
- Oracle Database Administration Case Studies
- Case Studies

Success Stories with Oracle Database Administration
Click HERE for Oracle APEX Administration Case Studies
Client: Leading independent, non profit research and development organization

Project: Oracle Database Training for the JUNO Mission
SkillBuilders’ John Watson began teaching the Oracle DBA team at this Research Organization starting in 2014; the training and mentoring is ongoing and continues to this day. They’ve attended training in Oracle RAC, Security, Data Guard, Tuning, RMAN and more. (Additionally, developers in the organization have attended Java training.) More recently they’ve hired us in a consulting role to assist with, for example, tricky RMAN issues. Almost nine years of successful services delivered and counting!
Testimonial from Chris
“We have worked with John Watson during on-site engagements as well as numerous online classes. His depth of knowledge and his ability to teach complex topics is simply outstanding. Both my colleague and I are seasoned DBAs but we always gained a vast amount of new knowledge to be applied in our daily tasks and corporate operations.”
Client: Silicon Valley Power
Silicon Valley Power has provided the city of Santa Clara California with electricity for 125 years.
Project: Configure MAA on Usage/Billing Oracle Database
The primary project was configuring MAA (Maximum Availability Architecture): RAC-to-RAC Data Guard, between two ODAs (Oracle Data Appliances). Secondary projects included configuring Data Guard between two Windows systems, apply the ODA full stack patches, and setup an RMAN catalog database to manage backups for the whole environment. This was a lot of work for a short engagement, but we did manage to complete the project within budget. The result is a highly resilient environment for various databases, critical for the effective management of electricity usage and billing.
Client: Foremost Farms
Foremost Farms is a milk solids processing business owned by the dairy farm families who supply our milk. That’s what it means to be a cooperative – and it’s why our farmer-members have a vested interest in providing the highest-quality milk possible. They own Foremost Farms.
Project: Research running Oracle Data Appliance (ODA) in Virtualized Environments
This project was about investigating the ODA virtualized architecture and understanding how best to use it with single instance databases. Not because of RAC, because of licences.
You can run ODA as “bare metal” or “virtualized”. Typically, people who installed ODA more than, say, two years ago run bare metal: the two ODA nodes run normal Linux and the databases reside on ASM disc groups. This is all straightforward and should present no surprises to the DBA or the SA. However, most people installing now follow Oracle’s recommendation and run it virtualized. The configuration can strike you as bizarre the first time you see it. The nodes run Xen Linux (Oracle’s version, of course) and everything happens in virtual machines. The databases reside on ACFS file systems, and are created from ACFS snapshots of preconfigured databases. The networking may take a while to get your head around. In general, the virtualized platform is probably the way to go, but you do need to be a bit of a wizard with Grid Infrastructure and Xen to understand what is going on. There are many GI facilities that can be used for fault tolerance and failover without RAC, which is not something that Is very well documented. Perhaps it should be.
Client: XIFIN
XIFIN is a health information technology company that leverages diagnostic information to improve the quality and economics of healthcare. The XIFIN technology platform facilitates connectivity and workflow automation for accessing and sharing clinical and financial diagnostic data, linking healthcare stakeholders in the delivery and reimbursement of care.
Project: Configure New Oracle RAC Database Environment plus Migrations and Consolidations
SkillBuilders Oracle Certified Master DBA John Watson assisted a medical insurer to move several Oracle databases supporting OLTP, BI, and batch workloads from single instance 11.2.0.4 to a three node 12.1.0.2 RAC, consolidating the applications into just two databases as we did it. The destination platform was Sun servers with infiniband networking and EMC VMAX storage: this is a pretty big environment with demanding service level agreements. We trained the DBA and Systems Admin staff and provided further support during the implementation. Problems encountered, identified, and resolved included storage issues and performance problems, and (surprise!) a couple of Oracle bugs.
Client: Shoe Carnival
Shoe Carnival is one of the nation’s largest family footwear retailers with its own brand of style. Headquartered in Evansville, Indiana, Shoe Carnival stores are located in 35 states and Puerto Rico, as well as online at shoecarnival.com.
Project: Oracle Data Guard Implementation on RAC Exadata
SkillBuilders was initially asked to get the in-house DBA team up-to-speed on Oracle Data Guard. So, SkillBuilders’ Oracle Certified Master DBA John Watson taught our Data Guard Administration class over one week to the in-house DBA team. After the class wrapped up, Shoe Carnival IT management, aware of the time pressure and critical nature of the project, asked John to implement their first Data Guard configuration. Specifically, configuring one of their production databases for Data Guard and RAC (RAC -> RAC between two Exadata’s) and of course thorough testing including switch-over (failover) and switch-back.
Client: Kyocera (formerly SLD Laser)
SLD Laser – Brighter. Smaller. Safer. LaserLight delivered. The next evolution in lighting has arrived, shining brighter and farther than ever before. LaserLight surpasses the limits of LED to deliver safe, high luminance white laser light from an incredibly small light source. It’s enabling entirely new opportunities with SLD Laser’s GaN laser technology.
Project: Design and Implement Secure Channels for Hybrid Database Connections in AWS for 24×7 Multi-State Communications
Starting point: SkillBuilders had previously designed and implemented an Oracle APEX stack on Amazon AWS for the company’s internet-facing APEX applications. The company also has critical information in Microsoft SQLServer Databases.
Now we were tasked with constructing secure communication channels for Oracle and SQLServer connections between the company’s headquarters and three remote locations.
Our team configured a VPN Tunnel between three of the customer’s remote facilities and the AWS subnet where SkillBuilders had previously deployed their Web Tier and DB machines in a private Virtual Private Cloud (VPC). The environment consisted of a Customer Gateway which describes the onsite router (in this case Cisco), its external ip, software & version, and their LAN subnet; this is then connected to an AWS Virtual Private Gateway which describes the AWS LAN, which are connected with a “Site-to-Site VPC Connection”.
These VPN Tunnels provide fully encrypted, 24×7, two-way traffic, including sqlnet & odbc connections between Oracle and Microsoft SQLServer Databases.
Large Advertising & Marketing Services Company
The project: build a secure Oracle APEX environment on Oracle Cloud for the company’s internet-facing applications.
Starting point: one virtual machine running a pre-created database with APEX installed End point: the full Apache/Tomcat/ORDS/APEX/database stack, SSL enabled Budget: 20 hours
As with any cloud provider, it takes a while to understand how to use the cloud infrastructure, particularly the networking and security. Once you have got past that, you can start work. First, the Oracle Enterprise Linux default configuration isn’t good enough. You need to install a graphical desktop, and also various RPMs and update OpenSSL. The firewall needs some configuration too. Then the usual: download and install Tomcat; download ORDS, configure and deploy to Tomcat; download the Apache http server plus the APR libraries and compile. We added an OpenVPN server for SQL*Net and ssh access, so that only the Apache listening ports would be exposed to the internet. Everything configured for automatic start on boot up.
All done within budget.
Mid-Size American City
Designed and implemented Oracle Database Standard Edition Failover via intelligent use of scripting and various OVM and networking facilities. Implemented OVM (Oracle Virtual Machine, Oracle’s take on Xen) at two sites, including a DR solution such that database VMs at either site can failover to the other in the event of problems with minimal data loss. Zero data loss and full automation were not possible as only Standard Edition licences were available, but intelligent use of scripting and various OVM and networking facilities give the client something very close to that. Each site had two physical machines: one (with magnetic discs) to run the OVM Manager and be an NFS server, the other (with mixed-use and write-intensive SSDs) to run the VMs to be used as database servers. Using NFS repositories, we directed backups and archives from each site to the other site. A minor complication was that for licence compliance we had to use hard partitioning to restrict the CPUs available to the database servers. In the event of a failure at one site, new VMs can be launched from templates at the surviving site and the databases restored onto them. Not Data Guard level resilience, but pretty darn good.
Brown University Administration
Since 2008, SkillBuilders has trained Brown University developers and Oracle Administrators in new skills vital to the Universities success. This includes Oracle Database performance tuning, new features, RMAN, Enterprise Manager / Grid Control and Grid Infrastructure. For developers SkillBuilders has provided education in Java Programming, Servlets, JSP, XML, Ruby on Rails and more.
SkillBuilders designed and installed active standby using Oracle Database Standard Edition, which supports Soraa production line. SkillBuilders also provides Oracle licenses, in this case saving Soraa tens of thousands (perhaps hundreds) by advising customer Standard Edition would meet customer requirements.
We also implemented RMAN backups, Alert/Incident monitoring and general database tuning. We remain on-call for support issues.
North Carolina Department of Labor
In 2012, SkillBuilders provided the Department of Labor in North Carolina state government with a turnkey APEX configuration, i.e. everything they needed to start deploying internal and internet-facing APEX applications. This included database installation and patching, APEX and APEX listener configuration, Java container (Glassfish) installation and configuration and assistance with firewall updates.
SkillBuilders continues to provide Oracle Database, APEX and infrastructure support; patches, upgrades and troubleshooting.
SkillBuilders provides ongoing Oracle Database advanced administration training. This includes Oracle Data Guard, Administration and Performance Tuning training. One student, who provided a perfect 5.0 score in his post-course evaluation, exclaimed, “I really enjoyed John as my instructor. He was extremely professional, personal, and deeply concerned about us understanding the concepts of in this course.”
Oracle Corporation
SkillBuilders teaches 12c Database new features to Oracle Corporation employees. We developed a custom course and have delivered it 12 times and counting! We also teach other subjects such as Oracle Database Performance Tuning and Data Guard Administration.
When Waters Corp software customers needed a tool to manage ASM disks, non-ASM disk, RMAN backups, imports/exports, monitor and send alerts, manage database users (e.g. password management), SkillBuilders developed an integrated Oracle APEX solution. The “Waters Database Manager”, or WDM, also provides other general management functions such as job scheduling and reporting.
Stretched (Extended Distance) Clusters / ASM
In 2010, Oracle Master John Watson was the development DBA (designer / implementer) behind the successful implementation of a stretched cluster (aka extended distance / geo-cluster). Here’s the story….
We all know what happens if the database that tracks passengers through security fails: the queues grow longer, and within minutes they stretch half way round the terminal. The database that tracks the baggage is as critical: thousands of bags stack up when they should be on the conveyor belts.
At this client (a large airport in Africa) it appeared that even Data Guard would not be adequate to keep things moving. Fast Start Failover with the Data Guard Broker is fast: it can initiate within seconds. But most DBAs will want to build in a delay of a few minutes. Then it takes time actually to switch over to the standby and reconnect all the sessions. That would have been too slow, given the speed with which chaos would escalate in that environment. And it would need Enterprise Edition licences.
RAC looks like the answer: near instantaneous failover of services and sessions from one instance to another if you lose a node. But it doesn’t protect you against losing the site. Or does it? Yes, if you set up a stretched cluster. At each of two airport terminals, we had a database server and a storage array, connected through a fiber switch. A separate ethernet gave the terminals on the security desks and the baggage scanners access, load balanced across both server nodes. ASM handled the mirroring. Losing a server node (not uncommon given erratic power suppliers and unreliable networks) caused all the broken sessions to reconnect (yes, we automated that) to the surviving node with a break in service time of only seconds. When the node came back online, ASM would re-synchronize the database copies. It really worked.
And, perhaps best of all, it worked with Standard Edition licences.
Manual Failover with Standard Edition
In 2013 we configured a manual failover on Standard Edition for an LED light manufacturer. This new company uses their Oracle database to keep their production lines moving – but the cost of Enterprise Edition and Advanced Data Guard is prohibitive and unnecessary, since our manual failover implementation will keep them up and running with 15 minutes of a failure on the primary server.
Advanced Queueing and Resource Manager
In 2008, Oracle Certified Master Administrator John Watson used his knowledge of Oracle Database features to create a clever solution to a performance problem. The client was maxed at 48,000 transactions per hour; John used Advanced Queuing and Resource manager to quadruple that. Read on…
SMSs (i.e. texts) make money – if you can process them fast enough!
The volume of SMS traffic is unbelievable. This isn’t just people voting for a Simon Cowell show: it is requests for ringtones, wallpapers, jokes, games, who knows what else. Millions of SMSs, and they all generate income. Or they should.
At this client (a cellphone network operator in Africa) they were processing maximum 48000 requests per hour. More than that could not go through the database: the sessions just hanged. When the SMS aggregators were throwing messages at peak rate to the application servers, the queues would shoot out, and eventually the application servers would start dropping messages. Bad, very bad: that meant not just losing a sale, but probably losing a customer.
What is the answer to a scalability problem? RAC, of course. Well, not always. We investigated the transactions, and the problem was enqueue contention: the business model relied on a pyramid hierarchical sales structure, so concurrent transactions at a low level would block each other at a higher level. We had to explain: RAC can’t help with TX enqueues, and might make things worse. The answer: re-design the transactions to use Advanced Queueing. The low level updates put a message on a queue, and commit. Fast. Then de-queueing every few seconds would process a group of a several hunded updates as one logical transaction. Problem solved: now processing up to 160000 requests per hour.
But they wanted more. Rolling out a banking application for transferring funds from one subscriber account to another, all with SMSs, looked like doubling the request rate. And the box was already running at around 90% utilization. It was trime to look at processing priorities, and what else was running on that box. The “what else” was reports. Such as analyzing the effect on sales of a radio advert – in real time. This is important and the information needs to be timely, but does it really matter if the analysis take three minutes instead of thirty seconds? This is where the Resource Manager comes into its own. Not widely used and understood (a lazy DBA can just throw more hardware and Oracle licences at the problem) but in this case perfect. By setting up appropriate priorities, every sales request would respond fast no matter what the workload, and at times the reports would degrade. Just another example of how enabling a couple of advanced features can save the need for more hardware and more licences.
We use cookies and browser activity to improve your experience, personalize content and ads, and analyze how our sites are used. For more information on how we collect and use this information, please review our Privacy Policy .
Database Administrator (DBA) Roles and Responsibilities in the Big Data Age

The database administrator (DBA) plays a key role in many companies. Today’s most valuable companies are the ones that make the best use of data. The database administrator is key to unlocking that value.
The tasks of database administration in the management of data and IT systems is a critical exercise, particularly in a time where data privacy regulation is a hot topic.
Database administrator roles and responsibilities have evolved over time, given the evolution of data types and sources , along with data storage options. Let’s review the current status of this career field and see what the future holds for database administrator roles.

What is a database administrator (DBA)?
A database administrator, or DBA for short, designs, implements, administers, and monitors data management systems and ensures consistency, quality, security, and compliance with rules and regulations.
The role of DBA has evolved into a mission-critical function. A DBA in database management involves the installing, configuring, monitoring, maintaining, and improving the performance of databases and data stores , according to Skills Framework for the Information Age–SFIA 8 . A solution architect may design the overall framework or structure of the databases, but the job of implementing and maintaining belongs to DBA roles.
(Read our data architecture explainer .)
Where do database administrators work?
The database administrator role has a place in nearly every organization, to at least some degree. The industries that employ the most people in database administration include tech fields such as computer systems design and information management.
Many also work in information-intensive businesses such as finance, insurance, and educational services. The management of companies and enterprises as a whole also drives the demand for DBAs.
Database administrator responsibilities
Now that we have defined what a database administrator is, we can take a deeper dive into the responsibilities that might be part of a database administrator job description. The day-to-day activities that a DBA performs may include:
- Creating and maintaining database standards and policies.
- Supporting database design, creation, and testing activities.
- Managing the database availability and performance, including incident and problem management.
- Administering database objects to achieve optimum utilization.
- Defining and implementing event triggers that will alert on potential database performance or integrity issues.
- Performing database housekeeping, such as tuning, indexing, etc.
- Monitoring usage, transaction volumes, response times, concurrency levels, etc.
- Implementing security measures and frameworks, while ensuring compliance.
- Identifying, reporting, and managing database security issues, audit trails, and forensics.
- Designing database backup, archiving, and storage strategy.
- Installing upgrades and patches to various apps.
Are you ready to harness the power of data? See how DataOps with BMC can transform your analytics. ›
What is the average wage for dbas.
Qualified people in this field, depending on the specifics of the various database administrator roles and the DBA tasks they handle, tend to earn on the high side on average. The median salary for a database administrator was $117,450 in May 2023. Those working in companies in the computer systems sector earned almost twice as much as those in educational services.
Database administrator requirements and education

At a bare minimum, database administrator job descriptions seek people who:
- Have a background in computer science, information technology, or engineering.
- Are conversant with structured query language (SQL) and relevant database technologies (whether proprietary or open source.)
- Understand coding and service management to some degree.
Relevant database technologies include SQL Server, MySQL, Oracle, IBM Db2, and MongoDB , among others. You don’t have to be certified in all of them, but a working knowledge of a few of them is required.
The European e-Competence framework ( e-CF ) outlines five associated competencies that the DBA should have. These competences are all proficiency level 3, on a scale of 1 to 5:
| e-CF Area | e-CF Competence | |
|---|---|---|
| Build | Application Development | Acts creatively to develop applications and to select appropriate technical options. Accounts for others’ development activities. Optimizes application development, maintenance, and performance by employing design patterns and by reusing proven solutions. |
| Component integration | Accounts for own and others’ actions in the integration process. Complies with appropriate standards and change control procedures to maintain integrity of the overall system functionality and . | |
| Run | Change Support | Ensures the integrity of the system by controlling the application of functional updates, software, or hardware additions and maintenance activities. Complies with budget requirements. |
| Information and Knowledge Management | Analyzes business processes and associated information requirements and provides the most appropriate information structure. | |
| Manage | Information Security Management | Evaluates security management measures and indicators and decides if compliant to information security policy. Investigates and instigates remedial measures to address any security breaches. |
You will need additional soft skills for success across database administrator roles and responsibilities:
- Business awareness and understanding of business requirements of IT
- Excellent problem-solving and analytical skills
- Good communication, teamwork, and negotiation skills
- Good organizational skills
- Flexibility and adaptability
- Excellent business relationship and user support skills
DBA career development
In mapping your career development plan, the following four areas of responsibility are defined in the Skills Framework for the Information Age (SFIA 8 :
Level 2 Database Administrator (Assist)
You will work under the guidance of someone with more experience, but will have some discretion in planning some of your work and resolving some issues. In this role, you will:
- Assist in database support activities.
- Collaborate with team members in addressing user needs.
- Contribute to issue resolution and discussions.
- Identify your own development opportunities and take advantage of chances to learn more.
Level 3 Database Administrator (Apply)
As you progress in experience and seniority, you will begin to work with greater autonomy. You will meet agreed milestones and will make judgments about responding to issues and when to escalate them to a higher level. DBA tasks at this level include:
- Performing standard database maintenance and administration tasks.
- Usings database management system software and tools to collect performance statistics.
- Interacting with customers, suppliers, and partners.
- Collaborating with others on the team, making substantive contributions.
Level 4 Database Administrator (Enable)
With an understanding of the general direction of your team, you have discretion in deciding what issues and assignments to prioritize. You will manage plans and schedules and keep track of how your team is meeting objectives and targets. People in this role:
- Develop and configure tools to enable automation of database administration tasks.
- Monitor performance statistics and create reports.
- Identify and investigate complex problems and issues and recommend corrective actions.
- Perform routine configuration, installation, and reconfiguration of database and related products.
Level 5 Database Administrator (Ensure, Advise)
You will need to be a self-starter and self-manager in this leadership role. You will be making decisions about assigning work, keeping to deadlines, and managing the budget. A database administrator job description for a DBA at this level may specify that they:
- Identify, evaluate, and manage the adoption of database administration tools and processes, including automation.
- Develop and maintain procedures and documentation for databases.
- Contribute to the setting of standards for definition, security, and integrity of database objects and ensure conformance to these standards.
- Manage database configuration including installing and upgrading software and maintaining relevant documentation.
- Monitor database activity and resource usage.
- Optimize database performance and plan for forecast resource needs.
Experience the power of efficient data workflow orchestration with Control-M! ›
Outlook for dbas.
Demand for data continues to explode and evolve. Jobs in this field are expected to grow by 8% between 2022 and 2032.
The idea of information organized in a logical framework is giving way to unstructured data in the form of text, images, sensor information, audio, and videos. Data generated from more sources, such as mobile devices, ecommerce, internet of things (IoT), artificial intelligence (AI), and social media.
The result is that the title of a “database administrator” is morphing into a “data administrator,” who manages structured (database) and unstructured (big data) data sets .
Since most digital organizations are no longer restricted to transactional data only, the modern DBA must be conversant with file, block and object storage solutions.
And because of the sheer volume of data, as well as the growing reliance on AI and machine learning solutions for data processing, most data is now stored in the cloud. You will need expertise in cloud architectures and technologies, including data lakes and big data solutions like Hadoop .
The rise of DevOps methodology as the preferred model for end-to-end product management means that you need a broad base of knowledge across several areas of specialization. You will likely work in an autonomous environment with platform engineers to develop automated self-service tools. Software developers can use the tools you develop to create the data solutions they require for their applications.
A career in database administration will also require you to add software engineering capabilities to your repertoire.
Leverage automation powered by AI and machine learning to provide world-class data management with BMC AMI Data. ›
Dbas must acknowledge data privacy.
Protecting data has become the focus of regulation across the world, with global enterprises being subject to a web of complex and intersecting laws and requirements.
Failure to comply with regulations, such as the sweeping GDPR laws that protect user/customer privacy rights , puts the reputation and data of your organization at risk and exposes your enterprise to hefty fines. For this reason, data management is now a critical corporate governance imperative.
To better comply with the cybersecurity challenge, various cybersecurity frameworks such as CISA , NIST , and ISO 27001 , and services that automate compliance have emerged. An expanding DBA role includes making sure your organization satisfies their requirements, is set up to prevent cybercrimes, can handle audits, and can handle demands for documentation.
Related reading
- BMC Machine Learning & Big Data Blog
- Top DBA Shell Scripts for Monitoring the Database
- What Is a Database Reliability Engineer (DBRE)?
- DataOps Explained: Understand how DataOps leverages analytics to drive actionable business insights
- Are IBM ® z/OS ® Db2 ® DBAs Vanishing?
- Today’s Best IT/Tech Certifications: A Complete Guide
Explore IT careers, roles, certifications, salaries & more!
This e-book give you a basic understanding of IT jobs, including tips for how to apply and interview for IT positions and how to stay sharp once you’ve embarked on your career.

These postings are my own and do not necessarily represent BMC's position, strategies, or opinion.
See an error or have a suggestion? Please let us know by emailing [email protected] .
Business, Faster than Humanly Possible
BMC works with 86% of the Forbes Global 50 and customers and partners around the world to create their future. With our history of innovation, industry-leading automation, operations, and service management solutions, combined with unmatched flexibility, we help organizations free up time and space to become an Autonomous Digital Enterprise that conquers the opportunities ahead. Learn more about BMC ›
You may also like

Linear Regression with Amazon AWS Machine Learning

Using Stored Procedures in Snowflake

Bias–Variance Tradeoff in Machine Learning: Concepts & Tutorials

How To Use Jupyter Notebooks with Apache Spark

If You Want to Be Data Driven, Pave the Way With DataOps

How To Publish Power BI Reports
About the author.
Joseph Mathenge
Joseph is a global best practice trainer and consultant with over 14 years corporate experience. His passion is partnering with organizations around the world through training, development, adaptation, streamlining and benchmarking their strategic and operational policies and processes in line with best practice frameworks and international standards. His specialties are IT Service Management, Business Process Reengineering, Cyber Resilience and Project Management.
- Questions? 877-634-9222
Case Studies
From small business to large enterprises, see how we’ve helped our clients gain value from their organizational data..
- content_search
Work with Us
Let’s have a conversation about what you need to succeed and how we can help get you there.
Work for Us
Where do you want to take your career? Explore exciting opportunities to join our team.
Database Case Studies
Database administration.
The client is a world-leading developer of clean, sustainable energy solutions. The client required SQL Server consultancy as we have significant experience of supporting high-throughput OLTP environments.
© Koderly 2024. All rights reserved. The Koderly® name and logo is a registered trademark of Koderly Limited. Koderly Limited is registered in England & Wales. Company Reg. No. 4070605 / VAT No. 757 9537 70
Japanese | English

HOME > Case Studies > Database Administration (Enterprise Services Industry)
- Database Administration (Enterprise Services Industry)
We handle database administration tasks on our clients’ projects; these include migrating data, reviewing backup environments, and conducting SQL performance tuning.

- No database administrators at the client company
- Reduced application performance as more data is stored
- Reduced responsiveness and difficulty connecting data with other systems
Our Solution
- Address the aforementioned challenges by working onsite as database administrators for a short period of time.
- Conduct performance tuning with the client's application team.
- Effectively use Oracle's distributed environment features to improve database linkage.

▲ Top of Page
Database Services
It infrastructure concierge services hands-on training services, case studies.
- Database System Migration (Enterprise Services Industry)
- Exadata Infrastructure Buildout (Enterprise Communications Industry)
- Consultation on Building a Production Management System (Enterprise Manufacturing Industry)
- Database System Maintenance and Support (Enterprise Distribution Industry)
- NOW EXPANDING OUR TEAM – SEE JOBS

- Case Studies
Remote database administration is more than a service – it’s a partnership.
- Optimizing SQL Server Performance with VMware vSphere
- How a Midsize Retailer Implemented Modern Analytics with Power BI and SQL Server
- Oracle Database Embedding Case Study (220kb)
- Lawson Migration to Db2 Case Study (135kb)
- Virtual-DBA Manage and Monitor Case Study (114kb)
- Healthcare Insurance SAAS Data Warehouse Case Study (292kb)
- Retail EDW Implementation Case Study (180kb)
- International Retail Company Db2 Database Support and Management (112kb)
Request More Info
Virtual-DBA uses cookies to personalize content and ads. Privacy Policy

Getting to know us
- Oracle Managed Services
- SQL Server Managed Services
- Google Managed Services
- Oracle Applications
- Oracle APEX
- API Development Services
- Oracle Cloud Infrastructure
- Microsoft Azure
- Google Cloud Platform
- Multi-cloud
- Machine Learning
- Business Intelligence
- Cloud Migration
- Engineered Systems
- Data Security
- Database Architecture
- Disaster Recovery
Managed Services
- Oracle Database
Application Services
- ISV Services
Cloud Services
Data science.
- Artificial Intelligence
Consulting Services
Technology solutions.
- Manufacturing
- Travel and Transport
- Software Vendors
Recent case study:
Oracle ebs cloud deployment.
Consolidating and Migrating assets into Oracle Cloud Infrastructure.
.png?width=250&name=stonewater-logo%20(1).png)
Most Visited Pages
- Oracle Licensing
- Oracle Cloud Calculator
- Oracle Cloud Migration
- Oracle Exadata Services
- Artificial Intelligence Services
- SQL Server Support
- Oracle Support
- Google Cloud Consultancy
- New Application Development
- Azure Virtual Desktop
- Case Studies
- Testimonials
DSP-Explorer acquires leading Oracle Applications Managed Services Provider, Claremont, to further extend its data management capabilities.

- Partner with DSP
- Customer Support Portal
- Customer Resources
The Key Responsibilities of a Database Administrator

What does a Database Administrator (DBA) actually do?
What does your Database Administrator (DBA) do?
Main concerns for Database administrators
A database administrator's (DBA) primary job is to ensure that data is available, protected from loss and corruption, and easily accessible as needed. Below are some of the chief responsibilities that make up the day-to-day work of a DBA. DSP deliver an outsourced DBA service in the UK, providing Oracle Support and SQL Server Support ; whilst mindset and toolset may be different, whether a database resides on-premise or in a Public / Private Cloud, the role of the DBA is not that different.
1. Software Installation and Maintenance
A DBA often collaborates on the initial installation and configuration of a new Oracle, SQL Server etc database. The system administrator sets up hardware and deploys the operating system for the database server, and then the DBA installs the database software and configures it for use. As updates and patches are required, the DBA handles this ongoing maintenance.
And if a new server is needed, the DBA handles the transfer of data from the existing system to the new platform.
2. Data Extraction, Transformation, and Loading
Known as ETL, data extraction, transformation, and loading refers to efficiently importing large volumes of data that have been extracted from multiple systems into a data warehouse environment.
This external data is cleaned up and transformed to fit the desired format so that it can be imported into a central repository.
3. Specialised Data Handling
Today’s databases can be massive and may contain unstructured data types such as images, documents, or sound and video files. Managing a very large database (VLDB) may require higher-level skills and additional monitoring and tuning to maintain efficiency.
4. Database Backup and Recovery
DBAs create backup and recovery plans and procedures based on industry best practices, then make sure that the necessary steps are followed. Backups cost time and money, so the DBA may have to persuade management to take necessary precautions to preserve data.
System admins or other personnel may actually create the backups, but it is the DBA’s responsibility to make sure that everything is done on schedule.
In the case of a server failure or other form of data loss, the DBA will use existing backups to restore lost information to the system. Different types of failures may require different recovery strategies, and the DBA must be prepared for any eventuality. With technology change, it is becoming ever more typical for a DBA to backup databases to the cloud, Oracle Cloud for Oracle Databases and MS Azure for SQL Server .
5. Security
A DBA needs to know potential weaknesses of the database software and the company’s overall system and work to minimise risks. No system is one hundred per cent immune to attacks, but implementing best practices can minimise risks.
In the case of a security breach or irregularity, the DBA can consult audit logs to see who has done what to the data. Audit trails are also important when working with regulated data.
6. Authentication
Setting up employee access is an important aspect of database security. DBAs control who has access and what type of access they are allowed. For instance, a user may have permission to see only certain pieces of information, or they may be denied the ability to make changes to the system.
7. Capacity Planning
The DBA needs to know how large the database currently is and how fast it is growing in order to make predictions about future needs. Storage refers to how much room the database takes up in server and backup space. Capacity refers to usage level.
If the company is growing quickly and adding many new users, the DBA will have to create the capacity to handle the extra workload.
8. Performance Monitoring
Monitoring databases for performance issues is part of the ongoing system maintenance a DBA performs. If some part of the system is slowing down processing, the DBA may need to make configuration changes to the software or add additional hardware capacity. Many types of monitoring tools are available, and part of the DBA’s job is to understand what they need to track to improve the system. 3rd party organisations can be ideal for outsourcing this aspect, but make sure they offer modern DBA support .
9. Database Tuning
Performance monitoring shows where the database should be tweaked to operate as efficiently as possible. The physical configuration, the way the database is indexed, and how queries are handled can all have a dramatic effect on database performance.
With effective monitoring, it is possible to proactively tune a system based on application and usage instead of waiting until a problem develops.
10. Troubleshooting
DBAs are on call for troubleshooting in case of any problems. Whether they need to quickly restore lost data or correct an issue to minimise damage, a DBA needs to quickly understand and respond to problems when they occur.
If you would like to know more about what the Oracle Support & SQL Server Support DBAs do here at DSP-Explorer, then please get in touch with us, or book a meeting...

If you liked this blog, make sure to check out the rest of our blogs here , which include content on Oracle Database, SQL Server, Google Cloud, Open Source Databases, Data Science and much more.
The fine art of being an Invisible DBA Service
The Opportunity Cost of using a Managed DBA Service

Case Studies Examples Scenarios Database System DBMS
Most of the time you see the case studies and scenario-based questions in the Database System (DBMS) paper. Keeping in view, I am sharing with you some of the case study base questions of the database course.
Examples of Case Studies and Scenarios questions from DBMS
- Examples of Case Studies and scenarios from the Database System.
- How you can make a database from the scenario mentioned below.
- How you can normalize the database tables from the case studies mentioned below.
- How to draw the Entity-relationship diagram from the given case study.
- How to draw the Data flow diagram from the case studies mentioned below.
- What database model is suitable for the case studies mentioned below.
- What kind of database users are suitable for the given case study.
- What kind of database redundancies and inconsistencies are possible in the given scenario.
- How You can write SQL Queries on the tables of the mentioned case study.
- Find the possible database keys from the tables of these case studies.
- Suggest the relationships among the tables of the given scenarios.
Vehicle information dissemination system for Cloud Android Project for BCS BSIT MCS BSSE
Gym and Fitness Management System Project IN C# for BCS BSIT MCS BSSE
HR Management System Project in C# and VB.NET for BCS BSIT MCS BSSE
Employees Attendance System via Fingerprint in C# and VB.NET for BCS BSIT MCS BSSE
Pharmacy Record Management System Project in PHP, ASP or C#.NET
Car information System using Android and Arduino final year Project for BSCS BSIT MCS BSSE
Agile File Master App final year project for BSCS BSIT MCS BSSE
Android Messenger App final year project for BSCS BSIT MCS BSSE
Android Call Recorder App final year project for BSCS BSIT MCS BSSE
Music Listening App final year project for BSCS BSIT MCS BSSE
Like mind matches Android application – Final year project for MCS
Financial Helper Using QR/Barcode Scanner Android Final year project for MCS BSCS BSSE
My Grocery List Mobile Application Project in Android
If you are still in reading the more case studies, then you can read 100+ case studies .
Related Posts:
- Case Studies Examples Scenarios OOP
- History of Database System (DBMS)
- Database Management System Architecture MCQs in DBMS
- Leadership Case Studies MCQs
- Ethical Dilemmas and Case Studies MCQs
- DBMS components and functions MCQs in DBMS
You must be logged in to post a comment.
Navigation Menu
Search code, repositories, users, issues, pull requests..., provide feedback.
We read every piece of feedback, and take your input very seriously.
Saved searches
Use saved searches to filter your results more quickly.
To see all available qualifiers, see our documentation .
- Notifications You must be signed in to change notification settings
A comprehensive collection of SQL case studies, queries, and solutions for real-world scenarios. This repository provides a hands-on approach to mastering SQL skills through a series of case studies, including table structures, sample data, and SQL queries.
tituHere/SQL-Case-Study
Folders and files.
| Name | Name | |||
|---|---|---|---|---|
| 1 Commit | ||||

- Database Consulting
- Database Migrations
- Performance Tuning
- Database Development
- SQL Database Consulting
- Security Services
- ETL Solutions
- Backup and disaster recovery
- Fully Managed Database Services
- MS SQL Server
- Managed Cloud
- Managed Security
- IT Support Costs
- IT Consulting
- Security Assessments
- PCI Compliance
- CCPA Compliance
- GDPR Compliance
- SOX Compliance
- Cloud Consulting
- Cloud Migration
- Microsoft Consulting
- Microsoft Teams
- Microsoft Azure
- IT Support Pricing
- GET A QUOTE!
HOW WE HELP OTHERS.
Below you will find just a small sample of the projects we accomplish each year.

Fiberutilities Group — FG
Fiberutilities Group, commonly know as ‘FG’ is a complete suite of offerings for medical and hospital locations. They provide network services, device inventory and management, and lastly the ability to help hospitals qualify for federal funding and subsidy. Everconnect worked with FG leadership and development team to ingest and process MANY different types of invoices.…

Leading Edge Admin
Leading Edge Admin is a Third Party Health Benefits Administration. They have access to a critical vendor database for some of their reporting. The issue was that they only had read only and could not create indexes, views, functions and stored procedures in the vendor database. This posed a problem for Leading Edge Admin as…


Barnhardt Manufacturing
Barnhardt Manufacturing has been in the material and upholstery business since the early 1900’s. Everconnect came into help them sink their financial software to their report applications. This required Everconnect to write hundreds of SSIS packages and tune many stored procedures and views in their existing database. Lastly Barnhardt was so comfortable with…

OMNIFlow Water
Omniflow Water was interested in working with Everconnect to setup a IOT data warehouse. This consisted of Azure hosting and configuration. Everconnect utilized Tuya APIs to collect IOT data from their water devices, create a database warehouse and built dashboards (and reports) for all the device info using PowerBI. Everconnect also styled these reports to…

Saint Francis Health System
Saint Francis Health System was interested in a full database inventory. We accomplished this by inventorying 300 database servers and nearly 3000 databases. We identified business owners and decommission candidates. This resulted in saving over 5TB of unneeded space and maintenance. This saves considerable costs and streamlines the Change Control process . BACK TO CASE…

Grandview was interested in working with Everconnect to setup a complete booking database and web application. This consisted of Azure hosting and configuration and importing existing booking data into the new booking system. This allowed Grandview to manage their data more efficiency and ensure reliability. BACK TO CASE STUDIES ShareShareTweetPin

Cforia has created a very impressive suite of finance and collections software. They have a large number of clients and impressive reputation. Everconnect worked on a monumental undertaking of taking one of their SQL Server databases and converting it to PostgreSQL. This includes all of the store procedure, function and view logic. Which consisted of…

Les Concierges
LesConcierges is the world’s premier provider of global concierge services and solutions. Everconnect was contracted to build a custom web application with sophisticated backed end database that could track customer needs, preferences and history. The system was written in MS .net with SQL server database. It interfaced with global travel systems and hospitality systems. Everconnect team built and managed…

Provider Resources INC
Provider Resources, Inc is a government contracted company that provides EHR (electronic health records) and medical coding reviews to the federal government. Everconnect built and developed database systems for PRI. Over the more then 3 year engagement, Everconnect built multiple web and database systems, created SSIS packages, successfully passed PII and PCI audits and managed the…

Skip Smashers
Skip Smashers is a company that was created specifically to provide data to the nation’s top professional people bounty hunters and investigators. Everconnect built the database that Skip Smashers uses today to track and find people evading bail. This database system was built on AWS. It is a multi terabyte encrypted database that tracks historical living data…

Relias Learning
Relias Learning is a corporate and HR training provider. Everconnect was contracted to optimize the database and add features to existing web application that tracks progress, completion and role requirements for all training. Relias had grown quicker then they anticipated and their database and application was suffering due to this fact. Everconnect was able to…

AVG Technologies
AVG Technologies is one of the global leaders in computer anti virus protection. Everconnect was contracted to setup monitoring, alerting and performance tuning for the production database systems. These databases were multi-terabyte and handled hundreds of millions of transactions a day from their over 350 million customers. The Everconnect team monitored and responded to alerts 24/7. …

State of California – CalWin
State of California – CalWin is an agency that provides medical, food and cash assistance to people and families in California. Everconnect came in to construct, execute and manage a project plan for database improvements. This included, ETL, High Availability, Disaster Recovery and database improvements. This was a project that took almost 2 years and…

Essence of Australia
Essense of Australia is a global leader in upscale wedding dresses. This global distribution requires inventory management databases that account for orders, supplies and cost. Everconnect came in to write a large number of SSIS packages to not only process inventory updates, but provide data warehouse metrics to provide aggregated and historical reports. Everconnect successfully…

Sonus Hearing
Sonus Hearing is a hearing aid manufacturer located in Orange CA. Everconnect came in to completely build an application and database to manage their whole process using modern web and database technologies. Prior to this they were using spreadsheets, phone calls and Access Database. This application allowed the company to track inventory attributes, doctor ordering…

Nexus World Services
Nexus World Services is a service provider for the hospitality industry. They provide a platform that allows hotels, travel providers and corporate entities’ to negotiate RFP’s (Request For Proposal). Everconnect came into to enhance their .net web application and SQL server database system. This made their platform more efficient and ultimately saved their customers valuable…

Next Wave Logistics
Next Wave Logistics works to provide solutions to MLM companies. The Everconnect team worked on their CGI platform. (Commission, Genealogy and Incentive). We built tables, stored procedures and SSIS packages to enhance the offerings to the customers. Also, Everconnect built and configured the database servers. To include linked servers and database mirroring. Everconnect successfully administered this environment…

Schoolzilla
Schoolzilla, collects school data and provides multiple-measures dashboard, provides a curated view of school district’s results in near real-time so that they can manage progress toward schools most important goals. Everconnect came on to setup database servers for Schoolzilla in the AWS cloud. This allowed for a High availability and Disaster recovery scenario that would protect customers valuable data.…
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Association between quantity and quality of carbohydrate intake and glaucoma: a cross-sectional study from the NHANES database
Affiliations.
- 1 Department of Ophthalmology, The First Affiliated Hospital of Xinjiang Medical University, 137 Liyushan South Road, Urumqi, 830054, Xinjiang Uygur Autonomous Region, China. [email protected].
- 2 Department of Ophthalmology, The First Affiliated Hospital of Xinjiang Medical University, 137 Liyushan South Road, Urumqi, 830054, Xinjiang Uygur Autonomous Region, China.
- PMID: 39192146
- DOI: 10.1007/s10792-024-03284-6
Background: Glaucoma is a public health problem among the worldwide population. Dietary as a modifiable factor have been reported to be associated with glaucoma. This study aimed to explore the association between quantity and quality of carbohydrate (CH) intake and glaucoma among U.S. adults.
Methods: In this cross-sectional study, data of participants aged ≥ 40 years old were extracted from the National Health and Nutrition Examination Survey (NHANES) 2005-2008. CH intake information were obtained by 24-h dietary recall interview. Glaucoma was defined by regraded disc images. Covariates included demographic information, physical examination, laboratory values, complications and nutrients intake. The weighted univariable and multivariate logistic regression models were used to assess the association between the quantity and quality of CH intake and glaucoma. Subgroup analyses based on the history of hypertension were further assessed the association.
Results: The weighted population included a total of 4789 participants, of whom 119 (2.48%) had glaucoma. After adjusting for age, adrenal cortical steroids, hypertension, chronic kidney diseases, diabetes and energy intake, high quantity (OR = 1.83, 95%CI: 1.08-3.11) and low quality (OR = 0.44, 95CI%: 0.20-0.98) of CH intake were associated with the higher odds of glaucoma. High quantity of CH intake (OR = 2.06, 95%CI: 1.15-3.69) was associated with the high odds of glaucoma in hypertension, while high quality of CH intake (fiber-to-CH ratio: OR = 0.23, 95%CI: 0.06-0.82; CH-to-fiber and fiber-to-added sugars ratio: OR = 0.10, 95%CI: 0.02-0.53) were associated with the lower odds of glaucoma in participants without hypertension.
Conclusion: In NAHNES 2005-2008, higher quantity and lower quality CH intake were associated with the high odds of glaucoma, especially among patients without hypertension. This study provides a theoretical basis for the health management of glaucoma patients from the perspective of dietary intake.
Keywords: Carbohydrate intake; Glaucoma; NHANES database; Quality; Quantity.
© 2024. The Author(s), under exclusive licence to Springer Nature B.V.
PubMed Disclaimer
- Stein JD, Khawaja AP, Weizer JS (2021) Glaucoma in adults-screening, diagnosis, and management: a review. JAMA 325(2):164–174. https://doi.org/10.1001/jama.2020.21899 - DOI - PubMed
- Mohan N, Chakrabarti A, Nazm N, Mehta R, Edward DP (2022) Newer advances in medical management of glaucoma. Indian J Ophthalmol 70(6):1920–1930. https://doi.org/10.4103/ijo.IJO_2239_21 - DOI - PubMed - PMC
- Bourne R, Price H, Taylor H, Leasher J, Keeffe J, Glanville J, Sieving PC, Khairallah M, Wong TY, Zheng Y, Mathew A, Katiyar S, Mascarenhas M, Stevens GA, Resnikoff S, Gichuhi S, Naidoo K, Wallace D, Kymes S, Peters C, Pesudovs K, Braithwaite T, Limburg H (2013) New systematic review methodology for visual impairment and blindness for the 2010 global burden of disease study. Ophthalmic Epidemiol 20(1):33–39. https://doi.org/10.3109/09286586.2012.741279 - DOI - PubMed - PMC
- Magkos F, Tetens I, Bügel SG, Felby C, Schacht SR, Hill JO, Ravussin E, Astrup A (2020) A perspective on the transition to plant-based diets: a diet change may attenuate climate change, but can it also attenuate obesity and chronic disease risk? Adv Nutr 11(1):1–9. https://doi.org/10.1093/advances/nmz090 - DOI - PubMed
- Corsello A, Pugliese D, Gasbarrini A, Armuzzi A (2020) Diet and nutrients in gastrointestinal chronic diseases. Nutrients 12(9):2693. https://doi.org/10.3390/nu12092693 - DOI - PubMed - PMC
Publication types
- Search in MeSH
LinkOut - more resources
- Genetic Alliance
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Voter Guide
- Michigan Politics
- John Carlisle
- M.L. Elrick
- Observer & Eccentric
M-STEP results database 2024: Find scores from your school district here
The Michigan Department of Education released results for the Michigan Student Test of Educational Progress (M-STEP) today .
Students took the test in the spring of the 2023-2024 school year. Students in grades 3-7 take the M-STEP test in English Language Arts (ELA) and math, while students in grade 8 take the PSAT in ELA and math.
Check the M-STEP scores from your school district using the database below (or tap here if you can't see it).
Search for 2024 M-STEP results by district or school
Contact Kristi Tanner: [email protected] and Lily Altavena: [email protected] .
- Open access
- Published: 27 August 2024
Developing a validated methodology for identifying clozapine treatment periods in electronic health records
- Aviv Segev ORCID: orcid.org/0000-0002-9550-3895 1 , 2 , 3 , 4 na1 ,
- Risha Govind ORCID: orcid.org/0000-0001-9925-7866 1 , 5 na1 ,
- Ebenezer Oloyede ORCID: orcid.org/0000-0003-1352-4017 6 , 7 ,
- Hamilton Morrin ORCID: orcid.org/0000-0002-7801-0212 2 , 8 ,
- Amelia Jewell ORCID: orcid.org/0000-0002-0887-2159 1 ,
- Rowena Jones ORCID: orcid.org/0000-0002-3426-1394 9 , 10 ,
- Laura Mangiaterra 1 , 2 , 11 ,
- Stefano Bonora 1 , 2 , 12 ,
- Ehtesham Iqbal ORCID: orcid.org/0000-0001-9477-9745 5 ,
- Robert Stewart ORCID: orcid.org/0000-0002-4435-6397 1 , 5 ,
- Matthew Broadbent 1 &
- James H. MacCabe ORCID: orcid.org/0000-0002-6754-1018 2 , 13
BMC Psychiatry volume 24 , Article number: 584 ( 2024 ) Cite this article
13 Accesses
Metrics details
Clozapine is the only recommended antipsychotic medication for individuals diagnosed with treatment-resistant schizophrenia. Unfortunately, its wider use is hindered by several possible adverse effects, some of which are rare but potentially life threatening. As such, there is a growing interest in studying clozapine use and safety in routinely collected healthcare data. However, previous attempts to characterise clozapine treatment have had low accuracy.
To develop a methodology for identifying clozapine treatment dates by combining several data sources and implement this on a large clinical database.
Non-identifiable electronic health records from a large mental health provider in London and a linked database from a national clozapine blood monitoring service were used to obtain information regarding patients' clozapine treatment status, blood tests and pharmacy dispensing records. A rule-based algorithm was developed to determine the dates of starting and stopping treatment based on these data, and more than 10% of the outcomes were validated by manual review of de-identified case note text.
A total of 3,212 possible clozapine treatment periods were identified, of which 425 (13.2%) were excluded due to insufficient data to verify clozapine administration. Of the 2,787 treatments remaining, 1,902 (68.2%) had an identified start-date. On evaluation, the algorithm identified treatments with 96.4% accuracy; start dates were 96.2% accurate within 15 days, and end dates were 85.1% accurate within 30 days.
Conclusions
The algorithm produced a reliable database of clozapine treatment periods. Beyond underpinning future observational clozapine studies, we envisage it will facilitate similar implementations on additional large clinical databases worldwide.
Peer Review reports
Introduction
Treatment-resistant schizophrenia (TRS) is associated with poor prognosis, long-term disability, and increased mortality [ 1 ]. The introduction of clozapine in the late 1950s provided clinicians with a unique option in the pharmacological treatment of individuals with TRS [ 2 , 3 ]. Despite its discovery many decades ago and the development of many drugs since then, clozapine remains the treatment of choice in TRS due to its superior efficacy [ 4 ]. Current evidence indicates that of the 30% of patients diagnosed with schizophrenia who do not respond to conventional antipsychotics, 50% will respond to clozapine [ 5 ]. Moreover, several studies have shown that clozapine yields the best prognosis versus other antipsychotics, not only for psychiatric clinical scales but also for broader health outcomes, including all-cause mortality [ 6 , 7 , 8 , 9 ].
Unfortunately, despite a considerable evidence-base for therapeutic benefits, clozapine is associated with a range of adverse effects, including potentially life-threatening events such as myocarditis, ileus and blood dyscrasias, mandating regular blood tests [ 10 , 11 ]. As such, there has been much interest in the study of clozapine: basic-science research, that attempts to elucidate the reasons for its superior efficacy or the mechanisms underlying its side effects [ 12 ], clinical and laboratory biomarkers to predict its efficacy [ 13 ] and clinical studies to better understand, detect and manage its adverse events [ 14 , 15 ]. Such insights may help to diminish underutilization of clozapine [ 16 ] and to prevent unnecessary clozapine cessation and the associated increased risk of relapse [ 17 ]. Many of these clinical observational studies rely on small, biased samples, and as such are disadvantaged by low statistical power and uncertainty around the generalizability of findings. In view of this, it is important to enable investigators to reliably study large, unbiased cohorts of patients prescribed clozapine, with accurate data on the dates when treatment was started and stopped.
South London and Maudsley NHS Foundation Trust (SLaM) is one of the largest mental health providers in Europe, catering to all secondary mental health care needs of over 1.3 million people spanning four London boroughs (Lambeth, Southwark, Lewisham, and Croydon). It contains clinical records of over 500,000 patients, including many individuals diagnosed with psychotic spectrum disorders, and patients who were or are currently prescribed clozapine. In the 2000s, SLaM records became digital and complete electronic health records (EHR) became available during 2006. In 2008, data from the SLaM EHR were made available to researchers through the Clinical Record Interactive Search (CRIS), which is a de-identified copy of the entire SLaM EHR [ 18 ]. The granularity of this type of data resource presents valuable opportunities for novel and informative observational studies. However, as with all real-world databases, there is the potential for input errors or missing data. Therefore, when using such data for research, data cleaning, validating, and processing of the desired cohort are required. Overcoming these challenges for clozapine pharmacoepidemiology requires a collaboration of clinicians, familiar with the patterns and protocols surrounding the usage of the medication, alongside informaticians, proficient in handling and analysing real-world big data. This paper describes the rationale, process and heuristics-based algorithms used to create a database of clozapine treatment periods, derived from CRIS at SLaM, to serve as a resource for large-scale retrospective clozapine studies. The generation of this database provides great potential for upcoming observational studies on clozapine. Beyond enabling studies on SLaM users, the heuristics and algorithms outlined in this paper can be adapted, with appropriate modifications, to suit any other extensive clinical database resembling CRIS in terms of data sources on an international scale. Consequently, it will facilitate the development of additional databases on clozapine treatment periods, thereby laying the groundwork for further research in diverse countries and psychiatric services.
The data sources – CRIS and ZTAS databases
The Clinical Record Interactive Search (CRIS), previously described, makes available all SLaM electronic health records for secondary analysis within a robust data security and governance framework [ 19 ].
The Zaponex Treatment Access System (ZTAS) is one of three mandatory blood monitoring service providers in the UK. All patients prescribed clozapine at SLaM are registered with ZTAS [ 20 ]. ZTAS has a database of all the mandatory blood test results and all the clozapine treatment-related statuses (e.g., on-treatment, discontinued etc.) assigned to each patient.
SLaM’s Clinical Data Linkage Service (CDLS) provides a secure data environment that allows CRIS to be linked with other external clinical and non-clinical databases, including ZTAS data, using individual matching but then discarding the identifiers, allowing the data to be made available in the same de-identified format as CRIS [ 21 ].
The linkage between CRIS and ZTAS, facilitated through CLDS, is the foundation of this cohort. The two databases were first linked in May 2016, followed by a refresh in October 2019. Therefore, the time frame for the current study starts with the establishment of ZTAS in 2004, and ends with its most recent linkage to CRIS, in October 2019.
Clinical aspects of clozapine prescription
There are several aspects of clozapine treatment that make it challenging to determine if and when clozapine treatments begin and end from the aforementioned databases. In clinical practice, there is often extensive discussion with the patient and treating team regarding the possibility of starting clozapine, for months or even years before the treatment is started. Thus, relying on natural language processing tools, which have shown success in identifying medications through textual references in medical records, may result in numerous false positives, particularly in the case of clozapine.
Patients may have single or multiple periods of clozapine treatment. Due to the adverse-effects profile of clozapine and its mandatory monitoring, any cessation of clozapine lasting more than 48 h requires re-initiation of the drug and blood monitoring as though for the first time [ 22 ]. Our algorithm aimed to identify each clozapine treatment period, even when several were recorded for the same patient. This was further complicated by the fact that patients may be prescribed clozapine for long periods but with infrequent clinical contacts, so the algorithm must infer whether there was a treatment break between two clinical contacts.
Another complication is that patients are sometimes registered with ZTAS but are ultimately not prescribed clozapine for various reasons (e.g., non-adherence, medical contraindications), or there may be a long delay between registration and receipt of the first dose.
Outline of algorithm
The first step was designed to confirm the validity of the treatment period, meaning that clozapine was indeed administered, rather than just intended to be prescribed. In addition, data were collected to define each treatment period, which involved identifying start- and end-dates. At the second stage, we used data from adjacent periods to further confirm clozapine administration, and to determine when two apparently separated treatments, were in fact one continuous treatment. Three data sources were used for this purpose (described in detail below): i) patients' recorded status, ii) blood test monitoring records, and iii) pharmacy dispensing records.
When devising the algorithm, it was decided to value precision over recall. Thus, the algorithm takes a conservative approach, even at the expense of missing potential treatment periods.
As part of the algorithm development, each heuristic implemented in this algorithm was examined separately. However, the validation and verification of the entire algorithm was done as a whole.
First data source – patient status
In clinical practice, registration with ZTAS is required for clozapine to be dispensed and administered. ZTAS receives notification and grants approval for each initiation of clozapine. When a clozapine treatment ends, the hospital pharmacy will report it to the ZTAS team, and if an additional clozapine treatment attempt is planned, re-registration with ZTAS is required. Possible patient statuses include "on-treatment", "interrupted", "discontinued", "transferred" or "non-rechallengeable" (and several variations of these). A patient’s status changes over time, and the dates of change are recorded, thus a history of dated status changes is stored. Thus, the status of the patient appears at face value to be a relatively robust and reliable dataset.
However, status was found to be inconsistently recorded in practice: some patients had multiple "on-treatment" entries, or multiple redundant "discontinuation" entries, or a confusing sequence of statuses. For example, if a patient's blood test returns with abnormal results, often a status of "interrupted" would appear on that day, as clozapine administration is paused. If an additional abnormal result re-occurs the following day, the patient's status would change to "discontinued". On the same day, or within a few days, usually after consultation between the ZTAS and clinical teams, the status would then change to "discontinued – final", and then "non-re-challengeable". As a result, each clozapine treatment period could be surrounded by many redundant and sometimes contradictory status entries. Accordingly, we classified all possible statuses to one of two groups – start-signals (e.g., "on-treatment") or stop-signals (e.g., "discontinued").
To overcome the problem of multiple and redundant entries, clozapine treatments were initially identified by locating the first start-signal status (per date), either as the first entry for the patient, or following a previous stop-signal. In the same manner, the end of the treatment was identified as the first stop-signal after a previous start-signal. Stop-signals were ignored if on the same day there was an additional start-signal, during an ongoing clozapine treatment period. The periods between the start- and stop-signals were defined as "tentative clozapine treatment periods", that need to be validated and examined. Tentative treatment periods of less than 7 days were excluded from the analysis. The rational for this exclusion stemmed from several reasons: it is likely that such very short treatment periods would not be significant to the study of clozapine; such a short window of treatment is more likely to represent the intention to administer clozapine, without the patient starting the treatment (or taking very few doses); and difficulty to identify markers for an automated verification for clozapine being administered.
There were several reasons why the start-signals and stop-signals could not be considered reliable on their own. Though the start-signals were designed to be assigned at the start of clozapine initiation, relying on patient status had limitations. Patients who were prescribed clozapine prior to the start date of the ZTAS database at our disposal, and who therefore were added to ZTAS during their clozapine treatment, had an inaccurate "start-signal". Similar problems occurred with patients who were registered for SLaM care after a transfer from another Trust in the UK or a different country whilst already receiving clozapine treatment. Another limitation was that the start-signal was an indicator of ZTAS approving a patient's clozapine treatment but did not necessarily indicate that the clozapine treatment was initiated. Delays in clozapine initiation could stem from different reasons, such as a patient’s refusal, physical deterioration, improvement in mental status, etc., and the actual commencement of clozapine dose titration might start weeks after a start-signal appeared in the status field. While clozapine treatment occurred outside the windows defined by the patient start- and stop-signals only in specific circumstances (described later), the presence of the window did not guarantee that clozapine was in fact administered, or that the start-signal corresponded to the actual administration start-date.
Another caveat was clerical errors of omission or commission. Errors of omission were particularly abundant in older patient records, where recording was less systematic. In such cases, a treatment could be evident in the clinical notes but have no preceding start-signal and therefore potentially missed in an algorithm relying on this. Errors of commission included incorrect status entries recorded. An example was a status entry of "transferred", despite the patient's records clearly showing that they remained under the care of SLaM, or "interrupted" despite the clinical records not indicating any problem or change in clozapine administration. Due to these limitations, it was necessary to address and integrate additional datasets.
Second data source – blood test monitoring
Blood monitoring information was used both for confirming the authenticity of the treatment period and for re-affirming actual start-dates. For each tentative clozapine treatment period, we established the pattern of blood test monitoring. To identify these patterns, we relied on the UK mandatory monitoring guidelines, which require weekly blood monitoring for 18 weeks, followed by fortnightly monitoring for an additional 34 weeks, after which monitoring is reduced to a monthly basis until the treatment is stopped [ 22 ]. Using the timing of blood tests, we aimed to identify several possible patterns of monitoring, with the following hierarchy: (1) Sustained weekly pattern (longer than 5 weeks); (2) Short weekly pattern (5 weeks or shorter); (3) Monthly pattern (of over 6 months); and (4) No pattern. The detailed criteria are elaborated in the supplementary material (S1).
ZTAS contains the results of blood tests and the date they were taken, but also the type of blood test in relation to the clozapine treatment period. The blood test that precedes actual administration is defined as "Baseline" (required for ZTAS approval of clozapine treatment). Tests during the clozapine treatment period are named "New". Tests that were entered retrospectively are defined as "Historical". Therefore, we used this information to further verify the actual start-date of the treatment period. When a "Baseline" blood test was recorded ± 10 days from a start-signal, it was regarded as re-affirming the actual start-date (as opposed to artificial start that, a label given to those starting clozapine prior to 2004 or having started this elsewhere prior to being transferred to SLaM).
Blood test monitoring records, when present, were considered a robust source of information. However, several caveats needed to be taken into consideration. The recording of blood tests in ZTAS did not systematically start with the establishment of ZTAS, and for several years was inconsistent between different service providers within SLaM. As such, a lack of blood test records did not mean blood was not taken. An additional problem was that the type of blood test was recorded improperly at times, and a "baseline" test might be labelled as "new". A third problem, limiting our ability to rely on blood test monitoring, was that blood could be drawn several times prior to clozapine initiation (due to the patient changing their mind about treatment, problematic results, etc.), or after clozapine cessation (mainly when attempting to verify neutrophil count normalization following neutropenia, per UK mandatory guidelines). However, the presence of blood test results outside the "treatment window" of start–end signals (as derived from a patient status) would help to detect errors of omission or commission in status records. A common example was lack of status for patients who were entered into ZTAS in the early days of the system when clerical errors were more likely to occur. In these cases, months and even years of repeated blood tests preceded the first status record. Therefore, in cases where blood tests were recorded more than three times prior to the first status, a new tentative "pre-status" treatment period was defined (though start and stop signals were missing). The reason for omitting cases with three or less blood tests was that it was common to see preparatory blood-results before commencing clozapine, preceding the start signal. In addition, analysing the authenticity of these very short periods was extremely challenging. On the other hand, even if those three blood tests represented part of a genuine clozapine treatment period, the inferred treatment period would not have been underestimated substantially. The pre-treatment period was defined as starting at the first blood test and ending at the last blood test preceding the first start-signal. Additional use of blood-monitoring was conducted to ascertain redundant stop signals. As per UK clozapine protocols, clozapine cessation should be followed by four follow-up blood tests. As such, 5 blood tests or less post a stop-signal was considered a per-protocol follow-up. Instances where more than five blood tests were identified outside of a tentative period were flagged and examined manually. Since there were only three such occurrences, it was determined that developing a specific algorithm to analyse these cases would be unnecessary, and thus they were disregarded.
Third data source – pharmacy dispensary records
SLaM Pharmacy records of clozapine dispensing, as with all other medications, are incorporated in the CRIS database and are completed both for inpatients and outpatients. Again, these had face validity as an ideal indication of clozapine administration; however, as with other data resources, they came with several caveats. Pharmacy records began inconsistently during the first years of wider SLaM record digitalization and records were consequently often missing in the first years of CRIS. Moreover, records were sometimes omitted due to technical or human errors. Conversely, dispensary records may exist even in cases where the patient did not receive the prescribed medication, often attributable to reasons such as patient non-compliance, although not limited to this factor alone. Therefore, the dispensary records could only serve as supporting evidence and were not sufficient to be used alone. We regarded dispensary records as re-affirming when at least 3 records of clozapine were recorded at 3 different dates, as a single dispensation might occur when a clozapine treatment did not commence (for example, due to patient reluctance).
Combining the three datasets
Using the described tiers of information, we devised an algorithm (Fig. 1 ) that classified each tentative clozapine treatment period into one of three possible categories:
Clozapine treatment period with identified start-date – in which we could have high certainty both that clozapine was administered and that the inferred start-date was a reliable one.
Clozapine treatment period with undetermined start-date – in which we had enough data to verify, with high level of certainty, that the patient did indeed receive clozapine, but a reliable start-date could not be established. For these treatment periods, there was no valid start-date, but rather a first known date of the treatment. These treatments could have been started only a few days before the first known date or, alternatively, years before it.
Unsubstantiated – In which there were insufficient data to ascertain that clozapine was given.

Clozapine treatment periods categorization per blood tests pattern, baseline type blood tests and pharmacy records
Of note, "pre-status" tentative treatment periods that were discovered and defined by blood tests records (i.e., treatment periods that were not created originally from start and end signals) were addressed during the categorization process in the same manner as status-based treatment periods.
Refining start-dates and end-dates
After the initial classification of treatment periods to these three categories, we implemented further rules to refine the start and end-date of each treatment period, using the treatment period's classification. These refinement rules were created to improve the accuracy of the start-date and the end-date, to overcome clerical errors in the early days of ZTAS, and to improve categorisation. The refinement rules are elaborated in the supplementary material (S2).
Merging clozapine treatment periods
After each treatment period was assigned a category and the start-date and end-date were refined, more information on treatment periods could be inferred using the adjacent periods. We examined the already-existing datasets, alongside the gaps between each period, in order to identify treatment periods that were wrongly identified by the algorithm as two separate periods. We have used clinical heuristics to merge periods, for example:
A clozapine treatment with an identified start-date cannot be a continuation of a previous period, unless it was truncated due to technical error, mandating the gap between the periods to be extremely short, and the first period to be relatively short.
Very short gaps between treatment periods (< 7 days) that do not entail even a short weekly pattern in the following treatment period (sometimes referred as "interrupted protocol"), are unlikely a new period and more often represent an error of commission or technical glitch. A common example would be a patient travelling abroad for more than 30 days and forgetting to send blood results. An "interrupted" status would then be added. Upon the patient's return, if clozapine was administered continuously, the status will change again to "on-treatment", representing the same treatment period, assigned for merging. If the patient stopped clozapine while abroad, the algorithm would recognize the start of a new weekly pattern of blood tests, thus categorizing the new treatment period as one with an identified start-date that would not be merged.
The complete set of rules by which treatment periods were merged are outlined in the supplementary material (S3).
Excluding clozapine treatment periods
Following the merging stage, unsubstantiated treatment periods that were not merged were excluded, and were disregarded in further analyses (except validation). Due to the various indications used, those periods were suspected to be "empty" treatment, meaning that no clozapine was given. These periods remained in the database, unlike the omitted periods, for two reasons:
They might be a focus of interest – for example, concerning the reasons that prevented the administration of clozapine.
Though suspected to be "empty", this impression relied mainly on the continuing lack of corroborative evidence. However, it was assumed that despite the absence of data, some were false negative treatments, i.e. clozapine was given.
Validation of results
After forming the new merged table of all clozapine treatment periods, at least 10% of the treatment periods were randomly selected and manually compared to their full text HER records by an experienced psychiatrist (AS). The accuracy of the start-date, end-date and the classification of the treatment periods were manually verified.
According to the ZTAS database, 2,056 SLaM patients were registered with ZTAS, 41 of whom had never had a blood test or an assigned status. ZTAS recorded 210,173 blood tests and 10,923 statuses for the remaining 2,015 SLaM patients. Patients had a mean of 103 blood tests (SD 76.6, range 1–341), 5 statuses (SD 4.8, range 1–41), and 108 pharmacy dispensaries (SD 98.3, range 1–571).
Figure 2 shows that 3,191 tentative treatment periods were first identified based on the start- and end-signals. An additional 1,241 tentative periods were identified after analysing the blood test data and pharmacy dispensary data. 693 tentative treatments were omitted; 30 (0.9%) were omitted because the period ended within 7 days, and 663 (53.4%) because they were "pre-status" periods with three or fewer blood tests. 510 tentative treatment periods were merged with adjacent periods based on the criteria outlined in Table S3. Glossary of main definitions is listed in Table 1 .

Results of categorization process, per each algorithmic step
After merging, re-categorizing, and refining, there were 3,212 treatment periods. Of these, 425 (13.2%) remained unsubstantiated treatment periods due to insufficient data to confirm that clozapine was given, and therefore were excluded.
In total, the algorithm identified 2,787 clozapine treatment periods: 1,902 (68.2%) with an identified start-date and 885 (31.8%) with an indeterminate start-date.
The 2,787 treatments belonged to 1,916 patients. The mean number of treatment periods per patient was 1.45 (median 1, interquartile range 1). 1,346 (70.0%) patients had only one treatment period, 373 (19.5%) had two, and 197 (10.3%) had 3–8 periods. 65.6% of the patients were male, 45.3% were of White ethnicity, and 41.7% were of Black ethnicity. The mean age at the point of the first known clozapine treatment was 39.0 (SD 12.1). Demographic characteristics of patients and treatment periods are displayed in Table 2 .
The final result of the algorithm was 2,787 clozapine treatment periods, which belonged to one of two categories: treatment periods with identified start dates and treatment periods with undetermined start-dates. Similar to the two types of start-points, the endpoints can also be categorised into two types: treatment periods with identified end-dates and treatment periods with undetermined end-dates. Treatments with identified end-dates are those ending with a clear end signal, for example, a "discontinued" status or the end of blood monitoring. Treatments with undetermined end-dates result from unavailable information due to patients being transferred outside of SLaM or treatments that remained ongoing at the end of the study window (October 2019). The proportions of treatments with each start-point and endpoint type are elaborated in Table 3 .
Validations
The validation process results of the algorithm reliability showed high level of accuracy, both in treatment periods' classification as well as in the determination of the periods' start and end-dates (Table 4 ).
This study describes the development process and implementation of an algorithm designed to identify clozapine treatment dates which can be used by researchers when conducting clozapine observational studies. By combining clinical experience with informatics expertise, we were able to create a complex algorithm relying on multiple datasets, each of which had severe limitations as a standalone source of data, but when judiciously combined, yielded highly accurate results.
The final database, which consisted of 2,787 clozapine treatment periods, can serve as an important resource for clozapine studies exploring its efficacy, safety, adherence, and other research area, which may aid to increase clozapine utilization and to prevent redundant clozapine cessation. The validation and verification process yielded very good results, showing that the carefully, specifically designed automated algorithm was successful in spotting "false" treatment periods, and was able to yield good accuracy in determining the start and stop-dates of each period.
It is common for real-world databases to suffer from missing, redundant, and falsely entered information. Errors are bound to occur, especially when the users contributing to this database are both numerous and heterogeneous in professional background (clinical, administrative, etc.). Prolonged development and implementation processes may further contribute to erroneous entries, as time-based changes yield non-uniform records. The algorithm presented in this study attempts to use both clinical insight as well as data-analysis procedures to overcome as many of these errors as possible.
The authors present this study as an example of what can be achieved through the multidisciplinary process of the algorithm creation, consisting of a continuous joint discussion between informaticians and clinicians. While the latter had brought their clinical expertise along with insight into the reality of clinical practice, the informaticians could translate those insights into the structure of the database and relay the numerous problems back to the clinicians for further exploration and feedback. Both the coding itself and the clinical deliberations were conducted collaboratively throughout the process.
Limitations
The main limitation of the methodology stems from missing data. The start-date was indeterminable for over 30% of the treatments. Despite the interplay between the three datasets and the encouraging validation results, missing data was present in all datasets, leading to misclassification or inaccurate dating. As dates might have shifted and valid treatment periods could have been excluded (or not recorded), epidemiological data should be interpreted with caution.
An additional limitation is the possible truncation of treatment periods. It was the authors’ intention to avoid over-merging (joining two separate treatment periods into one), thus risking over-truncation. The authors felt that future studies that might rely on this database, would preferentially accept possible redundant truncation and missing data, as opposed to falsely assumed data. A simple example is patients who were transferred to other hospital Trusts for periods of time between periods of SLaM care, so a break was recorded in the treatment period, often without available documentation whether clozapine treatment continued seamlessly, or was halted and then renewed. Another prominent example is treatment periods with undetermined start-date, which are recorded shortly after a previous clozapine treatment. One option to consider would be that the truncation is a mere technical fault, and that the two periods are actually a continuum. A second option would be that the subsequent treatment is a new clozapine period, for which the algorithm failed to identify a start-date. When the gap exceeded 2–3 months, a third explanation of a clozapine initiation in a different trust is also possible. To avoid redundant merging, it was decided not to merge these periods with the previous period. Many heuristics were examined in order to differentiate and decipher those instances, but none were proven to be sufficiently reliable. Even though the start-date can sometimes be ascertained over a relatively narrow timeframe (as it must occur after the previous end-date), it was decided to leave those labelled as "undetermined start-date" to mark the uncertainty arising. In several cases, for example, it was found that the previous treatment period had ended somewhat earlier than the attributed end-date, making the in-between-periods gap important enough to address these two treatment periods as separate. For example, a patient that was lost to follow-up and stopped taking clozapine might present to the emergency department, and a registration of "interrupted" status would then be recorded, along with the same day record of "new treatment" due to admission to a psychiatric ward.
Contrary to possible over-truncation of treatment periods, some treatment periods may contain several cycles of titration and re-titration. During validation, several incidents in which monthly blood tests returned to weekly pattern were preserved, suggesting clozapine cessation and re-titration. Sampling clinical notes in those dates showed that some of these cases are indeed interruptions or even "micro-interruptions" (while other may be a response to an abnormal blood test, does escalation, etc.). Future improvements of the algorithms can be devised to detect pattern changes, and better outline these conjoined treatment periods.
The validation process showed that 15% of the excluded treatments were false negative, meaning that clozapine was in fact administered in those periods. Though not many, these windows may still contain valuable information. The treatment periods mislabelled and mistakenly excluded were either too short for blood-pattern recognition or were commenced during the early years of the existence of ZTAS and CRIS early, when data was often not recorded properly.
It should be noted that while the database generated by the algorithm can be applied to various aspects of clozapine research, it is not necessarily representative of all clozapine users. The database includes all patients prescribed clozapine within SLaM, which may differ demographically from populations in other regions of the UK or worldwide. However, the significant over-representation of male patients in our sample has been observed in the UK with similar proportions and in other parts of the world, albeit to a lesser extent [ 23 , 24 ].We believe the implications of our study are twofold. The first, more concrete outcome is the creation of a robust clinical database that can facilitate further observational studies on clozapine. The second, albeit currently less tangible, result is the potential to adapt the heuristics and methodology of our algorithm for use in other large psychiatric services to produce additional clozapine databases. However, achieving this goal necessitates significant adaptations due to regulatory differences between countries [ 25 ], such as variations in blood monitoring frequencies and protocols, as well as differences in database structures, the availability of additional reliable datasets (such as dosage information), or the absence of datasets utilized in our methodology. A future area of interest is the development of an algorithm to identify clozapine-induced adverse effects, particularly in relation to clozapine doses. The development of such a tool can have diverse benefits for ensuring patient safety.
This paper describes a highly tailored algorithm developed through close collaboration between clinicians and data scientists. The combined expertise in clinical practices, particularly regarding the medication of interest, along with proficiency in data acquisition and analysis, facilitated the creation of an extensive database comprising clozapine treatment periods. Consequently, this paper presents two applicable products. Firstly, it introduces the described validated clozapine treatment database. Secondly, it presents a validated methodology for compiling clozapine treatment databases, which can be adapted to other large routine clinical databases, in the UK or globally, with necessary modifications to accommodate varying dispensing and blood monitoring regulations. These databases, as SLaM's clozapine database, may serve as a useful tool for researchers through two approaches. Firstly, it may serve as a platform for large dataset queries, for instance when exploring comparisons with other antipsychotics. Secondly, it may serve as a portal to specific sub-populations, which are often challenging to investigate, enabling the study of rare phenomena or clozapine-specific events. future endeavours should aim to include more detailed data, such as dosage information and adverse events.
Availability of data and materials
The data used in this study is available in the CRIS system, as well as the database created by this study.
However, CRIS data is available to researchers at SLaM only, due to patients' confidentiality. Access to it require authorization from SLaM BRC (Biomedical Research Center).
Kane JM, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019;80:18com12123.
Article PubMed Google Scholar
Crilly J. The history of clozapine and its emergence in the US market: a review and analysis. Hist Psychiatry. 2007;18:39–60.
Mortimer AM. Antipsychotic treatment in schizophrenia: Atypical options and NICE guidance. Eur Psychiatry. 2003;18:209–19.
Siskind D, Mccartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209:385–92.
Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62:772–7.
Article PubMed PubMed Central Google Scholar
McEvoy JP, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600–10.
Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96.
Article CAS PubMed Google Scholar
Vermeulen JM, et al. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1–12.5 Years. Schizophr Bull. 2019;45:315–29.
Hayes RD, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41:644.
Jakobsen MI, Schaug JP, Nielsen J, Simonsen E. Antipsychotic prescribing practices for outpatients with schizophrenia and reasons for non-clozapine treatment - Data from a Danish quality assessment audit. Nord J Psychiatry. 2023;77:481–90.
Legge SE, et al. Reasons for discontinuing clozapine: a cohort study of patients commencing treatment. Schizophr Res. 2016;174:113–9.
de Bartolomeis A, et al. Clozapine’s multiple cellular mechanisms: What do we know after more than fifty years? A systematic review and critical assessment of translational mechanisms relevant for innovative strategies in treatment-resistant schizophrenia. Pharmacol Ther. 2022;236:108236.
Samanaite R, et al. Biological predictors of clozapine response: a systematic review. Front Psychiatry. 2018;9:327.
Mijovic A, MacCabe JH. Clozapine-induced agranulocytosis. Ann Hematol. 2020;99:2477–82.
Article CAS PubMed PubMed Central Google Scholar
Segev A, et al. Clozapine-induced myocarditis: electronic health register analysis of incidence, timing, clinical markers and diagnostic accuracy. Br J Psychiatry. 2021;219:644–51.
Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69:224–7.
Leucht S, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–71.
Stewart R, et al. The South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLAM BRC) case register: development and descriptive data. BMC Psychiatry. 2009;9:51.
Fernandes AC, et al. Development and evaluation of a de-identification procedure for a case register sourced from mental health electronic records. BMC Med Inform Decis Mak. 2013;13:71.
ZTAS, Zaponex Treatment Access System. https://www.ztas.co.uk/ . Accessed 1 Aug 2024.
Perera G, et al. Cohort profile of the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLaM BRC) Case Register: current status and recent enhancement of an Electronic Mental Health Record-derived data resource. BMJ Open. 2016;6:e008721.
Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 14th ed. West Sussex: Wiley-Blackwell; 2021.
Wellesley Wesley E, et al. Gender disparities in clozapine prescription in a cohort of treatment-resistant schizophrenia in the South London and Maudsley case register. Schizophr Res. 2021;232:68–76.
Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Geographic and clinical variation in Clozapine use in the United States. Psychiatr Serv. 2014;65:186–92.
Oloyede E, et al. Clozapine haematological monitoring for neutropenia: a global perspective. Epidemiol Psychiatr Sci. 2022;31:e83.
South London and Maudsley Biomedical Research Centre Case Register - Health Research Authority. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/south-london-and-maudsley-biomedical-research-centre-case-register-3/ . Accessed 1 Aug 2024.
Download references
Acknowledgements
This paper represents independent research part funded by the NIHR Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. RS is part-funded by: i) the NIHR Maudsley Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King’s College London; ii) the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust; iii) UKRI – Medical Research Council through the DATAMIND HDR UK Mental Health Data Hub (MRC reference: MR/W014386); iv) the UK Prevention Research Partnership (Violence, Health and Society; MR-VO49879/1), an initiative funded by UK Research and Innovation Councils, the Department of Health and Social Care (England) and the UK devolved administrations, and leading health research charities. JHM receives funding from the National Institute for Health Research (NIHR) NIHR131157, NIHR150308, NIHR131175, and the Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Author information
Aviv Segev and Risha Govind contributed equally to this work.
Authors and Affiliations
NIHR Biomedical Research Centre, South London and Maudsley NHS Foundation Trust, London, UK
Aviv Segev, Risha Govind, Amelia Jewell, Laura Mangiaterra, Stefano Bonora, Robert Stewart & Matthew Broadbent
Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK
Aviv Segev, Hamilton Morrin, Laura Mangiaterra, Stefano Bonora & James H. MacCabe
Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
Shalvata Mental Center, Hod Hasharon, Israel
Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK
Risha Govind, Ehtesham Iqbal & Robert Stewart
Pharmacy Department, South London and Maudsley NHS Foundation Trust, London, UK
Ebenezer Oloyede
Department of Psychiatry, University of Oxford, Oxford, UK
Maudsley Training Programme, South London and Maudsley NHS Foundation Trust, London, UK
Hamilton Morrin
Birmingham and Solihull Mental Health Foundation Trust, Birmingham, UK
Rowena Jones
Institute for Mental Health, University of Birmingham, Birmingham, UK
Atkinson Morley Regional Neurosciences Centre, St. George’s Hospital, London, UK
Laura Mangiaterra
Department of Health Sciences, Università Degli Studi Di Milano, Milan, Italy
Stefano Bonora
National Psychosis Unit, South London and Maudsley NHS Trust, Beckenham, Kent, UK
James H. MacCabe
You can also search for this author in PubMed Google Scholar
Contributions
AS, RG and JM initiated and designed the study. AS, RG co-developed the algorithm. EO and JM assisted in devising the clinical heuristics. EI assisted in devising the algorithms. AJ designed and implemented the founding databases. AS, HM, RJ, LM and SB validated the results of the algorithm. RS, MB and JM has supervised and consulted the database creation. AS, RG, EO and HM drafted the manuscript. AJ, RS, MB and JM critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Aviv Segev .
Ethics declarations
Ethics approval and consent to participate.
The CRIS data, including the linked data used in this manuscript, has received research ethics approval for secondary analyses (Oxford REC C, reference 18/SC/0372). The CRIS Oversight Committee ensures that research conducted using health records is ethical and legal, and service users can opt-out of their CRIS data being used for research [ 26 ].
Consent for publication
Not relevant to the current manuscript.
Competing interests
RS declares research support received within the last 3 years from Janssen, GSK and Takeda. JHM has received investigator-initiated research funding from H Lundbeck. He has received research funding for clinical trials from H Lundbeck and Karuna Therapeutics. He has participated in advisory boards for H Lundbeck, LB Pharma, Newron Pharmaceuticals and Teva UK Ltd.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Segev, A., Govind, R., Oloyede, E. et al. Developing a validated methodology for identifying clozapine treatment periods in electronic health records. BMC Psychiatry 24 , 584 (2024). https://doi.org/10.1186/s12888-024-06022-5
Download citation
Received : 18 May 2024
Accepted : 14 August 2024
Published : 27 August 2024
DOI : https://doi.org/10.1186/s12888-024-06022-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Schizophrenia
BMC Psychiatry
ISSN: 1471-244X
- General enquiries: [email protected]
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
- Open access
- Published: 27 August 2024
Association between ursodeoxycholic acid use and COVID-19 in individuals with chronic liver disease: a nationwide case-control study in South Korea
- Sang Yi Moon 1 , 2 na1 ,
- Minkook Son 2 , 3 na1 ,
- Yeo Wool Kang 1 ,
- Myeongseok Koh 1 ,
- Jong Yoon Lee 1 &
- Yang Hyun Baek 1
Virology Journal volume 21 , Article number: 202 ( 2024 ) Cite this article
Metrics details
Conflicting evidence exists regarding the effects of ursodeoxycholic acid (UDCA) on coronavirus disease 2019 (COVID-19). This study investigates the association between UDCA administration and COVID-19 infection and its related outcomes in individuals with chronic liver disease (CLD).
A customized COVID-19 research database ( n = 3,485,376) was created by integrating data from the National Health Insurance Service (NHIS) and the Korea Disease Control and Prevention Agency’s COVID-19 databases. The study focused on patients diagnosed with COVID-19 in 2021, using the NHIS data from 365 days before diagnosis. To create comparable groups with and without UDCA administration before COVID-19, we used propensity score matching. The primary endpoint was the first confirmed positive result for severe acute respiratory syndrome coronavirus-2. In addition, we identified severe COVID-19-related outcomes. Subgroup analysis were conducted based on the dose of UDCA exposure.
Data from 74,074 individuals with CLD was analyzed. The participants’ average age was 57.5 years, and 52.1% (19,277) of those in each group were male. Those with prior UDCA exposure had a significantly lower risk of COVID-19 infection (adjusted OR: 0.80, 95% CI [0.76–0.85]) compared to the non-UDCA group. Additionally, the UDCA group had a lower risk of severe COVID-19 outcomes (adjusted OR: 0.67, 95% CI [0.46–0.98]). Subgroup analyses indicated that there was a decrease in COVID-19 infection and its related outcomes with increasing UDCA exposure dose.
Conclusions
Our large observational study highlights the potential use of readily available UDCA as an adjunctive therapy for COVID-19 in individuals with CLD.
Introduction
Since its declaration as a pandemic by the World Health Organization (WHO) in March 2020, COVID-19 has posed a significant challenge to public health, social stability, and the economy [ 1 , 2 ]. Notably, various factors, such as chronic comorbidities, complications, and demographics, can affect the outcomes of COVID-19 [ 3 , 4 ]. Specifically, individuals with chronic liver disease (CLD), particularly cirrhosis, have higher rates of morbidity and mortality from COVID-19 [ 5 , 6 , 7 , 8 , 9 ]. Vaccines and medications have been developed to reduce infection rates and prevent progression to severe disease; however, there is still a need for safer, more effective, and more accessible treatment options for individuals with CLD due to the limited duration of vaccine protection and potential side effects of medications [ 10 , 11 , 12 , 13 ].
Efforts were made to identify therapeutic targets through drug repurposing shortly after COVID-19 was declared a pandemic, leading to research into a prophylactic treatment approach by modulating angiotensin-converting enzyme 2 (ACE2), a critical host receptor of the virus [ 14 ]. Brevini et al. showed that ursodeoxycholic acid (UDCA), which has the farnesoid X receptor (FXR) antagonistic effects, downregulates ACE2 expression in experiments using animals and donor organs unsuitable for transplantation [ 15 ]. However, subsequent real-world retrospective studies on the relationship between UDCA intake and COVID-19 outcomes have yielded mixed results, with some studies showing positive effects [ 16 , 17 , 18 ] and others showing no significant impact [ 19 , 20 , 21 , 22 ].
This study explored the association between UDCA consumption and COVID-19 within a tailored South Korean COVID-19 cohort of 3,485,376 participants (including 580,896 COVID-19 cases and 2,904,480 controls). The investigation prioritized assessing the effects of UDCA consumption on COVID-19 susceptibility and its consequent outcomes among individuals with CLD within the cohort, while accounting for both the presence or absence of UDCA intake and its dosage, if applicable.
Data source and study population
A specialized COVID-19 research database was established for this investigation. This extensive repository amalgamates data from two primary origins: the National Health Insurance Service (NHIS) database, encompassing medical claims data for 97% of the Korean populace, and the database on COVID-19 confirmations and vaccinations administered by the Korea Disease Control and Prevention Agency [ 23 ]. The NHIS database furnishes a plethora of information, encompassing details regarding diagnoses, prescriptions, procedures, surgeries, insurance disbursements, and healthcare utilization for both inpatients and outpatients. It also incorporates invaluable health screening data, such as laboratory tests, physical measurements, and self-reported questionnaires concerning lifestyle habits. A tailored database was curated, incorporating data from patients diagnosed with COVID-19 between 2020 and 2021, alongside fivefold the number of controls matched for both sex and age with the diagnosed patients.
To ensure clear and efficient data analysis, our analysis only included patients diagnosed with COVID-19 between January 1, 2021, and December 31, 2021, due to the lack of definitive information on COVID-19 diagnosis dates in 2020. The date of diagnosis was defined as the index date, and only participants with NHIS data available from 365 days before the index date were included, particularly for health screening data. COVID-19-related outcomes were monitored until March 31, 2022, which customized the COVID-19 research database provided. Additionally, we utilized the International Classification of Diseases, 10th Revision (ICD-10) codes to differentiate between CLD subtypes. Finally, we matched the COVID-19 and control groups based on propensity scores. Moreover, to investigate the association between UDCA and COVID-19-related outcomes, we extracted individuals with CLD and COVID-19 and matched the event and control groups based on propensity scores.
UDCA exposure
UDCA exposure data encompassing UDCA prescription details (daily dose and duration of prescription) for the 365 days preceding the index date were retrieved. Cumulative exposure metrics, specifically cumulative defined daily dose (cDDD) and cumulative exposure duration (cED), were computed for each participant utilizing the World Health Organization’s established daily defined dose (DDD) of 750 mg/day for UDCA [ 24 ]. For analytical purposes, participants were stratified into two cohorts: those with prior UDCA exposure and those without. Moreover, participants were further segmented based on UDCA exposure duration using cDDD and cED. The study cohort was delineated into subgroups characterized by exposure durations of in less than one month (0.75 × 30 = 22.5 for cDDD, 30 days for cED), ≥ 1 month to < 3 months (0.75 × 90 = 67.5 for cDDD, 90 days for cED), and ≥ 3 months.
The primary endpoint encompassed the initial occurrence of a positive outcome for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) utilizing reverse transcriptase–polymerase chain reaction (RT-PCR) assays conducted on nasopharyngeal or oropharyngeal swabs. Apart from the principal outcome, we explored various other complications associated with COVID-19 as secondary endpoints. These included mortality attributable to COVID-19, instances of cardiopulmonary resuscitation (M15, M587), the requirement for mechanical ventilation (M585, M5860), renal replacement therapy (O70), extracorporeal membrane oxygenation (O190), and admission to an intensive care unit for critical care (AJ).
Demographic information, including age, sex, and income level, was extracted, with income level divided into four quartiles. Underlying diseases (hypertension, diabetes, and dyslipidemia) were assessed based on diagnoses recorded in the NHIS database up to 1 year prior to the COVID-19 diagnosis. Moreover, the Charlson comorbidity index (CCI) was utilized to gauge the burden of comorbidities [ 25 ]. CLD diagnoses were categorized as chronic viral infection, chronic liver disease, or liver cirrhosis utilizing ICD-10 codes [ 26 ]. Evaluated medications included those for hypertension and diabetes, statins, aspirin, antivirals for chronic hepatitis B, and hepatoprotective agents. Health screening results encompassed body mass index (BMI), systolic and diastolic blood pressure, fasting blood glucose, hemoglobin, glomerular filtration rate (GFR), and liver enzyme levels (aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transpeptidase). Additionally, participants’ current smoking status, alcohol consumption, and regular exercise habits were assessed via a self-reported questionnaire. Study participants were deemed vaccinated against COVID-19 if they had received at least one dose of any vaccine type. Supplementary Table 1 offers further details about the extracted covariates.
Statistical analysis
Baseline characteristics were expressed as mean ± standard deviation for continuous variables and as numbers with percentages (%) for categorical variables. Propensity score matching (PSM) was conducted at a 1:1 ratio, encompassing multiple covariates, such as sex, age, income level, underlying diseases, CCI, COVID-19 vaccination status, medications, BMI, systolic and diastolic blood pressure, fasting blood glucose, hemoglobin levels, GFR, liver enzyme levels, smoking status, alcohol consumption, and regular exercise habits. Exact matching was employed for sex, chronic viral infection, chronic liver disease, liver cirrhosis, COVID-19 vaccination status, antivirals for chronic hepatitis B, and hepatoprotective agents. However, greedy nearest neighbor matching was utilized for other variables, with a caliper set at 0.01 of the propensity scores. The standardized mean difference before and after PSM was utilized to assess the balance of covariate distribution between groups. Subsequently, odds ratios (ORs) and 95% confidence intervals (CIs) were computed through conditional logistic regression analysis post-matching. Additionally, multivariate-adjusted conditional logistic regression analysis was performed, incorporating the covariates. Statistical analyses were executed using SAS Enterprise Guide version 8.3 (SAS Institute Inc., Cary, NC, USA) and R 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria). A significance level of P < 0.05 was considered statistically significant.
Study population
This study utilized a customized COVID-19 research database comprising 3,485,376 participants, including 580,896 confirmed COVID-19 cases and 2,904,480 control participants. After excluding individuals with missing demographic information ( n = 73,857), prior COVID-19 infection before 2021 ( n = 34,819), incomplete health screening data within 1 year of the index date ( n = 2,321,109), or missing health screening data ( n = 6,368), 1,049,223 participants remained. From among these remaining participants, individuals diagnosed with CLD using ICD-10 codes were then identified ( n = 287,863).
PSM was employed to explore the association between UDCA exposure and COVID-19 infection. This technique matched participants with CLD in a 1:1 ratio to those with COVID-19 ( n = 37,037) and control groups ( n = 37,037) (Table 1 ). Subsequently, to investigate the relationship between UDCA exposure and COVID-19-related outcomes, another 1:1 PSM was performed within the previously matched group to separate participants with and without COVID-19-related outcomes (Baseline characteristics in Supplementary Table 2 ). The schematic diagram for this case-control study is presented in Fig. 1 . Table 1 presents the comparison results of the characteristics of the COVID-19 and control groups before and after PSM.
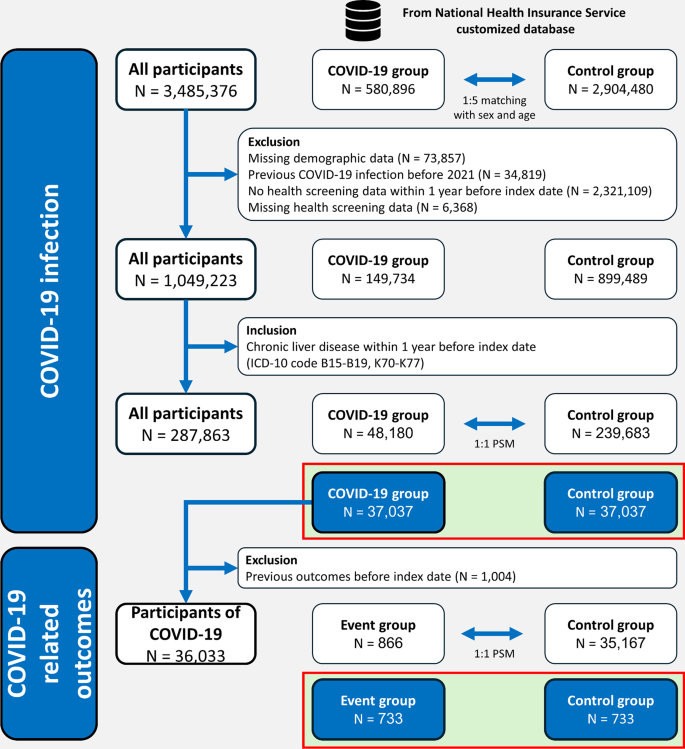
The flow of study population
COVID-19 infection according to UDCA exposure
Table 2 displays the OR and 95% CIs for COVID-19 infection in relation to UDCA exposure. Participants exposed to UDCA exhibited an adjusted OR of 0.80 for COVID-19 infection (95% CI [0.76–0.85], P-value < 0.001) compared with those in the non-exposure group. Upon stratification based on UDCA dose (with the cDDD < 22.5 group as the reference), the adjusted OR was 0.86 (95% CI [0.80–0.93], P-value < 0.001) for the 22.5 ≤ cDDD < 67.5 group and 0.83 (95% CI [0.77–0.90], P-value < 0.001) for the cDDD ≥ 67.5 group. Analogous outcomes were observed when analysing according to cED.
COVID-19-related outcomes according to UDCA exposure
Table 3 presents the association between UDCA exposure and COVID-19-related outcomes. Participants with UDCA exposure had an adjusted OR of 0.67 for COVID-19-related outcomes (95% CI [0.46–0.98], P-value: 0.04) compared with the non-exposure group. Following an analysis based on UDCA dose (using the cDDD < 22.5 group as reference), the adjusted OR was 0.89 (95% CI [0.50–1.58], P-value: 0.68) for the 22.5 ≤ cDDD < 67.5 group and 0.48 (95% CI [0.27–0.88], P-value: 0.02) for the cDDD ≥ 67.5 group. Similar results were found when an analysis based on cED was done. A forest plot analysis was employed to illustrate the findings presented in Tables 2 and 3 (Supplementary Fig. 1 ).
Sensitivity analysis
This study examined data from 365 days before the COVID-19 diagnosis, and the sensitivity analysis used data from 180 days before the COVID-19 diagnosis (Supplementary Tables 3 , 4 ). The sensitivity analysis was consistent with the main results.
A nationwide population-based cohort study utilizing a tailored COVID-19 research database encompassing 3.4 million individuals was employed to ascertain COVID-19 infection and its associated outcomes concerning UDCA exposure and dosage after PSM following the identification of individuals with CLD. Findings revealed a favorable correlation between COVID-19 infection and its related outcomes in the exposed group compared with the unexposed group (reference) (COVID-19 infection, adjusted OR: 0.80, 95% CI [0.76–0.85]; COVID-19-related outcomes, adjusted OR: 0.67, 95% CI [0.46–0.98]). To our knowledge, this study represents the most comprehensive investigation to date into the association between UDCA and COVID-19.
Prevention of COVID-19 is crucial for individuals with CLD. These individuals face an increased risk of severe complications from COVID-19, with those having cirrhosis experiencing particularly poor outcomes [ 5 , 6 , 7 , 8 , 9 ]. This is supported by findings from the National COVID Cohort Collaborative Study and the Veterans Affairs healthcare system, both of which independently reported that COVID-19 infection raises the risk of death within 30 days by 2.38 and 1.7 times, respectively, in individuals with cirrhosis compared to those without [ 6 , 9 ]. To further explore this association, we conducted a comparative analysis of COVID-19 infection and its outcomes between individuals with CLD and those with cirrhosis across our entire cohort, utilizing our customized COVID-19 research database. Our results align with previous studies [COVID-19 infection: without liver disease (reference); CLD, 1.11 ( p < 0.001); cirrhosis, 1.01 ( p = 0.88). COVID-19 related outcomes: without liver disease (reference); CLD, 1.79 ( p < 0.001); cirrhosis, 2.75 ( p < 0.001)] (Supplementary Table 5 ) [ 6 , 9 , 27 ]. Impairments in the complement system, macrophage activation, lymphocyte and neutrophil function, upregulated Toll-like receptors, and intestinal dysbiosis contribute to the increased susceptibility of individuals with CLD to viral infections. These factors trigger cytotoxic T-cell activation and dysregulation of the innate immune response, ultimately leading to liver damage and increased mortality [ 5 , 7 , 8 ].
UDCA is a well-established first-line treatment for primary biliary cholangitis [ 28 , 29 , 30 ]. It stimulates bile acid secretion and has shown immunomodulatory and anti-inflammatory effects in experimental studies. It also reduces oxidative stress and protects liver cells from apoptosis [ 31 , 32 , 33 , 34 , 35 , 36 ]. Recent research has identified a potential role for bile acids like UDCA in regulating COVID-19 infection, with a focus on the ACE2 receptor, which is a critical entry point for SARS-CoV-2 [ 37 , 38 ]. Experimental studies suggest that bile acids can act on this pathway in multiple ways: (1) hindering viral entry by disrupting the interaction between ACE2 and the spike protein, (2) influencing ACE2 activity, and potentially (3) regulating ACE2 expression [ 15 , 39 , 40 ]. Additionally, bile acids have shown promise in modulating the cytokine storm, an essential factor in the development of acute respiratory distress syndrome (ARDS), a severe complication of COVID-19 [ 41 , 42 ]. UDCA has a favorable safety profile and few side effects, making it a potential treatment to prevent infection and mitigate disease progression in patients with COVID-19 [ 43 ].
In a landmark decision on May 5, 2023, the WHO declared COVID-19 was no longer a global public health emergency, marking a turning point after a grueling 3-year battle [ 44 , 45 , 46 ]. This shift signifies that COVID-19 will transition from a pandemic to an endemic, managed alongside other prevalent illnesses. Factors contributing to this decision include rising herd immunity due to vaccination and natural infection, a reduced burden on healthcare systems, and decreased overall disease severity [ 44 , 45 , 46 ]. However, the WHO’s declaration does not signal the complete eradication of COVID-19. The emergence of new variants and the potential decline in vaccination rates pose significant challenges to ongoing management efforts [ 47 , 48 ]. Therefore, continued vigilance is essential. This is especially crucial for patients with pre-existing medical conditions that may make them more vulnerable to COVID-19 or for those living in low-income countries with low vaccination rates [ 49 ]. In such instances, UDCA can be used as an additional treatment to vaccines and conventional medications, and it has been proven to be affordable and accessible [ 50 , 51 ].
Limitations and strengths
This study has some limitations. First, the population’s demographic composition is predominantly from a single ethnic group. Second, the study cohort consisted mainly of individuals with CLD because UDCA was prescribed primarily to this group in South Korea. Therefore, it is difficult to explain the relationship between UDCA and COVID-19 in non-CLD groups, and the baseline characteristics of the study population differ from those of the general population. Third, identifying individuals with CLD relied solely on ICD-10 codes, which may not be perfectly accurate. Additionally, the available medical records only covered approximately 2 years. Fourth, there may be discrepancies between UCDA’s prescribed and actual usage. Patients with higher prescription rates and frequent hospital visits might focus more on preventing COVID-19, potentially influencing result interpretations [ 52 , 53 ]. Fifth, although various factors were adjusted for, misclassification and residual confounding factors may still be present. Sixth, the study did not obtain results regarding SARS-CoV-2 variants or reinfections. However, despite these limitations, we found a positive association between UDCA intake and COVID-19 infection and its related outcomes among 74,074 individuals with CLD who underwent PSM. Specifically, the analysis, stratified by the level of UDCA intake using data from the year before COVID-19 infection, revealed that higher UDCA intake, rather than simply its presence or absence, was associated with more beneficial effects. Unlike COVID-19 vaccines and medications, UDCA does not need to be re-studied for adverse effects, and its relatively low cost and accessibility make it feasible even in developing countries.
In reporting this study, we do not prioritize supplementing research findings with randomized controlled trials (RCTs), as is often suggested to complement observational studies. Conducting RCTs to investigate the association between UDCA and COVID-19 in the current situation, unlike during past severe pandemics, is unrealistic and of little significance. However, we aim to provide helpful information for patients with limited access to COVID-19 vaccines and medications by reporting positive outcomes of UDCA intake in patients with CLD using a large observational study. In addition, we hope that this study will contribute to the discussion of UDCA administration in situations with viruses similar to SARS-CoV-2 in the future [ 54 ].
This large-scale observational study has shown that UDCA can reduce COVID-19 infection and its related outcomes in individuals with CLD. These findings suggest that the readily available UDCA could be a valuable addition to the treatment regimens of individuals with CLD susceptible to COVID-19.
Data availability
No datasets were generated or analysed during the current study.
Bedford J, Enria D, Giesecke J, Heymann DL, Ihekweazu C, Kobinger G, Lane HC, Memish Z, Oh MD, Sall AA, et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015–8.
Article CAS PubMed PubMed Central Google Scholar
Naseer S, Khalid S, Parveen S, Abbass K, Song H, Achim MV. COVID-19 outbreak: impact on global economy. Front Public Health. 2022;10:1009393.
Article PubMed Google Scholar
Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855.
Choi YJ, Park JY, Lee HS, Suh J, Song JY, Byun MK, Cho JH, Kim HJ, Park HJ. Variable effects of underlying diseases on the prognosis of patients with COVID-19. PLoS ONE. 2021;16:e0254258.
Baldelli L, Marjot T, Barnes E, Barritt AS, Webb GJ, Moon AM. SARS-CoV-2 infection and liver disease: a review of Pathogenesis and outcomes. Gut Liver. 2023;17:12–23.
Article CAS PubMed Google Scholar
Ioannou GN, Liang PS, Locke E, Green P, Berry K, O’Hare AM, Shah JA, Crothers K, Eastment MC, Fan VS, Dominitz JA. Cirrhosis and severe Acute Respiratory Syndrome Coronavirus 2 infection in US veterans: risk of infection, hospitalization, Ventilation, and Mortality. Hepatology. 2021;74:322–35.
Spearman CW, Aghemo A, Valenti L, Sonderup MW. COVID-19 and the liver: a 2021 update. Liver Int. 2021;41:1988–98.
Marjot T, Webb GJ, Barritt ASt, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–64.
Ge J, Pletcher MJ, Lai JC. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487–e15011485.
Moon AM, Webb GJ, García-Juárez I, Kulkarni AV, Adali G, Wong DK, Lusina B, Dalekos GN, Masson S, Shore BM, et al. SARS-CoV-2 infections among patients with Liver Disease and Liver Transplantation who received COVID-19 vaccination. Hepatol Commun. 2022;6:889–97.
Cromer D, Steain M, Reynaldi A, Schlub TE, Khan SR, Sasson SC, Kent SJ, Khoury DS, Davenport MP. Predicting vaccine effectiveness against severe COVID-19 over time and against variants: a meta-analysis. Nat Commun. 2023;14:1633.
Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, De Rosa R, Corcione A, Durante-Mangoni E. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14:881–3.
Wong GL, Hui VW, Yip TC, Lui GC, Hui DS, Wong VW. Minimal risk of Drug-Induced Liver Injury with Molnupiravir and Ritonavir-Boosted Nirmatrelvir. Gastroenterology. 2023;164:151–3.
Gaziano L, Giambartolomei C, Pereira AC, Gaulton A, Posner DC, Swanson SA, Ho Y-L, Iyengar SK, Kosik NM, Vujkovic M, et al. Actionable druggable genome-wide mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27:668–76.
Brevini T, Maes M, Webb GJ, John BV, Fuchs CD, Buescher G, Wang L, Griffiths C, Brown ML, Scott WE, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature. 2023;615:134–42.
Li Y, Zhu N, Cui X, Lin Y, Li X. Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease. Front Cell Infect Microbiol. 2023;13:1178590.
John BV, Bastaich D, Webb G, Brevini T, Moon A, Ferreira RD, Chin AM, Kaplan DE, Taddei TH, Serper M, et al. Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis. J Intern Med. 2023;293:636–47.
Hu L, Zhang H, Huang C, Shen T, Feng Z, Mu F, Xu L, Lin Y, Yue C, Guo K, et al. Effect of Ursodeoxycholic Acid on preventing SARS-CoV-2 infection in patients with liver transplantation: a multicenter retrospective cohort study. Qjm; 2023.
Colapietro F, Angelotti G, Masetti C, Shiffer D, Pugliese N, De Nicola S, Carella F, Desai A, Ormas M, Calatroni M et al. Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients. Viruses : 2023, 15.
Liu T, Wang JS. Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children. Liver Int. 2023;43:1950–4.
Marrone G, Covino M, Merra G, Piccioni A, Amodeo A, Novelli A, Murri R, Pompili M, Gasbarrini A, Franceschi F. Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: a retrospective study of propensity score-matched cohorts. Liver Int. 2024;44:83–92.
Corpechot C, Verdoux M, Frank-Soltysiak M, Duclos-Vallée JC, Grimaldi L. Exploring the impact of ursodeoxycholic acid therapy on COVID-19 in a real-word setting. J Med Virol. 2024;96:e29418.
Nation Health Insurance Data Sharing Service. 2024. [ https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do ]
ATC/DDD, Index. 2024. [ https://atcddd.fhi.no/atc_ddd_index/code=A05AA02 ]
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82.
Lee SR, Lee HJ, Choi EK, Han KD, Jung JH, Cha MJ, Oh S, Lip GYH. Direct oral anticoagulants in patients with Atrial Fibrillation and Liver Disease. J Am Coll Cardiol. 2019;73:3295–308.
Fan VS, Dominitz JA, Eastment MC, Locke ER, Green P, Berry K, O’Hare AM, Shah JA, Crothers K, Ioannou GN. Risk factors for testing positive for severe Acute Respiratory Syndrome Coronavirus 2 in a National United States Healthcare System. Clin Infect Dis. 2021;73:e3085–94.
Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 Practice Guidance from the American Association for the study of Liver diseases. Hepatology. 2019;69:394–419.
Lee HA, Chang Y, Sung PS, Yoon EL, Lee HW, Yoo J-J, Lee Y-S, An J, Song DS, Cho YY, et al. Therapeutic mechanisms and beneficial effects of non-antidiabetic drugs in chronic liver diseases. Clin Mol Hepatol. 2022;28:425–72.
Article PubMed PubMed Central Google Scholar
Chang J-I, Kim JH, Sinn DH, Cho J-Y, Kim KM, Oh JH, Park Y, Sohn W, Goh MJ, Kang W, et al. Clinical outcomes and validation of Ursodeoxycholic Acid Response scores in patients with Korean primary biliary cholangitis: a Multicenter Cohort Study. Gut Liver. 2023;17:620–8.
Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012;36(Suppl 1):S3–12.
Oh AR, Bae JS, Lee J, Shin E, Oh BC, Park SC, Cha JY. Ursodeoxycholic acid decreases age-related adiposity and inflammation in mice. BMB Rep. 2016;49:105–10.
Takigawa T, Miyazaki H, Kinoshita M, Kawarabayashi N, Nishiyama K, Hatsuse K, Ono S, Saitoh D, Seki S, Yamamoto J. Glucocorticoid receptor-dependent immunomodulatory effect of ursodeoxycholic acid on liver lymphocytes in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G427–438.
Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–747.
El-Sherbiny GA, Taye A, Abdel-Raheem IT. Role of ursodeoxycholic acid in prevention of hepatotoxicity caused by Amoxicillin-clavulanic acid in rats. Ann Hepatol. 2009;8:134–40.
Mueller M, Castro RE, Thorell A, Marschall HU, Auer N, Herac M, Rodrigues CMP, Trauner M. Ursodeoxycholic acid: effects on hepatic unfolded protein response, apoptosis and oxidative stress in morbidly obese patients. Liver Int. 2018;38:523–31.
Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–19.
Fiorucci S, Urbani G, Biagioli M, Sepe V, Distrutti E, Zampella A. Bile acids and bile acid activated receptors in the treatment of Covid-19. Biochem Pharmacol 2023:115983.
Carino A, Moraca F, Fiorillo B, Marchianò S, Sepe V, Biagioli M, Finamore C, Bozza S, Francisci D, Distrutti E, et al. Hijacking SARS-CoV-2/ACE2 receptor Interaction by Natural and semi-synthetic Steroidal agents acting on functional pockets on the receptor binding domain. Front Chem. 2020;8:572885.
Fiorillo B, Marchianò S, Moraca F, Sepe V, Carino A, Rapacciuolo P, Biagioli M, Limongelli V, Zampella A, Catalanotti B, Fiorucci S. Discovery of bile acid derivatives as potent ACE2 activators by virtual screening and essential dynamics. J Chem Inf Model. 2022;62:196–209.
Ko WK, Lee SH, Kim SJ, Jo MJ, Kumar H, Han IB, Sohn S. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS ONE. 2017;12:e0180673.
Abdulrab S, Al-Maweri S, Halboub E. Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID-19-associated cytokine storm. Med Hypotheses. 2020;143:109897.
Prayitno K, Bhat M. Repurposing UDCA, an FXR inhibitor, to prevent SARS-Cov-2 infection. Gastroenterology. 2023;164:1019–20.
Contreras S, Iftekhar EN, Priesemann V. From emergency response to long-term management: the many faces of the endemic state of COVID-19. Lancet Reg Health Eur. 2023;30:100664.
Burki T. WHO ends the COVID-19 public health emergency. Lancet Respir Med. 2023;11:588.
Sarker R, Roknuzzaman ASM, Hossain MJ, Bhuiyan MA, Islam MR. The WHO declares COVID-19 is no longer a public health emergency of international concern: benefits, challenges, and necessary precautions to come back to normal life. Int J Surg. 2023;109:2851–2.
Hadj Hassine I. Covid-19 vaccines and variants of concern: a review. Rev Med Virol. 2022;32:e2313.
Sohan M, Hossain MJ, Islam MR. The SARS-CoV-2 Omicron (B.1.1.529) variant and effectiveness of existing vaccines: what we know so far. J Med Virol. 2022;94:1796–8.
Barouch DH. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med. 2022;387:1011–20.
Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009;360:1981–8.
Roy DN, Biswas M, Islam E, Azam MS. Potential factors influencing COVID-19 vaccine acceptance and hesitancy: a systematic review. PLoS ONE. 2022;17:e0265496.
Park HJ, Byun MK, Kim HJ, Ahn CM, Rhee CK, Kim K, Kim BY, Bae HW, Yoo KH. Regular follow-up visits reduce the risk for asthma exacerbation requiring admission in Korean adults with asthma. Allergy Asthma Clin Immunol. 2018;14:29.
Park HJ, Byun MK, Kim T, Rhee CK, Kim K, Kim BY, Ahn SI, Jo YU, Yoo KH. Frequent outpatient visits prevent exacerbation of Chronic Obstructive Pulmonary Disease. Sci Rep. 2020;10:6049.
Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, Baghbanzadeh M, Aghamohammadi N, Zhang W, Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020;49:717–26.
Download references
Acknowledgements
Not applicable.
This research was supported by Dong-A University Research Fund.
Author information
Sang Yi Moon and Minkook Son contributed equally to this work as first authors.
Authors and Affiliations
Department of Internal Medicine, Dong-A University College of Medicine, 32 Daesingongwon-ro, Seo-gu, Busan, 49201, South Korea
Sang Yi Moon, Yeo Wool Kang, Myeongseok Koh, Jong Yoon Lee & Yang Hyun Baek
Department of Data Sciences Convergence, Dong-A University Interdisciplinary Program, Busan, South Korea
Sang Yi Moon & Minkook Son
Department of Physiology, Dong-A University College of Medicine, Busan, South Korea
Minkook Son
You can also search for this author in PubMed Google Scholar
Contributions
Sang Yi Moon and Minkook Son contributed equally to this work as first authors. Dr. S. Moon, M. Son, and Y. Baek had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: S. Moon, M. SonAcquisition, analysis, or interpretation of data: S. Moon, M. Son. Drafting of the manuscript: S. Moon, M. Son. Critical review of the manuscript for important intellectual content: Y, Kang, Y. Baek. Statistical analysis: M. Son. Administrative, technical, or material support: Y. Baek. Supervision: Y. Baek. All authors reviewed the manuscript.
Corresponding author
Correspondence to Yang Hyun Baek .
Ethics declarations
Ethics approval and consent to participate.
The Dong-A University College of Medicine Institutional Review Board exempted this retrospective study from review due to its design (utilizing de-identified, publicly available clinical data for analysis) (DAUHIRB-EXP-23-026).
Consent for publication
Additional information.
This study used the database of the KDCA and the NHIS for policy and academic research. The research number of this study is KDCA-NHIS-2023-1-567. The KDCA is the Korea Disease Control and Prevention Agency, Republic of Korea. The NHIS is the National Health Insurance Service, Republic of Korea.
Competing interests
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Moon, S.Y., Son, M., Kang, Y.W. et al. Association between ursodeoxycholic acid use and COVID-19 in individuals with chronic liver disease: a nationwide case-control study in South Korea. Virol J 21 , 202 (2024). https://doi.org/10.1186/s12985-024-02464-1
Download citation
Received : 22 May 2024
Accepted : 07 August 2024
Published : 27 August 2024
DOI : https://doi.org/10.1186/s12985-024-02464-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Virology Journal
ISSN: 1743-422X
- Submission enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Since 2008, SkillBuilders has trained Brown University developers and Oracle Administrators in new skills vital to the Universities success. This includes Oracle Database performance tuning, new features, RMAN, Enterprise Manager / Grid Control and Grid Infrastructure. For developers SkillBuilders has provided education in Java Programming ...
A database administrator, or DBA for short, designs, implements, administers, and monitors data management systems and ensures consistency, quality, security, and compliance with rules and regulations. The role of DBA has evolved into a mission-critical function. A DBA in database management involves the installing, configuring, monitoring ...
Here are some topics related to Database Administration that you can study: Database management systems (DBMS): Learn about different types of DBMS, their features, and how to choose the right one for specific use cases. ... Learn the importance of regularly backing up databases and techniques to recover data in case of failures or disasters ...
Solutions. Analytics & Business Intelligence. Application Development & Integration. Application Implementation & Management. Application Performance Management. Cloud Migration & Management. Database Administration. Infrastructure Management & SysAdmin.
In this track you'll learn the key skills you need to set-up, design, maintain, and monitor your team's database. Dive in and learn the key SQL Server skills you need to securely manage access and monitor database performance using SQL server. SQL 24hrs 6 courses Statement of Accomplishment.
Read database case studies including database administration. SQL Server health check, and SQL Server Emergency Support. Koderly has 22+ years' experience ...
One of the challenges in database administration is to maintain a database with an optimized data structure. In large corporations where there are many databases, the database administrator (DBA) must execute a database structure analysis routine. In this context, this article presents a case study applied in the Brazilian Public Defender's Office (DPU) in Brazil, where there was a reduction ...
HOME > Case Studies > Database Administration (Enterprise Services Industry). Database Administration (Enterprise Services Industry) We handle database administration tasks on our clients' projects; these include migrating data, reviewing backup environments, and conducting SQL performance tuning.
The responsibilities of a database administrator include: • Ensuring that the database is up and running all the time. • Pre-implementation tasks such as feasibility study, capacity planning ...
Case Studies. Remote database administration is more than a service - it's a partnership. How a Midsize Retailer Implemented Modern Analytics with Power BI and SQL Server. Oracle Database Embedding Case Study (220kb) Lawson Migration to Db2 Case Study (135kb) Virtual-DBA Manage and Monitor Case Study (114kb)
Jon Cowling 05-Feb-2016 15:32:15. A database administrator's (DBA) primary job is to ensure that data is available, protected from loss and corruption, and easily accessible as needed. Below are some of the chief responsibilities that make up the day-to-day work of a DBA. DSP deliver an outsourced DBA service in the UK, providing Oracle Support ...
Virtualisation: A case study in database administration laboratory work Greg Cranitch and Michael Rees School of Information Technology Bond University, Australia This paper discusses the issues involved in using virtual machines to teach database administration concepts and the associated issues in a university student environment.
A database administrator (DBA) is a specialized computer systems administrator who's responsible for all activities related to maintaining an organization's database and keeping data secure. A DBA's priority is maintaining data integrity, keeping it available to users and preventing unauthorized access. They're responsible for understanding and ...
Most of the time you see the case studies and scenario-based questions in the Database System (DBMS) paper. Keeping in view, I am sharing with you some of the case study base questions of the database course. Examples of Case Studies and Scenarios questions from DBMS. Examples of Case Studies and scenarios from the Database System.
A comprehensive collection of SQL case studies, queries, and solutions for real-world scenarios. This repository provides a hands-on approach to mastering SQL skills through a series of case studies, including table structures, sample data, and SQL queries. - GitHub - tituHere/SQL-Case-Study: A comprehensive collection of SQL case studies, queries, and solutions for real-world scenarios.
Everconnect came on to setup database servers for Schoolzilla in the AWS cloud. This allowed for a High availability and Disaster recovery scenario that would protect customers valuable data.…. 1 2 3. This case study is a true story of how a database was created, maintained and used to solve a problem.
A case study involving database administration How database administration helps with making business decisions; Practice Exams. Final Exam Computer Science 303: Database Management Status: ...
This study aimed to explore the association between quantity and quality of carbohydrate (CH) intake and glaucoma among U.S. adults. Methods: In this cross-sectional study, data of participants aged ≥ 40 years old were extracted from the National Health and Nutrition Examination Survey (NHANES) 2005-2008. CH intake information were obtained ...
Check the M-STEP scores from your school district using the database below (or tap here if you can't see it). Search for 2024 M-STEP results by district or school
Background Clozapine is the only recommended antipsychotic medication for individuals diagnosed with treatment-resistant schizophrenia. Unfortunately, its wider use is hindered by several possible adverse effects, some of which are rare but potentially life threatening. As such, there is a growing interest in studying clozapine use and safety in routinely collected healthcare data. However ...
One of the challenges in database administration is to maintain a database with an optimized data structure. In large corporations where there are many databases, the database administrator (DBA) must execute a database structure analysis routine. In this context, this article presents a case study applied in the Brazilian Public Defender's Office (DPU) in Brazil, where there was a reduction ...
Conflicting evidence exists regarding the effects of ursodeoxycholic acid (UDCA) on coronavirus disease 2019 (COVID-19). This study investigates the association between UDCA administration and COVID-19 infection and its related outcomes in individuals with chronic liver disease (CLD). A customized COVID-19 research database (n = 3,485,376) was created by integrating data from the National ...