Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 June 2024

Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 2000 to 2020
- Vision Loss Expert Group of the Global Burden of Disease Study &
the GBD 2019 Blindness and Vision Impairment Collaborators
Eye ( 2024 ) Cite this article
264 Accesses
4 Altmetric
Metrics details
- Epidemiology
- Retinal diseases
To estimate global and regional trends from 2000 to 2020 of the number of persons visually impaired by diabetic retinopathy and their proportion of the total number of vision-impaired individuals.
Data from population-based studies on eye diseases between 1980 to 2018 were compiled. Meta-regression models were performed to estimate the prevalence of blindness (presenting visual acuity <3/60) and moderate or severe vision impairment (MSVI; <6/18 to ≥3/60) attributed to DR. The estimates, with 95% uncertainty intervals [UIs], were stratified by age, sex, year, and region.
In 2020, 1.07 million (95% UI: 0.76, 1.51) people were blind due to DR, with nearly 3.28 million (95% UI: 2.41, 4.34) experiencing MSVI. The GBD super-regions with the highest percentage of all DR-related blindness and MSVI were Latin America and the Caribbean (6.95% [95% UI: 5.08, 9.51]) and North Africa and the Middle East (2.12% [95% UI: 1.55, 2.79]), respectively. Between 2000 and 2020, changes in DR-related blindness and MSVI were greater among females than males, predominantly in the super-regions of South Asia (blindness) and Southeast Asia, East Asia, and Oceania (MSVI).
Conclusions
Given the rapid global rise in diabetes and increased life expectancy, DR is anticipated to persist as a significant public health challenge. The findings emphasise the need for gender-specific interventions and region-specific DR healthcare policies to mitigate disparities and prevent avoidable blindness. This study contributes to the expanding body of literature on the burden of DR, highlighting the need for increased global attention and investment in this research area.
Similar content being viewed by others

Chronic kidney disease and the global public health agenda: an international consensus

Peripheral blood mononuclear cell respiratory function is associated with progressive glaucomatous vision loss

How do I recognise and manage visual snow syndrome?
Introduction.
Diabetes mellitus (DM) and its complications are a major burden of disease around the world. DM has increased significantly in recent decades and will continue to rise in the next few decades, with a greater burden expected in low-middle income countries (LMICs) [ 1 ]. One of the most common microvascular complications of DM is diabetic retinopathy (DR). According to previous large-population based studies and meta-analyses, DR has been recognized as one of the most common causes of blindness and vision impairment among the working-age population; however, this is not true for some countries, such as the United Kingdom, due to the implementation of national DR strategies aimed at identifying and treating patients with this condition [ 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 ]. The Global Burden of Disease Study (GBD) began 30 years ago to systematically assess and scientifically report on critical health outcomes including DM and its complications. The findings are reported longitudinally and across various populations [ 10 ]. In 2020, DR was listed as one of the leading causes of global blindness among those aged 50 years and above [ 3 ]. Leasher et al. assessed changes in the prevalence of DR-related blindness and moderate or severe vision impairment (MSVI) from 1990 to 2010 [ 8 ]. Findings showed that DR accounted for 2.6% of all blindness and 1.9% of all MSVI in 2010, an increase from 2.1% and 1.3%, respectively, from 1990 [ 8 ]. Early detection and treatment interventions for DR can reduce the risk of severe visual loss by approximately 90% [ 11 ].
The Lancet Global Health Commission emphasised how improving eye health contributes to achieving the sustainable development goals (SDGs) of improving general health and well-being, reducing poverty and increasing work productivity, and improving education and equity [ 7 ]. Due to the unmet need of an ageing and growing population globally, eye health is a major public health concern that requires urgent attention to develop innovative treatments and deliver services on a large scale. Political commitment is necessary to act on eye health, particularly in low-resource settings [ 7 , 12 ].
The current meta-analysis provides an update of all available population-based studies from 2000 to 2020 to present estimates on the number of people (aged 50 years+) affected by DR-related blindness and DR-related MSVI. Additionally, we investigate the global and regional differences in the prevalence of DR-related blindness and MSVI, and differences by sex.
Materials/subjects and methods
Preparation of data included first a systematic review of published (between Jan 1, 1980, and Oct 1, 2018) population-based studies of vision impairment and blindness by the Vision Loss and Expert Group (VLEG) that also included gray literature sources. Eligible studies from this review were then combined with data from Rapid Assessment of Avoidable Blindness (RAAB) studies. Data from the US National Health and Nutrition Examination survey and the World Health Organization (WHO) Study on Global Ageing and Adult Health were contributed by the GBD team. More detailed methods are published elsewhere [ 3 , 13 ] and briefly discussed as follows.
In total, VLEG identified 137 studies and extracted data from 70 studies in their 2010 review, and additional 67 studies in their 2014–18 review. Studies were primarily national and subnational cross-sectional surveys. Additionally, the VLEG commissioned the preparation of 5-year age-disaggregated RAAB data from the RAAB repository. Studies were included if they met the following criteria: visual acuity data had to be measured using a test chart that could be mapped to the Snellen scale, and the sample had to be representative of the population. Self-report of vision loss was excluded. We used International Classification of Diseases 11 th (ICD-11) edition criteria for vision impairment, as used by WHO, which categorises people according to vision in the better eye on presentation, in which moderate vision impairment is defined as a visual acuity of 6/60 or better but less than 6/18, severe vision impairment as a visual acuity of 3/60 or better but less than 6/60, and blindness as a visual acuity of less than 3/60 or less than 10° visual field around central fixation (although the visual field definition is rarely used in population-based eye surveys) [ 14 ].
First, we separated raw data into vision-loss envelopes for all-cause mild, moderate, and severe vision impairment, and blindness. Data were input into a mixed-effects meta-regression tool developed by the Institute for Health Metrics and Evaluation (IHME) called MR-BRT (meta regression; Bayesian; regularized; trimmed) [ 15 ]. Presenting vision impairment was the reference definition for each level of severity. Undercorrected refractive error data were extracted directly from data sources where available, and otherwise calculated by subtracting best-corrected vision impairment from presenting vision impairment prevalence for each level of severity in studies that reported both measures for a given location, sex, age group, and year. All other causes were quantified as part of the best-corrected estimates of vision impairment at each level of severity.
We modeled distance vision impairment and blindness due to the following causes: cataract, undercorrected refractive error, age-related macular degeneration, myopic macular degeneration, glaucoma, diabetic retinopathy, and other causes of vision impairment (in aggregate). Minimum age for inclusion of data for these causes was set at 20 years for cataract and diabetic retinopathy, and 45 years for glaucoma and age-related macular degeneration. Other vision impairment estimates were combined with less prevalent causes of vision impairment to create a residual category (e.g., retinopathy of prematurity, corneal opacities or optic atrophy, trachoma).
We produced location, year, age, and sex-specific estimates of MSVI and blindness using Disease Modeling Meta-Regression (Dismod-MR) 2.1 [ 16 ]. The data processing steps are described elsewhere [ 3 ]. Briefly, Dismod-MR 2.1 models were run for all vision impairment by severity (moderate, severe, blindness) regardless of cause and, separately, for MSVI and blindness due to each modeled cause of vision impairment (e.g., MSVI due to cataract and blindness due to cataract). Then, models of MSVI due to specific causes were split into moderate and severe estimates using the ratio of overall prevalence in the all-cause moderate presenting vision impairment and severe presenting vision impairment models. Next, prevalence estimates for all causes by severity were scaled to the models of all-cause prevalence by severity. This produced final estimates by age, sex, year, and location for each individual cause of vision impairment by severity. We age-standardized our estimates using the GBD standard population [ 17 ].
According to our estimates from 2020, approximately 1.07 million (95% uncertainty intervals (UIs): 0.76, 1.51) people were blind and nearly 3.28 million (95% UI: 2.41, 4.34) had MSVI globally due to DR (Table 1 ). An estimated 462,000 males and 611,000 females of all ages, and 368,000 males and 494,000 females aged ≥50 years had DR-related blindness in 2020 (Table 2 ). The number of males and females (all ages) with DR-related MSVI in 2020 was 1.4 million and 1.8 million, respectively, whereas an estimated 1.3 million and 1.7 million people were aged 50 years and over (Table 3 ). Higher prevalence rates of DR-related blindness were seen among females aged 60 years and above, with the highest rates observed in people aged >95 years. Higher prevalence rates of DR-related blindness and MSVI were seen among females aged 60 years and above, with the highest rates observed in females aged >95 years.
DR caused 2.50% (95% UI: 1.77, 3.52) of blindness in 2020 worldwide. Regionally, the highest percentage of all DR-related blindness was found in Latin America and Caribbean (6.95% [95% UI: 5.08, 9.51]) and High-Income super-regions (5.37% [95% UI: 3.86, 7.55]) (Table 1 ). The super-regions with the lowest percentage of all DR-related blindness were Central Europe, Eastern Europe, and Central Asia (0.97% [95% UI: 0.67, 1.39]), and Sub-Saharan Africa (0.98% [95% UI: 0.69, 1.40]). DR caused 1.11% (95% UI: 0.82, 1.47) of MSVI in 2020 worldwide. North Africa and Middle East (2.12% [95% UI: 1.55, 2.79]), and Latin America and Caribbean (1.84% [95% UI: 1.36, 2.45]) were super-regions with the highest percentage of all MSVI due to DR (Table 1 ).
In 2020, the global age-standardized prevalence of DR-related blindness in those aged ≥50 years was 0.05% (95% UI: 0.03, 0.07) and 0.16% (95% UI: 0.12, 0.21) for DR-related MSVI (Table 1 ). The super-region with the highest age-standardized prevalence of DR-related blindness was Latin American and Caribbean (0.15% [95% UI: 0.10, 0.21]). The lowest age-standardized prevalence of DR-related blindness in 2020 was in Central Europe, Eastern Europe, and Central Asia (0.01% [95% UI: 0.01, 0.01]). The super-regions with the highest age-standardized prevalence of DR-related MSVI in 2020 were North Africa and Middle East (0.41% [95% UI: 0.30, 0.55]), and Latin America and the Caribbean (0.30% [95% UI: 0.22, 0.40]). The lowest estimates were found in the High-Income (0.08% [95% UI: 0.06, 0.11]) and Central Europe, Eastern Europe, and Central Asia (0.09% [95% UI: 0.07, 0.13]) super-regions (Table 1 ). Figure 1 presents the crude prevalence of blindness and MSVI due to DR in 2020 across super-regions.
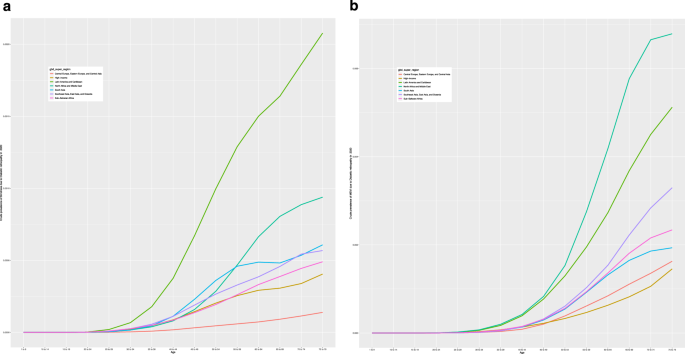
Crude prevalence of blindness and MSVI due to DR in 2020 by age, across seven world GBD super-regions. a Crude prevalence of blindness due to DR in 2020 by seven world GBD super-regions by age. The graph demonstrates an increase in prevalence with age, with notable variations between super-regions. The super-regions are represented by different coloured lines. b Crude prevalence of MSVI in 2020 by seven world GBD super-regions by age. Similar to ( a ), the prevalence increases with age, highlighting disparities among different super-regions. Each super-region is depicted by a distinct coloured line.
Between 2000 and 2020, the global percentage change in age-standardized prevalence of DR-related blindness among adults (≥50 years) showed different trends for males and females (Supplementary file, Table S1 ). For males, there was a minimal decrease of −0.10% (95% UI: −0.54, 0.34), while females experienced an increase of +12.89% (95% UI: 12.40, 13.38). An overall increase in the age-standardized prevalence of DR-related blindness among adults aged ≥50 years (both sexes) was found in South Asia (+25.66% [95% UI: 25.07, 26.24]), Southeast Asia, East Asia and Oceania (+15.36% [95% UI: 14.80, 15.92]) and Sub-Saharan Africa (+2.47% [95% UI: 2.01, 2.94]). An increase of +14.92% (95% UI: 14.39, 15.45) in age-standardized prevalence of DR-related blindness in South Asia from 2000 to 2020 was observed for males, whiles females experienced even greater gains with a rise of +34.68% (95% UI: 34.04, 35.32). In Southeast Asia, East Asia, and Oceania, the increase in age-standardized prevalence of DR-related blindness from 2000 to 2020 was +3.43% (95% UI: 2.94, 3.91) for males, compared to +26.34% (95% UI: 25.72, 26.97) for females. In Sub-Saharan Africa, although the overall age-standardized prevalence of DR-related blindness from 2000 to 2020 increased, a decrease was found among males (−12.46% [95% UI: −12.87, −12.04]) compared to females (+16.79% [95% UI: 16.27, 17.30]). All other super-regions demonstrated a decrease in the age-standardized prevalence of DR-related blindness (≥50 years) from 2000 to 2020 overall. In Central Europe, Eastern Europe and Central Asia, the age-standardized prevalence of DR-related blindness decreased by −21.99% (95% UI: −22.41, −21.58) for males compared to −3.15% (95% UI: −3.61, −2.70) for females. In Latin America and Caribbean, a decrease of −20.74% (95% UI: −21.06, −20.41) was observed in males, with a smaller decrease (−5.49% [95% UI: −5.86, −5.11]) among females. In the High-Income super-region, a reduction of −15.73% (95% UI: −16.13,−15.32) and −8.46% (95% UI: −8.83, −8.09) was found in males and females, respectively. Supplementary file contains Figs. ( S1 – S4 ) illustrating the total number of cases (males and females) with DR-related blindness and MSVI between 2000 and 2020, for all 21 GBD world regions, including the global total for comparison.
From 2000 to 2020, there was a decrease in the global percentage change in age-standardized prevalence of DR-related MSVI (≥50 years) among males (−0.93% [95% UI: −1.29, −0.56]), while females experienced an increase (+3.62% [95% UI: 3.25, 3.99]). Between 2000 and 2020, the super-region of Southeast Asia, East Asia, and Oceania showed an increase in the age-standardized prevalence of DR-related MSVI for both males (+1.17%, [95% UI: 0.79, 1.55]) and females (+3.33%, [95% UI: 2.95, 3.71]). In Sub-Saharan Africa, there was a decrease in the age-standardized prevalence of DR-related MSVI among males (−1.98%, [95% UI:−2.34, −1.63]), whereas females experienced an increase (+1.06%, [95% UI: 0.69, 1.42]). All other super-regions demonstrated a decrease in the age-standardized prevalence of DR-related MSVI (≥50 years) between 2000 and 2020 for both sexes. The super-region of North Africa and the Middle East showed the most notable decline in age-standardized DR-related MSVI for both sexes (−15.35% [−15.66, −15.05]). Among males, there was a decrease of −16.43% (95% UI: −16.73, −16.12), while females exhibited a −14.57% (95% UI: −14.88, −14.26) decrease (Supplementary file, Table S2 ).
The global percentage change in crude prevalence for DR-related blindness between 2000 and 2020 was +1.41% (95% UI: −0.96, 1.85) in males compared to a + 13.32% (95% UI: 12.83, 13.80) increase in females, and +7.90% (95% UI: 7.43, 8.36) overall. The percentage change in crude prevalence of DR-related MSVI was also higher among females (+3.56% (95% UI: 3.18, 3.93)) compared to males (+1.31% (95% UI: 0.93, 1.69)) globally (Supplementary file, Tables S1 , 2 ).
Although DR remains highly prevalent, the figures from 2020 show a slight decrease compared to those reported in 2010 [ 8 ]. In 2020, DR accounted for 2.5% of global blindness and 1.1% of MSVI, down from 2.6% and 1.9%, respectively, in 2010. Leasher et al. also showed that the highest age-standardized prevalence of DR-related blindness and MSVI was in the super-regions of North Africa/Middle East, Sub-Saharan Africa, and South Asia, while the lowest prevalence was in High-Income regions [ 8 ]. An increase in the numbers of people with DR-related blindness and MSVI with a relatively unchanged age-standardized prevalence from 2010 to 2020 may be attributed to the increasing population and average age in most regions, coupled with falling death rates.
Our study found that DR-related blindness has increased more among females than males in almost all super-regions. The largest sex-related inequalities were found in South Asia, Southeast Asia, East Asia and Oceania, and Sub-Saharan Africa. Though there are age-adjusted declines in DR prevalence for some super-regions, the overall global crude prevalence of both DR-related blindness and DR-related MSVI for males, females, and overall has increased globally due to aging and growth of the population. These figures represent the true burden of disease with which governments must contend.
The factors contributing to these gender disparities are multifaceted. One possible contributing factor is the difference in average life expectancy between women and men. As women tend to have a longer lifespan, they are consequently at greater risk of developing DM and DR. In LMICs, women may have poorer access to healthcare services compared to men [ 18 , 19 ]. Other factors that may contribute to disparities in eye health include, lack of access to information and resources, and lower literacy among females compared to males [ 20 , 21 , 22 ]. Pregnancy is another factor that can accelerate the progression of DR in women [ 23 ]. Finally, DR has been linked to intake of the retinal carotenoids lutein and zeaxanthin, and women are thought to have lower retinal levels of lutein and zeaxanthin [ 24 , 25 ]. The difference in retinal levels of lutein and zeaxanthin between men and women may be due to several factors including hormones, dietary patterns, and variances in metabolic processes [ 25 ]. Factors such as smoking might vary between women and men, contributing to differences in retinal levels. This requires further investigation to ascertain the precise causes behind the observed differences in retinal levels between men and women. Action is needed to improve female care and reduce the burden of DR-related blindness and MSVI.
Teo et al. estimated that there would be 103.12 million people with DR, 28.54 million people with vision-threatening DR, and 18.83 million people with clinically significant macular oedema in 2020 [ 26 ]. They found that the North America and Caribbean (NAC) and Middle East and North Africa (MENA) showed significantly higher prevalence of DR compared to other regions [ 26 ]. Similarly, our results show that the Latin America and Caribbean and North Africa, and Middle East super-regions demonstrated the highest prevalence of DR-related blindness and MSVI. This may be attributed to several factors such as limited access to quality healthcare services, increased DM cases, and inadequate management of DM. Although DR is estimated to affect over 100 million people globally, our data from 2020 suggests that less than 1.1 million are currently blind and less than 3.3 million are visually impaired. Compared to the 2010 data, 834,000 people were blind whereas 3.7 million were visually impaired [ 8 ]. The decline in the number of people with MSVI from 2010, despite an increase in DR-related blindness may be due to advancements in medical technology and treatments for DR. They play a role in preventing the progression of the disease to more severe stages, hence reducing the number of individuals with MSVI. Additionally, increased awareness about DM and its ocular complications might lead to earlier detection and intervention, which could prevent or mitigate MSVI cases despite the rise in DR-related blindness.
Blindness and MSVI can have a profound impact on quality of life, impairing both mental and physical health, and social independence [ 27 ]. As reported in the GBD Study 2019, blindness and low vision was ranked eighth (contributing 3·8% [95% UI 3·0, 4·9]) of all years lived with disability (YLDs) in people aged 50–69 years [ 13 ]. Among people aged 70 years and older, blindness and low vision was ranked fourth (contributing 6·4% [5·4, 7·4] of all YLDs) [ 13 ]. Furthermore, blindness and MSVI are associated with reduced economic, educational, and employment opportunities [ 28 , 29 , 30 ]. Economic productivity at the individual, family, community, and national level is important to sustainable development. An inability to work can diminish the productive capacity of the economy by reducing the workforce. Illness and disability can contribute to productivity losses through absenteeism from work, reduced productivity while at work or unemployment, including job loss and early retirement [ 28 , 29 , 30 , 31 ]. The Lancet Global Health Commission on Global Eye Health assessed the overall relative reduction in employment by working-aged people with blindness and MSVI [ 31 ]. They found that the global average relative reduction in employment of people with vision impairment was estimated to be 30.2% [ 31 ]. Since blindness and MSVI can have a large economic impact globally, more data on the employment status of people living with blindness and MSVI in all world regions, especially, LMICs needs to be available. Future research should explore more specifically how DR-related blindness and MSVI affect productivity losses and if there are relevant differences by sex.
We reviewed the literature to determine the economic burden of DR globally. According to UK estimates, DR has an annual cost of £379 million($476 million) for cases linked to type 2 DM, and almost £14 million ($17.6 million) for cases related to type 1 DM [ 32 ]. Economic modeling in the UK suggests that reducing the prevalence of type 2 DM-related DR by just 1% each year could save the UK economy £150 million ($188.6 million) by 2050 [ 32 ]. The estimated economic burden of DR in the United States is $0.5 billion [ 33 ], $3.91 billion in Germany [ 34 ], and $3.5 to 6.4 billion in the Latin America and the Caribbean region [ 35 ]. Further exploration of the economic burden in all world regions is necessary for agenda setting and policy planning in the future.
The VLEG populates and curates the Global Vision Database, a continuously updated, comprehensive, online database storing worldwide ophthalmic epidemiological information, including DR. By considering data from Jan 1st 1980 to Oct 1st 2018, the study covers a significant period, allowing for the assessment of trends and changes over time. The inclusion of gray literature enriches the database with unpublished data yet valuable data.
Our report provides an update on the worldwide and regional estimates for DR-related blindness and MSVI, including the changing patterns over time. It demonstrates that considerable regional differences and sex inequalities exist, highlighting areas that require particular attention such as low resource settings. These findings could aid further region-specific DR healthcare policies to prevent vision impairment, especially among females in the future.
Limitations
This meta-analysis has some limitations, such as potential publication bias and heterogeneity across studies. Due to the paucity of data across low burden regions, we may be over/under-estimating DR overall prevalence. While visual acuity is an important measure of visual function, it is not the only measure, and it is important to consider other methods of measuring visual impairment such as contrast sensitivity when assessing the prevalence of vision impairment. Nonetheless, our findings highlight the ongoing burden of DR-related vision impairment and underscore the need for effective prevention and management strategies.
Early detection and timely treatment are essential for preventing avoidable DR-related blindness and MSVI [ 36 , 37 ]. Between 2000 and 2020, high-income countries have made good progress in terms of reducing their DR-related blindness/MSVI which may be linked to improved risk factor control and advances in their screening and treatment services [ 7 , 38 , 39 ]. Despite this success, screening and treatment services still remain a challenge for super-regions such as Latin America (high prevalence of all DR-related blindness and MSVI ≥50 years old) [ 40 ]. While Sub-Saharan Africa might be anticipated to have a higher burden of DR compared to regions such as Latin America and Caribbean, Middle East, and North Africa, differences in population demographics, genetics, lifestyle, and DM management approaches contribute to varied prevalence rates. Under-reporting and insufficient data availability further complicate assessing the true extent of the issue. While healthcare resources are limited in Sub-Saharan Africa, certain areas within the region may have stronger healthcare infrastructure or targeted interventions that improve DR management compared to other LMICs. The global burden of DR is expected to remain high through 2045, disproportionately affecting countries in the Middle East and North Africa, and the Western Pacific [ 26 ]. Delivering innovative DR prevention and treatment strategies to improve global eye health is necessary. Screening for DR would also be much improved by the existence of population DM registers. Finally, our findings suggest the need for region-specific healthcare policies aimed at preventing vision loss, particularly among females.
Supplemental material is available at Eye’s website.
What was known before
Globally, in 2020, 1.07 million people were blind, and nearly 3.28 million were visually impaired by diabetic retinopathy.
What this study adds
The contribution of diabetic retinopathy and moderate and severe vision impairment (MSVI) by region and the change in this contribution between 2000 and 2020. The change in global age-standardized prevalence of DR-related blindness and MSVI between 2000 and 2020 and the differences by sex and region.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the coordinator of the Vision Loss Expert Group (Professor Rupert Bourne; [email protected]) upon reasonable request. Data are located in controlled access data storage at Anglia Ruskin University.
Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790.
Article CAS PubMed PubMed Central Google Scholar
Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Article PubMed PubMed Central Google Scholar
Bourne RRA, Steinmetz JD, Saylan M, Mersha AM, Weldemariam AH, Wondmeneh TG, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–60.
Article Google Scholar
Bourne RRA, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1:339–49.
Flaxman, Bourne RRA SR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–34.
Article PubMed Google Scholar
Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60:428–31.
Burton MJ, Ramke J, Marques AP, Bourne RRA, Congdon N, Jones I, et al. The lancet global health commission on global eye health: vision beyond 2020. Lancet Glob Health. 2021;9:e489–551.
Leasher JL, Bourne RRA, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39:1643–9.
Hashemi H, Rezvan F, Pakzad R, Ansaripour A, Yekta A, Ostadimoghaddam H, et al. Global and regional prevalence of diabetic retinopathy; a comprehensive systematic review and meta-analysis global and regional prevalence of diabetic retinopathy; a comprehensive systematic review and meta-analysis. Semin Ophthalmol. 2022;3:291–306.
Murray CJL. The Global Burden of Disease Study at 30 years. Nat Med. 2022;28:2019–26.
Article CAS PubMed Google Scholar
Safi H, Safi S, Hafezi-moghadam A, Ahmadieh H. Science direct early detection of diabetic retinopathy. Surv Ophthalmol. 2018;63:601–8.
Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–77.
Bourne RRA, Steinmetz JD, Flaxman S, Briant PS, Taylor HR, Resnikoff S, et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e130–43.
World Health Organization. International Classification of Diseases 11th Revision - The global standard for diagnostic health information [Internet]. 2023. Available from: https://icd.who.int/en .
Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22.
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
Vollset SE, Goren E, Yuan CW, Cao J, Smith AE, Hsiao T, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285–306.
Courtright P, Lewallen S. Improving gender equity in eye care: Advocating for the needs of women. Community Eye Health J. 2007;20:68–9.
Google Scholar
Xu Y, Wang A, Lin X, Xu J, Shan Y, Pan X, et al. Global burden and gender disparity of vision loss associated with diabetes retinopathy. Acta Ophthalmol. 2021;99:431–40.
Shrestha MK, Guo CW, Maharjan N, Gurung R, Ruit S. Health literacy of common ocular diseases in Nepal. BMC Ophthalmol. 2014;14:2.
Shrestha G, Sigdel R, Shrestha J, Sharma A, Shrestha R, Mishra S, et al. Awareness of eye health and diseases among the population of the hilly region of Nepal. J Ophthalmic Vis Res. 2018;13:461–9.
Quartuccio M, Simonsick EM, Langan S, Harris T, Sudore RL, Thorpe R, et al. The relationship of health literacy to diabetes status differs by sex in older adults. J Diabetes Complications. 2019;32:368–72.
Rahman W, Rahman FZ, Yassin S, Al-Suleiman SA, Rahman J. Progression of retinopathy during pregnancy in type 1 diabetes mellitus. Clin Exp Ophthalmol. 2007;35:231–6.
Hammond BR, Renzi-Hammond L. The influence of the macular carotenoids on women’s eye and brain health. Nutr Neurosci. 2023;8:720–6.
Bailey RL, Dog TL, Smith-Ryan AE, Das SK, Baker FC, Madak-Erdogan Z, et al. Sex differences across the life course: a focus on unique nutritional and health considerations among wom en . J Nutr. 2022;152:1597–610.
Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128:1580–91.
Kandel H, Nguyen V, Piermarocchi S, Ceklic L, Teo K, Arnalich-Montiel F, et al. Quality of life impact of eye diseases: a Save Sight Registries study. Clin Exp Ophthalmol. 2022;50:386–97.
Reddy PA, Congdon N, MacKenzie G, Gogate P, Wen Q, Jan C, et al. Effect of providing near glasses on productivity among rural Indian tea workers with presbyopia (PROSPER): a randomised trial. Lancet Glob Heal. 2018;6:e1019–27.
Frick KD, Foster A. The magnitude and cost of global blindness: An increasing problem that can be alleviated. Am J Ophthalmol. 2003;135:471–6.
Pezzullo L, Streatfeild J, Simkiss P, Shickle D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv Res. 2018;18:63.
Marques AP, Ramke J, Cairns J, Butt T, Zhang JH, Muirhead D, et al. Global economic productivity losses from vision impairment and blindness. EClinicalMedicine. 2021;35:100852.
Fight for Sight. Time to Focus. 2019.[cited 2023 Jan 10] Available from: https://www.fightforsight.org.uk/our-research/timetofocus/#:~:text=Time%20to%20Focus%20is%20the,loss%20and%20researchers%20in%20the .
Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–60.
Happich M, Reitberger U. The economic burden of diabetic retinopathy in Germany in 2002. Graedes Arch Clin Exp Ophthalmol. 2008;246:151–9.
Barcelo A, Arredondo A, Tobar AG, Segovia J. The cost of diabetes in Latin America and the Caribbean in 2015: evidence for decision and policy makers. J Glob Health. 2017;7:020410.
Ferris FL. Results of 20 years of research on the treatment of diabetic retinopathy. Prev Med. 1994;23:740–2.
Scanlon PH. The English National Screening Programme for diabetic retinopathy 2003–2016. Acta Diabetol. 2017;54:15–25.
Scanlon PH. The contribution of the English NHS Diabetic Eye Screening Programme to reductions in diabetes-related blindness, comparisons within Europe, and future challenges. Acta Diabetol. 2021;58:521–30.
Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7:140–9.
Fernandes AG, Ferraz AN, Brant R, Malerbi FK. Diabetic retinopathy screening and treatment through the Brazilian National Health Insurance. Sci Rep. 2022;12:13941.
Download references
Acknowledgements
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
This study was funded by Brien Holden Vision Institute, Fondation Thea, Fred Hollows Foundation, Bill & Melinda Gates Foundation, Lions Clubs International Foundation (LCIF), Sightsavers International, and University of Heidelberg.
Author information
These authors contributed equally: Rupert R. A. Bourne, Jaimie D. Steinmetz.
Authors and Affiliations
Centre for Public Health, Queens University Belfast, Belfast, Northern Ireland
Katie Curran & Tunde Peto
Department of Ophthalmology, Medical Faculty Mannheim, Heidelberg University, Heidelberg, Germany
Jost B. Jonas
Mass Eye and Ear, Harvard Medical School, Boston, MA, USA
David Friedman
University of Texas Southwestern Medical Center, Dallas, TX, USA
Judy E. Kim
Nova Southeastern University College for Optometry, Fort Lauderdale, FL, USA
Janet Leasher
Department of Ophthalmology, Cambridge University Hospitals, Cambridge, UK
Federal University of Sao Paolo, Sao Paolo/SP, Brazil
Arthur G. Fernandes
University of Calgary, Calgary/AB, Canada
School of Medicine, Vita-Salute San Raffaele University, Milan, Italy
Maria Vittoria Cicinelli
Department of Ophthalmology, IRCCS San Raffaele Scientific Institute, Milan, Italy
Scientific Institute San Raffaele Hospital, Vita-Salute University, Milan, Italy
Alessandro Arrigo
University of Poitiers, Poitiers, France
Nicolas Leveziel
CHU de Poitiers, Poitiers, France
Brien Holden Vision Institute, Sydney, NSW, Australia
Serge Resnikoff
School of Optometry and Vision Sciences, Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia
School of Population and Global Health, University of Melbourne, Carlton, VIC, Australia
Hugh R. Taylor
Vision and Eye Research Institute, Anglia Ruskin University, Cambridge, UK
Tabassom Sedighi, Rupert R. A. Bourne & Shahina Pardhan
Department of Computer Science, University of Oxford, Oxford, UK
Seth Flaxman
Ufa Eye Research Institute, Ufa, Russia
Mukkharram M. Bikbov
School of Life Course and Population Sciences, King’s College London, London, UK
Tasanee Braithwaite
The Medical Eye Unit, Guy’s and St Thomas’ NHS Foundation Trust, London, UK
University Hospital, Dijon, France
National University of Singapore, Singapore, Singapore
Ching-Yu Cheng
Singapore Eye Research Institute, Singapore, Singapore
University of Michigan, Ann Arbor, MI, USA
Monte A. Del Monte
Kellogg Eye Center, Ann Arbor, MI, USA
Institute for Social Research, University of Michigan, Ann Arbor, MI, USA
Joshua R. Ehrlich
Department of Ophthalmology and Visual Sciences, University of Michigan, Ann Arbor, MI, USA
Ribeirão Preto Medical School, University of São Paulo, São Paulo, Brazil
João M. Furtado
Institute of Ophthalmology UCL & NIHR Biomedical Research Centre, London, UK
Gus Gazzard
Stanford University, Stanford, CA, USA
M. Elizabeth Hartnett
Associated Ophthalmologists of Monastir, Monastir, Tunisia
Rim Kahloun
Department of Ophthalmology, Harvard University, Boston, MA, USA
John H. Kempen
Eye Unit, MyungSung Medical College, Addis Ababa, Ethiopia
Department of Ophthalmology, Addis Ababa University, Addis Ababa, Ethiopia
Sight for Souls, Bellevue, WA, USA
Fattouma Bourguiba University Hospital, University of Monastir, Monastir, Tunisia
Moncef Khairallah
Allen Foster Community Eye Health Research Centre, Gullapalli Pratibha Rao International Centre for Advancement of Rural Eye care, L.V. Prasad Eye Institute, Hyderabad, India
Rohit C. Khanna
Brien Holden Eye Research Centre, L.V. Prasad Eye Institute, Banjara Hills, Hyderabad, India
School of Optometry and Vision Science, University of New South Wales, Sydney, NSW, Australia
Rohit C. Khanna & Kovin S. Naidoo
University of Rochester, School of Medicine and Dentistry, Rochester, NY, USA
HelpMeSee, Instituto Mexicano de Oftalmologia, Santiago de Querétaro, Mexico
Van Charles Lansingh
University of Miami, Miami, FL, USA
University of Utah, Salt Lake City, UT, USA
African Vision Research Institute, University of KwaZulu-Natal (UKZN), Durban, South Africa
Kovin S. Naidoo
Suraj Eye Institute, Nagpur, India
Vinay Nangia
Institute of Optics and Optometry, University of Social Science, 121 Gdanska str., Lodz, 90-519, Poland
Michal Nowak
Medicine & Health, University of New South Wales, Sydney, NSW, Australia
Konrad Pesudovs
John Hopkins Wilmer Eye Institute, Baltimore, MD, USA
Pradeep Ramulu
1st Department of Ophthamology, Medical School, Aristotle University of Thessaloniki, Ahepa Hospital, Thessaloniki, Greece
Fotis Topouzis
University of Crete Medical School, Giofirakia, Greece
Mitiadis Tsilimbaris
Beijing Institute of Ophthamology, Beijing Tongren Hospital, Capital Medical University, Beijing Ophthamology and Visual Sciences Key Laboratory, Beijing, China
Ya Xing Wang
Beijing Institute of Ophthamology, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing, China
Ningli Wang
Centre for Public Health, Queen’s University Belfast, Belfast, UK
Vision and Eye Research Unit, Anglia Ruskin University, Cambridge, UK
Rupert Bourne
College of Optometry, Nova Southeastern University, Fort Lauderdale, FL, USA
Janet L. Leasher
Institute of Molecular and Clinical Ophthalmology Basel, Basel, Switzerland
Department of Ophthalmology, Heidelberg University, Mannheim, Germany
Mass Eye and Ear Department of Ophthalmology, Harvard Medical School, Boston, MA, USA
David S. Friedman
Department of Ophthalmology and Visual Sciences, Medical College of Wisconsin, Milwaukee, WI, USA
Department of Ophthalmology and Visual Sciences, Federal University of São Paulo, São Paulo, Brazil
School of Public Health, University of Technology Sydney, Sydney, NSW, Australia
Bright Opoku Ahinkorah
Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Hamid Ahmadieh
Department of Ophthalmology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan
Ayman Ahmed
Swiss Tropical and Public Health Institute, University of Basel, Basel, Switzerland
Department of Ophthalmology, University of Leipzig Medical Center, Leipzig, Germany
Ahmad Samir Alfaar
Department of Ophthalmology, Charité Medical University Berlin, Berlin, Germany
Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Louay Almidani
Doheny Image Reading and Research Lab (DIRRL) - Doheny Eye Institute, University of California Los Angeles, Los Angeles, CA, USA
Department of Population and Behavioural Sciences, University of Health and Allied Sciences, Ho, Ghana
Department of Medicine, University of Thessaly, Volos, Greece
Sofia Androudi
Health Management and Economics Research Center, Iran University of Medical Sciences, Tehran, Iran
Jalal Arabloo
Department of Applied Mathematics, University of Washington, Seattle, WA, USA
Aleksandr Y. Aravkin
Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA
Aleksandr Y. Aravkin, Paul Svitil Briant, Katrin Burkart, Kaleb Coberly, Xiaochen Dai, Stephen S. Lim, Tomislav Mestrovic, Ali H. Mokdad, Christopher J. L. Murray, Jaimie D. Steinmetz, Theo Vos & Peng Zheng
Department of Health Metrics Sciences, School of Medicine, University of Washington, Seattle, WA, USA
Aleksandr Y. Aravkin, Katrin Burkart, Xiaochen Dai, Stephen S. Lim, Awoke Misganaw, Ali H. Mokdad, Christopher J. L. Murray, Theo Vos & Peng Zheng
Department of Public Health, Debre Tabor University, Debre Tabor, Ethiopia
Mulu Tiruneh Asemu
Department of Neurovascular Research, Nested Knowledge, Inc., Saint Paul, MN, USA
Ahmed Y. Azzam
Faculty of Medicine, October 6 University, 6th of October City, Egypt
Department of Nursing, Saveh University of Medical Sciences, Saveh, Iran
Nayereh Baghcheghi
Big Data Institute - GRAM Project, University of Oxford, Oxford, UK
Freddie Bailey
Vocational School of Technical Sciences, Batman University, Batman, Türkiye
Mehmet Firat Baran
Miami Cancer Institute, Baptist Health South Florida, Miami, FL, USA
Mainak Bardhan
Heidelberg Institute of Global Health (HIGH), Heidelberg University, Heidelberg, Germany
Till Winfried Bärnighausen
T.H. Chan School of Public Health, Harvard University, Boston, MA, USA
Department of Epidemiology, University of Florida, Gainesville, FL, USA
Amadou Barrow
Department of Public & Environmental Health, University of The Gambia, Brikama, The Gambia
Department of Community Medicine and Family Medicine, All India Institute of Medical Sciences, Jodhpur, India
Pankaj Bhardwaj
School of Public Health, All India Institute of Medical Sciences, Jodhpur, India
Epidemiology Department, Ufa Eye Research Institute, Ufa, Russia
Mukharram Bikbov
Ophthalmology Department, Moorfields Eye Hospital NHS Foundation Trust, London, UK
International Centre for Eye Health, London School of Hygiene & Tropical Medicine, London, UK
Internal Medicine Department, Hospital Italiano de Buenos Aires (Italian Hospital of Buenos Aires), Buenos Aires, Argentina
Luis Alberto Cámera
Board of Directors, Argentine Society of Medicine, Buenos Aires, Argentina
Department of Addiction Medicine, Haukland University Hospital, Bergen, Norway
Omid Dadras
Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway
School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Amin Dehghan
USAID-JSI, Jimma University, Addis Ababa, Ethiopia
Berecha Hundessa Demessa
Department of Human Physiology, University of Gondar, Gondar, Ethiopia
Mengistie Diress
Department of Medicine, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Viet Nam
Thanh Chi Do
Department of Medicine, Can Tho University of Medicine and Pharmacy, Can Tho, Viet Nam
Thao Huynh Phuong Do
Department of Social Medicine and Health Care Organisation, Medical University “Prof. Dr. Paraskev Stoyanov”, Varna, Bulgaria
Klara Georgieva Dokova
Postgraduate Program in Epidemiology, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
Bruce B. Duncan
Department of Epidemiology and Medical Statistics, University of Ibadan, Ibadan, Nigeria
Michael Ekholuenetale
Faculty of Public Health, University of Ibadan, Ibadan, Nigeria
Faculty of Medicine, University of Tripoli, Tripoli, Libya
Muhammed Elhadi
Ophthalmic Epidemiology Research Center, Shahroud University of Medical Sciences, Shahroud, Iran
Mohammad Hassan Emamian
Department of Ophthalmology, University of California Los Angeles, Los Angeles, CA, USA
Mehdi Emamverdi
School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Hossein Farrokhpour & Sara Momtazmanesh
Endocrinology and Metabolism Research Institute, Non-Communicable Diseases Research Center (NCDRC), Tehran, Iran
Hossein Farrokhpour
Department of Environmental Health Engineering, Isfahan University of Medical Sciences, Isfahan, Iran
Ali Fatehizadeh
University Eye Clinic, University of Genoa, Genoa, Italy
Lorenzo Ferro Desideri
Division of Ophthalmology, University of São Paulo, Ribeirão Preto, Brazil
Department of Environmental Health, Wollo University, Dessie, Ethiopia
Mesfin Gebrehiwot
Ophthalmology Department, Tehran University of Medical Sciences, Tehran, Iran
Fariba Ghassemi
Department of Clinical Pharmacy, Haramaya University, Harar, Ethiopia
Mesay Dechasa Gudeta
Toxicology Department, Shriram Institute for Industrial Research, Delhi, India
Sapna Gupta
School of Medicine, Deakin University, Geelong, VIC, Australia
Veer Bala Gupta
Faculty of Medicine Health and Human Sciences, Macquarie University, Sydney, NSW, Australia
Vivek Kumar Gupta
Brain and Behavioral Sciences Program, University of Georgia, Athens, GA, USA
Billy Randall Hammond
Department of Nursing, Arak University of Medical Sciences, Arak, Iran
Mehdi Harorani
Department of Ophthalmology, Iran University of Medical Sciences, Karaj, Iran
Hamidreza Hasani
Independent Consultant, Santa Clara, CA, USA
Golnaz Heidari
Institute of Research and Development, Duy Tan University, Da Nang, Viet Nam
Mehdi Hosseinzadeh
Department of Computer Science, University of Human Development, Sulaymaniyah, Iraq
Department of Ophthalmology and Visual Science, Yale University, New Haven, CT, USA
John J. Huang
Institute for Physical Activity and Nutrition, Deakin University, Burwood, VIC, Australia
Sheikh Mohammed Shariful Islam
Sydney Medical School, University of Sydney, Sydney, NSW, Australia
Student Research Committee, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Nilofer Javadi
Department of Pediatrics, Isfahan University of Medical Sciences, Isfahan, Iran
Department of Ocular Epidemiology and Visual Health, Institute of Ophthalmology Foundation Conde de Valencia, Mexico City, Mexico
Aida Jimenez-Corona
Directorate General of Epidemiology, Mexico City, Mexico
Zoonoses Research Center, Islamic Azad University, Karaj, Iran
Mohammad Jokar
Department of Clinical Sciences, Jahrom University of Medical Sciences, Jahrom, Iran
Department of Economics, National Open University, Benin City, Nigeria
Charity Ehimwenma Joshua
Department of Oral and Maxillofacial Pathology, Krishna Vishwa Vidyapeeth (Deemed to be University), Karad, India
Vidya Kadashetti
Save Sight Institute, University of Sydney, Sydney, NSW, Australia
Himal Kandel & Yuyi You
Sydney Eye Hospital, South Eastern Sydney Local Health District, Sydney, NSW, Australia
Himal Kandel
Eye Research Center, Iran University of Medical Sciences, Tehran, Iran
Hengameh Kasraei
Health Policy Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Department of Pharmacology, All India Institute of Medical Sciences, Jodhpur, India
Rimple Jeet Kaur
Research Department, Better Vision Foundation Nepal, Kathmandu, Nepal
Sudarshan Khanal
Ophthalmology and Vision Science, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Zahra Khorrami
Social Determinants of Health Research Center, Saveh University of Medical Sciences, Saveh, Iran
Hamid Reza Koohestani
Department of Anthropology, Panjab University, Chandigarh, India
Kewal Krishan
Ophthalmology Department, Ministry of Health & Population, Aswan, Egypt
Mohammed Magdy Abd El Razek
Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
Vahid Mansouri
Department GF Ingrassia, University of Catania, Catania, Italy
Andrea Maugeri
University Centre Varazdin, University North, Varazdin, Croatia
Tomislav Mestrovic
National Data Management Center for Health, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
Awoke Misganaw
Optometry & Vision Sciences, Zahedan University of Medical Sciences, Zahedan, Iran
Hamed Momeni-Moghaddam
Eye Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
Non-communicable Diseases Research Center, Tehran University of Medical Sciences, Tehran, Iran
Sara Momtazmanesh
Department of Medical Laboratory Sciences, Adigrat University, Adigrat, Ethiopia
Hadush Negash
School of Medicine, Western Sydney University, Campbelltown, NSW, Australia
Uchechukwu Levi Osuagwu
Department of Optometry and Vision Science, University of KwaZulu-Natal, KwaZulu-Natal, South Africa
Global Health Governance Programme, University of Edinburgh, Edinburgh, UK
School of Dentistry, University of Leeds, Leeds, UK
Department of Genetics, Yale University, New Haven, CT, USA
Shrikant Pawar
Department of Statistics and Econometrics, Bucharest University of Economic Studies, Bucharest, Romania
Ionela-Roxana Petcu
Medical School, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Viet Nam
Hoang Tran Pham
Ophthalmology department, Isfahan University of Medical Sciences, Isfahan, Iran
Mohsen Pourazizi
Department of Neonatology, Case Western Reserve University, Cleveland, OH, USA
Ibrahim Qattea
Department of Population Science and Human Resource Development, University of Rajshahi, Rajshahi, Bangladesh
Mosiur Rahman
Multidisciplinary Laboratory Foundation University School of Health Sciences (FUSH), Foundation University, Islamabad, Pakistan
International Center of Medical Sciences Research (ICMSR), Islamabad, Pakistan
Applied Biomedical Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
Amirhossein Sahebkar
Biotechnology Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
Department of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Mohammad Amin Salehi
Department of Ophthalmology, Harvard Medical School, Boston, MA, USA
Maryam Shayan
Ophthalmic Research Center (ORC), Shahid Beheshti University of Medical Sciences, Tehran, Iran
Department of Veterinary Public Health and Preventive Medicine, Usmanu Danfodiyo University, Sokoto, Sokoto, Nigeria
Aminu Shittu
Aier Eye Hospital, Jinan university, Guangzhou, China
1st Department of Ophthalmology, Aristotle University of Thessaloniki, Thessaloniki, Greece
Department of Medicine, University of Crete, Heraklion, Greece
Aristidis Tsatsakis
Medical Genomics Research Department, King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
Muhammad Umair
Department of Life Sciences, University of Management and Technology, Lahore, Pakistan
School of Public Health, Zhejiang University, Zhejiang, China
Department of Public Health Science, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Macquarie Medical School, Macquarie University, Sydney, NSW, Australia
Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, CA, USA
Mikhail Sergeevich Zastrozhin
Addictology Department, Russian Medical Academy of Continuous Professional Education, Moscow, Russia
School of Medicine, Wuhan University, Wuhan, China
Zhi-Jiang Zhang
Vision Loss Expert Group of the Global Burden of Disease Study
- Katie Curran
- , Tunde Peto
- , Jost B. Jonas
- , David Friedman
- , Judy E. Kim
- , Janet Leasher
- , Ian Tapply
- , Arthur G. Fernandes
- , Maria Vittoria Cicinelli
- , Alessandro Arrigo
- , Nicolas Leveziel
- , Serge Resnikoff
- , Hugh R. Taylor
- , Tabassom Sedighi
- , Seth Flaxman
- , Mukkharram M. Bikbov
- , Tasanee Braithwaite
- , Alain Bron
- , Ching-Yu Cheng
- , Monte A. Del Monte
- , Joshua R. Ehrlich
- , João M. Furtado
- , Gus Gazzard
- , M. Elizabeth Hartnett
- , Rim Kahloun
- , John H. Kempen
- , Moncef Khairallah
- , Rohit C. Khanna
- , Van Charles Lansingh
- , Kovin S. Naidoo
- , Vinay Nangia
- , Michal Nowak
- , Konrad Pesudovs
- , Pradeep Ramulu
- , Fotis Topouzis
- , Mitiadis Tsilimbaris
- , Ya Xing Wang
- , Ningli Wang
- & Rupert R. A. Bourne
- , Rupert Bourne
- , Janet L. Leasher
- , David S. Friedman
- , Bright Opoku Ahinkorah
- , Hamid Ahmadieh
- , Ayman Ahmed
- , Ahmad Samir Alfaar
- , Louay Almidani
- , Hubert Amu
- , Sofia Androudi
- , Jalal Arabloo
- , Aleksandr Y. Aravkin
- , Mulu Tiruneh Asemu
- , Ahmed Y. Azzam
- , Nayereh Baghcheghi
- , Freddie Bailey
- , Mehmet Firat Baran
- , Mainak Bardhan
- , Till Winfried Bärnighausen
- , Amadou Barrow
- , Pankaj Bhardwaj
- , Mukharram Bikbov
- , Paul Svitil Briant
- , Katrin Burkart
- , Luis Alberto Cámera
- , Kaleb Coberly
- , Omid Dadras
- , Xiaochen Dai
- , Amin Dehghan
- , Berecha Hundessa Demessa
- , Mengistie Diress
- , Thanh Chi Do
- , Thao Huynh Phuong Do
- , Klara Georgieva Dokova
- , Bruce B. Duncan
- , Michael Ekholuenetale
- , Muhammed Elhadi
- , Mohammad Hassan Emamian
- , Mehdi Emamverdi
- , Hossein Farrokhpour
- , Ali Fatehizadeh
- , Lorenzo Ferro Desideri
- , Mesfin Gebrehiwot
- , Fariba Ghassemi
- , Mesay Dechasa Gudeta
- , Sapna Gupta
- , Veer Bala Gupta
- , Vivek Kumar Gupta
- , Billy Randall Hammond
- , Mehdi Harorani
- , Hamidreza Hasani
- , Golnaz Heidari
- , Mehdi Hosseinzadeh
- , John J. Huang
- , Sheikh Mohammed Shariful Islam
- , Nilofer Javadi
- , Aida Jimenez-Corona
- , Mohammad Jokar
- , Charity Ehimwenma Joshua
- , Vidya Kadashetti
- , Himal Kandel
- , Hengameh Kasraei
- , Rimple Jeet Kaur
- , Sudarshan Khanal
- , Zahra Khorrami
- , Hamid Reza Koohestani
- , Kewal Krishan
- , Stephen S. Lim
- , Mohammed Magdy Abd El Razek
- , Vahid Mansouri
- , Andrea Maugeri
- , Tomislav Mestrovic
- , Awoke Misganaw
- , Ali H. Mokdad
- , Hamed Momeni-Moghaddam
- , Sara Momtazmanesh
- , Christopher J. L. Murray
- , Hadush Negash
- , Uchechukwu Levi Osuagwu
- , Shahina Pardhan
- , Jay Patel
- , Shrikant Pawar
- , Ionela-Roxana Petcu
- , Hoang Tran Pham
- , Mohsen Pourazizi
- , Ibrahim Qattea
- , Mosiur Rahman
- , Umar Saeed
- , Amirhossein Sahebkar
- , Mohammad Amin Salehi
- , Maryam Shayan
- , Aminu Shittu
- , Jaimie D. Steinmetz
- , Aristidis Tsatsakis
- , Muhammad Umair
- , Hong Xiao
- , Mikhail Sergeevich Zastrozhin
- , Zhi-Jiang Zhang
- & Peng Zheng
Contributions
Please see Appendix for more detailed information about individual author contributions to the research, divided into the following categories: managing the overall research enterprise; writing the first draft of the manuscript; primary responsibility for applying analytical methods to produce estimates; primary responsibility for seeking, cataloguing, extracting, or cleaning data; designing or coding figures and tables; providing data or critical feedback on data sources; developing methods or computational machinery; providing critical feedback on methods or results; drafting the manuscript or revising it critically for important intellectual content; and managing the estimation or publications process.
Corresponding author
Correspondence to Rupert R. A. Bourne .
Ethics declarations
Competing interests.
GBD 2019 Blindness and Vision Impairment Collaborators : T W Bärnighausen reports grants or contracts from the European Union (Horizon 2020 and EIT Health), German Research Foundation (DFG), US National Institutes of Health, German Ministry of Education and Research, Alexander von Humboldt Foundation, Else-Kröner-Fresenius-Foundation, Wellcome Trust, Bill & Melinda Gates Foundation, KfW, UNAIDS, and WHO; consulting fees from KfW on the OSCAR initiative in Vietnam; participation on a Data Safety Monitoring Board or Advisory Board with NIH-funded study “Healthy Options” (PIs: Smith Fawzi, Kaaya) as Chair of the Data Safety and Monitoring Board, German National Committee on the “Future of Public Health Research and Education” as Chair of the scientific advisory board to the EDCTP Evaluation, Member of the UNAIDS Evaluation Expert Advisory Committee, National Institutes of Health Study Section Member on Population and Public Health Approaches to HIV/AIDS (PPAH), US National Academies of Sciences, Engineering, and Medicine’s Committee for the “Evaluation of Human Resources for Health in the Republic of Rwanda under the President’s Emergency Plan for AIDS Relief (PEPFAR)”, University of Pennsylvania (UPenn) Population Aging Research Center (PARC) as an External Advisory Board Member; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with the Global Health Hub Germany (which was initiated by the German Ministry of Health) as co-chair; all outside the submitted work. R Bourne reports support for the present manuscript to their institution, supporting the Vision Loss Expert Group, from the World Health Organization, the Brien Holden Vision Institute, Foundation Thea, Fred Hollows Foundation, Lions Clubs International Foundation; Bourne reports grants or contracts outside the submitted work to their institution supporting the Vision Loss Expert Group from Sightsavers International and University of Heidelberg. X Dai reports support for the present manuscript from the Institute for Health Metrics and Evaluation (University of Washington) for their salary. D S Friedman reports grants or contracts to their institution for research from Genentech; consulting fees from Abbvie, Kaliyope, Life Biosciences, Bausch and Lomb; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Thea Pharmaceuticals; support for attending meetings and travels from Thea Pharmaceuticals; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with Orbis International as a Member of the Board of Governors; all outside the submitted work. J M Furtado reports consulting fees outside the submitted work from SightFirst Latin America and Pan American Health Organization. V B Gupta reports grants or contracts from National Health and Medical Research Council (NHMRC) provided to Deakin University; outside the submitted work. V K Gupta reports grants or contracts from National Health and Medical Research Council (NHMRC) provided to Macquarie University; outside the submitted work. S M S Islam reports support for the present manuscript from the National Health and Medical Research Council (NHMRC, Australia) via an investigator grant and from the Heart Foundation of Australia via a Vanguard Grant. J E Kim reports consulting fees from Allergan, Apellis, Astellas, Bausch&Lomb, Clearside Biomedical, DORC, Genentech/Roche, Notal Vision, Outlook Therapeutics, and Regeneron; leadership or fiduciary roles in board, society, committee or advocacy groups, unpaid, with American Society of Retina Specialists, Macula Society, American Academy of Ophthalmology, and NAEVR/AEVR; receipt of equipment for research from Optos; all outside the submitted work. K Krishan reports non-financial support from the UGC Centre of Advanced Study, CAS II, awarded to the Department of Anthropology, Panjab University (Chandigarh, India); outside the submitted work. J L Leasher reports leadership or fiduciary roles in board, society, committee or advocacy groups, unpaid as a member of the National Eye Institute National Eye Health Education Program planning committee; outside the submitted work. J D Steinmetz reports support for the present manuscript from the Bill and Melinda Gates Foundation IHME funding for GBD analyses. Y Tan reports support for the present manuscript from the Department of Ophthalmology, The Third Xiangya Hospital, Central South University and the Postdoctoral Station of Clinical Medicine, The Third Xiangya Hospital, Central South University. F Topouzis reports grants or contracts from Thea Pharma Inc., Omikron, Pfizer, Alcon, AbbVie, Bayer, paid to their institution; consulting fees paid to them from Thea Pharma Inc., Omikron, Bausch & Lomb; participation on a Data Safety Monitoring Board or Advisory Board with Omikron, paid to them, and AbbVie and Roche, paid to their institution; leadership or fiduciary roles in board, society, committee or advocacy groups, unpaid, with European Glaucoma Society as President, Greek Glaucoma Society as President, and World Glaucoma Association as member of the Board of Governors; all outside the submitted work. Vision Loss Expert Group of the Global Burden of Disease Study : A Bron reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Théa. M A Del Monte reports support for attending meetings and/or travel from the University of Michigan, and leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid as past president of Costenbader Society. D Friedman reports receipt of equipment, materials, drugs, medical writing, gifts or other services from LumenisCorp (instrumental loan). J M Furtado reports consulting fees from Pan American Health Organization and from Lions Club International Foundation. G Gazzard reports consulting fees from Alcon Laboratories, Inc; Allergan, Inc; BELKIN Vision LTD; Carl Zeiss Meditec; Elios; Genentech/Roche; Reichert; Théa and ViaLase; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Alcon Laboratories, Inc; BELKIN Vision Ltd; Carl Zeiss Meditec; Elios and Ellex; participation on a Data Safety Monitoring Board or Advisory Board with Alcon Laboratories, Inc; Allergan, Inc; BELKIN Vision Ltd; Carl Zeiss Meditec; Elios and Visufarma; and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with Glaucoma UK Medical Advisory Board and UK&Eire Glaucoma Society as president. M E Hartnett reports support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc.) from Michael F. Marmor, M.D. Professor of Retinal Science and Disease as endowment to support salary; grants or contracts from any entity (from National Eye Institute R01 EY017011 and National Eye Institute R01 EY015130) as partial salary support; patents planned, issued or pending (WO2015123561A2 and WO2021062169A1); and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with Jack McGovern Coats’ Disease Foundation and as director of Women’s Eye Health and Macular Society Grant Review Chair. J H Kempen reports support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc.) from Mass Eye and Ear Global Surgery Program (as support of salary); grants or contracts from any entity from Sight for Souls (as support of salary); and stock or stock options with Betaliq and Tarsier (both as small equity owner). J E Kim reports consulting fees from Genentech/Roche, DORC, Notal Vision and Outlook Therapeutics (all as payment to J E Kim); participation on a Data Safety Monitoring Board or Advisory Board with Allergan, Amgen, Apellis, Bausch&Lomb, Clearside, Coherus, Novartis and Regeneron (all as participation on advisory board); leadership or fiduciary role in other borad, society, committee or advocacy group, paid or unpaid, with AAO, APRIS, ASRS, Macular Society and NAEVR/AEVR (all unpaid); and receipt of equipment, materials, drugs, medical writing, gifts or other services from Clearside and Genentech/Roche (both for medical writing). V C Lansingh reports consulting fees from HelpMeSee (as an employee); and support for attending meetings and/or travel from HelpMeSee (pay airfare and hotel). J Leasher reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with National Eye Institute (as a member) and National Eye Health Education Program planning committee (unpaid). M Nowak reports participation on a Data Safety Monitoring Board or Advisory Board with Vision Express Co. Poland as the chairman of medical advisory board of Vision Express Co. Poland. T Peto reports grants or contracts from any entity from Novartis (paid to institution); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer, Novartis and Roche (paid to T Peto); and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with European Association for Diabetic Eye Complications as current president (unpaid). P Ramulu reports grants or contracts from National Institute of Health and Perfuse Therapeutics; and consulting fees from Alcon and W. L. Gore. F Topouzis reports grants or contracts from Théa, Omikron, Pfizer, Alcon, Abbvie and Bayer (all paid to Institution); consulting fees from Omikron, Théa and Bausch & Lomb (all paid to Topouzis); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Omikron (paid to Topouzis), Abbvie and Roche (both paid to Institute); and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with European Glaucoma Society (as president), Greek Glaucoma Society (as president) and Board of Governors, World Glaucoma Association (all unpaid).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Fig s1: number of males (all ages) with msvi due to diabetic retinopathy in 2000 and 2020 by 21 gbd world regions, fig s2: number of females (all ages) with msvi due to diabetic retinopathy in 2000 and 2020 by 21 gbd world regions, fig s3: number of males (all ages) with blindness due to diabetic retinopathy in 2000 and 2020 by 21 gbd world regions, 41433_2024_3101_moesm6_esm.pdf.
Fig S4: Number of females (all ages) with blindness due to Diabetic retinopathy in 2000 and 2020 by 21 GBD world regions
Appendix: Contributions by Authors
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Vision Loss Expert Group of the Global Burden of Disease Study., the GBD 2019 Blindness and Vision Impairment Collaborators. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 2000 to 2020. Eye (2024). https://doi.org/10.1038/s41433-024-03101-5
Download citation
Received : 09 July 2023
Revised : 06 April 2024
Accepted : 19 April 2024
Published : 27 June 2024
DOI : https://doi.org/10.1038/s41433-024-03101-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
- Preventing Diabetes Problems
Diabetic Eye Disease
- Español
On this page:
What is diabetic eye disease?
How does diabetes affect my eyes, how common is diabetic eye disease, who is more likely to develop diabetic eye disease, what are the symptoms of diabetic eye disease, when should i see a doctor right away, how do doctors diagnose eye problems from diabetes, how do doctors treat diabetic eye disease, what can i do to protect my eyes, what if i already have some vision loss from diabetes.
Diabetic eye disease is a group of eye problems that can affect people with diabetes. These conditions include diabetic retinopathy, diabetic macular edema, cataracts, and glaucoma.
Over time, diabetes can cause damage to your eyes that can lead to poor vision or even blindness. But you can take steps to prevent diabetic eye disease, or keep it from getting worse, by taking care of your diabetes.
The best ways to manage your diabetes and keep your eyes healthy are to
- manage your blood glucose , blood pressure , and cholesterol , sometimes called the diabetes ABCs
- If you smoke, get help to quit smoking
- have a dilated eye exam once a year

Often, there are no warning signs of diabetic eye disease or vision loss when damage first develops. A full, dilated eye exam helps your doctor find and treat eye problems early—often before much vision loss can occur.
Diabetes affects your eyes when your blood glucose, also called blood sugar, is too high.
In the short term, you are not likely to have vision loss from high blood glucose. People sometimes have blurry vision for a few days or weeks when they’re changing their diabetes care plan or medicines. High glucose can change fluid levels or cause swelling in the tissues of your eyes that help you to focus, causing blurred vision. This type of blurry vision is temporary and goes away when your glucose level gets closer to normal.
If your blood glucose stays high over time, it can damage the tiny blood vessels in the back of your eyes. This damage can begin during prediabetes , when blood glucose is higher than normal, but not high enough for you to be diagnosed with diabetes. Damaged blood vessels may leak fluid and cause swelling. New, weak blood vessels may also begin to grow. These blood vessels can bleed into the middle part of the eye, lead to scarring, or cause dangerously high pressure inside your eye.
Most serious diabetic eye diseases begin with blood vessel problems. The four eye diseases that can threaten your sight are
Diabetic retinopathy
The retina is the inner lining at the back of each eye. The retina senses light and turns it into signals that your brain decodes, so you can see the world around you. Damaged blood vessels can harm the retina, causing a disease called diabetic retinopathy.
In early diabetic retinopathy, blood vessels can weaken, bulge, or leak into the retina. This stage is called nonproliferative diabetic retinopathy.
If the disease gets worse, some blood vessels close off, which causes new blood vessels to grow, or proliferate, on the surface of the retina. This stage is called proliferative diabetic retinopathy. These abnormal new blood vessels can lead to serious vision problems.

Diabetic macular edema
The part of your retina that you need for reading, driving, and seeing faces is called the macula . Diabetes can lead to swelling in the macula, which is called diabetic macular edema. Over time, this disease can destroy the sharp vision in this part of the eye, leading to partial vision loss or blindness. Macular edema usually develops in people who already have other signs of diabetic retinopathy.
Glaucoma is a group of eye diseases that can damage the optic nerve—the bundle of nerves that connects the eye to the brain. Diabetes doubles the chances of having glaucoma, which can lead to vision loss and blindness if not treated early.
Symptoms depend on which type of glaucoma you have. Learn more about glaucoma .

The lenses within our eyes are clear structures that help provide sharp vision—but they tend to become cloudy as we age. People with diabetes are more likely to develop cloudy lenses, called cataracts . People with diabetes can develop cataracts at an earlier age than people without diabetes. Researchers think that high glucose levels cause deposits to build up in the lenses of your eyes.

About one in three people with diabetes who are older than age 40 already have some signs of diabetic retinopathy. 1 Diabetic retinopathy is the most common cause of vision loss in people with diabetes. Each person’s outlook for the future, however, depends in large part on regular care. Finding and treating diabetic retinopathy early can reduce the risk of blindness by 95 percent.
Glaucoma and cataracts
Your chances of developing glaucoma or cataracts are about twice that of someone without diabetes.
Anyone with diabetes can develop diabetic eye disease. Your risk is greater with
- high blood glucose that is not treated
- high blood pressure that is not treated
High blood cholesterol and smoking may also raise your risk for diabetic eye disease.
Some groups are affected more than others. African Americans, American Indians and Alaska Natives, Hispanics/Latinos, Pacific Islanders, and older adults are at greater risk of losing vision or going blind from diabetes.
If you have diabetes and become pregnant, you can develop eye problems very quickly during your pregnancy. If you already have some diabetic retinopathy, it can get worse during pregnancy. Changes that help your body support a growing baby may put stress on the blood vessels in your eyes. Your health care team will suggest regular eye exams during pregnancy to catch and treat problems early and protect your vision.
Diabetes that occurs only during pregnancy, called gestational diabetes , does not usually cause eye problems. Researchers aren't sure why this is the case.
Your chances of developing diabetic eye disease increase the longer you have diabetes.
Often there are no early symptoms of diabetic eye disease. You may have no pain and no change in your vision as damage begins to grow inside your eyes, particularly with diabetic retinopathy.
When symptoms do occur, they may include
- blurry or wavy vision
- frequently changing vision—sometimes from day to day
- dark areas or vision loss
- poor color vision
- spots or dark strings (also called floaters)
- flashes of light
Talk with your eye doctor if you have any of these symptoms.
Call a doctor right away if you notice sudden changes to your vision, including flashes of light or many more spots (floaters) than usual. You also should see a doctor right away if it looks like a curtain is pulled over your eyes. These changes in your sight can be symptoms of a detached retina , which is a medical emergency.
Having a full, dilated eye exam is the best way to check for eye problems from diabetes. Your doctor will place drops in your eyes to widen your pupils. This allows the doctor to examine a larger area at the back of each eye, using a special magnifying lens. Your vision will be blurry for a few hours after a dilated exam.
Your doctor will also
- test your vision
- measure the pressure in your eyes
Your doctor may suggest other tests, too, depending on your health history.
Most people with diabetes should see an eye care professional once a year for a complete eye exam. Your own health care team may suggest a different plan, based on your type of diabetes and the time since you were first diagnosed.
Your doctor may recommend having eye exams more often than once a year, along with management of your diabetes. This means managing your diabetes ABCs, which include your A1c, blood pressure, and cholesterol; and quitting smoking. Ask your health care team what you can do to reach your goals.

Doctors may treat advanced eye problems with medicine, laser treatments, surgery, or a combination of these options.
Your doctor may treat your eyes with anti-VEGF medicine, such as aflibercept, bevacizumab, or ranibizumab. These medicines block the growth of abnormal blood vessels in the eye. Anti-VEGF medicines can also stop fluid leaks, which can help treat diabetic macular edema .
The doctor will inject an anti-VEGF medicine into your eyes during office visits. You'll have several treatments during the first few months, then fewer treatments after you finish the first round of therapy. Your doctor will use medicine to numb your eyes so you don’t feel pain. The needle is about the thickness of a human hair.
Anti-VEGF treatments can stop further vision loss and may improve vision in some people.
Laser Treatment
Laser treatment, also called photocoagulation, creates tiny burns inside the eye with a beam of light. This method treats leaky blood vessels and extra fluid, called edema. Your doctor usually provides this treatment during several office visits, using medicine to numb your eyes. Laser treatment can keep eye disease from getting worse, which is important to prevent vision loss or blindness. But laser treatment is less likely to bring back vision you’ve already lost compared with anti-VEGF medicines.
There are two types of laser treatment :
- Focal/grid laser treatment works on a small area of the retina to treat diabetic macular edema.
- Scatter laser treatment, also called panretinal photocoagulation (PRP), covers a larger area of the retina. This method treats the growth of abnormal blood vessels, called proliferative diabetic retinopathy.
Vitrectomy is a surgery to remove the clear gel that fills the center of the eye, called the vitreous gel. The procedure treats problems with severe bleeding or scar tissue caused by proliferative diabetic retinopathy. Scar tissue can force the retina to peel away from the tissue beneath it, like wallpaper peeling away from a wall. A retina that comes completely loose, or detaches, can cause blindness.
During vitrectomy, a clear salt solution is gently pumped into the eye to maintain eye pressure during surgery and to replace the removed vitreous. Vitrectomy is done in a surgery center or hospital with pain medicine.
Cataract Lens Surgery
In a surgery center or hospital visit, your doctor can remove the cloudy lens in your eye, where the cataract has grown, and replace it with an artificial lens. People who have cataract surgery generally have better vision afterward. After your eye heals, you may need a new prescription for your glasses. Your vision following cataract surgery may also depend on treating any damage from diabetic retinopathy or macular edema.
To prevent diabetic eye disease, or to keep it from getting worse, manage your diabetes ABCs: your A1c, blood pressure, and cholesterol; and quit smoking if you smoke. Read more information on how to manage diabetes .
Also, have a dilated eye exam at least once a year—or more often if recommended by your eye care professional. These actions are powerful ways to protect the health of your eyes—and can prevent blindness.
The sooner you work to manage your diabetes and other health conditions, the better. And, even if you’ve struggled in the past to manage your health, taking better care of yourself now can protect your eyes for the future. It’s never too late to begin.
Ask your eye care professional to help you find a low vision and rehabilitation clinic. Special eye care professionals can help you manage vision loss that cannot be corrected with glasses, contact lenses, medicine, or surgery. Special devices and training may help you make the most of your remaining vision so that you can continue to be active, enjoy hobbies, visit friends and family members, and live without help from others.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
The NIDDK would like to thank: Neil M. Bressler, MD, The Wilmer Eye Institute, Johns Hopkins University School of Medicine; Emily Chew, MD, National Eye Institute (NEI)

Deep Learning for Detection of Diabetic Eye Disease
November 29, 2016
Posted by Lily Peng MD PhD, Product Manager and Varun Gulshan PhD, Research Engineer
| Figure 1. Examples of retinal fundus photographs that are taken to screen for DR. The image on the left is of a healthy retina (A), whereas the image on the right is a retina with referable diabetic retinopathy (B) due a number of hemorrhages (red spots) present. |
| Figure 2. Performance of the algorithm (black curve) and eight ophthalmologists (colored dots) for the presence of referable diabetic retinopathy (moderate or worse diabetic retinopathy or referable diabetic macular edema) on a validation set consisting of 9963 images. The black diamonds on the graph correspond to the sensitivity and specificity of the algorithm at the high sensitivity and high specificity operating points. |
- General Science
- Health & Bioscience
- Machine Intelligence
Other posts of interest

June 26, 2024
- Generative AI ·
- Machine Intelligence ·
- Machine Perception

June 25, 2024
- Natural Language Processing ·
- Open Source Models & Datasets

June 18, 2024
- General Science ·
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Diabetic eye: associated diseases, drugs in clinic, and role of self-assembled carriers in topical treatment
Affiliation.
- 1 Departamento de Farmacología, Farmacia y Tecnología Farmacéutica, I+D Farma Group (GI-1645), Facultad de Farmacia and Health Research Institute of Santiago de Compostela (IDIS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain.
- PMID: 34253138
- DOI: 10.1080/17425247.2021.1953466
Introduction : Diabetes is a pandemic disease that causes relevant ocular pathologies. Diabetic retinopathy, macular edema, cataracts, glaucoma, or keratopathy strongly impact the quality of life of the patients. In addition to glycemic control, intense research is devoted to finding more efficient ocular drugs and improved delivery systems that can overcome eye barriers. Areas covered : The aim of this review is to revisit first the role of diabetes in the development of chronic eye diseases. Then, commercially available drugs and new candidates in clinical trials are tackled together with the pros and cons of their administration routes. Subsequent sections deal with self-assembled drug carriers suitable for eye instillation combining patient-friendly administration with high ocular bioavailability. Performance of topically administered polymeric micelles, liposomes, and niosomes for the management of diabetic eye diseases is analyzed in the light of ex vivo and in vivo results and outcomes of clinical trials. Expert opinion : Self-assembled carriers are being shown useful for efficient delivery of not only a variety of small drugs but also macromolecules (e.g. antibodies) and genes. Successful design of drug carriers may offer alternatives to intraocular injections and improve the treatment of both anterior and posterior segments diabetic eye diseases.
Keywords: Diabetic eye; clinical trials; liposomes; niosomes; polymeric micelles; topical ocular delivery.
PubMed Disclaimer
Similar articles
- Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. Onugwu AL, Nwagwu CS, Onugwu OS, Echezona AC, Agbo CP, Ihim SA, Emeh P, Nnamani PO, Attama AA, Khutoryanskiy VV. Onugwu AL, et al. J Control Release. 2023 Feb;354:465-488. doi: 10.1016/j.jconrel.2023.01.018. Epub 2023 Jan 19. J Control Release. 2023. PMID: 36642250 Review.
- Micelles: Promising Ocular Drug Carriers for Anterior and Posterior Segment Diseases. Durgun ME, Güngör S, Özsoy Y. Durgun ME, et al. J Ocul Pharmacol Ther. 2020 Jul/Aug;36(6):323-341. doi: 10.1089/jop.2019.0109. Epub 2020 Apr 20. J Ocul Pharmacol Ther. 2020. PMID: 32310723 Review.
- Topical application of polymeric nanomicelles in ophthalmology: a review on research efforts for the noninvasive delivery of ocular therapeutics. Grimaudo MA, Pescina S, Padula C, Santi P, Concheiro A, Alvarez-Lorenzo C, Nicoli S. Grimaudo MA, et al. Expert Opin Drug Deliv. 2019 Apr;16(4):397-413. doi: 10.1080/17425247.2019.1597848. Epub 2019 Apr 8. Expert Opin Drug Deliv. 2019. PMID: 30889977 Review.
- Delivery of therapeutics for deep-seated ocular conditions - status quo. Nguyen H, Eng S, Ngo T, Dass CR. Nguyen H, et al. J Pharm Pharmacol. 2018 Aug;70(8):994-1001. doi: 10.1111/jphp.12924. Epub 2018 Apr 19. J Pharm Pharmacol. 2018. PMID: 29675844 Review.
- Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. Mandal A, Bisht R, Rupenthal ID, Mitra AK. Mandal A, et al. J Control Release. 2017 Feb 28;248:96-116. doi: 10.1016/j.jconrel.2017.01.012. Epub 2017 Jan 11. J Control Release. 2017. PMID: 28087407 Free PMC article. Review.
- Oleogels for the ocular delivery of epalrestat: formulation, in vitro, in ovo, ex vivo and in vivo evaluation. Kattar A, Vivero-Lopez M, Concheiro A, Mudakavi R, Chauhan A, Alvarez-Lorenzo C. Kattar A, et al. Drug Deliv Transl Res. 2024 May 23. doi: 10.1007/s13346-024-01560-7. Online ahead of print. Drug Deliv Transl Res. 2024. PMID: 38780858
- Langmuir monolayer studies of non-ionic surfactants and DOTMA for the design of ophthalmic niosomes. Kattar A, V Lage E, Casas M, Concheiro A, Alvarez-Lorenzo C. Kattar A, et al. Heliyon. 2024 Feb 10;10(4):e25887. doi: 10.1016/j.heliyon.2024.e25887. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38380035 Free PMC article.
- AAV-Based Strategies for Treatment of Retinal and Choroidal Vascular Diseases: Advances in Age-Related Macular Degeneration and Diabetic Retinopathy Therapies. Castro BFM, Steel JC, Layton CJ. Castro BFM, et al. BioDrugs. 2024 Jan;38(1):73-93. doi: 10.1007/s40259-023-00629-y. Epub 2023 Oct 25. BioDrugs. 2024. PMID: 37878215 Free PMC article. Review.
- Formulation and Characterization of Epalrestat-Loaded Polysorbate 60 Cationic Niosomes for Ocular Delivery. Kattar A, Quelle-Regaldie A, Sánchez L, Concheiro A, Alvarez-Lorenzo C. Kattar A, et al. Pharmaceutics. 2023 Apr 14;15(4):1247. doi: 10.3390/pharmaceutics15041247. Pharmaceutics. 2023. PMID: 37111732 Free PMC article.
- An Update on Novel Ocular Nanosystems with Possible Benefits in the Treatment of Corneal Neovascularization. Zhang C, Yin Y, Zhao J, Li Y, Wang Y, Zhang Z, Niu L, Zheng Y. Zhang C, et al. Int J Nanomedicine. 2022 Oct 19;17:4911-4931. doi: 10.2147/IJN.S375570. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36267540 Free PMC article. Review.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Taylor & Francis
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

- Previous Article
- Next Article
Disparities in Prevalence
Disparities in rates of drd screening, interventions to improve drd screening rates, telemedicine to improve drd screening rates, acknowledgments, connected content.
In a special series of the ADA Journals' podcast Diabetes Core Update , host Dr. Neil Skolnik interviews special guests and authors of this clinical compendium issue. Listen now at Special Podcast Series: Focus on Diabetes or view the interviews on YouTube at A Practice Guide to Diabetes-Related Eye Care .
Disparities in Diabetes-Related Retinal Disease and Approaches to Improve Screening Rates
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Anjali R. Shah , Rebecca Wu; Disparities in Diabetes-Related Retinal Disease and Approaches to Improve Screening Rates. ADA Clinical Compendia 1 July 2022; 2022 (3): 16–19. https://doi.org/10.2337/db20223-16
Download citation file:
- Ris (Zotero)
- Reference Manager
According to the Centers for Disease Control and Prevention, an estimated 37.3 million people (11.3% of the U.S. population) have diabetes, and an additional 96 million people >18 years of age (38% of the U.S. adult population) have prediabetes (28). Sociodemographic factors such as race, ethnicity, income, education level, and insurance status have all been shown to affect diabetes prevalence rates (80–83). Ecological studies of diabetes prevalence have recently identified a “diabetes belt”: a region of the United States that encompasses 644 counties in mostly southern states in which diabetes prevalence is ≥11% (24). Research on these geographical disparities has shown that community factors such as racial/ethnic mix, income, and food environment all contribute to rates of diabetes. Diabetes-related retinal disease (DRD), the most common ocular complication of diabetes and a leading cause of blindness in the United States (84), also disproportionately affects certain populations.
Correlations between race/ethnicity and rates of DRD in the United States have been well established in multiple studies. Harris et al. published several studies in the 1990s that found that Blacks had significantly higher rates of DRD than non-Hispanic Whites. One study (85) reported that Black men were ~23% more likely to develop DRD than other race-sex groups, and another study (86) found that Blacks were more likely to develop DRD than Whites, with a calculated odds ratio (OR) of 2.96 after adjusting for glycemic control, blood pressure, and diabetes treatment. A third report by Harris et al. (87) showed that non-Hispanic Blacks and Mexican Americans not only have a higher prevalence of DRD compared to their White counterparts (46% and 84%, respectively), but also have higher rates of moderate and severe stages of DRD. This report concluded that, for Blacks, much of the difference in DRD rates could be attributed to higher levels of risk factors in that population.
Prevalence rates of diabetes in the Hispanic population are generally reported to be about twice that of non-Hispanic Whites (88). DRD is also noted to develop at higher rates in this population. The National Eye Institute reports that, in 2010, Hispanic Americans ≥50 years of age had the highest prevalence of DRD (8%) of any racial/ethnic group (84). Almost half of Latino people with diabetes have DRD, with reported rates of 46.9 and 48% in two large epidemiological studies (88,89). In addition, while the number of Americans with DRD is expected to nearly double between 2010 and 2050, Hispanic Americans are expected to experience the greatest rise in cases, with a nearly three-fold increase in that time frame (84). Figure 1 illustrates projected increases in DRD cases in the United States by race/ethnicity.

Projections of DRD in the United States for 2030 and 2050 (in millions).
Native Americans/Alaska Natives also have a prevalence of diagnosed diabetes that is about twice that of non-Hispanic Whites (1). Studies conducted in the 1980s and 1990s reported rates of DRD in these populations as high as 35–49%, but in a more recent study published in 2018, Bursell et al. (90) reported prevalence rates of DRD that were approximately half of those reported in earlier studies. They hypothesize that improvements in rates of DRD are associated with improvements in diabetes management.
Diabetes-related macular edema (DME), a vision-threatening stage of DRD, also disproportionately affects communities along racial/ethnic lines. Wong et al. (91) showed that the prevalence of DRD and DME was significantly higher in Blacks and Hispanics than in White and Chinese cohorts; however, despite the differences in rates of disease, their analysis found that race was not an independent predictor of the development of DRD. Another study (92) reported that the prevalence of DME was approximately threefold higher in non-Hispanic Blacks than in the non-Hispanic White population.
Proliferative diabetes-related retinal disease (PDR) is an advanced, vision-threatening stage of DRD. In a large database study, Malhotra et al. (93) reported that Black and Hispanic individuals had higher rates of PDR compared to their White and non-Hispanic counterparts. They also noted that Black and Hispanic people had worse visual acuity at initiation of treatment for vision-threatening disease compared to White and non-Hispanic people. Blacks and Hispanics had ORs of 1.23 and 1.71, respectively, for presenting with one level of DRD severity worse than White or non-Hispanic people. Black individuals presented with not only more severe DRD, but also significantly worse visual acuity.
Given the significant disparities in the rates of DRD, DME, and PDR, as well as differences in severity of disease at presentation, it is not surprising that visual impairment from DRD also disproportionately affects certain populations. Data from the Salisbury Eye Evaluation, reported two decades ago, showed that African Americans were four times more likely than Whites to suffer visual impairment from DRD (94).
In addition to race and ethnicity, there are significant correlations between other socioeconomic factors and prevalence of diabetes and DRD. A 2010 study (95) looked at prevalence of diabetes in Appalachian counties within the diabetes belt based on 3-year unemployment rate, per-capita income, and poverty rate. Counties were deemed “distressed” if they were in the bottom 10% of all counties in the country on these measures. The researchers found that residents of distressed Appalachian counties had 33% higher odds of having diabetes than those in non-Appalachian counties. The reasons for this disparity are likely complex and multifactorial, including higher rates of obesity, less physical activity, food insecurity, poor health literacy, and lack of access to care. A study of people with diabetes in North Carolina (96) reported that, in addition to increased prevalence in Blacks and individuals with a longer duration of diabetes, self-reported DRD was more common in adults who were not married or living with a partner, those with less than a high school education, those without health insurance, and adults with an annual household income <$25,000; these findings underscore the impact of socioeconomic status on rates of DRD.
Most people are asymptomatic in the early stages of diabetes-related eye disease. Nwanyanwu et al. (97) found that nearly 11% of people with type 2 diabetes were unaware of their DRD diagnosis, which represents an estimated 9.8 million individuals. Screening for DRD is a cost-effective way of identifying it early and providing opportunities for both systemic and vision-preserving interventions (98). Early detection and treatment of DRD can reduce severe vision loss by 94% (99), and up to 21% of people with type 2 diabetes may already have some degree of DRD at the time of their diabetes diagnosis (100). Despite these statistics, adherence to DRD screening guidelines is low, with one study demonstrating that 35% of its cohort with diabetes did not receive appropriate screening (99). Other estimates suggest that nearly half of all people with diabetes do not receive eye health screenings as recommended in guidelines. 2020 data from the National Committee of Quality Assurance (NCQA) show that less than 50% of individuals with commercial insurance, 50.6% of those with Medicaid, and less than 69% of those with Medicare underwent DRD screening as recommended (101).
Racial minority groups have lower rates of eye screening than non-Hispanic Whites. One study demonstrated that, from 2002 to 2009, while the screening rate for Whites increased from 56 to 59%, the screening rate in minorities decreased from 56 to 49% (102). Although not specific to people with diabetes, another study reported that African Americans were less likely than non-Hispanic Whites to receive any eye care examinations (103). In the Los Angeles Latino Eye Study (104), 65% of participants had not had guideline-recommended eye care for people with type 2 diabetes.
In addition to race and ethnicity, household income, education level, health literacy, and geographical location have all been shown to be significant barriers to meeting eye health screening guidelines. Lower income and education levels, rural residence, and lack of health insurance have all been linked to fewer visits to eye care professionals and fewer dilated eye exams, and all of these factors also contribute to the lower screening rates noted in minority populations (103–105).
Screening in youth is important because 20.1 and 7.2% of youth with newly diagnosed type 1 and type 2 diabetes, respectively, in a large U.S. managed-care network developed DRD during 3 years of follow-up (8). Disparities in DRD screening rates have also been documented in youth with diabetes. Thomas et al. (106) reported that 34.2% of the youth with type 1 or type 2 diabetes in their cohort had not had a prior diabetes-related eye exam. Being of non-White race and having Medicaid or other public insurance, lower household income, and parents with a high school education or less were all associated with being less likely to have had a prior eye exam. Another study involving youth with type 1 diabetes reported that White children were significantly more likely than Black children to be screened for DRD (OR 1.64) and that Black children in the study cohort were seven times more likely than White children to have public health insurance (107). The authors noted that youth who were not screened were more likely to have poorer diabetes control, suggesting that those who were not receiving eye exams were also the most at risk for DRD.
Numerous barriers to obtaining guideline-recommended screening exams have been documented, including patient-, physician-, and system-level factors ( Table 1 ) (103,105,108,109). It is important to note that, in addition to patient-level factors, several provider- and system-level factors can be addressed to improve DRD screening rates.
Barriers to Recommended DRD Screening
| Component . | Considerations . |
|---|---|
| Component . | Considerations . |
|---|---|
Various strategies have been implemented successfully to improve retinal screening rates. These have included patient and provider education programs, strategies to improve access to health care, computer-based registration or reminder systems, collaboration among organizations that provide retinal screenings, and the development of a community-based health care system (110). Interventions aimed specifically at non-White, low-income, and low–health literacy communities may also be effective (105).
Health education and reminder interventions focusing on both people with diabetes and their health care providers have been shown to improve screening rates (110–112). Educating patients about diabetes-related eye disease can help them understand the importance of regular screening and motivate them to participate more in their own care. Educating primary care providers about eye screening guidelines and improving provider-patient relationships by increasing providers' cultural competency may help to reduce the disparities in screening rates noted in minority populations. Walker et al. (113) increased DRD screening by 74% using a telephone intervention in a minority, low-income population. The telephone intervention, which was conducted by a bilingual interventionist, served to educate and motivate individuals about the importance of having an annual dilated eye examination and afforded the opportunity to discuss risk and the frequent lack of symptoms early in DRD and elicit and troubleshoot barriers. Another study (114) tested a health education intervention using a face-to-face session delivered in the local language, with pictorial educational materials in the local language, and telephone reminders. It found that personalized health education was the most important predictor of follow-through with screening referrals. Basch et al. (111) doubled the rate of ophthalmic examination among African Americans with diabetes from 27.3 to 54.7% using educational materials that included a low-literacy booklet, a motivational videotape, and telephone education and counseling.
At the system level, patient registries, collaboratives, and prompts within electronic medical record (EMR) systems have all been shown to help providers identify patients who are not getting recommended DRD screening (115,116). Kollipara et al. (116) increased screening rates in a large endocrinology clinic from 49 to 69% using a multifaceted approach that included a diabetes patient registry and decision-support tools within the EMR system. Use of the registry facilitated the identification of care gaps, and use of the EMR system facilitated patient outreach using bulk messaging through the patient portal as well as placement of referrals to ophthalmology and provided an efficient system for tracking the successful delivery of care.
The use of telemedicine using retinal imaging with remote interpretation by eye care specialists can increase DRD screening rates (117–119). Studies have shown a high degree of accuracy in detecting DRD by image-based telemedicine, with sensitivity of >80% and specificity of >90% in most studies (120). This approach has been implemented widely in many countries, including Singapore, China, and India (121), and is the standard for DRD screening in the United Kingdom (45). In 2014, Liew et al. (10) noted a decline in the absolute number and relative proportion of blindness certifications resulting from DRD/maculopathy among working-age adults after the 2003 introduction of the National Health Service Diabetic Eye Screening Programme in England. These authors also reported that, by 2009–2010, DRD/maculopathy was no longer the leading cause of certifiable blindness among working-age adults in England and Wales for the first time in at least five decades. In the United States, digital retinal imaging with remote interpretation has been implemented successfully by the Veterans Health Administration (VHA) and found to be cost-effective and to increase population reach (122).
The placement of digital imaging equipment in primary care offices for point-of-care testing can further reduce patient-level obstacles such as lack of transportation, inconvenience, and language barriers (123). This strategy decreases the travel distance and time required for DRD screening because it does not require a separate visit to a different location. In the Tribal Vision Project (124), people randomized to telemedicine were more likely to receive a DRD screening exam than those receiving traditional surveillance throughout a 6-month period.
Although studies of the VHA's teleretinal screening program have demonstrated cost-effectiveness (122), these results are not directly applicable to teleretinal programs implemented in community primary care clinics in the United States (125). The initial investment for retinal imaging devices and training may be prohibitively high for many primary care clinics without additional sources of funding. Although there is active research and development in the field of retinal imaging, a low-cost, validated, nonmydriatic retinal camera is not yet commercially available (103,125).
In addition, reimbursement for telemedicine Current Procedural Terminology (CPT) codes for retinal screening does not adequately reflect the work performed and is insufficient to cover the cost of most DRD telemedicine programs (103). In 2011, CPT codes 92227 and 92228 were introduced for remote imaging for detection of retinal disease. For individuals with no known retinal disease, CPT code 92227 (remote imaging for detection of retinal disease with analysis and report under physician supervision) has no compensation for physician work and is associated with very low reimbursement (average allowable amount <$16). CPT code 92228 (remote imaging for monitoring and management of active retinal disease with physician review, interpretation, and report) is used for people with active retinal disease and has slightly higher reimbursement (average allowable amount <$40). Despite the benefits of telemedicine for DRD screening, financial sustainability continues to be a major barrier to its widespread implementation (103,125).
Significant disparities exist in rates of both DRD and DRD screening. Black, Hispanic, and Native American populations are disproportionately affected, with higher rates of DRD and lower rates of DRD screening. Additionally, screening rates are affected by socioeconomic factors such as income, education level, insurance payor, and geographical location. Numerous patient-, physician-, and system-level factors contribute to these disparities, and various interventions have been shown to be effective in addressing barriers at each of these levels.
It is important for practitioners to recognize that socio-demographic factors play key roles in diabetes management and risk for complications such as DRD. Strong clinician-patient relationships and a better understanding of barriers faced by different racial/ethnic and socioeconomic groups will allow for targeted interventions such as providing people with literature in their native language and at an appropriate health literacy level, offering financial counseling, or engaging social work services to assist with transportation. System-level changes such as instituting EMR reminders and prompts and teleretinal imaging are also important strategies to improve rates of DRD screening. In particular, teleretinal imaging is an emerging and important strategy to address disparities in DRD disease burden, although financial sustainability remains a barrier to its widespread implementation. Recognition of the value of telemedicine services by payers and government agencies could lead to significant improvements in access to care and reductions in DRD disparities.
Editorial and project management services were provided by Debbie Kendall of Kendall Editorial in Richmond, VA.
Dualities of Interest
B.A.C. is a consultant for Genentech and Regeneron. S.A.R. is a speaker for Allergan, Inc., and VSP Vision Care. No other potential conflicts of interest relevant to this compendium were reported.
Author Contributions
All authors researched and wrote their respective sections. Lead author T.W.G. reviewed all content and is the guarantor of this work.
The opinions expressed are those of the authors and do not necessarily reflect those of VSP Vision Care, Regeneron, or the American Diabetes Association. The content was developed by the authors and does not represent the policy or position of the American Diabetes Association, any of its boards or committees, or any of its journals or their editors or editorial boards.
Email alerts
- Online ISSN 2771-6880
- Print ISSN 2771-6872
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Diabetic retinopathy
On this page
Risk factors, complications.
Diabetic retinopathy (die-uh-BET-ik ret-ih-NOP-uh-thee) is a diabetes complication that affects eyes. It's caused by damage to the blood vessels of the light-sensitive tissue at the back of the eye (retina).
At first, diabetic retinopathy might cause no symptoms or only mild vision problems. But it can lead to blindness.
The condition can develop in anyone who has type 1 or type 2 diabetes. The longer you have diabetes and the less controlled your blood sugar is, the more likely you are to develop this eye complication.
Products & Services
- A Book: Mayo Clinic Guide to Better Vision
- A Book: The Essential Diabetes Book
- A Book: The Mayo Clinic Diabetes Diet
You might not have symptoms in the early stages of diabetic retinopathy. As the condition progresses, you might develop:
- Spots or dark strings floating in your vision (floaters)
- Blurred vision
- Fluctuating vision
- Dark or empty areas in your vision
- Vision loss
When to see an eye doctor
Careful management of your diabetes is the best way to prevent vision loss. If you have diabetes, see your eye doctor for a yearly eye exam with dilation — even if your vision seems fine.
Developing diabetes when pregnant (gestational diabetes) or having diabetes before becoming pregnant can increase your risk of diabetic retinopathy. If you're pregnant, your eye doctor might recommend additional eye exams throughout your pregnancy.
Contact your eye doctor right away if your vision changes suddenly or becomes blurry, spotty or hazy.
More Information
- Screening for diabetic macular edema: How often?
- Spotting symptoms of diabetic macular edema
Over time, too much sugar in your blood can lead to the blockage of the tiny blood vessels that nourish the retina, cutting off its blood supply. As a result, the eye attempts to grow new blood vessels. But these new blood vessels don't develop properly and can leak easily.
There are two types of diabetic retinopathy:
Early diabetic retinopathy. In this more common form — called nonproliferative diabetic retinopathy (NPDR) — new blood vessels aren't growing (proliferating).
When you have nonproliferative diabetic retinopathy (NPDR), the walls of the blood vessels in your retina weaken. Tiny bulges protrude from the walls of the smaller vessels, sometimes leaking fluid and blood into the retina. Larger retinal vessels can begin to dilate and become irregular in diameter as well. NPDR can progress from mild to severe as more blood vessels become blocked.
Sometimes retinal blood vessel damage leads to a buildup of fluid (edema) in the center portion (macula) of the retina. If macular edema decreases vision, treatment is required to prevent permanent vision loss.
Advanced diabetic retinopathy. Diabetic retinopathy can progress to this more severe type, known as proliferative diabetic retinopathy. In this type, damaged blood vessels close off, causing the growth of new, abnormal blood vessels in the retina. These new blood vessels are fragile and can leak into the clear, jellylike substance that fills the center of your eye (vitreous).
Eventually, scar tissue from the growth of new blood vessels can cause the retina to detach from the back of your eye. If the new blood vessels interfere with the normal flow of fluid out of the eye, pressure can build in the eyeball. This buildup can damage the nerve that carries images from your eye to your brain (optic nerve), resulting in glaucoma.

In the early stages of diabetic retinopathy, the walls of the blood vessels in your retina weaken. Tiny bulges protrude from the vessel walls, sometimes leaking or oozing fluid and blood into the retina. Tissues in the retina may swell, producing white spots in the retina. As diabetic retinopathy progresses, new blood vessels may grow and threaten your vision.
- Reducing your risks of diabetic macular edema
Anyone who has diabetes can develop diabetic retinopathy. The risk of developing the eye condition can increase as a result of:
- Having diabetes for a long time
- Poor control of your blood sugar level
- High blood pressure
- High cholesterol
- Tobacco use
- Being Black, Hispanic or Native American
Diabetic retinopathy involves the growth of abnormal blood vessels in the retina. Complications can lead to serious vision problems:
Vitreous hemorrhage. The new blood vessels may bleed into the clear, jellylike substance that fills the center of your eye. If the amount of bleeding is small, you might see only a few dark spots (floaters). In more-severe cases, blood can fill the vitreous cavity and completely block your vision.
Vitreous hemorrhage by itself usually doesn't cause permanent vision loss. The blood often clears from the eye within a few weeks or months. Unless your retina is damaged, your vision will likely return to its previous clarity.
- Retinal detachment. The abnormal blood vessels associated with diabetic retinopathy stimulate the growth of scar tissue, which can pull the retina away from the back of the eye. This can cause spots floating in your vision, flashes of light or severe vision loss.
- Glaucoma. New blood vessels can grow in the front part of your eye (iris) and interfere with the normal flow of fluid out of the eye, causing pressure in the eye to build. This pressure can damage the nerve that carries images from your eye to your brain (optic nerve).
- Blindness. Diabetic retinopathy, macular edema, glaucoma or a combination of these conditions can lead to complete vision loss, especially if the conditions are poorly managed.
You can't always prevent diabetic retinopathy. However, regular eye exams, good control of your blood sugar and blood pressure, and early intervention for vision problems can help prevent severe vision loss.
If you have diabetes, reduce your risk of getting diabetic retinopathy by doing the following:
- Manage your diabetes. Make healthy eating and physical activity part of your daily routine. Try to get at least 150 minutes of moderate aerobic activity, such as walking, each week. Take oral diabetes medications or insulin as directed.
- Monitor your blood sugar level. You might need to check and record your blood sugar level several times a day — or more frequently if you're ill or under stress. Ask your doctor how often you need to test your blood sugar.
- Ask your doctor about a glycosylated hemoglobin test. The glycosylated hemoglobin test, or hemoglobin A1C test, reflects your average blood sugar level for the two- to three-month period before the test. For most people with diabetes, the A1C goal is to be under 7%.
- Keep your blood pressure and cholesterol under control. Eating healthy foods, exercising regularly and losing excess weight can help. Sometimes medication is needed, too.
- If you smoke or use other types of tobacco, ask your doctor to help you quit. Smoking increases your risk of various diabetes complications, including diabetic retinopathy.
- Pay attention to vision changes. Contact your eye doctor right away if your vision suddenly changes or becomes blurry, spotty or hazy.
Remember, diabetes doesn't necessarily lead to vision loss. Taking an active role in diabetes management can go a long way toward preventing complications.
- Does keeping a proper blood sugar level prevent diabetic macular edema and other eye problems?
Feb 21, 2023
- Diabetic retinopathy. National Eye Institute. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/diabetic-retinopathy. Accessed Feb. 19, 2021.
- AskMayoExpert. Diabetic retinopathy. Mayo Clinic, 2020.
- Fraser CE, et al. Diabetic retinopathy: Classification and clinical features. https://www.uptodate.com/contents/search. Accessed Feb. 22, 2021.
- Diabetic retinopathy. American Optometrics Association. https://www.aoa.org/healthy-eyes/eye-and-vision-conditions/diabetic-retinopathy?sso=y. Accessed Feb. 19, 2021.
- Fraser CE, et al. Diabetic retinopathy: Prevention and treatment. https://www.uptodate.com/contents/search. Accessed Feb. 22, 2021.
- The diabetes advisor: Eye exams for people with diabetes. American Diabetes Association. https://professional.diabetes.org/sites/professional.diabetes.org/files/media/Eyes_-_Eye_Tests_for_People_with_Diabetes.pdf. Accessed Feb. 25, 2021.
- Zhang HW, et al. Single herbal medicine for diabetic retinopathy (review). Cochrane Database of Systematic Reviews. 2018; doi:10.1002/14651858.CD007939.pub2.
- Nair AA, et al. Spotlight on faricimab in the treatment of wet age-related macular degeneration: Design, development and place in therapy. Drug Design, Development and Therapy. 2022; doi:10.2147/DDDT.S368963.
- Chodnicki KD (expert opinion). Mayo Clinic. Feb. 8, 2023.
- Diseases & Conditions
- Diabetic retinopathy symptoms & causes
News from Mayo Clinic

- Can medicine help prevent diabetic macular edema?
- Diabetic macular edema
- What is diabetic macular edema?
CON-XXXXXXXX
Your gift holds great power – donate today!
Make your tax-deductible gift and be part of the cutting-edge research and care that's changing medicine.

Image credit: Getty Images (Zorica Nastasic)
Cholesterol-lowering drug slows progression of eye disease in people with diabetes
The LENS trial has demonstrated that fenofibrate, a drug usually used to lower cholesterol, reduces the risk of progression of diabetic retinopathy by 27%. The results were announced today at the American Diabetes Association Scientific Sessions and published in NEJM Evidence .
Diabetes can cause damage to the small blood vessels at the back of the eye, a condition called diabetic retinopathy. Diabetic retinopathy is among the top five causes of visual loss worldwide and the only major cause to increase in recent decades.
Fenofibrate is a tablet that has been used to lower cholesterol for more than 30 years. Previous results from sub-studies of trials looking into treatments for heart disease had suggested that fenofibrate might be able to slow the progression of diabetic retinopathy but more conclusive results were needed.
Coordinated by Oxford Population Health, the LENS (Lowering Events in Non-proliferative retinopathy in Scotland) trial compared the effects of fenofibrate with a placebo (dummy tablet) on the progression of retinopathy in 1,151 adults with type 1 or type 2 diabetes in Scotland as part of the national routine diabetic eye screening programme. All of the participants had early to moderate diabetic retinopathy when they joined the trial.
Key findings:
• People who received fenofibrate had a 27% lower risk of needing to be referred for specialist care or treatment for diabetic retinopathy or maculopathy (a progressive eye disease that can lead to vision loss) over four years compared with people who were assigned to receive a placebo; • Treatment with fenofibrate was also associated with a lower risk of developing macular oedema (swelling at the back of the eye) and a lower risk of requiring treatment for retinopathy compared to placebo; • The benefits of fenofibrate were similar in people with both type 1 and type 2 diabetes, and in people with both normal and impaired kidney function.
Dr David Preiss , Associate Professor at Oxford Population Health and lead author of the study, said ‘Diabetic retinopathy remains a leading cause of visual loss. Good control of blood glucose is important but this is very difficult to achieve for many people, and there are few other treatments available. We need simple strategies that can be widely used to reduce the progression of diabetic eye disease. Fenofibrate may therefore provide a valuable addition to treat people with early to moderate diabetic retinopathy.’
Melville Henry, a LENS trial participant from Leven, said ‘Taking part in the trial was very easy; there was nothing to it really. I just had to follow the instructions and take the study tablets. I attended my local research clinic appointments at first and then I had regular telephone calls to ask about my progress.’
Linda Gillespie, a LENS trial participant from Kirkcaldy, said ‘I attended the clinic for diabetic eye screening anyway so taking part in the trial was extremely easy, I never had to think about it. If I had any questions, someone was always at the end of the phone. It was really important to me to take part in research because without trials like LENS we can’t move forward. The results of the trial might not help me but it might help someone else in the future.’
Dr Lucy Chambers, Head of Research Communications at Diabetes UK, said ‘Eye problems are a frightening and too frequent complication of diabetes. But acting early can stop the first signs of damage progressing into devastating sight loss. We’re excited by the positive results from this major trial of a new treatment to slow progression of eye damage, which has the potential to benefit many people with diabetes in the UK.’
LENS is being coordinated by Oxford Population Health, and was run in close partnership with the Universities of Glasgow, Aberdeen, Dundee and Edinburgh, and with NHS Scotland’s Diabetic Eye Screening Service. It was funded primarily by the National Institute for Health and Care Research’s Health Technology Assessment Programme.
The paper ' Effect of Fenofibrate on Progression of Diabetic Retinopathy ' is published in NEJM Evidence .
DISCOVER MORE
- Support Oxford's research
- Partner with Oxford on research
- Study at Oxford
- Research jobs at Oxford
You can view all news or browse by category
Diabetic Eye Disease Resources

Diabetic eye disease at a glance
- Diabetic eye diseases are a set of eye problems that can affect people with diabetes
- There are several kinds of diabetic eye diseases, and all of them can cause vision loss and blindness
- People with diabetes can protect their vision by getting eye exams at least once a year
- Controlling diabetes can help lower the risk of vision loss
More than 30 million people living in the United States have diabetes. Having diabetes increases the risk for vision loss and blindness from diabetic eye diseases. The most common diabetic eye disease is diabetic retinopathy, but people with diabetes are also at higher risk for diabetic macular edema (DME), cataract, and glaucoma.
The longer a person has diabetes, the greater their risk of developing diabetic eye disease. But the good news is that early detection and treatment can lower the risk of blindness by 95%. And managing diabetes — with diet, exercise, and medication — is the best way for people with diabetes to lower the risk of vision loss.
We need your help spreading the word about diabetic eye disease. Everyone with diabetes needs to know about the steps they can take to protect their vision.
Learn more about diabetic eye diseases:
Diabetic retinopathy
Cataract
Glaucoma
Browse NEHEP resources
Our educational resources will help you share information about steps people with diabetes can take to lower their risk of vision loss.
Hand out articles and fact sheets
Our articles and handouts make it easy to spread the word about diabetic eye disease. You can share them online or print and hand them out in your community.
Share infocards and infographics
Use these materials to share information about diabetic eye disease with older adults, community leaders, and partner organizations. Print them out to hang in common areas or post them on your website and social media.
Watch videos and webinars
Use our videos and webinars to help people learn about diabetic eye disease and vision loss.
Be part of Diabetic Eye Disease Month in November
Join the conversation in November — and share information about diabetic eye disease with your community all year long.
Spread the word on social media
Share our social media posts, videos, and infographics to help start a conversation in your community about diabetic eye disease.
Work with the media
Partnering with local newspapers, radio stations, and TV networks is a powerful way to reach people in your community — and we have tips and ready-to-use articles that make it easy.

Lead an information session
Use the educational module and materials in our Diabetes and Healthy Eyes toolkit in your information sessions.
Earn continuing education credits
NEI and experts in eye health and diabetes have developed a professional education initiative to help preserve the vision health of the millions of patients at risk of diabetic eye disease. These short, online activities fit into your busy schedule and have been accredited so that you can earn credits.
Last updated: February 13, 2023
- Introduction
- Conclusions
- Article Information
A, Diabetic macular edema (DME)/vision-threatening diabetic retinopathy (VTDR) was defined as diabetic macular edema, severe nonproliferative diabetic retinopathy (with or without diabetic macular edema), or proliferative diabetic retinopathy (with or without diabetic macular edema). B, Any DME was presented as a trend line (separate from DME/VTDR in A) and was characterized as any diagnosis of DME, by itself or with any stage of diabetic retinopathy. C, Non–vision-threatening diabetes-related eye disease was characterized as background diabetic retinopathy, nonproliferative diabetic retinopathy (not otherwise specified), unspecified diabetic retinopathy without macular edema, mild nonproliferative diabetic retinopathy (without DME), moderate nonproliferative diabetic retinopathy (without DME), diabetes with ophthalmic manifestations, or other diabetic ophthalmic complication.
Vision-threatening diabetic retinopathy (VTDR) was defined as severe nonproliferative diabetic retinopathy or proliferative diabetic retinopathy. DME indicates diabetic macular edema; VEGF, vascular endothelial growth factor.
DME/VTDR was defined as DME, severe nonproliferative diabetic retinopathy (with or without DME), or proliferative diabetic retinopathy (with or without DME). Presented separately is the prevalence of any DME, characterized as any diagnosis of DME by itself or with any stage of diabetic retinopathy.
DME/VTDR was defined as DME, severe nonproliferative diabetic retinopathy (with or without DME), or proliferative diabetic retinopathy (with or without DME). VEGF indicates vascular endothelial growth factor.
eTable 1. ICD-9 and ICD-10 diagnosis codes used to define diabetic macular edema or vision-threatening diabetic retinopathy (DME/VTDR)
eTable 2. ICD-9 and ICD-10 diagnosis codes used to define any diabetic macular edema (any DME)
eTable 3. ICD-9 and ICD-10 diagnosis codes used to define non-vision-threatening diabetes-related eye disease
eTable 4. List of procedure codes (Current Procedural Terminology and Healthcare Common Procedure Coding System) used to define 4 treatment types for diabetes-related eye disease
eTable 5. ICD-9 and ICD-10 diagnosis codes used to define vision-threatening diabetic retinopathy without diabetic macular edema (VTDR without DME)
eTable 6. ICD-9 and ICD-10 diagnosis codes used to define vision-threatening diabetic retinopathy with diabetic macular edema (VTDR with DME)
eTable 7. ICD-9 and ICD-10 diagnosis codes used to define any diabetic macular edema (any DME)
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Lundeen EA , Andes LJ , Rein DB, et al. Trends in Prevalence and Treatment of Diabetic Macular Edema and Vision-Threatening Diabetic Retinopathy Among Medicare Part B Fee-for-Service Beneficiaries. JAMA Ophthalmol. 2022;140(4):345–353. doi:10.1001/jamaophthalmol.2022.0052
Manage citations:
© 2024
- Permissions
Trends in Prevalence and Treatment of Diabetic Macular Edema and Vision-Threatening Diabetic Retinopathy Among Medicare Part B Fee-for-Service Beneficiaries
- 1 Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia
- 2 NORC at the University of Chicago, Chicago, Illinois
- 3 Economic & Valuation Services, KPMG LLP, McLean, Virginia
- 4 Division of Epidemiology and Clinical Applications, National Eye Institute, National Institutes of Health, Bethesda, Maryland
Question What are 10-year trends in annual prevalence of diagnosis and treatment of diabetic macular edema (DME) or vision-threatening diabetic retinopathy (VTDR) among patients 65 years and older with diabetes who are Medicare Part B fee-for-service beneficiaries?
Findings In this cross-sectional study, from 2009 to 2018, there was a 1.5-fold increase in annual prevalence of diagnosis (2.8% to 4.3%). Annual prevalence of anti–vascular endothelial growth factor injections doubled among those with DME (15.7% to 35.2%) and VTDR with DME (20.2% to 47.6%), while prevalence of laser photocoagulation and vitrectomy in 2018 was less than half that in 2009.
Meaning These findings show that treatments for DME or VTDR have changed dramatically among Medicare beneficiaries.
Importance While diabetes prevalence among US adults has increased in recent decades, few studies document trends in diabetes-related eye disease.
Objective To examine 10-year trends (2009-2018) in annual prevalence of Medicare beneficiaries with diabetes with a diagnosis of diabetic macular edema (DME) or vision-threatening diabetic retinopathy (VTDR) and trends in treatment.
Design, Setting, and Participants In this cross-sectional study using Centers for Medicare & Medicaid Services research identifiable files, data for patients 65 years and older were analyzed from claims. Beneficiaries were continuously enrolled in Medicare Part B fee-for-service (FFS) insurance for the calendar year and had a diagnosis of diabetes on 1 or more inpatient claims or 2 or more outpatient claims during the calendar year or a 1-year look-back period.
Main Outcomes and Measures Using diagnosis and procedure codes, annual prevalence was determined for beneficiaries with 1 or more claims for (1) any DME, (2) either DME or VTDR, and (3) anti–vascular endothelial growth factor (VEGF) injections, laser photocoagulation, or vitrectomy, stratified by any DME, VTDR with DME, and VTDR without DME. Racial and ethnic disparities in diagnosis and treatment are presented for 2018.
Results In 2018, 6 960 823 beneficiaries (27.4%) had diabetes; half were aged 65 to 74 years (49.7%), half (52.7%) were women, and 75.7% were non-Hispanic White. From 2009 to 2018, there was an increase in the annual prevalence of beneficiaries with diabetes who had 1 or more claims for any DME (1.0% to 3.3%) and DME/VTDR (2.8% to 4.3%). Annual prevalence of anti-VEGF increased, particularly among patients with any DME (15.7% to 35.2%) or VTDR with DME (20.2% to 47.6%). Annual prevalence of laser photocoagulation decreased among those with any DME (45.5% to 12.5%), VTDR with DME (54.0% to 20.3%), and VTDR without DME (22.5% to 5.8%). Among all 3 groups, prevalence of vitrectomy in 2018 was less than half that in 2009. Prevalence of any DME and DME/VTDR was highest among Hispanic beneficiaries (5.0% and 7.0%, respectively) and Black beneficiaries (4.5% and 6.2%, respectively) and lowest among non-Hispanic White beneficiaries (3.0% and 3.8%, respectively). Among those with DME/VTDR, anti-VEGF was most prevalent among non-Hispanic White beneficiaries (30.3%).
Conclusions and Relevance From 2009 to 2018, prevalence of DME or VTDR increased among Medicare Part B FFS beneficiaries alongside an increase in anti-VEGF treatment and a decline in laser photocoagulation and vitrectomy.
Diabetic retinopathy (DR) is a common microvascular complication of diabetes that results from high levels of blood glucose damaging retinal blood vessels. Diabetic retinopathy is the leading cause of incident blindness in US adults aged 20 to 74 years. 1 Vision-threatening DR (VTDR) includes severe nonproliferative DR (presence of intraretinal hemorrhages, microaneurysms, venous beading, or intraretinal microvascular abnormalities) and proliferative DR (presence of neovascularization or vitreous hemorrhage). Diabetic macular edema (DME), swelling in the macula caused by fluid leaking from retinal blood vessels, can occur with any stage of DR. 2 - 4 Diabetic retinopathy, VTDR, and DME affect 28.5%, 4.4%, and 3.8%, respectively, of US adults 40 years and older with diabetes. 2 , 5
Risk of developing VTDR is influenced by diabetes duration and glycemic control. 5 - 9 Interventions to increase glycemic control can prevent vision loss. 8 Individuals with diabetes are recommended to receive annual dilated eye examinations for early detection and timely treatment of DR. 10 - 13 The preferred treatment for proliferative DR, panretinal laser photocoagulation (ie, scatter laser surgery), 7 , 14 , 15 reduces the risk of moderate and severe vision loss by 50% in individuals with severe nonproliferative DR or proliferative DR. 8 The standard of care for non–center-involved DME, focal laser photocoagulation surgery, 15 reduces the risk of moderate vision loss by 50% to 70% in patients with macular edema. 8 In the early 2000s, physicians began using intravitreal injections of anti–vascular endothelial growth factor (VEGF) agents (ie, aflibercept, bevacizumab, and ranibizumab) for the treatment of center-involved DME. 15 In the last decade, anti-VEGF injections became the first-line treatment for DME because of their efficacy and ease of administration 16 and are included in the American Academy of Ophthalmology DR Preferred Practice Pattern. 15 A meta-analysis of 24 randomized clinical trials of anti-VEGF therapy in patients with DME and moderate vision loss found that aflibercept, bevacizumab, and ranibizumab were all more effective than laser photocoagulation for improving vision after 1 year. 16 For proliferative DR, 2 studies demonstrated that anti-VEGF injections can be alternatives to panretinal laser photocoagulation. 17 , 18 Other less commonly used treatments for DR include vitrectomy and retinal detachment repair.
Given these changes in DR treatment and the increase in diabetes prevalence among US adults (from 9.8% in 1999-2000 to 14.3% in 2017-2018), 19 , 20 a better understanding of trends in DR complications and treatment could help guide practice recommendations and guidelines. Previous observational studies of DR prevalence are limited by older data. The National Health and Nutrition Examination Survey (NHANES) last assessed DR in the period 2005 to 2008. 2 Medicare administrative claims provide an opportunity to evaluate DR prevalence and treatment among beneficiaries 65 years and older, the population at highest risk of DR. 21 Existing studies of DR in Medicare are older and do not capture recent changes in treatment options, such as anti-VEGF injections. 22 - 24 In addition, the prevalence of diabetes among Medicare fee-for-service (FFS) beneficiaries 68 years and older increased from 23.3% in 2001 to 32.2% in 2012, 25 highlighting the need to examine trends in diabetes complications in this population. In this article, we use new data from the Medicare 100% FFS file to examine 10-year trends in the annual prevalence of Medicare Part B beneficiaries 65 years and older with diabetes who have payment claims for DME or VTDR (hereafter DME/VTDR), the annual prevalence of treatment, and racial and ethnic disparities in the prevalence and treatment of DME/VTDR.
We analyzed 100% of the Centers for Medicare & Medicaid Services research identifiable files from 2009 to 2018 for beneficiaries 65 years and older who were enrolled in Medicare Part B FFS insurance. The analysis excluded beneficiaries enrolled in Medicare Advantage plans, which are not included in FFS claims. We restricted the sample to beneficiaries in each year with continuous Part B FFS enrollment for all 12 months. This study used a repeated cross-sections design, rather than following beneficiaries longitudinally, to present the annual prevalence of Medicare beneficiaries with 1 or more claims for DME/VTDR and the annual prevalence of treatment from 2009 to 2018. This research was considered exempt from institutional review board review under 45 Code of Federal Regulations 46.101[b][5], which covers Department of Health and Human Services research and demonstration projects that are designed to study, evaluate, or examine public benefit or service programs. Findings of this study are reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guidelines.
Using the Chronic Conditions Data Warehouse diabetes flag, beneficiaries were coded with a diabetes diagnosis if they had 1 or more inpatient codes or 2 or more different-day outpatient diagnosis codes (from the International Classification of Diseases, Ninth Revision, or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, hereafter ICD ) on claims during the calendar year or a 1-year look-back period. 26 The primary outcome was the annual national crude prevalence of Medicare Part B FFS beneficiaries with diabetes and 1 or more claims for DME/VTDR, defined as having an ICD code on a claim indicating a diagnosis of DME or VTDR. Prevalence was calculated as the number of continuously enrolled beneficiaries with diabetes who had 1 or more claims for DME/VTDR in the calendar year divided by the number of continuously enrolled beneficiaries with diabetes in that year. The annual crude national prevalence of DME/VTDR was calculated across the 10 years along with the corresponding prevalence count of beneficiaries.
Because of the emergence of new, effective therapies for DME, we separately present the annual prevalence of beneficiaries with 1 or more claims for DME, which included claims indicating DME alone or combined with any stage of DR (hereafter any DME). Lastly, we calculated the annual prevalence of beneficiaries with claims for non–vision-threatening diabetes-related eye disease, characterized as background DR, nonproliferative DR (not otherwise specified), unspecified DR without macular edema, mild nonproliferative DR (without DME), moderate nonproliferative DR (without DME), diabetes with ophthalmic manifestations, and other diabetic ophthalmic complications. eTables 1, 2, and 3 in the Supplement contain ICD diagnosis codes used to define these three groups: DME/VTDR, any DME, and non–vision-threatening diabetes-related eye disease.
A secondary outcome was treatment of vision-threatening diabetes-related eye disease. eTable 4 in the Supplement contains procedure codes ( Current Procedural Terminology and Healthcare Common Procedure Coding System) used to define 4 treatment types: anti-VEGF injections, laser photocoagulation, vitrectomy, and retinal detachment repair. Across the 10 years, we present the crude annual national prevalence of beneficiaries with diabetes and 1 or more claims for treatment, stratified by treatment type and by the form of vision-threatening disease: (1) any DME, (2) VTDR with DME (hereafter VTDR with DME), and (3) VTDR without DME (hereafter VTDR without DME). eTables 5, 6, and 7 in the Supplement contain the diagnosis codes used to define these groups. For each treatment, we calculated prevalence as the number of continuously enrolled beneficiaries with diabetes and vision-threatening disease who had 1 or more claims for treatment in the calendar year divided by the number of continuously enrolled beneficiaries with diabetes and vision-threatening disease in that year.
An additional analytic aim was to examine differences in the prevalence and treatment of DME/VTDR by race and ethnicity (American Indian/Alaska Native, Asian/Pacific Islander, Black, Hispanic, non-Hispanic White, and other races and ethnicities, a category that included any race or ethnicity named that did not fit in the preceding list). The Medicare enrollment database contains race and ethnicity information for beneficiaries from the Social Security Administration’s master beneficiary record, and Research Triangle Institute has developed an algorithm to improve the accuracy of this information. 27 We present the 2018 crude national prevalence of DME/VTDR, any DME, and the 4 treatment types by each race and ethnicity group. For simplicity, we present the prevalence of the 4 treatments by race and ethnicity among those with a diagnosis of DME/VTDR. We did not provide CIs or perform statistical testing because these data represent 100% of Medicare beneficiaries who met the inclusion criteria. All analyses were conducted using SAS Enterprise Guide (version 9.4; SAS Institute).
Among the 25 396 615 continuously enrolled Medicare Part B FFS beneficiaries 65 years and older in 2018, a total of 6 960 823 (27.4%) had a diabetes diagnosis ( Table ). Approximately half (49.7%) of these beneficiaries were aged 65 to 74 years and half (52.7%) were women. The majority of beneficiaries with diabetes were non-Hispanic White (75.7%) and qualified originally for Medicare by reaching age 65 years (84.5%). Characteristics of the population under study remained stable from 2009 to 2018.
The annual crude national prevalence of Medicare beneficiaries with diabetes who had 1 or more claims for DME/VTDR increased from 2.8% in 2009 to 4.3% in 2018 ( Figure 1 ). The number of beneficiaries with a claim for DME/VTDR increased from 184 520 to 301 550. The separate trendline for any DME shows an increase in prevalence from 1.0% of beneficiaries with diabetes in 2009 to 3.3% in 2018; the number of beneficiaries with a claim for any DME increased from 66 634 to 232 508. Conversely, the prevalence of beneficiaries with claims for non–vision-threatening diabetes-related eye disease decreased from 11.3% in 2009 to 7.3% in 2018.
From 2009 to 2018, the annual prevalence of anti-VEGF injections more than doubled among those with any DME (15.7% to 35.2%) and those with VTDR with DME (20.2% to 47.6%) ( Figure 2 ). The increase was smaller among those with VTDR without DME (5.2% to 7.4%). In contrast, the prevalence of laser photocoagulation decreased significantly among those with any DME (45.5% to 12.5%), VTDR with DME (54.0% to 20.3%), and VTDR without DME (22.5% to 5.8%). In each year from 2009 to 2018, more than half of the beneficiaries with VTDR with DME received either anti-VEGF injections or laser photocoagulation. Although vitrectomy and retinal detachment repair were uncommon, among all 3 groups, there was a decline in the prevalence of beneficiaries with claims for these procedures.
Differences by race and ethnicity were found. The 2018 prevalence of both DME/VTDR and any DME was highest among beneficiaries who were American Indian/Alaska Native (6.0% and 3.8%, respectively), Black (6.2% and 4.5%, respectively), and Hispanic (7.0% and 5.0%, respectively) ( Figure 3 ). Non-Hispanic White beneficiaries had the lowest prevalence of both DME/VTDR (3.8%) and any DME (3.0%). For treatment, the prevalence of beneficiaries with claims for anti-VEGF injections was highest among Hispanic (28.3%) and non-Hispanic White (30.3%) beneficiaries and lowest among beneficiaries who were American Indian/Alaska Native (23.4%), Black (24.3%), or other races and ethnicities (24.6%) ( Figure 4 ). The prevalence of laser photocoagulation was highest among Asian/Pacific Islander (12.2%), Black (12.5%), and Hispanic (15.2%) beneficiaries and lowest among American Indian/Alaska Native (9.3%) and non-Hispanic White (9.7%) beneficiaries. Retinal detachment repair and vitrectomy were infrequent (<4% prevalence) among all racial and ethnic groups.
From 2009 to 2018, we found a 54% increase in the annual prevalence of beneficiaries with claims for DME/VTDR (from 2.8% to 4.3%) and a 230% increase in the annual prevalence of beneficiaries with claims for any DME (from 1.0% to 3.3%) among Medicare Part B FFS beneficiaries 65 years and older with diabetes. During these years, Medicare added 117 000 beneficiaries with an annual claim for DME/VTDR. We observed a steep increase in prevalence between 2014 and 2016, following the 2012 Food and Drug Administration approval of ranibizumab for DME and coinciding with the 2014 approval of aflibercept for DME and the 2015 approval of ranibizumab and aflibercept for DR in patients with DME. This period also coincided with the 2015 transition from ICD-9 to ICD-10 coding, and we cannot discount the possibility that changes in ICD coding contributed to the observed trends. During the 10-year study period, there was a 35% decrease in the annual prevalence of beneficiaries with claims for non–vision-threatening diabetes-related eye disease. Although reasons are unknown for the observed increase in vision-threatening disease and decrease in non–vision-threatening disease, these trends could reflect worsening disease due to changes in glycemic control among the Medicare population with diabetes. A recent study found that glycemic control (hemoglobin A 1c <7%) among US adults 20 years and older with diabetes declined from 57.4% (95% CI, 52.9%-61.8%) in 2007 to 2010 to 50.5% (95% CI, 45.8%-55.3%) in 2015 to 2018, 28 potentially contributing to observed trends. We also cannot discount the possibility that these disease trends reflect changing patterns in screening and diagnosis of diabetes-related eye diseases or in medical coding for treatment.
We observed changes in treatment from 2009 to 2018. Among those with any DME or VTDR with DME, the annual prevalence of beneficiaries who had claims for anti-VEGF injections more than doubled. By 2018, nearly half of all beneficiaries who had VTDR with DME received anti-VEGF injections. This increase in anti-VEGF treatment corresponded to a sharp decline in the use of laser photocoagulation, as clinicians replaced laser photocoagulation in response to studies demonstrating superior efficacy of anti-VEGF injections for DME treatment. 16 While the present study did not capture visual acuity outcomes, studies have shown that improvements in visual acuity among patients with DME are superior with anti-VEGF treatment compared with laser photocoagulation, 16 and thus, the findings of the present study could translate to substantial improvements in visual acuity in the Medicare population. However, over time, this could result in increased racial and ethnic disparities in visual acuity, given the finding that receipt of anti-VEGF injections was lowest among beneficiaries who were American Indian/Alaska Native, Black, and other races and ethnicities. Procedures for retinal detachment repair and vitrectomy also declined during this period. Reasons for this finding are unknown; however, this trend could have been due to more aggressive treatment of diabetes-related eye diseases during this time period, leading to fewer severe outcomes.
There are few comparable studies examining the prevalence and treatment of DME/VTDR in the Medicare population. One study using Medicare Part B FFS data followed a sample (n = 20 325) of beneficiaries 65 years and older from 1991 to 1999 and found similar increases in the prevalence of vision-threatening forms of DR. 24 The prevalence of proliferative DR increased from 2.1% to 3.8%, and the prevalence of DME increased from 0.4% to 2.1%. 24 Another study using a nationally representative 5% sample of Medicare beneficiaries found that among patients with DME, use of laser photocoagulation decreased from 43% of patients in 2000 to 30% of patients in 2004, whereas use of intravitreal injection increased from 1% to 13% of patients. 29 Another study evaluated administrative claims from commercial health insurance and government insurance (Medicaid, Medicare, and Medicare Advantage) for patients with DME and found that the prevalence of receiving anti-VEGF treatments increased from 5.0% of patients in 2009 to 27.1% in 2014. 30 The study also found that anti-VEGF treatments, as a proportion of all DME treatments, increased from 11.6% in 2009 to 61.9% in 2014, whereas use of corticosteroids and focal laser procedures decreased from 6.1% to 2.8% and 75.3% to 24.0%, respectively. 30 Patients covered by Medicare Advantage, Medicaid, and Medicare received 31%, 24%, and 11% fewer anti-VEGF injections, respectively, compared with those with commercial health insurance. 30 Other studies have similarly documented exponential growth in the use of anti-VEGF treatment in the last 2 decades, although they are not directly comparable with our study because they included adults 18 years and older using combined data from commercial health insurance and Medicare Advantage 31 or used Medicare Part B data but did not distinguish between anti-VEGF used for age-related macular degeneration treatment vs diabetes-related eye diseases. 32 , 33
Our findings on racial and ethnic differences in DME/VTDR prevalence are consistent with those from investigations using data from population-based studies, 3 US nationally representative surveys, 2 , 5 and Medicare claims. 34 A previous study using the 2011 Medicare 5% claims files found that the prevalence of DR was higher in Asian (12.2%), Black (14.0%), Hispanic (17.3%), and Native American (16.6%) beneficiaries compared with White (10.4%) beneficiaries. 34 NHANES data for 2005 to 2008 also demonstrate a higher prevalence of vision-threatening DR among non-Hispanic Black individuals (9.3%) compared with non-Hispanic White individuals (3.2%) 2 ; a similar disparity was documented for DME. 5 These differences could be due to earlier age at diabetes diagnosis and longer disease duration among Black and Hispanic persons, 35 poorer glycemic control among Black and Hispanic persons with diabetes compared with White individuals, 36 - 38 or disparities in the quality of diabetes care experienced by Black and Hispanic patients. 39
Clinical reasons for observed racial and ethnic treatment differences are not known. The prevalence of diabetes among Medicare FFS beneficiaries 68 years and older is higher in Asian/Pacific Islander (43.5%), Black (47.4%), and Hispanic (46.3%) individuals compared with White (29.2%) individuals. 25 Previous research on management of diabetes among Medicare managed care beneficiaries found disparities in diabetes care, with Black patients less likely to receive hemoglobin A 1c screening, eye examinations, and cholesterol screening. 40 In addition, physicians’ experiences with the efficacy of different treatment modalities may influence treatment decisions. One recent study examined racial and ethnic differences in the efficacy of anti-VEGF treatment with bevacizumab and found that the percentage of patients with DME who had visual acuity improvement after 3 injections was lower for Black patients (34%) compared with Hispanic (55%) and White (59%) patients. 41 However, although racial and ethnic groups compared in this study were similar with respect to baseline hemoglobin A 1c , researchers were unable to account for differences in duration of diabetes and DME. Lastly, geographic differences in use of different treatment modalities for diabetes-related eye disease may contribute to racial and ethnic differences in treatment. Studies have documented geographic differences by US Census division in use of anti-VEGF treatment for Medicare beneficiaries with DME, 32 , 42 with the frequency of bevacizumab use highest in the Mountain division and the frequency of ranibizumab use highest in the Mid-Atlantic division. 42
In the absence of treatment, an individual who develops proliferative DR has a 50% chance of becoming blind (visual acuity 6/60 [meters] or 20/200 [feet] or less in the better-seeing eye) within 5 years. 43 - 45 Prevention of vision loss among patients with diabetes requires better management of diabetes as well as early detection and timely, effective treatment of DR and DME. Although Medicare Part B insurance covers annual eye examinations for beneficiaries with diabetes, 46 studies have shown that only about half of Medicare beneficiaries with diabetes have an annual eye examination. 47 - 49 Among Medicare beneficiaries with diabetes, the prevalence of annual eye examinations is lower among Black (48.9%) and Hispanic (48.2%) beneficiaries compared with non-Hispanic White (55.6%) beneficiaries. 47 This lower rate of screening among Black and Hispanic beneficiaries could indicate that the racial and ethnic disparities in DME/VTDR prevalence observed in the present study would be even higher if screening rates were equivalent among racial and ethnic groups.
This analysis is subject to several limitations. First, the analysis excludes the 29.5% of Medicare beneficiaries (on average in 2009-2018) enrolled in Medicare managed care plans, 50 which could have influenced the prevalence of DME/VTDR diagnoses and treatment. One study in Los Angeles County compared Medicare managed care patients with diabetes with Medicare FFS patients with diabetes and found that those insured by managed care were more likely to have DR and more likely to require treatment. 51 Thus, results of the present analysis may not be generalizable to all adults insured by Medicare, and the exclusion of beneficiaries enrolled Medicare Advantage plans may have resulted in lower rates of DME/VTDR diagnoses and treatment. Second, Medicare Part B FFS beneficiaries might have multiple insurers, and services reimbursed by a supplemental plan would not be recorded in Medicare claims, thereby underestimating the prevalence of both diagnoses and treatment. Third, the data presented are likely not representative of the American Indian/Alaska Native population because Medicare data do not include care provided by the Indian Health Service. Fourth, not requiring 2 years of continuous enrollment in Medicare Part B FFS for inclusion in the study may have resulted in some misclassification or underestimation of beneficiaries with diabetes. Fifth, in this analysis, we are unable to quantify the effect of switching from ICD-9 to ICD-10 coding in October 2015 on the prevalence of diagnoses.
We found that from 2009 to 2018, there was an increase in the annual prevalence of Medicare Part B FFS beneficiaries with diabetes who had claims for DME/VTDR, and by 2018, 1 in 25 beneficiaries with diabetes (more than 300 000 people) had vision-threatening disease. Treatment changed over the period, with anti-VEGF injections surpassing laser photocoagulation as the most commonly used treatment for DME/VTDR. We also documented racial and ethnic disparities in prevalence and treatment of vision-threatening diabetes-related eye disease in the Medicare population. While the prevalence of DME/VTDR is highest among American Indian/Alaska Native, Black, and Hispanic beneficiaries, non-Hispanic White beneficiaries have the highest annual prevalence of receiving anti-VEGF injections, the contemporary first-line treatment. Future studies could examine trends in these disparities over the last decade.
From 2005 to 2050, it is projected that there will be a nearly 3-fold increase in the United States in the number of people with DR (from 5.5 million to 16.0 million) and vision-threatening DR (from 1.2 million to 3.4 million). 52 Better understanding optimal treatment regimens and associated cost-effectiveness is vital, as DR was estimated to cost Medicare FFS $753 million in 2018. 53 While anti-VEGF has rapidly become the standard of care for DME and is cost-effective, 54 questions regarding the optimal duration of treatment and frequency of injections require further study. 7 In addition, future cost-benefit studies could estimate potential savings for Medicare resulting from increased adoption of anti-VEGF treatment and associated effects on disease progression and visual acuity. It is important for future research to examine barriers to eye care and treatment of DME/VTDR, particularly among racial and ethnic minority populations insured by Medicare.
Accepted for Publication: December 30, 2021.
Published Online: March 3, 2022. doi:10.1001/jamaophthalmol.2022.0052
Corresponding Author: Elizabeth A. Lundeen, PhD, Centers for Disease Control and Prevention, 4770 Buford Hwy NE, Mail Stop S107-3, Atlanta, GA 30341 ( [email protected] ).
Author Contributions : Drs Erdem and Gu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Lundeen, Saaddine, Chew.
Acquisition, analysis, or interpretation of data: Lundeen, Andes, Rein, Wittenborn, Erdem, Gu, Imperatore, Chew.
Drafting of the manuscript: Lundeen, Erdem.
Critical revision of the manuscript for important intellectual content: Lundeen, Andes, Rein, Wittenborn, Gu, Saaddine, Imperatore, Chew.
Statistical analysis: Lundeen, Andes, Rein, Erdem, Gu.
Obtained funding: Lundeen.
Administrative, technical, or material support: Lundeen, Wittenborn, Gu.
Supervision: Lundeen, Erdem, Saaddine, Imperatore.
Conflict of Interest Disclosures: Dr Rein reported grants from US Centers for Disease Control and Prevention (CDC) Vision Health Initiative during the conduct of the study. Mr Wittenborn reported grants from the CDC Vision Health Initiative during the conduct of the study. Dr Erdem reported grants from Health and Human Services and funding from NORC as a subcontract from a CDC cooperative agreement during the conduct of the study. Dr Gu reported funding from NORC as a subcontract from a US Centers for Disease Control and Prevention cooperative agreement during the conduct of the study. No other disclosures were reported.
Funding/Support: This study was led by epidemiologists from the US Centers for Disease Control and Prevention (CDC). Data analysis for this study was supported by funding from the CDC Vision Health Initiative (cooperative agreement U01DP006444, “Research to Enhance the US Vision and Eye Health Surveillance System”).
Role of the Funder/Sponsor: As coauthors, employees of the funding organization (Drs Lundeen, Andes, Saaddine, and Imperatore) participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The manuscript was reviewed and approved by the CDC scientific clearance process.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

- Research Areas, Centers & Programs
- Laboratories
- Research Cores
- Clinical Trials
- Office of Research Administration
- Technology & Innovations
- Clinical & Translational Research Center
- News & Breakthroughs
- Graduate Medical Education
- Graduate School of Biomedical Sciences
- Continuing Medical Education
- Professional Training Programs
- Women's Guild Simulation Center
- Center for the Arts and Humanities in Medicine
- Medical Library
- Campus Life
- Office of the Dean
- Academic Calendar
Ljubimov Lab
Donor human cornea for stem cell studies.
The Ljubimov Laboratory, under the direction of Alexander Ljubimov, PhD, director of the Eye Program at the Cedars-Sinai Regenerative Medicine Institute, is focused on diabetic eye disease. The goal of the Ljubimov Lab is to unravel human stem cell alterations in diabetic eye disease and their impact on disease development, in order to restore stem cell functions for future clinical translation. In the cornea, the aim is to normalize altered epithelial stem cells by gene therapy in organ cultures.
The Ljubimov Lab has also developed a new nano gene therapy for diabetic corneal disease. Recently, the lab's focus is on the development of a new induced pluripotent stem cell-driven source of corneal epithelial stem cells for limbal stem cell deficiency. Researchers in the Ljubimov Lab are investigating corneal markers that are epigenetically suppressed in diabetes.
The Ljubimov Laboratory is affiliated with the Regenerative Medicine Institute and Department of Biomedical Sciences .

Personal Statement
I am the director of the Board of Governors Regenerative Medicine Institute Eye Program, professor of biomedical sciences and neurosurgery and professor of medicine at the David Geffen School of Medicine at UCLA. My background is in cancer research, and I have studied ocular diabetes for the past 25 years."
Breakthrough Research Areas
Delayed wound healing, erosions, and keratitis — the most serious complications of diabetes in the cornea — are treated only symptomatically. The Ljubimov Laboratory has described a number of markers altered in diabetic corneas. Using adenoviral gene therapy, the lab has been able to correct aberrant wound healing and several marker protein expression abnormalities in human organ-cultured diabetic corneas.
Collaborations & Resources
Collaborations.
- Induced Pluripotent Stem Cell (iPSC) Core
- Nanomedicine Research Center
- Neurology & Neurosurgery
- Regenerative Medicine Institute Eye Program
- Svendsen Laboratory
- S. Wang Laboratory
Reagents and Resources
- The healthy and diabetic human eye tissues for Ljubimov Laboratory experiments are purchased from the National Disease Research Interchange (Philadelphia, Pennsylvania).
- Ezra Maguen, MD, ( American Eye Institute , Los Angeles) and Yaron Rabinowitz, MD, ( Cedars-Sinai ) are kindly supplying discard human corneoscleral rings for stem cell cultures under the approved Cedars-Sinai IRB protocols.
Meet Our Team
Learn more about the scientists, faculty members, investigators and other healthcare professionals of the Ljubimov Laboratory, whose dedicated efforts lead to groundbreaking discoveries.
Publications
Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells.
Leszczynska A, Kulkarni M, Ljubimov AV, Saghizadeh M.
Sci Rep. 2018 Oct 11;8(1):15173.
Concise review: stem cells for corneal wound healing.
Saghizadeh M, Kramerov AA, Svendsen CN, Ljubimov AV.
Stem Cells. 2017 Oct;35(10):2105-2114.
Covalent nano delivery systems for selective imaging and treatment of brain tumors.
Ljubimova JY, Sun T, Mashouf L, Ljubimov AV, Israel LL, Ljubimov VA, Falahatian V, Holler E.
Adv Drug Deliv Rev. 2017 Apr;113:177-200.
Contact the Ljubimov Lab
127 S. San Vicente Blvd. Advanced Health Sciences Pavilion, Suite A8106 Los Angeles, CA 90048
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Diabetic Eye Diseases: Overview and More
Diabetic eye diseases are eye problems that can affect you if you have diabetes . Some of these diseases, like diabetic retinopathy and diabetic macular edema , occur exclusively in those who have diabetes. Other eye problems like glaucoma and cataracts can occur in anyone, but your chances of developing them are higher when you have diabetes.
Diabetic eye diseases are increasing due to the growing number of people with diabetes. There are 34.2 million people with diabetes in the United States, or 10.5% of the total population. About 40% of those with diabetes develop diabetic retinopathy.
Here is more information about eye diseases associated with having diabetes.
Karl Tapales / Getty Images
Diabetic Retinopathy
Diabetic retinopathy is an eye disease that affects the light-sensitive part of the back of your eye, called the retina . When you have diabetic retinopathy, the retina’s blood vessels can leak, swell, and close off. The disease also can cause new blood vessels to grow on the retina’s surface.
Poorly controlled diabetes puts you at a higher risk for developing diabetic retinopathy. However, you’re also at higher risk the longer amount of time that you have had diabetes.
There are two types of diabetic retinopathy—nonproliferative and proliferative. Nonproliferative diabetic retinopathy is the early stage. Most people with type 1 or type 2 diabetes will eventually develop nonproliferative diabetic retinopathy. Proliferative retinopathy is the more advanced stage. It is less common, but it can threaten your vision.
Diabetic retinopathy is the most common eye disease associated with having diabetes. It also is the number-one cause of irreversible blindness in working-age Americans.
Diabetic retinopathy typically affects both eyes, but it does not always have symptoms. That’s why regular, comprehensive eye exams are crucial when you have diabetes.
When diabetic retinopathy does have symptoms, they include:
- Vision changes : For instance, you may have difficulty reading something or find it hard to see objects at a distance. These vision changes may happen inconsistently.
- Seeing dark spots or streaks : These can occur in the later stages of diabetic retinopathy and are caused by blood vessels in the retina that have started to bleed into the gel-like fluid in the center of the eye called the vitreous.
At the early stages of diabetic retinopathy, an eye doctor may monitor your eyes regularly but not use any treatment. As the disease develops, treatments used include:
- Injections in the eye of a type of medicine called anti-vascular endothelial growth factor (VEGF) can slow down the disease.
- Laser treatments can lower swelling and help blood vessels to become smaller and stop leaking.
- A vitrectomy is a type of surgery used if your eyes are bleeding a lot or there is scarring from the leaky blood vessels.
It is always helpful to control your blood sugar to avoid further effects from diabetic retinopathy and diabetes.
The treatments for diabetic retinopathy can help prevent further eye damage, but they typically do not restore vision loss. That’s another reason why prevention of the disease with regular eye exams is important. Your eye doctor can let you know how often you should have your eyes examined when you have diabetes. Many will recommend it once a year.
Diabetic Macular Edema
Diabetic macular edema refers to fluid that has built up in the center of the retina, in the area called the macula. The fluid makes the macula swell, affecting your vision.
Diabetic retinopathy is a common cause of diabetic macular edema. Macular edema also can occur after eye surgery. There are other causes of macular edema, but diabetic macular edema is specifically associated with having diabetes and diabetic retinopathy.
An estimated 750,000 people with diabetic retinopathy also have diabetic macular edema. Non-Hispanic Blacks are three times more likely to develop edema than non-Hispanic Whites, although this may be due to the higher prevalence of diabetes among Blacks.
Symptoms of diabetic macular edema include:
- You have blurry or wavy vision in the center of your eye. However, if you have diabetic macular edema in only one eye, you may not notice your blurry vision in that eye until it gets bad.
- Colors appear washed out or faded.
- You have problems reading.
Similar to diabetic retinopathy, treatments for diabetic macular edema include anti-VEGF injections and laser treatment. These can help block the blood vessels that may form and prevent leaky blood vessels in the retina. The treatments can help stop or delay further vision loss from diabetic macular edema.
When you have glaucoma , you have additional pressure in the eye that can cause damage to the optic nerve . This can cause vision damage and blindness if not treated.
Almost 3 million people in the United States have glaucoma. If you have diabetes, you are twice as likely to develop glaucoma as someone who does not have diabetes. The risk for glaucoma also increases with age and the longer you have had diabetes.
There are several different types of glaucoma. Open-angle glaucoma is most common among those with diabetes as well as among the general U.S. population. A less common type of glaucoma called neovascular glaucoma also has some association with diabetes due to the potential for abnormal blood vessel growth from diabetic retinopathy.
Glaucoma does not always have symptoms. This is yet another reason why you should see your eye doctor regularly for eye exams to help detect glaucoma or other diabetic eye diseases early on. If vision loss occurs, it will be your peripheral or side vision.
Treatment for glaucoma does not restore lost vision. However, the available treatments have expanded in recent years to offer more ways to preserve vision and reduce eye pressure. Those treatments include:
- Various types of eye drops, including prostaglandin analogs, beta blockers, and carbonic anhydrase inhibitors
- Various surgeries , including minimally invasive glaucoma surgery and a trabeculectomy
- Glaucoma drainage devices that help to release fluid from the eye
A cataract is a clouding of the lens in the eye. Cataracts are very common, especially as you get older. About 24.5 million Americans have a cataract, and there are two million cataract surgeries performed each year. When you have diabetes, you are more likely to develop a cataract and to do so at a younger age.
Initially, you may not notice any symptoms from a cataract. Over time, you may:
- Have cloudy vision
- Notice colors that appear faded
- Have to change your eyeglass prescription more often
- Notice lights that seem to be too bright
- Have problems seeing at night
Early on, an eye doctor may not recommend surgery for a cataract. You can make changes such as getting new glasses, using a magnifying lens, and wearing anti-glare sunglasses.
Eventually, you will likely need cataract surgery, which removes the cloudy lens and replaces it with an artificial lens. This artificial lens is called an intraocular lens. Ninety percent of those who have cataract surgery report seeing better after surgery.
A Word From Verywell
Diabetes does not just affect your blood sugar. It affects your whole body, including your eyes. Make sure to see an eye doctor regularly for eye exams to detect early signs of any eye diseases. Do your best to control your blood sugar for better overall health, including eye health.
Let your eye doctor know if you have eye symptoms such as changing vision. Doing so can help preserve your vision when you have diabetes and lessen your chances of developing diabetic eye diseases.
Centers for Disease Control and Prevention. National diabetes statistics report, 2020 .
National Eye Institute. Diabetic retinopathy .
American Diabetes Association. Eye complications .
National Eye Institute. Macular edema .
National Eye Institute. Glaucoma data and statistics .
Glaucoma Research Foundation. Glaucoma and your eyesight .
American Academy of Ophthalmology. Cataract surgery infographic .
National Eye Institute. Cataracts .
By Vanessa Caceres Caceres is a Florida-based health journalist with 15 years of experience. She holds a bachelor's degree in journalism and psychology and a master's degree in linguistics.

- For Ophthalmologists
- For Practice Management
- For Clinical Teams
- For Public & Patients
Museum of the Eye
- Latest Issue
- Back Issues (Nov. 2012 to present)
- Archive Highlights (pre Oct. 2012)
- Dr. Richard Mills' Opinions, 2002 to 2016
- Write For Us
- Corporate Lunches
- Corporate Webinars
- EyeNet Magazine
- / July 2024
- / Point-of-Care AI Screening for Diabetic Eye Disease
Log in to view this page
Point-of-care ai screening for diabetic eye disease.
- Mark Complete
Recognizing the need for more evidence of AI’s performance relative to human experts, Salongcay et al. conducted a prospective comparison study. They found that POC AI, within a defined protocol for mydriatic handheld retinal imaging, was effective in identifying referable DR.
All content on the Academy’s website is protected by copyright law and the Terms of Service . This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
- About the Academy
- Jobs at the Academy
- Financial Relationships with Industry
- Medical Disclaimer
- Privacy Policy
- Terms of Service
- Statement on Artificial Intelligence
- For Advertisers
- Ophthalmology Job Center
FOLLOW THE ACADEMY
Medical Professionals
Public & Patients
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 24, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Cholesterol-lowering drug slows progression of eye disease in people with diabetes: Clinical trial
by University of Oxford
The LENS trial has demonstrated that fenofibrate, a drug usually used to lower cholesterol, reduces the risk of progression of diabetic retinopathy by 27%. The results were announced at the American Diabetes Association Scientific Sessions and published in a paper titled, "Effect of Fenofibrate on Progression of Diabetic Retinopathy," published in NEJM Evidence .
Diabetes can cause damage to the small blood vessels at the back of the eye, a condition called diabetic retinopathy. Diabetic retinopathy is among the top five causes of visual loss worldwide and the only major cause to increase in recent decades.
Fenofibrate is a tablet that has been used to lower cholesterol for more than 30 years. Previous results from sub-studies of trials looking into treatments for heart disease had suggested that fenofibrate might be able to slow the progression of diabetic retinopathy but more conclusive results were needed.
Coordinated by Oxford Population Health, the LENS (Lowering Events in Non-proliferative retinopathy in Scotland) trial compared the effects of fenofibrate with a placebo (dummy tablet) on the progression of retinopathy in 1,151 adults with type 1 or type 2 diabetes in Scotland as part of the national routine diabetic eye screening program. All of the participants had early to moderate diabetic retinopathy when they joined the trial.
The results showed that people who received fenofibrate had a 27% lower risk of needing to be referred for specialist care or treatment for diabetic retinopathy or maculopathy (a progressive eye disease that can lead to vision loss ) over four years compared with people who were assigned to receive a placebo.
Treatment with fenofibrate was also associated with a lower risk of developing macular edema (swelling at the back of the eye) and a lower risk of requiring treatment for retinopathy compared to placebo, and the benefits of fenofibrate were similar in people with both type 1 and type 2 diabetes, and in people with both normal and impaired kidney function.
Dr. David Preiss, Associate Professor at Oxford Population Health and lead author of the study, said, "Diabetic retinopathy remains a leading cause of visual loss. Good control of blood glucose is important but this is very difficult to achieve for many people, and there are few other treatments available.
"We need simple strategies that can be widely used to reduce the progression of diabetic eye disease. Fenofibrate may therefore provide a valuable addition to treat people with early to moderate diabetic retinopathy ."
Melville Henry, a LENS trial participant from Leven, said, "Taking part in the trial was very easy; there was nothing to it really. I just had to follow the instructions and take the study tablets. I attended my local research clinic appointments at first and then I had regular telephone calls to ask about my progress."
Linda Gillespie, a LENS trial participant from Kirkcaldy, said, "I attended the clinic for diabetic eye screening anyway so taking part in the trial was extremely easy, I never had to think about it. If I had any questions, someone was always at the end of the phone. It was really important to me to take part in research because without trials like LENS we can't move forward. The results of the trial might not help me but it might help someone else in the future."
Dr. Lucy Chambers, Head of Research Communications at Diabetes UK, said, "Eye problems are a frightening and too frequent complication of diabetes. But acting early can stop the first signs of damage progressing into devastating sight loss. We're excited by the positive results from this major trial of a new treatment to slow progression of eye damage, which has the potential to benefit many people with diabetes in the U.K."
Explore further
Feedback to editors

Insurance coverage disruptions, challenges accessing care common amid Medicaid unwinding
Jun 29, 2024

Scientists developing a monoclonal antibody to neutralize Nipah virus one of the deadliest zoonotic pathogens

Researchers develop scalable synthesis of cancer-fighting compounds
Jun 28, 2024

New device inspired by python teeth may reduce the risk of rotator cuff re-tearing

Serotonin 2C receptor regulates memory in mice and humans: Implications for Alzheimer's disease

Fears of attack and no phone signal deter women trail runners, finds study

Creating supranormal hearing in mice

Visualizing core pathologies of Parkinson's disease and related disorders in live patients

Novel mechanism for targeting bone marrow adipocytes to prevent bone loss

Breakthrough research makes cancer-fighting viral agent more effective
Related stories.
Multicenter cohort study shows reduced risk of vision-threatening diabetic retinopathy from fenofibrate
Apr 27, 2022

Importance of routine eye care for patients with diabetes
Aug 18, 2023
Potential new treatment option for diabetic retinopathy could address the problem much earlier
Jun 18, 2024

Risk for diabetic retinopathy up for children with type 2 diabetes
Dec 3, 2021

Wegovy, Ozempic probably won't harm vision in people with diabetes, study finds
Nov 9, 2023

Statin therapy reduces risk for diabetic retinopathy in T2DM
Jan 11, 2019
Recommended for you

Researchers discover molecule's ability to suppress negative effects of type 2 diabetes, obesity

Most kids get antibiotics for pink eye, study shows. Experts say they're usually not needed
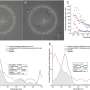
Novel photodynamic therapy method can eradicate ocular melanoma, study shows
Jun 27, 2024

Molecular mapping reveals tissue-specific gene regulation by diabetes-linked transcription factors
Jun 26, 2024

Study links gut microbiome changes to increased risk of type 2 diabetes
Jun 25, 2024

Fighting the late-night bright light could reduce risk of diabetes
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

- Health A to Z
- Alternative Medicine
- Blood Disorders
- Bone and Joint
- Cardiovascular Diseases
- Child Health
- Coronavirus
- Dental Care
- Digestive System
- Disabilities
- Drug Center
- Ear, Nose, and Throat
- Environmental Health
- Exercise And Fitness
- First Aid and Emergencies
- General Health
- Health Technology
- Hearing Loss
- Hypertension
- Infectious Disease
- LGBTQ Health
- Liver Health
- Men's Health
- Mental Health
- Pain Management
- Public Health
- Pulmonology
- Senior Health
- Sexual Health
- Skin Health
- Women's Health
- News For Medical Professionals
- Our Products
- Consumer News
- Physician's Briefing
- HealthDay TV
- Wellness Library
- HealthDay Living
- Conference Coverage
- Custom Products
- Health Writing
- Health Editing
- Video Production
- Medical Review
Cholesterol Med Might Slow Vision Loss in People With Diabetes

Key Takeaways
A well-known cholesterol drug appears to slow the progression of diabetic retinopathy
People taking fenofibrate had a 27% reduced progression of the eye disease
The drug also cut the risk of macular edema
TUESDAY, June 25, 2024 (HealthDay News) -- A well-established cholesterol-lowering drug appears to significantly slow the progression of a diabetes -related eye disease, a new trial shows.
Fenofibrate (Tricor) has been approved since 2004 as a means of lowering cholesterol. Now, this new study shows that fenofibrate also can reduce the progression of diabetic retinopathy by 27% compared to placebo.
The findings were published June 21 in the journal NEJM Evidence and presented simultaneously at the American Diabetes Association’s annual meeting in Orlando, Fla.
“Diabetic retinopathy remains a leading cause of visual loss and we need simple strategies that can be widely used to reduce the progression of diabetic eye disease,” said researcher David Preiss , an associate professor at Oxford Population Health in the UK.
The results from the new trial “suggest that fenofibrate may provide a valuable addition to treat people with diabetic retinopathy,” Preiss added in a meeting news release.
Diabetic retinopathy occurs when elevated blood sugar levels damage blood vessels in the back of the eye. The vessels start to swell and leak, eventually leading to blurry vision, blank spots and blindness.
For this study, researchers recruited 1,151 adults in Scotland who had developed early diabetic retinopathy or macular degeneration. They were randomly assigned to take either fenofibrate tablets or a placebo.
Over four years, nearly 23% of people taking fenofibrate had their eye disease worsen, compared with 29% on a placebo, results show.
Fenofibrate also cut the risk of developing macular edema, which is a potentially harmful swelling in the retina.
Participants’ progress will continue to be tracked, to better understand the long-term effects of fenofibrate on health, researchers said.
More information
The National Eye Institute has more on diabetic retinopathy .
SOURCE: American Diabetes Association, news release, June 21, 2024
What This Means For You
People with early-stage diabetic retinopathy should ask their doctor if fenofibrate might help them preserve their eyesight.
Related Stories


Aged Black Garlic Supports Glaucoma and New Eye-Related Research

What Is Aged Black Garlic?
Originating in East Asia, the recipe for black garlic is quite straightforward. Take fresh, raw garlic and ferment it between 140 degrees to 190 degrees Fahrenheit for 21 to 90 days. 1 2 3 This process induces the Maillard reaction, the chemical reaction between sugars and amino acids, which creates a scrumptious, complex flavor and turns the garlic dark brown. You can follow a recipe for making black garlic at home using a dehydrator, rice cooker, or slow cooker. Or, purchase black garlic in whole or powdered form. Black garlic oil is a foundational condiment and ingredient in Asian cooking.
How to Use Aged Black Garlic
As a superfood, black garlic chunks can be eaten straight from the jar for a healthy snack. Chefs treasure black garlic in recipes, such as:
- Garlic and mushroom risotto
- Vegan gravy
- Black garlic and tofu stir-fry
- Dipping sauce
Why Black Garlic Has Health Benefits
Garlic’s primary health-boosting compound is allicin, which is complemented by its high levels of antioxidants, 4 including flavonoids and polyphenols commonly found in fruits and vegetables. 5 Aged black garlic, in particular, contains higher concentrations of S-allyl-cysteine (SAC) compared to fresh garlic. SAC has been shown to significantly support cardiovascular health. Additionally, aged black garlic contains essential vitamins and minerals such as magnesium, calcium, phosphorus, selenium, and vitamins.
Aged Black Garlic and Vision Health
Glaucoma and retinal health.
Enhancing optic nerve blood flow is crucial for improving visual acuity in glaucoma patients. Research indicates that garlic supplements can be beneficial in this regard. One study found that garlic not only enhances visual acuity but also reduces central macular thickness (CMT) and lowers intraocular pressure (IOP). 6 This makes garlic a potential complementary treatment for individuals with diabetic macular edema. 7
Garlic’s ability to contract the ciliary muscles aids in the outflow of aqueous humor, thereby reducing IOP. 8 Additionally, consuming garlic may help prevent IOP spikes following anti-VEGF treatment.
Diabetes and Diabetic Retinopathy
Diabetic Macular Edema (DME) is a leading cause of vision loss in individuals with diabetic retinopathy. This condition arises from the accumulation of extracellular fluid in the retina, which disrupts the retinal cell structure. Studies have shown that garlic can mitigate the process of neurodegeneration in the neural retina, as evidenced by animal models. 9
Ocular ischemia, resulting from vascular endothelium damage, is a primary cause of diabetic retinopathy and neural retina damage. Garlic consumption is suggested to enhance visual acuity by increasing ocular blood flow and shielding the neural retina from oxidative stress.
Garlic serves as a complementary treatment for diabetic retinopathy and has been demonstrated to improve outcomes when used alongside anti-VEGF therapy for DME. 10 11
Note: Garlic has anticoagulant and antihypertensive properties and may increase the tendency to bleed in susceptible patients. It is important to check with your doctor before adding garlic to your supplement regimen, especially if you are on blood-thinning medication or have a heart condition.
Cardiovascular Health
Garlic is beneficial for cardiovascular health, helping to maintain optimal levels of blood pressure, cholesterol, and glucose. 12 One study revealed that consuming aged black garlic significantly improved eight different cardiovascular risk markers within just 12 weeks. 13 Another study found that aged black garlic increased HDL (good) cholesterol and reduced markers associated with heart disease. 14
Aged black garlic stands out as a potent superfood with extensive health benefits, particularly for cardiovascular and vision health. Its unique aging process enhances its medicinal properties, making it a valuable addition to a healthy diet. Whether you’re looking to improve your heart health, manage glaucoma, or support overall wellness, incorporating aged black garlic into your daily routine could be a game-changer. Always consult with your healthcare provider before making any significant changes to your supplement regimen or diet.
New Research
Macular degeneration and melatonin — new research.
Recent research from Case Western Reserve University School of Medicine and the Cole Eye Institute suggests that melatonin may lower the risk of age-related macular degeneration (AMD). In a cohort study involving 121,523 patients aged 50 and older with no prior history of AMD, taking melatonin was linked to a reduced risk of developing this eye condition. 15
Nitric Oxide and Glaucoma – New Research
A nitric oxide (NO)-donating prostaglandin analogue, NCX 470 0.1%, has been shown to be well-tolerated and more effective at lowering intraocular pressure (IOP) than latanoprost in patients with open-angle glaucoma or ocular hypertension. This finding comes from a study published in the American Journal of Ophthalmology. 16 NO aids in reducing eye pressure by enhancing the outflow of vitreous humor. 17 18 Additionally, NO influences intercellular junctions and plays a crucial role in vascular permeability, which may contribute to reducing trabecular outflow resistance. 19 20 Nitric oxide (NO) offers several benefits beyond eye health:
Cardiovascular Disease: NO improves conditions like angina pectoris and reverses ischemia. 21 As a potent vasodilator, NO acts as an endothelium-derived relaxing factor (EDRF), leading to the relaxation of smooth vascular muscles. 22
Gastrointestinal Health: NO helps reduce gastrointestinal spasms. 23
Systemic Role: NO plays a crucial role in various organ systems, including the cardiovascular, urogenital, respiratory, gastrointestinal, and immune systems. It is involved in vasodilation of smooth muscle, angiogenesis, platelet aggregation, and even regulates bone formation in the musculoskeletal system. 24
NEW PRODUCTS
Age-Less Formula 2oz
Age-less Eye and Body Package
Aged Black Garlic 30 vcaps
Berberine GT 60 vegcaps (M54381)
SUPPLEMENTS
Nitric Oxide Supplement
NMN Wonderfeel Capsul 60 vegcaps
Advanced Eye & Vision Support Formula (whole food) 60 vcaps
Dr. Grossman’s Meso Plus Retinal Support and Computer Eye Strain Formula with Astaxanthin 90 vcaps
Dr. Grossman’s Advanced Eye and Dr. G’s Whole Food Superfood Multi1 20 Vcap Combo – 2 months supply
Resveratrol (Trans) w/Quercetin 60 vegcaps
Dr. Grossman’s Bilberry/Ginkgo Combination 2oz (60ml)
Dr. Grossman’s Blood Vessel Control Formula 2oz
Recommended Books
Natural Eye Care: Your Guide to Healthy Vision and Healing
Natural Brain Support: Your Guide to Preventing and Treating Alzheimer’s, Dementia, and Other Related Diseases Naturally
Natural Parkinson’s Support: Your Guide to Preventing and Managing Parkinson’s
- Rais N, Ved A, Ahmad R, et al. S-Allyl-L-Cysteine — A garlic Bioactive: Physicochemical Nature, Mechanism, Pharmacokinetics, and health promoting activities. Journal of Functional Foods. 2023 2023/08/01/;107:105657. ↩
- Ryu JH, Kang D. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules. 2017 Jun 1;22(6). ↩
- Ahmed T, Wang CK. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules. 2021 Aug 19;26(16). ↩
- Choi I. S., Cha H. S., Lee Y. S. Physicochemical and antioxidant properties of black garlic. Molecules . 2014;19(10):16811–16823. doi: 10.3390/molecules191016811. ↩
- Ibid. Rais N, Ved A, Ahmad R, et al. ↩
- Chu T. C., Ogidigben M., Han J. C., Potter D. E. Allicin-induced hypotension in rabbit eyes. Journal of Ocular Pharmacology and Therapeutics . 1993;9(3):201–209. doi: 10.1089/jop.1993.9.201. ↩
- J Diabetes Res. 2022; 2022: 6620661. Published online 2022 Jul 14. doi: 10.1155/2022/6620661PMCID: PMC9303161 PMID: 35875346 Evaluation of the Effect of Garlic Tablet as a Complementary Treatment for Patients with Diabetic Retinopathy ↩
- Kampougeris G., Spyropoulos D., Mitropoulou A. Intraocular pressure rise after anti-VEGF treatment: prevalence, possible mechanisms and correlations. Journal of Current Glaucoma Practice. 2013;7(1):19–24. doi: 10.5005/jp-journals-10008-1132. ↩
- Arafat E. A., Youssef E. M., Khalaf H. A. The possible alleviating effect of garlic supplement on the neural retina in a rat model of hypercholesterolemia: a histological and immunohistochemical study. European Journal of Histochemistry: EJH . 2021;65(4) doi: 10.4081/ejh.2021.3322. ↩
- Lafuente M., Ortín L., Argente M., et al. Three-year outcomes in a randomized single-blind controlled trial of intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema. Retina. 2019;39(6):1083–1090. doi: 10.1097/IAE.0000000000002114 ↩
- Lafuente M., Ortín L., Argente M., et al. Combined intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema: two-year randomized single-blind controlled trial results. Retina. 2017;37(7):1277–1286. doi: 10.1097/IAE.0000000000001363. ↩
- https://www.cdc.gov/heartdisease/prevention.htm ↩
- Nirvanashetty S. High Potency Aged Garlic Extract reduces Cardiovascular Disease Risk Factors in Healthy Participants: A Randomized, Double-Blind, Placebo Controlled Study. Journal of Complementary and Alternative Medicine. 2023 September 20, 2023;6(1). ↩
- Nutrition. 2014 Sep;30(9):1034-9. doi: 10.1016/j.nut.2014.02.014. Epub 2014 Mar 12. ↩
- Jeong H, Shaia JK, Markle JC, Talcott KE, Singh RP. Melatonin and Risk of Age-Related Macular Degeneration. JAMA Ophthalmol. Published online June 06, 2024. doi:10.1001/jamaophthalmol.2024.1822 ↩
- Fechtner R, Mansberger S, Branch J, Mulaney J, Ziebell S, Lopez K, Hubatsch D. A Randomized, Controlled Comparison of NCX 470, a Nitric Oxide-Donating Bimatoprost, and Latanoprost in Subjects with Open-Angle Glaucoma or Ocular Hypertension: The MONT BLANC Study. Am J Ophthalmol. 2024 Mar 16;264:66-74. doi: 10.1016/j.ajo.2024.03.002. Epub ahead of print. PMID: 38499140. https://pubmed.ncbi.nlm.nih.gov/38499140/ ↩
- Dismuke WM, et al. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008;294(6):C1378–C1386. doi: 10.1152/ajpcell.00363.20 ↩
- Ellis D, et al. Characterization of soluble guanylate cyclase in NO-induced increases in aqueous humor outflow facility and in the trabecular meshwork. Investig Ophthalmol Vis Sci. 2009;50(4):1808–1813. doi: 10.1167/iovs.08-2750. ↩
- Moncada S, et al. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–142. ↩
- Predescu D, et al. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289(3):L371–L381. doi: 10.1152/ajplung.00175.2004. ↩
- Arnold W, et al. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci. 1977;1977:3203–3207. doi: 10.1073/pnas.74.8.3203. ↩
- Garcia-Calvo M, et al. Purification and reconstitution of the high-conductance, calcium activated potassium channel from tracheal smooth muscle. J Biol Chem. 1994;269(1994):676–682 ↩
- Michel T, Loscalzo J. Nitroglycerin and nitric oxide—a rondo of themes in cardiovascular therapeutics. N Engl J Med. 2015;373(18):1788–1789. doi: 10.1056/NEJMc1510178. ↩
- Antosova M, et al. Nitric oxide—important messenger in human body. Open J Mol Integr Physiol. 2012;02(03):98. doi: 10.4236/ojmip.2012.23014. ↩

IMAGES
VIDEO
COMMENTS
Between 2005 and 2014, Taiwan had a prevalence of 0.29 to 0.35% for blindness and poor vision and 3.75 to 3.95% for diabetic eye disease . From 14.3 per cent in 2006 to 15.9 per cent in 2013, DR was more common in Korea . Both analyses demonstrated that females having type 2 diabetes mellitus (4%) and (16.6-17%) had a greater preponderance of ...
The United States granted the most research funding for diabetic eye disease out of the seven countries assessed. The research objectives of grants focusing on diabetic retinopathy and diabetic macular edema differed by country. Additionally, the United States was dominant in terms of research output, publishing 17.53% of global papers about ...
Using a multidisciplinary approach, primary care physicians and eye care providers should follow evidence-based recommendations for screening and monitoring diabetic patients while working to improve patients' glycaemic index, blood pressure, and metabolic risk factors. Anti-vascular endothelial growth factor intravitreal injections in ...
In 2004, the Eye Diseases Prevalence Research Group (EDPRG) published meta-analytic estimates of the crude US prevalence of DR and VTDR for adults 40 years or older. 8 They found that 40.3% of adults with diabetes had DR and 8.2% had VTDR, corresponding to 4.1 million and 899 000 individuals, respectively. Among adults with diabetes, DR and ...
Diabetic retinopathy (DR) is one of the common complications of type 2 diabetes mellitus (T2DM) and is an irreversible and blinding eye disease. Depending on the degree of progression, DR is categorized as non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR), specifically mild non-proliferative diabetic ...
Clinical & Experimental Ophthalmology is an official RANZCO journal, publishing peer-reviewed papers covering all aspects of eye disease clinical practice and research. Abstract The prevalence of diabetes is rapidly increasing, and it is now the leading cause of blindness worldwide.
Researchers are working to identify risk factors for diabetes and diabetic retinopathy, and evaluate novel approaches to therapy that aim to preserve and restore vision for patients with diabetes. The Diabetic Eye Disease Team also oversees a broad program of clinical trials as part of the Diabetic Retinopathy Clinical Research Network (DRCR ...
Diabetic retinopathy can cause abnormal blood vessels to grow out of the retina and block fluid from draining out of the eye. This causes a type of glaucoma (a group of eye diseases that can cause vision loss and blindness). Retinal detachment. Diabetic retinopathy can cause scars to form in the back of your eye.
Data from population-based studies on eye diseases between 1980 to 2018 were compiled. ... The contribution of the English NHS Diabetic Eye Screening Programme to reductions in diabetes-related ...
Diabetes (DM) is the primary cause of visual disability in the United States and around the world. In the retina, DM leads to diabetic retinal disease (DRD), which can result in vision loss and blindness if not managed appropriately. 1. Since a key publication by Leber in 1875, 2.
Diabetic eye disease is a group of eye problems that can affect people with diabetes. These conditions include diabetic retinopathy, diabetic macular edema, cataracts, and glaucoma. Over time, diabetes can cause damage to your eyes that can lead to poor vision or even blindness. But you can take steps to prevent diabetic eye disease, or keep it ...
The researchers showed in a mouse model of diabetes that eye injections of a molecule called CD200Fc disrupts the activation of immune cells called microglia in the retina. Microglia, when confronted with high blood glucose levels, trigger a sequence of events that leads to inflammation and vision deterioration.
Deep Learning for Detection of Diabetic Eye Disease. November 29, 2016. Posted by Lily Peng MD PhD, Product Manager and Varun Gulshan PhD, Research Engineer. Diabetic retinopathy (DR) is the fastest growing cause of blindness, with nearly 415 million diabetic patients at risk worldwide. If caught early, the disease can be treated; if not, it ...
Diabetic retinopathy is best diagnosed with a comprehensive dilated eye exam. For this exam, drops placed in your eyes widen (dilate) your pupils to allow your doctor a better view inside your eyes. The drops can cause your close vision to blur until they wear off, several hours later. During the exam, your eye doctor will look for ...
Diabetes is a disease that affects the body's ability to produce or use insulin effectively to control blood sugar (glucose) levels. Too much glucose in the blood for a long time can cause damage in many parts of the body. Diabetes can damage the heart, kidneys and blood vessels. It damages small blood vessels in the eye as well.
Introduction: Diabetes is a pandemic disease that causes relevant ocular pathologies.Diabetic retinopathy, macular edema, cataracts, glaucoma, or keratopathy strongly impact the quality of life of the patients. In addition to glycemic control, intense research is devoted to finding more efficient ocular drugs and improved delivery systems that can overcome eye barriers.
Health education and reminder interventions focusing on both people with diabetes and their health care providers have been shown to improve screening rates (110-112). Educating patients about diabetes-related eye disease can help them understand the importance of regular screening and motivate them to participate more in their own care.
Overview. Diabetic retinopathy (die-uh-BET-ik ret-ih-NOP-uh-thee) is a diabetes complication that affects eyes. It's caused by damage to the blood vessels of the light-sensitive tissue at the back of the eye (retina). At first, diabetic retinopathy might cause no symptoms or only mild vision problems. But it can lead to blindness.
Diabetes can cause damage to the small blood vessels at the back of the eye, a condition called diabetic retinopathy. Diabetic retinopathy is among the top five causes of visual loss worldwide and the only major cause to increase in recent decades. Fenofibrate is a tablet that has been used to lower cholesterol for more than 30 years.
How the system learns. The mathematical algorithm is based on deep machine learning, a type of artificial intelligence (AI) technology in which a neural network "learns" to perform a task through repetition and self-correction. In this case, the authors reported, the computerized algorithm was trained with 128,175 human-graded fundus ...
Having diabetes increases the risk for vision loss and blindness from diabetic eye diseases. The most common diabetic eye disease is diabetic retinopathy, but people with diabetes are also at higher risk for diabetic macular edema (DME), cataract, and glaucoma. The longer a person has diabetes, the greater their risk of developing diabetic eye ...
Diabetic retinopathy (DR) is the leading cause of preventable blindness in the working-age population. The disease progresses slowly, and we can roughly differentiate two stages: early-stage (ESDR), in which there are mild retinal lesions and visual acuity is generally preserved, and advanced-stage (ASDR), in which the structural lesions are significant and visual acuity is compromised.
Key Points. Question What are 10-year trends in annual prevalence of diagnosis and treatment of diabetic macular edema (DME) or vision-threatening diabetic retinopathy (VTDR) among patients 65 years and older with diabetes who are Medicare Part B fee-for-service beneficiaries?. Findings In this cross-sectional study, from 2009 to 2018, there was a 1.5-fold increase in annual prevalence of ...
The Ljubimov Laboratory, under the direction of Alexander Ljubimov, PhD, director of the Eye Program at the Cedars-Sinai Regenerative Medicine Institute, is focused on diabetic eye disease. The goal of the Ljubimov Lab is to unravel human stem cell alterations in diabetic eye disease and their impact on disease development, in order to restore stem cell functions for future clinical translation.
Diabetic eye diseases are increasing due to the growing number of people with diabetes. There are 34.2 million people with diabetes in the United States, or 10.5% of the total population. ... Glaucoma Research Foundation. Glaucoma and your eyesight. American Academy of Ophthalmology. Cataract surgery infographic. National Eye Institute ...
Point-of-Care AI Screening for Diabetic Eye Disease. By Lynda Seminara Selected by Emily Y. Chew, MD Retina/Vitreous Read Article. Add to My Bookmarks. View; Mark Complete; Remove; Comments. Recognizing the need for more evidence of AI's performance relative to human experts, Salongcay et al. conducted a prospective comparison study. They ...
Dr. Lucy Chambers, Head of Research Communications at Diabetes UK, said, "Eye problems are a frightening and too frequent complication of diabetes. But acting early can stop the first signs of ...
TUESDAY, June 25, 2024 (HealthDay News) -- A well-established cholesterol-lowering drug appears to significantly slow the progression of a diabetes-related eye disease, a new trial shows.. Fenofibrate (Tricor) has been approved since 2004 as a means of lowering cholesterol. Now, this new study shows that fenofibrate also can reduce the progression of diabetic retinopathy by 27% compared to ...
A cholesterol-lowering drug may slow the progression of eye disease in people with diabetes, new research suggests. Diabetes can cause damage to the small blood vessels at the back of the eye, a ...
Recent research has uncovered the benefits of aged black garlic, particularly in relation to the cardiovascular system and the eyes, including glaucoma and diabetic retinopathy. From improving optic nerve blood flow and reducing intraocular pressure to boosting HDL cholesterol levels and mitigating heart disease risks, aged black garlic is a ...