An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancer Control
- v.28; Jan-Dec 2021

Cancer Biology, Epidemiology, and Treatment in the 21st Century: Current Status and Future Challenges From a Biomedical Perspective
Patricia piña-sánchez.
1 Oncology Research Unit, Oncology Hospital, Mexican Institute of Social Security, Mexico
Antonieta Chávez-González
Martha ruiz-tachiquín, eduardo vadillo, alberto monroy-garcía, juan josé montesinos, rocío grajales.
2 Department of Medical Oncology, Oncology Hospital, Mexican Institute of Social Security, Mexico
Marcos Gutiérrez de la Barrera
3 Clinical Research Division, Oncology Hospital, Mexican Institute of Social Security, Mexico
Hector Mayani
Since the second half of the 20th century, our knowledge about the biology of cancer has made extraordinary progress. Today, we understand cancer at the genomic and epigenomic levels, and we have identified the cell that starts neoplastic transformation and characterized the mechanisms for the invasion of other tissues. This knowledge has allowed novel drugs to be designed that act on specific molecular targets, the immune system to be trained and manipulated to increase its efficiency, and ever more effective therapeutic strategies to be developed. Nevertheless, we are still far from winning the war against cancer, and thus biomedical research in oncology must continue to be a global priority. Likewise, there is a need to reduce unequal access to medical services and improve prevention programs, especially in countries with a low human development index.
Introduction
During the last one hundred years, our understanding of the biology of cancer increased in an extraordinary way. 1 - 4 Such a progress has been particularly prompted during the last few decades because of technological and conceptual progress in a variety of fields, including massive next-generation sequencing, inclusion of “omic” sciences, high-resolution microscopy, molecular immunology, flow cytometry, analysis and sequencing of individual cells, new cell culture techniques, and the development of animal models, among others. Nevertheless, there are many questions yet to be answered and many problems to be solved regarding this disease. As a consequence, oncological research must be considered imperative.
Currently, cancer is one of the illnesses that causes more deaths worldwide. 5 According to data reported in 2020 by the World Health Organization (WHO), cancer is the second cause of death throughout the world, with 10 million deaths. 6 Clearly, cancer is still a leading problem worldwide. With this in mind, the objective of this article is to present a multidisciplinary and comprehensive overview of the disease. We will begin by analyzing cancer as a process, focusing on the current state of our knowledge on 4 specific aspects of its biology. Then, we will look at cancer as a global health problem, considering some epidemiological aspects, and discussing treatment, with a special focus on novel therapies. Finally, we present our vision on some of the challenges and perspectives of cancer in the 21 st century.
The Biology of Cancer
Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and epigenomic alterations that lead to cell transformation, the cells where these changes occur, and the processes of invasion and metastasis that, to an important degree, determine tumor aggressiveness.
Cancer Genomics
The genomics of cancer can be defined as the study of the complete sequence of DNA and its expression in tumor cells. Evidently, this study only becomes meaningful when compared to normal cells. The sequencing of the human genome, completed in 2003, was not only groundbreaking with respect to the knowledge of our gene pool, but also changed the way we study cancer. In the post-genomic era, various worldwide endeavors, such as the Human Cancer Genome Project , the Cancer Genome ATLAS (TCGA), the International Cancer Genome Consortium, and the Pan-Cancer Analysis Working Group (PCAWG), have contributed to the characterization of thousands of primary tumors from different neoplasias, generating more than 2.5 petabytes (10 15 ) of genomic, epigenomic, and proteomic information. This has led to the building of databases and analytical tools that are available for the study of cancer from an “omic” perspective, 7 , 8 and it has helped to modify classification and treatment of various neoplasms.
Studies in the past decade, including the work by the PCAWG, have shown that cancer generally begins with a small number of driving mutations (4 or 5 mutations) in particular genes, including oncogenes and tumor-suppressor genes. Mutations in TP53, a tumor-suppressor gene, for example, are found in more than half of all cancer types as an early event, and they are a hallmark of precancerous lesions. 9 - 12 From that point on, the evolution of tumors may take decades, throughout which the mutational spectrum of tumor cells changes significantly. Mutational analysis of more than 19 000 exomes revealed a collection of genomic signatures, some associated with defects in the mechanism of DNA repair. These studies also revealed the importance of alterations in non-coding regions of DNA. Thus, for example, it has been observed that various pathways of cell proliferation and chromatin remodeling are altered by mutations in coding regions, while pathways, such as WNT and NOTCH, can be disrupted by coding and non-coding mutations. To the present date, 19 955 genes that codify for proteins and 25 511 genes for non-coding RNAs have been identified ( https://www.gencodegenes.org/human/stats.html ). Based on this genomic catalogue, the COSMIC (Catalogue Of Somatic Mutations In Cancer) repository, the most robust database to date, has registered 37 288 077 coding mutations, 19 396 fusions, 1 207 190 copy number variants, and 15 642 672 non-coding variants reported up to August 2020 (v92) ( https://cosmic-blog.sanger.ac.uk/cosmic-release-v92/ ).
The genomic approach has accelerated the development of new cancer drugs. Indeed, two of the most relevant initiatives in recent years are ATOM (Accelerating Therapeutics for Opportunities in Medicine), which groups industry, government and academia, with the objective of accelerating the identification of drugs, 13 and the Connectivity Map (CMAP), a collection of transcriptional data obtained from cell lines treated with drugs for the discovery of functional connections between genes, diseases, and drugs. The CMAP 1.0 covered 1300 small molecules and more than 6000 signatures; meanwhile, the CMAP 2.0 with L1000 assay profiled more than 1.3 million samples and approximately 400 000 signatures. 14
The genomic study of tumors has had 2 fundamental contributions. On the one hand, it has allowed the confirmation and expansion of the concept of intratumor heterogeneity 15 , 16 ; and on the other, it has given rise to new classification systems for cancer. Based on the molecular classification developed by expression profiles, together with mutational and epigenomic profiles, a variety of molecular signatures have been identified, leading to the production of various commercial multigene panels. In breast cancer, for example, different panels have been developed, such as Pam50/Prosigna , Blue Print , OncotypeDX , MammaPrint , Prosigna , Endopredict , Breast Cancer Index , Mammostrat, and IHC4 . 17
Currently, the genomic/molecular study of cancer is more closely integrated with clinical practice, from the classification of neoplasms, as in tumors of the nervous system, 18 to its use in prediction, as in breast cancer. 17 Improvement in molecular methods and techniques has allowed the use of smaller amounts of biological material, as well as paraffin-embedded samples for genomic studies, both of which provide a wealth of information. 19 In addition, non-invasive methods, such as liquid biopsies, represent a great opportunity not only for the diagnosis of cancer, but also for follow-up, especially for unresectable tumors. 20
Research for the production of genomic information on cancer is presently dominated by several consortia, which has allowed the generation of a great quantity of data. However, most of these consortia and studies are performed in countries with a high human development index (HDI), and countries with a low HDI are not well represented in these large genomic studies. This is why initiatives such as Human Heredity and Health in Africa (H3Africa) for genomic research in Africa are essential. 21 Generation of new information and technological developments, such as third-generation sequencing, will undoubtedly continue to move forward in a multidisciplinary and complex systems context. However, the existing disparities in access to genomic tools for diagnosis, prognosis, and treatment of cancer will continue to be a pressing challenge at regional and social levels.
Cancer Epigenetics
Epigenetics studies the molecular mechanisms that produce hereditable changes in gene expression, without causing alterations in the DNA sequence. Epigenetic events are of 3 types: methylation of DNA and RNA, histone modification (acetylation, methylation, and phosphorylation), and the expression of non-coding RNA. Epigenetic aberrations can drive carcinogenesis when they alter chromosome conformation and the access to transcriptional machinery and to various regulatory elements (promoters, enhancers, and anchors for interaction with chromatin, for example). These changes may activate oncogenesis and silence tumor-suppressor mechanisms when they modulate coding and non-coding sequences (such as micro-RNAs and long-RNAs). This can then lead to uncontrolled growth, as well as the invasion and metastasis of cancer cells.
While genetic mutations are stable and irreversible, epigenetic alterations are dynamic and reversible; that is, there are several epigenomes, determined by space and time, which cause heterogeneity of the “epigenetic status” of tumors during their development and make them susceptible to environmental stimuli or chemotherapeutic treatment. 22 Epigenomic variability creates differences between cells, and this creates the need to analyze cells at the individual level. In the past, epigenetic analyses measured “average states” of cell populations. These studies revealed general mechanisms, such as the role of epigenetic marks on active or repressed transcriptional states, and established maps of epigenetic composition in a variety of cell types in normal and cancerous tissue. However, these approaches are difficult to use to examine events occurring in heterogeneous cell populations or in uncommon cell types. This has led to the development of new techniques that permit marking of a sequence on the epigenome and improvement in the recovery yield of epigenetic material from individual cells. This has helped to determine changes in DNA, RNA, and histones, chromatin accessibility, and chromosome conformation in a variety of neoplasms. 23 , 24
In cancer, DNA hypomethylation occurs on a global scale, while hypermethylation occurs in specific genomic loci, associated with abnormal nucleosome positioning and chromatin modifications. This information has allowed epigenomic profiles to be established in different types of neoplasms. In turn, these profiles have served as the basis to identify new neoplasm subgroups. For example, in triple negative breast cancer (TNBC), 25 and in hepatocellular carcinoma, 26 DNA methylation profiles have helped to the identification of distinct subgroups with clinical relevance. Epigenetic approaches have also helped to the development of prognostic tests to assess the sensitivity of cancer cells to specific drugs. 27
Epigenetic traits could be used to characterize intratumoral heterogeneity and determine the relevance of such a heterogeneity in clonal evolution and sensitivity to drugs. However, it is clear that heterogeneity is not only determined by genetic and epigenetic diversity resulting from clonal evolution of tumor cells, but also by the various cell populations that form the tumor microenvironment (TME). 28 Consequently, the epigenome of cancer cells is continually remodeled throughout tumorigenesis, during resistance to the activity of drugs, and in metastasis. 29 This makes therapeutic action based on epigenomic profiles difficult, although significant advances in this area have been reported. 30
During carcinogenesis and tumor progression, epigenetic modifications are categorized by their mechanisms of regulation ( Figure 1A ) and the various levels of structural complexity ( Figure 1B ). In addition, the epigenome can be modified by environmental stimuli, stochastic events, and genetic variations that impact the phenotype ( Figure 1C ). 31 , 32 The molecules that take part in these mechanisms/events/variations are therapeutic targets of interest with potential impact on clinical practice. There are studies on a wide variety of epidrugs, either alone or in combination, which improve antitumor efficacy. 33 However, the problems with these drugs must not be underestimated. For a considerable number of epigenetic compounds still being under study, the main challenge is to translate in vitro efficacy of nanomolar (nM) concentrations into well-tolerated and efficient clinical use. 34 The mechanisms of action of epidrugs may not be sufficiently controlled and could lead to diversion of the therapeutic target. 35 It is known that certain epidrugs, such as valproic acid, produce unwanted epigenetic changes 36 ; thus the need for a well-established safety profile before these drugs can be used in clinical therapy. Finally, resistance to certain epidrugs is another relevant problem. 37 , 38

Epigenetics of cancer. (A) Molecular mechanisms. (B) Structural hierarchy of epigenomics. (C) Factors affecting the epigenome. Modified from Refs. 31 and 32 .
As we learn about the epigenome of specific cell populations in cancer patients, a door opens to the evaluation of sensitivity tests and the search for new molecular markers for detection, prognosis, follow-up, and/or response to treatment at various levels of molecular regulation. Likewise, the horizon expands for therapeutic alternatives in oncology with the use of epidrugs, such as pharmacoepigenomic modulators for genes and key pathways, including methylation of promoters and regulation of micro-RNAs involved in chemoresponse and immune response in cancer. 39 There is no doubt that integrated approaches identifying stable pharmagenomic and epigenomic patterns and their relation with expression profiles and genetic functions will be more and more valuable in our fight against cancer.
Cancer Stem Cells
Tumors consist of different populations of neoplastic cells and a variety of elements that form part of the TME, including stromal cells and molecules of the extracellular matrix. 40 Such intratumoral heterogeneity becomes even more complex during clonal variation of transformed cells, as well as influence the elements of the TME have on these cells throughout specific times and places. 41 To explain the origin of cancer cell heterogeneity, 2 models have been put forward. The first proposes that mutations occur at random during development of the tumor in individual neoplastic cells, and this promotes the production of various tumor populations, which acquire specific growth and survival traits that lead them to evolve according to intratumor mechanisms of natural selection. 42 The second model proposes that each tumor begins as a single cell that possess 2 functional properties: it can self-renew and it can produce several types of terminal cells. As these 2 properties are characteristics of somatic stem cells, 43 the cells have been called cancer stem cells (CSCs). 44 According to this model, tumors must have a hierarchical organization, where self-renewing stem cells produce highly proliferating progenitor cells, unable to self-renew but with a high proliferation potential. The latter, in turn, give rise to terminal cells. 45 Current evidence indicates that both models may coexist in tumor progression. In agreement with this idea, new subclones could be produced as a result of a lack of genetic stability and mutational changes, in addition to the heterogeneity derived from the initial CSC and its descendants. Thus, in each tumor, a set of neoplastic cells with different genetic and epigenetic traits may be found, which would provide different phenotypic properties. 46
The CSC concept was originally presented in a model of acute myeloid leukemia. 47 The presence of CSCs was later proved in chronic myeloid leukemia, breast cancer, tumors of the central nervous system, lung cancer, colon cancer, liver cancer, prostate cancer, pancreatic cancer, melanoma, and cancer of the head and neck, amongst others. In all of these cases, detection of CSCs was based on separation of several cell populations according to expression of specific surface markers, such as CD133, CD44, CD24, CD117, and CD15. 48 It is noteworthy that in some solid tumors, and even in some hematopoietic ones, a combination of specific markers that allow the isolation of CSCs has not been found. Interestingly, in such tumors, a high percentage of cells with the capacity to start secondary tumors has been observed; thus, the terms Tumor Initiating Cells (TIC) or Leukemia Initiating Cells (LIC) have been adopted. 46
A relevant aspect of the biology of CSCs is that, just like normal stem cells, they can self-renew. Such self-renewal guarantees the maintenance or expansion of the tumor stem cell population. Another trait CSCs share with normal stem cells is their quiescence, first described in chronic myeloid leukemia. 49 The persistence of quiescent CSCs in solid tumors has been recently described in colorectal cancer, where quiescent clones can become dominant after therapy with oxaliplatin. 50 In non-hierarchical tumors, such as melanoma, the existence of slow-cycling cells that are resistant to antimitogenic agents has also been proved. 51 Such experimental evidence supports the idea that quiescent CSCs or TICs are responsible for both tumor resistance to antineoplastic drugs and clinical relapse after initial therapeutic success.
In addition to quiescence, CSCs use other mechanisms to resist the action of chemotherapeutic drugs. One of these is their increased numbers: upon diagnosis, a high number of CSCs are observed in most analyzed tumors, making treatment unable to destroy all of them. On the other hand, CSCs have a high number of molecular pumps that expulse drugs, as well as high numbers of antiapoptotic molecules. In addition, they have very efficient mechanisms to repair DNA damage. In general, these cells show changes in a variety of signaling pathways involved in proliferation, survival, differentiation, and self-renewal. It is worth highlighting that in recent years, many of these pathways have become potential therapeutic targets in the elimination of CSCs. 52 Another aspect that is highly relevant in understanding the biological behavior of CSCs is that they require a specific site for their development within the tissue where they are found that can provide whatever is needed for their survival and growth. These sites, known as niches, are made of various cells, both tumor and non-tumor, as well as a variety of non-cellular elements (extracellular matrix [ECM], soluble cytokines, ion concentration gradients, etc.), capable of regulating the physiology of CSCs in order to promote their expansion, the invasion of adjacent tissues, and metastasis. 53
It is important to consider that although a large number of surface markers have been identified that allow us to enrich and prospectively follow tumor stem cell populations, to this day there is no combination of markers that allows us to find these populations in all tumors, and it is yet unclear if all tumors present them. In this regard, it is necessary to develop new purification strategies based on the gene expression profiles of these cells, so that tumor heterogeneity is taken into account, as it is evident that a tumor can include multiple clones of CSCs that, in spite of being functional, are genetically different, and that these clones can vary throughout space (occupying different microenvironments and niches) and time (during the progression of a range of tumor stages). Such strategies, in addition to new in vitro and in vivo assays, will allow the development of new and improved CSC elimination strategies. This will certainly have an impact on the development of more efficient therapeutic alternatives.
Invasion and Metastasis
Nearly 90% of the mortality associated with cancer is related to metastasis. 54 This consists of a cascade of events ( Figure 2 ) that begins with the local invasion of a tumor into surrounding tissues, followed by intravasation of tumor cells into the blood stream or lymphatic circulation. Extravasation of neoplastic cells in areas distant from the primary tumor then leads to the formation of one or more micrometastatic lesions which subsequently proliferate to form clinically detectable lesions. 4 The cells that are able to produce metastasis must acquire migratory characteristics, which occur by a process known as epithelial–mesenchymal transition (EMT), that is, the partial loss of epithelial characteristics and the acquirement of mesenchymal traits. 55

Invasion and metastasis cascade. Invasion and metastasis can occur early or late during tumor progression. In either case, invasion to adjacent tissues is driven by stem-like cells (cancer stem cells) that acquire the epithelial–mesenchymal transition (EMT) (1). Once they reach sites adjacent to blood vessels, tumor cells (individually or in clusters) enter the blood (2). Tumor cells in circulation can adhere to endothelium and extravasation takes place (3). Other mechanisms alternative to extravasation can exist, such as angiopelosis, in which clusters of tumor cells are internalized by the endothelium. Furthermore, at certain sites, tumor cells can obstruct microvasculature and initiate a metastatic lesion right there. Sometimes, a tumor cells that has just exit circulation goes into an MET in order to become quiescent (4). Inflammatory signals can activate quiescent metastatic cells that will proliferate and generate a clinically detectable lesion (5).
Although several of the factors involved in this process are currently known, many issues are still unsolved. For instance, it has not yet been possible to monitor in vivo the specific moment when it occurs 54 ; the microenvironmental factors of the primary tumor that promote such a transition are not known with precision; and the exact moment during tumor evolution in which one cell or a cluster of cells begin to migrate to distant areas, is also unknown. The wide range of possibilities offered by intra- and inter-tumoral heterogeneity 56 stands in the way of suggesting a generalized strategy that could resolve this complication.
It was previously believed that metastasis was only produced in late stages of tumor progression; however, recent studies indicate that EMT and metastasis can occur during the early course of the disease. In pancreatic cancer, for example, cells going through EMT are able to colonize and form metastatic lesions in the liver in the first stages of the disease. 52 , 57 Metastatic cell clusters circulating in peripheral blood (PB) are prone to generate a metastatic site, compared to individual tumor cells. 58 , 59 In this regard, novel strategies, such as the use of micro-RNAs, are being assessed in order to diminish induction of EMT. 60 It must be mentioned, however, that the metastatic process seems to be even more complex, with alternative pathways that do not involve EMT. 61 , 62
A crucial stage in the process of metastasis is the intravasation of tumor cells (alone or in clusters) towards the blood stream and/or lymphatic circulation. 63 These mechanisms are also under intensive research because blocking them could allow the control of spreading of the primary tumor. In PB or lymphatic circulation, tumor cells travel to distant parts for the potential formation of a metastatic lesion. During their journey, these cells must stand the pressure of blood flow and escape interaction with natural killer (NK) cells . 64 To avoid them, tumor cells often cover themselves with thrombocytes and also produce factors such as VEGF, angiopoietin-2, angiopoietin-4, and CCL2 that are involved in the induction of vascular permeability. 54 , 65 Neutrophils also contribute to lung metastasis in the bloodstream by secreting IL-1β and metalloproteases to facilitate extravasation of tumor cells. 64
The next step in the process of metastasis is extravasation, for which tumor cells, alone or in clusters, can use various mechanisms, including a recently described process known as angiopellosis that involves restructuring the endothelial barrier to internalize one or several cells into a tissue. 66 The study of leukocyte extravasation has contributed to a more detailed knowledge of this process, in such a way that some of the proposed strategies to avoid extravasation include the use of integrin inhibitors, molecules that are vital for rolling, adhesion, and extravasation of tumor cells. 67 , 68 Another strategy that has therapeutic potential is the use of antibodies that strengthen vascular integrity to obstruct transendothelial migration of tumor cells and aid in their destruction in PB. 69
Following extravasation, tumor cells can return to an epithelial phenotype, a process known as mesenchymal–epithelial transition and may remain inactive for several years. They do this by competing for specialized niches, like those in the bone marrow, brain, and intestinal mucosa, which provide signals through the Notch and Wnt pathways. 70 Through the action of the Wnt pathway, tumor cells enter a slow state of the cell cycle and induce the expression of molecules that inhibit the cytotoxic function of NK cells. 71 The extravasated tumor cell that is in a quiescent state must comply with 2 traits typical of stem cells: they must have the capacity to self-renew and to generate all of the cells that form the secondary tumor.
There are still several questions regarding the metastatic process. One of the persisting debates at present is if EMT is essential for metastasis or if it plays a more important role in chemoresistance. 61 , 62 It is equally important to know if there is a pattern in each tumor for the production of cells with the capacity to carry out EMT. In order to control metastasis, it is fundamental to know what triggers acquisition of the migratory phenotype and the intrinsic factors determining this transition. Furthermore, it is essential to know if mutations associated with the primary tumor or the variety of epigenetic changes are involved in this process. 55 It is clear that metastatic cells have affinity for certain tissues, depending on the nature of the primary tumor (seed and soil hypothesis). This may be caused by factors such as the location and the direction of the bloodstream or lymphatic fluid, but also by conditioning of premetastatic niches at a distance (due to the large number of soluble factors secreted by the tumor and the recruitment of cells of the immune system to those sites). 72 We have yet to identify and characterize all of the elements that participate in this process. Deciphering them will be of upmost importance from a therapeutic point of view.
Epidemiology of Cancer
Cancer is the second cause of death worldwide; today one of every 6 deaths is due to a type of cancer. According to the International Agency for Research on Cancer (IARC), in 2020 there were approximately 19.3 million new cases of cancer, and 10 million deaths by this disease, 6 while 23.8 million cases and 13.0 million deaths are projected to occur by 2030. 73 In this regard, it is clear the increasing role that environmental factors—including environmental pollutants and processed food—play as cancer inducers and promoters. 74 The types of cancer that produce the greatest numbers of cases and deaths worldwide are indicated in Table 1 . 6
Total Numbers of Cancer Cases and Deaths Worldwide in 2020 by Cancer Type (According to the Global Cancer Observatory, IARC).
Data presented on this table were obtained from Ref. 6.
As shown in Figure 3 , lung, breast, prostate, and colorectal cancer are the most common throughout the world, and they are mostly concentrated in countries of high to very high human development index (HDI). Although breast, prostate, and colorectal cancer have a high incidence, the number of deaths they cause is proportionally low, mostly reflecting the great progress made in their control. However, these data also reveal the types of cancer that require further effort in prevention, precise early detection avoiding overdiagnosis, and efficient treatment. This is the case of liver, lung, esophageal, and pancreatic cancer, where the difference between the number of cases and deaths is smaller ( Figure 3B ). Social and economic transition in several countries has had an impact on reducing the incidence of neoplasms associated with infection and simultaneously produced an increase in the types related to reproductive, dietary, and hormonal factors. 75

Incidence and mortality for some types of cancer in the world. (A) Estimated number of cases and deaths in 2020 for the most frequent cancer types worldwide. (B) Incidence and mortality rates, normalized according to age, for the most frequent cancer types in countries with very high/& high (VH&H; blue) and/low and middle (L&M; red) Human Development Index (HDI). Data include both genders and all ages. Data according to https://gco.iarc.fr/today , as of June 10, 2021.
In the past 3 decades, cancer mortality rates have fallen in high HDI countries, with the exception of pancreatic cancer, and lung cancer in women. Nevertheless, changes in the incidence of cancer do not show the same consistency, possibly due to variables such as the possibility of early detection, exposure to risk factors, or genetic predisposition. 76 , 77 Countries such as Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the United Kingdom have reported a reduction in incidence and mortality in cancer of the stomach, colon, lung, and ovary, as well as an increase in survival. 78 Changes in modifiable risk factors, such as the use of tobacco, have played an important role in prevention. In this respect, it has been estimated that decline in tobacco use can explain between 35% and 45% of the reduction in cancer mortality rates, 79 while the fall in incidence and mortality due to stomach cancer can be attributed partly to the control of Helicobacter pylori infection. 80 Another key factor in the fall of mortality rates in developed countries has been an increase in early detection as a result of screening programs, as in breast and prostate cancer, which have had their mortality rates decreased dramatically in spite of an increase in their incidence. 76
Another important improvement observed in recent decades is the increase in survival rates, particularly in high HDI countries. In the USA, for example, survival rates for patients with prostate cancer at 5 years after initial diagnosis was 28% during 1947–1951; 69% during 1975–1977, and 100% during 2003–2009. Something similar occurred with breast cancer, with a 5-year survival rate of 54% in 1947–1951, 75% in 1975–1977, and 90% in 2003–2009. 81 In the CONCORD 3 version, age-standardize 5-year survival for patients with breast cancer in the USA during 2010–2014 was 90%, and 97% for prostate cancer patients. 82 Importantly, even among high HDI countries, significant differences have been identified in survival rates, being stage of disease at diagnosis, time for access to effective treatment, and comorbidities, the main factors influencing survival in these nations. 78 Unfortunately, survival rates in low HDI countries are significantly lower due to several factors, including lack of information, deficient screening and early detection programs, limited access to treatment, and suboptimal cancer registration. 82 It should be noted that in countries with low to middle HDI, neoplasms with the greatest incidence are those affecting women (breast and cervical cancer), which reflects not only a problem with access to health services, but also a serious inequality issue that involves social, cultural, and even religious obstacles. 83
Up to 42% of incident cases and 47% of deaths by cancer in the USA are due to potentially modifiable risk factors such as use of tobacco, physical activity, diet, and infection. 84 It has been calculated that 2.4 million deaths by cancer, mostly of the lung, can be attributed to tobacco. 73 In 2020, the incidence rate of lung cancer in Western Africa was 2.2, whereas in Polynesia and Eastern Asia was 37.3 and 34.4, respectively. 6 In contrast, the global burden of cancer associated with infection was 15.4%, but in Sub-Saharan Africa it was 30%. 85 Likewise, the incidence of cervical cancer in Eastern Africa was 40.1, in contrast with the USA and Canada that have a rate of 6.2. This makes it clear that one of the challenges we face is the reduction of the risk factors that are potentially modifiable and associated with specific types of cancer.
Improvement of survival rates and its disparities worldwide are also important challenges. Five-year survival for breast cancer—diagnosed during 2010-2014— in the USA, for example, was 90%, whereas in countries like South Africa it was 40%. 82 Childhood leukemia in the USA and several European countries shows a 5-year survival of 90%, while in Latin-American countries it is 50–76%. 86 Interestingly, there are neoplasms, such as pancreatic cancer, for which there has been no significant increase in survival, which remains low (5–15%) both in developed and developing countries. 82
Although data reported on global incidence and mortality gives a general overview on the epidemiology of cancer, it is important to note that there are great differences in coverage of cancer registries worldwide. To date, only 1 out of every 3 countries reports high quality data on the incidence of cancer. 87 For the past 50 years, the IARC has supported population-based cancer registries; however, more than one-third of the countries belonging to the WHO, mainly countries of low and middle income (LMIC), have no data on more than half of the 18 indicators of sustainable development goals. 88 High quality cancer registries only cover 4% of the population in Africa, 8% in Asia, and 7% in Latin America, contrasting with 83% in the USA and Canada, and 33% in Europe. 89 In response to this situation, the Global Initiative for Cancer Registry Development was created in 2012 to generate improved infrastructure to permit greater coverage and better quality registries, especially in countries with low and middle HDI. 88 It is expected that initiatives of this sort in the coming years will allow more and better information to guide strategies for the control of cancer worldwide, especially in developing regions. This will enable survival to be measured over longer periods of time (10, 15, or 20 years), as an effective measure in the control of cancer. The WHO has established as a target for 2025 to reduce deaths by cancer and other non-transmissible diseases by 25% in the population between the ages of 30–69; such an effort requires not only effective prevention measures to reduce incidence, but also more efficient health systems to diminish mortality and increase survival. At the moment, it is an even greater challenge because of the effects of the COVID-19 pandemic which has negatively impacted cancer prevention and health services. 90
Oncologic Treatments
A general perspective.
At the beginning of the 20th century, cancer treatment, specifically treatment of solid tumors, was based fundamentally on surgical resection of tumors, which together with other methods for local control, such as cauterization, had been used since ancient times. 91 At that time, there was an ongoing burst of clinical observations along with interventions sustained on fundamental knowledge about physics, chemistry, and biology. In the final years of the 19 th century and the first half of the 20th, these technological developments gave rise to radiotherapy, hormone therapy, and chemotherapy. 92 - 94 Simultaneously, immunotherapy was also developed, although usually on a smaller scale, in light of the overwhelming progress of chemotherapy and radiotherapy. 95
Thus began the development and expansion of disciplines based on these approaches (surgery, radiotherapy, chemotherapy, hormone therapy, and immunotherapy), with their application evolving ever more rapidly up to their current uses. Today, there is a wide range of therapeutic tools for the care of cancer patients. These include elements that emerged empirically, arising from observations of their effects in various medical fields, as well as drugs that were designed to block processes and pathways that form part of the physiopathology of one or more neoplasms according to knowledge of specific molecular alterations. A classic example of the first sort of tool is mustard gas, originally used as a weapon in war, 96 but when applied for medical purposes, marked the beginning of the use of chemicals in the treatment of malignant neoplasms, that is, chemotherapy. 94 A clear example of the second case is imatinib, designed specifically to selectively inhibit a molecular alteration in chronic myeloid leukemia: the Bcr-Abl oncoprotein. 97
It is on this foundation that today the 5 areas mentioned previously coexist and complement one another. The general framework that motivates this amalgam and guides its development is precision medicine, founded on the interaction of basic and clinical science. In the forecasts for development in each of these fields, surgery is expected to continue to be the fundamental approach for primary tumors in the foreseeable future, as well as when neoplastic disease in the patient is limited, or can be limited by applying systemic or regional elements, before and/or after surgical resection, and it can be reasonably anticipated for the patient to have a significant period free from disease or even to be cured. With regards to technology, intensive exploration of robotic surgery is contemplated. 98
The technological possibilities for radiotherapy have progressed in such a way that it is now possible to radiate neoplastic tissue with an extraordinary level of precision, and therefore avoid damage to healthy tissue. 99 This allows administration of large doses of ionizing radiation in one or a few fractions, what is known as “radiosurgery.” The greatest challenges to the efficacy of this approach are related to radio-resistance in certain neoplasms. Most efforts regarding research in this field are concentrated on understanding the underlying biological mechanisms of the phenomenon and their potential control through radiosensitizers. 100
“Traditional” chemotherapy, based on the use of compounds obtained from plants and other natural products, acting in a non-specific manner on both neoplastic and healthy tissues with a high proliferation rate, continues to prevail. 101 The family of chemotherapeutic drugs currently includes alkylating agents, antimetabolites, anti-topoisomerase agents, and anti-microtubules. Within the pharmacologic perspective, the objective is to attain a high concentration or activity of such molecules in specific tissues while avoiding their accumulation in others, in order to achieve an increase in effectiveness and a reduction in toxicity. This has been possible with the use of viral vectors, for example, that are able to limit their replication in neoplastic tissues, and activate prodrugs of normally nonspecific agents, like cyclophosphamide, exclusively in those specific areas. 102 More broadly, chemotherapy also includes a subgroup of substances, known as molecular targeted therapy, that affect processes in a more direct and specific manner, which will be mentioned later.
There is no doubt that immunotherapy—to be explored next—is one of the therapeutic fields where development has been greatest in recent decades and one that has produced enormous expectation in cancer treatment. 103 Likewise, cell therapy, based on the use of immune cells or stem cells, has come to complement the oncologic therapeutic arsenal. 43 Each and every one of the therapeutic fields that have arisen in oncology to this day continue to prevail and evolve. Interestingly, the foreseeable future for the development of cancer treatment contemplates these approaches in a joint and complementary manner, within the general framework of precision medicine, 104 and sustained by knowledge of the biological mechanisms involved in the appearance and progression of neoplasms. 105 , 106
Immunotherapy
Stimulating the immune system to treat cancer patients has been a historical objective in the field of oncology. Since the early work of William Coley 107 to the achievements reached at the end of the 20 th century, scientific findings and technological developments paved the way to searching for new immunotherapeutic strategies. Recombinant DNA technology allowed the synthesis of cytokines, such as interferon-alpha (IFN-α) and interleukin 2 (IL-2), which were authorized by the US Food and Drug Administration (FDA) for the treatment of hairy cell leukemia in 1986, 108 as well as kidney cancer and metastatic melanoma in 1992 and 1998, respectively. 109
The first therapeutic vaccine against cancer, based on the use of autologous dendritic cells (DCs), was approved by the FDA against prostate cancer in 2010. However, progress in the field of immunotherapy against cancer was stalled in the first decade of the present century, mostly due to failure of several vaccines in clinical trials. In many cases, application of these vaccines was detained by the complexity and cost involved in their production. Nevertheless, with the coming of the concept of immune checkpoint control, and the demonstration of the relevance of molecules such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), and programmed cell death molecule-1 (PD-1), immunotherapy against cancer recovered its global relevance. In 2011, the monoclonal antibody (mAb) ipilimumab, specific to the CTLA-4 molecule, was the first checkpoint inhibitor (CPI) approved for the treatment of advanced melanoma. 110 Later, inhibitory mAbs for PD-1, or for the PD-1 ligand (PD-L1), 111 as well as the production of T cells with chimeric receptors for antigen recognition (CAR-T), 112 which have been approved to treat various types of cancer, including melanoma, non-small cell lung cancer (NSCLC), head and neck cancer, bladder cancer, renal cell carcinoma (RCC), and hepatocellular carcinoma, among others, have changed the paradigm of cancer treatment.
In spite of the current use of anti-CTLA-4 and anti-PD-L1 mAbs, only a subgroup of patients has responded favorably to these CPIs, and the number of patients achieving clinical benefit is still small. It has been estimated that more than 70% of patients with solid tumors do not respond to CPI immunotherapy because either they show primary resistance, or after responding favorably, develop resistance to treatment. 113 In this regard, it is important to mention that in recent years very important steps have been taken to identify the intrinsic and extrinsic mechanisms that mediate resistance to CPI immunotherapy. 114 Intrinsic mechanisms include changes in the antitumor immune response pathways, such as faulty processing and presentation of antigens by APCs, activation of T cells for tumor cell destruction, and changes in tumor cells that lead to an immunosuppressive TME. Extrinsic factors include the presence of immunosuppressive cells in the local TME, such as regulatory T cells, myeloid-derived suppressor cells (MDSC), mesenchymal stem/stromal cells (MSCs), and type 2 macrophages (M2), in addition to immunosuppressive cytokines.
On the other hand, classification of solid tumors as “hot,” “cold,” or “excluded,” depending on T cell infiltrates and the contact of such infiltrates with tumor cells, as well as those that present high tumor mutation burden (TMB), have redirected immunotherapy towards 3 main strategies 115 ( Table 2 ): (1) Making T-cell antitumor response more effective, using checkpoint inhibitors complementary to anti-CTLA-4 and anti-PD-L1, such as LAG3, Tim-3, and TIGT, as well as using CAR-T cells against tumor antigens. (2) Activating tumor-associated myeloid cells including monocytes, granulocytes, macrophages, and DC lineages, found at several frequencies within human solid tumors. (3) Regulating the biochemical pathways in TME that produce high concentrations of immunosuppressive molecules, such as kynurenine, a product of tryptophan metabolism, through the activity of indoleamine 2,3 dioxygenase; or adenosine, a product of ATP hydrolysis by the activity of the enzyme 5’nucleotidase (CD73). 116
Current Strategies to Stimulate the Immune Response for Antitumor Immunotherapy.
Abbreviations: TME, tumor microenvironment; IL, interleukin; TNF, Tumor Necrosis Factor; TNFR, TNF-receptor; CD137, receptor–co-stimulator of the TNFR family; OX40, member number 4 of the TNFR superfamily; CD27/CD70, member of the TNFR superfamily; CD40/CD40L, antigen-presenting cells (APC) co-stimulator and its ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; STING, IFN genes-stimulator; RIG-I, retinoic acid inducible gene-I; MDA5, melanoma differentiation-associated protein 5; CDN, cyclic dinucleotide; ATP, adenosine triphosphate; HMGB1, high mobility group B1 protein; TLR, Toll-like receptor; HVEM, Herpes virus entry mediator; GITR, glucocorticoid-induced TNFR family-related gene; CTLA4, cytotoxic T lymphocyte antigen 4; PD-L1, programmed death ligand-1; TIGIT, T-cell immunoreceptor with immunoglobulin and tyrosine-based inhibition motives; CSF1/CSF1R, colony-stimulating factor-1 and its receptor; CCR2, Type 2 chemokine receptor; PI3Kγ, Phosphoinositide 3-Kinase γ; CXCL/CCL, chemokine ligands; LFA1, lymphocyte function-associated antigen 1; ICAM1, intercellular adhesion molecule 1; VEGF, vascular endothelial growth factor; IDO, indolamine 2,3-dioxigenase; TGF, transforming growth factor; LAG-3, lymphocyte-activation gene 3 protein; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; CD73, 5´nucleotidase; ARs, adenosine receptors; Selectins, cell adhesion molecules; CAR-T, chimeric antigen receptor T cell; TCR-T, T-cell receptor engineered T cell.
Apart from the problems associated with its efficacy (only a small group of patients respond to it), immunotherapy faces several challenges related to its safety. In other words, immunotherapy can induce adverse events in patients, such as autoimmunity, where healthy tissues are attacked, or cytokine release syndrome and vascular leak syndrome, as observed with the use of IL-2, both of which lead to serious hypotension, fever, renal failure, and other adverse events that are potentially lethal. The main challenges to be faced by immunotherapy in the future will require the combined efforts of basic and clinical scientists, with the objective of accelerating the understanding of the complex interactions between cancer and the immune system, and improve treatment options for patients. Better comprehension of immune phenotypes in tumors, beyond the state of PD-L1 and TME, will be relevant to increase immunotherapy efficacy. In this context, the identification of precise tumor antigenicity biomarkers by means of new technologies, such as complete genome sequencing, single cell sequencing, and epigenetic analysis to identify sites or subclones typical in drug resistance, as well as activation, traffic and infiltration of effector cells of the immune response, and regulation of TME mechanisms, may help define patient populations that are good candidates for specific therapies and therapeutic combinations. 117 , 118 Likewise, the use of agents that can induce specific activation and modulation of the response of T cells in tumor tissue, will help improve efficacy and safety profiles that can lead to better clinical results.
Molecular Targeted Therapy
For over 30 years, and based on the progress in our knowledge of tumor biology and its mechanisms, there has been a search for therapeutic alternatives that would allow spread and growth of tumors to be slowed down by blocking specific molecules. This approach is known as molecular targeted therapy. 119 Among the elements generally used as molecular targets there are transcription factors, cytokines, membrane receptors, molecules involved in a variety of signaling pathways, apoptosis modulators, promoters of angiogenesis, and cell cycle regulators. 120
Imatinib, a tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia, became the first targeted therapy in the final years of the 1990s. 97 From then on, new drugs have been developed by design, and today more than 60 targeted therapies have been approved by the FDA for the treatment of a variety of cancers ( Table 3 ). 121 This has had a significant impact on progression-free survival and global survival in neoplasms such as non-small cell lung cancer, breast cancer, renal cancer, and melanoma.
FDA Approved Molecular Targeted Therapies for the Treatment of Solid Tumors.
Abbreviations: mAb, monoclonal antibody; ALK, anaplastic lymphoma kinase; CDK, cyclin-dependent kinase; CTLA-4, cytotoxic lymphocyte antigen-4; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stroma tumor; mTOR, target of rapamycine in mammal cells; NSCLC, non-small cell lung carcinoma; PARP, poli (ADP-ribose) polimerase; PD-1, programmed death protein-1; PDGFR, platelet-derived growth factor receptor; PD-L1, programmed death ligand-1; ER, estrogen receptor; PR, progesterone receptor; TKR, tyrosine kinase receptors; SERM, selective estrogen receptor modulator; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor. Modified from Ref. [ 127 ].
Most drugs classified as targeted therapies form part of 2 large groups: small molecules and mAbs. The former are defined as compounds of low molecular weight (<900 Daltons) that act upon entering the cell. 120 Targets of these compounds are cell cycle regulatory proteins, proapoptotic proteins, or DNA repair proteins. These drugs are indicated based on histological diagnosis, as well as molecular tests. In this group there are multi-kinase inhibitors (RTKs) and tyrosine kinase inhibitors (TKIs), like sunitinib, sorafenib, and imatinib; cyclin-dependent kinase (CDK) inhibitors, such as palbociclib, ribociclib and abemaciclib; poli (ADP-ribose) polimerase inhibitors (PARPs), like olaparib and talazoparib; and selective small-molecule inhibitors, like ALK and ROS1. 122
As for mAbs, they are protein molecules that act on membrane receptors or extracellular proteins by interrupting the interaction between ligands and receptors, in such a way that they reduce cell replication and induce cytostasis. Among the most widely used mAbs in oncology we have: trastuzumab, a drug directed against the receptor for human epidermal growth factor-2 (HER2), which is overexpressed in a subgroup of patients with breast and gastric cancer; and bevacizumab, that blocks vascular endothelial growth factor and is used in patients with colorectal cancer, cervical cancer, and ovarian cancer. Other mAbs approved by the FDA include pembolizumab, atezolizumab, nivolumab, avelumab, ipilimumab, durvalumab, and cemiplimab. These drugs require expression of response biomarkers, such as PD-1 and PD-L1, and must also have several resistance biomarkers, such as the expression of EGFR, the loss of PTEN, and alterations in beta-catenin. 123
Because cancer is such a diverse disease, it is fundamental to have precise diagnostic methods that allow us to identify the most adequate therapy. Currently, basic immunohistochemistry is complemented with neoplastic molecular profiles to determine a more accurate diagnosis, and it is probable that in the near future cancer treatments will be based exclusively on molecular profiles. In this regard, it is worth mentioning that the use of targeted therapy depends on the existence of specific biomarkers that indicate if the patient will be susceptible to the effects of the drug or not. Thus, the importance of underlining that not all patients are susceptible to receive targeted therapy. In certain neoplasms, therapeutic targets are expressed in less than 5% of the diagnosed population, hindering a more extended use of certain drugs.
The identification of biomarkers and the use of new generation sequencing on tumor cells has shown predictive and prognostic relevance. Likewise, mutation analysis has allowed monitoring of tumor clone evolution, providing information on changes in canonic gene sequences, such as TP53, GATA3, PIK3CA, AKT1, and ERBB2; infrequent somatic mutations developed after primary treatments, like SWI-SNF and JAK2-STAT3; or acquired drug resistance mutations such as ESR1. 124 The study of mutations is vital; in fact, many of them already have specific therapeutic indications, which have helped select adequate treatments. 125
There is no doubt that molecular targeted therapy is one of the main pillars of precision medicine. However, it faces significant problems that often hinder obtaining better results. Among these, there is intratumor heterogeneity and differences between the primary tumor and metastatic sites, as well as intrinsic and acquired resistance to these therapies, the mechanisms of which include the presence of heterogeneous subclones, DNA hypermethylation, histone acetylation, and interruption of mRNA degradation and translation processes. 126 Nonetheless, beyond the obstacles facing molecular targeted therapy from a biological and methodological point of view, in the real world, access to genomic testing and specific drugs continues to be an enormous limitation, in such a way that strategies must be designed in the future for precision medicine to be possible on a global scale.
Cell Therapy
Another improvement in cancer treatment is the use of cell therapy, that is, the use of specific cells as therapeutic agents. This clinical procedure has 2 modalities: the first consists of replacing and regenerating functional cells in a specific tissue by means of stem/progenitor cells of a certain kind, 43 while the second uses immune cells as effectors to eliminate malignant cells. 127
Regarding the first type, we must emphasize the development of cell therapy based on hematopoietic stem and progenitor cells. 128 For over 50 years, hematopoietic cell transplants have been used to treat a variety of hematologic neoplasms (different forms of leukemia and lymphoma). Today, it is one of the most successful examples of cell therapy, including innovative modalities, such as haploidentical transplants, 129 as well as application of stem cells expanded ex vivo . 130 There are also therapies that have used immature cells that form part of the TME, such as MSCs. The replication potential and cytokine secretion capacity of these cells make them an excellent option for this type of treatment. 131 Neural stem cells can also be manipulated to produce and secrete apoptotic factors, and when these cells are incorporated into primary neural tumors, they cause a certain degree of regression. They can even be transfected with genes that encode for oncolytic enzymes capable of inducing regression of glioblastomas. 132
With respect to cell therapy using immune cells, several research groups have manipulated cells associated with tumors to make them effector cells and thus improve the efficacy and specificity of the antitumor treatment. PB leckocytes cultured in the presence of IL-2 to obtain activated lymphocytes, in combination with IL-2 administration, have been used in antitumor clinical protocols. Similarly, infiltrating lymphocytes from tumors with antitumor activity have been used and can be expanded ex vivo with IL-2. These lymphocyte populations have been used in immunomodulatory therapies in melanoma, and pancreatic and kidney tumors, producing a favorable response in treated patients. 133 NK cells and macrophages have also been used in immunotherapy, although with limited results. 134 , 135
One of the cell therapies with better projection today is the use of CAR-T cells. This strategy combines 2 forms of advanced therapy: cell therapy and gene therapy. It involves the extraction of T cells from the cancer patient, which are genetically modified in vitro to express cell surface receptors that will recognize antigens on the surface of tumor cells. The modified T cells are then reintroduced in the patient to aid in an exacerbated immune response that leads to eradication of the tumor cells ( Figure 4 ). Therapy with CAR-T cells has been used successfully in the treatment of some types of leukemia, lymphoma, and myeloma, producing complete responses in patients. 136

CAR-T cell therapy. (A) T lymphocytes obtained from cancer patients are genetically manipulated to produce CAR-T cells that recognize tumor cells in a very specific manner. (B) Interaction between CAR molecule and tumor antigen. CAR molecule is a receptor that results from the fusion between single-chain variable fragments (scFv) from a monoclonal antibody and one or more intracellular signaling domains from the T-cell receptor. CD3ζ, CD28 and 4-1BB correspond to signaling domains on the CAR molecule.
Undoubtedly, CAR-T cell therapy has been truly efficient in the treatment of various types of neoplasms. However, this therapeutic strategy can also have serious side effects, such as release of cytokines into the bloodstream, which can cause different symptoms, from high fever to multiorgan failure, and even neurotoxicity, leading to cerebral edema in many cases. 137 Adequate control of these side effects is an important medical challenge. Several research groups are trying to improve CAR-T cell therapy through various approaches, including production of CAR-T cells directed against a wider variety of tumor cell-specific antigens that are able to attack different types of tumors, and the identification of more efficient types of T lymphocytes. Furthermore, producing CAR-T cells from a single donor that may be used in the treatment of several patients would reduce the cost of this sort of personalized cell therapy. 136
Achieving wider use of cell therapy in oncologic diseases is an important challenge that requires solving various issues. 138 One is intratumor cell heterogeneity, including malignant subclones and the various components of the TME, which results in a wide profile of membrane protein expression that complicates finding an ideal tumor antigen that allows specific identification (and elimination) of malignant cells. Likewise, structural organization of the TME challenges the use of cell therapy, as administration of cell vehicles capable of recognizing malignant cells might not be able to infiltrate the tumor. This results from low expression of chemokines in tumors and the presence of a dense fibrotic matrix that compacts the inner tumor mass and avoids antitumor cells from infiltrating and finding malignant target cells.
Further Challenges in the 21st Century
Beyond the challenges regarding oncologic biomedical research, the 21 st century is facing important issues that must be solved as soon as possible if we truly wish to gain significant ground in our fight against cancer. Three of the most important have to do with prevention, early diagnosis, and access to oncologic medication and treatment.
Prevention and Early Diagnosis
Prevention is the most cost-effective strategy in the long term, both in low and high HDI nations. Data from countries like the USA indicate that between 40-50% of all types of cancer are preventable through potentially modifiable factors (primary prevention), such as use of tobacco and alcohol, diet, physical activity, exposure to ionizing radiation, as well as prevention of infection through access to vaccination, and by reducing exposure to environmental pollutants, such as pesticides, diesel exhaust particles, solvents, etc. 74 , 84 Screening, on the other hand, has shown great effectiveness as secondary prevention. Once population-based screening programs are implemented, there is generally an initial increase in incidence; however, in the long term, a significant reduction occurs not only in incidence rates, but also in mortality rates due to detection of early lesions and timely and adequate treatment.
A good example is colon cancer. There are several options for colon cancer screening, such as detection of fecal occult blood, fecal immunohistochemistry, flexible sigmoidoscopy, and colonoscopy, 139 , 140 which identify precursor lesions (polyp adenomas) and allow their removal. Such screening has allowed us to observe 3 patterns of incidence and mortality for colon cancer between the years 2000 and 2010: on one hand, an increase in incidence and mortality in countries with low to middle HDI, mainly countries in Asia, South America, and Eastern Europe; on the other hand, an increase in incidence and a fall in mortality in countries with very high HDI, such as Canada, the United Kingdom, Denmark, and Singapore; and finally a fall in incidence and mortality in countries like the USA, Japan, and France. The situation in South America and Asia seems to reflect limitations in medical infrastructure and a lack of access to early detection, 141 while the patterns observed in developed countries reveal the success, even if it may be partial, of that which can be achieved by well-structured prevention programs.
Another example of success, but also of strong contrast, is cervical cancer. The discovery of the human papilloma virus (HPV) as the causal agent of cervical cancer brought about the development of vaccines and tests to detect oncogenic genotypes, which modified screening recommendations and guidelines, and allowed several developed countries to include the HPV vaccine in their national vaccination programs. Nevertheless, the outlook is quite different in other areas of the world. Eighty percent of the deaths by cervical cancer reported in 2018 occurred in low-income nations. This reveals the urgency of guaranteeing access to primary and secondary prevention (vaccination and screening, respectively) in these countries, or else it will continue to be a serious public health problem in spite of its preventability.
Screening programs for other neoplasms, such as breast, prostate, lung, and thyroid cancer have shown outlooks that differ from those just described, because, among other reasons, these neoplasms are highly diverse both biologically and clinically. Another relevant issue is the overdiagnosis of these neoplasms, that is, the diagnosis of disease that would not cause symptoms or death in the patient. 142 It has been calculated that 25% of breast cancer (determined by mammogram), 50–60% of prostate cancer (determined by PSA), and 13–25% of lung cancer (determined by CT) are overdiagnosed. 142 Thus, it is necessary to improve the sensitivity and specificity of screening tests. In this respect, knowledge provided by the biology of cancer and “omic” sciences offers a great opportunity to improve screening and prevention strategies. All of the above shows that prevention and early diagnosis are the foundations in the fight against cancer, and it is essential to continue to implement broader screening programs and better detection methods.
Global Equity in Oncologic Treatment
Progress in cancer treatment has considerably increased the number of cancer survivors. Nevertheless, this tendency is evident only in countries with a very solid economy. Indeed, during the past 30 years, cancer mortality rates have increased 30% worldwide. 143 Global studies indicate that close to 70% of cancer deaths in the world occur in nations of low to middle income. But even in high-income countries, there are sectors of society that are more vulnerable and have less access to cancer treatments. 144 Cancer continues to be a disease of great social inequality.
In Europe, the differences in access to cancer treatment are highly marked. These treatments are more accessible in Western Europe than in its Eastern counterpart. 145 Furthermore, highly noticeable differences between high-income countries have been detected in the cost of cancer drugs. 146 It is interesting to note that in many of these cases, treatment is too costly and the clinical benefit only marginal. Thus, the importance of these problems being approached by competent national, regional, and global authorities, because if these new drugs and therapeutic programs are not accessible to the majority, progress in biomedical, clinical and epidemiological research will have a limited impact in our fight against cancer. We must not forget that health is a universal right, from which low HDI countries must not be excluded, nor vulnerable populations in nations with high HDI. The participation of a well-informed society will also be fundamental to achieve a global impact, as today we must fight not only against the disease, but also against movements and ideas (such as the anti-vaccine movement and the so-called miracle therapies) that can block the medical battle against cancer.
Final Comments
From the second half of the 20th century to the present day, progress in our knowledge about the origin and development of cancer has been extraordinary. We now understand cancer in detail in genomic, molecular, cellular, and physiological terms, and this knowledge has had a significant impact in the clinic. There is no doubt that a patient who is diagnosed today with a type of cancer has a better prospect than a patient diagnosed 20 or 50 years ago. However, we are still far from winning the war against cancer. The challenges are still numerous. For this reason, oncologic biomedical research must be a worldwide priority. Likewise, one of the fundamental challenges for the coming decades must be to reduce unequal access to health services in areas of low- to middle income, and in populations that are especially vulnerable, as well as continue improving prevention programs, including public health programs to reduce exposure to environmental chemicals and improve diet and physical activity in the general population. 74 , 84 Fostering research and incorporation of new technological resources, particularly in less privileged nations, will play a key role in our global fight against cancer.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Hector Mayani https://orcid.org/0000-0002-2483-3782
- Search by keyword
- Search by citation
Page 1 of 76
The troglitazone derivative EP13 disrupts energy metabolism through respiratory chain complex I inhibition in breast cancer cells and potentiates the antiproliferative effect of glycolysis inhibitntriors
The metabolism of cancer cells generally differs from that of normal cells. Indeed, most cancer cells have a high rate of glycolysis, even at normal oxygen concentrations. These metabolic properties can potent...
- View Full Text
Enhancing therapeutic efficacy in luminal androgen receptor triple-negative breast cancer: exploring chidamide and enzalutamide as a promising combination strategy
Extensive exploration of the molecular subtypes of triple-negative breast cancer (TNBC) is critical for advancing precision medicine. Notably, the luminal androgen receptor (LAR) subtype has attracted attentio...
Identification of fatty acids synthesis and metabolism-related gene signature and prediction of prognostic model in hepatocellular carcinoma
Fatty acids synthesis and metabolism (FASM)-driven lipid mobilization is essential for energy production during nutrient shortages. However, the molecular characteristics, physiological function and clinical p...
MiR-380 inhibits the proliferation and invasion of cholangiocarcinoma cells by silencing LIS1
The objective of this study was to determine the role and regulatory mechanism of miR-380 in cholangiocarcinoma.
Retraction Note: TMED3 promotes cell proliferation and motility in breast cancer and is negatively modulated by miR-188-3p
Comprehensive analyses of the cancer-associated fibroblast subtypes and their score system for prediction of outcomes and immunosuppressive microenvironment in prostate cancer.
Cancer-associated fibroblasts (CAFs) drive cancer progression and treatment failure on one hand, while their tumor-restraining functions are also observed on the other. Recent single cell RNA sequencing (scRNA...
Correction to: N6‑methyladenosine levels in peripheral blood RNA: a potential diagnostic biomarker for colorectal cancer
The original article was published in Cancer Cell International 2024 24 :96
Deciphering the prognostic features of bladder cancer through gemcitabine resistance and immune-related gene analysis and identifying potential small molecular drug PIK-75
Bladder cancer (BCa) stands out as a prevalent and highly lethal malignancy worldwide. Chemoresistance significantly contributes to cancer recurrence and progression. Traditional Tumor Node Metastasis (TNM) st...
CCDC25 suppresses clear cell renal cell carcinoma progression by LATS1/YAP-mediated regulation of the hippo pathway
Clear cell renal cell carcinoma (ccRCC) is one of the most prevalent renal cancers, and the molecular mechanisms underlying its progression are still not fully understood. The expression of CCDC25, a notably u...

Immune cell infiltration and drug response in glioblastoma multiforme: insights from oxidative stress-related genes
GBM, also known as glioblastoma multiforme, is the most prevalent and lethal type of brain cancer. The cell proliferation, invasion, angiogenesis, and treatment of gliomas are significantly influenced by oxida...
Biological roles of SLC16A1-AS1 lncRNA and its clinical impacts in tumors
Recent studies have increasingly highlighted the aberrant expression of SLC16A1-AS1 in a variety of tumor types, where it functions as either an oncogene or a tumor suppressor in the pathogenesis of different ...
β-hydroxybutyrate inhibits malignant phenotypes of prostate cancer cells through β-hydroxybutyrylation of indoleacetamide-N-methyltransferase
Prostate cancer (PCa) is one of the most prevalent cancers in men and is associated with high mortality and disability rates. β-hydroxybutyrate (BHB), a ketone body, has received increasing attention for its r...
The molecular subtyping and precision medicine in triple-negative breast cancer---based on Fudan TNBC classification
Triple-negative breast cancer (TNBC) is widely recognized as the most aggressive form of breast cancer, occurring more frequently in younger patients and characterized by high heterogeneity, early distant meta...
The molecular characteristics could supplement the staging system of pT2/T3N0M0 esophageal squamous cell carcinoma: a translational study based on a cohort with over 20 years of follow-up
This study aimed to construct a model based on 23 enrolled molecules to evaluate prognoses of pT2/3N0M0 esophageal squamous cell carcinoma (ESCC) patients with up to 20 years of follow-up.
RBP7 functions as a tumor suppressor in HR + breast cancer by inhibiting the AKT/SREBP1 pathway and reducing fatty acid
Increasing evidence proves that RBP7 plays a significant role in breast cancer (BC). The present study was aimed to investigate the mechanism of RBP7.
Low TYROBP expression predicts poor prognosis in multiple myeloma
Multiple myeloma (MM) is the second most common refractory hematologic cancer. Searching for new targets and prognostic markers for MM is significant.
Characterization of the biological and transcriptomic landscapes of bone marrow-derived mesenchymal stem cells in patients with multiple myeloma
Mesenchymal stem/stromal cells (MSCs) have been acknowledged as the most important stromal cells in the bone marrow (BM) microenvironment for physiologic hematopoiesis and the concomitant hematologic malignanc...
The upregulation of VGF enhances the progression of oral squamous carcinoma
Oral squamous cell carcinoma (OSCC) is a prevalent neoplasm worldwide, necessitating a deeper understanding of its pathogenesis. VGF nerve growth factor inducible (VGF), a neuropeptide, plays critical roles in...
Domperidone inhibits cell proliferation via targeting MEK and CDK4 in esophageal squamous cell carcinoma
Esophageal squamous cell carcinoma (ESCC) is one of the leading causes of digestive system tumor related death in the world. Unfortunately, effective chemopreventive agent is lack for patients with ESCC in cli...
LncRNA RASAL2-AS1 promotes METTL14-mediated m6A methylation in the proliferation and progression of head and neck squamous cell carcinoma
Long non-coding RNAs (lncRNAs) are key regulators of the 6-methyladenosine (m6A) epigenetic modification, playing a role in the initiation and progression of tumors. However, the regulatory mechanisms in head ...
Integrated analysis of disulfidptosis-related immune genes signature to boost the efficacy of prognostic prediction in gastric cancer
Gastric cancer (GC) remains a malignant tumor with high morbidity and mortality, accounting for approximately 1,080,000 diagnosed cases and 770,000 deaths worldwide annually. Disulfidptosis, characterized by t...
LncRNA WFDC21P interacts with SEC63 to promote gastric cancer malignant behaviors by regulating calcium homeostasis signaling pathway
Gastric cancer is currently estimated to be the fifth leading common cancer in the world, and responsible for about one million new cases and an estimated 769,000 cancer-related deaths each year. WFDC21P is lo...
Coordinated activation of DNMT3a and TET2 in cancer stem cell-like cells initiates and sustains drug resistance in hepatocellular carcinoma
Resistance to targeted therapies represents a significant hurdle to successfully treating hepatocellular carcinoma (HCC). While epigenetic abnormalities are critical determinants of HCC relapse and therapeutic...
Soluble Periostin is a potential surveillance biomarker for early and long-term response to chemotherapy in advanced breast cancer
Noninvasive biomarkers for the assessment of response to chemotherapy in advanced breast cancer (BCa) are essential for optimized therapeutic decision-making. We evaluated the potential of soluble Periostin (P...
Aptamers as an approach to targeted cancer therapy
Conventional cancer treatments can cause serious side effects because they are not specific to cancer cells and can damage healthy cells. Aptamers often are single-stranded oligonucleotides arranged in a uniqu...
Neighboring macrophage-induced alteration in the phenotype of colorectal cancer cells in the tumor budding area
A higher number of tumor buds in the invasive front of colorectal cancer (CRC) specimens has been shown to contribute to a poor prognosis in CRC patients. Because macrophages (Mφs) have been demonstrated to al...
Uncovering the cellular and omics characteristics of natural killer cells in the bone marrow microenvironment of patients with acute myeloid leukemia
Acute myeloid leukemia (AML) is a highly heterogeneous hematologic malignancy and the most frequently acute leukemia of stem cell precursors and the myeloid derivatives in adult. Longitudinal studies have indi...
Immune modulation in malignant pleural effusion: from microenvironment to therapeutic implications
Immune microenvironment and immunotherapy have become the focus and frontier of tumor research, and the immune checkpoint inhibitors has provided novel strategies for tumor treatment. Malignant pleural effusio...
Decoding contextual crosstalk: revealing distinct interactions between non-coding RNAs and unfolded protein response in breast cancer
Breast cancer is significantly influenced by endoplasmic reticulum (ER) stress, impacting both its initiation and progression. When cells experience an accumulation of misfolded or unfolded proteins, they acti...
Influential upregulation of KCNE4: Propelling cancer associated fibroblasts-driven colorectal cancer progression
Colorectal cancer (CRC) is a malignancy of remarkable heterogeneity and heightened morbidity. Cancer associated fibroblasts (CAFs) are abundant in CRC tissues and are essential for CRC growth. Here, we aimed t...
MicroRNAs as regulators of immune checkpoints in cancer immunotherapy: targeting PD-1/PD-L1 and CTLA-4 pathways
Immunotherapy has revolutionized cancer treatment by harnessing the power of the immune system to eliminate tumors. Immune checkpoint inhibitors (ICIs) block negative regulatory signals that prevent T cells fr...

Roles and mechanisms of aberrant alternative splicing in melanoma — implications for targeted therapy and immunotherapy resistance
Despite advances in therapeutic strategies, resistance to immunotherapy and the off-target effects of targeted therapy have significantly weakened the benefits for patients with melanoma.
Perspective view of allogeneic IgG tumor immunotherapy
Allogeneic tumors are eradicated by host immunity; however, it is unknown how it is initiated until the report in Nature by Yaron Carmi et al. in 2015. Currently, we know that allogeneic tumors are eradicated by ...
Impact of NDUFAF6 on breast cancer prognosis: linking mitochondrial regulation to immune response and PD-L1 expression
Breast cancer is a major global health concern, and there is a continuous search for novel biomarkers to predict its prognosis. The mitochondrial protein NDUFAF6, previously studied in liver cancer, is now bei...
Application status and optimization suggestions of tumor organoids and CAR-T cell co-culture models
Tumor organoids, especially patient-derived organoids (PDOs) exhibit marked similarities in histopathological morphology, genomic alterations, and specific marker expression profiles to those of primary tumour...
Mitochondrial respiratory chain component NDUFA4: a promising therapeutic target for gastrointestinal cancer
Gastrointestinal cancer, one of the most common cancers, continues to be a major cause of mortality and morbidity globally. Accumulating evidence has shown that alterations in mitochondrial energy metabolism a...
N 6 -methyladenosine levels in peripheral blood RNA: a potential diagnostic biomarker for colorectal cancer
N 6 -methyladenosine (m 6 A) is dysregulated in various cancers, including colorectal cancer (CRC). Herein, we assess the diagnostic potential of peripheral blood (PB) m 6 A levels in CRC.
The Correction to this article has been published in Cancer Cell International 2024 24 :126
Transmembrane protein 176B regulates amino acid metabolism through the PI3K-Akt-mTOR signaling pathway and promotes gastric cancer progression
The present study aimed to investigate the expression level, biological function, and underlying mechanism of transmembrane protein 176B (TMEM176B) in gastric cancer (GC).
Targeting HER2-positive breast cancer cells by a combination of dasatinib and BMS-202: Insight into the molecular pathways
Recent investigations have reported the benefits of using a tyrosine kinase inhibitor, dasatinib (DA), as well as programmed death-ligand 1 (PD-L1) inhibitors in the management of several solid tumors, includi...
RGS20 promotes non-small cell lung carcinoma proliferation via autophagy activation and inhibition of the PKA-Hippo signaling pathway
Novel therapeutic targets are urgently needed for treating drug-resistant non-small cell lung cancer (NSCLC) and overcoming drug resistance to molecular-targeted therapies. Regulator of G protein signaling 20 ...
A cuproptosis-related gene expression signature predicting clinical prognosis and immune responses in intrahepatic cholangiocarcinoma detected by single-cell RNA sequence analysis
Cholangiocarcinoma represents a malignant neoplasm originating from the hepatobiliary tree, with a subset of tumors developing inside the liver. Intrahepatic cholangiocarcinomas (ICC) commonly exhibit an asymp...
The circRNA hsa-circ-0013561 regulates head and neck squamous cell carcinoma development via the miR-7-5p/PDK3 axis
Circular RNAs (circRNAs) belong to a class of covalently closed single stranded RNAs that have been implicated in cancer progression. Former investigations showed that hsa-circ-0013561 is abnormally expressed ...
PPP2R1B abolishes colorectal cancer liver metastasis and sensitizes Oxaliplatin by inhibiting MAPK/ERK signaling pathway
Patients with colorectal cancer (CRC) with liver metastasis or drug resistance have a poor prognosis. Previous research has demonstrated that PPP2R1B inactivation results in the development of CRC. However, th...
The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: a promising therapeutic target
Cancer chemoresistance is a problematic dilemma that significantly restrains numerous cancer management protocols. It can promote cancer recurrence, spreading of cancer, and finally, mortality. Accordingly, en...

Dormancy of cutaneous melanoma
Many cancer-related deaths including melanoma result from metastases that develop months or years after the initial cancer therapy. Even the most effective drugs and immune therapies rarely eradicate all tumor...
LASS2 suppresses metastasis in multiple cancers by regulating the ferroptosis signalling pathway through interaction with TFRC
As a key enzyme in ceramide synthesis, longevity assurance homologue 2 (LASS2) has been indicated to act as a tumour suppressor in a variety of cancers. Ferroptosis is involved in a variety of tumour processes...
ACADL-YAP axis activity in non-small cell lung cancer carcinogenicity
The role of Acyl-CoA dehydrogenase long chain (ACADL) in different tumor types had different inhibiting or promoting effect. However, its role in non-small cell lung cancer (NSCLC) carcinogenicity is not clear.
SLC25A17 inhibits autophagy to promote triple-negative breast cancer tumorigenesis by ROS-mediated JAK2/STAT3 signaling pathway
SLC25A17, a peroxisomal solute carrier, has been implicated in various physiological and pathological processes. However, its precise roles and underlying mechanisms in triple-negative breast cancer (TNBC) rem...
Emerging trends in the coexistence of primary lung Cancer and hematologic malignancy: a comprehensive analysis of clinicopathological features and genetic abnormalities
The incidence of multiple primary cancers (MPC), especially involving primary lung cancer (PLC) and primary hematologic malignancies (PHM), is rising. This study aims to analyze clinicopathological features, g...
Genome-wide CRISPR screen identifies ESPL1 limits the response of gastric cancer cells to apatinib
Apatinib was the first anti-angiogenic agent approved for treatment of metastatic gastric cancer (GC). However, the emergence of resistance was inevitable. Thus investigating new and valuable off-target effect...

- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Contact Support for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
2022 Citation Impact 5.8 - 2-year Impact Factor 5.6 - 5-year Impact Factor 0.994 - SNIP (Source Normalized Impact per Paper) 1.145 - SJR (SCImago Journal Rank)
2023 Speed 34 days submission to first editorial decision for all manuscripts (Median) 110 days submission to accept (Median)
2023 Usage 2,067,719 downloads 1,052 Altmetric mentions
- More about our metrics
On the blog


PEAR-TREE: Understanding the usefulness of the PEAR-BIO platform in patients with kidney cancer
08 January 2024

Winners of the 2022 Cardio-Oncology Journal Prize
06 April 2023

Brain Tumor Awareness Month: Can proton beam therapy improve the long-term quality of life of patients with oligodendroglioma brain tumors?
22 March 2023
Institutional membership

Flowchart of the entire Mendelian Randomization analysis
Data sources
GWAS summary statistics for each immune cell are publicly available from the GWAS Catalog (accession numbers from GCST0001391 to GCST0002121) [ 12 , 17 ]. A total of 731 immune characteristic phenotypes were included in the data, which were divided into 7 immune cells according to classification, namely NK cells, myeloid cells, B cells, monocytes, Treg cells, cDC cells and mature T cells. RCC related GWAS data can be got from ieu [ 18 ]. The immunocyte-related GWAS data utilized in this study are based on a cohort of 6,602 participants of European descent from the central eastern coast of Sardinia, Italy. The RCC (Renal Cell Carcinoma)-related GWAS data employed in our analysis were sourced from IEU, comprising a total of 174,006 European ancestry samples, with a ncase count of 971.
Selection of instrumental variables (IVs)
In order to select suitable IVs, we followed the following criteria: (1) SNPs associated with genome-wide motif significance threshold ( P < 5 × 10 –8 ) were selected as candidate IVs. Unfortunately, only a very small fraction of SNPs was enrolled at that threshold, and we therefore used a second threshold (1 × 10 –5 ) to screen the IVs for more comprehensive results. For the screening of IVs in RCC, since the desired SNPs could not be screened at 5 × 10 –8 , we relaxed the criteria to 1 × 10 –5 ; (2) SNPS with low linkage disbalance (LD) and R 2 < 0.001(aggregation window size = 10,000 kb) were selected using the sample data of the European 1000 Genomes Project as the reference panel, and only SNPS with the lowest P -value were retained as candidate IVs; (3) Delete palindromic SNPS; (4) The F statistic of each SNP was calculated to evaluate the strength of IVs, and SNPS with F < 10 were excluded [ 19 ]. Detailed information on SNPs can be found in Supplementary Table 1 .
Statistical analysis
In this study, anti-variance weighting (IVW), simple model, MR Egger regression, weighted median and weighted modeling were used to explore the potential causal relationship between immune cells and RCC. In addition, we use MR-PRESSO analysis to identify and mitigate horizontal pleiotropy by eliminating significant outliers. Heterogeneity of individual SNP effects was assessed using the Cochran Q test [ 20 ]. The estimates are expressed as odds ratios (ORs) with 95% confidence intervals (ci), which indicate the average change in outcomes resulting from each exposure. In this study, IVW method was used as the main analysis method, and other methods were used as auxiliary proof [ 21 ]. These results only provide evidence of a causal relationship between exposure and outcomes, with no other explanation.
The analysis was mainly carried out using statistical software R. The R packages used in MR Analysis include various software packages such as TwoSampleMR, mendelanrandomization, MRPRESSO and ggplot2 for data processing and graphic painting. P < 0.05 was considered statistically significant.
Exploration of the causal effect of immune cells on RCC
To investigate the causal effect of immunophenotype on RCC, we used two-sample MR analysis with IVW method as the main analysis method. We detected that most immunophenotypes showed a protective trend against RCC: the OR for Activated & secreting CD4 regulatory T cells was 0.68 (95%CI = 0.48–0.96, P = 2.92 × 10 –2 ), and the OR for HLADR on CD14 + CD16 + monocyte was 0.84 (95%CI = 0.71–0.99, P = 3.92 × 10 –2 ), BAFF-R on IgD- CD38 + B cell OR of 0.69 (95%CI = 0.53–0.89, P = 5.74 × 10 –3 ), and the same trend was observed in the weighted median approach with an OR of 0.73 (95%CI = 0.55–0.97, P = 2.78 × 10 –2 ) and an OR of 0.59 (95%CI = 0.37–0.95, P = 2.87 × 10 –2 ) for CD19 on IgD + CD24- B cells, in addition to which we found that the protective effect of CD19 was also reflected in other types of B cells. In contrast, CD4-CD8-T cell was a risk factor for RCC, with an OR value of 0.84 (95%CI = 0.71–0.99, P = 3.92 × 10 –2 ), and the fact that CD4 + CD8 + T cell presented a protective effect against RCC also proved the above point (Fig. 2 , Table 1 , Supplementary Table 2 ). Heterogeneity and horizontal pleiotropy among the screened IVs were excluded by MR-Egger with the test of pleiotropy (Supplementary Table 3 ), and the stability of the results was also demonstrated by scatterplots and funnel plots (Supplementary Fig. 1 , Supplementary Fig. 2 ). MR analysis at another threshold also confirmed the reliability of our conclusions (Supplementary Table 4 ). MR analysis of the UKBB database as well as the FinnGen database also demonstrated the relevance of our conclusions (Supplementary Table 5 , Supplementary Table 6 ).
The forest plot shows the causal relationship between immune cell characteristics and RCC by different methods
Exploration of the causal effect of RCC on immune cells
To explore the causal effect of RCC on immune cells, we performed an inverse Mendelian randomization analysis, and we identified that the affected immune cells were mainly concentrated in T cells versus B cells. We found that with the onset of RCC can Plasma Blast-Plasma Cell expression levels (OR = 0.96, 95%CI = 0.92–1.00, P = 3.65 × 10 –2 ), and the same trend was observed in the MR-Egger method (OR = 0.90, 95%CI = 0.85–0.96, P = 4.78 × 10 –3 ).The expression level of HVEM on Effector Memory CD8 + T cell was also found to be increased (OR = 0.94, 95%CI = 0.88–1.00, P = 4.37 × 10 –2 ). As for B cell,we found that RCC was negatively correlated with the expression level of B cell, and the same trend was observed by IVW method (OR = 1.05, 95%CI = 1.01–1.09, P = 1.19 × 10 –2 ) and MR Egger (OR = 1.09, 95%CI = 1.02–1.16, P = 1.36 × 10 –2 ) method. Meanwhile, the estimation of the occurrence of RCC for CD8 + T cells was 1.04 (95%CI = 1.00–1.08, P = 2.83 × 10 –2 ), with the same trend, although the other methods were not statistically significant (Table 2 , Fig. 3 ).Effector Memory CD4 + T cells (OR = 1.04, 95% CI = 1.00–1.08, P = 4.51 × 10–2) and Effector Memory CD8 + T cell (OR = 1.04, 95%CI = 1.00–1.08, P = 4.51 × 10 –2 ) also observed a similar trend (Supplementary Table 7 ). And we excluded the presence of horizontal pleiotropy and heterogeneity by MR-Egger's intercept, MR-PRESSO's test, and heterogeneity test (Supplementary Table 8 ). Scatterplots and funnel plots also indicate the stability of the results (Supplementary Fig. 3 , Supplementary Fig. 4 ).
The forest plot shows the causal relationship between RCC and immune cell characteristics by different methods
Based on publicly available GWAS data, we categorized 731 immune cell traits into 7 categories and explored the causal relationship between them and RCC. We found that there are few MR analysis to explore the causal relationship between immune traits and RCC. In this study, we found a causal relationship between immune phenotypes such as T-cells, B-cells, and monocytes and the risk of developing RCC, as well as influencing the expression levels of some immune cells as RCC develops.
Our research revealed a positive correlation between the incidence of RCC and the elevated levels of CD4-CD8-T cells, whereas CD4 + CD8 + T cells would be the ones to show a protective effect. CD4-CD8-T cells, a type of regulatory T cells (Tregs), do not express NK cells markers. The function of regulatory T cells (Tregs) is mainly in maintaining immune cell homeostasis while suppressing anti-tumor immune responses [ 22 , 23 ]. Research has unequivocally demonstrated that CD4 + T cells possess a cytotoxic regimen that effectively eliminates malignant cells. Moreover, the functional capabilities of CD4 + T cells primarily revolve around the generation of cytokines, whereas the ability to directly eradicate target cells resides within the CD8 + T cell subset. Consequently, elevated quantities of CD4 + CD8 + T cells hold the potential to impede the development and advancement of tumors. This aligns perfectly with the outcomes of our investigation. Additionally, scientific evidence supports the utilization of CD4/8 cells as an immunotherapeutic strategy for renal cell carcinoma (RCC) [ 24 , 25 ]. Previous research has demonstrated that the survival rate of patients with RCC can be enhanced by having a higher proportion of tumor-infiltrating NK cells and Th1 markers (such as T-cells expressing HLA-DR +) [ 26 ]. Our study further reveals that there is a protective effect of HLA DR on the incidence of RCC by affecting the CD14 + CD16 + monocyte panel. HLA-DR is a cell surface receptor belonging to the MHC class II, which is encoded by the human leukocyte antigen complex on chromosome 6 region 6P21. In cases of chronic inflammation, the reduced expression of HLA-DR in monocytes confirms the anti-inflammatory role of this molecule and further supports the validity of our findings [ 12 , 27 ]. Our study suggests the possibility that HLA-DR could be a therapeutic target for RCC, other investigations have also illustrated a correlation between RCC and HLA ligands, suggesting that specific HLA-presenting peptides unique to ccRCC might serve as potential targets for immunotherapy [ 28 , 29 , 30 ].
BAFF-R is among the trio of receptors detected on fully developed B-cells, exhibiting abilities to proficiently eradicate diverse B-cell malignancies. Simultaneously, following genetic reconfiguration to foster the expression of CD19-specific chimeric antigen receptor (CAR), it can be effectively utilized for addressing progressive B-cell neoplasms, boasting robust antineoplastic outcomes. Currently, several investigations have illustrated the potential of CD19/BAFF-R as a novel therapeutic target for cancer [ 31 , 32 , 33 , 34 ], aligning with our own research findings that CD19 and BAFF-R on B-cells bestow protective properties. Bevacizumab, an extensively used anticancer medication, has been found to primarily target CD19, an essential gene-enriched pathway. Furthermore, it affectes T-cell impairment, particularly affecting CD19 [ 35 , 36 ]. CD19's impact on the effectiveness of sunitinib, another antitumor drug, has also been observed [ 37 ]. These findings highlight the potential of our discovery to offer innovative therapeutic targets for treating RCC.
This study explored the bidirectional causal analysis between immune cells and RCC through a two-sample Mendelian randomization study, which was statistically efficient because of the large sample size of the study population in this experiment. In addition, we used multiple MR analysis methods while excluding the effects of confounding factors, so our results are robust. Nowadays, immune-related studies on RCC are mainly focused on T cells, and our study can focus the immune studies on RCC among other immune cells and suggest new ideas and causal correlations. However, the study has some limitations. First, MR analysis can only reveal the causal association between the two, and molecular experiments are needed to confirm the mechanism by which immune cells influence the occurrence of RCC. Second, this is an overall data and lacks individual information to further stratify the population. Third, as to the mechanisms, it may not be caused by just one immune phenotype, but rather multiple immune phenotypes acting together, hence the discrepancy that arises when studying a single factor. Finally, we relaxed the screening criteria for IVs of RCC, and these may generate some false positives.
In conclusion, in this study we demonstrated a causal association between immune cells and RCC through bidirectional MR analysis, highlighting the complex pattern of interactions and interactions between the immune system and RCC. In addition, this study provides ideas for exploring the biological mechanisms between RCC and immune cells, and the found immune cells may become key molecules for early intervention in RCC development and treatment of RCC.
Availability of data and materials
Data is provided within the manuscript or supplementary information files. GWAS summary statistics for each immune cell are publicly available from the GWAS Catalog (accession numbers from GCST0001391 to GCST0002121) and RCC related GWAS data can be got from IEU ( https://gwas.mrcieu.ac.uk/ ) and FinnGen Consortium version R9 ( https://r9.finngen.fi/ ).
Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170(6 Pt 1):2163–72.
Article CAS PubMed Google Scholar
Ljungberg B, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol. 2022;82(4):399–410.
Article PubMed Google Scholar
Mao W, et al. Current status of research on exosomes in general and for the diagnosis and treatment of kidney cancer in particular. J Exp Clin Cancer Res. 2021;40(1):305.
Article PubMed PubMed Central Google Scholar
Siegel RL, et al. Cancer statistics 2023. CA Cancer J Clin. 2023;73(1):17–48.
Motzer RJ, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello BA, Derweesh IH, Desai A, Ged Y, George S, Gore JL, Haas N, Hancock SL, Kapur P, Kyriakopoulos C, Lam ET, Lara PN, Lau C, Lewis B, Madoff DC, Manley B, Michaelson MD, Mortazavi A, Nandagopal L, Plimack ER, Ponsky L, Ramalingam S, Shuch B, Smith ZL, Sosman J, Dwyer MA, Gurski LA, Motter A. Kidney Cancer, Version 3. 2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(6):804–34.
Dai S, et al. Intratumoral CXCL13+CD8+T cell infiltration determines poor clinical outcomes and immunoevasive contexture in patients with clear cell renal cell carcinoma. J Immuno Therapy of Cancer. 2021;9(2):e001823.
Article Google Scholar
Bi K, et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell. 2021;39(5):649–661.e5.
Article CAS PubMed PubMed Central Google Scholar
Krishna C, et al. Single-cell sequencing links multiregional immune landscapes and tissue-resident T cells in ccRCC to tumor topology and therapy efficacy. Cancer Cell. 2021;39(5):662–677.e6.
Senbabaoglu Y, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol. 2016;17(1):231.
Kansler ER, et al. Cytotoxic innate lymphoid cells sense cancer cell-expressed interleukin-15 to suppress human and murine malignancies. Nat Immunol. 2022;23(6):904–15.
Dantzer R, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56.
Wang C, et al. Causal role of immune cells in schizophrenia: Mendelian randomization (MR) study. BMC Psychiatry. 2023;23(1):590.
Huang D, et al. Association between COVID-19 and telomere length: A bidirectional Mendelian randomization study. J Med Virol. 2022;94(11):5345–53.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–6.
Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Research Synthesis Methods. 2019;10(4):486–96.
Wang Z, et al. Association between inflammatory bowel disease and periodontitis: A bidirectional two-sample Mendelian randomization study. J Clin Periodontol. 2023;50(6):736–43.
Orrù V, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(10):1036–45.
Kurki MI, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–18.
Li P, et al. Association between gut microbiota and preeclampsia-eclampsia: a two-sample Mendelian randomization study. BMC Medicine. 2022;20(1):443.
Fabiola Del Greco M, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–40.
Song J, et al. The causal links between gut microbiota and COVID-19: A Mendelian randomization study. J Med Viro. 2023;95(5):e28784.
Article CAS Google Scholar
Sharma A, et al. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3+ Regulatory T Cells (Tregs) in Human Cancers. Clin Cancer Res. 2019;25(4):1233–8.
Wu Z, et al. CD3+CD4-CD8-(Double-Negative) T Cells in Inflammation Immune Disorders and Cancer. Front Immunol. 2022;13:816005.
Oh DY, Fong L. Cytotoxic CD4+ T cells in cancer: Expanding the immune effector toolbox. Immunity. 2021;54(12):2701–11.
Wang Y, et al. Anti-CAIX BBζ CAR4/8 T cells exhibit superior efficacy in a ccRCC mouse model. Molecular Therapy - Oncolytics. 2022;24:385–99.
Geissler K, et al. Immune signature of tumor infiltrating immune cells in renal cancer. OncoImmunology. 2015;4(1):e985082.
Söderlund J, et al. Activation of brain interleukin-1β in schizophrenia. Mol Psychiatry. 2009;14(12):1069–71.
Reustle A, et al. Integrative-omics and HLA-ligandomics analysis to identify novel drug targets for ccRCC immunotherapy. Genome Medicine. 2020;12(1):32.
Jacquier A, et al. Tumor infiltrating and peripheral CD4+ILT2+ T cells are a cytotoxic subset selectively inhibited by HLA-G in clear cell renal cell carcinoma patients. Cancer Lett. 2021;519:105–16.
Tronik-Le Roux D, et al. The HLA-G immune checkpoint: a new immuno-stimulatory role for the α1-domain-deleted isoform. Cell Mol Life Sci. 2022;79(6):310.
Liu E, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6):545–53.
Dimitrios L, Wagner EF, Michael A, Pulsipher MA, Ahmed N, Mohamad Hamieh M, Hegde M, Ruella M, Savoldo B, Shah NN, Turtle CJ, Wayne AS, Abou-el-Enein A. Immunogenicity of CAR T cells in cancer therapy. Nat Rev Clin Oncol. 2021;18(6):379–93.
Xiuli Wang ZD, Awuah D, Chang WS, Cheng WA, Vyas V, Cha SC, Anderson AJ, Zhang T, Wang Z, Szymura SJ, Kuang BZ, Clark MC, Aldoss I, Forman SJ, Kwak LW, Qin H. CD19/BAFF-R dual-targeted CAR T cells for the treatment of mixed antigennegative variants of acute lymphoblastic leukemia. Leukemia. 2022;36(4):1015–24.
Wong DP, et al. A BAFF ligandbased CART cell targeting three receptors and multiple B cell cancers. Nat Commun. 2022;13(1):217.
Manzoni M, et al. Immunological Effects of Bevacizumab-Based Treatment in Metastatic Colorectal Cancer. Oncology. 2011;79(3–4):187–96.
Google Scholar
Lebedeva IV, et al. Prediction of novel target genes and pathways involved in bevacizumab-resistant colorectal cancer. Plos One. 2018;13(1):e0189582.
Lin Z, et al. Tumor infiltrating CD19+ B lymphocytes predict prognostic and therapeutic benefits in metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors. OncoImmunology. 2018;7(10):1–9.
Article PubMed Central Google Scholar
Download references
Acknowledgements
We want to acknowledge the participants and investigators of the FinnGen and IEU study.
This work was supported by the China Postdoctoral Science Foundation funded project (2022M711410); Jiangsu Province Postdoctoral Research Support Project (2021K595C), the Nanjing Postdoctoral Research Support Project (2021BSH204), Jiangsu Science and Technology Association Young Science and Technology Talents Lifting Project ((2021)082), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (22KJB320014), Jiangsu Province Hospital (the First Affiliated Hospital with Nanjing Medical University) Clinical Capacity Enhancement Project (JSPH-MC-2022–17), Open Project of Key Laboratory of Children’s Major Disease Research (JKLP202104), Open Project of Jiangsu Health Development Research Center (JSHD2021005), Jiangsu Science and Technology Think Tank Young Talent Program-Outstanding Youth Special (JSKJZK2023026), Foundation of Wuxi Municipal Health Commission(J202108), Medical Scientific Research Project of Jiangsu Provincial Health Commission (H2019041), and Jiangsu Province Capability Improvement Project through Science Technology and Education (ZDXK202219).
Author information
Zhongwen Lu, Yu Yin, and Tian Rao are equally contributed to this work.
Authors and Affiliations
The State Key Lab of Reproductive, Department of Urology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, 210009, China
Zhongwen Lu, Yu Yin, Tian Rao, Xinchi Xu, Kai Zhao, Zhanpeng Liu, Chao Qin & Min Tang
You can also search for this author in PubMed Google Scholar
Contributions
MT and CQ designed the research plan for this study. ZWL, YY and TR completed the manuscript writing. XCX, KZ and ZPL completed the image processing and data statistical analysis. All the authors approved the final version of the manuscript.
Corresponding authors
Correspondence to Chao Qin or Min Tang .
Ethics declarations
Ethics approval and consent to participate.
According to guidance received from our research ethics board, no ethics review is required for studies utilizing public data sets.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., supplementary material 3., supplementary material 4., supplementary material 5., supplementary material 6., supplementary material 7., supplementary material 8., supplementary material 9., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lu, Z., Yin, Y., Rao, T. et al. Interaction of immune cells with renal cancer development: Mendelian randomization (MR) study. BMC Cancer 24 , 439 (2024). https://doi.org/10.1186/s12885-024-12196-8
Download citation
Received : 14 January 2024
Accepted : 27 March 2024
Published : 09 April 2024
DOI : https://doi.org/10.1186/s12885-024-12196-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mendelian randomization
- Immune cells
- Tumor microenvironment
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Cancer Cell Biology Research
A dividing breast cancer cell.
Research in cancer cell biology seeks to define the biological basis underlying the differences between normal cells and cancerous cells. This includes studies of the fundamental mechanisms that drive pre-cancer states, oncogenic transformation, and that support tumor growth and behavior. Mechanistic understanding of this biology and the fundamental processes governing transformation, including the role of aging, gender, and ethnic disparities, are critical for identifying molecular targets for therapeutic or preventive interventions.
Research in this area is supported and directed by the Cancer Cell Biology Branch (CCBB) .
Cancer Cell Metabolism
Research in cancer cell metabolism focuses on altered cellular metabolic pathways that support the cancer phenotype, which is characterized by unchecked cell proliferation, resistance to metabolic and oxidative stress, evasion of programmed cell death, reduced dependence on growth factor signals, insensitivity to growth inhibitory signals, and resistance to therapeutic interventions.
Key research areas include:
- Oncogenic reprogramming of cellular metabolism (e.g., the Warburg Effect, glutamine addiction, upregulated/deregulated fatty acid metabolism)
- The links between protein translation, ribosome biogenesis, and metabolism
- Tumor metabolite profiling and characterization
- Regulation and mechanisms of nutrient, metabolic intermediate, and ion transport in cancer cells
Emerging areas in cancer metabolism include biological functions of metabolic intermediates, the molecular link between body homeostasis and cancer cell biology, mechanisms underlying the intersection between obesity and cancer, the metabolic plasticity of cancer cells, the mechanisms through which diet and fasting affect cancer initiation and maintenance, and the molecular mechanisms that lead to cancer cachexia.
Cancer Cell Stress Responses
Research in cancer cell stress responses focuses on the cell’s reaction to intrinsic and environmental stressors that determine whether a cell will die or adapt to survive. Examples of the types of stress included in this research area are oxidative stress, oncogenic stress, accumulation of unfolded or misfolded proteins, hypoxia, metal ions, chemotherapy, and inflammation.
- Mechanisms of cell death (e.g., apoptosis, necrosis/necroptosis, autophagy, anoikis, ferroptosis, and other forms of programmed/non-programmed cell death)
- Recycling of cellular components in response to stress (e.g., autophagy, mitophagy, lipophagy)
- ER stress and the unfolded protein response
- Exosome release as a mediator of cellular stress response and intercellular communications
- Altered processing of growth factors and their associated receptors
- Mechanisms of cellular control of toxic byproducts from biological processes (e.g., redox control)
Emerging areas relevant to this research include mechanisms of metal ions homeostasis, such as iron and copper, and their associated cellular targets and functions, and understanding the global effects of metal ions accumulation.
Organelle Biology
Research in the area of organelle biology investigates the mechanisms and role of dysregulated organelle biology in driving or supporting the cancer phenotype.
- Dysregulation of organelle biogenesis and function (e.g., mitochondria, endoplasmic reticulum, Golgi, lysosomes, lipid droplets, peroxisomes, endosomes, and cilia)
- Processing and trafficking of intracellular membranes and proteins
- Endocytosis and endosome sorting and recycling
- Interactions between nuclear-encoded oncogenic proteins and mitochondrial function
- Role of cell organelles in cancer-associated phenotypes
Emerging areas relevant to this research include regulation of mitochondrial growth and division, energy-independent functions of mitochondria, and the intersection between organelle structure/morphology and the phenotypic state or function of cancer cells.
Cancer Cell Cycle Control

Dr. Sita Kugel Investigates the Biology of Pancreatic Cancer and Cholangiocarcinoma
Cell cycle dysregulation is a hallmark of cancer, and cell cycle components have been aggressively
targeted in chemotherapeutic strategies. Research in this area focuses on altered cell cycle regulation and its contribution to oncogenic transformation and tumor maintenance.
- Characterization of factors that regulate cell cycle, mitosis, cytokinesis, centrosome duplication, and DNA replication in cancer cells
- Alternative, kinase-independent functions of cell cycle regulators
- Mechanisms that alter protein stability and function of cell cycle components in cancer cells
- Understanding the biological effects of cell cycle inhibitors in tumors, either alone or in combination with other therapies
Emerging areas relevant to this research include the elucidation of nutrient-sensing cell cycle checkpoints, understanding mechanisms that allow for the bypass of cell cycle checkpoints, and exploration of combination therapies with CDK inhibitors for certain cancers.
Post-transcriptional Regulations Influencing Cancer
Research in this area investigates the wide-ranging mechanisms and functional effects of post-transcriptional regulations that affect the cancer phenotype.
- Altered mechanisms and regulations of RNA stability, splicing, modifications, transport, and mRNA translation
- Regulation and mechanisms of alternative splicing in cancer
- The role of non-coding RNAs and RNA binding proteins in the regulation of splicing, modifications, transport, translation, and mRNA stability
- Translation factors that act as oncogenes or tumor suppressors
- Changes in protein maturation and stability, including diverse post-translation modifications (e.g., phosphorylation, acetylation, methylation, hydroxylation, ubiquitylation, sumoylation, neddylation, and glycosylation), as well as modifications of signaling effectors (e.g., promotors and drivers of tumorigenesis or cancer progression)
Emerging areas relevant to this research include the study of chemical modifications to RNAs and protein molecules, including writers, erasers, and readers of such modifications, that affect their stability, trafficking, RNA splicing and translation, and protein function, the development of novel technologies for efficient profiling of these modifications, and the interplay of different modifications and their alterations in cancer.
Basic Mechanisms of Cell Transformation
Research in this area includes mechanisms and effectors that govern the transition from normal cell to pre-cancer, early lesion, and cancer cell, as well as the identification of early biological events in transformation. Studies cover the role of tumor-initiating cells, field cancerization, and diverse signaling pathways governing cell fate determination and tumor formation. Research also examines the functions and regulations of oncogenes and tumor suppressor genes/proteins.
- Functional and molecular characterization of oncogenes and tumor suppressors and their affected pathways
- Oncogenic signal transduction and their rewiring
- The biology of tumor-initiating cells and cancer stem cells
- Role of developmental and cell differentiation programs in preneoplasia and cancer
- Senescence as an oncogenic or tumor suppressive mechanism, the relationship between quiescence and senescence states, and the relationship between senescence, aging, and cancer
Emerging areas relevant to this research include understanding lineage affiliation of stem and progenitor cells and its role in oncogenesis, characterizing the actual cell targets for oncogenic transformation, and deciphering the functional effects of multiple mutations in normal cells and their role in transformation.
Biospecimen Resources to Support Cancer Biology Research
Research in this area includes the development of projects that encompass the collection, storage, processing, and dissemination of human biological specimens—including nucleic acids and tissue arrays—and associated data for studies of human cancer biology, particularly early events in cancer formation and pre-neoplasia.
Stopping the cancer cells that thrive on chemotherapy – research into how pancreatic tumors adapt to stress could lead to a new treatment approach
Postdoctoral Scholar in Pathology, University of California, San Diego
Professor of Pathology, University of California, San Diego
Senior Scientist in Pathology, University of California, San Diego
Disclosure statement
David Cheresh receives funding from the NIH. He is a co-founder of Alpha Beta Therapeutics, Inc., a company creating new therapeutics to treat cancer, for which he also has equity and serves on the scientific advisory board.
Chengsheng Wu and Sara Weis do not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.
University of California, San Diego provides funding as a member of The Conversation US.
View all partners
As with weeds in a garden, it is a challenge to fully get rid of cancer cells in the body once they arise. They have a relentless need to continuously expand, even when they are significantly cut back by therapy or surgery. Even a few cancer cells can give rise to new colonies that will eventually outgrow their borders and deplete their local resources. They also tend to wander into places where they are not welcome, creating metastatic colonies at distant sites that can be even more difficult to detect and eliminate.
One explanation for why cancer cells can withstand such inhospitable environments and growing conditions is an old adage: What doesn’t kill them makes them stronger.
At the very earliest stage of tumor formation, even before cancer can be diagnosed, individual cancer cells typically find themselves in an environment lacking nutrients, oxygen or adhesive proteins that help them attach to an area of the body to grow. While most cancer cells will quickly die when faced with such inhospitable conditions, a small percentage can adapt and gain the ability to initiate a tumor colony that will eventually become malignant disease.
We are researchers studying how these microenvironmental stresses affect tumor initiation and progression. In our new study , we found that the harsh microenvironments of the body can push certain cancer cells to overcome the stress of being isolated and make them more adept at initiating and forming new tumor colonies. Moreover, these cancer cells may adapt even better in the inhospitable and stressful conditions they encounter while trying to establish metastases in other areas of the body or after they are challenged by treatment with chemotherapy or surgery.
Cancer cells overcoming isolation stress
We focused on pancreatic cancer , one of the most lethal cancers and one that is notoriously resistant to chemotherapy and often not curable with surgery. Almost 90% of pancreatic patients will succumb to cancer recurrence or metastasis within five years after diagnosis.
We wanted to study how tumor formation is affected by what we call “ isolation stress ,” when cells are deprived of nutrients or oxygen supply because of poor blood vessel formation or because they cannot benefit from making contact with nearby cancer cells. To study how cancer cells respond to these situations, we recreated different forms of isolation stress in cell cultures, in mice and in patient samples by depriving them of oxygen and nutrients or by exposing them to chemotherapeutic drugs. We then measured which genes were turned on or off in pancreatic cancer cells.
We found that pancreatic cancer cells challenged with conditions that mimic isolation stress gain a new receptor on their surface that unstressed cancer cells don’t typically have: lysophosphatidic acid receptor 4, or LPAR4 , a protein involved in tumor progression.
When we forced the cancer cells to produce LPAR4 on their surfaces, we found that they were able to form new tumor colonies two to eight times faster than average cancer cells under isolation stress conditions. Also, preventing cancer cells from gaining LPAR4 when they were stressed reduced their ability to form tumor colonies by 80% to 95%. These findings suggest that the ability of cancer cells to gain LPAR4 when they are exposed to stress is both necessary and sufficient to promote tumor initiation.
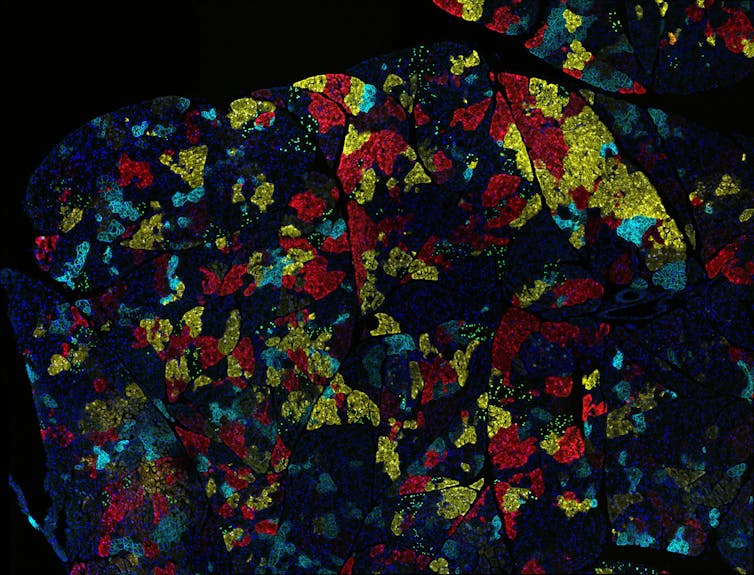
How does LPAR4 help build tumors?
We also found that LPAR4 helps cancer cells achieve tumor initiation by giving them the ability to produce a web of macromolecules, or an extracellular matrix network , that provides them an adhesive foothold within an otherwise inhospitable environment. By producing a halo of their own matrix, cancer cells with LPAR4 can start building their own tumor-supporting niche that provides a refuge from isolation stresses.
We determined that a key component of this extracellular matrix is fibronectin . When this protein binds to receptors called integrins on the surface of cells, it triggers a cascade of events that results in the expression of new genes promoting tumor initiation, stress tolerance and cancer progression. Eventually, other cancer cells are recruited into the fibronectin-rich matrix network, and a new satellite tumor colony starts to form.
Considering that tumor cells with LPAR4 can create their own tumor-supporting matrix on the fly, this suggests that LPAR4 may allow individual tumor cells to overcome isolation stress conditions and survive in the bloodstream, the lymphatic system involved in immune responses or distant organs as metastases.
Importantly, we found that isolation stress is not the only way to trigger LPAR4. Exposing pancreatic cancer cells to chemotherapy drugs, which are designed to impose stress upon cancer cells, also triggers an increase of LPAR4 on cancer cells. This finding might explain how such tumor cells could develop drug resistance.
Keeping cancer cells stressed
Understanding how to cut off the cascade of events that allows cancer cells to become stress-tolerant is important, because it provides a new area to explore for future treatments.
Our team is currently considering potential strategies to prevent cancer cells from utilizing the fibronectin matrix to gain stress tolerance, including drugs that can target the receptors that bind to fibronectin on the surface of tumor cells. One of these drugs, being developed by a company one of us co-founded, is poised to enter clinical trials soon. Other strategies include preventing cancer cells from gaining LPAR4 when they sense stress, or interfering with the signals that promote the generation of the fibronectin matrix.
For patients diagnosed with pancreatic cancer, there is a pressing need to discover how to improve the effectiveness of surgery or chemotherapy. Like combating weeds in your garden, this may require attacking the problem from multiple directions at once.
- Chemotherapy
- Pancreatic cancer
- Cancer treatment
- Tumour growth
- Cancer cell
- Cancer recurrence
- metastatic cancer

Research Fellow – Beyond The Resource Curse

Audience Development Coordinator (fixed-term maternity cover)

Lecturer (Hindi-Urdu)

Director, Defence and Security

Opportunities with the new CIEHF
Bacteria in cancer unmasked
Detailed catalogue of bacteria living in cancer metastases.
Researchers at the Netherlands Cancer Institute have compiled a detailed catalogue of bacteria living in cancer metastases. Having analyzed over 4000 tumors, they shed light on the diversity of these co-inhabitants and how they might interact with cancer cells and their surroundings. For example, certain bacteria were linked to a worse response to immunotherapy. This study paves the way to a better understanding of how bacteria help or hinder cancer (therapy), and how we can use this for patients' advantage. The researchers publish their findings today in the scientific journal Cell .
On and in our bodies live billions of microorganisms: bacteria, viruses and yeasts -- our microbiome. We need them, and they need us. Bacteria help us digest our food, for example, and cooperate with our immune system in the fight against pathogens. Gut bacteria in particular have been extensively studied, including in the context of cancer. For example, they can influence the effectiveness of immunotherapy and chemotherapy.
But these tiny co-inhabitants also house outside the gut. Bacteria are found in tumors, for example. With new techniques, researchers are getting better at finding out which microbes they are. But how bacteria get to a tumor and what exactly they do there remains largely unknown, making it unclear how important they are to disease and the effect of treatments.
26 cancer types
Because many patients eventually die from metastases, and many treatments target them, the research groups of Emile Voest and Lodewyk Wessels took a closer look at those metastases. After all, little was known about bacteria in these tumors. Together with their colleagues at, among others, the Netherlands Cancer Institute and Oncode Institute they have now mapped which bacteria are present in cancer metastases. Both groups are
In tissue from more than 4,000 metastases of 26 types of cancer, the researchers analyzed the code of the DNA present. From that genetic material you can see not only which human cells are there, but also which bacteria -- because these also have DNA. For this purpose they used clinical information and DNA data generated by Hartwig Medical Foundation.
With that unimaginably large mountain of information (400 terabytes), they used computer power to figure out which bacteria congregate in which places. This required a lot of clever programming, because there is relatively little bacterial DNA in such a piece of tissue.
"Surprisingly, it's not just metastases from colon cancer that contain a lot of bacteria," says researcher Thomas Battaglia. One might expect that because most of our bacteria reside in the colon, from where they could possibly travel along during metastasis to elsewhere in the body. "Also, which bacteria are present in a metastasis is strongly related to the location in the body, the conditions there, and the cancer type."
Therapy response
They also discovered a link between bacteria and therapy efficacy. Patients with lung cancer and Fusobacterium in their metastasis, for example, responded worse to immunotherapy than peers without that bacteria. Thomas: "We also noted that the more diverse the bacterial community, the more active the adjacent tumor cells."
"Our work opens doors for exploring new forms of treatments, for example against bacteria that might help the tumor," co-author Iris Mimpen says. "It helps us understand how the complex environment of tumors works, an environment in which all kinds of cells -- including bacteria -- live together and influence each other."
This research was financially supported by the AVL Foundation , KWF Dutch Cancer Society and Oncode Institute .
- Brain Tumor
- Colon Cancer
- Lung Cancer
- Microbiology
- Microbes and More
- Extreme Survival
- Immune system
- Prostate cancer
- Cervical cancer
- Colorectal cancer
- Breast cancer
- Esophageal cancer
Story Source:
Materials provided by Netherlands Cancer Institute . Note: Content may be edited for style and length.
Journal Reference :
- Thomas W. Battaglia, Iris L. Mimpen, Joleen J.H. Traets, Arne van Hoeck, Laurien J. Zeverijn, Birgit S. Geurts, Gijs F. de Wit, Michaël Noë, Ingrid Hofland, Joris L. Vos, Sten Cornelissen, Maartje Alkemade, Annegien Broeks, Charlotte L. Zuur, Edwin Cuppen, Lodewyk Wessels, Joris van de Haar, Emile Voest. A pan-cancer analysis of the microbiome in metastatic cancer . Cell , 2024; DOI: 10.1016/j.cell.2024.03.021
Cite This Page :
Explore More
- Pacific Cities Much Older Than Previously ...
- The Milky Way in Ancient Egyptian Mythology
- Physical Activity Best in the Evening
- How the Body Switches out of 'Fight' Mode
- New Drug Prevents Flu-Related Lung Damage
- 3D Mouth of an Ancient Jawless Fish
- Connecting Lab-Grown Brain Cells
- Device: Self-Healing Materials, Drug Delivery
- How We Perceive Bitter Taste
- Next-Generation Digital Displays
Trending Topics
Strange & offbeat.
- OU Homepage
- The University of Oklahoma
OU Research Details Adaptation Tactics of Pancreatic Cancer

Tumor cells find ways to thrive in acidic environment
OKLAHOMA CITY, OKLA. – Like alien invaders in a sci-fi movie, pancreatic cancer cells quickly adapt to the weapons used against them and find ways to survive, even in the harshest of conditions.
Understanding why is the aim of cancer scientists everywhere. A University of Oklahoma researcher recently advanced the knowledge of pancreatic cancer with a study about how it acclimates to and even thrives in a highly acidic environment. The research is published in the current issue of the journal Nature Cell Biology.
“Very few studies have tried to understand how that acidification contributes to tumor cell viability and what adaptations tumor cells undergo so that they are able to grow and sustain their aggressiveness,” said the study’s lead author, Pankaj Singh, Ph.D., professor and chair of the Department of Oncology Science in the OU College of Medicine.
Cancer is part of a tumor microenvironment – an ecosystem of normal cells, molecules and blood vessels that surround it, each affecting the other in ways both bad and good. In pancreatic cancer, the microenvironment is acidic because tumor cells consume a lot of the body’s glucose and then churn out an overabundance of lactic acid. As a whole, the tumor microenvironment is inhospitable because the cancer has deprived it of oxygen and nutrients due to its out-of-control growth. Yet pancreatic cancer constantly devises new ways to survive.
In his research, Singh discovered a series of steps that occur that allow pancreatic cancer to use the acidic environment to its advantage:
- Cancer-associated fibroblasts, a type of cell in the tumor, secrete a molecule called acetate.
- Pancreatic cancer cells use the acetate for “epigenetic reprogramming,” altering the way that genes work.
- One of those genes, called SAT1, is activated by acetate and essentially gives cancer cells the tools they need to better grow in acidic conditions.
Understanding the mechanisms that help tumor cells to adapt and thrive is the foundation for devising treatments that could stop the process somewhere along the way, Singh said. In this case, it may be possible to target SAT1 with a drug that can decrease the fitness of tumor cells to grow in the acidic microenvironment. Singh is continuing his studies by repurposing a pneumonia drug called pentamidine to test its effectiveness in decreasing pancreatic tumor aggressiveness in mice.
Pancreatic cancer remains one of the deadliest cancers – the five-year survival rate is just under 13%, according to the National Cancer Institute – and treatment options are few. Basic science research is crucial for continuing the unravel the devious nature of the cancer.
“This study highlights the fact that pancreatic tumor cells are not acting alone,” Singh said. “They have co-culprits in other cells, which they hijack and reprogram to their own advantage. By understanding these mechanisms, we can perhaps come up with better therapeutic approaches to target cancer.”
About the Project
The Nature Cell Biology paper can be accessed at 10.1038/s41556-024-01372-4. Singh also holds the Jim and Christy Everest Chair in Cancer Research and is Senior Director of Oncology Science for OU Health Stephenson Cancer Center.
About the University of Oklahoma
Founded in 1890, the University of Oklahoma is a public research university located in Norman, Oklahoma. As the state’s flagship university, OU serves the educational, cultural, economic and health care needs of the state, region and nation. OU was named the state’s highest-ranking university in U.S. News & World Report’s most recent Best Colleges list . For more information about the university, visit ou.edu .
More Research News
Ou researcher receives $3.1m grant for clean hydrogen technologies.
Hanping Ding, Ph.D., an assistant professor in the School of Aerospace and Mechanical Engineering at the University of Oklahoma, has been awarded a $3.1 million grant from the Hydrogen and Fuel Cell Technologies Office in the Department of Energy through the Bipartisan Infrastructure Law to further research in clean hydrogen production. The funding is part of a $750 million effort in President Biden’s Investing in American agenda.

Genetic Testing May Provide Improved Medication Therapy Safety and Efficacy for Pediatric Cystic Fibrosis Patients
Second-year University of Oklahoma pediatric resident Dr. Caroline Thompson has a professional and personal connection to cystic fibrosis patient care for children. A childhood friend had the disease and made a lasting impression on Thompson. Now, Thompson has received the Cystic Fibrosis Foundation’s Medical Resident Research Award for a pilot study evaluating pharmacogenomic-directed therapy for pediatric patients at the Oklahoma Cystic Fibrosis Center Tulsa.

Americans Supportive but Misinformed About Fusion Energy's Promise
Research led by Hank Jenkins-Smith, director of the Institute for Public Policy Research and Analysis at the University of Oklahoma, explores American adults’ perceptions of fusion energy. This first-of-its-kind study reveals broad public support from respondents, but their limited knowledge of the technology and frequent misconceptions could pose a challenge to those seeking to develop fusion energy in the U.S.

More OU News

- Accessibility
- Sustainability
- OU Job Search
- Legal Notices
- Resources and Offices
- OU Report It!
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 10 April 2024
How to supercharge cancer-fighting cells: give them stem-cell skills
- Sara Reardon 0
Sara Reardon is a freelance journalist based in Bozeman, Montana.
You can also search for this author in PubMed Google Scholar
A CAR T cell (orange; artificially coloured) attacks a cancer cell (green). Credit: Eye Of Science/Science Photo Library
Bioengineered immune cells have been shown to attack and even cure cancer , but they tend to get exhausted if the fight goes on for a long time. Now, two separate research teams have found a way to rejuvenate these cells: make them more like stem cells .
Both teams found that the bespoke immune cells called CAR T cells gain new vigour if engineered to have high levels of a particular protein. These boosted CAR T cells have gene activity similar to that of stem cells and a renewed ability to fend off cancer . Both papers were published today in Nature 1 , 2 .
The papers “open a new avenue for engineering therapeutic T cells for cancer patients”, says Tuoqi Wu, an immunologist at the University of Texas Southwestern in Dallas who was not involved in the research.
Reviving exhausted cells
CAR T cells are made from the immune cells called T cells, which are isolated from the blood of person who is going to receive treatment for cancer or another disease. The cells are genetically modified to recognize and attack specific proteins — called chimeric antigen receptors (CARs) — on the surface of disease-causing cells and reinfused into the person being treated.
But keeping the cells active for long enough to eliminate cancer has proved challenging, especially in solid tumours such as those of the breast and lung. (CAR T cells have been more effective in treating leukaemia and other blood cancers.) So scientists are searching for better ways to help CAR T cells to multiply more quickly and last longer in the body.
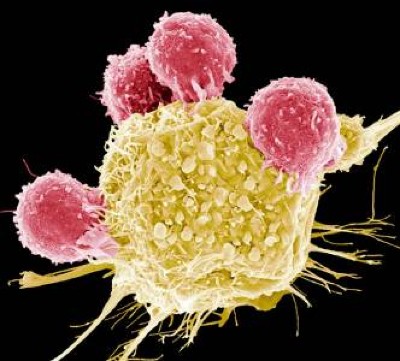
Cutting-edge CAR-T cancer therapy is now made in India — at one-tenth the cost
With this goal in mind, a team led by immunologist Crystal Mackall at Stanford University in California and cell and gene therapy researcher Evan Weber at the University of Pennsylvania in Philadelphia compared samples of CAR T cells used to treat people with leukaemia 1 . In some of the recipients, the cancer had responded well to treatment; in others, it had not.
The researchers analysed the role of cellular proteins that regulate gene activity and serve as master switches in the T cells. They found a set of 41 genes that were more active in the CAR T cells associated with a good response to treatment than in cells associated with a poor response. All 41 genes seemed to be regulated by a master-switch protein called FOXO1.
The researchers then altered CAR T cells to make them produce more FOXO1 than usual. Gene activity in these cells began to look like that of T memory stem cells, which recognize cancer and respond to it quickly.
The researchers then injected the engineered cells into mice with various types of cancer. Extra FOXO1 made the CAR T cells better at reducing both solid tumours and blood cancers. The stem-cell-like cells shrank a mouse’s tumour more completely and lasted longer in the body than did standard CAR T cells.
Master-switch molecule
A separate team led by immunologists Phillip Darcy, Junyun Lai and Paul Beavis at Peter MacCallum Cancer Centre in Melbourne, Australia, reached the same conclusion with different methods 2 . Their team was examining the effect of IL-15, an immune-signalling molecule that is administered alongside CAR T cells in some clinical trials. IL-15 helps to switch T cells to a stem-like state, but the cells can get stuck there instead of maturing to fight cancer.
The team analysed gene activity in CAR T cells and found that IL-15 turned on genes associated with FOXO1. The researchers engineered CAR T cells to produce extra-high levels of FOXO1 and showed that they became more stem-like, but also reached maturity and fought cancer without becoming exhausted. “It’s the ideal situation,” Darcy says.
Stem-cell and genetic therapies make a healthy marriage
The team also found that extra-high levels of FOXO1 improved the CAR T cells’ metabolism, allowing them to last much longer when infused into mice. “We were surprised by the magnitude of the effect,” says Beavis.
Mackall says she was excited to see that FOXO1 worked the same way in mice and humans. “It means this is pretty fundamental,” she says.
Engineering CAR T cells that overexpress FOXO1 might be fairly simple to test in people with cancer, although Mackall says researchers will need to determine which people and types of cancer are most likely to respond well to rejuvenated cells. Darcy says that his team is already speaking to clinical researchers about testing FOXO1 in CAR T cells — trials that could start within two years.
And Weber points to an ongoing clinical trial in which people with leukaemia are receiving CAR T cells genetically engineered to produce unusually high levels of another master-switch protein called c-Jun, which also helps T cells avoid exhaustion. The trial’s results have not been released yet, but Mackall says she suspects the same system could be applied to FOXO1 and that overexpressing both proteins might make the cells even more powerful.
doi: https://doi.org/10.1038/d41586-024-01043-2
Doan, A. et al. Nature https://doi.org/10.1038/s41586-024-07300-8 (2024).
Article Google Scholar
Chan, J. D. et al. Nature https://doi.org/10.1038/s41586-024-07242-1 (2024).
Download references
Reprints and permissions
Related Articles
- Medical research
ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D
Article 10 APR 24

FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy

Necroptosis blockade prevents lung injury in severe influenza
Biological age surges in survivors of childhood cancer
Research Highlight 11 APR 24
FOXO1 is a master regulator of memory programming in CAR T cells
The PARTNER trial of neoadjuvant olaparib in triple-negative breast cancer
Article 08 APR 24
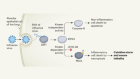
Blocking cell death limits lung damage and inflammation from influenza
News & Views 10 APR 24
mRNA drug offers hope for treating a devastating childhood disease
News 03 APR 24
Diabetes drug slows development of Parkinson’s disease
Postdoctoral Associate- Comparative Medicine
Houston, Texas (US)
Baylor College of Medicine (BCM)
Group Leader at Católica Biomedical Research Centre and Assistant or Associate Professor at Católica
Group Leader + Assistant/Associate Professor, tenure-track position in Biological and Biomedical Sciences, Data Science, Engineering, related fields.
Portugal (PT)
Católica Biomedical Research Centre
Faculty Positions at SUSTech Department of Biomedical Engineering
We seek outstanding applicants for full-time tenure-track/tenured faculty positions. Positions are available for both junior and senior-level.
Shenzhen, Guangdong, China
Southern University of Science and Technology (Biomedical Engineering)
Locum Associate or Senior Editor, Nature Cancer
To help us to build on the success of Nature Cancer we are seeking a motivated scientist with a strong background in any area of cancer research.
Berlin, Heidelberg or London - Hybrid working model
Springer Nature Ltd
Postdoctoral Research Fellows at Suzhou Institute of Systems Medicine (ISM)
ISM, based on this program, is implementing the reserve talent strategy with postdoctoral researchers.
Suzhou, Jiangsu, China
Suzhou Institute of Systems Medicine (ISM)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Português Br
- Journalist Pass
New study finds triple-negative breast cancer tumors with an increase in immune cells have lower risk of recurrence after surgery
Kelley Luckstein
Share this:

ROCHESTER, Minn. — A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
TNBC is a breast cancer subtype that does not respond to drugs that target the estrogen receptor or the HER2 protein. It grows rapidly, is more likely to spread beyond the breast before diagnosis and is more likely to recur than other breast cancers. TNBC represents about 15% of all breast cancers and is more common in younger people and in women of African American, Hispanic and Indian descent. Immune cells, also known as tumor-infiltrating lymphocytes, or TILs, are naturally existing immune system cells that can move from the bloodstream into a tumor and can recognize and destroy cancer cells.
"This is an important finding because it highlights that the abundance of TILs in breast tissue is a prognostic biomarker in people with early-stage triple-negative breast cancer, even when chemotherapy is not administered," says Roberto Leon-Ferre, M.D. , a breast medical oncologist at Mayo Clinic Comprehensive Cancer Center and first author of the study. "The study's findings may inspire future clinical trials to explore whether patients with a favorable prognosis (high TILs) can avoid intensive chemotherapy regimens."
"This meta-analysis confirms robustly the prognostic value of TILs that we have previously reported in TNBC patients treated with chemotherapy and expands it to patients treated without chemotherapy," says Sarah Flora Jonas, Ph.D., a statistician at Gustave Roussy and co-first author of the study. "Future studies may allow the use of this biomarker along with standard clinicopathological factors to inform treatment decisions in TNBC patients."
"Of interest, the first report suggesting that an increased number of immune cells being associated with better prognosis in breast cancer patients was described by doctors at Mayo Clinic more than 100 years ago," says Roberto Salgado, M.D., co-chair of the International Immuno-Oncology Biomarker Working Group; co-lead of the study; and pathologist from the Peter MacCallum Cancer Centre, Melbourne, Australia, and ZAS Hospitals, Antwerp, Belgium. "It took a global effort and a century later to reexamine this biomarker and bring it closer to application in patient care."
"TILs are not currently measured or reported in the routine examination of tissue samples of breast cancer," says co-senior author, Matthew Goetz, M.D. , a medical oncologist at Mayo Clinic Comprehensive Cancer Center and the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. "While prior studies have focused on measuring TILs in people treated with chemotherapy, this is the largest study to comprehensively demonstrate that the presence of TILs influences the natural behavior of breast cancer in people who have surgery and/or radiation with no additional medical treatment."
For this study, Mayo Clinic and Gustave Roussy researchers, in collaboration with the International Immuno-Oncology Biomarker Working Group, led 11 additional groups to collect data on 1,966 participants with early-stage TNBC who only underwent surgery with or without radiation therapy but did not receive chemotherapy. The participants had been followed for a median of 18 years. The results showed that higher levels of TILs in breast cancer tissue were associated with lower recurrence rates among participants with early-stage TNBC.
"Five years after surgery, 95% of participants with small tumors, stage 1 TNBC, and whose tumors had high TILs were alive, compared to 82% of patients whose tumors had low TILs. Importantly, the breast cancer recurrence rate was significantly lower among patients whose tumors had high TILs," says co-senior author, Stefan Michiels, Ph.D. , head of Oncostat team, Gustave Roussy, Inserm U1018, University Paris-Saclay. "With nearly 2,000 participants involved in the study, we have now assembled the largest international cohort across three continents of people with TNBC in which the primary treatment was surgery without chemotherapy."
"The results of this study could lead to a recommendation to include TILs in the pathology reports of early-stage TNBC worldwide, as it has the potential to inform clinicians and patients when they discuss treatment options," says Dr. Salgado.
Furthermore, this biomarker would only require a visual evaluation by a pathologist looking through a microscope, meaning there are no additional costs associated with identifying the presence of immune cells. This could be particularly beneficial to regions with limited resources, adds Dr. Leon-Ferre.
Most people with early-stage TNBC undergo chemotherapy either before or after surgery, including people with stage 1 breast cancer. Most people receive multiple chemotherapy drugs in combination, which can cause significant side effects. Currently, the main factors taken into consideration to determine the course of chemotherapy treatment for each person are the tumor size and the presence of lymph node metastases. However, the authors identified that the number of TILs further influences the risk of future recurrence.
The researchers plan to evaluate TILs as biomarkers in prospective clinical trials evaluating chemotherapy selection based on TIL levels. Ongoing efforts to conduct additional research with other potential biomarkers are underway.
For a complete list of authors, disclosures and funding, see the full paper here .
About Mayo Clinic Comprehensive Cancer Center Designated as a comprehensive cancer center by the National Cancer Institute , Mayo Clinic Comprehensive Cancer Center is defining new boundaries in possibility, focusing on patient-centered care, developing novel treatments, training future generations of cancer experts and bringing cancer research to communities. At Mayo Clinic Comprehensive Cancer Center, a culture of innovation and collaboration is driving research breakthroughs that are changing approaches to cancer prevention, screening and treatment, and improving the lives of cancer survivors.
About Mayo Clinic Mayo Clinic is a nonprofit organization committed to innovation in clinical practice, education and research, and providing compassion, expertise and answers to everyone who needs healing. Visit the Mayo Clinic News Network for additional Mayo Clinic news.
About Gustave Roussy Ranked as the leading French and European Cancer Centre and fourth in the world, Gustave Roussy is a centre with comprehensive expertise and is devoted entirely to patients suffering with cancer. The Institute is a founding member of the Paris Saclay Cancer Cluster. It is a source of diagnostic and therapeutic advances. It caters for almost 50,000 patients per year and its approach is one that integrates research, patient care and teaching. It is specialized in the treatment of rare cancers and complex tumors and it treats all cancers in patients of any age. Its care is personalized and combines the most advanced medical methods with an appreciation of the patient’s human requirements. In addition to the quality of treatment offered, the physical, psychological and social aspects of the patient’s life are respected. 4,100 professionals work on its two campuses: Villejuif and Chevilly-Larue. Gustave Roussy brings together the skills, which are essential for the highest quality research in oncology: 40% of patients treated are included in clinical studies. For further information: www.gustaveroussy.fr/en , Twitter , Facebook , LinkedIn , Instagram
Media contact:
- Kelley Luckstein, Mayo Clinic Communications, [email protected]
- CAR-T cell therapy helps man continue community advocacy Protect those eyes on the sky
Related Articles

IMAGES
COMMENTS
The Biology of Cancer. Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and ...
Research articles. Filter By: Article Type. All. All; Analysis (9) ... Schmid and colleagues show that pancreatic cancer cell colonization of the liver is accompanied by low-grade tissue injury ...
A new wave of research is unpicking the relationship between cancer and neurons — and looking for ways to stop the crosstalk. ... Related Articles. Cancer cells have 'unsettling' ability to ...
Acetate acts as a metabolic immunomodulator by bolstering T-cell effector function and potentiating antitumor immunity in breast cancer. Miller et al. show that blocking ACSS2 remodels acetate ...
The objective of this study was to determine the role and regulatory mechanism of miR-380 in cholangiocarcinoma. Zhicheng Wei, Bowen Xu, Yanjiang Yin, Jianping Chang, Zhiyu Li, Yefan Zhang, Xu Che and Xinyu Bi. Cancer Cell International 2024 24 :129. Research Published on: 6 April 2024.
Through self-renewal and differentiation, cancers are reconstructed by a dynamic subset of stem-like cells that enforce tumor heterogeneity and remodel the tumor microenvironment (TME). Through recent technology advances, we are now better equipped to investigate the fundamental role of cancer stem cells (CSCs) in cancer biology.
In this issue of Cancer Cell, Spitzer and colleagues demonstrate the role of IDH inhibitors on IDHmutant gliomas in reducing proliferation and enhancing cell differentiation toward an astrocytic-like state, thus altering neurodevelopmental pathways. Despite clinical promise, unresolved questions regarding mechanisms of action and resistance underline the need for further research for treatment ...
In this issue of Cancer Cell, Spitzer and colleagues demonstrate the role of IDH inhibitors on IDHmutant gliomas in reducing proliferation and enhancing cell differentiation toward an astrocytic-like state, thus altering neurodevelopmental pathways.Despite clinical promise, unresolved questions regarding mechanisms of action and resistance underline the need for further research for treatment ...
Alectinib in Resected ALK -Positive NSCLC. 2m 2s. Approximately 50% of patients with non-small-cell lung cancer (NSCLC) receive a diagnosis with early-stage or locally advanced disease (stage I ...
F. Castinetti and F. Borson-ChazotN Engl J Med 2023;389:1916-1918. Although medullary thyroid cancer accounts for less than 5% of thyroid cancers, it deserves attention because of its phenotypic ...
Conclusion: The expression of COL21A1 was significantly down-regulated in colon cancer tissues. Its down-regulation was correlated with immune cell infiltration and immunomodulatory molecule contents in colon cancer tissues. An in-depth investigation on COL21A1 may be beneficial for the immunotherapy of colon cancer. Full article
About the journal. Cancer Cell provides a high-profile forum to promote major advances in cancer research and oncology. The primary criterion for considering manuscripts is whether the studies provide major advances into answering important questions relevant to naturally occurring cancers. Cancer Cell particularly welcomes translational research.
NCI supports and directs cancer biology research through a variety of programs and approaches. For example: The Metastasis Research Network (MetNet) supports research to improve our understanding of how cancer spreads. Cancer metastasis is a complex, dynamic, nonlinear process. The network supports several specialized centers working ...
As the prevailing histologic subtype of kidney cancer, renal cell carcinoma (RCC) accounts for approximately 80 to 85% of all primary renal neoplasms [].The incidence of RCC has been steadily rising by 2% annually worldwide over the last twenty years [2, 3].It is estimated that there will be around 81,000 newly diagnosed cases and nearly 15,000 deaths of RCC in the United States in 2023 [].
This month, we launch a series of specially commissioned review and perspective articles on cancer cell biology, covering key topics and recent advances in understanding the cellular mechanisms ...
Cancer Cell. Cancer Cell provides a high-profile forum to promote major advances in cancer research and oncology. The primary criterion for considering manuscripts is whether the studies provide major advances into answering important questions relevant to naturally occurring cancers. More.
Credit: National Cancer Institute. Research in cancer cell biology seeks to define the biological basis underlying the differences between normal cells and cancerous cells. This includes studies of the fundamental mechanisms that drive pre-cancer states, oncogenic transformation, and that support tumor growth and behavior.
Exposing pancreatic cancer cells to chemotherapy drugs, which are designed to impose stress upon cancer cells, also triggers an increase of LPAR4 on cancer cells. This finding might explain how ...
from ScienceDaily. Researchers have compiled a detailed catalogue of bacteria living in cancer metastases. Having analyzed over 4000 tumors, they shed light on the diversity of these co ...
April 9, 2024. Mayo Clinic researchers will jump four hurdles to apply chimeric antigen receptor-T cell therapy (CAR-T cell therapy) to solid tumors in thyroid cancer. This regenerative immunotherapy has shown promising results in blood cancers, and new research is focused on using this treatment on more types of malignancies.
In his research, Singh discovered a series of steps that occur that allow pancreatic cancer to use the acidic environment to its advantage: Cancer-associated fibroblasts, a type of cell in the tumor, secrete a molecule called acetate. Pancreatic cancer cells use the acetate for "epigenetic reprogramming," altering the way that genes work.
Human colon cancer cells NIH/NCI Center for Cancer Research S AN DIEGO — Cancer cases among younger people have been rising for years, a trend researchers have struggled to explain.
IL-15 helps to switch T cells to a stem-like state, but the cells can get stuck there instead of maturing to fight cancer. The team analysed gene activity in CAR T cells and found that IL-15 ...
ROCHESTER, Minn. — A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
The promise has long been an affordable, personalized cancer vaccine that could train the immune system to recognize proteins from cancer cells and, subsequently, destroy the tumor.
St. Jude Children's Research Hospital. St. Jude Children's Research Hospital is leading the way the world understands, treats and cures childhood cancer, sickle cell disease, and other life-threatening disorders. It is the only National Cancer Institute-designated Comprehensive Cancer Center devoted solely to children. Treatments developed at St. Jude have helped push the overall childhood ...