- Open access
- Published: 13 January 2022

Evaluation of adverse effects/events of genetically modified food consumption: a systematic review of animal and human studies
- Chen Shen 1 ,
- Xiang-Chang Yin 2 ,
- Bo-Yang Jiao 3 ,
- Jing Li 4 ,
- Peng Jia 5 ,
- Xiao-Wen Zhang 1 ,
- Xue-Hao Cheng 6 ,
- Jian-Xin Ren 6 ,
- Hui-Di Lan 7 ,
- Wen-Bin Hou 1 ,
- Min Fang 1 ,
- Yu-Tong Fei 1 ,
- Nicola Robinson 1 , 8 &
- Jian-Ping Liu ORCID: orcid.org/0000-0002-0320-061X 1 , 9
Environmental Sciences Europe volume 34 , Article number: 8 ( 2022 ) Cite this article
46k Accesses
15 Citations
67 Altmetric
Metrics details
A systematic review of animal and human studies was conducted on genetically modified (GM) food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research.
Seven electronic databases were searched from January 1st 1983 till July 11th 2020 for in vivo, animal and human studies on the incidence of adverse effects/events of GM products consumption. Two authors independently identified eligible studies, assessed the study quality, and extracted data on the name of the periodical, author and affiliation, literature type, the theme of the study, publication year, funding, sample size, target population characteristics, type of the intervention/exposure, outcomes and outcome measures, and details of adverse effects/events. We used the Chi-square test to compare the adverse event reporting rates in articles funded by industry funding, government funding or unfunded articles.
One crossover trial in humans and 203 animal studies from 179 articles met the inclusion criteria. The study quality was all assessed as being unclear or having a high risk of bias. Minor illnesses were reported in the human trial. Among the 204 studies, 59.46% of adverse events (22 of 37) were serious adverse events from 16 animal studies (7.84%). No significant differences were found in the adverse event reporting rates either between industry and government funding ( χ 2 = 2.286, P = 0.131), industry and non-industry funding ( χ 2 = 1.761, P = 0.185) or funded and non-funded articles ( χ 2 = 0.491, P = 0.483). We finally identified 21 GM food-related adverse events involving 7 GM events (NK603 × MON810 maize, GTS 40-3-2 soybean, NK603 maize, MON863 maize, MON810 maize, MON863 × MON810 × NK603 maize and GM Shanyou 63 rice), which had all been on regulatory approval in some countries/regions.
Serious adverse events of GM consumption include mortality, tumour or cancer, significant low fertility, decreased learning and reaction abilities, and some organ abnormalities. Further clinical trials and long-term cohort studies in human populations, especially on GM food-related adverse events and the corresponding GM events, are still warranted. It suggests the necessity of labelling GM food so that consumers can make their own choice.
Introduction
Genetic modification is defined as introducing transgene(s) with desired traits into the recipient organism’s genome by recombinant deoxyribonucleic acid (DNA) technology, and therefore it does not occur naturally [ 1 , 2 , 3 ]. Genetically modified (GM) crops are thought to address food security, sustainability and climate change solutions by improving crop yields, conserving biodiversity, providing a better environment in terms of the insect-resistant and herbicide-tolerant traits, reducing CO 2 emissions and helping alleviate poverty through uplifting the economic situation [ 4 ]. Insect-resistant and herbicide-tolerant traits were first introduced into four types of crop, canola, cotton, maize and soybeans, at the beginning of GM production [ 5 ]. At present, the mainstream characteristics of new crops still pursue higher-yielding, more nutritious, pest- and disease-resistant and climate-smart to meet future demand for a yield increase of major crops such as wheat, rice and corn, due to the growing population [ 6 ].
Since 1996, the first year of commercialization of GM crops, 70 countries had adopted GM crops until 2018, including 26 countries that cumulatively planted 2.5 billion hectares of GM crops and an additional 44 countries that imported GM crops. During the 27 years (1992 to 2018), 4349 approvals for 387 GM events from 27 GM crops were granted by 70 countries involving 2063 for food (when the direct consumers are mainly humans), 1461 for feed (the products only intended for animal consumption) use and 825 for environmental release or cultivation [ 4 , 7 ]. The major agricultural product exporting countries like the U.S.A., Brazil and Argentina show over 90% adoption of biotech crops [ 4 ]. For GM animal products, biotech salmon, considered to be the first genetically engineered animal for human consumption, was approved by the United States Department of Agriculture and Food & Drug Administration in 2015 [ 8 ]. In addition, it is illegal to grow major GM food crops in China while there are substantial investments in biotechnology research and GM maize, soybeans, and canola are allowed to import and eat [ 9 ].
Genetically modified food, however, is an example of the controversial relation between the inherent uncertainty of the scientific approach and the need of consumers to use products resulting from scientific developments thought to be safe [ 10 ]. Significant health risks have not been reported in peer-reviewed studies on GM food safety/security, which may cause some publication bias [ 11 ] but with a few exceptions, like the most famous “Monarch Butterfly controversy” [ 12 ], "Pusztai case" [ 13 ] and the "Séralini case" [ 14 ]. Unexpected effects of GM crops were reported in these studies, occupying an important place in the pages of scientific journals. Nevertheless, the above controversies severely impacted the public image, leading to full or partial bans in 38 countries including the European Union [ 15 ].
The complexity of risk evaluation is shown in these conflicting results, and concerns about the citizen-consumers have been raised against GM food [ 10 ]. Of most concern, aroused from the controversial events and some research results, is the potential of carcinogenesis, teratogenesis [ 16 ], lethal effects and adverse influences on fertility. GM agriculture is now widely discussed in both positive and negative frames and currently serves as a hotbed of debate in the public and policymakers. Although there are some reports and evidence from human and animal studies on the potential health effects of GM food/feed, the evidence is not conclusive and public concerns have not been resolved.
We aimed to conduct a systematic review of animal and human studies on GM food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research.
This study was a systematic review of previously published studies, conducted and reported in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 17 ] guideline.
Search strategy
China National Knowledge Infrastructure (CNKI), Wanfang, VIP Database, Chinese Biomedical Database (SinoMed), PubMed, the Cochrane Library and Embase databases were searched from January, 1st, 1983 till July, 11th, 2020, using a predefined search strategy (Additional file 1 : Appendix S1). Reference lists of retrieved articles were also searched.
Eligibility criteria
Based on the evidence pyramid proposed by the Medical Center of State University of New York in 2001, we determined the type of research we included in the study. For a comprehensive evaluation of the literature, all in vivo animal studies and human studies (cross-sectional studies, case reports, case series, case–control studies, case–crossover studies, cohort studies, controlled clinical trials, including randomized trials, quasi-randomized trials and non-randomized trials) in multiple languages were included. Animal studies in all fields were included, that is, they could be clinical, agricultural and animal husbandry, veterinary medicine, life sciences, etc. Field studies were excluded.
The study population in animal studies was applied with inclusion criteria based on the categorization approach that highlights the actual use of them: laboratory animals and economical animals (livestock and aquatilia) were included, with no prespecified limitations on age, population, species/races, health status or others. Interventions/exposures of the genetically modified animal/plant/microorganism products included for animal/human ingestion referred to GM food, GM food ingredients and GM feed, regardless of their dosage or duration. The GM strain (line) and GM event were not limited. There was no restriction on whether controls were or were not included. The studies were excluded if they focused on the effects of GM food/feed on secondary or multilevel consumers in the food chain where GM food/feed was only consumed by primary consumers in the predator relationships. For instance, if non-GM fishes were fed with diet containing GM ingredients and then the fish was fed to the experimental cats, the study was excluded.
Outcomes focused on the incidence of adverse effects or adverse events in GM food/feed consumption, including primary outcomes on carcinogenesis, teratogenesis, lethal effect (all-cause mortality) and reproduction and secondary outcomes on other biomarkers were included. Toxicity studies of general toxicity studies (acute, sub-acute, sub-chronic, chronic and carcinogenicity toxicity studies) and specific toxicity studies (genotoxicity, reproductive and developmental toxicity, immunotoxicity and other toxicology studies) were included. Mortality in pups before weaning was considered as an outcome of reproductive toxicity but not as a lethal effect. Outcomes of adverse events in laboratory testing would not be included only when they could indicate tissue or organ toxicity. Outcomes of adverse events in breeding performance in animal husbandry studies, which focused on the economic benefits of the animal products, were included and these indicators were regarded as reproduction biomarkers in this research.
Outcomes of adverse events on growth performance, carcass traits, meat and fur production performance and meat quality for economic benefit evaluation of live stocks were excluded, of which the indicators included final body weight, weight gain, feed to gain ratio, half-eviscerated weight, eviscerated weight, percentage of eviscerated yield and muscle lean meat, sebum rate in some parts of the body, etc. Studies on the insecticidal effect of insect-resistant GM feed and outcomes of adverse events in gene fragments residual in the digestive tract were excluded. Besides, duplicate publications, studies with duplicate statistics, or references devoid of necessary information of participants, sample size, interventions/exposures or results were excluded.
Study selection and data extraction
Titles and abstracts of the retrieved articles were reviewed by 6 researchers in pair (C Shen, XC Yin, BY Jiao, J Peng, YZ Li, XH Cheng). 6 authors (C Shen, XC Yin, BY Jiao, JX Ren, J Li and XW Zhang) independently reviewed the full texts to identify the studies meeting eligibility criteria and then 8 researchers in pair (C Shen, XC Yin, BY Jiao, J Li, P Jia, XW Zhang, XH Cheng and JX Ren) independently extracted data from the included studies according to a predesignated extraction table. The discrepancies were resolved through consensus and if necessary, arbitrated by another author (JP Liu).
We extracted the name of the periodical, author and affiliation, literature type, the theme of the study, publication year, funding, sample size, target population characteristics, type of the intervention/exposure, outcomes and outcome measures. For those studies in which adverse effects/events occurred, details of interventions/exposures and control conditions (if any), dosage, duration, number of the generation, and the results were extracted.
Quality assessment
The methodological quality for animal studies was assessed, using criteria from the SYRCLE’s risk of bias tool for animal studies. The quality of animal studies was categorized into low risk of bias, unclear risk of bias, or high risk of bias according to the risk for each important outcome within included studies, including the adequacy of generation of the sequence generation, baseline characteristics, allocation concealment, random housing, blinding (performance bias), random outcome assessment, blinding (detection bias), incomplete outcome data, selective outcome reporting, or other sources of bias. The judgment of other risk of bias was based on whether there were contamination (pooling drugs), inappropriate influence of funders, unit of analysis errors, design-specific risks of bias or new animals added to the control and experimental groups to replace drop-outs from the original population.
Statistical synthesis and analyses
Statistical analyses were carried out using Microsoft Excel 2016 and SPSS 20.0. The findings were reported mainly in two parts, characteristics of the included studies and detailed information on the studies in which adverse effects/events occurred. Initially, descriptive statistics, frequencies, and percentages were calculated to summarize the data. Subsequently, studies that evaluated similar populations, interventions, controls (if any) and outcomes were pooled using a random-effects meta-analysis, and data from other studies were presented in tables and described in a narrative summary. The incidence of adverse events reported in articles funded by industry funding, government funding or unfunded articles were, respectively, counted and the Chi-square test was used for the comparisons.
Besides, we figured the incidence of serious adverse events (SAEs) by percentage. With reference to the Food and Drug Administration’s definition [ 18 ], our study defined SAEs as death, life-threatening, hospitalization (initial or prolonged), disability or permanent change, disruption, impairment or damage in a body function or structure (including cancer or tumour), in physical activities or quality of life, congenital anomaly or birth defect in the newborn child or pups, infertility or significant low in the number of deliveries or live birth rate than the non-GM commercial, conventional or blank controls, and an event resulting in intervention/treatment to prevent permanent impairment, damage or to prevent one of the other outcomes.
Meanwhile, the adverse events which cannot be ruled out that it has nothing to do with GM food (hereinafter abbreviated as GM food-related adverse events) were identified and the percentages under each outcome were calculated.
Description of studies
The flow diagram of the literature selection is shown in Fig. 1 . A total of 9668 records were identified, including 9584 from the initial search through seven databases and 84 from other sources. After removal of duplicates and exclusion of references by reading titles and abstracts, 455 full-text articles were screened and 276 references were excluded with reasons (seen in the flow chart). Finally, 204 studies from 179 articles [ 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 ] (153 journal articles, 22 dissertations, 3 conference proceedings and 1 unpublished report) were included in data synthesis, since there were more than one study conducted in each of the 2 included dissertations [ 107 , 127 ], 11 journal articles [ 19 , 33 , 35 , 63 , 67 , 88 , 102 , 118 , 132 , 172 , 184 ] and 1 unpublished report [ 32 ]. The included studies were of 203 in vivo animal studies and 1 crossover trial [ 97 ] in humans.
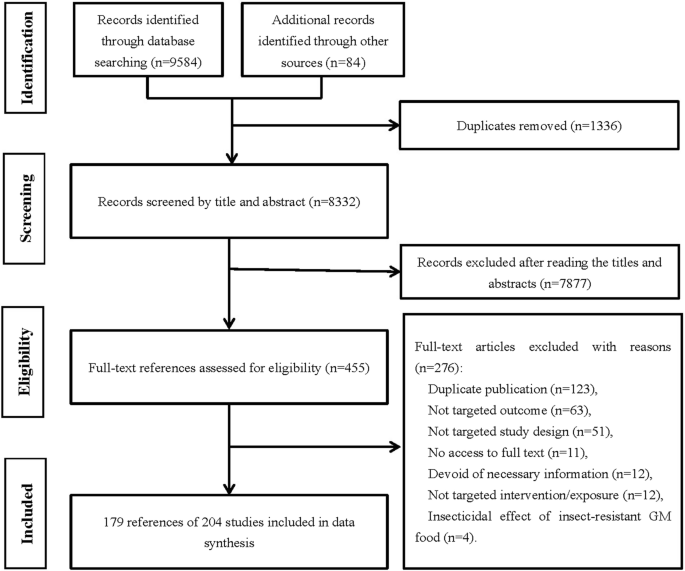
The flow of literature search and selection of studies on the safety of GM food
Study characteristics
Of the 179 included articles, 94 were in English [ 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 ], 83 were published in Chinese [ 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 ], and 2 in Japanese [ 196 , 197 ]. The earliest included reference dated back to 1998 [ 153 ] (shown in Fig. 2 ), after which the remaining articles were distributed from 2000 to 2020 (45 articles in the 2000s, while 131 in the 2010s and 2 in the 2020s). The year 2012 witnessed the largest volume of publication (n = 26 articles, 14.53%). For funding sources or sponsors (Additional file 1 : Appendix S2), in addition to 57 articles not mentioning the funding/sponsor (hereinafter as non-funded articles), there were 116 articles (64.8% of the 179 articles) supported by 56 kinds of government funding from 12 countries/government organizations and, still, 9 articles (5.03%) by 10 kinds of industry/institute funding sources/sponsors from 4 countries (America, Australia, French and German). Among them, 3 articles [ 29 , 62 , 74 ] claimed to have been funded or sponsored by both government and industry. China had undertaken the most government/school-level funding projects (39 of 56 projects, 69.64%).
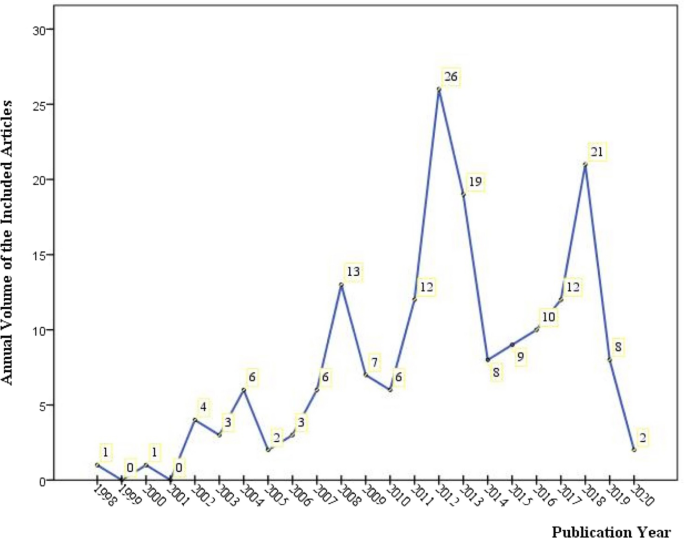
The publications (number of articles) on the safety of GM food by year
The periodicals that have published more than 5 included articles were Food and Chemical Toxicology (published 25 included articles), EFSA Journal (13), Regulatory Toxicology and Pharmacology (9), Journal of Hygiene Research (9) and Chinese Journal of Food Hygiene (8). 11 of 13 authors, who have published ten or more included studies, were from European Food Safety Authority and published 12 included articles as co-authors. They were Christina Tlustos (published 12 included articles), Claudia Bolognesi (12), Konrad Grob (12), Vittorio Silano (12), Andre Penninks (11), Gilles Riviere (11), Holger Zorn (11), Karl-Heinz Engel (11), Yi Liu (11), Natalia Kovalkovicova (10), Sirpa Karenlampi (10). In addition to the above 12 articles, the top 3 of the 11 authors who published five or more included studies was Yang Xiao-Guang (from Chinese Center for Disease Control and Prevention, published 11 included articles), Wang Jing (from Tianjin Centre for Disease Control and Prevention, published 10 included articles) and Zhuo Qin (from Chinese Center for Disease Control and Prevention, published 7 included articles). The top 5 affiliations which published included articles were Chinese Center for Disease Control and Prevention (published 16 included articles), Tianjin Centre for Disease Control and Prevention (12), European Food Safety Authority (12), National Chung Hsing University (10), International Rice Research Institute (9).
Of the 204 included studies, one was a double-blind crossover trial ( n = 36) in humans and the others were all animal studies. Individual sample sizes of the total 54,392 study population ranged from 4 (cats) [ 153 ] to 21,000 (Atlantic salmon) [ 23 ]. The studies involved 14 different kinds of animals (see Table 1 ). Apart from the most commonly used rats/mice (in 160 studies, 78.82%), pigs and chicks were two of the most extensively studied animals (in 23 studies, 11.33%). For themes of the 178 included animal studies, 158 were on clinical and 20 were on agricultural and animal husbandry. For the ones on clinical, 117 were on general toxicity (8 on acute, 6 sub-acute, 84 sub-chronic, 16 chronic toxicity, and still 3 on both acute, sub-acute and sub-chronic toxicity), 35 on specific toxicity (15 on reproductive and developmental toxicity, 16 on immunotoxicity, 3 on teratogenic effect and 1 on mutagenicity), 3 on allergenicity, 1 on learning and memory ability, 1 on athletic ability and 1 on both sub-chronic toxicity and allergenicity.
For interventions/exposures, 31 kinds of GM food were identified, including 18 kinds of GM plant food, 7 kinds of GM animal food and 6 kinds of GM microorganism food. Each included study covered one intervention/exposure, except for one study, Chen [ 29 ], that involved two kinds of GM products (sweet pepper and tomato) modified with the same gene (coat protein gene of cucumber mosaic virus), respectively, in two experimental groups. Maize, rice and soybean were the three most popular kinds of GM plant food (taken 79.38%) in research while milk/milk powder and animal-derived protein occupied the top two in GM animal food (56.25%). As for GM microorganism products, 5 kinds of food/feed enzyme derived from 5 different kinds of GM fungi or bacteria as well as 1 kind of microorganism-derived protein were among included studies.
Methodological quality of the animal studies
According to our predefined quality assessment criteria, all of the studies were identified as being unclear or having a high risk of bias (Fig. 3 ). None of the studies were reported to blind researchers from knowing which intervention each animal received. None of the studies reported prior sample-size calculation, 31 studies (15.27%) described wrong randomization procedures or did not mention the method of “randomization”, and 12 studies (5.91%) did not report adequate allocation concealment. 28 studies (13.79%) described that the groups were similar at baseline and 76 studies (37.44%) claimed that the housing conditions of animals from the various experimental groups were identical. 10 studies (4.93%) described randomly pick an animal during outcome assessment while 7 studies (3.45%) failed to select animals at random for outcome assessment. 88 studies (43.35%) completely used objective outcome indicators for outcome measurement. 185 studies (91.13%) reported consistent outcomes in the method and result sections while 5 studies did not, but none of the study protocols were available.
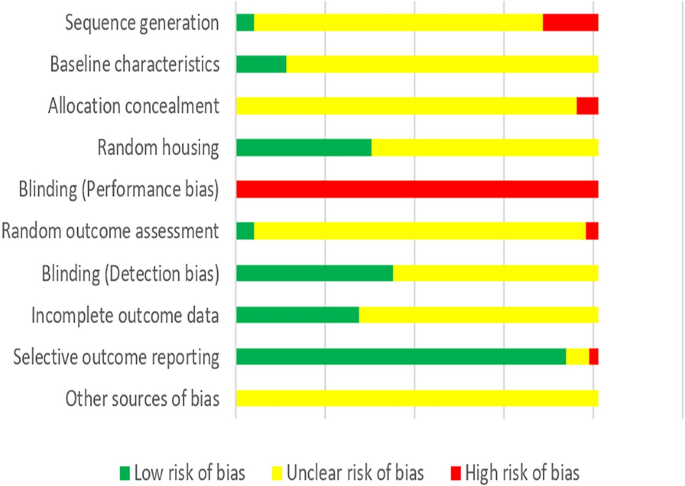
Risk of bias of the included animal studies
Incidence of adverse events/effects
No meta-analysis was conducted due to the significant heterogeneity of the primary studies. Among the 204 studies, a total of 29 studies (14.22%) from 23 articles reported 37 adverse events, involving 13 on mortality, 6 on reproductive toxicity, 3 on carcinogenesis and 15 on other biomarkers (including one human trial). It is worth noting that when, in one study, there were multiple aspects of adverse events on “other biomarkers”, we recorded it as 1 adverse event. Then, 22 serious adverse events (59.46% of adverse events) were identified in 16 studies (7.84% of the included studies and 55.17% of the studies reporting adverse events, marked in the tables with double asterisks). The SAEs mainly rested on mortality (13 studies), tumour or cancer (3), significant low in the number of pup deliveries (2), decreased learning and reaction abilities (1), severe stomach inflammation (1), intestinal adenoma lesions (1), and other pathology abnormalities (1) as hypertrophies and hyperplasia in mammary glands and pituitary, liver congestions and necrosis as well as severe chronic progressive nephropathies.
The incidence of adverse events reporting in government funding, industry funding and non-funded articles were 10.34% (12 of 116), 33.33% (3 of 9) and 15.79% (9 of 57), respectively. When comparing the adverse event reporting rates using the Chi-square test, we found that there were no significant differences either between industry funding and government funding ( χ 2 = 2.286, P = 0.131), industry funding and non-industry funding ( χ 2 = 1.761, P = 0.185) or funded and non-funded articles ( χ 2 = 0.491, P = 0.483).
Incidence of adverse events/effects in human trial
As for the human trial [ 97 ], shown in Table 2 , a randomized double-blind crossover design was conducted for acute consumption of two single breakfasts, with a 14-day washout period, containing either seed oil generated from transgenic Camelina sativa plants or commercially blended fish oil. 36 healthy people were randomly allocated into two groups and venous blood samples were collected after the postprandial session, 8 h after each meal. No follow-up was reported. No major adverse symptoms or health effects were reported but some unrelated minor illnesses for the 72 postprandial sessions from 36 participants, such as minor upper respiratory tract infections (2.78%), minor nose bleed (1.39%), pyelonephritis (1.39%) and headaches (8.33%).
Incidence of adverse events/effects in animal studies
For the 203 animal studies, 28 studies (13.79%) from 22 articles reported 36 adverse events, including 13 on mortality (Table 3 , 36.11%), 6 on reproductive toxicity ( Table 4 , 16.67%), 3 on carcinogenesis (Table 5 , 8.33%) and 14 on other biomarkers (Additional file 1 : Appendix S3, 38.89%).
All causes of death were included in this analysis and 11 of the 13 studies claimed that the mortality was not significantly different between the groups or had nothing to do with GM food. One study (Ermakova [ 37 ]) reported higher pup mortality in the Roundup-Ready soya (40.3.2 line) group compared with the controls. In Séralini [ 74 ] , the general cause of death was large mammary tumours in females and other organ problems in males. Besides, rats in the Roundup-tolerant GM NK603 maize groups were 2–3 times more likely to die than controls, and more rapidly.
With respect to effects on reproduction, 5 animal feeding studies were reported to trigger reproductive toxicity but one study (Cisterna [ 31 ]) claimed to have no substantial impact on fertility. The reproductive toxicity manifested in the significant low in the number of deliveries, survival rate (from birth to weaning), litter weight, litter size and weight of some organs in the pups. For example, in Ermakova I 2005, the rats fed with Roundup-Ready soya had a 55.6% pup mortality rate during lactation periods compared to 9% in the control of traditional soya and 6.8% in the reference group. The pups kept dying during the lactation period while pups from the control group only died during the first week. Cyran N 2008 a and Cyran N 2008 c [ 32 ] were two rat feeding studies reported in one article, both given NK603 × MON810 maize. A multi-generation study was conducted as Cyran N 2008 a while Cyran N 2008 c did a continuous breeding study. Both of them indicated that fewer sum of pups was born and weaned in the GM groups. Pup losses, in Cyran N 2008 a, overall generations were about twice as many pups lost as compared to the control group (14.59% vs 7.4%) but was not significantly different and significantly lower litter weight was also reported in Cyran N 2008 c.
Three mouse/rat feeding studies reported triggering cancers/tumours when Tang [ 156 ] attributed the incidence of the tumour to the elder age of rats. Séralini 2014 (on Roundup-tolerant GM maize) found that females in the treatment groups almost always developed large mammary tumours more often than and controls. As for males, 4 times larger palpable tumours than controls were presented which emerged up to 600 days earlier. Cyran 2008 b [ 32 ] revealed a life term study where mice in the three groups were fed with transgenic maize NK603xMON810 (from 33.0% in the diet), control isoline diet and GM-free Austrian corn reference diet, respectively. The survival rate was not significantly different while cancer (leucosis) was the common cause of death.
GM food-related adverse events
Among the 37 adverse events reported, 16 of them claimed to have nothing to do with GM food, while the rest 21 (from 17 studies) did not, still leaving the question open. The GM food-related adverse events existed in mortality (2 studies), reproductive toxicity (5), carcinogenesis (2), and other biomarkers (12).
By gathering evidence, we identified 3 kinds of GM food associated with adverse events, GM soybean, GM maize as well as GM rice. For the 17 studies involved in the GM food-related adverse events, 4 studies were absent of information on the GM event of their test substance and the remainder concentrated on 7 GM events (3 studies on NK603 × MON810 maize, 2 on GTS 40-3-2 soybean, 2 on NK603 maize, 2 on MON863 maize, 2 on MON810 maize, 1 on maize mixed with MON863 × MON810 × NK603, NK603 × MON810 and NK603 and 1 on GM Shanyou 63 rice). When searching in the GM Approval Database on the ISAAA website, we found that all of the first 6 GM events listed, all developed by Monsanto Company, had been on regulatory approval for food, feed and cultivation in multiple countries/regions, including the European Union. GM -39 Shanyou 63 was developed in China and given approval for food, feed, and cultivation only by China in 2009.
Summary of findings
We included 203 in vivo animal studies and 1 human trial, and all of the studies were identified as being unclear or having a high risk of bias. Overall, we reported two main findings. First, we identified 37 adverse events for GM food consumption while 22 of them (59.46%) were serious adverse events extracted from 16 animal studies (7.84%). SAEs were mortality, tumour or cancer, significantly low in the number of pup deliveries, decreased learning and reaction abilities, severe stomach inflammation, intestinal adenoma lesions, and other pathological abnormalities in the mammary glands, pituitary, liver and kidney.
Second, there were 21 GM food-related adverse events indicating that GM food may have effects on increased mortality (2 studies), reproductive toxicity (5 studies), which referred to significantly low fertility in parental generation and low survival rate, litter weight, litter size and weight of some organs in the pups, carcinogenesis (2 studies) and other biomarkers (12 studies). The effect-related GM food included 7 GM events (NK603 × MON810 maize, GTS 40-3-2 soybean, NK603 maize, MON863 maize, MON810 maize, MON863 × MON810 × NK603 maize and GM Shanyou 63 rice), which had all been on regulatory approval for food, feed and cultivation in some countries/regions.
Agreements and disagreements with other reviews
To our knowledge, there have been 3 previous systematic reviews (SRs) [ 198 , 199 , 200 ] and 6 conventional reviews [ 16 , 201 , 202 , 203 , 204 , 205 ] addressing similar research questions on the unexpected effects of GM food consumption. Keshani et al. [ 198 ], searching in 4 English databases, included experimental studies on GM crops’ potential effects on sperm parameters. The study finally included 7 rat feeding studies, which were all identified in our study, and indicated no harm to GM plants consumers. Edge et al. [ 199 ] addressed 30 review questions for including human studies, published in recent 20 years (1994–2014), on health effects of genetically engineered (GE) food crops, but found no human study on 25 questions. The remaining 5 questions, related to allergenicity and nutrient adequacy, were answered based on 21 human studies. The human studies were all excluded in our research because of no direct ingestion of GE food in the allergenicity assessment studies or no targeted outcomes in the nutrient assessment trial. To illustrate, the above-mentioned nutrient assessment clinical trial evaluated the effect of carrots containing twofold higher calcium content on calcium absorption and we thought it was not on outcome related to adverse events/effects. The conclusion of the research also supported that there were no clear adverse health effects associated with the consumption of GE food. Moreover, Dunn et al. [ 200 ] included both human and animal studies for examining the allergenicity of GM organisms and finally found 34 human studies and 49 animal studies eligible. In addition to 32 human studies which involved human serum for IgE binding or inhibition studies and not direct consumption of GM product, the rest 2 [ 206 , 207 ]studies were on actual ingestion of a GM food. However, they were not included in our research because of not targeted study type and unrelated outcomes. The conclusion agreed with the first two SRs that GM foods did not appear to be more allergenic than their conventional counterparts.
As for conventional reviews, Domingo showed special attention to the safety of GM food and published four literature reviews in 2000 [ 203 ], 2007 [ 204 ], 2011 [ 205 ] and 2016 [ 16 ]. Domingo searched two databases, PubMed and Scopus, to assess adverse/toxic effects of GM plants. In the latest updated review, he addressed the conclusion that GM soybeans, rice, corn/maize and wheat would be as safe as the parental species of these plants. However, our results may not be consistent with Domingo’s conclusion: we focus on a summarization of adverse events for GM food consumption through a systematic search in 7 databases; we identified 37 adverse events, 22 serious adverse events and 21 GM food-related adverse events; GM maize, soybean and rice with some specific GM events were all related to GM food-related adverse events. In addition, Domingo found a notable advance of studies published in scientific journals by biotechnology companies. Coincidentally, we did a Chi-square test to compare the adverse event reporting rates and found no significant differences between industry funding, government funding and non-funded articles. Besides, our systematic review validated Domingo’s findings that some GM plants were studied scarcely in recent years including GM potatoes discussed in the controversy of Pusztai case.
Strengths and limitations
In this review, a systematic search of major databases was conducted to identify all available studies in all languages on the adverse effects/events of GM food consumption. To make the inclusion and data synthesis comprehensive, both in vivo human and animal studies in all fields were included, with no limitations on the type of participant, type of intervention/exposure or whether control was included. The terms used for searching, containing all kinds of names of GM food, were based on a basic search on the internet by the researchers and the list was perfected as much as possible. With respect to additional searching, we went through multifarious news which reported controversy of GM food and thus we identified several hot studies by following the clue. In order to trace the potential conflicts of interest, we performed a Chi-square test for comparing adverse events report rates in articles funded by industry funding, government funding or unfunded articles, but found no statistical significance. Nevertheless, it was hard to conduct a quantitative data synthesis for the effects of GM food consumption on the adverse events because of the significant heterogeneity of the primary studies.
There are several limitations in this review. The methodological quality of the included studies is generally poor, which indicates a high or unclear risk of bias resulting from insufficient reporting of methodological components in the studies. Methodological quality may not be fully reflected based solely on the reporting of the manuscript. There were unclear descriptions of randomization procedures and a lack of blinding in all of the studies, which may have created potential performance biases and detection biases, as researchers might have been aware of the effects of interventions. The ability to perform meta-analysis was limited because of the heterogeneity of the participants, interventions (GM food in various GM events), comparisons, feeding doses, administration time, other exposure factors, and the variance of composite outcome measures used in the 204 included studies. When we did the manual search, we found that related publications were retracted sometimes, under the name of inadequate experimental designs or statistical analysis. For example, Séralini 2012 was retracted by Food and Chemical Toxicology , but subsequently republished in another journal [ 14 , 74 ]. This indicates that it was hard for us to find the original full-text papers of the retracted publications and articles provided by databases still have some unavoidable publication bias. The retraction on controversial researches may also cause the controversy for the public to doubt the reality of the studies published and to concern the safety of GM food. In addition, the lack of human studies is another key limitation of this research. As for the searching strategy, we did not include publication types as newspaper articles and comments. This was thought to be a limitation of this research because these sources may give us clues of related researches and can help us to do a manual search comprehensively. It is also an implication for future systematic reviews.
Implications for research
Future research should be conducted in humans, especially observational cohort studies. High-quality animal studies according to the ARRIVE reporting standard focusing on reproductive toxicity and carcinogenesis are still needed. Trials or studies should be registered prospectively, and be accessible. Furthermore, to address public concerns, future studies should focus on SAEs and GM food-related adverse events reported in this research such as NK603 maize, MON863 maize and MON810 maize. Meanwhile, some implications of findings still could be explored such as how GM food affects people’s eating habits, labelling of GM food and public choice. Some of the included studies conducted an intergenerational or multigenerational evaluation of the safety of GM food, but only two studies (Cyran N 2008 a and Cyran N 2008 c) in one article reported adverse events related to fertility. The differences in the results may be due to different interventions/exposures (GM food in certain GM events), laboratory animals, intervention/exposure time, experiment environment, etc. Therefore, it is necessary for subsequent studies to start with intergenerational or multigenerational research to verify the safety of GM food in terms of study design.
Serious adverse events accounted for 59.46% of the total 37 identified adverse events of GM consumption, which include: mortality, tumour or cancer, significantly lower number of pup deliveries, decreased learning and reaction abilities, and organ abnormalities in the stomach, intestinal adenoma, mammary glands, pituitary, liver and kidney. The interventions/exposures in the adverse event related studies emphasized on GM soybean, maize and rice in specific GM events. Animal studies occupy the lowest hierarchy of evidence, and there are flaws in study design and is not convincing enough. The evidence on the effect of GM consumption on humans is still insufficient. Further clinical trials and long-term cohort studies in human populations, especially on GM food-related adverse events and the corresponding GM events, are still warranted. It is better to prove the safety before they are approved for food consumption and it also suggests the necessity of labelling on GM food so that consumers can make their own choice.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
Genetically modified
Deoxyribonucleic acid
China National Knowledge Infrastructure
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Serious adverse event
Camelina sativa Seed oil
Blended fish oil
Body weight
Systematic reviews
Genetically engineered
Hundleby PA, Harwood WA (2019) Impacts of the EU GMO regulatory framework for plant genome editing. Food Energy Secur. https://doi.org/10.1002/fes3.161
Article Google Scholar
Sprink T, Eriksson D, Schiemann J et al (2016) Regulatory hurdles for genome editing: process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep 35:1493–1506. https://doi.org/10.1007/s00299-016-1990-2
Article CAS Google Scholar
Georges F, Ray H (2017) Genome editing of crops: a renewed opportunity for food security. GM Crops Food 8:1–12. https://doi.org/10.1080/21645698.2016.1270489
ISAAA (2018) Global status of commercialized biotech/GM Crops in 2018: biotech crops continue to help meet the challenges of increased population and climate change. ISAAA Brief No. 54 [Online]. http://www.isaaa.org/resources/publications/briefs/54/default.asp . Accessed 17 July 2020
Andrew J, Ismail NW, Djama M (2018) An overview of genetically modified crop governance, issues and challenges in Malaysia. J Sci Food Agric 98:12–17. https://doi.org/10.1002/jsfa.8666
Hickey LT, Hafeez AN, Robinson H et al (2019) Breeding crops to feed 10 billion. Nat Biotechnol 37(7):744–754. https://doi.org/10.1038/s41587-019-0152-9
Giraldo PA, Shinozuka H, Spangenberg GC et al (2019) Safety assessment of genetically modified feed: is there any difference from food? Front Plant Sci 10:1592. https://doi.org/10.3389/fpls.2019.01592
ISAAA (2016) Global status of commercialized biotech/GM crops: 2016. ISAAA Brief No. 52 [Online]. http://www.isaaa.org/resources/publications/briefs/52/default.asp . Accessed 17 July 2020
Pray C, Huang J, Hu R, Deng H et al (2018) Prospects for cultivation of genetically engineered food crops in China. Global Food Secur Agric Policy Econ Environ 16:133–137. https://doi.org/10.1016/j.gfs.2018.01.003
Martinelli L, Karbarz M, Siipi H (2013) Science, safety, and trust: the case of transgenic food. Croat Med J 54:91–96. https://doi.org/10.3325/cmj.2013.54.91
Klümper W, Qaim M (2014) A meta-analysis of the impacts of genetically modified crops. PLoS ONE. https://doi.org/10.1371/journal.pone.0111629
Losey JE, Rayor LS, Carter ME (1999) Transgenic pollen harms monarch larvae. Nature 399:214. https://doi.org/10.1038/20338
Ewen SW, Pusztai A (1999) Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet 354:1353–1354. https://doi.org/10.1016/S0140-6736(98)05860-7
Séralini GE, Clair E, Mesnage R et al (2012) Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize [retracted in: Food Chem Toxicol. 2014 Jan;63:244]. Food Chem Toxicol 50(11):4221–4231. https://doi.org/10.1016/j.fct.2012.08.005
Genetic Literacy Project (2017) Where are GMOs grown and banned? [Online]. https://gmo.geneticliteracyproject.org/FAQ/where-are-gmos-grownand-banned/ . Accessed 5 Sept 2020
Domingo JL (2016) Safety assessment of GM plants: an updated review of the scientific literature. Food Chem Toxicol 95:12–18. https://doi.org/10.1016/j.fct.2016.06.013 ( Epub 2016 Jun 16 )
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. BMJ. https://doi.org/10.1136/bmj.b2535
FDA (2016) What is a serious adverse event? [Online]. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event . Accessed 28 Sept 2020
Al-Harbi A, Lary S, Edwards MG et al (2019) A proteomic-based approach to study underlying molecular responses of the small intestine of Wistar rats to genetically modified corn (MON810). Transgenic Res 28(5–6):479–498. https://doi.org/10.1007/s11248-019-00157-y
Appenzeller LM, Munley SM, Hoban D et al (2009) Subchronic feeding study of grain from herbicide-tolerant maize DP-Ø9814Ø-6 in Sprague-Dawley rats. Food Chem Toxicol 47:2269–2280. https://doi.org/10.1016/j.fct.2009.06.014
Bai H, Wang Z, Hu R et al (2015) A 90-day toxicology study of meat from genetically modified sheep overexpressing TLR4 in Sprague-Dawley rats. PLoS ONE. https://doi.org/10.1371/journal.pone.0121636
Bakke-Mckellep AM, Koppang EO, Gunnes G et al (2007) Histological, digestive, metabolic, hormonal and some immune factor responses in Atlantic salmon, Salmo salar L, fed genetically modified soybeans. J Fish Dis 30:65–79. https://doi.org/10.1111/j.1365-2761.2007.00782.x
Bakke-McKellep AM, Sanden M, Danieli A et al (2008) Atlantic salmon ( Salmo salar L.) parr fed genetically modified soybeans and maize: histological, digestive, metabolic, and immunological investigations. Res Vet Sci 84:395–408. https://doi.org/10.1016/j.rvsc.2007.06.008
Buzoianu SG, Walsh MC, Rea MC et al (2012) Effect of feeding genetically modified Bt MON810 maize to ∼ 40-day-old pigs for 110 days on growth and health indicators. Animal 6:1609–1619. https://doi.org/10.1017/S1751731112000249
Buzoianu SG, Walsh MC, Rea MC et al (2012) Effects of feeding Bt maize to sows during gestation and lactation on maternal and offspring immunity and fate of transgenic material. PLoS ONE. https://doi.org/10.1371/journal.pone.0047851
Carman JA, Vlieger HR, Ver Steeg LJ et al (2013) A long-term toxicology study on pigs fed a combined genetically modified (GM) soy and GM maize diet. J Organic Systems 1:1–12
Google Scholar
Chen X, Gao MQ, Liang D et al (2017) Safety assessment of genetically modified milk containing human beta-defensin-3 on rats by a 90-day feeding study. Food Chem Toxicol 100:34–41. https://doi.org/10.1016/j.fct.2016.12.012
Chen YN, Hwang WZ, Fang TJ et al (2011) The impact of transgenic papaya (TPY10-4) fruit supplementation on immune responses in ovalbumin-sensitised mice. J Sci Food Agric 91:539–546. https://doi.org/10.1002/jsfa.4218
Chen ZL, Gu H, Li Y et al (2003) Safety assessment for genetically modified sweet pepper and tomato. Toxicology 188:297–307. https://doi.org/10.1016/s0300-483x(03)00111-2
Chukwudebe A, Privalle L, Reed A et al (2012) Health and nutritional status of Wistar rats following subchronic exposure to CV127 soybeans. Food Chem Toxicol 50:956–971. https://doi.org/10.1016/j.fct.2011.11.034
Cisterna B, Flach F, Vecchio L et al (2008) Can a genetically-modified organism-containing diet influence embryo development? A preliminary study on pre-implantation mouse embryos. Eur J Histochem 52:263–267. https://doi.org/10.4081/1226
Cyran N, Gülly C, Handl S et al (2008) Biological effects of transgenic maize NK603xMON810 fed in long term reproduction studies in mice. FiBL, Wien
de Vendômois JS, Roullier F, Cellier D et al (2009) A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci 5:706–726. https://doi.org/10.7150/ijbs.5.706
Delaney B, Appenzeller LM, Munley SM et al (2008) Subchronic feeding study of high oleic acid soybeans (Event DP-3Ø5423-1) in Sprague-Dawley rats. Food Chem Toxicol 46:3808–3817. https://doi.org/10.1016/j.fct.2008.10.003
EFSA Panel on Genetically Modified Organisms (GMO), Naegeli H, Birch AN et al (2018) Assessment of genetically modified soybean MON 87751 for food and feed uses under Regulation (EC) No 1829/2003 (application EFSA-GMO-NL-2014-121). EFSA J 16(8):e05346. https://doi.org/10.2903/j.efsa.2018.5346
El-Shamei ZS, Gab-Alla AA, Shatta AA (2012) Histopathological changes in some organs of male rats fed on genetically modified corn (Ajeeb YG). Am J Sci 10(8):684–696
Ermakova I (2005) Influence of genetically modified soya on the birth-weight and survival of rat pups. In: Epigenetics, transgenic plants& risk assessment.
Gao MQ, Zhang R, Yang Y et al (2018) A subchronic feeding safety evaluation of transgenic milk containing human β-defensin 3 on reproductive system of C57BL/6J mouse. Food Chem Toxicol 115:198–204. https://doi.org/10.1016/j.fct.2018.03.007
Gu J, Krogdahl Å, Sissener NH et al (2013) Effects of oral Bt-maize (MON810) exposure on growth and health parameters in normal and sensitised Atlantic salmon, Salmo salar L. Br J Nutr 109:1408–1423. https://doi.org/10.1017/S000711451200325X
Guo QY, He LX, Zhu H et al (2015) Effects of 90-day feeding of transgenic maize BT799 on the reproductive system in male Wistar rats. Int J Environ Res Public Health 12:15309–15320. https://doi.org/10.3390/ijerph121214986
Hammond B, Dudek R, Lemen J et al (2004) Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol 42:1003–1014. https://doi.org/10.1016/j.fct.2004.02.013
Hammond B, Lemen J, Dudek R et al (2006) Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem Toxicol 44:147–160. https://doi.org/10.1016/j.fct.2005.06.008
He XY, Tang MZ, Luo YB et al (2009) A 90-day toxicology study of transgenic lysine-rich maize grain (Y642) in Sprague-Dawley rats. Food Chem Toxicol 47:425–432. https://doi.org/10.1016/j.fct.2008.11.032
Healy C, Hammond B, Kirkpatrick J (2008) Results of a 13-week safety assurance study with rats fed grain from corn rootworm-protected, glyphosate-tolerant MON 88017 corn. Food Chem Toxicol 46:2517–2524. https://doi.org/10.1016/j.fct.2008.04.005
Ibrahim MA, Okasha EF (2016) Effect of genetically modified corn on the jejunal mucosa of adult male albino rat. Exp Toxicol Pathol 68:579–588. https://doi.org/10.1016/j.etp.2016.10.001
Kiliç A, Akay MT (2008) A three generation study with genetically modified Bt corn in rats: Biochemical and histopathological investigation. Food Chem Toxicol 46:1164–1170. https://doi.org/10.1016/j.fct.2007.11.016
Kiliçgün H, Gürsul C, Sunar M et al (2013) The comparative effects of genetically modified maize and conventional maize on rats. J Clin Anal Med 4:136–139
Lee NJ, Yang BC, Hwang JS et al (2010) Effects of cloned-cattle meat diet on reproductive parameters in pregnant rabbits. Food Chem Toxicol 48:871–876. https://doi.org/10.1016/j.fct.2009.12.025
Lee NJ, Yang BC, Im GS et al (2013) No long-term feeding toxicities on the health status in rats fed with cloned Korean native beef cattle (Hanwoo) meat. Toxicol Pathol 41:872–879. https://doi.org/10.1177/0192623312470762
Lin HT, Lee WC, Tsai YT et al (2016) Subchronic immunotoxicity assessment of genetically modified virus-resistant papaya in rats. J Agric Food Chem 64:5935–5940. https://doi.org/10.1021/acs.jafc.6b02242
Liu P, He X, Chen D et al (2012) A 90-day subchronic feeding study of genetically modified maize expressing Cry1Ac-M protein in Sprague-Dawley rats. Food Chem Toxicol 50:3215–3221. https://doi.org/10.1016/j.fct.2012.06.009
Liu Q, Yang W, Li M et al (2017) Effects of 60-week feeding diet containing Bt rice expressing the Cry1Ab protein on the offspring of inbred Wuzhishan Pigs fed the same diet. J Agric Food Chem 65:10300–10309. https://doi.org/10.1021/acs.jafc.7b04067
Liu S, Li CX, Feng XL et al (2013) Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. Food Chem Toxicol 57:314–321. https://doi.org/10.1016/j.fct.2013.04.00
Liu S, Liu HB, Wang HL et al (2019) Evaluation of behavioral profiles in mice fed with milk supplemented diets derived from human lactoferrin gene-modified cows. Regul Toxicol Pharmacol 104:133–140. https://doi.org/10.1016/j.yrtph.2019.03.008
Liu Y, Zhang S, Zhou Q et al (2020) Subchronic feeding toxicity studies of drought-tolerant transgenic wheat MGX11-10 in Wistar Han RCC rats. Food Chem Toxicol 137:111129. https://doi.org/10.1016/j.fct.2020.111129
MacKenzie SA, Lamb I, Schmidt J et al (2007) Thirteen week feeding study with transgenic maize grain containing event DAS-Ø15Ø7-1 in Sprague-Dawley rats. Food Chem Toxicol 45:551–562. https://doi.org/10.1016/j.fct.2006.09.016
Malatesta M, Boraldi F, Annovi G et al (2008) A long-term study on female mice fed on a genetically modified soybean: effects on liver ageing. Histochem Cell Biol 130(5):967–977. https://doi.org/10.1007/s00418-008-0476-x
Malatesta M, Caporaloni C, Gavaudan S et al (2002) Ultrastructural morphometrical and immunocytochemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean [published correction appears in Cell Struct Funct. 2002 Oct;27(5):399]. Cell Struct Funct 27(4):173–180. https://doi.org/10.1247/csf.27.173
Malatesta M, Caporaloni C, Rossi L et al (2002) Ultrastructural analysis of pancreatic acinar cells from mice fed on genetically modified soybean. J Anat 201(5):409–415. https://doi.org/10.1046/j.0021-8782.2002.00103.x
Mao J, Sun X, Cheng JH et al (2016) A 52-week safety study in cynomolgus macaques for genetically modified rice expressing Cry1Ab/1Ac protein. Food Chem Toxicol 95:1–11. https://doi.org/10.1016/j.fct.2016.06.015
Nouri-Ellouz O, Zeghal N, Makni S et al (2015) New food from a potato somatic hybrid: nutritional equivalence and safety assessment by a feeding study on rats. J Sci Food Agric 95(9):1911–1917. https://doi.org/10.1002/jsfa.6898
Oliva N, Florida Cueto-Reaño M, Trijatmiko KR et al (2020) Molecular characterization and safety assessment of biofortified provitamin A rice. Sci Rep 10(1):1376. https://doi.org/10.1038/s41598-020-57669-5
Papineni S, Golden RM, Thomas J (2017) The aryloxyalkanoate dioxygenase-12 (AAD-12) protein is not acutely toxic in mice[J]. Food Chem Toxicol 110:200–203. https://doi.org/10.1016/j.fct.2017.10.036
Papineni S, Murray JA, Ricardo E et al (2017) Evaluation of the safety of a genetically modified DAS-444Ø6-6 soybean meal and hulls in a 90-day dietary toxicity study in rats. Food Chem Toxicol 109(Pt 1):245–252. https://doi.org/10.1016/j.fct.2017.08.048
Papineni S, Passage JK, Ekmay RD et al (2018) Evaluation of 30% DAS-444Ø6-6 soybean meal in a subchronic rat toxicity study. Regul Toxicol Pharmacol 94:57–69. https://doi.org/10.1016/j.yrtph.2018.01.005
Poulsen M, Kroghsbo S, Schrøder M et al (2007) A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food Chem Toxicol 45(3):350–363. https://doi.org/10.1016/j.fct.2006.09.002
Qian ZY, Zhang SJ, Zhang L et al (2018) Subchronic toxicity study in rats evaluating genetically modified DAS-81419-2 soybean. Regul Toxicol Pharmacol 96:48–56. https://doi.org/10.1016/j.yrtph.2018.04.019
Qian ZY, Bultman J, Papineni S et al (2018) Safety evaluation of DAS-44406-6 soybeans in Wistar rats. Regul Toxicol Pharmacol 92:152–164. https://doi.org/10.1016/j.yrtph.2017.11.016
Tudisco R, Calabrò S, Cutrignelli MI et al (2015) Genetically modified soybean in a goat diet: Influence on kid performance. Small Rumin Res 126:67–74. https://doi.org/10.1016/j.smallrumres.2015.01.023
Richards HA, Han CT, Hopkins RG et al (2003) Safety assessment of recombinant green fluorescent protein orally administered to weaned rats. J Nutr 133(6):1909–1912. https://doi.org/10.1093/jn/133.6.1909
Sanden M, Ornsrud R, Sissener NH et al (2013) Cross-generational feeding of Bt ( Bacillus thuringiensis )-maize to zebrafish ( Danio rerio ) showed no adverse effects on the parental or offspring generations. Br J Nutr 110(12):2222–2233. https://doi.org/10.1017/S0007114513001748
Schrøder M, Poulsen M, Wilcks A et al (2007) A 90-day safety study of genetically modified rice expressing Cry1Ab protein ( Bacillus thuringiensis toxin) in Wistar rats. Food Chem Toxicol 45(3):339–349. https://doi.org/10.1016/j.fct.2006.09.001
Séralini GE, Cellier D, de Vendomois JS (2007) New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Arch Environ Contam Toxicol 52(4):596–602. https://doi.org/10.1007/s00244-006-0149-5
Séralini GE, Clair E, Mesnage R et al (2014) Republished study: long-term toxicity of a roundup herbicide and a roundup-tolerant genetically modified maize. Environ Sci Eur 26(1):14. https://doi.org/10.1186/s12302-014-0014-5
Sheng Y, Qi X, Liu Y et al (2014) Subchronic toxicity study in vivo and allergenicity study in vitro for genetically modified rice that expresses pharmaceutical protein (human serum albumin). Food Chem Toxicol 72:242–246. https://doi.org/10.1016/j.fct.2014.07.030
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (EFSA CEP Panel) et al (2019) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Bacillus licheniformis (strain NZYM-CE). EFSA J 17(4):e05685. https://doi.org/10.2903/j.efsa.2019.5685 ( Published 2019 Apr 30 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Bacillus subtilis (strain LMG S-24584). EFSA J 16(10):e05447. https://doi.org/10.2903/j.efsa.2018.5447 ( Published 2018 September 27 )
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), Silano V et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Aspergillus oryzae (strain NZYM-FA). EFSA J 16(11):e05480. https://doi.org/10.2903/j.efsa.2018.5480 ( Published 2018 Nov 16 )
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), Silano V et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Trichoderma reesei (strain DP-Nzd22). EFSA J 16(11):e05479. https://doi.org/10.2903/j.efsa.2018.5479 ( Published 2018 Nov 30 )
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (CEP) et al (2019) Safety evaluation of the food enzyme pullulanase from a genetically modified Bacillus licheniformis (strain DP-Dzp39). EFSA J 17(1):e05554. https://doi.org/10.2903/j.efsa.2019.5554 ( Published 2019 Jan 10 )
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (CEP) et al (2018) Safety evaluation of the food enzyme α-amylase from a genetically modified Aspergillus niger (strain NZYM-MC). EFSA J 16(10):e05451. https://doi.org/10.2903/j.efsa.2018.5451 ( Published 2018 Oct 31 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme alpha-amylase from a genetically modified Bacillus licheniformis (strain NZYM-AN). EFSA J 16(7):e05317. https://doi.org/10.2903/j.efsa.2018.5317 ( Published 2018 Jul 6 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme glucose oxidase from a genetically modified Aspergillus oryzae (strain NZYM-KP). EFSA J 16(7):e05319. https://doi.org/10.2903/j.efsa.2018.5319 ( Published 2018 Jul 6 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Aspergillus niger (strain XEA). EFSA J 16(4):e05228. https://doi.org/10.2903/j.efsa.2018.5228 ( Published 2018 Apr 27 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme aqualysin 1 from a genetically modified Bacillus subtilis (strain LMGS 25520). EFSA J 16(5):e05170. https://doi.org/10.2903/j.efsa.2018.5170 ( Published 2018 May 2 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme xylanase from a genetically modified Bacillus subtilis strain TD160(229). EFSA J 16(1):e05008. https://doi.org/10.2903/j.efsa.2018.5008 ( Published 2018 Jan 22 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of food enzyme xylanase from a genetically modified Bacillus subtilis (strain LMG S-27588). EFSA J 16(5):e05169. https://doi.org/10.2903/j.efsa.2018.5169 ( Published 2018 May 2 )
Talyn B, Lemon R, Badoella M et al (2019) Roundup ®, but not roundup-ready ® corn, increases mortality of drosophila melanogaster. Toxics. https://doi.org/10.3390/toxics7030038
Tang M, Xie T, Cheng W et al (2012) A 90-day safety study of genetically modified rice expressing rhIGF-1 protein in C57BL/6J rats [published correction appears in Transgenic Res. 2012 Aug;21(4):927]. Transgenic Res 21(3):499–510. https://doi.org/10.1007/s11248-011-9550-6
Tang X, Han F, Zhao K et al (2012) A 90-day dietary toxicity study of genetically modified rice T1C–1 expressing Cry1C protein in Sprague Dawley rats. PLoS ONE 7(12):e52507. https://doi.org/10.1371/journal.pone.0052507
Teshima R, Watanabe T, Okunuki H et al (2002) Effect of subchronic feeding of genetically modified corn (CBH351) on immune system in BN rats and B10A mice. Shokuhin Eiseigaku Zasshi 43(5):273–279. https://doi.org/10.3358/shokueishi.43.273
Trabalza Marinucci M, Brandi G, Rondini C (2008) A three-year longitudinal study on the effects of a diet containing genetically modified Bt176 maize on the health status and performance of sheep. Livest Sci 2008(113):178–190
Walsh MC, Buzoianu SG, Gardiner GE et al (2011) Fate of transgenic DNA from orally administered Bt MON810 maize and effects on immune response and growth in pigs. PLoS ONE 6(11):e27177. https://doi.org/10.1371/journal.pone.0027177
Walsh MC, Buzoianu SG, Gardiner GE et al (2012) Effects of short-term feeding of Bt MON810 maize on growth performance, organ morphology and function in pigs. Br J Nutr 107(3):364–371. https://doi.org/10.1017/S0007114511003011
Walsh MC, Buzoianu SG, Rea MC et al (2012) Effects of feeding Bt MON810 maize to pigs for 110 days on peripheral immune response and digestive fate of the cry1Ab gene and truncated Bt toxin. PLoS ONE 7(5):e36141. https://doi.org/10.1371/journal.pone.0036141
Wang X, He X, Zou S et al (2016) A subchronic feeding study of dicamba-tolerant soybean with the dmo gene in Sprague-Dawley rats. Regul Toxicol Pharmacol 77:134–142. https://doi.org/10.1016/j.yrtph.2016.02.001
West AL, Miles EA, Lillycrop KA et al (2019) Postprandial incorporation of EPA and DHA from transgenic Camelina sativa oil into blood lipids is equivalent to that from fish oil in healthy humans. Br J Nutr 121(11):1235–1246. https://doi.org/10.1017/S0007114519000825
Wu Y, Xu Y, Du Y et al (2017) Dietary safety assessment of genetically modified rice EH rich in β-carotene. Regul Toxicol Pharmacol 88:66–71. https://doi.org/10.1016/j.yrtph.2017.05.019
Xiao GJ, Jiang SW, Qian LL et al (2016) A 90-day feeding study in rats to assess the safety of genetically engineered pork. PLoS ONE 11(11):e0165843. https://doi.org/10.1371/journal.pone.0165843
Xie Z, Zou S, Xu W et al (2018) No subchronic toxicity of multiple herbicide-resistant soybean FG72 in Sprague-Dawley rats by 90-days feeding study. Regul Toxicol Pharmacol 94:299–305. https://doi.org/10.1016/j.yrtph.2018.02.004
Yang L, Sun Y, Wang Y et al (2014) Effects of dietary transgenic poplar leaf pellets on performance and tissues in rabbits. J Sci Food Agric 94(6):1163–1167. https://doi.org/10.1002/jsfa.6388
Yen GC, Lin HT, Cheng YH et al (2011) Food safety evaluation of papaya fruits resistant to papaya ring spot virus. J Food Drug Anal 19(2):269–377
CAS Google Scholar
Yong L, Liu YM, Jia XD et al (2012) Subchronic toxicity study of GH transgenic carp. Food Chem Toxicol 50(11):3920–3926. https://doi.org/10.1016/j.fct.2012.07.064
Zeljenková D, Aláčová R, Ondrejková J et al (2016) One-year oral toxicity study on a genetically modified maize MON810 variety in Wistar Han RCC rats (EU 7th Framework Programme project GRACE). Arch Toxicol 90(10):2531–2562. https://doi.org/10.1007/s00204-016-1798-4
Zeljenková D, Ambrušová K, Bartušová M et al (2014) Ninety-day oral toxicity studies on two genetically modified maize MON810 varieties in Wistar Han RCC rats (EU 7th Framework Programme project GRACE). Arch Toxicol 88(12):2289–2314. https://doi.org/10.1007/s00204-014-1374-8
Zhou C, Wang JW, Huang KL et al (2011) A 90-day safety study in Sprague-Dawley rats fed milk powder containing recombinant human lactoferrin (rhLF) derived from transgenic cloned cattle. Drug Chem Toxicol 34(4):359–368. https://doi.org/10.3109/01480545.2010.542465
Zhou XH, Dong Y, Xiao X et al (2011) A 90-day toxicology study of high-amylose transgenic rice grain in Sprague-Dawley rats. Food Chem Toxicol 49:3112–3118. https://doi.org/10.1016/j.fct.2011.09.024
Zhu HJ, Chen Y, Li YH et al (2015) A 90 day safety assessment of genetically modified rice expressing Cry1Ab/1Ac protein using an aquatic animal model [published correction appears in J Agric Food Chem. 2015 Aug 26;63(33):7462]. J Agric Food Chem 63(14):3627–3633. https://doi.org/10.1021/jf5055547
Zhu Y, He X, Luo Y et al (2013) A 90-day feeding study of glyphosate-tolerant maize with the G2-aroA gene in Sprague-Dawley rats. Food Chem Toxicol 51:280–287. https://doi.org/10.1016/j.fct.2012.09.008
Zou S, Tang M, He X et al (2015) A 90-day subchronic study of rats fed lean pork from genetically modified pigs with muscle-specific expression of recombinant follistatin. Regul Toxicol Pharmacol 73(2):620–628. https://doi.org/10.1016/j.yrtph.2015.09.009
Dong SS, Zhang DN, Zhang ZH et al (2019) Ecotoxicological effects of transgenic mCry1Ac maize (BT799) on zebrafish. Ying Yong Sheng Tai Xue Bao 30(8):2845–2853. https://doi.org/10.13287/j.1001-9332.201908.031
Hu Y, Piao J, Yang X et al (2012) Nutritional components and sub-chronic toxicity of genetically modified rice expressing human lactoferrin. Wei Sheng Yan Jiu 41(1):6–12. https://doi.org/10.2166/wst.2012.090
Bai H (2015) Bio-Safety assessment of transgenic sheep overexpressing oTLR4. PhD Thesis, China Agricultural University, Beijing, China
Bao ZK (2016) Safety assessment of GH-Transgenic dairy goats. MS Thesis, Nanjing Agricultural University, Nanjing, China
Cao ZH (2014) Safety assessment of transgenic Bt rice in growing pig diet. MS Thesis, Henan University of Science and Technology, Henan, China
Chen XP, Zhuo Q, Piao JH et al (2004) Immunotoxicologic assessment of transgenetic rice. J Hyg Res 33(1):77–80
Chu HH, Si QQ, Xu Y et al (2016) Effect of genetically modified soybean meal on the immune function, intestinal digestive enzyme activities and serum biochemical indexes of growing pigs. J Qingdao Agric Univ Nat Sci 33(02):119–123. https://doi.org/10.3969/J.ISSN.1674-148X.2016.02.008
Dai YN, Yang XZ, Liu Y et al (2018) Acute oral toxicity and 90 days feeding test of recombinant human lactoferrin. J Hyg Res 47(02):286–311
Du HF (2006) Safety assessment of transgenic rice used in broiler diet. PhD Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Feng XL, Wang HL, Li CX et al (2017) Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. J Hyg Res 29(1):19–25. https://doi.org/10.13590/j.cjfh.2017.01.005
Feng YQ, Hu J, Zhi Y et al (2013) The effect of exposure to transgenic Bt rice on the immune system of parental female rats. Chin J Food Hyg 25(4):298–302
Feng YQ, Wang EH, Zhi Y et al (2013) The effect of exposure to transgenic Bt rice on reproductive system of male offspring rats. Chin J Food Hyg 25(02):113–117
Guo MF (2018) A three generation study to evaluate reproductive and neurodevelopmental toxicity of genetically modified maize with Cry1Ab and epsps genes in SD rats. MS Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Hu J (2013) Establish of a rat model for development immunotoxicity and its application in safety evaluation of transgenic CryAb/Ac Rice. MS Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Huang Q, Xu HB, Gao F et al (2009) Anaphylactic reactions in WZS minipig orally induced by glycinin. J Hyg Res 38(05):531–534
Jia XD, Li N, Wang W et al (2005) Assessment of allergenicity of genetically modified rice S86 by BN rat model. Chin J Food Hyg 01:7–9. https://doi.org/10.13590/j.cjfh.2005.01.003
Li M (2012) Safety evaluation of recombinant herbicide-resistant protein AROa-CC-M and transgenic insect-resistant maize BT-799. MS Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Li M, Piao JH, Yang XG (2010) Subchronic toxicity test of genetically modified rice with double antisense starch-branching enzyme gene. J Hyg Res 39(04):436–443
Li R, Wang J, Jiang WL et al (2012) Effects of rice genetically modified with HJC-1 and G6-EPSPS genes on immunological parameters in Wuzhishan minipigs. J Environ Health 29(08):689–692. https://doi.org/10.16241/j.cnki.1001-5914.2012.08.017
Li YH, Piao JH, Chen XP et al (2004) Immunotoxicologic assessment on transgenic rice. China Public Health 20(04):20–22
Li YH, Piao JH, Zhuo Q et al (2004) Study on the teratogenicity effects of genetically modified rice with Xa21 on rats. J Hyg Res 33(06):710–712
Liang LQ, Wang J, Cao XC et al (2010) Toxicity analysis of common carp transferred salmon growth hormone gene. Food Sci 31(05):261–265
Liu HT, Wang CR, Liu L et al (2018) Acute toxicity of fresh leaves of insect resistant transgenic populus nigra to mice. J Anhui Agric Sci 46(01):94–136. https://doi.org/10.13989/j.cnki.0517-6611.2018.01.028
Liu HL, Wang J, Zeng Q et al (2012) Effects of rapeseed genetically modified with bar gene on immunological indicators in WZS minipigs. J Environ Health 29(11):977–980. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.020
Liu SS, Tan JZ, Sun Z et al (2011) Effects of glyphosate—resistant soybean meal on immune function of AA broilers. Chin J Anim Sci 47(13):41–46
Liu S, Wang XD, Feng XL et al (2013) Twenty-eight days feeding study on human lactoferrin expressed by cattle mammary bioreactor in mice. Chin J Public Health 29(02):230–232
Liu YH, Jiang SQ, Zhang J et al (2018) Subchronic toxicity of genetically modified Herbicide-resistant maize MON87427 with Cp4epsps gene in Wistar rats. J Public Health Prevent Med 29(06):17–20. https://doi.org/10.3969/j.issn.1006-2483.2018.06.004
Liu YF, Liu WH, He L et al (2008) Acute toxicity and mutagenic effect of transgenic rice on mice. J Hunan Univ Sci Technol Nat Sci Ed 23(04):111–117
Liu YF, Liu WH, He L et al (2008) Effects of resistant insects transgenic hybrid rice 21S/MSB on behavior and physiology of SD rats. Life Sci Res 12(03):257–261. https://doi.org/10.16605/j.cnki.1007-7847.2008.03.013
Lu MJ, Li F, Zhou GL et al (2008) Assessment of an anti-LeETR1 genetically modified tomato on reproductive-development toxicity and transferability of the transgene. J Toxicol 22(04):272–274. https://doi.org/10.16421/j.cnki.1002-3127.2008.04.006
Lu CB, Lin ZB, Zhang Y et al (2016) Effects of glyphosate-resistant transgenic soybean on physical enginery of male mice. Acta Agriculturae Zhejiangensis 28(07):1115–1120. https://doi.org/10.3969/j.issn.1004-1524.2016.07.04
Lu CB, Yang DY, Gao Z et al (2012) Safety assessment of reproductive system in male mice fed with genetically modified soybeans. J Yangzhou Univ Agric Life Sci Ed 33(01):23–27. https://doi.org/10.16872/j.cnki.1671-4652.2012.01.006
Lu CB, Zhang Y, Chen BH et al (2017) Effects of glyphosate-resistant transgenic soybean on in vitro fertilization of male mice with reproductive damage. Acta Agriculturae Zhejiangensis 29(06):910–916. https://doi.org/10.3969/j.issn.1004-1524.2017.06.08
Lu CB, Zhou W, Liu B et al (2013) Effects of transgenic soybean on reproductive system in male mice. Soybean Sci 32(01):119–123
Lv L, Guo J, Li SF et al (2013) Effects of transphytase gene maize on organ development and pathological changes of broilers. Chin J Anim Sci 49(05):31–34
Ma BT, Yuan XY, Wang XD et al (2017) Animal experiment of recombinant human lactoferrin based on the 28 days repeated oral toxicity. J Hyg Res 46(03):443–454
Ma YM, Wang J, Jiang WL et al (2012) Subchronic oral toxicity of genetically modified cottonseed with FBP7-iaaM gene in rats. J Environ Health 29(11):1001–1007. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.024
Qi XZ, Wang J, Zhou C et al (2010) Effect of transferred human lactoferrin milk powder on serum iron and ferritin in rats. Food Sci 31(23):340–343
Qin HF (2012) Safety assessment of rice genetically modified with Cry1Ac and sck feeding studies on broilers. PhD Thesis, Chinese Academy of Agricultural Sciences, Beijing, China.
Qiu ZL, Sun N, Wang J et al (2011) Sub-Chronic toxicity study of transgenic cottonseed in SD rats. Progr Mod Biomed 11(12):2215–2220. https://doi.org/10.13241/j.cnki.pmb.2011.12.010
Song LS, Gao GQ, Wei ZY et al (2017) Effects of Fat1 transgenic milk on the health and reproductive ability of mice. Lab Anim Sci 34(03):28–37
Song Y (2013) Establishment of immunotoxicity screening system in rodents and its application in immunotoxicity evaluation of pesticides and genetically modified foods. PhD Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Sun XW, Liang LQ, Yan XC et al (1998) Research on transgenic carp as food. High Technol Lett 03:3–5
Sun Z, Liu SS, Tan JZ et al (2011) Effects of glyphosate—resistant soybean meal on immune function of AA broilers. Chin J Anim Sci 23:836–841
Tan JZ (2011) The feed safety assessment of Glyphosate-Tolerant soybean meal in broilers. MS Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Tang XQ, Wang YF, Pei LJ et al (2019) Long-term toxicity study on transgenic rice T2A–1 with cry2A* gene. Chin J Food Hyg 31(6):510–516. https://doi.org/10.13590/j.jfh.2019.06.002
Tao R (2008) Detection of transgenic ingredients in feed and primary assessment on the safety of aquatic livestock fed transgenic soybean. MS Thesis, Chinese Academy of Sciences, Shandong, China
Wang EH (2014) A study to access effects of transgenic Cry1Ab/Ac Rice TT51 on reproductive and neural development in rats. PhD Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Wang EH, Yu Z, Fang HQ et al (2013) Effect of transgenic Bt rice TT51 on early physiological and neurological development of rats offspring. Chin J Food Hyg 25(06):485–488. https://doi.org/10.13590/j.cjfh.2013.06.008
Wang HM, Yin JY, Zhai WS, et al (2015) Toxic pathology of cynomolgus monkeys fed transgenic rice for 52 weeks. In: The 7th National Toxicology Conference of China Toxicology Society and the 8th Hubei Science and Technology Forum, Wuhan, China, pp 442–443
Wang J, Jiang WL, Wang XJ et al (2002) Toxicological safety evaluation of transgenic T5 line pepper. Chin J Urban Rural Ind Hyg 01:46
Wang J, Jiang WL, Wang Y et al (2012) Subacute oral toxicity of recombinant human lactoferrin from transgenic cows in rats on 28d. J Toxicol 26(05):393–397
Wang J, Li R, Liu HL (2012) Assessment of allergenicity of rice genetically modified with HJC-1 and G6-EPSPS genes by BN rats model. J Environ Health 29(11):967–970. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.014
Wang J, Liu HL, Zeng Q et al (2012) Subacute toxicity of rapeseed genetically modified with bar gene in WZS minipigs. J Environ Health 29(11):980–984. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.021
Wang J, Zhou C, Che HL et al (2010) Study on sub-chronic toxicity of powered milk containing transgenic lactoferrin on SD rats. Progr Mod Biomed 10(15):2809–2813. https://doi.org/10.13241/j.cnki.pmb.2010.15.002
Wang RY (2017) The effect of genetically modified feed on structure of mice testes. Livestock Poult Ind 28(06):6–7. https://doi.org/10.19567/j.cnki.1008-0414.2017.06.004
Wang R, Hu YC, Li M et al (2017) Study on the subchronic toxicity of transgenic DBN9978 herbicide resistant maize to rats. Food Nutr China 23(06):12–17
Wang XJ, Wang J, Liu HL et al (2012) Subchronic toxicity test of rice containing transgenic HJC-1 and G6-EPSPS in rats. J Environ Health 29(11):970–976. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.019
Wang Y (2011) Food safety assessment of genetically modified milk with human lactoferrin gene. MS Thesis, Tianjin Medical University, Tianjin, China
Wang Y, Lai WQ, Chen JG et al (2000) Toxicity of anti-herbicide gene (BAR) transgenic rice. J Hyg Res 29(03):141–142
Wu JH (2013) The nutritional, edible safety and efficacy assessment of genetically modified rice with human lactoferrin gene and its purified protein and the nutritional assessment of genetically modified wheat expressing GmDREB/TaDREB4 genes with drought-resistance. Ph.D. Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Wu P, Su YL, Zhang J et al (2003) Safety evaluation of transgenic tomato against Cucumber Mosaic Virus. J Capital Univ Med Sci 24(03):254–258
Xu YJ (2012) The forage safety assessment of genetically modified organism corn to weaning piglets. M.S. Thesis, Fujian Agriculture and Forestry University, Fujian, China
Yu T, Liu Y, Wang JW et al (2017) Teratogenic test of recombinant human lactoferrin in rats. J Toxicol 31(03):247–250
Yuan JQ (2015) The detection of the transgenic GTS40-3-2 related genes and their products of livestock products sold and toxicology study of Sprague-Dawley rats ( Rattus norvegicus ). Ph.D. Thesis, Shanxi Agricultural University, Shanxi, China
Zhang L, Cheng C, He N et al (2011) Study on subchronic toxicity of transgenic soybean with high oleic acid on rats. J Toxicol 25(05):391–394. https://doi.org/10.16421/j.cnki.1002-3127.2011.05.012
Zhang L, Wang J, Jiang SQ et al (2016) Subchronic toxicity of genetically modified corn with Cry1Ab/Cry2Aj and G10evo (EPSPS) genes in rats. J Environ Health 33(07):585–589
Zhang LL (2018) The Unintended Effects of Long-Term Intergenerational Feeding Transgenic Maize Diets to Pure Line White Leghorn Chickens on the Intestinal Health. M.S. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Zhang M, Zhuo Q, Tian Y et al (2012) Study on chronic toxicity of genetically modified rice expressing human lactoferrin. Chin J Food Hyg 24(06):391–394. https://doi.org/10.13590/j.cjfh.2012.06.008
Zhang Q (2014) Production of GH transgenic goat by somatic cell nuclear transfer. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China
Zhang WP, Li YY, Wang WG et al (2009) Detection of mutagenicity of chitinase and -1, 3 dextran gene in maize. Chin Remed Clin 9(05):405–407
Zhang ZY, Liu LJ, Zhang L et al (2010) Subchronic toxicity of Bt transgenic rice to mice. J Toxicol 24(02):126–129. https://doi.org/10.16421/j.cnki.1002-3127.2010.02.016
Zhao L, Zhang L, Zhang YY et al (2009) Immunotoxicological evaluation of transgenic soybean oil. China Health Care Nutr 11:5
Zhi Y, Liu HB, Di GY et al (2013) Genetic toxicity of transgenic human α-lactalbumin powdered milk. Carcinogen Teratogen Mutagen 25(02):124–133. https://doi.org/10.3969/j.issn.1004-616x.2013.02.010
Zhi Y, Liu HB, Di GY et al (2011) Study on sub-chronic toxicity of powered milk containing transgenic human α-lactalbumin. J Hyg Res 40(04):426–430. https://doi.org/10.19813/j.cnki.weishengyanjiu.2011.04.004
Zhong F (2013) Subchronic feeding study of transgenic BADH alfalfa on rabbits. M.S. Thesis, Shandong Agricultural University, Shandong, China
Zhou GL, Lu MJ, Chen YX et al (2007) Antisense LeETR1 transgenic tomato rats were fed for 4 weeks. J Toxicol 21(02):160–161
Zhou H (2012) Safety aeeseement of the phytase transgenic corn in the broiler diet. M.S. Thesis, Fujian Agriculture and Forestry University, Fujian, China
Zhou LG (2009) Research of high lysine transgenic paddy on broiler feed security. M.S. Thesis, Yangzhou University, Jiangsu, China
Zhou WL, Yang XF, Ping A et al (2014) Effects of transgenic Dwarf-mosaic-resistant maize on learning and memory abilities of rats. J Shanxi Agric Univ Nat Sci Ed 34(02):129–131. https://doi.org/10.13842/j.cnki.issn1671-8151.2014.02.006
Zhou XH (2012) Toxicological studies on the food safety of two transgenic rice. Ph.D. Thesis, Jiangsu University, Jiangsu, China
Zhou ZW, Wang DZ, Shen H et al (2012) Comprehensive evaluation on functions & safety of imported GM soybean using BDI-GS system. Soybean Sci 31(05):822–826
Zhu H, Zhu LY, Guo JY et al (2014) Safety evaluation of BT-799 corn on Wistar rats. Food Nutr China 20(09):63–67
Zhuo Q, Chen XP, Piao JH et al (2004) Experimental study on converting cowpea trypsin inhibitor rice for 90 days. J Hyg Res 33(02):176–179
Zhuo Q, Chen XP, Piao JH et al (2004) Study on teratogenic effect of cowpea trypsin inhibitor rice. J Hyg Res 33(01):74–77
Sakamoto Y, Tada Y, Fukumori N et al (2008) A 104-week feeding study of genetically modified soybeans in F344 rats. Shokuhin Eiseigaku Zasshi 49(4):272–282. https://doi.org/10.3358/shokueishi.49.272
Sakamoto Y, Tada Y, Fukumori N et al (2007) A 52-week feeding study of genetically modified soybeans in F344 rats. Shokuhin Eiseigaku Zasshi 48(3):41–50. https://doi.org/10.3358/shokueishi.48.41
Keshani P, Sharifi MH, Heydari MR et al (2020) The effect of genetically modified food on infertility indices: a systematic review study. Sci World J. https://doi.org/10.1155/2020/1424789
Edge MS, Kunkel ME, Schmidt J et al (2018) Evidence analysis library systematic review on advanced technology in food production. J Acad Nutr Diet 118(6):1106–1127. https://doi.org/10.1016/j.jand.2017.08.005
Dunn SE, Vicini JL, Glenn KC et al (2017) The allergenicity of genetically modified foods from genetically engineered crops: a narrative and systematic review. Ann Allergy Asthma Immunol 119(3):214–222. https://doi.org/10.1016/j.anai.2017.07.010
de Vos CJ, Swanenburg M (2018) Health effects of feeding genetically modified (GM) crops to livestock animals: a review. Food Chem Toxicol 117:3–12. https://doi.org/10.1016/j.fct.2017.08.031
Ricroch AE, Boisron A, Kuntz M (2014) Looking back at safety assessment of GM food/feed: an exhaustive review of 90-day animal feeding studies. Int J Biotechnology 13(4):230–256
Domingo Roig JL, Gómez Arnáiz M (2000) Riesgos sobre la salud de los alimentos modificados genéticamente: una revisión bibliográfica [Health risks of genetically modified foods: a literature review]. Revista espanola de salud publica 74(3):255–261
Domingo JL (2007) Toxicity studies of genetically modified plants: a review of the published literature. Crit Rev Food Sci Nutr 47(8):721–733. https://doi.org/10.1080/10408390601177670
Domingo JL, Giné Bordonaba J (2011) A literature review on the safety assessment of genetically modified plants. Environ Int 37(4):734–742. https://doi.org/10.1016/j.envint.2011.01.003
Teshima R, Watanabe T, Okunuki H et al (2002) Effect of subchronic feeding of genetically modified corn (CBH351) on immune system in BN rats and B10A mice. J Food Hyg Soc Jpn 43:273–279
Dubois AEJ, Pagliarani G, Brouwer RM et al (2015) First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar. Allergy 70:1406–1412
Download references
Acknowledgements
We appreciate Yi-Zhen Li for participating in screening the titles and abstracts .
This work was supported by Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202006). Prof. Nicola Robinson (visiting professor of Beijing University of Chinese Medicine) was funded by the International development and capacity enhancement of evidence-based Chinese medicine Project, Ministry of Science and Technology of the People's Republic of China, G20200001187.
Author information
Authors and affiliations.
Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, 11 Bei San Huan Dong Lu, Chaoyang District, Beijing, 100029, China
Chen Shen, Xiao-Wen Zhang, Wen-Bin Hou, Min Fang, Xun Li, Yu-Tong Fei, Nicola Robinson & Jian-Ping Liu
Beijing Institute of Radiation Medicine, Beijing, China
Xiang-Chang Yin
School of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Bo-Yang Jiao
Beijing Key Laboratory of the Innovative Development of Functional Staple and the Nutritional Intervention for Chronic Disease, China National Research Institute of Food & Fermentation Industries Co,. Ltd, Beijing, China
School of Life Sciences, Beijing University of Chinese Medicine, Beijing, China
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
Xue-Hao Cheng & Jian-Xin Ren
Ruikang Hospital, Guangxi University of Chinese Medicine, Guangxi, China
School of Health and Social Care, London South Bank University, London, SE1 OAA, UK
Nicola Robinson
Institute for Excellence in Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Jian-Ping Liu
You can also search for this author in PubMed Google Scholar
Contributions
All work was done by the authors. JPL and YTF conceived the study and revised the manuscript. CS contributed to data searching, screening and extraction, analysis of the data, drafted and revised the paper and approved the final version to be submitted. XCY, BYJ, JP, XHC, JXR, JL, XWZ, HDL, WBH and MF participated in identifying or screening the titles, abstracts and full-text screening and data extraction. XL, NR and JPL advised on the analysis of the data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jian-Ping Liu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix s1..
Search strategy applied in English language databases. Appendix S2. Funding sources or sponsors. Appendix S3. Adverse events/effects—other biomarkers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Shen, C., Yin, XC., Jiao, BY. et al. Evaluation of adverse effects/events of genetically modified food consumption: a systematic review of animal and human studies. Environ Sci Eur 34 , 8 (2022). https://doi.org/10.1186/s12302-021-00578-9
Download citation
Received : 07 July 2021
Accepted : 11 December 2021
Published : 13 January 2022
DOI : https://doi.org/10.1186/s12302-021-00578-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adverse event
- Genetically modified food
- Evidence-based medicine
- Systematic review
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 19 May 2020
Crop biotechnology and the future of food
- Michael A. Steinwand 1 &
- Pamela C. Ronald ORCID: orcid.org/0000-0002-4107-1345 1 , 2 , 3
Nature Food volume 1 , pages 273–283 ( 2020 ) Cite this article
4025 Accesses
73 Citations
68 Altmetric
Metrics details
- Agriculture
- Molecular engineering in plants
- Plant biotechnology
- Plant breeding
- Plant molecular biology
The global population continues to rise, as does the likelihood of reduced yields of major food crops due to the changing climate, thus making the development of genetically improved, stress-resilient crops a research priority. The convergence of low-cost genome sequencing with improved computational power and high-throughput molecular phenotyping technologies has accelerated the identification of genes underlying important agronomic traits relevant to food production and quality. Here, we discuss the evolution of plant improvement, and how researchers leverage genomic analyses and revolutionary new plant breeding technologies like site-directed nucleases to enhance food crop traits through agricultural biotechnology. Deployment of these products from the laboratory to the field remains hindered by biological and regulatory bottlenecks that require further development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Scott Camazine / Alamy Stock Photo (protein structure)
Similar content being viewed by others
Advancing crop genomics from lab to field
A CRISPR way for accelerating improvement of food crops
Genetic strategies for improving crop yields
Pingali, P. L. Green revolution: impacts, limits, and the path ahead. Proc. Natl Acad. Sci. USA 109 , 12302–12308 (2012).
ADS CAS PubMed Google Scholar
Wallace, J. G., Rodgers-Melnick, E. & Buckler, E. S. On the road to breeding 4.0: unraveling the good, the bad, and the boring of crop quantitative genomics. Annu. Rev. Genet. 52 , 421–444 (2018).
CAS PubMed Google Scholar
Hunter, M. C., Smith, R. G., Schipanski, M. E., Atwood, L. W. & Mortensen, D. A. Agriculture in 2050: recalibrating targets for sustainable intensification. Bioscience 67 , 386–391 (2017).
Google Scholar
Tilman, D., Balzer, C., Hill, J. & Befort, B. L. Global food demand and the sustainable intensification of agriculture. Proc. Natl Acad. Sci. USA 108 , 20260–20264 (2011).
Ray, D. K., Ramankutty, N., Mueller, N. D., West, P. C. & Foley, J. A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 3 , 1293 (2012).
ADS PubMed Google Scholar
Tietjen, B. et al. Climate change-induced vegetation shifts lead to more ecological droughts despite projected rainfall increases in many global temperate drylands. Glob. Chang. Biol. 23 , 2743–2754 (2017).
Hirabayashi, Y. et al. Global flood risk underclimate change. Nat. Clim. Chang. 3 , 816–821 (2013).
ADS Google Scholar
Rosenzweig, C. et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl Acad. Sci. USA 111 , 3268–3273 (2014).
Zhao, C. et al. Plausible rice yield losses under future climate warming. Nat. Plants 3 , 16202 (2017).
Myers, S. S. et al. Increasing CO 2 threatens human nutrition. Nature 510 , 139–142 (2014).
ADS CAS PubMed PubMed Central Google Scholar
Bebber, D. P., Ramotowski, M. A. T. & Gurr, S. J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 3 , 985–988 (2013).
Meyer, R. S. & Purugganan, M. D. Evolution of crop species: genetics of domestication and diversification. Nat. Rev. Genet. 14 , 840–852 (2013).
Olsen, K. M. & Wendel, J. F. A bountiful harvest: genomic insights into crop domestication phenotypes. Annu. Rev. Plant Biol. 64 , 47–70 (2013).
Hufford, M. B., Berny Mier y Teran, J. C. & Gepts, P. Crop biodiversity: an unfinished magnum opus of nature. Annu. Rev. Plant Biol. 70 , 727–751 (2019).
Studer, A., Zhao, Q., Ross-Ibarra, J. & Doebley, J. Identification of a functional transposon insertion in the maize domestication gene tb1 . Nat. Genet. 43 , 1160–1163 (2011).
CAS PubMed PubMed Central Google Scholar
Shang, Y. et al. Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 346 , 1084–1088 (2014).
Casañas, F., Simó, J., Casals, J. & Prohens, J. Toward an evolved concept of landrace. Front. Plant Sci. 8 , 145 (2017).
PubMed PubMed Central Google Scholar
Varshney, R. K. et al. Can genomics boost productivity of orphan crops? Nat. Biotechnol. 30 , 1172–1176 (2012).
Gaut, B. S., Díez, C. M. & Morrell, P. L. Genomics and the contrasting dynamics of annual and perennial domestication. Trends Genet. 31 , 709–719 (2015).
Moyers, B. T., Morrell, P. L. & McKay, J. K. Genetic costs of domestication and improvement. J. Hered. 109 , 103–116 (2018).
PubMed Google Scholar
Oladosu, Y. et al. Principle and application of plant mutagenesis in crop improvement: a review. Biotechnol. Biotechnol. Equip. 30 , 1–16 (2016).
CAS Google Scholar
Peng, J. et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400 , 256–261 (1999).
Rattanpal, H. S., Singh, G. & Gupta, M. Studies on mutation breeding in mandarin variety Kinnow. Curr. Sci. 116 , 483–487 (2019).
Dwivedi, S. L. et al. Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci. 21 , 31–42 (2016).
Mickelbart, M. V., Hasegawa, P. M. & Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 16 , 237–251 (2015).
Xu, K. & Mackill, D. J. A major locus for submergence tolerance mapped on rice chromosome 9. Mol. Breed. 2 , 219–224 (1996).
Xu, K., Xu, X., Ronald, P. C. & Mackill, D. J. A high-resolution linkage map of the vicinity of the rice submergence tolerance locus Sub1. Mol. Gen. Genet. 263 , 681–689 (2000).
Xu, K. et al. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442 , 705–708 (2006).
Ismail, A. M., Singh, U. S., Singh, S., Dar, M. H. & Mackill, D. J. The contribution of submergence-tolerant (Sub1) rice varieties to food security in flood-prone rainfed lowland areas in Asia. Field Crop. Res. 152 , 83–93 (2013).
Emerick, K. & Ronald, P. C. Sub1 rice: engineering rice for climate change. Cold Spring Harb. Perspect. Biol . https://doi.org/10.1101/cshperspect.a034637 (2019).
Li, Z. et al. The 3,000 rice genomes project. Gigascience 3 , 7 (2014).
Hendre, P. S. et al. African Orphan Crops Consortium (AOCC): status of developing genomic resources for African orphan crops. Planta 250 , 989–1003 (2019).
Jupe, F. et al. Resistance gene enrichment sequencing (RenSeq) enables reannotation of the NB-LRR gene family from sequenced plant genomes and rapid mapping of resistance loci in segregating populations. Plant J. 76 , 530–544 (2013).
Steuernagel, B. et al. Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 34 , 652–655 (2016).
Jouanin, A. et al. Development of the GlutEnSeq capture system for sequencing gluten gene families in hexaploid bread wheat with deletions or mutations induced by γ-irradiation or CRISPR/Cas9. J. Cereal Sci. 88 , 157–166 (2019).
Tardieu, F., Cabrera-Bosquet, L., Pridmore, T. & Bennett, M. Plant phenomics, from sensors to knowledge. Curr. Biol. 27 , R770–R783 (2017).
Lee, T. et al. AraNet v2: An improved database of co-functional gene networks for the study of Arabidopsis thaliana and 27 other nonmodel plant species. Nucleic Acids Res. 43 , D996–D1002 (2015).
Brooks, M. D. et al. Network Walking charts transcriptional dynamics of nitrogen signaling by integrating validated and predicted genome-wide interactions. Nat. Commun. 10 , 1569 (2019).
ADS PubMed PubMed Central Google Scholar
Lee, I. et al. Genetic dissection of the biotic stress response using a genome-scale gene network for rice. Proc. Natl Acad. Sci. USA 108 , 18548–18553 (2011).
Friso, G. & Van Wijk, K. J. Posttranslational protein modifications in plant metabolism. Plant Physiol. 169 , 1469–1487 (2015).
McWhite, C. D. et al. A pan-plant protein complex map reveals deep conservation and novel assemblies. Cell 181 , 460–474 (2020).
Huang, X. & Han, B. Natural variations and genome-wide association studies in crop plants. Annu. Rev. Plant Biol. 65 , 531–551 (2014).
Zhu, G., Gou, J., Klee, H. & Huang, S. Next-gen approaches to flavor-related metabolism. Annu. Rev. Plant Biol. 70 , 187–212 (2019).
Powell, A. L. T. et al. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 336 , 1711–1715 (2012).
Zhu, G. et al. Rewiring of the fruit metabolome in tomato breeding. Cell 172 , 249–261.e12 (2018).
Bauchet, G. et al. Identification of major loci and genomic regions controlling acid and volatile content in tomato fruit: implications for flavor improvement. New Phytol. 215 , 624–641 (2017).
Sauvage, C. et al. Genome-wide association in tomato reveals 44 candidate loci for fruit metabolic traits. Plant Physiol. 165 , 1120–1132 (2014).
Zhang, J. et al. Genome-wide association mapping for tomato volatiles positively contributing to tomato flavor. Front. Plant Sci. 6 , 1042 (2015).
Tieman, D. et al. A chemical genetic roadmap to improved tomato flavor. Science 355 , 391–394 (2017).
Zhao, J. et al. Meta-analysis of genome-wide association studies provides insights into genetic control of tomato flavor. Nat. Commun. 10 , 1534 (2019).
Crossa, J. et al. Genomic selection in plant breeding: methods, models, and perspectives. Trends Plant Sci. 22 , 961–975 (2017).
Singh, A., Ganapathysubramanian, B., Singh, A. K. & Sarkar, S. Machine learning for high-throughput stress phenotyping in plants. Trends Plant Sci. 21 , 110–124 (2016).
Zhang, Y., Malzahn, A. A., Sretenovic, S. & Qi, Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nat. Plants 5 , 778–794 (2019).
Puchta, H. The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J. Exp. Bot. 56 , 1–14 (2005).
Huang, T.-K. & Puchta, H. CRISPR/Cas-mediated gene targeting in plants: finally a turn for the better for homologous recombination. Plant Cell Rep. 38 , 443–453 (2019).
Hayut, S. F., Bessudo, C. M. & Levy, A. A. Targeted recombination between homologous chromosomes for precise breeding in tomato. Nat. Commun. 8 , 15605 (2017).
Swinnen, G., Goossens, A. & Pauwels, L. Lessons from domestication: targeting cis -regulatory elements for crop improvement. Trends Plant Sci. 21 , 506–515 (2016).
Modrzejewski, D. et al. What is the available evidence for the range of applications of genome-editing as a new tool for plant trait modification and the potential occurrence of associated off-target effects: a systematic map. Environ. Evid. 8 , 27 (2019).
Ma, X. et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8 , 1274–1284 (2015).
Rodríguez-Leal, D., Lemmon, Z. H., Man, J., Bartlett, M. E. & Lippman, Z. B. Engineering quantitative trait variation for crop improvement by genome editing. Cell 171 , 470–480.e8 (2017).
Weeks, D. P. Gene editing in polyploid crops: wheat, camelina, canola, potato, cotton, peanut, sugar cane, and citrus. Prog. Mol. Biol. Transl. Sci. 149 , 65–80 (2017).
Dederer, H.-G. & Hamburger, D. (eds) Regulation of Genome Editing in Plant Biotechnology. Regulation of Genome Editing in Plant Biotechnology (Springer, 2019).
Breitler, J.-C. et al. CRISPR/Cas9-mediated efficient targeted mutagenesis has the potential to accelerate the domestication of Coffea canephora . Plant Cell Tiss. Org. Cult. 134 , 383–394 (2018).
Trevaskis, B. Developmental pathways are blueprints for designing successful crops. Front. Plant Sci. 9 , 745 (2018).
Yang, Y. et al. Precise editing of CLAVATA genes in Brassica napus L. regulates multilocular silique development. Plant Biotechnol. J. 16 , 1322–1335 (2018).
Zheng, M. et al. Knockout of two BnaMAX1 homologs by CRISPR/Cas9-targeted mutagenesis improves plant architecture and increases yield in rapeseed ( Brassica napus L.). Plant Biotechnol. J. 18 , 644–654 (2019).
Zhou, J. et al. Multiplex QTL editing of grain-related genes improves yield in elite rice varieties. Plant Cell Rep. 38 , 475–485 (2019).
Huang, L. et al. Developing superior alleles of yield genes in rice by artificial mutagenesis using the CRISPR/Cas9 system. Crop J. 6 , 475–481 (2018).
Li, M. et al. Reassessment of the four yield-related genes Gn1a , DEP1 , GS3 , and IPA1 in rice using a CRISPR/Cas9 system. Front . Plant Sci. 7 , 377 (2016).
Miao, C. et al. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl Acad. Sci. USA 115 , 6058–6063 (2018).
Savary, S. et al. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3 , 430–439 (2019).
Pavan, S., Jacobsen, E., Visser, R. G. F. & Bai, Y. Loss of susceptibility as a novel breeding strategy for durable and broad-spectrum resistance. Mol. Breed. 25 , 1–12 (2010).
van Schie, C. C. N. & Takken, F. L. W. Susceptibility genes 101: how to be a good host. Annu. Rev. Phytopathol. 52 , 551–581 (2014).
Nekrasov, V. et al. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 7 , 482 (2017).
Wang, Y. et al. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 32 , 947–951 (2014).
Fister, A. S., Landherr, L., Maximova, S. N. & Guiltinan, M. J. Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao . Front. Plant Sci. 9 , 268 (2018).
Li, T., Liu, B., Spalding, M. H., Weeks, D. P. & Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 30 , 390–392 (2012).
Oliva, R. et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 37 , 1344–1350 (2019).
Peng, A. et al. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 15 , 1509–1519 (2017).
Chandrasekaran, J. et al. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 17 , 1140–1153 (2016).
Gomez, M. A. et al. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnol. J. 17 , 421–434 (2019).
Breiteneder, H. & Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 113 , 821–830 (2004).
Juhász, A. et al. Genome mapping of seed-borne allergens and immunoresponsive proteins in wheat. Sci. Adv. 4 , eaar8602 (2018).
Sánchez-León, S. et al. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 16 , 902–910 (2018).
García-Molina, M., Giménez, M., Sánchez-León, S. & Barro, F. Gluten free wheat: are we there? Nutrients 11 , 487 (2019).
PubMed Central Google Scholar
Dubois, A. E. J. et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1 -silenced apples cause fewer allergy symptoms than the wild-type cultivar. Allergy Eur. J. Allergy Clin. Immunol. 70 , 1406–1412 (2015).
Dodo, H. W., Konan, K. N., Chen, F. C., Egnin, M. & Viquez, O. M. Alleviating peanut allergy using genetic engineering: The silencing of the immunodominant allergen Ara h 2 leads to its significant reduction and a decrease in peanut allergenicity. Plant Biotechnol. J. 6 , 135–145 (2008).
Wakasa, Y., Hirano, K., Urisu, A., Matsuda, T. & Takaiwa, F. Generation of transgenic rice lines with reduced contents of multiple potential allergens using a null mutant in combination with an RNA silencing method. Plant Cell Physiol. 52 , 2190–2199 (2011).
Herman, E. M., Helm, R. M., Jung, R. & Kinney, A. J. Genetic modification removes an immunodominant allergen from soybean. Plant Physiol. 132 , 36–43 (2003).
Li, X. et al. Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front. Plant Sci. 9 , 559 (2018).
Li, R. et al. Multiplexed CRISPR/Cas9-mediated metabolic engineering of γ-aminobutyric acid levels in Solanum lycopersicum . Plant Biotechnol. J. 16 , 415–427 (2018).
Nonaka, S., Arai, C., Takayama, M., Matsukura, C. & Ezura, H. Efficient increase of γ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 7 , 7057 (2017).
Zhang, H. et al. Genome editing of upstream open reading frames enables translational control in plants. Nat. Biotechnol. 36 , 894–898 (2018).
Li, A. et al. Editing of an alpha-kafirin gene family increases digestibility and protein quality in sorghum. Plant Physiol. 177 , 1425–1438 (2018).
Haun, W. et al. Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 12 , 934–940 (2014).
Morineau, C. et al. Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa . Plant Biotechnol. J. 15 , 729–739 (2017).
Alexander, P. et al. Losses, inefficiencies and waste in the global food system. Agric. Syst. 153 , 190–200 (2017).
Bachem, C. W. B. et al. Antisense expression of polyphenol oxidase genes inhibits enzymatic browning in potato tubers. Nat. Biotechnol. 12 , 1101–1105 (1994).
Waltz, E. Nonbrowning GM apple cleared for market. Nature Biotechnology Blog http://blogs.nature.com/tradesecrets/2015/03/30/nonbrowning-gm-apple-cleared-for-market (2015).
Waltz, E. Gene-edited CRISPR mushroom escapes US regulation. Nature 532 , 293 (2016).
Re: Confirmation that PPO _ KO Potato is not a Regulated Article (United States Department of Agriculture, 2016); https://www.aphis.usda.gov/biotechnology/downloads/reg_loi/16-090-01_air_response_signed.pdf
Re: Confirmation of the Regulatory Status of Genome edited Lactuca sativa (Lettuce) (United States Department of Agriculture, 2019); https://www.aphis.usda.gov/biotechnology/downloads/reg_loi/18-243-01_air_response_signed.pdf
Non-browning lettuce is making its way to the market. AG Daily https://www.agdaily.com/news/non-browning-lettuce-market/ (3 June 2019).
Bruening, G. & Lyons, J. M. The case of the FLAVR SAVR tomato. Calif. Agric. 54 , 6–7 (2000).
Wang, D. et al. Characterisation of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato. Plant Physiol. 179 , 544–557 (2019).
Yang, L. et al. Silencing of SlPL, which encodes a pectate lyase in tomato, confers enhanced fruit firmness, prolonged shelf-life and reduced susceptibility to grey mould. Plant Biotechnol. J. 15 , 1544–1555 (2017).
Uluisik, S. et al. Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 34 , 950–952 (2016).
Mieulet, D. et al. Turning rice meiosis into mitosis. Cell Res. 26 , 1242–1254 (2016).
Wang, C. et al. Clonal seeds from hybrid rice by simultaneous genome engineering of meiosis and fertilization genes. Nat. Biotechnol. 37 , 283–286 (2019).
Khanday, I., Skinner, D., Yang, B., Mercier, R. & Sundaresan, V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 565 , 91–95 (2019).
Østerberg, J. T. et al. Accelerating the domestication of new crops: feasibility and approaches. Trends Plant Sci. 22 , 373–384 (2017).
Li, T. et al. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 36 , 1160–1163 (2018).
Zsögön, A. et al. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 36 , 1211–1216 (2018).
Chopra, R. et al. Identification and stacking of crucial traits required for the domestication of pennycress. Nat. Food 1 , 84–91 (2020).
Ribaut, J.-M. & Ragot, M. Modernising breeding for orphan crops: tools, methodologies, and beyond. Planta 250 , 971–977 (2019).
Lemmon, Z. H. et al. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 4 , 766–770 (2018).
Kwon, C., Heo, J., Lemmon, Z. H., Capua, Y. & Hutton, S. F. Rapid adaptation of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 38 , 182–188 (2020).
Bull, S. E. et al. Accelerated ex situ breeding of GBSS- and PTST1-edited cassava for modified starch. Sci. Adv. 4 , eaat6086 (2018).
Jørgensen, K. et al. Cassava plants with a depleted cyanogenic glucoside content in leaves and tubers. Distribution of cyanogenic glucosides, their site of synthesis and transport, and blockage of the biosynthesis by RNA interference technology. Plant Physiol. 139 , 363–374 (2005).
Tadele, Z. Orphan crops: their importance and the urgency of improvement. Planta 250 , 677–694 (2019).
Fernie, A. R. & Yan, J. De novo domestication: an alternative route toward new crops for the future. Mol. Plant 12 , 615–631 (2019).
Sunilkumar, G., Campbell, L. A. M., Puckhaber, L., Stipanovic, R. D. & Rathore, K. S. Engineering cottonseed for use in human nutrition by tissue-specific reduction of toxic gossypol. Proc. Natl Acad. Sci. USA 103 , 18054–18059 (2006).
Bradford, K. J., Van Deynze, A., Gutterson, N., Parrott, W. & Strauss, S. H. Regulating transgenic crops sensibly: lessons from plant breeding, biotechnology and genomics. Nat. Biotechnol. 23 , 439–444 (2005).
Napier, J. A., Haslam, R. P., Tsalavouta, M. & Sayanova, O. The challenges of delivering genetically modified crops with nutritional enhancement traits. Nat. Plants 5 , 563–567 (2019).
Brief 54: Global Status of Commercialized Biotech/GM Crops in 2018 (ISAAA, 2018).
GM Approval Database (ISAAA, accessed 1 October 2019); http://www.isaaa.org/gmapprovaldatabase/
Klümper, W. & Qaim, M. A meta-analysis of the impacts of genetically modified crops. PLoS One 9 , e111629 (2014).
Dively, G. P. et al. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl Acad. Sci. USA 115 , 3320–3325 (2018).
Kouser, S. & Qaim, M. Impact of Bt cotton on pesticide poisoning in smallholder agriculture: a panel data analysis. Ecol. Econ. 70 , 2105–2113 (2011).
Wu, F. Mycotoxin reduction in Bt corn: Potential economic, health, and regulatory impacts. Transgenic Res. 15 , 277–289 (2006).
Shelton, A. M. et al. Bt brinjal in Bangladesh: the first genetically engineered food crop in a developing country. Cold Spring Harb. Perspect. Biol . https://doi.org/10.1101/cshperspect.a034678 (2019).
Kawashima, C. G. et al. A pigeonpea gene confers resistance to Asian soybean rust in soybean. Nat. Biotechnol. 34 , 661–665 (2016).
La Concepcion, J. D., Franceschetti, M., Terauchi, R., Kamoun, S. & Banfield, M. Protein engineering expands the effector recognition profile of a rice NLR immune receptor. eLife 8 , e47713 (2019).
Wurtzel, E. T. et al. Revolutionizing agriculture with synthetic biology. Nat. Plants 5 , 1207–1210 (2019).
South, P. F., Cavanagh, A. P., Liu, H. W. & Ort, D. R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363 , eaat9077 (2019).
Dalal, J. et al. A photorespiratory bypass increases plant growth and seed yield in biofuel crop Camelina sativa . Biotechnol. Biofuels 8 , 175 (2015).
Manna, M., Achary, V. M. M., Islam, T., Agrawal, P. K. & Reddy, M. K. The development of a phosphite-mediated fertilization and weed control system for rice. Sci. Rep. 6 , 24942 (2016).
Pandeya, D. et al. Selective fertilization with phosphite allows unhindered growth of cotton plants expressing the ptxD gene while suppressing weeds. Proc. Natl Acad. Sci. USA 115 , E6946–E6955 (2018).
Mus, F. et al. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 82 , 3698–3710 (2016).
Ye, X. & Beyer, P. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287 , 303–305 (2000).
Paine, J. A. et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 23 , 482–487 (2005).
Paul, J.-Y. et al. Golden bananas in the field: elevated fruit pro-vitamin A from the expression of a single banana transgene. Plant Biotechnol. J. 15 , 520–532 (2017).
Zhu, Q. et al. From Golden Rice to aSTARice: bioengineering astaxanthin biosynthesis in rice endosperm. Mol. Plant 11 , 1440–1448 (2018).
Dong, O. X. et al. Marker-free carotenoid-enriched rice generated through targeted gene insertion using CRISPR-Cas9. Nat. Commun. 11 , 1178 (2020).
Ainley, W. M. et al. Trait stacking via targeted genome editing. Plant Biotechnol. J. 11 , 1126–1134 (2013).
Sun, Y., Li, J. & Xia, L. Precise genome modification via sequence-specific nucleases-mediated gene targeting for crop improvement. Front. Plant Sci. 7 , 1928 (2016).
Qi, Y. et al. Increasing frequencies of site-specific mutagenesis and gene targeting in Arabidopsis by manipulating DNA repair pathways. Genome Res. 23 , 547–554 (2013).
Baltes, N. J., Gil-Humanes, J., Cermak, T., Atkins, P. A. & Voytas, D. F. DNA replicons for plant genome engineering. Plant Cell 26 , 151–63 (2014).
Dahan-Meir, T. et al. Efficient in planta gene targeting in tomato using geminiviral replicons and the CRISPR/Cas9 system. Plant J. 95 , 5–16 (2018).
Gil-Humanes, J. et al. High-efficiency gene targeting in hexaploid wheat using DNA replicons and CRISPR/Cas9. Plant J. 89 , 1251–1262 (2017).
Altpeter, F. et al. Advancing crop transformation in the era of genome editing. Plant Cell 28 , 1510–1520 (2016).
Camacho, A., Van Deynze, A., Chi-Ham, C. & Bennett, A. B. Genetically engineered crops that fly under the US regulatory radar. Nat. Biotechnol. 32 , 1087–1091 (2014).
McHughen, A. & Smyth, S. US regulatory system for genetically modified [genetically modified organism (GMO), rDNA or transgenic] crop cultivars. Plant Biotechnol. J. 6 , 2–12 (2008).
Svitashev, S., Schwartz, C., Lenderts, B., Young, J. K. & Mark Cigan, A. Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat. Commun. 7 , 13274 (2016).
Liu, J. et al. Genome-scale sequence disruption following biolistic transformation in rice and maize. Plant Cell 31 , 368–383 (2019).
Jupe, F. et al. The complex architecture and epigenomic impact of plant T-DNA insertions. PLoS Genet. 15 , e1007819 (2019).
Lowe, K. et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 28 , 1998–2015 (2016).
Lowe, K. et al. Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cell. Dev. Biol. Plant 54 , 240–252 (2018).
Maher, M. F. et al. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 38 , 84–89 (2019).
Larkin, P. J. & Scowcroft, W. R. Somaclonal variation — a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 60 , 197–214 (1981).
Tang, X. et al. A large-scale whole-genome sequencing analysis reveals highly specific genome editing by both Cas9 and Cpf1 (Cas12a) nucleases in rice. Genome Biol. 19 , 84 (2018).
Li, J. et al. Whole genome sequencing reveals rare off‐target mutations and considerable inherent genetic or/and somaclonal variations in CRISPR /Cas9‐edited cotton plants. Plant Biotechnol. J. 17 , 858–868 (2019).
Fossi, M., Amundson, K., Kuppu, S., Britt, A. & Comai, L. Regeneration of Solanum tuberosum plants from protoplasts induces widespread genome instability. Plant Physiol. 180 , 78–86 (2019).
Han, Z. et al. Heritable epigenomic changes to the maize methylome resulting from tissue culture. Genetics 209 , 983–995 (2018).
Lee, K. & Seo, P. J. Dynamic epigenetic changes during plant regeneration. Trends Plant Sci. 23 , 235–247 (2018).
Stroud, H. et al. Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife 2 , e00354 (2013).
Wibowo, A. et al. Partial maintenance of organ-specific epigenetic marks during plant asexual reproduction leads to heritable phenotypic variation. Proc. Natl Acad. Sci. USA 115 , E9145–E9152 (2018).
Cunningham, F. J., Goh, N. S., Demirer, G. S., Matos, J. L. & Landry, M. P. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 36 , 882–897 (2018).
Demirer, G. S. et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 14 , 456–464 (2019).
Kwak, S.-Y. et al. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 14 , 447–455 (2019).
Zhao, X. et al. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat. Plants 3 , 956–964 (2017).
Kelliher, T. et al. One-step genome editing of elite crop germplasm during haploid induction. Nat. Biotechnol. 37 , 287–292 (2019).
Wang, B. et al. Development of a haploid-inducer mediated genome editing system for accelerating maize breeding. Mol. Plant 12 , 597–602 (2019).
Miller, J. K. & Bradford, K. J. The regulatory bottleneck for biotech specialty crops. Nat. Biotechnol. 28 , 1012–1014 (2010).
National Organic Standards Board Materials/GMO Subcommittee Proposal Excluded Methods Determinations (2019); https://www.ams.usda.gov/sites/default/files/media/MSExcludedMethodsProposaFall2019.pdf
National Organic Program Policy Memorandum 13 (USDA, 2013); https://www.ams.usda.gov/sites/default/files/media/NOP-PM-13-1-CellFusion.pdf
Ishii, T. & Araki, M. Consumer acceptance of food crops developed by genome editing. Plant Cell Rep. 35 , 1507–1518 (2016).
Urnov, F. D., Ronald, P. C. & Carroll, D. A call for science-based review of the European court’s decision on gene-edited crops. Nat. Biotechnol. 36 , 800–802 (2018).
Faure, J. D. & Napier, J. A. Europe’s first and last field trial of gene-edited plants? eLife 7 , e42379 (2018).
Secretary Perdue Issues USDA Statement on Plant Breeding Innovation USDA Press Release https://www.usda.gov/media/press-releases/2018/03/28/secretary-perdue-issues-usda-statement-plant-breeding-innovation (2018).
Waltz, E. With a free pass, CRISPR-edited plants reach market in record time. Nat. Biotechnol. 36 , 6–7 (2018).
Gheysen, G., Custers, R., Gheysen, G. & Custers, R. Why organic farming should embrace co-existence with cisgenic late blight–resistant potato. Sustainability 9 , 172 (2017).
Husaini, A. M. & Sohail, M. Time to redefine organic agriculture: can’t GM crops be certified as organics? Front. Plant Sci. 9 , 423 (2018).
Andersen, M. M. et al. Feasibility of new breeding techniques for organic farming. Trends Plant Sci. 20 , 426–434 (2015).
Scheufele, D. A. & Krause, N. M. Science audiences, misinformation, and fake news. Proc. Natl Acad. Sci. USA 116 , 7662–7669 (2019).
Hilscher, J., Bürstmayr, H. & Stoger, E. Targeted modification of plant genomes for precision crop breeding. Biotechnol. J. 12 , 1600173 (2017).
Download references
Acknowledgements
We apologize to those authors whose research could not be cited due to space limits. We thank H. Bartram for a careful reading of the manuscript. M.A.S. was supported by the Corteva Agriscience Open Innovation programme grant entitled “Gene Editing for Organic Agriculture.” P.C.R. was supported by grants from the US National Science Foundation (award no. 1237975), the Crary Social Ecology Fund, the Foundation for Food and Agricultural Research (award no. 534683) and the National Institutes of Health (GM122968).
Author information
Authors and affiliations.
Department of Plant Pathology, University of California, Davis, CA, USA
Michael A. Steinwand & Pamela C. Ronald
The UC Davis Genome Center, University of California, Davis, CA, USA
Pamela C. Ronald
Center on Food Security and the Environment, Stanford University, Stanford, CA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Pamela C. Ronald .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Steinwand, M.A., Ronald, P.C. Crop biotechnology and the future of food. Nat Food 1 , 273–283 (2020). https://doi.org/10.1038/s43016-020-0072-3
Download citation
Received : 22 October 2019
Accepted : 01 April 2020
Published : 19 May 2020
Issue Date : May 2020
DOI : https://doi.org/10.1038/s43016-020-0072-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Can plant hormonomics be built on simple analysis a review.
- Ondřej Vrobel
- Petr Tarkowski
Plant Methods (2023)
Osa-miR1861e Modulates Sodium Toxicity Responses in Rice Immature Grains via OsGST and OsPILS7b
- Tushar Khare
- Vitthal T. Barvkar
- Vinay Kumar
Journal of Plant Growth Regulation (2023)
Understanding the Origin and Evolution of Tea (Camellia sinensis [L.]): Genomic Advances in Tea
- Zai-Bao Zhang
Journal of Molecular Evolution (2023)
Target-oriented prioritization: targeted selection strategy by integrating organismal and molecular traits through predictive analytics in breeding
- Tingting Guo
- Jianbing Yan
Genome Biology (2022)
Estimating maize seedling number with UAV RGB images and advanced image processing methods
- Shuaibing Liu
- Xiuliang Jin
Precision Agriculture (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Genetically Modified Food Research Paper

This sample genetics research paper on genetically modified food features: 4000 words (approx. 13 pages) and a bibliography with 14 sources. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help. This is how your paper can get an A! Feel free to contact our writing service for professional assistance. We offer high-quality assignments for reasonable rates.
The topic of genetic modification of food has raised various ethical concerns among the consumers, environmentalist, farmers, scientists, and common citizens. The camps for and against the use of the modern technology in our food productions tend to base their arguments either on strict deontological moral principles or on the utilitarian calculation of overall harms and benefits. In this research paper the different ethical viewpoints are introduced and their feasibility analyzed.
Academic Writing, Editing, Proofreading, And Problem Solving Services
Get 10% off with 24start discount code, introduction.
This research paper discusses genetic modification of food. As the topic has raised many ethical concerns and points of view, it will examine different ethical issues as well as frameworks related to genetically modified organisms (GMOs) locally, regionally, and globally. The paper will introduce the methods of genetic modification and their history and current situation. Then it will discuss the various ethical approaches for and against genetic modification – including a discussion on known and potential benefits and harms of the genetic modification of organisms meant for human and animal consumption.
Conceptual Clarification
Genetic modification (GM) of food involves deliberate altering of the genetic material of plants and animals. It is an old agricultural practice carried on by farmers since early historical times. Recently, however, it has been improved by technology, i.e., genetic engineering and molecular biology. The term genetically modified foods (GMF) or genetically modified organisms is currently most commonly used to refer to crop plants created for human or animal consumption using these latest technologies.
Genetic modification in general describes the process by which scientists are able to pinpoint the individual gene which produces desired outcome, extract it, copy it, and insert it into another organism. Genetically engineered plants or animals are then generated by altering their genetic makeup. Usually genetically modified plants are tested in the laboratories for desired qualities. This testing is done by adding one or more genes to a plant’s genome using genetic engineering techniques (Freedman 2013; Fridovich-Kiehl and Diaz 2014).
Most direct genetic modification is done either by gene addition (cloning) or gene subtraction (genes are removed or inactivated). An example would be to isolate a gene responsible for drought tolerance and insert that gene into a different plant. Thus, the engineered organisms are sometimes also referred to as “transgenic organisms.”
History And Development
To some extent, humans have been involved in genetic modification for centuries. For example, larger cattle which produce more milk were bred to produce even larger offspring. Seeds from cereals and other crops that were more resilient and grew better were selected for planting the following year to produce better yields. This traditional process, however, has been slow and somewhat unreliable and inaccurate. The new technology that allows the scientists to take a gene from one living thing and put it directly into another plant or animal makes the desired changes more precise and happen in a much shorter time period. Currently the new technologies have created crops that are pest proof; disease, fungal, viral, and herbicide resistant; and weather (drought, cold, or hot) tolerant. It is also used to improve the nutritional content of crops and expand their storage. Also genetic modification has been used to improve taste, size, and color of food products. For example, so-called Golden Rice has been modified so that it gets an extra boost of vitamin A from a daffodil gene so that it produces almost 20 times the beta-carotene of previous varieties. This rice was intended for Asia, and the justification for this modification is that in this way, people who might have poor or restricted diet and cannot get enough vitamin A otherwise could get it directly from rice (FDA 2014, Fridovich-Kiel and Diaz 2014).
The discovery in 1946 that DNA can be transferred between organisms brought new elements to food production. The first genetically modified plant was produced in 1983 by using an antibiotic resistant tobacco plant. In 1994 the transgenic Flavr Savr tomato was approved by the US Food and Drug Administration (FDA) for marketing and commercial use in the USA. This genetic modification allowed the tomato to delay ripening after picking. Commercial sale of genetically modified crops began when Calgene first marketed its Flavr Savr tomato in 1996 (Fridovich-Kieh and Diaz 2014).
To date, most genetic modifications of foods have primarily focused on cash crops in high demand by farmers – such as soybean, corn, canola, and cottonseed oil. These have been engineered for resistance pathogens and herbicides as well as better nutrient profiles, as noted above. The most genetically modified food organisms are currently corn and soya. Corn that is used for food has been genetically modified to be resistant to various herbicides and to express a protein from Bacillus thuringiensis that kills certain insects. Nevertheless, it should be noted that since corn is used in various ways and processed into grits, meal and flour which for their part can be used in breakfast cereals, snack foods, baking mixes, etc.; genetic modification can be found in many different food products. Similarly soybeans are processed to different products that are used in a variety of foods, such as salad dressings soups, meat analogues, cheeses, nondairy creamer, desserts, infant formulas, breads, pasta, pet foods, etc.
According to various statistics, up to 90 % of the corn and soya beans grown in the USA have been genetically modified, and up to 60 % of all products on supermarket shelves could contain at least some GM soya (Freedman 2013; FridovichKieh and Diaz 2014). The percentage is lower in Europe, in Asia, and in Africa, but in reality it is very difficult to know exactly how far the genetic modification has spread across the globe.
GM livestock have also been experimentally developed. However, as of September 2014, there were no genetically modified animals approved for use as food, though GM salmon was still awaiting regulatory approval. In general according to FDA in the USA, many kinds of GE animals are currently in development. The largest class of GE animals is being developed for biopharm purposes – that is, they are intended to produce substances (e.g., in their milk or blood) that can be used as human or animal pharmaceuticals. Another group of GE animals are under development for use as sources of scarce cells, tissues, or organs for transplantation into humans (xenotransplant sources). Yet, others are intended for use as food and may be disease resistant or have improved nutritional or growth characteristics. Other developments include animals that produce high-value industrial or consumer products, such as highly specific antimicrobials against human and animal pathogens (e.g., E. coli 0157 or Salmonella). In general animals (e.g., goat) that are usually used for food production (e.g., milk) have already been genetically modified and approved by the FDA to produce nonfood products. (For details see FDA 2014; FridovichKiehl and Diaz 2014).
Currently, about only a tenth of world’s cropland includes GM plants. Four countries the USA, Canada, Brazil, and Argentina grow approximately 90% of the planet’s GM crops. Other Latin American countries are pushing away from the plants. In Africa there are test farms in some countries but not wide production. For example, Mauritius, South Africa, and Egypt are developing virus and pest-resistant transgenic sugarcane technologies. Other crops are tested also elsewhere in Africa (Freedman 2013).
Ethical Dimensions
From the point of view of global bioethics, genetic modification of food is an issue of central interest. After all, GM food has various transnational worldwide ethical dimensions. Firstly, GM has a global context, and many debates on its benefits and risks, whether health or environment related or economic, reach beyond national borders. Secondly, the responses to the use of genetic engineering in food production reach beyond borders as the movements for or against the GMF are not only national but also regional and international. Thirdly, many regulations that are set to control and test GMOs need to be based on international agreements.
Philosophical Ethics
In the philosophical ethics, the arguments for and against GM food vary often depending on the theoretical ethical starting point. Some deontological arguments may see the act of genetic modification to be wrong per se. This view is usually based on ideological or religious commitments. Genetic engineering may be seen, for example, as unnatural and, thus, immoral. In religious deontology, it could be seen as a human attempt to take the place of God – and thus, work against God’s will. These positions could be easily defeated by counter argumentation that points out that has God created human beings and gave them the ability to find scientific ways to improve and change nature. Consequently, the development of genetic engineering is in tandem with the God-given mandate to humankind to have dominion over other lesser creatures. Indeed, without fundamental theological assumptions, there do not seem to be strong categorical arguments against genetic modification. This is partly because genetic engineering has existed in one way or another for centuries if not thousands of years, for instance, the biblical account of Jacob “creating” cattle of rings raked, speckled, and spotted fleeces (Genesis 30:27:42).
On the other hand, reasoning along the lines of utilitarian ethics yields quite opposite conclusions on the rightness or wrongness of the overall consequences of genetic modification. Depending on whose utility we focus on and whether we calculate economic aspects, risks, or benefits and based on what information, we may get very different views on the moral desirability of GMF. Thus, while risks and benefits are at the core of the GMO debates, utilitarianism cannot currently provide very solid ethical argumentation either for or against genetic engineering – especially before all risks and benefits could be scientifically more accurately proven. Finally the tradition of Aristotelian virtue ethics may again see that human beings are changing the intrinsic telos of living organisms in a manner that prevents these organisms from flourishing naturally. Lastly, virtue ethics may also be interpreted to promote human beings realizing their full rational potential as inventors of these new technologies that merely enhance evolutionary tendencies of the nature (On philosophical ethical theories see for example Singer 1993).

Actual And Potential Harms And Benefits
As noted above, traditional moral theories do not necessarily provide a particularly solid or feasible framework for ethical argumentation for or against genetic modification of food. Thus, it is productive also to consider the actual benefits and risks that concern people in relation to the GMF.
The promoters of genetic modification of food appeal to the advantages that are achieved: better taste, larger sizes, better nutritional value, as well as economic benefits when the crops become resistant to pests, weeds, extreme weather conditions, etc. The overall benefit is mentioned to be the improved food production that would help to feed the poor across the world by providing more resistant crops with better nutritional values in places where the traditional farming techniques have not produced the needed results (WHO 2014).
Despite these benefits, there is still much opposition toward GMF. Politicians, administrators, “traditional” farmers, environmental movements, and just ordinary concerned consumers have raised several issues of concern. These vary from general concerns on environmental pollution, unintentional gene transfer to wild plants, possible creation of new viruses and toxins, etc. Below these risks are listed in more detail.
Firstly, there is a fear that genetic modification can lead to a loss of biodiversity if genetically modified organisms could have the potential to do unexpected harm to other plants and animals. The concern is that plants with new genes imported to them will accidentally crossbreed with wild plants and create harmful and unnatural effects in nontarget organisms (e.g., pesticideresistant super weeds or kill insects which are not pest but have a useful role in protecting plants). At worst, this could even lead to certain animal and wild plant species effectively being rendered extinct. This is not a fully unfounded concern as some laboratory tests have shown that pollen from GM maize in the USA damaged the caterpillars of the monarch butterfly. The studies on this case, however, are still highly controversial (see for details Winston 2002; Pewtrust 2003).
Secondly, where test crops have been planted in a country, there can be a definite danger of cross contamination with wild or non-GM plant strains. Many people are afraid that genetically modified foods may end up harming not only individual human beings but the environment at large. Even with very strict controls in place, it is impossible to prevent pollen from traveling on the wind from GM crops to other possible organic version of the same crop being grown nearby. Pollen could also be carried by insects. This could mean that in the end, all our food crops could contain a proportion of genetically modified elements. As consumers we would then lose our rights to choose GM food or not as it would be impossible to tell whether our food has been genetically modified at one stage or the other.
Thirdly, while the countries that are most affected by harsh weather conditions, arid lands, and famine could potentially be the greatest beneficiaries of GM foods, many regions with poverty and famine have not shown great enthusiasm for the newly developed crops.
In Africa there has been continuing resistance against adopting genetic modification in farming as many African governments warn that gene technologies will not help the local farmers. Instead they may destroy the diversity, the local knowledge, and the traditionally more sustainable agricultural systems. If these countries become dependent on the multinational companies who provide the seeds and related fertilizers and pesticides, the capacity to feed people in developing countries will diminish rather than increase. This feat may not be unfounded as multinational companies are there to make profits rather than feed the world and, thus, tend to have conditions for the commercial use of their seeds and crops. Multinational Monsanto that produces genetically modified crops, for example, is said to have had a terminator gene build in to them to prevent farmers from keeping seeds produced by their crops for the following year. Only recently this company was pressured to agree not to use this terminator technology in its crops (see, for example, Winston 2002).
Fourth, some of the specific fears expressed by opponents of GM technology include physical health risks such as alteration of nutritional quality of foods, potential toxicity and poisoning, possible antibiotics resistance, allergic reactions, etc. (Helferich and Winter 2010; Pusztai et al. 2003). The results on the actual and potential harms and benefit seem to be sometimes very contradictory, and it is difficult to know what we really should believe in.
Local, Regional, And Global Attitudes Toward GMF
In general, there appears to be a broad scientific consensus that food on the market derived from GM crops poses no greater risk to human health than conventional food. However, it is also fair to note that it might be too early to know for certain whether or not GM foods are harmful to human health or to the environment in the long run. This technology has been in use for too short a time to be able to assess and predict all possible unwanted side effects. What makes an accurate assessment even more difficult is the fact that the research reports on the safety of genetic modification produce contradictory results. It appears that their results depend on who is sponsoring the research and where the information comes from. While most available studies claim that GM foods have not harmed anyone directly, there are also reports that reveal harmful effects that have occurred.
Another ethical dimension of the research and studies on GMF is that the results may sometimes depend on the background politics and interests of certain involved parties. Thus, fully impartial information is still hard to get as the companies involved in genetic modification or distribution of genetically modified foods produce their own research and studies that focus on the benefits of GM technology and may undermine the risks. On the other hand, the opponents to the use of these technologies emphasize the risks and may exaggerate the potential harms.
Also, different countries and different regions across the world tend to have divided views on the GMF. They also focus on different ethical, political, and economic concerns. Governments in different countries have taken diverse approaches to assess and manage the risks associated with the use of genetic engineering technology in relation to genetically modified organisms. There are different regulations between countries and again particularly big difference between the USA and Europe. The European Commission has funded high number of research projects on the safety of GM crops. And while these studies have not found any high risk from GM group, the EU ministers are very cautious of letting new GM products in commercial markets and general consumption. The opinion, however, are split also within the EU. Particularly Germany, France, and Hungary have led the opposition, while other countries have been more favorable toward GMF. Traditionally Europe has been more conservative and has stronger opposition to the use of GMF than the USA. In the USA the big multinational companies located there, such as Monsanto, have produced genetically modified organisms and GM food for a long time for wide markets, and the consumers for the most part have welcomed the used technology and are willing to buy products with enhanced qualities. In Europe the markets are more reluctant to accept GMOs, and there are strict regulations to clearly label products “contaminated” with genetic modifications. Some critics say that many of the objections to GM food stem from politics rather than science, and they are motivated by an objection to large multinational corporations having enormous influence over the food production and distorting the agricultural markets and creating dependencies (see, e.g., Noussain et al. 2004, pp. 102–120; Pollack 2009, and also Monsanto 2014). From the point of view of global bioethics, many of these differences in views are related to the wider questions of (global and local) distributive justice. For example, African states and developing countries in general are particularly concerned about the economic dependency on multinational corporations. They also do not want to be used as testing grounds as they worry about the unknown health and environmental effects (Qaim 2009, pp. 667–669). However, institutionally they have not been able to follow up the implementation of regulations to control-related issues. Thus, while there is political resistance against the GMF, in many African and Asian countries, the general policy is not fully clear and GM products are not yet well regulated.
Country-specific regulations also often depend on the intended use of the products of the genetic engineering process. Countries which have legislation in place focus primarily on assessment of risks for consumer health and consumer rights. However, a crop not intended for food use is generally not reviewed by authorities responsible for food safety, and its use is followed as carefully as for food crops.
Legislation, Testing, And Labeling
In order to avoid any harmful effects of genetic modification of food, the safety assessment of GM foods usually investigates the following (1) direct health effect (toxicity), (2) tendencies to provoke allergic reaction (allergen city), (3) specific components thought to have nutritional or toxic properties, (4) the stability of the inserted gene, (5) nutritional effects associated with genetic modifications, and (6) any unintended effects which could result from the gene insertions.
One way to give the consumer at least a choice is the power to choose whether or not to buy GM products. Indeed the argument over the development, marketing, and selling GM foods has become a fierce political debate in recent years. Currently labeling can be mandatory up to a threshold GM content level (which varies between countries) or voluntary. In Canada and the USA, labeling GM food is voluntary, while in Europe all food including processed food or fee which contains greater than 0.9 % of GM contents is required to be labelled. Big companies that promote GM of foods such as Monsanto, General Mills, Pepsico, DuPont, Hershey, Cargill, Kellogg, Hormel, Kraft, Mars, Goya, Ocean Spray, and Nestle, as well as industrial food marketers, spend million on advertising to convince people to vote against labeling, while in many countries the consumer and environmental movements have pressured the government to make sure the GM products are clearly labeled (WHO 2014). Other ethical dilemmas may arise: [If the safety of GMFs is not a clear-cut issue, what are the ethical concerns associated with the power of choice? For instance, is it ethical for a government to allow its citizens to choose to buy products of uncertain risks and benefits? The non-labeling movement of GMFs tends to raise the question of trust.
In relation to international trade, according to WHO no specific international regulatory systems are currently in place. Several international organizations, however, are involved in developing protocols for GMOs. The Codex Alimentarius Commission (Codex) is the joint FAO/WHO body responsible for compiling the standards, codes of practice, guidelines, and recommendations. Codex is developing principles for the human health risk analysis of GM foods. The premise of these principles dictates a premarket assessment, performed on a case-by-case basis and including an evaluation of both direct effects (from the inserted gene) and unintended effects (that may arise as a consequence of inserting of the new gene). Codex principles do not have a binding effect on national legislation but are referred to specifically in the Sanitary and Phytosanitary Agreement of the World Trade Organization (SPS agreement) and can be used as a reference in case of trade disputes. The Cartagena Protocol on Biosafety (CPB), an environmental treaty legally binding for its parties, regulates trans-boundary movements of living modified organisms (MLOs). The cornerstone of the CPB is a requirement that exporters seek consent from importers before the first shipment of LMOs intended for release into the environments (WHO 2014).
From the point of view of global bioethics, many different ethical questions can be found in the debate for and against GMF: individual rights and health, environmental ethical issues, questions of distributive justice, issues of different worldviews and belief systems, etc. As GMF is a very complex issue and relatively little is known of its long-term consequences, it is important that we indeed learn more about the risks and benefits of the new technologies and their application. More studies needs to be carried out and preferably by independent research bodies rather than biotech companies or movement that are in principle against the GM technology. This is important in order to learn through impartial research about both short and long-term effects.
It is unlikely that GM products will be taken off the shelves or that the development of new GM organism would be stopped. Neither will the planting of crops cease. Thus, it is essential to continue testing the safety on new GM crops and follow any side effects of the current ones. The debate on GMF will likely continue, shaped by different views presented by governments, companies, and consumers. In this debate, clarity is often hindered when arguments are based on partial scientific evidence, on partial interests, on moral values, on politics, or on mere rhetoric.
Bibliography :
- Food and Drug Administration (FDA). (2014). General Q and A. https://www.fda.gov/
- Freedman, D. (2013). The truth about genetically modified food. Scientific American, 309, 3. https://www.scientificamerican.com/article/the-truth-about-genetically-modified-food/
- Fridovich-Keil, J. L, & Diaz, J. M. (2014). Genetically modified organism. Encyclopaedia Britannica. https://www.britannica.com/science/genetically-modified-organism .
- Helferich, W., & Winter, C. (Eds.). (2010). Food toxicology. Boca Raton: CRC Press.
- (2014).Commonly asked questions about the food safety of GMOs. http://www.monsanto.com/newsviews/pages/food-safety.aspx
- Noussain, C., Robin, S., & Ruffieux, B. (2004). Do consumers really refuse to buy genetically modified food? The Economic Journal, 114, 102–120.
- (2003). Three years later: Genetically engineered corn and the monarch butterfly controversy. https://www.pewtrusts.org/~/media/legacy/uploadedfiles/wwwpewtrustsorg/reports/food_and_biotechnology/vfbiotechmonarchpdf.pdf
- Pollack, M. (2009). When cooperation fails: The international law and politics of genetically modified foods. New York: Oxford University Press.
- Pusztai, A., Bardocz, S., & Ewen, S. (2003). Genetically modified foods: Potential human health effects. In J. P. F. D’Mello (Ed.), Food safety. Oxford/Cambridge: Cabi Publishing.
- Singer, P. (ed.). (1993). A Companion to Ethics. Oxford: Blackwell.
- Qaim, M. (2009). The economics of genetically modified crops. Annual Review of Resource Economics, 1, 665–693. https://www.annualreviews.org/doi/pdf/10.1146/annurev.resource.050708.144203
- Winston, M. (2002). Travels in the genetically modified zone. Harvard: Harvard University Press.
- World Health Organization (WHO). (2014). Frequently asked questions on genetically modified foods. https://www.who.int/foodsafety/areas_work/food-technology/faq-genetically-modified-food/en/
- Pence, G. E. (2002). The ethics of food: A reader for the twenty-first century. Lanham: Rowman and Littlefield.
ORDER HIGH QUALITY CUSTOM PAPER

- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Science and Research | Drugs
CDER Emerging Drug Safety Technology Program (EDSTP)
Introducing the emerging drug safety technology program (edstp).

The EDSTP is specifically focused on the use of artificial intelligence (AI) and other emerging technologies in pharmacovigilance (PV) and is part of the Center for Drug Evaluation and Research’s (CDER) multifaceted approach to enhance mutual learning of where and how specific innovations, such as AI, can best be used across the drug product lifecycle.
Emerging Technology's Potential in PV and Safety Surveillance
FDA has a longstanding commitment to ensure medicines marketed in the United States are safe through continued surveillance and research following approval. In the postmarket setting, regulated industry (per 21 CFR 314.80, 314.98, and 600.80) is obligated to review all adverse drug experience information received or otherwise obtained and submit reports to FDA. Both industry and regulatory authorities face challenges with timely and efficient collection, processing, and evaluation of single and aggregate patient safety data compounded by ever-increasing case volumes. Advances in emerging technology have the potential to address some of these challenges by creating more efficiencies within a PV system. For example, early adopters of AI are leveraging these emerging technologies to automate fundamental tasks (e.g., adverse event intake, data entry, and processing) with the intention to drive down associated administrative burden and costs. These technologies can also make safety surveillance more efficient and effective by capturing, aggregating, and analyzing larger and more diverse data sets.
Goals of the EDSTP
CDER recognizes industry’s interest in dialogue around AI capabilities that advance PV. Industry may have concerns that using such technologies could create uncertainties regarding satisfying regulatory obligations for PV. This is especially true while CDER familiarizes itself with new technologies (e.g., assessing their performance characteristics and efforts to validate and verify models) to determine how they may be evaluated within our regulatory framework.
In this context, CDER established the EDSTP with three goals:
- Serve as the central point of contact for discussion between industry and CDER on the use of AI and other emerging technologies in PV
- Enable knowledge management and knowledge transfer within FDA specific to the context of AI or other emerging technologies used in PV
- Understand the context of use of AI and other emerging technologies in PV to inform potential regulatory and policy approaches within PV
To help further these goals, CDER created the Emerging Drug Safety Technology Meeting (EDSTM) program , which will be administered by the EDSTP. FDA expects that increased communication with the broader pharmaceutical industry during EDSTMs will accelerate FDA’s understanding of how AI enabled tools and other emerging technologies are being used for PV, their performance characteristics, their associated risks and benefits, efforts to validate and verify relevant models, and barriers to implementation.
Questions and Answers
What is the purpose of the emerging drug safety technology meeting (edstm).
The EDSTM is a means by which eligible participants (see Eligibility Criteria ) can meet with CDER to share information about their use of AI and other emerging technologies and its potential application in PV. The goal of the meeting is to facilitate mutual learning and discussion on the opportunities and challenges with using such technologies in PV. Those selected for a meeting will meet with CDER to discuss their research, development, and/or use of AI and other emerging technologies in PV.
Which FDA representatives will be involved in the EDSTMs?
EDSTMs will be attended by members of CDER’s Emerging Drug Safety Technology Program, which includes representatives from CDER staff with experience in emerging drug safety technologies, pharmacovigilance activities, policy, and relevant inspection programs (e.g., Postmarketing Adverse Drug Experience (PADE) Compliance Program). The relevant interdisciplinary experts attending an EDSTM will depend on the nature of the topic proposed by the meeting requester. Additional experts from other centers or offices may participate as resources and time permit.
What topics are of interest to CDER for EDSTMs?
CDER is interested in a deeper understanding of AI and other emerging technologies that industry is exploring or has applied to PV activities. CDER is also interested in additional safety-related use cases involving emerging technology in PV, including but not limited to, signal detection and evaluation. The FDA AI/ML for Drug Development Discussion Paper references common uses of AI in PV, particularly for postmarket safety surveillance such as for the processing, evaluation, and submission of individual case safety reports (ICSRs) (see pages 8 – 9).
When exploring use cases of AI and other emerging technologies in PV with industry in EDSTMs, CDER is also interested in understanding how industry establishes the credibility and trustworthiness of AI models, including the following areas of consideration:
- Human-led governance, accountability, transparency, and explainability
- Data quality, reliability, representativeness, and bias mitigation
- Model development, performance, monitoring, and validation.
These areas of consideration are noted in the AI/ML for Drug Development Discussion Paper (see pages 17 – 23).
When is CDER accepting requests for EDSTMs? When are the submission deadlines?
CDER is now accepting requests for EDSTMs. Refer to the Submission Timeline and Process for quarterly submission deadlines. Requests will be reviewed on a quarterly basis for a total of up to nine participants in a 12-month period for the initial phase of the EDSTM.
For more information regarding CDER’s Emerging Drug Safety Technology Program (EDSTP), email [email protected] and include the subject line “EDSTP – General Inquiry”
In this Section

Emerging Drug Safety Technology Meeting (EDSTM) Program
Related information, focus area: artificial intelligence.
FDA aims to improve its understanding of AI’s potential and limitations. Learn more.
Artificial Intelligence and Machine Learning (AI/ML) for Drug Development
FDA recognizes the increased use of AI/ML throughout the drug development life cycle. Learn more.
Artificial Intelligence and Medical Products
Learn more about how the FDA is shaping the future of health care through the responsible and innovative integration of AI.
FDA Discussion Papers on AI/ML in Drug Development and Manufacturing
FDA recognizes the potential for AI/ML to enhance drug development in many ways. Learn more.
research paper
- December 2020

- Forman Christian College
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Ultra-Processed Foods and Health Outcomes: A Narrative Review
Leonie elizabeth.
1 School of Exercise and Nutrition Science, Deakin University, Geelong 3217, Australia; ua.ude.nikaed@ebazilel (L.E.); [email protected] (P.M.); [email protected] (P.B.)
Priscila Machado
2 Institute for Physical Activity and Nutrition, Deakin University, Geelong 3217, Australia
Marit Zinöcker
3 Department of Nutrition, Bjørknes University College, 0456 Oslo, Norway; [email protected]
Phillip Baker
Mark lawrence, associated data.
The nutrition literature and authoritative reports increasingly recognise the concept of ultra-processed foods (UPF), as a descriptor of unhealthy diets. UPFs are now prevalent in diets worldwide. This review aims to identify and appraise the studies on healthy participants that investigated associations between levels of UPF consumption and health outcomes. This involved a systematic search for extant literature; integration and interpretation of findings from diverse study types, populations, health outcomes and dietary assessments; and quality appraisal. Of 43 studies reviewed, 37 found dietary UPF exposure associated with at least one adverse health outcome. Among adults, these included overweight, obesity and cardio-metabolic risks; cancer, type-2 diabetes and cardiovascular diseases; irritable bowel syndrome, depression and frailty conditions; and all-cause mortality. Among children and adolescents, these included cardio-metabolic risks and asthma. No study reported an association between UPF and beneficial health outcomes. Most findings were derived from observational studies and evidence of plausible biological mechanisms to increase confidence in the veracity of these observed associations is steadily evolving. There is now a considerable body of evidence supporting the use of UPFs as a scientific concept to assess the ‘healthiness’ of foods within the context of dietary patterns and to help inform the development of dietary guidelines and nutrition policy actions.
1. Introduction
The concept of ultra-processed food (UPF) as a descriptor of unhealthy foods within dietary patterns is increasingly recognised in the nutrition literature [ 1 , 2 , 3 , 4 , 5 ] and authoritative reports [ 6 , 7 ]. Understanding of the contribution of UPFs to dietary quality and as a risk factor for diet-related diseases, disorders and conditions is rapidly emerging [ 8 ]. Yet, limited consideration has been given to UPF in strategies aiming to improve population health [ 9 ]. A crucial missing step in closing that gap is a review of the evidence base of the associations between UPF consumption and adverse health outcomes.
Dietary risk factors are leading contributors to the global burden of disease (GBD), responsible for an estimated 11 million deaths from non-communicable diseases (NCDs) (22% of all adult deaths) and 15% of disability life years (DALYs) lost in 2017 [ 10 ]. Leading contributors to diet-related deaths are cardiovascular disease (CVD), cancer and type 2 diabetes [ 10 ]. Contributors to DALYs from non-fatal chronic conditions include asthma, musculoskeletal conditions and mental health disorders [ 11 ].
Implicated dietary risk factors include certain nutrients, foods and dietary pattern exposures. Nutrient exposures include high amounts of sodium [ 10 , 12 ], saturated fat, trans-fat and added sugar [ 12 ]. Food exposures include low amounts of whole grains, fruit, vegetables, nuts and seeds [ 10 ] and fish [ 10 , 12 ], and high amounts of red meat, processed meat, potato chips and sugar-sweetened beverages (SSB) [ 12 , 13 ]. Dietary patterns include low scores on the Healthy Eating Index or Alternative Healthy Eating Index [ 14 ], or Mediterranean Dietary Pattern [ 15 ]; low adherence to the Dietary Approaches to Stop Hypertension diet [ 16 ]; or a high score on the Western dietary pattern [ 17 , 18 , 19 , 20 ].
In a novel approach to food categorization, NOVA (a name not an acronym) classifies foods and beverages ‘according to the extent and purpose of industrial processing’ [ 21 , 22 ], an aspect generally overlooked by public health nutrition science, policy and guidance. In 2009, a Brazilian research group, following studies on national trends over 25 years on household food acquisition and health implications [ 23 , 24 ], concluded diets containing high proportions of UPFs are intrinsically nutritionally unbalanced, harmful to health, or both [ 9 ]. This led to the development of the NOVA food classification system [ 25 ], which has since evolved [ 21 , 22 , 26 , 27 , 28 , 29 , 30 ].
The NOVA classification assigns foods to one of four groups, based on ‘the extent and purpose of industrial processing’ [ 21 ]: (1) ‘unprocessed or minimally processed foods’ (MPF), comprising edible parts of plants, animals or fungi without any processes applied to them or natural foods altered by minimal processing designed to preserve natural foods to make them suitable for storage, or to make them safe, edible or more palatable (e.g., fresh fruit, vegetables, grains, legumes, meat, milk); (2) processed culinary ingredients (PCI), which are substances extracted from group 1 (e.g., fats, oils, sugars and starches) or from nature (e.g., salt) used to cook and season MPF, not intended for consumption on their own; (3) processed foods (PF), where industrial products are made by adding PCI to MPF (e.g., canned vegetables in brine, fruit in syrup, cheese); and (4) UPFs, which are defined as ‘formulations of ingredients, mostly of exclusive industrial use, that result from a series of industrial processes (hence “ultra-processed”), many requiring sophisticated equipment and technology’ (e.g., sweet and savoury snacks, reconstituted meats, pizza dishes and confectionery, among others) [ 21 ]. Ingredients characteristic of UPFs include food substances of no or rare culinary use, including sugar, protein and oil derivatives (e.g., high-fructose corn syrup, maltodextrin, protein isolates, hydrogenated oil) and cosmetic additives (e.g., colours, flavours, flavour enhancers, emulsifiers, thickeners, and artificial sweeteners) designed to make the final product more palatable [ 21 ].
Since NOVA was established, nutrition researchers worldwide have increasingly implicated UPFs with poor dietary quality, and with adverse metabolic and health outcomes across a range of populations and country contexts [ 7 ]. Furthermore, UPFs have become dominant components in diets of populations worldwide [ 31 ], contributing up to more than 50% of energy intake in high-income countries [ 32 , 33 ], and up to 30% in middle-income countries [ 34 , 35 ], with consumption volumes rapidly increasing [ 36 , 37 , 38 ]. Because middle-income countries are home to the vast bulk of the world’s population, understanding the implications of rising UPF consumption for global human health is of utmost importance.
Several reviews have reported on UPFs and health outcomes [ 2 , 3 , 4 , 5 , 7 , 39 ]. However, despite the large and rapidly growing body of evidence linking UPFs with adverse health outcomes, the number of reviews and summarizing reports to date have been scarce, possibly delaying the inclusion of the ‘extent and purpose of industrial processing’ [ 21 ] as an independent factor for assessing the health potential of diets. As most dietary advice relies on systematic reviews and meta-analyses when reviewing evidence, a comprehensive review could be helpful in strengthening the evidence base and moving this field forward. To our knowledge, no review to date has employed a systematic search to identify all studies, without the restriction of health outcomes or study design.
The aim of this narrative review was to systematically identify and appraise the findings of studies on healthy participants (adults, adolescents and children) that have investigated associations between levels of UPF consumption and health outcomes.
2. Materials and Methods
A systematic search and narrative review method were adopted [ 40 , 41 ], involving four main steps: first, a systematic search process and application of inclusion and exclusion criteria; second, data extraction and synthesis of results; third, an analysis of key findings by narrative review; fourth, a quality appraisal procedure that included all studies. The method allowed a thorough search for extant literature, integration and interpretation of findings from diverse study types, populations, health outcomes, measurements and dietary assessments as well as quality appraisal. Checklists were used to ensure thoroughness of relevant components of the narrative review and systematic methods [ 42 , 43 , 44 ].
2.1. Search Process
The study utilised a systematic search process to ensure relevant studies were retrieved. Searches were performed in July 2019 using four databases—Medline, CINAHL, Global Health and Embase—and searching string variations of the following keywords: (ultra-process* or ultra process* or ultraprocess*) or (NOVA and “food classification”). Google Scholar citation searches and manual searches of reference lists were also conducted to identify relevant studies. A further EBSCO-host combined search was undertaken to identify any literature reviews and meta-analyses potentially missed by the initial searches. Additional searches of the electronic databases were performed in February 2020 and of Medline on 21 May 2020, to identify studies published in the interim. Records were downloaded to EndNote X8.2, and duplicates removed. Titles were first screened by title and abstract, then by full text against the inclusion and exclusion criteria given in Table 1 . Eligible articles were studies evaluating exposure (defined as ‘levels of consumption’) to ‘ultra-processed foods’ (defined as group 4 of the NOVA food classification system [ 21 ], described in Supplementary Material Table S1 ) and a ‘health outcome’ (defined as any disease, disorder or condition specified in the International Classification of Diseases, 11th Revision [ 45 ]). In this review, ‘food’ includes foods and beverages; ‘consumption’ includes national or household food availability and individual food intake. From a total of 851 papers, there were 263 identified studies on UPF and 43 for final inclusion in our review. Figure 1 presents a flow diagram adapted from PRISMA [ 43 , 44 ] of the search process.

Flow Diagram of Database Search and Article Eligibility (modified from PRISMA flow diagram [ 43 , 44 ]).
Inclusion and exclusion criteria used for screening studies.
| Description | |
|---|---|
| Inclusion criteria | |
| Exclusion criteria |
2.2. Data Extraction, Synthesis, Analysis and Quality Appraisal
Data were extracted on study details (author, publication date, study type, country and period of study) population (subjects, sample size), UPF exposure (food data extraction level, collection method, relative exposure assessment, NOVA reference), health outcomes (definition, data collection) and key findings. Studies were organised and tabulated into three classifications by specified health outcomes: (1) overweight, obesity and cardio-metabolic risks (hereafter referred to as ‘risks’), with ‘overweight and obesity’ also encompassing BMI, weight gain and related factors such as body fat percentage, body fat distribution, waist circumference; and ‘cardio-metabolic risks’ including high blood pressure, metabolic syndrome and relevant biomarkers for cardiovascular disease or type 2 diabetes; (2) diseases including cancer, cardiovascular disease and type 2 diabetes, and mortality (hereafter, referred to as ‘diseases’); and (3) other disorders and conditions (hereafter referred to as ‘disorders’). Studies on children and adolescents were considered separately to those on adults. In one study results on adults and adolescents were reported separately.
Data were extracted for the associations adjusted for potential confounders. Crude associations were extracted when the adjusted analysis was not performed. We presented only statistically significant associations (hereafter termed ‘associations’) in the written results and the relative risk of UPF exposure. For studies with a prospective design that also presented cross-sectional data, we presented the prospective results. There were varying terminologies describing body weight in the studies. We presented authors’ terminologies in Table 2 and Table 3 . In the results and discussion, we standardised to World Health Organization (WHO) definitions and BMI (kg/m 2 ) cut-off brackets for adults—(1) ‘overweight’ (BMI ≥ 25), to clarify this includes obesity; (2) ‘overweight (BMI 25–30)’ (BMI = 25.0–29.9), to clarify this excludes obesity; and (3) ‘obesity’ (BMI ≥ 30)—and WHO BMI-for-age z-scores for children, unless otherwise stated [ 46 , 47 , 48 ]. When more than one reference was included as NOVA reference, we assumed the most recent was applied to data classification.
Overweight, obesity and cardio-metabolic risks as outcomes in studies in adults *.
| Study Details | UPF Exposure | Outcomes | Results | |||||
|---|---|---|---|---|---|---|---|---|
| Publication Author(s) Year | Study Type (Year) Setting | Population (Number) | Extraction Level | Relative exposure [UPF Reference Year] | Data Collection Method | Health Outcome(s) (Study Definition) | Data Collection Method | Key Findings |
| Juul 2015 [ ] | Ecological (1960–2010) Sweden | Adults ≥18 years ( = −4000 household) | National + household sampling | : per capita UPF consumption + : UPF % share food purchase (kg or litre per capita per annum) [NOVA.2014] [ ] | : Swedish BOA net food ** available : 2-week purchase record by interview | BMI classified in prevalence overweight (BMI ≥ 25) and obesity (BMI ≥ 30) | National population statistics | From 1980 to 2008: rise in overweight prevalence for men from 35% to 54–56% and women from 26% to 39%; and obesity prevalence for men rose from 4.5% to 11% and for women from 5% to 10%. From 1960 to 2010 rise in UPF consumption of 142% tracks increase in overweight and obesity prevalence. |
| Monteiro 2017 [ ] | Ecological (1991–2008) Europe | Adults ≥ 18 years except Belgium ≥ 15 years ( = 19 countries) | Household (National Sample) | UPF % total E purchases (continuous) [NOVA.2018] [ ] | Belgium, Sweden, Germany = one month food ** purchase record; all others = 14 day record g/mL. | BMI classified in prevalence obesity (BMI ≥ 30) | National reports | UPF ranged 10.2–50.7% (median 26.4) of household total E in food purchases. Each 1% increase in UPF E availability was associated with 0.25% increase in obesity prevalence. |
| Vandevijvere 2019 [ ] | Ecological (Repeated cross-sectional) (2002–2014) Global | Adults ≥18 years ( = 80 countries) | National | UPF total sales (volume/capita) [NOVA.2018] [ ] | Volume sales of UPF (137 items from 212 food ** subgroups) | Mean population BMI | National reports | Increases in UPF volume sales/capita were directly associated with mean BMI trajectories. Every standard deviation increase in volume sales of UPF, mean BMI increased by 0.195 kg/m for men and 0.072 kg/m for women (drinks only), and 0.316 kg/m for men (foods only). |
| Canella 2014 [ ] | Cross-sectional (2008–2009) Brazil | All ages ( = 55,970 households; 190,159) individuals) | Household (National Sample) | UPF % total E purchases (quartiles) [NOVA.2012] [ ] | 7-day food ** purchase record | BMI classified in excess weight (BMI > 25), obesity (BMI > 30) WHO BMI for age Z scores [children] | Trained personnel | UPF contributed 25.5% of total E purchased. Participants living in household strata belonging to the upper quartile of UPF consumption had higher mean BMI (Z score) (β = 0.19; 95% CI 0.14, 0.25) prevalence of obesity (β = 3.72; 95% CI 2.50, 4.94) and prevalence of excess weight (β = 6.27; 95% CI 4.15, 8.39), compared with those in the lowest quartile. As UPF consumption rose from Quartile 1 to Quartile 4, the prevalence of excess weight rose from 34.1% to 43.9%, and prevalence of obesity rose from 9.8% to 13.1%. |
| Adams 2015 [ ] | Cross-sectional (2008–2012) UK | Adults > 18 years ( = 2174) | Individual (National Sample) | UPF % total E intake (continuous) [NOVA.2010] [ ] | 4-day food ** intake diary | BMI classified in overweight (BMI ≥ 25); obesity (BMI ≥ 30) | Trained personnel | UPF contributed 53% of total E intake. UPF consumption was not significantly associated with BMI, overweight and obesity, and obesity. |
| Louzada ‡ 2015 [ ] | Cross-sectional (2008–2009) Brazil | Adults > 20 years; children > 10 years ( = 30,243) | Individual (National Sample) | UPF % total E intake (quintiles) [NOVA.2012] [ ] | 2 × 24-h food ** intake record | BMI classified in excess weight (BMI ≥ 25), obesity (BMI ≥ 30) [adults]; WHO BMI for age Z scores [children] | Trained personnel | UPF contributed to 29.6% of total E intake. Individuals in the upper quintile of UPF intake had significantly higher BMI (0.94 kg/m ; 95% CI = 0.42, 1.47) and higher odds of being obese (OR = 1.98; 95% CI = 1.26, 3.12) compared with the lowest quintile. No significant association with excess weight was found. |
| Nardocci 2018 [ ] | Cross-sectional (2004–2005) Canada | Adults > 18 years (19,363) | Individual (National Sample) | UPF % total E intake (quintiles, and continuous) [NOVA2016.2018] [ , ] | 1 × 24-h recall | BMI classified in overweight (25.0 ≤ BMI < 30.0); obesity (BMI ≥ 30) | Trained personnel | UPF contributed 45.1% of total E intake. Individuals in highest quintile UPF intake significantly had higher odds of being obese (OR = 1.32, 95% CI 1.05, 1.57, and overweight (OR = 1.03; 95% CI 1.01, 1.07), compared with individuals in lowest quintile. |
| Juul 2018 [ ] | Cross-sectional (2005–2014) USA | Adults 20–64 years (15,977) | Individual (National sample) | UPF % total E intake (quintiles) [NOVA.2014] [ ] | 2 available 24-h recall or 1 day otherwise | BMI classified in overweight and obesity (BMI ≥ 25), obesity (BMI ≥ 30); WC classified in abdominal obesity (AO) [men ≥ 102 cm, women ≥ 88 cm) | Trained personnel | UPF contributed 56.1% of total E intake. Individuals in the highest quintile of UPF intake had significantly higher BMI (1.61 kg/m²; 95% CI 1.11, 2.10), and WC (4.07 cm, 95% CI 2.94, 5.19), and higher odds of having excess weight (OR = 1.48; 95% CI 1.25 to 1.76), obesity (OR = 1.53, 95% CI 1.29, 1.81), and abdominal obesity (OR = 1.62; 95% CI 1.39 to 1.89) compared with those in the lowest quintile. |
| Rauber 202 [ ] | Cross-sectional (2008–2016) UK | Adults 19−96 years ( = 6143) | Individual (National sample) | UPF % total E intake (quartiles) [NOVA.2019] [ ] | 4-day food ** intake diary | BMI classified in obesity (BMI ≥ 30). WC classified in AO | Trained personnel | UPF contributed 54.3% of total E intake. Individuals in the highest quartile of UPF intake had higher BMI (1.66 kg/m2; 95%CI 0.96, 2.36) and WC (3.56cm, 95% CI 1.79, 5.33), and higher odds of obesity (OR = 1.90, 95% CI 1.39, 2.61) compared with the lowest quartile. |
| Julia 2018 [ ] | Cross-sectional (2014) France | Adults Mean 43.8 years ( = 74,470) | Individual | UPF % total grams (quartiles) [NOVA.2016] [ , ] | 3 × 24 h records | BMI classified in overweight (25–29.9), obesity (≥30) | Self-report # | UPF contributed 18.4% of total weight intake, and 35.9% of total E intake. Higher consumption of UPF by % E intake was independently associated with overweight (p < 0.0001); and higher intake by energy-weighted UPF was independently associated with overweight, and obesity (both < 0.0001). |
| Silva 2018 [ ] | Cross-sectional (2008–2010) Brazil | Active and retired civil servants 35–64 years ( = 8977) | Individual | UPF % total E intake (quartiles) [NOVA.2016] [ ] | 114 item-FFQ | BMI classified in overweight (25.0-29.9); obesity (≥30); WC classified in increased WC (men ≥ 94; women ≥ 80); significantly increased WC (men ≥ 102; women ≥ 88) | Trained personnel | UPF contributed 22.7% of total E intake. Individuals in highest quartile UPF intake had significantly higher BMI (0.80 kg/m ; 95% CI 0.53, 1.07), WC (1.71 cm; 95% CI 1.02, 2.40), and higher odds of being overweight (OR = 1.31; 95% CI 1.13, 1.51), obese (OR = 1.41, 95% CI 1.18, 1.69), increased WC (OR = 1.31, 95% CI 0.96, 1.32), and significantly increased WC (OR = 1.41; 95% CI 1.20, 1.66), compared with individuals in the lowest quartile. |
| Da Silveira 2017 [ ] | Cross-sectional (2015) Brazil | Vegetarians > 16 years ( = 503) | Individual | UPF intake frequency (≥3 times per day) [DGB.2014] [ ] | FFQ (number of items not specified) | BMI classified in overweight BMI ≥ 25 (16–59 years), BMI ≥ 27 (≥60 years) | Self-report # | Higher intake of UPF (≥3 times/day) was independently associated with overweight (OR = 2.33; 95% CI 1.36, 4.03). |
| Ali 2020 [ ] | Cross-sectional (2018) Malaysia | Adults 18–59 years ( = 167) University personnel | Individual | UPF % total E intake (+continuous) [NOVA. 2018] [ ] | 2-day 24 h recall | BMI % Body fat | Trained personnel | UPF contributed 23 % of total E intake. No significant findings between ultra-processed food consumption BMI, body fat percent ( = 0.954). |
| Mendonca 2016 [ ] | Prospective Cohort (1999–2012) 8.9 years median follow-up Spain | Adults Mean 37.6 years ( = 8451) | Individual | UPF intake servings/day (quartiles) [NOVA.2016] [ ] | 136-item FFQ | BMI classified in overweight/obesity (BMI ≥ 25), obesity (BMI ≥ 30). | Self-report # | Participants in the highest quartile of UPF consumption were at a higher risk of developing overweight/obesity (HR = 1.26; 95% CI 1.10, 1.45) compared with those in the lowest quartile of consumption. |
| Canhada 2020 [ ] | Prospective Cohort (2008–2010) 3.8 years median follow-up Brazil | Adults 35–74 years ( = 11,827) | Individual | UPF % total E intake (quartiles) [NOVA 2016] [ ] | 114-item FFQ | Large weight gain (≥1·68 kg/year) Large WC gain (≥2·42 cm/year) Overweight/obesity (BMI ≥ 25 kg/m ) Obesity (BMI ≥ 30) | Trained personnel | UPF contributed 24.6% of total E intake. Participants in the highest quartile of UPF intake had greater risk of large weight (RR = 1.27; 95% CI 1.07, 1.50) and waist gains (RR = 1.33; 95% CI 1.12, 1.58), and of developing overweight/obesity (RR = 1.20; 95% CI 1.03, 1.40) compared with individuals in the lowest quartile. |
| Hall et al. 2019 [ ] | Randomised Controlled Trial (2018, 4 weeks) USA | Weight stable adults Mean 31.2 years ( = 20) | Individual | Whole diet UPF vs. MPF diet (ad libitum) [NOVA.2018] [ ] | Diets designed and analysed using ProNutra software | Energy Intake (kcal) Change in body weight (kg) | Trained personnel | Energy intake was greater during exposure to the UPF diet (508 ± 106 kcal/day; = 0.0001). Participants gained 0.9 ± 0.3 kg ( = 0.009) during the UPF diet, and lost 0.9 ± 0.3 kg ( = 0.007) during the MPF diet. |
| Lavigne- Robichaud 2017 [ ] | Cross-sectional (2005–2009) Canada | Adults ≥ 18 years ( = 811) | Individual | UPF total E % intake (quintiles) [NOVA.2010] [ ] | 1 × 24-h food ** recall | Metabolic syndrome (MetS) (≥3 factor: high WC, HT TAG, BG; low HDL-C) | Trained personnel | UPF contributed 51.9% of total E intake. Those in highest quintile of UPF intake significantly associated with higher prevalence of MetS (OR = 1.90; 95% CI 1.14), higher prevalence of reduced HDL-C (OR = 2.05; 95% CI 1.25, 3.38), elevated fasting plasma glucose (OR = 1.76, 95% CI 1.04, 2.97) compared with those in the lowest quintile. |
| Nasreddine 2018 [ ] | Cross-sectional (2014) Lebanon | Adults ≥18 years ( = 302) | Individual | UPF ‘pattern’ vs. MPF and PF ‘pattern’ (quartiles) [NOVA.2012] [ ] | 88-item FFQ | Metabolic syndrome (≥3 factors: high WC, HT, TAG, BG; low HDL-C) | Trained personnel | UPF vs. MPF were 36.5% vs. 27.1% of total E intake. Those in highest quartile MPF/PF significantly lower odds MetS (OR = 0.18, 95% CI 0.04, 0.77); hyperglycaemia (OR = 0.25, 95% CI 0.07, 0.98), low HDL-C (OR = 0.17, 95% CI 0.05, 0.60) compared with those in the lowest quartile. No significant association between MetS and UPF. |
| Lopes 2019 [ ] | Cross-sectional (2008–2010) Brazil | Adults 35–74 years ( = 8468) | Individual | UPF % total E intake (terciles) [NOVA 2016] [ ] | 114–item FFQ | C-reactive protein (CRP) level (mg/L) | Trained personnel | UPF contributed to 20% total E intake. Women in highest tercile UPF intake had higher levels of CRP (arithmetic mean = 1.14; 95% CI: 1.04–1.24) than lowest tercile of intake, no significance when controlling for BMI. No significant association was observed in men. |
| Martinez Steele 2019 [ ] | Cross-sectional (2009–2014) US | Adults ≥ 20 years ( = 6385) | Individual (National sample) | UPF Total E % intake (quintiles and continuous) [NOVA.2018.2019] [ , ] | 2 available ×24-h recall, or 1 day otherwise. | Metabolic syndrome (≥3 factor of high WC, HT, TAG, BG; low HDL) | Trained personnel | UPF contributed 55.5% of total E intake. The highest quintile of UPF consumption was associated with higher MetS prevalence (PR = 1.28; 95% CI 1.09, 1.50) compared with the lowest quintile of UPF consumption. Each 10% increase in the consumption of UPF was associated with 4% increase in MetS prevalence (PR = 1.04; 95% CI 1.02, 1.07) |
| Mendonca 2017 [ ] | Prospective Cohort (1999–2013) 9.1 years median follow-up Spain | Adult graduates ( = 14,790) | Individual | UPF E intake servings per day (tertiles) [NOVA.2016] [ ] | 136-item FFQ | Hypertension (BP: Systolic ≥ 140 mm Hg and/or Diastolic ≥ 90 mm Hg) | Self-report ξ | Participants in the highest tertile of UPF intake had higher risk of developing hypertension (HR = 1.21; 95% CI 1.06–1.37) compared with those in the lowest tertile of intake. |
Results are presented for adjusted associations for potential confounders and statistically significant associations. NOVA refers to the food classification system [ 21 ] or earlier versions, as referenced; * Includes studies on all ages; ** includes beverages; # anthropometrics; ξ reported medical diagnosis, medication, or BP readings, ‡ results for adolescents are presented in Table 3 ; UPF: ultra-processed food (includes foods and beverages); BOA: Board of Agriculture; BMI: Body Mass Index [weight (kilograms)/height (metres) 2 ]; E: energy in kilocalories or kilojoules; WHO: World Health Organisation; OR: odds ratio; CI: confidence interval; WC: waist circumference (cm); increased WC: (men ≥ 94; women ≥ 80; significantly increased WC (men ≥ 102; women ≥ 88); AO: abdominal obesity (men ≥ 102 cm; women ≥ 88 cm); FFQ: food frequency questionnaire; DGB: Dietary Guidelines for the Brazilian Population; HR: hazards ratio; RR: relative risk; MPF: unprocessed or minimally processed food; MetS: metabolic syndrome; HT: hypertension; TAG: triacylglycerol; BG: blood glucose; HDL-C: high density lipoprotein cholesterol; MPF and PF ‘pattern’: factor derived ‘pattern’ of mainly MPF and processed food (PF); CRP = C-reactive protein; BP = blood pressure.
Overweight, obesity and cardio-metabolic risks as outcome (children and adolescents).
| Study Details | UPF Exposure | Outcomes | Results | |||||
|---|---|---|---|---|---|---|---|---|
| Publication Author(s) Year | Study Type (Year) Setting | Population (Number) | Extraction Level | Relative exposure [UPF Reference Year] | Data Collection Method | Health Outcome (Study Definition) | Data Collection Method | Key Findings |
| Louzada * 2015 [ ] | Cross-sectional (2008-2009) Brazil | Children 10 to 19 years ( = 7534) | Individua (National Sample) | UPF % total E intake (quintiles) [NOVA.2012] [ ] | 2 × 24-h food ** intake record | WHO BMI-for-age Z-scores, in excess weight and obesity. | Trained personnel | UPF contribution ranged from ≤17% in lowest quintile to ≥52% in highest quintile. No significant association of UPF intake with mean BMI, excess weight or obesity was found. |
| Enes 2019 [ ] | Cross-sectional (2016) Brazil | Adolescents 10–18 years ( = 200) | Individual | UPF % total E intake (quartiles) [NOVA.2018] [ ] | 58-items FFQ | Overweight Obesity BMI-for-age Z-scores | Trained personnel | UPF contributed 50.6% of total E intake. No association with UPF and anthropometric indicators. |
| Cunha et al. 2018 [ ] | Prospective Cohort (2010–2012), 3 years median follow-up Brazil | Adolescents 15.7 years baseline, 17.6 years follow-up ( = 1035) | Individual | UPF intake (times/day) and daily E (kcal/day) [NOVA.2010] [ ] | 72-item FFQ | Trajectories of BMI (kg/m )% body fat | Trained personnel | Baseline UPF intake was 9.7–12.5 times/day (boys) and 10.9–13.1 times/day (girls). There was no significant difference in BMI and % body fat trajectories during follow-up. |
| Tavares 2012 [ ] | Cross-sectional (2006-2007) Brazil | Adolescents 12–19 years ( = 210) | Individual | UPF E intake (kJ) (quartiles)[NOVA.2009] [ ] | 90-item FFQ | Metabolic syndrome (MetS) (≥3 factor of high WC, HT, TAG, BG; low HDL) | Trained personnel (assumed) | Highest intake of UPF (>3rd quartile) was associated with higher MetS prevalence (PR = 2.49; = 0.012) than the lowest consumption. |
| Melo 2017 [ ] | Cross-sectional (2012) Brazil | Adolescents 14–19 years ( = 249) | Individual | UPF intake frequency (<3 per week vs. ≥3 per week) [DGB.2014] [ ] | 84- item FFQ | Excess weight (BMI-for-age) High waist circumference High blood pressure | Trained personnel | UPF intake was ≥ 3 × week in 46.2% of adolescents. MPF intake inversely associated with excess weight. UPF intake was not significantly associated with excess weight, high WC and high blood pressure. |
| Rauber 2015 [ ] | Prospective cohort (2001–2006) Brazil | Children 3–4 years at baseline; 7–8 years at follow-up ( = 345) | Individual | UPF % total E intake [NOVA.2014] [ , ] | 2 × 24-h recall | Changes in lipid concentrations | Trained personnel | UPF contributed 42.6% at pre-school, 49.2% at school age of % E intake. For every 1% increase E intake from UPF, total cholesterol increased 0.43 mg/dL ( = 0.046), and LDL-C increased 0.369 mg/dL ( = 0.047) from age 3–4 to 7–8 years. |
| Costa 2019 [ ] | Prospective Cohort (2001–2006), median follow-up age 4 to 8 years. Brazil | Children 4 years at baseline; 8 years at follow-up ( = 307) | Individual | UPF % total E intake [NOVA.2018] [ ] | 2 × 24-h recalls | Changes in BMI (kg/m ), Waist circumference (cm) Glucose profile and insulin resistance | Trained personnel | UPF contributed 41.8% preschool, 47.8% at school age of % E intake. Consumption of UPF consumption at age 4 was associated with increased delta waist circumference (B = 0.07 cm; 95% CI 0.01, 0.013) from age 4 to 8 years. No significant associations were observed for BMI, glucose profile and insulin resistance. |
| Leffa 2020 [ ] | Prospective cohort (2011–2015) Brazil | Children 3 years at baseline; 6 years at follow-up ( = 308) | Individual | UPF % total E intake [NOVA.2018.2019] [ , ] | 2 × 24-h recalls | Total cholesterol (TC) TAG | Trained personnel | UPF contributed 43.4% age 3 years, and 47.7% at age 6 years of % E intake. Those children in the highest tertile of consumption of UPF at age 3 had higher levels of TC (B = 0.22 mmol/L; 95 CI 0.04, 0.39) and TAG (B = 0.11 mmol/L; 95% CI 0.01, 0.20) at age 6 than those in the lowest tertile. |
Results are presented for adjusted associations for potential confounders and statistically significant associations. NOVA refers to the food classification system [ 21 ] or earlier versions, as referenced. * Also included in Table 2 ; ** food includes food and beverages; UPF: ultra-processed food (includes food and beverages); E: energy in kilocalories or kilojoules; WHO: World Health Organisation; BMI; Body Mass Index; FFQ; food frequency questionnaire; MetS; metabolic syndrome; WC: waist circumference; HT: hypertension; TAG: triacylglycerol; BG: blood glucose; HDL: high density lipoprotein; PR: prevalence; LDL: low-density lipoprotein; DGB: Dietary Guidelines for the Brazilian Population; TC: total cholesterol.
The analysis was addressed by a narrative review with a comprehensive exploration of general direct and negative associations in different health outcomes, associations within studies, differential effects of study types, populations and ages, exposure differences, and outcome measures. Objectivity was achieved by a quality assurance process, including group consensus; and for each article, a quality assessment was undertaken [ 49 , 50 , 51 ], as described in Supplementary Material Table S3 .
3.1. Overveiew of Identified Studies
Since the NOVA thesis was first published in 2009 [ 9 ] there have been 43 peer-reviewed studies reporting on UPF exposure and health outcomes that met this review’s eligibility criteria. The first study was published in 2012 [ 75 ] and 34 (79%) have been published since 2018. Studies were on adults ( n = 31) (six excluded elderly, two excluded adults < 45 years), children ages 3–11 ( n = 4), adolescents ages 10–19 ( n = 5), and mixed ages ( n = 3). Study types were ecological ( n = 3), cross-sectional ( n = 19), prospective cohort ( n = 19), case–control ( n = 1); and one randomised controlled trial (RCT). Studies were conducted in Brazil ( n = 16) [ 53 , 56 , 62 , 63 , 66 , 70 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 ], France ( n = 8) [ 61 , 83 , 84 , 85 , 86 , 87 , 88 , 89 ], Spain ( n = 6) [ 65 , 72 , 90 , 91 , 92 , 93 ], USA ( n = 4) [ 59 , 67 , 71 , 94 ], Canada ( n = 2) [ 57 , 68 ], UK ( n = 2) [ 55 , 60 ], and one each in Sweden [ 36 ], Lebanon [ 69 ] and Malaysia [ 64 ]. There was one study on 19 European countries [ 52 ], and one global study [ 37 ].
There were various levels at which researchers extracted food data. In the ecological studies, one study extracted at the household level and one combined household with national agriculture availability data [ 36 , 52 ] and the third, a global study on 80 countries, used national per capita sales volumes of UPF [ 37 ]. The remaining studies extracted data at the individual level, except one cross-sectional study which extracted at the household level [ 53 ]. Collection methods included sales-data ( n = 1), household purchase records (range 7 days to one month, one combined with agricultural data) ( n = 3), food-frequency questionnaire (FFQ) (range 88-880 items, one combined with 24-h recall) ( n = 17), 24hr recall ( n = 9), food intake records (from choice up to >3000 food items) ( n = 9), food diary ( n = 2), diet history interview ( n = 1); and a designed diet for the RCT ( n = 1). Relative UPF exposure was presented by per capita availability of sales by volume ( n = 1), % household energy purchased ( n = 3), % individual energy intake ( n = 18), daily energy intake ( n = 2), frequency(times/day)( n = 5), servings/day ( n = 3), % total grams ( n = 8), UPF ‘score’ ( n = 1), UPF ‘pattern’ versus MPF ‘pattern’ ( n = 1); and complete diet comparison (mainly UPF diet versus mainly MPF diet) for the RCT ( n = 1).
Health outcome data collection methods were from self-report questionnaires (on diagnoses, anthropometrics, medication use, or medical histories), measurement by trained personnel, diagnoses by medical practitioners, or extracted from national registries or statistical records.
3.2. Ultra-processed Food Consumption
In studies on adults reporting the proportion of total energy intake from UPF, Malaysia was lowest (23%) [ 64 ]. Higher levels of consumption were reported in Spain (24.4%) [ 92 ], Lebanon (27.1%) [ 69 ], Brazil (20–29.6%) [ 56 , 62 , 66 , 70 ], France (29.9–35.9%) [ 61 , 86 , 87 ], Canada (45.1–51.9%) [ 57 , 68 ], and UK (53–54.3%) [ 55 , 60 ]. The highest levels were reported in the USA (55.5–56.1%) [ 59 , 71 ]. In studies in adults reporting servings per day or frequency of UPF consumption, one study in USA reported a mean of 4 times a day [ 94 ], and one study in Spain reported 1.4–5.3 servings per day from the lowest to highest quartile of UPF intake [ 91 ]. In French studies reporting consumption by weight of food, UPF ranged from 14.4 to 18.7% of total [ 61 , 83 , 84 , 86 , 87 ].
In children and adolescents (Brazil only) the proportion of total energy intake from UPF was reported as 41.8–43.4% at ages 3–4 years, 47.7–49.2% at ages 6–8 years [ 77 , 78 , 79 ] and 50.6% in adolescents [ 73 ]. One study reported 46.2% of adolescents consumed UPF weekly (median ≥ 3 times per week) [ 76 ], and another study reported frequency intakes varied from 9.9 (private schools) to 14.5 (public schools) times per day [ 74 ].
3.3. Studies Using Overweight, Obesity and Cardio-metabolic Risks as Outcomes (Adults)
Table 2 reports the findings of studies that investigated associations of UPF exposure and overweight, obesity and cardio-metabolic risks in adults.
3.3.1. Overweight, Obesity and Related Factors
We identified 16 studies on adults investigating UPF exposure and ‘overweight’ (BMI ≥ 25), ‘overweight (BMI.25–30)’, obesity or related factors as outcomes. One study included all ages (including infants) and two studies included children or adolescents. Twelve studies reported direct associations with adverse health outcomes, one study showed no association and three studies showed mixed results (i.e., associations were not observed for all measures investigated or did not reach statistical significance).
There were three ecological studies identified: Juul et al. [ 36 ] analysed agricultural national food data and sampled approximately 4000 households. They found trends in food energy availability had risen in Sweden from 1980 to 2010, alongside a 142% increase in UPF portion in the diet. The prevalence of overweight rose in men (from 35% to 56%) and women (26% to 39%), and obesity rose in men (4.5% to 11%) and women (5% to 10%), closely tracking an increased share in food energy purchases from UPF [ 36 ]. Monteiro et al. [ 52 ] analysed budget surveys across 19 European countries. They found each percentage point increase in national household total food energy availability from UPF from 1991 to 2008 was associated with a higher national prevalence of obesity of 0.25% [ 52 ]. Vendevijvere et al. [ 37 ] investigated Euromonitor annual sales in 80 countries and found that increases in UPF volume sales were directly associated with population-level BMI trajectories. In drinks, every standard deviation increase (51 kg/capita in 2002) saw mean population BMI increase by 0.195 kg/m 2 for men ( p < 0.01) and 0.072 kg/m 2 for women ( p < 0.003). In foods, every standard deviation increase (40 kg/capita in 2002) saw mean population BMI increased by 0.316 kg/m 2 for men ( p < 0.001) with no significant association for women [ 37 ].
There were six cross-sectional studies on nationally representative samples. In Brazil, Canella et al. [ 53 ] found that household energy availability of UPF (purchased food items converted to kcal/day) was directly associated with average BMI and prevalence of excess weight or obesity. As UPF consumption rose from Quartile 1 to Quartile 4, the prevalence of excess weight rose from 34.1% to 43.9%, and prevalence of obesity rose from 9.8% to 13.1% [ 53 ]. In a second study in Brazil, Louzada et al. [ 56 ] found those in the highest quintile by percent energy intake of UPF had higher BMI and higher odds of being obese or overweight than those with the lowest quintile intake. For overweight individuals, the association was not significant [ 56 ]. In Canada, Nardocci et al. [ 57 ] found individuals in the highest quintile UPF by percent energy intake had a greater risk of obesity and overweight (BMI 25–30) compared with the lowest quintile [ 57 ]. In US adults aged 20–64 years from National Health and Nutrition Examination Survey 2005–2014 (NHANES), Juul et al. [ 59 ] found individuals in the highest quintile UPF by percent energy intake had higher BMI and waist circumference, and higher odds of overweight, obesity and abdominal obesity, with associations more pronounced in women [ 59 ]. In a UK study of 2174 adults, Adams et al. [ 55 ] found no significant association between UPF by percent energy intake and overweight or obesity. PCI was associated with the lowest odds of overweight and obesity, and MPF-CPI was associated with lower odds of being overweight [ 55 ]. In a second study in the UK on 6143 adults aged 19–96 years, Rauber et al. [ 60 ] found individuals in the highest quartile UPF by percent energy intake had higher BMI and waist circumference, and a higher odds of having obesity than those in the lowest quartile [ 60 ].
There were four cross-sectional studies on non-nationally representative samples. In Brazil, Silva et al. [ 62 ] assessed 8977 active and retired civil servants aged 35–62. They reported individuals in the highest quartile of percent energy intake compared with lowest quartile had higher BMI and waist circumference, as well as higher odds of being overweight (BMI 25–30), being obese, having increased waist circumference, or having significantly increased waist circumference [ 62 ]. In France, Julia et al. [ 61 ] studied 74,470 participants in a web-based Nutri-Santé cohort. They measured UPF intake by percent weight by quartiles and found UPF was associated with overweight (BMI 25–30), and energy-weighted quartiles were associated with overweight (BMI 25–30) and obesity [ 61 ]. In smaller studies, De Silveira et al. [ 63 ] examined vegetarians over 16 years old ( n = 503) and found overweight in high UPF consumers was 38.3% versus mean 23.5%. In Malaysia, Ali et al. [ 64 ] studied adults 18–59 years ( n = 167) and found no association between UPF and BMI or percentage body fat.
There were two prospective cohort studies. In Spain, Mendonca et al. [ 65 ] followed 8451 middle-aged graduates not overweight at baseline for a median of 8.9 years. Participants in the highest quartile of UPF intake (servings/day) had a 26% higher risk of developing overweight relative to the lowest quartile [ 65 ]. Considering those lost to follow-up, and those with repeated exposure measurement at 10-year follow-up, there remained a 24% risk and 19% risk, respectively [ 95 , 96 ]. In Brazil, Canhada et al. [ 66 ] followed 11,827 adults for 3.8 years. Participants in the highest quartile of UPF by percent energy intake had a 27% greater risk of developing ‘large weight’ and 33% risk of ‘waist gains’ than those in the lowest quintile. In those not overweight at baseline, there was a 20% risk of developing overweight compared to the lowest quartile. In crude analysis, for those overweight and not obese (BMI 25–30) at baseline there was an increased risk of developing obesity, but this did not reach significance in the fully adjusted model considering baseline BMI [ 66 ].
There was one randomised controlled crossover trial. In 20 US weight-stable adults free of disease at baseline (mean age 31.2 ± 1.6 years; mean BMI 27 ± 1.5 kg/m 2 ), Hall et al. [ 67 ] found that an a ultra-processed diet caused weight gain. Participants were provided two complete diets (either ultra-processed (81.3% energy from UPF) or unprocessed (0% UPF)) matched for calories, sugar, fat, sodium, fibre and macronutrients, in random order, in excess of daily energy requirements to consume ad libitum for 14 days each diet. Participants had a greater eating rate, consumed more energy (508 ± 106 kcal/day; p = 0.0001) and gained weight (0.9 ± 0.3 kg; p = 0.009) on the ultra-processed diet, and lost weight 0.9 ± 0.3 kg ( p = 0.007) during the unprocessed diet. Weight changes were highly correlated with energy intake ( r = 0.8, p < 0.0001) [ 67 ].
3.3.2. Cardio-Metabolic Risks
We identified five studies assessing cardio-metabolic risk factors in adults. Three showed an association of UPF exposure and adverse health outcome, one study reported mixed results, and one study had no observed association. There were four cross-sectional studies. On a representative sample of US adults, Martinez Steele et al. [ 71 ] found the highest quintile of UPF intake by percent energy was associated with 28% higher metabolic syndrome prevalence rate compared to the lowest quintile intake. Each 10% increase in consumption was associated with a 4% increase in metabolic syndrome prevalence. The association was strongest in younger adults [ 71 ]. In a study in Brazil of 8468 adults, Lopes et al. [ 70 ] found women in the highest tercile of UPF by percent energy intake had higher levels of C-reactive protein (CRP) than those in the lowest tercile. When controlling for BMI, the association was not statistically significant. No association was found in men [ 70 ]. In Canada, Lavigne-Robichard et al.’s [ 68 ] study of adults ( n = 811) found those in the highest quintile of UPF intake by percent energy had a higher prevalence of metabolic syndrome, reduced high-density lipoprotein cholesterol and elevated fasting glucose [ 68 ]. In a Lebanese study of adults ( n = 302), Nasreddine et al. [ 69 ] found those in medium/high adherence to a factor-derived MPF and PF ‘pattern’ vs. low adherence had lower odds of metabolic syndrome, hyperglycaemia and low HDL-C. No significant association was observed with metabolic syndrome and UPF ‘pattern’ [ 69 ]. There was one prospective cohort study. In Spain, Mendonca et al. [ 72 ] followed 14,790 adult graduates for a median 9.1 years. Participants in the highest tertile of UPF consumption (servings/day) had a 21% higher risk of developing hypertension than those in the lowest tertile [ 72 ].
3.4. Overweight, Obesity and Cardio-Metabolic Risks as Outcomes (Children and Adolescents)
There were eight studies on children and adolescents assessing UPF exposure and overweight, obesity body weight or cardio-metabolic risks as an outcome, which are presented in Table 3 . Three studies showed associations of UPF intake and outcomes, four showed no associations and one had mixed results. All studies were from Brazil.
There were three studies on overweight and obesity. In a nationally representative cross-sectional sample on children and adolescents aged 10–19, Louzada et al. [ 56 ] found no significant association with UPF and mean BMI, overweight/obesity or obesity. In a small cross-sectional study on adolescents aged 10–18 years ( n = 200), Enes et al. [ 73 ] found no association between UPF consumption and anthropometric indicators. In a prospective cohort study, Cunha et al. [ 74 ] followed 1035 adolescents (mean age 16 years) for three consecutive years and found no significant difference in BMI and percentage body fat trajectories.
There were five studies assessing cardio-metabolic risks. In a cross-sectional study on adolescents aged 12–19 years (n = 210), Tavares et al. [ 75 ] showed those with high UPF consumption (>Quartile 3) was associated with higher metabolic syndrome than those in the lowest quartile. In a second cross-sectional study on adolescents aged 14–19 years (n = 249), Melo et al. [ 76 ] found that while UPF was not associated with excess weight, hypertension or high waist circumference, consumption of MPF was inversely associated with excess weight. There were three prospective cohort studies. Following children aged 4–8 years ( n = 345), Rauber et al. [ 77 ] reported UPF consumption (by percent energy intake) at pre-school age was a predictor of higher total and LDL cholesterol at school age. In a follow-up of a previous RCT on 307 children aged 4–8 years, Costa et al. [ 78 ] found for every increase of 10% energy intake from UPF, delta waist circumference increased by 0.7 cm. Further, higher UPF consumption at pre-school age was a predictor of an increase in delta waist circumference from pre-school to school age. No association with fasting glucose or insulin was detected [ 78 ]. Leffer et al. [ 79 ] following young children, for three years found those children in the highest tertile of UPF energy intake at age 3 had higher levels of total cholesterol, and tri-acyl glycerol at age 6 than those in the lowest tertile [ 79 ].
3.5. Studies Using Diseases and Mortality as Outcomes
Table 4 reports the findings from studies that evaluated the association of UPF exposure and diseases and mortality as outcomes.
Diseases and mortality as outcomes.
| Study Details | UPF Exposure | Outcomes | Results | |||||
|---|---|---|---|---|---|---|---|---|
| Publication Author(s) Year | Study Type (Year) Setting | Population (Number) | Extraction Level | Relative exposure [UPF reference year] | Data Collection Method | Health Outcome | Data Collection Method | Key Findings |
| Queiroz 2018 [ ] | Case control study (2015) Brazil | Adult women Mean 53.1 years ( = 118) | Individual | UPF ≥ 5 day/week {NOVA.2010] [ ] | 98-item FFQ, 12-month recall | Breast cancer (BC) | Diagnosed BC | Regular consumption UPF (≥5 day/week) identified as risk factors for BC (OR = 2.35, 95% CI 1.08–5.12). |
| Fiolet 2018 [ ] | Prospective Cohort (2017), 5 years median follow-up France | Adults ≥ 18 years Mean 42.8 ( = 104,980) | Individual | UPF % g (quartiles) [NOVA 2018] [ ] | 3 × 24-h records | Overall, breast, prostate, and colorectal cancer | Self-report or/physician contact | UPF contribution in proportion of grams ranged from 18.7% lowest quartile to 32.3% in highest. A 10% increase in proportion of UPF consumption associated with a significant increase in overall (HR = 1.12; 95% CI 1.06; 1.18; for trend <0.001) and BC risk (HR = 1.11; 95% CI 1.02; 1.22, trend = 0.02). No significant associations were found for prostate and colorectal cancers ( = 0.8 and = 0.2, respectively). |
| Srour 2019 [ ] | Prospective cohort (2019), 5.2 years median follow-up France | Adults ≥18 years ( = 105,109) | Individual | UPF % grams (quartiles) [NOVA.2018] [ ] | 3 × 24 h records | Cardiovascular (CVD), coronary heart (CHD), cerebrovascular disease | Medical records, committee of doctors | UPF contribution averaged 17. 4% of total grams. A 10% increase in proportion of UPF consumption was associated with significant higher risk of overall CVD (HR = 1.12; 95% CI 1.05; −1.20, < 0.001); CHD (HR = 1.13; 95% CI 1.10; −1.24, = 0.02); and cerebrovascular disease (HR = 1.11; 95% CI 1.01–1.21; = 0.02). |
| Srour 2019 [ ] | Prospective cohort (2017), 6.0 years median follow-up France | Adults ≥ 18 Mean 42.7 years ( = 104,707) | Individual | UPF % g [NOVA.2018] [ ] | 3 × 24 h records | Type 2 Diabetes (T2D) | ICD-10 code or T2D medication | Mean UPF contribution was 17.3% by weight, and 29.95% by % E intake. A 10% increase in the proportion of UPF consumption was associated with a significant higher risk of T2D (HR = 1.15; 95% CI 1.06; 1.25; = 0.001). |
| Kim 2019 [ ] | Prospective cohort (2011), 19 years median follow-up USA | Adults ≥20 years ( = 11,898) | Individual | UPF frequency (times/day) (quartiles) [NOVA.2018] [ ] | 81-item FFQ, and 24-h recall | All-cause mortality (ACM) CVD mortality | National death index. CVD items 100–169 ICD-10 | Participants consumed UPF a mean 4 times/day. Individuals in the highest quartile of frequency of UPF consumption had significantly higher risk of ACM, (HR = 1.31; 95% CI 1.09; 1.58, -trend = 0.001). No significant association was observed with CVD mortality. |
| Schnabel 2019 [ ] | Prospective Cohort (2017), (median follow-up 7.1 years) France | Adults ≥ 45 years ( = 44,551) | Individual | UPF % g [NOVA 2018] [ ] | 3 × 24-h food record | ACM | National death registries. Causes by ICD-10 | UPF contributed 14.4% total weight, and 29.9% total E intake. A 10% increase in the proportion of UPF consumption was associated with a significant higher risk of ACM 1.14 (95% CI, 1.04–1.27; = 0.008). |
| Rico-Campà 2019 [ ] | Prospective Cohort (2014), (median follow-up 10.4 years) Spain | Adults 20−91 years ( = 19,899) | Individual | UPF servings/day (quartiles) [NOVA.2016] [ ] | 136-item FFQ | ACM CVD mortality Cancer mortality | Next of kin/Registries | UPF consumption ranged from 1.4 servings a day in lowest quintile to 5.3 servings a day in highest quintile. Individuals in the highest quartile of UPF consumption were at higher risk of ACM (HR = 1.62; 95% CI 1.13; 2.33) than those in the lowest quartile. No significant associations were found for cardiovascular and cancer mortality. |
| Blanco-Rojo 2019 [ ] | Prospective Cohort (2016), (mean follow-up 7.7 years) Spain | Adults Mean 46.9 years ( = 11,898) | Individual | UPF % total E intake (quartiles) [NOVA.2018] [ ] | 880-item FFQ | ACM | National Death Index | UPF contributed 24.4% total E intake. Individuals in the highest quartile of UPF consumption were at higher risk of ACM (HR = 1.46; 95% CI 1.04-2.05; trend = 0.03), than those in the lowest quartile. |
Results are presented for adjusted associations for potential confounders and statistically significant associations. NOVA refers to the food classification system [ 21 ] or earlier versions, as referenced; UPF: ultra-processed food (includes food and beverages); E:energy in calories or kilojoules; OR: odds ratio; FFQ: food frequency questionnaire; CI: confidence interval; HR: hazards ratio; food = food and beverages; BC = breast cancer; ICD-10:International Classification of Disease; CVDL cardiovascular disease, CHD: coronary heart disease; T2D:Type 2 diabetes; ACM: all-cause mortality.
3.5.1. Cancer
In a case–control study investigating breast cancer in Brazil, Quiroz et al. [ 81 ] matched 59 women with breast cancer to 59 non-cancer controls. They found regular consumption of UPF (>5 days/week) was identified as having 2.35 times higher odds of breast cancer [ 81 ]. In a longitudinal study, Fiolet et al. [ 84 ] found a higher incident rate of cancer with UPF exposure. For every 10% increment in the proportion of UPF in the diet (by percentage grams), there was a 12% higher risk for total cancers, and 11% increased risk for breast cancer [ 84 ].
3.5.2. Cardiovascular Disease
In a prospective cohort study on 105,109 adults over a median period of 5.2 years, Srour et al. [ 83 ] found a higher incident rate of all cardiovascular disease (CVD) in those with the highest intake of UPF consumption (by percentage weight). Those in the highest quartile intake had a 12%, 13% and 11% increased risk of all CVD, coronary heart disease and cerebrovascular disease, respectively [ 83 ].
3.5.3. Type 2 Diabetes
In France, Srour et al. [ 87 ] followed 104,707 participants free of type 1 or type 2 diabetes for a median 6.0 years. Participants with a higher proportional intake (by weight) of UPF in the diet had significantly higher risk of type 2 diabetes. A 10% increase in UPF in the diet was associated with a 15% higher risk of type 2 diabetes [ 87 ].
3.5.4. Mortality
In a mortality study of US adults aged over 20 years over a median follow-up of 19 years, Kim et al. [ 94 ] found those participants with the highest quartile of consumption of UPF (frequency/day) had a 31% higher risk of all-cause mortality. There was no observed association with CVD mortality [ 94 ]. In a study of 44,551 individuals over a median period of 7.1 years in France, Schnabel et al. [ 86 ] found that participants who consumed a higher proportion of UPF (by proportion of weight in grams) had a higher risk of all-cause mortality. Each 10% increment in proportion consumed was associated with a 14% higher risk of all-cause mortality [ 86 ]. In a prospective cohort study in Spain of 19,899 individuals, Rico-Campa et al. [ 91 ] found participants in the highest quartile of UPF consumption (by servings per day) had a 62% higher risk of all-cause mortality. For each additional serve, there was an 18% higher risk. No significant associations were found with CVD and cancer mortality [ 91 ]. In a second prospective cohort study in Spain, Blanco-Rojo et al. [ 92 ] followed 11,898 individuals for 7.7 median years. They found participants in the highest quartile of consumption (by percent energy) of UPF had a 44% higher risk of all-cause mortality. Iso-caloric substitution of UPF with MPF was associated with a decrease in mortality [ 92 ].
3.6. Studies Using Other Disorders and Conditions as Outcomes
Table 5 reports the findings of studies that investigated associations of UPF exposure and other disorders and conditions as outcomes.
Disorders and conditions as outcomes.
| Study Details | UPF Exposure | Outcomes | Results | |||||
|---|---|---|---|---|---|---|---|---|
| Publication Author(s) Year | Study Type (Year) Setting | Population (Number) | Extraction Level | Relative exposure [UPF Reference Year] | Data Collection Method | Health Outcome (Study Definition) | Data Collection Method | Key Findings |
| Schnabel 2018 [ ] | Cross-sectional (2013) France | Age ≥ 18 years (mean 50.4) ( = 33,343) | Individual | UPF % total grams (quartiles) [NOVA.2018] [ ] | 3 × 24-h records | Functional gastrointestinal disorders (Rome III criteria) | Self-report * | UPF contributed to 16% of total food intake by weight; 33.0% by total E intake. Individuals in the highest quartile of UPF intake had significantly higher risk of IBS (OR = 1.25; 95% CI 1.12; 1.39) and FDy (OR = 1.25; CI 95% 1.05; 1.47) but not FDy alone, compared with those in the lowest quartile. |
| Vasseur 2020 [ ] | Prospective cohort (2016) 2.3 years mean follow-up France | Adults ≥ 18 years (mean 43.3) ( = 105,832) | Individual | UPF % total grams (tertiles) [NOVA.2018] [ ] | 3 × 24 h records | Inflammatory bowel disease | Self-report ** | UPF contributed 17% food intake by weight in grams. No significant association was found with UPF consumption and IBD ( = 0.03). |
| Adjibade 2019 [ ] | Prospective Cohort (2012), 5.4 years mean follow-up France | Adults age 18–86 years (n= 26,730) | Individual | UPF % total grams (quartiles) [NOVA.2018] [ ] | 3 × 24 h records | Depression (CES-D scale) | Self-report * | UPF contributed 5% by weight in grams and 32% E intake. Individuals in the highest quartile of UPF intake had significantly higher risk of developing depressive symptoms (HR = 1.30; 95% CI 1.15–1.47) than those in the lowest quartile. Each 10% increase in UPF consumption was HR of 1.21 (95% CI, 1.15–1.27). |
| Gomez-Donoso, 2019 [ ] | Prospective cohort (2016), 10.3 years median follow-up Spain | Adults (mean 36.7 years) ( = 14,907) | Individual | UPF energy adjusted kcal/day (quartiles) [NOVA.2016] [ ] | 136–item FFQ | Depression | Self-report ** | Individuals in highest quartile UPF had significantly higher risk of depression (HR = 1.33; 95% CI 1.07–1.64); trend = 0.004), than individuals in lowest quartile of consumption, after confounder adjustment. |
| Sandoval-Insausti 2019 [ ] | Prospective Cohort (2008–2010), 3.5 years median follow-up Spain | Adults ≥ 60 years ( = 1822) | Individual | UPF intake % total E (quartiles) [NOVA2018] [ ] | Validated interview computerized diet history | Frailty (Fried’s criteria) | Trained personnel | UPF contributed mean of 19.3% total E intake. Individuals in the highest quartile of UPF intake had higher risk of frailty (OR = 3.67; 95% CI 2.00, 6.76) than those in the lowest quartile of intake. |
| Melo et al. 2018 [ ] | Cross-sectional (2012) Brazil | Grade 9 students ( = 109,104) | Individual | UPF score, intake per week (quintiles) [NOVA 2018] [ ] | 6-UPF sub-categories FFQ | Asthma, wheezing in past 12 months | Self-report * | Individuals in the highest quintile of the UPF intake score had higher odds of having asthma (OR = 1.27; 95% CI 1.15, 1.41) or wheezing (OR = 1.42; 95% CI 1.35 to 1.50), than those in the lowest quintile. |
| Azeredo 2020 [ ] | Prospective Cohort (2004–2010) Brazil | Children mean age 6.8 years baseline; 11.0 years at follow-up ( = 2190) | Individual | UPF % total E intake (quintiles) [NOVA.2018] [ ] | 55 (age 6) and 88 items (age 11) FFQ. | Wheezing, whistling or asthma in past 12 months | Self-report * | UPF contribution to total E intake was 42.3% at 6 years, and 33.7% at 11 years. Consumption of UPF at age 6 was not significantly associated with wheeze, asthma or severe asthma at age 11. |
Results are presented for adjusted associations for potential confounders and statistically significant associations. NOVA refers to the food classification system [ 21 ] or earlier versions, as referenced; food means foods and beverages; * self-report from questionnaire on condition, medical history, symptoms, medication use, and/or diagnosis by medical practitioner; ** questionnaire plus validation on sample laboratory test or interview; UPF: ultra-process food (includes food and beverages); E: energy in calories or kilojoules; OR: odds ratio; CI: confidence interval; IBS: irritable bowel syndrome; FDy: functional dyspepsia; IBD: inflammatory bowel disease; CES-D scale: Centre for Epidemiologic Studies Depression Scale; HR: hazards ratio; FFQ: food frequency questionnaire; HR: hazards ratio.
3.6.1. Gastrointestinal disorders
In a cross-sectional study in France of 33,343 adults, Schabel et al. [ 85 ] found participants in the highest quartile of UPF intake by percentage share (g/day) had a higher risk of irritable bowel syndrome (IBS)—either alone or when considering IBS and functional dyspepsia (FDy)—compared to those in the lowest quartile. There was no association of UPF intake with FDy alone [ 85 ]. In a prospective cohort study in France, Vasseur et al. [ 88 ] found no significant association with UPF intake and inflammatory bowel disease.
3.6.2. Depression
In a prospective cohort study in France, Adjiade et al. [ 89 ] followed 26,730 individuals without depressive symptoms at baseline by Centre for Epidemiological Studies Depression Scale (CES-D) scale) for mean 5.4 years. Participants in the highest quartile of UPF intake by percentage share (g/day) had a 31% increased risk of developing depression than those in the lowest quartile. An estimated 10% increase in UPF consumption was associated with a 21% increased risk of depression [ 89 ]. In Spain, Gomez-Donoso et al. [ 90 ] followed 14,907 university graduates free of depression (by diagnosis history) for a median 10.3 years. Participants in the highest quartile intake of UPF (percentage share g/day, energy-adjusted) had a 33% higher risk of developing depression than participants in the lowest quartile [ 90 ].
3.6.3. Frailty
In a prospective cohort study in Spain, Sandoval-Insausti et al. [ 93 ] followed 1822 adults >60 years free of frailty for 3.5 years. Those in the highest quartile of UPF intake (percent total energy) had 3.67 times higher odds of frailty than those in the lowest quartile [ 93 ].
3.6.4. Asthma
In a nationally representative sample of students aged 13–16 years, Melo et al. [ 80 ] found an association between a higher UPF score (based on consumption of selected sub-categories of UPF) and asthma in a dose-response manner. In a prospective cohort study in Brazil, Azerado et al. [ 82 ] followed 2190 children aged 6–11 years and found no association between UPF consumption (percent energy intake) and wheeze, asthma or severe asthma.
3.7. Quality Appraisal
The limitations and strengths found in the appraisal were used to support the critiques of studies in the discussion section. Further details are described in Supplementary Material Table S3 .
4. Discussion
This review identified 43 studies investigating associations between UPF exposure and various health outcomes and related risks, with most studies reporting more than one outcome. In 37 studies, there was at least one statistically significant association between UPF exposure and at least one adverse health outcome. No study reported an association between UPF exposure and beneficial health outcomes that reached statistical significance, were adjusted for covariates and―in prospective studies―were reported at follow-up. Beneficial outcomes were found associated with diets higher in MPF. The findings can be summarised and classified as follows: (1) in 21 studies assessing overweight, obesity and cardio-metabolic risks in adults, 15 reported significant associations and adverse health outcomes, four reported mixed associations (that is some adverse health outcomes, and some with no associations), and two found no significant associations; (2) in eight studies assessing disease or mortality, five found significant associations and adverse health outcomes, and three found mixed associations; (3) in seven studies investigating other disorders and conditions, five found only significant associations and adverse health outcomes, and two found no associations; (4) in eight studies reporting on overweight, obesity and cardio-metabolic risks in children and adolescents, three found significant associations with adverse health outcomes, two found mixed associations and three found no associations. Seven studies in adults found associations between diets high in MPF (or MPF combined with PCI or PF) and beneficial health outcomes. A study on mortality found iso-caloric substitution of UPF with MPF was associated with a decrease in mortality.
Exposure to UPF was found to be associated with population BMI trajectories, overweight and obesity prevalence as well as individual BMI, overweight including obesity (BMI ≥ 25), overweight excluding obesity (BMI 25–30), obesity, weight gain, abdominal obesity, metabolic syndrome, high blood pressure, blood lipids, blood glucose, type 2 diabetes, cardiovascular disease, coronary artery disease, cerebrovascular disease, overall cancers, breast cancer, frailty, IBS, depression and mortality (in adults), and asthma, blood lipids, metabolic syndrome and delta waist circumference (in children or adolescents). The studies that found no associations were assessing BMI, overweight, obesity, metabolic syndrome, inflammatory bowel disease, CRP (men), prostate and colorectal cancer, and mortality in CVD and cancer (in adults) or anthropometric indicators, high blood pressure, blood glucose and insulin resistance (in children). Although they showed no associations with UPF and investigated outcomes, two studies on adults and one on adolescents demonstrated that a higher intake of MPF-PCI had lower odds of overweight/obesity [ 55 ], a higher intake of MPF/PF ‘pattern’ had lower odds of metabolic syndrome [ 69 ], and MPF intake was inversely associated with overweight [ 76 ]. Three of the French studies found an association between diets higher in MPF consumption and lower risk of adverse health outcomes [ 83 , 84 , 87 ]. The RCT found participants lost weight and had improved bio-markers during the MPF diet phase [ 67 ].
4.1. Overweight, Obesity and Cardio-Metabolic Risks as Outcomes (Adults)
The majority of studies investigating UPF exposure and adverse health outcomes of overweight, obesity or cardio-metabolic risks showed associations. The WHO defines overweight and obesity as ‘abnormal or excessive fat accumulation that may impair health’ [ 48 ] and states that weight gain, at whatever the original weight, is a risk for disease [ 46 , 47 ]. Overweight and obesity are both significant risks for CVD, cancers and mortality, with body fat distribution, blood lipids, hypertension and blood glucose related risks [ 97 ]. Studies in individuals that measured only mean BMI, overweight and obesity [ 53 , 56 , 57 , 59 , 61 , 66 ] are indicative of risk. However, studies including body fat anthropometrics, abdominal obesity or biomarkers [ 59 , 60 , 62 , 67 ], the two prospective studies and RCT showing weight gain over time [ 65 , 66 , 67 ], the prospective study on hypertension [ 72 ], a study on metabolic syndrome on a nationally-presentative sample [ 71 ], and a smaller study [ 68 ] provide stronger support of the risk of UPF exposure. Moreover, the ecological studies tracing UPF purchase country comparisons [ 52 ] or time-trend analyses within [ 36 ] and between [ 37 ] countries, all showed an association with or trajectories of higher exposure to UPF and the risk to population prevalence rates or weight trajectories over time within or between countries.
The ecological studies’ strengths were the use of national obesity statistics [ 36 , 52 ], large standardised datasets [ 37 ] and nationally representative population surveys with complex sampling [ 52 ], which support an association of UPF availability and rising rates of global obesity and population mean BMIs. The strengths of the cross-sectional studies were in nationally representative samples ( n = 6) or large samples ( n = 2) with data extraction at individual intake, except one by household purchases [ 53 ] and outcome measurements by trained personnel in all studies except two [ 61 , 63 ] and partially in a third [ 57 ]. Critically, in two prospective cohort studies, Canhada et al. [ 66 ] and Mendonca et al. [ 65 ] demonstrated individuals not overweight or obese at baseline moved over time to BMI parameters of significant risk, with individuals in the highest quartile of UPF having the highest risk. The studies’ strengths were demonstrating both the risk over time for an individual, and group comparison of higher to lower UPF exposure. The Mendonca et al. study was on younger health professionals (mean age 37.6), a group who may be more motivated to follow a healthy diet pattern [ 65 ]. Results may not be applicable to the general population and in fact may be worse in those less motivated to follow a ‘healthy’ diet, which would make the association of UPF exposure and body weight gain even stronger. An interventional RCT study by Hall et al. [ 67 ] demonstrated weight gain on consumption of UPF diet and weight loss on an unprocessed diet. While whole diets are unable to be blinded, participants were blinded to glucose readings, and to weight change by wearing loose-fitting clothing.
Ecological studies were limited by methodologies unable to separate age and gender [ 36 , 52 ], the exclusion of food purchases outside the home or take-away food outlets [ 37 , 52 ], or time lags between the collection of survey data and obesity data of up to five years [ 52 ]. Ecological studies are considered a relatively weak study design due to the risk of bias in interpreting results that are observations at the population level, not necessarily translatable to individuals; and all cross-sectional studies are unable to determine causality and temporality and have the potential for confounders. In studies with mixed results, one study showed that in women (but not men), there was an association of UPF exposure with higher CRP, although not after controlling for BMI [ 70 ]. The authors posited that adiposity was driving the inflammation; however, it is also possible that inflammation could be driving adiposity [ 98 , 99 ] and that diet-induced inflammation could facilitate further pro-inflammatory events [ 98 , 100 ]. Three studies did not find any association of UPF exposure and overweight, obesity or cardio-metabolic risks. A Malaysian study on university personnel found no association with BMI or percentage body fat [ 64 ]. The study was limited by its small sample size and noted difficulties in the application of NOVA to the national food database. One study on a nationally representative sample in the UK showed no associations of UPF and body weight parameters [ 55 ]; whereas, the consumption of MPF-PCI pattern had lower odds of overweight and obesity. Likewise, in a study that showed no significant association with UPF and metabolic syndrome [ 69 ], there were lower odds of metabolic syndrome, low HDL and hyperglycaemia with higher MPF/PF intake. Both studies provide support for MPF diets over UPF. The UK study used an early NOVA classification and a more recent study found direct associations between UPF exposure and obesity [ 60 ].
4.2. Overweight, Obesity Cardio-Metabolic Risk as Outcomes (Children and Adolescents)
In investigations of cardio-metabolic risks in children and adolescents, three prospective studies following young children from ages three (or four) through to ages six (or eight) years showed a higher intake of UPF at age three (or four) predicted higher total cholesterol, LDL cholesterol, tri-acyl glycerol and/or increased delta waist circumference at age six (or eight) [ 77 , 78 , 79 ]. In cross-sectional studies, one study on adolescents showed an association of higher UPF intake and metabolic syndrome [ 75 ], and a second on adolescents showed no association with overweight, higher waist circumference and high blood pressure [ 76 ]. Three studies investigating overweight and obesity on older children and adolescents aged 10–19 years showed no association of UPF with anthropometrics measured [ 56 , 73 , 74 ]. Direct comparisons of the studies were difficult due to differing study designs, exposure measurements and outcomes and methodological weaknesses. Two of the studies reported quite high mean energy intakes of >4000 Kcal/day (>17,000 KJ) [ 73 , 74 ], and one study had a very small sample ( n = 200) with a low power of the statistical test [ 73 ]. In the prospective study there was a large drop-out rate of 43% [ 74 ]. The authors reported cross-sectional results and prospective changes were not statistically significant.
Children and adolescents have age-appropriate energy and nutrients needs for normal growth, development, cognitive advancement, physical activity and prevention of micro-nutrient deficiencies [ 101 ], in addition to consideration of risk for NCDs. The three studies on young children consistently showed a higher proportion of UPF were consumed at school age compared to preschool age and consistently showed direct associations between UPF consumption and cardio-metabolic or obesity risk. These provide strong support for an association between UPF consumption and cardio-metabolic risks in young children, and there is a concern that UPF consumption rises with age in childhood. The less strong associations in studies in older children and adolescents generally may be attributed to the role of the growth spurt in the pubertal years together with higher physical activity limiting excess weight. Nevertheless, dietary habits set in childhood and adolescence may be difficult to change, and there is a need to study post-pubertal young adults in future studies. The age bracket from post pre-school to pre-adolescence (~age 7–10) was under-represented in the studies reviewed. With the studies’ methodological differences and limitations, it is difficult to draw any firm conclusions in older children and adolescents.
4.3. Diseases and Mortality as Outcomes
There were four studies investigating UPF exposure and disease, and four studies investigating UPF and mortality. All of the studies showed a direct association with UPF intake and outcomes of breast cancer, total cancers, cardiovascular disease, coronary heart disease, cerebrovascular disease and mortality. There were no associations shown for prostate and colorectal cancer, cancer mortality and CVD mortality. Seven of the studies were prospective cohort studies and there was one case–control study in breast cancer cases compared to matched controls. Health outcome measurements were by validated scales of measure, medical records, confirmed diagnosed history and (for mortality) national death registries. All studies reported statistical analyses adjusting for appropriate covariates.
4.4. Studies that Investigated Other Disorders and Conditions as Outcomes
Seven studies investigated exposure to UPF and other disorders. Direct associations were observed with UPF intake and IBS, depression and frailty in adults [ 85 , 89 , 90 , 93 ]. Frailty, in turn, is a risk for stressors and less inclination for physical activity, and is in itself a risk for CVD [ 102 ]. One prospective study showed no association with inflammatory bowel disease. In the two studies investigating asthma, a prospective cohort study in children aged 6–11 years showed no association [ 82 ], and a cross-sectional study drawn from a representative sample of adolescents showed a direct association [ 80 ]. The studies are not directly comparable due to different study designs, age brackets and UPF definition, including 18 and 21 UPF groups in one study, and six in the other. While disorders and conditions are not considered main contributors to the global burden of death, they are main contributors to the burden of non-fatal illnesses and DALYs lost [ 11 ]. It is important to recognise the contribution diet—and in particular, UPF—is having on the reported incidence rates of these conditions.
4.5. Dietary Assessment, UPF Definitions and Confounding Variables
Most of the studies found UPF exposure was associated with one or more adverse health outcome. However, the differences in studies’ methodologies make comparisons between studies difficult.
Firstly, concerning the UPF definition, the original NOVA three-group (now four-group) food processing classification was first proposed in 2010 [ 9 , 25 ]. This was followed by development [ 26 , 27 , 29 , 54 ] and insertion into the dietary guidelines for the Brazilian population in 2014 [ 28 ]. An update occurred in 2016 [ 22 ], and further explanatory documents were published in 2018 and 2019 [ 21 , 30 ]. NOVA history has been described elsewhere [ 5 , 22 , 29 ]. The studies described in this review used these varying historical versions and additional studies on NOVA [ 33 , 58 ] as their reference for UPF definition, resulting in some differences in the foods classed as UPFs. Further details are provided in Supplementary Material Table S2 . For example, the studies using early definitions with only three food groups (‘ultra-processed food products’ contained the current groups three and four; that is, with cheese and all bread regarded as UPF and pasta regarded as PCI) [ 53 , 55 , 56 , 69 , 74 , 75 , 81 ], and artisanal bread included as UPF until 2014 [ 36 , 59 , 77 ], may not directly compare to studies using later definitions. Studies also ranged in the reporting of their application of NOVA from the provision of only a reference and brief description [ 70 , 75 ] or short food lists mismatched to reference [ 68 ] to detailed supplementary lists and/or explanations of analysis of national food databases of >3000 foods [ 61 ].
Secondly, against a broad range in the strengths and limitations of various data extraction methods [ 103 ], authors noted additional benefits or constraints of instruments in application to NOVA, and to national food databases. Food diaries (able to account for seasonal or weekly variations) [ 60 ] and 24-h recalls [ 59 , 78 ] record actual foods consumed and preparation methods, with fewer difficulties were noted in application than reported in the use of FFQs. FFQs included between 55 and 880 items and UPF ranged from 6 to >21 sub-groups, with application to NOVA constrained to foods listed. In several instances, foods were classified differently across surveys due to different food systems in country-specific contexts. For example, some FFQs do not separate the bread type and in the US and UK it is mainly UPF [ 60 , 104 ], whereas in France and Spain it is mainly artisanal [ 52 , 61 ]. Additional complexities included application to food databases with a lack of information on the differentiation of canned food into PF or UPF, databases disaggregating foods to nutrient content rather than processing type (e.g., frozen pizza disaggregated to component ingredients could inadvertently be classed as PF or PCI, and not correctly as UPF), and disaggregating handmade dishes (e.g., Bolognese pasta) into major food-items in the recipe (e.g., group 1, pasta) rather than underlying ingredients (pasta, meat, sauce, oil), which is the recommended approach [ 59 , 105 ].
Thirdly, most studies used percent energy intake as a measure of relative exposure of UPF, which is difficult to compare to studies that used frequencies (times per day), servings per day, or consumption of an UPF ‘pattern’. The French NutriNet-Santé studies used percentage weight per day, arguing the need to account for UPF that have no or less energy value (e.g., artificially sweetened beverages) than regular alternatives [ 61 , 83 , 84 , 85 , 86 , 87 , 88 ]. Ideally, prospective studies collect food data more than once, yet some studies provided no details or collected data only once [ 72 ]. Conversely, the NutriNet-Santé studies collected data by the use of three 24-h records every six months for two years to establish a baseline diet and UPF intake [ 61 , 83 , 84 , 85 , 86 , 87 , 88 ].
Lastly, adjustments for confounding variables differed among the studies. In particular, one ecological study did not have access to data on confounders [ 36 ]. Some studies did not adjust for total energy, which confounds the risk for weight gain which is in itself a risk for CVD, cancer and type 2 diabetes; or for physical activity [ 55 , 63 , 76 ] (or used proxies [ 56 ]), which confounds the risk for CVD. These factors make comparisons between studies difficult.
4.6. Consistency with Findings in Other Reviews and Studies
That UPF exposure has been found to be associated with numerous diseases and conditions across a broad range of ages, populations and settings, underlines the role of diet quality as assessed by the ‘extent and purpose of industrial processing’ (as defined by NOVA [ 21 ]) influencing human health. This finding is consistent with findings reported in other reviews [ 2 , 3 , 4 , 5 , 7 ]. An early narrative review [ 4 ] and a recent systematic review [ 5 ] investigating studies on UPF exposure and obesity and/or cardio-metabolic risks, and a systematic review investigating body fat in childhood [ 2 ], similarly found most studies showed direct associations. The recent review [ 5 ] and a systematic review of studies of children’s dietary intake in relation to Brazil’s Dietary Guidelines [ 106 ] also noted that the literature was marred by inconsistencies in study definitions and methods. However, our review found the more recent studies—particularly the French, Spanish, US and UK studies since 2018—which do not have the NOVA classification limitations as in earlier studies, have adjusted for confounders, and expert teams have evolved comprehensive processes for analysis and classification of national surveys and food-databases in the application of NOVA classification [ 37 , 59 , 60 , 61 , 65 , 67 , 71 , 72 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 ]. Our review found similar UPF consumption trends as previously reported of up to >50% in high income countries and up to 30% in middle income countries. Notably Spain (24.4%) and France (29.9–35.9%) had lower UPF intake than Canada, UK and the USA (45.1–56.1). In Brazil, children (41.4–49.2%) and adolescents (50.6%) had higher consumption rates than adults (20–29.6%). In our review, one prospective study in adolescents showed an association of UPF exposure and beneficial effects at baseline that did not reach statistical significance at follow-up [ 74 ]. In a book chapter reporting a study in Kenya, beneficial nutritional effects were found associated with UPF in children [ 107 ]. Food data, however, were extracted at the household level and estimated to individuals.
4.7. Proposed Mechanism of Effects
Evidence for the mechanisms underpinning the link between UPFs and adverse health outcomes is still emerging. Proposed mechanisms include a poor nutritional profile (i.e., UPFs are vectors for added sugars, sodium and trans-fats) and displacement of MPFs in the diet [ 33 , 34 , 35 , 58 , 108 , 109 , 110 , 111 , 112 , 113 , 114 ], higher glycaemic load and reduced gut–brain satiety signalling resulting from altered physical properties created by the processing of foods [ 115 , 116 , 117 , 118 ], carcinogens formed during high-temperature cooking (e.g., carbohydrate-rich foods with acrylamide) [ 119 , 120 ], and inflammatory responses linked with acellular nutrients and industrial food additives, gut microflora dysbiosis and increased intestinal permeability [ 98 , 121 , 122 ]. Certain properties of UPFs may promote overconsumption [ 123 ], including their often ubiquitous availability and convenience [ 124 , 125 , 126 ], palatability and quasi-addictiveness [ 127 , 128 ] and intensive marketing practices used to promote purchasing and consumption, especially among children and adolescents [ 129 , 130 , 131 ].
The NutriNet-Santé prospective studies investigating CVD and cancer found participants with high UPF exposure consumed diets higher in energy intake, sodium, lipids and carbohydrate, and participants in the CVD study had lower intakes of MPF, fruit, vegetables and fibre; in the study on type 2 diabetes, participants with higher UPF intake consumed diets higher in energy intake, sugar and sodium, and lower in wholegrain cereals, fruit, vegetables and fibre [ 83 , 84 , 87 ]. However, the studies reported associations of UPF exposure and the diseases remained even after adjusting for energy intake, salt, sugar/added sugar and Western dietary patterns (for CVD and cancer), and for a low ‘healthy’ diet pattern/diet quality (CVD and type 2 diabetes) or fibre (CVD). Those associations remained after adjustments, which suggest something other than nutritional factors may be contributing to the recorded observations. Moreover, in the CVD study, associations were observed for individual UPF food groups such as beverages, fats and sauces, meat, fish and eggs, sugary products and salty snacks; yet associations were stronger when the overall amount of UPF was considered rather than specific food groups. The authors postulated possible synergistic effects of many compounds in UPF. Similarly in other studies adjusting for overall diet ‘quality’, the associations between UPF exposure and adverse health outcomes or mortality remained [ 91 , 94 ].
In the RCT by Hall et al. [ 67 ], energy intake was greater during the UPF diet; the participants consumed more fat and carbohydrate, and gained weight and body fat. The diets were controlled for presented calories, energy density, macronutrients, sugar, sodium and fibre, providing causal evidence that something other than risk nutrients in UPFs causes energy intake and weight gain, and an increase in body fat with the consumption of a diet higher in UPFs. The study showed reduced secretion of the hunger hormone ghrelin, as well as increased levels of the satiety hormone PYY (peptide YY) with the unprocessed diet. Hence, relative to UPFs, unprocessed foods may stimulate more efficient regulation of biological mechanisms controlling hunger and satiety. The study also showed a reduction in the inflammatory bio-marker hs-CRP (highly sensitive CRP) with the unprocessed diet, and inflammation has been demonstrated to be coupled with satiety signals in animal studies [ 98 ].
Critics of NOVA have argued that ‘healthy dietary patterns’ are linked to nutrient intakes [ 132 ], and any adverse health effect seen in studies on UPF can be explained by nutritional factors [ 133 ] such as the presence of high levels of sugar, fat and salt. However, the literature indicates that the effects of ultra-processing on a food’s physical structure and chemical composition is independent of and therefore in addition to the effects of ‘risk’ or ‘positive’ nutrients present in the food [ 67 , 83 ]. Moreover, a defining characteristic of the NOVA classification is classifying added sugar, oils and fats, and salt as ingredients (Group 2). In UPF (Group 4), it is the high amount of these ingredients combined with processing techniques (deconstruction of the food matrix, removal of water) and addition of cosmetic additives, that explains the final characteristic of UPFs (high content of sugar, salt, fat; high energy density; and palatable) [ 21 ]. A further explanation of the mechanism by which UPFs influence health outcomes is that their inclusion in the diet displaces ‘protective’ MPFs, with low intakes of MPFs increasing the risk of CVD, cancer, type 2 diabetes and mortality [ 5 , 10 ].
4.8. Strength and Limitations
This is the first study to perform a systematic search for studies that have investigated UPF exposure and health outcomes, combined with a narrative review. The strengths of this review are the broad inclusion of study types, populations and health outcomes, a quality assurance process that involved a team working together and reaching consensus-based decisions, and undertaking a synthesis of the evidence to provide a coherent assessment of the role UPFs in influencing human health.
This review has limitations. We did not assess studies investigating participants with pre-existing conditions (including overweight or obesity) where UPF intake was considered an outcome. Interestingly such studies have similarly found direct associations of health conditions and UPF consumption [ 134 , 135 ]. The review was limited by the constraints within the studies under review which were mainly observational studies that cannot deduce cause and effect relationships. The smaller studies were difficult to compare to studies with national representative samples, yet were important representations of special groups or varying cultures [ 63 , 64 , 68 , 69 ].
4.9. Future Priorities for Nutrition Research and Policy Practice
With the rapidly growing number of studies reporting associations between UPFs and adverse health outcomes, and NCDs, there is an increasing need to take on board the contribution of these foods to the global burden of disease, as well as the need to act on multiple levels, including through government regulation, to improve the health prospects of current and future generations.
In the future, priority research activities to further develop the body of evidence for associations between UPF intake and health outcomes include continuing to analyse existing cohorts, conducting clinical trials, extending the cohort and clinical studies to a range of population groups, such as those in low- and middle-income countries, as well as childhood and adolescence years, to determine whether UPF influences on metabolism start before adulthood, and investigating plausible causal pathways to provide mechanistic understandings of the nature of the health associations. Further priorities include standardisation of application of the NOVA classification and analysed national databases, and a protocol for including confounder adjustment, especially weight parameters (with obesity being a known risk for CVD, cancer and type 2 diabetes) and low physical activity (a risk for CVD) [ 97 ].
With this solid evidence base the need for NOVA and UPF issues to be applied in policy practice, for example to food labelling and informing food procurement activities, is even more relevant. Food-based dietary guidelines and nutrition policy actions should consider incorporating the concept of ultra-processing to describe the healthiness of individual foods within the overall dietary pattern.
5. Conclusions
This review has shown that a high dietary intake of ultra-processed foods is associated with a range of adverse health outcomes, and non-communicable diseases, disorders and conditions, thereby bearing the potential to significantly influence the global burden of disease. Moreover, evidence suggests a higher risk of all-cause mortality with high consumption of ultra-processed foods. No study reported an association between UPF and beneficial health outcomes. The review has also shown beneficial outcomes were associated with diets higher in unprocessed and minimally processed foods. Although the majority of studies are of observational nature, the evidence base concerning plausible biological mechanisms supporting the observed associations is steadily evolving. The findings in this review support the notion that inferring health effects from individual nutrients and ingredients in foods is insufficient, and that industrial processing, and its extent and purpose, may add accuracy and reliability in predicting and explaining associations between foods and health outcomes. The considerable and growing body of evidence supporting the use of ultra-processed foods as a scientific concept to assess the ‘healthiness’ of foods within a dietary patterns context has the potential to improve future development of dietary guidelines as well as nutrition policy actions.
Abbreviations
| BMI | Body Mass Index |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| DALY | Disability life adjusted years |
| FFQ | Food frequency questionnaire |
| GBD | Global burden of disease |
| HDL | High-density lipoprotein cholesterol |
| IBS | Irritable bowel syndrome |
| LDL | Low density lipoprotein cholesterol |
| NCD | Non-communicable disease |
| MPF | Unprocessed and minimally processed foods |
| NHANES | National Health and Nutrition Examination Survey |
| PCI | Processed culinary ingredients |
| PF | Processed foods |
| SSB | Sugar-sweetened beverages |
| UPF | Ultra-processed foods |
| WHO | World Health Organization |
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/7/1955/s1 , Table S1: NOVA food groups definitions according to the extent and purpose of food processing, with examples; Table S2: NOVA ultra-processed food sub-groups reported in references in studies. Table S3: Quality assurance process.
Author Contributions
Conceptualization, M.L., P.B.; methodology, M.L., L.E., P.B., M.Z.; data curation, L.E., P.M.; writing—original draft preparation, L.E.; writing—review and editing, L.E., M.Z., P.B., P.M., M.L.; supervision, M.L., P.B.; project administration, M.L. All authors have read and agreed to the published version of the manuscript.
This research was supported by an Australian Research Council Discovery Project; DP190101323, ‘Reforming evidence synthesis and translation for food and nutrition policy’.
Conflicts of Interest
P.M. was co-author of an update of the NOVA classification [ 22 ], and has been co-author of published research using the classification, including one of the studies in this review [ 80 ], and stepped aside in the appraisal process of that article. All of the authors declare no conflict of interest.

IMAGES
VIDEO
COMMENTS
Abstract. Genetic modification is a special set of gene technology that alters the genetic machinery of such living organisms as animals, plants or microorganisms. Combining genes from different organisms is known as recombinant DNA technology and the resulting organism is said to be 'Genetically modified (GM)', 'Genetically engineered ...
A predictive study conducted by the International Food Policy Research Institute indicated that by 2050, ... This review paper presents a perspective of GMO technology, associated risks, and its current status. ... Unintended effects and their detection in genetically modified crops. Food and Chemical Toxicology, 42 (7), ...
The term "genetic modified organisms (GMO)" has become a controversial topic as its benefits for both food producers and consumers are companied by potential biomedical risks and environmental side effects. Increasing concerns from the public about GMO, particularly in the form of genetic modified (GM) foods, are aimed at the short- and ...
A series of extensive and long-term research has shown that the benefits of growing genetically modified crops in the fight against global food shortages and hunger have been significant. The steady increase in the global population has led researchers to focus on the benefits of developing genetically modified products, rather than the ...
A systematic review of animal and human studies was conducted on genetically modified (GM) food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research. Seven electronic databases were searched from January 1st 1983 till July 11th 2020 for in vivo, animal and human studies on the incidence of adverse effects/events of GM products ...
Genetic modification in plants was first recorded 10,000 years ago in Southwest Asia where humans first bred plants through artificial selection and selective breeding. Since then, advancements in agriculture science and technology have brought about the current GM crop revolution. GM crops are promising to mitigate current and future problems ...
Genetically modified (GM) foods are judged by the majority of scientists to be as safe for human consumption as conventionally grown foods 1,2, and have the potential to provide substantial ...
Aim. This review article mainly focuses on the importance, possible risks and state of public debate on genetic engineering particularly on genetically modified organisms (GMOs). During the last ...
Genetically modified (GM) technology is a highly controversial topic for today's global food consumer. The commercial development of GM crops began in 1996 with GM corn and has expanded every ...
A study in the USA on how best to label genetically modified and gene edited foods in compliance with the National Bioengineered Food Disclosure Standard which came into effect on January 1st, 2022 Citation 385 demonstrated that "a higher proportion of respondents choose a label if the Bioengineered label was disclosed using the approved ...
Modified lab strains deliver DNA of interest into susceptible plant cells, where it is randomly integrated into the host genome. ... the Foundation for Food and Agricultural Research (award no ...
The public perspective on genetically modified foods (GMFs) has been intensely debated and scrutinized. ... This paper studies the factors predicting GMF acceptance employing the representative ...
genetically modified foods may have hepatic, pancreatic, renal and reproductive effects on. humans although these claims have bee n. labelled as scientifically meaningless by. industries and food ...
In the European Union, EFSA has issued a completely separate guidance document for the risk assessment of food and feed from genetically modified plants (EFSA Citation 2011a). The US FDA also provides guidance on the types of information that are required to evaluate the safety of foods derived from genetically modified plants (US FDA Citation ...
In this chapter, the committee examines the evidence that substantiates or negates specific hypotheses and claims about the health risks and benefits associated with foods derived from genetically engineered (GE) crops. There are many reviews and official statements about the safety of foods from GE crops (for example, see Box 5-1), but to conduct a fresh examination of the evidence, the ...
The Health Effects of Genetically Modified Foods: a Brief Review. Int J Nutr Sci. 2021; 6(1): 1047. allows specific genes to be identified, isolated, copied, and introduced into other organisms in much more direct and controlled ways. The principal transgenic crops grown commercially in field are herbicide and insecticide resistant soybeans ...
Research Paper on GMOs. By Raymond Wu in Papers on December 10, 2018 . The Controversy of Genetically Modified Foods on Human Health. Genetically Modified Organisms (GMOs) are creatures in which their genetic make-up has been altered through genetic engineering or biotechnology in hopes of either obtaining favorable traits, eliminating ...
RESEARCH PAPER The influence of consumers' knowledge on their responses to genetically modified foods Hyesun Hwang a and Su-Jung Namb aDepartment of Consumer Science, Convergence Program for Social Innovation, College Sciences, Sungkyunkwan University, Seoul, Republic of Korea; bDepartment of Home Economics Education, College of Education, Jeonju University, Jeonju, Republic of Korea
In this study, we present the bibliometric trends emerging from research outputs on consumer perception and preference for genetically modified (GM) foods and policy prescriptions for enabling the consumption using VOSviewer visualization software. Consumers' positive response is largely influenced by the decision of the governments to ban or ...
Enzymatically modified starch offers a potential biodegradable coagulating agent. However, most of the research in this area mainly focuses on corn starch, leaving cassava starch underexplored. This study investigates the coagulation efficacy of cassava starch modified by α-amylase (AA), glucoamylase (GA), and their combination.
rice that contains b eta -carotene, the plant pigment that is the precursor of Vitamin A. This. rice, called "golden" rice because the ins erted beta - carotene t urns the grain a gold en. yellow ...
Food Research International provides a forum for the rapid dissemination of significant novel and high impact research in food science, technology, engineering and nutrition. The journal only publishes novel, high quality and high impact review papers, original research papers and letters to the editors, in the various disciplines encompassing the science and technology of food.
This sample genetics research paper on genetically modified food features: 4000 words (approx. 13 pages) and a bibliography with 14 sources. Browse other research paper examples for more inspiration. If you need a thorough research paper written according to all the academic standards, you can always turn to our experienced writers for help.
EDSTMs will be attended by members of CDER's Emerging Drug Safety Technology Program, which includes representatives from CDER staff with experience in emerging drug safety technologies ...
This research paper is about Genetically Modified Food (GM). The main purpose of writing this paper is to highlight the benefits of GM Food. It is an interesting topic for me because many people ...
In this review, 'food' includes foods and beverages; 'consumption' includes national or household food availability and individual food intake. From a total of 851 papers, there were 263 identified studies on UPF and 43 for final inclusion in our review. Figure 1 presents a flow diagram adapted from PRISMA [43,44] of the search process.
Access the portal of NASS, the official source of agricultural data and statistics in the US, and explore various reports and products.