

Systematic Review
- Library Help
- What is a Systematic Review (SR)?
Steps of a Systematic Review
- Framing a Research Question
- Developing a Search Strategy
- Searching the Literature
- Managing the Process
- Meta-analysis
- Publishing your Systematic Review
Forms and templates

Image: David Parmenter's Shop
- PICO Template
- Inclusion/Exclusion Criteria
- Database Search Log
- Review Matrix
- Cochrane Tool for Assessing Risk of Bias in Included Studies
• PRISMA Flow Diagram - Record the numbers of retrieved references and included/excluded studies. You can use the Create Flow Diagram tool to automate the process.
• PRISMA Checklist - Checklist of items to include when reporting a systematic review or meta-analysis
PRISMA 2020 and PRISMA-S: Common Questions on Tracking Records and the Flow Diagram
- PROSPERO Template
- Manuscript Template
- Steps of SR (text)
- Steps of SR (visual)
- Steps of SR (PIECES)
|
Image by | from the UMB HSHSL Guide. (26 min) on how to conduct and write a systematic review from RMIT University from the VU Amsterdam . , (1), 6–23. https://doi.org/10.3102/0034654319854352 . (1), 49-60. . (4), 471-475. (2020) (2020) - Methods guide for effectiveness and comparative effectiveness reviews (2017) - Finding what works in health care: Standards for systematic reviews (2011) - Systematic reviews: CRD’s guidance for undertaking reviews in health care (2008) |
|
| entify your research question. Formulate a clear, well-defined research question of appropriate scope. Define your terminology. Find existing reviews on your topic to inform the development of your research question, identify gaps, and confirm that you are not duplicating the efforts of previous reviews. Consider using a framework like or to define you question scope. Use to record search terms under each concept. It is a good idea to register your protocol in a publicly accessible way. This will help avoid other people completing a review on your topic. Similarly, before you start doing a systematic review, it's worth checking the different registries that nobody else has already registered a protocol on the same topic. - Systematic reviews of health care and clinical interventions - Systematic reviews of the effects of social interventions (Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies) - The protocol is published immediately and subjected to open peer review. When two reviewers approve it, the paper is sent to Medline, Embase and other databases for indexing. - upload a protocol for your scoping review - Systematic reviews of healthcare practices to assist in the improvement of healthcare outcomes globally - Registry of a protocol on OSF creates a frozen, time-stamped record of the protocol, thus ensuring a level of transparency and accountability for the research. There are no limits to the types of protocols that can be hosted on OSF. - International prospective register of systematic reviews. This is the primary database for registering systematic review protocols and searching for published protocols. . PROSPERO accepts protocols from all disciplines (e.g., psychology, nutrition) with the stipulation that they must include health-related outcomes. - Similar to PROSPERO. Based in the UK, fee-based service, quick turnaround time. - Submit a pre-print, or a protocol for a scoping review. - Share your search strategy and research protocol. No limit on the format, size, access restrictions or license.outlining the details and documentation necessary for conducting a systematic review: , (1), 28. |
| Clearly state the criteria you will use to determine whether or not a study will be included in your search. Consider study populations, study design, intervention types, comparison groups, measured outcomes. Use some database-supplied limits such as language, dates, humans, female/male, age groups, and publication/study types (randomized controlled trials, etc.). | |
| Run your searches in the to your topic. Work with to help you design comprehensive search strategies across a variety of databases. Approach the grey literature methodically and purposefully. Collect ALL of the retrieved records from each search into , such as , or , and prior to screening. using the and . | |
| - export your Endnote results in this screening software | Start with a title/abstract screening to remove studies that are clearly not related to your topic. Use your to screen the full-text of studies. It is highly recommended that two independent reviewers screen all studies, resolving areas of disagreement by consensus. |
| Use , or systematic review software (e.g. , ), to extract all relevant data from each included study. It is recommended that you pilot your data extraction tool, to determine if other fields should be included or existing fields clarified. | |
| Risk of Bias (Quality) Assessment - (download the Excel spreadsheet to see all data) | Use a Risk of Bias tool (such as the ) to assess the potential biases of studies in regards to study design and other factors. Read the to learn about the topic of assessing risk of bias in included studies. You can adapt ( ) to best meet the needs of your review, depending on the types of studies included. |
| - - - | Clearly present your findings, including detailed methodology (such as search strategies used, selection criteria, etc.) such that your review can be easily updated in the future with new research findings. Perform a meta-analysis, if the studies allow. Provide recommendations for practice and policy-making if sufficient, high quality evidence exists, or future directions for research to fill existing gaps in knowledge or to strengthen the body of evidence. For more information, see: . (2), 217–226. https://doi.org/10.2450/2012.0247-12 - Get some inspiration and find some terms and phrases for writing your manuscript - Automated high-quality spelling, grammar and rephrasing corrections using artificial intelligence (AI) to improve the flow of your writing. Free and subscription plans available. |
| - - | 8. Find the best journal to publish your work. Identifying the best journal to submit your research to can be a difficult process. To help you make the choice of where to submit, simply insert your title and abstract in any of the listed under the tab. |
Adapted from A Guide to Conducting Systematic Reviews: Steps in a Systematic Review by Cornell University Library
|
This diagram illustrates in a visual way and in plain language what review authors actually do in the process of undertaking a systematic review. |
This diagram illustrates what is actually in a published systematic review and gives examples from the relevant parts of a systematic review housed online on The Cochrane Library. It will help you to read or navigate a systematic review. |
Source: Cochrane Consumers and Communications (infographics are free to use and licensed under Creative Commons )
Check the following visual resources titled " What Are Systematic Reviews?"
- Video with closed captions available
- Animated Storyboard
|
Image: | - the methods of the systematic review are generally decided before conducting it.
Source: Foster, M. (2018). Systematic reviews service: Introduction to systematic reviews. Retrieved September 18, 2018, from |
- << Previous: What is a Systematic Review (SR)?
- Next: Framing a Research Question >>
- Last Updated: May 8, 2024 1:44 PM
- URL: https://lib.guides.umd.edu/SR
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- Systematic Review | Definition, Example, & Guide
Systematic Review | Definition, Example & Guide
Published on June 15, 2022 by Shaun Turney . Revised on November 20, 2023.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesize all available evidence. It answers a clearly formulated research question and explicitly states the methods used to arrive at the answer.
They answered the question “What is the effectiveness of probiotics in reducing eczema symptoms and improving quality of life in patients with eczema?”
In this context, a probiotic is a health product that contains live microorganisms and is taken by mouth. Eczema is a common skin condition that causes red, itchy skin.
Table of contents
What is a systematic review, systematic review vs. meta-analysis, systematic review vs. literature review, systematic review vs. scoping review, when to conduct a systematic review, pros and cons of systematic reviews, step-by-step example of a systematic review, other interesting articles, frequently asked questions about systematic reviews.
A review is an overview of the research that’s already been completed on a topic.
What makes a systematic review different from other types of reviews is that the research methods are designed to reduce bias . The methods are repeatable, and the approach is formal and systematic:
- Formulate a research question
- Develop a protocol
- Search for all relevant studies
- Apply the selection criteria
- Extract the data
- Synthesize the data
- Write and publish a report
Although multiple sets of guidelines exist, the Cochrane Handbook for Systematic Reviews is among the most widely used. It provides detailed guidelines on how to complete each step of the systematic review process.
Systematic reviews are most commonly used in medical and public health research, but they can also be found in other disciplines.
Systematic reviews typically answer their research question by synthesizing all available evidence and evaluating the quality of the evidence. Synthesizing means bringing together different information to tell a single, cohesive story. The synthesis can be narrative ( qualitative ), quantitative , or both.
Prevent plagiarism. Run a free check.
Systematic reviews often quantitatively synthesize the evidence using a meta-analysis . A meta-analysis is a statistical analysis, not a type of review.
A meta-analysis is a technique to synthesize results from multiple studies. It’s a statistical analysis that combines the results of two or more studies, usually to estimate an effect size .
A literature review is a type of review that uses a less systematic and formal approach than a systematic review. Typically, an expert in a topic will qualitatively summarize and evaluate previous work, without using a formal, explicit method.
Although literature reviews are often less time-consuming and can be insightful or helpful, they have a higher risk of bias and are less transparent than systematic reviews.
Similar to a systematic review, a scoping review is a type of review that tries to minimize bias by using transparent and repeatable methods.
However, a scoping review isn’t a type of systematic review. The most important difference is the goal: rather than answering a specific question, a scoping review explores a topic. The researcher tries to identify the main concepts, theories, and evidence, as well as gaps in the current research.
Sometimes scoping reviews are an exploratory preparation step for a systematic review, and sometimes they are a standalone project.
A systematic review is a good choice of review if you want to answer a question about the effectiveness of an intervention , such as a medical treatment.
To conduct a systematic review, you’ll need the following:
- A precise question , usually about the effectiveness of an intervention. The question needs to be about a topic that’s previously been studied by multiple researchers. If there’s no previous research, there’s nothing to review.
- If you’re doing a systematic review on your own (e.g., for a research paper or thesis ), you should take appropriate measures to ensure the validity and reliability of your research.
- Access to databases and journal archives. Often, your educational institution provides you with access.
- Time. A professional systematic review is a time-consuming process: it will take the lead author about six months of full-time work. If you’re a student, you should narrow the scope of your systematic review and stick to a tight schedule.
- Bibliographic, word-processing, spreadsheet, and statistical software . For example, you could use EndNote, Microsoft Word, Excel, and SPSS.
A systematic review has many pros .
- They minimize research bias by considering all available evidence and evaluating each study for bias.
- Their methods are transparent , so they can be scrutinized by others.
- They’re thorough : they summarize all available evidence.
- They can be replicated and updated by others.
Systematic reviews also have a few cons .
- They’re time-consuming .
- They’re narrow in scope : they only answer the precise research question.
The 7 steps for conducting a systematic review are explained with an example.
Step 1: Formulate a research question
Formulating the research question is probably the most important step of a systematic review. A clear research question will:
- Allow you to more effectively communicate your research to other researchers and practitioners
- Guide your decisions as you plan and conduct your systematic review
A good research question for a systematic review has four components, which you can remember with the acronym PICO :
- Population(s) or problem(s)
- Intervention(s)
- Comparison(s)
You can rearrange these four components to write your research question:
- What is the effectiveness of I versus C for O in P ?
Sometimes, you may want to include a fifth component, the type of study design . In this case, the acronym is PICOT .
- Type of study design(s)
- The population of patients with eczema
- The intervention of probiotics
- In comparison to no treatment, placebo , or non-probiotic treatment
- The outcome of changes in participant-, parent-, and doctor-rated symptoms of eczema and quality of life
- Randomized control trials, a type of study design
Their research question was:
- What is the effectiveness of probiotics versus no treatment, a placebo, or a non-probiotic treatment for reducing eczema symptoms and improving quality of life in patients with eczema?
Step 2: Develop a protocol
A protocol is a document that contains your research plan for the systematic review. This is an important step because having a plan allows you to work more efficiently and reduces bias.
Your protocol should include the following components:
- Background information : Provide the context of the research question, including why it’s important.
- Research objective (s) : Rephrase your research question as an objective.
- Selection criteria: State how you’ll decide which studies to include or exclude from your review.
- Search strategy: Discuss your plan for finding studies.
- Analysis: Explain what information you’ll collect from the studies and how you’ll synthesize the data.
If you’re a professional seeking to publish your review, it’s a good idea to bring together an advisory committee . This is a group of about six people who have experience in the topic you’re researching. They can help you make decisions about your protocol.
It’s highly recommended to register your protocol. Registering your protocol means submitting it to a database such as PROSPERO or ClinicalTrials.gov .
Step 3: Search for all relevant studies
Searching for relevant studies is the most time-consuming step of a systematic review.
To reduce bias, it’s important to search for relevant studies very thoroughly. Your strategy will depend on your field and your research question, but sources generally fall into these four categories:
- Databases: Search multiple databases of peer-reviewed literature, such as PubMed or Scopus . Think carefully about how to phrase your search terms and include multiple synonyms of each word. Use Boolean operators if relevant.
- Handsearching: In addition to searching the primary sources using databases, you’ll also need to search manually. One strategy is to scan relevant journals or conference proceedings. Another strategy is to scan the reference lists of relevant studies.
- Gray literature: Gray literature includes documents produced by governments, universities, and other institutions that aren’t published by traditional publishers. Graduate student theses are an important type of gray literature, which you can search using the Networked Digital Library of Theses and Dissertations (NDLTD) . In medicine, clinical trial registries are another important type of gray literature.
- Experts: Contact experts in the field to ask if they have unpublished studies that should be included in your review.
At this stage of your review, you won’t read the articles yet. Simply save any potentially relevant citations using bibliographic software, such as Scribbr’s APA or MLA Generator .
- Databases: EMBASE, PsycINFO, AMED, LILACS, and ISI Web of Science
- Handsearch: Conference proceedings and reference lists of articles
- Gray literature: The Cochrane Library, the metaRegister of Controlled Trials, and the Ongoing Skin Trials Register
- Experts: Authors of unpublished registered trials, pharmaceutical companies, and manufacturers of probiotics
Step 4: Apply the selection criteria
Applying the selection criteria is a three-person job. Two of you will independently read the studies and decide which to include in your review based on the selection criteria you established in your protocol . The third person’s job is to break any ties.
To increase inter-rater reliability , ensure that everyone thoroughly understands the selection criteria before you begin.
If you’re writing a systematic review as a student for an assignment, you might not have a team. In this case, you’ll have to apply the selection criteria on your own; you can mention this as a limitation in your paper’s discussion.
You should apply the selection criteria in two phases:
- Based on the titles and abstracts : Decide whether each article potentially meets the selection criteria based on the information provided in the abstracts.
- Based on the full texts: Download the articles that weren’t excluded during the first phase. If an article isn’t available online or through your library, you may need to contact the authors to ask for a copy. Read the articles and decide which articles meet the selection criteria.
It’s very important to keep a meticulous record of why you included or excluded each article. When the selection process is complete, you can summarize what you did using a PRISMA flow diagram .
Next, Boyle and colleagues found the full texts for each of the remaining studies. Boyle and Tang read through the articles to decide if any more studies needed to be excluded based on the selection criteria.
When Boyle and Tang disagreed about whether a study should be excluded, they discussed it with Varigos until the three researchers came to an agreement.
Step 5: Extract the data
Extracting the data means collecting information from the selected studies in a systematic way. There are two types of information you need to collect from each study:
- Information about the study’s methods and results . The exact information will depend on your research question, but it might include the year, study design , sample size, context, research findings , and conclusions. If any data are missing, you’ll need to contact the study’s authors.
- Your judgment of the quality of the evidence, including risk of bias .
You should collect this information using forms. You can find sample forms in The Registry of Methods and Tools for Evidence-Informed Decision Making and the Grading of Recommendations, Assessment, Development and Evaluations Working Group .
Extracting the data is also a three-person job. Two people should do this step independently, and the third person will resolve any disagreements.
They also collected data about possible sources of bias, such as how the study participants were randomized into the control and treatment groups.
Step 6: Synthesize the data
Synthesizing the data means bringing together the information you collected into a single, cohesive story. There are two main approaches to synthesizing the data:
- Narrative ( qualitative ): Summarize the information in words. You’ll need to discuss the studies and assess their overall quality.
- Quantitative : Use statistical methods to summarize and compare data from different studies. The most common quantitative approach is a meta-analysis , which allows you to combine results from multiple studies into a summary result.
Generally, you should use both approaches together whenever possible. If you don’t have enough data, or the data from different studies aren’t comparable, then you can take just a narrative approach. However, you should justify why a quantitative approach wasn’t possible.
Boyle and colleagues also divided the studies into subgroups, such as studies about babies, children, and adults, and analyzed the effect sizes within each group.
Step 7: Write and publish a report
The purpose of writing a systematic review article is to share the answer to your research question and explain how you arrived at this answer.
Your article should include the following sections:
- Abstract : A summary of the review
- Introduction : Including the rationale and objectives
- Methods : Including the selection criteria, search method, data extraction method, and synthesis method
- Results : Including results of the search and selection process, study characteristics, risk of bias in the studies, and synthesis results
- Discussion : Including interpretation of the results and limitations of the review
- Conclusion : The answer to your research question and implications for practice, policy, or research
To verify that your report includes everything it needs, you can use the PRISMA checklist .
Once your report is written, you can publish it in a systematic review database, such as the Cochrane Database of Systematic Reviews , and/or in a peer-reviewed journal.
In their report, Boyle and colleagues concluded that probiotics cannot be recommended for reducing eczema symptoms or improving quality of life in patients with eczema. Note Generative AI tools like ChatGPT can be useful at various stages of the writing and research process and can help you to write your systematic review. However, we strongly advise against trying to pass AI-generated text off as your own work.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Prospective cohort study
Research bias
- Implicit bias
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hindsight bias
- Affect heuristic
- Social desirability bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
A systematic review is secondary research because it uses existing research. You don’t collect new data yourself.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Turney, S. (2023, November 20). Systematic Review | Definition, Example & Guide. Scribbr. Retrieved June 18, 2024, from https://www.scribbr.com/methodology/systematic-review/
Is this article helpful?

Shaun Turney
Other students also liked, how to write a literature review | guide, examples, & templates, how to write a research proposal | examples & templates, what is critical thinking | definition & examples, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
Reference management. Clean and simple.
How to write a systematic literature review [9 steps]

What is a systematic literature review?
Where are systematic literature reviews used, what types of systematic literature reviews are there, how to write a systematic literature review, 1. decide on your team, 2. formulate your question, 3. plan your research protocol, 4. search for the literature, 5. screen the literature, 6. assess the quality of the studies, 7. extract the data, 8. analyze the results, 9. interpret and present the results, registering your systematic literature review, frequently asked questions about writing a systematic literature review, related articles.
A systematic literature review is a summary, analysis, and evaluation of all the existing research on a well-formulated and specific question.
Put simply, a systematic review is a study of studies that is popular in medical and healthcare research. In this guide, we will cover:
- the definition of a systematic literature review
- the purpose of a systematic literature review
- the different types of systematic reviews
- how to write a systematic literature review
➡️ Visit our guide to the best research databases for medicine and health to find resources for your systematic review.
Systematic literature reviews can be utilized in various contexts, but they’re often relied on in clinical or healthcare settings.
Medical professionals read systematic literature reviews to stay up-to-date in their field, and granting agencies sometimes need them to make sure there’s justification for further research in an area. They can even be used as the starting point for developing clinical practice guidelines.
A classic systematic literature review can take different approaches:
- Effectiveness reviews assess the extent to which a medical intervention or therapy achieves its intended effect. They’re the most common type of systematic literature review.
- Diagnostic test accuracy reviews produce a summary of diagnostic test performance so that their accuracy can be determined before use by healthcare professionals.
- Experiential (qualitative) reviews analyze human experiences in a cultural or social context. They can be used to assess the effectiveness of an intervention from a person-centric perspective.
- Costs/economics evaluation reviews look at the cost implications of an intervention or procedure, to assess the resources needed to implement it.
- Etiology/risk reviews usually try to determine to what degree a relationship exists between an exposure and a health outcome. This can be used to better inform healthcare planning and resource allocation.
- Psychometric reviews assess the quality of health measurement tools so that the best instrument can be selected for use.
- Prevalence/incidence reviews measure both the proportion of a population who have a disease, and how often the disease occurs.
- Prognostic reviews examine the course of a disease and its potential outcomes.
- Expert opinion/policy reviews are based around expert narrative or policy. They’re often used to complement, or in the absence of, quantitative data.
- Methodology systematic reviews can be carried out to analyze any methodological issues in the design, conduct, or review of research studies.
Writing a systematic literature review can feel like an overwhelming undertaking. After all, they can often take 6 to 18 months to complete. Below we’ve prepared a step-by-step guide on how to write a systematic literature review.
- Decide on your team.
- Formulate your question.
- Plan your research protocol.
- Search for the literature.
- Screen the literature.
- Assess the quality of the studies.
- Extract the data.
- Analyze the results.
- Interpret and present the results.
When carrying out a systematic literature review, you should employ multiple reviewers in order to minimize bias and strengthen analysis. A minimum of two is a good rule of thumb, with a third to serve as a tiebreaker if needed.
You may also need to team up with a librarian to help with the search, literature screeners, a statistician to analyze the data, and the relevant subject experts.
Define your answerable question. Then ask yourself, “has someone written a systematic literature review on my question already?” If so, yours may not be needed. A librarian can help you answer this.
You should formulate a “well-built clinical question.” This is the process of generating a good search question. To do this, run through PICO:
- Patient or Population or Problem/Disease : who or what is the question about? Are there factors about them (e.g. age, race) that could be relevant to the question you’re trying to answer?
- Intervention : which main intervention or treatment are you considering for assessment?
- Comparison(s) or Control : is there an alternative intervention or treatment you’re considering? Your systematic literature review doesn’t have to contain a comparison, but you’ll want to stipulate at this stage, either way.
- Outcome(s) : what are you trying to measure or achieve? What’s the wider goal for the work you’ll be doing?
Now you need a detailed strategy for how you’re going to search for and evaluate the studies relating to your question.
The protocol for your systematic literature review should include:
- the objectives of your project
- the specific methods and processes that you’ll use
- the eligibility criteria of the individual studies
- how you plan to extract data from individual studies
- which analyses you’re going to carry out
For a full guide on how to systematically develop your protocol, take a look at the PRISMA checklist . PRISMA has been designed primarily to improve the reporting of systematic literature reviews and meta-analyses.
When writing a systematic literature review, your goal is to find all of the relevant studies relating to your question, so you need to search thoroughly .
This is where your librarian will come in handy again. They should be able to help you formulate a detailed search strategy, and point you to all of the best databases for your topic.
➡️ Read more on on how to efficiently search research databases .
The places to consider in your search are electronic scientific databases (the most popular are PubMed , MEDLINE , and Embase ), controlled clinical trial registers, non-English literature, raw data from published trials, references listed in primary sources, and unpublished sources known to experts in the field.
➡️ Take a look at our list of the top academic research databases .
Tip: Don’t miss out on “gray literature.” You’ll improve the reliability of your findings by including it.
Don’t miss out on “gray literature” sources: those sources outside of the usual academic publishing environment. They include:
- non-peer-reviewed journals
- pharmaceutical industry files
- conference proceedings
- pharmaceutical company websites
- internal reports
Gray literature sources are more likely to contain negative conclusions, so you’ll improve the reliability of your findings by including it. You should document details such as:
- The databases you search and which years they cover
- The dates you first run the searches, and when they’re updated
- Which strategies you use, including search terms
- The numbers of results obtained
➡️ Read more about gray literature .
This should be performed by your two reviewers, using the criteria documented in your research protocol. The screening is done in two phases:
- Pre-screening of all titles and abstracts, and selecting those appropriate
- Screening of the full-text articles of the selected studies
Make sure reviewers keep a log of which studies they exclude, with reasons why.
➡️ Visit our guide on what is an abstract?
Your reviewers should evaluate the methodological quality of your chosen full-text articles. Make an assessment checklist that closely aligns with your research protocol, including a consistent scoring system, calculations of the quality of each study, and sensitivity analysis.
The kinds of questions you'll come up with are:
- Were the participants really randomly allocated to their groups?
- Were the groups similar in terms of prognostic factors?
- Could the conclusions of the study have been influenced by bias?
Every step of the data extraction must be documented for transparency and replicability. Create a data extraction form and set your reviewers to work extracting data from the qualified studies.
Here’s a free detailed template for recording data extraction, from Dalhousie University. It should be adapted to your specific question.
Establish a standard measure of outcome which can be applied to each study on the basis of its effect size.
Measures of outcome for studies with:
- Binary outcomes (e.g. cured/not cured) are odds ratio and risk ratio
- Continuous outcomes (e.g. blood pressure) are means, difference in means, and standardized difference in means
- Survival or time-to-event data are hazard ratios
Design a table and populate it with your data results. Draw this out into a forest plot , which provides a simple visual representation of variation between the studies.
Then analyze the data for issues. These can include heterogeneity, which is when studies’ lines within the forest plot don’t overlap with any other studies. Again, record any excluded studies here for reference.
Consider different factors when interpreting your results. These include limitations, strength of evidence, biases, applicability, economic effects, and implications for future practice or research.
Apply appropriate grading of your evidence and consider the strength of your recommendations.
It’s best to formulate a detailed plan for how you’ll present your systematic review results. Take a look at these guidelines for interpreting results from the Cochrane Institute.
Before writing your systematic literature review, you can register it with OSF for additional guidance along the way. You could also register your completed work with PROSPERO .
Systematic literature reviews are often found in clinical or healthcare settings. Medical professionals read systematic literature reviews to stay up-to-date in their field and granting agencies sometimes need them to make sure there’s justification for further research in an area.
The first stage in carrying out a systematic literature review is to put together your team. You should employ multiple reviewers in order to minimize bias and strengthen analysis. A minimum of two is a good rule of thumb, with a third to serve as a tiebreaker if needed.
Your systematic review should include the following details:
A literature review simply provides a summary of the literature available on a topic. A systematic review, on the other hand, is more than just a summary. It also includes an analysis and evaluation of existing research. Put simply, it's a study of studies.
The final stage of conducting a systematic literature review is interpreting and presenting the results. It’s best to formulate a detailed plan for how you’ll present your systematic review results, guidelines can be found for example from the Cochrane institute .

Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State

Systematic Reviews
- What is a Systematic Review?
What are the Steps of a Systematic Review?
- An Overview of the Systematic Review Process
- 1. Choose the Right Kind of Review
- 2. Formulate Your Question
- 3. Establish a Team
- 4. Develop a Protocol
- 5. Conduct the Search
- 6. Select Studies
- 7. Extract Data
- 8. Synthesize Your Results
- 9. Disseminate Your Report
- Request a Librarian Consultation
Consult With a Librarian

To make an appointment to consult with an HSL librarian on your systematic review, please read our Systematic Review Policy and submit a Systematic Review Consultation Request .
To ask a question or make an appointment for assistance with a narrative review, please complete the Ask a Librarian Form .
This video from the Yale University Medical Library provides a brief overview of the process of conducting a systematic review:
Check out the rest of Yale's video series on conducting systematic searches:
- Systematic Searches Series from Yale University
- << Previous: What is a Systematic Review?
- Next: 1. Choose the Right Kind of Review >>
- Last Updated: May 14, 2024 8:03 AM
- URL: https://hslguides.osu.edu/systematic_reviews

University of Pittsburgh Library System
- Collections
Course & Subject Guides
A guide to systematic reviews and evidence synthesis service @ uls.
- What is Evidence Synthesis?
- How can the ULS assist you with your Evidence Synthesis?
- What is a Systematic Review and where can you find them?
STEP 1. Identify your research question
Step 2. define inclusion and exclusion criteria, step 3. write a search strategy, step 4. register protocol, step 5. manage search results, step 6. select studies based on inclusion and exclusion criteria, step 7. extract data from included studies, step 7. assess quality of evidence in included studies, step 8. present results.
- Are there any guidelines or standards for publishing reviews?
- How can I get assistance with a review?
A well defined research question should address a gap in the current literature and is the essential starting point of your synthesis.
You can use the following frameworks to help construct your research question.
PICO for Quantitative Studies P Population/Problem I Intervention/Exposure C Comparison O Outcome Example: Is gabapentin (intervention), compared to placebo (comparison), effective in decreasing pain symptoms (outcome) in middle aged male amputees suffering phantom limb pain (population)?
PICo for Qualitative Studies P Population/Problem I Phenomenon of Interest Co Context Example: What are the experiences (phenomenon of interest) of caregivers providing home based care to patients with Alzheimer's disease (population) in Australia (context)?
SPICE S Setting P Perspective (for whom) I Intervention/Exposure C Comparison E Evaluation Example: What are the benefits (evaluation) of a doula (intervention) for low income mothers (perspective) in the developed world (setting) compared to no support (comparison)?
SPIDER S Sample PI Phenomenon of Interest D Design E Evaluation R Study Type Example: What are the experiences (evaluation) of wome n (sample) undergoing IVF treatment (phenomenon of interest) as assessed?
Design: questionnaire or survey or interview
Study Type: qualitative or mixed method
The above was adapted from Cornell University A Guide to Evidence Synthesis: 1. Develop a Research Question https://guides.library.cornell.edu/evidence-synthesis/research-question
After finalizing your research question but before you start your search, you need to define your inclusion and exclusion criteria. You must decide what contents an article MUST have before being included in the review. You also must determine which attributes would exclude an article from the review.

Image from the University of Melbourne Libguide Systematic Reviews https://unimelb.libguides.com/c.php?g=492361&p=3368110
Your search strategy must be exhaustive, encompasses multiple databases, include grey literature and be reproducible. PRISMA guidelines state that the full search strategy for at least one major database should be reported in an appendix and published along with the review ( http://www.prisma-statement.org /).
The University Library System provides access to a wide range of databases which can be accessed by subject on the A-Z database list . Most databases have controlled vocabulary (a certain way words and phrases are indexed) which is unique to the database. This may require using different terms for different databases." Given the complexity of the many indexing languages and rules governing the various databases, we recommend that early in the process you make use of an experienced research librarian who can examine your search strategy and help you choose citation databases relevant to your review question ." ( Aromataris, Edoardo PhD; Riitano, Dagmara BHSC, BA Systematic Reviews: Constructing a Search Strategy and Searching for Evidence, AJN, American Journal of Nursing: May 2014 - Volume 114 - Issue 5 - p 49-56 doi: 10.1097/01.NAJ.0000446779.99522.f6 )
Grey literature is produced outside of traditional publishing and distribution norms. This can included, among other things, white papers, government publications, working papers, preprints, unpublished trial data, and conference proceedings and abstracts. Grey literature can be found in some citation databases, as well as databases dedicated to grey literature.
Some databases dedicated to grey literature include:
- Grey Literature Report A report from the NY Academy of Medicine of gray literature published between 1999 - 2016
- Open Grey "Open access to 700.000 bibliographical references of grey literature (paper) produced in Europe and allows you to export records and locate the documents"
- GreySource "A selection of web-based resources on grey literature"
- Grey Matters Canadian Agency for Drugs and Technologies in Health provides a practical tool for searching health-related grey literature
Some sources for preprints include:
- ASAPbio Accelerating Science Publication in Biology preprint server directory
- OSF Preprints A searchable database of over 33 preprint repositories
- OAD Disciplinary Repositories Open Access Directory list of preprint depositories by subject
- OPENDOAR A "Global Directory of Open Access Repositories. You can search and browse through thousands of registered repositories based on a range of features"
An example of a complete and reproducible search strategy can be found in Appendix 1 of Petriwskyj, P. (2013). Family involvement in decision making for people with dementia in residential aged care: a systematic review of quantitative and qualitative evidence . JBI Database of Systematic Reviews and Implementation Reports , 11 (7), 131–282. https://doi.org/10.11124/jbisrir-2013-977
A protocol lists the objectives, methods, and outcomes of primary interest of the systematic review. Protocols promote transparency of methods and allows your peers to review how you will extract information to summarize the data. Registration of your protocol establishes your intent to conduct this review which may reduce the risk of others conducting similar reviews.
Here is an example of a published protocol
Mengesha, M.M., Ajema, D., Teshome, A. et al. The association between diagnosis disclosure and adherence to antiretroviral therapy among adolescents living with HIV in sub-Saharan Africa: a protocol for systematic review and meta-analysis. Syst Rev 9, 160 (2020). https://doi.org/10.1186/s13643-020-01420-8
Protocol Reporting Guidelines and Checklists
- Methodological Expectations of Cochrane Intervention Reviews (MECIR) Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates
- PRISMA for systematic review protocols PRISMA 2020 is an expanded 27-item checklist intended to facilitate the preparation and reporting of a robust protocol for the systematic review
- Cochrane Handbook 1.5 Protocol Development Lasserson TJ, Thomas J, Higgins JPT. Chapter 1: Starting a review. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Protocol Templates
- PROSPERO Registration Form
- Cochrane Qualitative Evidence Synthesis Template
- Collaboration for Environmental Evidence (CEE) Systematic Map Protocol Template
- Campbell Collaboration Template
- Collaboration for Environmental Evidence (CEE) Systematic Review Protocol Template
- Evidence Synthesis Protocol Template by Ghezzi-Kopel, K., & Porciello, J. found on Open Science Framework
- Systematic Review Protocol Template by Sarah Vistintini (Maritime SPOR SUPPORT Unit (MSSU))
- Open Science Framework Systematic Review Template Select Add New, and under step 2, select Generalized Systematic Review Registration
- Template from Warwick University You can use this template available from Warwick University to create your protocol
Protocol Registries
- Cochran Cochran protocols "contain information that defines the health problem and the intervention under investigation, how benefits and harms will be measured, and the type of appropriate study design"
- Campbell Collaboration "The Campbell Collaboration promotes positive social and economic change through the production and use of systematic reviews and other evidence synthesis for evidence-based policy and practice"
- PROSPERO "PROSPERO is an international database of prospectively registered systematic reviews in health and social care, welfare, public health, education, crime, justice, and international development, where there is a health related outcome"
- Open Science Framework Register your protocol on this free open platform
- Collaboration for Environmental Evidence (CEE) CEE seeks to promote and deliver evidence syntheses on issues of greatest concern to environmental policy and practice as a public service
- JBI "JBI is a global organization promoting and supporting evidence-based decisions that improve health and health service delivery."
- BioMed Central BioMed Central publishes a limited amount of protocols in Systematic Reviews
It is important to keep track of all search results from each database. The use of a template is recommended to capture the following information:
- Database Name
- Date Searched
- Keywords and Combination of Terms
- Search History
- Limiters (Language, Time Period, Publication Type)
- Number of Results
- Number of Duplicates
After running the search through a database, export the results to a citation manager. The method of export will depend on the database and the citation management tool used. Once all results from all sources are uploaded into a citation manager, you will need to de-duplicate the result list.
Visit our Library Guide, " Introduction to Citation Management " for an introduction to citation management tools and links to upcoming citation management workshops workshops.
Start with the screening of title and abstract to determine if a reference is relevant to your review. Obtain the full text of a reference if further screening is necessary. At least two reviewers will be needed to make a final determination on inclusion.
MECIR Box 4.6.c Relevant expectations for conduct of intervention reviews , Cochran Handbook

There are systematic review tools available to help with the screening process:
- Rayyan Web-based application to screen references and maintain systematic reviews. Rayyan also has a mobile app.
- Abstrackr Developed at Brown University, "Abstrackr is a free online tool to help you upload and organize the results of a literature search for a systematic review.
- CADIMA Web-based tool to manage your systematic review.
- PICO Portal Uses machine learning and artificial intelligence to facilitate deduplication, identify non-RCT articles and highlight keywords.
Tools which have a subscription cost
- Covidence This is the primary screening tool for Cochran Reviews. Covidence offers a FREE TRIAL for a single review containing 500 references or less. Single user and group pricing are available.
- JBISUMARI The System for the Unified Management of the Assessment and Review of Information is used in Joanna Briggs Institute Reviews and is available as an individual subscription.
- DistillerSR Commercially available systematic review software from Evidence Partners. DistillerSR offers various subscriptions, including a four month FREE subscription for students.
More tools for conducting systematic reviews can be found at the SR Toolbox
- SR Toolbox The Systematic Review Toolbox is a community-driven, searchable, web-based catalogue of tools that support various tasks within the systematic review and wider evidence synthesis process.
The reviewers must read the full text of the articles which were selected for inclusion in the review. The pertinent data must be extracted from each article. A standardized data extraction form should be used. An example of a data extraction form can be found below.
- Cochran Data and Extraction Assessment Form "This form can be used as a guide for developing your own data extraction form. Sections can be expanded and added, and irrelevant sections can be removed."
- Brown, U. (2003). A Framework for Developing a Coding Scheme for Meta-Analysis. Western Journal of Nursing Research, 25(2), 205–222.
If your review will contain a meta-analysis you may want to code the data in order to automate the statistical analysis process. Some systematic review software packages listed in step 6. can help you create coded data instruction forms. Instructions on designing a coded data extraction form can be found in the following article:
It is necessary to evaluate each study included in your review for bias. Cochran defines bias as "a systematic error, or deviation from the truth, in results or inferences. Biases can operate in either direction: different biases can lead to underestimation or overestimation of the true intervention effect". ( Cochran Handbook 8.2.1 )
Bias is evaluated on a level of risk. The risk of bias (RoB) can be demonstrated using a variety of tools:
- RoB 2 "Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) is the recommended tool to assess the risk of bias in randomized trials included in Cochrane Reviews. RoB 2 is structured into a fixed set of domains of bias, focussing on different aspects of trial design, conduct, and reporting. Within each domain, a series of questions ('signalling questions') aim to elicit information about features of the trial that are relevant to risk of bias. A proposed judgement about the risk of bias arising from each domain is generated by an algorithm, based on answers to the signalling questions. Judgement can be 'Low' or 'High' risk of bias, or can express 'Some concerns'."
- ROBINS-I ROBINS-I tool (“Risk Of Bias In Non-randomized Studies - of Interventions”) is concerned with evaluating the risk of bias (RoB) in the results of NRSIs that compare the health effects of two or more interventions. The types of NRSIs that can be evaluated using this tool are quantitative studies estimating the effectiveness (harm or benefit) of an intervention, which did not use randomization to allocate units (individuals or clusters of individuals) to comparison groups."
More information and an analysis of RoB tools can be found in the article:
Ma, L. L., Wang, Y. Y., Yang, Z. H., Huang, D., Weng, H., & Zeng, X. T. (2020). Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better?. Military Medical Research , 7 (1), 7. https://doi.org/10.1186/s40779-020-00238-8
Some study quality assessment tools include
- Critical Appraisal Skills Program Checklists Checklists from the Critical Appraisal Skills Program
- Study Quality Assessment Tools Tools available from NIH National Heart, Lung and Blood Institute
- Quality Assessment Tools Assessment tools from the NIH Office of Management
- Critical Appraisal Tools Worksheets to help you appraise the reliability, importance and applicability of clinical evidence from the Centre for Evidence Based Medicine
PRISMA provides a list of items to consider when reporting results.
- Study selection: Give numbers of studies screened, assessed for eligibility, & included in the review, with reasons for exclusions at each stage, ideally with a flow diagram.
- Study characteristics: For each study, present characteristics for which data were extracted (e.g., study size, PICOs, follow-up period) & provide the citations.
- Risk of bias within studies: Present data on risk of bias of each study &, if available, any outcome level assessment.
- Results of individual studies: For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates & confidence intervals, ideally with a forest plot.
- Synthesis of results: Present results of each meta-analysis done, including confidence intervals & measures of consistency.
- Risk of bias across studies: Present results of any assessment of risk of bias across studies.
- Additional analysis: Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression).
Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement
PRISMA Diagram Generators
- Flow Diagram Generator This is an updated version of the original PRISMA flow generator. Includes a downloadable PDF version.
- Flow Diagram PRISMA Contains both PDF & Word versions. From PRISMA.
Other Reporting Templates
- Equator Netowrk "Enhancing the QUAlity and Transparency Of health Research is an international initiative that seeks to improve the reliability and value of published health research literature by promoting transparent and accurate reporting and wider use of robust reporting guidelines"
Information in this section reproduced under a Creative Commons Attribution 4.0 license from the University of Michigan Libguide, " Systematic Reviews "
- << Previous: What is a Systematic Review and where can you find them?
- Next: Are there any guidelines or standards for publishing reviews? >>
- Last Updated: Apr 26, 2024 9:37 AM
- URL: https://pitt.libguides.com/SystematicReviews
Evidence Syntheses & Systematic Reviews: Steps in a Systematic Review
- Search & Document
- Databases & More
- Grey Literature
- Appraisal Tools & Checklists
- Useful Tools
- Types of Reviews
Steps in a Systematic Review
- How can I get assistance with my review?
- Check for existing reviews/protocols and complete an initial search of the literature.
Has a review on your topic already been completed? Has a protocol been registered? A librarian can help with an initial search which will answer this question and give you an idea of the literature on your topic.
- Formulate your question.
A clear and defined research question is an essential component of a systematic review. Formulating your research question is one of the most important steps in this process. This questions typically follow a framework. You can learn more about different types of frameworks here .
- Assemble a team.
Systematic reviews are dependent on teamwork. Most standards recommend, and even require, multiple reviewers to screen and assess bias. Also, studies have shown that the inclusion of librarians increase the quality of the systematic review. You also might need a statistician, particularly if you plan on conducting a meta-analysis as well.
- Develop a protocol. Think about registering it.
It is important to plan your research in advance of beginning your systematic search. A pre-established protocol should include research question, scope, extent of the review, screening process (including exclusion and inclusion criteria), data extraction, quality appraisal, and synthesis. Many protocols are published and establish rigor and transparency.
- Search for studies
The goal of a systematic review is to identify all relevant studies on your research question. Work with a librarian to design a comprehensive search strategy across multiple databases (at minimum three). Determine if you are going to use grey literature and then develop a methodical search for it. Choose a citation manager or other program to help with deduplication.
- Screening and selection of studies
Use the inclusion and exclusion criteria defined in your protocol, to determine which studies will be included in the appraisal. This process will remove studies that do not meet your criteria. The screening is performed twice; first with just title/abstracts, then to the full-text. This process is methodical and well-documented; each study at both stages should be screened by at least two members of the review team to minimize bias. A third reviewer is used to resolve conflicts.
- Quality appraisal.
At least two members will appraise the quality of the research in the included studies. This evaluation will include risk of bias using a standardized tool which can be adapted if need be.
- Extract data.
You will also extract the data from the relevant studies. You should use a spreadsheet or systematic review software. It is recommended to pilot your extraction tool with a small subset of your studies to make sure if fields are missing or need to be modified.
- Synthesize your results.
Your collected data must be combined into a coherent whole and accompanied by an analysis that conveys a deeper understanding of the body of evidence. All reviews will include a qualitative synthesis, and may or may not include a quantitative synthesis (also known as a meta-analysis).
- Disseminate your report.
Share your research findings in a clear and comprehensive manner using the appropriate medium.

This diagram illustrates the steps visually and in plain language, the steps authors do when completing a systematic review.
Further Reading
Clark, W. R., Clark, L. A., Raffo, D. M., & Williams, R. I. (2021). Extending Fisch and Block’s (2018) tips for a systematic review in management and business literature. Management Review Quarterly , 71 (1), 215–231. https://doi.org/10.1007/s11301-020-00184-8
Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Institute of Medicine (U.S.). (2011). Finding what works in health care: Standards for systematic reviews . National Academies Press. https://nap.nationalacademies.org/catalog/13059/finding-what-works-in-health-care-standards-for-systematic-reviews
Fisch, C., & Block, J. (2018). Six tips for your (systematic) literature review in business and management research. Management Review Quarterly , 68 (2), 103–106. https://doi.org/10.1007/s11301-018-0142-x
Mohamed Shaffril, H. A., Samsuddin, S. F., & Abu Samah, A. (2021). The ABC of systematic literature review: The basic methodological guidance for beginners. Quality & Quantity , 55 (4), 1319–1346. https://doi.org/10.1007/s11135-020-01059-6
Okoli, C. (2015). A guide to conducting a standalone systematic literature review. Communications of the Association for Information Systems , 37 (1). https://doi.org/10.17705/1CAIS.03743
Siddaway, A. P., Wood, A. M., & Hedges, L. V. (2019). How to do a systematic review: A best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annual Review of Psychology , 70 , 747–770. https://doi.org/10.1146/annurev-psych-010418-102803
Zawacki-Richter, O., Kerres, M., Bedenlier, S., Bond, M., & Buntins, K. (2020). Systematic reviews in educational research: Methodology, perspectives and application . Springer VS. https://proxy.lib.miamioh.edu/login?url=https://search.ebscohost.com/login.aspx?direct=true&AuthType=cookie,ip,url,uid,cpid&custid=s9002934&db=cat00344a&AN=mucat.b4716649&site=eds-live&scope=site&profile=eds_cat
- << Previous: Types of Reviews
- Next: How can I get assistance with my review? >>
- Last Updated: Jun 13, 2024 1:27 PM
- URL: https://libguides.lib.miamioh.edu/systematicreviews

- Duke NetID Login
- 919.660.1100
- Duke Health Badge: 24-hour access
- Accounts & Access
- Databases, Journals & Books
- Request & Reserve
- Training & Consulting
- Request Articles & Books
- Renew Online
- Reserve Spaces
- Reserve a Locker
- Study & Meeting Rooms
- Course Reserves
- Pay Fines/Fees
- Recommend a Purchase
- Access From Off Campus
- Building Access
- Computers & Equipment
- Wifi Access
- My Accounts
- Mobile Apps
- Known Access Issues
- Report an Access Issue
- All Databases
- Article Databases
- Basic Sciences
- Clinical Sciences
- Dissertations & Theses
- Drugs, Chemicals & Toxicology
- Grants & Funding
- Interprofessional Education
- Non-Medical Databases
- Search for E-Journals
- Search for Print & E-Journals
- Search for E-Books
- Search for Print & E-Books
- E-Book Collections
- Biostatistics
- Global Health
- MBS Program
- Medical Students
- MMCi Program
- Occupational Therapy
- Path Asst Program
- Physical Therapy
- Researchers
- Community Partners
Conducting Research
- Archival & Historical Research
- Black History at Duke Health
- Data Analytics & Viz Software
- Data: Find and Share
- Evidence-Based Practice
- NIH Public Access Policy Compliance
- Publication Metrics
- Qualitative Research
- Searching Animal Alternatives
Systematic Reviews
- Test Instruments
Using Databases
- JCR Impact Factors
- Web of Science
Finding & Accessing
- COVID-19: Core Clinical Resources
- Health Literacy
- Health Statistics & Data
- Library Orientation
Writing & Citing
- Creating Links
- Getting Published
- Reference Mgmt
- Scientific Writing
Meet a Librarian
- Request a Consultation
- Find Your Liaisons
- Register for a Class
- Request a Class
- Self-Paced Learning
Search Services
- Literature Search
- Systematic Review
- Animal Alternatives (IACUC)
- Research Impact
Citation Mgmt
- Other Software
Scholarly Communications
- About Scholarly Communications
- Publish Your Work
- Measure Your Research Impact
- Engage in Open Science
- Libraries and Publishers
- Directions & Maps
- Floor Plans
Library Updates
- Annual Snapshot
- Conference Presentations
- Contact Information
- Gifts & Donations
- What is a Systematic Review?
- Types of Reviews
- Manuals and Reporting Guidelines
- Our Service
- 1. Assemble Your Team
- 2. Develop a Research Question
- 3. Write and Register a Protocol
- 4. Search the Evidence
- 5. Screen Results
- 6. Assess for Quality and Bias
- 7. Extract the Data
- 8. Write the Review
- Additional Resources
- Finding Full-Text Articles
Systematic Review Steps

- << Previous: Our Service
- Next: 1. Assemble Your Team >>
- Last Updated: Jun 18, 2024 9:41 AM
- URL: https://guides.mclibrary.duke.edu/sysreview
- Duke Health
- Duke University
- Duke Libraries
- Medical Center Archives
- Duke Directory
- Seeley G. Mudd Building
- 10 Searle Drive
- [email protected]

- Langson Library
- Science Library
- Grunigen Medical Library
- Law Library
- Connect From Off-Campus
- Accessibility
- Gateway Study Center

Email this link
Systematic reviews & evidence synthesis methods.
- Schedule a Consultation / Meet our Team
- What is Evidence Synthesis?
- Types of Evidence Synthesis
- Evidence Synthesis Across Disciplines
- Finding and Appraising Existing Systematic Reviews
- 0. Preliminary Searching
- 1. Develop a Protocol
- 2. Draft your Research Question
- 3. Select Databases
- 4. Select Grey Literature Sources
- 5. Write a Search Strategy
- 6. Register a Protocol
- 7. Translate Search Strategies
- 8. Citation Management
- 9. Article Screening
- 10. Risk of Bias Assessment
- 11. Data Extraction
- 12. Synthesize, Map, or Describe the Results
- Open Access Evidence Synthesis Resources
Requirements for the Systematic Review Process
Systematic reviews are a huge endeavor, so here are a few requirements if you are thinking of employing this methodology:
- Systematic reviews require time . 12-24 months is usual from conception to submission.
- Systematic reviews require a team . Four (4) or more team members are recommended. A principal investigator, a second investigator, a librarian, and someone well-versed in statistics forms the basic team. Ideally the team might have another investigator and someone to coordinate all the moving pieces. Smaller teams are possible, three is the realistic minimum . Two investigators each wearing more than one hat and one librarian. Sometimes an investigator has the time and energy to coordinate. Occasionally one of the investigators is also a statistical guru.
- * An exception to this rule is an "empty review," which retrieves zero studies that meet the inclusion criteria. Empty reviews are relatively uncommon, but may be used to demonstrate a need for future research in an area. However, an empty review may instead indicate that the research question was defined too narrowly.
Why do a systematic review? A well done systematic review is a major contribution to the literature. But the requirements in time and effort are massive. Cochrane estimates one year from conception to completion. This does not including time for review, revision and publication. You need to assemble a team and they need to commit for the duration.
A good place to start is with a consultation with a librarian. Visit the " Schedule a Consultation " page to learn why.
- << Previous: Finding and Appraising Existing Systematic Reviews
- Next: 0. Preliminary Searching >>
- Last Updated: Jun 14, 2024 4:26 PM
- URL: https://guides.lib.uci.edu/evidence-synthesis
Off-campus? Please use the Software VPN and choose the group UCIFull to access licensed content. For more information, please Click here
Software VPN is not available for guests, so they may not have access to some content when connecting from off-campus.
- Open access
- Published: 01 August 2019
A step by step guide for conducting a systematic review and meta-analysis with simulation data
- Gehad Mohamed Tawfik 1 , 2 ,
- Kadek Agus Surya Dila 2 , 3 ,
- Muawia Yousif Fadlelmola Mohamed 2 , 4 ,
- Dao Ngoc Hien Tam 2 , 5 ,
- Nguyen Dang Kien 2 , 6 ,
- Ali Mahmoud Ahmed 2 , 7 &
- Nguyen Tien Huy 8 , 9 , 10
Tropical Medicine and Health volume 47 , Article number: 46 ( 2019 ) Cite this article
815k Accesses
304 Citations
94 Altmetric
Metrics details
The massive abundance of studies relating to tropical medicine and health has increased strikingly over the last few decades. In the field of tropical medicine and health, a well-conducted systematic review and meta-analysis (SR/MA) is considered a feasible solution for keeping clinicians abreast of current evidence-based medicine. Understanding of SR/MA steps is of paramount importance for its conduction. It is not easy to be done as there are obstacles that could face the researcher. To solve those hindrances, this methodology study aimed to provide a step-by-step approach mainly for beginners and junior researchers, in the field of tropical medicine and other health care fields, on how to properly conduct a SR/MA, in which all the steps here depicts our experience and expertise combined with the already well-known and accepted international guidance.
We suggest that all steps of SR/MA should be done independently by 2–3 reviewers’ discussion, to ensure data quality and accuracy.
SR/MA steps include the development of research question, forming criteria, search strategy, searching databases, protocol registration, title, abstract, full-text screening, manual searching, extracting data, quality assessment, data checking, statistical analysis, double data checking, and manuscript writing.
Introduction
The amount of studies published in the biomedical literature, especially tropical medicine and health, has increased strikingly over the last few decades. This massive abundance of literature makes clinical medicine increasingly complex, and knowledge from various researches is often needed to inform a particular clinical decision. However, available studies are often heterogeneous with regard to their design, operational quality, and subjects under study and may handle the research question in a different way, which adds to the complexity of evidence and conclusion synthesis [ 1 ].
Systematic review and meta-analyses (SR/MAs) have a high level of evidence as represented by the evidence-based pyramid. Therefore, a well-conducted SR/MA is considered a feasible solution in keeping health clinicians ahead regarding contemporary evidence-based medicine.
Differing from a systematic review, unsystematic narrative review tends to be descriptive, in which the authors select frequently articles based on their point of view which leads to its poor quality. A systematic review, on the other hand, is defined as a review using a systematic method to summarize evidence on questions with a detailed and comprehensive plan of study. Furthermore, despite the increasing guidelines for effectively conducting a systematic review, we found that basic steps often start from framing question, then identifying relevant work which consists of criteria development and search for articles, appraise the quality of included studies, summarize the evidence, and interpret the results [ 2 , 3 ]. However, those simple steps are not easy to be reached in reality. There are many troubles that a researcher could be struggled with which has no detailed indication.
Conducting a SR/MA in tropical medicine and health may be difficult especially for young researchers; therefore, understanding of its essential steps is crucial. It is not easy to be done as there are obstacles that could face the researcher. To solve those hindrances, we recommend a flow diagram (Fig. 1 ) which illustrates a detailed and step-by-step the stages for SR/MA studies. This methodology study aimed to provide a step-by-step approach mainly for beginners and junior researchers, in the field of tropical medicine and other health care fields, on how to properly and succinctly conduct a SR/MA; all the steps here depicts our experience and expertise combined with the already well known and accepted international guidance.
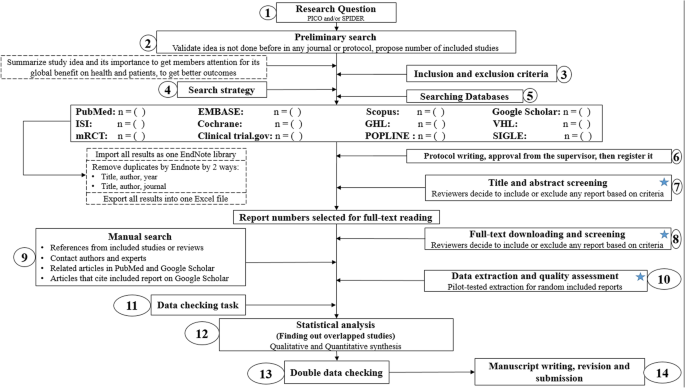
Detailed flow diagram guideline for systematic review and meta-analysis steps. Note : Star icon refers to “2–3 reviewers screen independently”
Methods and results
Detailed steps for conducting any systematic review and meta-analysis.
We searched the methods reported in published SR/MA in tropical medicine and other healthcare fields besides the published guidelines like Cochrane guidelines {Higgins, 2011 #7} [ 4 ] to collect the best low-bias method for each step of SR/MA conduction steps. Furthermore, we used guidelines that we apply in studies for all SR/MA steps. We combined these methods in order to conclude and conduct a detailed flow diagram that shows the SR/MA steps how being conducted.
Any SR/MA must follow the widely accepted Preferred Reporting Items for Systematic Review and Meta-analysis statement (PRISMA checklist 2009) (Additional file 5 : Table S1) [ 5 ].
We proposed our methods according to a valid explanatory simulation example choosing the topic of “evaluating safety of Ebola vaccine,” as it is known that Ebola is a very rare tropical disease but fatal. All the explained methods feature the standards followed internationally, with our compiled experience in the conduct of SR beside it, which we think proved some validity. This is a SR under conduct by a couple of researchers teaming in a research group, moreover, as the outbreak of Ebola which took place (2013–2016) in Africa resulted in a significant mortality and morbidity. Furthermore, since there are many published and ongoing trials assessing the safety of Ebola vaccines, we thought this would provide a great opportunity to tackle this hotly debated issue. Moreover, Ebola started to fire again and new fatal outbreak appeared in the Democratic Republic of Congo since August 2018, which caused infection to more than 1000 people according to the World Health Organization, and 629 people have been killed till now. Hence, it is considered the second worst Ebola outbreak, after the first one in West Africa in 2014 , which infected more than 26,000 and killed about 11,300 people along outbreak course.
Research question and objectives
Like other study designs, the research question of SR/MA should be feasible, interesting, novel, ethical, and relevant. Therefore, a clear, logical, and well-defined research question should be formulated. Usually, two common tools are used: PICO or SPIDER. PICO (Population, Intervention, Comparison, Outcome) is used mostly in quantitative evidence synthesis. Authors demonstrated that PICO holds more sensitivity than the more specific SPIDER approach [ 6 ]. SPIDER (Sample, Phenomenon of Interest, Design, Evaluation, Research type) was proposed as a method for qualitative and mixed methods search.
We here recommend a combined approach of using either one or both the SPIDER and PICO tools to retrieve a comprehensive search depending on time and resources limitations. When we apply this to our assumed research topic, being of qualitative nature, the use of SPIDER approach is more valid.
PICO is usually used for systematic review and meta-analysis of clinical trial study. For the observational study (without intervention or comparator), in many tropical and epidemiological questions, it is usually enough to use P (Patient) and O (outcome) only to formulate a research question. We must indicate clearly the population (P), then intervention (I) or exposure. Next, it is necessary to compare (C) the indicated intervention with other interventions, i.e., placebo. Finally, we need to clarify which are our relevant outcomes.
To facilitate comprehension, we choose the Ebola virus disease (EVD) as an example. Currently, the vaccine for EVD is being developed and under phase I, II, and III clinical trials; we want to know whether this vaccine is safe and can induce sufficient immunogenicity to the subjects.
An example of a research question for SR/MA based on PICO for this issue is as follows: How is the safety and immunogenicity of Ebola vaccine in human? (P: healthy subjects (human), I: vaccination, C: placebo, O: safety or adverse effects)
Preliminary research and idea validation
We recommend a preliminary search to identify relevant articles, ensure the validity of the proposed idea, avoid duplication of previously addressed questions, and assure that we have enough articles for conducting its analysis. Moreover, themes should focus on relevant and important health-care issues, consider global needs and values, reflect the current science, and be consistent with the adopted review methods. Gaining familiarity with a deep understanding of the study field through relevant videos and discussions is of paramount importance for better retrieval of results. If we ignore this step, our study could be canceled whenever we find out a similar study published before. This means we are wasting our time to deal with a problem that has been tackled for a long time.
To do this, we can start by doing a simple search in PubMed or Google Scholar with search terms Ebola AND vaccine. While doing this step, we identify a systematic review and meta-analysis of determinant factors influencing antibody response from vaccination of Ebola vaccine in non-human primate and human [ 7 ], which is a relevant paper to read to get a deeper insight and identify gaps for better formulation of our research question or purpose. We can still conduct systematic review and meta-analysis of Ebola vaccine because we evaluate safety as a different outcome and different population (only human).
Inclusion and exclusion criteria
Eligibility criteria are based on the PICO approach, study design, and date. Exclusion criteria mostly are unrelated, duplicated, unavailable full texts, or abstract-only papers. These exclusions should be stated in advance to refrain the researcher from bias. The inclusion criteria would be articles with the target patients, investigated interventions, or the comparison between two studied interventions. Briefly, it would be articles which contain information answering our research question. But the most important is that it should be clear and sufficient information, including positive or negative, to answer the question.
For the topic we have chosen, we can make inclusion criteria: (1) any clinical trial evaluating the safety of Ebola vaccine and (2) no restriction regarding country, patient age, race, gender, publication language, and date. Exclusion criteria are as follows: (1) study of Ebola vaccine in non-human subjects or in vitro studies; (2) study with data not reliably extracted, duplicate, or overlapping data; (3) abstract-only papers as preceding papers, conference, editorial, and author response theses and books; (4) articles without available full text available; and (5) case reports, case series, and systematic review studies. The PRISMA flow diagram template that is used in SR/MA studies can be found in Fig. 2 .
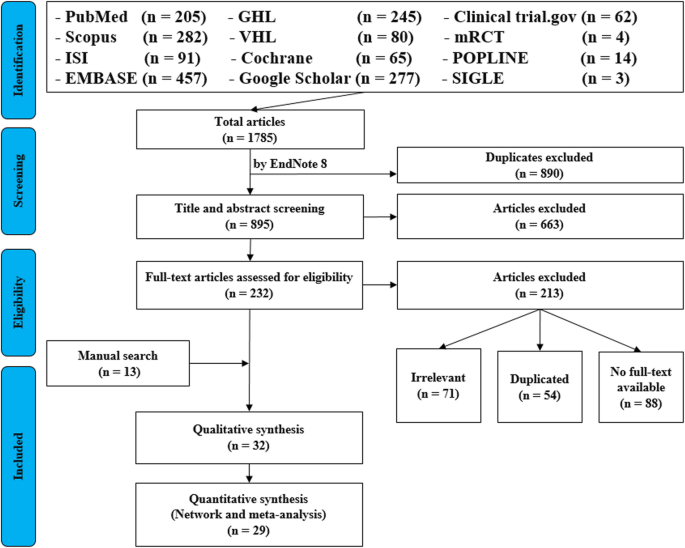
PRISMA flow diagram of studies’ screening and selection
Search strategy
A standard search strategy is used in PubMed, then later it is modified according to each specific database to get the best relevant results. The basic search strategy is built based on the research question formulation (i.e., PICO or PICOS). Search strategies are constructed to include free-text terms (e.g., in the title and abstract) and any appropriate subject indexing (e.g., MeSH) expected to retrieve eligible studies, with the help of an expert in the review topic field or an information specialist. Additionally, we advise not to use terms for the Outcomes as their inclusion might hinder the database being searched to retrieve eligible studies because the used outcome is not mentioned obviously in the articles.
The improvement of the search term is made while doing a trial search and looking for another relevant term within each concept from retrieved papers. To search for a clinical trial, we can use these descriptors in PubMed: “clinical trial”[Publication Type] OR “clinical trials as topic”[MeSH terms] OR “clinical trial”[All Fields]. After some rounds of trial and refinement of search term, we formulate the final search term for PubMed as follows: (ebola OR ebola virus OR ebola virus disease OR EVD) AND (vaccine OR vaccination OR vaccinated OR immunization) AND (“clinical trial”[Publication Type] OR “clinical trials as topic”[MeSH Terms] OR “clinical trial”[All Fields]). Because the study for this topic is limited, we do not include outcome term (safety and immunogenicity) in the search term to capture more studies.
Search databases, import all results to a library, and exporting to an excel sheet
According to the AMSTAR guidelines, at least two databases have to be searched in the SR/MA [ 8 ], but as you increase the number of searched databases, you get much yield and more accurate and comprehensive results. The ordering of the databases depends mostly on the review questions; being in a study of clinical trials, you will rely mostly on Cochrane, mRCTs, or International Clinical Trials Registry Platform (ICTRP). Here, we propose 12 databases (PubMed, Scopus, Web of Science, EMBASE, GHL, VHL, Cochrane, Google Scholar, Clinical trials.gov , mRCTs, POPLINE, and SIGLE), which help to cover almost all published articles in tropical medicine and other health-related fields. Among those databases, POPLINE focuses on reproductive health. Researchers should consider to choose relevant database according to the research topic. Some databases do not support the use of Boolean or quotation; otherwise, there are some databases that have special searching way. Therefore, we need to modify the initial search terms for each database to get appreciated results; therefore, manipulation guides for each online database searches are presented in Additional file 5 : Table S2. The detailed search strategy for each database is found in Additional file 5 : Table S3. The search term that we created in PubMed needs customization based on a specific characteristic of the database. An example for Google Scholar advanced search for our topic is as follows:
With all of the words: ebola virus
With at least one of the words: vaccine vaccination vaccinated immunization
Where my words occur: in the title of the article
With all of the words: EVD
Finally, all records are collected into one Endnote library in order to delete duplicates and then to it export into an excel sheet. Using remove duplicating function with two options is mandatory. All references which have (1) the same title and author, and published in the same year, and (2) the same title and author, and published in the same journal, would be deleted. References remaining after this step should be exported to an excel file with essential information for screening. These could be the authors’ names, publication year, journal, DOI, URL link, and abstract.
Protocol writing and registration
Protocol registration at an early stage guarantees transparency in the research process and protects from duplication problems. Besides, it is considered a documented proof of team plan of action, research question, eligibility criteria, intervention/exposure, quality assessment, and pre-analysis plan. It is recommended that researchers send it to the principal investigator (PI) to revise it, then upload it to registry sites. There are many registry sites available for SR/MA like those proposed by Cochrane and Campbell collaborations; however, we recommend registering the protocol into PROSPERO as it is easier. The layout of a protocol template, according to PROSPERO, can be found in Additional file 5 : File S1.
Title and abstract screening
Decisions to select retrieved articles for further assessment are based on eligibility criteria, to minimize the chance of including non-relevant articles. According to the Cochrane guidance, two reviewers are a must to do this step, but as for beginners and junior researchers, this might be tiresome; thus, we propose based on our experience that at least three reviewers should work independently to reduce the chance of error, particularly in teams with a large number of authors to add more scrutiny and ensure proper conduct. Mostly, the quality with three reviewers would be better than two, as two only would have different opinions from each other, so they cannot decide, while the third opinion is crucial. And here are some examples of systematic reviews which we conducted following the same strategy (by a different group of researchers in our research group) and published successfully, and they feature relevant ideas to tropical medicine and disease [ 9 , 10 , 11 ].
In this step, duplications will be removed manually whenever the reviewers find them out. When there is a doubt about an article decision, the team should be inclusive rather than exclusive, until the main leader or PI makes a decision after discussion and consensus. All excluded records should be given exclusion reasons.
Full text downloading and screening
Many search engines provide links for free to access full-text articles. In case not found, we can search in some research websites as ResearchGate, which offer an option of direct full-text request from authors. Additionally, exploring archives of wanted journals, or contacting PI to purchase it if available. Similarly, 2–3 reviewers work independently to decide about included full texts according to eligibility criteria, with reporting exclusion reasons of articles. In case any disagreement has occurred, the final decision has to be made by discussion.
Manual search
One has to exhaust all possibilities to reduce bias by performing an explicit hand-searching for retrieval of reports that may have been dropped from first search [ 12 ]. We apply five methods to make manual searching: searching references from included studies/reviews, contacting authors and experts, and looking at related articles/cited articles in PubMed and Google Scholar.
We describe here three consecutive methods to increase and refine the yield of manual searching: firstly, searching reference lists of included articles; secondly, performing what is known as citation tracking in which the reviewers track all the articles that cite each one of the included articles, and this might involve electronic searching of databases; and thirdly, similar to the citation tracking, we follow all “related to” or “similar” articles. Each of the abovementioned methods can be performed by 2–3 independent reviewers, and all the possible relevant article must undergo further scrutiny against the inclusion criteria, after following the same records yielded from electronic databases, i.e., title/abstract and full-text screening.
We propose an independent reviewing by assigning each member of the teams a “tag” and a distinct method, to compile all the results at the end for comparison of differences and discussion and to maximize the retrieval and minimize the bias. Similarly, the number of included articles has to be stated before addition to the overall included records.
Data extraction and quality assessment
This step entitles data collection from included full-texts in a structured extraction excel sheet, which is previously pilot-tested for extraction using some random studies. We recommend extracting both adjusted and non-adjusted data because it gives the most allowed confounding factor to be used in the analysis by pooling them later [ 13 ]. The process of extraction should be executed by 2–3 independent reviewers. Mostly, the sheet is classified into the study and patient characteristics, outcomes, and quality assessment (QA) tool.
Data presented in graphs should be extracted by software tools such as Web plot digitizer [ 14 ]. Most of the equations that can be used in extraction prior to analysis and estimation of standard deviation (SD) from other variables is found inside Additional file 5 : File S2 with their references as Hozo et al. [ 15 ], Xiang et al. [ 16 ], and Rijkom et al. [ 17 ]. A variety of tools are available for the QA, depending on the design: ROB-2 Cochrane tool for randomized controlled trials [ 18 ] which is presented as Additional file 1 : Figure S1 and Additional file 2 : Figure S2—from a previous published article data—[ 19 ], NIH tool for observational and cross-sectional studies [ 20 ], ROBINS-I tool for non-randomize trials [ 21 ], QUADAS-2 tool for diagnostic studies, QUIPS tool for prognostic studies, CARE tool for case reports, and ToxRtool for in vivo and in vitro studies. We recommend that 2–3 reviewers independently assess the quality of the studies and add to the data extraction form before the inclusion into the analysis to reduce the risk of bias. In the NIH tool for observational studies—cohort and cross-sectional—as in this EBOLA case, to evaluate the risk of bias, reviewers should rate each of the 14 items into dichotomous variables: yes, no, or not applicable. An overall score is calculated by adding all the items scores as yes equals one, while no and NA equals zero. A score will be given for every paper to classify them as poor, fair, or good conducted studies, where a score from 0–5 was considered poor, 6–9 as fair, and 10–14 as good.
In the EBOLA case example above, authors can extract the following information: name of authors, country of patients, year of publication, study design (case report, cohort study, or clinical trial or RCT), sample size, the infected point of time after EBOLA infection, follow-up interval after vaccination time, efficacy, safety, adverse effects after vaccinations, and QA sheet (Additional file 6 : Data S1).
Data checking
Due to the expected human error and bias, we recommend a data checking step, in which every included article is compared with its counterpart in an extraction sheet by evidence photos, to detect mistakes in data. We advise assigning articles to 2–3 independent reviewers, ideally not the ones who performed the extraction of those articles. When resources are limited, each reviewer is assigned a different article than the one he extracted in the previous stage.
Statistical analysis
Investigators use different methods for combining and summarizing findings of included studies. Before analysis, there is an important step called cleaning of data in the extraction sheet, where the analyst organizes extraction sheet data in a form that can be read by analytical software. The analysis consists of 2 types namely qualitative and quantitative analysis. Qualitative analysis mostly describes data in SR studies, while quantitative analysis consists of two main types: MA and network meta-analysis (NMA). Subgroup, sensitivity, cumulative analyses, and meta-regression are appropriate for testing whether the results are consistent or not and investigating the effect of certain confounders on the outcome and finding the best predictors. Publication bias should be assessed to investigate the presence of missing studies which can affect the summary.
To illustrate basic meta-analysis, we provide an imaginary data for the research question about Ebola vaccine safety (in terms of adverse events, 14 days after injection) and immunogenicity (Ebola virus antibodies rise in geometric mean titer, 6 months after injection). Assuming that from searching and data extraction, we decided to do an analysis to evaluate Ebola vaccine “A” safety and immunogenicity. Other Ebola vaccines were not meta-analyzed because of the limited number of studies (instead, it will be included for narrative review). The imaginary data for vaccine safety meta-analysis can be accessed in Additional file 7 : Data S2. To do the meta-analysis, we can use free software, such as RevMan [ 22 ] or R package meta [ 23 ]. In this example, we will use the R package meta. The tutorial of meta package can be accessed through “General Package for Meta-Analysis” tutorial pdf [ 23 ]. The R codes and its guidance for meta-analysis done can be found in Additional file 5 : File S3.
For the analysis, we assume that the study is heterogenous in nature; therefore, we choose a random effect model. We did an analysis on the safety of Ebola vaccine A. From the data table, we can see some adverse events occurring after intramuscular injection of vaccine A to the subject of the study. Suppose that we include six studies that fulfill our inclusion criteria. We can do a meta-analysis for each of the adverse events extracted from the studies, for example, arthralgia, from the results of random effect meta-analysis using the R meta package.
From the results shown in Additional file 3 : Figure S3, we can see that the odds ratio (OR) of arthralgia is 1.06 (0.79; 1.42), p value = 0.71, which means that there is no association between the intramuscular injection of Ebola vaccine A and arthralgia, as the OR is almost one, and besides, the P value is insignificant as it is > 0.05.
In the meta-analysis, we can also visualize the results in a forest plot. It is shown in Fig. 3 an example of a forest plot from the simulated analysis.
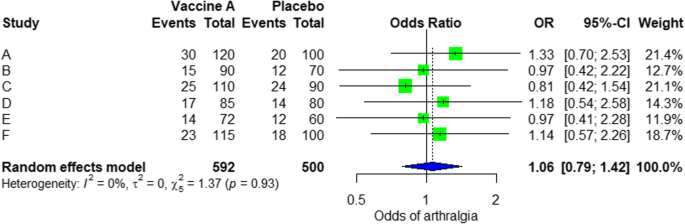
Random effect model forest plot for comparison of vaccine A versus placebo
From the forest plot, we can see six studies (A to F) and their respective OR (95% CI). The green box represents the effect size (in this case, OR) of each study. The bigger the box means the study weighted more (i.e., bigger sample size). The blue diamond shape represents the pooled OR of the six studies. We can see the blue diamond cross the vertical line OR = 1, which indicates no significance for the association as the diamond almost equalized in both sides. We can confirm this also from the 95% confidence interval that includes one and the p value > 0.05.
For heterogeneity, we see that I 2 = 0%, which means no heterogeneity is detected; the study is relatively homogenous (it is rare in the real study). To evaluate publication bias related to the meta-analysis of adverse events of arthralgia, we can use the metabias function from the R meta package (Additional file 4 : Figure S4) and visualization using a funnel plot. The results of publication bias are demonstrated in Fig. 4 . We see that the p value associated with this test is 0.74, indicating symmetry of the funnel plot. We can confirm it by looking at the funnel plot.
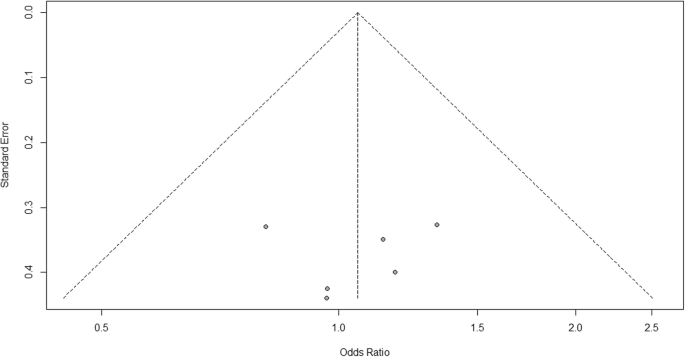
Publication bias funnel plot for comparison of vaccine A versus placebo
Looking at the funnel plot, the number of studies at the left and right side of the funnel plot is the same; therefore, the plot is symmetry, indicating no publication bias detected.
Sensitivity analysis is a procedure used to discover how different values of an independent variable will influence the significance of a particular dependent variable by removing one study from MA. If all included study p values are < 0.05, hence, removing any study will not change the significant association. It is only performed when there is a significant association, so if the p value of MA done is 0.7—more than one—the sensitivity analysis is not needed for this case study example. If there are 2 studies with p value > 0.05, removing any of the two studies will result in a loss of the significance.
Double data checking
For more assurance on the quality of results, the analyzed data should be rechecked from full-text data by evidence photos, to allow an obvious check for the PI of the study.
Manuscript writing, revision, and submission to a journal
Writing based on four scientific sections: introduction, methods, results, and discussion, mostly with a conclusion. Performing a characteristic table for study and patient characteristics is a mandatory step which can be found as a template in Additional file 5 : Table S3.
After finishing the manuscript writing, characteristics table, and PRISMA flow diagram, the team should send it to the PI to revise it well and reply to his comments and, finally, choose a suitable journal for the manuscript which fits with considerable impact factor and fitting field. We need to pay attention by reading the author guidelines of journals before submitting the manuscript.
The role of evidence-based medicine in biomedical research is rapidly growing. SR/MAs are also increasing in the medical literature. This paper has sought to provide a comprehensive approach to enable reviewers to produce high-quality SR/MAs. We hope that readers could gain general knowledge about how to conduct a SR/MA and have the confidence to perform one, although this kind of study requires complex steps compared to narrative reviews.
Having the basic steps for conduction of MA, there are many advanced steps that are applied for certain specific purposes. One of these steps is meta-regression which is performed to investigate the association of any confounder and the results of the MA. Furthermore, there are other types rather than the standard MA like NMA and MA. In NMA, we investigate the difference between several comparisons when there were not enough data to enable standard meta-analysis. It uses both direct and indirect comparisons to conclude what is the best between the competitors. On the other hand, mega MA or MA of patients tend to summarize the results of independent studies by using its individual subject data. As a more detailed analysis can be done, it is useful in conducting repeated measure analysis and time-to-event analysis. Moreover, it can perform analysis of variance and multiple regression analysis; however, it requires homogenous dataset and it is time-consuming in conduct [ 24 ].
Conclusions
Systematic review/meta-analysis steps include development of research question and its validation, forming criteria, search strategy, searching databases, importing all results to a library and exporting to an excel sheet, protocol writing and registration, title and abstract screening, full-text screening, manual searching, extracting data and assessing its quality, data checking, conducting statistical analysis, double data checking, manuscript writing, revising, and submitting to a journal.
Availability of data and materials
Not applicable.
Abbreviations
Network meta-analysis
Principal investigator
Population, Intervention, Comparison, Outcome
Preferred Reporting Items for Systematic Review and Meta-analysis statement
Quality assessment
Sample, Phenomenon of Interest, Design, Evaluation, Research type
Systematic review and meta-analyses
Bello A, Wiebe N, Garg A, Tonelli M. Evidence-based decision-making 2: systematic reviews and meta-analysis. Methods Mol Biol (Clifton, NJ). 2015;1281:397–416.
Article Google Scholar
Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. J R Soc Med. 2003;96(3):118–21.
Rys P, Wladysiuk M, Skrzekowska-Baran I, Malecki MT. Review articles, systematic reviews and meta-analyses: which can be trusted? Polskie Archiwum Medycyny Wewnetrznej. 2009;119(3):148–56.
PubMed Google Scholar
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579.
Gross L, Lhomme E, Pasin C, Richert L, Thiebaut R. Ebola vaccine development: systematic review of pre-clinical and clinical studies, and meta-analysis of determinants of antibody response variability after vaccination. Int J Infect Dis. 2018;74:83–96.
Article CAS Google Scholar
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, ... Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Giang HTN, Banno K, Minh LHN, Trinh LT, Loc LT, Eltobgy A, et al. Dengue hemophagocytic syndrome: a systematic review and meta-analysis on epidemiology, clinical signs, outcomes, and risk factors. Rev Med Virol. 2018;28(6):e2005.
Morra ME, Altibi AMA, Iqtadar S, Minh LHN, Elawady SS, Hallab A, et al. Definitions for warning signs and signs of severe dengue according to the WHO 2009 classification: systematic review of literature. Rev Med Virol. 2018;28(4):e1979.
Morra ME, Van Thanh L, Kamel MG, Ghazy AA, Altibi AMA, Dat LM, et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta-analysis. Rev Med Virol. 2018;28(3):e1977.
Vassar M, Atakpo P, Kash MJ. Manual search approaches used by systematic reviewers in dermatology. Journal of the Medical Library Association: JMLA. 2016;104(4):302.
Naunheim MR, Remenschneider AK, Scangas GA, Bunting GW, Deschler DG. The effect of initial tracheoesophageal voice prosthesis size on postoperative complications and voice outcomes. Ann Otol Rhinol Laryngol. 2016;125(6):478–84.
Rohatgi AJaiWa. Web Plot Digitizer. ht tp. 2014;2.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
Van Rijkom HM, Truin GJ, Van’t Hof MA. A meta-analysis of clinical studies on the caries-inhibiting effect of fluoride gel treatment. Carries Res. 1998;32(2):83–92.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Tawfik GM, Tieu TM, Ghozy S, Makram OM, Samuel P, Abdelaal A, et al. Speech efficacy, safety and factors affecting lifetime of voice prostheses in patients with laryngeal cancer: a systematic review and network meta-analysis of randomized controlled trials. J Clin Oncol. 2018;36(15_suppl):e18031-e.
Wannemuehler TJ, Lobo BC, Johnson JD, Deig CR, Ting JY, Gregory RL. Vibratory stimulus reduces in vitro biofilm formation on tracheoesophageal voice prostheses. Laryngoscope. 2016;126(12):2752–7.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355.
RevMan The Cochrane Collaboration %J Copenhagen TNCCTCC. Review Manager (RevMan). 5.0. 2008.
Schwarzer GJRn. meta: An R package for meta-analysis. 2007;7(3):40-45.
Google Scholar
Simms LLH. Meta-analysis versus mega-analysis: is there a difference? Oral budesonide for the maintenance of remission in Crohn’s disease: Faculty of Graduate Studies, University of Western Ontario; 1998.
Download references
Acknowledgements
This study was conducted (in part) at the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University, Japan.
Author information
Authors and affiliations.
Faculty of Medicine, Ain Shams University, Cairo, Egypt
Gehad Mohamed Tawfik
Online research Club http://www.onlineresearchclub.org/
Gehad Mohamed Tawfik, Kadek Agus Surya Dila, Muawia Yousif Fadlelmola Mohamed, Dao Ngoc Hien Tam, Nguyen Dang Kien & Ali Mahmoud Ahmed
Pratama Giri Emas Hospital, Singaraja-Amlapura street, Giri Emas village, Sawan subdistrict, Singaraja City, Buleleng, Bali, 81171, Indonesia
Kadek Agus Surya Dila
Faculty of Medicine, University of Khartoum, Khartoum, Sudan
Muawia Yousif Fadlelmola Mohamed
Nanogen Pharmaceutical Biotechnology Joint Stock Company, Ho Chi Minh City, Vietnam
Dao Ngoc Hien Tam
Department of Obstetrics and Gynecology, Thai Binh University of Medicine and Pharmacy, Thai Binh, Vietnam
Nguyen Dang Kien
Faculty of Medicine, Al-Azhar University, Cairo, Egypt
Ali Mahmoud Ahmed
Evidence Based Medicine Research Group & Faculty of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City, 70000, Vietnam
Nguyen Tien Huy
Faculty of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City, 70000, Vietnam
Department of Clinical Product Development, Institute of Tropical Medicine (NEKKEN), Leading Graduate School Program, and Graduate School of Biomedical Sciences, Nagasaki University, 1-12-4 Sakamoto, Nagasaki, 852-8523, Japan
You can also search for this author in PubMed Google Scholar
Contributions
NTH and GMT were responsible for the idea and its design. The figure was done by GMT. All authors contributed to the manuscript writing and approval of the final version.
Corresponding author
Correspondence to Nguyen Tien Huy .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:.
Figure S1. Risk of bias assessment graph of included randomized controlled trials. (TIF 20 kb)
Additional file 2:
Figure S2. Risk of bias assessment summary. (TIF 69 kb)
Additional file 3:
Figure S3. Arthralgia results of random effect meta-analysis using R meta package. (TIF 20 kb)
Additional file 4:
Figure S4. Arthralgia linear regression test of funnel plot asymmetry using R meta package. (TIF 13 kb)
Additional file 5:
Table S1. PRISMA 2009 Checklist. Table S2. Manipulation guides for online database searches. Table S3. Detailed search strategy for twelve database searches. Table S4. Baseline characteristics of the patients in the included studies. File S1. PROSPERO protocol template file. File S2. Extraction equations that can be used prior to analysis to get missed variables. File S3. R codes and its guidance for meta-analysis done for comparison between EBOLA vaccine A and placebo. (DOCX 49 kb)
Additional file 6:
Data S1. Extraction and quality assessment data sheets for EBOLA case example. (XLSX 1368 kb)
Additional file 7:
Data S2. Imaginary data for EBOLA case example. (XLSX 10 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Tawfik, G.M., Dila, K.A.S., Mohamed, M.Y.F. et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health 47 , 46 (2019). https://doi.org/10.1186/s41182-019-0165-6
Download citation
Received : 30 January 2019
Accepted : 24 May 2019
Published : 01 August 2019
DOI : https://doi.org/10.1186/s41182-019-0165-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Tropical Medicine and Health
ISSN: 1349-4147
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search

How to Write a Systematic Review of the Literature
Affiliations.
- 1 1 Texas Tech University, Lubbock, TX, USA.
- 2 2 University of Florida, Gainesville, FL, USA.
- PMID: 29283007
- DOI: 10.1177/1937586717747384
This article provides a step-by-step approach to conducting and reporting systematic literature reviews (SLRs) in the domain of healthcare design and discusses some of the key quality issues associated with SLRs. SLR, as the name implies, is a systematic way of collecting, critically evaluating, integrating, and presenting findings from across multiple research studies on a research question or topic of interest. SLR provides a way to assess the quality level and magnitude of existing evidence on a question or topic of interest. It offers a broader and more accurate level of understanding than a traditional literature review. A systematic review adheres to standardized methodologies/guidelines in systematic searching, filtering, reviewing, critiquing, interpreting, synthesizing, and reporting of findings from multiple publications on a topic/domain of interest. The Cochrane Collaboration is the most well-known and widely respected global organization producing SLRs within the healthcare field and a standard to follow for any researcher seeking to write a transparent and methodologically sound SLR. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), like the Cochrane Collaboration, was created by an international network of health-based collaborators and provides the framework for SLR to ensure methodological rigor and quality. The PRISMA statement is an evidence-based guide consisting of a checklist and flowchart intended to be used as tools for authors seeking to write SLR and meta-analyses.
Keywords: evidence based design; healthcare design; systematic literature review.
PubMed Disclaimer
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
- Suicidal Ideation. Harmer B, Lee S, Rizvi A, Saadabadi A. Harmer B, et al. 2024 Apr 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. 2024 Apr 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 33351435 Free Books & Documents.
- The future of Cochrane Neonatal. Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
- Systematic Reviews in Sports Medicine. DiSilvestro KJ, Tjoumakaris FP, Maltenfort MG, Spindler KP, Freedman KB. DiSilvestro KJ, et al. Am J Sports Med. 2016 Feb;44(2):533-8. doi: 10.1177/0363546515580290. Epub 2015 Apr 21. Am J Sports Med. 2016. PMID: 25899433 Review.
- The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Liberati A, et al. J Clin Epidemiol. 2009 Oct;62(10):e1-34. doi: 10.1016/j.jclinepi.2009.06.006. Epub 2009 Jul 23. J Clin Epidemiol. 2009. PMID: 19631507
- A systematic review and meta-analysis of balance training in patients with chronic ankle instability. Guo Y, Cheng T, Yang Z, Huang Y, Li M, Wang T. Guo Y, et al. Syst Rev. 2024 Feb 12;13(1):64. doi: 10.1186/s13643-024-02455-x. Syst Rev. 2024. PMID: 38347564 Free PMC article.
- Association between infection and the onset of giant cell arteritis and polymyalgia rheumatica: a systematic review and meta-analysis. Pacoureau L, Barde F, Seror R, Nguyen Y. Pacoureau L, et al. RMD Open. 2023 Nov;9(4):e003493. doi: 10.1136/rmdopen-2023-003493. RMD Open. 2023. PMID: 37949615 Free PMC article.
- From Social Rejection to Welfare Oblivion: Health and Mental Health in Juvenile Justice in Brazil, Colombia and Spain. Carbonell Á, Georgieva S, Navarro-Pérez JJ, Botija M. Carbonell Á, et al. Int J Environ Res Public Health. 2023 May 29;20(11):5989. doi: 10.3390/ijerph20115989. Int J Environ Res Public Health. 2023. PMID: 37297594 Free PMC article. Review.
- Why is didactic transposition in disaster education needed by prospective elementary school teachers? Noviana E, Syahza A, Putra ZH, Hadriana, Yustina, Erlinda S, Putri DR, Rusandi MA, Biondi Situmorang DD. Noviana E, et al. Heliyon. 2023 Apr 18;9(4):e15413. doi: 10.1016/j.heliyon.2023.e15413. eCollection 2023 Apr. Heliyon. 2023. PMID: 37128333 Free PMC article. Review.
- Comparative analysis of efficacy of different combination therapies of α-receptor blockers and traditional Chinese medicine external therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: Bayesian network meta-analysis. Zhang K, Zhang Y, Hong S, Cao Y, Liu C. Zhang K, et al. PLoS One. 2023 Apr 20;18(4):e0280821. doi: 10.1371/journal.pone.0280821. eCollection 2023. PLoS One. 2023. PMID: 37079509 Free PMC article.
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
Other Literature Sources
- scite Smart Citations

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Off-Campus Access
- Find Resources
- Research Support
- Reserve Space
- --> X
- YouTube
- HSHSL Updates RSS Feed
- Connective Issues Newsletter

601 West Lombard Street Baltimore MD 21201-1512 Reference: 410-706-7996 Circulation: 410-706-7928
Systematic Review Service: Steps in a Systematic Review
- Introduction
- Steps in a Systematic Review
- PRISMA and Other Standards
- Critical Appraisal of Studies
- What Type of Review is Right for You?
The Process
The following information is intended to be a general introduction to the process of conducting systematic reviews. For further details and guidance, refer to the Standards section of this guide.
For a video overview of the systematic review process, visit our tutorial.
1. Determine if a Systematic Review is Necessary
Before starting your review, determine if a systematic review is the best approach to answer your question. (What are other types of review to consider?)
- Will it fill a meaningful gap in existing literature?
- If you identify an existing review, assess its quality. (see box below)
- If a high quality review exists but was completed several years ago, a new review may be justified.
- Do you have the necessary time and resources to complete a systematic review?
3. Define Eligibility Criteria
One of the features that distinguishes a systematic review from a narrative review is the pre-specification of criteria for including and excluding studies in the review (eligibility criteria). Explicit criteria, based on the review’s scope and question(s), are used to include and exclude studies.
A large number of references (study titles and abstracts) will have been found at the searching stage of the review. A proportion of these will look as though they are relevant to the review's research questions. So, having explicit criteria against which to assess studies makes the process more efficient in terms of time.
More importantly, it also helps to avoid hidden bias by having clear consistent rules about which studies are being used to answer the review's specific research questions.
Each study needs to be compared against same criteria. To be included in the review, a study needs to meet all inclusion criteria and not meet any exclusion criteria. Inclusion/eligibility criteria iclude participants, interventions and comparisons and often study design. Outcomes are usually not part of the criteria, though some reviews do legitimately restrict eligibility to specific outcomes.
5. Search for Studies
A systematic search to identify studies must be comprehensive and it must strike a balance between recall and precision. In other words, don't expect to retrieve only relevant articles.
Most studies used in the review will be identified using electronic databases (e.g. PubMed), but identifying unpublished studies is important as well. Librarians can help to identify sources for finding grey literature.
Expect a large number of results. Depending on the scope of your topic, your search may result in as few as a couple hundred or as many as several thousand articles.
Some key steps in searching for studies include:
- Identifying databases to be searched.
- Identifying search terminology.
- Constructing and running database searches.
- Conducting hand searches of specialized journals.
- Searching reference lists of relevant studies.
- Contacting recognized experts working in the field.
- Searching relevant grey literature sources (e.g. clinical trials registers, conference proceedings).
It’s important to note that the IOM recommends working with a librarian to plan and executive your searches. Plan to meet with a librarian at the HSHSL to discuss your review.
7. Extract Data
Here, data simply refers to information about or details from a study including its methods and design, participants, setting, interventions, and results. Two researchers should independently extract data from each study in order to minimize errors and reduce the potential for bias. At a minimum, one researcher should extract data with a second person checking for accuracy.
To standardize the process and improve the validity of the results, time and thought should be given to creating a data extraction form. These can be created using general word processing software or spreadsheets, or you can use the data extraction features in Covidence .
It is important to collect enough information during this process to sufficiently assess each study. Collecting too much or too little information may be a waste of time and result in the omission of crucial data.
8. Assess the Quality of Studies
Assessment of study quality gives an indication of the strength of the evidence provided by your review. It helps determine whether the studies are vigorous enough to guide treatment, prevention, diagnostic or policy decisions.
Quality assessment of any study is likely to consider:
- Appropriateness of study design to the research objective
- Risk of bias
- Choice of outcome measure
- Statistical issues
- Quality of reporting
- Quality of the intervention
- Generalizability
From: Centre for Reviews and Dissemination, University of York (2008) Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care .
2. Clarify the Question
A clearly defined, focused review begins with a well-framed question. It provides the framework for the entire review.
- Clearly state the objectives of the review (what question are you trying to answer?)
- P opulation
- I nterventions
- C omparisons
- S tudy Design
Systematic Reviews are not limited to questions about effects of interventions, they may address trends, accuracy of diagnostic tests, effectiveness of programs, etc.
Ask yourself...
- Is the question answerable?
- Are there measurable constructs?
- Is it practical or relevant for policy/practice?
4. Create a Protocol
The review protocol sets out the methods to be used in the review and provides an explicit plan for your work. Decisions about the review question, inclusion criteria, search strategy, study selection, data extraction, quality assessment, data synthesis and plans for dissemination should be addressed. If modifications to the protocol are required, these should be clearly documented and justified. Modifications may arise from a clearer understanding of the review question, and should not be made because of an awareness of the results of individual studies.
Consider registering your protocol. PROSPERO , from the Centre for Reviews and Dissemination at the University of York, is an international database of registered reviews in health and social care. Key features from registered reviews and recorded and maintained as a permanent record. Registration helps to promote transparency in the review process and also reduces the potential for duplication.
6. Select Studies
A large quantity of studies needs to be assessed at this stage of the review. This process should involve at least two members of your group to help reduce bias. It is useful to develop an appropriate form to help select and keep track of articles that meet eligibility criteria. To be included, a study must meet all inclusion criteria and not meet any exclusion criteria.
Employ the following process:
- Examine titles/abstracts
- Retrieve the full-text of relevant studies
- Examine the full-text to determine eligibility
- Make final decisions on study inclusion
Remember to document your decisions! It is important to have a record of decisions made in order to ensure reproducibility and minimize errors.

This PRISMA flow diagram shows the number of studies remaining at each stage. It's a simple and useful way of documenting the study selection process and s hould be included in your final report.
9. Synthesize Data and Write the Report
Synthesis involves combining the results of the studies included in the review, summarizing their findings and drawing reliable conclusions based on the quality of the evidence. Synthesis may be done quantitatively using statistical techniques, such as a meta-analysis, or through a narrative approach.
In general, making recommendations for practice does not fall within the purview of systematic reviews. This is typically the domain of clinical practice guidelines. Systematic review authors can make conclusions about the need for further research or draw conclusions about the usefulness of an intervention.
When preparing your report or article, refer to the PRISMA Checklist . Many commissioning bodies and journals have adopted PRISMA as the required methods for reporting systematic reviews.
- << Previous: Introduction
- Next: Protocols >>
- Last Updated: Feb 1, 2023 3:10 PM
- URL: https://guides.hshsl.umaryland.edu/systematic
Projects Maintained by the HSHSL
- Alumni & Associates
- Congenital Heart Disease
- Corporate Services
- Embracing mHealth
- Faith Community Nursing
- Project SHARE Curriculum
- Citizen Science: Gearing Up For Discovery (edX)
- UMB Digital Archive
- Weise Gallery
University of Maryland Schools
- School of Dentistry
- Graduate School
- School of Law
- School of Medicine
- School of Nursing
- School of Pharmacy
- School of Social Work
Connective Issues E-Newsletter
Read Our Latest Issue
Join our subscribers!
Contact Us | Hours | Directions | Privacy Policy | Disclaimers | Supporting the Library | Suggestion Box | HSHSL Building Work Order | Web Accessibility | Diversity Statement
601 W. Lombard Street | Baltimore, Maryland 21201-1512 | 410-706-7995
Harvey Cushing/John Hay Whitney Medical Library
- Collections
- Research Help
YSN Doctoral Programs: Steps in Conducting a Literature Review
- Biomedical Databases
- Global (Public Health) Databases
- Soc. Sci., History, and Law Databases
- Grey Literature
- Trials Registers
- Data and Statistics
- Public Policy
- Google Tips
- Recommended Books
- Steps in Conducting a Literature Review
What is a literature review?
A literature review is an integrated analysis -- not just a summary-- of scholarly writings and other relevant evidence related directly to your research question. That is, it represents a synthesis of the evidence that provides background information on your topic and shows a association between the evidence and your research question.
A literature review may be a stand alone work or the introduction to a larger research paper, depending on the assignment. Rely heavily on the guidelines your instructor has given you.
Why is it important?
A literature review is important because it:
- Explains the background of research on a topic.
- Demonstrates why a topic is significant to a subject area.
- Discovers relationships between research studies/ideas.
- Identifies major themes, concepts, and researchers on a topic.
- Identifies critical gaps and points of disagreement.
- Discusses further research questions that logically come out of the previous studies.
APA7 Style resources
APA Style Blog - for those harder to find answers
1. Choose a topic. Define your research question.
Your literature review should be guided by your central research question. The literature represents background and research developments related to a specific research question, interpreted and analyzed by you in a synthesized way.
- Make sure your research question is not too broad or too narrow. Is it manageable?
- Begin writing down terms that are related to your question. These will be useful for searches later.
- If you have the opportunity, discuss your topic with your professor and your class mates.
2. Decide on the scope of your review
How many studies do you need to look at? How comprehensive should it be? How many years should it cover?
- This may depend on your assignment. How many sources does the assignment require?
3. Select the databases you will use to conduct your searches.
Make a list of the databases you will search.
Where to find databases:
- use the tabs on this guide
- Find other databases in the Nursing Information Resources web page
- More on the Medical Library web page
- ... and more on the Yale University Library web page
4. Conduct your searches to find the evidence. Keep track of your searches.
- Use the key words in your question, as well as synonyms for those words, as terms in your search. Use the database tutorials for help.
- Save the searches in the databases. This saves time when you want to redo, or modify, the searches. It is also helpful to use as a guide is the searches are not finding any useful results.
- Review the abstracts of research studies carefully. This will save you time.
- Use the bibliographies and references of research studies you find to locate others.
- Check with your professor, or a subject expert in the field, if you are missing any key works in the field.
- Ask your librarian for help at any time.
- Use a citation manager, such as EndNote as the repository for your citations. See the EndNote tutorials for help.
Review the literature
Some questions to help you analyze the research:
- What was the research question of the study you are reviewing? What were the authors trying to discover?
- Was the research funded by a source that could influence the findings?
- What were the research methodologies? Analyze its literature review, the samples and variables used, the results, and the conclusions.
- Does the research seem to be complete? Could it have been conducted more soundly? What further questions does it raise?
- If there are conflicting studies, why do you think that is?
- How are the authors viewed in the field? Has this study been cited? If so, how has it been analyzed?
Tips:
- Review the abstracts carefully.
- Keep careful notes so that you may track your thought processes during the research process.
- Create a matrix of the studies for easy analysis, and synthesis, across all of the studies.
- << Previous: Recommended Books
- Last Updated: Jan 4, 2024 10:52 AM
- URL: https://guides.library.yale.edu/YSNDoctoral

- Academic Writing Guides
How to Write a Literature Review? A Beginner’s Guide
Sooner or later in your academic path, you will be required to compose a literature review. So, it’s important to approach this task well-prepared and understand how to write a literature review inside out.
Are you interested in how to write lit review projects correctly and cover the subject comprehensively, from all angles? This article will explore the concept of review of literature , dwell on how to write a literature review in line with your professor’s expectations, and share a universal literature review template for your usage.
What Is the Purpose of a Literature Review?
To understand what should be included in a literature review , you need to understand its purpose and value in a larger work. A well-researched and written lit review usually addresses the following objectives:
- Inform . The fundamental purpose of any review of literature is to provide the foundation of knowledge on a specific topic or phenomenon. You explore what people have learned about it from prior studies and summarize those findings to inform your readers.
- Give credit . Another purpose of a lit review is to identify researchers who have contributed to the advancement of research on your chosen literature review topic and have produced the most valuable findings. This way, you pay tribute to those researchers and showcase your knowledge of the most considerable influencers.
- Identify gaps . By performing a thorough review of literature , you may not only discover what is known about your topic but also find out what it yet to be learned about it. As a result of reviewing the available evidence, you may identify gaps for addressing through your academic inquiry.
- Identify patterns . Those who know how to write a literature review can also effectively embrace data trends and patterns in the collected dataset. As a result, they can present a more nuanced analysis of the existing knowledge in your literature review and uncover dependencies that inform people’s understanding of certain phenomena and processes.
- Contextualize research . When you perform lit review writing, you can also create a spot for your own study within the broad field of your academic research interest. This way, you show to your readers that you can effectively navigate the landscape of your academic area.
These purposes lay the foundation for understanding how to write a literature review that will attain all academic goals. You simply need to use this list as your checklist for structuring an impactful lit review and including all vital data in it.
How to Write a Literature Review?
Now, we come to the main topic of this article – how to write a good literature review for dissertation projects, research papers, and other works. Follow the steps we’ve covered below to arrive at a consistent, logical piece of lit review .
Identify Relevant Sources
Any literature review writing starts with academic research. You should look for sources that explore your topic from various angles and provide valuable literature review findings to expand your knowledge on the subject. It’s best to look for subject-specific books first and then go through academic databases that publish journal articles. This way, you will start with the evidence of the highest reliability level and move on to expand your literature review dataset conveniently.
Screen Sources for Quality
The best solution on how to write a literature review without challenges is to rely on high-quality evidence. Your task is to research extensively in reliable academic databases to find peer-reviewed academic journals and books written by experts in your field. Don’t over-rely on online sources in your literature review, like blogs or opinion pieces, because they rarely possess the needed degree of credibility for an academic review. By choosing only industry-approved sources from qualified professionals, you can build a solid foundation for your writing and impress the audience.
Determine Data Patterns and Gaps
How to write a literature review of value for your readers? One of the best approaches is to go beyond mere summarization of what other researchers have found on the subject and to apply critical thinking and data categorization. This way, you will manage to uncover existing patterns and trends and examine those dependencies in your literature review. A systematic, critical approach is always evaluated much higher than a simple outline of what people say on your subject.
Draft an Outline
Now, it’s time to compose an outline for a literature review . The outline should include the main concepts you’re planning to cover in the literature review text and should structure the narrative consistently. By means of composing an outline before the actual writing process, you give yourself a hands-on roadmap for composing a logically flowing piece. As a result of using an outline, you will write the literature review faster and will avoid the risk of going off-topic.
Compose the Review
With a good and detailed outline, you should have no more problems or concerns about how to write a literature review . The writing process should go quickly and smoothly when you have all your evidence at your fingertips, categorized by themes and requiring only proper summarization in the text.
We recommend starting with a broad introduction to the topic and concepts related to it. You should give definitions and explain the topic’s features and components that require attention in the research process. After that, you may briefly outline the main sections of your review and then proceed to the exploration of each section in depth.
At times, your professor will give you a specific structure for review writing – such as the general introduction, coverage of theories, and then coverage of empirical evidence. At times, it may be a review of the data search strategy and a report on the identified resources that follow. In any case, you should follow the tutor’s prompt closely to ensure compliance with the task.
Make Use of This Generic Literature Review Template
Looking for a universal, ready-to-use literature review template ? Here is an effective literature review template that everyone can apply with minor tweaks to produce a high-quality review of literature .
LITERATURE REVIEW TEMPLATE
Introduction
- Introduce the topic of your literature review
- Examine its significance for your academic area
- Determine the scope of your literature review inquiry
- Give a brief outline of subtopics and sections included in your literature review
Body of the literature review
- Describe the subtopic and indicate how it relates to your literature review’s main idea
- Summarize the evidence available about it
- Compare the available data and voice your opinion
Conclusion
- Summarize the main points and findings from your literature review
- State the main contribution you have managed to achieve
- Identify the research gaps your literature review has revealed
Use this literature review template to pump your writing muscle and get ready for new literature review challenges.
More Pro Tips for Writing a Literature Review
If you’re still unsure about how to do a literature review with excellence, these pro tips may improve your understanding of this task type.
- Mind the audience . Understanding how to do a literature review for a research paper often has little to do with how to write literature review for thesis . This difference is explained by the fact that these types of academic work are of different lengths and pursue different scholarly goals. This way, you may need to cover only some basic seminal research in the review of literature for a research paper but will need to dig deeper into theoretical and applied research with deeper analysis and more critical thinking when dealing with a thesis.
- Mind the length . How long should a literature review be ? This is a vital question that you should answer before starting the outlining and writing process. Ask your professor if you’re not sure or apply the rule of thumb, where this section usually takes from 15% to 25% of the entire paper.
- Mind the structure . It’s important to cover all lit review aspects that your professor wants to see in the paper; otherwise, you risk getting a low grade even if your literature review is comprehensive and interesting. What should a literature review include ? In most cases, you will be required to cover some seminal research works in your literature review to show that you understand who the pioneers in the field are, and what contribution they have made to the topic’s exploration. Next, you should examine relevant theories that inform studies in your subject. At the end of the literature review, you should typically cite a variety of studies of applied nature, thus showing what empirical research is conducted in your academic field.
With these recommendations at your disposal, you’re sure to become much more proficient in how to do a lit review . If you need more help with a literature review project, welcome to use our professional and quick literature review writing service . Our experts know everything about how to write a literature review , so they will handle your literature review task with ease within the timeframe you set for them.

- Essay Writing Guides

- Systematic Review
- Open access
- Published: 14 June 2024
A systematic review and bioinformatic study on clinical, paraclinical, and genetic factors predisposing to stent restenosis following percutaneous coronary intervention
- Farzad Shahsanaei 1 ,
- Abdullah Gharibzadeh 1 ,
- Soudabeh Behrooj 1 ,
- Shahin Abbaszadeh 1 &
- Mahboobeh Nourmohammadi 1
BMC Cardiovascular Disorders volume 24 , Article number: 304 ( 2024 ) Cite this article
125 Accesses
Metrics details
Stent restenosis is a relatively common phenomenon among patients with coronary heart disease undergoing percutaneous coronary intervention (PCI). It seems that a set of clinical, laboratory, and even genetic factors make people susceptible to such a phenomenon and in fact, this is multi-factorial. We aimed to first determine the underlying clinical and laboratory risk factors for the occurrence of stent re-stenosis after PCI based on a systematic review study, and after that, through a bioinformatics study, to evaluate the related genes and microRNAs with the occurrence of stent re-stenosis.
In the first step, the manuscript databases including Medline, Web of Knowledge, Google Scholar, Scopus, and Cochrane were deeply searched by the two blinded investigators for all eligible studies based on the considered keywords to introduce clinical and laboratory determinants of stent re-stenosis. In the bioinformatic phase, and following a review of the literature to identify genes and microRNAs involved in restenosis, the interaction of each gene with other genes associated with stent re-stenosis was determined by GeneMANIA network analysis and Cytoscape software. Overall, 67 articles (including 40,789 patients) on clinical and biochemical predictors for stent restenosis and 25 articles on genetic determinants of this event were eligible for the final analysis. The predictors for this event were categorized into four subgroups patient-based parameters including traditional cardiovascular risk profiles, stent-based parameters including type and diametric characteristics of the stents used, coronary lesion-based parameters including several two target lesions and coronary involvement severity and laboratory-based parameters particularly related to activation of inflammatory processes. In the bioinformatic phase, we uncovered 42 genes that have been described to be involved in such a phenomenon considering a special position for genes encoding inflammatory cytokines. Also, 12 microRNAs have been pointed to be involved in targeting genes involved in stent re-stenosis.
Conclusions
The incidence of stent re-stenosis will be the result of a complex interaction of clinical risk factors, laboratory factors mostly related to the activation of inflammatory processes, and a complex network of gene-to-gene interactions.
Peer Review reports
Ischemic coronary heart disease is the result of an imbalance between blood distribution and tissue demand in the myocardial muscle. Coronary artery lumen narrowing due to atherosclerosis is responsible for about 98% of ischemic heart disease cases [ 1 ]. It should be noted that coronary heart disease mainly affects the age group of 35 to 65 years, and in an important part of society, conflict may occur at the level of young people. In addition, coronary heart disease accounts for 64% of all cardiovascular deaths. According to the published statistics, during the last decade, we have seen an increase in the morbidity caused by ischemic cardiovascular diseases [ 2 ]. Of course, it should be kept in mind that with the development of medicinal methods as well as therapeutic interventions such as angioplasty and coronary stenting, the frequency of morbidity and mortality cases caused by these diseases has decreased significantly [ 3 ]. The first case of stent implantation was performed in 1986 and after that percutaneous coronary intervention or PCI was listed as one of the standard treatment strategies for these disorders [ 4 ]. Today, these methods as endovascular treatments aimed at restoring coronary blood flow have led to the lives of millions of people. However, observations have shown that within 6 months to one year after successful coronary stenting, there is a possibility of angina recurrence due to restenosis of the stent [ 5 ]. This issue has even been reported for drug-eluting stents [ 6 ]. After initial coronary stenting, the prevalence of restenosis is between 20 and 30% [ 7 ]. This stent restenosis occurs for various clinical and even genetic reasons and it seems that a set of pathophysiological processes such as inflammatory processes, proliferation, genomic and epigenetic factors play a role in it [ 8 ]. But interestingly, the role of each of these factors can be very different in different societies. In particular, the impact of genetic factors is completely dependent on the demographic characteristics of that society. Today, all kinds of genes, genetic polymorphisms, and microRNAs have been identified and introduced in the incidence of stent re-stenosis, which, along with clinical risk factors, double the risk of this complication. We aimed to first determine the underlying clinical and laboratory risk factors for the occurrence of stent re-stenosis after PCI based on a systematic review study, and after that, through a bioinformatics study, to evaluate the related genes and microRNAs with the occurrence of stent re-stenosis.
Materials and methods
Systematic review phase.
The present systematic review and meta-analysis followed the guidelines for the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA). Firstly, two questions were suggested based on the author’s purposes “What are the main clinical determinants for stent re-stenosis?” and “What are the related and predictive genetic factors for re-stenosis?”. In the next step, the manuscript databases including Medline, Web of Knowledge, Google Scholar, Scopus, and Cochrane were deeply searched by the two blinded investigators for all eligible studies based on the considered keywords including “stent”, “stenosis”, “re-stenosis”, “predictor”, “risk factor”, “gene”, “genetics”, and “microRNA”. The inclusion criteria were considered to retrieve the studies: (1) the studies finally assessed clinical and/or genetic-based risk profiles related to stent re-stenosis, (2) due to the potential effects of other cardiac revascularization procedures such as coronary artery bypass grafting, those studies entering the patients who undergo such revascularization procedures or previous history of cardiac procedures were all excluded from analysis, (3) The studies were restricted to the English language, (4) the studies with unclear or irreproducible results were all excluded, (5) lack of access to the manuscripts full texts were also considered as the inclusion criteria unless the abstracts had enough data for our analysis, (6) case reports, case series, and review papers were all excluded. As shown in the flow diagram of the study selection (Fig. 1 ), 1536 articles were initially collected by database searching. After removing 13 articles due to evidence of duplication, 1523 records were primarily under-screened. Based on the titles and abstracts, 1426 records were excluded and the remaining 97 citations were assessed for further eligibility. Of those, 5 were also excluded due to the incompleteness of the data and contents. In the final, 67 articles on clinical and biochemical predictors for stent restenosis [ 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 ] (Table 1 ) and also 25 articles on genetic determinants of this event were eligible for the final analysis [ 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 ] (Table 2 ).
Data abstraction was independently performed by two un-blinded reviewers on structure collection forms without divergences in data collection. We resolved disagreements by consensus or by involving a third person. The study quality was evaluated based on the following criteria: (1) the systematic review and meta-analysis based on the questions primarily described and formulated; (2) inclusion and exclusion criteria predefined in the studies as eligibility criteria; (3) searching the literature performed on a systematic and comprehensive approach; (4) to minimize the bias, the full texts of the article were dually reviewed; (5) the quality of included studies were rated independently by the reviewers for appraising internal validity; (6) studies’ characteristics and findings were comprehensively listed; (7) the publication and risk of bias were listed; and (8) heterogeneity was also assessed. The risk of bias for each study was assessed using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions and also according to the QUADAS-2 tool. Any disagreement was resolved by discussion with the whole study team.
Bioinformatic phase
The details of bioinformatic processing to assess the genes and gene-gene interactions are described by Sheikhvatan et al. previously [ 102 ]. Briefly, the interaction of each gene with other genes associated with stent re-stenosis was determined by GeneMANIA software ( https://genemania.org/ ) indexing 2277 association networks containing more than 500 million interactions mapped to 163.599 genes in humans. The interactions were calculated based on FDR (False Discovery Rate) and coverage was classified under four categories (a) Shared protein domains, (b) Co-expression, (c) Co-localization, and (d) Genetic interactions. In this regard, a FDR ≤ 5% was considered to be significant. To design an integrated model of a gene interaction network, the Cytoscape software (version 3.6.1.0) was applied.
Findings of a systematic review
To assess the main correlates of stent re-stenosis based on applied keywords, in total 67 studies finally assessed that published from different countries between 2004 and 2022 (Table 1 ). According to our risk of bias assessment, all 67 studies yielded good quality and none of the citations was determined to have a high risk of bias therefore the pooled results should be persuasive. In total, 40,789 patients were assessed and scheduled for primary stenting for coronary artery disease and followed up for 6 to 36 months after the procedure for assessing the occurrence of stent restenosis and its main determinants. The predictors for this event might be categorized as the four subgroups including (1) patient-based parameters including history of diabetes mellitus, hypertension, hyperlipidemia, smoking, history of tenting, chronic renal failure, history of non-alcoholic fatty liver disease, higher age, medical history of COPD, history of PCI, higher body mass index, low physical activity, Type D personality, anger, and some nutritional habits including lower folate intake, low fruit intake, low vegetable intake, and low vitamin C ingestion; (2) stent-based parameters including type of stent (BMS versus DES), lower stent diameter, longer stent, (3) coronary lesion-based parameters including two target lesions, Gensini score, TIMI score, coronary artery calcium score, coronary artery diffuse disease, peripheral vascular lesions, bifurcation lesion, CHA2DS2-VASc score, calcified plaque volumes, plaque burden, remodeling index, multiple stenting, stents in left anterior descending artery (LAD), and SYNTAX score; and (4) laboratory-based parameters including Higher HbA1c level, higher HsCRP level, raised ApoB, MCV and MCH values, higher neutrophil to lymphocyte ratio, higher apoA-I, higher Homocysteine, higher IgE level, increased lipoprotein-associated phospholipase A2 (Lp-PLA2) and IL-6 levels, higher monocyte count, raised creatinine, raised blood uric acid, lower high-density lipoprotein, higher S100A12 level, higher postoperative homocysteine level, higher VLDL-C, higher PDW, higher BMP-2 level, higher lymphocyte-to-Monocyte Ratio, lower serum albumin, higher white blood cell and neutrophil counts, higher lipoprotein A, higher serum IL-33 serum level, higher serum total bilirubin, higher serum Cystatin C, higher fibrinogen levels, higher serum sLOX-1 level, higher serum IL-6, lower serum IL-10, lower adiponectin levels, higher plasma heparin cofactor II activity, and insulin resistance (Table 1 ).
Findings of bioinformatic study
By reviewing 25 articles on genes involved we uncovered 42 genes that have been described to be involved in such a phenomenon. A complex network of genes, gene-related polymorphisms, and microRNAs were shown to be involved in increasing the likelihood of stent restenosis (Table 2 ). According to the literature, the up-regulation of some genes including JUN, SP1, RAB14, RBBP5, IGF1R, PTPN1, DCAF10, CLTA, CAT, STAT5A, CD300A, CA1, NCF2, HBQ1, AHSP, SLC4A1, EPB42, ADRβ2, CDKN1B, M2BP, CAMLG, GALNT2, C11orf84, THOC5, SAMD11, PIK3R2 SOCS1, VEGF, A1166C, HMGB2, BCHE, A1166C, CYP2C19, RANTES, ALOX5AP, SERPINE1, AGTR1, and FGB have been indicated by using different gene assessment techniques. Also, the predictive roles of the expression of some genes related to interleukin production (IL-18, IL-6, IL-10, and IL-8) have been highlighted. To determine the central role of the powerful genes related to stent restenosis, functional interactions and functional relationships between spike genes were evaluated by applying the Genemania network and Cytoscapre analytical software. As shown in Fig. 1 , multiple pathways and gene-gene interactions seem to play a role in stent restenosis. In this context, many genes could interact with multi-pathway genes, but prominent gene interaction included co-expression (58.03%) followed by genetic interactions (13.28%). In this context, the main pathways activated in the background of this cluster based on FDR values were receptor signaling pathways via STAT with FDR value of 6.77e-8 (relevant genes of VEGFA, SOCS1, CCL5, IL10RA, IL18, STAT5A, and CD300A), cellular response to molecule of bacterial origin with FDR value of 6.77e-8 (relevant genes of HMGB2, IL1B, SERPINE1, CCL5, IL6, IL18, CXCL3, CXCL8) and response to lipopolysaccharide with FDR value of 6.77e-8 (relevant genes of HMGB2, IL1B, SERPINE1, CCL5, IL6, IL18, CXCL3, CXCL8). Along with gene polymorphisms and changing gene expression, some microRNAs were also assessed influencing genes and mRNA expressions that the studied microRNAs were shown in Table 1 . In this regard, the special place of miR-139-5p, miR-324-5p, miR-513a-5p, miR-513a-5p, miR-525-5p, miR-548b-5p and miR-1253 (targeting the genes of JUN, SP1, RAB14, RBBP5, IGF1R, PTPN1 and DCAF10 respectively), miR-126-3p (targeting PIK3R2), and miR-30b-5p (targeting 62 genes related to vascular remodeling and fibrosis) has been shown.
Stent re-stenosis after primary stenting in patients with acute coronary syndrome is an uncommon but multifactorial phenomenon. This phenomenon is created and expanded as a result of the interaction of a set of clinical and laboratory factors as well as genetic predisposing factors. Obviously, due to the multifactorial nature of this incident, it will not be possible to accurately determine its prevalence. On the other hand, for the same reasons, it will not be possible to accurately estimate the occurrence of such an event. In this regard and based on a review of the literature, a wide set of background factors are involved in the occurrence of stent restenosis. Among the clinical factors, the presence of classic underlying risk factors of cardiovascular diseases has been completely predictable, in such a way that the risk of stent re-stenosis is higher in elderly patients, obese patients, hypertensive and diabetic patients, patients with hyperlipidemia, as well as patients with The history of chronic renal failure as well as the history of ischemic heart disease have been completely predictable. Also, among the laboratory risk factors, a special place can be given to inflammatory markers, because the occurrence of atherosclerosis is also the result of the interaction between underlying risk factors and inflammatory factors, and such a process can also be predicted in the case of stent re-stenosis. The basis of the occurrence of such a complication has a strong link with the activation of the inflammatory cascade. Additionally, the genomic polymorphisms have also provided the basis for the emergence and spread of stent restenosis. In this direction and during the last two decades, efforts have been made to identify the genetic factors related to the occurrence of this event using various genetic techniques, to identify types of gene polymorphisms, changes in the expression of various genes, and also to identify microRNAs related to it, which play an important role in the changes of the targeted genes involved. There is growing evidence of genetic contribution to vascular remodeling and ultimately coronary calcification and atherosclerosis through extracellular matrix changes and also MicroRNAs involvement in endothelial cell and vascular smooth muscle dysfunction in diabetic patients which makes it an interesting topic to evaluate in the context of in-stent restenosis.

gene to gene interaction involving stent restenosis
Therefore, it seems that the incidence of stent re-stenosis will be the result of a complex interaction of clinical risk factors, laboratory factors mostly related to the activation of inflammatory processes, and a complex network of gene-to-gene interactions, and therefore, it will not be possible to indicate on only one or a limited number of predisposing factors. However, special attention to some background factors can be considered. For example, identifying and tracking increased expression or discovering polymorphisms related to genes encoding various types of inflammatory interleukins can provide a way for early diagnosis and prevention of this complication. It is also obvious that controlling the traditional risk factors of cardiovascular diseases will be successful in preventing the occurrence of such a complication.
Data availability
All data generated or analyzed during this study are included in this published article, and further detailed ones are available from the corresponding author on reasonable request.
Abbreviations
Percutaneous coronary intervention
Preferred Reporting Items for Systematic Review and Meta-Analysis
Bare-metal stent
Drug-eluting stent
World Health Organization. Cardiovascular diseases. http://www.who.int/mediacentre/factsheets/fs317/en/ . Accessed 9 February 2016.
Tardif J-C. Coronary artery disease in 2010. Eur Heart J. 2010;12(suppl C):C2–10. https://doi.org/10.1093/eurheartj/suq014 . [CrossRef] [Google Scholar].
Medical statistics [In Russian]. http://www.medinfo.kz/medstat.jsp . Accessed 9 February 2016.
Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316(12):701–6. [PubMed] [CrossRef] [Google Scholar].
Article CAS PubMed Google Scholar
Fischman D, Leon M, Baim D, Schatz R, Savage M, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study investigators. N Engl J Med. 1994;331(8):496–501. [PubMed] [CrossRef] [Google Scholar].
Spertus J, Nerella R, Kettlekamp R, House J, Marso S, Borkon A, Rumsfeld J. Risk of restenosis and health status outcomes for patients undergoing percutaneous coronary intervention versus coronary artery bypass graft surgery. Circulation. 2005;111(6):768–73. [PubMed] [CrossRef] [Google Scholar].
Article PubMed Google Scholar
Otsuki S, Sabate M. Drug-eluting stents and acute myocardial infarction: a lethal combination or friends? World J Cardiol. 2014;6(9):929–38. https://doi.org/10.4330/wjc.v6.i9.929 . [PMC free article] [PubMed] [CrossRef] [Google Scholar].
Article PubMed PubMed Central Google Scholar
Jukema J, Verschuren J, Ahmed T, Quax P. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;9(1):53–62. https://doi.org/10.1038/nrcardio.2011.132 . [PubMed] [CrossRef] [Google Scholar].
Sheikhvatan M, Chaichian S, Moazzami B. A systematic review and Bioinformatics Study on genes and micro-RNAs involving the Transformation of endometriosis into Ovarian Cancer. Microrna. 2020;9(2):101–11. https://doi.org/10.2174/2211536608666190917152104 .
Article CAS PubMed PubMed Central Google Scholar
Xiao Long Lin 1, Qiu Yu Li 1, Dong Hui Zhao 1, Jing Hua Liu 1, Qian Fan 1. Serum glycated albumin is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents: An observational study. Front Cardiovasc Med. 2022;9:943185. https://doi.org/10.3389/fcvm.2022.943185 . eCollection 2022.
Qiang Feng 1, Zhao Y 2, Wang H 1, Zhao J 2, Wang X. 2, Jianping Shi 1. A predictive model involving serum uric acid, C-reactive protein, diabetes, hypercholesteremia, and multiple lesions for restenosis risk in everolimus-eluting stent-treated coronary heart disease patients. Front Cardiovasc Med. 2022;9:857922. https://doi.org/10.3389/fcvm.2022.857922 . eCollection 2022.
Wang J. 1, Yuchun Yang 1, Lei Zhang 1, Pengyi He 1, Huyati Mu 1. Predictors of Stent Restenosis in Han and Uygur Patients with Coronary Heart Disease after PCI in the Xinjiang Region. Cardiol Res Pract. 2022;2022:7845108. https://doi.org/10.1155/2022/7845108 . eCollection 2022.
Gábor Csató 1, Nóra Erdei 1, Beatrix Ványai 2, Tímea Balla 1, Dániel Czuriga 1, Zoltán Csanádi 1, Zsolt Koszegi 1, István Édes 1, Gábor Tamás Szabó 1. Predictors of restenosis following percutaneous coronary stent implantation: The role of trimetazidine therapy. Front Cardiovasc Med. 2022;9:873899. https://doi.org/10.3389/fcvm.2022.873899 . eCollection 2022.
Xin W. # 1, Min Zhang # 2, Jie Cheng 3, Hua Zhou 4. Association of serum apoA-I with in-stent restenosis in coronary heart disease. BMC Cardiovasc Disord. 2022;22(1):355. https://doi.org/10.1186/s12872-022-02762-y .
Article CAS Google Scholar
Ping Wang YK 2, Liu Y 2, Zhang Y 3, Gao H, 2, Qilin Ma 4. Levels of plasma Quaking and cyclooxygenase-2 predict in-stent restenosis in patients with coronary artery disease after percutaneous coronary intervention. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47(6):739–747. https://doi.org/10.11817/j.issn.1672-7347.2022.210716 .
Jing Z. 1, Dayang Chai 1, Yuxiang Dai 2, Aichao Wang 1, Ting Yan 3, Shu Lu 1. Predictive Value Analysis of in-Stent Restenosis Within Three Years in Older Acute Coronary Syndrome Patients: A Two-Center Retrospective Study. Clin Appl Thromb Hemost. 2022 Jan-Dec;28:10760296221107888. https://doi.org/10.1177/10760296221107888 .
Jiqiang, Guo. 1 2 3, Ying Gao 1 2 3, Mohammad Ahmed 4, Pengfei Dong 4, Yuping Gao 2 3, Zhihua Gong 2 3, Jinwen Liu 2 3, Yajie Mao 2 3, Zhijie Yue 2 3, Qingli Zheng 1, Jiansheng Li 2 3, Jianrong Rong 2 3, Yongnian Zhou 2 3, Meiwen An 1, Linxia Gu 4, Jin Zhang 2. Serum homocysteine level predictive capability for severity of Restenosis Post Percutaneous Coronary intervention. Front Pharmacol. 2022;13:816059. https://doi.org/10.3389/fphar.2022.816059 . eCollection 2022.
Ming Yi 1 2 3, Xu W-HT. 2, Xiao Ke 1 2, Qiang Liu 2 4. Investigation Into the Risk Factors Related to In-stent Restenosis in Elderly Patients With Coronary Heart Disease and Type 2 Diabetes Within 2 Years After the First Drug-Eluting Stent Implantation. Front Cardiovasc Med. 2022;9:837330. https://doi.org/10.3389/fcvm.2022.837330 . eCollection 2022.
Jingmeng Liu 1, Qiujing Chen 1 2. Association of Circulating IgE and CML Levels with In-Stent Restenosis in Type 2 Diabetic Patients with Stable Coronary Artery Disease. J Cardiovasc Dev Dis. 2022;9(5):157. https://doi.org/10.3390/jcdd9050157 . Lin Lu 1 2, Qi Jin 1, Yangyang Bao 1, Tianyou Ling 1, Changjian Lin 1, Fenghua Ding 1, Xiaoqun Wang 1, Weifeng Shen 1 2, Ying Shen 1, Yang Dai 1 2, Liqun Wu 1.
Chen D 1, Lu XX. 2, Shengli Chen 1, Sunmei Lin 1. Predictive Value of Perioperative Cytokine Levels on the Risk for In-Stent Restenosis in Acute Myocardial Infarction Patients. Contrast Media Mol Imaging. 2022;2022:7832564. https://doi.org/10.1155/2022/7832564 . eCollection 2022.
Li S. 1, Hong Qiu 2, Zhaorong Lin 1, Lin Fan 1, Yongzhe Guo 1, Yujie Zhang 1, Lianglong Chen 1. The Early Predictive Value of Circulating Monocytes and Eosinophils in Coronary DES Restenosis. Front Cardiovasc Med. 2022;9:764622. https://doi.org/10.3389/fcvm.2022.764622 . eCollection 2022.
Yinhua Luo # 1, Ni Tan # 2, Jingbo Zhao 3, Yuanhong Li 3. A Nomogram for Predicting In-Stent Restenosis Risk in Patients Undergoing Percutaneous Coronary Intervention: A Population-Based Analysis. Int J Gen Med. 2022;15:2451–2461. https://doi.org/10.2147/IJGM.S357250 . eCollection 2022.
Yinhua L. # 1, Shengyu Cui # 2, Changjiang Zhang 2, Rui Huang 2, Jinbo Zhao 3, Ke Su 3, Dan Luo 3, Yuanhong Li 3. Prognostic Role of Fasting Remnant Cholesterol with In-Stent Restenosis After Drug-Eluting Stent Implantation. Int J Gen Med. 2022;15:1733–1742. https://doi.org/10.2147/IJGM.S348148 . eCollection 2022.
Zhang R 1, Tao Z. 1, Jing Gong 1, Zhenjun Ji 1, Mingming Yang 1, Genshan Ma 1, Yongjun Li 1. Albumin to globulin ratio was associated with in-stent restenosis and revascularization events after percutaneous coronary intervention. Clin Transl Sci. 2022;15(5):1187–1195. https://doi.org/10.1111/cts.13236 . Epub 2022 Feb 27.
Bo-Wen Chen 1, Jia-Jing Liu 1, Jun-Hui Xing 1, Heng-Dao Liu 1, Yu-Zhen Wei 1, Xiao-Fei Xue 1, He-Ping Gu 1, Hai-Long Tao 1. Analysis of the Correlation Between the Ratio of Monocytes to High-Density Lipoprotein Cholesterol and in-Stent Restenosis in Patients with Premature Coronary Heart Disease. Clin Appl Thromb Hemost. 2022 Jan-Dec;28:10760296221079334. https://doi.org/10.1177/10760296221079334 .
Xiaowen Z 1, Xu K, Yang X, Yang W, Zhang W, Jiang Y, Zhang Y, Qiu X, Shi H, Jiang L, Shen L, Ben He. Association between coronary artery calcium score and in-stent restenosis after drug-eluting stent implantation. Coron Artery Dis. 2022;33(4):284–94. https://doi.org/10.1097/MCA.0000000000001124 . Epub 2022 Jan 25.
Article Google Scholar
Mingrui Li. # 1 2, Jingyuan Hou # 3 4, Xiaodong Gu 3 4, Ruiqiang Weng 3 4, Zhixiong Zhong 5, Sudong Liu 6 7. Incidence and risk factors of in-stent restenosis after percutaneous coronary intervention in patients from southern China. Eur J Med Res. 2022;27(1):12. https://doi.org/10.1186/s40001-022-00640-z .
Huilin H. 1, Shijun Wang 1, Guanmin Tang 1, Changlin Zhai 1, Liang Shen 2. Impact of anemia on in-stent restenosis after percutaneous coronary intervention. BMC Cardiovasc Disord. 2021;21(1):548. https://doi.org/10.1186/s12872-021-02355-1 .
Masayuki Yoshimura 1 2. Seiji Umemoto 3 4, Reo Kawano 3, Mitsuyuki Hiromoto 5, Michio Yamada 6, Tatsuhiro Fujimura 1, Masakazu Tanaka 7, Tomoko Nao 8, Toshiro Miura 9, Masafumi Yano 1. Non-fasting Hypertriglyceridemia as an independent risk factor for coronary In-Stent restenosis after primary Bare Metal Stent Implantation in patients with coronary artery disease. Int Heart J. 2021;62(5):970–9. https://doi.org/10.1536/ihj.21-015 .
Min-Tao G. # 1, Bing Zhu # 2, Xiao-Cui Chen 1, Fen Liu 1, Xiang Xie 2, Xiao-Ming Gao 2, Xiang Ma 2, Zhen-Yan Fu 2, Yi-Tong Ma 3 4, Bang-Dang Chen 5 6. A prediction model based on platelet parameters, lipid levels, and angiographic characteristics to predict in-stent restenosis in coronary artery disease patients implanted with drug-eluting stents. Lipids Health Dis. 2021;20(1):118. https://doi.org/10.1186/s12944-021-01553-2 .
Ling Zhang YW # 1, Zhang Z 2. Hongyuan Liang 1, Liang Wu 1, Liang Ni 1, Guiju Gao 1, Di Yang 1, Hongxin Zhao 3, Jiang Xiao 4. Risk factors of in-stent restenosis among coronary artery disease patients with syphilis undergoing percutaneous coronary intervention: a retrospective study. BMC Cardiovasc Disord. 2021;21(1):438. https://doi.org/10.1186/s12872-021-02245-6 .
Wenbo H. # 1 2 3, Changwu Xu # 1 2 3, Xiaoying Wang 4, Jiyong Lei 1 2 3, Qinfang Qiu 1 2 3, Yingying Hu 1 2 3, Da Luo 5 6 7. Development and validation of a risk prediction nomogram for in-stent restenosis in patients undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. 2021;21(1):435. https://doi.org/10.1186/s12872-021-02255-4 .
Dan-Mihai. Alexandrescu 1 2, Ovidiu Mitu 1, Irina Iuliana Costache 1, Liviu Macovei 1, Ivona Mitu 3, Anca Alexandrescu 2, Catalina Arsenescu Georgescu 1. Risk factors associated with intra-stent restenosis after percutaneous coronary intervention. Exp Ther Med. 2021;22(4):1141. https://doi.org/10.3892/etm.2021.10575 . Epub 2021 Aug 9.
Hsin-Fu Lee 1, Yu-Wen Cheng 2, Jian-Rong Peng 3, Chiu-Yi Hsu 4, Chia-Hung Yang 3, Yi-Hsin Chan 5, Pao-Hsien Chu 6. Impact of chronic kidney disease on long-term outcomes for coronary in-stent restenosis after drug-coated balloon angioplasty. J Cardiol. 2021;78(6):564–570. doi: 10.1016/j.jjcc.2021.08.010. Epub 2021 Aug 26.
Guohua Sheng 1, Juan Zhou 2, Chi Zhang 3, Caijuan Wu 4, Kairong Huang 5, Xiaotong Qin 6, Jie Wu 4. Relationship between Lp-PLA2 and in-stent restenosis after coronary stenting: a 3-year follow-up study. Scott Med J. 2021;66(4):178–185. doi: 10.1177/00369330211034809. Epub 2021 Jul 28.
Praveen KG, 1, Jayaram Balachander 2. Predictor of in-stent restenosis in patients with drug-eluting stent (PRIDE)- a retrospective cohort study. Clin Investig Arterioscler. 2021 Jul-Aug;33(4):184–194. doi: 10.1016/j.arteri.2020.11.003. Epub 2021 Feb 20.
Pengfei Wang H, Qiao. 2, RuiJuan Wang 3, Ruitian Hou 3, Jingtao Guo 2. The characteristics and risk factors of in-stent restenosis in patients with percutaneous coronary intervention: what can we do. BMC Cardiovasc Disord. 2020;20(1):510. https://doi.org/10.1186/s12872-020-01798-2 .
Yini Wang SZ 2, Zhang G. 3, Bo Yu 4, Xueqin Gao 4, Zhenguo Dai 4, Xiuxian Yang 2, Xiaohui Qiu 2, Zhengxue Qiao 2, Jiawei Zhou 2, Ping Lin 5, Deyu Fang 6, Yanjie Yang 7. Association between type D personality and in-stent restenosis in patients treated with percutaneous coronary intervention: A mediation analysis of dietary patterns. J Psychosom Res. 2020;138:110244. https://doi.org/10.1016/j.jpsychores.2020.110244 . Epub 2020 Sep 10.
Hengyi Liang 1. Yuqi Cui 1 2, Haoran Bu 1, Hang Liu 3, Pengcheng Yan 1, Lianqun Cui 1, Liming Chen 1. Value of S100A12 in predicting in-stent restenosis in patients with coronary drug-eluting stent implantation. Exp Ther Med. 2020;20(1):211–8. https://doi.org/10.3892/etm.2020.8721 . Epub 2020 May 6.
Zhang G 1, Li S, Lin P, Chen Y. An analysis of factors related to the development of in-stent restenosis after percutaneous coronary intervention. Med (Baltim). 2020;99(5):e18915. https://doi.org/10.1097/MD.0000000000018915 .
Cui LT 1. The number of stents was an independent risk of stent restenosis in patients undergoing percutaneous coronary intervention. Med (Baltim). 2019;2(50):e18312. https://doi.org/10.1097/MD.0000000000018312 . Dan-Ping Liu 1, Ying-Ying Fu 1.
Lin S. 1, Chunyan Zhang 1, Yinghui Ju 1, Bin Tang 2, Meixiu Gu 1, Baishen Pan 1, Wei Guo 1, Beili Wang 1. Mean Corpuscular Volume Predicts In-Stent Restenosis Risk for Stable Coronary Artery Disease Patients Receiving Elective Percutaneous Coronary Intervention. Med Sci Monit. 2019;25:3976–3982. https://doi.org/10.12659/MSM.914654 .
Sheng Gang Zhao 1 2, Xu JJ. 2, Zhen Hao Tao 3, Lei Jin 4, Qin Liu 3, Wen Yue Zheng 5, Li Qin Jiang 2, Ning Fu Wang 1. CHA2DS2-Vasc score and CHA2DS2-Vasc-HS score are poor predictors of in-stent restenosis among patients with coronary drug-eluting stents. J Int Med Res. 2019;47(6):2533–2544. https://doi.org/10.1177/0300060519841836 . Epub 2019 Apr 30.
Gong Cheng 1 2, Feng-Jun. Factors Influencing Stent Restenosis After Percutaneous Coronary Intervention in Patients with Coronary Heart Disease: A Clinical Trial Based on 1-Year Follow-Up. Med Sci Monit. 2019;25:240–7. https://doi.org/10.12659/MSM.908692 . Chang 1, Yi Wang 1, Peng-Hua You 1, Hai-Chao Chen 1, Wen-Qi Han 1, Jun-Wei Wang 1, Ni-Er Zhong 1, Zhi-Qian Min 3.
Mojtaba Baktashian 1, Sara Saffar Soflaei 2, Negin Kosari 3, Mansoor Salehi 4, Alireza Khosravi 5, Maliheh Ahmadinejad 6, Mohsen Moohebati 7, Mahmood Ebrahimi 8, Farzad Rahmani 9, Ramin Khameneh-Bagheri 10, Mostafa Ahmadi 11, Fatemeh Sadabadi 12, Maryam Tayefi 13, Suzan Bazhdanzadeh 14, Gordon A Ferns 15, Seyed Mohammad Hashemi 16, Alireza Pasdar 17, Majid Ghayour-Mobarhan 18. Association of high level of hs-CRP with in-stent restenosis: A case-control study. Cardiovasc Revasc Med. 2019;20(7):583–587. https://doi.org/10.1016/j.carrev.2018.08.015 . Epub 2018 Aug 18.
Wang J-L. 1, Zheng Qin 1, Zhi-Jian Wang 1, Dong-Mei Shi 1, Yu-Yang Liu 1, Ying-Xin Zhao 1, Li-Xia Yang 1, Wan-Jun Cheng 1, Yu-Jie Zhou 1. New predictors of in-stent restenosis in patients with diabetes mellitus undergoing percutaneous coronary intervention with drug-eluting stent. J Geriatr Cardiol. 2018;15(2):137–145. https://doi.org/10.11909/j.issn.1671-5411.2018.02.011 .
Cheng-Ping H. 1, Yu Du 1, Yong Zhu 1, Chao Shi 1, Zheng Qin 1, Ying-Xin Zhao 1. Platelet Distribution Width on Admission Predicts In-Stent Restenosis in Patients with Coronary Artery Disease and Type 2 Diabetes Mellitus Treated with Percutaneous Coronary Intervention. Chin Med J (Engl). 2018;131(7):757–763. https://doi.org/10.4103/0366-6999.228247 .
Xie X-AW. 1, Xiu-Qi Li 1, Hui-Ting Wu 1, Xiang Wang 2. The value of serum visfatin in predicting in-stent restenosis of drug-eluting stents. Clin Chim Acta. 2018;479:20–24. https://doi.org/10.1016/j.cca.2018.01.004 . Epub 2018 Jan 3.
Yini Wang G 1, Liu X, Gao Z, Zhao L, Li W, Chen H, Tao B, Yu, Ping Lin. Prognostic value of type D personality for In-stent restenosis in coronary artery disease patients treated with drug-eluting stent. Psychosom Med. 2018;80(1):95–102. https://doi.org/10.1097/PSY.0000000000000532 .
Zheng Qin 1, Fang-Wu Zheng 2, Chuang Zeng 3, Kuo Zhou 1, Yu Geng 1, Jian-Long Wang 1, Yue-Ping Li 1, Qing-Wei Ji 1, Yu-Jie Zhou 4. Elevated Levels of Very Low-density Lipoprotein Cholesterol Independently Associated with In-stent Restenosis in Diabetic Patients after Drug-eluting Stent Implantation. Chin Med J (Engl). 2017;130(19):2326–2332. https://doi.org/10.4103/0366-6999.213575 .
Alparslan Kurtul 1. Usefulness of the CHA2DS2-VASc score in Predicting In-Stent restenosis among patients undergoing revascularization with Bare-Metal stents. Clin Appl Thromb Hemost. 2018;24(4):589–95. Epub 2017 Jul 4.
Yang W-PZ. 2, Li-Xiao Su 3, Yu Ning 4, Wan-Wan Wen 4, Man-Kun Xin 4, Xin Zhao 4, Ming Zhang 5. Association between plasma BMP-2 and in-stent restenosis in patients with coronary artery disease. Clin Chim Acta. 2017;471:150–153. https://doi.org/10.1016/j.cca.2017.05.033 . Epub 2017 May 27.
Yusuke Watanabe 1, Kensuke Takagi 1. Independent predictors of in-stent restenosis after drug-eluting stent implantation for ostial right coronary artery lesions. Int J Cardiol. 2017;240:108–13. https://doi.org/10.1016/j.ijcard.2017.04.083 . Epub 2017 Apr 26. Toru Naganuma 1, Hiroyoshi Kawamoto 1, Yusuke Fujino 1, Hisaaki Ishiguro 1, Satoko Tahara 1, Naoyuki Kurita 1, Koji Hosawa 1, Shotaro Nakamura 1, Sunao Nakamura 2.
Hiroshi Koiwaya 1. Predictors of Recurrent In-Stent Restenosis After Paclitaxel-Coated Balloon Angioplasty. Circ J. 2017;81(9):1286–92. https://doi.org/10.1253/circj.CJ-17-0095 . Epub 2017 Apr 28. Nozomi Watanabe 1, Nehiro Kuriyama 1, Shun Nishino 1, Kenji Ogata 1, Toshiyuki Kimura 1, Tatsuya Nakama 1, Hirohide Matsuura 1, Makoto Furugen 1, Yoshisato Shibata 1.
Samet Yilmaz 1. Mehmet Kadri Akboga 2, Dursun Aras 2, Serkan Topaloglu 2. Evaluation of the predictive value of CHA2DS2-VASc score for In-Stent restenosis. Angiology. 2018;69(1):38–42. Epub 2017 Mar 27.
Ying Zhou 1, Zhang H-W 2, Chen FT. 3, Tian-Wen Han 2, Ya-Hang Tan 4, Jia Zhou 5, Tao Zhang 2, Jing Jing 2, Yun-Dai Chen 2. Influence of increased epicardial adipose tissue volume on 1-year in-stent restenosis in patients who received coronary stent implantation. J Geriatr Cardiol. 2016;13(9):768–775. https://doi.org/10.11909/j.issn.1671-5411.2016.09.012 .
Christian Tesche 1, Carlo N, De Cecco R, Vliegenthart M, Duguay 4 AC. Stubenrauch 4, Russell D Rosenberg 5, Akos Varga-Szemes 4, Richard R Bayer 2nd 5, Junjie Yang 6, Ullrich Ebersberger 1, Moritz Baquet 7, David Jochheim 7, Ellen Hoffmann 8, Daniel H Steinberg 9, Salvatore A Chiaramida 9, U Joseph Schoepf 10. Coronary CT angiography-derived quantitative markers for predicting in-stent restenosis. J Cardiovasc Comput Tomogr. 2016 Sep-Oct;10(5):377 – 83. https://doi.org/10.1016/j.jcct.2016.07.005 . Epub 2016 Jul 6.
Sani Namik Murat 1. The Relationship Between Lymphocyte-to-Monocyte Ratio and Bare-Metal Stent In-Stent Restenosis in Patients With Stable Coronary Artery Disease. Clin Appl Thromb Hemost. 2017;23(3):235–40. doi: 10.1177/1076029615627340. Epub 2016 Jul 9. Mikail Yarlioglues 1, Ibrahim Etem Celik 1, Alparslan Kurtul 1, Mustafa Duran 1, Alparslan Kilic 1, Fatih Oksuz 1.
Dedi Wihanda 1, Alwi I, Yamin M, Shatri H, Mudjaddid E. Factors Associated with In-stent restenosis in patients following percutaneous coronary intervention. Acta Med Indones. 2015;47(3):209–15.
Google Scholar
Ibrahim Etem Celik 1, Mikail Yarlioglues 2, Alparslan Kurtul 2, Mustafa Duran 2, Cemal Koseoglu 2, Fatih Oksuz 2, Ozlem Aksoy 2, Sani Namik Murat 2. Preprocedural Albumin Levels and Risk of In-Stent Restenosis After Coronary Stenting With Bare-Metal Stent. Angiology. 2016;67(5):478 – 83. https://doi.org/10.1177/0003319715598084 . Epub 2015 Aug 3.
Osman Bolca 1, Güngör Bariş, Özcan KS, Fatma Ö, Karadeniz A, Sungur B, Köroğlu N, Bakhshyaliyev NS, Yelgeç. Baran Karataş, Göktürk İpek, Hale Yilmaz, Recep Öztürk. The neutrophil-to-lymphocyte ratio is associated with bare-metal stent restenosis in STEMI patients treated with primary PCI. Coron Artery Dis. 2015;26(5):402–8. https://doi.org/10.1097/MCA.0000000000000254 .
Choi S-HP 2, Park J-Y. 3, Ung Jeon 1, Hong-Seog Seo 2, Eung-Ju Kim 2, Jin-Oh Na 2, Cheol-Ung Choi 2, Jin-Won Kim 2, Hong-Euy Lim 2, Chang-Gyu Park 2, Dong-Joo Oh 2. Impact of high lipoprotein(a) levels on in-stent restenosis and long-term clinical outcomes of angina pectoris patients undergoing percutaneous coronary intervention with drug-eluting stents in Asian population. Clin Exp Pharmacol Physiol. 2015;42(6):588 – 95. https://doi.org/10.1111/1440-1681.12396 .
Samet Yılmaz S, Ünal Cağri, Yayla Özcan, Özeke D, Aras S, Topaloglu. Sınan Aydogdu. Usefulness of the platelet-to-lymphocyte ratio in predicting bare-metal stent restenosis. Scand Cardiovasc J. 2015;49(1):39–44. doi: 10.3109/14017431.2014.989537. Epub 2014 Dec 26.
Xu L-PZ, Wang L, Li H, Shao C-L, Gu H-B, Chan S-P, Xu H-F. Xiang-Jun Yang. Influence of insulin resistance on in-stent restenosis in patients undergoing coronary drug-eluting stent implantation after long-term angiographic follow-up. Coron Artery Dis. 2015;26(1):5–10. https://doi.org/10.1097/MCA.0000000000000170 .
Svitlana Demyanets 1, Jarai IT 2, Katharina M, Katsaros 3. Serdar Farhan 2, Anna Wonnerth 4, Thomas W Weiss 2, Johann Wojta 3, Walter S Speidl 4, Kurt Huber 5. An increase of interleukin-33 serum levels after coronary stent implantation is associated with coronary in-stent restenosis. Cytokine. 2014;67(2):65–70. https://doi.org/10.1016/j.cyto.2014.02.014 . Epub 2014 Mar 27.
Wu F-L, Liu W-Y, Zhao C-C, Chen C-X, Xie Y-Y, Wu S-J, Lin X-F, Chen Y-P. Danny Ka-Ho Wong, Man-Fung Yuen, Ming-Hua Zheng. Non-alcoholic fatty liver disease and risk of in-stent restenosis after bare metal stenting in native coronary arteries. Mol Biol Rep. 2014;1(7):4713–20. https://doi.org/10.1007/s11033-014-3342-z . Epub 2014 Apr 2.
Abdulmelik Yildiz 1, Tekiner F, Karakurt A, Sirin G, Duman D. Preprocedural red blood cell distribution width predicts bare metal stent restenosis. Coron Artery Dis. 2014;25(6):469–73. https://doi.org/10.1097/MCA.0000000000000105 .
Chunyu Zhao L 1, Yang L, Mao L, Zhong X, Li S, Wei. Cystatin C associates with the prediction of in-stent restenosis among patients receiving stent implantation: results of the 1-year follow-up. Coron Artery Dis. 2013;24(5):357–60. https://doi.org/10.1097/MCA.0b013e328361b3a4 .
Yutaka Aoyama 1, Hirayama H, Ishii H, Kobayashi K, Ishikawa K, Takigawa M, Nanasato M, Yoshida Y. Toru Aoyama, Daiji Yoshikawa, Tatsuaki Matsubara, Toyoaki Murohara. Impact of chronic kidney disease on a re-percutaneous coronary intervention for sirolimus-eluting stent restenosis. Coron Artery Dis. 2012;23(8):528–32. https://doi.org/10.1097/MCA.0b013e3283599463 .
Alessandro Lupi 1, Secco GG, Rognoni A, Rossi L, Lazzero M, Nardi F, Rolla R, Bellomo G. Angelo Sante Bongo, Carlo Di Mario. Plasma fibrinogen levels and restenosis after primary percutaneous coronary intervention. J Thromb Thrombolysis. 2012;33(4):308–17. https://doi.org/10.1007/s11239-011-0628-z .
Peter Scott Munk 1, Butt N. Alf Inge Larsen. Endothelial dysfunction predicts clinical restenosis after percutaneous coronary intervention. Scand Cardiovasc J. 2011;45(3):139–45. https://doi.org/10.3109/14017431.2011.564646 . Epub 2011 Mar 29.
Takashi Kuwano 1, Miura S-I, Shirai K, Ike A, Mori K, Shimizu T, Zhang B, Iwata A, Nishikawa H, Kawamura A, Keijiro Saku. Serum levels of bilirubin as an independent predictor of coronary in-stent restenosis: a new look at an old molecule. J Atheroscler Thromb. 2011;18(7):574–83. https://doi.org/10.5551/jat.6643 . Epub 2011 Mar 18.
Li B 1, Zhang Li-hua, Yang Xin-guo, Liu Y. Xiong-Tao Liu, Yin-Gang Ren. Postprocedural serum sLOX-1 levels are associated with coronary in-stent restenosis in patients with stable coronary artery disease. Coron Artery Dis. 2011;22(4):259–63. https://doi.org/10.1097/MCA.0b013e328344ede9 .
Aleksander Zurakowski 1, Wojakowski W, Dzielski T, Milewski K. Kinga Gościńska-Bis, Michał Tendera, Paweł Buszman. Plasma levels of C-reactive protein and interleukin-10 predict late coronary in-stent restenosis 6 months after elective stenting. Kardiol Pol. 2009;67(6):623–30.
Yoshinobu Kitta 1, Takano H, Nakamura T, Kodama Y, Umetani K, Fujioka D, Saito Y, Kawabata K-I, Obata J-E. Akira Mende, Tsuyoshi Kobayashi, Kiyotaka Kugiyama. Low adiponectin levels predict late in-stent restenosis after bare metal stenting in native coronary arteries. Int J Cardiol. 2008;131(1):78–82. https://doi.org/10.1016/j.ijcard.2007.09.004 . Epub 2008 Jan 4.
Nobuyuki Takamori 1, Azuma H, Kato M, Hashizume S, Aihara Ken-ichi, Akaike M, Tamura K, Matsumoto T. High plasma heparin cofactor II activity is associated with reduced incidence of in-stent restenosis after percutaneous coronary intervention. Circulation. 2004;109(4):481–6. https://doi.org/10.1161/01.CIR.0000109695.39671.37 . Epub 2004 Jan 26.
Linghong Song 1, Yufei Feng 1, Feng Tian 1 2, Xiaoang Liu 3, Shan Jin 1, Chengyan Wang 1, Wuyue Tang 1, Juncang Duan 4, Na Guo 1, Xihua Shen 1, Jianming Hu 1, Hong Zou 1, Wenyi Gu 5, Kejian Liu 1 6, Lijuan Pang 1 7. Integrated microarray for identifying the hub mRNAs and constructed miRNA-mRNA network in coronary in-stent restenosis. Physiol Genomics. 2022;54(10):371–379. https://doi.org/10.1152/physiolgenomics.00089.2021 . Epub 2022 Aug 15.
Chen M 1, Zhang YD 3, Liu S. 3. Identification of differentially expressed genes associated with coronary in-stent restenosis by integrated bioinformatics approaches. Ann Palliat Med. 2022;11(6):1940–1953. https://doi.org/10.21037/apm-21-2681 . Epub 2022 Feb 9.
Tarek A, Abdelaziz 1, Randa H, Mohamed M. Gad 1, Mohamed G Ghareeb 1, Sara F Saadawy 2. Single-nucleotide polymorphism of ADRβ2 and CDKN1B genes in Egyptian patients with coronary artery in-stent restenosis. Coron Artery Dis. 2022;33(4):277–283. https://doi.org/10.1097/MCA.0000000000001123 .
Le Yang H, Zhu. 2 3, Yuanyuan Sun 4, Pengcheng Yan 2, Xiaoning Song 2, Fayun Xu 1, Haitao Yuan 1 2, Liming Chen 1. Value of M2BP in predicting in-stent restenosis in patients after coronary drug-eluting stent implantation. Clin Cardiol. 2022;45(3):308–314. https://doi.org/10.1002/clc.23775 . Epub 2022 Jan 15.
Yen-Wen Liu 1 2, Huang M-S 1, Hsu L-W. 2, Hsien-Yuan Chang 1 2, Cheng-Han Lee 1, Chi-Ying Lee 3, Dao-Peng Chen 3, Yi-Heng Li 1, Ting-Hsin Chao 1, Pei-Fang Su 4, Meng-Ru Shen 5 6, Ping-Yen Liu 1 2. Genetic risk model for in-stent restenosis of second-and third-generation drug-eluting stents. iScience. 2021;24(9):103082. https://doi.org/10.1016/j.isci.2021.103082 . eCollection 2021 Sep 24.
Xianke Qiu 1, Wang J. 2, Zhongping Shi 2, Xiaojun Ji 2, Yiwei Huang 2, Haiyue Dai 2. Predictive value of miRNA-126 on in-stent restenosis in patients with coronary heart disease: A protocol for meta-analysis and bioinformatics analysis. Medicine (Baltimore). 2021;100(22):e25887. https://doi.org/10.1097/MD.0000000000025887 .
Encarnación G-C. 1 2 3, Isabel Mayoral-González 1 2, Francisco Jesús Morón 4, Mónica Fernández-Quero 3, Alejandro Domínguez-Rodríguez 1, Antonio Ordóñez 1 2, Tarik Smani 1 5. miR-30b-5p downregulation as a predictive biomarker of coronary In-Stent restenosis. Biomedicines. 2021;9(4):354. https://doi.org/10.3390/biomedicines9040354 .
Maheronnaghsh 1 M, Niktab 2 I, Enayati 3 S, Amoli 3 MM, Hosseini 4 SK, J Tavakkoly-Bazzaz 5. Differentially expressed miR-152, a potential biomarker for in-stent restenosis (ISR) in peripheral blood mononuclear cells (PBMCs) of coronary artery disease (CAD) patients. Nutr Metab Cardiovasc Dis. 2021;31(4):1137–47. https://doi.org/10.1016/j.numecd.2020.09.030 . Epub 2020 Oct 20.
Zhang J-JG 2, Liu Y-J. 2. Effects of miRNA-1,miRNA-21 in plasma on in-stent restenosis in patients with coronary heart disease and diabetes mellitus after percutaneous coronary intervention. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2018;34(4):304–308 384. https://doi.org/10.12047/j.cjap.5643.2018.070 .
Liang Zhou N, Wang. 1, Hong Li 1, Guoxin Tong 1, Jianmin Yang 1, Lei Lai 1, Hao Pan 1, Xianhua Ye 1, Jinyu Huang 1. SOCS1 gene promoter methylation status is associated with in-stent restenosis after percutaneous coronary intervention. Oncotarget. 2017;8(34):56959–56967. https://doi.org/10.18632/oncotarget.18398 . eCollection 2017 Aug 22.
Zsolt Bagyura 1. Loretta Kiss 1, Kristóf Hirschberg 1 2, Balázs Berta 1, Gábor Széplaki 1, Árpád Lux 1, Zsolt Szelid 1, Pál Soós 1, Béla Merkely 1. Association between VEGF Gene Polymorphisms and In-Stent restenosis after coronary intervention treated with Bare Metal Stent. Dis Markers. 2017;2017:9548612. https://doi.org/10.1155/2017/9548612 . Epub 2017 Mar 7.
Min Zhu 1 2. Association of seven renin angiotensin system gene polymorphisms with restenosis in patients following coronary stenting. J Renin Angiotensin Aldosterone Syst. 2017;18(1):1470320316688774. https://doi.org/10.1177/1470320316688774 . Minjun Yang 1 3, Jiangbo Lin 1 3, Huanhuan Zhu 1 3, Yifei Lu 1 3, Bing Wang 1 3, Yinshen Xue 1 3, Congfeng Fang 1 3, Lijiang Tang 1 3, Baohui Xu 4, Jianjun Jiang 1 3, Xiaofeng Chen 1 2 3.
Yu HH 1, Wang XQ 1, Zhang J. 1, Zhu Hui Liu 1, Wen Qi Pan 1, Ying Shen 1, Zheng Bin Zhu 1, Ling Jie Wang 1, Xiao Xiang Yan 1, Ke Yang 1, Rui Yan Zhang 1, Wei Feng Shen 1, Feng Hua Ding 1, Lin Lu 2. Association of Serum HMGB2 Levels With In-Stent Restenosis: HMGB2 Promotes Neointimal Hyperplasia in Mice With Femoral Artery Injury and Proliferation and Migration of VSMCs. Arterioscler Thromb Vasc Biol. 2017;37(4):717–729. https://doi.org/10.1161/ATVBAHA.116.308210 . Epub 2017 Feb 9.
L Pleva 1 2, Kovarova P 3 4, L Faldynova 5 6, P Plevova 7 8, S, Hilscherova. 9 10, J Zapletalova 11, P Kusnierova 12 13, P Kukla 14. The rs1803274 polymorphism of the BCHE gene is associated with an increased risk of coronary in-stent restenosis. BMC Cardiovasc Disord. 2015;15:135. https://doi.org/10.1186/s12872-015-0128-8 .
Li Y. 1, Fang Chen 1, Xiaoling Zhang 1, Yuechun Gao 1, Changyan Wu 1, Haiyan Li 1, Yuchen Zhang 1. Angiotensin type 1 receptor A1166C gene polymorphism is associated with endothelial dysfunction and in-stent restenosis after percutaneous coronary intervention. Int J Clin Exp Pathol. 2015;8(6):7350-7. eCollection 2015.
Younes Nozari 1, Vosooghi S, Boroumand M, Poorhosseini H, Nematipour E, Salarifar M, Kassaian SE, Amirzadegan A. Mohammad Alidoosti, Ali Mohammad Haji-Zeinali, Sepideh Saroukhani. The impact of cytochrome P450 2C19 polymorphism on the occurrence of one-year in-stent restenosis in patients who underwent percutaneous coronary intervention: a case-match study. Anatol J Cardiol. 2015;15(5):348–53. https://doi.org/10.5152/akd.2014.5418 .
Wenwei Liu Y 1, Liu H, Jiang X, Ding R, Zhu B, Li Y, Zhao. Plasma levels of interleukin 18, interleukin 10, and matrix metalloproteinase-9 and – 137G/C polymorphism of interleukin 18 are associated with incidence of in-stent restenosis after percutaneous coronary intervention. Inflammation. 2013;36(5):1129–35. https://doi.org/10.1007/s10753-013-9647-6 .
Jing Gao Y 1, Liu R-Z, Cui Y-M, Mao J, Zhou Q, Chen F-M, Zhao. Gui-Ming Yang. Relationship of interleukin-6-572 C/G promoter polymorphism and serum levels to post-percutaneous coronary intervention restenosis. Chin Med J (Engl). 2013;126(6):1019–25.
Jing Gao Rang-zhuang 1, Cui Y, Liu Yong-min, Mao J, Zhou Q, Chen Fu-mei, Zhao Gui-ming, Yang, Ting Liu. Relationship of interleukin-10 gene polymorphism with restenosis after percutaneous coronary intervention in Chinese. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2011;28(1):42–6. https://doi.org/10.3760/cma.j.issn.1003-9406.2011.01.010 .
Konstantina Vogiatzi 1, Apostolakis S, Voudris V, Thomopoulou S, Kochiadakis GE, Demetrios A, Spandidos. Interleukin 8 gene polymorphisms and susceptibility to restenosis after percutaneous coronary intervention. J Thromb Thrombolysis. 2010;29(1):134–40. https://doi.org/10.1007/s11239-009-0338-y .
Konstantina Vogiatzi 1, Voudris V, Apostolakis S, Kochiadakis GE, Thomopoulou S, Zaravinos A, Demetrios A, Spandidos. Genetic diversity of RANTES gene promoter and susceptibility to coronary artery disease and restenosis after percutaneous coronary intervention. Thromb Res. 2009;124(1):84–9. https://doi.org/10.1016/j.thromres.2008.12.043 . Epub 2009 Feb 7.
Svati HS 1, Hauser ER, Crosslin D, Wang L, Haynes C, Connelly J, Nelson S, Johnson J, Gadson S, Nelson CL, Seo D, Gregory S, Kraus WE, Granger CB. Pascal Goldschmidt-Clermont, L Kristin Newby. ALOX5AP variants are associated with in-stent restenosis after percutaneous coronary intervention. Atherosclerosis. 2008;201(1):148 – 54. https://doi.org/10.1016/j.atherosclerosis.2008.01.011 . Epub 2008 Feb 12.
Katsaros 1 KM, Speidl WS, Kastl SP, Zorn G, Huber K, Maurer G, Glogar D, Wojta J, Christ G. Plasminogen activator inhibitor-1 predicts coronary in-stent restenosis of drug-eluting stents. J Thromb Haemost. 2008;6(3):508–13. https://doi.org/10.1111/j.1538-7836.2007.02884.x . Epub 2007 Dec 25.
Jasper S, Wijpkema 1 PL, van Haelst PS, Monraats M, Bruinenberg AH, Zwinderman F, van der Zijlstra RJ, de Winter, Pieter AFM, Doevendans J, Waltenberger JW, Jukema. René A Tio. Restenosis after percutaneous coronary intervention is associated with the angiotensin-II type-1 receptor 1166A/C polymorphism but not with polymorphisms of angiotensin-converting enzyme, angiotensin-II receptor, angiotensinogen or heme oxygenase-1. Pharmacogenet Genomics. 2006;16(5):331-7. https://doi.org/10.1097/01.fpc.0000205001.07054.fa .
Pascalle S, Monraats 1 JS, Rana AH, Zwinderman, Moniek PM, de Maat JP, Kastelein, Willem RP, Agema, Pieter AF, Doevendans RJ, de Winter RenéA, Tio J, Waltenberger, Rune R, Frants A, van der Laarse, Ernst E, van der Wall JW, Jukema. -455G/A polymorphism and preprocedural plasma levels of fibrinogen show no association with the risk of clinical restenosis in patients with coronary stent placement. Thromb Haemost. 2005;93(3):564–9. https://doi.org/10.1160/TH04-11-0708 .
Sheikhvatan M, Chaichian S, Moazzami B. A systematic review and Bioinformatics Study on genes and micro-RNAs involving the Transformation of endometriosis into Ovarian Cancer. Microrna. 2020;9(2):101–11. https://doi.org/10.2174/2211536608666190917152104 . PMID: 31530272; PMCID: PMC7366012.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Cardiovascular Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
Farzad Shahsanaei, Abdullah Gharibzadeh, Soudabeh Behrooj, Shahin Abbaszadeh & Mahboobeh Nourmohammadi
You can also search for this author in PubMed Google Scholar
Contributions
F.S and S.B analyzed and interpreted the data. SH.A and A.GH was a major contributor in writing the manuscript. M.N revised the manuscript and supervised All the above from beginning to end. Authors read and approved the final manuscript.
Corresponding authors
Correspondence to Shahin Abbaszadeh or Mahboobeh Nourmohammadi .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shahsanaei, F., Gharibzadeh, A., Behrooj, S. et al. A systematic review and bioinformatic study on clinical, paraclinical, and genetic factors predisposing to stent restenosis following percutaneous coronary intervention. BMC Cardiovasc Disord 24 , 304 (2024). https://doi.org/10.1186/s12872-024-03955-3
Download citation
Received : 06 March 2024
Accepted : 22 May 2024
Published : 14 June 2024
DOI : https://doi.org/10.1186/s12872-024-03955-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Stent restenosis
- Bioinformatic
BMC Cardiovascular Disorders
ISSN: 1471-2261
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 18 June 2024
Patient safety in orthodontic care: a scoping literature review with proposal for terminology and future research agenda
- Nikolaos Ferlias 1 , 3 ,
- Ambrosina Michelotti 2 &
- Peter Stoustrup 1
BMC Oral Health volume 24 , Article number: 702 ( 2024 ) Cite this article
Metrics details
Knowledge about patient safety in orthodontics is scarce. Lack of standardisation and a common terminology hinders research and limits our understanding of the discipline. This study aims to 1) summarise current knowledge about patient safety incidents (PSI) in orthodontic care by conducting a systematic literature search, 2) propose a new standardisation of PSI terminology and 3) propose a future research agenda on patient safety in the field of orthodontics.
A systematic literature search was performed in the main online sources of PubMed, Web of Science, Scopus and OpenGrey from their inception to 1 July 2023. Inclusion criteria were based on the World Health Organization´s (WHO) research cycle on patient safety. Studies providing information about the cycle’s steps related to orthodontics were included. Study selection and data extraction were performed by two of the authors.
A total of 3,923 articles were retrieved. After review of titles and abstracts, 41 articles were selected for full-text review and 25 articles were eligible for inclusion. Seven provided information on the WHO’s research cycle step 1 (“measuring harm”), twenty-one on “understanding causes” (step 2) and twelve on “identifying solutions” (step 3). No study provided information on Steps 4 and 5 (“evaluating impact” or “translating evidence into safer care”).
Current evidence on patient safety in orthodontics is scarce due to a lack of standardised reporting and probably also under-reporting of PSIs. Current literature on orthodontic patient safety deals primarily with “measuring harms” and “understanding causes of patient safety”, whereas less attention has been devoted to initiatives “identifying solutions”, “evaluating impact” and “translating evidence into safer care”. The present project holds a proposal for a new categorisation, terminology and future research agenda that may serve as a framework to support future research and clinical initiatives to improve patient safety in orthodontic care.
Registration
PROSPERO (CRD42022371982).
Peer Review reports
Introduction
For decades, patient safety has been recognised as a healthcare discipline. However, the awareness-raising publication of “To Err Is Human” by the Institute of Medicine Committee on Quality of Health Care in the US drew considerable attention to this important aspect of healthcare [ 1 , 2 ]. In this publication, experts estimated that in the US in any given year as many as 98,000 people die from medical errors that occur in hospitals [ 1 ]. The definition of patient safety by the World Health Organization (WHO) from 2009 is: “the freedom for a patient from unnecessary harm or potential harm related to healthcare” [ 2 ]. Similarly, in their report, Kohn et al. recognised safety as “freedom from accidental injury” [ 1 ]. In this context, a patient safety incident (PSI) is an event or circumstance that could have resulted or did result in unnecessary harm to a patient [ 2 ].
Patient safety is a crucial aspect of healthcare that seeks to minimise preventable harm, accidents, complications and adverse events (AEs). AEs are defined as injuries resulting from poor management practices that could have been prevented but are not attributed to an underlying disease process [ 2 , 3 ]. The WHO classifies certain AEs as "never events", which are serious incidents that should not occur given the presence of strong systemic safety measures [ 4 ]. Never events can have a profound impact on patients, and their prevention is a key objective of healthcare organisations. In this context, patient safety aims to limit the impact of AEs adverse events and promote the avoidance of preventable harm.
Patient safety is a priority from the patient’s perspective, and for care providers it falls in line with the Hippocratic Oath ("primum non nocere"), which is an important element of modern healthcare. Patient safety initiatives analyse characteristics and features of healthcare systems that may lead to the occurrence of AEs. These features are latent risks that may be of any nature from a soft tissue laceration or a loose wire to inhalation of an orthodontic appliance [ 5 ]. Throughout most healthcare treatment courses, multiple latent risks exist and this makes patient safety multifactorial and complex. When an AE occurs, patient safety does not aim to punish but rather to investigate how and why the protective barriers failed [ 6 , 7 ].
Improving the quality of care is a road that passes through patient safety. Additionally, patient safety has additional psychosocial and financial benefits. Dealing with the consequences of an adverse event has an economic cost to the practitioner, the patient and society. By improving patient safety, dental practitioners increase their quality of care, which is associated with safer and better treatment outcomes [ 8 , 9 , 10 ]. In addition, it affords increased legal security by minimising the risk of legal claims [ 6 ].
Knowledge about patient safety in dental care and orthodontics in particular is scarce. The absence of patient safety guidelines in orthodontics is a major concern. This issue is further complicated by the absence of standardized terminology in the field, challenging the development of consistent safety protocols. Additionally, there is a noticeable lack of research and publications in this area, which hinders progress in developing effective, evidence-based strategies to ensure patient safety in orthodontic care [ 11 ]. Therefore, an urgent need exists for studies in the field of orthodontics in particular [ 2 , 3 , 12 ]. Among others, the lack of a common language among orthodontic caregivers ultimately hinders research and limits our understanding of the discipline [ 13 , 14 ]. The aims of this study were to 1) summarise current knowledge about PSIs in orthodontic care by performing a systematic literature search; 2) propose a new standardisation of PSI terminology; 3) propose a research agenda on patient safety in the field of orthodontics that may serve to further develop and provide direction for future research on the subject.
Materials and methods
Protocol and registration.
Prior to the initiation of the project, the study protocol was registered with PROSPERO (reg. no. CRD42022371982). No ethical approval was deemed necessary.
Search strategy
A systematic literature search was performed in the main online sources of MEDLINE (through PubMed), Web of Science, Scopus as well as the System for Information on Grey Literature in Europe (Open-Grey) from their inception to 1 July 2023. No language limitation was set in the search, and all types of eligible human studies were included.
The inclusion criteria for articles were based on the WHO research cycle on patient safety [ 15 , 16 ]. The various steps of the cycle aim to measure harm and identify causes while identifying solutions to improve patient safety. The ultimate goal is to translate evidence into safer care (Fig. 1 ). Only studies that provided relevant information in at least one of the following categories were eligible for inclusion in this systematic review:
Measuring harm: Studies characterising and/or reporting on the occurrence of AEs or orthodontic-related patient harm.
Understanding causes: Reports focusing on understanding causes leading to patient harm and AEs from orthodontic care.
Identifying solutions: Studies identifying solutions that are effective in reducing the occurrence of AEs and patient harm.
Evaluating impact: Studies evaluating the effectiveness of solutions in terms of impact, affordability and acceptability.

The World Health Organization’s research cycle on patient safety consisting of five steps with the main goal of measuring harm and its causes while identifying solutions and their impact. Ultimately, this evidence should lead to safer care with a set of actions and preventable measures
Only full-text articles were included. In addition, studies dealing with patient safety from a general dental-care perspective were included only if they were directly relevant to orthodontic care and the WHO research cycle. For example, although studies on oral surgery were excluded, wrong-tooth-extraction studies or articles investigating the light-curing safety on patients were included owing to their relevance to orthodontics.
The following MESH terms were used for the systematic search:
(((orthodontic*) OR (dental)) AND (patient safety)) AND ((((((((((((((((((((((((((harm) OR (risk*)) OR (malpractice)) OR (adverse event*)) OR (adverse effect*)) OR (never event*)) OR (iatrogenic)) OR (damage)) OR (incident*)) OR (accident*)) OR (delay* diagnos*)) OR (misdiagnosis)) OR (complication*)) OR (allerg*)) OR (infection)) OR (failure)) OR (error*)) OR (white spot lesion*)) OR (root resorption)) OR (relapse)) OR (decalcification)) OR (caries)) OR (periodontal disease)) OR (nerve damage)) OR (injury)) OR (temporomandibular joint dysfunction)).
Data extraction
After removal of duplicates, all results returned from the systematic literature search were initially screened by their title to establish their relevance. The second filtering decided relevance for inclusion based on the content of the abstract. Finally, the third filtering level was applied to the main text, and the remaining studies were then included in the review. All screening was performed independently by one of the authors (NF) and was later re-checked by another author (PS). Any disputes in study selection were addressed and resolved through discussion between the reviewing authors. On all included studies the main outcome/result was recorded. This was studies investigating prevalence (“measuring harm”- step 1) or assessing contributing factors (“understanding causes”-step 2). For all studies providing information on the cycle’s step 3 (“identifying solutions”), all recommended solutions to prevent harm were also noted. Due to the nature of the data in the included studies, no risk of bias assessment was possible. For the same reason, no quantitative synthesis and meta-analysis was performed. Based on these findings, the intention to conduct a systematic review was revised to a scoping literature review instead [ 17 ].
Study selection
A total of 3,923 studies were identified from the systematic search and imported into Excel (Microsoft®, USA) (PubMed n = 2,049, Web of Science n = 663, Scopus n = 1203 and OpenGrey n = 8). Among the 3,923 articles, 237 were deemed relevant according to the inclusion criteria after screening their titles. Filtering by abstracts, left 41 articles for inclusion after removal of the duplicates. In one case, the full-text of an article was unavailable and it was therefore excluded [ 18 ]. Three relevant articles found in the reference lists were also added [ 4 , 14 , 19 ]. Finally, 25 studies were included as they were found to provide information within any of the categories of the WHO’s research cycle on patient safety related to the orthodontic field (flowchart presented in Fig. 2 ).

PRISMA flowchart diagram of the systematic literature search and inclusion procedure
Study characteristics
Study characteristics are shown in Table 1 . Nine of the included papers were retrospective studies of AEs studying: eye wear protection and ocular trauma in orthodontic practice [ 19 ], clinical evaluation of a locking orthodontic facebow [ 20 ], adverse reactions to dental materials [ 3 ], case reports of latex allergy [ 21 ], wrong tooth extraction claims [ 4 ], dental and orthodontic PSIs in a UK register [ 7 ] and a Finnish register [ 8 ], adverse reactions to dental devices reported at the US Food and Drug Administration [ 9 ] and investigation of monomer release from orthodontic adhesives [ 22 ].
The remaining sixteen studies reported risk assessments of orthodontic procedures or materials. These included safety assessment of dental radiography [ 23 ], bonding of brackets under general anaesthesia [ 24 ], orthodontic facebows [ 10 ], mini-implants [ 12 , 25 , 26 ], soft-tissue lasers in orthodontics [ 13 ], effect of orthodontic treatment on patients’ diet [ 14 ], eye safety of curing lights [ 27 ], safety of metal fixed appliance during magnetic resonance imaging (MRI) [ 28 ], pulp safety of various types of curing lights [ 29 ], wrong tooth extraction in orthodontics [ 30 , 31 , 32 ], orthodontic treatment by identifying orthodontic never events [ 33 ] and complications after orthognathic surgery [ 34 ]. These studies identified risks in orthodontic procedures or materials and proposed solutions to manage and minimise these risks.
Study results
Measuring harm.
Seven of the studies included provided information in the first category of the WHO’s research cycle on patient safety, which is “measuring harm” [ 4 , 7 , 8 , 9 , 19 , 22 , 34 ]. Sims et al. conducted a postal survey on eye protection in the UK and found that ocular injuries were reported in 37.7% of all respondents involving orthodontists, assistants and patients [ 19 ]. Peleg et al. conducted a root-cause analysis of wrong-tooth extraction in 54 insurance claims in Israel and reported that in two thirds of all claims an identification error was the cause of the incorrect tooth extraction [ 4 ]. Also, a cross-sectional study on PSIs in the UK found that orthodontic PSIs accounted for 8.9% of all reported dental PSIs in the country [ 7 ]. Hebballi et al. investigated the frequency and types of AEs associated with dental devices as reported to the Food and Drug Administration and User Facility Device Experience (MAUDE) in the US [ 9 ]. They reported that orthodontic appliances and accessories accounted for 1% of all AEs involving dental devices. In a similar investigation in hospital and private settings in Finland, Hiivala et al. reported that orthodontic PSIs accounted for 3.6% of all dental PSIs [ 8 ]. Finally, a multi-centre retrospective review of orthognathic surgeries assessing complications and risk factors studied a population of 674 patients [ 34 ]. They reported that adverse events were rare (4.3%) with superficial incisional infection being the most common. They also concluded that the setting, the type of surgery as well as the patients’ ethnicity were identified as risk factors for some types of complications.
Understanding causes of harm & identifying solutions
Twenty-one of the included studies identified the underlying causes of AEs that caused patient harm (WHO’s Category 2 “Understanding the causes”) [ 3 , 4 , 7 , 10 , 12 , 13 , 14 , 19 , 20 , 21 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 34 ]. In addition, twelve studies identified possible solutions that may be effective in reducing the occurrence of AEs (WHO’s Cycle Category 3 “Identifying solutions”) [ 4 , 10 , 12 , 13 , 19 , 20 , 21 , 23 , 24 , 25 , 31 , 32 ]. These solutions included: health and safety instructions for eye-protection goggles to prevent ocular trauma [ 19 ], use of non-latex materials [ 21 ], clear instructions with a brief description of the tooth to be extracted addressed to the clinician using two different identification methods to prevent wrong-site extraction and use of a computerised checklist [ 4 , 31 , 32 ], use of facebows with a locking mechanism and self-releasing head strap to prevent injuries from headgear [ 10 , 20 ], suggestions to improve safety in dental radiography [ 23 ], use of rubber dam during bonding of brackets under general anaesthesia [ 24 ], recommendations to overcome failures and risks during placement, loading and removal of mini-implants [ 12 , 25 ] and, finally, instructions for safe use of soft-tissue lasers in orthodontics recommending that the clinician obtained appropriate training and certification, use of proper eye wear by all involved parties, obtaining informed consent and providing proper post-operative instructions [ 13 ].
None of the included studies provided information on how to evaluate the impact of such solutions or on how to translate evidence into safer care in terms of affordability and acceptability. Data synthesis and meta-analysis was not possible due to the heterogeneity of the different studies and the nature of the data.
Patient safety incidents in orthodontics
To our knowledge, this is the first systematic investigation of patient safety in orthodontics. The lack of evidence in the field manifests in our results. Twenty-five studies were included in this review and these studies were only peripherally related to orthodontics while providing some information based on the WHO’s research cycle. This cycle describes a process to identify solutions for enhancing patient safety and reducing patient harm. It consists of five steps representing the natural process for patient-safety initiatives. It seems that dentistry in general and orthodontics in particular have yet to take even the initial steps of the cycle (steps 1 and 2), which are to measure the harm and understand the causes of harm [ 16 ]. This is evident from the results as the included studies were either reviews of risks associated with specific orthodontic procedures (like mini-implant insertion, soft-tissue laser, facebow use, etc.) or retrospective reviews of AEs peripherally related to orthodontics (incidence of ocular trauma, adverse reactions to materials, etc.).
The results of this review document that current evidence relating to orthodontics is scarce. Without a basic understanding of PSIs and harms we cannot begin to understand the causes and identify solutions that will subsequently translate into safer care for our patients [ 16 ]. A major limitation to this is a trend towards potential under-reporting of PSIs in our field. In fact, a review of the National Patient Safety Agency (NPSA) database in the UK revealed that orthodontics is among the lowest reporting specialties along with dental surgery and paediatric dentistry [ 35 ]. A contributing factor in this may be the lesser severity of some PSIs in orthodontics, which may be smaller injuries like soft-tissue laceration from loose wires [ 16 ]. One way to overcome the underreporting issues may be effective keeping of patient records and clinical notes, which may prove an essential tool in clinical audits and will also underpin the reporting of more AEs [ 36 ]. Also, the lack of standardisation in terminology and reporting process of AEs makes it challenging if not impossible to summarise and categorise all PSIs in orthodontics, let alone analyse the data in depth.
Additionally, we hypothesise that an underreporting bias may exist between dental specialities. Dental implants are more expensive and dentists and/or patients may therefore report them more often when asking for replacements [ 9 ]. This leads, e.g., to many more reported PSIs for implants than for burs. Finally, another contributing factor in the lack of evidence on patient safety is the overlap found in some areas within dentistry. This makes it more challenging to precisely measure AEs in only one field. A clear example of this is the AE of wrong-tooth extraction for orthodontic reasons, which may fall in both the orthodontic and surgical category.
Standardisation and terminology
The lack of a standardised terminology and reporting of PSIs in orthodontics seems to hinder any effort to summarise and categorise PSIs, which could be a reasonable first research step to enhance our knowledge in this field. For future work in this field, we therefore suggest that PSIs related to orthodontics may be summarised into two main categories; local and systemic. Categorisation with subcategories and examples are shown in Table 2 . Terminology according to the WHO is proposed in Table 3 .
Local PSIs refer to any harm on dental tissues (root resorption, white spot lesions, pulp necrosis, caries) and soft tissues. This may be damage to both periodontal and surrounding soft tissues that could have been avoided (gingival recessions, soft tissue lacerations, local allergic reaction/contact dermatitis). In addition, local PSIs include treatment injuries with a negative effect on orofacial function. This may be development of lip catch as a result of orthodontic treatment. Finally, any harm related to any unwanted tooth movement is also included in this category. This may be unwanted tooth movement due to an active retainer.
Systemic PSIs refer to harm at a systemic level. This may be excessive pain and discomfort as a result of the orthodontic treatment due to a defective appliance or even hypersensitivity due to excessive interproximal reduction. In addition, systemic PSIs include potential emotional damage to patients. This may be development of general discomfort/odontophobia/mistrust towards the clinician or the healthcare system or deterioration of the oral health-related quality of life (OHRQoL). Systemic PSIs may be a result of delayed treatment initiation due to delayed/inadequate diagnosis. Finally, harm caused by poor cross-infection control, inhalation of orthodontic parts and extraction of a wrong tooth are also considered systemic PSIs.
Future research agenda
A proposal for a future research agenda in orthodontic patient safety is shown in Table 4 . The agenda is intended as inspiration to promote future research and development in patient safety in orthodontics. It should not be considered absolute as topics other than those listed may be of interest for future patient safety initiatives. Two main categories of studies are presented in Table 4 : Retrospective or prospective studies dealing with patient safety (26).
Retrospective studies are reactive in nature and focus on the incidence, characteristics and severity of PSIs using an acknowledged methodology such as journal file audit and root cause analysis (RCA) (26,27). They investigate PSIs that have already occurred with the intention of generating knowledge to promote learning and guidance for future patient safety initiatives. RCA allows us to focus on individual PSIs and investigate, through a comprehensive analysis, all the contributing factors that lead to the occurrence of an AE.
Conversely, prospective studies assess potential risks associated with a treatment, appliance or material. The methodology in these studies is failure mode and effects analysis (FMEA) (27,28). This approach is the analysis of a method, treatment, material or procedure by first creating a risk map and then implementing measures to reduce the likelihood or impact of a PSI (27–30).
Both intrinsic and extrinsic motivation are key factors in the establishment of safer future orthodontic care. Intrinsic motivation is shaped by professional ethics, norms and patient-reported outcomes and expectations [ 1 , 37 , 38 ]. The articles included in our review, however, mainly focused on the extrinsic motivation, which refers to the environment, policies and strategies that we may develop with the ultimate goal of improving patient safety in orthodontics.
In orthodontic patient safety research, a need exists to increase our focus on this aspect and on clinical routines and administrative, organisational and legal contexts. One strategy that may help us move in this direction is to establish excellent records and clinical notes through periodical audits [ 30 ]. This will help clinicians and/or patients report more AEs in future. Honest exchange of such information between health professionals is a necessary first step and a founding rock for safer care and further research. To achieve this, it is important to establish a non-blame culture with psychological safety and a feeling of partnership, enthusiasm and commitment to improving patient safety in orthodontics [ 36 ].
Research on patient safety is more advanced in other parts of healthcare than orthodontics. Even other fields of dentistry have taken steps in this direction with the creation of checklists, i.e. in endodontics, orofacial function and oral surgery [ 39 , 40 , 41 , 42 , 43 ]. Checklists seem to have a positive effect on patient safety [ 44 , 45 , 46 ]. Most of the checklists are adaptations of the WHO’s surgical checklist that is now used in a wide range of surgical specialties in medicine [ 47 ]. Adjusting this to fit our orthodontic needs and implementing it in daily practice may be an important step towards improving safety in orthodontics [ 48 ]. In the past decade, the WHO has published several guidelines and educational curricula to enhance the level of patient safety in healthcare in general [ 49 , 50 ]. These publications may provide a starting point for the spreading of local patient safety initiatives and the introduction of educational and organisational measures to further patient safety.
Some orthodontic societies seem to have taken steps towards patient safety, however all societies in different countries need to follow and implement policies for safer care. In its core patient safety is the purpose of audit and clinical governance. Amongst other, research is a vital element in this process. Nevertheless, a limitation in this could be that clinical governance might differ from one country to another.
Traditionally, patient safety was focused on rare types of incidents with a significant degree of harm referred to as “never events” in the literature [ 51 ]. However, in recent years, more efforts have been devoted to understanding the frequency and causes of PSIs that we assume occur more frequently than is reported today [ 51 ]. The perceived threshold determining what is considered a PSI may often be vague; and the border is not absolute, particularly as we come to understand patient safety better. It is important to emphasize that common side effects (e.g., root resorption) are not considered PSIs as these side effects may also occur when a patient has undergone an optimally performed course of treatment, unless, of course, these side effects were avoidable and appropriate measures had been adopted [ 52 ]. The extent of such side effects, however, can vary and probably depends on a wide range of factors (force magnitude, treatment duration) [ 53 ]. Excessive root resorption, however, may be considered a PSI if the risk factors were not assessed before initiating treatment and if precautionary measures were not taken in advance. A step towards safer orthodontics may be to incorporate such “risk maps” routinely in systematic reviews. For example, when a systematic review compares A to B, reporting just which of the two is more efficient or faster may be insufficient. The burden and the risk of harm to the patient should also be reported. This reporting may include anything that may be considered a PSI, from excessive root resorption to increased exposure to radiation, cytotoxicity, effect on patients’ OHRQoL, late diagnosis, overtreatment, gingival recessions or bone dehiscence, etc. A cultural change in the way we approach these “side effects” and further patient-centred research will improve patient safety in our field. In addition, in today's rapidly evolving technological landscape, where new advancements outpace research capabilities, emphasizing the safety of orthodontic materials is crucial while treatment decisions need to be patient-centred, based on their perspective [ 54 ].
Strengths and limitations
The strengths of this systematic review include an extensive literature search, a predefined protocol, a priori registration with PROSPERO and the adoption of a strict methodology at all study stages [ 55 ]. Also, the fact that there was no date or language limitation in the search, provided us with data that likely reflect the current understanding and knowledge about PSI in orthodontics. In addition, the proposed categorisation of PSIs in orthodontics and the future-agenda proposals may spark interest and lead to further research in the field of orthodontic patient safety.
Certain limitations need further consideration: mainly the inability to assess precise prevalence of orthodontic PSIs and categorise them accordingly. This inability is due to the poor current evidence and lack of standardisation and terminology and the fact that many PSIs are probably underreported. It can also be due to the fact that patient safety is a topic of increasing complexity, especially with the new risks arising directly from the use of new technologies [ 51 ]. Also, there is inherent risk of bias due to the nature of the studies included which were mostly retrospective [ 56 ]. Furthermore, in this study, the final selection of the included studies was consensus-based instead of individually assessing the suitability of the articles during the review process. Finally, despite thorough searching, there could be studies overlooked during the process, possibly originating from databases not encompassed in the search.
Current evidence on patient safety in orthodontics is scarce due to a lack of standardisation and potential under-reporting of PSIs. The current literature on orthodontic patient safety deals mostly with “measuring harms” and “understanding causes of patient safety”, whereas less attention has been devoted to initiatives “identifying solutions”, “evaluating impact” and “translating evidence into safer care”. The present project presents proposals for a new categorisation, terminology and a future research agenda that may serve as a framework to support future research and clinical initiatives to improve patient safety in orthodontic care.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human. Regul Toxicol Pharmacol. 2000;52:1–287.
Google Scholar
World Health Organization (WHO). Conceptual Framework for the International Classification for Patient Safety Final Technical Report. International Classification [Internet]. 2009;(January):3–101. Available from: http://www.who.int/patientsafety/taxonomy/ICPS_Statement_of_Purpose.pdf
Scott A, Egner W, Gawkrodger DJ, Hatton P V., Sherriff M, Van Noort R, et al. The national survey of adverse reactions to dental materials in the UK: A preliminary study by the UK Adverse Reactions Reporting Project. Vol. 196, British Dental Journal. Nature Publishing Group; 2004. p. 471–7.
Peleg O, Givot DMDN, Halamish-shani T, Taicher S. Wrong tooth extraction: root cause analysis. Br Dent J. 2011;210(4):163–163.
Article Google Scholar
Yamalik N, Perea PB. Patient safety and dentistry: What do we need to know? Fundamentals of patient safety, the safety culture and implementation of patient safety measures in dental practice. Int Dent J. 2012;62(4):189–96.
Article PubMed Google Scholar
Dehghanian D, Heydarpoor P, Attaran N, Khoshnevisan M. Clinical governance in general dental practice. Journal of International Oral Health. 2019;11(3):107–11.
Thusu S, Panesar S, Bedi R. Patient safety in dentistry - State of play as revealed by a national database of errors. Br Dent J. 2012;213(3):E3.
Article CAS PubMed Google Scholar
Hiivala N, Mussalo-Rauhamaa H, Tefke HL, Murtomaa H. An analysis of dental patient safety incidents in a patient complaint and healthcare supervisory database in Finland. Acta Odontol Scand [Internet]. 2016 Feb 17;74(2):81–9. Available from: https://www.tandfonline.com/doi/abs/ https://doi.org/10.3109/00016357.2015.1042040
Hebballi NB, Ramoni R, Kalenderian E, Delattre VF, Stewart DCL, Kent K, et al. The dangers of dental devices as reported in the food and drug administration manufacturer and user facility device experience database. J Am Dent Assoc. 2015;146(2):102–10.
Article PubMed PubMed Central Google Scholar
Samuels RHA, Brezniak N. Orthodontic facebows: Safety issues and current management. J Orthod. 2002;29(2):101–7.
Bailey E, Tickle M, Campbell S, O’Malley L. Systematic review of patient safety interventions in dentistry. BMC Oral Health. 2015;15(1):152.
Kravitz ND, Kusnoto B. Risks and complications of orthodontic miniscrews. Am J Orthod Dentofac Orthop. 2007;131(4):S43-51.
Kravitz ND, Kusnoto B. Soft-tissue lasers in orthodontics: An overview. Am J Orthod Dentofacial Orthop. 2008;133(4 SUPPL):S110-4.
Johal A, Abed Al Jawad F, Marcenes W, Croft N. Does orthodontic treatment harm children’s diets? J Dent. 2013;41(11):949–54.
World Health Organization. WHO patient safety research : better knowledge for safer care. 2009;12 p.
Tokede O, Walji M, Ramoni R, Rindal D, Worley D, Hebballi N, et al. Quantifying Dental Office–Originating Adverse Events: The Dental Practice Study Methods. J Patient Saf. 2017;Publish Ah(00):1–8.
Vaid N. Scoping studies: Should there be more in orthodontic literature? APOS Trends in Orthodontics. 2019;9(3):124–5.
Rak D. X-ray examinations in orthodontic diagnostics as a source of ionizing radiation. Bilten Udruzenja ortodonata Jugoslavije. Bulletin Orthod Soc Yugosl. 1989;22:37–48.
CAS Google Scholar
Sims AP, Roberts-Harry TJ, Roberts-Harry DP. The incidence and prevention of ocular injuries in orthodontic practice. Br J Orthod. 1993;20(4):339–43.
Samuels R, O’Neill J, Bhavra G, Hills D, Thomas P, Hug H, et al. A clinical evaluation of a locking orthodontic facebow. American J Orthod Dentofacial Orthop. 2000;117(3):344–50.
Article CAS Google Scholar
Raggio DP, Camargo LB, Naspitz GMCC, Bonifacio CC, Politano GT, Mendes FM, et al. Latex allergy in dentistry: Clinical cases report. J Clin Exp Dent. 2010;2(1):e55–9.
Bationo R, Jordana F, Boileau MJ, Colat-Parros J. Release of monomers from orthodontic adhesives. Am J Orthod Dentofac Orthop. 2016;150(3):491–8.
Abbott P. Are dental radiographs safe? Aust Dent J. 2000;45(3):208–13.
Chaushu S, Zeltser R, Becker A. Safe orthodontic bonding for children with disabilities during general anaesthesia. Eur J Orthod. 2000;22(3):225–8.
Suzuki M, Deguchi T, Watanabe H, Seiryu M, Iikubo M, Sasano T, et al. Evaluation of optimal length and insertion torque for miniscrews. Am J Orthod Dentofac Orthop. 2013;144(2):251–9.
Kuroda S, Tanaka E. Risks and complications of miniscrew anchorage in clinical orthodontics. Japan Dent Sci Rev. 2014;50:79–85.
McCusker N, Lee SM, Robinson S, Patel N, Sandy JR, Ireland AJ. Light curing in orthodontics; Should we be concerned? Dent Mater. 2013;29(6):e85-90.
Görgülü S, Ayyildiz S, Kamburoǧlu K, Gökçe S, Ozen T. Effect of orthodontic brackets and different wires on radiofrequency heating and magnetic field interactions during 3-T MRI. Dentomaxillofacial Radiol. 2014;43(2):20130356.
Mouhat M, Mercer J, Stangvaltaite L, Örtengren U. Light-curing units used in dentistry: factors associated with heat development—potential risk for patients. Clin Oral Investig. 2017;21(5):1687–96.
Anwar H, Waring D. Improving patient safety through a clinical audit spiral: prevention of wrong tooth extraction in orthodontics. Br Dent J. 2017;223(1):48–52.
Cullingham P, Saksena A, Pemberton MN. Patient safety: Reducing the risk of wrong tooth extraction. Br Dent J. 2017;222(10):759–63.
Jacob O, Gough E, Thomas H. Preventing wrong tooth extraction. Acta Stomatol Croat. 2021;55(3):316–24.
Jerrold L, Danoff-Rudick J. Never events in clinical orthodontic practice. Am J Orthod Dentofac Orthop. 2022;161(4):480–9.
Knoedler S, Baecher H, Hoch CC, Obed D, Matar DY, Rendenbach C, et al. Early Outcomes and Risk Factors in Orthognathic Surgery for Mandibular and Maxillary Hypo- and Hyperplasia: A 13-Year Analysis of a Multi-Institutional Database. J Clin Med. 2023;12(4):1444.
Bagley CHM, Panesar SS, Patel B, Cleary K, Pickles J. Safer cut: Revelations of surgical harm through a national database [Internet]. Vol. 71, British Journal of Hospital Medicine. MA Healthcare London; 2010. p. 484–5. Available from: https://www.magonlinelibrary.com/doi/10.12968/hmed.2010.71.9.78155
Yamalik N. Quality systems in dentistry Part 2. Quality assurance and improvement (QA/I) tools that have implications for dentistry. Int Dent J [Internet]. 2007;57(6):459–67. Available from: https://pubmed.ncbi.nlm.nih.gov/18265780/
Hua F. Dental patient-reported outcomes update 2022. J Evid Based Dent Pract. Mosby. 2023;23:1–6.
Tao Z, Zhao T, Ngan P, Qin D, Hua F, He H. The use of dental patient-reported outcomes among randomized controlled trials in orthodontics: a methodological study. J Evid Based Dent Pract. 2023;23(1): 101795.
Díaz-Flores-García V, Perea-Pérez B, Labajo-González E, Santiago-Sáez A, Cisneros-Cabello R. Proposal of a “Checklist” for endodontic treatment. J Clin Exp Dent. 2014;6(2):104–9.
Wright S, Ucer TC, Crofts G. The adaption and implementation of the WHO surgical safety checklist for dental procedures. Br Dent J. 2018;225(8):727–9.
Nenad MW, Halupa C, Spolarich AE, Gurenlian JAR. A Dental Radiography Checklist as a Tool for Quality Improvement. J Dent Hyg. 2016;90(6):386–93.
PubMed Google Scholar
Beddis HP, Davies SJ, Budenberg A, Horner K, Pemberton MN. Temporomandibular disorders, trismus and malignancy: Development of a checklist to improve patient safety. Br Dent J. 2014;217(7):351–5.
Schmitt CM, Buchbender M, Musazada S, Bergauer B, Neukam FW. Evaluation of Staff Satisfaction After Implementation of a Surgical Safety Checklist in the Ambulatory of an Oral and Maxillofacial Surgery Department and its Impact on Patient Safety. J Oral Maxillofac Surg. 2018;76(8):1616–39.
Wilson L, Walker L. The WHO surgical safety checklist: The evidence. J Perioper Pract [Internet]. 2009;19(10):362–4. Available from: https://journals.sagepub.com/doi/epdf/10.1177/175045890901901002
Weiser TG, Haynes AB, Dziekan G, Berry WR, Lipsitz SR, Gawande AA. Effect of A 19-item surgical safety checklist during urgent operations in a global patient population. Ann Surg. 2010;251(5):976–80.
Vats A, Vincent CA, Nagpal K, Davies RW, Darzi A, Moorthy K. Practical challenges of introducing WHO surgical checklist: UK pilot experience. BMJ (Online). 2010;340(7738):133–5.
World Health Organization. Tool and Resources [Internet]. WHO Surgical Safety Checklist. 2009. Available from: https://www.who.int/teams/integrated-health-services/patient-safety/research/safe-surgery/tool-and-resources
Clark S, Hamilton L. WHO surgical checklist: Needs to be customised by specialty. Vol. 340, BMJ (Online). British Medical Journal Publishing Group; 2010. p. 280.
World Health Organization (WHO). Global Patient Safety Action Plan 2021–2030 [Internet]. Vol. 53, World Health Organization. 2020. 1689–1699 p. Available from: https://www.who.int/teams/integrated-health-services/patient-safety/policy/global-patient-safety-action-plan
World Health Organization (WHO). Patient Safety Research course.2022; Available from: https://www.who.int/teams/integrated-health-services/patient-safety/guidance/patient-safety-research-course
Vincent C, Amalberti R. Safer Healthcare: Strategies for the Real World. Cham: Springer; 2016. p. 1–157.
Stoustrup P, Ferlias N. Patientskader i forbindelse med ortodonti Tandlaegebladet. 2022;126:812–22.
Yassir YA, McIntyre GT, Bearn DR. Orthodontic treatment and root resorption: An overview of systematic reviews. Eur J Orthod. 2021;43(4):442–56.
Alansari R, Vaiid N. Why do patients transition between orthodontic appliances? A qualitative analysis of patient decision-making. Orthod Craniofac Res. 2023;00:1–8.
Higgins, Julian PT and Green S. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training. Vol. 2, Handbook. 2011. p. 649.
OCEBM Table of Evidence Working Group = Jeremy Howick, Iain Chalmers (James Lind Library), Paul Glasziou, Trish Greenhalgh, Carl Heneghan, Alessandro Liberati, Ivan Moschetti, Bob Phillips, Hazel Thornton OG and MH. OCEBM Levels of Evidence — Centre for Evidence-Based Medicine (CEBM), University of Oxford [Internet]. 2011. p. 1. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
Download references
Author information
Authors and affiliations.
Section of Orthodontics, Department of Dentistry and Oral Health, Aarhus University, Aarhus, Denmark
Nikolaos Ferlias & Peter Stoustrup
Department of Neurosciences, Reproductive Sciences and Oral Sciences, Section of Orthodontics and Temporomandibular Disorders, University of Naples Federico II, Naples, Italy
Ambrosina Michelotti
Private Practice, Brighton, UK
Nikolaos Ferlias
You can also search for this author in PubMed Google Scholar
Contributions
NF: Conceptualization, Search strategy, Data synthesis, interpretation and analysis, Investigation, Methodology, Validation, Writing original draft, Writing review & editing. AM: Investigation, Methodology, Data interpretation and analysis, Supervision, Validation, Writing review & editing. PS: Conceptualization, Search strategy, Data synthesis, interpretation and analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing original draft, Writing review & editing.
Corresponding author
Correspondence to Nikolaos Ferlias .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ferlias, N., Michelotti, A. & Stoustrup, P. Patient safety in orthodontic care: a scoping literature review with proposal for terminology and future research agenda. BMC Oral Health 24 , 702 (2024). https://doi.org/10.1186/s12903-024-04375-7
Download citation
Received : 11 March 2024
Accepted : 14 May 2024
Published : 18 June 2024
DOI : https://doi.org/10.1186/s12903-024-04375-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Systematic review
- Patient safety
- Orthodontics
- Patient safety incidents
- Patient harm
- Adverse events
BMC Oral Health
ISSN: 1472-6831
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Image by TraceyChandler. Steps to conducting a systematic review. Quick overview of the process: Steps and resources from the UMB HSHSL Guide. YouTube video (26 min); Another detailed guide on how to conduct and write a systematic review from RMIT University; A roadmap for searching literature in PubMed from the VU Amsterdam; Alexander, P. A. (2020).
Reasons for inclusion and exclusion should be recorded. Step 3: Assessing the quality of studies. Study quality assessment is relevant to every step of a review. Question formulation (Step 1) and study selection criteria (Step 2) should describe the minimum acceptable level of design.
It provides detailed guidelines on how to complete each step of the systematic review process. ... Systematic review vs. literature review. A literature review is a type of review that uses a less systematic and formal approach than a systematic review. Typically, an expert in a topic will qualitatively summarize and evaluate previous work ...
Abstract. Performing a literature review is a critical first step in research to understanding the state-of-the-art and identifying gaps and challenges in the field. A systematic literature review is a method which sets out a series of steps to methodically organize the review. In this paper, we present a guide designed for researchers and in ...
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
Screen the literature. Assess the quality of the studies. Extract the data. Analyze the results. Interpret and present the results. 1. Decide on your team. When carrying out a systematic literature review, you should employ multiple reviewers in order to minimize bias and strengthen analysis.
Registration can be done on platforms like PROSPERO 5 for health and social care reviews or Cochrane 3 for interventions. Step 3: search. In the process of conducting a systematic review, a well-organized literature search is a pivotal step.
A comprehensive, transparent, and reproducible search of the literature is key to the validity of a systematic review's conclusions. Select studies . Appropriate studies will need to be selected from your search results based on the inclusion and exclusion criteria defined in your protocol.
The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information.
If your review will contain a meta-analysis you may want to code the data in order to automate the statistical analysis process. Some systematic review software packages listed in step 6. can help you create coded data instruction forms. Instructions on designing a coded data extraction form can be found in the following article:
This diagram illustrates the steps visually and in plain language, the steps authors do when completing a systematic review. Designed by Jessica Kaufman, Cochrane Consumers & Communication Review Group, Centre for Health Communication & Participation, La Trobe University, 2011.
What is a Systematic Review? Types of Reviews ; Manuals and Reporting Guidelines ; Our Service ; Steps in a Systematic Review. 1. Assemble Your Team ; 2. Develop a Research Question ; 3. Write and Register a Protocol ; 4. Search the Evidence ; 5. Screen Results ; 6. Assess for Quality and Bias ; 7. Extract the Data ; 8. Write the Review ...
Steps in a Systematic Review. 0. Preliminary Searching ; 1. Develop a Protocol ; 2. Draft your Research Question ... Systematic reviews require time. 12-24 months is usual from conception to submission. ... A well done systematic review is a major contribution to the literature. But the requirements in time and effort are massive.
A systematic review is a literature review that gathers all of the available evidence matching pre-specified eligibility criteria to answer a specific research question. It uses explicit, systematic methods, documented in a protocol, to minimize bias, provide reliable findings, and inform decision-making.
Detailed steps for conducting any systematic review and meta-analysis. We searched the methods reported in published SR/MA in tropical medicine and other healthcare fields besides the published guidelines like Cochrane guidelines {Higgins, 2011 #7} [] to collect the best low-bias method for each step of SR/MA conduction steps.Furthermore, we used guidelines that we apply in studies for all SR ...
The meticulous nature of the systematic review research methodology differentiates a systematic review from a narrative review (literature review or authoritative review). This paper provides a brief step by step summary of how to conduct a systematic review, which may be of interest for clinicians and researchers.
Method details Overview. A Systematic Literature Review (SLR) is a research methodology to collect, identify, and critically analyze the available research studies (e.g., articles, conference proceedings, books, dissertations) through a systematic procedure [12].An SLR updates the reader with current literature about a subject [6].The goal is to review critical points of current knowledge on a ...
Use a spreadsheet, or systematic review software, to extract all relevant data from each included study. It is recommended that you pilot your data extraction tool, to determine if other fields should be included or existing fields clarified. Evaluate the risk of bias of included studies . Use a Risk of Bias tool (such as the Cochrane RoB Tool ...
This article provides a step-by-step approach to conducting and reporting systematic literature reviews (SLRs) in the domain of healthcare design and discusses some of the key quality issues associated with SLRs. SLR, as the name implies, is a systematic way of collecting, critically evaluating, integrating, and presenting findings from across ...
A systematic search to identify studies must be comprehensive and it must strike a balance between recall and precision. In other words, don't expect to retrieve only relevant articles.. Most studies used in the review will be identified using electronic databases (e.g. PubMed), but identifying unpublished studies is important as well.
Systematic reviews that summarize the available information on a topic are an important part of evidence-based health care. There are both research and non-research reasons for undertaking a literature review. It is important to systematically review the literature when one would like to justify the need for a study, to update personal ...
A literature review is an integrated analysis-- not just a summary-- of scholarly writings and other relevant evidence related directly to your research question.That is, it represents a synthesis of the evidence that provides background information on your topic and shows a association between the evidence and your research question.
A literature review with an evaluation of existing evidence is often the first step in designing a research project. This article provides a systematic framework and guide for clinicians, researchers, and clinician-scientists to appraise the available literature to inform a clinical research project.
The rest of the paper is organized as follows; section 2 outlines the systematic review process. Section 3 presents the systematic protocol followed for the accumulation of the most relevant articles, assessment and systematic. Section 4 presents a summary of the results and findings of the review and discusses these accordingly.
Follow the steps we've covered below to arrive at a consistent, logical piece of lit review. ... This way, you will manage to uncover existing patterns and trends and examine those dependencies in your literature review. A systematic, critical approach is always evaluated much higher than a simple outline of what people say on your subject. ...
However, conducting a systematic review requires different steps that involve different tools and strategies. It can be difficult at times to access and utilize these resources. A researcher can understand and strategize a systematic review following the different steps outlined in this literature review.
The study quality was evaluated based on the following criteria: (1) the systematic review and meta-analysis based on the questions primarily described and formulated; (2) inclusion and exclusion criteria predefined in the studies as eligibility criteria; (3) searching the literature performed on a systematic and comprehensive approach; (4) to ...
A step towards safer orthodontics may be to incorporate such "risk maps" routinely in systematic reviews. For example, when a systematic review compares A to B, reporting just which of the two is more efficient or faster may be insufficient. The burden and the risk of harm to the patient should also be reported.
The ongoing digitization of the industrial sector has reached a pivotal juncture with the emergence of Digital Twins, offering a digital representation of physical assets and processes. One key aspect of those digital representations are simulation models, enabling a deeper insight in the assets current state and its characteristics. This paper asserts that the next evolutionary step in this ...