
Get instant access to detailed competitive research, SWOT analysis, buyer personas, growth opportunities and more for any product or business at the push of a button, so that you can focus more on strategy and execution.
Table of contents, creating a compounding pharmacy business plan.
- 13 May, 2024

Planning Your Compounding Pharmacy Business
When starting a compounding pharmacy business, careful planning is essential to lay the foundation for success. This section will guide you through two crucial aspects of the planning process: defining your niche and creating financial projections.
Defining Your Niche
Defining a clear niche is key to differentiate your compounding pharmacy from others and attract a specific target market. Consider the unique needs of your community and identify areas where you can provide specialized services. This can include compounding medications for specific patient populations, such as pediatrics, dermatology, hormone replacement therapy, or veterinary care.
By focusing on a niche, you can position your compounding pharmacy as a valuable resource for patients and healthcare providers, setting yourself apart from chain pharmacies. Emphasize the value and benefits of your customized medications and additional services, rather than competing solely on price. To promote your compounding services, develop cash-based marketing strategies and establish relationships with prescribers and patients in your community ( PCCA Blog ).
Financial Projections
Creating comprehensive financial projections is crucial to understand the potential profitability of your compounding pharmacy business. Financial projections should include estimates of revenue, expenses, and profitability over a specific period, typically three to five years.
When considering the financial aspect of your compounding pharmacy business, it’s important to note that compounding pharmacies can have higher net profit margins compared to retail pharmacies. According to Bryan Prescott, high-performing compounding-only pharmacies can achieve net profit margins of around 20%, significantly higher than the typical 3% net profit for retail pharmacies. Adding compounding services to an existing retail pharmacy can increase net profits by 8-12%.
To create accurate financial projections, consider factors such as the cost of acquiring or renting a suitable location, equipment and supply expenses, staffing costs, marketing expenses, and regulatory compliance costs. Conduct market research to estimate the demand for compounding services in your area and project your revenue based on potential customer volume and average transaction value.
It is also crucial to be aware of the challenges that independent compounding pharmacies may face, such as decreasing reimbursements and increasing DIR fees. However, by positioning your compounding pharmacy as a valuable clinical resource in the community, you can strengthen your financial position. The payoff for compounding pharmacies generally occurs within the first one to two years, with dedication and hard work leading to long-term financial success.
By carefully defining your niche and creating detailed financial projections, you can lay the groundwork for a successful compounding pharmacy business. These initial planning steps will help you establish a clear direction for your business and ensure its long-term profitability.
Essential Considerations
When planning to start a compounding pharmacy business, there are several essential considerations that must be taken into account. These include location selection, space planning, and ensuring safety.
Location Selection
Selecting the right location for your compounding pharmacy is crucial for its success. Considerations such as proximity to medical facilities, clinics, and hospitals, as well as the target customer base, should be taken into account. Additionally, assessing the competitive landscape and identifying any gaps in the market can help determine the ideal location.
It is important to note that regulations for compounding pharmacies can vary by state and locality. Familiarize yourself with the specific regulations for compounding pharmacies in your area to ensure compliance and to understand any additional requirements that may impact the choice of location.
Space Planning
Space planning is another critical aspect of setting up a compounding pharmacy. On average, a compounding pharmacy requires around 1,700 square feet of space. This space should be carefully designed to accommodate the various areas necessary for compounding, storage, dispensing, and administration.
Consider the layout and flow of the space to ensure efficient operations. Separate designated areas for compounding, quality control, storage of raw materials and finished products, and prescription filling. Adequate workspace, storage cabinets, and shelving should be incorporated to maintain organization and compliance with regulatory requirements.
Ensuring Safety
Ensuring safety is paramount in a compounding pharmacy. Compounded drugs have not undergone the same rigorous safety and efficacy testing as FDA-approved medications, making it crucial to maintain strict quality control measures.
To ensure patient and employee safety, it is important to adhere to proper sanitation and aseptic techniques. Inspections of compounding facilities have revealed concerning conditions, such as unsuitable sterilization methods and unhygienic practices near compounding areas. Operators should handle sterile drugs with proper protective measures to prevent contamination and patient harm.
Implementing a robust quality assurance program, including regular testing and monitoring, is essential. This helps maintain product integrity and ensures that compounded medications meet the required quality standards.
By carefully considering location selection, space planning, and safety measures, you can lay a solid foundation for your compounding pharmacy business. These essential considerations, along with a comprehensive compounding pharmacy business plan , will help guide your path to success in this specialized field of pharmacy.
Market Analysis and Trends
To create a successful compounding pharmacy business plan, it is crucial to conduct a thorough market analysis and stay updated on the latest trends in the industry. Understanding the compounding pharmacy market growth and implementing effective business strategies can contribute to the long-term success of your venture.
Compounding Pharmacy Market Growth
The compounding pharmacy market has experienced significant growth in recent years. From 2017 to 2022, the market witnessed growth from USD million to USD million, with a compound annual growth rate (CAGR) of %. It is estimated that the market will reach USD million by 2029.
Looking ahead, the global compounding pharmacy market is expected to continue its positive trajectory. Key players in the industry are implementing strategies that contribute to steady growth. The market is projected to experience significant growth between 2023 and 2030, with increasing opportunities for compounding pharmacies to thrive ( LinkedIn ).
To capitalize on this growth, it is essential to identify key market segments. The compounding pharmacy market has been divided into segments based on type, such as 503A compounding pharmacy and 503B compounding pharmacy, as well as application areas like pediatric, adult, geriatric, and veterinary. Understanding the specific needs and demands of these segments can help in tailoring your services and marketing strategies accordingly.
Business Strategies
Implementing effective business strategies is essential for success in the compounding pharmacy market. Several widely-used strategies can help boost market sales and attain a competitive advantage.
Market Segmentation : Dividing the market into distinct segments based on factors such as demographics, needs, and preferences allows for targeted marketing and personalized services. By understanding the unique requirements of different market segments, you can provide tailored solutions and enhance customer satisfaction.
Product Differentiation : Offering unique and innovative products can set your compounding pharmacy apart from competitors. By providing customized formulations, dosage forms, and delivery methods, you can cater to specific patient needs that may not be met by standard medications. This differentiation can attract a loyal customer base and contribute to business growth.
Cost Leadership : Implementing efficient operational processes and cost-effective pricing strategies can attract price-sensitive customers. By optimizing your supply chain, streamlining processes, and negotiating favorable agreements with suppliers, you can offer competitive pricing without compromising on quality.
Divergence : Introducing innovative services and diversifying revenue streams can help expand your business and reduce dependency on a single product or service. Consider offering additional services such as medication therapy management, medication synchronization, or partnering with healthcare providers to offer integrated care solutions.
To stay ahead of the competition and ensure your business plan aligns with the market trends, it is crucial to regularly monitor industry reports and news. This can provide insights into emerging technologies, regulatory changes, and evolving patient needs. By adapting your strategies and offerings accordingly, you can position your compounding pharmacy for long-term success in a dynamic market.
In the next sections, we will explore the regulatory landscape for compounding pharmacies, discuss financial projections, and provide insights on maximizing profitability through effective marketing strategies.
Regulatory Landscape
When starting a compounding pharmacy business, understanding the regulatory landscape is crucial. Compounded drugs, which are customized medications created by pharmacists to meet specific patient needs, are subject to regulations to ensure safety and effectiveness. In the United States, the regulatory framework for compounding pharmacies involves both FDA regulations and state board oversight.
FDA Regulations
Compounded drugs are technically considered “new drugs” under the Federal Food, Drug, and Cosmetic Act (FDCA) and are subject to its requirements. However, the FDA generally does not enforce new drug approval requirements for compounded drugs, as obtaining approval for each compounded drug produced for individual patients would be impractical for pharmacists ( NCBI Bookshelf ). Instead, the FDA provides oversight to compounded drugs through the issuance of a Compliance Policy Guide (CPG) and the Food and Drug Administration Modernization Act (FDAMA) of 1997.
Under Section 503A of the FDCA, pharmacists or physicians can compound drugs that meet certain conditions, such as having a valid prescription for an identified patient or being compounded in limited quantities based on a history of prescription orders. This section exempts compounded drugs from new drug approval, labeling, and certain current good manufacturing practice (CGMP) procedures. However, compounding pharmacies must comply with the conditions outlined in Section 503A and are primarily overseen by state boards of pharmacy.
Another category, known as 503B outsourcing facilities, was established under Section 503B of the FDCA. These facilities can voluntarily register with the FDA to compound drugs without patient-specific prescriptions and without restrictions on interstate distribution. Outsourcing facilities must comply with CGMP requirements, undergo inspections, and report adverse events. They are subject to more federal oversight than 503A compounding pharmacies and are required to register annually with the FDA ( NCBI Bookshelf ).
State Board Oversight
In addition to FDA regulations, compounding pharmacies are also subject to oversight by state boards of pharmacy. State boards play a crucial role in ensuring compliance with pharmacy practice acts, regulations, and guidelines specific to each state. They may have additional requirements for compounding pharmacies, including licensing, facility inspections, and adherence to specific compounding standards.
Pharmacists and pharmacy owners should familiarize themselves with the regulations and guidelines set forth by their respective state boards. Compliance with state regulations is essential to operate a compounding pharmacy legally and ethically. Staying informed about updates and changes in regulations is important to maintain compliance and provide safe and effective compounded medications to patients.
Understanding the regulatory landscape, including FDA regulations and state board oversight, is fundamental to establishing and operating a compounding pharmacy business. Compliance with these regulations ensures the safety and quality of compounded medications, providing peace of mind to both pharmacists and patients.
Financial Projection Model
A well-developed financial projection model is a crucial component of creating a comprehensive business plan for your compounding pharmacy. This model allows you to assess the financial viability and profitability of your business by projecting key financial outcomes over a specific period of time. The financial projection model provides valuable insights into the potential revenue, expenses, and overall financial performance of your compounding pharmacy.
Key Outputs
The Pharmacy Business 5-Year 3 Statement Financial Projection Model in Excel is an effective tool for preparing a financial projection for your compounding pharmacy. This model offers a versatile and user-friendly platform that covers various revenue streams, including retail sales, online sales, and clinic fees. It also includes a discounted cash flow valuation, enabling you to assess the value of your business over time.
The key outputs of this financial projection model include:
- Full financial statements (Income Statement, Balance Sheet, and Cash Flow Statement) on a monthly basis for up to 5 years.
- Annual summaries and a dashboard with various summaries, providing a concise overview of the financial performance.
- Compounded Annual Growth Rate (CAGR) calculations, which help you understand the growth trajectory of your compounding pharmacy.
- Key ratios that offer insights into the financial health and efficiency of your business.
- Charts displaying income statement and balance sheet projections, allowing for easy visualization and analysis.
- Revenue breakdowns by service category and volume, helping you assess the contribution of each revenue stream to your overall revenue.
These outputs provide a comprehensive view of your compounding pharmacy’s financial performance, allowing you to make informed decisions and adjustments to your business strategy as needed.
Customization Features
The financial projection model for a compounding pharmacy offers a range of customization features to tailor the model to your specific business needs. Some of the customizable inputs include:
- Business name and currency, allowing you to personalize the model to your compounding pharmacy’s identity and location.
- Projection period, enabling you to set the desired timeframe for your financial projections.
- Marketing costs, staff costs, and administrative costs, allowing you to input specific figures based on your business plan and market analysis.
- Sales tax applicability, ensuring accurate calculations and compliance with tax regulations.
- Opening balance sheet for existing businesses, allowing you to incorporate your current financial position into the projections.
- Income statement actuals for trend analysis, providing a basis for forecasting and evaluating future financial performance.
- Revenue assumptions and cost inputs, enabling you to estimate revenue streams and expenses based on market research and industry trends.
- Tax rates, dividend distributions, fixed assets, borrowings, and discount rate inputs, allowing you to adjust these variables based on your compounding pharmacy’s specific circumstances.
With these customization features, the financial projection model becomes a powerful tool that can be tailored to your compounding pharmacy’s unique requirements and goals.
By utilizing the financial projection model and customizing it to your compounding pharmacy, you can gain valuable insights into the financial aspects of your business. These insights will enable you to make informed decisions, plan for growth, and demonstrate the financial viability of your compounding pharmacy to potential investors or lenders.
Maximizing Profitability
When it comes to running a compounding pharmacy business, maximizing profitability is a key goal. By implementing effective strategies, pharmacists can ensure the financial success of their venture. Two important aspects to focus on are net profit insights and marketing strategies.
Net Profit Insights
According to Bryan Prescott, a pharmacist with experience in compounding pharmacies, adding compounding services to a pharmacy’s practice can significantly increase net profits. High-performing, compounding-only pharmacies have been known to operate with net profits of around 20%, which is considerably higher than the typical 3% net profit for retail pharmacies ( PCCA Blog ). The addition of compounding services can routinely increase net profits in the range of 8-12%.
To maximize net profit, it’s important to position the compounding pharmacy as a valuable clinical resource in the community. By offering customized medications, independent pharmacies can differentiate themselves from chain pharmacies and emphasize value over price. This can lead to improved profitability by attracting prescribers and patients who value the specialized services provided by compounding pharmacies.
Marketing Strategies
Effective marketing strategies are essential for promoting the compounding services offered by the pharmacy. Successful compounding pharmacy owners understand the importance of differentiating their products and services from chain pharmacies. By highlighting the value and clinical benefits of compounding, pharmacists can position their pharmacies as trusted resources for customized medications.
Cash-based marketing strategies play a crucial role in promoting compounding services. By targeting prescribers and patients who are willing to pay for specialized medications, pharmacists can generate demand and increase profitability. Effective communication about the benefits of compounding, both to prescribers and patients, is key in setting the compounding pharmacy apart from competitors ( PCCA Blog ).
Pharmacists considering opening a compounding-only or hybrid pharmacy (combining compounding and retail) should have some familiarity with compounding and understand the clinical possibilities it offers. Previous experience and the ability to effectively differentiate the pharmacy are crucial characteristics for success in the compounding pharmacy business. By leveraging their expertise and effectively communicating about their products and services, pharmacists can attract a loyal customer base and maximize profitability.
By focusing on net profit insights and implementing effective marketing strategies, pharmacists can maximize the profitability of their compounding pharmacy business. The combination of offering specialized compounding services and effective promotion can lead to financial success and establish the pharmacy as a valuable resource in the community.
Perform Deep Market Research In Seconds
Automate your competitor analysis and get market insights in moments
Create Your Account To Continue!
Automate your competitor analysis and get deep market insights in moments, stay ahead of your competition. discover new ways to unlock 10x growth., just copy and paste any url to instantly access detailed industry insights, swot analysis, buyer personas, sales prospect profiles, growth opportunities, and more for any product or business..

Connection denied by Geolocation Setting.
Reason: Blocked country:
The connection was denied because this country is blocked in the Geolocation settings.
Please contact your administrator for assistance.
- Chat with NCPA
Compounding
Pharmaceutical compounding has been a niche of pharmacists for hundreds of years. The practice has become more complex and regulated over time, but it still provides an excellent revenue opportunity for community pharmacies. There are numerous resources available about compounding including how to make it work in your business and how to comply with the myriad of laws and regulations. There are also many different degrees of compounding in a pharmacy setting, from nonsterile creams and tablets to sterile eye drops or other products. As you evaluate how compounding could fit into your current business operation, think about the following:
1. Consider your business . Is your pharmacy a traditional dispensing pharmacy or does it provide additional patient care services? Is there adequate space in the pharmacy for compounding? Do you have sufficient staff to support compounding services?
2. Consider your market . Are there other compounding pharmacies in the area? Are there physicians or veterinarians in the area that prescribe their patients compounded products? Are there enough patients near the pharmacy to support compounding services? What would differentiate your pharmacy over a competitor for compounding services?
3. Consider your customers . Have customers inquired about compounded products before? Does the patient population of your pharmacy frequently require compounded prescription drug products? Would starting compounding at your pharmacy help a significant amount of your patients and/or bring you a significant number of new patients?
Considering your pharmacy and the surrounding environment should give you a good idea of whether compounding services are needed in your area and whether starting them in your pharmacy would be a good decision for your business. Once you have decided to get into compounding pharmacy, you should also consider marketing, operations, management, and finances, as you would any other aspect of your business. Finally, you should become knowledgeable with all state and federal laws and regulations governing the practice of pharmacy as these relate to compounding.
There are many regulations for compounding because when managed improperly, pharmaceutical compounding can cause serious harm to patients. The United States Pharmacopeia (USP) sets standards for compounding which states and/or federal agencies may then choose to enforce. The USP General Chapters on compounding are considered the gold standard and should be adhered to in all compounding pharmacies even if your state has not adopted certain chapters. There are different standards for sterile, nonsterile, and hazardous drug compounding, so whichever you plan to provide in your pharmacy, ensure that you are adhering to all standards that apply. You may be surprised to know that some aspects of General Chapter <800> on hazardous drugs already apply to your pharmacy if your pharmacy supplies and handles even one hazardous drug. The General Chapters are frequently revised, so it is good to keep up with the updates as they come and make sure you are referring to the most recent version of the chapter.
- General Chapter <795> on Pharmaceutical Compounding of Nonsterile Preparations
- General Chapter <797> on Pharmaceutical Compounding of Sterile Preparations
- General Chapter <800> on Hazardous Drugs, Handling in Healthcare Settings
- General Chapter <1160> on Pharmaceutical Calculations in Prescription Compounding
The U.S. Food and Drug Administration provides guidance, compliance, and regulatory information for drug compounding. You should also become familiar with their information surrounding compounding if you want to start compounding services in your pharmacy. Some helpful pages from their website include laws and policies, oversight, and a question and answer page on the basics of compounding and the FDA.
It is important to also check the laws and regulations regarding compounding in pharmacies in your state. There can be some variation between states on things like requirements from the State Board of Pharmacy.
If you are unfamiliar with implementing compounding services or have questions about where to begin, besides contacting NCPA, another resource you may find helpful is the Professional Compounding Centers of America (PCCA) . You can find more information about what they do and how they can help at their website, which states that "PCCA is an independent compounding pharmacy's complete resource for fine chemicals, devices, equipment, training, and support."
As you can see, there are many moving parts to implementing compounding services in your pharmacy. However, the impact that doing so can have on your community and your business can make it all worthwhile. Beyond creating a plan for your store, following the guidance, standards, and regulations out there for compounding pharmacy, and potentially joining a compounding association, it is a great idea to get in touch with pharmacists and pharmacy owners who have implemented compounding services in their own stores. It can be incredibly helpful and beneficial to learn from someone who has done it before and has a working knowledge of how to succeed with compounding in a pharmacy. Reach out to contacts with this exposure, or contact us at NCPA and we can connect you with someone who has the knowledge and experience you are seeking.
Other Resources:
- Pharmacy Compounding Accreditation Board
- National Institute for Occupational Safety and Health (NIOSH) List of Antineoplastic and Other Hazardous Drugs
- CompoundingToday.com , a website brought to you by the International Journal of Pharmaceutical Compounding
- Compounding 2017 Preview
- USP 800: What you need to know for Community and LTC Pharmacy
Diversified Revenue Opportunities

Compounded Sterile Preparations Pharmacy

Compounded Sterile Preparations Pharmacy Specialty Certification (BCSCP)
Target Population: Pharmacists who are responsible for ensuring that sterile preparations meet the clinical needs of patients according to quality, safety, and environmental control requirements, regulations, and standards in all phases of preparation, storage, transportation, and administration.
Program Purpose: To validate that the pharmacist has the advanced knowledge and experience to ensure quality patient care and improve therapeutic outcomes and safety for medications that require sterile compounding.
Currently, there are more than 1,600 BPS Board-Certified Sterile Compounding Pharmacists.
Important Notice for Applicants:
USP approved and published updated versions of chapters <795> Pharmaceutical Compounding – Nonsterile Compounding and <797> Pharmaceutical Compounding – Sterile Preparations on November 1, 2022, and they are scheduled to become effective on November 1, 2023.
Recent updates to USP chapters <795> and <797> will be reflected on examination materials starting with the August/September 2023 examination window.
Chapter <800> became official on December 1, 2019, however, chapter <800> is considered informational by USP and not compendially applicable.
This information is offered by BPS to help reduce confusion regarding USP Chapters <795>, <797>, and <800>. There are other standards and regulations applicable to the Compounding Sterile Preparations Pharmacy examination. For the content outline for the BPS Compounded Sterile Preparations Pharmacy examination, click here .
For updates and timelines on the status of revisions to USP Chapters, click here: https://www.usp.org/compounding .
Compounded Sterile Preparations Pharmacy Specialty Council Members
The purpose of the BPS Specialty Councils is to develop standards and eligibility requirements for board certification, develop examinations and passing standards for certification, and review and approve professional development programs for recertification of board-certified pharmacists. Specialty council members are at the heart of the peer-reviewed and peer-developed nature of BPS Board Certification.

Hawkins is the Sterile Compounding Pharmacy Program Manager at the Lexington VA Health Care System (HCS) in Lexington, KY. She coordinates and oversees compounding activities, compliance, and quality assurance for the HCS’s main hospital and Community Living Center, as well as a variety of outpatient specialty clinics, including Urology, Ophthalmology, Infusion, Dermatology, and Chemotherapy. She represents her Veterans Integrated Services Network (VISN) on the National VA Compounded Drug workgroup. She has speaking experience at national and state-level conferences and has experience as an item writer, ghost writer, and a pharmacy compliance and patient safety consultant. She received her Doctor of Pharmacy from Ohio Northern University, has a teaching certificate from the University of Kentucky’s College of Pharmacy, and completed a PGY1 residency at Baptist Health Lexington. She is a graduate of the VISN 9 Midsouth Healthcare Network Leadership Institute.
Melton is an Adjunct Clinical Professor at the University of Michigan College of Pharmacy. He also works as a pharmacy specialist on the Compounding Compliance Team serving as a facilities management lead for over 20 sterile and non-sterile locations. He received his Doctorate of Pharmacy and Bachelors in Pharmaceutical Sciences from the University of Toledo.
Nicholas D. Baker, MPH, PharmD, BCPS, BCSCP is a Regional Director for Pharmacy Operations in northern California for Kaiser Permanente. He earned his Master of Public Health from San Jose State and his Doctor of Pharmacy degree from the University of the Pacific. His post graduate managed care residency training was completed at Kaiser Permanente in the central valley of California. He oversees pharmacy operations including budgeting, workflow design and implementation, policy development, regional training programs, complex supply chain logistics, business continuity planning, technology implementation, and resource planning for the ambulatory care infusion pharmacies in the region.
Robert Campbell, PharmD, BCSCP, currently serves as the Director, Standards Interpretation Group at The Joint Commission. In this role, Dr. Campbell is responsible for providing interpretation of Joint Commission standards in all Accreditation programs. He provides direction and leadership to surveyors and Standards Interpretation Group (SIG) staff addressing interpretation of standards. He also participates as a consultant in the development and revision of standards and supports ongoing accreditation services and special projects. Dr. Campbell also serves as the Director of Medication Management for the Joint Commission Enterprise. In this role, he functions as the subject matter expert for medication management related topics; assists with interpreting the intent of standards, as well as the development and revision of standards; provides guidance to organizations and Surveyors; and supports the accreditation and certification process across the Joint Commission Enterprise. Dr. Campbell continues to function as a Surveyor for The Joint Commission in the Hospital Accreditation and Critical Access Hospital Accreditation Programs, as well as a Reviewer in the Medication Compounding Certification Program to assess compliance with accreditation and certification program standards. He is a member of the Accreditation Council for the Joint Commission and the Co-Chair of the National Coordinating Council for Medication Error Reporting and Prevention.
Carroll is the Vice President of Pharmacy for Option Care Health, the nation’s largest independent provider for home infusion therapy. He has national responsibility for the companies 94 clean rooms as well as all sterile compounding related practices. Carroll has over 20 years in pharmacy leadership in both the home infusion and health system fields. He specializes in clean room and facility design as well as compliance with compounding and regulatory standards. Mike is a graduate of the University of Rhode Island where he received a Bachelor of Science degree. He sits on the Vermont Board of Pharmacy where he served as Chair for 2022-23.
Dr. Filibeck serves as Soleo Health’s, Vice President, Clinical Services and Quality, providing clinical pharmacy support for all Soleo Health branch locations. He received his Bachelor of Science in Pharmacy and Doctorate of Pharmacy degrees from the University of Michigan and his Master of Business Administration degree from Baldwin-Wallace College, Berea, Ohio. In his current role, Dr. Filibeck, along with other resources in the clinical services department, is responsible for developing policies/procedures, clinical protocols, reviewing clinical services, branch operational audits, regulatory and accreditation support. During his 40 years in the home and alternate site infusion industry, he has held a number of different roles, ranging from branch pharmacy manager to regional infusion manager for a large, national infusion company. He has been responsible for running the day-to-day operations (including reimbursement) of a hospital-based infusion program and has overseen clinical services in a large, multi-site region. Responsibilities have also included sales support, clinical protocol development, site evaluation and accreditation preparation. Dr. Filibeck has been very active in professional organizations including the American Society of Health-System Pharmacists and the National Home Infusion Association. He is also a member of AMCP, IgNS and NASP. Dr. Filibeck served on the USP Sterile Compounding Expert Panel from 2000 to 2010 during which time USP <797> was released (2004) and updated (2008). Filibeck has earned numerous recognitions, including ASHP Fellow and Distinguished Service Award and NHIA’s Volunteer of the Year Award and NHIA Fellow.
I am currently a pharmacy manager at Akron Children’s Hospital- Mahoning Valley Campus. I also act as the clinical specialist for our level 3 Neonatal Intensive Care Unit. I graduated from Ohio Northern University, received my PGY1 residency from Shands at the University of Florida, and my PGY2 from John Hopkins All Children’s Hospital. I am working toward my Masters in Business Administration. I live in Northeast Ohio with my husband and two teenage children. I look forward to working on the committee.
Dr. Hicks is a Director and Pharmacist in Charge at STAQ Pharma, an FDA registered Outsourcing Facility, where he is responsible for all compounding and manufacturing processes. Hicks specializes in cleanroom and facility design, automation and technology, and regulatory compliance. He received his Doctor of Pharmacy from the University of Toledo and spent 11 years in hospital and health-system pharmacy leadership before transitioning to industry. He enjoys the challenges of working in a highly regulated industry and believes the profession can both learn from and elevate pharmacy compounding towards cGMP.
Lazenby currently serves as Pharmacy Operations Manager at Howard University Hospital in Washington, DC. Previously he worked at The Queen’s Medical Center in Honolulu, Hawaii where he oversaw sterile compounding and chemotherapy operations and managed the related personnel for the state’s largest hospital and only Level 1 Trauma Center. Prior to the management post, he served as QMC’s Coordinator of Sterile Compounding and Hazardous Drug Operations and has been involved in multiple sterile compounding facility redesign projects. He earned his PharmD from West Virginia University.
Over the past thirteen years, Dr. Kane focused her career on compounding pharmacy. While working in community, institutional, and industry settings she developed extensive expertise on the evolving compounding regulation standards. Prior to beginning pharmacy school, Dr. Kane was laboratory manager for an independent community compounding pharmacy. Dr. Kane graduated from Chicago State University-College of Pharmacy with a PharmD degree in 2014. During her time there she focused her studies on improving the focus on compounding within the curriculum. She led the creation of compounding training videos and establishing CSU-COP as a competitor in the regional and national SPCC- Student Pharmacist Compounding Competition. After graduation, Dr. Kane expanded her knowledge by working on Phase 1 and Phase 2 clinical trials with a local drug manufacturer. Currently at University of Chicago, she is working on designing and constructing cleanroom spaces that meet the new cGMP and USP standards.
Denise Rodriguez has over two decades of leadership experience with dedication to the specialized field of compounding. In her current role as the Assistant Director of Pharmacy at The Methodist Hospital and Methodist Children’s Hospital in San Antonio, Texas, Denise is entrusted with oversight of regulatory and accreditation compliance for controlled substances, the safe handling of hazardous medications, nonsterile compounding, and sterile compounding. Denise previously served as Assistant Director for Compounding Quality and Compliance at The Johns Hopkins Hospital (JHH) in Baltimore, Maryland, leading the design, implementation, and oversight of a comprehensive quality assurance program to ensure the safe and effective compounding of medications at nine compounding facilities while upholding regulatory and accreditation requirements. During her tenure at JHH, Denise accomplished the effective integration of robotic enclosures, compounding workflow manager, and compounding compliance software. Denise’s additional achievements comprised of management of cleanroom suite renovation projects and chairing a compounding council to maintain quality control of compounding areas. Denise earned a Bachelor’s degree in pharmacy from Texas Southern University, a Master of Business Administration in Health Care Management at the Johns Hopkins Carey Business School, and is Board Certified as a Sterile Compounding Pharmacist.
Natalie Young serves as the Clinical Pharmacy Director at Vets Pets, overseeing pharmacy services for nearly 40 veterinary clinics and hospitals throughout North Carolina. Her primary focus is ensuring that all compounds meet the pinnacle of quality standards for their patients. With a significant background in pharmacy leadership, Natalie previously pioneered a sterile compounding veterinary-exclusive pharmacy in North Carolina. Under her guidance, this facility swiftly achieved accreditation and became synonymous with the highest compounding and regulatory standards. An alumna of the North Carolina School of Science and Mathematics, Natalie furthered her education at the University of North Carolina at Chapel Hill. Her dedication to the field is evident in her roles as the Compounding Chair for the American Pharmacists Association and the immediate Past-Chair of the American College of Veterinary Pharmacists. Moreover, she greatly enjoys mentoring the upcoming generation of pharmacists in her role as an Assistant Professor of Clinical Education at UNC Chapel Hill.
Eligibility Requirements for BCSCP
An applicant for board certification in Compounded Sterile Preparations Pharmacy must demonstrate all of the eligibility requirements listed below prior to sitting for the initial certification examination. Once all of the requirements below are met, an applicant will be deemed eligible to sit for the Compounded Sterile Preparations Pharmacy specialty certification examination. If an applicant achieves a passing score on the Compounded Sterile Preparations Pharmacy certification examination, they may use the designation Board-Certified Sterile Compounding Pharmacist, or BCSCP.
- Graduation from a pharmacy program accredited by the Accreditation Council for Pharmacy Education (ACPE) or a program outside the U.S. that qualifies the individual to practice in the jurisdiction.
- A current, active license/registration to practice pharmacy in the U.S. or another jurisdiction.
- Demonstration of practice experience through 4,000 hours of post-licensure or post-registration practice experience 1 in Compounded Sterile Preparations Pharmacy. The 4,000 hours may be earned in a variety of settings, such as accredited Compounded Sterile Preparations Pharmacy residency programs 2 . Practice hours in the activities listed in the Compounded Sterile Preparations Pharmacy Content Outline will be accepted as practice experience on an hour-for-hour basis.
1 All practice experience must be completed post-licensure/registration as a pharmacist. All applicants intending to demonstrate eligibility for any BPS certification examination utilizing the practice experience pathway must provide an attestation from their employer, on company letterhead, that verifies this experience accurately represents at least 50% of time spent in some or all of the activities defined by the applicable certification content outline. In addition, this practice experience must have occurred within the seven years immediately preceding the application. For more information, click here . A sample employer verification letter is available here .
2 American Society of Health-System Pharmacists (ASHP)-accredited/candidate status PGY1 pharmacy residency, residencies accredited under the ASHP Accreditation Standard for International Pharmacy Practice Residency Programs, or Canadian Pharmacy Residency Board (CPRB)-accredited Year 1 pharmacy residency.
The rationale for the appropriateness of the requirements for BPS certification programs are based upon the following:
- BPS recognizes individuals who graduate from a recognized school or college of pharmacy within the candidate’s jurisdiction. Those jurisdictions recognize and evaluate programs on the extent to which it accomplishes its stated goals and is consistent with the concept that pharmacy is a unique, personal service profession in the health science field. In the United States, the responsibility for recognizing schools and colleges of pharmacy falls to the Accreditation Council for Pharmacy Education (ACPE).
- The rationale for requiring licensure or registration of pharmacists within their jurisdiction is based upon the fact that for public protection, all pharmacists must be licensed or registered. This is considered a baseline requirement to be a pharmacist specialist. In the United States, BPS recognizes the licensure process administered by the National Association of Boards of Pharmacy (NABP). The National Association of Boards of Pharmacy (NABP) aims to ensure the public’s health and safety through its pharmacist license transfer and pharmacist competence assessment programs. NABP’s member boards of pharmacy are grouped into eight districts that include all 50 United States, the District of Columbia, Guam, Puerto Rico, the Virgin Islands, Bahamas, and all 10 Canadian provinces.
- The experiential component is required to help assure practical application of components of the specialty knowledge being certified. There are multiple pathways to meet the practice experience requirement. The faster eligibility pathways recognize accredited residencies through the American Society of Health System Pharmacists (ASHP). The ASHP residency accreditation program identifies and grants public recognition to practice sites having pharmacy residency training programs that have been evaluated and found to meet the qualifications of one of the ASHP’s residency accreditations standards. Thus, accreditation of a pharmacy residency program provides a means of assurance to residency applicants that a program meets certain basic requirements and is, therefore, an acceptable site for postgraduate training in pharmacy practice in organized health care.
- Passing the BPS pharmacy specialty examination helps assure knowledge consistent with the validated content outline for the BPS specialty.
The appropriateness of the BPS program requirements are consistent with the Council on Credentialing in Pharmacy’s Resource Paper titled: Scope of Contemporary Pharmacy Practice: Roles, Responsibilities, and Functions of Pharmacists and Pharmacy Technicians.
Upcoming Deadlines for Certification Examinations
Individuals who meet the eligibility requirements for the BCSCP examination can find more information about examination dates and fees for certification examinations here .
Candidate's Guide
The Candidate’s Guide is intended for use by pharmacists who are interested in becoming certified as specialists by BPS in any of the BPS-recognized specialty practice areas. To review critical information for BPS Certification Examinations, visit this page .
Content Outline for BCSCP Examinations
For the 2024 Examinations and forward , refer to the Compounded Sterile Preparations Pharmacy Content Outline found here for details.
The examination content outline is a product of a job analysis, also known as a role delineation study, that includes discussions with a panel of 15-20 subject matter experts who represent the specialty area. These experts determine the competencies required for safe and effective pharmacy practice in the specialty area and engage board-certified pharmacists through a validation survey for their endorsement of the identified competencies. The job analysis process is conducted every 5 years to help ensure that the competencies in the examination content outline reflect current pharmacy practice in the specialty area.
Click here to review the BCSCP Job Analysis Summary.
Important Resources
Preparatory courses for bcscp examinations.
- The study of journal articles, textbooks or other publications related to the Content Outline.
- Attendance at continuing education programs and courses in specialized pharmacy practice.
- Participation in study groups and examination preparation courses.
- Reviewing the sample examination items provided in order for candidates to familiarize themselves with the various item formats which are presented on the exam. Sample question performance should not be interpreted as an indicator of exam performance.
- American Pharmacists Association (202) 429-4125 https://www.pharmacist.com/Education/Board-Prep-Recertification/Sterile-Compounding
- American Society of Health-System Pharmacists (866) 279-0681 https://www.ashp.org/professional-development/board-certification-resources/compounded-sterile-preparations-pharmacy/compounded-sterile-prep-board-recertification
BPS partners with Prometric to provide the examination. BPS does not have any other partnerships for the certification or recertification application process. BPS partners with professional development program (PDP) providers to provide continuing pharmacy education (CPE) for recertification and the relationship is noted here. Any organization claiming a relationship with BPS for the application process or providing CPE labeled ‘BPS-approved’ outside of the organizations listed should be reported to BPS immediately.
Certification for Applicants Outside the U.S.
BPS would like to offer some helpful tips to candidates outside of the United States in order to make their application experience easier. To learn more about applying for board certification as a pharmacist outside of the U.S., visit this page .
Apply for ADA Accomodations
BPS complies with the relevant provisions of the Americans with Disabilities Act (ADA). For applicants looking to request special accommodations in their application process, more instructions can be found on this page .
Frequently Asked Questions
After review of the BPS Candidates Guide and specialty certification page, some applicants may still have questions. Visit this page to see frequently asked questions from pharmacists pursuing board certification like you!
Sample Examination Items
Sample items for bcscp examinations.
The sample examination items for BCSCP examinations are made available by BPS for the purposes of familiarizing certification candidates and other stakeholders with the structure and format of BPS certification examinations. This is not meant for use as a self-assessment. Performance on any of these items does not correlate with performance on the actual examination.
The content of these examples is meant to be illustrative of actual examination items, but these items do not appear on the certification examination and are not meant to identify the scope of the examination. For a more comprehensive indication of the scope of the certification examination, please refer to the BCSCP Exam Content Outline .
Examination items are in multiple-choice format. The great majority of examination items are multiple-choice with a single response from among four options. Some examinations may include a small percentage of items that require selection of multiple (three or four) responses from among a larger set of available (up to eight) options. Examinations items may also be supplemented by an image.
View the examination items down below.
A 503A compounding pharmacy at a health-system centralized compounding facility was inspected by the Food and Drug Administration (FDA) following a complaint. A FDA form 483 was issued to the pharmacy. What is the correct response from the pharmacy?
A hospital pharmacy received a shipment of antineoplastic hazardous drugs. Where should the shipment be unpacked?
Which organization regulates disinfectants by evaluating and registering manufacturer data submitted for microbiocidal activity, stability, toxicity, and contact time for labeling indicated uses?
When preparing a horizontal laminar airflow workbench for compounding a sterile product, compounding personnel should:
Compounding involving only transfer, measuring, and mixing manipulations using not more than three commercially manufactured sterile products and not more than two entries into any container describes what type of compounding risk level?
Which ingredient is incompatible and must not be added to a 3-in-1 parenteral nutrition admixture?
What is the most appropriate delivery route for a parenteral nutrition formulation with an osmolarity of 1,230 mOsm/L?
An emergency department nurse administered pancuronium instead of influenza vaccine to a patient. The patient experienced some respiratory depression but not permanent injuries. After a root cause analysis (RCA), the team determined the causes to be similar size and labels on the vials. Additionally, the look alike vials had been stored adjacent to each other in the refrigerator. What was the most appropriate action plan that resulted from the RCA?
How often must non-high risk compounding personnel demonstrate proficiency through hands-on testing/evaluation?
How often should personnel pass written and media-fill testing of aseptic work skills when working with sterile ingredients?
Share the quiz to show your results !
Subscribe to see your results, i got %%score%% of %%total%% right, recertification requirements for bcscp.
Pharmacists who earn the designation Board-Certified Sterile Compounding Pharmacist® (BCSCP) are required to maintain their certification over a seven-year period by completing one of the following recertification pathways:
Option One: Recertification Examination
- For BCSCP with certification beginning January 1, 2023 or earlier: Achieve a passing score on the recertification examination administered by BPS.
- For BCSCP with certification beginning January 1, 2024 or later: Achieve a passing score on the recertification examination administered by BPS and self-report 20 units of completed continuing professional development (CPD) in MyBPS. For more information on CPD, review the FAQ . To maintain an active certification in good standing, a minimum of two units of assessed CPE from BPS-approved professional development programs or self-reported CPD must be reported each year.
Option Two: Professional Development Program
- The American Pharmacists Association (APhA) , and/or
- The American Society of Health-Systems Pharmacists (ASHP) .
BCSCP may participate in recertification from any BPS-approved BCSCP programs.
Additionally, 20 units of continuing professional development (CPD) must be completed and self-reported in MyBPS. For more information on CPD, review the FAQ . To maintain an active certification in good standing, a minimum of two units of assessed CPE from BPS-approved professional development programs or self-reported CPD must be reported each year.
- BCSCP may participate in recertification from any BPS-approved BCSCP programs.
|
|
| |
| 2016 | 1/1/2017 | 12/31/2023 | 100 units assessed CPE via BPS-approved PDP |
| 2017 | 1/1/2018 | 12/31/2024 | 100 units assessed CPE via BPS-approved PDP |
| 2018 | 1/1/2019 | 12/31/2025 | 100 units assessed CPE via BPS-approved PDP |
| 2019 | 1/1/2020 | 12/31/2026 | 100 units assessed CPE via BPS-approved PDP |
| 2020 | 1/1/2021 | 12/31/2027 | 100 units assessed CPE via BPS-approved PDP |
| 2021 | 1/1/2022 | 12/31/2028 | 100 units assessed CPE via BPS-approved PDP |
| 2022 | 1/1/2023 | 12/31/2029 | 100 units assessed CPE via BPS-approved PDP |
| 2023 | 1/1/2024 | 12/31/2030 | 100 units (80 units assessed CPE via BPS-approved PDP + 20 units CPD) |
| 2024 onward | 1/1/2025 onward | 12/31/2031 onward | 100 units (80 units assessed CPE via BPS-approved PDP + 20 units CPD) |
For full details regarding recertification, please refer to the BPS Recertification Guide .
Board-Certified Sterile Compounding Pharmacists® are required to pay the BPS Annual Certification Maintenance fee of $125 each year for years one through six and the $400 recertification fee in year seven. Individuals with more than one BPS certification are assessed one BPS Annual Certification Maintenance Fee each year.
Upcoming Deadlines for Recertification
Candidates are required to recertify every 7 years. Certificants must submit their recertification application no later than the deadline of August 4. BPS encourages candidates to submit their recertification application as early as January 1 of their recertification year.
Candidates who intend to recertify via examination should note the availability of the recertification examination and related application deadlines. Candidates recertifying their BCSCP credential by examination can find more information about examination dates and fees for here .
Candidates who intend to recertify via continuing pharmacy education (CPE) MUST submit their recertification application by the deadline date of August 4 even if they have not completed their CPE requirements.
The deadline to complete the required CPE for recertification is December 31 for all specialties. The board-certified pharmacist is responsible for submitting an application that is completely and accurately filled out. Incomplete and/or unpaid applications will not be processed.
Recertification Guide
Cpe providers.
BCSCP with certification beginning January 1, 2023 or earlier: recertification via professional development program requires 100 units of assessed CPE from BPS-approved professional development programs offered by:
- The American Society of Health-Systems Pharmacists (ASHP) .
BCSCP with certification beginning January 1, 2024 or later: recertification via professional development program requires 100 units, comprised of 80 units of assessed CPE from BPS-approved professional development programs offered by:
After review of the BPS Recertification Guide and specialty page, some applicants may still have questions. Visit this page to see frequently asked questions from pharmacists renewing their board certification like you!
View the examination items down below.
Certification Verification
BPS offers the ability to search and verify a Board-Certified Pharmacist by name or credential number.

Board of Pharmacy Specialties
2215 Constitution Avenue, N.W. Washington, DC 20037-2985
202-946-5026 (Phone)

- Privacy Overview
- Strictly Necessary Cookies
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
Starting a sterile compounding pharmacy
Modular cleanroom expert Mecart provides a step-by-step guide to sterile compounding pharmacy as recommended by pharmacy regulatory inspectors
Deciding to build a sterile compounding pharmacy can be daunting, however experts at Mecart, the modular cleanroom manufacturer, are on hand to help - especially since its cleanrooms are recommended by the pharmacy regulatory inspectors.
Here are key insights from Mecart's experienced team when it comes to design a cleanroom for sterile compounding pharmacies.
1) Be aware of the applicable norms, standards and regulations
Approximately, there are three main aspects that determine which regulations apply to a compounding facility: the type of preparations that will be compounded (sterile or non-sterile, hazardous or non-hazardous); the geographic market in the compounding facility operates, both provincial/state and national (Canada, the US); and also the geographical markets in which the compounded preparations will be sold.
What type of products will be compounded?
- Non-Hazardous Sterile Compounding Preparations
- Hazardous Sterile Compounding Preparations
- Hazardous Non-Sterile Compounding Preparations
- Non-Hazardous Non-Sterile Compounding Preparations
Although it's not recommended, it is possible to compound both hazardous and non-hazardous preparations in two adjacent cleanrooms sharing the same anteroom and support zone, but strict conditions must be respected. Read the applicable norms for more information.
The most common regulations
- ISO 14644-1:2015
- The province/state of the pharmacy regulatory authority (eg. Ontario College of Pharmacists, College of Pharmacists of British Columbia, etc.)
In the following article, the focus will be on non-hazardous sterile compounding.
2) The typical layout for sterile compounding

Typical layout of a sterile compounding cleanroom
The areas reserved for compounding preparations must have at least two controlled, enclosed and distinct areas: a cleanroom (or buffer zone) in which the Primary Engineering Control (PEC) is located, and the anteroom. Some regulations strongly suggest a third room known as the support room, but this area is not necessarily enclosed nor is it classified.
Buffer Zone / Clean Room (ISO 7) The actual cleanroom or buffer zone/area is equipped with workstations. This is where the primary engineering control (PEC) (such as LAFW, BSC, CAI and CACI) is installed. To reduce the risk of introducing contaminants, the cleanroom must be isolated from the rest of the pharmacy as well as from unclassified areas. Walls, pass-throughs and doors serve to separate the areas. ISO Class 7 air quality must be maintained under dynamic operating conditions. To allow the pharmacist to observe activities being conducted inside, one or more observation windows must be installed. These windows are meant to reduce the non-essential back-and-forth entries into the control areas, as well as offering safety factors, (to be able to see what is going on inside the cleanroom in case of an emergency) and also for employees’ comfort, creating a more enjoyable, and thus productive work environment. Imagine being trapped in a room with no windows for hours on end!
Anteroom (ISO 7 or 8) The anteroom, also called ante-room, ante-area or antechamber, is a closed passage between the cleanroom and the support zone in which technicians perform support tasks (gowning, hand and forearm hygiene, labelling, etc.). It is equipped with two doors with a closing system that allows to open only one door at a time (interlock). It is actually a transitional space between the unclassified support zone and the cleanroom. The anteroom is usually equipped with a sink, cabinets and a bench, but equipment and activities in this area must be kept to a minimum. The anteroom can be engineered as an ISO 7 or ISO 8 environment depending on the risk level (HD or non-HD) of the sterile products being prepared in the critical area. Everything that enters the cleanroom must be taken through the anteroom.
Area for unpacking and storing (HD products) For hazardous product compounding, an unpacking and storing zone in a properly ventilated room with air being exhausted to the exterior may be required.
Mecart's top tip! Define the mechanical, electrical and plumbing requirements of the sterile compounding facility. The mechanical aspects focus on heating, cooling and ventilation, the electrical aspects focus on providing power to all outlets and appliances and the plumbing aspects focus on the delivery of water and the draining of waste water. Cleanrooms usually have their own electrical panel. It is important to ensure that the building can supply the electrical power. The cleanroom HVAC (cooling and humidifying) and the anteroom’s sink require drainage and water supply.
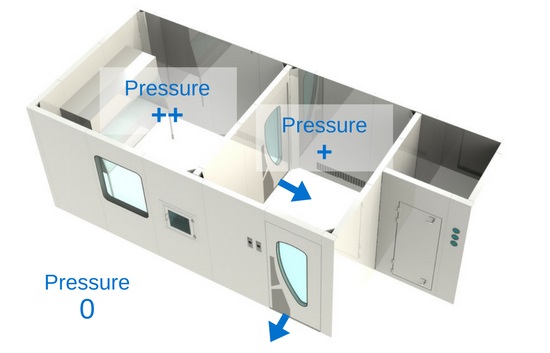
3) Pressure differential
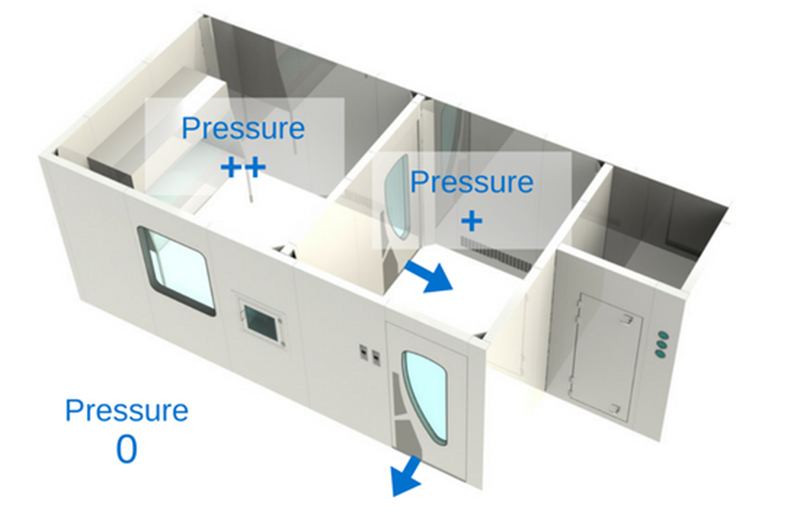
Pressure cascade differential in a cleanroom
The most important thing between these three zones is the pressure differential.
Cleanrooms are held in positive pressure, except when dealing with the compounding of hazardous preparations , which must be held in negative pressure. The most important thing between these three zones is the pressure differential. It is crucial that the pressure gradient of the clean zone be greater (at least 5 Pa) than the anteroom’s, and that the anteroom’s pressure gradient be greater (at least 5 Pa) than the support zone’s. Positive pressure will make the air flow out of the room instead of in. This means that the air in the cleanroom will have a tendency to leak out of the room, instead of in, thus preventing unfiltered air or air particulates from entering. That being said, in a hazardous compounding facility, it is the opposite: the pressure is negative in order to prevent air that might be contaminated by hazardous products from escaping the room. Therefore, the negative pressure pushes the air towards the “clean zone”.
Mecart's top tip! HVAC for cleanrooms are different from conventional ones. The HVAC system (cleanroom ventilation and filtration) must be specified by HVAC cleanroom specialists in order to choose and engineer the appropriate system and maintain the required parameters (pressure differential, temperature, relative humidity).
4) Heating, ventilation and air conditioning system (HVAC)
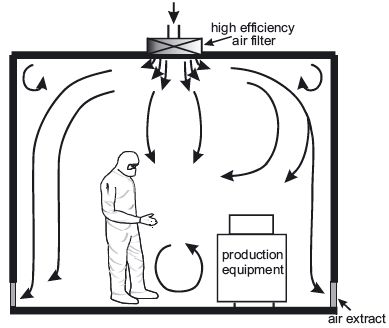
Non-unidirectional airflow
The HVAC system is the most important part of a cleanroom since it controls the supply of clean air to the cleanroom. The air intake comes from the ceiling, and is filtered through HEPA filters. The new air travels down the room, pushing the contaminated air to exits through return air intakes at the bottom of the walls. The air conditioning system is important for the employees’ comfort since wearing personal protective equipment can be quite hot. The air changes per hour (ACPH) must be of at least 30 in the cleanroom and of 20 ACPH in the anteroom, but may have to be greater depending on the size of the room, the number of people working inside, etc.
Mecart's top tips! Even though regulations recommend 30 changes per hour for the cleanroom and 20 for the anteroom, Mecart' standard practice is to design cleanroom HVAC with a slightly higher air changes per hour, for example 40 ACPH (instead of 30) for the cleanroom and 30 ACPH (instead of 20) for the anteroom. This higher rate increases the window of opportunity to notice a problem and fix it without having to stop operations inside the cleanroom. For example, if a HEPA filter gets dirty, the cleanroom’s higher ACPH will compensate and the cleanroom will remain compliant, giving the owner time to replace it. Another common example is if you decide to make small changes to the configuration inside your cleanroom. With higher air changes per hour, the air quality is less likely to be affected by the new configuration. Finally, a higher ACPH can also compensate for a human errors.
Another important aspect in cleanroom design and which is often forgotten is humidity. Most regulations do not mention anything about this functional parameter, however it is important for employees’ comfort. From Mecart's experience, the cleanroom tends to become very dry so they often suggest that clients should install a humidifier in their cleanrooms.
Featured companies
You may also like
Mecart converts old data centre into a cutting-edge semiconductor fab, trending articles.
- What is ISO 8 cleanroom classification? Each cleanroom class is denoted by a maximum concentration of particles per cubic meter or cubic foot of air. ISO 8 is the second lowest cleanroom classification
- Novartis and Sandoz support founding of Slovenian Cleanroom Society The professional association, Slovenian Cleanroom Society, has been founded to serve the cleanroom technology and contamination control sector in the country
- EU GMP Annex 1 compliance: should you get new equipment or upgrade? Buy a new machine or upgrade the existing equipment? In light of Annex 1, pharmaceutical manufacturers and contract manufacturing organisations are facing this question more than ever. Steffen Mueller discusses the solution Syntegon worked on with AJ Vaccines
Relevant companies
Upcoming event, mecart completes 300 sqft ips cell cleanroom, mecart announces $2m investment in the us, vaccine manufacturing facilities and cleanrooms explained, mecart cleanrooms obtains fm approval for cleanroom panels, six things to consider for a cgmp cell and gene therapy cleanroom, mecart supplies modular isolation units to montreal hospital.
New Catalog
Download our Cleanroom Catalog
Home / Learning center / Starting a Sterile Compounding Pharmacy – Part 1
Starting a Sterile Compounding Pharmacy

Considerations in Cleanroom Design
If you’ve decided to launch your sterile compounding pharmacy , but you don’t know anything about how to build the cleanroom , you can trust Mecart to help. Mecart cleanrooms are recommended by pharmacy regulatory inspectors. We have the know-how to build your sterile compounding cleanroom and we guarantee it will be compliant with required norms. In the past few years, we’ve been involved with several pharmacists who were looking for sterile compounding cleanrooms and we know how challenging a project like this can be.
In hopes of making things clearer to you, here are some insights about cleanroom design for sterile compounding pharmacies.
1) Be aware of the applicable norms, standards and regulations
2) the typical layout for sterile compounding, 3) pressure differential, 4) heating, ventilation and air conditioning system (hvac).
—> You might also like this article: Building a USP 800 Compliant Compounding Space
Which norms, standards, and regulations must you comply with? This is one of the first questions we ask our clients when they call for a cleanroom quote. Roughly speaking, there are three main aspects that determine which regulations apply to your compounding facility: the type of preparations that will be compounded (sterile or non-sterile, hazardous or non-hazardous); the geographic market in which you will operate, both provincial/state and national (Canada, USA); and also the geographical markets in which your compounded preparations will be sold.
What type of products will be compounded?
- Non-Hazardous Sterile Compounding Preparations
- Hazardous Sterile Compounding Preparations
- Hazardous Non-Sterile Compounding Preparations
- Non-Hazardous Non-Sterile Compounding Preparations
Although it is not recommended, it is possible to compound both hazardous and non-hazardous preparations in two adjacent clean rooms sharing the same anteroom and support zone, but strict conditions must be respected. Read the applicable norms for more information.
The most common regulations
- ISO 14644-1:2015
- Your province/state pharmacy regulatory authority (eg. Ontario College of Pharmacists , College of Pharmacists of British Columbia , etc.)
In the following article, the focus will be on non-hazardous sterile compounding.
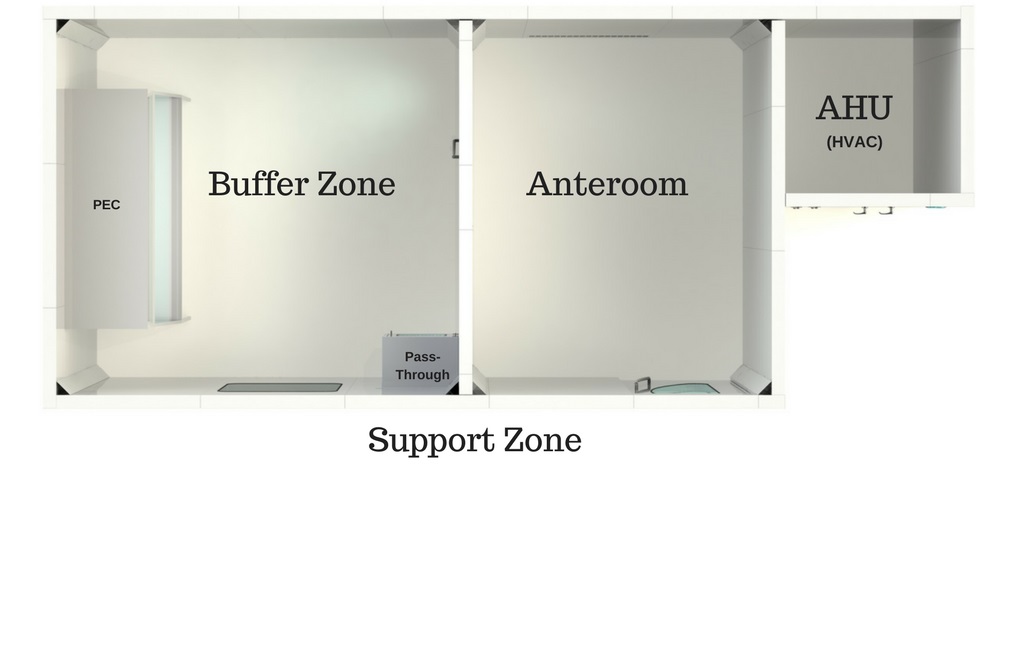
The areas reserved for compounding preparations must have at least two controlled, enclosed and distinct areas: a clean room (or buffer zone) in which the Primary Engineering Control (PEC) is located, and the anteroom. Some regulations strongly suggest a third room known as the support room, but this area is not necessarily enclosed nor is it classified.
- Buffer Zone / Clean Room (ISO 7)
The actual clean room or buffer zone/area is equipped with workstations. This is where the primary engineering control (PEC) (such as LAFW, BSC, CAI and CACI) is installed. To reduce the risk of introducing contaminants, the clean room must be isolated from the rest of the pharmacy as well as from unclassified areas. Walls , pass-throughs and doors serve to separate the areas. ISO Class 7 air quality must be maintained under dynamic operating conditions. To allow the pharmacist to observe activities being conducted inside, one or more observation windows must be installed. These windows are meant to reduce the non-essential back-and-forth entries into the control areas, as well as offering safety factors, (to be able to see what is going on inside the cleanroom in case of an emergency) and also for your employees’ comfort, creating a more enjoyable, and thus productive work environment. Imagine being trapped in a room with no windows for hours on end!
- Anteroom (ISO 7 or 8)
The anteroom, also called ante-room, ante-area or antechamber, is a closed passage between the clean room and the support zone in which technicians perform support tasks (gowning, hand and forearm hygiene, labelling, etc.). It is equipped with two doors with a closing system that allows to open only one door at a time (interlock). It is actually a transitional space between the unclassified support zone and the clean room. The anteroom is usually equipped with a sink, cabinets and a bench, but equipment and activities in this area must be kept to a minimum. The anteroom can be engineered as an ISO 7 or ISO 8 environment depending on the risk level (HD or non-HD) of the sterile products being prepared in the critical area. Everything that enters the clean room must be taken through the anteroom.
- Support Zone
The support zone is not mentioned in every regulation. It is an unclassified zone that may be enclosed or not, and is used for material storage, validation, entry of prescriptions, etc.
- Area for unpacking and storing (HD products)
For hazardous product compounding, an unpacking and storing zone in a properly ventilated room with air being exhausted to the exterior may be required.
Our team’s tips!
You must define the mechanical, electrical and plumbing requirements of the sterile compounding facility. The mechanical aspects focus on heating, cooling and ventilation, the electrical aspects focus on providing power to all outlets and appliances and the plumbing aspects focus on the delivery of water and the draining of waste water. Cleanrooms usually have their own electrical panel. It is important to ensure that the building can supply the electrical power. The cleanroom HVAC (cooling and humidifying) and the anteroom’s sink require drainage and water supply.
Need help with the design of your cleanroom?
Cleanrooms are held in positive pressure, except when dealing with the compounding of hazardous preparations, which must be held in negative pressure. The most important thing between these three zones is the pressure differential. It is crucial that the pressure gradient of the clean zone be greater (at least 5 Pa) than the anteroom’s, and that the anteroom’s pressure gradient be greater (at least 5 Pa) than the support zone’s. Positive pressure will make the air flow out of the room instead of in. What this means is that the air in the clean room will have a tendency to leak out of the room, instead of in, thus preventing unfiltered air or air particulates from entering. That being said, in a hazardous compounding facility, it is the opposite: the pressure is negative in order to prevent air that might be contaminated by hazardous products from escaping the room. Therefore, the negative pressure pushes the air towards the “clean zone”.
HVAC for cleanrooms are different from conventional ones. The HVAC system (cleanroom ventilation and filtration) must be specified by HVAC cleanroom specialists in order to choose and engineer the appropriate system and maintain the required parameters (pressure differential, temperature, relative humidity).
The HVAC system is the most important part of a cleanroom since it controls the supply of clean air to the cleanroom. The air intake comes from the ceiling , and is filtered through HEPA filters. The new air travels down the room, pushing the contaminated air to exits through return air intakes at the bottom of the walls. The air conditioning system is important for the employees’ comfort since wearing personal protective equipment can be quite hot. The air changes per hour (ACPH) must be of at least 30 in the clean room and of 20 ACPH in the anteroom, but may have to be greater depending on the size of the room, the number of people working inside, etc.
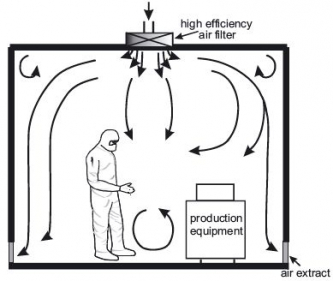
- Even though regulations recommend 30 changes per hour for the clean room and 20 for the anteroom, we always design our cleanroom HVAC with a slightly higher air changes per hour , for example 40 ACPH (instead of 30) for the clean room and 30 ACPH (instead of 20) for the anteroom. This higher rate gives you time to notice a problem and fix it without having to stop operations inside the cleanroom. For example, if a HEPA filter gets dirty, the cleanroom’s higher ACPH will compensate and your cleanroom will remain compliant, giving you time to replace it. Another common example is if you decide to make small changes to the configuration inside your cleanroom. With higher air changes per hour, the air quality is less likely to be affected by the new configuration. Finally, to err is human, therefore even though you have a strict operating protocol, a higher ACPH can also compensate for a human mistake.
- Another important aspect in cleanroom design and which is often forgotten is humidity . Most regulations do not mention anything about this functional parameter, however it is important for your employees’ comfort. From our experience, the clean room tends to become very dry. For this reason, we often suggest our clients to install a humidifier in their cleanrooms. You can be sure your employees will be thanking you for it.
The information, statements, opinions and analyses provided by MECART in this document is designed to provide helpful and educational information on the subjects discussed. The views and opinions expressed herein constitute the judgment of the authors and are subject to change without notice. MECART has made every reasonable effort to ensure the accuracy of all the information contained in this document. However, MECART makes no representations or warranties whatsoever whether express or implied as to the accuracy, validity, correctness, currentness, completeness, suitability for any purpose of such information. Any project is different in nature and the statements provided herein shall not be deemed appropriate or relied upon for any specific application or project without subsequent analysis, evaluation, verification and assessment. MECART hereby declines any liability for any error, damage, loss, injury or other consequence which may arise from use in any manner of any information contained in this document. The advice of a competent professional should be sought before entering in any project.
By continuing to use the site you agree to our privacy policy .

- Contact a Pharmacy Consultant
- Meet Our Team
- Our Partners
- Buying Group Services
- International Pharmacy Services
- Testimonials
- Videos & Webinars
- Pharmacy Operational Evaluations
- Pharmacy Location Feasibility Studies
- Buy A Pharmacy
- Selling Your Pharmacy
- Pharmacy Transfer Consulting
- Pharmacy Valuation Services
- Pharmacy Staffing (PA Owners Only)
- Turnkey Pharmacy Ownership Programs
- Health System Outpatient Pharmacy
- Are You Ready For Pharmacy Ownership?
- Open A Startup Pharmacy
- Fraud, Waste and Abuse Compliance Program
- Illinois Sexual Harassment Program
- Point of Care Testing
- USP795 Pharmaceutical Compounding – Nonsterile Preparations
- USP800 Hazardous Drug
- Controlled Drug and Red Flag Program
- COVID-19 and Bloodborne Pathogen Exposure Control Plan
- OIG and SAMS Exclusion Database Check
- Pharmacy Automation Program
- Hazard Communication Standard (OSHA)
- Cultural Competency and Non-Discrimination
- Track and Trace (DSCSA Compliance)
- Vaccine Best Practices
- Pharmacy Operations Manual
- License and Certification Expiration Tracking
- Medication Error Prevention and Documentation
- Medicare Part B Compliance (Drug and DMEPOS)
- New York State Sexual Harassment Program
- New York State HERO Act Program
- Pseudoephedrine Compliance Program
- Technician Protocols
- Form I-9 Compliance
- Employee Handbook Templates
- Pharmacy Group Compliance (GPO/PSAO)
NASI DMEPOS Accreditation
- Pharmacy Technician Immunization Training
- RX CHECK-UP™
How to Open a New Pharmacy
Opening an Independent Pharmacy from scratch is a challenging process. From choosing your location to forming a business entity, there’s much consider. In this article, we break down the steps you should follow when starting a Pharmacy, and provide you with information on the costs of opening a Pharmacy both legally and efficiently.
1. Choose a Location for Your Independent Pharmacy
Opening a Pharmacy begins with choosing the right location. You must consider factors including the location of your competitors, accessibility, parking availability, and the economic state of your trading area.
To begin, conduct a Pharmacy Feasibility Study , or site audit. Our Professional Site Feasibility Report evaluates the relevant market data to help you decide if you’re starting a Pharmacy in the right location – here’s how it works.
First, we gather as much information as possible on the area and competitors’ performance o determine if a new Pharmacy will be successful at the proposed location. Then, we run a closer analysis to determine some financial projections to better inform your decision-making. By investing some time and money at the outset, you are doing everything possible to ensure the success of your new Pharmacy venture.
You may be tempted to rely on the number of scripts written by physicians in the area to determine the right location for starting a Pharmacy. However, the majority of customers use Pharmacies located close to where they shop and live. Often physicians, especially specialists, draw patients from outside the community, and they’re unlikely to become your customers.
Rather than focusing solely on the number of local scripts written, focus instead on:
- the geographical trade area
- the population demographics within the trade area
Accurate demographics, considered alongside the average cost per script and the number of scripts filled per age range in that trade area, makes it possible to accurately formulate the amount of total prescription revenue available. Once you calculate this figure, you can then project how much of that revenue the Independent Pharmacy can reasonably expect to capture.
“Captured revenue” comes down to the number and type of competitors. Opening an Independent Pharmacy means convincing customers to switch from their current Pharmacy. How likely is that to happen? Usually, it is much harder to compete with another independent than it is with a major chain like a CVS or Rite Aid. Supermarket Pharmacies are tough competitors, because they have the advantage of the one-stop-shopping concept, which appeals to consumers.
Next, consider what the location itself offers. What can you offer to differentiate yourself from the existing competition? Note anything that impedes access to your Pharmacy, too, such as interstates, rivers, or natural terrain.
Once you build a complete picture of the trade area with the estimate of patients captured during the first two years, you can create realistic financial projections.
Project the amount of revenue, estimated gross profits, expenses, and if there’s a net operating profit or loss. With exceptions, most new Independent Pharmacies will operate at a loss for about 12-24 months before reaching a breakeven point.
2. Create a Business Plan
Before obtaining a start-up Pharmacy loan, you need a solid Pharmacy business plan. A good Pharmacy business plan should include:
- a mission statement
- an executive summary
- the services provided
- a location analysis
- profit projections
The costs of opening a Pharmacy vary, but include a full breakdown of costs and how much capital will be needed for construction, inventory, equipment, technology, employees, marketing, and operating expenses after opening to reach a breakeven point.
When you’re writing a business plan, less is generally better. Keep it concise and include any relevant, more detailed data supporting the opening of a Pharmacy as attachments. Remember, lenders only want to know how they’re getting their money back, so extraneous details are unnecessary. The experts at PRS Pharmacy Services can help you determine what to include in your plan.
3. Arrange Your Financing
Apply for a loan by presenting the business plan to lending institutions. A solid plan shows the bank that your proposed Independent Pharmacy is a sound investment, but be prepared to answer whatever follow-up questions your lender asks, including questions concerning your personal finances and Pharmacy management experience.
There are a few Pharmacy – specific banks that offer loans to start-up Pharmacies . These banks have experience in the Pharmacy sector and understand the loan process for a start-up Pharmacy better than most community banks. A typical start-up Pharmacy loan will be between $500,000-$700,000, and require a down payment of 20% cash. As stated earlier, most start-up Pharmacies will not break even point until 12-24 months post-opening, so starting a new Independent Pharmacy means having sufficient capital to cover costs and operating expenses until this point.
4. Set Up the Business Entity
Any new business needs to obtain a Federal Employer Identification Number (FEIN) through the IRS to operate and hire employees. In addition, whether you do it yourself or hire a firm, you must create a business entity, such as an LLC with S Corp tax designation to protect your personal assets from potential liabilities. Most business attorneys can assist with this process and guide you in the right direction.
5. Choose Your Wholesaler or Buying Group
Choosing a wholesaler is important, and Pharmacies need to do their homework and select the right partner for their business. Carefully consider what each wholesaler has to offer and how that fits into your Pharmacy’s business plan and objectives.
In almost all cases, it may be more beneficial to join a Pharmacy buying group . The buying group takes the total book of business for all its member Pharmacies combined to negotiate pricing and services with wholesalers and other vendors. When Pharmacies combine their purchase volume, they get the wholesalers to fight for their business, rather than the other way around, which gives them the power to demand a better deal. They also provide other benefits such as a generic drug program, return policy, and services to help run the Pharmacy more efficiently. Services provided vary per group, so select the one that best fits programs the Pharmacy plans on offering.
6. Implement Your Technology
When you’re opening up a Pharmacy, technology is vital. The basic technology you will need includes operating software, POS, printers and scanners, and internet and phone services. However, your technology shouldn’t stop there.
To run a Pharmacy efficiently, you need a system capable of supporting your backend processes such as workflow management, inventory control, financial reporting, and patient support services. This technology must be seamlessly implemented to improve your service offerings and streamline your day-to-day processes to increase profitability. Speak to your team, get a sense of what services they need to improve their efficiency, and schedule some demos from software providers.
Technological tools are a necessary cost of opening a Pharmacy. If you’re unsure how to choose the right technology solution for your business, consult a professional familiar with Pharmacy systems for advice.
7. Understand the Legal Protocols and Procedures Involved
Opening an Independent Pharmacy is a challenging process, especially given the number of state and federal laws and regulations you must follow. You must allow adequate time to fulfill your licensing and contracting obligations – otherwise, you’ll incur unnecessary expenses and difficulties.
- If you underestimate the time it takes to license your Pharmacy, you will suffer delays in opening.
- If you’re unaware of how long it takes to complete your PBM contracts, you will let customers down because you can’t handle their prescriptions, which means you’ll lose revenue.
- By underestimating how long it will take to open your Pharmacy, you could face various cash flow problems which disrupt your revenue potential.
- Finally, it’s very frustrating if you can’t open when planned because you haven’t completed your licensing requirements.
Pharmacy Permit Application
Before meeting with the landlord, architect, or construction manager, investigate/obtain your State Board of Pharmacy Rules and Regulations as they pertain to the application process, facility requirements, and inspection guidelines. You will need answers to questions like the following.
- What is the required Pharmacy equipment and reference library?
- Is a copy of the lease agreement or a wholesaler affidavit part of the application?
- What are the security, square footage and counseling area requirements?
- Does the computer need to be installed with the ability to print a label at the inspection?
- Do I need to submit a schematic drawing of the layout and design with the application?
- How long does it take to approve an application, schedule an inspection, and receive the Pharmacy permit number?
State Controlled Substance Registration (if Applicable)
Not all states require obtaining a state-controlled substance registration. Some states automatically provide this number along with your Pharmacy permit (with an additional fee), while others may require an additional application and submission to another agency. Understand what is involved and how to obtain this registration.
Drug Enforcement Administration (DEA)
The dea registration certificate requires obtaining a pharmacy permit and a state-controlled substance registration (if applicable) first. .
Reach out to the local DEA field office to inquire on their requirements for enrollment. In some areas, an inspection may be required and, if so, will add time onto the opening timelines.
National Provider Identifier (NPI)
The NPI is an identification number for health care providers. It’s used by all health plans. Most Pharmacists have their own NPI number;, however, for a start-up Pharmacy, you will need to obtain a facility NPI. This takes about 15 business days for enrollment.
National Council for Prescription Drug Programs (NCPDP)
This is a unique seven digit national identifier that assists Pharmacies in their interactions with Pharmacy payers and claim processors. You cannot request an enrollment application with a 3rd party payer until the NCPDP is obtained. The enrollment timeline is about 15 days.
Third Party Payers
Upon receipt of the federal and state licenses and the NCPDP number, you can now enroll in the PBM/third party contracts.
Part of the enrollment process is to find a Pharmacy Services Administration Organization (PSAO). Most buying groups or major wholesalers offer this service. This is a must to obtain the majority of third party enrollments in addition to negotiating reimbursements from payers as part of a large Pharmacy network.
Reach out to a PSAO while evaluating wholesalers to begin the application process. Enrollment can be finalized once the licenses are obtained. Please note that the following PBM’s are not included in any PSAO enrollment programs: Caremark, Cigna, Humana, Express Scripts, Optum Rx, State Medicaid, and Medicare Part B. It will be your responsibility to reach out to each PBM, request an application or enroll online and follow up for approval.
Allow approximately four months to complete enrollment for the non-PSAO plans.
Certificate of Liability Insurance
PBM’s will require a copy of this certificate in the amount of $1 million per incident/$3 million aggregate to be attached to the PBM application.
Surety Bond
You’ll need a $50,000 surety bond to enroll
in Medicare. Some private insurance prescription benefit programs will also require this bond.
The following enrollments can be found under the National Supplier Clearinghouse (NSC) website:
- Medicare Part B for drugs and biologics (855S)
- Medicare Part B for immunizations (855b)
- Medicare Part B DMEPOS will require accreditation
Medicaid is state- specific and, in some states, it could take between six and 9 months to enroll.
You can establish a timeline, and plan your opening date, as you work through the various protocols and licensing requirements.
8. Meet Your Compliance Requirements
Compliance and Credentialing will play a big part in opening an Independent Pharmacy.
Ensure you understand which laws, contractual requirements, and procedures apply to your Pharmacy, such as:
- General State Board Compliance
- DEA Controlled Drug Compliance
- State Controlled Drug Reporting
- Medicare Part D Fraud, Waste, And Abuse
- Medicare Part D Compliance Program
- USP 795 (Non-Sterile Compounding)
- USP 797 (Sterile Compounding)
- USP 800 (Handling of Hazardous Drugs)
- Third-Party Pharmacy Credentialing
- OSHA Hazardous Communication Standard
- OSHA Exposure Control Plan for Bloodborne Pathogens
- State Point of Care Testing for CLIA Waived Testing
- Drug Supply Chain Security Act
- Non-Discrimination (Section 1557 of the ACA)
- HR Requirements
How to Open an Independent Pharmacy
Starting a Pharmacy or opening an Independent Pharmacy is a complex process, requiring a significant investment in time, money, and resources. If this is your first Pharmacy and you’re unsure how to start a Pharmacy business, strongly consider hiring an experienced professional to assist you.
A Pharmacy Consultant with years of experience in opening Independent Pharmacies is a worthwhile investment in your success – so long as you choose a knowledgeable Consultant. Many so-called Pharmacy Consultants lack the knowledge or expertise to guide you effectively through the process.
Why You Should Choose PRS to Help With Opening an Independent Pharmacy
Starting a Pharmacy is a big undertaking, and even the smallest mistake can wreak havoc on your opening plans. To ensure a smooth Pharmacy opening and to maximize your ROI, you need the right team on your side.
PRS is the ONLY Pharmacy ownership consultant endorsed by NCPA, the Federation of Pharmacy Networks and over twenty buying groups representing more than 15,000 Independent Pharmacies. Why are we endorsed? Our vast experience in a variety of unique situations make us the BRAND name in Pharmacy Consulting, compliance, and brokerage . To date, PRS has sold, transferred , or opened over 500 Independent Pharmacies across all 50 states, and we are fully insured, licensed, and accredited.
If you choose PRS to show you how to start a Pharmacy business, you’ll work directly with one of our licensed Pharmacists. With over 20 years’ worth of experience, this Pharmacist will be your dedicated Project Manager, and they’ll help you every step of the way to make this stressful and complex process manageable.
Are You Ready to Open an Independent Pharmacy?
If you already have a location in mind, check out our Pharmacy Location Feasibility Study before you commit. If you don’t have a location ready yet, we can help – simply call us on 800-388-3688 to start the process.
For more information on how to open an Independent Pharmacy, or to find out more about working directly with one of our Pharmacy Consultants, reach out to our team for a free consultation – fill out the form below to get started.
" * " indicates required fields
P.O. BOX 852 201 Depot Street, Suite 200 Latrobe, PA 15650
Phone: 800-338-3688 Fax: 724-539-1388
COMPLIANCETrack
Staffing Client/Employee Portal
Privacy Policy
Terms & Conditions
Pharmacy Business Plan Template
Written by Dave Lavinsky
Pharmacy Business Plan
You’ve come to the right place to create your pharmacy business plan.
We have helped over 10,000 entrepreneurs and business owners create business plans and many have used them to start or grow their pharmacies.
Pharmacy Business Plan Example
Below is a sample pharmacy business plan and template to help you create each section of your pharmacy business plan.
Executive Summary
Business overview.
Healthy1 Pharmacy is a new independent retail pharmacy located in Charleston, South Carolina. The company is founded by Stephen Harris, a licensed pharmacist who has spent more than fifteen years working in the pharmacy industry. Stephen is confident that his strong communication skills combined with his keen attention to detail when preparing prescriptions will help him quickly grow a loyal customer base for his new pharmacy. Stephen has recruited a team of highly qualified professionals to help manage the day-to-day complexities of running a retail pharmacy including marketing, sales, customer service, financial reporting, and operations management.
Healthy1 Pharmacy will provide all of the products and services that are available at large retail chains, only with a better price and a small-town atmosphere. At Healthy1, sales associates and pharmacy technicians will get to know each customer by name and be able to offer a more personalized service not typically offered at larger pharmacies. Healthy1 Pharmacy will be a one-stop shop for any customer in need of a prescription, flu shot, OTC medication, and more.
Product Offering
The following are the services that Healthy1 Pharmacy will provide:
- Over-the-counter (OTC) medications
- Prescription medications
- Immunizations
- Travel medications
- Point-of-Care (POC) Tests
- Compounding
Customer Focus
Healthy1 Pharmacy will target all individuals in Charleston. The pharmacy will target multiple age groups from pediatric to geriatric. Healthy1 will also target patients needing prescriptions filled on a regular basis. No matter the customer, Healthy1 Pharmacy will deliver the best communication, service, and prices.
Management Team
Healthy1 Pharmacy will be owned and operated by Stephen Harris. He has recruited Emily Jackson, an experienced retail pharmacy manager to be his Store Manager and help to supervise the staff and run the day-to-day retail operations.
Stephen Harris is a licensed pharmacist with a Doctor of Pharmacy (PharmD) degree and more than fifteen years of experience working in the pharmacy industry. Stephen has been recognized by his former employer as a top performing pharmacist for five years in a row.
Emily Jackson has been a store manager at a local retail pharmacy for over a decade and has garnered a positive reputation for her exceptional organizational skills and leadership. Emily has worked in the pharmacy industry for so long, she understands all aspects required in running a successful retail pharmacy.
Success Factors
Healthy1 Pharmacy will be able to achieve success by offering the following competitive advantages:
- Friendly, knowledgeable, and highly qualified team of sales associates and pharmacy technicians who will assist customers, answer questions, and provide a personalized approach not found in larger pharmacies.
- Comprehensive array of products and services that includes everything you would expect from a large pharmacy, only with attentive customer service and lower prices.
- Healthy1 Pharmacy offers the best pricing in Charleston. The pharmacy’s pricing structure is the most cost effective compared to the competition.
Financial Highlights
Healthy1 Pharmacy is seeking $400,000 in debt financing to launch its new pharmacy. The funding will be dedicated towards securing the retail space and purchasing equipment, inventory, and supplies. Funding will also be dedicated towards three months of overhead costs to include payroll of the staff, rent, and marketing costs. The breakout of the funding is below:
- Pharmacy and retail space build-out: $100,000
- Equipment, supplies, inventory, and materials: $100,000
- Three months of overhead expenses (payroll, rent, utilities): $160,000
- Marketing costs: $25,000
- Working capital: $15,000
The following graph below outlines the pro forma financial projections for Healthy1 Pharmacy.
Company Overview
Who is healthy1 pharmacy.
Healthy1 Pharmacy is a newly established independent retail pharmacy located in Charleston, South Carolina. Healthy1 Pharmacy will provide all of the products and services that are available at large retail chains, only with lower prices and a small-town atmosphere. At Healthy1, sales associates and pharmacy technicians will get to know each customer by name and be able to offer a more personalized service not typically offered at larger pharmacies. Healthy1 Pharmacy will be a one-stop shop for any customer in need of a prescription, flu shot, OTC medication, and more. Healthy1 Pharmacy will be able to provide Charleson customers with the experience of a friendly, neighborhood drugstore while ensuring each prescription is handled with the highest standards of quality and care. The team of pharmacy technicians and sales associates will be highly qualified and experienced in helping customers find the right over-the-counter solutions for their individual needs as well as ensuring each prescription is filled accurately and efficiently.
Healthy1 Pharmacy History
Healthy1 is owned and operated by Stephen Harris, a licensed pharmacist with over fifteen years of experience working in the pharmacy industry. Stephen has worked for a large pharmacy chain and managed a team of pharmacy technicians for several years. Stephen’s tenure with the pharmacy chain combined with his pharmaceutical education has given him the skills and knowledge required to start his own pharmacy.
Since incorporation, Healthy1 Pharmacy has achieved the following milestones:
- Registered Healthy1 Pharmacy, LLC to transact business in the state of South Carolina.
- Has a contract in place to lease the retail storefront he will use for his pharmacy.
- Reached out to numerous contacts to include experienced pharmacy technicians and sales associates to advise them of the upcoming opportunities at his new pharmacy.
- Began recruiting the management team members including a store manager, an accountant/bookkeeper, and a marketing director.

Healthy1 Pharmacy Services
The following are the pharmacy products and services that Healthy1 Pharmacy will provide:
Industry Analysis
The pharmacy industry in the United States is valued at $534.2B and is projected to grow to $862B by 2028. Major market drivers include a growing number of types of diseases, an increasing percentage of people with chronic illnesses, an aging population, and higher healthcare costs.
There are more prescription medications being developed, and the demand for more prescriptions is growing faster than ever before. In 2020, there were an estimated 860M medications prescribed by physicians and 336M prescribed by hospitals. This prescription demand is resulting in more pharmacies opening across the U.S. The National Council for Prescription Drug Programs (NCPDP) reports an estimated 20,400 independent pharmacies in 2010. This number has grown to over 23,000 by 2019, a 12.9% increase in the number of independent pharmacies for that time period.
Industry operators in the pharmaceutical market can benefit from providing above average customer service, lower prices than competitors, and products or services that aren’t being offered elsewhere.
Customer Analysis
Demographic profile of target market.
Healthy1 Pharmacy will target all individuals in Charleston, South Carolina. The pharmacy will target multiple age groups from pediatric to geriatric. Healthy1 will also target patients needing prescriptions filled on a regular basis.
The precise demographics for Charleston, South Carolina are:
| Total | Percent | |
|---|---|---|
| Total population | 1,680,988 | 100% |
| Male | 838,675 | 49.9% |
| Female | 842,313 | 50.1% |
| 20 to 24 years | 114,872 | 6.8% |
| 25 to 34 years | 273,588 | 16.3% |
| 35 to 44 years | 235,946 | 14.0% |
| 45 to 54 years | 210,256 | 12.5% |
| 55 to 59 years | 105,057 | 6.2% |
| 60 to 64 years | 87,484 | 5.2% |
| 65 to 74 years | 116,878 | 7.0% |
| 75 to 84 years | 52,524 | 3.1% |
Customer Segmentation
Healthy1 will primarily target the following customer profiles:
- Parents of pediatric patients
- Geriatric patients
- People taking medications on a regular basis
- People needing compounding services
- People looking for vaccines or immunizations
- People who need lab tests
Competitive Analysis
Direct and indirect competitors.
Healthy1 Pharmacy will face competition from other companies with similar business profiles. A description of each competitor company is below.
Charleston Care Pharmacy
Charleston Care Pharmacy provides a wide variety of pharmacy products and services including OTC medications, lab testing, and compounding. Located in a senior neighborhood, Charleston Care Pharmacy specializes in serving the geriatric population, but welcomes customers of all ages. Charleston Care Pharmacy’s promise is to deliver effective communication, honesty, and integrity in every transaction. Charleston Care Pharmacy’s team of experienced pharmacy technicians assures customers are well taken care of and prescriptions are filled quickly and correctly.
FeelBetterNow Pharmacy
FeelBetterNowPharmacy is a Charleston-based neighborhood pharmacy that provides a full suite of services including compounding, vaccines, lab testing, local delivery, and more. The owners of FeelBetterNowPharmacy are licensed pharmacists who have extensive experience working for independent retail pharmacies so they understand what customers are looking for in a neighborhood pharmacy. Customers who choose FeelBetterNowPharmacy can rest assured they are getting the best quality products at reasonable prices.
Care Better Pharmacy
Care Better Pharmacy is a trusted Charleston pharmacy that is known for providing superior customer service. They are able to provide a one-stop shop for customers looking for convenient OTC and prescription medications, durable medical equipment, vaccines, and immunizations. Care Better Pharmacy is also able to serve customers in need of compounding, recurring prescriptions, and lab testing. They have expert pharmacy technicians to provide information about each medication and answer all of their customers’ questions.
Competitive Advantage
Healthy1 Pharmacy will be able to offer the following advantages over their competition:
- Healthy1 Pharmacy offers the best pricing in Charleston. The pharmacy offers its customers low prices on all of its products and services compared to the competition.
Marketing Plan
Brand & value proposition.
Healthy1 Pharmacy will offer the unique value proposition to its customers:
- Highly-qualified team of skilled pharmacy technicians and sales associates will be able to provide personalized customer service and ensure all prescriptions are handled with care.
- Unbeatable pricing to its clients – Healthy1 Pharmacy does not mark up its products and services at a large percentage. The pharmacy will offer the lowest prices guaranteed. If a customer finds a cheaper price elsewhere, Healthy1 Pharmacy will give the customer a better price.
Promotions Strategy
The promotions strategy for Healthy1 Pharmacy is as follows:
Healthy1 Pharmacy will create and maintain a company website that is well organized, informative, and lists all the products and services that Healthy1 is able to provide. The website will also list promotions and discounts, informative healthcare articles, and pharmacy-sponsored community events.
SEO/Google Marketing
The company’s marketing director will manage Healthy1’s website presence with SEO marketing tactics so that any time someone types in the Google or Bing search engine “Charleston pharmacy” or “pharmacy near me”, Healthy1 Pharmacy will be listed at the top of the search results.
Social Media Marketing
Healthy1 Pharmacy’s marketing director will also manage the company’s social media presence on several platforms including Instagram, Facebook, Twitter, YouTube, TikTok, and LinkedIn. The goal of the social media strategy is to attract new customers while engaging with current customers to encourage referrals, reviews, and feedback.
Content Marketing/Email Marketing
The company will post blogs and other promotional content on a regular basis with informative health and wellness information to keep people coming back. Healthy1 will post informative content on the website, social media platforms, and through email newsletters.
Professional Associations/Networking
Healthy1 Pharmacy will become a member of professional associations such as the Independent Retail Pharmacy Association, the National Community Pharmacists Association, and the Tennessee Pharmacists Association. The company will focus networking efforts on expanding its customer base.
Print Advertising
Healthy1 Pharmacy will invest in professionally designed print ads to display in programs or flyers at industry networking events, in magazines, direct mailers, and in local newspapers.
The pricing will be lower than competitors so customers feel they receive value when they choose Healthy1 products and services.
Operations Plan
The following will be the operations plan for Healthy1 Pharmacy.
Operation Functions:
- Stephen Harris will be the Owner and Lead Pharmacist. He will oversee pharmacy technicians. Stephen has spent the past year recruiting the following staff:
- Emily Jackson – Store Manager who will oversee all retail operations, sales associates, supplier relations, and inventory management.
- Jessica Johnson – Staff Accountant/Bookkeeper who will provide budgeting, tax payments, and financial reporting.
- Tim Thompson – Marketing Director who will provide marketing and sales campaigns for Healthy1 Pharmacy.
Milestones:
Healthy1 Pharmacy will have the following milestones complete in the next six months.
9/1/2022 – Finalize contract to lease the retail space.
9/15/2022 – Finalize personnel and staff employment contracts for the Healthy1 Pharmacy team.
10/1/2022 – Finalize contracts with suppliers.
10/15/2022 – Network at industry events and initiate the marketing and promotional campaign.
10/22/2022 – Begin moving into the Healthy1 Pharmacy storefront.
11/1/2022 – Healthy1 Pharmacy opens for business.
Stephen Harris is a licensed pharmacist with a Doctor of Pharmacy (PharmD) degree and more than fifteen years of experience working in the pharmacy industry. Stephen has been recognized for his commitment to excellence in filling prescriptions accurately and efficiently as well as his communication skills and positive rapport with customers.
Emily Jackson has been a store manager at another retail pharmacy for over a decade and has garnered a positive reputation for her exceptional organizational skills and leadership. Emily has worked in the pharmacy industry for so long, she understands all aspects required in running a successful retail pharmacy.
Financial Plan
Key revenue & costs.
The revenue drivers for Healthy1 Pharmacy are the fees charged to customers in exchange for the pharmacy’s products and services.
The cost drivers will be the overhead costs required in order to staff a pharmacy. The expenses will be the payroll cost, rent, utilities, inventory, supplies, and marketing materials.
Funding Requirements and Use of Funds
Healthy1 Pharmacy is seeking $400,000 in debt financing to launch its pharmacy business. The funding will be dedicated towards securing the retail space and purchasing equipment, inventory, and supplies. Funding will also be dedicated towards three months of overhead costs to include payroll of the staff, rent, and marketing costs. The breakout of the funding is below:
Key Assumptions
The following outlines the key assumptions required in order to achieve the revenue and cost numbers in the financials and in order to pay off the startup business loan.
- Number of prescriptions filled per month: 2,000
- Average fees collected each month: $50,000
- Retail lease per year: $100,000
Financial Projections
Income statement.
| FY 1 | FY 2 | FY 3 | FY 4 | FY 5 | ||
|---|---|---|---|---|---|---|
| Revenues | ||||||
| Total Revenues | $360,000 | $793,728 | $875,006 | $964,606 | $1,063,382 | |
| Expenses & Costs | ||||||
| Cost of goods sold | $64,800 | $142,871 | $157,501 | $173,629 | $191,409 | |
| Lease | $50,000 | $51,250 | $52,531 | $53,845 | $55,191 | |
| Marketing | $10,000 | $8,000 | $8,000 | $8,000 | $8,000 | |
| Salaries | $157,015 | $214,030 | $235,968 | $247,766 | $260,155 | |
| Initial expenditure | $10,000 | $0 | $0 | $0 | $0 | |
| Total Expenses & Costs | $291,815 | $416,151 | $454,000 | $483,240 | $514,754 | |
| EBITDA | $68,185 | $377,577 | $421,005 | $481,366 | $548,628 | |
| Depreciation | $27,160 | $27,160 | $27,160 | $27,160 | $27,160 | |
| EBIT | $41,025 | $350,417 | $393,845 | $454,206 | $521,468 | |
| Interest | $23,462 | $20,529 | $17,596 | $14,664 | $11,731 | |
| PRETAX INCOME | $17,563 | $329,888 | $376,249 | $439,543 | $509,737 | |
| Net Operating Loss | $0 | $0 | $0 | $0 | $0 | |
| Use of Net Operating Loss | $0 | $0 | $0 | $0 | $0 | |
| Taxable Income | $17,563 | $329,888 | $376,249 | $439,543 | $509,737 | |
| Income Tax Expense | $6,147 | $115,461 | $131,687 | $153,840 | $178,408 | |
| NET INCOME | $11,416 | $214,427 | $244,562 | $285,703 | $331,329 |
Balance Sheet
| FY 1 | FY 2 | FY 3 | FY 4 | FY 5 | ||
|---|---|---|---|---|---|---|
| ASSETS | ||||||
| Cash | $154,257 | $348,760 | $573,195 | $838,550 | $1,149,286 | |
| Accounts receivable | $0 | $0 | $0 | $0 | $0 | |
| Inventory | $30,000 | $33,072 | $36,459 | $40,192 | $44,308 | |
| Total Current Assets | $184,257 | $381,832 | $609,654 | $878,742 | $1,193,594 | |
| Fixed assets | $180,950 | $180,950 | $180,950 | $180,950 | $180,950 | |
| Depreciation | $27,160 | $54,320 | $81,480 | $108,640 | $135,800 | |
| Net fixed assets | $153,790 | $126,630 | $99,470 | $72,310 | $45,150 | |
| TOTAL ASSETS | $338,047 | $508,462 | $709,124 | $951,052 | $1,238,744 | |
| LIABILITIES & EQUITY | ||||||
| Debt | $315,831 | $270,713 | $225,594 | $180,475 | $135,356 | |
| Accounts payable | $10,800 | $11,906 | $13,125 | $14,469 | $15,951 | |
| Total Liability | $326,631 | $282,618 | $238,719 | $194,944 | $151,307 | |
| Share Capital | $0 | $0 | $0 | $0 | $0 | |
| Retained earnings | $11,416 | $225,843 | $470,405 | $756,108 | $1,087,437 | |
| Total Equity | $11,416 | $225,843 | $470,405 | $756,108 | $1,087,437 | |
| TOTAL LIABILITIES & EQUITY | $338,047 | $508,462 | $709,124 | $951,052 | $1,238,744 |
Cash Flow Statement
| FY 1 | FY 2 | FY 3 | FY 4 | FY 5 | ||
|---|---|---|---|---|---|---|
| CASH FLOW FROM OPERATIONS | ||||||
| Net Income (Loss) | $11,416 | $214,427 | $244,562 | $285,703 | $331,329 | |
| Change in working capital | ($19,200) | ($1,966) | ($2,167) | ($2,389) | ($2,634) | |
| Depreciation | $27,160 | $27,160 | $27,160 | $27,160 | $27,160 | |
| Net Cash Flow from Operations | $19,376 | $239,621 | $269,554 | $310,473 | $355,855 | |
| CASH FLOW FROM INVESTMENTS | ||||||
| Investment | ($180,950) | $0 | $0 | $0 | $0 | |
| Net Cash Flow from Investments | ($180,950) | $0 | $0 | $0 | $0 | |
| CASH FLOW FROM FINANCING | ||||||
| Cash from equity | $0 | $0 | $0 | $0 | $0 | |
| Cash from debt | $315,831 | ($45,119) | ($45,119) | ($45,119) | ($45,119) | |
| Net Cash Flow from Financing | $315,831 | ($45,119) | ($45,119) | ($45,119) | ($45,119) | |
| Net Cash Flow | $154,257 | $194,502 | $224,436 | $265,355 | $310,736 | |
| Cash at Beginning of Period | $0 | $154,257 | $348,760 | $573,195 | $838,550 | |
| Cash at End of Period | $154,257 | $348,760 | $573,195 | $838,550 | $1,149,286 |
What Is a Pharmacy Business Plan?
A pharmacy business plan is a plan to start and/or grow your pharmacy business. Among other things, it outlines your business concept, identifies your target customers, presents your marketing plan and details your financial projections.
You can easily complete your pharmacy business plan using our Pharmacy Business Plan Template here .
What are the Main Types of Pharmacy Businesses?
There are a number of different kinds of self storage business , some examples include: Pharmacy, Home Care Pharmacy, Mail Order Pharmacy and Compounding Pharmacy.
How Do You Get Funding for Your Pharmacy Business Plan?
Pharmacy businesses are often funded through small business loans. Personal savings, credit card financing and angel investors are also popular forms of funding.
What are the Steps To Start a Pharmacy Business?
Starting a pharmacy business can be an exciting endeavor. Having a clear roadmap of the steps to start a business will help you stay focused on your goals and get started faster.
1. Develop A Pharmacy Business Plan - The first step in starting a business is to create a detailed pharmacy business plan that outlines all aspects of the venture. This should include potential market size and target customers, the services or products you will offer, pricing strategies and a detailed financial forecast.
2. Choose Your Legal Structure - It's important to select an appropriate legal entity for your pharmacy business. This could be a limited liability company (LLC), corporation, partnership, or sole proprietorship. Each type has its own benefits and drawbacks so it’s important to do research and choose wisely so that your pharmacy business is in compliance with local laws.
3. Register Your Pharmacy Business - Once you have chosen a legal structure, the next step is to register your pharmacy business with the government or state where you’re operating from. This includes obtaining licenses and permits as required by federal, state, and local laws.
4. Identify Financing Options - It’s likely that you’ll need some capital to start your pharmacy business, so take some time to identify what financing options are available such as bank loans, investor funding, grants, or crowdfunding platforms.
5. Choose a Location - Whether you plan on operating out of a physical location or not, you should always have an idea of where you’ll be based should it become necessary in the future as well as what kind of space would be suitable for your operations.
6. Hire Employees - There are several ways to find qualified employees including job boards like LinkedIn or Indeed as well as hiring agencies if needed – depending on what type of employees you need it might also be more effective to reach out directly through networking events.
7. Acquire Necessary Pharmacy Equipment & Supplies - In order to start your pharmacy business, you'll need to purchase all of the necessary equipment and supplies to run a successful operation.
8. Market & Promote Your Business - Once you have all the necessary pieces in place, it’s time to start promoting and marketing your pharmacy business. This includes creating a website, utilizing social media platforms like Facebook or Twitter, and having an effective Search Engine Optimization (SEO) strategy. You should also consider traditional marketing techniques such as radio or print advertising.
Learn more about how to start a successful pharmacy business:
- How to Start a Pharmacy Business
Where Can I Get a Pharmacy Business Plan PDF?
You can download our free pharmacy business plan template PDF here . This is a sample pharmacy business plan template you can use in PDF format.
Sterile Compounding Pharmacist Board Certification Review and Recertification Activities
APhA has partnered with the U.S. Pharmacopeia (USP), the organization responsible for setting standards for sterile compounding, to plan and develop a comprehensive, innovative and professional development program for the Board of Pharmacy Specialties (BPS) Specialty in Compounded Sterile Preparations Pharmacy. This program is designed to meet the needs of practitioners seeking a BCSCP designation or to provide recertification credit to those who have earned the credential.
APhA is approved by BPS as a provider for the recertification of BCSCP.
Register today – May USP Exam Review Live Webinars
May 7, 2024 live webinar: pharmaceutical compounding—sterile preparations – 6.5 credit hours.
This live webinar describes the scope of USP <797>, including 2024 chapter updates, outlines the responsibilities of compounders, defines the criteria for component selection, and describes the facility requirements for sterile compounding. This webinar will take place on May 7, 2024 from 8:30 a.m. – 4:30 p.m. ET.
Learn more and register
May 8, 2024 Live Webinar: Hazardous Drugs—Handling in Healthcare Settings – 6.5 credit hours
This live webinar describes the scope of USP <800> by outlining quality standards and responsibilities of personnel for handling hazardous drugs (HDs) to help ensure patient and health care worker safety and environmental protection. The webinar will take place on May 8, 2024, from 8:30 a.m. – 4:30 p.m. ET.
Currently available Sterile Compounding Recertification activities
The recertification products below feature interactive activities, archived webinars, and several courses offered in conjunction with the US Pharmacopeia (USP). BCSCP recertification and ACPE credit is available upon successful completion of the corresponding exam for each activity. Per BPS requirements, only one opportunity to pass the exam is allowed.
Coming Soon: APhA2024 Sterile Compounding Recertification Conference: BCSCP Recert Credit
Learn practical information about how to ensure regulatory compliance, quality control and training your staff in the most efficient manner from experts practicing in the field of sterile compounding. This course package offers current BCSCP specialists an opportunity to earn 7 hours of board recertification and ACPE credit. These sessions were held live during the APhA 2024 annual meeting and were recorded. The recorded course and assessment will be available for BPS credit until March 22,2025. To earn recertification credit, you must successfully complete the assessment. Expired: APhA2023 Trending Topics in Sterile Compounding: BCSCP Recert Credit
Register Now
BCSCP Review: USP <797> Sterile Preparations:
This new 6.5-hour BCSCP accredited course, aims to improve your knowledge in the implementation of USP <797> Sterile Preparations. The course describes the scope of USP <797>, outlines the responsibilities of compounders, defines the criteria for component selection and describes the facility requirements for sterile compounding.
This course was produced in conjunction with the US Pharmacopeia.
BCSCP Review: USP <800> Hazardous Drugs
This 6.5-hour series of BCSCP-accredited course aims to improve your knowledge in the implementation of USP <800> Hazardous Drugs. The course describes the standards to protect personnel, patients, and the environment when handling hazardous drugs (HDs). Handling HDs includes, but is not limited to, the receipt, storage, compounding, dispensing, administration, and disposal of sterile and nonsterile products and preparations. The course provides an overview of the chapter and describes personnel responsibilities and educational expectations. It also covers facility design, engineering control, and documentation requirements.
BCSCP Recert: USP <797> Pharmaceutical Compounding — Sterile Preparations for Compounding Professionals
This 6.5-hour online interactive recertification course is intended for Board Certified Sterile Compounding Pharmacists (BCSCP) seeking to earn recertification credit for their BPS certification. Developed in partnership with the U.S. Pharmacopeia (USP), this course describes the scope of USP <797>, outlines the responsibilities of compounders, defines the criteria for component selection, and describes the facility requirements for sterile compounding.
BCSCP Recert: USP <800> Hazardous Drugs — Handling in Healthcare Settings for Compounding Professionals
This 6.5-hour online interactive recertification course is intended for Board Certified Sterile Compounding Pharmacists (BCSCP) seeking to earn recertification credit for their BPS certification. Developed in partnership with the U.S. Pharmacopeia (USP), this training describes the scope of USP <800> by outlining quality standards and responsibilities of personnel for handling hazardous drugs (HDs) to help ensure patient and health care worker safety and environmental protection.

Compounding Sterile Preparations
List Price: $165.00
Member Price: $125.00
Page Count: 488
The essential sterile compounding reference every pharmacist needs—now with important updates. From its inception, Compounding Sterile Preparations has served as both a professional reference guide for pharmacists and pharmacy technicians and a student textbook covering the regulation, technique, and quality assurance of compounding sterile preparations.
NOTE: The link below allows you to download the ePub file. If you want the PDF files, click on Table of Contents, browse the chapters by clicking on the drop-down symbol ^, select a chapter, and you will see the DOWNLOAD PDF orange button in the upper right. Most ePub files can be opened in eBook readers, like the B&N Nook and Kobo eReader. These files have to be converted to .Mobi format before they are usable on the Amazon Kindle device or app. For your computer, the easiest way to open an ePub file is to double-click on it and let your PC decide which default application should open the file. If no program opens it, then you probably do not have an application installed that can view ePub files. ePub files can also be opened on a computer with various free programs including Adobe Digital Editions. If you have access to this title you can download the ePub here:
- Compounding Sterile Preparations (EPUB 6.00 MB)
- Compounding Sterile Preparations (PDF 23.9 MB)
The latest edition of Compounding Sterile Preparations by Ryan A. Forrey, Lindsey B. Amerine, and Angela W. Yaniv reflects the latest advancements in the field, providing you with an indispensable resource to navigate the complex landscape of sterile compounding.
New in this Edition:
- Updated Standards: All chapters have undergone extensive revisions to align with the most recent literature and the revised USP Chapter <797> standards.
- USP Chapter <825>: Now includes information on radiopharmaceutical compounding in USP Chapter <825>.
- Expanded Knowledge Base: Two brand-new chapters covering allergenic extracts and Corrective and Preventative Action (CAPA) plans.
- Enhanced Content: Several chapters have been expanded to provide you with a comprehensive understanding of critical topics, including establishing beyond-use dates; receipt, storage, movement, and disposal of compounded sterile preparation components and finished preparations in the pharmacy; secondary engineering controls; point-of-care compounding and preparation for administration; biologicals, biosimilars, and vaccines; and handling and compounding of hazardous drugs.
Ryan A. Forrey, PharmD, MS, BCSCP, FFIP, FASHP
Ryan A. Forrey is Global Clinical Solutions Director for Surgical Complication Solutions at Becton, Dickinson and Company. He is also a Clinical Assistant Professor at The Ohio State University College of Pharmacy.
Lindsey B. Amerine, PharmD, MS, BCPS, CPEL, FASHP
Lindsey B. Amerine is System Executive Director of Pharmacy for UNC Health and HSPAL Residency Coordinator at UNC Medical Center. She is also an Associate Professor of Clinical Education at the UNC Eshelman School of Pharmacy.
Angela W. Yaniv, PharmD, BCSCP
Angela W. Yaniv is Director of Pharmacy, Sterile Products at the Cleveland Clinic. She is also a Clinical Assistant Professor of Pharmacy Practice at Northeast Ohio Medical University College of Pharmacy.
5-Star Review
The book's purpose is to put into one compendium all that is needed to know about sterile compounding practice, making it one of the most comprehensive and readable books about compounding in the pharmacy literature.
It is easy to imagine someone with little experience in this area reading this text and getting a very good idea of not only the practice of compounding but also the equipment and the rules necessary to govern it. (Lawrence P Carey, BS, PharmD, Doodys Review Service)
© 2024 American Society of Health-System Pharmacists. All Rights Reserved.
Powered by: PubFactory
- [185.66.14.236]
- 185.66.14.236
Character limit 500 /500

The basic configuration of the compounding rooms is this—an ante room sandwiched between the USP 797 and USP 800 laboratories. Pharmacy personnel enter the dirty side of the ante room where they don their lab garb, then cross to the clean side where they scrub prior to entering either lab.
To enable communication between the highly regulated labs and the broader work area of the pharmacy, the USP 797 and USP 800 rooms each have a glass front and phone to maintain visual and auditory communication between the spaces. Additionally, a chamber lock pass-through is used between labs and processing area to enable IVs and other drugs to be transferred while still maintaining pressure in the USP 797 and USP 800 rooms.
The specifics of each lab and how they differ are summarized below.
USP 797 Pharmacy Design
Because USP 797 clean rooms involve sterile compounding, it’s paramount to keep contaminants out of the lab while pharmacists and technicians prepare these drugs. To create a sterile environment with consistent pressure and temperature, we work with hospitals to achieve the following:
- USP 797 Entry. The door to the USP 797 swings out (into the ante room) to reduce entry of particulates into the clean room.
- Equipment. One or two chemical hoods (depending on capacity) for preparation of IV solutions, parenteral nutrition (TPN) bags, and the like.
- Consistent Temperature. Thermostats are read outside the USP 797 room so personnel can regularly log temperatures and ensure consistent environmental conditions, another USP and Board of Pharmacy requirement.
- Positive Pressure. Sophisticated mechanical systems continuously push air into the room to blow out airborne contaminants. Pressure, similar to temperature, is recorded daily and can be read from outside the room.
USP 800 Pharmacy Design
USP 800 design requirements prioritize staff safety. Because compounding pharmacists handle hazardous drugs in USP 800 labs, the room is designed to contain and remove chemical contamination.
- USP 800 Entry. To contain harsh drugs and contaminants in the lab, the USP 800 door swings into the room.
- Equipment. Compounding hoods and refrigerators used to process and store hazardous drugs.
- Consistent Temperature. Again, constant environmental conditions are key, so an external thermostat provides daily readings to ensure consistency.
- Storage. All hazardous drugs must be contained, so the room must be sized to house adequate medication refrigeration and shelving.
- Negative Pressure. To keep the flow of contamination in one direction, air is pulled out of the room and directly exhausted outside. Again, because design focuses on safety, it's important that advanced mechanical systems continually pull air out of the room. As another layer of precaution, HEPA filters are used in the ceiling to trap harmful particles. Similar to the USP 797 setup, the pressure gauge is outside the room for easy access and readings.
While all of this sounds terribly sterile, these codes and Board of Pharmacy regulations are actually keeping everyone on their toes. Codes can change quickly and dramatically, forcing big changes in pharmacy design and operations.
Better Design Solutions
To navigate these changes, we work hand-in-hand with pharmacies and Lean personnel to ensure compliance, analyze processes, and support operations with better design solutions.
Our work with Presbyterian Healthcare Services (PHS) has given us the opportunity to work with them on numerous pharmacies, many of which have required extensive renovation while maintaining operations. PHS has not only been quick to adopt the new codes in their pharmacies, but they have also refined their operations to better support the large volume of pharmaceuticals they deliver to patients.
At Rust Medical Center , for instance, PHS is pushing to create a one-stop shop for patients to more easily receive infusion, oncology, and other care and reduce risk to patients who have compromised immune systems.
This model requires a lot from the pharmacy. To help manage the large volume and distribution of medications, the pharmacy at Rust is expanding to accommodate two hoods in both the USP 797 and 800 rooms, and the footprint is being rearranged for processing and delivery breakdown tasks. Construction is phased and fast-tracked to minimize disruption to operations.
In addition to rearranging the footprint, the work surfaces and lighting are designed according to task. For instance, if there is a highly repetitive task where someone is standing and moving around, high countertops are specified. Conversely, seated tasks require lower countertops. Proper lighting is also paramount to reduce eye strain and human errors.
Padded mats and cooler temperatures in labs where personnel must wear coats, gloves, and hats also enhance comfort for a better working environment.
Pharmacies at Presbyterian Hospital in downtown Albuquerque, Presbyterian Espanola Hospital, Presbyterian Kaseman Hospital, Plains Region Medical Center, Trigg Memorial Hospital, and other facilities in PHS's network are being similarly renovated according to Board of Pharmacy regulations and best practices for healthcare facility design, putting Presbyterian Healthcare Services at the forefront of state-of-the-art pharmacy design and operations.
Because PHS is refining their pharmacy processes and looking at the bigger picture of how their pharmacies can work together, they are able to improve workflow, consolidate processes, and ultimately provide better care for patients.
The goal of these new codes and changes in pharmacy design and operations is always meant to enhance patient safety and well-being. As health care continues to advance, hospitals and pharmacies will continue to renovate their spaces to refine operations and provide more and better options for communities.
Contributors:

Dekker Perich Sabatini's dedicated team of healthcare architects is committed to evidence-based design, enhanced patient care, Lean design principles, and the well-being of patients, staff, and visitors. For more information about Board of Pharmacy regulations and healthcare facility design, contact John Laur .
Read our minds.
Sign up for our email.
Related Content
In public safety, passion makes perfect, tunable lighting in a special needs preschool, an architect’s perspective on market uncertainty.

Designing more hospitality in hospital.
Urban design solutions are not a cure-all when it comes to rural healthcare needs.
Explore DPS Projects

If the office fits…
Project: Gap Offices

Maximizing the potential for learning
Project: Capitan Middle & High School

Classrooms that reach outside
Project: Cottonwood Elementary School
Trust Our Expertise

Learning Opportunities in Outdoor Environments

Weaving Safety and Security into Every Building

Sustainable Building Materials
Our insights in your inbox..
- Pharmaceutical Engineering Magazine
- Online Exclusives
- Special Reports
- Facilities & Equipment
- Information Systems
- Product Development
- Production Systems
- Research + Development
- Supply Chain
- White Papers
- iSpeak Blog
- Editorial Calendar
- Article of the Year
- Submit an Article
- Editorial Team
New ISPE Guide Provides Support for 503A Compounding Community

A recently released guidance document, the ISPE Guide: 503A Compounding – Regulatory Basis and Industry Good Practices for Pharmacies , summarizes and contextualizes key information from US governmental bodies relevant to 503A pharmacies. The resource is intended to benefit the existing pharmacy compounding industry, those considering entering that space, or third parties working with pharmacy compounders, such as hospitals. The guide is designed to help the compounding community better understand existing requirements and good industry practices, thus enabling them to deliver safer drug products. 1
- 1 ISPE. ISPE Guide: 503A Compounding – Regulatory Basis and Industry Good Practices for Pharmacies. ISPE; 2024. https://ispe.org/publications/guidance-documents/guide-503a-compounding.
Although most US patients receive their medical prescriptions via traditional manufacturers, the role of compounding pharmacies is increasing. The US compounding pharmacies market was estimated to be over $5 billion in 2023, with the 503A segment accounting for almost three-fourths of this. The total market is expected to increase to over $10 billion in the next decade. 2

By law, compounding pharmacies cannot make generics of US Food and Drug Administration (US FDA)-approved and marketed products, except in the case of published drug shortages. Compounding pharmacies can also produce versions of products that have been discontinued by manufacturers, and both drug shortages and discontinuations have led to increased demand for compounded products. 2 Pain management, hormone replacement, dermatology applications, and nutritional supplements have made up a significant portion of the compounding market, but a variety of other drugs would be categorized as compounded products as well. 3
Parties looking to enter the compounding space have sometimes found it challenging to synthesize, interpret, and implement existing guidance on compounding pharmacies, which are regulated differently than mass-produced traditional products.
Under the original US Federal Food, Drug, and Cosmetic Act (FDCA), traditional prescription drug manufacturers are required to submit evidence of the safety and efficacy of drugs, e.g., through clinical trials and other requirements. However, individual compounding pharmacies were initially excluded from these federal mandates. 4
To this day, states are the main regulators for most prescription-based drug compounding practices, but the US federal government does play some role in requirements and oversight. In 1997, Congress created an additional section to the FDCA, Section 503A, which applies directly to compounding pharmacies.
In 2013, Congress passed the Drug Quality and Security ACT (DQSA) to further clarify the US FDA’s authority in oversight of the compounded drug market, partly in response to an outbreak of fungal meningitis traced to a large-scale drug compounding facility. The act both amended 503A and added another section to the FDCA, Section 503B, which would apply to larger compounding facilities. 4
503A pharmacies are smaller facilities which compound medications for individual patients based on a doctor’s prescription. In contrast, 503B compounders are registered as outsourcing facilities which must follow additional regulations. They can manufacture large batches of compounded drugs to be sold to healthcare facilities, without necessarily compounding for patient specific prescriptions. 4
The recently released ISPE Guide provides advice and insights pertaining specifically to compounding under FDCA Section 503A. It reviews key details and provides additional context surrounding the criteria set out by the United States Pharmacopoeia (USP), chapters 795, 797, 800, and 1163, and sections of other chapters to which all 503A pharmacies are expected to adhere. Additionally, although it does not provide individual state-based guidance, it clarifies the authority of State Boards of Pharmacy and their coordination with the US FDA.1
The ISPE Guide: 503A Compounding – Regulatory Basis and Industry Good Practices for Pharmacies provides general background on compounding, sterile and nonsterile drugs, general compounding requirements such as those pertaining to prescriptions and personnel, quality management systems, training, material sourcing, and facilities and equipment. It also includes information on environmental controls, quality, and maintenance, as well as product testing and the handling, storage, and transport of compounded products.1
Another key benefit of the guide is its inclusion of suggested Standard Operating Procedures (SOPs) lists. These outline the requirements that need to be performed to satisfy the relevant USP chapters for pharmaceutical compounding. 1
This ISPE 503A compounding guide provides complementary information to the ISPE 503B compounding guide released in 2023: ISPE Guide: 503B Compounding – Regulatory Basis and Industry Good Practices for Outsourcing Facilities . The latter guide provides targeted information for outsourcing facilities, which must follow additional requirements. 5
The information provided in the ISPE guides on compounding are a significant first step in ISPE’s efforts to provide resources for the compounding industry. The Community of Practice (CoP) that ISPE has developed for compounding further emphasizes their recognition of this segment of the pharmaceutical industry as critical to patient care and creates a collaborative space for bringing together individuals and organizations in the compounding industry to share best practices and address the ever-evolving practice of compounding. Rebecca Welton Senior Consultant, Regulatory Affairs Lachman Consultant Services, Inc. Co-secretary of the Pharmaceutical Compacting Community of Practice (CoP), and lead author of the ISPE guides on 503A compounding and 503B compounding
- 2 a b c Biospace. U.S. compounding pharmacies market size, share, CAGR, Report 2024 to 2033. April 15, 2024. https://www.biospace.com/article/releases/u-s-compounding-pharmacies-market-size-share-cagr-report-2024-to-2033/
- 3 Global Market Insights. Compounding pharmacies market size. August 2022. https://www.gminsights.com/industry-analysis/compounding-pharmacies-market
- 4 a b c National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy; Jackson LM, Parker RM, Mattison DR, editors. 3, Regulatory framework for compounded preparations. The Clinical Utility of Compounded Bioidentical Hormone Therapy: A Review of Safety, Effectiveness, and Use. Washington (DC): National Academies Press (US); 2020 Jul 1. https://www.ncbi.nlm.nih.gov/books/NBK562888/
- 1
- 5 ISPE. ISPE Guide: 503B Compounding – Regulatory Basis and Industry Good Practices for Outsourcing Facilities. ISPE; 2023. https://ispe.org/publications/guidance-documents/guide-503b-compounding
- 'Share on Twitter'
About the Author

Related Articles

We've had the opportunity to interview Hilal Yamaner, an active ISPE Emerging Leader and member of ISPE Women in Pharma ® . Throughout this member spotlight, she shares her journey with ISPE, her perspective and passion for AI integration, the work she’s put in to planning a related ISPE Women in Pharma panel session at the upcoming
On 17 April 2024 at the 2024 ISPE Europe Annual Conference in Lisbon, Portugal, a panel discussion titled “Harmonization to Break Down Barriers to Robust Supply Chains” featured an international panel of experts who discussed drug recalls, drug shortages, risks that can lead to poor drug product quality, quality risk management, Annex 1 implementation and its effect on the supply chain,...

Eli Lilly has recently achieved a remarkable milestone with the completion of its new synthetic peptide manufacturing facility/platform at its facility in Kinsale, Ireland. This cutting-edge project not only enhances production capabilities but also exemplifies a commitment to innovation and safety. Below is an overview which delves into the details of this groundbreaking achievement and...
You are using an outdated browser. Please upgrade your browser to improve your experience.
- Pharmacy Technicians
About Pharmacy Technicians
- Advanced Pharmacy Technician Roles Toolkits
Sterile Compounding Technician Toolkit
- -1 ? window.location.search.substring(1) : '')"> Add To Favorites
Sterile compounding is a complex specialty that allows pharmacy technicians to work in an advanced role. There are many different practice settings such as hospitals, infusion centers, compounding facilities and more. In general, a sterile compounding role involves creating IV infusions in a clean room setting using aseptic technique, a process that allows the end medication to be sterile enough for injection into the patient. These roles require absolute precision and a strong attention to detail as small mistakes can have devastating consequences. Compared to a typical outpatient pharmacy role, such as in a community pharmacy, the sterile compounding technician works much more independently.
Sterile compounding roles require in-depth knowledge of the various state, federal, and non-governmental guidelines that regulate the practice. Some of the most important regulations are those created by the United States Pharmacopeia (USP), a scientific nonprofit organization. These regulations are categorized into three main sections, based on the type of compounding done. They are USP <795> for nonsterile compounding, USP <797> for sterile compounding, and USP 800, for hazardous compounding.
The resources below will help familiarize you with the different practice settings, competencies, and expectations that come with an advanced role position in sterile compounding as a pharmacy technician.
Job Descriptions
Below are some real-life examples of job descriptions for pharmacy technician sterile compounding roles. The level of education and experience sought by employers, as well as what the role description are included. As mentioned, there are many different roles for compounding technicians, and these represent only a small portion of those roles.
- Advanced Compounding Solutions Job Description
- UnitedHealth Group Home Infusion Sterile Compounding Job Description
- Massachusetts General Compounding Technician Job Description
Sterile Compounding
This section includes links to better demonstrate what sterile compounding looks like on a day-to-day basis; from actual compounding to structural layouts of IV Rooms, and automated devices that assist in the start-to-finish accuracy of compounding products. For a simple introduction to compounding, and how the technician demonstrates and explains the importance of practicing aseptic technique, follow the links below.
- Aseptic Technique Procedures [Video]
- Sterile Compounding [Video]
As previously mentioned, there are three main regulations within sterile compounding. Each section is unique in its own way through guidelines, specific location for compounding, and how these locations may be organized in space. To better understand the spatial set up of an IV room that contains both USP <797> and USP <800> utilization, follow the link below.
- Clean Room Design [Video]
It is very important to keep the compounding environments sterile, so that the medications reach the patients safely for administration. In order to hold this regulation of high standard, there are specific techniques to be practiced. For proper cleaning techniques and products, the following article is useful in outlining how to clean the IV rooms and the order in which to use specific products.
- Cleaning Products and Procedures [PDF]
Automation devices can assist in sterile compounding, increasing the accuracy of prepared medications. The following link explains the BD Pyxis IV Prep program which uses gravimetric & barcode verification along with electronic documentation.
- BD Pyxis IV Prep [Video]
Training and Education
Formal training and education programs are important for pharmacy technicians to be able to demonstrate their expertise through formal certification. The below programs are designed for pharmacy technicians who already working in a compounding practice setting.
Practical Training in Compounding Sterile Preparations Certificate
Description: This self-guided, online program is a PTCB-recognized sterile compounding education/training program for those who are pursuing the PTCB Compounded Sterile Preparation Technician® (CSPT®) certification.
The program provides 29 hours of ACPE continuing education for pharmacists and technicians, incorporating recorded presentations, readings, video demonstrations, and exercises in curricular modules. The program covers sterile product preparation practice standards and regulations, pharmacy calculations, facilities and equipment, cleanroom personnel behaviors and expectations, sterile compounding components and procedures, stability, sterility, non-sterile to sterile compounding, hazardous drugs, and all aspects of handling from material receipt to final check or disposal. After completing all of the modules, participants should be proficient in the fundamental concepts required to ensure safe and compliant sterile product preparation.
Target Audience : Pharmacist and Technicians
C ost: $445 (ASHP Member), $545 (Non-ASHP Member)
Organization: ASHP
CSPT Certification
Description: Many pharmacy technicians are responsible for sterile compounding, the preparation of medications in a sterile environment to prevent contamination. Becoming a Certified Compounded Sterile Preparation Technician® (CSPT®) demonstrates a CPhT’s knowledge and skill as a specialized pharmacy technician as well as their commitment to the role they play in ensuring medication safety. Earning the CSPT Certification provides the opportunity to be recognized by your employer and colleagues for successfully meeting PTCB’s rigorous requirements for this advanced credential.
Eligibility Criteria: An active PTCB CPhT must qualify under one of the following pathways:
Pathway 1: Completion of, or enrollment in, a PTCB-Recognized Education/Training Program for the CSPT Program AND one year of full-time continuous compounded sterile preparation (CSP) work experience.
Pathway 2: Three years of full-time continuous compounded sterile preparation (CSP) work experience.
Cost: $199
BUNDLE Pharmacy technicians who enroll in ASHP’S Sterile Product Preparation Certificate Program and complete PTCB’s Compounded Sterile Preparation Technician® (CSPT® ) Certification save $219 and receive a one-year ASHP technician membership.
Continuing Education
As an ASHP technician member, you have access to the PharmacyTechCE catalog, a collection of continuing education resources that are available at a discount or at no additional cost. Below are some compounding related CE activities that will help you learn more about the role and what it entails.
Pharmacy Technicians: Getting Started in Sterile Compounding
Description: Sterile compounding is a unique challenge for pharmacy technicians and is an exciting professional path. This webinar presents information about sterile compounding, what it is, the minimum requirements to sterile compound, and in what settings this training is useful. Entry level and advanced positions will be discussed. This presentation describes sterile compounding, identifies what education and training is needed by pharmacy technicians related to sterile compounding, and discusses the practice settings where sterile compounding is utilized and required.
Cost: Free to ASHP technician members and PharmacytechCE subscribers
CE Credits: 1.0 hours
Sterile Compounding Technology: Pharmacy Technicians Lead the Adoption of Best Practices
Description: Over the past 10 years there has been growth and maturity in the compounding technology market and increased uptake in the use of sterile compounding technologies by pharmacies. Pharmacists and pharmacy technicians, like other healthcare providers, are increasingly feeling the pressure of a reduced workforce during the pandemic. Many are also experiencing burnout which can directly impact compounding success. Together, pharmacists and technicians must address these challenges to improve the safety of sterile compounding practices as well as continue to increase the safe use of technology in the process. This presentation describes common challenges faced by technicians using technology in the sterile compounding process and the role that technicians play in designing a safe workflow plan. In addition, the new sterile compounding best practice guidelines for 2022 from ISMP are reviewed as part of the program.
What’s All the Fuss About? Safe Handling of Hazardous Drugs – USP
Description: Since the first ASHP guidelines on handling hazardous drugs were published in 1983, it is known that hazardous drugs have the potential to negatively affect anyone that is inadvertently exposed to them – occupational exposure in the health care setting being a primary concern. Many studies have been done that have demonstrated the long term effects of being exposed to hazardous drugs, especially if not handled appropriately. To reduce unintended harm to those who handle hazardous drugs, all institutions that handle hazardous drugs will be required to follow the revised USP standards, scheduled to be enforceable starting November 2023. This presentation begins with an overview of basic medication safety concepts and will discuss how pharmacy technicians are uniquely skilled to prevent many medication errors throughout the medication use process. The presentation will also discuss tools pharmacy technicians can use to apply medication safety concepts to recognize or prevent medication errors in their practice.
Related Links
- Pharmacy Technician Career Overview
- Pharmacy Technician Resume/CV Development
- Pharmacy Technician Development
- Advanced Pharmacy Technician Roles
- Frequently Asked Questions
- Helpful Resources
- Midyear Clinical Meeting 2022
- Match Day Resources
- continuing education
- drug shortages
- policy positions and guidelines
- for presenters
- get involved in a meeting
We noticed that you are in Europe. Would you like to go to the EMEA - English site?
Not all products are available or approved for use in each region. Please go to the EMEA site for all relevant products.

- Compounding Pharmacy /
- USP Standards /
- PPE & Garbing /
- USP <797> /
- Sterile Compounding
Understanding the Impact of USP Chapter Updates on Compounding Pharmacies
Contec healthcare.
Share This Post:
The latest updates to USP Chapter <797> have brought significant changes to the landscape of sterile compounding pharmacies and cleanroom operations. In this blog post, we'll explore the key updates from the chapter and discuss their implications for pharmacies. You can also learn more about USP Chapter <797> Compliant Cleaning Procedures here .
Key Update 1: Sterile Products within the PEC
One of the most notable changes is the requirement for sterile products within the Primary Engineering Control (PEC). Previously, only alcohol used in compounding needed to be sterile, but now, both the disinfectant and wipes/applicators must be sterile.
Key Update 2: Frequency of Cleaning and Disinfecting
Chapter 7 specifies the required cleaning frequency and locations for compounding areas. In a Primary Engineering Control (PEC), sterile 70% IPA must be applied after cleaning and disinfecting or following the use of a one-step disinfectant cleaner or sporicidal disinfectant, to remove any residue. Sterile 70% IPA must also be applied immediately before starting compounding. During compounding, sterile 70% IPA must be applied to the horizontal work surface, including removable work trays, of the PEC at least every 30 minutes if the process takes 30 minutes or less. If the process exceeds 30 minutes, disinfection must occur immediately after compounding.
Key Update 3: Garbing Requirements
Section 7 of the chapter addresses cleaning and disinfecting activities being performed by trained and appropriately garbed personnel. Staff must use facility-approved agents and procedures, which must be described in written SOPs. The frequency, method(s), and location(s) of cleaning, disinfecting, and applying sporicidal disinfectants must be established in written SOPs. The minimum contact time must be followed for each of the cleaning, disinfecting, and sporicidal disinfectants used.
Key Update 4: Introduction of Items into Cleanrooms
The chapter now includes specific guidelines for introducing items into cleanrooms and segregated compounding areas (SCAs). Items must be wiped with a disinfectant, sterile IPA, or sporicidal agent before being brought into the cleanroom to minimize the risk of contamination. This step is essential for maintaining a state-of-control for compounding activities.
Key Update 5: Support for Implementing Change
To assist pharmacies in implementing these changes, Contec Healthcare is offering a range of resources and support. From training videos to task sheets and online learning platforms, we are committed to helping pharmacies navigate the transition smoothly. You can access these sources here: Resource Center | Contec Healthcare (contecinc.com)
In conclusion, these updates to the USP Chapter have significant implications for compounding pharmacies. By adhering to these new requirements for sterility, cleaning, and garbing, pharmacies can ensure the safety and quality of compounded medications. With support from Contec Healthcare, pharmacies can successfully navigate these changes and continue to provide essential healthcare services to their patients.
For more information on USP Chapter <797> visit our USP web experience here.

At Contec® Healthcare, we know clean counts most — especially in critical operations like yours where you improve patient outcomes.
Related posts.

Optimizing Sterile Compounding: USP <797> Compliant PEC Solutions
Sterile disinfectants: part one.

5 Elements to Maintain a Microbial State of Control
Speak with an expert.
Using our Contec Locator tool, you’ll be able to find the expert associated with your industry and geographic region. Fill out the information in the boxes below to contact a Contec expert who will help find the Contec products that are right for you.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Drug Safety and Availability
FDA alerts patients and health care professionals not to use compounded products intended to be sterile from Prescription Labs Inc. dba Greenpark Compounding Pharmacy
[9/29/2021] Update: FDA is alerting veterinarians and animal owners/caretakers not to use compounded products intended to be sterile from Greenpark Compounding Pharmacy. Animal owners/caretakers who have received compounded drugs produced by Greenpark Compounding Pharmacy and have concerns should contact their veterinarian as appropriate. Veterinarians and animal owners/caretakers can report adverse events or side effects related to the use of these products. Please visit How to Report Animal Drug and Device Side Effects and Product Problems for more information.
[9/17/2021] FDA is alerting patients and health care professionals not to use compounded products intended to be sterile, produced and distributed nationwide by Prescription Labs Inc. doing business as Greenpark Compounding Pharmacy, Houston, Texas, due to lack of sterility assurance. Administration of non-sterile products intended to be sterile may result in serious and potentially life-threatening infections or death.
Health care professionals and patients should immediately check their medical supplies, quarantine any products marketed as sterile from Greenpark Compounding Pharmacy, and not administer or provide them to patients.
FDA investigators recently inspected Greenpark Compounding Pharmacy’s facility during July and August 2021 and observed conditions which could cause the compounded drugs to be contaminated or otherwise pose risks to patients.
On August 18 and August 31, 2021, the compounder initiated a recall of several lots of a compounded ophthalmic drug. On September 2, 2021, FDA recommended Greenpark Compounding Pharmacy expand its recall to all unexpired compounded drugs intended to be sterile and stop sterile production until it implements adequate corrective actions. To date, the company has refused to recall all unexpired compounded drug products intended to be sterile or to cease compounding of all sterile drugs.
Patients who have received compounded drugs produced by Greenpark Compounding Pharmacy and have concerns should contact their health care professional.
FDA is not aware of any adverse reactions associated with the use of compounded drugs from Greenpark Compounding Pharmacy. FDA encourages health care professionals and patients to report adverse reactions or quality problems experienced with the use of these drugs to the FDA’s MedWatch Adverse Event Reporting program:
- Complete and submit the report online at FDA MedWatch
- MedWatch Voluntary Report frequently asked questions
Opening Hours : M-F 9am – 5:30pm | Sat 9am-1pm | Sun: Closed – On the grounds of St Agnes Hospital

410-644-8400
Ext 0 – to reach pharmacy Ext 4 – to reach compounding
Voshell’s Pharmacy
Semaglutide – A Revolution in Weight Management
INTRODUCTORY OFFER
Semaglutide injections starting only at $279/month*.
Experience the transformative power of Semaglutide and embark on a journey toward a revitalized self. For a limited time, we’re making this journey even more accessible.
To take advantage, simply mention this special to the pharmacist when you call or complete our online form!
*for the first month only
What is Semaglutide?
Semaglutide is a medication primarily designed for the treatment of type 2 diabetes but has shown significant promise in aiding weight loss. By mimicking the functions of a natural hormone, it helps regulate your blood sugar and slows down digestion, making you feel full and eat less.
Why Choose Voshell’s for Your Semaglutide Prescription?
- Expertise: With years of experience in compounding sterile specialized prescriptions, we ensure the highest standards of safety, accuracy, and quality.
- Tailored Solutions: We customize dosages and formulations to meet the unique needs of every patient.
- Secure Online Refills: Hassle-free prescription refills from the comfort of your home. Your medication, delivered to your doorstep.
- Licensed in Three States: Proudly serving clients in Pennsylvania, Maryland, and Delaware.
- Consultation Services: Have questions? Our trained pharmacists are here to guide you every step of the way.
EFFECTIVE WEIGHT MANAGEMENT
Studies have shown a significant reduction in body weight among participants., controlled appetite, feel full with fewer cravings., enhanced blood sugar regulation, helpful for those with type 2 diabetes., how to get started with semaglutide.
- Consult Your Doctor: Ensure Semaglutide is right for you. Discuss potential benefits, side effects, and dosage.
- Call Voshell’s and Place Your Order: Once prescribed, use our simple online form or give us a call to place your order or refill your prescription.
- Delivery at Your Doorstep: With discreet packaging and swift delivery, get your medication without any hassles.

What Our Patients Say
“The package and the video were very helpful. I’ve lost 2 lbs.” – Kate
“The pharmacy has been so helpful!.” – Maureen
“So far so good. So grateful for the better price.” – Larcia
“The semiglutide has been working really well. I was initially feeling impatient with first set of doses which were .1 per week because I wasn’t really losing weight. But I’m now on the second week of the .5 dosage and can feel the effects and it’s kept my appetite down effectively. Looking back, I’m glad I had the initial four small doses to make sure my body adjusted to the medicine. I didn’t have any adverse reactions. Thank you! – Keerthi”
“A very positive experience since beginning the Semaglutide. I have had none of the GI side effects associated with Ozempic and I attribute this to the addition of the vitamin b12. Semaglutide definitely makes you feel fuller quicker by slowing the digestion of food. Voshell’s is always great to work with and the Pharmacists are very knowledgeable and helpful I recommend without reservation.” -Susan
“Great customer service!!” – L. Johnson
“Great pharmacy!” – Sharon
“I got my medicine in 5 minutes had just left the drs could hardly walk. and the people that work there are very nice” – L. Galster
“Friendly staff that takes the time to make sure you understand anything you’ve got a question about and pretty quick with your order.” – L. Eaton
“It feels like I’m dealing with people that know me, unlike the big chains. I would recommend them to anyone really he best service I’ve ever had pharmacy wise.” – J. Kondas
“I love this pharmacy I refuse to go anywhere the know the patients and they get u in n out.” – D. Allen
“Great people are awesome.” – A. Ford
“Awesome staff! Willing to help in any capacity. I highly recommend this place. 5 stars.” – J. Jackson
Semaglutide FAQs
Q: How is Semaglutide administered? A: Semaglutide is typically administered as a subcutaneous injection, which means it’s injected under the skin. It’s generally given once a week at a time that’s most convenient for the patient.
Q: How does Semaglutide aid in weight loss? A: Semaglutide acts on receptors in the brain that control appetite. It helps people feel fuller for longer, thereby reducing overall food intake and leading to weight loss.
Q: Can Semaglutide be used with other weight loss medications? A: It’s essential to discuss with your healthcare provider about any other medications you are taking. While Semaglutide can be used in conjunction with some other medications, there may be specific interactions to be aware of.
Q: Who should not take Semaglutide? A: People with a history of medullary thyroid carcinoma, those with Multiple Endocrine Neoplasia syndrome type 2, or those allergic to Semaglutide or any of its ingredients should not take this medication. Always consult with your physician to determine if this medication is right for you.
Q: Are there any dietary restrictions while taking Semaglutide? A: No specific dietary restrictions are associated with Semaglutide, but a balanced diet combined with regular exercise can enhance the medication’s weight loss benefits.
Q: How soon can I expect to see results after starting Semaglutide? A: Weight loss results can vary from person to person. Some patients report noticing changes as early as a few weeks into treatment, while for others, it might take a few months. It’s essential to stay consistent with the medication and follow your doctor’s recommendations.
Q: Is it safe to drink alcohol while on Semaglutide? A: While Semaglutide doesn’t have a direct interaction with alcohol, alcohol can lower blood sugar levels. It’s advisable to discuss your alcohol consumption habits with your healthcare provider to determine what’s safe for you.
Q: What happens if I miss a dose? A: If you miss a dose, take it as soon as you remember if it’s within five days of the missed dose. If it’s been more than five days, skip the missed dose and administer the next injection on the regularly scheduled day. Always check with your healthcare provider for guidance on missed doses.
Q: Can I take Semaglutide if I’m pregnant or planning to become pregnant? A: The effects of Semaglutide during pregnancy are not fully known. If you’re pregnant, planning to become pregnant, or breastfeeding, consult with your physician before starting or continuing the medication.
Remember, while these FAQs provide general information, it’s always best to consult with a healthcare professional about your specific situation and needs.
To fill your Semaglutide prescription, reach out to our team:
- Phone: 410-644-8400 (ext. 4 for compounding)
- Shipping: FAST shipping to Maryland, Delaware, and Pennsylvania
- Location: 3455 Wilkens Ave, Ste 103, Baltimore, MD 21229
Connect with us on Facebook !
- College of Pharmacy
- Location Location
- Contact Contact
- Colleges and Schools
Faculty and Staff
- Michael Shtutman
Michael Shtutman, Ph.D.
| Associate Professor Director, Functional Genomics Core | |
| Drug Discovery & Biomedical Sciences (DDBS) College of Pharmacy | |
| 803-777-8988 | |
| College of Pharmacy 715 Sumter Street - CLS 713A Columbia, SC 29208 |

Ph.D. Experimental Oncology, Cancer Research Center, Moscow, Russia, 1996 M.S. Biotechnology, Moscow Mendeleev Chemical - Technological Institute, 1988
Postdoctoral Fellowship Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot, Israel, 1997-1999
Michael Shtutman, received his Ph.D. in Cancer Research Center in Moscow, Russia in 1996 and completed his postdoctoral fellowship at the Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot, Israel, in 1999. Since 2011, he has received grant funding from the National Institutes of Health for his research laboratory, as well as in collaboration with other academic institutions.
Dr. Shtutman is director of the NIH-funded Functional Genomics Core , part of the COBRE Center for Targeted Therapeutics , which supports multiple genomics-centered projects across the University of South Carolina and statewide, such as single-cell transcriptomics application and genome polymorphism analysis.
Research Interests
- Cancer research
- HIV research
- Neurodegenerative diseases
- Drug repurposing
- Artificial intelligence (AI)
- Induced pluripotent stem cells
- Genomics and Transcriptomics
Research Lab
Our laboratory's primary interest is the discovery of druggable target genes and the repurposing of existing drugs for the treatment of cancer and neurodegenerative diseases. With advanced genomics and functional screening approaches, we discovered several candidates: DDX24, IGSF8, and COPZ2 for anti-cancer drug development.
Currently, our research has centered on the application of Artificial Intelligence (AI) for drug repurposing with a focus on the discovery of the treatment of HIV-associated neurocognitive disorder. In collaboration with the University of Delaware, the laboratory developed MOLIER and AGATHA, AI-based systems for automatic hypothesis generation through the mining of biomedical literature. This system was applied for the discovery of compounds for HIV-associated neurocognitive disorders' (HAND) treatment repurposing, including FDA-approved drugs and small molecules in the late stage of clinical development.
We are using neuronal cultures and induced pluripotent stem cells (iPSCs) as a disease model and applying transcriptomics, including single-cell transcriptomics and proteomics approaches to discover the molecular mechanisms of the drug's activity.
Selected Publications
Marina Aksenova, Justin Sybrandt, Biyun Cui, Vitali Sikirzhytski, Hao Ji, Diana Odhiambo, Mathew Lucius, Jill R. Turner, Eugenia Broude, Edsel Pena, Sofia Lizarraga, Jun Zhu, Ilya Safro, Michael D Wyatt, Michael Shtutman "Inhibition of the DDX3 prevents HIV-1 Tat and cocaine-induced neurotoxicity by targeting microglia activation", Journal of Neuroimmune Pharmacology, 2020 Jun;15(2):209-223
Celia Cui, B., V. Sikirzhytski, M. Aksenova, M.D. Lucius, G.H. Levon, Z.T. Mack, C. Pollack, D. Odhiambo, E. Broude, S.B. Lizarraga, M.D. Wyatt, and M. Shtutman . Pharmacological inhibition of DEAD-Box RNA Helicase 3 attenuates stress granule assembly. Biochem. Pharmacol. 2020, 114280. PMID: 33049245.
Gasparian A, Aksenova M, Oliver D, Levina E, Doran R, Lucius M, Piroli G, Oleinik N, Ogretmen B, Mythreye K, Frizzell N, Broude E, Wyatt MD, Shtutman . Depletion of COPI in cancer cells: the role of reactive oxygen species in the induction of lipid accumulation, noncanonical lipophagy and apoptosis. M. Mol Biol Cell. 2022 Dec 1;33(14):ar135. doi: 10.1091/mbc.E21-08-0420. Epub 2022 Oct 12. PMID: 36222847
J. Sybrandt, M. Shtutman , I. Safro "MOLIERE: Automatic Biomedical Hypothesis Generation System", Proceedings of the 23rd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, pp. 1633-1642, 2017
Challenge the conventional. Create the exceptional. No Limits.
Compounding

A compounding pharmacy makes custom medication tailored to individual needs. For example:
- If you are allergic to an inactive ingredient in a prescribed medication, such as a preservative or dye, our pharmacist can make that medication without the inactive ingredient.
- If your child cannot swallow pills, we can make a liquid option that she can ingest more easily.
- If you take hormones, your body may react better to bioidentical hormone therapy.
At The Medicine Shoppe® Pharmacy, our focus is on your health and wellness. Talk to our pharmacist about using compounding to tailor your medication to your unique needs. Together with your doctor, we can find a treatment that works for you.

IMAGES
VIDEO
COMMENTS
This doesn't mean a retail pharmacy that adds compounding to its practice will go from 3% to 20% net profit, but he does routinely see the addition of compounding increase net profit significantly. "We measure this with a lot of the pharmacies that send us data," Bryan says, "and what we find is net profits in the range of 8-12% very ...
According to Bryan Prescott, high-performing compounding-only pharmacies can achieve net profit margins of around 20%, significantly higher than the typical 3% net profit for retail pharmacies. Adding compounding services to an existing retail pharmacy can increase net profits by 8-12%. To create accurate financial projections, consider factors ...
Compounding also broadens the pharmacy's customer base by capturing a special market share within independent pharmacy. "Compounding can be a good niche service and a good source of revenue," Hauser said. "The more specialized you become, there's a need to provide compounds to a wider geographical area.".
There are different standards for sterile, nonsterile, and hazardous drug compounding, so whichever you plan to provide in your pharmacy, ensure that you are adhering to all standards that apply. You may be surprised to know that some aspects of General Chapter <800> on hazardous drugs already apply to your pharmacy if your pharmacy supplies ...
Hawkins is the Sterile Compounding Pharmacy Program Manager at the Lexington VA Health Care System (HCS) in Lexington, KY. She coordinates and oversees compounding activities, compliance, and quality assurance for the HCS's main hospital and Community Living Center, as well as a variety of outpatient specialty clinics, including Urology, Ophthalmology, Infusion, Dermatology, and Chemotherapy.
The United States Pharmacopeia (USP) provides guidelines that inform pharmacies on best practices. They recently published their latest revisions to the compounding USP chapters <797> and <795>, which will be made official on November 1, 2023. As the leading provider of sterile and nonsterile compounding resources in the industry, we are dedicated to providing our members and customers with ...
Developed through the ASHP Council on Pharmacy Management and approved by the ASHP Board of Directors on June 6, 2015. These guidelines supersede the ASHP Guidelines on Outsourcing Sterile Compounding Services dated January 14, 2010. The revision of this document was led by Patricia C. Kienle, M.P.A., FASHP.
1) Be aware of the applicable norms, standards and regulations. Approximately, there are three main aspects that determine which regulations apply to a compounding facility: the type of preparations that will be compounded (sterile or non-sterile, hazardous or non-hazardous); the geographic market in the compounding facility operates, both ...
PCAB, Pharmacy Compounding Accreditation Board. 1970 1980 1990 1995 2000 2005 2010 1971—Deadly nationwide nosocomial infection outbreak 1992—FDA Compliance Guide, USP ... The sterile compounding area (ante and buffer areas) may be constructed of either hard- or soft-walled enclosures, and ) ...
2) The typical layout for sterile compounding. The areas reserved for compounding preparations must have at least two controlled, enclosed and distinct areas: a clean room (or buffer zone) in which the Primary Engineering Control (PEC) is located, and the anteroom. Some regulations strongly suggest a third room known as the support room, but ...
FOR COMPOUNDING STERILE PREPARATIONS Organizational practices employed for compounding sterile preparations are in compliance with USP standards related to sterile compounding. The organization implements well-defined policies and procedures to guide the compounding of sterile preparations.
Before obtaining a start-up Pharmacy loan, you need a solid Pharmacy business plan. A good Pharmacy business plan should include: a mission statement; an executive summary; ... USP 795 (Non-Sterile Compounding) USP 797 (Sterile Compounding) USP 800 (Handling of Hazardous Drugs) Third-Party Pharmacy Credentialing;
A pharmacy business plan is a plan to start and/or grow your pharmacy business. Among other things, it outlines your business concept, identifies your target customers, presents your marketing plan and details your financial projections. You can easily complete your pharmacy business plan using our Pharmacy Business Plan Template here.
USP 797 SCOPE AND PURPOSE. • Sterile compounding. • Combining, admixing, diluting, pooling, reconstituting, repackaging or other alterations of a drug or bulk drug substance to create a sterile compound. • Purpose of the chapter is to minimize harm • Aseptic technique must be followed • Administration is out of scope.
Sterile Compounding Pharmacist Board Certification Review and Recertification Activities. APhA has partnered with the U.S. Pharmacopeia (USP), the organization responsible for setting standards for sterile compounding, to plan and develop a comprehensive, innovative and professional development program for the Board of Pharmacy Specialties (BPS) Specialty in Compounded Sterile Preparations ...
16 hours live CE credit. This is a comprehensive introductory sterile compounding training course, which includes a review of USP Chapter <797>. This course was developed to train pharmacists and pharmacy technicians in aseptic technique and the basics of sterile compounding based on USP <797> standards. The live two-day workshop will review ...
Member Price: $125.00. Page Count: 488. The essential sterile compounding reference every pharmacist needs—now with important updates. From its inception, Compounding Sterile Preparations has served as both a professional reference guide for pharmacists and pharmacy technicians and a student textbook covering the regulation, technique, and ...
The basic configuration of the compounding rooms is this—an ante room sandwiched between the USP 797 and USP 800 laboratories. Pharmacy personnel enter the dirty side of the ante room where they don their lab garb, then cross to the clean side where they scrub prior to entering either lab. To enable communication between the highly regulated labs and the broader work area of the pharmacy ...
The National Pharmacy Strategy (NPS) is a 10-year plan to transform the delivery of pharmaceutical care and medication management in Singapore that was approved by the Ministry of Health (MOH). In 2017, MOH approved the implementation of the Hub-and-Spoke compounding and distribution model to provide
A recently released guidance document, the ISPE Guide: 503A Compounding - Regulatory Basis and Industry Good Practices for Pharmacies, summarizes and contextualizes key information from US governmental bodies relevant to 503A pharmacies. The resource is intended to benefit the existing pharmacy compounding industry, those considering entering that space, or third parties working with ...
Sterile Compounding Technician Toolkit. Sterile compounding is a complex specialty that allows pharmacy technicians to work in an advanced role. There are many different practice settings such as hospitals, infusion centers, compounding facilities and more. In general, a sterile compounding role involves creating IV infusions in a clean room ...
In conclusion, these updates to the USP Chapter have significant implications for compounding pharmacies. By adhering to these new requirements for sterility, cleaning, and garbing, pharmacies can ensure the safety and quality of compounded medications. With support from Contec Healthcare, pharmacies can successfully navigate these changes and ...
DNA Pharmacy Services, Inc. (dba Palm Beach Compounding), Jupiter, FL - 503A Facility - Ceased sterile compounding FMD 145 Letter Issued 03/05/2019 Untitled Letter Issued 05/08/2018
FDA is alerting patients and health care professionals not to use compounded products intended to be sterile, produced and distributed nationwide by Prescription Labs Inc. doing business as ...
To fill your Semaglutide prescription, reach out to our team: Phone: 410-644-8400 (ext. 4 for compounding) Shipping: FAST shipping to Maryland, Delaware, and Pennsylvania. Location: 3455 Wilkens Ave, Ste 103, Baltimore, MD 21229. Connect with us on !
College of Pharmacy: Email: [email protected]: Phone: 803-777-8988: Office: College of Pharmacy 715 Sumter Street - CLS 713A Columbia, SC 29208: ... Business; Education; Engineering and Computing; The Graduate School; Hospitality, Retail and Sport Management; Information and Communications; Law; Medicine (Columbia)
Compounding provides an innovative way for pharmacists to customize medications to fit the needs of their patients. The art of compounding utilizes modern medicine while still holding true to the roots of the profession of pharmacy. Compounding pharmacies can produce unique dosage forms based on patient preferences and/or restrictions.
26 Sterile Compounding jobs available in Moscow, IA on Indeed.com. Apply to Pharmacy Technician, Pharmacy Technician II, Process Technician and more!