- Library Catalogue

Formatting your thesis: Tables, figures, illustrations

On this page
Tables, figures, illustration requirements and tips, table specifications, figures and other image specifications, image resolution and formatting, using images and objects from other publications.
- Include captions/titles/headings for tables, figures, and other illustrations as paragraph text. This allows captions and headings to be populated into the Table of Contents (ToC) or the lists that appear after the ToC.
- The maximum width for objects on a portrait page is 6 inches (15.24 cm).
- Text wrapping should be set to “In Line with Text” (no wrapping).
- notes, if any
- Source notes or footnotes for tables/figures/illustrations are inserted manually. Insert the note in the paragraph directly below the table or figure.
- Font: Arial Narrow 11pt (default), Arial Narrow 10pt [minimum size].
- To change the font or line spacing for tables see the Thesis Template Instructions .
- Font: Text in image files should follow the overall Font Specifications and be large enough to be read when inserted into the document. The font in images should appear to be the same size as the text in your thesis.
- For example, an image 6 inches wide should be 1800 pixels wide to produce an equivalent resolution of 300 ppi. 6 inches X 300ppi = 1800px.
- For best results, insert images as flattened .tif, .png, or high quality .jpg files.
- Crop as closely as possible around the image to remove blank space and maximize the size. This can be done in Word or in an image editor like Photoshop or Fireworks.
- Landscape images on a portrait page should be rotated with the top of the image to the left.
If your thesis incorporates images, photos, maps, diagrams, etc., not created by you, copyright permission must be obtained from the copyright holder of those works to use their content within your thesis. A copy of each permission must be uploaded to the Thesis Registration System.
See Copyright at SFU for instructions on how to obtain copyright permissions.

- Mardigian Library
- Subject Guides
Formatting Your Thesis or Dissertation with Microsoft Word
- Tables and Figures
- Introduction
- Copyright Page
- Dedication, Acknowledgements, & Preface
- Headings and Subheadings
- Citations and Bibliography
- Page Numbers
- Rotated (Landscape) Pages
- Table of Contents
- Lists of Tables and Figures
- List of Abbreviations
- Some Things to Watch For
- PDF with Embedded Fonts
Tables and figures
Many theses include tables and figures. Most often, they are added to the thesis as images, but sometimes you might want to add some as a linked Excel file. And, the way that captions are added to figures and tables differs between APA and IEEE style. The videos below are lengthy, so they start with a table of contents so you can jump to the section that you need.
Tables and figures - IEEE style
This video demonstrates a modified IEEE style for tables and figures that most CECS students use.
- << Previous: Page Numbers
- Next: Rotated (Landscape) Pages >>
- Last Updated: Mar 21, 2024 2:35 PM
- URL: https://guides.umd.umich.edu/Word_for_Theses
Call us at 313-593-5559
Chat with us
Text us: 313-486-5399
Email us your question

- 4901 Evergreen Road Dearborn, MI 48128, USA
- Phone: 313-593-5000
- Maps & Directions
- M+Google Mail
- Emergency Information
- UM-Dearborn Connect
- Wolverine Access
- Directories
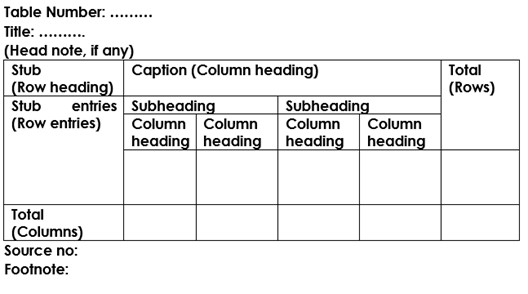
- A figure is a graphic illustration of information, such as a line drawing, a graph, a map, a photograph, a plate, or a chart.
- A table is a graphic that contains a systematic arrangement of facts or numbers in rows and columns.
- All figures and tables must be numbered and have a descriptive caption, including figures and tables in the appendix.
- All figures and tables must be listed in the List of Figures or List of Tables.
- Figure numbers and captions appear below the figure.
- Table numbers and captions appear above the table.
- Captions are always single spaced, including the entry in the List of Figures or List of Tables.
- Do not make the mistake of choosing one style for your table captions and another style for your figure captions (i.e. do not make your table captions flush with the left margin and make your figure captions centered on the page).
- Do not split a caption over 2 pages. If the caption will not fit, you will need to select one of the options below to fill the white space or handle oversize figures/tables.
- Captions must be in the same font (e.g., Times Roman)as the main text of the thesis.
- Captions must be the same size font (i.e.10-12 point) as the main text of the thesis (do not make captions smaller).
- Captions are NOT in bold font
- Captions are NOT in italic font
Numbering a figure or table
- Figures and tables are numbered consecutively throughout the text of the thesis.
- For example, The 1st figure in chapter 2 would be Figure 2.1 & the next figure would be Figure 2.2, etc.
- Related figures may be identified either by using the same number with a lower case letter (Figure 4.3a, Figure 4.3b), or by different numbers (Figure 4.3, Figure 4.4).
- Numbers for figures or tables that appear in appendices are preceded by the capital letter identifying the appendix, as in Figure A.3 or Table C.2.
- Do not make the mistake of numbering your figures and tables in the main text as 2.1, then numbering your figures and tables in the appendices as 2-1.
Placing a figure or table
- More than one table or figure may appear in sequence on a single page, if they represent sequential information.
- Use consistent line spacing to separate the figure or table from the preceding and following text.
- When caption text is too close to the main text, it can be difficult for the reader to tell the difference between the caption and the main text.
- Text must come before and after figures and tables, not top, around the side and under.
- Figures and tables must be placed one after the other (top of page to bottom of page)
- the caption for that table or illustration is centered (horizontally and vertically) alone on a separate preceding page.
- followed on the next page by the full-page table or figure
- first part will be labeled with (a) + the full caption (i.e. Figure 2.1a + caption)
- Do not split a single part (one image) figure over multiple pages.
- first part will be labeled normally (i.e. Table 2.1 + full caption
- each consecutive page will be labeled with the Table + number + Continued (i.e. Table 2.1 Continued). If the table lists sources, the sources appear at the bottom of the first page.
- Stand alone figures or tables must be centered on the page both horizontally and vertically.
- More than one figure or table on a page does not make a stand alone figure or table, so if you have more than one figure or table on the page, you they either need to fill the page, or you need to add main text to the page.
- A figure or table too large to fit within the 6- inch by 9-inch text area may be reduced, but its caption font must be the same size and style as that used for the text in the rest of the thesis.
- A large figure or table that will not fit on a page and is not integral to the thesis may be uploaded as a supplemental file.
Landscape Figures and Tables
- Figures and tables that are formatted wider than they are high may be turned to fit on the page within all margins (landscape mode).
- The entire illustration, including its caption and sources, is turned so that the top of the illustration is parallel to and just inside the regular binding-side text margin (landscape orientation).
- The page number should be placed at the bottom of the figure or table, on the 11”side of the page (landscape orientation).
- This allows for optimum viewing on a computer screen.
Referring to a figure or table in the text
- All figures and tables included in the thesis must be referred to in the text of the thesis. The first reference in the text to a figure or table must precede it.
- If you cannot place a figure or table immediately after the text referencing the figure/table, tell the reader the page where figure/table can be found. (i.e. See Figure 7.1 on page 10)
- If two or more figures are referred to consecutively on one page, then they must follow on the page or the next pages consecutively.
- The text reference should identify a figure or table by number (e.g., write, “See Figure 7.1”), rather than by a relative location (e.g. do not write, “In the following figure . . .”).
The Graduate College at the University of Illinois at Urbana-Champaign
Figures and tables.
Refer to the Sample (Straight Numbering) or Sample (Decimal Numbering) pages as you read through this section.
A thesis may include tables, figures, photographs, musical examples, charts, graphs, line drawings, maps, and other illustrative materials. In addition, a thesis may include statements such as definitions, corollaries, lemmas, theorems, propositions, and schemes. For the following discussion about numbering and placement, these items will be called figures.
- All figures must fit within the minimum 1-inch margins. Theses with any material extending beyond these margins will not be accepted for deposit.
- Figures must be numbered consecutively throughout the entire thesis. Students may choose from one of two options:
- A straight sequence (1, 2, 3, etc.). (Using this method, continue figure numbering from the previous chapter. Do not re-start the numbering at 1.)
- The decimal system (1.1, 1.2, 1.3, 2.1, 2.2, etc.), in which the first digit corresponds to the chapter number and the digit after the decimal point is the figure number within the chapter. (Do not number figures by section (e.g., 1.2.1, 1.3.2, or the like. Using this method, figures in Appendix A, B, C, and so forth would be numbered A.1, A.2, B.1, B.2, C.1 and so on.)
- Each type of content (figures, tables, etc.) should be numbered independently.
- Figure captions may be single-spaced and are not required to be set in the same font style or size as that of the main text.
- Figure captions should appear on the same page as the figure to which they refer.
Figure Placement
- Figures should not be placed out of the order in which they are numbered.
- If figures are grouped at the end of a chapter or the main text, each figure may be placed on a separate page.
- Multiple figures may be placed on the same page as long as doing so does not compromise their legibility.
- Figures grouped at the end of a chapter are considered a section, the first page of which should display the appropriate section heading (i.e., “Figures”, “Tables”, “Figures and Tables”, etc.).
- Figures grouped at the end of the main text are considered a chapter, the first page of which should display the appropriate chapter title (i.e., “Figures”, “Tables”, “Figures and Tables”, etc.).
- Do not add figure callouts (i.e., <place Figure 2.1 about here>) anywhere in the thesis.
Multiple-Page Figures
- Every page containing part of a multiple-page figure must include at least an abbreviated figure label such as “Figure 2.5 (cont.)”.
- The figure caption is only required to appear once; an abbreviated label may be used on subsequent pages.
Rotated Figures
- Page numbers, headings, captions, and titles may be rotated with the figure.
Color Figures
Use of color in the thesis is acceptable. However, because theses may be converted to black-and-white if they are archived on microfilm or partially reproduced on a black-and-white printer, labels, symbols and other data within figures should be identified through other means than the use of color.
Images, figures and tables
- Important information
Figure setup
Table setup.
- Full citation and copyright statement
- For decoration
- Referring to images
In APA style,
- Tables are visual displays of text or data in columns and rows.
- Figure refers to all illustrations except tables including graphs, photos, screenshots, drawings, maps, infographics and images .
Figures and tables can add visual appeal and make your work more understandable.
All figures and tables you have created, adapted or reproduced in your assignment should be:
- set up in APA style (see Figure setup and Table setup ); and
- referred to in the text of your assignment (e.g. Figure 1 shows...).
If you wish to use an image for decorative purposes only and will not refer to it in your text, see For decoration .
All figures and tables included in your assessments are presumed to be your own work unless you state otherwise. As personal images and data are not retrievable, they do not require a full citation and copyright statement nor an entry in your reference list.
When using other people's images in your work there are important factors to consider. Before using an image from a public website, a book or other source, you need to ensure your use of it falls within any limitations set by the copyright or licence. For more information see Full citation and copyright statement .
If you need to cite an image in your assignment (but not reproduce it), see Referring to images .
For more information about questions to ask when deciding to use an image and how to find images:
- Finding images The Library's guide on finding images for your assignments.
- Copyright for students (La Trobe University) Discover what is protected by copyright and how to decide what materials can be used for your studies.
Title of Figure
[Insert figure here]
Figure number
- All figures are numbered as they appear in-text.
- The word Figure and the figure number are in bold, e.g. Figure 1 .
- Figures are numbered in a separate sequence to any tables.
- If you include a figure in your document you should refer to it in your text, e.g. Figure 1 shows... (not 'figure above' or 'figure below')
- The Title is written in italics below the Figure number .
- Give the figure a short clear descriptive title in Title Case .
- The Note is immediately below each figure.
- A note is included if you need to explain the figure or its contents.
- If the figure (or the data you have used to create the figure) is reprinted or adapted from another source the note must include a full citation and a copyright statement .
For more information on how to set up a figure in APA:
- Figure Setup How to set up a figure using APA 7 formatting, including where to place figures in your assignment.
- Sample figures Sample APA 7 figures.
Title of Table
[Insert table here]
Table number
- All tables are numbered as they appear in-text.
- The word Table and the table number are in bold, e.g. Table 1 .
- Tables are numbered in a separate sequence to any figures.
- If you include a figure in your document you should refer to it in your text, e.g. Table 1 shows... (not 'table above' or 'table below')
- The Title is written in italics below the Table number .
- Give the table a short clear descriptive title in Title Case .
- The Note is immediately below each table.
- A note is included if you need to explain the table or its contents.
- If the table (or the data you have used to create the table) is reprinted or adapted from another source the note must include a full citation and a copyright statement .
For more information on how to set up a table in APA:
- Table setup How to set up a table using APA 7 formatting, including where to place tables in your assignment.
- Sample tables Sample tables in APA 7.
If the figure or table (or data you have used to create it) is reprinted (reproduced) or adapted from another source you must include a full citation and a copyright statement in the Note section.
Full citation
- The full citation is used as an in-text citation and includes all elements of the reference in the order of title , author , year of publication and source , determined by where you reprinted or adapted it from.
- Use From when you are reproducing an image / figure as is (i.e. you haven't made any changes).
- Use Adapted from when you are reproducing an image / figure or data from another source and you have changed it for your own purposes, e.g. put data into a graph or table of your own, joined two images together.
- See the Figure examples and Table examples in the boxes below to see how the full citation should be written if the image or data is coming from an article , book , or webpage .
Copyright statement
- Copyright Year by Name of Copyright holder , e.g. Copyright 2020 by La Trobe University.
- Creative Commons Licence abbreviation (with link) , e.g. CC BY 4.0 .
- In the public domain . This statement can only be used if a resource is no longer subject to copyright. A resource is not 'In the public domain' just because it is freely available on the internet.
You must also include a full citation in your Reference List.
Please note if your work is going to be published , the copyright statement must be followed by the permission statement , e.g. Reprinted [or Adapted] with permission. You can ONLY add a permission statement if permission has been sought and granted. Please refer to your supervisor and or the La Trobe University Copyright Officer for more information.
If you are using an image purely for decorative purposes in a presentation or poster (i.e. you are not referring to it in your text), you may not need to include a figure number, title, note, full citation or copyright statement. This will depend on the licencing details of the image as some images (including personal images) require no attribution . However, it is good practice to add a full citation and the copyright statement or CC licence (i.e. attribution) for anything that is not your own.
The La Trobe University Copyright Officer suggests using images from the following public domain image banks for this purpose however, it is very important to check the licence details of each image:
For further information:
- Clip art or stock images referencing Information regarding the special requirements for using clip art and stock images in APA style assignments.
Source: Publication Manual of the American Psychological Association (7th ed., pp. 346-347).
Figure examples
- From an article
- From a book
- From an image database
- From a webpage
Source: Publication Manual of the American Psychological Association (7th ed., pp. 225 – 250, 389 – 391); APA Style Sample Figures .
Source: Publication Manual of the American Psychological Association (7th ed., pp. 225-250, 389-391); APA Style Sample Figures .
Table examples
Source: Publication Manual of the American Psychological Association (7th ed., pp. 195-224, 389-391 ); APA Style Sample Tables .
- << Previous: Government publications
- Next: Indigenous knowledges >>
- Graduate School
- Current Students
- Dissertation & Thesis Preparation
Formatting Requirements
Tables, figures and illustrations.
Tables, figures, illustrations, and other such items should be identified with the word "Table", "Figure", or other appropriate descriptor, and include a title and/or caption. The title or caption must be included in the List of Tables, List of Figures, or other list.
You must use a consistent format for titles and captions of tables, figures, illustrations, and other such items throughout the thesis.
- lettering in tables and figures should be at least 2 mm high to ensure that the information is easy to read
- tables, figures, illustrations, and other such items must have titles or captions, and must be numbered
- titles or captions can go either above or below the table, figure, or illustration
- headings must be repeated on the second and subsequent pages of tables that split over two pages or more
- tables should be split at an appropriate place, e.g. just before a new subheading
- the format for titles and captions of tables, figures, illustrations, and other such items must be consistent throughout the thesis.
Tables, figures, illustrations and other such items must be numbered consecutively in order of appearance within the thesis.
There are two methods for numbering Tables, Figures and other items:
- sequentially throughout the thesis, e.g. 1, 2, 3…
- chapter number first, then numbered sequentially within each chapter, e.g.:
Tables in Chapter 1: Table 1.1, 1.2, 1.3…
Figures in Chapter 3: Figure 3.1, 3.2, 3.3…
Whichever method you choose, the numbering style must be the same for all numbered items; for example: Table 1.1 and Figure 1.3, or Table 1 and Figure 3, not Table 1 and Figure 1.3.
Tables and figures in the Appendices may be labelled A1, A2, etc.
There are three acceptable locations for tables and figures:
- within the chapter immediately following first reference to them
- grouped at the end of the relevant chapter
- grouped at the end of the thesis before the bibliography
Whichever method you choose, you must be consistent.
If your tables and figures are grouped at the end of the thesis, you must include an entry in the table of contents that directs the reader to their location.
If the caption for a figure, table, etc., will not fit on the same page as its accompanying illustration, place the illustration on a separate page.
Reproducing and Reducing
Copying and/or reducing the size of figures (e.g. charts, drawings, graphs, photographs, maps, etc.) may make certain images illegible. After reduction, all lettering must be large enough to fulfill the font size requirements, and must be clear and readable.
Copyrighted Material
If you remove copyrighted tables, figures, or illustrations from your thesis you must insert the following at the spot where the table, figure, or illustration previously appeared:
- A statement that the material has been removed because of copyright restrictions
- A description of the material and the information it contained, plus a link to an online source if one is available
- A full citation of the original source of the material
See the Theses and Dissertations Guide “ Unable to get Permission? ”
- Why Grad School at UBC?
- Graduate Degree Programs
- Application & Admission
- Info Sessions
- Research Supervisors
- Research Projects
- Indigenous Students
- International Students
- Tuition, Fees & Cost of Living
- Newly Admitted
- Student Status & Classification
- Student Responsibilities
- Supervision & Advising
- Managing your Program
- Health, Wellbeing and Safety
- Professional Development
- Final Doctoral Exam
- Final Dissertation & Thesis Submission
- Life in Vancouver
- Vancouver Campus
- Graduate Student Spaces
- Graduate Life Centre
- Life as a Grad Student
- Graduate Student Ambassadors
- Meet our Students
- Award Opportunities
- Award Guidelines
- Minimum Funding Policy for PhD Students
- Killam Awards & Fellowships
- Policies & Procedures
- Information for Supervisors
- Dean's Message
- Leadership Team
- Strategic Plan & Priorities
- Vision & Mission
- Equity, Diversity & Inclusion
- Initiatives, Plans & Reports
- Graduate Education Analysis & Research
- Media Enquiries
- Newsletters
- Giving to Graduate Studies
Strategic Priorities
- Strategic Plan 2019-2024
- Improving Student Funding
- Promoting Excellence in Graduate Programs
- Enhancing Graduate Supervision
- Advancing Indigenous Inclusion
- Supporting Student Development and Success
- Reimagining Graduate Education
- Enriching the Student Experience
Initiatives
- Public Scholars Initiative
- 3 Minute Thesis (3MT)
- PhD Career Outcomes
- Great Supervisor Week

- Manuscript Preparation
How to Use Tables and Figures effectively in Research Papers
- 3 minute read
- 40.2K views
Table of Contents
Data is the most important component of any research. It needs to be presented effectively in a paper to ensure that readers understand the key message in the paper. Figures and tables act as concise tools for clear presentation . Tables display information arranged in rows and columns in a grid-like format, while figures convey information visually, and take the form of a graph, diagram, chart, or image. Be it to compare the rise and fall of GDPs among countries over the years or to understand how COVID-19 has impacted incomes all over the world, tables and figures are imperative to convey vital findings accurately.
So, what are some of the best practices to follow when creating meaningful and attractive tables and figures? Here are some tips on how best to present tables and figures in a research paper.
Guidelines for including tables and figures meaningfully in a paper:
- Self-explanatory display items: Sometimes, readers, reviewers and journal editors directly go to the tables and figures before reading the entire text. So, the tables need to be well organized and self-explanatory.
- Avoidance of repetition: Tables and figures add clarity to the research. They complement the research text and draw attention to key points. They can be used to highlight the main points of the paper, but values should not be repeated as it defeats the very purpose of these elements.
- Consistency: There should be consistency in the values and figures in the tables and figures and the main text of the research paper.
- Informative titles: Titles should be concise and describe the purpose and content of the table. It should draw the reader’s attention towards the key findings of the research. Column heads, axis labels, figure labels, etc., should also be appropriately labelled.
- Adherence to journal guidelines: It is important to follow the instructions given in the target journal regarding the preparation and presentation of figures and tables, style of numbering, titles, image resolution, file formats, etc.
Now that we know how to go about including tables and figures in the manuscript, let’s take a look at what makes tables and figures stand out and create impact.
How to present data in a table?
For effective and concise presentation of data in a table, make sure to:
- Combine repetitive tables: If the tables have similar content, they should be organized into one.
- Divide the data: If there are large amounts of information, the data should be divided into categories for more clarity and better presentation. It is necessary to clearly demarcate the categories into well-structured columns and sub-columns.
- Keep only relevant data: The tables should not look cluttered. Ensure enough spacing.
Example of table presentation in a research paper
For comprehensible and engaging presentation of figures:
- Ensure clarity: All the parts of the figure should be clear. Ensure the use of a standard font, legible labels, and sharp images.
- Use appropriate legends: They make figures effective and draw attention towards the key message.
- Make it precise: There should be correct use of scale bars in images and maps, appropriate units wherever required, and adequate labels and legends.
It is important to get tables and figures correct and precise for your research paper to convey your findings accurately and clearly. If you are confused about how to suitably present your data through tables and figures, do not worry. Elsevier Author Services are well-equipped to guide you through every step to ensure that your manuscript is of top-notch quality.

- Research Process
What is a Problem Statement? [with examples]

What is the Background of a Study and How Should it be Written?
You may also like.

Make Hook, Line, and Sinker: The Art of Crafting Engaging Introductions

Can Describing Study Limitations Improve the Quality of Your Paper?

A Guide to Crafting Shorter, Impactful Sentences in Academic Writing

6 Steps to Write an Excellent Discussion in Your Manuscript

How to Write Clear and Crisp Civil Engineering Papers? Here are 5 Key Tips to Consider

The Clear Path to An Impactful Paper: ②

The Essentials of Writing to Communicate Research in Medicine

Changing Lines: Sentence Patterns in Academic Writing
Input your search keywords and press Enter.
Guidelines for Using Figures and Tables in Your Research Manuscript [Free Downloadable Guide Included]
- Research Process
- Peer Review
In this article and downloadable resource, you will find information to help you determine what you should do if you would like to republish an image that has been previously published elsewhere by you or by another researcher, or publish an image that is similar to one previously published elsewhere.
Updated on February 13, 2019

If you created a figure or table to support your research, you may think that you are able to use it in as many publications as you want if the data are relevant. That is not always the case, however. Usually, the copyright permissions policies of the original publisher will determine if and how you may use it in future publications.
Use the questions below as a guide, or download this helpful resource created jointly by AJE and the Endocrine Society to find out if you need permission to use a figure and how you should provide attribution.
Are you using an original figure or table?
An original figure or table is one that you created and has not been published. If you would like to include an original figure or table in your manuscript, you do not need to ask permission or use attribution to use it. You can simply include the figure or table with your research manuscript when you submit it to the journal.
Are you using a reproduced figure or table?
A reproduced figure or table is an exact copy of a figure or table that has already been published by a journal or book, both in print and online. The figure may be one that was published in your manuscript, or it may be from another researcher's manuscript. A reproduced figure or table is not an original. To use a reproduced figure or table in a manuscript, you must receive permission from the owner of the copyright of the original figure or table, and you must also include attribution to the original source in your manuscript next to the reproduced figure or table. The owner of the copyright will often be the publisher of the original figure or table. In some journals, such as Endocrine Society journals, figures and tables used in most types of article may not be reproduced or adapted; only in reviews and mini-reviews may reproduced or adapted figures and tables be used.
Are you using an adapted figure or table?
An adapted figure or table is one that looks similar to a figure or table that has been previously published but has been changed slightly. To use an adapted figure or table in your manuscript, you must also obtain permission from the copyright owner (usually the original publisher) and provide attribution to the source that published the original figure or table. This must be done even if you created the original figure or table from which the new one has been adapted.
When you consider what figures and tables you would like to include with your manuscript, using an original figure is always the easiest, and many publishers require original figures for most article types. However, there may be data in a figure or table that has already been published that are essential to the discussion in the manuscript you're preparing. If this happens, and if the publisher allows the use of already published material, you have the option of using a reproduced or adapted version of the original figure or table and should consider which of these three types would work best for what you are communicating. If you decide to use already published material in a reproduced or adapted figure or table, it will be necessary to obtain the appropriate permissions and provide the correct attribution in the legend.

Theresa Somerville, BA
See our "Privacy Policy"
- Thesis & Dissertation Editing
- Books and Journal Articles
- Coaching and Consultation
- Research Assistance
- Quantitative
- Qualitative
- Document Review Service
- Meet The Team
- Client Testimonials
- Join Our Team
- Get In Touch
- Make Payment

APA Style: Tables, Figures, & Appendices
9th September 2020

APA Style: Tables, Figures, & Appendices
It is very likely that you'll be using tables, figures, or appendices in your thesis. While each of these elements is optional, they are meant to improve the readers’ understanding of your thesis’s content. The following tips and pointers on tables, figures, and appendices will help you determine when each should be used, as well as how and where they should appear per APA style.
Tables are especially helpful when presenting a great deal of numerical data at once. A well-organized table can effectively deliver vast amounts of information in an easy-to-read manner. Per APA guidelines , all tables included in your thesis or dissertation should be necessary—if you can deliver the information clearly in the body of your text, you should avoid using a table. Each table should also include at least two columns (vertical axis) or rows (horizontal axis) per APA style (i.e. if your data can be presented in a single row or column, it should be delivered in paragraph form instead of a table). Tables are meant to supplement your content, which means that all tables must be referenced within the narrative of your paper. Explain how each table relates to your content and be sure to highlight what the reader will learn from looking at the table. Additionally, a reader should be able to make sense of your table without having read your text, so be sure to define all abbreviations and symbols in a note beneath the data. Your tables should be numbered sequentially and each one should appear as near to where it is mentioned in the text as possible. Additionally, do not let your table span more than one page, if possible. Formatting your table:
- The table may be single- or double-spaced, but consistency across all tables is key.
- Each table must have a brief title explaining its contents left aligned, below the table number, italicized and in title case.
- Every column must have a brief heading.
- Vertical lines are not permitted per APA formatting style. Only horizontal lines may be used to improve readability.
- All numerical data must be presented consistently (use the same number of decimal places and unit of measurement for each column).
- Write the word “Note:” below the table to provide abbreviation meanings and probability level values.
Example of a properly formatted table:
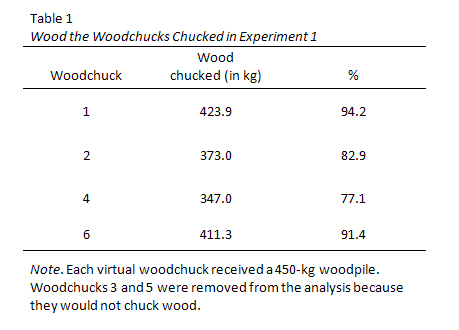
Figure 1. Screenshot of a table formatted per APA style . Reprinted from “The Grammar of Mathematics: Percentage or %?,” by T. McAdoo, 2011, APA Style Blog. Retrieved from http://blog.apastyle.org/apastyle/2011/11/the-grammar-of-mathematics-percentage.html
Per APA style, figures are all types of visual elements other than tables. This includes photographs, graphs, and charts. Similar to tables, figures must be necessary and supplement your content. Figures should also be numbered sequentially. When creating a figure, simplicity is key. Keeping your figure legible and clear for the reader is more important than eye-catching graphics. Your figure, whether it be a bar graph, scatter plot, or other visual graphic, should be easy to understand and read. Your figures should fit within your paper’s margins. The font used within your figure should be sans serif, consistently sized, and between eight and fourteen point. Resist the temptation to include a title within the figure itself. Instead, use a caption above the figure to provide the name of the figure, as well as any pertinent information. Your caption should include the figure number, bold and left aligned on the first line, followed by a brief but explanatory title, written in italics, title case, and left aligned on the double-spaced second line. For example: Figure 2
Sample Population by Age and Gender Figures must also be mentioned within the narrative of your text.
Appendices are ideal for including detailed or additional information that supplements your paper but would be distracting if placed within the text. For example, your appendices might include your instrument of measurement, an informed consent letter, or interview questions. Basically, anything too large or unwieldy to be placed in your text can be presented in the appendices. Each appendix should stand on its own, with each one appearing on a new page at the very end of your dissertation. If you have only one appendix, place the word “Appendix” at the top, centered ( not bolded, underlined, italicized, or in quotes). If you have multiple appendices, order them alphabetically; the first should be titled “Appendix A,” the second Appendix B, and so on. Additionally, each appendix must have a title. The title appears below “Appendix” in title case. For example:
APA Style Resources
Obviously, the complete Publication Manual of the American Psychological Association, 7th Edition is the authoritative resource on the topic. The APA style website is also an excellent resource for specific situations regarding tables, figures, and appendices.
Need Help Formatting Tables, Figures, and Appendices per APA Style?
If you’d rather not review an exhaustive style manual, or if you find that you lack the skills to efficiently develop or format tables, figures, and appendices per APA style, we are here to help! We have PhD-educated APA style experts on staff who can develop or format all visual aspects of your dissertation or thesis. Please feel free to call or e-mail us at any time for a free quote. Phone: +442039928489 Email: [email protected]
American Psychological Association. (2019). Publication manual of the American Psychological Association (7th ed.). Washington, DC: American Psychological Association. McAdoo, T. (2011, November 17). The grammar of mathematics: Percentage or %? Retrieved from http://blog.apastyle.org/apastyle/2011/11/the-grammar-of-mathematics-percentage.html Purdue Online Writing Lab. Tables and Figures. Retrieved 09 September, 2020 from https://owl.purdue.edu/owl/research_and_citation/apa_style/apa_formatting_and_style_guide/apa_tables_and_figures.html
Tagged under: APA 6th Edition APA Style APA Style Help APA format APA formatting APA tables dissertation editing services dissertation formatting services thesis editing services
Initially I had trouble writing my Project scope for my DBA as I have an undergraduate in English and an MBA which meant I could write essays at a business report level but not a doctorate level. I reached out to Dr Leach at a Thesis Editor and she spoke with me over the phone several times trying to understand what I needed. She also put together a quick turnaround package as my deadline was very close by. Ultimately, she scheduled an appointment with an editor with many years of experience who guided me back onto the right path. My supervisors at university had only helped me a little but the Thesis Editor team have supported me entirely. I am not only grateful but will be working with Thesis Editor for the entirety of my DBA - the next 4 years. Thank you Dr Leach and thank you to the Thesis Editor team for a wonderful experience.
Read More Client Testimonials
Thesis Editor
+44 20 3992 8489 info@thesis-editor.co.uk Hamilton House, Mabledon Place, London, WC1H 9BB
© 2024 Thesis Editor Ltd , all rights reserved.
Academic Integrity Policy Policies, Terms & Conditions Referral Rewards Privacy Policy Site map
- SpringerLink shop
Figures and tables
Figures and tables (display items) are often the quickest way to communicate large amounts of complex information that would be complicated to explain in text.
Many readers will only look at your display items without reading the main text of your manuscript. Therefore, ensure your display items can stand alone from the text and communicate clearly your most significant results.
Display items are also important for attracting readers to your work. Well designed and attractive display items will hold the interest of readers, compel them to take time to understand a figure and can even entice them to read your full manuscript.
Finally, high-quality display items give your work a professional appearance . Readers will assume that a professional-looking manuscript contains good quality science. Thus readers may be more likely to trust your results and your interpretation of those results.
When deciding which of your results to present as display items consider the following questions:
- Are there any data that readers might rather see as a display item rather than text?
- Do your figures supplement the text and not just repeat what you have already stated?
- Have you put data into a table that could easily be explained in the text such as simple statistics or p values?
Tables are a concise and effective way to present large amounts of data. You should design them carefully so that you clearly communicate your results to busy researchers.
The following is an example of a well-designed table:
- Clear and concise legend/caption
- Data divided into categories for clarity
- Sufficient spacing between columns and rows
- Units are provided
- Font type and size are legible
Our support team is here to help you daily via chat, WhatsApp, email, or phone between 9:00 a.m. to 11:00 p.m. CET.
Our APA experts default to APA 7 for editing and formatting. For the Citation Editing Service you are able to choose between APA 6 and 7.
Yes, if your document is longer than 20,000 words, you will get a sample of approximately 2,000 words. This sample edit gives you a first impression of the editor’s editing style and a chance to ask questions and give feedback.
How does the sample edit work?
You will receive the sample edit within 24 hours after placing your order. You then have 24 hours to let us know if you’re happy with the sample or if there’s something you would like the editor to do differently.
Read more about how the sample edit works
Yes, you can upload your document in sections.
We try our best to ensure that the same editor checks all the different sections of your document. When you upload a new file, our system recognizes you as a returning customer, and we immediately contact the editor who helped you before.
However, we cannot guarantee that the same editor will be available. Your chances are higher if
- You send us your text as soon as possible and
- You can be flexible about the deadline.
Please note that the shorter your deadline is, the lower the chance that your previous editor is not available.
If your previous editor isn’t available, then we will inform you immediately and look for another qualified editor. Fear not! Every Scribbr editor follows the Scribbr Improvement Model and will deliver high-quality work.
Yes, our editors also work during the weekends and holidays.
Because we have many editors available, we can check your document 24 hours per day and 7 days per week, all year round.
If you choose a 72 hour deadline and upload your document on a Thursday evening, you’ll have your thesis back by Sunday evening!
Yes! Our editors are all native speakers, and they have lots of experience editing texts written by ESL students. They will make sure your grammar is perfect and point out any sentences that are difficult to understand. They’ll also notice your most common mistakes, and give you personal feedback to improve your writing in English.
Every Scribbr order comes with our award-winning Proofreading & Editing service , which combines two important stages of the revision process.
For a more comprehensive edit, you can add a Structure Check or Clarity Check to your order. With these building blocks, you can customize the kind of feedback you receive.
You might be familiar with a different set of editing terms. To help you understand what you can expect at Scribbr, we created this table:
View an example
When you place an order, you can specify your field of study and we’ll match you with an editor who has familiarity with this area.
However, our editors are language specialists, not academic experts in your field. Your editor’s job is not to comment on the content of your dissertation, but to improve your language and help you express your ideas as clearly and fluently as possible.
This means that your editor will understand your text well enough to give feedback on its clarity, logic and structure, but not on the accuracy or originality of its content.
Good academic writing should be understandable to a non-expert reader, and we believe that academic editing is a discipline in itself. The research, ideas and arguments are all yours – we’re here to make sure they shine!
After your document has been edited, you will receive an email with a link to download the document.
The editor has made changes to your document using ‘Track Changes’ in Word. This means that you only have to accept or ignore the changes that are made in the text one by one.
It is also possible to accept all changes at once. However, we strongly advise you not to do so for the following reasons:
- You can learn a lot by looking at the mistakes you made.
- The editors don’t only change the text – they also place comments when sentences or sometimes even entire paragraphs are unclear. You should read through these comments and take into account your editor’s tips and suggestions.
- With a final read-through, you can make sure you’re 100% happy with your text before you submit!
You choose the turnaround time when ordering. We can return your dissertation within 24 hours , 3 days or 1 week . These timescales include weekends and holidays. As soon as you’ve paid, the deadline is set, and we guarantee to meet it! We’ll notify you by text and email when your editor has completed the job.
Very large orders might not be possible to complete in 24 hours. On average, our editors can complete around 13,000 words in a day while maintaining our high quality standards. If your order is longer than this and urgent, contact us to discuss possibilities.
Always leave yourself enough time to check through the document and accept the changes before your submission deadline.
Scribbr is specialised in editing study related documents. We check:
- Graduation projects
- Dissertations
- Admissions essays
- College essays
- Application essays
- Personal statements
- Process reports
- Reflections
- Internship reports
- Academic papers
- Research proposals
- Prospectuses
Calculate the costs
The fastest turnaround time is 24 hours.
You can upload your document at any time and choose between three deadlines:
At Scribbr, we promise to make every customer 100% happy with the service we offer. Our philosophy: Your complaint is always justified – no denial, no doubts.
Our customer support team is here to find the solution that helps you the most, whether that’s a free new edit or a refund for the service.
Yes, in the order process you can indicate your preference for American, British, or Australian English .
If you don’t choose one, your editor will follow the style of English you currently use. If your editor has any questions about this, we will contact you.
Doctoral Thesis Guidelines
Introduction
Preparing to Submit the Thesis
Application for the Degree Oral Final Examination – Signature Page
Online Submission of the Thesis
ETDs @ ProQuest ORCID Harvard Author Agreement Redaction Embargoes Surveys
Distribution of the Thesis
Open Access After Submission Bound Thesis Fee Additional Bound Copies
Copyright and Publishing Considerations
Understanding Your Copyright and Fair Use Copyright Registration Acknowledging the Work of Others Use of Copyrighted Material Steps for Using Published and To-Be Published Work
Formatting Guidelines
Text Margins Pagination Title Title Page Abstract Body of Thesis Figures and Tables Footnotes Bibliography Supplemental Material
Citation & Style Guides
Thesis Submission Checklist
INTRODUCTION All DrPH degree candidates at the Harvard Chan School are required to successfully complete and submit a thesis to qualify for degree conferral. This website provides information on the requirements for how to format your thesis, how to submit your thesis, and how your thesis will be distributed. Please follow the submission and formatting guidelines provided here. Back to top
PREPARING TO SUBMIT THE THESIS The electronic submission of your thesis and the original Signature Page are due on the dates specified on the Harvard Chan School’s Academic Calendar Summary for each degree awarding period (November, March, and May). These items must be submitted using the ETDs @ ProQuest tool in order for the degree to be voted. No exceptions will be made to this rule. Back to top
Application for the Degree There are three degree granting periods: November, March, and May. To apply for graduation, students must complete the Application for Degree on the my.Harvard portal by the deadline posted on the Harvard Chan School’s Academic Calendar .
Deadline extensions are not possible. Students who miss the deadline must apply for the subsequent degree conferral date (November, March, or May). The student is responsible for meeting submission deadlines. Back to top
Oral Final Examination — Signature Page All Doctoral Committee members are required to sign the Signature Page at the time of the Doctoral Final Oral Examination indicating their final approval of the thesis.
A scanned copy of the Signature Page should appear before the title page of the PDF online submission of the thesis; no page number should be assigned to the Signature Page. The title on the Signature Page must read exactly as it does on the title page of the thesis. The Signature Page will be included in all copies of the thesis.
Click here for instructions on how to merge the Signature Page into the thesis PDF.
The Signature Page for DrPH students must be formatted as follows:
This Doctoral Thesis, [ Title of Doctoral Project ], presented by [ Student’s Name ], and Submitted to the Faculty of The Harvard T.H. Chan School of Public Health in Partial Fulfillment of the Requirements for the Degree of Doctor of Public Health , has been read and approved by:
______________________________________ (typed name below line – signature above)
________________________________________ (typed name below the line – signature above)
Date : [ Doctoral Project Official Approval Date (month day, year) ]
Back to top
ONLINE SUBMISSION OF THE THESIS
ETDs @ ProQuest All DrPH candidates are required to submit a digital copy of the thesis to the Registrar’s Office as a PDF file via ETDs @ ProQuest by the deadline established for each degree conferral date. Theses must be submitted in their final format, as described in the section Formatting Guidelines . Students must check their formatting carefully before submitting. Formatting errors will prevent the students’ theses from being accepted and approved.
The online-submission tool can be found at: http://www.etdadmin.com/hsph.harvard
A how-to video for submitting a thesis via ETDs is available on the Countway Library website .
ORCID ETDs @ ProQuest supports ORCIDs. ORCIDs are persistent digital identifiers that link you to your professional activity. You may register for an ORCID either before or during submission if you do not yet have one. To do so, you may go here .
The Harvard Library ORCID page provides information about the value of having an ORCID iD and how Harvard plans to use ORCID data. Additionally, please visit the Harvard ORCID Connect site to connect your existing ORCID iD to Harvard University.
Harvard Author Agreement When submitting work through ETDs @ ProQuest, you will be consenting to the Harvard Author Agreement , which grants the University a non-exclusive license to preserve, reproduce, and display the work. This license, which is the same the Harvard Chan School faculty use under the School’s Open Access Policy, does not constrain your rights to publish your work subsequently. Back to top
Redaction Very few theses require redaction, which is the process of obscuring or removing sensitive information for distribution. ETDs @ ProQuest does support redacted versioning for these very rare cases where there is sensitive or potentially harmful material in the thesis (e.g., commercially sensitive information, sensitive personal data, risk of harmful retribution, etc.).
If your work is one such rare instance, then you may select the “I think I need to submit a redacted version of my thesis” on the file upload screen. You will then be prompted to contact the Office for Scholarly Communication, which will help you with your request. Back to top
Embargoes To forestall any potential challenges that a student may face in the publication process (e.g., if the candidate has a publication pending with a publisher or has previously published some of the content in the thesis and there is a publisher’s embargo that must be honored), the Harvard Chan School has instituted a default one-year embargo for submissions through ETDs @ ProQuest. The embargo starts on the date of the thesis submission deadline. With an embargo, the full text of the thesis will be unavailable for view or download for a limited period of time. The citation and abstract for the work, however, will be publicly available.
If a student would like to make her/his work available immediately by opting out of the embargo process, she/he may do so by selecting the No Embargo option during the submission process.
If, due to extenuating circumstances, a student is required to embargo part or all of their work beyond one year, she/he must request an extension during the submission process. An extension can be requested for up to two years. This request is subject to the approval of the student’s department chair(s) and the University Librarian.
Any embargo applied to the DASH version of the thesis will be applied to the Countway Library and Harvard Chan School department versions of the work.
Students do not need to take any action to remove an embargo. The embargo will automatically be lifted in DASH at the end of the selected and approved period. If a student would like to change the duration of his/her embargo request, then please contact the Registrar’s Office at [email protected] or 617-432-1032. Back to top
Surveys The School of Public Health is asked to participate in the Survey of Earned Doctorates. This is an annual census of research doctorate recipients in the United States. Data collected from these surveys are used to make federal policy decisions regarding graduate education.
Students are required to complete the Survey of Earned Doctorates upon submission of their thesis. A Certificate of Completion will be sent to you, as well as to the Registrar’s Office.
Please click here to complete your survey.
DISTRIBUTION OF THE THESIS
Open Access For information on open access, we recommend the Office of Scholarly Communication’s (OSC) Director Peter Suber’s brief introduction . He has also written about providing open access to theses . The OSC has produced several videos of Harvard faculty and students discussing open access. Two may be of particular interest: the first features Professors Gary King and Stuart Shieber , and the second features a recent Harvard graduate, Ben Finio . Back to top
After Submission Once you have applied for your degree and submitted your thesis online, it is checked for compliance by the Registrar’s Office and, if accepted, is piped to the following downstream systems:
- DASH : Your work will be sent to DASH (Digital Access to Scholarship at Harvard), Harvard’s open access repository. Search engines index DASH, which means your work will be more discoverable and more frequently cited. You will be making DASH access decisions for your work at the point of submission. This will be the access copy of the thesis.
- HOLLIS : The metadata about your work will be sent to HOLLIS . This will make your work discoverable through the Harvard Library catalog.
- DRS2 : Your work will be stored in Harvard Library’s digital preservation repository, DRS2 . This will be the preservation copy of the thesis.
By default, theses will be made available through DASH one year after students submit their theses via ETDs @ Harvard for degree completion (see Embargoes ). DASH is operated by Harvard Library’s Office for Scholarly Communication and is the University’s central service for openly distributing Harvard’s scholarly output.
Note that any embargo applied to the DASH version of the thesis will be applied to the Countway Library and department versions of the work. Back to top
Bound Thesis Fee Currently we are not receiving bound thesis copies. Doctoral students will not be charged bound thesis fees. Back to top
Additional Bound Copies Students may secure extra copies of their work for their own purposes. These additional copies may be purchased through Acme Bookbinding . or through ETDs @ ProQuest . Back to top
COPYRIGHT AND PUBLISHING CONSIDERATIONS
Understanding Your Copyright and Fair Use The Office for Scholarly Communication has created copyright-related resources for your reference.
The first addresses your copyrights and identifies some considerations when publishing (see “ Planning to publish? ”). It is important that you envision any future use you may like to make of your work. Any publishing contract you sign can affect your potential future uses, such as use in teaching, posting your work online on either a personal or departmental website, or any potential future publication. Before you sign a publication agreement, you can negotiate with a publisher to secure licensing terms that best suit your needs. It is important that you read any contract you sign and keep a copy for your own records.
The second resource discusses fair use (see “ Fair use ”), what it is, the laws that have determined its shape over time, and tips for ensuring that use of third-party material (including quotes, images, music, film, etc.) in your thesis is fair. Back to top
Copyright Registration Your work is copyrighted as soon as it is fixed in a tangible form. You are not required to register your copyright with the U.S. Copyright Office to enjoy protection of your work. However, if you choose to do so, you may register your work with the Copyright Office online . Back to top
Acknowledging the Work of Others Students are responsible for acknowledging any facts, ideas, or materials of others used in their own work. Students should refer to the statement on Academic Dishonesty and Plagiarism in the Harvard Chan School’s Student Handbook . Back to top
Use of Copyrighted Material A thesis is a scholarly work, and as such use of third party material is often essential. Fair use applies to the reproduction of any third party material, including your own previously published work, that you may use in your thesis.
If you have questions about copyright and fair use, please contact the Office for Scholarly Communication . Back to top
Steps for Using Published and To-Be Published Work When submitting an article for publication that you intend to use in your thesis, you should secure permission to do so (along with permission to reuse your own work as you would like) from your publisher in your publishing agreement. If the default contract does not let you retain these rights already, then you should use an author addendum to secure these rights (see “ Planning to publish? ”).
You may use your own previously published material as part of your thesis with the permission of the publisher. Again, refer to your publication agreement for details. If your contract does not specify these rights, then contact the publisher to negotiate this use. Back to top
FORMATTING GUIDELINES The following are instructions on how to format your thesis. If, after reading the instructions here, you have additional questions about the requirements, please contact the Registrar’s Office at (617) 432-1032; [email protected]. Back to top
Text All text should be double-spaced on one side of the page with footnotes single-spaced. The font size should be at least 10 point, but no larger than 12 point. The font and font size should be consistent throughout. All text should be black. Back to top | Back to Formatting Guidelines
Margins The margins of the thesis must be 1 inch on all sides. Back to top | Back to Formatting Guidelines
Pagination Students’ theses must follow the pagination guidelines as illustrated below. It is customary not to have a page number on the page containing a chapter/paper heading. Drawings, charts, graphs, and photographs should be referred to as figures and should be numbered consecutively within the text of the thesis with Arabic numerals. Each figure should carry a suitable caption; e.g., Fig. 42. Arrangement of Experimental Equipment. Check pagination carefully and account for all pages.
All page numbers should be consecutive and centered at either the bottom or top of the page. Back to top | Back to Formatting Guidelines
Title The title of the thesis should be brief and should indicate the general subject treated. Nine words are usually sufficient to describe the investigation. Students are strongly encouraged to embed keywords into their title, so that the title will be retrievable on computerized listings. Back to top | Back to Formatting Guidelines
Title Page The title page must contain the following information, well-spaced and centered on the page:
For DrPH Students:
TITLE OF DOCTORAL THESIS
STUDENT’S NAME
A Doctoral Thesis Submitted to the Faculty of
The Harvard T.H. Chan School of Public Health
in Partial Fulfillment of the Requirements
for the Degree of Doctor of Public Health
Harvard University
Boston, Massachusetts.
Date (the month in which degree will be awarded, year of graduation (e.g., May 2021)
Back to top | Back to Formatting Guidelines
Abstract The abstract should not exceed 350 words. It should immediately follow the Title Page, and should state the problem, describe the methods and procedures used, and give the main results or conclusions of the research. The abstract should be double-spaced. The author’s name and the title of the thesis, as well as the name of the thesis advisor, should be included on the abstract page. The author’s name should be right justified, the title of the thesis centered, and “Thesis Advisor: Dr. ____________” should be left-justified at the top of the abstract page.
Thesis Advisor: Dr. [Advisor’s name] [Author’s name]
[Title of thesis]
The text of the abstract, not to exceed 350 words, should be double-spaced. The first line of each paragraph is indented. Full justification of the text is not recommended.
Students will also be required to submit a text version of the abstract via the online-submission tool. Back to top | Back to Formatting Guidelines
Body of Thesis The thesis should consist of manuscripts suitable for publication in a scientific medium appropriate to the candidate’s field and/or approved reprints of the published work(s) (see Steps for Using Published and To-Be Published Work and Use of Copyrighted Material ).
Technical appendices should be added where necessary to demonstrate full development of the thesis material. Papers published under joint authorship are acceptable provided the candidate has contributed a major part to the investigation. The degree candidate is expected to be senior author on at least one of the papers. In the case of manuscripts published under joint authorship, the co-authors or the advisor may be consulted by the readers or the CAD to clarify the nature and extent of the candidate’s contribution. In addition to evaluating the quality and significance of the work, those responsible for accepting the thesis [the Department(s) and Doctoral Project Committee] may determine whether the format is suitable for publication in a scientific medium appropriate to the degree candidate’s field(s). Back to top | Back to Formatting Guidelines
Figures and Tables Figures and tables must be placed as close as possible to their first mention in the text. They may be placed on a page with no text above or below, or they may be placed directly in the text. If a figure or table is alone on a page with no narrative, it should be centered within the margins of the page.
Figures and tables referred to in the text may not be placed at the end of the chapter or at the end of the thesis. Figure and table numbering must be either continuous throughout the thesis or by paper (e.g., 1.1, 1.2, 2.1, 2.2). For example, there cannot be two figures designated in a thesis as “Figure 5.”
Headings of tables should be placed at the top of the table. While there are no specific rules for the format of table headings and figure captions, a consistent format must be used throughout the thesis. (See Citation and Style Guides )
Captions of figures should be placed at the bottom of the figure. If the figure takes up the entire page, the figure caption should be placed alone on the preceding page and centered vertically and horizontally within the margins. Each page receives a separate page number. When a figure or table title is on a preceding page, the second and subsequent pages of the figure or table should say, for example, “Figure 5 (Continued).” In such an instance, the list of figures or tables will list the page number containing the title. The word “Figure” should be written in full (not abbreviated), and the “F” should be capitalized (e.g., Figure 5). In instances where the caption continues on a second page, the “(Continued)” notation should appear on the second and any subsequent page. The figure/table and the caption are viewed as one entity and the numbering should show correlation between all pages. Each page must include a header.
Horizontal figures and tables must be positioned correctly and bound at the top, so that the top of the figure or table will be at the left margin (leave a 1 inch margin on the long edge of the paper above the top of the table).
Figure and table headings/captions are placed with the same orientation as the figure or table when on the same page. When on a separate page, headings/captions are always placed in vertical orientation, regardless of the orientation of the figure or table. Page numbers are always placed as if the figure were vertical on the page.
Figures created with software are acceptable if the figures are clear and legible. Legends and titles created by the same process as the figures will be accepted if they too are clear, legible, and run at least 10 or 12 characters per inch. Otherwise, legends and captions should be printed with the same font used in the text. Back to top | Back to Formatting Guidelines
Footnotes Footnotes are reserved for substantive additions to the text and should be indicated by an asterisk in the text. Extensive use of footnotes is not encouraged. The footnote should be placed at the bottom of the page. A horizontal line of at least two inches should be typed above the first footnote on any page. Footnotes should be placed so that at least one inch is left at the bottom of the page. Use single-spacing within footnotes. Back to top | Back to Formatting Guidelines
Bibliography To document the sources of information, a bibliography must be included at the end of the papers or thesis. References may be numbered or listed alphabetically. If references in the bibliography are numbered, then corresponding in-text references should be indicated by listing the number in parentheses after the name of the author.
Bibliographic Example:
23. Gibbs, C.S.: Filterable virus carriers. J. Bact., 23, 1932, 113.
In-Text Example:
“. . . as Gibbs (23) has stated.”
The initial number should be omitted if references are listed alphabetically.
Within any bibliographic section there should be consistency and adherence to an acceptable journal style for a bibliography. Each reference in the bibliography must contain the name of the author, title of the paper, name of publication, volume, date, and first page.
More than one publication by the same author in the same year should be indicated both in the bibliography and in the text by the use of underlined letters, etc., after the date of publication. The standard system of abbreviation used by the Quarterly Cumulative Index should be followed for the abbreviations of journal titles.
If students’ individual papers have different bibliographic styles, then it is not necessary to change the bibliographic style of one to match the other. Consistency within each bibliographic section is the most important element. Back to top | Back to Formatting Guidelines
Supplemental Material Supplemental figures and tables must be placed at the end of each chapter/paper in an appendix. If additional digital information (including text, audio, video, image, or datasets) will accompany the main body of the thesis, then it should be uploaded as supplemental material via the ETDs @ Harvard online submission tool. Back to top | Back to Formatting Guidelines
CITATION & STYLE GUIDES
- The Chicago Manual of Style. 16th ed. Chicago, IL: University of Chicago Press, 2003.
- Crews, Kenneth D. Copyright Law and the Doctoral Dissertation. Ann Arbor, MI: ProQuest, 2000.
- Day, Robert A. and Barbara Gastel. How to Write & Publish a Scientific Paper. 6th ed. Westport, CT: Greenwood, 2006.
- MLA Style Manual and Guide to Scholarly Publishing. 3rd ed. New York, NY: Modern Language Association of America, 2008. Strunk, William. The Elements of Style. 4th ed. New York, NY: Penguin Press, 2005.
- Publication Manual of the American Psychological Association. 6th ed. Washington, DC: American Psychological Association, 2010.
- Turabian, Kate L. A Manual for Writers of Term Papers, Theses, and Dissertations. Chicago
- Guides to Writing, Editing, and Publishing. 7th ed. Chicago, IL: University of Chicago Press, 2007.
THESIS SUBMISSION CHECKLIST ☐ Is the Signature Page unnumbered and positioned as the first page of the PDF file? ☐ Is there a blank page after the Signature Page? ☐ Does the body of the thesis begin with Page 1? ☐ Is the pagination continuous? Are all pages included? ☐ Is every page of the thesis correctly numbered? ☐ Is the placement of page numbers centered throughout the manuscript? ☐ Is the Title Page formatted correctly? ☐ Is the author’s name, in full, on the Title Page of the thesis and the abstract? ☐ Does the author’s name read the same on both and does it match the Signature Page? ☐ Is the abstract included after the Title Page? ☐ Does the abstract include the title of the thesis, the author’s name, and the thesis advisor(s)’ name? ☐ Is the title on the abstract the same as that on the title page? ☐ Are the margins 1” on all sides? ☐ Is the font size 10-12 point? ☐ Are all charts, graphs, and other illustrative materials perfectly legible? ☐ Do lengthy figures and tables include the “(Continued)” notation? ☐ Has all formatting been checked? ☐ Is the Survey of Earned Doctorates completed? ☐ Has the Survey of Earned Doctorates’ confirmation email or certificate been uploaded to ETDs @ Harvard?
News from the School

Bethany Kotlar, PhD '24, studies how children fare when they're born to incarcerated mothers

Soccer, truffles, and exclamation points: Dean Baccarelli shares his story

Health care transformation in Africa highlighted at conference

COVID, four years in

The Plagiarism Checker Online For Your Academic Work
Start Plagiarism Check
Editing & Proofreading for Your Research Paper
Get it proofread now
Online Printing & Binding with Free Express Delivery
Configure binding now
- Academic essay overview
- The writing process
- Structuring academic essays
- Types of academic essays
- Academic writing overview
- Sentence structure
- Academic writing process
- Improving your academic writing
- Titles and headings
- APA style overview
- APA citation & referencing
- APA structure & sections
- Citation & referencing
- Structure and sections
- APA examples overview
- Commonly used citations
- Other examples
- British English vs. American English
- Chicago style overview
- Chicago citation & referencing
- Chicago structure & sections
- Chicago style examples
- Citing sources overview
- Citation format
- Citation examples
- College essay overview
- Application
- How to write a college essay
- Types of college essays
- Commonly confused words
- Definitions
- Dissertation overview
- Dissertation structure & sections
- Dissertation writing process
- Graduate school overview
- Application & admission
- Study abroad
- Master degree
- Harvard referencing overview
- Language rules overview
- Grammatical rules & structures
- Parts of speech
- Punctuation
- Methodology overview
- Analyzing data
- Experiments
- Observations
- Inductive vs. Deductive
- Qualitative vs. Quantitative
- Types of validity
- Types of reliability
- Sampling methods
- Theories & Concepts
- Types of research studies
- Types of variables
- MLA style overview
- MLA examples
- MLA citation & referencing
- MLA structure & sections
- Plagiarism overview
- Plagiarism checker
- Types of plagiarism
- Printing production overview
- Research bias overview
- Types of research bias
- Example sections
- Types of research papers
- Research process overview
- Problem statement
- Research proposal
- Research topic
- Statistics overview
- Levels of measurment
- Frequency distribution
- Measures of central tendency
- Measures of variability
- Hypothesis testing
- Parameters & test statistics
- Types of distributions
- Correlation
- Effect size
- Hypothesis testing assumptions
- Types of ANOVAs
- Types of chi-square
- Statistical data
- Statistical models
- Spelling mistakes
- Tips overview
- Academic writing tips
- Dissertation tips
- Sources tips
- Working with sources overview
- Evaluating sources
- Finding sources
- Including sources
- Types of sources
Your Step to Success
Plagiarism Check within 10min
Printing & Binding with 3D Live Preview
List Of Figures And Tables For Your Dissertation
How do you like this article cancel reply.
Save my name, email, and website in this browser for the next time I comment.

The list of figures and tables in a research paper , thesis, or dissertation provides a structured overview of graphic elements included in the paper. This list guides readers to find specific graphs, images, tables, or charts effortlessly. The process of compiling this list needs more than just listing the captions; it also requires proper formatting and sequencing in line with academic guidelines. This article explores creating a well-structured list of figures and tables with examples.
Inhaltsverzeichnis
- 1 List of Figures and Tables – In a Nutshell
- 2 Definition: List of figures and tables
- 3 Creating the list of figures and tables in Word
- 4 Example list of figures and tables
- 5 List of figures and tables: Additional lists
List of Figures and Tables – In a Nutshell
The American Psychological Association publishes the APA style guide, which aims to:
- Facilitate concise academic and scholarly communication worldwide.
- Act as a reference for the various components and conventions of scientific and technical writing.
- Improve the readability of documents.
Definition: List of figures and tables
Tables show numerical values or text arranged in rows and columns. In contrast, figures typically consist of graphs, illustrations, or drawings.
The APA style guide defines figures as graphical displays other than tables, including photographs, graphics, charts, and non-textual information.
Suppose a dissertation contains one or more tables or figures. In that case, the APA guide specifies including a list of figures and tables as appropriate.
Every list of figures and tables includes a tabulated, numerical enumeration of the titles of each relevant item. This uniform and consistent approach enables dissertation readers – including examiners – to quickly scan and locate the sources, findings, and key points in long documents.
By following APA recommendations to make a list of figures and tables, college and university students can present their dissertations correctly.
List of Tables
Table 1 Title of Table One ……………………………………………………………………………..2 Table 2 Title of Table Two .…………………………………………………………………………….3 Table 3 Title of Table ‘Three ………………………………………………………………………….3
List of Figures
Figure 1 Title of Figure One …………………………………………………………………………..4 Figure 2 Title of Figure Two …………………………………………………………………………..5 Figure 3 Title of Figure Three ………………………………………………………………………..5
This article will delve into how to include a list of figures and tables in APA style in your dissertation.
Creating the list of figures and tables in Word
Creating a list of figures and tables is straightforward in most word processing software, such as Microsoft Word.
- Firstly, we must add captions to each figure or table. The figure number goes in bold above the figure (e.g. Figure 1). Then, the figure title appears as one double-spaced line below the figure number in italics in title case, i.e. with the first letter of major words capitalized.
- Next, use the command on the “References” menu to complete the detailed settings you require. On confirming, the software will create the list sorted by page number and include it in your document.
Note: It is essential to eschew plagiarism if you are creating a list of figures and tables based on copying from another document.
Also, remember that the source document settings and format may affect how the table looks in your new paper: font style, page number conventions, margin widths, etc.
- Firstly, we must add captions to each figure or table. The figure number goes in bold above the figure (e.g., Figure 1). Then, the figure title appears as one double-spaced line below the figure number in italics in title case, i.e., with the first letter of major words capitalized.
Further information on formatting standards for a list of figures and tables are on pages 225 to 250 of the APA Publication Manual 7th Edition (2020).
Example list of figures and tables

List of figures and tables: Additional lists
Other lists you might consider including in a dissertation are:
- A list of abbreviations
- A table of contents
After the title, approval signature, and copyright page(s) as applicable, we recommend you arrange the pages of a dissertation in the following order:
- Table of Contents
Occasionally, research results or lengthy analyses may extend to hundreds of rows. Instead of including all the detail, a clickable link or URL (universal resource locator) to an online version may be preferable.
We recommend opting for a data repository or an arXiv location, as privately hosted websites may change or disappear.
Best practice guidelines advocate the long-term availability of datasets for at least five years after publication. 2 Resources such as nature.com publish details of storage options by scientific field.
How do you list tables in a dissertation?
Your list of figures and tables comes after the table of contents. If both lists are present, the list of titles appears before the list of figures.
What are figure keys?
Figure legends (also known as keys) explain uncommon symbols used in the figure image. They should appear within the borders of the figure.
What are figure notes?
Figure notes explain, describe, clarify, or supplement the information in the image. Only some figures include notes, as and when necessary.
Where do I position notes for figures or tables?
According to the APA style guide, notes appear below the figure or table. Use double line spacing and left justification.
We use cookies on our website. Some of them are essential, while others help us to improve this website and your experience.
- External Media
Individual Privacy Preferences
Cookie Details Privacy Policy Imprint
Here you will find an overview of all cookies used. You can give your consent to whole categories or display further information and select certain cookies.
Accept all Save
Essential cookies enable basic functions and are necessary for the proper function of the website.
Show Cookie Information Hide Cookie Information
Statistics cookies collect information anonymously. This information helps us to understand how our visitors use our website.
Content from video platforms and social media platforms is blocked by default. If External Media cookies are accepted, access to those contents no longer requires manual consent.
Privacy Policy Imprint
- Open access
- Published: 01 April 2024
Association of anthropometric measures with all-cause and cause-specific mortality in US adults: revisiting the obesity paradox
- Shan Li 1 , 2 na1 ,
- Zhiqing Fu 1 , 2 na1 &
- Wei Zhang 2 , 3
BMC Public Health volume 24 , Article number: 929 ( 2024 ) Cite this article
Metrics details
Previous studies have shown that the obesity paradox exists in a variety of clinical settings, whereby obese individuals have lower mortality than their normal-weight counterparts. It remains unclear whether the association between obesity and mortality risk varies by anthropometric measures. The purpose of this study is to examine the association between various anthropometric measures and all-cause and cause-specific mortality in US adults.
This cohort study included data from the National Health and Nutrition Examination Survey between 2009 and 2018, with a sample size of 28,353 individuals weighted to represent 231 million US adults. Anthropometric measurements were obtained by trained technicians using standardized methods. Mortality data were collected from the date of enrollment through December 31, 2019. Weighted Cox proportional hazards models, restricted cubic spline curves, and cumulative incidence analyses were performed.
A total of 2091 all-cause deaths, 606 cardiovascular deaths, 519 cancer deaths, and 966 other-cause deaths occurred during a median follow-up of 5.9 years. The association between body mass index (BMI) and mortality risk was inversely J-shaped, whereas the association between waist-to-height ratio (WHtR) and mortality risk was positively J-shaped. There was a progressive increase in the association between the WHtR category and mortality risk. Compared with the reference category of WHtR < 0.5, the estimated hazard ratio (HR) for all-cause mortality was 1.004 (95% confidence interval [CI] 1.001–1.006) for WHtR 0.50–0.59, 1.123 (95% CI 1.120–1.127) for WHtR 0.60–0.69, 1.591 (95% CI 1.584–1.598) for WHtR 0.70–0.79, and 2.214 (95% CI 2.200–2.228) for WHtR ≥ 0.8, respectively. Other anthropometric indices reflecting central obesity also showed that greater adiposity was associated with higher mortality.
Conclusions
Anthropometric measures reflecting central obesity were independently and positively associated with mortality risk, eliminating the possibility of an obesity paradox.
Graphical Abstract
Peer Review reports
Introduction
Obesity is a growing public health concern, with the global prevalence predicted to reach 14% in men and 20% in women by 2030 [ 1 ]. In the United States, the prevalence of obesity will be as high as 47% in both men and women by that time [ 1 ]. The etiology of obesity is multifactorial and includes biology, genetics, socioeconomics, environmental factors, and access to healthcare resources [ 2 ]. Strong evidence suggests that obesity has deleterious effects on glucolipid homeostasis, blood pressure, systemic inflammation, and oxidative stress, thereby increasing the risk of various pathophysiological conditions such as diabetes, atherosclerosis, hypertension, musculoskeletal disorders, and certain cancers, as well as predisposing to premature death [ 3 , 4 , 5 , 6 , 7 ]. However, the existing literature reports that obese individuals have better survival than their normal-weight counterparts in a variety of clinical settings, a phenomenon known as the obesity-survival paradox [ 8 , 9 , 10 ]. This counterintuitive relationship may make it difficult to clarify the link between obesity and metabolic pathology and may send confusing messages to healthcare professionals and policymakers, potentially leading to hesitancy in controlling weight and adopting healthy lifestyles.
Several assessment tools have been used clinically to define excess body fat, including anthropometric, bioelectrical impedance analysis, densitometric and imaging-based methods [ 2 ]. Body mass index (BMI) is the most used anthropometric measure reflecting overall obesity. The obesity-survival paradox is typically documented using BMI as an evaluative indicator [ 8 , 10 , 11 ]. Epidemiological and genetic evidence suggests that the systemic metabolic risks of obesity depend not only on the amount of fat, but also on its distribution, and that central obesity, mainly the accumulation of abdominal or visceral fat, contributes to major cardiometabolic abnormalities and total mortality [ 12 , 13 , 14 , 15 ]. However, BMI has inherent limitations in defining adiposity because it can differentiate neither body compositions nor regional fat distribution, which weakens its credibility in predicting obesity-related metabolic risks and leads to heterogeneity or even conflicting epidemiologic relevance. Consequently, other anthropometric indices have been developed, including waist circumference, waist-to-height ratio (WHtR), waist-to-hip ratio (WHR), and body composition measured by dual-energy X-ray absorptiometry (DXA), which may be better surrogates for reflecting central obesity. However, the available evidence on the association between central obesity indices and mortality risk is not sufficient, so it remains unclear whether the obesity paradox reflected by BMI is a real existence or an artifact of anthropometric measures. It is of concern that the persistence of conflicting findings on the relationship between obesity and survival may misinterpret the considerable efforts toward weight control.
Therefore, we conducted this study in US adults from the 2009–2018 National Health and Nutrition Examination Survey (NHANES). The objectives of this study were to (i) examine the association of BMI and WHtR with all-cause and cause-specific mortality, using them as proxies for overall obesity and central obesity, respectively, (ii) characterize the association of other anthropometric indices reflecting overall obesity or central obesity with mortality risk, and (iii) attempt to explore the possible reasons for the discrepancy between anthropometric measures and the outcomes according to the correlation between anthropometric indices and DXA-based visceral fat measurements. We hypothesize that the obesity paradox may not be real but an artifact of anthropometric measures.
Study population
NHANES is a historical, nationally representative survey of the U.S. civilian noninstitutionalized population. The survey uses a multistage stratified probability cluster sampling design and incorporates participant weights to ensure accuracy in reflecting the demographics of the U.S. Census during the same time period [ 16 ]. We extracted data from five consecutive cycles of the NHANES database (2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018). The study was conducted in accordance with the Declaration of Helsinki. All adult participants provided written informed consent, and all NHANES protocols were approved by the National Center for Health Statistics (NCHS) Ethics Review Board [ 17 ]. Of the 49,693 adults who participated in the 5 NHANES cycles, 21,340 were excluded because they were younger than 18 years ( n = 19,341), pregnant ( n = 247), had missing weight or height data ( n = 1,596), had a BMI less than 10 kg/m 2 or greater than 60 kg/m 2 ( n = 73), and had missing follow-up information ( n = 83). Finally, 28,353 individuals were included in the analysis (Supplementary material Figure S 1 ).
Anthropometric measures
Participants wore disposable examination gowns and baseline weight, height and waist circumference were measured by trained health technicians to ensure methodological consistency. Waist circumference was measured at the uppermost edge of the right and left ilium, and waist circumference data were available for 26,998 individuals. The following anthropometric measures were examined, including overall obesity indices (BMI, body surface area [BSA], and standardized weight percentage) and central obesity indices (WHtR, waist circumference, body roundness index [BRI], weight-adjusted-waist index [WWI], relative fat mass [RFM], and body shape index [BSI]). The overall obesity indices were calculated based on weight and height, while the central obesity indices were calculated based on waist circumference and height (see Graphical abstract). According to the World Health Organization (WHO) criteria, individuals were divided into five BMI categories: underweight, < 18.5 kg/m 2 ; normal weight, 18.5–24.9 kg/m 2 ; overweight, 25–29.9 kg/m 2 ; class I obesity, 30–34.9 kg/m 2 ; and class II or III obesity, ≥ 35 kg/m 2 . For analyses using the WHtR, individuals were categorized as follows: < 0.50, 0.50–0.59, 0.60–0.69, 0.70–0.79, ≥ 0.80, with WHtR < 0.5 being the normal range. DXA-based visceral adipose tissue (VAT) measurements were available for 12,792 individuals, and VAT area and mass were measured at the L4 and L5 intervertebral spaces.
The primary outcome was all-cause mortality, and the secondary outcomes were cardiovascular mortality, cancer mortality and other-cause mortality. Mortality data from the date of enrollment through December 31, 2019 were obtained by linking the NHANES dataset to death certificate records from the National Death Index (NDI) provided by the National Center for Health Statistics (NCHS) [ 18 ]. Cause-specific mortality was defined based on the recorded NCHS underlying classification of death (UCOD). Cardiovascular deaths were defined as deaths from heart disease and deaths from cerebrovascular disease. Cancer deaths were defined as deaths due to malignant neoplasms.
A wide range of covariates were considered, including age, sex, ethnicity, education, marital status, poverty income ratio, smoking status, alcohol consumption, systolic blood pressure, heart rate, BMI (for WHtR analyses) or waist circumference (for BMI analyses), atherosclerotic cardiovascular disease (ASCVD), diabetes mellitus, chronic obstructive pulmonary disease (COPD), cancer, aspirin, lipid-lowering drugs, hypoglycemic agents, and laboratory measurements (white blood cell count, hemoglobin, albumin, creatinine, urea nitrogen, glycohemoglobin, total cholesterol, and high-density lipoprotein cholesterol [HDL-C]). Demographic and health information was collected by experienced interviewers using a computer-assisted personal interview (CAPI) system and reviewed for completeness, consistency, and logicality to ensure data quality. Physical examinations were performed at a dedicated mobile examination center (MEC) using a uniform methodology and laboratory measurements were performed using the Beckman Coulter DxH 800 instrument for complete blood counts, and the Roche Cobas 6000 (c501 module) analyzer for standard biochemistry indices.
Statistical analysis
All analyses were performed using R software (version 4.2.0) and EmpowerStats (X&Y Solutions, Inc., Boston, MA). Statistical significance was defined as a 2-tailed p-value < 0.05. Sample weights, stratification, and clustering were incorporated in all analyses to account for unequal selection and nonresponse probabilities. Baseline characteristics were expressed as means with standard deviations (SDs), medians with interquartile ranges (IQRs), or numbers with percentages and were compared by one-way analysis of variance, Kruskal–Wallis test, and chi-squared test. Data on covariates were more than 93% complete (Supplementary material Table S 1 ). Missing values were imputed using chained equation multiple imputation ( n = 5 data sets).
Restricted cubic spline curves based on Cox models were used to visualize the continuous association between BMI or WHtR and mortality. The inflection points of the mortality risk were estimated and the effect sizes before and after which were reported. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of all-cause and cause-specific mortality for categorical and continuous WHtR. Proportional hazard assumptions were tested and confirmed by Schoenfeld's residual estimates and log(time) plots. Cumulative all-cause mortality for the BMI and WHtR groups was estimated using the Kaplan–Meier method, with the interval from the date of examination to the date of death or the end of follow-up as the time scale. Fine and Gray competing risk models were used for cause-specific mortality, with deaths from the two remaining causes as competing outcomes. The associations between other anthropometric indices and mortality were also visualized using restricted cubic spline curves. Linear regression fitting and Pearson's correlation coefficient were used to test the correlation between anthropometric indices and VAT measurements.
Sensitivity analyses were performed. First, we examined the association between other anthropometric indices (BSA, standardized weight percentage, waist circumference, BRI, WWI, RFM and BSI) as continuous variables and outcomes using the COX proportional hazards models. Second, we performed stratified analyses with subgroups of interest, including age, sex, ethnicity, and presence of diabetes mellitus. Third, individuals with less than 1 year of follow-up were excluded to minimize the potential bias for reverse causality. Fourth, complete case analyses were performed using only complete data for all covariates to assess whether missing data distorted the current results.
The sample included 28,353 individuals from the 2009–2018 NHANES data sets, weighted to represent 231 million US adults. During a median follow-up of 5.9 years, 2091 (7.4%) all-cause deaths, 606 (2.2%) cardiovascular deaths, 519 (1.8%) cancer deaths, and 966 (3.4%) other-cause deaths were recorded, respectively. BMI, BSA and standardized weight percentage were available for all individuals. Waist circumference was available for 95.2% of individuals, as were WHtR, BRI, WWI, RFM, and BSI. Of the total population, 32.1% were classified as overweight and 37.8% as obese, with a mean BMI of 29.0 (SD 6.8) kg/m 2 , while 83.0% had a WHtR outside the normal range of < 0.5, with a mean WHtR of 0.6 (SD 0.1). Individuals with higher BMI or WHtR were older, more likely to be non-Hispanic blacks, less likely to be current smokers, had a higher prevalence of ASCVD and diabetes mellitus, and had higher white blood cell and lower HDL-C levels. All-cause mortality increased progressively with increasing WHtR, while the opposite was present for BMI (Table 1 and Supplementary material Table S 2 ).
Association between BMI or WHtR and mortality
In restricted cubic spline analyses, there was an inversely J-shaped association between continuous BMI and mortality, with risk inflection points for all-cause mortality, cardiovascular mortality, cancer mortality, and other-cause mortality at BMIs of 27.6, 25.0, 25.3, and 29.2 kg/m 2 , respectively. The risk of death decreased sharply before the inflection point and remained almost constant thereafter. Conversely, there was a positively J-shaped association between continuous WHtR and mortality, with risk inflection points for all-cause mortality, cardiovascular mortality, and other-cause mortality occurring at WHtRs of 0.61, 0.58, and 0.62, respectively. The risk of death remained stable until the inflection point and then increased sharply and significantly. No significant association was found between WHtR and cancer death (Fig. 1 ). In Cox proportional hazards analyses, there was a gradual increase in the association between the WHtR category and all-cause mortality, cardiovascular mortality, and other-cause mortality. In addition, when WHtR was examined as a continuous variable, each 0.1 increase in WHtR was associated with a 36.8%, 43.7%, and 47.6% increase in all-cause, cardiovascular, and other-cause mortality, respectively (Table 2 ).
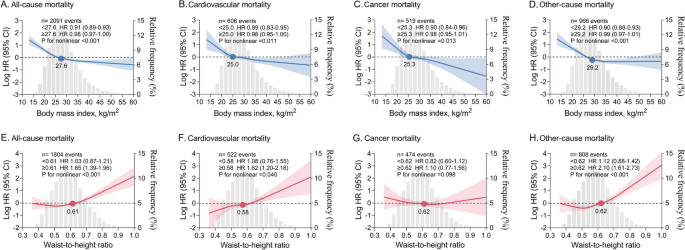
Nonlinear association between continuous BMI or WHtR and mortality. A-D BMI and mortality. E – H WHtR and mortality. HRs (solid lines) and 95% CIs (shaded areas) are based on weighted restricted cubic splines. The gray areas in the background show the distributions (histograms) of BMI or WHtR in the population. Solid dots represent risk inflection points for nonlinear associations. Effect size for per unit change in BMI (1 kg/m 2 ) and WHtR (0.1) before and after the inflection point are shown separately. Models were adjusted for age, sex, ethnicity, waist circumference (for BMI analysis) or BMI (for WHtR analysis), education level, marital status, poverty income ratio, smoking status, alcohol consumption, systolic blood pressure, heart rate, ASCVD, diabetes mellitus, COPD, cancer, aspirin, lipid-lowering drugs, hypoglycemic agents, and laboratory measurements (white blood cell count, hemoglobin, albumin, creatinine, urea nitrogen, glycohemoglobin, total cholesterol, and HDL-C). ASCVD, atherosclerotic cardiovascular disease. COPD, chronic obstructive pulmonary disease. HDL-C, high-density lipoprotein cholesterol. HR, hazard ratio. CI, confidence interval
Cumulative mortality according to BMI or WHtR groups
The cumulative mortality curve showed a gradual decrease in mortality among groups with higher BMI. Specifically, the underweight group had the highest mortality, followed by the normal weight group, while the groups with overweight and obesity had the lowest mortality. Cumulative morbidity for WHtR showed the opposite pattern. Cumulative all-cause, cardiovascular, and other-cause deaths progressively increased in groups with incrementally higher WHtR, whereas there was no evidence that higher WHtR was associated with higher cumulative cancer mortality (Fig. 2 ).
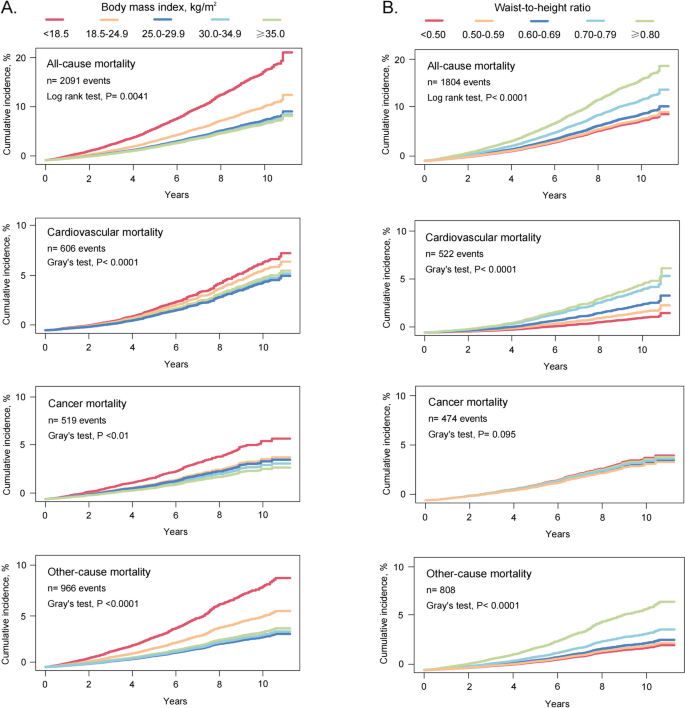
Cumulative mortality by BMI or WHtR groups. Cumulative incidence for mortality was estimated according to ( A ) BMI and ( B ) WHtR groups, and all-cause and cause-specific mortality was followed up to December 31, 2019. The Fine and Gray competing risk models were used for cause-specific mortality, with deaths from the remaining two causes as competing risks
The concordance between BMI and WHtR
Although a strong correlation between BMI and WHtR was observed ( r 2 = 0.839), there were significant differences in agreement between subcategories. Specifically, 97.6% (489 out of 501) of underweight individuals had WHtRs within the normal range, whereas less than half of normal-weight individuals (49.7%, 3807 out of 7653) had WHtRs < 0.5, with the remaining half having WHtRs between 0.5 and 0.7. The concordance between BMI and WHtR was quite high among overweight and obese individuals, most of whom had WHtRs above the normal range (Fig. 3 ).
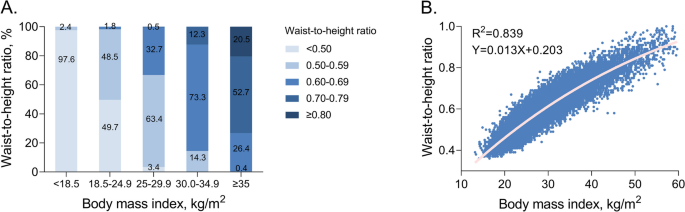
Correlation between BMI and WHtR categories. A WHtR percentage across BMI categories. B Correlation between BMI and WHtR categories
Correlation between anthropometric indices and VAT measurements
In addition to BMI and WHtR, we also examined the correlation of body weight and waist circumference with VAT, as the latter two are the basis for calculating the overall and central obesity indices, respectively. Overall, Pearson's correlations between anthropometric indices and VAT measures were modest. However, the correlations between VAT measures and WHtR or waist circumference were stronger than those with BMI or body weight, ranging from r 2 = 0.566–0.614 for WHtR and waist circumference to r 2 = 0.426–0.473 for BMI and body weight (Fig. 4 ).
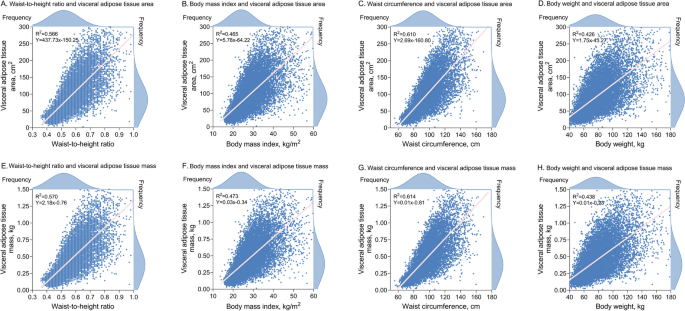
Correlation between anthropometric indices and visceral adipose tissue measurements Pink lines show linear regressions of anthropometric indices on visceral adipose area ( A - D ) and linear regressions of anthropometric indices on visceral adipose mass ( E – H )
Other anthropometric indices and mortality
Other central obesity indices (waist circumference, BRI, WWI, RFM and BSI) also had positively J-shaped associations with all-cause, cardiovascular, and other-cause mortality, but no significant associations with cancer mortality (Fig. 5 ). Other overall obesity indices (BSA and standardized body weight percentage) had inversely J-shaped associations with mortality, whereas the association between BSA and cancer death did not appear to be significant (Supplementary material Figure S 2 ).
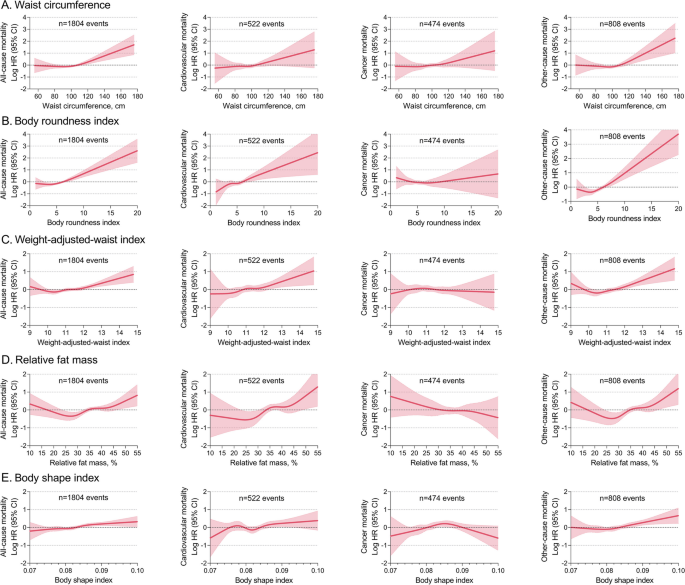
Nonlinear association between other central obesity indices and mortality HRs (solid lines) and 95% CIs (shaded areas) are based on weighted restricted cubic splines. The models were adjusted for age, sex, ethnicity, BMI, education, marital status, poverty income ratio, smoking status, alcohol consumption, systolic blood pressure, heart rate, ASCVD, diabetes mellitus, COPD, cancer, aspirin, lipid-lowering drugs, hypoglycemic agents, and laboratory measurements (white blood cell count, hemoglobin, albumin, creatinine, urea nitrogen, glycohemoglobin, total cholesterol, and HDL-C). ASCVD, atherosclerotic cardiovascular disease. COPD, chronic obstructive pulmonary disease. HDL-C, high-density lipoprotein cholesterol. HR, hazard ratio. CI, confidence interval
Sensitivity analysis
The results of the sensitivity analyses were generally consistent with those of the primary analyses. First, using the COX proportional hazards models, other central obesity indices (waist circumference, BRI, WWI, RFM and BSI) were positively associated with all-cause, cardiovascular, and other-cause mortality. In contrast, other overall obesity indices (BSA and standardized body weight percentage) were negatively associated with mortality (Supplementary material Table S 3 ). Second, in subgroups stratified by age, sex, ethnicity, and diabetes, the association pattern between continuous BMI or WHtR and mortality was consistent with the primary analysis (Supplementary material Figures S 3 -S 6 ). Third, exclusion of individuals with less than 1 year of follow-up did not substantially change the results (Supplementary material Figure S 7 ). Fourth, a complete case analysis showed that missing data did not distort the current findings (Supplementary material Figure S 8 ).
In a large nationally representative cohort of US adults, we examined the association between various anthropometric measures and all-cause and cause-specific mortality with a maximum follow-up of 11.3 years. We found that BMI and WHtR had diametrically opposite associations with mortality risk. The association between BMI and mortality was inversely J-shaped, whereas the association between WHtR and mortality was positively J-shaped. Other anthropometric indices of overall obesity also suggested a negative association between obesity and mortality, whereas none of the central obesity indices supported such a counterintuitive relationship. The current findings suggest that the obesity paradox may be an artifact of anthropometric measures, and that central obesity indices were independently and positively associated with all-cause death, cardiovascular death, and death from diseases other than cancer, eliminating the possibility that the obesity paradox exists.
The obesity-survival paradox has been previously reported in studies of critically ill patients, the elderly, and the general population [8-10]. A meta-analysis involving 218,532 patients with cardiovascular disease also demonstrated that total mortality was lower in overweight and obese patients than in normal weight patients, with a hazard ratio of approximately 0.70 [ 19 ]. Using BMI as an anthropometric indicator, we replicated this counterintuitive association through an inversely J-shaped pattern, whereby the risk of death decreased gradually within the initial units of BMI and then reached a plateau. All the nadirs of mortality risk were in the overweight range, lending further credence that a higher BMI may be protective for survival. In addition, when other indices of overall obesity were examined, the results were similar to those of the BMI, suggesting that overall obesity indices derived from weight-based calculations are consistent in estimating mortality risk.
Previous findings on the association between central obesity indices and adverse outcomes have been heterogeneous, with some studies reporting J-shaped or monotonic positive associations and others showing negative or null associations [ 20 , 21 , 22 , 22 ]. Methodologically, some of these studies did not address the hard endpoint of mortality, some focused on all-cause mortality and lacked data on cause-specific mortality, and some analyzed only a single anthropometric index. Our results provide evidence on these unaddressed issues. We found a positively J-shaped association between WHtR and all-cause, cardiovascular, and other-cause mortality, independent of BMI. The risk inflection point occurred around a WHtR of 0.6, slightly above the currently recommended threshold of 0.5, with slight change in the risk of death until the inflection point, followed by a sharp and linear increase. This positive association pattern was consistently observed for other anthropometric indices of central obesity, including waist circumference, BRI, WWI, RFM and BSI, albeit slightly attenuated for RFM and BSI. The current findings are in accordance with those of several recent large studies revealing an independent positive association between central obesity indices and adverse outcomes (e.g., premature mortality, heart failure hospitalization, cardiometabolic risk), some of which used a Mendelian randomized design to infer causality [ 23 , 24 , 25 ]. We did not find a substantial association between WHtR and cancer mortality. The association between obesity and cancer incidence and mortality varies by cancer site. The International Agency for Research on Cancer (IARC) has reported seven cancers for which there is compelling evidence of a dose–response relationship with obesity, including cancers of esophagus, colorectum, pancreas, cardia, liver, gallbladder, and kidney [ 26 ]. However, the three most common cancers in the current cohort were breast (15.4%), prostate (15.3%), and skin cancers (14.8%), which accounted for nearly half of individuals with cancers. This may explain our inability to find a clear association between obesity and cancer death in this nationally representative population.
The divergent association pattern may be due to differences in population-level risk classification using different anthropometric measures. In the present study, despite a strong linear correlation between BMI and WHtR, the consideration of WHtR resulted in a significant reclassification of individuals with normal weight. Only half of the normal weight individuals fell within the normal range of WHtR, suggesting that the pathophysiological milieu of the remaining half may be overlooked. There was less misclassification of overweight or obese individuals, with 98% having a WHtR greater than 0.5. A minority of overweight or obese individuals have a normal WHtR, which may be due to increased muscle mass rather than fat accumulation. These individuals, known as metabolically healthy obese (MHO), have been previously documented [ 27 ]. Our findings suggest that BMI is not sufficient to identify the high-risk phenotype for central obesity as defined by the WHtR, especially in those with normal weight (underestimation of risk). Previous evidence has also shown that high-risk characteristics for central obesity include a higher ratio of visceral-to-subcutaneous adipose tissue, a larger waist circumference, and a higher ratio of waist circumference to hip or leg circumference [ 28 ], which can be captured by central obesity indices rather than BMI alone.
Epidemiological and genetic evidence has shown that the regional distribution of fat may be more important than its absolute mass in predicting obesity-related metabolic risk [ 14 , 25 , 29 ]. Computed tomography (CT) and magnetic resonance imaging (MRI) allow accurate quantification of the body compositions at each level, thereby identifying subcutaneous adipose tissue (e.g., gluteal and thigh fat) and VAT (e.g., intra-abdominal and ectopic fat) [ 30 , 31 ]. However, CT involves ionizing radiation and MRI is time consuming, both are expensive and require specially trained personnel to perform. DXA serves as a viable alternative with low radiation exposure and low cost, and has been validated by CT and MRI in identifying the high-risk metabolic phenotype [ 32 , 33 ]. We found moderate correlations between anthropometric indices and VAT measurements based on DXA, it is in line with the expectation that an anthropometric indicator that can only make a rough estimate of fat distribution. Specifically, WHtR and waist circumference had stronger correlations with VAT measurements than BMI, whereas body weight had the weakest correlation, and the correlation coefficients were in agreement with previous studies [ 32 ]. Mechanistically, subcutaneous adipose tissue plays a critical role in energy storage and thermoregulation, and when its storage capacity is saturated, adipotoxic VAT deposition occurs. VAT exerts adipocyte biological effects through increased secretion of pro-inflammatory adipokines and decreased secretion of anti-inflammatory adipocytokines [ 2 , 34 ]. Consequently, VAT creates an atherogenic, diabetogenic, and inflammatory milieu leading to downstream metabolic dysregulation and cardiovascular damage [ 35 ]. Because routine measurement of VAT may be impractical, the use of alternative anthropometric indicators as simple estimates in clinical practice is promising. The stronger correlation between WHtR and VAT measurements compared to BMI may partly explain why WHtR provides a better estimate for adverse outcomes.
The growing obesity epidemic is associated with substantial mortality, morbidity, and health care expenditures. Therefore, obesity has been included in the global targets for the control of non-communicable diseases (NCD) [ 36 ]. Based on current research, we have several considerations. First, it is imperative to implement comprehensive and effective prevention strategies that focus on promoting healthy lifestyles and controlling excessive weight gain. However, the existence of the obesity-survival paradox may cause confusion and hesitation among the public and policy makers. Our findings suggest that the obesity paradox may be an artifact of anthropometric measures rather than an actual biological advantage of excess fat storage, which dispels concerns that being overweight or obese improves survival over being normal weight. Second, susceptibility to obesity-related metabolic risk may be mediated by visceral fat, and anthropometric measures of central obesity provide independent and additive information beyond BMI in characterizing adverse risk. A growing number of obesity professional societies have recommended that central obesity indices (waist circumference or WHtR) should be routinely used alongside BMI for the stratification and management of obesity [ 15 , 37 ]. Third, accurate assessment of obesity requires consideration of the validity, feasibility, and standardization of assessment indicators. Measuring waist circumference alone is inadequate because it does not consider the effect of height, which is significantly and inversely associated with health risks such as cardiovascular disease and cancer [ 38 , 39 ]. Waist-hip ratio is a valid indicator for considering both VAT and lower-body subcutaneous adipose tissue. However, hip circumference is less readily available, making waist-to-hip ratio less practical. The WHtR corrects waist circumference by height, normalizing the threshold to 0.5, regardless of gender, age, and ethnicity. This simplifies the health message to the notion that waist circumference should not exceed half of one's height, offering a more feasible and pragmatic measure for both health professionals and the general population. Finally, further research should focus on whether the adoption of these anthropometric metrics can meaningfully enhance risk prediction algorithms beyond traditional measurements, and whether these anthropometric indicators can serve as valid targets for risk reduction.
Strengths and limitations
We applied the weights in each of the models to account for oversampling of minority groups, survey nonresponse, and post-stratification adjustments. Baseline anthropometric measurements were completed by trained technicians rather than self-reported height and weight, thereby mitigating anthropometric bias. A wide range of covariates were adjusted to maximize consideration of confounding factors. Mortality events were provided by the NCHS using an enhanced linkage algorithm that allowed for 98.5% matching accuracy. Several limitations should be noted. First, due to the inherent limitations of the observational study, we cannot prove a causality relationship. Second, despite our efforts to comprehensively adjust for confounders, residual confounders may still exist. However, the statistical E-values for the associations between WHtR > 0.8 and all-cause, cardiovascular, and other-cause mortality were 3.83, 6.64 and 7.14, respectively, implying that the unmeasured confounders should have an association with the exposure (WHtR > 0.8) and outcome (mortality) comparable to these values to negate the current results. Third, although we excluded individuals with less than 1 year of follow-up to rule out reverse causation and obtain similar risk estimates, complete elimination of reverse causation cannot be achieved because individuals may survive with a disease for a longer period before succumbing to it. Fourth, DXA data were available for only half of the cohort, so the correlation between anthropometric indices and VAT measures should be interpreted with caution.
Anthropometric measures reflecting central obesity were independently and positively associated with mortality risk, eliminating the possibility of an obesity paradox. WHtR provides additional information beyond BMI and can be used as a valid anthropometric indicator for physical examination screening.
Availability of data and materials
The NHANES data that support the findings of this study are available on the Center for Disease Control web site ( https://www.cdc.gov/nchs/nhanes/ ).
World Obesity Atlas 2022, World Obesity Federation. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022 . Accessed in May 2022.
González-Muniesa P, Mártinez-González MA, Hu FB, et al. Obesity Nature reviews Disease primers. 2017;3:17034.
Article PubMed Google Scholar
Jayedi A, Rashidy-Pour A, Khorshidi M, Shab-Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose-response meta-analysis of more than 2 3 million participants. Obesity reviews an official journal of the international association for the study of obesity. 2018;19(5):654–67.
Article CAS PubMed Google Scholar
Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet (London, England). 2014;383(9921):970–83.
Compston JE, Flahive J, Hosmer DW, et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(2):487–93.
Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ (Clinical research ed). 2017;356:j477.
Afshin A, Forouzanfar MH, Reitsma MB, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377(1):13–27.
Lv Y, Mao C, Gao X, et al. The obesity paradox is mostly driven by decreased noncardiovascular disease mortality in the oldest old in China: a 20-year prospective cohort study. Nature aging. 2022;2(5):389–96.
Sakr Y, Alhussami I, Nanchal R, et al. Being Overweight Is Associated With Greater Survival in ICU Patients: Results From the Intensive Care Over Nations Audit. Crit Care Med. 2015;43(12):2623–32.
Lu JL, Molnar MZ, Naseer A, Mikkelsen MK, Kalantar-Zadeh K, Kovesdy CP. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol. 2015;3(9):704–14.
Article PubMed PubMed Central Google Scholar
Schetz M, De Jong A, Deane AM, et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–69.
Chandramouli C, Tay WT, Bamadhaj NS, et al. Association of obesity with heart failure outcomes in 11 Asian regions: A cohort study. PLoS Med. 2019;16(9):e1002916.
Article CAS PubMed PubMed Central Google Scholar
Tsujimoto T, Kajio H. Abdominal Obesity Is Associated With an Increased Risk of All-Cause Mortality in Patients With HFpEF. J Am Coll Cardiol. 2017;70(22):2739–49.
Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13(3):275–86.
Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16(3):177–89.
Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). About the National Health and Nutrition Examination Survey (NHANES). Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm . Accessed on 21 Nov 2022.
Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). NCHS Research Ethics Review Board (ERB) Approval. Available online: https://www.cdc.gov/nchs/nhanes/irba98.htm . Accessed on 21 Nov 2022.
Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). NCHS Public-use Linked Mortality Files. Available online: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm . Accessed on 21 Nov 2022.
Niedziela J, Hudzik B, Niedziela N, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol. 2014;29(11):801–12.
Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk? evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12(9):680–7.
Carmienke S, Freitag MH, Pischon T, et al. General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr. 2013;67(6):573–85.
Chen Y, Yang Y, Jiang H, Liang X, Wang Y, Lu W. Associations of BMI and Waist Circumference with All-Cause Mortality: A 22-Year Cohort Study. Obesity (Silver Spring, Md). 2019;27(4):662–9.
Jayedi A, Soltani S, Zargar MS, Khan TA, Shab-Bidar S. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ (Clinical research ed). 2020;370:m3324.
PubMed PubMed Central Google Scholar
Butt JH, Petrie MC, Jhund PS, et al. Anthropometric measures and adverse outcomes in heart failure with reduced ejection fraction: revisiting the obesity paradox. Eur Heart J. 2023;44(13):1136–53.
Emdin CA, Khera AV, Natarajan P, et al. Genetic Association of Waist-to-Hip Ratio With Cardiometabolic Traits, Type 2 Diabetes, and Coronary Heart Disease. JAMA. 2017;317(6):626–34.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer-Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8.
Phillips CM. Metabolically healthy obesity across the life course: epidemiology, determinants, and implications. Ann N Y Acad Sci. 2017;1391(1):85–100.
Yaghootkar H, Scott RA, White CC, et al. Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes. 2014;63(12):4369–77.
Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr Res Rev. 2010;23(2):247–69.
Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131–41.
Neeland IJ, Poirier P, Després JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137(13):1391–406.
Kaul S, Rothney MP, Peters DM, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring, Md). 2012;20(6):1313–8.
Borga M, West J, Bell JD, et al. Advanced body composition assessment: from body mass index to body composition profiling. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2018;66(5):1–9.
Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. 2022;185(3):419–46.
Neeland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–25.
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet (London, England). 2016;387(10026):1377–96.
National Institute for Health and Care Excellence: Guidelines. Obesity: identification, assessment and management. In: London: National Institute for Health and Care Excellence (NICE). 2023.
Google Scholar
Paajanen TA, Oksala NK, Kuukasjärvi P, Karhunen PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur Heart J. 2010;31(14):1802–9.
Davey Smith G, Hart C, Upton M, et al. Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. J Epidemiol Community Health. 2000;54(2):97–103.
Download references
Acknowledgements
Not Applicable.
This research received no external funding.
Author information
Shan Li and Zhiqing Fu are contributed equally to this work.
Authors and Affiliations
Department of Cardiology, Second Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, 100853, China
Shan Li & Zhiqing Fu
National Clinical Research Center for Geriatric Diseases, Chinese People’s Liberation Army General Hospital, Beijing, 100853, China
Shan Li, Zhiqing Fu & Wei Zhang
Department of Outpatient, The Second Medical Center, Chinese People’s Liberation Army General Hospital, Beijing, 100853, China
You can also search for this author in PubMed Google Scholar
Contributions
S.L. has fully obtained all of the data and take responsibility for the integrity of the data and the accuracy of the analysis. S.L. and Z.F. contribute to the conceptualization and study design. S.L. and W.Z. contribute to the acquisition, statistical analysis and interpretation of the data. W.Z. and Z.F. contribute to the drafting of the manuscript. All authors are responsible for the critical revision of the manuscript for important content.
Corresponding author
Correspondence to Shan Li .
Ethics declarations
Ethics approval and consent of participate.
All the NHANES protocols were approved by the National Center for Health Statistics ethics review board (Protocol #2005–06, effective beginning October 26, 2004; Protocol #2011–17, effective beginning October 26, 2010; Protocol #2018–01, effective beginning October 26, 2017). Written informed consent was obtained from all adult participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, S., Fu, Z. & Zhang, W. Association of anthropometric measures with all-cause and cause-specific mortality in US adults: revisiting the obesity paradox. BMC Public Health 24 , 929 (2024). https://doi.org/10.1186/s12889-024-18418-9
Download citation
Received : 28 November 2023
Accepted : 22 March 2024
Published : 01 April 2024
DOI : https://doi.org/10.1186/s12889-024-18418-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Central obesity
- Overall obesity
- Obesity paradox
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 26 March 2024
Real-world association between systemic corticosteroid exposure and complications in US patients with severe asthma
- Thomas B Casale 1 ,
- Thomas Corbridge 2 ,
- Guillaume Germain 3 ,
- François Laliberté 3 ,
- Sean D MacKnight 3 ,
- Julien Boudreau 3 ,
- Mei S Duh 4 &
- Arijita Deb 5
Allergy, Asthma & Clinical Immunology volume 20 , Article number: 25 ( 2024 ) Cite this article
126 Accesses
Metrics details
Systemic corticosteroid (SCS) use remains widespread among patients with severe asthma, despite associated complications.
Evaluate the association between cumulative SCS exposure and SCS-related complications in severe asthma.
This retrospective, longitudinal study used claims data from the Optum Clinformatics Data Mart database (GSK ID: 214469). Eligible patients (≥ 12 years old) had an asthma diagnosis and were divided into two cohorts: SCS use and non/burst-SCS use. Patients in the SCS use cohort had a claim for a daily prednisone-equivalent dose ≥ 5 mg SCS following ≥ 6 months of continuous SCS use; those in the non/burst-SCS cohort had no evidence of continuous SCS use and had a non-SCS controller/rescue medication initiation claim. For each cohort, the date of the qualifying claim was the index date. SCS users were further stratified by SCS use during each quarter of follow-up: low (≤ 6 mg/day), medium (> 6–12 mg/day), high (> 12 mg/day), and continuous high (≥ 20 mg/day for 90 days). SCS-related complications were evaluated in the quarter following SCS exposure. The adjusted odds ratios (OR) of experiencing SCS-related complications during follow-up in each of the SCS use groups versus the non/burst SCS cohort were calculated using generalized estimating equations models.
SCS and non/burst-SCS use cohorts included 7473 and 89,281 patients (mean follow-up: 24.6 and 24.2 months), respectively. Compared with the non/burst-SCS use cohort, medium, high, and continuous high SCS use was associated with greater odds of any SCS-related complication (adjusted OR [95% confidence interval]: 1.30 [1.21, 1.39], 1.49 [1.35, 1.64] and 1.63 [1.40, 1.89], respectively) including increased acute gastrointestinal, cardiovascular, and immune system-related complications, and chronic cardiovascular, metabolic/endocrine, central nervous system, bone-/muscle-related, ophthalmologic, and hematologic/oncologic complications. Low-dose SCS use was also associated with significantly increased odds of acute gastrointestinal and immune system-related complications, and chronic bone-/muscle-related and hematologic/oncologic complications versus the non/burst-SCS use cohort.
SCS use, even at low doses, is associated with increased risk of SCS-related complications among patients with severe asthma.
Patients with severe asthma, accounting for 3–10% of the total asthma population, require treatment with high-dose inhaled corticosteroids (ICS) plus a second controller and/or SCS to control symptoms [ 1 , 2 ]. However, their asthma can remain uncontrolled despite these therapies [ 1 , 2 ]. Historically, a primary component of severe asthma management has been maintenance treatment with and/or rescue bursts of SCS, which includes both oral corticosteroids (OCS) and parenteral corticosteroids delivered via subcutaneous and intravenous routes [ 2 , 3 , 4 ]. However, the adverse effects of short- and long-term SCS use are well documented and include osteoporosis, bone fractures, cardiovascular disease, gastrointestinal conditions, impaired immune response, alterations in glucose and lipid metabolism, and psychiatric disturbances [ 5 , 6 , 7 , 8 , 9 , 10 ]. Importantly, while the risk of SCS-related complications has been shown to increase in a dose-dependent manner [ 6 ], even patients with low SCS exposure are at greater risk of SCS-related complications than non-users [ 8 ]. In addition to the substantial clinical burden associated with SCS-related complications, the increased risk also translates to a greater economic burden in patients with severe asthma, with higher annual healthcare costs and healthcare resource utilization (HCRU) in SCS users versus non-users [ 5 , 6 , 8 , 9 ].
The Global Initiative for Asthma (GINA) guideline (2023) acknowledges the substantial adverse effects of OCS use and recommends only considering maintenance OCS as a last resort [ 2 ]. Several biologic agents have been approved for the treatment of severe asthma, including omalizumab, reslizumab, tezepelumab, mepolizumab, benralizumab, and dupilumab [ 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ]. These agents have demonstrated OCS-sparing effects both in clinical trials and in real-world settings [ 16 , 19 , 20 , 22 , 23 , 24 ]. While the cost effectiveness of biologics has been debated [ 25 ], and reductions in exacerbations requiring hospitalization are the primary driver of cost effectiveness with biologic therapy [ 26 , 27 ], chronic OCS reduction has also been reported to contribute to the lifetime discounted cost effectiveness of biologic therapy [ 28 ]. Despite the introduction of biologics in several countries worldwide, evidence suggests that patients remain unnecessarily reliant on OCS for the management of asthma [ 29 ]. Currently, there are limited data available on the association between cumulative SCS exposure and related complications since the introduction of biologic therapies; therefore, updated assessments are needed.
The objective of this real-world, retrospective study was to evaluate the association between cumulative SCS exposure and SCS-related complications and HCRU in patients with severe asthma, stratified by SCS use.
Data source
This was a retrospective, longitudinal study using medical, and pharmacy claims data from the Optum Clinformatics Data Mart (CDM) database (GSK ID: 214469). Optum CDM covers approximately 15–20 million annual lives of UnitedHealth Group members in all US census regions and includes data from January 1, 2014, to December 31, 2020. The database comprises both commercial and Medicare Advantage health plan data. This study used fully de-identified data compliant with the Health Insurance Portability and Accountability Act; therefore, informed consent, ethics committee or institutional review board approval was not required.
Study design
The study design is outlined in Fig. 1 . Two cohorts were defined: the SCS use and non/burst-SCS use cohorts (see Patients section for eligibility criteria of each cohort). The index date for patients in the SCS use cohort was the date of the first pharmacy or medical claim for SCS with a daily prednisone-equivalent dose ≥ 5 mg following 6 months of continuous SCS use (defined as ≥ 5 mg/day of SCS for 6 months without any gap of > 14 days between the last day of supply of a claim and the next claim; Fig. 1 ).
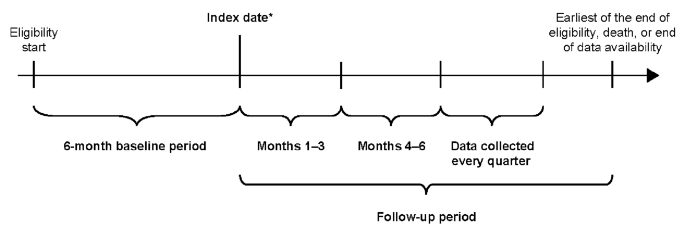
Study design. *First pharmacy/medical claim date for SCS ≥ 5 mg/day following 6 months of continuous (≥ 5 mg/day for 6 months without any gap > 14 days between claims) SCS use (SCS use cohort) or date of initiation of any non-SCS asthma controller/rescue medication (non/burst-SCS use cohort). SCS, systemic corticosteroid
The 6-month period of continuous SCS use, and the ≥ 5 mg daily prednisone equivalent threshold were used for consistency with prior retrospective studies and a randomized clinical trial [ 6 , 7 , 8 , 16 ]. The index date for patients in the non/burst-SCS use cohort was defined as the date of initiation of any asthma controller or rescue medication other than SCS (Fig. 1 ); if more than one asthma medication other than SCS was taken among patients in the non/burst-SCS cohort, the index date was randomly assigned among the asthma medication initiation dates. The index date for the non/burst-SCS cohort was selected randomly among asthma medication initiation dates instead of selecting the first asthma initiation date to avoid indexing non/burst-SCS patients at a systematically earlier timepoint in their treatment journey relative to the SCS cohort.
Data were collected for the 6-month period preceding the index date (baseline period; for evaluation of demographic and clinical characteristics) and for a variable-length, follow-up period. The length of the follow-up period spanned from the index date up to the end of eligibility, death, or end of data availability, whichever occurred first.
To account for the change in cumulative SCS exposure over time, the follow-up period was segmented into quarterly (90-day) intervals, and only complete quarters of follow-up were assessed. The association between cumulative SCS exposure (measured as the average daily dose received from index up to the first day of each quarter) and study outcomes was evaluated repeatedly for each quarter of follow-up. The first quarter post-index was reserved for the assessment of SCS exposure, whereas outcomes were evaluated starting in the second quarter post-index.
Eligible patients were ≥ 12 years of age on the index date, had ≥ 6 months of continuous eligibility both before and after the index date and had ≥ 2 primary or secondary asthma diagnosis codes any time before or on the index date. International Classification of Diseases (ICD)-9/10-clinical modification (CM) diagnosis codes are included in Additional File 1 (Supplementary Table 1 ). Patients with severe asthma included in the SCS use cohort were required to have ≥ 6 months of continuous use of oral or parenteral SCS (see Study design section for definition of continuous use) with a daily dose equivalent to ≥ 5 mg prednisone, and ≥ 1 dispensing or administration of SCS with a daily dose equivalent to ≥ 5 mg prednisone after the previous 6 months of continuous SCS use. The non/burst-SCS use cohort included patients with asthma and no claim for any OCS or parenteral SCS used to treat asthma at any time during their eligibility period, except for those received as part of an outpatient (OP) or emergency room (ER) exacerbation, and with ≥ 1 dispensing for a controller or rescue medication for asthma other than SCS. OP/ER exacerbations were defined as an asthma-related OP or ER visit with ≥ 1 claim for an SCS within − 4/+5 days of the encounter. Asthma-related visits were identified as those with a claim with a primary diagnosis of asthma.
Patients from both cohorts were excluded if they had ≥ 1 primary or secondary diagnosis of cancer of the respiratory and intrathoracic system any time before or on the index date, or if they had ≥ 1 primary or secondary diagnosis for other conditions where OCS is commonly used, including rheumatoid arthritis, Crohn’s disease, ulcerative colitis, systemic lupus erythematosus, or multiple sclerosis, at any time during the study period.
The primary outcome was the frequency of SCS-related complications (any, acute, and chronic) during the follow-up period, identified based on the presence of associated diagnosis codes (list of all SCS-related complications defined in Additional File 1 ; Supplementary Table 2 ). Secondary outcomes included HCRU rates due to SCS-related complications during the follow-up period, and SCS treatment patterns during the follow-up period (SCS use cohort only). HCRU due to SCS-related complications included IP visits (hospitalizations and skilled nursing facilities), ER visits, OP visits, and other visits (including home services and hospice visits), and were identified with a primary or secondary diagnosis code for any SCS-related complication. Patient demographics and characteristics at baseline were also described.
For the assessment of SCS-related complications and HCRU due to SCS-related complications, patients in the SCS use cohort were stratified into mutually exclusive subgroups based on SCS exposure, measured as the average daily dose (prednisone-equivalent) from the index date up to the first day of each measurement quarter using an open-cohort approach. Further, patients were classified into low (≤ 6 mg/day), medium (> 6–12 mg/day), and high (> 12 mg/day) SCS exposure subgroups. In addition, patients using ≥ 20 mg/day of SCS for 90 days (assessed in the 90 days preceding each quarter) were included in a non-mutually exclusive continuous high-dose subgroup [ 30 , 31 , 32 ]. Finally, patients in the SCS use cohort with ≥ 3 SCS bursts or ≥ 4 SCS bursts between the index date and the first day of each quarter were included in two further non-mutually exclusive subgroups. An SCS burst was defined as a pharmacy or medical claim for SCS with 2–28 days of supply and an average daily dose equivalent to prednisone ≥ 20 mg. Multiple SCS bursts fewer than 14 days apart were considered as a single SCS burst.
Statistical analysis
Patient baseline demographics and characteristics were assessed descriptively, with mean, standard deviation (SD), and median values for the continuous variables, and frequencies and proportions for the categorical variables. Differences between the SCS use and non/burst-SCS use cohorts were assessed using standardized differences, with a difference between cohorts ≥ 10% considered important [ 33 ].
The frequency of SCS-related complications and the rates of HCRU due to SCS-related complications during the follow-up period were analyzed using adjusted odds ratios (OR) with 95% confidence intervals (CI) calculated from generalized estimating equations (GEE) models using a binomial distribution for complications, and a Poisson distribution for HCRU. Adjusted GEE models controlled for the following baseline covariates: age, sex, year of index date, region, insurance plan type, physician specialty, Quan-Charlson comorbidity index (CCI) score [ 34 ], respiratory and other medications (≥ 5% prevalence in either cohort), asthma-related exacerbations (IP and OP/ER) during baseline and on the index date, all-cause and asthma-related HCRU and costs (IP, ER, OP, and other visit components), Elixhauser and asthma-related comorbidities (≥ 5% prevalence in either cohort), and baseline SCS-related complications (≥ 5% prevalence in either cohort).
SCS treatment patterns were assessed per quarter and were also reported over the entire follow-up period. Metrics evaluated over the entire follow-up period were reported per patient per quarter and were weighted by each patient’s length of observation (in complete quarters). A sensitivity analysis was conducted comparing SCS complications among the low-dose SCS use subgroup versus the non/burst-SCS use cohort after removing patients with any SCS exposure during follow-up from the non/burst-SCS user cohort (i.e., patients with SCS exposure as part of an OP or ER exacerbation during the follow-up).
Patient population
Overall, 7473 patients in the SCS use cohort and 89,281 in the non/burst-SCS use cohort met the eligibility criteria and were included in the analysis (Additional File 2 ; Supplementary Fig. 1 ).
Compared with the non/burst-SCS use cohort, the SCS use cohort was older, had a greater proportion of Medicare enrollees, and had a lower proportion of patients managed by primary care physicians at index date (Table 1 ).
During the 6-month baseline period, the SCS use cohort also had a higher mean Quan-CCI score, and more frequent medication use (including controllers and rescue medications) compared with the non/burst-SCS use cohort (Table 2 ). In general, all-cause, and asthma-related HCRU was higher in the SCS use cohort compared with the non/burst-SCS use cohort, with more frequent all-cause and asthma-related IP and OP visits (Table 2 ). The associated all-cause and asthma-related healthcare costs followed a similar trend (Additional File 1 ; Supplementary Table 3 ). In the SCS use cohort, the mean numbers of IP exacerbations and OP/ER asthma exacerbations during baseline were higher than in the non/burst-SCS use cohort (Table 2 ).
Baseline chronic SCS-related complications, including metabolic and endocrine complications, cardiovascular complications, and bone- and muscle-related complications, were more frequent in the SCS use versus non/burst-SCS use cohort (Table 2 ). Acute bone- and muscle-related complications occurred in similar proportions in both cohorts. Patients in the SCS use cohort also more frequently had hypertension, diabetes, and cardiac arrhythmias compared with those in the non/burst-SCS use cohort (Additional File 1 ; Supplementary Table 3 ).
SCS exposure
The mean length of post-index follow-up period was similar in the SCS use cohort (24.6 months) and the non/burst-SCS use cohort (24.2 months) (Table 1 ). The number of patients in both cohorts decreased with follow-up length. Of the 7473 patients in the SCS use cohort at index, 5818 (77.9%) were observed for ≥ 1 year post-index, 3257 (43.6%) for ≥ 2 years post-index, and 1684 (22.5%) for ≥ 3 years post-index (Additional File 1 ; Supplementary Table 4 ). During the first quarter of follow-up (Months 1–3), the SCS use cohort included 3175 (42.5%) patients with low SCS exposure, 2532 (33.9%) patients with medium SCS exposure, and 1766 (23.6%) patients with high SCS exposure, and 452 (6.0%) patients had continuous high exposure. These proportions remained similar throughout the follow-up period. The proportion of patients in the SCS use cohort with ≥ 3 and ≥ 4 SCS bursts was initially low (0.8% and 0.0%, respectively) and increased throughout the follow-up period to 66.7% and 56.4%, respectively, at Months 73–75.
SCS-related complications during the follow-up period
Patients with medium, high, and continuous high SCS dose, along with patients with ≥ 3 and ≥ 4 SCS bursts, had significantly greater odds (adjusted OR: 1.30–1.63) of developing any SCS-related complication than the non/burst-SCS use cohort (Fig. 2 ). Numerically, the greatest odds for developing any SCS-related complication were in patients with continuous high SCS dose (Fig. 2 ).
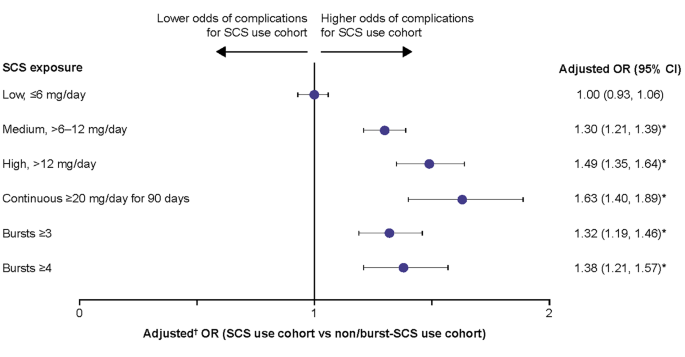
Odds of any SCS-related complications during the follow-up period due to SCS exposure. Prednisone-equivalent SCS dose; * p -value < 0.05; † from multivariate GEE models adjusting for patient demographics and baseline clinical characteristics (see Methods for covariates). CI, confidence interval; GEE, generalized estimating equations; OR, odds ratio; SCS, systemic corticosteroid
Patients with medium, high, and continuous high SCS dose, along with those with ≥ 3 and ≥ 4 SCS bursts, had significantly greater odds of developing acute gastrointestinal (adjusted OR: 1.25–1.57), cardiovascular (adjusted OR: 1.35–1.84), and immune system-related (adjusted OR: 1.22–1.47) complications than the non/burst-SCS use cohort (Fig. 3 ). Patients with low-dose SCS also had significantly greater odds of developing acute gastrointestinal (adjusted OR: 1.09) and immune system-related (adjusted OR: 1.09) complications than the non/burst-SCS use cohort (Fig. 3 ).
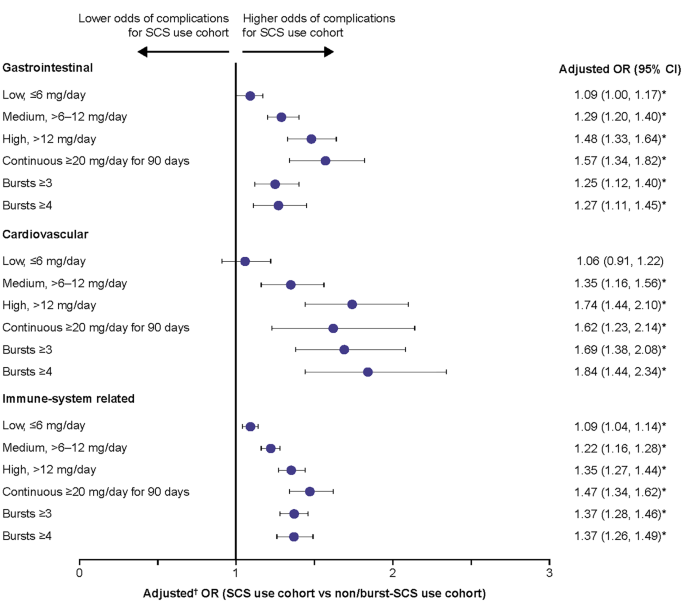
Odds of acute SCS-related complications during the follow-up period due to SCS exposure. Prednisone-equivalent SCS dose; * p -value < 0.05; † from multivariate GEE models adjusting for patient demographics/baseline clinical characteristics (see Methods for covariates). CI, confidence interval; GEE, generalized estimating equations; OR, odds ratio; SCS, systemic corticosteroid; SD standard deviation
For chronic SCS-related complications, patients with medium, high, and continuous high SCS dose, along with patients with ≥ 3 and ≥ 4 SCS bursts, had significantly higher odds of developing any of the complications assessed versus the non/burst-SCS use cohort, with the exception of central nervous system and ophthalmologic complications in the medium-dose subgroup (Fig. 4 ). In the low-dose subgroup, the odds of developing a bone and muscle-related or hematologic/oncologic complication were significantly increased versus the non/burst-SCS use cohort. The highest ORs were observed for hematologic/oncologic complications. The increased odds of chronic complications were dose-dependent, with the greatest odds for complications in patients with continuous high SCS dose. Overall proportions of patients with complications within each category are shown in Additional File 1 , Supplementary Table 5 . In the sensitivity analysis excluding patients with any SCS exposure during follow-up, patients with low SCS dose (≤ 6 mg/day) had increased odds of most SCS-related complications when compared with the non/burst-SCS user cohort (Additional File 2 ; Supplementary Fig. 2 ).
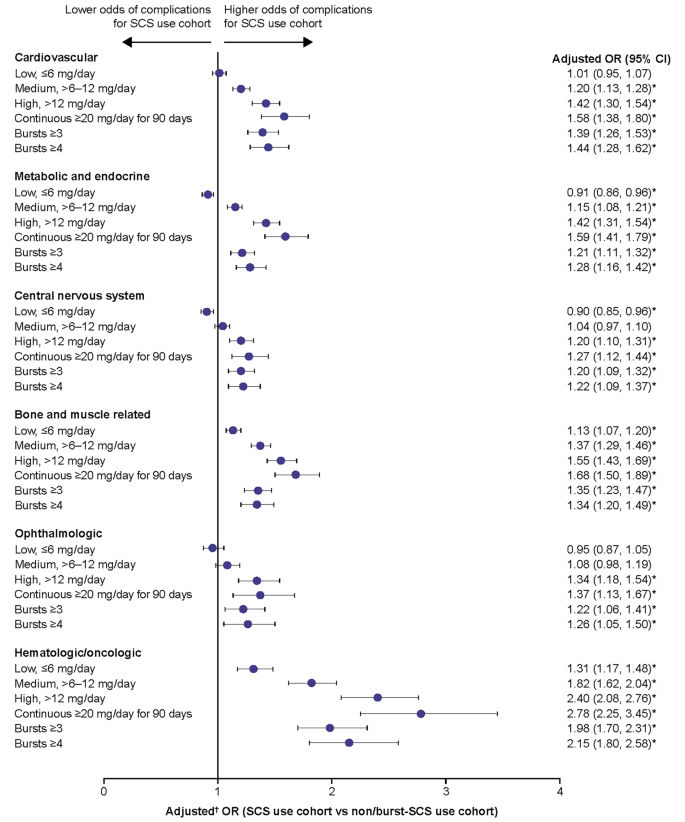
Odds of chronic SCS-related complications during the follow-up period due to SCS exposure. Prednisone-equivalent SCS dose; * p -value < 0.05; † from multivariate GEE models adjusting for patient demographics and baseline clinical characteristics (see Methods for covariates). CI, confidence interval; GEE, generalized estimating equations; OR, odds ratio; SCS, systemic corticosteroid
HCRU due to SCS-related complications
The rates of all types of HCRU with a diagnosis code for SCS-related complications during the follow-up period were higher in patients with medium, high, and continuous high SCS dose, and in those with ≥ 3 and ≥ 4 SCS bursts, compared with the non/burst-SCS use cohort (adjusted rate ratio [RR]: 1.14–2.12) (Fig. 5 ). Patients with a low SCS dose had a statistically significantly higher rate of IP visits (adjusted RR: 1.27) and other visits (adjusted RR 1.13) than those in the non/burst-SCS use cohort, although the adjusted RR was lower than for patients with medium, high, and continuous high SCS dose.
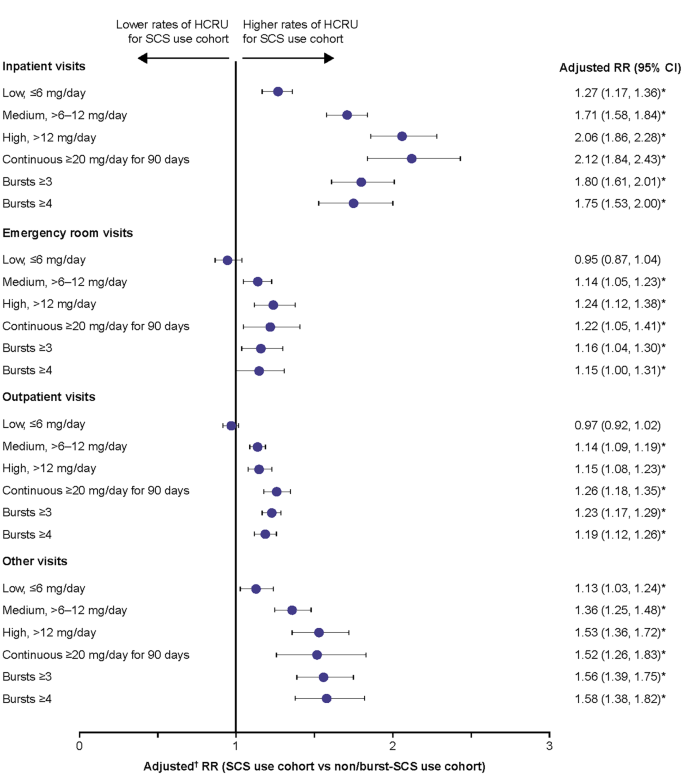
Rate of HCRU due to SCS-related complications during the follow-up period. Prednisone-equivalent SCS dose; * p -value < 0.05; † from multivariate GEE models adjusting for patient demographics/baseline clinical characteristics (see Methods for covariates). CI, confidence interval; GEE, generalized estimating equations; HCRU, healthcare resource utilization; RR, rate ratio; SCS, systemic corticosteroid
SCS treatment patterns in the SCS use cohort
In the SCS use cohort, the mean daily dose of SCS gradually decreased between Months 1–3 (9.4 mg/day) and Months 28–30 (to 6.5 mg/day), then stabilized at approximately 6–7 mg/day for the remainder of the follow-up period (Additional File 1 ; Supplementary Table 6 ).
This real-world retrospective study in patients with severe asthma using data from a large US claims database found that both burst and chronic SCS use, even at low (≤ 6 mg/day) doses, significantly increases the odds of developing SCS-related complications. Similarly, burst and chronic SCS use in this population was linked with significantly higher rates of HCRU due to SCS-related complications, with even low chronic SCS doses associated with more frequent IP and other HCRU visits. These data support existing evidence of the well-documented and wide-reaching adverse effects associated with SCS use by highlighting a dose-dependent increase in the risk of complications among patients with severe asthma who continue to be frequent users of SCS therapy [ 5 , 6 , 7 , 8 , 9 , 10 , 29 ]. They also suggest that even low doses of SCS are associated with complications and that complications occur with both maintenance and burst SCS use. Further, this study provides updated insight into the association between SCS use with related complications and HCRU in the era of biologic therapy.
In patients with a medium SCS dose or higher, including those with continuous high SCS dose and those with ≥ 3 or ≥ 4 SCS bursts, the odds of developing any SCS-related complication were 1.3–1.6 times greater than in the non/burst SCS use cohort. Notably, the increase in the risk of developing SCS-related complications was dose-dependent, with the greatest increase observed for patients with continuous high SCS use. These results build on previous findings that demonstrated the increased risk of SCS-related complications with greater SCS use in patients with asthma [ 5 , 6 , 7 , 8 ]. For example, in a retrospective claims analysis conducted in the US between 1997 and 2013, patients with medium (> 6–12 mg/day) and high (> 12 mg/day) SCS exposure had significantly higher risks of several different SCS-related complications versus those with low (≤ 6 mg/day) exposure, with ORs by complication type ranging from 1.29 to 2.12 for medium-dose users and 1.23 to 1.96 for high-dose users [ 7 ]. A further analysis of this dataset found a significant dose-response relationship between SCS exposure and any SCS-related complication, with ORs versus non-users increasing from 2.03 in low-dose (≤ 6 mg/day) users to 3.64 in high-dose (> 12 mg/day) users [ 8 ]. Similarly, in another retrospective claims analysis conducted in the US between 2003 and 2014, the odds of developing SCS-related complications increased in a dose-dependent manner with ORs compared with non-users ranging from 2.50 in patients with low SCS exposure (< 5 mg/day) to 3.32 in patients with high exposure (> 10 mg/day) [ 6 ]. The odds of developing any SCS-related complication reported in our study were lower in comparison to the studies conducted in 1997–2013 and 2003–2014 [ 6 , 8 ], which may reflect the ongoing shift from regular use of SCS within asthma management. Together these data highlight the significant association between SCS dose and SCS-related complications in the asthma population and support the guideline-recommended aim of minimizing SCS use in these patients [ 2 ].
In line with previous findings [ 6 , 7 , 8 ], the current study found dose-dependent increases in the risk of acute gastrointestinal, cardiovascular and immune-system related complications as well as chronic cardiovascular, metabolic and endocrine, central nervous system, bone- and muscle-related, ophthalmologic, and hematologic/oncologic complications. Notably, even patients with low SCS exposure had increased risk of developing acute gastrointestinal and immune-system related complications as well as chronic bone- and muscle-related and hematologic/oncologic complications compared with the non/burst SCS use cohort. These findings are largely consistent with those from a previous focused literature review observing an increased risk of these complications with low-dose SCS versus no exposure among patients with severe asthma [ 35 ]. For other complications with low-dose versus no SCS exposure, results have been more mixed, with two studies finding no increase in the risk of cardiovascular complications, and for metabolic and ophthalmologic events, one study each showing a positive association and no association; [ 35 ] this largely accords with the lack of a significantly increased risk for these complications with low-dose SCS use in the current study. However, when patients with any SCS exposure during the follow-up period were excluded from the non/burst-SCS use cohort in a sensitivity analysis, the odds of all except ophthalmologic SCS-related complications were increased in the low-dose SCS subgroup compared with the modified non/burst-SCS use cohort. Overall, these data indicate an increased risk of complications even among those with low SCS use.
In addition to the increased risk of SCS-related complications, this study found higher rates of HCRU associated with SCS-related complications among patients with medium-dose SCS use or greater, with RRs versus non/burst SCS users ranging between 1.14 and 2.12. While there was some indication that higher SCS exposure correlated with higher HCRU than lower exposure, the dose-response relationship was not as clear as for SCS-related complications. An association between increased SCS exposure and higher HCRU due to SCS-related complications has also been found in previous retrospective claim studies [ 6 , 7 , 8 ]. Two of these studies also found that, compared with no SCS use even low SCS doses were associated with an increased rate of IP and other HCRU visits, consistent with the current study [ 6 , 8 ]. Interestingly, Dalal et al., also found that low doses of SCS were associated with an increased rate of OP and ER visits compared with no SCS use [ 6 ]. Together, these findings indicate that beyond SCS-related complications, even low doses of SCS are associated with higher HCRU, suggesting a need for alternative corticosteroid-sparing management strategies among patients with severe asthma.
In the current study, baseline data generally showed higher comorbidity and concomitant medication use in SCS users versus non/burst-SCS users as well as higher all-cause and asthma-related healthcare costs. The proportion of SCS users without any controller therapy use appeared high at nearly 30%, which may indicate that some patients in the current study were using SCS as their controller therapy. These results appeared to align with other studies in asthma which did not require patients to be taking controller therapy. For example, in one claims analysis of patients with non-allergic asthma, 21.5% received fixed-dose ICS/ long-acting β2-agonists (LABA) during a 12-month baseline period, which was lower than the 42.5% during a 6-month baseline period in the current study [ 36 ]. While we did not collect data on healthcare costs during the follow-up period, the baseline differences may indicate that follow-up costs would also have been higher in SCS users, although their relationship with SCS-related complications is unclear. Outside of this study, it has been shown that healthcare costs are higher in SCS versus non-SCS users, with the costs associated with SCS-related complications increasing with SCS dose [ 5 , 6 , 7 , 8 , 9 ]. When combined with the adverse effects associated with SCS use, the higher healthcare costs among SCS users may be a further supporting factor for the use of SCS-sparing medications, such as biologics, in patients with severe asthma [ 16 , 19 , 20 , 22 , 24 ]. Furthermore, the cost effectiveness of biologic treatments has been demonstrated in a number of studies, particularly when treatment is targeted to specific responder populations [ 27 , 28 , 37 , 38 ].
There are several limitations to the current study. As with other claims-based analyses, the data used were collected for payment rather than research purposes and are subject to coding limitations and may contain data entry errors. In particular, the categorization of complications as acute or chronic was based on the presence of an associated diagnosis code, which is contingent upon the treating physician’s judgement. As noted previously, the same caveat applies to HCRU given that encounters with a diagnosis code indicative of an SCS-related complication could also be due to uncontrolled asthma. Also, the presence of a dispensed medication does not indicate that the medication was taken as prescribed, nor that it was taken on the date of dispensing. In addition, patients with SCS bursts only or no bursts were not evaluated separately in this analysis. It should also be noted that eligibility for the non/burst-SCS use cohort precluded continuous SCS exposure for ≥ 6 months (the same criteria that defined patients in the SCS cohort as having severe asthma); consequently, differences in asthma severity between cohorts may have potentially impacted outcome differences between cohorts. Next, as ICS was not included in the definition of SCS use, it is possible that corticosteroid exposure was underestimated. However, since the bioavailability of ICS is lower than that of SCS, the impact of this exclusion is expected to be small. Additionally, the claims database used in this analysis includes data from 2014, prior to approval of several biologics for severe asthma in the US [ 11 , 15 , 17 , 18 ]. However, the vast majority of patients included in the study had index dates after 2015. Therefore, the inclusion of data from 2014 may not have had a substantial effect on the relevance of this data for understanding the impact of SCS use since the introduction of biologic therapies. Finally, results may not be generalizable beyond the commercial and Medicare insurance population used. Nonetheless, these data provide valuable information on the burden of SCS use in patients with severe asthma and support a reduction of their use wherever possible in this population.
Conclusions
In conclusion, these findings add to the currently available evidence on the risks of SCS use for patients with severe asthma and characterize the continued impact of SCS use for healthcare providers, policy makers, and payers. As such, they provide further support for the use of SCS-sparing therapies such as biologics for the treatment of severe asthma.
Data availability
To access data for GSK sponsored research, please submit an enquiry via www.gsk-studyregister.com/en/ .
Abbreviations
confidence interval
Charlson comorbidity index
Clinformatics Data Mart
Clinical Modification
emergency room
generalized estimating equations
Global Initiative for Asthma
healthcare resource utilization
International Classification of Diseases
inhaled corticosteroid
long-acting β2-agonist
long-acting muscarinic antagonist
oral corticosteroids
short-acting β2-agonist
short-acting muscarinic antagonist
systemic corticosteroid
standard deviation
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73.
Article CAS PubMed Google Scholar
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention., 2023. Available from: https://ginasthma.org/wp-content/uploads/2023/07/GINA-2023-Full-report-23_07_06-WMS.pdf . [Last accessed August, 2023].
Sadatsafavi M, Khakban A, Tavakoli H, Ehteshami-Afshar S, Lynd LD, FitzGerald JM. Trends in oral corticosteroids use in severe asthma: a 14-year population-based study. Respir Res. 2021;22:103.
Article CAS PubMed PubMed Central Google Scholar
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention., 2006. Available from: https://ginasthma.org/wp-content/uploads/2019/01/2006-GINA.pdf [Last accessed December, 2022].
Bleecker ER, Menzies-Gow AN, Price DB, Bourdin A, Sweet S, Martin AL, et al. Systematic literature review of systemic corticosteroid use for Asthma Management. Am J Respir Crit Care Med. 2020;201:276–93.
Article PubMed PubMed Central Google Scholar
Dalal AA, Duh MS, Gozalo L, Robitaille MN, Albers F, Yancey S, et al. Dose-response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Managed care Specialty Pharm. 2016;22:833–47.
Article Google Scholar
Lefebvre P, Duh MS, Lafeuille MH, Gozalo L, Desai U, Robitaille MN, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1488–95.
Lefebvre P, Duh MS, Lafeuille MH, Gozalo L, Desai U, Robitaille MN, et al. Burden of systemic glucocorticoid-related complications in severe asthma. Curr Med Res Opin. 2017;33:57–65.
Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39:2216–29.
Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33:1413–32.
Article PubMed Google Scholar
AstraZeneca. Fasenra Highlights of Prescribing Information., 2019. Available from: https://www.azpicentral.com/fasenra/fasenra.pdf . [Last accessed July, 2022].
AstraZeneca. Prescribing Information AZTEZSPIRE. 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf . [Last accessed August, 2023].
Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384:1800–9.
Castro M, Mathur S, Hargreave F, Boulet L-P, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a Randomized, Placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–32.
Teva Pharmaceuticals, Prescribing Information CINQAIR. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf . [Last accessed August, 2022].
Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–97.
Genentech XOLAIR, Prescribing Information. 2021. Available from: https://www.gene.com/download/pdf/xolair_prescribing.pdf . [Last accessed August, 2022].
GSK. Nucala Highlights of Prescribing Information. 2022. Available from: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL-IFU-COMBINED.PDF . [Last accessed July, 2022].
Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of Benralizumab in severe asthma. N Engl J Med. 2017;376:2448–58.
Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of Dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–85.
Regeneron, Dupixent highlights of prescribing Information. 2022. Available from: https://www.regeneron.com/downloads/dupixent_fpi.pdf . [Last accessed July, 2022].
Siergiejko Z, Świebocka E, Smith N, Peckitt C, Leo J, Peachey G, et al. Oral corticosteroid sparing with omalizumab in severe allergic (IgE-mediated) asthma patients. Curr Med Res Opin. 2011;27:2223–8.
Calzetta L, Aiello M, Frizzelli A, Bertorelli G, Rogliani P, Chetta A. Oral corticosteroids dependence and biologic drugs in severe asthma: myths or facts? A systematic review of real-world evidence. Int J Mol Sci 2021; 22.
Casale TB, Burnette A, Bourdin A, Howarth P, Hahn B, Stach-Klysh A, et al. Oral corticosteroid-sparing effects of mepolizumab in severe eosinophilic asthma: evidence from randomized controlled trials and real-world studies. Ther Adv Respir Dis. 2022;16:17534666221107313.
Azzano P, Dufresne É, Poder T, Bégin P. Economic considerations on the usage of biologics in the allergy clinic. Allergy. 2021;76:191–209.
Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–35.
McQueen RB, Sheehan DN, Whittington MD, van Boven JFM, Campbell JD. Cost-effectiveness of biological asthma treatments: a systematic review and recommendations for future economic evaluations. PharmacoEconomics. 2018;36:957–71.
ICER. Biologic Therapies for Treatment of Asthma Associated with Type 2 Inflammation: Effectiveness, Value, and Value-Based Price Benchmarks. 2018. Available from: https://icer.org/wp-content/uploads/2020/10/Asthma-Revised-Report-FOR-PUBLICATION-11.13.2018.pdf . [Last accessed August, 2022].
van der Meer A-N, de Jong K, Ferns M, Widrich C, ten Brinke A. Overuse of oral corticosteroids in Asthma is often underdiagnosed and inadequately addressed. J Allergy Clin Immunol Pract. 2022;10:2093–8.
Bengtson LGS, Yu Y, Wang W, Cao F, Hulbert EM, Wolbeck R, et al. Inhaled corticosteroid-containing treatment escalation and outcomes for patients with asthma in a U.S. Health Care Organization. J Managed care Specialty Pharm. 2017;23:1149–59.
Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford). 2011;50:1982–90.
Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Effects of Age and Disease Severity on systemic corticosteroid responses in Asthma. Am J Respir Crit Care Med. 2017;195:1439–48.
Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf. 2008;17:1202–17.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9.
Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J 2018; 52.
Lafeuille MH, Gravel J, Figliomeni M, Zhang J, Lefebvre P. Burden of illness of patients with allergic asthma versus non-allergic asthma. J Asthma: Official J Association Care Asthma. 2013;50:900–7.
Anderson WC 3rd, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122:367–72.
Padilla-Galo A, Garcia-Ruiz AJ, Levy Abitbol RC, Olveira C, Rivas-Ruiz F, Garcia-Agua Soler N, et al. Real-life cost-effectiveness of benralizumab in patients with severe asthma. Respir Res. 2021;22:163.
Download references
Acknowledgements
This study was funded by GSK (GSK ID: 214469). Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating, and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Katherine Crossland, PhD, at Fishawack Indicia, UK, part of Avalere Health, and was funded by GSK.
This study was funded by GSK (GSK ID: 214469). Medical writing support was funded by GSK.
Author information
Authors and affiliations.
Division of Allergy/Immunology, University of South Florida, Tampa, FL, USA
Thomas B Casale
US Medical Affairs, GSK, Durham, NC, USA
Thomas Corbridge
Health Economics and Outcomes Research, Groupe d’analyse, Montréal, QC, Canada
Guillaume Germain, François Laliberté, Sean D MacKnight & Julien Boudreau
Health Economics and Outcomes Research, Analysis Group Inc, Boston, MA, USA
Value Evidence and Outcomes, GSK, Upper Providence, PA, USA
Arijita Deb
You can also search for this author in PubMed Google Scholar
Contributions
TCo and MB contributed to the conception and design and the data analysis and interpretation. AD contributed to the acquisition of data and the data analysis and interpretation. GG, FL, SM, JB, and MSD contributed to data analysis and interpretation. All authors contributed to the development of the manuscript and approval of the final draft to be published. All authors reviewed and revised the manuscript critically for important intellectual content, agreed to submit to the current journal, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All authors had access to the study data.
Corresponding author
Correspondence to Arijita Deb .
Ethics declarations
Ethics approval and consent to participate.
This study used fully de-identified data compliant with the Health Insurance Portability and Accountability Act; therefore, informed consent, ethics committee or institutional review board approval was not required.
Consent for publication
This study used fully de-identified data compliant with the Health Insurance Portability and Accountability Act; therefore, informed consent for publication was not required.
Competing interests
TBCa has received consulting and speaking fees from GSK independent of this activity. TCo and AD are GSK employees and hold GSK shares. GG, FL, SDM, JB, and MSD are employees of Analysis Group, which received funding from GSK to complete this study.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1. Additional File 1: Supplementary Tables.docx
Supplementary material 2. additional file 2: supplementary figures.docx, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Casale, T.B., Corbridge, T., Germain, G. et al. Real-world association between systemic corticosteroid exposure and complications in US patients with severe asthma. Allergy Asthma Clin Immunol 20 , 25 (2024). https://doi.org/10.1186/s13223-024-00882-y
Download citation
Received : 24 November 2023
Accepted : 20 February 2024
Published : 26 March 2024
DOI : https://doi.org/10.1186/s13223-024-00882-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Systemic corticosteroid
- Systemic corticosteroid-related complication
- Gastrointestinal
- Cardiovascular
- Central nervous system
- Ophthalmologic
Allergy, Asthma & Clinical Immunology
ISSN: 1710-1492
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Generate accurate APA citations for free
- Knowledge Base
- Citing tables and figures from other sources in APA Style
Citing Tables and Figures in APA Style | Format & Examples
Published on November 6, 2020 by Jack Caulfield . Revised on December 27, 2023.
When you reprint or adapt a table or figure from another source, the source should be acknowledged in an in-text citation and in your reference list . Follow the format for the source type you took the table or figure from.
You also have to include a copyright statement in a note beneath the table or figure. The example below shows how to cite a figure from a journal article .
Table of contents
Citing tables and figures, including a copyright note, examples from different source types, frequently asked questions about apa style citations.
Tables and figures taken from other sources are numbered and presented in the same format as your other tables and figures . Refer to them as Table 1, Figure 3, etc., but include an in-text citation after you mention them to acknowledge the source.
You should also include the source in the reference list. Follow the standard format for the source type you took the table or figure from.
Scribbr Citation Checker New
The AI-powered Citation Checker helps you avoid common mistakes such as:
- Missing commas and periods
- Incorrect usage of “et al.”
- Ampersands (&) in narrative citations
- Missing reference entries
As well as a citation and reference, when you reproduce a table or figure in your own work, you also need to acknowledge the source in a note directly below it.
The image below shows an example of a table with a copyright note.
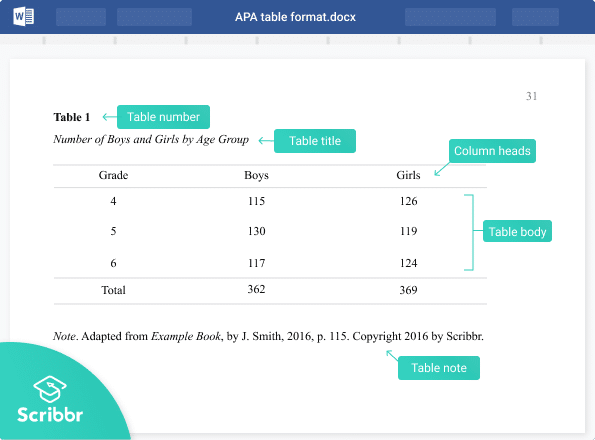
If you’ve reproduced a table or figure exactly, start the note with “From …” If you’ve adapted it in some way for your own purposes (e.g. incorporating part of a table or figure into a new table or figure in your paper), write “Adapted from …”
This is followed by information about the source (title, author, year, publisher, and location), and then copyright information at the end.
Types of copyright and permission
A source will either be under standard copyright, under a Creative Commons license, or in the public domain. You need to state which of these is the case.
Under standard copyright, you sometimes also need permission from the publisher to reprint or adapt materials. If you sought and obtained permission, mention this at the end of the note.
Look for information on copyright and permissions from the publisher. If you’re having trouble finding this information, consult your supervisor for advice.
- From a journal article
- From a website
- From a book
Copyright information can usually be found wherever the table or figure was published. For example, for a diagram in a journal article , look on the journal’s website or the database where you found the article. Images found on sites like Flickr are listed with clear copyright information.
If you find that permission is required to reproduce the material, be sure to contact the author or publisher and ask for it.
APA doesn’t require you to include a list of tables or a list of figures . However, it is advisable to do so if your text is long enough to feature a table of contents and it includes a lot of tables and/or figures .
A list of tables and list of figures appear (in that order) after your table of contents, and are presented in a similar way.
If you adapt or reproduce a table or figure from another source, you should include that source in your APA reference list . You should also include copyright information in the note for the table or figure, and include an APA in-text citation when you refer to it.
Tables and figures you created yourself, based on your own data, are not included in the reference list.
In most styles, the title page is used purely to provide information and doesn’t include any images. Ask your supervisor if you are allowed to include an image on the title page before doing so. If you do decide to include one, make sure to check whether you need permission from the creator of the image.
Include a note directly beneath the image acknowledging where it comes from, beginning with the word “ Note .” (italicized and followed by a period). Include a citation and copyright attribution . Don’t title, number, or label the image as a figure , since it doesn’t appear in your main text.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Caulfield, J. (2023, December 27). Citing Tables and Figures in APA Style | Format & Examples. Scribbr. Retrieved April 1, 2024, from https://www.scribbr.com/apa-examples/citing-tables-figures/
Is this article helpful?

Jack Caulfield
Other students also liked, how to format tables and figures in apa style, how to cite an image in apa style, setting up the apa reference page | formatting & references (examples), scribbr apa citation checker.
An innovative new tool that checks your APA citations with AI software. Say goodbye to inaccurate citations!

IMAGES
VIDEO
COMMENTS
To do this, follow these steps: Navigate to the References tab, and click "Insert Caption," which you can find in the Captions group. Give your caption a name. In the Label list, you can select the label that best describes your figure or table, or make your own by selecting "New Label.". Next, you can insert the list of tables and ...
Tables, figures, illustration requirements and tips. Include captions/titles/headings for tables, figures, and other illustrations as paragraph text. This allows captions and headings to be populated into the Table of Contents (ToC) or the lists that appear after the ToC. The maximum width for objects on a portrait page is 6 inches (15.24 cm).
Lists of figures and tables are often not required, and they aren't particularly common. They specifically aren't required for APA Style, though you should be careful to follow their other guidelines for figures and tables. If you have many figures and tables in your thesis or dissertation, include one may help you stay organised.
Many theses include tables and figures. Most often, they are added to the thesis as images, but sometimes you might want to add some as a linked Excel file. And, the way that captions are added to figures and tables differs between APA and IEEE style.
All figures and tables included in the thesis must be referred to in the text of the thesis. The first reference in the text to a figure or table must precede it. If the figure or table is on a separate page, then the reference to it should be on the preceding text page.
A thesis may include tables, figures, photographs, musical examples, charts, graphs, line drawings, maps, and other illustrative materials. In addition, a thesis may include statements such as definitions, corollaries, lemmas, theorems, propositions, and schemes. For the following discussion about numbering and placement, these items will be ...
Referring to images. In APA style, Tables are visual displays of text or data in columns and rows. Figure refers to all illustrations except tables including graphs, photos, screenshots, drawings, maps, infographics and images. Figures and tables can add visual appeal and make your work more understandable.
Tables, figures, illustrations, and other such items should be identified with the word "Table", "Figure", or other appropriate descriptor, and include a title and/or caption. The title or caption must be included in the List of Tables, List of Figures, or other list. You must use a consistent format for titles and captions of tables, figures, illustrations, and other such items throughout the ...
So, the tables need to be well organized and self-explanatory. Avoidance of repetition: Tables and figures add clarity to the research. They complement the research text and draw attention to key points. They can be used to highlight the main points of the paper, but values should not be repeated as it defeats the very purpose of these elements.
An original figure or table is one that you created and has not been published. If you would like to include an original figure or table in your manuscript, you do not need to ask permission or use attribution to use it. You can simply include the figure or table with your research manuscript when you submit it to the journal.
Figures. Per APA style, figures are all types of visual elements other than tables. This includes photographs, graphs, and charts. Similar to tables, figures must be necessary and supplement your content. Figures should also be numbered sequentially. When creating a figure, simplicity is key. Keeping your figure legible and clear for the reader ...
Figures and tables. Figures and tables (display items) are often the quickest way to communicate large amounts of complex information that would be complicated to explain in text. Many readers will only look at your display items without reading the main text of your manuscript. Therefore, ensure your display items can stand alone from the text ...
Read more about lists of figures and tables. List of abbreviations. If you have used a lot of industry- or field-specific abbreviations in your thesis, you should include them in an alphabetized list of abbreviations. This way, your readers can easily look up any meanings they aren't familiar with. Read more about lists of abbreviations. Glossary
7. You appear to start from an assumption of reading on screen. If some actually wants to read a thesis (as opposed to looking something up in it) there's a fair chance they're working on paper. An examiner for example. A list of figures/list of tableswill help them track down some of the important material quickly.
Lists of figures and tables are often not required, and they aren't particularly common. They specifically aren't required for APA Style, though you should be careful to follow their other guidelines for figures and tables. If you have many figures and tables in your thesis or dissertation, include one may help you stay organised.
The Signature Page will be included in all copies of the thesis. ... Figures and tables referred to in the text may not be placed at the end of the chapter or at the end of the thesis. Figure and table numbering must be either continuous throughout the thesis or by paper (e.g., 1.1, 1.2, 2.1, 2.2). For example, there cannot be two figures ...
Figures and tables other than outlook are usually included in data/methods, results, or discussion, but not in abstract or intro. For thesis, the story is different. Introduction of a thesis can sometimes be even a review paper you have published in the past, so figures, tables, videos, etc are all fine unless your school specifies otherwise.
The list of figures and tables in a research paper, thesis, or dissertation provides a structured overview of graphic elements included in the paper. This list guides readers to find specific graphs, images, tables, or charts effortlessly. The process of compiling this list needs more than just listing the captions; it also requires proper formatting and sequencing in line with academic ...
Lists of figures and tables are often not required, and aren't particularly common. They specifically aren't required for APA-Style, though you should be careful to follow their other guidelines for figures and tables. If you have many figures and tables in your thesis or dissertation, include one may help you stay organized.
figure, etc. For example, a table numbered 2.3 would indicate that it is the third table to appear in Chapter 2. Main headings: Headings that indicate a new chapter or main section of the document. Examples include ABSTRACT, CHAPTER 5, SELECTED BIBLIOGRAPHY, LIST OF FIGURES, and chapter titles (or main sections if not using chapters). Main headings
Association between BMI or WHtR and mortality. In restricted cubic spline analyses, there was an inversely J-shaped association between continuous BMI and mortality, with risk inflection points for all-cause mortality, cardiovascular mortality, cancer mortality, and other-cause mortality at BMIs of 27.6, 25.0, 25.3, and 29.2 kg/m 2, respectively.The risk of death decreased sharply before the ...
Towns in Kalidāsa's Kāvyas and the Thesis of Urban Decay. Kesavan Veluthat [email protected] View all authors and affiliations. ... Figures and tables Figures & Media Tables. View Options. Get access. ... Expand Table. Show all View all authors and affiliations.
Patient population. Overall, 7473 patients in the SCS use cohort and 89,281 in the non/burst-SCS use cohort met the eligibility criteria and were included in the analysis (Additional File 2; Supplementary Fig. 1).. Compared with the non/burst-SCS use cohort, the SCS use cohort was older, had a greater proportion of Medicare enrollees, and had a lower proportion of patients managed by primary ...
Tables and figures taken from other sources are numbered and presented in the same format as your other tables and figures. Refer to them as Table 1, Figure 3, etc., but include an in-text citation after you mention them to acknowledge the source. In-text citation example. The results in Table 1 (Ajzen, 1991, p. 179) show that ….