Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Newborn screening timeliness quality improvement initiative: Impact of national recommendations and data repository
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Center for Public Health Innovation, CI International, Littleton, Colorado, United States of America, Department of Epidemiology, Colorado School of Public Health, University of Colorado Denver, Aurora, Colorado, United States of America
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing
Roles Data curation, Investigation, Writing – review & editing
Affiliation Association of Public Health Laboratories, Silver Spring, Maryland, United States of America
Roles Conceptualization, Data curation, Funding acquisition, Resources, Writing – review & editing
Roles Conceptualization, Funding acquisition, Resources, Writing – review & editing
Roles Resources, Writing – review & editing
Affiliation Maternal and Child Health Bureau, Health Resources and Services Administration, U.S. Department of Health and Human Services Rockville, Rockville, Maryland, United States of America
Affiliation Department of Pediatrics, Willis-Knighton Health System, Tulane University School of Medicine, Shreveport, Louisiana, United States of America
Roles Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft
Affiliations Center for Public Health Innovation, CI International, Littleton, Colorado, United States of America, Department of Community and Behavioral Health, Colorado School of Public Health, University of Colorado Denver, Aurora, Colorado, United States of America
- Marci K. Sontag,
- Joshua I. Miller,
- Sarah McKasson,
- Ruthanne Sheller,
- Sari Edelman,
- Careema Yusuf,
- Sikha Singh,
- Deboshree Sarkar,
- Joseph Bocchini,

- Published: April 2, 2020
- https://doi.org/10.1371/journal.pone.0231050
- Peer Review
- Reader Comments
Newborn screening (NBS) aims to achieve early identification and treatment of affected infants prior to onset of symptoms. The timely completion of each step (i.e., specimen collection, transport, testing, result reporting), is critical for early diagnosis. Goals developed by the Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) for NBS timeliness were adopted (time-critical results reported by five days of life, and non-time-critical results reported by day seven), and implemented into a multi-year quality improvement initiative (NewSTEPS 360) aimed to decrease the time to result reporting and intervention.
The NBS system from specimen collection through reporting of results was assessed (bloodspot specimen collection, specimen shipping, sample testing, and result reporting). Annual data from 25 participating NBS programs were analyzed; the medians (and interquartile range, IQR) of state-specific percent of specimens that met the goal are presented.
The percent of specimens collected before 48 hours of life increased from 95% (88–97%) in 2016 to 97% (IQR 92–98%) in 2018 for the 25 states, with 20 (80%) of programs collecting more than 90% of the specimens within 48 hours of birth. Approximately 41% (IQR 29–57%) of specimens were transported within one day of collection. Time-critical result reporting in the first five days of life improved from 49% (IQR 26–74%) in 2016 to 64% (42%-71%) in 2018, and for non-time critical results from 64% (IQR 58%-78%) in 2016 to 81% (IQR 68–91%) in 2018. Laboratories open seven days a week in 2018 reported 95% of time-critical results within five days, compared to those open six days (62%), and five days (45%).
NBS programs that participated in NewSTEPs 360 made great strides in improving timeliness; however, ongoing quality improvement efforts are needed in order to ensure all infants receive a timely diagnosis.
Citation: Sontag MK, Miller JI, McKasson S, Sheller R, Edelman S, Yusuf C, et al. (2020) Newborn screening timeliness quality improvement initiative: Impact of national recommendations and data repository. PLoS ONE 15(4): e0231050. https://doi.org/10.1371/journal.pone.0231050
Editor: Jacobus P. van Wouwe, TNO, NETHERLANDS
Received: September 27, 2019; Accepted: March 15, 2020; Published: April 2, 2020
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: Data cannot be shared publicly because of signed memoranda of understanding between state newborn screening programs and the Association of Public Health Laboratories. Data requests are reviewed by the NewSTEPs Data Review Workgroup (contact via [email protected] ) for individuals who meet the criteria for access to state-level data.
Funding: The project described in this article was funded by the Department of Health and Human Services, Health Resources and Services Administration under Cooperative Agreements #UG8MC28554 [MKS] and #U22MC24078 [JO] and the Cystic Fibrosis Foundation under Grant Number SONTAG16Q10 [MKS]. The HRSA provided support in the form of salaries for authors [MKS, JIM, SM, CY, RS, SE, SS, JO, YKG], but did not have any additional role in the study design, or data collection and analysis. CI international provided support in the form of salaries for some of the authors [MKS, JIM, YKG]. The specific roles of these authors are articulated in the ‘author contributions’ section. The HSRA did however participate in the decision to publish as well as in the preparation of the manuscript. The CFF and CI International did not have any role in the study design, or data collection and analysis, the decision to publish, or preparation of the manuscript.
Competing interests: The authors have read the journal's policy and have the following competing interests: MKS, JIM, and YKG are paid employees of CI International. The Cystic Fibrosis Foundation provided support for this study under Grant Number SONTAG16Q10 [MKS]. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products to declare.
Abbreviations: ACHDNC, Advisory Committee on Heritable Disorders in Newborns and Children; ACMG, American College of Medical Genetics and Genomics; DBS, Dried Blood Spot; HHS, U.S. Department of Health and Human Services; HIT, Health Information Technology; HRSA, Health Resources and Services Administration; MOU, Memorandum of Understanding; NBS, Newborn Screening; NewSTEPs, Newborn Screening Technical assistance and Evaluation Program; QI, Quality Indicator; RUSP, Recommended Uniform Screening Panel
Introduction
Newborn screening.
Newborn screening (NBS) is a public health program that aims to identify newborns at risk for serious life-altering disorders in the first week of life. The NBS process is composed of multiple components ( Fig 1 ) that must work in a coordinated and efficient manner to allow for early medical intervention before significant and irreversible damage occurs.[ 1 ] Hospital staff, midwives, and other clinical personnel collect, package, and ship the dried blood spot NBS specimen through commercial or private couriers to be delivered to a state, regional, or private NBS laboratory. Once the specimen is received at the laboratory, testing is completed and results are reported to the appropriate medical personnel who confirm or rule out a diagnosis and initiate the required intervention as appropriate.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Newborn screening (NBS) is a complex system that involves the collection of specimens at birthing facilities, transportation of specimens to the NBS public health laboratory for testing and communicating results to health care providers and families. Each step needs to occur in a timely manner in order to prevent infant mortality and morbidity. NewSTEPs 360 supported state/territorial NBS programs to address challenges associated with the pre-analytical and analytical phases of the NBS process by implementing various activities, including 1) providing education to birthing centers and midwives about the importance of timely collection and shipment of specimens; 2) shortening transit time by optimizing shipping methods; 3) expanding laboratory operating hours to decrease the time from specimen receipt to results reporting; 4) improving the efficiency of laboratory workflows; and 5) developing a health information technology infrastructure to improve the transmission of electronic demographic information, laboratory orders, and results between the NBS laboratory and health care providers.
https://doi.org/10.1371/journal.pone.0231050.g001
Timeliness in newborn screening
Timely identification of newborns affected by core disorders on the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) Recommended Uniform Screening Panel (RUSP) is critical.[ 2 , 3 ] The ACHDNC has the mission to reduce morbidity and mortality due to heritable disorders in newborns and children, and provides recommendations to guide and strengthen the newborn screening system. While early detection has always been the goal of NBS, the expansion of the list of screened disorders in the late 1990s and early 2000s to include those identified via tandem-mass spectrometry with a short pre-symptomatic window has led to an increased urgency to detect affected newborns as quickly as possible.[ 4 , 5 ] NBS timeliness recommendations were first published in 2006 by the American College of Medical Genetics and Genomics (ACMG) and included specifications that all specimens should arrive at the NBS laboratory within three days of collection, and that results be reported within two days of specimen receipt and within five days of specimen collection.[ 1 ]
In 2013, based on public comment, the ACHDNC decided to review policies and practices relating to timeliness of NBS in the United States. In support of this work, the Society for Inherited Metabolic Disorders (SIMD) classified 16 of 35 disorders included on the RUSP as time-critical, requiring immediate medical attention. [ 6 ] Based on methodologies in practice, published literature and expert opinion, in 2015, the ACHDNC developed five timeframes for conducting newborn screening ( Table 1 ). [ 7 ]
https://doi.org/10.1371/journal.pone.0231050.t001
NewSTEPs 360
In February 2015, the Health Resources and Services Administration (HRSA) funded a collaborative improvement and innovation network to support multidisciplinary teams in improving newborn screening timeliness. This project was called NewSTEPs 360. Supplemental funding to support this project was also provided by the Cystic Fibrosis Foundation. Under NewSTEPs 360, NBS programs were convened to identify and overcome barriers to timely NBS through technical and financial assistance. NewSTEPs 360 was built upon the foundation of the HRSA-funded Newborn Screening Technical assistance and Evaluation Program (NewSTEPs)[ 8 ], which included access to the NewSTEPs Data Repository.
The NewSTEPs Data Repository collects data on NBS system components with the goal of supporting quality improvement initiatives and providing comparative data at the state, regional, and national levels. NBS programs that voluntarily enter data into the repository have access to their own data plus de-identified, aggregate data from other participating programs. Data elements collected in the repository include NBS program information (e.g., disorders screened, fees, policies, program structure, etc.), quality indicators (QI) for each stage of the NBS process at the programmatic level, and case data [ 9 ] on infants with a confirmed diagnosis of a disorder detected by NBS.
NewSTEPs adopted a panel of eight quality indicators that measure newborn screening program performance that were developed by the broader newborn screening community, including newborn screening laboratorians, follow-up specialists, and clinical providers.[ 10 ] A subset of the panel of QIs that reflect timeliness outcomes were collected as part of NewSTEPs 360 ( Table 2 ). Each NBS program participating in NewSTEPs 360 was assigned a continuous quality improvement (CQI) coach who met with the NBS program team monthly to identify challenges and opportunities for improvement in this subset of QIs.
Excerpted from NBS quality indicator panel. [ 10 ].
https://doi.org/10.1371/journal.pone.0231050.t002
This study summarizes the impact of implementing quality improvement efforts in participating NBS programs to attain national timeliness recommendations for newborn screening. We evaluated the timeliness of initial specimen collection, delivery from the birthing center to the NBS laboratory, reporting of results for both time-critical and non-time-critical disorders, and the overall reporting of all NBS results. In a subset of programs, we also assessed the timeliness of diagnosis and medical intervention. Finally, we analyzed the impact of individual program activities to improve timeliness.
Participants in NewSTEPs 360
Twenty-eight US state and territorial NBS programs were selected to participate in NewSTEPs 360 via two rounds of a competitive application process. State newborn screening programs applied through an internet-based application process, identifying the challenges within their programs, and proposed corresponding quality improvement initiatives. Baseline data were required from all applicants. Sixteen applicants representing 20 states were selected for participation in 2015 and a second cohort of eight programs joined in 2016. Participating programs actively engaged in a continuous quality improvement framework to improve timeliness by developing individual improvement projects at the programmatic level. Funding for NewSTEPs 360 was provided by HRSA (UG8MC28554, 9/1/15–8/31/18, no-cost extension through 8/31/19). Supplemental funding was provided by the Cystic Fibrosis Foundation (SONTAG16Q10). The infrastructure for NewSTEPs is funded through HRSA (U22MC24078). NewSTEPs activities were deemed to be public health quality improvement and not human subject research by the Colorado Multiple Institutional Review Board.
Data collection
Newsteps data repository and data security..
The repository is a centralized web-based platform that can be accessed by registered users via a 128-bit secure socket layer (SSL) encryption. Registration for the NewSTEPs repository is open to all interested parties, however access to state specific data elements is restricted to individuals working in the state newborn screening system. NewSTEPs requires that NBS programs have a signed Memorandum of Understanding (MOU) with the Association of Public Health Laboratories (APHL) in order to enter Quality Indicator (QI) and case data into the repository. Review of QI and case-level data are limited by role-based access control that was assigned at the individual NBS program level, whereas review of programmatic NBS program information (i.e. operating hours, policies and procedures, state demographics) is available to the public.
Quality indicator data.
NewSTEPs 360 participants were required to provide monthly data for the QIs associated with timeliness,[ 10 ] which was aggregated at the annual level for cross-year comparison. To further encourage data entry and accuracy, CQI coaches frequently reviewed the data using visualization tools and discussed progress or barriers during the monthly or bi-monthly team coaching calls.
Quality indicator benchmarks.
Benchmarks were adapted directly from the ACHDNC timeliness goal recommendations ( https://www.hrsa.gov/advisory-committees/heritable-disorders/newborn-screening-timeliness.html ). Few newborn screening programs were able to meet the ambitious recommendation stated by the ACHDNC the NBS specimens should be received at the laboratory as soon as possible, ideally within 24 hours of collection. In response to this, NewSTEPs created an additional benchmark of two calendar days to assess time elapsed from specimen collection to receipt at the laboratory as an intermediary step. For purposes of our analysis, we equated 24-hours to the next calendar day. Assessing timing of specimen receipt per calendar day is in better alignment with the typical newborn screening laboratory approach. Programs typically test specimens after scheduled shipments have arrived on a given day, shipments arriving earlier in the day may not be tested earlier than those arriving right before the scheduled testing time, making calendar day a more meaningful metric than hours of delivery.
Further, an additional metric was added to assess the time from specimen receipt at the laboratory to results being reporting. This added metric helps identify opportunities for improving laboratory processes that could affect overall timeliness. The additional benchmark was calculated based upon the benchmarks set by the ACHDNC for other timeliness outcomes. The calculation of specimen receipt to reporting results are provided in Table 3 .
https://doi.org/10.1371/journal.pone.0231050.t003
The NewSTEPs Data Repository collects basic demographic and diagnostic information on all newborns with a disorder diagnosed through NBS in the US. Continuous timeliness measures were collected on each confirmed case to understand the factors that lead to shortened intervention times Case data is collected in the year following the birth of an infant to allow for the diagnostic process to be completed; cases entered for 2015–2017 by NewSTEPs 360 programs were included in this analysis.
NewSTEPs has implemented the following definitions for intervention and diagnosis, with disorder-specific definitions available[ 11 ]:
- Time to medical intervention: The first time a medical professional acts to change the course of care for an infant. Intervention may occur via phone or clinic visit. This may also include the date a decision was made NOT to change course for the infant.
- Time to diagnosis: The time elapsed from birth until a biochemical or molecular test result on a specimen taken from the infant that confirm the NBS result reported.
Strategies employed to improve timeliness.
NewSTEPs 360 guided participating NBS programs through CQI activities via training, personalized coaching, and interactive learning sessions between NBS programs. To support team development and growth, a Plan-Do-Study-Act (PDSA) personality tool was developed to support team growth and team members’ roles within their programs ( S1 File ). The tool is a short quiz that helps a team to determine if members naturally affiliate with one functional component of the PDSA cycle to better understand how the team functions together; subsequent discussions led teams to identify strategies to maximize team productivity when engaging the PDSA cycle. Programmatic activities varied ( Table 4 ) from educational strategies for birthing facilities and health care providers to increasing courier services and operating hours (Timeliness Toolkit for Expanding Newborn Screening Services– S2 File ) and improving health information technology (HIT) systems (Building Block Guide— S3 File ). Successes and failures were shared within the participating programs to facilitate the continuous quality improvement environment.
* Each Strategy is Linked to a Corresponding QI Solution in Fig 1 .
https://doi.org/10.1371/journal.pone.0231050.t004
Statistical analysis
Monthly quality improvement data reported for January 2016 –December 2018 were converted to annual metrics and were included in the analysis. Timeliness Quality Indicator (QI) data were collected for the purposes of program improvement on a national level and are not intended for formal statistical analysis. Each participating state newborn screening program provides data for this repository with the intent of informing decisions to improve newborn screening systems. NewSTEPs 360 was a quality improvement initiative that was not powered to detect statistical differences. Further, small cell sizes for individual improvement categories may result in spurious significant results. As a result, descriptive statistics and graphical displays were created, presenting the changes in the percent of specimens meeting ACHDNC benchmarks for time elapsed from birth to specimen collection, collection to laboratory receipt, laboratory receipt to reporting out NBS results, and birth to reporting out NBS results. Additional investigations of the timeliness indicators were completed, stratified by days of operation and type of laboratory (local state laboratory vs. external [regional or private]). Differences in individual case data were tested using non-parametric Wilcoxon-ranked sum tests, and a significance level of 0.05 was set. Data were analyzed using SAS version 9.4 (Cary, NC), and displayed using Tableau Desktop (Seattle, WA, copyright 2019). Results of the analysis do not display state or territory names with the intent to protect NBS programs from the release of sensitive information.
The Colorado Multiple Institutional Review Board determined that the newborn screening quality improvement initiaitives led developed through NewSTEPs are not human subject research.
Timeliness quality indicators
Twenty-eight newborn screening programs participated in NewSTEPs 360; 25 provided complete data for birth to specimen collection (2016–2018), and a subset of those programs provided data for each of the other timeliness QIs ( Table 5 ). Participating programs that provided data for the three years are presented, although some programs were not able to provide data for all of the QIs due to systems challenges and laboratory information management systems structure that did not allow for data collection or retrieval at the program level. Progression toward meeting the ACHDNC timeliness goals for all QIs was achieved in most programs, demonstrated through individual trajectories representing the percent of specimens that met the goal ( Fig 2A–2C ). The median of all programs for each indicator demonstrated improvement in all indicators across all three years, described in detail below.
Data are presented for each state program individually, with box plots overlaid to demonstrate national trends. Box and whisker plots display the median of the percent of specimens that met the benchmark for each program, with upper and lower quartiles, and range. Panel A: Percent of specimens collected within 48 hours of birth, Panel B: Percent of specimens received at the laboratory within one day of collection (next calendar day), Panel C: Percent of specimens received at the laboratory within two calendar days of collection.
https://doi.org/10.1371/journal.pone.0231050.g002
For example: in 2016, half of the programs reported that at least 95.1% of the specimens met the goal of birth to specimen collection.
https://doi.org/10.1371/journal.pone.0231050.t005
Specimen collection and transportation.
Programs successfully collected initial specimens within the first 48 hours of birth, with stepwise increases demonstrated each year ( Fig 2A , Table 5 ; median of the programs’ percent of specimens that met benchmark in 2016: 95.1%, 2017: 96.4%, 2018: 97.0%). In 2018, more than 90% of specimens were collected in the 48-hour time frame in 19 programs (n = 19/25, 76%) and more than 95% were collected within 48 hours in 14 programs (n = 14/25, 56%). Specimens deemed to be unsatisfactory for analysis by the NBS laboratory were flagged for recollection as they could result in delayed testing and subsequent reporting of results. The percent of specimens deemed unsatisfactory for analysis by state laboratories was 1.3% in 2016, 1.3% in 2017, and 1.5% in 2018 (medians of all programs). Further, NBS cards that were submitted without complete essential demographic information could have also delayed testing and reporting of results. Variable definitions between NBS programs and changes in the definitions of the required information within state programs made these data difficult to summarize for programmatic trends.
The program median for the time from collection to receipt at the NBS laboratory ( Fig 2B , Table 5 ) on the next calendar day was 40.0% in 2016, 39.4% in 2017 and 41.8% in 2018. Allowing two calendar days after collection to receipt ( Fig 2C ), the program median of specimens which met the guideline increased to 74.3% in 2016, to 80.9% in 2017 and 81% in 2018.
Reporting of NBS results
The percentage of specimens with reporting times that met the benchmarks improved both in individual state trajectories and in program medians over all three years ( Fig 3A , Table 5 ). The timely reporting of NBS results for time-critical disorders within two days of receipt at the laboratory improved from a program median of 65.5% in 2016 to 75.8% in 2018. Similarly, the program median for non-time-critical result reporting within four days of laboratory receipt improved from 80.2% in 2016 to 93.5% in 2018. Reporting of all NBS results from laboratory receipt improved from 90.3% in 2016 to 94.2% in 2018.
Data are presented for each state program individually, with box plots overlaid to demonstrate national trends. Box and whisker plots display the median of the percent of specimens that met the benchmark for each program, with upper and lower quartiles, and range. Panel A: Percent of specimens with results reported out for time-critical results within two days of receipt at lab (top), non-time-critical reported out within four days of receipt (middle), and all results reported out within four days of receipt (bottom); Panel B: Percent of specimens with results reported out for time-critical results within five days of birth (top), non-time-critical reported out within seven days of birth (middle), and all results reported out within seven days after birth (bottom).
https://doi.org/10.1371/journal.pone.0231050.g003
The elapsed time from birth to result reporting showed improvements in each category as well ( Fig 3B , Table 5 ). Time-critical results reported within five days of birth started at 48.9% in 2016 and increased to 63.5% by 2018; reporting of non-time-critical results within seven days of birth improved from 64.3% to 80.9%. The program median for reporting all NBS results within seven days of birth did not demonstrate change over this period (88.9% in 2106 to 89.5% in 2018).
Timeliness data for cases with a confirmed diagnosis.
The 25 participating NBS programs that provided data for NewSTEPs 360 reported 1,713 cases with a confirmed diagnosis of a disorder identified by newborn screening for the years 2016–2018 (288 time-critical cases; 1,425 non-time-critical cases). Individual specimen collection times are consistent with ACHDNC timeliness goals ( Table 6 ). The median report time for time-critical disorders (five days) was earlier (p < 0.0001) than non-time-critical disorders (seven days), and both are in alignment with the timeliness goals. The resulting intervention and diagnosis times are earlier for time-critical disorders than non-time-critical (p<0.0001).
https://doi.org/10.1371/journal.pone.0231050.t006
Individual-level data from diagnosed cases demonstrate that at least 50% of the specimens were collected, received, and results were reported within the ACHDNC recommended period. Intervention and diagnosis occur earlier in infants with time-critical disorders compared to infants with non-time-critical disorders, reflecting the expedited nature of laboratory processes within the laboratories related to time-critical disorders.
Laboratory operating hours.
We found that laboratory operating hours are a critical factor associated with improved specimen delivery times, timely testing, and efficient reporting of results. Each state reported wheteher they were open 5, 6, or 7 days, along with the activites performed on those days. During the NewSTEPs 360 program, two participating NBS programs increased the number of days their laboratories were open, and multiple programs added or adjusted the hours of operation to align with the delivery of samples. By 2018, six of the 25 NBS laboratories were open five days a week, 13 were open six days a week, and six were open all seven days. Activities performed on a given day of the week by laboratories vary ( Table 7 ) due to staff training, availability, and internal policy decisions. For example, programs may report being open 7 dyas a week while not reporting non-time-critical results due to an agreement with clinical specialists to wait until regular business hours to avoid prolonged waiting times for families with a presumptive positive for non-time-critical disorders. These internal decision may impact the timelienss of individual reporting, but no links to clinical outcomes can be made.
https://doi.org/10.1371/journal.pone.0231050.t007
The percentage of specimens reported out within the ACHDNC recommended benchmarks were improved in laboratories with seven days operations compared to those with five or six-day operations ( Fig 4 ). The median percent of programs with specimen results reported out for time-critical disorders within five days of life was greater than 80% in all years for laboratories open seven days a week, while the median in laboratories open six days a week did not reach 65%, and in those open five days a week the median failed to reach 50% of specimens. The median percent of programs with specimen results reported in a timely manner increased for all laboratories regardless of operating days for both non-time-critical disorders and all results in seven days; however, laboratories open seven days reported results earlier than those open six or five days.
Box and whisker plots display the median of the percent of specimens that met the benchmark for each program, with upper and lower quartiles, and range. Panel A: Percent of specimens with results reported out for time-critical results within five days of life at programs open five days (left), six days a week (middle) and seven days a week (right). Panel B: Percent of specimens with results reported for non-time-critical results within seven days of life at programs open five days (left), six days a week (middle) and seven days a week (right). Panel C: Percent of specimens with all results reported within seven days of life at programs open five days (left), six days a week (middle) and seven days a week (right).
https://doi.org/10.1371/journal.pone.0231050.g004
Most NBS programs participating in NewSTEPs 360 have a laboratory housed within their state public health department (n = 18, recorded in 2018), while others contract with external laboratories (n = 7, recorded in 2018). The external laboratory may be managed by a private or commercial entity, or it may be housed within another state public health department. The percent of specimens with results reported within the recommended time frames from birth for both individual state laboratories and external laboratories for 2016–2018 demonstrate improvement for time-critical, non-time-critical, and all result reporting ( Fig 5 ) suggesting that both state and private labs can achieve the same success in newborn screening timeliness.
Box and whisker plots display the median of the percent of specimens that met the benchmark for each program, with upper and lower quartiles, and range. Panel A: Percent of specimens with results reported out for time-critical results within five days of birth for state labs (left) and external labs (right); Panel B: Percent of specimens with non-time-critical results reported out within seven days of birth for state labs (left) and external labs (right); Panel C: Percent of specimens with all results reported out within seven days after birth for state labs (left) and external labs (right).
https://doi.org/10.1371/journal.pone.0231050.g005
The overarching ACHDNC timeliness goals are designed to achieve the earliest diagnosis and intervention for infants with time-critical and non-time-critical disorders identified through newborn screening. [ 7 ] The introduction of national timeliness goals, paired with a continuous quality improvement program has led to improved times in reporting results to the clinical community, and earlier intervention of affected infants for NBS programs which participated in NewSTEPs 360. NewSTEPs 360 has demonstrated that NBS programs can make progress toward reaching these goals on a population level in a relatively short time through an organized, focused quality improvement effort tailored to the needs of individual states; however, there is room for system improvement.
State efforts to improve timeliness
During NewSTEPs 360 participation, state programs improved the percentage of specimens collected within ACHDNC’s recommended collection time of before 48 hours of life, the percentage of specimens received within two days of collection, and the percentage of results reported out by recommended guidelines. Programs achieved this through different approaches, including (1) implementing educational campaigns with birthing facilities, (2) increasing laboratory hours of operation and workforce schedules, (3) expanding courier service to deliver specimens to the NBS laboratory, (4) changes in laboratory testing methods, (5) using electronic ordering and results reporting with birthing centers, and (6) changes in regulations to require earlier collection. However, the trajectory of improvement and percent improvement varied among participants.
The timely collection of a newborn screening specimen at the birthing facility allows for earlier analysis and reporting. Regulations in three participating states were changed to reflect the shorter national guidelines of 24–48 hours for collection, and remarkable improvements were seen in those states. Additionally, participating NewSTEPs 360 programs developed educational materials, videos, online modules and in-person training sessions to ensure the staff collecting the specimens were knowledgeable about the importance of proper and timely collection and shipping.
NBS laboratories have historically operated during normal working hours on weekdays. However, the increased urgency of many of the new disorders added to the newborn screening panel has changed the paradigm.[ 4 ] Many NBS laboratories have shifted their work days to include Saturdays and/or Sundays and extended or modified operating hours throughout the week. Programs within NewSTEPs 360 pursued changes in operating hours, seeking additional funding, increased fees, and modified work schedules for employees. Continued efforts to increase operating hours so that babies receive the same services independent of the day-of-the-week they were born will decrease the risk of tragic outcomes for individual families. [ 12 ]
Most NBS programs are still working to achieve the goal of specimens being delivered to the newborn screening testing laboratory within one or two calendar days of collection. This has been accomplished by individual states through improved shipping from birthing centers, expanded courier systems, increased communication with the couriers, and increased laboratory operating hours to accept specimens. Based upon the largest gaps in timeliness identified in NewSTEPs 360, the best potential for timeliness gains includes increasing the number of days that laboratories are open, adding weekend and holiday couriers, and improving courier services for the transportation of specimens from birthing facilities to newborn screening laboratories.
Improvements in laboratory processes internal to the program were implemented across participating programs with the goal of improving timeliness. For example, one program identified a delay in reporting due to the timing of hemoglobinopathy results, which delayed the reporting of all results. The program changed their incubation and workflow process so that all results could be reported in a timely manner. In another program, a concerted effort was placed on improving the demographic data entry from the dried blood spot cards to improve timely data acquisition and reporting.
Health Information Technology (HIT) solutions hold promise for continuing to improve newborn screening timeliness. Many programs have instituted electronic solutions for data sharing, including electronic orders to improve demographic data transfer, electronic transfer of data, result reporting, and electronic faxing of results. One program implemented electronic ordering of dried blood spot tests, decreasing the time to verify information and initiate testing, initially in four hospitals, then more broadly across the state. The Building Blocks guide provides guidance to NBS programs to implement HIT solutions that can improve timely orders and reporting of results ( S3 File ).
Increased data entry into repository through utilization
One change that was seen as part of NewSTEPs 360 was an increase in data entry in the NewSTEPs data repository. As part of NewSTEPs 360, the repository was configured so that participating states could enter Quality Indicator data monthly vs just yearly. Further, CQI coaches encouraged monthly data entry and tracking. Options to upload data to ease manual entry were also provided, including direct upload of comma-separated-values files (.CSV), and direct assistance with data manipulation within states. NewSTEPs 360 participants utilized real-time data analysis in partnership with their quality improvement coach. The NewSTEPs Data Repository and infographics have been utilized by NBS programs to advocate for additional resources at the local level. For example, programs shared the NewSTEPs 360 data infographics with program leadership to demonstrate the improvements in timeliness metrics that were gained from adding operating hours or couriers, including weekend/holiday couriers. Conversely, other programs were able to demonstrate that they lagged behind the other participating programs and identified resource needs that could help to improve outcomes.
Unintended consequences
While improving timeliness in newborn screening was the ultimate goal of the NewSTEPs 360 program, timeliness efforts may have unintended consequences. Analytic cutoffs have typically been developed based upon age-based normal ranges for infants who are 24–48 hours of life, and testing infants earlier may impact the accuracy of the tests. Decreasing the accuracy of the screen may result in a high number of specimens flagged for follow-up testing, more infants sent for diagnostic testing, and an increase in false negatives, although this was not reported in this study. Further, earlier screening may limit the time to educate parents in the hospital prior to collecting the screen.
NBS programs have limited resources. Additional demands on staff to meet timeliness goals can limit the time that programs have to improve other program outcomes. Further, changes in programmatic and individual performance expectations may impact staff morale, which in turn affects staff retention. State programs may need to advocate for additional resources to meet timeliness goals, and the other requirements of the NBS program to meet the needs of its newborn population and provide the best outcomes for newborns with a disorder identified by NBS.
Limitations
The results of this analysis are limited to NewSTEPs 360 funded programs, yet most NBS programs are engaged in activities to achieve ACHDNC timeliness goals and beyond. States NBS programs applied to participate in the NewSTEPs 360 program, potentially introducing a selection bias as they may not be a representative sample of all NBS programs Additionally, NBS program variation in NBS data collection may limit interpretation of QI timeliness data. For instance, the ACHDNC timeliness goals apply to first specimens collected, but some programs were unable to differentiate between first and subsequent specimens, which can result in longer reported timeframes than programs reporting data for first specimens only. Some programs also complete second-tier testing to improve the specificity of the screen, but potentially delaying the final result reporting. In addition, not all programs were able to collect the necessary time stamp of specimen receipt at the laboratory electronically, resulting in limited data reporting for some of the outcomes. There is also significant variability in the definitions of required data elements on the dried blood spot card, making interpretation across programs difficult. Finally, only a subset of programs provided case level data, limiting the generalizability of the results.
Conclusions
Newborn screening is one of the most successful public health programs in the US.[ 13 ] While states have clear authority with regard to NBS program oversight and monitoring, there is a federal role in supporting states in the implementation of the various components of the newborn screening system and ensuring timely diagnosis and management. The ACHDNC, public health departments, clinical specialists, birthing facilities, midwives, primary care providers, and parents have partnered to improve the newborn screening system. Improving timeliness of reporting of results has been a critical focus.
The individualized approach within NewSTEPs 360 allowed coaches to customize the support provided to the state newborn screening program and, whenever possible, connect one program with another who had shown success in an area. We believe that this structure strengthened the effectiveness of the program. In addition, the NewSTEPs Data Repository played a key role in the success of programs because it allowed (1) participating programs and CQI coaches to identify areas of needs, (2) the NewSTEPs 360 leadership to identify and meet educational needs of the larger group, and (3) the newborn screening community to see the gains made in timeliness.
The achievements of the NBS programs participating in this continuous quality improvement project in partnership with NewSTEPs 360 are the result of the ongoing support by the broader newborn screening community and its commitment to the newborns it serves. Continued success will depend upon that network of support.
Supporting information
https://doi.org/10.1371/journal.pone.0231050.s001
https://doi.org/10.1371/journal.pone.0231050.s002
https://doi.org/10.1371/journal.pone.0231050.s003
Acknowledgments
Disclaimer: The views expressed in this publication are solely the opinions of the authors and do not necessarily reflect the official policies of the U.S. Department of Health and Human Services or the Health Resources and Services Administration, nor does mention of the department or agency names imply endorsement by the U.S. Government.
- View Article
- PubMed/NCBI
- Google Scholar
- 7. Newborn Screening Timeliness Goals ww.hrsa.gov/advisory-committees/heritable-disorders: Health Resources and Services Administration; 2015 [updated 09/2017; cited 2019 6/25/2019].
- 10. NewSTEPs Quality Indicator Source Document https://www.newsteps.org/quality-indicators: NewSTEPs, a Program of the Association of Public Health Laboratories; [cited 2019 6/26/2019].
- 11. NewSTEPs Case Definition Toolkit [website]. https://www.newsteps.org/case-definitions: NewSTEPs, a Program of the Association of Public Health Laboratories; [cited 2019 6/25/2019].
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Quality Improvement Article
- Published: 23 March 2021
Improving timeliness of newborn screens in the neonatal intensive care unit: a quality improvement initiative
- Kelechi Ikeri ORCID: orcid.org/0000-0002-1482-0320 1 ,
- Vilmaris Quinones Cardona ORCID: orcid.org/0000-0001-5384-2429 1 , 2 &
- Ogechukwu R. Menkiti ORCID: orcid.org/0000-0002-0852-2626 1 , 2
Journal of Perinatology volume 41 , pages 1166–1173 ( 2021 ) Cite this article
1298 Accesses
1 Citations
1 Altmetric
Metrics details
- Outcomes research
- Paediatrics
Despite the established utility of newborn screening tests (NBS), achieving timely specimen transit is a challenge for neonatal intensive care units (NICU).
This project was conducted between September 2017 and July 2020 using the Plan-Do-Study-Act (PDSA) tool. Our primary aim was to increase the percent of NBS samples reaching the state laboratory within 1 day of collection by 20% by April 2020. Process, outcome, and balancing measures were monitored.
Five hundred and eighty-five NBS were collected. There was special cause variation with improvement in the percent of samples received within 1 day of collection from 28 to 77%. Special cause variation was also observed in the process measures without an increase in the percent of unacceptable samples.
Conclusions
Standardizing the NBS collection processes by adopting a sample collection window and same day courier pickup ensures timely specimen transit without adversely affecting the quality of samples collected.
Similar content being viewed by others

Optimizing the hospital discharge process to facilitate family-centered care for well newborns
Kelechi Ikeri, Kristen Noles, … Michael Zayek
Frequency of diagnostic errors in the neonatal intensive care unit: a retrospective cohort study
Grant J. Shafer, Hardeep Singh, … Kanekal Suresh Gautham
Newborn bloodspot screening in the time of COVID-19
Ronda F. Greaves, James Pitt, … John Christodoulou
Introduction
The newborn screen (NBS) program is one of the largest public health initiatives involving newborns in the United States. Since the development of screening tests for phenylketonuria by Robert Guthrie in the 1960s, the number of diseases detected by this program has expanded. Currently, testing is available for more than 50 disorders in most states and to be effective, all involved processes and steps must be efficient and timely [ 1 ].
Traditionally, the NBS process has been divided into three phases. The time from birth to NBS sample arrival at the testing laboratory is termed the pre-analytic phase. The analytic phase includes all events in the laboratory through generation of results. The post-analytic phase encompasses all the processes from reporting and receipt of results to the completion of the follow up actions and closure of each case [ 2 ].
Timeliness of the NBS process is important to reduce morbidity and mortality. Time-critical metabolic conditions like amino acid disorders require immediate treatment, with hours of delay resulting in increased risk of mortality [ 3 ]. Diagnostic tests for most time-critical conditions have long turnaround times (TATs) hence the NBS serves as an important early screening tool.
The NewSTEPS 360 program was developed in 2015 to support state NBS programs in an effort to improve timeliness. This initiative, funded by the Health Resources and Services Administration, has improved various quality benchmarks among state participants [ 2 ].
Current NBS timeliness goals established by the Advisory Committee on Heritable Disorders in Newborn and Children (ACHDNC) in April 2015 are recognized nationally as quality indicators [ 2 , 3 ]. The sole purpose of these goals is to ensure timely screening in the presymptomatic phase thereby reducing disability, morbidity, and mortality. These benchmarks are standards for specimen collection, transit and result reporting. For specimen transit, the specific goal is for all samples to reach the state laboratory ideally within one calendar day [ 2 ]. Despite improvements in overall timeliness, few NewSTEPs 360 participants have met the ACHDNC target of timely transit of specimens to state laboratories. In the United States between 2016 and 2018, only 39–42% of specimens from NBS programs in 25 participating states were received within one calendar day despite the implementation of suggested interventions [ 2 ].
For nonparticipating centers like ours, achieving timeliness goals also remain a challenge.
During the baseline period (September 2017–April 2019), only 34% of the samples were same day courier pickups with 28% arriving at the state laboratory within one calendar day. Also, 76% of filter papers were sent with missing information. These delays could result in devastating consequences such as in cases of congenital hypothyroidism where delayed treatment could lead to poor neurodevelopmental outcomes [ 4 , 5 ]. In our unit, two infants with presumptive alpha-L-iduronidase deficiency had results reported at 18 and 22 days after sample collection causing delays in initiating appropriate management.
Specific aims
Our SMART (Specific, Measurable, Achievable, Realistic, and Timely) AIM was to increase the percent of samples reaching the state laboratory within 1 calendar day of collection by 20% (from 28 to 48%) by April 2020.
This quality improvement project was conducted between September 2017 and July 2020 in the neonatal intensive care unit (NICU) at St. Christopher’s Hospital for Children using the Model for Improvement’s Plan-Do-Study-Act (PDSA) tool to gather knowledge, test and implement interventions. This unit is a 39-bed level IV out-born tertiary referral center. Clinical providers include neonatology attending physicians, neonatology fellows, nurse practitioners, physician assistants, nurses and rotating pediatric residents. All patients admitted to the NICU are transferred from other hospitals and initial samples are customarily obtained prior to transfer. State regulations stipulate that samples should not be collected before 24 h of life because of higher false negative rates and increased potential to miss life-threatening metabolic conditions [ 3 ]. Most of our NBS samples are repeat specimens.
The unit utilizes an electronic health record (EHR) system and the NBS can be checked as part of a power plan or ordered separately. After EHR order entry by the provider, the NBS sample is collected by the patient’s nurse who hands it to the unit secretary. Safe keeping of NBS filter papers, pre- and post-sample collection, processing, and subsequent dispatch to the hospital laboratory is ensured by the unit secretary. The hospital has a central laboratory that processes the NBS samples after receipt from the NICU and a unique tracking number is generated by laboratory personnel prior to courier shipping. Samples are shipped to the state laboratory on 6 calendar days per week excluding Sundays. Specimens drawn on Sundays are stored in the hospital laboratory until courier pickup the following day. All samples in Pennsylvania are subsequently processed at the PerkinElmer laboratory in Pittsburgh, PA. Data on individual specimen TAT, percent of unacceptable samples, and percent of samples with missing information are provided to the NICU Medical Director by the state laboratory on a monthly basis.
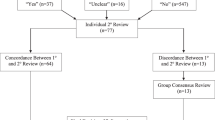
Process mapping identified barriers and process redundancies (Supplementary Fig. 1 ). Feedback from key stakeholders in the NBS program shed light on the common causes of delay: erratic timing of sample collection, inconsistent collection techniques, lack of standardized sample processing, and inconsistent pickup by courier services. A driver diagram was created to identify primary drivers and formulate interventions required to reduce delays (Fig. 1 ).
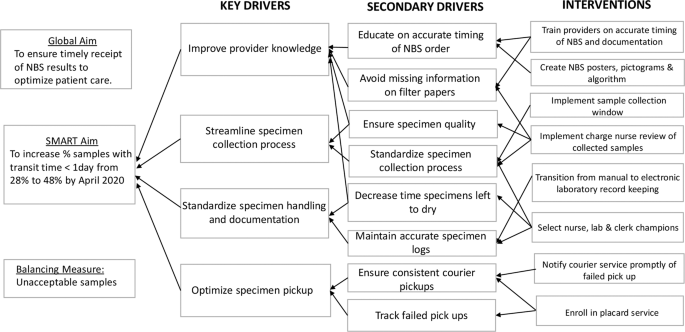
Key Driver Diagram summarizing the project aim, drivers and interventions required to achieve smart aim.
Interventions
We assembled a quality improvement team comprising two neonatology attending physicians, a neonatology fellow as well as a pediatric resident, nursing, clerical, and laboratory staff champions. Interventions were targeted at problems identified in the various phases of the NBS process and implemented through a series of PDSA cycles. Problems with timing of specimen collection were addressed through educational sessions, use of the NBS algorithm, the NBS calendar and implementation of a sample collection window. Nursing in-service, the use of visual reminders and review of filter papers by the nursing champion were aimed at standardizing sample collection techniques. The placard system was introduced to tackle inconsistencies with courier pickup.
Educational sessions
These sessions were conducted to prepare providers for upcoming changes to the NBS process and to fill gaps in knowledge of appropriate NBS sampling times based on the current Pennsylvania guidelines. Informal provider education began in April 2019 and a formal educational series was started in June 2019 during the weekly NICU conference. Brief sessions were subsequently conducted monthly for pediatric residents on the first day of their NICU rotation.
NBS algorithm, calendar, and handoff
Despite ongoing educational interventions, providers experienced difficulties in identifying and appropriately timing repeat NBS tests. A NBS admission algorithm was designed to help providers navigate these challenging situations. Educational presentations were revised to include this algorithm.
The NBS calendar and documentation in handoff sheets were introduced to facilitate appropriate timing of repeat samples. Providers were required to manually enter the patient’s name below the scheduled test date on the calendar. This information was shared with nursing and clerical champions to facilitate timely filter paper preparation. Providers were also asked to document the dates of upcoming NBS in the patient handoff.
Sample collection window
This intervention was implemented to address erratic timing of specimen collection. Review of baseline data provided by the state laboratory revealed a higher percent of samples with transit time less than one calendar day in February 2019. Most of these samples were collected between 3 a.m. and 11 a.m. and preferentially timed by the physician team to coincide with other morning laboratory samples to minimize additional heel sticks. Further study showed that morning sample collection also allowed for adequate sample preparation and processing before the stipulated 4 p.m. courier pickup. We hypothesized that modification of sample collection time to a morning window would improve transit time by eliminating redundancies in handling prior to shipping. Through implementation of PDSA ramp to drive improvement (Fig. 2 ), a morning collection time was implemented in May 2019. This intervention was modified in June 2019 to include a narrower collection window from 8 to 10 a.m. in routine situations so that samples were collected and processed in the same shift to minimize handoffs. This collection window was required to fall within 24–48 h of life, with the exception of repeat samples collected beyond 48 h of life. However, providers were reminded to obtain NBS samples prior to emergency transfusion of packed red blood cells, even if the patient was <24 h old and the timing of collection fell outside of the window. In these instances, repeat samples were obtained following state guidelines.
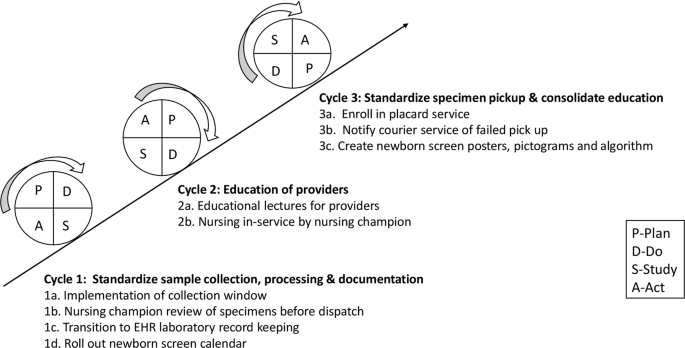
PDSA testing ramp for improvement illustrating the PDSA cycles implemented to achieve SMART Aim.
Nursing in-service and visual reminders
To standardize sample collection techniques, nursing staff in-service on sample collection and processing were organized by the nursing champions. NBS posters and pictograms were created and placed in strategic NICU areas to serve as visual reminders and to provide information on proper sampling methods [ 6 ].
Nursing champion review of filter papers
To address the high percent of samples sent to the state laboratory with missing information, starting in June 2019, filter paper samples were required to be reviewed by the available nursing champion or unit secretary. This served as quality assurance prior to processing and dispatch to the hospital laboratory.
Placard service
Local laboratory records showed inconsistent sample pickups by the courier. A placard service was requested in June 2019 and successfully implemented in September 2019 at no extra cost. It involved placement of a provided barcoded plaque on the NBS courier collection box and courier personnel scanning at sample pickup time. This allowed for real time team notification of successful pickup. Failed pickups were promptly identified and immediately rescheduled online by the fellow physician team member. Information on failed pickups was relayed to the courier company contact to aid in root cause analysis, avoid recurrences and foster resolution.
Process measures included the percent of samples obtained within the instituted collection window and the percent of dried blood spot filter papers sent with missing information or incompletely filled. Data entry and documentation compliance was monitored through review of patients’ EHR, handoff sheets, the NBS calendar and laboratory records. We also tracked the percent of samples picked up on the same day by the courier service and the monthly percent of NBS samples reaching the state laboratory within 1 day of collection. To determine the impact of our interventions on the entire NBS process, we monitored the percent of samples with TAT <3 days. TAT was defined as time from sample collection to result reporting. Specimen transit time and TAT data on each sample was provided to a designated physician monthly by the state laboratory.
To further highlight clinical relevance, we compared the percent of patients with presumptive diagnosis made <3, 4, and 5 calendar days after sample collection pre- and post-intervention as an outcome measure.
We recognized that the implementation of a strict sample collection window could inadvertently provoke undue stress among nursing staff potentially affecting the quality of specimens obtained. For this reason, the percentage of unacceptable samples was chosen as a balancing measure. Unacceptable samples were defined by the laboratory as: quantity not sufficient for testing, oversaturated, diluted, discolored, clotted, double spotted or contaminated specimens, specimens not soaked through to the back of filter paper, and specimens mailed while blood is wet or got wet in transit [ 6 ].
Study of interventions
Process and balancing measures were represented using annotated P statistical process control (SPC) charts. The outcome measure was represented on a bar graph comparing pre- and post-intervention data. QI-Macros 2019 was used to analyze and generate SPC charts. Centerlines and 3-σ control limits were defined using standard approaches. Special cause variation was detected and established rules were applied when at least eight consecutive points were above or below the center line, one or more data points fell beyond the control limit or six consecutive points trended in either direction [ 7 , 8 ]. Centerlines were adjusted based on detection of special cause signal [ 9 ]. This initiative was reviewed by the Drexel University Institutional Review Board and determined to not meet the definition of human subject research.
A total of 585 NBS samples were collected: 361 samples during the 20-month baseline period from September 2017 to April 2019, and 224 samples during the intervention period between May 2019 and July 2020. All NBS samples were included and there were no missing or lost samples. There was special cause variation in the percent of samples collected between 8 and 10 a.m. from a baseline of 4.6 to 78% after implementation of the sample collection window (Fig. 3A ). Monthly fluctuations in this measure reflected either noncompliance or higher volume of acute cases with emergent need for blood transfusion. There was special cause variation and a center-line shift in filter papers missing information with a decrease from 76.2 to 17% (Fig. 3B ). Provider documentation compliance was 92.2% with the admission note, 83.1% with the handoff sheets, and 66.4% with the calendar after interventions.
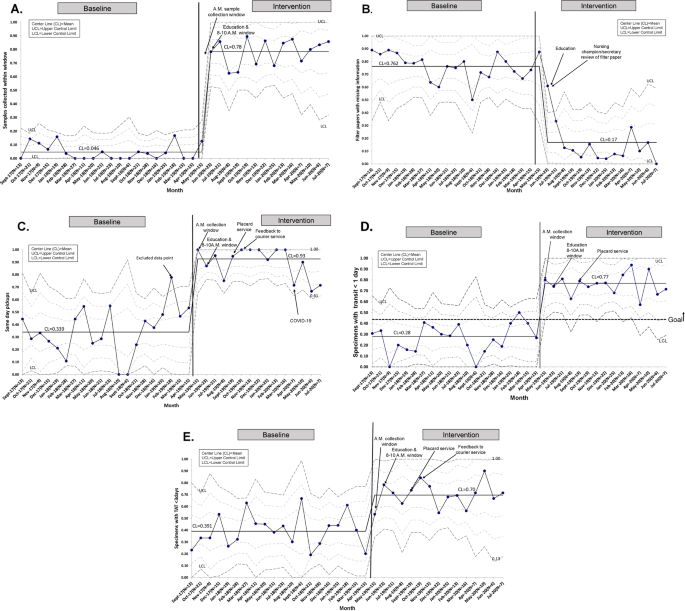
A Specimens collected within the designated window. B Filter papers with missing information. C Same day pickups. D Specimen with transit time <1 day. E Specimen turnaround times <3 days.
For same day courier pickup, initial review showed special cause variation in the baseline data with one data point (February 2019) falling above the upper control limit. Further study showed that most NBS samples obtained that month were timed with collection of other morning samples at the preference of the on-service physician. This data point was not incorporated into the calculation of the center line or control limits. With interventions, special cause variation was observed in same day pickup with improvement from 33.9 to 93% (Fig. 3C ). After sequential process changes, there was special cause variation with center-line shift in the percent of samples reaching the state laboratory within 1 day from 28 to 77%, exceeding our goal of 48% (Fig. 3D ). Special cause variation was also observed in the percent of samples with TAT <3 days with improvement from 39.1 to 70% (Fig. 3E ).
Compared to baseline, a higher percent of patients were presumptively diagnosed at <3, 4, and 5 days of sample collection respectively following interventions (Fig. 4 ).
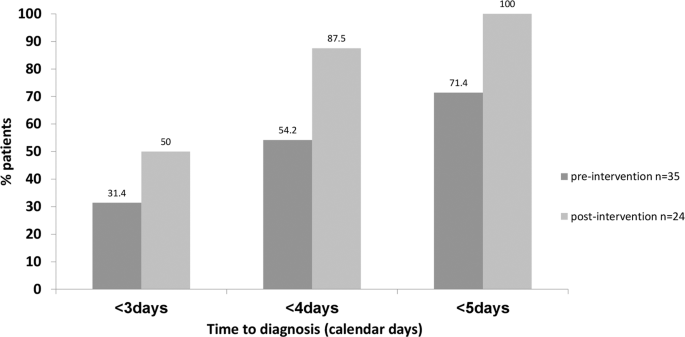
Percent of patients who were presumptively diagnosed in <3, 4 and 5 days from NBS collection pre and post-intervention.
Regarding the balancing measure, the percent of unacceptable specimens remained minimal with a mean of 4% (Fig. 5 ).
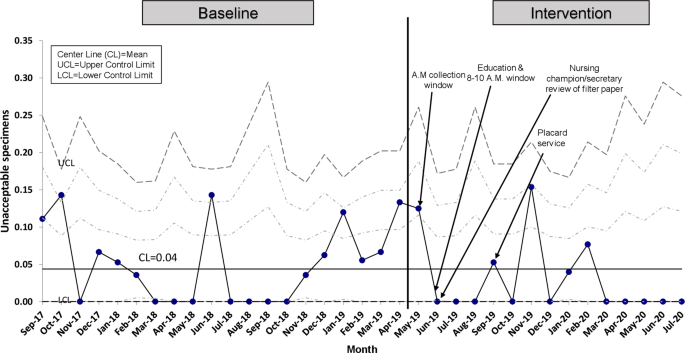
Percent of patients with unacceptable NBS specimens.
Timeliness of the NBS system facilitates early diagnosis of critical and noncritical medical conditions allowing for prompt initiation of management strategies. Preventing NBS process delays is critical to achieving timeliness goals. ACHDNC timeliness goals apply to initial specimens collected and are reported in relation to time of birth [ 2 ]. For Level IV out-born NICUs where most NBS samples collected are repeat specimens, computing timeliness data in reference to the time of collection is more reflective of the NBS process.
Achieving timeliness of specimen transit remains a challenge even for NewSTEPs 360 participating centers. Sontag et al. reported only 41% of samples arrived at the laboratory within 1 calendar day [ 2 ]. A recent study assessing the Michigan NBS program also identified the time between specimen collection and receipt by the state laboratory as a significant bottleneck in the process. The authors concluded that modifying sample pickup time to 9 p.m. could reduce specimen transit delays [ 10 ]. In our initiative, we demonstrated that improved specimen transit time is attainable with a pickup as early as 4 p.m. without comprising specimen quality. Afternoon pickup allowed for same day morning sample collection and ample time for processing (3 h drying time and in-house laboratory processing). To achieve same day pickup, a strategic modification to a morning sample collection window was essential. A challenge we faced in the adherence to this collection window was the need for emergency transfusions among high acuity patients altering the timing of specimen collection. We also encountered courier service interruptions imposed by the COVID-19 pandemic leading to a transient decrease in same day pickup in April 2020. Despite these challenges, the overall timeliness was not adversely affected likely due to the cumulative effect of other implemented interventions. The improvement in the percent of samples with transit time <1 calendar day to 77% is particularly important for time-critical metabolic conditions. As a result of pre-analytic improvement in timeliness, a higher percent of presumptive positive cases was promptly detected allowing for earlier subspecialty referrals and treatment.
Samples sent with missing information also cause delays in the NBS process. Strategies adopted by NewSTEPs 360 programs to tackle this problem focused solely on education of providers [ 2 ]. In comparison, we demonstrated a significant reduction in the percent of samples with missing information through education in addition to the implementation of filter paper review prior to dispatch.
Our balancing measure, as defined by the percent of unacceptable samples, did not increase with our interventions.
Limitations
This quality improvement project was conducted in a Level IV NICU with specialized resources, a dedicated in-house laboratory and established courier service which may limit its generalizability. However, creative interventions such as a establishing a sample collection window and optimization of courier services can be more readily incorporated in the workflow of all level NICUs with invested leadership and staff.
Our work focused on the pre-analytic phase of the NBS process. We recognize that problems in any of the three phases of NBS processing can lead to delayed result reporting despite local efforts to improve timeliness. However, we demonstrated that directed efforts to the pre-analytic phase improved overall timeliness and further work is necessary in the other steps to further impact our TAT. In terms of the data itself, we had no means of assessing the degree to which each identified factor contributed to TAT delays. This information would have been useful for determining priority of interventions.
This quality initiative serves as a roadmap for improving NBS timeliness in the NICU and demonstrates success through implementation of strategic interventions addressing barriers to timely specimen transit. More importantly, this initiative highlights earlier detection of presumptive positive cases is attainable without adversely affecting the quality of samples collected or comprehensiveness of information required for processing in a Level IV NICU. Continued surveillance is crucial for long-term sustainability, hence appointed champions will continue to drive project aims and the sample collection window will be incorporated into local clinical practice guidelines. Future directions include expanding these efforts to the local outpatient pediatric and special needs clinic, modification of sample collection window to ensure synchrony with clinic workflow and training of clinic staff on specimen collection.
Further work is needed to understand the role of the analytic and post-analytic phases in overall timeliness.
Kronn D. Navigating newborn screening in the NICU: a user’s guide. Neoreviews. 2019;20:e280–91.
Article Google Scholar
Sontag MK, Miller JI, Mckasson S, Sheller R, Edelman S, Yusuf C, et al. (2020) Newborn screening timeliness quality improvement initiative: impact of national recommendations and data repository. PLoS ONE. 2020;15:e0231050.
Article CAS Google Scholar
Newborn Screening Timeliness Goals. Health resources and service administration. 2015. https://www.hrsa.gov/advisory-committees/heritable-disorders/newborn-screening-timeliness .
Wassner AJ. Congenital hypothyroidism. Clin Perinatol. 2018;45:1–18.
Weiner A, Oberfield S, Vuguin P. The laboratory features of congenital hypothyroidism and approach to therapy. Neoreviews. 2020;21:e37–44.
Solutions for Newborn Screening. Perkin Elmer; 2020. https://newbornscreening.perkinelmer.com .
Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and health improvement. Qual Saf Health Care. 2003;12:458–64.
Provost LP, Murray S. The health care data guide: learning from data for improvement. Hoboken, NJ: Wiley; 2011.
Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32:2113–26.
Cochran AL, Tarini BA, Kleyn M, Zayas-Caban G. Newborn screening collection and delivery processes in Michigan birthing hospitals: strategies to improve timeliness. Matern Child Health J. 2018;22:1436–43.
Download references
Acknowledgements
The authors acknowledge the tremendous efforts of our key champions Jillian Taylor MD, Sharon Leonardo RN, Leann Mason RN, Milagros Pina, Jeanette Rodriguez, Terrilyn Rynkiewicz, Nikki Beckel, and Rebecca Bausinger. We also want to thank the entire NICU team who made implementation and sustainability of this initiative possible.
Author information
Authors and affiliations.
St. Christopher’s Hospital for Children, Philadelphia, PA, USA
Kelechi Ikeri, Vilmaris Quinones Cardona & Ogechukwu R. Menkiti
Drexel University College of Medicine, Philadelphia, PA, USA
Vilmaris Quinones Cardona & Ogechukwu R. Menkiti
You can also search for this author in PubMed Google Scholar
Contributions
KI conceptualized and designed the study, collected data, participated in the analysis, and drafted the initial paper. VQC conceptualized and designed the study, assisted and supervised data collection, carried out analysis, reviewed the data, critically reviewed, and revised the paper for important intellectual content. ORM conceptualized and designed the study, assisted in data collection, critically reviewed, and revised the paper for intellectual content. All authors approved the final paper as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Correspondence to Vilmaris Quinones Cardona .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental figure 1, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Ikeri, K., Quinones Cardona, V. & Menkiti, O.R. Improving timeliness of newborn screens in the neonatal intensive care unit: a quality improvement initiative. J Perinatol 41 , 1166–1173 (2021). https://doi.org/10.1038/s41372-021-00985-z
Download citation
Received : 21 October 2020
Revised : 14 January 2021
Accepted : 03 February 2021
Published : 23 March 2021
Issue Date : May 2021
DOI : https://doi.org/10.1038/s41372-021-00985-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Enhancing specimen collection skills for dried blood spots through an immersive virtual learning environment: a cross-sectional study.
- Hafsa Majid
- Aysha Habib Khan
BMC Research Notes (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Texas Health Steps
Newborn screening, section 1: the purpose of newborn screening.
This section includes:
The Screening Tests
Blood specimen and handling procedures.
- 5 Top Ways to Avoid Unsatisfactory Specimens (“UNSAT”)
Timing of Specimen Collection
Specimen collection and submission, remote data services, limitations of newborn screening, parental right to request destruction of blood spot samples.
- Increase text size
- Decrease text size
- Choose the two time frames within which newborn screening must be conducted.
- Given a case study, indicate one procedure for collection and handling of newborn blood specimens.
Newborn screening is recognized internationally as an essential, preventive public health program for early identification of disorders in newborns that can affect their long-term health. The most important goal of newborn screening is to identify infants with treatable conditions before they become symptomatic.
In 2006, the American College of Medical Genetics (ACMG) recommended testing 29 core conditions (including hearing screening) to help identify potentially treatable disorders within days of birth. This panel is now referred to as the Recommended Uniform Screening Panel as recommended by the Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Recently, Severe Combined Immunodeficiency (SCID) has been added to the uniform panel. Texas has not implemented statewide screening for SCID at this time, but the Newborn Screening program is participating in a pilot study to determine the ideal testing methodologies, cutoff levels, and incidence rates in Texas. The Secretary’s Advisory Committee has also recommended that Critical Congenital Heart Disease be screened for in all newborns by pulse oximetry; however, Texas has not implemented screening for this condition at this time.
Many of the disorders screened for in these tests are rare but serious, and may cause irreparable damage in the first days and weeks of life. Newborn screening can:
- Detect a serious, congenital disorder before symptoms present.
- Lead to diagnosis and treatment that can prevent serious problems, including cognitive and developmental delays, and death.
- Detect carriers of certain genetic disorders.
Early detection, diagnosis, and treatment can also lead to reduced medication, hospitalizations, and mortality. For some of the disorders, changes in diet or other interventions can prevent lifelong consequences.
In Texas, all newborns are screened for 28 specific metabolic, endocrine, hematologic, and genetic disorders at 24 to 48 hours after birth regardless of feeding status and/or before any transfusions. Every infant must receive another screening between 1 and 2 weeks of age, as some cases may only be detected in the second screen.
The Texas Department of State Health Services (DSHS) Newborn Screening Program identifies a significant number of diagnosed cases from the second screen, after the first screen is normal. In some conditions, due to the stress of birth, the first screen may be out-of-range with a normal second screen.
Both required screens are performed on a small sample of blood collected from a newborn’s heel. The DSHS Laboratory in Austin evaluates all blood samples collected for newborn screening. In addition to specimen collection, testing, and lab reporting, newborn screening in Texas consists of clinical care coordination (formerly called case management) and follow-up of diagnosis and treatment.
Laboratory services are a federally mandated component of Early and Periodic Screening, Diagnosis, and Treatment (EPSDT), in Texas called Texas Health Steps. EPSDT preventive care services are available to Medicaid-eligible children who are birth through 20 years of age. The Texas Health Steps Periodicity Schedule designates age-specific medical checkup components. The second newborn screening is a required component for the two-week checkup. At any time up to 12 months of age, if a child comes in for a scheduled checkup and the results of the newborn screen are not available, a blood sample for screening must be obtained during the checkup.
All Texas newborns are screened for congenital hearing disorders. The newborn hearing screening is conducted in the birth facility using either otoacoustic emissions (OAE) or auditory brainstem response (ABR) techniques. A newborn must pass the hearing screen for both ears, or a second birth screening must be done before discharge from the birthing facility. If the second screening is not passed, the newborn is referred to a pediatric audiologist for definitive outpatient screening. The goal of the Texas Early Hearing Detection and Intervention (TEHDI) Program is to have the initial screen completed by 1 month of age, outpatient diagnosis by 3 months of age, and intervention by 6 months of age.
For more information about hearing screening, enroll in the Texas Health Steps provider education module titled Newborn Hearing Screening .
The collection process requires precise handling of all blood samples as well as strict attention to detail in collecting specimens at the required intervals. This ten-step illustrated guide provides directions for correct blood specimen collection and handling procedures for a newborn screen.

Illustrated guide used with permission from Whatman, a trademark of GE Healthcare Companies. ©2009–2010.
This short video further explains how to correctly collect and prepare samples for a newborn screen.
- play/pause the video
- mute/unmute the video
- rewind and stop the video
Please install the latest version of Flash to view the video.
Video produced by the Texas State Department of Health Services and the Texas Health and Human Services Commission.
Five Top Ways to Avoid Unsatisfactory Specimens (“UNSAT”)
- Apply blood from one side while viewing from the other side to ensure complete saturation of the entire circle.
- Complete one circle at a time and fill all five circles.
- Allow 4 hours to dry, then send the specimen to DSHS as soon as possible.
- Double check for accurate and complete demographic information.
- Check expiration dates on collection kits.
Examples of Unsatisfactory Blood Spots
Blood did not Soak Through Paper - Incomplete saturation.
Blood did not Completely Fill Specimen Circles.
Specimen Appears Contaminated or Discolored (Light area in center from possible alcohol contamination).

Specimen Appears Contaminated or Discolored. (Improper Drying)
Blood was Caked, Clotted, or Layered onto the Filter Paper.

Filter Paper is Scratched from the Possible use of Capillary Tubes.
Serum Separation due to Improper Drying or Specimen Collection.

Blood spot sample images used with permission from the DSHS Newborn Screening Laboratory.
The initial blood specimen must be obtained after the newborn is 24 hours old, but before he or she is 48 hours old, regardless of feeding status and/or before transfusions. If the newborn is scheduled for discharge from the birth facility before these criteria are met, the blood specimen must be obtained immediately before discharge. When the infant is between 7 and 14 days of age, a second blood specimen must be collected by the child’s physician or other health-care practitioner. If an out-of-range result occurs on either of the two screenings, prompt follow-up is critical.
Although the timing of specimen collection is straightforward in full-term, healthy newborn infants, the primary care provider should be aware of factors that may influence the results of a particular screening test, including gestational and postnatal age, early discharge, diet, transfusions, and total parenteral nutrition (TPN).
For an infant who presents to a provider for the first time after 14 days of age without having had a second specimen taken, the specimen should be collected at the first opportunity. Please be advised that for older children, the screen may not detect all disorders. The concept of the medical home—the patient’s primary point of contact when accessing health care—was developed by the American Academy of Pediatrics (AAP) and promoted by Texas Health Steps. It establishes responsibility by a primary care provider for submitting the newborn blood specimen and responding to the outcome if out-of-range. The definition of a medical home is one in which care is accessible, family-centered, continuous, comprehensive, compassionate, and culturally competent. It is a partnership between a child, the child’s family or caregiver, and the child’s primary health-care setting. The primary health-care setting can be a physician’s office, a hospital outpatient clinic, a school-based health center, a community health center, or a health department clinic.
For more information about the medical home topic, enroll in the Texas Health Steps provider education module titled Introduction to the Medical Home .
As Texas Health Steps is a voluntary preventive care program for Medicaid-eligible children, a patient might seek services only for acute care issues. Medicaid providers should review their patients’ records for newborn screening results at any time up to 12 months of age to assure the screenings were completed and the results did not need to be addressed.
Screening Supplies
The newborn screening sample must be collected using specific supplies. The required specimen collection kit consists of a specialized filter paper collection device, a form for recording patient demographic information, a parent information sheet, a parent form about residual use and storage of blood spots, and envelopes. These kits must be ordered from the DSHS Laboratory using the DSHS Order Form for Newborn Screening Supplies. Click the following link to access the order form online . For additional information on the specimen collection kits, contact DSHS Laboratory Supply at 512-776-7661 or fax 512-776-7672.
Each kit is licensed by the U.S. Food and Drug Administration and contains a lot number and an expiration date. A kit must not be used past its expiration date. Any specimen collected on an expired form will be rejected as unsatisfactory and will need to be recollected.
Required Information
Certain demographic information must be submitted to the DSHS Laboratory with the infant blood sample. Incorrect, invalid, incomplete, or illegible information can result in a need to redraw blood and can delay the timely provision of follow-up testing and treatment that affected infants may need.
The following information is required to complete the submission:
- Infant’s name: first and last.
- Mother’s name: first, last, and maiden name.
- Gender of infant.
- Birth date and time.
- Date and time of specimen collection.
- Patient identification data, such as a medical record number and parent’s current address and telephone number.
- Birth weight in grams. (Use the online Newborn Screening Case Management Publications Order Form for a copy of the Newborn Screening Program Weight Conversion Chart).
- Infant diet at time of screen: breastfeeding, bottle-feeding, TPN, or combination.
- Submitter’s identification number and address.
- Name and telephone number of infant’s primary care provider. (This is needed so that DSHS can quickly contact the infant’s health-care provider in case of out-of-range newborn screening results).
- First-screen serial number for second and subsequent screens only. (This is used to link the second screen specimen to the first screen specimen. The first screen serial number will be on the tear-off sheet given to parents at the time the first screen is collected).
In circumstances when information is unavailable, such as in the case of foster care or the mother’s incarceration, use the best information available for contact in the case of an emergency. This may be a caseworker’s cell phone number, adoption agency name, or grandparent’s information.
Record all required information on each infant’s specimen collection card, taking care not to obscure, contaminate, or otherwise interfere with the circles designated for the placement of the blood specimen on the cards.
Timely Submission of Specimens
Specimens must be tested within a specific timeframe to ensure accurate test results. All specimens should be mailed within 24 hours of collection. Holding a specimen for batch mailing will delay testing and follow-up of out-of-range results. In addition, if the specimen is too old to test, it will be rejected as unsatisfactory and will need to be recollected.
To avoid submitting “too-old-to-test” specimens, mail the dried blood spot cards as soon as possible. Do not save them for batch mailing.
Care in Screening
All blood specimen collection instructions provided in the kit must be followed exactly. Some tests may not be accurate if the specimens are collected outside the appropriate timeframe or if they are collected haphazardly. Because some of the disorders for which newborns are screened can cause irreparable harm within days after birth if follow-up care is not promptly provided, it is essential to meet all screening requirements for both tests.
Safety and Accuracy Protocols
Make sure that:
- All of your collection kits are up to date by checking the expiration date.
- Your samples are not contaminated in any way during storage, collection, or preparation for submission.
- All demographic data on the infant and mother is complete, accurate, and legible.
- The first blood sample is collected at 24 to 48 hours after birth.
- The second blood sample is collected at 1 to 2 weeks after birth.
- All samples have been completely dried for at least four hours on a flat, non-absorbent surface before being placed in an envelope for mailing.
- All blood samples are sent to the DSHS Newborn Screening Laboratory within 24 hours of collection.
Ignoring any of these key elements can cause a delay in a complete screen and in providing screening results. Such delays slow the delivery of essential health-care services to infants who may be affected by the disorders for which screening is provided.
Unsatisfactory Specimens
If for some reason the newborn screen result is reported as "unsatisfactory" (or "UNSAT"), a repeat screen must be done as soon as possible.
Access to Test Results
Typically, it takes three to five days after receipt of the samples in the laboratory to obtain test results on all 28 disorders. In addition to calling the DSHS Newborn Screening Laboratory at 512-776-7333 to request laboratory reports, the newborn’s medical home may access screening results through the Newborn Screening Web Application and the Voice Response System (VRS) .
With an Internet connection and Adobe Reader 8 or higher , authorized users of the Newborn Screening Web Application can immediately access NBS result reports on a 24/7 basis. With the proper information, users can access any newborn screening results reported by the laboratory since 02/08/2007. This includes access to newborn screening results for specimens submitted by other facilities.
Results are available through this system as soon as they are reported by DSHS, and facilities can avoid waiting for reports to be delivered through the mail. To obtain authorization to access the system, users are required to complete and submit a Facility Security Agreement for each facility and Web User Access Agreement forms for each user. These forms can be downloaded from the NBS Remote Data Services Forms web page. Once the security agreements are received and processed, DSHS provides a username and password.
NBS result reports can also be accessed 24/7 through the NBS Voice Response System by calling 888-963-7111, Ext. 7300. To access the service, you will need your submitter ID number and PIN. To obtain a PIN, users may send an email request to [email protected] or call 888-963-7111, Ext. 6988.
Demographic Data Entry
The Newborn Screening Web Application also allows authorized users to electronically submit patient demographic information. Use of this system can reduce errors in data interpretation and transcription. In addition, the system includes data validations that ensure accuracy and completeness of information prior to submission. These features can reduce the number of specimens rejected due to missing, illegible, or invalid demographics.
Results from the newborn screening panel should not be considered diagnostic. Recommended follow-up testing (diagnostic testing or for a limited number of conditions, a repeat screen) should be performed after an out-of-range NBS result.
Newborn screening panels do not include all possible congenital conditions. Find information on additional available (supplemental) screening at Baby’s First Test .
An in-range newborn screening result does not eliminate the possibility that a clinically symptomatic child may have one of the screened-for congenital conditions. In addition, some screened conditions may present with clinical deterioration before notification of newborn screening results. Therefore, when an infant has signs or symptoms that are suggestive of, or consistent with, one of the disorders that can be detected by newborn screening, this congenital condition should still be considered.
Signs and Symptoms May Include:
- Poor feeding
- Failure to thrive
- Developmental delay
- Hepatosplenomegaly
- Persistent jaundice
- Persistent hypoglycemia
- Metabolic acidosis
- Hyperkalemia
- Hyponatremia
Newborn screening may fail to identify some children who actually have the condition (false negative), cause parental anxiety after false-positive results, or detect disorders for which treatment is not effective.
There are circumstances, in addition, under which the primary care provider might have cause for concern that newborn screening was not performed. These circumstances include, but are not limited to, home births, emergency births, hospital transfers, and international adoption. In such cases, the provider should complete a screen, and if clinical symptoms exist, conduct a diagnostic workup.
A Texas law implemented in 2009 allows the parents, managing conservator, or legal guardian of a newborn to request destruction of their baby’s newborn screening blood spot card following testing. Under the law, providers are required to provide the parents or guardian with a disclosure/directive to destroy form (named “Use and Storage of Newborn Screening Blood Spot Cards”), which is attached to the specimen collection kit. Texas Health and Safety Code § 33.0111d-e.
This video webinar provides additional information on newborn screening and disclosure for providers.
Video produced by the Texas State Department of Health Services.
The current Texas Newborn Screening collection kit includes the disclosure/directive to destroy form on page 2 in English and Spanish. Disclosure/directive forms are available in English, Spanish, and Vietnamese on the DSHS Laboratory Services Newborn Screening web page .
The verification checkbox for the provider is located in the bottom right-hand corner of page 3 of the collection kit.

Form provided by the DSHS Newborn Screening Laboratory
New Blood Spot Parental Decision Process to Take Effect on June 1, 2012
A new law passed during the 82nd Texas Legislative Session in 2011 changes the current opt-out process for all residual specimen uses to an opt-in process for long-term storage and possible research uses outside of DSHS. Starting June 1, 2012, parents and guardians of newborns will be presented with, and asked to complete and return, a new form titled “Parental Decision for Storage and Use of Newborn Screening Blood Spot Cards.”
For more information about the use and storage of dried blood spots after the newborn screening test is complete, click the following link to the DSHS Newborn Screening Laboratory web page or call the Newborn Screening Laboratory at 888-963-7111, ext. 7333.
The parents of Lupita, a 12-day-old newborn, bring her in for her second newborn screening specimen collection. When you tell them about the disclosure/directive to destroy form and hand it to them, they said they already filled one out at the hospital when Lupita’s first “heelstick” was done and that they do not need another form.
Should you give them the form?
Your answer is correct! Even if the parents or guardian do not opt to have the blood spot card destroyed, the health-care provider must provide the form each time the infant is screened. However, it is true that the parent does not need to send the form to DSHS a second time.
Your answer is incorrect. Try again.
I verify that I've read this entire lesson.
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article

INTRODUCTION
Limitations of this statement, limitations of newborn screening, the algorithms, 3- to 5-day-old visit, concern that newborn screening was not conducted, parents decline newborn screening, provide parent education, order newborn screening, obtain waiver, flag the charts of unscreened patients, results received, 2- to 4-week visit, are newborn screening results available, call for newborn screening results, screening results, document in-range screening results and reassure family, reorder newborn screening, consult act sheets and state newborn screening program, condition identified, document false out-of-range results and reassure parents, identify the child as a child with special health care needs and initiate chronic care management, implementing the algorithm, role of the medical home, role of the subspecialist, recommendations, preparing the practice, collaboration with other health care professionals, prenatal health care professionals, hospitals and other birthing facilities, pediatric medical subspecialists, state systems, state newborn screening advisory committees, national partnerships, national medical specialty organizations, including the aap, conclusions, newborn screening authoring committee, writing consultant, appendix 1 2005 acmg recommended screening panel, appendix 2., appendix 3., appendix 4 contact information for state newborn screening programs, appendix 5: documenting refusal to have infants undergo newborn screening, acknowledgement, newborn screening expands: recommendations for pediatricians and medical homes—implications for the system.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Newborn Screening Authoring Committee; Newborn Screening Expands: Recommendations for Pediatricians and Medical Homes—Implications for the System. Pediatrics January 2008; 121 (1): 192–217. 10.1542/peds.2007-3021
Download citation file:
- Ris (Zotero)
- Reference Manager
Advances in newborn screening technology, coupled with recent advances in the diagnosis and treatment of rare but serious congenital conditions that affect newborn infants, provide increased opportunities for positively affecting the lives of children and their families. These advantages also pose new challenges to primary care pediatricians, both educationally and in response to the management of affected infants. Primary care pediatricians require immediate access to clinical and diagnostic information and guidance and have a proactive role to play in supporting the performance of the newborn screening system. Primary care pediatricians must develop office policies and procedures to ensure that newborn screening is conducted and that results are transmitted to them in a timely fashion; they must also develop strategies to use should these systems fail. In addition, collaboration with local, state, and national partners is essential for promoting actions and policies that will optimize the function of the newborn screening systems and ensure that families receive the full benefit of them.
It's another busy day in pediatric practice, even before you receive the telephone call from the state newborn screening program. One of your newborn patients has an out-of-range result * on the screen for a rare but serious congenital condition. “Now what?” you wonder, as you begin to take down the notes. What additional testing is needed? What is the treatment regimen, and when does it begin? What do you tell the parents? And, what do you do about the rest of your schedule?
In the past decade, new technologies have led to a rapid expansion in the number of congenital conditions that are targeted in state newborn screening programs. As newborn screening programs expand, the likelihood increases that individual pediatricians will one day receive an out-of-range screening result for an unfamiliar congenital condition for one of their patients.
In 2005, the American Academy of Pediatrics (AAP) endorsed a report from the American College of Medical Genetics (ACMG), which recommended that all states screen newborn infants for a core panel of 29 treatable congenital conditions and an additional 25 conditions that may be detected by screening (Appendix 1 ). 1 The Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children (ACHDGDNC) † also adopted that report. Some states are now screening for more than 50 congenital conditions, many of which are rare and unfamiliar to pediatricians and other primary health care professionals. In the foreseeable future, screening programs will likely adopt screening technologies that will further expand the number of conditions screened and tests offered.
The ACMG, with the support of the Health Resources and Services Administration (HRSA) Maternal and Child Health Bureau (MCHB), has developed and maintains Web-based resources it calls action (ACT) sheets to guide pediatricians through preliminary responses to out-of-range newborn screening results. These brief reference resources provide a focused, single-page summary of differential diagnoses, descriptions of the condition, actions to be taken by the pediatrician, diagnostic evaluation, clinical considerations, reporting requirements, and links to additional resources. ACT sheets are designed to be supplemented by state-specific information regarding referral resources. Many state-program Web sites have additional program-specific educational information; links to these program Web sites are readily accessible through an interactive map maintained by the National Newborn Screening and Genetics Resource Center ( http://genes-r-us.uthscsa.edu/resources/consumer/statemap.htm ).
Advances in newborn screening technologies and the availability of resources such as ACT sheets are aimed at improving health outcomes for affected children. To optimize this potential, primary care pediatricians (PCPs) must effectively engage the newborn screening program in their state. PCPs who treat patients who routinely cross state borders for care will likely engage multiple newborn screening programs.
The primary goals of this statement are to:
delineate the responsibilities of PCPs and pediatric medical subspecialists within the newborn screening program;
introduce 2 algorithms that, together, outline a clear and efficient pathway through the process of fulfilling those responsibilities; and
outline resources that will support PCPs in addressing these responsibilities.
In addition to these primary goals, this statement addresses the steps that individual PCPs and practices must take to prepare for these responsibilities. We also recognize the significant roles other health care professionals and agencies have on the newborn screening system and identify ways these other entities can support PCPs and improve newborn screening and, therefore, advance improved health outcomes for newborns across the nation.
State newborn screening systems vary in their specific structure, procedures, and practices; this statement is focused on the core elements that are common to most state newborn screening systems. Newborn screening is increasingly being offered by commercial laboratories that market directly to parents and pediatric health care professionals. These programs introduce another layer of variation, which is beyond the scope of this statement.
Adequate funding of all aspects of newborn screening systems is necessary to ensure optimal performance of the system. This statement includes some general recommendations to promote such funding, and the AAP supports efforts to address financing for the nation's newborn screening systems and their constituent parts. Detailed recommendations for addressing the myriad challenges of system financing lie beyond the purview of this document. ‡
It is important to emphasize that newborn screening panels do not include all possible congenital conditions, and results for conditions on the panel should not be considered diagnostic. Thus, an in-range newborn screening result does not eliminate the possibility that a clinically symptomatic child has a congenital condition. Congenital conditions must be considered whenever an infant has signs or symptoms that are suggestive of (or consistent with) one of the disorders that can be detected by newborn screening.
An important goal of newborn screening is to identify infants with treatable congenital conditions before they become symptomatic. However, clinicians who care for children must be aware that some screened conditions may present with clinical deterioration before notification of newborn screening results. Pediatricians and emergency care physicians are often among the first health care professionals to encounter symptomatic infants, so they should be knowledgeable about the newborn screening program, ACT sheets for suspected conditions, and local or regional pediatric medical subspecialists to whom infants can be referred. The state newborn screening program usually can provide information about suspected conditions and expedite the newborn's follow-up confirmatory testing and care.
The PCP plays several significant roles in the newborn screening system. In addition to responding to out-of-range newborn screening results, the PCP serves as a central source of education for parents regarding multiple aspects of the newborn screening system; the PCP also has responsibility for ensuring that newborn screening has been conducted, which can include providing education and encouragement to parents who decline screening. Finally, the PCP must ensure coordinated and comprehensive care for children affected by congenital conditions that are identified through newborn screening. The medical home provides a model for such care; the algorithms presented here address the specific roles of a medical home provider within the newborn screening system (Figs 1 and 2 ).
The AAP 2 and Bright Futures 3 recommend neonatal follow-up visits in a child's medical home shortly after hospital discharge (3 to 5 days of life) and again by 1 month of age to ensure adequate weight gain, resolve neonatal concerns such as hyperbilirubinemia, and address parental questions. At the 3- to 5-day-old visit, the PCP should check for circumstances suggesting that newborn screening might not have been conducted.
In most cases, newborn screening will occur as a result of standing orders at a hospital or birthing facility. In these cases, the PCP can address other aspects of the visit.
There are circumstances, however, under which the PCP might have cause for concern that the newborn screening was not performed. These circumstances include, but are not limited to, home births, emergency births, hospital transfers, and international adoption. In addition, although most states mandate newborn screening, most jurisdictions provide parents with the right of refusal (see “Parents Decline Newborn Screening?”).
If available discharge papers do not indicate that the newborn screening has been performed, the PCP should make arrangements for specimen acquisition.
If parental refusal is the reason that newborn screening has not been conducted, or if parents refuse newborn screening suggested by the PCP, the PCP should discuss the possible implications of nontesting and supply the parents with printed materials on newborn screening. Educational materials for parents and PCPs can be accessed through the AAP Web site ( www.medicalhomeinfo.org/screening/newborn.html ).
Parent concerns and questions should be addressed fully, and a discussion of the general benefits and limited risks of newborn screening is recommended. More familiar conditions, such as congenital hearing loss, phenylketonuria, and sickle cell disease, may be used as examples.
If parental permission is obtained, arrangements for specimen acquisition should be made immediately, and newborn screening should be ordered.
Newborn screening is conducted through the state newborn screening program, and protocols for ordering the screening vary by state. Contact information for each state's newborn screening program is available (see Appendix 4 and http://genes-r-us.uthscsa.edu/resources/consumer/statemap.htm ).
If parental permission is not obtained, parents or guardians should be asked to sign a waiver that documents their decision to decline newborn screening. In many cases, parents already will have signed a waiver at the hospital. PCPs should document the additional conversation and the parents’ decision in the patient's chart and may wish to include a waiver signed in the PCP's office. A sample waiver form is included as Appendix 5 ; appropriate waiver forms should also be available through the state health department.
In addition to documenting the discussion of newborn screening and the parents’ refusal to consent to the screening, PCPs should flag the chart of any patients who are not screened so that the lack of screening will be taken into account should any subsequent concerns emerge regarding the child's growth or development. Vomiting, poor growth, seizures, developmental delay, lethargy, recurrent pneumonia, or poor feeding should prompt an evaluation that includes consideration of heritable conditions.
The chart note should also prompt the pediatrician to return to the question of newborn screening on subsequent visits to determine if the parents have changed their minds. The usefulness of screening after the neonatal period varies by condition, and use of state newborn screening systems for older infants varies by program.
Special Circumstances
For cases in which newborn screening is delayed because of previous parental refusal, because the infant was receiving total parenteral nutrition, or because of circumstances such as international adoption or an older infant entering care, the PCP should consult with the state newborn screening program regarding the availability and usefulness of the newborn screening protocol.
Newborn screening may not be ordered or may require an additional specimen in the case of preterm births, transfusion before screening, and other circumstances. 4 In these cases, the PCP should consult with a neonatal specialist.
In every circumstance, until and unless newborn screening is conducted, the patient's chart should be flagged to ensure that the lack of newborn screening is considered during ongoing care.
In the case of an invalid or out-of-range screening result, the pediatrician identified on the newborn screening card should be called by the state newborn screening program in accordance with the urgency of the need for clinical intervention. In-range results are often transmitted by mail and should arrive before the 2- to 4-week visit.
The PCP cannot assume a “no news is good news” approach with regard to newborn screening. Delays or procedural failures at hospitals, state laboratories, other facilities, or within the newborn screening program may result in late or lost results. An infant's medical follow-up may not occur as planned, or newborn screening results may go directly to the child's birth facility instead of the infant's medical home.
Office staff should check routinely for newborn screening results before the 2- to 4-week visit and pursue missing results before the visit. Using electronic-chart prompts or paper-chart templates for newborn visits will remind office staff to seek out newborn screening results.
If newborn screening results are not available before the 2- to 4-week visit, the PCP should contact the state newborn screening program or the birthing facility for the results. An increasing number of state newborn screening programs have automated interactive telephone- or Internet-based systems through which pediatric offices can check for newborn screening results at any time.
Occasionally, newborn screening results may be sent to the nonprimary physician; a physician who provides hospital or perinatal care for the infant may be noted on the newborn screening card even if he or she is not the infant's medical home physician. Clerical or other errors also may result in a physician who is unconnected to the child receiving the newborn screening results. However, the name on the card implies responsibility for the results, and physicians who receive results for patients who are no longer in their care should collaborate with the state newborn screening program and, in some instances, the hospital or birthing facility to locate the infant's family and/or current provider and to proceed with appropriate follow-up until the responsibility for subsequent care is clearly established. Physicians who receive results for patients with whom they or their colleagues have had no interaction should also notify the state newborn screening program immediately.
The state newborn screening program will report results to the child's physician of record as being in range, invalid, or out of range. Appropriate responses to each of these results are discussed in the next sections.
In-range newborn screening results should be noted in the infant's chart and shared with the parents or guardians. In reassuring the family, the PCP should keep in mind that newborn screening does not rule out congenital conditions that are not included in the panel and does not absolutely guarantee the absence of the conditions that are screened. The PCP might note, however, that false in-range results are quite rare and the family can be reassured that their child is unlikely to be affected by conditions for which screening was performed.
Nine states (Arizona, Colorado, Delaware, Nevada, New Mexico, Oregon, Texas, Utah, and Wyoming) mandate an additional screening when the infant is 1 to 2 weeks old on the basis of the belief that a second screening is necessary to identify the maximum number of children with genetic disorders. A second screening is recommended for all infants in several other states, and approximately 25% of all US newborn infants currently receive 2 screenings. The relevance of second screenings for endocrinopathies is the subject of a study currently being designed by the MCHB. PCPs should familiarize themselves with their state's policies and procedures. If a second screening is ordered, it can be introduced and explained to parents within the context of state policies and the current limitations to newborn screening technologies discussed previously.
If the specimen is invalid (eg, collected too early, inadequate specimen, poor drying or application technique, inadequate or illegible patient information), the infant's newborn screening must be reordered and blood redrawn. This screen should be completed promptly to optimize the availability of results. PCPs must be familiar with local protocols for rescreening and should contact parents immediately to direct them to the site at which the second blood specimen will be obtained.
An out-of-range result on the newborn screening panel is not a diagnosis. However, some congenital conditions can be rapidly fatal in infants who appeared entirely healthy a few days earlier; thus, out-of-range screening results should always lead to prompt action by the PCP.
If the state newborn screening program does not provide the ACT sheet specific to the condition for which an out-of-range result was obtained, the PCP should download it ( www.acmg.net/resources/policies/ACT/condition-analyte-links.htm ).
The ACT sheet should be reviewed and followed in its entirety, but the most important actions are highlighted. These actions include:
when to contact the family;
whom to consult and whether an appointment is needed immediately;
when the patient must be seen by the PCP;
whether additional confirmatory testing is needed and what tests should be conducted;
whether treatment is necessary and what treatment to initiate;
how to educate parents about the condition; and
when findings need to be reported back to the newborn screening program.
In addition to following ACT sheet recommendations, the PCP should consult with the state newborn screening program regarding out-of-range results. The state program should be familiar with local or regional experts for the conditions on their screening panels. In some states, the programs fund subspecialty clinics to conduct diagnostic evaluations and provide short-term and/or long-term subspecialty care to infants with out-of-range screening results.
After an out-of-range screening result is obtained, confirmatory testing and/or definitive consultation with subspecialists are required before a final diagnosis can be made.
To increase the sensitivity of a population screening test for rare conditions (and hopefully minimize the number of false in-range results missed), false out-of-range results are expected to occur, and false out-of-range results are significantly more frequent than true out-of-range results for most newborn screening tests. However, given the seriousness of the congenital conditions included in the newborn screening panel, the PCP must avoid complacency in the face of out-of-range results. Until confirmatory testing and/or definitive consultation with subspecialists can be accomplished, all out-of-range results must be taken very seriously.
In addition to true or false out-of-range results, confirmatory tests may identify the child as a carrier of the condition or may lead to an indeterminate result.
Carriers are individuals who are heterozygous for an autosomal-recessive condition and are usually not at risk of health problems themselves, although this may vary with the condition. Many state programs notify the PCP that the infant has been identified as a carrier, and it may be the responsibility of the PCP to disclose and discuss these results with the parents.
Knowledge of carrier status has 2 implications. First, because most of the conditions tested for on newborn screening are autosomal-recessive in inheritance, it is highly probable that at least 1 of the parents is a carrier also, and both parents might be carriers. If both parents are carriers, they have a 1-in-4 chance with each pregnancy of having an affected child. Alerting parents to the carrier status of their child serves to alert them that they may be at increased risk of having an affected infant with their next pregnancy. (When newborn screening results lead to genetic testing of the parents, pediatricians should be aware that misattributed paternity could be identified. Discussion with a geneticist or genetic counselor about how to manage these sensitive results may be helpful.)
The second implication of identifying a newborn as a carrier is that the infant will be at an increased risk of bearing an affected child when he or she achieves reproductive age if his or her future partner also is a carrier for the same condition. The risk is largely determined by the prevalence of the condition within the population, and additional genetic counseling may be warranted.
Occasionally, confirmatory diagnostic test results will not result in a definitive diagnosis. Uncertain results can be distressing to parents and PCPs, so thorough consultation with a subspecialist is essential. Unfortunately, indeterminate results may not be possible to resolve without more knowledge about some of these conditions and longer-term follow-up of these children.
At this point, it is incumbent on the PCP and the subspecialist to maintain an ongoing collaboration and continue to monitor the infant for signs and symptoms of a suspected condition. Children with uncertain results should have their chart identified for close monitoring. Good communication between the PCP and the consulting subspecialist is essential at this point to ensure that a unified message is conveyed to parents.
In the event that the initial out-of-range result proves to be a false out-of-range result, the PCP can provide reassurance to parents. However, research that evaluated parents of infants with false out-of-range results has suggested that 5% to 20% of these parents will persist in their concerns about the health of their children for months or years after screening. 5 – 9 Therefore, PCPs should not take the event of a false out-of-range result too lightly and may wish to discuss this issue with parents on subsequent visits to provide additional reassurance and eliminate any misconceptions.
To lay the foundation for comprehensive and collaborative care, it is critical during this time of uncertainty that the parents and family of the neonate be provided with condition-specific information and support as they await final clarification of the child's diagnosis and begin to plan for treatment and management. Parents are usually intensely anxious about the health of their child while the diagnosis is being pursued, and increasingly, parents are adept at tapping into resources on the Internet about specific conditions. Frequent, specific, and supportive communication from the PCP will help to avoid confusion and build trust. Appropriate materials for distribution to parents have been produced by the AAP ( www.aap.org ), the American College of Obstetricians and Gynecologists (ACOG [ www.acog.org ]), and MCHB/HRSA ( www.mchb.hrsa.gov/screening ). State newborn screening programs may also make educational materials available to health care professionals.
Any child who is given a diagnosis of a significant medical condition should be identified by the medical home physician as a child with special health care needs. Such a child should be entered into the practice's children with special health care needs registry, and chronic condition management should be initiated. Chronic care management provides proactive care for children with special health care needs, including condition-related office visits, written care plans, explicit comanagement with subspecialists, appropriate patient education, and effective information systems for monitoring and tracking the child's condition. 10
Regardless of diagnosis, every child needs a medical home to ensure coordinated and comprehensive care such that all of the medical, psychosocial, and educational needs of the child and family are met successfully within the local community. The PCP is responsible for providing a medical home.
Some conditions identified by newborn screening are relatively mild and or/transitory, and others have a wide spectrum of severity from asymptomatic to life-threatening crises. Plans for continuing care should be made in consultation with the family and appropriate subspecialists in light of the condition affecting the child and the severity of its manifestation. In some cases, the PCP may provide all or most of the ongoing care.
In other cases, the family may view their subspecialist as their primary care physician. Although a subspecialist may provide substantial ongoing care for a child who has been diagnosed with a severe and complex condition, the PCP retains the responsibility for providing a central source of “family centered, accessible, continuous, coordinated, comprehensive, compassionate, and culturally effective” care for the family. 11 The parents and family should be encouraged to maintain their relationship with their PCP. This relationship is critical, especially for cases in which the subspecialist is located at some distance from the family. In a crisis, the PCP may be the only available provider with knowledge of the child; he or she must have up-to-date information regarding the child's treatment.
The complex nature of many conditions identified by newborn screening may require care by a team of medical subspecialists, therapists, nutritionists, and educators. 12 The PCP and other professionals involved in the child's care must collaborate in the provision of acute care for illness or injury; surveillance of growth and development; anticipatory guidance to the family; immunizations; communications with schools, social services, and camps; transitions in care; and communication with other care professionals. In any case, clearly defined roles may help to reduce redundancies of services and prevent fragmentation of care.
The medical home should actively engage public and private resources to aid in the management of chronic conditions. Public health nursing provided through some state public health departments’ maternal and child health programs often has a role in assisting PCPs, subspecialists, and families of children with conditions that are diagnosed through newborn screening. The level of public health nursing may vary from simply providing information and referrals to assisting with chronic condition management for a family.
If there is not a local health department or nursing service, PCPs may contact their state maternal and child health department (Title V) and the directors of programs for children with special health care needs through the state department of public health to obtain information on the availability of local family services; the state department of education for contacts with school nurses; and the early intervention agency (Individuals With Disabilities Education Act Part C) for contact information for the local early childhood connections program. Although the state resources for public health vary greatly from state to state, almost all communities have one or all of these resources available for families. The national organization Family Voices ( www.familyvoices.org ) can provide information on local organizations and agencies that can offer resources to families with children with special health care needs and can assist families in accessing community services.
For additional information regarding care coordination, see the AAP policy statement “Care Coordination in the Medical Home: Integrating Health and Related Systems of Care for Children With Special Health Care Needs.” 13
For most of the conditions that may be detected through newborn screening, the subspecialist will confirm the diagnosis, develop the treatment plan, educate the family about the treatment, monitor treatment, identify complications related to the disease process that may require additional referral, and work with other consultants in coordination of care. When acute illness exacerbates the condition, the PCP should work with the subspecialist to diagnose the acute illness and manage it appropriately to reduce morbidity.
Some children with conditions identified through newborn screening will have long-term sequelae that will require ongoing subspecialty management despite appropriate early intervention. Many of these children will have mild neurodevelopmental disabilities that may present as learning difficulties, attention-deficit/hyperactivity disorder, or other behavioral problems. However, in some instances, more significant cognitive and motor deficits and/or problems that adversely affect the child's feeding skills and respiratory status may be present. It is essential that the PCP provide ongoing screening and surveillance for these developmental disabilities.
Even with appropriate treatment, patients with certain conditions identified through newborn screening can undergo metabolic decompensation during an acute febrile illness. PCPs need to be aware of the initial clinical signs and laboratory abnormalities that may be found when metabolic decompensation occurs and be able to provide immediate intervention to stabilize the child until more specific advice can be obtained from the appropriate treating subspecialist. Effective communication among subspecialists as well as between each subspecialist and the PCP is essential for optimal long-term management of these children. 14 Long-term responsibilities of the subspecialist, in collaboration with the PCP, include:
Providing genetic counseling and evaluation: Because the majority of conditions diagnosed through newborn screening are hereditary, genetic evaluation and counseling will be necessary for the parents. Older siblings may be affected with the condition but not yet symptomatic; diagnostic studies may be indicated for the siblings, and other relatives may wish to undergo carrier testing. The PCP, the state newborn screening program, and subspecialists are jointly responsible for ensuring that referral for genetic services occurs.
Providing ongoing parent education and links to available resources: Resources for managing the condition should be made available to the patients and their families. Subspecialists, the PCP, and the state newborn screening program should collaborate in making appropriate referrals to programs for children with special health care needs, childhood early intervention programs, community-based support services, and additional subspecialists who are needed to evaluate and manage associated disabilities. Information from disease-specific advocacy organizations, along with parent brochures and guidance for child health care professionals, may be available through the subspecialist. The Genetic Alliance, a coalition of advocacy groups, serves as another national resource for parents ( www.geneticalliance.org ). The National Library of Medicine also has material on every condition in the expanded ACMG-recommended panel ( http://ghr.nlm.nih.gov ).
Assisting in the transition to adult care: When transition to adult care is appropriate, the subspecialist will work with the PCP to identify a new team of physicians to care for the young adult. As adolescence proceeds, additional genetic counseling and preparation for family planning are appropriate.
Before receiving notice of an out-of-range newborn screening result from their state newborn screening system, PCPs can take several steps to enhance their ability to successfully address their roles and responsibilities within the newborn screening program.
PCPs should familiarize themselves with their state newborn screening program via available (online) resources or, if necessary, by contacting the state program. PCPs should develop some familiarity with the conditions being screened and basic operations of their state newborn screening program, including protocols for retesting invalid screening results and conducting second screenings. PCPs should identify the person(s) with whom they should consult in the case of an out-of-range screening result and ensure that contact information is readily available.
State-specific contact information for regional pediatric medical subspecialists should be collected and kept on file in the PCP's office.
Procedures to address several steps of the algorithm should be developed in advance. These procedures include:
updating contact information for the state newborn screening program and regional pediatric medical subspecialists;
identifying children who are most likely not to have had newborn screening;
confirming receipt of newborn screening results on all patients;
obtaining newborn screening results when they are not received from the state program;
documenting parental refusal of newborn screening; and
obtaining newborn screening specimens in the case of lost, delayed, or invalid results (the CPT code for retesting is 84030, and the diagnosis code is 270.10; PCPs should check with insurers to assess reimbursement).
PCPs should establish registries to identify, follow, and provide chronic condition management for children with special health care needs.
Educational materials regarding newborn screening should be on hand to distribute to expectant parents, parents who may decline newborn screening, and parents whose child's screening returns an out-of-range or inconclusive result. These materials should be available in languages and at literacy levels appropriate to all patients served. Appropriate materials for distribution to parents have been produced by the AAP ( www.medicalhomeinfo.org/screening/newborn.html ), ACOG ( www.acog.org ), and MCHB/HRSA ( www.mchb.hrsa.gov/screening ). State newborn screening programs may also make educational materials available to health care professionals.
Care coordination plays an essential role in ongoing efforts to integrate health and related systems of care for children and youth with special health care needs. 15 Becoming aware of available resources, being involved in the care coordination process, and developing unique care coordination approaches within one's own practice and community and in relationship with existing tertiary care centers are essential for providing optimal care for children with special health care needs. Families, PCPs, and other professionals can collaborate meaningfully to provide effective coordinated care. 13 , 15
PCPs are also encouraged to participate in state, regional, or national registries; quality assurance programs; and/or research projects designed to enhance the care of children with the rare and complex conditions included in the newborn screening panel. They are also encouraged to seek opportunities for additional training and learning about state newborn screening programs and the conditions for which infants are screened and to work with their local AAP chapter and state newborn screening advisory committee (SNSAC) to advance the quality and effectiveness of the newborn screening system at the state and federal levels.
The goals of ensuring the successful operation of the newborn screening system and advancing optimal care for infants and their families cannot be accomplished by PCPs alone. Effective collaboration and communication among PCPs and other clinicians and among the systems of care that engage the newborn screening system will ensure the best outcomes for infants and families. In light of this necessary collaboration, recommendations have been developed for prenatal health care professionals, hospitals and other birthing facilities, pediatric medical subspecialists, states and SNSACs, and federal agencies.
The prenatal period provides an ideal opportunity to begin to educate a family regarding the importance of newborn screening and the risks and benefits of early identification of the conditions identified through screening. The ACOG Committee on Genetics has asserted that “[o]bstetricians need to be aware of the status of newborn screening in their states and should be prepared to address questions or refer their patients to appropriate sources for additional information.” 16 The following specific steps can help bring the awareness and knowledge of the obstetrician to bear in preparing a family for newborn screening and promoting the function of the newborn screening system.
Prenatal health care professionals are ideally positioned to educate expectant parents about the newborn screening program in conjunction with the prenatal screening program. The obstetrician is encouraged to begin the education early enough to allow patients the opportunity to ask questions that will assist them in understanding the purpose of newborn screening, its implementation, and the importance of test results and follow-up. Concise, clear, and comprehensive educational materials and/or video presentations already in existence should be made available to expectant parents during the prenatal period. Appropriate materials are available from the AAP ( www.medicalhomeinfo.org/screening/newborn.html ) and the National Library of Medicine ( ghr.nlm.nih.gov/nbs ).
Prenatal health care professionals should strongly encourage prospective parents to identify a medical home for their infant early in pregnancy. When the mother presents for postpartum care, the prenatal health care professional can further support the medical home by inquiring about the infant's well-being and follow-up care.
If an infant is lost to follow-up to the newborn screening program, prenatal health care professionals should assist in locating the family.
In most cases, it is the facility at which the infant is delivered that is initially responsible for processing the newborn screening specimen. It is essential that these facilities have policies and procedures in place to ensure high-quality specimen processing and prompt delivery to the designated screening laboratory.
Particular attention should be brought to the development of protocols for:
Repeat screening of invalid specimens.
Documenting parental refusal to consent to newborn screening: Parents should be asked to sign a waiver form that documents not only their refusal to consent to newborn screening but also their understanding of the program and its purpose and the risks associated with their refusal.
Adequate training of clinical and laboratory staff and quality assurance programs focused on high-quality specimen processing: Appropriate and complete information regarding the infant, contact information, and medical follow-up must be gathered and submitted with specimens.
Assisting public health authorities in locating infants who are lost to follow-up: If the infant's medical home is not clearly identified, the facility at which the child was born should assume responsibility for notifying the family of an out-of-range screening result and referring for additional diagnostic testing and subspecialty care.
Identification of the medical home or site of medical follow-up should be established as a condition for discharge.
Discharge materials should clearly indicate whether newborn screening was conducted and should identify the PCP and the in-hospital managing physician for later contact, if needed.
Hospitals and other birthing facilities should ensure the availability of printed and/or video educational materials, presented in concise and understandable language, to all families, including those whose primary language is not English. These materials should address the purpose of newborn screening, the risks and benefits associated with newborn screening, and the consequences of delaying or refusing newborn screening.
Opportunities for further discussion or questions should be made available with either the family's chosen PCP or staff members who are knowledgeable about the screening process and the conditions for which screening is conducted.
Pediatric medical subspecialists play several roles in the care of children who have out-of-range results from newborn screening: they conduct confirmatory testing, care for the primary condition of infants who are affected by congenital diseases, and collaborate in the care of children with disabilities associated with some of the diseases identified through newborn screening. In fulfillment of these roles:
Pediatric medical subspecialists should assist the state newborn screening program in the development of educational materials for the public, families, PCPs, the state newborn screening program, and policy makers on specific conditions identified by newborn screening.
Pediatric medical subspecialists should serve on their SNSAC.
Pediatric medical subspecialists should respond promptly to requests for diagnostic and management services to infants with out-of-range screening results and children with conditions identified by newborn screening. Findings from clinic visits, laboratory studies, imaging studies, and diet and medication changes should be communicated promptly to the PCP, state newborn screening programs, other pediatric medical subspecialists, and the family (as appropriate).
Pediatric medical subspecialists should underscore the importance of maintaining a medical home relationship with the PCP for the infant identified with a condition through newborn screening.
Pediatric medical subspecialists should assist in the identification of associated disabilities and appropriate referral to other subspecialists for management.
Pediatric medical subspecialists should assist in the development of condition-specific protocols for the treatment of acute illness or injury and in the development of the child's care plan for school, activity restrictions, and special feeding/diet programs. Pediatric medical subspecialists should also work with the PCP, the family, and other subspecialists to delineate each person's role in managing acute illnesses, establishing relationships with schools and therapists, providing immunizations, working with social services and camps, and maintaining contact with insurers.
Pediatric medical subspecialists should provide ongoing education to the family and PCP about new developments and treatments for the condition and associated disabilities.
Pediatric medical subspecialists should work with the PCP and other subspecialists in identifying appropriate adult health care professionals for the transition to adult care.
The state's role in newborn screening is to design, coordinate, and manage an effective newborn screening system. It has traditionally been the state's responsibility to oversee key aspects of the newborn screening system, including initial screening, confirmation of diagnosis, and coordination of short-term follow-up for infants with out-of-range screening results as well as longer-term care for children with special health care needs. Ultimately, the state must maintain an adequate public health infrastructure to ensure that every newborn infant receives appropriate care.
The AAP Newborn Screening Task Force set forth a broad agenda for state newborn screening systems in its statement published in 2000. 17 In addition to addressing the recommendations that follow, states are urged to consult that AAP statement for guidance in developing and supporting an effective and comprehensive newborn screening system.
To ensure the appropriate and effective function of newborn screening systems, the following recommendations must be addressed immediately:
1. States must monitor specimen collection and transmission of information between screening hospitals, the testing laboratory, and individual practitioners.
2. Identification of the follow-up medical home must be required on all newborn screening specimens. 16 , 18
3. Laboratory collection and handling procedures must be clearly delineated at every site at which newborn screens are obtained or processed. State newborn screening laboratories are expected to maintain up-to-date technology and procedures and be prepared to implement recommended changes in the newborn screening process. 11
4. Practical mechanisms should be established for retesting infants whose newborn screening results are indeterminate/invalid regardless of the cause.
5. Procedures should be adopted to ensure that the medical home is notified of out-of-range screening results by telephone on a schedule consistent with the urgency of the need for intervention. In the case of urgent out-of-range results, a designated medical subspecialist may be notified in addition to the medical home; the newborn screening program may need to contact the family if efforts to contact physicians are not successful.
6. Procedures should be adopted to ensure that in-range and invalid screening results are available to the medical home within 2 weeks of an infant's birth.
7. When out-of-range screen results are reported, the appropriate updated ACT sheet (or equivalent) and state-specific referral information should be forwarded immediately to the PCP.
8. States must have policies and procedures in place to locate children who have not established a medical home and to ensure that all newborn infants with out-of-range screening results receive appropriate diagnostic follow-up and subspecialty care.
9. States must provide clinicians with contact information for their newborn screening program coordinator and ensure that clinicians are updated promptly should any changes occur.
10. Public health agencies and maternal and child health programs should assist with care coordination for patients with special health care needs and their families.
Because states play a significant educational role in the newborn screening system, the following are recommended:
11. With direction from the SNSAC, states should develop and facilitate distribution of clear and concise educational materials for families at prenatal visits and in the hospital at the time of delivery. Condition-specific materials must be developed for families whose infants have out-of-range screening results; these materials include an explanation of test results, appropriate educational materials on the tested condition, referral for additional diagnostic testing, and referral for subspecialty care. Educational materials developed by the AAP, ACOG, and HRSA/MCHB may be used and/or supplemented with materials developed by the state. These materials can be accessed at www.medicalhomeinfo.org/screening/newborn.html or mchb.hrsa.gov/screening.
12. The state must develop educational information for medical professionals that outlines their responsibilities in the newborn screening process.
Finally, there are a number of steps that can be taken to improve the operation of the newborn screening system, including the following:
13. To prevent delays in processing when screening occurs on the weekend, the newborn screening laboratory responsible for state screening should operate at least 6 days a week, with coverage for holidays. Rapid turnaround time for results is essential for prompt diagnosis and treatment of metabolic conditions.
14. Information systems through which clinicians could directly download newborn screening results should be developed. Policies and regulations must be developed concurrently to protect privacy and confidentiality rights.
15. States should develop and implement information systems that facilitate the tracking of infants across state lines through communication and integration of data across newborn screening systems.
16. States must develop and implement policies that allow for interstate licensure and practice of medicine (including the use of telemedicine) to facilitate consultation and communication to underserved areas and ensure the free flow of information across state lines. There is a shortage of pediatric medical subspecialists across the country and a complete absence from more sparsely populated regions. This challenge must be addressed cooperatively by the states.
17. States should ensure the availability of ongoing care for infants with out-of-range screening results who lack health insurance and for those whose insurance does not provide coverage for necessary services and treatments. Medically required diets and vitamins are among the treatments often excluded from coverage provided by third-party payers. 19
18. To promote greater understanding of the effects and benefits of the newborn screening system, states should develop information systems that are capable of tracking the multitude of performance measures for the newborn screening system and long-term outcomes of children with special health care needs identified through newborn screening. Performance measures include diagnosis for and treatment of infants with out-of-range screening results, cases missed by newborn screening, false out-of-range result rates, time to diagnosis, parental involvement and satisfaction, the social and psychological effects on families of infants with out-of-range and false out-of-range results, and family access to appropriate and necessary services. Data to support the analysis of cost-effectiveness and cost benefit should also be collected.
19. To provide national data for newborn screening system quality assurance and program comparison, state programs should contribute timely case findings and laboratory data to the national newborn screening data-collection system operated by the National Newborn Screening and Genetics Resource Center ( www2.uthscsa.edu/nnsis ).
20. SNSACs should be authorized in each state to help implement and ensure the establishment of principles of universal access, clinician and community education, remedial surveillance for accountability, and quality of services for all infants. SNSACs should be chartered with appropriate authority and provided adequate support to effectively fulfill the roles outlined as follows.
SNSACs should comprise a balanced, representative, and diverse membership. Representation by diverse families and societal leaders should be balanced by members of the health care community, including clinicians in practice, representatives of hospitals and professional organizations, and public health experts, including the laboratories and the state. A diverse clinician representation would include pediatricians, obstetricians, family physicians, and nurse and midwife practitioners. In addition, the panel must have access to expert medical subspecialists, health care researchers, and biostatisticians.
SNSACs should cooperate with the US Department of Health and Human Services ACHDGDNC and other federal agencies to promote consistency in newborn screening throughout the nation.
SNSACs must work to advance state support and development of the newborn screening system, with particular attention to:
efforts to use health information technology to advance clinician and family access to information about newborn screening as well as screening and follow-up services;
optimization and accurate interpretation of privacy laws;
implementation of a systems approach based on the Institute of Medicine principles for patient-centered safety, effectiveness, efficiency, timeliness, and equity 20 ;
efforts to provide unfettered access, through both print and electronic media, to understandable education materials for families with diverse reading and language abilities; and
development and distribution of resources for PCPs.
SNSACs must address identified challenges of fragmented service delivery as well as geographic, cultural, social, and financing barriers across county and state lines.
SNSACs should promote a statewide report on newborn health status for identifiable conditions and a national newborn health report that provides data on incidence, outcome, and community participation.
SNSACs must develop a mechanism for receiving feedback from parents, medical home practitioners, and subspecialists on the appropriateness of including particular conditions in the newborn screening program. This feedback should then be transmitted to the ACHDGDNC.
Each SNSAC is encouraged to develop its own charter and seek statutory establishment and state support.
Although states remain responsible for newborn screening systems, federal agencies and national organizations play a significant role in the newborn screening system and in supporting families of children with genetic conditions. Strengthening national partnerships between federal agencies and professional, nonprofit, and family organizations provides the opportunity for a coordinated effort to increase the services offered to children with genetic and congenital conditions in all stages of diagnosis, treatment, and follow-up. There are 4 critical points of partnership for these groups: collaboration, funding, oversight, and follow-up.
Collaboration
1. Health care professionals, nonprofit agencies, state and federal public health programs, and families should seek to build relationships with other groups that focus on the newborn screening system. Relationships can be fostered through partnering on national initiatives, inviting other perspectives to serve on project advisory committees, and establishing a systematic method of receiving feedback from families.
2. Research should be performed on all aspects of newborn screening systems, including parent and provider education, results management, laboratory quality, residual specimen storage and use, and, most importantly, efficacy of newborn screening for each proposed condition. A national research agenda for newborn screening should be outlined. Input from federal agencies, professional associations, nonprofit organizations, and family support organizations should be coordinated. Multistate or national collaborations are often necessary to recruit a sufficient number of affected infants to understand the clinical spectrum of the disease and to compare treatment strategies. Collaboration will be key in conducting this research.
3. National partnerships should be developed and coordinated to support state newborn screening systems and encourage coordination, effective collaboration, and decrease duplication.
4. Adequate third-party reimbursement, grant applications, nonprofit fundraising efforts, and other sources of funding for newborn screening programs should be pursued by those who seek to improve the newborn screening system. Funding for the components of the newborn screening system and long-term care of children with genetic conditions comes from a variety of sources including screening fees, federal programs, state programs, nonprofit fundraising, insurance companies, and others, and such funding is critical at all levels.
5. Because ongoing research in the areas of education, results management, laboratory quality, and identifying and treating genetic diseases is important as the world of newborn screening continues to expand, funding for the implementation of these research projects should be provided.
6. Because establishing and funding a 24-hour hotline for access to online state-specific newborn screening program contact information can be useful in supporting state newborn screening programs, physicians, and families, a dedicated newborn screening hotline should be considered as part of preparing for national emergencies, natural disasters, or other circumstances.
7. Funding should be provided for demonstration projects directed toward strengthening the communication process between pediatricians and the newborn screening program. These efforts can include the development of telemedicine, effective health information exchanges, and linked information systems to facilitate the communication process.
8. Because the increased level of services required to comanage and coordinate care for patients with special needs identified through newborn screening can pose a significant financial burden for the PCP and the subspecialist, appropriate CPT coding that is aimed at enhanced reimbursement for chronic condition management should be developed.
9. ACHDGDNC policies and activities should promote and facilitate uniformity across newborn screening programs, promote coordination between state newborn screening programs, support public health infrastructure for these programs, monitor the quality of these programs, and coordinate and promote research efforts related to newborn screening.
10. The ACHDGDNC should promote federal interagency collaboration and federal agency collaboration with state public health newborn screening programs to encourage coordination and effective collaboration between federal and state agencies.
11. Family involvement in all levels of newborn screening and follow-up care is important and should be encouraged. Families can give feedback on services provided, make suggestions on improving systems of care, advocate for needed services, and support other families that are going through similar situations.
12. Appropriate treatment and chronic condition management for children with congenital conditions should be ensured. Federal agencies, state newborn screening programs, and others can collaborate to create a national definition for follow-up to newborn screening systems.
13. Because enrolling children onto long-term research studies can provide the opportunity to test new treatments and better understand the natural history of chronic conditions, federal agencies and national organizations should promote opportunities for such research and create materials to educate parents about research in general and specific opportunities to participate in research.
National medical specialty organizations and their state chapters can play specific roles in the continued development of the collaboration necessary to ensure optimal performance of the newborn screening system throughout the country.
They should maintain communication with and participation on the ACHDGDNC to provide information to their constituencies and communicate any concerns to the ACHDGDNC.
They should foster education regarding newborn screening and promote pediatric medical subspecialties that focus on metabolic diseases among medical students and residents.
They should promote the development and implementation of a Health Plan Employer Data and Information Set (HEDIS) measure on newborn screening.
They should comment on the appropriateness of adding new tests to the core screening panel, ensuring that any newborn screening provides clear benefit to all children screened and to their families. These comments should be presented to the ACHDGDNC for consideration and adoption.
Advances in newborn screening technology, coupled with recent advances in the diagnosis and treatment of rare but serious congenital conditions that affect newborn infants, provide increased opportunities for positively affecting the lives of children and their families. These advantages, however, also pose new challenges to PCPs, both educationally and in response to the management of affected infants.
To respond appropriately, PCPs require immediate access to clinical and diagnostic information and guidance; ACT sheets from the ACMG are a valuable source of such guidance. PCPs, however, have a proactive role to play in supporting the performance of the newborn screening system and ensuring the successful completion of their responsibilities to the program. PCPs must develop office policies and procedures to ensure that newborn screening is conducted and that results are transmitted to them in a timely fashion. PCPs must also develop strategies to use should these systems fail.
The newborn screening system extends well beyond the PCP's office, and many other stakeholders are essential for ensuring that the system functions well and supporting PCPs in their role within the system. The system is challenged by error, lack of education or information on the part of families and health care professionals, and systemic challenges such as the national shortage of pediatric medical subspecialists and barriers inherent in state licensing requirements. Lack of universal health care coverage and limited funding for newborn screening programs present additional significant challenges.
State and federal entities, hospitals, prehospital health care professionals, pediatricians, and pediatric medical subspecialists should act collaboratively to address these challenges or reduce their effects on the newborn screening system. AAP chapters and individual pediatricians should work together with the AAP and SNSACs to promote actions and policies that will optimize the function of newborn screening systems and ensure that children and families receive the full benefit of them.
E. Stephen Edwards, MD, Chairperson
Vinod K. Bhutani, MD
Committee on Fetus and Newborn
Jeffrey Botkin, MD
Committee on Bioethics
Barbara Deloian, PhD, RN, CPNP
Bright Futures Early Childhood Expert Panel
Stephen Deputy, MD
Section on Neurology
Timothy Geleske, MD
Medical Home Initiatives for Children With Special Needs Project Advisory Committee
Joseph H. Hersh, MD
Council on Children With Disabilities
Celia Kaye, MD, PhD
Committee on Genetics
Jennifer Lail, MD
Quality improvement expert
Michele A. Lloyd-Puryear, MD, PhD
Maternal and Child Health Bureau
Michael Watson, PhD
American College of Medical Genetics
Aaron Carroll, MD, MS
Medical Informatician
Melissa Capers
Anne Gramiak, MPH
Jennifer Mansour
OA indicates disorders of organic acid metabolism; FAO, disorders of fatty acid metabolism; AA, disorders of amino acid metabolism; CoA, coenzyme A.

MCH indicates Maternal Child Health; CSHCN, children with special health care needs; NBS, newborn screening; SC DHEC, South Carolina Department of Health and Environmental Control.
Despite the best efforts of health care professionals to educate parents and guardians about the need to have their infants undergo newborn screening and the importance of newborn screening in the early identification of certain diseases, some parents and guardians will decline to have their infants undergo newborn screening.
All parents and guardians should be informed about the purpose of and need for newborn screening, the risks and benefits of newborn screening, and the consequences of late diagnosis of certain conditions that would have been identified earlier through newborn screening. The use of this or a similar form that demonstrates the importance you place on newborn screening and focuses attention on the unnecessary risk for which a parent or guardian is accepting responsibility may, in some instances, induce a wavering parent or guardian to accept your recommendation.
Disclaimer . This form may be used as a template for such documentation, but it should not be used without obtaining legal advice from a qualified health care attorney about the use of the form in your practice. Moreover, completion of a form, in and of itself, never substitutes for good risk communication, nor would it provide absolute immunity from liability. For instances in which parents or guardians refuse newborn screening, health care professionals should take advantage of their ongoing relationship with the family and revisit the discussion on subsequent visits. Documentation in the medical chart of such follow-up discussions is strongly recommended, and the template, therefore, makes provision for this documentation.
This form may be duplicated or changed to suit your needs and your patients’ needs and should be reviewed with your health care attorney before use. It will be available on the AAP Web 7 site ( www.aap.org/bookstore ).

Algorithm 1. NBS indicates newborn screening program (see Appendix 2 ).

Algorithm 2. NBS indicates newborn screening program; CSHCN, child with special health care needs (see Appendix 3 ).
All clinical reports from the American Academy of Pediatrics automatically expire 5 years after publication unless reaffirmed, revised, or retired at or before that time.
The guidance in this report does not indicate an exclusive course of treatment or serve as a standard of medical care. Variations, taking into account individual circumstances, may be appropriate.
A note about language: although physicians often think of screening results as being “normal/abnormal” or “negative/positive,” laboratories use the more specific language of “in range” and “out of range” to report results. We felt that it was appropriate to use and promote this language for the sake of clarity and consistency. For ease of reading, we use “parent” as a generic term to connote the adult who is responsible for a child's health care; we recognize that adults other than the biological parent may serve in this role. Where implications of congenital disorders are discussed, these obviously affect only those persons who are related biologically. In some circumstances, a primary care physician may suggest that the biological parent be contacted regarding congenital conditions, even if that parent is not the current primary caregiver for the child.
A federal advisory committee to the Secretary of Health and Human Services, the ACHDGDNC advises and guides the Secretary regarding the most appropriate application of universal newborn screening tests, technologies, policies, guidelines, and programs for effectively reducing morbidity and mortality in newborns and children having or being at risk for heritable disorders.
For guidance on the Current Procedural Terminology (CPT) codes appropriate for use in the care of children who are identified as having congenital disorders, PCPs should refer to Rappo MA, Rappo PD. A special issue: coding for children with special health care needs. AAP Pediatric Coding Newsletter . January 2007. Available at: http://coding.aap.org/newsletterarchive.aspx .
This project was funded by the Health Resources and Services Administration's Maternal and Child Health Bureau, through a contract in the American College of Medical Genetics cooperative agreement (U22 MC 03957-01-00). The views expressed in this report are those of the American Academy of Pediatrics, not necessarily that of HRSA/MCHB.
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Newborn Screening Collection and Delivery Processes in Michigan Birthing Hospitals: Strategies to Improve Timeliness
Affiliations.
- 1 Biostatistics and Medical Informatics, University of Wisconsin-Madison, Madison, USA.
- 2 Department of Pediatrics, University of Iowa, Iowa City, USA.
- 3 Department of Pediatrics, University of Michigan, Ann Arbor, USA.
- 4 Michigan Department of Health and Human Services, Lansing, MI, USA.
- 5 Industrial and Systems Engineering, University of Wisconsin-Madison, 1513 University Avenue, Madison, WI, 53706, USA. [email protected].
- PMID: 29616441
- DOI: 10.1007/s10995-018-2524-z
Objectives This study aimed to determine which steps in the newborn screening collection and delivery processes contribute to delays and identify strategies to improve timeliness. Methods Data was analyzed from infants (N = 94,770) who underwent newborn screening at 83 hospitals in Michigan between April 2014 and March 2015. Linear mixed effects models estimated effects of hospital and newborn characteristics on times between steps in the process, whereas simulation explored how to improve timeliness through adjustments to schedules for the state laboratory and for specimen pickup from hospitals. Results Time from collection to receipt of arrival to the state laboratory varied greatly with collection timing (P < 0.001), with specimens collected on Friday or Saturday delayed an average of 9-12 h compared to other specimens. Simulation estimates shifting specimen pickup from 6 p.m. Sunday-Friday to 9 p.m. Sunday-Friday could lead to an additional 12.6% of specimens received by the Michigan laboratory within 60 h of birth. Conclusions for Practice The time between when a specimen is collected and received by the laboratory can be a significant bottleneck in the newborn screening process. Modifying hospital pickup schedules appears to be a simple way to improve timeliness.
Keywords: Genetic testing; Newborn screening; Newborn screening process; Simulation model.
- Blood Specimen Collection / standards*
- Computer Simulation*
- Datasets as Topic
- Genetic Testing* / standards
- Infant, Newborn
- Neonatal Screening / methods*
- Neonatal Screening / organization & administration*
- Time Factors
Grants and funding
- 72453/Robert Wood Johnson Foundation
WARNING: We do not support Internet Explorer. It is not secure and will not work correctly. Please come back using a newer web browser.
Newborn Screening
Did you know that early newborn screening saves lives every year, blood tests, using a few drops of blood from a newborn's heel, can help detect genetic, endocrine, and metabolic disorders and defects allowing for early intervention and preventing death and disability., clsi newborn screening laboratory standards and products provide important test collection instructions, as well as information on methods for the detection of metabolic and hereditary disorders..

The APHL/ISNS Newborn Screening Symposium took place 15-19 October 2023 in Sacramento, CA. Click for more information .
Featured products | view and purchase our most recently published standards.

Newborn Screening Follow-up and Education, 3rd Edition
This guideline describes the basic principles, scope, and range of follow-up and education activities within the newborn screening program and system.

Newborn Screening for X-Linked Adrenoleukodystrophy, 1st Edition
This guideline discusses the detection of X-linked. adrenoleukodystrophy by population-based newborn screening using dried blood spot specimens to measure C26:0-lysophosphatidylcholine.

Dried Blood Spot Specimen Collection for Newborn Screening, 7th Edition
This standard highlights specimen collection methods, discusses acceptable techniques for applying blood drops or aliquots to the filter paper section of the specimen collection device, and provides instructions on proper specimen drying, handling, and transport to ensure quality specimens are consistently obtained for newborn screening analysis.

Newborn Screening for Hemoglobinopathies, 1st Edition
This guideline describes the recommended protocols for detecting hemoglobinopathies and thalassemias by population-based newborn screening using dried blood spot specimens. Early, presymptomatic detection to identify newborns with abnormal hemoglobins is critical because it improves treatment effectiveness.
More From CLSI

NBS01OL Free Video
Latest Insights: September is Newborn Screening Awareness Month
In 1963, as a result of a discovery by Dr. Robert Guthrie, newborns could finally be screened and receive treatment for a disease before symptoms appeared. Dr. Guthrie and his colleague published a paper outlining their discovery.

Shop all our Newborn Screening documents and resources here
Related blog posts, recent technologies for newborn screening.
Newborn screening (NBS) is an important component of public health that helps health care providers identify hereditary and metabolic disorders in newborns and enables early therapeutic intervention, which can be lifesaving or altering. Newborn screening programs are typically state- or country-based public health programs. Today, newer and more complex technology is increasingly being integrated into NBS.
The Importance of Prenatal and Newborn Screening
Prenatal screening tests are performed during pregnancy to determine the likelihood of a baby having specific birth defects. Most tests are performed during the first or second trimester and are noninvasive.
Improving Accuracy of Newborn Screening for Cystic Fibrosis
Cystic fibrosis newborn screening accuracy is essential for early diagnosis and treatment, which has been shown to improve outcomes. In a seven year study of 16 sibling pairs with cystic fibrosis, it was found that early initiation of therapy (before age 1) was beneficial.
The Importance of Newborn Screening Standards
Newborn blood spot screening is the practice of testing newborns for certain harmful or potentially fatal diseases that aren't apparent at birth. A simple blood test can diagnose these rare conditions.
Related Resources

CLSI uses cookies to ensure the best website experience. Continuing without changing cookie settings assumes you consent to our use of cookies on this device. You can change these settings at any time, but that may impair functionality on our websites. Review our privacy policy .
- Research Note
- Open access
- Published: 04 January 2024
Enhancing specimen collection skills for dried blood spots through an immersive virtual learning environment: a cross-sectional study
- Hafsa Majid 1 ,
- Lena Jafri 1 ,
- Shanzay Rehman 1 ,
- Azeema Jamil 1 ,
- Fatima Khanam 2 ,
- Nadir Shah 3 ,
- Nasir Ali Khan 1 &
- Aysha Habib Khan 1
BMC Research Notes volume 17 , Article number: 16 ( 2024 ) Cite this article
397 Accesses
Metrics details
The quality of dried blood spot (DBS) specimens impacts newborn screening (NBS) results, hence proper training is crucial for DBS specimen collection. To address this, a training module for Allied Health Professionals (AHPs) and nurses was created on Moodle, a virtual learning environment (VLE). The purpose of this research was to determine the feasibility and effectiveness of this module.
Methodology
Participants were trained on-site (March to December 2019), through online training sessions (January to June 2020), and the two training strategies were compared. Data analysis included the total number of participants, cost-effectiveness, trainer engagement, and the number of unacceptable samples collected by nurses/AHPs trained by the two strategies.
A total of 55 nurses/AHPs were trained on-site, while 79 nurses/AHPs completed the online module and received certificates through online VLE-based training. The trainer engagement and cost were more for onsite training. After online training, the specimen rejection rate was reduced from 0.84% (44 rejected out of 5220 total specimens collected) to 0.38% (15/3920).
Conclusions
This study shows that using VLE-based DBS specimen collection training is feasible and effective for training nurses and AHPs.
Peer Review reports
Introduction
The goal of newborn screening (NBS) is to identify infants with treatable disorders whose symptoms and signs are not present at birth [ 1 ]. The preferred sample for NBS testing is a dried blood spot (DBS) specimen because it is simple to collect, less traumatic for newborns, has less onerous storage and transport requirements, and can be used for multiple tests from a single DBS card [ 2 ]. However, because the target analyte concentration in DBS specimens is very low, it is necessary to use assays with very low analytical sensitivity, and a high-quality DBS specimen is required [ 3 , 4 ].
For the NBS test, a DBS sample is collected from heel prick by nurses or allied health professionals (AHPs) in inpatient settings [ 5 ]. Improper collection, specimen contamination, incomplete drying, and transporting at an inappropriate temperature can all degrade the quality of the DBS specimen [ 6 , 7 ]. As a result, these nurses or AHPs must understand ‘what constitutes a good quality specimen’ as well as ‘how to collect, store, and transport a DBS specimen’. So that DBS specimen quality issues do not impact NBS results, because specimen rejection causes delayed reporting affecting the overall NBS program performance [ 8 ].
Nurses and AHPs are typically trained on-site for DBS sample collection and evaluated for competence. These nurses/AHPs then collect DBS specimens and transport them to the clinical laboratory, where the quality of the specimen is evaluated by a trained technologist upon receipt at the clinical laboratory for analysis [ 8 ]. However, these on-site training sessions, and competence assessments, take up a significant amount of faculty time and must be repeated for recruits or retraining of the already existing pool of nurses/AHPs. Moreover, only a restricted number of trainees can be accommodated in a single session.
In this era of technological advancements, virtual learning environments (VLEs) are becoming increasingly popular for teaching and training, offering innovative educational tools and platforms [ 9 ]. These asynchronous teaching strategies allow trainees to fulfill learning objectives at their own pace and time. A training course was created on ‘Moodle’ a VLE, to train nurses/AHPs for DBS specimen collection. This study aimed to determine the acceptability and feasibility of the VLE as a training tool for DBS specimen collection.
This cross-sectional study was conducted at the Biochemical Genetics Laboratory (BGL) in the Department of Pathology and Laboratory Medicine at Aga Khan University. The project team consisted of a Chemical Pathologist, a BGL supervisor, an eLearning developer, and a nurse manager. Ethical approval for the study was obtained from the institute’s ethical review committee (ID # 2022-7346-20976). All participants gave written consent by electronic signature to participate in the study.
The study was conducted in four hospitals affiliated with Aga Khan University, including Stadium Road Hospital (a tertiary care center) and three maternal and child hospitals, Karimabad Hospital, Garden Hospital, and Kharadar Hospital. At these hospitals, full-term newborn babies were admitted to the well-baby nursery of their respective hospitals, while premature and critically ill newborns, were admitted to the Neonatal Intensive Care Unit (NICU), and DBS specimens were collected for NBS by nurses/AHPs. These nurses and AHPs were trained for DBS specimen collection, where the AHPs included phlebotomists and NICU technicians.
The onsite training was provided to nurses and AHPs specifically at the Stadium Road Hospital, and their competence was assessed afterward. Alternatively, an online training course was developed on “Moodle,“ a VLE, nurses, and AHPs from all three hospitals were trained through this online platform, followed by an assessment. The key performance indicators (KPIs) for the two training methods, including the number of unacceptable samples, were then compared. The clinical laboratory was responsible for receiving and analyzing all DBS specimens to monitor this KPI.
Onsite training for the DBS specimen collection
Only nurses and AHPs at the Stadium Road Hospital received physical or on-site training. The trainers for these sessions were an NBS expert and a BGL scientist, and a total of three sessions were conducted (Fig. 1 ). Each session lasted half a day and included instructional lectures/videos on DBS specimen collection, an overview of the institute’s current NBS program, relevant policies, and diagnostic protocols for the disorders, followed by a discussion and a question-answer session. Additionally, the training involved a demonstration by expert trainers on the proper collection, drying, and transportation of DBS specimens. Following the demonstration, the nurses’/AHPs’ performance was directly observed, and their competence in the procedure was assessed.

Onsite training of allied health professionals for dried blood spot collection
Online module development on VLE for DBS specimen collection
An online course was created on Moodle for DBS specimen collection training. The Clinical and Laboratory Standards Institute (CLSI) NBS01-A6 guidelines and ‘Laboratory General,‘ and ‘Clinical Biochemical Genetics’ Checklists of the College of American Pathologist Standards were used to create all content [ 10 , 11 ]. The group developed a contextual curriculum that included instructional materials such as videos, a presentation on the NBS program at the Aga Khan University, and a DBS manual including specimen collection site, procedure, specimen handling, characteristics of good and bad quality specimens, frequency of training and criteria for retraining. The DBS manual also included contact information for relevant individuals, such as well-baby nursery nursing managers, neonatal intensive care units, BGL technologists, Paediatric endocrine and metabolic team representatives, and NBS experts.
For knowledge assessment, a quiz was created, and competency assessment forms were uploaded to Moodle. After a trainee received 100% on the quiz and passed the competence assessment, the certificate was generated. To ensure content validity and reliability of instructional materials, two non-team members piloted the module to evaluate both the content and the course. The objective was to assess the difficulty, reliability, and accuracy of both the quizzes and study material, ensuring their suitability before implementing the module.
VLE-based training for DBS specimen collection
This module was implemented from January to June 2020, and nurses were enrolled to participate in the training module. The train-the-trainer approach was employed for this module, where four trainers were carefully selected to perform a competence assessment. Before introducing the module to the participants, these trainers read the material, and completed quizzes, after direct observation of DBS specimen collection on a newborn baby, the BGL scientist assessed their competence. These trainers then enrolled and trained other nurses in their respective areas for DBS specimen collection on Moodle. The decision to use the train-the-trainer approach was motivated by the large number of learners involved. Scalability, efficiency, and uniformity in the training process were attained by teaching a small number of trainers who could then assess the competence of a broader audience.
Statistical analysis
Analysis was performed using Excel 2020. The feasibility was evaluated based on the number of nurses/AHPs trained; the cost of online/onsite courses; the time of trainer for developing content, implementing the online course, or conducting onsite sessions, and the ratio of nurses/AHPs trained to trainer time. To assess the effectiveness of training, unacceptable DBS specimens received by the nurses/AHPs trained by the two strategies were compared. A specimen was labeled as unacceptable if any of the following criteria was met, incomplete drying, in sufficient quantity, clotting or layering, presence of serum rings, or contamination.
During the three on-site sessions, 53 nurses and 2 AHPs from the well-baby nursery and NICU underwent training, and their competence was evaluated by trainers. Over the following twelve months, the nurses and AHPs were monitored to assess the occurrence of unacceptable specimens. Table 1 provides information on the associated costs, trainer engagement, and the percentage of unacceptable specimens collected by nurses/AHPs trained via onsite sessions.
In Jan 2021, an online training module was developed on Moodle. The trainers included NBS experts (n = 4), and BGL scientists (n = 2). Over a period of six months, 120 nurses/AHPs were enrolled in the online training module (supplemental Fig. 1 ). Among the participants, there were 106 nurses from four different hospitals [Stadium Road Hospital (n = 3), Karimabad (n = 26), Garden (n = 39), and Kharadar (n = 38)]. While 14 AHPs, including NICU technicians and phlebotomists, were also enrolled. The participants attempted the knowledge assessment quizzes, a total of 234 times with 222 completed attempts. Out of the total, only, 104 scored 100%, 48% (n = 50) attempted the quiz once, 23% (n = 24) twice, 13.5% (n = 14) three times, and 15.4% (n = 16) more than three times. The quiz took approximately 7.2 min (4–60) to complete, and the median score was 17.3 (18 − 2.4). The total number of quiz attempts showed a positive correlation with the quiz score (r 0.33, p-value 0.001), but an inverse correlation with the total number of attempts (r -0.13, p-value 0.049). After achieving a perfect score, 79 nurses or AHPs (75.9%) cleared on competence assessment and received training certificates. The KPI was monitored for 6 months after the online training via Moodle. Table 1 provides information on the associated costs, trainer engagement, and the percentage of unacceptable specimens collected by nurses/AHPs trained via onsite and online sessions.
The KPI improved after VLE-based training, Table 1 . However, the report comparing the two strategies for this KPI was not statistically significant (p-value 0.3384). Table 2 presents a detailed comparison of the two training methods for DBS specimen collection.
This study encompassed both AHPs and Nurses since both play a crucial role in collecting dried blood spot specimens. In our clinical setup, AHPs primarily included phlebotomists and NICU technicians. Although nursing is not typically classified as an AHP according to the Association of Schools of Allied Health Professionals [ 12 ], we included both nurses and AHPs in this study due to their involvement in DBS specimen collection. On-site training posed challenges in terms of resource allocation and time commitment from trainers. To address these issues, an online course was developed as a solution to overcome these limitations and facilitate more accessible training methods.
According to our study findings, the VLE-based platform was feasible and effective in training nurses or AHPs for DBS specimen collection. The online course was more cost-effective, had improved accessibility, flexibility for trainees to access at their own pace, scalability with minimal infrastructure cost, and training a large number of participants with minimal trainer engagement. Another factor could be the availability of downloaded educational resources during the online modules, allowing AHPs to reference them as needed. However, certain limitations were no face-to-face interaction with the trainers and technological requirements, such as good Wi-Fi connectivity, and devices that can support VLE platforms. Similar findings were reported in a study by Al Rawashdeh AZ et al., which reported that online learning strategies improve accessibility and flexibility but decreases teacher-student interaction, leading to social isolation [ 13 ].
The quality indicator for the unacceptable DBS specimen improved after the online training module was implemented, with the percentage of unacceptable specimens decreasing by more than half, from 0.84 to 0.38%. This could be because we were able to train a greater number of trainees with minimal logistic requirements and trainer engagement using the VLE-based training. Similar findings were reported by MacRae D JM et al. that online training is flexible, scalable, can train a large number of trainees, and trainees can complete these courses at their own pace. The participants have complete control over their learning and can go back and read up on areas where they believe they are lacking, since the course content can be accessed at any point in time. Moreover, they can devote more time to a specific topic while skipping over irrelevant content [ 14 ].
According to a study by Veenhof H et al., the utilization of a web-based application for training in DBS specimen collection resulted in a decrease in the number of unacceptable specimens and an increase in the percentage of satisfactory specimens to 95.9%. The purpose of this application was to provide an objective evaluation of the quality of DBS specimens [ 15 ]. The current study also demonstrated similar findings, with a reduction of over 70% in the proportion of unacceptable specimens. Another study conducted by Allen MA et al. reported similar findings, they evaluated the acceptability and feasibility of web-based multimedia training for DBS specimen collection. Their findings showed that 96% of the collected specimens were of good quality and usable. As a result, they concluded that this training method is viable for instructing non-healthcare providers in the self-collection of specimens [ 16 ].
DBS specimens are not only utilized for routine patient testing but are also gaining popularity in research applications. The primary advantage of DBS specimen collection is its user-friendly nature, allowing it to be conducted outside of hospital settings by non-healthcare personnel. They are being used for various purposes such as drug analysis, toxicology studies, viral load testing, and routine chemistry analyses [ 17 , 18 ]. Currently, many NBS programs are preserving surplus DBS samples for future utilization in research, including method validation for new biomarkers or conducting repeat analyses and testing [ 19 ]. However, the accuracy of the results is directly associated with the quality of the DBS specimen. Therefore, proper training of nurses and AHPs becomes critical for obtaining good-quality DBS specimens [ 20 ]. The study’s findings suggest the use of VLE-based training for DBS specimen collecting to promote standardized and high-quality specimen collection. Online training modules on VLE systems provide a practical and efficient alternative to training large groups of learners and can be more cost-effective than on-site training sessions when considerations such as trainer involvement and associated costs are considered.
The study’s findings highlight the effectiveness of VLE-based platforms in delivering DBS specimen collection training to nurses/AHPs, which is crucial for ensuring the availability of high-quality specimens for accurate specimen collection. VLE-based training offers scalability and flexibility, enabling the training of a larger number of students while minimizing logistical constraints and trainer involvement.
Limitations
Limitations of this study include the relatively small sample size, as only 55 nurses/AHPs were trained on-site and 79 completed the online module. The study duration of March 2019 to June 2020 might not capture long-term effects or variations over time. Also, the curriculum developed was contextual so the study may not be generalizable. Hence, studies with larger sample sizes, longer duration, and diverse settings are needed to validate the findings and address these limitations.
Data Availability
The data set used and analyzed in the current study is available from the corresponding author upon reasonable request. Temporary access to the training module on Moodle ( https://vle.aku.edu/course/view.php?id=2637 ) can be provided on request.
Abbreviations
- Virtual learning environment
dried blood spot
biochemical genetics laboratory
neonatal intensive care unit
Clinical laboratory standards institute
key performance indicator
El-Hattab AW, Almannai M, Sutton VR. Newborn screening: history, current status, and future directions. Pediatr Clin North Am. 2018;65(2):389–405.
Article PubMed Google Scholar
Moat SJGR, Carling RS. Use of dried blood spot specimens to monitor patients with inherited metabolic disorders. Int J Neonatal Screen Int J Neonatal Screen. 2020;6(2):26.
McDade TWWS, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;1(44):899–925.
Article Google Scholar
Malsagova K, Kopylov A, Stepanov A, Butkova T, Izotov A, Kaysheva A. Dried blood spot in Laboratory: directions and prospects. Diagnostics (Basel). 2020;10(4).
Ikeri K, Quinones Cardona V, Menkiti OR. Improving timeliness of newborn screens in the neonatal intensive care unit: a quality improvement initiative. J Perinatol. 2021;41(5):1166–73.
Article PubMed PubMed Central Google Scholar
Winter TLA, Hannemann A, Nauck M, Müller C. Contamination of dried blood spots–an underestimated risk in newborn screening. CCLM. 2018;56(2):278–84.
Article CAS PubMed Google Scholar
Weiss LM, B-SM, Myck M, Nocoń K, Oczkowska M, Topór-Mądry R, Andersen-Ranberg K, Börsch-Supan A. Blood collection in the field–results and lessons from the Polish test study. Health and socio-economic status over the life course: De Gruyter Oldenbourg; 2019.
Google Scholar
El-Enein ANO. Nurses’ performance and knowledge regarding newborn screening program for congenital hypothyroidism, EL-Behaira Governorate. J High Inst Public Health. 2011;41(1):98–111.
Castro MD, Tumibay GM. A literature review: efficacy of online learning courses for higher education institution using meta-analysis. Educ Inform Technol. 2021;26:1367–85.
Clinical and Laboratory Standards Institute, Clinical and Laboratory Standards Institute. Blood collection on filter paper for newborn screening programs; approved standard. CLSI Document NBS01-A6. 2013.
College of American Pathologists. (2022). Laboratory General and Clinical Biochemical Genetics Checklist. In Laboratory Accreditation Program (LAP). Retrieved from [ https://documents.cap.org/documents/cap-accreditation-checklists.pdf ].
Turnbull C, Grimmer-Somers K, Kumar S, May E, Law D, Ashworth E. Allied, scientific and complementary health professionals: a new model for Australian allied health. Aust Health Rev. 2009;33(1):27–37.
Al Rawashdeh AZ, Mohammed EY, Al Arab AR, Alara M, Al-Rawashdeh B. Advantages and disadvantages of using e-learning in university education: analyzing students’ perspectives. Electron J E-learning. 2021;19(3):107–17.
MacRae DJM, Tyerman J, Luctkar-Flude M. Investing in Engagement: integrating virtual learning experiences across an undergraduate nursing program. Clin Simul Nurs. 2021;52:17–32.
Veenhof H, Koster RA, Brinkman R, Senturk E, Bakker SJL, Berger SP, et al. Performance of a web-based application measuring spot quality in dried blood spot sampling. Clin Chem Lab Med. 2019;57(12):1846–53.
Allen AM, Lundeen K, Murphy SE, Spector L, Harlow BL. Web-delivered Multimedia Training materials for the Self-Collection of dried blood spots: a formative project. JMIR Form Res. 2018;2(2):e11025.
Lim MD. Dried Blood Spots for Global Health Diagnostics and Surveillance: opportunities and challenges. Am J Trop Med Hyg. 2018;99(2):256–65.
Villanueva ME, Camilli E, Chirillano AC, Cufré JA, de Landeta MC, Rigacci LN, Velazco VM, Pighin AF. Teaching instrumental analytical chemistry during COVID-19 times in a developing country: asynchronous versus synchronous communication. J Chem Educ. 2020;97(9):2719–22.
Article CAS Google Scholar
Olney RSMC, Ojodu JA, Lindegren ML, Hannon WH. Storage and use of residual dried blood spots from state newborn screening programs. J Pediatr. 2006;148(5):618–22.
Sikombe KHC, Musukuma K, Sharma A, Padian N, Holmes C, Czaicki N, Simbeza S, Somwe P, Bolton-Moore C, Sikazwe I. Accurate dried blood spots collection in the community using non-medically trained personnel could support scaling up routine viral load testing in resource limited settings. PLoS ONE. 2019;14(10).
Download references
Acknowledgements
We wanted to acknowledge Saba Musharraf from the Department of Solutions and Innovations for her support in the maintenance of the Moodle shell.
This is a non-funded project.
Author information
Authors and affiliations.
Department of Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan
Hafsa Majid, Lena Jafri, Shanzay Rehman, Azeema Jamil, Nasir Ali Khan & Aysha Habib Khan
Secondary Hospitals, Aga Khan University, Karachi, Pakistan
Fatima Khanam
IT Academics and Computing, Aga Khan University, Karachi, Pakistan
You can also search for this author in PubMed Google Scholar
Contributions
HM designed the study, executed the project, and wrote the initial manuscript; SR, LJ & AHK critically reviewed the manuscript for intellectual content. AJ, NAK & FK, trained nurses and reviewed the manuscript, NS developed the online course on Moodle.
Corresponding author
Correspondence to Hafsa Majid .
Ethics declarations
Ethics approval and consent to participate.
The study was done as per Helsinki’s ethical code, and the ethical review committee’s approval was not required. The exemption was sought from Aga Khan University’s Ethical Review Committee ERC ID: (2022-7346-20976). All participants gave written informed consent by electronic signature to participate in the study.
Consent for publication
Consent to publish was taken from the survey respondents and focus group discussion participants. All participants gave written informed consent by electronic signature for publication of identifying images.
Competing interests
Authors declared no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Majid, H., Jafri, L., Rehman, S. et al. Enhancing specimen collection skills for dried blood spots through an immersive virtual learning environment: a cross-sectional study. BMC Res Notes 17 , 16 (2024). https://doi.org/10.1186/s13104-023-06584-9
Download citation
Received : 01 March 2023
Accepted : 19 October 2023
Published : 04 January 2024
DOI : https://doi.org/10.1186/s13104-023-06584-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dried blood spot
- Allied health professionals
BMC Research Notes
ISSN: 1756-0500
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Newborn Screening Archives as a Specimen Source for Epidemiologic Studies: Feasibility and Potential for Bias
To evaluate the feasibility of obtaining dried blood spots (DBS) from newborn screening archives for subjects in epidemiologic studies and using these specimens for genotyping, and to evaluate the potential for bias in their use.
We attempted to locate DBS at Washington State's archives for 230 participants in a previous case-control study of childhood cancer, who were born 1978-1990. We compared characteristics of children for whom we did and did not locate specimens, and attempted genetic polymorphism analyses (11 polymorphisms, 82-480 bp amplicons).
We retrieved specimens for 203 (88%) children, including 199 (94%) born in months when a DBS catalog was available. Among the latter, the proportion with specimens located varied by birth place (e.g., hospital, home), maternal education and prenatal smoking, but did not vary significantly by race/ethnicity. All genotyping assays were completed for all specimens, and among controls genotype distributions were in Hardy-Weinberg equilibrium and similar to previous reports.
Conclusions:
Newborn screening archives have potential to provide specimens for epidemiologic studies conducting genotyping and perhaps other assays, but the possibility that reliance on these resources could bias risk estimates must be considered.
Introduction
Many countries universally screen infants for selected in-born disorders using Guthrie cards, specially formulated filter paper used to absorb approximately 13 mm spots of blood collected shortly after birth. In many regions these dried blood spots (DBS) are stored, and epidemiologists have recognized the potential value of these population-based archives [ 1 - 3 ]. Most commonly, anonymous random specimens have been used, for example to estimate the population prevalence of HTLV-I antibodies [ 4 ], or to provide a control group in a study of childhood cancer and glutathione-S transferase (GST) polymorphisms [ 5 ]. Occasionally, researchers have used such archives to collect specimens for specific individuals participating in new or existing epidemiologic studies, the potential benefits including cost-efficiency, reduced subject burden, inclusion of deceased subjects, and use of pre-diagnosis specimens. Examples include a large prospective cohort study evaluating the maternal-fetal transmission of toxoplasmosis [ 6 ], case-control studies of childhood cancer and chemical metabolism genes [ 7 , 8 ], and case series demonstrating genetic alterations associated with childhood leukemia [ 9 ]. DBS have also been used to measure possible indicators of infection [ 10 - 12 ], inflammation [ 13 , 14 ], nutritional status [ 15 - 17 ], and environmental exposure [ 18 , 19 ].
Previously we demonstrated the feasibility of collecting DBS archived for up to 25 years for child participants from an existing case-control study (brain tumors), and using these specimens for genotyping [ 7 ]. Here we detail our methods and assess the potential for bias in risk estimates introduced by reliance on archived DBS. This has not been examined previously, and future studies using these specimens likely will focus on childhood diseases, especially often fatal ones, as did we.
Subject identification
Institutional Review Board approvals were received from the Fred Hutchinson Cancer Research Center and the Washington State Department of Health (WDOH) prior to the conduct of this study. We included children from an earlier case-control study of brain tumors [ 20 ]. Briefly, cases were diagnosed <20 years of age, during 1984-1991, while living in the Seattle-Puget Sound region of Washington State covered by a population-based cancer registry. Controls in the same region were identified by random digit dialing [ 21 ], frequency matched to cases 2:1 by sex and age. Of eligible children, 415 participated (134 (74%) cases and 281 (79%) controls, Figure 1 ). Mothers of all subjects and 83% of fathers were interviewed following informed consent obtained in writing or via telephone.

Children for whom an archived dried blood spot (DBS) was sought, and number for whom a DBS was located, overall and by presence of specimen cataloguing
WDOH screens >99% of Washington newborns, and archives all DBS for 21.5 years without special climate controls. Using the date, hospital and city of residence at birth for children in the original case-control study, we identified those born after 1977 (1978-90) to Washington state residents — children for whom a DBS card might have remained at the WDOH Newborn Screening Program archives. Birth in a hospital in Washington state was not required because WDOH recommends a second card be collected in the physician's office a week or two after birth (about 80-90% newborns participated during the study years). Of the 415 children in the original study, 230 (55%) were born after 1977 to Washington state residents ( Figure 1 ).
Specimen collection
We abstracted data on these 230 eligible children's sex, birth date, and hospital/location of birth; and the first and last name (at the time of the interview) of the child, mother, and father. Blind to case status, WDOH staff matched the list with the catalog of DBS cards (N=212), or for births before May 1979 searched uncatalogued cards manually (N=18, Figure 1 ). When the card was located, staff double-checked the match, and verified at least one complete (fully saturated without punches) DBS would remain in the archives. A complete (when available) or partial DBS was clipped from the card (or two cards, when a unique match could not be determined). For quality assurance, 21 duplicate or quadruplicate specimens for a convenience sample of 6% cases and 6% controls were collected.
Maintenance of confidentiality
Specimens were labeled only with a new randomly assigned identification number. Prior to release from the archives, specimens were “anonymized” [ 22 - 24 ]. We created a file with non-identifying interview data linked to the new identification number. We then destroyed the link with the identifiers, and the list used for matching.
The University of Washington Center for Ecogenetics and Environmental Health Functional Genomics Core Laboratory used approximately one third of each DBS (six 3mm punches) for genotyping. DNA was extracted using a commercial kit [ 7 ]; GSTM1 and GSTT1 null status were determined using a single assay (215, 268 and 480 bp amplicons) with co-amplification of a region of the β-globin gene to verify double-nulls [ 25 ]; and nine single nucleotide polymorphisms ( GSTP1 I105V , CYP2D6 *3 (2549delA), CYP2E1 *5 (RsaI, C-1053T), EPHX1 Y113H , EPHX1 H139R , PON1 C-108T , PON1 Q192R , PON1 L55M and PON2 S311C ) were determined using separate TaqMan™ assays (82-227 bp amplicons) [ 7 , 25 , 26 ]. These polymorphisms affect xenobiotic metabolism.
Statistical analysis
With Fisher's two-sided exact chi-square test in Stata [ 27 ] we: compared characteristics of children with and without specimens located (to examine the potential for a biased sample), checked Hardy-Weinberg equilibrium of genotypes (to examine assay accuracy with these specimens/potential for environmental contamination), and among controls compared genotypes with selected demographic characteristics (to examine whether sample composition might influence risk estimates).
At least one specimen was located for 203 (88%) subjects for whom we attempted to locate a DBS — 199 (94%) born in catalogued months, and four (22%) born in earlier, uncatalogued months ( Figure 1 ).
Specimen and match quality
Of the 203 children with a DBS, we included 13 (6% eligible subjects) by accepting a partial specimen, and three (1% eligible subjects, one singleton and two with a same-sex twin) by collecting specimens for two equally well matching children ( Table 1 ). For 172 (85%), the birth date, hospital, sex, mother's first name, and a family last name on the DBS card(s) agreed with the list provided to WDOH. An additional 11 (5%) fully matched except that the card was collected at a doctor's office in the birth city, instead of the hospital. For the remaining 20 matches, one part of the birth date (N=6), sex (N=4), last name (N=4), mother's first name (N=2), or multiple variables (N=4) did not match or were missing, but for 14 (70%), additional information supported the match (e.g., child's first name on the card).
Specimen and match quality for subjects for whom a dried blood spot was located
Factors associated with failure to locate a DBS
Just over half of children for whom a specimen could not be located (14 children, 6% eligible subjects) were born during uncatalogued months. Twelve of the other 13 unlocated children had one of the following characteristics: five (2% eligible subjects) were born in the earliest three-year period when a catalogue existed but was indexed by the child's last name only (not computerized); five (2% eligible subjects) were born in a hospital that sent DBS to an out-of-state laboratory; one (0.4% eligible subjects) was born in a neighboring state; and one (0.4% eligible subjects) was born at home. Consistent with this, among the 212 (92%) children born in catalogued months, the proportion with DBS located was markedly greater for children born in an in-state non-federal hospital (97%) than elsewhere (30%, p<0.0001, Table 2 ; includes 2/2 children born in a birth center, 1/2 born at home, and none in federal/out-of-state hospitals). It was somewhat better among children with interviewed fathers (95% vs. 80% without paternal interviews, p=0.02) or mothers who did not smoke during pregnancy (96% vs. 85% with mothers who smoked, p=0.02). It appeared slightly better among cases (98% vs. 92% controls, p=0.07), children whose mothers had a college degree (100% vs. 92% without a degree, p=0.04), those living at the same residence at birth and interview (98% vs. 92% who moved, p=0.14), and Hispanic or non-white children (100% vs. 93% of non-Hispanic white children), although the latter comparison was based on particularly small numbers (p=1.0). Multivariate analyses were not possible, but among the 13 children without DBS, all four whose fathers were not interviewed were born in an in-state non-federal hospital, while prenatal smoking was prevalent regardless of birth location (3/6 born in an in-state non-federal hospital, 3/7 born elsewhere, data not shown).
Proportion dried blood spots (DBS) collected among subjects born in a catalogued month, by selected characteristics of subjects
We completed all assays for all children. Genotyping confirmed that the two possible matches for one singleton control were from two individuals with different genotypes for some polymorphisms, leaving 136 (99.3%) controls and all 66 cases. Agreement between the primary and replicate specimens was 99.1%.
Among the 136 controls, 52.2% were GSTM1 null, 15.4% were GSTT1 null, and 10.3% were double-null ( Table 3 ). Variant allele frequencies for the other polymorphisms were 0.018-0.44, and all genotype frequencies were in Hardy-Weinberg equilibrium. Results for all polymorphisms were similar to those reported previously for healthy Caucasians. Eight (6%) controls had Hispanic or non-white parent(s), and compared to controls with two non-Hispanic white parents, they more often carried PON1 192R (p<0.01) and CYP2E1 *5 (p=0.06) alleles (data not shown), consistent with previous observations [ 28 - 31 ].
Genotyping results using archived dried blood spots for control children
Abbreviations: n/a=not applicable; GST=glutathione S-transferase; CYP=cytochrome P450; EPHX=microsomal epoxide hydrolase; PON=paraoxonase
Among non-Hispanic white controls, children of mothers with some college education were more likely than other children to carry the CYP2E1 *5 allele (p=0.04) and the PON1 -108C allele (p=0.07); and children of mothers who smoked during pregnancy were more likely than other children to carry the EPHX1 139H allele (p=0.12). Genotyping data were only available for three children born outside a hospital, precluding consideration of this factor.
We collected DBS from newborn screening archives for 94% of children born in months when DBS cards were catalogued, similar to a study in another state [ 8 ]. All our genotyping assays were completed for all children with DBS, with genotype/allele frequencies similar to studies using DNA from fresh blood [ 25 , 28 , 29 , 32 - 34 ]. These results are encouraging, given that many states archive DBS for several years [ 35 ] and might allow them to be used for research [ 2 ]. It is important, however, to consider how bias might be introduced.
Screening participation
In Washington state, newborn screening is nearly universal [ 35 ], but here and elsewhere non-participation is unlikely to be random. Unscreened children are perhaps more likely to have been born at home, died shortly after birth, or have parents who object to standard medical care. The one child without a specimen and born at home perhaps was not screened.
Archive coverage
We sought a DBS for all children born to all state residents, even those born out of state or in hospitals that sent screening cards to another state, because these subjects could have participated in the recommended secondary screening in-state. Excluding them a priori would have been inappropriate because they likely differed from others. We located none, affecting our collection effort somewhat. In regions where residents more commonly cross state lines for childbirth, attempting to access the neighboring state's archives may minimize bias that could otherwise be introduced by incomplete archive coverage.
Matching information
The mother's first name and child's date and hospital of birth were often adequate for matching. Important exceptions were the short periods when the DBS catalog was unavailable or searchable only by last name. Some children's last names may have changed between birth and interview. Children without a specimen located were more likely to have changed residence since birth (likely associated with a name change) and their fathers were less likely to have been interviewed (providing additional matching information).
In rare instances we were unable to narrow matches to a single screened child. For each we collected a DBS from each of the two matching children, and retained their assay results when concordant, because such children may differ from others (e.g. common name, incomplete matching information, large birth hospital, twin).
With such archives one cannot rule out collecting a DBS for the wrong subject. However, our match quality was generally excellent, suggesting this problem may be minimal.
These potential sources of bias related to matching are probably less common in studies accessing archives with computerized catalogues searchable by several variables, and that can determine the child's birth name prior to matching via interview or linkage to other data (e.g. birth certificates).
Specimen availability
A DBS was available from all located cards, but had we not accepted a partial DBS, we would have excluded several children, and potentially introduced bias. Specimens may be less available for subjects with only one card, fewer spots collected per card (because of birth year or other factors), ‘positive’ newborn screening program results, or with parents requesting the specimen not be archived/used for research.
Specimen usability
Despite storage for 25 years, at times without air conditioning, all specimens yielded genotyping results. However, the climate in western Washington is moderate, and our assays mainly required short amplicons. In a separate study in another state using similar methods on DBS of similar age, GST genotyping was completed for 97% of children born in the later years, but only 3% born in earlier years, when specimens reached high temperatures during storage [ 8 ]. Nevertheless, DNA in DBS stored in tropical conditions for at least ten years was stable enough for genotyping of all of specimens with assays requiring 255, 674 and 1039 bp amplicons [ 36 ]. However, only 60% of slightly older specimens in that study were usable for the assay with the longest amplicon, consistent with another report [ 3 ] that genotype might not be determinable for all subjects for assays requiring long amplicons. Specimen age and storage conditions might also affect the ability to obtain results for assays assessing something other than genotype.
Assay accuracy
All the genotype/allele frequencies observed among controls were consistent with previous reports and in Hardy-Weinberg equilibrium, and there was no evidence of under-representation of GSTT1 expressers (480 bp, the longest amplicon) or over-representation of GSTM1 expressers or double-null children (215-268 bp, shorter amplicons). It appears that any DNA fragmentation or environmental contamination (e.g. from existing punches [ 37 ] or contact with adjacent cards [ 12 ]) were not sufficient to alter results, even though the DBS cards were stored in direct contact with one another. However, the TaqMan assays we used each required amplicons of a single, modest length, and considered the relative amount of each allele, not just their presence. In addition, we flame-sterilized the punch between specimens.
Assays measuring characteristics other than genotype might be less accurate with dried than with fresh blood, and accuracy might also depend on storage time and conditions. Some assays might also be less resilient to possible contamination of the DBS, although potential counter strategies exist (e.g. comparing amounts of viral DNA on index and adjacent DBS cards to determine which is truly positive [ 12 ], and comparing amounts of environmental chemicals in blood-saturated vs. blood-free portions of the card).
Potential effect on risk estimates
Some potential sources of bias could affect risk estimates by altering the composition of the study groups. Our results indicate that potentially important differences (e.g., maternal smoking and education) may exist in children for whom archived DBS cannot be located. Other studies have observed non-random differences between children for whom a located specimen can and cannot be assayed [ 8 , 36 ]. The magnitude and direction of the effect, if any, on risk estimates might be difficult to predict. We located DBS for a somewhat greater percentage of cases than controls, possibly because proportionally more cases had fathers interviewed, and none had out-of-state specimens. Further, some characteristics associated with locating a specimen, maternal education and possibly smoking, were also associated with genotype. If these associations applied equally for cases and controls for whom we failed to locate a DBS (largely born to mothers without a college degree), we might expect a slight under-representation of, for example, the PON1 −108T allele among both cases and controls in a study focused on this polymorphism [ 7 ]. And, because proportionally more control DBS specimens were not located, an odds ratio of 1.7 for case status in relation to the PON1 −108T allele might be biased slightly upward. In another study using DBS [ 8 ], in which birth year was strongly associated with ability to obtain genotyping results, cases and controls were matched on birth date, so even if there were racial/ethnic changes in the population over time, presumably risk estimates were not materially affected.
Bias in the form of measurement error would arise if results from dried blood, or that stored for a long time in non-optimal conditions, are not as accurate as those from fresh blood. This did not appear to occur with our genotyping, but may with other assays. If the resulting measurement error does not differ between comparison groups, risk estimates may be biased toward null.
The effect of other potential sources of bias could be less straightforward. For example, it seems unlikely that environmental contamination could cause true heterozygotes (TaqMan assays), double expressers (GST assays), and viral-DNA-positive subjects to be misclassified. For the remaining subjects (those truly homozygous, GST null, and viral-DNA-negative, respectively), it would be more difficult to rule out the possibility that environmental contamination could alter assay results (e.g. [ 12 ]). If such misclassification occurred and was non-differential, one would generally expect risk estimates to be biased toward null (e.g. if based on inherently dichotomous categories such as viral DNA positive/negative), but exceptions exist (e.g. odds ratios based only on the two homozygous categories would not be biased). Similarly, bias may or may not occur if the wrong specimen is obtained. For example, in our previous study using population-based controls, unconfounded odds ratios might be similar to those observed even had we obtained the wrong specimen for all controls; our risk estimates were very similar when we used randomly selected archive controls [ 7 ]. Only in the absence of a true association might cases' specimens be substituted in this manner without bias. However, studies that track match quality can assess whether questionable matches affect risk estimates.
Most potential sources of bias related to reliance on archived DBS are likely minimal, or can be reduced. In some situations reliance on archived DBS might reduce bias in risk estimates, compared to approaches in which specimens are obtained after diagnosis and only from surviving individuals. The potential for bias does not preclude the use of these specimens for genotyping and perhaps other assays, but careful consideration of how bias might enter into results is warranted.
Acknowledgements
This work supported by NIEHS T32ES07262, NIEHS P30ES07033 and 1 R03 CA106011 from the National Institutes of Health; and contract N01-CN-05230 from the National Cancer Institute. We thank the Washington State Department of Health Newborn Screening Program for their generous participation in this project, especially Mr. Michael Glass and Mr. Michael Ginder; the University of Washington Center for Ecogenetics and Environmental Health Functional Genomics Core Laboratory, especially Dr. Federico M. Farin and Ms. Hannah-Malia A. Viernes; and Ms. Randi Niemer.
Abbreviations and Acronyms
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.

COMMENTS
Case Study 10-3: Newborn Screening Specimen Collection. Judy is a seasoned phlebotomist who has recently changed employers and now works in a busy women's hospital where lots of babies are born and lots of newborn screening specimens are collected. Judy has never really liked collecting newborn screening specimens and tries to avoid being the ...
Newborn screening process, illustrating specimen collection through result reporting. Newborn screening (NBS) is a complex system that involves the collection of specimens at birthing facilities, transportation of specimens to the NBS public health laboratory for testing and communicating results to health care providers and families.
Background Newborn screening (NBS) aims to achieve early identification and treatment of affected infants prior to onset of symptoms. The timely completion of each step (i.e., specimen collection, transport, testing, result reporting), is critical for early diagnosis. Goals developed by the Secretary of Health and Human Services' Advisory Committee on Heritable Disorders in Newborns and ...
Despite the established utility of newborn screening tests (NBS), achieving timely specimen transit is a challenge for neonatal intensive care units (NICU). This project was conducted between ...
The Philippine newborn bloodspot screening (NBS) program began in 1996 with 24 hospitals and was formalized by legislation in 2004. The NBS panel was recently expanded to include a number of additional hereditary congenital conditions. Expertise and experiences from other NBS programs already screening for hemoglobinopathies were essential to ...
Case Study. The parents of Lupita, a 12-day-old newborn, bring her in for her second newborn screening specimen collection. When you tell them about the disclosure/directive to destroy form and hand it to them, they said they already filled one out at the hospital when Lupita's first "heelstick" was done and that they do not need another ...
19. To provide national data for newborn screening system quality assurance and program comparison, state programs should contribute timely case findings and laboratory data to the national newborn screening data-collection system operated by the National Newborn Screening and Genetics Resource Center (www2.uthscsa.edu/nnsis).
Newborn screening officials described challenges including short staffing across the entire program, collection and transport of specimens, interrupted follow-up activities, and pilot study recruitment. To address these challenges, state programs implemented a wide variety of solutions to maintain the high standards of newborn screening.
Newborn screening is the largest genetic testing effort for newborns in the U.S. Its purpose is to identify newborns who are at risk for metabolic, endocrine, or hematologic disorders. A review of ...
Objectives This study aimed to determine which steps in the newborn screening collection and delivery processes contribute to delays and identify strategies to improve timeliness. Methods Data was analyzed from infants (N = 94,770) who underwent newborn screening at 83 hospitals in Michigan between April 2014 and March 2015.
CLSI NBS01 Dried Blood Spot Specimen Collection for Newborn Screening, 7th Edition. This standard highlights specimen collection methods, discusses acceptable techniques for applying blood drops or aliquots to the filter paper section of the specimen collection device, and provides instructions on proper specimen drying, handling, and transport to ensure quality specimens are consistently ...
Specimen cards are shipped quarterly to most birth hospitals, to the attention of the designated hospital employee. To obtain additional blood specimen cards at no charge: Visit our website at www.wadsworth.org and fill out a supply request form. E-mail a request to [email protected]. Call (518) 473-7552.
Newborn screening was established over 50 years ago to identify cases of disorders that were serious, urgent, and treatable, mirroring the criteria of Wilson and Jungner. In the last decade, conditions have been added to newborn screening that do not strictly meet these criteria, and genomic newborn screening is beginning to be discussed.
A second newborn screening specimen should be collected at 2-6 weeks of age (4 weeks optimal) on all full term infants with a normal first test screen. 2. If the first test specimen was collected when the infant was greater than one week of age but less than two ... Collect the specimen on the proper Newborn Screening collection form. 3 ...
November 2011. Screening newborns for certain treatable genetic or congenital conditions during the first 24 to 48 hours of birth is a public health responsibility. Effective laboratory testing of ...
Objective The quality of dried blood spot (DBS) specimens impacts newborn screening (NBS) results, hence proper training is crucial for DBS specimen collection. To address this, a training module for Allied Health Professionals (AHPs) and nurses was created on Moodle, a virtual learning environment (VLE). The purpose of this research was to determine the feasibility and effectiveness of this ...
Newborn screening is a state-run healthcare initiative that encompasses the process of parental education, infant screening, appropriate follow-up, diagnostic testing, disease management, and continued evaluation.[1] The newborn screen itself is a specific set of laboratory evaluations and point-of-care examinations performed on newborn infants in an attempt to identify clinically occult but ...
Instructions for specimen collection can also be found on the back of the specimen collection form. Updated copy of Religious refusal form. Newborn screening has the potential to save or greatly improve the life of a newborn; therefore, newborn screening is mandatory in New York State (Public Health Law Section 2500-a, 10 NYCRR Section 69-1.4).
Abstract. Routine second screening of most newborns at 8-14 days of life for a panel of newborn conditions occurs in 12 U.S. states, while newborns in the other states typically undergo only a single routine newborn screen. The study objective was to evaluate screening consequences for primary congenital hypothyroidism (CH) in one- and two ...
SPECIMEN COLLECTION. The ADH Newborn Screening Program uses standards developed by the Clinical and Laboratory Standards Institute (CLSI ) for blood collection on filter paper specifically for newborn screening programs. The goal of this standard is to ensure the quality of blood spots collected from newborns. Poor quality specimens
We collected DBS from newborn screening archives for 94% of children born in months when DBS cards were catalogued, similar to a study in another state . All our genotyping assays were completed for all children with DBS, with genotype/allele frequencies similar to studies using DNA from fresh blood [ 25 , 28 , 29 , 32 - 34 ].