An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Compositional and functional properties of milk and dairy products derived from cows fed pasture or concentrate-based diets
Affiliations.
- 1 Food Chemistry and Technology, Teagasc Food Research Centre, Cork, Ireland.
- 2 School of Food and Nutritional Sciences, University College Cork, Cork, Ireland.
- PMID: 33949109
- DOI: 10.1111/1541-4337.12751
Worldwide milk production is predominantly founded on indoor, high-concentrate feeding systems, whereas pasture-based feeding systems are most common in New Zealand and Ireland but have received greater attention recently in countries utilizing conventional systems. Consumer interest in 'pasture-fed' dairy products has also increased, arising from environmental, ethical, and nutritional concerns. A substantial body of research exists describing the effect of different feeding strategies on the composition of milk, with several recent studies focusing on the comparison of pasture- and concentrate-based feeding regimes. Significant variation is typically observed in the gross composition of milk produced from different supplemental feeds, but various changes in the discrete composition of macromolecular components in milk have also been associated with dietary influence, particularly in relation to the fatty acid profile. Changes in milk composition have also been shown to have implications for milk and dairy product processability, functionality and sensory properties. Methods to determine the traceability of dairy products or verify marketing claims such as 'pasture-fed' have also been established, based on compositional variation due to diet. This review explores the effects of feed types on milk composition and quality, along with the ultimate effect of diet-induced changes on milk and dairy product functionality, with particular emphasis placed on pasture- and concentrate-based feeding systems.
Keywords: Bovine diet; concentrate; milk composition; pasture; processing.
© 2021 The Authors. Comprehensive Reviews in Food Science and Food Safety published by Wiley Periodicals LLC on behalf of Institute of Food Technologists.
PubMed Disclaimer
Similar articles
- Invited review: A 2020 perspective on pasture-based dairy systems and products. Moscovici Joubran A, Pierce KM, Garvey N, Shalloo L, O'Callaghan TF. Moscovici Joubran A, et al. J Dairy Sci. 2021 Jul;104(7):7364-7382. doi: 10.3168/jds.2020-19776. Epub 2021 Apr 15. J Dairy Sci. 2021. PMID: 33865573 Review.
- Using estimated nutrient intake from pasture to formulate supplementary concentrate mixes for grazing dairy cows. Douglas ML, Auldist MJ, Wright MM, Marett LC, Russo VM, Hannah MC, Garcia SC, Wales WJ. Douglas ML, et al. J Dairy Sci. 2021 Apr;104(4):4350-4361. doi: 10.3168/jds.2020-19383. Epub 2021 Jan 28. J Dairy Sci. 2021. PMID: 33516549
- The effect of by-product inclusion and concentrate feeding rate on milk production and composition, pasture dry matter intake, and nitrogen excretion of mid-late lactation spring-calving cows grazing a perennial ryegrass-based pasture. Condren SA, Kelly AK, Lynch MB, Boland TM, Whelan SJ, Grace C, Rajauria G, Pierce KM. Condren SA, et al. J Dairy Sci. 2019 Feb;102(2):1247-1256. doi: 10.3168/jds.2018-14970. Epub 2018 Dec 20. J Dairy Sci. 2019. PMID: 30580955
- Quality characteristics, chemical composition, and sensory properties of butter from cows on pasture versus indoor feeding systems. O'Callaghan TF, Faulkner H, McAuliffe S, O'Sullivan MG, Hennessy D, Dillon P, Kilcawley KN, Stanton C, Ross RP. O'Callaghan TF, et al. J Dairy Sci. 2016 Dec;99(12):9441-9460. doi: 10.3168/jds.2016-11271. Epub 2016 Oct 19. J Dairy Sci. 2016. PMID: 27771086
- Invited review: production and digestion of supplemented dairy cows on pasture. Bargo F, Muller LD, Kolver ES, Delahoy JE. Bargo F, et al. J Dairy Sci. 2003 Jan;86(1):1-42. doi: 10.3168/jds.S0022-0302(03)73581-4. J Dairy Sci. 2003. PMID: 12613846 Review.
- Multi-Target Feeding-Behavior Recognition Method for Cows Based on Improved RefineMask. Li X, Gao R, Li Q, Wang R, Liu S, Huang W, Yang L, Zhuo Z. Li X, et al. Sensors (Basel). 2024 May 8;24(10):2975. doi: 10.3390/s24102975. Sensors (Basel). 2024. PMID: 38793830 Free PMC article.
- Combined Supplementation of Two Selenium Forms (Organic and Inorganic) and Iodine in Dairy Cows' Diet to Obtain Enriched Milk, Cheese, and Yogurt. Azorín I, Madrid J, Martínez-Miró S, López M, López MB, López MJ, Hernández F. Azorín I, et al. Animals (Basel). 2024 May 2;14(9):1373. doi: 10.3390/ani14091373. Animals (Basel). 2024. PMID: 38731376 Free PMC article.
- Production, Composition and Nutritional Properties of Organic Milk: A Critical Review. Linehan K, Patangia DV, Ross RP, Stanton C. Linehan K, et al. Foods. 2024 Feb 11;13(4):550. doi: 10.3390/foods13040550. Foods. 2024. PMID: 38397527 Free PMC article. Review.
- HS-GC-IMS and HS-SPME/GC-MS coupled with E-nose and E-tongue reveal the flavors of raw milk from different regions of China. Chi X, Zhang Y, Zheng N, Wang J, Liu H. Chi X, et al. Curr Res Food Sci. 2023 Dec 30;8:100673. doi: 10.1016/j.crfs.2023.100673. eCollection 2024. Curr Res Food Sci. 2023. PMID: 38269357 Free PMC article.
- The "Crosstalk" between Microbiota and Metabolomic Profile of Kefalograviera Cheese after the Innovative Feeding Strategy of Dairy Sheep by Omega-3 Fatty Acids. Tzora A, Nelli A, Kritikou AS, Katsarou D, Giannenas I, Lagkouvardos I, Thomaidis NS, Skoufos I. Tzora A, et al. Foods. 2022 Oct 11;11(20):3164. doi: 10.3390/foods11203164. Foods. 2022. PMID: 37430914 Free PMC article.
- Adler, S. A., Dahl, A. V., Jensen, S. K., Thuen, E., Gustavsson, A. M., & Steinshamn, H. (2013). Fatty acid composition, fat-soluble vitamin concentrations and oxidative stability in bovine milk produced on two pastures with different botanical composition. Livestock Science, 154, 93-102.
- Agabriel, C., Cornu, A., Journal, C., Sibra, C., Grolier, P., & Martin, B. (2007). Tanker milk variability according to farm feeding practices: Vitamins A and E, carotenoids, color, and terpenoids. Journal of Dairy Science, 90, 4884-4896.
- Agenäs, S., Holtenius, K., Griinari, M., & Burstedt, E. (2002). Effects of turnout to pasture and dietary fat supplementation on milk fat composition and conjugated linoleic acid in dairy cows. Acta Agriculturae Scandinavica, Section A - Animal Science, 52, 25-33.
- Akkerman, M., Larsen, L. B., Sørensen, J., & Poulsen, N. A. (2019). Natural variations of citrate and calcium in milk and their effects on milk processing properties. Journal of Dairy Science, 102, 6830-6841.
- Al Attabi, Z., D'Arcy, B., & Deeth, H. (2009). Volatile sulphur compounds in UHT milk. Critical Reviews in Food Science and Nutrition, 49, 28-47.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources, other literature sources.
- scite Smart Citations

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
| Property | Value |
|---|---|
| Status | |
| Version | |
| Ad File | |
| Disable Ads Flag | |
| Environment | |
| Moat Init | |
| Moat Ready | |
| Contextual Ready | |
| Contextual URL | |
| Contextual Initial Segments | |
| Contextual Used Segments | |
| AdUnit | |
| SubAdUnit | |
| Custom Targeting | |
| Ad Events | |
| Invalid Ad Sizes |
Access provided by
Login to your account
If you don't remember your password, you can reset it by entering your email address and clicking the Reset Password button. You will then receive an email that contains a secure link for resetting your password
If the address matches a valid account an email will be sent to __email__ with instructions for resetting your password
Download started.
- PDF [334 KB] PDF [334 KB]
- Add To Online Library Powered By Mendeley
- Add To My Reading List
- Export Citation
- Create Citation Alert
A 100-Year Review: Progress on the chemistry of milk and its components
- John A. Lucey John A. Lucey Correspondence Corresponding author Contact Affiliations Center for Dairy Research, University of Wisconsin–Madison, Madison 53706 Search for articles by this author
- Don Otter Don Otter Affiliations Center for Dairy Research, University of Wisconsin–Madison, Madison 53706 Search for articles by this author
- David S. Horne David S. Horne Affiliations Center for Dairy Research, University of Wisconsin–Madison, Madison 53706 Search for articles by this author
- milk protein
- functionality
- dairy chemistry
INTRODUCTION
- Full Text PDF
- Google Scholar
- Harper W.J.
- Brunner R.J.
- Scopus (49)
- Larson B.L.
- McMeekin T.L.
- Swanson A.M.
- Whitnah C.H.
- Whitney R. McL.
- Scopus (25)
- Farrell Jr., H.M.
- Jimenez-Flores R.
- Butler J.E.
- Creamer L.K.
- Hollar C.M.
- Ng-Kwai-Hang K.F.
- Swaisgood H.E.
- Scopus (1010)
- Richmond H.D.
- Bauman D.E.
- Mather I.H.
- Scopus (297)
A CENTURY OF PROGRESS IN DAIRY CHEMISTRY
Advances in analytical techniques.
- Nitschmann H.
- Scopus (30)
- Bloomfield V.A.
- Scopus (99)
- Scopus (485)
- Kumosinski T.F.
- Scopus (168)
- Haugaard G.
- Pettinati J.D.
- Scopus (13)
Milk Proteins
- Gordon W.G.
- Scopus (65)
- Scopus (469)
- Scopus (157)
- Scopus (333)
- Brodkorb A.
- Scopus (302)
Fractionation and Identification of Individual Milk Proteins
- Linderstrøm-Lang K.
- Mellander O.
- von Hippel P.H.
- Scopus (171)
- Scopus (78)
- Aschaffenburg R.
- Scopus (153)
- Groves M.L.
- Scopus (28)
- Kaminogawa S.
- Mizobuchi H.
- Yamauchi K.
- Hofmann C.J.
- Chibber B.A.K.
- Tomich J.M.
- Keenan T.W.
- Scopus (112)
- Silanikove N.
- Rowland S.J.
- Scopus (369)
Physical and Chemical Properties of Proteins
- Hutton J.T.
- Scopus (57)
- Scopus (158)
- Scopus (62)
- Schauperl M.
- Podewitz M.
- Waldner B.J.
- Scopus (22)
- Srinivasan M.
- Scopus (145)
Calcium Binding
- Scopus (91)
- Scopus (18)
- Scopus (87)
Micelle Models
- Shimmin P.D.
- Scopus (56)
- Scopus (100)
- Stothart P.H.
- Cebula D.J.
- McGann T.C.A.
- Buchheim W.
- Kearney R.D.
- Richardson T.
- Scopus (58)
- Schmidt D.G.
- De Kruif C.G.
- Scopus (432)
- Scopus (588)
Nutritional Aspects of Milk Proteins
- Pellegrino L.
- Cattaneo S.
- Hogenboom J.A.
- Scopus (23)
- Rutherfurd S.M.
- Fanning A.C.
- Miller B.J.
- Moughan P.J.
- Scopus (276)
- Scopus (660)
- Beltrán-Barrientos L.M.
- Hernández-Mendoza A.
- Torres-Llanez M.J.
- González-Córdova A.F.
- Vallejo-Córdoba B.
- Scopus (124)
Functionality of Milk Proteins and Development of New Ingredients
- Mangino M.E.
- Scopus (59)
- Schmidt R.H.
- Packard V.S.
- Morris H.A.
- Scopus (127)
- de Wit J.N.
- Scopus (437)
- Melachouris N.
- Huffman L.M.
- Scopus (96)
- Scopus (29)
- Scopus (371)
- Jensen R.G.
- Sampugna J.
- Scopus (11)
- Scopus (202)
- Parodi P.W.
- Scopus (172)
- Lovegrove J.A.
- Gijsbers L.
- Givens D.I.
- Soedamah-Muthu A.S.
- Scopus (274)
- Palmquist D.L.
- Beaulieu A.D.
- Barbano D.M.
- Scopus (713)
- Whittier E.O.
- Scopus (48)
- Haworth W.N.
- Denton W.L.
- Brodbeck U.
- Whiteman M.
- Yarwood R.J.
- Scopus (37)
Other Milk Components
- McCollum E.V.
- Steenbock H.
- Scopus (90)
- Backstrand J.R.
- Scopus (98)
- Pasteurized Milk Ordinance
- Shahani K.M.
- Parry Jr., R.M.
- Zittle C.A.
- Babcock S.M.
- Russell H.L.
- Jouan P.-N.
- Gauthier S.F.
- Laforest J.-P.
- Brossmer R.
- Scopus (61)
- Scopus (38)
Physical Equilibria and Chemistry of Milk
- Scopus (386)
- Sommer H.H.
- Scopus (17)
- Scopus (72)
- White J.C.D.
- Davies D.T.
- Scopus (43)
- Scopus (67)
- Scopus (51)
- Augustin M.A.
- Scopus (52)
SUMMARY AND FUTURE DIRECTIONS
Acknowledgments.
| Date | Milestone | Reference |
|---|---|---|
| 1921 | Immunoglobulins are shown to be transferred from colostrum to the blood of newborn calves. | 1921; 49: 115-118 |
| 1925 | Casein is shown to be heterogeneous. | |
| 1925 | Lactoperoxidase is first purified. | 1925; 19 (16743483): 175-187 |
| 1927 | Correct structure of lactose is proposed. | 1927; 1927: 544-548 |
| 1929 | Hormones in milk are described for the first time. | 1929; 19: 39-101 |
| 1930 | α-Lactalbumin is crystallized and isolated. | Cited in Milk Proteins, Chemistry and Molecular Biology. Vol. 2. Academic Press, New York, NY1971: 331-365 |
| 1934 | β-Lactoglobulin is isolated. | Cited in Milk Proteins, Chemistry and Molecular Biology. Vol. 2. Academic Press, New York, NY1971: 331-365 |
| 1935 | Evidence shows presence of conjugated linoleic acid in fatty acids from butter. | Cited in 1977; 60: 1550-1553 |
| 1938 | Milk is first fortified with fat-soluble vitamin D | |
| 1939 | Iron-containing red protein (lactoferrin) is observed in milk (but not named “lactoferrin” until 1961). | 1939; 23: 55-99 |
| 1946 | Immunoglobulins from milk and colostrum are isolated and characterized. | |
| 1950 | Bovine serum albumin is isolated. | Cited in Milk Proteins, Chemistry and Molecular Biology. Vol. 2. Academic Press, New York, NY1971: 331-365 |
| 1955 | β-Lactoglobulin is shown to have 2 major genetic variants. | 1955; 176 (13244664): 218-219 |
| 1956 | α - and κ-casein are first identified. | 1956; 78: 4576-4582 |
| 1956 | Sialyl oligosaccharides are first reported. | 1956; 89: 2013-2025 |
| 1959 | Mechanism proposed for formation of milk fat globule in lactating cells. | |
| 1966 | Cholesterol is identified in milk. | 1966; 7 (5947986): 27-37 |
| 1969 | α -Casein fractionated into α - and α -caseins | -casein complex of bovine milk. 1969; 36: 259-268 |
| 1971–1972 | Sequences for major caseins reported | |
| 1977 | The conjugated linoleic acid -9, -11 C18:2 is characterized. | 1977; 60: 1550-1553 |
| 1978 | Skim and low-fat milks are first fortified with vitamin A. | |
| 1998 | Dual-bonding model for micelle assembly is proposed, explaining how κ-casein acts as a chain polymerization terminator, and thus obtains a surface location. | 1998; 8: 171-177 |
| 2002 | Docosahexaenoic acid (DHA) and arachidonic acid (ARA) are first added to infant formula. | |
| 2006 | Milk fat globule membrane proteome is published. | 2006; 73 (16834814): 406-416 |
| 2008 | Bovine milk glycome is published. | 2008; 91 (18832198): 3768-3778 |
| 2009 | Metabolomics analysis of bovine milk using liquid chromatography-mass spectrometry. |
- Open table in a new tab
- Clarenburg R.
- Chaikoff I.L.
- Reinhardt T.A.
- Lippolis J.D.
- Scopus (226)
- Sorensen M.
- Sorensen S.P.L.
- DePeters E.J.
- German J.B.
- Lebrilla C.B.
- Scopus (208)
Article info
Publication history.
This review is part of a special issue of the Journal of Dairy Science commissioned to celebrate 100 years of publishing (1917–2017).
Identification
DOI: https://doi.org/10.3168/jds.2017-13250
User license

For non-commercial purposes:
- Read, print & download
- Text & data mine
- Translate the article
Not Permitted
- Reuse portions or extracts from the article in other works
- Redistribute or republish the final article
- Sell or re-use for commercial purposes
ScienceDirect
- View Large Image
- Download Hi-res image
- Download .PPT
Related Articles
- Access for Developing Countries
- Articles and Issues
- Articles In Press
- Current Issue
- List of Issues
- Supplements
- For Authors
- Instructions to Authors
- Permissions
- Researcher Academy
- Submit Manuscript
- For Reviewers
- Review a Manuscript
- Scientific Sections in the Journal
- Journal Info
- About the Journal
- About Open Access
- Abstracting/Indexing
- Contact Information
- Content Alerts
- Editorial Board
- Journal Editors
- Display Advertisers
- Recruitment Advertising
- Membership Benefits
- ADSA Meetings
- ADSA Member Access
- Collections
- Editor's Choice
- Meeting Abstracts
- JDS Club 100
- Twitter / X
The content on this site is intended for healthcare professionals and researchers across all fields of science.
- Privacy Policy
- Terms and Conditions
- Accessibility
- Help & Contact

Session Timeout (2:00)
Your session will expire shortly. If you are still working, click the ‘Keep Me Logged In’ button below. If you do not respond within the next minute, you will be automatically logged out.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Author Biographies
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Production, composition and nutritional properties of organic milk: a critical review.

Graphical Abstract
1. Introduction
2. an introduction to organic milk production, organic milk production regulations, 3. milk production systems, 3.1. conventional systems, 3.1.1. traditional system, 3.1.2. intensive system, 3.2. organic system.
| Milk Production System | ||
|---|---|---|
| Management Practice | Organic | Conventional |
| Pasture access | Required | Not required |
| Nutrition | All feed must be certified organic | Concentrate feed |
| Antibiotics use | In emergencies, for veterinary indication | Allowed, for veterinary indication |
| Parasiticide use | In emergencies, for veterinary indication | Allowed, for veterinary indication |
| Growth Hormone use | Prohibited | Allowed, for veterinary indication |
| Weed Management | Crop rotation, hand weeding, mulches | Chemical Herbicides |
| Pest Management | Crop rotation, Companion Planting, trap crops, promotion of beneficial insects and natural predators | Chemical Pesticides |
| Green House Gas Emissions | Lower per unit of area | Higher per unit of area |
| Fertilizers | Organic fertilizers only | High dependence on synthetic NPK fertilizers |
| Genetically Modified Organisms | Prohibited | Allowed |
| Synthetic food Additives | Prohibited | Allowed |
| Milk Yields | Lower on average | Higher on average |
| Shelf Life | Higher on average | Lower on average |
| Product Price | Higher on average | Lower on average |
| Soil Impact | Reduced soil loss, increased organic matter, water-holding capacity and microbial diversity | Increased soil loss and erosion, lower water holding capacity, lower carbon storage and microbial diversity |
| Water Consumption | Lower | Higher |
| Energy Usage | Low intensity of energy use (higher energy efficiency) | High intensity of non-renewable energy use (agrochemicals, machinery, water pumping etc.) |
| Impact on Landscape | Larger floral and faunal biodiversity. Diverse agricultural landscapes | Loss of biodiversity in agricultural landscapes, Unified agricultural landscapes (monocultures) |
4. Impact of Production Systems on Farm Performance and Raw Milk Composition
4.1. milk yield, 4.2. udder health and somatic cell count (scc), 4.3. microbiological quality, 4.4. mastitis, 4.5. volatile organic compounds, 4.6. protein, 4.7. vitamins, 4.8. carbohydrates, 4.10. minerals and heavy metals.
| Organic System | Conventional Systems | ||
|---|---|---|---|
| Proteins | Organic Milk | Traditional Milk | Intensive Milk |
| Total Protein (%) | 3.1–3.26 | 3.1–3.24 | 3.48 |
| Casein (%) | 2.54 | 2.52 | 2.78 |
| Whey protein (%) | 0.72–0.84 | 0.72–0.84 | 0.70–0.82 |
| β-Lactoglobulin (g/L) | 3.32–3.35 | 3.26–3.58 | 3.01–3.28 |
| α-Lactalbumin (g/L) | 1.07–1.19 | 1.05–1.21 | 0.98–1.14 |
| Bovine serum albumin (g/L) | 0.43 | 0.44 | 0.41–0.49 |
| Lactoferrin (mg/L) | 123.8–125.9 | 109.80–130.62 | 94.01–121.23 |
| Lysozyme (µg/L) | 11.14 | 9.92–10.71 | 6.90–12.13 |
| Vitamins | Organic Milk | Traditional Milk | Intensive Milk |
| Vitamin A (retinol) (mg/L) | 0.468–0.800 | 0.410–0.556 | 0.347–0.465 |
| β-carotene (mg/L) | 0.195–0.580 | 0.231–0.252 | 0.175–0.190 |
| Vitamin E (α-tocopherol) (mg/L) | 1.358–2.655 | 1.656–1.953 | 1.075–1.302 |
| Vitamin D (cholecalciferol) (μg/L) | 0.461–0.768 | 0.610–1.212 | 0.589–0.700 |
| Carbohydrates | Organic Milk | Traditional Milk | Intensive Milk |
| Lactose (%) | 4.80–5 | 4.7–5 | nd |
| 3 Hex (Trisa) (m/z) | 60.82–61.11 | 51.37–55.86 | nd |
| 3 Hex, 1 NeuAc (m/z) | 11.83–14.60 | 9.24–12.42 | nd |
| 4 Hex, 1 HexNAc (m/z) | 0.87–0.93 | 0.63–0.69 | nd |
| 3 Hex, 2 HexNAc (m/z) | 0.31–0.33 | 0.25 | nd |
| Fat | Organic Milk | Traditional Milk | Intensive Milk |
| Fat (%) | 3.7–4 | 3.8–4 | 3.8–4 |
| SFAs (g/100 g) | 66.28 | 59.03–64.74 | 67.69–71.41 |
| MUFAs (g/100 g) | 26.11–34.07 | 30.33–32.16 | 21.87–28.15 |
| Oleic acid (c9 C18:1) | 20 | 16.10–22.66 | 16.16–17.20 |
| Vaccenic acid (t11 C18:1) (g/100 g) | 1.22–2.00 | 1.18–7.00 | 0.80–2.00 |
| PUFAs (g/100 g) | 3.85–5.36 | 3.69–5.32 | 1.65–3.77 |
| Eicosapentaenoic acid, EPA (C20:5 n-3) (g/100 g) | 0.05 | 0.08 | 0.05 |
| Conjugated linoleic acid, CLA (cis9 trans11) (g/100 g) | 0.83–1.53 | 0.54–0.93 | 0.42–1.19 |
| Linoleic acid, LA (C18:2 n-6) (g/100 g) | 0.59–2.08 | 1.17–2.18 | 1.4–2.39 |
| α-linolenic acid, ALA (C18:3 n-3) (g/100 g) | 0.44–1.05 | 0.49–1.25 | 0.39–0.42 |
| γ-linolenic acid, GLA (C18:3 n-6) (g/100 g) | 0.11 | 0.13 | 0.12 |
| Proportion 18:3n3: 18:3n6 | 1.35 | 0.60–2.77 | 1.26 |
| Minerals and Heavy Metals | Organic Milk | Conventional Milk | |
| Calcium (mg/L) | 971.33–1161 | 1170–1417.76 | |
| Iron (mg/L) | 0.26–0.67 | 0.26–0.47 | |
| Manganese (mg/L) | 0.023–0.047 | 0.022–0.139 | |
| Copper (mg/L) | 0.023–0.084 | 0.038–0.161 | |
| Iodine(mg/L) | 0.013–0.283 | 0.071–6.540 | |
| Aluminium (mg/L) | 0.76 | 0.63 | |
| Potassium (mg/L) | 1509–1896.92 | 1514–1844.37 | |
| Sodium (mg/L) | 366.59 | 476.35 | |
| Magnesium (mg/L) | 86.21 | 113.87–118.50 | |
| Zinc (mg/L) | 2.86–3.96 | 2.96–4.39 | |
| Selenium (mg/L) | 0.002–0.020 | 0.008–0.040 | |
| Cobalt (mg/L) | 0.001 | 0.001 | |
| Strontium (mg/L) | 0.166 | 0.202 | |
5. Perceived Health Benefits of Organic and Conventional Milk
6. global market for organic milk products, 7. future challenges and perspectives, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- OECD-FAO. Agricultural Outlook 2018–2027. Available online: https://www.fao.org/documents/card/en/c/I9166EN (accessed on 1 February 2024).
- FAO. The Global Dairy Sector: Facts. 2019. Available online: http://www.dairydeclaration.org/Portals/153/Content/Documents/DDOR%20Global%20Dairy%20Facts%202019.pdf (accessed on 1 February 2024).
- Fusco, V.; Chieffi, D.; Fanelli, F.; Logrieco, A.F.; Cho, G.S.; Kabisch, J.; Böhnlein, C.; Franz, C.M.A.P. Microbial quality and safety of milk and milk products in the 21st century. Compr. Rev. Food Sci. Food Saf. 2020 , 19 , 2013–2049. [ Google Scholar ] [ CrossRef ]
- Cimmino, F.; Catapano, A.; Petrella, L.; Villano, I.; Tudisco, R.; Cavaliere, G. Role of Milk Micronutrients in Human Health. Front. Biosci. -Landmark 2023 , 28 , 41. [ Google Scholar ] [ CrossRef ]
- Lin, T.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Bioactives in bovine milk: Chemistry, technology, and applications. Nutr. Rev. 2021 , 79 , 48–69. [ Google Scholar ] [ CrossRef ]
- Meier, M.S.; Stoessel, F.; Jungbluth, N.; Juraske, R.; Schader, C.; Stolze, M. Environmental impacts of organic and conventional agricultural products–Are the differences captured by life cycle assessment? J. Environ. Manag. 2015 , 149 , 193–208. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhu, Z.; Chu, F.; Dolgui, A.; Chu, C.; Zhou, W.; Piramuthu, S. Recent advances and opportunities in sustainable food supply chain: A model-oriented review. Int. J. Prod. Res. 2018 , 56 , 5700–5722. [ Google Scholar ] [ CrossRef ]
- Gomiero, T. Food quality assessment in organic vs. conventional agricultural produce: Findings and issues. Appl. Soil Ecol. 2018 , 123 , 714–728. [ Google Scholar ] [ CrossRef ]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016 , 2 , 1–8. [ Google Scholar ] [ CrossRef ]
- Knorr, D. Organic agriculture and foods: Advancing process-product integrations. Crit. Rev. Food Sci. Nutr. 2023 , 1–13. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lambotte, M.; De Cara, S.; Brocas, C.; Bellassen, V. Organic farming offers promising mitigation potential in dairy systems without compromising economic performances. J. Environ. Manag. 2023 , 334 , 117405. [ Google Scholar ] [ CrossRef ]
- Organic Agriculture: FAQ. 2021. Available online: http://www.fao.org/organicag/oa-faq/oa-faq1/en/ (accessed on 1 February 2024).
- Yu, Y.; He, Y. Information disclosure decisions in an organic food supply chain under competition. J. Clean. Prod. 2021 , 292 , 125976. [ Google Scholar ] [ CrossRef ]
- Kumar, D.; Willer, H.; Ravisankar, N.; Kumar, A. Current Scenario of Organic Farming Worldwide. In Transforming Organic Agri-Produce into Processed Food Products ; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 1–24. [ Google Scholar ]
- David, R.A.R.; Silva, M.A.d.; Lopes, C.F.; Ferreira, M.C.d.Q.; Carvalho, F.V.d.B.; Santos, C.A.F.d.; Lima, N.S.; Vieira, T.A.d.S.; Moreira, T.M.d.O.; Moreira, E.d.O. O LEITE ORGÂNICO: ASPECTOS GERAIS E COLABORAÇÃO PARA O DESENVOLVIMENTO SUSTENTÁVEL. Avanços Ciênc. Tecnol. Aliment. 2021 , 5 , 267–284. [ Google Scholar ]
- Nechaev, V.; Mikhailushkin, P.; Alieva, A. Trends in demand on the organic food market in the European countries. In Proceedings of the 2018 International Scientific Conference “Investment, Construction, Real Estate: New Technologies and Special-Purpose Development Priorities”, Irkutsk, Russia, 26–27 April 2018; p. 07008. [ Google Scholar ]
- Giampieri, F.; Mazzoni, L.; Cianciosi, D.; Alvarez-Suarez, J.M.; Regolo, L.; Sánchez-González, C.; Capocasa, F.; Xiao, J.; Mezzetti, B.; Battino, M. Organic vs conventional plant-based foods: A review. Food Chem. 2022 , 383 , 132352. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Willer, H.; Lernoud, J. The World of Organic Agriculture. STATISTICS and Emerging Trends 2023 ; Research Institute of Organic Agriculture FiBL: Frick, Switzerland; IFOAM-Organics International: Bonn, Germany, 2023. [ Google Scholar ]
- Kapsdorferova, Z.; Čereš, M.; ŠVikruhovÁ, P.; ZÁBojnÍKovÁ, V.; Kataniková, R. Challenges and innovative approaches in the agricultural and food industry and changing consumer behaviour in the milk and milk products market: Case of Slovakia. Agric. Econ./Zemědělská Ekon. 2023 , 69 . [ Google Scholar ] [ CrossRef ]
- Kumar, S.R.; Prajapati, S.; Parambil, J.V. Current Status of Organic Processed Food Products in the World. In Transforming Organic Agri-Produce into Processed Food Products: Post-COVID-19 Challenges and Opportunitie ; CRC Press: Boca Raton, FL, USA, 2023. [ Google Scholar ]
- Grodkowski, G.; Gołębiewski, M.; Slósarz, J.; Grodkowska, K.; Kostusiak, P.; Sakowski, T.; Puppel, K. Organic Milk Production and Dairy Farming Constraints and Prospects under the Laws of the European Union. Animals 2023 , 13 , 1457. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Manuelian, C.L.; Penasa, M.; da Costa, L.; Burbi, S.; Righi, F.; De Marchi, M. Organic livestock production: A bibliometric review. Animals 2020 , 10 , 618. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brodziak, A.; Wajs, J.; Zuba-Ciszewska, M.; Król, J.; Stobiecka, M.; Jańczuk, A. Organic versus Conventional Raw Cow Milk as Material for Processing. Animals 2021 , 11 , 2760. [ Google Scholar ] [ CrossRef ]
- Średnicka-Tober, D.; Barański, M.; Seal, C.J.; Sanderson, R.; Benbrook, C.; Steinshamn, H.; Gromadzka-Ostrowska, J.; Rembiałkowska, E.; Skwarło-Sońta, K.; Eyre, M. Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: A systematic literature review and meta-and redundancy analyses. Br. J. Nutr. 2016 , 115 , 1043–1060. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Manuelian, C.L.; Vigolo, V.; Burbi, S.; Righi, F.; Simoni, M.; De Marchi, M. Detailed comparison between organic and conventional milk from Holstein-Friesian dairy herds in Italy. J. Dairy Sci. 2022 , 105 , 5561–5572. [ Google Scholar ] [ CrossRef ]
- Liu, N.; Parra, H.A.; Pustjens, A.; Hettinga, K.; Mongondry, P.; Van Ruth, S.M. Evaluation of portable near-infrared spectroscopy for organic milk authentication. Talanta 2018 , 184 , 128–135. [ Google Scholar ] [ CrossRef ]
- Gomes, S.I.F.; van Bodegom, P.M.; van Agtmaal, M.; Soudzilovskaia, N.A.; Bestman, M.; Duijm, E.; Speksnijder, A.; van Eekeren, N. Microbiota in dung and milk differ between organic and conventional dairy farms. Front. Microbiol. 2020 , 11 , 1746. [ Google Scholar ] [ CrossRef ]
- Rozenberg, S.; Body, J.-J.; Bruyere, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.-P.; Gielen, E.; Goemaere, S.; Kaufman, J.-M. Effects of dairy products consumption on health: Benefits and beliefs—A commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016 , 98 , 1–17. [ Google Scholar ] [ CrossRef ]
- Barański, M.; Rempelos, L.; Iversen, P.O.; Leifert, C. Effects of organic food consumption on human health; the jury is still out! Food Nutr. Res. 2017 , 61 , 1287333. [ Google Scholar ] [ CrossRef ]
- Butler, G.; Stergiadis, S. Organic milk: Does it confer health benefits? In Milk and Dairy Foods ; Elsevier: Amsterdam, The Netherlands, 2020; pp. 121–143. [ Google Scholar ]
- Calabro, G.; Vieri, S. Limits and potential of organic farming towards a more sustainable European agri-food system. Br. Food J. 2023 , 126 , 223–236. [ Google Scholar ] [ CrossRef ]
- Hirsch, S.; Koppenberg, M. Market power and profitability of organic versus conventional dairy farmers in the EU. In Proceedings of the 2023 Annual Meeting, Washington, DC, USA, 23–25 July 2023. [ Google Scholar ]
- Kononets, Y.; Konvalina, P.; Bartos, P.; Smetana, P. The evolution of organic food certification. Front. Sustain. Food Syst. 2023 , 7 , 1167017. [ Google Scholar ] [ CrossRef ]
- Dinçer, M.A.M.; Arslan, Y.; Okutan, S.; Dil, E. An inquiry on organic food confusion in the consumer perception: A qualitative perspective. Br. Food J. 2023 , 125 , 1420–1436. [ Google Scholar ] [ CrossRef ]
- Banerjee, M.; Shanthakumar, S. International and National Policies on Organic Agriculture. In Transforming Organic Agri-Produce into Processed Food Products: Post-COVID-19 Challenges and Opportunitie ; CRC Press: Boca Raton, FL, USA, 2023. [ Google Scholar ]
- Schwendel, B.H.; Wester, T.J.; Morel, P.C.H.; Tavendale, M.H.; Deadman, C.; Shadbolt, N.M.; Otter, D.E. Invited review: Organic and conventionally produced milk—An evaluation of factors influencing milk composition. J. Dairy Sci. 2015 , 98 , 721–746. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Adesogan, A.T.; Dahl, G.E. MILK Symposium Introduction: Dairy production in developing countries. J. Dairy Sci. 2020 , 103 , 9677–9680. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Reardon, T.; Lu, L.; Zilberman, D. Links among innovation, food system transformation, and technology adoption, with implications for food policy: Overview of a special issue. Food Policy 2019 , 83 , 285–288. [ Google Scholar ] [ CrossRef ]
- Clay, N.; Garnett, T.; Lorimer, J. Dairy intensification: Drivers, impacts and alternatives. Ambio 2020 , 49 , 35–48. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cele, L.P.; Hennessy, T.; Thorne, F. Evaluating farm and export competitiveness of the Irish dairy industry: Post-quota analysis. Compet. Rev. Int. Bus. J. 2022 , 32 , 1–20. [ Google Scholar ] [ CrossRef ]
- McAuliffe, S.; Gilliland, T.J.; Hennessy, D. Comparison of pasture-based feeding systems and a total mixed ration feeding system on dairy cow milk production. In Proceedings of the 26th General Meeting of the European Grassland Federation, Trondheim, Norway, 4–8 September 2016. [ Google Scholar ]
- Smid, A.-M.C.; Weary, D.M.; Von Keyserlingk, M.A.G. The influence of different types of outdoor access on dairy cattle behavior. Front. Vet. Sci. 2020 , 7 , 257. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Joubran, A.M.; Pierce, K.M.; Garvey, N.; Shalloo, L.; O’Callaghan, T.F. Invited review: A 2020 perspective on pasture-based dairy systems and products. J. Dairy Sci. 2021 , 104 , 7364–7382. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Magan, J.B.; O′Callaghan, T.F.; Kelly, A.L.; McCarthy, N.A. Compositional and functional properties of milk and dairy products derived from cows fed pasture or concentrate-based diets. Compr. Rev. Food Sci. Food Saf. 2021 , 20 , 2769–2800. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016 , 99 , 9424–9440. [ Google Scholar ] [ CrossRef ]
- O’Callaghan, T.F.; Faulkner, H.; McAuliffe, S.; O’Sullivan, M.G.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; Stanton, C.; Ross, R.P. Quality characteristics, chemical composition, and sensory properties of butter from cows on pasture versus indoor feeding systems. J. Dairy Sci. 2016 , 99 , 9441–9460. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- O’Callaghan, T.F.; Mannion, D.T.; Hennessy, D.; McAuliffe, S.; O’Sullivan, M.G.; Leeuwendaal, N.; Beresford, T.P.; Dillon, P.; Kilcawley, K.N.; Sheehan, J.J. Effect of pasture versus indoor feeding systems on quality characteristics, nutritional composition, and sensory and volatile properties of full-fat Cheddar cheese. J. Dairy Sci. 2017 , 100 , 6053–6073. [ Google Scholar ] [ CrossRef ]
- Timlin, M.; Fitzpatrick, E.; McCarthy, K.; Tobin, J.T.; Murphy, E.G.; Pierce, K.M.; Murphy, J.P.; Hennessy, D.; O’Donovan, M.; Harbourne, N. Impact of varying levels of pasture allowance on the nutritional quality and functionality of milk throughout lactation. J. Dairy Sci. 2023 , 106 , 6597–6622. [ Google Scholar ] [ CrossRef ]
- Schingoethe, D.J. A 100-Year Review: Total mixed ration feeding of dairy cows. J. Dairy Sci. 2017 , 100 , 10143–10150. [ Google Scholar ] [ CrossRef ]
- van den Pol-van Dasselaar, A.; Hennessy, D.; Isselstein, J. Grazing of dairy cows in Europe—An in-depth analysis based on the perception of grassland experts. Sustainability 2020 , 12 , 1098. [ Google Scholar ] [ CrossRef ]
- Charlton, G.L.; Rutter, S.M.; East, M.; Sinclair, L.A. Preference of dairy cows: Indoor cubicle housing with access to a total mixed ration vs. access to pasture. Appl. Anim. Behav. Sci. 2011 , 130 , 1–9. [ Google Scholar ] [ CrossRef ]
- Legrand, A.L.; Von Keyserlingk, M.A.G.; Weary, D.M. Preference and usage of pasture versus free-stall housing by lactating dairy cattle. J. Dairy Sci. 2009 , 92 , 3651–3658. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Armbrecht, L.; Lambertz, C.; Albers, D.; Gauly, M. Does access to pasture affect claw condition and health in dairy cows? Vet. Rec. 2018 , 182 , 79. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Firth, C.L.; Laubichler, C.; Schleicher, C.; Fuchs, K.; Käsbohrer, A.; Egger-Danner, C.; Köfer, J.; Obritzhauser, W. Relationship between the probability of veterinary-diagnosed bovine mastitis occurring and farm management risk factors on small dairy farms in Austria. J. Dairy Sci. 2019 , 102 , 4452–4463. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mee, J.F.; Boyle, L.A. Assessing whether dairy cow welfare is “better” in pasture-based than in confinement-based management systems. N. Z. Vet. J. 2020 , 68 , 168–177. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Morales-Almaráz, E.; Soldado, A.; González, A.; Martínez-Fernández, A.; Domínguez-Vara, I.; de la Roza-Delgado, B.; Vicente, F. Improving the fatty acid profile of dairy cow milk by combining grazing with feeding of total mixed ration. J. Dairy Res. 2010 , 77 , 225–230. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rodríguez-Bermúdez, R.; Miranda, M.; Baudracco, J.; Fouz, R.; Pereira, V.; López-Alonso, M. Breeding for organic dairy farming: What types of cows are needed? J. Dairy Res. 2019 , 86 , 3–12. [ Google Scholar ] [ CrossRef ]
- Santoni, M.; Verdi, L.; Imran Pathan, S.; Napoli, M.; Dalla Marta, A.; Dani, F.R.; Pacini, G.C.; Ceccherini, M.T. Soil microbiome biomass, activity, composition and CO 2 emissions in a long-term organic and conventional farming systems. Soil Use Manag. 2023 , 39 , 588–605. [ Google Scholar ] [ CrossRef ]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of organic farming for achieving sustainability in agriculture. Farming Syst. 2023 , 1 , 100005. [ Google Scholar ] [ CrossRef ]
- Orjales, I.; Lopez-Alonso, M.; Miranda, M.; Alaiz-Moretón, H.; Resch, C.; López, S. Dairy cow nutrition in organic farming systems. Comparison with the conventional system. Animal 2019 , 13 , 1084–1093. [ Google Scholar ] [ CrossRef ]
- Health, E.P.o.A.; Animal, W.; Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; et al. Welfare of dairy cows. EFSA J. 2023 , 21 , e07993. [ Google Scholar ]
- Wagner, K.; Brinkmann, J.; Bergschmidt, A.; Renziehausen, C.; March, S. The effects of farming systems (organic vs. conventional) on dairy cow welfare, based on the Welfare Quality ® protocol. Animal 2021 , 15 , 100301. [ Google Scholar ] [ CrossRef ]
- Åkerfeldt, M.P.; Gunnarsson, S.; Bernes, G.; Blanco-Penedo, I. Health and welfare in organic livestock production systems—A systematic mapping of current knowledge. Org. Agric. 2021 , 11 , 105–132. [ Google Scholar ] [ CrossRef ]
- Xiong, W.; Sun, Y.; Zeng, Z. Antimicrobial use and antimicrobial resistance in food animals. Environ. Sci. Pollut. Res. 2018 , 25 , 18377–18384. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Foutz, C.A.; Godden, S.M.; Bender, J.B.; Diez-Gonzalez, F.; Akhtar, M.; Vatulin, A. Exposure to antimicrobials through the milk diet or systemic therapy is associated with a transient increase in antimicrobial resistance in fecal Escherichia coli of dairy calves. J. Dairy Sci. 2018 , 101 , 10126–10141. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ager, E.; Carvalho, T.; Silva, E.; Ricke, S.; Hite, J. Global trends in antimicrobial resistance on organic and conventional farms. bioRxiv 2023 , 13 , 22608. [ Google Scholar ] [ CrossRef ]
- Lorenz, K.; Lal, R. Organic Agriculture and Greenhouse Gas Emissions. In Organic Agriculture and Climate Change ; Springer: Berlin/Heidelberg, Germany, 2022; pp. 129–175. [ Google Scholar ]
- Brito, A.F.; Silva, L.H.P. Symposium review: Comparisons of feed and milk nitrogen efficiency and carbon emissions in organic versus conventional dairy production systems. J. Dairy Sci. 2020 , 103 , 5726–5739. [ Google Scholar ] [ CrossRef ]
- Średnicka-Tober, D.; Obiedzińska, A.; Kazimierczak, R.; Rembiałkowska, E. Environmental impact of organic vs. conventional agriculture-a review. J. Res. Appl. Agric. Eng. 2016 , 61 , 204–211. [ Google Scholar ]
- Kilcawley, K.N.; Faulkner, H.; Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P. Factors influencing the flavour of bovine milk and cheese from grass based versus non-grass based milk production systems. Foods 2018 , 7 , 37. [ Google Scholar ] [ CrossRef ]
- Król, J.; Brodziak, A.; Topyła, B. The nutritional value of the milk of Simmental cows in relation to the season and production system. Anim. Prod. Rev. 2016 , 6 , 20–24. [ Google Scholar ]
- Sapbamrer, R.; Thammachai, A. A systematic review of factors influencing farmers’ adoption of organic farming. Sustainability 2021 , 13 , 3842. [ Google Scholar ] [ CrossRef ]
- Poudel, S.P.; Acharya, R.; Chetri, D.K. Somatic cell count: An indicator of intramammary infection in dairy animals. Matrix Sci. Pharma 2021 , 5 , 49–53. [ Google Scholar ] [ CrossRef ]
- Stocco, G.; Summer, A.; Cipolat-Gotet, C.; Zanini, L.; Vairani, D.; Dadousis, C.; Zecconi, A. Differential somatic cell count as a novel indicator of milk quality in dairy cows. Animals 2020 , 10 , 753. [ Google Scholar ] [ CrossRef ]
- Neculai-Valeanu, A.-S.; Ariton, A.-M. Udder health monitoring for prevention of bovine mastitis and improvement of milk quality. Bioengineering 2022 , 9 , 608. [ Google Scholar ] [ CrossRef ]
- Moradi, M.; Omer, A.K.; Razavi, R.; Valipour, S.; Guimarães, J.T. The relationship between milk somatic cell count and cheese production, quality and safety: A review. Int. Dairy J. 2021 , 113 , 104884. [ Google Scholar ] [ CrossRef ]
- Orjales, I.; Lopez-Alonso, M.; Miranda, M.; Rodríguez-Bermúdez, R.; Rey-Crespo, F.; Villar, A. The main factors affecting somatic cell count in organic dairy farming. Span. J. Agric. Res. 2017 , 15 , e06SC02. [ Google Scholar ] [ CrossRef ]
- Orjales, I.; López-Alonso, M.; Rodríguez-Bermúdez, R.; Rey-Crespo, F.; Villar, A.; Miranda, M. Use of homeopathy in organic dairy farming in Spain. Homeopathy 2016 , 105 , 102–108. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Orjales, I.; López-Alonso, M.; Rodríguez-Bermúdez, R.; Rey-Crespo, F.; Villar, A.; Miranda, M. Is lack of antibiotic usage affecting udder health status of organic dairy cattle? J. Dairy Res. 2016 , 83 , 464–467. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- FDA. Grade “A” Pasteurized Milk Ordinance ; FDA: Silver Spring, MD, USA, 2017.
- Martin, N.H.; Evanowski, R.L.; Wiedmann, M. Invited review: Redefining raw milk quality—Evaluation of raw milk microbiological parameters to ensure high-quality processed dairy products. J. Dairy Sci. 2023 , 106 , 1502–1517. [ Google Scholar ] [ CrossRef ]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020 , 8 , 103. [ Google Scholar ]
- Dean, C.J.; Slizovskiy, I.B.; Crone, K.K.; Pfennig, A.X.; Heins, B.J.; Caixeta, L.S.; Noyes, N.R. Investigating the cow skin and teat canal microbiomes of the bovine udder using different sampling and sequencing approaches. J. Dairy Sci. 2021 , 104 , 644–661. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dahlberg, J.; Williams, J.E.; McGuire, M.A.; Peterson, H.K.; Östensson, K.; Agenäs, S.; Dicksved, J.; Waller, K.P. Microbiota of bovine milk, teat skin, and teat canal: Similarity and variation due to sampling technique and milk fraction. J. Dairy Sci. 2020 , 103 , 7322–7330. [ Google Scholar ] [ CrossRef ]
- Dean, C.J.; Deng, Y.; Wehri, T.J.; Ray, T.; Peña-Mosca, F.; Crooker, B.A.; Godden, S.M.; Caixeta, L.S.; Noyes, N. The impact of kit, environment and sampling contamination on the observed microbiome of bovine milk. bioRxiv 2023 . [ Google Scholar ] [ CrossRef ]
- Guo, W.; Liu, S.; Khan, M.Z.; Wang, J.; Chen, T.; Alugongo, G.M.; Li, S.; Cao, Z. Bovine milk microbiota: Key players, origins, and potential contributions to early-life gut development. J. Adv. Res. 2023 . [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hagey, J.V.; Bhatnagar, S.; Heguy, J.M.; Karle, B.M.; Price, P.L.; Meyer, D.; Maga, E.A. Fecal microbial communities in a large representative cohort of California dairy cows. Front. Microbiol. 2019 , 10 , 1093. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Loor, J.J.; Elolimy, A.A.; McCann, J.C. Dietary impacts on rumen microbiota in beef and dairy production. Anim. Front. 2016 , 6 , 22–29. [ Google Scholar ] [ CrossRef ]
- Zhang, R.; Huo, W.; Zhu, W.; Mao, S. Characterization of bacterial community of raw milk from dairy cows during subacute ruminal acidosis challenge by high-throughput sequencing. J. Sci. Food Agric. 2015 , 95 , 1072–1079. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mbareche, H.; Veillette, M.; Bilodeau, G.J.; Duchaine, C. Fungal bioaerosols at five dairy farms: A novel approach to describe workers’ exposure. bioRxiv 2018 , 308825. [ Google Scholar ] [ CrossRef ]
- Guzzon, R.; Carafa, I.; Tuohy, K.; Cervantes, G.; Vernetti, L.; Barmaz, A.; Larcher, R.; Franciosi, E. Exploring the microbiota of the red-brown defect in smear-ripened cheese by 454-pyrosequencing and its prevention using different cleaning systems. Food Microbiol. 2017 , 62 , 160–168. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tommasoni, C.; Fiore, E.; Lisuzzo, A.; Gianesella, M. Mastitis in Dairy Cattle: On-Farm Diagnostics and Future Perspectives. Animals 2023 , 13 , 2538. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Angelopoulou, A.; Holohan, R.; Rea, M.C.; Warda, A.K.; Hill, C.; Ross, R.P. Bovine mastitis is a polymicrobial disease requiring a polydiagnostic approach. Int. Dairy J. 2019 , 99 , 104539. [ Google Scholar ] [ CrossRef ]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021 , 41 , 107–136. [ Google Scholar ] [ CrossRef ]
- Peña-Mosca, F.; Dean, C.; Machado, V.; Fernandes, L.; Pinedo, P.; Doster, E.; Heins, B.; Sharpe, K.; Ray, T.; Feijoo, V.; et al. Investigation of intramammary infections in primiparous cows during early lactation on organic dairy farms. J. Dairy Sci. 2023 , 106 , 9377–9392. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pol, M.; Ruegg, P.L. Relationship between antimicrobial drug usage and antimicrobial susceptibility of gram-positive mastitis pathogens. J. Dairy Sci. 2007 , 90 , 262–273. [ Google Scholar ] [ CrossRef ]
- Cicconi-Hogan, K.M.; Gamroth, M.; Richert, R.; Ruegg, P.L.; Stiglbauer, K.E.; Schukken, Y.H. Risk factors associated with bulk tank standard plate count, bulk tank coliform count, and the presence of Staphylococcus aureus on organic and conventional dairy farms in the United States. J. Dairy Sci. 2013 , 96 , 7578–7590. [ Google Scholar ] [ CrossRef ]
- Tikofsky, L.L.; Barlow, J.W.; Santisteban, C.; Schukken, Y.H. A comparison of antimicrobial susceptibility patterns for Staphylococcus aureus in organic and conventional dairy herds. Microb. Drug Resist. 2003 , 9 , 39–45. [ Google Scholar ] [ CrossRef ]
- Hamilton, C.; Emanuelson, U.; Forslund, K.; Hansson, I.; Ekman, T. Mastitis and related management factors in certified organic dairy herds in Sweden. Acta Vet. Scand. 2006 , 48 , 1–7. [ Google Scholar ] [ CrossRef ]
- Valle, P.S.; Lien, G.; Flaten, O.; Koesling, M.; Ebbesvik, M. Herd health and health management in organic versus conventional dairy herds in Norway. Livest. Sci. 2007 , 112 , 123–132. [ Google Scholar ] [ CrossRef ]
- Richert, R.M.; Cicconi, K.M.; Gamroth, M.J.; Schukken, Y.H.; Stiglbauer, K.E.; Ruegg, P.L. Risk factors for clinical mastitis, ketosis, and pneumonia in dairy cattle on organic and small conventional farms in the United States. J. Dairy Sci. 2013 , 96 , 4269–4285. [ Google Scholar ] [ CrossRef ]
- Hardeng, F.; Edge, V.L. Mastitis, ketosis, and milk fever in 31 organic and 93 conventional Norwegian dairy herds. J. Dairy Sci. 2001 , 84 , 2673–2679. [ Google Scholar ] [ CrossRef ]
- Mullen, K.A.E.; Sparks, L.G.; Lyman, R.L.; Washburn, S.P.; Anderson, K.L. Comparisons of milk quality on North Carolina organic and conventional dairies. J. Dairy Sci. 2013 , 96 , 6753–6762. [ Google Scholar ] [ CrossRef ]
- Ueda, Y.; Asakuma, S.; Miyaji, M.; Akiyama, F. Effect of time at pasture and herbage intake on profile of volatile organic compounds of dairy cow milk. Anim. Sci. J. 2016 , 87 , 117–125. [ Google Scholar ] [ CrossRef ]
- Aprea, E.; Biasioli, F.; Carlin, S.; Endrizzi, I.; Gasperi, F. Investigation of volatile compounds in two raspberry cultivars by two headspace techniques: Solid-phase microextraction/gas chromatography—Mass spectrometry (SPME/GC−MS) and proton-transfer reaction−mass spectrometry (PTR−MS). J. Agric. Food Chem. 2009 , 57 , 4011–4018. [ Google Scholar ] [ CrossRef ]
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Quantification of trace volatile sulfur compounds in milk by solid-phase microextraction and gas chromatography–pulsed flame photometric detection. J. Dairy Sci. 2006 , 89 , 2919–2927. [ Google Scholar ] [ CrossRef ]
- Calvo, M.M.; de la Hoz, L. Flavour of heated milks. A review. Int. Dairy J. 1992 , 2 , 69–81. [ Google Scholar ] [ CrossRef ]
- Urbach, G. Effect of feed on flavor in dairy foods. J. Dairy Sci. 1990 , 73 , 3639–3650. [ Google Scholar ] [ CrossRef ]
- Smigic, N.; Djekic, I.; Tomasevic, I.; Stanisic, N.; Nedeljkovic, A.; Lukovic, V.; Miocinovic, J. Organic and conventional milk–insight on potential differences. Br. Food J. 2017 , 119 , 366–376. [ Google Scholar ] [ CrossRef ]
- Schwendel, B.H.; Wester, T.J.; Morel, P.C.H.; Fong, B.; Tavendale, M.H.; Deadman, C.; Shadbolt, N.M.; Otter, D.E. Pasture feeding conventional cows removes differences between organic and conventionally produced milk. Food Chem. 2017 , 229 , 805–813. [ Google Scholar ] [ CrossRef ]
- Liu, N.; Koot, A.; Hettinga, K.; De Jong, J.; van Ruth, S.M. Portraying and tracing the impact of different production systems on the volatile organic compound composition of milk by PTR-(Quad) MS and PTR-(ToF) MS. Food Chem. 2018 , 239 , 201–207. [ Google Scholar ] [ CrossRef ]
- Clarke, H.J.; Griffin, C.; Rai, D.K.; O’Callaghan, T.F.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Dietary compounds influencing the sensorial, volatile and phytochemical properties of bovine milk. Molecules 2019 , 25 , 26. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alothman, M.; Hogan, S.A.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; O’Donovan, M.; Tobin, J.; Fenelon, M.A.; O’Callaghan, T.F. The “grass-fed” milk story: Understanding the impact of pasture feeding on the composition and quality of bovine milk. Foods 2019 , 8 , 350. [ Google Scholar ] [ CrossRef ]
- Gallina Toschi, T.; Bendini, A.; Barbieri, S.; Valli, E.; Cezanne, M.L.; Buchecker, K.; Canavari, M. Organic and conventional nonflavored yogurts from the Italian market: Study on sensory profiles and consumer acceptability. J. Sci. Food Agric. 2012 , 92 , 2788–2795. [ Google Scholar ] [ CrossRef ]
- Bloksma, J.; Adriaansen-Tennekes, R.; Huber, M.; van de Vijver, L.P.L.; Baars, T.; de Wit, J. Comparison of organic and conventional raw milk quality in the Netherlands. Biol. Agric. Hortic. 2008 , 26 , 69–83. [ Google Scholar ] [ CrossRef ]
- Manzocchi, E.; Martin, B.; Bord, C.; Verdier-Metz, I.; Bouchon, M.; De Marchi, M.; Constant, I.; Giller, K.; Kreuzer, M.; Berard, J. Feeding cows with hay, silage, or fresh herbage on pasture or indoors affects sensory properties and chemical composition of milk and cheese. J. Dairy Sci. 2021 , 104 , 5285–5302. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brodziak, A.; Król, J.; Litwińczuk, Z.; Barłowska, J. Differences in bioactive protein and vitamin status of milk from certified organic and conventional farms. Int. J. Dairy Technol. 2018 , 71 , 321–332. [ Google Scholar ] [ CrossRef ]
- Kiczorowska, B.; Samolińska, W.; Marczuk, J.; Winiarska-Mieczan, A.; Klebaniuk, R.; Kowalczuk-Vasilev, E.; Kiczorowski, P.; Zasadna, Z. Comparative effects of organic, traditional, and intensive production with probiotics on the fatty acid profile of cow’s milk. J. Food Compos. Anal. 2017 , 63 , 157–163. [ Google Scholar ] [ CrossRef ]
- Qin, N.; Faludi, G.; Beauclercq, S.; Pitt, J.; Desnica, N.; Pétursdóttir, Á.; Newton, E.E.; Angelidis, A.; Givens, I.; Juniper, D. Macromineral and trace element concentrations and their seasonal variation in milk from organic and conventional dairy herds. Food Chem. 2021 , 359 , 129865. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Singh, A.; Duche, R.T.; Wandhare, A.G.; Sian, J.K.; Singh, B.P.; Sihag, M.K.; Singh, K.S.; Sangwan, V.; Talan, S.; Panwar, H. Milk-derived antimicrobial peptides: Overview, applications, and future perspectives. Probiotics Antimicrob. Proteins 2023 , 15 , 44–62. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brodziak, A.; Król, J.; Litwińczuk, Z.; Florek, M. Bioactive compound levels and sensory quality of partially skimmed organic yoghurts: Effects of the milk treatment, production season and starter culture. Int. J. Dairy Technol. 2021 , 74 , 139–147. [ Google Scholar ] [ CrossRef ]
- Król, J.; Brodziak, A.; Zaborska, A.; Litwińczuk, Z. Comparison of whey proteins and lipophilic vitamins between four cow breeds maintained in intensive production system. Mljekarstvo/Dairy 2017 , 67 . [ Google Scholar ] [ CrossRef ]
- Wójcik-Saganek, A. Nutritional Value and Technological Suitability of Farm Milk Together with a Technical and Economic Analysis of the Efficiency of Its Production. Doctoral Dissertation, The University of Life Sciences in Lublin, Lublin, Poland, 2019. [ Google Scholar ]
- Zhao, X.; Xu, X.-X.; Liu, Y.; Xi, E.-Z.; An, J.-J.; Tabys, D.; Liu, N. The in vitro protective role of bovine lactoferrin on intestinal epithelial barrier. Molecules 2019 , 24 , 148. [ Google Scholar ] [ CrossRef ]
- Anand, R.; Mohan, L.; Bharadvaja, N. Disease prevention and treatment using β-carotene: The ultimate provitamin A. Rev. Bras. Farmacogn. 2022 , 32 , 491–501. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A.; Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Vitamins in milk and dairy products. In Dairy Chemistry and Biochemistry ; Springer: Berlin/Heidelberg, Germany, 2015; pp. 271–297. [ Google Scholar ]
- Liao, S.; Omage, S.O.; Börmel, L.; Kluge, S.; Schubert, M.; Wallert, M.; Lorkowski, S. Vitamin E and metabolic health: Relevance of interactions with other micronutrients. Antioxidants 2022 , 11 , 1785. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant activity of milk and dairy products. Animals 2022 , 12 , 245. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: Antioxidant action, animal health, and product quality—Invited review. Animals 2022 , 12 , 3279. [ Google Scholar ] [ CrossRef ]
- Kuczyńska, B.; Nałęcz-Tarwacka, T.; Puppel, K.; Gołębiewski, M.; Grodzki, H.; Slósarz, J. The content of bioactive components in milk depending on cow feeding model in certified ecological farms. J. Res. Appl. Agric. Eng 2011 , 56 , 7–13. [ Google Scholar ]
- Fong, B.; Ma, K.; McJarrow, P. Quantification of bovine milk oligosaccharides using liquid chromatography–selected reaction monitoring–mass spectrometry. J. Agric. Food Chem. 2011 , 59 , 9788–9795. [ Google Scholar ] [ CrossRef ]
- Kiely, L.J.; Busca, K.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Molecular strategies for the utilisation of human milk oligosaccharides by infant gut-associated bacteria. FEMS Microbiol. Rev. 2023 , 47 , fuad056. [ Google Scholar ] [ CrossRef ]
- Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-chain fatty acids—An underexplored class of dairy-derived fatty acids. Nutrients 2020 , 12 , 2875. [ Google Scholar ] [ CrossRef ]
- O’Callaghan, T.F. The benefits of pasture-based dairy. Ir. Food 2019 , 1 , 34–35. [ Google Scholar ]
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of fatty acids in milk fat and the influence of selected factors on their variability—A review. Molecules 2018 , 23 , 1636. [ Google Scholar ] [ CrossRef ]
- Tzamaloukas, O.; Neofytou, M.C.; Simitzis, P.E.; Miltiadou, D. Effect of farming system (organic vs. conventional) and season on composition and fatty acid profile of bovine, caprine and ovine milk and retail Halloumi cheese produced in Cyprus. Foods 2021 , 10 , 1016. [ Google Scholar ] [ CrossRef ]
- Vanbergue, E.; Peyraud, J.L.; Ferlay, A.; Miranda, G.; Martin, P.; Hurtaud, C. Effects of feeding level, type of forage and milking time on milk lipolytic system in dairy cows. Livest. Sci. 2018 , 217 , 116–126. [ Google Scholar ] [ CrossRef ]
- Liu, S.; Zhang, R.; Kang, R.; Meng, J.; Ao, C. Milk fatty acids profiles and milk production from dairy cows fed different forage quality diets. Anim. Nutr. 2016 , 2 , 329–333. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Benbrook, C.M.; Davis, D.R.; Heins, B.J.; Latif, M.A.; Leifert, C.; Peterman, L.; Butler, G.; Faergeman, O.; Abel-Caines, S.; Baranski, M. Enhancing the fatty acid profile of milk through forage-based rations, with nutrition modeling of diet outcomes. Food Sci. Nutr. 2018 , 6 , 681–700. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Benbrook, C.M.; Butler, G.; Latif, M.A.; Leifert, C.; Davis, D.R. Organic production enhances milk nutritional quality by shifting fatty acid composition: A United States–wide, 18-month study. PLoS ONE 2013 , 8 , e82429. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tsiafoulis, C.G.; Papaemmanouil, C.; Alivertis, D.; Tzamaloukas, O.; Miltiadou, D.; Balayssac, S.; Malet-Martino, M.; Gerothanassis, I.P. NMR-based μetabolomics of the lipid fraction of organic and conventional bovine milk. Molecules 2019 , 24 , 1067. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ellis, K.A.; Innocent, G.; Grove-White, D.; Cripps, P.; McLean, W.G.; Howard, C.V.; Mihm, M. Comparing the fatty acid composition of organic and conventional milk. J. Dairy Sci. 2006 , 89 , 1938–1950. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Collomb, M.; Bisig, W.; Bütikofer, U.; Sieber, R.; Bregy, M.; Etter, L. Fatty acid composition of mountain milk from Switzerland: Comparison of organic and integrated farming systems. Int. Dairy J. 2008 , 18 , 976–982. [ Google Scholar ] [ CrossRef ]
- Butler, G.; Stergiadis, S.; Seal, C.; Eyre, M.; Leifert, C. Fat composition of organic and conventional retail milk in northeast England. J. Dairy Sci. 2011 , 94 , 24–36. [ Google Scholar ] [ CrossRef ]
- Adler, S.A.; Jensen, S.K.; Govasmark, E.; Steinshamn, H. Effect of short-term versus long-term grassland management and seasonal variation in organic and conventional dairy farming on the composition of bulk tank milk. J. Dairy Sci. 2013 , 96 , 5793–5810. [ Google Scholar ] [ CrossRef ]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.-i.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019 , 10 , 4007. [ Google Scholar ] [ CrossRef ]
- Khiaosa-Ard, R.; Klevenhusen, F.; Soliva, C.R.; Kreuzer, M.; Leiber, F. Transfer of linoleic and linolenic acid from feed to milk in cows fed isoenergetic diets differing in proportion and origin of concentrates and roughages. J. Dairy Res. 2010 , 77 , 331–336. [ Google Scholar ] [ CrossRef ]
- Ferreiro, T.; Gayoso, L.; Rodríguez-Otero, J.L. Milk phospholipids: Organic milk and milk rich in conjugated linoleic acid compared with conventional milk. J. Dairy Sci. 2015 , 98 , 9–14. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Davis, H.; Chatzidimitriou, E.; Leifert, C.; Butler, G. Evidence that forage-fed cows can enhance milk quality. Sustainability 2020 , 12 , 3688. [ Google Scholar ] [ CrossRef ]
- Bauman, D.E.; Lock, A.L.; Conboy Stephenson, R.; Linehan, K.; Ross, R.P.; Stanton, C. Conjugated linoleic acid: Biosynthesis and nutritional significance. In Advanced Dairy Chemistry ; Springer: Cham, Switzerland, 2020; Volume 2, pp. 67–106. [ Google Scholar ]
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.-E. Conjugated linoleic acid (CLA) as a functional food: Is it beneficial or not? Food Res. Int. 2023 , 172 , 113158. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Manuelian, C.L.; Penasa, M.; Visentin, G.; Zidi, A.; Cassandro, M.; De Marchi, M. Mineral composition of cow milk from multibreed herds. Anim. Sci. J. 2018 , 89 , 1622–1627. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Litwińczuk, Z.; Koperska, N.; Chabuz, W.; Kędzierska-Matysek, M. Basic chemical composition and mineral content of the milk of cows of various breeds raised on organic farms and on traditional farms using intensive and traditional feeding systems. Med. Weter. 2018 , 74 , 309–313. [ Google Scholar ] [ CrossRef ]
- Stergiadis, S.; Qin, N.; Faludi, G.; Beauclercq, S.; Pitt, J.; Desnica, N.; Pétursdóttir, Á.H.; Newton, E.E.; Angelidis, A.E.; Givens, I. Mineral concentrations in bovine milk from farms with contrasting grazing management. Foods 2021 , 10 , 2733. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kwiatkowski, C.A.; Harasim, E. Chemical properties of soil in four-field crop rotations under organic and conventional farming systems. Agronomy 2020 , 10 , 1045. [ Google Scholar ] [ CrossRef ]
- Rodríguez-Bermúdez, R.; López-Alonso, M.; Miranda, M.; Fouz, R.; Orjales, I.; Herrero-Latorre, C. Chemometric authentication of the organic status of milk on the basis of trace element content. Food Chem. 2018 , 240 , 686–693. [ Google Scholar ] [ CrossRef ]
- López-Alonso, M.; Rey-Crespo, F.; Herrero-Latorre, C.; Miranda, M. Identifying sources of metal exposure in organic and conventional dairy farming. Chemosphere 2017 , 185 , 1048–1055. [ Google Scholar ] [ CrossRef ]
- Boudebbouz, A.; Boudalia, S.; Bousbia, A.; Habila, S.; Boussadia, M.I.; Gueroui, Y. Heavy metals levels in raw cow milk and health risk assessment across the globe: A systematic review. Sci. Total Environ. 2021 , 751 , 141830. [ Google Scholar ] [ CrossRef ]
- Varol, M.; Sünbül, M.R. Macroelements and toxic trace elements in muscle and liver of fish species from the largest three reservoirs in Turkey and human risk assessment based on the worst-case scenarios. Environ. Res. 2020 , 184 , 109298. [ Google Scholar ] [ CrossRef ]
- Ziarati, P.; Shirkhan, F.; Mostafidi, M.; Zahedi, M.T. An overview of the heavy metal contamination in milk and dairy products. Acta Sci. Pharm. Sci. 2018 , 2 , 1–14. [ Google Scholar ]
- Zwierzchowski, G.; Ametaj, B.N. Minerals and heavy metals in the whole raw milk of dairy cows from different management systems and countries of origin: A meta-analytical study. J. Agric. Food Chem. 2018 , 66 , 6877–6888. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Seğmenoğlu, M.S.; Baydan, E. Comparison of heavy metal levels of organic and conventional milk and milk products in Turkey. Turk. J. Agric. -Food Sci. Technol. 2021 , 9 , 696–700. [ Google Scholar ] [ CrossRef ]
- Stergiadis, S.; Berlitz, C.B.; Hunt, B.; Garg, S.; Givens, D.I.; Kliem, K.E. An update to the fatty acid profiles of bovine retail milk in the United Kingdom: Implications for nutrition in different age and gender groups. Food Chem. 2019 , 276 , 218–230. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, N.; Pustjens, A.M.; Erasmus, S.W.; Yang, Y.; Hettinga, K.; van Ruth, S.M. Dairy farming system markers: The correlation of forage and milk fatty acid profiles from organic, pasture and conventional systems in the Netherlands. Food Chem. 2020 , 314 , 126153. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ormston, S.; Qin, N.; Faludi, G.; Pitt, J.; Gordon, A.W.; Theodoridou, K.; Yan, T.; Huws, S.A.; Stergiadis, S. Implications of organic dairy management on herd performance and milk fatty acid profiles and interactions with season. Foods 2023 , 12 , 1589. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Soedamah-Muthu, S.S.; Guo, J. Dairy consumption and cardiometabolic diseases: Evidence from prospective studies. In Milk and Dairy Foods ; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–28. [ Google Scholar ]
- Wang, W.; Wu, Y.; Zhang, D. Association of dairy products consumption with risk of obesity in children and adults: A meta-analysis of mainly cross-sectional studies. Ann. Epidemiol. 2016 , 26 , 870–882.e872. [ Google Scholar ] [ CrossRef ]
- Chen, M.; Pan, A.; Malik, V.S.; Hu, F.B. Effects of dairy intake on body weight and fat: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012 , 96 , 735–747. [ Google Scholar ] [ CrossRef ]
- Booth, A.O.; Huggins, C.E.; Wattanapenpaiboon, N.; Nowson, C.A. Effect of increasing dietary calcium through supplements and dairy food on body weight and body composition: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015 , 114 , 1013–1025. [ Google Scholar ] [ CrossRef ]
- Soedamah-Muthu, S.S.; De Goede, J. Dairy consumption and cardiometabolic diseases: Systematic review and updated meta-analyses of prospective cohort studies. Curr. Nutr. Rep. 2018 , 7 , 171–182. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tong, X.; Dong, J.Y.; Wu, Z.W.; Li, W.; Qin, L.Q. Dairy consumption and risk of type 2 diabetes mellitus: A meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2011 , 65 , 1027–1031. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- O’Sullivan, T.A.; Hafekost, K.; Mitrou, F.; Lawrence, D. Food sources of saturated fat and the association with mortality: A meta-analysis. Am. J. Public Health 2013 , 103 , e31–e42. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Drouin-Chartier, J.-P.; Li, Y.; Ardisson Korat, A.V.; Ding, M.; Lamarche, B.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in dairy product consumption and risk of type 2 diabetes: Results from 3 large prospective cohorts of US men and women. Am. J. Clin. Nutr. 2019 , 110 , 1201–1212. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; De Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016 , 103 , 1111–1124. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Guo, J.; Astrup, A.; Lovegrove, J.A.; Gijsbers, L.; Givens, D.I.; Soedamah-Muthu, S.S. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: Dose–response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2017 , 32 , 269–287. [ Google Scholar ] [ CrossRef ]
- Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J. Nutr. 2016 , 115 , 737–750. [ Google Scholar ] [ CrossRef ]
- Chen, G.-C.; Wang, Y.; Tong, X.; Szeto, I.M.Y.; Smit, G.; Li, Z.-N.; Qin, L.-Q. Cheese consumption and risk of cardiovascular disease: A meta-analysis of prospective studies. Eur. J. Nutr. 2017 , 56 , 2565–2575. [ Google Scholar ] [ CrossRef ]
- Power, M.L.; Heaney, R.P.; Kalkwarf, H.J.; Pitkin, R.M.; Repke, J.T.; Tsang, R.C.; Schulkin, J. The role of calcium in health and disease. Am. J. Obstet. Gynecol. 1999 , 181 , 1560–1569. [ Google Scholar ] [ CrossRef ]
- Kalkwarf, H.J.; Khoury, J.C.; Lanphear, B.P. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am. J. Clin. Nutr. 2003 , 77 , 257–265. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Huncharek, M.; Muscat, J.; Kupelnick, B. Impact of dairy products and dietary calcium on bone-mineral content in children: Results of a meta-analysis. Bone 2008 , 43 , 312–321. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wallace, T.C.; Bailey, R.L.; Lappe, J.; O’Brien, K.O.; Wang, D.D.; Sahni, S.; Weaver, C.M. Dairy intake and bone health across the lifespan: A systematic review and expert narrative. Crit. Rev. Food Sci. Nutr. 2021 , 61 , 3661–3707. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rizzoli, R. Dairy products and bone health. Aging Clin. Exp. Res. 2022 , 99 , 1256S–1262S. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions. J. Nutr. 2020 , 150 , 663–671. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ralston, R.A.; Truby, H.; Palermo, C.E.; Walker, K.Z. Colorectal cancer and nonfermented milk, solid cheese, and fermented milk consumption: A systematic review and meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2014 , 54 , 1167–1179. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zang, J.; Shen, M.; Du, S.; Chen, T.; Zou, S. The association between dairy intake and breast cancer in Western and Asian populations: A systematic review and meta-analysis. J. Breast Cancer 2015 , 18 , 313–322. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dong, J.-Y.; Zhang, L.; He, K.; Qin, L.-Q. Dairy consumption and risk of breast cancer: A meta-analysis of prospective cohort studies. Breast Cancer Res. Treat. 2011 , 127 , 23–31. [ Google Scholar ] [ CrossRef ]
- Lumsden, A.L.; Mulugeta, A.; Hyppönen, E. Milk consumption and risk of twelve cancers: A large-scale observational and Mendelian randomisation study. Clin. Nutr. 2023 , 42 , 1–8. [ Google Scholar ] [ CrossRef ]
- Palupi, E.; Jayanegara, A.; Ploeger, A.; Kahl, J. Comparison of nutritional quality between conventional and organic dairy products: A meta-analysis. J. Sci. Food Agric. 2012 , 92 , 2774–2781. [ Google Scholar ] [ CrossRef ]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. Oléagineux Corps Gras Lipides 2010 , 17 , 267–275. [ Google Scholar ] [ CrossRef ]
- Kummeling, I.; Thijs, C.; Huber, M.; van de Vijver, L.P.L.; Snijders, B.E.P.; Penders, J.; Stelma, F.; Van Ree, R.; van den Brandt, P.A.; Dagnelie, P.C. Consumption of organic foods and risk of atopic disease during the first 2 years of life in the Netherlands. Br. J. Nutr. 2008 , 99 , 598–605. [ Google Scholar ] [ CrossRef ]
- Christensen, J.S.; Asklund, C.; Skakkebæk, N.E.; Jørgensen, N.; Andersen, H.R.; Jørgensen, T.M.; Olsen, L.H.; Høyer, A.P.; Moesgaard, J.; Thorup, J. Association between organic dietary choice during pregnancy and hypospadias in offspring: A study of mothers of 306 boys operated on for hypospadias. J. Urol. 2013 , 189 , 1077–1082. [ Google Scholar ] [ CrossRef ]
- Murali, A.P.; Trząskowska, M.; Trafialek, J. Microorganisms in Organic Food-Issues to Be Addressed. Microorganisms 2023 , 11 , 1557. [ Google Scholar ] [ CrossRef ]
- Sadiq, M.; Paul, J.; Bharti, K. Dispositional traits and organic food consumption. J. Clean. Prod. 2020 , 266 , 121961. [ Google Scholar ] [ CrossRef ]
- Rana, J.; Paul, J. Consumer behavior and purchase intention for organic food: A review and research agenda. J. Retail. Consum. Serv. 2017 , 38 , 157–165. [ Google Scholar ] [ CrossRef ]
- Precedence Research. Organic Dairy Market. 2022. Available online: https://www.precedenceresearch.com/organic-dairy-market#:~:text=The%20global%20organic%20dairy%20market,10.1%25%20from%202022%20to%202030.&text=The%20organic%20dairy%20products%20are,reared%20using%20organic%20farming%20techniques (accessed on 1 February 2024).
- Barkema, H.W.; von Keyserlingk, M.A.G.; Kastelic, J.P.; Lam, T.J.G.M.; Luby, C.; Roy, J.P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited review: Changes in the dairy industry affecting dairy cattle health and welfare. J. Dairy Sci. 2015 , 98 , 7426–7445. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Carlson, A.; Greene, C.; Raszap Skorbiansky, S.; Hitaj, C.; Ha, K.; Cavigelli, M.; Ferrier, P.; McBride, W. US Organic Production, Markets, Consumers, and Policy, 2000–21 ; United States Department of Agriculture (USDA): Washington, DC, USA, 2023.
- Willer, H.; Schaack, D.; Lernoud, J. Organic farming and market development in Europe and the European Union. In The World of Organic Agriculture. Statistics and Emerging Trends 2019 ; Research Institute of Organic Agriculture FiBL: Frick, Switzerland; IFOAM-Organics International: Bonn, Germany, 2019; pp. 217–254. [ Google Scholar ]
- Willer, H.; Sorensen, N.; Yussefi-Menzler, M. The world of organic agriculture 2008: Summary. In the World of Organic Agriculture ; Routledge: London, UK, 2010; pp. 15–22. [ Google Scholar ]
- EU Agricultural Outlook 2021-31: Lower Demand for Feed to Impact Arable Crops. December 2021. Available online: https://agriculture.ec.europa.eu/news/eu-agricultural-outlook-2021-31-lower-demand-feed-impact-arable-crops-2021-12-09_en (accessed on 1 February 2024).
- Maji, S.; Meena, B.S.; Paul, P.; Rudroju, V. Prospect of organic dairy farming in India: A review. Asian J. Dairy Food Res. 2017 , 36 , 1–8. [ Google Scholar ] [ CrossRef ]
- Mahesh, M.S. Integrated organic farming and organic milk production: Opportunities and challenges in India. Indian Dairym. 2013 , 65 , 56–60. [ Google Scholar ]
- KPMG. Global Organic Milk Production Market Report. Available online: https://ciorganicos.com.br/wp-content/uploads/2020/09/global-organic-milk-production-market-report.pdf (accessed on 1 February 2024).
- Managi, S.; Yamamoto, Y.; Iwamoto, H.; Masuda, K. Valuing the influence of underlying attitudes and the demand for organic milk in Japan. Agric. Econ. 2008 , 39 , 339–348. [ Google Scholar ] [ CrossRef ]
- McBride, W.D. Characteristics, Costs, and Issues for Organic Dairy Farming ; DIANE Publishing: Collingdale, PA, USA, 2010; Volume 82. [ Google Scholar ]
- Sabunevica, S.; Zagorska, J. Organic Milk as Medium for Lactic Acid Bacteria Growth: A Review. Rural Sustain. Res. 2023 , 49 , 73–86. [ Google Scholar ] [ CrossRef ]
| Country | Pasture Access | Nutrition | Antibiotics Use | Organic Conversion Period | Regulation |
|---|---|---|---|---|---|
| European Union | Year-round, weather permitting | ≥60% of daily dry matter intake must consist of roughage, fresh or dried fodder, or silage. | Permitted under veterinary recommendation. ≥2 day milk withdrawal. ≥3 treatments or ≥1 treatment (if productive lifecycle is <1 y) will cause animal to lose its organic status. | Land conversion period of 24-months. Animals must be under organic management ≥6 months. | Regulation (EU) 2018/848 of the European Parliament and of the Council. |
| United States | ≥120 days annually | ≥30% of daily dry matter intake must come from pasture during grazing season. | Prohibited. Usage will cause animal to lose its organic status. | Animals must be under organic management ≥12 months. | Organic foods production act provisions 2023. |
| Canada | ≥120 days annually | ≥30% of daily dry matter intake must come from pasture during grazing season. 60% of dry matter intake consists of hay, fresh/dried fodder, or silage. | Permitted under veterinary recommendation. ≥30 day milk withdrawal. ≥2 treatments, 12 month transition period before regaining organic status. | Animals must be under organic management ≥12 months. | Organic Production Systems General Principles and Management Standards 2021. |
| Japan | ≥2 days per week, year-round | ≥50% of daily dry matter intake must consist of roughage, fresh or dried fodder, or silage. | Permitted under veterinary recommendation. | Animals must be under organic management ≥6 months. | Japanese Agricultural Standard for Organic Livestock Products, 2018. |
| New Zealand | ≥150 days annually | ≥50% of daily dry matter intake must consist of roughage, fresh or dried fodder, or silage. | Prohibited. Usage will cause animal to lose its organic status. | Animals must be under organic management ≥12 months. | AsureQuality Organic Standard For Primary Producers, 2018. |
| Australia | Year-round, weather permitting | 100% of daily dry matter intake must be sourced from organic or bio-dynamic feed. | Permitted under veterinary recommendation. 180 day transition period before regaining organic status. | Animals must be under organic management ≥6 months. | National Standard for Organic and Bio-Dynamic Produce, 2022. |
| China | Year-round, weather permitting | ≥60% of daily dry matter intake must consist of roughage, fresh or dried fodder, or silage. | Permitted under veterinary recommendation. | Animals must be under organic management ≥6 months. | China Organic Standard GB/T 19630-2019. |
| India | Year-round, weather permitting | ≥85% of daily dry matter intake must be sourced from organic feed | Permitted under veterinary recommendation. | Land conversion period of 24 months. Animals must be under organic management ≥6 months. | Agricultural and Processed Food Products Export Development Authority (APEDA) 2018. |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Linehan, K.; Patangia, D.V.; Ross, R.P.; Stanton, C. Production, Composition and Nutritional Properties of Organic Milk: A Critical Review. Foods 2024 , 13 , 550. https://doi.org/10.3390/foods13040550
Linehan K, Patangia DV, Ross RP, Stanton C. Production, Composition and Nutritional Properties of Organic Milk: A Critical Review. Foods . 2024; 13(4):550. https://doi.org/10.3390/foods13040550
Linehan, Kevin, Dhrati V. Patangia, Reynolds Paul Ross, and Catherine Stanton. 2024. "Production, Composition and Nutritional Properties of Organic Milk: A Critical Review" Foods 13, no. 4: 550. https://doi.org/10.3390/foods13040550
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Composition of Milk
Cite this chapter.

- Robert Jenness
2694 Accesses
28 Citations
Milk is secreted by all species of mammals to supply nutrition and immunological protection to the young. It performs these functions with a large array of distinctive compounds. Interspecies differences in the quantitative composition of milk (Jenness and Sloan 1970) probably reflect differences in the metabolic processes of the lactating mother and in the nutritive requirements of the suckling young.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Unable to display preview. Download preview PDF.
Similar content being viewed by others

Production and Utilization of Milk
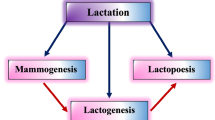
Chemical Composition of Human and Mammalian Milk (A Review)
Milk proteins.
Allen, R. J. L. 1940. The estimation of phosphorus. Biochem. J. 34 , 858–865.
CAS Google Scholar
Archibald, J. G. 1958. Trace elements in milk: A review. Dairy Sci. Abstr. 20 , 712–725, 798–808.
Google Scholar
Armstrong, T. V. 1959. Variations in the gross composition of milk as related to the breed of the cow: A review and critical evaluation of literature of the United States and Canada. J. Dairy Sci. 42 , 1–19.
Armstrong, M. D. and Yates, K. N. 1963. Free amino acids in milk. Proc. Soc. Exp. Biol. Med. 113 , 680–683.
Babajimopoulos, M. and Mikolajcik, E. M. 1977. Quantification of selected serum proteins of milk by immunological procedures. J. Dairy Sci 60 , 721–725.
Barry, J. M. and Rowland, S. J. 1953. Variations in the ionic and lactose concentrations of milk. Biochem. J. 54 , 575–578.
Basu, K. P., Paul, T. M., Shroff, N. B. and Rahman, M. A. 1962. Composition of Milk and Ghee . Indian Counc. Agr. Res. Report Series No. 8.
Beebe, J. M. and Gilpin, R. K. 1983. Determination of α -and β -lactose in dairy products by totally aqueous liquid chromatography. Anal. Chim. Acta 146 , 255–259.
Bell, J. W. and Stone, W. K. 1979. Rapid separation of whey proteins by cellulose acetate electrophoresis. J. Dairy Sci. 62 , 502–504.
Bican, P. and Blanc, B. 1982. Milk protein analysis—a high-performance chromatography study. Milchwissenschaft 37 , 592–593.
Bingham, R. J., Huber, J. D. and Aurand, L. W. 1967. Thioctic acid in milk. J. Dairy Sci. 50 , 318–323.
Blanc, B. 1981. Biochemical aspects of human milk—comparison with bovine milk. Wld. Rev. Nutr. Dietet. 36 , 1–89.
Bonnier, G., Hansson, A. and Jarl, F. 1946. Studies in the variations of the calory content of milk. Acta Agr. Suecana 2 , 159–169.
Booth, A. N., Robbins, D. J. and Dunkley, W. L. 1962. Occurrence of salicyluric acid in milk. Nature (Lond.) 194 , 290–291.
Booy, C. J., Klijn, C. J. and Posthumus, G. 1962. The rapid estimation of the protein content of milk. Dairy Sci. Abstr. 24 , 223–228, 275–279.
Bortree, A. L., Carroll, E. J. and Schalm, O. W. 1962. Whey protein patterns of milk from cows with experimentally produced mastitis. J. Dairy Sci. 45 , 1465–1471.
Brewington, C. R., Parks, O. W. and Schwartz, D. P. 1974. Conjugated compounds in cow’s milk. II. J. Agr. Food. Chem. 22 , 293–294.
Brons, C. and Olieman, C. 1983. Study of the high-performance liquid chromatographic separation of reducing sugars, applied to the determination of lactose in milk. J. Chromatog. 259 , 79–86.
Butler, J. E. 1974. Immunoglobulins of the mammary secretions. In: Lactation: A Comprehensive Treatise , Vol. 3. B. L. Larson and V. R. Smith (Editors). Academic Press, New York.
Byun, S. M. and Jenness, R. 1982. Estimation of free myoinositol in milks of various species and its source in milk of rats ( Rattus norvegicus ) J. Dairy Sci. 65 , 531–536.
Carroll, E. J., Schalm, O. W. and Lasmanis, J. 1963. Experimental coliform ( Aerobacter aerogenes ) mastitis: Distribution of whey proteins during the early acute phase. J. Dairy Sci. 46 , 1236–1242.
Cerbulis, J. and Farrell, H. M., Jr. 1975. Composition of milks of dairy cattle. I. Protein, lactose, and fat contents and distribution of protein fraction. J. Dairy Sci. 58 , 817–827.
Cerbulis, J. and Farrell, H. M., Jr. 1976. Composition of the milks of dairy cattle. II. Ash, calcium, magnesium and phosphorus. J. Dairy Sci. 59 , 589–593.
Cobble, J. W. and Herman, H. A. 1951. The Influence of Environmental Temperature on the Composition of the Milk of the Dairy Cow . Mo. Agr. Exp. Sta. Res. Bull 485.
Cole, D. D., Harper, W. J. and Hankinson, C. L. 1961. Observations on ammonia and volatile amines in milk. J. Dairy Sci 44 , 171–173.
Dagley, S. 1974. Citrate—UV spectrophotometric determination. In:Methods of Enzymatic Analysis , 2nd ed. H. U. Bergmeyer (Editor). Academic Press, New York.
Davies, D. T. 1974. The quantitative partition of the albumin fraction of milk serum proteins by gel chromatography. J. Dairy Res. 41 , 217–228.
Davies, D. T. and Law, A. J. R. 1977. An improved method for the quantitative fractionation of casein mixtures using ion-exchange chromatography. J. Dairy Res. 44 , 213–221.
Davies, D. T. and White, J. C. D. 1960. The use of ultrafiltration and dialysis in isolating the aqueous phase of milk and in determining the partition of milk constituents between the aqueous and disperse phases. J. Dairy Res. 27 , 171–190.
Davies, J. G. 1952. The chemical composition of milk between 1900 and 1950. Analyst 77 , 494–524.
Dawson, R. R. and Rook, J. A. F. 1972. A note on the influence of stage of lactation on the response in lactose content of milk to a change of plane of energy nutrition in the cow. J. Dairy Res. 39 , 107–111.
deKoning, P. J. and Wijand, H. P. 1965. The effect of sugars and heat treatment on the determination of N -acetyl neuraminic acid in milk. Neth. Milk Dairy J. 19 , 73–81.
Deutsch, A. and Samuelsson, E. G. 1958. Amino acids and low molecular weight aminoacid derivatives in cows’ milk. Int. Dairy Cong. 1958. 3 , 1650–1652.
Devery-Pocius, J. E. and Larson, B. L. 1983. Age and previous lactations as factors in the amount of bovine colostral immunoglobulins. J. Dairy Sci. 66 , 221–226.
Diosady, L. L., Bergen, I. and Harwalkar, V. R. 1980. High performance liquid chromatography of whey proteins. Milchwissenschaft 35 , 671–674.
Ebner, K. E. and Schanbacher, F. L. 1974. Biochemistry of lactose and related carbohydrates. In: Lactation: A Comprehensive Treatise ., Vol. 2. B. L. Larson and V. R. Smith (Editors) Academic Press, New York.
Eigel, W. N., Butler, J. E., Ernstrom, C. A., Farrell, H. M. Jr., Harwalker, V. R., Jenness, R. and Whitney, R. McL. 1984. Nomenclature of the proteins of cow’s milk. Fifth revision. J. Dairy Sci. 67 , 1599–1631.
Ellenberger, H. B., Newlander, J. A. and Jones, C. H. 1950. Variations in the Calcium and Phosphorus Contents of Cow’s Milk . Vt. Agr. Exp. Sta. Bull. 556.
Erfle, J. D., Fisher, L. J. and Sauer, F. 1970. Carnitine and acetyl-carnitine in the milk of normal and ketotic cows. J. Dairy Sci. 53 , 486–492.
FAO Production Yearbook . 1979. Vol. 33. Food and Agriculture Organization of the United Nations.
Faulkner, A., Chaiybutr, N., Peaker, M., Carrick, D. I. and Kuhn, N. J. 1981. Metabolic significance of milk glucose. J. Dairy Res. 48 , 51–56.
Faulkner, A. and Clapperton, J. L. 1981. Changes in the concentration of some minor constituents of milk from cows fed low or high-fat diets. Comp. Biochem. Physiol 68A 281–283.
Frayer, J. M. 1940. The Dissolved Gases in Milk and Dye Reduction . Vt. Agr. Expt. Sta. Bull 461.
Gala, R. R., Forsyth, I. A. and Turney, A. 1980. Milk prolactin is biologically active. Life Sci. 26 , 987–993.
Ganguli, N. C. and Iya, K. K. 1963. The occurrence of sugar phosphates in milk. Ind. J. Chem. 1 , 145–146.
Garrett, O. F. and Overman, O. R. 1940. Mineral composition of colostral milk. J. Dairy Sci. 23 , 13–17.
Gaull, G. E., Jensen, R. G., Rassin, D. K. and Malloy, M. 1982. Human milk as food. Adv. Perinatal Med. 2 , 47–120.
Gaunt, S. N. 1980. Genetic variation in the yields and contents of milk constituents. In: Factors Affecting the Yield and Contents of Milk Constituents of Commercial Importance . P. C. Moore and J. A. F. Rook (Editors). Internat. Dairy Fed. Doc. 125.
Gil, A. and Sanchez-Mendina, F. 1981. Acid-soluble nucleotides of cow’s, goat’s and sheep’s milks at different stages of lactation. J. Dairy Res. 48 , 35–44.
Grant, D. R. and Patel, R. R. 1980. Changes of protein composition of milk by ratio of roughage to concentrate. J. Dairy Sci. 63 , 756–761.
Griffiths, T. W. and Featherstone, J. 1957. Variations in the solids-not-fat content of milk. Investigations into the nature of the solids-not-f at problems in the West Midlands. J. Dairy Res. 24 , 201–209.
Grimbleby, F. H. 1956. The determination of lactose in milk. J. Dairy Res. 229–237.
Goulden, J. D. S. 1964. Analysis of milk by infra-red absorption. J. Dairy Res. 31 , 273–284.
Guidry, A. J. and Pearson, R. E. 1979. Improved methodology for quantitative determination of serum and milk proteins by single radial immuno-diffusion. J. Dairy Sci. 62 , 1252–1257.
Guthrie, E. S. 1946. The results of deaeration on the oxygen, vitamin C and oxidized flavors of milk. J. Dairy Sci. 29 , 359–369.
Han, K. and Boulange, M. 1963. Determination of thiocyanate in milk by a technique of immunodiffusion. Clin. Chim. Acta 8 , 779–785.
Harper, W. J., Gould, I. A. and Hankinson, C. L. 1961. Observations on the free volatile acids in milk. J. Dairy Sci. 44 , 1764–1765.
Harper, W. J. and Huber, R. M. 1956. Some carbonyl compounds in raw milk. J. Dairy Sci. 39 , 1609.
Hartman, A. M. and Dryden, L. P. 1974. The vitamins in milk and milk products. In:Fundamentals of Dairy Chemistry , 2nd ed. B. H. Webb, A. H. Johnson and J. A. Alford (Editors). AVI Publishing Co. Westport, Conn.
Hazum, E., Sabatka, J. J., Chang, K. J., Brent, D. A., Findlay, J. W. A. and Cuatrecasas, P. 1981. Morphine in cow and human milk: Could dietary morphine constitute a ligand for specific morphine ( µ ) receptors? Science 213 , 1010–1012.
Heinemann, B. 1944. The determination of citric acid in milk products by cerate oxidimetry. J. Dairy Sci. 27 , 377–384.
Herreid, E. O. and Francis, J. 1949. The effect of handling and processing of milk on its oxygen content. J. Dairy Sci. 32 , 202–208.
Herrington, B. L., Sherbon, J. W., Ledford, R. A. and Houghton, G. E. 1972. Composition of milk in New York State. New York’s Food Life Sci. Bull. 18 , 1–23.
Hinton, C. L. and Macara, T. 1927. The determination of aldose sugars by means of chloramine-T, with special reference to the analysis of milk products. Analyst 52 , 668–688.
Hoff, J. E. 1963. Determination of N-acetylglucosamine-1-phosphate and N -acetylgluco-samine in milk. J. Dairy Sci. 46 , 573–574.
Hoff, J. E. and Wick, E. L., 1963. Acid soluble phosphates in cow milk. J. Food Sci. 28 , 510–518.
Holt, C., Dalgleish, D. G. and Jenness, R. 1981. Calculation of the ion equilibria in milk diffusate and comparison with experiment. Anal Biochem. 113 , 154–163.
Horwitz, W. (Editor). 1980. Official Methods of Analysis , 13th ed. Association of Official Analytical Chemists, Washington, D.C.
Janse, L. C. 1950. Composition of Friesian milk. Neth. Milk Dairy J. 4 , 1–9.
Jenness, R. 1974. The composition of milk. In: Lactation: A Comprehensive Treatise , Vol. 3. B.L. Larson and V.R. Smith (Editors). Academic Press, New York.
Jenness, R. 1979. The composition of human milk. Semin. Perinatol 3 , 225–239.
Jenness, R. 1980. Composition and characteristics of goat milk: A review, 1968–1979. J. Dairy Sci. 63 , 1605–1630.
Jenness, R., Regehr, E. A. and Sloan, R. E. 1964. Comparative biochemical studies of milks. II. Dialyzable carbohydrates. Comp. Biochem. Physiol. 13 , 339–352.
Jenness, R. and Sloan, R. E. 1970. The composition of milks of various species. A review. Dairy Sci. Abstr. 32 , 599–612.
Johansson, I., Korkman, N. and Nelson, N. J. 1952. Studies on udder evacuation of dairy cows I and II. Acta Agr. Scand. 3 , 43–81, 82–102.
Johke, T. 1963. Acid-soluble nucleotides of colostrum, milk and mammary gland. J. Biochem. 54 , 388–397.
Johnson, A. H. 1975. The composition of milk. In: Fundamentals of Dairy Chemistry , 2nd ed. B. H. Webb, A. H. Johnson and J. A. Alford (Editors) AVI Publishing Co., Wesport, Conn.
Kiermeier, F. and Freisfeld, I. 1965. Neuraminic acid content of cows’ milk. Z. Lebensmut. Untersuch. Forsch., 128 , 207–217.
Knodt, C. B., Shaw, J. C. and White, G. C. 1942. Studies on ketosis in dairy cattle. II. Blood and urinary acetone bodies of dairy cattle in relation to parturition, lactation, gestation and breed. J. Dairy Sci. 25 , 851–860.
Kolbata, A., Ziro, S. and Kida, M. 1962. The acid-soluble nucleotides of milk. J. Biochem. 51 , 277–287.
Koldovsky, O. 1980. Hormones in milk. Life Sci. 26 , 1833–1836.
Koops, J. 1965. Rapid turbidimetric determination of inorganic sulphate in milk. Neth. Milk Dairy J. 19 , 59–62.
Kreula, M. and Virtanen, A. I. 1956. α-keto acids in cow’s milk. 14th Int. Dairy Cong. 1 , 802–806.
Kroger, M. 1974. General environmental contaminants occurring in milk. In: Lactation: A Comprehensive Treatise , Vol. 3. B. L. Larson and V. R. Smith (Editors). Academic Press, New York.
Kumar, F. A., Ferchmin, P. A. and Caputto, R. 1965. Isolation and identification of a lactose phosphate ester from cow colostrum. Biochem. Biophys. Res. Commun. 20 , 60–62.
Kuramoto, S., Jenness, R., Coulter, S. T. and Choi, R. P. 1959. Standardization of the Harland-Ashworth test for whey protein nitrogen. J. Dairy Sci. 42 , 28–38.
Kurz, G. and Wallenfels, K. 1974. D-Galactose. UV-assay with galactose dehydrogenase. In:Methods of Enzymatic Analysis , 2nd éd. H. U. Bergmeyer (Editor). Academic Press, New York.
Laben, R. C. 1963. Factors responsible for variation in milk composition. J. Dairy Sci. 46 , 1293–1301.
Langen, H. de. 1967. Determination of DNA in milk. Aust. J. Dairy Technol. 22 , 36–40.
Larson, B. L. and Hageman, E. C. 1963. Determination of α -lactalbumin in complex systems. J. Dairy Sci. 46 , 14–18.
Larson, B. L. and Hegarty, H. M. 1977. Orotic acid and pyrimidine nucleotides in ruminant milks. J. Dairy Sci. 60 , 1223–1229.
Larson, B. L. and Twarog, J. M. 1961. Determination of β -lactoglobulin in complex systems by a simple immunological procedure. J. Dairy Sci. 44 , 1843–1856.
Lawrence, A. J. 1970. The thiocyanate content of milk. 18th Internat. Dairy Congr. IE. 99.
Laxminarayan, H. and Dastur, N. N. 1968. Buffaloes’ milk and milk products. Dairy Sci. Abstr. 30 , 177–186, 231–241.
Leece, J. G. and Legates, J. E. 1959. Changes in the paper electrophoretic whey-protein pattern of cows with acute mastitis. J. Dairy Sci. 42 , 698–704.
Legates, J. E. 1960. Genetic and environmental factors affecting the solids-not-fat composition of milk. J. Dairy Sci. 43 , 1527–1532.
Lengemann, F. W., Wentworth, R. A. and Comar, C. L. 1974. Physiological and biochemical aspects of the accumulation of contaminant radionuclides in milk. In: Lactation: A Comprehensive Treatise , Vol 3. B. L. Larson and V. R. Smith (Editors). Academic Press, New York.
Ling, E. R. 1956. A Textbook of Dairy Chemistry , 3rd ed. Vol. 2, Practical . Chapman Hall, London.
Macy, I. G., Kelly, H. J. and Sloan, R. E. 1953. The Composition of Milks . Nat. Acad. Sci.-Nat. Res. Counc. Publ. 254.
Marier, J. R. and Boulet, M. 1959. Direct analysis of lactose in milk and serum. J. Dairy Sci. 42 , 1390–1391.
Marsili, R. T., Ostapenko, H., Simmons, R. E. and Green, D. E. 1981. High performance liquid chromatographic determination of organic acids in dairy products. J. Food Sci. 46 , 52–57.
Me Dowall, F. H. 1936. The determination of carbon dioxide in biological fluids, more particularly milk and cream. Analyst 61 , 472–473.
McDowell, A. K. R. 1941. The estimation of lactose in milk. J. Dairy Res. 12 , 131–138.
McGeown, M. G. and Malpress, F. H. 1952. Studies on the synthesis of lactose by the mammary gland. 2. The sugar phosphate esters of milk. Biochem. J. 52 , 606–611.
Meun, D. H. C. and Smith, K. C. 1968. A microphosphate method. Anal. Biochem. 26 , 364–368.
Moore, J. H. and Rook, J. A. F. (Editors). 1980. Factors affecting the yields and contents of milk constituents of commercial importance. Internat. Dairy Fed. Bulletin Doc. 125 , 1–167.
Morr, C. V., Harper, W. J. and Gould, I. A. 1957. Some organic acids in raw and heated skim milk. J. Dairy Sci. 40 , 964–972.
Morrissey, P. A. 1973. The iV-acetyl neuraminic acid content of the milk of various species. J. Dairy Res. 40 , 421–425.
Murthy, G. K. 1974. Trace elements in milk. CRC Crit. Rev. Environmental Control 4 , 1–37.
Murthy, G. K. and Rhea, U. 1967. Determination of major cations in milk by atomic absorption spectrophotometry. J. Dairy Sci. 50 , 313–317.
Nickerson, T. A. 1960. Chemical composition of milk. J. Dairy Sci. 60 , 598–606.
Nickerson, T. A., Moore, E. E. and Zimmer, A. A. 1964. Spectrophotometric determination of calcium in milk using 2,2’-(ethanediylidene-dinitrilo) diphenol (glyoxal bis (2-hydroxyanil). Anal. Chem. 36 , 1676–1677.
Noll, C. I. and Supplee, G. C. 1941. Factors affecting the gas content of milk. J. Dairy Sci. 24 , 993–1013.
Overman, O. R. 1945. Monthly variations in the composition of milk. J. Dairy Sci. 28 , 305–309.
Overman, O. R., Garrett, O. F., Wright, K. E. and Sanmann, F. P. 1939. Composition of the Milk of Brown Swiss Cows . Ill. Agr. Expt. Sta. Bull. 457.
Overman, O. R., Keirs, R. J. and Craine, E. M. 1953. Composition of Herd Milk of the Brown Swiss Breed . Ill. Agr. Exp. Sta. Bull. 567.
Packard, V. S. 1982. Human Milk and Infant Formula . Academic Press, New York.
Parkash, S. and Jenness, R. 1968. The composition and characteristics of goats’ milk: A review. Dairy Sci. Abstr. 30 , 67–87.
Parkhie, M. R., Gilmore, L. O. and Fechheimer, N. S. 1966. Effect of successive lactations, gestation, and season of calving on constituents of cows’ milk. J. Dairy Sci. 49 , 1410–1415.
Parks, O. W., Schwartz, D. P., Nelson, K. and Allen, C. 1967. Evidence for kynurenine in milk. J. Dairy Sci. 50 , 10–11.
Parrish, D. B., Wise, G. H., Hughes, E. S. and Atkeson, F. W. 1948. Properties of the colostrum of the dairy cow. II. Effect of prepartal rations upon the nitrogenous constituents. J. Dairy Sci. 31 , 889–895.
Parrish, D. B., Wise, G. H., Hughes, E. S. and Atkeson, F. W. 1950. Properties of the colostrum of the dairy cow. V. Yield, specific gravity and concentrations of total solids and its various components of colostrum and early milk. J. Dairy Sci. 33 , 457–465.
Patton, S., Forss, D. A. and Day, E. A. 1956. Methyl sulfide and the flavor of milk. J. Dairy Sci. 39 , 1469–1470.
Patton, S. and Potter, F. E. 1956. The presence of α -ketoglutaric acid in milk. J. Dairy Sci. 39 , 611–612.
Perry, N. A. and Doan, F. J. 1950. A picric acid method for the simultaneous determination of lactose and sucrose in dairy products. J. Dairy Sci. 33 , 176–185.
Pope, G. S. and Swinburne, J. K. 1980. Hormones in milk: Their physiological significance and value as diagnostic aids. J. Dairy Res. 47 , 427–449.
Powell, E. B. 1939. Some relations of the roughage intake to the composition of milk. J. Dairy Sci. 22 , 453–454.
Powell, E. B. 1941. Progress report on the relation of the ration to the composition of milk. J. Dairy Sci. 24 , 504–505.
Ramos, M. and Juarez, M. 1981. The composition of ewe’s and goat’s milk. Internat. Dairy Fed. Bull. Doc. 140 , 1–19.
Rassin, D. K., Sturman, J. A. and Gaull, G. E. 1978. Taurine and other free amino acids in milk of man and other mammals. Early Human Dev. 2 , 1–13.
Reinart, A. and Nesbitt, J. M. 1956A. The distribution of nitrogen in milk in Manitoba. 14th Int. Dairy Cong. 1 , 925–933.
Reinart, A. and Nesbitt, J. M. 1956B. The composition of milk in Manitoba. 14th Int. Dairy Cong. 1 , 946–956.
Reinart, A. and Nesbitt, J. M. 1956C. The lactose content of milk in Manitoba. 14th Int. Dairy Cong. 1 , 957–964.
Reineccius, G. A., Kavanagh, T. E. and Keeney, P. G. 1970. Identification and quantitation of free neutral carbohydrates in milk products by gas-liquid chromatography and mass spectrometry. J. Dairy Sci. 53 , 1018–1022.
Reithel, F. J. and Venkataraman, R. 1956. Lactose in the Sapotaceace. Science 123 , 1083–1084.
Richardson, T., McGann, T. C. A. and Kearney, R. D. 1980. Levels and location of adenosine 5’-tri-phosphate in bovine milk. J. Dairy Res. 47 , 91–96.
Rook, J. A. F. 1961. Variations in the chemical composition of the milk of the cow. Dairy Sci. Abstr. 23 , 251–258, 303–308.
Rowland, S. J. 1938. The determination of the nitrogen distribution in milk. J. Dairy Res. 9 , 42–46.
Rowland, S. J., Neave, F. K., Dodd, F. H. and Oliver, J. 1959. The effect of Staphlphococ-cus pyogenes infections on milk secretion. 15th Int. Dairy Cong. 1 , 121–127.
Sanders, G. P. 1939. The determination of chloride in milk. J. Dairy Sci. 22 , 841–851.
Sanguansermsri, J., Gyorgy, P. and Zilliken, F. 1974. Polyamines in human and cow’s milk. Am. J. Clin. Nutr. 27 , 859–865.
Scanlan, R. A., Lindsay, R. C., Libbey, L. M. and Day, E. A. 1968. Heat-induced volatile compounds in milk. J. Dairy Sci. 51 , 1001–1007.
Schalm, D. W., Carroll, E. J. and Jain, N. C., 1971. Bovine Mastitis . Lea & Febiger, Philadelphia.
Schwartz, D. P. and Pallansch, M. J. 1962A. Identification of some nitrogenous constituents of cow’s milk by ion exchange and paper chromatrography. J. Ag. Food Chem. 10 , 86–89.
Schwartz, D. P. and Pallansch, M. J. 1962B. Occurrence of phenylacetyl-glutamine in cow’s milk. Nature (Lond.) 194 , 186.
Sharp, P. F. and Struble, E. B. 1935. Period of lactation and the direct titratable chloride value of milk. J. Dairy Sci. 18 , 527–538.
Smith, A. C. 1964. The carbon dioxide content of milk during handling, processing and storage and its effect upon the freezing point. J. Milk Food Tech. 27 , 38–41.
Snelson, J. J. (Editor). 1979. Chemical Residues in Milk and Milk Products . International Dairy Federation Bulletin, Document 113, 1–69.
Snoswell, A. M. and Linzell, J. L. 1975. Carnitine secretion into milk of ruminants. J. Dairy Res. 42 , 371–380.
Spinelli, F. 1946. Indican in cow and goat milks. Boll. Soc. Ital. Sper. 21 , 210–211.
Sumner, J. B. 1944. A method for the colorimetric determination of phosphorus. Science 100 , 413.
Swaisgood, H. (Editor). 1975. Methods of Gel Electrophoresis of Milk Proteins . Am. Dairy Sci. Assn. Champaign, Ill.
Swope, F. C. and Brunner, J. R. 1965. Identification of ribonucleic acid in the fat globule membrane. J. Dairy Sci. 48 , 1705–1707.
Swope, F. C., Brunner, J. R. and Vadhera, D. V. 1965. Riboflavin and its natural derivatives in the fat-globule membrane. J. Dairy Sci. 48 , 1707–1708.
Tamsma, A. and Tarassuk, N. P., 1957. Removal of oxygen from evaporated milk with glucose oxidase. J. Dairy Sci. 40 , 1181–1188.
Thomas, P. C. 1980. Influence of nutrition on the yield and content of protein in milk: Dietary protein and energy supply. In: Factors Affecting the Yields and Content of Milk Constituents of Commercial Importance . J. H. Moore and J. A. F. Rook (Editors). Internat. Dairy Fed. Doc. 125.
Tocher, J. F. 1925. Variations in the Composition of Milk . H. M. Stationery Office, London.
Touchberry, R. W. 1974. Environmental and genetic factors in the development and maintenance of lactation. In: Lactation: A Comprehensive Treatise , Vol. 3. B.L. Larson and V. R. Smith (Editors). Academic Press, New York.
Underwood, E. J. 1977. Trace Elements in Human and Animal Nutrition , 4th ed. Academic Press, New York.
U.S. Dept. Agr. 1980. Federal and State Standards for the Composition of Milk Products . Agr. Handbook 51. Government Printing Office, Washington, D.C.
van der Have, A. J., Deen, J. R. and Mulder, H. 1979. The composition of cow’s milk. 1. The composition of separate milkings of individual cows. Neth. Milk Dairy J. 33 , 65–81.
Virtanen, A. I. 1966. Milk production of cows on protein-free feed. Science 153 , 1603–1614.
Vogel, J. and Deshusses, J. 1965. Polarographic estimation of small amounts of benzoic acid: The application of the method for the estimation of benzoic acid in normal milk and yoghurt. Mitt. Geb. Lebensmut. Untersuch Hyg. 56 , 63–67. ( Dairy Sci. Abstr. 27 , 2944, 1965.)
Waite, R. 1961. A note on a method of overcoming the effect of udder disease or injury in experiments involving milk yield and composition. J. Dairy Res. 28 , 75–79.
Waite, R. and Patterson, J. A. 1959. The composition of bulk milk measured on Scottish milk-recorded farms. J. Soc. Dairy Technol. 12 , 117–122.
Walstra, P. and Jenness, R. 1984. Dairy Chemistry and Physics . John Wiley & Sons, New York.
Walstra, P. and Mulder, H. 1964. Gravimetric methods for the determination of the fat content of milk and milk products. V. Comparison of methods. Neth. Milk Dairy J. 18 , 237–242.
Wayman, O., Johnson, H. D., Merilan, C. P. and Berry, I. L. 1962. Effect of ad libitum or force-feeding of two rations on lactating dairy cows subject to temperature stress. J. Dairy Sci. 45 , 1472–1478.
Wenner, V. R. 1958. Rapid determination of milk salts and ions. I. Determination of sodium, potassium, magnesium and calcium by flame spectrophotometry. J. Dairy Sci. 41 , 761–768.
West, D. W. and Towers, G. E. 1976. Cellulose acetate electrophoresis of casein proteins. Anal Biochem. 75 , 58–66.
Wheelock, J. V. 1980. Influence of physiological factors on the yields and contents of milk constituents. In: Factors Affecting the Yields and Contents of Milk Constituents of Commercial Importance . J. H. Moore and J. A. F. Rook (Editors). Internat. Dairy Fed. Doc. 125.
Wheelock, J. V, Rook, J. A. F. and Dodd, F. H. 1965. The effect of milking throughout the whole of pregnancy on the composition of cow’s milk. J. Dairy Res. 32 , 249–254.
White, J. C. D. and Davies, D. T. 1958. The relation between the chemical composition of milk and the stability of the caseinate complex. I. General introduction, description of samples, methods and chemical composition of samples. J. Dairy Res. 25 , 236–255.
White, J. C. D. and Davies, D. T. 1962. The determination of calcium and magnesium in milk and milk diffusate. J. Dairy Res. 29 , 285–296.
White, J. C. D. and Davies, D. T. 1963. The determination of citric acid in milk and milk sera. J. Dairy Res. 30 , 171–189.
Whittlestone, W. G. 1953. Variations in the fat content of milk throughout the milking process. J. Dairy Res. 20 , 146–153.
Wilcox, C. J., Pfau, K. O., Mather, R. E. and Bartlett, J. W. 1959. Genetic and environmental influences upon solids-not-fat content of cow’s milk. J. Dairy Sci. 42 , 1132–1146.
Williams, K. I. H., Burstein, S. H. and Layne, D. S. 1966. Dimethyl sulfone: Isolation from cows’ milk. Proc. Soc. Exp. Biol. Med. 122 , 865–866.
Wolfschoon-Pombo, A. and Klostermeyer, H. 1981. The NPN-fraction of cow milk I. Amount and composition. Milchwissenschaft 36 , 598–600.
Wong, N. P. and Patton, S. 1962. Identification of some volatile compounds related to the flavor of milk and cream. J. Dairy Sci. 45 , 724–728.
Wrenn, T. R., Bitman, J., Cecil, H. C. and Gilliam, D. R. 1963. Histamine concentration in blood, milk and urine of dairy cattle. J. Dairy Sci. 46 , 1243–1245.
Zarembski, P. M. and Hodgkinson, A. 1962. The determination of oxalic acid in food. Analyst 87 , 698–702.
Zulak, I. M., Patton, S. and Hammerstedt, R. H. 1976. Adenosine triphosphate in milk. J. Dairy Sci. 59 , 1388–1391.
Download references
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Agricultural Research Service, U.S. Department of Agriculture, USA
Noble P. Wong
Department of Biochemistry, University of Minnesota, USA
Robert Jenness
Department of Chemistry and Biochemistry, University of Maryland, USA
Mark Keeney
Department of Food Science, University of Wisconsin, USA
Elmer H. Marth
Rights and permissions
Reprints and permissions
Copyright information
© 1988 Van Nostrand Reinhold Company Inc.
About this chapter
Jenness, R. (1988). Composition of Milk. In: Wong, N.P., Jenness, R., Keeney, M., Marth, E.H. (eds) Fundamentals of Dairy Chemistry. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-7050-9_1
Download citation
DOI : https://doi.org/10.1007/978-1-4615-7050-9_1
Publisher Name : Springer, Boston, MA
Print ISBN : 978-0-442-20489-1
Online ISBN : 978-1-4615-7050-9
eBook Packages : Springer Book Archive
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Composition of Milk of Different Farm Animal: A Review
American Journal of Pure and Applied Biosciences.
17 Pages Posted: 16 Mar 2021 Last revised: 27 May 2021
Hassen Yusuf Bekere
Haramaya University

Mohammedsham Husen
Haramaya university,college of veterinary medicine.; Tulo offices of livestock and fisheries development
Date Written: July 7, 2020
Milk is a key contributor to improving nutrition and food security particularly in developing countries. Milk composition of mammalian species varies widely with reference to genetic, physiological, nutritional factors and environmental conditions. It is necessary to know the maximum value addition in the dairy food chain as the nutrient, to not only determine the dietary value of milk for human consumption, but also help to define market strategy for various classes of consumer like; growing children, nursing mothers, young people involved in hard jobs or elderly people. The use of cow, camel, goat, buffalo milk gained worldwide acceptance and importance throughout the world, while consumption of mare milk popular only in Western Europe. Goat milk prescribed by many doctors for children, who are sensitive to cow milk, and is an alternative for people who are allergic to cow milk. Goat milk is very useful for people suffering from problems such as acidity, eczema, asthma, migraine, colitis, stomach ulcer, digestive disorder, liver and gallbladder diseases and stress-related symptoms such as insomnia, constipation and neurotic indigestion. For some people with digestive difficulties, goat’s milk can be easily digested. Camel milk is an important source of proteins for the people living in the arid lands of the world camel milk is considered to have anti-cancer, hypo-allergenic and anti- diabetic properties. Buffalo milk is a natural product that can be consumed like any other milk. It is one of the richest products from a compositional point of view and characterized by higher fat, total solids, proteins, caseins, and lactose and ash contents than cow, goat, camel and human milk. The major constituents of buffalo milk are higher in concentration than that of human, cow, goat and camel milk with respect to nutritional values. In addition to its advantage as a rich source of nutrients, a recent study indicated that subjects with cow milk allergy are able to tolerate buffalo milk. Therefore, this review is aimed to explore composition of milk of farm animals and to make awareness on availability of different sources of milks.
Keywords: Milk Composition, Farm Animal, Nutrition of Milk, Enzymes in Milk
Suggested Citation: Suggested Citation
Haramaya University ( email )
OR Ethiopia +251910723442 (Phone)
Mohammedsham Husen (Contact Author)
Tulo offices of livestock and fisheries development ( email ).
Haramaya Haramaya University Haramaya, Oromia 138,Direda Ethiopia (251)-255-530-313 (Phone) +(251)-255-530-325 (Fax)
HOME PAGE: http://www.haramaya.edu.et
Haramaya university,college of veterinary medicine. ( email )
Do you have a job opening that you would like to promote on ssrn, paper statistics, related ejournals, food politics & sociology ejournal.
Subscribe to this fee journal for more curated articles on this topic
Agriculture Products eJournal
Animal science ejournal, agricultural food science & food groups ejournal, food chemistry ejournal.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 26 June 2024
Exploring the impact of maternal factors and dietary habits on human milk oligosaccharide composition in early breastfeeding among Mexican women
- Víctor H. Urrutia-Baca 1 ,
- Janet A. Gutiérrez-Uribe 1 ,
- Perla A. Ramos-Parra 2 ,
- Astrid Domínguez-Uscanga 1 ,
- Nora A. Rodriguez-Gutierrez 3 ,
- Karla L. Chavez-Caraza 3 ,
- Ilen Martinez-Cano 4 ,
- Alicia S. Padilla-Garza 3 ,
- Elias G. Ruiz-Villarreal 4 ,
- Francisca Espiricueta-Candelaria 1 &
- Cristina Chuck-Hernández 1
Scientific Reports volume 14 , Article number: 14685 ( 2024 ) Cite this article
564 Accesses
Metrics details
- Health care
Human milk oligosaccharides (HMOs) promote adequate intestinal microbiota development and favor the immune system's maturation and cognitive development. In addition to non-modifiable factors, HMOs composition can be influenced by other factors like body mass index and eating habits, but the reports are discrepant. The aim of this work was to describe the correlation between maternal factors and HMOs concentration in colostrum in 70 women from northeastern Mexico categorized into women with normal weight and women with overweight or obesity. The absolute concentration of six HMOs were significantly lower in women with overweight or obesity compared to women with normal weight (LNFPI p = 0.0021, 2’-FL p = 0.0304, LNT p = 0.0492, LNnT p = 0.00026, 3’-SL p = 0.0476, 6’-SL p = 0.00041). Another main finding was that the frequency of consumption of food groups such as vegetables, fruits and meats was positively correlated to specific HMOs (Poblano chili and 2’-FL; r s = 0.702, p = 0.0012; Orange or tangerine and 3-FL; r s = 0.428, p = 0.0022; Chicken and 2'-FL; r s = 0.615, p = 0.0039). This study contributes to the elucidation of how maternal factors influence the composition of HMOs and opens possibilities for future research aimed at mitigating overweight or obesity, consequently improving the quality of human milk.
Similar content being viewed by others

Associations between human milk oligosaccharides and infant growth in a Bangladeshi mother–infant cohort
Human milk oligosaccharides, infant growth, and adiposity over the first 4 months of lactation

Association of human milk oligosaccharides and nutritional status of young infants among Bangladeshi mother–infant dyads
Introduction.
Health authorities around the world have recognized the nutritional, physiological, and protective benefits of breastfeeding, such as the tendency to reduce the risk of type 2 diabetes, obesity, allergies, celiac disease, necrotizing enterocolitis (NEC), gastrointestinal tract infections, and some cancers. HMOs are multifunctional glycans naturally present in human milk and are particularly interesting for their quantity and structural diversity 1 . So far, more than 200 individual molecular species of HMOs and more than 100 structures have been reported 2 . HMOs contain glucose (Glc), galactose (Gal), N -acetylglucosamine (GlcNac), fucose (Fuc), and N -acetylneuraminic acid (Neu5Ac) and are formed by a reducing lactose core that can be extended enzymatically by lacto- N -biose (Galβ-1,3-GlcNAc, type 1 LacNAc) or N-acetyllactosamine (Gal-β1,4-GlcNAc, type 2 LacNAc) motifs. These structures can be further decorated by the addition of Fuc residues in α1,2-, α1,3-, and α1,4-linkages or Neu5Ac residues in α2,3- and α2,6-linkages.
Since oligosaccharide chains can be either fucosylated or sialylated, they are usually classified into three main categories: neutral non-fucosylated, neutral fucosylated, and HMOs containing sialic acid. HMOs promote the colonization of beneficial microbes in the infant's gut, such as beneficial Bifidobacterium species, preventing the growth of other harmful bacteria and contributing to the development of the immune system 3 . HMOs have been shown to have anti-bacterial, anti-viral, and anti-inflammatory effects and have proven beneficial toward the protection from NEC 4 , 5 . The benefits of HMOs may extend beyond infancy to the development of cognitive functions, making them the focus of intense current scientific research.
In addition to genetic factors, environmental factors such as geographical location, season of delivery, and maternal diet and maternal characteristics such as age, ethnicity, parity, mode of delivery, and maternal nutritional status affects HMOs concentration and composition 6 , 7 , 8 , 9 . Body mass index (BMI) at the beginning of pregnancy has been related to differences in the concentration of HMOs. Some studies have reported that overweight and obesity may positively or negatively correlate with the concentration of specific oligosaccharides 7 , 8 , 10 , 11 , 12 . McGuire et al. 7 , in 410 breastfeeding women from an international cohort (Spanish, Swedish, Peruvian, North American, Ethiopian, Gambian, Ghanaian, and Kenyan) found that maternal weight and BMI were positively associated with 2′-FL and fucosyl-lacto- N -hexaose (FLNH); maternal weight was positively correlated with lacto- N -fucopentaose III (LNFP III) and difucosyl-lacto- N -tetraose (DFLNT). Conversely, maternal weight and BMI were inversely correlated with LNnT and disialyl-lacto-N-tetraose (DSLNT). Azad et al. 8 , observed that lacto- N -hexaose (LNH) was lower in women with overweight compared to women with normal weight while DFLNT was higher in women with obesity. Isganaitis et al. 12 , found that three HMOs along with other seven metabolites concentrations were significantly different in the milk of one month infant mothers from Oklahoma (USA) with overweight or obesity compared to normal weight at one month after delivery; particularly Lacto- N -fucopentaose-I (LNFP-I) and 2’-FL contents were reduced by 62% and 38%, respectively, while Lacto-N-fucopentaose-II (LNFP-II) or Lacto- N -fucopentaose-III (LNFP-III) increased by 65% 12 .
In another study in a European cohort, women with overweight had significantly higher concentrations of 3’-SL, 6’-galactosyl-lactose (6’-GL) and DSLNT at day 2; 6’-SL at day 17; and lacto-N-fucopentaose-V (LNFP-V) at day 90 and 120 than women with normal weight. In addition, lower concentrations of LNnT (at day 2 postpartum), lacto-N-tetraose (LNT; at day 30 and 90 postpartum), and LNFP-V (at day 60 postpartum) were observed in women with overweight or obesity compared to women with normal weight 10 . Ferreira et al. 11 , in 101 Brazilian mothers reported a direct correlation between LNnT and prepregnancy weight and BMI and an inverse correlation with 3-fucosyllactose (3-FL), LNFP-III and DFLNH at 2–8 days. However, a study reported that prepregnancy BMI was not associated with the concentration of HMOs in Hispanic mothers living in Los Angeles, CA, USA 13 .
To understand the influence of obesity on the breast milk composition, Saben et al. 14 , were the first to evaluate the relationship between obesity-associated maternal factors (as hyperglycemia, hyperinsulinemia, and insulin resistance) and HMOs in 136 US mothers. They observed a negative association between third trimester fasting plasma glucose and insulin with total HMO-bound sialic acid and concentrations of the sialylated HMOs 3’-SL and DSLNT in non-secretors (women who are unable to produce 2’-FL and LNFPI in breast milk) at two months postpartum. In secretors, difucosyllactose and LNFP-II concentrations increased, and sialyllacto- N -tetraose c (LSTc) and sialyllacto- N -tetraose b (LSTb) decreased as insulin sensitivity increased 14 .
A recent study discovered significant associations with changes in maternal food intake during lactation. Higher cheese, egg, fruits and vegetables consumption has been positively correlated with HMO concentrations in secretory mothers (for example, 2'-FL and LNT + LNnT), while fish consumption has been negatively associated with LNFP-V 15 . Previous studies have also demonstrated the association of food consumption with HMOs composition with contradictory results between them 8 , 15 , 16 , 17 .
Undoubtedly, the discrepancy between the different reports invites new research on the influence of modifiable factors on HMOs. The aim of the present study was to describe the correlation between maternal factors and HMOs concentrations at the beginning of breastfeeding.
Participant demographics, medical history, and anthropometrics: mother–child pairs
In the present study, 70 mothers and their newborn children were recruited. Of the participating mothers, 94.3% were secretors (HMOs production status). They had an average age of 23.0 ± 5.2 years at delivery (Table 1 ). Regarding their demographics, 80% resided in the Monterrey N.L. Mexico metropolitan area, 60% had middle school as their highest level of completed education, and 90% of the mothers were employed in various unspecified occupations. Medically, 10% had a history of high blood pressure and 5.7% of cholesterolemia or dyslipidemia; none had a history of kidney disease. Cholesterolemia or dyslipidemia was found to be associated with overweight or obesity in mothers (p = 0.05); the four women who had this medical history were in the overweight or obesity group, as detailed in Table 1 .
Regarding the data associated with pregnancy and childbirth, 67.1% of births were spontaneous at the beginning, and 77.1% of the deliveries were eutocic. According to their conclusion, each delivery resulted in the birth of a single child. The average number of previous births compared to the current was 1.3 ± 1.4 children. Based on the anthropometric data in all participants, the prepregnancy BMI was 24.5 ± 5.9 kg m -2 , and the BMI at delivery was 28.3 ± 5.7 kg m -2 with a total weight gain of 9.8 ± 7.1 kg. The prepregnancy BMI and BMI at delivery were significantly higher in women with overweight or obesity compared to the women with normal weight, as expected (Table 1 ). Furthermore, the diastolic and systolic blood pressures in all participants were 69.4 ± 11.1 mm Hg and 113.0 ± 11.2 mm Hg, respectively. Systolic blood pressure was significantly higher (p = 0.01) in women with overweight or obesity (116.4 ± 9.4 mm Hg) compared to women with normal weight (109.7 ± 11.8 mm Hg). However, 4/36 (11.1%) and 3/34 (8.8%) showed a diastolic blood pressure above 80 mm Hg in women with normal weight and women with overweight or obesity, respectively. On the other hand, 6/36 (16.6%) and 9/34 (26.5%) showed a systolic blood pressure above 120 mm Hg in women with normal weight and women with overweight or obesity, respectively. Regarding to another maternal factors such as age at delivery, residence, maximum level of studies, occupation, high blood pressure medical history, mode of delivery, parity, diastolic blood pressure and weight gain, no differences were found between the groups (women with overweight or obesity vs. women with normal weight; p > 0.05), as shown in Table 1 .
Data were obtained from all participating mothers' newborns, 60% were female, and 95.7% did not show congenital anomalies. The weight and height at birth were 3.2 ± 0.4 kg and 49.2 ± 2.1 cm, respectively. Of newborns, 68.6% were normal weight, and 81.4% were normal height. The weight of the newborns was significantly higher in women with overweight or obesity compared to women with normal weight (p = 0.024). In addition, the average birth head circumference was 33.6 ± 1.3 cm. Regarding neonatal screening, APGAR and Silverman-Andersen scores were normal in most newborns, and hearing screening was positive (a positive result indicates correct hearing at birth) in 45.7% (Table 1 ). Regarding birth height, birth head circumference, sex, congenital anomalies, APGAR and Silverman-Andersen scores, and hearing screening, no differences were found between the groups (p > 0.05).
HMOs profiling and individual concentrations between the study groups
The total HMOs content was significantly lower in the group of women with overweight or obesity with 11.7 ± 3.4 g L −1 compared to women with normal weight with 15.9 ± 4.7 g L −1 (p < 0.001). These concentrations were based on the sum of 2'-FL, 3-FL, LNT, LNnT, LNFPI, 3'-SL, and 6'-SL, as the most representative HMOs (about 90%) in breast milk (Fig. 1 and Supplementary Table 2 ). In all participants, about 63% of the total HMOs were fucosylated neutrals (Fig. 1 a), followed by 21.9% non-fucosylated neutrals (Fig. 1 b) and 15.1% acidic (Fig. 1 c).

Comparison of HMO concentrations in colostrum samples from Mexican mothers with normal weight and overweight/obesity. ( a ) Neutral fucosylated, ( b ) neutral no fucosylated and ( c ) acidic. The data is displayed in a grouped boxplot with connect mean line and the p values were obtained using the non-parametric Mann–Whitney U test. Significance levels are denoted as * for p < 0.05, ** for p < 0.01 and *** for p < 0.001; n.s. not significant. NW women with normal weight, OW/OB women with overweight/obesity, 2’-FL 2’-fucosyllactose, 3’-SL 3’-sialyllactose, 6’-SL 6’-sialyllactose, 3-FL 3-fucosyllactose, LNFPI lacto- N -fucopentaose I, LNnT lacto- N -neotetraose, LNT lacto- N -tetraose.
There was a significant decrease in the concentration of six of the seven HMOs in women with overweight or obesity compared to women with normal weight. For the neutral fucosylated HMOs, the concentrations of 2’-FL were 5.2 ± 1.8 g L −1 , and LNFPI were 2.7 ± 1.9 g L −1 in women with normal weight compared to women with overweight or obesity with 4.4 ± 1.4 g L −1 and 1.7 ± 0.9 g L −1 , respectively (Fig. 1 a). In addition, the acidic HMOs 3’-SL and 6’-SL showed concentrations of 1.9 ± 1.3 g L −1 and 1.1 ± 0.5 g L −1 in women with normal weight compared to women with overweight or obesity with 1.2 ± 1.5 g L −1 and 0.5 ± 0.3 g L −1 , respectively (Fig. 1 c). In the case of non-fucosylated neutral HMOs, the concentrations of LNT and LNnT were 2.7 ± 1.8 g L −1 and 0.8 ± 0.5 g L −1 in women with normal weight compared to women with overweight or obesity with 1.8 ± 1.5 g L −1 and 0.5 ± 0.3 g L −1 , respectively (Fig. 1 b).
Correlations between HMOs concentrations and maternal factors
Three main horizontal clusters were observed among maternal factors in the hierarchical analysis (Fig. 2 ). The first was composed of age, parity, prepregnancy weight and BMI, and weight and BMI at delivery, while the second was composed of prepregnancy height, height at delivery and weight gain, and the third group composed by diastolic and systolic blood pressure. Only the factors grouped in the first cluster showed correlations with HMOs. HMOs were arranged in two main vertical clusters; the first formed by LNT, 3-FL and 2'-FL while the second by LNFPI, LNnT, 6'-SL, total HMOs and 3'-SL. Negative correlations associated with age, previous births, prepregnancy weight, prepregnancy BMI, weight at delivery, and BMI at delivery were observed with HMOs concentrations at the beginning of lactation.

Heatmap and dendrogram of Spearman’s correlation coefficients between HMOs concentrations and factors including age, anthropometrics, and previous births. Red squares indicate negative correlations, while blue squares indicate positive correlations. Significance levels are denoted as * for p < 0.05, ** for p < 0.01 and *** for p < 0.001. PP prepregnancy, BP blood pressure.
Age showed a weak negative correlation with LNnT ( r s = −0.254; p < 0.05) and 3’-SL ( r s = −0.242; p < 0.05). The number of previous births showed a weak negative correlation with LNnT ( r s = −0.295; p < 0.05), 3’-SL ( r s = −0.397; p < 0.001), and 6’-SL ( r s = −0.268; p < 0.05), but a weak positive correlation with LNT ( r s = 0.266; p < 0.05), as shown in Fig. 2 .
Pre-pregnancy weight and BMI were weak negatively correlated with total HMOs ( r s = −0.349 and ρ = −0.347; p < 0.01 both), 6’-SL (pre-pregnancy weight: r s = −0.391; p < 0.001) and moderate negatively correlated 6’-SL (pre-pregnancy: r s = −0.407; p < 0.001) and LNnT ( r s = −0.407 and r s = −0.426; p < 0.001 both). In addition, weight and BMI at delivery were moderate negatively correlated with total HMOs ( r s = −0.444 and r s = −0.451; p < 0.001 both), 6'-SL (ρ = −0.462 and ρ = −0.487; p < 0.001 both) and LNnT (ρ = −0.458 and ρ = −0.491; p < 0.001 both), and weak negatively correlated with 3’-SL ( r s = −0.298 and r s = −0.302; p < 0.05 both) and LNFPI ( r s = −0.284 and r s = −0.312; p < 0.05 and p < 0.01), as shown in Fig. 2 .
Correlations between HMOs and frequency of food group consumption
The foods with the highest total weekly consumption frequencies of each food group were whole milk (7.4 ± 6.1), orange or tangerine (7.4 ± 10.0), onion (10.3 ± 9.2), egg-warm or boiled (7.0 + 8.1), fresh fish (2.3 ± 3.0), beans prepared at home-cooked (5.8 ± 6.0), breakfast cereal: fruit flavor—froot loops (8.5 ± 7.8), corn atole—with milk (3.3 ± 5.7), water (24.0 ± 12.4), pasta soup—broth (3.4 ± 4.2), Tortilla (purchased) or factory-made tortilla (29.0 ± 23.3), and salt or seasoning with salt added to the foods (14.1 ± 8.5). In addition, some differences in the total weekly consumption frequencies of specific foods between the study groups were observed, as shown in Supplementary Table 3 .
In the dairy foods group, a strong and very strong positive correlation was only observed between the natural drinkable yogurt consumption and two HMOs: LNnT ( r s = 0.756) and 6’-SL ( r s = 0.850). On the other hand, strong negative correlations of the natural whole yogurt ( r s = −0.624) and whole yogurt with fruits ( r s = −0.647) with LNT concentrations were observed. Panela or fresh or cottage cheese consumption was moderate negatively associated with the concentration of acidic HMOs 3-SL ( r s = −0.421) and 6’-SL ( r s = −0.404). In addition, semi or ripened cheese consumption was weak negatively correlated to LNnT ( r s = −0.323) and total HMOs ( r s = −0.317) concentrations, and moderate negatively correlated to 6’-SL ( r s = −0.443), as shown in Table 2 .
In the fruit group, we found that the consumption of seven different types of fruits was associated with changes in the concentration of HMOs. Guava consumption showed a moderate negative correlation with the level of LNT ( r s = −0.551), whereas from weak to strong positive correlations were: (1) orange or tangerine with LNFPI and 3-FL; (2) apple or pear with LNnT, 3’-SL, 6’-SL, and total HMOs; (3) mango with LNnT, 3’-SL and 6’-SL; (4) strawberries with LNFPI, LNnT, 6’-SL and total HMOs; (5) grapes with 3-FL; and (6) crystallized or dried fruits with 3-FL (Table 2 ).
Results like those previously described were observed in the vegetable group, seven different vegetables showed positive correlations with the concentrations of specific HMOs. The consumption of frozen vegetables moderate negatively correlated with the LNFPI concentrations ( r s = −0.498). From weak to strong positive correlations are described as follows: (1) green leaves with LNnT; (2) carrot with 3-FL; (3) lettuce with up to four HMOs (LNFPI, LNnT, 3’-SL, and 6’-SL) and total HMOs; (4) nopales (cactus) with up to four HMOs (LNFPI, LNnT, 3’-SL, and 6’-SL); (5) cucumber with 3-FL; (6) avocado with LNFPI and LNnT; and (7) poblano chili with LNnT, 6’-SL, 3-FL and total HMOs.
The consumption of beef, pork, turkey or mixed sausage, pork or turkey ham or mortadella and egg were weak positively associated with the concentrations of total HMOs ( r s = 0.269), 3-FL ( r s = 0.256), and LNnT ( r s = 0.311), respectively. Chicken was moderate postively correlated with 2’-FL ( r s = 0.615). Pork meat consumption was weak positively correlated to LNFPI ( r s = 0.391) and total HMOs ( r s = 0.382), and moderate positively correlated to 2’-FL ( r s = 0.432).
In the fish group, tuna or sardine showed a weak positive correlation with the concentrations of LNnT ( r s = 0.374), whereas that some seafood showed a moderate positive correlation with LNT ( r s = 0.453).
Beans prepared at home, cooked, and refried showed a weak ( r s = 0.366) and moderate ( r s = 0.597) positive correlations with 3-FL concentrations, respectively).
In the cereals and tubers groups, whole grain bread and sweet bread both showed a moderate ( r s = 0.515) and weak ( r s = 0.342) positive correlation with 3-FL, respectively; whereas that potatoes ( r s = −0.389; half fried piece or half a potato pancake) showed a weak negative correlation and breakfast cereal ( r s = −0.912; fruit flavor; froot loops) showed a very strong negative correlation with 3-FL.
In the corn food, appetizers with vegetables (such as sopes, quesadillas, tlacoyos, gorditas, and enchiladas; not tacos) fried and without frying were moderate positively correlated with 3-FL ( r s = 0.509) and strong negatively correlated with 6’-SL ( r s = −0.660), respectively.
In the beverages group, the consumption of natural fruit waters with sugar was strong positively correlated with 3-FL ( r s = 0.708) and total HMOs ( r s = 0.664), unsweetened industrialized flavored beverages with 6’-SL ( r s = 0.673), and fruit nectars or fruit pulp industrialized with sugar were moderate positively associated with LNnT ( r s = 0.405) and 3’-SL ( r s = 0.443), while weak positively correlated to 6’-SL ( r s = 0.328) concentrations.
Soups with pasta (pasta soup) and tortilla (corn flour tortilla) groups were weak and strong positively correlated to 3’-SL ( r s = 0.369) and LNnT ( r s = 0.712) concentrations, respectively.
In the miscellaneous group, dried chili was strong and very strong positively associated with LNFPI ( r s = 0.7127) and 3-FL ( r s = 0.81742) concentrations, respectively; soy sauce, Worcestershire sauce, or liquid food seasonings were very strong positively associated with 3’-SL ( r s = 0.84515). The correlations between all food groups and HMOs are shown in Supplementary Table 4 .
In addition, a statistical difference (p = 0.008) was observed in total daily calories between women with normal weight (4133.1 ± 1142.4 kCal) and women with overweight or obesity (3391.1 ± 1038.8 kCal). However, total daily calories and concentrations of each of the HMOs were not correlated.
The focus of the study was to describe the correlation between maternal factors and HMO concentration in colostrum samples. The absolute concentrations of LNFPI, 2’-FL, LNT, LNnT, 3’-SL, and 6’-SL were significantly lower in women with overweight or obesity compared to women with normal weight. In addition, from weak to moderate correlations between maternal age, previous births, prepregnancy weight, prepregnancy BMI, weight at delivery, and BMI at delivery, and HMOs concentrations at the beginning of lactation were observed. The frequency of consumption of food groups such as vegetables (poblano chili and 2’-FL; rs = 0.702, p = 0.0012), fruits (orange or tangerine and 3-FL; rs = 0.428, p = 0.0022) and meats (chicken and 2'-FL; rs = 0.615, p = 0.0039) was positively correlated to specific HMOs from moderate to strong correlations.
During pregnancy, many studies suggest that a medical history of diseases associated with lipid metabolism is linked to increased BMI 18 . Hernández-Higareda et al. 19 , conducted a study on 600 Mexican women from Guadalajara, Jalisco to identify pregnancy-related diseases associated with obesity. Like the present study, they observed a significant association between a medical history of cholesterolemia or dyslipidemia and overweight or obesity in the participating women. Although the size of the samplein the present study is limited, the average systolic blood pressure was also significantly higher in women with overweight or obesity. Studies in other countries and continents have previously reported this association 20 , 21 , 22 .
Being overweight or obese during pregnancy not only increases the risk of health complications in the woman but also affects the perinatal health of the newborn. Taoudi et al. 23 , studied 90 participants from Témara, Morocco, aged between 18 and 43 years and found a significant positive correlation between gestational BMI and newborn birth weight ( r s = 0.29; p < 0.001). A recent study in 1,112 Mexican women, reported that overweight marginally decreased the probability of having a low birth weight. In the present study, we observed that children born to women with overweight or obesity have a significant increase in birth weight compared to children born to women with normal weight 24 , 25 .
Approximately 15–20% of women worldwide do not express the FUT2 gene and are considered non-secretors related to a lower diversity and concentration of fucosylated HMOs such as 2’-FL and LNFPI 26 , 27 . In the present study, we observed a low prevalence (5.7%) of the non-secretor state of mothers. The proportion of non-secretors and secretor women vary between each report and geographic location. A proportion of 0%, 32%, 36%, and 37% of non-secretors has been reported in Bolivia, USA, Gambia, and South Africa, respectively 28 , 29 , 30 , 31 . However, this can vary significantly in different regions within the same country due to the diversity of ethnic origins 15 .
Isganaitis et al. 12 , analyzed the relationships between maternal obesity and human milk metabolites in 31 mothers from Oklahoma, USA and found that LNFPI was reduced by about 62% in the breast milk of women with overweight or obesity compared to women with normal weight at one month of breastfeeding (p = 0.007). In the present study we observed a similar percentage reduction in LNFPI (63%) in the colostrum of women with overweight or obesity compared to women with normal weight although in the previous study their HMOs quantifications were relative by untargeted metabolomics. However, our results differ from the previous study in that no significant reduction in 2'-FL was observed compared to the 38% they observed 12 . Another study in 78 Brazilian mothers, observed that secretor women with overweight (BMI 25–29.9 kg m −2 ) had a significantly higher concentration of 2'-FL (p = 0.030) and lower 3'-FL concentration (p = 0.011) than secretor women with normal weight (BMI 18.5–24.9 kg m −2 ) about one month postpartum 32 . The results of that study, using a similar LC–MS analytical platform, were consistent with the present study in which 3-FL was found to be significantly reduced in women with overweight or obesity (p = 0.011). A fact to highlight is that the HMO content is highest in colostrum and tends to decrease over the course of lactation. These two studies were conducted in women at one month of lactation where 2’-FL can be reduced by close to 68% and 3-FL may be increased by around 215%.
In a study conducted by McGuire et al. 7 , maternal weight and BMI were negatively correlated with LNnT ( r s = −0.16 and r s = −0.21, respectively) and positively correlated with 2′-fucosyllactose ( r s = 0.20 for both) in 410 healthy women from different countries at about three months postpartum. In addition, Samuel et al. 10 , found that women with overweight had significantly higher concentrations of 3′-SL (at day 2; colostrum), 6′-SL (at day 17) while lower concentrations of LNnT (at day 2; colostrum) and LNT (at day 30 and 90) compared to women with normal weight (p < 0.05 for all) in 370 women from seven European countries. These two reports were consistent with results found in the present study, where the concentrations of LNT and LNnT were lower in women with overweight or obesity compared to women with normal weight, although McGuire et al. 7 used LC coupled to a duo ion-trap mass spectrometer for quantitative analysis. However, the increase of 3’-SL) concentration observed in women with overweight at day 2 of lactation by Samuel et al. 10 disagrees with those found in the present study; LNnT was reduced in overweight women as was in the present study. Another study in 322 women from Brazil, prepregnancy BMI was moderate negatively correlated with 3-FL ( r s = − 0.5), consistent with our study but discrepant in that prepregancy BMI was moderate positively correlated with LNnT ( r s = 0.4) at 2–8 days of lactation 11 .
The impact of other maternal factors (maternal age, mode of delivery, parity, and blood pressure) on the composition of HMOs remains unclear. Austin et al. 33 , conducted a cross-sectional study of 446 Chinese women in different stages of lactation (from 0–4 days to 4–8 months) and observed that the mode of delivery had no impact on HMOs composition. However, the study conducted by Samuel et al. 10 , observed that women giving birth through cesarean section (dystocic) had significantly higher concentrations of LNT (colostrum; at day 2) and 6′-SL (at day 30), and significantly lower concentrations of 2′-FL, 3′-SL, 6′-GL, LNFP III, LNnDFH and LNFP II (colostrum; at day 2) compared to those giving birth by vaginal delivery (eutocic). The present study is consistent only with those reported with Austin et al. 33 in colostrum samples (< 48 h). Ferreira et al. 11 , reported that parity was moderate positively correlated with LNFP II ( r s = 0.4), DFLNT ( r s = 0.4), LNH ( r s = 0.4) and FDSLNH ( r s = 0.4) in colostrum samples at 2–8 days of lactation. Unlike the present study where parity showed a weak positive correlation with LNT and a weak negative correlation with 3'-SL, 6'-SL and LNnT.
McGuire et al. 7 , found that maternal age was weak negatively correlated with concentrations of LNnT, LSTc, and DSLNH ( r s = − 0.14, r s = − 0.17, and r s = − 0.15, respectively) and was weak positively correlated with the concentration of FLNH ( r s = 0.15) from two weeks to five months of lactation. In the present study, maternal age was also weak negatively associated with LNnT concentrations at < 48 h of lactation.
In another studies, Fan et al. 15 , studied 468 pregnant women from Illinois USA and found that maternal age was weak positively correlated with relative abundances of 3-FL ( r s = 0.12, p = 0.019), DFLNHb ( r s = 0.11, p = 0.034), and IFLNH III ( r s = 0.1, p = 0.046), and weak negatively correlated with abundances of p-LNH ( r s = − 0.13, p = 0.011) and IFLNH I ( r s = − 0.13, p = 0.012) at 6 week of lactation. A study in 3,407 Canadian mothers conducted by Azad et al. 8 , lower concentrations of DFLNT and higher concentrations of LNnT and LNT in older mothers, while lower concentrations of 3-FL were observed in multiparous mothers and the mode of delivery was not associated with HMOs concentrations at 3–4 month of lactation 8 . In a longitudinal study of 116 Chinese mothers conducted by Wang et al. 34 , maternal age was weak negatively correlated with LNT + LNnT ( r s = − 0.29), LNFPI ( r s = − 0.21), but was weak positively correlated with 2′-FL ( r s = 0.22) only in secretor women at 1–5 days of lactation. In addition, they found a weak negative correlation between parity and LNFPI ( r s = − 0.20) at 1–5 days of lactation. Tonon et al. 32 , observed a strong positive correlation between parity and LNT + LNnT ( r s = 0.79) and LNFPI ( r s = 0.79) levels and a strong negative correlation between parity and 3′-FL ( r s = − 0.79) concentrations in secretor mothers (Se+) from 17 to 76 days of lactation.
Our results were consistent with the obtained by McGuire et al. 7 , and Wang et al. 34 , who observed a negative correlation between maternal age and LNnT concentrations. Regarding parity and composition of HMOs, our results are aligned with those obtained by Tonon et al. 32 , where parity showed a positive correlation with LNT concentrations. In addition, like Azad et al. 8 and Samuel et al. 10 , no statistical differences were observed between the delivery mode and the concentrations of each of the HMOs evaluated in the present study.
Only a few studies investigated the impact of maternal diet on HMOs composition. Hallam et al. 35 , evaluated the effect of high-protein and high-prebiotic-fiber maternal diets on the composition of oligosaccharides in breast milk in maternal rats and observed an increase in both neutral and acidic oligosaccharides. Although the previous study is an animal model, our results show that the frequency of consumption of foods rich in fiber such as vegetables and legumes (beans) were positively correlated with the concentrations of HMOs.
In an interventional study using two different cohorts from Baylor, Texas tested the effect of glucose or galactose-enriched diet for 30–57 h (n = 7) or a high-fat or high-carbohydrate diets for 8 days (n = 7) on HMOs. HMO-bound fucose concentration was reduced with the glucose-enriched diet 16 . These results contrast with those found in the present study where we observed that the frequency of consumption of beverages rich in sugar increases the concentrations of fucosylated HMOs such as LNFPI and 3-FL.
Nutmeg is the richest in myristic acid (a food not typical in the Mexican diet), but we can find it in quantities 30–50 times lower in dairy products (such as yogurt), fish (such as tuna or sardines) and eggs 36 . This could explain the positive correlations observed in the present study between foods containing myristic acid and concentrations of LNT or LNnT. A source of stearic acid of plant origin is cereals (corn, rice, and others) which could be related to the findings of this study where negative correlations of 2'-FL and 6'-SL in consumers of breakfast cereal (fruit flavor) and appetizers with vegetables (such as sopes, quesadillas, tlacoyos, gorditas, and enchiladas; corn-based), respectively were observed.
Another study by Seppo et al. 37 , showed that supplementation with probiotics ( Lactobacillus rhamnosus GG, Lactobacillus rhamnosus LC705, Bifidobacterium breve Bb99, and Propionibacterium freudenreichii subspecies shermanii JS) during the last three weeks of pregnancy increased the concentrations of 3-FL and 3′-SL in colostrum from 1223 pregnant mothers from Helsinki, Finland. In the present study, foods containing probiotics such as natural yogurt showed a positive correlation with LNnT and 6'-SL concentrations. However, the probiotic bacteria contained in this dairy food were not specified in the present study.
Fan et al. 15 , reported that higher cheese consumption was correlated with a higher amount of 2′-FL (p = 0.046) in breast milk at six weeks postpartum. In that same study, increased egg consumption was associated with greater LNT + LNnT abundance (p = 0.012). These results were contrary to those found in the present study, where the consumption of panela, fresh or cottage cheese, and semi or ripened cheeses showed a negative correlation with 3’-SL, 6’-SL, and LNnT concentrations. However, egg consumption was positively correlated with LNnT concentrations, consistent with what was reported by Fan et al. 15 . Quin et al. 38 , found that ingested carbohydrates (simple sugars and dietary fiber) were positively correlated with the relative levels of Galactose and Fucose present in HMOs with the decrease of Neu5Ac and Neu5Gc. Furthermore, they observed similar correlations for the total amount of fruit ingested, an important dietary source of sugars and fiber (a known source of Fucose). They observed significant positive correlations for Galactose and Fucose, while Neu5Ac levels were significantly lower in HMOs biosynthesized by women consuming large amounts of fruit. They observed that only cheese intake was positively associated with Neu5Gc levels and HMOs concentrations. These results are consistent with those found in the present study, showed that the consumption of different types of fruit was positively associated with the concentrations of all HMOs studied except 2'-FL.
Quin et al. 38 demonstrated that Neu5Ac obtained from the diet from red meat and products derived from cow's milk (such as cheese) positively influences the concentrations of HMOs. Those above could explain the positive correlation observed in the present study between the consumption of red meat (pork meat and beef) and the concentrations of total HMOs.
Our study has some limitations, with the main restriction being the small sample size, which limits the power to detect differences between groups. Despite this, we found very revealing statistical differences consistent with those reported in the literature. In addition, the study was a single-center study, limiting the generalizability to other settings. However, regional studies where overweight and obesity are very prevalent among women and school-age children are necessary to improve our understanding of the influence of dietary habits, BMI, and other maternal factors on the composition of breast milk. The present study is the first to be carried out in Mexico, a country where about 30% of adults have obesity with a greater impact on women. The results of the present study show the impact of obesity on the composition of breast milk is significative, which would suggest consequences for the infant from birth.
In conclusion, the influence of overweight and obesity as a modifiable factor associated with lower concentrations of 2’-FL, LNFPI, 3’-SL, 6’-SL, LNT and LNnT at the beginning of lactation could affect the quality of breast milk and deprive the newborn of its associated health benefits. The frequency of consumption of certain food groups, such as fruits, vegetables, and meats, can increase the concentrations of specific HMOs. The present study opens the possibility of new research aimed to improve the quality of breast milk through nutritional intervention based on healthy eating and BMI control for overweight/obesity prevention.
Study design
The present was a cross-sectional, descriptive, prospective, and single-center study that was carried out at the Hospital Regional Materno Infantil de Alta Especialidad (HRMIAE) in Monterrey, Nuevo León, Mexico, over nine months from February 2023 to October 2023.
Ethical approval
This study was conducted according to the ethical principles for medical research involving human subjects outlined in the Helsinki Declaration. Institutional review board approval (approval number: DEISC-PR-190122074; Hospital Regional Materno Infantil de Alta Especialidad) was obtained, and the participants provided written informed consent before participating in the study.
Recruitment, eligibility criteria, and study groups
In the present study, 70 mothers from 18 to 49 years of age were recruited, and a signed written informed consent was previously obtained. Recruitment was in the rooming-in area (where recovering mothers and newborns stay together until their hospital discharge) at the HRMIAE, where the first contact was established with the participants. Then, the resident doctor or social service intern evaluated compliance with the following eligibility:
Inclusion criteria: (a) women with a full-term pregnancy (≥ 38 weeks); (b) Mexican nationality; (c) healthy; (d) 18 to 49 years of age; (e) that the weight and height record is in the pregnancy control record from the first trimester; (f) patient without a history of any diabetes.
Exclusion criteria: (a) history of antibiotic use in the three months before delivery; (b) prolonged exposure to antibiotics (> 3 weeks) during pregnancy; (c) immunosuppressive or immunomodulatory therapy with corticosteroids; (d) history of vegan, lacto-ovo-vegetarian or exclusion diet (for example ketogenic diet); (e) history of bariatric surgery; (f) exposure to antineoplastic drugs; (g) histamine-H2 receptor antagonists or proton pump inhibitors or monoclonal antibodies; (h) history of mental illness (those that make it impossible to take the sample); (i) history of alcohol, tobacco or drug use during pregnancy; j) neonates with fetal distress or contact with meconium; (k) neonates who were administered an antibiotic at birth; (l) patients who do not have reliable weight and height information during prenatal care; (m) patient with a history of metabolic diseases (gestational diabetes, diabetes mellitus type 1 and 2).
Elimination criteria: (a) antibiotics for more than 24 h after delivery; (b) intensive care (mother or baby) or any condition that prevents the collection of breast milk. (c) Patients who are unable for some reason to continue breastfeeding.
Mothers were categorized into two study groups (1: women with normal weight and 2: women with overweight or obesity) based on their pre-pregnancy BMI.The permanence in the study group until the conclusion of pregnancy was determined by total weight gain. This classification followed the 2009 IOM/NRC guidelines, which detail weight gain recommendations for women with singleton and twin fetuses in Table 2 39 . For example, women who had the diagnosis of normal weight (18.5–24.9 kg m −2 ), based on prepregnancy BMI and gained normal weight during pregnancy (11.3–15.9 kg) were classified as "women with normal weight", but if gained excessive weight during pregnancy was classified as "women with overweight or obesity." On the other hand, women who had the diagnosis of overweight (25.0–29.9 kg m −2 ) or obesity (≥ 30.0 kg m −2 ), based on prepregnancy BMI, but if gained normal weight during pregnancy (6.8–11.3 kg for overweight and 5–9 kg for obesity) remained classified as "women with overweight or obesity".
In addition, women were categorized according to their secretory status based on the ability to produce 2’-FL and LNFPI in breast milk.
Data and sample collection
Socio-demographic data (age, residence, maximum level of studies, occupation), medical history, and anthropometric data were collected from all participants, and a food consumption frequency questionnaire was applied, which is a validated tool for the Mexican population within the nutritional evaluation that allows measuring caloric consumption and the portion consumed according to the frequency of consumption of food groups; total weekly frequency of food group consumption was calculated by multiplying the daily by the weekly frequency. Food consumption frequency data were collected at the same time as the colostrum sample collection and reflect the frequency in the last week (7 days).
In addition, relevant data associated with the delivery and newborn data were collected, such as anthropometrics, basic metabolic screening, and hearing screening routinely performed on all newborns. All information was collected using a data collection instrument .
About sample collection, 1 mL of breast milk samples (colostrum; < 48 h postpartum) were collected from all participating women with the help of medical personnel from the HRMIAE Human Milk Bank. After collection, the samples were immediately stored frozen at −20 °C. Finally, the samples were transported to the Centro de Biotecnología FEMSA of the Tecnologico de Monterrey and stored under the same freezing conditions until processing.
Absolute quantification of human milk oligosaccharides
Sample preparation.
The samples were prepared following the methodology previously validated in our laboratory by Urrutia Baca et al. 40 . 50 µL of each colostrum sample was diluted with 150 µL water and vortex mixed. Next, 50 µL of the mixture was transferred to 1.5 mL new tubes and mixed with 450 µL of water, and the mixture was centrifuged at 12,000× g for 30 min at 4 °C. Then, 300 µL of the lower aqueous phase was transferred to 1.5 mL tubes and mixed with 900 µL of acetonitrile. The mixture was sonicated for 10 min, incubated for 60 min at 4 °C, centrifuged at 12,000× g for 30 min at 4 °C, and the supernatant was recovered. For removal of residual protein, 500 µL of the supernatant was ultracentrifuged using tubes with a 3 kDa molecular weight cut-off membrane (Vivaspin 500, 3 kDa MWCO; GE28-9322-18; Sartorius, Stonehouse, UK) for 50 min at 7500× g , 4 °C. In this step, a carbohydrate fraction was obtained and filtered through a 0.22 μm nylon before UPLC–MS/MS analysis.
UPLC–MS/MS analysis
Calibration curves were made to establish the concentration ranges for quantification. For 2’-FL, 3-FL, and LNFPI, there were seven concentration points from 1 µg to 100 µg mL −1 , and for 6’-SL, 3’-SL, LNT, and LNnT there were six concentration points from 1 to 50 µg mL −1 . The linearity equations and the determination coefficient were obtained for both calibration curves.
The experiments were performed using an Acquity UPLC system (Waters, Milford, MA) equipped with Micromass Quattro Premier XE Mass Spectrometer (Waters, Milford, MA). An ACQUITY UPLC BEH Amide Column (Waters; 1.7 μm, 2.1 × 100 mm) coupled to an ACQUITY UPLC BEH Amide pre-column (Waters; 1.7 μm, 2.1 × 5 mm) was used for chromatographic separation adjusted at 40 °C using a binary gradient. The mobile phase A and B consisted of 10 mM ammonium formate in water and 99.9% acetonitrile, respectively. The following UPLC gradient was used: 0 − 2 min from 98 to 95% B, 2–3 min to 65% B, 3–8.5 min to 55% A, 8.5–11 min to 55% A, 11–11.2 min to 98% A, 11.2–14 min to 98% B. The flow rate was set to 0.4 mL min −1 .
Mass spectrometric analyses were performed with a triple quadrupole spectrometer equipped with an electrospray ionization source (ESI). ESI conditions were as follows: Capillary 4 kV, Cone 50 V, Extractor 3 V, RF Lens 0 V, Source temperature 150 °C, desolvation temperature 300 °C, gas flow desolvation 720 L h −1 , gas flow cone 300 L h −1 . High-purity argon was used as collision gas. The mass spectrometer was operated in negative-ion, multiple reaction monitoring (MRM) mode. Our work team previously optimized MRM conditions (Supplementary Table 1 ) and carried out the validation of the method applied in the present study 40 .
Statistical analysis
The distribution of data was determined for each study variable by Kolmogorov–Smirnov test. Next, associations were established between the mother and the newborn variables based on the study groups. Student’s t-test and Chi-square with Fisher’s Exact Test were used for continuous and categorical variables, respectively. The concentration values of each HMOs, total, and profile showed no normal distribution; therefore, a non-parametric Mann–Whitney U test was used to explore the differences between the study groups. The correlation between HMOs concentrations and factors including age, anthropometrics, previous births, and frequency of food group consumption were calculated by Spearman's correlation test; Spearman's correlation coefficient > 0 indicates a positive correlation and < 0 indicates a negative correlation. The maternal variables were organized into clusters on the X axis and the concentrations of each specific HMOs were organized on the Y axis by hierarchical cluster analysis based on ρ values. Finally, the total frequency of consumption of each food group was calculated and then correlated with the concentrations of each HMOs by Spearman's correlation test. For all statistical tests, a p-value less than 0.05 was considered significant. Finally, the box charts, heatmaps, and dendrograms were generated using Origin v2023.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Wang, J. et al. Current advances in structure–function relationships and dose-dependent effects of human milk oligosaccharides. J. Agric. Food Chem. 70 , 6328–6353. https://doi.org/10.1021/acs.jafc.2c01365 (2022).
Article CAS PubMed Google Scholar
Kijner, S., Kolodny, O. & Yassour, M. Human milk oligosaccharides and the infant gut microbiome from an eco-evolutionary perspective. Curr. Opin. Microbiol. https://doi.org/10.1016/j.mib.2022.102156 (2022).
Article PubMed Google Scholar
Okburan, G. & Kızıler, S. Human milk oligosaccharides as prebiotics. Pediatr. Neonatol. 64 , 231–238. https://doi.org/10.1016/j.pedneo.2022.09.017 (2023).
Donovan, S. M. & Comstock, S. S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 69 , 41–51. https://doi.org/10.1159/000452818 (2016).
Article Google Scholar
Sodhi, C. P. et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 89 , 91–101. https://doi.org/10.1038/s41390-020-0852-3 (2020).
Biddulph, C. et al. Human milk oligosaccharide profiles and associations with maternal nutritional factors: A scoping review. Nutrients https://doi.org/10.3390/nu13030965 (2021).
Article PubMed PubMed Central Google Scholar
McGuire, M. K. et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 105 , 1086–1100. https://doi.org/10.3945/ajcn.116.139980 (2017).
Article CAS PubMed PubMed Central Google Scholar
Azad, M. B. et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J. Nutr. 148 , 1733–1742. https://doi.org/10.1093/jn/nxy175 (2018).
Rousseaux, A. et al. Human milk oligosaccharides: Their effects on the host and their potential as therapeutic agents. Front. Immunol. https://doi.org/10.3389/fimmu.2021.680911 (2021).
Samuel, T. M. et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. https://doi.org/10.1038/s41598-019-48337-4 (2019).
Ferreira, A. L. et al. Human milk oligosaccharide profile variation throughout postpartum in healthy women in a Brazilian cohort. Nutrients https://doi.org/10.3390/nu12030790 (2020).
Isganaitis, E. et al. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 110 , 111–120. https://doi.org/10.1093/ajcn/nqy334 (2019).
Berger, P. K. et al. Human milk oligosaccharides and hispanic infant weight gain in the first 6 months. Obesity 28 , 1519–1525. https://doi.org/10.1002/oby.22884 (2020).
Saben, J. L., Abraham, A., Bode, L., Sims, C. R. & Andres, A. Third-trimester glucose homeostasis in healthy women is differentially associated with human milk oligosaccharide composition at 2 months postpartum by secretor phenotype. Nutrients https://doi.org/10.3390/nu12082209 (2020).
Fan, Y., Vinjamuri, A., Tu, D., Lebrilla, C. B. & Donovan, S. M. Determinants of human milk oligosaccharides profiles of participants in the STRONG kids 2 cohort. Front. Nutr. https://doi.org/10.3389/fnut.2023.1105668 (2023).
Seferovic, M. D. et al. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. https://doi.org/10.1038/s41598-020-79022-6 (2020).
Selma-Royo, M. et al. Maternal diet is associated with human milk oligosaccharide profile. Mol. Nutr. Food Res. https://doi.org/10.1002/mnfr.202200058 (2022).
Alston, M. C., Redman, L. M. & Sones, J. L. An overview of obesity, cholesterol, and systemic inflammation in preeclampsia. Nutrients https://doi.org/10.3390/nu14102087 (2022).
Hernández-Higareda, S. et al. Maternal metabolic diseases related to pre-pregnancy overweight and obesity in Mexican women with high risk pregnancy. Cirugía Cirujanos (English Edition) 85 , 292–298. https://doi.org/10.1016/j.circen.2017.08.003 (2017).
Anwar, R., Razzaq, K. & Parveen, N. Maternal obesity and obstetric outcome in pregnant women attending Pns Shifa Karachi. Pak. Armed Forces Med. J. 71 , S245-249. https://doi.org/10.51253/pafmj.v71iSuppl-1.2837 (2021).
Abraham, T. & Romani, A. M. P. The relationship between obesity and pre-eclampsia: Incidental risks and identification of potential biomarkers for pre-eclampsia. Cells https://doi.org/10.3390/cells11091548 (2022).
Lewandowska, M., Więckowska, B. & Sajdak, S. Pre-pregnancy obesity, excessive gestational weight gain, and the risk of pregnancy-induced hypertension and gestational diabetes mellitus. J. Clin. Med. https://doi.org/10.3390/jcm9061980 (2020).
Taoudi, F. et al. Study of the prevalence of obesity and its association with maternal and neonatal characteristics and morbidity profile in a population of Moroccan pregnant women. J. Nutr. Metab. 1–10 , 2021. https://doi.org/10.1155/2021/6188847 (2021).
Lewandowska, M. Maternal obesity and risk of low birth weight, fetal growth restriction, and macrosomia: Multiple analyses. Nutrients https://doi.org/10.3390/nu13041213 (2021).
Ikedionwu, C. A. et al. Pre-pregnancy maternal obesity, macrosomia, and risk of stillbirth: A population-based study. Eur. J. Obstet. Gynecol. Reprod. Biol. 252 , 1–6. https://doi.org/10.1016/j.ejogrb.2020.06.004 (2020).
Cheema, A. S. et al. Human milk oligosaccharides and bacterial profile modulate infant body composition during exclusive breastfeeding. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23052865 (2022).
Pérez-Escalante, E. et al. Human milk oligosaccharides as bioactive compounds in infant formula: Recent advances and trends in synthetic methods. Crit. Rev. Food Sci. Nutr. 62 , 181–214. https://doi.org/10.1080/10408398.2020.1813683 (2020).
Docq, S., Spoelder, M., Wang, W. & Homberg, J. R. The protective and long-lasting effects of human milk oligosaccharides on cognition in mammals. Nutrients https://doi.org/10.3390/nu12113572 (2020).
Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 22 , 1147–1162. https://doi.org/10.1093/glycob/cws074 (2012).
Vinjamuri, A. et al. Human milk oligosaccharide compositions illustrate global variations in early nutrition. J. Nutr. 152 , 1239–1253. https://doi.org/10.1093/jn/nxac027 (2022).
Fiese, B. H. et al. The STRONG Kids 2 Birth Cohort Study: A cell-to-society approach to dietary habits and weight trajectories across the first 5 years of life. Curr. Dev. Nutr. https://doi.org/10.1093/cdn/nzz007 (2019).
Tonon, M., de Morais, K. B., Abrão, M. F. V., Miranda, A. C. & Morais, A. B. Maternal and infant factors associated with human milk oligosaccharides concentrations according to secretor and Lewis phenotypes. Nutrients. https://doi.org/10.3390/nu11061358 (2019).
Austin, S. et al. Temporal change of the content of 10 oligosaccharides in the milk of Chinese Urban mothers. Nutrients https://doi.org/10.3390/nu8060346 (2016).
Wang, M. et al. Neutral human milk oligosaccharides are associated with multiple fixed and modifiable maternal and infant characteristics. Nutrients https://doi.org/10.3390/nu12030826 (2020).
Hallam, M. C., Barile, D., Meyrand, M., German, J. B. & Reimer, R. A. Maternal high-protein or high-prebiotic-fiber diets affect maternal milk composition and gut microbiota in rat dams and their offspring. Obesity 22 , 2344–2351. https://doi.org/10.1002/oby.20849 (2014).
Beare-Rogers, J. L., Dieffenbacher, A. & Holm, J. V. Lexicon of lipid nutrition (IUPAC technical report). Pure Appl. Chem. 73 , 685–744. https://doi.org/10.1351/pac200173040685 (2001).
Article CAS Google Scholar
Seppo, A. E. et al. Association of maternal probiotic supplementation with human milk oligosaccharide composition. JAMA Pediatrics https://doi.org/10.1001/jamapediatrics.2018.4835 (2019).
Quin, C. et al. Influence of sulfonated and diet-derived human milk oligosaccharides on the infant microbiome and immune markers. J. Biol. Chem. 295 , 4035–4048. https://doi.org/10.1074/jbc.RA119.011351 (2020).
Rasmussen, K. M., Catalano, P. M. & Yaktine, A. L. New guidelines for weight gain during pregnancy: What obstetrician/gynecologists should know. Curr. Opin. Obstet. Gynecol. 21 , 521–526. https://doi.org/10.1097/GCO.0b013e328332d24e (2009).
Urrutia-Baca, V. H., Chuck-Hernández, C., Gutiérrez-Uribe, J., Ramos-Parra, P. A. & Licona-Cassani, C. Development and validation of a versatile analytical method for absolute quantification of seven oligosaccharides in human, bovine, and goat milk. Heliyon https://doi.org/10.1016/j.heliyon.2023.e22475 (2023).
Download references
Acknowledgements
The authors thank the Integrative Biology Unit (IBU) and the Healthy Food Unit (HFU) of the Institute for Obesity Research (IOR), Centro de Investigación y Desarrollo de Proteinas (CIDPRO) and Centro de Biotecnología FEMSA of the Tecnologico de Monterrey, and Hospital Regional Materno Infantil de Alta Especialidad (HRMIAE) of the Secretaria de Salud, Nuevo Leon, Mexico for supporting the development of this study. The authors thank Dr. Ana Lucía Araujo (Resident of Pediatrics) for her support in obtaining Institutional Review Board approval for this study (HRMIAE and Secretaria de Salud, Nuevo León, México). The authors also thank and recognize the incredible contribution of the HRMIAE Human Milk Bank health personnel during the collection of colostrum samples in the present study.
This work was supported by the Challenge-Based Research Funding 2022 of Tecnologico de Monterrey [I008-IOR001-C6-T1-E, November 3, 2022].
Author information
Authors and affiliations.
Tecnologico de Monterrey, Institute for Obesity Research, Ave. Eugenio Garza Sada 2501, 64849, Monterrey, NL, Mexico
Víctor H. Urrutia-Baca, Janet A. Gutiérrez-Uribe, Astrid Domínguez-Uscanga, Francisca Espiricueta-Candelaria & Cristina Chuck-Hernández
Tecnologico de Monterrey, Escuela de Ingeniería y Ciencias, Ave. Eugenio Garza Sada 2501, 64849, Monterrey, NL, Mexico
Perla A. Ramos-Parra
Tecnologico de Monterrey, Escuela de Medicina y Ciencias de la Salud, Ave. Ignacio Morones Prieto 3000, 64710, Monterrey, NL, Mexico
Nora A. Rodriguez-Gutierrez, Karla L. Chavez-Caraza & Alicia S. Padilla-Garza
Facultad de Medicina, Universidad Autónoma de Nuevo León, Ave. Dr. José Eleuterio González 235, 64460, Monterrey, NL, Mexico
Ilen Martinez-Cano & Elias G. Ruiz-Villarreal
You can also search for this author in PubMed Google Scholar
Contributions
V.H.U.B., C.C.H., J.G.U., and P.A.R.P. contributed to the conception and design of the study, the interpretation of the results, and the drafting of the manuscript. A.D.U., N.A.R.G., K.L.C.C., A.S.P.G., I.M.C., E.G.R.V., and F.E.C. conducted the acquisition of data. V.H.U.B. conducted the statistical analyses. All authors have read and approved the final version of the manuscript.
Corresponding author
Correspondence to Cristina Chuck-Hernández .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary tables., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Urrutia-Baca, V.H., Gutiérrez-Uribe, J.A., Ramos-Parra, P.A. et al. Exploring the impact of maternal factors and dietary habits on human milk oligosaccharide composition in early breastfeeding among Mexican women. Sci Rep 14 , 14685 (2024). https://doi.org/10.1038/s41598-024-63787-1
Download citation
Received : 12 December 2023
Accepted : 03 June 2024
Published : 26 June 2024
DOI : https://doi.org/10.1038/s41598-024-63787-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Food Nutr Res

Milk and dairy products: good or bad for human health? An assessment of the totality of scientific evidence
Tanja kongerslev thorning.
1 Department of Nutrition, Exercise and Sports, Faculty of Science, University of Copenhagen, Copenhagen, Denmark
Tine Tholstrup
Sabita s. soedamah-muthu.
2 Division of Human Nutrition, Wageningen University, Wageningen, The Netherlands
3 Centre for Food, Nutrition and Health, University of Reading, Reading, UK
Arne Astrup
There is scepticism about health effects of dairy products in the public, which is reflected in an increasing intake of plant-based drinks, for example, from soy, rice, almond, or oat.
This review aimed to assess the scientific evidence mainly from meta-analyses of observational studies and randomised controlled trials, on dairy intake and risk of obesity, type 2 diabetes, cardiovascular disease, osteoporosis, cancer, and all-cause mortality.
The most recent evidence suggested that intake of milk and dairy products was associated with reduced risk of childhood obesity. In adults, intake of dairy products was shown to improve body composition and facilitate weight loss during energy restriction. In addition, intake of milk and dairy products was associated with a neutral or reduced risk of type 2 diabetes and a reduced risk of cardiovascular disease, particularly stroke. Furthermore, the evidence suggested a beneficial effect of milk and dairy intake on bone mineral density but no association with risk of bone fracture. Among cancers, milk and dairy intake was inversely associated with colorectal cancer, bladder cancer, gastric cancer, and breast cancer, and not associated with risk of pancreatic cancer, ovarian cancer, or lung cancer, while the evidence for prostate cancer risk was inconsistent. Finally, consumption of milk and dairy products was not associated with all-cause mortality. Calcium-fortified plant-based drinks have been included as an alternative to dairy products in the nutrition recommendations in several countries. However, nutritionally, cow's milk and plant-based drinks are completely different foods, and an evidence-based conclusion on the health value of the plant-based drinks requires more studies in humans.
The totality of available scientific evidence supports that intake of milk and dairy products contribute to meet nutrient recommendations, and may protect against the most prevalent chronic diseases, whereas very few adverse effects have been reported.
Several media stories and organisations claim that dairy increases risk of chronic diseases including obesity, type 2 diabetes, cardiovascular disease, osteoporosis, and cancer. Therefore, there is an increasing scepticism among the general consumers about the health consequences of eating dairy products. This is reflected in an increasing consumption of plant-based drinks, for example, based on soy, rice, almond, or oats. Dairy is an essential part of the food culture in the Nordic countries; thus, inclusion of milk and dairy products in the diet may be natural for many Nordic individuals. The major causes of loss of disease-free years in the Nordic countries today are type 2 diabetes, cardiovascular diseases, and cancers. Moreover, the increasing prevalence of obesity greatly increases the risk of these chronic diseases. Given the increasing prevalence of these chronic diseases, it is critically important to understand the health effects of milk and dairy products in the diet. Accordingly, this narrative review presents the latest evidence from meta-analyses and systematic reviews of observational studies and randomised controlled trials on dairy intake (butter excluded) and risk of obesity, type 2 diabetes, cardiovascular disease, osteoporosis, and cancer as well as all-cause mortality.
We aim to answer the key questions: 1) For the general consumer, will a diet with milk and dairy products overall provide better or worse health, and increase or decrease risk of major diseases and all-cause mortality than a diet with no or low content of milk and dairy products? 2) Is it justified to recommend the general lactose-tolerant population to avoid consumption of milk and dairy products? 3) Is there scientific evidence to substantiate that replacing milk with plant-based drinks will improve health?
Obesity and type 2 diabetes
A large share of the on-going increase in prevalence of type 2 diabetes is driven by the obesity epidemic ( 1 , 2 ), and it is therefore relevant to assess the role of milk and dairy products for body weight control. Childhood overweight and obesity worldwide is a major contributor to the current obesity epidemic, and childhood obesity frequently tracks into adulthood ( 3 ). Therefore, early prevention of childhood obesity is important. A meta-analysis showed that among children in the pre-school and school age, there was no association between dairy intake and adiposity ( 4 ). However, there was a modestly protective effect in adolescence. A recent meta-analysis by Lu et al. ( 5 ) found that children in the highest dairy intake group were 38% less likely to be overweight or obese compared to those in the lowest dairy intake group. An increase in dairy intake of one serving per day was associated with a 0.65% lower body fat and a 13% lower risk of overweight or obesity.
Milk and dairy products are good sources of high-quality protein. Protein is important during weight loss and subsequent weight maintenance due to the high satiating effect which helps to prevent over-consumption of energy and thereby reduces body fat stores ( 6 , 7 ). Furthermore, dairy protein is a good source of essential amino acids for muscle protein synthesis and thus helps to maintain the metabolically active muscle mass during weight loss ( 8 ). Meta-analyses support that in adults, dairy products facilitate weight loss and improve body composition, that is, reduce body fat mass and preserve lean body mass during energy restriction and in short-term studies ( 9 – 11 ). The effect of an increased dairy consumption on body weight in long-term studies (>1 year) and in energy balance studies is less convincing ( 10 , 11 ). This is likely due to the opposing effects of dairy on body composition, that is, reduction of fat mass and preservation of lean body mass.
Meta-analyses assessing the role of intake of milk and dairy products on risk of type 2 diabetes have consistently found no or a slight beneficial effect of dairy intake on diabetes risk ( 12 – 15 ). This is consistent with a Mendelian randomisation study using genetic polymorphisms for the lactase gene, which showed that milk intake assessed by lactose tolerance was not associated with risk of type 2 diabetes or obesity ( 16 ). The most recent meta-analysis on dairy intake and diabetes incidence included 22 cohort studies with a total of 579,832 subjects and 43,118 type 2 diabetes cases ( 17 ). An inverse association between total dairy and yoghurt intake and risk of type 2 diabetes was reported although there was no association with milk intake. The benefits of fermented dairy products (cheese and yoghurt) in relation to type 2 diabetes may be due to their effect on the gut microbiota ( 18 , 19 ). Other studies have identified that whey protein (primarily in milk and yoghurt) can reduce postprandial plasma glucose concentration in type 2 diabetic subjects ( 20 ). This effect may be due to the branched chain amino acids in the whey protein fraction, particularly leucine which has been shown to induce a greater stimulation of glucose-dependent insulinotropic polypeptide (GIP), but not glucagon like peptide 1 (GLP-1), compared to other amino acids ( 21 ). The GIP response is possibly a key factor in the higher insulin response and the subsequent lowering of blood glucose seen after whey ingestion, at least in healthy subjects. In addition to the insulinotropic effect of milk, a recent study has indicated that dairy may also improve insulin sensitivity ( 22 ).
Conclusion on obesity and type 2 diabetes
A diet high in milk and dairy products reduces the risk of childhood obesity and improves body composition in adults. This likely contributes to lower the risk of developing type 2 diabetes. Additionally, dairy product consumption during energy restriction facilitates weight loss, whereas the effect of dairy intake during energy balance is less clear. Finally, there is increasing evidence suggesting that especially the fermented dairy products, cheese and yoghurt, are associated with a reduced risk of type 2 diabetes.
Cardiovascular disease
Low-fat, calcium-rich dairy products are generally considered to lower blood pressure. This was supported by a meta-analysis of six observational studies, whereas no association was found with intake of high-fat dairy products ( 23 ). High-fat dairy products are known to increase high density lipoprotein (HDL)- and low density lipoprotein (LDL)-cholesterol concentrations. The latter normally predicts risk of cardiovascular disease ( 24 ), but this may depend on the size of the LDL-cholesterol particles. Small, dense LDL particles are more atherogenic than their larger counterparts ( 25 – 28 ) due to their lower affinity for the LDL-receptor and higher susceptibility to oxidation ( 29 ). In agreement, some of the fatty acids typically found in milk and dairy products have been associated with less small, dense LDL particles (4:0–10:0 and 14:0 in the diet, and 15:0 and 17:0 in serum phospholipids) ( 30 ). In addition, the minerals in milk and dairy products have been shown to attenuate the LDL-response to high-fat dairy intake ( 31 , 32 ).
Among high-fat dairy products, cheese in particular does not seem to increase LDL-cholesterol to the extent expected, based on the high content of saturated fat ( 33 ). When compared to habitual diet with a lower total and saturated fat content ( 33 ), or compared to diets with lower total fat content but higher content of high-GI carbohydrates ( 34 , 35 ), a high intake of cheese was found not to increase LDL-cholesterol. A meta-analysis of randomised controlled trials studying the effect of cheese consumption compared with other foods on blood lipids and lipoproteins showed that cheese caused lower total cholesterol, LDL-cholesterol, and HDL-cholesterol concentrations compared with butter ( 36 ). Compared with milk, however, there was no statistically significant difference in blood lipids ( 32 , 37 ). Several meta-analyses have been conducted on the relationship between intake of milk and dairy products and risk of developing cardiovascular disease. There was no consistent association between milk or dairy intake and cardiovascular disease, coronary heart disease or stroke in a meta-analysis by Soedamah-Muthu et al. ( 38 ). In a recent update, including a higher number of prospective cohort studies, there was a significant inverse association between milk intake and stroke, with a 7% lower risk of stroke per 200 ml milk/day, but considerable heterogeneity. Further, stratification for Asian and Western countries showed a more marked reduction in risk in Asian than in Western countries. This is consistent with a previous meta-analysis by Hu et al. ( 39 ) showing a non-linear dose–response relationship between milk intake and risk of stroke, with the highest risk reduction of 7–8% with a milk intake of 200–300 ml/day. Also, the meta-analyses by Hu et al. ( 39 ) and de Goede et al. ( 40 ) both showed an inverse association between cheese intake and stroke, however only borderline significant in the latter. Accordingly, another meta-analysis on dairy and cardiovascular disease found that intake of cheese and milk as well as yoghurt was inversely associated with cardiovascular disease risk ( 41 ). A later meta-analysis by Qin et al. ( 42 ) found that dairy intake was associated with a 12% lower risk of cardiovascular disease, and 13% lower risk of stroke as compared to individuals with no or a low dairy consumption ( 42 ). Likewise, a recent and comprehensive meta-analysis, including 31 cohort studies, suggested that a high dairy intake was associated with a 9% lower risk of stroke, whereas no association was found with total cardiovascular disease or coronary heart disease ( 43 ). Moreover, a high intake of cheese was associated with an 8% lower risk of coronary heart disease and a 13% lower risk of stroke. In addition, high plasma levels of the saturated fatty acid C 17:0, which primarily originates from dairy, were found to be associated with a reduced risk of coronary heart disease ( 44 ). Finally, a meta-analysis by O'Sullivan et al. ( 45 ) found no indication of total dairy intake or any specific dairy product being associated with an increased cardiovascular mortality. Studies are emerging showing that dairy products, particularly the low-fat types, cluster within a healthy dietary pattern ( 46 ), and therefore, the risk of residual confounding in the observational studies cannot be ruled out.
In accordance with the latest meta-analyses presented above, the latest Nordic Nutrition Recommendations have concluded that high consumption of low-fat milk products is associated with reduced risk of hypertension and stroke ( 47 ).
Conclusion on cardiovascular disease
The overall evidence indicates that a high intake of milk and dairy products, that is, 200–300 ml/day, does not increase the risk of cardiovascular disease. Specifically, there is an inverse association with risk of hypertension and stroke.
Bone health and osteoporosis
Milk and dairy products contain a number of nutrients that are required for building strong bones in childhood and for their maintenance during adulthood with the aim to reduce osteoporosis and bone fractures in older age ( 48 ). The European Commission has concluded that protein, calcium, phosphorus, magnesium, manganese, zinc, vitamin D, and vitamin K are necessary for maintaining normal bones (European Commission regulation 2012). With the exception of vitamin D, these nutrients are all present in significant amounts in milk and dairy products.
Osteoporosis has been described as a ‘paediatric disease with geriatric consequences’ as low milk, and hence, low mineral intake during childhood and adolescence has been associated with significantly increased risk of osteoporotic fractures in middle and older age, particularly in women ( 49 , 50 ). A recent study indicated that in children and adolescents, except for those with very low calcium intakes, magnesium intake may be more important than calcium in relation to bone development ( 51 ). Calcium intake was found not to be significantly associated with total bone mineral content or density, whereas intake of magnesium and the amount absorbed were key predictors of bone mass. The extent to which these results can be extrapolated to the general population is uncertain, but milk and dairy products are important sources of magnesium and hence important supporters of bone growth during adolescence. In a meta-analysis by Huncharek et al. ( 52 ), dairy products, with or without vitamin D supplementation, increased total body and lumbar spine bone mineral content in children with low baseline dairy intake, whereas no effect was found for children with a high baseline dairy intake. Thus, there may be a threshold above which increasing intake of dairy products or dairy-calcium does not additionally benefit bone mineral content or density in children.
In adults, interactions between calcium, phosphorus, protein and vitamin D reduce bone resorption and increase bone formation, thereby attenuating age-related bone loss ( 53 ). Possibly due to the complex interaction between nutrients and the multifactorial nature of bone fractures, it has been difficult to establish whether or not a low intake of milk and dairy in adulthood increases the risk of osteoporosis and bone fractures. Hence, to date, meta-analyses have not supported a protective effect of milk and dairy intake in adulthood on risk of osteoporosis and bone fractures ( 54 , 55 ). Nevertheless a recent systematic review concluded that calcium and dairy are important contributors to bone health in adults ( 56 ).
In the 2015–2020 Dietary Guidelines for Americans, it was stated that ‘Healthy eating patterns include fat-free and low-fat (1%) dairy, including milk, yoghurt, cheese, or fortified soy beverages (commonly known as “soymilk”). Those who are unable or choose not to consume dairy products should consume foods that provide the range of nutrients generally obtained from dairy, including protein, calcium, potassium, magnesium, vitamin D, and vitamin A (e.g. fortified soy beverages)’. Although the focus is on achieving the nutrient requirements by foods rather than supplements, plant-based beverages typically contain inorganic chemical forms of calcium, which may actually increase cardiovascular risk ( 56 , 57 ). As calcium in dairy is organic, milk and dairy products should still be considered the superior sources of calcium ( 58 ). Yet, future studies need to address whether or not vitamin D fortification of dairy products is crucial for these to have a positive effect on bone fracture risk.
Conclusion on bone health and osteoporosis
The present evidence suggests a positive effect of milk and dairy intake on bone health in childhood and adolescence, but with only limited evidence on bone health in adulthood and on the risk of bone fractures in older age.
In population studies, dairy has been associated positively and negatively with various cancers, but most have been based on limited evidence and very few findings remain robust. Dairy products contain a variety of bioactive compounds that could exert both positive and negative effects on carcinogenesis. The positive effects may be related to the content of calcium, lactoferrin, and fermentation products, whereas the negative effects could be linked to the content of insulin-like growth factor I (IGF-1) ( 59 ). The World Cancer Research Fund (WCRF) continuously and systematically reviews the evidence on diet and physical activity in relation to prevention of cancer, and specific areas are updated when new evidence has emerged.
Colorectal cancer is the second most common cause of death among cancers in developed countries. Even though colorectal tumourigenesis is a complex process, epidemiological and experimental data indicate that milk and dairy products have a chemopreventive role in the pathogenesis. In the 2011 WCRF report on colorectal cancer, it was concluded that consumption of milk and calcium probably reduces the risk of this cancer ( 60 ). Likewise, in meta-analyses, dairy intake has consistently been associated with a decreased risk of colorectal cancer ( 61 , 62 ) and colon cancer ( 63 ). The most recent meta-analysis by Ralston et al. ( 64 ) reported 26% lower colon cancer risk in males consuming 525 g of milk per day, whereas no association was found in females.
The link between dairy intake and colorectal cancer is considered to be mainly due to the calcium derived from dairy, with a 24% risk reduction with a dairy-calcium intake of 900 mg/day ( 65 ). The proposed mechanisms behind this are calcium binding to secondary bile acids and ionised fatty acids, thereby reducing their proliferative effects in the colorectal epithelium ( 66 ). Also, calcium may influence multiple intracellular pathways leading to differentiation in normal cells and apoptosis in transformed cells ( 67 ). Accordingly, a number of studies have reported reduced cell proliferation in the colon and rectum with intake of calcium and dairy products ( 68 – 72 ).
In the 2010 WCRF report on breast cancer, it was concluded that the evidence for dairy intake and risk of breast cancer is non-conclusive ( 73 ). In accordance with a meta-analysis from 2011 on prospective cohort studies ( 74 ), a recent meta-analysis by Zang et al. ( 75 ), however, suggested that a high (>600 g/d) and modest (400–600 g/d) dairy intake was associated with a reduced risk of breast cancer (10% and 6%, respectively) compared with a low dairy intake (<400 g/d). Within dairy subgroups, particularly yoghurt and low-fat dairy were found to be inversely associated with the risk of developing breast cancer. As calcium and vitamin D supplementation was previously shown to reduce risk of breast cancer in the Women's Health Initiative ( 76 ), these nutrients could be involved in the underlying mechanisms.
According to the 2014 WCRF report on prostate cancer, dairy may be associated with a limited-suggestive increased risk of prostate cancer, but the current evidence is limited ( 77 ). However, this conclusion was substantiated by the most recent meta-analysis by Aune et al. ( 78 ), which suggested that a high intake of dairy products, milk, low-fat milk, cheese, and calcium were associated with a 3–9% increased risk of prostate cancer. The mechanism behind this was suggested to be an increased circulating concentration of IGF-1, which has been previously shown to be associated with an increased prostate cancer risk ( 79 ).
The 2015 WCRF report on bladder cancer suggested that the evidence for milk and dairy on bladder cancer risk was inconsistent and inconclusive ( 80 ). Two meta-analyses on milk intake and bladder cancer risk have suggested a decreased risk of bladder cancer with a high intake of milk ( 61 , 81 ). Others have found no association between milk and dairy intake and risk of bladder cancer risk ( 82 ), but none have suggested an adverse effect.
Of the cancer types for which the associations with dairy intake were not presented in the WCRF reports, recent meta-analyses have suggested no association between dairy intake and risk of ovarian cancer ( 83 ), lung cancer ( 84 , 85 ), or pancreatic cancer ( 86 ) and an inverse association between dairy intake and risk of gastric cancer in Europe and the United States ( 87 ).
Studies in lactose-intolerant individuals
In a limited number of subjects, potential differences in cancer risk and mortality between lactose-tolerant and lactose-intolerant individuals (self-reported or assessed by polymorphisms for the lactase gene) have been reported under the assumption that lactose-intolerant individuals consume less milk. However, there may also be other differences between these two groups that need to be taken into consideration, for example, genetics, ethnicity, other dietary habits, smoking, physical activity, and socio-economic factors.
Bácsi et al. ( 88 ) examined the role of genetically determined differences in the ability to degrade lactose and showed that subjects with deficiencies in the genes coding for lactase (i.e. subjects not drinking milk due to intolerance) had an increased risk of colorectal cancer. This supports the ability of dairy products to reduce colorectal cancer risk and the causality of this relation. In the European EPIC study, the hypothesis that the genetically determined lactose tolerance was associated with elevated dairy product intake and increased prostate cancer risk was examined ( 89 ). The study included 630 men with prostate cancer and 873 matched control participants. Dairy product consumption was assessed by diet questionnaires, and intake of milk and total dairy products varied significantly by lactase genotype, with an almost twofold higher intake in lactose-tolerant compared to lactose-intolerant subjects. However, the lactase variant was not found to be significantly associated with prostate cancer risk. This indicates that residual confounding may have biased the associations observed between milk and dairy intake and prostate cancer risk in the observational studies included in a previous meta-analysis ( 78 ).
Ji et al. ( 90 ) investigated Swedish subjects with self-reported lactose intolerance and found a lower risk of lung, breast, and ovarian cancers compared to lactose-tolerant subjects. Unfortunately, no information about milk intake, or other genetic, ethnic, lifestyle (diet, smoking and physical activity), and behavioural characteristics were reported. Also, self-reported lactose intolerance may not be comparable to genetically determined lactose intolerance. Due to potential bias in the design and the lack of control for known confounders, it is impossible to conclude about the relationship with dairy intake. Also, these findings are in contrast with the additional literature suggesting no or an inverse association between dairy intake and risk of breast cancer ( 74 , 75 ), ovarian cancer ( 83 , 91 ), and lung cancer ( 84 , 85 ).
Conclusion on cancer
According to WCRF reports and the latest meta-analyses, consumption of milk and dairy products probably protects against colorectal cancer, bladder cancer, gastric cancer, and breast cancer. Dairy intake does not seem to be associated with risk of pancreatic cancer, ovarian cancer, or lung cancer, whereas the evidence for prostate cancer risk is inconsistent. In women, dairy offers significant and robust health benefits in reducing the risk of the common and serious colorectal cancer and, possibly, also the risk of breast cancer. In men, the benefit of the protective effect of milk and dairy on the common and serious colorectal cancer is judged to outweigh a potentially increased risk of prostate cancer.
All-cause mortality
In medical research, the term ‘all-cause mortality’ implies all causes of death. There are many individual studies reporting that a high consumption of milk and dairy products is associated with decreased mortality ( 92 ), unchanged mortality ( 93 ), or even increased mortality ( 94 ). However, based on meta-analyses of observational cohort studies, there is no evidence to support the view that milk and dairy product intake is associated with all-cause mortality ( 45 , 95 ). In a meta-analysis, O'Sullivan et al. ( 45 ) studied whether intake of milk and dairy products as food sources of saturated fat was related to all-cause mortality, cancer mortality, and cardiovascular mortality. Neither total dairy intake nor intake of any specific dairy products was found to be associated with all-cause mortality. In the most recent meta-analysis including 12 observational studies of milk intake and mortality, there were no consistent associations between milk intake and all-cause or cause-specific mortality ( 95 ).
Conclusion on all-cause mortality
The evidence from observational studies confirms that there is no association between consumption of milk and dairy products and all-cause mortality.
Comparison of nutrient content and health aspects of milk and plant-based drinks
In recent decades, the market for milk and dairy substitute drinks based on, for example, soy, rice, oats, or almonds has expanded, and calcium-fortified plant-based drinks have become part of the nutrition recommendations as alternatives to milk in several countries, such as the United States, Sweden, Australia, and Brazil. Among the plant-based milk substitutes, soy drink dominates the market in the Western world, but the emerging of other plant-based drinks has influenced the market for soy drink ( 96 ).
The nutrient density of plant-based milk substitutes varies considerably between and within types, and their nutritional properties depend on the raw material used, the processing, the fortification with vitamins and minerals, and the addition of other ingredients such as sugar and oil. Soy drink is the only plant-based milk substitute that approximates the protein content of cow's milk, whereas the protein contents of the drinks based on oat, rice, and almonds are extremely low, and the recent review of Mäkinen et al. ( 96 ) emphasises the importance of consumer awareness of such low-protein contents. Moreover, there are now cases of severe nutritional deficiencies in children being reported as a result of inappropriate consumption of plant-based drinks ( 97 , 98 ).
Despite the fact that most of the plant-based drinks are low in saturated fat and cholesterol, some of these products have higher energy contents than whole milk due to a high content of oil and added sugar. Some plant-based drinks have a sugar content equal to that of sugar-sweetened beverages, which have been linked to obesity, reduced insulin sensitivity ( 99 ), increased liver, muscle, and visceral fat content as well as increased blood pressure, and increased concentrations of triglyceride and cholesterol in the blood ( 100 , 101 ). Analyses of several commercially available plant-based drinks carried out at the Technical University of Denmark showed a generally higher energy content and lower contents of iodine, potassium, phosphorus, and selenium in the plant-based drinks compared to semi-skimmed milk ( 102 ). Also, rice drinks are known to have a high content of inorganic arsenic, and soy drinks are known to contain isoflavones with oestrogen-like effects. Consequently, The Danish Veterinary and Food Administration concluded that the plant-based drinks cannot be recommended as full worthy alternatives to cow's milk ( 102 ), which is consistent with the conclusions drawn by the Swedish National Food Agency ( 103 ).
The importance of studying whole foods instead of single nutrients is becoming clear as potential nutrient–nutrient interactions may affect the metabolic response to the whole food compared to its isolated nutrients. As the plant-based drinks have undergone processing and fortification, any health effects of natural soy, rice, oats, and almonds cannot be directly transferred to the drinks, but need to be studied directly. Only a few studies have compared the effects of cow's milk with plant-based drinks as whole foods on disease risk markers ( 104 – 108 ). However, none of these have included commercially available drinks or disease endpoints. Therefore, the evidence is currently insufficient to conclude that plant-based drinks possess health benefits above those of milk and dairy products. Until more research has been conducted and a scientifically sound conclusion can be drawn, health authorities should be cautious about recommending plant-based drinks as acceptable substitutes to cow's milk for the general population.
Conclusion on nutrient content and health aspects of milk and plant-based drinks
Cow's milk and plant-based drinks are completely different products, both regarding nutrient content and presumably also health effects. Although there are concerns about children consuming the low-protein drinks, further evidence-based assessment of the nutritional and health value of the plant-based drinks must await more studies in humans.
Answers to the key questions
Key question 1: For the general consumer, will a diet with milk and dairy products overall provide better or worse health, and increase or decrease risk of major diseases and all-cause mortality than a diet with no or low content of milk and dairy products?
Consumption of dairy products is associated with an overall reduced risk of cardiometabolic diseases and some cancers, whereas only very few adverse effects have been reported ( Fig. 1 ). Dairy products may therefore have the potential to reduce the burden of the most prevalent chronic diseases in the population and to substantially reduce the health care costs for society ( 109 ). Consumption of dairy products is part of the dietary recommendations in several nations, for example, Sweden, Denmark, and United States. A general recommendation to reduce the intake of dairy products in individuals who actually tolerate them may be counterproductive for health and could therefore increase health care expenses. However, more emphasis should be on the foods which dairy replaces in the diet. In addition, as most of the conducted meta-analyses are on observational data, residual confounding cannot be ruled out, and it is also possible that milk and dairy intake in these studies could be just a marker of diets of higher nutritional quality.

Overall effect/association between dairy product intake and health outcomes. ↓ favourable effect/association; ↑adverse effect/association; → no effect/association.
Key question 2: Is it justified to recommend the general lactose-tolerant population to avoid the consumption of milk and dairy products?
In the Nordic countries, as few as 2% of the population has primary lactase deficiency and can be classified as lactose-intolerant individuals ( 110 ). Yet, most lactose-intolerant adults can tolerate one glass of milk or a scoop of ice cream. Cheeses have negligible lactose contents, and the lactose in yoghurt is digested more efficiently than other dairy sources due to the bacterial lactase present in yoghurt which facilitates lactose digestion ( 111 ). Therefore, fermented dairy products, that is, yoghurt and most cheeses (cottage cheese, as well as soft and hard cheeses), can be tolerated by lactose-intolerant individuals without symptoms ( 111 , 112 ).
The same applies to cow's milk protein allergy that typically occurs in 0.1–2.0% of children in the Nordic countries and Europe ( 113 ). Among children with verified cow's milk-specific IgE who were re-evaluated 1 year after diagnosis, 69% tolerated cow's milk at re-evaluation ( 114 ). Thus, the condition is generally seen to resolve in children. To warn the general population against dairy consumption based on rare milk allergies would be equivalent to warn against foods, such as peanuts or seafood due to the fact that a small subset of the population is allergic to these foods.
Key question 3: Is there scientific evidence to substantiate that replacing milk and dairy products with plant-based drinks will improve health?
Cow's milk and plant-based drinks are not nutritionally comparable foods. As only a few studies have investigated the health effects of replacing cow's milk with plant-based drinks and none have focused on commercially available drinks or on disease endpoints, the effect of this replacement can only be speculated on. There have, however, been individual cases reporting illness in children consuming low-protein plant-based drinks, but an evidence-based final assessment of the health value of plant-based drinks compared to cow's milk must await more studies in humans.
Overall conclusions regarding intake of milk and dairy products and health
Our review of the totality of available scientific evidence supports that intake of milk and dairy products contributes to meeting nutrient recommendations and may protect against the most prevalent, chronic non-communicable diseases, whereas very few adverse effects have been reported.
Conflicts of interest and funding
Tanja Kongerslev Thorning has no conflicts of interest to declare. Anne Raben is recipient of research funding from the Dairy Research Institute, Rosemont, IL, USA and the Danish Agriculture & Food Council.Tine Tholstrup is recipient of research grants from the Danish Dairy Research Foundation and the Dairy Research Institute, Rosemont, IL. The sponsors had no role in design and conduct of the studies, data collection and analysis, interpretation of the data, decision to publish, or preparation of the manuscripts. Sabita S. Soedamah-Muthu received funding from the Global Dairy Platform, Dairy Research Institute and Dairy Australia for meta-analyses on cheese and blood lipids and on dairy and mortality. The sponsors had no role in design and conduct of the meta-analyses, data collection and analysis, interpretation of the data, decision to publish, or preparation of the manuscripts. Ian Givens is recipient of research grants from UK Biotechnology and Biological Sciences Research Council (BBSRC), UK Medical Research Council (MRC), Arla Foods UK, AAK-UK (both in kind), The Barham Benevolent Foundation, Volac UK, DSM Switzerland and Global Dairy Platform. He is a consultant for The Bio-competence Centre of Healthy Dairy Products, Tartu, Estonia, and in the recent past for The Dairy Council (London). Arne Astrup is recipient of research grants from Arla Foods, DK; Danish Dairy Research Foundation; Global Dairy Platform; Danish Agriculture & Food Council; GEIE European Milk Forum, France. He is member of advisory boards for Dutch Beer Knowledge Institute, NL; IKEA, SV; Lucozade Ribena Suntory Ltd, UK; McCain Foods Limited, USA; McDonald's, USA; Weight Watchers, USA. He is a consultant for Nestlé Research Center, Switzerland; Nongfu Spring Water, China. Astrup receives honoraria as Associate Editor of American Journal of Clinical Nutrition , and for membership of the Editorial Boards of Annals of Nutrition and Metabolism and Annual Review of Nutrition . He is recipient of travel expenses and/or modest honoraria (<$2,000) for lectures given at meetings supported by corporate sponsors. He received financial support from dairy organisations for attendance at the Eurofed Lipids Congress (2014) in France and the meeting of The Federation of European Nutrition Societies (2015) in Germany.

IMAGES
VIDEO
COMMENTS
Comparative Compositions of Cattle and Non-cattle Milks. The comparative compositions of milk from different species have been extensively reviewed in previous studies (5, 17-19).The milk from different species vary in composition (Table 1).Protein, fat, lactose, and minerals are the four major components in all milks, irrespective of the species (); the composition of milk within the same ...
1 INTRODUCTION. Milk provides a comprehensive source of nutrition for mammalian neonates. Although milk composition varies substantially between species, the milk of other species can be consumed by mature humans and children of sufficient renal development (Ziegler, 2007), providing a greater distribution of essential macro- and micro-nutrients than most other food sources (Drewnowski, 2005).
1. Introduction. Milk is among the most versatile and valuable foods in the food industry. In 2018, global milk production reached 843 billion liters, with an estimated value of USD 307 billion and is projected to grow by 22% by 2027 [].Approximately 80% of yearly milk production comes from cows, with the rest from other dairy animals like buffaloes, goats, camels, and sheep, according to the ...
Milk is a considerable resource of products whose composition varies. Four components are dominant in quantitative terms: water, fat, protein and lactose; while the minor components are minerals ...
This paper will review the major scientific advances in manipulation of milk composition over the last 25 yr. A multitude of factors influence the final composition of milk including genetics and breed of animal, environment, stage of lactation, parity, and nutrition of the cow. Although all of these factors work in combination to determine the ...
We identified 32 studies on the composition of human milk in the United States and Canada conducted in the 5-y period from 2017 to 2022, which suggests a rapid increase in human milk research compared to the 28 studies identified by Wu et al. [8] in the 36-y period from 1980 to 2016.
A substantial body of research exists describing the effect of different feeding strategies on the composition of milk, with several recent studies focusing on the comparison of pasture- and concentrate-based feeding regimes. Significant variation is typically observed in the gross composition of milk produced from different supplemental feeds ...
Understanding the chemistry of milk and its components is critical to the production of consistent, high-quality dairy products as well as the development of new dairy ingredients. Over the past 100 yr we have gone from believing that milk has only 3 protein fractions to identifying all the major and minor types of milk proteins as well as discovering that they have genetic variants.
4.6 Genetic influences on milk fat concentrations and fatty acid profiles 70 4.7 Influence of feeds, feeding regimes, pasture and stage of lactation on milk lipids and their levels 71 4.8 Digestion of milk fat 72 4.9 Nutritional effects of milk fatty acids 72 4.10 Evidence for effects of milk fat on CVD from prospective cohort studies 74
The composition of non-fat solids of skim milk is: 52.15% lactose, 38.71% protein (31.18% casein, 7.53% whey protein), 1.08% fat, and 8.06% ash. Fresh milk from a healthy cow is practically free from bacteria, but it must be protected against contamination as soon as it leaves the udder.
Milk composition determines the economic feasibility of processing (i.e., the yield of butter, or cheese obtained per kg of milk) and affects the quality of dairy products (Chen et al., 2014). Low protein percentage has been reported in a handful of studies investigating milk composition in Kenya (Kabui et al., 2015; Ondieki et al., 2017).
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Milk composition (lactose ...
This study describes the development of a database, called MilkyBase, of the biochemical composition of human milk. The data were selected, digitized and curated partly by machine-learning, partly ...
Cow's milk: Composition, nutritional, biological an d cardioprotective benefits. Aicha BENYAHIA-MOSTEFAOUI, Myriem LAMRI-SENH ADJI. Laboratoire de Nutrition Clinique et Métabolique (LNCM ...
As regards the milk consumption by older adults, it meets the needs of the body in the best way. because its nutrients are in digestible form. One liter (1 L) of milk provides 700 calories and ...
Milk is one of the most valuable products in the food industry with most milk production throughout the world being carried out using conventional management, which includes intensive and traditional systems. The intensive use of fertilizers, antibiotics, pesticides and concerns regarding animal health and the environment have given increasing importance to organic dairy and dairy products in ...
1. Introduction. Human milk from the infant's own mother is the optimal source of nutrition for infants because: (1) it provides core required nutrients, including proteins, lipids, carbohydrates and minerals; (2) it provides bioactive factors, especially immunological factors, to protect infants from invading microorganisms (Ballard & Morrow, 2013; Lee et al., 2018); (3) it helps the ...
Variations in the gross composition of milk as related to the breed of the cow: A review and critical evaluation of literature of the United States and Canada. J. Dairy Sci. 42, 1-19. CAS Google Scholar. Armstrong, M. D. and Yates, K. N. 1963. Free amino acids in milk. Proc. Soc. Exp. Biol. Med. 113, 680-683.
For some people with digestive difficulties, goat's milk can be easily digested. Camel milk is an important source of proteins for the people living in the arid lands of the world camel milk is considered to have anti-cancer, hypo-allergenic and anti- diabetic properties. Buffalo milk is a natural product that can be consumed like any other milk.
J. G. LINN. Milk composition is economically important to milk producers and processors and nutritionally important to consumers. It has been known for years that variations in milk composition occur; however, the composition of milk marketed nationally has been rather constant over the last 15 years, averaging 3.6 percent fat, 3.2 percent protein, and 4.7 percent lactose (Young et al., 1986).
Human milk oligosaccharides (HMOs) promote adequate intestinal microbiota development and favor the immune system's maturation and cognitive development. In addition to non-modifiable factors ...
The average mean values of lactose in% are shown in Table 3. From the results it can be seen that lactose content (%) as part of the chemical composition of milk, in herd in the first lactation varies from 4.39% to 4.54% where the coefficient of variation ranges from 1.86 to 5.66%. In herd in the second lactation, the average lactose content ...
This is the reason for including zinc in the composition of indicators assessing the nutritional quality of the diet i.e., calories-for-nutrient (CFN), and naturally nutrient rich (NNR) score [76,77,78,90]. According to our research, the share of milk and dairy products in the supply of potassium is almost 12%, with a significant share of milk.
This paper aimed to estimate the heritability and genetic correlations for processability (milk coagulation properties, cheese yield, and heat stability), milk production, and milk composition of dairy sheep in a New Zealand flock. Test-day records from 169 ewes were obtained during the 2021-2022 season.
Breast milk macronutrients are provided by milk synthesized in lactocytes, mother's diet and maternal stores. Research Aim. This study was conducted to investigate the relationship between the body composition of mothers and the anthropometric characteristics of the baby and the nutritional content of breast milk. Method
Obesity and type 2 diabetes. A large share of the on-going increase in prevalence of type 2 diabetes is driven by the obesity epidemic (1, 2), and it is therefore relevant to assess the role of milk and dairy products for body weight control.Childhood overweight and obesity worldwide is a major contributor to the current obesity epidemic, and childhood obesity frequently tracks into adulthood ().