancer and papillary thyroid cancer.
Complications
Thyroid cancer that comes back.
Thyroid cancer can return despite successful treatment, and it can even come back if you've had your thyroid removed. This could happen if cancer cells spread beyond the thyroid before it's removed.
Most thyroid cancers aren't likely to recur, including the most common types of thyroid cancer — papillary thyroid cancer and follicular thyroid cancer. Your health care provider can tell you if your cancer has an increased risk of recurring based on the particulars of your cancer.
Recurrence is more likely if your cancer is aggressive or if it grows beyond your thyroid. When thyroid cancer recurrence happens, it's usually found in the first five years after your initial diagnosis.
Thyroid cancer that comes back still has a good prognosis. It's often treatable, and most people will have successful treatment.
Thyroid cancer may recur in:
- Lymph nodes in the neck
- Small pieces of thyroid tissue left behind during surgery
- Other areas of the body, such as the lungs and bones
Your health care provider may recommend periodic blood tests or thyroid scans to check for signs that your cancer has returned. At these appointments, your provider may ask if you've experienced any signs and symptoms of thyroid cancer recurrence, such as:
- A lump in the neck
- Trouble swallowing
- Voice changes, such as hoarseness
Thyroid cancer that spreads (metastasizes)
Thyroid cancer sometimes spreads to nearby lymph nodes or to other parts of the body. The cancer cells that spread might be found when you're first diagnosed or they might be found after treatment. The great majority of thyroid cancers don't ever spread.
When thyroid cancer spreads, it most often travels to:
Thyroid cancer that spreads might be detected on imaging tests, such as CT and MRI, when you're first diagnosed with thyroid cancer. After successful treatment, your health care provider might recommend follow-up appointments to look for signs that your thyroid cancer has spread. These appointments might include nuclear imaging scans that use a radioactive form of iodine and a special camera to detect thyroid cancer cells.
Doctors aren't sure what causes the gene changes that lead to most thyroid cancers, so there's no way to prevent thyroid cancer in people who have an average risk of the disease.
Prevention for people with a high risk
Adults and children with an inherited gene that increases the risk of medullary thyroid cancer may consider thyroid surgery to prevent cancer (prophylactic thyroidectomy). Discuss your options with a genetic counselor who can explain your risk of thyroid cancer and your treatment options.
Prevention for people near nuclear power plants
A medication that blocks the effects of radiation on the thyroid is sometimes provided to people living near nuclear power plants in the United States. The medication (potassium iodide) could be used in the unlikely event of a nuclear reactor accident. If you live within 10 miles of a nuclear power plant and are concerned about safety precautions, contact your state or local emergency management department for more information.
Thyroid cancer care at Mayo Clinic
Living with thyroid cancer?
Connect with others like you for support and answers to your questions in the Thyroid Cancer support group on Mayo Clinic Connect, a patient community.
Thyroid Cancer Discussions

11 Replies Fri, May 17, 2024

6 Replies Fri, May 17, 2024

16 Replies Fri, May 17, 2024
- AskMayoExpert. Differentiated thyroid cancer (adult). Mayo Clinic; 2018.
- Niederhuber JE, et al., eds. Cancer of the endocrine system. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Jan. 21, 2022.
- Melmed S, et al. Nontoxic diffuse goiter, nodular thyroid disorders and thyroid malignancies. In: Williams Textbook of Endocrinology. 14th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Jan. 21, 2022.
- AskMayoExpert. Medullary thyroid cancer. Mayo Clinic; 2021.
- Thyroid carcinoma. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1470. Accessed Jan. 21, 2022.
- Vaccarella S, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. The New England Journal of Medicine. 2016; doi:10.1056/NEJMp1604412.
- AskMayoExpert. Multiple endocrine neoplasia type 2. Mayo Clinic; 2021.
- Suteau V, et al. Sex bias in differentiated thyroid cancer. International Journal of Molecular Sciences. 2021; doi:10.3390/ijms222312992.
- Health Education & Content Services (Patient Education). Follow-up for low-risk thyroid cancer. Mayo Clinic; 2018.
- Frequently asked questions about potassium iodide. U.S. Nuclear Regulatory Commission. https://www.nrc.gov/about-nrc/emerg-preparedness/about-emerg-preparedness/potassium-iodide/ki-faq.html. Accessed Jan. 25, 2022.
- Wang TS, et al. Thyroidectomy. https://www.uptodate.com/contents/search. Accessed Jan. 20, 2022.
- Thyroid cancer. Cancer.Net. https://www.cancer.net/cancer-types/thyroid-cancer. Accessed Feb. 7, 2022.
- Health Education & Content Services (Patient Education). Thyroid surgery. Mayo Clinic; 2018.
- Schumm MA, et al. Frequency of hormone replacement after lobectomy for differentiated thyroid cancer. Endocrine Practice. 2021; doi:10.1016/j.eprac.2021.01.004.
- I-131 radiotherapy. Society of Nuclear Medicine and Molecular Imaging. https://www.snmmi.org/AboutSNMMI/Content.aspx?ItemNumber=10563. Accessed Jan. 25, 2022.
- Palliative care. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1454. Accessed Jan. 25, 2022.
- Sosa JA, et al. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Annals of Surgery. 1998; doi:10.1097/00000658-199809000-00005.
- Jensen NA. Allscripts EPSi. Mayo Clinic. Nov. 11, 2021.
- Creagan ET (expert opinion). Mayo Clinic. Feb. 26, 2022.
- Thyroid cancer FAQs
Associated Procedures
- Chemotherapy
- Needle biopsy
- Palliative care
- Radiation therapy
- Thyroidectomy
News from Mayo Clinic
- Unleashing CAR-T cell therapy to destroy solid tumors in thyroid cancer April 09, 2024, 12:30 p.m. CDT
- What you should know about thyroid cancer in adolescents, young adults Sept. 20, 2022, 03:00 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Thyroid cancer.
Kenny Lee ; Catherine Anastasopoulou ; Chandriya Chandran ; Sebastiano Cassaro .
Affiliations
Last Update: May 1, 2023 .
- Continuing Education Activity
Thyroid cancer is a malignancy arising from the thyroid parenchymal cells. Its incidence is steadily increasing worldwide, while the mortality rate has remained stable over the past several years. The clinical behavior of thyroid cancer is highly variable, from indolent, slowly progressing tumors to highly aggressive tumors with high mortality rates. There are various new cutting-edge treatment options for advanced thyroid cancer, while there is also evidence against the overtreatment of low-risk thyroid cancers. Hence, a thorough understanding of the types of thyroid cancer and its management is of paramount importance in providing the appropriate treatment to the patient. This activity reviews the incidence, etiology, pathophysiology, diagnosis, and treatment of thyroid cancer and highlights the role of interprofessional communication in optimizing the care of these patients.
- Outline the epidemiology and risk factors of thyroid cancer.
- Describe the clinical presentation and detailed histopathology of the different types of thyroid cancer.
- Explain the different treatment options for patients with thyroid cancer.
- Highlight the importance of interprofessional teams in coordinating care to optimize outcomes for patients with thyroid cancer.
- Introduction
Thyroid cancer is a malignancy of the thyroid parenchymal cells. The thyroid parenchyma consists of two major cell types, the thyroid follicular cells that give rise to differentiated thyroid cancer(DTC) and the parafollicular or C-cells that give rise to medullary thyroid carcinoma (MTC). DTC comprises papillary thyroid cancer(PTC), follicular thyroid cancer(FTC), and Hurthle cell cancer which account for 90-95% of all thyroid malignancies. MTC accounts for around 1 to 2%, and anaplastic thyroid carcinoma accounts for less than 1% of all thyroid cancers [1]
Familial occurrence of thyroid cancer is approximately 5% for PTC and FTC and 15 to 30% for MTC. [2] Over the last decades, the incidence of papillary thyroid cancer has increased worldwide, mostly due to early detection and advanced imaging technology with the risk of overdetection. [3] Mutations and translocations in the genes coding the mitogen-activated protein kinase (MAPK) cellular signaling pathway have been implicated in the genetic basis of most thyroid cancers. [4]
Some of the common mutations are as follows:
PTC - Point mutation in the BRAF gene leading to BRAF V600E mutant kinase is the most common mutation leading to PTC (29 to 69%) and PTC-associated anaplastic thyroid cancer (0 to 12%). [5] Translocation of the RET-papillary thyroid cancer(RET/PTC) occurs in about 7% of PTC. [6] Mutations in RAS proto-oncogene occur in 10-20% of follicular variant PTC (FVPTC). [2]
FTC - Mutations in RAS proto-oncogene are most common in FTC (40 to 50%). Translocation in PAX8–peroxisome proliferator-activated receptor γ (PPARγ) has been identified in around 30 to 35% of FTC. [7]
Anaplastic - Inactivating mutation of the p53 tumor suppressor gene has been identified in addition to early inactivating mutations in about 50 to 80% of the cases with anaplastic thyroid cancer. [8] [9] [10] Also, 66% of anaplastic thyroid cancers have been identified to harbor mutations in the CTNNB1 gene. [5] RAS mutations are also associated with 20 to 40% of anaplastic thyroid cancers.
MTC - Germline mutations of RET proto-oncogene in inherited forms of MTC (approximately 25% of MTC) and RAS mutations in 25% of MTC. [11]
Several other uncommon gene mutations have been associated with the development of thyroid cancer, such as TERT mutations, especially highly aggressive PTC. [12] DTC can be inherited by autosomal dominant inheritance or appear as a part of tumor-susceptibility syndromes. [13]
Risk factors: Female sex, a family history of thyroid cancer, and radiation exposure of the thyroid gland during childhood are the major risk factors associated with DTC. [14] [15] [16] [15] [14] A recent study showed that thyroid cancer affects both genders equally, as seen in autopsy reports, but it might be detected in women more frequently than men. The difference could be explained by access to medical care. [17]
- Epidemiology
Thyroid cancer represents 1% to 4% of all malignancies and is the fifth most common cancer in women in the United States. [14] It has a female preponderance of around 3:1. [18] There has been a steady rise in the incidence of thyroid cancer globally; particularly, PTC detection has risen by 240% in the last three decades. [19] This increase in the incidence has been observed in both genders and among all races and is thought to be primarily due to an increasing trend in the rate of diagnostic imaging. [20] [21]
PTC is the most common endocrine cancer, responsible for 96% of all new and 66.8% of deaths due to endocrine cancers. [22] As was mentioned earlier, most thyroid cancers derive from the follicular epithelium, with PTC and FTC being far more common than anaplastic thyroid cancer. [23] [24]
- Histopathology
PTC: Microscopically, the unique characteristic feature of PTC is papillae formation. A papilla consists of layers of tumor cells surrounding a fibrovascular core. Follicles are typically absent in classic PTC. Typical cellular histomorphology includes cells with large and clear nuclei with finely granular chromatin, often described as ground-glass or "Orphan Annie-eyed" nuclei with nuclear grooving and intranuclear inclusion bodies. Psammoma bodies, which are calcified clumps of cells likely derived from necrosed papillae, are also common. [25]
Some variants of PTC do not form papillae and are termed follicular variants of PTC, provided they still have the nuclear features of PTC. Variants of PTC such as tall cell variant, columnar variant, insular carcinoma, and diffuse sclerosing variant are more aggressive than classic PTC and are termed thyroid cancers with intermediate differentiation. [26]
FTC: The histological features of FTC can be highly variable, from a well-differentiated follicular pattern to a poorly differentiated pattern with marked nuclear atypia, absence of follicles, extensive capsular or vascular invasion, and solid growth. The latter changes are associated with a poor prognosis. [27] As described above, features that are characteristic of PTC should be absent. Differentiating a follicular carcinoma from a benign follicular adenoma can only be made based on extracapsular and/or vascular invasion. FTC is further classified as minimally invasive, encapsulated, angioinvasive, and widely invasive, depending on the extent of invasion.
Hurthle cell carcinoma: This is characterized by the occurrence of eosinophilic oxyphilic cells with abundant cytoplasm (oncocytes) and prominent nucleoli. [28]
MTC: Given its origin from the parafollicular C cells, its histological features are the presence of spindle-shaped cells with no follicle formation. Amyloid deposition and calcitonin immunoreactivity are typically present.
Anaplastic thyroid carcinoma: The usual histologic variants are spindle-cell, pleomorphic giant cell, and squamoid variants. [29] Most of these cancers can consist of a mixed morphology of 2 or 3 variants. Atypical mitosis and numerous mitotic figures are very common. These cancers are less likely to stain for thyroid transcription factor 1 (TTF1), PAX 8, or thyroglobulin.
- History and Physical
The most common presenting feature in DTC is either neck swelling (detected by the patient or a clinician) or incidentally detected thyroid nodules on neck imaging. The risk of malignancy of a thyroid nodule in the general population is around 5 to 10%, with the risk being higher in men and extremes of age. [30]
A careful history and physical examination will help to differentiate low-risk vs. high-risk nodules, although these signs and symptoms lack specificity. Aspects in the patient's history that could be concerning for malignancy include a sudden increase in the size of the nodule with pressure symptoms such as hoarseness of voice, dysphagia, dyspnea, or Horner's Syndrome, as well as a family history of thyroid cancer, childhood irradiation to the head and neck region, or the occurrence of systemic effects such as weight loss and fatigue. On physical examination of the neck, firmness of the nodule, immobility, and the presence of neck lymph nodes should trigger suspicion for malignancy and lead to further evaluation.
On the other hand, anaplastic thyroid cancer can present as a rapidly enlarging neck mass and rapid occurrence of compressive symptoms of the aerodigestive tract. Some patients can present with constitutional symptoms such as fever, weight loss, and anorexia. [31] [32]
A thyroid function panel is the most appropriate initial evaluation in a patient with a thyroid nodule. A hyperthyroid state often correlates with a lower risk of malignancy; in such patients, a radionuclide uptake scan is indicated. If the nodule/nodules are identified as hyperfunctioning, fine-needle aspiration biopsy (FNAB) should generally be avoided. This is because these nodules are rarely malignant, and the FNAB results for hyperfunctioning nodules are often inaccurate. [30]
Evaluation of the thyroid nodule when biochemically euthyroid or hypothyroid should begin with a high-resolution diagnostic thyroid ultrasound. This can help assess the nodules for high-risk features, detect additional nodules not felt on physical examination, evaluate for neck lymph nodes, and guide FNAB if warranted. High-risk features on ultrasound include a significant increase in size from prior imaging, hypoechogenicity, irregular margins, size taller than wide, microcalcifications, a solid internal structure, extra-thyroidal extension, and central vascularity. The features associated with a lower risk of malignancy are a purely cystic nodule, spongiform appearance, comet tail shadowing, and peripheral vascularity. The decision to subject a nodule for FNAB should be based on these radiological criteria with guidance from Thyroid Imaging Reporting and Data System (TIRADS) or American Thyroid Association (ATA) criteria, but importance should be given to clinical indications irrespective of imaging criteria. [33] [34] [35]
Limitations of FNAB
Of note, the diagnostic accuracy of FNAB depends on the skill of the person performing the procedure as well as the pathologist interpreting the results, and it ranges between 70 to 97%. Approximately 17 to 20% of FNAB are classified as insufficient samples. [36] Also, since FNAB allows for the analysis of individual cellular features and not the overall architecture of the nodule, it is excellent for diagnosing PTC, but it cannot detect capsular or vascular invasions by FTC. As a result, while FNA can categorize certain findings as suspicious for FTC, a diagnosis of FTC can only be made from the final pathology after surgical resection. [37] The same applies to Hurthle cell neoplasms.
Bethesda System for Reporting Thyroid Cytopathology
The FNAB result is usually reported by the Bethesda Criteria for Reporting Thyroid Cytology, which stratifies the biopsy results based on the cytology, and recommends a further course of action. [38]
Bethesda Category 1 is indicative of non-diagnostic FNAB; re-aspiration is indicated.
Bethesda Category 2 is suggestive of a benign nodule that can be followed clinically with periodic thyroid ultrasound as warranted.
Bethesda Categories 3 (Atypia of undetermined significance, AUS or Follicular lesion of undetermined significance, FLUS) and 4 (follicular neoplasm, FN or suspicious for follicular neoplasm) suggests that the inclusion or exclusion of thyroid cancer is not clear, and these patients may benefit from repeat FNAB (Category 3), molecular testing, or lobectomy (Categories 3 and 4).
Bethesda Categories 5 (Suspicious for malignancy) and 6 (Malignant) usually require surgery.
Role of Molecular Testing in Thyroid Cancer
Molecular testing is generally used in Bethesda categories 3 and 4, where cytology is indeterminate. [34] Recently, testing for single mutations such as BRAF V600E or RET/PTC translocations was performed. While these tests yield good specificity (100%), their sensitivity is usually poor (50 to 60%). [39] [40]
Molecular Diagnostic Approaches [41]
- Gene mutation profiling panel- such as the 7-gene panel that detects multiple genes including BRAF V600E, HRAS codon 61, KRAS codons 12/13, and NRAS codon 61 point mutations, and RET/PTC1, PAX8/PPARγ, and RET/PTC3 translocations which account for approximately 70% of thyroid cancer. [41] This panel has improved the sensitivity and the negative predictive of these tests to >90%. [42] Therefore, mutation tests are good rule-in tests for thyroid cancer. [43]
- A 167-Gene expression classifier provides a strong negative predictive value, while its positive predictive value is only around 50%. [41] Hence, gene expression classifier testing is a good rule-out test for thyroid cancer.
However, recently a large multi-gene panel of mutation markers has been introduced, further improving sensitivity and specificity. [43] [44]
While CT and MRI scans are not routine modalities in evaluating thyroid nodules for malignancies, their use may be appropriate in assessing local spread in more advanced diseases or those with enlarged cervical nodes in association with a suspicious mass. [45] A CT scan should be advised for patients with thyroid mass with extension into the substernal region confirmed by ultrasound or plain radiographs.
- Treatment / Management
Papillary and Follicular Thyroid Cancers
Surgical Treatment
Surgical resection remains the main treatment modality of both PTC and FTC, followed by radioiodine ablation (RAI ablation) when indicated and suppression therapy with thyroid hormone. [34] [46] Systemic radiation and chemotherapy seldom play a significant role in treatment, although they may be used in advanced cases refractory to conventional methods.
To minimize the risk of complications, specifically recurrent laryngeal nerve injury and hypoparathyroidism, surgery is recommended, performed by experienced, "high-volume" thyroid surgeons. [47]
Pre-operative neck ultrasound is pivotal in deciding the appropriate surgical procedure. Surgical resection can be hemithyroidectomy or total thyroidectomy, with or without lymph node dissection. The choice of surgery depends on tumor size, presence of lymph node metastasis, extra-thyroidal extension, age of the patient, and the presence or absence of co-morbid conditions. In patients with locally advanced disease, additional imaging of the neck is advised.
A thyroid lobectomy is preferred for unilateral DTC < 1 cm, without any extra-thyroidal or lymph node invasion, unless there are clear indications for total thyroidectomy, such as childhood head and neck irradiation or a strong family history of thyroid cancer. Lately, there is also a trend for just active surveillance without immediate surgery, but more studies are needed to show the difference, if any, in the outcomes and prognosis. [48]
For tumor sizes between 1 and 4 cm with no extrathyroidal or lymphatic invasion, the procedure of choice can either be a total thyroidectomy or lobectomy, depending on patient preferences and risk factors, as described above. This decision should be made with the patient aware that a completion thyroidectomy may be necessary depending on pathology results.
For tumors > 4 cm or tumors with extra-thyroidal or lymph node invasion, a total thyroidectomy is the preferred surgical procedure as there is a high risk of multifocal carcinoma in such cancers. It is also intended to facilitate RAI ablation and future surveillance with thyroglobulin as a tumor marker.
The decision for lymph node dissection should be made on a patient-by-patient basis, and there is still a lot of controversy about the proven survival benefit of prophylactic node dissection. Regardless, all patients with proven or suspected PTC should undergo a thorough examination of both the central and lateral neck for possible nodal metastasis. The lateral neck compartments are not routinely entered in thyroidectomy and should be assessed preoperatively with ultrasound and subsequent FNAB if there is a concern for lymphatic spread. If pathologic nodes are confirmed, an ipsilateral neck dissection should be carried out, with a formal clearance of defined lymph node compartments as opposed to isolated "berry-picking" of diseased nodes. The central neck lymph nodes are difficult to assess preoperatively due to their location. A careful inspection and palpation of the central neck should be performed at the time of surgery, with subsequent compartmental neck dissection if abnormal nodes are found.
Postsurgical Risk Stratification
Postsurgical risk stratification must be performed to determine the need for additional treatment, especially with RAI ablation. The TNM (Tumor, Node, Metastasis) risk stratification by the American Joint Commission on Cancer(AJCC) predicts disease-specific mortality, while the American Thyroid Association (ATA) risk stratification system, which is widely used, helps predict the persistence or recurrence of residual cancer. [49]
The ATA system classifies patients as low, intermediate, or high risk based on clinicopathologic findings, including but not limited to tumor size, histologic type, vascular or lymph node involvement, local invasion, distant metastasis, the extent of tumor resection, post-operative thyroglobulin levels, and post-operative radioiodine uptake outside of the thyroid gland. [34] [50] [34]
The TNM-AJCC system accounts for factors such as the tumor size, the presence and extent of extra-thyroidal invasion, the number of nodal metastases, and whether there is the presence of distal metastasis. Age is a significant factor in predicting mortality in thyroid cancer patients, and its role is also significant in staging the disease. Patients under 55 years old at the time of diagnosis will receive a stage II diagnosis at the most. [51]
Radioiodine (RAI) Ablation Therapy
RAI therapy after thyroidectomy is used for remnant ablation of normal residual thyroid tissue, as adjuvant therapy for subclinical micrometastases, or as treatment of apparent local or distant metastasis. [52] High-risk and some selected intermediate-risk patients, per the ATA risk stratification system, will benefit from RAI ablation. Patients who are candidates for RAI therapy should maintain a low iodine diet for 1 to 2 weeks before the treatment to ensure iodine depletion of the cells; they should also be cautioned against large iodine administrations such as through iodinated contrast or amiodarone to improve the avidity of the thyroid follicular cells to iodine. RAI ablation works best when thyroid hormone has been withdrawn, with a goal thyroid-stimulating hormone (TSH) of 30mIU/liter or higher. This level of TSH can be achieved either through the withdrawal of thyroid hormones or the administration of exogenous recombinant human TSH.
Thyroid Hormone Suppression Therapy
Thyroid hormone suppression therapy to suppress TSH and thereby potentially minimize its stimulation of thyroid cancer growth is recommended in most patients after surgery. For patients with ATA high-risk, the goal TSH should be no more than 0.1m IU/liter, and for patients in the intermediate-risk category, the goal TSH should be between 0.1 and 0.5 mIU/liter. For the ATA low-risk category, a goal TSH between 0.5 and 2.0 mIU/liter is acceptable. [53] [54] [53]
Persistent or Recurrent Disease
For recurrent minimal iodine-avid disease, RAI ablation is the preferred therapy. For invasive neck disease, surgical resection is recommended. Percutaneous ethanol injection [55] has been tried for cervical lymph node metastasis. For small distant metastasis to bones or lungs, radiofrequency ablation has been used. Other treatment options are external beam radiation and systemic chemotherapy.
Systemic Chemotherapy
Systemic chemotherapy is usually only considered in a group of carefully selected patients with a high metastatic disease burden or rapidly progressive metastatic disease despite the above treatment (Iodide-refractory). Because of the significant adverse effects associated with such therapy, it should be considered only when the associated benefits exceed the risks. [34]
Systemic chemotherapy for DTC is preferably administered through a clinical trial. The common agents of choice are the kinase inhibitor class of drugs such as anti-angiogenic multi-targeted kinase inhibitors (aaMKI- lenvatinib, sorafenib), BRAF kinase inhibitors (vemurafenib, dabrafenib), MEK inhibitors (trametinib, cobimetinib), NTR kinase inhibitors (larotrectinib), and RET inhibitor (selpercatinib). [56] The choice of agent depends on the occurrence of specific gene mutations or signaling irregularities such as those described above. For patients with no identifiable mutations, aaMKIs are the recommended first-line therapy.
Dynamic Risk Stratification
After the initial postsurgical risk stratification and appropriate treatment as above, patients should be re-stratified during each follow-up visit depending on their response to therapy into one of the following clinical outcomes: 1. Excellent response, 2. Biochemical incomplete response, 3. Structural incomplete response, 4. Indeterminate response. [57] [58] [59]
Monitoring in the first postsurgical year primarily involves a thyroid ultrasound scan every 6 to 12 months and TSH and thyroglobulin levels every 3 to 6 months, depending on risk. For higher-risk patients, additional imaging such as CT scan, MRI, FDG-PET, or whole-body radioiodine scanning is required.
After one year, the frequency of monitoring depends on the dynamic risk stratification.
Medullary Thyroid Cancer
Surgical therapy that includes total thyroidectomy with resection of local and regional metastases is the mainstay of treatment for MTC. In most patients with confirmed MTC and no evidence of pre-operative cervical lymph node metastasis on ultrasound, prophylactic central lymph node dissection should be performed at the time of the total thyroidectomy. Patients with confirmed lateral zone nodal metastases should receive lateral compartment dissection, central neck dissection, and total thyroidectomy. Serum calcitonin, carcinoembryonic antigen, and biochemical testing for coexisting hyperparathyroidism and pheochromocytoma should be performed. Patients should be monitored long-term with serial calcitonin levels, neck ultrasound, and physical examination. Of note, as MTC is not of follicular origin, there is no role for radioiodine ablation or TSH suppression in its management. [60] For refractory MTC, systemic chemotherapy with kinase inhibitors has been shown to be beneficial. RET-specific kinase inhibitors are preferred in patients with RET mutation, while in patients negative for RET mutation, aaMKIs are the preferred drugs.
Anaplastic Thyroid Cancer
In patients diagnosed with anaplastic thyroid cancer, BRAF V600E mutation testing and staging are performed. Resectable disease is surgically removed, followed by specific BRAF kinase inhibitors in patients with BRAF V600E mutations. Other patients received targeted radiation treatment and cytotoxic chemotherapy after surgery. However, distant metastases are common in patients even at initial diagnosis due to their rapidly progressive course, and local invasion into the trachea or vasculature may occur, making it unresectable. Mortality is near 100% for these cancers, and a conservative surgical approach for palliation can be considered in such high-risk patients.
- Differential Diagnosis
- Benign thyroid nodule
- Toxic nodular goiter
- Primary thyroid lymphoma
- Cervical lymphadenopathy
The prognosis of thyroid cancer varies greatly, depending on its type, tumor size, the extent of metastasis, patient's age, and amenability to resection. The prognosis is generally good, with up to 95% 5-year survival rate for patients of all ages and races. Poor prognostic factors include large tumor size, the presence of extra-thyroidal extensions or metastases, older age, or unfavorable tumor types such as undifferentiated cancer. [61]
- Complications
Untreated thyroid cancer can be locally invasive into the airway, esophagus, or other nearby neurovascular structures. Distant metastasis most commonly involves the lung, bone, and other soft tissue structures.
Both thyroid lobectomy and total thyroidectomy carry the potential for neurovascular injuries, with the most common involving the recurrent laryngeal nerve, leading to hoarseness of voice and potentially respiratory failure with bilateral injuries.
Treatment of thyroid cancer during pregnancy, mostly with thyroidectomy, did not show any significant increase in the complications of pregnancy. [62]
- Consultations
- Thyroid surgeon
- Endocrinologist
- Radiologist
- Pathologist
- Deterrence and Patient Education
As previously discussed, patients with risk factors, such as prior irradiation to the head and neck area and a family history of thyroid cancer, should be screened for thyroid cancer, especially when such a patient presents with thyroid nodules with or without pressure symptoms.
- Enhancing Healthcare Team Outcomes
Thyroid cancer, as discussed, can have highly varied manifestations, from a clinically indolent low-risk disease that can be managed with only active surveillance to a highly aggressive metastatic disease that needs extensive surgical resection with or without systemic chemotherapy.
Hence, managing a patient with thyroid cancer is a highly individualized process taking into account the patient's risk of recurrence and preferences (after being educated about the different treatment strategies and risks vs. benefits of each). A close collaboration between all interprofessional team members, including but not limited to the thyroid surgeon, the endocrinologist, the pathologist, the radiologist, and possibly the oncologist, plays a vital role in providing the most appropriate treatment for the patient while avoiding overtreatment at the same time. Nursing staff should ensure the patient is involved and comfortable every step of the way, from pre-operative planning to treatment and postoperative monitoring. When pursuing chemotherapy, a specialized oncology pharmacist is also a valuable addition to the interprofessional team. Interprofessional teamwork relies on open communication channels between all team members, and the maintaining of meticulous records so that all professionals involved in the case have access to the same updated patient information and can reach out to other team members if they see anything that requires their attention. This type of interprofessional care coordination combined with open information sharing will yield the best patient outcomes. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Kenny Lee declares no relevant financial relationships with ineligible companies.
Disclosure: Catherine Anastasopoulou declares no relevant financial relationships with ineligible companies.
Disclosure: Chandriya Chandran declares no relevant financial relationships with ineligible companies.
Disclosure: Sebastiano Cassaro declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Lee K, Anastasopoulou C, Chandran C, et al. Thyroid Cancer. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Radioactive Iodine for Thyroid Malignancies. [StatPearls. 2024] Radioactive Iodine for Thyroid Malignancies. Palot Manzil FF, Kaur H. StatPearls. 2024 Jan
- Thyroid malignancies: a New Zealand South Island thyroid clinic experience 1995-2006. [N Z Med J. 2008] Thyroid malignancies: a New Zealand South Island thyroid clinic experience 1995-2006. Brownlie B, Mercer P, Turner J, Allison R. N Z Med J. 2008 Aug 8; 121(1279):36-45. Epub 2008 Aug 8.
- Vascular endothelial growth factor expression is higher in differentiated thyroid cancer than in normal or benign thyroid. [J Clin Endocrinol Metab. 1997] Vascular endothelial growth factor expression is higher in differentiated thyroid cancer than in normal or benign thyroid. Soh EY, Duh QY, Sobhi SA, Young DM, Epstein HD, Wong MG, Garcia YK, Min YD, Grossman RF, Siperstein AE, et al. J Clin Endocrinol Metab. 1997 Nov; 82(11):3741-7.
- Review Simultaneous Occurrence of Medullary Thyroid Carcinoma and Papillary Thyroid Carcinoma: A Case Series with Literature Review. [Curr Oncol. 2023] Review Simultaneous Occurrence of Medullary Thyroid Carcinoma and Papillary Thyroid Carcinoma: A Case Series with Literature Review. Fallahi P, Patrizio A, Stoppini G, Elia G, Ragusa F, Paparo SR, Balestri E, Mazzi V, Botrini C, Varricchi G, et al. Curr Oncol. 2023 Nov 30; 30(12):10237-10248. Epub 2023 Nov 30.
- Review Overview of the 2022 WHO Classification of Thyroid Neoplasms. [Endocr Pathol. 2022] Review Overview of the 2022 WHO Classification of Thyroid Neoplasms. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M, Tallini G, et al. Endocr Pathol. 2022 Mar; 33(1):27-63. Epub 2022 Mar 14.
Recent Activity
- Thyroid Cancer - StatPearls Thyroid Cancer - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
Home > Patients, Caregivers, and Advocates > About Cancer > Cancers > Thyroid Cancer
Thyroid Cancer

Thyroid cancer is cancer of the butterfly-shaped gland at the base of the throat. The thyroid uses iodine, a mineral found in some foods and in iodized salt, to help make several hormones that control heart rate, body temperature, metabolism, and the amount of calcium in the blood.
There are four main types of thyroid cancer: papillary thyroid cancer, follicular thyroid cancer, medullary thyroid cancer, and anaplastic thyroid cancer. According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program , approximately 44,020 people in the United States will be diagnosed with thyroid cancer in 2024, and about 2,170 will die of the disease. Thyroid cancer is highly treatable, and the five-year relative survival rate is estimated at 98.4 percent.
Thyroid cancer is much more common among women than it is among men. Age and exposure to radiation are also risk factors.
Source: National Cancer Institute

You can support the AACR's mission by contacting your representatives and senators and advocating for increased funding for lifesaving cancer research.
Accessing a Clinical Trial During the Pandemic

What It’s Like to Get a “C” in Graduate School

Striving for a Full Life with Metastatic Cancer
- September is Thyroid Cancer Awareness Month
- Ovarian Cancer
- Testicular Cancer
- Introduction
- Conclusions
- Article Information
AJCC indicates American Joint Committee on Cancer Cancer Staging Manual, 8th Edition; ATA, American Thyroid Association; ATC, anaplastic thyroid carcinoma; CMTC, cribriform morular thyroid carcinoma; FTC, follicular thyroid carcinomas; IEFVPTC, invasive encapsulated follicular variant papillary thyroid carcinoma; MTC, medullary thyroid carcinomas; NA, not applicable; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; OCA, oncocytic carcinomas of the thyroid; and PTC, papillary thyroid carcinomas.
AUS indicates atypia of undetermined significance; FN, follicular neoplasm; ND, nondiagnostic; and SFM, suspicious for malignancy.
ATC indicates anaplastic thyroid carcinoma; AUS, atypia of undetermined significance; FN, follicular neoplasm; IEFVPTC, invasive encapsulated follicular variant papillary thyroid carcinoma; ND, nondiagnostic; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; OCA, oncocytic carcinomas of the thyroid; PTC, papillary thyroid carcinomas; and SFM, suspicious for malignancy.
eTable 1. Association of Variant Allele Fraction of RAS and BRAF V600E and TERT Promoter Variants With Clinicohistopathologic Features of Well-Differentiated Thyroid Tumors
eTable 2. Surgical Pathology Follow-up of Variant Allele Fraction Assays of RAS , BRAF V600E, and TERT Promoter Variants on the Residual FNA Biopsy Specimens From Thyroid Nodules
eTable 3. Performance of Variant Allele Fraction Assays of RAS and BRAF and TERT Variants in Differentiating Malignancy Among Thyroid Surgical Tumors and Preoperative Thyroid Nodules
eTable 4. Direct Cost of Laboratory Developed Tests for Variant Allele Fraction Assays
eFigure 1. Detection and Quantification of HRAS , NRAS , and KRAS Variants by dPCR Assays and Verification by Sanger Sequencing
eFigure 2. Variant Allele Fraction Distributions Across 3 RAS Gene Isoforms in Thyroid Tumors and Different Histology Diagnoses
eFigure 3. Clinicomolecular Characteristics of Interpatient Variabilities of RAS , BRAF V600E, and TERT Promoter Variants at the Variant Allele Fraction Level in Papillary Thyroid Carcinomas (PTC) Classified by The 2017 WHO Classification of Thyroid Neoplasms
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Fu G , Chazen RS , MacMillan C , Witterick IJ. Discriminating Interpatient Variabilities of RAS Gene Variants for Precision Detection of Thyroid Cancer. JAMA Netw Open. 2024;7(5):e2411919. doi:10.1001/jamanetworkopen.2024.11919
Manage citations:
© 2024
- Permissions
Discriminating Interpatient Variabilities of RAS Gene Variants for Precision Detection of Thyroid Cancer
- 1 Alex and Simona Shnaider Research Laboratory in Molecular Oncology, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Sinai Health, Toronto, Ontario, Canada
- 2 Department of Pathology and Laboratory Medicine, Mount Sinai Hospital, Sinai Health and University of Toronto, Toronto, Ontario, Canada
- 3 Joseph and Mildred Sonshine Family Centre for Head and Neck Diseases, Mount Sinai Hospital, Sinai Health, Toronto, Ontario, Canada
- 4 Department of Otolaryngology-Head and Neck Surgery, Mount Sinai Hospital, Sinai Health and University of Toronto, Toronto, Ontario, Canada
Question Is discrimination of interpatient variabilities of RAS gene variants associated with improved accuracy in malignancy diagnosis among thyroid nodules?
Findings This diagnostic study of 620 patients, including 438 surgically resected thyroid tumor tissues and 249 thyroid nodule fine-needle aspiration biopsies, delineated interpatient differences in RAS variants at the variant allele fraction (VAF) levels, ranging from 0.15% to 51.53%. While RAS variants alone, regardless of the extent of variation, were associated with low-risk thyroid cancer in 88.8% of tumor samples, they did not definitively distinguish malignancy of an unknown tumor; however, detection of interpatient variabilities of RAS , BRAF, and TERT promoter variants in combination could assist in classifying indeterminate thyroid nodules.
Meaning These findings suggest that discrimination of interpatient differences in genomic variants could facilitate precision cancer detection, including preoperative malignancy diagnosis and stratification of low-risk tumors from high-risk ones, among patients with indeterminate thyroid nodules.
Importance Interpatient variabilities in genomic variants may reflect differences in tumor statuses among individuals.
Objectives To delineate interpatient variabilities in RAS variants in thyroid tumors based on the fifth World Health Organization classification of thyroid neoplasms and assess their diagnostic significance in cancer detection among patients with thyroid nodules.
Design, Setting, and Participants This prospective diagnostic study analyzed surgically resected thyroid tumors obtained from February 2016 to April 2022 and residual thyroid fine-needle aspiration (FNA) biopsies obtained from January 2020 to March 2021, at Mount Sinai Hospital, Toronto, Ontario, Canada. Data were analyzed from June 20, 2022, to October 15, 2023.
Exposures Quantitative detection of interpatient disparities of RAS variants (ie, NRAS, HRAS , and KRAS ) was performed along with assessment of BRAF V600E and TERT promoter variants (C228T and C250T) by detecting their variant allele fractions (VAFs) using digital polymerase chain reaction assays.
Main Outcomes and Measures Interpatient differences in RAS , BRAF V600E, and TERT promoter variants were analyzed and compared with surgical histopathologic diagnoses. Malignancy rates, sensitivity, specificity, positive predictive values, and negative predictive values were calculated.
Results A total of 438 surgically resected thyroid tumor tissues and 249 thyroid nodule FNA biopsies were obtained from 620 patients (470 [75.8%] female; mean [SD] age, 50.7 [15.9] years). Median (IQR) follow-up for patients who underwent FNA biopsy analysis and subsequent resection was 88 (50-156) days. Of 438 tumors, 89 (20.3%) were identified with the presence of RAS variants, including 51 (11.6%) with NRAS , 29 (6.6%) with HRAS , and 9 (2.1%) with KRAS . The interpatient differences in these variants were discriminated at VAF levels ranging from 0.15% to 51.53%. The mean (SD) VAF of RAS variants exhibited no significant differences among benign nodules (39.2% [11.2%]), noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTPs) (25.4% [14.3%]), and malignant neoplasms (33.4% [13.8%]) ( P = .28), although their distribution was found in 41.7% of NIFTPs and 50.7% of invasive encapsulated follicular variant papillary thyroid carcinomas ( P < .001). RAS variants alone, regardless of a low or high VAF, were significantly associated with neoplasms at low risk of tumor recurrence (60.7% of RAS variants vs 26.9% of samples negative for RAS variants; P < .001). Compared with the sensitivity of 54.2% (95% CI, 48.8%-59.4%) and specificity of 100% (95% CI, 94.8%-100%) for BRAF V600E and TERT promoter variant assays, the inclusion of RAS variants into BRAF and TERT promoter variant assays improved sensitivity to 70.5% (95% CI, 65.4%-75.2%), albeit with a reduction in specificity to 88.8% (95% CI, 79.8%-94.1%) in distinguishing malignant neoplasms from benign and NIFTP tumors. Furthermore, interpatient differences in 5 gene variants ( NRAS, HRAS, KRAS, BRAF, and TERT ) were discriminated in 54 of 126 indeterminate FNAs (42.9%) and 18 of 76 nondiagnostic FNAs (23.7%), and all tumors with follow-up surgical pathology confirmed malignancy.
Conclusions and Relevance This diagnostic study delineated interpatient differences in RAS variants present in thyroid tumors with a variety of histopathological diagnoses. Discrimination of interpatient variabilities in RAS in combination with BRAF V600E and TERT promoter variants could facilitate cytology examinations in preoperative precision malignancy diagnosis among patients with thyroid nodules.
Thyroid cancer, especially papillary thyroid cancer (PTC), has experienced a rapid increase in incidence since the 1980s 1 and is primarily diagnosed through ultrasonographic examinations and fine-needle aspiration (FNA) biopsy of suspicious nodules. 2 , 3 However, approximately 30% of FNAs exhibit an indeterminate diagnosis, and 10% of findings are nondiagnostic. 4 Patients with indeterminate thyroid nodule findings usually undergo diagnostic surgery, with 20% to 30% of nodules being detected as malignant. Thus, up to 70% to 80% of patients with indeterminate nodules found histologically benign have undergone unnecessary surgical procedures. Patients with nondiagnostic cytological findings are typically recommended for a repeat FNA, with 13% of nodules detected as being malignant. 4 Cancer arises along with genetic alterations. Molecular assays of FNA specimens are being increasingly used to enhance preoperative diagnostic accuracy for patients with indeterminate cytological findings and avoid unnecessary surgery for benign thyroid nodules. 2 , 5
RAS is the most frequently variated gene family in human cancer. Approximately 19% of patients with cancer harbor activating variations from 3 RAS gene isoforms: NRAS (OMIM 164790 ) in 17% of patients, HRAS (OMIM 190020 ) in 7% of patients, or KRAS (OMIM 190070 ) in 75% of patients. 6 Similarly, RAS variants are the second most common alterations in thyroid nodules, with NRAS variants being the dominant isoform followed by HRAS and KRAS . 7 - 10 In thyroid tumors, RAS gene variations are detected in tumors spanning a wide spectrum of histological diagnoses, with a prevalence of 10% to 30% in PTC, 11 - 13 40% to 50% in follicular thyroid carcinomas (FTCs), 14 , 15 12% to 85% in follicular adenoma or hyperplasia, and 5% to 46% in noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTPs). 14 , 16 Indeterminate thyroid nodules carrying RAS variants have shown malignancy rates varying from 9% to 83%, 7 - 10 , 17 and such discrepancies can be primarily attributed to the use of small patient cohorts in these studies. Despite the widespread application of RAS variants in panel tests, assays of RAS variants often yield inconclusive results in detecting malignancy of thyroid nodules, frequently leading to a diagnostic surgery. 2 , 12 , 18 On the contrary, BRAF V600E and TERT promoter variants (C228T and C250T) are the most frequently detected genetic variants in thyroid nodules, providing a more definitive basis for cancer diagnosis. 19 - 21
Interpatient variabilities in genomic variants may reflect differences in tumor statuses among individuals. 20 However, the diagnostic impact of discriminating interpatient variabilities of RAS variants on cancer detection remains unclear, particularly under the 2022 updated fifth World Health Organization (WHO) classification of thyroid neoplasms. 22 In alignment with the WHO classification, the 2023 Bethesda System for Reporting Thyroid Cytopathology (BSRTC) 23 has updated nomenclature for each of the 6 diagnostic categories: I, nondiagnostic (ND); II, benign; III, atypia undetermined significance (AUS); IV, follicular neoplasm (FN); V, suspicious for malignancy (SFM); and VI, malignant. 23 Currently, the methods of detecting RAS variations are mainly based on polymerase chain reaction (PCR) and Sanger sequencing or next-generation sequencing (NGS). This study aimed to delineate interpatient disparities of RAS variants in thyroid tissues by quantifying variant allele fraction (VAF) using digital PCR (dPCR) assays and to examine their diagnostic associations with the preoperative detection of malignancy among patients with thyroid nodules.
This prospective diagnostic study was reviewed and approved by the Sinai Health Research Ethics Bord. All patients provided written informed consent, and samples were deidentified for data analysis. Data are reported in alignment with the Standards for Reporting of Diagnostic Accuracy ( STARD ) reporting guideline.
A total of 438 thyroid tissue specimens were obtained from surgically resected thyroid tumors with a maximum dimension of 1 cm or larger from 436 consecutive patients who underwent surgery between February 1, 2016, and April 4, 2022, and 249 FNA specimens were collected from 234 consecutive patients who underwent biopsy procedures between January 22, 2020, and March 2, 2021, at Mount Sinai Hospital, Sinai Health, Toronto, Canada. All surgical tissue specimens sampled were quickly placed in liquid nitrogen and transferred to −80 °C for long-term preservation. As for preoperative biopsies, all FNAs were routinely obtained under ultrasonographic guidance using a 23-gauge needle and subjected to CytoLyte (Hologic) fixation. After cytological examination according to the BSRTC, 4 , 23 the leftover materials of a total of 249 FNA biopsies were collected and stored at 4 °C until DNA purification. These preoperative biopsies primarily included ND and indeterminate (BSRTC categories I, III, IV, and V) specimens, along with some malignant (BSRTC category VI) and benign (BSRTC category II) specimens. A follow-up of thyroid nodules was conducted among patients who had previously undergone FNA procedures and subsequently underwent surgery. The patient clinical records, surgical pathology reports, and hematoxylin and eosin–stained sections were reviewed. The final histological diagnoses were made in accordance with the fifth WHO classification of thyroid neoplasms 22 and Protocol for the Examination of Specimens From Patients With Carcinomas of the Thyroid Gland. 24 Patients with cancer were further stratified as having low, intermediate, or high risk of recurrence based on the 2015 American Thyroid Association guidelines. 25
Molecular assays for the most prevalent RAS variants of 3 RAS genes, NRAS (Q61R or Q61K), HRAS (Q61R or Q61K), and KRAS (G12C, G12D, G12V, G12A, or G13D), were developed using locked nucleic acid probe–based droplet dPCR by following the strategy and procedures recently established for the VAF assays of BRAF V600E and TERT promoter variants (C228T and C250T). 20 , 26 The details of DNA extraction, dPCR assays, and verification of RAS variants using PCR and Sanger sequencing were documented in the eMethods in Supplement 1 .
Data were summarized as frequencies and percentages for categorical variables and means and SDs for continuous variables. Blinded central review–based 2022 WHO histologic classification and 2023 BSRTC were used as the reference standard. 22 , 23 The continuous parametric variables were compared by t test or 1-way analysis of variance test. Associations between molecular status and the clinicopathological characteristics were assessed by χ 2 or Fisher exact test with 95% CIs. Statistical tests were conducted using SPSS software version 22.0 (IBM). P values were 2-sided, with P < .05 considered statically significant. Data were analyzed from June 20, 2022, to October 15, 2023.
A total of 438 surgically resected thyroid tumor tissues and 249 thyroid nodule FNA biopsies were obtained from 620 patients (470 [75.8%] female; mean [SD] age, 50.7 [15.9] years). Of 438 thyroid tumors, 431 were follicular cell-derived neoplasms, comprising 77 benign tumors (thyroid follicular nodular disease, follicular adenoma, or oncocytic adenoma), 12 NIFTPs, and 343 malignant neoplasms, including 258 PTCs, 67 invasive encapsulated follicular variant PTCs (IEFVPTCs), 5 FTCs, 10 oncocytic carcinomas of the thyroid (OCAs), and 3 anaplastic thyroid carcinomas (ATCs). The cohort also included 5 medullary thyroid carcinomas (MTCs) and 2 cribriform morular thyroid carcinomas (CMTCs) ( Figure 1 and Table 1 ; eTable 1 in Supplement 1 ). Hence the surgical tumor cohort exhibited a high tumor malignancy rate of 79.7% (95% CI, 75.5%-83.3%). In a separate cohort of 249 FNA biopsies, there were 76 (30.5%) with ND findings, 126 (50.6%) with indeterminate findings, 34 (13.7%) with malignant findings, and 13 (5.2%) with benign findings ( Figure 2 and Table 2 ; eTable 2 in Supplement 1 ). The indeterminate FNAs comprised 83 AUS (65.9%), 26 FN (20.6%), and 17 SFM (13.5%).
Molecular VAF assays were developed for the quantitative detection of RAS variants at single-nucleotide resolution positive for NRAS , HRAS , and KRAS in tumor tissues but not in the adjacent healthy tissue, which were verified by Sanger sequencing (eFigure 1 in Supplement 1 ). Of 438 tumors that underwent surgery, 89 (20.3%) were identified with the presence of RAS variants, including 51 (11.6%) with NRAS , 29 (6.6%) with HRAS , and 9 (2.1%) with KRAS variants, in mutually exclusive existence from each other ( Figure 1 and Table 1 ). When compared with the 3 RAS gene isoforms across all tumor subtypes, the profiles of interpatient variabilities were delineated at the VAF levels ranging from 0.15% to 51.53%, specifically from 0.59% to 51.53% for NRAS , from 0.36% to 43.56% for HRAS , and from 0.15% to 46.64% for KRAS variants, with no significant difference among 3 isoforms ( P = .16) ( Figure 1 ; eFigure 2 in Supplement 1 ). Of these variants, 84 (94.4%) exhibited a VAF of greater than 1% and 5 showed a VAF of less than 1%, with 1 NRAS , 2 HRAS , and 2 KRAS variants. RAS variants were found in 5 benign neoplasms (6.4%), 5 NIFTPs (41.7%), and 79 malignant neoplasms (22.6%) ( P < .001). Of 79 malignant neoplasms, 41 (51.9%) were PTCs, from 15.9% of total PTCs; 34 (43.0%) were IEFVPTCs, from 50.7% of total IEFVPTCs; and 4 (5.3%) comprised 1 each of FTCs, OCAs, ATCs, and MTCs, from 17.4% of all these carcinomas ( P < .001) ( Figure 1 and Table 1 ). Detection of RAS variants yielded a sensitivity of 22.6% (95% CI, 18.3%-27.0%), specificity of 88.8% (95% CI, 82.2%-95.3%), positive predictive value (PPV) of 88.8% (95% CI, 82.2%-95.3%), and negative predictive value (NPV) of 22.6% (95% CI, 18.3%-27.0%) in distinguishing malignant neoplasms from benign and NIFTP tumors (eTable 3 in Supplement 1 ). Notably, the VAF distribution of RAS variants was not statistically different among benign, NIFTP, and malignant neoplasms, as well as between PTCs and IEFVPTCs (eFigure 2 in Supplement 1 ), despite a high incidence of RAS variants in both NIFTPs (5 of 12 tumors [41.7%]) and IEFVPTCs (34 of 67 tumors [50.8%]). RAS variants, whether at a low or high VAF, were significantly associated with tumors undergoing partial thyroidectomy, with tumors absent for extrathyroidal extension, lymph node metastasis, capsular invasion, lymphatic invasion, or perineural invasion ( Table 1 ). In addition, RAS variants alone had a significant association with a low-risk recurrence of thyroid carcinomas ( Table 1 ).
Of 340 well-differentiated thyroid carcinomas, 77 (22.6%) were detected with RAS variants, including 46 (13.5%) with NRAS , 25 (7.4%) with HRAS , and 6 (1.8%) with KRAS . In addition, interpatient variabilities of BRAF V600E and TERT promoter variants (C228T and C250T) were detected in 173 (50.9%) and 55 (16.2%) carcinomas, respectively, with 45 (13.2%) of them in coexistence. Hence, there were 100 carcinomas (29.4%) with neither RAS nor BRAF V600E or TERT promoter variants ( Figure 1 ; eTable 1 in Supplement 1 ). RAS variants were distributed in 41 PTCs, including 34 classical subtypes (CPTCs), 3 infiltrative follicular subtypes (IFPTCs), and 4 tall, hobnail, or columnar cell subtypes (thcPTCs); 34 IEFVPTCs; and 1 each of FTC and OCA ( P < .001) (eTable 1 in Supplement 1 ). Of 41 RAS variant PTCs, 6 (14.6%) coharbored BRAF V600E alone: 4 in CPTCs and 1 in each of IFPTC and thcPTC; 4 (9.8%) coharbored TERT promoter variants alone: 3 in CPTCs and 1 in thcPTC; and 2 (4.9%) coharbored both BRAF V600E and TERT promoter variants: 1 in each of CPTC and thcPTC. Of 34 RAS variant IEFVPTCs, 5 (14.7%) coexisted with BRAF V600E alone and 1 (2.9%) coexisted with both BRAF V600E and TERT promoter variants. For an additional 2 carcinomas with RAS variants, 1 in ATC was found coexisting with both BRAF V600E and TERT promoter variants, and the other in MTC coexisting with BRAF V600E alone. Of 57 malignant tumors harboring RAS variants alone, 29 (50.9%) were found in PTCs, with 26 CPTCs, 2 IFPTCs, and 1 thcPTC, and 28 (49.1%) were found in IEFVPTCs. No RAS variants were detected in the 2 CMTC tumors, but 1 CMTC tumor presented with the coexistence of BRAF V600E and TERT promoter variants. The inclusion of RAS variants into BRAF and TERT variant assays reached a sensitivity of 70.5% (95% CI, 65.4%-75.2%) and a specificity of 88.8% (95% CI, 79.8%-94.1%), with a PPV of 96.1% (95% CI, 92.7%-98.0%) and an NPV of 43.4% (95% CI, 36.2%-50.9%) in distinguishing malignant neoplasms from benign and NIFTP tumors. This represents a 30.2% increase in sensitivity but a 11.2% decrease in specificity compared with BRAF and TERT variant assays alone, which had a sensitivity of 54.2% (95% CI, 48.8%-59.4%) and specificity of 100% (95% CI, 94.8%-100%) (eTable 3 in Supplement 1 ).
VAF assays of 249 residual FNA specimens identified 36 specimens (14.5%) with RAS variants with interpatient variabilities (including 23 FNAs [9.2%] with NRAS , 10 FNAs [4.0%] with HRAS , and 3 FNAs [1.2%] with KRAS) , 50 specimens (20.1%) with BRAF V600E, and 25 FNAs (10.0%) with TERT promoter variants ( Figure 2 and Table 2 ). Of 36 FNA specimens with RAS variants, 28 (77.8%) had RAS variants alone in various BSRTC categories (4 ND, 9 AUS, 1 FN, 5 SFM, 5 malignant, and 1 benign); 5 (13.9%) coexisted with BRAF V600E: 1 AUS, 2 SFM, and 2 malignant; and 3 (8.3%) coexisted with TERT promoter variants: 1 FN and 2 SFM. Interpatient differences in the 5 gene variants ( NRAS, HRAS, KRAS, BRAF , and TERT ) were detected in 54 of 126 indeterminate FNAs (42.9%) and 18 of 76 ND FNAs (23.7%). During a median (IQR) follow-up of 88 (50-156) days for patients who underwent resections, VAF assays of 71 residual FNAs achieved a sensitivity of 56.6% (95% CI, 42.4%-69.9%), specificity of 100% (95% CI, 85.9%-100%), PPV of 100% (95% CI, 85.9%-100%), and NPV of 43.9% (95% CI, 28.8%-60.1%) in differentiating malignancy based on their surgical pathological findings (eTable 3 in Supplement 1 ). Of these FNAs, 12 (16.9%) had RAS variants (9 with RAS variants alone and 3 coexisting with BRAF V600E). Histopathologic outcomes confirmed all 12 (25.4%) nodules were malignant neoplasms, including 5 CPTCs and 7 IEFVPTCs ( Figure 3 ; eTable 2 in Supplement 1 ). All FNAs with RAS variants coexisting with BRAF V600E (except for the patient with AUS, who was not available for follow-up) were subsequently found as IEFVPTC. In addition, among 18 nodules (25.4%) identified without RAS variants but with BRAF V600E or TERT promoter variants in the prior FNAs (9 with BRAF V600E alone, 3 with TERT promoter alone, and 4 in coexistence with both variants), 14 were subsequently found as PTC, with 12 for CPTC and 2 for thcPTC; 2 were found as ATC; and 1 each was found as IEFVPTC and OCA. Among 41 nodules (57.8%) identified with neither RAS , BRAF V600E, nor TERT promoter variants, 17 were benign tumors, 1 was NIFTP, 14 were CPTCs, and 9 were IEFVPTCs. Of note, 1 nodular goiter with an NRAS variant in its prior FNA was later confirmed as CPTC. Compared with the 5 gene variants detected in the matched surgical specimens, VAF assays on residual FNA biopsies exhibited a high agreement (κ = 0.799; P < .001) ( Figure 3 ) and demonstrated a sensitivity of 87.1% (95% CI, 69.2%-95.8%), specificity of 92.5% (95% CI, 78.5%-98.0%), PPV of 90.0% (95% CI, 72.3%-97.4%), and NPV of 90.2% (95% CI, 75.9%-96.8%).
In this diagnostic study, interpatient variabilities in RAS variants were delineated in thyroid tumors with VAFs ranging from 0.15% to 51.53% using sensitive VAF assays. While RAS variants alone, regardless of the VAF levels, were associated with thyroid cancer in 88.8% of thyroid nodules harboring such variants, they did not definitively distinguish malignant tumors from NIFTP and benign ones. However, they did facilitate the stratification of low-risk tumors from high-risk ones among malignant neoplasms. Furthermore, interpatient differences in the 5 gene variants were discriminated in 42.9% of indeterminate FNAs, 23.7% ND FNAs, and all FNAs with follow-up surgical pathology-confirmed malignancy.
Currently, molecular assays of RAS variants do not effectively risk stratify tumors due to their limited sensitivities and specificities. 27 , 28 In our study, the sensitive VAF assays identified substantial interpatient differences in the most common RAS gene variants, including 57.3% of NRAS variants in predominance, 33.7% of HRAS variants, and 9.0% of KRAS variants. In a comparable PTC cohort from The Cancer Genome Atlas study, the prevalence of RAS variants was 12.9% in PTCs, including 8.5% with NRAS , 3.5% with HRAS , and 1.0% with KRAS , based on NGS assays. 11 In contrast, our study observed a prevalence of 23.1% for RAS variants in PTCs, classified by the 2017 WHO classification, 29 including 13.5% with NRAS , 7.4% with HRAS , and 2.2% with KRAS (eFigure 3 in Supplement 1 ), suggesting that VAF assays revealed higher frequencies of RAS variants in thyroid neoplasms. Hence, significant discrepancies from different methods of detecting genomic variants may result in false-negative results or missed diagnoses of clinical significance, particularly when methods with lower sensitivities are used. 28 , 30 In addition, a high agreement observed in VAF assays between residual FNA biopsies and matched surgical specimens underscores the clinical significance of using residual specimens. At a direct cost of $12.36 per laboratory-developed test reaction coupled with a turnaround time within 8 hours from specimen receipt to result (eTable 4 in Supplement 1 ), this approach facilitates the timely and rapid delivery of molecular results concurrently with cytological examination on the same source biopsies, holding promise as an effective addition to existing protocols for personalized thyroid cancer care.
High rates of RAS variants were identified in lesions exhibiting follicular architecture, such as NIFTP (41.7%) and IEFVPTC (50.7%). It is noteworthy that 70.6% of CPTCs carrying RAS variants exhibited a predominantly follicular growth pattern, with most of them presenting encapsulation. Unfortunately, discriminating variant differences did not improve the stratification power of RAS variants in distinguishing between malignant neoplasms and NIFTPs, follicular adenomas, or oncocytic adenomas, nor between lesions exhibiting differential follicular architecture, such as NIFTP and IEFVPTC neoplasms. The limited effectiveness of RAS variants in stratifying these histological types may be attributed to their close similarity in gene expression profiles. 27 , 31 , 32 Moreover, low VAF events of RAS variations, including those at VAF less than 1%, were associated with an equally high risk of cancer as high VAF events. This finding aligns with that of a 2017 study that reported an equivalent malignancy rate in RAS variants detected at VAF less than 10% compared with variants detected at VAF greater than 10%. 8 Further studies are needed to elucidate the biological role and clinical significance of the different extents of RAS variations in tumor development. 33 - 35
The widespread implementation of molecular assays as routine cancer diagnosis remains a challenge. First, interpretation of genomic variations can be complex and may vary due to interpatient differences in such variants. 36 - 38 RAS variants alone, including the low VAF events, do not confirm the malignancy of an unknown tumor; therefore, they should not solely dictate clinical decisions. 39 However, RAF variants do enhance the stratification of low-risk tumors, 12 , 27 aiding in informing the extent of operation. Second, BRAF V600E and TERT promoter variants were detected exclusively in malignant tumors and exhibited a stronger association with aggressive tumor behaviors, aligning with our prior findings and those of other studies. 20 , 21 , 40 , 41 The inclusion of RAS variants into BRAF V600E and TERT promoter variant assays significantly enhanced the sensitivity for malignancy detection, albeit with a trade-off of reduced specificity. In addition, RAS variants coexisting with BRAF V600E and/or TERT promoter variants tend to be enriched in high-risk cancers, such as thcPTC, FTC, OCA, ATC, and MTC. 42 - 44 Third, search for novel molecular markers is needed to screen the rest 29.5% of malignant tumors and 64.4% of thyroid nodules that did not have BRAF V600E, TERT promoter variants, or RAS variants. Hence, leveraging NGS with a high-fidelity read capability may help identify additional actionable molecular alterations for detecting malignancy among tumors negative for RAS, BRAF , and TERT variants.
This study has some limitations. This study was conducted at a single center, where our surgical tumor cohort exhibited a high tumor malignancy rate, potentially contributing to the observed high prevalence of RAS variants in malignant tumors. With its ultrasensitivity in absolute quantification, VAF assay stands out as a favorable choice for testing known and definitive biomarkers, be they single variants or a small panel of variants, particularly when dealing with low variant levels. However, VAF assay has a relatively limited capacity of detecting multiple genomic variants in a single reaction. To reinforce the clinical utility of our findings, further larger-scale multicenter validation is necessary, using sensitive VAF assays targeting RAS in conjunction with other genomic variants.
This diagnostic study delineated interpatient variabilities of RAS variants in thyroid tumors with various histopathological diagnoses. These findings suggest that discrimination of interpatient differences in RAS in combination with BRAF V600E and TERT promoter variants could facilitate cytology examinations in preoperative precision malignancy diagnosis among patients with thyroid nodules.
Accepted for Publication: March 18, 2024.
Published: May 17, 2024. doi:10.1001/jamanetworkopen.2024.11919
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Fu G et al. JAMA Network Open .
Corresponding Author: Guodong Fu, PhD, Mount Sinai Hospital, Sinai Health, 600 University Ave, Toronto, ON M5G 1X5, Canada ( [email protected] ; [email protected] ).
Author Contributions: Dr Fu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Fu, Witterick.
Acquisition, analysis, or interpretation of data: Fu, Chazen, MacMillan.
Drafting of the manuscript: Fu.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Fu.
Obtained funding: Fu.
Administrative, technical, or material support: Fu, Chazen.
Supervision: Fu, Witterick.
Conflict of Interest Disclosures: Dr Witterick reported owning stock in Proteocyte Diagnositcs, serving on an advisory board for Sanofi Canada, and receiving personal fees from Sanofi Canada, GSK Canada, and Medtronic Canada outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by The Harry Barberian Research Fund from the Department of Otolaryngology–Head & Neck Surgery of the University of Toronto (Dr Fu) and the Mount Sinai Hospital Foundation of Toronto Da Vinci Gala Fundraiser (Dr Witterick).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: David Nguyen, BASc, and the pathologists’ assistants at the Department of Pathology and Laboratory Medicine, Mount Sinai Hospital, Sinai Health, Toronto, Ontario, Canada, assisted in the collection of thyroid tumor specimens and clinical information without compensation. The Cytopathology Laboratory of the Department of Pathology and Laboratory Medicine, Mount Sinai Hospital, Toronto, Ontario, Canada, helped with the collection of the residual fine-needle aspiration materials without compensation. We appreciate the participation of all the patients in this study.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

WHO 2022 updates on follicular cell and c-cell derived thyroid neoplasm
Affiliations.
- 1 Department of Pathology, All India Institute of Medical Sciences, Rajkot, Gurjat, India.
- 2 King George's Medical University, Lucknow, India.
- 3 Department of Pathology, Sharda Hospital, Greater Noida, India.
- PMID: 38737660
- PMCID: PMC11080517
- DOI: 10.25122/jml-2023-0270
The latest edition of the WHO Classification of thyroid tumors was released in 2022 and incorporates novel concepts vital to patient management. Thyroid follicular nodular disease is a term used to collectively represent a wide variety of benign and non-neoplastic lesions, including both clonal and non-clonal proliferations that manifest clinically as multinodular goiter. Thyroid neoplasms develop from follicular cells and can be either benign, low-risk, or malignant. To avoid classifying all lesions under 1 cm in diameter as low-risk illnesses, the new classification method highlights the need for subtyping papillary thyroid cancer based on histomorphologic indicators rather than tumor size. Formerly known as the cribriform-morular variety of papillary thyroid carcinoma, this tumor is now more commonly referred to by its more accurate name, cribriform-morular thyroid carcinoma. Its histogenesis is unknown. Similar to the traditional definition of 'poorly differentiated thyroid carcinoma' according to the Turin criteria, the newly defined 'differentiated high-grade thyroid carcinoma' encompasses papillary thyroid cancer, follicular thyroid carcinomas, and oncocytic carcinomas with high-grade characteristics linked to worse prognosis. The squamous cell subtype of anaplastic thyroid cancer has also recently been characterized as a distinct morphologic pattern. In this article, we will discuss the latest revision to the World Health Organization's classification system for thyroid cancer.
Keywords: C-cell neoplasm; Follicular cell neoplasm; Thyroid neoplasm.
© 2024 by the authors.
- Adenocarcinoma, Follicular* / pathology
- Thyroid Neoplasms* / classification
- Thyroid Neoplasms* / diagnosis
- Thyroid Neoplasms* / pathology
- World Health Organization*
Management of low-risk papillary thyroid cancer. Minimally-invasive treatments dictate a further paradigm shift?
- Mini Review
- Open access
- Published: 20 May 2024
Cite this article
You have full access to this open access article

- E. Papini 1 ,
- R. Guglielmi 1 ,
- R. Novizio 2 ,
- A. Pontecorvi 2 &
- C. Durante 3
Current management options for PTMC include lobo-isthmectomy and active surveillance (AS). Recently, ultrasound-guided minimally invasive procedures (MITs) are offered as a nonsurgical therapy for PTMC because they do not require hospitalization and general anaesthesia, and do not result in loss of thyroid function or cosmetic damage. MITs are reported to consistently provide, mostly in large retrospective series of patients, a rapid, safe, and cost-effective way to eradicate low-risk thyroid malignancies. However, conclusive data from well-conducted prospective studies on the histologically-proven completeness of tumor ablation and the long-term clinical advantages versus AS are still lacking.
This study aimed to evaluate the efficacy and safety of ultrasound-guided minimally invasive treatments (MITs) for PTMC in comparison to traditional surgical methods and active surveillance, and to assess their role in current clinical practice.
A structured literature review was conducted using keywords related to PTMC, MIT, and comparative techniques. Studies were evaluated based on treatment modality, patient selection, follow-up duration, complication rates, and clinical outcomes.
MITs have shown promising results in the management of PTMC. These treatments offer several advantages over surgery, such as reduced use of surgical resources, lower costs, minimal work disruption, and fewer major complications. However, there are still limitations, including the need for long-term surveillance and the potential risk of incomplete tumor ablation.
Conclusions
MITs represent a promising non-surgical option for managing low-risk PTMC, especially for patients ineligible for or refusing surgery. Despite favorable outcomes, more robust prospective data are needed to confirm their long-term benefits and completeness of tumor ablation. Interdisciplinary discussions and thorough patient education on the advantages and limitations of MITs are crucial for informed decision-making.
Avoid common mistakes on your manuscript.
The diagnosis of papillary thyroid carcinoma (PTC) has become more frequent during the last decades due to the widespread availability of neck imaging techniques, routine use of ultrasound (US)-guided fine needle aspiration biopsy (FNA), and, presumably, to the actual increase in the incidence of differentiated thyroid tumors [ 1 ]. Almost half of these cancers are under 10 mm in size and slow-growing, often incidental, and associated with a favorable prognosis. The majority of these tumors are low-risk papillary thyroid carcinomas (PTC), lacking aggressive histological features, extrathyroidal spread, nodal or distant metastasis, and worrisome mutations. These very low-risk cancers are generally referred to as papillary thyroid microcarcinomas (PTMC) in the literature, although the 2022 WHO classification does not consider them as distinct pathological conditions [ 2 ].
Currently, there is robust evidence supporting a minimalistic approach to the management of PTMC. The recommended surgical treatment for unifocal PTMC is lobo-isthmectomy but active surveillance (AS) is increasingly being proposed as an alternative management option. Several long-term studies, conducted in various thyroid centers across different countries, have demonstrated the safety of clinical and US monitoring without immediate intervention [ 3 , 4 , 5 , 6 , 7 ]. Recently, a third management option has been proposed to prevent the risk of overtreatment of clinically insignificant tumors and is now being tested worldwide [ 8 ]. Minimally invasive treatments (MIT), performed under US guidance, are established as effective and safe therapeutic tools for symptomatic benign thyroid nodules [ 9 , 10 , 11 , 12 ]. MITs are now being considered also as a possible nonsurgical therapy for PTMC because they do not require hospitalization, general anaesthesia, and do not result in loss of thyroid function or cosmetic damage [ 9 , 11 , 12 , 13 ]. Over the last 10 years, several clinical studies have shown that thermal ablation procedures—performed either with laser (LTA), radiofrequency (RFA), or microwaves (MWA) devices—can be used to selectively ablate PTMC with a diameter of up to 10 mm. Thus, the choice of the most appropriate management—surveillance, thermal ablation, or surgery—for these typically indolent tumors necessitates a thorough assessment of individual needs, clinical circumstances, and preferences.
Aim of the present paper is to assess the level of evidence for the outcomes of MITs in the treatment of PTMC, in order to evaluate the advantages and limitations of these innovative techniques in comparison to surgery and AS and to define their present role in current clinical practice.
To conduct the literature review on the management of Papillary Thyroid Microcarcinoma (PTMC), especially focusing on minimally invasive treatments, a structured search strategy using both specific keywords and Boolean operators has been employed. Keywords such as “PTMC”, “Papillary Thyroid Microcarcinoma”, “Thermal ablation”, “Laser”, “Radiofrequency”, “Microwave”, “Active Surveillance” guided the initial search. The search included Boolean combinations such as (“Papillary Thyroid Microcarcinoma” OR “PTMC” OR “Low-risk Papillary Thyroid Carcinoma”) AND (“minimally invasive procedures” OR “thermal ablation” OR “minimally invasive treatment” OR “ablation” OR “ablat*”) and Thyroid NEAR/3 Ablation to focus on articles discussing specific techniques used in the minimally invasive approach. Additionally, the comparison between surgical and non-surgical techniques has been explored using the string “Thyroid Surgery” VS “Non-Surgical Techniques” AND “PTMC”, allowing for a focused retrieval of studies comparing these approaches. For broader technology-based searches, (“Papillary Thyroid Microcarcinoma” OR “PTMC” OR “PTCM” OR “Papillary Thyroid Carcinoma”) AND (“laser” OR “LTA” OR “LT” OR “radiofrequency” OR “RFA” OR “microwaves” OR “MWA”) has been used to include various technologies applied in PTMC treatments. To investigate the long-term efficacy and patient-centered outcomes, strings such as “Active Surveillance” AND (“Papillary Thyroid Microcarcinoma” OR “PTMC” OR “PTCM” OR “Papillary Thyroid Carcinoma”) AND “outcomes” and (“Cost-Effectiveness” OR “Quality of Life”) AND (“Thermal Ablation” OR “Minimally Invasive”) AND “Thyroid Cancer”.
Available evidence
After the initial feasibility studies and pilot trials [ 14 , 15 , 16 ], several studies, performed with various minimally invasive technologies, investigated the efficacy, safety, and long-term outcomes of these treatment. Table 1 includes the main studies on the use of MIT for low-risk PTMC and summarizes modalities of treatment, patients’ selection, follow-up duration, complications rate, and clinical outcomes.
Advantages of minimally invasive treatments versus surgery
The benefits of thermal ablation in the management of low-risk PTMC are potentially relevant and encourage considering them as an alternative to surgery in selected conditions:
No use of surgical resources. Reducing the number of thyroid surgeries for low-risk PTMC could led to more appropriate allocation of resources and time towards surgery for patients with advanced thyroid cancer or large goiter that cause local pressure symptoms [ 9 , 10 , 11 , 12 ].
Low cost of the procedure. Due to the absence of general anaesthesia, surgical staff employment, and in-hospital stay, a relevant decrease of the costs may be achieved in comparison to thyroidectomy [ 17 , 18 ].
Minimal loss of working days. The persistence of local symptoms is generally limited to 24–48 h and the general clinical conditions and working ability are only minimally affected by MIT [ 18 ].
Low risk of major complications. Lower occurrence of peri-operatory complications and of permanent dysphonia are consistently reported in comparison to surgery [ 19 ].
Loss of thyroid function. Replacement therapy is only anecdotally required after MIT while is necessary, over time, in a sizable number of patients treated with lobectomy [ 19 , 20 ].
Absence of cosmetic damage. Cervical scars are avoided without the need for complex, and more expensive, trans-axillary or trans-oral surgical techniques [ 18 , 20 , 21 , 22 ].
Advantages of minimally invasive treatments versus AS
A few advantages can be attributed to MITs due to the following considerations:
A linear growth of PTMC volume may occur in about 8% of AS patients and may eventually prompt a delayed surgical treatment [ 6 , 23 ].
A minority of patients, especially young subjects, may develop over time cervical lymph node metastases that could require a more extensive, and potentially more disfiguring, neck surgery [ 23 ].
A substantial number of patients in AS programs eventually undergo thyroidectomy for reasons unrelated to cancer growth, mostly anxiety due to the awareness of harboring an untreated malignancy [ 24 ].
It can be speculated that only a limited number of centers can offer a reliable life-long clinical and US surveillance for a steadily increasing number of patients.
A conclusive definition of the role of MITs as a first line therapeutic option in the management of PTMC still has limitations that should be specifically addressed in future trials:
Completeness of tumor ablation . The histological confirmation of the complete ablation of malignant tissue is based on few anecdotal cases and on minute series of patients who, for unrelated reasons, underwent thyroidectomy after MITs [ 15 , 25 ]. Only few papers report the regular use of FNA or core needle biopsy (CNB) for ruling out the persistence of viable tumor cells in the treated area (Table 2 ). A retrospective cohort study, performed with long-term US follow up and systematic assessment with CNB of the ablation zone provided reassuring information [ 26 ]. Conversely, the risk of an incomplete ablation cannot be excluded with certainty in the majority of MIT studies which used US evaluation alone for the clinical outcome assessment. This issue is of pivotal importance because the eradication of any viable tumor cell represents the major advantage of MITs versus AS in the management of PTMC. The challenges of achieving complete tumor ablation are further compounded by the following factors. First, nearly all the available series include a relevant number of very small size (5 mm or less in diameter) PTMC (Table 3 ). Due to the need of a 2 mm circumferential safety margin of ablation around the PTMC circumference [ 8 ] the risk of an oncologically incomplete ablation rapidly increases with the increment in tumor size and is, consequently, much greater for tumors close to 10 mm. Second, the location of PTMC in areas difficult to be treated—close to the trachea, major vessels, or laryngeal nerve course—may hamper the radicality of the ablation even after a well conducted hydrodissection. Third, in case of incomplete ablation, the sonographic changes induced by MIT might hinder the persistence or recurrence of the treated PTMC.
Risk of complications . Head-to-head randomized prospective studies comparing MITs vs lobectomy are lacking. The current evidence is mostly based on retrospective studies comparing two cohorts of patients after non-randomized enrolment (Table 4 ). Besides this methodological limitation, most available studies compare thermal ablation, performed with different technologies and variable modalities, to surgery performed with lobectomy, total thyroidectomy, or thyroidectomy with central compartment resection. Differences in complications are most significant when comparing MITs to the most extensive surgical options, favoring MITs, but diminish when compared to lobectomy, the current recommended choice for managing unifocal carcinomas.
Quality of life . Surgical interventions are expected to negatively affect quality of life. and a better tolerability of MIT procedures may be postulated. Yet, controlled studies comparing the peri-operative and long-term impact on the QoL of the three management alternatives with validated and internationally accepted questionnaires are scarce.
Long-term surveillance . Surgery rules out the risk of tumor recurrence in the affected lobe and provides accurate information about tumor multifocality, extrathyroidal extension, aggressive histology, or clinically significant lymph node involvement. If these data, which conclusively define the risk of recurrence, are lacking, a prolonged though not intensive clinical and sonographic follow-up is required after thermal ablation, similarly to AS.
Access to treatment : a limited number of centers currently offer MIT for treating PTMC and specific training courses and certifications are not available in most countries.
Conclusions for clinical practice
Thermal ablation is a promising approach for non-surgical management of low-risk PTMC. Minimally invasive treatments could provide a rapid, safe, and cost-effective way to eradicate these common malignancies. However, conclusive data from well-conducted prospective studies on the histologically-proven completeness of tumor ablation and the long-term clinical advantages versus active surveillance are still lacking.
Presently, MIT should be considered in high-volume thyroid centers for patients with PTMC who are not candidates for surgery or refuse it, but still seek treatment to decrease the risk of progressive growth or extrathyroidal spread of their malignancy over time.
The three available therapeutic options—lobectomy, thermal ablation, and active surveillance—should always be discussed in an interdisciplinary manner. This discussion should be based not only on the preliminary clinical and US staging but also on the patient’s preferences, available resources, and local expertise.
Patients undergoing MIT for PTMC should be fully informed about the advantages and limitations of the procedure. Due to the presently incomplete level of evidence, long-term clinical and US follow-up is still required.
D.S.A. McLeod, L. Zhang, C. Durante, D.S. Cooper, Contemporary debates in adult papillary thyroid cancer management. Endocr. Rev. 40 (6), 1481–1499 (2019). https://doi.org/10.1210/er.2019-00085 .
Article PubMed Google Scholar
Z.W. Baloch, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr. Pathol. 33 (1), 27–63 (2022). https://doi.org/10.1007/s12022-022-09707-3 .
M.J. Jeon, W.G. Kim, K.W. Chung, J.H. Baek, W.B. Kim, Y.K. Shong, Active surveillance of papillary thyroid microcarcinoma: where do we stand? Eur. Thyroid J. 8 (6) (2019). https://doi.org/10.1159/000503064 .
R.M. Tuttle et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol. Head Neck Surg. 143 (10) (2017). https://doi.org/10.1001/jamaoto.2017.1442 .
H.S. Oh et al. Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid (2018). https://doi.org/10.1089/thy.2018.0263 .
H. Oda, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid 26 (1), 150–155 (2016). https://doi.org/10.1089/thy.2015.0313 .
Article PubMed PubMed Central Google Scholar
E. Molinaro et al. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: experience at a single Italian Center. J. Clin. Endocrinol. Metab. 105 (3), p. e172–e180 (2020). https://doi.org/10.1210/clinem/dgz113 .
G. Mauri et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur. Thyroid J. 10 (3) (2021). https://doi.org/10.1159/000516469 .
E. Papini, H. Monpeyssen, A. Frasoldati, L. Hegedüs, 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in Benign thyroid nodules. Eur. Thyroid J. 9 (4) (2020). https://doi.org/10.1159/000508484 .
L.A. Orloff, et al. Radiofrequency ablation and related ultrasound‐guided ablation technologies for treatment of benign and malignant thyroid disease: an international multidisciplinary consensus statement. Head Neck 44 (3), 633–660 (2022). https://doi.org/10.1002/hed.26960 .
C.F. Sinclair et al. General principles for the safe performance, training and adoption of ablation techniques for benign thyroid nodules: an American Thyroid Association Statement. Thyroid® (2023). https://doi.org/10.1089/thy.2023.0281 .
J.H. Kim et al. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J. Radiol. 19 (4) (2018). https://doi.org/10.3348/kjr.2018.19.4.632 .
C. Durante et al. European Thyroid Association clinical practice guidelines for thyroid nodule management. Eur. Thyroid J. 12 (5) (2023). https://doi.org/10.1530/ETJ-23-0067 .
E. Papini et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid 21 (8) (2011). https://doi.org/10.1089/thy.2010.0447 .
R. Valcavi, S. Piana, G.S. Bortolan, R. Lai, V. Barbieri, R. Negro, Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid 23 (12) (2013). https://doi.org/10.1089/thy.2013.0279 .
W. Zhou, S. Jiang, W. Zhan, J. Zhou, S. Xu, L. Zhang, Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: preliminary results. Eur. Radiol. 27 (7), 2934–2940 (2017). https://doi.org/10.1007/s00330-016-4610-1 .
Gestione del nodulo benigno della tiroide causa di sintomi locali. https://www.associazionemediciendocrinologi.it/images/pubblicazioni/pos-stat/ultrashort-LGnodulo-tiroideo.pdf . Accessed 19 Aug 2023.
G. Papi, R. Novizio, M. Brunetti, G. Mauri, Impact of the introduction of minimally invasive treatments of the thyroid (MITT) for benign thyroid nodules in an Italian hospital: a cost-minimization analysis. Endocrine (2023). https://doi.org/10.1007/s12020-023-03403-w .
J.F. Wang et al. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin. Med. J. 130 (11) (2017). https://doi.org/10.4103/0366-6999.206347 .
A. Bergenfelz et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3660 patients. Langenbecks Arch. Surg. 393 (5) (2008). https://doi.org/10.1007/s00423-008-0366-7 .
C.M. Pacella et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients, J. Clin. Endocrinol. Metab. 100 (10) (2015). https://doi.org/10.1210/jc.2015-1964 .
J.H. Baek et al. Complications encountered in the treatment of benign thyroid nodules with us-guided radiofrequency ablation: a multicenter study. Radiology 262 (1) (2012). https://doi.org/10.1148/radiol.11110416 .
Y. Ito, A. Miyauchi, M. Kihara, T. Higashiyama, K. Kobayashi, A. Miya, Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 24 (1), 27–34 (2014). https://doi.org/10.1089/thy.2013.0367 .
Article CAS PubMed PubMed Central Google Scholar
A. Miyauchi, Y. Ito, H. Oda, Insights into the management of papillary microcarcinoma of the thyroid. Thyroid 28 (1), 23–31 (2018). https://doi.org/10.1089/thy.2017.0227 .
M. Tong, S. Li, Y. Li, Y. Li, Y. Feng, Y. Che, Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int. J. Hyperth. 36 (1) (2019). https://doi.org/10.1080/02656736.2019.1700559 .
X. Li et al. Sonographic evolution and pathologic findings of papillary thyroid cancer after radiofrequency ablation: a five-year retrospective cohort study. Thyroid® 34 (1), 54–63 (2024). https://doi.org/10.1089/thy.2023.0415 .
B. Xu, N.-M. Zhou, W.-T. Cao, S.-Y. Gu, Comparative study on operative trauma between microwave ablation and surgical treatment for papillary thyroid microcarcinoma. World J. Clin. Cases 6 (15), 936–943 (2018). https://doi.org/10.12998/wjcc.v6.i15.936 .
D. Teng, G. Sui, C. Liu, Y. Wang, Y. Xia, H. Wang, Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J. Cancer Res. Clin. Oncol. 144 (4), 771–779 (2018). https://doi.org/10.1007/s00432-018-2607-7 .
D.-K. Teng et al. Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine 64 (1), 109–117 (2019). https://doi.org/10.1007/s12020-019-01868-2 .
Article CAS PubMed Google Scholar
J. Li, Y. Liu, J. Liu, P. Yang, X. Hu, L. Qian, A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int. J. Hyperth. 36 (1), 639–645 (2019). https://doi.org/10.1080/02656736.2019.1626492 .
Article Google Scholar
W. Zhou, X. Ni, S. Xu, L. Zhang, Y. Chen, W. Zhan, Ultrasound-guided laser ablation versus surgery for solitary papillary thyroid microcarcinoma: a retrospective study. Int. J. Hyperth. 36 (1), 896–903 (2019). https://doi.org/10.1080/02656736.2019.1649475 .
L. Wang et al. Safety and efficacy of ultrasound-guided percutaneous thermal ablation in treating low-risk papillary thyroid microcarcinoma: a pilot and feasibility study. J. Cancer Res. Ther. 15 (7), 1522 (2019). https://doi.org/10.4103/jcrt.JCRT_214_19 .
M. Zhang et al. Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: results of over 5 years follow-up. Thyroid 30 (3), 408–417 (2020). https://doi.org/10.1089/thy.2019.0147 .
W.-W. Yue et al. US-guided microwave ablation of low-risk papillary thyroid microcarcinoma: longer-term results of a prospective study. J. Clin. Endocrinol. Metab. 105 (6), 1791–1800 (2020). https://doi.org/10.1210/clinem/dgaa128 .
S.J. Cho, S.M. Baek, H.K. Lim, K.D. Lee, J.M. Son, J.H. Baek, Long-term follow-up results of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: more than 5-year follow-up for 84 tumors. Thyroid 30 (12), 1745–1751 (2020). https://doi.org/10.1089/thy.2020.0106 .
J. Xiao et al. Efficacy and safety of ultrasonography-guided radiofrequency ablation for the treatment of T1bN0M0 papillary thyroid carcinoma: a retrospective study. Int. J. Hyperth. 37 (1), 392–398 (2020). https://doi.org/10.1080/02656736.2020.1752945 .
X.-J. Cao et al. Efficacy and safety of thermal ablation for treatment of solitary T1N0M0 papillary thyroid carcinoma: a multicenter retrospective study. Radiology 300 (1), 209–216 (2021). https://doi.org/10.1148/radiol.2021202735 .
X.-J. Cao et al. Ultrasound-guided thermal ablation for papillary thyroid microcarcinoma: a multicenter retrospective study. Int. J. Hyperth. 38 (1), 916–922 (2021). https://doi.org/10.1080/02656736.2021.1936218 .
Article CAS Google Scholar
X. Wang et al. Analysis and evaluation of the efficacy of ultrasound-guided microwave ablation for papillary thyroid microcarcinoma. Int. J. Hyperth. 38 (1), 1476–1485 (2021). https://doi.org/10.1080/02656736.2021.1988152 .
J. Xiao et al. Ultrasonography-guided radiofrequency ablation for the treatment of T2N0M0 papillary thyroid carcinoma: a preliminary study. Int. J. Hyperth. 38 (1), 402–408 (2021). https://doi.org/10.1080/02656736.2021.1895332 .
L. Zheng et al. Thermal ablation for papillary thyroid microcarcinoma located in the isthmus: a study with 3 years of follow-up. Future Oncol. 18 (4), 471–480 (2022). https://doi.org/10.2217/fon-2021-0463 .
X. Li et al. Ultrasound-guided thermal ablation of Bethesda IV thyroid nodules: a pilot study. Front. Endocrinol. 12 (2021). https://doi.org/10.3389/fendo.2021.674970 .
J. Wu et al. A feasibility study of microwave ablation for papillary thyroid cancer close to the thyroid capsule. Int. J. Hyperth. 38 (1), 1217–1224 (2021). https://doi.org/10.1080/02656736.2021.1962549 .
G. Mauri et al. Image-guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an Italian experience, Front. Endocrinol. 11 (2021). https://doi.org/10.3389/fendo.2020.575152 .
L. Yan, M. Zhang, Q. Song, Y. Luo, Ultrasound-guided radiofrequency ablation versus thyroid lobectomy for low-risk papillary thyroid microcarcinoma: a propensity-matched cohort study of 884 patients. Thyroid 31 (11), 1662–1672 (2021). https://doi.org/10.1089/thy.2021.0100 .
L. Yong-ping et al. The value of ultrasound guided laser ablation in papillary thyroid recurrence carcinoma: a retrospective, single center study from China. Front. Endocrinol. 13 (2022). https://doi.org/10.3389/fendo.2022.946966 .
L. Zhang, G.P. Zhang, W.W. Zhan, W. Zhou, The feasibility and efficacy of ultrasound-guided percutaneous laser ablation for multifocal papillary thyroid microcarcinoma. Front. Endocrinol. 13 (2022). https://doi.org/10.3389/fendo.2022.921812 .
Z.-L. Zhao et al. Upgraded hydrodissection and its safety enhancement in microwave ablation of papillary thyroid cancer: a comparative study. Int. J. Hyperth. 40 (1) (2023). https://doi.org/10.1080/02656736.2023.2202373 .
L. Zheng et al. Microwave ablation vs. surgery for papillary thyroid carcinoma with minimal sonographic extrathyroid extension: a multicentre prospective study. Eur. Radiol. 33 (1), 233–243 (2022). https://doi.org/10.1007/s00330-022-08962-6 .
L. Yan et al. Long-term outcomes of ultrasound-guided thermal ablation for the treatment of solitary low-risk papillary thyroid microcarcinoma. Ann. Surg. 277 (5), 846–853 (2023). https://doi.org/10.1097/SLA.0000000000005800 . May.
Z. Han et al. Safety and efficacy of microwave ablation for the treatment of low-risk papillary thyroid microcarcinoma: a prospective multicenter study. Eur. Radiol. 33 (11), 7942–7951 (2023). https://doi.org/10.1007/s00330-023-09802-x .
Download references
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and affiliations.
Department of Endocrinology and Metabolism, Regina Apostolorum Hospital, Albano, Rome, Italy
E. Papini & R. Guglielmi
Department of Endocrinology, Catholic University of The Sacred Heart, Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy
R. Novizio & A. Pontecorvi
Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy
You can also search for this author in PubMed Google Scholar
Contributions
All authors equally contributed to the manuscript.
Corresponding author
Correspondence to C. Durante .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Papini, E., Guglielmi, R., Novizio, R. et al. Management of low-risk papillary thyroid cancer. Minimally-invasive treatments dictate a further paradigm shift?. Endocrine (2024). https://doi.org/10.1007/s12020-024-03864-7
Download citation
Received : 25 March 2024
Accepted : 05 May 2024
Published : 20 May 2024
DOI : https://doi.org/10.1007/s12020-024-03864-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Papillary thyroid cancer
- Minimally invasive procedures
- Thermal ablation
- Radiofrequency
- Find a journal
- Publish with us
- Track your research
Thyroid Research
Aims and scope.
Thyroid Research is an online journal that aims to present the newest knowledge related to thyroid hormones, thyroid diseases and any related fields. The journal’s regular readership includes researchers, clinicians and healthcare providers across the world.
Thyroid Research encompasses a wide range of thyroidology topics including, diagnosis, pharmacological and other treatment methods, invasive treatment, physiological mechanisms of thyroid hormone action and regulation, immunological aspects, genetics, new guidelines in disease management, thyroid related diseases and complications.
Unraveling thyroid cancer management: from molecular insights to clinical practice
Edited by: Antonio Matrone, MD, PhD, University of Pisa, Italy Submission Status: Open until 31 October 2024
The thyroid in pregnancy and infertility - challenges, diagnostics, and treatment
Sofie Bliddal, MD, PhD, Copenhagen University Hospital, Denmark
- Most accessed
Multi-element analysis of metals in human pathological and unchanged thyroid glands – pilot study
Authors: Aleksandra Kuzan, Justyna Rewak-Soroczyńska, Marta Kardach, Emilia Królewicz, Krzysztof Kaliszewski and Rafał Wiglusz
A case of hyalinizing trabecular tumor of the thyroid: diagnostic significance of PAX8-GLIS3 fusion
Authors: Shuto Hayashi, Nobuyuki Bandoh, Shogo Baba, Misaki Hayashi, Takashi Goto, Miki Takahara, Yasutaka Kato, Eriko Aimono and Hiroshi Nishihara
TERT RNAscope analysis of sub-centimetric papillary thyroid carcinomas and synchronous lymph node metastases
Authors: Marie-Lisa Eich, Wiebke Jeske, Uschi Zenz, Costanza Chiapponi, Christina Alidousty, Sabine Merkelbach-Bruse, Reinhard Büttner and Anne M. Schultheis
Novel molecular typing reveals the risk of recurrence in patients with early-stage papillary thyroid cancer
Authors: Mingyu Sun, Bingqing Zhao, Tao Chen, Lijun Yao, Xiaoxin Li, Shaojun Hu, Chengling Chen, Xinbao Gao and Chuangang Tang
Evaluating the progression to abnormal thyrotropin in euthyroid preconception women: a population-based study
Authors: Rili Gao, Xinyi Lyu, Ying Yang, Jinrong Fu, Chuanyu Zhao, Haixia Guan and Xu Ma
Most recent articles RSS
View all articles
The upper limit for TSH during pregnancy: why we should stop using fixed limits of 2.5 or 3.0 mU/l
Authors: Tim I. M. Korevaar
Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis
Authors: Theodore T Zava and David T Zava
The thyroid gland and the process of aging; what is new?
Authors: Adam Gesing, Andrzej Lewiński and Małgorzata Karbownik-Lewińska
The mechanisms of atrial fibrillation in hyperthyroidism
Authors: Agata Bielecka-Dabrowa, Dimitri P Mikhailidis, Jacek Rysz and Maciej Banach
Hashimoto thyroiditis is more frequent than expected when diagnosed by cytology which uncovers a pre-clinical state
Authors: Anca Staii, Sarah Mirocha, Kristina Todorova-Koteva, Simone Glinberg and Juan C Jaume
Most accessed articles RSS
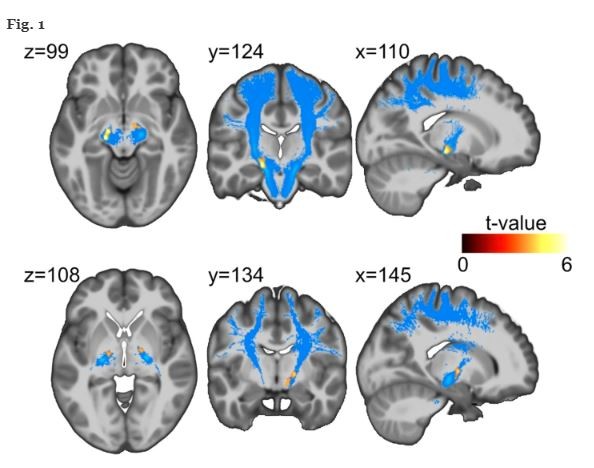
Featured Article: Changes in brain structure in subjects with resistance to thyroid hormone due to THRB mutations
Being critical for brain development and neurocognitive function thyroid hormones may have an effect on behaviour and brain structure. Our exploratory study aimed to delineate the influence of mutations in the thyroid hormone receptor (TR) ß gene on brain structure.
Featured Article: Thyroid storm in pregnancy: a review
There is a critical need to recognize thyroid storm during pregnancy and initiate proper medical interventions promptly.
Thyroid Research’s 15th anniversary
Join our 15th anniversary celebration by discovering Thyroid Research’s collection of most influential articles. We would like to thank everyone who helped to achieve the goals of Thyroid Research.

- About cancer
- Get involved
- Our research
- Funding for researchers
- Cancer types
- Cancer in general
- Causes of cancer
- Coping with cancer
- Health professionals
- Do your own fundraising
- By cancer type
- By cancer subject
- Our funding schemes
- Applying for funding
- Managing your research grant
- How we deliver our research
- Find a shop
- Shop online
- Our eBay shop
- Our organisation
- Current jobs
- Cancer news
Thyroid cancer
The thyroid is a small butterfly shaped gland that makes and releases hormones. It’s found at the front, lower part of your neck, just behind the small hollow where your collar bones meet.
Thyroid cancer is quite rare. It’s more common in women than in men. There are different types of thyroid cancer and your treatment depends on what type you have.
What is thyroid cancer?
Thyroid cancer is when abnormal cells in the thyroid gland start to divide and grow in an uncontrolled way.
Symptoms of thyroid cancer
The symptoms of thyroid cancer include a lump in your neck, a hoarse voice, a sore throat or difficulty in swallowing.
Getting diagnosed with thyroid cancer
You usually start by seeing your GP. They will ask about your symptoms. They might refer you to a specialist and organise tests.
Survival for thyroid cancer
Survival for thyroid cancer depends upon the type and stage of your thyroid cancer. Survival is generally very good for papillary and follicular thyroid cancers.
Stages and types of thyroid cancer
The type of thyroid cancer refers to the type of cell the cancer started in. The stage of a cancer tells you its size and whether it has spread.
Treatment for thyroid cancer
Possible treatments include surgery, radiotherapy, targeted drugs and chemotherapy. What treatment you have depends on your type and stage of thyroid cancer.
Research and clinical trials for thyroid cancer
Researchers are looking at ways to improve the treatment for thyroid cancer.
Living with thyroid cancer
Practical and emotional support is available to help you cope with thyroid cancer.
Risks and causes of thyroid cancer
Some factors might increase your risk of developing thyroid cancer. These include your age, being very overweight and some non cancerous thyroid conditions.
It’s a worrying time for many people and we want to be there for you whenever - and wherever - you need us. Cancer Chat is our fully moderated forum where you can talk to others affected by cancer, share experiences, and get support. Cancer Chat is free to join and available 24 hours a day.
Visit the Cancer Chat forum
About Cancer generously supported by Dangoor Education since 2010.

Find a clinical trial
Search our clinical trials database for all cancer trials and studies recruiting in the UK
Cancer Chat forum
Talk to other people affected by cancer
Nurse helpline 0808 800 4040
Questions about cancer? Call freephone 9 to 5 Monday to Friday or email us
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 13, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Variations in telomere lengthening genes may predispose some people to papillary thyroid cancer
by Johns Hopkins University
Johns Hopkins Medicine researchers say they have found that specific variations in three genes related to maintaining the length of telomeres—the protective DNA endcaps on chromosomes—may explain up to 4.5% of papillary thyroid cancers. Papillary thyroid cancer is the most common form of thyroid cancer.
The findings, published online April 29 in The American Journal of Human Genetics , follow previous research results from the Johns Hopkins scientists that very long telomeres are linked to development of certain cancers.
"This study provides a better understanding of what may predispose some people to papillary thyroid cancer, including multiple individuals in families," says Mary Armanios, M.D., professor of oncology at the Johns Hopkins Kimmel Cancer Center, professor of genetic medicine, molecular biology and genetics, and pathology at the Johns Hopkins University School of Medicine, and director of the Telomere Center at Johns Hopkins.
"We now may be able to identify people who could benefit from closer monitoring for secondary cancers common among this population."
Of 18 people with variants in three telomere -related genes, 15 (83%) developed a second cancer. Most frequently, these cancers were melanoma, sarcoma and cancer of lymphocytes (such as lymphoma and multiple myeloma). Their family members , who carried the same gene variants, were also prone to papillary thyroid cancers as well as other malignancies.
However, Armanios cautions that more work is needed to understand how to interpret these sequence variants and the role of telomere length, and genetic counseling and testing is best reserved for people with papillary tumors and secondary cancers, and those with a family history of thyroid cancers.
Armanios adds, this research contributes to mounting evidence of the role of long telomeres and telomere lengthening as risk factors for development of cancer.
Thyroid cancer occurs in more than 40,000 people in the U.S. each year and is one of the top 10 cancers in women in the country. Early stage disease is nearly always treatable. Most (about 80%) of these cancers are papillary thyroid cancers.
Scientists have known that 5%–10% of papillary thyroid cancers are heritable in some families, and that people with the condition may be prone to developing other cancers.
The researchers say the findings suggest that a mechanism underlying this risk may be longer telomeres.
The scientists analyzed the genetic sequence of five genes related to telomere maintenance in 200 people with papillary thyroid cancer from 189 families who had volunteered to be included in a registry at the Ohio State University.
About one-quarter of the 200 people had hereditary thyroid cancer or secondary cancers to thyroid tumors, or were males who developed thyroid cancer at a young age. They found nine people from seven families (4.5% of the 200) had genetic variations in at least one of three genes (POT1, TINF2, or ACD) already linked to telomere maintenance.
Of the people with those gene variants, the scientists measured their telomere lengths and discovered that five of them had very long telomeres—longer than 90% of most people—and three had ultra-long telomeres—longer than 99% of the population.
In another group of 270 people with papillary thyroid cancer not included in the hereditary cancer registry, four of the 270 (1.5%) had variants in those same three genes .
Researchers Emily DeBoy, Anna Nicosia, Sandya Liyanarachchi and Sheila Iyer from Johns Hopkins, and Manisha Shah, Matthew Ringel and Pamela Brock from the Ohio State University contributed to the study.
Explore further
Feedback to editors
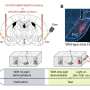
Male and female mice exhibit different empathic behaviors to others' pain
14 minutes ago
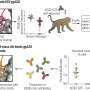
A boost for HIV vaccine research: Studies present comprehensive platform for validating next steps
15 minutes ago

Researchers develop wireless electronic suture for postoperative long-term monitoring of soft tissue
37 minutes ago

Researchers suggest brain scans for babies reduce risk of stroke later in life
Matcha mouthwash shown to inhibit bacteria that cause periodontitis

AI can help improve ER admission decisions, study finds

Why nightmares and 'daymares' could be early warning signs of autoimmune disease
15 hours ago

Yoga and meditation-induced altered states of consciousness are common in the general population, study says
17 hours ago
Second Phase 3 clinical trial again shows dupilumab lessens disease in COPD patients with type 2 inflammation

Study sheds light on bacteria associated with pre-term birth
18 hours ago
Related Stories

Long telomeres, the endcaps on DNA, not the fountain of youth once thought, and scientists may now know why
May 4, 2023
A 20-year study may upend long-held theory about chromosomes and cancer
Mar 30, 2023
Consumer Health: Understanding thyroid cancer risk
Jan 26, 2022

Treating thyroid cancer
Oct 2, 2023
Gene self-correction in 'chromosome caps' can beat mutations, help prevent blood cancers
Sep 8, 2021
Charting a new path for pediatric thyroid cancer treatment
Mar 1, 2022
Recommended for you
Benign nail condition linked to rare syndrome that greatly increases cancer risk
23 hours ago
Motivated by lack of diversity in neurological studies, scientists look to ensure equity in brain research
Scientists use new approach to disable brain cancer cells' ability to survive
22 hours ago

New mechanisms underlying tumor variety in brain cancers discovered
Body's 'message in a bottle' delivers targeted cancer treatment
Researchers reveal promising treatment target for resistant brain cancer
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Advances in Thyroid Cancer Research. Micrograph of medullary thyroid carcinoma. NCI-funded researchers are working to advance our understanding of how to treat thyroid cancer. The four main types of thyroid cancer are papillary , follicular , medullary , and anaplastic thyroid cancer. Most thyroid cancers are found at an early stage and can be ...
Thyroid cancer is the ninth most common cancer worldwide, the seventh most common cancer in US women, and the most common cancer in adolescents and adults younger than 40 years. 1,4 Data from the Surveillance, Epidemiology, and End Results 9 (SEER-9) cancer registry program shows that the incidence of thyroid cancer has increased from 1974-1977 ...
The past 5-10 years have brought in a new era in the care of patients with thyroid cancer, with the introduction of transformative diagnostic and management options. Several international ultrasound-based thyroid nodule risk stratification systems have been developed with the goal of reducing unnecessary biopsies. Less invasive alternatives to surgery for low-risk thyroid cancer, such as ...
Important research into thyroid cancer is being done right now in many university hospitals, medical centers, and other institutions around the country. Each year, scientists find out more about what causes the disease, how to prevent it, and how to improve treatment. In past years, for example, evidence has grown showing the benefits of ...
Guidelines from multiple societies across the world reflect these changes, which focus on taking a more individualized approach to clinical management. In this review, we discuss the current more personalized approach to patients with follicular cell-derived thyroid cancer and point toward areas of future research still needed in the field.
Thyroid cancer is the most common endocrine malignancy with almost one million people living with thyroid cancer in the United States. Although early-stage well-differentiated thyroid cancers account for the majority of thyroid cancers on diagnosis and have excellent survival rates, the incidence of advanced-stage disease has increased over the past few years and confers poorer prognosis ...
Research Highlights 02 Nov 2023 ... Routine post-operative administration of RAI is no longer indicated in patients with low risk thyroid cancer and might instead be used selectively in patients ...
Genomic data from patients with thyroid cancer, combined with information on mutation-specific mechanisms from experimental models, is transforming the thyroid cancer research field. This Review ...
Physiologically relevant preclinical mouse models and human correlative research studies will inform development of the next stage of immune-based therapies for patients with advanced thyroid cancer.
By Susan Buckles. Mayo Clinic researchers will jump four hurdles to apply chimeric antigen receptor-T cell therapy (CAR-T cell therapy) to solid tumors in thyroid cancer. This regenerative immunotherapy has shown promising results in blood cancers, and new research is focused on using this treatment on more types of malignancies.
Displaying 34 studies. Retrospective Review of Patients with Thyroid Disorders at Mayo Clinic Rochester, MN. The purpose of this study is to determine the optimal approach to the diagnosis and treatment of patients with thyroid disorders. A Study of the Impact of the Terminology Used to Identify Papillary Thyroid Cancer on the Treatment Options ...
There are four main types of thyroid cancer: papillary (the most common), follicular, medullary, and anaplastic. The four types differ in how aggressive they are. Start here to find information on thyroid cancer treatment, screening, research, and statistics.
The incidence of thyroid cancer continues to increase, representing the 5th most common cancer type in the USA today (Sherman, Lancet 361(9356):501-11, 2003). The current study sought to analyze the global burden of thyroid cancer utilizing the publicly accessible GLOBOCAN database. ... Previous research has revealed that 50% of thyroid ...
Clinical trials for thyroid cancer include testing new medications, including drugs known as targeted therapy. As explained in Types of Treatment, targeted therapy is a treatment that targets specific genes, proteins, or the tissue environment that contributes to cancer growth and survival. In addition, researchers are looking at new ...
Age-related variation in thyroid function - a narrative review highlighting important implications for research and clinical practice. Thyroid hormones are key determinants of health and well-being. Normal thyroid function is defined according to the standard 95% confidence interval of the disease-free population.
Thyroid cancer is the most common malignancy of the endocrine system, representing 3.8% of all new cancer cases in the United States and is the ninth most common cancer overall. The American Cancer Society estimates that 62,450 people in the United States will be diagnosed with thyroid cancer in 2015, and 1950 deaths will result from the ...
Thyroid Cancer(Papillary and Follicular) Thyroid cancer is relatively uncommon compared to other cancers. In the United States, it is estimated that in 2021 approximately 44,000 people will receive a new diagnosis of thyroid cancer, compared to over 280,000 with breast cancer and over 150,000 with colon cancer. However, despite this ...
Anaplastic thyroid cancer tends to occur in people older than 60. It can cause severe signs and symptoms, such as neck swelling that worsens very quickly and may lead to difficulty breathing and swallowing. Medullary thyroid cancer. This rare type of thyroid cancer begins in thyroid cells called C cells, which produce the hormone calcitonin.
Thyroid cancer is a malignancy of the thyroid parenchymal cells. The thyroid parenchyma consists of two major cell types, the thyroid follicular cells that give rise to differentiated thyroid cancer(DTC) and the parafollicular or C-cells that give rise to medullary thyroid carcinoma (MTC). DTC comprises papillary thyroid cancer(PTC), follicular thyroid cancer(FTC), and Hurthle cell cancer ...
Thyroid cancer is cancer of the butterfly-shaped gland at the base of the throat. The thyroid uses iodine, a mineral found in some foods and in iodized salt, to help make several hormones that control heart rate, body temperature, metabolism, and the amount of calcium in the blood. There are four main types of thyroid cancer: papillary thyroid ...
Thyroid cancer, especially papillary thyroid cancer (PTC), has experienced a rapid increase in incidence since the 1980s 1 and is primarily diagnosed through ultrasonographic examinations and fine-needle aspiration (FNA) biopsy of suspicious nodules. 2,3 However, approximately 30% of FNAs exhibit an indeterminate diagnosis, and 10% of findings ...
Help us end cancer as we know it, for everyone. Donate with Confidence. If you have thyroid cancer or are close to someone who does, knowing what to expect can help you cope. Here you can find out all about thyroid cancer, including risk factors, symptoms, how it is found, and how it is treated.
Thyroid neoplasms develop from follicular cells and can be either benign, low-risk, or malignant. To avoid classifying all lesions under 1 cm in diameter as low-risk illnesses, the new classification method highlights the need for subtyping papillary thyroid cancer based on histomorphologic indicators rather than tumor size.
The diagnosis of papillary thyroid carcinoma (PTC) has become more frequent during the last decades due to the widespread availability of neck imaging techniques, routine use of ultrasound (US)-guided fine needle aspiration biopsy (FNA), and, presumably, to the actual increase in the incidence of differentiated thyroid tumors [].Almost half of these cancers are under 10 mm in size and slow ...
Thyroid Research is an online journal that aims to present the newest knowledge related to thyroid hormones, thyroid diseases and any related fields. The journal's regular readership includes researchers, clinicians and healthcare providers across the world. Thyroid Research encompasses a wide range of thyroidology topics including, diagnosis, pharmacological and other treatment methods ...
Thyroid cancer is when cells in the thyroid divide and grow in an uncontrolled way. Find out about symptoms, diagnosis, treatment, survival and coping. ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee.
Armanios adds, this research contributes to mounting evidence of the role of long telomeres and telomere lengthening as risk factors for development of cancer. Thyroid cancer occurs in more than ...