- Sample Research

FREE 10+ Clinical Research Proposal Samples in MS Word | PDF

“Physicians must keep current with the clinical information to practice evidence-based medicine…to answer many of their clinical questions, physicians need access to reports of original research. This requires the reader to critically appraise the design, conduct, and analysis of each study and subsequently interpret the results.” These words emphasized from the study of Donna Windish and others. In this article, we will discuss beneficial steps of creating a proposal for your clinical research , plus we have various examples of a research proposal template for you to use. Keep on reading!
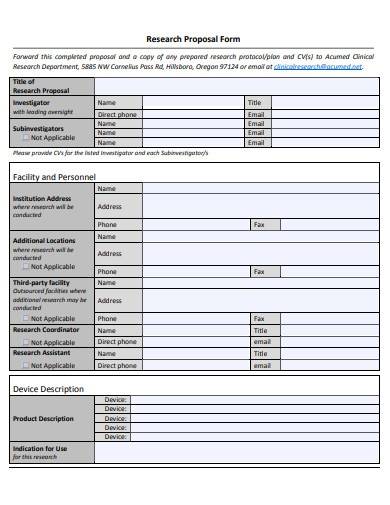
Clinical Research Proposal
Free 10+ clinical research proposal samples, 1. clinical research proposal template, 2. clinical research proposal form, 3. clinical program research proposal, 4. sample clinical research proposal, 5. medical research proposal assignment, 6. medical research proposal template, 7. sample clinical research proposal template, 8. clinical research project proposal, 9. medical research request proposal, 10. simple clinical research proposal form, 11. formal clinical research proposal, what is a clinical research proposal, how to write a clinical research proposal , 1. determine the research problem and set realistic goals, 2. collect and analyze resources and data , 3. explain your methodology, 4. critically review and evaluate the study, what is the purpose of clinical research, what are the different types of clinical research, how to create an effective clinical study, what type of study is a clinical trial.

Size: 43 KB

Size: 92 KB

Size: 75 KB

Size: 20 KB

Size: 591 KB

Size: 106 KB

Size: 191 KB

Size: 102 KB

Size: 186 KB
Size: 41 KB
A proposal for a clinical research is a valuable document that demonstrates primary targets, objectives, and methods or strategies established by the clinical researchers, medicine professionals, healthcare practitioners or scientists in encouraging potential sponsors or medical associations for the implementation of a particular clinical study or trial.
Working as a diligent clinical researcher, you need to fully learn and apply the integral process and skills, enhance your grade, and significantly design a useful end product. In this matter, we suggest that you follow the steps below while freely using one of our sample research proposals in this article:
The first step that you need to accomplish is determining the research problem so that you can set up realistic goals, and objectives in creating a proposal for your clinical research. The book “ Essentials of Clinical Research ” stated that “research questions are generated in a variety of settings such as during journal reading, hospital rounds, discussions with colleagues, seminars, and lectures. Those questions can be refined into a research idea and, after further review of the literature, ultimately developed into a hypothesis.”
Additionally, the book “ Designing Clinical Research ” described good research questions that came from medical articles and conferences, from critical thinking about clinical practices and problems, from applying new methods to old issues.
The second step that you need to do is collecting and analyzing important resources and necessary data for your clinical study. These systems have been utilized widely in clinical trials to support randomization of research subjects and to collect essential data for the research study. “ The Data Book: Collection and Management of Research Data ” explained that the accuracy and validity of data have a direct effect on the conclusions drawn from them. That’s why data management and research are inevitably connected.
Thoroughly explain the various kinds of methods and strategies that you are planning to use for your research study . According to the book “ Clinical Trial Methodology ”, clinical trial methodology includes all methods required for the protection of participants in a clinical trial and all methods that are fundamental to provide a valid inference about the objective of the trial. So, you must show a logical chronological order of the methods in all aspects of your clinical trial.
Based on the book ‘Evaluating Clinical Research”, obtaining a special knowledge of biostatistics may help a research evaluator to properly analyze the design of a clinical research trial. Medical and healthcare practitioners should be able to focus on patient issues rather than the statistical and methodological issues. So, you need to be able to critically review what you read.
Clinical research is important to create top-quality data so that healthcare and medical institutions are able to set up reasonable decisions. By doing a thorough clinical research, many scientific questions can be answered, and solve crucial health issues in our society.
Some different types of clinical research are prevention trials, treatment trials, case control studies, cohort studies, pilot and feasibility studies, cross sectional studies, and many more.
To create an effective clinical study, you need to maintain simplicity and order in your work. Recruit patients and professionals who are integral for the study. Plan accurately the four stages with the aim of your clinical trial. Set up a sufficient budget and resources plan.
An article published by National Institute of Aging (NIA) explained that “clinical trials are research studies performed in people that are targeted at analyzing a medical, surgical, or behavioral intervention.” Thus, it is an essential method by researchers to know if a new treatment is safe and effective in people.
Ike Skelton once said: “Modern medical advances have helped millions of people live longer, healthier lives. We owe these improvements to decades of investment in medical research.” Therefore, writing a proposal for your clinical research is a significant stepping stone for you to make things happen especially in the near future of healthcare and medical science. So, we recommend that you get a clinical research proposal template today to begin your clinical research study proposal or trial proposal!
Related Posts
Free 22+ letter of support samples in pdf ms word, free 9+ healthcare marketing plan samples in pdf ms word, free 32 white papers in pdf, free 9+ sample social work cover letter templates in ms word, free 10+ investigator brochure templates in ai indesign | ms ..., free 8+ product monograph samples in pdf ms word, free 7+ letter of intent medical school in pdf, free 8+ sample cover letter for medical assistant in ms word, free 7+ stakeholder management strategy samples in pdf doc, free 61+ sop templates in pdf ms word, free 7+ management proposal samples in ms word pdf, free 9+ sample manufacturing project reports in pdf ms word ..., free 9+ sample need assessment templates in ms word pdf, scientific report templates, free 7+ sample forensic report templates in ms word pdf, free 25+ sample business proposals in pdf ms word | pages ..., free 7+ sample catering proposals in pdf ms word | google ..., free 8+ sample professional proposal templates in pdf ms ..., free 12+ sample research reports in ms word apple pages ....

ISP Proposal Samples
You must have Adobe Acrobat Reader to view or print these PDF files. Click the button below to download a free copy:
Section 'Sub' Navigation:
- Introduction
- Role of Committee
- Expectations
- Past ISP Titles and Chairs
- Sample Proposals
- Helpful Hints
- Proposal Review Process
- Time Frames
- Completing the ISP
- Elective Credit
- Contact ISP Coordinator
- AudioVisual Support
- Body Donation Program
- Central Administration
- Educational Technology
- Professional Development Center
- Simulation Training Center
- MedEd Site Index
Page 'Breadcrumb' Navigation:
Site 'Main' Navigation:
- ASA Divisional Offices/Centers
- Admissions Office
- Financial Aid Office
- Student Affairs Office
- Diversity & Community Partnerships
- Global Health & Academic Concentration
- Medical Scientist Training Program
- Problem Based Learning Recruitment
- Schedules and Calendars
- Visiting Senior Students
- SOM Block Schedule
- UGME Divisional Offices/Centers
- ACA Preceptor
- Educational Support Services
- ISP Handbook
- Medical Education Technology and Evaluation
- Medical Teaching Laboratory
- Credentials Verification
- House Officer
- Benefits & Liability Insurance
- Medical License, DEA Registration & EPCS Enrollment
- Visiting Residents
- Program Coordinators & Directors
- Policies & Notices
- Anonymous Feedback
- GME Orientation
- Continuing Medical Education
- Alumni Affairs
- Business Office
- Equipment Replacement
- MedEd Support Services
- Medical Education Technology
- Medical Education
- School of Medicine
- UC San Diego Health System
- UC San Diego Health Sciences
Official Web Site of the University of California, San Diego. Copyright © 2024 Regents of the University of California. All rights reserved. Webmaster
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Starting the research process
- How to Write a Research Proposal | Examples & Templates
How to Write a Research Proposal | Examples & Templates
Published on October 12, 2022 by Shona McCombes and Tegan George. Revised on November 21, 2023.
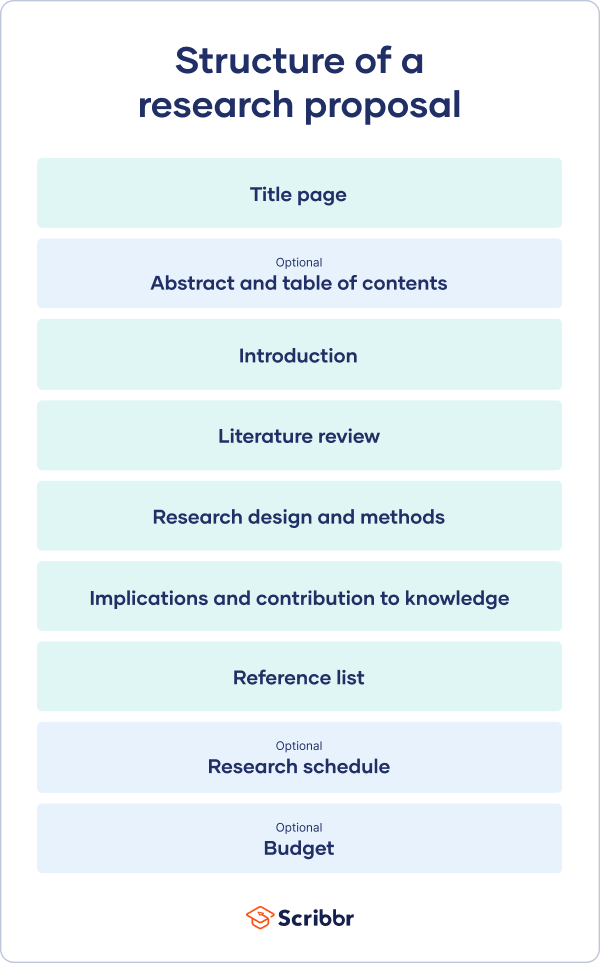
A research proposal describes what you will investigate, why it’s important, and how you will conduct your research.
The format of a research proposal varies between fields, but most proposals will contain at least these elements:
Introduction
Literature review.
- Research design
Reference list
While the sections may vary, the overall objective is always the same. A research proposal serves as a blueprint and guide for your research plan, helping you get organized and feel confident in the path forward you choose to take.
Table of contents
Research proposal purpose, research proposal examples, research design and methods, contribution to knowledge, research schedule, other interesting articles, frequently asked questions about research proposals.
Academics often have to write research proposals to get funding for their projects. As a student, you might have to write a research proposal as part of a grad school application , or prior to starting your thesis or dissertation .
In addition to helping you figure out what your research can look like, a proposal can also serve to demonstrate why your project is worth pursuing to a funder, educational institution, or supervisor.
Research proposal length
The length of a research proposal can vary quite a bit. A bachelor’s or master’s thesis proposal can be just a few pages, while proposals for PhD dissertations or research funding are usually much longer and more detailed. Your supervisor can help you determine the best length for your work.
One trick to get started is to think of your proposal’s structure as a shorter version of your thesis or dissertation , only without the results , conclusion and discussion sections.
Download our research proposal template
Prevent plagiarism. Run a free check.
Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We’ve included a few for you below.
- Example research proposal #1: “A Conceptual Framework for Scheduling Constraint Management”
- Example research proposal #2: “Medical Students as Mediators of Change in Tobacco Use”
Like your dissertation or thesis, the proposal will usually have a title page that includes:
- The proposed title of your project
- Your supervisor’s name
- Your institution and department
The first part of your proposal is the initial pitch for your project. Make sure it succinctly explains what you want to do and why.
Your introduction should:
- Introduce your topic
- Give necessary background and context
- Outline your problem statement and research questions
To guide your introduction , include information about:
- Who could have an interest in the topic (e.g., scientists, policymakers)
- How much is already known about the topic
- What is missing from this current knowledge
- What new insights your research will contribute
- Why you believe this research is worth doing
As you get started, it’s important to demonstrate that you’re familiar with the most important research on your topic. A strong literature review shows your reader that your project has a solid foundation in existing knowledge or theory. It also shows that you’re not simply repeating what other people have already done or said, but rather using existing research as a jumping-off point for your own.
In this section, share exactly how your project will contribute to ongoing conversations in the field by:
- Comparing and contrasting the main theories, methods, and debates
- Examining the strengths and weaknesses of different approaches
- Explaining how will you build on, challenge, or synthesize prior scholarship
Following the literature review, restate your main objectives . This brings the focus back to your own project. Next, your research design or methodology section will describe your overall approach, and the practical steps you will take to answer your research questions.
To finish your proposal on a strong note, explore the potential implications of your research for your field. Emphasize again what you aim to contribute and why it matters.
For example, your results might have implications for:
- Improving best practices
- Informing policymaking decisions
- Strengthening a theory or model
- Challenging popular or scientific beliefs
- Creating a basis for future research
Last but not least, your research proposal must include correct citations for every source you have used, compiled in a reference list . To create citations quickly and easily, you can use our free APA citation generator .
Some institutions or funders require a detailed timeline of the project, asking you to forecast what you will do at each stage and how long it may take. While not always required, be sure to check the requirements of your project.
Here’s an example schedule to help you get started. You can also download a template at the button below.
Download our research schedule template
If you are applying for research funding, chances are you will have to include a detailed budget. This shows your estimates of how much each part of your project will cost.
Make sure to check what type of costs the funding body will agree to cover. For each item, include:
- Cost : exactly how much money do you need?
- Justification : why is this cost necessary to complete the research?
- Source : how did you calculate the amount?
To determine your budget, think about:
- Travel costs : do you need to go somewhere to collect your data? How will you get there, and how much time will you need? What will you do there (e.g., interviews, archival research)?
- Materials : do you need access to any tools or technologies?
- Help : do you need to hire any research assistants for the project? What will they do, and how much will you pay them?
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
Methodology
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
Once you’ve decided on your research objectives , you need to explain them in your paper, at the end of your problem statement .
Keep your research objectives clear and concise, and use appropriate verbs to accurately convey the work that you will carry out for each one.
I will compare …
A research aim is a broad statement indicating the general purpose of your research project. It should appear in your introduction at the end of your problem statement , before your research objectives.
Research objectives are more specific than your research aim. They indicate the specific ways you’ll address the overarching aim.
A PhD, which is short for philosophiae doctor (doctor of philosophy in Latin), is the highest university degree that can be obtained. In a PhD, students spend 3–5 years writing a dissertation , which aims to make a significant, original contribution to current knowledge.
A PhD is intended to prepare students for a career as a researcher, whether that be in academia, the public sector, or the private sector.
A master’s is a 1- or 2-year graduate degree that can prepare you for a variety of careers.
All master’s involve graduate-level coursework. Some are research-intensive and intend to prepare students for further study in a PhD; these usually require their students to write a master’s thesis . Others focus on professional training for a specific career.
Critical thinking refers to the ability to evaluate information and to be aware of biases or assumptions, including your own.
Like information literacy , it involves evaluating arguments, identifying and solving problems in an objective and systematic way, and clearly communicating your ideas.
The best way to remember the difference between a research plan and a research proposal is that they have fundamentally different audiences. A research plan helps you, the researcher, organize your thoughts. On the other hand, a dissertation proposal or research proposal aims to convince others (e.g., a supervisor, a funding body, or a dissertation committee) that your research topic is relevant and worthy of being conducted.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. & George, T. (2023, November 21). How to Write a Research Proposal | Examples & Templates. Scribbr. Retrieved April 9, 2024, from https://www.scribbr.com/research-process/research-proposal/
Is this article helpful?
Shona McCombes
Other students also liked, how to write a problem statement | guide & examples, writing strong research questions | criteria & examples, how to write a literature review | guide, examples, & templates, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”

Basic Methods Handbook for Clinical Orthopaedic Research pp 249–254 Cite as
How to Write a Winning Clinical Research Proposal?
- Christian Lattermann 8 &
- Janey D. Whalen 9
- First Online: 02 February 2019
2313 Accesses
This chapter addresses general approaches towards writing a clinical research proposal. The landscape in research has changed in the last decade and has become more translational in nature. Therefore, research proposals are read and evaluated by scientists from various fields that may not be intricately familiar with specific techniques and technique-related jargon. This chapter is designed to address the need for more translational and reviewer-friendly proposal writing and is built on errors and mistakes commonly seen in research proposals.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Shapiro FR. The Yale book of quotations, section: Blaise Pascal. New Haven: Yale University Press; 2006. p. 583.
Google Scholar
Download references
Author information
Authors and affiliations.
Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Christian Lattermann
Icartilage.com, Boston, MA, USA
Janey D. Whalen
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christian Lattermann .
Editor information
Editors and affiliations.
UPMC Rooney Sports Complex, University of Pittsburgh, Pittsburgh, PA, USA
Volker Musahl
Department of Orthopaedics, Sahlgrenska Academy, Gothenburg University, Sahlgrenska University Hospital, Gothenburg, Sweden
Jón Karlsson
Department of Orthopaedic Surgery and Traumatology, Kantonsspital Baselland (Bruderholz, Laufen und Liestal), Bruderholz, Switzerland
Michael T. Hirschmann
McMaster University, Hamilton, ON, Canada
Olufemi R. Ayeni
Hospital for Special Surgery, New York, NY, USA
Robert G. Marx
Department of Orthopaedic Surgery, NorthShore University HealthSystem, Evanston, IL, USA
Jason L. Koh
Institute for Medical Science in Sports, Osaka Health Science University, Osaka, Japan
Norimasa Nakamura
Rights and permissions
Reprints and permissions
Copyright information
© 2019 ISAKOS
About this chapter
Cite this chapter.
Lattermann, C., Whalen, J.D. (2019). How to Write a Winning Clinical Research Proposal?. In: Musahl, V., et al. Basic Methods Handbook for Clinical Orthopaedic Research. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-58254-1_27
Download citation
DOI : https://doi.org/10.1007/978-3-662-58254-1_27
Published : 02 February 2019
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-662-58253-4
Online ISBN : 978-3-662-58254-1
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Center for Clinical and Translational Science (CCaTS)
Postdoctoral master's degree program, research proposal.
The mentored research experience is a key component of the Postdoctoral Master's Degree Program in clinical and translational science.
Students develop their research proposals in CTSC 5010: Clinical Research Proposal Development, with a deadline based on the academic cycle listed below. Members of the CCaTS Scientific Review Group review research proposals, and the Master's and Certificate Programs Executive Committee approves them.
Include these items in your proposal packet:
- Research Proposal Packet Cover Page (PDF) .
- The proposal. Research proposals are approved by the CCaTS Master's and Certificate Programs Executive Committee and Mayo Clinic Graduate School of Biomedical Sciences (MCGSBS). Follow the Research Proposal Guidelines (PDF) , and construct your specific aims in a way that clearly defines the two or three publications resulting from the proposal.
- Research Proposal Project Timeline form .
- Translational Impact Statement. In one paragraph or a maximum of one page, describe the translational gap that your project will fill. You may include key literature that helped you identify that gap.
- Names. Your name should be first.
- Member expertise. Stick to the expertise that has value for this specific proposal.
- Help collect data?
- Train the student leader on necessary skills?
- Provide mentorship for the student leader for their research?
- Communication. How will the team meet, for example, in person or online? How often? How will the team resolve differences of opinion? What is the structure of meetings? Be clear about how you are situated in this structure.
- Final statement. Include a statement such as, "This team brings together fields of expertise that uniquely allow us to achieve [define a positive outcome]."
- Audience. Remember that you want to be writing for a broad readership. Limit your use of jargon and strive for clarity whenever possible
- Current curricula vitae for you and your mentor.
Thesis Advisory Committee (TAC) form. Complete the Proposed Master Thesis Advisory Committee form (PDF) . All TAC members are expected to provide scientific peer review and approve your proposal prior to submission. After your TAC has been approved by the CCaTS Master’s and Certificate Programs Executive Committee, the program staff will submit the MCGSBS TAC e-form on your behalf.
Read more about the Thesis Advisory Committee .
Verification statement from your department or division. This statement must document that peer review of the proposal has taken place and that the proposed work is scientifically sound and clinically meaningful. The minute excerpt from your department or division research committee or minute excerpt used for submission to the Mayo Clinic Institutional Review Board (IRB) may be used.
Note: If your project is deemed nontherapeutic and minimal risk — for example, a retrospective chart review — the IRB does not require divisional review and the CCaTS Minimal Risk Study form (PDF) is used in lieu of department or division verification.
If the proposal is part of a larger project, submit the abstract and documentation of scientific peer review of the larger project (that is, the minute excerpt from the appropriate research committee or funding agency).
- IRB approval email notification. This email notification documents that IRB review of your proposal was completed and approved. If the IRB has not yet approved your proposal, submit the email notification when approved. If your proposal is part of a larger project, please submit the IRB approval for the larger project.
- Research Proposal Potential Reviewers (PDF) .
Research proposal review criteria
The CCaTS Master's and Certificate Programs Executive Committee considers whether the thesis that will result from the proposal has the potential to meet the prescribed thesis standards and is feasible within the time frame identified. Proposals are reviewed by the CCaTS Scientific Review Group using these criteria:
- Clinical or translational research. Does the project meet the definition of clinical or translational research? If an animal model is being studied, has the scholar justified its significance to human disease?
- Scientific peer review. Is the hypothesis significant? Is the study design valid? Is the data collection and analysis plan consistent with the study goal? Is there statistical justification?
- IRB review. Has IRB approval for the project been obtained, or is it pending? If the scholar's project is part of a larger project, has documentation been provided showing IRB approval for the larger project?
- Authorship. Will the scholar be the first author on publications resulting from this project? If the scholar's project is part of a larger, multiauthor study, has the scholar explained the contribution to authorship? Will the project result in at least one publication?
- Feasibility. Is there sufficient funding for the project? Is there sufficient time to collect data and recruit participants? Does the project align with the scholar's research interests?
- Recruitment plan. Has a satisfactory subject recruitment plan, including a timeline, been provided?
- Current status. Has the status of the project been provided? For example, "Fifty percent of the data are collected, with data collection expected to be complete in six months."
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Clinical Trials
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- Search Menu
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Literature
- Classical Reception
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Papyrology
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Archaeology
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Emotions
- History of Agriculture
- History of Education
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Evolution
- Language Reference
- Language Acquisition
- Language Variation
- Language Families
- Lexicography
- Linguistic Anthropology
- Linguistic Theories
- Linguistic Typology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Modernism)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Media
- Music and Religion
- Music and Culture
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Science
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Politics
- Law and Society
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Clinical Neuroscience
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Toxicology
- Medical Oncology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Ethics
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Security
- Computer Games
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Psychology
- Cognitive Neuroscience
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Ethics
- Business Strategy
- Business History
- Business and Technology
- Business and Government
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic History
- Economic Systems
- Economic Methodology
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Behaviour
- Political Economy
- Political Institutions
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Political Theory
- Politics and Law
- Public Policy
- Public Administration
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Developmental and Physical Disabilities Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous chapter
- Next chapter >
87Chapter 4 Writing your research proposal
- Published: November 2011
- Cite Icon Cite
- Permissions Icon Permissions
In many ways it’s a reflection of yourself as a researcher and an insight into your proposed work. A poorly written proposal has the ability to wreck a project and embarrass the researcher before it has even begun. Similarly, a well-constructed proposal bodes well for the success of the project and displays the researcher in a good light amongst their peers and supervisors. The research proposal identifies: • What the topic is, both in terms of background and the individual area of interest. • What you plan to accomplish and why it needs doing. • What in particular you are trying to find out, i.e. the research question. • How you will get the answer to your question, i.e. your methodology. • What others will learn from it and why it is worth learning. • How long it will take. • How much money it will cost. Through your research proposal you are attempting to convince potential supporters that your project is worth doing, you are scientifically competent to run it, and are in possession of the necessary management skills to ensure its completion. The proposal concisely describes the key elements of the study process, although in sufficient depth to permit evaluation. It is a stand-alone document that must contain evidence of an answerable question, demonstrate your grasp of the literature, and also clearly show that your methodology is sound. A research time-table is required to demonstrate a realistic appreciation of how the study will progress through time. The research proposal serves many purposes to many different parties. Amongst these purposes, some of the key ones are: • Acting as a route map and timetable for all involved in your project. • Giving a clear overview of your planned work to ensure favourable decision at ethical review. • Gaining funding to carry out your proposed study. • Securing a place to undertake a higher scientific degree. • Being an opportunity to ‘blow your own trumpet’ on paper. Although there are several bodies who will be obliged to see your proposal, there is a reasonable chance it will end up being wider read than this, so a coherent piece of work will reflect well on you.
Signed in as
Institutional accounts.
- GoogleCrawler [DO NOT DELETE]
- Google Scholar Indexing
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
Institutional access
- Sign in with a library card Sign in with username/password Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Sign in through your institution
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Sign in with a library card
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Advertisement

- Previous Issue
- Previous Article
- Next Article
Preparation of the Investigator for a Proposal
The research proposal, insights into the reviewer's perspective, conclusions, writing successful research proposals for medical science .
(Schwinn) Professor of Anesthesiology and Surgery; Associate Professor of Pharmacology/Cancer Biology, Duke University Medical Center; Senior Fellow, Duke Pepper Aging Center.
(DeLong) Associate Professor, Division of Biometry and Medical Informatics, Duke University Medical Center.
(Shafer) Staff Anesthesiologist, Palo Alto VA Health Care System; Associate Professor of Anesthesia, Stanford University.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
- Search Site
Debra A. Schwinn , Elizabeth R. DeLong , Steven L. Shafer; Writing Successful Research Proposals for Medical Science . Anesthesiology 1998; 88:1660–1666 doi: https://doi.org/10.1097/00000542-199806000-00031
Download citation file:
- Ris (Zotero)
- Reference Manager
HIGH-QUALITY research proposals are required to obtain funds for the basic and clinical sciences. In this era of diminishing revenues, the ability to compete successfully for peer-reviewed research money is essential to create and maintain scientific programs. Ideally, the essentials of “grantsmanship” are learned through observation and participation in grant preparation, but the training environment experienced by most physicians typically focuses on clinical skills. Most physicians are never exposed to a research environment and therefore do not learn how to write grants. The result is that many clinical studies, even when designed by skilled clinicians and those that address important clinical questions, often do not compete successfully with proposals written by basic scientists. This creates a perception that clinical studies are not favorably viewed by research review committees. The opposite is probably closer to the truth; research review committees are very keen to fund excellent clinical research. Although greater numbers of researchers with Ph.D. degrees have applied for National Institutes of Health (NIH) grants compared with researchers with M.D. degrees over the last 10 yr, funding rates (percent applications funded) have remained approximately the same for these investigators ( Figure 1 ; 1995 success rates: all degrees, 6,759 [26.8%]; M.D. - Ph.D., 370 [23.1%]; M.D., 1,518 [28.1%]; Ph.D., 4,746 [26.8%]; other degree, 125 [23.1%]).[section]
![clinical research proposal pdf Figure 1. Overall success rates for NIH funding of scientific applications, 1986 - 1995. No difference in funding rate is observed between applicants holding M.D. versus Ph.D. degrees. As the success rate for first-time applications was 11.3% in 1993, it is apparent that resubmission of a revised application significantly increases the overall chance of having research proposal ultimately funded.[section]](https://asa2.silverchair-cdn.com/asa2/content_public/journal/anesthesiology/88/6/10.1097_00000542-199806000-00031/4/m_31ff1.png?Expires=1714478590&Signature=DF7-UG0u~UJKg~wyOGmJ4waYoPjUxVIrlVBxQ0~ghz-Jf-oIsRlCqxm4Qt3wH4M-l6wWrzOwEaqCekVUFcbGhitKEhM8DIDKCOI~6XmRDAUa~phZZz2tt9Pl7RaAw2tnyOQTOVU-cDkRhxGNGTz1BeeAZ9Tg4v73jLJ6ltntapoy6Sp0kQBf0iHxYPBO3RDE~ds4jxXNEMLjNbzXSIWapjuJyb1rSYj0j9tRPeFq6UHlMddud6m4R67UnrD5KsRECAkYP14B9KZFv06k7IiCdsDfrxfswUJ2jY3gDlsQDLYqKaaXRWiCHHeE4fNLtu5jR-DXfly1vlt4igxeVh8sjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Figure 1. Overall success rates for NIH funding of scientific applications, 1986 - 1995. No difference in funding rate is observed between applicants holding M.D. versus Ph.D. degrees. As the success rate for first-time applications was 11.3% in 1993, it is apparent that resubmission of a revised application significantly increases the overall chance of having research proposal ultimately funded.[section]
Capable medical researchers ultimately write research proposals for funding by the NIH. Standards of excellence for NIH grants are high (only the top [almost equal to] 20% of grants are funded). Research questions posed must be hypothesis driven; the investigator must be qualified to perform the study; and preliminary evidence should be presented demonstrating that the research is feasible and will answer the questions posed. The goal of this article is to review important elements of successful research proposals, with emphasis on funding sources available to the anesthesiology community. Two important anesthesia-specific organizations exist to support anesthesia research - The Foundation for Anesthesia Education and Research (FAER, an organization under the auspices of the American Society of Anesthesiologists) and the International Anesthesiology Research Society (IARS).
Successful applications for research support from FAER and IARS have many of the characteristics of grants funded by the NIH and other peer-reviewed funding sources. These characteristics include (1) a highly qualified investigator(s);(2) for junior investigators, a mentor with a successful track record in scientific investigation, peer-reviewed funding, and mentorship of fellows and faculty;(3) a supportive academic environment; and (4) a scientifically sound proposal. Each of these characteristics is discussed in the subsequent sections.
Training of the Investigator
One of the most important components of a successful research proposal is a well-trained investigator. Training in clinical anesthesia is not training in research methodology or scientific thinking; it does not prepare an individual for a career in investigation. Although obvious for basic science research, clinical research also requires commitment of a minimum of 1 yr of dedicated training with a good mentor, and more typically 2 - 3 yr in the field of the proposed research. The applicant also needs to demonstrate commitment to a career in investigation. Several years of scientific training is the first demonstration of such commitment. Research proposals must document institutional support for nonclinical time, and the investigator must provide evidence that this time has been used wisely and will continue to be dedicated to the proposed research.
The research proposal must document a track record of productivity by the investigator. This expectation increases as the training and career of the investigator progresses. Fellowship awards do not have an expectation of prior research training, so publications from prior research are not expected. At the fellowship level, outstanding letters of recommendation, undergraduate and medical school performance, and related accomplishments are most important. Because previous training is not required of the fellowship applicant, prior success of the mentor (publications and track record with previous trainees) weighs heavily in the fellowship review. For junior faculty, peer-reviewed publications are expected from the fellowship period. Young Investigator Annoucements (from FAER) and several new IARS awards require several years as a successful junior faculty member, so expectations of demonstrated research success are further increased. The investigator must demonstrate (1) rigorous training, (2) commitment to research, (3) an appropriate career path, and (4) a track record of productive work. None of these are trivial issues, and none can be easily accomplished without making a commitment to research early in the academic career.
The quality of the mentor is another important aspect of awards granted to fellows and junior faculty. Identification of a mentor is explicitly required for FAER and certain junior level NIH grant applications. First and foremost, the mentor must be a successful investigator. Criteria for this include a track record of publication in the area of the proposed research, continued peer-reviewed funding, and a history of successfully training young investigators. Although mentorship is not considered heavily in more senior grant applications, input from a more experienced investigator often remains beneficial throughout one's career (as we can personally attest to). In addition to the mentor, high-quality coinvestigators, collaborators, and consultants also play important roles in strengthening a research proposal.
Environment
Good research is best accomplished in a supportive, cooperative environment. Because of the changing climate of clinical medicine, researchers (both clinical and basic science) face increasing pressure to minimize research time. It is not possible to become a successful investigator in one's spare time. Documentation of adequate nonclinical time for research (not for committee meetings or other unrelated tasks) is essential. Receiving funding at a junior level often enables the department to match funds or to guarantee nonclinical time to the budding investigator. In general, the more non-clinical time available to an investigator, the more competitive the application.
Other important elements of the environment include people, space, and institutional resources. People include mentors, consultants who can help with specific methodologies, statistical support, helpful colleagues, experienced technicians, a clinical research team, and a dedicated chairperson. There must be adequate space for performing the proposed studies, office space for research personnel, and storage space for equipment and supplies. Institutional resources include related departmental and interdepartmental seminar series, a critical mass of investigators in a related area, instrument development and repair shops, and necessary laboratory space and common facilities.
Criteria for a sound research proposal are the same whether the proposal is submitted to NIH, FAER, IARS, or other funding sources. In crafting a proposal, it is essential to consider the perspective of the reviewer; therefore, items of interest to the reviewer are listed after general definition of the grant proposal.
Review committees receive dozens of grants. NIH study sections may review as many as 150 proposals during one session. Typically, only two or three reviewers are assigned to read each grant in detail, but everyone is expected to read each abstract. Hence, the abstract is often one of the most important parts of the research proposal. The abstract should address the significance of the question and the overall topic, state the hypothesis, and point out key preliminary data. Additionally, the abstract should provide a synopsis of methodologies planned. In the end, the reviewer must be convinced that the applicant is uniquely (or ideally) suited to undertake this important study by the end of this concise paragraph.
Body of the Grant
Specific Aims. The specific aims section is critically important in a scientific proposal. It is here that the investigator crystallizes the overall goal of the research and states specific hypotheses.
Beginning with the specific aims, the proposal must be well written and logically organized. A poorly organized grant application is difficult to review, even if the science is otherwise excellent. Typically, the specific aims begin with a short introduction (one paragraph), followed by a formally stated hypothesis. The hypothesis must be answerable by the research methods proposed. Generally, two or three specific aims are outlined with subheadings where appropriate. Organization of the specific aims is often temporal, starting with a proposed mechanism or the first set of studies in a clinical project. In general, the specific aims section should be no longer than one page.
Background and Significance. The background section provides an opportunity to bring reviewers up to date on current research in the area of the proposal. This section should summarize succinctly studies from the literature and related work published by the investigator. The most crucial aspect of the background is to build a case for significance of the proposed research regarding the ultimate clinical application or mechanistic understanding. Ideally, the background section should demonstrate that the current proposal is a logical extension of previous studies in the field and will provide new information and novel insights. In general, the background section should be about one fourth of the length of the grant proposal.
Preliminary Data. Preliminary data provide the opportunity for the investigator to demonstrate his or her ability to perform the proposed research. The goal in presenting preliminary data is to convince the reviewer that the investigator is capable of performing the proposed studies and that the mechanisms proposed are plausible. Good preliminary data support novel (or even unlikely) hypotheses. Each experimental method proposed should be accompanied by preliminary data demonstrating facility and expertise with related preparations. For example, if the investigator proposes using a specific electrophysiologic technique to study an ion channel, evidence demonstrating that this technique has been used by the investigator with other ion channels and a Figure showingresults from pilot experiments on the channel of interest would suffice. In clinical studies, demonstration of a working investigative team and the ability to enroll a given number of patients per week is helpful. Figures or tables help to convey the message in a succinct manner. They also conserve space in the proposal and create a more impressive effect. Although it is best if the applicant has generated his or her own preliminary data, for training awards, preliminary data from the mentor's laboratory is entirely appropriate. An effective way to organize preliminary data is to present it in the same order as the specific aims (e.g., C.1 preliminary data corresponds to A.1 specific aims, C.2 preliminary data corresponds to A.2 specific aims, etc.). Presentation of preliminary data usually takes about one fourth to one third of the length of the grant application.
Methods. The methods are the guts of the research proposal. Unfortunately, many investigators run out of steam by the time they reach the methods, leaving reviewers unconvinced by the proposed methodology. Ideally, the model being investigated should be broken down into simple, logical components, each accompanied by a description of specific experiments/interventions to be performed. The investigator should assume that at least one reviewer is an expert in each method presented. Therefore, enough detail should be provided to convince an expert that the experiment or technique is being performed properly. Methods presented as a list of recipes, requiring the reviewer to guess which method applies to each study, are recipes for disaster. Individual experimental techniques should be state of the art. In addition, approaching a problem from several angles is often helpful. “Lingo” of the field should be avoided; it is very annoying to reviewers to have to look up unexplained abbreviations or to have models alluded to rather than described. For training grants, methods should involve techniques currently being performed in the laboratory of the mentor. An effective way to organize the methods section is to follow the same order as the preliminary data and specific aims sections (e.g., D.1 methods corresponds to C.1 preliminary data and A.1 specific aims, etc.).
The methods sections should include a description of the design, conduct, and analysis of each study being proposed. Common errors in design include lack of specification of primary outcome, lack of randomization or blinding in clinical trials, inadequate justification of sample size, failure to adjust the total study number for expected dropouts/failed experiments or patient refusal, and use of single drug doses or concentrations rather than development of dose - response or concentration - response relations. Common errors in conducting research include lack of confirmation of drug concentrations, inadequate reproducibility of final results, lack of standardization of procedures, inadequate follow-up, incomplete data recording, and overall lack of organization.
Inadequate or inappropriate statistical methods can be a major weakness of a grant proposal. Many investigators feel confident with all aspects of their methods except the statistical section. Because statistical issues underlie the design and analysis strategy for every study, the input of a biostatistician is essential in planning the research and writing the grant application. Statistical considerations include specification of the primary end points that drive power calculations. Common statistical errors in research proposals include lack of sample size/power calculations, treating continuous variables as dichotomous, repeated t tests when a more comprehensive modeling approach should be taken, application of statistical tests that assume normality without verifying assumptions, failure to consider covariate effects, and failure to distinguish between interindividual and intraindividual variability. The investigator should be familiar with the concept of statistical power and be prepared to estimate some of the quantities needed to formulate an alternative hypothesis appropriately. The statistical analysis should be clearly outlined with specific methodology directed toward the hypotheses of the study. A statistical reviewer is unlikely to be convinced by a statement that “appropriate statistical methodology will be used” or by a barrage of nonspecific statistical jargon. At least one full paragraph (and sometimes an entire page) of the research proposal should be devoted to statistical analysis. Often several smaller statistics sections are appropriately included after each method is presented.
Even the best methods have potential problems and weaknesses. It is critical that the methods section discuss potential problems that may be encountered during the study and state how the investigator proposes to deal with these problems creatively. Reviewers tend to be impressed when the investigator presents potential problems that never occurred to them, because it suggests that the investigator is an expert in this area of research. A time line and organizational plan (who will be responsible for what) should also be included in the methods section so the reviewers can determine whether the investigator is being realistic in his or her approach. The methods section is typically one third to one half of the length of the entire grant proposal.
Introduction to Revised Application. Because so few grant applications are funded on their first submission (11.5% in 1993), the new investigator should not be unduly alarmed if his or her application is not funded. When a grant application has been unsuccessful, an investigator should revise the application and reapply, even if the original score was “noncompetitive”(meaning the grant was in the lower 50% of applications). Often the reviewers suggest key changes that will improve the application significantly. When submitting a revised application, an introduction (placed before the specific aims section) is used to discuss how criticisms of the original grant have been addressed in the revised proposal. Because the reviewer's comments are intended to be helpful, it is important to address each concern carefully in the revised proposal (changed text should be highlighted in the revised application by italic, bold, or identifying lines in the margin), with changes outlined in the introduction section. Angry responses to reviewers do not facilitate funding of the revised application. Remember that reviewers usually have a copy of the prior review, and they expect corrections or, when appropriate, an explanation of why you have chosen not to incorporate some suggestions from a prior review. Time taken to revise an application is well spent; as Figure 1 demonstrates, investigators who persist in revising and resubmitting their applications have an increased chance ([almost equal to] 20% with no previous NIH support, [almost equal to] 35% if previously funded) of ultimately being funded.[section]
In writing a research grant, it is helpful to consider the reviewer's perspective. Key features considered by reviewers include significance, approach, and feasibility. It is wise for the investigator to reread his or her application before submission with these features in mind. The NIH recently has published two documents on-line that discuss review criteria; examination of these documents before submission of a research proposal may prove helpful. These include the Report of the Committee on Rating Grant Applications[double vertical bar] and Review Criteria for Rating Unsolicited Research Grants.#
Significance
First and foremost, is the investigator asking an important question? There are two general ways research studies can be significant. The first is to demonstrate clinical significance. The litmus test for clinical significance is whether the proposed research will improve patient care. The second is elucidation of fundamental mechanisms underlying disease or biologic processes. The ideal research question succeeds in being significant in both areas.
The reviewer assesses whether the research plan can support or refute the stated hypothesis. In addition, the reviewer assesses whether the methodologies used provide adequate or, better yet, elegant approaches to the problem. Recently, the NIH has mandated an increasing emphasis on innovation in research. [1] **
Review committees generally are composed of individuals with expertise in many scientific areas. Additionally, study sections often retain outside reviewers with expertise in the proposed research area. The investigator should assume that his or her methods will be critiqued by at least one expert. Therefore, the investigator should not propose a method that would strike the world's expert in the field as being simplistic, inappropriate, or nonsensical, because the world's expert just might be one of the reviewers. Conversely, some reviewers do not have expertise in the proposed area of research. To ensure that the nonexpert is convinced of the validity and importance of proposed methodologies, the overall proposal should be written with a logical flow of ideas that build from basic to sophisticated concepts. Beginning each portion of the methods section with a short introduction for the nonexpert, followed by a more detailed description of the proposed methods, is an effective strategy to address the needs of both expert and nonexpert reviewers.
Feasibility
The investigator must convince reviewers that the chosen approach is feasible. Preliminary data provide the best demonstration of feasibility. Feasibility is often demonstrated by a track record of publications or peer-reviewed grant support for the applicant or mentor using the proposed experimental approach. Feasibility also can be demonstrated by appropriate statistical analysis of the proposal. For example, a power analysis and corresponding data on the number of patients with the required characteristics at the investigator's institution helps convince reviewers that a clinical study is feasible.
Anesthesiology Funding Sources
Funding for research performed by anesthesiologists is available from many sources. Because the discipline of anesthesiology overlaps many other fields, anesthesiologists have the opportunity to apply for research funds from agencies as diverse as the American Academy of Pediatrics, American Cancer Society, American Heart Association (national and local), American Thoracic Society, American Society for Regional Anesthesiology, critical care societies, Department of Veterans Affairs, National Science Foundation, Shriners, Society for Cardiovascular Anesthesiology, Society for Obstetrics and Perinatology, National Aeronautics and Space Aviation, NIH, and many other private foundations. Grants from FAER and IARS are available specifically to the anesthesiology community.
It is important that anesthesiologists continue to apply for NIH grants. For fiscal year 1996, the NIH awarded 149 research grants (including career development grants, R29, R01, and program project grants) to departments of anesthesiology, totaling $21 million in direct costs ([almost equal to]$31 million in total costs). Because of the diversity of research projects in anesthesiology, these grants were awarded by 14 different institutes, centers, and divisions within the NIH. In analyzing data for three recent review sessions (June 1996, October 1996, and February 1997) from the surgery, anesthesiology, and trauma study section, 26% of anesthesiology applications scored in the top 20th percentile, and 31% scored in the top 25th percentile; clearly no bias exists against anesthesiology in this predominantly surgical study section, at least in this limited sample (Alison Cole, anesthesiology representative for the National Institute of General Medicine Science at the NIH, personal communication, December, 1997). Table 1
Table 1. Number of Recipients of NIH Research Project Annoucements

A brief list of funding opportunities available to anesthesiologists early in their career is shown in Table 2 . Several sites are available on the World Wide Web ( Table 3 ) to facilitate access to grant/training resources for anesthesiologists. We have created an additional website ( http://pkpd.icon.palo-alto.med.va.gov/grants/grants.htm ), which provides access to more comprehensive lists of funding agencies and direct links to funding sources. This website also contains example grants designed to illustrate the grant writing principles discussed in this article.
Table 2. Potential Funding Sources

Table 3. Grant/Training Resources on the WWW

Successful grant applications require a well-trained investigator who carefully outlines a hypothesis-driven research proposal. Unique to FAER and IARS research committees is that the reviewers are mostly investigators and practicing anesthesiologists. These reviewers fully appreciate the importance of clinical research and enthusiastically support high-quality clinical studies. Although descriptive clinical studies are interesting to practicing clinicians, from a scientific perspective, clinical research must be driven by testable hypotheses. Without a testable hypothesis, clinical research cannot pass the test of adequate significance required for funding.
It is our hope that by demystifying the grant writing and review process that more anesthesiologists will be encouraged to submit proposals for research funding. As part of this effort, we strongly encourage residents and fellows interested in research careers to obtain adequate research training and to apply for appropriate fellowship/junior faculty awards early in their careers.
[section] NIH Extramural Data and Trends, Fiscal Years 1986 - 1995. Bethesda, Office of Reports and Analysis (component of the Office of Extramural Research), National Institutes of Health. (Published on-line and periodically updated. http://www.nih.gov/grants/award/award.htm ).
[double vertical bar] Report of the Committee on Rating Grant Applications. Revised 5/17/96. Bethesda, National Institutes of Health. (Published on-line. http://www.nih.gov/grants/peer/rga.pdf ).
# Review Criteria for Rating Unsolicited Research Grants. NIH Guide, Vol. 26, No. 22, 6/27/97. Bethesda, National Institutes of Health. (Published on-line. http://www.nih.gov/grants/guide/1997/97.06.27/notice-review-criter9.html ).
** Brown KS: A winning strategy for grant application: Focus on impact. The Scientist 1997; April 8:13–4
Citing articles via
Most viewed, email alerts, related articles, social media, affiliations.
- ASA Practice Parameters
- Online First
- Author Resource Center
- About the Journal
- Editorial Board
- Rights & Permissions
- Online ISSN 1528-1175
- Print ISSN 0003-3022
- Anesthesiology
- ASA Monitor

- Terms & Conditions Privacy Policy
- Manage Cookie Preferences
- © Copyright 2024 American Society of Anesthesiologists
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Research Proposal Example/Sample
Detailed Walkthrough + Free Proposal Template
If you’re getting started crafting your research proposal and are looking for a few examples of research proposals , you’ve come to the right place.
In this video, we walk you through two successful (approved) research proposals , one for a Master’s-level project, and one for a PhD-level dissertation. We also start off by unpacking our free research proposal template and discussing the four core sections of a research proposal, so that you have a clear understanding of the basics before diving into the actual proposals.
- Research proposal example/sample – Master’s-level (PDF/Word)
- Research proposal example/sample – PhD-level (PDF/Word)
- Proposal template (Fully editable)
If you’re working on a research proposal for a dissertation or thesis, you may also find the following useful:
- Research Proposal Bootcamp : Learn how to write a research proposal as efficiently and effectively as possible
- 1:1 Proposal Coaching : Get hands-on help with your research proposal

FAQ: Research Proposal Example
Research proposal example: frequently asked questions, are the sample proposals real.
Yes. The proposals are real and were approved by the respective universities.
Can I copy one of these proposals for my own research?
As we discuss in the video, every research proposal will be slightly different, depending on the university’s unique requirements, as well as the nature of the research itself. Therefore, you’ll need to tailor your research proposal to suit your specific context.
You can learn more about the basics of writing a research proposal here .
How do I get the research proposal template?
You can access our free proposal template here .
Is the proposal template really free?
Yes. There is no cost for the proposal template and you are free to use it as a foundation for your research proposal.
Where can I learn more about proposal writing?
For self-directed learners, our Research Proposal Bootcamp is a great starting point.
For students that want hands-on guidance, our private coaching service is recommended.

Psst… there’s more!
This post is an extract from our bestselling Udemy Course, Research Proposal Bootcamp . If you want to work smart, you don't want to miss this .
You Might Also Like:
I am at the stage of writing my thesis proposal for a PhD in Management at Altantic International University. I checked on the coaching services, but it indicates that it’s not available in my area. I am in South Sudan. My proposed topic is: “Leadership Behavior in Local Government Governance Ecosystem and Service Delivery Effectiveness in Post Conflict Districts of Northern Uganda”. I will appreciate your guidance and support
GRADCOCH is very grateful motivated and helpful for all students etc. it is very accorporated and provide easy access way strongly agree from GRADCOCH.
Proposal research departemet management
I am at the stage of writing my thesis proposal for a masters in Analysis of w heat commercialisation by small holders householdrs at Hawassa International University. I will appreciate your guidance and support
please provide a attractive proposal about foreign universities .It would be your highness.
comparative constitutional law
Kindly guide me through writing a good proposal on the thesis topic; Impact of Artificial Intelligence on Financial Inclusion in Nigeria. Thank you
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
Clinical Research Proposal

In the time when the sea was the avenue through which a nation can conquer the world, Britain believed that a strong naval fleet of almost 2,000 men could make a dent out of Spain’s stronghold in America. After surviving the storms and long days at sea, the people did not expect that they would fall prey to a silent killer amongst them. Sailors had been dying from the most common maritime disease that had claimed the lives of countless others—scurvy. It was only later learned that the disease could be cured with citrus fruits, thanks to the physician James Lind. Years after the treatment for scurvy was available, Britain became a naval superpower, and the English language started to be introduced to the rest of the world. Lind’s observation was the first of many clinical research that changed the world.
7+ Clinical Research Proposal Templates and Samples
However revolutionary, there are downsides to fresh fruits as the go-to cure for the disease. Citrus fruits generally do not last long, especially then when there was no refrigerator nor electricity. The extract contains a volatile compound in its natural form. Heat and long periods of storage destroy the therapeutic activity of the substance, and the compound itself. It was much later that vitamin C was synthetically produced as tablets and capsules. Before a drug reaches the patients, it undergoes a series of tests and clinical trials that ensure that the drug is safe and significantly effective. Clinical research deals with treatments and services for the benefit of society. Clinical researchers are working on several treatment and prevention options to improve and advance medicine and human health. Their revolutionary ideas start with a research proposal for succeeding tests and validations.
1. Research Proposal Template

- Google Docs
Size: A4 & US Letter Sizes
Clinical research covers studies that deal with the improvement of health products and services. Each study proposes news or reviews the effect of the present method or product aimed to better the health quality of people. For the research to proceed to the testing for efficacy and safety for human consumption, researchers first have to convince a board or committee concerned that the study is worth pursuing. The research proposal should sway the respective authority to approve and support the activity. It should show that you are competent and capable of conducting the desired research undertaking. To help you in preparing for your proposal, you can download the sample Research Proposal Template shown above.
2. Research Project Proposal Template

A research proposal is a declaration of intent to pursue a research direction, pending the approval and endorsement of proper channels. It is also a ticket for a financial grant by government agencies and private institutions. The document introduces the study, its objectives, and its significance to the scientific body and society. It also includes an overview of the research methods and techniques that the researchers plan to perform, the project timeline , as well as a breakdown of possible expenses that will be incurred during the conduct of the study. You don’t start from scratch when you get a copy of the Research Project Proposal Template we’ve included above.
3. Brief Research Proposal Template

The document is a summary of the study. The problem statement and the methodology used for the research must be clear. The researchers should have a general idea of how their study will be conducted. This will be evident in the methods proposed. The research methodology and the schedule of activities should be feasible and realistic. This means that the materials, equipment, and facilities are available for intended use, and the results are obtainable at an expected deadline. When needed, you can resort to a Brief Research Proposal Template with customizable content, as shown above.
4. One-Page Proposal Template

Size: A4, US
One of the essential elements in a clinical research proposal should be a well-written and timely research question that your study will focus on. Your objectives should be aligned with your thesis statement . In the same manner, your methods should closely follow the study’s objectives. The proposal is an outline of your strategy. And like any strategy, there should be thorough planning before the execution. The document will guide you while conducting research. You will be able to monitor your progress and if you have answered your problem statement and objectives. Gather all these important elements in one short research proposal. Luckily, you may use the One-Page Proposal Template we’ve embedded above. Try it out now!
5. Nursing Research Proposal Template

A research proposal should indicate the background of the study. It should also include a review of related literature about the problem that you plan on addressing. The literature review should point to previous significant works that the researchers would use as a reference citing in their study. This applies when creating a nursing research or case study too. With the help of the Nursing Research Proposal Template shown above, crafting an informative and accurate research proposal while in nursing school will be easier.

6. Free Clinical Research Proposal Sample

Size: 232 KB
A good research proposal , like this sample, provides the reader with a research problem that talks about a current issue, the realistic goal of your research, and a well-explained methodology that will allow other researchers to replicate and verify your study. The specific amount and measurements, for example, should be indicated in your procedure. You must report the statistical analysis that you will perform on your data. Your statistical analysis will influence your results. Therefore, the reader must be informed on how you will treat your data. If you do not include a literature review, present a list of credible references that you will be using throughout your study.
7. Research Proposal Example

Size: 102 KB
For your reference on what makes for a compelling research proposal, you can download the sample Research Proposal Example embedded above. It is made by a person who qualified for the research program of the Mississippi State Department of Health. The document features a title that catches the attention of the reader. Because a person reads the title first, it can create an urge for the reader to keep reading what the writer has to say. The more content is read, the higher the chance that the proposal can sway support to your side. The research problem is relevant to the medical field and society, and the writer explains why it should be addressed. The writer expressed his or her plan on how the study will be performed. You can model your research proposal after this sample if you intend to try for a research program. Take note of the proposal guidelines for other agencies, though, as the requirements may be different.
8. Blank Research Proposal Template

Size: 114 KB
Now that you are ready to prepare a convincing research proposal, you can use the Blank Research Proposal Template featured above. It guides you on what to include in the document. You can look for a current issue or problem in the news. Identify a field or a broad topic you wish to study, and read on scientific journals about it. As you read, you can find gaps in the literature. You can also refer to the recommendations in some research articles for help in your next research topic. You can create a flow chart along the way so you will have a guide on your project. Remember the tips you have learned in this article and incorporate them into your proposal.
Proposal Maker
Text prompt
- Instructive
- Professional
Generate a proposal for a new school recycling program
Compose a proposal for a school field trip to a science museum.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Clin Diagn Res
- v.10(11); 2016 Nov
Protocol Writing in Clinical Research
Azzam al-jundi.
1 Professor, Department of Orthodontics, King Saud bin Abdul Aziz University for Health Sciences, College of Dentistry, Riyadh, Kingdom of Saudi Arabia.
Salah Sakka
2 Associate Professor, Department of Oral Surgery, Al Farabi Dental College, Riyadh, Kingdom of Saudi Arabia.
Writing a research proposal is probably one of the most challenging and difficult task as research is a new area for the majority of postgraduates and new researchers. The purpose of this article is to summarize the most important steps and necessary guidelines for producing a standard research protocol. Academic and administrative success of any project is usually determined by acquiring a grant for the related field of research. Hence, the quality of a protocol is primarily required to achieve success in this scientific competition.
What is A Protocol?
Clinical research is conducted according to a plan (a protocol) or an action plan. The protocol demonstrates the guidelines for conducting the trial. It illustrates what will be made in the study by explaining each essential part of it and how it is carried out. It also describes the eligibility of the participants, the length of the study, the medications and the related tests.
A protocol is directed by a chief researcher. The health of the participants’ will be regularly checked by members of the research team to ultimately ensure the study’s safety and effectiveness.
Purpose of a Research Proposal
[ Table/Fig-1 ] outlines the key aims of the protocol.
[Table/Fig-1]:
Aims of the protocol.
Benefits of the Proposal to a Researcher
[ Table/Fig-2 ] outlines the key benefits of the protocol.
[Table/Fig-2]:
Benefits of the protocol.
The key points of the proposal should include justification for the need of the project and a detailed plan for the investigation [ 1 , 2 ]:
What is the question? (Hypothesis) What is it to be investigated?
- Why is the study important? (Significance)
- Where and when it will take place?
- What is the methodology? (Procedures and methods to be used).
- How are you going to do it? (Research design)
- Proposed time table and budget.
- Resources required (technical, scientific, and financial).
Drafting the protocol correctly will increase the likelihood that the conclusions drawn from the research are scientifically sound. Recommendations and suggestions should be sought from colleagues and experts so that researchers can develop their plans. However, once the study is launched, the protocol should not be altered during the progression of the study or trials. If the changes during progress of study are minor, then that part of the study should be excluded from the analysis. Unless unexpected complications occur during the conduct of the trial, it is advisable to reconsider and rewrite the protocol where the whole process is started again provided that the original research topic is still considered to be relevant. If complications are anticipated, it is suitable to run a pilot study, to check the feasibility of the study and find answers to the potential areas of the trial.
What is A Protocol Review?
Clinical trials must be approved and monitored by an Institutional Review Board that ensures that the risks are negligible and are worth any potential benefits. It is an independent committee that consists of physicians, dentists, statisticians, and members of the community.
The committee ensures that clinical trials are ethical and that the rights of all participants are protected. The board must initially approve and periodically review the research.
Components of a Research Protocol: The topics that should be covered in a protocol are shown in [ Table/Fig-3 ] [ 3 , 4 ].
[Table/Fig-3]:
The components of the protocol.
Writing The Protocol
Protocol writing allows the researcher to review and critically evaluate the published literature on the interested topic, plan and review the project steps and serves as a guide throughout the investigation. The proposal is an inevitable document that enables the researcher to monitor the progress of the project [ 5 ].
1) Title of the Study: Title of proposal should be accurate, short, concise, and identify [ 2 , 6 ].
What is the study about, Who are the targets, Where is the setting of the study and When it is launched, if applicable-
It should make the main objective clear, convey the main purpose of the research and mention the target population. Carry maximum information about the topic in a few words; it is a good practice to keep the title to within 12-15 words. It should convey the idea about the area of research and what methods are going to be used in a compact, relevant, accurate, attractive, easy to understand, and informative way.
2) Administrative Details: The following administrative details and a protocol content summary should follow the title page:
- Contents page list of relevant sections and sub-sections with corresponding page number.
- Signature page is signed by senior members of the research team and dated to confirm that the version concerned has been approved by them.
- Contact details for the research team members listing postal, e-mail addresses and telephone numbers.
3) Project Summary: The summary should be distinctive, concise and should sum up all the essentials of the protocol.
4) Introduction (Background): The background to the project should be concise and refer to the subject straight forwardly. In writing the review, attention should be drawn to the positives, negatives and limitations of the studies quoted [ 7 – 9 ]. Introduction is concluded by explaining how the present study will benefit the community. The literature review should logically lead to the statement of the aims of the proposed project and end with the aims and objectives of the study. The review should include the most recent publications in the field and the topic of the research is selected only after completing the literature review and finding some gaps in it.
Introduction should briefly answer the importance of the topic, the gaps/lacunae in the literature, the purpose of the study and benefits for the society, from the study.
The research question should be described precisely and concisely. It is going to be the basis of designing the project. The definition of the problem should be clear so that a reader can straight forwardly recognize the real meaning of it.
5) Study Objectives (Aims): The aims should be explicitly stated. These should be confined to the intention of the project and they should arise from the literature review. State the goal you need to achieve.
The study aims or objectives emerge from the study questions/ hypothesis. They are answers to what are the possible responses to the research question or hypothesis under analysis and measure. Aims should be logical and coherent, feasible, concise, realistic, considering local conditions, phrased to clearly meet the purpose of the study and related to what the specific research is intended to accomplish. For example, to evaluate knowledge level regarding dental caries in primary school children in KSA (this is not detailed). The following should be added: Causes, treatment, preventive measures, etc.
The objectives should be (SMART objective): Specific, Measurable, Achievable, Relevant and Time based [ 10 ].
Specific Aims: Details of each objective that will finally lead to the achievement of the goal should be stated. Specific aims one by one should be listed concisely. It is good practice not to include too many aims in the study (2-5 best); too many objectives often lead to inaccurate and poorly defined results. Furthermore, aims should be achievable, realistic and specific with no general and ambiguous statements. They should be stated in action verbs that illustrate their purpose: i.e., “to determine, to compare, to verify, to calculate, to reduce, to describe, etc.”
Secondary Objectives (Optional): These are referred to as ancillary and minor objectives that could be studied during the course of the study.
The formulation of objectives helps to focus the study and to avoid the collection of any unnecessary data and hence organize the study in clear and distinct stages.
Hypothesis: It is a statement based on sound scientific theory that recognizes the predicted correlation between two or additional assessable variables [ 11 ]. It is always developed in response to the purpose statement or to answer the research questions posed. Furthermore, hypothesis transforms research questions into a format amendable to testing or into a statement that predicts an expected outcome.
Types of hypothesis statements:
- Null hypothesis: A null hypothesis is a statement that there is no actual relationship between variables (H0 or HN). It may be read as there is no difference between the groups to be compared and no relationship between the exposure and outcome under investigation. H0 states the contradictory of what the researchers expect. The final conclusion of the investigators will either keep a null hypothesis or reject it in support of an alternative hypothesis. It does not essentially mean that H0 is accurate when not rejecting it as there might not be an adequate proof against it.
- Alternative hypothesis: An alternative hypothesis is a statement that suggests a potential outcome that the researcher may expect (H1 or HA). This hypothesis is derived from previous studies where an evident difference between the groups to be compared is present. It is recognized only when a null hypothesis is rejected. Practically, hypotheses are stated in the null form, because they have their inferential statistics. Such hypotheses of no difference will be challenged by researchers and the result of the statistical testing gives the probability that the hypothesis of no difference is true or false [ 12 ].
Aims should be logically linked and arranged according to the tested hypothesis statement.
- Research question: Is there a difference in fluoride release between the Compomer and Glass- ionomer cement?
- Null Hypothesis: There is no difference in fluoride release between the Compomer and Glass- ionomer cement.
- Alternate Hypothesis: There is a difference in fluoride release between the Compomer and Glass-ionomer cement.
The statement of the problem should provide a summary of exactly what the project is trying to achieve.
- What exactly do you want to study?
- Why is it worth studying?
- Does the proposed study have theoretical and/or practical significance?
- Does it contribute to a new understanding of a phenomenon? (i.e., Does it address new or little known material or does it treat familiar material in a new way or does it challenge an existing understanding or extend existing knowledge?)
The justification of the research should be a convincing statement for the need to do it:
- How does the research relate to the priorities of the region and the country?
- What knowledge and information will be obtained?
- What is the ultimate purpose that the knowledge obtained from the study will serve?
- How will the results be disseminated?
- How will the results be used, and who will be the beneficiaries?
6) Methods and Materials: It should describe in detail the ‘Where’, ‘Who’, ‘How’ the research will be conducted. It explains the study design and procedures and techniques used to achieve the proposed objectives. It defines the variables and demonstrates in detail how the variables will be measured. It details the proposed methodology for data gathering and processing.
Methodology composes an important part of the protocol. It assures that the hypothesis will be confirmed or rejected. It also refers to a thorough strategy to attain the objectives [ 13 ].
The methods and materials are divided into various subheadings:
a) Study design (cross-sectional, case-control, intervention study, RCT, etc.): Proper explanation should be given as to why a particular design was chosen (on the basis of proposed objectives and availability of resources).
A study design is in fact the researcher’s general plan to acquire the answer (s) to the hypothesis being tested. Here, strategies will be applied to develop balanced, correct, objective and meaningful information [ 14 ]. It explains the methods that will be used to collect and analyze data. Proper selection of the study design is important to attain reliable and valid scientific results.
Ethics, logistic concerns, economic features and scientific thorough-ness will determine the design of the study. Here, a chief concern is given to the legality of the results including potential bias mystifying issues.
Randomized controlled clinical trial is the best to document a causal relationship between an exposure and its outcome [ Table/Fig-4 ].
[Table/Fig-4]:
Suitable research design depends on the purpose of the study.
b) Study population (Study subjects): Where are you going to do the research and who is the study population (why doing research in this place and why selecting this population?).
It describes in detail about the study subjects, all aspects of the selection procedure and sample size calculation. Proper definition of eligibility, inclusion, exclusion and discontinuation criteria of the study subjects should be stated. Allocation of subjects to study arms should be explained and described in details bearing in mind the concealment and randomization process [ 15 , 16 ].
c) Sample size: Sample size calculation is recommended for economical and ethical reasons [ 16 – 18 ]. The calculation of the sample size must be explained including the power of the sample. The sampling technique should be mentioned, e.g., randomization that will be used in order to obtain a representative sample for your target population. Each step involved in the recruitment of the study subjects should be described according to the selection criteria (inclusion and exclusion criteria).
“Informed consent” should be mentioned (Permission granted in full knowledge of the possible consequences).
d) Proposed intervention: Full description of proposed intervention should be given. Here, all the activities and actions should be recorded and thoroughly explained in their order of occurrence.
- When using drugs, both scientific and brand name should be mentioned followed by the name of the manufacturing company, city, and country. Drug route, dosage, frequency of administration, and total duration of treatment with the drug should be mentioned.
- When using apparatus its name should be given followed by the name of the manufacturer, city and country.
Involved personnel should precisely define:
- Who will be responsible for the interventions?
- What activities each personnel will perform and with what frequency and intensity?
e) Data collection methods, instruments used:
Data collection tools are:
- Retrospective data (medical records) [ 19 ]
- Questionnaires [ 20 ]
- Interviews (Structured, Semi-Structured)
- Laboratory test (literature or personal knowledge should be referenced, if established test, or description should be provided in details, if not established)
- Clinical examinations
- Description of instruments, tools used for data collection, as well as the methods used to test the validity and reliability of the instrument should be provided [ 21 ].
7) Data Management and Analysis Plan: This section should be written following statistical advice from a statistician. The analysis plan and which statistical tests will be used to check the significance to the research question/hypothesis with appropriate references should be described. Names of variables that will be used in the analyses and the name of statistical analysis that will be performed to assess the outcome should be listed [ 22 – 25 ].
If computer programs are to be applied, it is important to mention the software used and its version.
8) Project Management: Work plan-A work plan is an outline of activities of all the phases of the research to be carried out according to an anticipated time schedule.
Proper time table for accomplishing each major step of the study should be defined. Assigning time frame to each step in the trial will be helpful in organizing the structure of the research trial. The personnel (investigators, assistants, laboratory technicians etc.) involved in the study or data collection should be properly trained.
9) Strengths and Limitations: It is important to mention the strengths or limitations of the study, i.e., what study can achieve or cannot achieve is important, so as to prevent wasteful allocation of resources.
10) Ethical Considerations (Issues for Ethical Review and Approvals): It should indicate whether the procedures to be followed are in accord with the Declaration of Helsinki. In any case, study should not start unless approval from ethics committee is received [ 26 ].
The following points should be explained:
- The benefits and risks for the subjects involved. The physical, social and psychological implications of the research.
- Details of the information to be given to the study patients including alternative treatments/approaches.
- Information should be provided on the free informed consent of the participants. Information form should contain: Justification for research, outline of study, risks, confidentiality, and voluntary participation should be told patients about the freedom to withdraw from the study whenever they wish to. Confidentiality indicates how the personal information obtained from the patient will be kept secret (Data safety).
11) Operational Planning and Budgeting (Budget Summary): Outline the budget requirement showing head wise expenditure for the study-manpower, transportation, instruments, laboratory tests, and cost of the drug. Budget estimate is to be attached in the annexure. All costs including personnel, consumables, equipment, supplies, communication, and funds for patients and data processing are all included in the budget. Each item should be justified.
12) Reference System: Referencing is the regular method of recognizing information taken from other researchers’ work. A proper citation will enable the readers to follow-up any reference of interest. Plagiarism refers to claiming and acquiring someone else’s ideas, an action that is considered a criminal action.
Failure to reference an idea that you have found in your research, or to acknowledge the work of other team members in a team assignment falls under the category of plagiarism. Therefore referencing is an extremely important aspect of the research protocol. The two most commonly used citation systems in clinical writing are the Vancouver system and the Harvard system [ 27 , 28 ]. The choice of referencing system is dependent upon the funding organizations where the research protocol is being submitted. These frequently identify their preferred system of referencing and this should be strictly adhered to. The most common style used in the dental literature is Vancouver style.
13) Annexure:
The following annexes are to be attached at the end of the protocol:
- Informed consent form.
- Letters from ethics committees.
- Study questionnaire (copies of any questionnaires or draft questionnaires).
- Case Record Forms (CRFs).
- Budget details.
- Curriculum Vitae (CV) of the chief investigator and co-investigator and their role in the study. It will ensure that the role of each investigator is well defined.
How to Judge A Good Protocol?
The protocol should adequately answer the research question. The research design must be sound enough to yield the expected knowledge. It should provide enough detail (methodology) that can allow another investigator to do the study and arrive at comparable conclusions. Here, the proposed number of participants is reasonably justified and the scientific design is adequately described.
Common Mistakes (Common Pitfalls to Avoid)
Incorporating insufficient elements regarding proposed studies and inadequate explanation for the implication of the problem must be shunned as well as suggesting far more work than can be practically done during the study period. Furthermore, underpowered sample size should be justified, invalid or unreliable instrumentation should be tested and improper statistics should be adequately analyzed.
The most difficult stage of conducting a research project is the preparation of a protocol that results in a short yet comprehensive document that clearly summarizes the project. Such proposal is considered successful when it is clear, free of typographical errors, accurate and easy to read.
It is important to understand the steps in developing a research protocol in order to perform an appropriate study and obtain reliable results. Extra time spent to write a good protocol will save failures at a later stage besides helping analysis. If the protocol is poorly prepared and not adhered to, it is unlikely that the project will yield the information that you hope for and in all probability the chances of selling your idea to the reviewers of a granting agency would be less.
Financial or Other Competing Interests
19+ SAMPLE Clinical Research Proposal in PDF

Clinical Research Proposal
19+ sample clinical research proposal, what is a clinical research proposal, when writing a clinical research proposal, what should you keep in mind, elements of clinical research proposal, steps in writing a clinical research proposal, how long should a research proposal be, why write a research proposal, why must you submit a research proposal.

Clinical Research Project Proposal

Center For Clinical and Translational Science Research Proposal

Clinical Research Proposal Process Improvement Project

Clinical Research Project Proposal Form

Clinical Research Skills Proposal

Clinical Research Investigator Proposal

Clinical Research Proposal Example

Clinical Research Proposal in PDF

Clinical Research Certificate Program Proposal

Basic Clinical Research Proposal

Clinical Research Grant Proposal

Clinical Nursing Research Grant Proposal

Clinical Research Ethica Proposal

Printable Clinical Research Proposal

Simple Clinical Research Proposal

Clinical Research Grant Proposal Form

Clinical Trials Research Proposal

Clinical Research Grant Application Proposal
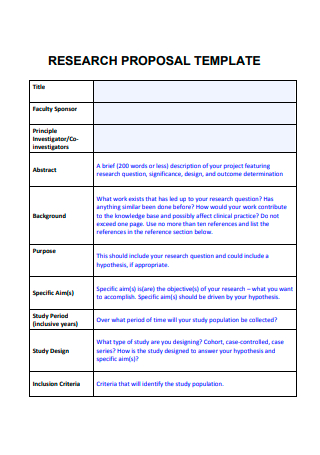
Clinical Research Proposal Template

Clinical Nursing Research Proposal
1. your target audience, 2. pitfalls that could occur, 3. data and research, 1. define the statement of the problem, 2. present your proposal, 3. extend your research, 4. create a schedule and a budget plan, 5. indicate conclusion, 6. verify that your proposal is error-free, share this post on your network, file formats, word templates, google docs templates, excel templates, powerpoint templates, google sheets templates, google slides templates, pdf templates, publisher templates, psd templates, indesign templates, illustrator templates, pages templates, keynote templates, numbers templates, outlook templates, you may also like these articles, 25+ sample construction company proposal in ms word.
Navigating the intricate world of construction demands a seasoned company with a proven track record. Our comprehensive guide on the Construction Company Proposal is your blueprint to understanding the…
8+ SAMPLE Drama Proposal in PDF

Julia Child said: “Drama is very important in life: You have to come on with a bang. You never want to go out with a whimper. Everything can have…
browse by categories
- Questionnaire
- Description
- Reconciliation
- Certificate
- Spreadsheet
Information
- privacy policy
- Terms & Conditions
- ESCMID Global
- escmid global
- study groups
- observership
- summer school
Research Grants
Every year, ESCMID allocates around 800k EUR year to propel ground-breaking research projects in the fields of Clinical Microbiology (CM) and Infectious Diseases (ID). We provide individual research grants to aspiring young investigators and as a part of our ESCMID Study Groups (SGs), a complimentary benefit available to ESCMID members. These grants aim to drive collaboration and innovation, pushing CM and ID to new levels and positioning ESCMID as a pioneer in the future of healthcare excellence.
Individual Research Grant
ESCMID Research Grants help in nurturing the next generation of researchers starting out in CM and ID, marking an important start in their journey of research funding. These grants offer a unique opportunity to explore Bacterial Infections & Diseases during even years and Fungal/Viral/Parasitic Infections & Diseases during odd years.
Funding and payment
ESCMID’s research grants offer up to 20K EUR per project. The grant can be used for the entirety or specific aspects of your project. Upon the signing of the grant agreement, 50% of the allocated funds are issued to the grantee’s institution, with the remaining 50% released upon the submission and approval of the final reports (refer to reporting below for more details). ESCMID also reserves up to 50K EUR each for two exceptional projects. Recognised by our esteemed reviewers for their outstanding potential, selected projects have the possibility to receive this additional funding to propel their topics further.
ESCMID Förderverein Grants for Research in AMR and Emerging Infections
As part of the new ESCMID strategy, the ESCMID Förderverein grant enhances funding for research projects focusing on Antimicrobial Resistance (AMR) or Emerging Infectious Diseases (EID). This support benefits both individual researchers and SGs . As part of this initiative, four additional grants are awarded annually. To qualify, projects must address the challenges posed by AMR and/or EID, aligning with the specified theme for the year. Applications are integrated into our standard grant calls, with clear indications provided in the application form.
Project criteria
Your research project can include laboratory investigations, clinical studies, experimental animal studies, or a combination of approaches. In 2024, the research grant programme will welcome projects focused on bacterial infections and diseases (including diagnostics, pathogenesis, susceptibility and resistance, stewardship, and vaccines. Projects usually last from 12-24 months, starting from the year the grant is received. Should your project align with the specified criteria, it will undergo a rigorous peer review process, with the most promising projects being selected through a meticulous ranking of applications and research proposals. Please note that projects that do not align with the established criteria will be disqualified without a peer review.
Eligibility criteria
Qualify for our research grant opportunities by meeting the following criteria:
- Serve as the principal investigator for the proposed research project
- Hold a valid ESCMID Young Scientist Membership (YSM) or ESCMID low- or middle-income country (LMIC) membership*
- Have a medical degree (MD, MBBS, PharmD, or equivalent), a PhD, or be actively enrolled in a PhD programme
Applicants who have received any ESCMID Research Grant (Individual, Study Group or SG Collaboration) may only apply for a subsequent grant in the year after official completion of the first project. Additionally, you cannot be awarded more than one ESCMID Research Grant (Individual, Study Group or SG Collaboration) in a single year or be listed as principal investigator in more than one concurrent project. Please be aware that members of the ESCMID Executive Committee (EC) are not eligible to apply for research grants or provide support letters for applications. *We invite ESCMID LMIC members to apply if they are still in training or completing their training (PhD no more than eight years post-graduation, MD or MD/PhD no more than five years from completion of clinical training or speciality). If this opportunity sparks your interest, we encourage you to contact ESCMID Office and submit documents proving your status as early as possible, no later than two weeks before the application deadline.
What you need to apply
If you meet the above criteria, we welcome you to apply. To ensure a seamless and efficient application process, all documentation must be submitted electronically via our online application form. As you prepare to submit, please have the following documents readily available.
A single PDF document (labelled “YourSurname”.pdf; in Calibri font, size 11, with 2 cm margins, max. 4MB) including the following key elements:
- Abstract of your research project (max. 750 words; with 3-5 keywords that best characterise your project). Please also include the abstract and keywords separately in the online form (in addition to including them in the PDF document) to help facilitate reviewer selection.
- Introduction/Background (max. 500 words)
- Objectives (max. 300 words)
- Purpose and potential impact of your project (max. 700 words)
- Research plan (max. 2,000 words) including design, material and methods, data analysis, project timeline, co-investigators and their contribution to the project; (references/bibliography are not included in word limit)
- Budget including personnel, equipment, consumables (max. 1,500 words; please use tables wherever possible)
- Description of your present research (max. 750 words)
- Your CV (max. 1,500 words) demonstrating how your skills align with the project's requirements. Alternatively, support letters must confirm that adequate mentoring is available to ensure the project’s success. We advise discussing with your supporters to determine the most suitable strategy for your situation.
- Your publication list , highlighting your most relevant publications from the past five years to help reviewers assess your experience and competencies in relation to the project you are submitting (max. of 10)
Two signed letters of support (labelled “YourSurname”_support1.pdf and “YourSurname”_support2.pdf; max. 4MB each).
- The supporters you select to participate in this research journey know you professionally and can judge your work. They may include an advisor of yours during training, or any other previous collaboration partners, but cannot be your current direct supervisor and/or anyone from your same department.
- Projects missing signed letters of support will not be accepted
Potential reviewers: As part of the application process, at least two suitable reviewers should be suggested. Conditions for reviewers include not being directly involved in the project proposal, not having the same affiliation as you, and if possible, not from the same country either. Should there be any individual you prefer not to have as a reviewer, kindly inform us accordingly.
A colour photograph (labelled “YourSurname_Firstname”.tiff, .eps or .jpg; max. 4MB), measuring at least 4 x 6 cm with a 300 dpi resolution, suitable for publication.
You can ensure a smooth submission process by first verifying you have the correct documents and file types before upload. After the deadline, changes will unfortunately be impossible, so we strongly recommend ensuring all details are correct before submission.
Writing your application
For full transparency about the review process, you will find the instructions and rating criteria used to assess your application here. Here are some helpful guidance for writing your research grant application:
- Write as concisely as possible, you do not need to reach the maximum word counts
- Use bullet points to facilitate review
- If English is not your first language, consider having your text proofread before submission
- Clearly specify where funding will be used if your project is selected for a grant, especially if your project combines different funding sources (please note that omission will lead to rejection)
Within six months following the completion of your project, you must submit your financial and scientific reports. We will be happy to provide you with templates and thorough guidelines to support your report writing. Your financial report should illustrate a full breakdown of how the provided funds were used, accompanied by the signature of your head of department. Your scientific report should capture your research journey in a maximum of 1,000 words, covering the key elements listed below:
- A short introduction summarising the current level of knowledge for non-specialists
- Your study hypothesis
- An overview of what was funded by your ESCMID Grant
- A summary of the main findings – potentially including how the data contributed to other projects
- A conclusion on your findings
Should a published scientific article accompany your report, we welcome a concise version of your submission, with references directing to the relevant sections within the article. Please do consider the article should transparently disclose any funding from alternative sources.
Financial report template Scientific report template
Publication
Grant recipients must acknowledge ESCMID in all scientific publications using the wording “This study/project has been funded by a European Society of Clinical Microbiology and Infectious Diseases (ESCMID) research grant to (initials of grantee) in (year of grant)”. Grantees should first consider publishing in one of the Society’s official journals Clinical Microbiology and Infection (CMI) or CMI Communications for initial submission of manuscripts, but alternative journals may be considered depending on the scope of the project. We ask grantees to please share with the ESCMID Office any published scientific articles stemming from their grant-funded projects, regardless of whether the project has concluded, even after all reports have been submitted. These articles are a source of pride for our Society and will be continuously showcased on our official website and in our annual ESCMID yearbook. In this way, we will be able to keep celebrating your research accomplishments and contribution to the global advancement of knowledge in the fields of CM and ID.
Study Group Research Grant
Dive into cutting-edge research with ESCMID SG research grants, tailored for CM and ID related projects. Our SGs cover a vast range of themes, ensuring that there are no limits on the topics eligible for funding each year. Membership of a SG is free and open to all ESCMID members, and will propel your research to new heights of excellence.
With up to 30K EUR offered to cover project costs, ESCMID SG research grants can help you support the entirety or specific elements of your project. Upon signing, ESCMID will assign the designated grant amount. This sum can be used to settle invoices or be transferred directly to your institution for the project's needs.
As part of the new ESCMID strategy, the ESCMID Förderverein Grant enhances funding for research projects focusing on Antimicrobial Resistance (AMR) or Emerging Infectious Diseases (EID). This support benefits both individual researchers and SGs . As part of this initiative, two additional grants are awarded annually. To qualify, projects must address the challenges posed by AMR and/or EID, aligning with the specified theme for the year. Applications are integrated into our standard grant calls, with clear indications provided in the application form.
Your research project can encompass a range of dynamic approaches, including laboratory investigations, clinical studies, experimental animal studies, or a combination of these approaches. As we value collaboration, your research project should be conducted in partnership with at least two separate institutions across two different countries. Designed for a maximum duration of two years, your project should start after the grant agreement is signed. The full support and engagement of all SGs involved is mandatory.
Applicants who have received any ESCMID Research Grant (Individual, Study Group or SG Collaboration) may only apply for a subsequent grant in the year after official completion of the first project. Additionally, you cannot be awarded more than one ESCMID Research Grant (Individual, Study Group or SG Collaboration) in a single year or be listed as principal investigator in more than one concurrent project. Please be aware that members of the ESCMID Executive Committee (EC) are not eligible to apply for research grants or provide support letters for applications. Qualify for our research grant opportunities by meeting the following criteria. Each SG may apply with a maximum of two research projects, as indicated below:
- Either one project as a single SG applicant + one project in collaboration with another SG
- Or two projects, each in collaboration with another SG
Two projects as a single SG cannot be accepted as these grants aim to boost interconnectivity across a wide range of topic areas.
- As the applicant, you should be the principal investigator for the proposed research
- Each SG involved should also fully support the proposal by written approval of their respective study group EC
- To qualify, you should be an ESCMID Full, LMIC or YSM, and a member of at least one of the involved SG
To ensure ease and efficiency through the application process, all documentation must be submitted electronically via our online application form. As you prepare to submit, please have the following documents readily available:
A single PDF document (labelled “YourSurname_StudyGroup”.pdf; in Calibri font, size 11, with 2 cm margins, max 4MB, up to 6 pages) including the following key elements:
- Summary of your research (max. 1 page including Gantt chart)
- Introduction (scientific background, objectives, rationale, and purpose of your project)
- Research plan (including project design, tasks of the involved teams, material and methods, and data analysis)
- Importance of the project for the supporting SG (max. 1/2 page)
- Teams involved and their roles (at least two teams from two different countries) (max. 1/2 page)
- Budget , including costs for personnel, equipment, and consumables (max. 1/2 page)
- Description of your present research (max. 1/2 page)
- Your CV and list of relevant publications (max. 2 pages)
A support letter (labelled “YourSurname_support”.pdf; max 4MB) signed by the Chairperson, Secretary, and Treasurer of the SG involved in the proposal. The letter should clearly state the title of your project, your name as the applicant, and your respective SG.
Importantly, where collaboration between two study groups will be occurring, a single scanned file containing two support letters, one for each involved study group, is required.
A colour photograph (labelled “YourSurname_Firstname”.tiff, .eps or .jpg; max 4MB), measuring at least 4 x 6 cm with a 300 dpi resolution, suitable for publication.
Evaluation process
The Scientific Affairs Subcommittee (SAS) and potentially external peer-reviewers will be taking an extensive look at your proposal, focusing on the following key areas:
Scientific and/or technological excellence
- Soundness of concept and quality of objectives
- State-of-the-art progress
- Quality and effectiveness of the scientific/technological methodology and work plan
Quality and efficiency of implementation and budgeting
- Overall appropriateness of collaborating partners
- Overall quality and relevant experience of individual partners
- Overall suitability and justification of the grant resources for the respective project (budget, staff, and equipment)
- Overall timing and scope of work
Parity assessment
- Geographic distribution of partners
- Gender balance
In making the final decision, please note we may consider other concurrently submitted projects as well as previously approved or ongoing projects within the same SG.
Within 6 months following the completion of your project, you must submit your two-page report summarising your findings. Here is what the report should include:
- Summary overview: a short introduction for non-specialists recapping the current level of knowledge on the topic and any gaps which prompted the research
- Study hypothesis
- Main findings
- Conclusions from the investigation
- Presentations or publications originating from this project (oral/poster)
If peer-reviewed publications covering the funded project are published, please disregard the above report requirement. Further information, together with the ESCMID templates for the scientific and financial reports, will be provided after signing the grant agreement.
Study Group Collaboration Grant
The Study Group Collaboration Grant is a larger-scale grant for innovative research projects in clinical microbiology and infectious diseases organised by an experienced PI with the support of at least three ESCMID SGs collaborating. Please do not apply, if you do not have full support from three or more of ESCMID's SGs. SG membership is free and open to all ESCMID members.
The maximum amount granted per project is 180K €. This includes any potential overhead costs charged by your institution. ESCMID’s funding may support the entire project or part of it. After signing the grant agreement, ESCMID will set aside the amount that can either be used to pay invoices or be transferred to your institution for project use.
We believe that collaboration is key, this is why your project must be supported by at least three ESCMID SGs and be carried out in partnership with institutions from at least two different countries. In addition, each project should include members from each participating SG, ensuring diverse expertise and participation. Your research should encompass a broad range of methodologies, including laboratory investigations, clinical studies, and experimental animal research. Designed for a maximum duration of two years, your project should be of high quality, with a focus on innovation and networking. Please note that projects that do not align with the established criteria will be disqualified without a peer review.
- Be the principal investigator for the proposed research, with previous experience in conducting large research projects
- Have full support of the Chairperson, Secretary, and Treasurer of each SG, with their written approval
- Be an active ESCMID Full, YSM, or LMIC member
Please note that applications are not accepted from companies/start-up research firms. Applicants who have received any ESCMID Research Grant (Individual, Study Group or SG Collaboration) may only apply for a subsequent grant in the year after official completion of the first project. Additionally, you cannot be awarded more than one ESCMID Research Grant (Individual, Study Group or SG Collaboration) in a single year or be listed as principal investigator in more than one concurrent project. Please be aware that members of the ESCMID Executive Committee (EC) are not eligible to apply for research grants or provide support letters for applications.
To ensure ease and efficiency through the application process, all documentation must be submitted electronically via our online application form. As you prepare to submit, please ensure the following documents readily available:
- Completed application form based on the criteria above (labelled “YourSurname”_application.pdf; max 4MB)
- Your CV as the primary investigator along with the list of your top 10 publications (labelled “YourSurname”_CV.pdf; max 4MB) (max 2 pages)
- Support letters signed by the Chairperson, Secretary, and Treasurer of the SGs involved in the proposal (electronic signatures allowed). The letters must include the title of the project, your name as the applicant, and the SGs involved (labelled “YourSurname”_support.pdf; max 4MB).
- A colour photograph (labelled “YourSurname_Firstname”.tiff, .eps or .jpg; max 4MB), measuring at least 4 x 6 cm with a 300 dpi resolution, suitable for publication
- Optional : supplemental data or graphical abstracts to support the proposal (PDF format; max 4 MB)
We are happy to specify SGs can be part of multiple submissions.
The SAS and potentially external peer-reviewers will be taking an extensive look at your proposal, focusing on the following key areas:
Entry criteria
- Your research project should include a translational component
- The roles of each SG should be clearly defined, and responsibilities appropriately split
Scientific factors
- Clarity and objectives of the proposal (10 points)
- Importance and relevance to ID and CM (10 points)
- Adequacy of preliminary work (10 points)
- Suitability of methods for the project (10 points)
- Innovation of the proposal (10 points)
- Sufficiency of research infrastructure for success (10 points)
- Scientific excellence and proposal feasibility (10 points)
Network considerations
- Inclusion and appropriateness of external collaborators (5 points)
- Priority for projects with partners in Low- or Lower-middle-income countries* (5 points). *As defined by the world bank classification
Logistical components
- Clear definition of all funding sources and realistic pricing for consumables, equipment, and personnel (10 points)
- Clear definition of the team and their roles (5 points)
- Realistic and detailed proposed timeline (5 points)
Total possible score: 100 points
We appreciate high-quality applications aligned with the scoring criteria outlined above. Innovation and networking are key, and as such, we look forward to seeing how your projects incorporate these elements. If it is judged that none of the applications for a given year meet our quality standards, ESCMID may decide to withhold funding.
- Summary overview: a short introduction for non-specialist readers summarising the current level of knowledge on the topic at hand, and any gaps which prompted the research
As a proud provider of financial support for selected research projects, ESCMID kindly requests grant recipients acknowledge the Society in all scientific publications using the wording “This study/project has been funded by a European Society of Clinical Microbiology and Infectious Diseases (ESCMID) research grant to [initials of grantee] in [year of grant]”. Grantees should first consider publishing in one of the Society’s official journals Clinical Microbiology and Infection (CMI) or CMI Communications for initial submission of manuscripts, but alternative journals may be considered depending on the scope of the project. We ask grantees to please share with the ESCMID Office any published scientific articles stemming from their grant-funded projects, regardless of whether the project has concluded, even after all reports have been submitted. These articles are a source of pride for our Society and will be continuously showcased on our official website and in our annual ESCMID yearbook. In this way, we will be able to keep celebrating your research accomplishments and contribution to the global advancement of knowledge in the fields of CM and ID.
- Our Program Divisions
- Our Three Academies
- Government Affairs
- Statement on Diversity and Inclusion
- Our Study Process
- Conflict of Interest Policies and Procedures
- Project Comments and Information
- Read Our Expert Reports and Published Proceedings
- Explore PNAS, the Official Scientific Journal of NAS
- Access Transportation Research Board Publications
- Coronavirus Disease 2019 (COVID-19)
- Diversity, Equity, and Inclusion
- Economic Recovery
- Fellowships and Grants
- Publications by Division
- Division of Behavioral and Social Sciences and Education
- Division on Earth and Life Studies
- Division on Engineering and Physical Sciences
- Gulf Research Program
- Health and Medicine Division
- Policy and Global Affairs Division
- Transportation Research Board
- National Academy of Sciences
- National Academy of Engineering
- National Academy of Medicine
- Publications by Topic
- Agriculture
- Behavioral and Social Sciences
- Biography and Autobiography
- Biology and Life Sciences
- Computers and Information Technology
- Conflict and Security Issues
- Earth Sciences
- Energy and Energy Conservation
- Engineering and Technology
- Environment and Environmental Studies
- Food and Nutrition
- Health and Medicine
- Industry and Labor
- Math, Chemistry, and Physics
- Policy for Science and Technology
- Space and Aeronautics
- Surveys and Statistics
- Transportation and Infrastructure
- Searchable Collections
- New Releases
VIEW LARGER COVER
Advancing Clinical Research with Pregnant and Lactating Populations
Overcoming real and perceived liability risks.
Congress called on the National Academies to convene a committee to examine the real and perceived risks of liability arising from research conducted with pregnant and lactating women. The resulting report, Clinical Research with Pregnant and Lactating Populations: Overcoming Real and Perceived Liability Risks, explores and finds limited evidence of legal liability for inclusion of pregnant and lactating women in clinical research, contradicting perceptions of heightened liability. The committee also makes recommendations that could lead to a more robust evidence base about the safety and efficacy of medications for pregnant and lactating women that would facilitate more informed decision making regarding care while mitigating liability.
RESOURCES AT A GLANCE
- Policy Brief for FDA
- Policy Brief for Congress
- Policy Brief for HHS
- Policy Brief for NIH
- Infographic on Dissuasive Factors for Inclusion
- Interactive Overview
- ↓ Scroll Down for More Resources
- Health and Medicine — Women's Health
- Health and Medicine — Health Sciences
- Health and Medicine — Policy, Reviews and Evaluations
Suggested Citation
National Academies of Sciences, Engineering, and Medicine. 2024. Advancing Clinical Research with Pregnant and Lactating Populations: Overcoming Real and Perceived Liability Risks . Washington, DC: The National Academies Press. https://doi.org/10.17226/27595. Import this citation to: Bibtex EndNote Reference Manager
Publication Info
What is skim.
The Chapter Skim search tool presents what we've algorithmically identified as the most significant single chunk of text within every page in the chapter. You may select key terms to highlight them within pages of each chapter.
Overview Video
Copyright information.
The National Academies Press (NAP) has partnered with Copyright Clearance Center's Marketplace service to offer you a variety of options for reusing NAP content. Through Marketplace, you may request permission to reprint NAP content in another publication, course pack, secure website, or other media. Marketplace allows you to instantly obtain permission, pay related fees, and print a license directly from the NAP website. The complete terms and conditions of your reuse license can be found in the license agreement that will be made available to you during the online order process. To request permission through Marketplace you are required to create an account by filling out a simple online form. The following list describes license reuses offered by the NAP through Marketplace:
- Republish text, tables, figures, or images in print
- Post on a secure Intranet/Extranet website
- Use in a PowerPoint Presentation
- Distribute via CD-ROM
Click here to obtain permission for the above reuses. If you have questions or comments concerning the Marketplace service, please contact:
Marketplace Support International +1.978.646.2600 US Toll Free +1.855.239.3415 E-mail: [email protected] marketplace.copyright.com
To request permission to distribute a PDF, please contact our Customer Service Department at [email protected] .
What is a prepublication?
An uncorrected copy, or prepublication, is an uncorrected proof of the book. We publish prepublications to facilitate timely access to the committee's findings.
What happens when I pre-order?
The final version of this book has not been published yet. You can pre-order a copy of the book and we will send it to you when it becomes available. We will not charge you for the book until it ships. Pricing for a pre-ordered book is estimated and subject to change. All backorders will be released at the final established price. As a courtesy, if the price increases by more than $3.00 we will notify you. If the price decreases, we will simply charge the lower price. Applicable discounts will be extended.
Downloading and Using eBooks from NAP
What is an ebook.
An ebook is one of two file formats that are intended to be used with e-reader devices and apps such as Amazon Kindle or Apple iBooks.
Why is an eBook better than a PDF?
A PDF is a digital representation of the print book, so while it can be loaded into most e-reader programs, it doesn't allow for resizable text or advanced, interactive functionality. The eBook is optimized for e-reader devices and apps, which means that it offers a much better digital reading experience than a PDF, including resizable text and interactive features (when available).
Where do I get eBook files?
eBook files are now available for a large number of reports on the NAP.edu website. If an eBook is available, you'll see the option to purchase it on the book page.
View more FAQ's about Ebooks
Types of Publications
Consensus Study Report: Consensus Study Reports published by the National Academies of Sciences, Engineering, and Medicine document the evidence-based consensus on the study’s statement of task by an authoring committee of experts. Reports typically include findings, conclusions, and recommendations based on information gathered by the committee and the committee’s deliberations. Each report has been subjected to a rigorous and independent peer-review process and it represents the position of the National Academies on the statement of task.
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Clinical Reasoning of a Generative Artificial Intelligence Model Compared With Physicians
- 1 Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts
- 2 Department of Medicine, Massachusetts General Hospital, Boston
- 3 Department of Pulmonary and Critical Care, Brigham and Women’s Hospital, Boston, Massachusetts
Large language models (LLMs) have shown promise in clinical reasoning, but their ability to synthesize clinical encounter data into problem representations remains unexplored. 1 - 3 We compared an LLM’s reasoning abilities against human performance using standards developed for physicians.
Read More About
Cabral S , Restrepo D , Kanjee Z, et al. Clinical Reasoning of a Generative Artificial Intelligence Model Compared With Physicians. JAMA Intern Med. Published online April 01, 2024. doi:10.1001/jamainternmed.2024.0295
Manage citations:
© 2024
Artificial Intelligence Resource Center
Best of JAMA Network 2022
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
steps. It begins with selecting a study topic, reviewing. the literature, setting goals, choosing a study design and. appropriate statistical tools, and formulating a research proposal. to obtain ...
INTRODUCTION Contd. Writing an academic project proposal requires that one demonstrates an understanding of a specific problem within ones discipline. A Clinical research is concerned with the study of health and illness in people. It helps us how learn how to prevent, diagnose and treat illness.
What is a Clinical Research Proposal? A proposal for a clinical research is a valuable document that demonstrates primary targets, objectives, and methods or strategies established by the clinical researchers, medicine professionals, healthcare practitioners or scientists in encouraging potential sponsors or medical associations for the implementation of a particular clinical study or trial.
Research Proposal Template Biomedical Research: General Page 2 Methods . Human Subjects This section describes your proposed study population and, if the study is prospective, how you will identify and enroll participants and obtain their informed consent. The bulleted list gives examples of elements you may need to describe (the first three items
ISP Proposal Samples. Analysis of a. Scientific or. Medical Problem. Community Service & Leadership. Medical Education. Scientific Research. Focused Clinical Multidisciplinary. SMP1 - [71 kb]
Research proposal examples. Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We've included a few for you below. Example research proposal #1: "A Conceptual Framework for Scheduling Constraint Management" Example research proposal #2: "Medical Students as Mediators of ...
and only a minority of grant proposals receive funding. In partic-ular, funding for patient-oriented research lags behind that allocated for basic science research. Grant writing is a skill of fundamental importance to the clinical researcher, and conducting high-quality clinical research requires funds received through suc-cessful grant proposals.
3.3 Material/Methods/Study Design. This is the most technical part of your proposal and should be written as such. Hence, there is no need to "tell a story" but stay short, brief and to the point. In the following is a short bullet point list of elements that should be include in your material/method section:
Include these items in your proposal packet: Research Proposal Packet Cover Page (PDF). The proposal. Research proposals are approved by the CCaTS Master's and Certificate Programs Executive Committee and Mayo Clinic Graduate School of Biomedical Sciences (MCGSBS). Follow the Research Proposal Guidelines (PDF), and construct your specific aims in a way that clearly defines the two or three ...
The research proposal serves many purposes to many different parties. Amongst these purposes, some of the key ones are: • Acting as a route map and timetable for all involved in your project. • Giving a clear overview of your planned work to ensure favourable decision at ethical review.
• What is your research question and if relevant, your specific hypothesis? (testable prediction) • For QI/PI, this is your aim statement. The aim statement should include your population, location, outcome measure, amount of change expected in your outcome measure, and date of expected change result. 7. Research Methods
Clinical research is necessary to establish the safety and effective-ness of specifi c health and medical products and practices. Much of ... mulate proposals and guidelines for research in the fi eld of drug de-velopment. These reports formed the basis for WHO's "Guidelines for good clinical practice (GCP) for trials on pharmaceutical ...
Successful grant applications require a well-trained investigator who carefully outlines a hypothesis-driven research proposal. Unique to FAER and IARS research committees is that the reviewers are mostly investigators and practicing anesthesiologists. These reviewers fully appreciate the importance of clinical research and enthusiastically ...
It puts the proposal in context. 3. The introduction typically begins with a statement of the research problem in precise and clear terms. 1. The importance of the statement of the research problem 5: The statement of the problem is the essential basis for the construction of a research proposal (research objectives, hypotheses, methodology ...
Detailed Walkthrough + Free Proposal Template. If you're getting started crafting your research proposal and are looking for a few examples of research proposals, you've come to the right place. In this video, we walk you through two successful (approved) research proposals, one for a Master's-level project, and one for a PhD-level ...
of heavy clinical months and anticipate when you'll have less time to work on your project. Consider working backwards from abstract submission deadlines. 9. Resources Required • Personnel to be involved and their time (e.g., statistical help, survey design, qualitative analysis) • Research equipment • Budget (if applicable) 10.
clinical research handbook will be available for physicians and PIs starting in January 2021. This. clinical handbook starts by discussing various ways for the clinical studies to be organized and. executed, including a step-by-step approach to research documentation while managing. regulatory and ethical concerns in research.
Clinical researchers are working on several treatment and prevention options to improve and advance medicine and human health. Their revolutionary ideas start with a research proposal for succeeding tests and validations. 1. Research Proposal Template. Details. File Format. Google Docs. MS Word. Pages.
Initial Proposal Concept Form (MS Word, 39K) - This form should be used to advocate for an initiative by the Division of Geriatrics and Clinical Gerontology (DGCG) for a clinical trial or trials that exceed $2 million in direct costs in any year of funding. DGCG Clinical Trials Advisory Panel, a task force of the National Advisory Council on ...
Protocol writing allows the researcher to review and critically evaluate the published literature on the interested topic, plan and review the project steps and serves as a guide throughout the investigation. The proposal is an inevitable document that enables the researcher to monitor the progress of the project [ 5 ].
Take a look at our sample clinical research proposal, which has been included in this post. 1. Define the Statement of the Problem. To convince your audience that your topic is a medical concern that should be treated as soon as possible, you must explain why you believe this is the case. This is the section of the medical research proposal ...
Hyun Jung Kim, hk9746, Biomedical Engineering. Purpose. The purpose of the proposed research is to collect tissue, blood and fecal samples from patients undergoing standard of care for their gastrointestinal disease, including Inflammatory Bowel Disease (IBD), and Colorectal Cancer (CRC). Tissue and blood samples will be obtained during ...
Clinical trial proposals submitted in response to a call for proposals are subject to the same formal and legal requirements (including e.g. the mandatory information in part A and the structure and page limitations of part B of the proposal) as any other proposal. This also applies to the electronic submission format.
Qualitative research methods are increasingly recognized for their importance in healthcare-related research, particularly in contextualizing social and cultural realities that impact human behavior (Al-Busaidi et al., 2008; Renjith et al., 2021).There is a growing interest in and acceptance of qualitative research approaches in the health sciences, both as stand-alone methodologies and ...
The Study Group Collaboration Grant is a larger-scale grant for innovative research projects in clinical microbiology and infectious diseases organised by an experienced PI with the support of at least three ESCMID SGs collaborating. ... supplemental data or graphical abstracts to support the proposal (PDF format; max 4 MB) We are happy to ...
3 Strategies to Reduce Harm Through Clinical Research: 81-122: 4 Mitigating Liability Associated with Clinical Research: 123-148: 5 Dissuasive and Persuasive Factors for the Inclusion of Pregnant and Lactating Women in Clinical Research: 149-178: 6 Recommendations: 179-206: Appendix A: Public Meeting Agendas: 207-216
This cross-sectional study assesses the ability of a large language model to process medical data and display clinical reasoning compared with the ability ... Future research should assess clinical reasoning of the LLM-physician interaction, as LLMs will more likely augment, not replace, the human reasoning process. ... Get unlimited access and ...
This award provides funding for pre-clinical research in aging biology and geroscience in the United . States, Canada, and Europe. The HF-GRO Awards are for basic and translational research only (not clinical . trials). The program provides support for research projects in the basic biology of aging, as well as
Research Program and establish a strategic research plan that would expand, intensify, expedite, and coordinate NIH's Long COVID activities. This plan would be published within 6 months of the development of this research program and be updated annually. o The Director of the Long COVID Research Program must be someone
1 M100 standard in the table refers to Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed. CLSI supplement M100; 2023. Dated: April 4, 2024. Lauren K. Roth, Associate Commissioner for Policy. [FR Doc. 2024-07495 Filed 4-8-24; 8:45 am] BILLING CODE 4164-01-P