Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 11 September 2008

Cancer stem cells in solid tumours: accumulating evidence and unresolved questions
- Jane E. Visvader 1 &
- Geoffrey J. Lindeman 1 , 2
Nature Reviews Cancer volume 8 , pages 755–768 ( 2008 ) Cite this article
29k Accesses
2717 Citations
24 Altmetric
Metrics details
The cancer stem cell (CSC) hypothesis is an attractive model to account for the functional heterogeneity that is commonly observed in solid tumours. It proposes a hierarchical organization of cells within the tumour, in which a subpopulation of stem-like cells is responsible for sustaining tumour growth.
The first evidence for CSCs came from acute myeloid leukaemia. There is now increasing evidence for CSCs in a variety of solid tumours (both mouse and human), provided through transplantation studies using prospectively isolated tumour cells.
The frequency of CSCs in solid tumours is highly variable, reflecting biological variation as well as technical issues. Technical issues include the purity of solid tumour cell fractionation, the requirement for more definitive markers and the challenges associated with xenotransplantation. Ultimately it will be necessary to study CSCs and potential heterogeneity within this population at a clonal level through 'cell tagging'.
Not all solid tumours will follow the CSC model of heterogeneity. Some may conform to the clonal evolution model, in which a dominant population of proliferating cells drives tumorigenesis.
Metastatic CSCs may exist, with properties distinct from primary CSCs.
The concept of CSCs has significant clinical implications: CSCs have been shown to be more resistant to chemotherapy and radiotherapy.
Recent reports, primarily for haematopoietic malignancies, suggest that CSCs can be selectively targeted without ablating normal stem cell function.
Solid tumours are an enormous cancer burden and a major therapeutic challenge. The cancer stem cell (CSC) hypothesis provides an attractive cellular mechanism to account for the therapeutic refractoriness and dormant behaviour exhibited by many of these tumours. There is increasing evidence that diverse solid tumours are hierarchically organized and sustained by a distinct subpopulation of CSCs. Direct evidence for the CSC hypothesis has recently emerged from mouse models of epithelial tumorigenesis, although alternative models of heterogeneity also seem to apply. The clinical relevance of CSCs remains a fundamental issue but preliminary findings indicate that specific targeting may be possible.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Feasibility of functional precision medicine for guiding treatment of relapsed or refractory pediatric cancers
Arlet M. Acanda De La Rocha, Noah E. Berlow, … Diana J. Azzam

Spatial transcriptomics reveals discrete tumour microenvironments and autocrine loops within ovarian cancer subclones
Elena Denisenko, Leanne de Kock, … Alistair R. R. Forrest
Human lung cancer harbors spatially organized stem-immunity hubs associated with response to immunotherapy
Jonathan H. Chen, Linda T. Nieman, … Nir Hacohen
Heppner, G. H. & Miller, B. E. Tumor heterogeneity: biological implications and therapeutic consequences. Cancer Metastasis Rev. 2 , 5–23 (1983).
Article CAS PubMed Google Scholar
Southam, C. M. & Brunschwig, A. Quantitative studies of autotransplantation of human cancer. Cancer 14 , 971–978 (1961).
Article Google Scholar
Furth, J. & Kahn, M. C. The transmission of leukemia in mice with a single cell. Am J. Cancer 31 , 276–282 (1937).
Google Scholar
Hewitt, H. B. Studies of the dissemination and quantitative transplantation of a lymphocytic leukaemia of CBA mice. Br. J. Cancer 12 , 378–401 (1958).
Article CAS PubMed PubMed Central Google Scholar
Hamburger, A. W. & Salmon, S. E. Primary bioassay of human tumor stem cells. Science 197 , 461–463 (1977).
Bonnet, D. & Dick, J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 3 , 730–737 (1997).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414 , 105–111 (2001).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194 , 23–28 (1976). A seminal paper describing the clonal evolution of tumour cell populations involving stepwise selection of cells through the acquisition of genetic changes.
Campbell, L. L. & Polyak, K. Breast tumor heterogeneity: cancer stem cells or clonal evolution? Cell Cycle 6 , 2332–2338 (2007).
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367 , 645–648 (1994).
Barabe, F., Kennedy, J. A., Hope, K. J. & Dick, J. E. Modeling the initiation and progression of human acute leukemia in mice. Science 316 , 600–604 (2007). This study reveals that leukaemia stem cells have the potential to evolve with time from a primitive cell type to one containing rearranged immunoglobulin H genes. One implication of this work is that CSCs themselves may be subject to clonal evolution.
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406 , 532–535 (2000).
Huntly, B. J. et al. MOZ–TIF2, but not BCR–ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell 6 , 587–596 (2004).
Krivtsov, A. V. et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL–AF9. Nature 442 , 818–822 (2006).
Somervaille, T. C. & Cleary, M. L. Identification and characterization of leukemia stem cells in murine MLL–AF9 acute myeloid leukemia. Cancer Cell 10 , 257–268 (2006).
Cozzio, A. et al. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 17 , 3029–3035 (2003).
Chen, W. et al. Malignant transformation initiated by Mll – AF9 : gene dosage and critical target cells. Cancer Cell 13 , 432–440 (2008).
Jamieson, C. H. et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 351 , 657–667 (2004).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100 , 3983–3988 (2003). This paper provides the first description of the prospective purification of tumour-initiating cells from a solid malignancy, breast cancer.
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432 , 396–401 (2004). The first demonstration of CSCs in brain tumours through the use of CD133 for prospective isolation.
Bao, S. et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 66 , 7843–7848 (2006).
Beier, D. et al. CD133 + and CD133 − glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 67 , 4010–4015 (2007).
Taylor, M. D. et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 8 , 323–335 (2005).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444 , 756–760 (2006).
Piccirillo, S. G. et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444 , 761–765 (2006). These studies reveal that CSCs in gliomas appear to have different properties from the bulk of the population. Reference 24 shows that they are more radioresistant and reference 25 demonstrates that they are responsive to BMP-induced differentiation.
O'Brien, C. A., Pollett, A., Gallinger, S. & Dick, J. E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445 , 106–110 (2007).
Ricci-Vitiani, L. et al. Identification and expansion of human colon-cancer-initiating cells. Nature 445 , 111–115 (2007).
Hermann, P. C. et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1 , 313–323 (2007). These findings support the concept of a distinct metastatic CSC with important implications for designing drugs that specifically target the metastatic CSC.
Uchida, N. et al. Direct isolation of human central nervous system stem cells. Proc. Natl Acad. Sci. USA 97 , 14720–14725 (2000).
Lee, A. et al. Isolation of neural stem cells from the postnatal cerebellum. Nature Neurosci. 8 , 723–729 (2005).
Oshima, Y. et al. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology 132 , 720–732 (2007).
Dalerba, P. et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl Acad. Sci. USA 104 , 10158–10163 (2007).
Ginestier, C. et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1 , 555–567 (2007).
Wright, M. H. et al. Brca1 breast tumors contain distinct CD44 + /CD24 − and CD133 − cells with cancer stem cell characteristics. Breast Cancer Res. 10 , R10 (2008).
Article PubMed PubMed Central CAS Google Scholar
Schatton, T. et al. Identification of cells initiating human melanomas. Nature 451 , 345–349 (2008). This study reveals that expression of the CSC marker and drug transporter protein ABCB5 in melanoma correlates with clinical progression.
Kern, S. E. & Shibata, D. The fuzzy math of solid tumor stem cells: a perspective. Cancer Res. 67 , 8985–8988 (2007).
Bonnefoix, T., Bonnefoix, P., Verdiel, P. & Sotto, J. J. Fitting limiting dilution experiments with generalized linear models results in a test of the single-hit Poisson assumption. J. Immunol. Methods 194 , 113–119 (1996).
Zeppernick, F. et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin. Cancer Res. 14 , 123–129 (2008).
Patrawala, L. et al. Highly purified CD44 + prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 25 , 1696–1708 (2006).
Patrawala, L. et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2 + and ABCG2 − cancer cells are similarly tumorigenic. Cancer Res. 65 , 6207–6219 (2005).
Collins, A. T., Berry, P. A., Hyde, C., Stower, M. J. & Maitland, N. J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65 , 10946–10951 (2005).
Eramo, A. et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 15 , 504–514 (2008).
Neering, S. J. et al. Leukemia stem cells in a genetically defined murine model of blast-crisis CML. Blood 110 , 2578–2585 (2007).
Yilmaz, O. H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441 , 475–482 (2006).
Kelly, P. N., Dakic, A., Adams, J. M., Nutt, S. L. & Strasser, A. Tumor growth need not be driven by rare cancer stem cells. Science 317 , 337 (2007). This paper has challenged the CSC hypothesis, following the observation that three mouse models of leukaemia and lymphoma are maintained by a dominant cell population. The authors posit that xenotransplantation may select for tumour cells capable of surviving in a foreign environment.
Cho, R. W. et al. Isolation and molecular characterization of cancer stem cells in MMTV– Wnt-1 murine breast tumors. Stem Cells 26 , 364–371 (2008).
Vaillant, F., Asselin-Labat, M. L., Shackleton, M., Lindeman, G. J. and Visvader, J. E. The mammary progenitor marker CD61/b3integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. (in the press).
Zhang, M. et al. Identification of tumor-initiating cells in a p53 null mouse model of breast cancer. Cancer Res. 68 , 4674–4682 (2008).
Malanchi, I. et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signalling. Nature 452 , 650–653 (2008). References 46–49 provide definitive evidence for the existence of CSCs in syngeneic mouse models of mammary and skin tumorigenesis. They further suggest that normal stem and progenitor markers have utility in the identification and isolation of CSCs.
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nature Rev. Cancer 2 , 442–454 (2002).
Article CAS Google Scholar
Mani, S. A. et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc. Natl Acad. Sci. USA 104 , 10069–10074 (2007).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438 , 820–827 (2005).
Yang, Z. F. et al. Significance of CD90 + cancer stem cells in human liver cancer. Cancer Cell 13 , 153–166 (2008).
Light, R. W., Erozan, Y. S. & Ball, W. C. Jr. Cells in pleural fluid. Their value in differential diagnosis. Arch. Intern. Med. 132 , 854–860 (1973).
Liu, R. et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N. Engl. J. Med. 356 , 217–226 (2007).
Shipitsin, M. et al. Molecular definition of breast tumor heterogeneity. Cancer Cell 11 , 259–273 (2007).
Shmelkov, S. V. et al. CD133 expression is not restricted to stem cells, and both CD133 and CD133 metastatic colon cancer cells initiate tumors. J. Clin. Invest. 118 , 2111–2120 (2008).
CAS PubMed PubMed Central Google Scholar
Carpenter, G. & Cohen, S. Epidermal growth factor. Annu. Rev. Biochem. 48 , 193–216 (1979).
Rifkin, D. B. & Moscatelli, D. Recent developments in the cell biology of basic fibroblast growth factor. J. Cell Biol. 109 , 1–6 (1989).
Kuperwasser, C. et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc. Natl Acad. Sci. USA 101 , 4966–4971 (2004). This study represents an important step in establishing humanized mouse models for solid tumours, demonstrating that a species-specific stromal niche is important for the growth of human epithelial cells.
Gupta, P. B. et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 67 , 2062–2071 (2007).
Takenaka, K. et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nature Immunol. 8 , 1313–1323 (2007).
Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz- scid IL2Rγ null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174 , 6477–6489 (2005).
Galli, R. et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 64 , 7011–7021 (2004).
Li, C. et al. Identification of pancreatic cancer stem cells. Cancer Res. 67 , 1030–1037 (2007).
Bissell, M. J. & Labarge, M. A. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell 7 , 17–23 (2005).
Mehta, R. R., Graves, J. M., Hart, G. D., Shilkaitis, A. & Das Gupta, T. K. Growth and metastasis of human breast carcinomas with Matrigel in athymic mice. Breast Cancer Res. Treat. 25 , 65–71 (1993).
Henson, B. et al. An orthotopic floor-of-mouth model for locoregional growth and spread of human squamous cell carcinoma. J. Oral Pathol. Med. 36 , 363–370 (2007).
Prokhorova, T. A. et al. Teratoma formation by human embryonic stem cells is site-dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 7 Apr 2008 (doi:10.1089/scd.2007.0266).
Marshall, G. P. 2nd, Reynolds, B. A. & Laywell, E. D. Using the neurosphere assay to quantify neural stem cells in vivo . Curr. Pharm. Biotechnol. 8 , 141–145 (2007).
Reynolds, B. A. & Rietze, R. L. Neural stem cells and neurospheres — re-evaluating the relationship. Nature Meth. 2 , 333–336 (2005). The sphere assay, originally developed for neural cells, has formed an important basis for the development of an in vitro assay to study both normal stem and progenitor cells and tumour-initiating cells in a variety of solid tumours including brain (reference 73) and breast (reference 78).
Kondo, T. & Raff, M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289 , 1754–1757 (2000).
Hemmati, H. D. et al. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl Acad. Sci. USA 100 , 15178–15183 (2003).
Clement, V., Sanchez, P., de Tribolet, N., Radovanovic, I. & Ruiz i Altaba, A. HEDGEHOG–GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 17 , 165–172 (2007).
Diamandis, P. et al. Chemical genetics reveals a complex functional ground state of neural stem cells. Nature Chem. Biol. 3 , 268–273 (2007).
Beier, D. et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 68 , 5706–5715 (2008).
Yu, F. et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 131 , 1109–1123 (2007).
Dontu, G. et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17 , 1253–1270 (2003).
Ishikawa, F. et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nature Biotechnol. 25 , 1315–1321 (2007).
Ito, K. et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature 453 , 1072–1078 (2008).
Shachaf, C. M. et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature 431 , 1112–1117 (2004).
Guzman, M. L. et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 105 , 4163–4169 (2005).
Jin, L., Hope, K. J., Zhai, Q., Smadja-Joffe, F. & Dick, J. E. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nature Med. 12 , 1167–1174 (2006).
Article PubMed CAS Google Scholar
Krause, D. S., Lazarides, K., von Andrian, U. H. & Van Etten, R. A. Requirement for CD44 in homing and engraftment of BCR–ABL-expressing leukemic stem cells. Nature Med. 12 , 1175–1180 (2006).
Lee, J. et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell 13 , 69–80 (2008).
Walkley, C. R. et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor γ deficiency. Cell 129 , 1097–1110 (2007).
Calabrese, C. et al. A perivascular niche for brain tumor stem cells. Cancer Cell 11 , 69–82 (2007). This study and reference 21 suggest that CSCs in tumours are maintained by an aberrant vascular niche and that glioblastoma CSCs have potent angiogenic activity.
Folkins, C. et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 67 , 3560–3564 (2007).
Hambardzumyan, D. et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo . Genes Dev. 22 , 436–448 (2008).
Blazek, E. R., Foutch, J. L. & Maki, G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133 − cells, and the CD133 + sector is enlarged by hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 67 , 1–5 (2007).
Phillips, T. M., McBride, W. H. & Pajonk, F. The response of CD24 −/low /CD44 + breast cancer-initiating cells to radiation. J. Natl Cancer Inst. 98 , 1777–1785 (2006).
Article PubMed Google Scholar
Woodward, W. A. et al. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc. Natl Acad. Sci. USA 104 , 618–623 (2007).
Al-Hajj, M. Cancer stem cells and oncology therapeutics. Curr. Opin. Oncol. 19 , 61–64 (2007).
PubMed Google Scholar
Li, X. et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl Cancer Inst. 100 , 672–679 (2008). References 77 and 94 provide evidence for a subpopulation of chemotherapy-resistant cancer-initiating cells in breast cancer patients.
Shafee, N. et al. Cancer stem cells contribute to cisplatin resistance in Brca1 / p53 -mediated mouse mammary tumors. Cancer Res. 68 , 3243–3250 (2008).
Dylla, S. J. et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE 3 , e2428 (2008).
Todaro, M. et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 1 , 389–402 (2007).
Johnstone, R. W., Cretney, E. & Smyth, M. J. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood 93 , 1075–1085 (1999).
CAS PubMed Google Scholar
Blair, A., Hogge, D. E., Ailles, L. E., Lansdorp, P. M. & Sutherland, H. J. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo . Blood 89 , 3104–3112 (1997).
Jordan, C. T. et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 14 , 1777–1784 (2000).
Chang, H. H., Hemberg, M., Barahona, M., Ingber, D. E. & Huang, S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature 453 , 544–547 (2008).
Hope, K. J., Jin, L. & Dick, J. E. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature Immunol. 5 , 738–743 (2004).
Kleinsmith, L. J. & Pierce, G. B., Jr. Multipotentiality of single embryonal carcinoma cells. Cancer Res. 24 , 1544–1551 (1964).
Lowe, S. W. & Sherr, C. J. Tumor suppression by Ink4a - Arf : progress and puzzles. Curr. Opin. Genet. Dev. 13 , 77–83 (2003).
Molofsky, A. V., He, S., Bydon, M., Morrison, S. J. & Pardal, R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16 Ink4a and p19 Arf senescence pathways. Genes Dev. 19 , 1432–1437 (2005).
Pardal, R., Molofsky, A. V., He, S. & Morrison, S. J. Stem cell self-renewal and cancer cell proliferation are regulated by common networks that balance the activation of proto-oncogenes and tumor suppressors. Cold Spring Harb. Symp. Quant. Biol. 70 , 177–185 (2005).
Leung, C. et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 428 , 337–341 (2004).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434 , 843–850 (2005).
He, X. C., Zhang, J. & Li, L. Cellular and molecular regulation of hematopoietic and intestinal stem cell behavior. Ann. N. Y. Acad. Sci. 1049 , 28–38 (2005).
Haramis, A. P. et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303 , 1684–1686 (2004).
He, X. C. et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–β-catenin signaling. Nature Genet. 36 , 1117–1121 (2004).
Ming Kwan, K., Li, A. G., Wang, X. J., Wurst, W. & Behringer, R. R. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genesis 39 , 10–25 (2004).
Ayyanan, A. et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc. Natl Acad. Sci. USA 103 , 3799–3804 (2006).
Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. Nature 439 , 84–88 (2006).
Zhao, C. et al. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo . Cancer Cell 12 , 528–541 (2007).
Mizrak, D., Brittan, M. & Alison, M. R. CD133: molecule of the moment. J. Pathol. 214 , 3–9 (2008).
Prince, M. E. et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl Acad. Sci. USA 104 , 973–978 (2007).
Wu, C. et al. Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Res. 67 , 8216–8222 (2007).
Clarke, M. F. et al. Cancer stem cells — perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 66 , 9339–9344 (2006).
Singh, S. K. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63 , 5821–5828 (2003).
Download references
Acknowledgements
We sincerely apologize to those authors whose papers we could not cite owing to space constraints. We are grateful to J. Adams for discussions and P. Maltezos for expert help with the figures. This work was supported by the Victorian Breast Cancer Research Consortium and the National Health and Medical Research Council (Australia). We also acknowledge support from the National Breast Cancer Foundation (Australia), the Susan G. Komen Breast Cancer Foundation, the US Department of Defense, the Australian Stem Cell Centre and the Australian Cancer Research Foundation.
Author information
Authors and affiliations.
VBCRC Laboratory, The Walter and Eliza Hall Institute of Medical Research, Melbourne, 3050, Victoria, Australia
Jane E. Visvader & Geoffrey J. Lindeman
Department of Medical Oncology, Royal Melbourne Hospital, Parkville, 3050, Victoria, Australia
Geoffrey J. Lindeman
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jane E. Visvader .
Related links
National cancer institute.
breast cancer
colon cancer
ependymomas
glioblastoma multiforme
lung cancer
medulloblastoma
ovarian cancer
pancreatic carcinoma
prostate cancer
National Cancer Institute Drug Dictionary
bevacizumab
cyclophosphamide
doxorubicin
gemcitabine
temozolomide
FURTHER INFORMATION
J. E. Visvader's homepage
G. J. Lindeman's homepage
Owing to the limited amount of tumour material it is necessary to establish xenografts. This involves limited passaging of the tumour, preferably in an orthotopic location, in immunocompromised mice such as NOD-SCID strains. The validity of using xenografts has been documented for many different tumour types. The engraftment rate can be variable, dependent on the tumour type.
Both normal and cancerous cells from numerous organs can be expanded as non-adherent sphere-like cellular aggregates in serum-free media containing EGF and FGF2.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Visvader, J., Lindeman, G. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8 , 755–768 (2008). https://doi.org/10.1038/nrc2499
Download citation
Published : 11 September 2008
Issue Date : October 2008
DOI : https://doi.org/10.1038/nrc2499
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Beyond the barrier: the immune-inspired pathways of tumor extravasation.
- Sara Di Russo
- Francesca Romana Liberati
- Alessio Paone
Cell Communication and Signaling (2024)
Construction and in vitro evaluation of pH-sensitive nanoparticles to reverse drug resistance of breast cancer stem cells
- Yanhong Wang
Discover Oncology (2024)
Production of a Ribosome-Displayed Mouse scFv Antibody Against CD133, Analysis of Its Molecular Docking, and Molecular Dynamic Simulations of Their Interactions
- Sepideh Ghani
- Mojgan Bandehpour
- Bahram Kazemi
Applied Biochemistry and Biotechnology (2024)
Histological transformation to signet-ring cell carcinoma in a patient with clinically aggressive poorly differentiated adenocarcinoma of the ascending colon after response to chemotherapy plus cetuximab: a case report
- Hideki Nagano
- Shigekazu Ohyama
- Mikiko Kobayashi
World Journal of Surgical Oncology (2023)
Post-translational modification of CDK1–STAT3 signaling by fisetin suppresses pancreatic cancer stem cell properties
- Xiaodong Xu
- Shengnan Jia
Cell & Bioscience (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Advertisement
- Next Article
Introduction
The cancer stem cell hypothesis, implications of the cancer stem cell hypothesis: a paradigm shift in thinking about carcinogenesis and our approach to cancer prevention and therapy, acknowledgments, cancer stem cells: an old idea—a paradigm shift.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
- Version of Record February 17 2006
Max S. Wicha , Suling Liu , Gabriela Dontu; Cancer Stem Cells: An Old Idea—A Paradigm Shift. Cancer Res 15 February 2006; 66 (4): 1883–1890. https://doi.org/10.1158/0008-5472.CAN-05-3153
Download citation file:
- Ris (Zotero)
- Reference Manager
Although the concept that cancers arise from “stem cells” or “germ cells” was first proposed about 150 years ago, it is only recently that advances in stem cell biology have given new impetus to the “cancer stem cell hypothesis.” Two important related concepts of this hypothesis are that ( a ) tumors originate in either tissue stem cells or their immediate progeny through dysregulation of the normally tightly regulated process of self-renewal. As a result of this, ( b ) tumors contain a cellular subcomponent that retains key stem cell properties. These properties include self-renewal, which drives tumorigenesis, and differentiation albeit aberrant that contributes to cellular heterogeneity. Recent experimental evidence in a variety of tumors has lent strong support to the cancer stem cell hypothesis that represents a paradigm shift in our understanding of carcinogenesis and tumor cell biology. This hypothesis has fundamental implications for cancer risk assessment, early detection, prognostication, and prevention. Furthermore, the current development of cancer therapeutics based on tumor regression may have produced agents that kill differentiated tumor cells while sparing the rare cancer stem cell population. The development of more effective cancer therapies may thus require targeting this important cell population. (Cancer Res 2006; 66(4): 1883-90)
In a thought-provoking article published in Fortune in 2004, Leaf, a cancer survivor, poses the question, “Are we losing the war on cancer?” ( 1 ). In this article, he reviews data on the progress made since the “war on cancer” was declared in 1961. Over this time, there have clearly been dramatic advances in the treatment of such diseases as childhood leukemia, Hodgkin's disease, and testicular cancer. Furthermore, the overall mortality for some of the common epithelial malignances, such as breast cancer and prostate cancer, have been declining recently largely due to advances in early detection and prevention. However, as Leaf points out, for the four most common epithelial malignancies (lung, breast, prostate, and colon cancers), the survival of patients with metastatic disease has not changed significantly over the past several decades. Despite these statistics, there is considerable optimism in the cancer research community that new targeted therapies will significantly improve on the results of empiric-based therapeutics. The ability to specifically target pathways deranged in cancer raises the hope of developing therapies with enhanced specificity and decreased toxicity. However, as our ability to attack specific targets increases, a fundamental question remains, “Are we targeting the right cells”? Evidence is accumulating that most, if not all, malignancies are driven by “a cancer stem cell compartment.” Furthermore, these cancer stem cells may be inherently resistant to our current therapeutic approaches. The cancer stem cell hypothesis has fundamental implications for understanding the biology of carcinogenesis as well as for developing new strategies for cancer prevention as well as new therapies for advanced disease. In this commentary, we will discuss the cancer stem cell hypothesis, including recent evidence supporting its validity, and the implications of this model for cancer prevention and therapy.
All tissues in the body are derived from organ-specific stem cells that are defined by their capacity to undergo self-renewal as well as to differentiate into the cell types that comprise each organ. These tissue-specific stem cells are distinguished from embryonic stem cells in that their differentiation is largely restricted to cell types within a particular organ. The cancer stem cell hypothesis has two separate but related components. The first component concerns the cellular origin of tumors, including the question of whether tumors arise from tissue stem cells. A second related component of this hypothesis is that tumors are driven by cellular components that display “stem cell properties.” The concept that cancer might arise from a rare population of cells with stem cell properties was proposed about 150 years ago ( 2 – 5 ). Over 40 years ago, it was postulated that tissue-specific stem cells may be the cell of origin of cancer ( 6 ). Over 30 years ago, Pierce ( 7 ) proposed that cancers represented a maturation arrest of stem cells. The concept that tumors contain cell populations with stem cell properties was also suggested by in vitro “clonogenic assays” that showed subpopulations of tumor cells with increased proliferative capacity as shown by colony formation in in vitro assays using cells isolated from tumor specimens ( 8 ). A major limitation of these studies, however, was that they measured in vitro proliferation rather than true self-renewal. In addition, it has been observed that the production of human tumor xenografts in animal models required a relatively large number of cells. However, it was unclear whether this was due to the inefficiency of these cells in promoting tumor growth or to the existence of rare subpopulations within a tumor that were uniquely tumorigenic in these systems.
Evidence supporting the cancer stem cell hypothesis has gained impetus due to recent advances in stem cell biology and the development of new animal models to measure self-renewal and more directly test the validity of this hypothesis. The concept that cancers arise from the transformation of stem cells is appealing for several reasons. Stem cells by their long-lived nature are subject to the accumulation of multiple mutations that are required for carcinogenesis. For example, women exposed to atomic bomb radiation in Hiroshima and Nagasaki developed breast cancer approximately 20 to 30 years after exposure ( 9 ). Mutations found in these women's breast cancers are consistent with those known to be induced by radiation ( 9 ). Furthermore, women exposed to radiation during late adolescents had the highest susceptibility to breast cancer development. This is thought to be the period when the mammary gland has the highest number of stem cells ( 10 ). Further evidence that stem cells may play a role in carcinogenesis is the observation that normal stem cells and cancer cells share several important properties. These include ( a ) the capacity for self-renewal, ( b ) the ability to differentiate, ( c ) active telomerase expression, ( d ) activation of antiapoptotic pathways, ( e ) increased membrane transporter activity, and ( f ) the ability to migrate and metastasize. Indeed, properties, such as anchorage independence, which have been thought to be a hallmark of transformed cells, have recently been described by us and others as a property of normal tissue stem cells ( 11 – 13 ). One of the key early events in transformation may be the dysregulation of the normally highly regulated process of self-renewal. Stem cells are the only cells capable of undergoing self-renewal divisions. In the steady state, these divisions are asymmetric in which a stem cell is able to produce an exact copy of itself as well as a daughter cell that undergoes differentiation into the lineages found in differentiated tissues. During stem cell expansion and tumorigenesis, stem cells may undergo symmetric divisions in which stem cells produce two identical stem cell progeny, thus allowing for stem cell expansion (ref. 14 ; Fig. 1 ). During normal development, stem cell self-renewal is regulated by signals from the surrounding stem cell “niche.” As has been elegantly shown in bone marrow transplantation models, a single hematopoietic stem cell introduced into a lethally irradiated mouse is able to repopulate the stem cell compartment resulting in reconstitution of the entire hematopoietic system. Extensive expansion in the stem cell population stops when this pool is replenished, illustrating the tight control of this process. We and others have hypothesized that deregulation of this self-renewal process leading to stem cell expansion may be a key early event in carcinogenesis. Recently, the pathways that regulate the self-renewal of normal stem cells, including Wnt, Notch, and Hedgehog, have begun to be elucidated. These signaling pathways have been implicated in regulating the self-renewal of hematopoietic, neuronal, and mammary stem cells ( 14, 15 ). The dysregulation of each of these pathways in rodent models leads to tumorigenesis. Furthermore, there is substantial evidence that dysregulation of these pathways also plays an important role in human carcinogenesis. Defects in the Wnt signaling pathway are seen early in colon cancer carcinogenesis. Alterations in Hedgehog signaling were first shown in human basal carcinomas of the skin ( 16 ). More recently, evidence for dysregulation of this pathway has been reported in human pancreatic, gastric, prostate, and breast carcinomas ( 17, 18 ). Alterations in Notch signaling have been observed in human T-cell acute lymphoblastic leukemia, cervical cancer, and breast cancer ( 19 – 23 ).

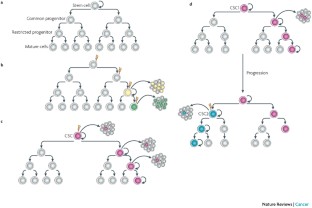
Stem cells in normal development, tissue homeostasis, and carcinogenesis. A, during normal development, symmetric stem cell self-renewal results in stem cell expansion. This process is tightly regulated by components of the stem cell niche. Stem cells differentiate into a transient amplifying population that undergoes further proliferation and lineage commitment followed by cell migration, terminal cell differentiation, and apoptosis of fully differentiated cells. B, during normal tissue homeostasis, asymmetric self-renewal of stem cells results in stem cell maintenance. Proliferation and differentiation of transient amplifying progenitor cells replaces normal cell loss resulting in tissue homeostasis. C, carcinogenesis may be initiated by stem cell expansion via symmetric self-renewal. Unlike normal organogenesis, this process is dysregulated resulting in cancer stem cell expansion. Aberrant differentiation of these cells generates tumor heterogeneity. Further mutations or epigenetic changes may accompany tumor invasion and metastasis. Metastases require the dissemination of cancer stem cells that may remain dormant and be reactivated resulting in tumor recurrence. In contrast, dissemination of differentiated tumor cells produces only micrometastasis that do not progress.
Recent studies have suggested that tumors may arise from progenitor cells and tissue stem cells. Transformation of these cells may require that they acquire the stem cell property of self-renewal. In support of this hypothesis, Jamieson et al. showed that chronic myelogenous leukemia (CML) blast crisis may originate in hematopoietic progenitor cells as a consequence of dysregulated Wnt signaling, allowing these cells to self-renew, a property normally restricted to hematopoietic stem cells ( 24 ). Similarly, by transfecting purified populations of hematopoietic progenitor cells, Kelly and Gilliland showed that AML-ETO may induce transformation of myeloid progenitor cells enabling them to acquire the property of self-renewal ( 25 ). We have recently proposed that human breast cancers may arise from the transformation of either mammary stem cells or early progenitor cells resulting in production of breast cancers with distinct molecular and clinical phenotypes ( 26 ). This concept is also consistent with recent descriptions in transgenic mouse models of mammary tumorigenesis, which suggest that distinct oncogenes may affect different stem and progenitor cells resulting in phenotypic differences in mammary tumors ( 27 ).
The second major component of the cancer stem cell hypothesis is that tumors contain and are “driven” by cellular components that display stem cell properties. This concept has gained substantial experimental support recently with the development of animal models that have permitted the direct assessment of stem cell properties of tumor cell subpopulations. These models have shown that prospectively identifiable subpopulations of tumor cells display the defining stem cell properties of self-renewal and differentiation. Self-renewal drives tumorigenesis, whereas differentiation (albeit aberrant in tumors) contributes to tumor phenotypic heterogeneity. In 1997, Dick et al. showed that the ability to transfer human leukemias into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice was retained in a small population of cancer stem cells that like their normal counterparts displayed the cell surface phenotype CD34 + CD38 − . These cells that comprised <1 in 10,000 leukemia cells could transfer the human leukemia into NOD/SCID mice, whereas the introduction of thousands of fold higher numbers of cells that did not bear this phenotype were nontumorigenic. Furthermore, the leukemias that were produced recapitulated the histologic phenotype found in the original tumor ( 28 ). More recently, this group used cellular marking studies to show that leukemic stem cells like their normal counterparts are heterogeneous with varying degrees of self-renewal potential. These findings suggest that leukemic stem cells, like their normal counterparts, exist in a hierarchy that is developmentally regulated. This supports the hypothesis that leukemic stem cells originate from the transformation of normal hematopoietic stem cells ( 29 ). Using a similar approach, in collaboration with Michael Clarke, we showed that human breast cancers contain a cell population characterized by the expression of the cell surface markers CD44 + CD24 low lin− that have stem cell characteristics. As few as 200 of these cells, which comprise between 1% and 10% of the total cell population, are able to form tumors when implanted in NOD/SCID mice ( 30 ). In contrast, 20,000 cells isolated from the same tumor that do not display this cell surface phenotype are unable to form tumors. Furthermore, consistent with a stem cell model, cancer stem cells are able to generate tumors that recapitulate the phenotypic heterogeneity found in the initial tumor.
Confirming and extending our findings, Ponti et al. recently reported that, in addition to being tumorigenic, CD44 + CD24 − human breast cancer cells form tumor mammospheres in vitro , a property that we described previously for normal mammary stem/progenitor cells ( 31, 32 ). Furthermore, the stem cell phenotype of these tumor cells was suggested by their expression of the stem cell markers Oct-4 as well as by the absence of Cx43 expression. Interestingly, these cells also produced vascular endothelial growth factor (VEGF) and were highly angiogenic ( 31 ). Lending further support to the cancer stem cell hypothesis and extending its generality, three groups have independently shown the existence of a cancer stem cell compartment in human brain tumors. These cancer stem cells, like their normal counterparts, are able to form neurospheres in vitro and express the neural stem cell markers CD133 and nestin. Furthermore, as few as 100 of these cells were able to transfer the tumors when injected intracranially into NOD/SCID mice ( 33, 34 ). In contrast, 10 5 CD133 − cells engrafted but did not produce a tumor. The tumors produced by the CD133 + cells recapitulated the phenotypic heterogeneity found in the initial tumor ( 33 ). Evidence for existence of a clonogenic subpopulation of cells in human multiple myeloma was recently reported by Matsui et al. ( 35 ). Multiple myeloma cells express syndecan-1 (CD138). However, a small subpopulation resembling postgerminal center B cells were CD138 − . Only the CD138 − cells were clonogenic in vitro and in NOD/SCID mice ( 35 ). In the prostate, Xin et al. showed that stem cell antigen-1 (Sca-1) enriches for a prostate regenerating cell in mouse model and genetic perturbations of PTEN/AKT produced prostate cancer associated with a dramatic increase in Sca-1 + cells ( 36 ). Further evidence for the existence of a cell population with stem cell properties in prostate cancer has been reported by Richardson et al. They found that normal human prostate stem cells expressed CD133 ( 37 ). Furthermore, they identified a subpopulation of cells in human prostate cancer characterized as CD44 + /α 2 β 1 hi /CD133 + with stem cell properties. As few as 500 cells with this phenotype that constituted 0.1% of total tumor cells formed tumors in NOD/SCID mice, whereas 5 × 10 5 CD44 − cells failed to form tumors ( 38 ). Evidence for the existence of cancer stem cells in lung cancer has recently been presented by Kim et al. ( 39 ). They identified bronchial alveolar stem cells present at the bronchial alveolar duct junction. These cells exhibited the stem cell properties of self-renewal and multilineage differentiation. These stem cells could be transformed by K-ras in vitro and could form tumor in mice ( 39 ).
Submitted for publication.
Models of carcinogenesis. “Stochastic models” of carcinogenesis hold that transformation results from random mutation and subsequent clonal selection. In this model, any cell may be the target of carcinogenesis. The stem cell model of carcinogenesis, in contrast, suggests that cancers originate in tissue stem or progenitor cells probably through dysregulation of self-renewal pathways. This leads to expansion of this cell population that then may undergo further genetic or epigenetic changes to become fully transformed. In addition, epigenetic changes normally involved in cell differentiation contribute to the cellular phenotypic heterogeneity found in tumors. This model represents a paradigm shift in our thinking and has fundamental consequences for understanding the biology of carcinogenesis as well as important clinical implications for early detection, prevention, and therapy of human malignancies. These implications are summarized in Fig. 2 .

Clinical implications of cancer stem cell model. The cancer stem cell model has important implications for cancer risk reduction, early detection, prevention, and treatment. Interventions that reduce normal stem cell number may decrease cancer risk. Detection of factors secreted by initiated stem cells may allow for the earlier detection of cancers. Interventions that induce apoptosis or differentiation of initiated stem cells may be effective in cancer prevention. Conventional cancer therapies, including cytotoxic agents, selectively destroy differentiated cancer cells, sparing the cancer stem cell compartment resulting in cancer recurrence at primary or metastatic sites. Therapies that selectively eliminate cancer stem cells leave residual nontumorigenic cells resulting in potential cancer cures.
Biological implications. The cancer stem cell hypothesis has important biological implications for the development of animal models of carcinogenesis as well as for understanding key biological processes, such as stromal-epithelial interactions and metastasis. Although there has been considerable progress in the development of mouse models of human cancer, in many cases, these models fail to recapitulate human disease. Many transgenic models use tissue-specific promoters to drive oncogene expression. However, these tissue-specific genes may be expressed only in differentiated cells. If stem cells or their immediate progeny are the true targets of transforming events, then the expression of oncogenes in more differentiated cells may fail to recapitulate actual carcinogenic processes. There is recent evidence that the expression of oncogenes in primitive cells using direct transfection technologies results in a fundamentally different phenotype than expression of the same genes driven by tissue-specific promoters. Welm et al. showed that expression of c-Met and c-Myc driven by the mammary-specific promoter mouse mammary tumor virus fails to produce carcinomas, whereas these genes transduced into primitive cells via a stem cell virus produced mammary carcinomas ( 40 ). Kim et al. have developed an animal model that targets normal lung stem cells to produce adenocarcinomas that resemble those found in human lung cancers ( 39 ).
In preparation.
Implications for cancer risk assessment, early detection, molecular profiling, and prevention. The cancer stem cell model has important implications for many aspects of cancer risk assessment and prevention. If cancer stem cells or their immediate progeny are the targets for transformation, then cancer risk may be directly related to the number of stem cell targets. Pathways that influence target number may thus influence cancer risk. For example, it has been suggested that a previously unrecognized function of the hereditary breast cancer gene BRCA1 may be in the regulation of normal breast stem cell function ( 45 ). An important regulator of stem cell self-renewal of both normal and transformed stem cells is the polycomb gene Bmi-1 ( 46 – 48 ). It has recently been shown that Bmi-1 induced down-regulation of P-16 plays an important role in the regulation of hematopoietic and neuronal stem cell self-renewal ( 47, 49 ). Interestingly, recent studies by Holst et al. have suggested that one of the earliest events in carcinogenesis of the breast may be the silencing of P-16 expression by gene methylation ( 50 ). Together, these studies suggest that Bmi-1 may regulate normal stem cell self-renewal through down-regulation of P-16. During carcinogenesis, the silencing of this gene through methylation may result in the constitutive expansion of the stem cell population. In a similar manner, dysregulation of Wnt signaling may allow for the expansion of colonic stem cells during early colon cancer carcinogenesis.
The stem cell model also has important implications for the development of markers for the early detection of cancer. Most currently used tumor markers, such as prostate-specific antigen for prostate cancer or CA125 for ovarian cancer, are the products of differentiated cells within tumors. If tumors are to be detected during earlier stages of carcinogenesis, it may be necessary to characterize and detect markers made by the cancer stem cell populations. There has been considerable excitement generated by studies that show that important clinical prognostic and predictive information can be obtained from determining the molecular expression profile of tumors. This is consistent with the hypothesis that these molecular profiles represent the cell of origin as well as the differentiation pattern produced by subsequent oncogenic events. We have proposed previously that the molecular classifications of human breast cancers by gene expression analysis may reflect different cellular origins of these subtypes ( 51 ). If tumors are driven by a stem cell component, then elucidation of gene signatures characteristic of these stem cells may provide important prognostic information. In support of this, Glinsky et al. developed an 11-gene signature whose expression was regulated by the stem cell self-renewal gene Bmi-1. Remarkably, expression of this “stem cell gene” signature was associated with a poor prognosis for 10 different types of human malignancies ( 52 ). These studies summarized in an accompanying editorial, “Stem Cell-ness: A Magic Marker for Cancer” ( 53 ), provide strong evidence for the clinical relevance of the cancer stem cell hypothesis. Despite the important prognostic value of tumor profiling, the cancer stem cell hypothesis predicts that there will be considerably less value in using molecular profiling to identify new therapeutic targets. If cancer stem cells comprise only a minor fraction of total tumor cells and if these cells drive tumorigenesis, then the profiling of purified populations of cancer stem cells may identify more important therapeutic targets than profiling the entire tumor.
The cancer stem cell hypothesis suggests avenues for cancer prevention. If stem cells are the targets of transformation, then strategies that reduce stem cell number might reduce cancer risk. The use of tamoxifen in primary breast cancer prevention might occur through such a mechanism. Furthermore, if early events in carcinogenesis involve expansion of the stem cell pool, then interventions that induce either apoptosis or differentiation with a loss of self-renewal capacity in these cells represent a rational therapeutic approach to cancer prevention. Although the concept of differentiation therapy for cancer is not new ( 54 ), development of agents that can specifically target initiated stem cells may provide opportunities to intervene at the earliest stages of carcinogenesis before significant genetic instability occurs. This highlights the importance of elucidating the pathways that control differentiation and survival in these cells.
Implications for cancer therapeutics. The cancer stem cell model has fundamental implications for the development of new cancer therapeutic agents. Antineoplastic agents have largely been developed through testing in animal models as well as phase II human trials. In both of these, the measured outcome has been shrinkage of tumors. Tumor response is usually defined in the clinic as the shrinkage of a tumor by at least 50%. However, if cancer stem cells are inherently resistant to therapeutic agents and if these cells comprise only a minority of the tumor cell population, then shrinkage of tumors may reflect the effects of these agents on the differentiated cells in a tumor rather than the cancer stem cell component. This may explain why in clinical trials for advanced cancers, tumor regression often does not translate into clinically significant increases in patient survival. This has been shown in many tumor types, including solid tumors and multiple myeloma, where patient survival does not correlate with changes in the M-protein levels ( 55 ). If the cancer stem cell hypothesis is valid, then we may need to devise new experimental paradigms other than assessment of tumor regression for the evaluation of antineoplastic agents. To develop therapies that target the cancer stem cell population, it will be important to find and validate intermediate end points that predict ultimate patient survival. For instance, future clinical trial design may use such intermediate end points as time to tumor progression following delivery of an agent that can target cancer stem cells.
Therapeutic resistance of cancer stem cells. By virtue of their fundamental importance in organogenesis, normal stem cells have evolved mechanisms that promote their survival and resistance to apoptosis. For example, during normal mammary involution following lactation, there is massive apoptosis of differentiated cells, whereas stem cells are spared and regenerate the gland during subsequent pregnancies. Inherent resistance of normal stem cells to apoptosis is also observed in patients undergoing cytotoxic chemotherapy. When patients are given nonmyeloablative doses of cytotoxic chemotherapy, they experience a transient decrease in their WBC counts. This is caused by apoptosis of differentiated neutrophils and myeloid precursors. Stem cells in the bone marrow are not ablated by these doses of chemotherapy and are able to regenerate a normal hematopoietic system after several weeks. Similarly, many of the gastrointestinal side effects of chemotherapy are caused by induction of apoptosis in differentiating colonic epithelial cells. These dying cells are regenerated by gut stem cells that survive these chemotherapeutic insults. Just as normal stem cells may be resistant to the induction of apoptosis by cytotoxic agents and radiation therapy, cancer stem cells may display increased resistance to these agents compared with more differentiated cells that comprise the bulk of tumors. Supporting this concept, Guzman et al. have shown that leukemic stem cells are more resistant to chemotherapy than are the more differentiated myeloblastic cells that constitute the vast majority of cells in leukemia ( 56 ). Similarly, Matsui et al. have shown that myeloma stem cells are resistant to many therapies being used to treat myeloma including chemotherapy and the proteosome inhibitor Velcade ( 35, 57 ). There are several molecular mechanisms that may account for the resistance to apoptosis of cancer stem cells. These include ( a ) cell cycle kinetics. Many cancer stem cells are not cycling and are in G 0 and thus resistant to cell cycle–specific chemotherapy agents ( 58 ). ( b ) DNA replication and repair mechanisms. Stem cells may be resistant to DNA-damaging agents by virtue of being able to undergo asynchronous DNA synthesis in addition to displaying enhanced DNA repair ( 59 – 63 ). ( c ) During asynchronous DNA synthesis, the parental “immortal” DNA strand always segregates with the stem cell and not the differentiating progeny. This process may be regulated by P53 ( 64 ). This prevents the stem cell compartment from accumulating mutations associated with replication or from being affected by DNA-damaging agents. ( d ) Antiapoptotic proteins. Stem cells express higher levels of antiapoptotic proteins, such as members of the Bcl-2 family and inhibitors of apoptosis, than do differentiated cells ( 65 ). ( e ) Transporter proteins. Stem cells express high levels of transporter proteins, such as ABCG2 (BCRP), as well as P-glycoprotein. The development of effective immunologic approaches to cancer therapy may also be affected by the existence of cancer stem cells. Many of these therapies have involved targeting cells that express tumor-specific antigens. These antigens may be selectively expressed on differentiated tumor cells. Cancer stem cells that do not express these antigens may thus be spared by these immunologic interventions.
The concept of cancer stem cells also has implications for the development of targeted therapies. Arguably, the most successful targeted therapy has been the development of imatinib that targets BCR-Abl in patients with CML. The vast majority of patients with early stages of CML are put into a remission by administration of imatinib. However, recent studies have suggested that although imatinib may target differentiated and progenitor CML cells, it does not eliminate CML stem cells that harbor this mutation. Following withdrawal of imatinib in animal models or the development of a resistant clone in patients, the disease reappears with kinetics predicted by a stem cell model ( 66 ). These studies suggest that the cure of CML will require the elimination of BCR-Abl containing CML stem cells.
If the ultimate cure of various cancers depends on the elimination of cancer stem cells, one can question why several malignancies, such as testicular carcinoma in men and choriocarcinoma in women, are curable with chemotherapy even in advanced disease, whereas the majority of common epithelial malignancies are not. One might speculate that the stem cell component of testicular carcinoma and choriocarcinoma are inherently different from those in other tissues, because these tumors arise in germ cells. Indeed, chemotherapy treatment of these tumors often produces residual masses that are benign teratomas composed of differentiated cells. An understanding of the inherent differences between stem cells of testicular cancer and choriocarcinoma compared with those from other tumors may provide new clues for the development of therapies for more common tumor types.
Opportunities for new therapeutics. The cancer stem cell model suggests that it may be necessary to alter the current paradigm in drug development. Eradication of cancers may require the targeting and elimination of cancer stem cells. Thus, one must devise strategies that can selectively kill these cancer stem cells while sparing normal stem cells, such as those in the gut and bone marrow. This represents a challenge because many pathways, such as those involved in self-renewal, are shared by cancer stem cells and their normal counterparts. However, a variety of recent studies using animal models that have targeted these pathways indicate the feasibility of this approach. For example, Notch signaling requires processing by the enzyme γ-secretase. An inhibitor of this enzyme has been recently shown to have activity against breast cancers that over express Notch1 ( 67, 68 ). Agents targeting Hedgehog signaling have recently been shown to have antineoplastic activity. The Hedgehog inhibitor cyclopamine that specifically inhibits Hedgehog signaling was used to treat animals bearing a variety of tumor xenografts. Cyclopamine caused dramatic regression of tumors that did not recur following cessation of treatment. Furthermore, at least over brief periods, the administration of these agents seemed to be nontoxic ( 17 ). A Hedgehog pathway inhibitor, HhAntag, with greater activity than cyclopamine has recently been shown to block medulloblastoma formation in a transgenic mouse model ( 69 ). These studies support the feasibility of selectively targeting the cancer stem cell population. The elimination of this key cell population may result in improved therapeutic outcomes for patients with even advanced cancers.
In the accompanying article, Hill presents his views that the current evidence is not conclusive regarding the existence of cancer stem cells in solid tumors. Although we agree that much remains to be learned about tumor stem cells, we feel that the substantial biological and clinical implications of this model justify intensive research in this area. Hill points to several theoretical and methodologic questions regarding the experimental evidence for the existence of cells with stem cell properties in solid tumors. He points out that the relative inefficiency of transferring human tumors to xenografts may be due to inherent inefficiencies in the systems rather than tumor subpopulations that differ in their tumorigenicity. We believe that the recent prospective identification of solid tumor stem cells in a variety of malignancies, including breast cancer, brain cancer, and prostate cancer, provide strong evidence that “not all cancer cells are equal.” Only a relatively small percentage of cells with characteristic cell surface markers are able to be serially passaged in immunocompromised mice, a demonstration of their self-renewal capacity. We do, however, agree with Hill that tumor stem cells may themselves be heterogeneous with varying self-renewal capacity. Indeed, as stated in our article, this has recently been shown for human leukemias. We also agree with his statement that the microenvironment is important in determining the behavior of transplanted cells. In our review, we stress the importance of the stem cell niche in normal stem cell function and the tumor microenvironment in tumor growth and progression. For this reason, solid tumor xenograft models of breast and brain cancer have used orthotopic installation of tumors to the mammary fat pad or brain, respectively. Hill contends that genetic instability drives tumor development, so that the relationship between stem cell behavior and differentiation might change during tumor progression. Although genetic instability undoubtedly plays an important role in tumor progression, molecular profiling studies suggest that the biological behavior of tumors is inherent in the initial tumor. This is more consistent with a tumor stem cell model in which tumor behavior is largely determined by the cell of origin and its genetic profile. Hill also speculates that differentiated tumor cells that have “lost the ability to manifest as cancer stem cells might regain the ability next week or next month.” However, we are unaware of any direct evidence of de-differentiation of tumor cells. In xenograft models, differentiated tumor cells from tumors fail to form tumors when transplanted even after long periods of observation. Finally, it is important to distinguish between markers that may serve to identify tumor stem cells from molecules that play important roles in stem cell behavior. In many cases, the markers present on tumor stem cells mimic that of their normal stem cell counterparts. Thus, the argument that proteolytic digestion of solid tumors changes cell surface markers has no direct bearing on the behavior of these cells when they are introduced into immunocompromised mice.
In summary, cancer stem cells were first described in human leukemias. Accumulating evidence in a variety of solid tumors suggest that these tumors may also be driven by a subset of cells that display stem cell properties. Further studies should lead to a greater understanding of the biology of these cells with significant implications for cancer treatment and prevention.
Note: Max Wicha has financial holdings and is a scientific advisor for OncoMed Pharmaceuticals.
Grant support: NIH grants R01-CA101860 and P30CA46592, Department of Defense grant BC030214, and The Susan G. Koman Foundation grant PDF0503599.
Citing articles via
Email alerts.
- Online First
- Collections
- Online ISSN 1538-7445
- Print ISSN 0008-5472

AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Do cancer stem cells exist? A pilot study combining a systematic review with the hierarchy-of-hypotheses approach
Roles Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Biology, Chemistry, Pharmacy, Institute of Biology, Freie Universität Berlin, Berlin, Germany
Roles Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing
Affiliations Department of Biology, Chemistry, Pharmacy, Institute of Biology, Freie Universität Berlin, Berlin, Germany, Leibniz-Institute of Freshwater Ecology and Inland Fisheries (IGB), Freie Universität Berlin, Berlin, Germany, Berlin-Brandenburg Institute of Advanced Biodiversity Research (BBIB), Freie Universität Berlin, Berlin, Germany
- Isabelle Bartram,
- Jonathan M. Jeschke

- Published: December 13, 2019
- https://doi.org/10.1371/journal.pone.0225898
- Reader Comments
The phenomenon of cancer cell heterogeneity has been explained by different hypotheses, each entailing different therapy strategies. The most recent is the cancer stem cell model, which says that tumourigenicity and self-renewal are restricted to rare stem cell-like cancer cells. Since its conception, conflicting evidence has been published. In this study, we tested the applicability of a new approach developed in the field of ecology, the hierarchy-of-hypotheses approach, for the Cancer Stem Cell hypothesis. This approach allows to structure a broad concept into more specific sub-hypotheses, which in turn can be connected to available empirical studies. To generate a dataset with empirical studies, we conducted a systematic literature review in the Web of Science limited to the first 1000 publications returned by the search. From this pool, 51 publications were identified that tested whether a cell sub-population had cancer stem cell properties. By classifying the studies according to: (1) assessed indicators, (2) experimental assays and (3) model cancer cells used, we built a hierarchical structure of sub-hypotheses. The empirical tests from the selected studies were subsequently assigned to this hierarchy of hypotheses, and the percentage of supporting, undecided and questioning evidence was calculated for each sub-hypothesis, as well as additional experimental characteristics. Our approach successfully allowed us to determine that within our dataset, the empirical support for the CSC hypothesis was only 49.0%. The support of different sub-hypotheses was highly variable. Most noticeable, the conception that putative cancer stem cells are a rare subset of cells could not be confirmed by most studies (13.5% support). The empirical support varied also between types of cancer, animal models and cell isolation method used. For the first time, this study showed the applicability of the hierarchy-of-hypotheses approach for synthesizing and evaluating empirical evidence for a broad hypothesis in the field of bio-medical research.
Citation: Bartram I, Jeschke JM (2019) Do cancer stem cells exist? A pilot study combining a systematic review with the hierarchy-of-hypotheses approach. PLoS ONE 14(12): e0225898. https://doi.org/10.1371/journal.pone.0225898
Editor: Daotai Nie, Southern Illinois University School of Medicine, UNITED STATES
Received: May 29, 2019; Accepted: November 14, 2019; Published: December 13, 2019
Copyright: © 2019 Bartram, Jeschke. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: This study has been financially supported by Freie Universität Berlin. Further support was provided by the Deutsche Forschungsgemeinschaft (DFG) to JMJ (grants JE 288/9-1, JE 288/9-2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The search for new cancer therapies is exacerbated by the fact that cancer is a highly heterogeneous disease. Cancer cells do not only vary phenotypically between patients and affected organs, but already within single tumours. This phenomenon was first explained by a clonal or stochastic model of cancer, where the heterogeneity of the cells is due to continuing mutagenesis [ 1 , 2 , 3 ]. Here, all cells have tumourigenic potential and, under the right external conditions, can re-establish a tumour. Early on it was challenged by the observation that in different kinds of cancers only a very small fraction of tumour cells proliferated when seeded in vitro or in vivo in mice [ 4 , 5 ] and that only certain primary tumour cells are capable to form metastasis in distant sites [ 6 ].
When Bonnet & Dick showed that in Acute Myeloid Leukaemia (AML), this subset of cells had an exclusive phenotype (CD34+/CD38–) [ 7 ], a new model to explain cancer cell heterogeneity was proposed: cancer stem cells (CSC) [ 8 , 9 ]. Here, a tumour is seen as an “abnormal organ” to which “the principles of normal stem cell biology can be applied” [ 8 ]. Analogous to normal tissues, the heterogeneous cell population of a tumour is exclusively replenished by multipotent CSCs that are able to self-renew and give rise to phenotypically different cells through asymmetric cell divisions. This concept is supported by the observation that many of the pathways that regulate self-renewal in stem cells were found to be active in cancer cells, such as the Wnt, the Sonic hedgehog (Shh) and the Notch pathway [ 8 , 10 ]. It also offers an explanation to the riddle why some cancer patients stay, seemingly cancer free, in remission for prolonged episodes of time before they relapse. Quiescence, a property of stem cells, would give CSCs the ability to survive treatment with chemotherapy in a dormant state to later re-enter the cell cycle and repopulate a tumour cell population [ 11 ].
While similar evidence was found for many cancer types, the CSC model has since been challenged as well. For each of the unique characteristics that were used to define CSCs as a separate population of cells (rarity, specific surface markers, tumourigenicity, differentiation potential, unlimited capacity for self-renewal, resistance against chemotherapy), contradictory evidence was found [ 12 ]. Additionally, further research has indicated that xenotransplantation, the “gold standard for in vivo testing” of the CSC model [ 11 ], might not be sufficient proof for an exclusive tumourigenicity of cells identified as CSCs. Quitana et al. found a surprising 25% of melanoma cells to be capable of tumourigenesis when they were injected into even more immunocompromised NOD/SCID IL2Rγnull mice (NGS) [ 13 , 14 ]. These tumourigenic cells could not be defined by CD133 or any other previously identified CSC marker and were phenotypically highly heterogeneous. It is thus arguable whether previous studies simply selected for cells with a unique capability to proliferate in mice rather than tumourigenic potential in humans. Another explanation for the conflicting results could be that some forms of cancer are indeed hierarchically organized with CSCs at the top, while others, like melanoma, are not.
Furthermore, a combination of both models is possible where CSCs do exist and are clonally selected, explaining the co-existence of different phenotypes of CSCs in studies on AML and glioblastoma [ 15 , 16 ]. Or maybe CSCs do exist but rather describe a transient state of cancer cells, which are able to switch their phenotype [ 17 , 18 ].
The two theoretical models are highly relevant because they result in different therapeutic strategies [ 19 ]. In the stochastic model, drugs must have a cytotoxic effect on all the heterogeneous cancer cell types of a tumour. In contrast, the existence of CSCs would have the attractive implication that it is only necessary to target a small fraction of cells in order to eradicate cancer from a patient. Additionally, stem cells have mechanisms of chemotherapy resistance such as a high expression of ABC transporters that if shared with CSCs need to be addressed in therapy development [ 9 ]. Accordingly, several new therapeutic strategies have been developed in the last years based on the assumption that CSCs are the drivers of cancer pathogenesis and progression. A focal area of research on these therapies has been the development of monoclonal antibodies or vaccines against the identified CSC markers such as CD133 or CD44, and small molecule inhibitors of receptors expressed predominantly by CSCs [ 20 , 21 , 22 ], although some researchers have criticized this strategy [ 12 , 19 , 23 , 24 ].
The hierarchy-of-hypotheses approach (HoH)
While much basic research exists on CSC, a comprehensive synthesis and re-evaluation of the available evidence is currently lacking. Thus, even while CSC-specific therapies are already being developed and clinically tested [ 25 ], it remains contested whether CSC exist or not. As with most topics in biomedical basic research, the existing reviews on CSC are traditional narrative reviews that do not specify the process by which the authors have selected the included studies. On the other hand, formal meta-analysis sensu stricto can only be applied to research results that are either given in the same effect size (e.g. Hedges’ g) or are reasonably transformable to the same effect size [ 26 ]. Even if it is mathematically possible to express results in the same effect-size metric, it is not always clear if this is advisable, as effect sizes and their interpretation can sometimes genuinely vary among model systems, methods or spatiotemporal scales. To address these challenges of research synthesis, a method was recently developed within the field of ecology. This method is called the hierarchy-of-hypotheses (HoH) approach. One of its purposes is to help systematically analyse and contextualize empirical data and information. Thus far, the approach has been applied to test popular concepts in the field of invasion ecology for their actual empirical support [ 27 , 28 , 29 , 30 ]. The approach can be combined with formal meta-analytical methods.
In the HoH approach, the overarching hypothesis is divided into sub-hypotheses to create a hierarchical structure into which every empirical study in question is then sorted. By assessing the amount of support for each sub-hypothesis on the lowest hierarchical level, the overall support for higher-level sub-hypotheses and the overarching hypothesis can be assessed. The HoH approach allows to reduce complex hypotheses and makes it possible to visualize the network of relationships between (sub-) hypotheses, thereby also pointing out current research gaps [ 31 ]. Thus, an HoH analysis of existing experimental data on CSCs will make it possible to gain conceptual clarity and systematically test empirical support for this important and influential hypothesis on a large scale.
This study is a first feasibility test of the HoH approach in the biomedical research field, specifically for basic CSC research. Its objective is to investigate if the HoH approach is a useful method to structure the highly variable existing evidence surrounding the CSC model, and if it is helpful to test its different sub-hypotheses, e.g. the individual characteristics that are used to define CSC. Applying the HoH approach, it should also be possible to assess the amount of support for the CSC model in different research contexts such as type of cancer, used model organism or cell lines.
Materials & methods
To generate a dataset with all available empirical studies on cancer heterogeneity, we conducted a systematic literature review following the PRISMA-P 2015 guidelines for systematic reviews and meta-analyses (see PRISMA diagram in S1 Fig ; PRISMA checklist in S1 Table ) [ 32 ]. First, we systematically searched the Web of Science (WoS) that tested the broad definition of the CSC hypothesis. Using the terms “cancer stem cells” or “tumor-initiating cells” or “tumour-initiating cells” or “tumorigenic cells” or “tumourigenic cells” or “tumor heterogeneity” or “tumour heterogeneity" on 6 September 2016. As this work is a first test whether the HoH approach is a useful method for CSC research synthesis in principle, we limited the scope of the study to the first 1000 publications (sorted by publication date) out of a total of 14,291 publications returned by the search. The truly relevant publications were identified by their titles and abstracts (returning 115 possibly relevant publications) and then thoroughly reading the publications in question. In this way, we identified a sample of 51 publications that tested whether a certain cell sub-population had CSC properties–independently of whether they were called as such or not. We thereby relied on the consensus definition of stem cells from the AARC cancer stem cell workshop [ 32 ], which defines a CSC as “a cell within a tumour that possess the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumour.” Additional characteristics ascribed to CSCs when the concept was conceived and still implemented by current studies are rarity and the expression of stem cell markers [ 8 , 33 ]. We thus selected all studies that tested whether a subset of cancer cells complied with these characteristics compared to bulk tumour cells. Studies that only used cells the authors defined as CSCs and did not compare them to the rest of the cancer cell population were rejected. We also excluded theoretical (modelling) studies and reviews. In other words, the HoH analysis only included primary empirical studies.
From each of these 51 publications, we extracted the separate empirical tests the authors had performed, and recorded for each of them in a database: cancer type, model cancer cells (primary tumour cells, cell line, patient-derived xenograft …), animal model, CSC isolation method, indicator, assay, number of patient samples or cell lines tested, and result of the respective test.
To build an HoH of the CSC model, we defined different sub-hypotheses by classifying the evidence on three levels: (1) indicators assessed to test the existence of CSCs, (2) experimental assay and (3) model cancer cells used. On the 1st level of sub-hypotheses, the different indicators that are used to define CSCs in contrast to the bulk of cancer cells are located. As outlined above, these are the indicators ubiquitously used as defining characteristics of CSCs [ 8 , 33 , 34 ]. Further down, the 2nd level of sub-hypotheses contains the type of assays used to measure the respective indicators. For instance, differences in tumourigenicity between certain cancer cell subsets can be assessed by different empirical tests: by comparing the overall tumourigenic capacity of cell subsets (as measured by respective latency, number of tumours induced or overall tumour volume) after subcutaneous injections or orthotopic transplantations of cancer cells in vivo; or by comparing the frequency of tumour-inducing cells determined by limiting dilution injections in vivo. On the 3rd level of sub-hypotheses, the different origins of cells used in the assays are discriminated: human or other species, cultured or primary cancer tissue samples.
Finally, each empirical test recorded in the systematic review above was assigned to the sub-hypotheses at the lowest level, and was classified as either supporting, being undecided or questioning the sub-hypothesis in question (if the evidence was conflicting because e.g. the results from different cell lines tested were contradictory, the test was classified as “undecided”). For this scoring approach, we considered primarily effect sizes and the results of statistical significance tests given by the respective publications. The following specifications were made: the characteristic “rarity” is a major aspect of the CSC concept, but is not statistically evaluated in most studies. Here, a percentage of cells under 1% of the whole cell population was defined as “rare”. Another specification was necessary for assessing the support from expression analysis of RNA or protein markers. In many papers, several markers are assessed and therefore we set a cut-off at more than 50% of measured genes or proteins that had to be significantly differentially expressed to be counted as “supporting” evidence. If no measured stem-cell marker was differentially expressed, the evidence was counted as “questioning”, in-between as “undecided”. Many tumourigenicity assays only included a very small number of mice, and study authors often did not calculate statistical significance. But the evidence was still counted as “supporting” under the condition that tumours did form when the cell set defined as CSC was injected, while no tumours formed in mice when bulk tumour cells were injected. Please note that the HoH approach can also be combined with a fully quantitative meta-analytic framework if effect sizes are comparable, but this was not the case here, thus we applied the semi-quantitative scoring approach outlined in this paragraph.
To evaluate the support for the CSC model, the percentage of supporting, undecided and questioning evidence was calculated for each sub-hypothesis on all levels of the HoH. To account for the fact that most studies included several empirical tests, which are thus not independent of each other, each publication was only included once for each sub-hypothesis or the main hypothesis. Additionally, the amount of support was calculated in the same manner for different types of cancer, different model organisms and types of cancer cells used. We used a Mann-Whitney U-test to test for significant differences between the empirical support for sub-hypotheses and between different kinds of cancer etc. where the number of studies was ≥5. Statistical tests were performed using the software PAST version 3.23 (March 2019) [ 35 ].
The 51 relevant publications that we identified studied putative CSCs in very different kinds of cancer using different model systems and different laboratory methods. As the authors tested several components of the CSC hypothesis in each study, the resulting number of empirical tests was 174 (on average 3–4 tests per study). A table with all empirical tests analysed is included in the S2 Table . We then assigned these tests to the respective sub-hypotheses and evaluated the empirical support for each of them and, due to the HoH approach, also for broader, higher-level sub-hypotheses. Fig 1 outlines the HoH structure for the CSC hypothesis, including the number of empirical tests summarized on each level and the amount of support for each sub-hypothesis.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
The sub-hypotheses were defined by classifying the evidence on three levels: (1) indicators assessed to test the existence of CSCs, (2) experimental assay and (3) model cancer cells used. The empirical tests of analysed 51 studies were sorted into the HoH. Green indicates that more than 50% of the studies supported the sub-hypothesis; red indicates that more than 50% questioned the sub-hypothesis; yellow indicates sub-hypotheses for which neither supporting nor questioning evidence reached 50%. The number of studies with supporting / undecided / questioning evidence is indicated for each hypothesis in parentheses.
https://doi.org/10.1371/journal.pone.0225898.g001
The detailed results of the HoH analysis are illustrated in Fig 2 . Within the 51 studies, the overall empirical support was 49.0% and highly variable for each second-level sub-hypothesis ( Fig 2A ). Most noticeable, the conception that putative CSCs are a rare subset of cells (for this analysis defined as 1% of tumour cells or less) could not be confirmed by most studies (13.5% support). The amount of supporting evidence was significantly higher in all other sub-hypotheses on the second level of the HoH.
The empirical evidence of each evaluated test was categorized as either supporting (S), being undecided (U) or questioning (Q), based on the data reported in the respective studies. N is the numbers of studies that tested the sub-hypotheses. Where the level of empirical support was significantly different (p<0.05, Mann Whitney U-tests), the respective p-value is specified.
https://doi.org/10.1371/journal.pone.0225898.g002
The analysis also indicates that the support for the CSC concept varies between what cells were used–while most experiments using primary tumour cells did not support the CSC model, those that used cultured cells mostly confirmed it ( Fig 2B ). While more than half of the studies that used cells that grew selectively in stem-cell medium or expressed CD90 as putative CSCs found supporting evidence, studies that used other surface markers or side population staining did not. The level of support for the CSC model also varied greatly by which cancer type was investigated. While all studies from research on glioblastoma multiforme and ovarian cancer found evidence for the existence of CSCs, most studies in our dataset that investigated breast cancer did not ( Fig 2D ). Likewise, the support varied with the mouse model used in the studies. While most tumourigenicity tests using the NOD/SCID mouse model could confirm a CSC subset (75.0%), the level of support from tests using the more immunodeficient NGS mouse model was low (25.0%, Fig 2E ).
Applying the HoH approach to studies in CSC research revealed highly interesting results, but of course a drawback of this pilot study is that only a fraction of the available literature was analysed. Extrapolating from this analysis, another 700–800 relevant publications are available in the literature.
Our results demonstrate that a comprehensive evaluation of all CSC studies available would be very valuable for the research community. For example, the notion that CSCs are a rare subset of cells within a tumour could face challenge if most tests reject this hypothesis also in a larger dataset. This would have implications for the idea that it is possible to develop drugs targeting a small number of CSCs to eradicate cancer from a patient. Also, several of the markers like CD133, which was thought by some to be a universal CSC marker [ 22 ], failed to define CSCs in a significant portion of empirical tests. Possibly the CSC phenotype is defined by different markers in different cancer types after all. A future lager dataset should also record cancer sub-types, as it is possible that even sub-types could feature different CSC markers.
In our analysis, the majority of tumourigenicity assays did show that the ability to form tumours was indeed limited to the subset of putative CSCs. But as others have observed [ 14 ], outcomes varied greatly by the used mouse model. This finding was not associated with different experimental contexts, e.g. different cancer types. Thus, the mouse model itself potentially influences the experimental outcome. The often used NOD-SCID mice are highly immunocompromised, but still tumour tissues often fail to engraft as some immune function remains. NGS mice additionally bear a mutation in the interleukin-2 gamma chain receptor that renders them highly receptive to engraftment of human cells [ 36 ]. Our results indicate that tumourigenicity assays used in the evaluated studies could simply select for cells that are good at evading the immune system of immunocompromised mice, not cells uniquely able to generate new tumours. Furthermore, experiments that used primary human cells from patients more often failed to prove the existence of CSCs in tumourigenicity tests. In contrast, the majority of experiments using cultured cells supported the CSC model. Replicated in a larger dataset, this could challenge the notion that cultured cells can be used as a simple substitute for gaining knowledge on tumours in patients. This is of course not a new observation–a recent study showed that the breast cancer cell line MCF7, which was also used in five studies in our dataset, is very different from the original patient sample and has become highly unreliable due to its many genetic and epigenetic alterations gained in culture [ 37 ].
If these results can be confirmed in a larger analysis, the implications for CSC research would be immense. One explanation for our findings could be that CSC is an unstable tumour cell state. Although when the CSC model was conceived it was (and still is) unclear how CSC emerge, the original model does not suggest that CSC is a highly plastic phenotype. Since then, some authors have proposed a redefinition where tumour cells can shift back and forth between CSC and non-CSC phenotype in response to environmental conditions as their findings did not match the model [ 17 , 18 ]. In our HoH, we applied the original hypothesis to first synthesise all available evidence and settle whether this original CSC model holds true–or which sub-hypotheses of the model are supported by evidence, and under which experimental conditions the CSC model can be corroborated. In a second step, a new model could then be proposed and tested. This approach would provide the urgently needed clarity of terminology, as the term CSC at this point has different meanings to different scientists.
In the field of invasion ecology, the HoH approach has helped to demonstrate that several intuitively appealing and widely used hypotheses are actually not supported by the majority of available empirical studies [ 30 ]. Similarly, this form of analysis could provide a useful tool to let go of disproven, but appealing hypotheses in the biomedical field–or provide a substantial empirical basis for their support. Additionally, the HoH analysis proved to be a useful tool to structure and categorize the seemingly heterogeneous research results surrounding the concept of CSCs. Up close, the 51 studies analysed all included very similar experiments to test the same characteristics. While it would have been difficult to summarize the unstructured results, the HoH analysis made it possible to compare different contexts and evaluate the support for the overarching hypotheses of the CSC model.
Basic research has recently been under much discussion for a possible “reproducibility crisis” with one culprit being the lack of published “negative” data, meaning results that do not show statistical differences or pointing in the “wrong” direction [ 38 , 39 , 40 , 41 ]. There has been a special concern for published basic cancer research after two pharmaceutical companies revealed that they could only reproduce a fraction of landmark studies [ 42 ]. In our preliminary analysis, we noticed that a major part of publications used different cell lines for different assays without giving an explanation. A possible reason for this phenomenon is that they performed the assays with all cell lines, but only presented the data that demonstrated a significant difference. Of course, we do not know if that was really the case. Such a practice would have a highly distorting effect on the available evidence that would be mirrored even in the most comprehensive HoH analysis. Thus, an ideal HoH would also include unpublished results. A solution could be an interactive open-access online platform where researchers can enter their data and in turn receive detailed information on overall support for the different sub-hypotheses in the exact context they are investigating or plan to investigate.
A further challenge that is not unique to the HoH approach, but shared by all quantitative and semi-quantitative meta-analyses sensu lato is the question of interdependence. Some publications include multiple tests of different sub-hypotheses, which are obviously not independent of each other. Other authors publish such tests of different sub-hypotheses in several smaller publications, which are again not independent of each other. Frequently, the same data are re-used in different publications, sometimes by different author teams. Studies based on different data but done by the same people are also not independent of each other. Even studies from different research groups are not necessarily independent of each other, as scientists influence each other through educational, institutional and specialization ties [ 43 ]. Co-author network analyses from different scientific disciplines revealed that relationships between researchers are highly clustered in “small worlds” where knowledge and resources are shared [ 44 ]. While knowledge flows between scientists have long been a study subject within social and data sciences by means of network analysis [ 43 , 45 , 46 ], to our knowledge these insights have not yet been integrated into meta-analyses on biomedical (or any other natural science) research. A first effort in this direction is a recent study from the field of biogeography [ 47 ] in which there was indeed a statistical association between the group of authors conducting a study and the outcome of the study. In further developing the HoH approach, these and other factors could be included in an “independence weight” of study outcomes, e.g. effect sizes reported in a study.
Supporting information
S1 fig. prisma 2009 flow diagram..
From Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi: 10.1371/journal.pmed1000097 . For more information, visit http://www.prisma-statement.org .
https://doi.org/10.1371/journal.pone.0225898.s001
S1 Table. PRISMA 2009 checklist.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6(7): e1000097. doi: 10.1371/journal.pmed1000097
https://doi.org/10.1371/journal.pone.0225898.s002
S2 Table. Dataset of empirical tests.
Tests and results from 51 studies testing the Cancer Stem Cell hypothesis that were included in our analysis.
https://doi.org/10.1371/journal.pone.0225898.s003
Acknowledgments
We appreciate helpful comments and suggestions by Claudia Baldus.
- View Article
- PubMed/NCBI
- Google Scholar
- 26. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Converting among effect sizes. In: Introduction to Meta-Analysis. West Sussex, England: John Wiley & Sons, Ltd.; 2009: 45–49.
- 30. Jeschke JM, Heger T. Invasion Biology: Hypotheses and Evidence. Wallingford, UK: CABI; 2018.
- 45. Crane D. Invisible Colleges: Diffusion of Knowledge in Scientific Communities. Chicago, USA: University of Chicago Press; 1972.
- Around the Practice
- Between the Lines
- Contemporary Concepts
- Readout 360
- Insights from Experts at Mayo Clinic on Translating Evidence to Clinical Practice
- Optimizing Outcomes in Patients with HER2+ Metastatic Breast Cancer

- Conferences
- Publications
Growing Evidence Supports Stem Cell Hypothesis of Cancer
During the past 18 months, researchers have developed substantial evidence supporting the notion that stem cells play a critical role in the development of at least some cancers, their progression, and the prognosis of patients, including breast, brain, lung, and prostate cancer, multiple myeloma, and melanoma.
BETHESDA, MarylandDuring the past 18 months, researchers have developed substantial evidence supporting the notion that stem cells play a critical role in the development of at least some cancers, their progression, and the prognosis of patients, including breast, brain, lung, and prostate cancer, multiple myeloma, and melanoma.
"The idea of stem cells in cancer is a very old one, but it is only recently that scientists have had experimental models that actually validate this," Max Wicha, MD, professor of internal medicine and director of the University of Michigan Comprehensive Cancer Center, Ann Arbor, said at a meeting of the National Cancer Advisory Board (NCAB). "It represents a paradigm shift in how we need to approach cancer because it has very wide clinical implications."
The stem cell hypothesis challenges the classic stochastic model, which holds that cancer results from a random mutation in a cell that reproduces and eventually forms a malignant neoplasm. In contrast, the stem cell model suggests that in many instances, stem cells or their immediate progeny are the cells transformed during carcinogenesis, and that only these cells are capable of self-replication within a tumor. All other cells in a cancer have lost their ability to self-renew and are in various stages of differentiation.
Moreover, if these differentiated cells should escape the primary tumor and travel to other parts of the body, they would not grow metastases of clinical consequence. This means that once a cancer develops, its growth is driven by a small number of cellsperhaps as few as 100which have the two distinguishing properties of stem cells, namely the ability to make exact copies of themselves and to differentiate.
In the Michigan team's scenario, a stem cell that reaches a distant site might settle in a microenvironment that fails to support its immediate proliferation. This could explain the dormancy of tumors and their late emergence when the environment becomes right to put the cell back into cycle.
"If the stem cell model is correct, then we have to reexamine, in a very critical way, the preclinical models for therapeutic development," Dr. Wicha said. "We have to look at the endpoints for clinical trials, which may not be adequate because the tumor stem cells may be resistant to these therapies. And we think effective therapies will need to target the tumor stem cell population while sparing normal stem cells. Our laboratory and others are working on potential strategies to target this cancer stem cell population." Dr. Wicha is one of the founders of OncoMed Pharmaceuticals, a California-based biotech company that is developing technology to target cancer stem cells.
Five years ago, Canadian scientists suggested that stem cells were key players in leukemia. Dr. Wicha, working with Michael F. Clarke, MD, now at the Stanford University Comprehensive Cancer Center, began investigating the role of stem cells in breast cancer.
Dr. Wicha and his colleagues have isolated stem cells in the breast and characterized how they change as normal breast tissue becomes cancerous. They have concluded that the cells involved are mammary stem cells whose ability to differentiate is limited to the cell types that occur in the breast. These cells appear to undergo mutation as the result of a genetic instability or exposure to some damaging environmental cause, which, in turn, destabilizes the process that regulates the cells' self-renewal ability.
"More importantly for therapeutics, there is now emerging evidence that both normal stem cells and these transformed counterparts are highly resistant to our therapies, which has lots of implications," Dr. Wicha said. "Even the metastasis of cancer is probably related to the homing of stem cells, in that both normal and transformed stem cells use very similar receptors as they spread to distant sites."
The Michigan researchers employed cell markers to eventually identify two variations of a cell designated B38+CD44, an extracellular matrix receptor, that differed by having or not having the marker CD24, an adhesion molecule. Using NOD/SCID mice, the researchers made a surprising discovery. Mice injected with as many as 20,000 CD24-positive cells from human breast tumors failed to develop cancers. However, mice injected with CD24-negative cells all developed cancerous tumors within 12 weeks, even with as few as 200 of the cells injected. "If you take unsorted cells, you have to put in about 50,000 cells to get tumors," Dr. Wicha said.
Flow cytometry studies of cells positive and negative for CD24 have ruled out the possibility that the tumors that developed in the animals resulted from the inadvertent selection and injection of a highly metastatic clone, he said.
Investigators have identified several signaling pathways, eg, Notch, Hedgehog, Bmi-1, and Wnt, believed to be involved in stem cell self-renewal and tumorigenesis, which have yielded insights into the normal and abnormal regulation of the cells. Researchers have begun sorting out how the pathways may go awry and produce an expanded pool of stem cells that, in turn, provides additional targets for further transforming events. In studies of the Hedgehog pathway, Dr. Wicha and his colleagues have created human stroma in mice using human mammary stem cells obtained from tissue removed from women during reduction mammoplasties. When they perturbed the pathway, the mice developed human ductal carcinoma in situ, which suggests that a skewed Hedgehog signaling pathway plays a role in early breast cancer and may provide a target for halting or reversing a cell's momentum to malignancy.
The Michigan team also has identified Bmi-1, an important transcription factor, as one pathway that appears to regulate the unique renewal and differentiation properties of stem cells in normal and cancerous stem cells. Now they are trying to determine how its deregulation might contribute to the growth and spread of cancer.
"These stem cells have a very different genetic profile in terms of gene expression than do the vast majority of cells that form the bulk of the tumor," Dr. Wicha said. "What is clear is that patients who had the so-called stem cell profile had a very poor survival in comparison to those who did not have the stem cell phenotype."
Other researchers have made the same finding in other cancers, "suggesting a commonality of stem cells in a variety of cancers that carry this prognostic indication," he added. "The molecular profiling studies, which have been quite exciting, are actually telling us what the stem cell of the tumor is and the differentiated progeny that is produced."
Implications for Oncology
The stem cell model, if confirmed, carries enormous implications for oncology. In Dr. Wicha's view, identifying and eliminating mutated stem cells, or forcing them to differentiate, may one day become an important prevention strategy. Moreover, the genetic profiles may reflect the genetic make-up of the stem cell from which a tumor arose.
From their work, he and his colleagues have proposed that basal breast cancers, which have a poor prognosis, result from a stem cell that is estrogen-receptor (ER) negative. ER-positive breast tumors may have either a bad or good prognosis. ER-positive luminal B breast tumors, which have a very poor prognosis, appear driven by a stem cell that itself is ER negative. The stem cell driving luminal A tumors and their good prognoses apparently is ER positive.
Thus, effective therapy and, perhaps, prevention would need to focus on the stem cell underlying a cancer. Killing a tumor's differentiated cells but not its driving stem cells may explain why patients have tumor shrinkage that has no impact on their survival. "We need to be targeting the stem cell population," Dr. Wicha said. "And we believe that in certain tumors, like testicular cancer, that are curable by chemotherapy, that is exactly what happens."
Survival Outcomes Improve With TIL Presence in TNBC
An increased TIL count in the breast tissue was associated with improved distant recurrence-free survival in patients with triple-negative breast cancer.

Achieving Health Equity in Lung Cancer Surgery
Rian M. Hasson Charles, MD, MPH, FACS, discusses advances in equitable lung cancer screening and her experiences as a woman in thoracic oncology.
Updates in Breast Cancer Care From the 41st Annual Miami Breast Cancer Conference
Several presentations from the 41st Annual Miami Breast Cancer Conference focused on new and evolving data in the space, according to Neil M. Iyengar, MD.

Managing CDK4/6 Inhibitor, ADC Toxicity in Metastatic Breast Cancer
Sarah Donahue, MPH, NP, speaks to the importance of communicating potential adverse effects associated with treatments such as CDK4/6 inhibitors to patients with breast cancer.

72 A Novel Biosignature for Early- Stage Invasive Breast Cancer to Predict Radiotherapy Benefit and Assess Recurrence Risk for Patients Treated With Breast- Conserving Surgery

ODAC Approves MRD End Point in Multiple Myeloma Trials
Minimal residual disease can now be considered an end point in trials leading to accelerated approvals by the FDA for multiple myeloma.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

The cancer stem cell hypothesis: failures and pitfalls
Affiliation.
- 1 Department of Neurosurgery, University of Florida, Gainesville, Florida, USA. [email protected]
- PMID: 21135745
- DOI: 10.1227/NEU.0b013e3181ff9eb5
Based on the clonal evolution model and the assumption that the vast majority of tumor cells are able to propagate and drive tumor growth, the goal of cancer treatment has traditionally been to kill all cancerous cells. This theory has been challenged recently by the cancer stem cell (CSC) hypothesis, that a rare population of tumor cells, with stem cell characteristics, is responsible for tumor growth, resistance, and recurrence. Evidence for putative CSCs has been described in blood, breast, lung, prostate, colon, liver, pancreas, and brain. This new hypothesis would propose that indiscriminate killing of cancer cells would not be as effective as selective targeting of the cells that are driving long-term growth (ie, the CSCs) and that treatment failure is often the result of CSCs escaping traditional therapies.The CSC hypothesis has gained a great deal of attention because of the identification of a new target that may be responsible for poor outcomes of many aggressive cancers, including malignant glioma. As attractive as this hypothesis sounds, especially when applied to tumors that respond poorly to current treatments, we will argue in this article that the proposal of a stemlike cell that initiates and drives solid tissue cancer growth and is responsible for therapeutic failure is far from proven. We will present the point of view that for most advanced solid tissue cancers such as glioblastoma multiforme, targeting a putative rare CSC population will have little effect on patient outcomes. This review will cover problems with the CSC hypothesis, including applicability of the hierarchical model, inconsistencies with xenotransplantation data, and nonspecificity of CSC markers.
Publication types
- Neoplasms / pathology*
- Neoplasms / therapy
- Neoplastic Stem Cells / pathology*
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Life (Basel)

The Concept of “Cancer Stem Cells” in the Context of Classic Carcinogenesis Hypotheses and Experimental Findings
In this Commentary , the operational definition of cancer stem cells or cancer initiating cells includes the ability of certain cells, found in a heterogeneous mixture of cells within a tumor, which are able to sustain growth of that tumor. However, that concept of cancer stem cells does not resolve the age-old controversy of two opposing hypotheses of the origin of the cancer, namely the stem cell hypothesis versus the de-differentiation or re-programming hypothesis. Moreover, this cancer stem concept has to take into account classic experimental observations, techniques, and concepts, such as the multi-stage, multi-mechanism process of carcinogenesis; roles of mutagenic, cytotoxic and epigenetic mechanisms; the important differences between errors of DNA repair and errors of DNA replication in forming mutations; biomarkers of known characteristics of normal adult organ-specific stem cells and of cancer stem cells; and the characteristics of epigenetic mechanisms involved in the carcinogenic process. In addition, vague and misleading terms, such as carcinogens, immortal and normal cells have to be clarified in the context of current scientific facts. The ultimate integration of all of these historic factors to provide a current understanding of the origin and characteristics of a cancer stem cell , which is required for a rational strategy for prevention and therapy for cancer, does not follow a linear path. Lastly, it will be speculated that there exists evidence of two distinct types of cancer stem cells , one that has its origin in an organ-specific adult stem cell that is ‘initiated’ in the stem cell stage, expressing the Oct4A gene and not expressing any connexin gene or having functional gap junctional intercellular communication (GJIC). The other cancer stem cell is derived from a stem cell that is initiated early after the Oct4A gene is suppressed and the connexin gene is expressed, which starts early differentiation, but it is blocked from terminal differentiation.
“ The biochemistry of cancer is a problem that obligates the investigator to combine the reductionalistic approaches of the molecular biologists with the holistic requirements of hierarchies within the organism. The cancer problem is not merely a cell problem, it is a problem of cell interactions, not only within tissues but also with distal cells in other tissues ” [ 1 ] . “Some would argue that the search for the origin and treatment of this disease will continue over the next quarter century in much the same manner as it already has in the recent past, by adding further layers of complexity to a scientific literature that is already complex beyond measure. But we anticipate otherwise: those researching the cancer [or any other human disease] problem will be practicing a dramatically different type of science than we have experience over the last 25 years. Surely much of this change will be apparent on the technical level. But ultimately the more fundamental change will be conceptual” [ 2 ]. “Personalized medicine is the latest promise of a gene-centered biomedicine to provide custom-tailored to the specific needs of patients. Although surrounded by much hype, personalized medicine lacks the empirical and theoretical foundations necessary to render it a long-term perspective . In particular, the role of genetic data and the relationship between causal understanding, prediction, prevention and treatment of a disease need clarifying” [ 3 ].
1. Introduction: How Some Historic Experimental Findings and Hypotheses of Cancer Shaped Today’s Concept of Cancer Stem Cells
These three introductory quotes embody much of my concern in this Commentary as it concerns the concept of cancer stem cells . They span decades of cancer research by different disciplinarians, involving years of experimental research and philosophical reflection of their experiences of their field.
In this Commentary , I rely on those giants of cancer research and my own research experience of 50 years, to try to make sense of both great discoveries and ideas, as well as confusing and often contradictory uses of terms and concepts. With that as a framework of what is to follow in a non-linear historical fashion, I will try to use both experimental findings and concepts, as well as my own historic and philosophical musings, to generate a view of cancer stem cells that is testable.
Because it has long been known that cells derived from a patient’s cancer could outlast the patient, from whom they were derived, and could be perpetuated either in vitro or in experimental animals, it was assumed to have developed the property of immortality during the initiation of the carcinogenic process. Take for example, the HeLa cell line, derived from Henrietta Lang, which has been studied in laboratories all over the world and has been subject of thousands of research papers [ 4 ]. Consequently, one objective of many studies was to induce immortality in normal cells, in vitro, in order to determine the mechanism by which this happens and to use this protocol to determine if any physical, chemical or biological agent might be a carcinogen . Although there was some initial success using rodent cells for this purpose [ 5 ], trying to immortalize normal human cells met with failure [ 6 , 7 , 8 ]. When the new concept of oncogenes was introduced, genetically-engineering normal rodent cells with specific DNA sequences led the way to get insights on the immortalizing normal human cells [ 9 , 10 , 11 ].
However, before the next breakthrough that seemed to provide another view of the immortalizing process, the concept of cancer stem cells has to be viewed from the perspective of many classical experimental animal cancer studies and those from epidemiology. In many of today’s studies of cancer stem cells , these classic studies have been largely ignored. When Percivall Potts correlated the unusually high frequency of scrotal cancers in chimney sweepers and the soot from the combustion of fuel [ 12 ], a link was formed to chemicals in the soot that seemed to be carcinogen. One school of thought was that these chemicals must have an irreversible effect on the genome (DNA) and later studies did show some of these chemicals could attack the DNA [ 13 ]. However, at that time, animal experiments [ 14 , 15 ], in general, seemed to indicate that the carcinogenic process was not a one-hit process, by which a single normal cell could be irreversibly altered to become an invasive, metastatic cancer cell [ 16 , 17 , 18 ]. The new concept emerged suggesting that carcinogenesis was a multi-step, multi-mechanism process, consisting of an initiation of a single “normal” cell to become “immortal”, followed by a promotion event over a long period of regular exposures at a threshold level to clonally amplify this single “initiated” cell into a benign tumor, which then transitioned to become an invasive and metastatic cell by the progression process.
Another important concept that added to this classic understanding of this initiation/promotion/progression process was that, even though all the cells in the tumor appeared to be heterogeneous in terms of their genotypes and phenotypes, they were derived from a single common “normal” ancestor [ 19 , 20 ].
Added to this new concept were two opposing hypotheses as to which single cell gave rise to these cancer cells. The stem cell hypothesis [ 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ], and the de-differentiation or re-programming hypothesis [ 29 ] emerged. The late Dr. Van R. Potter conceptualized the stem cell hypothesis as: “Oncogeny as partially-blocked ontogeny” [ 1 ].
2. The Stem Cell versus the De-Differentiation Hypotheses: The Origin of the “Cancer Stem Cell”
Now back to how the concept of cancer stem cells emerged and how the current controversy of the origin of the cancer stem cells seems to be unresolved. In the history of science, the intellectual journey from the starting idea of any explanation for a disease causation to the current hypothesis is never a linear journey. Ideas that ultimately get to the current state come by jumps, starts, rejection or refinement of disproven paradigms, parallel experimental, methodological or conceptual advances in different disciplines, and of course, serendipity. To finally merge all these disconnected ideas, it takes what was once said: “ Research is to see what everyone has seen and think what nobody has thought” [ 30 ]. To make clear, several words have been used in the history of trying to understand the mechanisms of carcinogenesis, namely, normal cells, carcinogens and immortal , are now seen as very misleading. As will be shown later, what is the normal cell that is being converted to ultimately become a cancer stem cell ? What is a carcinogen, a concept that implies an agent that can induce all three phases of the multi-step, multi-mechanism process of carcinogenesis, even after a single exposure? Last, is a cell being classified as immortal due to an induced change in the phenotype caused by some irreversible process, such as a mutation, or by this mutation causing the cell to stay in its natural immortal state. These are important issues that need to be resolved.
While this brief review of the history of carcinogenesis is not linear in time, in retrospect, the early events now make sense in view of recent characterizations of stem cells. Recall the early concept that stem cells might be the target cells for the start of the carcinogenic process; when this concept was developed, no one had even isolated or characterized a real stem cell. The idea of stem cells in developmental biology and embryology was a reasonable logical concept. After it was shown that in vitro experimental approaches, using soft agar growth, might help us to understand the mechanisms of carcinogenesis, the induction of apparent tumors that appeared when normal cells were exposed to some agent (physical, chemical, biological). To test if these abnormal looking clones of cells are really tumorigenic, they were placed in soft agar or injected back into an immune-deficient rodent. If tumors appeared after this procedure, the agent that brought about the in vitro and in vivo tumors was assumed to be a carcinogen and probably a mutagen. However, if only a few cells of the soft agar clones were used to be injected in the immune-deficient rodent, no tumors were seen in vivo, unless large numbers were injected. At the time of these early experiments, no one had any idea why this was the case. In addition, several in vitro assays to determine if an agent that contributed to this conversion of a “normal” cell to one that gave rise to a tumor in an immune-deficient rodent, it was assumed to be a mutagen or genotoxicant. The Ames assay and many other so-called mutation assays were shown to be non-consistent with one another for a number of reasons. Even later, when real mutations at the molecular level were found, both in vitro and in vivo, in the cells shown to be tumorigenic, problems of interpretation again arose, since the DNA studies were not determined to be either the genomic DNA or mitochondrial DNA or that the methods of isolating the DNA might have contributed to the measurements. In brief, major challenges to the interpretation of these mutation assays have been discussed [ 31 , 32 ].
One early observation was that of discrepancies in these in vitro assays to find transformed cells, few of which actually cite this paper; a study was designed to determine why, from lab to lab, or day to day in the same lab using identical chemicals and cells, as well as protocols, dramatically different results were found [ 33 ]. To make this long story short, it was shown, using a pool of Syrian embryo cells to test the presumptive chemical carcinogen, that if the population of normal cells had a few cells that seemed to have no contact-inhibition, one could obtain transformed cells after the application of the presumptive carcinogen . If the population from this pool of Syrian hamster embryo cells did not have in its population the type of no-contact inhibited cells, no amount of the presumptive carcinogen would induce transformed cells. The clue was that only the few cells that did not have contact inhibition gave rise to transformed cells. The clue was contact inhibition [ 34 ].
In another disciplinary field, the work of Werner Loewenstein and Kanno [ 35 ], as well as the freeze fracture pioneer Dr. J.P. Revel [ 36 ], fused the fields of electrophysiology and electron microscopy to identify a structure of cell membranes (gap junctions) to a physiological function of this structure to synchronizing both metabolic and electrotonic functions of cells in tissues. Later, Borek and Sachs [ 37 ] and Borek et al. [ 38 ] noted that normal cells, which had gap junctions, could contact inhibit or have growth control and differentiate, as well as have the potential to become senescent. On the other hand, cancer cells that do not contact-inhibit or have growth control, cannot terminally differentiate, but were immortal , and also had no functional gap junctional intercellular communication [ 39 ].
The terms, senescent , immortal and normal cells appear, again and again, in the cancer literature. However, it has now been shown that normal primary human fibroblasts cells would, through replicative replication, senesce after about 50 cell passages in vitro [ 40 ]. However, later it was shown that, given the manner by which human primary biopsies that gave rise to these fibroblasts were grown at ambient oxygen levels, they followed Hayflick’s observation. Yet, if these primary fibroblasts were grown at very low oxygen levels, they could be passaged much longer [ 41 , 42 , 43 , 44 , 45 ]. It was as though oxygen was a toxic agent to some cells in the population that were needed for their sustained growth. Even later, it was shown in our laboratory that early passages of skin fibroblasts contained adult stem cells [ 46 , 47 ]. Our results, which are currently unpublished, have shown that very early primary human fibroblasts express a key stem cell marker, Oct4A. Could oxygen levels affect the stem cell state of stemness?
3. Clues to Prove the Stem Cell versus De-Differentiation Hypotheses of the Origin of Cancer Stem Cells
Having these published observations in several disciplines in mind, in the context of my laboratory wanting to resolve the issue as to whether the Stem Cell hypothesis or the De-differentiation hypothesis was correct as being the origin of the “cancer stem cell” hypothesis, the question was: “ How could a normal stem cell in any tissue/organ survive without being forced to differentiate by close differentiated offspring that had functional gap junctions?” Only two possible explanations seemed reasonable. First, the stem cells were sequestered by some physical barrier that prevents contact with these gap junction-expressing cells, or second, these stem cells did not express their connexin, or gap junction, genes. We then designed what we called the kiss of death assay [ 48 ].
This assay was based on disassociating all the cells of a normal human organ biopsy, which contained three kinds of cells, namely, a few rare organ-specific adult stem cells, the large numbers of progenitor cells and the terminally differentiated cells. We assumed that the stem cells did not have either expressed connexin genes or have any functional gap junctions. The progenitor cells would have functional gap junctions, while the terminally differentiated cells might or might not have gap junctions, but they could not divide. Next, with approximately a million of these disassociated cells, they were placed on a confluent mat of normal human fibroblast, which were lethally irradiated and were unable to proliferate. Once the progenitor cells attached to the confluent mat of fibroblasts, they formed gap junctions with the proliferative- disabled fibroblasts and eventually died. The terminally differentiated cells never formed any clones on the mat. Since they either died by apoptosis or because they could not proliferate. On the other hand, after a week, a few small clones of cells appeared to be proliferating. After these clones were removed, they were tested for expressed connexins and functional gap junctions. None were found. These cells were then exposed to various differentiating agents, and then they expressed connexin genes, had functional gap junctions and ultimately differentiated (see Figure 3 in [ 48 ]).
Later, when it was shown that one of the biomarker genes of embryonic stem cells was the Oct4 gene [ 49 , 50 , 51 ], our lab had several organ-specific adult stem cells (kidney, breast, pancreas, and later, liver). We tested them for expressed Oct4 and non-expressed connexin genes [ 52 ]. This confirmed our hypothesis that the clones we isolated via the kiss of death assay and later other techniques [ 53 ] were, in fact, true adult stem cells. We decided that only two of our 20,000+ genes needed to be tested as biomarkers for any stem cells. Oct4 is required for maintaining stemness and the connexin genes and functional gap junctions are required for differentiation, growth control, apoptosis [ 54 ] and senescence.
4. A Test for Stem Cell and De-Differentiation Hypotheses for the Origin of the “Cancer Stem Cells”
Even though speculations and experimental tests were reported to support the stem cell hypothesis [ 22 , 23 , 24 , 25 , 26 , 27 ], no reports used a single isolated human adult stem cell to put these hypotheses to a test. Using normal human adult stem cells as the target cell of the initiation/promotion/progression carcinogenic in vitro process, we tested these cells for the expression of Oct4A and for functional gap junctional intercellular communication [ 23 ]. Oct4A was expressed but no connexin43 was expressed and there was no functional gap junctions in the human breast stem cells. We then tested whether these human breast adult stem cells could be differentiated into breast epithelial cells and whether Oct4A was still expressed and whether the connexin43 was expressed, and if they had functional gap junctions. These differentiated breast epithelial cells had no expressed Oct4A, but did express connexin43 and had functional gap junctions (see Figure 1 in [ 23 ]).
Next, the normal human adult breast stem cells and the differentiated breast epithelial cells were transfected with the large T gene of the SV-40 virus; only a few clones of proliferating breast stem cells were obtained. These cells still expressed the Oct4A gene and they had no functional gap junctions. These cells were apparently immortal , but not tumorigenic when tested in immune-suppressed mice. No immortalized cells were derived from those differentiated breast epithelial cells, confirming what many previous studies had shown, that to immortalize normal differentiated cells was either difficult or impossible [ 6 , 7 , 8 ].
The immortalized human breast stem cells were now X ray-irradiated, and a few clones that formed soft agar clones were isolated. These were tested for tumorigenicity in immune-deficient mice and they formed slowly or weakly growing tumors. Next, these X-irradiated cloned cells were treated with the ErB2/Neu gene and several clones that had significant rapid growth in soft agar were tested for tumorigenicity in immune-suppressed mice. In this case, the tumors were very tumorigenic and still expressed Oct4A gene and did not have functional gap junctional intercellular communication.
The take-home message of this experiment on a clonally-derived series of adult human breast stem cells showed that the tumorigenic breast cell line was directly derived from the normal adult stem cell that expressed the Oct4A gene and did not have functional gap junctions. In other words, the biomarker gene, Oct4A, was not induced by the carcinogenic process, but remained expressed from the start of the initiating event. Moreover, the initiating event blocked the differentiation process, as the late Dr. Potter predicted (“Oncogeny as partially blocked ontogeny” [ 1 ]). In addition, since the original stem cells are naturally “immortal” until they are terminally differentiated or become mortal, the so-called immortalizing viruses, e.g., SV40, are not immortalizing a normal mortal cell, they are blocking mortalization . These types of immortalizing viruses should be re-named. In other words, this experiment adds to those speculated hypotheses and actual direct experiments that strongly suggest the stem cell hypothesis is the correct hypothesis for the origin of cancers.
The recent demonstration of the isolation of induced pluripotent stem cells has given renewed support for re-programming or the de-differentiation of normal differentiated somatic cells to become “immortal”, or embryonic-like [ 55 ]. While no one can doubt that genetically-engineering a population of normal differentiated somatic cells with the Yamanaka embryonic genes, includingOct4, cannot produce “iPS” cells. However, there is now a legitimate reason to challenge the interpretation that re-programming took place. Clearly, that original population contained a few fibroblast stem cells [ 46 , 47 ]. These few adult fibroblast stem cells that, when transfected with these embryonic genes, now had both their own endogenous Oct4 gene expressed, but also those of the exogenous Oct4 that were introduced in its genome. That gave these few fibroblast adult stem cells the growth advantage over the somatic differentiated non-Oct4 expressing cells. Therefore, those using “iPS” cells for all kinds of experiments are really using normal fibroblast stem cells with their genome altered by the exogenous embryonic genes. In effect, these “iPS” cells are not really the result of “re-programming”, but rather the selection of pre-existing adult stem cells of the original primary culture of human tissue.
Now, one has to demonstrate if these tumorigenic cancer cells that were derived from a single normal immortal adult specific stem cell contain the cancer stem cells.
5. Characteristics of the Cancer Stem Cells
Today, these terms, cancer stem cell or cancer-initiating cell , are defined, operationally, as the cell that has the ability to sustain the long-term growth of a tumor, having all the characteristics of a tumor from which it was derived. One of the first clues came from a creative experiment, using Hoechst dye to stain cells of a tumor [ 56 ]. When these cells were placed in a cell sorter, two populations of cells were obtained, one fluorescing and a small population that did not incorporate the dye, hence not fluorescing. These latter population were classified as side population cells. These side population cells were shown to develop into tumors, manifesting the same characteristics as the tumor from which they were derived. Hence this procedure to isolate cancer-initiating or cancer stem cells became the operational definition of the cancer stem cells . These cells did not retain the Hoechst dye because they expressed functional drug transporter genes. Therefore, the Hoechst dye-containing cells or the cancer non-stem cells did not express those genes; hence, the dye, which binds to DNA, indicated that toxic chemicals could enter these types of cells and be killed. On the other hand, the side population cells would be resistant to potential toxic chemicals. Hence, this is a significant clue as to why most anti-cancer drugs, designed to kill cancer cells, can only kill the cancer non-stem cells but not the cancer stem cells .
At the time, one had to view this finding from an evolutionary vantage point. If during early evolution of the multi-cellular organism, when the stem cell appeared, that organism, with its few stem cells and many progenitor and terminally differentiated cells, if they were exposed to some toxic agent, and all cell types were equally susceptible to the toxic agent, the organism would not survive. The stem cells are needed for growth wound repair, tissue damage and the natural attrition of dying cells. Evolution selected for the stem cells to be able to be maintained in a low oxygen micro-environment, have various anti-oxidant systems to protect against free radicals, and to have both a nuclear membrane to act as another barrier to free radical production of the mitochondria of its neighboring differentiated daughters [ 57 , 58 ] and DNA repair enzymes [ 59 ].
6. Are All “Cancer Stem Cells” Identical?
The cancer stem cell of the breast, colon, liver, pancreas, etc. would have specific organ-specific markers. However, do they share some common markers that make them a cancer stem cell ? As pointed out previously, if the cancer stem cell is derived from a normal organ-specific adult stem cell, and if one marker, namely, the Oct4A gene, is shared by all cancer stem cells , as seems to be indicated by many published papers [ 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 ], then it would seem that some strategy should be developed to find ways to either shut down this gene in order to force the cells to differentiate or apoptose. In addition, if the connexin genes and functional gap junctions are not found in either the normal stem cells or cancer stem cells , agents that might induce the connexin genes to be expressed could induce either differentiation or apoptosis. One such example of this happening has been demonstrated [ 78 ].
An additional series of observations have, however, complicated this simple solution. In a series of many canine tumor types, Oct4 was detected in over 95% of these tumors [ 79 ]. Yet, there were a few canine tumors where no Oct4 marker was detected in the tumor cell population. Again, to complicate the situation, virtually all tumors are found in two states, namely, the embryonic-like phenotype, or in the quasi-differentiated phenotype (basal cell skin carcinoma or squamous skin carcinoma; polyp-type colon carcinoma and the flat-type or embryonic-type colon carcinoma; lung small cell carcinoma or the non-small cell carcinoma, etc.). To my knowledge, no one has examined the cancer stem cell of either type of tumors of any organ to determine if the Oct4A is not expressed, but some connexin gene is expressed in the partially differentiated stem cell, and if cancer stem cells of the embryonic-type organ tumor have the Oct4A gene expressed and no connexin gene or functional gap junctional intercellular communication is found. If that prediction is confirmed, then only these two genes (possibly the drug transporter genes) need to be monitored.
In a recent paper, it has been predicted, based on many arguments presented here, that there exist two cancer stem cells [ 80 , 81 ]. Therefore, using any cancer therapy protocol against one type will not work against the other type in the same organ. Currently, there is no explanation as to why some cancer stem cells do not express the Oct4A gene, yet are still derived from the normal organ-specific adult stem cell, as are the Oct4A expressing cancer stem cells . One potential explanation is that, as an adult stem cell starts to differentiate, it starts to turn off the expression of Oct4A; it is initiated in this transition state. The connexin gene is expressed and it starts to differentiate, but does not achieve the ability to terminally differentiate (i.e., asymmetrical cell division is inhibited, but the symmetrical cell division is still functioning). In these cases, since it was shown that gap junction can be inhibited if some oncogene is also expressed, thus blocking the gap junction function. Only future experiments to test this hypothesis will affirm or deny its validity.
Last, one of the new insights that has emerged in the search for ways to target the cancer stem cells in a cancer patient is to find ways to minimize the unintended consequences of the toxic effects of the therapies being used. One of the side effects of killing cancer cells by radiation or chemotherapeutic cytotoxicants is that it triggers a cytokine storm [ 82 , 83 ], which is a natural response for an organism to repair tissue or the loss of dying cells. These various cytokines have been shown to modulate gap junctional intercellular communication [ 84 ].
Since the drug metformin has already been shown to protect against chemicals that can inhibit gap junctional intercellular communication to cause enhanced cell proliferation [ 85 ], and since metformin has already been shown to target cancer stem cells in a three dimensional human breast organoid [ 86 ], we predicted that using it together with any anti-cancer therapy to minimize both the side consequences of the therapy on the non-cancer cells, as well as to help sensitize targeting the cancer stem cells, would seem to be a rational strategy. Several reviews of the literature seems to have provided mixed results, with some studies showing no effects, others some negative effects, and some demonstrating positive effects [ 87 , 88 , 89 , 90 ]. Since metformin seems to act in a similar, but not identical, way to other chemicals that protect cells from agents that can inhibit gap junction intercellular communication, such as resveratrol, CAPE, green tea components, licorice components, caffeine, lycopene [ 91 ], as well as lovastatin [ 92 ], melatonin [ 86 ] and others, understanding all the different mechanisms by which agents can modulate gap junctional intercellular communication is critical. Phorbol esters, a powerful gap junction inhibitor, work by a very different biochemical cellular mechanism than does DDT. There are both receptor- and receptor-independent mechanisms by which inhibitors of gap junction function, for example low dose estrogen verses high dose estrogen, have different effects of triggering intracellular signaling. The mixed clinical trials might simply be due to not understanding the specific factors involved, namely, the individual’s genetics, gender, development stage, the specific intra-cellular signaling pathways that are triggered by the agents being used, dose to be used, timing with the anti-cancer therapy, knowing which of the two types of cancer stem cells is in the patient’s tumors, as well as the time of day the therapy is administered. If ever there was a support for precision medicine or personalized medicine, this could be an example. This is seen in the context of the definition of personalized or precision medicine which refers to a medical approach in which diseases are diagnosed, prevented, and treated according to the context of each patient’s unique genetics, history, and lifestyle. Treatments are optimized and side effects are reduced, and this drastically reduces the overall cost of healthcare to society.
7. The Ultimate Problem of Designing an Anti-Cancer Agent That Targets These Cancer Stem Cells
Strategically, one would like to prevent any future cancer over the treatment of an existing tumor or the wide-spread metastatic cancer. However, there are many obstacles that have to be overcome. The first is the problem of the initiation of a single normal cell (in this case, it is assumed that a normal cell is an adult organ-specific adult stem cell), because initiation, while preventable to a degree, it is not possible to eliminate all initiating events or a mutation in a critical cancer-associated gene responsible for blocking asymmetrical cell division of the organ-specific stem cell. One needs to not be exposed to too much UV light from the sun or to not sit on a uranium pile. However, mutations can be produced not only by the genomic DNA being damaged ( errors in DNA repair ) [ 93 , 94 , 95 , 96 , 97 ], but also by errors in DNA replication [ 98 ]. Consequently, even in the absence of genomic DNA damage, every time a stem cell replicates during a growth spurt, hormone growth factor, or cytokine stimulation during wound healing, cell death of a tissue and tissue removal, there is a finite probability that a “spontaneous” mutation in one of those initiating genes would occur.
Probably one of the most convincing proofs that a specific oncogene mutation in a tumor associated with lung cancer is the demonstration that mutations in non-smokers’ lung cancer cell’s Ha-ras oncogene were identical to the mutation in that gene found in lung tumor cells of smokers [ 99 ]. As an important side observation, it was a classic chemical found in cigarette smoke that was shown not to be a genotoxicant or mutagen, but rather an agent that increased the transformation frequency of baby hamster embryonic cells [ 100 , 101 , 102 ]. Later, studies on any of the predominant aromatic hydrocarbons in cigarette smoke showed them to be tumor promoters, but not initiators [ 103 , 104 ]. When using assays to detect agents that could reversibly inhibit gap junctional intercellular communication and shown that they were epigenetic-acting chemicals, the most predominate aromatic hydrocarbon was a non-mutagenic, 1-methyl anthracene [ 105 ]. Therefore, tying this set of observations together, it seems that lung cancers of non-smokers and smokers might be the result of a spontaneous mutation, caused by an error of DNA replication in a gene that blocks asymmetric cell division of a stem cell of the lung, which was promoted by endogenous or exogenous epigenetic chemicals.
Returning to the major problem of killing the cancer stem cells , how can an agent be designed to be given in vivo to target the cancer stem cell of a benign or malignant cancer? It is now accepted that all tumors are a heterogeneous mixture of non-cancer stem cells [ 106 ] and a few cancer stem cells, together with normal stromal cells and invasive immune cells. In general, there is a very wide range of genotypes (both chromosomal and gene-wise) in the cancer non-stem cells. To date, there is no solid evidence that the deviation chromosomal/gene mutations and chromosome instability, let alone epigenetic deviations of the initiated cells during its evolution, were responsible for the origin of the cancer stem cell.
First, as was pointed out before, we will never eliminate the origin of the initiated adult stem cell because it is usually surrounded by, and communicating with, its normal differentiated progenitor or differentiated daughters. Under those conditions, these communicating normal cells are sending signals to induce a normal phenotype in the initiated cell, in spite of having a critical mutation in a cancer-associated gene. This allows the “initiated cell” to escape the immune system, as it appears to be “normal” or self-like.
However, when these cell–cell communication-coupled cells are exposed to agents and conditions that can inhibit this communication process, these initiated cells can clone multiply to form those benign lesions, such as a papilloma of the skin, enzyme altered foci of the liver, polyp of the colon or nodule in the breast. Not all of these lesions will go on to form an invasive and metastatic tumor. In fact, some might even be blocked from further development or even regress [ 107 ]. However, a few of these lesions will have a cell within the benign lesions that will have acquired the “hallmarks of cancer” [ 2 , 108 ].
Faced with the problem of targeting any agent that might induce differentiation, apoptosis or cytotoxicity in vivo of any organ-specific tumor, one has to deal with a tumor that is a heterogeneous mixture of cancer non-stem cells, cancer stem cells and various normal stromal and immune cells. Moreover, a chemical anti-cancer drug or inducer of an immune response has to find its way to those cancer stem cells. In addition, any cancer stem cell -targeted drug must not attack the normal stem cells of the body. One can imagine the complex cell–cell interactions between all of these different cell types, changing the normal gene expressions and phenotypes of those cells that are absent of those interactions. The apparent strategy is to design at least a multiple approach, first to kill the sensitive cancer non-stem cells, and then to eliminate at least some of the barriers to any cancer stem cell -targeted agent. However, remember that the death of those non-cancer stem cells will be releasing various cytokine-like chemicals that could cause the stimulation of the resistant and surviving cancer stem cells. Using stem cells grown in three-dimensions to mimic tissue organization, both tumor promoters and anti-cancer agents have been used to target the cancer stem cells [ 81 , 109 ].
In this Commentary, an examination of the origin of the cancer stem cell concept was developed by viewing historical experimental observations, techniques and concepts that have led to the operational concept that these cancer stem cells are those responsible for sustaining the growth of any tumor. Challenges to the understanding of what is the cancer stem cell , and from which cell it originated, have come from terms, such as normal cells, immortal, and carcinogens . These terms were examined in the context of several major classic concepts, such as the multi-stage, multi-mechanism process of carcinogenesis, and the demonstration of epigenetic agents as being the driver of this multi-stage, multi-mechanism process. A major challenge to the concept that re-programming of somatic differentiated cells exists during the immortalization of a normal cell. Characterization of several markers of isolated stem cells, such as Oct4A and connexin genes, in both normal adult stem cells and in cancer stem cells , has led to the potential strategy of targeting the cancer stem cells in a heterogeneous mixture of cancer cells in all tumors. Last, it has been speculated that there exist two different kinds of cancer stem cells, which suggests two very different kinds of anti-cancer drug strategy must be developed.
Acknowledgments
While I fully acknowledge all the work, discussions and intellectual contributions of my former students, postdoctoral fellows, and international Visiting Scholars and collaborating colleagues during my 50 years of research, I take full responsibility of all the speculative claims made in this manuscript.
As a Professor Emeritus, I received no grants (governmental, Foundation, or institutional) that supported the development of this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Conflicts of interest.
The author has no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
That adult stem cells give rise to cancer is an attractive hypothesis, given that the classic multistep model of carcinogenesis requires a long-lived cell in which multiple genetic hits can occur ...
According to the cancer stem cell hypothesis, only cancer stem cells have self-renewal ability and the rest of the tumor cells would die out without being replenished from the cancer stem cells. ... Zheng X, Shen G, Yang X, et al. Most C6 cells are cancer stem cells: evidence from clonal and population analyses. Cancer Res. 2007; 67:3691-3697 ...
Empirical evidence regarding sub-hypotheses and further aspects of the Cancer Stem Cell hypothesis. The empirical evidence of each evaluated test was categorized as either supporting (S), being undecided (U) or questioning (Q), based on the data reported in the respective studies. N is the numbers of studies that tested the sub-hypotheses.
Abstract. There is a growing body of evidence that supports the idea that malignant tumors are initiated and maintained by a population of tumor cells that share similar biologic properties to normal adult stem cells. This model, the cancer stem cell (CSC) hypothesis, is based on the observation that tumors, like adult tissues, arise from cells ...
The cancer stem cell hypothesis: a work in progress Brenton Thomas Tan*, Christopher Yongchul Park*, Laurie Elizabeth Ailles and ... There is a growing body of evidence that supports the idea that ...
The cancer stem cell (CSC) hypothesis provides an attractive cellular mechanism to account for the therapeutic refractoriness and dormant behaviour exhibited by many of these tumours.
The cancer stem cell hypothesis posits that tumor growth is driven by a rare subpopulation of cells, designated cancer stem cells (CSC). Studies supporting this theory are based in large part on xenotransplantation experiments wherein human cancer cells are grown in immunocompromised mice and only CSC, often constituting less than 1% of the malignancy, generate tumors.
There is a growing body of evidence that supports the idea that malignant tumors are initiated and maintained by a population of tumor cells that share similar biologic properties to normal adult stem cells. This model, the cancer stem cell (CSC) hypothesis, is based on the observation that tumors, like adult tissues, arise from cells that exhibit the ability to self-renew as well as give rise ...
Evidence supporting the cancer stem cell hypothesis has gained impetus due to recent advances in stem cell biology and the development of new animal models to measure self-renewal and more directly test the validity of this hypothesis. The concept that cancers arise from the transformation of stem cells is appealing for several reasons.
A stem cell theory of cancer provides greater universality, interconnectivity, and utility. Although genetic defects are pivotal, cellular context is paramount. When it concerns tumor heterogeneity, perhaps we need to revisit the conventional wisdom of precision medicine and revise our current practice of targeted therapy in cancer care.
Common cancer theories hold that tumor is an uncontrolled somatic cell proliferation caused by the progressive addition of random mutations in critical genes that control cell growth. Nevertheless, various contradictions related to the mutation theory have been reported previously. These events may …
The phenomenon of cancer cell heterogeneity has been explained by different hypotheses, each entailing different therapy strategies. The most recent is the cancer stem cell model, which says that tumourigenicity and self-renewal are restricted to rare stem cell-like cancer cells. Since its conception, conflicting evidence has been published. In this study, we tested the applicability of a new ...
There is a growing body of evidence that supports the idea that malignant tumors are initiated and maintained by a population of tumor cells that share similar biologic properties to normal adult stem cells. This model, the cancer stem cell (CSC) hypothesis, is based on the observation that tumors, like adult tissues, arise from cells that ...
Central to the heterogeneity of human malignancies is the existence of cancer stem cells (CSCs), a rare fraction of slow-cycling cancer cells that have the ability of self-renewal, pluripotency and chemo-resistance. In the presumptive CSC model, only the CSCs are tumorigenic and can produce all of the cells necessary to repopulate a tumor ...
In recent years, there has been an increasing amount of evidence to support a CSC phenotype in human lung cancer. 19, 20, 21 Many of these markers have also been found in other tumors and indeed in normal stem cells. One such phenotype is the so-called side population (SP) cells, which are capable of excluding Hoechst 33342 dye by ABC transporters. In addition, cells expressing the cell ...
Over the past ten years, the cancer stem cell (CSC) hypothesis has produced an enormous amount of interest, optimism, and debate in the cancer research community. ... evidence generated using the xenotransplantation approach was potentially null, given it . 5 had been carried out by transplanting human cancer into mice models. In other words, the
This theory has been challenged recently by the cancer stem cell (CSC) hypothesis, that a rare population of tumor cells, with stem cell characteristics, is responsible for tumor growth, resistance, and recurrence. Evidence for putative CSCs has been described in blood, breast, lung, prostate, colon, liver, pancreas, and brain.
The first evidence of a solid tumor cancer stem-like cell followed in 2002 with the discovery of a clonogenic, ... There are two theories to explain the phenotypic and metabolic heterogeneity of CSCs; clonal variation and cancer stem cell theory. While former theory dictates the role of genetic, epigenetic and micro environment where tumour ...
The stem cell hypothesis challenges the classic stochastic model, which holds that cancer results from a random mutation in a cell that reproduces and eventually forms a malignant neoplasm. In contrast, the stem cell model suggests that in many instances, stem cells or their immediate progeny are the cells transformed during carcinogenesis, and ...
In order to fully appreciate the cancer stem cell hypothesis and its implications for therapy, it is critical to understand basic concepts of normal stem cell biology. Most human tissues are composed of rapidly dividing cells that continually die or are lost and that are replenished through tightly regulated mechanisms. ... Evidence for this ...
This theory has been challenged recently by the cancer stem cell (CSC) hypothesis, that a rare population of tumor cells, with stem cell characteristics, is responsible for tumor growth, resistance, and recurrence. Evidence for putative CSCs has been described in blood, breast, lung, prostate, colon, liver, pancreas, and brain.
This theory — called the Knudson's 'two-hit' paradigm — was first formulated in 1971 and proposed that these genes must be inactivated permanently in our cells before cancer can arise."
The presence of stem cell in cancer were further supported in other ... To link the possible roles of inflammation and CSC in the cancer initiation, evidence can be drawn from the changes in the microenvironment within the stem cell niche. ... inflammation and cancer stem cells: a hypothesis for a paradigm change and new targets in cancer ...
The tumor microenvironment (TME) plays an important role in the process of tumorigenesis, regulating the growth, metabolism, proliferation, and invasion of cancer cells, as well as contributing to tumor resistance to the conventional chemoradiotherapies. Several types of cells with relatively stable phenotypes have been identified within the TME, including cancer-associated fibroblasts (CAFs ...
1. Introduction: How Some Historic Experimental Findings and Hypotheses of Cancer Shaped Today's Concept of Cancer Stem Cells. These three introductory quotes embody much of my concern in this Commentary as it concerns the concept of cancer stem cells.They span decades of cancer research by different disciplinarians, involving years of experimental research and philosophical reflection of ...