Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Consensus Statement
- Published: 05 October 2023

Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine
- Deirdre K. Tobias 1 , 2 na1 ,
- Jordi Merino 3 , 4 , 5 na1 ,
- Abrar Ahmad 6 na1 ,
- Catherine Aiken 7 , 8 na1 ,
- Jamie L. Benham 9 na1 ,
- Dhanasekaran Bodhini ORCID: orcid.org/0000-0001-7194-0639 10 na1 ,
- Amy L. Clark 11 ,
- Kevin Colclough 12 na1 ,
- Rosa Corcoy 13 , 14 , 15 ,
- Sara J. Cromer 4 , 16 , 17 na1 ,
- Daisy Duan ORCID: orcid.org/0000-0002-4392-3206 18 na1 ,
- Jamie L. Felton 19 , 20 , 21 na1 ,
- Ellen C. Francis 22 na1 ,
- Pieter Gillard 23 na1 ,
- Véronique Gingras 24 , 25 na1 ,
- Romy Gaillard 26 na1 ,
- Eram Haider 27 na1 ,
- Alice Hughes ORCID: orcid.org/0000-0003-1352-4447 12 na1 ,
- Jennifer M. Ikle 28 , 29 na1 ,
- Laura M. Jacobsen 30 na1 ,
- Anna R. Kahkoska 31 na1 ,
- Jarno L. T. Kettunen ORCID: orcid.org/0000-0002-9995-698X 32 , 33 , 34 na1 ,
- Raymond J. Kreienkamp 4 , 5 , 16 , 35 na1 ,
- Lee-Ling Lim 36 , 37 , 38 na1 ,
- Jonna M. E. Männistö 39 , 40 na1 ,
- Robert Massey 27 na1 ,
- Niamh-Maire Mclennan 41 na1 ,
- Rachel G. Miller ORCID: orcid.org/0000-0003-1845-8477 42 ,
- Mario Luca Morieri 43 , 44 na1 ,
- Jasper Most 45 na1 ,
- Rochelle N. Naylor 46 na1 ,
- Bige Ozkan 47 , 48 na1 ,
- Kashyap Amratlal Patel ORCID: orcid.org/0000-0002-9240-8104 12 na1 ,
- Scott J. Pilla 49 , 50 na1 ,
- Katsiaryna Prystupa 51 , 52 na1 ,
- Sridharan Raghavan 53 , 54 na1 ,
- Mary R. Rooney 47 , 55 na1 ,
- Martin Schön 51 , 52 , 56 , 57 na1 ,
- Zhila Semnani-Azad 2 na1 ,
- Magdalena Sevilla-Gonzalez 16 , 17 , 58 na1 ,
- Pernille Svalastoga 59 , 60 na1 ,
- Wubet Worku Takele 61 na1 ,
- Claudia Ha-ting Tam ORCID: orcid.org/0000-0002-9169-0013 38 , 62 , 63 na1 ,
- Anne Cathrine B. Thuesen 3 na1 ,
- Mustafa Tosur 64 , 65 , 66 na1 ,
- Amelia S. Wallace 47 , 55 na1 ,
- Caroline C. Wang 55 na1 ,
- Jessie J. Wong 67 na1 ,
- Jennifer M. Yamamoto 68 na1 ,
- Katherine Young 12 na1 ,
- Chloé Amouyal 69 , 70 ,
- Mette K. Andersen 3 ,
- Maxine P. Bonham 71 ,
- Mingling Chen 72 ,
- Feifei Cheng 73 ,
- Tinashe Chikowore 17 , 74 , 75 , 76 ,
- Sian C. Chivers 77 ,
- Christoffer Clemmensen ORCID: orcid.org/0000-0003-2456-9667 3 ,
- Dana Dabelea 78 ,
- Adem Y. Dawed ORCID: orcid.org/0000-0003-0224-2428 27 ,
- Aaron J. Deutsch ORCID: orcid.org/0000-0001-6750-5335 5 , 16 , 17 ,
- Laura T. Dickens 79 ,
- Linda A. DiMeglio ORCID: orcid.org/0000-0002-8033-6078 19 , 20 , 21 , 80 ,
- Monika Dudenhöffer-Pfeifer 6 ,
- Carmella Evans-Molina ORCID: orcid.org/0000-0001-7764-8663 19 , 20 , 21 , 81 ,
- María Mercè Fernández-Balsells 82 , 83 ,
- Hugo Fitipaldi 6 ,
- Stephanie L. Fitzpatrick 84 ,
- Stephen E. Gitelman 85 ,
- Mark O. Goodarzi ORCID: orcid.org/0000-0001-6364-5103 86 , 87 ,
- Jessica A. Grieger 88 , 89 ,
- Marta Guasch-Ferré 2 , 90 ,
- Nahal Habibi 88 , 89 ,
- Torben Hansen ORCID: orcid.org/0000-0001-8748-3831 3 ,
- Chuiguo Huang 38 , 62 ,
- Arianna Harris-Kawano 19 , 20 , 21 ,
- Heba M. Ismail 19 , 20 , 21 ,
- Benjamin Hoag 91 , 92 ,
- Randi K. Johnson 93 , 94 ,
- Angus G. Jones ORCID: orcid.org/0000-0002-0883-7599 12 , 95 ,
- Robert W. Koivula 96 ,
- Aaron Leong 4 , 17 , 97 ,
- Gloria K. W. Leung 71 ,
- Ingrid M. Libman 98 ,
- Kai Liu 88 ,
- S. Alice Long ORCID: orcid.org/0000-0002-0281-1240 99 ,
- William L. Lowe Jr ORCID: orcid.org/0000-0001-9467-3422 100 ,
- Robert W. Morton 101 , 102 , 103 ,
- Ayesha A. Motala 104 ,
- Suna Onengut-Gumuscu ORCID: orcid.org/0000-0002-6563-8334 105 ,
- James S. Pankow ORCID: orcid.org/0000-0001-7076-483X 106 ,
- Maleesa Pathirana 88 , 89 ,
- Sofia Pazmino 107 ,
- Dianna Perez 19 , 20 , 21 ,
- John R. Petrie 108 ,
- Camille E. Powe 4 , 16 , 17 , 109 ,
- Alejandra Quinteros 88 ,
- Rashmi Jain 110 , 111 ,
- Debashree Ray ORCID: orcid.org/0000-0002-0979-2935 55 , 112 ,
- Mathias Ried-Larsen ORCID: orcid.org/0000-0002-8388-5291 113 , 114 ,
- Zeb Saeed 115 ,
- Vanessa Santhakumar 1 ,
- Sarah Kanbour 49 , 116 ,
- Sudipa Sarkar 49 ,
- Gabriela S. F. Monaco 19 , 20 , 21 ,
- Denise M. Scholtens ORCID: orcid.org/0000-0002-8252-7863 117 ,
- Elizabeth Selvin ORCID: orcid.org/0000-0002-3539-2070 47 , 55 ,
- Wayne Huey-Herng Sheu 118 , 119 , 120 ,
- Cate Speake ORCID: orcid.org/0000-0003-1480-4272 121 ,
- Maggie A. Stanislawski 93 ,
- Nele Steenackers 107 ,
- Andrea K. Steck 122 ,
- Norbert Stefan ORCID: orcid.org/0000-0002-2186-9595 52 , 56 , 123 ,
- Julie Støy 124 ,
- Rachael Taylor 125 ,
- Sok Cin Tye 126 , 127 ,
- Gebresilasea Gendisha Ukke 61 ,
- Marzhan Urazbayeva 65 , 128 ,
- Bart Van der Schueren 107 , 129 ,
- Camille Vatier 130 , 131 ,
- John M. Wentworth 132 , 133 , 134 ,
- Wesley Hannah 135 , 136 ,
- Sara L. White 77 , 137 ,
- Gechang Yu 38 , 62 ,
- Yingchai Zhang 38 , 62 ,
- Shao J. Zhou 89 , 138 ,
- Jacques Beltrand 139 , 140 ,
- Michel Polak 139 , 140 ,
- Ingvild Aukrust 59 , 141 ,
- Elisa de Franco 12 ,
- Sarah E. Flanagan 12 ,
- Kristin A. Maloney ORCID: orcid.org/0000-0002-8607-1146 142 ,
- Andrew McGovern 12 ,
- Janne Molnes 59 , 141 ,
- Mariam Nakabuye 3 ,
- Pål Rasmus Njølstad ORCID: orcid.org/0000-0003-0304-6728 59 , 60 ,
- Hugo Pomares-Millan 6 , 143 ,
- Michele Provenzano 144 ,
- Cécile Saint-Martin 145 ,
- Cuilin Zhang 146 , 147 ,
- Yeyi Zhu 148 , 149 ,
- Sungyoung Auh 150 ,
- Russell de Souza 102 , 151 ,
- Andrea J. Fawcett 152 , 153 ,
- Chandra Gruber 154 ,
- Eskedar Getie Mekonnen 155 , 156 ,
- Emily Mixter 157 ,
- Diana Sherifali 102 , 158 ,
- Robert H. Eckel 159 ,
- John J. Nolan 160 , 161 ,
- Louis H. Philipson 157 ,
- Rebecca J. Brown 150 na2 ,
- Liana K. Billings 162 , 163 na2 ,
- Kristen Boyle ORCID: orcid.org/0000-0001-9689-3322 78 na2 ,
- Tina Costacou 42 ,
- John M. Dennis ORCID: orcid.org/0000-0002-7171-732X 12 na2 ,
- Jose C. Florez ORCID: orcid.org/0000-0002-1730-9325 4 , 5 , 16 , 17 na2 ,
- Anna L. Gloyn ORCID: orcid.org/0000-0003-1205-1844 28 , 29 , 164 na2 ,
- Maria F. Gomez ORCID: orcid.org/0000-0001-6210-3142 6 , 165 na2 ,
- Peter A. Gottlieb 122 na2 ,
- Siri Atma W. Greeley 166 na2 ,
- Kurt Griffin 111 , 167 na2 ,
- Andrew T. Hattersley ORCID: orcid.org/0000-0001-5620-473X 12 , 95 na2 ,
- Irl B. Hirsch 168 na2 ,
- Marie-France Hivert ORCID: orcid.org/0000-0001-7752-2585 4 , 169 , 170 na2 ,
- Korey K. Hood 67 na2 ,
- Jami L. Josefson 152 na2 ,
- Soo Heon Kwak ORCID: orcid.org/0000-0003-1230-0919 171 na2 ,
- Lori M. Laffel 172 na2 ,
- Siew S. Lim 61 na2 ,
- Ruth J. F. Loos 3 , 173 na2 ,
- Ronald C. W. Ma ORCID: orcid.org/0000-0002-1227-803X 38 , 62 , 63 na2 ,
- Chantal Mathieu 23 na2 ,
- Nestoras Mathioudakis 49 na2 ,
- James B. Meigs 17 , 97 , 174 na2 ,
- Shivani Misra ORCID: orcid.org/0000-0003-2886-0726 175 , 176 na2 ,
- Viswanathan Mohan ORCID: orcid.org/0000-0001-5038-6210 177 na2 ,
- Rinki Murphy ORCID: orcid.org/0000-0002-0043-2423 178 , 179 , 180 na2 ,
- Richard Oram ORCID: orcid.org/0000-0003-3581-8980 12 , 95 na2 ,
- Katharine R. Owen ORCID: orcid.org/0000-0003-3982-1407 96 , 181 na2 ,
- Susan E. Ozanne ORCID: orcid.org/0000-0001-8753-5144 182 na2 ,
- Ewan R. Pearson ORCID: orcid.org/0000-0001-9237-8585 27 na2 ,
- Wei Perng 78 na2 ,
- Toni I. Pollin 142 , 183 na2 ,
- Rodica Pop-Busui 184 ,
- Richard E. Pratley 185 na2 ,
- Leanne M. Redman 186 na2 ,
- Maria J. Redondo 64 , 65 na2 ,
- Rebecca M. Reynolds 41 na2 ,
- Robert K. Semple ORCID: orcid.org/0000-0001-6539-3069 41 , 187 na2 ,
- Jennifer L. Sherr ORCID: orcid.org/0000-0001-9301-3043 188 na2 ,
- Emily K. Sims ORCID: orcid.org/0000-0002-4393-954X 19 , 20 , 21 na2 ,
- Arianne Sweeting 189 , 190 na2 ,
- Tiinamaija Tuomi ORCID: orcid.org/0000-0002-8306-6202 32 , 33 , 34 na2 ,
- Miriam S. Udler ORCID: orcid.org/0000-0003-3824-9162 4 , 5 , 16 , 17 na2 ,
- Kimberly K. Vesco 191 na2 ,
- Tina Vilsbøll 192 , 193 na2 ,
- Robert Wagner ORCID: orcid.org/0000-0002-6120-0191 51 , 52 , 194 na2 ,
- Stephen S. Rich ORCID: orcid.org/0000-0003-3872-7793 105 na2 &
- Paul W. Franks ORCID: orcid.org/0000-0002-0520-7604 2 , 6 , 96 , 103 na2
Nature Medicine volume 29 , pages 2438–2457 ( 2023 ) Cite this article
27k Accesses
235 Altmetric
Metrics details
- Medical research
- Translational research
Precision medicine is part of the logical evolution of contemporary evidence-based medicine that seeks to reduce errors and optimize outcomes when making medical decisions and health recommendations. Diabetes affects hundreds of millions of people worldwide, many of whom will develop life-threatening complications and die prematurely. Precision medicine can potentially address this enormous problem by accounting for heterogeneity in the etiology, clinical presentation and pathogenesis of common forms of diabetes and risks of complications. This second international consensus report on precision diabetes medicine summarizes the findings from a systematic evidence review across the key pillars of precision medicine (prevention, diagnosis, treatment, prognosis) in four recognized forms of diabetes (monogenic, gestational, type 1, type 2). These reviews address key questions about the translation of precision medicine research into practice. Although not complete, owing to the vast literature on this topic, they revealed opportunities for the immediate or near-term clinical implementation of precision diabetes medicine; furthermore, we expose important gaps in knowledge, focusing on the need to obtain new clinically relevant evidence. Gaps include the need for common standards for clinical readiness, including consideration of cost-effectiveness, health equity, predictive accuracy, liability and accessibility. Key milestones are outlined for the broad clinical implementation of precision diabetes medicine.
Similar content being viewed by others

Principal component-based clinical aging clocks identify signatures of healthy aging and targets for clinical intervention

Genome-wide association studies

Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial
Diabetes is a major global problem, with many hundreds of millions of people affected by the disease, many of whom are undiagnosed. The major burden of diabetes is exerted through the development of life-threatening complications, often involving damage to large and small blood vessels. The disease is currently classified into several types of diabetes. The two most common forms are type 1 diabetes (T1D), an autoimmune disease accounting for ~2% of all forms of diabetes worldwide 1 , and type 2 diabetes (T2D), which accounts for most of the remaining cases. Rare ‘monogenic’ forms of diabetes also exist, with gestational diabetes mellitus (GDM) being an additional category (Box 1 ). A major challenge with most diabetes is that it is heterogeneous in etiology, clinical presentation and prognosis. Understanding and leveraging this heterogeneity is a core objective of precision diabetes medicine (Fig. 1 ).
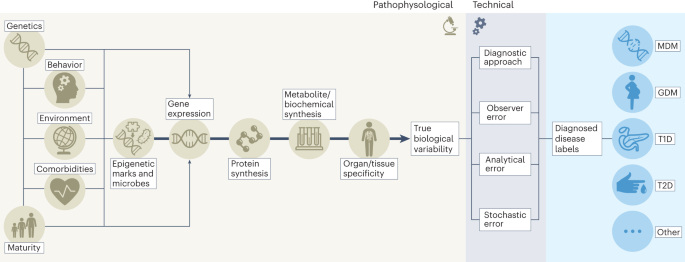
The success of precision diabetes medicine will be enhanced by successfully leveraging heterogeneity in diabetes. To do so will require parsing ‘signal’ from ‘noise’; the figure illustrates the key sources of heterogeneity within each of these domains.
This second international consensus report from the Precision Medicine in Diabetes Initiative (PMDI) summarizes the comprehensive systematic reviews and resulting consensus among the PMDI consortium for the pillars of precision medicine prevention, diagnosis, treatment and prognosis 2 across monogenic diabetes mellitus (MDM), GDM, T1D and T2D 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 (Fig. 2 ). The objectives of the PMDI consortium were to identify (1) where current evidence supports the application of precision approaches in diabetes prevention and care, and (2) key gaps where additional and/or higher quality evidence is needed before precision medicine can be implemented. Areas of consensus for these objectives are reflected in key milestones put forth to support the evidence-based and scalable implementation of precision diabetes medicine within the next decade.
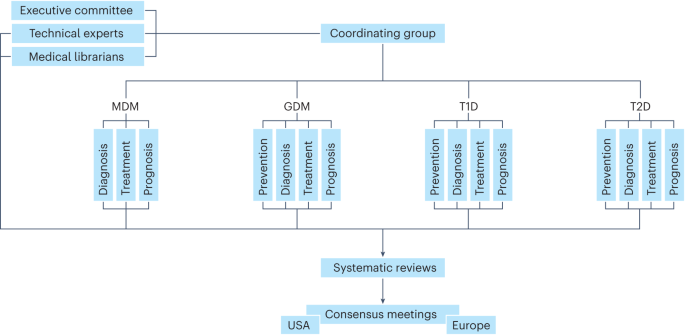
Organizational structure of the PMDI consortium during the systematic review and consensus report processes.
The PMDI was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes to address the untenable health and economic burdens of diabetes prevention and care 17 . The first consensus report on precision medicine in diabetes published in 2020 (ref. 2 ) highlighted that precision medicine involves tailored diagnostics or therapeutics (for prevention or treatment) applied to population subgroups sharing similar characteristics, thereby minimizing error and risk while maximizing efficacy (Box 2 ). Four key pillars of precision medicine were also defined: prevention, diagnosis, treatment and prognosis, which can be applied throughout the life course (Fig. 3 ).
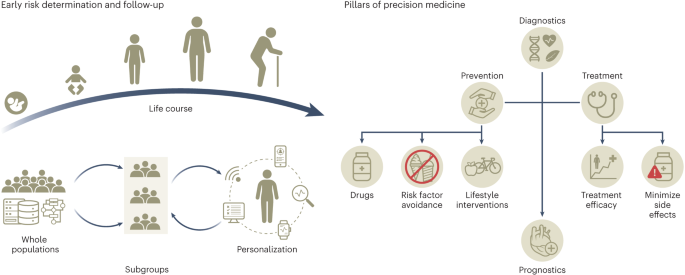
The collection of deep phenotypic, environmental and social data through the life course will allow the generation of prediction algorithms to identify individuals with shared characteristics that are more likely to benefit from targeted screening and preventive and treatment strategies for the prevention, diagnosis and management of diabetes.
The data inputs, technologies and tools for subgroup characterization are incredibly diverse and readiness for valid and cost-effective implementation in diabetes medicine varies widely. The first consensus report concluded with a call for a rigorous review elucidating effective precision medicine strategies, areas of promise and notable gaps across MDM, GDM, T1D and T2D to inform an evidence-based road map to optimize the integration of precision medicine into the global response to the diabetes crisis.
The key findings of this second consensus report are that, within the areas examined, several actionable and near-actionable examples of precision diabetes medicine exist. However, the quality of data is generally low, and few studies have been explicitly designed to test precision medicine hypotheses. There is also a dearth of relevant, high-quality research in people of non-European ancestry, hindering the development and implementation of precision diabetes medicine in many of the most heavily burdened populations worldwide.
Box 1 Contemporary diagnostic definitions of the established forms of diabetes
Based on the ADA Standards of Care 2022, diabetes can be classified into the following general categories:
T1D is a disease caused by autoimmune damage of the insulin-producing β-cells of the pancreatic islets, usually leading to absolute endogenous insulin deficiency, including latent autoimmune diabetes of adulthood.
T2D is a disease characterized by a progressive loss of adequate β-cell insulin secretion frequently in the presence of excess adiposity and insulin resistance.
GDM is a disease characterized by persistent hyperglycemia, often diagnosed in the second or third trimester of pregnancy, which was not determined to be prepregnancy diabetes.
Other rarer types of diabetes include:
MDM, which represents a rare form of diabetes due to specific genetic defects that cause β-cell dysfunction with minimal or no defects in insulin action and include neonatal diabetes and maturity-onset diabetes of the young.
Secondary forms of diabetes, such as diabetes due to other causes such as the exocrine pancreas (for example, cystic fibrosis) and pancreatitis and drug- or chemical-induced diabetes (such as with glucocorticoid use, in the treatment of HIV/AIDS or after organ transplantation).
Box 2 Revisions to definitions described in the first PMDI consensus report
Several definitions used in the first consensus report on precision diabetes medicine 2 are revised here to (1) highlight key benchmarks used to determine the success of precision diabetes medicine in practice and (2) distinguish individual-level processes that can be objectively quantified and incorporated into prediction models from those that cannot, yet are integral to the transfer of medical or health recommendations to recipients.
The following terms are revised:
Precision medicine:
From: “Precision (or stratified) medicine emphasizes tailoring diagnostics or therapeutics (prevention or treatment) to subgroups of populations sharing similar characteristics, thereby minimizing error and risk while maximizing efficacy” 2
To: “Precision medicine focuses on minimizing errors and improving accuracy in medical decisions and health recommendations. It seeks to maximize efficacy, cost-effectiveness, safety, access for those in need and compliance compared with contemporary evidence-based medicine. Precision medicine emphasizes tailoring diagnostics or therapeutics (prevention or treatment) to subgroups of populations sharing similar characteristics.”
Personalized and individualized medicine:
From: both terms are used interchangeably, defined as: “the final step in the process of translating knowledge into practice” 2
To: “The use of a person’s own data to objectively gauge the efficacy, safety, and tolerability of therapeutics, and, subjectively, to tailor health recommendations and/or medical decisions to the individual’s preferences, circumstances, and capabilities” 61 .
Evidence evaluation process
After the first consensus report in 2020 (ref. 2 ), the PMDI executive committee established the PMDI consortium to represent each of the precision medicine pillars within the four types of diabetes across 15 working groups. The consortium comprises >200 clinical and research investigators across all career stages and domains of diabetes expertise, residing in 28 countries across four continents (see author list). A separate cross-cutting methodology working group provided critical training and guidance and the executive committee provided strategic direction and administrative oversight. The working groups were supported by administrative staff (C.G., E.M., P.S.) and medical librarians (M.B., K.A.).
Working groups were tasked with defining the key research questions that would need to be addressed for precision diabetes medicine to be implemented into practice by 2030. The first consensus report opted for this specific timeline to instill a sense of urgency while allowing time for proof-of-conduct research to be undertaken.
Systematic literature review protocols were developed by the working groups for the priority research questions, with principles for procedure, synthesis and consensus reporting outlined by the methodology working group to ensure a rigorous and consistent process. Working groups were permitted to generate expert opinion statements for hypotheses that did not reach the level of priority for a full systematic review. All systematic review protocols were prospectively registered on the PROSPERO database 18 , 19 . The full methods, results and working group conclusions are described in the individual systematic reviews 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 .
Synthesis of evidence
The following summarizes the results and synthesis reported in the supporting series of systematic evidence reviews from the PMDI consortium for the second consensus report 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 .
MDM results from a mutation in a single gene 20 . It can be diagnosed in the neonatal period (neonatal diabetes) or typically, but not exclusively, before the age of 45 years. MDM diagnosed outside the neonatal period has historically been known as maturity-onset diabetes of the young. There are autosomal dominant, recessive and maternally inherited forms as well as varieties that arise from de novo mutations and chromosome abnormalities 20 . Although MDM is rare, collectively it accounts for up to 5% of diabetes and presents opportunities for precision medicine 20 . Despite the clinical benefits of making a diagnosis of MDM, many patients are misdiagnosed with T1D or T2D owing to overlapping clinical features.
Precision diagnosis
We systematically reviewed the evidence underpinning two priority questions for precision diagnosis of MDM 7 : (1) who should be tested, and (2) how should they be tested? Our eligible literature review included 98 studies among pediatric or adult populations of testing criteria and 32 studies of testing methods among individuals with suspected neonatal diabetes mellitus or MDM.
Based on our evidence synthesis, the data support precision diagnostics testing for (1) neonatal diabetes in all infants aged <1 year diagnosed with diabetes, (2) MDM in individuals aged <30 years without obesity who are islet cell autoantibody-negative with detectable C-peptide, (3) GCK - related hyperglycemia in women with GDM without overweight or obesity and fasting glucose >5.5 mmol l −1 and (4) GCK- related hyperglycemia in young individuals without obesity with persistent mild fasting hyperglycemia regardless of family history. Ethnic-specific body mass index (BMI) thresholds should be used to determine overweight and obesity.
Testing modalities include the use of (1) targeted next-generation sequencing for neonatal diabetes and MDM, (2) targeted genetic panels with all known causes of MDM, including mitochondrial diabetes, detection of known noncoding mutations, and copy-number variants, (3) a comprehensive panel that includes all recessively inherited genes, particularly in populations with high rates of consanguinity, (4) a separate multiplex ligation-dependent probe amplification assay for copy-number variants detection or genotyping assay (such as pyrosequencing) for detection of m.3243A>G, (5) a methylation-based assay, such as methylation-specific multiplex ligation-dependent probe amplification for neonatal diabetes testing, since 6q24 imprinting defects are a common cause of transient neonatal diabetes mellitus, and (6) rapid Sanger sequencing of GCK in suspected GCK- related hyperglycemia, and the KCNJ11 , ABCC8 and INS genes in suspected neonatal diabetes.
Additional considerations for causality, penetrance, reporting, disparities in testing access and barriers to genetic testing and follow-up of causal variant reporting were qualitatively reviewed. We noted a critical gap across the literature addressing access to genetic testing for MDM to mitigate health disparities, including concerns with replication and external validation in non-European ancestry populations. There was inconsistent measurement of islet autoantibodies and C-peptide diabetes diagnosis under the age of 45 years with ancestry-appropriate T1D genetic risk score data. We encourage the development of guidelines tailored to additional MDM types and genes, de-identified case-sharing platforms to gather the evidence to evaluate pathogenicity and deep mutational scanning maps of MDM genes for variant classification. The clinical guidance for genetic counseling, subsequent referrals and family testing, as well as research on the outcomes of implementation will also be essential to maximize precision diagnostic approaches for monogenic forms of diabetes.
Precision treatment
Precision treatment of MDM is potentially optimized by characterizing an individual’s molecular genetic subtype and pathophysiology; thus, we reviewed the evidence for comparative effectiveness of therapies among populations with specific monogenic subtypes of β-cell diabetes and severe insulin resistance 6 .
Most diabetes that occurs at ages <6 months is monogenic neonatal diabetes. Sulfonylureas were recently established as the most effective treatment for neonatal diabetes due to a potassium channel mutation 21 . Our systematic review for the effects of noninsulin treatments included 19 studies in individuals with 6q24-related transient neonatal diabetes 4 ; all studies graded as having moderate or serious risk of bias. In some, but not all, studies, sulfonylurea use during the neonatal period improved diabetes outcomes, allowing cessation of insulin, and was well tolerated. Evidence for the efficacy of a variety of noninsulin therapies was more consistent later in life during a relapse phase. We reviewed 32 studies in individuals with SLC19A2 -related neonatal diabetes, also known as thiamine-responsive megaloblastic anemia, all with moderate or serious risk of bias. Most studies described some potential benefits of thiamine, such as reduction or cessation of insulin use and/or improved glycemic control, with no reports of adverse effects. We concluded that the current evidence is low quality for clinical guidance on use of noninsulin therapy with 6q24-related diabetes or thiamine in thiamine-responsive megaloblastic anemia.
We evaluated the effects of therapies for HNF1A diabetes, HNF4A diabetes and GCK -related hyperglycemia reported in 34 studies, including four randomized controlled trials (RCTs). Sulfonylureas are effective specifically in HNF1A diabetes for glycemic control, more so than in individuals with T2D. For HNF1A and HNF4A diabetes, transitioning from insulin or other noninsulin therapies to sulfonylureas may improve glycemic control. Some experimental studies demonstrate glinides and glucagon-like peptide-1 receptor agonists (GLP1-RAs) may be alternatives to sulfonylureas for HNF1A diabetes, as well as dipeptidyl peptidase 4 inhibitors as augmentative therapy. For GCK -related hyperglycemia, published case series indicate that treatment should not be given and can be discontinued.
Monogenic disorders of severe insulin resistance include generalized lipodystrophy caused by mutations in AGPAT2 and BSCL2 , partial lipodystrophy with mutations in LMNA and PPARG , and pathogenic variants in the INSR gene. Safety and efficacy of recombinant human leptin (metreleptin) and thiazolidinediones were analyzed in populations with lipodystrophy syndromes and recombinant insulin-like growth factor-1 in INSR mutation carriers. Of 43 nonrandomized experiments and cause series included for review, most individuals had partial lipodystrophy, some had generalized lipodystrophy and few carried INSR mutations. This evidence had moderate or serious risk of bias. Response to metreleptin was described in subgroups with familial partial lipodystrophy and congenital generalized lipodystrophy, where treatment was related to lower triglycerides in aggregated lipodystrophy, partial lipodystrophy and generalized lipodystrophy, as well as those with LMNA , PPARG , AGPAT2 or BSCL2 mutations. HbA1c levels decreased in all but AGPAT2 . Thiazolidinediones lowered triglycerides and HbA1c levels in aggregated lipodystrophy, and triglycerides in LMNA but not PPARG . Response to insulin-like growth factor-1, alone or in combination with IGFBP3, lowered HbA1c levels. There were very few adverse events reported for any therapies, possibly due to small samples sizes and underreporting.
Precision prognosis
We reviewed evidence describing the incidence and severity of diabetes-related microvascular and macrovascular complications in populations with permanent neonatal diabetes due to pathogenic variants in KCNJ11 and ABCC8 , and MDM due to pathogenic variants in HNF1A , HNF4A and GCK 6 . Extra-pancreatic complications (for example, hepatic adenomas in HNF1A diabetes, developmental delay, epilepsy and neonatal diabetes syndrome) were beyond the scope of the review.
Individuals with most forms of MDM are at high risk of microvascular and macrovascular complications, impacted by poor glycemic control. Many studies focused on younger populations diagnosed with neonatal diabetes, where rates of severe microvascular complications were low. Their risk may be in part mitigated by improved glycemic control in neonatal diabetes related to pathogenic variants in the KCNJ11 and ABCC8 genes and HNF1A diabetes and HNF4A diabetes, where precision therapy with sulfonylureas is available. Additional long-term follow-up studies will be important to understand the natural progression of microvascular and macrovascular complications in permanent neonatal diabetes mellitus from mutations in KCNJ11 and ABCC8 .
From 78 articles, retinopathy and microalbuminuria were reported in cases with neonatal diabetes mellitus, but progressive retinopathy and severe renal disease were uncommon. Populations with isolated GCK -related hyperglycemia have overall very low rates of diabetes-related complications. Indeed, microvascular complications were very rare in cohort studies of populations with GCK- related hyperglycemia and prolonged disease duration (>50 years).
Recent studies of HNF1A diabetes reported lower rates of complications compared to those published earlier (for example, for retinopathy, 17% in recent versus 47% earlier; for cardiovascular disease (CVD), 7% in recent years versus 16% earlier). In recent studies, rates of microangiopathic complications observed were less than in T1D, although rates were similar or higher among these populations in older studies. More recent studies of patients with HNF1A diabetes and HNF4A diabetes show improved prognosis of diabetes microvascular and macrovascular complications, likely reflecting an earlier molecular diagnosis, tighter treatment targets and higher rates of precision therapy.
GDM is abnormal glucose tolerance with onset or first recognition during pregnancy. GDM is the most common metabolic complication of pregnancy. Unlike most other forms of diabetes, the onset of GDM is rapid and typically resolves after delivery. Nevertheless, the short- and long-term health risks that GDM poses to the mother and offspring can be substantial, underscoring the importance of widely available screening, diagnosis and effective treatment.
Precision prevention
We systematically reviewed results of 116 interventions on GDM prevention, including diet and/or exercise (diet n = 16; exercise n = 17; diet and exercise combined n = 59), metformin ( n = 13) and supplements such as myoinositol/inositol, probiotics and fish oil ( n = 12) 9 . We considered interventions initiated in the preconception or antenatal period and reporting GDM among its outcomes for prevention efficacy.
In our meta-analyses, lifestyle interventions led to lower incidence of GDM compared with control care: diet only by 25%, exercise only by 31%, and combined diet and exercise by 18% (moderate-to-low-quality evidence). Metformin reduced GDM by 34%, and myoinositol/inositol supplements by 61%; however, this evidence was rated very low quality. Only seven trials initiated interventions in the preconception period. Metformin interventions implemented in the preconception period had better GDM risk reduction when compared to those initiated during pregnancy. For exercise-only interventions, greater risk reduction for GDM was seen in studies enrolling women with a BMI in the normal range. Combined diet and exercise interventions were more effective in GDM reduction among women with overweight or obesity, without polycystic ovary syndrome, without history of prior GDM and with advanced maternal age at pregnancy. Metformin was relatively more effective in preventing GDM among women with a history of polycystic ovary syndrome, with older maternal age and with higher fasting blood glucose at enrollment. Parity, education and employment status, race and history of having a large for gestational age infant did not appreciably impact the effectiveness of interventions. These findings came primarily from comparing effect estimates across trials with different participant characteristics rather than from within-study analyses stratified by participant characteristics. Overall, the strength of evidence for GDM risk reduction with the use of lifestyle modification, metformin and myoinositol/inositol is moderate to very low. Moreover, few data were available to determine which individual characteristics might predict who would benefit most from a given type of intervention.
Future research should include interventions in early pregnancy with sufficient sample size to assess GDM as a primary outcome as well as to provide results stratified by pertinent participant characteristics, including social and environmental factors, clinical traits and other novel risk factors to predict the effectiveness of GDM prevention programs.
The overarching goal of the GDM precision diagnosis working group was to review evidence of precision markers beyond glycemic level (that is, information about a person’s pathophysiology, environment and/or context) that might help refine the diagnosis of GDM. Through the lens of clinical translation, we investigated the evidence supporting GDM subtypes and etiologic or pathologic heterogeneity, as well as associations with adverse perinatal outcomes 14 . The systematic review and meta-analysis focused on observational studies evaluating maternal and fetal anthropometry, clinical and sociocultural/environmental risk factors, genetics, omics and nonglycemic biomarkers that could identify subgroups of individuals with diagnosed GDM at differentially higher risk of adverse pregnancy outcomes. Of 137 studies included, 68 studies evaluated maternal anthropometry as a potential modifier or precision marker related to pregnancy outcomes. The meta-analysis among a subset of studies reporting on maternal BMI in relation to risk for neonatal large for gestational age and/or macrosomia. Forty-nine studies evaluated maternal clinical or sociocultural factors, and 30 studies evaluated nonglycemic biomarkers, lipids and insulin sensitivity/secretion indices. Few studies incorporated fetal anthropometry (11 studies), risk-prediction models with multiple variables (six studies) or genetics/genomics and other omics (five studies).
Anthropometry measures were the most analyzed risk factor with outcomes among pregnancies complicated by GDM. Meta-analyses demonstrated that women with GDM and overweight/obesity have two to three higher risk for neonatal macrosomia or neonatal large for gestational age. Larger birth size is the leading risk factor for birth trauma (shoulder dystocia) and emergency C-section. Regarding nonglycemic biochemical markers ( n = 33 studies), lipids and insulin resistance or secretion indices were the most studied, with elevated maternal triglycerides and insulin resistance generally associated with greater risk of neonatal large for gestational age and macrosomia; study findings were inconsistent. Studies reporting on genetics and omics were scarce. Few studies described risk-prediction models with multiple variables. Traditional GDM risk factors, such as advanced maternal age, parity, prior history of GDM or family history of diabetes, were not consistent markers of adverse perinatal outcomes in women with GDM. There was sparse evidence to support conclusions for the role of race, ethnicity or country of origin as precision markers, given high heterogeneity across studies, and that data interpretation is dependent on sociocultural context. Very few studies investigated diet, physical activity or psychological health as precision markers for diagnosis of GDM.
For most of the precision markers (other than BMI), it will be necessary to conduct validation and replication studies in adequately powered studies of people representing the diversity of target populations. For precision biomarkers, validated, standardized and affordable assays are required for broad adoption by clinical laboratories. There is a need to identify and test different clinical decision and management strategies if a precision diagnostic identifies a woman at high risk of perinatal complications. Finally, for modifiable precision markers (for example, lipid levels, insulin sensitivity), novel interventions should be developed and validated that specifically target these markers during pregnancy.
It is unknown whether precision treatment of GDM could improve maternal and/or offspring outcomes. We conducted a systematic review of evidence for precision markers of GDM treatment success to determine (1) which precision approaches in addition to standard of care can enable achievement of glucose targets with lifestyle measures alone, and (2) which characteristics predict whether glucose targets can be achieved in women treated with diet and lifestyle alone, and in women receiving oral agents 10 .
Only two studies reporting personalized approaches of tailoring lifestyle-based treatments in GDM met the inclusion criteria for review, with variable findings for prepregnancy BMI or excessive gestational weight gain as precision markers for intervention efficacy and implementation. For predictors of escalation with the need for pharmacological interventions, 48 studies were included, and 34 studies were included in meta-analyses. Precision markers for successful GDM management with lifestyle measures without the need for additional pharmacological therapy (insulin, metformin and/or glyburide; 34 studies) were (1) younger maternal age, (2) nulliparity, (3) lower BMI, (4) no previous history of GDM, (5) lower levels of HbA1c, fasting glucose and postchallenge glucose concentrations (at 1, 2 and 3 h), (6) no family history of diabetes, (7) later gestation of diagnosis of GDM, and (8) no previous macrosomia. Similar precision markers for successful treatment with metformin and/or glyburide without requiring supplementary insulin were found with the addition of later gestation of initiation of the oral agent (12 studies). Data were lacking to identify precision markers of responses to one agent versus another. Only two studies included genetics or omics as potential markers for treatment escalation. The studies were limited by the predominant focus on high-income settings and the small sample sizes.
Overall, based on findings from moderate-to-good quality, key maternal characteristics were identified that may be used to build prediction models for pharmacological GDM treatment. Precision markers for GDM treatment are usually available from routine clinical measures; however, it is unknown whether other precision markers could be identified (for example, genetics or omics) or whether these can be implemented in clinical practice. Future studies should be appropriately powered and designed to assess individual precision markers or algorithms incorporating multiple precision markers. Validation and replication in diverse populations are lacking and are also needed.
GDM incurs health risks to both a mother and her offspring, not only during pregnancy and at delivery but also over the longer term. The systematic evidence evaluation focused on studies describing predictors of postpartum and long-term cardiometabolic outcomes in women with GDM and their offspring 5 . The evidence synthesis focused on prognostic endpoints of T2D and CVD for women with prior GDM, as well as anthropometric features and preclinical cardiometabolic biomarkers among offspring exposed to GDM in utero. We included 89 studies of which 55 reported on maternal outcomes (52 observational, three RCTs) and 45 reported on offspring outcomes (37 observational, eight RCTs).
Collectively, studies reported that women with a history of GDM are at higher risk of T2D and CVD, with a dose-dependent relationship between degree of pregnancy hyperglycemia and these outcomes. Similarly, offspring born to women with more severe GDM had more adiposity and higher risk of being overweight or of obesity across the life span. GDM severity was also associated with greater risk of incident T2D and CVD among women and with an unfavorable cardiometabolic profile in offspring later in life. Broadly, the relationships between GDM severity and the maternal/offspring outcomes were robust to adjustment for gestational week at diagnosis, offspring birth size and family-level socioeconomic status; however, failing to adjust for maternal BMI and lifestyle factors was a concerning source of bias for the relationships of GDM with offspring outcomes.
Some studies considered whether the type of treatment needed to achieve glycemic targets in women with GDM is a precision marker for long-term outcomes. Treatment with insulin, but not lifestyle, had a worse prognosis for both mothers and offspring; however, this apparent effect could be due to the prescription of insulin when GDM is ‘more severe’, which may be partly due to confounding by indication. Unfavorable maternal and child outcomes associated with GDM history (exposure) were modified by lifestyle. For maternal outcomes, the primary risk mitigators were healthy diet and regular moderate-to-vigorous physical activity. For offspring outcomes, the offspring’s diet and physical activity modified cardiometabolic risk. Greater exclusivity and longer duration of breastfeeding attenuated cardiometabolic risk among GDM-exposed offspring, although this literature was less robust than that for reduction of the risk for T2D in breastfeeding women with prior GDM. There is presently very limited evidence about the role of omics biomarkers and polygenic scores (for T2D or CVD) in women with prior GDM.
Despite the above insights, studies regarding GDM prognostic factors indicative of future maternal and offspring cardiometabolic health are generally low quality. Most current literature describes retrospective studies leveraging registry data and observational cohort studies; inferring causal relationships from these data about prognostic factors is hampered by risk of confounding and reverse causation (attributable, for example, to preexisting conditions and other pregnancy characteristics).
T1D results from autoimmune-induced destruction of the pancreatic β-cells, requiring insulin treatment for survival. While representing ~2% of all forms of diabetes worldwide 1 , T1D has a large healthcare cost owing to the early age at onset for many affected, the high cost of insulin and related technologies (insulin infusion pumps, continuous glucose monitors (CGM), hybrid closed loop systems) and elevated risk of both microvascular and macrovascular complications. Growing insights into the pathogenesis of T1D motivated the classification of the disease in different stages, with stage 0 being presence of 1 autoantibody in people at high genetic risk, stage 1 and 2 being the presence of two or more islet autoantibodies in normo- (stage 1) or dysglycemia (stage 2) and clinical diabetes (stage 3). Etiologic heterogeneity is recognized in both children and adults.
A key question in T1D is whether individual characteristics or biomarkers can be used to identify those most likely to respond to disease-modifying therapy before clinical T1D onset (stage 3). We conducted a systematic review of RCTs focused on the identification of features associated with treatment response published over the past 25 years 13 . Multiple trials were identified that compared disease-modifying agents, mostly immunotherapies, to placebo. A formal meta-analysis was not conducted given the heterogeneity of interventions and approaches. Of 75 manuscripts extracted for review, 15 described prevention trials with the remainder focused on treatment in the recent- or stage 3-onset period.
Prevention trials generally enrolled individuals at elevated genetic risk, typically based on the presence of a first-degree relative with T1D and/or with islet autoimmunity, with or without changes in β-cell function (stages 0 to 3). Studies commonly used time-to-diabetes as an outcome. Stage 3 studies used more consistent eligibility criteria and frequently tested C-peptide area under the curve as a primary outcome. Fifty-seven studies, including primary trials and longitudinal follow-up of trials, performed precision analyses, specifically testing features associated with treatment response. Analyses tested the associations of many features with treatment response, most commonly age, measures of β-cell function and/or an immune phenotype.
Overall, the RCTs received high-quality ratings and were graded to have a low risk of bias; however, precision prevention analyses had lower quality rankings. Reasons for this were that studies typically did not prespecify an analytic plan, had inconsistent reporting of key methodologic details (for example, sample size or a correction for multiple comparisons) and tended to report only positive (that is, statistically significant) findings. There is large interest in precision features associated with treatment response to disease-modifying therapy in T1D; however, most analyses were exploratory without follow-up with prespecified prospective analyses.
We recommend that future studies are powered to undertake multiple prespecified analyses to permit statistically robust testing of features associated with treatment response (for example, through stratified effects or biomarker–treatment interactions). These data will be required for the effective identification and implementation of precision approaches to disease-modifying therapies.
Islet autoantibodies are validated predictors of disease progression and are being incorporated into clinical practice. We focused this systematic evidence review 13 on determining whether autoantibodies help stratify subgroups across four settings: (1) disease progression before stage 3 diagnosis, (2) disease presentation/stage 3 diagnosis, (3) disease progression after stage 3 diagnosis and (4) response to disease-modifying interventions.
We identified 151 publications, 90 relevant to progression before stage 3, 44 for heterogeneity at stage 3, 11 for progression after stage 3 and 13 for interventions. While insulin autoantibodies are commonly the first to appear before diagnosis in younger children, the presence of IA-2 autoantibodies corresponds with faster disease progression. Interactions between high-risk HLA alleles (for example, HLA-DRB1*03:01 and HLA-DRB1*04:01), the number and types of islet autoantibodies, and age at seroconversion are most often used in models (or added to existing models) to predict disease. The replacement of traditional radio-binding assays with electrochemiluminescent assays improved the sensitivity of some autoantibody testing and identified high-risk subgroups (individuals with two or more autoantibodies) who were previously low risk (positive for a single autoantibody).
At stage 3 diagnosis, the presence of specific autoantibodies correlated with age, suggesting that the inciting antigens are different in younger, compared to older, individuals. The primary antibodies at seroconversion often disappeared at the time of stage 3 diagnosis. There was weak evidence that declining islet autoantibody titers and the number of autoantibodies after stage 3 diagnosis corresponded to preserved residual C-peptide level, thereby not supporting the use of islet autoantibodies to define heterogeneity in metabolic outcomes. Evidence of islet autoantibody features to predict response to disease-modifying therapies was modest, making the impact of a specific antigen (and its corresponding autoantibody) less significant. For clinical implementation, the grade of evidence is limited by the reports (1) being mainly from European ancestry populations, (2) rarely correcting analyses for multiple comparisons, (3) consisting of observational measurements from RCTs limited to observational endpoints and (4) inconsistently reporting on assay methods and validation.
These results suggest that islet autoantibodies are useful to define heterogeneity in T1D before stage 3 diagnosis, and that benefit will be gained by incorporating age and genetics into risk scores. Thoughtfully designed, prospective trials are needed to apply these observations and develop precision medicine approaches to diagnosis and treatment. In a corresponding paper 13 , a methods checklist is proposed to ensure reproducibility and applicability of islet autoantibody-based research.
Treatment of clinical, stage 3 T1D includes insulin therapy, adjunctive agents, nutrition, exercise, behavioral health, glycemic targets and transitions of care; however, in the last decade, the major development for people living with T1D has been in technology. This systematic evidence review focused on whether diabetes management technologies impact clinically relevant outcomes, based on differences between subpopulations 11 .
A systematic review of 71 peer-reviewed RCTs and related secondary/extension studies with at least 50 participants from the past 10 years concluded that novel technologies (ranging from isolated CGM, decision-support tools, continuous subcutaneous insulin infusion pumps, to advanced hybrid closed loop systems) have resulted in lower HbA1c levels, increased CGM-defined time-in-range glucose between 70–180 mg dl −1 , reduced hypoglycemia risk and improved person-reported outcomes. The broad array of technologies permits the application of individualized treatment plans for people living with T1D but limits cross-trial comparisons or meta-analyses. CGM use among very young children reduced the risk of hypoglycemia and lowered parental distress while having minimal impact on HbA1c level and time-in-range when compared to self-monitoring of blood glucose. Technologies that include sensor-augmented pump therapy, predictive low glucose suspend pumps and automated insulin-delivery systems improved hypoglycemia, time-in-range and HbA1c levels across all age groups. Age of the individual should be considered in clinical decisions related to technology use. Variation in baseline glycemic status (for example, suboptimal versus targeted HbA1c level) did not consistently impact these outcomes.
Important limitations of published trials were identified. There is limited availability of preplanned or well-powered analyses in subgroups (for example, children, older adults or people with advanced complications). While the quality of evaluated RCTs is high with a low risk of bias, high-quality data related to these subpopulations is needed, and results are considered exploratory until appropriately powered studies are conducted and findings are adjusted for multiple comparisons. Confounding variables, including (1) access to technologies, (2) education with device initiation, (3) concomitant behavioral modifications and (4) frequent contact with the healthcare team are rarely described in enough detail to assess their impact.
The landmark Diabetes Control and Complications Trial demonstrated that intensive glucose control effectively prevents microvascular complications in individuals with uncomplicated and recent-onset T1D (<5 years), as well as those with more than 5 years since diagnosis and mild nonproliferative diabetic retinopathy 22 , 23 . However, glucose control accounts for only 50% of T1D complication risk 24 , 25 , 26 , 27 . Moreover, certain subgroups, including the elderly, young children and people with hypoglycemia unawareness or autonomic neuropathy, may be harmed from severe hypoglycemia resulting from tight glucose control 28 . This highlights the importance of identifying additional nonglycemic interventions to mitigate complication risk for all people living with T1D.
Epidemiological studies have identified additional (epi-)genetic, biological and phenotypic traits in people with T1D at higher risk for kidney, eye and neurological complications and a worse prognosis. Presence of risk factors traditionally associated with CVD and T2D such as overweight, dyslipidemia, hypertension and smoking also contribute to the development of advanced stages of kidney disease, peripheral neuropathy, retinopathy and CVD in T1D 25 , 29 , 30 , 31 . Although cholesterol and blood pressure levels outside of current clinical targets increase complication risk in T1D 32 , 33 , T1D-specific recommendations are lacking.
Recent evidence has identified distinct changes in lipid and amino acid metabolism that predict earlier, more rapid kidney function decline in T1D 34 . Additionally, socioeconomic and psychological factors play a role in microvascular complications 35 , 36 , 37 , 38 . Although evidence from RCTs is absent, blood pressure control with renin-angiotensin-aldosterone system (RAAS) inhibition and the use of statins for hyperlipidemia are promising therapies against progression to hard endpoints (for example, end-stage renal disease or CVD); however, there is a scarcity of evidence that precision medicine alters prognosis in T1D.
A rare example of precision prognosis in T1D relates to the haptoglobin ( HP ) genotype. In the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study, coronary artery disease (CAD) risk was comparable between the intensive and conventional therapy groups with the HP 2-2 genotype 39 . CAD risk was greatly reduced with intensive therapy in noncarriers of HP 2-2. Similarly, although better glycemic control was associated with lower CAD incidence in the EDC study, a residual risk related to HP 2-2 was observed 39 , 40 .
After the Diabetes Control and Complications Trial, there has been little evidence from RCTs for evaluating the impact of tight glycemic control in specific subgroups with respect to complications; however, clinical precision medicine is utilized in the prognosis and choice of therapy for people with T1D. More sophisticated decision tools, based on deeper genetic and phenotypic profiling in multi-ethnic cohorts are needed to improve personalized prognosis in T1D.
Approximately 500 million people worldwide are estimated to have T2D 41 , which is predicted to rise to 1.3 billion by 2050 (ref. 42 ). A diagnosis of T2D is one of exclusion, occurring when other plausible explanations for chronically elevated blood glucose have been considered and dismissed. This high degree of uncertainty and potential heterogeneity presents major challenges for the prevention and treatment of T2D.
Large-scale RCTs demonstrate that dietary or lifestyle interventions can delay the progression to T2D. However, there is large interindividual variability in response to preventive interventions 43 . Identifying predictors of response to interventions and the characteristics of people who would be most likely to benefit remain high priorities and are key focus areas for precision prevention in T2D 15 . This systematic review identified 33 trials focused on lifestyle interventions ( n = 24 studies), dietary modification ( n = 4 studies) and dietary supplementation ( n = 5 studies). From the 33 trials, there were 80 post hoc stratified analyses based on demographic, clinical, social or molecular factors.
Sociodemographic characteristics such as age, sex, race/ethnicity or socioeconomic status were not found to significantly affect response to intervention. We found evidence, albeit of low quality, that individuals with poorer health status at baseline, in particular prediabetes, tend to benefit more from lifestyle and dietary interventions than healthier individuals. Studies that stratified on body size at baseline reported inconsistent observations, with some showing that those with a lower BMI benefited more from intervention, whereas other studies found no difference according to body size. There was suggestive evidence that individuals who smoke and those with lower levels of physical activity at baseline benefited less from a lifestyle program, whereas no such interactions were reported for dietary or supplement interventions. There was little evidence that genetic factors or biomarkers attenuated or exacerbated the effects of these interventions.
Although our systematic review included intervention studies, most of which were RCTs with low risk of confounding, we evaluated certainty of post hoc stratification analyses. This suggested that statistical power was often limited. Further, most did not adjust for individual-level risk factors.
Although T2D can be prevented or delayed in tightly controlled clinical trials, adherence to lifestyle or diet modifications in real-world settings is often suboptimal. Thus, to maximize success of precision prevention interventions it will be important to incorporate methods tailored to the individual that enhance adherence.
In this systematic review 8 , evidence was assessed for optimization of T2D diagnosis through subclassification using (1) approaches involving ‘simple’ categorization of clinical characteristics such as biomarkers, imaging or other routinely available parameters and (2) ‘complex’ approaches involving machine learning applied to omic and genomic data.
Current data on the clinical value of T2D subclassification come predominantly from populations of European ancestry. Though glycemic measures are used to diagnose T2D, several nonglycemic measures were consistently applied to subclassify disease, including BMI, homeostatic model assessment of insulin resistance, C-peptide and lipid profiles.
Simple T2D subclassification approaches focused on data including pancreatic autoantibodies, BMI, measures related to pancreatic β-cell function, age at diagnosis, lipid profiles, oral glucose tolerance test measures and cardiovascular features. The study designs, specific cutoffs and outcomes were heterogeneous, with no study replicated or meeting high Grading of Recommendations Assessment, Development and Evaluation (GRADE) quality.
Complex approaches yielded some reproducible subtypes of T2D. The most frequently replicated subtypes were the clusters first described by Ahlqvist et al. 44 , which were replicated in 22 studies, including people of diverse ancestries. These studies used k-means clustering applied to exposures assessed close to diabetes diagnosis: age, HbA1c, BMI, homeostatic model assessment of β-cell function, homeostatic model assessment (2) of insulin resistance and GAD-65 antibody. The four nonautoimmune diabetes subtypes described were severe insulin-deficient diabetes, severe insulin-resistant diabetes, mild obesity-related diabetes and mild age-related diabetes. Associations of these subtypes with clinical outcomes, including glycaemia, microvascular and macrovascular outcomes and death, were replicated in 12 studies. There was also replication of genetic subtypes of T2D from Udler et al. 45 , with associations with clinical features seen in multiple cohorts of European ancestry.
Subclassification strategies for T2D have been associated with meaningful clinical outcomes. However, evidence supporting the clinical application of these subclassification approaches is of moderate quality at best. Further ancestry-inclusive and high-quality evidence is needed. In contrast to simple approaches, the clinical application of machine-learning-derived approaches may require real-time computation of subphenotype classification of an individual with T2D, and necessary computing resources may be unavailable in some settings.
This systematic evidence review 3 focused on two of the most recently introduced antihyperglycemic drug classes, SGLT2 inhibitors (SGLT2i) and GLP1-RAs. These drugs have been shown in RCTs to not only reduce glycemia but also to lower the risk of renal and CVD outcomes among high-risk individuals with T2D. Other therapeutics were not included in this evaluation owing to the complexity and volume of this literature.
The population of those with T2D is heterogenous in its demographics, clinical features and prognosis; thus, there may be differences in response to one or both of these drug classes. A systematic review was conducted to identify individual-level demographic, clinical or biological biomarkers associated with heterogeneous glycemia, CVD and renal outcome in individuals with T2D treated with SLGT2i or GLP1-RA.
For SGLT2i, 339 full-text articles were screened, which yielded 101 studies for evaluation; for GLP1-RA, 161 full-text articles were screened, yielding 75 studies for evaluation. These studies predominantly represent secondary analyses of industry-funded, placebo-controlled trials, or meta-analyses of these trials, with a few observational studies. The most common stratification variables were demographics, baseline HbA1c, obesity and preexisting CVD or nephropathy.
Overall, limited evidence was found for robust modification of the effects of GLP1-RA or SGLT2i on glycemia, renal or CVD outcomes by these features. For SGLT2i, reduced baseline renal function was associated with lesser glycemic response, while a higher baseline HbA1c level was associated with greater glycemic response. For GLP1-RA, a lower β-cell function was associated with a lesser glycemic response. Generally, the strength of evidence was modest, largely reflecting a lack of studies designed and sufficiently powered to address the question of treatment effect heterogeneity.
This systematic review included a meta-analysis 16 to combine evidence from longitudinal studies of individuals with T2D for markers predicting CVD and evaluated their predictive utility beyond current practice. After full-text review, 416 studies were analyzed with 77% focusing on nongenetic biomarkers, 12% on genetic biomarkers and 11% on risk scores.
There were 195 novel nongenetic biomarkers for CVD, of which 134 (69%) had a net positive number of studies showing a significant adjusted association. Of these, 12 biomarkers showed improvement in c-statistic, net reclassification index or integrated discrimination index consistently in more than one study. Considering the results of our pooled meta-analyses, nonpooled analyses, evidence for improved prediction performance indicators and risk of bias, we found high predictive utility for N-terminal pro b-type natriuretic peptide (high evidence), troponin T and triglyceride-glucose (moderate evidence), moderate predictive utility for coronary computed tomography angiography and single-photon emission computed tomography (low evidence) and pulse wave velocity (moderate evidence), and low predictive utility for C-reactive protein (moderate evidence), coronary artery calcium score, galectin-3 (Gal-3), troponin I, carotid plaque and growth differentiation factor-15 (low evidence).
Among the 48 genetics studies, 79 genetic biomarkers were evaluated for CVD outcomes, 29 having a net positive number of studies with a significant association. Three genetic biomarkers demonstrated promise: rs10911021 in GLUL , genetic risk score (GRS) for CAD and isoform e4 in APOE . Only the GRS for CAD showed improvement in all three performance indicators in a single study. A few studies employed different GRSs using up to 204 variants from 160 distinct loci derived from the general population that were externally validated, demonstrating improvements in CVD risk reclassification and significant enhancements in discrimination indices. Most studies, however, were conducted in European ancestry populations, with a few of Asian ancestry and very little or no representation of most other ethnicities. Some studies report a relative integrated discrimination index >6%, suggesting adequate predictive utility for the GRS for CAD, but this will need to be confirmed in appropriately designed ad hoc trials, to confirm clinical utility and transferability to other ancestries.
Risk scores showed overall modest discrimination, and model performance tended to decline when validated in countries that differed from the derivation cohort. Most studies focused on baseline characteristics and did not account for time-varying factors that may modify CVD risk, such as medications.
In summary, the highest predictive utility was found for N-terminal pro b-type natriuretic peptide, troponin T, triglyceride-glucose and GRS for CAD, with NT-proBNP having the highest level of evidence. Prospective studies evaluating prognostic biomarkers and risk scores as clinical decision-support tools in T2D are scarce, as is information on their cost-effectiveness. Our findings illustrate the need for development and validation of prognostic markers for CVD in diverse populations of people with T2D to promote equity in precision diabetes care.
Consensus on implementation of precision diabetes medicine
The PMDI consortium working groups convened over two in-person meetings to deliver consensus across the precision medicine pillars and diabetes domains. Their main objectives were identifying evidence to support immediate clinical applications of precision medicine approaches and the gaps to address otherwise. Although the framework we outlined using the pillars of precision medicine may help structure approaches in research and practice, there will be overlap between pillars in some settings. For example, ‘precision diagnostics’ may focus on identifying diabetes subtypes that are treatment-dependent, and within ‘precision prognostics’ there may be elements of ‘precision prevention.’ Thus, while these pillars may help with implementation of precision medicine in both research and practice settings, they should not be considered monolithic.
Promising applications of precision medicine in diabetes
Through the systematic reviews of prioritized diabetes research questions, we identified cases where the available evidence supports the use of a precision medicine approach. Research for MDM has witnessed progress for precision diagnosis, underscored by major advances in the availability and affordability of next-generation sequencing technologies for genetic testing.
In women with a GDM pregnancy, factors reflecting severity of GDM, including higher serum glucose values or the number of time points with elevated serum glucose at the diagnostic oral glucose tolerance test, earlier gestational age at diagnosis and insulin treatment requirement predicted GDM treatment success and risk of long-term prognostic outcomes for mother 46 and offspring 47 . Precision prevention, diagnosis and treatment research should leverage information provided by readily available clinical measures to support GDM care. Published evidence shows that maternal BMI, insulin sensitivity and secretion and dyslipidemia may enhance precision diagnostic tools 14 . Beyond this, there is a need for further research to develop and validate algorithms predicting GDM treatment success or risk of complications using traditional clinical factors possibly combined with novel markers such as metabolomics, paving the way for precision treatment and prognosis tools.
In T1D, clinical prevention strategies are increasingly informed by the primary etiology and progression in those at elevated genetic risk. Recent approval by the US Food and Drug Administration (FDA) of an anti-CD3 monoclonal compound (teplizumab) for use in stage 2 T1D has provided evidence of slowing, if not blocking, disease progression. Heterogeneity in response to preventive therapies represents a major opportunity for research and clinical investigation. In T1D, genetic risk has been defined largely using data from pediatric-onset populations of European ancestry individuals, aiding the development of GRS in this group that, when coupled with islet autoantibody testing, predicts disease development and aids diagnosis.
Precision medicine in T2D includes refining the subclassification of diabetes into pathophysiologically and clinically meaningful disease subtypes 44 or the development of probabilistic scoring algorithms to assert likely ‘archetypes’ 48 . The methods and data inputs to these derivations are diverse, often with machine learning unsupervised clustering methods applied to clinical and genomics or other omic data. The application of such approaches to the clinical setting is likely to require further refinement of classification models, as only about a third of people with diabetes can be reliably subclassified currently, and people tend to drift between diabetes subtypes as their disease progresses 48 , making longer-term prognosis challenging. The most promising precision approaches to T2D treatment, however, are to use individual patient-level features to predict differential treatment outcomes 49 . There is now robust evidence that routine clinical features and pharmacogenetic biomarkers alter glycemic response for all major drug classes after metformin, supported by the recent prospective TriMaster trial 50 . Development of treatment decision-support tools prioritizing routine clinical features would provide a low-cost and equitable approach to T2D precision treatment that may be of special utility in global regions where access to essential diabetes medications is very limited.
Research gaps to accelerate precision medicine in diabetes
A key finding of this consensus report is that trials explicitly designed to test precision medicine hypotheses are needed, particularly those that yield clinically translatable findings. Incorporating trials explicitly designed to test precision medicine hypotheses in the drug development pipeline will be important if treatment recommendations for these drugs are to be meaningfully optimized. For this to succeed, engagement with regulatory authorities will be required. These and other supporting studies should consider whether markers of treatment heterogeneity are part of causal process, or noncause predictors of such effects. Although noncausal markers may be adequate for the purpose of prediction, where the marker is the intervention target, it should lie on the causal pathway. Determining causal mechanisms will also be important in research focused on understanding biological heterogeneity and interactions, which may, for example, include novel target discovery efforts.
As much of the current precision diabetes medicine research has been conducted in people of European ancestry living in high-income settings, there is a pressing need to broaden the scope to include other ethnic, geographic and cultural groups, particularly those who are most vulnerable. Correspondingly, there is also a need to better understand the impact of precision medicine on disparities, to help ensure gaps are closed and not inadvertently widened.
Across the pillars of MDM, there is a need to develop improved differential diagnosis of MDM that can masquerade as either T1D or T2D. As MDM encompasses several genetic variants in genes involved in glucose metabolism, it is important to consider complementary approaches for clinical translation, including genetic counseling, cascade testing and open sharing of confirmed mutations in a standardized global platform. More studies are needed to determine the efficacy of treatments for specific monogenic forms of diabetes, with focus on extra-pancreatic effects of MDM.
GDM is heterogeneous in etiology and prognosis, arguing for more precise prevention, diagnosis and treatment, as well as continued investigation of prognostic implications in both women and offspring throughout the life course. The clinical translation of precision medicine in GDM will require new studies that test precision interventions targeting the physiological processes characterized by these biomarkers; such studies will also need to demonstrate improved health outcomes in women and/or their offspring. Dynamic biomarker assessments in pregnancy and postpartum will also be required, ideally with point-of-care testing, since GDM, unlike other types of diabetes, unfolds and exerts its effects rapidly. Many GDM prevention trials have intervened relatively late in pregnancy and reported variable outcomes on maternal and offspring health 51 . Interventions starting in early pregnancy or preconception may be more impactful; success in identifying who should be the focus of interventions, the specific nature of those interventions and when they should occur may be enhanced by precision prediction models. Because the impact of the intrauterine environment on the fetus is plausibly mediated by epigenetic modifications to fetal deoxyribonucleic acid, the characterization of cell-free fetal deoxyribonucleic acid using maternal plasma 52 may prove useful in the development of precision GDM medicine. This would also facilitate identification of fetal carrier status for pregnant women with MDM, potentially aiding management decisions.
With T1D, there is substantial heterogeneity in age, presence and type of islet autoantibodies, and genetic risk in those who transition to clinical (stage 3) disease, impacting diagnosis and prediction. Critical gaps remain in both non-European ancestry and adult-onset groups. Although improved glycemic control has aided in the reduction of proportion and impact of complications of T1D, evidence suggests that not all groups benefit from tight glycemic control, in part due to the risks associated with hypoglycemia. For T1D, the most promising areas for immediate clinical implementation include genetic risk classification, screening for islet autoantibodies (particularly at an early age and, potentially, later in life) and the ability to detect those at risk of progression, thereby affording the opportunity to utilize an immune intervention to delay or prevent progression to stage 3 T1D, recognizing that such therapies must also be cost-effective. Continued development of pharmacologic agents and technologies to minimize risk of microvascular and macrovascular complications in those living with T1D remains essential. Further, the availability of an effective and cost-effective disease-modifying intervention for early detection and prevention in those at risk, with use of better therapeutics, and recommendations for control of microvascular and macrovascular complications is essential in those living with T1D.
As with many other areas of precision diabetes medicine, much of the evidence for T2D precision medicine is of weak quality, focusing mainly on populations of European ancestry, and with a dearth of adequate validation. Papers reporting studies on precision medicine in T2D that claim translational potential often lack key metrics to allow benchmarking against current standards of care such as measures of predictive accuracy and cost-effective analyses. There is also a need for prospectively designed precision medicine trails that focus on validating key hypotheses pertaining to, for example, a stratified treatment response.
Road map and milestones for global precision medicine in diabetes
Recommendations, derived from the systematic reviews underpinning this consensus report and through two in-person consensus meetings, are shown in Table 1 .
Reporting precision medicine research
Barriers to determining the clinical relevance of published research for precision diabetes medicine are that published reports rarely provide key details regarding a priori hypotheses, statistical tests for heterogeneity, number of events observed, statistical power to evaluate interactions, and more. Often these metrics are unclear, leading to misinterpretation of results. This has been the case with some of the diabetes subtyping that relies on hard clustering methods, where the individual-level probabilities of a person having a specific diabetes subtype are often low, such that treating a person based on their ‘subtype’ would often be ineffective. Nevertheless, much of the popular narrative has focused on using this type of subtyping to transform individual-level treatment.
Information that should be described in precision medicine research publications, particularly when citing evidence said to be of relevance for clinical translation, includes:
Measures of discriminative or predictive accuracy and calibration accuracy (both ideally in independent datasets) of precision medicine models
Measures of variance and central tendency
Effect estimates and risk ratios with 95% confidence intervals (not merely P values)
The units underlying effect estimates and risk ratios (for example, mmol/allele or risk/allele)
For unsupervised clustering, classification probabilities (for example, relative entropy statistic)
The use of machine learning and deep learning is becoming increasingly popular in precision medicine-facing research. However, as population-specific features (including prevalence of diabetes, risk factors and cultural and lifestyle features) are important in determination of diagnosis, treatment and prognosis, these algorithms need to be tailored to the community being served. In addition, it is often not possible to determine how outputs from such models were derived, which may increase the risk of misinterpretation or inadequate understanding of the potential risks associated with deployment of algorithms in clinical practice. Thus, methods to interpret and validate results from machine-/deep-learning models are likely needed for the safe and meaningful translation of precision medicine research into clinical practice.
Regulatory requirements
The successful deployment of precision medicine may require regulatory bodies to adopt new approaches for product approval. This will entail new guidance about the processes for obtaining approval to commercialize new therapeutics and diagnostics, including when and under what circumstances the use of a new drug must be preceded by and/or accompanied by a diagnostic or screening test. If the healthcare system is to secure the full benefits of precision medicine, it must provide full and fair reimbursement for new technologies, products and services, based on market principles to the extent possible.
Diagnostics include clinical decision-support software. In 2022, the FDA provided updated guidance on the regulation of this type of software 53 , which distinguishes between nondevice clinical decision-support software and software as a medical device with more stringent regulatory requirements. Software as a medical device is intended to provide high-level clinical decision-support software, have direct impacts on patient care and typically requires FDA clearance or approval before it can be marketed. On the other hand, nondevice clinical decision-support software does not require clearance; applying the criteria to distinguish between the two can be challenging. A new risk-prediction tool for CVD in people with T2D developed using machine learning in a large electronic health record dataset could qualify as either software as a medical device or nondevice clinical decision-support software. The tool may guide clinical decision-making, which would qualify more as a nondevice clinical decision-support software, but also has the potential to impact patient care by assisting in making treatment decisions for people at risk of developing CVD. If a risk score is considered nondevice clinical decision-support software, it will not require FDA clearance or approval, making the process of bringing it to market easier and faster. However, if it is considered software as a medical device, the regulatory requirements will be more stringent and the development process will likely be more costly and time consuming. Making a clear distinction between nondevice clinical decision-support software and software as a medical device is crucial for precision medicine, as the development and implementation of biomarkers, risk scores and other precision tools rely on the interpretation of the FDA guidance.
Meeting standards of care
Diabetes is a multifaceted disease that is impacted by broad societal issues and has ramifications beyond the affected individual. Consequently, healthcare systems face significant challenges in providing effective and equitable care. While precision medicine promises to provide more personalized care for people at risk of and with diabetes, it requires effective implementation in healthcare systems to enhance disease prevention, management and prognosis. However, healthcare systems are currently struggling to provide care following current practice guidelines in diabetes. Gaps in adherence to current standards of care, which incorporate relatively simplistic personalized approaches, highlight the need for research to understand strategies for deploying precision medicine. While this may introduce additional levels of complexity if poorly managed, precision medicine may reduce complexity by improving or replacing suboptimally functioning processes and improving cost-effectiveness. Nevertheless, healthcare service optimization through precision medicine remains an understudied topic and represents an area in need of investment.
Commercialization versus access
The commercialization of precision medicine through direct-to-consumer platforms or products for diabetes often involves complex trade-offs. Companies investing in precision diabetes medicine need to protect their intellectual property with patents to prevent infringement. However, this can lead to high costs for recipients, potentially widening healthcare disparities. In addition, companies must ensure the financial viability of their products to continue research and development, which may lead to pricing barriers that restrict access for those most in need. To balance commercialization and accessibility, companies may need to make their products more affordable and accessible through partnerships with healthcare providers, insurers and government funding. Workflow complexity within the clinical environment is a barrier to commercialization; embedding precision medicine decision-support systems within the electronic medical record system may improve adoption by clinicians, but there may be additional proprietary considerations such as the electric medical system record vendor requiring a share of the profits. Ultimately, achieving a balance between commercialization and accessibility is crucial, considering the ethical, social and economic implications of precision diabetes medicine approaches. The balance point will vary depending on the sociocultural setting where the product or service is deployed.
Health equity and mitigating disparities
Precision medicine has the potential to improve health equity if the solutions it provides are optimized to the target populations, accessible to those in need, cost-effective and safe. Precision medicine solutions developed in one population may not transfer adequately to another 54 . Moreover, developing such solutions will require high-quality data for the target populations, as imprecise data are likely to obstruct the development of precision medicine. This is true of many technologies 55 and data of relevance to precision medicine. For example, at present, most genetic data and other omics data used in genome-wide studies of diabetes come from people of European ancestry living in high-income countries 56 , yet the burden of diabetes is growing most quickly in other regions and ethnicities 41 .
Although genomic medicine is an important component of precision medicine and is relatively easy to deploy owing to the robustness of deoxyribonucleic acid samples and the stability of a person’s germline genetic variation across the life course 57 , overreliance on this single technology may contribute to inequities in health. This is because not all diabetes subtypes are equally heritable or understood 58 . This may be especially problematic in non-European ancestry populations, where genomics research is less advanced.
The systematic evidence reviews conducted as part of this report reinforce the need for increased ethnic, geographic and sociodemographic diversity in precision medicine research. Nevertheless, there are major genomic and precision medicine initiatives underway in some regions of Asia 59 , Africa 60 and the Middle East that will help offset this imbalance. On the other hand, most low- and middle-income countries currently lack adequately resourced and structured efforts to generate such data, despite a compelling rationale to develop precision medicine solutions for these populations. Thus, a key marker of the success of precision diabetes medicine will be its accessibility and cost-effectiveness where it is most needed; given that the burden of diabetes is growing most rapidly in the global south, the development and implementation of precision diabetes medicine in these regions should be prioritized.
Liabilities
A precision medicine approach for diabetes should also consider potential liabilities. Liabilities could relate to both underutilizing recommendations, for example with an omission to assess for MDM in a person with a high-risk score, or potentially restricting access to certain treatments if underpowered subgroup analyses suggest limited utility. As precision medicine approaches should apply to all individuals at risk of, or living with, diabetes, the global implementation of these approaches will need to be shown as economically beneficial to the global community, a task that could be difficult in countries with historically limited resources allocated to healthcare. Further consideration of potential liability remains an area for ongoing evaluation.
Future research priorities
Key future research priorities include (1) meta-analyses of existing clinical trials using individual-level data to ensure adequate power and re-analysis of existing trials with attention placed on determining treatment effect heterogeneity, (2) novel clinical trial designs to test a priori hypotheses regarding treatment heterogeneity, particularly those with two or more active comparators to inform clinically relevant decisions, (3) discovery and evaluation of novel genetic and nonstandard biomarkers and (4) integration of combinations of clinically accessible features to enhance prediction of response and selection of optimal therapies.
Precision diabetes medicine is currently largely aspirational. Nevertheless, it is a potentially highly practical and economically viable alternative to current practices for diabetes prevention, diagnosis, treatment and prognostics, encompassing wide-ranging data about exposures and outcomes. In this second international consensus report, we summarize findings of 15 systematic reviews and expert opinions in prioritized areas of precision diabetes medicine. This effort was wide-reaching and multifaceted, yet of necessity not entirely comprehensive given the enormity of the diabetes field. The results show clear progress in the implementation of precision diabetes medicine, while illuminating many knowledge gaps. This review highlights the importance of specific diagnoses, and how etiological complexity and diagnostic uncertainty must be appropriately dealt with as part of this process. While precision diagnoses help determine treatment choices, there remains a great need for additional biomarkers and other signposts to guide precision prevention, therapeutics and prognostics. The dearth of data from non-European ancestry populations will need to be addressed to help ensure equity in precision diabetes medicine.
Green, A. et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia 64 , 2741–2750 (2021).
Article PubMed PubMed Central Google Scholar
Chung, W. K. et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 43 , 1617–1635 (2020).
Article CAS PubMed PubMed Central Google Scholar
Young, K. G. et al. Treatment effect heterogeneity following type 2 diabetes treatment with GLP1-receptor agonists and SGLT2-inhibitors: a systematic review. Commun. Med. https://doi.org/10.1038/s43856-023-00359-w (2023).
Semple, R. K., Patel, K. A., Auh, S., ADA/EASD PMDI & Brown, R. J. Genotype-stratified treatment for monogenic insulin resistance: a systematic review. Commun. Med. https://doi.org/10.1038/s43856-023-00368-9 (2023).
Semnani-Azad, Z. et al. Predictors and risk factors of short-term and long-term outcomes among women with gestational diabetes mellitus (GDM) and their offspring: moving toward precision prognosis? Preprint at medRxiv https://doi.org/10.1101/2023.04.14.23288199 (2023).
Naylor R. N. et al. Systematic review of treatment of beta-cell monogenic diabetes. Preprint at medRxiv https://doi.org/10.1101/2023.05.12.23289807 (2023).
Murphy, R. et al. The use of precision diagnostics for monogenic diabetes: a systematic review and expert opinion. Commun. Med. https://doi.org/10.1038/s43856-023-00369-8 (2023).
Misra, S. et al. Precision subclassification of type 2 diabetes: a systematic review. Commun. Med. https://doi.org/10.1038/s43856-023-00360-3 (2023).
Lim, S. et al. Participant characteristics in the prevention of gestational diabetes as evidence for precision medicine: a systematic review and meta-analysis. Commun. Med. https://doi.org/10.1038/s43856-023-00366-x (2023).
Benham J. L. et al. Precision gestational diabetes treatment: a systematic review and meta-analyses. Commun. Med. https://doi.org/10.1038/s43856-023-00371-0 (2023).
Jacobsen L. M. et al. Utility and precision evidence of technology in the treatment of type 1 diabetes: a systematic review. Commun. Med. https://doi.org/10.1038/s43856-023-00358-x (2023).
Felton J. L. et al. Precision diagnostics: using islet autoantibodies to characterize heterogeneity in type 1 diabetes. Preprint at medRxiv https://doi.org/10.1101/2023.04.18.23288756 (2023).
Felton, J. L. et al. Disease-modifying therapies and features linked to treatment response in type 1 diabetes prevention: a systematic review. Commun. Med. https://doi.org/10.1038/s43856-023-00357-y (2023).
Francis E. C. et al. Refining the diagnosis of gestational diabetes mellitus: a systematic review to inform efforts in precision medicine. Preprint at medRxiv https://doi.org/10.1101/2023.04.19.23288627 (2023).
Bodhini D. et al. Impact of individual and environmental factors on dietary or lifestyle interventions to prevent type 2 diabetes development: a systematic review. Commun. Med. https://doi.org/10.1038/s43856-023-00363-0 (2023).
Ahmad, A. et al. Precision prognostics for cardiovascular disease in type 2 diabetes: a systematic review and meta-analysis. Preprint at medRxiv https://doi.org/10.1101/2023.04.26.23289177 (2023).
Nolan, J. J. et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diabetes Care 45 , 261–266 (2022).
Sideri, S., Papageorgiou, S. N. & Eliades, T. Registration in the international prospective register of systematic reviews (PROSPERO) of systematic review protocols was associated with increased review quality. J. Clin. Epidemiol. 100 , 103–110 (2018).
Article PubMed Google Scholar
Booth, A. et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst. Rev. 1 , 2 (2012).
Zhang, H., Colclough, K., Gloyn, A. L. & Pollin, T. I. Monogenic diabetes: a gateway to precision medicine in diabetes. J. Clin. Invest. 131 , e142244 (2021).
Garcin, L. et al. Neonatal diabetes due to potassium channel mutation: response to sulfonylurea according to the genotype. Pediatr. Diabetes 21 , 932–941 (2020).
Article CAS PubMed Google Scholar
Nathan, D. M. & Group, D. E. R. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 37 , 9–16 (2014).
Diabetes, C. et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329 , 977–986 (1993).
Article Google Scholar
Braffett, B. H. et al. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) dtudy. Diabetes 69 , 1000–1010 (2020).
Svensson, E. et al. Early glycemic control and magnitude of HbA(1c) reduction predict cardiovascular events and mortality: population-based cohort study of 24,752 metformin initiators. Diabetes Care 40 , 800–807 (2017).
Lachin, J. M. et al. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial–revisited. Diabetes 57 , 995–1001 (2008).
The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 44 , 968–983 (1995).
McCracken, A., Gilster, S., Connerton, E., Canfield, H. & Painter-Romanello, M. Developing a tool to measure functional changes in advanced dementia. Nursingconnections 6 , 55–66 (1993).
CAS PubMed Google Scholar
Hainsworth, D. P. et al. Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC study. Diabetes Care 42 , 875–882 (2019).
Perkins, B. A. et al. Risk factors for kidney disease in type 1 diabetes. Diabetes Care 42 , 883–890 (2019).
Tesfaye, S. et al. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 352 , 341–350 (2005).
Rawshani, A. et al. Relative prognostic importance and optimal levels of risk factors for mortality and cardiovascular outcomes in type 1 diabetes mellitus. Circulation 139 , 1900–1912 (2019).
Guo, J. et al. Optimal blood pressure thresholds for minimal coronary artery disease risk in type 1 diabetes. Diabetes Care 42 , 1692–1699 (2019).
Afshinnia, F. et al. Circulating free fatty acid and phospholipid signature predicts early rapid kidney function decline in patients with type 1 diabetes. Diabetes Care 44 , 2098–2106 (2021).
Mizokami-Stout, K. et al. Symptomatic diabetic autonomic neuropathy in type 1 diabetes (T1D): findings from the T1D exchange. J. Diabetes Complicat. 36 , 108148 (2022).
Putnam, N. M. et al. Neuropsychological outcomes in individuals with type 1 and type 2 diabetes. Front Endocrinol. (Lausanne) 13 , 834978 (2022).
Jeyam, A. et al. Diabetic neuropathy is a substantial burden in people with type 1 diabetes and is strongly associated with socioeconomic disadvantage: a population-representative study from Scotland. Diabetes Care 43 , 734–742 (2020).
Mizokami-Stout, K. R. et al. The contemporary prevalence of diabetic neuropathy in type 1 diabetes: findings from the T1D exchange. Diabetes Care 43 , 806–812 (2020).
Orchard, T. J. et al. Haptoglobin 2-2 genotype and the risk of coronary artery disease in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). J. Diabetes Complicat. 30 , 1577–1584 (2016).
Costacou, T., Evans, R. W. & Orchard, T. J. Glycaemic control modifies the haptoglobin 2 allele-conferred susceptibility to coronary artery disease in Type 1 diabetes. Diabet. Med 33 , 1524–1527 (2016).
Sun, H. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin. Pr. 183 , 109119 (2022).
Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402 , 203–234 (2023).
Galaviz, K. I. et al. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care 41 , 1526–1534 (2018).
Ahlqvist, E. et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 6 , 361–369 (2018).
Udler, M. S. et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. 15 , e1002654 (2018).
Farrar, D. et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ 354 , i4694 (2016).
Hoodbhoy, Z. et al. The impact of maternal preeclampsia and hyperglycemia on the cardiovascular health of the offspring: a systematic review and meta-analysis. Am. J. Perinatol. 40 , 363–374 (2023).
Wesolowska-Andersen, A. et al. Four groups of type 2 diabetes contribute to the etiological and clinical heterogeneity in newly diagnosed individuals: an IMI DIRECT study. Cell Rep. Med 3 , 100477 (2022).
Dennis, J. M., Shields, B. M., Henley, W. E., Jones, A. G. & Hattersley, A. T. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 7 , 442–451 (2019).
Shields, B. M. et al. Patient stratification for determining optimal second-line and third-line therapy for type 2 diabetes: the TriMaster study. Nat. Med. 29 , 376–383 (2023).
Teede, H. J. et al. Association of antenatal diet and physical activity-based interventions with gestational weight gain and pregnancy outcomes: a systematic review and meta-analysis. JAMA Intern Med 182 , 106–114 (2022).
Lo, Y. M. D., Han, D. S. C., Jiang, P. & Chiu, R. W. K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 372 , eaaw3616 (2021).
US FDA. Guidance document: clinical decision support software – guidance for industry and food and drug administration staff. https://www.fda.gov/media/109618/ (2022).
Kamiza, A. B. et al. Transferability of genetic risk scores in African populations. Nat. Med. 28 , 1163–1166 (2022).
Salamanca-Buentello, F. & Daar, A. S. Nanotechnology, equity and global health. Nat. Nanotechnol. 16 , 358–361 (2021).
Fitipaldi, H. & Franks, P. W. Ethnic, gender and other sociodemographic biases in genome-wide association studies for the most burdensome non-communicable diseases: 2005-2022. Hum. Mol. Genet 32 , 520–532 (2023).
Franks, P. W. et al. Technological readiness and implementation of genomic-driven precision medicine for complex diseases. J. Intern. Med. 290 , 602–620 (2021).
Mansour Aly, D. et al. Genome-wide association analyses highlight etiological differences underlying newly defined subtypes of diabetes. Nat. Genet. 53 , 1534–1542 (2021).
Wong, E. et al. The Singapore national precision medicine strategy. Nat. Genet. 55 , 178–186 (2023).
Chikowore, T., Kamiza, A. B., Oduaran, O. H., Machipisa, T. & Fatumo, S. Non-communicable diseases pandemic and precision medicine: is Africa ready? EBioMedicine 65 , 103260 (2021).
Franks, P. W. et al. Precision medicine for cardiometabolic disease: a framework for clinical translation. Lancet Diabetes Endocrinol. (2023). https://doi.org/10.1016/S2213-8587(23)00165-1
Download references
Acknowledgements
We thank P. Siming (Department of Clinical Sciences, Lund University, Malmö, Sweden) for administrative support and M. Björklund and K. Aronsson (Faculty of Medicine Library, Lund University, Sweden) for Covidence support. We thank L. Schwingshackl (University of Freiburg, Germany) for advice on systematic review methods and ADA for administrative support. Funding: The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University, Sweden (PI: P.W. Franks) for which technical support was provided by M. Björklund and K. Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), University of Chicago (IL, USA) and the American Diabetes Association (Washington DC, USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person consensus meetings (PI: L. Phillipson, University of Chicago, IL, USA). The opinions expressed herein do not necessarily reflect those of the American Diabetes Association, the European Association for the Study of Diabetes, Novo Nordisk Foundation, National Institutes of Health (NIH), or any other society, institute, or foundation. J. Merino was partially supported by funding from the American Diabetes Association (award no. 7-21-JDFM-005) and the NIH (grant nos. P30 DK40561 and UG1 HD107691); S.J.C. is supported by a Junior Faculty Development Award from the American Diabetes Association (award no. 7-21-JDFM-005); D. Duan is supported by NIH grant no. K23DK133690; E.C.F. received grant support from NIH/NICHD grant no. K99108272-02 and NIH/NHLBI grant no. R25HL146166-05; J.M.I. was supported by NIH (grant no. K08 DK133676-01); A.R.K. is supported by the National Center for Advancing Translational Sciences, NIH, through grant no. KL2TR002490. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. A.R.K. also reports receiving research grants from the Diabetes Research Connection and the American Diabetes Association, and a research prize from the National Academy of Medicine, outside the submitted work; R.J.K. is supported by NIGMS grant no. T32GM774844 and Pediatric Endocrine Society Rising Star Award; N.-M.M. is supported by grants from the NIDDK (grant nos. R01DK125780 and R01DK134955); M.L.M. is supported by the Italian Ministry of Health grant no. GR-2019-12369702; R.N.N. was supported by ADA grant nos. 7-22-ICTSPM-17, R01DK104942 and U54DK118612; B.O. is supported by American Heart Association (grant no. 20SFRN35120152); K.A.P. is funded by the Wellcome Trust (grant no. 219606/Z/19/Z) and the National Institute for Health Research (NIHR) Exeter Biomedical Research Centre, Exeter, UK; S.J.P. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. K23DK128572); S.R. is funded by US Department of Veterans Affairs Award no. IK2-CX001907 and a Webb-Waring Biomedical Research Award from the Boettcher Foundation; M.S.-G. is supported by the American Diabetes Association (grant no. 9-22-PDFPM-04) and NIH (grant no. 5UM1DK078616-14); W.W.T. has received funding from the Monash Graduate and International Tuition Scholarship and the Australian Government Research Training Program Scholarship; M.T. is supported by grant no. K23-DK129821 from NIH/NIDDK; A.S.W. is supported by NIH/NHLBI grant no. T32HL007024; M.C. has received funding from the Monash Graduate and International Tuition Scholarship and the Australian Government Research Training Program Scholarship; T.C. is an international training fellow supported by the Wellcome Trust (grant no. 214205/Z/18/Z); S.C.C. is funded by Diabetes UK Sir George Alberti fellowship (grant no. 21/0006277); A.J.D. is supported by NIH/NIDDK grant no. T32DK007028; S.E.G. currently receives research funding from the NIH: NIDDK (TrialNet, grant no. 2U01DK106993-02) and NIAID (Immune Tolerance Network, grant no. FY20ITN372), and from Provention Bio; M.O.G. is supported by the Eris M. Field Chair in Diabetes Research and NIH grant no. P30-DK063491; J.A.G. is supported by a NHMRC Ideas Grant (APP: 2000905); H.M.I. was supported by NIH/NIDDK grant no. K23DK129799; Pilot and Feasibility Award, CDMD, NIH/NIDDK grant no. P30 DK097512; grant no. 2021258 from the Doris Duke Charitable Foundation through the COVID-19 Fund to Retain Clinical Scientists collaborative grant program and grant no. 62288 from the John Templeton Foundation; R.K.J. is funded by NIH grant no. R03-DK127472 and The Leona M. and Harry B. Helmsley Charitable Trust (grant no. 2103-05094); A.L. is supported by grant no. 2020096 from the Doris Duke Foundation and the American Diabetes Association grant no. 7-22-ICTSPM-23; D.R. is supported by NIH/NIDDK grant no. R21DK125888 and other grants from the NIH; E.S. is supported by NIH/NHLBI grant no. K24 HL152440 and other grants from the NIH; W.H.-H.S. obtained funding from NHRI, Taiwan (grant nos. MG-112-PP-18 and MG-112-PP-19); M.A.S. is supported by NIH grant no. K01/NHLBI HL157658; G.G.U. is funded by the Monash Graduate Scholarship and Monash International Tuition Scholarship; J.M.W is funded by NHMRC Ideas Grant; S.L.W. is supported by a research grant no. MR/W003740/1 from the Medical Research Council; J.B. is funded by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases; E.D.F. is a Diabetes UK RD Lawrence Fellow (19/005971); S.E.F. has a Wellcome Trust Senior Research Fellowship (grant no. 223187/Z/21/Z); K.A.M. was supported by NIH grant no. U54DK118612 and NIH/NICHD grant no. U24HD093486; J. Molnes is funded by the Norwegian Diabetes Association; P.R.N. was supported by grants from the European Research Council (grant no. 293574), the Trond Mohn Foundation (Mohn Center for Diabetes Precision Medicine, grant no. TMS2022TMT01), the Research Council of Norway (grant no. 240413) and the Novo Nordisk Foundation (grant no. 54741); T.C. was supported by grant nos. R01HL130153 and R01DK034818; J.M.D. is supported by Research England’s Expanding Excellence in England (E3) fund; J.C.F. is supported by NIH grant no. K24 HL157960; A.L.G. is a Wellcome Trust Senior Fellow (200837/Z/16/Z) and is also supported by NIDDK (award no. UM-1DK126185); M.F.G. is supported by the Swedish Heart-Lung Foundation (grant no. 20190470), Swedish Research Council (EXODIAB, grant nos. 2009-1039 and 2018-02837), Swedish Foundation for Strategic Research (LUDC-IRC, grant no. 15-0067) and EU H2020-JTI-lMl2-2015-05 (grant agreement no. 115974 - BEAt-DKD); M.-F.H. was supported by the American Diabetes Association Pathways Award no. 1-15-ACE-26; J.L.J. is funded by the NIH (grant no. 5R01DK118403); L.M.L. is supported by National Institute of Healths grant no. P30DK036836; S.S.L. is funded by the Australian National Health and Medical Research Council (NHMRC) Fellowship; C.C., J. Merino, A.C.B.T., M.K.A., M.G.-F., T.H. and R.J.F.L. acknowledge that The Novo Nordisk Foundation Center for Basic Metabolic Research is supported by and unrestricted grant from the Novo Nordisk Foundation (grant no. NNF18CC0034900); R.C.W.M. acknowledges support from the Research Grants Council of the Hong Kong Special Administrative Region (grant no. CU R4012-18), the Croucher Foundation Senior Medical Research Fellowship and University Grants Committee Research Grants Matching Scheme and Research Committee Postdoctoral Fellowship Scheme of the Chinese University of Hong Kong; J.B.M. reports funding from NIH grant nos. U01 DK078616 and R01 HL151855; S.M. has a personal award from Wellcome Trust Career Development scheme (grant no. 223024/Z/21/Z) and is supported by the NIHR Imperial Biomedical Research Centre; K.R.O. is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health; S.E.O. is funded by the British Heart Foundation (grant no. RG/17/12/33167) and the Medical Research Council (grant no. MC_UU_00014/4); T.I.P. was supported by NIH grant nos. U54DK118612, NIH/NICHD U24HD093486 and NIH/NHGRI U01HG007775; L.M.R. is funded by the National Institute of Health (grant no. 5R01DK124806); relevant funding for M.J.R. includes NIH grant no. R01 DK124395 and NIH grant no. 1 R01 DK121843-01; R.M.R. acknowledges the support of the British Heart Foundation (grant no. RE/18/5/34216); T.T. is supported by The Folkhalsan Research Foundation and The Academy of Finland/University of Helsinki (grant nos. 312072 and 336826); M.S.U. is supported by an NIH grant no. K23DK114551 and the Doris Duke Foundation Clinical Scientist Development Award; S.S.R. is supported by NIH/NIDDK grant no. R01DK122586 and other grants from the NIH; P.W.F. is supported by research grants from the European Commission (grant no. ERC-CoG_NASCENT-681742) and the Swedish Research Council (grant nos. 2014-03529 and 2019-01348).
Author information
These authors contributed equally: Deirdre K. Tobias, Jordi Merino, Abrar Ahmad, Catherine Aiken, Jamie L. Benham, Dhanasekaran Bodhini, Kevin Colclough, Sara J. Cromer, Daisy Duan, Jamie L. Felton, Ellen C. Francis, Pieter Gillard, Véronique Gingras, Romy Gaillard, Eram Haider, Alice Hughes, Jennifer M. Ikle, Laura M. Jacobsen, Anna R. Kahkoska, Jarno L. T. Kettunen, Raymond J. Kreienkamp, Lee-Ling Lim, Jonna M. E. Männistö, Robert Massey, Niamh-Maire Mclennan, Mario Luca Morieri, Jasper Most, Rochelle N. Naylor, Bige Ozkan, Kashyap Amratlal Patel, Scott J. Pilla, Katsiaryna Prystupa, Sridaran Raghaven, Mary R. Rooney, Martin Schön, Zhila Semnani-Azad, Magdalena Sevilla-Gonzalez, Pernille Svalastoga, Wubet Worku Takele, Claudia Ha-ting Tam, Anne C. Baun Thuesen, Mustafa Tosur, Amelia S. Wallace, Caroline C. Wang, Jessie J. Wong, Jennifer M. Yamamoto, Katherine Young.
These authors jointly supervised this work: Rebecca J. Brown, Liana K. Billings, Kristen Boyle, John M. Dennis, Jose C. Florez, Anna L. Gloyn, Maria F. Gomez, Peter A. Gottlieb, Siri Atma W. Greeley, Kurt Griffin, Andrew T. Hattersley, Irl B. Hirsch, Marie-France Hivert, Korey K. Hood, Jami L. Josefson, Soo Heon Kwak, Lori M. Laffel, Siew S. Lim, Ruth J. F. Loos, Ronald C. W. Ma, Chantal Mathieu, Nestoras Mathioudakis, James B. Meigs, Shivani Misra, Viswanathan Mohan, Rinki Murphy, Richard Oram, Katharine R. Owen, Susan E. Ozanne, Ewan R. Pearson, Wei Perng, Toni I. Pollin, Richard E. Pratley, Leanne M. Redman, Maria J. Redondo, Rebecca M. Reynolds, Robert K. Semple, Jennifer L. Sherr, Emily K. Sims, Arianne Sweeting, Tiinamaija Tuomi, Miriam S. Udler, Kimberly K. Vesco, Tina Vilsbøll, Robert Wagner, Stephen S. Rich, Paul W. Franks.
Authors and Affiliations
Division of Preventative Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Deirdre K. Tobias & Vanessa Santhakumar
Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Deirdre K. Tobias, Zhila Semnani-Azad, Marta Guasch-Ferré & Paul W. Franks
Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Jordi Merino, Anne Cathrine B. Thuesen, Mette K. Andersen, Christoffer Clemmensen, Torben Hansen, Mariam Nakabuye & Ruth J. F. Loos
Diabetes Unit, Endocrine Division, Massachusetts General Hospital, Boston, MA, USA
Jordi Merino, Sara J. Cromer, Raymond J. Kreienkamp, Aaron Leong, Camille E. Powe, Jose C. Florez, Marie-France Hivert & Miriam S. Udler
Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA
Jordi Merino, Raymond J. Kreienkamp, Aaron J. Deutsch, Jose C. Florez & Miriam S. Udler
Department of Clinical Sciences, Lund University Diabetes Centre, Lund University, Malmö, Sweden
Abrar Ahmad, Monika Dudenhöffer-Pfeifer, Hugo Fitipaldi, Hugo Pomares-Millan, Maria F. Gomez & Paul W. Franks
Department of Obstetrics and Gynaecology, The Rosie Hospital, Cambridge, UK
Catherine Aiken
NIHR Cambridge Biomedical Research Centre, University of Cambridge, Cambridge, UK
Departments of Medicine and Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada
Jamie L. Benham
Department of Molecular Genetics, Madras Diabetes Research Foundation, Chennai, India
Dhanasekaran Bodhini
Division of Pediatric Endocrinology, Department of Pediatrics, Saint Louis University School of Medicine, SSM Health Cardinal Glennon Children’s Hospital, St. Louis, MO, USA
Amy L. Clark
Department of Clinical and Biomedical Sciences, University of Exeter Medical School, Exeter, UK
Kevin Colclough, Alice Hughes, Kashyap Amratlal Patel, Katherine Young, Angus G. Jones, Elisa de Franco, Sarah E. Flanagan, Andrew McGovern, John M. Dennis, Andrew T. Hattersley & Richard Oram
CIBER-BBN, ISCIII, Madrid, Spain
Rosa Corcoy
Institut d’Investigació Biomèdica Sant Pau (IIB SANT PAU), Barcelona, Spain
Departament de Medicina, Universitat Autònoma de Barcelona, Bellaterra, Spain
Programs in Metabolism and Medical & Population Genetics, Broad Institute, Cambridge, MA, USA
Sara J. Cromer, Raymond J. Kreienkamp, Magdalena Sevilla-Gonzalez, Aaron J. Deutsch, Camille E. Powe, Jose C. Florez & Miriam S. Udler
Department of Medicine, Harvard Medical School, Boston, MA, USA
Sara J. Cromer, Magdalena Sevilla-Gonzalez, Tinashe Chikowore, Aaron J. Deutsch, Aaron Leong, Camille E. Powe, Jose C. Florez, James B. Meigs & Miriam S. Udler
Division of Endocrinology, Diabetes and Metabolism, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA
Jamie L. Felton, Linda A. DiMeglio, Carmella Evans-Molina, Arianna Harris-Kawano, Heba M. Ismail, Dianna Perez, Gabriela S. F. Monaco & Emily K. Sims
Herman B Wells Center for Pediatric Research, University School of Medicine, Indianapolis, IN, USA
Center for Diabetes and Metabolic Diseases, Indiana University School of Medicine, Indianapolis, IN, USA
Department of Biostatistics and Epidemiology, Rutgers School of Public Health, Piscataway, NJ, USA
Ellen C. Francis
University Hospital Leuven, Leuven, Belgium
Pieter Gillard & Chantal Mathieu
Department of Nutrition, Université de Montréal, Montreal, Quebec, Quebec, Canada
Véronique Gingras
Research Center, Sainte-Justine University Hospital Center, Montreal, Quebec, Quebec, Canada
Department of Pediatrics, Erasmus Medical Center, Rotterdam, the Netherlands
Romy Gaillard
Division of Population Health & Genomics, School of Medicine, University of Dundee, Dundee, UK
Eram Haider, Robert Massey, Adem Y. Dawed & Ewan R. Pearson
Department of Pediatrics, Stanford School of Medicine, Stanford University, Stanford, CA, USA
Jennifer M. Ikle & Anna L. Gloyn
Stanford Diabetes Research Center, Stanford School of Medicine, Stanford University, Stanford, CA, USA
University of Florida, Gainesville, FL, USA
Laura M. Jacobsen
Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Anna R. Kahkoska
Helsinki University Hospital, Abdominal Centre/Endocrinology, Helsinki, Finland
Jarno L. T. Kettunen & Tiinamaija Tuomi
Folkhalsan Research Center, Helsinki, Finland
Institute for Molecular Medicine Finland FIMM, University of Helsinki, Helsinki, Finland
Department of Pediatrics, Division of Endocrinology, Boston Children’s Hospital, Boston, MA, USA
Raymond J. Kreienkamp
Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
Lee-Ling Lim
Asia Diabetes Foundation, Hong Kong SAR, China
Department of Medicine & Therapeutics, Chinese University of Hong Kong, Hong Kong SAR, China
Lee-Ling Lim, Claudia Ha-ting Tam, Chuiguo Huang, Gechang Yu, Yingchai Zhang & Ronald C. W. Ma
Departments of Pediatrics and Clinical Genetics, Kuopio University Hospital, Kuopio, Finland
Jonna M. E. Männistö
Department of Medicine, University of Eastern Finland, Kuopio, Finland
Centre for Cardiovascular Science, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Niamh-Maire Mclennan, Rebecca M. Reynolds & Robert K. Semple
Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA, USA
Rachel G. Miller & Tina Costacou
Metabolic Disease Unit, University Hospital of Padova, Padova, Italy
Mario Luca Morieri
Department of Medicine, University of Padova, Padova, Italy
Department of Orthopedics, Zuyderland Medical Center, Sittard-Geleen, The Netherlands
Jasper Most
Departments of Pediatrics and Medicine, University of Chicago, Chicago, IL, USA
Rochelle N. Naylor
Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Bige Ozkan, Mary R. Rooney, Amelia S. Wallace & Elizabeth Selvin
Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins School of Medicine, Baltimore, MD, USA
Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
Scott J. Pilla, Sarah Kanbour, Sudipa Sarkar & Nestoras Mathioudakis
Department of Health Policy and Management, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA
Scott J. Pilla
Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University Düsseldorf, Düsseldorf, Germany
Katsiaryna Prystupa, Martin Schön & Robert Wagner
German Center for Diabetes Research (DZD), Neuherberg, Germany
Katsiaryna Prystupa, Martin Schön, Norbert Stefan & Robert Wagner
Section of Academic Primary Care, US Department of Veterans Affairs Eastern Colorado Health Care System, Aurora, CO, USA
Sridharan Raghavan
Department of Medicine, University of Colorado School of Medicine, Aurora, CO, USA
Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Mary R. Rooney, Amelia S. Wallace, Caroline C. Wang, Debashree Ray & Elizabeth Selvin
Institute of Diabetes Research and Metabolic Diseases (IDM), Helmholtz Center Munich, Neuherberg, Germany
Martin Schön & Norbert Stefan
Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, Bratislava, Slovakia
Martin Schön
Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, Boston, MA, USA
Magdalena Sevilla-Gonzalez
Mohn Center for Diabetes Precision Medicine, Department of Clinical Science, University of Bergen, Bergen, Norway
Pernille Svalastoga, Ingvild Aukrust, Janne Molnes & Pål Rasmus Njølstad
Children and Youth Clinic, Haukeland University Hospital, Bergen, Norway
Pernille Svalastoga & Pål Rasmus Njølstad
Eastern Health Clinical School, Monash University, Melbourne, Victoria, Australia
Wubet Worku Takele, Gebresilasea Gendisha Ukke & Siew S. Lim
Laboratory for Molecular Epidemiology in Diabetes, Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong SAR, China
Claudia Ha-ting Tam, Chuiguo Huang, Gechang Yu, Yingchai Zhang & Ronald C. W. Ma
Hong Kong Institute of Diabetes and Obesity, The Chinese University of Hong Kong, Hong Kong SAR, China
Claudia Ha-ting Tam & Ronald C. W. Ma
Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA
Mustafa Tosur & Maria J. Redondo
Division of Pediatric Diabetes and Endocrinology, Texas Children’s Hospital, Houston, TX, USA
Mustafa Tosur, Marzhan Urazbayeva & Maria J. Redondo
Children’s Nutrition Research Center, USDA/ARS, Houston, TX, USA
Mustafa Tosur
Stanford University School of Medicine, Stanford, CA, USA
Jessie J. Wong & Korey K. Hood
Internal Medicine, University of Manitoba, Winnipeg, Manitoba, Canada
Jennifer M. Yamamoto
Department of Diabetology, APHP, Paris, France
Chloé Amouyal
Sorbonne Université, INSERM, NutriOmic team, Paris, France
Department of Nutrition, Dietetics and Food, Monash University, Melbourne, Victoria, Australia
Maxine P. Bonham & Gloria K. W. Leung
Monash Centre for Health Research and Implementation, Monash University, Clayton, Victoria, Australia
Mingling Chen
Health Management Center, The Second Affiliated Hospital of Chongqing Medical University, Chongqing Medical University, Chongqing, China
Feifei Cheng
MRC/Wits Developmental Pathways for Health Research Unit, Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Tinashe Chikowore
Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Sydney Brenner Institute for Molecular Bioscience, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Department of Women and Children’s Health, King’s College London, London, UK
Sian C. Chivers & Sara L. White
Lifecourse Epidemiology of Adiposity and Diabetes (LEAD) Center, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Dana Dabelea, Kristen Boyle & Wei Perng
Section of Adult and Pediatric Endocrinology, Diabetes and Metabolism, Kovler Diabetes Center, University of Chicago, Chicago, IL, USA
Laura T. Dickens
Department of Pediatrics, Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN, USA
Linda A. DiMeglio
Richard L. Roudebush VAMC, Indianapolis, IN, USA
Carmella Evans-Molina
Biomedical Research Institute Girona, IdIBGi, Girona, Spain
María Mercè Fernández-Balsells
Diabetes, Endocrinology and Nutrition Unit Girona, University Hospital Dr Josep Trueta, Girona, Spain
Institute of Health System Science, Feinstein Institutes for Medical Research, Northwell Health, Manhasset, NY, USA
Stephanie L. Fitzpatrick
University of California at San Francisco, Department of Pediatrics, Diabetes Center, San Francisco, CA, USA
Stephen E. Gitelman
Division of Endocrinology, Diabetes and Metabolism, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Mark O. Goodarzi
Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Adelaide Medical School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, South Australia, Australia
Jessica A. Grieger, Nahal Habibi, Kai Liu, Maleesa Pathirana & Alejandra Quinteros
Robinson Research Institute, The University of Adelaide, Adelaide, South Australia, Australia
Jessica A. Grieger, Nahal Habibi, Maleesa Pathirana & Shao J. Zhou
Department of Public Health and Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Marta Guasch-Ferré
Division of Endocrinology and Diabetes, Department of Pediatrics, Sanford Children’s Hospital, Sioux Falls, SD, USA
Benjamin Hoag
University of South Dakota School of Medicine, E Clark St, Vermillion, SD, USA
Department of Biomedical Informatics, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Randi K. Johnson & Maggie A. Stanislawski
Department of Epidemiology, Colorado School of Public Health, Aurora, CO, USA
Randi K. Johnson
Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK
Angus G. Jones, Andrew T. Hattersley & Richard Oram
Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford, Oxford, UK
Robert W. Koivula, Katharine R. Owen & Paul W. Franks
Division of General Internal Medicine, Massachusetts General Hospital, Boston, MA, USA
Aaron Leong & James B. Meigs
UPMC Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA
Ingrid M. Libman
Center for Translational Immunology, Benaroya Research Institute, Seattle, WA, USA
S. Alice Long
Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
William L. Lowe Jr
Department of Pathology & Molecular Medicine, McMaster University, Hamilton, Ontario, Canada
Robert W. Morton
Population Health Research Institute, Hamilton, Ontario, Canada
Robert W. Morton, Russell de Souza & Diana Sherifali
Department of Translational Medicine, Medical Science, Novo Nordisk Foundation, Hellerup, Denmark
Robert W. Morton & Paul W. Franks
Department of Diabetes and Endocrinology, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
Ayesha A. Motala
Center for Public Health Genomics, Department of Public Health Sciences, University of Virginia, Charlottesville, VA, USA
Suna Onengut-Gumuscu & Stephen S. Rich
Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN, USA
James S. Pankow
Department of Chronic Diseases and Metabolism, Clinical and Experimental Endocrinologyó, KU Leuven, Leuven, Belgium
Sofia Pazmino, Nele Steenackers & Bart Van der Schueren
School of Health and Wellbeing, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK
John R. Petrie
Department of Obstetrics, Gynecology, and Reproductive Biology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Camille E. Powe
Sanford Children’s Specialty Clinic, Sioux Falls, SD, USA
Rashmi Jain
Department of Pediatrics, Sanford School of Medicine, University of South Dakota, Sioux Falls, SD, USA
Rashmi Jain & Kurt Griffin
Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Debashree Ray
Centre for Physical Activity Research, Rigshospitalet, Copenhagen, Denmark
Mathias Ried-Larsen
Institute for Sports and Clinical Biomechanics, University of Southern Denmark, Odense, Denmark
Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, Indiana University School of Medicine, Indianapolis, IN, USA
AMAN Hospital, Doha, Qatar
Sarah Kanbour
Department of Preventive Medicine, Division of Biostatistics, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Denise M. Scholtens
Institute of Molecular and Genomic Medicine, National Health Research Institutes, Zhunan, Taiwan
Wayne Huey-Herng Sheu
Divsion of Endocrinology and Metabolism, Taichung Veterans General Hospital, Taichung, Taiwan
Division of Endocrinology and Metabolism, Taipei Veterans General Hospital, Taipei, Taiwan
Center for Interventional Immunology, Benaroya Research Institute, Seattle, WA, USA
Cate Speake
Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Andrea K. Steck & Peter A. Gottlieb
University Hospital of Tübingen, Tübingen, Germany
Norbert Stefan
Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus, Denmark
University of Newcastle, Newcastle upon Tyne, UK
Rachael Taylor
Sections on Genetics and Epidemiology, Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA
Sok Cin Tye
Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, the Netherlands
Gastroenterology, Baylor College of Medicine, Houston, TX, USA
Marzhan Urazbayeva
Department of Endocrinology, University Hospitals Leuven, Leuven, Belgium
Bart Van der Schueren
Sorbonne University, Inserm U938, Saint-Antoine Research Centre, Institute of Cardiometabolism and Nutrition, Paris, France
Camille Vatier
Department of Endocrinology, Diabetology and Reproductive Endocrinology, Assistance Publique-Hôpitaux de Paris, Saint-Antoine University Hospital, National Reference Center for Rare Diseases of Insulin Secretion and Insulin Sensitivity (PRISIS), Paris, France
Royal Melbourne Hospital Department of Diabetes and Endocrinology, Parkville, Victoria, Australia
John M. Wentworth
Walter and Eliza Hall Institute, Parkville, Victoria, Australia
University of Melbourne Department of Medicine, Parkville, Victoria, Australia
Deakin University, Melbourne, Victoria, Australia
Wesley Hannah
Department of Epidemiology, Madras Diabetes Research Foundation, Chennai, India
Department of Diabetes and Endocrinology, Guy’s and St Thomas’ Hospitals NHS Foundation Trust, London, UK
Sara L. White
School of Agriculture, Food and Wine, University of Adelaide, Adelaide, South Australia, Australia
Shao J. Zhou
Institut Cochin, Inserm U 10116, Paris, France
Jacques Beltrand & Michel Polak
Pediatric Endocrinology and Diabetes, Hopital Necker Enfants Malades, APHP Centre, Université de Paris, Paris, France
Department of Medical Genetics, Haukeland University Hospital, Bergen, Norway
Ingvild Aukrust & Janne Molnes
Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
Kristin A. Maloney & Toni I. Pollin
Department of Epidemiology, Geisel School of Medicine at Dartmouth, Hanover, NH, USA
Hugo Pomares-Millan
Nephrology, Dialysis and Renal Transplant Unit, IRCCS—Azienda Ospedaliero-Universitaria di Bologna, Alma Mater Studiorum University of Bologna, Bologna, Italy
Michele Provenzano
Department of Medical Genetics, AP-HP Pitié-Salpêtrière Hospital, Sorbonne University, Paris, France
Cécile Saint-Martin
Global Center for Asian Women’s Health, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Cuilin Zhang
Department of Obstetrics and Gynecology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Kaiser Permanente Northern California Division of Research, Oakland, CA, USA
Department of Epidemiology and Biostatistics, University of California, San Francisco, CA, USA
National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA
Sungyoung Auh & Rebecca J. Brown
Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada
Russell de Souza
Ann & Robert H. Lurie Children’s Hospital of Chicago, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Andrea J. Fawcett & Jami L. Josefson
Department of Clinical and Organizational Development, Chicago, IL, USA
Andrea J. Fawcett
American Diabetes Association, Arlington, VA, USA
Chandra Gruber
College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Eskedar Getie Mekonnen
Global Health Institute, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium
Department of Medicine and Kovler Diabetes Center, University of Chicago, Chicago, IL, USA
Emily Mixter & Louis H. Philipson
School of Nursing, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada
Diana Sherifali
Division of Endocrinology, Metabolism, Diabetes, University of Colorado, Aurora, CO, USA
Robert H. Eckel
Department of Clinical Medicine, School of Medicine, Trinity College Dublin, Dublin, Ireland
John J. Nolan
Department of Endocrinology, Wexford General Hospital, Wexford, Ireland
Division of Endocrinology, NorthShore University HealthSystem, Skokie, IL, USA
Liana K. Billings
Department of Medicine, Prtizker School of Medicine, University of Chicago, Chicago, IL, USA
Department of Genetics, Stanford School of Medicine, Stanford University, Stanford, CA, USA
Anna L. Gloyn
Faculty of Health, Aarhus University, Aarhus, Denmark
Maria F. Gomez
Departments of Pediatrics and Medicine and Kovler Diabetes Center, University of Chicago, Chicago, IL, USA
Siri Atma W. Greeley
Sanford Research, Sioux Falls, SD, USA
Kurt Griffin
University of Washington School of Medicine, Seattle, WA, USA
Irl B. Hirsch
Department of Population Medicine, Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA, USA
Marie-France Hivert
Department of Medicine, Universite de Sherbrooke, Sherbrooke, Quebec, Canada
Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Republic of Korea
Soo Heon Kwak
Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA
Lori M. Laffel
Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Ruth J. F. Loos
Broad Institute, Cambridge, MA, USA
James B. Meigs
Division of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Shivani Misra
Department of Diabetes & Endocrinology, Imperial College Healthcare NHS Trust, London, UK
Department of Diabetology, Madras Diabetes Research Foundation & Dr. Mohan’s Diabetes Specialities Centre, Chennai, India
Viswanathan Mohan
Department of Medicine, Faculty of Medicine and Health Sciences, University of Auckland, Auckland, New Zealand
Rinki Murphy
Auckland Diabetes Centre, Te Whatu Ora Health New Zealand, Auckland, New Zealand
Medical Bariatric Service, Te Whatu Ora Counties, Health New Zealand, Auckland, New Zealand
Oxford NIHR Biomedical Research Centre, University of Oxford, Oxford, UK
Katharine R. Owen
University of Cambridge, Metabolic Research Laboratories and MRC Metabolic Diseases Unit, Wellcome-MRC Institute of Metabolic Science, Cambridge, UK
Susan E. Ozanne
Department of Epidemiology & Public Health, University of Maryland School of Medicine, Baltimore, MD, USA
Toni I. Pollin
Department of Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI, USA
Rodica Pop-Busui
AdventHealth Translational Research Institute, Orlando, FL, USA
Richard E. Pratley
Pennington Biomedical Research Center, Baton Rouge, LA, USA
Leanne M. Redman
MRC Human Genetics Unit, Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK
Robert K. Semple
Yale School of Medicine, New Haven, CT, USA
Jennifer L. Sherr
Faculty of Medicine and Health, University of Sydney, Sydney, New South Wales, Australia
Arianne Sweeting
Department of Endocrinology, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia
Kaiser Permanente Northwest, Kaiser Permanente Center for Health Research, Portland, OR, USA
Kimberly K. Vesco
Clinial Research, Steno Diabetes Center Copenhagen, Herlev, Denmark
Tina Vilsbøll
Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Department of Endocrinology and Diabetology, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany
Robert Wagner
You can also search for this author in PubMed Google Scholar
Contributions
P.W.F. was the PMDI second international consensus report chair. R.H.E., J.J.N., L.H.P., S.R. and P.W.F. planned the work. D.K.T., C.G., S.S.R. and P.W.F. coordinated the work. D.T., R.J.dS. and D.S. provided technical guidance on systematic reviews. R.J.B., L.K.B., K.B., T.C., J.M.D., J.C.F., A.L.G., M.F.G., P.A.G., S.A.W.G., K.G., A.T.H., I.B.H., M.-F.H., K.K.H., J.L.J., S.H.K., L.M.L., S.S.L., R.J.F.L., R.C.W.M., C.M., N.M., J. Merino, S.M., V.M., R.M., R.O., K.R.O., S.E.O., E.R.P., W.P., T.I.P., R.P.-B., R.E.P., L.M.R., M.J.R., R.M.R., R.K.S., J.L.S., E.K.S., A.S., T.T., M.S.U., K.K.V., T.V. and R.W. were responsible for systematic review working group leadership. D.T., J. Merino, J.J.N., L.H.P., R.J.B., L.K.B., K.B., T.C., J.D., J.C.F., A.L.G., M.F.G., P.A.G., S.A.W.G., K.G., A.T.H., I.B.H., M.-F.H., K.K.H., J.L.J., S.H.K., L.M.L., S.S.L., R.J.F.L., R.C.W.M., C.M., N.M., J. Molnes, S.M., V.M., R.M., R.O., K.R.O., S.E.O., E.R.P., W.P., T.I.P., R.P.-B., R.E.P., L.M.R., M.J.R., R.M.R., R.K.S., J.L.S., E.K.S., A.S., T.T., M.S.U., K.K.V., T.V., R.W., S.S.R. and P.W.F. wrote the manuscript. J. Merino, M.-F.H., S.S.R. and P.W.F. revised the manuscript. All authors reviewed and approved the submitted version of this manuscript.
Corresponding author
Correspondence to Paul W. Franks .
Ethics declarations
Competing interests.
The following authors report unrelated disclosures. J. Merino. is an Associate Editor of Diabetologia; S.J.C. reports a close family member employed by a Johnson & Johnson company; A.R.K. is supported by the National Center for Advancing Translational Sciences, NIH, through grant KL2TR002490. A.R.K. also reports receiving research grants from the Diabetes Research Connection and the American Diabetes Association, and a research prize from the National Academy of Medicine, outside the submitted work; J.L.T.K. declares lecture fees from Novo Nordisk, international conference costs covered by Medtronic, AstraZeneca and Novo Nordisk; L.-L.L. reports receiving research grants via her academic institution and serving on an advisory board for Boehringer Ingelheim, Novartis, Novo Nordisk, Viatris Pharmaceutical and Zuellig Pharma. L.-L.L. has received speaking honoraria from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Roche, Sanofi, Servier, Viatris Pharmaceutical and Zuellig Pharma for scientific talks over which she had full control of the content; R.M. has received consulting or speaker honoraria from Eli Lilly, Novo Nordisk and Boeringer Engelheim; M.L.M. has received lecture, consultancy, or advisory board fees from Amarin, Amgen, Eli Lilly, Merck Sharp & Dohme, Mylan, Novo Nordisk, Novartis, Servier and SlaPharma; C.E.-M. reports serving on advisory boards for Provention Bio, Isla Technologies, MaiCell Technologies, Avotres, DiogenyX and Neurodon; receiving in-kind research support from Bristol Myers Squibb and Nimbus Pharmaceuticals; receiving investigator-initiated grants from Lilly Pharmaceuticals and Astellas Pharmaceuticals; and having patent (16/291,668) Extracellular Vesicle Ribonucleic Acid (RNA) Cargo as a Biomarker of Hyperglycaemia and Type 1 Diabetes and provisional patent (63/285,765) Biomarker for Type 1 Diabetes (PDIA1 as a biomarker of β cell stress); S.E.G. has been on advisory boards for Abata, Avotres, Genentech, GentiBio, Provention Bio, Sana Biotechnology, Sanofi and is a DSMB member for INNODIA (MELD, ATG), Diamyd (DIAGNODE-3, GAD) and JDRF (TADPOL, DFMO); M.O.G. has served on an advisory board for Nestle Health Science; A.L. reports a close family member employed by Merck & Co., Inc.; C.E.P. is an Associate Editor of Diabetes Care, receives payments from Wolters Kluwer for UpToDate chapters on diabetes in pregnancy and has received payments for consulting and speaking from Mediflix Inc; E.S. is a Deputy Editor of Diabetes Care and a member of the editorial board of Diabetologia and receives payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate on measurements of glycemic control and screening tests for type 2 diabetes; W.H.H.S. has been an Advisor and/or Speaker for AstraZeneca, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals, Daiichi Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Sanofi-Aventis, Takeda Pharmaceutical Company; J.M.W. reports a research collaboration with ENABLE Biosciences; R.J.B. has received research support from Amryt, Third Rock Ventures, Ionis and Regeneron; L.K.B. has received consulting honoraria from Bayer, Novo Nordisk, Sanofi, Lilly and Xeris; J.C.F. has received speaking honoraria from AstraZeneca and Novo Nordisk for scientific talks over which he had full control of content; A.L.G.’s spouse holds stock options in Roche and is an employee of Genentech; M.F.G. has received financial and nonfinancial (in kind) support from Boehringer Ingelheim Pharma GmbH, JDRF International, Eli Lilly, AbbVie, Sanofi-Aventis, Astellas, Novo Nordisk A/S, Bayer AG, within EU grant H2020-JTI-lMl2-2015-05 (grant agreement number 115974 - BEAt-DKD); and also from Novo Nordisk, Pfizer, Follicum, Coegin Pharma, Abcentra, Probi, Johnson & Johnson, within a project funded by the Swedish Foundation for Strategic Research on precision medicine in diabetes (LUDC-IRC no. 15-0067). M.F.G. has received personal consultancy fees from Lilly and Tribune Therapeutics AB. R.dS. has served as an external resource person to the World Health Organization’s Nutrition Guidelines Advisory Group on trans fats, saturated fats, and polyunsaturated fats. The WHO paid for his travel and accommodation to attend meetings from 2012 to 2017 to present and discuss this work. He has presented updates of this work to the WHO in 2022. He has also done contract research for the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism, and Diabetes, Health Canada, and the World Health Organization for which he received remuneration. He has received speaker’s fees from the University of Toronto, and McMaster Children’s Hospital. He has served as an independent director of the Helderleigh Foundation (Canada). He serves as a member of the Nutrition Science Advisory Committee to Health Canada (Government of Canada), co-chair of the Method working group of the ADA/EASD Precision Medicine in Diabetes group and is a co-opted member of the Scientific Advisory Committee on Nutrition (SACN) Subgroup on the Framework for the Evaluation of Evidence (Public Health England). He has held grants from the Canadian Institutes of Health Research, Canadian Foundation for Dietetic Research, Population Health Research Institute, and Hamilton Health Sciences Corporation as a principal investigator, and is a co-investigator on several funded team grants from the Canadian Institutes of Health Research. P.A.G. consults or has consulted for Provention Bio and Viacyte. He is the cofounder of IM Therapeutics and serves as CMO on the Board as well as being a shareholder. P.A.G. has also received research support from Helmsley Foundation, Nova Pharmaceuticals, Imcyse, Provention Bio, Intrexon T1D Partners and Novartis. All payments have been made to the University of Colorado; R.C.W.M. has received research grants from AstraZeneca, Bayer, Novo Nordisk, Pfizer, Roche Diagnostics (HK) Ltd, Tricida Inc, and consultancy/speaker honorarium from AstraZeneca, Boehringer Ingelheim, Bayer and Merck. All proceeds have been donated to the Chinese University of Hong Kong to support diabetes research. R.C.W.M. is a cofounder of GemVCare, a technology start-up initiated with support from the Hong Kong Government Innovation and Technology Commission and its Technology Start-up Support Scheme for Universities (TSSSU); C.M. serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Imcyse, Insulet, Zealand Pharma, Avotres, Mannkind, Sandoz and Vertex. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for C.M. from Medtronic, Imcyse, Novo Nordisk, Sanofi and ActoBio Therapeutics; C.M. serves or has served on the speakers’ bureau for Novo Nordisk, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, AstraZeneca and Novartis. Financial compensation for these activities has been received by KU Leuven. C.M. has been an Advisor and/or Speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi-Aventis. C.M. is president of EASD (all external support of EASD is to be found on www.easd.org ); N.S. is Senior Associate Editor of Diabetes and has received speaking honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer and Sanofi for scientific talks over which he had full control of content; J.B.M. is an Academic Associate for Quest Inc. Diagnostics R&D; S.M. has investigator-initiated funding from DexCom, has received speaker fees from Sanofi for a scientific talk over which she had full control of content and serves on the Board of Trustees for the Diabetes Research & Wellness Foundation (UK); V.M. has acted as consultant and speaker, received research or educational grants from Novo Nordisk, MSD, Eli Lilly, Novartis, Boehringer Ingelheim, Lifescan J&J, Sanofi-Aventis, Roche Diagnostics, Abbott and from several Indian pharmaceutical companies including USV, Dr. Reddy’s Laboratories and Sun Pharma. E.R.P. has received speaking honoraria from Lilly, Novo Nordisk and Illumina for scientific talks over which he had full control of content; J.L.S. serves or has served on advisory panels for Bigfoot Biomedical, Cecelia Health, Insulet Corportation, Medtronic Diabetes, StartUp Health Diabetes Moonshot and Vertex. She has served as a consultant to Abott Diabetes, Bigfoot Biomedical, Insulet, Medtronic Diabetes and Zealand. Yale School of Medicine has received research support for JLS from Abott Diabetes, JAEB Center for Health Research, JDRF, Insulet, Medtronic, NIH and Provention Bio; M.S.U. reports an unpaid collaborator with AstraZeneca; K.K.V. reports that her institution received funding from Pfizer Independent Grants for Learning and Change for an unrelated study. R.W. declares lecture fees from Novo Nordisk, Sanofi and Eli Lilly. He served on an advisory board for Akcea Therapeutics, Daiichi Sankyo, Sanofi, Eli Lilly and Novo Nordisk; P.W.F. has received consulting fees from Zoe Ltd and research grants from multiple pharmaceutical companies; R.W.M. and P.W.F. are employees of the Novo Nordisk Foundation, a private philanthropic enterprise foundation based in Denmark supporting research and education in the life sciences. All other authors report no disclosures.
Peer review
Peer review information.
Nature Medicine thanks Sisse Ostrowski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Joao Monteiro, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Tobias, D.K., Merino, J., Ahmad, A. et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat Med 29 , 2438–2457 (2023). https://doi.org/10.1038/s41591-023-02502-5
Download citation
Received : 17 May 2023
Accepted : 14 July 2023
Published : 05 October 2023
Issue Date : October 2023
DOI : https://doi.org/10.1038/s41591-023-02502-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Simple Steps to Preventing Diabetes

Keeping weight in check, being active, and eating a healthy diet can help prevent most cases of type 2 diabetes.
If type 2 diabetes were an infectious disease, passed from one person to another, public health officials would say we’re in the midst of an epidemic. This difficult disease is striking an ever-growing number of adults, and with the rising rates of childhood obesity, it has become more common in youth, especially among certain ethnic groups ( learn more about diabetes, including the other types and risk factors ).
The good news is that prediabetes and type 2 diabetes are largely preventable. About 9 in 10 cases in the U.S. can be avoided by making lifestyle changes. These same changes can also lower the chances of developing heart disease and some cancers. The key to prevention can be boiled down to five words: Stay lean and stay active.
What if I already have diabetes?
Simple steps to lowering your risk, control your weight.
Excess weight is the single most important cause of type 2 diabetes. Being overweight increases the chances of developing type 2 diabetes seven-fold. Being obese makes you 20 to 40 times more likely to develop diabetes than someone with a healthy weight. [1]
Losing weight can help if your weight is above the healthy-weight range. Losing 7-10% of your current weight can cut your chances of developing type 2 diabetes in half.
Get moving—and turn off the television
Inactivity promotes type 2 diabetes. [2] Working your muscles more often and making them work harder improves their ability to use insulin and absorb glucose. This puts less stress on your insulin-making cells. So trade some of your sit-time for fit-time.
Long bouts of hot, sweaty exercise aren’t necessary to reap this benefit. Findings from the Nurses’ Health Study and Health Professionals Follow-up Study suggest that walking briskly for a half hour every day reduces the risk of developing type 2 diabetes by 30%. [3,4] More recently, The Black Women’s Health Study reported similar diabetes-prevention benefits for brisk walking of more than 5 hours per week. [5] This amount of exercise has a variety of other benefits as well. And even greater cardiovascular and other advantages can be attained by more, and more intense, exercise.
Television-watching appears to be an especially-detrimental form of inactivity: Every two hours you spend watching TV instead of pursuing something more active increases the chances of developing diabetes by 20%; it also increases the risk of heart disease (15%) and early death (13%). [6] The more television people watch, the more likely they are to be overweight or obese, and this seems to explain part of the TV viewing-diabetes link. The unhealthy diet patterns associated with TV watching may also explain some of this relationship.
Tune Up Your Diet
Four dietary changes can have a big impact on the risk of type 2 diabetes.
There is convincing evidence that diets rich in whole grains protect against diabetes, whereas diets rich in refined carbohydrates lead to increased risk [7]. In the Nurses’ Health Studies I and II, for example, researchers looked at the whole grain consumption of more than 160,000 women whose health and dietary habits were followed for up to 18 years. Women who averaged 2-3 servings of whole grains a day were 30% less likely to have developed type 2 diabetes than those who rarely ate whole grains. [8] When the researchers combined these results with those of several other large studies, they found that eating an extra two servings of whole grains a day decreased the risk of type 2 diabetes by 21%.
Whole grains don’t contain a magical nutrient that fights diabetes and improves health. It’s the entire package—elements intact and working together—that’s important. The bran and fiber in whole grains make it more difficult for digestive enzymes to break down the starches into glucose. This leads to lower, slower increases in blood sugar and insulin, and a lower glycemic index. As a result, they stress the body’s insulin-making machinery less, and so may help prevent type 2 diabetes. [9] Whole grains are also rich in essential vitamins, minerals, and phytochemicals that may help reduce the risk of diabetes.
In contrast, white bread, white rice, mashed potatoes, donuts, bagels, and many breakfast cereals have what’s called a high glycemic index and glycemic load . That means they cause sustained spikes in blood sugar and insulin levels, which in turn may lead to increased diabetes risk. [9] In China, for example, where white rice is a staple, the Shanghai Women’s Health Study found that women whose diets had the highest glycemic index had a 21% higher risk of developing type 2 diabetes, compared with women whose diets had the lowest glycemic index. [10] Similar findings were reported in the Black Women’s Health Study. [11]
More recent findings from the Nurses Health Studies I and II and the Health Professionals Follow-Up Study suggest that swapping whole grains for white rice could help lower diabetes risk: Researchers found that women and men who ate the most white rice—five or more servings a week—had a 17% higher risk of diabetes than those who ate white rice less than one time a month. People who ate the most brown rice—two or more servings a week—had an 11% lower risk of diabetes than those who rarely ate brown rice. Researchers estimate that swapping whole grains in place of even some white rice could lower diabetes risk by 36%. [12]
Like refined grains, sugary beverages have a high glycemic load, and drinking more of this sugary stuff is associated with increased risk of diabetes. In the Nurses’ Health Study II, women who drank one or more sugar-sweetened beverages per day had an 83% higher risk of type 2 diabetes, compared with women who drank less than one sugar-sweetened beverage per month. [13]
Combining the Nurses’ Health Study results with those from seven other studies found a similar link between sugary beverage consumption and type 2 diabetes. For every additional 12-ounce serving of sugary beverage that people drank each day, their risk of type 2 diabetes rose 25%. [14] Studies also suggest that fruit drinks— powdered drinks, fortified fruit drinks, or juices—are not the healthy choice that food advertisements often portray them to be. Women in the Black Women’s Health study who drank two or more servings of fruit drinks a day had a 31% higher risk of type 2 diabetes, compared with women who drank less than one serving a month. [15]
How do sugary drinks lead to this increased risk? Weight gain may explain the link. In both the Nurses’ Health Study II and the Black Women’s Health Study, women who drank more sugary drinks gained more weight than women who cut back on sugary drinks. [13,15] Several studies show that children and adults who drink soda or other sugar-sweetened beverages are more likely to gain weight than those who don’t. [15-17] and that switching from these to water or unsweetened beverages can reduce weight. [18] Even so, weight gain caused by sugary drinks may not completely explain the increased diabetes risk. There is mounting evidence that sugary drinks contribute to chronic inflammation, high triglycerides, decreased “good” (HDL) cholesterol, and increased insulin resistance, all of which are risk factors for diabetes. [19]
What to drink in place of the sugary stuff? Water is an excellent choice. Coffee and tea are also good calorie-free substitutes for sugared beverages (as long as you don’t load them up with sugar and cream). And there’s convincing evidence that coffee may help protect against diabetes; [20,21] emerging research suggests that tea may hold diabetes-prevention benefits as well, but more research is needed.
There’s been some controversy over whether artificially sweetened beverages are beneficial for weight control and, by extension, diabetes prevention. [22] Some studies have found that people who regularly drink diet beverages have a higher risk of diabetes than people who rarely drink such beverages, [23,24] but there could be another explanation for those findings. People often start drinking diet beverages because they have a weight problem or a family history of diabetes; studies that don’t adequately account for these other factors may make it wrongly appear as though the diet soda led to the increased diabetes risk. A long-term analysis on data from 40,000 men in the Health Professionals Follow-up Study found that drinking one 12-ounce serving of diet soda a day did not appear to increase diabetes risk. [25] So, in moderation diet beverages can be a sugary-drink alternative for adults.
The types of fats in your diet can also affect the development of diabetes. Healthful fats, such as the polyunsaturated fats found in liquid vegetable oils, nuts, and seeds can help ward off type 2 diabetes. [26] Trans fats do just the opposite. [1,27] These harmful fats were once found in many kinds of margarine, packaged baked goods, fried foods in most fast-food restaurants, and any product that listed “partially hydrogenated vegetable oil” on the label. Eating polyunsaturated fats from fish—also known as “long chain omega 3” or “marine omega 3” fats—does not protect against diabetes, even though there is much evidence that these marine omega 3 fats help prevent heart disease. [28] If you already have diabetes, eating fish can help protect you against a heart attack or dying from heart disease. [29]
The evidence is growing stronger that eating red meat (beef, pork, lamb) and processed red meat (bacon, hot dogs, deli meats) increases the risk of diabetes, even among people who consume only small amounts. A meta-analysis combined findings from the Nurses’ Health Studies I and II, the Health Professionals Follow-up Study, and six other long-term studies. The researchers looked at data from roughly 440,000 people, about 28,000 of whom developed diabetes during the course of the study. [30] They found that eating just one 3-ounce serving of red meat daily—say, a steak that’s about the size of a deck of cards—increased the risk of type 2 diabetes by 20%. Eating even smaller amounts of processed red meat each day—just two slices of bacon, one hot dog, or the like—increased diabetes risk by 51%.
The good news from this study: Swapping out red meat or processed red meat for a healthier protein source , such as nuts, low-fat dairy, poultry, or fish, or for whole grains lowered diabetes risk by up to 35%. Not surprisingly, the greatest risk reductions came from ditching processed red meat.
How meat is cooked may matter too . A study of three large cohorts followed for 12-16 years—including more than 289,000 men and women from the Nurses’ Health Studies and the Health Professionals Follow-up Study—found that participants who most frequently ate meats and chicken cooked at high temperatures were 1.5 times more likely to develop type 2 diabetes, compared with those who ate the least. [31] An increased risk of weight gain and developing obesity in the frequent users of high-temperature cooking methods may have contributed to the development of diabetes.
Why do these types of meat appear to boost diabetes risk? It may be that the high iron content of red meat diminishes insulin’s effectiveness or damages the cells that produce insulin. The high levels of sodium and nitrites (preservatives) in processed red meats may also be to blame. Red and processed meats are a hallmark of the unhealthful “Western” dietary pattern, which seems to trigger diabetes in people who are already at genetic risk. [32]
Furthermore, a related body of research has suggested that plant-based dietary patterns may help lower type 2 diabetes risk, and more specifically, those who adhere to predominantly healthy plant-based diets may have a lower risk of developing type 2 diabetes than those who follow these diets with lower adherence:
- A 2019 meta-analysis that included health data from 307,099 participants with 23,544 cases of type 2 diabetes examined adherence to an “overall” predominantly plant-based diet (which could include a mix of healthy plant-based foods such as fruits, vegetables, whole grains, nuts, and legumes, but also less healthy plant-based foods such as potatoes, white flour, and sugar, and modest amounts of animal products). The researchers also looked at “healthful” plant-based diets, which were defined as those emphasizing healthy plant-based foods, with lower consumption of unhealthy plant-based foods. They found that people with the highest adherence to overall predominantly plant-based diets had a 23% lower risk of type 2 diabetes compared to those with weaker adherence to the diets. The researchers also found that the association was strengthened for those who ate healthful plant-based diets [41]
Don’t smoke
Add type 2 diabetes to the long list of health problems linked with smoking. Smokers are roughly 50% more likely to develop diabetes than nonsmokers, and heavy smokers have an even higher risk. [33]
Light to moderate alcohol consumption
Evidence has consistently linked moderate alcohol consumption with reduced risk of heart disease. The same may be true for type 2 diabetes. Moderate amounts of alcohol—up to a drink a day for women, up to two drinks a day for men—increases the efficiency of insulin at getting glucose inside cells. And some studies indicate that moderate alcohol consumption decreases the risk of type 2 diabetes. [1, 34-39], but excess alcohol intake actually increases the risk. If you already drink alcohol, the key is to keep your consumption in the moderate range, as higher amounts of alcohol could increase diabetes risk. [40] If you don’t drink alcohol, there’s no need to start—you can get the same benefits by losing weight, exercising more, and changing your eating patterns.
Beyond individual behavior
Type 2 diabetes is largely preventable by taking several simple steps: keeping weight under control, exercising more, eating a healthy diet, and not smoking. Yet it is clear that the burden of behavior change cannot fall entirely on individuals. Families, schools, worksites, healthcare providers, communities, media, the food industry, and government must work together to make healthy choices easy choices. For links to evidence-based guidelines, research reports, and other resources for action, visit our diabetes prevention toolkit .
- Diabetes – overview, types, and risk factors
- Asian Diabetes Prevention Initiative
- Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. New England journal of medicine . 2001 Sep 13;345(11):790-7.
- Rana JS, Li TY, Manson JE, Hu FB. Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes care . 2007 Jan 1;30(1):53-8.
- Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation . 2003 May 20;107(19):2435-9.
- Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA . 1999 Oct 20;282(15):1433-9.
- Krishnan S, Rosenberg L, Palmer JR. Physical activity and television watching in relation to risk of type 2 diabetes: the Black Women’s Health Study. American journal of epidemiology . 2008 Dec 4;169(4):428-34.
- Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA . 2011 Jun 15;305(23):2448-55.
- AlEssa H, Bupathiraju S, Malik V, Wedick N, Campos H, Rosner B, Willett W, Hu FB. Carbohydrate quality measured using multiple quality metrics is negatively associated with type 2 diabetes. Circulation . 2015;1:31.
- de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS medicine . 2007 Aug 28;4(8):e261.
- Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA . 2002 May 8;287(18):2414-23.
- Villegas R, Liu S, Gao YT, Yang G, Li H, Zheng W, Shu XO. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Archives of internal medicine . 2007 Nov 26;167(21):2310-6.
- Krishnan S, Rosenberg L, Singer M, Hu FB, Djoussé L, Cupples LA, Palmer JR. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Archives of Internal Medicine . 2007 Nov 26;167(21):2304-9.
- Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, Hu FB. White rice, brown rice, and risk of type 2 diabetes in US men and women. Archives of internal medicine . 2010 Jun 14;170(11):961-9.
- Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA . 2004 Aug 25;292(8):927-34.
- Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes care . 2010 Nov 1;33(11):2477-83.
- Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Archives of internal medicine . 2008 Jul 28;168(14):1487-92.
- Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. The Lancet . 2001 Feb 17;357(9255):505-8.
- Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. American journal of public health . 2007 Apr;97(4):667-75.
- Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics . 2006 Mar 1;117(3):673-80.
- Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation . 2010 Mar 23;121(11):1356-64.
- Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Archives of internal medicine . 2009 Dec 14;169(22):2053-63.
- Van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: a prospective cohort study in younger and middle-aged US women. Diabetes care . 2006 Feb 1;29(2):398-403.
- Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. European journal of clinical nutrition . 2007 Jun;61(6):691.
- Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome. Circulation . 2008 Feb 12;117(6):754-61.
- Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation . 2007 Jul 31;116(5):480-8.
- De Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. The American journal of clinical nutrition . 2011 Mar 23;93(6):1321-7.
- Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Progress in lipid research . 2009 Jan 1;48(1):44-51.
- Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. New England Journal of Medicine . 2006 Apr 13;354(15):1601-13.
- Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. The American journal of clinical nutrition . 2009 Jul 22;90(3):613-20.
- Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain ω-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation . 2003 Apr 15;107(14):1852-7.
- Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. The American journal of clinical nutrition . 2011 Aug 10;94(4):1088-96.
- Liu G, Zong G, Wu K, Hu Y, Li Y, Willett WC, Eisenberg DM, Hu FB, Sun Q. Meat cooking methods and risk of type 2 diabetes: results from three prospective cohort studies. Diabetes care . 2018 May 1;41(5):1049-60.
- Qi L, Cornelis MC, Zhang C, Van Dam RM, Hu FB. Genetic predisposition, Western dietary pattern, and the risk of type 2 diabetes in men. The American journal of clinical nutrition . 2009 Mar 11;89(5):1453-8.
- Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA . 2007 Dec 12;298(22):2654-64.
- Djoussé L, Biggs ML, Mukamal KJ, Siscovick DS. Alcohol consumption and type 2 diabetes among older adults: the Cardiovascular Health Study. Obesity . 2007 Jul;15(7):1758-65.
- Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ . 1995 Mar 4;310(6979):555-9.
- Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes care . 2005 Mar 1;28(3):719-25.
- Conigrave KM, Hu BF, Camargo CA, Stampfer MJ, Willett WC, Rimm EB. A prospective study of drinking patterns in relation to risk of type 2 diabetes among men. Diabetes . 2001 Oct 1;50(10):2390-5.
- Mukamal KJ, Conigrave KM, Mittleman MA, Camargo Jr CA, Stampfer MJ, Willett WC, Rimm EB. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. New England Journal of Medicine . 2003 Jan 9;348(2):109-18.
- Joosten MM, Grobbee DE, van der A DL, Verschuren WM, Hendriks HF, Beulens JW. Combined effect of alcohol consumption and lifestyle behaviors on risk of type 2 diabetes. The American journal of clinical nutrition . 2010 Apr 21;91(6):1777-83.
- Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes care . 2009 Nov 1;32(11):2123-32.
- Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern Med. Published online July 22, 2019.
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Diabetes prevention research: a systematic review of external validity in lifestyle interventions
Affiliation.
- 1 Prevention Research Collaboration, University of Sydney, Australia. [email protected]
- PMID: 22813687
- DOI: 10.1016/j.amepre.2012.04.017
Context: Type 2 diabetes is a major contributor to disease burden globally. A number of systematic reviews support the efficacy of lifestyle interventions in preventing Type 2 diabetes in adults; however, relatively little attention has been paid to the generalizability of study findings. This study systematically reviews the reporting of external validity components and generalizability of diabetes prevention studies.
Evidence acquisition: Lifestyle intervention studies for the prevention of Type 2 diabetes in adults with at least 6 months' follow-up, published between 1990 and 2011, were identified through searches of major electronic databases. External validity reporting was rated using an assessment tool, and all analysis was undertaken in 2011.
Evidence synthesis: A total of 31 primary studies (n=95 papers) met the selection criteria. All studies lacked full reporting on external validity elements. Description of the study sample, intervention, delivery agents, and participant attrition rates were reported by most studies. However, few studies reported on the representativeness of individuals and settings, methods for recruiting settings and delivery agents, costs, and how interventions could be institutionalized into routine service delivery. It is uncertain to what extent the findings of diabetes prevention studies apply to men, socioeconomically disadvantaged individuals, those living in rural and remote communities, and to low- and middle-income countries.
Conclusions: Reporting of external validity components in diabetes prevention studies needs to be enhanced to improve the evidence base for the translation and dissemination of these programs into policy and practice.
Copyright © 2012 American Journal of Preventive Medicine. Published by Elsevier Inc. All rights reserved.
PubMed Disclaimer
- Research to inform policy in diabetes prevention: a work in progress. Ackermann RT. Ackermann RT. Am J Prev Med. 2012 Aug;43(2):225-7. doi: 10.1016/j.amepre.2012.05.002. Am J Prev Med. 2012. PMID: 22813690 No abstract available.
Similar articles
- Applying RE-AIM to Evaluate the External Validity of Weight Gain Prevention Interventions in Young Adults: A Systematic Review. Haire-Joshu D, Morshed AB, Phad A, Johnston S, Tabak RG. Haire-Joshu D, et al. J Public Health Manag Pract. 2021 Mar-Apr 01;27(2):154-165. doi: 10.1097/PHH.0000000000001159. J Public Health Manag Pract. 2021. PMID: 32332487 Free PMC article.
- Cultural adaptation of a peer-led lifestyle intervention program for diabetes prevention in India: the Kerala diabetes prevention program (K-DPP). Mathews E, Thomas E, Absetz P, D'Esposito F, Aziz Z, Balachandran S, Daivadanam M, Thankappan KR, Oldenburg B. Mathews E, et al. BMC Public Health. 2018 Jan 4;17(1):974. doi: 10.1186/s12889-017-4986-0. BMC Public Health. 2018. PMID: 29298703 Free PMC article. Clinical Trial.
- Poor quality of external validity reporting limits generalizability of overweight and/or obesity lifestyle prevention interventions in young adults: a systematic review. Partridge SR, Juan SJ, McGeechan K, Bauman A, Allman-Farinelli M. Partridge SR, et al. Obes Rev. 2015 Jan;16(1):13-31. doi: 10.1111/obr.12233. Epub 2014 Nov 18. Obes Rev. 2015. PMID: 25407633 Review.
- Review of external validity reporting in childhood obesity prevention research. Klesges LM, Dzewaltowski DA, Glasgow RE. Klesges LM, et al. Am J Prev Med. 2008 Mar;34(3):216-23. doi: 10.1016/j.amepre.2007.11.019. Am J Prev Med. 2008. PMID: 18312810 Review.
- The Logan Healthy Living Program: a cluster randomized trial of a telephone-delivered physical activity and dietary behavior intervention for primary care patients with type 2 diabetes or hypertension from a socially disadvantaged community--rationale, design and recruitment. Eakin EG, Reeves MM, Lawler SP, Oldenburg B, Del Mar C, Wilkie K, Spencer A, Battistutta D, Graves N. Eakin EG, et al. Contemp Clin Trials. 2008 May;29(3):439-54. doi: 10.1016/j.cct.2007.10.005. Epub 2007 Nov 4. Contemp Clin Trials. 2008. PMID: 18055274 Clinical Trial.
- The effectiveness of interventions during the first 1,000 days to improve energy balance-related behaviors or prevent overweight/obesity in children from socio-economically disadvantaged families of high-income countries: a systematic review. Lioret S, Harrar F, Boccia D, Hesketh KD, Kuswara K, Van Baaren C, Maritano S, Charles MA, Heude B, Laws R. Lioret S, et al. Obes Rev. 2023 Jan;24(1):e13524. doi: 10.1111/obr.13524. Epub 2022 Nov 17. Obes Rev. 2023. PMID: 36394375 Free PMC article. Review.
- Questioning the ethics of evidence-based practice for Indigenous health and social settings in Australia. Luke J, Verbunt E, Zhang A, Bamblett M, Johnson G, Salamone C, Thomas D, Eades S, Gubhaju L, Kelaher M, Jones A. Luke J, et al. BMJ Glob Health. 2022 Jun;7(6):e009167. doi: 10.1136/bmjgh-2022-009167. BMJ Glob Health. 2022. PMID: 35680132 Free PMC article. Review.
- Identification of tools used to assess the external validity of randomized controlled trials in reviews: a systematic review of measurement properties. Jung A, Balzer J, Braun T, Luedtke K. Jung A, et al. BMC Med Res Methodol. 2022 Apr 6;22(1):100. doi: 10.1186/s12874-022-01561-5. BMC Med Res Methodol. 2022. PMID: 35387582 Free PMC article. Review.
- Health professional-delivered obesity prevention interventions during the first 1,000 days: A systematic review of external validity reporting. Hennessy M, Heary C, Laws R, Van Rhoon L, Toomey E, Wolstenholme H, Byrne M. Hennessy M, et al. HRB Open Res. 2019 Jul 19;2:14. doi: 10.12688/hrbopenres.12924.2. eCollection 2019. HRB Open Res. 2019. PMID: 32002513 Free PMC article.
- Participant recruitment into a community-based diabetes prevention trial in India: Learnings from the Kerala Diabetes Prevention Program. Sathish T, Aziz Z, Absetz P, Thankappan KR, Tapp RJ, Balachandran S, Shetty SS, Oldenburg B. Sathish T, et al. Contemp Clin Trials Commun. 2019 May 21;15:100382. doi: 10.1016/j.conctc.2019.100382. eCollection 2019 Sep. Contemp Clin Trials Commun. 2019. PMID: 31193921 Free PMC article.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Elsevier Science
- Genetic Alliance
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Providing Care
- Living with Diabetes
- Clinical Guidance
- DSMES for Health Care Providers
- Prevent Type 2 Diabetes: Talking to Your Patients About Lifestyle Change
- Employers and Insurers
- Community-based Organizations (CBOs)
- Toolkits for Diabetes Educators and Community Health Workers
- National Diabetes Statistics Report
- Reports and Publications
- Data and Statistics
- Current Research Projects
Related Topics:
- View All Home
- National Diabetes Prevention Program
- State, Local, and National Partner Diabetes Programs for Public Health
- Diabetes Self-Management Education and Support (DSMES) Toolkit
Preventing Type 2 Diabetes
- Prediabetes is a serious health condition, and 1 in 3 US adults has it.
- With early action, you can reverse prediabetes to prevent or delay type 2 diabetes.
- Find out if you have a high risk for prediabetes by taking the 1-minute risk test.

What is prediabetes?
Before developing type 2 diabetes, most people have prediabetes. This is when their blood sugar is higher than normal but not high enough yet for a type 2 diabetes diagnosis. In the United States, about 98 million adults have prediabetes; that's 1 in 3 people.
There are usually no signs when you have prediabetes, which is why 81% of people don't know they have it. You can have prediabetes for years and not know.
Ready to find out where you stand? Take the 1-minute prediabetes risk test . If your score shows you have a high risk of prediabetes, visit your doctor for a simple blood test to confirm your result.
You can reverse prediabetes
Risk factors.
Anyone can develop prediabetes at any age, but you may have a higher risk if you:
- Have a family history of type 2 diabetes.
- Are over age 45.
- Have overweight or obesity.
- Had gestational diabetes (diabetes when pregnant).
- Have high blood pressure.
- Are a Hispanic/Latino, Black, Asian American, or Native American person.
Join the lifestyle change program
If your doctor confirms you have prediabetes, join the CDC-recognized National Diabetes Prevention Program (National DPP) lifestyle change program. You'll learn how to make lasting lifestyle changes to prevent or delay type 2 diabetes:
- Work with a trained Lifestyle Coach, who will help you take small, manageable steps that fit into your life.
- Discover how to eat healthy and add more physical activity into your day.
- Find out how to manage stress, stay motivated, and solve problems to maintain your progress.
Ready to get started?
Find a lifestyle change program near you or online.
Diabetes is a chronic disease that affects how your body turns food into energy. About 1 in 10 Americans has diabetes.
For Everyone
Health care providers, public health.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- News Archive
Story of Discovery: Progress on the Pathway to Prevention of Type 1 Diabetes
Type 1 diabetes is a devastating illness where the body’s ability to produce the pancreas-derived hormone insulin is lost, requiring people with the disease to administer insulin daily for survival. Even with this burdensome treatment, people with type 1 diabetes are at risk for life-threatening complications. Research shows that the incidence of type 1 diabetes is on the rise in the United States, so identifying ways to prevent type 1 diabetes in those at risk is critical, in parallel with efforts to cure the disease in those who have been diagnosed. The story of type 1 diabetes—which is still being written as key questions and challenges remain— involves geneticists, epidemiologists, molecular and cellular biologists, immunologists, endocrinologists, bioengineers, researchers in other fields, and patient participants. This multifaceted and collaborative approach has resulted in valuable new knowledge that is moving us closer to a long-standing goal of type 1 diabetes prevention.
IDENTIFYING THOSE AT RISK TO DEVELOP TYPE 1 DIABETES
Preventing type 1 diabetes requires not only a successful therapy, but also the ability to identify those who are at risk of developing the disease. But answering the deceptively simple question of “who is at risk?” required a multi-pronged research approach.
One of the first steps was to understand the disease better. Early on, scientists searched for a toxin or infectious agent that caused type 1 diabetes. However, some observed that people with type 1 diabetes sometimes had other disorders related to abnormal hormone levels or function (endocrine disorders), particularly those associated with autoimmunity, leading scientists, after decades of studying the disease, to hypothesize that type 1 diabetes was an autoimmune disease. Autoimmune diseases result when a person’s immune system does not properly distinguish between “self” and “non-self” and inappropriately targets and attacks the body’s own organs, tissues, and cells. One component of an immune attack is antibodies, produced by an immune cell type called B lymphocytes. Self-directed antibodies are called “autoantibodies,” and their presence in the blood can indicate an autoimmune process.
In the early 1970s, researchers found that, by using blood from people with multiple autoimmune endocrine disorders, including type 1 diabetes, they could detect a specific autoantibody response to insulin-producing pancreatic islet tissue. Later research demonstrated that antibodies that react with islet cells could be found in the majority of people with newly diagnosed type 1 diabetes. Further research has identified more than four different autoantibodies specifically enriched in people with type 1 diabetes. One of the earliest autoantibodies to appear, most commonly in younger children, is directed against insulin. Early studies of families with type 1 diabetes in the 1970s led to the observation that the disease often appeared in siblings, indicating that there could be a genetic component to the disease. NIDDK-supported scientists and others soon discovered that human leukocyte antigen (HLA) gene alleles (variant forms of a gene required for the function of another immune cell type—T cells) were associated with type 1 diabetes. With the use of modern tools for genetic analysis, we now know that HLA accounts for approximately 50 percent of the heritability of type 1 diabetes. Additionally, NIDDK-supported researchers and others have since identified more than 50 other genetic loci that contribute to type 1 diabetes susceptibility, accounting for nearly 90 percent of genetic risk in the Caucasian population, which is most affected by type 1 diabetes. Many of these genes are known to be involved in the immune response, further strengthening the understanding of type 1 diabetes as an autoimmune disease. These exciting findings set the stage for efforts to identify those at risk to develop type 1 diabetes and to test immune-modulating therapies to prevent the disease.
A WINDOW OF OPPORTUNITY TO PREVENT TYPE 1 DIABETES
Until the discovery of autoantibodies, it was generally assumed that type 1 diabetes had an acute onset whose first clinical symptoms were the sudden appearance of metabolic abnormalities as a result of the loss of insulin in previously healthy people. Prevention would be difficult in such a disease, as there would be no warning before the clinical appearance of the disease and identifying at-risk individuals would not be possible. Not only did the revelation that type 1 diabetes was an autoimmune disease mean that autoantibodies could possibly be used to identify those at risk before the manifestation of clinical symptoms, but it also suggested that a window of opportunity for prevention might exist. Destruction of the insulin-producing β (beta) cells (which are in the pancreatic islet cell clusters) by an errant immune attack might happen over time, rather than immediately, and perhaps this destruction could be delayed or stopped altogether, preserving the precious remaining β cells.
NIDDK-supported scientists and others spent the 1980s studying cohorts of people that had these autoantibodies in their blood but had not been clinically diagnosed with diabetes to determine whether the appearance of autoantibodies preceded loss of insulin and if they indicated the early stages of type 1 diabetes. In one study, NIDDK-supported investigators followed a set of triplets and a set of twins, each with one person with type 1 diabetes. These people were studied for nearly 2 decades. Over that time, one triplet and one twin—neither of whom had diabetes at the start of the study—first developed autoantibodies and then onset of clinically overt disease, allowing scientists to document the slow, progressive loss of insulin before the onset of clinical diabetes.
In another study, NIDDK-supported scientists screened over 1,700 first-degree relatives (parents, siblings, and offspring) of people with type 1 diabetes for the presence of islet-cell autoantibodies. Only 16 of those screened had the autoantibodies, but 2 of those developed type 1 diabetes in the next 2 years, compared to 1 of the 1,700 without antibodies. In addition, the researchers examined the insulin response in 12 of the relatives with autoantibodies and found that 6 of these individuals had low insulin responses, an indicator of diminished β cell function. Results from these and similar studies contributed to the growing body of evidence that islet-cell autoantibodies were predictors of type 1 diabetes and that β cell destruction was not an immediate event. These studies also provided key information on how screening programs could be designed to identify people and assess their risk, setting the stage for trials to prevent type 1 diabetes.
SETTING THE STAGE FOR A LARGE¬SCALE PREVENTION TRIAL
For a first test of type 1 diabetes prevention, researchers turned to a familiar candidate: insulin. Studies in animal models, as well as small pilot studies in humans, suggested that insulin could delay type 1 diabetes development. It was thought that administering low-dose insulin to an at-risk person before the disease progressed could induce protective immunity that might slow or prevent the immune system’s attack. In 1994, the NIDDK-supported Diabetes Prevention Trial-Type 1 Diabetes (DPT-1) began screening first- and second-degree relatives to identify eligible participants for a clinical trial to test this hypothesis. More than 84,000 people were screened; about 340 were found positive for autoantibodies, had more than an estimated 50 percent chance to develop type 1 diabetes in the next 5 years, and elected to participate in a study testing injectable insulin for prevention of type 1 diabetes. Participants were studied for an average of about 3.5 years, and this clinical trial concluded in 2001. DPT-1 also tested the effect of orally administered insulin in relatives who had an estimated 26 to 50 percent chance of developing type 1 diabetes in the next 5 years. Over 370 participants were studied for an average of 4.3 years in that trial, which concluded in 2003. Although both injectable and oral insulin were very safe, with negligible side effects, neither was found to delay or prevent type 1 diabetes.
Despite the negative results, the DPT-1 was a success in other ways. DPT-1 researchers estimated participant risk using the presence of islet-cell antibodies, insulin response to glucose tests, and the presence or absence of specific HLA alleles, validating these predictive tools and demonstrating that it was possible to identify a cohort of people at high risk for type 1 diabetes. DPT-1 also demonstrated that large type 1 diabetes prevention trials were feasible in at-risk family members of individuals with type 1 diabetes, establishing a path for future prevention trials, just in time for the emergence of new agents that would require testing.
CREATING A NETWORK FOR PREVENTION TRIALS: TYPE 1 DIABETES TRIALNET
As the DPT-1 was concluding, the continued need for a network of investigators and sites to conduct trials of promising therapies to prevent type 1 diabetes became evident. These trials would require screening of large numbers of people to identify those who would be eligible to participate. Additionally, a coordinated and collaborative effort would accelerate progress in this field. Thus, in 2001, NIDDK launched Type 1 Diabetes TrialNet. Since its start nearly 2 decades ago, TrialNet has become an international network of clinical research centers, affiliate sites, a hub, and a coordinating center that involves hundreds of scientists and staff and, most importantly, thousands of participants. TrialNet has conducted multiple studies of agents to delay progression of type 1 diabetes in people with and at risk for the disease, as well as contributed key insights into understanding the type 1 diabetes disease process.
REFINING RISK AND STAGING PROGRESSION OF TYPE 1 DIABETES
The ability to accurately assess those at risk for type 1 diabetes is critical to identify participants for prevention trials and to ensure that as many people as possible can benefit, if and when new prevention strategies are proven effective. To refine and quantify type 1 diabetes risk, NIDDK-supported researchers pooled data from multiple studies and, in 2013, reported that the majority of children who had multiple islet autoantibodies in their blood progressed to the disease over the next 15 years, suggesting that prevention studies focus on this high-risk population.
Data from DPT-1, TrialNet, and other studies revealed that progression to clinical type 1 diabetes proceeds through distinct stages prior to onset of symptoms. This formed the basis for a recommendation from TrialNet, JDRF, the Endocrine Society, and the American Diabetes Association for a type 1 diabetes staging classification in at-risk individuals. This staging provides a framework for the research and development of preventive therapies (see Figure 1): stage 1 is defined as the presence of two or more different types of islet autoantibodies with normal blood glucose (sugar) levels and is considered early type 1 diabetes; stage 2 diabetes is the presence of two or more autoantibodies but with abnormal blood glucose levels without symptoms; and stage 3 is when clinical diagnosis has been reached and symptoms of type 1 diabetes are usually present. TrialNet’s prevention trials enroll individuals with pre-clinical (stage 1 and 2) type 1 diabetes, and TrialNet’s new-onset trials enroll participants in early stage 3 diabetes.

The combination of these efforts led to the following understanding of type 1 diabetes disease risk: 35 percent of people in stage 1 and 70 percent of people in stage 2 will progress to clinical diabetes within 3 to 5 years of identification. The lifetime risk for developing clinical type 1 diabetes from stage 1 or 2 nears 100 percent. In the future, risk assessment could take into account an individual’s genetic makeup and their environmental exposures to determine risk even before autoantibodies appear.
Of note, most new cases of type 1 diabetes occur in people who have no affected relatives. There is currently no way to identify these people other than by conducting population-wide genetic screening, which is not currently feasible. Therefore, for now, research has demonstrated that the most efficient way to identify people at risk for type 1 diabetes is to screen first- and second-degree relatives of people with the disease due to their 15-fold increased risk for developing the disease compared to the general population. To date, TrialNet has screened more than 200,000 relatives and screens more than 15,000 annually to identify at-risk individuals for enrollment in trials. More than 7,000 people have enrolled in TrialNet’s Pathway to Prevention Study (an observational study for relatives with autoantibodies) and/or have participated in a TrialNet trial.
IDENTIFYING CANDIDATE THERAPIES TO TEST
One of the challenges of clinical trials is balancing the potential benefits against the risks. There is risk associated with introducing an agent, particularly one that modulates the immune system and may have serious side effects, into a healthy person— albeit one who will eventually develop clinical diabetes but has not yet done so—especially when the participants are children. Therefore, careful consideration is paramount in deciding which agents are the most promising and should be tested in prevention trials. With that in mind, TrialNet looks for agents that have been tested for safety in animal models, in pilot studies in people, or have been tested (or even approved for use) in people with other autoimmune diseases or conditions before a larger prevention trial is considered.
One of the first agents to emerge as a possible candidate for therapy was an antibody known as anti-CD3, which modifies the function of T cells, but does not dramatically deplete them. In studies of mouse models of type 1 diabetes, anti-CD3 agents have consistently reversed diabetes at the onset of symptoms. In a small clinical trial funded by NIDDK and reported in 2002, scientists found that an anti¬CD3 agent, teplizumab, preserved some insulin production after 1 year with no severe side effects in people recently diagnosed with type 1 diabetes. Following these results, multiple larger and longer studies confirmed that teplizumab treatment delayed the loss of insulin production, including one done by the National Institute of Allergy and Infectious Diseases’ Immune Tolerance Network, an important TrialNet partner.
To continue to make progress towards a more effective, durable and safe therapy for type 1 diabetes, TrialNet has tested additional immune-modifying agents in people newly diagnosed with type 1 diabetes. For example, in 2009, TrialNet reported that rituximab, which is approved as a cancer therapy and destroys B lymphocytes, slowed disease progression for 6 to 9 months. Another TrialNet trial tested the drug abatacept, which acts on T cells and is an approved therapy for rheumatoid arthritis. The results showed that participants who received abatacept had higher insulin production than those who received placebo after 2 years. In 2018, TrialNet reported that another immune system suppressant, low-dose anti-thymocyte globulin (ATG), delayed the loss of insulin production and improved blood glucose control for up to 2 years. All of these drugs can have significant side effects and each had only temporary benefit, likely due in part to immune cell regeneration when the treatments ended. However, these positive results showed that many immune-modulating therapies could slow the disease, indicating that these agents could be tested in prevention trials, where their effects could be more beneficial.
DEMONSTRATION THAT TYPE 1 DIABETES CAN BE PREVENTED
Teplizumab, with several positive trials in people with newly diagnosed type 1 diabetes, was chosen by TrialNet as the first agent to test for disease prevention. TrialNet began a trial in 2011 and, in 2019, reported that teplizumab delayed onset of clinical type 1 diabetes in people at high risk (stage 2) for an average of 2 years (see advance and Patient Profile in this chapter). This exciting discovery provides the first evidence that the onset of clinical type 1 diabetes can be delayed with early preventive treatment. Participants are being followed to determine the durability of the effect, but these results have important implications for people, particularly youth. Treatment with teplizumab could give at-risk individuals 2 years free of type 1 diabetes and insulin administration; 2 years that they do not have to check blood glucose levels; and 2 more years of good health towards preventing or delaying diabetes complications. Based on TrialNet’s results, the U.S. Food and Drug Administration gave teplizumab “Breakthrough Therapy Designation” to expedite its development and review.
THE FUTURE FOR PREVENTION TRIALS OF TYPE 1 DIABETES
Much remains to be explored about teplizumab and other immune-modifying drugs so that more effective treatments can be designed. First and foremost, we need to understand more about the mechanisms of autoimmune pathogenesis and how individual people respond to therapies. From the beginning, TrialNet has engaged in mechanistic research, collecting blood samples from people enrolled in trials and analyzing them for the specific mechanistic effects of treatment. Building on these data, TrialNet has designed a new prevention trial that will combine two agents that showed benefit to newly diagnosed participants in previous trials and that affect complementary immune pathways. Alternative dosing regimens, testing agents even earlier in at-risk people (i.e., stage 1), and other types of combination trials all present exciting opportunities to build on this advance. Additionally, TrialNet currently has two other single-agent prevention studies under way: one testing abatacept (see earlier) and one testing the drug hydroxychloroquine, both of which are already used to reduce symptoms and progression of other autoimmune diseases. There are also many other promising therapies in TrialNet’s pipeline, with even more expected in the future as new knowledge is uncovered by TrialNet’s mechanistic work and through other NIDDK-supported research efforts focused on the underlying mechanisms of type 1 diabetes development. With continued research, the goal of preventing type 1 diabetes—permanently and in anyone who could develop the disease—now seems possible after decades of contributions from countless scientists and, most importantly, the trial participants who never gave up hope.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
- Complications
Type 2 Diabetes Medication and Treatment
Learn about lifestyle changes, medication, and insulin treatment
Bariatric Surgery
- Next in Type 2 Diabetes Guide Coping With Type 2 Diabetes
Type 2 diabetes medications include metformin, sulfonylureas, alpha-glucosidase inhibitors, and other medications that change the way your body uses insulin and glucose. Not everyone with type 2 diabetes needs medication, however. Many people can manage their condition with healthy lifestyle changes alone.
There's no one-size-fits-all treatment for type 2 diabetes . The key to managing this increasingly common condition is piecing together a treatment plan that fits the individual.
This article looks at diabetes medication and some of the other approved ways to manage and treat type 2 diabetes.
Lifestyle Changes
Type 2 diabetes is a condition that affects the way your body uses insulin, a hormone that helps regulate your blood sugar. It can often be treated by changing or beginning certain lifestyle practices. This is nearly always the first step in treating type 2 diabetes.
Weight loss is one of the primary goals of these lifestyle changes. A loss of just 5% to 10% of total body weight can have a dramatic effect on blood sugar levels in people with type 2 diabetes.
Some of the lifestyle changes to manage type 2 diabetes include:
- Quitting smoking
It's especially important to reduce your carbohydrate intake. This can lead to weight loss, improved blood sugar control, and lower levels of triglycerides . Triglycerides are fats associated with an increased risk of heart disease.
There's no such thing as an official " diabetes diet ." There are a variety of approaches to eating and nutrition that have been found to be helpful, though, including:
- The Plate Method: This is an easy way to help manage your blood sugar. It emphasizes non-starchy vegetables , whole grains, lean protein, and fiber. Fiber, in particular, can help to slow increases in blood sugar levels. Specific percentages of the plate are dedicated to certain foods.
- Carb consistency: Carbohydrates impact blood sugar more than protein and fat. Taking in the same amount of carbs at each meal will help keep glucose levels steady. For example, this might mean consistently eating 45 grams of carbs for breakfast and lunch, 15 grams of carbs for a between-meal snack, and 60 grams of carbs for dinner each day.
- Limiting foods that dramatically increase blood sugar levels: These include fruit juice, refined and processed carbohydrates like white bread and pasta, and sugary sweets such as cookies, cake, and candy. It's typically fine to eat two or three servings of fresh whole fruit per day.
Besides these basic guidelines, there's evidence that dramatically reducing carbohydrates can have a profound and positive impact on type 2 diabetes.
In one study, people with obesity and type 2 diabetes who followed a very carb-restricted diet for six months had lower hemoglobin A1C results. This test is a measure of blood sugar over a period of two to three months. Participants on the carb-restricted diet also lost more weight than those who followed a reduced-calorie diet. Both groups exercised regularly and had the support of group meetings.
This is just one study, however. Always consult your healthcare provider and/or a dietitian who specializes in diabetes before making major dietary changes.
Regular exercise is critical for managing type 2 diabetes. Physical activity burns calories and may contribute to weight loss. Exercise can also have a direct impact on blood sugar control because insulin resistance is closely linked to increased fat and decreased muscle mass. When you have insulin resistance, your body doesn't use insulin the way it's supposed to.
Insulin helps your cells use blood glucose for energy. Muscle cells use insulin far more efficiently than fat. By building muscle and burning fat, you can help better control your blood glucose levels.
The American Diabetes Association (ADA) recommends the following exercise guidelines for adults with type 2 diabetes:
- 150 minutes or more of moderate-to-vigorous aerobic activity weekly: Spread this out over at least three days, with no more than two days in a row without activity.
- Two to three sessions of resistance exercise per week on nonconsecutive days: This includes things like weight training or bodyweight exercises.
- For older adults, two to three sessions per week of flexibility and balance training: This includes activities like yoga and tai chi.
The ADA also recommends that people with type 2 diabetes not sit for prolonged periods of time. Aim to get up and move about every 30 minutes or so.
Quitting Smoking
People who smoke are 30% to 40% more likely to develop type 2 diabetes than nonsmokers. Even using smokeless tobacco can increase diabetes risk. What's more, smokers with diabetes are more likely to develop serious complications .
People with diabetes who stop smoking see rapid improvements in their diabetes symptoms and overall health.
There are many approaches to smoking cessation . Discuss the options with a healthcare provider or certified diabetes educator (CDE). This will help you find the strategy that is most likely to work for you.
Type 2 Diabetes Medications
Type 2 diabetes treatment sometimes includes medication. This is usually the next step when dietary changes, exercise, and weight loss aren't enough to control blood sugar levels.
Some diabetes medications are taken orally, and others are injected.
Medication is not meant to be used as a substitute for healthy lifestyle changes. Because some diabetes medications can cause a drop in blood sugar before or after exercise, however, it is important to know how your body responds to exercise and how your medications may affect you.
Here are some of the drugs that have been approved by the Food and Drug Administration (FDA) for treating type 2 diabetes.
Metformin lowers the amount of glucose being produced by the liver. It also makes the body more sensitive to insulin. Metformin is classified as a biguanide . It is the most commonly used diabetic drug.
Metformin is usually the first medication prescribed to treat type 2 diabetes. Most people stay on metformin as other medications are added, as long as it is well tolerated.
Metformin can cause gastrointestinal side effects including:
- Stomach pain
- Indigestion
- Constipation
In recent years, metformin products have been recalled several times due to higher-than-acceptable levels of a potentially cancer-causing contaminant called N-nitrosodimethylamine (NDMA) 410 in some formulations. If you're concerned about the metformin product you're using, speak to your healthcare provider. Remember that stopping metformin without a replacement can pose serious health risks to patients with type 2 diabetes.
Sulfonylureas
Sulfonylureas are the oldest class of oral diabetes medications. They work by stimulating the pancreas to release more insulin into the bloodstream. They include:
- Glucotrol (glipizide)
- Glynase (glyburide)
- Amaryl (glimepiride); also in combination with Duetact (pioglitazone)
Thiazolidinediones
Thiazolidinediones sensitize muscle and fat cells to accept insulin more readily. These drugs pose certain health risks that will need to be considered before they are prescribed.
- Actos (pioglitazone); also in combination with alogliptin as Oseni; with metformin as Actoplus Met; and with glimepiride as Duetact
Alpha-Glucosidase Inhibitors
Alpha-glucosidase inhibitors delay the conversion of carbohydrates to glucose during digestion. This helps to regulate blood glucose levels and prevent sugars from peaking too high.
- Glyset (miglitol)
Meglitinides
Meglitinides help stimulate insulin production when glucose is present in the blood. They are not as effective if blood sugar levels are low.
- Repaglinide
- Nateglinide
The brand names Prandin (repaglinide) and Starlix (nateglinide) have been discontinued, but the generic medications are still available.
DPP-4 Inhibitors
Dipeptidyl peptidase-4 (DPP-4) is an enzyme that destroys hormones called incretins. These hormones help the body produce more insulin when needed. DPP-4 inhibitors work by blocking this enzyme.
- Januvia (sitagliptin); also in combination with metformin as Janumet and with ertugliflozin as Steglujan
- Onglyza (saxagliptin); also in combination with metformin as Kombiglyze XR; with dapagliflozin as Qtern; and with metformin and dapagliflozin as Qternmet
- Tradjenta (linagliptin); also in combination with metformin as Jentadueto and with empagliflozin as Glyxambi
- Nesina (alogliptin); also in combination with metformin as Kazano and with pioglitazone as Oseni
It's important to note that in August 2015, the FDA added a warning and precaution about a potential side effect of DPP-4 inhibitors: severe and potentially disabling joint pain. If you are taking a medication that contains a DPP-4 inhibitor and develop joint pain, let your healthcare provider know right away. You may need to switch to a different medication.
SSGT-2 Inhibitors
Selective sodium-glucose transporter-2 (SSGT-2) inhibitors lower blood sugar by causing the kidneys to remove glucose from the body through urine.
- Farxiga (dapagliflozin); also in combination with saxagliptin as Qtern; with saxagliptin and metformin as Qternmet XR; and with metformin as Xigduo XR
- Jardiance (empagliflozin); also in combination with empagliflozin and linagliptin as Glyxami and with empagliflozin and metformin as Synjardy
- Steglatro (ertugliflozin); also in combination with ertugliflozin and metformin as Segluromet and ertugliflozin and sitagliptin as Steglujan
- Invokana (canagliflozin); also in combination with metformin as Invokamet
Taking canagliflozin can increase the risk of amputation of a toe, foot, or leg due to infection or other complications. Call your healthcare provider right away if you have any pain; tenderness; sores; ulcers; a swollen, warm, reddened area in your leg or foot; fever or chills; or other signs and symptoms of infection.
Glucagon-Like Peptide (GLP-1) Receptor Protein
Rybelsus (semaglutide) oral tablet was approved as the first and only oral GLP-1 to improve control of blood sugar in adults with type 2 diabetes.
Kerendia (Finerenone)
This drug is prescribed to people who have type 2 diabetes and kidney disease. It helps reduce the risk of kidney failure, heart attack, and heart failure.
Combination Therapy
Trijardy XR (empagliflozin/linagliptin/metformin hydrochloride extended-release) was approved in January 2020 as the only triple combination oral therapy for type 2 diabetes. Trijardy combines Jardiance, Tradjenta, and metformin hydrochloride in one extended-release pill taken once daily.
Incretin Mimetics
Also known as GLP-1 receptor agonists, incretin mimetics are injectable medications that stimulate the production of insulin. They also slow the rate of digestion so that glucose enters the blood more slowly.
- Byetta, BYDUREON (exenatide), BYDUREON BCise (exenatide extended-release)
- Victoza, Saxenda (liraglutide); also in combination with insulin degludec as Xultophy
- Trulicity (dulaglutide)
- Lyxumia (lixisenatide)
- Ozempic (semaglutide)
Mounjaro (Tirzepatide)
In May 2022, the FDA approved the once-weekly injection, Mounjaro (tirzepatide). Mounjaro is the only dual-acting glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist approved to improve glycemic control in adults with type 2 diabetes in addition to diet and exercise.
The FDA updated the label of Ozempic to note the potential increased risk of intestinal blockage. The condition, called ileus, occurs when there are problems pushing food through the intestine and can cause build-up and blockage there. The weight loss drug Wegovy, which has the same active ingredient as Ozempic, and the diabetes drug Mounjaro, already listed ileus on their safety labels.
Amylin is a hormone released by the pancreas at the same time as insulin. It inhibits the secretion of glucagon. Glucagon is another pancreatic hormone that prevents blood glucose levels from dropping too low. Amylin also slows the rate at which food is emptied from the stomach and helps promote a feeling of fullness after eating.
As with insulin, people with type 2 diabetes do not produce normal amounts of amylin. It's believed that replacing amylin helps control blood sugar levels. A synthetic, injectable version of amylin called Symlin (pramlintide acetate) was approved by the FDA in March 2005.
Type 2 diabetes treatment plans sometimes include supplemental insulin, but not always. Although supplemental insulin is vital to managing type 1 diabetes, it is only necessary for certain people with type 2 diabetes. This typically includes:
- People who already had very high blood sugar levels when they were diagnosed
- Those who are very insulin-resistant
- People who haven't been able to control blood sugar with oral medicine, diet, and exercise
Your insulin regimen will be tailored to your needs. Some people may need to take long-acting insulin in the morning that will work throughout the day. Others will benefit most from short-acting or rapid-acting insulin taken at mealtime. Others may need both.
There are several delivery options for injectable insulin. The most common is an insulin pen. This is a device fitted with a small needle. Other options include:
- A basic needle and syringe
- An insulin pump or patch that is attached to the body
A synthetic form of insulin called Semglee (insulin glargine) is available. This long-acting form can be substituted for the more expensive Lantus (insulin glargine).
There is also an inhalable insulin called Afrezza (insulin human). This type of insulin is faster-acting than other types.
Blood Glucose Monitoring
For people with type 2 diabetes who take insulin, blood glucose monitoring may be essential for a number of reasons. For example:
- It can provide a picture of how well treatment is working.
- It can tell you how your blood sugar levels are affected by food and physical activity.
If you take multiple injections of insulin throughout the day, you will likely need to take a blood sugar reading before meals and at bedtime. If you take only a long-acting insulin 410 , you may just need to test twice a day, before breakfast and before dinner.
Monitoring is done with a device called a blood glucose meter , or glucometer. This device can measure blood sugar based on a single drop of blood taken from a fingertip. Most devices are designed to do single tests, but there are some that provide continuous glucose monitoring .
If you are very overweight, your healthcare provider may recommend bariatric surgery as part of your type 2 diabetes treatment. This type of weight loss surgery changes the structure of your digestive system.
There are several types of bariatric surgery, but the Roux-en-Y gastric bypass tends to have the greatest effect on blood sugar. During this procedure, the gastrointestinal tract is changed so food bypasses most of the stomach and upper portion of the small intestine.
People with type 2 diabetes with a body mass index (BMI) greater than 35 are candidates for bariatric surgery. Keep in mind, however, that although BMI is still widely used in the medical community (it’s an inexpensive and quick way to analyze a person’s potential health status and outcomes), it is a dated, flawed measure . BMI does not take into account factors such as body composition , ethnicity, sex, race, and age.
In studies of bariatric surgery performed on more than 135,000 people with type 2 diabetes, the results were significant. Nearly 90% of subjects:
- Had lower blood sugar
- Were able to reduce the dosage of medication
- Experienced improvements in health problems caused by diabetes
What's more, 78% of patients went into remission after losing weight as a result of surgery.
According to the ASMBS, Roux-en-Y gastric bypass can lead to "remission of type 2 diabetes in 80% of patients and improvement of the disease in an additional 15% of patients."
As with any surgical procedure, bariatric surgery has risks. It also requires significant lifestyle changes. People who undergo weight loss surgery are required to follow a specific nutrition plan that's high in protein and limits refined carbs and added sugars. They also must commit to taking nutritional supplements.
Given the potential complications associated with type 2 diabetes, particularly for people who are obese, the benefits of bariatric surgery may outweigh the risks.
There are many options for managing type 2 diabetes. The first step is usually lifestyle changes like diet and exercise. Quitting smoking is another important way to improve your diabetes symptoms .
When lifestyle changes don't work, there are oral and injectible medications available to help you manage your type 2 diabetes. For some people, daily insulin may be necessary. If you take insulin, you will likely need to monitor your blood glucose levels regularly so you know how well your treatment is working and how your blood sugar levels are impacted by food and exercise.
For people with type 2 diabetes who have a body mass index greater than 35, bariatric surgery may be an option.
American Diabetes Association Professional Practice Committee. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of Care in Diabetes -2024 . Diabetes Care . 2024;47(Suppl 1):S145-S157. doi:10.2337/dc24-S008
Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report . Diabetes Care . 2019;42(5):731–54. doi:10.2337/dci19-0014
Bowen ME, Cavanaugh KL, Wolff K, et al. The diabetes nutrition education study randomized controlled trial: A comparative effectiveness study of approaches to nutrition in diabetes self-management education . Patient Educ Couns . 2016;99(8):1368-1376. doi:10.1016/j.pec.2016.03.017
Centers for Disease Control and Prevention. Diabetes meal planning .
American Diabetes Association Professional Practice Committee. 5. Facilitating positive health behaviors and well-being to improve health outcomes: Standards of Care in Diabetes -2024 [published correction appears in Diabetes Care. 2024 Feb 05;:]. Diabetes Care . 2024;47(Suppl 1):S77-S110. doi:10.2337/dc24-S005
Tay J, Luscombe-Marsh ND, Thompson CH, et al. A very low-carbohydrate, low–saturated fat diet for type 2 diabetes management: a randomized trial . Diabetes Care 2014;37(11):2909-2918. doi:10.2337/dc14-0845
ElSayed NA, Aleppo G, Aroda VR, et al. 5. Facilitating positive health behaviors and well-being to improve health outcomes: Standards of care in diabetes—2023 . Diabetes Care . 2023;46(Supple 1):S68-S96. doi:10.2337/dc23-S005
Raman A, Peiffer JJ, Hoyne GF, Lawler NG, Currie AJ, Fairchild TJ. Effect of exercise on acute postprandial glucose concentrations and interleukin-6 responses in sedentary and overweight males . Appl Physiol Nutr Metab . 2018;43(12):1298-1306. doi:10.1139/apnm-2018-0160
Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association . Diabetes Care . 2016;39(11):2065-2079. doi:10.2337/dc16-1728
Carlsson S, Andersson T, Araghi M, et al. Smokeless tobacco (snus) is associated with an increased risk of type 2 diabetes: results from five pooled cohorts . J Intern Med . 2017;281(4):398-406. doi:10.1111/joim.12592
Centers for Disease Control and Prevention. Smoking and diabetes .
Shahar J, Hamdy O. Medication and exercise interactions: considering and managing hypoglycemia risk . Diabetes Spectr . 2015;28(1):64-67. doi:10.2337/diaspect.28.1.64
American Diabetes Association. Medication management: what are my options ?
National Health Service. Understanding medication .
American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Care in Diabetes -2024 . Diabetes Care . 2024;47(Suppl 1):S158-S178. doi:10.2337/dc24-S009
MedlinePlus. Metformin .
Kalra S, Bahendeka S, Sahay R, et al. Consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2 diabetes mellitus - International Task Force . Indian J Endocrinol Metab . 2018;22(1):132–57. doi:10.4103/ijem.IJEM_556_17
ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: Standards of care in diabetes—2023 . Diabetes Care . 2023;46(Suppl 1):S140-S157. doi:10.2337/dc23-S009
American Journal of Managed Care. FDA approves Kerendia for patients with CKD associated with T2D .
Trijardy XR. Prescribing information .
Hieronymus L, Griffin S. Role of amylin in type 1 and type 2 diabetes . Diabetes Educ . 2015;41(1_suppl):47S-56S. doi:10.1177%2F0145721715607642
Federal Food and Drug Administration. FDA approves first interchangeable biosimilar insulin product for treatment of diabetes .
Klonoff DC. Afrezza inhaled insulin: the fastest-acting FDA-approved insulin on the market has favorable properties . J Diabetes Sci Technol . 2014;8(6):1071-1073. doi:10.1177/1932296814555820
Centers for Disease Control and Prevention. Manage blood sugar .
American Society for Metabolic and Bariatric Surgery. Surgery for diabetes .
Centers for Disease Control and Prevention. New CDC report: More than 100 million Americans have diabetes or prediabetes .
Ostenson CG, Hilding A, Grill V, et al. High consumption of smokeless tobacco ("snus") predicts increased risk of type 2 diabetes in a 10-year prospective mtudy of middle-aged swedish men . Scand J Public Health . 2012;40(8):730–7. doi:10.1177/1403494812459814
U.S. Food and Drug Administration. FDA drug safety communication: FDA warns that DPP-4 Inhibitors for type 2 diabetes may cause severe joint pain .
By Debra Manzella, RN Debra Manzella, MS, RN, is a corporate clinical educator at Catholic Health System in New York with extensive experience in diabetes care.
Factors Influencing the Acceptance or Rejection of Dietary and Body Norm Systems Favorable to the Prevention and Control of Type 2 Diabetes Among Sub-Saharan Africa migrants: A Scoping Review
- Published: 05 July 2024
Cite this article

- Gisèle Mandiangu Ntanda 1 , 2 , 3 ,
- Drissa Sia 1 , 2 ,
- Idrissa Beogo 3 , 4 ,
- Aurélie Baillot 3 , 5 ,
- Eric Tchouaket Nguemeleu 1 ,
- Lisa Merry 2 , 6 ,
- Jean Ramdé 7 ,
- Kettly Pinchinat Jean-Charles 2 &
- Léonel Philibert 3 , 8
15 Accesses
Explore all metrics
Introduction
The systems of dietary and body that favor the prevention and control of type 2 diabetes (T2D) go against what is vital for most of the migrant population, exposing them to conflicts of norms that are difficult to reconcile. The purpose of this scoping review is to identify factors that may influence the acceptance or rejection of dietary and body norm systems favorable to the prevention and control of T2D by sub-Saharan Africa migrants living with T2D.
An electronic search of studies from 2011 to 2022, published in English, Italian, French, or Portuguese was conducted in seven databases and in gray literature. The selection of articles was done independently and blindly by six teams of two researchers in accordance with the inclusion and exclusion criteria defined by the PICO.
Seven studies were included. The results show several factors influencing the acceptance or rejection of dietary and body norms systems favorable to the prevention and control of T2D among the migrants from sub-Saharan Africa, mainly social network, income, availability, and affordability of foods, among others.
Given the paucity of studies available on factors influencing the acceptance or rejection of body norm systems favorable to the prevention and control of T2D by sub-Saharan Africa migrants living with T2D, further studies are needed to better document these factors. A better understanding of these factors and their influence on the well-being of migrant people from sub-Saharan Africa living with T2D could help guide policy, research, and interventions so that they are better adapted to the realities of these populations.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Perceptions of dietary intake amongst Black, Asian and other minority ethnic groups in high-income countries: a systematic review of qualitative literature
Systematic mapping review of the factors influencing dietary behaviour in ethnic minority groups living in europe: a dedipac study.
Barriers and facilitators to healthy eating in disadvantaged adults living in the UK: a scoping review
Data availability.
Data availability statement: Further details regarding the data analyzed during the current review are available from the corresponding author on reasonable request.
Organisation mondiale de la santé (OMS). Rapport mondial sur le diabète. Résumé d'orientation. World Health Organization 2016. Retrieved October 10, 2020 from. www.who.int/diabetes/global-report .
Fédération Internationale du Diabète (FID). Plan mondial contre le diabète 2011-2021. Fédération Internationale du Diabète (FID) 2011. Retrieved January 12, 2020 from. https://www.agisante-gard.org/a/610/plan-mondiale-contre-le-diabete-2011-2021/ .
Cheng AYY. Lignesdirectrices de pratique clinique 2013 de l’Association canadienne du diabète pour la prévention et le traitement du diabète au Canada: Introduction. Can J Diabetes. 2013. S361-S364.
Robyn H. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada: Introduction. Can J Diabetes. 2018;42:S1–5.
Article Google Scholar
Creatore MI, et al. Age-and sex-related prevalence of diabetes mellitus among immigrants to Ontario, Canada. Cmaj. 2010;182(8):781–9.
Article PubMed PubMed Central Google Scholar
Adhikari R, Sanou D. Risk factors of diabetes in Canadian immigrants: a synthesis of recent literature. Can J Diabetes. 2012;36(3):142–50.
Nanhou V, Bernèche F, L’état de santé des immigrants du Québec at-il changé au cours des années 2000 par rapport à celui des canadiens de naissance?: une vue d’ensemble à partir d’indicateurs-clés. 2014, Institut de la statistique du Québec.
Gatineau M, Mathrani S. Ethnicity and obesity in the UK. Perspect Public Health. 2011;131(4):159.
Article PubMed Google Scholar
Creatore MI, et al. Association of neighborhood walkability with change in overweight, obesity, and diabetes. JAMA. 2016;315(20):2211–20.
Article CAS PubMed Google Scholar
Veenstra G, Patterson AC. Black–white health inequalities in Canada. J Immigr Minor Health. 2016;18(1):51–7.
Agence de la santé publique du Canada, Réseau pancanadien de santé publique, Statistique Canada et Institut canadien d’information sur la santé, Outil de données sur les inégalités en santé à l'échelle du Canada. Une initiative commune de l'Agence de la santé publique du Canada, du Réseau pancanadien de santé publique, de Statistique Canada et de l’Institut canadien d’information sur la santé. 2019: Ottawa.
Creatore MI, et al. Age- and sex-related prevalence of diabetes mellitus among immigrants to Ontario, Canada. CMAJ: Can Med Assoc J = J l’Assoc Med Can. 2010;182(8):781.
Agence de la santé publique du Canada, Principales inégalités en santé, un portrait national. 2018, Agence de la santé publique du Canada: Ottawa.
Diabète Canada, Le diabète au Canada : Document d’information 2021: Ottawa. Retrieved October 2022 from https://www.diabetes.ca/DiabetesCanadaWebsite/media/Advocacy-and-Policy/Backgrounder/2021_Backgrounder_Canada_French_FINAL.pdf .
García-Pérez L-E, et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–94.
García-Ramírez M, et al. Building healthcare stakeholder coalitions: a community psychology approach to user involvement for migrant populations. Inequalities in health care for migrants and ethnic minorities. 2012: p. 188–204. Retrieved october 2021 from. https://www.researchgate.net/profile/SoniaPlaza/publication/265042208_Building_healthcare_stakeholder_coalitions_A_community_psychology_approach_to_user_involvement_for_migrant_populations/links/5522691a0cf2f9c13052b98d/Building-healthcare-stakeholder-coalitions-A-community-psychology-approach-to-user-involvement-for-migrant-populations.pdf .
Steyn NP, et al. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 2004;7(1a):147–65.
Roy B. Sang sucré, pouvoirs codés, médecine amère: diabète et processus de construction identitaire: les dimensions socio-politiques du diabète chez les Innus de Pessamit. 2002: Presses Université Laval.
Sanou D, et al. Acculturation and nutritional health of immigrants in Canada: a scoping review. J Immigr Minor Health. 2014;16(1):24–34.
Pillarella S. 4.5 Acculturation alimentaire des immigrants récents de l’Afrique de l’Ouest francophone établis à Montréal: Une analyse écologique. Médias et le façonnement des normes en matière de santé, Presses de l’Université du Québec: Québec City, QC, Canada, 2006, p. 235-254. Retrieved on novembrer 2022 from. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=0b9849f1fcbba6a8a656acb301d22299a254ab96#page=253 .
Ntanda GM. Du naturalisme au vitalisme: construire autrement le diabète de type 2 chez les migrants originaires de l’Afrique subsaharienne. 2022. Corpus.Ulaval. Retrieved january 2023 from. https://corpus.ulaval.ca/entities/publication/cd011eb7-7382-4745-9a91-20c0ec3af014.
Ntanda GM, Roy B. Corps sain… vue d’Afrique | Corps malade… vue d’Amérique, in SpiritualitéSanté. 2020. Retrieved on april 2021 from. https://www.chudequebec.ca/a-propos-de-nous/publications/revues-en-ligne/spiritualite-sante/dossiers/titre-2/corps-sain%E2%80%A6-vue-d%E2%80%99afrique-corps-malade%E2%80%A6-vue-d%E2%80%99am.aspx .
Ilunga Tshiswaka D, et al. Perceptions of dietary habits and risk for type 2 diabetes among Congolese immigrants. J ournal of diabetes research , 2017(1), 4736176.
Puoane T, Tsolekile L, Steyn N. Perceptions about body image and sizes among black African girls living in Cape Town. Ethnicity & disease, 2010, 20(1), 29-34.
Van Olmen J, et al. Content, participants and outcomes of three diabetes care programmes in three low and middle income countries. Prim Care Diabetes. 2015;9(3):196–202.
Boëtsch G. Le corps entre normes biologiques et normes sociales . Communication presented at a conference, Octobre 8, 2013, France. Retrieved, January 20,2020 from. https://studylibfr.com/doc/2733576/le-corps-entre-normes-biologiques-et-normes-socialesin . Conférence du 8 octobre 2013. 2013.
Worms F. Le vitalisme critique, ou le seil réalisme qui vaille. In E.Alloa & É During (eds). Chose en soi. Métaphysique du réalisme, 2018, Presses Universitaires de France: Paris.
Organisation internationale pour les migrations (OIM). État de la migration dans le monde 2013: le bien-être des immigrants et le développement. 2013, Organisation internationale pour les migrations (OIM). Genève. Retrieved, october 8, 2023 from. https://publications.iom.int/system/files/pdf/wmr2013_fr_0.pdf.
Osei-Kwasi HA, et al. Systematic mapping review of the factors influencing dietary behaviour in ethnic minority groups living in Europe: a DEDIPAC study. Int J Behav Nutr Phys Act. 2016;13(1):1–17.
Alzubaidi H, et al. The relationships between illness and treatment perceptions with adherence to diabetes self-care: a comparison between Arabic-speaking migrants and Caucasian English-speaking patients. Diabetes Res Clin Pract. 2015;110(2):208–17.
Hyman I, et al. Self-management, health service use and information seeking for diabetes care among recent immigrants in Toronto. Chronic Dis Inj Can. 2012;33(1) 12–18. https://pubmed.ncbi.nlm.nih.gov/23294917/ .
Marzona I, et al. Prevalence and management of diabetes in immigrants resident in the Lombardy Region: the importance of ethnicity and duration of stay. Acta Diabetol. 2018;55(4):355–62.
Moise RK, et al. Diabetes knowledge, management, and prevention among Haitian immigrants in Philadelphia. Diabetes Educ. 2017;43(4):341–7.
Njeru JW, et al. Stories for change: development of a diabetes digital storytelling intervention for refugees and immigrants to minnesota using qualitative methods. BMC Public Health. 2015;15:1311.
Njeru JW, et al. High rates of diabetes mellitus, pre-diabetes and obesity among Somali immigrants and refugees in Minnesota: a retrospective chart review. J Immigr Minor Health. 2016;18(6):1343–9.
Njeru JW, et al. Diabetes knowledge, attitudes and behaviors among Somali and Latino immigrants. J Immigr Minor Health. 2016;18(6):1432–40.
Dinca-Panaitescu M, et al. The dynamics of the relationship between diabetes incidence and low income: longitudinal results from Canada’s National Population Health Survey. Maturitas. 2012;72(3):229–35.
Dinca-Panaitescu S, et al. Diabetes prevalence and income: results of the Canadian Community Health Survey. Health Policy. 2011;99(2):116–23.
Liburd, L. C. Diabetes and health disparities: community-based approaches for racial and ethnic populations . New York: Springer Publishing Company, 2009. https://www.researchgate.net/publication/242531110_Diabetes_and_Health_Disparities_Community-Based_Approaches_for_Racial_and_Ethnic_Populations .
Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Ntanda GM, et al. Factors influencing the acceptance or rejection of dietary and body norm systems favorable to the prevention and control of type 2 diabetes among Sub-Saharan Africa migrants: a scoping review protocol. 2023. Retrieved december 20, 2023 from. https://www.researchsquare.com/article/rs-2574407/v1 .
International Organization of Migration (IOM). Glossary on migration. 2019. Retrieved december 20, 2020 from. https://publications.iom.int/system/files/pdf/iml_34_glossary.pdf .
Jobin L, Pigeon M, Bédard L. La santé et ses déterminants: mieux comprendre pour mieux agir. Ministère de la Santé et des Services sociaux (MSSS), Québec: 2012;26p. Retrieved january 8, 2020, from. https://publications.msss.gouv.qc.ca/msss/fichiers/2011/11-202-06.pdf .
Mikkonen J, Raphael D. Déterminants sociaux de la santé: les réalités canadiennes. 2011. Toronto, École de gestion et de politique de la santé de l’Université de York, 2010. Retrieved October 9, 2021 from. https://thecanadianfacts.org/Les_realites_canadiennes-Flyer.pdf .
Galbete C, et al. Food consumption, nutrient intake, and dietary patterns in Ghanaian migrants in Europe and their compatriots in Ghana. Food Nutr Res. 2017;61(1):1341809.
Von Bertalanffy L. The history and status of general systems theory. Acad Manag J. 1972;15(4):407–26.
Sia D, et al. Interventions facilitating access to perinatal care for migrant women without medical insurance: a scoping review protocol. PLoS ONE. 2022;17(3):e0265232.
Article CAS PubMed PubMed Central Google Scholar
Osei-Kwasi HA, et al. Acculturation and food intake among Ghanaian migrants in Europe: findings from the RODAM Study. J Nutr Educ Behav. 2020;52(2):114–25.
Mbombo-Dweba, T. P., Mbajiorgu, C. A., Agyepong, A. O., & Oguttu, J. W. Food consumption patterns of sub-Saharan African immigrants residing in Gauteng Province, South Africa. Applied Ecology & Environmental Research , 2017, 15(4). Retrieved september 9, 2020 from. https://www.aloki.hu/pdf/1504_10231038.pdf .
McElrone M, et al. Barriers and facilitators to food security among adult Burundian and Congolese refugee females resettled in the US. Ecol Food Nutr. 2019;58(3):247–64.
Okafor MC, Carter-Pokras OD, Zhan M. Greater dietary acculturation (dietary change) is associated with poorer current self-rated health among African immigrant adults. J Nutr Educ Behav. 2014;46(4):226–35.
Ngoubene-Atioky AJ, Williamson-Taylor C. Culturally based health assumptions in Sub-Saharan African immigrants: body mass index predicting self-reported health status. J Health Psychol. 2019;24(6):750–60.
Mbombo-Dweba TP et al. Assessment of dietary challenges faced by Sub-Saharan immigrants residing in Gauteng province: a pilot study. J Consum Sci. 2017. Retrieved december 10, 2020 from. file:///C:/Users/mandgi01/Downloads/ajol-file-journals_397_articles_158695_submission_proof_158695-4729-412810-1-10-20170713.pdf .
Goulão B, Santos O, Carmo I. The impact of migration on body weight: a review. Cad Saude Publica. 2015;31:229–45.
Ngoubene-Atioky AJ, et al. Migration trends and dietary patterns in Sub-Saharan African adult immigrants: a comparative analysis of populations in France, the UK, and the USA. Migr Stud. 2021;9(3):1116–43.
Akresh IR, Frank R. Health selection among new immigrants. Am J Public Health. 2008;98(11):2058–64.
Roshania R, Narayan KV, Oza-Frank R. Age at arrival and risk of obesity among US immigrants. Obesity. 2008;16(12):2669–75.
Pillarella S. L’acculturation alimentaire des immigrants récents de l’Afrique de l’ouest francophone établis à 3 Montréal : une analyse écologique. Montréal: Université du Québec à Montréal; 2006.
Google Scholar
Shi Y, Lukomskyj N, Allman-Farinelli M. Food access, dietary acculturation, and food insecurity among international tertiary education students: a scoping review protocol. JBI Evid Synth. 2020;18(9):2090–7.
Kivuyo NG, Sharma S. Dietary acculturation among African emigrant students in India: determinants and problems. Public Health Nutr. 2020;23(13):2402–9.
Pillarella S. L’acculturation alimentaire des immigrants récents de l’Afrique de l’ouest francophone établis à Montréal : une analyse écologique. Montréal: Université du Québec à Montréal; 2006.
Patil CL, Hadley C, Nahayo PD. Unpacking dietary acculturation among new Americans: results from formative research with African refugees. J Immigr Minor Health. 2009;11(5):342–58.
Statistique Canada. Revenu et faible revenu des particuliers en 2020: répercussions de la première année de la pandémie de COVID-19. 2020. Retrieved september 10, 2021 from. https://statistique.quebec.ca/fr/produit/publication/revenu-faible-revenu-particuliers-2020-repercussions-pandemie-covid-19-analyse .
Larocque ML-CP-I, Kègle MS-PL. Le diabète à Montréal: un problème qui continue de prendre de l’ampleur. 2015. Retrieved november 10, 2016 from. https://policycommons.net/artifacts/2061749/le-diabete-a-montreal/2814840/ .
Pereira CA, Larder N, Somerset S. Food acquisition habits in a group of African refugees recently settled in Australia. Health Place. 2010;16(5):934–41.
Mahmoodi M, George RM, Gokhale D. Dietary acculturation of international students in Pune, India: a cross-sectional study. Nutr Health. 2022;28(2):271–6.
Dualle, M. A., Robinette, L. M., & Hatsu, I. E. Food Related Challenges and Mental Health Among U.S. African Migrants: A Narrative Review. Journal of immigrant and minority health, 2023, 26(2) , 371–384. https://doi.org/10.1007/s10903-023-01512-2 .
Okafor MT, et al. The relationship of language acculturation (English proficiency) to current self-rated health among African immigrant adults. J Immigr Minor Health. 2013;15(3):499–509.
Ngongalah, L., Rankin, J., Rapley, T., Odeniyi, A., Akhter, Z., & Heslehurst, N. Dietary and Physical Activity Behaviours in African Migrant Women Living in High Income Countries: A Systematic Review and Framework Synthesis. Nutrients , 2018; 10(8 ):1017. https://doi.org/10.3390/nu10081017 .
Toselli S, Rinaldo N, Gualdi-Russo E. Body image perception of African immigrants in Europe. Glob Health. 2016;12(1):48.
Pradeilles R, et al. Body size preferences for women and adolescent girls living in Africa: a mixed-methods systematic review. Public Health Nutr. 2022;25(3):738–59.
Bosire EN, et al. ‘I’d say I’m fat, I’m not obese’: obesity normalisation in urban-poor South Africa. Public Health Nutr. 2020;23(9):1515–26.
Micklesfield LK, et al. Socio-cultural, environmental and behavioural determinants of obesity in black South African women. Cardiovasc J Afr. 2013;24(9–10):369–75.
Batis C, et al. Food acculturation drives dietary differences among Mexicans, Mexican Americans, and Non-Hispanic Whites. J Nutr. 2011;141(10):1898–906.
Mude W, Nyanhanda T. Food behaviours and eating habits among Sub-Saharan African migrant mothers of school-aged children in South Australia. J Migr Health. 2023;7:100149.
Renzaho AM, Swinburn B, Burns C. Maintenance of traditional cultural orientation is associated with lower rates of obesity and sedentary behaviours among African migrant children to Australia. Int J Obes (Lond). 2008;32(4):594–600.
Antecol H, Bedard K. Unhealthy assimilation: why do immigrants converge to American health status levels? Demography. 2006;43(2):337–60.
Delavari M, et al. Acculturation and obesity among migrant populations in high income countries–a systematic review. BMC Public Health. 2013;13:458.
Download references
Acknowledgements
Special thanks to Sherpa University Institute for funding.
The study was funded by the Sherpa University Institute (Sherpa postdoctoral fellowship competition 2022–2023). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and affiliations.
Department of Nursing, Université du Québec en Outaouais, Saint-Jérôme, QC, Canada
Gisèle Mandiangu Ntanda, Drissa Sia & Eric Tchouaket Nguemeleu
Institut Universitaire Sherpa, Montréal, QC, Canada
Gisèle Mandiangu Ntanda, Drissa Sia, Lisa Merry & Kettly Pinchinat Jean-Charles
Institut du Savoir de L’hôpital Montfort-Recherche, Ottawa, ON, Canada
Gisèle Mandiangu Ntanda, Idrissa Beogo, Aurélie Baillot & Léonel Philibert
School of Nursing, Faculty of Health Sciences, University of Ottawa, Ottawa, ON, Canada
Idrissa Beogo
Department of Nursing, Université du Québec en Outaouais, Gatineau, QC, Canada
Aurélie Baillot
Faculty of Nursing, Université de Montréal, Montréal, Canada
Département des fondements et pratiques en éducation | Faculté des sciences de l’éducation, Université Laval, Québec, Canada
Pôle Pluralité Humaine, Université de L’Ontario Français, Toronto, Canada
Léonel Philibert
You can also search for this author in PubMed Google Scholar
Contributions
All authors agreed on the publication of this manuscript.
○ Funding acquisition: Gisèle Mandiangu Ntanda (GMN)
○ Project administration: Gisèle Mandiangu Ntanda (GMN)
○ Conceptualization: Gisèle Mandiangu Ntanda (GMN)
○ Who performed the literature search? Gisèle Mandiangu Ntanda (GMN) and Kettly Pinchinat Jean Charles (KPJC)
○ Writing—original draft: Gisèle Mandiangu Ntanda (GMN)
○ Writing—review & editing: Gisèle Mandiangu Ntanda (GMN), Drissa Sia (DS), Idrissa Beogo (IB), Aurélie Baillot (AB), Eric Tchouaket Nguemeleu (ETN), Lisa Merry (LM), Léonel Philibert (LP), Jean Ramdé (JR), Kettly Pinchinat Jean Charles (KPJC)
○ Validation: Gisèle Mandiangu Ntanda (GMN), Drissa Sia (DS), Idrissa Beogo (IB), Aurélie Baillot (AB), Eric Tchouaket Nguemeleu (ETN), Lisa Merry (LM), Léonel Philibert (LP), Jean Ramdé (JR), Kettly Pinchinat Jean Charles (KPJC)
Corresponding author
Correspondence to Gisèle Mandiangu Ntanda .
Ethics declarations
Registration.
This scoping review has been registered in Research Registry (8433; https://www.researchregistry.com/browse-the-registry#home/ ).
Ethics Approval and Consent to Participate
This protocol is part of a research project that has received ethical approval from the ethics committee of the Université du Québec en Outaouais in October 2022.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions

About this article
Ntanda, G.M., Sia, D., Beogo, I. et al. Factors Influencing the Acceptance or Rejection of Dietary and Body Norm Systems Favorable to the Prevention and Control of Type 2 Diabetes Among Sub-Saharan Africa migrants: A Scoping Review. J. Racial and Ethnic Health Disparities (2024). https://doi.org/10.1007/s40615-024-02072-3
Download citation
Received : 10 February 2024
Revised : 18 June 2024
Accepted : 23 June 2024
Published : 05 July 2024
DOI : https://doi.org/10.1007/s40615-024-02072-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Corporality
- Sub-Saharan Africa
- Type 2 diabetes
- Find a journal
- Publish with us
- Track your research

- Previous Article
- Next Article
Review of DPP Translations for Underserved Populations
Acknowledgments, duality of interest, author contributions, the diabetes prevention program for underserved populations: a brief review of strategies in the real world.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Mona AuYoung , Tannaz Moin , Caroline R. Richardson , Laura J. Damschroder; The Diabetes Prevention Program for Underserved Populations: A Brief Review of Strategies in the Real World. Diabetes Spectr 1 November 2019; 32 (4): 312–317. https://doi.org/10.2337/ds19-0007
Download citation file:
- Ris (Zotero)
- Reference Manager
IN BRIEF This review highlights examples of the translation of the Diabetes Prevention Program (DPP) to underserved populations. Here, underserved populations are defined as groups whose members are at greater risk for health conditions such as diabetes but often face barriers accessing treatment. Strategies to develop and evaluate future DPP translations are discussed.
The Diabetes Prevention Program (DPP) has been successfully translated across many real-world settings since the results of the landmark study were published ( 1 ). Some populations are at relatively higher risk for type 2 diabetes, are less likely to have access to resources to prevent type 2 diabetes, or are medically underserved, so it is important to consider the effectiveness of the DPP lifestyle change intervention within these specific groups. This article reviews studies that have translated the DPP into these underserved populations, including racial/ethnic minorities, rural populations, and populations with low socioeconomic status (SES). The prevalence of type 2 diabetes among racial/ethnic minorities (8.0–15.1%) is greater than that of non-Hispanic whites (7.4%) ( 2 ). However, there is variation within racial/ethnic groups and by region. Although the Centers for Disease Control and Prevention (CDC) reported the prevalence of type 2 diabetes among American Indians and Alaska Natives as 15.1%, this includes a rate of 6.0% for Alaska Natives and 22.2% for American Indians in the Southwest ( 2 ).
The relationship between SES and type 2 diabetes incidence and prevalence is complex because of other confounding circumstances (e.g., health care access, opportunities to exercise, and access to healthy foods) and overlapping risk factors ( 3 ). Racial/ethnic minorities now make up 21% of rural populations; their health status is poorer than those of both rural whites and urban minorities ( 4 ). Poverty is more prevalent in rural and inner-city communities, further increasing the risk of diabetes within these communities ( 4 ). Therefore, increasing engagement and retention in the DPP lifestyle change intervention is crucial among these high-risk groups. Some DPP translation studies have included populations with multiple categories of risk factors, in which case they are referenced in multiple categories as appropriate. This review discusses 1 ) how the DPP has been adapted for different underserved populations and 2 ) strategies for how to adapt and assess future translations of the DPP for other populations. This article highlights some of the work done to provide the DPP to underserved populations, but it also aims to highlight approaches for assessing findings from these translations and to emphasize the need to share information more broadly with others.
Other articles have reviewed DPP translations in different settings ( 5 ), for different racial/ethnic groups ( 6 ), for level of cultural adaptation by theoretical frameworks ( 7 ), and for degree of cultural adaptation and implementation strategy ( 8 ) and have proposed a framework for evaluating the effectiveness of cultural tailoring ( 9 ). This work discusses strategies for tailoring and implementing the DPP and broadening definitions of underserved populations to racial/ethnic minorities, rural populations, and individuals with low SES. The focus is on studies that have specified that they translated the DPP. These studies were identified through a literature search using the search terms “diabetes prevention program” and “underserved” or “minority” or “ethnic” or “tailored” or “low income” or “rural” in PubMed and Google Scholar. Studies identified through other articles on DPP translations are also included.
DPP Tailored for Racial/Ethnic Minorities
The previously described CDC report on diabetes rates among minorities had limited data on more specific categories of populations ( 2 ). For example, the 2014 Native Hawaiian and Pacific Islander (NHPI) National Health Interview Survey showed that 15.2% of NHPI adults had diabetes, ranging from 14.2% for Native Hawaiians to 22.1% for Samoans ( 10 ). This level of granularity is important for understanding different levels of risk and outcomes, especially when studying different racial and ethnic groups. A 2012 meta-analysis of DPPs ( 11 ) found an average of 4–5% weight loss at 12 months. A 2011 systematic review ( 5 ) found a range of 2.7–6% weight loss within DPP translations (compared to almost 7% in the original DPP study). Within these outcomes, however, there appear to be racial/ethnic disparities. The original DPP study population was notably 45% minorities, including 22% African Americans. However, the DPP was less effective for African Americans ( 1 , 12 ) relative to other racial/ethnic groups in the sample; African Americans averaged only half of the overall average weight loss, and weight loss outcomes were smallest for African American women ( 13 ).
Most studies described using stakeholder or community feedback to inform their translations and implementation process. This process helped to identify preferred settings for classes, delivery, and content. The more common adaptations were a group-based approach, the use of peer coaches, and a shortened number of sessions delivered ( 6 , 8 , 9 ), sometimes due to concerns about feasibility ( 14 ). Community settings (e.g., churches and recreation centers) were chosen for their cultural value or common use for community gatherings. Some programs built the setting into the delivery (e.g., recruiting church members or scheduling classes right after church), whereas other programs recruited more broadly in underserved neighborhoods ( 15 , 16 ).
Common characteristics for peer coaches included bilingual skills, a racial/ethnic match to participants, and being from the local community. Peers were used to help build trust and have coaches that could relate to participants; most programs with peer coaches reported successful weight outcomes for participants ( 9 , 11 ). Some challenges ( 9 ) arose when coaches were only available on a part-time basis ( 17 ), which limited their ability to engage with participants, and also when there was not consistency in the content being delivered (i.e., the coaches each designed their own curriculum) ( 18 ). Stakeholder feedback also led to content modifications such as adding topics that the community found relevant (e.g., how to eat healthy on a low income and how to discuss personal matters with a doctor) ( 19 ) and adding activities (e.g., providing a food guide to use on a supermarket tour and holding practice walking sessions with pedometers) ( 20 ) to address gaps in knowledge or existing barriers to lifestyle changes.
DPP for Rural Populations
Rural areas have a higher prevalence of type 2 diabetes (17% greater than in urban areas) but face limited access to diabetes management programs (62% of nonmetropolitan counties do not have diabetes self-management education and support programs) ( 21 ). This problem is compounded by the lower ratio of providers to patients. Although 17% (59 million) of the U.S. population lives in rural areas, only 9% of doctors and 16% of registered nurses practice in such settings ( 4 ). Although rural stakeholders noted diabetes as their third highest health priority (behind nutrition and weight status), access to health care remains the greatest need ( 4 ). A systematic review and meta-analysis by Joiner et al. ( 22 ) found a wide range of eHealth DPP translations delivered through the Internet, mobile phones (applications or text messages), DVDs, interactive voice response telephone calls, videoconferencing, and video-on-demand programs. The authors categorized the interventions into stand-alone, behavioral support from a remote counselor, and behavioral support from an in-person counselor and found average percentage weight losses of 3.34, 4.31, and 4.65%, respectively. However, across these studies, participants were mostly female, college-educated, and white. The authors recognized a need for additional studies with more diverse populations, rural residents, and those with less education.
One such study ( 23 ) compared outcomes for an in-person and a telehealth DPP in Montana. A telehealth site was chosen in each of seven different towns (an average of 83 miles from the main health care center). The DPP classes were provided on site at one main health care center and simultaneously broadcast at one of the telehealth sites (the telehealth sites rotated over time). There were no significant differences between onsite and telehealth participants, respectively, in terms of attendance or meeting the 7% weight loss goal (38 vs. 41%). It was estimated that the average telehealth participant cost $125 less than an onsite participant (on top of an estimated $810 savings in participant travel costs).
Before their larger study, there was a pilot study to test the feasibility of the telehealth DPP, in which the onsite and telehealth groups had similar rates of attendance and weight loss (46 and 50%, respectively, met the 7% weight loss goal) ( 23 ).
To implement the telehealth DPP, there were again partnerships developed and coordination done before the start of the intervention. The main site had to get buy-in from the telehealth sites and ensure that they had equipment capable of hosting the telehealth sessions (usually existing telemedicine networks). Each telehealth site also needed a local site coordinator to weigh participants, set up rooms, conduct surveys, and collect and mail participant log books (due to unreliable Internet or cell phone access), while the main health care center provided the program materials. The onsite and telehealth classes were held simultaneously, so the onsite coaches had to be conscious of the need to make sure all class demonstrations were visible for the camera so the telehealth participants could see them. Because of limited community resources for participants, the lifestyle coaches established partnerships, including a local motel pool for water aerobics classes, a local high school for cooking classes, and a local grocery store that started offering $10 bags of produce ( 24 ).
An estimated 4.7 million veterans live in rural areas, and a larger proportion of rural veterans (58% rural vs. 37% urban) enroll in the Veterans Administration (VA) health care system, even though they may not live near their closest VA medical center ( 25 ). More than half of rural veterans are ≥65 years of age and earn less than $35,000 annually, and 27% do not have home Internet access. These veterans are more likely to have health conditions such as diabetes. A multisite demonstration of the DPP within the VA, both in person and online, had promising weight loss outcomes (average of 3% weight loss at 12 months) ( 26 ). This program also demonstrated the feasibility and effectiveness of an online DPP, despite at least some participants being relatively new to both computer and Internet use.
The online VA DPP was completely virtual; a live coach communicated electronically, and weights were collected through a wireless Bluetooth scale. The online VA DPP vendor ensured that veterans were assigned to cohorts with at least one other veteran member in this study. There were benefits to the in-person DPP as well, with anecdotal data at one site about the positive impact of having a peer (fellow veteran) as a coach.
Damschroder et al. ( 27 ) describes using a hybrid type 3 implementation framework, a design in which the primary focus is on testing the implementation strategy for a program because it is believed to have an impact on the program’s effectiveness, but that also includes assessment of program outcomes. In addition to assessing the implementation process at the different VA sites and fidelity to the original DPP curriculum, they also studied the effectiveness of the DPP relative to usual care. The online DPP enrolled participants from four different VA sites around the country, so another VA site served as the coordinating center to manage the collection and tracking of participant surveys and other study details. The coordinating center staff also visited each site to assess the fidelity of content delivered to participants by session.
DPP Tailored for SES
Low-income individuals often face access issues when it comes to health care and health-promoting resources, so they may be less likely to get screened for type 2 diabetes or to live near options for healthy eating or physical activity. As previously stated, type 2 diabetes risk factors related to SES (e.g., educational level and income) are often related to other risk factors such as race/ethnicity and rural location. The following studies each took a different approach in designing DPP programs.
Fontil et al. ( 28 ) described a collaboration between researchers and a digital health company to modify the DPP for low-income safety net clinic patients. They used focus groups and interviews in English and Spanish to modify the content (for general and health literacy) and the online platform. Content modifications included using simpler terms and providing health advice or examples that were more relevant or realistic, such as recommending dancing or playing sports instead of gym memberships or yoga classes. Additional tools (e.g., video tutorials and handouts with computer screenshots) were created to help with the online process of signing up and navigating the program. Some participants needed assistance setting up email accounts, and others rarely checked their email, despite reporting frequent Internet use. A conference call was added to the beginning of the program to help orient participants and build connections within the cohort. Weight loss outcomes are being analyzed ( 29 ).
Similarly, the Power Up for Health program ( 16 ) was implemented in accessible locations and provided participants with membership to local recreation centers. However, the memberships were underutilized, and post-intervention interviews revealed that participants wished they had had class time to exercise or been given demonstrations of exercises ( 30 ). This desire was not limited to exercise; participants also said they would have appreciated cooking demonstrations, help with meal planning, and information about outside resources to help with sustaining behavior changes. Overall, participants averaged 3.8% weight loss (ranging from 1.3 to 6.2% by site) ( 31 ).
DPP Tailored for Sex
Although one program was specifically tailored to men ( 16 , 30 ) and another program by default served mostly men ( 26 , 27 ), there is little literature on lifestyle programs designed specifically for men and their health needs. Most studies on weight loss or lifestyle change have large samples of women. Studies that use group-based programs may deter male participants who view these sessions as female-oriented (e.g., Weight Watchers). Compounding this issue is the reticence of men to actively seek health care (especially prevention).
There can also be a cultural preference for sex-specific groups ( 32 ); while assessing cultural preferences for the delivery of the DPP translation within an Arab-American community, focus group participants noted a preference to have separate groups for men and women. Within that study, 44% met the 7% weight loss goal (59% lost at least 5% baseline weight). Within the VA, which has a majority of men, tailored DPP groups for women only also found success, with an average of 5.24% weight loss ( 33 ).
To create a DPP that men would attend, Power Up for Health ( 16 ) started with focus groups, discussions with community leaders, and an advisory panel to help adapt the curriculum before piloting their work. Some focus group participants expressed concern about being able to fully share and discuss issues with women in the group. The male community leader noted that societal expectations around masculinity could make conversations difficult. Interviews conducted after the program ended revealed that participants appreciated having men-only groups that were composed of fellow minorities because they felt like they could trust them and relate more. They also appreciated having coaches that had personal experience with diabetes or weight issues.
Other underserved populations are not fully discussed in this article because of space constraints rather than a lack of importance. For example, although a history of gestational diabetes is often part of eligibility criteria for these interventions, there are few programs available for postpartum women (especially minority women, who receive less diabetes screening) ( 34 ). The few studies that exist unsurprisingly report difficulty with engagement due to the competing demands of being a new mother, although one ongoing study ( 34 ) is incorporating tailored health coaching calls to try to address that barrier. Individuals with severe mental illness are at risk for obesity because of the psychotropic medications used for treatment and high rates of sedentary behavior and unhealthy diet. One translation of the DPP into a community mental health organization demonstrated its feasibility, although with minimal weight loss ( 35 ). Unlike the previous studies discussed, in which the overall number of sessions or timeline was condensed to reduce participant burden, stakeholders here reported the need for more time to process information and practice strategies.
The DPP continues to be translated for use in many diverse populations, with program staff making adaptations to tailor program content and structure to specific populations or regional barriers and needs. As technology continues to evolve, there may be more options for delivering the DPP even more widely, as long as there is Internet or mobile network access. Fortunately, DPP modifications do not appear to affect weight loss outcomes ( 36 ); for example, peer coaches have been shown to be just as effective as medical or allied health care providers and require lower program costs ( 11 ). Most of the DPP translations reviewed here included formative work to determine what aspects of the DPP to adapt; many used community-based participatory research (CBPR) methods as guidance ( 8 , 9 ). CBPR methods can be valuable when tailoring the DPP for any population—not just racial/ethnic minorities ( 37 ). In addition to learning more about common barriers or group preferences, there is the opportunity to include key stakeholders throughout the research process.
Many of the studies reviewed used one or more of the following as a part of their formative research: focus groups, community advisory boards, and stakeholder interviews (e.g., community leaders). Although not discussed in detail, the authors described having relationships with community partners and other organizations to conduct this formative work. Furthermore, having direct conversations with members of underserved populations may reveal regional or population differences that might differ from those described in the current literature. For example, as previously mentioned, a population with severe mental illness requires more, not fewer, sessions. Time to build relationships and trust and to show the value of the programs such as the DPP is important, especially in populations that do not usually have access to care or have often had negative experiences with health care. One example of this can be found in the study by Jaber et al. ( 32 ), in which participants who declined the intervention could choose to participate in the educational arm instead. After completing the educational arm, participants were again given the option to enroll in the intervention, and interest was higher than expected (78% decided to enroll).
Fewer of the studies described their implementation process or guiding framework. There are common challenges in real-world implementation related to recruitment and retention, program delivery, and continuation or expansion of the program after initial funding ends. As more translations of the DPP are conducted within underserved populations, it is even more important to share their implementation findings in addition to the health outcomes they achieve to gain a better understanding of why certain strategies may work or how to make improvements. Although populations and regions have their unique characteristics and differences, considering the strategies used by others can be helpful.
M.A.Y. was supported by the Clinical and Translational Science Award (CTSA) #UL1 TR002550 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The contents of this article are solely the responsibility of the authors and do not necessarily reflect the views of the CTSA NIH or the Veterans Health Administration.
No potential conflicts of interest relevant to this article were reported.
M.A.Y. conducted the literature search and wrote the manuscript. T.M. identified additional references and reviewed and edited the manuscript. C.R.R. reviewed the manuscript. L.J.D. reviewed and edited the manuscript. M.A.Y. is the guarantor of this work and, as such, had full access to all of the articles cited in this review and takes responsibility for the integrity and accuracy of the review.

Email alerts
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Trends in...
Trends in cardiovascular disease incidence among 22 million people in the UK over 20 years: population based study
- Related content
- Peer review
- Geert Molenberghs , professor 4 ,
- Geert Verbeke , professor 4 ,
- Francesco Zaccardi , associate professor 5 ,
- Claire Lawson , associate professor 5 ,
- Jocelyn M Friday , data scientist 1 ,
- Huimin Su , PhD student 2 ,
- Pardeep S Jhund , professor 1 ,
- Naveed Sattar , professor 6 ,
- Kazem Rahimi , professor 3 ,
- John G Cleland , professor 1 ,
- Kamlesh Khunti , professor 5 ,
- Werner Budts , professor 1 7 ,
- John J V McMurray , professor 1
- 1 School of Cardiovascular and Metabolic Health, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK
- 2 Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium
- 3 Deep Medicine, Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, UK
- 4 Interuniversity Institute for Biostatistics and statistical Bioinformatics (I-BioStat), Hasselt University and KU Leuven, Belgium
- 5 Leicester Real World Evidence Unit, Diabetes Research Centre, University of Leicester, Leicester, UK
- 6 College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, UK
- 7 Congenital and Structural Cardiology, University Hospitals Leuven, Belgium
- Correspondence to: N Conrad nathalie.conrad{at}kuleuven.be (or @nathalie_conrad on X)
- Accepted 1 May 2024
Objective To investigate the incidence of cardiovascular disease (CVD) overall and by age, sex, and socioeconomic status, and its variation over time, in the UK during 2000-19.
Design Population based study.
Setting UK.
Participants 1 650 052 individuals registered with a general practice contributing to Clinical Practice Research Datalink and newly diagnosed with at least one CVD from 1 January 2000 to 30 June 2019.
Main outcome measures The primary outcome was incident diagnosis of CVD, comprising acute coronary syndrome, aortic aneurysm, aortic stenosis, atrial fibrillation or flutter, chronic ischaemic heart disease, heart failure, peripheral artery disease, second or third degree heart block, stroke (ischaemic, haemorrhagic, and unspecified), and venous thromboembolism (deep vein thrombosis or pulmonary embolism). Disease incidence rates were calculated individually and as a composite outcome of all 10 CVDs combined and were standardised for age and sex using the 2013 European standard population. Negative binomial regression models investigated temporal trends and variation by age, sex, and socioeconomic status.
Results The mean age of the population was 70.5 years and 47.6% (n=784 904) were women. The age and sex standardised incidence of all 10 prespecified CVDs declined by 19% during 2000-19 (incidence rate ratio 2017-19 v 2000-02: 0.80, 95% confidence interval 0.73 to 0.88). The incidence of coronary heart disease and stroke decreased by about 30% (incidence rate ratios for acute coronary syndrome, chronic ischaemic heart disease, and stroke were 0.70 (0.69 to 0.70), 0.67 (0.66 to 0.67), and 0.75 (0.67 to 0.83), respectively). In parallel, an increasing number of diagnoses of cardiac arrhythmias, valve disease, and thromboembolic diseases were observed. As a result, the overall incidence of CVDs across the 10 conditions remained relatively stable from the mid-2000s. Age stratified analyses further showed that the observed decline in coronary heart disease incidence was largely restricted to age groups older than 60 years, with little or no improvement in younger age groups. Trends were generally similar between men and women. A socioeconomic gradient was observed for almost every CVD investigated. The gradient did not decrease over time and was most noticeable for peripheral artery disease (incidence rate ratio most deprived v least deprived: 1.98 (1.87 to 2.09)), acute coronary syndrome (1.55 (1.54 to 1.57)), and heart failure (1.50 (1.41 to 1.59)).
Conclusions Despite substantial improvements in the prevention of atherosclerotic diseases in the UK, the overall burden of CVDs remained high during 2000-19. For CVDs to decrease further, future prevention strategies might need to consider a broader spectrum of conditions, including arrhythmias, valve diseases, and thromboembolism, and examine the specific needs of younger age groups and socioeconomically deprived populations.
Introduction
Since the 1970s, the prevention of coronary disease, both primary and secondary, has improved considerably, largely attributable to public health efforts to control risk factors, such as antismoking legislation, and the widespread use of drugs such as statins. 1 2
Improvements in mortality due to heart disease have, however, stalled in several high income countries, 3 and reports suggest that the incidence of heart disease might even be increasing among younger people. 4 5 6 Conversely, along with coronary heart disease, other cardiovascular conditions are becoming relatively more prominent in older people, altering the profile of cardiovascular disease (CVD) in ageing societies. The importance of non-traditional risk factors for atherosclerotic diseases, such as socioeconomic deprivation, has also been increasingly recognised. Whether socioeconomic deprivation is as strongly associated with other CVDs as with atherosclerosis is uncertain, but it is important to understand as many countries have reported an increase in socioeconomic inequalities. 7
Large scale epidemiological studies are therefore needed to investigate secular trends in CVDs to target future preventive efforts, highlight the focus for future clinical trials, and identify healthcare resources required to manage emerging problems. Existing comprehensive efforts, such as statistics on CVD from leading medical societies or the Global Burden of Diseases studies, have helped toward this goal, but reliable age standardised incidence rates for all CVDs, how these vary by population subgroups, and changes over time are currently not available. 8 9 10
We used a large longitudinal database of linked primary care, secondary care, and death registry records from a representative sample of the UK population 11 12 to assess trends in the incidence of 10 of the most common CVDs in the UK during 2000-19, and how these differed by sex, age, socioeconomic status, and region.
Data source and study population
We used anonymised electronic health records from the GOLD and AURUM datasets of Clinical Practice Research Datalink (CPRD). CPRD contains information on about 20% of the UK population and is broadly representative of age, sex, ethnicity, geographical spread, and socioeconomic deprivation. 11 12 It is also one of the largest databases of longitudinal medical records from primary care in the world and has been validated for epidemiological research for a wide range of conditions. 11 We used the subset of CPRD records that linked information from primary care, secondary care from Hospital Episodes Statistics (HES admitted patient care and HES outpatient) data, and death certificates from the Office for National Statistics (ONS). Linkage was possible for a subset of English practices, covering about 50% of the CPRD records. Data coverage dates were 1 January 1985 to 31 December 2019 for primary care data (including drug prescription data), 1 April 1997 to 30 June 2019 for secondary care data, and 2 January 1998 to 30 May 2019 for death certificates.
Included in the study were men and women registered with a general practice for at least one year during the study period (1 January 2000 to 30 June 2019) whose records were classified by CPRD as acceptable for use in research and approved for HES and ONS linkage.
Study endpoints
The primary endpoint was the first presentation of CVD as recorded in primary or secondary care. We investigated 10 CVDs: acute coronary syndrome, aortic aneurysm, aortic stenosis, atrial fibrillation or flutter, chronic ischaemic heart disease, heart failure, peripheral artery disease, second or third degree heart block, stroke (ischaemic, haemorrhagic, or unspecified), and venous thromboembolism (deep vein thrombosis or pulmonary embolism). We defined incident diagnoses as the first record of that condition in primary care or secondary care regardless of its order in the patient’s record.
Diseases were considered individually and as a composite outcome of all 10 CVDs combined. For the combined analyses, we calculated the primary incidence (considering only the first recorded CVD in each patient, reflecting the number of patients affected by CVDs) and the total incidence (considering all incident CVD diagnoses in each patient, reflecting the cumulative number of CVD diagnoses). We performed sensitivity analyses including diagnoses recorded on death certificates.
To identify diagnoses, we compiled a list of diagnostic codes based on the coding schemes in use in each data source following previously established methods. 13 14 15 We used ICD-10 (international classification of diseases, 10th revision) codes for diagnoses recorded in secondary care, ICD-9 (international classification of diseases, ninth revision) (in use until 31 December 2000) and ICD-10 codes for diagnoses recorded on death certificates (used in sensitivity analyses only), the UK Office of Population Censuses and Surveys classification (OPCS-4) for procedures performed in secondary care settings, and a combination of Read, SNOMED, and local EMIS codes for diagnoses recorded in primary care records (see supplementary table S1). 16 Supplementary texts S1, S2, and S3 describe our approach to the generation of the diagnostic code list as well as considerations and sensitivity analyses into the validity of diagnoses recorded in UK electronic health records.
We selected covariates to represent a range of known cardiovascular risk factors. For clinical data, including systolic and diastolic blood pressure, smoking status, cholesterol (total:high density lipoprotein ratio), and body mass index (BMI), we abstracted data from primary care records as the most recent measurement within two years before the incident CVD diagnosis. BMI was categorised as underweight (<18.5), normal (18.5-24.9), overweight (25-29.9), and obesity (≥30). Information on the prevalence of chronic kidney disease, dyslipidaemia, hypertension, and type 2 diabetes was obtained as the percentage of patients with a diagnosis recorded in their primary care or secondary care record at any time up to and including the date of a first CVD diagnosis. Patients’ socioeconomic status was described using the index of multiple deprivation 2015, 17 a composite measure of seven dimensions (income, employment, education, health, crime, housing, living environment) and provided by CPRD. Measures of deprivation are calculated at small area level, covering an average population of 1500 people, and are presented in fifths, with the first 20% and last 20% representing the least and most deprived areas, respectively. We extracted information on ethnicity from both primary and secondary care records, and we used secondary care data when records differed. Ethnicity was grouped into four categories: African/Caribbean, Asian, white, and mixed/other. Finally, we extracted information on cardiovascular treatments (ie, aspirin and other antiplatelets, alpha adrenoceptor antagonists, aldosterone antagonists/mineralocorticoid receptor antagonists, angiotensin converting enzyme inhibitors, angiotensin II receptor antagonists, beta blockers, calcium channel blockers, diuretics, nitrates, oral anticoagulants, and statins) as the number of patients with at least two prescriptions of each drug class within six months after incident CVD, among patients alive and registered with a general practitioner 30 days after the diagnosis. Supplementary table S2 provides a list of substances included in each drug class. Prescriptions were extracted from primary care records up to 31 December 2019.
Statistical analyses
Categorical data for patient characteristics are presented as frequencies (percentages), and continuous data are presented as means and standard deviations (SDs) for symmetrically distributed data or medians and interquartile ranges (IQRs) for non-symmetrically distributed data, over the whole CVD cohort and stratified by age, sex, socioeconomic status, region, and calendar year of diagnosis. For variables with missing entries, we present numbers and percentages of records with missing data. For categorical variables, frequencies refer to complete cases.
Incidence rates of CVD were calculated by dividing the number of incident diagnoses by the number of patient years in the cohort. Category specific rates were computed separately for subgroups of age, sex, socioeconomic status, region, and calendar year of diagnosis. Age calculations were updated for each calendar year. To ensure calculations referred to incident diagnoses, we excluded individuals, from both the numerator and the denominator populations, with a disease of interest diagnosed before the study start date (1 January 2000), or within the first 12 months of registration with their general practice. Time at risk started at the latest of the patient’s registration date plus 12 months, 30 June of their birth year, or study start date; and stopped at the earliest of death, transfer out of practice, last collection date of the practice, incidence of the disease of interest, or linkage end date (30 June 2019). Disease incidence was standardised for age and sex 18 using the 2013 European standard population 19 in five year age bands up to age 90 years.
Negative binomial regression models were used to calculate overall and category specific incidence rate ratios and corresponding 95% confidence intervals (CIs). 20 Models were adjusted for calendar year of diagnosis, age (categorised into five years age bands), sex, socioeconomic status, and region. We chose negative binomial models over Poisson models to account for potential overdispersion in the data. Sensitivity analyses comparing Poisson and negative binomial models showed similar results.
Study findings are reported according to the RECORD (reporting of studies conducted using observational routinely collected health data) recommendations. 21 We performed statistical analyses in R, version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
No patients or members of the public were directly involved in this study owing to constraints on funding and time.
A total of 22 009 375 individuals contributed data between 1 January 2000 and 30 June 2019, with 146 929 629 patient years of follow-up. Among those we identified 2 906 770 new CVD diagnoses, affecting 1 650 052 patients. Mean age at first CVD diagnosis was 70.5 (SD 15.0) years, 47.6% (n=784 904) of patients were women, and 11.6% (n=191 421), 18.0% (n=296 554), 49.7% (n=820 892), and 14.2% (n=233 833) of patients had a history of chronic kidney disease, dyslipidaemia, hypertension, and type 2 diabetes, respectively, at the time of their first CVD diagnosis ( table 1 ).
Characteristics of patients with a first diagnosis of CVD, 2000-19. Values are number (percentage) unless stated otherwise
- View inline
During 2017-19, the most common CVDs were atrial fibrillation or flutter (age-sex standardised incidence 478 per 100 000 person years), heart failure (367 per 100 000 person years), and chronic ischaemic heart disease (351 per 100 000 person years), followed by acute coronary syndrome (190 per 100 000 person years), venous thromboembolism (183 per 100 000 person years), and stroke (181 per 100 000 patient years) ( fig 1 ).

Incidence of a first diagnosis of cardiovascular disease per 100 000 person years, 2000-19. Incidence rates are age-sex standardised to the 2013 European standard population. Any cardiovascular disease refers to the primary incidence of cardiovascular disease across the10 conditions investigated (ie, number of patients with a first diagnosis of cardiovascular disease). See supplementary table S4 for crude incidence rates by age and sex groups. IRR=incidence rate ratio
- Download figure
- Open in new tab
- Download powerpoint
Temporal trends
The primary incidence of CVDs (ie, the number of patients with CVD) decreased by 20% during 2000-19 (age-sex standardised incidence rate ratio 2017-19 v 2000-02: 0.80 (95% CI 0.73 to 0.88)). However, the total incidence of CVD (ie, the total number of new CVD diagnoses) remained relatively stable owing to an increasing number of subsequent diagnoses among patients already affected by a first CVD (incidence rate ratio 2017-19 v 2000-02: 1.00 (0.91 to 1.10)).
The observed decline in CVD incidence was largely due to declining rates of atherosclerotic diseases, in particular acute coronary syndrome, chronic ischaemic heart disease, and stroke, which decreased by about 30% during 2000-19. The incidence of peripheral artery disease also declined, although more modestly (incidence rate ratio 2017-19 v 2000-02: 0.89 (0.80 to 0.98)) ( fig 1 ).
The incidence of non-atherosclerotic heart diseases increased at varying rates, with incidence of aortic stenosis and heart block more than doubling over the study period (2017-19 v 2000-02: 2.42 (2.13 to 2.74) and 2.22 (1.99 to 2.46), respectively) ( fig 1 ). These increasing rates of non-atherosclerotic heart diseases balanced the reductions in ischaemic diseases so that the overall incidence of CVD across the 10 conditions appeared to reach a plateau and to remain relatively stable from 2007-08 (incidence rate ratio 2017-19 v 2005-07: 1.00 (0.91 to 1.10)) ( fig 2 ).

Age standardised incidence of cardiovascular disease by sex, 2000-19. Any cardiovascular disease refers to the primary incidence of cardiovascular disease across the 10 conditions investigated (ie, number of patients with a first diagnosis of cardiovascular disease). IRR=incidence rate ratio
Age stratified analyses further showed that the observed decrease in incidence of chronic ischaemic heart disease, acute coronary syndrome, and stroke was largely due to a reduced incidence in those aged >60 years, whereas incidence rates in those aged <60 years remained relatively stable ( fig 3 and fig 4 ).

Sex standardised incidence of cardiovascular disease in all age groups. Any cardiovascular disease refers to the primary incidence of cardiovascular disease across the 10 conditions investigated (ie, number of patients with a first diagnosis of cardiovascular disease)

Sex standardised incidence of cardiovascular diseases by age subgroups <69 years. Any cardiovascular disease refers to the primary incidence of cardiovascular disease across the 10 conditions investigated (ie, number of patients with a first diagnosis of cardiovascular disease)
Age at diagnosis
CVD incidence was largely concentrated towards the end of the life span, with a median age at diagnosis generally between 65 and 80 years. Only venous thromboembolism was commonly diagnosed before age 45 years ( fig 5 ). Over the study period, age at first CVD diagnosis declined for several conditions, including stroke (on average diagnosed 1.9 years earlier in 2019 than in 2000), heart block (1.3 years earlier in 2019 than in 2000), and peripheral artery disease (1 year earlier in 2019 than in 2000) (see supplementary figure S1). Adults with a diagnosis before age 60 years were more likely to be from lower socioeconomic groups and to have a higher prevalence of several risk factors, including obesity, smoking, and high cholesterol levels (see supplementary table S3).

Incidence rates of cardiovascular diseases calculated by one year age bands and divided into a colour gradient of 20 quantiles to reflect incidence density by age. IQR=interquartile range
Incidence by sex
Age adjusted incidence of all CVDs combined was higher in men (incidence rate ratio for women v men: 1.46 (1.41 to 1.51)), with the notable exception of venous thromboembolism, which was similar between men and women. The incidence of aortic aneurysms was higher in men (3.49 (3.33 to 3.65)) ( fig 2 ). The crude incidence of CVD, however, was similar between men and women (1069 per 100 000 patient years and 1176 per 100 000 patient years, respectively), owing to the higher number of women in older age groups. Temporal trends in disease incidence were generally similar between men and women ( fig 2 ).
Incidence by socioeconomic status
The most deprived socioeconomic groups had a higher incidence of any CVDs (incidence rate ratio most deprived v least deprived: 1.37 (1.30 to 1.44)) ( fig 6 ). A socioeconomic gradient was observed across almost every condition investigated. That gradient did not decrease over time, and it was most noticeable for peripheral artery disease (incidence rate ratio most deprived v least deprived: 1.98 (1.87 to 2.09)), acute coronary syndrome (1.55 (1.54 to 1.57)), and heart failure (1.50 (1.41 to 1.59)). For aortic aneurysms, atrial fibrillation, heart failure, and aortic stenosis, socioeconomic inequalities in disease incidence appeared to increase over time.

Age-sex standardised incidence rates of cardiovascular diseases by socioeconomic status (index of multiple deprivation 2015). Any cardiovascular disease refers to the primary incidence of cardiovascular disease across the 10 conditions investigated (ie, number of patients with a first diagnosis of cardiovascular disease). Yearly incidence estimates were smoothed using loess (locally estimated scatterplot smoothing) regression lines
Regional differences
Higher incidence rates were seen in northern regions (north west, north east, Yorkshire and the Humber) of England for all 10 conditions investigated, even after adjusting for socioeconomic status. Aortic aneurysms and aortic stenosis had the strongest regional gradients, with incidence rates about 30% higher in northern regions compared with London. Geographical variations remained modest, however, and did not appear to change considerably over time (see supplementary figure S2).
Sensitivity analyses
In sensitivity analyses that used broader disease definitions, that included diagnoses recorded on death certificates, that relied on longer lookback periods for exclusion of potentially prevalent diagnoses, or that were restricted to diagnoses recorded during hospital admissions, temporal trends in disease incidence appeared similar (see supplementary figures S3-S6).
Secondary prevention treatments
The proportion of patients using statins and antihypertensive drugs after a first CVD diagnosis increased over time, whereas the use of non-dihydropyridines calcium channel blockers, nitrates, and diuretics decreased over time. Non-vitamin K antagonist oral anticoagulants increasingly replaced vitamin K anticoagulants (see supplementary figure S7).
The findings of this study suggest that important changes occurred in the distribution of CVDs during 2000-19 and that several areas are of concern. The incidence of non-atherosclerotic heart diseases was shown to increase, the decline in atherosclerotic disease in younger people was stalling, and socioeconomic inequalities had a substantial association across almost every CVD investigated.
Implications for clinical practice and policy
Although no causal inference can be made from our data, the decline in rates of ischaemic diseases coincided with reductions in the prevalence of risk factors such as smoking, hypertension, and raised cholesterol levels in the general population over the same period, 22 and this finding suggests that efforts in the primary and secondary prevention of atherosclerotic diseases have been successful. The decline in stroke was not as noticeable as that for coronary heart disease, which may reflect the rising incidence of atrial fibrillation. The variation in trends for peripheral artery disease could be due to differences in risk factors (eg, a stronger association with diabetes), the multifaceted presentations and causes, and the introduction of systematic leg examinations for people with diabetes. 23 24
All the non-atherosclerotic diseases, however, appeared to increase during 2000-19. For some conditions, such as heart failure, the observed increase remained modest, whereas for others, such as aortic stenosis and heart block, incidence rates doubled. All analyses in this study were standardised for age and sex, to illustrate changes in disease incidence independently of changes in population demographics. Whether these trends solely reflect increased awareness, access to diagnostic tests, or even screening (eg, for abdominal aortic aneurysm 25 ) and coding practices, is uncertain. Reductions in premature death from coronary heart disease may have contributed to the emergence of these other non-atherosclerotic CVDs. Regardless, the identification of increasing numbers of people with these problems has important implications for health services, especially the provision of more surgical and transcatheter valve replacement, pacemaker implantation, and catheter ablation for atrial fibrillation. Importantly, these findings highlight the fact that for many cardiovascular conditions such as heart block, aortic aneurysms, and non-rheumatic valvular diseases, current medical practice remains essentially focused on the management of symptoms and secondary prevention and that more research into underlying causes and possible primary prevention strategies is needed. 26 27
These varying trends also mean that the contribution of individual CVDs towards the overall burden has changed. For example, atrial fibrillation or flutter are now the most common CVDs in the UK. Atrial fibrillation is also a cause (and consequence) of heart failure, and these two increasingly common problems may amplify the incidence of each other. Venous thromboembolism and heart block also appeared as important contributors to overall CVD burden, with incidence rates similar to those of stroke and acute coronary syndrome, yet both receive less attention in terms of prevention efforts.
The stalling decline in the rate of coronary heart disease in younger age groups is of concern, has also been observed in several other high income countries, and may reflect rising rates of physical inactivity, obesity, and type 2 diabetes in young adults. 4 6 28 The stalled decline suggests prevention approaches may need to be expanded beyond antismoking legislation, blood pressure control, and lipid lowering interventions to include the promotion of physical activity, weight control, and use of new treatments shown to reduce cardiovascular risk in people with type 2 diabetes. 29 Although CVD incidence is generally low in people aged <60 years, identifying those at high risk of developing CVD at a young age and intervening before problems occur could reduce premature morbidity and mortality and have important economic implications.
Our study further found that socioeconomic inequalities may contribute to CVD burden, and that this association is not restricted to selected conditions but is visible across most CVDs. The reasons behind the observed increase in risk in relation to socioeconomic inequalities are likely to be multifactorial and to include environmental, occupational, psychosocial, and behavioural risk factors, including established cardiovascular risk factors such as smoking, obesity, nutrition, air pollution, substance misuse, and access to care. 30 How these findings apply to different countries is likely to be influenced by socioeconomic structures and healthcare systems, although health inequalities have been reported in numerous countries. 30 One important factor in the present study is that access to care is free at the point of care in the UK, 31 and yet socioeconomic inequalities persist despite universal health coverage and they did not appear to improve over time. Independently of the specificities of individual countries, our findings highlight the importance of measuring and considering health inequalities and suggest that dealing with the social determinants of health—the conditions under which people are born, live, work, and age—could potentially bring substantial health improvements across a broad range of chronic conditions.
Finally, our results reflect disease incidence based on diagnostic criteria, screening practices, availability, and accuracy of diagnostic tests in place at a particular time and therefore must be interpreted within this context. 32 Several of the health conditions investigated are likely to being sought and detected with increased intensity over the study period. For example, during the study period the definition of myocardial infarction was revised several times, 33 34 35 and high sensitivity troponins were progressively introduced in the UK from 2010. These more sensitive markers of cardiac injury are thought to have increased the detection rates for less severe disease. 36 37 Similarly, increased availability of computed tomography may have increased detection rates for stroke. 38 These changes could have masked an even greater decline in these conditions than observed in the present study. Conversely, increased use of other biochemical tests (such as natriuretic peptides) and more sensitive imaging techniques might have increased the detection of other conditions. 39 40 41 The implementation of a screening programme for aortic aneurysm and incentive programmes aimed at improving coding practices, including the documentation of CVD, associated risk factors and comorbidities, and treatment of these, are also likely to have contributed to the observed trends. 25 42 43 As a result, the difference in incidence estimates and prevalence of comorbidities over time may not reflect solely changes in the true incidence but also differences in ascertainment of people with CVD. 44 Nonetheless, long term trends in large and unconstrained populations offer valuable insights for healthcare resource planning and for the design of more targeted prevention strategies that could otherwise not be answered by using smaller cohorts, cross sectional surveys, or clinical trials; and precisely because they are based on routinely reported diagnoses they are more likely to capture the burden of disease as experienced by doctors and health services.
Strengths and limitations of this study
A key strength of this study is its statistical power, with >140 million person years of data. The large size of the cohort allowed us to perform incidence calculations for a broad spectrum of conditions, and to examine the influence of age, sex, and socioeconomic status as well as trends over 20 years. One important limitation of our study was the modest ethnic diversity in our cohort and the lack of information on ethnicity for the denominator population, which precluded us from stratifying incidence estimates by ethnic group. Our analyses were also limited by the unavailability or considerable missingness of additional variables potentially relevant to the development of CVD, such as smoking, body mass index, imaging data, women specific cardiovascular risk factors (eg, pregnancy associated hypertension and gestational diabetes), and blood biomarkers. Further research may also need to consider an even wider spectrum of CVDs, including individual types of valve disease, pregnancy related conditions, and infection related heart diseases. Research using databases with electronic health records is also reliant on the accuracy of clinical coding input by doctors in primary care as part of a consultation, or in secondary care as part of a hospital admission. We therefore assessed the validity of diagnoses in UK electronic health records data and considered it to be appropriate in accordance with the >200 independent validation studies reporting an average positive predictive value of about 90% for recorded diagnoses. 45 Observed age distributions were also consistent with previous studies and added to the validity of our approach. Nevertheless, our results must be interpreted within the context and limitations of routinely collected data from health records, diagnostic criteria, screening practices, the availability and accuracy of diagnostic tests in place at that time, and the possibility that some level of miscoding is present or that some bias could have been introduced by restricting the cohort to those patients with at least 12 months of continuous data.
Conclusions
Efforts to challenge the notion of the inevitability of vascular events with ageing, and evidence based recommendations for coronary heart disease prevention, have been successful and can serve as a model for other non-communicable diseases. Our findings show that it is time to expand efforts to improve the prevention of CVDs. Broadening research and implementation efforts in both primary and secondary prevention to non-atherosclerotic diseases, tackling socioeconomic inequalities, and introducing better risk prediction and management among younger people appear to be important opportunities to tackle CVDs.
What is already known on this topic
Recent data show that despite decades of declining rates of cardiovascular mortality, the burden from cardiovascular disease (CVD) appears to have stalled in several high income countries
What this study adds
This observational study of a representative sample of 22 million people from the UK during 2000-19 found reductions in CVD incidence to have been largely restricted to ischaemic heart disease and stroke, and were paralleled by a rising number of diagnoses of cardiac arrhythmias, valve disease, and thromboembolic events
Venous thromboembolism and heart block were important contributors to the overall burden of CVDs, with incidence rates similar to stroke and acute coronary syndromes
Improvements in rates of coronary heart disease almost exclusively appeared to benefit those aged >60 years, and the CVD burden in younger age groups appeared not to improve
Ethics statements
Ethical approval.
This study was approved by the Clinical Practice Research Datalink Independent Scientific Advisory Committee.
Data availability statement
Access to Clinical Practice Research Datalink (CPRD) data is subject to a license agreement and protocol approval process that is overseen by CPRD’s research data governance process. A guide to access is provided on the CPRD website ( https://www.cprd.com/data-access ) To facilitate the subsequent use and replication of the findings from this study, aggregated data tables are provided with number of events and person years at risk by individual condition and by calendar year, age (by five year age band), sex, socioeconomic status, and region (masking field with fewer than five events, as per CPRD data security and privacy regulations) on our GitHub repository ( https://github.com/nathalieconrad/CVD_incidence ).
Acknowledgments
We thank Hilary Shepherd, Sonia Coton, and Eleanor L Axson from the Clinical Practice Research Datalink for their support and expertise in preparing the dataset underlying these analyses.
Contributors: NC and JJVM conceived and designed the study. NC, JJVM, GM, and GV designed the statistical analysis plan and NC performed the statistical analysis. All authors contributed to interpreting the results, drafting the manuscript, and the revisions. NC, GM, and GV had permission to access the raw data and NC and GM verified the raw data. All authors gave final approval of the version to be published and accept responsibility to submit the manuscript for publication. NC and JJVM accept full responsibility for the conduct of the study, had access to aggregated data, and controlled the decision to publish. They are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by a personal fellowship from the Research Foundation Flanders (grant No 12ZU922N), a research grant from the European Society of Cardiology (grant No App000037070), and the British Heart Foundation Centre of Research Excellence (grant No RE/18/6/34217). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: NC is funded by a personal fellowship from the Research Foundation Flanders and a research grant from the European Society of Cardiology. JMF, PSJ, JGC, NS, and JJVM are supported by British Heart Foundation Centre of Research Excellence. PSJ and JJVM are further supported by the Vera Melrose Heart Failure Research Fund. JJVM has received funding to his institution from Amgen and Cytokinetics for his participation in the steering sommittee for the ATOMIC-HF, COSMIC-HF, and GALACTIC-HF trials and meetings and other activities related to these trials; has received payments through Glasgow University from work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardurion, Dal-Cor, GlaxoSmithKline, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos; and has received personal lecture fees from the Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Alkem Metabolics, Eris Lifesciences, Lupin, ProAdWise Communications, Servier Director, and Global Clinical Trial Partners. NS declares consulting fees or speaker honorariums, or both, from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Hanmi Pharmaceuticals, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi; and grant support paid to his university from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics. KK has acted as a consultant or speaker or received grants for investigator initiated studies for Astra Zeneca, Bayer, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Oramed Pharmaceuticals, Roche, and Applied Therapeutics. KK is supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC). CL is funded by an NIHR Advanced Research Fellowship (NIHR-300111) and supported by the Leicester BRC. PSJ has received speaker fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals, and Intas Pharmaceuticals; has received advisory board fees from AstraZeneca, Boehringer Ingelheim, and Novartis; has received research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices; his employer, the University of Glasgow, has been remunerated for clinical trial work from AstraZeneca, Bayer, Novartis, and Novo Nordisk; and is the Director of Global Clinical Trial Partners. HS is supported by the China Scholarship Council. Other authors report no support from any organisation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Transparency: The lead author (NC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Results from this study will be shared with patient associations and foundations dedicated to preventing cardiovascular diseases, such as the European Heart Network and the American Heart Association. To reach the public, findings will also be press released alongside publication of this manuscript. Social media (eg, X) will be used to draw attention to the work and stimulate debate about its findings. Finally, the underlying developed algorithms will be freely available for academic use at https://github.com/nathalieconrad/CVD_incidence .
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/ .
- ↵ Institute for Health Metrics and Evaluation. Gobal Burden of Diseases Viz Hub. 2023. https://vizhub.healthdata.org/gbd-compare/
- Ananth CV ,
- Rutherford C ,
- Rosenfeld EB ,
- O’Flaherty M ,
- Allender S ,
- Scarborough P ,
- Andersson C ,
- Abdalla SM ,
- Mensah GA ,
- Johnson CO ,
- GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group
- Almarzooq ZI ,
- American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee
- Townsend N ,
- Atlas Writing Group, European Society of Cardiology
- Herrett E ,
- Gallagher AM ,
- Bhaskaran K ,
- ↵ Wolf A, Dedman D, Campbell J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. International Journal of Epidemiology. 2019 Mar 11 [cited 2019 Mar 22]; https://academic.oup.com/ije/advance-article/doi/10.1093/ije/dyz034/5374844
- Verbeke G ,
- Molenberghs G ,
- Ferguson LD ,
- ↵ Medicines and Healthcare products Regulatory Agency. What coding systems are used in CPRD data? 2023. https://www.cprd.com/defining-your-study-population
- ↵ Department for Communities and Local Government (DCLG). The English Index of Multiple Deprivation 2015: Guidance. 2015; pp1-7. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015
- ↵ Kirkwood B, Sterne J. Essential medical statistics. 2010.
- ↵ Eurostat’s task force. Revision of the European Standard Population Report. 2013.
- Benchimol EI ,
- Guttmann A ,
- RECORD Working Committee
- ↵ NHS Digital. Health Survey for England, 2021 part 1. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2021
- Criqui MH ,
- ↵ Health and Social Care Information Centre. Quality and Outcomes Framework - Indicators 2011-12. 2011. https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/quality-and-outcomes-framework-2011-12
- Jacomelli J ,
- Summers L ,
- Stevenson A ,
- Earnshaw JJ
- Vahanian A ,
- Beyersdorf F ,
- ESC/EACTS Scientific Document Group
- Glikson M ,
- Nielsen JC ,
- Kronborg MB ,
- ESC Scientific Document Group
- Stevenson C ,
- Peeters A ,
- Federici M ,
- Schultz WM ,
- Verbakel JY ,
- Thygesen K ,
- Alpert JS ,
- Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction
- Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction
- Sandoval Y ,
- High-STEACS investigators
- Camargo ECS ,
- Singhal AB ,
- Wiener RS ,
- Schwartz LM ,
- Sappler N ,
- Roalfe AK ,
- Lay-Flurrie SL ,
- Ordóñez-Mena JM ,
- Herbert A ,
- Wijlaars L ,
- Zylbersztejn A ,
- Cromwell D ,
- ↵ NHS Digital. Quality and Outcomes Framework (QOF), 2019-20. Indicator definitions. 2020. https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2019-20
- Pronovost PJ
- Thomas SL ,
- Schoonen WM ,
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Popul Health Manag

Implementation of Diabetes Prevention in Health Care Organizations: Best Practice Recommendations
Janet williams.
1 Improving Health Outcomes, American Medical Association, Chicago, Illinois, USA.
Neha Sachdev
2 David Geffen School of Medicine, UCLA and VA, Los Angeles, California, USA.
Kate Kirley
3 David Geffen School of Medicine, UCLA, Los Angeles, California, USA.
Tannaz Moin
O. kenrik duru, kimberly d. brunisholz.
4 Healthcare Delivery Institute, Intermountain Healthcare, Murray, Utah, USA.
Elizabeth Joy
5 Wellness and Nutrition, Intermountain Healthcare, Salt Lake City, Utah, USA.
Gina C. Aquino
6 Henry Ford Macomb Hospital, Clinton Township, Michigan, USA.
Ameldia R. Brown
7 Faith and Community Health, Henry Ford Health System, Clinton Township, Michigan, USA.
Christopher O'Connell
8 Ambulatory Division, Henry Ford Macomb Hospital, Clinton Township, Michigan, USA.
9 Department of Family Medicine and Preventive Medicine, Loma Linda University Health, Redlands, California, USA.
Holly Craig-Buckholtz
10 Diabetes and Outpatient Wound Care Services, Loma Linda University Medical Center, Loma Linda, California, USA.
Patricia W. Witherspoon
11 University of South Carolina Family Medicine Residency, Columbia, South Carolina, USA.
Cindy Bruett
12 Diabetes Prevention Program, Community Health & Well-Being, Trinity Health, Livonia, Michigan, USA.
Approximately 1 in 3 American adults has prediabetes, a condition characterized by blood glucose levels that are above normal, not in the type 2 diabetes ranges, and that increases the risk of developing type 2 diabetes. Evidence-based treatments can be used to prevent or delay type 2 diabetes in adults with prediabetes. The American Medical Association (AMA) has collaborated with health care organizations across the country to build sustainable diabetes prevention strategies. In 2017, the AMA formed the Diabetes Prevention Best Practices Workgroup (DPBP) with representatives from 6 health care organizations actively implementing diabetes prevention. Each organization had a unique strategy, but all included the National Diabetes Prevention Program lifestyle change program as a core evidence-based intervention. DPBP established the goal of disseminating best practices to guide other health care organizations in implementing diabetes prevention and identifying and managing patients with prediabetes. Workgroup members recognized similarities in some of their basic steps and considerations and synthesized their practices to develop best practice recommendations for 3 strategy maturity phases. Recommendations for each maturity phase are classified into 6 categories: (1) organizational support; (2) workforce and funding; (3) promotion and dissemination; (4) clinical integration and support; (5) evaluation and outcomes; (6) and program. As the burden of chronic disease grows, prevention must be prioritized and integrated into health care. These maturity phases and best practice recommendations can be used by any health care organization committed to diabetes prevention. Further research is suggested to assess the impact and adoption of diabetes prevention best practices.
Introduction
Diabetes mellitus is one of the nation's most prevalent chronic diseases, currently affecting more than 34 million Americans 1 and leading to increasing economic and social burdens. At the same time, approximately 1 in 3 American adults has prediabetes, 1 a condition that is characterized by blood glucose levels that are above normal but not high enough to be diagnosed as type 2 diabetes. Individuals with prediabetes are at increased risk of progression to type 2 diabetes, yet more than 84% are unaware that they have this condition. 1
Type 2 diabetes can potentially be prevented or delayed in adults with prediabetes through evidence-based treatments. The landmark US Diabetes Prevention Program (DPP) study demonstrated that intensive lifestyle intervention was effective at reducing the incidence of type 2 diabetes. At approximately 3 years follow-up, the incidence of diabetes was 58% lower among those who received intensive lifestyle intervention compared to those who received placebo treatment. 2 Since this study, lifestyle interventions for the prevention of type 2 diabetes have been successfully translated and delivered in a variety of settings and modalities. 3–8 Based on evidence from the DPP study and subsequent translational studies, Congress authorized the Centers for Disease Control and Prevention (CDC) to establish the National Diabetes Prevention Program (National DPP) lifestyle change program in 2010 to address the rising incidence of type 2 diabetes. 9 The program is a structured and group-based intensive behavioral change program designed to help adults with overweight or obesity who are at risk for type 2 diabetes to prevent or delay its onset. During the first 4 years (February 2012 through January 2016) of program implementation, 14,747 adults were enrolled and attended a median of 14 sessions over an average of 172 days. 10 As of April 2019, more than 324,000 individuals have participated in the National DPP lifestyle change program offered by more than 3000 partner organizations. 11 Currently, there are more than 1800 in-person, online, and/or distance learning lifestyle change programs offered by health care organizations, community-based organizations, and digital health providers registered with the National Diabetes Recognition Program. 12
American Medical Association Diabetes Prevention Workgroup
The American Medical Association (AMA) established the prevention of type 2 diabetes as a long-term strategic goal in 2012 and has collaborated with health care organizations across the country to build sustainable diabetes prevention strategies. In 2017, the AMA formed the Diabetes Prevention Best Practices Workgroup (DPBP) with representatives from 6 health care organizations actively implementing diabetes prevention: Henry Ford Health System; Intermountain Healthcare; Loma Linda University Health; University of South Carolina School of Medicine-Columbia Campus; Trinity Health; and University of California, Los Angeles, (UCLA) Health.* DPBP established the goal of disseminating best practices to guide other health care organizations in implementing diabetes prevention strategies that identify and manage patients with prediabetes. Each organization had a unique strategy, but all included the National DPP lifestyle change program as a core evidence-based intervention. Organizations delivering the National DPP lifestyle change program must meet national standards to ensure fidelity and quality, including the use of certified coaches and curriculum and close tracking of participant physical activity minutes and weight. Recognition as a DPP lifestyle change program by the CDC requires achievement of specific performance metrics. At the time of manuscript submission, these metrics included an average weight loss of 5% and minimum engagement standards among participants. The DPBP organizations are all fully recognized by the CDC, indicating that these standards and performance metrics are being successfully met and sustained over time.
Although there were varying models of implementation at each organization, it became clear that some basic steps and considerations were common among these diverse systems. With this awareness, the DPBP synthesized the best practice implementation recommendations that will be presented in the following sections for other health care organizations. This process spanned 2 years and included in-person meetings, conference calls, and semi-structured interviews with teams from each DPBP member organization. These teams consisted of individuals with varied professional qualifications, including endocrinology, primary care medicine, sports medicine, physical therapy, nutrition, osteopathic medicine, cardiovascular health, medical administration, research, community health, and nursing. Common activities conducted at DPBP institutions formed the foundation for the recommendations. The diverse geographic locations and patient populations served by DPBP members and their multidisciplinary professional backgrounds support the broad applicability of the recommendations. Each DPBP member collected metrics specific to her/his organization's diabetes prevention strategy and maturity phase.
Implementation Maturity Phases
Implementing diabetes prevention at a system level usually involves several stages over time. DPBP grouped implementation-structured activities into 3 strategy maturity phases: (1) Getting Started, (2) Planning for Growth, and (3) Advancing Innovation ( Tables 1–3 ). Although the three phases interconnect, each has distinct and specific characteristics that can propel the organization into the next phase, and activities may repeat themselves in each phase.
Best Practice Recommendations for Getting Started Maturity Phase
| Key focus areas are to obtain organizational support, secure workforce and funding resources, and begin offering a National Diabetes Prevention Program (National DPP) lifestyle change program. |
| • Align diabetes prevention strategy (strategy) goals and expected outcomes with the organization's strategic plan and mission. • Use available data, such as Community Health Needs Assessment results and community and patient diabetes data, to illustrate the return on investment and anticipated improvement in health outcomes from the strategy. • Form an interdisciplinary leadership/advisory group to guide strategy and increase visibility. |
| • Develop a budget and estimate the short-term and long-term costs of the strategy; conduct networking to secure the necessary resources. • Identify the available workforce and build an interdisciplinary project team to execute strategy activities. • Identify existing short-term and long-term funding sources, such as community benefit dollars, grants, and insurer benefits. |
| • Develop a communications and outreach plan along with key messages about strategy for the entire organization and community. • Share success stories and outcomes from strategy implementation early, often, and in many venues. • Use available existing materials and educational resources, such as posters, flyers, and handouts. |
| • Identify and recruit clinical champions, including providers and other care team members. • Adapt processes to increase prediabetes identification and management, and facilitate referrals to a National DPP lifestyle change program. • Determine the process to integrate clinical decision-support tools and other health information technology, and begin to engage key stakeholders. |
| • Define the goals of the strategy and the criteria for success. • Develop quantitative and qualitative assessments of strategy progress that include informal feedback from key stakeholders, such as patients, physicians, and lifestyle change program coaches. • Determine the metrics to assess the current state of identification and management of patients with prediabetes, and use the results to guide activities. |
| • Obtain guidance and technical assistance as needed for adhering to the CDC standards and achieving recognition; establish processes for collecting and submitting required CDC reporting metrics. • Monitor lifestyle change program outcomes and identify areas of success and areas for improvement. • Incorporate structured onboarding and skills development for coordinators and coaches. • Develop quality assurance methods, such as structured performance feedback for coaches, for delivery of a lifestyle change program. |
Reproduced with permission from the American Medical Association. This Table may be photocopied noncommercially by physicians, educators, and other health care professionals to use for educational purposes. Please address all other permissions to the AMA. Notwithstanding publication in Population Health Management, AMA retains all of its copyright and other intellectual property rights in the foregoing.
© 2020 American Medical Association. All rights reserved.
AMA, American Medical Association; CDC, Centers for Disease Control and Prevention; DPP, Diabetes Prevention Program.
Best Practice Recommendations for Planning for Growth Maturity Phase
| Key focus areas are to increase and systemize clinical engagement, increase overall awareness of strategy, and expand program and prediabetes management. |
| • Continue to cultivate leadership support for the diabetes prevention strategy (strategy) through regular updates and results. • Query stakeholders to determine ways to increase support for the strategy, and adjust activities based on feedback. • Pilot a quality improvement initiative or incentive for diabetes prevention. |
| • Identify additional business units and departments to engage in the strategy, such as clinical operations. • Estimate resources needed to increase reach and spread of the strategy; develop a cost-effective, feasible plan for expansion. • Secure ongoing funding of the strategy, such as community health and benefits budgets. |
| • Use marketing and communications to increase overall awareness of the strategy both inside and outside the organization. • Create or identify forums to share strategy benefits and outcomes. • Highlight aggregate outcomes from program participation, such as reduction in weight and increase in physical activity. |
| • Leverage existing champions and recruit additional champions to expand awareness and clinical engagement in the strategy. • Provide education to all clinical care teams on the identification and management of patients with prediabetes; consider offering training on shared decision-making and counseling techniques. • Integrate and optimize clinical decision-support tools and health information tools for prediabetes, such as referral platforms. • Improve and standardize referral and bidirectional feedback processes between clinical care teams and lifestyle change program providers. |
| • Begin to collect and monitor clinical metrics, such as the number of patients with prediabetes who receive a referral to a National Diabetes Prevention Program (National DPP) lifestyle change program. • Expand the initial qualitative and quantitative evaluation methods. • Continue to monitor the progress and impact of the strategy. |
| • Automate processes for collecting and submitting required metrics for program recognition; continue to regularly monitor the delivery quality and metrics of the lifestyle change program. • Establish an ongoing coach, staff a professional development program, and offer additional skills training, such as motivational interviewing. • Select and certify coaches to become master trainers for the lifestyle change program. • Consider expanding program offerings, such as group physical activity opportunities, based on participant requests and needs. |
AMA, American Medical Association; DPP, Diabetes Prevention Program.
Best Practice Recommendations for Advancing Innovation Maturity Phase
| Key focus areas are to share achievements and ensure the sustainability of strategy and improvements. |
| • Ensure continued visibility and provide regular updates on the diabetes prevention strategy (strategy) to the organization's leadership. • Adopt system-wide goals or incentives for diabetes prevention that align vertically and laterally (eg, leadership goals align with clinical care team goals). |
| • Use an established advisory group, champions, and project team for other prevention initiatives. • Monitor operational costs and maintain the cost-effectiveness of the strategy. • Secure additional funding sources for the strategy, such as reimbursement for the National Diabetes Prevention Program (National DPP) lifestyle change program through insurance coverage or employer benefits. |
| • Continue to highlight success stories that demonstrate the benefit of the strategy to the organization and the larger community. • Externally publish and present learnings and results of the strategy. • Advocate for diabetes prevention locally and nationally through such activities as writing commentaries, white papers, or legislative briefings or responding to open comments for programs and policies. |
| • Provide regular reporting to care teams on metrics related to prediabetes identification and management; address any negative trends, such as decreased program referral rates. • Use the entire care team to identify and manage patients with prediabetes. • Offer multiple evidence-based treatment options for patients with prediabetes. |
| • Track population-level outcomes and additional health outcomes, such as reductions in blood glucose levels or the incidence of diabetes. • Revise existing metrics and evaluation methods as needed. • Solicit ongoing feedback on the strategy from all stakeholders. • Consider data exchange with external sources, such as health plans and state health departments, to improve local and national efforts related to diabetes prevention. |
| • Create a multidirectional communication flow and enable care coordination between the lifestyle change program, clinical care teams, patients, and other service organizations to address participant needs. • Continue to monitor the quality and process the efficiency of the lifestyle change program. • Offer advanced skills training or cross-train coaches to deliver other programs. • Monitor and address coordinator and coach attrition. |
The Getting Started phase ( Table 1 ) is the start-up period during which an organization obtains organizational support and commits to establishing a diabetes prevention strategy that offers treatment options for prediabetes, such as a CDC-recognized lifestyle change program, secures the necessary workforce and funding, and establishes a National DPP lifestyle change program offering. Planning for Growth ( Table 2 ) is the subsequent phase during which an organization advances the strategy by increasing overall awareness, building infrastructure, expanding clinical engagement, offering the National DPP lifestyle change program to additional sites, or further developing the program curricula and coaches to expand program reach and enrollment. The Advancing Innovation phase ( Table 3 ) occurs when diabetes prevention becomes part of routine clinical operations for an organization and the focus is on population management and sustainability. At this point, strategy milestones and processes can be broadly shared and insights from implementation can be applied to other quality improvement initiatives.
As an organization completes each maturity phase, the reach and population effects of a strategy likely will increase; however, benefits of a strategy are seen in all phases as patients with prediabetes receive an evidence-based intervention. Although the maturity phases are sequential, the timing for each phase is variable. Organizations may opt to remain in one phase longer, or some organizations may require less time than others to execute a phase, depending on prior experience with diabetes prevention. For example, an organization that has an established CDC-recognized National DPP lifestyle change program may progress through Getting Started within a few weeks, whereas an organization that is starting a new program may need months to progress in this phase.
DPBP outlined best practice implementation recommendations for each maturity phase, which are presented in Tables 1 – 3 . The recommendations are classified into 6 overarching categories:
Organizational support recommendations encompass implementation activities that assist with obtaining leadership buy-in, demonstrating alignment with organizational mission, and sharing the expected or actual impact and return on investment from implementing diabetes prevention. Workforce and funding recommendations focus on securing and maintaining the resources and team members needed to execute and sustain a diabetes prevention strategy. Interdisciplinary teams are essential and include ambulatory clinical care team members, data analysts, researchers, clinical operations personnel, health coaches, and diabetes educators as potential core team members. Promotion and dissemination recommendations concentrate on raising awareness of a strategy, sharing success stories, and publicizing and/or publishing results within and outside an organization. Evaluation and outcomes recommendations center on measuring the impact and progress of the strategy and supporting the collection of quantitative and qualitative metrics and data. Clinical integration and support recommendations outline activities to increase engagement from clinical care teams and improve the identification, referral numbers, and management of patients with prediabetes. Program recommendations support the activities associated with the launch and expansion of a high-quality National DPP lifestyle change program offering or collaboration with an external community-based National DPP lifestyle change program.
When planning or executing within these 6 overarching categories, certain foundational structural processes and principles apply throughout all implementation phases and activities. DPBP noted that although variability among health care organizations in patient demographics exists, leadership teams must ensure throughout the planning and implementation process that from historically marginalized/minoritized communities are receiving the benefits of the diabetes prevention strategy. It is essential to apply a health equity lens in the development of all diabetes prevention activities and processes. The purpose of an equity lens is to be deliberately inclusive as an organization makes decisions on process and outcomes. This also ensures that patients with prediabetes are identified and managed with culturally competent care throughout all diabetes prevention phases.
Other foundational processes include the optimization of health information and digital health technology to ensure that the diabetes prevention strategy is linked to the continuum of care for each patient. To successfully integrate clinical decision support tools and other health information technology, the identification of key stakeholders within the organization needs to be applied consistently throughout the maturity phases.
Implementation Road Map: Demonstrating Best Practice
The best practice implementation recommendations developed by DPBP can be used by health care organizations as a road map in each maturity phase.
Getting started phase
During the Getting Started phase, obtaining organizational support and establishing the necessary resources for workforce and funding are often the initial requisite steps, and assessing existing resources can be helpful. For example, the Henry Ford Health System team identified an established group of faith-based nurses to deliver the National DPP lifestyle change program. The nurses were already embedded in the community and training them as lifestyle coaches allowed the team to begin offering the program in many locations. Loma Linda University Health team members included faculty and students from the university's School of Public Health as well as fitness center staff who delivered the program, and clinical care case managers who recruited eligible patients.
To help gain initial buy-in across the organization, existing data such as local diabetes prevalence rates can be highlighted. Trinity Health used results from its Community Health Needs Assessment to incorporate funding for National DPP lifestyle change program offerings into its community health and benefits budget.
Stakeholder engagement is critical because diverse groups (in and out of the organization) can synergistically help make the case for implementing and sustaining diabetes prevention services. In the case of UCLA Health, the diabetes prevention team was able to form a partnership with departments that are not traditionally linked to clinical care or clinical operations, such as campus recreation services, occupational health, and human resources. This team diversity helped achieve broad organizational support.
Planning for growth phase
In the Planning for Growth phase, clinical engagement and endorsement, integration of digital health tools, and dissemination of strategy processes and metrics can drive expansion. Engaging clinical champions and educating care teams can raise overall awareness of a diabetes prevention strategy. Thus, partnership with clinical champions increases needed buy-in from frontline clinical providers who may help identify, refer, and encourage patients to participate in the National DPP lifestyle change program offering. Training members of care teams on specific counseling or communication techniques to address prediabetes with patients also can improve the overall identification and management of prediabetes. At UCLA Health, pharmacists engaged in a shared decision-making process with identified patients on their prediabetes treatment options; patients who participated in this process had an increased uptake of the National DPP lifestyle change program and/or metformin. 13
Incorporating digital health tools to support systematic identification and management of prediabetes, including referrals to programs, also can drive further clinical engagement. For example, Loma Linda University Health experienced an uptrend in referrals to the National DPP lifestyle change program when an electronic referral order was made available and providers were educated on the National DPP lifestyle change program as a resource for their patients. The Henry Ford Health System also recognized the potential role technology could play in advancing its strategy and implemented a diabetes prevention module within its electronic health record that included best practice alerts, an electronic referral to its National DPP lifestyle change program, and a prediabetes registry. Processes for National DPP lifestyle change program referrals and bidirectional feedback between program providers and care teams were refined and standardized to maximize efficiency and utility. Collectively, these changes led to a significant increase in the number of clinical referrals and improved patient outcomes.
Another strategy emphasized by DPBP is to increase support from key system stakeholders for diabetes prevention by consistently sharing data and metrics regarding program processes and outcomes. For example, University of South Carolina Family Medicine implemented a quality improvement project with its residents that focused on ensuring all patients eligible for abnormal glucose screening were receiving the necessary laboratory testing and that those with prediabetes were formally diagnosed and counseled on treatment options. The team recognized that emphasizing identification along with program referral was necessary to the success of its strategy and used data to help drive improvement in prediabetes identification and management.
The Planning for Growth phase also presents new opportunities, such as additional skills training for lifestyle coaches, to build capacity and longevity of a National DPP lifestyle change program offering. Trinity Health has trained its lifestyle coaches in motivational interviewing to improve participant engagement and retention, whereas UCLA Health and the Henry Ford Health System have internal master trainers to train new coaches in their organizations.
Programs also may augment and enhance their offerings to meet participant needs. For example, Loma Linda University Health provided participants with free memberships to its fitness center, and lifestyle coaches led group physical activity for participants interested in exercising together after regularly scheduled program sessions.
Advancing innovation phase
In the Advancing Innovation phase, strategy sustainability is a key focus. By this phase, diabetes prevention should be part of routine clinical processes of care, and organizations should be offering a variety of treatment options for prediabetes. For example, Intermountain Healthcare developed a care process model for its entire system that includes the National DPP lifestyle change program, an introductory prediabetes educational session, medical nutrition therapy, and pharmacotherapy as options in managing patients with prediabetes.
The Advancing Innovation phase is also an appropriate time for health care organizations to use promotion and dissemination to broadly share strategy achievements. Complex mixed method evaluation and outcomes tracking can help organizations demonstrate long-term sustainability of a strategy. Intermountain Healthcare developed a method to track the conversion rates of patients with prediabetes to type 2 diabetes to demonstrate the lasting benefit of this work. This sophisticated evaluation builds in opportunities to test and adapt the strategy activities to meet the changing health care landscape.
Many DPBP members have presented or published details of their diabetes prevention strategies at national conferences and in peer-reviewed journals, 13–22 whereas others have disseminated their results in less formal ways. These range from ongoing presentations at internal medical group summits, to huddle discussions, to participation in prevention workgroups such as the DPBP.
As the burden of chronic disease in the United States and worldwide grows, prevention must be prioritized and integrated into health care. The recent public health emergency (PHE) and COVID-19 pandemic have demonstrated the need to prioritize prevention of chronic disease, health equity, and investing in new models of delivery. During the PHE, DPBP members continued to support and engage in diabetes prevention activities, pivoting to offer the National DPP lifestyle change program using virtual platforms to maintain offerings and observed continued clinical and participant engagement. Previous and future publications from DPBP organizations may offer more details about each strategy and results.
More work is needed to explore innovation and advance equity within diabetes prevention. The maturity phases and best practice implementation recommendations outlined herein can be used by any health care organization committed to diabetes prevention to launch and sustain an effective strategy and improve the health of patients and communities. Further research is suggested to assess the impact and adoption of diabetes prevention best practices.
*Diabetes Prevention Best Practices Workgroup Members and Health Care Organizations Represented
Gina C. Aquino, MSN, RN, CHSP, RN, Henry Ford Health System; Ameldia R. Brown, M.Div., BSN, RN, Henry Ford Health System; Christopher O'Connell, DO, CPE, Henry Ford Health System; Elizabeth Joy, MD, MPH, Intermountain Healthcare; Kimberly D. Brunisholz PhD, MST, Intermountain Healthcare; Tannaz Moin, MD, MBA, MSHS, University of California, Los Angeles, Health; O. Kenrik Duru, MD, MSHS, University of California, Los Angeles, Health; Holly Craig-Buckholtz, MBA, BSN, RN, Loma Linda University Health; Brenda Rea MD, DrPH, PT, RD, Loma Linda University Health; Patricia W Witherspoon, MD, FAAFP, University of South Carolina; Cindy Bruett, Trinity Health.
Acknowledgments
The authors would like to acknowledge the following individuals for their contributions to this manuscript: Jaime Dircksen, Vice President, Community Health and Well-Being, Trinity Health; Chuck Carter, MD, FAAFP, Academic Vice Chair, Clinical Professor, Department of Family and Preventive Medicine, and Medical Director, South Carolina Center for Rural and Primary Healthcare, University of South Carolina School of Medicine-Columbia; Kevin Taylor, MD, MS, Medical Director, IHA Towsley Primary Care and Geriatrics; Shannon Haffey, MHSA, Director of Payer and Payment Strategies, Improving Health Outcomes, American Medical Association; Karen Kmetik, PhD, Group Vice President, Health Outcomes, American Medical Association; and Annalynn Skipper, PhD, RD, Author Service Manager, Health and Science, American Medical Association. We also thank Lori O'Keefe for assisting with the writing and editing of this manuscript.
Authors' Contributions
Ms.Williams: manuscript conception and drafting, data collection, analysis and interpretation, critical review and revisions, and final approval of the version to be published. Dr. Sachdev: manuscript conception and drafting, data collection, analysis and interpretation, critical review and revisions, and final approval of the version to be published. Dr. Kirley: manuscript conception and drafting, critical review and revisions, and final approval of the version to be published. Dr. Moin: drafting, critical review and revisions, and final approval of the version to be published. Dr. Duru: drafting, critical review and revisions, and final approval of the version to be published. Ms. Sill: drafting, critical review and revisions, and final approval of the version to be published. Dr. Brunisholz: drafting, critical review and revisions, and final approval of the version to be published. Dr. Joy: drafting, critical review and revisions, and final approval of the version to be published. Ms. Aquino: provided revisions and final approval of the version to be published. Ms. Brown: provided final approval of the version to be published. Dr. O'Connell: provided revisions and final approval of the version to be published. Dr. Rea: provided revisions and final approval of the version to be published. Ms. Craig-Buckholtz: provided revisions and final approval of the version to be published. Dr. Witherspoon: provided revisions and final approval of the version to be published. Ms. Bruett: provided revisions and final approval of the version to be published.
Author Disclosure Statement
The authors declare that there are no conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the American Medical Association.
Funding Information
No funding was received for this article.
COVID-19 infections by race: What's behind the health disparities?
Why are some people more at risk of being affected by coronavirus disease 2019 (covid-19).
Factors in a person's life or community can raise the risk of being affected by COVID-19.
Having other health conditions and barriers to medical care can change the risk of getting COVID-19 or getting very sick from COVID-19. Other factors include where a person lives, the work a person does and beliefs a person has about medical care.
COVID-19 may cause illness in some groups more than others because of how society treats the group.
Together these factors are called social determinants of health.
Discrimination
Unfair and unjust treatment based on race, age, ethnicity, gender or other traits can play a part in poor health. Discrimination affects all aspects of health starting with the world around a person. It also can affect a person's access to healthcare professionals, diagnosis of illness and treatment.
Other medical conditions
The stress of dealing with racial discrimination can take a toll on the body. Diagnosis of heart disease, obesity, diabetes, high blood pressure, and kidney or liver disease is linked to the stress of racial discrimination.
A person with any of these diseases, due to racism or other causes, has a higher risk of severe illness with COVID-19.
Access to healthcare
Members of some racial and ethnic groups are more likely to face barriers to getting healthcare. For example, some people may not have health insurance.
Based on U.S. census data, about 7% of non-Hispanic white adults and adults of Asian descent were uninsured in 2022. The rate was about 11% for Black adults and about 23% for Hispanic adults in that same year.
Where people live can make it hard to avoid getting COVID-19 or to get COVID-19 treatment. People in rural areas may not have access to healthcare. And people in areas with a dense population may find it hard to stay physically apart from others.
Beliefs about medical care
Groups who distrust the healthcare system may be less likely to get a COVID-19 vaccine or get help for COVID-19 or other illnesses.
Type of work
Having an essential job that can't be done remotely can raise the risk of catching the virus that causes COVID-19. The risk also is higher if you have to come in contact with lots of people.
COVID-19 health disparities
In the early years of the COVID-19 pandemic, American Indian and Alaska Native people, non-Hispanic Black people and Hispanic people had higher rates of infection and COVID-19 deaths compared with those of non-Hispanic white people.
Black and Hispanic people in the United States also had higher chances of needing care in the hospital for COVID-19.
Early pandemic data suggested that American Indian and Alaska Native people were four times more likely to need hospital care for COVID-19 than were non-Hispanic white people.
By 2021, the rate of infection and death for non-Hispanic white people had risen and closed the gap between the groups. In April 2024, non-Hispanic white people had the highest rate of death compared with that of other race and ethnicities.
Taken together, the COVID-19 pandemic shows how disease can raise the risk of illness based on factors that can be prevented. The pandemic highlights the need to promote the health and well-being of people with a higher than average risk of disease.
Daniel C. DeSimone, M.D.
- COVID-19: How can I protect myself?
- Sex and COVID-19
- What is health equity? Centers for Disease Control and Prevention. https://www.cdc.gov/healthequity/whatis/index.html. Accessed June 4, 2024.
- Discrimination. Healthy People 2030. https://health.gov/healthypeople/priority-areas/social-determinants-health/literature-summaries/discrimination. Accessed June 4, 2024.
- Goldman L, et al., eds. In: Goldman-Cecil Medicine. 27th ed. Elsevier; 2024. https://www.clinicalkey.com. Accessed June 4, 2024.
- People with certain medical conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed April 19, 2022.
- Mackey K, et al. Racial and ethnic disparities in COVID-19 — Related infections, hospitalizations, and deaths: A systematic Review. Annals of Internal Medicine. 2020; doi:10.7326/M20-6306.
- Lundberg, et al. COVID-19 Mortality by race and ethnicity in US metropolitan and nonmetropolitan areas, March 2020 to February 2022. Journal of the American Medical Association Network Open. 2023; doi:10.1001/jamanetworkopen.2023.11098.
- COVID-19 Monthly death rates per 100,000 population by age group, race and ethnicity, and sex. Centers for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#demographicsovertime. Accessed June 4, 2024.
- Keisler-Starkey K, et al. Health insurance coverage in the United States: 2022. U.S. Census Bureau. https://www.census.gov/library/publications/2023/demo/p60-281.html. Accessed June 17, 2024.
Products and Services
- A Book: Endemic - A Post-Pandemic Playbook
- Begin Exploring Women's Health Solutions at Mayo Clinic Store
- A Book: Future Care
- Antibiotics: Are you misusing them?
- COVID-19 and vitamin D
- Convalescent plasma therapy
- Coronavirus disease 2019 (COVID-19)
- Herd immunity and respiratory illness
- COVID-19 and pets
- COVID-19 and your mental health
- COVID-19 antibody testing
- COVID-19, cold, allergies and the flu
- Long-term effects of COVID-19
- COVID-19 tests
- COVID-19 drugs: Are there any that work?
- COVID-19 in babies and children
- COVID-19 travel advice
- COVID-19 vaccine: Should I reschedule my mammogram?
- COVID-19 vaccines for kids: What you need to know
- COVID-19 vaccines
- COVID-19 variant
- COVID-19 vs. flu: Similarities and differences
- COVID-19: Who's at higher risk of serious symptoms?
- Debunking coronavirus myths
- Different COVID-19 vaccines
- Extracorporeal membrane oxygenation (ECMO)
- Fever: First aid
- Fever treatment: Quick guide to treating a fever
- Fight coronavirus (COVID-19) transmission at home
- Honey: An effective cough remedy?
- How do COVID-19 antibody tests differ from diagnostic tests?
- How to measure your respiratory rate
- How to take your pulse
- How to take your temperature
- How well do face masks protect against COVID-19?
- Is hydroxychloroquine a treatment for COVID-19?
- Loss of smell
- Mayo Clinic Minute: You're washing your hands all wrong
- Mayo Clinic Minute: How dirty are common surfaces?
- Multisystem inflammatory syndrome in children (MIS-C)
- Nausea and vomiting
- Pregnancy and COVID-19
- Safe outdoor activities during the COVID-19 pandemic
- Safety tips for attending school during COVID-19
- Shortness of breath
- Thermometers: Understand the options
- Treating COVID-19 at home
- Unusual symptoms of coronavirus
- Vaccine guidance from Mayo Clinic
- Watery eyes
- COVID-19 infections by race Whats behind the health disparities
Your gift holds great power – donate today!
Make your tax-deductible gift and be part of the cutting-edge research and care that's changing medicine.
- Study Protocol
- Open access
- Published: 05 July 2024
Strategic use of resources to enhance colorectal cancer screening for patients with diabetes (SURE: CRC4D) in federally qualified health centers: a protocol for hybrid type ii effectiveness-implementation trial
- Denalee M. O’Malley 1 , 2 ,
- Benjamin F. Crabtree 1 , 2 ,
- Srivarsha Kaloth 1 ,
- Pamela Ohman-Strickland 1 , 2 , 3 ,
- Jeanne Ferrante 1 , 2 ,
- Shawna V. Hudson 1 , 2 &
- Anita Y. Kinney 2 , 3
BMC Primary Care volume 25 , Article number: 242 ( 2024 ) Cite this article
30 Accesses
Metrics details
Persons with diabetes have 27% elevated risk of developing colorectal cancer (CRC) and are disproportionately from priority health disparities populations. Federally qualified health centers (FQHCs) struggle to implement CRC screening programs for average risk patients. Strategies to effectively prioritize and optimize CRC screening for patients with diabetes in the primary care safety-net are needed.
Guided by the Exploration, Preparation, Implementation and Sustainment Framework, we conducted a stakeholder-engaged process to identify multi-level change objectives for implementing optimized CRC screening for patients with diabetes in FQHCs. To identify change objectives, an implementation planning group of stakeholders from FQHCs, safety-net screening programs, and policy implementers were assembled and met over a 7-month period. Depth interviews ( n = 18–20) with key implementation actors were conducted to identify and refine the materials, methods and strategies needed to support an implementation plan across different FQHC contexts. The planning group endorsed the following multi-component implementation strategies: identifying clinic champions, development/distribution of patient educational materials, developing and implementing quality monitoring systems, and convening clinical meetings. To support clinic champions during the initial implementation phase, two learning collaboratives and bi-weekly virtual facilitation will be provided. In single group, hybrid type 2 effectiveness-implementation trial, we will implement and evaluate these strategies in a in six safety net clinics ( n = 30 patients with diabetes per site). The primary clinical outcomes are: (1) clinic-level colonoscopy uptake and (2) overall CRC screening rates for patients with diabetes assessed at baseline and 12-months post-implementation. Implementation outcomes include provider and staff fidelity to the implementation plan, patient acceptability, and feasibility will be assessed at baseline and 12-months post-implementation.
Study findings are poised to inform development of evidence-based implementation strategies to be tested for scalability and sustainability in a future hybrid 2 effectiveness-implementation clinical trial. The research protocol can be adapted as a model to investigate the development of targeted cancer prevention strategies in additional chronically ill priority populations.
Trial registration
This study was registered in ClinicalTrials.gov (NCT05785780) on March 27, 2023 (last updated October 21, 2023).
Patients with diabetes mellitus have an estimated 27% elevated lifetime risk of developing colorectal cancer (CRC), and are disproportionately from priority health disparities populations (e.g., low-income, Non-Hispanic Black and Hispanic) [ 1 , 2 ]. Nationally, guideline concordant receipt of CRC screening for patients with diabetes is not significantly different for women with diabetes (57% vs. patients without diabetes 58%) and is significantly higher among men with diabetes (63% vs. patients with diabetes 58%) [ 3 ]. CRC screening for patients with diabetes, who do not have other indications of high risk (e.g., family history of CRC, polyp removal during colonoscopy, personal history of CRC, inflammatory bowel disease) are advised to follow the average risk screening recommendations [ 4 ]. Federally qualified health centers (FQHCs) primarily serve as primary care for priority health disparities populations and struggle to sustainably implement CRC screening programs for average-risk patients which includes patients with diabetes. CRC screening uptake in FQHCs populations has been consistently lower (44.1%) than the national average for average risk, age-eligible adults (67.3%) [ 5 ].
Persons receiving diabetes care in FQHCs have elevated health risks overall and higher rates of poverty and low-income status than the general population [ 6 ]. Ten percent of FQHC patients have a diabetes diagnosis and more than a third within this group have uncontrolled diabetes (HbA1c > 9%). Failure to implement preventive CRC screenings translates to an average of 6.5 years of lost life for patients subsequently diagnosed with CRC [ 7 ]. Moreover, this contributes to greater burden for patients with diabetes who are diagnosed with CRC who suffer greater morbidity, all-cause mortality, and cancer-specific mortality compared to CRC patients [ 8 , 9 , 10 ]. Therefore, efforts to prioritize CRC screening for patients with diabetes are needed in primary care safety-net settings.
Multiple evidence-based CRC screening tests are available which complicates implementation. The U.S. Preventive Services Taskforce (USPSTF) recommends CRC screening in adults aged 45–75, with multiple screening options available including non-invasive stool based testing: high sensitivity guaiac fecal occult blood tests (gFOBT), fecal immunochemical test (FIT), FIT plus stool DNA testing (FIT-DNA); and direct visualization tests: colonoscopy, computed tomography (CT) colography, and flexible sigmoidoscopy (FS) (with or without FIT) (see Table 1 for intervals) [ 4 ]. Colonoscopy and FS, have been shown to reduce mortality by (68% and 28%, respectively). FIT and FOBT are associated with 13–33% mortality reductions. Stool-based testing mortality reductions require sustained annual adherence. [ 11 , 12 , 13 , 14 ]. Research has shown that failures to screen at all, to screen at appropriate intervals, and to follow-up on abnormal results are associated with risk of CRC death [ 15 ].
Given major differences in mortality reduction benefits, temporal intervals for retesting, costs, and patient burden, controversies have emerged surrounding the pros and cons of testing methods [ 16 , 17 ]. Colonoscopy and FS allow for polypectomies, which can prevent CRC [ 18 , 19 ]; however, FS is not widely used in the U.S, because colonoscopy evaluates the entire colon, can be done every 10 years, and is associated with a greater mortality reduction [ 20 ]. A re-analysis of the USPSTF data suggest that prevention, through the removal of polyps during colonoscopy, is the sole mechanism of CRC mortality reductions [ 19 ]. Colonoscopy is thus the “gold standard,” despite critiques about the rigor of this evidence (e.g., indirect and observational). [ 21 , 22 , 23 , 24 ]. In FQHCs, non-invasive tests are emphasized and colonoscopies are often a second line-screening based on abnormal gFOBT/FIT findings. [ 25 ]. Non-invasive tests are emphasized because these are less costly, require less time (and time off of work), less complicated to complete, do not require transportation, and are guideline concordant [ 26 ]. Despite stool based testing’s acceptability, US-based trials in FQHCs designed to increase annual adherence to stool-based testing have reported low screening adherence over three years (10.4–16.4%) [ 27 , 28 , 29 ].
Prioritizing colonoscopy with longer testing intervals in under-resourced FQHCs for patients with diabetes introduces fewer opportunities for care breakdowns, is guideline concordant, and prevents CRC by removing premalignant colonic polyps. Guided by the Exploration, Preparation, Implementation and Sustainment [ 30 ]. (EPIS) framework, this research study will develop and evaluate targeted CRCs screening strategies for patients with diabetes in safety-net settings. This study addresses known implementation challenges using a “designing for dissemination” approach [ 31 , 32 , 33 ] that attends to important contextual, organizational capacity and patient complexity factors that impact CRC screening program implementation in clinics and uptake among patients with diabetes.
Conceptual framework
The design of this study was guided by the EPIS framework. EPIS is an evidence-based practice (EBP) implementation framework that includes four defined phases for assessment of inner and outer contextual factors that influence EBP implementation (see Table 2 ). For this study, the EBP is CRC screening uptake among age eligible patients with diabetes. Exploration is the act of identifying patient needs and the availability of EBPs to address identified needs, and the decision to adopt evidence into practice based on fit within the inner clinical context. During this phase, the adaptations to the evidence are based on system, organization, and individual patient factors. Preparation includes planning implementation, inventorying proposed challenges, and developing strategies to overcome anticipated barriers. A critical component of this phase is the planning of implementation strategies to support EBP utilization in the next two phases and to address organizational climate to ensure that EBPs will be supported, expected, and rewarded. During the clinical trial, this study focuses on implementation, the process of assuring and balancing fidelity to the EBP delivered with adaptations needed to assure program success. Sustainment focuses on maintenance and program and factors impacting implementation over the long haul. EPIS considers innovation factors, which are the characteristics of the EBP being implemented. The innovation-EBP fit considers if the EBP fits the patient, provider, and organizational needs. Innovation factors are assessed and can be adapted to maximize the fit of an EBP while maintaining the core elements of the intervention to retain fidelity.
Methods and design
Identifying multi-level change levers: a multi-method stakeholder informed approach.
Earlier phases of this research focused on the Exploration and Preparation phases, while the current protocol describes the intervention implementation and its evaluation. During the exploration phase, a secondary analysis was conducted of a nationally representative data set to identify patient level determinants of CRC screening uptake overall (i.e., with any test) and test-specific uptake among individuals with diabetes. We explored disparities in uptake overall and testing type based on race, ethnicity, income, and educational status. Additionally, a scoping literature review was performed to identify evidence-based interventions and implementation strategies for CRC screening and diabetes management in FQHCs. Based on this scoping review, we identified additional interventions and implementation strategies, using the Expert Recommendations for Implementing Change (ERIC) taxonomy [ 34 ]. A list of interventions and implementation strategies was compiled related to diabetes management processes to expand an existing measure that was developed and used to evaluate the use of evidence-based intervention and implementation for CRC in FQHCs [ 35 ].
For the preparation phase of the formative research, we used implementation mapping, an iterative process that incorporates community based participatory research principles [ 36 , 37 ]. An Implementation Planning Group (IPG) was assembled to represent a diversity of implementation actors (e.g., clinicians, state-level decision makers, screening safety-net programs) who work in and with FQHCs. The goal of the IPG, which met 5 times over a six-month period, was to develop shared understandings of the research problem based on empirical knowledge from the national survey analysis, the scoping review of the literature, and local knowledge of the IPG members about patient population and clinic system capacities. The IPG group identified and prioritized the selection of implementation strategies to improve CRC screening uptake for patients with diabetes. The IPG and research team iterated an implementation plan specifying multi-level change objectives and implementation determinants to develop supports to help prioritize CRC screening implementation for patients with diabetes.
Guided by the insights of the exploration and preparation phases, we developed the Strategic Use of Resources for Enhanced ColoRectal Cancer Screening in Patients with Diabetes (SURE: CRC4D) implementation toolkit, which includes tailorable materials and protocols that will be tested in a single arm, hybrid type 2 effectiveness-implementation single arm clinical trial. The objectives of this trial are to:
Determine the effectiveness of the SURE: CRC4D multi-component implementation strategies to increase CRC screening uptake among patients with diabetes.
Evaluate the fidelity, feasibility, and acceptability of SURE: CRC4D implementation.
Refine the SURE: CRC4D toolkit based on multi-level user feedback and conduct an evaluation to promote scalability and sustainable use.
Study participants and setting
This single arm trial will be conducted in six FQHC clinical sites in New Jersey. Eligibility criteria for the FQHC clinics include: (1) provide care to at least 450 patients aged 50–74 years; (2) 10% of patient population previously diagnosed with diabetes; (3) located in New Jersey; and, 3) clinical and administrative leadership willing to engage in the intervention and research requirements (interviews, data validation, process evaluation). Implementation outcomes will be assessed using mixed methods guided by the EPIS constructs (see Table 2 ). The methods of this study have been reported using Standard Protocol Items: Recommendation for Interventional Trials (SPIRIT) guidelines (Supplemental file 1 ).
During the implementation a clinic-based registry of patients eligible for CRC screening will be developed for each clinic at baseline and updated at six and 12 months post-baseline. Patient eligibility criteria will include: (1) patients not up-to-date or due for CRC screening [ 4 ]. based on electronic health record (EHR) documentation (e.g. FOBT/FIT test in last year, flexible sigmoidoscopy within 4 years, or colonoscopy within 9 years), (2) previous diagnosis of type II diabetes, (3) age-eligible for CRC screening (45–74 years of age) and (4) ) FIT/FOBT that has been ordered for more than 6 months that has not been completed or a sigmoidoscopy or colonoscopy referral that has not been completed for 12 or more months. Patients are excluded if they have EHR documentation medical conditions not concordant with standard CRC screening intervals (e.g. prior CRC diagnosis, inflammatory bowel disease, renal failure, etc.) [ 4 ].
The NJ Primary Care Research Network (NJPCRN) will recruit eligible clinics for participation. The NJPCRN is an Agency for Healthcare Research and Quality recognized practice-based research in primary care practices. The NJPRN will contact FQHCs that participated in previous research and ask IPG members to make introductions with their FQHC leadership networks. Emails with study flyers will be sent to the FQHC with follow-up telephone outreach. This protocol has been approved by the Rutgers University Institutional Review Board (Pro2020002075). All study participants will be asked to provide informed consent prior to participation in all phase of this research. We expect the distribution of patient participants to reflect the racial/ethnic diversity of the FQHCs recruited, who predominantly serve low-income, racial, and ethnic minority populations.
Implementation strategies
The goal of SURE: CRC4D is to enable FQHC clinics to adopt strategies to optimize the use of evidence based colorectal cancer screenings (See Table 1 ) uptake for patients with type II diabetes. To accomplish this, multi-level, multi-component implementation strategies (see Table 3 ) will be utilized. The core components of this implementation effort includes the identification and engagement of 2–3 clinic change champions, who will participate in two virtual learning collaborative events [ 38 , 39 , 40 , 41 ] and lead the change effort in the clinic aided by bi-weekly virtual practice facilitator support [ 38 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ]. The SURE: CRC4D toolkit will include guidance on pulling data to develop and implement quality monitoring systems to provide regular audit/feedback to the clinic, patient educational materials in English and Spanish and dissemination materials for clinical meetings to orient other clinic members to the change process being implemented to optimize CRC screening for patients with diabetes [ 50 , 51 ]. Clinic champions will tailor toolkit resources as clinics may have different electronic medical records, type and composition of staff, clinical workflows, and standing clinical team meetings.
The implementation will be rolled out over a 12-week period. Initially, clinics will be asked to identify 1–3 clinic champions, with at least one clinician (i.e., physician, advanced practice nurse, physician assistant) per team. Each team will meet with the external practice change facilitator approximately two weeks prior to the initial learning collaborative. This initial facilitation meeting is introductory, with the goal of encouraging clinic champions to reflect about the current clinic CRC screening strategies and diabetes care management processes prior to the 1st virtual learning collaborative. Clinical champions will attend the 1- hour virtual learning collaborative, where the materials in the SURE: CRC4D toolkit will be provided and reviewed, and each clinic team will formulate practice change goals. Teams will decide on how to deploy the toolkit strategies at their FQHC sites over the course of the next ten weeks. The practice facilitator will support the clinic champion team in the development, implementation, and refinement of the local practice change plan. The champions will meet with the practice facilitator every two weeks for 8 weeks (4 times). During this time, the plan will be refined and adjusted based on feedback from clinic leaders and practice staff members and identified strengths and barriers that are encountered during the implementation effort. At week 10, a second learning collaborative will be virtually convened, providing a forum where the different clinic teams can share their successes and obstacles during the development and execution of their plan. This forum will foster cross-team learning and idea generation that can inform the refinement of the SURE: CRC4D toolkit and sustainability of practice change efforts. Two weeks after the second learning collaborative, a final virtual facilitation meeting will be held to reflect and refine the practice plan to support sustainability.
Evaluation of the effectiveness and implementation of SURE: CRC4D
The effectiveness and implementation of SURE: CRC4D will be evaluated using a mixed method learning evaluation strategy, where ongoing data collection and analysis are used to refine implementation to optimize adoption of CRC screening for patients with diabetes [ 52 , 53 ]. This evaluation is designed to address two research questions: (1) are the adapted implementation strategies clinically effective in increasing CRC screening rates for patients with diabetes; and, (2) are the implementation strategies feasible and acceptable to implementers (e.g., clinicians and staff) and patients in FQHCs? This evaluation builds an evidence base about the effectiveness of the implementation strategies in a real-world context and allows for the collection of data that can be used to refine the implementation toolkit for a larger scale, definitive cluster randomized controlled trial. Guided by EPIS, contextual factors were selected based on suggestions from clinical stakeholders, community partners, and previous literature suggesting they may influence implementation success [ 54 , 55 , 56 ] (see Table 4 ). The following assessments and measures will be collected to evaluate the trial:
Organizational assessments
Guided by EPIS, contextual factors will be evaluated at baseline and 1 year-post implementation. Medical Directors or the Chief Operating Officer of each clinic will be asked to complete a web-based survey called the Clinic Organizational Information Form (COIF). This survey assesses Implementation Climate and History of Implementation related to CRC screening and diabetes management [ 35 ]. Additionally, patient demographics, management strategies, and payor mix are collected using this survey for each clinic.
Clinic staff measures and assessments
The Clinic Staff Questionnaire (CSQ) will be administered to all practice clinicians and staff members at baseline and 12-month post-implementation. The clinic team measures include Medical Provider and Staff Background and history with the organization. Additionally, Change Process Capability will be measured, specifically “previous history of change,” and “ability to initiate and sustain change.” [ 57 ]. These two measures have been identified these as key mechanisms for successful organizational change and its wide use in cardiovascular care implementation [ 58 , 59 , 60 ]. Additional practice-based measures will include: Adaptive Reserve a feature of resilient organizations shown to be associated with practice-level implementation of CRC screening, will be measured in the CSQ with the validated 23-item scale [ 57 , 61 ]. The CSQ will also include the Implementation Leadership Scale (ILS), a brief psychometrically strong measure that contains 12-items with four subscales of proactive, knowledgeable, supportive, and perseverant leadership [ 62 ].
Process data outcomes
Learning collaborative and facilitation phone calls will be audio recorded and transcribed to document issues that arose during the implementation process. Additionally, qualitative interviews will be conducted at baseline and beginning at 6 months post implementation. We will select key implementers (3–4 individuals per site) to assess perceptions of organizational readiness to change, leadership style and additional facility characteristics (e.g., assets and deficits of location, satisfaction with ease of access to facility, etc.). Staff and clinician perceptions of the SURE: CRC4D implementations’ feasibility and acceptability will also assessed asking providers and staff to describe their implementation experiences. The interviews will probe stakeholder perceptions of change in their organizations, systems, and factors that they think impacted implementation. Staff or provider fidelity will be assessed based on the clinic-level proportion of eligible patients who were (1) contacted based on implementation protocol and (2) completed any CRC screening at 1 year.
Patient level: clinical effectiveness outcome and implementation assessment
The primary outcome variables to assess clinical effectiveness will be the clinic level proportion of patients with diabetes who: (a) receive any CRC screening and (b) complete a colonoscopy at 12 months from baseline. An exploratory analysis will assess clinic-level CRC screening completion by glucose control (controlled vs. uncontrolled, i.e., HbA1c > 9 at 12 months). Patient level data is collected in aggregate and will include no identified personal health information.
Patient acceptability will be assessed through the assessment of patient rates of opting-out and non-adherence of CRC screening. This rate will be based on the proportion of CRC screening among patients with diabetes compared to overall eligible patient population (without diabetes) in each clinic.
Data analyses
Qualitative analysis.
On a quarterly basis, we will analyze data from each clinic site using a comparative case analysis [ 63 ]. Organizational level data and interview transcripts will be organized, read and coded in ATLAS.ti. Data will be analyzed on an ongoing basis, and a working summary of emergent findings will be updated as incoming data is added. As a validity check of qualitative results, we will check relevant data interpretations against all new data using a constant comparison approach [ 64 ]. We will note similarities and differences of implementation feasibility between practice sites based on clinic characteristics and from data provided in interviews. Each quarter all quantitative and qualitative results will be summarized in brief reports to be shared with the research team for reflections on any changes needed. These analyses represent ongoing monitoring and feedback to inform refinements of the implementation strategy and clinical trial procedures to refine implementation strategy to better fit local needs and contexts.
Quantitative analysis
Descriptive statistics will be used to summarize patient and clinic characteristics. We will declare our intervention a success if at least 25% of those unscreened are screened at 12-month follow-up in this difficult to reach population. We will declare the optimization of screening a success if 15% of those unscreened are screened with a colonoscopy or flexible sigmoidoscopy at 12-months. Overall improvement metrics are comparable to improvements in previous CRC screening implementation studies in FQHCs [ 65 , 66 ]. At baseline, we will calculate average screening rates and their confidence intervals across all practice sites in intent to treat analyses and at 12-months we will assess screening rates and their confidence intervals for all sites. The confidence intervals will be compared to 25%. We will compare differences in CRC screening by glucose control, sex, and race/ethnicity.
Power calculations
The value of information method [ 67 ] was utilized to select a sample size balancing the costs and feasibility goals of the trial. This sample size (e.g., six clinic sites, assuming at least n = 30 patient in each) is sufficient to generate preliminary estimates of the estimated effect (80% confidence interval) of the implementation strategy on CRC screeningrates [ 68 , 69 ]. In developing the power calculation, we assume equal numbers of patients ( n = 50) per clinic (the anticipated number of eligible patients, n = 450 CRC screening eligible, with > 10% diabetes diagnosis). Of those with diabetes, we expect 40% to be up-to-date with screening guidelines based on the average rate of CRC screening in FQHCs [ 70 ]. Thus, the target sample size is n = 30 patients in each FQHC.
This study aims to optimize CRC screening using the engagement of multi-level stakeholders (patients, clinicians, staff in FQHCs) and using an implementation mapping during the exploration and preparation phases prior to implementation [ 37 ]. This project is innovative in several key ways. Regarding conceptual innovation, few studies have included CRC screening as a component of diabetes care prior to CRC diagnosis [ 71 , 72 ], while many have focused on improving CRC screening for average risk adults in FQHCs [ 65 , 66 , 73 , 74 , 75 , 76 , 77 , 78 , 79 ].An EPIS framework systematic review concluded that attention to planning EBP use is “infrequent though critical [ 80 ].” FQHC implementation of CRC screening programs focus on achieving the Uniform Data System (UDS) targets, which do not distinguish patients at greater risk for CRC in the “average-risk” patient population [ 70 , 81 ]. Metrics for UDS CRC screening program are also cross-sectional and collected as separate metrics unrelated to diabetes care or annual stool-based testing adherence. For FIT and FOBT stool-based CRC screening strategies to be clinically effective and for their mortality reductions to be realized sustained annual adherence is required, which has been proven difficult to accomplish in safety-net primary care settings [ 12 , 13 ]. Additionally, few FQHCs formally assess factors related screening prior to implementing improvement interventions [ 82 ]. This study aims to optimize CRC prevention using the engagement of multi-level stakeholders (patients, clinicians, staff in FQHCs) and using an implementation mapping during the exploration and preparation phases prior to implementation [ 37 ].
Despite being the most studied evidence-based cancer screening in the National Institutes of Health implementation science portfolio, no systematic studies have integrated CRC screening and diabetes evidence-based approaches to prioritize preventive care for patients with diabetes in the primary care safety-net. To date, research has focused on overall CRC guideline adherence, relying on an ‘all boats rise’ approach despite the failures of such strategies to achieve improvements in chronic disease targets [ 83 ]. In contrast, this study focuses on optimizing CRC screening using targeted implementation strategies to address disparities among individuals with diabetes to promote health equity.
Study findings are poised to inform the develop scalable, equitable approaches to CRC screening in safety-net primary care settings. If successful, next steps will include testing the scalability and sustainability in federally qualified health centers nationally. Further, this approach can be adapted as a model to investigate the development of targeted cancer prevention strategies in additional chronically ill priority populations.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- Colorectal cancer
Computed tomography
Fecal immunochemical tests
Fecal immunochemical test stool DNA testing
Federally qualified health centers
Flexible sigmoidoscopy
Guaiac fecal occult blood tests
Implementation Planning Group
Electronic health record
Evidence-based practice
Expert Recommendations for Implementing Change
Exploration, Preparation, Implementation, Sustainment
New Jersey Primary Care Research Network
CRC4D: Strategic Use of Resources for Enhanced ColoRectal Cancer Screening in Patients with Diabetes
Standard Protocol Items: Recommendation for Interventional Trials
Uniform Data System
U.S. Preventive Services Taskforce
Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13(6):814–23.
Article PubMed Google Scholar
Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519–27.
Miller EA, Tarasenko YN, Parker JD, Schoendorf KC. Diabetes and colorectal cancer screening among men and women in the USA: National Health interview survey: 2008, 2010. Cancer Causes Control. 2014;25(5):553–60. https://doi.org/10.1007/s10552-014-0360-z .
Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965–77. https://doi.org/10.1001/jama.2021.6238 . PubMed PMID: 34003218.
May FP, Yang L, Corona E, Glenn BA, Bastani R. Disparities in Colorectal Cancer Screening in the United States before and after implementation of the Affordable Care Act. Clinical Gastroenterology and Hepatology; 2019.
Huang ES, Zhang Q, Brown SE, Drum ML, Meltzer DO, Chin MH. The cost-effectiveness of improving diabetes care in U.S. federally qualified community health centers. Health Serv Res. 2007;42(6 Pt 1):2174-93; discussion 294–323. doi: 10.1111/j.1475-6773.2007.00734.x. PubMed PMID: 17995559; PMCID: PMC2151395.
Liu P-H, Wang J-D, Keating NL. Expected years of life lost for six potentially preventable cancers in the United States. Prev Med. 2013;56(5):309–13. https://doi.org/10.1016/j.ypmed.2013.02.003 .
Boakye D, Rillmann B, Walter V, Jansen L, Hoffmeister M, Brenner H. Impact of comorbidity and frailty on prognosis in colorectal cancer patients: A systematic review and meta-analysis. Cancer Treat Rev. 2018;64. https://doi.org/10.1016/j.ctrv.2018.02.003 . Epub 2018/02/10. :30 – 9.
Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26(11):863–76. https://doi.org/10.1007/s10654-011-9617-y .
Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum. 2013;56(11):1304–19. https://doi.org/10.1097/DCR.0b013e3182a479f9 .
Article PubMed PubMed Central Google Scholar
Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–14. https://doi.org/10.1056/NEJMoa1300720 . PubMed PMID: 24047060.
Article CAS PubMed Google Scholar
Jensen CD, Corley DA, Quinn VP, Doubeni CA, Zauber AG, Lee JK, Zhao WK, Marks AR, Schottinger JE, Ghai NR, Lee AT, Contreras R, Klabunde CN, Quesenberry CP, Levin TR, Mysliwiec PA. Fecal immunochemical test program performance over 4 rounds of Annual Screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456–63. https://doi.org/10.7326/M15-0983 . PubMed PMID: 26811150; PMCID: PMC4973858.
Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, Munoz R, Lau C, Somsouk M, El-Nachef N, Hayward RA. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–82. https://doi.org/10.1001/archinternmed.2012.332 . Epub 2012/04/12.
Shroff J, Thosani N, Batra S, Singh H, Guha S. Reduced incidence and mortality from colorectal cancer with flexible-sigmoidoscopy screening: a meta-analysis. World J Gastroenterol. 2014;20(48):18466–76. https://doi.org/10.3748/wjg.v20.i48.18466 . PubMed PMID: 25561818.
Doubeni CA, Fedewa SA, Levin TR, Jensen CD, Saia C, Zebrowski AM, Quinn VP, Rendle KA, Zauber AG, Becerra-Culqui TA, Mehta SJ, Fletcher RH, Schottinger J, Corley DA. Modifiable failures in the colorectal Cancer screening process and their Association with risk of death. Gastroenterology. 2019;156(1):63–e746. https://doi.org/10.1053/j.gastro.2018.09.040 .
Gorin SS. Multilevel approaches to reducing Diagnostic and Treatment Delay in Colorectal Cancer. Annals Family Med. 2019;17(5):386–9.
Article Google Scholar
Breen N, Skinner CS, Zheng Y, Inrig S, Corley DA, Beaber EF, Garcia M, Chubak J, Doubeni C, Quinn VP, Haas JS, Li CI, Wernli KJ, Klabunde CN. Time to follow-up after Colorectal Cancer Screening by Health Insurance Type. Am J Prev Med. 2019;56(5):e143–52. https://doi.org/10.1016/j.amepre.2019.01.005 .
Swartz AW, Eberth JM, Josey MJ, Strayer SM. Ann Intern Med. 2017;167(8):602–3. https://doi.org/10.7326/m17-0859 . Epub 2017/08/23. Reanalysis of All-Cause Mortality in the U.S. Preventive Services Task Force 2016 Evidence Report on Colorectal Cancer Screening.
Swartz AW, Eberth JM, Strayer SM. Preventing colorectal cancer or early diagnosis: Which is best? A re-analysis of the U.S. Preventive Services Task Force Evidence Report. Prev Med. 2019;118. https://doi.org/10.1016/j.ypmed.2018.10.014 . Epub 2018/10/28. :104 – 12.
Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, Smith N, Whitlock EP. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(23):2576–94.
Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JMA, Parkin DM, Wardle J, Duffy SW, Cuzick J. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–33. https://doi.org/10.1016/S0140-6736(10)60551-X .
Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, Bresalier R, Andriole GL, Buys SS, Crawford ED, Fouad MN, Isaacs C, Johnson CC, Reding DJ, O’Brien B, Carrick DM, Wright P, Riley TL, Purdue MP, Izmirlian G, Kramer BS, Miller AB, Gohagan JK, Prorok PC, Berg CD. Colorectal-Cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366(25):2345–57. https://doi.org/10.1056/NEJMoa1114635 . PubMed PMID: 22612596.
Article CAS PubMed PubMed Central Google Scholar
Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, Andreoni B, Arrigoni A, Bisanti L, Casella C, Crosta C, Falcini F, Ferrero F, Giacomin A, Giuliani O, Santarelli A, Visioli CB, Zanetti R, Atkin WS, Senore C, Group atSW. Once-only sigmoidoscopy in Colorectal Cancer Screening: follow-up findings of the Italian randomized controlled Trial—SCORE. JNCI: J Natl Cancer Inst. 2011;103(17):1310–22. https://doi.org/10.1093/jnci/djr284 .
Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, Eide TJ, Skovlund E, Schneede J, Tveit KM, Hoff G. Effect of flexible Sigmoidoscopy Screening on Colorectal Cancer incidence and mortality: a Randomized Clinical Trial. JAMA. 2014;312(6):606–15. https://doi.org/10.1001/jama.2014.8266 .
Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, Imamura Y, Willett WC, Rosner BA, Fuchs CS, Giovannucci E, Ogino S, Chan AT. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095–105. https://doi.org/10.1056/NEJMoa1301969 . PubMed PMID: 24047059.
Gwede CK, Davis SN, Quinn GP, Koskan AM, Ealey J, Abdulla R, Vadaparampil ST, Elliott G, Lopez D, Shibata D, Roetzheim RG, Meade CD. Tampa Bay Community Cancer Network P. making it work: Health Care Provider perspectives on strategies to increase colorectal Cancer screening in federally qualified Health centers. J Cancer Educ. 2013;28(4):777–83. https://doi.org/10.1007/s13187-013-0531-8 .
Arnold CL, Rademaker A, Wolf MS, Liu D, Lucas G, Hancock J, Davis TC. Final results of a 3-Year literacy-informed intervention to promote Annual Fecal Occult Blood Test Screening. J Community Health. 2016;41(4):724–31. https://doi.org/10.1007/s10900-015-0146-6 . PubMed PMID: 26769026; PMCID: PMC4930386.
Singal AG, Gupta S, Skinner CS, Ahn C, Santini NO, Agrawal D, Mayorga CA, Murphy C, Tiro JA, McCallister K, Sanders JM, Bishop WP, Loewen AC, Halm EA. Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: a Randomized Clinical Trial. JAMA. 2017;318(9):806–15. https://doi.org/10.1001/jama.2017.11389 . PubMed PMID: 28873161; PMCID: PMC5648645.
Green BB, Anderson ML, Cook AJ, Chubak J, Fuller S, Meenan RT, Vernon SW. A centralized mailed program with stepped increases of support increases time in compliance with colorectal cancer screening guidelines over 5 years: a randomized trial. Cancer. 2017;123(22):4472–80. https://doi.org/10.1002/cncr.30908 . PubMed PMID: 28753230; PMCID: PMC5673524.
Aarons GA, Hurlburt M, Horwitz SM. Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health. 2011;38(1):4–23. https://doi.org/10.1007/s10488-010-0327-7 . Epub 2011/01/05.
Holtrop JS, Rabin BA, Glasgow RE. Dissemination and implementation science in primary Care Research and Practice: contributions and opportunities. J Am Board Fam Med. 2018;31(3):466–78. https://doi.org/10.3122/jabfm.2018.03 . .170259. PubMed PMID: 29743229.
Dearing JW, Kreuter MW. Designing for diffusion: how can we increase uptake of cancer communication innovations? Patient Educ Couns. 2010;81 Suppl:S100-10. https://doi.org/10.1016/j.pec.2010.10.013 . PubMed PMID: 21067884; PMCID: 3000559.
Brownson RC, Jacobs JA, Tabak RG, Hoehner CM, Stamatakis KA. Designing for dissemination among public health researchers: findings from a national survey in the United States. Am J Public Health. 2013;103(9):1693–9. https://doi.org/10.2105/AJPH.2012.301165 . PubMed PMID: 23865659; PMCID: 3966680.
Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, Proctor EK, Kirchner JE. A refined compilation of implementation strategies: results from the Expert recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10(1):21. https://doi.org/10.1186/s13012-015-0209-1 .
Adams SA, Rohweder CL, Leeman J, Friedman DB, Gizlice Z, Vanderpool RC, Askelson N, Best A, Flocke SA, Glanz K, Ko LK, Kegler M. Use of evidence-based interventions and implementation strategies to increase colorectal Cancer screening in federally qualified Health centers. J Community Health. 2018;43(6):1044–52. https://doi.org/10.1007/s10900-018-0520-2 . PubMed PMID: 29770945; PMCID: PMC6239992.
Wallerstein N, Duran B. Community-based participatory research contributions to intervention research: the intersection of science and practice to improve health equity. Am J Public Health. 2010;100(Suppl 1). https://doi.org/10.2105/ajph.2009.184036 . Epub 2010/02/12. :S40-6.
Fernandez ME, Ten Hoor GA, van Lieshout S, Rodriguez SA, Beidas RS, Parcel G, Ruiter RAC, Markham CM, Kok G. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health. 2019;7:158. https://doi.org/10.3389/fpubh.2019.00158 . Epub 2019/07/06.
Balasubramanian BA, Chase SM, Nutting PA, Cohen DJ, Strickland PA, Crosson JC, Miller WL, Crabtree BF, Team US. Using learning teams for reflective adaptation (ULTRA): insights from a team-based change management strategy in primary care. Ann Fam Med. 2010;8(5):425–32. https://doi.org/10.1370/afm.1159 . Epub 2010/09/17.
Mittman BS. Creating the evidence base for quality improvement collaboratives. Ann Intern Med. 2004;140(11):897–901. https://doi.org/10.7326/0003-4819-140-11-200406010-00011 . Epub 2004/06/03.
Bate JOV, Cleary P, Cretin P, Gustafson S, McInnes D, McLeod K, Molfenter H, Plsek T, Robert P, Shortell G, Wilson S. Quality collaboratives: lessons from research. Qual Saf Health Care. 2002;11(4):345–51. PubMed PMID: 12468695; PMCID: PMC1757995.
Shaw EK, Chase SM, Howard J, Nutting PA, Crabtree BF. More black box to explore: how quality improvement collaboratives shape practice change. J Am Board Fam Med. 2012;25(2):149–57. https://doi.org/10.3122/jabfm.2012.02.110090 . PubMed PMID: 22403195; PMCID: PMC3362133. Epub 2012/03/10.
Chase SM, Crabtree BF, Stewart EE, Nutting PA, Miller WL, Stange KC, Jaen CR. Coaching strategies for enhancing practice transformation. Fam Pract. 2015;32(1):75–81. https://doi.org/10.1093/fampra/cmu062 . Epub 2014/10/05.
Chase SM, Miller WL, Shaw E, Looney A, Crabtree BF. Meeting the challenge of practice quality improvement: a study of seven family medicine residency training practices. Acad Med. 2011;86(12):1583–9. https://doi.org/10.1097/ACM.0b013e31823674fa . Epub 2011/10/28.
Dickinson WP, Dickinson LM, Nutting PA, Emsermann CB, Tutt B, Crabtree BF, Fisher L, Harbrecht M, Gottsman A, West DR. Practice facilitation to improve diabetes care in primary care: a report from the EPIC randomized clinical trial. Ann Fam Med. 2014;12(1):8–16. https://doi.org/10.1370/afm.1591 . Epub 2014/01/22.
Howard J, Shaw EK, Clark E, Crabtree BF. Up close and (inter)personal: insights from a primary care practice’s efforts to improve office relationships over time, 2003–2009. Qual Manag Health Care. 2011;20(1):49–61. https://doi.org/10.1097/QMH.0b013e31820311e6 . Epub 2010/12/31.
Nutting PA, Crabtree BF, Stewart EE, Miller WL, Palmer RF, Stange KC, Jaen CR. Effect of facilitation on practice outcomes in the National Demonstration Project model of the patient-centered medical home. Ann Fam Med. 2010;8. https://doi.org/10.1370/afm.1119 . PubMed PMID: 20530393; PMCID: PMC2885723. Suppl 1:S33-44; S92Epub 2010/06/16.
Shaw E, Looney A, Chase S, Navalekar R, Stello B, Lontok O, Crabtree B. In the moment’: an analysis of Facilitator Impact during a quality improvement process. Group Facil. 2010;10:4–16. Epub 2010/01/01. PubMed PMID: 22557936; PMCID: PMC3340924.
PubMed PubMed Central Google Scholar
Shaw EK, Ohman-Strickland PA, Piasecki A, Hudson SV, Ferrante JM, McDaniel RR Jr., Nutting PA, Crabtree BF. Effects of facilitated team meetings and learning collaboratives on colorectal cancer screening rates in primary care practices: a cluster randomized trial. Ann Fam Med. 2013;11(3):220–8. https://doi.org/10.1370/afm.1505 . S1-8. Epub 2013/05/22.
Agulnik A, Boykin D, O’Malley DM, Price J, Yang M, McKone M, Curran G, Ritchie MJ. Virtual facilitation best practices and research priorities: a scoping review. Implement Sci Commun. 2024;5(1):16. https://doi.org/10.1186/s43058-024-00551-6 .
Jamtvedt G, Young JM, Kristoffersen DT, O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006;2CD000259. https://doi.org/10.1002/14651858.CD000259.pub2 . Epub 2006/04/21.
Hysong SJ, Best RG, Pugh JA. Audit and feedback and clinical practice guideline adherence: making feedback actionable. Implement Sci. 2006;1:9. https://doi.org/10.1186/1748-5908-1-9 . Epub 2006/05/26.
Balasubramanian BA, Cohen DJ, Davis MM, Gunn R, Dickinson LM, Miller WL, Crabtree BF, Stange KC. Learning evaluation: blending quality improvement and implementation research methods to study healthcare innovations. Implement Sci. 2015;10:31. https://doi.org/10.1186/s13012-015-0219-z . Epub 2015/04/19.
Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci. 2013;8:117. https://doi.org/10.1186/1748-5908-8-117 . Epub 2013/10/04.
Aarons GA, Green AE, Trott E, Willging CE, Torres EM, Ehrhart MG, Roesch SC. The roles of System and Organizational Leadership in system-wide evidence-based intervention sustainment: a mixed-method study. Adm Policy Ment Health. 2016;43(6):991–1008. PubMed PMID: 27439504; PMCID: 5494253.
Smith JD, Polaha J. Using implementation science to guide the integration of evidence-based family interventions into primary care. Fam Syst Health. 2017;35(2):125–35. https://doi.org/10.1037/fsh0000252 . PubMed PMID: 28617015; PMCID: 5473179.
Stirman SW, Gutner CA, Langdon K, Graham JR. Bridging the Gap between Research and Practice in Mental Health Service settings: an overview of developments in implementation theory and research. Behav Ther. 2016;47(6):920–36. PubMed PMID: 27993341.
Jaen CR, Crabtree BF, Palmer RF, Ferrer RL, Nutting PA, Miller WL, Stewart EE, Wood R, Davila M, Stange KC. Methods for evaluating practice change toward a patient-centered medical home. Ann Fam Med. 2010;8(Suppl 1):S9–20. S92. Epub 2010/06/16. doi: 8/Suppl_1/S9 [pii]. 1370/afm. 1108. PubMed PMID: 20530398; PMCID: 2885721.
Li R, Simon J, Bodenheimer T, Gillies RR, Casalino L, Schmittdiel J, Shortell SM. Organizational factors affecting the adoption of diabetes care management processes in physician organizations. Diabetes Care. 2004;27(10):2312–6. Epub 2004/09/29. doi: 27/10/2312 [pii]. PubMed PMID: 15451893.
Rittenhouse DR, Casalino LP, Gillies RR, Shortell SM, Lau B. Measuring the medical home infrastructure in large medical groups. Health Aff (Millwood). 2008;27(5):1246–58. https://doi.org/10.1377/hlthaff.27.5.1246 . Epub 2008/09/11.
EvidenceNOW: Change Process Capability Questionnaire (CPC-Q). Scoring Guidance. Content last reviewed February 2019. Agency for Healthcare Research and Quality. Rockville, MD. https://www.ahrq.gov/evidencenow/results/research/cpcq-scoring.html .
Nutting PA, Crabtree BF, Miller WL, Stewart EE, Stange KC, Jaén CR. Journey to the patient-centered medical home: a qualitative analysis of the experiences of practices in the National Demonstration Project. Ann Fam Med. 2010;8(Suppl 1). https://doi.org/10.1370/afm.1075 . PubMed PMID: 20530394; PMCID: PMC2885728. S45-56; s92.
Aarons GA, Ehrhart MG, Farahnak LR. The implementation Leadership Scale (ILS): development of a brief measure of unit level implementation leadership. Implement Sci. 2014;9(1):45. https://doi.org/10.1186/1748-5908-9-45 . Epub 20140414.
Yin RK. Case study research and applications. Sage Thousand Oaks, CA; 2018.
Glaser BG. The constant comparative method of qualitative analysis. Soc Probl. 1965;12(4):436–45.
Coronado GD, Petrik AF, Vollmer WM, Taplin SH, Keast EM, Fields S, Green BB. Effectiveness of a Mailed Colorectal Cancer Screening Outreach Program in Community Health clinics: the STOP CRC Cluster Randomized Clinical Trial. JAMA Intern Med. 2018;178(9):1174–81. https://doi.org/10.1001/jamainternmed.2018.3629 .
Coronado GD, Rivelli JS, Fuoco MJ, Vollmer WM, Petrik AF, Keast E, Barker S, Topalanchik E, Jimenez R. Effect of Reminding Patients to Complete Fecal Immunochemical Testing: A Comparative Effectiveness Study of Automated and Live Approaches. Journal of general internal medicine. 2018;33(1):72 – 8. Epub 2017/10/10. https://doi.org/10.1007/s11606-017-4184-x . PubMed PMID: 29019046.
Rothery C, Strong M, Koffijberg HE, Basu A, Ghabri S, Knies S, Murray JF, Schmidler GDS, Steuten L, Fenwick E. Value of information analytical methods: report 2 of the ISPOR value of information analysis emerging good practices task force. Value Health. 2020;23(3):277–86.
Bacchetti P. Current sample size conventions: flaws, harms, and alternatives. BMC Med. 2010;8:17-. https://doi.org/10.1186/1741-7015-8-17 . PubMed PMID: 20307281.
Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci. 2011;4(5):332–7. https://doi.org/10.1111/j.1752-8062.2011.00347 . .x. PubMed PMID: 22029804.
Administration HRaS. Health Center Program. 2019.
Hong YR, Sonawane KB, Holcomb DR, Deshmukh AA. Effect of multimodal information delivery for diabetes care on colorectal cancer screening uptake among individuals with type 2 diabetes. Prev Med Rep. 2018;11:89–92. https://doi.org/10.1016/j.pmedr.2018.05.008 . Epub 2018/07/10.
Kiran T, Kopp A, Moineddin R, Glazier RH. Longitudinal evaluation of physician payment reform and team-based care for chronic disease management and prevention. CMAJ. 2015;187(17):E494–502. https://doi.org/10.1503/cmaj.150579 . Epub 2015/09/21.
Taplin SH, Haggstrom D, Jacobs T, Determan A, Granger J, Montalvo W, Snyder WM, Lockhart S, Calvo A. Implementing colorectal cancer screening in community health centers: addressing cancer health disparities through a regional cancer collaborative. Med Care. 2008;46(9 Suppl 1):S74–83. https://doi.org/10.1097/MLR.0b013e31817fdf68 . PubMed PMID: 18725837.
Coury J, Schneider JL, Rivelli JS, Petrik AF, Seibel E, D’Agostini B, Taplin SH, Green BB, Coronado GD. Applying the Plan-Do-Study-Act (PDSA) approach to a large pragmatic study involving safety net clinics. BMC Health Serv Res. 2017;17(1):411. https://doi.org/10.1186/s12913-017-2364-3 . Epub 2017/06/21.
Davis T, Arnold C, Rademaker A, Bennett C, Bailey S, Platt D, Reynolds C, Liu D, Carias E, Bass P III. Improving colon cancer screening in community clinics. Cancer. 2013;119(21):3879–86.
Davis SN, Christy SM, Chavarria EA, Abdulla R, Sutton SK, Schmidt AR, Vadaparampil ST, Quinn GP, Simmons VN, Ufondu CB, Ravindra C, Schultz I, Roetzheim RG, Shibata D, Meade CD, Gwede CK. A randomized controlled trial of a multicomponent, targeted, low-literacy educational intervention compared with a nontargeted intervention to boost colorectal cancer screening with fecal immunochemical testing in community clinics. Cancer. 2017;123(8):1390–400. https://doi.org/10.1002/cncr.30481 . Epub 2016/12/01.
Gwede CK, Sutton SK, Chavarria EA, Gutierrez L, Abdulla R, Christy SM, Lopez D, Sanchez J, Meade CD. A culturally and linguistically salient pilot intervention to promote colorectal cancer screening among latinos receiving care in a federally qualified Health Center. Health Educ Res. 2019;34(3):310–20. https://doi.org/10.1093/her/cyz010 . PubMed PMID: 30929015.
Dolan NC, Ramirez-Zohfeld V, Rademaker AW, Ferreira MR, Galanter WL, Radosta J, Eder MM, Cameron KA. The effectiveness of a physician-only and physician-patient intervention on Colorectal Cancer Screening discussions between providers and African American and latino patients. J Gen Intern Med. 2015;30(12):1780–7. https://doi.org/10.1007/s11606-015-3381-8 . Epub 2015/05/19.
Baker DW, Brown T, Buchanan DR, Weil J, Balsley K, Ranalli L, Lee JY, Cameron KA, Ferreira MR, Stephens Q. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–41.
Moullin JC, Dickson KS, Stadnick NA, Rabin B, Aarons GA. Systematic review of the Exploration, Preparation, implementation, sustainment (EPIS) framework. Implement Science: IS. 2019;14(1):1. https://doi.org/10.1186/s13012-018-0842-6 . PubMed PMID: 30611302.
Riehman KS, Stephens RL, Henry-Tanner J, Brooks D. Evaluation of Colorectal Cancer Screening in federally qualified Health centers. Am J Prev Med. 2018;54(2):190–6. https://doi.org/10.1016/j.amepre.2017.10.007 .
Leeman J, Askelson N, Ko LK, Rohweder CL, Avelis J, Best A, Friedman D, Glanz K, Seegmiller L, Stradtman L, Vanderpool RC. Understanding the processes that Federally Qualified Health Centers use to select and implement colorectal cancer screening interventions: a qualitative study. Transl Behav Med. 2019. https://doi.org/10.1093/tbm/ibz023 . PubMed PMID: 30794725. Epub 2019/02/23.
Calman NS, Hauser D, Schussler L, Crump C. A risk-based intervention approach to eliminate diabetes health disparities. Prim Health care Res Dev. 2018;19(5):518–22.
Download references
Acknowledgements
We would like to thank our FQHC participants for informing the intervention and their contributions to this work.
This research is supported by the National Cancer Institute (K99 CA256043/R00CA256043) The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by the funders.
Author information
Authors and affiliations.
Department of Family Medicine and Community Health, Research Division, Rutgers Robert Wood Johnson Medical School, 303 George Street, Rm 309, New Brunswick, NJ, USA
Denalee M. O’Malley, Benjamin F. Crabtree, Srivarsha Kaloth, Pamela Ohman-Strickland, Jeanne Ferrante & Shawna V. Hudson
Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA
Denalee M. O’Malley, Benjamin F. Crabtree, Pamela Ohman-Strickland, Jeanne Ferrante, Shawna V. Hudson & Anita Y. Kinney
Department of Biostatistics and Epidemiology, School of Public Health, Rutgers University, New Brunswick, NJ, USA
Pamela Ohman-Strickland & Anita Y. Kinney
You can also search for this author in PubMed Google Scholar
Contributions
D.O. conceptualized study and acquired funding; D.O and S.K. Writing – original draft; BFC, SK, PO, JF, SVH, AK reviewed, edited, and approved the manuscript.
Corresponding author
Correspondence to Denalee M. O’Malley .
Ethics declarations
Ethics approval and consent to participate.
This protocol has been approved by the Rutgers University Institutional. Review Board (Pro2020002075). All study participants will be asked to provide informed consent prior to participation in any phase of this research. All authors have completed ethics training. This research will be performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
O’Malley, D.M., Crabtree, B.F., Kaloth, S. et al. Strategic use of resources to enhance colorectal cancer screening for patients with diabetes (SURE: CRC4D) in federally qualified health centers: a protocol for hybrid type ii effectiveness-implementation trial. BMC Prim. Care 25 , 242 (2024). https://doi.org/10.1186/s12875-024-02496-0
Download citation
Received : 18 May 2024
Accepted : 26 June 2024
Published : 05 July 2024
DOI : https://doi.org/10.1186/s12875-024-02496-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Federally qualified health center
- Diabetes mellitus
- Hybrid 2 effectiveness-implementation
- Implementation planning
- Primary care safety-net
BMC Primary Care
ISSN: 2731-4553
- General enquiries: [email protected]

IMAGES
COMMENTS
Prevention of type 2 diabetes (T2D) is a great challenge worldwide. The aim of this evidence synthesis was to summarize the available evidence in order to update the European Association for the Study of Diabetes (EASD) clinical practice guidelines for nutrition therapy. We conducted a systematic review and, where appropriate, meta-analyses of ...
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. The Lancet Diabetes & Endocrinology 2015;3(11):866-875.
The European multi-center study of diabetes prevention by acarbose (STOP-NIDM) revealed that a 3-year-long acarbose drug intervention can decrease the risk of type 2 diabetes in people with IGT by 36%. 20 As previously mentioned, the reported studies were carried out in patients with abnormal glucose metabolism.
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
However, type 2 diabetes makes up more than 90% of diagnosed diabetes cases in the United States. 35 Thus, our findings largely reflect risk-factor treatment and control in those with type 2 diabetes.
Looking at a huge amount of data from the NHS Diabetes Prevention Programme, the paper concludes that these interventions represent a viable diabetes prevention strategy. Research article: Lemp et al.
Purpose of Review This article highlights foundational evidence, translation studies, and current research behind type 2 diabetes prevention efforts worldwide, with focus on high-risk populations, and whole-population approaches as catalysts to global prevention. Recent Findings Continued focus on the goals of foundational lifestyle change program trials and their global translations, and the ...
The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623-634.
This second international consensus report on precision diabetes medicine summarizes the findings from a systematic evidence review across the key pillars of precision medicine (prevention ...
The nutrition Consensus Report and four featured papers (2-5) in the special section on nutrition in this issue of Diabetes Care focus on nutrition therapy and medical nutrition therapy (MNT) in the management and prevention of diabetes.The Consensus Report, which is intended to update and replace the 2014 American Diabetes Association (ADA) nutrition position statement (), examines ...
Avoid foods that are "bad carbohydrates" — high in sugar with little fiber or nutrients: white bread and pastries, pasta from white flour, fruit juices, and processed foods with sugar or high-fructose corn syrup. 4. Eat healthy fats. Fatty foods are high in calories and should be eaten in moderation.
Prevention of type 2 diabetes (T2D) is a great challenge worldwide. The aim of this evidence synthesis was to summarize the available evidence in order to update the European Association for the Study of Diabetes (EASD) clinical practice guidelines for nutrition therapy. We conducted a systematic review and, where appropriate, meta-analyses of ...
We estimate that a population-wide strategy would reduce the number of diabetes cases by 60,000-85,000 in 2025 from an estimated 2 million cases under the status quo scenario. A high-risk prevention strategy would result in 106,000 to 150,000 fewer cases of diabetes in 2025, and surgically induced weight loss would result in 3,000-6,000 ...
Four dietary changes can have a big impact on the risk of type 2 diabetes. 1. Choose whole grains and whole grain products over refined grains and other highly processed carbohydrates. 2. Skip the sugary drinks, and choose water, coffee, or tea instead. 3. Choose healthy fats. 4.
Preventing Chronic Disease (PCD) is a peer-reviewed electronic journal established by the National Center for Chronic Disease Prevention and Health Promotion. PCD provides an open exchange of information and knowledge among researchers, practitioners, policy makers, and others who strive to improve the health of the public through chronic disease prevention.
Evidence acquisition: Lifestyle intervention studies for the prevention of Type 2 diabetes in adults with at least 6 months' follow-up, published between 1990 and 2011, were identified through searches of major electronic databases. External validity reporting was rated using an assessment tool, and all analysis was undertaken in 2011.
Efficacy of Lifestyle Interventions for Diabetes Prevention or Delay. Data from randomized controlled trials of individuals with IGT unequivocally show that lifestyle modification reduces diabetes incidence, improves glycemic control, and has beneficial effects on diabetes risk factors and its complications. 6-12 In the largest diabetes prevention study (n=3234), the U.S. Diabetes Prevention ...
Before developing type 2 diabetes, most people have prediabetes. This is when their blood sugar is higher than normal but not high enough yet for a type 2 diabetes diagnosis. In the United States, about 98 million adults have prediabetes; that's 1 in 3 people. There are usually no signs when you have prediabetes, which is why 81% of people don ...
The primary outcome is the development of diabetes, diagnosed by fasting or post-challenge plasma glucose concentrations meeting the 1997 American Diabetes Association criteria. The 3,000 participants will provide 90% power to detect a 33% reduction in an expected diabetes incidence rate of at least 6.5% per year in the placebo group.
Figure 1: Windows for Prevention of Type 1 Diabetes (T1D): This graphic illustrates how type 1 diabetes progresses. Genetic risk, combined with an unknown environmental trigger (s), is followed by inappropriate activation of the immune system to attack the insulin-producing β cells. The appearance of more than one islet-cell autoantibody in a ...
Researchers unveil comprehensive youth diabetes dataset and interactive portal to boost research and prevention strategies. ScienceDaily . Retrieved July 4, 2024 from www.sciencedaily.com ...
The stated goals of the Larry L. Hillblom Islet Research Center are to bring together a group of leading scientists to work in the center as a team focused on islet research with the overall goal of contributing toward, while providing leadership in, the worldwide efforts that will eventually lead to the prevention and cure of diabetes.
American Diabetes Association Professional Practice Committee. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S145-S157. doi:10.2337/dc24-S008
Research on Lifestyle Interventions to Prevent/Delay Type 2 Diabetes. The Diabetes Prevention Program (DPP) is the largest efficacy trial providing evidence that type 2 diabetes can be prevented or delayed in those at high risk ( 4 ). This research study, led by the National Institutes of Health, is a landmark trial.
Furthermore, this review highlighted the lack of data on factors likely to influence the acceptance or rejection of food and body norm systems conducive to the prevention and control of type 2 diabetes among migrants from SSA. Studies on these factors are needed. Future research could better document these factors.
The Diabetes Prevention Program (DPP) has been successfully translated across many real-world settings since the results of the landmark study were published ().Some populations are at relatively higher risk for type 2 diabetes, are less likely to have access to resources to prevent type 2 diabetes, or are medically underserved, so it is important to consider the effectiveness of the DPP ...
Objective To investigate the incidence of cardiovascular disease (CVD) overall and by age, sex, and socioeconomic status, and its variation over time, in the UK during 2000-19. Design Population based study. Setting UK. Participants 1 650 052 individuals registered with a general practice contributing to Clinical Practice Research Datalink and newly diagnosed with at least one CVD from 1 ...
As the burden of chronic disease grows, prevention must be prioritized and integrated into health care. These maturity phases and best practice recommendations can be used by any health care organization committed to diabetes prevention. Further research is suggested to assess the impact and adoption of diabetes prevention best practices.
The stress of dealing with racial discrimination can take a toll on the body. Diagnosis of heart disease, obesity, diabetes, high blood pressure, and kidney or liver disease is linked to the stress of racial discrimination. A person with any of these diseases, due to racism or other causes, has a higher risk of severe illness with COVID-19.
The research protocol can be adapted as a model to investigate the development of targeted cancer prevention strategies in additional chronically ill priority populations. ... identified and prioritized the selection of implementation strategies to improve CRC screening uptake for patients with diabetes. The IPG and research team iterated an ...